
TEBUCONAZOLE
First draft prepared by
E. Bosshard,
Federal Office of Public Health, Division of Food Science,
Schwerzenbach, Switzerland
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Embryotoxicity and teratogenicity
Genotoxicity
Special studies
Skin and eye irritation and skin sensitization
Cataract induction
Studies on metabolites
Acute toxicity
Short-term toxicity
Embryotoxicity and teratogenicity
Genotoxicity
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Tebuconazole is a triazole fungicide which inhibits ergosterol
biosynthesis. It was evaluated for the first time by the present
Meeting.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution and excretion
Rats were treated with single doses of 2 or 20 mg/kg bw per day
of tebuconazole labelled with 14C, either uniformly in the phenyl
ring or in the 3,5-triazole ring, with or without pretreatment with
unlabelled compound. Tebuconazole was excreted predominantly in the
bile and faeces: Male rats with biliary fistulae excreted about 90%
of the dose with the bile, and faecal excretion within 72 h after
administration was about 80% of the applied dose in males and about
65% in females; urinary excretion amounted to about 16% of the
applied dose in males and about 35% in females. The amount excreted
was not related to the administered dose. These results indicate
that enterohepatic recirculation occurs in intact animals. The sex
difference in excretion was observed only with the phenyl-labelled
compound. After administration of triazole-labelled tebuconazole,
about 70-80% of the administered dose was excreted in faeces and up
to 25% in urine. Maximal plasma concentrations were reached 0.5-2 h
after administration. The plasma concentration of radioactivity rose
more slowly and terminal elimination from plasma proceeded faster
after administration of the high dose. Less than 1% of the
administered dose was recovered in the tissues two to three days
after administration; liver contained the most tissue residues. Male
animals in all groups had higher residue levels than females (Weber,
1987, 1988; Chopade, 1992).
Uniformly labelled [14C-phenyl]-tebuconazole was administered
to a lactating goat at a dose of 15 mg/kg bw per day for three
consecutive days. Organ and milk samples were analysed 2 h after the
last dose. Residue levels were highest in kidney (4 ppm) and liver
(5 ppm); less than 0.1 ppm of residues were found in fat, muscle and
milk (Lee & Wood, 1987).
The excretion of tebuconazole was studied in laying hens. Of a
dose of 10 mg/kg bw per day administered orally on three consecutive
days, about 80% was excreted within the first 3.5 h. One-half hour
after the last dose, 8 ppm were found in liver, 6 ppm in kidney and
0.15 ppm in eggs (Lee et al., 1988; Ecker & Weber, 1991).
The permeability of human and rat skin in vitro to a series
of 25% w/w EC formulations of tebuconazole was determined using
14C-tebuconazole and two reference compounds, [4-14C]-
hydrocortisone and [4-14C]-testosterone. Within 24 h, up to 37% of
a dose of 1.25 g/l tebuconazole in water had permeated human skin,
as compared with about 22% of testosterone and up to 5% of
hydrocortisone. In the rat, permeation by tebuconazole was lower
than that by testosterone but higher than that by hydrocortisone
(Brain, 1991).
Groups of rats were treated dermally with doses of 0.01, 0.1, 1
or 10 mg/rat of 14C-tebuconazole in ethanol, corresponding to
actual doses of 0.604, 5.86, 52.4 or 547 µg/cm2. Radioactivity was
measured in the urine, faeces, blood and carcass and at the
application site 0.5, 1, 2, 4, 8 and 24 h after treatment to provide
an estimate of skin absorption. At doses up to 52.4 µg/cm2, about
60% of the applied dose was absorbed within 24 h; at the highest
dose, about 12% was absorbed through the skin. Most of the dose was
absorbed within the first few hours of application (Eigenberg,
1991).
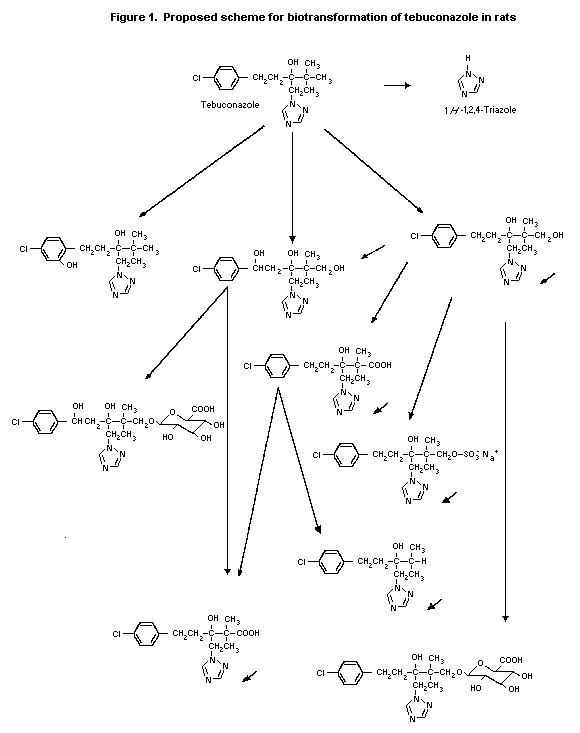
(b) Biotransformation
In the study described above in which rats were treated with
tebuconazole labelled with 14C either in the phenyl ring or in the
3,5-triazole ring, with or without pretreatment with unlabelled
compound (Weber, 1987, 1988; Chopade, 1992), the main metabolites
were the oxidation products of one of the methyl groups of the
tertiary butyl moiety, i.e. the alcohol and the carboxylic acid.
Metabolism in female animals resulted preferentially in simple
oxidation products (e.g. hydroxy and carboxy metabolites) and then
conjugation to the glucuronide and sulfate, with only minor cleavage
of the triazole moiety. In male animals, the primary oxidation
products were further oxidized to triol and keto acid derivatives;
in addition, cleavage of triazole occurred, as indicated in trials
with triazole-labelled compound. The free triazole accounted for
about 5% in the urine of the males and 1.5% in that of females.
Parent compound was found in only minor amounts (Ecker et al.,
1987; Weber, 1987, 1988; Chopade, 1992). The proposed
biotransformation pathway in rats is shown in Figure 1.
In the study described above in lactating goats, the metabolic
pathway was similar to that found in rats. The major metabolite
identified was the tert-butyl alcohol derivative and its
conjugate; the parent compound was also found (Lee & Wood, 1987).
In the study in laying hens described above, hydroxylation of
the tert-butyl group followed by conjugation to the sulfate was
the major metabolic pathway (Lee et al., 1988; Ecker & Weber,
1991).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of tebuconazole is summarized in Table 1.
Table 1. Acute toxicity of tebuconazole
Species Sex Route LD50 (mg/kg bw) Reference
or LC50 (mg/m3)a
Rat (fasted) M Oral > 5000 Heimann & Pauluhn, 1983
F 3930
Rat M Oral 4260 Heimann & Pauluhn, 1983
F 3350
Rat (fasted) M Oral 4000 Ohta, 1991a
F 1700
Rat M Intraperitoneal 750 Heimann & Pauluhn, 1983
F 395
Rat M&F Dermal (24 h) > 5000 Heimann & Pauluhn, 1983
Rat M&F Dermal (24 h) > 2000 Ohta, 1991b
Rat M&F Inhalation (4 h)b > 818 Heimann & Pauluhn, 1983
Inhalation (5x6 h)b > 240
Rat M&F Inhalation (4 h); aerosol > 371 Pauluhn, 1988
Inhalation (4 h); dust > 5093
Mouse (fasted) M Oral 1615 Heimann & Pauluhn, 1983
F 3023
Mouse (fasted) M Oral 2800 Ohta, 1991c
F 5200
Rabbit (fasted) M&F Oral > 1000 Heimann & Pauluhn, 1983
Dog (fasted) M&F Oral 625-1250 Hoffmann, 1983a
Sheep (fasted) M&F Oral 625-1250 Hoffmann, 1983b
a Concentrations determined analytically
b In ethanol and polyethylene glycol
The symptoms of acute poisoning described in rats consisted of
sedation, locomotor incoordination, spastic gait and emaciation.
A study was conducted in male rats to study possible
combination effects of tebuconazole with either triadimenol or
dichlofluanid. A slightly superadditive effect was observed in
combination with triadimenol but not with dichlofluanid (Flucke,
1987).
 (b) Short-term toxicity
Rats
Groups of 20 male and 20 female Wistar rats (Bor: WISW) were
given tebuconazole at doses of 0, 30, 100 or 300 mg/kg bw per day by
intubation for four weeks and observed during a four-week recovery
period. The dose of 300 mg/kg bw per day caused mild lethargy and
reduced body-weight gain during the treatment period. Doses of 100
mg/kg bw per day and above decreased the haemoglobin concentration
and haematocrit values. In females at 300 mg/kg bw per day, the
leukocyte count was increased. Clinical chemistry revealed slight,
not statistically significant, increases in the activities of
glutamate-oxalate and glutamate-pyruvate transaminases in males at
300 mg/kg bw per day, and marked increases in liver enzyme
activities (glutamate-oxalate and glutamate-pyruvate transaminases
and alkaline phosphatase) in females at the same dose. Treatment at
100 and 300 mg/kg bw per day induced the microsomal enzyme system,
and the activities of N- and O-demethylases and cytochrome P450
and the triglyceride concentration in liver were increased. All
these changes were reversible. Urinalyses revealed no abnormal
findings. At 100 and 300 mg/kg bw per day, the weights of the liver
and spleen were increased in animals of each sex and the weight of
the kidney in females. Histopathological findings at 300 mg/kg bw
per day consisted of fatty changes in the liver and bile-duct
proliferation in females; enlargement of the centrilobular
hepatocytes was found in male rats. Histopathological changes were
also found in the adrenal cortex, consisting of proliferated
endothelial cells and an increased incidence of fat vacuoles.
Sclerosis of the red pulp of the spleen, associated with
sideropenia, was observed in males at 300 mg/kg bw per day;
sideropenia was also found in females at 100 mg/kg bw per day. The
NOAEL was 30 mg/kg bw per day on the basis of changes in
haematological and clinical chemical parameters and organ weights at
higher doses (Heimann & Kaliner, 1984).
Groups of 10 male and 10 female Wistar rats (Bor: WISW) were
exposed to nominal aerosol concentrations of tebuconazole at 0, 5,
50 or 500 mg/m3 in polyethylene glycol by head and nose exposure
for 6 h/day, five days per week for three weeks (15 days). The
analytical concentrations were 0, 1.2, 11 and 156 mg/m3. About 90%
of the particle mass had an aerodynamic diameter of < 5 µm. The
treatment had no effect on mortality rate, body-weight gain,
haematological or clinical chemical parameters or organ weights;
urinalysis showed no abnormal findings. Rats treated with 156
mg/m3 had piloerection after each exposure. Mixed-function
oxidases in the liver were induced. At the end of the study,
N-demethylase activity in the liver was increased in animals of
each sex at the highest dose. Males in this group also had increased
O-demethylase activity. No treatment-related gross or
histopathological alterations were observed. The NOAEL was 10.6
mg/m3, equivalent to about 2 mg/kg bw per day, on the basis of
liver enzyme induction at the highest concentration (Pauluhn, 1985,
1987).
Groups of 10 male and 10 female rats (Wistar, BOR:WISW) were
given dietary concentrations of tebuconazole (purity, 93.4%; 4.8%
symmetrical isomer) at 0, 100, 400 or 1600 ppm, equal to 9, 35 or
172 mg/kg bw per day, for 13 weeks. The treatment had no effect on
appearance or behaviour or the findings of ophthalmic or
haematological examinations or urinalyses. Food intake was increased
in animals of each sex at 1600 ppm. Retardation of body-weight gain
was observed at 400 ppm in females during the first six weeks and at
1600 ppm in animals of each sex. Two animals at 1600 ppm died; no
deaths occurred in the other dose groups. Few of the changes in
clinical chemistry showed a dose-related or time-consistent pattern
and were regarded as toxicologically insignificant. An increase in
urea concentration at 1600 ppm and a decrease in triglyceride
concentrations in animals at 400 ppm and above were seen only after
the first four weeks and not at the end of the study. Pronounced
increases in N-demethylase activity and cytochrome P450 content
were found in male animals at 1600 ppm, and increased liver weights
were seen in females at this dose. Histopathological examination
revealed an increased incidence of intra-plasmatic vacuoles in the
cells of the zona fasciculata of the adrenals (probably lipid
accumulation) in some females at 400 ppm and in all females at 1600
ppm. This effect was less pronounced in males because of a higher
background incidence of adrenal vacuole formation in control animals
(Bomhard & Schilde, 1986). The NOAEL was 100 ppm, equal to 9 mg/kg
bw per day, on the basis of retardation of body-weight gain and
histopathological changes in the adrenals at higher doses
Rabbits
Groups of 18 male and 18 female HC New Zealand white rabbits
received tebuconazole at doses of 0, 50 or 250 mg/kg bw per day on
the shorn skin for 6 h daily for three weeks. The treatment was
tolerated with no effects (Heimann & Schilde, 1984). In an
additional study, a dose of 1000 mg/kg bw per day was also tolerated
with no systemic or local effects (Heimann & Schilde, 1988).
Dogs
Groups of four male and four female beagle dogs were given
tebuconazole (purity, 93.4%; 4.8% symmetric isomer) at dietary
concentrations of 0, 200, 1000 or 5000 ppm (equal to 9, 45 or 220
mg/kg bw per day for males and females combined) over 13 weeks. One
dog at 5000 ppm was found dead after the first dose, with no
previous clinical signs. Treatment did not affect body temperature,
pulse rate or neurological signs. Food consumption was reduced at
1000 and 5000 ppm, and body-weight gain was retarded in these
groups; a few dogs at 1000 ppm and most of those at 5000 ppm had a
deteriorated nutritional status. Ophthalmic examination revealed
lens opacities in all animals at 5000 ppm, which first appeared
after seven weeks of treatment. An increase in thrombocyte count was
found at 5000 ppm, and at the end of the study six of eight animals
at this dose had marked anisocytosis. Alkaline phosphatase activity
in plasma showed a retarded age-induced fall at 1000 ppm and an
increase at 5000 ppm. N-Demethylase activity in the liver had
increased slightly by the end of the study in animals at 1000 ppm
and had increased markedly in those at 5000 ppm. The cytochrome P450
content of the liver was also elevated at 5000 ppm. In the group at
the highest dose, a shift in the composition of serum proteins was
observed, with a slightly lower albumin content and a simultaneous
increase in the ß-globulin content. Urinalyses revealed no
treatment-related effects. Changes in organ weights did not follow a
consistent pattern, except that an increase in spleen weights was
seen in animals of each sex at 5000 ppm. Histopathological
examination confirmed lens degeneration, indicating the induction of
cataracts in animals at 5000 ppm. Other histopathological
alterations observed in this group included slightly increased
accumulation of ferriferrous pigments in Kupffer cells of the liver
and of siderocytes in spleen. The NOAEL was 200 ppm, equal to 9
mg/kg bw per day, on the basis of reduced body-weight gain and food
consumption and liver enzyme induction at 1000 ppm and higher. The
NOAEL for cataract induction was 1000 ppm, equal to 45 mg/kg bw per
day (von Keutz & Schilde, 1987a).
In a one-year study, groups of four male and four female beagle
dogs were given tebuconazole (purity, 96.9%) at dietary
concentrations of 0, 40, 200 or 1000 ppm (weeks 1-39) and 2000 ppm
(weeks 40-52), equal to 2, 10 or 60 mg/kg bw per day in males and
females combined. Concentrations of up to 2000 ppm did not affect
survival rate, appearance, behaviour or organ weights, and
haematological examination and urinalyses showed no changes. Body
temperature, pulse rates and reflexes were normal, as were food and
water consumption and body-weight gain. Ophthalmic examination
revealed lens opacities in two dogs at 200 ppm and one at 1000 ppm;
the opacities appeared between weeks 26 and 32 and were of the same
intensity at all subsequent examinations. In the single dog at 1000
ppm which showed this ocular change, corneal opacity was also found,
which persisted until the end of the study, whereas the lens
opacities disappeared after week 32 of treatment. The lack of a
dose-response relationship with regard to lens opacities does not
preclude an association with treatment but may be due to the small
number of animals in the group and differences in individual
sensitivity. None of the other animals in this group had lens stars;
in single animals, faint lens stars were already present before
treatment started but did not become more pronounced with the
treatment. Incipient lens stars observed in animals at 40 and 200
ppm also remained stable, and most disappeared before the end of the
treatment period. Lens stars are physiological structures found
occasionally in juvenile dogs. Clinical chemical analyses revealed
slight, dose-related changes in the activity of alkaline
phosphatase: Whereas the age-dependent reduction in activity was
similar in control animals and in those at 40 and 200 ppm, the mean
activity in animals at 1000/2000 ppm indicated slight induction,
resulting in a retardation in the physiological fall in alkaline
phosphatase activity. The activity of N-demethylase and the
triglyceride content of the liver were slightly increased in animals
at 1000/2000 ppm. Most of the gross pathological findings, such as a
dose-related increase in the incidence of livers with marked
lobulation in animals treated with 200 ppm and above, were not
correlated with histopathological alterations. Histopathological
findings included intracytoplasmic vacuoles in cells of the zona
fasciculata of the adrenals in animals at 200 and 1000/2000 ppm and
slight siderosis in the spleen in most animals at 1000/2000 ppm. The
NOAEL was 40 ppm, equal to 2 mg/kg bw per day, on the basis of
cataract formation and histopathological changes in the adrenals at
higher doses (von Keutz & Schilde, 1987b).
In a second one-year study conducted at lower doses, groups of
12 male and 12 female beagle dogs were given tebuconazole (purity,
96%) at dietary concentrations of 0, 100 or 150 ppm, equal to 3 or
4.5 mg/kg bw per day for males and females combined. The treatment
did not affect mortality, body-weight gain, food consumption,
biochemical, haematological or urinary parameters, ophthalmoscopic
findings, gross pathological appearance or organ weights. The only
histopathological alteration was slight hypertrophy of adrenal zona
fasciculata cells in all animals at 150 ppm; only one control animal
had similar changes. The enlargement was accompanied by an increased
incidence of large fatty vacuoles. The NOAEL was 100 ppm, equal to 3
mg/kg bw per day, on the basis of histopathological alterations in
the adrenals at the higher dose. The NOAEL for cataract induction
was 150 ppm, equal to 4.5 mg/kg bw per day (Porter et al., 1989).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female NMRI mice were given
tebuconazole (purity, 95%) at dietary concentrations of 0, 20, 60 or
180 ppm, equal to 6, 18 or 53 mg/kg bw per day, for 21 months.
Groups of 10 treated animals of each sex were sacrificed after one
year of treatment. Appearance, behaviour, mortality, food
consumption and body weight were not affected by the treatment. In
animals treated at 180 ppm, reduced erythrocyte count and
haemoglobin and haematocrit values were observed, particularly at
the end of the first year of the study. Sporadic alterations in
clinical chemical parameters were observed in all groups. The few
dose-dependent changes consisted of an increase in total bilirubin
concentration in females treated with concentrations of 60 ppm and
higher and decreased cholesterol concentrations in plasma at 180
ppm. Gross pathological examination showed no treatment-related
effects. Liver weights were increased in animals at 180 ppm at
interim sacrifice and at the end of the study. Histopathological
examination revealed a slight increase in the prevalence of
lipid-containing periportal vacuoles in the livers of animals
treated with 60 or 180 ppm at both interim and terminal sacrifice.
No increase in tumour incidence was found. The NOAEL was 20 ppm,
equal to 6 mg/kg bw per day, on the basis of histopathological
changes in the liver (Bomhard & Ramm, 1988a).
In a similar study using higher doses, groups of 50 male and 50
female NMRI mice were maintained on a diet containing tebuconazole
(purity, 95%) at concentrations of 0, 500 or 1500 ppm, equal to 85
or 280 mg/kg bw per day, for 21 months. Groups of 10 animals per sex
and dose were scheduled for interim sacrifice after 12 months. The
mortality rate was not affected by treatment. The incidence of
animals with a 'distended abdomen' (probably due to liver
enlargement) was increased among those at 1500 ppm. A dose-dependent
reduction in body-weight gain was observed in males at 500 and 1500
ppm and in females at 1500 ppm, particularly during the first half
of the study. Food intake tended to be higher in treated animals
than in controls. Alterations in haematological parameters were
found in both treated groups: At 500 ppm and above, reduced mean
cell volume and haematocrit values and elevated mean cell
haemoglobin concentrations were found in males, and thrombocyte
counts were elevated in females. Thromboplastin time was reduced in
both treated groups. At 1500 ppm, erythrocyte counts, haemoglobin
content and haematocrit values were markedly reduced and thrombocyte
counts were elevated, particularly in males. Leukocyte counts were
also increased.
Parameters of clinical chemistry that showed dose-related
changes in both treated groups included pronounced increases in the
activities of glutamate-oxalate and glutamate-pyruvate transaminases
and alkaline phosphatase, particularly at 1500 ppm. Cholesterol,
bilirubin and albumin levels were reduced at 500 and 1500 ppm, but
there was no clear dose-response relationship. Liver weights were
increased in a dose-related fashion at both doses, and adrenal
weights were elevated in females at 1500 ppm. The gross pathological
evidence for treatment-related effects on the liver consisted of
enlargement and pale appearance of the liver in both treated groups
and some enhanced lobulation. Capsular thickening and swelling and
an increased incidence of nodular masses were also found at 1500
ppm. Histopathological examination revealed treatment-related
alterations, mainly in the liver. The non-neoplastic findings
consisted of single-cell and focal necroses, focal inflammation,
hepatocytic degeneration, hepatocytic hyerplasia and hypertrophy,
fatty vacuolation, bile-duct hyperplasia and steatoses, oval-cell
proliferation, extramedullary haematopoiesis and pigment
accumulation in Kupffer cells. Many of these alterations were found
at both 500 and 1500 ppm.
No evidence was found for a compound-related effect on the
incidence of benign or malignant tumours after one year of
treatment. Increased incidences of tumour-bearing animals were seen,
however, at 1500 ppm after 21 months of treatment. Increases were
seen in the incidences of both benign and malignant tumours in males
and of malignant tumours in females. Statistically significant
increases in tumour incidence were found only in the liver. An
increased incidence of adenomas was observed at 1500 ppm, with 36%
(versus 6% in controls) in males and 4% (versus 0 in controls) in
females; the incidences of carcinomas at this dose were 21% (versus
0 in controls) in males and 26% (versus 2% in controls) in females.
The incidences of hepatocellular tumours in animals at 1500 ppm were
reported to be above those in historical control mice of this
strain. The only reported incidences were 0-12% for adenoma and
0-10% for carcinoma in males. The NOAEL was < 500 ppm, equal to <
85 mg/kg bw per day, on the basis of reduced body-weight gain and
histopathological changes in the liver. The NOAEL for
carcinogenicity was 500 ppm, equal to 85 mg/kg bw per day, on the
basis of an increased incidence of liver tumours at 1500 ppm
(Bomhard, 1991).
Rats
Groups of 50 male and 50 female Wistar rats (Bor:WISW) were
given tebuconazole (purity, 95%) in the diet at concentrations of 0,
100, 300 or 1000 ppm, equal to 5, 16 or 55 mg/kg bw per day, for two
years. Groups of 10 animals per sex and dose were similarly treated
and killed at interim sacrifice after 12 months of treatment.
Treatment did not affect mortality, appearance, general behaviour of
the animals or haematological parameters, and urinalyses showed no
changes; no treatment-related damage to the eyes was found.
Body-weight gains were lower than those of the control animals at
doses of 300 ppm and above. Food consumption was increased among
females at 1000 ppm, and water consumption was decreased in females
at 300 ppm and above. Clinical examinations revealed several
statistically significant changes but no consistent dose-related
pattern indicative of an association with treatment, with the
probable exception of a marginal increase in the activity of alanine
aminotransferase at 1000 ppm. Decreased triglyceride concentration
was found in female animals at 1000 ppm, and an increase in spleen
weight was found in these animals. Gross pathological examination
did not provide any indication of treatment-related effects.
Histopathological examination revealed an increased incidence of
pigmented Kupffer cells in liver and increased haemosiderin
accumulation in the spleen of females at 1000 ppm.
The incidence of C-cell adenomas and carcinomas of the thyroid
was increased in all treated males in comparison with that in
controls (0/50), with 2/49 (4%) at 100 ppm, 3/50 (6%) at 300 ppm and
3/50 (6%) at 1000 ppm There was no clear dose-response relationship,
and the rates were within the range of spontaneously occurring
thyroid C-cell tumours in male Wistar rats. The mean historical
incidences of interstitial-cell and C-cell adenomas grouped with
parafollicular tumours were reported to be 7.4% (0-19.3) in males
and 8.4% (2.5-21.2) in females (Bomhard et al., 1986). The
incidence of 0 in concurrent controls is unusually low. In female
rats, a higher frequency of endometrial adenocarcinoma was found in
comparison with controls (0/50), with incidences of 3/50 at 100 ppm,
2/50 at 300 ppm and 1/50 at 1000 ppm These incidences were also
small and not dose-related. The reported historical incidence of
uterine adenocarcinomas varied considerably, from 0 to 14.4%, with a
mean incidence of 6.3% (Bomhard et al., 1986). The fact that the
endometrial tumours found in this study differ morphologically from
the typical uterine carcinomas that occur spontaneously in this rat
strain precludes a direct comparison, either qualitatively or
quantitatively. The absence of a dose-response relationship may
indicate, however, that these findings were not treatment-related.
The NOAEL was 100 ppm, equal to 5 mg/kg bw per day, on the basis of
reduced body-weight gain at higher doses (Bomhard & Ramm, 1988b).
