
FENPROPIMORPH
First draft prepared by
P.H. van Hoeven-Arentzen and A.J. Deijns
National Institute of Public Health and Environmental Protection,
Bilthoven, Netherlands
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Embryotoxicity and teratogenicity
Genotoxicity
Special studies
Skin and eye irritation and skin sensitization
Delayed neuropathy
Liver function and cholinesterase inhibition
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Fenpropimorph is a morpholine fungicide with systemic activity,
which is based on interference with sterol biosynthesis in the
target fungus. Fenpropimorph was considered for the first time by
the present Meeting.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution and excretion
[14C-morpholine]-Fenpropimorph (radiochemical purity, > 99%)
was administered to six female Füllinsdorfer albino rats at 50 mg/kg
bw by gavage. Two animals were used as controls. Urine and faeces
were sampled after 6 (only urine), 24, 48, 72 and 96 h. In a second
experiment, two males and two females were treated in the same way,
and radioactivity was measured in urine, faeces and expired air up
to 72 h. At the end of the collection period, the animals in both
experiments were killed, and residual activity was measured in
blood, liver, intestinal tract, spleen, brain, kidney, heart,
gonads, fat, bone (only in the first experiment), lungs (only in the
second experiment) and residual carcass. In the first experiment,
64-72% of the administered radioactivity had been excreted by the
time of sacrifice at 96 h, with 37-45% in faeces and 21-34% in
urine; the residues in most tissues amounted to 0.3-1.9 mg/kg
fenpropimorph equivalents, but the liver content of radiolabelled
residue was 7.3 mg/kg. No residues were found in intestinal tract
tissue or contents. In the second experiment, 9-12% of the
radioactivity was found in expired air. In contrast to the results
of the first experiment, 14-29% was found in faeces and 28-43% in
urine. At the end of this study (72 h), the amounts of residues in
the tissues (1.4-3.4 mg/kg) were slightly higher than in the first
experiment; the total residue in the residual carcass was about
13.5% of the administered dose. High residue levels were found in
liver (12.9 mg/kg), lungs (6.3-15.9 mg/kg) and intestinal tract
tissue (25.2 mg/kg) (von der Mühll & Gätzi, 1979).
An extensive study of pharmacokinetics and metabolism was
performed with 14C-fenpropimorph. Groups of five male and five
female Wistar rats received the radiolabelled compound as a single
oral dose of 1.25 or 100 mg/kg bw, as repeated oral doses of 1.25
mg/kg bw for seven days or as a single intravenous administration of
1.25 mg/kg bw. Both phenyl- and morpholine-labelled compounds were
tested by oral administration of 100 mg/kg bw, but only
phenyl-labelled compound was tested at other dosages. A group of
five male and five female animals was pretreated for 14 days with
unlabelled compound and then treated once with phenyl-labelled
compound at 1.25 mg/kg bw. Three male and three female rats with
bile-duct cannulas received a single oral dose of 1.25 mg/kg bw.
Maximal concentrations of radioactivity were seen in plasma and
blood 4-8 h after the single oral administration of phenyl-labelled
fenpropimorph at 1.25 mg/kg bw. Elimination from blood and plasma
followed first-order kinetics, with half-lives ranging from 16 to 24
h. Nearly complete excretion (92-105%) was observed via urine and
faeces within 96 h, mostly within 48 h. Males excreted a total of
33-51% of the administered radioactivity in the urine and 32-49% in
the faeces, and females excreted 24-44% in urine and 56-71% in
faeces. The animals with bile-duct cannulas excreted large amounts
in bile: males excreted 61-77% in bile, about 23% in urine and about
7% in faeces, and females excreted 76-83% in bile, about 16% in
urine and about 1% in faeces. The relatively low levels in urine and
the high levels in bile in these animals indicate that under normal
conditions enterohepatic circulation occurs. At the high dose, 100
mg/kg bw, there was no significant difference in the rate or route
of elimination. After intravenous administration, males excreted
about 44% in faeces and 52% in urine, and females excreted 49% in
faeces and 44% in urine. Administration of morpholine-labelled
compound resulted in a small fraction of radioactivity (1.5-1.8%) in
expired air.
Residual radioactivity was generally low 96 h after oral
administration of a single dose of 1.25 mg/kg bw. Total residual
radioactivity was about 1.5% of the administered dose in the
digestive tract, about 2% in organs and tissues and 1.1% in residual
carcass. The levels in most organs and tissues were 0.007-0.03
mg/kg; higher concentrations of radiolabelled residue were found
only in liver (0.196-0.278 mg/kg), fat (0.013-0.104 mg/kg) and the
digestive tract (0.109-0.153 mg/kg). After treatment with 100 mg/kg
bw, the levels were correspondingly higher, with residue levels of
12.5-17.1 mg/kg in liver, 6.4-9.8 mg/kg in fat and 11.7-12.3 mg/kg
in the digestive tract. Females also had a slightly higher
concentration in the ovary and uterus (2.8 mg/kg) than in most other
organs and tissues (0.3-1.6 mg/kg). The total residual radioactivity
in the digestive tract, tissues, organs and residual carcass,
however, was 0.8-2% of the administered dose. A comparable
distribution pattern was found for morpholine-labelled compound.
After a single intravenous administration of 1.25 mg/kg bw, the
total residual radioactivity was about 3.6% of the administered dose
in the digestive tract, 2.6% in tissues and organs and 2.7% in the
residual carcass. The residual radioactivity in fat, liver and the
digestive tract ranged from 0.353 mg/kg in the digestive tract to
0.759 mg/kg in fat; ovary and uterus contained 0.139 mg/kg; the
residue levels in all other organs and tissues ranged from 0.015 to
0.062 mg/kg. Pretreatment with unlabelled compound did not affect
the rate or route of excretion nor the level of residual
radioactivity in organs and tissues.
In the study in which seven consecutive doses of radiolabelled
compound were administered and blood and tissue samples were taken
up to 72 h after the last dose, residual radioactivity in all organs
and tissues, blood and plasma reached maximal levels 4 h after the
last dose in males and 4-8 h after the last dose in females. The
maximal levels were generally 0.15-0.77 mg/kg, but higher levels
were found in the liver (13.8 mg/kg), kidney (1.2 mg/kg) and plasma
(1.3 mg/l). Elimination in blood and tissues followed first-order
kinetics. The half-lives in plasma and blood were comparable to
those after exposure to a single dose, ranging from 19 to 32 h; the
half-lives in the various tissues ranged from 22 to 29 h, except in
fat, for which half-lives of 41 h in males and 58 h were calculated
in females (van Dijk & Vogel, 1989).
Two lactating goats kept in metabolism cages received
14C-morpholine-labelled fenpropimorph (radiochemical purity, >
97%) in gelatine capsules at 1.5 mg/day for 10 consecutive days,
equal to a daily intake of 0.025 mg/kg bw. Blood samples were
collected before each administration and at several times after the
last dose, and the goats were milked twice daily; they were last
milked 24 h after the final dose and were then killed. Most of the
administered dose was excreted in urine and faeces. During the first
24 h, urinary excretion amounted to 12-13% of the first dose; faecal
excretion was 16-24%. Cumulative excretion in faeces reached a
plateau at the end of the study, at 56-59% of the total radiolabel
administered. In urine, a plateau of about 25-29% was reached at day
8. The concentration of radioactivity in blood reached a maximum
after five days of treatment and then remained constant at about 7
ng/ml fenpropimorph equivalents in plasma and 5 ng/ml in whole
blood. Concentrations in milk followed the plasma levels and reached
a fairly constant steady-state level of about 7 ng/ml after five
days of dosing. About 1.7% of the administered radioactivity was
eliminated in the milk. Little residual radioactivity was generally
seen (total percentage not given). The levels were highest in the
liver (89-103 ng/g) and kidney (about 29 ng/g); in other tissues,
the levels did not exceed 12 ng/g. Muscle contained 4 ng/g
fenpropimorph equivalents. High concentrations of radiolabelled
residue were detected in the bile of both animals (911 and 1618
ng/g), indicating that biliary excretion is significant (Hawkins &
Jackson, 1980a,b).
Two lactating goats received five daily oral doses of 56 mg/kg
bw 14C-labelled fenpropimorph by intubation, equal to dietary
concentrations of 2236 and 1421 ppm. One animal was treated with
phenyl-labelled compound (radiochemical purity, > 98%) and the
other with morpholine-labelled compound (radiochemical purity, >
99%). The animals were kept in metabolism cages, and urine, faeces
and milk were collected; blood samples were taken 1 h after each
administration and up to 5 h after the final dose, at which time the
animals were killed. During the first 24 h of the study, urinary and
faecal excretion amounted to about 19% of the first dose with the
phenyl label and 12% with the morpholine label. Total excretion (5 h
after the last administration) was 35% (phenyl label) and 50%
(morpholine label) of the amount of radiolabel administered, with 14
and 21% in urine and 20 and 29% in faeces. Only small amounts of
radioactivity were eliminated in milk (0.06% of the phenyl label and
0.3% of the morpholine label); the maximal concentrations of
fenpropimorph equivalents were 9.7 mg/l after the fourth
administration of the phenyl label and 23.1 mg/l after the fifth
administration of the morpholine label. After administration of the
phenyl label, the radioactivity in whole blood increased over time:
the concentrations 1 h after the first dosing were 6.7 mg/l in blood
and 9.3 mg/l in plasma, and those 1 h after the last dosing were
52.8 mg/l and 87.7 mg/l. After administration of the morpholine
label, a plateau was reached at about 17 mg/l in blood and 23 mg/l
in plasma after the fourth treatment. At sacrifice, residual
radioactivity determined in organs and tissues amounted to 4.5% of
the total administered phenyl label and 4.2% of the morpholine
label. The highest levels of residual phenyl and morpholine label
were found in the liver (140 and 124 mg/kg), kidney (233 and 53
mg/kg) and bile (10 128 and 4240 mg/kg). Concentrations of 4-20
mg/kg fenpropimorph equivalents were detected in all other organs
examined (Ritter & Vogel, 1989).
Groups of 10 white Leghorn hens were treated with
14C-fenpropimorph by crop intubation at daily doses of 4 mg/kg bw
for five consecutive days, equivalent to a dietary exposure of about
50 ppm. One group received phenyl-labelled compound (radiochemical
purity, 99.2%) and the other the morpholine label (radiochemical
purity, 94.9%). Eggs and excreta were collected; 5 h after the final
administration, the birds were killed and residual radioactivity was
determined in organs and tissues. During the treatment period, 83.1%
(phenyl label) and 79.1% (morpholine label) of the administered
radiolabel were collected in the excreta; only 0.2% of the
phenyl-labelled dose and 0.4% of the morpholine-labelled dose were
found in eggs. The concentration of phenyl-labelled fenpropimorph
equivalents in the whites of the eggs reached a plateau (0.155-0.215
mg/kg) two days after the start of treatment; no clear plateau was
reachedwith the morpholine label, and the concentration varied from
0.316 to 0.611 mg/kg. In the yolks, the concentration increased
slowly with time and was 0.832 mg/kg (phenyl) and 2.963 mg/kg
(morpholine label) at the time of sacrifice. The total levels of
residues in organs, tissues and blood were 3.1% of the administered
dose of phenyl-labelled compound and 3.6% of the morpholine-labelled
compound. The concentration was highest in the liver (2.751 and
3.920 mg/kg fenpropimorph equivalents with the two labels,
respectively) and kidneys (2.808 and 2.369 mg/kg); 1.431 mg/l
(phenyl) and 1.119 mg/l (morpholine) were detected in blood. The
levels in other organs ranged from 0.243 to 1.450 mg/kg. The ovaries
of hens treated with morpholine-labelled compound contained 4.1
mg/kg fenpropimorph equivalents (Ritter, 1989).
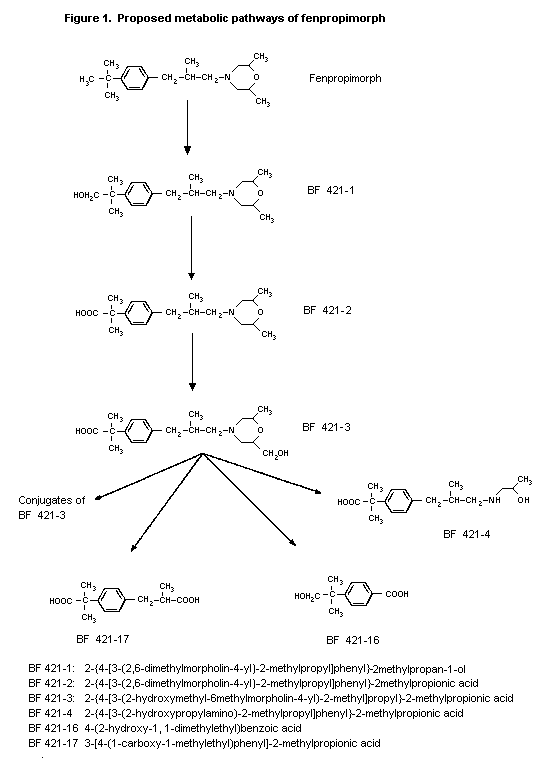
(b) Biotransformation
Biotransformation of morpholine-labelled fenpropimorph was
investigated in female rats. Five metabolite fractions were isolated
from urine, which amounted to less than 4% of the administered
radioactvity. A volatile compound in one of these fractions was
identified as 2,6-dimethylmorpholine; the other compounds were all
oxidation products. No parent compound was found in the urine. In
the faeces, parent compound represented 3.2% of the administered
radioactivity. Three metabolites were isolated but not identified
(Pryde et al., 1979,1980).
In the extensive study of the pharmacokinetics and metabolism
of fenpropimorph in rats described above (van Dijk & Vogel, 1989),
no parent compound was found in urine or faecal extracts after
treatment with 1.25 or 100 mg/kg bw. After single oral doses of 100
mg/kg bw of either phenyl- or morpholine-labelled compound, the
major urinary metabolite was
2-{4-[3-(2-hydroxymethyl-6-methylmorpholin-4-yl)-2-methylpropyl]phen
yl}-2-methylpropionic acid (BF 421-3) (37-50% of the radioactivity
recovered in urine), and various conjugates of BF 421-3 accounted
for 13-18%. Other metabolites found were the less polar metabolite
2-{4-[3-(2,6-dimethylmorpholin-4-yl)-2-methylpropyl]phenyl}-2-methyl
-propionic acid (BF 421-2) (accounting for 11-20%),
4-(2-hydroxy-1,1-dimethylethyl)benzoic acid (BF 421-16),
3-[4-(1-carboxy-1-methylethyl)phenyl]-2-methylpropionic acid (BF
421-17) (each accounting for 7-11%) and U3.1, probably identical to
2-{4-[3-(2-hydroxypropylamino)-2-methylpropyl]phenyl}-2-methylpropio
nic acid (BF 421-4), which accounted for 22% in males and 8% in
females. After a single oral administration of 1.25 mg/kg bw of the
phenyl-labelled compound, more BF 421-3 was excreted in the
conjugated moiety, representing 47-67% of the recovered
radioactivity in urine. A higher percentage of conjugates (39-46%)
was also observed after the single intravenous administration.
Little fenpropimorph was excreted as conjugates in the urine of the
bile-cannulated rats. Somewhat larger amounts of conjugates (about
25%) and of the metabolites BF 421-16 and 17 (about 29%) were
excreted after repeated oral administrations. The patterns of
metabolites in faeces were comparable after all dosages. Eight
metabolite fractions were isolated from the extractable portion of
the faeces of the animals that received 100 mg/kg bw (either label),
of which five were identified: BF 421-3 (accounting for 14-35% of
the extractable faecal radiolabel), several of its conjugates
(accounting for a total of 12-20%), BF 421-2 (accounting for 11-35%)
and BF 421-16 and 17. In organs and plasma, the major metabolite of
both labelled compounds was BF 421-2, which accounted for 51-76% of
the recovered radiolabel in liver, 24-43% in kidney and 52-69% in
plasma. BF 421-3 was found only after administration of
phenyl-labelled compound and represented 4-14% of the recovered
radiolabel in liver, kidney and plasma. Two unidentified
morpholine-labelled metabolite fractions were observed in liver and
kidney, which accounted for 7-19% and about 14% of the recovered
radiolabel, respectively. BF 421-16 and 17 (about 10% of the
recovered radiolabel) were also found in kidney (van Dijk & Vogel,
1989).
In the study of Ritter and Vogel (1989), described above, the
urine of the goat treated with phenyl-labelled fenpropimorph
contained up to 10 metabolites, which represented 2-20% of the
recovered radiolabel; BF 421-3 accounted for 4.5%. The three major
fractions, which accounted for 16, 17 and 20%, could not be
identified. In faeces sampled after the first to the fourth
administrations, the major metabolite, accounting for 38% of the
recovered radioactivity, was 2-{4-[3-(2,6-dimethylmorpholin-4-yl)-
2-methylpropyl]phenyl}-2-methylpropan-1-ol (BF 421-1); BF 421-3
accounted for 9%, and five other metabolites, which could not be
identified, accounted for 2-13%. Two additional metabolite fractions
were found in faeces sampled 5 h after the last administration. Two
of seven fractions were identified as BF 421-1 and 2, each
accounting for about 12%; all of the other fractions accounted for
4-18%. Three metabolites were detected in the protein-free plasma
fraction: the major one was BF 421-2, accounting for 51% of the
total radiolabel in plasma. BF 421-3 accounted for 3% and the other
for 7%. Up to eight metabolites were detected in the urine of the
goat treated with morpholine-labelled compound. The major metabolite
(accounting for 43.4% of the recovered radioactivity) was identified
as BF 421-3; the unidentified fractions accounted for 2-20%. BF
421-1, 2 and 3 were identified in faeces, BF 421-2 accounting for
53-60% of the radioactivity. All of the other metabolite fractions
accounted for 3-12%. BF 421-2 and 3 were detected in the
protein-free plasma fraction, accounting for 58 and 3% of the total
radiolabel in plasma. Two unknown fractions accounted for 2 and 5%.
Six (phenyl) and five (morpholine) metabolite fractions were
detected in the whey of the milk of both goats, accounting for 76
and 66% of the radioactivity recovered in milk. BF 421-2 and 3 were
identified in the milk of the goat treated with the
morpholine-labelled compound, accounting for 17 and 7%,
respectively. Several metabolites were also identified in tissues
and organs: The main metabolite found in liver and muscle was BF
421-2, and several of its conjugates were found in liver. BF 421-2
and 3 were identified in kidney and fat, and BF 421-1 was detected
in fat. The parent compound was not found in urine, faeces, milk,
plasma or any tissues or organs (Ritter & Vogel, 1989).
In the study of Ritter (1989) in hens, described above, the
extractability of the radioactivity in excreta and tissues and
organs differed according to whether the phenyl or morpholine label
had been administered. Binding of radioactivity to the whites of
eggs from hens was 20% with the phenyl label and 78% with the
morpholine label. In yolk, most of the total radioactive residues
were not associated with proteins (63% for phenyl label and 67% for
morpholine label). In plasma, a slightly higher percentage of
radiolabel was associated with protein in birds treated with the
morpholine label (17%) than in those given the phenyl label (7%).
Less radiolabel could be extracted from organs and tissues of birds
treated with the morpholine label than from those given the phenyl
label, the unextractable portions being 24% (morpholine) and 13%
(phenyl) in liver, 30 and 13% in kidney, 33 and 3% in muscle, 31 and
12% in gizzard, 3 and 1% in fat and 8 and 4% in skin. Various
metabolite fractions were found in organs and tissues, ranging from
three in fat to 10 in liver. The pattern of metabolites was
comparable with the two labels. Identification of the metabolites
was hindered by the presence of very high amounts of fat in the
extracts, owing to the lipophilicity of the test compound and of
most of its metabolites. In the hens treated with the phenyl label,
parent compound was detected in kidney (11%), the metabolite BF
421-2 in plasma and kidney and BF 421-3 in kidney. In the hens
treated with the morpholine label, BF 421-1 was found in plasma and
BF 421-2 in plasma and liver (Ritter, 1989).
The major metabolic pathways of fenpropimorph are outlined in
Figure 1.
 2. Toxicological studies
(a) Acute toxicity
The results of tests for the acute toxicity in rats of
fenpropimorph administered by various routes are summarized in Table
1. Fenpropimorph is slightly toxic after dermal application and
slightly to moderately toxic after oral administration or
inhalation. Common signs of toxicity included sedation, lethargy,
dyspnoea and ataxia, which often intensified to coma. Signs of local
irritation were observed after oral administration (irritation of
stomach and intestines) and intraperitoneal treatment (irritation of
peritoneum).
Table 1. Acute toxicity of fenpropimorph in rats
Strain Sex Route LD50 (mg/kg bw) Purity Reference
or LC50 (mg/m3) (%)
Outbred albino M Oral 2090 98.5 Camponovo, 1983
F 1467
Sprague-Dawley M&F Oral 3515 NR Leuschner, 1978a
Sprague-Dawley M&F Dermal 4291a NR Leuschner, 1978b
Sprague-Dawley M&F Intraperitoneal 2465 NR Leuschner, 1978c
Sprague-Dawley CD M Inhalationb > 8500 NR Jackson & Clark, 1980
F 5200 NR
Wistar M&F Inhalationc > 3580d 98.5 Keller, 1983
NR, not reported
a Local effects were slight erythema during the first few days, exsiccation of the
skin, formation of rhagades and loss of hair. Before death or the end of the 14 days
of observation, partial growth of hair was seen, but there was no healing of
exsiccation or rhagades.
b Nose only; 85-90% of particles were less than 5 µm.
c Nose only; 23% of particles were less than 7 µm.
d No mortality; slight dyspnoea and ruffled fur were observed during and shortly
after exposure.
(b) Short-term toxicity
Rats
Groups of 20 male and 20 female Sprague-Dawley rats were given
diets containing 0, 100, 250, 625 or 1600 ppm fenpropimorph (purity,
99.1%), equivalent to 0, 10, 25, 62.5 or 160 mg/kg bw, for four
weeks. After that time, 10 male and 10 female animals from each
group were kept on normal diet for a two-week recovery period. No
effects were observed on mortality or on urinary parameters.
Deteriorated general condition, ruffled fur and reddening of the
upper and lower lips were recorded in animals at 1600 ppm.
Significantly reduced food consumption and body weight were found in
males and females at 1600 ppm and in females at 625 ppm. The
haemoglobin concentration was significantly reduced in all groups.
Significant, dose-related reductions in the concentrations of
triglycerides, calcium, glucose and total proteins were found in
males in all groups; in females, the total protein concentration was
reduced in animals at doses > 625 ppm. A significant,
dose-related decrease in cholesterol level and a significant,
dose-related increase in alanine aminotransferase level were
observed in males at doses > 625 ppm and in females at doses >
1600 ppm. The bilirubin concentration was increased in males at
doses > 250 ppm and in females at > 625 ppm. In all dose
groups, significant, dose-related increases in relative and absolute
liver weights were seen in animals of each sex, with no lesions. In
males, absolute and relative thyroid weights were increased at doses
> 250 ppm. At 1600 ppm, mucosal hyperkeratosis of the oesophagus
and oedema of the forestomach were found. Almost all of the
parameters returned to control levels after the recovery period,
except that the liver weights of females in the highest dose group
remained high (Kirsch et al., 1980a).
Four groups of 30 male and 30 female Sprague-Dawley rats were
exposed to fenpropimorph (purity, 91.1%) at doses of 0, 6.25, 12.5
or 25 ppm for three months (equal to 0, 0.38, 0.77 or 1.54 mg/kg bw
per day for males and 0, 0.46, 0.92 or 1.78 mg/kg bw per day for
females). Ten male and 10 female animals from each group were then
allowed to recover for six weeks. Clinical signs, food consumption
and body weight were not affected, and haematology, urinalysis and
histopathology showed no effect. Two females--one control and one at
25 ppm--died during the experiment. Plasma cholinesterase levels
were significantly decreased at 12.5 and 25 ppm in animals of each
sex, but there was no clear relationship with dose: the middle-dose
group had decreases of 75-89% of the control value, and the
high-dose group had decreases of 72-94%. Erythrocyte cholinesterase
levels were significantly decreased in females in all dose groups.
As there were large variations within the groups (owing partly to
methodological problems), no clear dose-response relationship and no
clear effects in males, the relevance of this finding is unclear.
Brain cholinesterase levels were not determined. The level of
triglycerides was increased in males, and total protein level was
increased in both males and females at 25 ppm. Relative liver
weights were significantly increased at doses > 12.5 ppm in both
males and females; absolute liver weights were also increased in
females at the high dose. In males, the relative weights of the
thyroid and adrenal glands were increased at 25 ppm. After recovery,
all values returned to control levels. The NOAEL was 6.25 ppm, equal
to 0.38 mg/kg bw per day (Kirsch et al., 1979).
Groups of 10 male and 10 female Sprague-Dawley rats were
exposed to fenpropimorph (purity, 90.3%; dissolved in 78% ethanol
and deionized water, with 50% of particles with a median aerodynamic
diameter of < 1.2 µm) at concentrations of 10, 40 or 160 mg/m3
air for 6 h/day, five days/week for four weeks. One control group
was exposed to fresh air and the other to the solvent. No effects
were seen on body weight, mortality or haematological parameters.
With increasing dose levels, crusts were observed around the nose,
which increased in incidence (0 in controls, 3 at 10 mg/m3, 13 at
40 mg/m3 and 20 at 160 mg/m3) and severity. In all groups, the
plasma cholinesterase concentration was decreased (by up to 19% in
males and 55% in females at the highest dose); no effects were found
on brain or erythrocyte cholinesterase levels. The activities of
alkaline phosphatase and alanine aminotransferase were increased at
doses > 40 mg/m3 in animals of each sex, and the level of
aspartate aminotransferase was increased in males given the highest
dose. Cholesterol levels were decreased in males and females in the
highest dose group. Absolute and relative liver weights were
increased in females at 40 and 160 mg/m3, and the relative liver
weight was increased in males in the highest dose group. Clear
effects of the substance were observed on histopathological
examination of the skin and respiratory tract. Focal skin lesions
(inflammation, hyperkeratosis) were found around the opening of the
nose, and increased incidences of focal basal-cell hyperplasia were
observed in the trachea, the bifurcation and the mainstream bronchi
at doses > 40 mg/m3 in animals of each sex. The
no-observed-adverse-effect concentration for systemic and irritating
effects was 10 mg/m3 (Klimisch et al., 1981).
In a limited experiment, groups of two male and two female
Sprague-Dawley rats received repeated dermal applications of
fenpropimorph (purity, 91%; dissolved in 1% aqueous methylcellulose)
on 3 x 4-cm areas of the clipped, abraded dorsal skin at
concentrations of 5, 10, 30 or 60% w/v (equal to 91, 182, 546 or
1092 mg/kg bw per day), which were maintained under an occlusive
dressing for 6 h/day for 21 consecutive days. After each
application, the skin was washed with 75% ethanol and dried. Skin
lesions were recorded in all animals, which increased with the dose
from slight to severe. The treatment was terminated at day 14
because of the severity of the skin lesions. Females treated with
60% w/v had hunched posture, lethargy and decreased respiratory
rate. All animals (except males at 5% w/v) showed body-weight loss.
Subcutaneous bruising and pitting and hyperaemia of subcutaneous
blood vessels were found in females at 30 or 60% w/v and in males at
60% w/v (Elliot & Mallard, 1981).
Rabbits
In range-finding studies, 54 New Zealand white rabbits received
repeated dermal doses of the active ingredient (purity, 91%;
dissolved in distilled water) or of a 750 EC formulation (BAS
42100F, emulsifiable concentrate containing 750 g/l fenpropimorph)
for 6 h/day for 21 days at various doses, on intact or abraded skin
and with different decontaminants. Application of the active
ingredient at doses of 11.4-1365 mg/kg bw (0.5-60% w/v) in 1%
aqueous methylcellulose produced severe dermal reactions. Time of
onset and severity varied with the dose. Treatment with doses >
34 mg/kg bw had to be terminated after 4-12 days. Generally, rabbits
treated with the formulation had less skin irritation than those
treated with the active ingredient, and the onset of reactions was
delayed (Elliot & Mallard, 1981).
Groups of 10 male and 10 female New Zealand white rabbits
received the 750 EC formulation (suspended in distilled water) at
concentrations of 0, 0.05, 0.15 or 0.5% w/v (equal to 0, 0.85, 2.6
or 8.5 mg/kg bw per dayof the active ingredient) for 6 h/day, on
five days/week for three weeks. No dose-related effects were
observed on macroscopy or haematological examination, and no changes
were seen in plasma, erythrocyte or brain cholinesterase levels or
organ weights. At the highest dose, food consumption and body
weights of females were decreased, but these signs were probably
related to the occurrence of enteritis in four animals in this
group. A dose-related increase in the incidence and severity of
dermal reactions was observed in all treated males and in females at
doses > 2.6 mg/kg bw per day. Slight erythema was seen in vehicle
controls. During the study, six rabbits died and six were killed in
extremis because of enteritis. Histological examination revealed
epidermal hyperplasia, hyperkeratinization and increased
inflammatory cell infiltration. Incidental findings of focal
parakeratosis, scab formation, epidermal ulceration and vascular
congestion of the dermis were described in animals at the two
highest doses. No systemic effects were observed. Irritation was
observed in all treated males and in females at concentrations >
0.15% w/v (Elliot et al., 1981).
Dogs
Groups of four male and four female beagle dogs were given
diets containing 0, 50, 100, 200 or 400 ppm fenpropimorph (purity,
91.1%) for 13 weeks (equivalent to 0, 1.25, 2.5, 5 or 10 mg/kg bw).
No effects were observed on clinical parameters, food consumption,
body weight, mortality or plasma cholinesterase level, and
haematological and urine analyses and histopathological examination
also showed no effects. In the group fed 400 ppm, the levels of
alkaline phosphatase and aspartate aminotransferase were increased
in males, and the level of alanine aminotransferase was increased in
both males and females. The absolute and relative weights of the
pituitary gland and the relative weight of the adrenal gland were
reduced in females at 400 ppm. The NOAEL was 200 ppm, equivalent to
5 mg/kg bw per day (Kirsch et al., 1980b).
