
CHLORFENVINPHOS
First draft prepared by
M.F.A. Wouters & P.H. van Hoeven-Arentzen
National Institute of Public Health and Environmental Protection,
Bilthoven, Netherlands
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Embryotoxicity and teratogenicity
Genotoxicity
Special studies
Skin and eye irritation and skin sensitization
Cholinesterase inhibition and related
pharmacological and neurotoxic effects
Hormone concentrations
Porphyrigenic potential
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Chlorfenvinphos was evaluated by the Joint Meeting in 1971
(Annex I, reference 16), which allocated an ADI of 0-0.002 mg/kg bw.
The technical material contains not less than 90% chlorfenvinphos
with an approximate E:Z isomer ratio of 1:8.6; a typical sample
contains 9.5% E and 82.3% Z isomer.
The compound was re-evaluated by the present Meeting as a
result of the CCPR periodic review programme. This monograph
summarizes the data received since the previous evaluation,
comprising short-term studies in mice and dogs, long-term studies in
mice and rats, a study of reproductive toxicity in rats and studies
on embryotoxicity and teratogenicity in rats and rabbits. It also
includes relevant studies from the previous monograph (Annex I,
reference 17).
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution and excretion
Six male and six female rats (strain not specified) given a
dose of 2 ppm of [14C-vinyl]-chlorfenvinphos (3.0 µCi/mg) excreted
52-77% of the radiolabel in urine within 24 h. An additional 6.1-26%
was excreted during the next 24 h. Faecal elimination comprised 11%
of the radiolabel, and a further 1.4% was excreted via the lungs
within 96 h, by which time the entire dose had been excreted. In the
same study, two male and two female dogs (strain not specified) fed
capsules containing 0.3 ppm [14C-vinyl]-chlorfenvinphos (8.0 µCi)
excreted 86% (83-91%) of the administered radiolabel in urine and
4.1% in faeces within 24 h (Hutson et al., 1967; Annex I,
reference 17).
After administration of 25-30 ppm to young rats (strain not
specified), unchanged chlorfenvinphos was detected in peripheral
blood. A dog that received 88 mg/kg in feed had similar
concentrations of unchanged chlorfenvinphos in peripheral blood
after a similar interval (Hutson & Hathway, 1967; Annex I, reference
17).
After intramuscular injection of a single 233-mg dose of
14C-chlorfenvinphos (647 µCi) into a lactating cow, only 0.2% of
the radiolabel appeared in the milk, mainly during the first two
milkings. Unchanged chlorfenvinphos represented 75% of the
radiolabel (Hunter, 1969a; Annex I, reference 17).
Oral administration of a single 12.5-mg dose of
14C-chlorfenvinphos (35.1 µCi) to an adult man resulted in rapid
elimination of radiolabel in the urine: 72% of the dose was excreted
within 4.5 h and 94% within 24 h (Hutson, 1969; Annex I, reference
17).
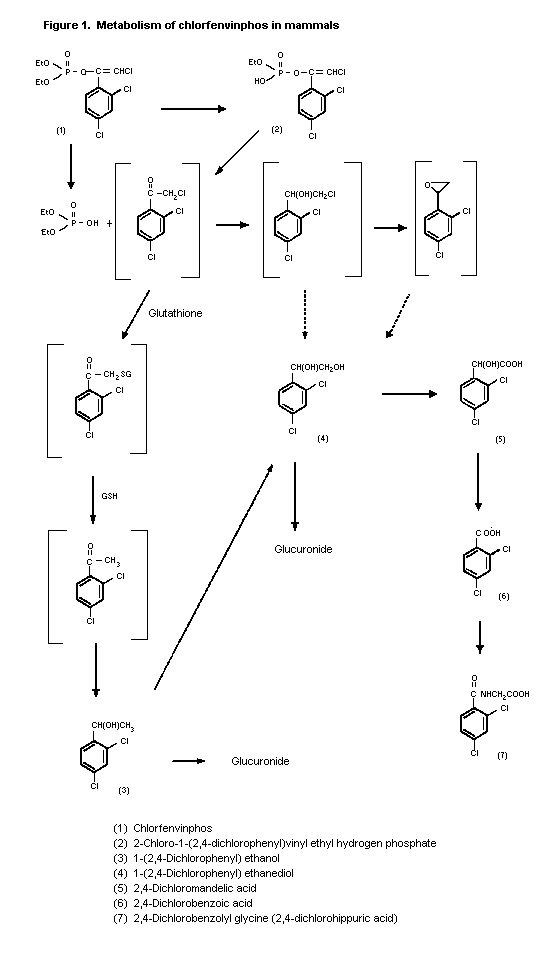
(b) Biotransformation
Oral administration of 2 ppm 14C-chlorfenvinphos to rats
(strain not specified) was followed by complete metabolism of the
compound. The urinary metabolites were 2-chloro-1-(2,4-
dichlorophenyl)vinylethyl hydrogen phosphate (32% of administered
radiolabel), [1-(2,4-dichlorophenyl)ethyl-ß-D-glucopyranosid]uronic
acid (41%), 2,4-dichloromandelic acid (7.0%),
2,4-dichlorophenylethanediol glucuronide (2.6%) and
2,4-dichlorohippuric acid (4.3%) (Hutson et al., 1967; Annex I,
reference 17).
14C-Chlorfenvinphos was also completely metabolized in dogs
(strain not specified) that received 0.26 ppm. The proportions of
urinary metabolites were as follows: 2-chloro-1-(2,4-
dichlorophenyl)vinylethyl hydrogen phosphate, 70%; [1-(2,4-
dichlorophenyl)ethyl-ß-D-glucopyranosid]uronic acid, 3.6%;
2,4-dichloromandelic acid, 13%; 2,4-dichlorophenyl-ethanediol
glucuronide, 2.7%. 2,4-Dichlorohippuric acid was not detected
(Hutson et al., 1967; Annex I, reference 17).
The milk of a lactating cow given an intramuscular injection of
0.58 mg/kg bw 14C-chlorfenvinphos contained 0.2% of the
radiolabel, of which 75% was unchanged chlorfenvinphos; there were
also small amounts of 2,4-dichloro-acetophenone,
1-(2,4-dichlorophenyl)ethanol and 2,4-dichloromandelic acid. The
urinary metabolites comprised 1-(2,4-dichlorophenyl)ethanol and
1-(2,4-dichlorophenyl)ethanediol. The glucuronides of these
compounds were not detected (Hunter, 1969a; Annex I, reference 17).
Five metabolites were identified in the urine of an adult man
following administration of a single oral dose of 12.5 mg
14C-chlorfenvinphos. These were
2-chloro-1-(2,4-dichlorophenyl)vinylethyl hydrogen phosphate, 24%;
2,4-dichloromandelic acid, 24%; [1-(2,4-dichlorophenyl)-ethyl-ß-D-
glucopyranosid]uronic acid, 2,4-dichlorophenylethanediol glucuronide
and 2,4-dichlorobenzoyl glycine (Hutson, 1969; Annex I, reference
17).
Pretreatment of CFE rats for 12 days with 200 ppm dieldrin, an
inducer of the monooxygenase system, afforded a 10-fold protection
against the acute toxic effects of chlorfenvinphos. At a dose of 2.5
mg/kg bw chlorfenvinphos, dieldrin pretreatment brought about
minimal changes in the metabolic profile; however, in rats treated
with 13 mg/kg bw chlorfenvinphos, animals that had not received
dieldrin pretreatment showed clinical signs of intoxication, while
the pretreated rats had a four-fold increase in the yield of
de-ethyl-chlorfenvinphos and more rapid urinary excretion (66%
compared with 16% without pretreatment during the first 12 h)
(Hutson & Wright, 1980).
The acute oral toxicity of chlorfenvinphos in Fischer 344 rats
was also reduced by pretreatment with chlorfenvinphos. Protection
was seen after 8 h and became maximal 24 h after oral pretreatment
at a dose of 15 mg/kg bw. A maximal three-fold increase in the
LD50 was seen, but there were no changes in the type of toxic
signs or time of death. The protection was related to a decrease in
the plasma concentration of chlorfenvinphos and to reduced
inhibition of brain cholinesterase. The same single oral
pretreatment increased chlorfenvinphos metabolism to 178% in the
hepatic microsomal fraction and increased various indicators of
cytochrome P450-related biotransormation activity. Pharmacokinetic
analysis of protection against the acute toxicity of succeeding
doses suggested that the decrease in plasma concentration was
correlated to the induction of the hepatic biotransformation and
possibly to a greater uptake of chlorfenvinphos by the liver and
reduced uptake by the intestines (Ikeda et al., 1990, 1991, 1992).
(c) Effects on enzymes and other biochemical parameters
The de-ethylation of radiolabelled chlorfenvinphos in vitro
was investigated in liver slices from mice, rats, rabbits and dogs.
In all of the preparations, chlorfenvinphos was converted to
2-chloro-1-(2,4-dichlorophenyl)vinylethyl hydrogen phosphate. The
catalysing enzyme was located in the microsomal fraction of liver
homogenates and was found to be dependent on NADPH and oxygen. The
mechanism of dealkylation involved hydroxylation of the alpha-carbon
atom of an alkyl group, which was removed as the corresponding
aldehyde. An inverse correlation was observed between the oral
LD50 and the rate of de-ethylation, the conversion rates being 1
in rats, 8 in mice, 24 in rabbits and 88 in dogs (Donninger et
al., 1972).
The rates of oxidative metabolism (O-de-ethylation) by liver
preparations from rats, rabbits and human beings in vitro have
also been compared. As in the study described above, rats had a much
lower conversion rate than rabbits. When the results were expressed
in terms of cytochrome P450, detoxification of chlorfenvinphos by
human liver enzymes was almost as effective as that by rabbit
enzymes (Hutson & Logan, 1986). A proposed metabolic pathway is
shown in Figure 1.
2. Toxicological studies
(a) Acute toxicity
A striking intra-species difference in acute toxicity is seen,
the oral LD50 values being 9.6-39 mg/kg bw for rats, 117-200 mg/kg
bw for mice, 125-250 mg/kg bw for guinea-pigs, 300-1000 mg/kg bw for
rabbits and > 12 000 mg/kg bw for dogs. The dermal LD50 was
30-108 mg/kg bw for rats and 412-4700 mg/kg bw for rabbits. The
symptoms recorded were typical of anticholinesterase activity (Annex
I, reference 17). In male and female Charles River CD rats, the oral
LD50 of 93.1% pure chlorfenvinphos was 31 mg/kg bw and the dermal
LD50 was 420 mg/kg bw. The effects observed included tremor,
fasciculation, salivation, splayed gait, unkempt appearance,
chromodacryorrhoea, piloerection, hunched posture, salivation,
hypothermia, tachypnoea and twitching (Gardner, 1992).
Pretreatment of rats (strain not specified) with 200 ppm
dieldrin (equivalent to 10 mg/kg bw per day) for 12 days afforded a
10-fold protection against the acute oral toxic effect of
chlorfenvinphos (Hutson & Wright, 1980).
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female NMRI mice were treated with
chlorfenvinphos (purity, 92.8%) at doses of 0, 1, 10, 100 or 1000
ppm in the diet (equal to 0.18, 1.9, 18 or 188 mg/kg bw per day for
males and 0.21, 2.1, 21 or 226 mg/kg bw per day for females) for 28
days. Treatment did not affect survival, body weight or
ophthalmoscopic end-points, and macroscopic and microscopic
examinations showed no effect. Females at the highest dose had a
slight but significant increase in food consumption during the last
two weeks of the study, but relative food consumption was not
significantly increased. The concentration of serum albumin was
slightly increased in males fed 100 or 1000 ppm, but the increase
was not related to dose; the total protein concentration was
increased in males fed 1000 ppm. Plasma cholinesterase activity was
inhibited in both males and females, by 18-19% in those fed 10 ppm,
by 79-82% in those treated with 100 ppm and by 94-95% in those at
1000 ppm. Erythrocyte cholinesterase activity was inhibited by 30%
in males fed 100 ppm and by 65-66% in males and females fed 1000
ppm. Brain cholinesterase activity was inhibited by 50-54% in
animals of each sex fed 1000 ppm; in females, activity was inhibited
by about 30% in those fed 100 ppm, by 20% in those fed 10 ppm and by
26% in those fed 1 ppm (not related to dose); in males, 8%
inhibition was seen in those fed 100 ppm and 22% in those fed 10
ppm. At 1000 ppm, males had increased relative weights of liver and
kidney, and females showed increased relative weights of liver and
heart. The NOAEL was 1 ppm, equal to 0.18 mg/kg bw per day (Tennekes
et al., 1991).
Dogs
In a range-finding study, groups of two male and two female
pure-bred beagle dogs were fed diets containing 0, 3, 100 or 3000
ppm chlorfenvinphos (equal to 0.12, 3.9 or 105 mg/kg bw per day) for
four weeks. No effect was seen on mortality, clinical signs, body
weight or ophthalmoscopic or haematological end-points; urinalysis
and macroscopic and microscopic examinations also showed no effect.
The food intake of two dogs fed 3000 ppm was reduced during the
first week of treatment and remained reduced in one dog
throughoutthestudy. In dogs fed 100 or 3000 ppm, a dose-related
reduction in plasma cholinesterase activity was observed; those fed
3000 ppm also had a reduction in erythrocyte cholinesterase activity
and a slight increase in liver weight (Allen et al., 1992).
Groups of four male and four female pure-bred beagle dogs were
fed diets containing 0, 3, 100 or 3000 ppm chlorfenvinphos (equal to
0.1, 2.8 or 92 mg/kg bw per day for males and 0.1, 3.1 or 93 mg/kg
bw per day for females) for 52 weeks. No effects were found on
mortality, clinical signs, ophthalmoscopic or haematological
parameters or brain cholinesterase activity; urinalysis and
macroscopic and microscopic examinations also showed no effect. Food
consumption of animals fed 3000 ppm was reduced during the first
four to five weeks, and body-weight loss was recorded for most of
these animals during the first five to six weeks of treatment;
however, both food consumption and body weight subsequently returned
to normal. In males fed this dose, the plasma magnesium
concentration was decreased at 52 weeks; in females, the plasma
chloride concentration was increased at 26 and 52 weeks, and albumin
was decreased at 4, 13 and 52 weeks. Plasma cholinesterase activity
was inhibited by 71-95% in dogs fed 100 or 3000 ppm, and the effect
was dose-related. In animals fed 3 ppm, a significant 31% reduction
in plasma cholinesterase activity was observed in males at 52 weeks
and a 7-10% reduction in females at weeks 8-13. Erythrocyte
cholinesterase activity was markedly reduced (75-83%) in animals of
each sex fed 3000 ppm from week 4 onwards. At this dose, the
relative adrenal weight was increased in males and the relative
thyroid weight was increased in females. The NOAEL in this study was
100 ppm, equal to 2.8 mg/kg bw per day (Allen et al., 1993).
Figure 1. Metabolism of chlorfenvinphos in mammals
 (c) Long-term toxicity and carcinogenicity
Mice
In a study designed to determine carcinogenic potential,
chlorfenvinphos (purity, 92.8%) was administered for 24 months to
groups of 74 male and 74 female NMRI mice at 1, 25 or 625 ppm in the
diet, equal to 0.15, 3.7 or 93 mg/kg bw per day for males and to
0.2, 5.0 or 119 mg/kg bw per day for females. Twelve mice of each
sex from each group were sacrificed after 52 weeks of treatment; all
females were killed after 90 weeks of treatment and all males after
104 weeks. Clinical signs, food consumption, body weight,
differential leukocyte count, cholinesterase activity and organ
weights were observed, and macroscopic and microscopic examinations
were undertaken. Plasma cholinesterase activity was inhibited in
animals of each sex fed 25 ppm (38-50% inhibition) or 625 ppm
(87-96%). In males and females fed 625 ppm, erythrocyte
cholinesterase activity was decreased by 18-66% and brain
cholinesterase activity by 25-46%. Microscopic examination at
termination of the experiment showed changes in the adrenal cortex
of males fed 625 ppm, which included increased incidence and
intensity of ceroid pigment and focal hypertrophy and increased
severity of nodular hyperplasia. Tumour incidence was not enhanced.
No effects were observed at 25 ppm, equal to 3.7 mg/kg bw per day
(Schmid et al., 1993).
Rats
In a study of both chronic toxicity and carcinogenicity, groups
of 50 male and 50 female Wistar rats were fed chlorfenvinphos
(purity, 90.5%) at 0.3, 1, 3 or 30 ppm in the diet (equivalent to
0.015, 0.05, 0.15 or 1.5 mg/kg bw per day) for 24 months. The
control group consisted of 100 rats of each sex. Clinical signs,
body weight and food consumption were observed. At termination,
organ weights, haematological parameters and the results of clinical
chemistry, urinalysis and macroscopic and microscopic examinations
were recorded. Additional groups of 12 rats of each sex were
examined after 6, 12 and 18 months for haematological and clinical
chemical parameters including cholinesterase activity; after
sacrifice, the organs were weighed and animals fed 0, 0.3 or 30 ppm
chlorfenvinphos were examined macroscopically and histologically.
Plasma cholinesterase activity was inhibited after six months in all
treated animals but thereafter only in rats fed 3 or 30 ppm. In
males and females fed 30 ppm, erythrocyte cholinesterase activity
was inhibited by about 60% and brain cholinesterase activity by
about 45%; erythrocyte cholinesterase activity was inhibited by
about 17% in females fed 1 or 3 ppm after 12 months' exposure. In
all groups and at all intervals during the study, minor but
significant changes were seen in the concentrations of alpha-, ß-
and gamma-globulins. No dose-related or significant macroscopic or
histological changes were observed, and tumour incidences were not
enhanced. The NOAEL in this study was 3 ppm, equivalent to 0.15
mg/kg bw per day (Pickering, 1980).