(d) Reproductive toxicity
Rats
Groups of 25 male and 25 female Wistar rats (Bor:WISW) were
treated with tebuconazole (purity, 95%) at dietary concentrations of
0, 100, 300 or 1000 ppm, equal to 7, 22 or 72 mg/kg bw per day, over
two generations. Treatment did not affect appearance, behaviour,
general condition or mortality rates, and fertility and gestation
indexes were not altered. At 1000 ppm, the body-weight gain of the
parent animals was reduced; food consumption was reduced only among
males at the highest dose in the F0 generation and in animals of
each sex in the F1 generation. Reductions in the mean litter size
at birth, in the viability index (survival until day 5 after birth)
and in the lactation index were seen at 1000 ppm, and mean birth
weights (only in the F1a generation) and body-weight gain (in both
generations) were reduced. None of the pups had grossly apparent
malformations, and no treatment-related organotoxic effects were
noted on gross and microscopic examination. The NOAEL was 300 ppm,
equal to 22 mg/kg bw per day, on the basis of reduced body-weight
gain in parents and pups and reduced litter size at the highest dose
(Eiben, 1987).
(e) Embryotoxicity and teratogenicity
Mice
Groups of 25 female NMRI/ORIG Kisslegg mice were treated by
gavage with tebuconazole (purity, 93.6%) at a dose of 0, 10, 30 or
100 mg/kg bw per day on days 6-15 of gestation. The treatment did
not affect mortality rates, body-weight gain or pregnancy rates of
the maternal animals. At 30 mg/kg bw per day and higher, a
dose-related increase in the incidence of runts was observed, with
20/234 (8.6%) at 30 mg/kg bw per day and 26/202 (13%) at 100 mg/kg
bw per day; there were 4/234 (2%) at 10 mg/kg bw per day and 5/236
(2%) in the control group. An increased incidence of malformations
was found at 100 mg/kg bw per day, 13/202 (6.5%), which consisted
most frequently of cleft palates and individual cases of
micrognathia, rib fusion and spinal dysplasia; the incidence of
malformations in the control group was 1/236 (0.4%). The incidence
of cleft palate at this dose, 6/202 (3%), was also markedly higher
than the mean incidence in historical controls, 0.7%. The NOAEL for
maternal toxicity and clinical signs was 100 mg/kg bw per day and
that for embryotoxicity and teratogenicity was 10 mg/kg bw per day
(Renhof, 1988a).
In a study to investigate maternal toxicity further, groups of
10 female NMRI/ORIG Kisslegg mice were treated by gavage with
tebuconazole (purity, 97.4%) at doses of 0, 10, 20, 30 or 100 mg/kg
bw per day on days 6-15 of gestation. Haematological and clinical
chemical tests showed no effect of treatment on mortality rates or
body weights of the maternal animals; slight reductions in the
haematocrit and mean corpuscular volume were seen at 30 and 100
mg/kg bw per day, but with no clear dose-response relationship. The
activities of alanine and aspartate transaminases showed slight,
usually statistically significant increases in all treated groups
but with no consistent relation to dose. Triglyceride levels were
increased at 100 mg/kg bw per day. Liver weights were increased at
all doses, again with no clear dose-response relationship.
Histopathological investigation revealed cytoplasmic vacuolation of
the liver at 100 mg/kg bw per day, which was correlated with an
increase in lipid content. The dose of 100 mg/kg bw per day thus
produced pronounced liver toxicity; the changes in liver enzyme
activities, unrelated to dose, in all treated groups indicate that
maternal toxicity may have been induced at 10 mg/kg bw per day. The
embrotoxic and teratogenic effects thus occur at maternally toxic
doses. The NOAEL for maternal toxicity was < 10 mg/kg bw per day
(Renhof & Karbe, 1988).
Groups of 25 female NMRI/HAN mice were given tebuconazole
(purity, 96-98%; different batches used for main and supplementary
study) at daily dermal doses of 0, 100, 300 or 1000 mg/kg bw per day
for 6 h/day on days 6-15 of gestation. Treatment had no effect on
mortality rates, body weight, food consumption or reproductive
parameters (numbers of corpora lutea, implantations, resorptions and
live fetuses). Examination of the litters revealed no
treatment-related effect on mean body weight, and no abnormal
findings were detected in the viscera. The frequency of external
abnormalities (cleft palate, malposition of hind legs, exencephaly
and 'tail cranial bended') at 1000 mg/kg bw per day was not
different from that in the control group, with 3% in the controls,
3% at 100 mg/kg bw per day, 1.8% at 300 mg/kg bw per day and 5.3% at
1000 mg/kg bw per day. The increase at the highest dose was due to a
higher incidence of cleft palate, with 11/285 (3.9%) at 1000 mg/kg
bw per day and 6/301 (2%) in the control group. The incidence of
cleft palate in control animals in an independent study was 1.6%.
Skeletal examination showed an increased number of supernumerary
ribs at 1000 mg/kg bw per day. The NOAEL was 1000 mg/kg bw per day
for maternal toxicity and clinical signs and 300 mg/kg bw per day
for embryotoxicity and teratogenicity (Becker et al., 1990).
Since treatment-related effects on the fetuses appeared to have
occurred in the absence of maternal toxicity, a study was initiated
that included biochemical and histopathological examinations.
Tebuconazole was administered in the same way as described above to
groups of 10 mated females. As in the first study, no deaths
occurred and no clinical signs were observed in animals treated at
1000 mg/kg bw per day; the treatment had no effect on body-weight
gain, food consumption or reproductive parameters. Clinical
biochemical examinations revealed increased alanine transaminase
activity at 1000 mg/kg bw per day; there was also increased
cytochrome P450 content and N-demethylase activity in liver tissue
at > 300 mg/kg bw per day and a marginal increase in
O-demethylase activity. None of these parameters showed a
dose-response relationship. There was a dose-related reduction in
relative adrenal weights in all dose groups, which was statistically
significant at 1000 mg/kg bw per day; absolute adrenal weights were
also reduced in all treatment groups, but not in relation to dose.
No effect was seen on liver weights, but histological examination
revealed fatty changes in periportal areas in most mice at 300 mg/kg
bw per day and in all mice at 1000 mg/kg bw per day. The NOAEL was
100 mg/kg bw per day on the basis of fatty changes in the liver at
higher doses (Becker et al., 1990).
Rats
In a range-finding study, groups of 25 female Wistar rats were
treated by gavage with tebuconazole (purity, 99.5%) at doses of 0 or
100 mg/kg bw per day on days 6-15 of gestation. The treatment had no
effect on mortality rates, but body-weight gain was retarded. The
fertilization rate was not reduced by treatment, but most litter
parameters (such as number of implantations, litter size and losses)
indicated an adverse effect. More runts and fetuses with
malformations (micrognathia, hydronephrosis and hydroureter) were
found in treated animals. The NOAEL for maternal toxicity,
embryotoxicity and teratogenicity was < 100 mg/kg bw per day
(Renhof, 1984).
In a similar study, groups of 25 female Wistar rats were
treated by gavage with tebuconazole (purity, 93%) at doses of 0, 10,
30 or 100 mg/kg bw per day on days 6-15 of gestation. No
treatment-related deaths occurred. At 30 and 100 mg/kg bw per day,
dose-related reductions in weight gain were observed throughout the
treatment period; at 100 mg/kg bw per day, retarded body-weight gain
was observed throughout gestation. Effects on litter parameters
included an increased number of losses and a decrease in mean fetal
weight at 100 mg/kg bw per day. At this dose there were also more
runts and fetuses with malformations (mostly microphthalmia): the
runt incidence was 36%, compared with 7% in controls, and the
incidence of fetuses with malformations was 7%, compared with 2% in
controls. The NOAEL for maternal toxicity was 10 mg/kg bw per day
and that for embrotoxicity and teratogenicity was 30 mg/kg bw per
day (Renhof, 1985a).
Groups of 25 femaleWistar rats were treated by gavage with
tebuconazole (purity, 98.3%) at doses of 0, 30, 60 or 120 mg/kg bw
per day on days 6-15 of gestation. The dose of 30 mg/kg bw per day
affected neither maternal nor fetal parameters. At 60 mg/kg bw per
day and above, food consumption was reduced and a dose-related loss
of body weight was seen at the beginning of the treatment period.
Liver weights showed a dose-related increase at 60 and 120 mg/kg bw
per day. Reproductive parameters (numbers of corpora lutea and
implantations) were not adversely affected. At 120 mg/kg bw per day,
resorptions occurred more frequently, and consequently there were
fewer live fetuses than in the control group; mean fetal body
weights were lower. This dose induced increased incidences of
visceral alterations (consisting of excess fluid in the thoracic
cavity), supernumerary ribs, non-ossified cervical vertebrae and
incompletely ossified sternebrae, indicating retardation of fetal
development. The NOAEL was 30 mg/kg bw per day for maternal toxicity
and 60 mg/kg bw per day for embryotoxicity (Becker et al., 1988a).
Groups of 25 female Wistar rats were treated dermally with
tebuconazole (purity, 97.4%) at doses of 0, 100, 300 or 1000 mg/kg
bw per day for 6 h/day on days 6-15 of gestation. The treatment was
tolerated with no observed effects on dams or fetuses with respect
to the mortality rate, body-weight gain, gestation rate or other
reproductive parameters, such as numbers of corpora lutea,
resorptions and live fetuses. There was no indication of a
treatment-related effect on the incidence or spectrum of
malformations (Renhof, 1988b).
Rabbits
Groups of 15 female Himalayan CHBB:HM rabbits were treated by
gavage with tebuconazole (purity, 93%) at doses of 0, 3, 10 or 30
mg/kg bw per day on days 6-18 of gestation. Dams given the highest
dose had reduced body-weight gain during the treatment period.
Reproductive and fetal parameters were not adversely affected, and
no treatment-related increases in the incidence of malformations
were observed. The NOAEL was 10 mg/kg bw per day for maternal
toxicity and 30 mg/kg bw per day for embryotoxicity and
teratogenicity (Renhof, 1985b).
Groups of 16 female CHIN hybrid rabbits were treated by gavage
with tebuconazole at doses of 0, 10, 30 or 100 mg/kg bw per day on
days 6-18 of gestation. Treatment-related effects were seen at 100
mg/kg bw per day, consisting of reduced body-weight gain and food
consumption, increased mean post-implantation loss and slightly
reduced mean fetal body weight. External examination of fetuses
showed an increased incidence of malformations, including peromelia
(the most frequent malformation), agenesis of claws and cleft
palate. Of 90 fetuses born to dams treated at 100 mg/kg bw per day,
eight (9%) were malformed, and five of these (6%) had peromelia.
Peromelia was seen in none of a total of 346 fetuses in the other
three groups combined. At 100 mg/kg bw per day, the percentage of
fetuses with absent or incomplete phalangeal ossification was also
increased. In combination with the reduction in mean fetal body
weight, these findings indicate delayed maturation of fetuses at the
highest dose. The NOAEL was 30 mg/kg bw per day for maternal
toxicity, embryotoxicity and teratogenicity (Becker et al.,
1988b).
(f) Genotoxicity
Numerous studies have been conducted on the genotoxicity of
tebuconazole. The results are summarized in Table 2. No genotoxic
activity was found in any study.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Tebuconazole did not irritate the skin of rabbits, and
administration of 50 mg into the conjunctival sac did not induce
irritation of the eye (Heimann & Pauluhn, 1983; Sheets, 1988;
Märtins, 1990). In another study, instillation of 100 mg into the
conjunctival sac of rabbits caused slight irritation of the
conjunctiva (Eigenberg, 1988).
Tebuconazole did not reveal skin-sensitizing potential in a
maximization test in guinea-pigs (Heimann, 1983). The negative
result was confirmed in two further tests for sensitization in
guinea-pigs, but with the less sensitive Buehler method (Heimann,
1987; Sheets, 1990).
Table 2. Results of tests for the genotoxicity of tebuconazole
End-point Test system Concentration Purity Results Reference
of tebuconazole (%)
In vitro
Reverse S. typhimurium TA98, 20-12 500 µg/platea 97 Negative Herbold, 1983a,
mutation 100, 1535, 1587 1990
Reverse S. typhimurium TA98, 37.5-2400 µg/plateb 96.6 Negative Herbold, 1988a,b
mutation 100, 1535, 1537, 1538
Reverse S. typhimurium 15.6-500 µg/plate 98 Negative Ohta, 1991d
mutation E. coli (WPuvrA) 31.3-1000 µg/platec
156-5000 µg/plated
Forward Chinese hamster 80-100 µg/mle 96.6 Negative Lehn, 1988
mutation ovary cells 12.5-200 µg/mlc
(hprt locus)
Recombination B. subtilis (spores) 0.3-20 µg/disc 98 Negativec,d Ohta, 1992
repair capacity H17 (rec+), M45 (rec-)
Cytogenicity Human lympho cytes 3-30 µg/mlc 96.6 Negative Herbold, 1988c
30-300 µg/mld
DNA polymerase E. coli 625-10 000 µg/plate 97.1 Negativec,d Herbold, 1983b
repair capacity (K12)p 3478 (pol-)
W 3110 (pol+)
Unscheduled Rat hepatocytes 0.5-25.2 µg/ml 96.5 Negative Cifone, 1988
DNA synthesis
Sister chromatid Chinese hamster 4-30 µg/mlc 96.5 Negative Putman, 1987
exchange ovary cells 15-120 µg/mld
Table 2 (contd)
End-point Test system Concentration Purity Results Reference
of tebuconazole (%)
In vivo
Micronucleus Mouse bone marrow Single oral dose: 2000, 95.3 Negative Herbold, 1985
formation 500 or 200 mg/kg bwf
Dominant lethal Male mouse Single oral dose: 93.5 Negative Herbold, 1986
mutation 2000 mg/kg bw
a Toxic at doses > 500 µg/plate
b Toxic at doses > 60 µg/plate
c In the absence of metabolic activation
d In the presence of metabolic activation
e Toxic at doses > 75 µg/ml with and without activation
f Doses reduced in second and third trials owing to inhibition of erythropoiesis by the test compound
(ii) Cataract induction
Groups of four male and four female Forest of Dean cats
received whole-body exposure to tebuconazole (purity, 95.8%) in
polyethylene glycol and ethanol at mean analytical concentrations of
61 or 309 mg/m3 (99% of particles with an aerodynamic diameter of
<5 µm) for 6 h/day for four weeks, followed by an observation
period of 15 weeks. Control animals were exposed to aerosols of
either the vehicle or about 20 mg/m3 Sclex (KNJ 0953) (positive
controls). The treatment did not induce clinical symptoms and did
not affect mortality rates or body-weight gain. Cataracts due to
lens fibre degeneration were found in all animals in the positive
control group during the observation period. Exposure to
tebuconazole did not result in cataract induction, but three females
at 309 mg/m3 and one positive control animal had yellow-tinged
spots along the lens fissure. Examination of 42 untreated female
cats aged 7-12 months showed that these ocular changes were not
common spontaneous alterations; no such finding was seen in vehicle
controls. The etiology of this finding and its toxicological
relevance remain unclear. The NOAEL was 61 mg/m3, equivalent to
about 5 mg/kg bw per day, on the basis of ocular effects other than
cataracts of unknown etiology at the highest concentration (Märtins
et al., 1990).
Groups of four female beagle dogs were treated by head-nose
exposure to tebuconazole (purity, 97.1%) in polyethylene glycol and
ethanol at target aerosol concentrations of 0, 150 or 800 mg/m3
for 4 h/day for six weeks and observed for eight weeks. The
analytical concentrations were 163 and 914 mg/m3; about 90% of the
particles had an aerodynamic diameter of < 3 µm. No vehicle
control group was included in the study. The treatment did not
affect mortality rates. Most animals at 914 mg/m3 began salivating
immediately after exposure, and single animals also made tussive
noises; these effects were reversed within 2 h. Retardation of
body-weight gain was observed in both treated groups during the
second half of the study, but with no dose-effect relationship. A
lung function test revealed a marginal decrease in the mean minute
volume in both treated groups and a slight reduction in the mean
partial pressure of oxygen. The body temperature of treated animals
was reduced during exposure, probably due to central nervous system
depression caused by the ethanol vehicle. Increased spleen weight
observed at 914 mg/m3 was the only change in organ weights. No
gross pathological or histopathological changes were observed, and
ophthalmic examinations gave no indication of cataract formation.
The NOAEL was 163 mg/m3, equivalent to 23 mg/kg bw per day, on the
basis of clinical effects. The NOAEL for cataract induction was 914
mg/m3, equivalent to 125 mg/kg bw per day (Märtins, 1991).
3. Studies on metabolites
(a) Acute toxicity
The acute toxic effects of several metabolites of tebuconazole
are summarized in Table 3.
Table 3. Acute toxicity of metabolites of tebuconazole
Metabolite Species Sex Route LD50 (mg/kg bw)a Reference
Carboxy metabolite Rat F Oral > 4000 Astroff & Hagen, 1992
Hydroxy metabolite Rat F Oral > 5000 Sheets & Phillips, 1992
1H-1,2,4-triazole Rat M Oral 1375 Flucke, 1978
Rat M&F Oral 1650 Thyssen & Kimmerle, 1976
a Concentrations determined analytically
(b) Short-term toxicity
In a three-month study, groups of 15 male and 15 female Wistar
rats were fed diets containing 1 H-1,2,4-triazole (purity, 99.6%)
at concentrations of 0, 100, 500 or 2500 ppm, equal to 8, 38 or 212
mg/kg bw per day. Appearance, mortality rates, behaviour, clinical
chemistry parameters and the results of urinalysis, thyroid function
tests (protein-bound iodine) and gross pathological examination were
not affected by the treatment. At 2500 ppm, temporary convulsions
occurred; body-weight gain and food consumption were reduced,
particularly in males; and haematological parameters (haemoglobin
concentration, haematocrit, mean corpuscular volume and mean
corpuscular haemoglobin) were reduced in males. Histopathological
changes, confined to the liver, consisted of fatty accumulation in
three males at 2500 ppm (Bomhard et al., 1979).
(c) Embryotoxicity and teratogenicity
Groups of 25 pregnant Bor:WISW Wistar rats were treated orally
with 1,2,4-triazole (purity, 95.3%) on days 6-15 of gestation at
doses of 0, 10, 30 or 100 mg/kg bw per day. Treatment at 100 mg/kg
bw per day caused a reduction in body-weight gain of the dams,
reduced fetal weight and an increased number of runts. At the same
dose, the incidence of malformations, consisting mainly of eye
deformities such as anophthalmia and micro-phthalmia, was increased
over that in concurrent or historical controls (Renhof, 1988c).
In a similar study, groups of 25 pregnant Bor:WISW Wistar rats
were treated orally with 1,2,4-triazole at doses of 0, 100 or 200
mg/kg bw per day. The treatment impaired body-weight gain in both
treated groups but was statistically significant only at 200 mg/kg
bw per day. At this dose, an increased number of resorptions and a
reduction in fetal weight were observed. In both treated groups, a
dose-related increase in the incidence of runts was observed; the
incidence of malformations (mainly hydronephrosis and cleft palate)
was increased only at 200 mg/kg bw per day (Renhof, 1988d).
(d) Genotoxicity
The 1 H-1,2,4-triazole metabolite of tebuconazole (purity,
99.7%) was tested for its ability to induce reverse mutation in
Salmonella typhimurium strains TA98, TA100, TA1535 and TA1537.
Doses of 10-5000 µg/plate were ineffective, in either the presence
or the absence of metabolic activation (Poth, 1989).
4. Observations in humans
Employees working in the production of tebuconazole, who were
under continous medical supervision, did not show exposure-related
effects (Kollert, 1987).
Comments
After oral administration of tebuconazole to rats, 65-80% of
the dose was eliminated by the biliary and faecal route, whereas
elimination in urine amounted to about 16-35%. Males had a greater
biliary and faecal elimination than females. Biotransformation
proceeded by oxidation reactions, resulting in hydroxy, carboxy,
triol and ketoacid metabolites and conjugates as well as triazole.
Administration of acute oral doses to rats induced sedation,
abnormal gait and emaciation. Tebuconazole has low acute toxicity
and has been classified by WHO (1992) as unlikely to present an
acute hazard in normal use.
In a 13-week study, rats were fed tebuconazole at
concentrations of 0, 100, 400 or 1600 ppm. The NOAEL was 100 ppm,
equal to 9 mg/kg bw per day, on the basis of retardation of
body-weight gain and histopathological changes in the adrenal glands
at higher doses.
In two one-year studies in dogs fed diets containing 0, 40, 200
or 1000 ppm or 0, 100 or 150 ppm, an NOAEL of 100 ppm for the two
studies combined, equal to 3 mg/kg bw per day, was determined on the
basis of histopathological alterations in the adrenal glands at 150
ppm and above and cataract production at 200 ppm and above.
Two 21-month studies of toxicity and carcinogenicity were
conducted in mice, at dietary concentrations of 0, 20, 60 or 180 ppm
or 0, 500 or 1500 ppm. The NOAEL was 20 ppm, equal to 6 mg/kg bw per
day, on the basis of fatty changes in the liver. At 1500 ppm,
pronounced liver toxicity and an increased incidence of liver
tumours were observed. This tumorigenic potential is not considered
relevant to humans.
In a two-year study of toxicity and carcinogenicity in rats
treated at dietary concentrations of 0, 100, 300 or 1000 ppm, the
NOAEL was 100 ppm, equal to 5 mg/kg bw per day, on the basis of
reduced body-weight gain at higher doses. There was no evidence of
carcinogenicity.
In a two-generation study in rats fed dietary concentrations of
0, 100, 300 or 1000 ppm, the NOAEL was 300 ppm, equal to 22 mg/kg bw
per day, on the basis of reduced body-weight gain in the parental
generation and adverse effects on litters at higher dose.
Teratogenicity was investigated in mice, rats and rabbits. In
mice, doses of 0, 10, 20, 30 or 100 mg/kg bw per day were
administered. Increased enzyme activities in liver were observed at
all doses but were not dose-related, so that there was no clear
NOAEL for maternal toxicity. In mice fed doses of 0, 10, 30 or 100
mg/kg bw per day, a higher incidence of runts was seen at 30 mg/kg
bw per day and a higher incidence of malformations (mainly cleft
palate) at 100 mg/kg bw per day. The NOAEL for embryotoxicity and
teratogenicity was thus 10 mg/kg bw per day.
In rats treated with doses of 0, 10, 30 or 100 mg/kg bw per day
or 0, 30, 60 or 120 mg/kg bw per day, the NOAEL was 10 mg/kg bw per
day for maternal toxicity on the basis of reduced body-weight gain
at higher doses. Embryotoxicity and an increased incidence of
malformations (mainly microphthalmia) and visceral and skeletal
variations were found at 100 mg/kg bw per day and above, giving an
NOAEL for embryotoxicity and teratogenicity of 60 mg/kg bw per day.
In rabbits treated at doses of 0, 3, 10 or 30 mg/kg bw per day
or 0, 10, 30 or 100 mg/kg bw per day, the NOAELs were 10 mg/kg bw
per day for maternal toxicity and 30 mg/kg bw per day for
embryotoxicity and teratogenicity. At 100 mg/kg bw per day,
embryotoxicity and an increased incidence of external malformations
(mainly peromelia) were observed.
Tebuconazole has been studied in a range of tests for
genotoxicity in vivo and in vitro. The Meeting concluded that
there was no evidence of genotoxicity.
An ADI was established on the basis of an NOAEL of 100 ppm in a
one-year dietary study in dogs and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 20 ppm, equal to 6 mg/kg bw per day (21-month study
of toxicity and carcinogenicity)
< 10 mg/kg bw per day (maternal toxicity in a study
of teratogenicity)
10 mg/kg bw per day (embryotoxicity in a study of
teratogenicity)
Rat: 100 ppm, equal to 5 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
10 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
60 mg/kg bw per day (embryotoxicity in a study of
teratogenicity)
Rabbit: 10 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
30 mg/kg bw per day (embryotoxicity in a study of
teratogenicity)
Dog: 100 ppm, equal to 3 mg/kg bw per day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Astroff, A.B. & Hagen, L.L. (1992) Acute toxicity study with HWG
2443 (a metabolite of Tebuconazole Folicur(TM)) in female rats.
Unpublished report Ref. No. 92-012-PB, prepared by Miles Inc.,
Agricultural Division, Toxicology, Stillwell, KS, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Becker, H., Vogel, W. & Terrier, C. (1988a) Embryotoxicity study
(including teratogenicity) with HWG 1608 technical in the rat.
Unpublished report Ref. No. R 4451, prepared by Research &
Consulting Co. AG (RCC), Itingen, Switzerland. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Becker, H., Vogel, W. & Terrier, C. (1988b) Embryotoxicity study
(including teratogenicity) with HWG 1608 technical in the rabbit.
Unpublished report Ref. No. R 4323, prepared by Research &
Consulting Co. AG (RCC), Itingen, Switzerland. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Becker, H., Vogel, O., Terrier, C., Biedermann, K. & Luetkemeier, H.
(1990) Embryotoxicity study (including teratogenicity) with HWG 1608
technical in the mouse (dermal application). Unpublished report Ref.
No. R 5116, prepared by Research & Consulting Co. AG (RCC), Itingen,
Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomhard, E. (1991) HWG 1608--Toxic dose range carcinogenicity study
in NMRI mice (supplement to study T 601 89 53 with administration in
diet over a 21-month period). Unpublished report Ref. No. 16376 A,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomhard, E. & Ramm, W. (1988a) HWG 1608--Study for cancerogenicity
in NMRI mice (administration in diet for up to twenty-one months).
Unpublished report Ref. No. 16376, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomhard, E. & Ramm, W. (1988b) HWG 1608--Study for chronic toxicity
and cancerogenicity in Wistar rats (administration in diet for two
years). Unpublished report Ref. No. 16375, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E. & Schilde, B. (1986) HWG 1608--Subchronic toxicological
study with rats (feeding for thirteen weeks). Unpublished report
Ref. No. 15211, prepared by Bayer AG, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Bomhard, E., Löser, E. & Schilde, B. (1979) 1,2,4-Triazole:
Subchronic toxicological study with rats (feeding study over three
months). Unpublished report Ref. No. 8667, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E., Karbe, E. & Loeser, E. (1986) Spontaneous tumours of
2000 Wistar TNO/W. 70 rats in two-year carcinogenicity studies.
J.E.P.T.O. 7: 1/2, pp. 35-52.