Groups of four male and four female beagle dogs were given
diets containing 25, 100 or 400 ppm fenpropimorph (purity, >
94.7%) for 12 months (equal to a daily intake of 0.8, 3.2 or 12.3
mg/kg bw per day for males, and 0.8, 3.2 or 13.2 mg/kg bw per day
for females). Six males and six females were used as controls. No
effects were observed on clinical signs, food consumption, body
weight, mortality or plasma, erythrocyte or brain cholinesterase
levels; haematological, ophthalmological, urinary and
histopathological examinations also showed no effect. At the highest
dose, the level of alkaline phosphatase was increased in males and
that of alanine aminotransferase in females. The relative weights of
kidneys and adrenal glands were increased in females. The NOAEL was
100 ppm, equal to 3.2 mg/kg bw per day (Hellwig et al., 1990).
(c) Long-term toxicity and carcinogenicity
Mice
In a study of carcinogenicity, fenpropimorph (purity, 92.5%)
was administered in the diet to groups of 60 male and 60 female
Charles River CD-1 mice at doses of 0, 5, 30, 150 or 1000 ppm (equal
to 0.5, 3.0, 16 or 106 mg/kg bw per day in males and 0.5, 3.5, 17 or
118 mg/kg bw per day in females). Treatment was suspended after 95
weeks, when the survival rates in the various groups ranged from 44
to 68%. Ten animals of each sex per group were sacrificed after 52
and 95 weeks; the remaining mice were kept on normal diet until
terminal sacrifice at weeks 103 (males) and 104 (females). The
withdrawal period was introduced in order to investigate the
reversibility of the inhibition of erythrocyte cholinesterase.
No dose-related effects were observed on clinical signs,
mortality, food consumption or efficiency of food use. Body-weight
gain was decreased in males at the highest dose, but no significant
decrease was observed in females. The haemoglobin concentration was
decreased in males at the highest dose (significant at 95 weeks,
tendency at 51 weeks). The erythrocyte cholinesterase level was
decreased in females, by 26% at 150 ppm and by 29% at 1000 ppm;
plasma and brain cholinesterase levels were not affected (decreased
brain cholinesterase was observed in all males at 53 weeks but not
after 96 weeks). Relative liver weights were increased in males at
1000 ppm at 52 weeks, and relative liver and kidney weights were
increased in females at the highest dose after 95 weeks. Amyloid
deposits were increased in adrenal glands, kidneys and thyroid
glands of animals of each sex at the highest dose. Tumour incidences
were not enhanced. At the end of the withdrawal period, erythrocyte
cholinesterase levels and organ weights were similar to control
values. No effects were observed at 30 ppm, equal to 3 mg/kg bw per
day (Hunter et al., 1982a).
Rats
In a study of chronic toxicity and carcinogenicity, groups of
75 male and 75 female Charles River CD rats were exposed to
fenpropimorph (purity, 92.5%) in the diet at doses of 0, 5, 10, 50
or 250 ppm (equal to 0.2, 0.3, 1.7 or 8.8 mg/kg bw per day for males
and 0.2, 0.4, 2.1 or 11.2 mg/kg bw per day for females) for 104-115
weeks. Of the 60 animals of each sex in the main group, 10 were
killed at 52 weeks; the rest were maintained for evaluation of
carcinogenic effects and were killed at week 107 (females) or 115
(males). Groups of 15 rats of each sex were examined by haematology,
blood chemistry and urinalysis.
No dose-related effects were observed on clinical signs,
mortality or water consumption, and ophthalmoscopy, haematology,
blood chemistry, urinalysis and macroscopy showed no changes; there
were no neoplastic findings. Females at the highest dose had
decreased food consumption throughout the experiment. Food
conversion efficiency was decreased in animals of each sex at the
highest dose, and body-weight gain was lower in those at 50 and 250
ppm (although not significant in males at 50 ppm). The plasma
cholinesterase activity was decreased (by up to 29% in males and 41%
in females) at 250 ppm and in females also at 50 ppm. At interim
sacrifice, a significant but slight decrease (12%) in brain
cholinesterase activity was observed in females at the high dose. At
the end of the study, a small, dose-related decrease in brain
cholinesterase activity was observed in males at all doses: by 23%
at 5 ppm, 24% at 10 ppm, 30% at 50 ppm and 40% at 250 ppm; in
females, slight decreases were observed at doses of 10 ppm and
above: by 9% (not significant) at 10 ppm, 13% at 50 ppm and 12% at
250 ppm. At termination, however, the brain cholinesterase activity
in the control animals was 21-23% lower than at interim sacrifice.
No effects were observed on erythrocyte cholinesterase activities.
Relative liver weights were increased throughout the study in males
at > 50 ppm, and enlargement of hepatocytes was seen in animals
of each sex at that dose. Multinucleated cells (in males and
females), cellular pleomorphism (in males) and increased numbers of
foci of altered hepatocytes were found at the highest dose. No
tumorigenic effects were observed. As the effects on brain
cholinesterase in males were seen only at the end of the study and
not at interim sacrifice, there was no clear dose-response
relationship at the two lower doses and, remarkably, erythrocyte
cholinesterase activities were not affected at any time (the
decrease in males at the two lowest doses was considered not to be
toxicologically relevant). The NOAEL was 10 ppm, equal to 0.3 mg/kg
bw per day (Hunter et al., 1982b).
(d) Reproductive toxicity
Rats
In a two-generation study of reproductive toxicity,
fenpropimorph (purity, 92.5%) was administered to groups of 12 male
and 24 female Sprague-Dawley rats at dietary doses of 0, 6.25, 12.5
or 25 ppm, equivalent to 0.31, 0.62 or 1.25 mg/kg bw per day. At
least 100 days after the beginning of treatment, F0 animals were
mated in order to produce an F1 litter. On day 21 after birth, 12
males and 24 females were selected as the F1 parents and were kept
on the diet for at least 120 days before they were mated. The study
was terminated after weaning of the F2 litter. Pups were observed
for the usual parameters and also for physiological development
(erection of the auricles, eruption of incisors, development of fur,
opening of the auditory canal and of the eyes) and behavioural
effects (gripping reflex, pupillary reflex, hearing test). In
females of the F0 generation at the highest dose, a slight but
significant increase in food consumption was seen during pregnancy
and in body-weight gain during the entire study period. The
percentage of stillborn pups was slightly but significantly
increased at 25 ppm, with incidences of 1.1% in controls, 4.3% at
6.25 ppm, 2.7% at 12.5 ppm and 6.2% at 25 ppm. A slight but
significant decrease in relative heart weight was observed in males
at 25 ppm. F1 pups at the highest dose showed decreased body
weight from day 14 after birth (not significant in males) and
slightly retarded unfolding of the auricle. Increased relative
weights of the kidney (males) and brain (both sexes) were observed
at 25 ppm. At 25 ppm, F1 male parents had increased relative liver
weights, and females had decreased relative brain weights. In F2
pups, development of fur and opening of the eyes were slightly
retarded at 25 ppm, and one female pup at this dose had
microphthalmia, which was not considered to be related to treatment.
The effects observed at the high dose were considered to be
marginal. The NOAEL was 12.5 ppm, equivalent to 0.62 mg/kg bw per
day (Merkle et al., 1982).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 18 pregnant Sprague-Dawley rats were given
fenpropimorph (purity, 92.5%; dissolved in olive oil) by gavage at
doses of 0, 2.5, 10, 40 or 160 mg/kg bw per day from day 15 of
gestation until day 21 of lactation. The dams were examined for
clinical symptoms, deaths, body weight, litter size, delivery
behaviour, numbers of viable and dead fetuses, implantation rate,
macroscopic appearance and organ weights. Pups were observed for
body weight, sex, clinical signs, physiological development, deaths,
behaviour, viability, macroscopic appearance, organ weights and
skeletal anomalies. At 160 mg/kg bw per day, diarrhoea, salivation,
trembling, reddening of the urogenital area, vaginal bleeding and a
penetrating odour of urine were observed; four of the dams died
during the treatment. Decreased body-weight gain was observed in
dams at doses > 40 mg/kg bw per day. At 160 mg/kg bw per day,
relative heart and kidney weights were decreased, care of pups was
unsatisfactory, and the mean number of viable fetuses was decreased
(1.7 versus 11.7 in the control group), in association with a
considerable increase in the number of dead fetuses. At 160 mg/kg bw
per day, the body weights and weight gain of the pups were reduced,
and erection of the auricle, development of fur and opening of the
eyes and auditory canal were delayed. Increased pup mortality was
observed at doses > 40 mg/kg bw per day, by 25.4% at 40 mg/kg bw
per day and 50.0% at 160 mg/kg bw per day, as compared with 16.3% in
controls. The gripping reflex was decreased in female pups at doses
> 40 mg/kg bw per day. At the highest dose, relative heart and
spleen weights were decreased and relative weights of the liver and
kidney were increased in male pups. In female pups at the highest
dose, the relative weights of the heart, spleen and liver were
increased and the relative kidney weight was decreased. Because few
pups at this dose survived, however, the effects on organ weights
could not be assessed properly. The NOAEL for maternal and
developmental toxicity was 10 mg/kg bw per day (Hofmann & Merkle,
1979a).
Groups of 26-31 pregnant Sprague-Dawley rats were given
fenpropimorph (purity, 92.5%; dissolved in olive oil) by gavage at
doses of 0, 2.5, 10, 40 or 160 mg/kg bw per day on days 6-15 of
gestation. Dams were observed for clinical symptoms, body weight,
mortality, macroscopic appearance of internal organs, conception
rate and numbers of corpora lutea, viable implantations and dead
implantations (early, intermediate and late resorptions). Fetuses
were examined for weight, length, placental weight and external,
skeletal and visceral signs. Vaginal bleeding was observed in one
control, one animal at 10 mg/kg bw per day, three at 40 mg/kg bw per
day and 16 at 160 mg/kg bw per day. A dose-related reduction in
body-weight gain was observed in dams at doses > 40 mg/kg bw per
day during the treatment period and at 160 mg/kg bw per day during
the whole observation period, starting from day 6. At 160 mg/kg bw
per day, the body weights of the dams were reduced and the number of
viable fetuses was decreased, in association with an increased
number of dead implants. The weight and length of the fetuses were
reduced, and placental weight was increased. Irreversible structural
changes, including cleft palate (14/274) and inferior brachygnathia
(1/274), were observed at the highest dose. The NOAELs in this study
were 10 mg/kg bw per day for maternal toxicity and 40 mg/kg bw per
day for embryo- and fetotoxicity and teratogenicity (Hofmann &
Merkle, 1979b).
Rabbits
Groups of 15 female Himalayan rabbits were given fenpropimorph
(purity, 92.5%) by gavage at doses of 0, 2.4, 12.0, 36 or 60 mg/kg
bw per day in 0.5% carboxymethylcellulose (5 ml) during days 6-18 of
gestation. No dose-related effects on the number of corpora lutea,
preimplantation loss or conception rate were observed in dams, and
no effects were seen in fetuses on visceral examination. Diarrhoea
was seen in all groups, which increased in incidence and severity
with the dose. At 60 mg/kg bw per day, severe diarrhoea, salivation,
apathy, a greenish mucous discharge from the nose and encrustations
in the vaginal region and snout were seen, and convulsions were
observed before the deaths of 11 animals. Food consumption and body
weight were reduced. Macroscopic examination of the animals that
died at this dose showed dilatation of the right heart and
congestive hyperaemia; the clinical findings were confirmed.
Absolute uterine weights were reduced at 60 mg/kg bw per day, and
the numbers of early resorptions and dead fetuses were increased
such that only one fetus survived. This fetus had several
abnormalities, including syndactyly on the forelegs, an anomalous
position of the hindlegs and micromelia, and reduced weight and
length; furthermore, the placental weight was increased, and the
individual sternebrae were fused. At 36 mg/kg bw per day, the dams
had clinical signs that were less severe and occurred at a lower
incidence than in the highest dose group. Two animals aborted and
three were killed in extremis. The numbers of dead implantations
(due mainly to early resorptions) were slightly increased at this
dose. Six fetuses had pseudoankylosis. The NOAEL for maternal,
embryo- and fetotoxicity was 12 mg/kg bw per day (Zeller & Merkle,
1980).
In another study, groups of 20 pregnant Russian Chbb:HM rabbits
were gavaged intragastrically with fenpropimorph (purity, 95.6%; in
0.5% aqueous sodium carboxymethylcellulose) at doses of 0, 7.5, 15
or 30 mg/kg bw per day on days 7-19 of gestation. No effects were
observed on mortality, numbers of corpora lutea or live or dead
fetuses, sex ratio, numbers of early or late resorptions or total
postimplantation loss. Treatment-related effects were found only at
30 mg/kg bw per day. Swelling of the anus was observed in dams; food
consumption, body-weight gain, uterine weight and weights of male
fetuses were decreased. Fetuses had an increased total number of
malformations (21/116 in four litters) and anomalies (36/116 in 13
litters). The malformations occurred mainly in the litters of three
dams which showed marked signs of toxicity during treatment. Twenty
of 116 fetuses in three litters had shortened fore- and hindlimbs,
and 4/116 fetuses in two litters had a cleft palate. One of these
fetuses also had exencephaly and open eye, and another had gastro-
and cranioschisis, an asymmetric skull and oedema of the trunk. One
fetus in another litter had a diaphragmatic hernia. Furthermore,
abnormal positions of forelimbs were observed in 25/116 fetuses (in
seven litters) and of the hindlimbs in 8/116 fetuses (in three
litters). The external findings were confirmed by skeletal
examination. The NOAEL was 15 mg/kg bw per day for maternal
toxicity, embryotoxicity, fetotoxicity and teratogenicity (Marty,
1993).
(f) Genotoxicity
The results of tests for the genotoxicity of fenpropimorph are
summarized in Table 2.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
The iritating properties of fenpropimorph to the skin were
examined in five studies in rabbits. Three male and three female New
Zealand white rabbits were administered 0.5 ml of technical-grade
fenpropimorph (purity not specified) on intact and abraded skin for
24 h. Slight erythema was observed in all rabbits directly after
exposure. This reaction increased to well-defined erythema in one
animal after 48 h and in two others at 96 h. Very slight oedema was
observed in one animal after 48 h and in four animals after 96 h. No
signs of irritation were visible after 14 days. The irritating
response of abraded skin was comparable (Leuschner, 1979d).
Three female and one male New Zealand white rabbits were
administered 0.5 ml of technical-grade fenpropimorph (purity not
specified) on intact skin for 4 h and observed at 4 h and after 1,
2, 7 and 14 days. Slight erythema was observed in all rabbits after
one day, and this reaction increased to well-defined erythema in
three rabbits by day 7. Very slight oedema was observed in two
animals at that time. Signs of irritation were no longer visible
after 14 days (Leuschner, 1980).
Six rabbits (sex and strain not specified) were administered
0.5 ml fenpropimorph (purity not specified) on intact and abraded
skin for 24 h and observed at 24 and 72 h and after eight days.
Well-defined erythema and slight oedema were observed in all rabbits
directly after exposure; this reaction increased in severity, and
five of six animals showed necrosis after eight days. The irritating
response of abraded skin was comparable (Liggett & Wilson, 1980a).
Six rabbits (sex and strain not specified) were administered
0.5 ml fenpropimorph (purity not specified) on intact skin for 4 h
and observed at 4, 24, 48 and 72 h and after eight days.
Well-defined erythema and slight oedema were observed in all rabbits
directly after exposure, which persisted until the end of the study.
Skin necrosis was seen in one animal at 48 h, and necrosis or deep
injuries were observed in three animals on day 8 (Liggett & Wilson,
1980b).
Table 2. Results of tests for the genotoxicity of fenpropimorph
End-point Test system Concentration Purity Results Reference
of fenpropimorph (%)
In vitro
Reverse S. typhimurium TA98, 4-2500 µg/plate in 91 Negativea Zeller & Engelhardt,
mutation 100, 1535, 1537, 1538 ethanol; no toxicity 1980
Mitotic gene S. cerevisiae 0.4, 2 or 10 mg/ml 92.5 Negativea Bootman & Lodge, 1981
conversion, D7 in ethanol
reciprocal
crossing over,
mutation,
aneuploidy
Chromosomal Human 0.16-1.66 µg/ml 92.5 Negativea Mosesso & Nunziata,
aberration lymphocytes 5-50 µg/ml Negativeb 1982
Unscheduled Rat hepatocytes 0.1-10 µg/ml in 94.7 Negative Cifone, 1988
DNA ethanol; toxic
synthesis from 5 µg/ml
In vivo
Micronucleus Male Swiss mice 0, 1 or 4 ml/kg bw 92.5 Negative Siou & Conau, 1979d
formation in two oral
administrations
Dominant lethal Male NMRI 0, 212.5 or 425 mg/kg bw; 92.5 Negative Engelhardt & Zeller,
mutation mice single intraperitoneal 1979
injection; toxic at 425
mg/kg bw
a In the absence of metabolic activation
h In the presence of metabolic activation
A dose of 0.5 ml of fenpropimorph (purity, > 91%) was applied
to the intact dorsal skin of four white Vienna rabbits for 3 min or
4 h, and animals were observed up to day 8 after treatment.
Fenpropimorph caused slight to moderate erythema and oedema after
either application. The reactions intensified, and two of four
rabbits had necrosis eight days after the 4-h exposure. In the 3-min
test, animals had necrotic-like skin changes and/or scaling
(Grundler, 1980a).
Fenpropimorph was considered severely irritating and corrosive
to rabbit skin.
The irritancy of fenpropimorph to the eye was examined in three
white Vienna rabbits in a study in which 0.1 ml of fenpropimorph
(purity, > 91%) was instilled into the conjunctival sac of the
right eye. Observations were made up to day 8 after instillation.
Slight to distinct conjunctival redness was observed in all rabbits.
Fenpropimorph was considered to be irritating to the rabbit eye,
since slight irritation was still seen at the end of the study
(Grundler, 1980b).
The sensitizing properties of the compound were examined in two
studies in guinea-pigs. Fenpropimorph (purity not specified) was
applied to the shaved skin of 20 guinea-pigs five days per week for
three weeks, and the application site was left uncovered. Challenge
applications were given on days 21 and 35 of the study. A control
group of 10 animals received the challenge doses only. Slight skin
irritation was observed in 9/10 control animals and 8/20 test
animals. Since there was no difference in the frequency or intensity
of the skin reaction after challenge, fenpropimorph was considered
not to be sensitizing (Klecak et al., 1983).
A maximization test was performed in 12 male Pirbright white
guinea-pigs. During the induction period, the animals were injected
intradermally with 25% fenpropimorph (purity not specified). Five
animals served as a control group. One week later, 50% fenpropimorph
was applied topically, and a challenge application of 25%
fenpropimorph was given 14 days later. The substance did not
sensitize the skin (Gelbke, 1979).
(ii) Delayed neuropathy
The delayed neurotoxicity of fenpropimorph was investigated in
hens using doses based on the results of a preliminary test for the
LD50. Groups of 10 adult hens (20 at the highest dose) were
treated with fenpropimorph (purity, 92.5%; dissolved in olive oil)
in single oral doses of 0, 425, 850 or 1700 mg/kg bw by gavage and
then observed for 21 days. Treated birds were pretreated with
2-pyridine aldoxime methyl chloride and atropine. One group of
controls received only olive oil; a positive control group was
treated with tri- ortho-cresyl phosphate at 500 mg/kg bw in corn
oil. The birds were examined for general health, body weight, food
consumption, mortality and ataxia; after being killed, they
underwent a macroscopic examination and histopathological
observation of the spinal cord and sciatic nerve. Decreased body
weight and reduced food consumption were observed at the two highest
doses during the first three days, and symptoms of weakness and
unsteadiness were observed in all birds at 1700 mg/kg bw and in some
at 850 mg/kg bw. Two of 10 birds at 850 mg/kg bw and 8/20 at 1700
mg/kg bw died. No signs of delayed neurotoxicity were observed in
treated birds (Roberts et al., 1980).
(iii) Liver function and cholinesterase inhibition
In order to study the effects of fenpropimorph on liver
function and cholinesterase inhibition, groups of 40-100 female
Sprague-Dawley rats received a single oral dose of 0, 420, 1240 or
2290 mg/kg bw. Groups of 10 animals were killed after 1, 3, 8 and 15
days, and blood samples were taken. Clinical chemistry included
blood gas analysis and estimation of plasma, erythrocyte and brain
cholinesterase activity; the liver and brain were weighed, and
liver, brain, pancreas and affected organs were examined macro- and
microscopically. The incidence and severity of toxic signs increased
with the dose, and 13 of 100 animals at the highest dose died. Mean
body-weight gain was reduced at the middle and high doses by days 3
and 8 but had returned to normal by day 15. Reduced concentrations
of triglyceride and cholesterol were observed for the first three
days in all treated groups and up to day 8 at the highest dose. The
effect on cholesterol was overcompensated (increased) in the
middle-dose group at day 8 and in the high-dose group at day 15. The
bilirubin level was increased up to day 8 at the middle and high
doses. Alanine aminotransferase activity was increased in the
highest-dose group by day 8 and that of aspartate aminotransferase
by day 3. A reduction in plasma cholinesterase (by up to 51% of the
control value at the high dose) was observed in all treated groups
on days 8 and 15. Erythrocyte cholinesterase activity was not
affected. A slight but significant decrease in brain cholinesterase
activity (by up to 79% of control value at the high dose) was
observed after one, three and eight days at the middle and high
doses but had returned to control levels by the end of the study.
The relative liver weights were increased in all treated animals
from day 3 onwards, and the relative brain weight was increased at
the high dose by days 3 and 8. The effects on organ weights were not
accompanied by histological anomalies. At the end of the study, the
animals given the highest dose had focal skin changes (scaling,
alopecia areata) (Jäckh et al., 1980).
The effect of fenpropimorph (purity not specified) on hepatic
drug-metabolizing enzymes was investigated in groups of 16 male
Spraque-Dawley rats by giving them dietary doses of 0, 50, 250 or
1600 ppm (equivalent to 0, 2.5, 12.5 or 80 mg/kg bw per day) for 14
days. A positive control group was treated with phenobarbital at 50
mg/kg bw per day by gastric gavage. Fenpropimorph induced aniline
hydroxylase, glucuronyl transferase and glutathione S-transferase at
doses > 250 ppm; ethylmorphine N-demethylase was induced at 50
ppm. Phenobarbital sleeping time was reduced at doses > 50 ppm.
The effects were generally of similar magnitude as those in rats
treated with 50 mg/kg bw per day phenobarbital (Hawkins et al.,
1981).
The effect on plasma cholinesterase of a single administration
of fenpropimorph (purity 99.1%) was examined in groups of 20 (40 at
the highest dose) female Sprague-Dawley rats. The compound was
administered at 0, 200, 650 or 2000 mg/kg bw in a 0.5% aqueous
solution of carboxymethylcellulose by a single intraperitoneal
injection; animals were then observed for 72 h, and blood was
sampled at 2, 6, 24 and 72 h. As rats at the highest dose were
comatous, the final samples had to be taken at sacrifice after 30 or
50 h. Blood samples taken after 2 and 6 h showed increased aspartate
aminotransferase activity and bilirubin concentrations in all
treated groups. Plasma cholinesterase was unaffected after 2, 6 and
24 h; after 72 h, dose-related reductions were seen at 200 mg/kg bw
(53% reduction) and at 650 mg/kg bw (64% reduction). Absolute liver
weights were increased at doses > 200 mg/kg bw; and at doses >
650 mg/kg bw peritonitis, perihepatitis and fatty deposits in the
liver were found (Kirsch et al., 1980c).
The effect of fenpropimorph metabolites on cholinesterase
inhibition was studied in vitro. The activity of
acetylcholinesterase from Electrophorus electricus and of plasma
cholinesterase from rats and dogs was decreased by compounds with a
ß-ethanolamine structure, which are structural analogues of a
metabolite of fenpropimorph, N-[3-[4-(1,1-dimethyl-
2-carboxyethyl)phenyl]-2-methyl]propylethanolammonium ion (BF
421-4). The compound tested, which is closely related to
fenpropimorph, showed no inhibitory activity. On the basis of the
results of this study, the authors concluded that fenpropimorph
itself is not directly responsible for the inhibitory effect on
cholinesterase and that the metabolite is responsible for the
effects seen in rats in vivo (Deckardt & Hildebrand, 1980).
3. Observations in humans
Between 1984 and 1991, five cases of dermal irritation of
various grades and one case of Quincke's oedema after eye contact
were recorded at BASF AG, Ludwigshafen, Germany. Two incidents in
which a fenpropimorph formulation was ingested by children aged two
and five passed with no sequelae (Adolph, 1992).
Comments
After oral administration to rats, goats and chickens,
fenpropimorph was rapidly absorbed, distributed and excreted. In
rats and goats, enterohepatic circulation plays an important role.
The half-life in plasma and blood varied from 16 to 24 h. In all
three species, the levels of residues in tissues were relatively
low. No accumulation was detected in organs or tissues; only minor
amounts appeared in milk and eggs. The excretion pattern (rate and
route) was not significantly influenced by the administration route,
number of exposures, species, sex or dose.
Fenpropimorph was extensively metabolized in all three species
studied (rat, goat and chickens), and a small fraction of the parent
compound was found only in chickens. The first metabolic steps
involve the oxidation of the side-chains of the phenyl and the
morpholine rings. Further metabolic steps occur, as indicated by the
detection of many (mostly unidentified) metabolites. In addition,
after administration of the morpholine-labelled molecule to rats,
14CO2 was expired, indicating degradation of the morpholine ring.
Fenpropimorph is slightly to moderately toxic to rats after
acute oral exposure. WHO (1992) has classified fenpropimorph as
unlikely to present an acute hazard in normal use.
In a four-week study of toxicity, rats were given dietary doses
of 0, 100, 250, 625 or 1600 ppm fenpropimorph. Increased liver
weights and reduced haemoglobin were observed in all dose groups. No
NOAEL could be defined. In a three-month study of toxicity, rats
were exposed to fenpropimorph in the diet at levels of 0, 6.25, 12.5
or 25 ppm. On the basis of increased liver weights at doses >
12.5 ppm, the NOAEL was 6.25 ppm, equal to 0.38 mg/kg bw per day.
Dogs were fed diets containing 0, 50, 100, 200 or 400 ppm
fenpropimorph for 13 weeks, or 25, 100 or 400 ppm for 12 months. At
the highest dose, the serum activities of alkaline phosphatase and
both aspartate and alanine aminotransferase were increased and some
slight effects on organ weights were seen. The NOAEL was 200 ppm,
equivalent to 5 mg/kg bw per day, in the 13-week study and 100 ppm,
equal to 3.2 mg/kg bw per day, in the 12-month study.
In a study of carcinogenicity, fenpropimorph was administered
in the diet to mice at doses of 0, 5, 30, 150 or 1000 ppm for 95
weeks (followed by a recovery period). At the highest level, the
main effects were decreased body-weight gain, decreased haemoglobin
in males and increased relative liver weights. In females,
erythrocyte cholinesterase activity was decreased by 26% at 150 ppm
and 29% at 1000 ppm. No effect was found on brain cholinesterase,
and there was no evience of carcinogenicity. The NOAEL was 30 ppm,
equal to 3 mg/kg bw per day.
In a two-year study of toxicity and carcinogenicity, rats were
fed doses of 0, 5, 10, 50 or 250 ppm fenpropimorph in the diet. The
effects seen at 50 ppm and higher were decreased brain
cholinesterase activity and increased relative liver weights in
males, and enlarged hepatocytes in animals of each sex. There was no
evidence of carcinogenicity. The NOAEL was 10 ppm, equal to 0.3
mg/kg bw per day.
A two-generation study of reproductive toxicity was performed
in which rats received dietary doses of 0, 6.25, 12.5 or 25 ppm
fenpropimorph. Several marginal effects were observed, but only in
the group receiving the highest dose. In the F1 generation, an
increased number of stillborn pups was observed, and the pups had
decreased body weight and retarded unfolding of the auricle. In F2
pups, development of the fur and opening of the eyes were retarded.
The NOAEL in this study was 12.5 ppm, equivalent to 0.6 mg/kg bw per
day.
In order to investigate peri- and postnatal effects, pregnant
rats were exposed by gavage to fenpropimorph at doses of 0, 2.5, 10,
40 or 160 mg/kg bw per day from day 15 of gestation to day 21 of
lactation. At the highest dose, many toxic effects were observed in
dams and pups, including unsatisfactory general state and pup care,
decreased body weight, increased number of dead fetuses, increased
pup mortality and diminished physical and behavioural development.
At 40 mg/kg bw per day, the body weights of dams, pup mortality and
gripping reflex (female pups) were affected. The NOAEL for maternal
and developmental toxicity was 10 mg/kg bw per day.
Rats were exposed by gavage to fenpropimorph at doses of 0,
2.5, 10, 40 or 160 mg/kg bw per day on days 6-15 of gestation.
Maternal toxicity, as evidenced by reduced body-weight gain, vaginal
bleeding and an increased number of dead implants (only at 160 mg/kg
bw per day), was observed at doses > 40 mg/kg bw per day. At the
highest dose, the weight and length of the fetuses were reduced, and
irreversible structural changes, such as cleft palate and inferior
brachygnathia, were observed. The NOAEL was 10 mg/kg bw per day for
maternal toxicity and 40 mg/kg bw per day for embryo- and
fetotoxicity and teratogenicity.
Two studies of teratogenicity were performed with rabbits
treated orally. In the first study (with doses of 0, 2.4, 12, 36 or
60 mg/kg bw per day during days 6-18 of gestation), severe maternal
toxicity was observed at 60 mg/kg bw per day; 11 dams died. Only one
fetus with several anomalies survived at this dose. At 36 mg/kg bw
per day, most of the effects were less severe and occurred at a
lower incidence; six fetuses had pseudoankylosis. The NOAEL was 12
mg/kg bw per day for both maternal and embryo- and fetotoxicity.