(d) Reproductive toxicity
Rats
In a range-finding study, 10 male and 10 female Wistar rats
were exposed to 0, 5, 25 or 125 ppm chlorfenvinphos (purity, 92.8%)
mixed with microgranulated feed (equivalent to 0.25, 1.3 or 6.3
mg/kg bw per day) during a three-week period before mating and
throughout mating, gestation and lactation. After they had been
weaned, F1 pups were reared for a further week on their test
diets. The body-weight gain of males and females fed 125 ppm was
slightly reduced. Inhibition of erythrocyte and brain cholinesterase
activity was observed in females fed 5, 25 or 125 ppm and in males
fed 25 or 125 ppm. Plasma cholinesterase activity was inhibited only
in females. Reproductive parameters, including mating, gestation,
parturition, pregnancy rate, lactation and litter characteristics,
were not affected (Dotti et al., 1993a).
In a two-generation study of reproductive toxicity, groups of
25 male and 25 female Wistar rats were treated with 0, 1, 10 or 100
ppm chlorfenvinphos (purity, 92.8%) mixed with microgranulated diet,
equivalent to 0, 0.05, 0.5 or 5 mg/kg bw per day. The diets were fed
to the parental generation during growth, mating, gestation and
lactation and to one litter per generation. Clinical signs, body
weight, food consumption, cholinesterase activity (only in the
parent generation), mating performance and reproductive parameters
were observed. The parents were examined histologically; the pups
were sexed and examined for gross malformations, and the numbers of
stillborn and live pups were recorded. One male fed 100 ppm died on
day 10, and reduced body-weight gain and food consumption were noted
in males and females of the parental and F1 generations at this
dose. A slight decrease in food consumption was observed in F1
males and parental and F1 females fed 10 ppm. Plasma
cholinesterase activity was decreased by 14-78% in males and females
fed 10 or 100 ppm, and erythrocyte cholinesterase activation was
inhibited by 35-63% in males and females fed 100 ppm. Brain
cholinesterase activity was decreased by 32% in males and 38% in
females fed 100 ppm and by 17% in females fed 10 ppm. In the
parental generation, post-implantation loss was higher in animals
fed 10 or 100 ppm than in controls, and postnatal loss was increased
in rats fed 100 ppm; breeding loss up to the end of lactation was
higher in animals fed 10 or 100 ppm. In the F1 generation,
breeding loss was higher than that in controls in animals fed 100
ppm. At this dose, retardation of body-weight gain was noted in both
F1 and F2 pups during the lactation period. The NOAEL in this
study was 1 ppm, equivalent to 0.05 mg/kg bw per day (Dotti et
al., 1993b).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 25 pregnant specific-pathogen-free CrL:COBS CD(SD)BR
rats were treated by gavage with chlorfenvinphos (purity, > 90%) at
0, 0.3, 1 or 3 mg/kg bw per day in corn oil on days 6-15 of
pregnancy. The doses were determined in a preliminary study in which
doses of 3, 6 and 10 mg/kg bw per day caused dose-related loss of
body weight. The rats were sacrificed on day 20 of gestation. No
effects were found on food consumption, pregnancy rate or litter
parameters, including numbers of fetal deaths, implants, corpora
lutea and pre- and post-implantation losses, litter weight, fetal
weight and gravid uteri, or on the incidences of malformations,
visceral and skeletal anomalies and variations. The only clinical
effect seen was the occurrence of small faeces in animals fed 3
mg/kg bw per day from days 3-4 until the end of treatment;
body-weight gain was slightly decreased in animals fed 3 mg/kg bw
per day on days 6-20. There was no indication of irreversible
structural effects. The NOAEL for maternal toxicity in this study
was 1 mg/kg bw per day, and that for developmental toxicity was 3
mg/kg bw per day (Mayfield & John, 1986).
Rabbits
Pregnant banded Dutch rabbits (21-22 per group) received three
capsules containing chlorfenvinphos (purity not specified) at 25, 50
or 100 mg/kg bw per day in corn oil (total volume, 200 µl) on days
6-18 of gestation. A control group of 32 females was used. The
rabbits were sacrificed on day 28 of gestation. No effects were
found on mortality, clinical signs or mean numbers of corpora lutea,
implantations, resorptions or fetal deaths. Body-weight gain and
food consumption were reduced in dams fed 100 mg/kg bw per day on
days 6-12 of gestation. Plasma and erythrocyte cholinesterase
activities were decreased in dams at all doses, erythrocyte activity
being decreased by 55-75%. Brain cholinesterase activity was not
measured. Pre-implantation losses occurred in dams fed 25 or 100
mg/kg bw per day. No differences in the incidences of visceral or
skeletal malformations or variations between the groups were
observed that could be attributed to treatment. There was no
indication of irreversible structural effects. No NOAEL could be
established for maternal toxicity; the NOAEL for embryo- and
fetotoxicity was 100 mg/kg bw per day (Dix et al., 1979).
In a limited experiment with rats, rabbits and hamsters, doses
of 50 mg/kg bw per day and higher increased the incidence of open
eyes and oedema in hamster dams and decreased the weight and length
of hamster fetuses (Dzierzawski & Minta, 1979).
(f) Genotoxicity
The results of tests for the genotoxicity of chlorfenvinphos
are summarized in Table 1.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Chlorfenvinphos (purity, 93.1%), administered to three New
Zealand white rabbits as a 4-h semi-occluded topical application of
0.5 ml undiluted compound, caused very slight erythema within 1 h
after removal of the dressing. The erythema persisted for at least
48 h after treatment, but all overt effects disappeared within seven
days. The compound was considered slightly irritating to rabbit skin
(Gardner, 1992).
The skin sensitizing potential of chlorfenvinphos was examined
in two studies in guinea-pigs. In the first study, 10 male and 10
female Tunstall breeding unit 'P' guinea-pigs received an
intradermal injection of 0.5% (mol/volume) chlorfinvenphos (purity,
91.3%) during the induction period, a topical application of the
undiluted test compound one week later and a challenge application
of undiluted compound two weeks after that. A control group of five
male and five female animals was used. No effect was seen in the
test animals 24 or 48 h after removal of the challenge patches
(Rose, 1982).
In the second study, 10 male and 10 female Dunkin-Hartley
guinea-pigs were given an intradermal induction dose of 2%
(mol/volume) chlorfenvinphos (purity, 93.1%) and undiluted compound
for the topical and challenge applications. A control group of five
male and five female animals was used. Ten of 20 test animals showed
a positive response after 24 h and nine of the same animals after 48
h. None of the control animals responded (Gardner, 1992).
Instillation of 0.1 ml undiluted chlorfenvinphos (purity,
93.1%) into the eyes of three New Zealand white rabbits caused
marked constriction of the pupil, slight conjunctival redness,
slight chemosis and ocular discharge. All signs were reversed within
24 h. The compound is not considered to irritate the eye (Gardner,
1992).
Table 1. Results of tests for the genotoxicity of chlorfenvinphos
End-point Test system Concentration Purity Results Reference
of chlorfenvinphos (%)
In vitro
Reverse mutation S. typhimurium TA98, 25-2025 µg/platea NR Negativeb,c Arni & Müller, 1979
100, 1535, 1537
Reverse mutation E. coli WP2, WP2 hcr, 250 µg/plate NR Negatived Shirasu et al., 1976
S. typhimurium TA1535,
1536, 1537, 1538
Reverse mutation S. typhimurium TA98, Up to 5000 µg/plate NR Negativec,d Moriya et al., 1983
100, 1535, 1537, 1538
Reverse mutation S. typimurium TA100 Up to 5000 µg/plate NR Positivec,e Moriya et al., 1983
Reverse mutation S. typhimurium TA98, Up to 5000 µg/plate NR Negativec,e Moriya et al., 1983
1535, 1537, 1538
Reverse mutation S. typhimurium TA98, 0.2-2000 µg/plate 90.5 Negativeb,c Brooks, 1978
100, 1538
Reverse mutation E. coli B/r WP2 NR > 99 Negativec,d Dean, 1971
Reverse mutation E. coli WP2, WP2 uvrA 0.2-2000 g/plate 90.5 Negativec Brooks, 1978
Reverse mutation E. coli WP2 hcr Up to 5000 µg/plate NR Negativeb,c Moriya et al., 1983
Mitotic gene Saccharomyces 0.001-5.0 mg/ml; toxic 90.5 Negativec,d Brooks, 1978
conversion cerevisiae JD1 at 0.5, 1.0, 5.0 mg/ml
Chromosomal Human lymphocytes 18.8-300 µg/platea,d 93.1 Negativeb,c Strasser & Arni,
aberrations 15.6-250 µg/platea,e 1988
Table 1 (contd)
End-point Test system Concentration Purity Results Reference
of chlorfenvinphos (%)
In vivo
Chromosomal Chinese hamster 25 or 50 mg/kg bw 90.5 Negativec Dean, 1978
aberrations bone marrow per day for 2 days
Dominant lethal CD1 mice (male) 10, 20 or 40 mg/kg bw 90.5 Negativec Dean & Hend, 1978
mutation in DMSO
NR, nor reported; DMSO, dimethyl sulfoxide
a It is not clear whether chlorfenvinphos was tested: the substance was coded C8949.
b In the presence and absence of metabolic activation
c Positive controls were used.
d In the absence of metabolic activation
e In the presence of metabolic activation
(ii) Cholinesterase inhibition and related pharmacological
and neurotoxic effects
After oral administration of chlorfenvinphos at 15 mg/kg bw to
Fischer 344 rats, brain cholinesterase activity was maximally
inhibited (to about 20% of the control value) at 4 h; the inhibition
lasted more than 24 h after administration (Ikeda et al., 1990).
Groups of 10 male and 10 female Wistar rats were fed dietary
concentrations of 0, 0.3, 1, 3 or 30 ppm chlorfenvinphos for four
weeks, or received the same treatments and then received control
diet for four weeks. Administration of 30 ppm resulted in decreased
activity of plasma, erythrocyte and brain cholinesterase. Feeding of
the control diet for four weeks reversed the inhibition of plasma,
erythrocyte and brain cholinesterase activities, resulting in values
of 100, 88 and 91%, respectively (Pickering, 1978).
In a comparison of the lethality of chlorfenvinphos after
intravenous and oral administration, Sprague-Dawley rats given
lethal doses by either route showed typical signs of
anticholinesterase poisoning and marked reductions in brain and
erythrocyte cholinesterase activity. Anaesthetized and conscious
rats administered chlorfenvinphos intravenously had hypertension and
apnoea. After oral administration, breathing stopped before
cessation of heart beats (Takahashi et al., 1991).
The effects of chlorfenvinphos on spontaneous
electroencephalographic, electromyo-graphic and cholinesterase
activity in brain and red blood cells was examined in male Wistar
rats. Single oral doses of 1 mg/kg bw had no effect. Doses over 2
mg/kg bw induced dose-related inhibition of the cholinesterase
activity in brain and erythrocytes. Maximal inhibition of brain
cholinesterase activity was seen 3 h after treatment and lasted for
more than 72 h. Electroencephalography showed a prominent arousal
pattern, and the appearance of slow-wave sleep and para-sleep was
markedly depressed. The duration of the arousal pattern was
proportional to the dose; however, the awake-sleep cycle returned to
the control rate on the second day, and an increase in para-sleep
occurred on the third day (Osumi et al., 1975).
In 30 Sprague-Dawley rats fed a diet containing 150 ppm
chlorfenvinphos (equivalent to 7.5 mg/kg bw per day) for three
months, no effect was seen on the amplitude of the muscular response
to nerve stimulation. Electrophysiological effects were signalled
firstly by a prolonged negative potential after the direct muscle
response to a single nerve impulse and secondly by repetitive
activity. These abnormalities became more marked with time. Double
and repetitive stimulation reduced or abolished the prolonged
negative potential and repetitive activity (Maxwell & LeQuesne,
1982).
Oral administration to male Wistar rats of chlorfenvinphos at
50% of the LD50 caused maximal inhibition of brain cholinesterase
activity (to about 15% of the control value) between 2 and 3 h after
treatment. A significant reduction in brain norepinephrine level was
seen within 15 min, but no consistent diminution was seen
subsequently. During exposure at 5% of the LD50 for 12 weeks,
brain cholinesterase activity was inhibited to 60-80% of control
values and brain norepinephrine content was significantly decreased,
with maximal depletion at week 8 (Brzezinski & Wysocka-Paraszewska,
1980).
Cholinesterase activity in plasma, erythrocytes and different
parts of the brain, open-field behaviour and response to change in a
'T' maze were investigated in two groups of male Wistar rats after
administration of a single intraperitoneal injection of 1 or 3 mg/kg
bw chlorfenvinphos. Cholinesterase activity was strongly inhibited,
to a similar degree, in the blood and brain, with a mean inhibition
of 80% in rats at the high dose and 50% in those at the low dose 3 h
after treatment. Reversal of the inhibition proceeded at similar
rates in the blood and brain and was complete within 96 h with the
low dose and within 14 days with the high dose. No changes in
negotiating the 'T' maze that suggested impairment of short-term
memory were observed up to 14 days after treatment. In the
open-field test, a decrease in short-term memory was manifested by
the absence of increased locomotor and exploratory activity in
response to a new object, only in rats administered 3 mg/kg bw. This
finding suggests that behavioural disturbances last longer than
recovery of cholinesterase activity (Socko et al., 1989).
(iii) Hormone concentrations
In Wistar rats given chlorfenvinphos as a single oral dose of
6.15 mg/kg bw, a significant increase in corticosterone
concentration was seen after 1 and 3 h and in aldosterone
concentration 1-6 h after treatment. Plasma corticosteroid levels
were maximal within 1 h, when brain cholinesterase activity was only
slightly inhibited (Osicka-Koprowska et al., 1984).
(iv) Porphyrigenic potential
Chlorfenvinphos induced porphyria in primary cultures of
chicken embryo liver cells. Its porphyrigenic potential was markedly
increased by prior treatment of the cells with a compound that
induces drug metabolizing enzymes (Koeman et al., 1980).
3. Observations in humans
Workers in India were studied while applying chlorfenvinphos to
paddy rice over a period of six days; they were not wearing
protective clothing. Workers applying an emulsifiable concentrate
had decreased plasma cholinesterase activity but no change in
erythrocyte cholinesterase activity. Workers applying granules
containing 10% chlorfenvinphos had no reduction in cholinesterase
activity (Blok et al., 1977).
A group of 33 workers employed in a chlorfenvinphos
manufacturing plant were monitored medically for eight years
(1967-75). Two control groups were used. The exposed group had
slightly lower haemoglobin levels and slightly lower cholinesterase
activity in plasma and erythrocytes. Some changes in
neurophysiological parameters (lower electromyographic voltages,
associated with slower conduction velocities) were observed, but
these were reversed when the workers were removed from exposure
(Ottevanger, 1976).
Workers involved in the manufacture of chlorfenvinphos were
monitored in 1985-86 in a routine programme in which blood and urine
were collected and cholinesterase activity was measured in whole
blood, plasma and erythrocytes. A few cases of depressed plasma
cholinesterase were recorded, which were not, however, correlated
with increases in the urinary concentrations of diethylphosphate and
monoethylphosphate metabolites (Eadsforth, 1987).
Comments
The main step in the biotransformation of chlorfenvinphos is
detoxification by de-ethylation into the corresponding
phosphodiester; microsomal enzymes play an important role.
Interspecies differences in the rate of metabolism of
chlorfenvinphos were found in vitro. Oxidative metabolism by human
liver enzymes was comparable to that of rabbit liver enzymes.
Single oral doses of chlorfenvinphos were very toxic to rats,
toxic to mice and guinea-pigs and moderately to slightly toxic to
rabbits and dogs. The clinical signs observed were consistent with
cholinesterase inhibition and included tremors, fasciculation and
salivation. Pretreatment with enzyme inducers can increase the acute
oral LD50. Interspecies differences in the acute oral LD50
reflect the activity of the hepatic metabolizing enzymes involved in
the detoxification of chlorfenvinphos. WHO (1992) has classified
chlorfenvinphos as extremely hazardous.
In a four-week study in which mice were fed 0, 1, 10, 100 or
1000 ppm chlorfenvinphos in the diet, plasma and erythrocyte
cholinesterase activities were inhibited in animals fed 100 or 1000
ppm. Brain cholinesterase activity was inhibited in males fed 10 or
1000 ppm and in females at all doses; thus, no NOAEL could be
established for female mice. The NOAEL in males was 1 ppm, equal to
0.18 mg/kg bw per day.
In a one-year study in which dogs were fed 0, 3, 100 or 3000
ppm in the diet, inhibition of erythrocyte cholinesterase activity
and increased relative adrenal weight were seen in males; in
females, increased relative thyroid weight was seen in those fed the
highest dose. The NOAEL was 100 ppm, equal to 2.8 mg/kg bw per day.
In a study of the carcinogenicity of chlorfenvinphos, in which
mice were fed 0, 1, 25 or 625 ppm in the diet, no increase in tumour
incidence was observed. Erythrocyte and brain cholinesterase
activity was inhibited in mice fed the highest dose. The NOAEL was
25 ppm, equal to 3.7 mg/kg bw per day.
In a two-year study of toxicity and carcinogenicity in rats fed
diets containing 0, 0.3, 1, 3 or 30 ppm, erythrocyte and brain
cholinesterase activity was inhibited in those fed 30 ppm. There was
no evidence of carcinogenicity. The NOAEL was 3 ppm, equivalent to
0.15 mg/kg bw per day.
In a two-generation study of reproductive toxicity in rats fed
diets containing 0, 1, 10 or 100 ppm, reduced brain cholinesterase
activity, post-implantation loss and breeding losses were observed
in animals fed 10 or 100 ppm. The NOAEL was 1 ppm, equivalent to
0.05 mg/kg bw per day.