Brain, K.R. (1991) In vitro skin permeability of tebuconazole.
Unpublished report Ref. No. M 7591, prepared by An-eX Analytical
Services Ltd., Cardiff, United Kingdom. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Chopade, H.M. (1992) Addendum I. [Phenyl-U-14C] HWG 1608: Study of
biokinetic behavior in the rat. Response to EPA requests and
inquiries. Unpublished report Ref. No. MR 97439-1 Metab I/2,
prepared by Miles Inc., Stilwell, KS, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Cifone, M.A. (1988) Mutagenicity test on HWG 1608 techn. in the rat
primary hepatocyte unscheduled DNA synthesis assay. Unpublished
report Ref. No. R 4111a, prepared by Hazelton Laboratories America
Inc., Kensington, MD, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Ecker, W. & Weber, H. (1991) [Chlorophenyl-U-14C] tebuconazole
absorption, distribution, excretion and metabolism in laying hens.
Unpublished report Ref. No. PF 3587, prepared by Bayer AG,
Leverkusen, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Ecker, W., Brauner, A., Klein, O. & Weber, H. (1987) Folicur:
Metabolism part of the general metabolism study in the rat.
Unpublished report Ref. No. PF 2907 and MR 97438. Metab I/3,
prepared by Bayer AG, Leverkusen, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Eiben, R. (1987) HWG 1608--Two-generation study in rats. Unpublished
report Ref. No. 16223, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Eigenberg, D.A. (1988) Primary eye irritation of Folicur (HWG 1608)
technical in albino rabbits. Unpublished report Ref. No. 1003,
prepared by Mobay Corp., Stilwell, KS, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Eigenberg, D.A. (199) Dermal absorption of 14C HWG 1608 technical
in rats. Unpublished report Ref. No. MR 97470. Metab I/5, prepared
by Mobay Corp., Stilwell, KS, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1978) Determination of LD 50--Compound: 1,2,4-Triazole.
Unpublished letter report by Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1987) HWG 1608 and KWG 0519 (c.n. Triadimenol)/HWG 1608
and KUE 13032c (c.n. Dichlofluanid) combination toxicity study.
Unpublished report Ref. No. 15747, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1983) HWG 1608--Study for skin-sensitising effect on
guinea pigs. Unpublished report Ref. No. 12024, prepared by Bayer
AG, Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K.G. (1987) HWG 1608 technical--Study of skin sensitization
effect on guinea pigs (Buehler patch test). Unpublished report Ref.
No. 16238, prepared by Bayer AG, Wuppertal, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Heimann, K.G. & Kaliner, G. (1984) HWG 1608--Study of the subacute
oral toxicity to rats. Unpublished report Ref. No. 13028, prepared
by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Heimann, K.G. & Pauluhn, J. (1983) HWG 1608 study for acute
toxicity. Unpublished report Ref. No. 12168, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K.G. & Schilde, B. (1984) HWG 1608--Subacute study of
dermal toxicity to rabbits. Unpublished report Ref. No. 12669,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Heimann, K.G. & Schilde, B. (1988) HWG 1608 technical--Subacute
dermal study of toxicity to rabbits. Unpublished report Ref. No.
12669a (Addendum to report No. 12669), prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B.A. (1983a) HWG 1608--Salmonella/microsome test to
evaluate for point mutagenic effect. Unpublished report Ref. No.
12086, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1983b) HWG 1608--Pol test on E. coli to evaluate for
harmful effects on DNA. Unpublished report Ref. No. 11902, prepared
by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B.A. (1985) HWG 1608--Micronucleus test on the mouse to
evaluate for mutagenic effect. Unpublished report Ref. No. 13159,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1986) HWG 1608--Dominant lethal test on the male
mouse to evaluate for mutagenic effect. Unpublished report Ref. No.
14985, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1988a) HWG 160--Salmonella/microsome test to evaluate
for point mutagenic effects. Unpublished report Ref. No. 16383,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1988b) HWG 1608--Salmonella/microsome test using TA
1538 to evaluate for point mutagenic effects (addendum to final
report Ref. No. 16383). Unpublished report Ref. No. 16383 A,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1988c) In vitro cytogenetic study with human
lymphocytes for the detection of induced clastogenic effects.
Unpublished report Ref. No. 16395, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1990) HWG 1608--Salmonella/microsome test for
point-mutagenic effects (appendix to Report 12086). Unpublished
report Ref. No. 12086A, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1983a) HWG 1608 acute toxicity to the dog after oral
administration. Unpublished report Ref. No. 11974, prepared by Bayer
AG, Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hoffmann, K. (1983b) HWG 1608 acute toxicity to the sheep after oral
administration. Unpublished report Ref. No. 11970, prepared by Bayer
AG, Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
von Keutz, E. & Schilde, B. (1987a) HWG 1608--Subchronic study of
toxicity to dogs with oral administration (thirteen-weeks feeding
study). Unpublished report Ref. No. 15763, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
von Keutz, E. & Schilde, B. (1987b) HWG 1608--Study of chronic
toxicity to dogs after oral administration (twelve-month feeding
study). Unpublished report Ref. No. 16211, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kollert, W. (1987) Report dated 10 November 1987, HWE 1608--Internal
experiments, Bayer Medical Department, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Lee, S.G.K. & Wood, S.E. (1987) The metabolism of Folicur in dairy
goats. Unpublished report Ref. No. MR 94882, prepared by Mobay
Corporation, Kansas City, MO, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lee, S.G.K., Hanna, L.A., Johnston, K., Wood, S.E. & Leimkuehler,
W.M. (1988) The metabolism of 14C-Folicur in chickens. Unpublished
report Ref. No. 87 156, prepared by Mobay Corp., Kansas City, MO,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lehn, H. (1988) HWG 1608--Mutagenicity study for the detection of
induced forward mutations in the CHO-HGPRT assay in vitro.
Unpublished report Ref. No. 16749, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Märtins, T. (1990) HWG 1608--Study for skin and eye
irritation/corrosion in rabbits. Unpublished report Ref. No. 12168 B
(addendum report to report No. 12168), prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Märtins, T. (1991) HWG 1608 (tebuconazole)--Subacute inhalation
toxicity to dogs--Study for cataracts. Unpublished report Ref. No.
20884, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Märtins, T., Pauluhn, J. & Krötlinger, F. (1990) HWG 1608
(tebuconazole)--Subacute inhalation toxicity to cats--Study for
cataracts. Unpublished report Ref. No. 19644, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Ohta, K. (1991a) HWG 1608 technical--Acute oral toxicity study on
rats. Study No. 91A016. Unpublished report prepared by Nihon Bayer
Agrochem K.K., Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Ohta, K. (1991b) HWG 1608 technical--Acute dermal toxicity study on
rats. Study No. 91A012. Unpublished report prepared by Nihon Bayer
Agrochem K.K. Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Ohta, K. (1991c) HWG 1608 technical--Acute oral toxicity study on
mice. Study No. 91A017. Unpublished report prepared by Nihon Bayer
Agrochem K.K., Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Ohta, K. (1991d) HWG 1608--Reverse mutation assay (Salmonella
typhimurium and Escherichia coli). Unpublished report Ref. No. RA
91036, prepared by Nikon Bayer Agrochem K.K., Tokyo, Japan.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ohta, K. (1992) HWG 1608--Rec- assay with spores in the bacterial
system. Unpublished report Ref. No. RA 92007, prepared by Nihon
Bayer Agrochem K.K., Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Pauluhn, J. (1985) HWG 1608--Study for subacute inhalation toxicity
to rat for three weeks (exposure 15 x 6 hours). Unpublished report
Ref. No. 13305, prepared by Bayer AG, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1987) HWG 1608--Study for subacute inhalation toxicity
to rat for three weeks (exposure 15 x 6 hours). Histopathological
examinations. Unpublished report Ref. No. 13305A (addendum to Ref.
No. 13305), prepared by Bayer AG, Wuppertal, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1988) HWG 1608--Study for acute inhalation toxicity to
the rat to OECD-guideline No. 403. Unpublished report Ref. No.
16345, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Porter, M.C., Jasty, V., Troup, C.M. & Hartnagel, R.E. (1989) Safety
evaluation of HWG 1608: Chronic (1 year) feeding study in dogs.
Unpublished report Ref. No. R 4781, prepared by Toxicology
Department, Miles Inc., Elkhart, IN, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Poth, A. (1989) Salmonella typhimurium reverse mutation assay with
1H-1,2,4-triazole. Unpublished report Ref. No. R 4859, prepared by
Cytotest Cell Research GMBH & Co. KG, Rossdorf, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Putman, D.L. (1987) HWG 1608--Sister chromatid exchange assay in
Chinese hamster ovary (CHO) cells. Unpublished report Ref. No. 953,
prepared by Microbiological Associates Inc., Bethesda, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1984) HWG 1608--Study for embryotoxic effects on rats
after oral administration. Unpublished report Ref. No. 12457,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1985a) HWG 1608--Study for embryotoxic effects on rats
after oral administration. Unpublished report Ref. No. 13273,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1985b) HWG 1608--Study for embryotoxic effects on
rabbits after oral administration. Unpublished report Ref. No.
13287, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Renhof, M. (1988a) HWG 1608--Study for embryotoxic effects on mice
following oral administration. Unpublished report Ref. No. 16527,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1988b) HWG 1608--Study for embryotoxic effects on rats
after dermal administration. Unpublished report Ref. No. 17089,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1988c) Triazole--Investigations into embryotoxic effects
on rats after oral administration. Unpublished report No. 17401,
Ref. No. 50 19 339, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1988d) Triazole--Investigations into embryotoxic effects
on rats after oral administration. Unpublished report No. 17402,
Supplement to Ref. No. T 50 19 339, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. & Karbe, E. (1988) HWG 1608--Supplementary study for
maternal toxicity on mice following oral administration. Unpublished
report Ref. No. 16511, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Sheets, L.P. (1988) Primary dermal irritation of technical grade
Folicur in rabbits. Unpublished report Ref. No. 1066, prepared by
Mobay Corp., Stilwell, KS, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Sheets, L.P. (1990) Dermal sensitization study with technical grade
tebuconazole (Folicur) in guinea pigs. Unpublished report Ref. No.
5052, prepared by Mobay Corp., Stilwell, KS, USA. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Sheets, L.P. & Phillips, S.D. (1992) Acute oral toxicity study with
HWG 2061 (a metabolite of tebuconazole Folicur(TM)) in female
rats. Unpublished report Ref. No. 6685, prepared by Miles Inc.,
Agricultural Division, Toxicology, Stilwell, KS, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1976) 1,2,4-Triazole--Occupational
toxicology study. Unpublished report Ref. No. 5926, prepared by
Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Weber, H. (1987) [Phenyl-U-14C] HWG 1608: Study of biokinetic
behaviour in the rat. Unpublished report Ref. No. PF 2859. Metab
II/15, prepared by Bayer AG, Leverkusen, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Weber, H. (1988) [Phenyl-U-14C] HWG 1608:
Ganzkörperautoradiographische Verteilung der Radioaktivität in der
Ratte. Unpublished report Ref. No. PF 2962. Metab II/16, prepared by
Bayer AG, Leverkusen, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
(b) Short-term toxicity
Rats
Groups of 20 male and 20 female Wistar rats (Bor: WISW) were
given tebuconazole at doses of 0, 30, 100 or 300 mg/kg bw per day by
intubation for four weeks and observed during a four-week recovery
period. The dose of 300 mg/kg bw per day caused mild lethargy and
reduced body-weight gain during the treatment period. Doses of 100
mg/kg bw per day and above decreased the haemoglobin concentration
and haematocrit values. In females at 300 mg/kg bw per day, the
leukocyte count was increased. Clinical chemistry revealed slight,
not statistically significant, increases in the activities of
glutamate-oxalate and glutamate-pyruvate transaminases in males at
300 mg/kg bw per day, and marked increases in liver enzyme
activities (glutamate-oxalate and glutamate-pyruvate transaminases
and alkaline phosphatase) in females at the same dose. Treatment at
100 and 300 mg/kg bw per day induced the microsomal enzyme system,
and the activities of N- and O-demethylases and cytochrome P450
and the triglyceride concentration in liver were increased. All
these changes were reversible. Urinalyses revealed no abnormal
findings. At 100 and 300 mg/kg bw per day, the weights of the liver
and spleen were increased in animals of each sex and the weight of
the kidney in females. Histopathological findings at 300 mg/kg bw
per day consisted of fatty changes in the liver and bile-duct
proliferation in females; enlargement of the centrilobular
hepatocytes was found in male rats. Histopathological changes were
also found in the adrenal cortex, consisting of proliferated
endothelial cells and an increased incidence of fat vacuoles.
Sclerosis of the red pulp of the spleen, associated with
sideropenia, was observed in males at 300 mg/kg bw per day;
sideropenia was also found in females at 100 mg/kg bw per day. The
NOAEL was 30 mg/kg bw per day on the basis of changes in
haematological and clinical chemical parameters and organ weights at
higher doses (Heimann & Kaliner, 1984).
Groups of 10 male and 10 female Wistar rats (Bor: WISW) were
exposed to nominal aerosol concentrations of tebuconazole at 0, 5,
50 or 500 mg/m3 in polyethylene glycol by head and nose exposure
for 6 h/day, five days per week for three weeks (15 days). The
analytical concentrations were 0, 1.2, 11 and 156 mg/m3. About 90%
of the particle mass had an aerodynamic diameter of < 5 µm. The
treatment had no effect on mortality rate, body-weight gain,
haematological or clinical chemical parameters or organ weights;
urinalysis showed no abnormal findings. Rats treated with 156
mg/m3 had piloerection after each exposure. Mixed-function
oxidases in the liver were induced. At the end of the study,
N-demethylase activity in the liver was increased in animals of
each sex at the highest dose. Males in this group also had increased
O-demethylase activity. No treatment-related gross or
histopathological alterations were observed. The NOAEL was 10.6
mg/m3, equivalent to about 2 mg/kg bw per day, on the basis of
liver enzyme induction at the highest concentration (Pauluhn, 1985,
1987).
Groups of 10 male and 10 female rats (Wistar, BOR:WISW) were
given dietary concentrations of tebuconazole (purity, 93.4%; 4.8%
symmetrical isomer) at 0, 100, 400 or 1600 ppm, equal to 9, 35 or
172 mg/kg bw per day, for 13 weeks. The treatment had no effect on
appearance or behaviour or the findings of ophthalmic or
haematological examinations or urinalyses. Food intake was increased
in animals of each sex at 1600 ppm. Retardation of body-weight gain
was observed at 400 ppm in females during the first six weeks and at
1600 ppm in animals of each sex. Two animals at 1600 ppm died; no
deaths occurred in the other dose groups. Few of the changes in
clinical chemistry showed a dose-related or time-consistent pattern
and were regarded as toxicologically insignificant. An increase in
urea concentration at 1600 ppm and a decrease in triglyceride
concentrations in animals at 400 ppm and above were seen only after
the first four weeks and not at the end of the study. Pronounced
increases in N-demethylase activity and cytochrome P450 content
were found in male animals at 1600 ppm, and increased liver weights
were seen in females at this dose. Histopathological examination
revealed an increased incidence of intra-plasmatic vacuoles in the
cells of the zona fasciculata of the adrenals (probably lipid
accumulation) in some females at 400 ppm and in all females at 1600
ppm. This effect was less pronounced in males because of a higher
background incidence of adrenal vacuole formation in control animals
(Bomhard & Schilde, 1986). The NOAEL was 100 ppm, equal to 9 mg/kg
bw per day, on the basis of retardation of body-weight gain and
histopathological changes in the adrenals at higher doses
Rabbits
Groups of 18 male and 18 female HC New Zealand white rabbits
received tebuconazole at doses of 0, 50 or 250 mg/kg bw per day on
the shorn skin for 6 h daily for three weeks. The treatment was
tolerated with no effects (Heimann & Schilde, 1984). In an
additional study, a dose of 1000 mg/kg bw per day was also tolerated
with no systemic or local effects (Heimann & Schilde, 1988).
Dogs
Groups of four male and four female beagle dogs were given
tebuconazole (purity, 93.4%; 4.8% symmetric isomer) at dietary
concentrations of 0, 200, 1000 or 5000 ppm (equal to 9, 45 or 220
mg/kg bw per day for males and females combined) over 13 weeks. One
dog at 5000 ppm was found dead after the first dose, with no
previous clinical signs. Treatment did not affect body temperature,
pulse rate or neurological signs. Food consumption was reduced at
1000 and 5000 ppm, and body-weight gain was retarded in these
groups; a few dogs at 1000 ppm and most of those at 5000 ppm had a
deteriorated nutritional status. Ophthalmic examination revealed
lens opacities in all animals at 5000 ppm, which first appeared
after seven weeks of treatment. An increase in thrombocyte count was
found at 5000 ppm, and at the end of the study six of eight animals
at this dose had marked anisocytosis. Alkaline phosphatase activity
in plasma showed a retarded age-induced fall at 1000 ppm and an
increase at 5000 ppm. N-Demethylase activity in the liver had
increased slightly by the end of the study in animals at 1000 ppm
and had increased markedly in those at 5000 ppm. The cytochrome P450
content of the liver was also elevated at 5000 ppm. In the group at
the highest dose, a shift in the composition of serum proteins was
observed, with a slightly lower albumin content and a simultaneous
increase in the ß-globulin content. Urinalyses revealed no
treatment-related effects. Changes in organ weights did not follow a
consistent pattern, except that an increase in spleen weights was
seen in animals of each sex at 5000 ppm. Histopathological
examination confirmed lens degeneration, indicating the induction of
cataracts in animals at 5000 ppm. Other histopathological
alterations observed in this group included slightly increased
accumulation of ferriferrous pigments in Kupffer cells of the liver
and of siderocytes in spleen. The NOAEL was 200 ppm, equal to 9
mg/kg bw per day, on the basis of reduced body-weight gain and food
consumption and liver enzyme induction at 1000 ppm and higher. The
NOAEL for cataract induction was 1000 ppm, equal to 45 mg/kg bw per
day (von Keutz & Schilde, 1987a).
In a one-year study, groups of four male and four female beagle
dogs were given tebuconazole (purity, 96.9%) at dietary
concentrations of 0, 40, 200 or 1000 ppm (weeks 1-39) and 2000 ppm
(weeks 40-52), equal to 2, 10 or 60 mg/kg bw per day in males and
females combined. Concentrations of up to 2000 ppm did not affect
survival rate, appearance, behaviour or organ weights, and
haematological examination and urinalyses showed no changes. Body
temperature, pulse rates and reflexes were normal, as were food and
water consumption and body-weight gain. Ophthalmic examination
revealed lens opacities in two dogs at 200 ppm and one at 1000 ppm;
the opacities appeared between weeks 26 and 32 and were of the same
intensity at all subsequent examinations. In the single dog at 1000
ppm which showed this ocular change, corneal opacity was also found,
which persisted until the end of the study, whereas the lens
opacities disappeared after week 32 of treatment. The lack of a
dose-response relationship with regard to lens opacities does not
preclude an association with treatment but may be due to the small
number of animals in the group and differences in individual
sensitivity. None of the other animals in this group had lens stars;
in single animals, faint lens stars were already present before
treatment started but did not become more pronounced with the
treatment. Incipient lens stars observed in animals at 40 and 200
ppm also remained stable, and most disappeared before the end of the
treatment period. Lens stars are physiological structures found
occasionally in juvenile dogs. Clinical chemical analyses revealed
slight, dose-related changes in the activity of alkaline
phosphatase: Whereas the age-dependent reduction in activity was
similar in control animals and in those at 40 and 200 ppm, the mean
activity in animals at 1000/2000 ppm indicated slight induction,
resulting in a retardation in the physiological fall in alkaline
phosphatase activity. The activity of N-demethylase and the
triglyceride content of the liver were slightly increased in animals
at 1000/2000 ppm. Most of the gross pathological findings, such as a
dose-related increase in the incidence of livers with marked
lobulation in animals treated with 200 ppm and above, were not
correlated with histopathological alterations. Histopathological
findings included intracytoplasmic vacuoles in cells of the zona
fasciculata of the adrenals in animals at 200 and 1000/2000 ppm and
slight siderosis in the spleen in most animals at 1000/2000 ppm. The
NOAEL was 40 ppm, equal to 2 mg/kg bw per day, on the basis of
cataract formation and histopathological changes in the adrenals at
higher doses (von Keutz & Schilde, 1987b).
In a second one-year study conducted at lower doses, groups of
12 male and 12 female beagle dogs were given tebuconazole (purity,
96%) at dietary concentrations of 0, 100 or 150 ppm, equal to 3 or
4.5 mg/kg bw per day for males and females combined. The treatment
did not affect mortality, body-weight gain, food consumption,
biochemical, haematological or urinary parameters, ophthalmoscopic
findings, gross pathological appearance or organ weights. The only
histopathological alteration was slight hypertrophy of adrenal zona
fasciculata cells in all animals at 150 ppm; only one control animal
had similar changes. The enlargement was accompanied by an increased
incidence of large fatty vacuoles. The NOAEL was 100 ppm, equal to 3
mg/kg bw per day, on the basis of histopathological alterations in
the adrenals at the higher dose. The NOAEL for cataract induction
was 150 ppm, equal to 4.5 mg/kg bw per day (Porter et al., 1989).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female NMRI mice were given
tebuconazole (purity, 95%) at dietary concentrations of 0, 20, 60 or
180 ppm, equal to 6, 18 or 53 mg/kg bw per day, for 21 months.
Groups of 10 treated animals of each sex were sacrificed after one
year of treatment. Appearance, behaviour, mortality, food
consumption and body weight were not affected by the treatment. In
animals treated at 180 ppm, reduced erythrocyte count and
haemoglobin and haematocrit values were observed, particularly at
the end of the first year of the study. Sporadic alterations in
clinical chemical parameters were observed in all groups. The few
dose-dependent changes consisted of an increase in total bilirubin
concentration in females treated with concentrations of 60 ppm and
higher and decreased cholesterol concentrations in plasma at 180
ppm. Gross pathological examination showed no treatment-related
effects. Liver weights were increased in animals at 180 ppm at
interim sacrifice and at the end of the study. Histopathological
examination revealed a slight increase in the prevalence of
lipid-containing periportal vacuoles in the livers of animals
treated with 60 or 180 ppm at both interim and terminal sacrifice.
No increase in tumour incidence was found. The NOAEL was 20 ppm,
equal to 6 mg/kg bw per day, on the basis of histopathological
changes in the liver (Bomhard & Ramm, 1988a).
In a similar study using higher doses, groups of 50 male and 50
female NMRI mice were maintained on a diet containing tebuconazole
(purity, 95%) at concentrations of 0, 500 or 1500 ppm, equal to 85
or 280 mg/kg bw per day, for 21 months. Groups of 10 animals per sex
and dose were scheduled for interim sacrifice after 12 months. The
mortality rate was not affected by treatment. The incidence of
animals with a 'distended abdomen' (probably due to liver
enlargement) was increased among those at 1500 ppm. A dose-dependent
reduction in body-weight gain was observed in males at 500 and 1500
ppm and in females at 1500 ppm, particularly during the first half
of the study. Food intake tended to be higher in treated animals
than in controls. Alterations in haematological parameters were
found in both treated groups: At 500 ppm and above, reduced mean
cell volume and haematocrit values and elevated mean cell
haemoglobin concentrations were found in males, and thrombocyte
counts were elevated in females. Thromboplastin time was reduced in
both treated groups. At 1500 ppm, erythrocyte counts, haemoglobin
content and haematocrit values were markedly reduced and thrombocyte
counts were elevated, particularly in males. Leukocyte counts were
also increased.
Parameters of clinical chemistry that showed dose-related
changes in both treated groups included pronounced increases in the
activities of glutamate-oxalate and glutamate-pyruvate transaminases
and alkaline phosphatase, particularly at 1500 ppm. Cholesterol,
bilirubin and albumin levels were reduced at 500 and 1500 ppm, but
there was no clear dose-response relationship. Liver weights were
increased in a dose-related fashion at both doses, and adrenal
weights were elevated in females at 1500 ppm. The gross pathological
evidence for treatment-related effects on the liver consisted of
enlargement and pale appearance of the liver in both treated groups
and some enhanced lobulation. Capsular thickening and swelling and
an increased incidence of nodular masses were also found at 1500
ppm. Histopathological examination revealed treatment-related
alterations, mainly in the liver. The non-neoplastic findings
consisted of single-cell and focal necroses, focal inflammation,
hepatocytic degeneration, hepatocytic hyerplasia and hypertrophy,
fatty vacuolation, bile-duct hyperplasia and steatoses, oval-cell
proliferation, extramedullary haematopoiesis and pigment
accumulation in Kupffer cells. Many of these alterations were found
at both 500 and 1500 ppm.
No evidence was found for a compound-related effect on the
incidence of benign or malignant tumours after one year of
treatment. Increased incidences of tumour-bearing animals were seen,
however, at 1500 ppm after 21 months of treatment. Increases were
seen in the incidences of both benign and malignant tumours in males
and of malignant tumours in females. Statistically significant
increases in tumour incidence were found only in the liver. An
increased incidence of adenomas was observed at 1500 ppm, with 36%
(versus 6% in controls) in males and 4% (versus 0 in controls) in
females; the incidences of carcinomas at this dose were 21% (versus
0 in controls) in males and 26% (versus 2% in controls) in females.
The incidences of hepatocellular tumours in animals at 1500 ppm were
reported to be above those in historical control mice of this
strain. The only reported incidences were 0-12% for adenoma and
0-10% for carcinoma in males. The NOAEL was < 500 ppm, equal to <
85 mg/kg bw per day, on the basis of reduced body-weight gain and
histopathological changes in the liver. The NOAEL for
carcinogenicity was 500 ppm, equal to 85 mg/kg bw per day, on the
basis of an increased incidence of liver tumours at 1500 ppm
(Bomhard, 1991).
