
CLETHODIM
First draft prepared by
M. Caris,
Bureau of Chemical Safety,
Health Canada, Ottawa, Canada
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Embryotoxicity and teratogenicity
Genotoxicity
Special studies
Skin and eye irritation and skin sensitization
Liver cytochrome induction
Studies on metabolites
Acute toxicity
Short-term toxicity
Embryotoxicity and teratogenicity
Genotoxicity
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Clethodim is a new, selective cyclohexanedione herbicide which
is effective against a wide range of annual and perennial grasses
and has little or no activity against broadleaf weeds and sedges. It
exerts its activity by inhibiting acetyl coenzyme A carboxylase, an
enzyme common to the pathways of biosynthesis of fatty acids and
flavonoids. Interference occurs at the acetyl coenzyme A-malonyl
coenzyme A transferase site (Rendina & Felts, 1988).
Clethodim was considered for the first time by the WHO Expert
Group at the 1994 Joint Meeting on Pesticide Residues.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution and excretion
Male and female Crl:CD(SD)BR rats were given single oral doses
of [propyl-1-14C]-clethodim at 4.4 or 468 mg/kg bw or unlabelled
test material at 4.5 mg/kg bw per day for 14 consecutive days before
treatment with a single radiolabelled dose of 4.8 mg/kg bw.
Elimination was rapid: 94-98% of the administered dose was excreted
within 48 h after treatment. The principal route of excretion was
the urine (87-93%), and a smaller percentage (9-17%) of the
radioactivity was eliminated in the faeces. The mean amount of
radioactivity excreted in expired air as carbon dioxide represented
0.5-1% of the administered dose. Although the elimination patterns
were similar in all groups, the rate of elimination was somewhat
faster in animals that were administered the single low dose of 4.4
mg/kg bw (98% eliminated within 40 h) than in those given the single
high dose of 468 mg/kg bw (98% within 50 h). No differences in
elimination rate were seen for animals of either sex administered
repeated low doses of clethodim. Seven days after treatment, the
total amount of radiolabel recovered from organs and tissues was
less than 1% of the administered dose. The highest residual tissue
concentrations were found in the adrenals, kidney and liver. There
were no significant dose-related or sex-specific differences in
tissue distribution, when expressed as a proportion of the dose
administered, and there was no evidence of bioaccumulation (Rose et
al., 1988a).
Groups of 8-10 white Leghorn laying hens were given
[cyclohexene-4,6-14C]-clethodim at 0, 2.1 or 51.3 mg/kg bw per day
for five consecutive days and were sacrificed about 4 h after the
final treatment. Radioanalysis showed that 78% of the low dose and
85% of the high dose of administered radioactivity was excreted in
the faeces; 1.9% of the low dose and 4.2% of the high dose were
found in the tissues. The radiolabel was distributed in the tissues
in the following order of decreasing concentration: gastrointestinal
tract > kidney > liver. In eggs, 0.1% of the low dose and 0.3% of
the high dose were found; the levels were highest in egg whites,
intermediate in shells and lowest in yolks (Lee et al., 1988).
A single lactating goat received [propyl-1-14C]-clethodim at
1.16 mg/kg bw per day in alfalfa diet, in three equal daily doses of
14.2 mg for three days and then a single dose of 14.2 mg on the
fourth day, for a total of 10 doses. One female goat served as
control. The treated goat was sacrificed 4 h after the final dose.
Clethodim was rapidly absorbed: the peak blood radiocarbon
concentration (0.273 ppm) was achieved within the first hour after
the initial dose. The mean amount of radiolabel recovered in the
urine represented 56% of the administered dose and that in the
faeces, 34%. The total amount of radiolabel found in the milk, blood
and tissues represented less than 1% of the amount administered.
Milk contained only 0.14%; the peak concentration (0.035 ppm) was
attained after the sixth dose. The tissues contained 0.37% and the
blood, 0.22% of the dose. The highest residual tissue concentrations
were detected in the liver and kidney (Rose et al., 1988b).
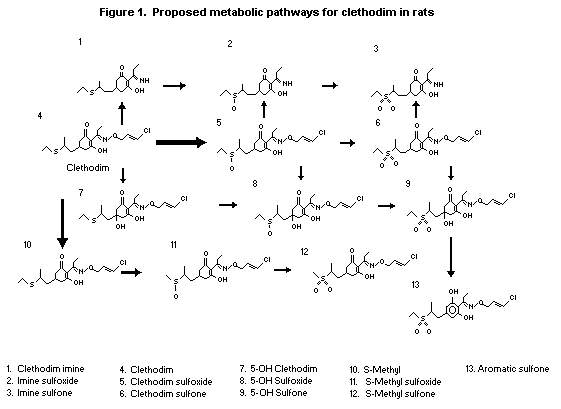
(b) Biotransformation
The proposed metabolic pathways for clethodim in rats are
depicted in Figure 1. It is postulated that once clethodim has been
absorbed it can be (i) oxidized to clethodim sulfoxide (dominant
process), (ii) converted to the S-methyl via a sulfonium cation
intermediate, (iii) cleaved at the oxime N-O bond to generate imine
or (iv) hydroxylated at the 5 position.
In the study described above in which male and female
Crl:CD(SD)BR rats were given single oral doses of [propyl-1-
14C]-clethodim or unlabelled material (Rose et al., 1988a), a
supplementary group of male rats were given a single oral dose of
450 mg/kg bw radiolabelled compound to ensure a sufficiently large
quantity of labelled metabolites. Urine and faeces were collected
and analysed for the parent compound and its metabolites. The
metabolic profiles of males and females in all dose groups were
remarkably similar. Nine urinary metabolites were identified and
characterized. The major metabolite was clethodim sulfoxide
(representing 65-75% of the administered dose), and smaller amounts
were found of the imine sulfoxide (6-13%), the sulfone (1-3%), the
5-hydroxy sulfoxide/sulfone (0.5-1.5%) and the oxazole sulfone
(0-5%). The primary faecal metabolites, which accounted for more
than 1% of the radiolabel, were identified as clethodim sulfoxide
and the imine sulfoxide. Minor (present as < 1% of the dose)
urinary and faecal metabolites were the oxazole sulfoxide, the
demethyl sulfoxide and the aromatic sulfone. Other minor faecal
metabolites were clethodim sulfone, the trione sulfoxide and
c-olefins. Parent clethodim accounted for about 1% of the
administered dose (Rose et al., 1988a).
 Two groups of eight white Leghorn laying hens were given daily
doses of [cyclohexene-4,6-14C]-clethodim at 2.1 or 51.3 mg/kg bw
per day by capsule for five consecutive days. The hens were
sacrificed about 4 h after the final dose, and tissues were
collected for analysis. Two major metabolites, clethodim sulfone and
clethodim sulfoxide, were identified in tissues and eggs. Clethodim
sulfone accounted for up to 57% of tissue levels of radiolabel,
whereas clethodim sulfoxide accounted for 10-31%. Clethodim
sulfoxide was the principal metabolite in egg white (26-82%) and
yolk (25-37%); parent clethodim was detected in significantly
smaller amounts in both tissues and eggs. The proposed metabolic
pathway in chickens was different from and considered to be simpler
than that observed in rats and goats, since none of the imine,
5-hydroxy or S-methyl analogues was detected in chickens (Lee et
al., 1988).
In the study described above in which a lactating goat received
[propyl-1-14C]-clethodim (Rose et al., 1988b), hind- and
forequarter muscle, peritoneal and subcutaneous fat, liver, kidneys,
heart and blood were collected to allow characterization of
metabolites. The major urinary metabolite was clethodim sulfoxide,
which accounted for 67% of the urinary radiocarbon. Other urinary
radiolabelled components were identified as clethodim (3-27%) and
the demethyl sulfoxide (12-18%), S-methyl (7-13%), imine sulfoxide
(1.5-2.8%), sulfone (1.5-2.2%) and 5-hydroxy sulfoxide (0-3%). In
the milk, about half of the radiocarbon could be extracted into
organic solvents and occurred in clethodim, clethodim sulfoxide and
clethodim demethyl sulfoxide; the other half of the radiocarbon was
water soluble and was shown to be 14C-lactose. In blood and
tissues, the maximal extractable radiocarbon residues accounted for
77-95% of the radiolabel and were identified as clethodim and the
sulfoxide, demethyl sulfoxide, imine sulfoxide, sulfone and
5-hydroxy sulfone. The proposed metabolic pathway in goats was
essentially the same as that proposed for rats (Rose et al.,
1988b).
2. Toxicological studies
(a) Acute toxicity
Technical-grade clethodim has been tested for acute toxicity in
mice, rats and rabbits. The results are summarized in Table 1.
Clethodim was virtually non-toxic after oral or dermal
administration to rabbits or after inhalation by rats. Slight to
moderate toxicity was observed in mice and rats treated orally, with
LD50 levels of 1360-2570 mg/kg bw. Clethodim administered
intraperitoneally was moderately toxic to rats, with a combined
LD50 for the two sexes of 1117 mg/kg bw.
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female Charles River CD-1 mice were
fed diets containing technical-grade clethodim (purity, 83.3%) for
four weeks at 100, 250, 625, 1500 or 4000 ppm, equivalent to 0, 15,
38, 94, 225 or 600 mg/kg bw per day. A slight anaemic process was
manifested in male mice as a decreased erythrocyte count at >
1500 ppm, decreased haemoglobin at > 625 ppm and lowered
haematocrit at 4000 ppm. Increased liver weights, which were
correlated microscopically with centrilobular hypertrophy, were
observed in males fed 1500 ppm and in animals of each sex treated at
the highest dose of 4000 ppm. An increased incidence of focal
coagulative necrosis of the liver was recorded in males treated at
> 1500 ppm. Treatment appeared to have no effect on survival,
clinical signs, body weight or food consumption. The NOAEL was 250
ppm, equivalent to 38 mg/kg bw per day, on the basis of the
dose-related decrease in erythroid parameters at dietary levels >
625 ppm (Cox & Slabik, 1986).
Rats
Technical-grade clethodim (purity, 83.2%) was applied in
carboxymethylcellulose and Tween 80 to the skin of groups of six
male and six female Crl:CD BR rats at doses corresponding to 0, 10,
100 or 1000 mg/kg bw per day for 6 h/day, five days per week for
four weeks, for a total of 21 exposures. A dose-related increase in
the occurrence of skin irritation was observed at all dose levels.
Clinical signs seen at 1000 mg/kg bw per day were described as
anogenital discharge and staining of the fur, alopecia and red nasal
discharge. At the highest dose, group mean body weights,
corresponding weight gains and feed efficiency values were decreased
in males, and increased liver weights were recorded in females. In
males, a slight increase was observed in kidney regeneration, which
was described as a minimal multifocal lesion. Although this finding
was observed only in treated rats, evidence of chronic progressive
nephropathy is not uncommon in this strain of rat and was not
considered to represent a treatment-related effect. The NOAEL for
systemic toxicity under the conditions of this study was thus 100
mg/kg bw per day (Cushman et al., 1987a).
Table 1. Acute toxicity of technical-grade clethodim in male and female rodents
Species, Route and vehicle LD50 Purity Reference
strain (mg/kg bw) (%)
Mouse, CD-1 Oral; M: 2570 83.3 Cox & Zoetis, 1986
Tween 80 + CMC F: 2430 (technical)
Rat, Crl:CD Oral; M: 1630 83.3 Cushman et al., 1986d
(SD)BR Tween 80 + CMC (technical)
Rat, Crl:CD Intraperitoneal; M: 1040 NR Cox, 1988
(SD)BR Tween 80 + CMC F: 1200
Rat, Inhalation M&F: 83.3 Griffis et al., 1986
Sprague-Dawley > 3.9 mg/l (technical)
Rabbit, New Oral; M: > 2000 and 82.5 Dougherty et al., 1989
Zealand Tween 80 + CMC < 5000 (technical)
white F: > 5000
Rabbit, New Dermal M&F: > 5000 83.3 Cushman et al., 1987b
Zealand (technical)
white
NR, not reported; Tween 80, 0.5% polyoxyethylenesorbitan monooleate; CMC,
carboxymethylcellulose
In a preliminary five-week study, groups of 10 male and 10
female Crl:CD(SD)BR rats were treated with technical-grade clethodim
(purity, 83.4%) at dietary levels of 0, 5, 200, 1000, 4000 or 8000
ppm, equal to 0.3, 13, 66, 261 or 515 mg/kg bw per day for males and
0.3, 14, 71, 291 or 554 mg/kg bw per day for females.
Treatment-related effects were decreased body-weight gain and food
consumption (particularly during the first week of the study) in
animals of each sex at 4000 and 8000 ppm; increased liver weights at
> 4000 ppm; and slight to mild centrilobular hypertrophy in males
at 1000 ppm and in animals of each sex at 4000 and 8000 ppm.
Dose-related, statistically significant decreases in haemoglobin
concentration were noted in males at > 1000 ppm and in
haematocrit in males at > 4000 ppm. Raised cholesterol
concentrations were recorded in males receiving 8000 ppm, and raised
serum uric acid levels were seen in females given > 4000 ppm.
Treatment had no effect on survival, clinical signs or urinary
parameters. The NOAEL in this study was 200 ppm, equal to 13 mg/kg
bw per day, on the basis of decreases in erythroid parameters and
centrilobular hypertrophy of the liver at 1000 ppm and more (Cisson
& Eisenlord, 1986).
Technical-grade clethodim (purity, 84%) was administered to
groups of 12 male and 12 female Crl:CD(SD)BR rats for 13 weeks at
dietary levels of 0, 50, 500, 2500 or 5000 ppm, equal to 2, 25, 134
or 280 mg/kg bw per day for males and 3, 30, 160 or 340 mg/kg bw per
day for females. In order to study the reversibility of potential
treatment-related effects, an additional 12 rats of each sex were
assigned to the groups receiving 0, 2500 and 5000 ppm and were kept
for a six-week recovery period after the end of treatment with
clethodim. Significantly decreased body weights and weight gains
were recorded in males treated with 2500 ppm and in animals of each
sex treated with 5000 ppm. Decreased mean food consumption was noted
in both males and females at 5000 ppm. Significantly ( p < 0.05)
increased concentrations of cholesterol, total protein and globulin
recorded in males fed 5000 ppm were within the normal control range
of variability. Increased liver weights and increased incidence and
severity of centrilobular hypertrophy of the liver were noted in
males and females fed 2500 and 5000 ppm. A slightly increased
incidence of renal focal regeneration was seen in males treated at
> 2500 ppm. The pathologist did not consider this finding to be
related to treatment; rather, these lesions were regarded as typical
of the earliest minimal stage of spontaneous chronic progressive
nephropathy. Treatment did not affect survival, clinical signs,
ophthalmoscopic or haematological parameters or the results of
urinalysis. No treatment-related effects were seen after the
six-week recovery period. The NOAEL was 500 ppm, equal to 25 mg/kg
bw per day, as determined by an increased incidence of hepatic
centrilobular hypertrophy and decreased body-weight gain in animals
of each sex treated at 2500 ppm and above (Dougherty et al.,
1987).
Dogs
Groups of four male and four female beagle dogs received
gelatin capsules containing technical-grade clethodim (purity,
83.3%) orally at 0, 1, 25, 75 or 125 mg/kg bw per day for 13 weeks.
Treatment affected blood chemistry in females, which had increased
cholesterol levels at 75 mg/kg bw per day and above and increased
alkaline phosphatase activity at 125 mg/kg bw per day. An increased
globulin concentration, with a concomitant decrease in the
albumin:globulin ratio, was noted in males treated at 125 mg/kg bw
per day. Significantly ( p < 0.05) increased total leukocyte
counts were seen in males and decreased activated partial
thromboplastin time was seen in females after one month at 75 and
125 mg/kg bw per day. As no similar differences from control values
were seen, however, after two or three months of treatment, and the
only differences seen were in one sex, the significance of these
findings is doubtful. Increased liver weights were recorded in males
and females fed 75 or 125 mg/kg bw per day. Slight increases in the
severity of cytoplasmic vesiculation and vacuolation of the
centrilobular hepatocytes were seen in animals of each sex treated
at 125 mg/kg bw per day. Treatment did not affect survival, clinical
signs, ophthalmoscopic parameters, body weight or food consumption.
The NOAEL was 25 mg/kg bw per day on the basis of elevated
cholesterol levels and increased liver weights at 75 mg/kg bw per
day and above (Daly & Knezevich, 1987).
In a one-year study, groups of six male and six female beagle
dogs were administered technical-grade clethodim (purity, 83.3%) in
gelatin capsules at doses of 0, 1, 75 or 200-300 mg/kg bw per day.
In the absence of any signs of overt toxicity, the high dose of 200
mg/kg bw per day was increased after seven weeks of treatment to 300
mg/kg bw per day. Significant effects on haematological parameters
were seen, expressed as increased platelet and leukocyte counts in
females at 75 mg/kg bw per day and in males and females at 300 mg/kg
bw per day. At 300 mg/kg bw per day, erythroid parameters
(erythrocyte, haemoglobin and haematocrit values) were decreased in
animals of each sex, and reticulocyte and segmented neutrophil
counts were increased in females. Treatment-related effects on blood
chemistry were seen as increased levels of cholesterol,
triglycerides, alkaline phosphatase and alanine aminotransferase in
males and females at 300 mg/kg bw per day. Decreased serum glucose
concentrations were noted in both males and females at the high dose
and in females treated at 75 mg/kg bw per day. Increased liver
weights were recorded in animals of each sex at 75 and 300 mg/kg bw
per day. Significantly ( p < 0.05) increased thyroid weights seen
in males at 300 mg/kg bw per day were not correlated with any
microscopic changes. Histopathological alterations in animals of
each sex treated at 300 mg/kg bw per day included hepatocellular
enlargement, increased granular pigment (which was not associated
with bile, iron or acid-fast lipofuscin) and slight hypercellularity
of the bone marrow; the last finding was also observed in one male
and one female at 75 mg/kg bw per day. The significance of a
slightly increased incidence of pigment (not otherwise
characterized) in the spleen of females receiving 75 or 300 mg/kg bw
per day was uncertain. Treatment did not affect survival, clinical
signs, ophthalmoscopic parameters, body weight, food consumption or
the results of urinalysis. The NOAEL was 1 mg/kg bw per day on the
basis of treatment-related effects on haematological and blood
chemical parameters, increased liver weight and hypercellularity of
the bone marrow at 75 mg/kg bw per day and above (Cox & Zoetis,
1988a).
(c) Long-term toxicity and carcinogenicity
Mice
Technical-grade clethodim (purity, 83.3%) was administered in
the diet to groups of 60 male and 60 female Crl:CD-1 (ICR)BR mice
for 78 weeks at 0, 20, 200, 1000 or 2000-3000 ppm, equivalent to 3,
30, 150 or 300-450 mg/kg bw per day. The high dose was increased to
3000 ppm after 15 weeks of treatment, when haematological assessment
did not show the significant decrease in erythrocyte parameters seen
in the four-week preliminary study. Ten mice of each sex were
sacrificed after 52 weeks of treatment in each group for interim
assessment. A significant ( p < 0.05) trend to decreased survival
was noted among males and females fed 3000 ppm, with survival at 78
weeks of 58, 66, 60, 52 and 32% males and 67, 84, 80, 59 and 48%
females, respectively. The predominant cause of death at this dose
was increased incidence and severity of systemic amyloidosis.
Decreased erythrocyte counts were recorded in animals of each sex at
3000 ppm, with concomitant decreases in haemoglobin and haematocrit
values in males. After 52 weeks of treatment, increased liver
weights and centrilobular hypertrophy of the liver were observed in
males at 1000 ppm and in males and females at 3000 ppm. Increased
pigment, described as morphologically compatible with haemosiderin
and bile, was noted in males at 3000 ppm. After 78 weeks of
treatment, the hepatic changes included increased liver weights in
females at 3000 ppm, centrilobular hypertrophy and increased pigment
in both males and females at 1000 and 3000 ppm and bile-duct
hyperplasia in males at > 1000 ppm. An increased incidence of
multifocal, amphophilic alveolar lung macrophages was also observed
in animals of each sex treated at > 1000 ppm. No significant
effect was seen on body weight, food consumption or clinical signs.
The NOAEL was 200 ppm, equivalent to 30 mg/kg bw per day, on the
basis of hepatic changes, notably centrilobular hypertrophy,
increased pigment and bile-duct hyperplasia, and an increased
incidence of alveolar macrophages in the lungs of mice treated at
1000 ppm and above. There was no evidence that clethodim has
carcinogenic potential (Cox & Zoetis, 1988b).
Rats
Groups of 65 male and 65 female Crl:CD (SD)BR rats were
administered technical-grade clethodim (purity, 83%) in the diet at
levels of 0, 5, 20, 500 or 2500 ppm, equal to 0.2, 0.6, 16 or 86
mg/kg bw per day for males and 0.2, 0.7, 21 or 110 mg/kg bw per day
for females, for two years. Ten rats of each sex in each group were
sacrificed after one year. After two years, 47, 49, 46, 50 and 33%
of males and 55, 58, 51, 40 and 49% of females were still alive in
the five dose groups, respectively. Treatment did not affect cause
or time of death, clinical signs, ophthalmoscopic or haematological
parameters or the results of clinical chemistry or urinalysis.
Body-weight gain and food consumption were significantly decreased
in animals of each sex during the first year of treatment with 2500
ppm. Food intake, calculated relative to body weight, was slightly
greater in males and females at 2500 ppm than in the other treated
groups. At interim sacrifice after one year, increased liver weights
and slight to mild centrilobular hypertrophy were noted in rats of
each sex fed 2500 ppm. Increased liver weights seen in females at
500 ppm were not correlated with any microscopically discernible
change. At terminal sacrifice, liver weights of females at 2500 ppm
were increased; males had no significant increase in liver weight,
and no treatment-related centrilobular hypertrophy was seen in
animals of either sex. Females treated at 2500 ppm had a slightly
greater (12%) incidence of binucleated cells in the liver than the
controls (2%), but the effect was of uncertain toxicological
significance. Clethodim at dietary levels up to 3000 ppm showed no
evidence of carcinogenic potential. The NOAEL was 500 ppm, equal to
16 mg/kg bw per day, on the basis of decreased body-weight gain,
decreased food intake, increased liver weight and associated
centrilobular hypertrophy at 2500 ppm (Dougherty et al., 1988a).
(d) Reproductive toxicity
Rats
In a preliminary study, groups of eight male and eight female
Crl:CD(SD)BR rats were fed technical-grade clethodim (purity, 83.3%)
at dietary levels of 0, 500, 2000 or 5000 ppm, equivalent to 25, 100
or 250 mg/kg bw per day, for one week before and during mating.
Males were sacrificed after mating, whereas females were treated
throughout gestation and up to day 7 of the lactation period for a
single litter. Parental animals of each sex that received 5000 ppm
had decreased body-weight gain and food intake ( p < 0.05, only in
males). Mean pup weights and corresponding weight gains were
decreased at all dietary levels. No adverse effects on reproductive
parameters were evident at the highest dose (McKensie, 1986).
In a two-generation (one litter per generation) study, groups
of 30 male and 30 female Crl:COBS CD(SD) rats were treated with
technical-grade clethodim (purity, 83%) at dietary levels of 0, 5,
20, 500 or 2500 ppm, equal to 0.1, 1.4, 39 or 190 mg/kg bw per day
for males and 0.2, 1.8, 45 or 220 mg/kg bw per day for females. Mean
body weight was decreased at 2500 ppm in both males and females of
the F1 generation and in males of the F0 generation. Occasional
significant ( p < 0.05) decreases in food consumption were
recorded in F1 males and females treated with 2500 ppm. Treatment
had no effect on parental survival, clinical signs, organ weights or
histopathological manifestations; there were no adverse effects on
reproductive indices (mating, fertility, pregnancy and gestation);
and there was no effect on litter size, sex ratio, pup weight or pup
viability. A significant ( p < 0.05) increase in the number of
stillborn pups was observed in the litters of F1 dams treated at
2500 ppm; however, this finding was not considered to be related to
treatment, since the value was within the historical control range,
and no similar increase was seen in the subsequent F2 litter. The
NOAEL for parental systemic toxicity was 500 ppm, equal to 39 mg/kg
bw per day, on the basis of decreased body weights and food
consumption at 2500 ppm. The NOAEL for adverse effects on
reproduction in the F0 and F1 generations was 2500 ppm, the
highest dietary level tested, equal to 190 mg/kg bw per day (Tellone
et al., 1987).
(e) Embryotoxicity and teratogenicity
Rats
In a preliminary range-finding study, groups of 10 mated female
Crl:CD(SD) rats were given technical-grade clethodim (purity, 82.6%)
in Tween 80 and carboxymethylcellulose by gavage at 0, 50, 150, 300
or 500 mg/kg bw per day during days 6-15 of gestation. The day on
which evidence of mating was observed was designated day 0 of
gestation. Clinical signs of toxicity were an increased incidence of
excessive salivation in females at 300 and 500 mg/kg bw per day, and
slightly decreased body-weight gain, decreased mean number of
uterine implantations and decreased mean fetal body weights at 500
mg/kg bw per day. External examination of the fetuses failed to
reveal any gross findings related to treatment (Schroeder & Daly,
1986).
Groups of 25 mated female Crl:CD(COBS) rats were treated with
technical-grade clethodim (purity, 82.6%) in Tween 80 and
carboxymethylcellulose daily by gavage at 0, 10, 100, 350 or 700
mg/kg bw per day during days 6-15 of gestation. The day on which
evidence of mating was observed was designated day 0 of gestation.
Treatment with clethodim at 350 mg/kg bw per day and more decreased
maternal body weight. Toxicity was manifested at 700 mg/kg bw per
day as increased mortality (20%), decreased food intake and clinical
signs of poor physical condition, including red nasal discharge,
chromodacryorrhoea and staining of the anogenital region. Excessive
salivation was observed in females treated at 350 and 700 mg/kg bw
per day. Significantly decreased fetal weights and increased
incidences of fetuses with skeletal variations or at least one
ossification variation were recorded at 350 mg/kg bw per day and
more. The skeletal variations were apparent as definite delays in
ossification and were identified by incompletely or unossified
vertebral elements (thoracic, sacral, caudal) and unossified fifth
and/or sixth sternebrae. Treatment at the highest dose, 700 mg/kg bw
per day, induced a significant increase in the number of fetuses
(8/221; 3.6%) and litters (6/18; 33.2%) with external malformations,
comprising primarily an increased incidence (seven fetuses in six
litters) of tail defects characterized by short, filamentous or
absent tails. An imperforated anus was recorded in two fetuses in
two litters at this dose. Visceral malformations (exencephaly,
defects of the lung, aortic arch and large intestine, absence of
kidneys, bladder or ureter) were observed in four fetuses in three
litters of dams exposed to 700 mg/kg bw per day but not in
concurrent or historical controls (5032 fetuses in 806 litters; 38
studies). Three of the four fetuses with visceral malformations also
had external malformations. The NOAEL for maternal and developmental
toxicity was 100 mg/kg bw per day. The NOAEL for teratogenicity was
350 mg/kg bw per day on the basis of an increased incidence of
malformations, particularly tail defects, observed at the maternally
toxic and lethal dose of 700 mg/kg bw per day (Schroeder & Daly,
1987).