In two studies of teratogenicity--one in rats and one in
rabbits--doses that were maternally toxic were not embryotoxic and
there was no indication of teratogenicity. In rats, the NOAEL was 1
mg/kg bw per day for maternal toxicity and 3 mg/kg bw per day, the
highest dose tested, for developmental toxicity. In rabbits, no
NOAEL could be established for maternal toxicity (< 25 mg/kg bw);
the NOAEL for developmental toxicity was > 100 mg/kg bw per day.
Chlorfenvinphos was mutagenic in only one study, in Salmonella
typhimurium TA100 in the presence of an exogenous metabolic system
at doses of at least 1000 µg per plate. It was inactive in other
bacterial tests and did not induce mitotic conversion in yeast or
chromosomal aberrations in human lymphocytes in vitro.
Chlorfenvinphos did not induce chromosomal aberrations in Chinese
hamster bone-marrow cells or dominant lethal effects in male mice
in vivo. The Meeting concluded that chlorfenvinphos was not
genotoxic.
Delayed neurotoxicity in chickens has not been evaluated.
Observations in humans were not suitable for use in estimating
an ADI.
An ADI was established on the basis of an NOAEL of 0.05 mg/kg
bw per day in a two-generation study of reproductive toxicity in
rats and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 3.7 mg/kg bw per day (two-year study of
carcinogenicity)
Rat: 3 ppm, equivalent to 0.15 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
1 ppm, equivalent to 0.05 mg/kg bw per day (two-generation
study of reproductive toxicity)
1 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Rabbit: < 25 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Dog: 100 ppm, equal to 2.8 mg/kg bw (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Study of delayed neurotoxicity in chickens, with estimation of
neuropathy target esterase
2. Further observations in humans
3. Studies of pharmacokinetics in mammals in vivo
References
Allen, T.R., Corney, S.J., Frei, T., Luetkemeier, H., Biedermann, K.
& Springall, C.J. (1992) 4-Week oral range-finding toxicity
(feeding) study with chlorfenvinphos in the dog. RCC Project No.
271934. Unpublished report from Research and Consulting Co.,
Itingen, Switzerland. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Allen, T.R., Corney, S.J., Frei, T., Luetkemeier, H., Biedermann, K.
& Springall, C.J. (1993) 52-Week oral toxicity (feeding) study with
chlorfenvinphos in the dog. RCC Project No. 271945. Unpublished
report from Research and Consulting Co., Itingen, Switzerland.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Arni, P. & Müller, D. (1979) Salmonella/mammalian microsome
mutagenicity test with C8949. Test for mutagenic properties in
bacteria. Experiment no. 78/2589. Unpublished report from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Blok, A.C., Mann, A.H. & Robinson, J. (1977) Organophosphorus
insecticide exposure of sprayers under field conditions in rice in
India. I. Birlane (chlorfenvinphos). TOX 77-005. Unpublished report
from Shell International Research Maatschappy, Toxicology Division.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Brooks, T.M. (1978) Toxicity studies with chlorfenvinphos:
mutagenicity studies with chlorfenvinphos in micro-organisms. Group
Research Report TLGR.00142.78. Unpublished report from Shell
Research Ltd, London. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Brzezinski, J. & Wysocka-Paruszewska, B. (1980) Neurochemical
alterations in rat brain as a test for studying the neurotoxicity of
organophosphorus insecticides. Arch. Toxicol., Suppl. 4, 475-478.
Dean, B.J. (1971) The mutagenic effect of organophosphate
insecticides on Escherichia coli. Group Research Report
TLGR.0034.71. Unpublished report from Shell Research Ltd, London.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Dean, B.J. (1978) Toxicity studies with chlorfenvinphos: chromosome
studies on bone marrow cells of Chinese hamsters after two daily
oral doses of chlorfenvinphos. Group Research Report TLGR.0087.78.
Unpublished report from Shell Research Ltd, London. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Dean, B.J. & Hend, R.W. (1978) Toxicity studies with
chlorfenvinphos: dominant lethal assay in male mice after single
oral doses of chlorfenvinphos. Group Research Report TLGR.0063.78.
Unpublished report from Shell Research Ltd, London. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Dix, K.M., Cassidy, S.L. & Vilkauls, J. (1979) Toxicity of
chlorfenvinphos: teratological studies in rabbits given
chlorfenvinphos orally. Group Research Report TLGR.79.105.
Unpublished report from Shell Research Ltd, London. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Donninger, C., Hutson, D.H. & Pickering, B.A. (1972) The oxidative
dealkylation of insecticidal phosphoric acid triesters by mammalian
liver enzymes. Biochem. J., 126, 701-707.
Dotti, A., Kinder, J., Luetkemeier, H. & Biedermann, K. (1993a)
Chlorfenvinphos. Preliminary study to the two-generation
reproduction study in the rat. RCC Project No. 271822. Unpublished
report from Research and Consulting Co., Itingen Switzerland.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Dotti, A., Kinder, J., Luetkemeier, H. & Biedermann, K. (1993b) Two
generation reproduction study with chlorvinphos in the rat. RCC
Project No. 271833. Unpublished report from Research and Consulting
Co., Itingen, Switzerland. Submitted to WHO by American Cyanamid
Co., Princeton, NJ, USA.
Dzierzawski, A. & Minta, M. (1979) Embryotoxic effects of
chlorfenvinphos and bromfenvinphos in laboratory animals. Bull.
Vet. Inst. Pulawy, 23, 1-2, 32-42.
Eadsforth, C.V. (1987) Biological monitoring of exposure of
operators during manufacture of Birlane in CAS, location CMDU-N,
during 1985-6. Unpublished report from Shell Biomedical Laboratory,
SNR/SNC Pernis. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Gardner, J. (1992) Birlane technical: acute oral and dermal dermal
toxicity in rat, skin and eye irritancy in rabbit and skin
sensitization potential in guinea pig. Unpublished report from Shell
Research Ltd, Sittingbourne Research Centre, Sittingbourne, United
Kingdom. Submitted to WHO by American Cyanamid Co., Princeton, NJ,
USA.
Hutson, D.H. & Wright, A.S. (1980) The effect of hepatic microsomal
monooxygenase induction on the metabolism and toxicity of the
organophosphorus insecticide chlorfenvinphos. Chem. Biol.
Interactions, 31, 93-101.
Hutson, D.H. & Logan, C.J. (1986) Detoxification of the
organophosphorus insecticide chlorfenvinphos by rat, rabbit and
human liver enzymes. Xenobiotica, 16, 87-93.
Ikeda, T., Kojima, T., Yoshida, M., Takahashi, H., Tsuda, S. &
Shirasu, Y. (1990) Pretreatment of rats with an organophosphorus
insecticide, chlorfenvinphos, protects against subsequent challenge
with the same compound. Fundam. Appl. Toxicol., 14, 560-567.
Ikeda, T., Tsuda, S. & Shirasu, Y. (1991) Metabolic induction of the
hepatic cytochrome P450 system by chlorfenvinphos in rats. Fundam.
Appl. Toxicol., 17, 361-367.
Ikeda, T., Tsuda, S. & Shirasu, Y. (1992) Pharmacokinetic analysis
of protection by organophosphorus insecticide, chlorfenvinphos,
against the toxicity of its succeeding dosage in rats. Fundam.
Appl. Toxicol., 18, 299-306.
Koeman, J.H., Debets, F.M.H. & Strik, J.J.T.W.A. (1980) The
porphyrogenic potential of pesticides with special emphasis on
organophosphorus compounds. In: Tordoir, W.F. & van Heemstra,
E.A.H., eds, Field Worker Exposure during Pesticide Application,
Amsterdam, Elsevier Scientific Publishing Co., pp. 157-162.
Maxwell, J.C. & LeQuesne, P.M. (1982) Neuromuscular effects of
chronic administration of two organophosphorus insecticides to rats.
Neurotoxicology, 3, 1-10.
Mayfield, R. & John, D.M. (1986) Effect of Birlane on pregnancy of
the rat. HCR Report No. SLL 84/851593. Unpublished report from
Huntingdon Research Centre, . Submitted to WHO by American Cyanamid
Co., Princeton, NJ, USA.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. &
Shirasu, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116, 185-216.
Osicka-Koprowska, A., Lipska, M. & Wysocka-Paruszewska, B. (1984)
Effects of chlorfenvinphos on plasma corticosterone and aldosterone
levels in rats. Arch. Toxicol., 55, 68-69.
Osumi, Y., Fujiwara, H., Oishi, R. & Takaori, S. (1975) Central
cholinergic activation by chlorfenvinphos, an organophosphate, in
the rat. Jpn. J. Pharmacol., 25, 47-54.
Ottevanger, C.F. (1976) An epidemiological and toxicological study
of occupational exposure to an organphosphorus pesticide. University
of Amsterdam, MD Thesis. Rotterdam, Phoenix & den Oudsten.
Pickering, C.E. (1978) Toxicity of chlorfenvinphos: the
reversibility of cholinesterase and aliesterase inhibition produced
by feeding chlorfenvinphos. Unpublished report from Shell Research,
TLGR0169.78, Sittingbourne United Kingdom. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA
Pickering, R.G. (1980) Toxicity studies on the insecticide
chlorfenvinphos. A two year feeding study in rats. Budget ref.
50070712. Unpublished report from Shell Research Ltd, London.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Rose, G.P. (1982) Toxicology of Birlane: the skin sensitizing
potential of Birlane. Project No. 226/82. Unpublished report from
Shell Research Ltd, Sittingbourne Research Centre, Sittingbourne,
United Kingdom. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Schmid, H., Probst, D., Luetkemeier, H., Weber, K., Wilson, J.T.,
Springall, C.J. & Biedermann, K. (1993) Oncogenicity (feeding) study
with chlorfenvinphos in the mouse. Final Report. RCC Project No.
265926. Unpublished report from Research and Consulting Co.,
Itingen, Switzerland. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening system of pesticides in the microbial system.
Mutat. Res., 40, 19-30.
Socko, R., Gralewicz, S. & Gorny, R. (1989) Neurotoxicity of
chlorphenvinphos, an organo-phosphorus pesticide: effects on blood
and brain cholinesterase activity, open field, behavior and
response-to-change in a 'T' maze in rats. Polish J. Occup. Med.,
2, 294-308.
Strasser, F. & Arni, P. (1988) Chromosome studies on human
lymphocytes. Test material C8949 tech. Test no.: 871201. Unpublished
report from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Takahashi, H., Kojima, T., Ikeda T., Tsuda S. & Shirasu Y. (1991)
Differences in the mode of lethality produced through intravenous
and oral administration of organophosphorus insecticides in rats.
Fundam. Appl. Toxicol., 16, 459-468.
Tennekes, H., Janiak, T., Stucki, H.P., Probst, D., Luetkemeier, H.,
Vogel, O., Schlotke, B., Biedermann, K. & Heusner, W. (1991) 28-Day
range-finding (feeding) study with chlorfenvinphos in the mouse. RCC
Project No. 243202. Unpublished report from Research and Consulting
Co., Itingen, Switzerland. Submitted to WHO by American Cyanamid
Co., Princeton, NJ, USA.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
(c) Long-term toxicity and carcinogenicity
Mice
In a study designed to determine carcinogenic potential,
chlorfenvinphos (purity, 92.8%) was administered for 24 months to
groups of 74 male and 74 female NMRI mice at 1, 25 or 625 ppm in the
diet, equal to 0.15, 3.7 or 93 mg/kg bw per day for males and to
0.2, 5.0 or 119 mg/kg bw per day for females. Twelve mice of each
sex from each group were sacrificed after 52 weeks of treatment; all
females were killed after 90 weeks of treatment and all males after
104 weeks. Clinical signs, food consumption, body weight,
differential leukocyte count, cholinesterase activity and organ
weights were observed, and macroscopic and microscopic examinations
were undertaken. Plasma cholinesterase activity was inhibited in
animals of each sex fed 25 ppm (38-50% inhibition) or 625 ppm
(87-96%). In males and females fed 625 ppm, erythrocyte
cholinesterase activity was decreased by 18-66% and brain
cholinesterase activity by 25-46%. Microscopic examination at
termination of the experiment showed changes in the adrenal cortex
of males fed 625 ppm, which included increased incidence and
intensity of ceroid pigment and focal hypertrophy and increased
severity of nodular hyperplasia. Tumour incidence was not enhanced.
No effects were observed at 25 ppm, equal to 3.7 mg/kg bw per day
(Schmid et al., 1993).
Rats
In a study of both chronic toxicity and carcinogenicity, groups
of 50 male and 50 female Wistar rats were fed chlorfenvinphos
(purity, 90.5%) at 0.3, 1, 3 or 30 ppm in the diet (equivalent to
0.015, 0.05, 0.15 or 1.5 mg/kg bw per day) for 24 months. The
control group consisted of 100 rats of each sex. Clinical signs,
body weight and food consumption were observed. At termination,
organ weights, haematological parameters and the results of clinical
chemistry, urinalysis and macroscopic and microscopic examinations
were recorded. Additional groups of 12 rats of each sex were
examined after 6, 12 and 18 months for haematological and clinical
chemical parameters including cholinesterase activity; after
sacrifice, the organs were weighed and animals fed 0, 0.3 or 30 ppm
chlorfenvinphos were examined macroscopically and histologically.
Plasma cholinesterase activity was inhibited after six months in all
treated animals but thereafter only in rats fed 3 or 30 ppm. In
males and females fed 30 ppm, erythrocyte cholinesterase activity
was inhibited by about 60% and brain cholinesterase activity by
about 45%; erythrocyte cholinesterase activity was inhibited by
about 17% in females fed 1 or 3 ppm after 12 months' exposure. In
all groups and at all intervals during the study, minor but
significant changes were seen in the concentrations of alpha-, ß-
and gamma-globulins. No dose-related or significant macroscopic or
histological changes were observed, and tumour incidences were not
enhanced. The NOAEL in this study was 3 ppm, equivalent to 0.15
mg/kg bw per day (Pickering, 1980).
(d) Reproductive toxicity
Rats
In a range-finding study, 10 male and 10 female Wistar rats
were exposed to 0, 5, 25 or 125 ppm chlorfenvinphos (purity, 92.8%)
mixed with microgranulated feed (equivalent to 0.25, 1.3 or 6.3
mg/kg bw per day) during a three-week period before mating and
throughout mating, gestation and lactation. After they had been
weaned, F1 pups were reared for a further week on their test
diets. The body-weight gain of males and females fed 125 ppm was
slightly reduced. Inhibition of erythrocyte and brain cholinesterase
activity was observed in females fed 5, 25 or 125 ppm and in males
fed 25 or 125 ppm. Plasma cholinesterase activity was inhibited only
in females. Reproductive parameters, including mating, gestation,
parturition, pregnancy rate, lactation and litter characteristics,
were not affected (Dotti et al., 1993a).
In a two-generation study of reproductive toxicity, groups of
25 male and 25 female Wistar rats were treated with 0, 1, 10 or 100
ppm chlorfenvinphos (purity, 92.8%) mixed with microgranulated diet,
equivalent to 0, 0.05, 0.5 or 5 mg/kg bw per day. The diets were fed
to the parental generation during growth, mating, gestation and
lactation and to one litter per generation. Clinical signs, body
weight, food consumption, cholinesterase activity (only in the
parent generation), mating performance and reproductive parameters
were observed. The parents were examined histologically; the pups
were sexed and examined for gross malformations, and the numbers of
stillborn and live pups were recorded. One male fed 100 ppm died on
day 10, and reduced body-weight gain and food consumption were noted
in males and females of the parental and F1 generations at this
dose. A slight decrease in food consumption was observed in F1
males and parental and F1 females fed 10 ppm. Plasma
cholinesterase activity was decreased by 14-78% in males and females
fed 10 or 100 ppm, and erythrocyte cholinesterase activation was
inhibited by 35-63% in males and females fed 100 ppm. Brain
cholinesterase activity was decreased by 32% in males and 38% in
females fed 100 ppm and by 17% in females fed 10 ppm. In the
parental generation, post-implantation loss was higher in animals
fed 10 or 100 ppm than in controls, and postnatal loss was increased
in rats fed 100 ppm; breeding loss up to the end of lactation was
higher in animals fed 10 or 100 ppm. In the F1 generation,
breeding loss was higher than that in controls in animals fed 100
ppm. At this dose, retardation of body-weight gain was noted in both
F1 and F2 pups during the lactation period. The NOAEL in this
study was 1 ppm, equivalent to 0.05 mg/kg bw per day (Dotti et
al., 1993b).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 25 pregnant specific-pathogen-free CrL:COBS CD(SD)BR
rats were treated by gavage with chlorfenvinphos (purity, > 90%) at
0, 0.3, 1 or 3 mg/kg bw per day in corn oil on days 6-15 of
pregnancy. The doses were determined in a preliminary study in which
doses of 3, 6 and 10 mg/kg bw per day caused dose-related loss of
body weight. The rats were sacrificed on day 20 of gestation. No
effects were found on food consumption, pregnancy rate or litter
parameters, including numbers of fetal deaths, implants, corpora
lutea and pre- and post-implantation losses, litter weight, fetal
weight and gravid uteri, or on the incidences of malformations,
visceral and skeletal anomalies and variations. The only clinical
effect seen was the occurrence of small faeces in animals fed 3
mg/kg bw per day from days 3-4 until the end of treatment;
body-weight gain was slightly decreased in animals fed 3 mg/kg bw
per day on days 6-20. There was no indication of irreversible
structural effects. The NOAEL for maternal toxicity in this study
was 1 mg/kg bw per day, and that for developmental toxicity was 3
mg/kg bw per day (Mayfield & John, 1986).