In the second study, rabbits were exposed by gavage to
fenpropimorph at doses of 0, 7.5, 15 or 30 mg/kg bw per day on days
7-19 of gestation. Maternal and embryo- and fetotoxicity were
observed only at 30 mg/kg bw per day. An increased total number of
malformations and anomalies was observed at this dose. The main
irreversible structural changes were cleft palate and shortened
fore- and hindlimbs. The NOAEL in this study was 15 mg/kg bw per day
for maternal toxicity, embryo- and fetotoxicity and teratogenicity.
Fenpropimorph has been adequately tested for genotoxicity in a
range of tests in vitro and in vivo. The Meeting concluded that
it was not genotoxic.
No sign of delayed neuropathy was observed in chickens treated
with fenpropimorph.
An ADI was established on the basis of the NOAEL of 10 ppm,
equal to 0.3 mg/kg bw per day, in the two-year study of toxicity and
carcinogenicity in rats, and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 30 ppm, equal to 3 mg/kg bw per day (95-week
carcinogenicity study)
Rat: 10 ppm, equal to 0.3 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
12.5 ppm, equivalent to 0.6 mg/kg bw per day
(two-generation study of reproductive toxicity)
10 mg/kg bw per day (maternal toxicity in study of
teratogenicity)
40 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of teratogenicity)
Rabbit: 15 mg/kg bw per day (maternal, embryo- and
fetotoxicity and teratogenicity in study of
teratogenicity)
Dog: 100 ppm, equal to 3.2 mg/kg bw per day (one-year
study of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Adolph, W.H. (1992) Fenpropimorph review. Memorandum from Dr Adolph
concerning medical data (DOA/WH-H308). Unpublished review dated 1
February 1992 from BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Bootman, J. & Lodge, D.C. (1981) Fenpropimorph: assessment of its
capacity to induce genetic damage in Saccharomyces cerevisiae.
Project No. 81/BAS008/015. Unpublished report dated 13 January 1981
from Life Science Research, Stock, United Kingdom. Submitted to WHO
by Ciba-Geigy AG, Basel, Switzerland.
Camponovo, F. (1983) Acute oral toxicity of Ro-14-3169/070 in rats.
Project No. B-104 800. Unpublished report dated 3 January 1983 from
F. Hoffmann-La Roche Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy AG, Switzerland.
Cifone, M.A. (1988) Mutagenicity test on fenpropimorph in the rat;
unscheduled DNA synthesis assay. Project No. 10001-0-447.
Unpublished report dated 8 June 1988 from Hazleton Laboratories
America Inc., Kensington, MD, USA. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Deckardt, K. & Hildebrand, B. (1980) In vitro investigations on the
cholinesterase inhibition by metabolites of morpholine derivatives.
Unpublished report dated 29 October 1980 from Department of
Industrial Hygiene and Toxicology, BASF Aktiengesellschaft,
Ludwigshafen, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
van Dijk, A. & Vogel, W. (1989) 14C-Ro 14-3169: Absorption,
distribution, excretion and metabolism after single intravenous,
single oral and repeated oral administration to the rat. Project No.
063641. Unpublished report dated 24 August 1989 from Research and
Consulting Co., Itingen, Switzerland. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Elliott, P.H. & Mallard, J.R. (1981) The effect of repeated
application of Reg. No. 108 406 and BAS 421 00F to the skin of
rabbits and of Reg. No. 108 406 to the skin of rats. Project No. BSF
325/80715/SA1. Unpublished report dated 22 June 1981 from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Elliott, P.H., Mallard, J.R., Street, A.E., Gibson, W.A., Offer,
J.M., Buckley, P. & Prentice, D.E. (1981) The effect of repeated
application of BAS 421 00F to the skin of rabbits for three weeks.
Project No. BSF 359/801090/SB. Unpublished report dated 2 June 1981
from Huntingdon Research Centre, Huntingdon, United Kingdom.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Engelhardt, G. & Zeller, H. (1979) Report on the investigation of
Reg. No. 108 406 in the dominant lethal test on male mice after
single intraperitoneal administration. Unpublished report dated 12
March 1979 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Gelbke, H.-P. (1979) Dermal sensitization study on the guinea pig
according to the maximization test. Unpublished report dated 6
September 1979 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Grundler, O.J. (1980a) Report on the study of the corrosive effect
of 'Reg. No. 108 406' on the rabbit in the 4-hour test and in the
3-minute test. Unpublished report dated 10 January 1980 from
Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Grundler, O.J. (1980b) Report on the study of the primary irritation
of 'Reg. No. 108 406' on the eye of white rabbits. Unpublished
report dated 10 January 1980 from Department of Industrial Hygiene
and Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hawkins, D.R. & Jackson A.J.S. (1980a) Residues of radioactivity in
milk after oral administration of 14C-BAS 421 F to goats. Project
No. BSF 348/80539. Unpublished report dated 30 July 1980 from
Huntingdon Research Centre, Huntingdon, United Kingdom. Submitted to
WHO by Ciba-Geigy AG, Basel, Switzerland.
Hawkins, D.R. & Jackson A.J.S. (1980b) Excretion of radioactivity
after oral administration of 14C-BAS 421F to goats. Project No.
BSF 348/80545. Unpublished report dated 1 August 1980 from
Huntingdon Research Centre, Huntingdon, United Kingdom. Submitted to
WHO by Ciba-Geigy AG, Basel, Switzerland.
Hawkins, D.R., Down, W.H., Ballard, S.A., Prentice, D.E., Edmonson,
N.A. & Street, A.E. (1981) The effect of BAS 108 306 F on hepatic
drug-metabolizing enzyme activity in the rat. Project no. BSF
368/81203. Unpublished report dated 21 May 1981 from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Hellwig, J., Deckardt, K., Freisberg, K.O. & Hildebrand, B. (1990)
Report on the study of the toxicity of Reg. No. 108 406 in beagle
dogs, administration via the diet over 12 months. Project No.
33D0133/87021. Unpublished report dated 29 June 1990 from Department
of Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hofmann, H.T. & Merkle, J. (1979a) Study of the perinatal and
postnatal toxicity of 4-[3-[4-(1,1-dimethylethyl)phenyl]-
2-methyl]propyl-2,6(cis)-dimethylmorpholine on rats. Unpublished
report dated 16 January 1979 from Department of Industrial Hygiene
and Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hofmann, H.T. & Merkle, J. (1979b) Investigation to determine the
prenatal toxicity of 4-[3-[4-(1,1-dimethylethyl)phenyl]-
2-methyl]propyl-2,6(cis)-dimethylmorpholine on rats. Unpublished
report dated 12 March 1979 from Department of Industrial Hygiene
and Toxicology, BASF Aktien-gesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hunter, B., Hayman, R.A., Heywood, R., Street, A.E., Prentice, D.E.,
Offer, J.M., Owen, R.A. & Gibson, W.A. (1982a) Reg. No. 108 406:
Assessment of potential tumorigenic effects in prolonged dietary
administration to mice. Project No. BSF 320/81746. Unpublished
report dated 28 June 1982 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Hunter, B., Barnard, A.V., Hayman, R.A., Street, A.E., Heywood, R.,
Prentice, D.E., Isaacs, K. & Gibson, W.A. (1982b) Reg. No. 108 406:
Assessment of potential tumorigenic and toxic effects in prolonged
dietary administration to rats. Project No. BSF 308/81138.
Unpublished report dated 12 March 1982 from Huntingdon Research
Centre, Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Jackson, G.C. & Clark, G.C. (1980) Fenpropimorph Reg No. 108 406:
Acute inhalation toxicity in rats, 4 hour exposure. Project No. BSF
356/80413. Unpublished report dated 6 June 1980 from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Jäckh, Deckardt, K., Gembardt, C., Gelbke H.-P., Hildebrand, B. &
Ruff, M. (1980) Study on the effects of Reg. No. 108 406 (DMM) in
rats after single oral administration by gavage. Unpublished report
dated 21 November 1980 from Department of Industrial Hygiene and
Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Keller, P. (1983) Four hour aerosol inhalation toxicity study (LC50)
with Ro 14-3169-070 in rats. Project No. 014703. Unpublished report
dated 11 February 1983 from Research and Consulting Co., Itingen,
Switzerland. Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Gembardt, C., Gelbke H.-P., Hildebrand, B.
& Byrt, B.E. (1979) Study of the toxicity of Reg. No. 108 406 on
rats in a 3-months feeding experiment. Unpublished report dated 26
November 1979 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Freisberg, K.O., Mirea, D., Schulz, V. &
Byrt, B.E. (1980a) Study of the toxicity of Reg. No. 108 406 in rats
in a 4-week feeding study. Unpublished report dated 30 June 1980
from Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Freisberg, K.O., Gembardt, C., Schulz, V.
& Ruff, M. (1980b) Study of the toxicity of Reg. No. 108 406 in
beagle dogs in a 3-months feeding study. Unpublished report dated 28
April 1980 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Gembardt, C., Jäckh, Gelbke, H.-P.,
Hildebrand, B. & Ruff, M. (1980c) Report on the study of the
cholinesterase inhibition of Reg. No. 108 406 in rats after single
intraperitoneal administration. Unpublished report dated 24 October
1980 from Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Klecak, G., Belcis, D. & Schneider, M.M. (1983) Open epicutaneous
test. Project No. 2489a. Unpublished report dated 4 January 1983
from F. Hoffmann-La Roche Ltd, Basel, Switzerland. Submitted to WHO
by Ciba-Geigy AG, Basel, Switzerland.
Klimisch, H.J., Deckardt, K., Gembardt, C., Hildebrand, B. & Ruff,
M. (1981) Study of the subchronic inhalation toxicity of Reg. No.
108 406 in Sprague Dawley rats (4-week aerosol study). Unpublished
report dated 5 August 1981 from Department of Industrial Hygiene and
Toxicology, BASF Aktien-gesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Leuschner, F. (1978a) The acute oral toxicity of the preparation
Reg. No. 108 406 in rats. Project No. 78/164-1. Unpublished report
dated 8 June 1978 from Laboratorium für Pharmakologie und
Toxikologie, Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Leuschner, F. (1978b) The acute local toxicity of the preparation
Reg. No. 108 406 in rats. Project No. 78/164-1. Unpublished report
dated 31 July 1978 from Laboratorium für Pharmakologie und
Toxikologie, Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Leuschner, F. (1978c) The acute intraperitoneal toxicity of the
preparation Reg. No. 108 406 in rats. Project No. 78/164-1.
Unpublished report dated 8 June 1978 from Laboratorium für
Pharmakologie und Toxikologie, Hamburg, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Leuschner, F. (1979d) Tolerance of the intact and abraded rabbit
skin of the preparation Reg. No. 108 406. Unpublished report dated
19 January 1979 from Laboratorium für Pharmakologie und Toxikologie,
Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
Leuschner, F. (1980) Examination on causticity of fenpropimorph
techn. in rabbits during a 4-hours test. Unpublished report dated 10
April 1980 from Laboratorium für Pharmakologie und Toxikologie,
Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
Liggett, M.P. & Wilson, J.C. (1980a) Irritant effects of Reg. No.
108 406 on rabbit skin. Project No. 80401 D/BSF 357/SE. Unpublished
report dated 8 July 1980 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Liggett, M.P. & Wilson, J.C. (1980b) Irritant effects of Reg. No.
108 406 on rabbit skin. Project No. 80401 D/BSF 358/SE. Unpublished
report dated 8 July 1980 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Marty, J. (1993) CGA 101'031 technical: Rabbit oral teratogenicity.
Project No. 923154. Unpublished report dated July 1993 from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Merkle, J., Freisberg, K.O., Hildebrand, B. & Ruff, M. (1982) Report
on a reproduction study with 4-[3-[4-(1,1-dimethylethyl)phenyl]-
2-methyl]propyl-2,6(cis)-dimethylmorpholine (Reg. No. 108 406) in
rats after oral administration (feeding). Two-generation study.
Unpublished report dated 22 February 1982 from Department of
Industrial Hygiene and Toxicology, BASF Aktiengesellschaft,
Ludwigshafen, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
Mosesso, P. & Nunziata, A. (1982) Report on experiment of chromosome
aberrations in human lymphocytes with and without metabolic
activation on WNT 81/200 (Fenpropimorph) of BASF AG, Ludwigshafen
(Germany). Project No. CRF 282/M. Unpublished report dated 16 March
1982 from Centro Ricerca Farmaceutica SPA, Pomezia (Roma), Italy.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
von der Mühll, F. & Gätzi, M. (1979) 14C-Ro-14-3169/005: Rat
balance study after oral administration. Unpublished report dated 14
June 1979 from F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Pryde, A., Etterli, M. & Oesterfelt, G. (1979) Metabolism of
14C-Ro14-3169/005 in the rat. Unpublished report dated 28
September 1979 from F. Hoffmann-La Roche Ltd., Basel, Switzerland.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Pryde, A., Etterli, M. & Oesterfelt, G. (1980) Metabolism of
14C-Ro14-3169/005 in the rat: Additional results. Unpublished
report dated 14 January 1980 from F. Hoffmann-La Roche Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Ritter, A. (1989) Distribution, excretion and metabolism of
14C-BAS 421 F after repeated oral administration to laying hens.
Project No. 081314. Unpublished report dated 9 November 1989 from
Research and Consulting Co., Itingen, Switzerland. Submitted to WHO
by Ciba-Geigy AG, Basel, Switzerland.
Ritter, A. & Vogel, W. (1989) Distribution, degradation and
excretion of 14C-BAS 421 F after repeated oral administration to
lactating goats. Project No. 081303. Unpublished report dated 13
October 1989 from Research and Consulting Co., Itingen, Switzerland.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Roberts, N.L., Fairley, C., Prentice, D.E. & Wight, D.G.D. (1980)
The acute oral toxicity (LD50) and neurotoxic effects of Reg. No.
108 406 to the domestic hen. Project No. BSF 336/80382. Unpublished
report dated 8 October 1980 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Siou, G. & Conau, L. (1979) Research on the mutagenous action of
fenpropimorph by means of the micronucleus test technique.
Unpublished report dated 23 April 1979 from Cabinet d'Etudes et de
Recherche en Toxicologie Industrielle (CERTI), Versailles, France.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Zeller, H. & Engelhardt, G. (1980) Report on the study of Reg. No.
108 406 in the Ames test. Unpublished report dated 8 December 1980
from Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Zeller, H. & Merkle, J. (1980) Study to determine the prenatal
toxicity of 4-[3-[4-(1,1-dimethyl-ethyl)phenyl]-2-
methyl]propyl-2,6(cis)-dimethylmorpholine in rabbits. Unpublished
report dated 7 May 1980 from Department of Industrial Hygiene and
Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted
to WHO by Ciba-Geigy AG, Basel, Switzerland.
2. Toxicological studies
(a) Acute toxicity
The results of tests for the acute toxicity in rats of
fenpropimorph administered by various routes are summarized in Table
1. Fenpropimorph is slightly toxic after dermal application and
slightly to moderately toxic after oral administration or
inhalation. Common signs of toxicity included sedation, lethargy,
dyspnoea and ataxia, which often intensified to coma. Signs of local
irritation were observed after oral administration (irritation of
stomach and intestines) and intraperitoneal treatment (irritation of
peritoneum).
Table 1. Acute toxicity of fenpropimorph in rats
Strain Sex Route LD50 (mg/kg bw) Purity Reference
or LC50 (mg/m3) (%)
Outbred albino M Oral 2090 98.5 Camponovo, 1983
F 1467
Sprague-Dawley M&F Oral 3515 NR Leuschner, 1978a
Sprague-Dawley M&F Dermal 4291a NR Leuschner, 1978b
Sprague-Dawley M&F Intraperitoneal 2465 NR Leuschner, 1978c
Sprague-Dawley CD M Inhalationb > 8500 NR Jackson & Clark, 1980
F 5200 NR
Wistar M&F Inhalationc > 3580d 98.5 Keller, 1983
NR, not reported
a Local effects were slight erythema during the first few days, exsiccation of the
skin, formation of rhagades and loss of hair. Before death or the end of the 14 days
of observation, partial growth of hair was seen, but there was no healing of
exsiccation or rhagades.
b Nose only; 85-90% of particles were less than 5 µm.
c Nose only; 23% of particles were less than 7 µm.
d No mortality; slight dyspnoea and ruffled fur were observed during and shortly
after exposure.
(b) Short-term toxicity
Rats
Groups of 20 male and 20 female Sprague-Dawley rats were given
diets containing 0, 100, 250, 625 or 1600 ppm fenpropimorph (purity,
99.1%), equivalent to 0, 10, 25, 62.5 or 160 mg/kg bw, for four
weeks. After that time, 10 male and 10 female animals from each
group were kept on normal diet for a two-week recovery period. No
effects were observed on mortality or on urinary parameters.
Deteriorated general condition, ruffled fur and reddening of the
upper and lower lips were recorded in animals at 1600 ppm.
Significantly reduced food consumption and body weight were found in
males and females at 1600 ppm and in females at 625 ppm. The
haemoglobin concentration was significantly reduced in all groups.
Significant, dose-related reductions in the concentrations of
triglycerides, calcium, glucose and total proteins were found in
males in all groups; in females, the total protein concentration was
reduced in animals at doses > 625 ppm. A significant,
dose-related decrease in cholesterol level and a significant,
dose-related increase in alanine aminotransferase level were
observed in males at doses > 625 ppm and in females at doses >
1600 ppm. The bilirubin concentration was increased in males at
doses > 250 ppm and in females at > 625 ppm. In all dose
groups, significant, dose-related increases in relative and absolute
liver weights were seen in animals of each sex, with no lesions. In
males, absolute and relative thyroid weights were increased at doses
> 250 ppm. At 1600 ppm, mucosal hyperkeratosis of the oesophagus
and oedema of the forestomach were found. Almost all of the
parameters returned to control levels after the recovery period,
except that the liver weights of females in the highest dose group
remained high (Kirsch et al., 1980a).
Four groups of 30 male and 30 female Sprague-Dawley rats were
exposed to fenpropimorph (purity, 91.1%) at doses of 0, 6.25, 12.5
or 25 ppm for three months (equal to 0, 0.38, 0.77 or 1.54 mg/kg bw
per day for males and 0, 0.46, 0.92 or 1.78 mg/kg bw per day for
females). Ten male and 10 female animals from each group were then
allowed to recover for six weeks. Clinical signs, food consumption
and body weight were not affected, and haematology, urinalysis and
histopathology showed no effect. Two females--one control and one at
25 ppm--died during the experiment. Plasma cholinesterase levels
were significantly decreased at 12.5 and 25 ppm in animals of each
sex, but there was no clear relationship with dose: the middle-dose
group had decreases of 75-89% of the control value, and the
high-dose group had decreases of 72-94%. Erythrocyte cholinesterase
levels were significantly decreased in females in all dose groups.
As there were large variations within the groups (owing partly to
methodological problems), no clear dose-response relationship and no
clear effects in males, the relevance of this finding is unclear.
Brain cholinesterase levels were not determined. The level of
triglycerides was increased in males, and total protein level was
increased in both males and females at 25 ppm. Relative liver
weights were significantly increased at doses > 12.5 ppm in both
males and females; absolute liver weights were also increased in
females at the high dose. In males, the relative weights of the
thyroid and adrenal glands were increased at 25 ppm. After recovery,
all values returned to control levels. The NOAEL was 6.25 ppm, equal
to 0.38 mg/kg bw per day (Kirsch et al., 1979).
Groups of 10 male and 10 female Sprague-Dawley rats were
exposed to fenpropimorph (purity, 90.3%; dissolved in 78% ethanol
and deionized water, with 50% of particles with a median aerodynamic
diameter of < 1.2 µm) at concentrations of 10, 40 or 160 mg/m3
air for 6 h/day, five days/week for four weeks. One control group
was exposed to fresh air and the other to the solvent. No effects
were seen on body weight, mortality or haematological parameters.
With increasing dose levels, crusts were observed around the nose,
which increased in incidence (0 in controls, 3 at 10 mg/m3, 13 at
40 mg/m3 and 20 at 160 mg/m3) and severity. In all groups, the
plasma cholinesterase concentration was decreased (by up to 19% in
males and 55% in females at the highest dose); no effects were found
on brain or erythrocyte cholinesterase levels. The activities of
alkaline phosphatase and alanine aminotransferase were increased at
doses > 40 mg/m3 in animals of each sex, and the level of
aspartate aminotransferase was increased in males given the highest
dose. Cholesterol levels were decreased in males and females in the
highest dose group. Absolute and relative liver weights were
increased in females at 40 and 160 mg/m3, and the relative liver
weight was increased in males in the highest dose group. Clear
effects of the substance were observed on histopathological
examination of the skin and respiratory tract. Focal skin lesions
(inflammation, hyperkeratosis) were found around the opening of the
nose, and increased incidences of focal basal-cell hyperplasia were
observed in the trachea, the bifurcation and the mainstream bronchi
at doses > 40 mg/m3 in animals of each sex. The
no-observed-adverse-effect concentration for systemic and irritating
effects was 10 mg/m3 (Klimisch et al., 1981).
In a limited experiment, groups of two male and two female
Sprague-Dawley rats received repeated dermal applications of
fenpropimorph (purity, 91%; dissolved in 1% aqueous methylcellulose)
on 3 x 4-cm areas of the clipped, abraded dorsal skin at
concentrations of 5, 10, 30 or 60% w/v (equal to 91, 182, 546 or
1092 mg/kg bw per day), which were maintained under an occlusive
dressing for 6 h/day for 21 consecutive days. After each
application, the skin was washed with 75% ethanol and dried. Skin
lesions were recorded in all animals, which increased with the dose
from slight to severe. The treatment was terminated at day 14
because of the severity of the skin lesions. Females treated with
60% w/v had hunched posture, lethargy and decreased respiratory
rate. All animals (except males at 5% w/v) showed body-weight loss.
Subcutaneous bruising and pitting and hyperaemia of subcutaneous
blood vessels were found in females at 30 or 60% w/v and in males at
60% w/v (Elliot & Mallard, 1981).
Rabbits
In range-finding studies, 54 New Zealand white rabbits received
repeated dermal doses of the active ingredient (purity, 91%;
dissolved in distilled water) or of a 750 EC formulation (BAS
42100F, emulsifiable concentrate containing 750 g/l fenpropimorph)
for 6 h/day for 21 days at various doses, on intact or abraded skin
and with different decontaminants. Application of the active
ingredient at doses of 11.4-1365 mg/kg bw (0.5-60% w/v) in 1%
aqueous methylcellulose produced severe dermal reactions. Time of
onset and severity varied with the dose. Treatment with doses >
34 mg/kg bw had to be terminated after 4-12 days. Generally, rabbits
treated with the formulation had less skin irritation than those
treated with the active ingredient, and the onset of reactions was
delayed (Elliot & Mallard, 1981).
Groups of 10 male and 10 female New Zealand white rabbits
received the 750 EC formulation (suspended in distilled water) at
concentrations of 0, 0.05, 0.15 or 0.5% w/v (equal to 0, 0.85, 2.6
or 8.5 mg/kg bw per dayof the active ingredient) for 6 h/day, on
five days/week for three weeks. No dose-related effects were
observed on macroscopy or haematological examination, and no changes
were seen in plasma, erythrocyte or brain cholinesterase levels or
organ weights. At the highest dose, food consumption and body
weights of females were decreased, but these signs were probably
related to the occurrence of enteritis in four animals in this
group. A dose-related increase in the incidence and severity of
dermal reactions was observed in all treated males and in females at
doses > 2.6 mg/kg bw per day. Slight erythema was seen in vehicle
controls. During the study, six rabbits died and six were killed in
extremis because of enteritis. Histological examination revealed
epidermal hyperplasia, hyperkeratinization and increased
inflammatory cell infiltration. Incidental findings of focal
parakeratosis, scab formation, epidermal ulceration and vascular
congestion of the dermis were described in animals at the two
highest doses. No systemic effects were observed. Irritation was
observed in all treated males and in females at concentrations >
0.15% w/v (Elliot et al., 1981).
Dogs
Groups of four male and four female beagle dogs were given
diets containing 0, 50, 100, 200 or 400 ppm fenpropimorph (purity,
91.1%) for 13 weeks (equivalent to 0, 1.25, 2.5, 5 or 10 mg/kg bw).
No effects were observed on clinical parameters, food consumption,
body weight, mortality or plasma cholinesterase level, and
haematological and urine analyses and histopathological examination
also showed no effects. In the group fed 400 ppm, the levels of
alkaline phosphatase and aspartate aminotransferase were increased
in males, and the level of alanine aminotransferase was increased in
both males and females. The absolute and relative weights of the
pituitary gland and the relative weight of the adrenal gland were
reduced in females at 400 ppm. The NOAEL was 200 ppm, equivalent to
5 mg/kg bw per day (Kirsch et al., 1980b).
Groups of four male and four female beagle dogs were given
diets containing 25, 100 or 400 ppm fenpropimorph (purity, >
94.7%) for 12 months (equal to a daily intake of 0.8, 3.2 or 12.3
mg/kg bw per day for males, and 0.8, 3.2 or 13.2 mg/kg bw per day
for females). Six males and six females were used as controls. No
effects were observed on clinical signs, food consumption, body
weight, mortality or plasma, erythrocyte or brain cholinesterase
levels; haematological, ophthalmological, urinary and
histopathological examinations also showed no effect. At the highest
dose, the level of alkaline phosphatase was increased in males and
that of alanine aminotransferase in females. The relative weights of
kidneys and adrenal glands were increased in females. The NOAEL was
100 ppm, equal to 3.2 mg/kg bw per day (Hellwig et al., 1990).
(c) Long-term toxicity and carcinogenicity
Mice
In a study of carcinogenicity, fenpropimorph (purity, 92.5%)
was administered in the diet to groups of 60 male and 60 female
Charles River CD-1 mice at doses of 0, 5, 30, 150 or 1000 ppm (equal
to 0.5, 3.0, 16 or 106 mg/kg bw per day in males and 0.5, 3.5, 17 or
118 mg/kg bw per day in females). Treatment was suspended after 95
weeks, when the survival rates in the various groups ranged from 44
to 68%. Ten animals of each sex per group were sacrificed after 52
and 95 weeks; the remaining mice were kept on normal diet until
terminal sacrifice at weeks 103 (males) and 104 (females). The
withdrawal period was introduced in order to investigate the
reversibility of the inhibition of erythrocyte cholinesterase.
No dose-related effects were observed on clinical signs,
mortality, food consumption or efficiency of food use. Body-weight
gain was decreased in males at the highest dose, but no significant
decrease was observed in females. The haemoglobin concentration was
decreased in males at the highest dose (significant at 95 weeks,
tendency at 51 weeks). The erythrocyte cholinesterase level was
decreased in females, by 26% at 150 ppm and by 29% at 1000 ppm;
plasma and brain cholinesterase levels were not affected (decreased
brain cholinesterase was observed in all males at 53 weeks but not
after 96 weeks). Relative liver weights were increased in males at
1000 ppm at 52 weeks, and relative liver and kidney weights were
increased in females at the highest dose after 95 weeks. Amyloid
deposits were increased in adrenal glands, kidneys and thyroid
glands of animals of each sex at the highest dose. Tumour incidences
were not enhanced. At the end of the withdrawal period, erythrocyte
cholinesterase levels and organ weights were similar to control
values. No effects were observed at 30 ppm, equal to 3 mg/kg bw per
day (Hunter et al., 1982a).
Rats
In a study of chronic toxicity and carcinogenicity, groups of
75 male and 75 female Charles River CD rats were exposed to
fenpropimorph (purity, 92.5%) in the diet at doses of 0, 5, 10, 50
or 250 ppm (equal to 0.2, 0.3, 1.7 or 8.8 mg/kg bw per day for males
and 0.2, 0.4, 2.1 or 11.2 mg/kg bw per day for females) for 104-115
weeks. Of the 60 animals of each sex in the main group, 10 were
killed at 52 weeks; the rest were maintained for evaluation of
carcinogenic effects and were killed at week 107 (females) or 115
(males). Groups of 15 rats of each sex were examined by haematology,
blood chemistry and urinalysis.
No dose-related effects were observed on clinical signs,
mortality or water consumption, and ophthalmoscopy, haematology,
blood chemistry, urinalysis and macroscopy showed no changes; there
were no neoplastic findings. Females at the highest dose had
decreased food consumption throughout the experiment. Food
conversion efficiency was decreased in animals of each sex at the
highest dose, and body-weight gain was lower in those at 50 and 250
ppm (although not significant in males at 50 ppm). The plasma
cholinesterase activity was decreased (by up to 29% in males and 41%
in females) at 250 ppm and in females also at 50 ppm. At interim
sacrifice, a significant but slight decrease (12%) in brain
cholinesterase activity was observed in females at the high dose. At
the end of the study, a small, dose-related decrease in brain
cholinesterase activity was observed in males at all doses: by 23%
at 5 ppm, 24% at 10 ppm, 30% at 50 ppm and 40% at 250 ppm; in
females, slight decreases were observed at doses of 10 ppm and
above: by 9% (not significant) at 10 ppm, 13% at 50 ppm and 12% at
250 ppm. At termination, however, the brain cholinesterase activity
in the control animals was 21-23% lower than at interim sacrifice.
No effects were observed on erythrocyte cholinesterase activities.
Relative liver weights were increased throughout the study in males
at > 50 ppm, and enlargement of hepatocytes was seen in animals
of each sex at that dose. Multinucleated cells (in males and
females), cellular pleomorphism (in males) and increased numbers of
foci of altered hepatocytes were found at the highest dose. No
tumorigenic effects were observed. As the effects on brain
cholinesterase in males were seen only at the end of the study and
not at interim sacrifice, there was no clear dose-response
relationship at the two lower doses and, remarkably, erythrocyte
cholinesterase activities were not affected at any time (the
decrease in males at the two lowest doses was considered not to be
toxicologically relevant). The NOAEL was 10 ppm, equal to 0.3 mg/kg
bw per day (Hunter et al., 1982b).