Rabbits
In a range-finding study, groups of eight artificially
inseminated female New Zealand white Hra: specific-pathogen-free
rabbits were treated by gavage with clethodim (purity, 83.3%) in
Tween 80 and carboxymethylcellulose at 0, 50, 150, 300 or 500 mg/kg
bw per day on days 7-19 of gestation. The day of insemination was
designated day 0 of gestation. Maternal toxicity was seen at all
doses as decreased body-weight gain and food consumption. An
increased incidence of alopecia was recorded at 150 mg/kg bw per day
and more. Dried faeces and gastrointestinal lesions (hairball and/or
ulceration of the gastric mucosa) were observed at doses of 300
mg/kg bw per day and more. In the groups given 300 and 500 mg/kg bw
per day, two of seven pregnant rabbits died, apparently due to
concomitant weight loss, decreased food intake, gastrointestinal
lesions and/or abortion. In the group given 500 mg/kg bw per day,
four of seven rabbits aborted, and one rabbit delivered prematurely.
Data on litters of dams given 500 mg/kg bw per day could not
effectively be evaluated, as only one female survived to term; dams
given 300 mg/kg bw per day had an increased incidence of resorption
and decreased litter weights when compared with the controls
(Dearlove et al., 1986).
Groups of 20 artificially inseminated female New Zealand white
Hra specific-pathogen-free rabbits were administered
technical-grade clethodim (purity, 83.3%) in Tween 80 and sodium
carboxymethylcellulose by gavage at doses of 0, 25, 100 or 300 mg/kg
bw per day on days 7-19 of gestation. The day of insemination was
designated day 0 of gestation. Mean body weights, corresponding
weight gains and food consumption were decreased in dams given 100
or 300 mg/kg bw per day. An increased incidence of dried faeces was
also noted at 100 mg/kg bw per day and more; the presence of a red
substance on the cage pans of the dams at 300 mg/kg bw per day was
considered to be related to gastrointestinal and/or rectal
irritation. Treatment had no adverse effect on survival, the
abortion rate, pre- or post-implantation loss, the number of live
fetuses, fetal weight or the fetal sex ratio. A slight increase in
the incidence of minor skeletal alterations, comprising angulation
of one or both hyoid alae, irregular ossification of the skull and
nasal passages, was observed at 300 mg/kg bw per day; however, in
the absence of a clear dose-response relationship, no direct
correlation with treatment could be concluded. Clethodim was not
teratogenic at doses up to 300 mg/kg bw per day. The NOAEL for
maternal toxicity was 25 mg/kg bw per day, and that for
developmental toxicity was 300 mg/kg bw per day (Dearlove et al.,
1987).
(f) Genotoxicity
The results of assays for mutagenicity of technical-grade
clethodim are presented in Table 2. The compound did not induce gene
mutation in bacteria in vitro. Clethodim of unknown purity
(Putnam, 1986a) induced chromosomal aberrations in Chinese hamster
ovary cells in the absence of exogenous metabolic activation, but a
purified preparation did not (Putnam, 1986b). Clethodim at doses
sufficiently high to cause acute lethality did not induce
micronuclei in bone-marrow cells of rats treated in vivo, and it
did not induce unscheduled DNA synthesis in hepatocytes of mice
treated in vivo.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Technical-grade clethodim (purity, 83.2%) was applied at a dose
of 0.5 ml under occluded conditions to two intact and two abraded
sites on the shorn backs of each of 12 female New Zealand white
rabbits for 4 h. Slight erythema, observed immediately after dosing,
was followed 24-72 h later by slight-to-severe erythema and
slight-to-moderate oedema. General clearance was seen by 14 days.
The primary irritation score was 0.7. The test material was
considered to be slightly irritating to rabbit skin (Cushman et
al., 1986b).
Undiluted technical-grade clethodim (purity, 83.3%) was
instilled at a dose of 0.1 ml into the conjunctival sac of one eye
of each of nine male New Zealand white rabbits, the other eye
serving as a control. The eyes of three of the nine rabbits were
rinsed after a 30-sec exposure. No corneal opacity or iritis was
observed. In the six rabbits with unrinsed eyes, conjunctival
irritation was moderate to severe 1 h after dosing and slight to
moderate 24 h after dosing; all eyes were clear after 72 h. In the
rinsed eyes, conjunctival irritation was moderate at 1 h and slight
at 24 h; all eyes were clear at 48 h. The test material was
considered to be mildly irritating to the rabbit eye (Cushman et
al., 1986a).
Table 2. Genotoxicity of technical-grade clethodim
End-point Test system Concentration Purity (%) Resultsa Reference
In vitro
Reverse mutation S. typhimurium TA98, 0.1-10 mg/plate 83.3 Negativea Carver et al.,
100, 1535, 1537 (technical) 1986b
E. coli WP2 uvrA
S. typhimurium TA98, 0.1-10 mg/plate 83.3 Negativea Carver et al.,
100, 1535, 1537 (technical) 1986a
Reverse mutation S. typhimurium TA98, 0, 0.1, 0.33, 1.0, 91.1 Negativea Machado et al.,
100, 1535, 1537 3.33, 10 mg/plate (technical) 1990
E. coli WP2 uvrA
Chromosomal Chinese hamster 0.03, 0.1, 0.3, 1.0 Ál/ml NR Negativeb Putnam, 1986a
aberration ovary CHO-K1 0.6, 0.8, 1.0, 1.2 Ál/ml Positivec at
CCI 61 cells 1.0, 1.2 Ál/ml
Chromosomal Chinese hamster 0.03, 0.1, 0.3, 1.0 Ál/ml 96.1 Negativea Putnam, 1986b
aberration ovary CHO-K1 0.6, 0.8, 1.0, 1.2 Ál/ml (purified)
CCI 61 cells
In vivo
Chromosomal Male and female 0, 150, 500, 1500 83.3 Negative Putnam, 1987
aberration Sprague- mg/kg bw, (technical)
Dawley rats single oral dose
Unscheduled Male B6C3F1 0, 100, 1000, 5000 83.3 Negative Mirsalis &
DNA synthesis mice mg/kg bw, gavage (technical) Steinmetz, 1986
NR, not reported
a In the presence and absence of metabolic activation
b In the presence of metabolic activation
c In the absence of metabolic activation
Technical-grade clethodim (purity, 83.4%) did not appear to
sensitize the skin of male Hartley albino guinea-pigs when tested by
the modified Buehler test (Cushman et al., 1986c).
(ii) Liver cytochrome induction
Groups of eight male Crl:CD BR rats were treated by gavage with
technical-grade clethodim (purity, 83.8%) in carboxymethylcellulose
and Tween 80 at 0 or 250 mg/kg bw per day for 21 consecutive days.
Liver samples taken at sacrifice were assayed for cytochrome P450
activity. Treatment did not affect survival, clinical signs or body
weight. The weights of the livers of animals of each sex were
significantly increased (20-23%), and enlarged livers were observed
upon gross examination of two treated animals. Although the mean
cytochrome P450 and hepatic protein contents were significantly
greater in treated animals than controls, the mean concentrations of
hepatic cytochrome P450 per milligram of protein or per gram of
liver were not significantly increased. These results may reflect
increased liver weight and do not provide evidence that clethodim
induces liver cytochrome P450 (Beatty et al., 1989).
3. Studies on metabolites
The imine sulfone and the 5-hydroxy sulfone of clethodim
occurred in larger amounts in plants than in rats (Rose et al.,
1988a). Ancillary studies on these metabolites are summarized in
Table 3.
(a) Acute toxicity
Five female Crl:CD(SD)BR rats were given a single oral dose of
1.4 g/kg bw of the imine sulfone of clethodim. One animal had
decreased motor activity 4 h after dosing and was dead the next day
(Dougherty et al., 1988b).
Groups of five female Crl:CD (SD)BR rats received the 5-hydroxy
sulfone of clethodim or technical-grade clethodim as a single oral
dose of 1.4 g/kg bw. There were no clinical signs of toxicity, and
all animals treated with the 5-hydroxy sulfone survived the 14-day
observation period after dosing. All five rats treated with
clethodim showed overt signs of toxicity, noted as salivation,
decreased motor activity, collapse, hyperactivity, tremors,
diarrhoea, dehydration and nasal, ocular, oral and anogenital
discharge, and all died within three days of dosing (Dougherty et
al., 1988c).
(b) Short-term toxicity
The imine sulfone of clethodim was administered daily in the
diet to groups of 10 male and 10 female Crl:CD (SD)BR rats at 0,
100, 1000 or 8000 ppm, equal to 6.7, 71 or 600 mg/kg bw per day, for
five weeks. Body weight and food consumption were significantly
decreased during the first week of treatment in males given 8000
ppm. Other effects observed only at 8000 ppm were increased serum
cholesterol levels in animals of each sex, increased reticulocyte
counts in males and increased liver weights in males and females in
the absence of related histopathological alterations. Treatment did
not affect survival, clinical signs or ophthalmoscopic parameters.
The NOAEL was 1000 ppm, equal to 71 mg/kg bw per day (Beatty et
al., 1988a).
Groups of 10 male and 10 female Crl:CD (SD)BR rats were fed the
5-hydroxy sulfone of clethodim daily at dietary concentrations of 0,
100, 1000 or 8000 ppm, equal to 5.9, 68 or 590 mg/kg bw per day, for
five weeks. No signs of toxicity were seen at any dose. On the basis
of the absence of any effects on survival, clinical signs, body
weight, food consumption, haematological, blood chemical or
histopathological changes, or organ weights, the NOAEL was greater
than 8000 ppm, equal to 590 mg/kg bw per day (Beatty et al.,
1988b).
Table 3. Effects of clethodim and its imine sulfone and 5-hydroxy sulfone
Study Clethodim Imine sulfone 5-Hydroxy sulfone
Acute toxicity; 3/5 deaths (Cushman et al., 1986d) 1/5 deaths 0/5 deaths
female rat; 5/5 deaths (Dougherty et al., 1988c) (Dougherty et al., 1988b) (Dougherty et al.,
1.4 g/kg bw 1988bc)
Five-week dietary; Decreased body weight and Decreased body weight and No treatment-related
male & female rats food intake, slight anaemia, food intake, increased effects; NOAEL,
increased liver weights, liver weights, cholesterol > 588mg/kg bw per day
liver hypertrophy at 4000 and and reticulocytes at 8000 (Beatty et al., 1988b)
8000 ppm; anaemia, liver ppm. NOAEL, 71 mg/kg bw per
hypertrophy at 1000 ppm day (Beatty et al., 1988a)
NOAEL, 13 mg/kg bw per day
(Cisson & Eisenlord, 1986)
Teratogenicity; rats Maternal and fetal toxicity Maternal toxicity at 100 Clinical signs and
at 350 and 700 mg/kg bw per and 700 mg/kg bw per day; slightly decreased
day; NOAEL, 100 mg/kg bw fetal effects at 700 mg/kg fetal body weights at
per day. Increased incidence bw per day 700 mg/kg bw per day
of tail malformations at NOAEL, 10 mg/kg bw NOAEL, 100 mg/kg
700 mg/kg bw per day per day (Hoberman & per day (Hoberman &
(Schroeder & Daly, 1987) Christian, 1988a) Christian, 1988b)
Microbial gene Negative Negative Negative
mutation (Carver et al., 1986a,b) (Carver et al., 1988) (Carver eta[., 1987)
Chromosomal Positive in absence of Negative Negative
aberration, metabolic activation (Putnam, 1988a) (Putnam, 1988b)
CHO cells (Putnam, 1986a)
(c) Embryotoxicity and teratogenicity
Groups of 10 pregnant female Crl:CD (SD)BR rats were treated by
gavage with the imine sulfone of clethodim in Tween 80 and
carboxymethylcellulose at doses of 0, 10, 100 or 700 mg/kg bw per
day during days 6-15 of gestation. Maternal toxicity was seen at the
high dose of 700 mg/kg bw per day, manifested as excessive
salivation and decreased body weight and food consumption.
Significant decreases in maternal body weight were also recorded at
100 mg/kg bw per day. At 700 mg/kg bw per day, fetal weights were
decreased, and there were increased incidences of fetuses and
litters with cervical ribs and delayed sternal ossification. The
NOAEL for maternal toxicity was 10 mg/kg bw per day on the basis of
reduced body weights at 100 mg/kg bw per day and more. An increased
incidence of fetal variations at the maternally toxic dose of 700
mg/kg bw per day resulted in an NOAEL of 100 mg/kg bw per day for
developmental toxicity (Hoberman & Christian, 1988a).
Groups of 10 pregnant female Crl:CD (SD)BR rats were
administered the 5-hydroxy sulfone of clethodim in
carboxymethylcellulose and Tween 80 by gavage at 0, 10, 100 or 700
mg/kg bw per day on days 6-15 of gestation. At 700 mg/kg bw per day,
one female showed excessive salivation and two females had rales; a
slight decrease in fetal body weights in this group, although not
statistically significant, was possibly related to treatment. No
teratogenicity was seen at any dose. The NOAEL for maternal and
fetal toxicity was 100 mg/kg bw per day (Hoberman & Christian,
1988b).
(d) Genotoxicity
Neither the imine sulfone nor the 5-hydroxy sulfone of
clethodim induced gene mutation in Salmonella typhimurium TA98,
TA100, TA1535 or TA1537 or in Escherichia coli WP2 uvrA in the
presence or absence of metabolic activation. Neither metabolite
induced chromosomal aberrations in Chinese hamster ovary cells.
4. Observations in humans
No information was available.
Comments
Clethodim was readily absorbed by rats after oral
administration. Excretion was rapid and occurred predominantly via
the urine, with lesser amounts eliminated in the faeces and as
carbon dioxide. There were no significant dose-related or
sex-specific differences in elimination pattern or tissue
distribution, and there was no evidence of bioaccumulation.
The dominant metabolic pathway proposed for clethodim in rats
is oxidation to clethodim sulfoxide. Other postulated pathways are
cleavage of the N-O bond to form the imine, conversion of the
S-ethyl group to S-methyl, formation of the oxazole, and
hydroxylation at the 5 position. The urinary metabolites identified
were the sulfoxides and sulfones of clethodim and 5-hydroxy
clethodim, the sulfoxides of the imine and S-methyl clethodim and
the sulfone of the oxazole.
Clethodim had slight to moderate acute oral toxicity in the rat
and mouse. WHO has not classified clethodim with regard to toxic
hazard.
The primary effect of treatment with clethodim after short- and
long-term dietary exposure in the mouse, rat and dog was on the
liver. Hepatic effects were manifest as increased liver weights and
centrilobular hypertrophy. A study in rats administered clethodim at
250 mg/kg bw per day did not provide evidence for liver cytochrome
P450 induction.
In a four-week study in mice given dietary concentrations of 0,
100, 250, 625, 1500 or 4000 ppm, the NOAEL was 250 ppm, equivalent
to 38 mg/kg bw per day on the basis of evidence of slight anaemia at
625 ppm and above. In a 78-week study of toxicity and
carcinogenicity in which mice received dietary levels of 0, 20, 200,
1000 or 2000-3000 ppm, the NOAEL was 200 ppm, equivalent to 30 mg/kg
bw per day, on the basis of hepatic effects and an increased
incidence of alveolar lung macrophages at 1000 ppm and above.
In a five-week study in which rats received dietary levels of
0, 5, 200, 1000, 4000 or 8000 ppm, the NOAEL was 200 ppm, equal to
13 mg/kg bw per day, on the basis of liver hypertrophy and evidence
of slight anaemia at 1000 ppm and above. A 13-week study at dietary
concentrations of 0, 50, 500, 2500 or 5000 ppm and a two-year
feeding study at dietary levels of 0, 5, 20, 500 or 2500 ppm in rats
revealed an NOAEL of 500 ppm, equal to 25 mg/kg bw per day in the
first study and equal to 16 mg/kg bw per day in the second. The
NOAEL was based on decreased body weights and liver hypertrophy at
dietary levels of 2500 ppm and above.
In dogs, 13-week oral administration of capsules of clethodim
at doses of 0, 1, 25, 75 or 125 mg/kg bw per day resulted in an
NOAEL of 25 mg/kg bw per day, on the basis of increased liver weight
and cholesterol levels at 75 mg/kg bw per day and above. In a
one-year study at doses of 0, 1, 75 or 200-300 mg/kg bw per day, the
NOAEL was 1 mg/kg bw per day on the basis of treatment-related
effects on the liver at 75 mg/kg bw per day and above.
Clethodim was not carcinogenic when fed to mice or rats at
dietary levels of up to 2500 ppm.
A two-generation study of reproductive toxicity in rats treated
with clethodim at dietary levels of 0, 5, 20, 500 or 2500 ppm did
not reveal any adverse effects on reproduction. The NOAEL was 500
ppm, equal to 39 mg/kg bw per day, on the basis of decreased
parental body weights and food consumption at 2500 ppm.
Studies of teratogenicity were conducted with clethodim in rats
at doses of 0, 10, 100, 350 or 700 mg/kg bw per day and in rabbits
at 0, 25, 100 or 300 mg/kg bw per day. In rats, the NOAEL for
maternal toxicity was 100 mg/kg bw per day. Fetal toxicity was
observed at the maternally toxic doses of 350 and 700 mg/kg bw per
day. An increased incidence of malformations, specifically tail
defects (short, filamentous or absent tails), was observed at the
maternally toxic and lethal high dose of 700 mg/kg bw per day. In
rabbits, maternal toxicity was observed at 100 and 300 mg/kg bw per
day, resulting in an NOAEL of 25 mg/kg bw per day. There were no
adverse effects on the rabbit fetus and no evidence of teratogenic
potential at doses up to 300 mg/kg bw per day.
Clethodim was adequately tested for genotoxicity in a series of
assays in vivo and in vitro. The Meeting concluded that
clethodim was not genotoxic.
Although the imine sulfone and 5-hydroxy sulfone metabolites of
clethodim have been reported to occur in higher amounts in plants
than in animals, neither of the metabolites was more toxic than the
parent, clethodim, in studies of acute or short-term toxicity and
teratogenicity. There was similarly no evidence of genotoxic
potential.
An ADI was established on the basis of the NOAEL of 1 mg/kg bw
per day from the one-year study in dogs and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 200 ppm, equivalent to 30 mg/kg bw per day (78-week
study of toxicity and carcinogenicity)
Rat: 500 ppm, equal to 16 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 25 mg/kg bw per day (study of teratogenicity)
Dog: 1 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Observations in humans
References
Beatty, P.W., Bagos, A.D., Richter, W.R. & Wong, Z.A. (1988a)
Five-week oral toxicity study in rats with RE-47719 (SX-1800).
Project No. 2949. Unpublished report dated 15 November 1988 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Beatty, P.W., Bagos, A.C., Richter, W.R. & Wong, Z.A. (1988b)
Five-week oral toxicity study in rats with RE-51228 (SX-1803).
Project No. 2950. Unpublished report dated 15 November 1988 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Beatty, P.W., Brorby, G.P. & Wong, Z.A. (1989) The potential of
RE-45601 Technical (SX-1688) to induce cytochrome P-450 following
21-day oral administration in male rats. Project No. 2784.
Unpublished report dated 16 November 1989 from Chevron Environmental
Health Center Inc., Richmond, CA, USA. Submitted to WHO by Tomen
Pacific Agro Co., San Francisco, CA, USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1986a)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-45601 Technical (83% purity, SC-1688). Project No. SOCAL
2505. Unpublished report dated 13 January 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1986b)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-45601 Technical (83% purity, SC-1688). Project No. CEHC
2555. Unpublished report dated 28 April 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1987)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-51228. Project No. 2856. Unpublished report dated 7 December
1987 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1988)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-47719. Project No. 2948. Unpublished report dated 19 October
1988 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Cisson, C.M. & Eisenlord, G.H. (1986) Five-week pilot feeding study
in rats with RE-45601 Technical (SX-1653). Project No. SOCAL 2457.
Unpublished report dated 7 October 1986 from Chevron Environmental
Health Center Inc., Richmond, CA, USA. Submitted to WHO by Tomen
Pacific Agro Co., San Francisco, CA, USA.
Cox, R.H. (1988) Acute intraperitoneal toxicity study in rats with
Chevron RE-45601 Technical (SX-1688). Project No. 2107-151.
Unpublished revised report dated 6 April 1988 from Hazelton
Laboratories America Inc., Vienna, VA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Cox, R.H. & Slabik, C.M. (1986) Four-week subchronic oral toxicity
study in mice with Chevron RE-45601 Technical. Project No. 2107-140.
Unpublished report dated 21 July 1986 from Hazelton Laboratories
America Inc., Vienna, VA, USA. Submitted to WHO by Tomen Pacific
Agro Co., San Francisco, CA, USA.
Cox, R.H. & Zoetis, T. (1986) Acute oral toxicity study in mice with
Chevron RE-45601 Technical. Project No. 2107-143. Unpublished report
dated 16 September 1986 from Hazelton Laboratories America Inc.,
Vienna, VA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Cox, R.H. & Zoetis, T. (1988b) One-year oral toxicity study in dogs
with Chevron RE-45601 Technical (SX-1688). Project No. 2107-153.
Unpublished report dated 28 October 1988 from Hazelton Laboratories
America Inc., Vienna, VA, USA. Submitted to WHO by Tomen Pacific
Agro Co., San Francisco, CA, USA.
Cox, R.H. & Zoetis, T. (1988b) Chronic oral oncogenicity study in
mice with Chevron RE-45601 Technical (SX-1688). Project No.
2107-145. Unpublished report dated 25 October 1988 from Hazelton
Laboratories America Inc., Vienna, VA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Thompson, J.D. & Wong, Z.A. (1986a) The acute eye
irritation potential of RE-45601 Technical (SX-1688). Project No.
CEHC 2511. Unpublished report dated 1 October 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Korenaga, G.L. & Wong, Z.A. (1986b) The four-hour
skin irritation potential of RE-45601 Technical (SX-1688). Project
No. CEHC 2512. Unpublished report dated 18 December 1986 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Cooper, P.N. & Wong, Z.A. (1986c) Modified Buehler
Test for the skin sensitization potential of RE-45601 Technical
(SX-1688). Project No. CEHC 2514. Unpublished report dated 15
December 1986 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Cushman, J.R., Easter, M.D. & Wong, Z.A. (1986d) The acute oral
toxicity of RE-45601 Technical (SX-1688) in adult male and female
rats. Project No. SOCAL 2498. Unpublished report dated 27 June 1986
from Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Hedgecock, J.H. & Wong, Z.A. (1987a) Four-week
repeated dose dermal toxicity study in rats with RE-45601 Technical
(SX-1688). Project No. CEHC 2552. Unpublished report dated 20
November 1987 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Cushman, J.R., Korenaga, G.L. & Wong, Z.A. (1987b) The acute dermal
toxicity of RE-45601 Technical (SX-1688) in adult male and female
rabbits. Project No. CEHC 2510. Unpublished report dated 7 January
1987 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Daly, I.W. & Knezevich, A.L. (1987) Ninety-day subchronic oral
toxicity study in dogs with Chevron RE-45601 Technical. Project No.
85-2999. Unpublished report dated 26 February 1987 from Bio/dynamics
Inc., East Millstone, NJ, USA. Submitted to WHO by Tomen Pacific
Agro Co., San Francisco, CA, USA.
Dearlove, G.E., Hoberman, A.M. & Christian, M.S. (1986) Pilot
teratology study in rabbits with Chevron RE-45601 Technical. Project
No. 303-007P. Unpublished report dated 15 July 1986 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Dearlove, G.E., Hoberman, A.M. & Christian, M.S. (1987) Teratology
study in rabbits with Chevron RE-45601 Technical. Project No.
303-007. Unpublished report dated 30 January 1987 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Dougherty, K.K., Eisenlord, G.H., Low, S.S. & Wong, Z.A. (1987)
13-Week oral toxicity study in rats with RE-45601 Technical
(SX-1688). Project No. SOCAL 2501. Unpublished report dated 13
February 1987 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Eisenlord, G.H. & Wong, Z.A. (1988a) Combined
chronic toxicity/oncogenicity study in rats with RE-45601 Technical
(SX-1688). Project No. SOCAL 2500. Unpublished report dated 1
November 1988 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Duncan, R.A. & Wong, Z.A. (1988b) The comparative
acute oral toxicity of RE-47719 (SX-1800) and RE-45601 (SX-1688) in
adult female rats. Project No. 2952. Unpublished report dated 21
October 1988 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Duncan, R.A. & Wong, Z.A. (1988c) The comparative
acute oral toxicity of RE-51228 (SX-1796) and RE-45601 (SX-1688) in
adult female rats. Project No. 2951. Unpublished report dated 21
October 1988 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Waid, L.J. & Wong, Z.A. (1989) The acute oral
toxicity of RE-45601 Technical (SX-1688) in adult male and female
rabbits. Project No. CEHC 2992. Unpublished report dated 21 June
1989 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Griffis, L.C., Bruce, E.D. & Wong, Z.A. (1986) The acute inhalation
toxicity of RE-45601 Technical (SX-1688) in rats. Project No. CEHC
2513. Unpublished report dated 7 November 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Hoberman, A.M. & Christian, M.S. (1988a) Oral teratogenicity and
developmental toxicity screen in rats with RE-47719. Project No.
303-012. Unpublished report dated 8 November 1988 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Hoberman, A.M. & Christian, M.S. (1988b) Oral teratogenicity and
developmental toxicity screen in rats with RE-51228. Project No.