Rabbits
Pregnant banded Dutch rabbits (21-22 per group) received three
capsules containing chlorfenvinphos (purity not specified) at 25, 50
or 100 mg/kg bw per day in corn oil (total volume, 200 µl) on days
6-18 of gestation. A control group of 32 females was used. The
rabbits were sacrificed on day 28 of gestation. No effects were
found on mortality, clinical signs or mean numbers of corpora lutea,
implantations, resorptions or fetal deaths. Body-weight gain and
food consumption were reduced in dams fed 100 mg/kg bw per day on
days 6-12 of gestation. Plasma and erythrocyte cholinesterase
activities were decreased in dams at all doses, erythrocyte activity
being decreased by 55-75%. Brain cholinesterase activity was not
measured. Pre-implantation losses occurred in dams fed 25 or 100
mg/kg bw per day. No differences in the incidences of visceral or
skeletal malformations or variations between the groups were
observed that could be attributed to treatment. There was no
indication of irreversible structural effects. No NOAEL could be
established for maternal toxicity; the NOAEL for embryo- and
fetotoxicity was 100 mg/kg bw per day (Dix et al., 1979).
In a limited experiment with rats, rabbits and hamsters, doses
of 50 mg/kg bw per day and higher increased the incidence of open
eyes and oedema in hamster dams and decreased the weight and length
of hamster fetuses (Dzierzawski & Minta, 1979).
(f) Genotoxicity
The results of tests for the genotoxicity of chlorfenvinphos
are summarized in Table 1.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Chlorfenvinphos (purity, 93.1%), administered to three New
Zealand white rabbits as a 4-h semi-occluded topical application of
0.5 ml undiluted compound, caused very slight erythema within 1 h
after removal of the dressing. The erythema persisted for at least
48 h after treatment, but all overt effects disappeared within seven
days. The compound was considered slightly irritating to rabbit skin
(Gardner, 1992).
The skin sensitizing potential of chlorfenvinphos was examined
in two studies in guinea-pigs. In the first study, 10 male and 10
female Tunstall breeding unit 'P' guinea-pigs received an
intradermal injection of 0.5% (mol/volume) chlorfinvenphos (purity,
91.3%) during the induction period, a topical application of the
undiluted test compound one week later and a challenge application
of undiluted compound two weeks after that. A control group of five
male and five female animals was used. No effect was seen in the
test animals 24 or 48 h after removal of the challenge patches
(Rose, 1982).
In the second study, 10 male and 10 female Dunkin-Hartley
guinea-pigs were given an intradermal induction dose of 2%
(mol/volume) chlorfenvinphos (purity, 93.1%) and undiluted compound
for the topical and challenge applications. A control group of five
male and five female animals was used. Ten of 20 test animals showed
a positive response after 24 h and nine of the same animals after 48
h. None of the control animals responded (Gardner, 1992).
Instillation of 0.1 ml undiluted chlorfenvinphos (purity,
93.1%) into the eyes of three New Zealand white rabbits caused
marked constriction of the pupil, slight conjunctival redness,
slight chemosis and ocular discharge. All signs were reversed within
24 h. The compound is not considered to irritate the eye (Gardner,
1992).
Table 1. Results of tests for the genotoxicity of chlorfenvinphos
End-point Test system Concentration Purity Results Reference
of chlorfenvinphos (%)
In vitro
Reverse mutation S. typhimurium TA98, 25-2025 µg/platea NR Negativeb,c Arni & Müller, 1979
100, 1535, 1537
Reverse mutation E. coli WP2, WP2 hcr, 250 µg/plate NR Negatived Shirasu et al., 1976
S. typhimurium TA1535,
1536, 1537, 1538
Reverse mutation S. typhimurium TA98, Up to 5000 µg/plate NR Negativec,d Moriya et al., 1983
100, 1535, 1537, 1538
Reverse mutation S. typimurium TA100 Up to 5000 µg/plate NR Positivec,e Moriya et al., 1983
Reverse mutation S. typhimurium TA98, Up to 5000 µg/plate NR Negativec,e Moriya et al., 1983
1535, 1537, 1538
Reverse mutation S. typhimurium TA98, 0.2-2000 µg/plate 90.5 Negativeb,c Brooks, 1978
100, 1538
Reverse mutation E. coli B/r WP2 NR > 99 Negativec,d Dean, 1971
Reverse mutation E. coli WP2, WP2 uvrA 0.2-2000 g/plate 90.5 Negativec Brooks, 1978
Reverse mutation E. coli WP2 hcr Up to 5000 µg/plate NR Negativeb,c Moriya et al., 1983
Mitotic gene Saccharomyces 0.001-5.0 mg/ml; toxic 90.5 Negativec,d Brooks, 1978
conversion cerevisiae JD1 at 0.5, 1.0, 5.0 mg/ml
Chromosomal Human lymphocytes 18.8-300 µg/platea,d 93.1 Negativeb,c Strasser & Arni,
aberrations 15.6-250 µg/platea,e 1988
Table 1 (contd)
End-point Test system Concentration Purity Results Reference
of chlorfenvinphos (%)
In vivo
Chromosomal Chinese hamster 25 or 50 mg/kg bw 90.5 Negativec Dean, 1978
aberrations bone marrow per day for 2 days
Dominant lethal CD1 mice (male) 10, 20 or 40 mg/kg bw 90.5 Negativec Dean & Hend, 1978
mutation in DMSO
NR, nor reported; DMSO, dimethyl sulfoxide
a It is not clear whether chlorfenvinphos was tested: the substance was coded C8949.
b In the presence and absence of metabolic activation
c Positive controls were used.
d In the absence of metabolic activation
e In the presence of metabolic activation
(ii) Cholinesterase inhibition and related pharmacological
and neurotoxic effects
After oral administration of chlorfenvinphos at 15 mg/kg bw to
Fischer 344 rats, brain cholinesterase activity was maximally
inhibited (to about 20% of the control value) at 4 h; the inhibition
lasted more than 24 h after administration (Ikeda et al., 1990).
Groups of 10 male and 10 female Wistar rats were fed dietary
concentrations of 0, 0.3, 1, 3 or 30 ppm chlorfenvinphos for four
weeks, or received the same treatments and then received control
diet for four weeks. Administration of 30 ppm resulted in decreased
activity of plasma, erythrocyte and brain cholinesterase. Feeding of
the control diet for four weeks reversed the inhibition of plasma,
erythrocyte and brain cholinesterase activities, resulting in values
of 100, 88 and 91%, respectively (Pickering, 1978).
In a comparison of the lethality of chlorfenvinphos after
intravenous and oral administration, Sprague-Dawley rats given
lethal doses by either route showed typical signs of
anticholinesterase poisoning and marked reductions in brain and
erythrocyte cholinesterase activity. Anaesthetized and conscious
rats administered chlorfenvinphos intravenously had hypertension and
apnoea. After oral administration, breathing stopped before
cessation of heart beats (Takahashi et al., 1991).
The effects of chlorfenvinphos on spontaneous
electroencephalographic, electromyo-graphic and cholinesterase
activity in brain and red blood cells was examined in male Wistar
rats. Single oral doses of 1 mg/kg bw had no effect. Doses over 2
mg/kg bw induced dose-related inhibition of the cholinesterase
activity in brain and erythrocytes. Maximal inhibition of brain
cholinesterase activity was seen 3 h after treatment and lasted for
more than 72 h. Electroencephalography showed a prominent arousal
pattern, and the appearance of slow-wave sleep and para-sleep was
markedly depressed. The duration of the arousal pattern was
proportional to the dose; however, the awake-sleep cycle returned to
the control rate on the second day, and an increase in para-sleep
occurred on the third day (Osumi et al., 1975).
In 30 Sprague-Dawley rats fed a diet containing 150 ppm
chlorfenvinphos (equivalent to 7.5 mg/kg bw per day) for three
months, no effect was seen on the amplitude of the muscular response
to nerve stimulation. Electrophysiological effects were signalled
firstly by a prolonged negative potential after the direct muscle
response to a single nerve impulse and secondly by repetitive
activity. These abnormalities became more marked with time. Double
and repetitive stimulation reduced or abolished the prolonged
negative potential and repetitive activity (Maxwell & LeQuesne,
1982).
Oral administration to male Wistar rats of chlorfenvinphos at
50% of the LD50 caused maximal inhibition of brain cholinesterase
activity (to about 15% of the control value) between 2 and 3 h after
treatment. A significant reduction in brain norepinephrine level was
seen within 15 min, but no consistent diminution was seen
subsequently. During exposure at 5% of the LD50 for 12 weeks,
brain cholinesterase activity was inhibited to 60-80% of control
values and brain norepinephrine content was significantly decreased,
with maximal depletion at week 8 (Brzezinski & Wysocka-Paraszewska,
1980).
Cholinesterase activity in plasma, erythrocytes and different
parts of the brain, open-field behaviour and response to change in a
'T' maze were investigated in two groups of male Wistar rats after
administration of a single intraperitoneal injection of 1 or 3 mg/kg
bw chlorfenvinphos. Cholinesterase activity was strongly inhibited,
to a similar degree, in the blood and brain, with a mean inhibition
of 80% in rats at the high dose and 50% in those at the low dose 3 h
after treatment. Reversal of the inhibition proceeded at similar
rates in the blood and brain and was complete within 96 h with the
low dose and within 14 days with the high dose. No changes in
negotiating the 'T' maze that suggested impairment of short-term
memory were observed up to 14 days after treatment. In the
open-field test, a decrease in short-term memory was manifested by
the absence of increased locomotor and exploratory activity in
response to a new object, only in rats administered 3 mg/kg bw. This
finding suggests that behavioural disturbances last longer than
recovery of cholinesterase activity (Socko et al., 1989).
(iii) Hormone concentrations
In Wistar rats given chlorfenvinphos as a single oral dose of
6.15 mg/kg bw, a significant increase in corticosterone
concentration was seen after 1 and 3 h and in aldosterone
concentration 1-6 h after treatment. Plasma corticosteroid levels
were maximal within 1 h, when brain cholinesterase activity was only
slightly inhibited (Osicka-Koprowska et al., 1984).
(iv) Porphyrigenic potential
Chlorfenvinphos induced porphyria in primary cultures of
chicken embryo liver cells. Its porphyrigenic potential was markedly
increased by prior treatment of the cells with a compound that
induces drug metabolizing enzymes (Koeman et al., 1980).
3. Observations in humans
Workers in India were studied while applying chlorfenvinphos to
paddy rice over a period of six days; they were not wearing
protective clothing. Workers applying an emulsifiable concentrate
had decreased plasma cholinesterase activity but no change in
erythrocyte cholinesterase activity. Workers applying granules
containing 10% chlorfenvinphos had no reduction in cholinesterase
activity (Blok et al., 1977).
A group of 33 workers employed in a chlorfenvinphos
manufacturing plant were monitored medically for eight years
(1967-75). Two control groups were used. The exposed group had
slightly lower haemoglobin levels and slightly lower cholinesterase
activity in plasma and erythrocytes. Some changes in
neurophysiological parameters (lower electromyographic voltages,
associated with slower conduction velocities) were observed, but
these were reversed when the workers were removed from exposure
(Ottevanger, 1976).
Workers involved in the manufacture of chlorfenvinphos were
monitored in 1985-86 in a routine programme in which blood and urine
were collected and cholinesterase activity was measured in whole
blood, plasma and erythrocytes. A few cases of depressed plasma
cholinesterase were recorded, which were not, however, correlated
with increases in the urinary concentrations of diethylphosphate and
monoethylphosphate metabolites (Eadsforth, 1987).
Comments
The main step in the biotransformation of chlorfenvinphos is
detoxification by de-ethylation into the corresponding
phosphodiester; microsomal enzymes play an important role.
Interspecies differences in the rate of metabolism of
chlorfenvinphos were found in vitro. Oxidative metabolism by human
liver enzymes was comparable to that of rabbit liver enzymes.
Single oral doses of chlorfenvinphos were very toxic to rats,
toxic to mice and guinea-pigs and moderately to slightly toxic to
rabbits and dogs. The clinical signs observed were consistent with
cholinesterase inhibition and included tremors, fasciculation and
salivation. Pretreatment with enzyme inducers can increase the acute
oral LD50. Interspecies differences in the acute oral LD50
reflect the activity of the hepatic metabolizing enzymes involved in
the detoxification of chlorfenvinphos. WHO (1992) has classified
chlorfenvinphos as extremely hazardous.
In a four-week study in which mice were fed 0, 1, 10, 100 or
1000 ppm chlorfenvinphos in the diet, plasma and erythrocyte
cholinesterase activities were inhibited in animals fed 100 or 1000
ppm. Brain cholinesterase activity was inhibited in males fed 10 or
1000 ppm and in females at all doses; thus, no NOAEL could be
established for female mice. The NOAEL in males was 1 ppm, equal to
0.18 mg/kg bw per day.
In a one-year study in which dogs were fed 0, 3, 100 or 3000
ppm in the diet, inhibition of erythrocyte cholinesterase activity
and increased relative adrenal weight were seen in males; in
females, increased relative thyroid weight was seen in those fed the
highest dose. The NOAEL was 100 ppm, equal to 2.8 mg/kg bw per day.
In a study of the carcinogenicity of chlorfenvinphos, in which
mice were fed 0, 1, 25 or 625 ppm in the diet, no increase in tumour
incidence was observed. Erythrocyte and brain cholinesterase
activity was inhibited in mice fed the highest dose. The NOAEL was
25 ppm, equal to 3.7 mg/kg bw per day.
In a two-year study of toxicity and carcinogenicity in rats fed
diets containing 0, 0.3, 1, 3 or 30 ppm, erythrocyte and brain
cholinesterase activity was inhibited in those fed 30 ppm. There was
no evidence of carcinogenicity. The NOAEL was 3 ppm, equivalent to
0.15 mg/kg bw per day.
In a two-generation study of reproductive toxicity in rats fed
diets containing 0, 1, 10 or 100 ppm, reduced brain cholinesterase
activity, post-implantation loss and breeding losses were observed
in animals fed 10 or 100 ppm. The NOAEL was 1 ppm, equivalent to
0.05 mg/kg bw per day.
In two studies of teratogenicity--one in rats and one in
rabbits--doses that were maternally toxic were not embryotoxic and
there was no indication of teratogenicity. In rats, the NOAEL was 1
mg/kg bw per day for maternal toxicity and 3 mg/kg bw per day, the
highest dose tested, for developmental toxicity. In rabbits, no
NOAEL could be established for maternal toxicity (< 25 mg/kg bw);
the NOAEL for developmental toxicity was > 100 mg/kg bw per day.
Chlorfenvinphos was mutagenic in only one study, in Salmonella
typhimurium TA100 in the presence of an exogenous metabolic system
at doses of at least 1000 µg per plate. It was inactive in other
bacterial tests and did not induce mitotic conversion in yeast or
chromosomal aberrations in human lymphocytes in vitro.
Chlorfenvinphos did not induce chromosomal aberrations in Chinese
hamster bone-marrow cells or dominant lethal effects in male mice
in vivo. The Meeting concluded that chlorfenvinphos was not
genotoxic.
Delayed neurotoxicity in chickens has not been evaluated.
Observations in humans were not suitable for use in estimating
an ADI.
An ADI was established on the basis of an NOAEL of 0.05 mg/kg
bw per day in a two-generation study of reproductive toxicity in
rats and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 3.7 mg/kg bw per day (two-year study of
carcinogenicity)
Rat: 3 ppm, equivalent to 0.15 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
1 ppm, equivalent to 0.05 mg/kg bw per day (two-generation
study of reproductive toxicity)
1 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Rabbit: < 25 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Dog: 100 ppm, equal to 2.8 mg/kg bw (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Study of delayed neurotoxicity in chickens, with estimation of
neuropathy target esterase
2. Further observations in humans
3. Studies of pharmacokinetics in mammals in vivo
References
Allen, T.R., Corney, S.J., Frei, T., Luetkemeier, H., Biedermann, K.
& Springall, C.J. (1992) 4-Week oral range-finding toxicity
(feeding) study with chlorfenvinphos in the dog. RCC Project No.
271934. Unpublished report from Research and Consulting Co.,
Itingen, Switzerland. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Allen, T.R., Corney, S.J., Frei, T., Luetkemeier, H., Biedermann, K.
& Springall, C.J. (1993) 52-Week oral toxicity (feeding) study with
chlorfenvinphos in the dog. RCC Project No. 271945. Unpublished
report from Research and Consulting Co., Itingen, Switzerland.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Arni, P. & Müller, D. (1979) Salmonella/mammalian microsome
mutagenicity test with C8949. Test for mutagenic properties in
bacteria. Experiment no. 78/2589. Unpublished report from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Blok, A.C., Mann, A.H. & Robinson, J. (1977) Organophosphorus
insecticide exposure of sprayers under field conditions in rice in
India. I. Birlane (chlorfenvinphos). TOX 77-005. Unpublished report
from Shell International Research Maatschappy, Toxicology Division.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Brooks, T.M. (1978) Toxicity studies with chlorfenvinphos:
mutagenicity studies with chlorfenvinphos in micro-organisms. Group
Research Report TLGR.00142.78. Unpublished report from Shell
Research Ltd, London. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Brzezinski, J. & Wysocka-Paruszewska, B. (1980) Neurochemical
alterations in rat brain as a test for studying the neurotoxicity of
organophosphorus insecticides. Arch. Toxicol., Suppl. 4, 475-478.
Dean, B.J. (1971) The mutagenic effect of organophosphate
insecticides on Escherichia coli. Group Research Report
TLGR.0034.71. Unpublished report from Shell Research Ltd, London.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Dean, B.J. (1978) Toxicity studies with chlorfenvinphos: chromosome
studies on bone marrow cells of Chinese hamsters after two daily
oral doses of chlorfenvinphos. Group Research Report TLGR.0087.78.