Rats
Groups of 50 male and 50 female Wistar rats (Bor:WISW) were
given tebuconazole (purity, 95%) in the diet at concentrations of 0,
100, 300 or 1000 ppm, equal to 5, 16 or 55 mg/kg bw per day, for two
years. Groups of 10 animals per sex and dose were similarly treated
and killed at interim sacrifice after 12 months of treatment.
Treatment did not affect mortality, appearance, general behaviour of
the animals or haematological parameters, and urinalyses showed no
changes; no treatment-related damage to the eyes was found.
Body-weight gains were lower than those of the control animals at
doses of 300 ppm and above. Food consumption was increased among
females at 1000 ppm, and water consumption was decreased in females
at 300 ppm and above. Clinical examinations revealed several
statistically significant changes but no consistent dose-related
pattern indicative of an association with treatment, with the
probable exception of a marginal increase in the activity of alanine
aminotransferase at 1000 ppm. Decreased triglyceride concentration
was found in female animals at 1000 ppm, and an increase in spleen
weight was found in these animals. Gross pathological examination
did not provide any indication of treatment-related effects.
Histopathological examination revealed an increased incidence of
pigmented Kupffer cells in liver and increased haemosiderin
accumulation in the spleen of females at 1000 ppm.
The incidence of C-cell adenomas and carcinomas of the thyroid
was increased in all treated males in comparison with that in
controls (0/50), with 2/49 (4%) at 100 ppm, 3/50 (6%) at 300 ppm and
3/50 (6%) at 1000 ppm There was no clear dose-response relationship,
and the rates were within the range of spontaneously occurring
thyroid C-cell tumours in male Wistar rats. The mean historical
incidences of interstitial-cell and C-cell adenomas grouped with
parafollicular tumours were reported to be 7.4% (0-19.3) in males
and 8.4% (2.5-21.2) in females (Bomhard et al., 1986). The
incidence of 0 in concurrent controls is unusually low. In female
rats, a higher frequency of endometrial adenocarcinoma was found in
comparison with controls (0/50), with incidences of 3/50 at 100 ppm,
2/50 at 300 ppm and 1/50 at 1000 ppm These incidences were also
small and not dose-related. The reported historical incidence of
uterine adenocarcinomas varied considerably, from 0 to 14.4%, with a
mean incidence of 6.3% (Bomhard et al., 1986). The fact that the
endometrial tumours found in this study differ morphologically from
the typical uterine carcinomas that occur spontaneously in this rat
strain precludes a direct comparison, either qualitatively or
quantitatively. The absence of a dose-response relationship may
indicate, however, that these findings were not treatment-related.
The NOAEL was 100 ppm, equal to 5 mg/kg bw per day, on the basis of
reduced body-weight gain at higher doses (Bomhard & Ramm, 1988b).
(d) Reproductive toxicity
Rats
Groups of 25 male and 25 female Wistar rats (Bor:WISW) were
treated with tebuconazole (purity, 95%) at dietary concentrations of
0, 100, 300 or 1000 ppm, equal to 7, 22 or 72 mg/kg bw per day, over
two generations. Treatment did not affect appearance, behaviour,
general condition or mortality rates, and fertility and gestation
indexes were not altered. At 1000 ppm, the body-weight gain of the
parent animals was reduced; food consumption was reduced only among
males at the highest dose in the F0 generation and in animals of
each sex in the F1 generation. Reductions in the mean litter size
at birth, in the viability index (survival until day 5 after birth)
and in the lactation index were seen at 1000 ppm, and mean birth
weights (only in the F1a generation) and body-weight gain (in both
generations) were reduced. None of the pups had grossly apparent
malformations, and no treatment-related organotoxic effects were
noted on gross and microscopic examination. The NOAEL was 300 ppm,
equal to 22 mg/kg bw per day, on the basis of reduced body-weight
gain in parents and pups and reduced litter size at the highest dose
(Eiben, 1987).
(e) Embryotoxicity and teratogenicity
Mice
Groups of 25 female NMRI/ORIG Kisslegg mice were treated by
gavage with tebuconazole (purity, 93.6%) at a dose of 0, 10, 30 or
100 mg/kg bw per day on days 6-15 of gestation. The treatment did
not affect mortality rates, body-weight gain or pregnancy rates of
the maternal animals. At 30 mg/kg bw per day and higher, a
dose-related increase in the incidence of runts was observed, with
20/234 (8.6%) at 30 mg/kg bw per day and 26/202 (13%) at 100 mg/kg
bw per day; there were 4/234 (2%) at 10 mg/kg bw per day and 5/236
(2%) in the control group. An increased incidence of malformations
was found at 100 mg/kg bw per day, 13/202 (6.5%), which consisted
most frequently of cleft palates and individual cases of
micrognathia, rib fusion and spinal dysplasia; the incidence of
malformations in the control group was 1/236 (0.4%). The incidence
of cleft palate at this dose, 6/202 (3%), was also markedly higher
than the mean incidence in historical controls, 0.7%. The NOAEL for
maternal toxicity and clinical signs was 100 mg/kg bw per day and
that for embryotoxicity and teratogenicity was 10 mg/kg bw per day
(Renhof, 1988a).
In a study to investigate maternal toxicity further, groups of
10 female NMRI/ORIG Kisslegg mice were treated by gavage with
tebuconazole (purity, 97.4%) at doses of 0, 10, 20, 30 or 100 mg/kg
bw per day on days 6-15 of gestation. Haematological and clinical
chemical tests showed no effect of treatment on mortality rates or
body weights of the maternal animals; slight reductions in the
haematocrit and mean corpuscular volume were seen at 30 and 100
mg/kg bw per day, but with no clear dose-response relationship. The
activities of alanine and aspartate transaminases showed slight,
usually statistically significant increases in all treated groups
but with no consistent relation to dose. Triglyceride levels were
increased at 100 mg/kg bw per day. Liver weights were increased at
all doses, again with no clear dose-response relationship.
Histopathological investigation revealed cytoplasmic vacuolation of
the liver at 100 mg/kg bw per day, which was correlated with an
increase in lipid content. The dose of 100 mg/kg bw per day thus
produced pronounced liver toxicity; the changes in liver enzyme
activities, unrelated to dose, in all treated groups indicate that
maternal toxicity may have been induced at 10 mg/kg bw per day. The
embrotoxic and teratogenic effects thus occur at maternally toxic
doses. The NOAEL for maternal toxicity was < 10 mg/kg bw per day
(Renhof & Karbe, 1988).
Groups of 25 female NMRI/HAN mice were given tebuconazole
(purity, 96-98%; different batches used for main and supplementary
study) at daily dermal doses of 0, 100, 300 or 1000 mg/kg bw per day
for 6 h/day on days 6-15 of gestation. Treatment had no effect on
mortality rates, body weight, food consumption or reproductive
parameters (numbers of corpora lutea, implantations, resorptions and
live fetuses). Examination of the litters revealed no
treatment-related effect on mean body weight, and no abnormal
findings were detected in the viscera. The frequency of external
abnormalities (cleft palate, malposition of hind legs, exencephaly
and 'tail cranial bended') at 1000 mg/kg bw per day was not
different from that in the control group, with 3% in the controls,
3% at 100 mg/kg bw per day, 1.8% at 300 mg/kg bw per day and 5.3% at
1000 mg/kg bw per day. The increase at the highest dose was due to a
higher incidence of cleft palate, with 11/285 (3.9%) at 1000 mg/kg
bw per day and 6/301 (2%) in the control group. The incidence of
cleft palate in control animals in an independent study was 1.6%.
Skeletal examination showed an increased number of supernumerary
ribs at 1000 mg/kg bw per day. The NOAEL was 1000 mg/kg bw per day
for maternal toxicity and clinical signs and 300 mg/kg bw per day
for embryotoxicity and teratogenicity (Becker et al., 1990).
Since treatment-related effects on the fetuses appeared to have
occurred in the absence of maternal toxicity, a study was initiated
that included biochemical and histopathological examinations.
Tebuconazole was administered in the same way as described above to
groups of 10 mated females. As in the first study, no deaths
occurred and no clinical signs were observed in animals treated at
1000 mg/kg bw per day; the treatment had no effect on body-weight
gain, food consumption or reproductive parameters. Clinical
biochemical examinations revealed increased alanine transaminase
activity at 1000 mg/kg bw per day; there was also increased
cytochrome P450 content and N-demethylase activity in liver tissue
at > 300 mg/kg bw per day and a marginal increase in
O-demethylase activity. None of these parameters showed a
dose-response relationship. There was a dose-related reduction in
relative adrenal weights in all dose groups, which was statistically
significant at 1000 mg/kg bw per day; absolute adrenal weights were
also reduced in all treatment groups, but not in relation to dose.
No effect was seen on liver weights, but histological examination
revealed fatty changes in periportal areas in most mice at 300 mg/kg
bw per day and in all mice at 1000 mg/kg bw per day. The NOAEL was
100 mg/kg bw per day on the basis of fatty changes in the liver at
higher doses (Becker et al., 1990).
Rats
In a range-finding study, groups of 25 female Wistar rats were
treated by gavage with tebuconazole (purity, 99.5%) at doses of 0 or
100 mg/kg bw per day on days 6-15 of gestation. The treatment had no
effect on mortality rates, but body-weight gain was retarded. The
fertilization rate was not reduced by treatment, but most litter
parameters (such as number of implantations, litter size and losses)
indicated an adverse effect. More runts and fetuses with
malformations (micrognathia, hydronephrosis and hydroureter) were
found in treated animals. The NOAEL for maternal toxicity,
embryotoxicity and teratogenicity was < 100 mg/kg bw per day
(Renhof, 1984).
In a similar study, groups of 25 female Wistar rats were
treated by gavage with tebuconazole (purity, 93%) at doses of 0, 10,
30 or 100 mg/kg bw per day on days 6-15 of gestation. No
treatment-related deaths occurred. At 30 and 100 mg/kg bw per day,
dose-related reductions in weight gain were observed throughout the
treatment period; at 100 mg/kg bw per day, retarded body-weight gain
was observed throughout gestation. Effects on litter parameters
included an increased number of losses and a decrease in mean fetal
weight at 100 mg/kg bw per day. At this dose there were also more
runts and fetuses with malformations (mostly microphthalmia): the
runt incidence was 36%, compared with 7% in controls, and the
incidence of fetuses with malformations was 7%, compared with 2% in
controls. The NOAEL for maternal toxicity was 10 mg/kg bw per day
and that for embrotoxicity and teratogenicity was 30 mg/kg bw per
day (Renhof, 1985a).
Groups of 25 femaleWistar rats were treated by gavage with
tebuconazole (purity, 98.3%) at doses of 0, 30, 60 or 120 mg/kg bw
per day on days 6-15 of gestation. The dose of 30 mg/kg bw per day
affected neither maternal nor fetal parameters. At 60 mg/kg bw per
day and above, food consumption was reduced and a dose-related loss
of body weight was seen at the beginning of the treatment period.
Liver weights showed a dose-related increase at 60 and 120 mg/kg bw
per day. Reproductive parameters (numbers of corpora lutea and
implantations) were not adversely affected. At 120 mg/kg bw per day,
resorptions occurred more frequently, and consequently there were
fewer live fetuses than in the control group; mean fetal body
weights were lower. This dose induced increased incidences of
visceral alterations (consisting of excess fluid in the thoracic
cavity), supernumerary ribs, non-ossified cervical vertebrae and
incompletely ossified sternebrae, indicating retardation of fetal
development. The NOAEL was 30 mg/kg bw per day for maternal toxicity
and 60 mg/kg bw per day for embryotoxicity (Becker et al., 1988a).
Groups of 25 female Wistar rats were treated dermally with
tebuconazole (purity, 97.4%) at doses of 0, 100, 300 or 1000 mg/kg
bw per day for 6 h/day on days 6-15 of gestation. The treatment was
tolerated with no observed effects on dams or fetuses with respect
to the mortality rate, body-weight gain, gestation rate or other
reproductive parameters, such as numbers of corpora lutea,
resorptions and live fetuses. There was no indication of a
treatment-related effect on the incidence or spectrum of
malformations (Renhof, 1988b).
Rabbits
Groups of 15 female Himalayan CHBB:HM rabbits were treated by
gavage with tebuconazole (purity, 93%) at doses of 0, 3, 10 or 30
mg/kg bw per day on days 6-18 of gestation. Dams given the highest
dose had reduced body-weight gain during the treatment period.
Reproductive and fetal parameters were not adversely affected, and
no treatment-related increases in the incidence of malformations
were observed. The NOAEL was 10 mg/kg bw per day for maternal
toxicity and 30 mg/kg bw per day for embryotoxicity and
teratogenicity (Renhof, 1985b).
Groups of 16 female CHIN hybrid rabbits were treated by gavage
with tebuconazole at doses of 0, 10, 30 or 100 mg/kg bw per day on
days 6-18 of gestation. Treatment-related effects were seen at 100
mg/kg bw per day, consisting of reduced body-weight gain and food
consumption, increased mean post-implantation loss and slightly
reduced mean fetal body weight. External examination of fetuses
showed an increased incidence of malformations, including peromelia
(the most frequent malformation), agenesis of claws and cleft
palate. Of 90 fetuses born to dams treated at 100 mg/kg bw per day,
eight (9%) were malformed, and five of these (6%) had peromelia.
Peromelia was seen in none of a total of 346 fetuses in the other
three groups combined. At 100 mg/kg bw per day, the percentage of
fetuses with absent or incomplete phalangeal ossification was also
increased. In combination with the reduction in mean fetal body
weight, these findings indicate delayed maturation of fetuses at the
highest dose. The NOAEL was 30 mg/kg bw per day for maternal
toxicity, embryotoxicity and teratogenicity (Becker et al.,
1988b).
(f) Genotoxicity
Numerous studies have been conducted on the genotoxicity of
tebuconazole. The results are summarized in Table 2. No genotoxic
activity was found in any study.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Tebuconazole did not irritate the skin of rabbits, and
administration of 50 mg into the conjunctival sac did not induce
irritation of the eye (Heimann & Pauluhn, 1983; Sheets, 1988;
Märtins, 1990). In another study, instillation of 100 mg into the
conjunctival sac of rabbits caused slight irritation of the
conjunctiva (Eigenberg, 1988).
Tebuconazole did not reveal skin-sensitizing potential in a
maximization test in guinea-pigs (Heimann, 1983). The negative
result was confirmed in two further tests for sensitization in
guinea-pigs, but with the less sensitive Buehler method (Heimann,
1987; Sheets, 1990).
Table 2. Results of tests for the genotoxicity of tebuconazole
End-point Test system Concentration Purity Results Reference
of tebuconazole (%)
In vitro
Reverse S. typhimurium TA98, 20-12 500 µg/platea 97 Negative Herbold, 1983a,
mutation 100, 1535, 1587 1990
Reverse S. typhimurium TA98, 37.5-2400 µg/plateb 96.6 Negative Herbold, 1988a,b
mutation 100, 1535, 1537, 1538
Reverse S. typhimurium 15.6-500 µg/plate 98 Negative Ohta, 1991d
mutation E. coli (WPuvrA) 31.3-1000 µg/platec
156-5000 µg/plated
Forward Chinese hamster 80-100 µg/mle 96.6 Negative Lehn, 1988
mutation ovary cells 12.5-200 µg/mlc
(hprt locus)
Recombination B. subtilis (spores) 0.3-20 µg/disc 98 Negativec,d Ohta, 1992
repair capacity H17 (rec+), M45 (rec-)
Cytogenicity Human lympho cytes 3-30 µg/mlc 96.6 Negative Herbold, 1988c
30-300 µg/mld
DNA polymerase E. coli 625-10 000 µg/plate 97.1 Negativec,d Herbold, 1983b
repair capacity (K12)p 3478 (pol-)
W 3110 (pol+)
Unscheduled Rat hepatocytes 0.5-25.2 µg/ml 96.5 Negative Cifone, 1988
DNA synthesis
Sister chromatid Chinese hamster 4-30 µg/mlc 96.5 Negative Putman, 1987
exchange ovary cells 15-120 µg/mld
Table 2 (contd)
End-point Test system Concentration Purity Results Reference
of tebuconazole (%)
In vivo
Micronucleus Mouse bone marrow Single oral dose: 2000, 95.3 Negative Herbold, 1985
formation 500 or 200 mg/kg bwf
Dominant lethal Male mouse Single oral dose: 93.5 Negative Herbold, 1986
mutation 2000 mg/kg bw
a Toxic at doses > 500 µg/plate
b Toxic at doses > 60 µg/plate
c In the absence of metabolic activation
d In the presence of metabolic activation
e Toxic at doses > 75 µg/ml with and without activation
f Doses reduced in second and third trials owing to inhibition of erythropoiesis by the test compound
(ii) Cataract induction
Groups of four male and four female Forest of Dean cats
received whole-body exposure to tebuconazole (purity, 95.8%) in
polyethylene glycol and ethanol at mean analytical concentrations of
61 or 309 mg/m3 (99% of particles with an aerodynamic diameter of
<5 µm) for 6 h/day for four weeks, followed by an observation
period of 15 weeks. Control animals were exposed to aerosols of
either the vehicle or about 20 mg/m3 Sclex (KNJ 0953) (positive
controls). The treatment did not induce clinical symptoms and did
not affect mortality rates or body-weight gain. Cataracts due to
lens fibre degeneration were found in all animals in the positive
control group during the observation period. Exposure to
tebuconazole did not result in cataract induction, but three females
at 309 mg/m3 and one positive control animal had yellow-tinged
spots along the lens fissure. Examination of 42 untreated female
cats aged 7-12 months showed that these ocular changes were not
common spontaneous alterations; no such finding was seen in vehicle
controls. The etiology of this finding and its toxicological
relevance remain unclear. The NOAEL was 61 mg/m3, equivalent to
about 5 mg/kg bw per day, on the basis of ocular effects other than
cataracts of unknown etiology at the highest concentration (Märtins
et al., 1990).
Groups of four female beagle dogs were treated by head-nose
exposure to tebuconazole (purity, 97.1%) in polyethylene glycol and
ethanol at target aerosol concentrations of 0, 150 or 800 mg/m3
for 4 h/day for six weeks and observed for eight weeks. The
analytical concentrations were 163 and 914 mg/m3; about 90% of the
particles had an aerodynamic diameter of < 3 µm. No vehicle
control group was included in the study. The treatment did not
affect mortality rates. Most animals at 914 mg/m3 began salivating
immediately after exposure, and single animals also made tussive
noises; these effects were reversed within 2 h. Retardation of
body-weight gain was observed in both treated groups during the
second half of the study, but with no dose-effect relationship. A
lung function test revealed a marginal decrease in the mean minute
volume in both treated groups and a slight reduction in the mean
partial pressure of oxygen. The body temperature of treated animals
was reduced during exposure, probably due to central nervous system
depression caused by the ethanol vehicle. Increased spleen weight
observed at 914 mg/m3 was the only change in organ weights. No
gross pathological or histopathological changes were observed, and
ophthalmic examinations gave no indication of cataract formation.
The NOAEL was 163 mg/m3, equivalent to 23 mg/kg bw per day, on the
basis of clinical effects. The NOAEL for cataract induction was 914
mg/m3, equivalent to 125 mg/kg bw per day (Märtins, 1991).
3. Studies on metabolites
(a) Acute toxicity
The acute toxic effects of several metabolites of tebuconazole
are summarized in Table 3.
Table 3. Acute toxicity of metabolites of tebuconazole
Metabolite Species Sex Route LD50 (mg/kg bw)a Reference
Carboxy metabolite Rat F Oral > 4000 Astroff & Hagen, 1992
Hydroxy metabolite Rat F Oral > 5000 Sheets & Phillips, 1992
1H-1,2,4-triazole Rat M Oral 1375 Flucke, 1978
Rat M&F Oral 1650 Thyssen & Kimmerle, 1976
a Concentrations determined analytically
(b) Short-term toxicity
In a three-month study, groups of 15 male and 15 female Wistar
rats were fed diets containing 1 H-1,2,4-triazole (purity, 99.6%)
at concentrations of 0, 100, 500 or 2500 ppm, equal to 8, 38 or 212
mg/kg bw per day. Appearance, mortality rates, behaviour, clinical
chemistry parameters and the results of urinalysis, thyroid function
tests (protein-bound iodine) and gross pathological examination were
not affected by the treatment. At 2500 ppm, temporary convulsions
occurred; body-weight gain and food consumption were reduced,
particularly in males; and haematological parameters (haemoglobin
concentration, haematocrit, mean corpuscular volume and mean
corpuscular haemoglobin) were reduced in males. Histopathological
changes, confined to the liver, consisted of fatty accumulation in
three males at 2500 ppm (Bomhard et al., 1979).
(c) Embryotoxicity and teratogenicity
Groups of 25 pregnant Bor:WISW Wistar rats were treated orally
with 1,2,4-triazole (purity, 95.3%) on days 6-15 of gestation at
doses of 0, 10, 30 or 100 mg/kg bw per day. Treatment at 100 mg/kg
bw per day caused a reduction in body-weight gain of the dams,
reduced fetal weight and an increased number of runts. At the same
dose, the incidence of malformations, consisting mainly of eye
deformities such as anophthalmia and micro-phthalmia, was increased
over that in concurrent or historical controls (Renhof, 1988c).
In a similar study, groups of 25 pregnant Bor:WISW Wistar rats
were treated orally with 1,2,4-triazole at doses of 0, 100 or 200
mg/kg bw per day. The treatment impaired body-weight gain in both
treated groups but was statistically significant only at 200 mg/kg
bw per day. At this dose, an increased number of resorptions and a
reduction in fetal weight were observed. In both treated groups, a
dose-related increase in the incidence of runts was observed; the
incidence of malformations (mainly hydronephrosis and cleft palate)
was increased only at 200 mg/kg bw per day (Renhof, 1988d).
(d) Genotoxicity
The 1 H-1,2,4-triazole metabolite of tebuconazole (purity,
99.7%) was tested for its ability to induce reverse mutation in
Salmonella typhimurium strains TA98, TA100, TA1535 and TA1537.
Doses of 10-5000 µg/plate were ineffective, in either the presence
or the absence of metabolic activation (Poth, 1989).
4. Observations in humans
Employees working in the production of tebuconazole, who were
under continous medical supervision, did not show exposure-related
effects (Kollert, 1987).
Comments
After oral administration of tebuconazole to rats, 65-80% of
the dose was eliminated by the biliary and faecal route, whereas
elimination in urine amounted to about 16-35%. Males had a greater
biliary and faecal elimination than females. Biotransformation
proceeded by oxidation reactions, resulting in hydroxy, carboxy,
triol and ketoacid metabolites and conjugates as well as triazole.
Administration of acute oral doses to rats induced sedation,
abnormal gait and emaciation. Tebuconazole has low acute toxicity
and has been classified by WHO (1992) as unlikely to present an
acute hazard in normal use.
In a 13-week study, rats were fed tebuconazole at
concentrations of 0, 100, 400 or 1600 ppm. The NOAEL was 100 ppm,
equal to 9 mg/kg bw per day, on the basis of retardation of
body-weight gain and histopathological changes in the adrenal glands
at higher doses.
In two one-year studies in dogs fed diets containing 0, 40, 200
or 1000 ppm or 0, 100 or 150 ppm, an NOAEL of 100 ppm for the two
studies combined, equal to 3 mg/kg bw per day, was determined on the
basis of histopathological alterations in the adrenal glands at 150
ppm and above and cataract production at 200 ppm and above.
Two 21-month studies of toxicity and carcinogenicity were
conducted in mice, at dietary concentrations of 0, 20, 60 or 180 ppm
or 0, 500 or 1500 ppm. The NOAEL was 20 ppm, equal to 6 mg/kg bw per
day, on the basis of fatty changes in the liver. At 1500 ppm,
pronounced liver toxicity and an increased incidence of liver
tumours were observed. This tumorigenic potential is not considered
relevant to humans.
In a two-year study of toxicity and carcinogenicity in rats
treated at dietary concentrations of 0, 100, 300 or 1000 ppm, the
NOAEL was 100 ppm, equal to 5 mg/kg bw per day, on the basis of
reduced body-weight gain at higher doses. There was no evidence of
carcinogenicity.
In a two-generation study in rats fed dietary concentrations of
0, 100, 300 or 1000 ppm, the NOAEL was 300 ppm, equal to 22 mg/kg bw
per day, on the basis of reduced body-weight gain in the parental
generation and adverse effects on litters at higher dose.
Teratogenicity was investigated in mice, rats and rabbits. In
mice, doses of 0, 10, 20, 30 or 100 mg/kg bw per day were
administered. Increased enzyme activities in liver were observed at
all doses but were not dose-related, so that there was no clear
NOAEL for maternal toxicity. In mice fed doses of 0, 10, 30 or 100
mg/kg bw per day, a higher incidence of runts was seen at 30 mg/kg
bw per day and a higher incidence of malformations (mainly cleft
palate) at 100 mg/kg bw per day. The NOAEL for embryotoxicity and
teratogenicity was thus 10 mg/kg bw per day.
In rats treated with doses of 0, 10, 30 or 100 mg/kg bw per day
or 0, 30, 60 or 120 mg/kg bw per day, the NOAEL was 10 mg/kg bw per
day for maternal toxicity on the basis of reduced body-weight gain
at higher doses. Embryotoxicity and an increased incidence of
malformations (mainly microphthalmia) and visceral and skeletal
variations were found at 100 mg/kg bw per day and above, giving an
NOAEL for embryotoxicity and teratogenicity of 60 mg/kg bw per day.
In rabbits treated at doses of 0, 3, 10 or 30 mg/kg bw per day
or 0, 10, 30 or 100 mg/kg bw per day, the NOAELs were 10 mg/kg bw
per day for maternal toxicity and 30 mg/kg bw per day for
embryotoxicity and teratogenicity. At 100 mg/kg bw per day,
embryotoxicity and an increased incidence of external malformations
(mainly peromelia) were observed.
Tebuconazole has been studied in a range of tests for
genotoxicity in vivo and in vitro. The Meeting concluded that
there was no evidence of genotoxicity.