303-010. Unpublished report dated 8 November 1988 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Lee, S.G., Maier, K.M., Tamichi, E.H. & Tucker, B.V. (1988)
[Ring-4,6-14C] clethodim: A radiocarbon metabolism study in laying
hens. Project No. MEF-0089. Unpublished report dated 30 December
1988 from Chevron Chemical Company, Ortho Agricultural Chemicals
Division, Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro
Co., San Francisco, CA, USA.
Machado, M.L., Lubeck, S.G. & Wilkenfeld, R.M. (1990)
Microbial/microsome reverse mutation plate incorporation assay with
RE-45601 Technical (SX-1845). Project No. CEHC 3146. Unpublished
report dated 20 August 1990 from Chevron Environmental Health Center
Inc., Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co.,
San Francisco, CA, USA.
McKensie, K.M. (1986) Pilot rat reproduction study with Chevron
RE-45601 Technical. Project No. 6183-102. Unpublished report dated
16 December 1986 from Hazelton Laboratories America Inc., Madison,
WI, USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco,
CA, USA.
Mirsalis, J.C. & Steinmetz, K.L. (1986) In vivo--in vitro
hepatocyte DNA repair assay: in vitro evaluation of unscheduled
DNA synthesis (UDS) following oral administration of Chevron
RE-45601 Technical to B6C3F1 Mice. Project No. LSC-1960.
Unpublished report dated August 1986 from SRI International, Meno
Park, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Putnam, D.L. (1986a) Chromosome aberrations in Chinese hamster ovary
(CHO) cells with Chevron RE-45601 Technical. Project No. T4529.337.
Unpublished report dated August 1986 from Microbiological Associates
Inc., Bethesda, MD, USA. Submitted to WHO by Tomen Pacific Agro Co.,
San Francisco, CA, USA.
Putnam, D.L. (1986b) Chromosome aberrations in Chinese hamster ovary
(CHO) cells with purified Chevron RE-45601. Project No. T429.337.
Unpublished report dated 15 December 1986 from Microbiological
Associates Inc., Bethesda, MD, USA. Submitted to WHO by Tomen
Pacific Agro Co., San Francisco, CA, USA.
Putnam, D.L. (1987) Cytogenetics assay in bone marrow cells of rats
following acute oral exposure to RE-45601 Technical. Project No.
T5172.105. Unpublished report dated 25 February 1987 from
Microbiological Associates Inc., Bethesda, MD, USA. Submitted to WHO
by Tomen Pacific Agro Co., San Francisco, CA, USA.
Putnam, D.L. (1988a) Chromosome aberrations in Chinese hamster ovary
(CHO) cells: RE-47719. Project No. T8226.337003. Unpublished report
dated 10 November 1988 from Microbiological Associates Inc.,
Bethesda, MD, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Putnam, D.L. (1988b) Chromosome aberrations in Chinese hamster ovary
(CHO) cells: RE-51228. Project No. T8227.337003. Unpublished report
dated 10 November 1988 from Microbiological Associates Inc.,
Bethesda, MD, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Rendina, A.R. & Felts, J.M. (1988) Cyclohexanedione herbicides are
selective and potent inhibitors of acetyl-CoA carboxylase from
grasses. Plant Physiol., 86, 983-986.
Rose, A.F., Griffis, L.C., Suzuki, J.P., Bruce, E.D., Primer, R.L.,
Wong, Z.A. & Tucker, B.V. (1988a) The in vivo metabolism of
[propyl-1-14C] clethodim in rats. Project No. MEF-0086/CEHC 2515.
Unpublished report dated 22 December 1988 from Chevron Chemical Co.,
Ortho Agricultural Chemicals Division, Richmond, CA, USA. Submitted
to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Rose, A.F., Suzuki, J.P., Primer, R.L. & Tucker, B.V. (1988b) The
in vivo metabolism of [propyl-1-14C] clethodim in a lactating
goat. Project No. MEF-0038. Unpublished report dated 29 December
1988 from Chevron Chemical Co., Ortho Agricultural Chemicals
Division, Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro
Co., San Francisco, CA, USA.
Schroeder, R.E. & Daly, I.W. (1986) A pilot teratology study in rats
with Chevron RE-45601 Technical. Project No. 85-3032. Unpublished
report dated 8 September 1986 from Bio/dynamics Inc., East
Millstone, NJ, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Schroeder, R.E. & Daly, I.W. (1987) Teratology study in rats with
Chevron RE-45601 Technical. Project No. 86-3042. Unpublished report
dated 20 July 1987 from Bio/dynamics Inc., East Millstone, NJ, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Tellone, C.I., Hardy, L.M. & Richter, W.R. (1987) Two-generation
(one litter) reproduction study in rats with RE-45601 Technical.
Project No. CEHC 2596. Unpublished report dated 27 August 1987 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Two groups of eight white Leghorn laying hens were given daily
doses of [cyclohexene-4,6-14C]-clethodim at 2.1 or 51.3 mg/kg bw
per day by capsule for five consecutive days. The hens were
sacrificed about 4 h after the final dose, and tissues were
collected for analysis. Two major metabolites, clethodim sulfone and
clethodim sulfoxide, were identified in tissues and eggs. Clethodim
sulfone accounted for up to 57% of tissue levels of radiolabel,
whereas clethodim sulfoxide accounted for 10-31%. Clethodim
sulfoxide was the principal metabolite in egg white (26-82%) and
yolk (25-37%); parent clethodim was detected in significantly
smaller amounts in both tissues and eggs. The proposed metabolic
pathway in chickens was different from and considered to be simpler
than that observed in rats and goats, since none of the imine,
5-hydroxy or S-methyl analogues was detected in chickens (Lee et
al., 1988).
In the study described above in which a lactating goat received
[propyl-1-14C]-clethodim (Rose et al., 1988b), hind- and
forequarter muscle, peritoneal and subcutaneous fat, liver, kidneys,
heart and blood were collected to allow characterization of
metabolites. The major urinary metabolite was clethodim sulfoxide,
which accounted for 67% of the urinary radiocarbon. Other urinary
radiolabelled components were identified as clethodim (3-27%) and
the demethyl sulfoxide (12-18%), S-methyl (7-13%), imine sulfoxide
(1.5-2.8%), sulfone (1.5-2.2%) and 5-hydroxy sulfoxide (0-3%). In
the milk, about half of the radiocarbon could be extracted into
organic solvents and occurred in clethodim, clethodim sulfoxide and
clethodim demethyl sulfoxide; the other half of the radiocarbon was
water soluble and was shown to be 14C-lactose. In blood and
tissues, the maximal extractable radiocarbon residues accounted for
77-95% of the radiolabel and were identified as clethodim and the
sulfoxide, demethyl sulfoxide, imine sulfoxide, sulfone and
5-hydroxy sulfone. The proposed metabolic pathway in goats was
essentially the same as that proposed for rats (Rose et al.,
1988b).
2. Toxicological studies
(a) Acute toxicity
Technical-grade clethodim has been tested for acute toxicity in
mice, rats and rabbits. The results are summarized in Table 1.
Clethodim was virtually non-toxic after oral or dermal
administration to rabbits or after inhalation by rats. Slight to
moderate toxicity was observed in mice and rats treated orally, with
LD50 levels of 1360-2570 mg/kg bw. Clethodim administered
intraperitoneally was moderately toxic to rats, with a combined
LD50 for the two sexes of 1117 mg/kg bw.
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female Charles River CD-1 mice were
fed diets containing technical-grade clethodim (purity, 83.3%) for
four weeks at 100, 250, 625, 1500 or 4000 ppm, equivalent to 0, 15,
38, 94, 225 or 600 mg/kg bw per day. A slight anaemic process was
manifested in male mice as a decreased erythrocyte count at >
1500 ppm, decreased haemoglobin at > 625 ppm and lowered
haematocrit at 4000 ppm. Increased liver weights, which were
correlated microscopically with centrilobular hypertrophy, were
observed in males fed 1500 ppm and in animals of each sex treated at
the highest dose of 4000 ppm. An increased incidence of focal
coagulative necrosis of the liver was recorded in males treated at
> 1500 ppm. Treatment appeared to have no effect on survival,
clinical signs, body weight or food consumption. The NOAEL was 250
ppm, equivalent to 38 mg/kg bw per day, on the basis of the
dose-related decrease in erythroid parameters at dietary levels >
625 ppm (Cox & Slabik, 1986).
Rats
Technical-grade clethodim (purity, 83.2%) was applied in
carboxymethylcellulose and Tween 80 to the skin of groups of six
male and six female Crl:CD BR rats at doses corresponding to 0, 10,
100 or 1000 mg/kg bw per day for 6 h/day, five days per week for
four weeks, for a total of 21 exposures. A dose-related increase in
the occurrence of skin irritation was observed at all dose levels.
Clinical signs seen at 1000 mg/kg bw per day were described as
anogenital discharge and staining of the fur, alopecia and red nasal
discharge. At the highest dose, group mean body weights,
corresponding weight gains and feed efficiency values were decreased
in males, and increased liver weights were recorded in females. In
males, a slight increase was observed in kidney regeneration, which
was described as a minimal multifocal lesion. Although this finding
was observed only in treated rats, evidence of chronic progressive
nephropathy is not uncommon in this strain of rat and was not
considered to represent a treatment-related effect. The NOAEL for
systemic toxicity under the conditions of this study was thus 100
mg/kg bw per day (Cushman et al., 1987a).
Table 1. Acute toxicity of technical-grade clethodim in male and female rodents
Species, Route and vehicle LD50 Purity Reference
strain (mg/kg bw) (%)
Mouse, CD-1 Oral; M: 2570 83.3 Cox & Zoetis, 1986
Tween 80 + CMC F: 2430 (technical)
Rat, Crl:CD Oral; M: 1630 83.3 Cushman et al., 1986d
(SD)BR Tween 80 + CMC (technical)
Rat, Crl:CD Intraperitoneal; M: 1040 NR Cox, 1988
(SD)BR Tween 80 + CMC F: 1200
Rat, Inhalation M&F: 83.3 Griffis et al., 1986
Sprague-Dawley > 3.9 mg/l (technical)
Rabbit, New Oral; M: > 2000 and 82.5 Dougherty et al., 1989
Zealand Tween 80 + CMC < 5000 (technical)
white F: > 5000
Rabbit, New Dermal M&F: > 5000 83.3 Cushman et al., 1987b
Zealand (technical)
white
NR, not reported; Tween 80, 0.5% polyoxyethylenesorbitan monooleate; CMC,
carboxymethylcellulose
In a preliminary five-week study, groups of 10 male and 10
female Crl:CD(SD)BR rats were treated with technical-grade clethodim
(purity, 83.4%) at dietary levels of 0, 5, 200, 1000, 4000 or 8000
ppm, equal to 0.3, 13, 66, 261 or 515 mg/kg bw per day for males and
0.3, 14, 71, 291 or 554 mg/kg bw per day for females.
Treatment-related effects were decreased body-weight gain and food
consumption (particularly during the first week of the study) in
animals of each sex at 4000 and 8000 ppm; increased liver weights at
> 4000 ppm; and slight to mild centrilobular hypertrophy in males
at 1000 ppm and in animals of each sex at 4000 and 8000 ppm.
Dose-related, statistically significant decreases in haemoglobin
concentration were noted in males at > 1000 ppm and in
haematocrit in males at > 4000 ppm. Raised cholesterol
concentrations were recorded in males receiving 8000 ppm, and raised
serum uric acid levels were seen in females given > 4000 ppm.
Treatment had no effect on survival, clinical signs or urinary
parameters. The NOAEL in this study was 200 ppm, equal to 13 mg/kg
bw per day, on the basis of decreases in erythroid parameters and
centrilobular hypertrophy of the liver at 1000 ppm and more (Cisson
& Eisenlord, 1986).
Technical-grade clethodim (purity, 84%) was administered to
groups of 12 male and 12 female Crl:CD(SD)BR rats for 13 weeks at
dietary levels of 0, 50, 500, 2500 or 5000 ppm, equal to 2, 25, 134
or 280 mg/kg bw per day for males and 3, 30, 160 or 340 mg/kg bw per
day for females. In order to study the reversibility of potential
treatment-related effects, an additional 12 rats of each sex were
assigned to the groups receiving 0, 2500 and 5000 ppm and were kept
for a six-week recovery period after the end of treatment with
clethodim. Significantly decreased body weights and weight gains
were recorded in males treated with 2500 ppm and in animals of each
sex treated with 5000 ppm. Decreased mean food consumption was noted
in both males and females at 5000 ppm. Significantly ( p < 0.05)
increased concentrations of cholesterol, total protein and globulin
recorded in males fed 5000 ppm were within the normal control range
of variability. Increased liver weights and increased incidence and
severity of centrilobular hypertrophy of the liver were noted in
males and females fed 2500 and 5000 ppm. A slightly increased
incidence of renal focal regeneration was seen in males treated at
> 2500 ppm. The pathologist did not consider this finding to be
related to treatment; rather, these lesions were regarded as typical
of the earliest minimal stage of spontaneous chronic progressive
nephropathy. Treatment did not affect survival, clinical signs,
ophthalmoscopic or haematological parameters or the results of
urinalysis. No treatment-related effects were seen after the
six-week recovery period. The NOAEL was 500 ppm, equal to 25 mg/kg
bw per day, as determined by an increased incidence of hepatic
centrilobular hypertrophy and decreased body-weight gain in animals
of each sex treated at 2500 ppm and above (Dougherty et al.,
1987).
Dogs
Groups of four male and four female beagle dogs received
gelatin capsules containing technical-grade clethodim (purity,
83.3%) orally at 0, 1, 25, 75 or 125 mg/kg bw per day for 13 weeks.
Treatment affected blood chemistry in females, which had increased
cholesterol levels at 75 mg/kg bw per day and above and increased
alkaline phosphatase activity at 125 mg/kg bw per day. An increased
globulin concentration, with a concomitant decrease in the
albumin:globulin ratio, was noted in males treated at 125 mg/kg bw
per day. Significantly ( p < 0.05) increased total leukocyte
counts were seen in males and decreased activated partial
thromboplastin time was seen in females after one month at 75 and
125 mg/kg bw per day. As no similar differences from control values
were seen, however, after two or three months of treatment, and the
only differences seen were in one sex, the significance of these
findings is doubtful. Increased liver weights were recorded in males
and females fed 75 or 125 mg/kg bw per day. Slight increases in the
severity of cytoplasmic vesiculation and vacuolation of the
centrilobular hepatocytes were seen in animals of each sex treated
at 125 mg/kg bw per day. Treatment did not affect survival, clinical
signs, ophthalmoscopic parameters, body weight or food consumption.
The NOAEL was 25 mg/kg bw per day on the basis of elevated
cholesterol levels and increased liver weights at 75 mg/kg bw per
day and above (Daly & Knezevich, 1987).
In a one-year study, groups of six male and six female beagle
dogs were administered technical-grade clethodim (purity, 83.3%) in
gelatin capsules at doses of 0, 1, 75 or 200-300 mg/kg bw per day.
In the absence of any signs of overt toxicity, the high dose of 200
mg/kg bw per day was increased after seven weeks of treatment to 300
mg/kg bw per day. Significant effects on haematological parameters
were seen, expressed as increased platelet and leukocyte counts in
females at 75 mg/kg bw per day and in males and females at 300 mg/kg
bw per day. At 300 mg/kg bw per day, erythroid parameters
(erythrocyte, haemoglobin and haematocrit values) were decreased in
animals of each sex, and reticulocyte and segmented neutrophil
counts were increased in females. Treatment-related effects on blood
chemistry were seen as increased levels of cholesterol,
triglycerides, alkaline phosphatase and alanine aminotransferase in
males and females at 300 mg/kg bw per day. Decreased serum glucose
concentrations were noted in both males and females at the high dose
and in females treated at 75 mg/kg bw per day. Increased liver
weights were recorded in animals of each sex at 75 and 300 mg/kg bw
per day. Significantly ( p < 0.05) increased thyroid weights seen
in males at 300 mg/kg bw per day were not correlated with any
microscopic changes. Histopathological alterations in animals of
each sex treated at 300 mg/kg bw per day included hepatocellular
enlargement, increased granular pigment (which was not associated
with bile, iron or acid-fast lipofuscin) and slight hypercellularity
of the bone marrow; the last finding was also observed in one male
and one female at 75 mg/kg bw per day. The significance of a
slightly increased incidence of pigment (not otherwise
characterized) in the spleen of females receiving 75 or 300 mg/kg bw
per day was uncertain. Treatment did not affect survival, clinical
signs, ophthalmoscopic parameters, body weight, food consumption or
the results of urinalysis. The NOAEL was 1 mg/kg bw per day on the
basis of treatment-related effects on haematological and blood
chemical parameters, increased liver weight and hypercellularity of
the bone marrow at 75 mg/kg bw per day and above (Cox & Zoetis,
1988a).
(c) Long-term toxicity and carcinogenicity
Mice
Technical-grade clethodim (purity, 83.3%) was administered in
the diet to groups of 60 male and 60 female Crl:CD-1 (ICR)BR mice
for 78 weeks at 0, 20, 200, 1000 or 2000-3000 ppm, equivalent to 3,
30, 150 or 300-450 mg/kg bw per day. The high dose was increased to
3000 ppm after 15 weeks of treatment, when haematological assessment
did not show the significant decrease in erythrocyte parameters seen
in the four-week preliminary study. Ten mice of each sex were
sacrificed after 52 weeks of treatment in each group for interim
assessment. A significant ( p < 0.05) trend to decreased survival
was noted among males and females fed 3000 ppm, with survival at 78
weeks of 58, 66, 60, 52 and 32% males and 67, 84, 80, 59 and 48%
females, respectively. The predominant cause of death at this dose
was increased incidence and severity of systemic amyloidosis.
Decreased erythrocyte counts were recorded in animals of each sex at
3000 ppm, with concomitant decreases in haemoglobin and haematocrit
values in males. After 52 weeks of treatment, increased liver
weights and centrilobular hypertrophy of the liver were observed in
males at 1000 ppm and in males and females at 3000 ppm. Increased
pigment, described as morphologically compatible with haemosiderin
and bile, was noted in males at 3000 ppm. After 78 weeks of
treatment, the hepatic changes included increased liver weights in
females at 3000 ppm, centrilobular hypertrophy and increased pigment
in both males and females at 1000 and 3000 ppm and bile-duct
hyperplasia in males at > 1000 ppm. An increased incidence of
multifocal, amphophilic alveolar lung macrophages was also observed
in animals of each sex treated at > 1000 ppm. No significant
effect was seen on body weight, food consumption or clinical signs.
The NOAEL was 200 ppm, equivalent to 30 mg/kg bw per day, on the
basis of hepatic changes, notably centrilobular hypertrophy,
increased pigment and bile-duct hyperplasia, and an increased
incidence of alveolar macrophages in the lungs of mice treated at
1000 ppm and above. There was no evidence that clethodim has
carcinogenic potential (Cox & Zoetis, 1988b).
Rats
Groups of 65 male and 65 female Crl:CD (SD)BR rats were
administered technical-grade clethodim (purity, 83%) in the diet at
levels of 0, 5, 20, 500 or 2500 ppm, equal to 0.2, 0.6, 16 or 86
mg/kg bw per day for males and 0.2, 0.7, 21 or 110 mg/kg bw per day
for females, for two years. Ten rats of each sex in each group were
sacrificed after one year. After two years, 47, 49, 46, 50 and 33%
of males and 55, 58, 51, 40 and 49% of females were still alive in
the five dose groups, respectively. Treatment did not affect cause
or time of death, clinical signs, ophthalmoscopic or haematological
parameters or the results of clinical chemistry or urinalysis.
Body-weight gain and food consumption were significantly decreased
in animals of each sex during the first year of treatment with 2500
ppm. Food intake, calculated relative to body weight, was slightly
greater in males and females at 2500 ppm than in the other treated
groups. At interim sacrifice after one year, increased liver weights
and slight to mild centrilobular hypertrophy were noted in rats of
each sex fed 2500 ppm. Increased liver weights seen in females at
500 ppm were not correlated with any microscopically discernible
change. At terminal sacrifice, liver weights of females at 2500 ppm
were increased; males had no significant increase in liver weight,
and no treatment-related centrilobular hypertrophy was seen in
animals of either sex. Females treated at 2500 ppm had a slightly
greater (12%) incidence of binucleated cells in the liver than the
controls (2%), but the effect was of uncertain toxicological
significance. Clethodim at dietary levels up to 3000 ppm showed no
evidence of carcinogenic potential. The NOAEL was 500 ppm, equal to
16 mg/kg bw per day, on the basis of decreased body-weight gain,
decreased food intake, increased liver weight and associated
centrilobular hypertrophy at 2500 ppm (Dougherty et al., 1988a).
(d) Reproductive toxicity
Rats
In a preliminary study, groups of eight male and eight female
Crl:CD(SD)BR rats were fed technical-grade clethodim (purity, 83.3%)
at dietary levels of 0, 500, 2000 or 5000 ppm, equivalent to 25, 100
or 250 mg/kg bw per day, for one week before and during mating.
Males were sacrificed after mating, whereas females were treated
throughout gestation and up to day 7 of the lactation period for a
single litter. Parental animals of each sex that received 5000 ppm
had decreased body-weight gain and food intake ( p < 0.05, only in
males). Mean pup weights and corresponding weight gains were
decreased at all dietary levels. No adverse effects on reproductive
parameters were evident at the highest dose (McKensie, 1986).
In a two-generation (one litter per generation) study, groups
of 30 male and 30 female Crl:COBS CD(SD) rats were treated with
technical-grade clethodim (purity, 83%) at dietary levels of 0, 5,
20, 500 or 2500 ppm, equal to 0.1, 1.4, 39 or 190 mg/kg bw per day
for males and 0.2, 1.8, 45 or 220 mg/kg bw per day for females. Mean
body weight was decreased at 2500 ppm in both males and females of
the F1 generation and in males of the F0 generation. Occasional
significant ( p < 0.05) decreases in food consumption were
recorded in F1 males and females treated with 2500 ppm. Treatment
had no effect on parental survival, clinical signs, organ weights or
histopathological manifestations; there were no adverse effects on
reproductive indices (mating, fertility, pregnancy and gestation);
and there was no effect on litter size, sex ratio, pup weight or pup
viability. A significant ( p < 0.05) increase in the number of
stillborn pups was observed in the litters of F1 dams treated at
2500 ppm; however, this finding was not considered to be related to
treatment, since the value was within the historical control range,
and no similar increase was seen in the subsequent F2 litter. The
NOAEL for parental systemic toxicity was 500 ppm, equal to 39 mg/kg
bw per day, on the basis of decreased body weights and food
consumption at 2500 ppm. The NOAEL for adverse effects on
reproduction in the F0 and F1 generations was 2500 ppm, the
highest dietary level tested, equal to 190 mg/kg bw per day (Tellone
et al., 1987).
(e) Embryotoxicity and teratogenicity
Rats
In a preliminary range-finding study, groups of 10 mated female
Crl:CD(SD) rats were given technical-grade clethodim (purity, 82.6%)
in Tween 80 and carboxymethylcellulose by gavage at 0, 50, 150, 300
or 500 mg/kg bw per day during days 6-15 of gestation. The day on
which evidence of mating was observed was designated day 0 of
gestation. Clinical signs of toxicity were an increased incidence of
excessive salivation in females at 300 and 500 mg/kg bw per day, and
slightly decreased body-weight gain, decreased mean number of
uterine implantations and decreased mean fetal body weights at 500
mg/kg bw per day. External examination of the fetuses failed to
reveal any gross findings related to treatment (Schroeder & Daly,
1986).
Groups of 25 mated female Crl:CD(COBS) rats were treated with
technical-grade clethodim (purity, 82.6%) in Tween 80 and
carboxymethylcellulose daily by gavage at 0, 10, 100, 350 or 700
mg/kg bw per day during days 6-15 of gestation. The day on which
evidence of mating was observed was designated day 0 of gestation.
Treatment with clethodim at 350 mg/kg bw per day and more decreased
maternal body weight. Toxicity was manifested at 700 mg/kg bw per
day as increased mortality (20%), decreased food intake and clinical
signs of poor physical condition, including red nasal discharge,
chromodacryorrhoea and staining of the anogenital region. Excessive
salivation was observed in females treated at 350 and 700 mg/kg bw
per day. Significantly decreased fetal weights and increased
incidences of fetuses with skeletal variations or at least one
ossification variation were recorded at 350 mg/kg bw per day and
more. The skeletal variations were apparent as definite delays in
ossification and were identified by incompletely or unossified
vertebral elements (thoracic, sacral, caudal) and unossified fifth
and/or sixth sternebrae. Treatment at the highest dose, 700 mg/kg bw
per day, induced a significant increase in the number of fetuses
(8/221; 3.6%) and litters (6/18; 33.2%) with external malformations,
comprising primarily an increased incidence (seven fetuses in six
litters) of tail defects characterized by short, filamentous or
absent tails. An imperforated anus was recorded in two fetuses in
two litters at this dose. Visceral malformations (exencephaly,
defects of the lung, aortic arch and large intestine, absence of
kidneys, bladder or ureter) were observed in four fetuses in three
litters of dams exposed to 700 mg/kg bw per day but not in
concurrent or historical controls (5032 fetuses in 806 litters; 38
studies). Three of the four fetuses with visceral malformations also
had external malformations. The NOAEL for maternal and developmental
toxicity was 100 mg/kg bw per day. The NOAEL for teratogenicity was
350 mg/kg bw per day on the basis of an increased incidence of
malformations, particularly tail defects, observed at the maternally
toxic and lethal dose of 700 mg/kg bw per day (Schroeder & Daly,
1987).
Rabbits
In a range-finding study, groups of eight artificially
inseminated female New Zealand white Hra: specific-pathogen-free
rabbits were treated by gavage with clethodim (purity, 83.3%) in
Tween 80 and carboxymethylcellulose at 0, 50, 150, 300 or 500 mg/kg
bw per day on days 7-19 of gestation. The day of insemination was
designated day 0 of gestation. Maternal toxicity was seen at all
doses as decreased body-weight gain and food consumption. An
increased incidence of alopecia was recorded at 150 mg/kg bw per day
and more. Dried faeces and gastrointestinal lesions (hairball and/or
ulceration of the gastric mucosa) were observed at doses of 300
mg/kg bw per day and more. In the groups given 300 and 500 mg/kg bw
per day, two of seven pregnant rabbits died, apparently due to
concomitant weight loss, decreased food intake, gastrointestinal
lesions and/or abortion. In the group given 500 mg/kg bw per day,
four of seven rabbits aborted, and one rabbit delivered prematurely.