Unpublished report from Shell Research Ltd, London. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Dean, B.J. & Hend, R.W. (1978) Toxicity studies with
chlorfenvinphos: dominant lethal assay in male mice after single
oral doses of chlorfenvinphos. Group Research Report TLGR.0063.78.
Unpublished report from Shell Research Ltd, London. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Dix, K.M., Cassidy, S.L. & Vilkauls, J. (1979) Toxicity of
chlorfenvinphos: teratological studies in rabbits given
chlorfenvinphos orally. Group Research Report TLGR.79.105.
Unpublished report from Shell Research Ltd, London. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Donninger, C., Hutson, D.H. & Pickering, B.A. (1972) The oxidative
dealkylation of insecticidal phosphoric acid triesters by mammalian
liver enzymes. Biochem. J., 126, 701-707.
Dotti, A., Kinder, J., Luetkemeier, H. & Biedermann, K. (1993a)
Chlorfenvinphos. Preliminary study to the two-generation
reproduction study in the rat. RCC Project No. 271822. Unpublished
report from Research and Consulting Co., Itingen Switzerland.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Dotti, A., Kinder, J., Luetkemeier, H. & Biedermann, K. (1993b) Two
generation reproduction study with chlorvinphos in the rat. RCC
Project No. 271833. Unpublished report from Research and Consulting
Co., Itingen, Switzerland. Submitted to WHO by American Cyanamid
Co., Princeton, NJ, USA.
Dzierzawski, A. & Minta, M. (1979) Embryotoxic effects of
chlorfenvinphos and bromfenvinphos in laboratory animals. Bull.
Vet. Inst. Pulawy, 23, 1-2, 32-42.
Eadsforth, C.V. (1987) Biological monitoring of exposure of
operators during manufacture of Birlane in CAS, location CMDU-N,
during 1985-6. Unpublished report from Shell Biomedical Laboratory,
SNR/SNC Pernis. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Gardner, J. (1992) Birlane technical: acute oral and dermal dermal
toxicity in rat, skin and eye irritancy in rabbit and skin
sensitization potential in guinea pig. Unpublished report from Shell
Research Ltd, Sittingbourne Research Centre, Sittingbourne, United
Kingdom. Submitted to WHO by American Cyanamid Co., Princeton, NJ,
USA.
Hutson, D.H. & Wright, A.S. (1980) The effect of hepatic microsomal
monooxygenase induction on the metabolism and toxicity of the
organophosphorus insecticide chlorfenvinphos. Chem. Biol.
Interactions, 31, 93-101.
Hutson, D.H. & Logan, C.J. (1986) Detoxification of the
organophosphorus insecticide chlorfenvinphos by rat, rabbit and
human liver enzymes. Xenobiotica, 16, 87-93.
Ikeda, T., Kojima, T., Yoshida, M., Takahashi, H., Tsuda, S. &
Shirasu, Y. (1990) Pretreatment of rats with an organophosphorus
insecticide, chlorfenvinphos, protects against subsequent challenge
with the same compound. Fundam. Appl. Toxicol., 14, 560-567.
Ikeda, T., Tsuda, S. & Shirasu, Y. (1991) Metabolic induction of the
hepatic cytochrome P450 system by chlorfenvinphos in rats. Fundam.
Appl. Toxicol., 17, 361-367.
Ikeda, T., Tsuda, S. & Shirasu, Y. (1992) Pharmacokinetic analysis
of protection by organophosphorus insecticide, chlorfenvinphos,
against the toxicity of its succeeding dosage in rats. Fundam.
Appl. Toxicol., 18, 299-306.
Koeman, J.H., Debets, F.M.H. & Strik, J.J.T.W.A. (1980) The
porphyrogenic potential of pesticides with special emphasis on
organophosphorus compounds. In: Tordoir, W.F. & van Heemstra,
E.A.H., eds, Field Worker Exposure during Pesticide Application,
Amsterdam, Elsevier Scientific Publishing Co., pp. 157-162.
Maxwell, J.C. & LeQuesne, P.M. (1982) Neuromuscular effects of
chronic administration of two organophosphorus insecticides to rats.
Neurotoxicology, 3, 1-10.
Mayfield, R. & John, D.M. (1986) Effect of Birlane on pregnancy of
the rat. HCR Report No. SLL 84/851593. Unpublished report from
Huntingdon Research Centre, . Submitted to WHO by American Cyanamid
Co., Princeton, NJ, USA.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. &
Shirasu, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116, 185-216.
Osicka-Koprowska, A., Lipska, M. & Wysocka-Paruszewska, B. (1984)
Effects of chlorfenvinphos on plasma corticosterone and aldosterone
levels in rats. Arch. Toxicol., 55, 68-69.
Osumi, Y., Fujiwara, H., Oishi, R. & Takaori, S. (1975) Central
cholinergic activation by chlorfenvinphos, an organophosphate, in
the rat. Jpn. J. Pharmacol., 25, 47-54.
Ottevanger, C.F. (1976) An epidemiological and toxicological study
of occupational exposure to an organphosphorus pesticide. University
of Amsterdam, MD Thesis. Rotterdam, Phoenix & den Oudsten.
Pickering, C.E. (1978) Toxicity of chlorfenvinphos: the
reversibility of cholinesterase and aliesterase inhibition produced
by feeding chlorfenvinphos. Unpublished report from Shell Research,
TLGR0169.78, Sittingbourne United Kingdom. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA
Pickering, R.G. (1980) Toxicity studies on the insecticide
chlorfenvinphos. A two year feeding study in rats. Budget ref.
50070712. Unpublished report from Shell Research Ltd, London.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Rose, G.P. (1982) Toxicology of Birlane: the skin sensitizing
potential of Birlane. Project No. 226/82. Unpublished report from
Shell Research Ltd, Sittingbourne Research Centre, Sittingbourne,
United Kingdom. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Schmid, H., Probst, D., Luetkemeier, H., Weber, K., Wilson, J.T.,
Springall, C.J. & Biedermann, K. (1993) Oncogenicity (feeding) study
with chlorfenvinphos in the mouse. Final Report. RCC Project No.
265926. Unpublished report from Research and Consulting Co.,
Itingen, Switzerland. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening system of pesticides in the microbial system.
Mutat. Res., 40, 19-30.
Socko, R., Gralewicz, S. & Gorny, R. (1989) Neurotoxicity of
chlorphenvinphos, an organo-phosphorus pesticide: effects on blood
and brain cholinesterase activity, open field, behavior and
response-to-change in a 'T' maze in rats. Polish J. Occup. Med.,
2, 294-308.
Strasser, F. & Arni, P. (1988) Chromosome studies on human
lymphocytes. Test material C8949 tech. Test no.: 871201. Unpublished
report from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Takahashi, H., Kojima, T., Ikeda T., Tsuda S. & Shirasu Y. (1991)
Differences in the mode of lethality produced through intravenous
and oral administration of organophosphorus insecticides in rats.
Fundam. Appl. Toxicol., 16, 459-468.
Tennekes, H., Janiak, T., Stucki, H.P., Probst, D., Luetkemeier, H.,
Vogel, O., Schlotke, B., Biedermann, K. & Heusner, W. (1991) 28-Day
range-finding (feeding) study with chlorfenvinphos in the mouse. RCC
Project No. 243202. Unpublished report from Research and Consulting
Co., Itingen, Switzerland. Submitted to WHO by American Cyanamid
Co., Princeton, NJ, USA.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
 (c) Long-term toxicity and carcinogenicity
Mice
In a study designed to determine carcinogenic potential,
chlorfenvinphos (purity, 92.8%) was administered for 24 months to
groups of 74 male and 74 female NMRI mice at 1, 25 or 625 ppm in the
diet, equal to 0.15, 3.7 or 93 mg/kg bw per day for males and to
0.2, 5.0 or 119 mg/kg bw per day for females. Twelve mice of each
sex from each group were sacrificed after 52 weeks of treatment; all
females were killed after 90 weeks of treatment and all males after
104 weeks. Clinical signs, food consumption, body weight,
differential leukocyte count, cholinesterase activity and organ
weights were observed, and macroscopic and microscopic examinations
were undertaken. Plasma cholinesterase activity was inhibited in
animals of each sex fed 25 ppm (38-50% inhibition) or 625 ppm
(87-96%). In males and females fed 625 ppm, erythrocyte
cholinesterase activity was decreased by 18-66% and brain
cholinesterase activity by 25-46%. Microscopic examination at
termination of the experiment showed changes in the adrenal cortex
of males fed 625 ppm, which included increased incidence and
intensity of ceroid pigment and focal hypertrophy and increased
severity of nodular hyperplasia. Tumour incidence was not enhanced.
No effects were observed at 25 ppm, equal to 3.7 mg/kg bw per day
(Schmid et al., 1993).
Rats
In a study of both chronic toxicity and carcinogenicity, groups
of 50 male and 50 female Wistar rats were fed chlorfenvinphos
(purity, 90.5%) at 0.3, 1, 3 or 30 ppm in the diet (equivalent to
0.015, 0.05, 0.15 or 1.5 mg/kg bw per day) for 24 months. The
control group consisted of 100 rats of each sex. Clinical signs,
body weight and food consumption were observed. At termination,
organ weights, haematological parameters and the results of clinical
chemistry, urinalysis and macroscopic and microscopic examinations
were recorded. Additional groups of 12 rats of each sex were
examined after 6, 12 and 18 months for haematological and clinical
chemical parameters including cholinesterase activity; after
sacrifice, the organs were weighed and animals fed 0, 0.3 or 30 ppm
chlorfenvinphos were examined macroscopically and histologically.
Plasma cholinesterase activity was inhibited after six months in all
treated animals but thereafter only in rats fed 3 or 30 ppm. In
males and females fed 30 ppm, erythrocyte cholinesterase activity
was inhibited by about 60% and brain cholinesterase activity by
about 45%; erythrocyte cholinesterase activity was inhibited by
about 17% in females fed 1 or 3 ppm after 12 months' exposure. In
all groups and at all intervals during the study, minor but
significant changes were seen in the concentrations of alpha-, ß-
and gamma-globulins. No dose-related or significant macroscopic or
histological changes were observed, and tumour incidences were not
enhanced. The NOAEL in this study was 3 ppm, equivalent to 0.15
mg/kg bw per day (Pickering, 1980).
(d) Reproductive toxicity
Rats
In a range-finding study, 10 male and 10 female Wistar rats
were exposed to 0, 5, 25 or 125 ppm chlorfenvinphos (purity, 92.8%)
mixed with microgranulated feed (equivalent to 0.25, 1.3 or 6.3
mg/kg bw per day) during a three-week period before mating and
throughout mating, gestation and lactation. After they had been
weaned, F1 pups were reared for a further week on their test
diets. The body-weight gain of males and females fed 125 ppm was
slightly reduced. Inhibition of erythrocyte and brain cholinesterase
activity was observed in females fed 5, 25 or 125 ppm and in males
fed 25 or 125 ppm. Plasma cholinesterase activity was inhibited only
in females. Reproductive parameters, including mating, gestation,
parturition, pregnancy rate, lactation and litter characteristics,
were not affected (Dotti et al., 1993a).
In a two-generation study of reproductive toxicity, groups of
25 male and 25 female Wistar rats were treated with 0, 1, 10 or 100
ppm chlorfenvinphos (purity, 92.8%) mixed with microgranulated diet,
equivalent to 0, 0.05, 0.5 or 5 mg/kg bw per day. The diets were fed
to the parental generation during growth, mating, gestation and
lactation and to one litter per generation. Clinical signs, body
weight, food consumption, cholinesterase activity (only in the
parent generation), mating performance and reproductive parameters
were observed. The parents were examined histologically; the pups
were sexed and examined for gross malformations, and the numbers of
stillborn and live pups were recorded. One male fed 100 ppm died on
day 10, and reduced body-weight gain and food consumption were noted
in males and females of the parental and F1 generations at this
dose. A slight decrease in food consumption was observed in F1
males and parental and F1 females fed 10 ppm. Plasma
cholinesterase activity was decreased by 14-78% in males and females
fed 10 or 100 ppm, and erythrocyte cholinesterase activation was
inhibited by 35-63% in males and females fed 100 ppm. Brain
cholinesterase activity was decreased by 32% in males and 38% in
females fed 100 ppm and by 17% in females fed 10 ppm. In the
parental generation, post-implantation loss was higher in animals
fed 10 or 100 ppm than in controls, and postnatal loss was increased
in rats fed 100 ppm; breeding loss up to the end of lactation was
higher in animals fed 10 or 100 ppm. In the F1 generation,
breeding loss was higher than that in controls in animals fed 100
ppm. At this dose, retardation of body-weight gain was noted in both
F1 and F2 pups during the lactation period. The NOAEL in this
study was 1 ppm, equivalent to 0.05 mg/kg bw per day (Dotti et
al., 1993b).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 25 pregnant specific-pathogen-free CrL:COBS CD(SD)BR
rats were treated by gavage with chlorfenvinphos (purity, > 90%) at
0, 0.3, 1 or 3 mg/kg bw per day in corn oil on days 6-15 of
pregnancy. The doses were determined in a preliminary study in which
doses of 3, 6 and 10 mg/kg bw per day caused dose-related loss of
body weight. The rats were sacrificed on day 20 of gestation. No
effects were found on food consumption, pregnancy rate or litter
parameters, including numbers of fetal deaths, implants, corpora
lutea and pre- and post-implantation losses, litter weight, fetal
weight and gravid uteri, or on the incidences of malformations,
visceral and skeletal anomalies and variations. The only clinical
effect seen was the occurrence of small faeces in animals fed 3
mg/kg bw per day from days 3-4 until the end of treatment;
body-weight gain was slightly decreased in animals fed 3 mg/kg bw
per day on days 6-20. There was no indication of irreversible
structural effects. The NOAEL for maternal toxicity in this study
was 1 mg/kg bw per day, and that for developmental toxicity was 3
mg/kg bw per day (Mayfield & John, 1986).
Rabbits
Pregnant banded Dutch rabbits (21-22 per group) received three
capsules containing chlorfenvinphos (purity not specified) at 25, 50
or 100 mg/kg bw per day in corn oil (total volume, 200 µl) on days
6-18 of gestation. A control group of 32 females was used. The
rabbits were sacrificed on day 28 of gestation. No effects were
found on mortality, clinical signs or mean numbers of corpora lutea,
implantations, resorptions or fetal deaths. Body-weight gain and
food consumption were reduced in dams fed 100 mg/kg bw per day on
days 6-12 of gestation. Plasma and erythrocyte cholinesterase
activities were decreased in dams at all doses, erythrocyte activity
being decreased by 55-75%. Brain cholinesterase activity was not
measured. Pre-implantation losses occurred in dams fed 25 or 100
mg/kg bw per day. No differences in the incidences of visceral or
skeletal malformations or variations between the groups were
observed that could be attributed to treatment. There was no
indication of irreversible structural effects. No NOAEL could be
established for maternal toxicity; the NOAEL for embryo- and
fetotoxicity was 100 mg/kg bw per day (Dix et al., 1979).
In a limited experiment with rats, rabbits and hamsters, doses
of 50 mg/kg bw per day and higher increased the incidence of open
eyes and oedema in hamster dams and decreased the weight and length
of hamster fetuses (Dzierzawski & Minta, 1979).
(f) Genotoxicity
The results of tests for the genotoxicity of chlorfenvinphos
are summarized in Table 1.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Chlorfenvinphos (purity, 93.1%), administered to three New
Zealand white rabbits as a 4-h semi-occluded topical application of
0.5 ml undiluted compound, caused very slight erythema within 1 h
after removal of the dressing. The erythema persisted for at least
48 h after treatment, but all overt effects disappeared within seven
days. The compound was considered slightly irritating to rabbit skin
(Gardner, 1992).
The skin sensitizing potential of chlorfenvinphos was examined
in two studies in guinea-pigs. In the first study, 10 male and 10
female Tunstall breeding unit 'P' guinea-pigs received an
intradermal injection of 0.5% (mol/volume) chlorfinvenphos (purity,
91.3%) during the induction period, a topical application of the
undiluted test compound one week later and a challenge application
of undiluted compound two weeks after that. A control group of five
male and five female animals was used. No effect was seen in the
test animals 24 or 48 h after removal of the challenge patches
(Rose, 1982).
In the second study, 10 male and 10 female Dunkin-Hartley
guinea-pigs were given an intradermal induction dose of 2%
(mol/volume) chlorfenvinphos (purity, 93.1%) and undiluted compound
for the topical and challenge applications. A control group of five
male and five female animals was used. Ten of 20 test animals showed
a positive response after 24 h and nine of the same animals after 48
h. None of the control animals responded (Gardner, 1992).
Instillation of 0.1 ml undiluted chlorfenvinphos (purity,
93.1%) into the eyes of three New Zealand white rabbits caused
marked constriction of the pupil, slight conjunctival redness,
slight chemosis and ocular discharge. All signs were reversed within
24 h. The compound is not considered to irritate the eye (Gardner,
1992).