An ADI was established on the basis of an NOAEL of 100 ppm in a
one-year dietary study in dogs and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 20 ppm, equal to 6 mg/kg bw per day (21-month study
of toxicity and carcinogenicity)
< 10 mg/kg bw per day (maternal toxicity in a study
of teratogenicity)
10 mg/kg bw per day (embryotoxicity in a study of
teratogenicity)
Rat: 100 ppm, equal to 5 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
10 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
60 mg/kg bw per day (embryotoxicity in a study of
teratogenicity)
Rabbit: 10 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
30 mg/kg bw per day (embryotoxicity in a study of
teratogenicity)
Dog: 100 ppm, equal to 3 mg/kg bw per day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Astroff, A.B. & Hagen, L.L. (1992) Acute toxicity study with HWG
2443 (a metabolite of Tebuconazole Folicur(TM)) in female rats.
Unpublished report Ref. No. 92-012-PB, prepared by Miles Inc.,
Agricultural Division, Toxicology, Stillwell, KS, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Becker, H., Vogel, W. & Terrier, C. (1988a) Embryotoxicity study
(including teratogenicity) with HWG 1608 technical in the rat.
Unpublished report Ref. No. R 4451, prepared by Research &
Consulting Co. AG (RCC), Itingen, Switzerland. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Becker, H., Vogel, W. & Terrier, C. (1988b) Embryotoxicity study
(including teratogenicity) with HWG 1608 technical in the rabbit.
Unpublished report Ref. No. R 4323, prepared by Research &
Consulting Co. AG (RCC), Itingen, Switzerland. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Becker, H., Vogel, O., Terrier, C., Biedermann, K. & Luetkemeier, H.
(1990) Embryotoxicity study (including teratogenicity) with HWG 1608
technical in the mouse (dermal application). Unpublished report Ref.
No. R 5116, prepared by Research & Consulting Co. AG (RCC), Itingen,
Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomhard, E. (1991) HWG 1608--Toxic dose range carcinogenicity study
in NMRI mice (supplement to study T 601 89 53 with administration in
diet over a 21-month period). Unpublished report Ref. No. 16376 A,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomhard, E. & Ramm, W. (1988a) HWG 1608--Study for cancerogenicity
in NMRI mice (administration in diet for up to twenty-one months).
Unpublished report Ref. No. 16376, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomhard, E. & Ramm, W. (1988b) HWG 1608--Study for chronic toxicity
and cancerogenicity in Wistar rats (administration in diet for two
years). Unpublished report Ref. No. 16375, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E. & Schilde, B. (1986) HWG 1608--Subchronic toxicological
study with rats (feeding for thirteen weeks). Unpublished report
Ref. No. 15211, prepared by Bayer AG, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Bomhard, E., Löser, E. & Schilde, B. (1979) 1,2,4-Triazole:
Subchronic toxicological study with rats (feeding study over three
months). Unpublished report Ref. No. 8667, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E., Karbe, E. & Loeser, E. (1986) Spontaneous tumours of
2000 Wistar TNO/W. 70 rats in two-year carcinogenicity studies.
J.E.P.T.O. 7: 1/2, pp. 35-52.
Brain, K.R. (1991) In vitro skin permeability of tebuconazole.
Unpublished report Ref. No. M 7591, prepared by An-eX Analytical
Services Ltd., Cardiff, United Kingdom. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Chopade, H.M. (1992) Addendum I. [Phenyl-U-14C] HWG 1608: Study of
biokinetic behavior in the rat. Response to EPA requests and
inquiries. Unpublished report Ref. No. MR 97439-1 Metab I/2,
prepared by Miles Inc., Stilwell, KS, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Cifone, M.A. (1988) Mutagenicity test on HWG 1608 techn. in the rat
primary hepatocyte unscheduled DNA synthesis assay. Unpublished
report Ref. No. R 4111a, prepared by Hazelton Laboratories America
Inc., Kensington, MD, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Ecker, W. & Weber, H. (1991) [Chlorophenyl-U-14C] tebuconazole
absorption, distribution, excretion and metabolism in laying hens.
Unpublished report Ref. No. PF 3587, prepared by Bayer AG,
Leverkusen, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Ecker, W., Brauner, A., Klein, O. & Weber, H. (1987) Folicur:
Metabolism part of the general metabolism study in the rat.
Unpublished report Ref. No. PF 2907 and MR 97438. Metab I/3,
prepared by Bayer AG, Leverkusen, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Eiben, R. (1987) HWG 1608--Two-generation study in rats. Unpublished
report Ref. No. 16223, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Eigenberg, D.A. (1988) Primary eye irritation of Folicur (HWG 1608)
technical in albino rabbits. Unpublished report Ref. No. 1003,
prepared by Mobay Corp., Stilwell, KS, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Eigenberg, D.A. (199) Dermal absorption of 14C HWG 1608 technical
in rats. Unpublished report Ref. No. MR 97470. Metab I/5, prepared
by Mobay Corp., Stilwell, KS, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1978) Determination of LD 50--Compound: 1,2,4-Triazole.
Unpublished letter report by Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1987) HWG 1608 and KWG 0519 (c.n. Triadimenol)/HWG 1608
and KUE 13032c (c.n. Dichlofluanid) combination toxicity study.
Unpublished report Ref. No. 15747, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1983) HWG 1608--Study for skin-sensitising effect on
guinea pigs. Unpublished report Ref. No. 12024, prepared by Bayer
AG, Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K.G. (1987) HWG 1608 technical--Study of skin sensitization
effect on guinea pigs (Buehler patch test). Unpublished report Ref.
No. 16238, prepared by Bayer AG, Wuppertal, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Heimann, K.G. & Kaliner, G. (1984) HWG 1608--Study of the subacute
oral toxicity to rats. Unpublished report Ref. No. 13028, prepared
by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Heimann, K.G. & Pauluhn, J. (1983) HWG 1608 study for acute
toxicity. Unpublished report Ref. No. 12168, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K.G. & Schilde, B. (1984) HWG 1608--Subacute study of
dermal toxicity to rabbits. Unpublished report Ref. No. 12669,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Heimann, K.G. & Schilde, B. (1988) HWG 1608 technical--Subacute
dermal study of toxicity to rabbits. Unpublished report Ref. No.
12669a (Addendum to report No. 12669), prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B.A. (1983a) HWG 1608--Salmonella/microsome test to
evaluate for point mutagenic effect. Unpublished report Ref. No.
12086, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1983b) HWG 1608--Pol test on E. coli to evaluate for
harmful effects on DNA. Unpublished report Ref. No. 11902, prepared
by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B.A. (1985) HWG 1608--Micronucleus test on the mouse to
evaluate for mutagenic effect. Unpublished report Ref. No. 13159,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1986) HWG 1608--Dominant lethal test on the male
mouse to evaluate for mutagenic effect. Unpublished report Ref. No.
14985, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1988a) HWG 160--Salmonella/microsome test to evaluate
for point mutagenic effects. Unpublished report Ref. No. 16383,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1988b) HWG 1608--Salmonella/microsome test using TA
1538 to evaluate for point mutagenic effects (addendum to final
report Ref. No. 16383). Unpublished report Ref. No. 16383 A,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1988c) In vitro cytogenetic study with human
lymphocytes for the detection of induced clastogenic effects.
Unpublished report Ref. No. 16395, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1990) HWG 1608--Salmonella/microsome test for
point-mutagenic effects (appendix to Report 12086). Unpublished
report Ref. No. 12086A, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1983a) HWG 1608 acute toxicity to the dog after oral
administration. Unpublished report Ref. No. 11974, prepared by Bayer
AG, Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hoffmann, K. (1983b) HWG 1608 acute toxicity to the sheep after oral
administration. Unpublished report Ref. No. 11970, prepared by Bayer
AG, Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
von Keutz, E. & Schilde, B. (1987a) HWG 1608--Subchronic study of
toxicity to dogs with oral administration (thirteen-weeks feeding
study). Unpublished report Ref. No. 15763, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
von Keutz, E. & Schilde, B. (1987b) HWG 1608--Study of chronic
toxicity to dogs after oral administration (twelve-month feeding
study). Unpublished report Ref. No. 16211, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kollert, W. (1987) Report dated 10 November 1987, HWE 1608--Internal
experiments, Bayer Medical Department, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Lee, S.G.K. & Wood, S.E. (1987) The metabolism of Folicur in dairy
goats. Unpublished report Ref. No. MR 94882, prepared by Mobay
Corporation, Kansas City, MO, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lee, S.G.K., Hanna, L.A., Johnston, K., Wood, S.E. & Leimkuehler,
W.M. (1988) The metabolism of 14C-Folicur in chickens. Unpublished
report Ref. No. 87 156, prepared by Mobay Corp., Kansas City, MO,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lehn, H. (1988) HWG 1608--Mutagenicity study for the detection of
induced forward mutations in the CHO-HGPRT assay in vitro.
Unpublished report Ref. No. 16749, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Märtins, T. (1990) HWG 1608--Study for skin and eye
irritation/corrosion in rabbits. Unpublished report Ref. No. 12168 B
(addendum report to report No. 12168), prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Märtins, T. (1991) HWG 1608 (tebuconazole)--Subacute inhalation
toxicity to dogs--Study for cataracts. Unpublished report Ref. No.
20884, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Märtins, T., Pauluhn, J. & Krötlinger, F. (1990) HWG 1608
(tebuconazole)--Subacute inhalation toxicity to cats--Study for
cataracts. Unpublished report Ref. No. 19644, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Ohta, K. (1991a) HWG 1608 technical--Acute oral toxicity study on
rats. Study No. 91A016. Unpublished report prepared by Nihon Bayer
Agrochem K.K., Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Ohta, K. (1991b) HWG 1608 technical--Acute dermal toxicity study on
rats. Study No. 91A012. Unpublished report prepared by Nihon Bayer
Agrochem K.K. Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Ohta, K. (1991c) HWG 1608 technical--Acute oral toxicity study on
mice. Study No. 91A017. Unpublished report prepared by Nihon Bayer
Agrochem K.K., Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Ohta, K. (1991d) HWG 1608--Reverse mutation assay (Salmonella
typhimurium and Escherichia coli). Unpublished report Ref. No. RA
91036, prepared by Nikon Bayer Agrochem K.K., Tokyo, Japan.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ohta, K. (1992) HWG 1608--Rec- assay with spores in the bacterial
system. Unpublished report Ref. No. RA 92007, prepared by Nihon
Bayer Agrochem K.K., Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Pauluhn, J. (1985) HWG 1608--Study for subacute inhalation toxicity
to rat for three weeks (exposure 15 x 6 hours). Unpublished report
Ref. No. 13305, prepared by Bayer AG, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1987) HWG 1608--Study for subacute inhalation toxicity
to rat for three weeks (exposure 15 x 6 hours). Histopathological
examinations. Unpublished report Ref. No. 13305A (addendum to Ref.
No. 13305), prepared by Bayer AG, Wuppertal, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1988) HWG 1608--Study for acute inhalation toxicity to
the rat to OECD-guideline No. 403. Unpublished report Ref. No.
16345, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Porter, M.C., Jasty, V., Troup, C.M. & Hartnagel, R.E. (1989) Safety
evaluation of HWG 1608: Chronic (1 year) feeding study in dogs.
Unpublished report Ref. No. R 4781, prepared by Toxicology
Department, Miles Inc., Elkhart, IN, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Poth, A. (1989) Salmonella typhimurium reverse mutation assay with
1H-1,2,4-triazole. Unpublished report Ref. No. R 4859, prepared by
Cytotest Cell Research GMBH & Co. KG, Rossdorf, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Putman, D.L. (1987) HWG 1608--Sister chromatid exchange assay in
Chinese hamster ovary (CHO) cells. Unpublished report Ref. No. 953,
prepared by Microbiological Associates Inc., Bethesda, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1984) HWG 1608--Study for embryotoxic effects on rats
after oral administration. Unpublished report Ref. No. 12457,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1985a) HWG 1608--Study for embryotoxic effects on rats
after oral administration. Unpublished report Ref. No. 13273,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1985b) HWG 1608--Study for embryotoxic effects on
rabbits after oral administration. Unpublished report Ref. No.
13287, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Renhof, M. (1988a) HWG 1608--Study for embryotoxic effects on mice
following oral administration. Unpublished report Ref. No. 16527,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1988b) HWG 1608--Study for embryotoxic effects on rats
after dermal administration. Unpublished report Ref. No. 17089,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1988c) Triazole--Investigations into embryotoxic effects
on rats after oral administration. Unpublished report No. 17401,
Ref. No. 50 19 339, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1988d) Triazole--Investigations into embryotoxic effects
on rats after oral administration. Unpublished report No. 17402,
Supplement to Ref. No. T 50 19 339, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. & Karbe, E. (1988) HWG 1608--Supplementary study for
maternal toxicity on mice following oral administration. Unpublished
report Ref. No. 16511, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Sheets, L.P. (1988) Primary dermal irritation of technical grade
Folicur in rabbits. Unpublished report Ref. No. 1066, prepared by
Mobay Corp., Stilwell, KS, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Sheets, L.P. (1990) Dermal sensitization study with technical grade
tebuconazole (Folicur) in guinea pigs. Unpublished report Ref. No.
5052, prepared by Mobay Corp., Stilwell, KS, USA. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Sheets, L.P. & Phillips, S.D. (1992) Acute oral toxicity study with
HWG 2061 (a metabolite of tebuconazole Folicur(TM)) in female
rats. Unpublished report Ref. No. 6685, prepared by Miles Inc.,
Agricultural Division, Toxicology, Stilwell, KS, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1976) 1,2,4-Triazole--Occupational
toxicology study. Unpublished report Ref. No. 5926, prepared by
Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Weber, H. (1987) [Phenyl-U-14C] HWG 1608: Study of biokinetic
behaviour in the rat. Unpublished report Ref. No. PF 2859. Metab
II/15, prepared by Bayer AG, Leverkusen, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Weber, H. (1988) [Phenyl-U-14C] HWG 1608:
Ganzkörperautoradiographische Verteilung der Radioaktivität in der
Ratte. Unpublished report Ref. No. PF 2962. Metab II/16, prepared by
Bayer AG, Leverkusen, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
 (b) Short-term toxicity
Rats
Groups of 20 male and 20 female Wistar rats (Bor: WISW) were
given tebuconazole at doses of 0, 30, 100 or 300 mg/kg bw per day by
intubation for four weeks and observed during a four-week recovery
period. The dose of 300 mg/kg bw per day caused mild lethargy and
reduced body-weight gain during the treatment period. Doses of 100
mg/kg bw per day and above decreased the haemoglobin concentration
and haematocrit values. In females at 300 mg/kg bw per day, the
leukocyte count was increased. Clinical chemistry revealed slight,
not statistically significant, increases in the activities of
glutamate-oxalate and glutamate-pyruvate transaminases in males at
300 mg/kg bw per day, and marked increases in liver enzyme
activities (glutamate-oxalate and glutamate-pyruvate transaminases
and alkaline phosphatase) in females at the same dose. Treatment at
100 and 300 mg/kg bw per day induced the microsomal enzyme system,
and the activities of N- and O-demethylases and cytochrome P450
and the triglyceride concentration in liver were increased. All
these changes were reversible. Urinalyses revealed no abnormal
findings. At 100 and 300 mg/kg bw per day, the weights of the liver
and spleen were increased in animals of each sex and the weight of
the kidney in females. Histopathological findings at 300 mg/kg bw
per day consisted of fatty changes in the liver and bile-duct
proliferation in females; enlargement of the centrilobular
hepatocytes was found in male rats. Histopathological changes were
also found in the adrenal cortex, consisting of proliferated
endothelial cells and an increased incidence of fat vacuoles.
Sclerosis of the red pulp of the spleen, associated with
sideropenia, was observed in males at 300 mg/kg bw per day;
sideropenia was also found in females at 100 mg/kg bw per day. The
NOAEL was 30 mg/kg bw per day on the basis of changes in
haematological and clinical chemical parameters and organ weights at
higher doses (Heimann & Kaliner, 1984).
Groups of 10 male and 10 female Wistar rats (Bor: WISW) were
exposed to nominal aerosol concentrations of tebuconazole at 0, 5,
50 or 500 mg/m3 in polyethylene glycol by head and nose exposure
for 6 h/day, five days per week for three weeks (15 days). The
analytical concentrations were 0, 1.2, 11 and 156 mg/m3. About 90%
of the particle mass had an aerodynamic diameter of < 5 µm. The
treatment had no effect on mortality rate, body-weight gain,
haematological or clinical chemical parameters or organ weights;
urinalysis showed no abnormal findings. Rats treated with 156
mg/m3 had piloerection after each exposure. Mixed-function
oxidases in the liver were induced. At the end of the study,
N-demethylase activity in the liver was increased in animals of
each sex at the highest dose. Males in this group also had increased
O-demethylase activity. No treatment-related gross or
histopathological alterations were observed. The NOAEL was 10.6
mg/m3, equivalent to about 2 mg/kg bw per day, on the basis of
liver enzyme induction at the highest concentration (Pauluhn, 1985,
1987).
Groups of 10 male and 10 female rats (Wistar, BOR:WISW) were
given dietary concentrations of tebuconazole (purity, 93.4%; 4.8%
symmetrical isomer) at 0, 100, 400 or 1600 ppm, equal to 9, 35 or
172 mg/kg bw per day, for 13 weeks. The treatment had no effect on
appearance or behaviour or the findings of ophthalmic or
haematological examinations or urinalyses. Food intake was increased
in animals of each sex at 1600 ppm. Retardation of body-weight gain
was observed at 400 ppm in females during the first six weeks and at
1600 ppm in animals of each sex. Two animals at 1600 ppm died; no
deaths occurred in the other dose groups. Few of the changes in
clinical chemistry showed a dose-related or time-consistent pattern
and were regarded as toxicologically insignificant. An increase in
urea concentration at 1600 ppm and a decrease in triglyceride
concentrations in animals at 400 ppm and above were seen only after
the first four weeks and not at the end of the study. Pronounced
increases in N-demethylase activity and cytochrome P450 content
were found in male animals at 1600 ppm, and increased liver weights
were seen in females at this dose. Histopathological examination
revealed an increased incidence of intra-plasmatic vacuoles in the
cells of the zona fasciculata of the adrenals (probably lipid
accumulation) in some females at 400 ppm and in all females at 1600
ppm. This effect was less pronounced in males because of a higher
background incidence of adrenal vacuole formation in control animals
(Bomhard & Schilde, 1986). The NOAEL was 100 ppm, equal to 9 mg/kg
bw per day, on the basis of retardation of body-weight gain and
histopathological changes in the adrenals at higher doses
Rabbits
Groups of 18 male and 18 female HC New Zealand white rabbits
received tebuconazole at doses of 0, 50 or 250 mg/kg bw per day on
the shorn skin for 6 h daily for three weeks. The treatment was
tolerated with no effects (Heimann & Schilde, 1984). In an
additional study, a dose of 1000 mg/kg bw per day was also tolerated
with no systemic or local effects (Heimann & Schilde, 1988).
Dogs
Groups of four male and four female beagle dogs were given
tebuconazole (purity, 93.4%; 4.8% symmetric isomer) at dietary
concentrations of 0, 200, 1000 or 5000 ppm (equal to 9, 45 or 220
mg/kg bw per day for males and females combined) over 13 weeks. One
dog at 5000 ppm was found dead after the first dose, with no
previous clinical signs. Treatment did not affect body temperature,
pulse rate or neurological signs. Food consumption was reduced at
1000 and 5000 ppm, and body-weight gain was retarded in these
groups; a few dogs at 1000 ppm and most of those at 5000 ppm had a
deteriorated nutritional status. Ophthalmic examination revealed
lens opacities in all animals at 5000 ppm, which first appeared
after seven weeks of treatment. An increase in thrombocyte count was
found at 5000 ppm, and at the end of the study six of eight animals
at this dose had marked anisocytosis. Alkaline phosphatase activity
in plasma showed a retarded age-induced fall at 1000 ppm and an
increase at 5000 ppm. N-Demethylase activity in the liver had
increased slightly by the end of the study in animals at 1000 ppm
and had increased markedly in those at 5000 ppm. The cytochrome P450
content of the liver was also elevated at 5000 ppm. In the group at
the highest dose, a shift in the composition of serum proteins was
observed, with a slightly lower albumin content and a simultaneous
increase in the ß-globulin content. Urinalyses revealed no
treatment-related effects. Changes in organ weights did not follow a
consistent pattern, except that an increase in spleen weights was
seen in animals of each sex at 5000 ppm. Histopathological
examination confirmed lens degeneration, indicating the induction of
cataracts in animals at 5000 ppm. Other histopathological
alterations observed in this group included slightly increased
accumulation of ferriferrous pigments in Kupffer cells of the liver
and of siderocytes in spleen. The NOAEL was 200 ppm, equal to 9
mg/kg bw per day, on the basis of reduced body-weight gain and food
consumption and liver enzyme induction at 1000 ppm and higher. The
NOAEL for cataract induction was 1000 ppm, equal to 45 mg/kg bw per
day (von Keutz & Schilde, 1987a).
In a one-year study, groups of four male and four female beagle
dogs were given tebuconazole (purity, 96.9%) at dietary
concentrations of 0, 40, 200 or 1000 ppm (weeks 1-39) and 2000 ppm
(weeks 40-52), equal to 2, 10 or 60 mg/kg bw per day in males and
females combined. Concentrations of up to 2000 ppm did not affect
survival rate, appearance, behaviour or organ weights, and
haematological examination and urinalyses showed no changes. Body
temperature, pulse rates and reflexes were normal, as were food and
water consumption and body-weight gain. Ophthalmic examination
revealed lens opacities in two dogs at 200 ppm and one at 1000 ppm;
the opacities appeared between weeks 26 and 32 and were of the same
intensity at all subsequent examinations. In the single dog at 1000
ppm which showed this ocular change, corneal opacity was also found,
which persisted until the end of the study, whereas the lens
opacities disappeared after week 32 of treatment. The lack of a
dose-response relationship with regard to lens opacities does not
preclude an association with treatment but may be due to the small
number of animals in the group and differences in individual
sensitivity. None of the other animals in this group had lens stars;
in single animals, faint lens stars were already present before
treatment started but did not become more pronounced with the
treatment. Incipient lens stars observed in animals at 40 and 200
ppm also remained stable, and most disappeared before the end of the
treatment period. Lens stars are physiological structures found
occasionally in juvenile dogs. Clinical chemical analyses revealed
slight, dose-related changes in the activity of alkaline
phosphatase: Whereas the age-dependent reduction in activity was
similar in control animals and in those at 40 and 200 ppm, the mean
activity in animals at 1000/2000 ppm indicated slight induction,
resulting in a retardation in the physiological fall in alkaline
phosphatase activity. The activity of N-demethylase and the
triglyceride content of the liver were slightly increased in animals
at 1000/2000 ppm. Most of the gross pathological findings, such as a
dose-related increase in the incidence of livers with marked
lobulation in animals treated with 200 ppm and above, were not
correlated with histopathological alterations. Histopathological
findings included intracytoplasmic vacuoles in cells of the zona
fasciculata of the adrenals in animals at 200 and 1000/2000 ppm and
slight siderosis in the spleen in most animals at 1000/2000 ppm. The
NOAEL was 40 ppm, equal to 2 mg/kg bw per day, on the basis of
cataract formation and histopathological changes in the adrenals at
higher doses (von Keutz & Schilde, 1987b).
In a second one-year study conducted at lower doses, groups of
12 male and 12 female beagle dogs were given tebuconazole (purity,
96%) at dietary concentrations of 0, 100 or 150 ppm, equal to 3 or
4.5 mg/kg bw per day for males and females combined. The treatment
did not affect mortality, body-weight gain, food consumption,
biochemical, haematological or urinary parameters, ophthalmoscopic
findings, gross pathological appearance or organ weights. The only
histopathological alteration was slight hypertrophy of adrenal zona
fasciculata cells in all animals at 150 ppm; only one control animal
had similar changes. The enlargement was accompanied by an increased
incidence of large fatty vacuoles. The NOAEL was 100 ppm, equal to 3
mg/kg bw per day, on the basis of histopathological alterations in
the adrenals at the higher dose. The NOAEL for cataract induction
was 150 ppm, equal to 4.5 mg/kg bw per day (Porter et al., 1989).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female NMRI mice were given
tebuconazole (purity, 95%) at dietary concentrations of 0, 20, 60 or
180 ppm, equal to 6, 18 or 53 mg/kg bw per day, for 21 months.
Groups of 10 treated animals of each sex were sacrificed after one
year of treatment. Appearance, behaviour, mortality, food
consumption and body weight were not affected by the treatment. In
animals treated at 180 ppm, reduced erythrocyte count and
haemoglobin and haematocrit values were observed, particularly at
the end of the first year of the study. Sporadic alterations in
clinical chemical parameters were observed in all groups. The few
dose-dependent changes consisted of an increase in total bilirubin
concentration in females treated with concentrations of 60 ppm and
higher and decreased cholesterol concentrations in plasma at 180
ppm. Gross pathological examination showed no treatment-related
effects. Liver weights were increased in animals at 180 ppm at
interim sacrifice and at the end of the study. Histopathological
examination revealed a slight increase in the prevalence of
lipid-containing periportal vacuoles in the livers of animals
treated with 60 or 180 ppm at both interim and terminal sacrifice.
No increase in tumour incidence was found. The NOAEL was 20 ppm,
equal to 6 mg/kg bw per day, on the basis of histopathological
changes in the liver (Bomhard & Ramm, 1988a).