Data on litters of dams given 500 mg/kg bw per day could not
effectively be evaluated, as only one female survived to term; dams
given 300 mg/kg bw per day had an increased incidence of resorption
and decreased litter weights when compared with the controls
(Dearlove et al., 1986).
Groups of 20 artificially inseminated female New Zealand white
Hra specific-pathogen-free rabbits were administered
technical-grade clethodim (purity, 83.3%) in Tween 80 and sodium
carboxymethylcellulose by gavage at doses of 0, 25, 100 or 300 mg/kg
bw per day on days 7-19 of gestation. The day of insemination was
designated day 0 of gestation. Mean body weights, corresponding
weight gains and food consumption were decreased in dams given 100
or 300 mg/kg bw per day. An increased incidence of dried faeces was
also noted at 100 mg/kg bw per day and more; the presence of a red
substance on the cage pans of the dams at 300 mg/kg bw per day was
considered to be related to gastrointestinal and/or rectal
irritation. Treatment had no adverse effect on survival, the
abortion rate, pre- or post-implantation loss, the number of live
fetuses, fetal weight or the fetal sex ratio. A slight increase in
the incidence of minor skeletal alterations, comprising angulation
of one or both hyoid alae, irregular ossification of the skull and
nasal passages, was observed at 300 mg/kg bw per day; however, in
the absence of a clear dose-response relationship, no direct
correlation with treatment could be concluded. Clethodim was not
teratogenic at doses up to 300 mg/kg bw per day. The NOAEL for
maternal toxicity was 25 mg/kg bw per day, and that for
developmental toxicity was 300 mg/kg bw per day (Dearlove et al.,
1987).
(f) Genotoxicity
The results of assays for mutagenicity of technical-grade
clethodim are presented in Table 2. The compound did not induce gene
mutation in bacteria in vitro. Clethodim of unknown purity
(Putnam, 1986a) induced chromosomal aberrations in Chinese hamster
ovary cells in the absence of exogenous metabolic activation, but a
purified preparation did not (Putnam, 1986b). Clethodim at doses
sufficiently high to cause acute lethality did not induce
micronuclei in bone-marrow cells of rats treated in vivo, and it
did not induce unscheduled DNA synthesis in hepatocytes of mice
treated in vivo.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Technical-grade clethodim (purity, 83.2%) was applied at a dose
of 0.5 ml under occluded conditions to two intact and two abraded
sites on the shorn backs of each of 12 female New Zealand white
rabbits for 4 h. Slight erythema, observed immediately after dosing,
was followed 24-72 h later by slight-to-severe erythema and
slight-to-moderate oedema. General clearance was seen by 14 days.
The primary irritation score was 0.7. The test material was
considered to be slightly irritating to rabbit skin (Cushman et
al., 1986b).
Undiluted technical-grade clethodim (purity, 83.3%) was
instilled at a dose of 0.1 ml into the conjunctival sac of one eye
of each of nine male New Zealand white rabbits, the other eye
serving as a control. The eyes of three of the nine rabbits were
rinsed after a 30-sec exposure. No corneal opacity or iritis was
observed. In the six rabbits with unrinsed eyes, conjunctival
irritation was moderate to severe 1 h after dosing and slight to
moderate 24 h after dosing; all eyes were clear after 72 h. In the
rinsed eyes, conjunctival irritation was moderate at 1 h and slight
at 24 h; all eyes were clear at 48 h. The test material was
considered to be mildly irritating to the rabbit eye (Cushman et
al., 1986a).
Table 2. Genotoxicity of technical-grade clethodim
End-point Test system Concentration Purity (%) Resultsa Reference
In vitro
Reverse mutation S. typhimurium TA98, 0.1-10 mg/plate 83.3 Negativea Carver et al.,
100, 1535, 1537 (technical) 1986b
E. coli WP2 uvrA
S. typhimurium TA98, 0.1-10 mg/plate 83.3 Negativea Carver et al.,
100, 1535, 1537 (technical) 1986a
Reverse mutation S. typhimurium TA98, 0, 0.1, 0.33, 1.0, 91.1 Negativea Machado et al.,
100, 1535, 1537 3.33, 10 mg/plate (technical) 1990
E. coli WP2 uvrA
Chromosomal Chinese hamster 0.03, 0.1, 0.3, 1.0 Ál/ml NR Negativeb Putnam, 1986a
aberration ovary CHO-K1 0.6, 0.8, 1.0, 1.2 Ál/ml Positivec at
CCI 61 cells 1.0, 1.2 Ál/ml
Chromosomal Chinese hamster 0.03, 0.1, 0.3, 1.0 Ál/ml 96.1 Negativea Putnam, 1986b
aberration ovary CHO-K1 0.6, 0.8, 1.0, 1.2 Ál/ml (purified)
CCI 61 cells
In vivo
Chromosomal Male and female 0, 150, 500, 1500 83.3 Negative Putnam, 1987
aberration Sprague- mg/kg bw, (technical)
Dawley rats single oral dose
Unscheduled Male B6C3F1 0, 100, 1000, 5000 83.3 Negative Mirsalis &
DNA synthesis mice mg/kg bw, gavage (technical) Steinmetz, 1986
NR, not reported
a In the presence and absence of metabolic activation
b In the presence of metabolic activation
c In the absence of metabolic activation
Technical-grade clethodim (purity, 83.4%) did not appear to
sensitize the skin of male Hartley albino guinea-pigs when tested by
the modified Buehler test (Cushman et al., 1986c).
(ii) Liver cytochrome induction
Groups of eight male Crl:CD BR rats were treated by gavage with
technical-grade clethodim (purity, 83.8%) in carboxymethylcellulose
and Tween 80 at 0 or 250 mg/kg bw per day for 21 consecutive days.
Liver samples taken at sacrifice were assayed for cytochrome P450
activity. Treatment did not affect survival, clinical signs or body
weight. The weights of the livers of animals of each sex were
significantly increased (20-23%), and enlarged livers were observed
upon gross examination of two treated animals. Although the mean
cytochrome P450 and hepatic protein contents were significantly
greater in treated animals than controls, the mean concentrations of
hepatic cytochrome P450 per milligram of protein or per gram of
liver were not significantly increased. These results may reflect
increased liver weight and do not provide evidence that clethodim
induces liver cytochrome P450 (Beatty et al., 1989).
3. Studies on metabolites
The imine sulfone and the 5-hydroxy sulfone of clethodim
occurred in larger amounts in plants than in rats (Rose et al.,
1988a). Ancillary studies on these metabolites are summarized in
Table 3.
(a) Acute toxicity
Five female Crl:CD(SD)BR rats were given a single oral dose of
1.4 g/kg bw of the imine sulfone of clethodim. One animal had
decreased motor activity 4 h after dosing and was dead the next day
(Dougherty et al., 1988b).
Groups of five female Crl:CD (SD)BR rats received the 5-hydroxy
sulfone of clethodim or technical-grade clethodim as a single oral
dose of 1.4 g/kg bw. There were no clinical signs of toxicity, and
all animals treated with the 5-hydroxy sulfone survived the 14-day
observation period after dosing. All five rats treated with
clethodim showed overt signs of toxicity, noted as salivation,
decreased motor activity, collapse, hyperactivity, tremors,
diarrhoea, dehydration and nasal, ocular, oral and anogenital
discharge, and all died within three days of dosing (Dougherty et
al., 1988c).
(b) Short-term toxicity
The imine sulfone of clethodim was administered daily in the
diet to groups of 10 male and 10 female Crl:CD (SD)BR rats at 0,
100, 1000 or 8000 ppm, equal to 6.7, 71 or 600 mg/kg bw per day, for
five weeks. Body weight and food consumption were significantly
decreased during the first week of treatment in males given 8000
ppm. Other effects observed only at 8000 ppm were increased serum
cholesterol levels in animals of each sex, increased reticulocyte
counts in males and increased liver weights in males and females in
the absence of related histopathological alterations. Treatment did
not affect survival, clinical signs or ophthalmoscopic parameters.
The NOAEL was 1000 ppm, equal to 71 mg/kg bw per day (Beatty et
al., 1988a).
Groups of 10 male and 10 female Crl:CD (SD)BR rats were fed the
5-hydroxy sulfone of clethodim daily at dietary concentrations of 0,
100, 1000 or 8000 ppm, equal to 5.9, 68 or 590 mg/kg bw per day, for
five weeks. No signs of toxicity were seen at any dose. On the basis
of the absence of any effects on survival, clinical signs, body
weight, food consumption, haematological, blood chemical or
histopathological changes, or organ weights, the NOAEL was greater
than 8000 ppm, equal to 590 mg/kg bw per day (Beatty et al.,
1988b).
Table 3. Effects of clethodim and its imine sulfone and 5-hydroxy sulfone
Study Clethodim Imine sulfone 5-Hydroxy sulfone
Acute toxicity; 3/5 deaths (Cushman et al., 1986d) 1/5 deaths 0/5 deaths
female rat; 5/5 deaths (Dougherty et al., 1988c) (Dougherty et al., 1988b) (Dougherty et al.,
1.4 g/kg bw 1988bc)
Five-week dietary; Decreased body weight and Decreased body weight and No treatment-related
male & female rats food intake, slight anaemia, food intake, increased effects; NOAEL,
increased liver weights, liver weights, cholesterol > 588mg/kg bw per day
liver hypertrophy at 4000 and and reticulocytes at 8000 (Beatty et al., 1988b)
8000 ppm; anaemia, liver ppm. NOAEL, 71 mg/kg bw per
hypertrophy at 1000 ppm day (Beatty et al., 1988a)
NOAEL, 13 mg/kg bw per day
(Cisson & Eisenlord, 1986)
Teratogenicity; rats Maternal and fetal toxicity Maternal toxicity at 100 Clinical signs and
at 350 and 700 mg/kg bw per and 700 mg/kg bw per day; slightly decreased
day; NOAEL, 100 mg/kg bw fetal effects at 700 mg/kg fetal body weights at
per day. Increased incidence bw per day 700 mg/kg bw per day
of tail malformations at NOAEL, 10 mg/kg bw NOAEL, 100 mg/kg
700 mg/kg bw per day per day (Hoberman & per day (Hoberman &
(Schroeder & Daly, 1987) Christian, 1988a) Christian, 1988b)
Microbial gene Negative Negative Negative
mutation (Carver et al., 1986a,b) (Carver et al., 1988) (Carver eta[., 1987)
Chromosomal Positive in absence of Negative Negative
aberration, metabolic activation (Putnam, 1988a) (Putnam, 1988b)
CHO cells (Putnam, 1986a)
(c) Embryotoxicity and teratogenicity
Groups of 10 pregnant female Crl:CD (SD)BR rats were treated by
gavage with the imine sulfone of clethodim in Tween 80 and
carboxymethylcellulose at doses of 0, 10, 100 or 700 mg/kg bw per
day during days 6-15 of gestation. Maternal toxicity was seen at the
high dose of 700 mg/kg bw per day, manifested as excessive
salivation and decreased body weight and food consumption.
Significant decreases in maternal body weight were also recorded at
100 mg/kg bw per day. At 700 mg/kg bw per day, fetal weights were
decreased, and there were increased incidences of fetuses and
litters with cervical ribs and delayed sternal ossification. The
NOAEL for maternal toxicity was 10 mg/kg bw per day on the basis of
reduced body weights at 100 mg/kg bw per day and more. An increased
incidence of fetal variations at the maternally toxic dose of 700
mg/kg bw per day resulted in an NOAEL of 100 mg/kg bw per day for
developmental toxicity (Hoberman & Christian, 1988a).
Groups of 10 pregnant female Crl:CD (SD)BR rats were
administered the 5-hydroxy sulfone of clethodim in
carboxymethylcellulose and Tween 80 by gavage at 0, 10, 100 or 700
mg/kg bw per day on days 6-15 of gestation. At 700 mg/kg bw per day,
one female showed excessive salivation and two females had rales; a
slight decrease in fetal body weights in this group, although not
statistically significant, was possibly related to treatment. No
teratogenicity was seen at any dose. The NOAEL for maternal and
fetal toxicity was 100 mg/kg bw per day (Hoberman & Christian,
1988b).
(d) Genotoxicity
Neither the imine sulfone nor the 5-hydroxy sulfone of
clethodim induced gene mutation in Salmonella typhimurium TA98,
TA100, TA1535 or TA1537 or in Escherichia coli WP2 uvrA in the
presence or absence of metabolic activation. Neither metabolite
induced chromosomal aberrations in Chinese hamster ovary cells.
4. Observations in humans
No information was available.
Comments
Clethodim was readily absorbed by rats after oral
administration. Excretion was rapid and occurred predominantly via
the urine, with lesser amounts eliminated in the faeces and as
carbon dioxide. There were no significant dose-related or
sex-specific differences in elimination pattern or tissue
distribution, and there was no evidence of bioaccumulation.
The dominant metabolic pathway proposed for clethodim in rats
is oxidation to clethodim sulfoxide. Other postulated pathways are
cleavage of the N-O bond to form the imine, conversion of the
S-ethyl group to S-methyl, formation of the oxazole, and
hydroxylation at the 5 position. The urinary metabolites identified
were the sulfoxides and sulfones of clethodim and 5-hydroxy
clethodim, the sulfoxides of the imine and S-methyl clethodim and
the sulfone of the oxazole.
Clethodim had slight to moderate acute oral toxicity in the rat
and mouse. WHO has not classified clethodim with regard to toxic
hazard.
The primary effect of treatment with clethodim after short- and
long-term dietary exposure in the mouse, rat and dog was on the
liver. Hepatic effects were manifest as increased liver weights and
centrilobular hypertrophy. A study in rats administered clethodim at
250 mg/kg bw per day did not provide evidence for liver cytochrome
P450 induction.
In a four-week study in mice given dietary concentrations of 0,
100, 250, 625, 1500 or 4000 ppm, the NOAEL was 250 ppm, equivalent
to 38 mg/kg bw per day on the basis of evidence of slight anaemia at
625 ppm and above. In a 78-week study of toxicity and
carcinogenicity in which mice received dietary levels of 0, 20, 200,
1000 or 2000-3000 ppm, the NOAEL was 200 ppm, equivalent to 30 mg/kg
bw per day, on the basis of hepatic effects and an increased
incidence of alveolar lung macrophages at 1000 ppm and above.
In a five-week study in which rats received dietary levels of
0, 5, 200, 1000, 4000 or 8000 ppm, the NOAEL was 200 ppm, equal to
13 mg/kg bw per day, on the basis of liver hypertrophy and evidence
of slight anaemia at 1000 ppm and above. A 13-week study at dietary
concentrations of 0, 50, 500, 2500 or 5000 ppm and a two-year
feeding study at dietary levels of 0, 5, 20, 500 or 2500 ppm in rats
revealed an NOAEL of 500 ppm, equal to 25 mg/kg bw per day in the
first study and equal to 16 mg/kg bw per day in the second. The
NOAEL was based on decreased body weights and liver hypertrophy at
dietary levels of 2500 ppm and above.
In dogs, 13-week oral administration of capsules of clethodim
at doses of 0, 1, 25, 75 or 125 mg/kg bw per day resulted in an
NOAEL of 25 mg/kg bw per day, on the basis of increased liver weight
and cholesterol levels at 75 mg/kg bw per day and above. In a
one-year study at doses of 0, 1, 75 or 200-300 mg/kg bw per day, the
NOAEL was 1 mg/kg bw per day on the basis of treatment-related
effects on the liver at 75 mg/kg bw per day and above.
Clethodim was not carcinogenic when fed to mice or rats at
dietary levels of up to 2500 ppm.
A two-generation study of reproductive toxicity in rats treated
with clethodim at dietary levels of 0, 5, 20, 500 or 2500 ppm did
not reveal any adverse effects on reproduction. The NOAEL was 500
ppm, equal to 39 mg/kg bw per day, on the basis of decreased
parental body weights and food consumption at 2500 ppm.
Studies of teratogenicity were conducted with clethodim in rats
at doses of 0, 10, 100, 350 or 700 mg/kg bw per day and in rabbits
at 0, 25, 100 or 300 mg/kg bw per day. In rats, the NOAEL for
maternal toxicity was 100 mg/kg bw per day. Fetal toxicity was
observed at the maternally toxic doses of 350 and 700 mg/kg bw per
day. An increased incidence of malformations, specifically tail
defects (short, filamentous or absent tails), was observed at the
maternally toxic and lethal high dose of 700 mg/kg bw per day. In
rabbits, maternal toxicity was observed at 100 and 300 mg/kg bw per
day, resulting in an NOAEL of 25 mg/kg bw per day. There were no
adverse effects on the rabbit fetus and no evidence of teratogenic
potential at doses up to 300 mg/kg bw per day.
Clethodim was adequately tested for genotoxicity in a series of
assays in vivo and in vitro. The Meeting concluded that
clethodim was not genotoxic.
Although the imine sulfone and 5-hydroxy sulfone metabolites of
clethodim have been reported to occur in higher amounts in plants
than in animals, neither of the metabolites was more toxic than the
parent, clethodim, in studies of acute or short-term toxicity and
teratogenicity. There was similarly no evidence of genotoxic
potential.
An ADI was established on the basis of the NOAEL of 1 mg/kg bw
per day from the one-year study in dogs and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 200 ppm, equivalent to 30 mg/kg bw per day (78-week
study of toxicity and carcinogenicity)
Rat: 500 ppm, equal to 16 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 25 mg/kg bw per day (study of teratogenicity)
Dog: 1 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Observations in humans
References
Beatty, P.W., Bagos, A.D., Richter, W.R. & Wong, Z.A. (1988a)
Five-week oral toxicity study in rats with RE-47719 (SX-1800).
Project No. 2949. Unpublished report dated 15 November 1988 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Beatty, P.W., Bagos, A.C., Richter, W.R. & Wong, Z.A. (1988b)
Five-week oral toxicity study in rats with RE-51228 (SX-1803).
Project No. 2950. Unpublished report dated 15 November 1988 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Beatty, P.W., Brorby, G.P. & Wong, Z.A. (1989) The potential of
RE-45601 Technical (SX-1688) to induce cytochrome P-450 following
21-day oral administration in male rats. Project No. 2784.
Unpublished report dated 16 November 1989 from Chevron Environmental
Health Center Inc., Richmond, CA, USA. Submitted to WHO by Tomen
Pacific Agro Co., San Francisco, CA, USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1986a)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-45601 Technical (83% purity, SC-1688). Project No. SOCAL
2505. Unpublished report dated 13 January 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1986b)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-45601 Technical (83% purity, SC-1688). Project No. CEHC
2555. Unpublished report dated 28 April 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1987)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-51228. Project No. 2856. Unpublished report dated 7 December
1987 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1988)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-47719. Project No. 2948. Unpublished report dated 19 October
1988 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Cisson, C.M. & Eisenlord, G.H. (1986) Five-week pilot feeding study
in rats with RE-45601 Technical (SX-1653). Project No. SOCAL 2457.
Unpublished report dated 7 October 1986 from Chevron Environmental
Health Center Inc., Richmond, CA, USA. Submitted to WHO by Tomen
Pacific Agro Co., San Francisco, CA, USA.
Cox, R.H. (1988) Acute intraperitoneal toxicity study in rats with
Chevron RE-45601 Technical (SX-1688). Project No. 2107-151.
Unpublished revised report dated 6 April 1988 from Hazelton
Laboratories America Inc., Vienna, VA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Cox, R.H. & Slabik, C.M. (1986) Four-week subchronic oral toxicity
study in mice with Chevron RE-45601 Technical. Project No. 2107-140.
Unpublished report dated 21 July 1986 from Hazelton Laboratories
America Inc., Vienna, VA, USA. Submitted to WHO by Tomen Pacific
Agro Co., San Francisco, CA, USA.
Cox, R.H. & Zoetis, T. (1986) Acute oral toxicity study in mice with
Chevron RE-45601 Technical. Project No. 2107-143. Unpublished report
dated 16 September 1986 from Hazelton Laboratories America Inc.,
Vienna, VA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Cox, R.H. & Zoetis, T. (1988b) One-year oral toxicity study in dogs
with Chevron RE-45601 Technical (SX-1688). Project No. 2107-153.
Unpublished report dated 28 October 1988 from Hazelton Laboratories
America Inc., Vienna, VA, USA. Submitted to WHO by Tomen Pacific
Agro Co., San Francisco, CA, USA.
Cox, R.H. & Zoetis, T. (1988b) Chronic oral oncogenicity study in
mice with Chevron RE-45601 Technical (SX-1688). Project No.
2107-145. Unpublished report dated 25 October 1988 from Hazelton
Laboratories America Inc., Vienna, VA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Thompson, J.D. & Wong, Z.A. (1986a) The acute eye
irritation potential of RE-45601 Technical (SX-1688). Project No.
CEHC 2511. Unpublished report dated 1 October 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Korenaga, G.L. & Wong, Z.A. (1986b) The four-hour
skin irritation potential of RE-45601 Technical (SX-1688). Project
No. CEHC 2512. Unpublished report dated 18 December 1986 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Cooper, P.N. & Wong, Z.A. (1986c) Modified Buehler
Test for the skin sensitization potential of RE-45601 Technical
(SX-1688). Project No. CEHC 2514. Unpublished report dated 15
December 1986 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Cushman, J.R., Easter, M.D. & Wong, Z.A. (1986d) The acute oral
toxicity of RE-45601 Technical (SX-1688) in adult male and female
rats. Project No. SOCAL 2498. Unpublished report dated 27 June 1986
from Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Hedgecock, J.H. & Wong, Z.A. (1987a) Four-week
repeated dose dermal toxicity study in rats with RE-45601 Technical
(SX-1688). Project No. CEHC 2552. Unpublished report dated 20
November 1987 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Cushman, J.R., Korenaga, G.L. & Wong, Z.A. (1987b) The acute dermal
toxicity of RE-45601 Technical (SX-1688) in adult male and female
rabbits. Project No. CEHC 2510. Unpublished report dated 7 January
1987 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Daly, I.W. & Knezevich, A.L. (1987) Ninety-day subchronic oral
toxicity study in dogs with Chevron RE-45601 Technical. Project No.
85-2999. Unpublished report dated 26 February 1987 from Bio/dynamics
Inc., East Millstone, NJ, USA. Submitted to WHO by Tomen Pacific
Agro Co., San Francisco, CA, USA.
Dearlove, G.E., Hoberman, A.M. & Christian, M.S. (1986) Pilot
teratology study in rabbits with Chevron RE-45601 Technical. Project
No. 303-007P. Unpublished report dated 15 July 1986 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Dearlove, G.E., Hoberman, A.M. & Christian, M.S. (1987) Teratology
study in rabbits with Chevron RE-45601 Technical. Project No.
303-007. Unpublished report dated 30 January 1987 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Dougherty, K.K., Eisenlord, G.H., Low, S.S. & Wong, Z.A. (1987)
13-Week oral toxicity study in rats with RE-45601 Technical
(SX-1688). Project No. SOCAL 2501. Unpublished report dated 13
February 1987 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Eisenlord, G.H. & Wong, Z.A. (1988a) Combined
chronic toxicity/oncogenicity study in rats with RE-45601 Technical
(SX-1688). Project No. SOCAL 2500. Unpublished report dated 1
November 1988 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Duncan, R.A. & Wong, Z.A. (1988b) The comparative
acute oral toxicity of RE-47719 (SX-1800) and RE-45601 (SX-1688) in
adult female rats. Project No. 2952. Unpublished report dated 21
October 1988 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Duncan, R.A. & Wong, Z.A. (1988c) The comparative
acute oral toxicity of RE-51228 (SX-1796) and RE-45601 (SX-1688) in
adult female rats. Project No. 2951. Unpublished report dated 21
October 1988 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Waid, L.J. & Wong, Z.A. (1989) The acute oral
toxicity of RE-45601 Technical (SX-1688) in adult male and female
rabbits. Project No. CEHC 2992. Unpublished report dated 21 June
1989 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Griffis, L.C., Bruce, E.D. & Wong, Z.A. (1986) The acute inhalation
toxicity of RE-45601 Technical (SX-1688) in rats. Project No. CEHC
2513. Unpublished report dated 7 November 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Hoberman, A.M. & Christian, M.S. (1988a) Oral teratogenicity and
developmental toxicity screen in rats with RE-47719. Project No.
303-012. Unpublished report dated 8 November 1988 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Hoberman, A.M. & Christian, M.S. (1988b) Oral teratogenicity and
developmental toxicity screen in rats with RE-51228. Project No.
303-010. Unpublished report dated 8 November 1988 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Lee, S.G., Maier, K.M., Tamichi, E.H. & Tucker, B.V. (1988)
[Ring-4,6-14C] clethodim: A radiocarbon metabolism study in laying
hens. Project No. MEF-0089. Unpublished report dated 30 December
1988 from Chevron Chemical Company, Ortho Agricultural Chemicals
Division, Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro
Co., San Francisco, CA, USA.
Machado, M.L., Lubeck, S.G. & Wilkenfeld, R.M. (1990)
Microbial/microsome reverse mutation plate incorporation assay with
RE-45601 Technical (SX-1845). Project No. CEHC 3146. Unpublished
report dated 20 August 1990 from Chevron Environmental Health Center
Inc., Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co.,
San Francisco, CA, USA.
McKensie, K.M. (1986) Pilot rat reproduction study with Chevron
RE-45601 Technical. Project No. 6183-102. Unpublished report dated
16 December 1986 from Hazelton Laboratories America Inc., Madison,
WI, USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco,
CA, USA.
Mirsalis, J.C. & Steinmetz, K.L. (1986) In vivo--in vitro
hepatocyte DNA repair assay: in vitro evaluation of unscheduled
DNA synthesis (UDS) following oral administration of Chevron
RE-45601 Technical to B6C3F1 Mice. Project No. LSC-1960.
Unpublished report dated August 1986 from SRI International, Meno
Park, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Putnam, D.L. (1986a) Chromosome aberrations in Chinese hamster ovary
(CHO) cells with Chevron RE-45601 Technical. Project No. T4529.337.
Unpublished report dated August 1986 from Microbiological Associates
Inc., Bethesda, MD, USA. Submitted to WHO by Tomen Pacific Agro Co.,
San Francisco, CA, USA.
Putnam, D.L. (1986b) Chromosome aberrations in Chinese hamster ovary
(CHO) cells with purified Chevron RE-45601. Project No. T429.337.
Unpublished report dated 15 December 1986 from Microbiological
Associates Inc., Bethesda, MD, USA. Submitted to WHO by Tomen
Pacific Agro Co., San Francisco, CA, USA.