Table 1. Results of tests for the genotoxicity of chlorfenvinphos
End-point Test system Concentration Purity Results Reference
of chlorfenvinphos (%)
In vitro
Reverse mutation S. typhimurium TA98, 25-2025 µg/platea NR Negativeb,c Arni & Müller, 1979
100, 1535, 1537
Reverse mutation E. coli WP2, WP2 hcr, 250 µg/plate NR Negatived Shirasu et al., 1976
S. typhimurium TA1535,
1536, 1537, 1538
Reverse mutation S. typhimurium TA98, Up to 5000 µg/plate NR Negativec,d Moriya et al., 1983
100, 1535, 1537, 1538
Reverse mutation S. typimurium TA100 Up to 5000 µg/plate NR Positivec,e Moriya et al., 1983
Reverse mutation S. typhimurium TA98, Up to 5000 µg/plate NR Negativec,e Moriya et al., 1983
1535, 1537, 1538
Reverse mutation S. typhimurium TA98, 0.2-2000 µg/plate 90.5 Negativeb,c Brooks, 1978
100, 1538
Reverse mutation E. coli B/r WP2 NR > 99 Negativec,d Dean, 1971
Reverse mutation E. coli WP2, WP2 uvrA 0.2-2000 g/plate 90.5 Negativec Brooks, 1978
Reverse mutation E. coli WP2 hcr Up to 5000 µg/plate NR Negativeb,c Moriya et al., 1983
Mitotic gene Saccharomyces 0.001-5.0 mg/ml; toxic 90.5 Negativec,d Brooks, 1978
conversion cerevisiae JD1 at 0.5, 1.0, 5.0 mg/ml
Chromosomal Human lymphocytes 18.8-300 µg/platea,d 93.1 Negativeb,c Strasser & Arni,
aberrations 15.6-250 µg/platea,e 1988
Table 1 (contd)
End-point Test system Concentration Purity Results Reference
of chlorfenvinphos (%)
In vivo
Chromosomal Chinese hamster 25 or 50 mg/kg bw 90.5 Negativec Dean, 1978
aberrations bone marrow per day for 2 days
Dominant lethal CD1 mice (male) 10, 20 or 40 mg/kg bw 90.5 Negativec Dean & Hend, 1978
mutation in DMSO
NR, nor reported; DMSO, dimethyl sulfoxide
a It is not clear whether chlorfenvinphos was tested: the substance was coded C8949.
b In the presence and absence of metabolic activation
c Positive controls were used.
d In the absence of metabolic activation
e In the presence of metabolic activation
(ii) Cholinesterase inhibition and related pharmacological
and neurotoxic effects
After oral administration of chlorfenvinphos at 15 mg/kg bw to
Fischer 344 rats, brain cholinesterase activity was maximally
inhibited (to about 20% of the control value) at 4 h; the inhibition
lasted more than 24 h after administration (Ikeda et al., 1990).
Groups of 10 male and 10 female Wistar rats were fed dietary
concentrations of 0, 0.3, 1, 3 or 30 ppm chlorfenvinphos for four
weeks, or received the same treatments and then received control
diet for four weeks. Administration of 30 ppm resulted in decreased
activity of plasma, erythrocyte and brain cholinesterase. Feeding of
the control diet for four weeks reversed the inhibition of plasma,
erythrocyte and brain cholinesterase activities, resulting in values
of 100, 88 and 91%, respectively (Pickering, 1978).
In a comparison of the lethality of chlorfenvinphos after
intravenous and oral administration, Sprague-Dawley rats given
lethal doses by either route showed typical signs of
anticholinesterase poisoning and marked reductions in brain and
erythrocyte cholinesterase activity. Anaesthetized and conscious
rats administered chlorfenvinphos intravenously had hypertension and
apnoea. After oral administration, breathing stopped before
cessation of heart beats (Takahashi et al., 1991).
The effects of chlorfenvinphos on spontaneous
electroencephalographic, electromyo-graphic and cholinesterase
activity in brain and red blood cells was examined in male Wistar
rats. Single oral doses of 1 mg/kg bw had no effect. Doses over 2
mg/kg bw induced dose-related inhibition of the cholinesterase
activity in brain and erythrocytes. Maximal inhibition of brain
cholinesterase activity was seen 3 h after treatment and lasted for
more than 72 h. Electroencephalography showed a prominent arousal
pattern, and the appearance of slow-wave sleep and para-sleep was
markedly depressed. The duration of the arousal pattern was
proportional to the dose; however, the awake-sleep cycle returned to
the control rate on the second day, and an increase in para-sleep
occurred on the third day (Osumi et al., 1975).
In 30 Sprague-Dawley rats fed a diet containing 150 ppm
chlorfenvinphos (equivalent to 7.5 mg/kg bw per day) for three
months, no effect was seen on the amplitude of the muscular response
to nerve stimulation. Electrophysiological effects were signalled
firstly by a prolonged negative potential after the direct muscle
response to a single nerve impulse and secondly by repetitive
activity. These abnormalities became more marked with time. Double
and repetitive stimulation reduced or abolished the prolonged
negative potential and repetitive activity (Maxwell & LeQuesne,
1982).
Oral administration to male Wistar rats of chlorfenvinphos at
50% of the LD50 caused maximal inhibition of brain cholinesterase
activity (to about 15% of the control value) between 2 and 3 h after
treatment. A significant reduction in brain norepinephrine level was
seen within 15 min, but no consistent diminution was seen
subsequently. During exposure at 5% of the LD50 for 12 weeks,
brain cholinesterase activity was inhibited to 60-80% of control
values and brain norepinephrine content was significantly decreased,
with maximal depletion at week 8 (Brzezinski & Wysocka-Paraszewska,
1980).
Cholinesterase activity in plasma, erythrocytes and different
parts of the brain, open-field behaviour and response to change in a
'T' maze were investigated in two groups of male Wistar rats after
administration of a single intraperitoneal injection of 1 or 3 mg/kg
bw chlorfenvinphos. Cholinesterase activity was strongly inhibited,
to a similar degree, in the blood and brain, with a mean inhibition
of 80% in rats at the high dose and 50% in those at the low dose 3 h
after treatment. Reversal of the inhibition proceeded at similar
rates in the blood and brain and was complete within 96 h with the
low dose and within 14 days with the high dose. No changes in
negotiating the 'T' maze that suggested impairment of short-term
memory were observed up to 14 days after treatment. In the
open-field test, a decrease in short-term memory was manifested by
the absence of increased locomotor and exploratory activity in
response to a new object, only in rats administered 3 mg/kg bw. This
finding suggests that behavioural disturbances last longer than
recovery of cholinesterase activity (Socko et al., 1989).
(iii) Hormone concentrations
In Wistar rats given chlorfenvinphos as a single oral dose of
6.15 mg/kg bw, a significant increase in corticosterone
concentration was seen after 1 and 3 h and in aldosterone
concentration 1-6 h after treatment. Plasma corticosteroid levels
were maximal within 1 h, when brain cholinesterase activity was only
slightly inhibited (Osicka-Koprowska et al., 1984).
(iv) Porphyrigenic potential
Chlorfenvinphos induced porphyria in primary cultures of
chicken embryo liver cells. Its porphyrigenic potential was markedly
increased by prior treatment of the cells with a compound that
induces drug metabolizing enzymes (Koeman et al., 1980).
3. Observations in humans
Workers in India were studied while applying chlorfenvinphos to
paddy rice over a period of six days; they were not wearing
protective clothing. Workers applying an emulsifiable concentrate
had decreased plasma cholinesterase activity but no change in
erythrocyte cholinesterase activity. Workers applying granules
containing 10% chlorfenvinphos had no reduction in cholinesterase
activity (Blok et al., 1977).
A group of 33 workers employed in a chlorfenvinphos
manufacturing plant were monitored medically for eight years
(1967-75). Two control groups were used. The exposed group had
slightly lower haemoglobin levels and slightly lower cholinesterase
activity in plasma and erythrocytes. Some changes in
neurophysiological parameters (lower electromyographic voltages,
associated with slower conduction velocities) were observed, but
these were reversed when the workers were removed from exposure
(Ottevanger, 1976).
Workers involved in the manufacture of chlorfenvinphos were
monitored in 1985-86 in a routine programme in which blood and urine
were collected and cholinesterase activity was measured in whole
blood, plasma and erythrocytes. A few cases of depressed plasma
cholinesterase were recorded, which were not, however, correlated
with increases in the urinary concentrations of diethylphosphate and
monoethylphosphate metabolites (Eadsforth, 1987).
Comments
The main step in the biotransformation of chlorfenvinphos is
detoxification by de-ethylation into the corresponding
phosphodiester; microsomal enzymes play an important role.
Interspecies differences in the rate of metabolism of
chlorfenvinphos were found in vitro. Oxidative metabolism by human
liver enzymes was comparable to that of rabbit liver enzymes.
Single oral doses of chlorfenvinphos were very toxic to rats,
toxic to mice and guinea-pigs and moderately to slightly toxic to
rabbits and dogs. The clinical signs observed were consistent with
cholinesterase inhibition and included tremors, fasciculation and
salivation. Pretreatment with enzyme inducers can increase the acute
oral LD50. Interspecies differences in the acute oral LD50
reflect the activity of the hepatic metabolizing enzymes involved in
the detoxification of chlorfenvinphos. WHO (1992) has classified
chlorfenvinphos as extremely hazardous.
In a four-week study in which mice were fed 0, 1, 10, 100 or
1000 ppm chlorfenvinphos in the diet, plasma and erythrocyte
cholinesterase activities were inhibited in animals fed 100 or 1000
ppm. Brain cholinesterase activity was inhibited in males fed 10 or
1000 ppm and in females at all doses; thus, no NOAEL could be
established for female mice. The NOAEL in males was 1 ppm, equal to
0.18 mg/kg bw per day.
In a one-year study in which dogs were fed 0, 3, 100 or 3000
ppm in the diet, inhibition of erythrocyte cholinesterase activity
and increased relative adrenal weight were seen in males; in
females, increased relative thyroid weight was seen in those fed the
highest dose. The NOAEL was 100 ppm, equal to 2.8 mg/kg bw per day.
In a study of the carcinogenicity of chlorfenvinphos, in which
mice were fed 0, 1, 25 or 625 ppm in the diet, no increase in tumour
incidence was observed. Erythrocyte and brain cholinesterase
activity was inhibited in mice fed the highest dose. The NOAEL was
25 ppm, equal to 3.7 mg/kg bw per day.
In a two-year study of toxicity and carcinogenicity in rats fed
diets containing 0, 0.3, 1, 3 or 30 ppm, erythrocyte and brain
cholinesterase activity was inhibited in those fed 30 ppm. There was
no evidence of carcinogenicity. The NOAEL was 3 ppm, equivalent to
0.15 mg/kg bw per day.
In a two-generation study of reproductive toxicity in rats fed
diets containing 0, 1, 10 or 100 ppm, reduced brain cholinesterase
activity, post-implantation loss and breeding losses were observed
in animals fed 10 or 100 ppm. The NOAEL was 1 ppm, equivalent to
0.05 mg/kg bw per day.
In two studies of teratogenicity--one in rats and one in
rabbits--doses that were maternally toxic were not embryotoxic and
there was no indication of teratogenicity. In rats, the NOAEL was 1
mg/kg bw per day for maternal toxicity and 3 mg/kg bw per day, the
highest dose tested, for developmental toxicity. In rabbits, no
NOAEL could be established for maternal toxicity (< 25 mg/kg bw);
the NOAEL for developmental toxicity was > 100 mg/kg bw per day.
Chlorfenvinphos was mutagenic in only one study, in Salmonella
typhimurium TA100 in the presence of an exogenous metabolic system
at doses of at least 1000 µg per plate. It was inactive in other
bacterial tests and did not induce mitotic conversion in yeast or
chromosomal aberrations in human lymphocytes in vitro.
Chlorfenvinphos did not induce chromosomal aberrations in Chinese
hamster bone-marrow cells or dominant lethal effects in male mice
in vivo. The Meeting concluded that chlorfenvinphos was not
genotoxic.
Delayed neurotoxicity in chickens has not been evaluated.
Observations in humans were not suitable for use in estimating
an ADI.
An ADI was established on the basis of an NOAEL of 0.05 mg/kg
bw per day in a two-generation study of reproductive toxicity in
rats and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 3.7 mg/kg bw per day (two-year study of
carcinogenicity)
Rat: 3 ppm, equivalent to 0.15 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
1 ppm, equivalent to 0.05 mg/kg bw per day (two-generation
study of reproductive toxicity)
1 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Rabbit: < 25 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Dog: 100 ppm, equal to 2.8 mg/kg bw (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Study of delayed neurotoxicity in chickens, with estimation of
neuropathy target esterase
2. Further observations in humans
3. Studies of pharmacokinetics in mammals in vivo
References
Allen, T.R., Corney, S.J., Frei, T., Luetkemeier, H., Biedermann, K.
& Springall, C.J. (1992) 4-Week oral range-finding toxicity
(feeding) study with chlorfenvinphos in the dog. RCC Project No.
271934. Unpublished report from Research and Consulting Co.,
Itingen, Switzerland. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Allen, T.R., Corney, S.J., Frei, T., Luetkemeier, H., Biedermann, K.
& Springall, C.J. (1993) 52-Week oral toxicity (feeding) study with
chlorfenvinphos in the dog. RCC Project No. 271945. Unpublished
report from Research and Consulting Co., Itingen, Switzerland.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Arni, P. & Müller, D. (1979) Salmonella/mammalian microsome
mutagenicity test with C8949. Test for mutagenic properties in
bacteria. Experiment no. 78/2589. Unpublished report from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Blok, A.C., Mann, A.H. & Robinson, J. (1977) Organophosphorus
insecticide exposure of sprayers under field conditions in rice in
India. I. Birlane (chlorfenvinphos). TOX 77-005. Unpublished report
from Shell International Research Maatschappy, Toxicology Division.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Brooks, T.M. (1978) Toxicity studies with chlorfenvinphos:
mutagenicity studies with chlorfenvinphos in micro-organisms. Group
Research Report TLGR.00142.78. Unpublished report from Shell
Research Ltd, London. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Brzezinski, J. & Wysocka-Paruszewska, B. (1980) Neurochemical
alterations in rat brain as a test for studying the neurotoxicity of
organophosphorus insecticides. Arch. Toxicol., Suppl. 4, 475-478.
Dean, B.J. (1971) The mutagenic effect of organophosphate
insecticides on Escherichia coli. Group Research Report
TLGR.0034.71. Unpublished report from Shell Research Ltd, London.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Dean, B.J. (1978) Toxicity studies with chlorfenvinphos: chromosome
studies on bone marrow cells of Chinese hamsters after two daily
oral doses of chlorfenvinphos. Group Research Report TLGR.0087.78.
Unpublished report from Shell Research Ltd, London. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Dean, B.J. & Hend, R.W. (1978) Toxicity studies with
chlorfenvinphos: dominant lethal assay in male mice after single
oral doses of chlorfenvinphos. Group Research Report TLGR.0063.78.
Unpublished report from Shell Research Ltd, London. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Dix, K.M., Cassidy, S.L. & Vilkauls, J. (1979) Toxicity of
chlorfenvinphos: teratological studies in rabbits given
chlorfenvinphos orally. Group Research Report TLGR.79.105.
Unpublished report from Shell Research Ltd, London. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Donninger, C., Hutson, D.H. & Pickering, B.A. (1972) The oxidative
dealkylation of insecticidal phosphoric acid triesters by mammalian
liver enzymes. Biochem. J., 126, 701-707.
Dotti, A., Kinder, J., Luetkemeier, H. & Biedermann, K. (1993a)
Chlorfenvinphos. Preliminary study to the two-generation
reproduction study in the rat. RCC Project No. 271822. Unpublished
report from Research and Consulting Co., Itingen Switzerland.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Dotti, A., Kinder, J., Luetkemeier, H. & Biedermann, K. (1993b) Two
generation reproduction study with chlorvinphos in the rat. RCC
Project No. 271833. Unpublished report from Research and Consulting
Co., Itingen, Switzerland. Submitted to WHO by American Cyanamid
Co., Princeton, NJ, USA.
Dzierzawski, A. & Minta, M. (1979) Embryotoxic effects of
chlorfenvinphos and bromfenvinphos in laboratory animals. Bull.
Vet. Inst. Pulawy, 23, 1-2, 32-42.
Eadsforth, C.V. (1987) Biological monitoring of exposure of
operators during manufacture of Birlane in CAS, location CMDU-N,
during 1985-6. Unpublished report from Shell Biomedical Laboratory,
SNR/SNC Pernis. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Gardner, J. (1992) Birlane technical: acute oral and dermal dermal
toxicity in rat, skin and eye irritancy in rabbit and skin
sensitization potential in guinea pig. Unpublished report from Shell
Research Ltd, Sittingbourne Research Centre, Sittingbourne, United
Kingdom. Submitted to WHO by American Cyanamid Co., Princeton, NJ,
USA.
Hutson, D.H. & Wright, A.S. (1980) The effect of hepatic microsomal
monooxygenase induction on the metabolism and toxicity of the
organophosphorus insecticide chlorfenvinphos. Chem. Biol.
Interactions, 31, 93-101.
Hutson, D.H. & Logan, C.J. (1986) Detoxification of the
organophosphorus insecticide chlorfenvinphos by rat, rabbit and
human liver enzymes. Xenobiotica, 16, 87-93.
Ikeda, T., Kojima, T., Yoshida, M., Takahashi, H., Tsuda, S. &
Shirasu, Y. (1990) Pretreatment of rats with an organophosphorus
insecticide, chlorfenvinphos, protects against subsequent challenge
with the same compound. Fundam. Appl. Toxicol., 14, 560-567.
Ikeda, T., Tsuda, S. & Shirasu, Y. (1991) Metabolic induction of the
hepatic cytochrome P450 system by chlorfenvinphos in rats. Fundam.
Appl. Toxicol., 17, 361-367.
Ikeda, T., Tsuda, S. & Shirasu, Y. (1992) Pharmacokinetic analysis
of protection by organophosphorus insecticide, chlorfenvinphos,
against the toxicity of its succeeding dosage in rats. Fundam.
Appl. Toxicol., 18, 299-306.