(d) Reproductive toxicity
Rats
In a two-generation study of reproductive toxicity,
fenpropimorph (purity, 92.5%) was administered to groups of 12 male
and 24 female Sprague-Dawley rats at dietary doses of 0, 6.25, 12.5
or 25 ppm, equivalent to 0.31, 0.62 or 1.25 mg/kg bw per day. At
least 100 days after the beginning of treatment, F0 animals were
mated in order to produce an F1 litter. On day 21 after birth, 12
males and 24 females were selected as the F1 parents and were kept
on the diet for at least 120 days before they were mated. The study
was terminated after weaning of the F2 litter. Pups were observed
for the usual parameters and also for physiological development
(erection of the auricles, eruption of incisors, development of fur,
opening of the auditory canal and of the eyes) and behavioural
effects (gripping reflex, pupillary reflex, hearing test). In
females of the F0 generation at the highest dose, a slight but
significant increase in food consumption was seen during pregnancy
and in body-weight gain during the entire study period. The
percentage of stillborn pups was slightly but significantly
increased at 25 ppm, with incidences of 1.1% in controls, 4.3% at
6.25 ppm, 2.7% at 12.5 ppm and 6.2% at 25 ppm. A slight but
significant decrease in relative heart weight was observed in males
at 25 ppm. F1 pups at the highest dose showed decreased body
weight from day 14 after birth (not significant in males) and
slightly retarded unfolding of the auricle. Increased relative
weights of the kidney (males) and brain (both sexes) were observed
at 25 ppm. At 25 ppm, F1 male parents had increased relative liver
weights, and females had decreased relative brain weights. In F2
pups, development of fur and opening of the eyes were slightly
retarded at 25 ppm, and one female pup at this dose had
microphthalmia, which was not considered to be related to treatment.
The effects observed at the high dose were considered to be
marginal. The NOAEL was 12.5 ppm, equivalent to 0.62 mg/kg bw per
day (Merkle et al., 1982).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 18 pregnant Sprague-Dawley rats were given
fenpropimorph (purity, 92.5%; dissolved in olive oil) by gavage at
doses of 0, 2.5, 10, 40 or 160 mg/kg bw per day from day 15 of
gestation until day 21 of lactation. The dams were examined for
clinical symptoms, deaths, body weight, litter size, delivery
behaviour, numbers of viable and dead fetuses, implantation rate,
macroscopic appearance and organ weights. Pups were observed for
body weight, sex, clinical signs, physiological development, deaths,
behaviour, viability, macroscopic appearance, organ weights and
skeletal anomalies. At 160 mg/kg bw per day, diarrhoea, salivation,
trembling, reddening of the urogenital area, vaginal bleeding and a
penetrating odour of urine were observed; four of the dams died
during the treatment. Decreased body-weight gain was observed in
dams at doses > 40 mg/kg bw per day. At 160 mg/kg bw per day,
relative heart and kidney weights were decreased, care of pups was
unsatisfactory, and the mean number of viable fetuses was decreased
(1.7 versus 11.7 in the control group), in association with a
considerable increase in the number of dead fetuses. At 160 mg/kg bw
per day, the body weights and weight gain of the pups were reduced,
and erection of the auricle, development of fur and opening of the
eyes and auditory canal were delayed. Increased pup mortality was
observed at doses > 40 mg/kg bw per day, by 25.4% at 40 mg/kg bw
per day and 50.0% at 160 mg/kg bw per day, as compared with 16.3% in
controls. The gripping reflex was decreased in female pups at doses
> 40 mg/kg bw per day. At the highest dose, relative heart and
spleen weights were decreased and relative weights of the liver and
kidney were increased in male pups. In female pups at the highest
dose, the relative weights of the heart, spleen and liver were
increased and the relative kidney weight was decreased. Because few
pups at this dose survived, however, the effects on organ weights
could not be assessed properly. The NOAEL for maternal and
developmental toxicity was 10 mg/kg bw per day (Hofmann & Merkle,
1979a).
Groups of 26-31 pregnant Sprague-Dawley rats were given
fenpropimorph (purity, 92.5%; dissolved in olive oil) by gavage at
doses of 0, 2.5, 10, 40 or 160 mg/kg bw per day on days 6-15 of
gestation. Dams were observed for clinical symptoms, body weight,
mortality, macroscopic appearance of internal organs, conception
rate and numbers of corpora lutea, viable implantations and dead
implantations (early, intermediate and late resorptions). Fetuses
were examined for weight, length, placental weight and external,
skeletal and visceral signs. Vaginal bleeding was observed in one
control, one animal at 10 mg/kg bw per day, three at 40 mg/kg bw per
day and 16 at 160 mg/kg bw per day. A dose-related reduction in
body-weight gain was observed in dams at doses > 40 mg/kg bw per
day during the treatment period and at 160 mg/kg bw per day during
the whole observation period, starting from day 6. At 160 mg/kg bw
per day, the body weights of the dams were reduced and the number of
viable fetuses was decreased, in association with an increased
number of dead implants. The weight and length of the fetuses were
reduced, and placental weight was increased. Irreversible structural
changes, including cleft palate (14/274) and inferior brachygnathia
(1/274), were observed at the highest dose. The NOAELs in this study
were 10 mg/kg bw per day for maternal toxicity and 40 mg/kg bw per
day for embryo- and fetotoxicity and teratogenicity (Hofmann &
Merkle, 1979b).
Rabbits
Groups of 15 female Himalayan rabbits were given fenpropimorph
(purity, 92.5%) by gavage at doses of 0, 2.4, 12.0, 36 or 60 mg/kg
bw per day in 0.5% carboxymethylcellulose (5 ml) during days 6-18 of
gestation. No dose-related effects on the number of corpora lutea,
preimplantation loss or conception rate were observed in dams, and
no effects were seen in fetuses on visceral examination. Diarrhoea
was seen in all groups, which increased in incidence and severity
with the dose. At 60 mg/kg bw per day, severe diarrhoea, salivation,
apathy, a greenish mucous discharge from the nose and encrustations
in the vaginal region and snout were seen, and convulsions were
observed before the deaths of 11 animals. Food consumption and body
weight were reduced. Macroscopic examination of the animals that
died at this dose showed dilatation of the right heart and
congestive hyperaemia; the clinical findings were confirmed.
Absolute uterine weights were reduced at 60 mg/kg bw per day, and
the numbers of early resorptions and dead fetuses were increased
such that only one fetus survived. This fetus had several
abnormalities, including syndactyly on the forelegs, an anomalous
position of the hindlegs and micromelia, and reduced weight and
length; furthermore, the placental weight was increased, and the
individual sternebrae were fused. At 36 mg/kg bw per day, the dams
had clinical signs that were less severe and occurred at a lower
incidence than in the highest dose group. Two animals aborted and
three were killed in extremis. The numbers of dead implantations
(due mainly to early resorptions) were slightly increased at this
dose. Six fetuses had pseudoankylosis. The NOAEL for maternal,
embryo- and fetotoxicity was 12 mg/kg bw per day (Zeller & Merkle,
1980).
In another study, groups of 20 pregnant Russian Chbb:HM rabbits
were gavaged intragastrically with fenpropimorph (purity, 95.6%; in
0.5% aqueous sodium carboxymethylcellulose) at doses of 0, 7.5, 15
or 30 mg/kg bw per day on days 7-19 of gestation. No effects were
observed on mortality, numbers of corpora lutea or live or dead
fetuses, sex ratio, numbers of early or late resorptions or total
postimplantation loss. Treatment-related effects were found only at
30 mg/kg bw per day. Swelling of the anus was observed in dams; food
consumption, body-weight gain, uterine weight and weights of male
fetuses were decreased. Fetuses had an increased total number of
malformations (21/116 in four litters) and anomalies (36/116 in 13
litters). The malformations occurred mainly in the litters of three
dams which showed marked signs of toxicity during treatment. Twenty
of 116 fetuses in three litters had shortened fore- and hindlimbs,
and 4/116 fetuses in two litters had a cleft palate. One of these
fetuses also had exencephaly and open eye, and another had gastro-
and cranioschisis, an asymmetric skull and oedema of the trunk. One
fetus in another litter had a diaphragmatic hernia. Furthermore,
abnormal positions of forelimbs were observed in 25/116 fetuses (in
seven litters) and of the hindlimbs in 8/116 fetuses (in three
litters). The external findings were confirmed by skeletal
examination. The NOAEL was 15 mg/kg bw per day for maternal
toxicity, embryotoxicity, fetotoxicity and teratogenicity (Marty,
1993).
(f) Genotoxicity
The results of tests for the genotoxicity of fenpropimorph are
summarized in Table 2.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
The iritating properties of fenpropimorph to the skin were
examined in five studies in rabbits. Three male and three female New
Zealand white rabbits were administered 0.5 ml of technical-grade
fenpropimorph (purity not specified) on intact and abraded skin for
24 h. Slight erythema was observed in all rabbits directly after
exposure. This reaction increased to well-defined erythema in one
animal after 48 h and in two others at 96 h. Very slight oedema was
observed in one animal after 48 h and in four animals after 96 h. No
signs of irritation were visible after 14 days. The irritating
response of abraded skin was comparable (Leuschner, 1979d).
Three female and one male New Zealand white rabbits were
administered 0.5 ml of technical-grade fenpropimorph (purity not
specified) on intact skin for 4 h and observed at 4 h and after 1,
2, 7 and 14 days. Slight erythema was observed in all rabbits after
one day, and this reaction increased to well-defined erythema in
three rabbits by day 7. Very slight oedema was observed in two
animals at that time. Signs of irritation were no longer visible
after 14 days (Leuschner, 1980).
Six rabbits (sex and strain not specified) were administered
0.5 ml fenpropimorph (purity not specified) on intact and abraded
skin for 24 h and observed at 24 and 72 h and after eight days.
Well-defined erythema and slight oedema were observed in all rabbits
directly after exposure; this reaction increased in severity, and
five of six animals showed necrosis after eight days. The irritating
response of abraded skin was comparable (Liggett & Wilson, 1980a).
Six rabbits (sex and strain not specified) were administered
0.5 ml fenpropimorph (purity not specified) on intact skin for 4 h
and observed at 4, 24, 48 and 72 h and after eight days.
Well-defined erythema and slight oedema were observed in all rabbits
directly after exposure, which persisted until the end of the study.
Skin necrosis was seen in one animal at 48 h, and necrosis or deep
injuries were observed in three animals on day 8 (Liggett & Wilson,
1980b).
Table 2. Results of tests for the genotoxicity of fenpropimorph
End-point Test system Concentration Purity Results Reference
of fenpropimorph (%)
In vitro
Reverse S. typhimurium TA98, 4-2500 µg/plate in 91 Negativea Zeller & Engelhardt,
mutation 100, 1535, 1537, 1538 ethanol; no toxicity 1980
Mitotic gene S. cerevisiae 0.4, 2 or 10 mg/ml 92.5 Negativea Bootman & Lodge, 1981
conversion, D7 in ethanol
reciprocal
crossing over,
mutation,
aneuploidy
Chromosomal Human 0.16-1.66 µg/ml 92.5 Negativea Mosesso & Nunziata,
aberration lymphocytes 5-50 µg/ml Negativeb 1982
Unscheduled Rat hepatocytes 0.1-10 µg/ml in 94.7 Negative Cifone, 1988
DNA ethanol; toxic
synthesis from 5 µg/ml
In vivo
Micronucleus Male Swiss mice 0, 1 or 4 ml/kg bw 92.5 Negative Siou & Conau, 1979d
formation in two oral
administrations
Dominant lethal Male NMRI 0, 212.5 or 425 mg/kg bw; 92.5 Negative Engelhardt & Zeller,
mutation mice single intraperitoneal 1979
injection; toxic at 425
mg/kg bw
a In the absence of metabolic activation
h In the presence of metabolic activation
A dose of 0.5 ml of fenpropimorph (purity, > 91%) was applied
to the intact dorsal skin of four white Vienna rabbits for 3 min or
4 h, and animals were observed up to day 8 after treatment.
Fenpropimorph caused slight to moderate erythema and oedema after
either application. The reactions intensified, and two of four
rabbits had necrosis eight days after the 4-h exposure. In the 3-min
test, animals had necrotic-like skin changes and/or scaling
(Grundler, 1980a).
Fenpropimorph was considered severely irritating and corrosive
to rabbit skin.
The irritancy of fenpropimorph to the eye was examined in three
white Vienna rabbits in a study in which 0.1 ml of fenpropimorph
(purity, > 91%) was instilled into the conjunctival sac of the
right eye. Observations were made up to day 8 after instillation.
Slight to distinct conjunctival redness was observed in all rabbits.
Fenpropimorph was considered to be irritating to the rabbit eye,
since slight irritation was still seen at the end of the study
(Grundler, 1980b).
The sensitizing properties of the compound were examined in two
studies in guinea-pigs. Fenpropimorph (purity not specified) was
applied to the shaved skin of 20 guinea-pigs five days per week for
three weeks, and the application site was left uncovered. Challenge
applications were given on days 21 and 35 of the study. A control
group of 10 animals received the challenge doses only. Slight skin
irritation was observed in 9/10 control animals and 8/20 test
animals. Since there was no difference in the frequency or intensity
of the skin reaction after challenge, fenpropimorph was considered
not to be sensitizing (Klecak et al., 1983).
A maximization test was performed in 12 male Pirbright white
guinea-pigs. During the induction period, the animals were injected
intradermally with 25% fenpropimorph (purity not specified). Five
animals served as a control group. One week later, 50% fenpropimorph
was applied topically, and a challenge application of 25%
fenpropimorph was given 14 days later. The substance did not
sensitize the skin (Gelbke, 1979).
(ii) Delayed neuropathy
The delayed neurotoxicity of fenpropimorph was investigated in
hens using doses based on the results of a preliminary test for the
LD50. Groups of 10 adult hens (20 at the highest dose) were
treated with fenpropimorph (purity, 92.5%; dissolved in olive oil)
in single oral doses of 0, 425, 850 or 1700 mg/kg bw by gavage and
then observed for 21 days. Treated birds were pretreated with
2-pyridine aldoxime methyl chloride and atropine. One group of
controls received only olive oil; a positive control group was
treated with tri- ortho-cresyl phosphate at 500 mg/kg bw in corn
oil. The birds were examined for general health, body weight, food
consumption, mortality and ataxia; after being killed, they
underwent a macroscopic examination and histopathological
observation of the spinal cord and sciatic nerve. Decreased body
weight and reduced food consumption were observed at the two highest
doses during the first three days, and symptoms of weakness and
unsteadiness were observed in all birds at 1700 mg/kg bw and in some
at 850 mg/kg bw. Two of 10 birds at 850 mg/kg bw and 8/20 at 1700
mg/kg bw died. No signs of delayed neurotoxicity were observed in
treated birds (Roberts et al., 1980).
(iii) Liver function and cholinesterase inhibition
In order to study the effects of fenpropimorph on liver
function and cholinesterase inhibition, groups of 40-100 female
Sprague-Dawley rats received a single oral dose of 0, 420, 1240 or
2290 mg/kg bw. Groups of 10 animals were killed after 1, 3, 8 and 15
days, and blood samples were taken. Clinical chemistry included
blood gas analysis and estimation of plasma, erythrocyte and brain
cholinesterase activity; the liver and brain were weighed, and
liver, brain, pancreas and affected organs were examined macro- and
microscopically. The incidence and severity of toxic signs increased
with the dose, and 13 of 100 animals at the highest dose died. Mean
body-weight gain was reduced at the middle and high doses by days 3
and 8 but had returned to normal by day 15. Reduced concentrations
of triglyceride and cholesterol were observed for the first three
days in all treated groups and up to day 8 at the highest dose. The
effect on cholesterol was overcompensated (increased) in the
middle-dose group at day 8 and in the high-dose group at day 15. The
bilirubin level was increased up to day 8 at the middle and high
doses. Alanine aminotransferase activity was increased in the
highest-dose group by day 8 and that of aspartate aminotransferase
by day 3. A reduction in plasma cholinesterase (by up to 51% of the
control value at the high dose) was observed in all treated groups
on days 8 and 15. Erythrocyte cholinesterase activity was not
affected. A slight but significant decrease in brain cholinesterase
activity (by up to 79% of control value at the high dose) was
observed after one, three and eight days at the middle and high
doses but had returned to control levels by the end of the study.
The relative liver weights were increased in all treated animals
from day 3 onwards, and the relative brain weight was increased at
the high dose by days 3 and 8. The effects on organ weights were not
accompanied by histological anomalies. At the end of the study, the
animals given the highest dose had focal skin changes (scaling,
alopecia areata) (Jäckh et al., 1980).
The effect of fenpropimorph (purity not specified) on hepatic
drug-metabolizing enzymes was investigated in groups of 16 male
Spraque-Dawley rats by giving them dietary doses of 0, 50, 250 or
1600 ppm (equivalent to 0, 2.5, 12.5 or 80 mg/kg bw per day) for 14
days. A positive control group was treated with phenobarbital at 50
mg/kg bw per day by gastric gavage. Fenpropimorph induced aniline
hydroxylase, glucuronyl transferase and glutathione S-transferase at
doses > 250 ppm; ethylmorphine N-demethylase was induced at 50
ppm. Phenobarbital sleeping time was reduced at doses > 50 ppm.
The effects were generally of similar magnitude as those in rats
treated with 50 mg/kg bw per day phenobarbital (Hawkins et al.,
1981).
The effect on plasma cholinesterase of a single administration
of fenpropimorph (purity 99.1%) was examined in groups of 20 (40 at
the highest dose) female Sprague-Dawley rats. The compound was
administered at 0, 200, 650 or 2000 mg/kg bw in a 0.5% aqueous
solution of carboxymethylcellulose by a single intraperitoneal
injection; animals were then observed for 72 h, and blood was
sampled at 2, 6, 24 and 72 h. As rats at the highest dose were
comatous, the final samples had to be taken at sacrifice after 30 or
50 h. Blood samples taken after 2 and 6 h showed increased aspartate
aminotransferase activity and bilirubin concentrations in all
treated groups. Plasma cholinesterase was unaffected after 2, 6 and
24 h; after 72 h, dose-related reductions were seen at 200 mg/kg bw
(53% reduction) and at 650 mg/kg bw (64% reduction). Absolute liver
weights were increased at doses > 200 mg/kg bw; and at doses >
650 mg/kg bw peritonitis, perihepatitis and fatty deposits in the
liver were found (Kirsch et al., 1980c).
The effect of fenpropimorph metabolites on cholinesterase
inhibition was studied in vitro. The activity of
acetylcholinesterase from Electrophorus electricus and of plasma
cholinesterase from rats and dogs was decreased by compounds with a
ß-ethanolamine structure, which are structural analogues of a
metabolite of fenpropimorph, N-[3-[4-(1,1-dimethyl-
2-carboxyethyl)phenyl]-2-methyl]propylethanolammonium ion (BF
421-4). The compound tested, which is closely related to
fenpropimorph, showed no inhibitory activity. On the basis of the
results of this study, the authors concluded that fenpropimorph
itself is not directly responsible for the inhibitory effect on
cholinesterase and that the metabolite is responsible for the
effects seen in rats in vivo (Deckardt & Hildebrand, 1980).
3. Observations in humans
Between 1984 and 1991, five cases of dermal irritation of
various grades and one case of Quincke's oedema after eye contact
were recorded at BASF AG, Ludwigshafen, Germany. Two incidents in
which a fenpropimorph formulation was ingested by children aged two
and five passed with no sequelae (Adolph, 1992).
Comments
After oral administration to rats, goats and chickens,
fenpropimorph was rapidly absorbed, distributed and excreted. In
rats and goats, enterohepatic circulation plays an important role.
The half-life in plasma and blood varied from 16 to 24 h. In all
three species, the levels of residues in tissues were relatively
low. No accumulation was detected in organs or tissues; only minor
amounts appeared in milk and eggs. The excretion pattern (rate and
route) was not significantly influenced by the administration route,
number of exposures, species, sex or dose.
Fenpropimorph was extensively metabolized in all three species
studied (rat, goat and chickens), and a small fraction of the parent
compound was found only in chickens. The first metabolic steps
involve the oxidation of the side-chains of the phenyl and the
morpholine rings. Further metabolic steps occur, as indicated by the
detection of many (mostly unidentified) metabolites. In addition,
after administration of the morpholine-labelled molecule to rats,
14CO2 was expired, indicating degradation of the morpholine ring.
Fenpropimorph is slightly to moderately toxic to rats after
acute oral exposure. WHO (1992) has classified fenpropimorph as
unlikely to present an acute hazard in normal use.
In a four-week study of toxicity, rats were given dietary doses
of 0, 100, 250, 625 or 1600 ppm fenpropimorph. Increased liver
weights and reduced haemoglobin were observed in all dose groups. No
NOAEL could be defined. In a three-month study of toxicity, rats
were exposed to fenpropimorph in the diet at levels of 0, 6.25, 12.5
or 25 ppm. On the basis of increased liver weights at doses >
12.5 ppm, the NOAEL was 6.25 ppm, equal to 0.38 mg/kg bw per day.
Dogs were fed diets containing 0, 50, 100, 200 or 400 ppm
fenpropimorph for 13 weeks, or 25, 100 or 400 ppm for 12 months. At
the highest dose, the serum activities of alkaline phosphatase and
both aspartate and alanine aminotransferase were increased and some
slight effects on organ weights were seen. The NOAEL was 200 ppm,
equivalent to 5 mg/kg bw per day, in the 13-week study and 100 ppm,
equal to 3.2 mg/kg bw per day, in the 12-month study.
In a study of carcinogenicity, fenpropimorph was administered
in the diet to mice at doses of 0, 5, 30, 150 or 1000 ppm for 95
weeks (followed by a recovery period). At the highest level, the
main effects were decreased body-weight gain, decreased haemoglobin
in males and increased relative liver weights. In females,
erythrocyte cholinesterase activity was decreased by 26% at 150 ppm
and 29% at 1000 ppm. No effect was found on brain cholinesterase,
and there was no evience of carcinogenicity. The NOAEL was 30 ppm,
equal to 3 mg/kg bw per day.
In a two-year study of toxicity and carcinogenicity, rats were
fed doses of 0, 5, 10, 50 or 250 ppm fenpropimorph in the diet. The
effects seen at 50 ppm and higher were decreased brain
cholinesterase activity and increased relative liver weights in
males, and enlarged hepatocytes in animals of each sex. There was no
evidence of carcinogenicity. The NOAEL was 10 ppm, equal to 0.3
mg/kg bw per day.
A two-generation study of reproductive toxicity was performed
in which rats received dietary doses of 0, 6.25, 12.5 or 25 ppm
fenpropimorph. Several marginal effects were observed, but only in
the group receiving the highest dose. In the F1 generation, an
increased number of stillborn pups was observed, and the pups had
decreased body weight and retarded unfolding of the auricle. In F2
pups, development of the fur and opening of the eyes were retarded.
The NOAEL in this study was 12.5 ppm, equivalent to 0.6 mg/kg bw per
day.
In order to investigate peri- and postnatal effects, pregnant
rats were exposed by gavage to fenpropimorph at doses of 0, 2.5, 10,
40 or 160 mg/kg bw per day from day 15 of gestation to day 21 of
lactation. At the highest dose, many toxic effects were observed in
dams and pups, including unsatisfactory general state and pup care,
decreased body weight, increased number of dead fetuses, increased
pup mortality and diminished physical and behavioural development.
At 40 mg/kg bw per day, the body weights of dams, pup mortality and
gripping reflex (female pups) were affected. The NOAEL for maternal
and developmental toxicity was 10 mg/kg bw per day.
Rats were exposed by gavage to fenpropimorph at doses of 0,
2.5, 10, 40 or 160 mg/kg bw per day on days 6-15 of gestation.
Maternal toxicity, as evidenced by reduced body-weight gain, vaginal
bleeding and an increased number of dead implants (only at 160 mg/kg
bw per day), was observed at doses > 40 mg/kg bw per day. At the
highest dose, the weight and length of the fetuses were reduced, and
irreversible structural changes, such as cleft palate and inferior
brachygnathia, were observed. The NOAEL was 10 mg/kg bw per day for
maternal toxicity and 40 mg/kg bw per day for embryo- and
fetotoxicity and teratogenicity.
Two studies of teratogenicity were performed with rabbits
treated orally. In the first study (with doses of 0, 2.4, 12, 36 or
60 mg/kg bw per day during days 6-18 of gestation), severe maternal
toxicity was observed at 60 mg/kg bw per day; 11 dams died. Only one
fetus with several anomalies survived at this dose. At 36 mg/kg bw
per day, most of the effects were less severe and occurred at a
lower incidence; six fetuses had pseudoankylosis. The NOAEL was 12
mg/kg bw per day for both maternal and embryo- and fetotoxicity.
In the second study, rabbits were exposed by gavage to
fenpropimorph at doses of 0, 7.5, 15 or 30 mg/kg bw per day on days
7-19 of gestation. Maternal and embryo- and fetotoxicity were
observed only at 30 mg/kg bw per day. An increased total number of
malformations and anomalies was observed at this dose. The main
irreversible structural changes were cleft palate and shortened
fore- and hindlimbs. The NOAEL in this study was 15 mg/kg bw per day
for maternal toxicity, embryo- and fetotoxicity and teratogenicity.
Fenpropimorph has been adequately tested for genotoxicity in a
range of tests in vitro and in vivo. The Meeting concluded that
it was not genotoxic.
No sign of delayed neuropathy was observed in chickens treated
with fenpropimorph.
An ADI was established on the basis of the NOAEL of 10 ppm,
equal to 0.3 mg/kg bw per day, in the two-year study of toxicity and
carcinogenicity in rats, and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 30 ppm, equal to 3 mg/kg bw per day (95-week
carcinogenicity study)
Rat: 10 ppm, equal to 0.3 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
12.5 ppm, equivalent to 0.6 mg/kg bw per day
(two-generation study of reproductive toxicity)
10 mg/kg bw per day (maternal toxicity in study of
teratogenicity)
40 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of teratogenicity)
Rabbit: 15 mg/kg bw per day (maternal, embryo- and
fetotoxicity and teratogenicity in study of
teratogenicity)
Dog: 100 ppm, equal to 3.2 mg/kg bw per day (one-year
study of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Adolph, W.H. (1992) Fenpropimorph review. Memorandum from Dr Adolph
concerning medical data (DOA/WH-H308). Unpublished review dated 1
February 1992 from BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Bootman, J. & Lodge, D.C. (1981) Fenpropimorph: assessment of its
capacity to induce genetic damage in Saccharomyces cerevisiae.
Project No. 81/BAS008/015. Unpublished report dated 13 January 1981
from Life Science Research, Stock, United Kingdom. Submitted to WHO
by Ciba-Geigy AG, Basel, Switzerland.
Camponovo, F. (1983) Acute oral toxicity of Ro-14-3169/070 in rats.
Project No. B-104 800. Unpublished report dated 3 January 1983 from
F. Hoffmann-La Roche Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy AG, Switzerland.
Cifone, M.A. (1988) Mutagenicity test on fenpropimorph in the rat;
unscheduled DNA synthesis assay. Project No. 10001-0-447.
Unpublished report dated 8 June 1988 from Hazleton Laboratories
America Inc., Kensington, MD, USA. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Deckardt, K. & Hildebrand, B. (1980) In vitro investigations on the
cholinesterase inhibition by metabolites of morpholine derivatives.
Unpublished report dated 29 October 1980 from Department of
Industrial Hygiene and Toxicology, BASF Aktiengesellschaft,
Ludwigshafen, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
van Dijk, A. & Vogel, W. (1989) 14C-Ro 14-3169: Absorption,
distribution, excretion and metabolism after single intravenous,
single oral and repeated oral administration to the rat. Project No.
063641. Unpublished report dated 24 August 1989 from Research and
Consulting Co., Itingen, Switzerland. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Elliott, P.H. & Mallard, J.R. (1981) The effect of repeated
application of Reg. No. 108 406 and BAS 421 00F to the skin of
rabbits and of Reg. No. 108 406 to the skin of rats. Project No. BSF
325/80715/SA1. Unpublished report dated 22 June 1981 from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Elliott, P.H., Mallard, J.R., Street, A.E., Gibson, W.A., Offer,
J.M., Buckley, P. & Prentice, D.E. (1981) The effect of repeated
application of BAS 421 00F to the skin of rabbits for three weeks.
Project No. BSF 359/801090/SB. Unpublished report dated 2 June 1981
from Huntingdon Research Centre, Huntingdon, United Kingdom.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Engelhardt, G. & Zeller, H. (1979) Report on the investigation of
Reg. No. 108 406 in the dominant lethal test on male mice after
single intraperitoneal administration. Unpublished report dated 12
March 1979 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Gelbke, H.-P. (1979) Dermal sensitization study on the guinea pig
according to the maximization test. Unpublished report dated 6
September 1979 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Grundler, O.J. (1980a) Report on the study of the corrosive effect
of 'Reg. No. 108 406' on the rabbit in the 4-hour test and in the
3-minute test. Unpublished report dated 10 January 1980 from
Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Grundler, O.J. (1980b) Report on the study of the primary irritation
of 'Reg. No. 108 406' on the eye of white rabbits. Unpublished
report dated 10 January 1980 from Department of Industrial Hygiene
and Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hawkins, D.R. & Jackson A.J.S. (1980a) Residues of radioactivity in
milk after oral administration of 14C-BAS 421 F to goats. Project
No. BSF 348/80539. Unpublished report dated 30 July 1980 from
Huntingdon Research Centre, Huntingdon, United Kingdom. Submitted to
WHO by Ciba-Geigy AG, Basel, Switzerland.
Hawkins, D.R. & Jackson A.J.S. (1980b) Excretion of radioactivity
after oral administration of 14C-BAS 421F to goats. Project No.