Putnam, D.L. (1987) Cytogenetics assay in bone marrow cells of rats
following acute oral exposure to RE-45601 Technical. Project No.
T5172.105. Unpublished report dated 25 February 1987 from
Microbiological Associates Inc., Bethesda, MD, USA. Submitted to WHO
by Tomen Pacific Agro Co., San Francisco, CA, USA.
Putnam, D.L. (1988a) Chromosome aberrations in Chinese hamster ovary
(CHO) cells: RE-47719. Project No. T8226.337003. Unpublished report
dated 10 November 1988 from Microbiological Associates Inc.,
Bethesda, MD, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Putnam, D.L. (1988b) Chromosome aberrations in Chinese hamster ovary
(CHO) cells: RE-51228. Project No. T8227.337003. Unpublished report
dated 10 November 1988 from Microbiological Associates Inc.,
Bethesda, MD, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Rendina, A.R. & Felts, J.M. (1988) Cyclohexanedione herbicides are
selective and potent inhibitors of acetyl-CoA carboxylase from
grasses. Plant Physiol., 86, 983-986.
Rose, A.F., Griffis, L.C., Suzuki, J.P., Bruce, E.D., Primer, R.L.,
Wong, Z.A. & Tucker, B.V. (1988a) The in vivo metabolism of
[propyl-1-14C] clethodim in rats. Project No. MEF-0086/CEHC 2515.
Unpublished report dated 22 December 1988 from Chevron Chemical Co.,
Ortho Agricultural Chemicals Division, Richmond, CA, USA. Submitted
to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Rose, A.F., Suzuki, J.P., Primer, R.L. & Tucker, B.V. (1988b) The
in vivo metabolism of [propyl-1-14C] clethodim in a lactating
goat. Project No. MEF-0038. Unpublished report dated 29 December
1988 from Chevron Chemical Co., Ortho Agricultural Chemicals
Division, Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro
Co., San Francisco, CA, USA.
Schroeder, R.E. & Daly, I.W. (1986) A pilot teratology study in rats
with Chevron RE-45601 Technical. Project No. 85-3032. Unpublished
report dated 8 September 1986 from Bio/dynamics Inc., East
Millstone, NJ, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Schroeder, R.E. & Daly, I.W. (1987) Teratology study in rats with
Chevron RE-45601 Technical. Project No. 86-3042. Unpublished report
dated 20 July 1987 from Bio/dynamics Inc., East Millstone, NJ, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Tellone, C.I., Hardy, L.M. & Richter, W.R. (1987) Two-generation
(one litter) reproduction study in rats with RE-45601 Technical.
Project No. CEHC 2596. Unpublished report dated 27 August 1987 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
 Two groups of eight white Leghorn laying hens were given daily
doses of [cyclohexene-4,6-14C]-clethodim at 2.1 or 51.3 mg/kg bw
per day by capsule for five consecutive days. The hens were
sacrificed about 4 h after the final dose, and tissues were
collected for analysis. Two major metabolites, clethodim sulfone and
clethodim sulfoxide, were identified in tissues and eggs. Clethodim
sulfone accounted for up to 57% of tissue levels of radiolabel,
whereas clethodim sulfoxide accounted for 10-31%. Clethodim
sulfoxide was the principal metabolite in egg white (26-82%) and
yolk (25-37%); parent clethodim was detected in significantly
smaller amounts in both tissues and eggs. The proposed metabolic
pathway in chickens was different from and considered to be simpler
than that observed in rats and goats, since none of the imine,
5-hydroxy or S-methyl analogues was detected in chickens (Lee et
al., 1988).
In the study described above in which a lactating goat received
[propyl-1-14C]-clethodim (Rose et al., 1988b), hind- and
forequarter muscle, peritoneal and subcutaneous fat, liver, kidneys,
heart and blood were collected to allow characterization of
metabolites. The major urinary metabolite was clethodim sulfoxide,
which accounted for 67% of the urinary radiocarbon. Other urinary
radiolabelled components were identified as clethodim (3-27%) and
the demethyl sulfoxide (12-18%), S-methyl (7-13%), imine sulfoxide
(1.5-2.8%), sulfone (1.5-2.2%) and 5-hydroxy sulfoxide (0-3%). In
the milk, about half of the radiocarbon could be extracted into
organic solvents and occurred in clethodim, clethodim sulfoxide and
clethodim demethyl sulfoxide; the other half of the radiocarbon was
water soluble and was shown to be 14C-lactose. In blood and
tissues, the maximal extractable radiocarbon residues accounted for
77-95% of the radiolabel and were identified as clethodim and the
sulfoxide, demethyl sulfoxide, imine sulfoxide, sulfone and
5-hydroxy sulfone. The proposed metabolic pathway in goats was
essentially the same as that proposed for rats (Rose et al.,
1988b).
2. Toxicological studies
(a) Acute toxicity
Technical-grade clethodim has been tested for acute toxicity in
mice, rats and rabbits. The results are summarized in Table 1.
Clethodim was virtually non-toxic after oral or dermal
administration to rabbits or after inhalation by rats. Slight to
moderate toxicity was observed in mice and rats treated orally, with
LD50 levels of 1360-2570 mg/kg bw. Clethodim administered
intraperitoneally was moderately toxic to rats, with a combined
LD50 for the two sexes of 1117 mg/kg bw.
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female Charles River CD-1 mice were
fed diets containing technical-grade clethodim (purity, 83.3%) for
four weeks at 100, 250, 625, 1500 or 4000 ppm, equivalent to 0, 15,
38, 94, 225 or 600 mg/kg bw per day. A slight anaemic process was
manifested in male mice as a decreased erythrocyte count at >
1500 ppm, decreased haemoglobin at > 625 ppm and lowered
haematocrit at 4000 ppm. Increased liver weights, which were
correlated microscopically with centrilobular hypertrophy, were
observed in males fed 1500 ppm and in animals of each sex treated at
the highest dose of 4000 ppm. An increased incidence of focal
coagulative necrosis of the liver was recorded in males treated at
> 1500 ppm. Treatment appeared to have no effect on survival,
clinical signs, body weight or food consumption. The NOAEL was 250
ppm, equivalent to 38 mg/kg bw per day, on the basis of the
dose-related decrease in erythroid parameters at dietary levels >
625 ppm (Cox & Slabik, 1986).
Rats
Technical-grade clethodim (purity, 83.2%) was applied in
carboxymethylcellulose and Tween 80 to the skin of groups of six
male and six female Crl:CD BR rats at doses corresponding to 0, 10,
100 or 1000 mg/kg bw per day for 6 h/day, five days per week for
four weeks, for a total of 21 exposures. A dose-related increase in
the occurrence of skin irritation was observed at all dose levels.
Clinical signs seen at 1000 mg/kg bw per day were described as
anogenital discharge and staining of the fur, alopecia and red nasal
discharge. At the highest dose, group mean body weights,
corresponding weight gains and feed efficiency values were decreased
in males, and increased liver weights were recorded in females. In
males, a slight increase was observed in kidney regeneration, which
was described as a minimal multifocal lesion. Although this finding
was observed only in treated rats, evidence of chronic progressive
nephropathy is not uncommon in this strain of rat and was not
considered to represent a treatment-related effect. The NOAEL for
systemic toxicity under the conditions of this study was thus 100
mg/kg bw per day (Cushman et al., 1987a).
Table 1. Acute toxicity of technical-grade clethodim in male and female rodents
Species, Route and vehicle LD50 Purity Reference
strain (mg/kg bw) (%)
Mouse, CD-1 Oral; M: 2570 83.3 Cox & Zoetis, 1986
Tween 80 + CMC F: 2430 (technical)
Rat, Crl:CD Oral; M: 1630 83.3 Cushman et al., 1986d
(SD)BR Tween 80 + CMC (technical)
Rat, Crl:CD Intraperitoneal; M: 1040 NR Cox, 1988
(SD)BR Tween 80 + CMC F: 1200
Rat, Inhalation M&F: 83.3 Griffis et al., 1986
Sprague-Dawley > 3.9 mg/l (technical)
Rabbit, New Oral; M: > 2000 and 82.5 Dougherty et al., 1989
Zealand Tween 80 + CMC < 5000 (technical)
white F: > 5000
Rabbit, New Dermal M&F: > 5000 83.3 Cushman et al., 1987b
Zealand (technical)
white
NR, not reported; Tween 80, 0.5% polyoxyethylenesorbitan monooleate; CMC,
carboxymethylcellulose
In a preliminary five-week study, groups of 10 male and 10
female Crl:CD(SD)BR rats were treated with technical-grade clethodim
(purity, 83.4%) at dietary levels of 0, 5, 200, 1000, 4000 or 8000
ppm, equal to 0.3, 13, 66, 261 or 515 mg/kg bw per day for males and
0.3, 14, 71, 291 or 554 mg/kg bw per day for females.
Treatment-related effects were decreased body-weight gain and food
consumption (particularly during the first week of the study) in
animals of each sex at 4000 and 8000 ppm; increased liver weights at
> 4000 ppm; and slight to mild centrilobular hypertrophy in males
at 1000 ppm and in animals of each sex at 4000 and 8000 ppm.
Dose-related, statistically significant decreases in haemoglobin
concentration were noted in males at > 1000 ppm and in
haematocrit in males at > 4000 ppm. Raised cholesterol
concentrations were recorded in males receiving 8000 ppm, and raised
serum uric acid levels were seen in females given > 4000 ppm.
Treatment had no effect on survival, clinical signs or urinary
parameters. The NOAEL in this study was 200 ppm, equal to 13 mg/kg
bw per day, on the basis of decreases in erythroid parameters and
centrilobular hypertrophy of the liver at 1000 ppm and more (Cisson
& Eisenlord, 1986).
Technical-grade clethodim (purity, 84%) was administered to
groups of 12 male and 12 female Crl:CD(SD)BR rats for 13 weeks at
dietary levels of 0, 50, 500, 2500 or 5000 ppm, equal to 2, 25, 134
or 280 mg/kg bw per day for males and 3, 30, 160 or 340 mg/kg bw per
day for females. In order to study the reversibility of potential
treatment-related effects, an additional 12 rats of each sex were
assigned to the groups receiving 0, 2500 and 5000 ppm and were kept
for a six-week recovery period after the end of treatment with
clethodim. Significantly decreased body weights and weight gains
were recorded in males treated with 2500 ppm and in animals of each
sex treated with 5000 ppm. Decreased mean food consumption was noted
in both males and females at 5000 ppm. Significantly ( p < 0.05)
increased concentrations of cholesterol, total protein and globulin
recorded in males fed 5000 ppm were within the normal control range
of variability. Increased liver weights and increased incidence and
severity of centrilobular hypertrophy of the liver were noted in
males and females fed 2500 and 5000 ppm. A slightly increased
incidence of renal focal regeneration was seen in males treated at
> 2500 ppm. The pathologist did not consider this finding to be
related to treatment; rather, these lesions were regarded as typical
of the earliest minimal stage of spontaneous chronic progressive
nephropathy. Treatment did not affect survival, clinical signs,
ophthalmoscopic or haematological parameters or the results of
urinalysis. No treatment-related effects were seen after the
six-week recovery period. The NOAEL was 500 ppm, equal to 25 mg/kg
bw per day, as determined by an increased incidence of hepatic
centrilobular hypertrophy and decreased body-weight gain in animals
of each sex treated at 2500 ppm and above (Dougherty et al.,
1987).
Dogs
Groups of four male and four female beagle dogs received
gelatin capsules containing technical-grade clethodim (purity,
83.3%) orally at 0, 1, 25, 75 or 125 mg/kg bw per day for 13 weeks.
Treatment affected blood chemistry in females, which had increased
cholesterol levels at 75 mg/kg bw per day and above and increased
alkaline phosphatase activity at 125 mg/kg bw per day. An increased
globulin concentration, with a concomitant decrease in the
albumin:globulin ratio, was noted in males treated at 125 mg/kg bw
per day. Significantly ( p < 0.05) increased total leukocyte
counts were seen in males and decreased activated partial
thromboplastin time was seen in females after one month at 75 and
125 mg/kg bw per day. As no similar differences from control values
were seen, however, after two or three months of treatment, and the
only differences seen were in one sex, the significance of these
findings is doubtful. Increased liver weights were recorded in males
and females fed 75 or 125 mg/kg bw per day. Slight increases in the
severity of cytoplasmic vesiculation and vacuolation of the
centrilobular hepatocytes were seen in animals of each sex treated
at 125 mg/kg bw per day. Treatment did not affect survival, clinical
signs, ophthalmoscopic parameters, body weight or food consumption.
The NOAEL was 25 mg/kg bw per day on the basis of elevated
cholesterol levels and increased liver weights at 75 mg/kg bw per
day and above (Daly & Knezevich, 1987).
In a one-year study, groups of six male and six female beagle
dogs were administered technical-grade clethodim (purity, 83.3%) in
gelatin capsules at doses of 0, 1, 75 or 200-300 mg/kg bw per day.
In the absence of any signs of overt toxicity, the high dose of 200
mg/kg bw per day was increased after seven weeks of treatment to 300
mg/kg bw per day. Significant effects on haematological parameters
were seen, expressed as increased platelet and leukocyte counts in
females at 75 mg/kg bw per day and in males and females at 300 mg/kg
bw per day. At 300 mg/kg bw per day, erythroid parameters
(erythrocyte, haemoglobin and haematocrit values) were decreased in
animals of each sex, and reticulocyte and segmented neutrophil
counts were increased in females. Treatment-related effects on blood
chemistry were seen as increased levels of cholesterol,
triglycerides, alkaline phosphatase and alanine aminotransferase in
males and females at 300 mg/kg bw per day. Decreased serum glucose
concentrations were noted in both males and females at the high dose
and in females treated at 75 mg/kg bw per day. Increased liver
weights were recorded in animals of each sex at 75 and 300 mg/kg bw
per day. Significantly ( p < 0.05) increased thyroid weights seen
in males at 300 mg/kg bw per day were not correlated with any
microscopic changes. Histopathological alterations in animals of
each sex treated at 300 mg/kg bw per day included hepatocellular
enlargement, increased granular pigment (which was not associated
with bile, iron or acid-fast lipofuscin) and slight hypercellularity
of the bone marrow; the last finding was also observed in one male
and one female at 75 mg/kg bw per day. The significance of a
slightly increased incidence of pigment (not otherwise
characterized) in the spleen of females receiving 75 or 300 mg/kg bw
per day was uncertain. Treatment did not affect survival, clinical
signs, ophthalmoscopic parameters, body weight, food consumption or
the results of urinalysis. The NOAEL was 1 mg/kg bw per day on the
basis of treatment-related effects on haematological and blood
chemical parameters, increased liver weight and hypercellularity of
the bone marrow at 75 mg/kg bw per day and above (Cox & Zoetis,
1988a).
(c) Long-term toxicity and carcinogenicity
Mice
Technical-grade clethodim (purity, 83.3%) was administered in
the diet to groups of 60 male and 60 female Crl:CD-1 (ICR)BR mice
for 78 weeks at 0, 20, 200, 1000 or 2000-3000 ppm, equivalent to 3,
30, 150 or 300-450 mg/kg bw per day. The high dose was increased to
3000 ppm after 15 weeks of treatment, when haematological assessment
did not show the significant decrease in erythrocyte parameters seen
in the four-week preliminary study. Ten mice of each sex were
sacrificed after 52 weeks of treatment in each group for interim
assessment. A significant ( p < 0.05) trend to decreased survival
was noted among males and females fed 3000 ppm, with survival at 78
weeks of 58, 66, 60, 52 and 32% males and 67, 84, 80, 59 and 48%
females, respectively. The predominant cause of death at this dose
was increased incidence and severity of systemic amyloidosis.
Decreased erythrocyte counts were recorded in animals of each sex at
3000 ppm, with concomitant decreases in haemoglobin and haematocrit
values in males. After 52 weeks of treatment, increased liver
weights and centrilobular hypertrophy of the liver were observed in
males at 1000 ppm and in males and females at 3000 ppm. Increased
pigment, described as morphologically compatible with haemosiderin
and bile, was noted in males at 3000 ppm. After 78 weeks of
treatment, the hepatic changes included increased liver weights in
females at 3000 ppm, centrilobular hypertrophy and increased pigment
in both males and females at 1000 and 3000 ppm and bile-duct
hyperplasia in males at > 1000 ppm. An increased incidence of
multifocal, amphophilic alveolar lung macrophages was also observed
in animals of each sex treated at > 1000 ppm. No significant
effect was seen on body weight, food consumption or clinical signs.
The NOAEL was 200 ppm, equivalent to 30 mg/kg bw per day, on the
basis of hepatic changes, notably centrilobular hypertrophy,
increased pigment and bile-duct hyperplasia, and an increased
incidence of alveolar macrophages in the lungs of mice treated at
1000 ppm and above. There was no evidence that clethodim has
carcinogenic potential (Cox & Zoetis, 1988b).
Rats
Groups of 65 male and 65 female Crl:CD (SD)BR rats were
administered technical-grade clethodim (purity, 83%) in the diet at
levels of 0, 5, 20, 500 or 2500 ppm, equal to 0.2, 0.6, 16 or 86
mg/kg bw per day for males and 0.2, 0.7, 21 or 110 mg/kg bw per day
for females, for two years. Ten rats of each sex in each group were
sacrificed after one year. After two years, 47, 49, 46, 50 and 33%
of males and 55, 58, 51, 40 and 49% of females were still alive in
the five dose groups, respectively. Treatment did not affect cause
or time of death, clinical signs, ophthalmoscopic or haematological
parameters or the results of clinical chemistry or urinalysis.
Body-weight gain and food consumption were significantly decreased
in animals of each sex during the first year of treatment with 2500
ppm. Food intake, calculated relative to body weight, was slightly
greater in males and females at 2500 ppm than in the other treated
groups. At interim sacrifice after one year, increased liver weights
and slight to mild centrilobular hypertrophy were noted in rats of
each sex fed 2500 ppm. Increased liver weights seen in females at
500 ppm were not correlated with any microscopically discernible
change. At terminal sacrifice, liver weights of females at 2500 ppm
were increased; males had no significant increase in liver weight,
and no treatment-related centrilobular hypertrophy was seen in
animals of either sex. Females treated at 2500 ppm had a slightly
greater (12%) incidence of binucleated cells in the liver than the
controls (2%), but the effect was of uncertain toxicological
significance. Clethodim at dietary levels up to 3000 ppm showed no
evidence of carcinogenic potential. The NOAEL was 500 ppm, equal to
16 mg/kg bw per day, on the basis of decreased body-weight gain,
decreased food intake, increased liver weight and associated
centrilobular hypertrophy at 2500 ppm (Dougherty et al., 1988a).
(d) Reproductive toxicity
Rats
In a preliminary study, groups of eight male and eight female
Crl:CD(SD)BR rats were fed technical-grade clethodim (purity, 83.3%)
at dietary levels of 0, 500, 2000 or 5000 ppm, equivalent to 25, 100
or 250 mg/kg bw per day, for one week before and during mating.
Males were sacrificed after mating, whereas females were treated
throughout gestation and up to day 7 of the lactation period for a
single litter. Parental animals of each sex that received 5000 ppm
had decreased body-weight gain and food intake ( p < 0.05, only in
males). Mean pup weights and corresponding weight gains were
decreased at all dietary levels. No adverse effects on reproductive
parameters were evident at the highest dose (McKensie, 1986).
In a two-generation (one litter per generation) study, groups
of 30 male and 30 female Crl:COBS CD(SD) rats were treated with
technical-grade clethodim (purity, 83%) at dietary levels of 0, 5,
20, 500 or 2500 ppm, equal to 0.1, 1.4, 39 or 190 mg/kg bw per day
for males and 0.2, 1.8, 45 or 220 mg/kg bw per day for females. Mean
body weight was decreased at 2500 ppm in both males and females of
the F1 generation and in males of the F0 generation. Occasional
significant ( p < 0.05) decreases in food consumption were
recorded in F1 males and females treated with 2500 ppm. Treatment
had no effect on parental survival, clinical signs, organ weights or
histopathological manifestations; there were no adverse effects on
reproductive indices (mating, fertility, pregnancy and gestation);
and there was no effect on litter size, sex ratio, pup weight or pup
viability. A significant ( p < 0.05) increase in the number of
stillborn pups was observed in the litters of F1 dams treated at
2500 ppm; however, this finding was not considered to be related to
treatment, since the value was within the historical control range,
and no similar increase was seen in the subsequent F2 litter. The
NOAEL for parental systemic toxicity was 500 ppm, equal to 39 mg/kg
bw per day, on the basis of decreased body weights and food
consumption at 2500 ppm. The NOAEL for adverse effects on
reproduction in the F0 and F1 generations was 2500 ppm, the
highest dietary level tested, equal to 190 mg/kg bw per day (Tellone
et al., 1987).
(e) Embryotoxicity and teratogenicity
Rats
In a preliminary range-finding study, groups of 10 mated female
Crl:CD(SD) rats were given technical-grade clethodim (purity, 82.6%)
in Tween 80 and carboxymethylcellulose by gavage at 0, 50, 150, 300
or 500 mg/kg bw per day during days 6-15 of gestation. The day on
which evidence of mating was observed was designated day 0 of
gestation. Clinical signs of toxicity were an increased incidence of
excessive salivation in females at 300 and 500 mg/kg bw per day, and
slightly decreased body-weight gain, decreased mean number of
uterine implantations and decreased mean fetal body weights at 500
mg/kg bw per day. External examination of the fetuses failed to
reveal any gross findings related to treatment (Schroeder & Daly,
1986).
Groups of 25 mated female Crl:CD(COBS) rats were treated with
technical-grade clethodim (purity, 82.6%) in Tween 80 and
carboxymethylcellulose daily by gavage at 0, 10, 100, 350 or 700
mg/kg bw per day during days 6-15 of gestation. The day on which
evidence of mating was observed was designated day 0 of gestation.
Treatment with clethodim at 350 mg/kg bw per day and more decreased
maternal body weight. Toxicity was manifested at 700 mg/kg bw per
day as increased mortality (20%), decreased food intake and clinical
signs of poor physical condition, including red nasal discharge,
chromodacryorrhoea and staining of the anogenital region. Excessive
salivation was observed in females treated at 350 and 700 mg/kg bw
per day. Significantly decreased fetal weights and increased
incidences of fetuses with skeletal variations or at least one
ossification variation were recorded at 350 mg/kg bw per day and
more. The skeletal variations were apparent as definite delays in
ossification and were identified by incompletely or unossified
vertebral elements (thoracic, sacral, caudal) and unossified fifth
and/or sixth sternebrae. Treatment at the highest dose, 700 mg/kg bw
per day, induced a significant increase in the number of fetuses
(8/221; 3.6%) and litters (6/18; 33.2%) with external malformations,
comprising primarily an increased incidence (seven fetuses in six
litters) of tail defects characterized by short, filamentous or
absent tails. An imperforated anus was recorded in two fetuses in
two litters at this dose. Visceral malformations (exencephaly,
defects of the lung, aortic arch and large intestine, absence of
kidneys, bladder or ureter) were observed in four fetuses in three
litters of dams exposed to 700 mg/kg bw per day but not in
concurrent or historical controls (5032 fetuses in 806 litters; 38
studies). Three of the four fetuses with visceral malformations also
had external malformations. The NOAEL for maternal and developmental
toxicity was 100 mg/kg bw per day. The NOAEL for teratogenicity was
350 mg/kg bw per day on the basis of an increased incidence of
malformations, particularly tail defects, observed at the maternally
toxic and lethal dose of 700 mg/kg bw per day (Schroeder & Daly,
1987).
Rabbits
In a range-finding study, groups of eight artificially
inseminated female New Zealand white Hra: specific-pathogen-free
rabbits were treated by gavage with clethodim (purity, 83.3%) in
Tween 80 and carboxymethylcellulose at 0, 50, 150, 300 or 500 mg/kg
bw per day on days 7-19 of gestation. The day of insemination was
designated day 0 of gestation. Maternal toxicity was seen at all
doses as decreased body-weight gain and food consumption. An
increased incidence of alopecia was recorded at 150 mg/kg bw per day
and more. Dried faeces and gastrointestinal lesions (hairball and/or
ulceration of the gastric mucosa) were observed at doses of 300
mg/kg bw per day and more. In the groups given 300 and 500 mg/kg bw
per day, two of seven pregnant rabbits died, apparently due to
concomitant weight loss, decreased food intake, gastrointestinal
lesions and/or abortion. In the group given 500 mg/kg bw per day,
four of seven rabbits aborted, and one rabbit delivered prematurely.
Data on litters of dams given 500 mg/kg bw per day could not
effectively be evaluated, as only one female survived to term; dams
given 300 mg/kg bw per day had an increased incidence of resorption
and decreased litter weights when compared with the controls
(Dearlove et al., 1986).
Groups of 20 artificially inseminated female New Zealand white
Hra specific-pathogen-free rabbits were administered
technical-grade clethodim (purity, 83.3%) in Tween 80 and sodium
carboxymethylcellulose by gavage at doses of 0, 25, 100 or 300 mg/kg
bw per day on days 7-19 of gestation. The day of insemination was
designated day 0 of gestation. Mean body weights, corresponding
weight gains and food consumption were decreased in dams given 100
or 300 mg/kg bw per day. An increased incidence of dried faeces was
also noted at 100 mg/kg bw per day and more; the presence of a red
substance on the cage pans of the dams at 300 mg/kg bw per day was
considered to be related to gastrointestinal and/or rectal
irritation. Treatment had no adverse effect on survival, the
abortion rate, pre- or post-implantation loss, the number of live
fetuses, fetal weight or the fetal sex ratio. A slight increase in
the incidence of minor skeletal alterations, comprising angulation
of one or both hyoid alae, irregular ossification of the skull and
nasal passages, was observed at 300 mg/kg bw per day; however, in
the absence of a clear dose-response relationship, no direct
correlation with treatment could be concluded. Clethodim was not
teratogenic at doses up to 300 mg/kg bw per day. The NOAEL for
maternal toxicity was 25 mg/kg bw per day, and that for
developmental toxicity was 300 mg/kg bw per day (Dearlove et al.,
1987).