Koeman, J.H., Debets, F.M.H. & Strik, J.J.T.W.A. (1980) The
porphyrogenic potential of pesticides with special emphasis on
organophosphorus compounds. In: Tordoir, W.F. & van Heemstra,
E.A.H., eds, Field Worker Exposure during Pesticide Application,
Amsterdam, Elsevier Scientific Publishing Co., pp. 157-162.
Maxwell, J.C. & LeQuesne, P.M. (1982) Neuromuscular effects of
chronic administration of two organophosphorus insecticides to rats.
Neurotoxicology, 3, 1-10.
Mayfield, R. & John, D.M. (1986) Effect of Birlane on pregnancy of
the rat. HCR Report No. SLL 84/851593. Unpublished report from
Huntingdon Research Centre, . Submitted to WHO by American Cyanamid
Co., Princeton, NJ, USA.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. &
Shirasu, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116, 185-216.
Osicka-Koprowska, A., Lipska, M. & Wysocka-Paruszewska, B. (1984)
Effects of chlorfenvinphos on plasma corticosterone and aldosterone
levels in rats. Arch. Toxicol., 55, 68-69.
Osumi, Y., Fujiwara, H., Oishi, R. & Takaori, S. (1975) Central
cholinergic activation by chlorfenvinphos, an organophosphate, in
the rat. Jpn. J. Pharmacol., 25, 47-54.
Ottevanger, C.F. (1976) An epidemiological and toxicological study
of occupational exposure to an organphosphorus pesticide. University
of Amsterdam, MD Thesis. Rotterdam, Phoenix & den Oudsten.
Pickering, C.E. (1978) Toxicity of chlorfenvinphos: the
reversibility of cholinesterase and aliesterase inhibition produced
by feeding chlorfenvinphos. Unpublished report from Shell Research,
TLGR0169.78, Sittingbourne United Kingdom. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA
Pickering, R.G. (1980) Toxicity studies on the insecticide
chlorfenvinphos. A two year feeding study in rats. Budget ref.
50070712. Unpublished report from Shell Research Ltd, London.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Rose, G.P. (1982) Toxicology of Birlane: the skin sensitizing
potential of Birlane. Project No. 226/82. Unpublished report from
Shell Research Ltd, Sittingbourne Research Centre, Sittingbourne,
United Kingdom. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Schmid, H., Probst, D., Luetkemeier, H., Weber, K., Wilson, J.T.,
Springall, C.J. & Biedermann, K. (1993) Oncogenicity (feeding) study
with chlorfenvinphos in the mouse. Final Report. RCC Project No.
265926. Unpublished report from Research and Consulting Co.,
Itingen, Switzerland. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening system of pesticides in the microbial system.
Mutat. Res., 40, 19-30.
Socko, R., Gralewicz, S. & Gorny, R. (1989) Neurotoxicity of
chlorphenvinphos, an organo-phosphorus pesticide: effects on blood
and brain cholinesterase activity, open field, behavior and
response-to-change in a 'T' maze in rats. Polish J. Occup. Med.,
2, 294-308.
Strasser, F. & Arni, P. (1988) Chromosome studies on human
lymphocytes. Test material C8949 tech. Test no.: 871201. Unpublished
report from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Takahashi, H., Kojima, T., Ikeda T., Tsuda S. & Shirasu Y. (1991)
Differences in the mode of lethality produced through intravenous
and oral administration of organophosphorus insecticides in rats.
Fundam. Appl. Toxicol., 16, 459-468.
Tennekes, H., Janiak, T., Stucki, H.P., Probst, D., Luetkemeier, H.,
Vogel, O., Schlotke, B., Biedermann, K. & Heusner, W. (1991) 28-Day
range-finding (feeding) study with chlorfenvinphos in the mouse. RCC
Project No. 243202. Unpublished report from Research and Consulting
Co., Itingen, Switzerland. Submitted to WHO by American Cyanamid
Co., Princeton, NJ, USA.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
(c) Long-term toxicity and carcinogenicity
Mice
In a study designed to determine carcinogenic potential,
chlorfenvinphos (purity, 92.8%) was administered for 24 months to
groups of 74 male and 74 female NMRI mice at 1, 25 or 625 ppm in the
diet, equal to 0.15, 3.7 or 93 mg/kg bw per day for males and to
0.2, 5.0 or 119 mg/kg bw per day for females. Twelve mice of each
sex from each group were sacrificed after 52 weeks of treatment; all
females were killed after 90 weeks of treatment and all males after
104 weeks. Clinical signs, food consumption, body weight,
differential leukocyte count, cholinesterase activity and organ
weights were observed, and macroscopic and microscopic examinations
were undertaken. Plasma cholinesterase activity was inhibited in
animals of each sex fed 25 ppm (38-50% inhibition) or 625 ppm
(87-96%). In males and females fed 625 ppm, erythrocyte
cholinesterase activity was decreased by 18-66% and brain
cholinesterase activity by 25-46%. Microscopic examination at
termination of the experiment showed changes in the adrenal cortex
of males fed 625 ppm, which included increased incidence and
intensity of ceroid pigment and focal hypertrophy and increased
severity of nodular hyperplasia. Tumour incidence was not enhanced.
No effects were observed at 25 ppm, equal to 3.7 mg/kg bw per day
(Schmid et al., 1993).
Rats
In a study of both chronic toxicity and carcinogenicity, groups
of 50 male and 50 female Wistar rats were fed chlorfenvinphos
(purity, 90.5%) at 0.3, 1, 3 or 30 ppm in the diet (equivalent to
0.015, 0.05, 0.15 or 1.5 mg/kg bw per day) for 24 months. The
control group consisted of 100 rats of each sex. Clinical signs,
body weight and food consumption were observed. At termination,
organ weights, haematological parameters and the results of clinical
chemistry, urinalysis and macroscopic and microscopic examinations
were recorded. Additional groups of 12 rats of each sex were
examined after 6, 12 and 18 months for haematological and clinical
chemical parameters including cholinesterase activity; after
sacrifice, the organs were weighed and animals fed 0, 0.3 or 30 ppm
chlorfenvinphos were examined macroscopically and histologically.
Plasma cholinesterase activity was inhibited after six months in all
treated animals but thereafter only in rats fed 3 or 30 ppm. In
males and females fed 30 ppm, erythrocyte cholinesterase activity
was inhibited by about 60% and brain cholinesterase activity by
about 45%; erythrocyte cholinesterase activity was inhibited by
about 17% in females fed 1 or 3 ppm after 12 months' exposure. In
all groups and at all intervals during the study, minor but
significant changes were seen in the concentrations of alpha-, ß-
and gamma-globulins. No dose-related or significant macroscopic or
histological changes were observed, and tumour incidences were not
enhanced. The NOAEL in this study was 3 ppm, equivalent to 0.15
mg/kg bw per day (Pickering, 1980).
(d) Reproductive toxicity
Rats
In a range-finding study, 10 male and 10 female Wistar rats
were exposed to 0, 5, 25 or 125 ppm chlorfenvinphos (purity, 92.8%)
mixed with microgranulated feed (equivalent to 0.25, 1.3 or 6.3
mg/kg bw per day) during a three-week period before mating and
throughout mating, gestation and lactation. After they had been
weaned, F1 pups were reared for a further week on their test
diets. The body-weight gain of males and females fed 125 ppm was
slightly reduced. Inhibition of erythrocyte and brain cholinesterase
activity was observed in females fed 5, 25 or 125 ppm and in males
fed 25 or 125 ppm. Plasma cholinesterase activity was inhibited only
in females. Reproductive parameters, including mating, gestation,
parturition, pregnancy rate, lactation and litter characteristics,
were not affected (Dotti et al., 1993a).
In a two-generation study of reproductive toxicity, groups of
25 male and 25 female Wistar rats were treated with 0, 1, 10 or 100
ppm chlorfenvinphos (purity, 92.8%) mixed with microgranulated diet,
equivalent to 0, 0.05, 0.5 or 5 mg/kg bw per day. The diets were fed
to the parental generation during growth, mating, gestation and
lactation and to one litter per generation. Clinical signs, body
weight, food consumption, cholinesterase activity (only in the
parent generation), mating performance and reproductive parameters
were observed. The parents were examined histologically; the pups
were sexed and examined for gross malformations, and the numbers of
stillborn and live pups were recorded. One male fed 100 ppm died on
day 10, and reduced body-weight gain and food consumption were noted
in males and females of the parental and F1 generations at this
dose. A slight decrease in food consumption was observed in F1
males and parental and F1 females fed 10 ppm. Plasma
cholinesterase activity was decreased by 14-78% in males and females
fed 10 or 100 ppm, and erythrocyte cholinesterase activation was
inhibited by 35-63% in males and females fed 100 ppm. Brain
cholinesterase activity was decreased by 32% in males and 38% in
females fed 100 ppm and by 17% in females fed 10 ppm. In the
parental generation, post-implantation loss was higher in animals
fed 10 or 100 ppm than in controls, and postnatal loss was increased
in rats fed 100 ppm; breeding loss up to the end of lactation was
higher in animals fed 10 or 100 ppm. In the F1 generation,
breeding loss was higher than that in controls in animals fed 100
ppm. At this dose, retardation of body-weight gain was noted in both
F1 and F2 pups during the lactation period. The NOAEL in this
study was 1 ppm, equivalent to 0.05 mg/kg bw per day (Dotti et
al., 1993b).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 25 pregnant specific-pathogen-free CrL:COBS CD(SD)BR
rats were treated by gavage with chlorfenvinphos (purity, > 90%) at
0, 0.3, 1 or 3 mg/kg bw per day in corn oil on days 6-15 of
pregnancy. The doses were determined in a preliminary study in which
doses of 3, 6 and 10 mg/kg bw per day caused dose-related loss of
body weight. The rats were sacrificed on day 20 of gestation. No
effects were found on food consumption, pregnancy rate or litter
parameters, including numbers of fetal deaths, implants, corpora
lutea and pre- and post-implantation losses, litter weight, fetal
weight and gravid uteri, or on the incidences of malformations,
visceral and skeletal anomalies and variations. The only clinical
effect seen was the occurrence of small faeces in animals fed 3
mg/kg bw per day from days 3-4 until the end of treatment;
body-weight gain was slightly decreased in animals fed 3 mg/kg bw
per day on days 6-20. There was no indication of irreversible
structural effects. The NOAEL for maternal toxicity in this study
was 1 mg/kg bw per day, and that for developmental toxicity was 3
mg/kg bw per day (Mayfield & John, 1986).
Rabbits
Pregnant banded Dutch rabbits (21-22 per group) received three
capsules containing chlorfenvinphos (purity not specified) at 25, 50
or 100 mg/kg bw per day in corn oil (total volume, 200 µl) on days
6-18 of gestation. A control group of 32 females was used. The
rabbits were sacrificed on day 28 of gestation. No effects were
found on mortality, clinical signs or mean numbers of corpora lutea,
implantations, resorptions or fetal deaths. Body-weight gain and
food consumption were reduced in dams fed 100 mg/kg bw per day on
days 6-12 of gestation. Plasma and erythrocyte cholinesterase
activities were decreased in dams at all doses, erythrocyte activity
being decreased by 55-75%. Brain cholinesterase activity was not
measured. Pre-implantation losses occurred in dams fed 25 or 100
mg/kg bw per day. No differences in the incidences of visceral or
skeletal malformations or variations between the groups were
observed that could be attributed to treatment. There was no
indication of irreversible structural effects. No NOAEL could be
established for maternal toxicity; the NOAEL for embryo- and
fetotoxicity was 100 mg/kg bw per day (Dix et al., 1979).
In a limited experiment with rats, rabbits and hamsters, doses
of 50 mg/kg bw per day and higher increased the incidence of open
eyes and oedema in hamster dams and decreased the weight and length
of hamster fetuses (Dzierzawski & Minta, 1979).
(f) Genotoxicity
The results of tests for the genotoxicity of chlorfenvinphos
are summarized in Table 1.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Chlorfenvinphos (purity, 93.1%), administered to three New
Zealand white rabbits as a 4-h semi-occluded topical application of
0.5 ml undiluted compound, caused very slight erythema within 1 h
after removal of the dressing. The erythema persisted for at least
48 h after treatment, but all overt effects disappeared within seven
days. The compound was considered slightly irritating to rabbit skin
(Gardner, 1992).
The skin sensitizing potential of chlorfenvinphos was examined
in two studies in guinea-pigs. In the first study, 10 male and 10
female Tunstall breeding unit 'P' guinea-pigs received an
intradermal injection of 0.5% (mol/volume) chlorfinvenphos (purity,
91.3%) during the induction period, a topical application of the
undiluted test compound one week later and a challenge application
of undiluted compound two weeks after that. A control group of five
male and five female animals was used. No effect was seen in the
test animals 24 or 48 h after removal of the challenge patches
(Rose, 1982).
In the second study, 10 male and 10 female Dunkin-Hartley
guinea-pigs were given an intradermal induction dose of 2%
(mol/volume) chlorfenvinphos (purity, 93.1%) and undiluted compound
for the topical and challenge applications. A control group of five
male and five female animals was used. Ten of 20 test animals showed
a positive response after 24 h and nine of the same animals after 48
h. None of the control animals responded (Gardner, 1992).
Instillation of 0.1 ml undiluted chlorfenvinphos (purity,
93.1%) into the eyes of three New Zealand white rabbits caused
marked constriction of the pupil, slight conjunctival redness,
slight chemosis and ocular discharge. All signs were reversed within
24 h. The compound is not considered to irritate the eye (Gardner,
1992).
Table 1. Results of tests for the genotoxicity of chlorfenvinphos
End-point Test system Concentration Purity Results Reference
of chlorfenvinphos (%)
In vitro
Reverse mutation S. typhimurium TA98, 25-2025 µg/platea NR Negativeb,c Arni & Müller, 1979
100, 1535, 1537
Reverse mutation E. coli WP2, WP2 hcr, 250 µg/plate NR Negatived Shirasu et al., 1976
S. typhimurium TA1535,
1536, 1537, 1538
Reverse mutation S. typhimurium TA98, Up to 5000 µg/plate NR Negativec,d Moriya et al., 1983
100, 1535, 1537, 1538
Reverse mutation S. typimurium TA100 Up to 5000 µg/plate NR Positivec,e Moriya et al., 1983
Reverse mutation S. typhimurium TA98, Up to 5000 µg/plate NR Negativec,e Moriya et al., 1983
1535, 1537, 1538
Reverse mutation S. typhimurium TA98, 0.2-2000 µg/plate 90.5 Negativeb,c Brooks, 1978
100, 1538
Reverse mutation E. coli B/r WP2 NR > 99 Negativec,d Dean, 1971
Reverse mutation E. coli WP2, WP2 uvrA 0.2-2000 g/plate 90.5 Negativec Brooks, 1978
Reverse mutation E. coli WP2 hcr Up to 5000 µg/plate NR Negativeb,c Moriya et al., 1983
Mitotic gene Saccharomyces 0.001-5.0 mg/ml; toxic 90.5 Negativec,d Brooks, 1978
conversion cerevisiae JD1 at 0.5, 1.0, 5.0 mg/ml
Chromosomal Human lymphocytes 18.8-300 µg/platea,d 93.1 Negativeb,c Strasser & Arni,
aberrations 15.6-250 µg/platea,e 1988
Table 1 (contd)
End-point Test system Concentration Purity Results Reference
of chlorfenvinphos (%)
In vivo
Chromosomal Chinese hamster 25 or 50 mg/kg bw 90.5 Negativec Dean, 1978
aberrations bone marrow per day for 2 days
Dominant lethal CD1 mice (male) 10, 20 or 40 mg/kg bw 90.5 Negativec Dean & Hend, 1978
mutation in DMSO
NR, nor reported; DMSO, dimethyl sulfoxide
a It is not clear whether chlorfenvinphos was tested: the substance was coded C8949.
b In the presence and absence of metabolic activation
c Positive controls were used.
d In the absence of metabolic activation
e In the presence of metabolic activation
(ii) Cholinesterase inhibition and related pharmacological
and neurotoxic effects
After oral administration of chlorfenvinphos at 15 mg/kg bw to
Fischer 344 rats, brain cholinesterase activity was maximally
inhibited (to about 20% of the control value) at 4 h; the inhibition
lasted more than 24 h after administration (Ikeda et al., 1990).
Groups of 10 male and 10 female Wistar rats were fed dietary
concentrations of 0, 0.3, 1, 3 or 30 ppm chlorfenvinphos for four
weeks, or received the same treatments and then received control
diet for four weeks. Administration of 30 ppm resulted in decreased
activity of plasma, erythrocyte and brain cholinesterase. Feeding of
the control diet for four weeks reversed the inhibition of plasma,
erythrocyte and brain cholinesterase activities, resulting in values
of 100, 88 and 91%, respectively (Pickering, 1978).
In a comparison of the lethality of chlorfenvinphos after
intravenous and oral administration, Sprague-Dawley rats given
lethal doses by either route showed typical signs of
anticholinesterase poisoning and marked reductions in brain and
erythrocyte cholinesterase activity. Anaesthetized and conscious
rats administered chlorfenvinphos intravenously had hypertension and
apnoea. After oral administration, breathing stopped before
cessation of heart beats (Takahashi et al., 1991).
The effects of chlorfenvinphos on spontaneous
electroencephalographic, electromyo-graphic and cholinesterase
activity in brain and red blood cells was examined in male Wistar
rats. Single oral doses of 1 mg/kg bw had no effect. Doses over 2
mg/kg bw induced dose-related inhibition of the cholinesterase
activity in brain and erythrocytes. Maximal inhibition of brain
cholinesterase activity was seen 3 h after treatment and lasted for
more than 72 h. Electroencephalography showed a prominent arousal
pattern, and the appearance of slow-wave sleep and para-sleep was
markedly depressed. The duration of the arousal pattern was
proportional to the dose; however, the awake-sleep cycle returned to
the control rate on the second day, and an increase in para-sleep
occurred on the third day (Osumi et al., 1975).