In a similar study using higher doses, groups of 50 male and 50
female NMRI mice were maintained on a diet containing tebuconazole
(purity, 95%) at concentrations of 0, 500 or 1500 ppm, equal to 85
or 280 mg/kg bw per day, for 21 months. Groups of 10 animals per sex
and dose were scheduled for interim sacrifice after 12 months. The
mortality rate was not affected by treatment. The incidence of
animals with a 'distended abdomen' (probably due to liver
enlargement) was increased among those at 1500 ppm. A dose-dependent
reduction in body-weight gain was observed in males at 500 and 1500
ppm and in females at 1500 ppm, particularly during the first half
of the study. Food intake tended to be higher in treated animals
than in controls. Alterations in haematological parameters were
found in both treated groups: At 500 ppm and above, reduced mean
cell volume and haematocrit values and elevated mean cell
haemoglobin concentrations were found in males, and thrombocyte
counts were elevated in females. Thromboplastin time was reduced in
both treated groups. At 1500 ppm, erythrocyte counts, haemoglobin
content and haematocrit values were markedly reduced and thrombocyte
counts were elevated, particularly in males. Leukocyte counts were
also increased.
Parameters of clinical chemistry that showed dose-related
changes in both treated groups included pronounced increases in the
activities of glutamate-oxalate and glutamate-pyruvate transaminases
and alkaline phosphatase, particularly at 1500 ppm. Cholesterol,
bilirubin and albumin levels were reduced at 500 and 1500 ppm, but
there was no clear dose-response relationship. Liver weights were
increased in a dose-related fashion at both doses, and adrenal
weights were elevated in females at 1500 ppm. The gross pathological
evidence for treatment-related effects on the liver consisted of
enlargement and pale appearance of the liver in both treated groups
and some enhanced lobulation. Capsular thickening and swelling and
an increased incidence of nodular masses were also found at 1500
ppm. Histopathological examination revealed treatment-related
alterations, mainly in the liver. The non-neoplastic findings
consisted of single-cell and focal necroses, focal inflammation,
hepatocytic degeneration, hepatocytic hyerplasia and hypertrophy,
fatty vacuolation, bile-duct hyperplasia and steatoses, oval-cell
proliferation, extramedullary haematopoiesis and pigment
accumulation in Kupffer cells. Many of these alterations were found
at both 500 and 1500 ppm.
No evidence was found for a compound-related effect on the
incidence of benign or malignant tumours after one year of
treatment. Increased incidences of tumour-bearing animals were seen,
however, at 1500 ppm after 21 months of treatment. Increases were
seen in the incidences of both benign and malignant tumours in males
and of malignant tumours in females. Statistically significant
increases in tumour incidence were found only in the liver. An
increased incidence of adenomas was observed at 1500 ppm, with 36%
(versus 6% in controls) in males and 4% (versus 0 in controls) in
females; the incidences of carcinomas at this dose were 21% (versus
0 in controls) in males and 26% (versus 2% in controls) in females.
The incidences of hepatocellular tumours in animals at 1500 ppm were
reported to be above those in historical control mice of this
strain. The only reported incidences were 0-12% for adenoma and
0-10% for carcinoma in males. The NOAEL was < 500 ppm, equal to <
85 mg/kg bw per day, on the basis of reduced body-weight gain and
histopathological changes in the liver. The NOAEL for
carcinogenicity was 500 ppm, equal to 85 mg/kg bw per day, on the
basis of an increased incidence of liver tumours at 1500 ppm
(Bomhard, 1991).
Rats
Groups of 50 male and 50 female Wistar rats (Bor:WISW) were
given tebuconazole (purity, 95%) in the diet at concentrations of 0,
100, 300 or 1000 ppm, equal to 5, 16 or 55 mg/kg bw per day, for two
years. Groups of 10 animals per sex and dose were similarly treated
and killed at interim sacrifice after 12 months of treatment.
Treatment did not affect mortality, appearance, general behaviour of
the animals or haematological parameters, and urinalyses showed no
changes; no treatment-related damage to the eyes was found.
Body-weight gains were lower than those of the control animals at
doses of 300 ppm and above. Food consumption was increased among
females at 1000 ppm, and water consumption was decreased in females
at 300 ppm and above. Clinical examinations revealed several
statistically significant changes but no consistent dose-related
pattern indicative of an association with treatment, with the
probable exception of a marginal increase in the activity of alanine
aminotransferase at 1000 ppm. Decreased triglyceride concentration
was found in female animals at 1000 ppm, and an increase in spleen
weight was found in these animals. Gross pathological examination
did not provide any indication of treatment-related effects.
Histopathological examination revealed an increased incidence of
pigmented Kupffer cells in liver and increased haemosiderin
accumulation in the spleen of females at 1000 ppm.
The incidence of C-cell adenomas and carcinomas of the thyroid
was increased in all treated males in comparison with that in
controls (0/50), with 2/49 (4%) at 100 ppm, 3/50 (6%) at 300 ppm and
3/50 (6%) at 1000 ppm There was no clear dose-response relationship,
and the rates were within the range of spontaneously occurring
thyroid C-cell tumours in male Wistar rats. The mean historical
incidences of interstitial-cell and C-cell adenomas grouped with
parafollicular tumours were reported to be 7.4% (0-19.3) in males
and 8.4% (2.5-21.2) in females (Bomhard et al., 1986). The
incidence of 0 in concurrent controls is unusually low. In female
rats, a higher frequency of endometrial adenocarcinoma was found in
comparison with controls (0/50), with incidences of 3/50 at 100 ppm,
2/50 at 300 ppm and 1/50 at 1000 ppm These incidences were also
small and not dose-related. The reported historical incidence of
uterine adenocarcinomas varied considerably, from 0 to 14.4%, with a
mean incidence of 6.3% (Bomhard et al., 1986). The fact that the
endometrial tumours found in this study differ morphologically from
the typical uterine carcinomas that occur spontaneously in this rat
strain precludes a direct comparison, either qualitatively or
quantitatively. The absence of a dose-response relationship may
indicate, however, that these findings were not treatment-related.
The NOAEL was 100 ppm, equal to 5 mg/kg bw per day, on the basis of
reduced body-weight gain at higher doses (Bomhard & Ramm, 1988b).
(d) Reproductive toxicity
Rats
Groups of 25 male and 25 female Wistar rats (Bor:WISW) were
treated with tebuconazole (purity, 95%) at dietary concentrations of
0, 100, 300 or 1000 ppm, equal to 7, 22 or 72 mg/kg bw per day, over
two generations. Treatment did not affect appearance, behaviour,
general condition or mortality rates, and fertility and gestation
indexes were not altered. At 1000 ppm, the body-weight gain of the
parent animals was reduced; food consumption was reduced only among
males at the highest dose in the F0 generation and in animals of
each sex in the F1 generation. Reductions in the mean litter size
at birth, in the viability index (survival until day 5 after birth)
and in the lactation index were seen at 1000 ppm, and mean birth
weights (only in the F1a generation) and body-weight gain (in both
generations) were reduced. None of the pups had grossly apparent
malformations, and no treatment-related organotoxic effects were
noted on gross and microscopic examination. The NOAEL was 300 ppm,
equal to 22 mg/kg bw per day, on the basis of reduced body-weight
gain in parents and pups and reduced litter size at the highest dose
(Eiben, 1987).
(e) Embryotoxicity and teratogenicity
Mice
Groups of 25 female NMRI/ORIG Kisslegg mice were treated by
gavage with tebuconazole (purity, 93.6%) at a dose of 0, 10, 30 or
100 mg/kg bw per day on days 6-15 of gestation. The treatment did
not affect mortality rates, body-weight gain or pregnancy rates of
the maternal animals. At 30 mg/kg bw per day and higher, a
dose-related increase in the incidence of runts was observed, with
20/234 (8.6%) at 30 mg/kg bw per day and 26/202 (13%) at 100 mg/kg
bw per day; there were 4/234 (2%) at 10 mg/kg bw per day and 5/236
(2%) in the control group. An increased incidence of malformations
was found at 100 mg/kg bw per day, 13/202 (6.5%), which consisted
most frequently of cleft palates and individual cases of
micrognathia, rib fusion and spinal dysplasia; the incidence of
malformations in the control group was 1/236 (0.4%). The incidence
of cleft palate at this dose, 6/202 (3%), was also markedly higher
than the mean incidence in historical controls, 0.7%. The NOAEL for
maternal toxicity and clinical signs was 100 mg/kg bw per day and
that for embryotoxicity and teratogenicity was 10 mg/kg bw per day
(Renhof, 1988a).
In a study to investigate maternal toxicity further, groups of
10 female NMRI/ORIG Kisslegg mice were treated by gavage with
tebuconazole (purity, 97.4%) at doses of 0, 10, 20, 30 or 100 mg/kg
bw per day on days 6-15 of gestation. Haematological and clinical
chemical tests showed no effect of treatment on mortality rates or
body weights of the maternal animals; slight reductions in the
haematocrit and mean corpuscular volume were seen at 30 and 100
mg/kg bw per day, but with no clear dose-response relationship. The
activities of alanine and aspartate transaminases showed slight,
usually statistically significant increases in all treated groups
but with no consistent relation to dose. Triglyceride levels were
increased at 100 mg/kg bw per day. Liver weights were increased at
all doses, again with no clear dose-response relationship.
Histopathological investigation revealed cytoplasmic vacuolation of
the liver at 100 mg/kg bw per day, which was correlated with an
increase in lipid content. The dose of 100 mg/kg bw per day thus
produced pronounced liver toxicity; the changes in liver enzyme
activities, unrelated to dose, in all treated groups indicate that
maternal toxicity may have been induced at 10 mg/kg bw per day. The
embrotoxic and teratogenic effects thus occur at maternally toxic
doses. The NOAEL for maternal toxicity was < 10 mg/kg bw per day
(Renhof & Karbe, 1988).
Groups of 25 female NMRI/HAN mice were given tebuconazole
(purity, 96-98%; different batches used for main and supplementary
study) at daily dermal doses of 0, 100, 300 or 1000 mg/kg bw per day
for 6 h/day on days 6-15 of gestation. Treatment had no effect on
mortality rates, body weight, food consumption or reproductive
parameters (numbers of corpora lutea, implantations, resorptions and
live fetuses). Examination of the litters revealed no
treatment-related effect on mean body weight, and no abnormal
findings were detected in the viscera. The frequency of external
abnormalities (cleft palate, malposition of hind legs, exencephaly
and 'tail cranial bended') at 1000 mg/kg bw per day was not
different from that in the control group, with 3% in the controls,
3% at 100 mg/kg bw per day, 1.8% at 300 mg/kg bw per day and 5.3% at
1000 mg/kg bw per day. The increase at the highest dose was due to a
higher incidence of cleft palate, with 11/285 (3.9%) at 1000 mg/kg
bw per day and 6/301 (2%) in the control group. The incidence of
cleft palate in control animals in an independent study was 1.6%.
Skeletal examination showed an increased number of supernumerary
ribs at 1000 mg/kg bw per day. The NOAEL was 1000 mg/kg bw per day
for maternal toxicity and clinical signs and 300 mg/kg bw per day
for embryotoxicity and teratogenicity (Becker et al., 1990).
Since treatment-related effects on the fetuses appeared to have
occurred in the absence of maternal toxicity, a study was initiated
that included biochemical and histopathological examinations.
Tebuconazole was administered in the same way as described above to
groups of 10 mated females. As in the first study, no deaths
occurred and no clinical signs were observed in animals treated at
1000 mg/kg bw per day; the treatment had no effect on body-weight
gain, food consumption or reproductive parameters. Clinical
biochemical examinations revealed increased alanine transaminase
activity at 1000 mg/kg bw per day; there was also increased
cytochrome P450 content and N-demethylase activity in liver tissue
at > 300 mg/kg bw per day and a marginal increase in
O-demethylase activity. None of these parameters showed a
dose-response relationship. There was a dose-related reduction in
relative adrenal weights in all dose groups, which was statistically
significant at 1000 mg/kg bw per day; absolute adrenal weights were
also reduced in all treatment groups, but not in relation to dose.
No effect was seen on liver weights, but histological examination
revealed fatty changes in periportal areas in most mice at 300 mg/kg
bw per day and in all mice at 1000 mg/kg bw per day. The NOAEL was
100 mg/kg bw per day on the basis of fatty changes in the liver at
higher doses (Becker et al., 1990).
Rats
In a range-finding study, groups of 25 female Wistar rats were
treated by gavage with tebuconazole (purity, 99.5%) at doses of 0 or
100 mg/kg bw per day on days 6-15 of gestation. The treatment had no
effect on mortality rates, but body-weight gain was retarded. The
fertilization rate was not reduced by treatment, but most litter
parameters (such as number of implantations, litter size and losses)
indicated an adverse effect. More runts and fetuses with
malformations (micrognathia, hydronephrosis and hydroureter) were
found in treated animals. The NOAEL for maternal toxicity,
embryotoxicity and teratogenicity was < 100 mg/kg bw per day
(Renhof, 1984).
In a similar study, groups of 25 female Wistar rats were
treated by gavage with tebuconazole (purity, 93%) at doses of 0, 10,
30 or 100 mg/kg bw per day on days 6-15 of gestation. No
treatment-related deaths occurred. At 30 and 100 mg/kg bw per day,
dose-related reductions in weight gain were observed throughout the
treatment period; at 100 mg/kg bw per day, retarded body-weight gain
was observed throughout gestation. Effects on litter parameters
included an increased number of losses and a decrease in mean fetal
weight at 100 mg/kg bw per day. At this dose there were also more
runts and fetuses with malformations (mostly microphthalmia): the
runt incidence was 36%, compared with 7% in controls, and the
incidence of fetuses with malformations was 7%, compared with 2% in
controls. The NOAEL for maternal toxicity was 10 mg/kg bw per day
and that for embrotoxicity and teratogenicity was 30 mg/kg bw per
day (Renhof, 1985a).
Groups of 25 femaleWistar rats were treated by gavage with
tebuconazole (purity, 98.3%) at doses of 0, 30, 60 or 120 mg/kg bw
per day on days 6-15 of gestation. The dose of 30 mg/kg bw per day
affected neither maternal nor fetal parameters. At 60 mg/kg bw per
day and above, food consumption was reduced and a dose-related loss
of body weight was seen at the beginning of the treatment period.
Liver weights showed a dose-related increase at 60 and 120 mg/kg bw
per day. Reproductive parameters (numbers of corpora lutea and
implantations) were not adversely affected. At 120 mg/kg bw per day,
resorptions occurred more frequently, and consequently there were
fewer live fetuses than in the control group; mean fetal body
weights were lower. This dose induced increased incidences of
visceral alterations (consisting of excess fluid in the thoracic
cavity), supernumerary ribs, non-ossified cervical vertebrae and
incompletely ossified sternebrae, indicating retardation of fetal
development. The NOAEL was 30 mg/kg bw per day for maternal toxicity
and 60 mg/kg bw per day for embryotoxicity (Becker et al., 1988a).
Groups of 25 female Wistar rats were treated dermally with
tebuconazole (purity, 97.4%) at doses of 0, 100, 300 or 1000 mg/kg
bw per day for 6 h/day on days 6-15 of gestation. The treatment was
tolerated with no observed effects on dams or fetuses with respect
to the mortality rate, body-weight gain, gestation rate or other
reproductive parameters, such as numbers of corpora lutea,
resorptions and live fetuses. There was no indication of a
treatment-related effect on the incidence or spectrum of
malformations (Renhof, 1988b).
Rabbits
Groups of 15 female Himalayan CHBB:HM rabbits were treated by
gavage with tebuconazole (purity, 93%) at doses of 0, 3, 10 or 30
mg/kg bw per day on days 6-18 of gestation. Dams given the highest
dose had reduced body-weight gain during the treatment period.
Reproductive and fetal parameters were not adversely affected, and
no treatment-related increases in the incidence of malformations
were observed. The NOAEL was 10 mg/kg bw per day for maternal
toxicity and 30 mg/kg bw per day for embryotoxicity and
teratogenicity (Renhof, 1985b).
Groups of 16 female CHIN hybrid rabbits were treated by gavage
with tebuconazole at doses of 0, 10, 30 or 100 mg/kg bw per day on
days 6-18 of gestation. Treatment-related effects were seen at 100
mg/kg bw per day, consisting of reduced body-weight gain and food
consumption, increased mean post-implantation loss and slightly
reduced mean fetal body weight. External examination of fetuses
showed an increased incidence of malformations, including peromelia
(the most frequent malformation), agenesis of claws and cleft
palate. Of 90 fetuses born to dams treated at 100 mg/kg bw per day,
eight (9%) were malformed, and five of these (6%) had peromelia.
Peromelia was seen in none of a total of 346 fetuses in the other
three groups combined. At 100 mg/kg bw per day, the percentage of
fetuses with absent or incomplete phalangeal ossification was also
increased. In combination with the reduction in mean fetal body
weight, these findings indicate delayed maturation of fetuses at the
highest dose. The NOAEL was 30 mg/kg bw per day for maternal
toxicity, embryotoxicity and teratogenicity (Becker et al.,
1988b).
(f) Genotoxicity
Numerous studies have been conducted on the genotoxicity of
tebuconazole. The results are summarized in Table 2. No genotoxic
activity was found in any study.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Tebuconazole did not irritate the skin of rabbits, and
administration of 50 mg into the conjunctival sac did not induce
irritation of the eye (Heimann & Pauluhn, 1983; Sheets, 1988;
Märtins, 1990). In another study, instillation of 100 mg into the
conjunctival sac of rabbits caused slight irritation of the
conjunctiva (Eigenberg, 1988).
Tebuconazole did not reveal skin-sensitizing potential in a
maximization test in guinea-pigs (Heimann, 1983). The negative
result was confirmed in two further tests for sensitization in
guinea-pigs, but with the less sensitive Buehler method (Heimann,
1987; Sheets, 1990).
Table 2. Results of tests for the genotoxicity of tebuconazole
End-point Test system Concentration Purity Results Reference
of tebuconazole (%)
In vitro
Reverse S. typhimurium TA98, 20-12 500 µg/platea 97 Negative Herbold, 1983a,
mutation 100, 1535, 1587 1990
Reverse S. typhimurium TA98, 37.5-2400 µg/plateb 96.6 Negative Herbold, 1988a,b
mutation 100, 1535, 1537, 1538
Reverse S. typhimurium 15.6-500 µg/plate 98 Negative Ohta, 1991d
mutation E. coli (WPuvrA) 31.3-1000 µg/platec
156-5000 µg/plated
Forward Chinese hamster 80-100 µg/mle 96.6 Negative Lehn, 1988
mutation ovary cells 12.5-200 µg/mlc
(hprt locus)
Recombination B. subtilis (spores) 0.3-20 µg/disc 98 Negativec,d Ohta, 1992
repair capacity H17 (rec+), M45 (rec-)
Cytogenicity Human lympho cytes 3-30 µg/mlc 96.6 Negative Herbold, 1988c
30-300 µg/mld
DNA polymerase E. coli 625-10 000 µg/plate 97.1 Negativec,d Herbold, 1983b
repair capacity (K12)p 3478 (pol-)
W 3110 (pol+)
Unscheduled Rat hepatocytes 0.5-25.2 µg/ml 96.5 Negative Cifone, 1988
DNA synthesis
Sister chromatid Chinese hamster 4-30 µg/mlc 96.5 Negative Putman, 1987
exchange ovary cells 15-120 µg/mld
Table 2 (contd)
End-point Test system Concentration Purity Results Reference
of tebuconazole (%)
In vivo
Micronucleus Mouse bone marrow Single oral dose: 2000, 95.3 Negative Herbold, 1985
formation 500 or 200 mg/kg bwf
Dominant lethal Male mouse Single oral dose: 93.5 Negative Herbold, 1986
mutation 2000 mg/kg bw
a Toxic at doses > 500 µg/plate
b Toxic at doses > 60 µg/plate
c In the absence of metabolic activation
d In the presence of metabolic activation
e Toxic at doses > 75 µg/ml with and without activation
f Doses reduced in second and third trials owing to inhibition of erythropoiesis by the test compound
(ii) Cataract induction
Groups of four male and four female Forest of Dean cats
received whole-body exposure to tebuconazole (purity, 95.8%) in
polyethylene glycol and ethanol at mean analytical concentrations of
61 or 309 mg/m3 (99% of particles with an aerodynamic diameter of
<5 µm) for 6 h/day for four weeks, followed by an observation
period of 15 weeks. Control animals were exposed to aerosols of
either the vehicle or about 20 mg/m3 Sclex (KNJ 0953) (positive
controls). The treatment did not induce clinical symptoms and did
not affect mortality rates or body-weight gain. Cataracts due to
lens fibre degeneration were found in all animals in the positive
control group during the observation period. Exposure to
tebuconazole did not result in cataract induction, but three females
at 309 mg/m3 and one positive control animal had yellow-tinged
spots along the lens fissure. Examination of 42 untreated female
cats aged 7-12 months showed that these ocular changes were not
common spontaneous alterations; no such finding was seen in vehicle
controls. The etiology of this finding and its toxicological
relevance remain unclear. The NOAEL was 61 mg/m3, equivalent to
about 5 mg/kg bw per day, on the basis of ocular effects other than
cataracts of unknown etiology at the highest concentration (Märtins
et al., 1990).
Groups of four female beagle dogs were treated by head-nose
exposure to tebuconazole (purity, 97.1%) in polyethylene glycol and
ethanol at target aerosol concentrations of 0, 150 or 800 mg/m3
for 4 h/day for six weeks and observed for eight weeks. The
analytical concentrations were 163 and 914 mg/m3; about 90% of the
particles had an aerodynamic diameter of < 3 µm. No vehicle
control group was included in the study. The treatment did not
affect mortality rates. Most animals at 914 mg/m3 began salivating
immediately after exposure, and single animals also made tussive
noises; these effects were reversed within 2 h. Retardation of
body-weight gain was observed in both treated groups during the
second half of the study, but with no dose-effect relationship. A
lung function test revealed a marginal decrease in the mean minute
volume in both treated groups and a slight reduction in the mean
partial pressure of oxygen. The body temperature of treated animals
was reduced during exposure, probably due to central nervous system
depression caused by the ethanol vehicle. Increased spleen weight
observed at 914 mg/m3 was the only change in organ weights. No
gross pathological or histopathological changes were observed, and
ophthalmic examinations gave no indication of cataract formation.
The NOAEL was 163 mg/m3, equivalent to 23 mg/kg bw per day, on the
basis of clinical effects. The NOAEL for cataract induction was 914
mg/m3, equivalent to 125 mg/kg bw per day (Märtins, 1991).
3. Studies on metabolites
(a) Acute toxicity
The acute toxic effects of several metabolites of tebuconazole
are summarized in Table 3.
Table 3. Acute toxicity of metabolites of tebuconazole
Metabolite Species Sex Route LD50 (mg/kg bw)a Reference
Carboxy metabolite Rat F Oral > 4000 Astroff & Hagen, 1992
Hydroxy metabolite Rat F Oral > 5000 Sheets & Phillips, 1992
1H-1,2,4-triazole Rat M Oral 1375 Flucke, 1978
Rat M&F Oral 1650 Thyssen & Kimmerle, 1976
a Concentrations determined analytically
(b) Short-term toxicity
In a three-month study, groups of 15 male and 15 female Wistar
rats were fed diets containing 1 H-1,2,4-triazole (purity, 99.6%)
at concentrations of 0, 100, 500 or 2500 ppm, equal to 8, 38 or 212
mg/kg bw per day. Appearance, mortality rates, behaviour, clinical
chemistry parameters and the results of urinalysis, thyroid function
tests (protein-bound iodine) and gross pathological examination were
not affected by the treatment. At 2500 ppm, temporary convulsions
occurred; body-weight gain and food consumption were reduced,
particularly in males; and haematological parameters (haemoglobin
concentration, haematocrit, mean corpuscular volume and mean
corpuscular haemoglobin) were reduced in males. Histopathological
changes, confined to the liver, consisted of fatty accumulation in
three males at 2500 ppm (Bomhard et al., 1979).
(c) Embryotoxicity and teratogenicity
Groups of 25 pregnant Bor:WISW Wistar rats were treated orally
with 1,2,4-triazole (purity, 95.3%) on days 6-15 of gestation at
doses of 0, 10, 30 or 100 mg/kg bw per day. Treatment at 100 mg/kg
bw per day caused a reduction in body-weight gain of the dams,
reduced fetal weight and an increased number of runts. At the same
dose, the incidence of malformations, consisting mainly of eye
deformities such as anophthalmia and micro-phthalmia, was increased
over that in concurrent or historical controls (Renhof, 1988c).
In a similar study, groups of 25 pregnant Bor:WISW Wistar rats
were treated orally with 1,2,4-triazole at doses of 0, 100 or 200
mg/kg bw per day. The treatment impaired body-weight gain in both
treated groups but was statistically significant only at 200 mg/kg
bw per day. At this dose, an increased number of resorptions and a
reduction in fetal weight were observed. In both treated groups, a
dose-related increase in the incidence of runts was observed; the
incidence of malformations (mainly hydronephrosis and cleft palate)
was increased only at 200 mg/kg bw per day (Renhof, 1988d).
(d) Genotoxicity
The 1 H-1,2,4-triazole metabolite of tebuconazole (purity,
99.7%) was tested for its ability to induce reverse mutation in
Salmonella typhimurium strains TA98, TA100, TA1535 and TA1537.
Doses of 10-5000 µg/plate were ineffective, in either the presence
or the absence of metabolic activation (Poth, 1989).
4. Observations in humans
Employees working in the production of tebuconazole, who were
under continous medical supervision, did not show exposure-related
effects (Kollert, 1987).
Comments
After oral administration of tebuconazole to rats, 65-80% of
the dose was eliminated by the biliary and faecal route, whereas
elimination in urine amounted to about 16-35%. Males had a greater
biliary and faecal elimination than females. Biotransformation
proceeded by oxidation reactions, resulting in hydroxy, carboxy,
triol and ketoacid metabolites and conjugates as well as triazole.
Administration of acute oral doses to rats induced sedation,
abnormal gait and emaciation. Tebuconazole has low acute toxicity
and has been classified by WHO (1992) as unlikely to present an
acute hazard in normal use.
In a 13-week study, rats were fed tebuconazole at
concentrations of 0, 100, 400 or 1600 ppm. The NOAEL was 100 ppm,
equal to 9 mg/kg bw per day, on the basis of retardation of
body-weight gain and histopathological changes in the adrenal glands
at higher doses.