(f) Genotoxicity
The results of assays for mutagenicity of technical-grade
clethodim are presented in Table 2. The compound did not induce gene
mutation in bacteria in vitro. Clethodim of unknown purity
(Putnam, 1986a) induced chromosomal aberrations in Chinese hamster
ovary cells in the absence of exogenous metabolic activation, but a
purified preparation did not (Putnam, 1986b). Clethodim at doses
sufficiently high to cause acute lethality did not induce
micronuclei in bone-marrow cells of rats treated in vivo, and it
did not induce unscheduled DNA synthesis in hepatocytes of mice
treated in vivo.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Technical-grade clethodim (purity, 83.2%) was applied at a dose
of 0.5 ml under occluded conditions to two intact and two abraded
sites on the shorn backs of each of 12 female New Zealand white
rabbits for 4 h. Slight erythema, observed immediately after dosing,
was followed 24-72 h later by slight-to-severe erythema and
slight-to-moderate oedema. General clearance was seen by 14 days.
The primary irritation score was 0.7. The test material was
considered to be slightly irritating to rabbit skin (Cushman et
al., 1986b).
Undiluted technical-grade clethodim (purity, 83.3%) was
instilled at a dose of 0.1 ml into the conjunctival sac of one eye
of each of nine male New Zealand white rabbits, the other eye
serving as a control. The eyes of three of the nine rabbits were
rinsed after a 30-sec exposure. No corneal opacity or iritis was
observed. In the six rabbits with unrinsed eyes, conjunctival
irritation was moderate to severe 1 h after dosing and slight to
moderate 24 h after dosing; all eyes were clear after 72 h. In the
rinsed eyes, conjunctival irritation was moderate at 1 h and slight
at 24 h; all eyes were clear at 48 h. The test material was
considered to be mildly irritating to the rabbit eye (Cushman et
al., 1986a).
Table 2. Genotoxicity of technical-grade clethodim
End-point Test system Concentration Purity (%) Resultsa Reference
In vitro
Reverse mutation S. typhimurium TA98, 0.1-10 mg/plate 83.3 Negativea Carver et al.,
100, 1535, 1537 (technical) 1986b
E. coli WP2 uvrA
S. typhimurium TA98, 0.1-10 mg/plate 83.3 Negativea Carver et al.,
100, 1535, 1537 (technical) 1986a
Reverse mutation S. typhimurium TA98, 0, 0.1, 0.33, 1.0, 91.1 Negativea Machado et al.,
100, 1535, 1537 3.33, 10 mg/plate (technical) 1990
E. coli WP2 uvrA
Chromosomal Chinese hamster 0.03, 0.1, 0.3, 1.0 Ál/ml NR Negativeb Putnam, 1986a
aberration ovary CHO-K1 0.6, 0.8, 1.0, 1.2 Ál/ml Positivec at
CCI 61 cells 1.0, 1.2 Ál/ml
Chromosomal Chinese hamster 0.03, 0.1, 0.3, 1.0 Ál/ml 96.1 Negativea Putnam, 1986b
aberration ovary CHO-K1 0.6, 0.8, 1.0, 1.2 Ál/ml (purified)
CCI 61 cells
In vivo
Chromosomal Male and female 0, 150, 500, 1500 83.3 Negative Putnam, 1987
aberration Sprague- mg/kg bw, (technical)
Dawley rats single oral dose
Unscheduled Male B6C3F1 0, 100, 1000, 5000 83.3 Negative Mirsalis &
DNA synthesis mice mg/kg bw, gavage (technical) Steinmetz, 1986
NR, not reported
a In the presence and absence of metabolic activation
b In the presence of metabolic activation
c In the absence of metabolic activation
Technical-grade clethodim (purity, 83.4%) did not appear to
sensitize the skin of male Hartley albino guinea-pigs when tested by
the modified Buehler test (Cushman et al., 1986c).
(ii) Liver cytochrome induction
Groups of eight male Crl:CD BR rats were treated by gavage with
technical-grade clethodim (purity, 83.8%) in carboxymethylcellulose
and Tween 80 at 0 or 250 mg/kg bw per day for 21 consecutive days.
Liver samples taken at sacrifice were assayed for cytochrome P450
activity. Treatment did not affect survival, clinical signs or body
weight. The weights of the livers of animals of each sex were
significantly increased (20-23%), and enlarged livers were observed
upon gross examination of two treated animals. Although the mean
cytochrome P450 and hepatic protein contents were significantly
greater in treated animals than controls, the mean concentrations of
hepatic cytochrome P450 per milligram of protein or per gram of
liver were not significantly increased. These results may reflect
increased liver weight and do not provide evidence that clethodim
induces liver cytochrome P450 (Beatty et al., 1989).
3. Studies on metabolites
The imine sulfone and the 5-hydroxy sulfone of clethodim
occurred in larger amounts in plants than in rats (Rose et al.,
1988a). Ancillary studies on these metabolites are summarized in
Table 3.
(a) Acute toxicity
Five female Crl:CD(SD)BR rats were given a single oral dose of
1.4 g/kg bw of the imine sulfone of clethodim. One animal had
decreased motor activity 4 h after dosing and was dead the next day
(Dougherty et al., 1988b).
Groups of five female Crl:CD (SD)BR rats received the 5-hydroxy
sulfone of clethodim or technical-grade clethodim as a single oral
dose of 1.4 g/kg bw. There were no clinical signs of toxicity, and
all animals treated with the 5-hydroxy sulfone survived the 14-day
observation period after dosing. All five rats treated with
clethodim showed overt signs of toxicity, noted as salivation,
decreased motor activity, collapse, hyperactivity, tremors,
diarrhoea, dehydration and nasal, ocular, oral and anogenital
discharge, and all died within three days of dosing (Dougherty et
al., 1988c).
(b) Short-term toxicity
The imine sulfone of clethodim was administered daily in the
diet to groups of 10 male and 10 female Crl:CD (SD)BR rats at 0,
100, 1000 or 8000 ppm, equal to 6.7, 71 or 600 mg/kg bw per day, for
five weeks. Body weight and food consumption were significantly
decreased during the first week of treatment in males given 8000
ppm. Other effects observed only at 8000 ppm were increased serum
cholesterol levels in animals of each sex, increased reticulocyte
counts in males and increased liver weights in males and females in
the absence of related histopathological alterations. Treatment did
not affect survival, clinical signs or ophthalmoscopic parameters.
The NOAEL was 1000 ppm, equal to 71 mg/kg bw per day (Beatty et
al., 1988a).
Groups of 10 male and 10 female Crl:CD (SD)BR rats were fed the
5-hydroxy sulfone of clethodim daily at dietary concentrations of 0,
100, 1000 or 8000 ppm, equal to 5.9, 68 or 590 mg/kg bw per day, for
five weeks. No signs of toxicity were seen at any dose. On the basis
of the absence of any effects on survival, clinical signs, body
weight, food consumption, haematological, blood chemical or
histopathological changes, or organ weights, the NOAEL was greater
than 8000 ppm, equal to 590 mg/kg bw per day (Beatty et al.,
1988b).
Table 3. Effects of clethodim and its imine sulfone and 5-hydroxy sulfone
Study Clethodim Imine sulfone 5-Hydroxy sulfone
Acute toxicity; 3/5 deaths (Cushman et al., 1986d) 1/5 deaths 0/5 deaths
female rat; 5/5 deaths (Dougherty et al., 1988c) (Dougherty et al., 1988b) (Dougherty et al.,
1.4 g/kg bw 1988bc)
Five-week dietary; Decreased body weight and Decreased body weight and No treatment-related
male & female rats food intake, slight anaemia, food intake, increased effects; NOAEL,
increased liver weights, liver weights, cholesterol > 588mg/kg bw per day
liver hypertrophy at 4000 and and reticulocytes at 8000 (Beatty et al., 1988b)
8000 ppm; anaemia, liver ppm. NOAEL, 71 mg/kg bw per
hypertrophy at 1000 ppm day (Beatty et al., 1988a)
NOAEL, 13 mg/kg bw per day
(Cisson & Eisenlord, 1986)
Teratogenicity; rats Maternal and fetal toxicity Maternal toxicity at 100 Clinical signs and
at 350 and 700 mg/kg bw per and 700 mg/kg bw per day; slightly decreased
day; NOAEL, 100 mg/kg bw fetal effects at 700 mg/kg fetal body weights at
per day. Increased incidence bw per day 700 mg/kg bw per day
of tail malformations at NOAEL, 10 mg/kg bw NOAEL, 100 mg/kg
700 mg/kg bw per day per day (Hoberman & per day (Hoberman &
(Schroeder & Daly, 1987) Christian, 1988a) Christian, 1988b)
Microbial gene Negative Negative Negative
mutation (Carver et al., 1986a,b) (Carver et al., 1988) (Carver eta[., 1987)
Chromosomal Positive in absence of Negative Negative
aberration, metabolic activation (Putnam, 1988a) (Putnam, 1988b)
CHO cells (Putnam, 1986a)
(c) Embryotoxicity and teratogenicity
Groups of 10 pregnant female Crl:CD (SD)BR rats were treated by
gavage with the imine sulfone of clethodim in Tween 80 and
carboxymethylcellulose at doses of 0, 10, 100 or 700 mg/kg bw per
day during days 6-15 of gestation. Maternal toxicity was seen at the
high dose of 700 mg/kg bw per day, manifested as excessive
salivation and decreased body weight and food consumption.
Significant decreases in maternal body weight were also recorded at
100 mg/kg bw per day. At 700 mg/kg bw per day, fetal weights were
decreased, and there were increased incidences of fetuses and
litters with cervical ribs and delayed sternal ossification. The
NOAEL for maternal toxicity was 10 mg/kg bw per day on the basis of
reduced body weights at 100 mg/kg bw per day and more. An increased
incidence of fetal variations at the maternally toxic dose of 700
mg/kg bw per day resulted in an NOAEL of 100 mg/kg bw per day for
developmental toxicity (Hoberman & Christian, 1988a).
Groups of 10 pregnant female Crl:CD (SD)BR rats were
administered the 5-hydroxy sulfone of clethodim in
carboxymethylcellulose and Tween 80 by gavage at 0, 10, 100 or 700
mg/kg bw per day on days 6-15 of gestation. At 700 mg/kg bw per day,
one female showed excessive salivation and two females had rales; a
slight decrease in fetal body weights in this group, although not
statistically significant, was possibly related to treatment. No
teratogenicity was seen at any dose. The NOAEL for maternal and
fetal toxicity was 100 mg/kg bw per day (Hoberman & Christian,
1988b).
(d) Genotoxicity
Neither the imine sulfone nor the 5-hydroxy sulfone of
clethodim induced gene mutation in Salmonella typhimurium TA98,
TA100, TA1535 or TA1537 or in Escherichia coli WP2 uvrA in the
presence or absence of metabolic activation. Neither metabolite
induced chromosomal aberrations in Chinese hamster ovary cells.
4. Observations in humans
No information was available.
Comments
Clethodim was readily absorbed by rats after oral
administration. Excretion was rapid and occurred predominantly via
the urine, with lesser amounts eliminated in the faeces and as
carbon dioxide. There were no significant dose-related or
sex-specific differences in elimination pattern or tissue
distribution, and there was no evidence of bioaccumulation.
The dominant metabolic pathway proposed for clethodim in rats
is oxidation to clethodim sulfoxide. Other postulated pathways are
cleavage of the N-O bond to form the imine, conversion of the
S-ethyl group to S-methyl, formation of the oxazole, and
hydroxylation at the 5 position. The urinary metabolites identified
were the sulfoxides and sulfones of clethodim and 5-hydroxy
clethodim, the sulfoxides of the imine and S-methyl clethodim and
the sulfone of the oxazole.
Clethodim had slight to moderate acute oral toxicity in the rat
and mouse. WHO has not classified clethodim with regard to toxic
hazard.
The primary effect of treatment with clethodim after short- and
long-term dietary exposure in the mouse, rat and dog was on the
liver. Hepatic effects were manifest as increased liver weights and
centrilobular hypertrophy. A study in rats administered clethodim at
250 mg/kg bw per day did not provide evidence for liver cytochrome
P450 induction.
In a four-week study in mice given dietary concentrations of 0,
100, 250, 625, 1500 or 4000 ppm, the NOAEL was 250 ppm, equivalent
to 38 mg/kg bw per day on the basis of evidence of slight anaemia at
625 ppm and above. In a 78-week study of toxicity and
carcinogenicity in which mice received dietary levels of 0, 20, 200,
1000 or 2000-3000 ppm, the NOAEL was 200 ppm, equivalent to 30 mg/kg
bw per day, on the basis of hepatic effects and an increased
incidence of alveolar lung macrophages at 1000 ppm and above.
In a five-week study in which rats received dietary levels of
0, 5, 200, 1000, 4000 or 8000 ppm, the NOAEL was 200 ppm, equal to
13 mg/kg bw per day, on the basis of liver hypertrophy and evidence
of slight anaemia at 1000 ppm and above. A 13-week study at dietary
concentrations of 0, 50, 500, 2500 or 5000 ppm and a two-year
feeding study at dietary levels of 0, 5, 20, 500 or 2500 ppm in rats
revealed an NOAEL of 500 ppm, equal to 25 mg/kg bw per day in the
first study and equal to 16 mg/kg bw per day in the second. The
NOAEL was based on decreased body weights and liver hypertrophy at
dietary levels of 2500 ppm and above.
In dogs, 13-week oral administration of capsules of clethodim
at doses of 0, 1, 25, 75 or 125 mg/kg bw per day resulted in an
NOAEL of 25 mg/kg bw per day, on the basis of increased liver weight
and cholesterol levels at 75 mg/kg bw per day and above. In a
one-year study at doses of 0, 1, 75 or 200-300 mg/kg bw per day, the
NOAEL was 1 mg/kg bw per day on the basis of treatment-related
effects on the liver at 75 mg/kg bw per day and above.
Clethodim was not carcinogenic when fed to mice or rats at
dietary levels of up to 2500 ppm.
A two-generation study of reproductive toxicity in rats treated
with clethodim at dietary levels of 0, 5, 20, 500 or 2500 ppm did
not reveal any adverse effects on reproduction. The NOAEL was 500
ppm, equal to 39 mg/kg bw per day, on the basis of decreased
parental body weights and food consumption at 2500 ppm.
Studies of teratogenicity were conducted with clethodim in rats
at doses of 0, 10, 100, 350 or 700 mg/kg bw per day and in rabbits
at 0, 25, 100 or 300 mg/kg bw per day. In rats, the NOAEL for
maternal toxicity was 100 mg/kg bw per day. Fetal toxicity was
observed at the maternally toxic doses of 350 and 700 mg/kg bw per
day. An increased incidence of malformations, specifically tail
defects (short, filamentous or absent tails), was observed at the
maternally toxic and lethal high dose of 700 mg/kg bw per day. In
rabbits, maternal toxicity was observed at 100 and 300 mg/kg bw per
day, resulting in an NOAEL of 25 mg/kg bw per day. There were no
adverse effects on the rabbit fetus and no evidence of teratogenic
potential at doses up to 300 mg/kg bw per day.
Clethodim was adequately tested for genotoxicity in a series of
assays in vivo and in vitro. The Meeting concluded that
clethodim was not genotoxic.
Although the imine sulfone and 5-hydroxy sulfone metabolites of
clethodim have been reported to occur in higher amounts in plants
than in animals, neither of the metabolites was more toxic than the
parent, clethodim, in studies of acute or short-term toxicity and
teratogenicity. There was similarly no evidence of genotoxic
potential.
An ADI was established on the basis of the NOAEL of 1 mg/kg bw
per day from the one-year study in dogs and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 200 ppm, equivalent to 30 mg/kg bw per day (78-week
study of toxicity and carcinogenicity)
Rat: 500 ppm, equal to 16 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 25 mg/kg bw per day (study of teratogenicity)
Dog: 1 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Observations in humans
References
Beatty, P.W., Bagos, A.D., Richter, W.R. & Wong, Z.A. (1988a)
Five-week oral toxicity study in rats with RE-47719 (SX-1800).
Project No. 2949. Unpublished report dated 15 November 1988 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Beatty, P.W., Bagos, A.C., Richter, W.R. & Wong, Z.A. (1988b)
Five-week oral toxicity study in rats with RE-51228 (SX-1803).
Project No. 2950. Unpublished report dated 15 November 1988 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Beatty, P.W., Brorby, G.P. & Wong, Z.A. (1989) The potential of
RE-45601 Technical (SX-1688) to induce cytochrome P-450 following
21-day oral administration in male rats. Project No. 2784.
Unpublished report dated 16 November 1989 from Chevron Environmental
Health Center Inc., Richmond, CA, USA. Submitted to WHO by Tomen
Pacific Agro Co., San Francisco, CA, USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1986a)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-45601 Technical (83% purity, SC-1688). Project No. SOCAL
2505. Unpublished report dated 13 January 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1986b)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-45601 Technical (83% purity, SC-1688). Project No. CEHC
2555. Unpublished report dated 28 April 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1987)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-51228. Project No. 2856. Unpublished report dated 7 December
1987 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1988)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-47719. Project No. 2948. Unpublished report dated 19 October
1988 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Cisson, C.M. & Eisenlord, G.H. (1986) Five-week pilot feeding study
in rats with RE-45601 Technical (SX-1653). Project No. SOCAL 2457.
Unpublished report dated 7 October 1986 from Chevron Environmental
Health Center Inc., Richmond, CA, USA. Submitted to WHO by Tomen
Pacific Agro Co., San Francisco, CA, USA.
Cox, R.H. (1988) Acute intraperitoneal toxicity study in rats with
Chevron RE-45601 Technical (SX-1688). Project No. 2107-151.
Unpublished revised report dated 6 April 1988 from Hazelton
Laboratories America Inc., Vienna, VA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Cox, R.H. & Slabik, C.M. (1986) Four-week subchronic oral toxicity
study in mice with Chevron RE-45601 Technical. Project No. 2107-140.
Unpublished report dated 21 July 1986 from Hazelton Laboratories
America Inc., Vienna, VA, USA. Submitted to WHO by Tomen Pacific
Agro Co., San Francisco, CA, USA.
Cox, R.H. & Zoetis, T. (1986) Acute oral toxicity study in mice with
Chevron RE-45601 Technical. Project No. 2107-143. Unpublished report
dated 16 September 1986 from Hazelton Laboratories America Inc.,
Vienna, VA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Cox, R.H. & Zoetis, T. (1988b) One-year oral toxicity study in dogs
with Chevron RE-45601 Technical (SX-1688). Project No. 2107-153.
Unpublished report dated 28 October 1988 from Hazelton Laboratories
America Inc., Vienna, VA, USA. Submitted to WHO by Tomen Pacific
Agro Co., San Francisco, CA, USA.
Cox, R.H. & Zoetis, T. (1988b) Chronic oral oncogenicity study in
mice with Chevron RE-45601 Technical (SX-1688). Project No.
2107-145. Unpublished report dated 25 October 1988 from Hazelton
Laboratories America Inc., Vienna, VA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Thompson, J.D. & Wong, Z.A. (1986a) The acute eye
irritation potential of RE-45601 Technical (SX-1688). Project No.
CEHC 2511. Unpublished report dated 1 October 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Korenaga, G.L. & Wong, Z.A. (1986b) The four-hour
skin irritation potential of RE-45601 Technical (SX-1688). Project
No. CEHC 2512. Unpublished report dated 18 December 1986 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Cooper, P.N. & Wong, Z.A. (1986c) Modified Buehler
Test for the skin sensitization potential of RE-45601 Technical
(SX-1688). Project No. CEHC 2514. Unpublished report dated 15
December 1986 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Cushman, J.R., Easter, M.D. & Wong, Z.A. (1986d) The acute oral
toxicity of RE-45601 Technical (SX-1688) in adult male and female
rats. Project No. SOCAL 2498. Unpublished report dated 27 June 1986
from Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Hedgecock, J.H. & Wong, Z.A. (1987a) Four-week
repeated dose dermal toxicity study in rats with RE-45601 Technical
(SX-1688). Project No. CEHC 2552. Unpublished report dated 20
November 1987 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Cushman, J.R., Korenaga, G.L. & Wong, Z.A. (1987b) The acute dermal
toxicity of RE-45601 Technical (SX-1688) in adult male and female
rabbits. Project No. CEHC 2510. Unpublished report dated 7 January
1987 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Daly, I.W. & Knezevich, A.L. (1987) Ninety-day subchronic oral
toxicity study in dogs with Chevron RE-45601 Technical. Project No.
85-2999. Unpublished report dated 26 February 1987 from Bio/dynamics
Inc., East Millstone, NJ, USA. Submitted to WHO by Tomen Pacific
Agro Co., San Francisco, CA, USA.
Dearlove, G.E., Hoberman, A.M. & Christian, M.S. (1986) Pilot
teratology study in rabbits with Chevron RE-45601 Technical. Project
No. 303-007P. Unpublished report dated 15 July 1986 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Dearlove, G.E., Hoberman, A.M. & Christian, M.S. (1987) Teratology
study in rabbits with Chevron RE-45601 Technical. Project No.
303-007. Unpublished report dated 30 January 1987 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Dougherty, K.K., Eisenlord, G.H., Low, S.S. & Wong, Z.A. (1987)
13-Week oral toxicity study in rats with RE-45601 Technical
(SX-1688). Project No. SOCAL 2501. Unpublished report dated 13
February 1987 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Eisenlord, G.H. & Wong, Z.A. (1988a) Combined
chronic toxicity/oncogenicity study in rats with RE-45601 Technical
(SX-1688). Project No. SOCAL 2500. Unpublished report dated 1
November 1988 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Duncan, R.A. & Wong, Z.A. (1988b) The comparative
acute oral toxicity of RE-47719 (SX-1800) and RE-45601 (SX-1688) in
adult female rats. Project No. 2952. Unpublished report dated 21
October 1988 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Duncan, R.A. & Wong, Z.A. (1988c) The comparative
acute oral toxicity of RE-51228 (SX-1796) and RE-45601 (SX-1688) in
adult female rats. Project No. 2951. Unpublished report dated 21
October 1988 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Waid, L.J. & Wong, Z.A. (1989) The acute oral
toxicity of RE-45601 Technical (SX-1688) in adult male and female
rabbits. Project No. CEHC 2992. Unpublished report dated 21 June
1989 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Griffis, L.C., Bruce, E.D. & Wong, Z.A. (1986) The acute inhalation
toxicity of RE-45601 Technical (SX-1688) in rats. Project No. CEHC
2513. Unpublished report dated 7 November 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Hoberman, A.M. & Christian, M.S. (1988a) Oral teratogenicity and
developmental toxicity screen in rats with RE-47719. Project No.
303-012. Unpublished report dated 8 November 1988 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Hoberman, A.M. & Christian, M.S. (1988b) Oral teratogenicity and
developmental toxicity screen in rats with RE-51228. Project No.
303-010. Unpublished report dated 8 November 1988 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Lee, S.G., Maier, K.M., Tamichi, E.H. & Tucker, B.V. (1988)
[Ring-4,6-14C] clethodim: A radiocarbon metabolism study in laying
hens. Project No. MEF-0089. Unpublished report dated 30 December
1988 from Chevron Chemical Company, Ortho Agricultural Chemicals
Division, Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro
Co., San Francisco, CA, USA.
Machado, M.L., Lubeck, S.G. & Wilkenfeld, R.M. (1990)
Microbial/microsome reverse mutation plate incorporation assay with
RE-45601 Technical (SX-1845). Project No. CEHC 3146. Unpublished
report dated 20 August 1990 from Chevron Environmental Health Center
Inc., Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co.,
San Francisco, CA, USA.
McKensie, K.M. (1986) Pilot rat reproduction study with Chevron
RE-45601 Technical. Project No. 6183-102. Unpublished report dated
16 December 1986 from Hazelton Laboratories America Inc., Madison,
WI, USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco,
CA, USA.
Mirsalis, J.C. & Steinmetz, K.L. (1986) In vivo--in vitro
hepatocyte DNA repair assay: in vitro evaluation of unscheduled
DNA synthesis (UDS) following oral administration of Chevron
RE-45601 Technical to B6C3F1 Mice. Project No. LSC-1960.
Unpublished report dated August 1986 from SRI International, Meno
Park, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Putnam, D.L. (1986a) Chromosome aberrations in Chinese hamster ovary
(CHO) cells with Chevron RE-45601 Technical. Project No. T4529.337.
Unpublished report dated August 1986 from Microbiological Associates
Inc., Bethesda, MD, USA. Submitted to WHO by Tomen Pacific Agro Co.,
San Francisco, CA, USA.
Putnam, D.L. (1986b) Chromosome aberrations in Chinese hamster ovary
(CHO) cells with purified Chevron RE-45601. Project No. T429.337.
Unpublished report dated 15 December 1986 from Microbiological
Associates Inc., Bethesda, MD, USA. Submitted to WHO by Tomen
Pacific Agro Co., San Francisco, CA, USA.
Putnam, D.L. (1987) Cytogenetics assay in bone marrow cells of rats
following acute oral exposure to RE-45601 Technical. Project No.
T5172.105. Unpublished report dated 25 February 1987 from
Microbiological Associates Inc., Bethesda, MD, USA. Submitted to WHO
by Tomen Pacific Agro Co., San Francisco, CA, USA.
Putnam, D.L. (1988a) Chromosome aberrations in Chinese hamster ovary
(CHO) cells: RE-47719. Project No. T8226.337003. Unpublished report
dated 10 November 1988 from Microbiological Associates Inc.,
Bethesda, MD, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Putnam, D.L. (1988b) Chromosome aberrations in Chinese hamster ovary
(CHO) cells: RE-51228. Project No. T8227.337003. Unpublished report
dated 10 November 1988 from Microbiological Associates Inc.,
Bethesda, MD, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Rendina, A.R. & Felts, J.M. (1988) Cyclohexanedione herbicides are
selective and potent inhibitors of acetyl-CoA carboxylase from
grasses. Plant Physiol., 86, 983-986.
Rose, A.F., Griffis, L.C., Suzuki, J.P., Bruce, E.D., Primer, R.L.,
Wong, Z.A. & Tucker, B.V. (1988a) The in vivo metabolism of
[propyl-1-14C] clethodim in rats. Project No. MEF-0086/CEHC 2515.
Unpublished report dated 22 December 1988 from Chevron Chemical Co.,
Ortho Agricultural Chemicals Division, Richmond, CA, USA. Submitted
to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Rose, A.F., Suzuki, J.P., Primer, R.L. & Tucker, B.V. (1988b) The
in vivo metabolism of [propyl-1-14C] clethodim in a lactating
goat. Project No. MEF-0038. Unpublished report dated 29 December
1988 from Chevron Chemical Co., Ortho Agricultural Chemicals
Division, Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro
Co., San Francisco, CA, USA.