In 30 Sprague-Dawley rats fed a diet containing 150 ppm
chlorfenvinphos (equivalent to 7.5 mg/kg bw per day) for three
months, no effect was seen on the amplitude of the muscular response
to nerve stimulation. Electrophysiological effects were signalled
firstly by a prolonged negative potential after the direct muscle
response to a single nerve impulse and secondly by repetitive
activity. These abnormalities became more marked with time. Double
and repetitive stimulation reduced or abolished the prolonged
negative potential and repetitive activity (Maxwell & LeQuesne,
1982).
Oral administration to male Wistar rats of chlorfenvinphos at
50% of the LD50 caused maximal inhibition of brain cholinesterase
activity (to about 15% of the control value) between 2 and 3 h after
treatment. A significant reduction in brain norepinephrine level was
seen within 15 min, but no consistent diminution was seen
subsequently. During exposure at 5% of the LD50 for 12 weeks,
brain cholinesterase activity was inhibited to 60-80% of control
values and brain norepinephrine content was significantly decreased,
with maximal depletion at week 8 (Brzezinski & Wysocka-Paraszewska,
1980).
Cholinesterase activity in plasma, erythrocytes and different
parts of the brain, open-field behaviour and response to change in a
'T' maze were investigated in two groups of male Wistar rats after
administration of a single intraperitoneal injection of 1 or 3 mg/kg
bw chlorfenvinphos. Cholinesterase activity was strongly inhibited,
to a similar degree, in the blood and brain, with a mean inhibition
of 80% in rats at the high dose and 50% in those at the low dose 3 h
after treatment. Reversal of the inhibition proceeded at similar
rates in the blood and brain and was complete within 96 h with the
low dose and within 14 days with the high dose. No changes in
negotiating the 'T' maze that suggested impairment of short-term
memory were observed up to 14 days after treatment. In the
open-field test, a decrease in short-term memory was manifested by
the absence of increased locomotor and exploratory activity in
response to a new object, only in rats administered 3 mg/kg bw. This
finding suggests that behavioural disturbances last longer than
recovery of cholinesterase activity (Socko et al., 1989).
(iii) Hormone concentrations
In Wistar rats given chlorfenvinphos as a single oral dose of
6.15 mg/kg bw, a significant increase in corticosterone
concentration was seen after 1 and 3 h and in aldosterone
concentration 1-6 h after treatment. Plasma corticosteroid levels
were maximal within 1 h, when brain cholinesterase activity was only
slightly inhibited (Osicka-Koprowska et al., 1984).
(iv) Porphyrigenic potential
Chlorfenvinphos induced porphyria in primary cultures of
chicken embryo liver cells. Its porphyrigenic potential was markedly
increased by prior treatment of the cells with a compound that
induces drug metabolizing enzymes (Koeman et al., 1980).
3. Observations in humans
Workers in India were studied while applying chlorfenvinphos to
paddy rice over a period of six days; they were not wearing
protective clothing. Workers applying an emulsifiable concentrate
had decreased plasma cholinesterase activity but no change in
erythrocyte cholinesterase activity. Workers applying granules
containing 10% chlorfenvinphos had no reduction in cholinesterase
activity (Blok et al., 1977).
A group of 33 workers employed in a chlorfenvinphos
manufacturing plant were monitored medically for eight years
(1967-75). Two control groups were used. The exposed group had
slightly lower haemoglobin levels and slightly lower cholinesterase
activity in plasma and erythrocytes. Some changes in
neurophysiological parameters (lower electromyographic voltages,
associated with slower conduction velocities) were observed, but
these were reversed when the workers were removed from exposure
(Ottevanger, 1976).
Workers involved in the manufacture of chlorfenvinphos were
monitored in 1985-86 in a routine programme in which blood and urine
were collected and cholinesterase activity was measured in whole
blood, plasma and erythrocytes. A few cases of depressed plasma
cholinesterase were recorded, which were not, however, correlated
with increases in the urinary concentrations of diethylphosphate and
monoethylphosphate metabolites (Eadsforth, 1987).
Comments
The main step in the biotransformation of chlorfenvinphos is
detoxification by de-ethylation into the corresponding
phosphodiester; microsomal enzymes play an important role.
Interspecies differences in the rate of metabolism of
chlorfenvinphos were found in vitro. Oxidative metabolism by human
liver enzymes was comparable to that of rabbit liver enzymes.
Single oral doses of chlorfenvinphos were very toxic to rats,
toxic to mice and guinea-pigs and moderately to slightly toxic to
rabbits and dogs. The clinical signs observed were consistent with
cholinesterase inhibition and included tremors, fasciculation and
salivation. Pretreatment with enzyme inducers can increase the acute
oral LD50. Interspecies differences in the acute oral LD50
reflect the activity of the hepatic metabolizing enzymes involved in
the detoxification of chlorfenvinphos. WHO (1992) has classified
chlorfenvinphos as extremely hazardous.
In a four-week study in which mice were fed 0, 1, 10, 100 or
1000 ppm chlorfenvinphos in the diet, plasma and erythrocyte
cholinesterase activities were inhibited in animals fed 100 or 1000
ppm. Brain cholinesterase activity was inhibited in males fed 10 or
1000 ppm and in females at all doses; thus, no NOAEL could be
established for female mice. The NOAEL in males was 1 ppm, equal to
0.18 mg/kg bw per day.
In a one-year study in which dogs were fed 0, 3, 100 or 3000
ppm in the diet, inhibition of erythrocyte cholinesterase activity
and increased relative adrenal weight were seen in males; in
females, increased relative thyroid weight was seen in those fed the
highest dose. The NOAEL was 100 ppm, equal to 2.8 mg/kg bw per day.
In a study of the carcinogenicity of chlorfenvinphos, in which
mice were fed 0, 1, 25 or 625 ppm in the diet, no increase in tumour
incidence was observed. Erythrocyte and brain cholinesterase
activity was inhibited in mice fed the highest dose. The NOAEL was
25 ppm, equal to 3.7 mg/kg bw per day.
In a two-year study of toxicity and carcinogenicity in rats fed
diets containing 0, 0.3, 1, 3 or 30 ppm, erythrocyte and brain
cholinesterase activity was inhibited in those fed 30 ppm. There was
no evidence of carcinogenicity. The NOAEL was 3 ppm, equivalent to
0.15 mg/kg bw per day.
In a two-generation study of reproductive toxicity in rats fed
diets containing 0, 1, 10 or 100 ppm, reduced brain cholinesterase
activity, post-implantation loss and breeding losses were observed
in animals fed 10 or 100 ppm. The NOAEL was 1 ppm, equivalent to
0.05 mg/kg bw per day.
In two studies of teratogenicity--one in rats and one in
rabbits--doses that were maternally toxic were not embryotoxic and
there was no indication of teratogenicity. In rats, the NOAEL was 1
mg/kg bw per day for maternal toxicity and 3 mg/kg bw per day, the
highest dose tested, for developmental toxicity. In rabbits, no
NOAEL could be established for maternal toxicity (< 25 mg/kg bw);
the NOAEL for developmental toxicity was > 100 mg/kg bw per day.
Chlorfenvinphos was mutagenic in only one study, in Salmonella
typhimurium TA100 in the presence of an exogenous metabolic system
at doses of at least 1000 µg per plate. It was inactive in other
bacterial tests and did not induce mitotic conversion in yeast or
chromosomal aberrations in human lymphocytes in vitro.
Chlorfenvinphos did not induce chromosomal aberrations in Chinese
hamster bone-marrow cells or dominant lethal effects in male mice
in vivo. The Meeting concluded that chlorfenvinphos was not
genotoxic.
Delayed neurotoxicity in chickens has not been evaluated.
Observations in humans were not suitable for use in estimating
an ADI.
An ADI was established on the basis of an NOAEL of 0.05 mg/kg
bw per day in a two-generation study of reproductive toxicity in
rats and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 3.7 mg/kg bw per day (two-year study of
carcinogenicity)
Rat: 3 ppm, equivalent to 0.15 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
1 ppm, equivalent to 0.05 mg/kg bw per day (two-generation
study of reproductive toxicity)
1 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Rabbit: < 25 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Dog: 100 ppm, equal to 2.8 mg/kg bw (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Study of delayed neurotoxicity in chickens, with estimation of
neuropathy target esterase
2. Further observations in humans
3. Studies of pharmacokinetics in mammals in vivo
References
Allen, T.R., Corney, S.J., Frei, T., Luetkemeier, H., Biedermann, K.
& Springall, C.J. (1992) 4-Week oral range-finding toxicity
(feeding) study with chlorfenvinphos in the dog. RCC Project No.
271934. Unpublished report from Research and Consulting Co.,
Itingen, Switzerland. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Allen, T.R., Corney, S.J., Frei, T., Luetkemeier, H., Biedermann, K.
& Springall, C.J. (1993) 52-Week oral toxicity (feeding) study with
chlorfenvinphos in the dog. RCC Project No. 271945. Unpublished
report from Research and Consulting Co., Itingen, Switzerland.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Arni, P. & Müller, D. (1979) Salmonella/mammalian microsome
mutagenicity test with C8949. Test for mutagenic properties in
bacteria. Experiment no. 78/2589. Unpublished report from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Blok, A.C., Mann, A.H. & Robinson, J. (1977) Organophosphorus
insecticide exposure of sprayers under field conditions in rice in
India. I. Birlane (chlorfenvinphos). TOX 77-005. Unpublished report
from Shell International Research Maatschappy, Toxicology Division.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Brooks, T.M. (1978) Toxicity studies with chlorfenvinphos:
mutagenicity studies with chlorfenvinphos in micro-organisms. Group
Research Report TLGR.00142.78. Unpublished report from Shell
Research Ltd, London. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Brzezinski, J. & Wysocka-Paruszewska, B. (1980) Neurochemical
alterations in rat brain as a test for studying the neurotoxicity of
organophosphorus insecticides. Arch. Toxicol., Suppl. 4, 475-478.
Dean, B.J. (1971) The mutagenic effect of organophosphate
insecticides on Escherichia coli. Group Research Report
TLGR.0034.71. Unpublished report from Shell Research Ltd, London.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Dean, B.J. (1978) Toxicity studies with chlorfenvinphos: chromosome
studies on bone marrow cells of Chinese hamsters after two daily
oral doses of chlorfenvinphos. Group Research Report TLGR.0087.78.
Unpublished report from Shell Research Ltd, London. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Dean, B.J. & Hend, R.W. (1978) Toxicity studies with
chlorfenvinphos: dominant lethal assay in male mice after single
oral doses of chlorfenvinphos. Group Research Report TLGR.0063.78.
Unpublished report from Shell Research Ltd, London. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Dix, K.M., Cassidy, S.L. & Vilkauls, J. (1979) Toxicity of
chlorfenvinphos: teratological studies in rabbits given
chlorfenvinphos orally. Group Research Report TLGR.79.105.
Unpublished report from Shell Research Ltd, London. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Donninger, C., Hutson, D.H. & Pickering, B.A. (1972) The oxidative
dealkylation of insecticidal phosphoric acid triesters by mammalian
liver enzymes. Biochem. J., 126, 701-707.
Dotti, A., Kinder, J., Luetkemeier, H. & Biedermann, K. (1993a)
Chlorfenvinphos. Preliminary study to the two-generation
reproduction study in the rat. RCC Project No. 271822. Unpublished
report from Research and Consulting Co., Itingen Switzerland.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Dotti, A., Kinder, J., Luetkemeier, H. & Biedermann, K. (1993b) Two
generation reproduction study with chlorvinphos in the rat. RCC
Project No. 271833. Unpublished report from Research and Consulting
Co., Itingen, Switzerland. Submitted to WHO by American Cyanamid
Co., Princeton, NJ, USA.
Dzierzawski, A. & Minta, M. (1979) Embryotoxic effects of
chlorfenvinphos and bromfenvinphos in laboratory animals. Bull.
Vet. Inst. Pulawy, 23, 1-2, 32-42.
Eadsforth, C.V. (1987) Biological monitoring of exposure of
operators during manufacture of Birlane in CAS, location CMDU-N,
during 1985-6. Unpublished report from Shell Biomedical Laboratory,
SNR/SNC Pernis. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Gardner, J. (1992) Birlane technical: acute oral and dermal dermal
toxicity in rat, skin and eye irritancy in rabbit and skin
sensitization potential in guinea pig. Unpublished report from Shell
Research Ltd, Sittingbourne Research Centre, Sittingbourne, United
Kingdom. Submitted to WHO by American Cyanamid Co., Princeton, NJ,
USA.
Hutson, D.H. & Wright, A.S. (1980) The effect of hepatic microsomal
monooxygenase induction on the metabolism and toxicity of the
organophosphorus insecticide chlorfenvinphos. Chem. Biol.
Interactions, 31, 93-101.
Hutson, D.H. & Logan, C.J. (1986) Detoxification of the
organophosphorus insecticide chlorfenvinphos by rat, rabbit and
human liver enzymes. Xenobiotica, 16, 87-93.
Ikeda, T., Kojima, T., Yoshida, M., Takahashi, H., Tsuda, S. &
Shirasu, Y. (1990) Pretreatment of rats with an organophosphorus
insecticide, chlorfenvinphos, protects against subsequent challenge
with the same compound. Fundam. Appl. Toxicol., 14, 560-567.
Ikeda, T., Tsuda, S. & Shirasu, Y. (1991) Metabolic induction of the
hepatic cytochrome P450 system by chlorfenvinphos in rats. Fundam.
Appl. Toxicol., 17, 361-367.
Ikeda, T., Tsuda, S. & Shirasu, Y. (1992) Pharmacokinetic analysis
of protection by organophosphorus insecticide, chlorfenvinphos,
against the toxicity of its succeeding dosage in rats. Fundam.
Appl. Toxicol., 18, 299-306.
Koeman, J.H., Debets, F.M.H. & Strik, J.J.T.W.A. (1980) The
porphyrogenic potential of pesticides with special emphasis on
organophosphorus compounds. In: Tordoir, W.F. & van Heemstra,
E.A.H., eds, Field Worker Exposure during Pesticide Application,
Amsterdam, Elsevier Scientific Publishing Co., pp. 157-162.
Maxwell, J.C. & LeQuesne, P.M. (1982) Neuromuscular effects of
chronic administration of two organophosphorus insecticides to rats.
Neurotoxicology, 3, 1-10.
Mayfield, R. & John, D.M. (1986) Effect of Birlane on pregnancy of
the rat. HCR Report No. SLL 84/851593. Unpublished report from
Huntingdon Research Centre, . Submitted to WHO by American Cyanamid
Co., Princeton, NJ, USA.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. &
Shirasu, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116, 185-216.
Osicka-Koprowska, A., Lipska, M. & Wysocka-Paruszewska, B. (1984)
Effects of chlorfenvinphos on plasma corticosterone and aldosterone
levels in rats. Arch. Toxicol., 55, 68-69.
Osumi, Y., Fujiwara, H., Oishi, R. & Takaori, S. (1975) Central
cholinergic activation by chlorfenvinphos, an organophosphate, in
the rat. Jpn. J. Pharmacol., 25, 47-54.
Ottevanger, C.F. (1976) An epidemiological and toxicological study
of occupational exposure to an organphosphorus pesticide. University
of Amsterdam, MD Thesis. Rotterdam, Phoenix & den Oudsten.
Pickering, C.E. (1978) Toxicity of chlorfenvinphos: the
reversibility of cholinesterase and aliesterase inhibition produced
by feeding chlorfenvinphos. Unpublished report from Shell Research,
TLGR0169.78, Sittingbourne United Kingdom. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA
Pickering, R.G. (1980) Toxicity studies on the insecticide
chlorfenvinphos. A two year feeding study in rats. Budget ref.
50070712. Unpublished report from Shell Research Ltd, London.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Rose, G.P. (1982) Toxicology of Birlane: the skin sensitizing
potential of Birlane. Project No. 226/82. Unpublished report from
Shell Research Ltd, Sittingbourne Research Centre, Sittingbourne,
United Kingdom. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Schmid, H., Probst, D., Luetkemeier, H., Weber, K., Wilson, J.T.,
Springall, C.J. & Biedermann, K. (1993) Oncogenicity (feeding) study
with chlorfenvinphos in the mouse. Final Report. RCC Project No.
265926. Unpublished report from Research and Consulting Co.,
Itingen, Switzerland. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening system of pesticides in the microbial system.
Mutat. Res., 40, 19-30.
Socko, R., Gralewicz, S. & Gorny, R. (1989) Neurotoxicity of
chlorphenvinphos, an organo-phosphorus pesticide: effects on blood
and brain cholinesterase activity, open field, behavior and
response-to-change in a 'T' maze in rats. Polish J. Occup. Med.,
2, 294-308.
Strasser, F. & Arni, P. (1988) Chromosome studies on human
lymphocytes. Test material C8949 tech. Test no.: 871201. Unpublished
report from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Takahashi, H., Kojima, T., Ikeda T., Tsuda S. & Shirasu Y. (1991)
Differences in the mode of lethality produced through intravenous
and oral administration of organophosphorus insecticides in rats.
Fundam. Appl. Toxicol., 16, 459-468.
Tennekes, H., Janiak, T., Stucki, H.P., Probst, D., Luetkemeier, H.,
Vogel, O., Schlotke, B., Biedermann, K. & Heusner, W. (1991) 28-Day
range-finding (feeding) study with chlorfenvinphos in the mouse. RCC
Project No. 243202. Unpublished report from Research and Consulting
Co., Itingen, Switzerland. Submitted to WHO by American Cyanamid
Co., Princeton, NJ, USA.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