BSF 348/80545. Unpublished report dated 1 August 1980 from
Huntingdon Research Centre, Huntingdon, United Kingdom. Submitted to
WHO by Ciba-Geigy AG, Basel, Switzerland.
Hawkins, D.R., Down, W.H., Ballard, S.A., Prentice, D.E., Edmonson,
N.A. & Street, A.E. (1981) The effect of BAS 108 306 F on hepatic
drug-metabolizing enzyme activity in the rat. Project no. BSF
368/81203. Unpublished report dated 21 May 1981 from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Hellwig, J., Deckardt, K., Freisberg, K.O. & Hildebrand, B. (1990)
Report on the study of the toxicity of Reg. No. 108 406 in beagle
dogs, administration via the diet over 12 months. Project No.
33D0133/87021. Unpublished report dated 29 June 1990 from Department
of Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hofmann, H.T. & Merkle, J. (1979a) Study of the perinatal and
postnatal toxicity of 4-[3-[4-(1,1-dimethylethyl)phenyl]-
2-methyl]propyl-2,6(cis)-dimethylmorpholine on rats. Unpublished
report dated 16 January 1979 from Department of Industrial Hygiene
and Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hofmann, H.T. & Merkle, J. (1979b) Investigation to determine the
prenatal toxicity of 4-[3-[4-(1,1-dimethylethyl)phenyl]-
2-methyl]propyl-2,6(cis)-dimethylmorpholine on rats. Unpublished
report dated 12 March 1979 from Department of Industrial Hygiene
and Toxicology, BASF Aktien-gesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hunter, B., Hayman, R.A., Heywood, R., Street, A.E., Prentice, D.E.,
Offer, J.M., Owen, R.A. & Gibson, W.A. (1982a) Reg. No. 108 406:
Assessment of potential tumorigenic effects in prolonged dietary
administration to mice. Project No. BSF 320/81746. Unpublished
report dated 28 June 1982 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Hunter, B., Barnard, A.V., Hayman, R.A., Street, A.E., Heywood, R.,
Prentice, D.E., Isaacs, K. & Gibson, W.A. (1982b) Reg. No. 108 406:
Assessment of potential tumorigenic and toxic effects in prolonged
dietary administration to rats. Project No. BSF 308/81138.
Unpublished report dated 12 March 1982 from Huntingdon Research
Centre, Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Jackson, G.C. & Clark, G.C. (1980) Fenpropimorph Reg No. 108 406:
Acute inhalation toxicity in rats, 4 hour exposure. Project No. BSF
356/80413. Unpublished report dated 6 June 1980 from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Jäckh, Deckardt, K., Gembardt, C., Gelbke H.-P., Hildebrand, B. &
Ruff, M. (1980) Study on the effects of Reg. No. 108 406 (DMM) in
rats after single oral administration by gavage. Unpublished report
dated 21 November 1980 from Department of Industrial Hygiene and
Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Keller, P. (1983) Four hour aerosol inhalation toxicity study (LC50)
with Ro 14-3169-070 in rats. Project No. 014703. Unpublished report
dated 11 February 1983 from Research and Consulting Co., Itingen,
Switzerland. Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Gembardt, C., Gelbke H.-P., Hildebrand, B.
& Byrt, B.E. (1979) Study of the toxicity of Reg. No. 108 406 on
rats in a 3-months feeding experiment. Unpublished report dated 26
November 1979 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Freisberg, K.O., Mirea, D., Schulz, V. &
Byrt, B.E. (1980a) Study of the toxicity of Reg. No. 108 406 in rats
in a 4-week feeding study. Unpublished report dated 30 June 1980
from Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Freisberg, K.O., Gembardt, C., Schulz, V.
& Ruff, M. (1980b) Study of the toxicity of Reg. No. 108 406 in
beagle dogs in a 3-months feeding study. Unpublished report dated 28
April 1980 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Gembardt, C., Jäckh, Gelbke, H.-P.,
Hildebrand, B. & Ruff, M. (1980c) Report on the study of the
cholinesterase inhibition of Reg. No. 108 406 in rats after single
intraperitoneal administration. Unpublished report dated 24 October
1980 from Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Klecak, G., Belcis, D. & Schneider, M.M. (1983) Open epicutaneous
test. Project No. 2489a. Unpublished report dated 4 January 1983
from F. Hoffmann-La Roche Ltd, Basel, Switzerland. Submitted to WHO
by Ciba-Geigy AG, Basel, Switzerland.
Klimisch, H.J., Deckardt, K., Gembardt, C., Hildebrand, B. & Ruff,
M. (1981) Study of the subchronic inhalation toxicity of Reg. No.
108 406 in Sprague Dawley rats (4-week aerosol study). Unpublished
report dated 5 August 1981 from Department of Industrial Hygiene and
Toxicology, BASF Aktien-gesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Leuschner, F. (1978a) The acute oral toxicity of the preparation
Reg. No. 108 406 in rats. Project No. 78/164-1. Unpublished report
dated 8 June 1978 from Laboratorium für Pharmakologie und
Toxikologie, Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Leuschner, F. (1978b) The acute local toxicity of the preparation
Reg. No. 108 406 in rats. Project No. 78/164-1. Unpublished report
dated 31 July 1978 from Laboratorium für Pharmakologie und
Toxikologie, Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Leuschner, F. (1978c) The acute intraperitoneal toxicity of the
preparation Reg. No. 108 406 in rats. Project No. 78/164-1.
Unpublished report dated 8 June 1978 from Laboratorium für
Pharmakologie und Toxikologie, Hamburg, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Leuschner, F. (1979d) Tolerance of the intact and abraded rabbit
skin of the preparation Reg. No. 108 406. Unpublished report dated
19 January 1979 from Laboratorium für Pharmakologie und Toxikologie,
Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
Leuschner, F. (1980) Examination on causticity of fenpropimorph
techn. in rabbits during a 4-hours test. Unpublished report dated 10
April 1980 from Laboratorium für Pharmakologie und Toxikologie,
Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
Liggett, M.P. & Wilson, J.C. (1980a) Irritant effects of Reg. No.
108 406 on rabbit skin. Project No. 80401 D/BSF 357/SE. Unpublished
report dated 8 July 1980 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Liggett, M.P. & Wilson, J.C. (1980b) Irritant effects of Reg. No.
108 406 on rabbit skin. Project No. 80401 D/BSF 358/SE. Unpublished
report dated 8 July 1980 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Marty, J. (1993) CGA 101'031 technical: Rabbit oral teratogenicity.
Project No. 923154. Unpublished report dated July 1993 from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Merkle, J., Freisberg, K.O., Hildebrand, B. & Ruff, M. (1982) Report
on a reproduction study with 4-[3-[4-(1,1-dimethylethyl)phenyl]-
2-methyl]propyl-2,6(cis)-dimethylmorpholine (Reg. No. 108 406) in
rats after oral administration (feeding). Two-generation study.
Unpublished report dated 22 February 1982 from Department of
Industrial Hygiene and Toxicology, BASF Aktiengesellschaft,
Ludwigshafen, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
Mosesso, P. & Nunziata, A. (1982) Report on experiment of chromosome
aberrations in human lymphocytes with and without metabolic
activation on WNT 81/200 (Fenpropimorph) of BASF AG, Ludwigshafen
(Germany). Project No. CRF 282/M. Unpublished report dated 16 March
1982 from Centro Ricerca Farmaceutica SPA, Pomezia (Roma), Italy.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
von der Mühll, F. & Gätzi, M. (1979) 14C-Ro-14-3169/005: Rat
balance study after oral administration. Unpublished report dated 14
June 1979 from F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Pryde, A., Etterli, M. & Oesterfelt, G. (1979) Metabolism of
14C-Ro14-3169/005 in the rat. Unpublished report dated 28
September 1979 from F. Hoffmann-La Roche Ltd., Basel, Switzerland.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Pryde, A., Etterli, M. & Oesterfelt, G. (1980) Metabolism of
14C-Ro14-3169/005 in the rat: Additional results. Unpublished
report dated 14 January 1980 from F. Hoffmann-La Roche Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Ritter, A. (1989) Distribution, excretion and metabolism of
14C-BAS 421 F after repeated oral administration to laying hens.
Project No. 081314. Unpublished report dated 9 November 1989 from
Research and Consulting Co., Itingen, Switzerland. Submitted to WHO
by Ciba-Geigy AG, Basel, Switzerland.
Ritter, A. & Vogel, W. (1989) Distribution, degradation and
excretion of 14C-BAS 421 F after repeated oral administration to
lactating goats. Project No. 081303. Unpublished report dated 13
October 1989 from Research and Consulting Co., Itingen, Switzerland.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Roberts, N.L., Fairley, C., Prentice, D.E. & Wight, D.G.D. (1980)
The acute oral toxicity (LD50) and neurotoxic effects of Reg. No.
108 406 to the domestic hen. Project No. BSF 336/80382. Unpublished
report dated 8 October 1980 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Siou, G. & Conau, L. (1979) Research on the mutagenous action of
fenpropimorph by means of the micronucleus test technique.
Unpublished report dated 23 April 1979 from Cabinet d'Etudes et de
Recherche en Toxicologie Industrielle (CERTI), Versailles, France.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Zeller, H. & Engelhardt, G. (1980) Report on the study of Reg. No.
108 406 in the Ames test. Unpublished report dated 8 December 1980
from Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Zeller, H. & Merkle, J. (1980) Study to determine the prenatal
toxicity of 4-[3-[4-(1,1-dimethyl-ethyl)phenyl]-2-
methyl]propyl-2,6(cis)-dimethylmorpholine in rabbits. Unpublished
report dated 7 May 1980 from Department of Industrial Hygiene and
Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted
to WHO by Ciba-Geigy AG, Basel, Switzerland.
 2. Toxicological studies
(a) Acute toxicity
The results of tests for the acute toxicity in rats of
fenpropimorph administered by various routes are summarized in Table
1. Fenpropimorph is slightly toxic after dermal application and
slightly to moderately toxic after oral administration or
inhalation. Common signs of toxicity included sedation, lethargy,
dyspnoea and ataxia, which often intensified to coma. Signs of local
irritation were observed after oral administration (irritation of
stomach and intestines) and intraperitoneal treatment (irritation of
peritoneum).
Table 1. Acute toxicity of fenpropimorph in rats
Strain Sex Route LD50 (mg/kg bw) Purity Reference
or LC50 (mg/m3) (%)
Outbred albino M Oral 2090 98.5 Camponovo, 1983
F 1467
Sprague-Dawley M&F Oral 3515 NR Leuschner, 1978a
Sprague-Dawley M&F Dermal 4291a NR Leuschner, 1978b
Sprague-Dawley M&F Intraperitoneal 2465 NR Leuschner, 1978c
Sprague-Dawley CD M Inhalationb > 8500 NR Jackson & Clark, 1980
F 5200 NR
Wistar M&F Inhalationc > 3580d 98.5 Keller, 1983
NR, not reported
a Local effects were slight erythema during the first few days, exsiccation of the
skin, formation of rhagades and loss of hair. Before death or the end of the 14 days
of observation, partial growth of hair was seen, but there was no healing of
exsiccation or rhagades.
b Nose only; 85-90% of particles were less than 5 µm.
c Nose only; 23% of particles were less than 7 µm.
d No mortality; slight dyspnoea and ruffled fur were observed during and shortly
after exposure.
(b) Short-term toxicity
Rats
Groups of 20 male and 20 female Sprague-Dawley rats were given
diets containing 0, 100, 250, 625 or 1600 ppm fenpropimorph (purity,
99.1%), equivalent to 0, 10, 25, 62.5 or 160 mg/kg bw, for four
weeks. After that time, 10 male and 10 female animals from each
group were kept on normal diet for a two-week recovery period. No
effects were observed on mortality or on urinary parameters.
Deteriorated general condition, ruffled fur and reddening of the
upper and lower lips were recorded in animals at 1600 ppm.
Significantly reduced food consumption and body weight were found in
males and females at 1600 ppm and in females at 625 ppm. The
haemoglobin concentration was significantly reduced in all groups.
Significant, dose-related reductions in the concentrations of
triglycerides, calcium, glucose and total proteins were found in
males in all groups; in females, the total protein concentration was
reduced in animals at doses > 625 ppm. A significant,
dose-related decrease in cholesterol level and a significant,
dose-related increase in alanine aminotransferase level were
observed in males at doses > 625 ppm and in females at doses >
1600 ppm. The bilirubin concentration was increased in males at
doses > 250 ppm and in females at > 625 ppm. In all dose
groups, significant, dose-related increases in relative and absolute
liver weights were seen in animals of each sex, with no lesions. In
males, absolute and relative thyroid weights were increased at doses
> 250 ppm. At 1600 ppm, mucosal hyperkeratosis of the oesophagus
and oedema of the forestomach were found. Almost all of the
parameters returned to control levels after the recovery period,
except that the liver weights of females in the highest dose group
remained high (Kirsch et al., 1980a).
Four groups of 30 male and 30 female Sprague-Dawley rats were
exposed to fenpropimorph (purity, 91.1%) at doses of 0, 6.25, 12.5
or 25 ppm for three months (equal to 0, 0.38, 0.77 or 1.54 mg/kg bw
per day for males and 0, 0.46, 0.92 or 1.78 mg/kg bw per day for
females). Ten male and 10 female animals from each group were then
allowed to recover for six weeks. Clinical signs, food consumption
and body weight were not affected, and haematology, urinalysis and
histopathology showed no effect. Two females--one control and one at
25 ppm--died during the experiment. Plasma cholinesterase levels
were significantly decreased at 12.5 and 25 ppm in animals of each
sex, but there was no clear relationship with dose: the middle-dose
group had decreases of 75-89% of the control value, and the
high-dose group had decreases of 72-94%. Erythrocyte cholinesterase
levels were significantly decreased in females in all dose groups.
As there were large variations within the groups (owing partly to
methodological problems), no clear dose-response relationship and no
clear effects in males, the relevance of this finding is unclear.
Brain cholinesterase levels were not determined. The level of
triglycerides was increased in males, and total protein level was
increased in both males and females at 25 ppm. Relative liver
weights were significantly increased at doses > 12.5 ppm in both
males and females; absolute liver weights were also increased in
females at the high dose. In males, the relative weights of the
thyroid and adrenal glands were increased at 25 ppm. After recovery,
all values returned to control levels. The NOAEL was 6.25 ppm, equal
to 0.38 mg/kg bw per day (Kirsch et al., 1979).
Groups of 10 male and 10 female Sprague-Dawley rats were
exposed to fenpropimorph (purity, 90.3%; dissolved in 78% ethanol
and deionized water, with 50% of particles with a median aerodynamic
diameter of < 1.2 µm) at concentrations of 10, 40 or 160 mg/m3
air for 6 h/day, five days/week for four weeks. One control group
was exposed to fresh air and the other to the solvent. No effects
were seen on body weight, mortality or haematological parameters.
With increasing dose levels, crusts were observed around the nose,
which increased in incidence (0 in controls, 3 at 10 mg/m3, 13 at
40 mg/m3 and 20 at 160 mg/m3) and severity. In all groups, the
plasma cholinesterase concentration was decreased (by up to 19% in
males and 55% in females at the highest dose); no effects were found
on brain or erythrocyte cholinesterase levels. The activities of
alkaline phosphatase and alanine aminotransferase were increased at
doses > 40 mg/m3 in animals of each sex, and the level of
aspartate aminotransferase was increased in males given the highest
dose. Cholesterol levels were decreased in males and females in the
highest dose group. Absolute and relative liver weights were
increased in females at 40 and 160 mg/m3, and the relative liver
weight was increased in males in the highest dose group. Clear
effects of the substance were observed on histopathological
examination of the skin and respiratory tract. Focal skin lesions
(inflammation, hyperkeratosis) were found around the opening of the
nose, and increased incidences of focal basal-cell hyperplasia were
observed in the trachea, the bifurcation and the mainstream bronchi
at doses > 40 mg/m3 in animals of each sex. The
no-observed-adverse-effect concentration for systemic and irritating
effects was 10 mg/m3 (Klimisch et al., 1981).
In a limited experiment, groups of two male and two female
Sprague-Dawley rats received repeated dermal applications of
fenpropimorph (purity, 91%; dissolved in 1% aqueous methylcellulose)
on 3 x 4-cm areas of the clipped, abraded dorsal skin at
concentrations of 5, 10, 30 or 60% w/v (equal to 91, 182, 546 or
1092 mg/kg bw per day), which were maintained under an occlusive
dressing for 6 h/day for 21 consecutive days. After each
application, the skin was washed with 75% ethanol and dried. Skin
lesions were recorded in all animals, which increased with the dose
from slight to severe. The treatment was terminated at day 14
because of the severity of the skin lesions. Females treated with
60% w/v had hunched posture, lethargy and decreased respiratory
rate. All animals (except males at 5% w/v) showed body-weight loss.
Subcutaneous bruising and pitting and hyperaemia of subcutaneous
blood vessels were found in females at 30 or 60% w/v and in males at
60% w/v (Elliot & Mallard, 1981).
Rabbits
In range-finding studies, 54 New Zealand white rabbits received
repeated dermal doses of the active ingredient (purity, 91%;
dissolved in distilled water) or of a 750 EC formulation (BAS
42100F, emulsifiable concentrate containing 750 g/l fenpropimorph)
for 6 h/day for 21 days at various doses, on intact or abraded skin
and with different decontaminants. Application of the active
ingredient at doses of 11.4-1365 mg/kg bw (0.5-60% w/v) in 1%
aqueous methylcellulose produced severe dermal reactions. Time of
onset and severity varied with the dose. Treatment with doses >
34 mg/kg bw had to be terminated after 4-12 days. Generally, rabbits
treated with the formulation had less skin irritation than those
treated with the active ingredient, and the onset of reactions was
delayed (Elliot & Mallard, 1981).
Groups of 10 male and 10 female New Zealand white rabbits
received the 750 EC formulation (suspended in distilled water) at
concentrations of 0, 0.05, 0.15 or 0.5% w/v (equal to 0, 0.85, 2.6
or 8.5 mg/kg bw per dayof the active ingredient) for 6 h/day, on
five days/week for three weeks. No dose-related effects were
observed on macroscopy or haematological examination, and no changes
were seen in plasma, erythrocyte or brain cholinesterase levels or
organ weights. At the highest dose, food consumption and body
weights of females were decreased, but these signs were probably
related to the occurrence of enteritis in four animals in this
group. A dose-related increase in the incidence and severity of
dermal reactions was observed in all treated males and in females at
doses > 2.6 mg/kg bw per day. Slight erythema was seen in vehicle
controls. During the study, six rabbits died and six were killed in
extremis because of enteritis. Histological examination revealed
epidermal hyperplasia, hyperkeratinization and increased
inflammatory cell infiltration. Incidental findings of focal
parakeratosis, scab formation, epidermal ulceration and vascular
congestion of the dermis were described in animals at the two
highest doses. No systemic effects were observed. Irritation was
observed in all treated males and in females at concentrations >
0.15% w/v (Elliot et al., 1981).
Dogs
Groups of four male and four female beagle dogs were given
diets containing 0, 50, 100, 200 or 400 ppm fenpropimorph (purity,
91.1%) for 13 weeks (equivalent to 0, 1.25, 2.5, 5 or 10 mg/kg bw).
No effects were observed on clinical parameters, food consumption,
body weight, mortality or plasma cholinesterase level, and
haematological and urine analyses and histopathological examination
also showed no effects. In the group fed 400 ppm, the levels of
alkaline phosphatase and aspartate aminotransferase were increased
in males, and the level of alanine aminotransferase was increased in
both males and females. The absolute and relative weights of the
pituitary gland and the relative weight of the adrenal gland were
reduced in females at 400 ppm. The NOAEL was 200 ppm, equivalent to
5 mg/kg bw per day (Kirsch et al., 1980b).
Groups of four male and four female beagle dogs were given
diets containing 25, 100 or 400 ppm fenpropimorph (purity, >
94.7%) for 12 months (equal to a daily intake of 0.8, 3.2 or 12.3
mg/kg bw per day for males, and 0.8, 3.2 or 13.2 mg/kg bw per day
for females). Six males and six females were used as controls. No
effects were observed on clinical signs, food consumption, body
weight, mortality or plasma, erythrocyte or brain cholinesterase
levels; haematological, ophthalmological, urinary and
histopathological examinations also showed no effect. At the highest
dose, the level of alkaline phosphatase was increased in males and
that of alanine aminotransferase in females. The relative weights of
kidneys and adrenal glands were increased in females. The NOAEL was
100 ppm, equal to 3.2 mg/kg bw per day (Hellwig et al., 1990).
(c) Long-term toxicity and carcinogenicity
Mice
In a study of carcinogenicity, fenpropimorph (purity, 92.5%)
was administered in the diet to groups of 60 male and 60 female
Charles River CD-1 mice at doses of 0, 5, 30, 150 or 1000 ppm (equal
to 0.5, 3.0, 16 or 106 mg/kg bw per day in males and 0.5, 3.5, 17 or
118 mg/kg bw per day in females). Treatment was suspended after 95
weeks, when the survival rates in the various groups ranged from 44
to 68%. Ten animals of each sex per group were sacrificed after 52
and 95 weeks; the remaining mice were kept on normal diet until
terminal sacrifice at weeks 103 (males) and 104 (females). The
withdrawal period was introduced in order to investigate the
reversibility of the inhibition of erythrocyte cholinesterase.
No dose-related effects were observed on clinical signs,
mortality, food consumption or efficiency of food use. Body-weight
gain was decreased in males at the highest dose, but no significant
decrease was observed in females. The haemoglobin concentration was
decreased in males at the highest dose (significant at 95 weeks,
tendency at 51 weeks). The erythrocyte cholinesterase level was
decreased in females, by 26% at 150 ppm and by 29% at 1000 ppm;
plasma and brain cholinesterase levels were not affected (decreased
brain cholinesterase was observed in all males at 53 weeks but not
after 96 weeks). Relative liver weights were increased in males at
1000 ppm at 52 weeks, and relative liver and kidney weights were
increased in females at the highest dose after 95 weeks. Amyloid
deposits were increased in adrenal glands, kidneys and thyroid
glands of animals of each sex at the highest dose. Tumour incidences
were not enhanced. At the end of the withdrawal period, erythrocyte
cholinesterase levels and organ weights were similar to control
values. No effects were observed at 30 ppm, equal to 3 mg/kg bw per
day (Hunter et al., 1982a).
Rats
In a study of chronic toxicity and carcinogenicity, groups of
75 male and 75 female Charles River CD rats were exposed to
fenpropimorph (purity, 92.5%) in the diet at doses of 0, 5, 10, 50
or 250 ppm (equal to 0.2, 0.3, 1.7 or 8.8 mg/kg bw per day for males
and 0.2, 0.4, 2.1 or 11.2 mg/kg bw per day for females) for 104-115
weeks. Of the 60 animals of each sex in the main group, 10 were
killed at 52 weeks; the rest were maintained for evaluation of
carcinogenic effects and were killed at week 107 (females) or 115
(males). Groups of 15 rats of each sex were examined by haematology,
blood chemistry and urinalysis.
No dose-related effects were observed on clinical signs,
mortality or water consumption, and ophthalmoscopy, haematology,
blood chemistry, urinalysis and macroscopy showed no changes; there
were no neoplastic findings. Females at the highest dose had
decreased food consumption throughout the experiment. Food
conversion efficiency was decreased in animals of each sex at the
highest dose, and body-weight gain was lower in those at 50 and 250
ppm (although not significant in males at 50 ppm). The plasma
cholinesterase activity was decreased (by up to 29% in males and 41%
in females) at 250 ppm and in females also at 50 ppm. At interim
sacrifice, a significant but slight decrease (12%) in brain
cholinesterase activity was observed in females at the high dose. At
the end of the study, a small, dose-related decrease in brain
cholinesterase activity was observed in males at all doses: by 23%
at 5 ppm, 24% at 10 ppm, 30% at 50 ppm and 40% at 250 ppm; in
females, slight decreases were observed at doses of 10 ppm and
above: by 9% (not significant) at 10 ppm, 13% at 50 ppm and 12% at
250 ppm. At termination, however, the brain cholinesterase activity
in the control animals was 21-23% lower than at interim sacrifice.
No effects were observed on erythrocyte cholinesterase activities.
Relative liver weights were increased throughout the study in males
at > 50 ppm, and enlargement of hepatocytes was seen in animals
of each sex at that dose. Multinucleated cells (in males and
females), cellular pleomorphism (in males) and increased numbers of
foci of altered hepatocytes were found at the highest dose. No
tumorigenic effects were observed. As the effects on brain
cholinesterase in males were seen only at the end of the study and
not at interim sacrifice, there was no clear dose-response
relationship at the two lower doses and, remarkably, erythrocyte
cholinesterase activities were not affected at any time (the
decrease in males at the two lowest doses was considered not to be
toxicologically relevant). The NOAEL was 10 ppm, equal to 0.3 mg/kg
bw per day (Hunter et al., 1982b).
(d) Reproductive toxicity
Rats
In a two-generation study of reproductive toxicity,
fenpropimorph (purity, 92.5%) was administered to groups of 12 male
and 24 female Sprague-Dawley rats at dietary doses of 0, 6.25, 12.5
or 25 ppm, equivalent to 0.31, 0.62 or 1.25 mg/kg bw per day. At
least 100 days after the beginning of treatment, F0 animals were
mated in order to produce an F1 litter. On day 21 after birth, 12
males and 24 females were selected as the F1 parents and were kept
on the diet for at least 120 days before they were mated. The study
was terminated after weaning of the F2 litter. Pups were observed
for the usual parameters and also for physiological development
(erection of the auricles, eruption of incisors, development of fur,
opening of the auditory canal and of the eyes) and behavioural
effects (gripping reflex, pupillary reflex, hearing test). In
females of the F0 generation at the highest dose, a slight but
significant increase in food consumption was seen during pregnancy
and in body-weight gain during the entire study period. The
percentage of stillborn pups was slightly but significantly
increased at 25 ppm, with incidences of 1.1% in controls, 4.3% at
6.25 ppm, 2.7% at 12.5 ppm and 6.2% at 25 ppm. A slight but
significant decrease in relative heart weight was observed in males
at 25 ppm. F1 pups at the highest dose showed decreased body
weight from day 14 after birth (not significant in males) and
slightly retarded unfolding of the auricle. Increased relative
weights of the kidney (males) and brain (both sexes) were observed
at 25 ppm. At 25 ppm, F1 male parents had increased relative liver
weights, and females had decreased relative brain weights. In F2
pups, development of fur and opening of the eyes were slightly
retarded at 25 ppm, and one female pup at this dose had
microphthalmia, which was not considered to be related to treatment.
The effects observed at the high dose were considered to be
marginal. The NOAEL was 12.5 ppm, equivalent to 0.62 mg/kg bw per
day (Merkle et al., 1982).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 18 pregnant Sprague-Dawley rats were given
fenpropimorph (purity, 92.5%; dissolved in olive oil) by gavage at
doses of 0, 2.5, 10, 40 or 160 mg/kg bw per day from day 15 of
gestation until day 21 of lactation. The dams were examined for
clinical symptoms, deaths, body weight, litter size, delivery
behaviour, numbers of viable and dead fetuses, implantation rate,
macroscopic appearance and organ weights. Pups were observed for
body weight, sex, clinical signs, physiological development, deaths,
behaviour, viability, macroscopic appearance, organ weights and
skeletal anomalies. At 160 mg/kg bw per day, diarrhoea, salivation,
trembling, reddening of the urogenital area, vaginal bleeding and a
penetrating odour of urine were observed; four of the dams died
during the treatment. Decreased body-weight gain was observed in
dams at doses > 40 mg/kg bw per day. At 160 mg/kg bw per day,
relative heart and kidney weights were decreased, care of pups was
unsatisfactory, and the mean number of viable fetuses was decreased
(1.7 versus 11.7 in the control group), in association with a
considerable increase in the number of dead fetuses. At 160 mg/kg bw
per day, the body weights and weight gain of the pups were reduced,
and erection of the auricle, development of fur and opening of the
eyes and auditory canal were delayed. Increased pup mortality was
observed at doses > 40 mg/kg bw per day, by 25.4% at 40 mg/kg bw
per day and 50.0% at 160 mg/kg bw per day, as compared with 16.3% in
controls. The gripping reflex was decreased in female pups at doses
> 40 mg/kg bw per day. At the highest dose, relative heart and
spleen weights were decreased and relative weights of the liver and
kidney were increased in male pups. In female pups at the highest
dose, the relative weights of the heart, spleen and liver were
increased and the relative kidney weight was decreased. Because few
pups at this dose survived, however, the effects on organ weights
could not be assessed properly. The NOAEL for maternal and
developmental toxicity was 10 mg/kg bw per day (Hofmann & Merkle,
1979a).
Groups of 26-31 pregnant Sprague-Dawley rats were given
fenpropimorph (purity, 92.5%; dissolved in olive oil) by gavage at
doses of 0, 2.5, 10, 40 or 160 mg/kg bw per day on days 6-15 of
gestation. Dams were observed for clinical symptoms, body weight,
mortality, macroscopic appearance of internal organs, conception
rate and numbers of corpora lutea, viable implantations and dead
implantations (early, intermediate and late resorptions). Fetuses
were examined for weight, length, placental weight and external,
skeletal and visceral signs. Vaginal bleeding was observed in one
control, one animal at 10 mg/kg bw per day, three at 40 mg/kg bw per
day and 16 at 160 mg/kg bw per day. A dose-related reduction in
body-weight gain was observed in dams at doses > 40 mg/kg bw per
day during the treatment period and at 160 mg/kg bw per day during
the whole observation period, starting from day 6. At 160 mg/kg bw
per day, the body weights of the dams were reduced and the number of
viable fetuses was decreased, in association with an increased
number of dead implants. The weight and length of the fetuses were
reduced, and placental weight was increased. Irreversible structural
changes, including cleft palate (14/274) and inferior brachygnathia
(1/274), were observed at the highest dose. The NOAELs in this study
were 10 mg/kg bw per day for maternal toxicity and 40 mg/kg bw per
day for embryo- and fetotoxicity and teratogenicity (Hofmann &
Merkle, 1979b).