Schroeder, R.E. & Daly, I.W. (1986) A pilot teratology study in rats
with Chevron RE-45601 Technical. Project No. 85-3032. Unpublished
report dated 8 September 1986 from Bio/dynamics Inc., East
Millstone, NJ, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Schroeder, R.E. & Daly, I.W. (1987) Teratology study in rats with
Chevron RE-45601 Technical. Project No. 86-3042. Unpublished report
dated 20 July 1987 from Bio/dynamics Inc., East Millstone, NJ, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Tellone, C.I., Hardy, L.M. & Richter, W.R. (1987) Two-generation
(one litter) reproduction study in rats with RE-45601 Technical.
Project No. CEHC 2596. Unpublished report dated 27 August 1987 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Two groups of eight white Leghorn laying hens were given daily
doses of [cyclohexene-4,6-14C]-clethodim at 2.1 or 51.3 mg/kg bw
per day by capsule for five consecutive days. The hens were
sacrificed about 4 h after the final dose, and tissues were
collected for analysis. Two major metabolites, clethodim sulfone and
clethodim sulfoxide, were identified in tissues and eggs. Clethodim
sulfone accounted for up to 57% of tissue levels of radiolabel,
whereas clethodim sulfoxide accounted for 10-31%. Clethodim
sulfoxide was the principal metabolite in egg white (26-82%) and
yolk (25-37%); parent clethodim was detected in significantly
smaller amounts in both tissues and eggs. The proposed metabolic
pathway in chickens was different from and considered to be simpler
than that observed in rats and goats, since none of the imine,
5-hydroxy or S-methyl analogues was detected in chickens (Lee et
al., 1988).
In the study described above in which a lactating goat received
[propyl-1-14C]-clethodim (Rose et al., 1988b), hind- and
forequarter muscle, peritoneal and subcutaneous fat, liver, kidneys,
heart and blood were collected to allow characterization of
metabolites. The major urinary metabolite was clethodim sulfoxide,
which accounted for 67% of the urinary radiocarbon. Other urinary
radiolabelled components were identified as clethodim (3-27%) and
the demethyl sulfoxide (12-18%), S-methyl (7-13%), imine sulfoxide
(1.5-2.8%), sulfone (1.5-2.2%) and 5-hydroxy sulfoxide (0-3%). In
the milk, about half of the radiocarbon could be extracted into
organic solvents and occurred in clethodim, clethodim sulfoxide and
clethodim demethyl sulfoxide; the other half of the radiocarbon was
water soluble and was shown to be 14C-lactose. In blood and
tissues, the maximal extractable radiocarbon residues accounted for
77-95% of the radiolabel and were identified as clethodim and the
sulfoxide, demethyl sulfoxide, imine sulfoxide, sulfone and
5-hydroxy sulfone. The proposed metabolic pathway in goats was
essentially the same as that proposed for rats (Rose et al.,
1988b).
2. Toxicological studies
(a) Acute toxicity
Technical-grade clethodim has been tested for acute toxicity in
mice, rats and rabbits. The results are summarized in Table 1.
Clethodim was virtually non-toxic after oral or dermal
administration to rabbits or after inhalation by rats. Slight to
moderate toxicity was observed in mice and rats treated orally, with
LD50 levels of 1360-2570 mg/kg bw. Clethodim administered
intraperitoneally was moderately toxic to rats, with a combined
LD50 for the two sexes of 1117 mg/kg bw.
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female Charles River CD-1 mice were
fed diets containing technical-grade clethodim (purity, 83.3%) for
four weeks at 100, 250, 625, 1500 or 4000 ppm, equivalent to 0, 15,
38, 94, 225 or 600 mg/kg bw per day. A slight anaemic process was
manifested in male mice as a decreased erythrocyte count at >
1500 ppm, decreased haemoglobin at > 625 ppm and lowered
haematocrit at 4000 ppm. Increased liver weights, which were
correlated microscopically with centrilobular hypertrophy, were
observed in males fed 1500 ppm and in animals of each sex treated at
the highest dose of 4000 ppm. An increased incidence of focal
coagulative necrosis of the liver was recorded in males treated at
> 1500 ppm. Treatment appeared to have no effect on survival,
clinical signs, body weight or food consumption. The NOAEL was 250
ppm, equivalent to 38 mg/kg bw per day, on the basis of the
dose-related decrease in erythroid parameters at dietary levels >
625 ppm (Cox & Slabik, 1986).
Rats
Technical-grade clethodim (purity, 83.2%) was applied in
carboxymethylcellulose and Tween 80 to the skin of groups of six
male and six female Crl:CD BR rats at doses corresponding to 0, 10,
100 or 1000 mg/kg bw per day for 6 h/day, five days per week for
four weeks, for a total of 21 exposures. A dose-related increase in
the occurrence of skin irritation was observed at all dose levels.
Clinical signs seen at 1000 mg/kg bw per day were described as
anogenital discharge and staining of the fur, alopecia and red nasal
discharge. At the highest dose, group mean body weights,
corresponding weight gains and feed efficiency values were decreased
in males, and increased liver weights were recorded in females. In
males, a slight increase was observed in kidney regeneration, which
was described as a minimal multifocal lesion. Although this finding
was observed only in treated rats, evidence of chronic progressive
nephropathy is not uncommon in this strain of rat and was not
considered to represent a treatment-related effect. The NOAEL for
systemic toxicity under the conditions of this study was thus 100
mg/kg bw per day (Cushman et al., 1987a).
Table 1. Acute toxicity of technical-grade clethodim in male and female rodents
Species, Route and vehicle LD50 Purity Reference
strain (mg/kg bw) (%)
Mouse, CD-1 Oral; M: 2570 83.3 Cox & Zoetis, 1986
Tween 80 + CMC F: 2430 (technical)
Rat, Crl:CD Oral; M: 1630 83.3 Cushman et al., 1986d
(SD)BR Tween 80 + CMC (technical)
Rat, Crl:CD Intraperitoneal; M: 1040 NR Cox, 1988
(SD)BR Tween 80 + CMC F: 1200
Rat, Inhalation M&F: 83.3 Griffis et al., 1986
Sprague-Dawley > 3.9 mg/l (technical)
Rabbit, New Oral; M: > 2000 and 82.5 Dougherty et al., 1989
Zealand Tween 80 + CMC < 5000 (technical)
white F: > 5000
Rabbit, New Dermal M&F: > 5000 83.3 Cushman et al., 1987b
Zealand (technical)
white
NR, not reported; Tween 80, 0.5% polyoxyethylenesorbitan monooleate; CMC,
carboxymethylcellulose
In a preliminary five-week study, groups of 10 male and 10
female Crl:CD(SD)BR rats were treated with technical-grade clethodim
(purity, 83.4%) at dietary levels of 0, 5, 200, 1000, 4000 or 8000
ppm, equal to 0.3, 13, 66, 261 or 515 mg/kg bw per day for males and
0.3, 14, 71, 291 or 554 mg/kg bw per day for females.
Treatment-related effects were decreased body-weight gain and food
consumption (particularly during the first week of the study) in
animals of each sex at 4000 and 8000 ppm; increased liver weights at
> 4000 ppm; and slight to mild centrilobular hypertrophy in males
at 1000 ppm and in animals of each sex at 4000 and 8000 ppm.
Dose-related, statistically significant decreases in haemoglobin
concentration were noted in males at > 1000 ppm and in
haematocrit in males at > 4000 ppm. Raised cholesterol
concentrations were recorded in males receiving 8000 ppm, and raised
serum uric acid levels were seen in females given > 4000 ppm.
Treatment had no effect on survival, clinical signs or urinary
parameters. The NOAEL in this study was 200 ppm, equal to 13 mg/kg
bw per day, on the basis of decreases in erythroid parameters and
centrilobular hypertrophy of the liver at 1000 ppm and more (Cisson
& Eisenlord, 1986).
Technical-grade clethodim (purity, 84%) was administered to
groups of 12 male and 12 female Crl:CD(SD)BR rats for 13 weeks at
dietary levels of 0, 50, 500, 2500 or 5000 ppm, equal to 2, 25, 134
or 280 mg/kg bw per day for males and 3, 30, 160 or 340 mg/kg bw per
day for females. In order to study the reversibility of potential
treatment-related effects, an additional 12 rats of each sex were
assigned to the groups receiving 0, 2500 and 5000 ppm and were kept
for a six-week recovery period after the end of treatment with
clethodim. Significantly decreased body weights and weight gains
were recorded in males treated with 2500 ppm and in animals of each
sex treated with 5000 ppm. Decreased mean food consumption was noted
in both males and females at 5000 ppm. Significantly ( p < 0.05)
increased concentrations of cholesterol, total protein and globulin
recorded in males fed 5000 ppm were within the normal control range
of variability. Increased liver weights and increased incidence and
severity of centrilobular hypertrophy of the liver were noted in
males and females fed 2500 and 5000 ppm. A slightly increased
incidence of renal focal regeneration was seen in males treated at
> 2500 ppm. The pathologist did not consider this finding to be
related to treatment; rather, these lesions were regarded as typical
of the earliest minimal stage of spontaneous chronic progressive
nephropathy. Treatment did not affect survival, clinical signs,
ophthalmoscopic or haematological parameters or the results of
urinalysis. No treatment-related effects were seen after the
six-week recovery period. The NOAEL was 500 ppm, equal to 25 mg/kg
bw per day, as determined by an increased incidence of hepatic
centrilobular hypertrophy and decreased body-weight gain in animals
of each sex treated at 2500 ppm and above (Dougherty et al.,
1987).
Dogs
Groups of four male and four female beagle dogs received
gelatin capsules containing technical-grade clethodim (purity,
83.3%) orally at 0, 1, 25, 75 or 125 mg/kg bw per day for 13 weeks.
Treatment affected blood chemistry in females, which had increased
cholesterol levels at 75 mg/kg bw per day and above and increased
alkaline phosphatase activity at 125 mg/kg bw per day. An increased
globulin concentration, with a concomitant decrease in the
albumin:globulin ratio, was noted in males treated at 125 mg/kg bw
per day. Significantly ( p < 0.05) increased total leukocyte
counts were seen in males and decreased activated partial
thromboplastin time was seen in females after one month at 75 and
125 mg/kg bw per day. As no similar differences from control values
were seen, however, after two or three months of treatment, and the
only differences seen were in one sex, the significance of these
findings is doubtful. Increased liver weights were recorded in males
and females fed 75 or 125 mg/kg bw per day. Slight increases in the
severity of cytoplasmic vesiculation and vacuolation of the
centrilobular hepatocytes were seen in animals of each sex treated
at 125 mg/kg bw per day. Treatment did not affect survival, clinical
signs, ophthalmoscopic parameters, body weight or food consumption.
The NOAEL was 25 mg/kg bw per day on the basis of elevated
cholesterol levels and increased liver weights at 75 mg/kg bw per
day and above (Daly & Knezevich, 1987).
In a one-year study, groups of six male and six female beagle
dogs were administered technical-grade clethodim (purity, 83.3%) in
gelatin capsules at doses of 0, 1, 75 or 200-300 mg/kg bw per day.
In the absence of any signs of overt toxicity, the high dose of 200
mg/kg bw per day was increased after seven weeks of treatment to 300
mg/kg bw per day. Significant effects on haematological parameters
were seen, expressed as increased platelet and leukocyte counts in
females at 75 mg/kg bw per day and in males and females at 300 mg/kg
bw per day. At 300 mg/kg bw per day, erythroid parameters
(erythrocyte, haemoglobin and haematocrit values) were decreased in
animals of each sex, and reticulocyte and segmented neutrophil
counts were increased in females. Treatment-related effects on blood
chemistry were seen as increased levels of cholesterol,
triglycerides, alkaline phosphatase and alanine aminotransferase in
males and females at 300 mg/kg bw per day. Decreased serum glucose
concentrations were noted in both males and females at the high dose
and in females treated at 75 mg/kg bw per day. Increased liver
weights were recorded in animals of each sex at 75 and 300 mg/kg bw
per day. Significantly ( p < 0.05) increased thyroid weights seen
in males at 300 mg/kg bw per day were not correlated with any
microscopic changes. Histopathological alterations in animals of
each sex treated at 300 mg/kg bw per day included hepatocellular
enlargement, increased granular pigment (which was not associated
with bile, iron or acid-fast lipofuscin) and slight hypercellularity
of the bone marrow; the last finding was also observed in one male
and one female at 75 mg/kg bw per day. The significance of a
slightly increased incidence of pigment (not otherwise
characterized) in the spleen of females receiving 75 or 300 mg/kg bw
per day was uncertain. Treatment did not affect survival, clinical
signs, ophthalmoscopic parameters, body weight, food consumption or
the results of urinalysis. The NOAEL was 1 mg/kg bw per day on the
basis of treatment-related effects on haematological and blood
chemical parameters, increased liver weight and hypercellularity of
the bone marrow at 75 mg/kg bw per day and above (Cox & Zoetis,
1988a).
(c) Long-term toxicity and carcinogenicity
Mice
Technical-grade clethodim (purity, 83.3%) was administered in
the diet to groups of 60 male and 60 female Crl:CD-1 (ICR)BR mice
for 78 weeks at 0, 20, 200, 1000 or 2000-3000 ppm, equivalent to 3,
30, 150 or 300-450 mg/kg bw per day. The high dose was increased to
3000 ppm after 15 weeks of treatment, when haematological assessment
did not show the significant decrease in erythrocyte parameters seen
in the four-week preliminary study. Ten mice of each sex were
sacrificed after 52 weeks of treatment in each group for interim
assessment. A significant ( p < 0.05) trend to decreased survival
was noted among males and females fed 3000 ppm, with survival at 78
weeks of 58, 66, 60, 52 and 32% males and 67, 84, 80, 59 and 48%
females, respectively. The predominant cause of death at this dose
was increased incidence and severity of systemic amyloidosis.
Decreased erythrocyte counts were recorded in animals of each sex at
3000 ppm, with concomitant decreases in haemoglobin and haematocrit
values in males. After 52 weeks of treatment, increased liver
weights and centrilobular hypertrophy of the liver were observed in
males at 1000 ppm and in males and females at 3000 ppm. Increased
pigment, described as morphologically compatible with haemosiderin
and bile, was noted in males at 3000 ppm. After 78 weeks of
treatment, the hepatic changes included increased liver weights in
females at 3000 ppm, centrilobular hypertrophy and increased pigment
in both males and females at 1000 and 3000 ppm and bile-duct
hyperplasia in males at > 1000 ppm. An increased incidence of
multifocal, amphophilic alveolar lung macrophages was also observed
in animals of each sex treated at > 1000 ppm. No significant
effect was seen on body weight, food consumption or clinical signs.
The NOAEL was 200 ppm, equivalent to 30 mg/kg bw per day, on the
basis of hepatic changes, notably centrilobular hypertrophy,
increased pigment and bile-duct hyperplasia, and an increased
incidence of alveolar macrophages in the lungs of mice treated at
1000 ppm and above. There was no evidence that clethodim has
carcinogenic potential (Cox & Zoetis, 1988b).
Rats
Groups of 65 male and 65 female Crl:CD (SD)BR rats were
administered technical-grade clethodim (purity, 83%) in the diet at
levels of 0, 5, 20, 500 or 2500 ppm, equal to 0.2, 0.6, 16 or 86
mg/kg bw per day for males and 0.2, 0.7, 21 or 110 mg/kg bw per day
for females, for two years. Ten rats of each sex in each group were
sacrificed after one year. After two years, 47, 49, 46, 50 and 33%
of males and 55, 58, 51, 40 and 49% of females were still alive in
the five dose groups, respectively. Treatment did not affect cause
or time of death, clinical signs, ophthalmoscopic or haematological
parameters or the results of clinical chemistry or urinalysis.
Body-weight gain and food consumption were significantly decreased
in animals of each sex during the first year of treatment with 2500
ppm. Food intake, calculated relative to body weight, was slightly
greater in males and females at 2500 ppm than in the other treated
groups. At interim sacrifice after one year, increased liver weights
and slight to mild centrilobular hypertrophy were noted in rats of
each sex fed 2500 ppm. Increased liver weights seen in females at
500 ppm were not correlated with any microscopically discernible
change. At terminal sacrifice, liver weights of females at 2500 ppm
were increased; males had no significant increase in liver weight,
and no treatment-related centrilobular hypertrophy was seen in
animals of either sex. Females treated at 2500 ppm had a slightly
greater (12%) incidence of binucleated cells in the liver than the
controls (2%), but the effect was of uncertain toxicological
significance. Clethodim at dietary levels up to 3000 ppm showed no
evidence of carcinogenic potential. The NOAEL was 500 ppm, equal to
16 mg/kg bw per day, on the basis of decreased body-weight gain,
decreased food intake, increased liver weight and associated
centrilobular hypertrophy at 2500 ppm (Dougherty et al., 1988a).
(d) Reproductive toxicity
Rats
In a preliminary study, groups of eight male and eight female
Crl:CD(SD)BR rats were fed technical-grade clethodim (purity, 83.3%)
at dietary levels of 0, 500, 2000 or 5000 ppm, equivalent to 25, 100
or 250 mg/kg bw per day, for one week before and during mating.
Males were sacrificed after mating, whereas females were treated
throughout gestation and up to day 7 of the lactation period for a
single litter. Parental animals of each sex that received 5000 ppm
had decreased body-weight gain and food intake ( p < 0.05, only in
males). Mean pup weights and corresponding weight gains were
decreased at all dietary levels. No adverse effects on reproductive
parameters were evident at the highest dose (McKensie, 1986).
In a two-generation (one litter per generation) study, groups
of 30 male and 30 female Crl:COBS CD(SD) rats were treated with
technical-grade clethodim (purity, 83%) at dietary levels of 0, 5,
20, 500 or 2500 ppm, equal to 0.1, 1.4, 39 or 190 mg/kg bw per day
for males and 0.2, 1.8, 45 or 220 mg/kg bw per day for females. Mean
body weight was decreased at 2500 ppm in both males and females of
the F1 generation and in males of the F0 generation. Occasional
significant ( p < 0.05) decreases in food consumption were
recorded in F1 males and females treated with 2500 ppm. Treatment
had no effect on parental survival, clinical signs, organ weights or
histopathological manifestations; there were no adverse effects on
reproductive indices (mating, fertility, pregnancy and gestation);
and there was no effect on litter size, sex ratio, pup weight or pup
viability. A significant ( p < 0.05) increase in the number of
stillborn pups was observed in the litters of F1 dams treated at
2500 ppm; however, this finding was not considered to be related to
treatment, since the value was within the historical control range,
and no similar increase was seen in the subsequent F2 litter. The
NOAEL for parental systemic toxicity was 500 ppm, equal to 39 mg/kg
bw per day, on the basis of decreased body weights and food
consumption at 2500 ppm. The NOAEL for adverse effects on
reproduction in the F0 and F1 generations was 2500 ppm, the
highest dietary level tested, equal to 190 mg/kg bw per day (Tellone
et al., 1987).
(e) Embryotoxicity and teratogenicity
Rats
In a preliminary range-finding study, groups of 10 mated female
Crl:CD(SD) rats were given technical-grade clethodim (purity, 82.6%)
in Tween 80 and carboxymethylcellulose by gavage at 0, 50, 150, 300
or 500 mg/kg bw per day during days 6-15 of gestation. The day on
which evidence of mating was observed was designated day 0 of
gestation. Clinical signs of toxicity were an increased incidence of
excessive salivation in females at 300 and 500 mg/kg bw per day, and
slightly decreased body-weight gain, decreased mean number of
uterine implantations and decreased mean fetal body weights at 500
mg/kg bw per day. External examination of the fetuses failed to
reveal any gross findings related to treatment (Schroeder & Daly,
1986).
Groups of 25 mated female Crl:CD(COBS) rats were treated with
technical-grade clethodim (purity, 82.6%) in Tween 80 and
carboxymethylcellulose daily by gavage at 0, 10, 100, 350 or 700
mg/kg bw per day during days 6-15 of gestation. The day on which
evidence of mating was observed was designated day 0 of gestation.
Treatment with clethodim at 350 mg/kg bw per day and more decreased
maternal body weight. Toxicity was manifested at 700 mg/kg bw per
day as increased mortality (20%), decreased food intake and clinical
signs of poor physical condition, including red nasal discharge,
chromodacryorrhoea and staining of the anogenital region. Excessive
salivation was observed in females treated at 350 and 700 mg/kg bw
per day. Significantly decreased fetal weights and increased
incidences of fetuses with skeletal variations or at least one
ossification variation were recorded at 350 mg/kg bw per day and
more. The skeletal variations were apparent as definite delays in
ossification and were identified by incompletely or unossified
vertebral elements (thoracic, sacral, caudal) and unossified fifth
and/or sixth sternebrae. Treatment at the highest dose, 700 mg/kg bw
per day, induced a significant increase in the number of fetuses
(8/221; 3.6%) and litters (6/18; 33.2%) with external malformations,
comprising primarily an increased incidence (seven fetuses in six
litters) of tail defects characterized by short, filamentous or
absent tails. An imperforated anus was recorded in two fetuses in
two litters at this dose. Visceral malformations (exencephaly,
defects of the lung, aortic arch and large intestine, absence of
kidneys, bladder or ureter) were observed in four fetuses in three
litters of dams exposed to 700 mg/kg bw per day but not in
concurrent or historical controls (5032 fetuses in 806 litters; 38
studies). Three of the four fetuses with visceral malformations also
had external malformations. The NOAEL for maternal and developmental
toxicity was 100 mg/kg bw per day. The NOAEL for teratogenicity was
350 mg/kg bw per day on the basis of an increased incidence of
malformations, particularly tail defects, observed at the maternally
toxic and lethal dose of 700 mg/kg bw per day (Schroeder & Daly,
1987).
Rabbits
In a range-finding study, groups of eight artificially
inseminated female New Zealand white Hra: specific-pathogen-free
rabbits were treated by gavage with clethodim (purity, 83.3%) in
Tween 80 and carboxymethylcellulose at 0, 50, 150, 300 or 500 mg/kg
bw per day on days 7-19 of gestation. The day of insemination was
designated day 0 of gestation. Maternal toxicity was seen at all
doses as decreased body-weight gain and food consumption. An
increased incidence of alopecia was recorded at 150 mg/kg bw per day
and more. Dried faeces and gastrointestinal lesions (hairball and/or
ulceration of the gastric mucosa) were observed at doses of 300
mg/kg bw per day and more. In the groups given 300 and 500 mg/kg bw
per day, two of seven pregnant rabbits died, apparently due to
concomitant weight loss, decreased food intake, gastrointestinal
lesions and/or abortion. In the group given 500 mg/kg bw per day,
four of seven rabbits aborted, and one rabbit delivered prematurely.
Data on litters of dams given 500 mg/kg bw per day could not
effectively be evaluated, as only one female survived to term; dams
given 300 mg/kg bw per day had an increased incidence of resorption
and decreased litter weights when compared with the controls
(Dearlove et al., 1986).
Groups of 20 artificially inseminated female New Zealand white
Hra specific-pathogen-free rabbits were administered
technical-grade clethodim (purity, 83.3%) in Tween 80 and sodium
carboxymethylcellulose by gavage at doses of 0, 25, 100 or 300 mg/kg
bw per day on days 7-19 of gestation. The day of insemination was
designated day 0 of gestation. Mean body weights, corresponding
weight gains and food consumption were decreased in dams given 100
or 300 mg/kg bw per day. An increased incidence of dried faeces was
also noted at 100 mg/kg bw per day and more; the presence of a red
substance on the cage pans of the dams at 300 mg/kg bw per day was
considered to be related to gastrointestinal and/or rectal
irritation. Treatment had no adverse effect on survival, the
abortion rate, pre- or post-implantation loss, the number of live
fetuses, fetal weight or the fetal sex ratio. A slight increase in
the incidence of minor skeletal alterations, comprising angulation
of one or both hyoid alae, irregular ossification of the skull and
nasal passages, was observed at 300 mg/kg bw per day; however, in
the absence of a clear dose-response relationship, no direct
correlation with treatment could be concluded. Clethodim was not
teratogenic at doses up to 300 mg/kg bw per day. The NOAEL for
maternal toxicity was 25 mg/kg bw per day, and that for
developmental toxicity was 300 mg/kg bw per day (Dearlove et al.,
1987).
(f) Genotoxicity
The results of assays for mutagenicity of technical-grade
clethodim are presented in Table 2. The compound did not induce gene
mutation in bacteria in vitro. Clethodim of unknown purity
(Putnam, 1986a) induced chromosomal aberrations in Chinese hamster
ovary cells in the absence of exogenous metabolic activation, but a
purified preparation did not (Putnam, 1986b). Clethodim at doses
sufficiently high to cause acute lethality did not induce
micronuclei in bone-marrow cells of rats treated in vivo, and it
did not induce unscheduled DNA synthesis in hepatocytes of mice
treated in vivo.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Technical-grade clethodim (purity, 83.2%) was applied at a dose
of 0.5 ml under occluded conditions to two intact and two abraded
sites on the shorn backs of each of 12 female New Zealand white
rabbits for 4 h. Slight erythema, observed immediately after dosing,
was followed 24-72 h later by slight-to-severe erythema and
slight-to-moderate oedema. General clearance was seen by 14 days.
The primary irritation score was 0.7. The test material was
considered to be slightly irritating to rabbit skin (Cushman et
al., 1986b).
Undiluted technical-grade clethodim (purity, 83.3%) was
instilled at a dose of 0.1 ml into the conjunctival sac of one eye
of each of nine male New Zealand white rabbits, the other eye
serving as a control. The eyes of three of the nine rabbits were
rinsed after a 30-sec exposure. No corneal opacity or iritis was
observed. In the six rabbits with unrinsed eyes, conjunctival
irritation was moderate to severe 1 h after dosing and slight to
moderate 24 h after dosing; all eyes were clear after 72 h. In the
rinsed eyes, conjunctival irritation was moderate at 1 h and slight
at 24 h; all eyes were clear at 48 h. The test material was
considered to be mildly irritating to the rabbit eye (Cushman et
al., 1986a).