In two one-year studies in dogs fed diets containing 0, 40, 200
or 1000 ppm or 0, 100 or 150 ppm, an NOAEL of 100 ppm for the two
studies combined, equal to 3 mg/kg bw per day, was determined on the
basis of histopathological alterations in the adrenal glands at 150
ppm and above and cataract production at 200 ppm and above.
Two 21-month studies of toxicity and carcinogenicity were
conducted in mice, at dietary concentrations of 0, 20, 60 or 180 ppm
or 0, 500 or 1500 ppm. The NOAEL was 20 ppm, equal to 6 mg/kg bw per
day, on the basis of fatty changes in the liver. At 1500 ppm,
pronounced liver toxicity and an increased incidence of liver
tumours were observed. This tumorigenic potential is not considered
relevant to humans.
In a two-year study of toxicity and carcinogenicity in rats
treated at dietary concentrations of 0, 100, 300 or 1000 ppm, the
NOAEL was 100 ppm, equal to 5 mg/kg bw per day, on the basis of
reduced body-weight gain at higher doses. There was no evidence of
carcinogenicity.
In a two-generation study in rats fed dietary concentrations of
0, 100, 300 or 1000 ppm, the NOAEL was 300 ppm, equal to 22 mg/kg bw
per day, on the basis of reduced body-weight gain in the parental
generation and adverse effects on litters at higher dose.
Teratogenicity was investigated in mice, rats and rabbits. In
mice, doses of 0, 10, 20, 30 or 100 mg/kg bw per day were
administered. Increased enzyme activities in liver were observed at
all doses but were not dose-related, so that there was no clear
NOAEL for maternal toxicity. In mice fed doses of 0, 10, 30 or 100
mg/kg bw per day, a higher incidence of runts was seen at 30 mg/kg
bw per day and a higher incidence of malformations (mainly cleft
palate) at 100 mg/kg bw per day. The NOAEL for embryotoxicity and
teratogenicity was thus 10 mg/kg bw per day.
In rats treated with doses of 0, 10, 30 or 100 mg/kg bw per day
or 0, 30, 60 or 120 mg/kg bw per day, the NOAEL was 10 mg/kg bw per
day for maternal toxicity on the basis of reduced body-weight gain
at higher doses. Embryotoxicity and an increased incidence of
malformations (mainly microphthalmia) and visceral and skeletal
variations were found at 100 mg/kg bw per day and above, giving an
NOAEL for embryotoxicity and teratogenicity of 60 mg/kg bw per day.
In rabbits treated at doses of 0, 3, 10 or 30 mg/kg bw per day
or 0, 10, 30 or 100 mg/kg bw per day, the NOAELs were 10 mg/kg bw
per day for maternal toxicity and 30 mg/kg bw per day for
embryotoxicity and teratogenicity. At 100 mg/kg bw per day,
embryotoxicity and an increased incidence of external malformations
(mainly peromelia) were observed.
Tebuconazole has been studied in a range of tests for
genotoxicity in vivo and in vitro. The Meeting concluded that
there was no evidence of genotoxicity.
An ADI was established on the basis of an NOAEL of 100 ppm in a
one-year dietary study in dogs and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 20 ppm, equal to 6 mg/kg bw per day (21-month study
of toxicity and carcinogenicity)
< 10 mg/kg bw per day (maternal toxicity in a study
of teratogenicity)
10 mg/kg bw per day (embryotoxicity in a study of
teratogenicity)
Rat: 100 ppm, equal to 5 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
10 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
60 mg/kg bw per day (embryotoxicity in a study of
teratogenicity)
Rabbit: 10 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
30 mg/kg bw per day (embryotoxicity in a study of
teratogenicity)
Dog: 100 ppm, equal to 3 mg/kg bw per day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Astroff, A.B. & Hagen, L.L. (1992) Acute toxicity study with HWG
2443 (a metabolite of Tebuconazole Folicur(TM)) in female rats.
Unpublished report Ref. No. 92-012-PB, prepared by Miles Inc.,
Agricultural Division, Toxicology, Stillwell, KS, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Becker, H., Vogel, W. & Terrier, C. (1988a) Embryotoxicity study
(including teratogenicity) with HWG 1608 technical in the rat.
Unpublished report Ref. No. R 4451, prepared by Research &
Consulting Co. AG (RCC), Itingen, Switzerland. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Becker, H., Vogel, W. & Terrier, C. (1988b) Embryotoxicity study
(including teratogenicity) with HWG 1608 technical in the rabbit.
Unpublished report Ref. No. R 4323, prepared by Research &
Consulting Co. AG (RCC), Itingen, Switzerland. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Becker, H., Vogel, O., Terrier, C., Biedermann, K. & Luetkemeier, H.
(1990) Embryotoxicity study (including teratogenicity) with HWG 1608
technical in the mouse (dermal application). Unpublished report Ref.
No. R 5116, prepared by Research & Consulting Co. AG (RCC), Itingen,
Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomhard, E. (1991) HWG 1608--Toxic dose range carcinogenicity study
in NMRI mice (supplement to study T 601 89 53 with administration in
diet over a 21-month period). Unpublished report Ref. No. 16376 A,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomhard, E. & Ramm, W. (1988a) HWG 1608--Study for cancerogenicity
in NMRI mice (administration in diet for up to twenty-one months).
Unpublished report Ref. No. 16376, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomhard, E. & Ramm, W. (1988b) HWG 1608--Study for chronic toxicity
and cancerogenicity in Wistar rats (administration in diet for two
years). Unpublished report Ref. No. 16375, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E. & Schilde, B. (1986) HWG 1608--Subchronic toxicological
study with rats (feeding for thirteen weeks). Unpublished report
Ref. No. 15211, prepared by Bayer AG, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Bomhard, E., Löser, E. & Schilde, B. (1979) 1,2,4-Triazole:
Subchronic toxicological study with rats (feeding study over three
months). Unpublished report Ref. No. 8667, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E., Karbe, E. & Loeser, E. (1986) Spontaneous tumours of
2000 Wistar TNO/W. 70 rats in two-year carcinogenicity studies.
J.E.P.T.O. 7: 1/2, pp. 35-52.
Brain, K.R. (1991) In vitro skin permeability of tebuconazole.
Unpublished report Ref. No. M 7591, prepared by An-eX Analytical
Services Ltd., Cardiff, United Kingdom. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Chopade, H.M. (1992) Addendum I. [Phenyl-U-14C] HWG 1608: Study of
biokinetic behavior in the rat. Response to EPA requests and
inquiries. Unpublished report Ref. No. MR 97439-1 Metab I/2,
prepared by Miles Inc., Stilwell, KS, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Cifone, M.A. (1988) Mutagenicity test on HWG 1608 techn. in the rat
primary hepatocyte unscheduled DNA synthesis assay. Unpublished
report Ref. No. R 4111a, prepared by Hazelton Laboratories America
Inc., Kensington, MD, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Ecker, W. & Weber, H. (1991) [Chlorophenyl-U-14C] tebuconazole
absorption, distribution, excretion and metabolism in laying hens.
Unpublished report Ref. No. PF 3587, prepared by Bayer AG,
Leverkusen, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Ecker, W., Brauner, A., Klein, O. & Weber, H. (1987) Folicur:
Metabolism part of the general metabolism study in the rat.
Unpublished report Ref. No. PF 2907 and MR 97438. Metab I/3,
prepared by Bayer AG, Leverkusen, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Eiben, R. (1987) HWG 1608--Two-generation study in rats. Unpublished
report Ref. No. 16223, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Eigenberg, D.A. (1988) Primary eye irritation of Folicur (HWG 1608)
technical in albino rabbits. Unpublished report Ref. No. 1003,
prepared by Mobay Corp., Stilwell, KS, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Eigenberg, D.A. (199) Dermal absorption of 14C HWG 1608 technical
in rats. Unpublished report Ref. No. MR 97470. Metab I/5, prepared
by Mobay Corp., Stilwell, KS, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1978) Determination of LD 50--Compound: 1,2,4-Triazole.
Unpublished letter report by Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1987) HWG 1608 and KWG 0519 (c.n. Triadimenol)/HWG 1608
and KUE 13032c (c.n. Dichlofluanid) combination toxicity study.
Unpublished report Ref. No. 15747, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1983) HWG 1608--Study for skin-sensitising effect on
guinea pigs. Unpublished report Ref. No. 12024, prepared by Bayer
AG, Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K.G. (1987) HWG 1608 technical--Study of skin sensitization
effect on guinea pigs (Buehler patch test). Unpublished report Ref.
No. 16238, prepared by Bayer AG, Wuppertal, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Heimann, K.G. & Kaliner, G. (1984) HWG 1608--Study of the subacute
oral toxicity to rats. Unpublished report Ref. No. 13028, prepared
by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Heimann, K.G. & Pauluhn, J. (1983) HWG 1608 study for acute
toxicity. Unpublished report Ref. No. 12168, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K.G. & Schilde, B. (1984) HWG 1608--Subacute study of
dermal toxicity to rabbits. Unpublished report Ref. No. 12669,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Heimann, K.G. & Schilde, B. (1988) HWG 1608 technical--Subacute
dermal study of toxicity to rabbits. Unpublished report Ref. No.
12669a (Addendum to report No. 12669), prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B.A. (1983a) HWG 1608--Salmonella/microsome test to
evaluate for point mutagenic effect. Unpublished report Ref. No.
12086, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1983b) HWG 1608--Pol test on E. coli to evaluate for
harmful effects on DNA. Unpublished report Ref. No. 11902, prepared
by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B.A. (1985) HWG 1608--Micronucleus test on the mouse to
evaluate for mutagenic effect. Unpublished report Ref. No. 13159,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1986) HWG 1608--Dominant lethal test on the male
mouse to evaluate for mutagenic effect. Unpublished report Ref. No.
14985, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1988a) HWG 160--Salmonella/microsome test to evaluate
for point mutagenic effects. Unpublished report Ref. No. 16383,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1988b) HWG 1608--Salmonella/microsome test using TA
1538 to evaluate for point mutagenic effects (addendum to final
report Ref. No. 16383). Unpublished report Ref. No. 16383 A,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1988c) In vitro cytogenetic study with human
lymphocytes for the detection of induced clastogenic effects.
Unpublished report Ref. No. 16395, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1990) HWG 1608--Salmonella/microsome test for
point-mutagenic effects (appendix to Report 12086). Unpublished
report Ref. No. 12086A, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1983a) HWG 1608 acute toxicity to the dog after oral
administration. Unpublished report Ref. No. 11974, prepared by Bayer
AG, Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hoffmann, K. (1983b) HWG 1608 acute toxicity to the sheep after oral
administration. Unpublished report Ref. No. 11970, prepared by Bayer
AG, Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
von Keutz, E. & Schilde, B. (1987a) HWG 1608--Subchronic study of
toxicity to dogs with oral administration (thirteen-weeks feeding
study). Unpublished report Ref. No. 15763, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
von Keutz, E. & Schilde, B. (1987b) HWG 1608--Study of chronic
toxicity to dogs after oral administration (twelve-month feeding
study). Unpublished report Ref. No. 16211, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kollert, W. (1987) Report dated 10 November 1987, HWE 1608--Internal
experiments, Bayer Medical Department, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Lee, S.G.K. & Wood, S.E. (1987) The metabolism of Folicur in dairy
goats. Unpublished report Ref. No. MR 94882, prepared by Mobay
Corporation, Kansas City, MO, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lee, S.G.K., Hanna, L.A., Johnston, K., Wood, S.E. & Leimkuehler,
W.M. (1988) The metabolism of 14C-Folicur in chickens. Unpublished
report Ref. No. 87 156, prepared by Mobay Corp., Kansas City, MO,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lehn, H. (1988) HWG 1608--Mutagenicity study for the detection of
induced forward mutations in the CHO-HGPRT assay in vitro.
Unpublished report Ref. No. 16749, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Märtins, T. (1990) HWG 1608--Study for skin and eye
irritation/corrosion in rabbits. Unpublished report Ref. No. 12168 B
(addendum report to report No. 12168), prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Märtins, T. (1991) HWG 1608 (tebuconazole)--Subacute inhalation
toxicity to dogs--Study for cataracts. Unpublished report Ref. No.
20884, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Märtins, T., Pauluhn, J. & Krötlinger, F. (1990) HWG 1608
(tebuconazole)--Subacute inhalation toxicity to cats--Study for
cataracts. Unpublished report Ref. No. 19644, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Ohta, K. (1991a) HWG 1608 technical--Acute oral toxicity study on
rats. Study No. 91A016. Unpublished report prepared by Nihon Bayer
Agrochem K.K., Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Ohta, K. (1991b) HWG 1608 technical--Acute dermal toxicity study on
rats. Study No. 91A012. Unpublished report prepared by Nihon Bayer
Agrochem K.K. Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Ohta, K. (1991c) HWG 1608 technical--Acute oral toxicity study on
mice. Study No. 91A017. Unpublished report prepared by Nihon Bayer
Agrochem K.K., Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Ohta, K. (1991d) HWG 1608--Reverse mutation assay (Salmonella
typhimurium and Escherichia coli). Unpublished report Ref. No. RA
91036, prepared by Nikon Bayer Agrochem K.K., Tokyo, Japan.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ohta, K. (1992) HWG 1608--Rec- assay with spores in the bacterial
system. Unpublished report Ref. No. RA 92007, prepared by Nihon
Bayer Agrochem K.K., Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Pauluhn, J. (1985) HWG 1608--Study for subacute inhalation toxicity
to rat for three weeks (exposure 15 x 6 hours). Unpublished report
Ref. No. 13305, prepared by Bayer AG, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1987) HWG 1608--Study for subacute inhalation toxicity
to rat for three weeks (exposure 15 x 6 hours). Histopathological
examinations. Unpublished report Ref. No. 13305A (addendum to Ref.
No. 13305), prepared by Bayer AG, Wuppertal, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1988) HWG 1608--Study for acute inhalation toxicity to
the rat to OECD-guideline No. 403. Unpublished report Ref. No.
16345, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Porter, M.C., Jasty, V., Troup, C.M. & Hartnagel, R.E. (1989) Safety
evaluation of HWG 1608: Chronic (1 year) feeding study in dogs.
Unpublished report Ref. No. R 4781, prepared by Toxicology
Department, Miles Inc., Elkhart, IN, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Poth, A. (1989) Salmonella typhimurium reverse mutation assay with
1H-1,2,4-triazole. Unpublished report Ref. No. R 4859, prepared by
Cytotest Cell Research GMBH & Co. KG, Rossdorf, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Putman, D.L. (1987) HWG 1608--Sister chromatid exchange assay in
Chinese hamster ovary (CHO) cells. Unpublished report Ref. No. 953,
prepared by Microbiological Associates Inc., Bethesda, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1984) HWG 1608--Study for embryotoxic effects on rats
after oral administration. Unpublished report Ref. No. 12457,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1985a) HWG 1608--Study for embryotoxic effects on rats
after oral administration. Unpublished report Ref. No. 13273,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1985b) HWG 1608--Study for embryotoxic effects on
rabbits after oral administration. Unpublished report Ref. No.
13287, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Renhof, M. (1988a) HWG 1608--Study for embryotoxic effects on mice
following oral administration. Unpublished report Ref. No. 16527,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1988b) HWG 1608--Study for embryotoxic effects on rats
after dermal administration. Unpublished report Ref. No. 17089,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1988c) Triazole--Investigations into embryotoxic effects
on rats after oral administration. Unpublished report No. 17401,
Ref. No. 50 19 339, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1988d) Triazole--Investigations into embryotoxic effects
on rats after oral administration. Unpublished report No. 17402,
Supplement to Ref. No. T 50 19 339, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. & Karbe, E. (1988) HWG 1608--Supplementary study for
maternal toxicity on mice following oral administration. Unpublished
report Ref. No. 16511, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Sheets, L.P. (1988) Primary dermal irritation of technical grade
Folicur in rabbits. Unpublished report Ref. No. 1066, prepared by
Mobay Corp., Stilwell, KS, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Sheets, L.P. (1990) Dermal sensitization study with technical grade
tebuconazole (Folicur) in guinea pigs. Unpublished report Ref. No.
5052, prepared by Mobay Corp., Stilwell, KS, USA. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Sheets, L.P. & Phillips, S.D. (1992) Acute oral toxicity study with
HWG 2061 (a metabolite of tebuconazole Folicur(TM)) in female
rats. Unpublished report Ref. No. 6685, prepared by Miles Inc.,
Agricultural Division, Toxicology, Stilwell, KS, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1976) 1,2,4-Triazole--Occupational
toxicology study. Unpublished report Ref. No. 5926, prepared by
Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Weber, H. (1987) [Phenyl-U-14C] HWG 1608: Study of biokinetic
behaviour in the rat. Unpublished report Ref. No. PF 2859. Metab
II/15, prepared by Bayer AG, Leverkusen, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Weber, H. (1988) [Phenyl-U-14C] HWG 1608:
Ganzkörperautoradiographische Verteilung der Radioaktivität in der
Ratte. Unpublished report Ref. No. PF 2962. Metab II/16, prepared by
Bayer AG, Leverkusen, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
(b) Short-term toxicity
Rats
Groups of 20 male and 20 female Wistar rats (Bor: WISW) were
given tebuconazole at doses of 0, 30, 100 or 300 mg/kg bw per day by
intubation for four weeks and observed during a four-week recovery
period. The dose of 300 mg/kg bw per day caused mild lethargy and
reduced body-weight gain during the treatment period. Doses of 100
mg/kg bw per day and above decreased the haemoglobin concentration
and haematocrit values. In females at 300 mg/kg bw per day, the
leukocyte count was increased. Clinical chemistry revealed slight,
not statistically significant, increases in the activities of
glutamate-oxalate and glutamate-pyruvate transaminases in males at
300 mg/kg bw per day, and marked increases in liver enzyme
activities (glutamate-oxalate and glutamate-pyruvate transaminases
and alkaline phosphatase) in females at the same dose. Treatment at
100 and 300 mg/kg bw per day induced the microsomal enzyme system,
and the activities of N- and O-demethylases and cytochrome P450
and the triglyceride concentration in liver were increased. All
these changes were reversible. Urinalyses revealed no abnormal
findings. At 100 and 300 mg/kg bw per day, the weights of the liver
and spleen were increased in animals of each sex and the weight of
the kidney in females. Histopathological findings at 300 mg/kg bw
per day consisted of fatty changes in the liver and bile-duct
proliferation in females; enlargement of the centrilobular
hepatocytes was found in male rats. Histopathological changes were
also found in the adrenal cortex, consisting of proliferated
endothelial cells and an increased incidence of fat vacuoles.
Sclerosis of the red pulp of the spleen, associated with
sideropenia, was observed in males at 300 mg/kg bw per day;
sideropenia was also found in females at 100 mg/kg bw per day. The
NOAEL was 30 mg/kg bw per day on the basis of changes in
haematological and clinical chemical parameters and organ weights at
higher doses (Heimann & Kaliner, 1984).
Groups of 10 male and 10 female Wistar rats (Bor: WISW) were
exposed to nominal aerosol concentrations of tebuconazole at 0, 5,
50 or 500 mg/m3 in polyethylene glycol by head and nose exposure
for 6 h/day, five days per week for three weeks (15 days). The
analytical concentrations were 0, 1.2, 11 and 156 mg/m3. About 90%
of the particle mass had an aerodynamic diameter of < 5 µm. The
treatment had no effect on mortality rate, body-weight gain,
haematological or clinical chemical parameters or organ weights;
urinalysis showed no abnormal findings. Rats treated with 156
mg/m3 had piloerection after each exposure. Mixed-function
oxidases in the liver were induced. At the end of the study,
N-demethylase activity in the liver was increased in animals of
each sex at the highest dose. Males in this group also had increased
O-demethylase activity. No treatment-related gross or
histopathological alterations were observed. The NOAEL was 10.6
mg/m3, equivalent to about 2 mg/kg bw per day, on the basis of
liver enzyme induction at the highest concentration (Pauluhn, 1985,
1987).
Groups of 10 male and 10 female rats (Wistar, BOR:WISW) were
given dietary concentrations of tebuconazole (purity, 93.4%; 4.8%
symmetrical isomer) at 0, 100, 400 or 1600 ppm, equal to 9, 35 or
172 mg/kg bw per day, for 13 weeks. The treatment had no effect on
appearance or behaviour or the findings of ophthalmic or
haematological examinations or urinalyses. Food intake was increased
in animals of each sex at 1600 ppm. Retardation of body-weight gain
was observed at 400 ppm in females during the first six weeks and at
1600 ppm in animals of each sex. Two animals at 1600 ppm died; no
deaths occurred in the other dose groups. Few of the changes in
clinical chemistry showed a dose-related or time-consistent pattern
and were regarded as toxicologically insignificant. An increase in
urea concentration at 1600 ppm and a decrease in triglyceride
concentrations in animals at 400 ppm and above were seen only after
the first four weeks and not at the end of the study. Pronounced
increases in N-demethylase activity and cytochrome P450 content
were found in male animals at 1600 ppm, and increased liver weights
were seen in females at this dose. Histopathological examination
revealed an increased incidence of intra-plasmatic vacuoles in the
cells of the zona fasciculata of the adrenals (probably lipid
accumulation) in some females at 400 ppm and in all females at 1600
ppm. This effect was less pronounced in males because of a higher
background incidence of adrenal vacuole formation in control animals
(Bomhard & Schilde, 1986). The NOAEL was 100 ppm, equal to 9 mg/kg
bw per day, on the basis of retardation of body-weight gain and
histopathological changes in the adrenals at higher doses
Rabbits
Groups of 18 male and 18 female HC New Zealand white rabbits
received tebuconazole at doses of 0, 50 or 250 mg/kg bw per day on
the shorn skin for 6 h daily for three weeks. The treatment was
tolerated with no effects (Heimann & Schilde, 1984). In an
additional study, a dose of 1000 mg/kg bw per day was also tolerated
with no systemic or local effects (Heimann & Schilde, 1988).
Dogs
Groups of four male and four female beagle dogs were given
tebuconazole (purity, 93.4%; 4.8% symmetric isomer) at dietary
concentrations of 0, 200, 1000 or 5000 ppm (equal to 9, 45 or 220
mg/kg bw per day for males and females combined) over 13 weeks. One
dog at 5000 ppm was found dead after the first dose, with no
previous clinical signs. Treatment did not affect body temperature,
pulse rate or neurological signs. Food consumption was reduced at
1000 and 5000 ppm, and body-weight gain was retarded in these
groups; a few dogs at 1000 ppm and most of those at 5000 ppm had a
deteriorated nutritional status. Ophthalmic examination revealed
lens opacities in all animals at 5000 ppm, which first appeared
after seven weeks of treatment. An increase in thrombocyte count was
found at 5000 ppm, and at the end of the study six of eight animals
at this dose had marked anisocytosis. Alkaline phosphatase activity
in plasma showed a retarded age-induced fall at 1000 ppm and an
increase at 5000 ppm. N-Demethylase activity in the liver had
increased slightly by the end of the study in animals at 1000 ppm
and had increased markedly in those at 5000 ppm. The cytochrome P450
content of the liver was also elevated at 5000 ppm. In the group at
the highest dose, a shift in the composition of serum proteins was
observed, with a slightly lower albumin content and a simultaneous
increase in the ß-globulin content. Urinalyses revealed no
treatment-related effects. Changes in organ weights did not follow a
consistent pattern, except that an increase in spleen weights was
seen in animals of each sex at 5000 ppm. Histopathological
examination confirmed lens degeneration, indicating the induction of
cataracts in animals at 5000 ppm. Other histopathological
alterations observed in this group included slightly increased
accumulation of ferriferrous pigments in Kupffer cells of the liver
and of siderocytes in spleen. The NOAEL was 200 ppm, equal to 9
mg/kg bw per day, on the basis of reduced body-weight gain and food
consumption and liver enzyme induction at 1000 ppm and higher. The
NOAEL for cataract induction was 1000 ppm, equal to 45 mg/kg bw per
day (von Keutz & Schilde, 1987a).
In a one-year study, groups of four male and four female beagle
dogs were given tebuconazole (purity, 96.9%) at dietary
concentrations of 0, 40, 200 or 1000 ppm (weeks 1-39) and 2000 ppm
(weeks 40-52), equal to 2, 10 or 60 mg/kg bw per day in males and
females combined. Concentrations of up to 2000 ppm did not affect
survival rate, appearance, behaviour or organ weights, and
haematological examination and urinalyses showed no changes. Body
temperature, pulse rates and reflexes were normal, as were food and
water consumption and body-weight gain. Ophthalmic examination
revealed lens opacities in two dogs at 200 ppm and one at 1000 ppm;
the opacities appeared between weeks 26 and 32 and were of the same
intensity at all subsequent examinations. In the single dog at 1000
ppm which showed this ocular change, corneal opacity was also found,
which persisted until the end of the study, whereas the lens
opacities disappeared after week 32 of treatment. The lack of a
dose-response relationship with regard to lens opacities does not
preclude an association with treatment but may be due to the small
number of animals in the group and differences in individual
sensitivity. None of the other animals in this group had lens stars;
in single animals, faint lens stars were already present before
treatment started but did not become more pronounced with the
treatment. Incipient lens stars observed in animals at 40 and 200
ppm also remained stable, and most disappeared before the end of the
treatment period. Lens stars are physiological structures found
occasionally in juvenile dogs. Clinical chemical analyses revealed
slight, dose-related changes in the activity of alkaline
phosphatase: Whereas the age-dependent reduction in activity was
similar in control animals and in those at 40 and 200 ppm, the mean
activity in animals at 1000/2000 ppm indicated slight induction,
resulting in a retardation in the physiological fall in alkaline
phosphatase activity. The activity of N-demethylase and the
triglyceride content of the liver were slightly increased in animals
at 1000/2000 ppm. Most of the gross pathological findings, such as a
dose-related increase in the incidence of livers with marked
lobulation in animals treated with 200 ppm and above, were not
correlated with histopathological alterations. Histopathological
findings included intracytoplasmic vacuoles in cells of the zona
fasciculata of the adrenals in animals at 200 and 1000/2000 ppm and
slight siderosis in the spleen in most animals at 1000/2000 ppm. The
NOAEL was 40 ppm, equal to 2 mg/kg bw per day, on the basis of
cataract formation and histopathological changes in the adrenals at
higher doses (von Keutz & Schilde, 1987b).