Rabbits
Groups of 15 female Himalayan rabbits were given fenpropimorph
(purity, 92.5%) by gavage at doses of 0, 2.4, 12.0, 36 or 60 mg/kg
bw per day in 0.5% carboxymethylcellulose (5 ml) during days 6-18 of
gestation. No dose-related effects on the number of corpora lutea,
preimplantation loss or conception rate were observed in dams, and
no effects were seen in fetuses on visceral examination. Diarrhoea
was seen in all groups, which increased in incidence and severity
with the dose. At 60 mg/kg bw per day, severe diarrhoea, salivation,
apathy, a greenish mucous discharge from the nose and encrustations
in the vaginal region and snout were seen, and convulsions were
observed before the deaths of 11 animals. Food consumption and body
weight were reduced. Macroscopic examination of the animals that
died at this dose showed dilatation of the right heart and
congestive hyperaemia; the clinical findings were confirmed.
Absolute uterine weights were reduced at 60 mg/kg bw per day, and
the numbers of early resorptions and dead fetuses were increased
such that only one fetus survived. This fetus had several
abnormalities, including syndactyly on the forelegs, an anomalous
position of the hindlegs and micromelia, and reduced weight and
length; furthermore, the placental weight was increased, and the
individual sternebrae were fused. At 36 mg/kg bw per day, the dams
had clinical signs that were less severe and occurred at a lower
incidence than in the highest dose group. Two animals aborted and
three were killed in extremis. The numbers of dead implantations
(due mainly to early resorptions) were slightly increased at this
dose. Six fetuses had pseudoankylosis. The NOAEL for maternal,
embryo- and fetotoxicity was 12 mg/kg bw per day (Zeller & Merkle,
1980).
In another study, groups of 20 pregnant Russian Chbb:HM rabbits
were gavaged intragastrically with fenpropimorph (purity, 95.6%; in
0.5% aqueous sodium carboxymethylcellulose) at doses of 0, 7.5, 15
or 30 mg/kg bw per day on days 7-19 of gestation. No effects were
observed on mortality, numbers of corpora lutea or live or dead
fetuses, sex ratio, numbers of early or late resorptions or total
postimplantation loss. Treatment-related effects were found only at
30 mg/kg bw per day. Swelling of the anus was observed in dams; food
consumption, body-weight gain, uterine weight and weights of male
fetuses were decreased. Fetuses had an increased total number of
malformations (21/116 in four litters) and anomalies (36/116 in 13
litters). The malformations occurred mainly in the litters of three
dams which showed marked signs of toxicity during treatment. Twenty
of 116 fetuses in three litters had shortened fore- and hindlimbs,
and 4/116 fetuses in two litters had a cleft palate. One of these
fetuses also had exencephaly and open eye, and another had gastro-
and cranioschisis, an asymmetric skull and oedema of the trunk. One
fetus in another litter had a diaphragmatic hernia. Furthermore,
abnormal positions of forelimbs were observed in 25/116 fetuses (in
seven litters) and of the hindlimbs in 8/116 fetuses (in three
litters). The external findings were confirmed by skeletal
examination. The NOAEL was 15 mg/kg bw per day for maternal
toxicity, embryotoxicity, fetotoxicity and teratogenicity (Marty,
1993).
(f) Genotoxicity
The results of tests for the genotoxicity of fenpropimorph are
summarized in Table 2.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
The iritating properties of fenpropimorph to the skin were
examined in five studies in rabbits. Three male and three female New
Zealand white rabbits were administered 0.5 ml of technical-grade
fenpropimorph (purity not specified) on intact and abraded skin for
24 h. Slight erythema was observed in all rabbits directly after
exposure. This reaction increased to well-defined erythema in one
animal after 48 h and in two others at 96 h. Very slight oedema was
observed in one animal after 48 h and in four animals after 96 h. No
signs of irritation were visible after 14 days. The irritating
response of abraded skin was comparable (Leuschner, 1979d).
Three female and one male New Zealand white rabbits were
administered 0.5 ml of technical-grade fenpropimorph (purity not
specified) on intact skin for 4 h and observed at 4 h and after 1,
2, 7 and 14 days. Slight erythema was observed in all rabbits after
one day, and this reaction increased to well-defined erythema in
three rabbits by day 7. Very slight oedema was observed in two
animals at that time. Signs of irritation were no longer visible
after 14 days (Leuschner, 1980).
Six rabbits (sex and strain not specified) were administered
0.5 ml fenpropimorph (purity not specified) on intact and abraded
skin for 24 h and observed at 24 and 72 h and after eight days.
Well-defined erythema and slight oedema were observed in all rabbits
directly after exposure; this reaction increased in severity, and
five of six animals showed necrosis after eight days. The irritating
response of abraded skin was comparable (Liggett & Wilson, 1980a).
Six rabbits (sex and strain not specified) were administered
0.5 ml fenpropimorph (purity not specified) on intact skin for 4 h
and observed at 4, 24, 48 and 72 h and after eight days.
Well-defined erythema and slight oedema were observed in all rabbits
directly after exposure, which persisted until the end of the study.
Skin necrosis was seen in one animal at 48 h, and necrosis or deep
injuries were observed in three animals on day 8 (Liggett & Wilson,
1980b).
Table 2. Results of tests for the genotoxicity of fenpropimorph
End-point Test system Concentration Purity Results Reference
of fenpropimorph (%)
In vitro
Reverse S. typhimurium TA98, 4-2500 µg/plate in 91 Negativea Zeller & Engelhardt,
mutation 100, 1535, 1537, 1538 ethanol; no toxicity 1980
Mitotic gene S. cerevisiae 0.4, 2 or 10 mg/ml 92.5 Negativea Bootman & Lodge, 1981
conversion, D7 in ethanol
reciprocal
crossing over,
mutation,
aneuploidy
Chromosomal Human 0.16-1.66 µg/ml 92.5 Negativea Mosesso & Nunziata,
aberration lymphocytes 5-50 µg/ml Negativeb 1982
Unscheduled Rat hepatocytes 0.1-10 µg/ml in 94.7 Negative Cifone, 1988
DNA ethanol; toxic
synthesis from 5 µg/ml
In vivo
Micronucleus Male Swiss mice 0, 1 or 4 ml/kg bw 92.5 Negative Siou & Conau, 1979d
formation in two oral
administrations
Dominant lethal Male NMRI 0, 212.5 or 425 mg/kg bw; 92.5 Negative Engelhardt & Zeller,
mutation mice single intraperitoneal 1979
injection; toxic at 425
mg/kg bw
a In the absence of metabolic activation
h In the presence of metabolic activation
A dose of 0.5 ml of fenpropimorph (purity, > 91%) was applied
to the intact dorsal skin of four white Vienna rabbits for 3 min or
4 h, and animals were observed up to day 8 after treatment.
Fenpropimorph caused slight to moderate erythema and oedema after
either application. The reactions intensified, and two of four
rabbits had necrosis eight days after the 4-h exposure. In the 3-min
test, animals had necrotic-like skin changes and/or scaling
(Grundler, 1980a).
Fenpropimorph was considered severely irritating and corrosive
to rabbit skin.
The irritancy of fenpropimorph to the eye was examined in three
white Vienna rabbits in a study in which 0.1 ml of fenpropimorph
(purity, > 91%) was instilled into the conjunctival sac of the
right eye. Observations were made up to day 8 after instillation.
Slight to distinct conjunctival redness was observed in all rabbits.
Fenpropimorph was considered to be irritating to the rabbit eye,
since slight irritation was still seen at the end of the study
(Grundler, 1980b).
The sensitizing properties of the compound were examined in two
studies in guinea-pigs. Fenpropimorph (purity not specified) was
applied to the shaved skin of 20 guinea-pigs five days per week for
three weeks, and the application site was left uncovered. Challenge
applications were given on days 21 and 35 of the study. A control
group of 10 animals received the challenge doses only. Slight skin
irritation was observed in 9/10 control animals and 8/20 test
animals. Since there was no difference in the frequency or intensity
of the skin reaction after challenge, fenpropimorph was considered
not to be sensitizing (Klecak et al., 1983).
A maximization test was performed in 12 male Pirbright white
guinea-pigs. During the induction period, the animals were injected
intradermally with 25% fenpropimorph (purity not specified). Five
animals served as a control group. One week later, 50% fenpropimorph
was applied topically, and a challenge application of 25%
fenpropimorph was given 14 days later. The substance did not
sensitize the skin (Gelbke, 1979).
(ii) Delayed neuropathy
The delayed neurotoxicity of fenpropimorph was investigated in
hens using doses based on the results of a preliminary test for the
LD50. Groups of 10 adult hens (20 at the highest dose) were
treated with fenpropimorph (purity, 92.5%; dissolved in olive oil)
in single oral doses of 0, 425, 850 or 1700 mg/kg bw by gavage and
then observed for 21 days. Treated birds were pretreated with
2-pyridine aldoxime methyl chloride and atropine. One group of
controls received only olive oil; a positive control group was
treated with tri- ortho-cresyl phosphate at 500 mg/kg bw in corn
oil. The birds were examined for general health, body weight, food
consumption, mortality and ataxia; after being killed, they
underwent a macroscopic examination and histopathological
observation of the spinal cord and sciatic nerve. Decreased body
weight and reduced food consumption were observed at the two highest
doses during the first three days, and symptoms of weakness and
unsteadiness were observed in all birds at 1700 mg/kg bw and in some
at 850 mg/kg bw. Two of 10 birds at 850 mg/kg bw and 8/20 at 1700
mg/kg bw died. No signs of delayed neurotoxicity were observed in
treated birds (Roberts et al., 1980).
(iii) Liver function and cholinesterase inhibition
In order to study the effects of fenpropimorph on liver
function and cholinesterase inhibition, groups of 40-100 female
Sprague-Dawley rats received a single oral dose of 0, 420, 1240 or
2290 mg/kg bw. Groups of 10 animals were killed after 1, 3, 8 and 15
days, and blood samples were taken. Clinical chemistry included
blood gas analysis and estimation of plasma, erythrocyte and brain
cholinesterase activity; the liver and brain were weighed, and
liver, brain, pancreas and affected organs were examined macro- and
microscopically. The incidence and severity of toxic signs increased
with the dose, and 13 of 100 animals at the highest dose died. Mean
body-weight gain was reduced at the middle and high doses by days 3
and 8 but had returned to normal by day 15. Reduced concentrations
of triglyceride and cholesterol were observed for the first three
days in all treated groups and up to day 8 at the highest dose. The
effect on cholesterol was overcompensated (increased) in the
middle-dose group at day 8 and in the high-dose group at day 15. The
bilirubin level was increased up to day 8 at the middle and high
doses. Alanine aminotransferase activity was increased in the
highest-dose group by day 8 and that of aspartate aminotransferase
by day 3. A reduction in plasma cholinesterase (by up to 51% of the
control value at the high dose) was observed in all treated groups
on days 8 and 15. Erythrocyte cholinesterase activity was not
affected. A slight but significant decrease in brain cholinesterase
activity (by up to 79% of control value at the high dose) was
observed after one, three and eight days at the middle and high
doses but had returned to control levels by the end of the study.
The relative liver weights were increased in all treated animals
from day 3 onwards, and the relative brain weight was increased at
the high dose by days 3 and 8. The effects on organ weights were not
accompanied by histological anomalies. At the end of the study, the
animals given the highest dose had focal skin changes (scaling,
alopecia areata) (Jäckh et al., 1980).
The effect of fenpropimorph (purity not specified) on hepatic
drug-metabolizing enzymes was investigated in groups of 16 male
Spraque-Dawley rats by giving them dietary doses of 0, 50, 250 or
1600 ppm (equivalent to 0, 2.5, 12.5 or 80 mg/kg bw per day) for 14
days. A positive control group was treated with phenobarbital at 50
mg/kg bw per day by gastric gavage. Fenpropimorph induced aniline
hydroxylase, glucuronyl transferase and glutathione S-transferase at
doses > 250 ppm; ethylmorphine N-demethylase was induced at 50
ppm. Phenobarbital sleeping time was reduced at doses > 50 ppm.
The effects were generally of similar magnitude as those in rats
treated with 50 mg/kg bw per day phenobarbital (Hawkins et al.,
1981).
The effect on plasma cholinesterase of a single administration
of fenpropimorph (purity 99.1%) was examined in groups of 20 (40 at
the highest dose) female Sprague-Dawley rats. The compound was
administered at 0, 200, 650 or 2000 mg/kg bw in a 0.5% aqueous
solution of carboxymethylcellulose by a single intraperitoneal
injection; animals were then observed for 72 h, and blood was
sampled at 2, 6, 24 and 72 h. As rats at the highest dose were
comatous, the final samples had to be taken at sacrifice after 30 or
50 h. Blood samples taken after 2 and 6 h showed increased aspartate
aminotransferase activity and bilirubin concentrations in all
treated groups. Plasma cholinesterase was unaffected after 2, 6 and
24 h; after 72 h, dose-related reductions were seen at 200 mg/kg bw
(53% reduction) and at 650 mg/kg bw (64% reduction). Absolute liver
weights were increased at doses > 200 mg/kg bw; and at doses >
650 mg/kg bw peritonitis, perihepatitis and fatty deposits in the
liver were found (Kirsch et al., 1980c).
The effect of fenpropimorph metabolites on cholinesterase
inhibition was studied in vitro. The activity of
acetylcholinesterase from Electrophorus electricus and of plasma
cholinesterase from rats and dogs was decreased by compounds with a
ß-ethanolamine structure, which are structural analogues of a
metabolite of fenpropimorph, N-[3-[4-(1,1-dimethyl-
2-carboxyethyl)phenyl]-2-methyl]propylethanolammonium ion (BF
421-4). The compound tested, which is closely related to
fenpropimorph, showed no inhibitory activity. On the basis of the
results of this study, the authors concluded that fenpropimorph
itself is not directly responsible for the inhibitory effect on
cholinesterase and that the metabolite is responsible for the
effects seen in rats in vivo (Deckardt & Hildebrand, 1980).
3. Observations in humans
Between 1984 and 1991, five cases of dermal irritation of
various grades and one case of Quincke's oedema after eye contact
were recorded at BASF AG, Ludwigshafen, Germany. Two incidents in
which a fenpropimorph formulation was ingested by children aged two
and five passed with no sequelae (Adolph, 1992).
Comments
After oral administration to rats, goats and chickens,
fenpropimorph was rapidly absorbed, distributed and excreted. In
rats and goats, enterohepatic circulation plays an important role.
The half-life in plasma and blood varied from 16 to 24 h. In all
three species, the levels of residues in tissues were relatively
low. No accumulation was detected in organs or tissues; only minor
amounts appeared in milk and eggs. The excretion pattern (rate and
route) was not significantly influenced by the administration route,
number of exposures, species, sex or dose.
Fenpropimorph was extensively metabolized in all three species
studied (rat, goat and chickens), and a small fraction of the parent
compound was found only in chickens. The first metabolic steps
involve the oxidation of the side-chains of the phenyl and the
morpholine rings. Further metabolic steps occur, as indicated by the
detection of many (mostly unidentified) metabolites. In addition,
after administration of the morpholine-labelled molecule to rats,
14CO2 was expired, indicating degradation of the morpholine ring.
Fenpropimorph is slightly to moderately toxic to rats after
acute oral exposure. WHO (1992) has classified fenpropimorph as
unlikely to present an acute hazard in normal use.
In a four-week study of toxicity, rats were given dietary doses
of 0, 100, 250, 625 or 1600 ppm fenpropimorph. Increased liver
weights and reduced haemoglobin were observed in all dose groups. No
NOAEL could be defined. In a three-month study of toxicity, rats
were exposed to fenpropimorph in the diet at levels of 0, 6.25, 12.5
or 25 ppm. On the basis of increased liver weights at doses >
12.5 ppm, the NOAEL was 6.25 ppm, equal to 0.38 mg/kg bw per day.
Dogs were fed diets containing 0, 50, 100, 200 or 400 ppm
fenpropimorph for 13 weeks, or 25, 100 or 400 ppm for 12 months. At
the highest dose, the serum activities of alkaline phosphatase and
both aspartate and alanine aminotransferase were increased and some
slight effects on organ weights were seen. The NOAEL was 200 ppm,
equivalent to 5 mg/kg bw per day, in the 13-week study and 100 ppm,
equal to 3.2 mg/kg bw per day, in the 12-month study.
In a study of carcinogenicity, fenpropimorph was administered
in the diet to mice at doses of 0, 5, 30, 150 or 1000 ppm for 95
weeks (followed by a recovery period). At the highest level, the
main effects were decreased body-weight gain, decreased haemoglobin
in males and increased relative liver weights. In females,
erythrocyte cholinesterase activity was decreased by 26% at 150 ppm
and 29% at 1000 ppm. No effect was found on brain cholinesterase,
and there was no evience of carcinogenicity. The NOAEL was 30 ppm,
equal to 3 mg/kg bw per day.
In a two-year study of toxicity and carcinogenicity, rats were
fed doses of 0, 5, 10, 50 or 250 ppm fenpropimorph in the diet. The
effects seen at 50 ppm and higher were decreased brain
cholinesterase activity and increased relative liver weights in
males, and enlarged hepatocytes in animals of each sex. There was no
evidence of carcinogenicity. The NOAEL was 10 ppm, equal to 0.3
mg/kg bw per day.
A two-generation study of reproductive toxicity was performed
in which rats received dietary doses of 0, 6.25, 12.5 or 25 ppm
fenpropimorph. Several marginal effects were observed, but only in
the group receiving the highest dose. In the F1 generation, an
increased number of stillborn pups was observed, and the pups had
decreased body weight and retarded unfolding of the auricle. In F2
pups, development of the fur and opening of the eyes were retarded.
The NOAEL in this study was 12.5 ppm, equivalent to 0.6 mg/kg bw per
day.
In order to investigate peri- and postnatal effects, pregnant
rats were exposed by gavage to fenpropimorph at doses of 0, 2.5, 10,
40 or 160 mg/kg bw per day from day 15 of gestation to day 21 of
lactation. At the highest dose, many toxic effects were observed in
dams and pups, including unsatisfactory general state and pup care,
decreased body weight, increased number of dead fetuses, increased
pup mortality and diminished physical and behavioural development.
At 40 mg/kg bw per day, the body weights of dams, pup mortality and
gripping reflex (female pups) were affected. The NOAEL for maternal
and developmental toxicity was 10 mg/kg bw per day.
Rats were exposed by gavage to fenpropimorph at doses of 0,
2.5, 10, 40 or 160 mg/kg bw per day on days 6-15 of gestation.
Maternal toxicity, as evidenced by reduced body-weight gain, vaginal
bleeding and an increased number of dead implants (only at 160 mg/kg
bw per day), was observed at doses > 40 mg/kg bw per day. At the
highest dose, the weight and length of the fetuses were reduced, and
irreversible structural changes, such as cleft palate and inferior
brachygnathia, were observed. The NOAEL was 10 mg/kg bw per day for
maternal toxicity and 40 mg/kg bw per day for embryo- and
fetotoxicity and teratogenicity.
Two studies of teratogenicity were performed with rabbits
treated orally. In the first study (with doses of 0, 2.4, 12, 36 or
60 mg/kg bw per day during days 6-18 of gestation), severe maternal
toxicity was observed at 60 mg/kg bw per day; 11 dams died. Only one
fetus with several anomalies survived at this dose. At 36 mg/kg bw
per day, most of the effects were less severe and occurred at a
lower incidence; six fetuses had pseudoankylosis. The NOAEL was 12
mg/kg bw per day for both maternal and embryo- and fetotoxicity.
In the second study, rabbits were exposed by gavage to
fenpropimorph at doses of 0, 7.5, 15 or 30 mg/kg bw per day on days
7-19 of gestation. Maternal and embryo- and fetotoxicity were
observed only at 30 mg/kg bw per day. An increased total number of
malformations and anomalies was observed at this dose. The main
irreversible structural changes were cleft palate and shortened
fore- and hindlimbs. The NOAEL in this study was 15 mg/kg bw per day
for maternal toxicity, embryo- and fetotoxicity and teratogenicity.
Fenpropimorph has been adequately tested for genotoxicity in a
range of tests in vitro and in vivo. The Meeting concluded that
it was not genotoxic.
No sign of delayed neuropathy was observed in chickens treated
with fenpropimorph.
An ADI was established on the basis of the NOAEL of 10 ppm,
equal to 0.3 mg/kg bw per day, in the two-year study of toxicity and
carcinogenicity in rats, and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 30 ppm, equal to 3 mg/kg bw per day (95-week
carcinogenicity study)
Rat: 10 ppm, equal to 0.3 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
12.5 ppm, equivalent to 0.6 mg/kg bw per day
(two-generation study of reproductive toxicity)
10 mg/kg bw per day (maternal toxicity in study of
teratogenicity)
40 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of teratogenicity)
Rabbit: 15 mg/kg bw per day (maternal, embryo- and
fetotoxicity and teratogenicity in study of
teratogenicity)
Dog: 100 ppm, equal to 3.2 mg/kg bw per day (one-year
study of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Adolph, W.H. (1992) Fenpropimorph review. Memorandum from Dr Adolph
concerning medical data (DOA/WH-H308). Unpublished review dated 1
February 1992 from BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Bootman, J. & Lodge, D.C. (1981) Fenpropimorph: assessment of its
capacity to induce genetic damage in Saccharomyces cerevisiae.
Project No. 81/BAS008/015. Unpublished report dated 13 January 1981
from Life Science Research, Stock, United Kingdom. Submitted to WHO
by Ciba-Geigy AG, Basel, Switzerland.
Camponovo, F. (1983) Acute oral toxicity of Ro-14-3169/070 in rats.
Project No. B-104 800. Unpublished report dated 3 January 1983 from
F. Hoffmann-La Roche Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy AG, Switzerland.
Cifone, M.A. (1988) Mutagenicity test on fenpropimorph in the rat;
unscheduled DNA synthesis assay. Project No. 10001-0-447.
Unpublished report dated 8 June 1988 from Hazleton Laboratories
America Inc., Kensington, MD, USA. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Deckardt, K. & Hildebrand, B. (1980) In vitro investigations on the
cholinesterase inhibition by metabolites of morpholine derivatives.
Unpublished report dated 29 October 1980 from Department of
Industrial Hygiene and Toxicology, BASF Aktiengesellschaft,
Ludwigshafen, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
van Dijk, A. & Vogel, W. (1989) 14C-Ro 14-3169: Absorption,
distribution, excretion and metabolism after single intravenous,
single oral and repeated oral administration to the rat. Project No.
063641. Unpublished report dated 24 August 1989 from Research and
Consulting Co., Itingen, Switzerland. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Elliott, P.H. & Mallard, J.R. (1981) The effect of repeated
application of Reg. No. 108 406 and BAS 421 00F to the skin of
rabbits and of Reg. No. 108 406 to the skin of rats. Project No. BSF
325/80715/SA1. Unpublished report dated 22 June 1981 from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Elliott, P.H., Mallard, J.R., Street, A.E., Gibson, W.A., Offer,
J.M., Buckley, P. & Prentice, D.E. (1981) The effect of repeated
application of BAS 421 00F to the skin of rabbits for three weeks.
Project No. BSF 359/801090/SB. Unpublished report dated 2 June 1981
from Huntingdon Research Centre, Huntingdon, United Kingdom.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Engelhardt, G. & Zeller, H. (1979) Report on the investigation of
Reg. No. 108 406 in the dominant lethal test on male mice after
single intraperitoneal administration. Unpublished report dated 12
March 1979 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Gelbke, H.-P. (1979) Dermal sensitization study on the guinea pig
according to the maximization test. Unpublished report dated 6
September 1979 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Grundler, O.J. (1980a) Report on the study of the corrosive effect
of 'Reg. No. 108 406' on the rabbit in the 4-hour test and in the
3-minute test. Unpublished report dated 10 January 1980 from
Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Grundler, O.J. (1980b) Report on the study of the primary irritation
of 'Reg. No. 108 406' on the eye of white rabbits. Unpublished
report dated 10 January 1980 from Department of Industrial Hygiene
and Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hawkins, D.R. & Jackson A.J.S. (1980a) Residues of radioactivity in
milk after oral administration of 14C-BAS 421 F to goats. Project
No. BSF 348/80539. Unpublished report dated 30 July 1980 from
Huntingdon Research Centre, Huntingdon, United Kingdom. Submitted to
WHO by Ciba-Geigy AG, Basel, Switzerland.
Hawkins, D.R. & Jackson A.J.S. (1980b) Excretion of radioactivity
after oral administration of 14C-BAS 421F to goats. Project No.
BSF 348/80545. Unpublished report dated 1 August 1980 from
Huntingdon Research Centre, Huntingdon, United Kingdom. Submitted to
WHO by Ciba-Geigy AG, Basel, Switzerland.
Hawkins, D.R., Down, W.H., Ballard, S.A., Prentice, D.E., Edmonson,
N.A. & Street, A.E. (1981) The effect of BAS 108 306 F on hepatic
drug-metabolizing enzyme activity in the rat. Project no. BSF
368/81203. Unpublished report dated 21 May 1981 from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Hellwig, J., Deckardt, K., Freisberg, K.O. & Hildebrand, B. (1990)
Report on the study of the toxicity of Reg. No. 108 406 in beagle
dogs, administration via the diet over 12 months. Project No.
33D0133/87021. Unpublished report dated 29 June 1990 from Department
of Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hofmann, H.T. & Merkle, J. (1979a) Study of the perinatal and
postnatal toxicity of 4-[3-[4-(1,1-dimethylethyl)phenyl]-
2-methyl]propyl-2,6(cis)-dimethylmorpholine on rats. Unpublished
report dated 16 January 1979 from Department of Industrial Hygiene
and Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hofmann, H.T. & Merkle, J. (1979b) Investigation to determine the
prenatal toxicity of 4-[3-[4-(1,1-dimethylethyl)phenyl]-
2-methyl]propyl-2,6(cis)-dimethylmorpholine on rats. Unpublished
report dated 12 March 1979 from Department of Industrial Hygiene
and Toxicology, BASF Aktien-gesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hunter, B., Hayman, R.A., Heywood, R., Street, A.E., Prentice, D.E.,
Offer, J.M., Owen, R.A. & Gibson, W.A. (1982a) Reg. No. 108 406:
Assessment of potential tumorigenic effects in prolonged dietary
administration to mice. Project No. BSF 320/81746. Unpublished
report dated 28 June 1982 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Hunter, B., Barnard, A.V., Hayman, R.A., Street, A.E., Heywood, R.,
Prentice, D.E., Isaacs, K. & Gibson, W.A. (1982b) Reg. No. 108 406:
Assessment of potential tumorigenic and toxic effects in prolonged
dietary administration to rats. Project No. BSF 308/81138.
Unpublished report dated 12 March 1982 from Huntingdon Research
Centre, Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Jackson, G.C. & Clark, G.C. (1980) Fenpropimorph Reg No. 108 406:
Acute inhalation toxicity in rats, 4 hour exposure. Project No. BSF
356/80413. Unpublished report dated 6 June 1980 from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Jäckh, Deckardt, K., Gembardt, C., Gelbke H.-P., Hildebrand, B. &
Ruff, M. (1980) Study on the effects of Reg. No. 108 406 (DMM) in
rats after single oral administration by gavage. Unpublished report
dated 21 November 1980 from Department of Industrial Hygiene and
Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Keller, P. (1983) Four hour aerosol inhalation toxicity study (LC50)
with Ro 14-3169-070 in rats. Project No. 014703. Unpublished report
dated 11 February 1983 from Research and Consulting Co., Itingen,
Switzerland. Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Gembardt, C., Gelbke H.-P., Hildebrand, B.
& Byrt, B.E. (1979) Study of the toxicity of Reg. No. 108 406 on
rats in a 3-months feeding experiment. Unpublished report dated 26
November 1979 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Freisberg, K.O., Mirea, D., Schulz, V. &
Byrt, B.E. (1980a) Study of the toxicity of Reg. No. 108 406 in rats
in a 4-week feeding study. Unpublished report dated 30 June 1980
from Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Freisberg, K.O., Gembardt, C., Schulz, V.
& Ruff, M. (1980b) Study of the toxicity of Reg. No. 108 406 in
beagle dogs in a 3-months feeding study. Unpublished report dated 28
April 1980 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Gembardt, C., Jäckh, Gelbke, H.-P.,
Hildebrand, B. & Ruff, M. (1980c) Report on the study of the
cholinesterase inhibition of Reg. No. 108 406 in rats after single
intraperitoneal administration. Unpublished report dated 24 October
1980 from Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Klecak, G., Belcis, D. & Schneider, M.M. (1983) Open epicutaneous
test. Project No. 2489a. Unpublished report dated 4 January 1983
from F. Hoffmann-La Roche Ltd, Basel, Switzerland. Submitted to WHO
by Ciba-Geigy AG, Basel, Switzerland.
Klimisch, H.J., Deckardt, K., Gembardt, C., Hildebrand, B. & Ruff,
M. (1981) Study of the subchronic inhalation toxicity of Reg. No.
108 406 in Sprague Dawley rats (4-week aerosol study). Unpublished
report dated 5 August 1981 from Department of Industrial Hygiene and
Toxicology, BASF Aktien-gesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Leuschner, F. (1978a) The acute oral toxicity of the preparation
Reg. No. 108 406 in rats. Project No. 78/164-1. Unpublished report
dated 8 June 1978 from Laboratorium für Pharmakologie und
Toxikologie, Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Leuschner, F. (1978b) The acute local toxicity of the preparation
Reg. No. 108 406 in rats. Project No. 78/164-1. Unpublished report
dated 31 July 1978 from Laboratorium für Pharmakologie und
Toxikologie, Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Leuschner, F. (1978c) The acute intraperitoneal toxicity of the
preparation Reg. No. 108 406 in rats. Project No. 78/164-1.