Table 2. Genotoxicity of technical-grade clethodim
End-point Test system Concentration Purity (%) Resultsa Reference
In vitro
Reverse mutation S. typhimurium TA98, 0.1-10 mg/plate 83.3 Negativea Carver et al.,
100, 1535, 1537 (technical) 1986b
E. coli WP2 uvrA
S. typhimurium TA98, 0.1-10 mg/plate 83.3 Negativea Carver et al.,
100, 1535, 1537 (technical) 1986a
Reverse mutation S. typhimurium TA98, 0, 0.1, 0.33, 1.0, 91.1 Negativea Machado et al.,
100, 1535, 1537 3.33, 10 mg/plate (technical) 1990
E. coli WP2 uvrA
Chromosomal Chinese hamster 0.03, 0.1, 0.3, 1.0 Ál/ml NR Negativeb Putnam, 1986a
aberration ovary CHO-K1 0.6, 0.8, 1.0, 1.2 Ál/ml Positivec at
CCI 61 cells 1.0, 1.2 Ál/ml
Chromosomal Chinese hamster 0.03, 0.1, 0.3, 1.0 Ál/ml 96.1 Negativea Putnam, 1986b
aberration ovary CHO-K1 0.6, 0.8, 1.0, 1.2 Ál/ml (purified)
CCI 61 cells
In vivo
Chromosomal Male and female 0, 150, 500, 1500 83.3 Negative Putnam, 1987
aberration Sprague- mg/kg bw, (technical)
Dawley rats single oral dose
Unscheduled Male B6C3F1 0, 100, 1000, 5000 83.3 Negative Mirsalis &
DNA synthesis mice mg/kg bw, gavage (technical) Steinmetz, 1986
NR, not reported
a In the presence and absence of metabolic activation
b In the presence of metabolic activation
c In the absence of metabolic activation
Technical-grade clethodim (purity, 83.4%) did not appear to
sensitize the skin of male Hartley albino guinea-pigs when tested by
the modified Buehler test (Cushman et al., 1986c).
(ii) Liver cytochrome induction
Groups of eight male Crl:CD BR rats were treated by gavage with
technical-grade clethodim (purity, 83.8%) in carboxymethylcellulose
and Tween 80 at 0 or 250 mg/kg bw per day for 21 consecutive days.
Liver samples taken at sacrifice were assayed for cytochrome P450
activity. Treatment did not affect survival, clinical signs or body
weight. The weights of the livers of animals of each sex were
significantly increased (20-23%), and enlarged livers were observed
upon gross examination of two treated animals. Although the mean
cytochrome P450 and hepatic protein contents were significantly
greater in treated animals than controls, the mean concentrations of
hepatic cytochrome P450 per milligram of protein or per gram of
liver were not significantly increased. These results may reflect
increased liver weight and do not provide evidence that clethodim
induces liver cytochrome P450 (Beatty et al., 1989).
3. Studies on metabolites
The imine sulfone and the 5-hydroxy sulfone of clethodim
occurred in larger amounts in plants than in rats (Rose et al.,
1988a). Ancillary studies on these metabolites are summarized in
Table 3.
(a) Acute toxicity
Five female Crl:CD(SD)BR rats were given a single oral dose of
1.4 g/kg bw of the imine sulfone of clethodim. One animal had
decreased motor activity 4 h after dosing and was dead the next day
(Dougherty et al., 1988b).
Groups of five female Crl:CD (SD)BR rats received the 5-hydroxy
sulfone of clethodim or technical-grade clethodim as a single oral
dose of 1.4 g/kg bw. There were no clinical signs of toxicity, and
all animals treated with the 5-hydroxy sulfone survived the 14-day
observation period after dosing. All five rats treated with
clethodim showed overt signs of toxicity, noted as salivation,
decreased motor activity, collapse, hyperactivity, tremors,
diarrhoea, dehydration and nasal, ocular, oral and anogenital
discharge, and all died within three days of dosing (Dougherty et
al., 1988c).
(b) Short-term toxicity
The imine sulfone of clethodim was administered daily in the
diet to groups of 10 male and 10 female Crl:CD (SD)BR rats at 0,
100, 1000 or 8000 ppm, equal to 6.7, 71 or 600 mg/kg bw per day, for
five weeks. Body weight and food consumption were significantly
decreased during the first week of treatment in males given 8000
ppm. Other effects observed only at 8000 ppm were increased serum
cholesterol levels in animals of each sex, increased reticulocyte
counts in males and increased liver weights in males and females in
the absence of related histopathological alterations. Treatment did
not affect survival, clinical signs or ophthalmoscopic parameters.
The NOAEL was 1000 ppm, equal to 71 mg/kg bw per day (Beatty et
al., 1988a).
Groups of 10 male and 10 female Crl:CD (SD)BR rats were fed the
5-hydroxy sulfone of clethodim daily at dietary concentrations of 0,
100, 1000 or 8000 ppm, equal to 5.9, 68 or 590 mg/kg bw per day, for
five weeks. No signs of toxicity were seen at any dose. On the basis
of the absence of any effects on survival, clinical signs, body
weight, food consumption, haematological, blood chemical or
histopathological changes, or organ weights, the NOAEL was greater
than 8000 ppm, equal to 590 mg/kg bw per day (Beatty et al.,
1988b).
Table 3. Effects of clethodim and its imine sulfone and 5-hydroxy sulfone
Study Clethodim Imine sulfone 5-Hydroxy sulfone
Acute toxicity; 3/5 deaths (Cushman et al., 1986d) 1/5 deaths 0/5 deaths
female rat; 5/5 deaths (Dougherty et al., 1988c) (Dougherty et al., 1988b) (Dougherty et al.,
1.4 g/kg bw 1988bc)
Five-week dietary; Decreased body weight and Decreased body weight and No treatment-related
male & female rats food intake, slight anaemia, food intake, increased effects; NOAEL,
increased liver weights, liver weights, cholesterol > 588mg/kg bw per day
liver hypertrophy at 4000 and and reticulocytes at 8000 (Beatty et al., 1988b)
8000 ppm; anaemia, liver ppm. NOAEL, 71 mg/kg bw per
hypertrophy at 1000 ppm day (Beatty et al., 1988a)
NOAEL, 13 mg/kg bw per day
(Cisson & Eisenlord, 1986)
Teratogenicity; rats Maternal and fetal toxicity Maternal toxicity at 100 Clinical signs and
at 350 and 700 mg/kg bw per and 700 mg/kg bw per day; slightly decreased
day; NOAEL, 100 mg/kg bw fetal effects at 700 mg/kg fetal body weights at
per day. Increased incidence bw per day 700 mg/kg bw per day
of tail malformations at NOAEL, 10 mg/kg bw NOAEL, 100 mg/kg
700 mg/kg bw per day per day (Hoberman & per day (Hoberman &
(Schroeder & Daly, 1987) Christian, 1988a) Christian, 1988b)
Microbial gene Negative Negative Negative
mutation (Carver et al., 1986a,b) (Carver et al., 1988) (Carver eta[., 1987)
Chromosomal Positive in absence of Negative Negative
aberration, metabolic activation (Putnam, 1988a) (Putnam, 1988b)
CHO cells (Putnam, 1986a)
(c) Embryotoxicity and teratogenicity
Groups of 10 pregnant female Crl:CD (SD)BR rats were treated by
gavage with the imine sulfone of clethodim in Tween 80 and
carboxymethylcellulose at doses of 0, 10, 100 or 700 mg/kg bw per
day during days 6-15 of gestation. Maternal toxicity was seen at the
high dose of 700 mg/kg bw per day, manifested as excessive
salivation and decreased body weight and food consumption.
Significant decreases in maternal body weight were also recorded at
100 mg/kg bw per day. At 700 mg/kg bw per day, fetal weights were
decreased, and there were increased incidences of fetuses and
litters with cervical ribs and delayed sternal ossification. The
NOAEL for maternal toxicity was 10 mg/kg bw per day on the basis of
reduced body weights at 100 mg/kg bw per day and more. An increased
incidence of fetal variations at the maternally toxic dose of 700
mg/kg bw per day resulted in an NOAEL of 100 mg/kg bw per day for
developmental toxicity (Hoberman & Christian, 1988a).
Groups of 10 pregnant female Crl:CD (SD)BR rats were
administered the 5-hydroxy sulfone of clethodim in
carboxymethylcellulose and Tween 80 by gavage at 0, 10, 100 or 700
mg/kg bw per day on days 6-15 of gestation. At 700 mg/kg bw per day,
one female showed excessive salivation and two females had rales; a
slight decrease in fetal body weights in this group, although not
statistically significant, was possibly related to treatment. No
teratogenicity was seen at any dose. The NOAEL for maternal and
fetal toxicity was 100 mg/kg bw per day (Hoberman & Christian,
1988b).
(d) Genotoxicity
Neither the imine sulfone nor the 5-hydroxy sulfone of
clethodim induced gene mutation in Salmonella typhimurium TA98,
TA100, TA1535 or TA1537 or in Escherichia coli WP2 uvrA in the
presence or absence of metabolic activation. Neither metabolite
induced chromosomal aberrations in Chinese hamster ovary cells.
4. Observations in humans
No information was available.
Comments
Clethodim was readily absorbed by rats after oral
administration. Excretion was rapid and occurred predominantly via
the urine, with lesser amounts eliminated in the faeces and as
carbon dioxide. There were no significant dose-related or
sex-specific differences in elimination pattern or tissue
distribution, and there was no evidence of bioaccumulation.
The dominant metabolic pathway proposed for clethodim in rats
is oxidation to clethodim sulfoxide. Other postulated pathways are
cleavage of the N-O bond to form the imine, conversion of the
S-ethyl group to S-methyl, formation of the oxazole, and
hydroxylation at the 5 position. The urinary metabolites identified
were the sulfoxides and sulfones of clethodim and 5-hydroxy
clethodim, the sulfoxides of the imine and S-methyl clethodim and
the sulfone of the oxazole.
Clethodim had slight to moderate acute oral toxicity in the rat
and mouse. WHO has not classified clethodim with regard to toxic
hazard.
The primary effect of treatment with clethodim after short- and
long-term dietary exposure in the mouse, rat and dog was on the
liver. Hepatic effects were manifest as increased liver weights and
centrilobular hypertrophy. A study in rats administered clethodim at
250 mg/kg bw per day did not provide evidence for liver cytochrome
P450 induction.
In a four-week study in mice given dietary concentrations of 0,
100, 250, 625, 1500 or 4000 ppm, the NOAEL was 250 ppm, equivalent
to 38 mg/kg bw per day on the basis of evidence of slight anaemia at
625 ppm and above. In a 78-week study of toxicity and
carcinogenicity in which mice received dietary levels of 0, 20, 200,
1000 or 2000-3000 ppm, the NOAEL was 200 ppm, equivalent to 30 mg/kg
bw per day, on the basis of hepatic effects and an increased
incidence of alveolar lung macrophages at 1000 ppm and above.
In a five-week study in which rats received dietary levels of
0, 5, 200, 1000, 4000 or 8000 ppm, the NOAEL was 200 ppm, equal to
13 mg/kg bw per day, on the basis of liver hypertrophy and evidence
of slight anaemia at 1000 ppm and above. A 13-week study at dietary
concentrations of 0, 50, 500, 2500 or 5000 ppm and a two-year
feeding study at dietary levels of 0, 5, 20, 500 or 2500 ppm in rats
revealed an NOAEL of 500 ppm, equal to 25 mg/kg bw per day in the
first study and equal to 16 mg/kg bw per day in the second. The
NOAEL was based on decreased body weights and liver hypertrophy at
dietary levels of 2500 ppm and above.
In dogs, 13-week oral administration of capsules of clethodim
at doses of 0, 1, 25, 75 or 125 mg/kg bw per day resulted in an
NOAEL of 25 mg/kg bw per day, on the basis of increased liver weight
and cholesterol levels at 75 mg/kg bw per day and above. In a
one-year study at doses of 0, 1, 75 or 200-300 mg/kg bw per day, the
NOAEL was 1 mg/kg bw per day on the basis of treatment-related
effects on the liver at 75 mg/kg bw per day and above.
Clethodim was not carcinogenic when fed to mice or rats at
dietary levels of up to 2500 ppm.
A two-generation study of reproductive toxicity in rats treated
with clethodim at dietary levels of 0, 5, 20, 500 or 2500 ppm did
not reveal any adverse effects on reproduction. The NOAEL was 500
ppm, equal to 39 mg/kg bw per day, on the basis of decreased
parental body weights and food consumption at 2500 ppm.
Studies of teratogenicity were conducted with clethodim in rats
at doses of 0, 10, 100, 350 or 700 mg/kg bw per day and in rabbits
at 0, 25, 100 or 300 mg/kg bw per day. In rats, the NOAEL for
maternal toxicity was 100 mg/kg bw per day. Fetal toxicity was
observed at the maternally toxic doses of 350 and 700 mg/kg bw per
day. An increased incidence of malformations, specifically tail
defects (short, filamentous or absent tails), was observed at the
maternally toxic and lethal high dose of 700 mg/kg bw per day. In
rabbits, maternal toxicity was observed at 100 and 300 mg/kg bw per
day, resulting in an NOAEL of 25 mg/kg bw per day. There were no
adverse effects on the rabbit fetus and no evidence of teratogenic
potential at doses up to 300 mg/kg bw per day.
Clethodim was adequately tested for genotoxicity in a series of
assays in vivo and in vitro. The Meeting concluded that
clethodim was not genotoxic.
Although the imine sulfone and 5-hydroxy sulfone metabolites of
clethodim have been reported to occur in higher amounts in plants
than in animals, neither of the metabolites was more toxic than the
parent, clethodim, in studies of acute or short-term toxicity and
teratogenicity. There was similarly no evidence of genotoxic
potential.
An ADI was established on the basis of the NOAEL of 1 mg/kg bw
per day from the one-year study in dogs and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 200 ppm, equivalent to 30 mg/kg bw per day (78-week
study of toxicity and carcinogenicity)
Rat: 500 ppm, equal to 16 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 25 mg/kg bw per day (study of teratogenicity)
Dog: 1 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Observations in humans
References
Beatty, P.W., Bagos, A.D., Richter, W.R. & Wong, Z.A. (1988a)
Five-week oral toxicity study in rats with RE-47719 (SX-1800).
Project No. 2949. Unpublished report dated 15 November 1988 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Beatty, P.W., Bagos, A.C., Richter, W.R. & Wong, Z.A. (1988b)
Five-week oral toxicity study in rats with RE-51228 (SX-1803).
Project No. 2950. Unpublished report dated 15 November 1988 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Beatty, P.W., Brorby, G.P. & Wong, Z.A. (1989) The potential of
RE-45601 Technical (SX-1688) to induce cytochrome P-450 following
21-day oral administration in male rats. Project No. 2784.
Unpublished report dated 16 November 1989 from Chevron Environmental
Health Center Inc., Richmond, CA, USA. Submitted to WHO by Tomen
Pacific Agro Co., San Francisco, CA, USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1986a)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-45601 Technical (83% purity, SC-1688). Project No. SOCAL
2505. Unpublished report dated 13 January 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1986b)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-45601 Technical (83% purity, SC-1688). Project No. CEHC
2555. Unpublished report dated 28 April 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1987)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-51228. Project No. 2856. Unpublished report dated 7 December
1987 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Carver, J.H., Machado, M.L. & Richter, W.R. (1988)
Microbial/mammalian microsome mutagenicity plate incorporation assay
with RE-47719. Project No. 2948. Unpublished report dated 19 October
1988 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Cisson, C.M. & Eisenlord, G.H. (1986) Five-week pilot feeding study
in rats with RE-45601 Technical (SX-1653). Project No. SOCAL 2457.
Unpublished report dated 7 October 1986 from Chevron Environmental
Health Center Inc., Richmond, CA, USA. Submitted to WHO by Tomen
Pacific Agro Co., San Francisco, CA, USA.
Cox, R.H. (1988) Acute intraperitoneal toxicity study in rats with
Chevron RE-45601 Technical (SX-1688). Project No. 2107-151.
Unpublished revised report dated 6 April 1988 from Hazelton
Laboratories America Inc., Vienna, VA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Cox, R.H. & Slabik, C.M. (1986) Four-week subchronic oral toxicity
study in mice with Chevron RE-45601 Technical. Project No. 2107-140.
Unpublished report dated 21 July 1986 from Hazelton Laboratories
America Inc., Vienna, VA, USA. Submitted to WHO by Tomen Pacific
Agro Co., San Francisco, CA, USA.
Cox, R.H. & Zoetis, T. (1986) Acute oral toxicity study in mice with
Chevron RE-45601 Technical. Project No. 2107-143. Unpublished report
dated 16 September 1986 from Hazelton Laboratories America Inc.,
Vienna, VA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Cox, R.H. & Zoetis, T. (1988b) One-year oral toxicity study in dogs
with Chevron RE-45601 Technical (SX-1688). Project No. 2107-153.
Unpublished report dated 28 October 1988 from Hazelton Laboratories
America Inc., Vienna, VA, USA. Submitted to WHO by Tomen Pacific
Agro Co., San Francisco, CA, USA.
Cox, R.H. & Zoetis, T. (1988b) Chronic oral oncogenicity study in
mice with Chevron RE-45601 Technical (SX-1688). Project No.
2107-145. Unpublished report dated 25 October 1988 from Hazelton
Laboratories America Inc., Vienna, VA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Thompson, J.D. & Wong, Z.A. (1986a) The acute eye
irritation potential of RE-45601 Technical (SX-1688). Project No.
CEHC 2511. Unpublished report dated 1 October 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Korenaga, G.L. & Wong, Z.A. (1986b) The four-hour
skin irritation potential of RE-45601 Technical (SX-1688). Project
No. CEHC 2512. Unpublished report dated 18 December 1986 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Cooper, P.N. & Wong, Z.A. (1986c) Modified Buehler
Test for the skin sensitization potential of RE-45601 Technical
(SX-1688). Project No. CEHC 2514. Unpublished report dated 15
December 1986 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Cushman, J.R., Easter, M.D. & Wong, Z.A. (1986d) The acute oral
toxicity of RE-45601 Technical (SX-1688) in adult male and female
rats. Project No. SOCAL 2498. Unpublished report dated 27 June 1986
from Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Cushman, J.R., Hedgecock, J.H. & Wong, Z.A. (1987a) Four-week
repeated dose dermal toxicity study in rats with RE-45601 Technical
(SX-1688). Project No. CEHC 2552. Unpublished report dated 20
November 1987 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Cushman, J.R., Korenaga, G.L. & Wong, Z.A. (1987b) The acute dermal
toxicity of RE-45601 Technical (SX-1688) in adult male and female
rabbits. Project No. CEHC 2510. Unpublished report dated 7 January
1987 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Daly, I.W. & Knezevich, A.L. (1987) Ninety-day subchronic oral
toxicity study in dogs with Chevron RE-45601 Technical. Project No.
85-2999. Unpublished report dated 26 February 1987 from Bio/dynamics
Inc., East Millstone, NJ, USA. Submitted to WHO by Tomen Pacific
Agro Co., San Francisco, CA, USA.
Dearlove, G.E., Hoberman, A.M. & Christian, M.S. (1986) Pilot
teratology study in rabbits with Chevron RE-45601 Technical. Project
No. 303-007P. Unpublished report dated 15 July 1986 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Dearlove, G.E., Hoberman, A.M. & Christian, M.S. (1987) Teratology
study in rabbits with Chevron RE-45601 Technical. Project No.
303-007. Unpublished report dated 30 January 1987 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Dougherty, K.K., Eisenlord, G.H., Low, S.S. & Wong, Z.A. (1987)
13-Week oral toxicity study in rats with RE-45601 Technical
(SX-1688). Project No. SOCAL 2501. Unpublished report dated 13
February 1987 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Eisenlord, G.H. & Wong, Z.A. (1988a) Combined
chronic toxicity/oncogenicity study in rats with RE-45601 Technical
(SX-1688). Project No. SOCAL 2500. Unpublished report dated 1
November 1988 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Duncan, R.A. & Wong, Z.A. (1988b) The comparative
acute oral toxicity of RE-47719 (SX-1800) and RE-45601 (SX-1688) in
adult female rats. Project No. 2952. Unpublished report dated 21
October 1988 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Duncan, R.A. & Wong, Z.A. (1988c) The comparative
acute oral toxicity of RE-51228 (SX-1796) and RE-45601 (SX-1688) in
adult female rats. Project No. 2951. Unpublished report dated 21
October 1988 from Chevron Environmental Health Center Inc.,
Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Dougherty, K.K., Waid, L.J. & Wong, Z.A. (1989) The acute oral
toxicity of RE-45601 Technical (SX-1688) in adult male and female
rabbits. Project No. CEHC 2992. Unpublished report dated 21 June
1989 from Chevron Environmental Health Center Inc., Richmond, CA,
USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA,
USA.
Griffis, L.C., Bruce, E.D. & Wong, Z.A. (1986) The acute inhalation
toxicity of RE-45601 Technical (SX-1688) in rats. Project No. CEHC
2513. Unpublished report dated 7 November 1986 from Chevron
Environmental Health Center Inc., Richmond, CA, USA. Submitted to
WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Hoberman, A.M. & Christian, M.S. (1988a) Oral teratogenicity and
developmental toxicity screen in rats with RE-47719. Project No.
303-012. Unpublished report dated 8 November 1988 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Hoberman, A.M. & Christian, M.S. (1988b) Oral teratogenicity and
developmental toxicity screen in rats with RE-51228. Project No.
303-010. Unpublished report dated 8 November 1988 from Argus
Research Laboratories Inc., Horsham, PA, USA. Submitted to WHO by
Tomen Pacific Agro Co., San Francisco, CA, USA.
Lee, S.G., Maier, K.M., Tamichi, E.H. & Tucker, B.V. (1988)
[Ring-4,6-14C] clethodim: A radiocarbon metabolism study in laying
hens. Project No. MEF-0089. Unpublished report dated 30 December
1988 from Chevron Chemical Company, Ortho Agricultural Chemicals
Division, Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro
Co., San Francisco, CA, USA.
Machado, M.L., Lubeck, S.G. & Wilkenfeld, R.M. (1990)
Microbial/microsome reverse mutation plate incorporation assay with
RE-45601 Technical (SX-1845). Project No. CEHC 3146. Unpublished
report dated 20 August 1990 from Chevron Environmental Health Center
Inc., Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro Co.,
San Francisco, CA, USA.
McKensie, K.M. (1986) Pilot rat reproduction study with Chevron
RE-45601 Technical. Project No. 6183-102. Unpublished report dated
16 December 1986 from Hazelton Laboratories America Inc., Madison,
WI, USA. Submitted to WHO by Tomen Pacific Agro Co., San Francisco,
CA, USA.
Mirsalis, J.C. & Steinmetz, K.L. (1986) In vivo--in vitro
hepatocyte DNA repair assay: in vitro evaluation of unscheduled
DNA synthesis (UDS) following oral administration of Chevron
RE-45601 Technical to B6C3F1 Mice. Project No. LSC-1960.
Unpublished report dated August 1986 from SRI International, Meno
Park, CA, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Putnam, D.L. (1986a) Chromosome aberrations in Chinese hamster ovary
(CHO) cells with Chevron RE-45601 Technical. Project No. T4529.337.
Unpublished report dated August 1986 from Microbiological Associates
Inc., Bethesda, MD, USA. Submitted to WHO by Tomen Pacific Agro Co.,
San Francisco, CA, USA.
Putnam, D.L. (1986b) Chromosome aberrations in Chinese hamster ovary
(CHO) cells with purified Chevron RE-45601. Project No. T429.337.
Unpublished report dated 15 December 1986 from Microbiological
Associates Inc., Bethesda, MD, USA. Submitted to WHO by Tomen
Pacific Agro Co., San Francisco, CA, USA.
Putnam, D.L. (1987) Cytogenetics assay in bone marrow cells of rats
following acute oral exposure to RE-45601 Technical. Project No.
T5172.105. Unpublished report dated 25 February 1987 from
Microbiological Associates Inc., Bethesda, MD, USA. Submitted to WHO
by Tomen Pacific Agro Co., San Francisco, CA, USA.
Putnam, D.L. (1988a) Chromosome aberrations in Chinese hamster ovary
(CHO) cells: RE-47719. Project No. T8226.337003. Unpublished report
dated 10 November 1988 from Microbiological Associates Inc.,
Bethesda, MD, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Putnam, D.L. (1988b) Chromosome aberrations in Chinese hamster ovary
(CHO) cells: RE-51228. Project No. T8227.337003. Unpublished report
dated 10 November 1988 from Microbiological Associates Inc.,
Bethesda, MD, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Rendina, A.R. & Felts, J.M. (1988) Cyclohexanedione herbicides are
selective and potent inhibitors of acetyl-CoA carboxylase from
grasses. Plant Physiol., 86, 983-986.
Rose, A.F., Griffis, L.C., Suzuki, J.P., Bruce, E.D., Primer, R.L.,
Wong, Z.A. & Tucker, B.V. (1988a) The in vivo metabolism of
[propyl-1-14C] clethodim in rats. Project No. MEF-0086/CEHC 2515.
Unpublished report dated 22 December 1988 from Chevron Chemical Co.,
Ortho Agricultural Chemicals Division, Richmond, CA, USA. Submitted
to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Rose, A.F., Suzuki, J.P., Primer, R.L. & Tucker, B.V. (1988b) The
in vivo metabolism of [propyl-1-14C] clethodim in a lactating
goat. Project No. MEF-0038. Unpublished report dated 29 December
1988 from Chevron Chemical Co., Ortho Agricultural Chemicals
Division, Richmond, CA, USA. Submitted to WHO by Tomen Pacific Agro
Co., San Francisco, CA, USA.
Schroeder, R.E. & Daly, I.W. (1986) A pilot teratology study in rats
with Chevron RE-45601 Technical. Project No. 85-3032. Unpublished
report dated 8 September 1986 from Bio/dynamics Inc., East
Millstone, NJ, USA. Submitted to WHO by Tomen Pacific Agro Co., San
Francisco, CA, USA.
Schroeder, R.E. & Daly, I.W. (1987) Teratology study in rats with
Chevron RE-45601 Technical. Project No. 86-3042. Unpublished report
dated 20 July 1987 from Bio/dynamics Inc., East Millstone, NJ, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
Tellone, C.I., Hardy, L.M. & Richter, W.R. (1987) Two-generation
(one litter) reproduction study in rats with RE-45601 Technical.
Project No. CEHC 2596. Unpublished report dated 27 August 1987 from
Chevron Environmental Health Center Inc., Richmond, CA, USA.
Submitted to WHO by Tomen Pacific Agro Co., San Francisco, CA, USA.