Unpublished report dated 8 June 1978 from Laboratorium für
Pharmakologie und Toxikologie, Hamburg, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Leuschner, F. (1979d) Tolerance of the intact and abraded rabbit
skin of the preparation Reg. No. 108 406. Unpublished report dated
19 January 1979 from Laboratorium für Pharmakologie und Toxikologie,
Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
Leuschner, F. (1980) Examination on causticity of fenpropimorph
techn. in rabbits during a 4-hours test. Unpublished report dated 10
April 1980 from Laboratorium für Pharmakologie und Toxikologie,
Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
Liggett, M.P. & Wilson, J.C. (1980a) Irritant effects of Reg. No.
108 406 on rabbit skin. Project No. 80401 D/BSF 357/SE. Unpublished
report dated 8 July 1980 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Liggett, M.P. & Wilson, J.C. (1980b) Irritant effects of Reg. No.
108 406 on rabbit skin. Project No. 80401 D/BSF 358/SE. Unpublished
report dated 8 July 1980 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Marty, J. (1993) CGA 101'031 technical: Rabbit oral teratogenicity.
Project No. 923154. Unpublished report dated July 1993 from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Merkle, J., Freisberg, K.O., Hildebrand, B. & Ruff, M. (1982) Report
on a reproduction study with 4-[3-[4-(1,1-dimethylethyl)phenyl]-
2-methyl]propyl-2,6(cis)-dimethylmorpholine (Reg. No. 108 406) in
rats after oral administration (feeding). Two-generation study.
Unpublished report dated 22 February 1982 from Department of
Industrial Hygiene and Toxicology, BASF Aktiengesellschaft,
Ludwigshafen, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
Mosesso, P. & Nunziata, A. (1982) Report on experiment of chromosome
aberrations in human lymphocytes with and without metabolic
activation on WNT 81/200 (Fenpropimorph) of BASF AG, Ludwigshafen
(Germany). Project No. CRF 282/M. Unpublished report dated 16 March
1982 from Centro Ricerca Farmaceutica SPA, Pomezia (Roma), Italy.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
von der Mühll, F. & Gätzi, M. (1979) 14C-Ro-14-3169/005: Rat
balance study after oral administration. Unpublished report dated 14
June 1979 from F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Pryde, A., Etterli, M. & Oesterfelt, G. (1979) Metabolism of
14C-Ro14-3169/005 in the rat. Unpublished report dated 28
September 1979 from F. Hoffmann-La Roche Ltd., Basel, Switzerland.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Pryde, A., Etterli, M. & Oesterfelt, G. (1980) Metabolism of
14C-Ro14-3169/005 in the rat: Additional results. Unpublished
report dated 14 January 1980 from F. Hoffmann-La Roche Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Ritter, A. (1989) Distribution, excretion and metabolism of
14C-BAS 421 F after repeated oral administration to laying hens.
Project No. 081314. Unpublished report dated 9 November 1989 from
Research and Consulting Co., Itingen, Switzerland. Submitted to WHO
by Ciba-Geigy AG, Basel, Switzerland.
Ritter, A. & Vogel, W. (1989) Distribution, degradation and
excretion of 14C-BAS 421 F after repeated oral administration to
lactating goats. Project No. 081303. Unpublished report dated 13
October 1989 from Research and Consulting Co., Itingen, Switzerland.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Roberts, N.L., Fairley, C., Prentice, D.E. & Wight, D.G.D. (1980)
The acute oral toxicity (LD50) and neurotoxic effects of Reg. No.
108 406 to the domestic hen. Project No. BSF 336/80382. Unpublished
report dated 8 October 1980 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Siou, G. & Conau, L. (1979) Research on the mutagenous action of
fenpropimorph by means of the micronucleus test technique.
Unpublished report dated 23 April 1979 from Cabinet d'Etudes et de
Recherche en Toxicologie Industrielle (CERTI), Versailles, France.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Zeller, H. & Engelhardt, G. (1980) Report on the study of Reg. No.
108 406 in the Ames test. Unpublished report dated 8 December 1980
from Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Zeller, H. & Merkle, J. (1980) Study to determine the prenatal
toxicity of 4-[3-[4-(1,1-dimethyl-ethyl)phenyl]-2-
methyl]propyl-2,6(cis)-dimethylmorpholine in rabbits. Unpublished
report dated 7 May 1980 from Department of Industrial Hygiene and
Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted
to WHO by Ciba-Geigy AG, Basel, Switzerland.
2. Toxicological studies
(a) Acute toxicity
The results of tests for the acute toxicity in rats of
fenpropimorph administered by various routes are summarized in Table
1. Fenpropimorph is slightly toxic after dermal application and
slightly to moderately toxic after oral administration or
inhalation. Common signs of toxicity included sedation, lethargy,
dyspnoea and ataxia, which often intensified to coma. Signs of local
irritation were observed after oral administration (irritation of
stomach and intestines) and intraperitoneal treatment (irritation of
peritoneum).
Table 1. Acute toxicity of fenpropimorph in rats
Strain Sex Route LD50 (mg/kg bw) Purity Reference
or LC50 (mg/m3) (%)
Outbred albino M Oral 2090 98.5 Camponovo, 1983
F 1467
Sprague-Dawley M&F Oral 3515 NR Leuschner, 1978a
Sprague-Dawley M&F Dermal 4291a NR Leuschner, 1978b
Sprague-Dawley M&F Intraperitoneal 2465 NR Leuschner, 1978c
Sprague-Dawley CD M Inhalationb > 8500 NR Jackson & Clark, 1980
F 5200 NR
Wistar M&F Inhalationc > 3580d 98.5 Keller, 1983
NR, not reported
a Local effects were slight erythema during the first few days, exsiccation of the
skin, formation of rhagades and loss of hair. Before death or the end of the 14 days
of observation, partial growth of hair was seen, but there was no healing of
exsiccation or rhagades.
b Nose only; 85-90% of particles were less than 5 µm.
c Nose only; 23% of particles were less than 7 µm.
d No mortality; slight dyspnoea and ruffled fur were observed during and shortly
after exposure.
(b) Short-term toxicity
Rats
Groups of 20 male and 20 female Sprague-Dawley rats were given
diets containing 0, 100, 250, 625 or 1600 ppm fenpropimorph (purity,
99.1%), equivalent to 0, 10, 25, 62.5 or 160 mg/kg bw, for four
weeks. After that time, 10 male and 10 female animals from each
group were kept on normal diet for a two-week recovery period. No
effects were observed on mortality or on urinary parameters.
Deteriorated general condition, ruffled fur and reddening of the
upper and lower lips were recorded in animals at 1600 ppm.
Significantly reduced food consumption and body weight were found in
males and females at 1600 ppm and in females at 625 ppm. The
haemoglobin concentration was significantly reduced in all groups.
Significant, dose-related reductions in the concentrations of
triglycerides, calcium, glucose and total proteins were found in
males in all groups; in females, the total protein concentration was
reduced in animals at doses > 625 ppm. A significant,
dose-related decrease in cholesterol level and a significant,
dose-related increase in alanine aminotransferase level were
observed in males at doses > 625 ppm and in females at doses >
1600 ppm. The bilirubin concentration was increased in males at
doses > 250 ppm and in females at > 625 ppm. In all dose
groups, significant, dose-related increases in relative and absolute
liver weights were seen in animals of each sex, with no lesions. In
males, absolute and relative thyroid weights were increased at doses
> 250 ppm. At 1600 ppm, mucosal hyperkeratosis of the oesophagus
and oedema of the forestomach were found. Almost all of the
parameters returned to control levels after the recovery period,
except that the liver weights of females in the highest dose group
remained high (Kirsch et al., 1980a).
Four groups of 30 male and 30 female Sprague-Dawley rats were
exposed to fenpropimorph (purity, 91.1%) at doses of 0, 6.25, 12.5
or 25 ppm for three months (equal to 0, 0.38, 0.77 or 1.54 mg/kg bw
per day for males and 0, 0.46, 0.92 or 1.78 mg/kg bw per day for
females). Ten male and 10 female animals from each group were then
allowed to recover for six weeks. Clinical signs, food consumption
and body weight were not affected, and haematology, urinalysis and
histopathology showed no effect. Two females--one control and one at
25 ppm--died during the experiment. Plasma cholinesterase levels
were significantly decreased at 12.5 and 25 ppm in animals of each
sex, but there was no clear relationship with dose: the middle-dose
group had decreases of 75-89% of the control value, and the
high-dose group had decreases of 72-94%. Erythrocyte cholinesterase
levels were significantly decreased in females in all dose groups.
As there were large variations within the groups (owing partly to
methodological problems), no clear dose-response relationship and no
clear effects in males, the relevance of this finding is unclear.
Brain cholinesterase levels were not determined. The level of
triglycerides was increased in males, and total protein level was
increased in both males and females at 25 ppm. Relative liver
weights were significantly increased at doses > 12.5 ppm in both
males and females; absolute liver weights were also increased in
females at the high dose. In males, the relative weights of the
thyroid and adrenal glands were increased at 25 ppm. After recovery,
all values returned to control levels. The NOAEL was 6.25 ppm, equal
to 0.38 mg/kg bw per day (Kirsch et al., 1979).
Groups of 10 male and 10 female Sprague-Dawley rats were
exposed to fenpropimorph (purity, 90.3%; dissolved in 78% ethanol
and deionized water, with 50% of particles with a median aerodynamic
diameter of < 1.2 µm) at concentrations of 10, 40 or 160 mg/m3
air for 6 h/day, five days/week for four weeks. One control group
was exposed to fresh air and the other to the solvent. No effects
were seen on body weight, mortality or haematological parameters.
With increasing dose levels, crusts were observed around the nose,
which increased in incidence (0 in controls, 3 at 10 mg/m3, 13 at
40 mg/m3 and 20 at 160 mg/m3) and severity. In all groups, the
plasma cholinesterase concentration was decreased (by up to 19% in
males and 55% in females at the highest dose); no effects were found
on brain or erythrocyte cholinesterase levels. The activities of
alkaline phosphatase and alanine aminotransferase were increased at
doses > 40 mg/m3 in animals of each sex, and the level of
aspartate aminotransferase was increased in males given the highest
dose. Cholesterol levels were decreased in males and females in the
highest dose group. Absolute and relative liver weights were
increased in females at 40 and 160 mg/m3, and the relative liver
weight was increased in males in the highest dose group. Clear
effects of the substance were observed on histopathological
examination of the skin and respiratory tract. Focal skin lesions
(inflammation, hyperkeratosis) were found around the opening of the
nose, and increased incidences of focal basal-cell hyperplasia were
observed in the trachea, the bifurcation and the mainstream bronchi
at doses > 40 mg/m3 in animals of each sex. The
no-observed-adverse-effect concentration for systemic and irritating
effects was 10 mg/m3 (Klimisch et al., 1981).
In a limited experiment, groups of two male and two female
Sprague-Dawley rats received repeated dermal applications of
fenpropimorph (purity, 91%; dissolved in 1% aqueous methylcellulose)
on 3 x 4-cm areas of the clipped, abraded dorsal skin at
concentrations of 5, 10, 30 or 60% w/v (equal to 91, 182, 546 or
1092 mg/kg bw per day), which were maintained under an occlusive
dressing for 6 h/day for 21 consecutive days. After each
application, the skin was washed with 75% ethanol and dried. Skin
lesions were recorded in all animals, which increased with the dose
from slight to severe. The treatment was terminated at day 14
because of the severity of the skin lesions. Females treated with
60% w/v had hunched posture, lethargy and decreased respiratory
rate. All animals (except males at 5% w/v) showed body-weight loss.
Subcutaneous bruising and pitting and hyperaemia of subcutaneous
blood vessels were found in females at 30 or 60% w/v and in males at
60% w/v (Elliot & Mallard, 1981).
Rabbits
In range-finding studies, 54 New Zealand white rabbits received
repeated dermal doses of the active ingredient (purity, 91%;
dissolved in distilled water) or of a 750 EC formulation (BAS
42100F, emulsifiable concentrate containing 750 g/l fenpropimorph)
for 6 h/day for 21 days at various doses, on intact or abraded skin
and with different decontaminants. Application of the active
ingredient at doses of 11.4-1365 mg/kg bw (0.5-60% w/v) in 1%
aqueous methylcellulose produced severe dermal reactions. Time of
onset and severity varied with the dose. Treatment with doses >
34 mg/kg bw had to be terminated after 4-12 days. Generally, rabbits
treated with the formulation had less skin irritation than those
treated with the active ingredient, and the onset of reactions was
delayed (Elliot & Mallard, 1981).
Groups of 10 male and 10 female New Zealand white rabbits
received the 750 EC formulation (suspended in distilled water) at
concentrations of 0, 0.05, 0.15 or 0.5% w/v (equal to 0, 0.85, 2.6
or 8.5 mg/kg bw per dayof the active ingredient) for 6 h/day, on
five days/week for three weeks. No dose-related effects were
observed on macroscopy or haematological examination, and no changes
were seen in plasma, erythrocyte or brain cholinesterase levels or
organ weights. At the highest dose, food consumption and body
weights of females were decreased, but these signs were probably
related to the occurrence of enteritis in four animals in this
group. A dose-related increase in the incidence and severity of
dermal reactions was observed in all treated males and in females at
doses > 2.6 mg/kg bw per day. Slight erythema was seen in vehicle
controls. During the study, six rabbits died and six were killed in
extremis because of enteritis. Histological examination revealed
epidermal hyperplasia, hyperkeratinization and increased
inflammatory cell infiltration. Incidental findings of focal
parakeratosis, scab formation, epidermal ulceration and vascular
congestion of the dermis were described in animals at the two
highest doses. No systemic effects were observed. Irritation was
observed in all treated males and in females at concentrations >
0.15% w/v (Elliot et al., 1981).
Dogs
Groups of four male and four female beagle dogs were given
diets containing 0, 50, 100, 200 or 400 ppm fenpropimorph (purity,
91.1%) for 13 weeks (equivalent to 0, 1.25, 2.5, 5 or 10 mg/kg bw).
No effects were observed on clinical parameters, food consumption,
body weight, mortality or plasma cholinesterase level, and
haematological and urine analyses and histopathological examination
also showed no effects. In the group fed 400 ppm, the levels of
alkaline phosphatase and aspartate aminotransferase were increased
in males, and the level of alanine aminotransferase was increased in
both males and females. The absolute and relative weights of the
pituitary gland and the relative weight of the adrenal gland were
reduced in females at 400 ppm. The NOAEL was 200 ppm, equivalent to
5 mg/kg bw per day (Kirsch et al., 1980b).
Groups of four male and four female beagle dogs were given
diets containing 25, 100 or 400 ppm fenpropimorph (purity, >
94.7%) for 12 months (equal to a daily intake of 0.8, 3.2 or 12.3
mg/kg bw per day for males, and 0.8, 3.2 or 13.2 mg/kg bw per day
for females). Six males and six females were used as controls. No
effects were observed on clinical signs, food consumption, body
weight, mortality or plasma, erythrocyte or brain cholinesterase
levels; haematological, ophthalmological, urinary and
histopathological examinations also showed no effect. At the highest
dose, the level of alkaline phosphatase was increased in males and
that of alanine aminotransferase in females. The relative weights of
kidneys and adrenal glands were increased in females. The NOAEL was
100 ppm, equal to 3.2 mg/kg bw per day (Hellwig et al., 1990).
(c) Long-term toxicity and carcinogenicity
Mice
In a study of carcinogenicity, fenpropimorph (purity, 92.5%)
was administered in the diet to groups of 60 male and 60 female
Charles River CD-1 mice at doses of 0, 5, 30, 150 or 1000 ppm (equal
to 0.5, 3.0, 16 or 106 mg/kg bw per day in males and 0.5, 3.5, 17 or
118 mg/kg bw per day in females). Treatment was suspended after 95
weeks, when the survival rates in the various groups ranged from 44
to 68%. Ten animals of each sex per group were sacrificed after 52
and 95 weeks; the remaining mice were kept on normal diet until
terminal sacrifice at weeks 103 (males) and 104 (females). The
withdrawal period was introduced in order to investigate the
reversibility of the inhibition of erythrocyte cholinesterase.
No dose-related effects were observed on clinical signs,
mortality, food consumption or efficiency of food use. Body-weight
gain was decreased in males at the highest dose, but no significant
decrease was observed in females. The haemoglobin concentration was
decreased in males at the highest dose (significant at 95 weeks,
tendency at 51 weeks). The erythrocyte cholinesterase level was
decreased in females, by 26% at 150 ppm and by 29% at 1000 ppm;
plasma and brain cholinesterase levels were not affected (decreased
brain cholinesterase was observed in all males at 53 weeks but not
after 96 weeks). Relative liver weights were increased in males at
1000 ppm at 52 weeks, and relative liver and kidney weights were
increased in females at the highest dose after 95 weeks. Amyloid
deposits were increased in adrenal glands, kidneys and thyroid
glands of animals of each sex at the highest dose. Tumour incidences
were not enhanced. At the end of the withdrawal period, erythrocyte
cholinesterase levels and organ weights were similar to control
values. No effects were observed at 30 ppm, equal to 3 mg/kg bw per
day (Hunter et al., 1982a).
Rats
In a study of chronic toxicity and carcinogenicity, groups of
75 male and 75 female Charles River CD rats were exposed to
fenpropimorph (purity, 92.5%) in the diet at doses of 0, 5, 10, 50
or 250 ppm (equal to 0.2, 0.3, 1.7 or 8.8 mg/kg bw per day for males
and 0.2, 0.4, 2.1 or 11.2 mg/kg bw per day for females) for 104-115
weeks. Of the 60 animals of each sex in the main group, 10 were
killed at 52 weeks; the rest were maintained for evaluation of
carcinogenic effects and were killed at week 107 (females) or 115
(males). Groups of 15 rats of each sex were examined by haematology,
blood chemistry and urinalysis.
No dose-related effects were observed on clinical signs,
mortality or water consumption, and ophthalmoscopy, haematology,
blood chemistry, urinalysis and macroscopy showed no changes; there
were no neoplastic findings. Females at the highest dose had
decreased food consumption throughout the experiment. Food
conversion efficiency was decreased in animals of each sex at the
highest dose, and body-weight gain was lower in those at 50 and 250
ppm (although not significant in males at 50 ppm). The plasma
cholinesterase activity was decreased (by up to 29% in males and 41%
in females) at 250 ppm and in females also at 50 ppm. At interim
sacrifice, a significant but slight decrease (12%) in brain
cholinesterase activity was observed in females at the high dose. At
the end of the study, a small, dose-related decrease in brain
cholinesterase activity was observed in males at all doses: by 23%
at 5 ppm, 24% at 10 ppm, 30% at 50 ppm and 40% at 250 ppm; in
females, slight decreases were observed at doses of 10 ppm and
above: by 9% (not significant) at 10 ppm, 13% at 50 ppm and 12% at
250 ppm. At termination, however, the brain cholinesterase activity
in the control animals was 21-23% lower than at interim sacrifice.
No effects were observed on erythrocyte cholinesterase activities.
Relative liver weights were increased throughout the study in males
at > 50 ppm, and enlargement of hepatocytes was seen in animals
of each sex at that dose. Multinucleated cells (in males and
females), cellular pleomorphism (in males) and increased numbers of
foci of altered hepatocytes were found at the highest dose. No
tumorigenic effects were observed. As the effects on brain
cholinesterase in males were seen only at the end of the study and
not at interim sacrifice, there was no clear dose-response
relationship at the two lower doses and, remarkably, erythrocyte
cholinesterase activities were not affected at any time (the
decrease in males at the two lowest doses was considered not to be
toxicologically relevant). The NOAEL was 10 ppm, equal to 0.3 mg/kg
bw per day (Hunter et al., 1982b).
(d) Reproductive toxicity
Rats
In a two-generation study of reproductive toxicity,
fenpropimorph (purity, 92.5%) was administered to groups of 12 male
and 24 female Sprague-Dawley rats at dietary doses of 0, 6.25, 12.5
or 25 ppm, equivalent to 0.31, 0.62 or 1.25 mg/kg bw per day. At
least 100 days after the beginning of treatment, F0 animals were
mated in order to produce an F1 litter. On day 21 after birth, 12
males and 24 females were selected as the F1 parents and were kept
on the diet for at least 120 days before they were mated. The study
was terminated after weaning of the F2 litter. Pups were observed
for the usual parameters and also for physiological development
(erection of the auricles, eruption of incisors, development of fur,
opening of the auditory canal and of the eyes) and behavioural
effects (gripping reflex, pupillary reflex, hearing test). In
females of the F0 generation at the highest dose, a slight but
significant increase in food consumption was seen during pregnancy
and in body-weight gain during the entire study period. The
percentage of stillborn pups was slightly but significantly
increased at 25 ppm, with incidences of 1.1% in controls, 4.3% at
6.25 ppm, 2.7% at 12.5 ppm and 6.2% at 25 ppm. A slight but
significant decrease in relative heart weight was observed in males
at 25 ppm. F1 pups at the highest dose showed decreased body
weight from day 14 after birth (not significant in males) and
slightly retarded unfolding of the auricle. Increased relative
weights of the kidney (males) and brain (both sexes) were observed
at 25 ppm. At 25 ppm, F1 male parents had increased relative liver
weights, and females had decreased relative brain weights. In F2
pups, development of fur and opening of the eyes were slightly
retarded at 25 ppm, and one female pup at this dose had
microphthalmia, which was not considered to be related to treatment.
The effects observed at the high dose were considered to be
marginal. The NOAEL was 12.5 ppm, equivalent to 0.62 mg/kg bw per
day (Merkle et al., 1982).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 18 pregnant Sprague-Dawley rats were given
fenpropimorph (purity, 92.5%; dissolved in olive oil) by gavage at
doses of 0, 2.5, 10, 40 or 160 mg/kg bw per day from day 15 of
gestation until day 21 of lactation. The dams were examined for
clinical symptoms, deaths, body weight, litter size, delivery
behaviour, numbers of viable and dead fetuses, implantation rate,
macroscopic appearance and organ weights. Pups were observed for
body weight, sex, clinical signs, physiological development, deaths,
behaviour, viability, macroscopic appearance, organ weights and
skeletal anomalies. At 160 mg/kg bw per day, diarrhoea, salivation,
trembling, reddening of the urogenital area, vaginal bleeding and a
penetrating odour of urine were observed; four of the dams died
during the treatment. Decreased body-weight gain was observed in
dams at doses > 40 mg/kg bw per day. At 160 mg/kg bw per day,
relative heart and kidney weights were decreased, care of pups was
unsatisfactory, and the mean number of viable fetuses was decreased
(1.7 versus 11.7 in the control group), in association with a
considerable increase in the number of dead fetuses. At 160 mg/kg bw
per day, the body weights and weight gain of the pups were reduced,
and erection of the auricle, development of fur and opening of the
eyes and auditory canal were delayed. Increased pup mortality was
observed at doses > 40 mg/kg bw per day, by 25.4% at 40 mg/kg bw
per day and 50.0% at 160 mg/kg bw per day, as compared with 16.3% in
controls. The gripping reflex was decreased in female pups at doses
> 40 mg/kg bw per day. At the highest dose, relative heart and
spleen weights were decreased and relative weights of the liver and
kidney were increased in male pups. In female pups at the highest
dose, the relative weights of the heart, spleen and liver were
increased and the relative kidney weight was decreased. Because few
pups at this dose survived, however, the effects on organ weights
could not be assessed properly. The NOAEL for maternal and
developmental toxicity was 10 mg/kg bw per day (Hofmann & Merkle,
1979a).
Groups of 26-31 pregnant Sprague-Dawley rats were given
fenpropimorph (purity, 92.5%; dissolved in olive oil) by gavage at
doses of 0, 2.5, 10, 40 or 160 mg/kg bw per day on days 6-15 of
gestation. Dams were observed for clinical symptoms, body weight,
mortality, macroscopic appearance of internal organs, conception
rate and numbers of corpora lutea, viable implantations and dead
implantations (early, intermediate and late resorptions). Fetuses
were examined for weight, length, placental weight and external,
skeletal and visceral signs. Vaginal bleeding was observed in one
control, one animal at 10 mg/kg bw per day, three at 40 mg/kg bw per
day and 16 at 160 mg/kg bw per day. A dose-related reduction in
body-weight gain was observed in dams at doses > 40 mg/kg bw per
day during the treatment period and at 160 mg/kg bw per day during
the whole observation period, starting from day 6. At 160 mg/kg bw
per day, the body weights of the dams were reduced and the number of
viable fetuses was decreased, in association with an increased
number of dead implants. The weight and length of the fetuses were
reduced, and placental weight was increased. Irreversible structural
changes, including cleft palate (14/274) and inferior brachygnathia
(1/274), were observed at the highest dose. The NOAELs in this study
were 10 mg/kg bw per day for maternal toxicity and 40 mg/kg bw per
day for embryo- and fetotoxicity and teratogenicity (Hofmann &
Merkle, 1979b).
Rabbits
Groups of 15 female Himalayan rabbits were given fenpropimorph
(purity, 92.5%) by gavage at doses of 0, 2.4, 12.0, 36 or 60 mg/kg
bw per day in 0.5% carboxymethylcellulose (5 ml) during days 6-18 of
gestation. No dose-related effects on the number of corpora lutea,
preimplantation loss or conception rate were observed in dams, and
no effects were seen in fetuses on visceral examination. Diarrhoea
was seen in all groups, which increased in incidence and severity
with the dose. At 60 mg/kg bw per day, severe diarrhoea, salivation,
apathy, a greenish mucous discharge from the nose and encrustations
in the vaginal region and snout were seen, and convulsions were
observed before the deaths of 11 animals. Food consumption and body
weight were reduced. Macroscopic examination of the animals that
died at this dose showed dilatation of the right heart and
congestive hyperaemia; the clinical findings were confirmed.
Absolute uterine weights were reduced at 60 mg/kg bw per day, and
the numbers of early resorptions and dead fetuses were increased
such that only one fetus survived. This fetus had several
abnormalities, including syndactyly on the forelegs, an anomalous
position of the hindlegs and micromelia, and reduced weight and
length; furthermore, the placental weight was increased, and the
individual sternebrae were fused. At 36 mg/kg bw per day, the dams
had clinical signs that were less severe and occurred at a lower
incidence than in the highest dose group. Two animals aborted and
three were killed in extremis. The numbers of dead implantations
(due mainly to early resorptions) were slightly increased at this
dose. Six fetuses had pseudoankylosis. The NOAEL for maternal,
embryo- and fetotoxicity was 12 mg/kg bw per day (Zeller & Merkle,
1980).
In another study, groups of 20 pregnant Russian Chbb:HM rabbits
were gavaged intragastrically with fenpropimorph (purity, 95.6%; in
0.5% aqueous sodium carboxymethylcellulose) at doses of 0, 7.5, 15
or 30 mg/kg bw per day on days 7-19 of gestation. No effects were
observed on mortality, numbers of corpora lutea or live or dead
fetuses, sex ratio, numbers of early or late resorptions or total
postimplantation loss. Treatment-related effects were found only at
30 mg/kg bw per day. Swelling of the anus was observed in dams; food
consumption, body-weight gain, uterine weight and weights of male
fetuses were decreased. Fetuses had an increased total number of
malformations (21/116 in four litters) and anomalies (36/116 in 13
litters). The malformations occurred mainly in the litters of three
dams which showed marked signs of toxicity during treatment. Twenty
of 116 fetuses in three litters had shortened fore- and hindlimbs,
and 4/116 fetuses in two litters had a cleft palate. One of these
fetuses also had exencephaly and open eye, and another had gastro-
and cranioschisis, an asymmetric skull and oedema of the trunk. One
fetus in another litter had a diaphragmatic hernia. Furthermore,
abnormal positions of forelimbs were observed in 25/116 fetuses (in
seven litters) and of the hindlimbs in 8/116 fetuses (in three
litters). The external findings were confirmed by skeletal
examination. The NOAEL was 15 mg/kg bw per day for maternal
toxicity, embryotoxicity, fetotoxicity and teratogenicity (Marty,
1993).
(f) Genotoxicity
The results of tests for the genotoxicity of fenpropimorph are
summarized in Table 2.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
The iritating properties of fenpropimorph to the skin were
examined in five studies in rabbits. Three male and three female New
Zealand white rabbits were administered 0.5 ml of technical-grade
fenpropimorph (purity not specified) on intact and abraded skin for
24 h. Slight erythema was observed in all rabbits directly after
exposure. This reaction increased to well-defined erythema in one
animal after 48 h and in two others at 96 h. Very slight oedema was
observed in one animal after 48 h and in four animals after 96 h. No
signs of irritation were visible after 14 days. The irritating
response of abraded skin was comparable (Leuschner, 1979d).
Three female and one male New Zealand white rabbits were
administered 0.5 ml of technical-grade fenpropimorph (purity not
specified) on intact skin for 4 h and observed at 4 h and after 1,
2, 7 and 14 days. Slight erythema was observed in all rabbits after
one day, and this reaction increased to well-defined erythema in
three rabbits by day 7. Very slight oedema was observed in two
animals at that time. Signs of irritation were no longer visible
after 14 days (Leuschner, 1980).
Six rabbits (sex and strain not specified) were administered
0.5 ml fenpropimorph (purity not specified) on intact and abraded
skin for 24 h and observed at 24 and 72 h and after eight days.
Well-defined erythema and slight oedema were observed in all rabbits
directly after exposure; this reaction increased in severity, and
five of six animals showed necrosis after eight days. The irritating
response of abraded skin was comparable (Liggett & Wilson, 1980a).
Six rabbits (sex and strain not specified) were administered
0.5 ml fenpropimorph (purity not specified) on intact skin for 4 h
and observed at 4, 24, 48 and 72 h and after eight days.
Well-defined erythema and slight oedema were observed in all rabbits
directly after exposure, which persisted until the end of the study.
Skin necrosis was seen in one animal at 48 h, and necrosis or deep
injuries were observed in three animals on day 8 (Liggett & Wilson,
1980b).
Table 2. Results of tests for the genotoxicity of fenpropimorph
End-point Test system Concentration Purity Results Reference
of fenpropimorph (%)
In vitro
Reverse S. typhimurium TA98, 4-2500 µg/plate in 91 Negativea Zeller & Engelhardt,
mutation 100, 1535, 1537, 1538 ethanol; no toxicity 1980
Mitotic gene S. cerevisiae 0.4, 2 or 10 mg/ml 92.5 Negativea Bootman & Lodge, 1981
conversion, D7 in ethanol
reciprocal
crossing over,
mutation,
aneuploidy
Chromosomal Human 0.16-1.66 µg/ml 92.5 Negativea Mosesso & Nunziata,
aberration lymphocytes 5-50 µg/ml Negativeb 1982
Unscheduled Rat hepatocytes 0.1-10 µg/ml in 94.7 Negative Cifone, 1988
DNA ethanol; toxic
synthesis from 5 µg/ml
In vivo
Micronucleus Male Swiss mice 0, 1 or 4 ml/kg bw 92.5 Negative Siou & Conau, 1979d
formation in two oral
administrations
Dominant lethal Male NMRI 0, 212.5 or 425 mg/kg bw; 92.5 Negative Engelhardt & Zeller,
mutation mice single intraperitoneal 1979
injection; toxic at 425
mg/kg bw
a In the absence of metabolic activation
h In the presence of metabolic activation
A dose of 0.5 ml of fenpropimorph (purity, > 91%) was applied
to the intact dorsal skin of four white Vienna rabbits for 3 min or
4 h, and animals were observed up to day 8 after treatment.
Fenpropimorph caused slight to moderate erythema and oedema after
either application. The reactions intensified, and two of four
rabbits had necrosis eight days after the 4-h exposure. In the 3-min
test, animals had necrotic-like skin changes and/or scaling
(Grundler, 1980a).
Fenpropimorph was considered severely irritating and corrosive
to rabbit skin.
The irritancy of fenpropimorph to the eye was examined in three
white Vienna rabbits in a study in which 0.1 ml of fenpropimorph
(purity, > 91%) was instilled into the conjunctival sac of the
right eye. Observations were made up to day 8 after instillation.
Slight to distinct conjunctival redness was observed in all rabbits.
Fenpropimorph was considered to be irritating to the rabbit eye,
since slight irritation was still seen at the end of the study
(Grundler, 1980b).
The sensitizing properties of the compound were examined in two
studies in guinea-pigs. Fenpropimorph (purity not specified) was
applied to the shaved skin of 20 guinea-pigs five days per week for
three weeks, and the application site was left uncovered. Challenge
applications were given on days 21 and 35 of the study. A control
group of 10 animals received the challenge doses only. Slight skin
irritation was observed in 9/10 control animals and 8/20 test
animals. Since there was no difference in the frequency or intensity
of the skin reaction after challenge, fenpropimorph was considered
not to be sensitizing (Klecak et al., 1983).
A maximization test was performed in 12 male Pirbright white
guinea-pigs. During the induction period, the animals were injected
intradermally with 25% fenpropimorph (purity not specified). Five
animals served as a control group. One week later, 50% fenpropimorph
was applied topically, and a challenge application of 25%
fenpropimorph was given 14 days later. The substance did not
sensitize the skin (Gelbke, 1979).
(ii) Delayed neuropathy
The delayed neurotoxicity of fenpropimorph was investigated in
hens using doses based on the results of a preliminary test for the
LD50. Groups of 10 adult hens (20 at the highest dose) were
treated with fenpropimorph (purity, 92.5%; dissolved in olive oil)
in single oral doses of 0, 425, 850 or 1700 mg/kg bw by gavage and
then observed for 21 days. Treated birds were pretreated with
2-pyridine aldoxime methyl chloride and atropine. One group of
controls received only olive oil; a positive control group was
treated with tri- ortho-cresyl phosphate at 500 mg/kg bw in corn
oil. The birds were examined for general health, body weight, food
consumption, mortality and ataxia; after being killed, they
underwent a macroscopic examination and histopathological
observation of the spinal cord and sciatic nerve. Decreased body
weight and reduced food consumption were observed at the two highest
doses during the first three days, and symptoms of weakness and
unsteadiness were observed in all birds at 1700 mg/kg bw and in some
at 850 mg/kg bw. Two of 10 birds at 850 mg/kg bw and 8/20 at 1700
mg/kg bw died. No signs of delayed neurotoxicity were observed in
treated birds (Roberts et al., 1980).
(iii) Liver function and cholinesterase inhibition
In order to study the effects of fenpropimorph on liver
function and cholinesterase inhibition, groups of 40-100 female
Sprague-Dawley rats received a single oral dose of 0, 420, 1240 or
2290 mg/kg bw. Groups of 10 animals were killed after 1, 3, 8 and 15
days, and blood samples were taken. Clinical chemistry included
blood gas analysis and estimation of plasma, erythrocyte and brain
cholinesterase activity; the liver and brain were weighed, and
liver, brain, pancreas and affected organs were examined macro- and
microscopically. The incidence and severity of toxic signs increased
with the dose, and 13 of 100 animals at the highest dose died. Mean
body-weight gain was reduced at the middle and high doses by days 3
and 8 but had returned to normal by day 15. Reduced concentrations
of triglyceride and cholesterol were observed for the first three
days in all treated groups and up to day 8 at the highest dose. The
effect on cholesterol was overcompensated (increased) in the
middle-dose group at day 8 and in the high-dose group at day 15. The
bilirubin level was increased up to day 8 at the middle and high
doses. Alanine aminotransferase activity was increased in the
highest-dose group by day 8 and that of aspartate aminotransferase
by day 3. A reduction in plasma cholinesterase (by up to 51% of the
control value at the high dose) was observed in all treated groups
on days 8 and 15. Erythrocyte cholinesterase activity was not
affected. A slight but significant decrease in brain cholinesterase
activity (by up to 79% of control value at the high dose) was
observed after one, three and eight days at the middle and high
doses but had returned to control levels by the end of the study.
The relative liver weights were increased in all treated animals
from day 3 onwards, and the relative brain weight was increased at
the high dose by days 3 and 8. The effects on organ weights were not
accompanied by histological anomalies. At the end of the study, the
animals given the highest dose had focal skin changes (scaling,
alopecia areata) (Jäckh et al., 1980).
The effect of fenpropimorph (purity not specified) on hepatic
drug-metabolizing enzymes was investigated in groups of 16 male
Spraque-Dawley rats by giving them dietary doses of 0, 50, 250 or
1600 ppm (equivalent to 0, 2.5, 12.5 or 80 mg/kg bw per day) for 14
days. A positive control group was treated with phenobarbital at 50
mg/kg bw per day by gastric gavage. Fenpropimorph induced aniline
hydroxylase, glucuronyl transferase and glutathione S-transferase at
doses > 250 ppm; ethylmorphine N-demethylase was induced at 50
ppm. Phenobarbital sleeping time was reduced at doses > 50 ppm.
The effects were generally of similar magnitude as those in rats
treated with 50 mg/kg bw per day phenobarbital (Hawkins et al.,
1981).
The effect on plasma cholinesterase of a single administration
of fenpropimorph (purity 99.1%) was examined in groups of 20 (40 at
the highest dose) female Sprague-Dawley rats. The compound was
administered at 0, 200, 650 or 2000 mg/kg bw in a 0.5% aqueous
solution of carboxymethylcellulose by a single intraperitoneal
injection; animals were then observed for 72 h, and blood was
sampled at 2, 6, 24 and 72 h. As rats at the highest dose were
comatous, the final samples had to be taken at sacrifice after 30 or
50 h. Blood samples taken after 2 and 6 h showed increased aspartate
aminotransferase activity and bilirubin concentrations in all
treated groups. Plasma cholinesterase was unaffected after 2, 6 and
24 h; after 72 h, dose-related reductions were seen at 200 mg/kg bw
(53% reduction) and at 650 mg/kg bw (64% reduction). Absolute liver
weights were increased at doses > 200 mg/kg bw; and at doses >
650 mg/kg bw peritonitis, perihepatitis and fatty deposits in the
liver were found (Kirsch et al., 1980c).
The effect of fenpropimorph metabolites on cholinesterase
inhibition was studied in vitro. The activity of
acetylcholinesterase from Electrophorus electricus and of plasma
cholinesterase from rats and dogs was decreased by compounds with a
ß-ethanolamine structure, which are structural analogues of a
metabolite of fenpropimorph, N-[3-[4-(1,1-dimethyl-
2-carboxyethyl)phenyl]-2-methyl]propylethanolammonium ion (BF
421-4). The compound tested, which is closely related to
fenpropimorph, showed no inhibitory activity. On the basis of the
results of this study, the authors concluded that fenpropimorph
itself is not directly responsible for the inhibitory effect on
cholinesterase and that the metabolite is responsible for the
effects seen in rats in vivo (Deckardt & Hildebrand, 1980).
3. Observations in humans
Between 1984 and 1991, five cases of dermal irritation of
various grades and one case of Quincke's oedema after eye contact
were recorded at BASF AG, Ludwigshafen, Germany. Two incidents in
which a fenpropimorph formulation was ingested by children aged two
and five passed with no sequelae (Adolph, 1992).
Comments
After oral administration to rats, goats and chickens,
fenpropimorph was rapidly absorbed, distributed and excreted. In
rats and goats, enterohepatic circulation plays an important role.
The half-life in plasma and blood varied from 16 to 24 h. In all
three species, the levels of residues in tissues were relatively
low. No accumulation was detected in organs or tissues; only minor
amounts appeared in milk and eggs. The excretion pattern (rate and
route) was not significantly influenced by the administration route,
number of exposures, species, sex or dose.
Fenpropimorph was extensively metabolized in all three species
studied (rat, goat and chickens), and a small fraction of the parent
compound was found only in chickens. The first metabolic steps
involve the oxidation of the side-chains of the phenyl and the
morpholine rings. Further metabolic steps occur, as indicated by the
detection of many (mostly unidentified) metabolites. In addition,
after administration of the morpholine-labelled molecule to rats,
14CO2 was expired, indicating degradation of the morpholine ring.
Fenpropimorph is slightly to moderately toxic to rats after
acute oral exposure. WHO (1992) has classified fenpropimorph as
unlikely to present an acute hazard in normal use.
In a four-week study of toxicity, rats were given dietary doses
of 0, 100, 250, 625 or 1600 ppm fenpropimorph. Increased liver
weights and reduced haemoglobin were observed in all dose groups. No
NOAEL could be defined. In a three-month study of toxicity, rats
were exposed to fenpropimorph in the diet at levels of 0, 6.25, 12.5
or 25 ppm. On the basis of increased liver weights at doses >
12.5 ppm, the NOAEL was 6.25 ppm, equal to 0.38 mg/kg bw per day.
Dogs were fed diets containing 0, 50, 100, 200 or 400 ppm
fenpropimorph for 13 weeks, or 25, 100 or 400 ppm for 12 months. At
the highest dose, the serum activities of alkaline phosphatase and
both aspartate and alanine aminotransferase were increased and some
slight effects on organ weights were seen. The NOAEL was 200 ppm,
equivalent to 5 mg/kg bw per day, in the 13-week study and 100 ppm,
equal to 3.2 mg/kg bw per day, in the 12-month study.
In a study of carcinogenicity, fenpropimorph was administered
in the diet to mice at doses of 0, 5, 30, 150 or 1000 ppm for 95
weeks (followed by a recovery period). At the highest level, the
main effects were decreased body-weight gain, decreased haemoglobin
in males and increased relative liver weights. In females,
erythrocyte cholinesterase activity was decreased by 26% at 150 ppm
and 29% at 1000 ppm. No effect was found on brain cholinesterase,
and there was no evience of carcinogenicity. The NOAEL was 30 ppm,
equal to 3 mg/kg bw per day.
In a two-year study of toxicity and carcinogenicity, rats were
fed doses of 0, 5, 10, 50 or 250 ppm fenpropimorph in the diet. The
effects seen at 50 ppm and higher were decreased brain
cholinesterase activity and increased relative liver weights in
males, and enlarged hepatocytes in animals of each sex. There was no
evidence of carcinogenicity. The NOAEL was 10 ppm, equal to 0.3
mg/kg bw per day.
A two-generation study of reproductive toxicity was performed
in which rats received dietary doses of 0, 6.25, 12.5 or 25 ppm
fenpropimorph. Several marginal effects were observed, but only in
the group receiving the highest dose. In the F1 generation, an
increased number of stillborn pups was observed, and the pups had
decreased body weight and retarded unfolding of the auricle. In F2
pups, development of the fur and opening of the eyes were retarded.
The NOAEL in this study was 12.5 ppm, equivalent to 0.6 mg/kg bw per
day.
In order to investigate peri- and postnatal effects, pregnant
rats were exposed by gavage to fenpropimorph at doses of 0, 2.5, 10,
40 or 160 mg/kg bw per day from day 15 of gestation to day 21 of
lactation. At the highest dose, many toxic effects were observed in
dams and pups, including unsatisfactory general state and pup care,
decreased body weight, increased number of dead fetuses, increased
pup mortality and diminished physical and behavioural development.
At 40 mg/kg bw per day, the body weights of dams, pup mortality and
gripping reflex (female pups) were affected. The NOAEL for maternal
and developmental toxicity was 10 mg/kg bw per day.
Rats were exposed by gavage to fenpropimorph at doses of 0,
2.5, 10, 40 or 160 mg/kg bw per day on days 6-15 of gestation.
Maternal toxicity, as evidenced by reduced body-weight gain, vaginal
bleeding and an increased number of dead implants (only at 160 mg/kg
bw per day), was observed at doses > 40 mg/kg bw per day. At the
highest dose, the weight and length of the fetuses were reduced, and
irreversible structural changes, such as cleft palate and inferior
brachygnathia, were observed. The NOAEL was 10 mg/kg bw per day for
maternal toxicity and 40 mg/kg bw per day for embryo- and
fetotoxicity and teratogenicity.
Two studies of teratogenicity were performed with rabbits
treated orally. In the first study (with doses of 0, 2.4, 12, 36 or
60 mg/kg bw per day during days 6-18 of gestation), severe maternal
toxicity was observed at 60 mg/kg bw per day; 11 dams died. Only one
fetus with several anomalies survived at this dose. At 36 mg/kg bw
per day, most of the effects were less severe and occurred at a
lower incidence; six fetuses had pseudoankylosis. The NOAEL was 12
mg/kg bw per day for both maternal and embryo- and fetotoxicity.
In the second study, rabbits were exposed by gavage to
fenpropimorph at doses of 0, 7.5, 15 or 30 mg/kg bw per day on days
7-19 of gestation. Maternal and embryo- and fetotoxicity were
observed only at 30 mg/kg bw per day. An increased total number of
malformations and anomalies was observed at this dose. The main
irreversible structural changes were cleft palate and shortened
fore- and hindlimbs. The NOAEL in this study was 15 mg/kg bw per day
for maternal toxicity, embryo- and fetotoxicity and teratogenicity.
Fenpropimorph has been adequately tested for genotoxicity in a
range of tests in vitro and in vivo. The Meeting concluded that
it was not genotoxic.
No sign of delayed neuropathy was observed in chickens treated
with fenpropimorph.
An ADI was established on the basis of the NOAEL of 10 ppm,
equal to 0.3 mg/kg bw per day, in the two-year study of toxicity and
carcinogenicity in rats, and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 30 ppm, equal to 3 mg/kg bw per day (95-week
carcinogenicity study)
Rat: 10 ppm, equal to 0.3 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
12.5 ppm, equivalent to 0.6 mg/kg bw per day
(two-generation study of reproductive toxicity)
10 mg/kg bw per day (maternal toxicity in study of
teratogenicity)
40 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of teratogenicity)
Rabbit: 15 mg/kg bw per day (maternal, embryo- and
fetotoxicity and teratogenicity in study of
teratogenicity)
Dog: 100 ppm, equal to 3.2 mg/kg bw per day (one-year
study of toxicity)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Adolph, W.H. (1992) Fenpropimorph review. Memorandum from Dr Adolph
concerning medical data (DOA/WH-H308). Unpublished review dated 1
February 1992 from BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Bootman, J. & Lodge, D.C. (1981) Fenpropimorph: assessment of its
capacity to induce genetic damage in Saccharomyces cerevisiae.
Project No. 81/BAS008/015. Unpublished report dated 13 January 1981
from Life Science Research, Stock, United Kingdom. Submitted to WHO
by Ciba-Geigy AG, Basel, Switzerland.
Camponovo, F. (1983) Acute oral toxicity of Ro-14-3169/070 in rats.
Project No. B-104 800. Unpublished report dated 3 January 1983 from
F. Hoffmann-La Roche Ltd, Basel, Switzerland. Submitted to WHO by
Ciba-Geigy AG, Switzerland.
Cifone, M.A. (1988) Mutagenicity test on fenpropimorph in the rat;
unscheduled DNA synthesis assay. Project No. 10001-0-447.
Unpublished report dated 8 June 1988 from Hazleton Laboratories
America Inc., Kensington, MD, USA. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Deckardt, K. & Hildebrand, B. (1980) In vitro investigations on the
cholinesterase inhibition by metabolites of morpholine derivatives.
Unpublished report dated 29 October 1980 from Department of
Industrial Hygiene and Toxicology, BASF Aktiengesellschaft,
Ludwigshafen, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
van Dijk, A. & Vogel, W. (1989) 14C-Ro 14-3169: Absorption,
distribution, excretion and metabolism after single intravenous,
single oral and repeated oral administration to the rat. Project No.
063641. Unpublished report dated 24 August 1989 from Research and
Consulting Co., Itingen, Switzerland. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Elliott, P.H. & Mallard, J.R. (1981) The effect of repeated
application of Reg. No. 108 406 and BAS 421 00F to the skin of
rabbits and of Reg. No. 108 406 to the skin of rats. Project No. BSF
325/80715/SA1. Unpublished report dated 22 June 1981 from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Elliott, P.H., Mallard, J.R., Street, A.E., Gibson, W.A., Offer,
J.M., Buckley, P. & Prentice, D.E. (1981) The effect of repeated
application of BAS 421 00F to the skin of rabbits for three weeks.
Project No. BSF 359/801090/SB. Unpublished report dated 2 June 1981
from Huntingdon Research Centre, Huntingdon, United Kingdom.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Engelhardt, G. & Zeller, H. (1979) Report on the investigation of
Reg. No. 108 406 in the dominant lethal test on male mice after
single intraperitoneal administration. Unpublished report dated 12
March 1979 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Gelbke, H.-P. (1979) Dermal sensitization study on the guinea pig
according to the maximization test. Unpublished report dated 6
September 1979 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Grundler, O.J. (1980a) Report on the study of the corrosive effect
of 'Reg. No. 108 406' on the rabbit in the 4-hour test and in the
3-minute test. Unpublished report dated 10 January 1980 from
Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Grundler, O.J. (1980b) Report on the study of the primary irritation
of 'Reg. No. 108 406' on the eye of white rabbits. Unpublished
report dated 10 January 1980 from Department of Industrial Hygiene
and Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hawkins, D.R. & Jackson A.J.S. (1980a) Residues of radioactivity in
milk after oral administration of 14C-BAS 421 F to goats. Project
No. BSF 348/80539. Unpublished report dated 30 July 1980 from
Huntingdon Research Centre, Huntingdon, United Kingdom. Submitted to
WHO by Ciba-Geigy AG, Basel, Switzerland.
Hawkins, D.R. & Jackson A.J.S. (1980b) Excretion of radioactivity
after oral administration of 14C-BAS 421F to goats. Project No.
BSF 348/80545. Unpublished report dated 1 August 1980 from
Huntingdon Research Centre, Huntingdon, United Kingdom. Submitted to
WHO by Ciba-Geigy AG, Basel, Switzerland.
Hawkins, D.R., Down, W.H., Ballard, S.A., Prentice, D.E., Edmonson,
N.A. & Street, A.E. (1981) The effect of BAS 108 306 F on hepatic
drug-metabolizing enzyme activity in the rat. Project no. BSF
368/81203. Unpublished report dated 21 May 1981 from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Hellwig, J., Deckardt, K., Freisberg, K.O. & Hildebrand, B. (1990)
Report on the study of the toxicity of Reg. No. 108 406 in beagle
dogs, administration via the diet over 12 months. Project No.
33D0133/87021. Unpublished report dated 29 June 1990 from Department
of Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hofmann, H.T. & Merkle, J. (1979a) Study of the perinatal and
postnatal toxicity of 4-[3-[4-(1,1-dimethylethyl)phenyl]-
2-methyl]propyl-2,6(cis)-dimethylmorpholine on rats. Unpublished
report dated 16 January 1979 from Department of Industrial Hygiene
and Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hofmann, H.T. & Merkle, J. (1979b) Investigation to determine the
prenatal toxicity of 4-[3-[4-(1,1-dimethylethyl)phenyl]-
2-methyl]propyl-2,6(cis)-dimethylmorpholine on rats. Unpublished
report dated 12 March 1979 from Department of Industrial Hygiene
and Toxicology, BASF Aktien-gesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Hunter, B., Hayman, R.A., Heywood, R., Street, A.E., Prentice, D.E.,
Offer, J.M., Owen, R.A. & Gibson, W.A. (1982a) Reg. No. 108 406:
Assessment of potential tumorigenic effects in prolonged dietary
administration to mice. Project No. BSF 320/81746. Unpublished
report dated 28 June 1982 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Hunter, B., Barnard, A.V., Hayman, R.A., Street, A.E., Heywood, R.,
Prentice, D.E., Isaacs, K. & Gibson, W.A. (1982b) Reg. No. 108 406:
Assessment of potential tumorigenic and toxic effects in prolonged
dietary administration to rats. Project No. BSF 308/81138.
Unpublished report dated 12 March 1982 from Huntingdon Research
Centre, Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Jackson, G.C. & Clark, G.C. (1980) Fenpropimorph Reg No. 108 406:
Acute inhalation toxicity in rats, 4 hour exposure. Project No. BSF
356/80413. Unpublished report dated 6 June 1980 from Huntingdon
Research Centre, Huntingdon, United Kingdom. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Jäckh, Deckardt, K., Gembardt, C., Gelbke H.-P., Hildebrand, B. &
Ruff, M. (1980) Study on the effects of Reg. No. 108 406 (DMM) in
rats after single oral administration by gavage. Unpublished report
dated 21 November 1980 from Department of Industrial Hygiene and
Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Keller, P. (1983) Four hour aerosol inhalation toxicity study (LC50)
with Ro 14-3169-070 in rats. Project No. 014703. Unpublished report
dated 11 February 1983 from Research and Consulting Co., Itingen,
Switzerland. Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Gembardt, C., Gelbke H.-P., Hildebrand, B.
& Byrt, B.E. (1979) Study of the toxicity of Reg. No. 108 406 on
rats in a 3-months feeding experiment. Unpublished report dated 26
November 1979 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Freisberg, K.O., Mirea, D., Schulz, V. &
Byrt, B.E. (1980a) Study of the toxicity of Reg. No. 108 406 in rats
in a 4-week feeding study. Unpublished report dated 30 June 1980
from Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Freisberg, K.O., Gembardt, C., Schulz, V.
& Ruff, M. (1980b) Study of the toxicity of Reg. No. 108 406 in
beagle dogs in a 3-months feeding study. Unpublished report dated 28
April 1980 from Department of Industrial Hygiene and Toxicology,
BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Kirsch, P., Deckardt, K., Gembardt, C., Jäckh, Gelbke, H.-P.,
Hildebrand, B. & Ruff, M. (1980c) Report on the study of the
cholinesterase inhibition of Reg. No. 108 406 in rats after single
intraperitoneal administration. Unpublished report dated 24 October
1980 from Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Klecak, G., Belcis, D. & Schneider, M.M. (1983) Open epicutaneous
test. Project No. 2489a. Unpublished report dated 4 January 1983
from F. Hoffmann-La Roche Ltd, Basel, Switzerland. Submitted to WHO
by Ciba-Geigy AG, Basel, Switzerland.
Klimisch, H.J., Deckardt, K., Gembardt, C., Hildebrand, B. & Ruff,
M. (1981) Study of the subchronic inhalation toxicity of Reg. No.
108 406 in Sprague Dawley rats (4-week aerosol study). Unpublished
report dated 5 August 1981 from Department of Industrial Hygiene and
Toxicology, BASF Aktien-gesellschaft, Ludwigshafen, Germany.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Leuschner, F. (1978a) The acute oral toxicity of the preparation
Reg. No. 108 406 in rats. Project No. 78/164-1. Unpublished report
dated 8 June 1978 from Laboratorium für Pharmakologie und
Toxikologie, Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Leuschner, F. (1978b) The acute local toxicity of the preparation
Reg. No. 108 406 in rats. Project No. 78/164-1. Unpublished report
dated 31 July 1978 from Laboratorium für Pharmakologie und
Toxikologie, Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Leuschner, F. (1978c) The acute intraperitoneal toxicity of the
preparation Reg. No. 108 406 in rats. Project No. 78/164-1.
Unpublished report dated 8 June 1978 from Laboratorium für
Pharmakologie und Toxikologie, Hamburg, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Leuschner, F. (1979d) Tolerance of the intact and abraded rabbit
skin of the preparation Reg. No. 108 406. Unpublished report dated
19 January 1979 from Laboratorium für Pharmakologie und Toxikologie,
Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
Leuschner, F. (1980) Examination on causticity of fenpropimorph
techn. in rabbits during a 4-hours test. Unpublished report dated 10
April 1980 from Laboratorium für Pharmakologie und Toxikologie,
Hamburg, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
Liggett, M.P. & Wilson, J.C. (1980a) Irritant effects of Reg. No.
108 406 on rabbit skin. Project No. 80401 D/BSF 357/SE. Unpublished
report dated 8 July 1980 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Liggett, M.P. & Wilson, J.C. (1980b) Irritant effects of Reg. No.
108 406 on rabbit skin. Project No. 80401 D/BSF 358/SE. Unpublished
report dated 8 July 1980 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Marty, J. (1993) CGA 101'031 technical: Rabbit oral teratogenicity.
Project No. 923154. Unpublished report dated July 1993 from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Ciba-Geigy
AG, Basel, Switzerland.
Merkle, J., Freisberg, K.O., Hildebrand, B. & Ruff, M. (1982) Report
on a reproduction study with 4-[3-[4-(1,1-dimethylethyl)phenyl]-
2-methyl]propyl-2,6(cis)-dimethylmorpholine (Reg. No. 108 406) in
rats after oral administration (feeding). Two-generation study.
Unpublished report dated 22 February 1982 from Department of
Industrial Hygiene and Toxicology, BASF Aktiengesellschaft,
Ludwigshafen, Germany. Submitted to WHO by Ciba-Geigy AG, Basel,
Switzerland.
Mosesso, P. & Nunziata, A. (1982) Report on experiment of chromosome
aberrations in human lymphocytes with and without metabolic
activation on WNT 81/200 (Fenpropimorph) of BASF AG, Ludwigshafen
(Germany). Project No. CRF 282/M. Unpublished report dated 16 March
1982 from Centro Ricerca Farmaceutica SPA, Pomezia (Roma), Italy.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
von der Mühll, F. & Gätzi, M. (1979) 14C-Ro-14-3169/005: Rat
balance study after oral administration. Unpublished report dated 14
June 1979 from F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Pryde, A., Etterli, M. & Oesterfelt, G. (1979) Metabolism of
14C-Ro14-3169/005 in the rat. Unpublished report dated 28
September 1979 from F. Hoffmann-La Roche Ltd., Basel, Switzerland.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Pryde, A., Etterli, M. & Oesterfelt, G. (1980) Metabolism of
14C-Ro14-3169/005 in the rat: Additional results. Unpublished
report dated 14 January 1980 from F. Hoffmann-La Roche Ltd, Basel,
Switzerland. Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Ritter, A. (1989) Distribution, excretion and metabolism of
14C-BAS 421 F after repeated oral administration to laying hens.
Project No. 081314. Unpublished report dated 9 November 1989 from
Research and Consulting Co., Itingen, Switzerland. Submitted to WHO
by Ciba-Geigy AG, Basel, Switzerland.
Ritter, A. & Vogel, W. (1989) Distribution, degradation and
excretion of 14C-BAS 421 F after repeated oral administration to
lactating goats. Project No. 081303. Unpublished report dated 13
October 1989 from Research and Consulting Co., Itingen, Switzerland.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
Roberts, N.L., Fairley, C., Prentice, D.E. & Wight, D.G.D. (1980)
The acute oral toxicity (LD50) and neurotoxic effects of Reg. No.
108 406 to the domestic hen. Project No. BSF 336/80382. Unpublished
report dated 8 October 1980 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Ciba-Geigy AG,
Basel, Switzerland.
Siou, G. & Conau, L. (1979) Research on the mutagenous action of
fenpropimorph by means of the micronucleus test technique.
Unpublished report dated 23 April 1979 from Cabinet d'Etudes et de
Recherche en Toxicologie Industrielle (CERTI), Versailles, France.
Submitted to WHO by Ciba-Geigy AG, Basel, Switzerland.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Zeller, H. & Engelhardt, G. (1980) Report on the study of Reg. No.
108 406 in the Ames test. Unpublished report dated 8 December 1980
from Department of Industrial Hygiene and Toxicology, BASF
Aktiengesellschaft, Ludwigshafen, Germany. Submitted to WHO by
Ciba-Geigy AG, Basel, Switzerland.
Zeller, H. & Merkle, J. (1980) Study to determine the prenatal
toxicity of 4-[3-[4-(1,1-dimethyl-ethyl)phenyl]-2-
methyl]propyl-2,6(cis)-dimethylmorpholine in rabbits. Unpublished
report dated 7 May 1980 from Department of Industrial Hygiene and
Toxicology, BASF Aktiengesellschaft, Ludwigshafen, Germany. Submitted
to WHO by Ciba-Geigy AG, Basel, Switzerland.