In a second one-year study conducted at lower doses, groups of
12 male and 12 female beagle dogs were given tebuconazole (purity,
96%) at dietary concentrations of 0, 100 or 150 ppm, equal to 3 or
4.5 mg/kg bw per day for males and females combined. The treatment
did not affect mortality, body-weight gain, food consumption,
biochemical, haematological or urinary parameters, ophthalmoscopic
findings, gross pathological appearance or organ weights. The only
histopathological alteration was slight hypertrophy of adrenal zona
fasciculata cells in all animals at 150 ppm; only one control animal
had similar changes. The enlargement was accompanied by an increased
incidence of large fatty vacuoles. The NOAEL was 100 ppm, equal to 3
mg/kg bw per day, on the basis of histopathological alterations in
the adrenals at the higher dose. The NOAEL for cataract induction
was 150 ppm, equal to 4.5 mg/kg bw per day (Porter et al., 1989).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female NMRI mice were given
tebuconazole (purity, 95%) at dietary concentrations of 0, 20, 60 or
180 ppm, equal to 6, 18 or 53 mg/kg bw per day, for 21 months.
Groups of 10 treated animals of each sex were sacrificed after one
year of treatment. Appearance, behaviour, mortality, food
consumption and body weight were not affected by the treatment. In
animals treated at 180 ppm, reduced erythrocyte count and
haemoglobin and haematocrit values were observed, particularly at
the end of the first year of the study. Sporadic alterations in
clinical chemical parameters were observed in all groups. The few
dose-dependent changes consisted of an increase in total bilirubin
concentration in females treated with concentrations of 60 ppm and
higher and decreased cholesterol concentrations in plasma at 180
ppm. Gross pathological examination showed no treatment-related
effects. Liver weights were increased in animals at 180 ppm at
interim sacrifice and at the end of the study. Histopathological
examination revealed a slight increase in the prevalence of
lipid-containing periportal vacuoles in the livers of animals
treated with 60 or 180 ppm at both interim and terminal sacrifice.
No increase in tumour incidence was found. The NOAEL was 20 ppm,
equal to 6 mg/kg bw per day, on the basis of histopathological
changes in the liver (Bomhard & Ramm, 1988a).
In a similar study using higher doses, groups of 50 male and 50
female NMRI mice were maintained on a diet containing tebuconazole
(purity, 95%) at concentrations of 0, 500 or 1500 ppm, equal to 85
or 280 mg/kg bw per day, for 21 months. Groups of 10 animals per sex
and dose were scheduled for interim sacrifice after 12 months. The
mortality rate was not affected by treatment. The incidence of
animals with a 'distended abdomen' (probably due to liver
enlargement) was increased among those at 1500 ppm. A dose-dependent
reduction in body-weight gain was observed in males at 500 and 1500
ppm and in females at 1500 ppm, particularly during the first half
of the study. Food intake tended to be higher in treated animals
than in controls. Alterations in haematological parameters were
found in both treated groups: At 500 ppm and above, reduced mean
cell volume and haematocrit values and elevated mean cell
haemoglobin concentrations were found in males, and thrombocyte
counts were elevated in females. Thromboplastin time was reduced in
both treated groups. At 1500 ppm, erythrocyte counts, haemoglobin
content and haematocrit values were markedly reduced and thrombocyte
counts were elevated, particularly in males. Leukocyte counts were
also increased.
Parameters of clinical chemistry that showed dose-related
changes in both treated groups included pronounced increases in the
activities of glutamate-oxalate and glutamate-pyruvate transaminases
and alkaline phosphatase, particularly at 1500 ppm. Cholesterol,
bilirubin and albumin levels were reduced at 500 and 1500 ppm, but
there was no clear dose-response relationship. Liver weights were
increased in a dose-related fashion at both doses, and adrenal
weights were elevated in females at 1500 ppm. The gross pathological
evidence for treatment-related effects on the liver consisted of
enlargement and pale appearance of the liver in both treated groups
and some enhanced lobulation. Capsular thickening and swelling and
an increased incidence of nodular masses were also found at 1500
ppm. Histopathological examination revealed treatment-related
alterations, mainly in the liver. The non-neoplastic findings
consisted of single-cell and focal necroses, focal inflammation,
hepatocytic degeneration, hepatocytic hyerplasia and hypertrophy,
fatty vacuolation, bile-duct hyperplasia and steatoses, oval-cell
proliferation, extramedullary haematopoiesis and pigment
accumulation in Kupffer cells. Many of these alterations were found
at both 500 and 1500 ppm.
No evidence was found for a compound-related effect on the
incidence of benign or malignant tumours after one year of
treatment. Increased incidences of tumour-bearing animals were seen,
however, at 1500 ppm after 21 months of treatment. Increases were
seen in the incidences of both benign and malignant tumours in males
and of malignant tumours in females. Statistically significant
increases in tumour incidence were found only in the liver. An
increased incidence of adenomas was observed at 1500 ppm, with 36%
(versus 6% in controls) in males and 4% (versus 0 in controls) in
females; the incidences of carcinomas at this dose were 21% (versus
0 in controls) in males and 26% (versus 2% in controls) in females.
The incidences of hepatocellular tumours in animals at 1500 ppm were
reported to be above those in historical control mice of this
strain. The only reported incidences were 0-12% for adenoma and
0-10% for carcinoma in males. The NOAEL was < 500 ppm, equal to <
85 mg/kg bw per day, on the basis of reduced body-weight gain and
histopathological changes in the liver. The NOAEL for
carcinogenicity was 500 ppm, equal to 85 mg/kg bw per day, on the
basis of an increased incidence of liver tumours at 1500 ppm
(Bomhard, 1991).
Rats
Groups of 50 male and 50 female Wistar rats (Bor:WISW) were
given tebuconazole (purity, 95%) in the diet at concentrations of 0,
100, 300 or 1000 ppm, equal to 5, 16 or 55 mg/kg bw per day, for two
years. Groups of 10 animals per sex and dose were similarly treated
and killed at interim sacrifice after 12 months of treatment.
Treatment did not affect mortality, appearance, general behaviour of
the animals or haematological parameters, and urinalyses showed no
changes; no treatment-related damage to the eyes was found.
Body-weight gains were lower than those of the control animals at
doses of 300 ppm and above. Food consumption was increased among
females at 1000 ppm, and water consumption was decreased in females
at 300 ppm and above. Clinical examinations revealed several
statistically significant changes but no consistent dose-related
pattern indicative of an association with treatment, with the
probable exception of a marginal increase in the activity of alanine
aminotransferase at 1000 ppm. Decreased triglyceride concentration
was found in female animals at 1000 ppm, and an increase in spleen
weight was found in these animals. Gross pathological examination
did not provide any indication of treatment-related effects.
Histopathological examination revealed an increased incidence of
pigmented Kupffer cells in liver and increased haemosiderin
accumulation in the spleen of females at 1000 ppm.
The incidence of C-cell adenomas and carcinomas of the thyroid
was increased in all treated males in comparison with that in
controls (0/50), with 2/49 (4%) at 100 ppm, 3/50 (6%) at 300 ppm and
3/50 (6%) at 1000 ppm There was no clear dose-response relationship,
and the rates were within the range of spontaneously occurring
thyroid C-cell tumours in male Wistar rats. The mean historical
incidences of interstitial-cell and C-cell adenomas grouped with
parafollicular tumours were reported to be 7.4% (0-19.3) in males
and 8.4% (2.5-21.2) in females (Bomhard et al., 1986). The
incidence of 0 in concurrent controls is unusually low. In female
rats, a higher frequency of endometrial adenocarcinoma was found in
comparison with controls (0/50), with incidences of 3/50 at 100 ppm,
2/50 at 300 ppm and 1/50 at 1000 ppm These incidences were also
small and not dose-related. The reported historical incidence of
uterine adenocarcinomas varied considerably, from 0 to 14.4%, with a
mean incidence of 6.3% (Bomhard et al., 1986). The fact that the
endometrial tumours found in this study differ morphologically from
the typical uterine carcinomas that occur spontaneously in this rat
strain precludes a direct comparison, either qualitatively or
quantitatively. The absence of a dose-response relationship may
indicate, however, that these findings were not treatment-related.
The NOAEL was 100 ppm, equal to 5 mg/kg bw per day, on the basis of
reduced body-weight gain at higher doses (Bomhard & Ramm, 1988b).
(d) Reproductive toxicity
Rats
Groups of 25 male and 25 female Wistar rats (Bor:WISW) were
treated with tebuconazole (purity, 95%) at dietary concentrations of
0, 100, 300 or 1000 ppm, equal to 7, 22 or 72 mg/kg bw per day, over
two generations. Treatment did not affect appearance, behaviour,
general condition or mortality rates, and fertility and gestation
indexes were not altered. At 1000 ppm, the body-weight gain of the
parent animals was reduced; food consumption was reduced only among
males at the highest dose in the F0 generation and in animals of
each sex in the F1 generation. Reductions in the mean litter size
at birth, in the viability index (survival until day 5 after birth)
and in the lactation index were seen at 1000 ppm, and mean birth
weights (only in the F1a generation) and body-weight gain (in both
generations) were reduced. None of the pups had grossly apparent
malformations, and no treatment-related organotoxic effects were
noted on gross and microscopic examination. The NOAEL was 300 ppm,
equal to 22 mg/kg bw per day, on the basis of reduced body-weight
gain in parents and pups and reduced litter size at the highest dose
(Eiben, 1987).
(e) Embryotoxicity and teratogenicity
Mice
Groups of 25 female NMRI/ORIG Kisslegg mice were treated by
gavage with tebuconazole (purity, 93.6%) at a dose of 0, 10, 30 or
100 mg/kg bw per day on days 6-15 of gestation. The treatment did
not affect mortality rates, body-weight gain or pregnancy rates of
the maternal animals. At 30 mg/kg bw per day and higher, a
dose-related increase in the incidence of runts was observed, with
20/234 (8.6%) at 30 mg/kg bw per day and 26/202 (13%) at 100 mg/kg
bw per day; there were 4/234 (2%) at 10 mg/kg bw per day and 5/236
(2%) in the control group. An increased incidence of malformations
was found at 100 mg/kg bw per day, 13/202 (6.5%), which consisted
most frequently of cleft palates and individual cases of
micrognathia, rib fusion and spinal dysplasia; the incidence of
malformations in the control group was 1/236 (0.4%). The incidence
of cleft palate at this dose, 6/202 (3%), was also markedly higher
than the mean incidence in historical controls, 0.7%. The NOAEL for
maternal toxicity and clinical signs was 100 mg/kg bw per day and
that for embryotoxicity and teratogenicity was 10 mg/kg bw per day
(Renhof, 1988a).
In a study to investigate maternal toxicity further, groups of
10 female NMRI/ORIG Kisslegg mice were treated by gavage with
tebuconazole (purity, 97.4%) at doses of 0, 10, 20, 30 or 100 mg/kg
bw per day on days 6-15 of gestation. Haematological and clinical
chemical tests showed no effect of treatment on mortality rates or
body weights of the maternal animals; slight reductions in the
haematocrit and mean corpuscular volume were seen at 30 and 100
mg/kg bw per day, but with no clear dose-response relationship. The
activities of alanine and aspartate transaminases showed slight,
usually statistically significant increases in all treated groups
but with no consistent relation to dose. Triglyceride levels were
increased at 100 mg/kg bw per day. Liver weights were increased at
all doses, again with no clear dose-response relationship.
Histopathological investigation revealed cytoplasmic vacuolation of
the liver at 100 mg/kg bw per day, which was correlated with an
increase in lipid content. The dose of 100 mg/kg bw per day thus
produced pronounced liver toxicity; the changes in liver enzyme
activities, unrelated to dose, in all treated groups indicate that
maternal toxicity may have been induced at 10 mg/kg bw per day. The
embrotoxic and teratogenic effects thus occur at maternally toxic
doses. The NOAEL for maternal toxicity was < 10 mg/kg bw per day
(Renhof & Karbe, 1988).
Groups of 25 female NMRI/HAN mice were given tebuconazole
(purity, 96-98%; different batches used for main and supplementary
study) at daily dermal doses of 0, 100, 300 or 1000 mg/kg bw per day
for 6 h/day on days 6-15 of gestation. Treatment had no effect on
mortality rates, body weight, food consumption or reproductive
parameters (numbers of corpora lutea, implantations, resorptions and
live fetuses). Examination of the litters revealed no
treatment-related effect on mean body weight, and no abnormal
findings were detected in the viscera. The frequency of external
abnormalities (cleft palate, malposition of hind legs, exencephaly
and 'tail cranial bended') at 1000 mg/kg bw per day was not
different from that in the control group, with 3% in the controls,
3% at 100 mg/kg bw per day, 1.8% at 300 mg/kg bw per day and 5.3% at
1000 mg/kg bw per day. The increase at the highest dose was due to a
higher incidence of cleft palate, with 11/285 (3.9%) at 1000 mg/kg
bw per day and 6/301 (2%) in the control group. The incidence of
cleft palate in control animals in an independent study was 1.6%.
Skeletal examination showed an increased number of supernumerary
ribs at 1000 mg/kg bw per day. The NOAEL was 1000 mg/kg bw per day
for maternal toxicity and clinical signs and 300 mg/kg bw per day
for embryotoxicity and teratogenicity (Becker et al., 1990).
Since treatment-related effects on the fetuses appeared to have
occurred in the absence of maternal toxicity, a study was initiated
that included biochemical and histopathological examinations.
Tebuconazole was administered in the same way as described above to
groups of 10 mated females. As in the first study, no deaths
occurred and no clinical signs were observed in animals treated at
1000 mg/kg bw per day; the treatment had no effect on body-weight
gain, food consumption or reproductive parameters. Clinical
biochemical examinations revealed increased alanine transaminase
activity at 1000 mg/kg bw per day; there was also increased
cytochrome P450 content and N-demethylase activity in liver tissue
at > 300 mg/kg bw per day and a marginal increase in
O-demethylase activity. None of these parameters showed a
dose-response relationship. There was a dose-related reduction in
relative adrenal weights in all dose groups, which was statistically
significant at 1000 mg/kg bw per day; absolute adrenal weights were
also reduced in all treatment groups, but not in relation to dose.
No effect was seen on liver weights, but histological examination
revealed fatty changes in periportal areas in most mice at 300 mg/kg
bw per day and in all mice at 1000 mg/kg bw per day. The NOAEL was
100 mg/kg bw per day on the basis of fatty changes in the liver at
higher doses (Becker et al., 1990).
Rats
In a range-finding study, groups of 25 female Wistar rats were
treated by gavage with tebuconazole (purity, 99.5%) at doses of 0 or
100 mg/kg bw per day on days 6-15 of gestation. The treatment had no
effect on mortality rates, but body-weight gain was retarded. The
fertilization rate was not reduced by treatment, but most litter
parameters (such as number of implantations, litter size and losses)
indicated an adverse effect. More runts and fetuses with
malformations (micrognathia, hydronephrosis and hydroureter) were
found in treated animals. The NOAEL for maternal toxicity,
embryotoxicity and teratogenicity was < 100 mg/kg bw per day
(Renhof, 1984).
In a similar study, groups of 25 female Wistar rats were
treated by gavage with tebuconazole (purity, 93%) at doses of 0, 10,
30 or 100 mg/kg bw per day on days 6-15 of gestation. No
treatment-related deaths occurred. At 30 and 100 mg/kg bw per day,
dose-related reductions in weight gain were observed throughout the
treatment period; at 100 mg/kg bw per day, retarded body-weight gain
was observed throughout gestation. Effects on litter parameters
included an increased number of losses and a decrease in mean fetal
weight at 100 mg/kg bw per day. At this dose there were also more
runts and fetuses with malformations (mostly microphthalmia): the
runt incidence was 36%, compared with 7% in controls, and the
incidence of fetuses with malformations was 7%, compared with 2% in
controls. The NOAEL for maternal toxicity was 10 mg/kg bw per day
and that for embrotoxicity and teratogenicity was 30 mg/kg bw per
day (Renhof, 1985a).
Groups of 25 femaleWistar rats were treated by gavage with
tebuconazole (purity, 98.3%) at doses of 0, 30, 60 or 120 mg/kg bw
per day on days 6-15 of gestation. The dose of 30 mg/kg bw per day
affected neither maternal nor fetal parameters. At 60 mg/kg bw per
day and above, food consumption was reduced and a dose-related loss
of body weight was seen at the beginning of the treatment period.
Liver weights showed a dose-related increase at 60 and 120 mg/kg bw
per day. Reproductive parameters (numbers of corpora lutea and
implantations) were not adversely affected. At 120 mg/kg bw per day,
resorptions occurred more frequently, and consequently there were
fewer live fetuses than in the control group; mean fetal body
weights were lower. This dose induced increased incidences of
visceral alterations (consisting of excess fluid in the thoracic
cavity), supernumerary ribs, non-ossified cervical vertebrae and
incompletely ossified sternebrae, indicating retardation of fetal
development. The NOAEL was 30 mg/kg bw per day for maternal toxicity
and 60 mg/kg bw per day for embryotoxicity (Becker et al., 1988a).
Groups of 25 female Wistar rats were treated dermally with
tebuconazole (purity, 97.4%) at doses of 0, 100, 300 or 1000 mg/kg
bw per day for 6 h/day on days 6-15 of gestation. The treatment was
tolerated with no observed effects on dams or fetuses with respect
to the mortality rate, body-weight gain, gestation rate or other
reproductive parameters, such as numbers of corpora lutea,
resorptions and live fetuses. There was no indication of a
treatment-related effect on the incidence or spectrum of
malformations (Renhof, 1988b).
Rabbits
Groups of 15 female Himalayan CHBB:HM rabbits were treated by
gavage with tebuconazole (purity, 93%) at doses of 0, 3, 10 or 30
mg/kg bw per day on days 6-18 of gestation. Dams given the highest
dose had reduced body-weight gain during the treatment period.
Reproductive and fetal parameters were not adversely affected, and
no treatment-related increases in the incidence of malformations
were observed. The NOAEL was 10 mg/kg bw per day for maternal
toxicity and 30 mg/kg bw per day for embryotoxicity and
teratogenicity (Renhof, 1985b).
Groups of 16 female CHIN hybrid rabbits were treated by gavage
with tebuconazole at doses of 0, 10, 30 or 100 mg/kg bw per day on
days 6-18 of gestation. Treatment-related effects were seen at 100
mg/kg bw per day, consisting of reduced body-weight gain and food
consumption, increased mean post-implantation loss and slightly
reduced mean fetal body weight. External examination of fetuses
showed an increased incidence of malformations, including peromelia
(the most frequent malformation), agenesis of claws and cleft
palate. Of 90 fetuses born to dams treated at 100 mg/kg bw per day,
eight (9%) were malformed, and five of these (6%) had peromelia.
Peromelia was seen in none of a total of 346 fetuses in the other
three groups combined. At 100 mg/kg bw per day, the percentage of
fetuses with absent or incomplete phalangeal ossification was also
increased. In combination with the reduction in mean fetal body
weight, these findings indicate delayed maturation of fetuses at the
highest dose. The NOAEL was 30 mg/kg bw per day for maternal
toxicity, embryotoxicity and teratogenicity (Becker et al.,
1988b).
(f) Genotoxicity
Numerous studies have been conducted on the genotoxicity of
tebuconazole. The results are summarized in Table 2. No genotoxic
activity was found in any study.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Tebuconazole did not irritate the skin of rabbits, and
administration of 50 mg into the conjunctival sac did not induce
irritation of the eye (Heimann & Pauluhn, 1983; Sheets, 1988;
Märtins, 1990). In another study, instillation of 100 mg into the
conjunctival sac of rabbits caused slight irritation of the
conjunctiva (Eigenberg, 1988).
Tebuconazole did not reveal skin-sensitizing potential in a
maximization test in guinea-pigs (Heimann, 1983). The negative
result was confirmed in two further tests for sensitization in
guinea-pigs, but with the less sensitive Buehler method (Heimann,
1987; Sheets, 1990).
Table 2. Results of tests for the genotoxicity of tebuconazole
End-point Test system Concentration Purity Results Reference
of tebuconazole (%)
In vitro
Reverse S. typhimurium TA98, 20-12 500 µg/platea 97 Negative Herbold, 1983a,
mutation 100, 1535, 1587 1990
Reverse S. typhimurium TA98, 37.5-2400 µg/plateb 96.6 Negative Herbold, 1988a,b
mutation 100, 1535, 1537, 1538
Reverse S. typhimurium 15.6-500 µg/plate 98 Negative Ohta, 1991d
mutation E. coli (WPuvrA) 31.3-1000 µg/platec
156-5000 µg/plated
Forward Chinese hamster 80-100 µg/mle 96.6 Negative Lehn, 1988
mutation ovary cells 12.5-200 µg/mlc
(hprt locus)
Recombination B. subtilis (spores) 0.3-20 µg/disc 98 Negativec,d Ohta, 1992
repair capacity H17 (rec+), M45 (rec-)
Cytogenicity Human lympho cytes 3-30 µg/mlc 96.6 Negative Herbold, 1988c
30-300 µg/mld
DNA polymerase E. coli 625-10 000 µg/plate 97.1 Negativec,d Herbold, 1983b
repair capacity (K12)p 3478 (pol-)
W 3110 (pol+)
Unscheduled Rat hepatocytes 0.5-25.2 µg/ml 96.5 Negative Cifone, 1988
DNA synthesis
Sister chromatid Chinese hamster 4-30 µg/mlc 96.5 Negative Putman, 1987
exchange ovary cells 15-120 µg/mld
Table 2 (contd)
End-point Test system Concentration Purity Results Reference
of tebuconazole (%)
In vivo
Micronucleus Mouse bone marrow Single oral dose: 2000, 95.3 Negative Herbold, 1985
formation 500 or 200 mg/kg bwf
Dominant lethal Male mouse Single oral dose: 93.5 Negative Herbold, 1986
mutation 2000 mg/kg bw
a Toxic at doses > 500 µg/plate
b Toxic at doses > 60 µg/plate
c In the absence of metabolic activation
d In the presence of metabolic activation
e Toxic at doses > 75 µg/ml with and without activation
f Doses reduced in second and third trials owing to inhibition of erythropoiesis by the test compound
(ii) Cataract induction
Groups of four male and four female Forest of Dean cats
received whole-body exposure to tebuconazole (purity, 95.8%) in
polyethylene glycol and ethanol at mean analytical concentrations of
61 or 309 mg/m3 (99% of particles with an aerodynamic diameter of
<5 µm) for 6 h/day for four weeks, followed by an observation
period of 15 weeks. Control animals were exposed to aerosols of
either the vehicle or about 20 mg/m3 Sclex (KNJ 0953) (positive
controls). The treatment did not induce clinical symptoms and did
not affect mortality rates or body-weight gain. Cataracts due to
lens fibre degeneration were found in all animals in the positive
control group during the observation period. Exposure to
tebuconazole did not result in cataract induction, but three females
at 309 mg/m3 and one positive control animal had yellow-tinged
spots along the lens fissure. Examination of 42 untreated female
cats aged 7-12 months showed that these ocular changes were not
common spontaneous alterations; no such finding was seen in vehicle
controls. The etiology of this finding and its toxicological
relevance remain unclear. The NOAEL was 61 mg/m3, equivalent to
about 5 mg/kg bw per day, on the basis of ocular effects other than
cataracts of unknown etiology at the highest concentration (Märtins
et al., 1990).
Groups of four female beagle dogs were treated by head-nose
exposure to tebuconazole (purity, 97.1%) in polyethylene glycol and
ethanol at target aerosol concentrations of 0, 150 or 800 mg/m3
for 4 h/day for six weeks and observed for eight weeks. The
analytical concentrations were 163 and 914 mg/m3; about 90% of the
particles had an aerodynamic diameter of < 3 µm. No vehicle
control group was included in the study. The treatment did not
affect mortality rates. Most animals at 914 mg/m3 began salivating
immediately after exposure, and single animals also made tussive
noises; these effects were reversed within 2 h. Retardation of
body-weight gain was observed in both treated groups during the
second half of the study, but with no dose-effect relationship. A
lung function test revealed a marginal decrease in the mean minute
volume in both treated groups and a slight reduction in the mean
partial pressure of oxygen. The body temperature of treated animals
was reduced during exposure, probably due to central nervous system
depression caused by the ethanol vehicle. Increased spleen weight
observed at 914 mg/m3 was the only change in organ weights. No
gross pathological or histopathological changes were observed, and
ophthalmic examinations gave no indication of cataract formation.
The NOAEL was 163 mg/m3, equivalent to 23 mg/kg bw per day, on the
basis of clinical effects. The NOAEL for cataract induction was 914
mg/m3, equivalent to 125 mg/kg bw per day (Märtins, 1991).
3. Studies on metabolites
(a) Acute toxicity
The acute toxic effects of several metabolites of tebuconazole
are summarized in Table 3.
Table 3. Acute toxicity of metabolites of tebuconazole
Metabolite Species Sex Route LD50 (mg/kg bw)a Reference
Carboxy metabolite Rat F Oral > 4000 Astroff & Hagen, 1992
Hydroxy metabolite Rat F Oral > 5000 Sheets & Phillips, 1992
1H-1,2,4-triazole Rat M Oral 1375 Flucke, 1978
Rat M&F Oral 1650 Thyssen & Kimmerle, 1976
a Concentrations determined analytically
(b) Short-term toxicity
In a three-month study, groups of 15 male and 15 female Wistar
rats were fed diets containing 1 H-1,2,4-triazole (purity, 99.6%)
at concentrations of 0, 100, 500 or 2500 ppm, equal to 8, 38 or 212
mg/kg bw per day. Appearance, mortality rates, behaviour, clinical
chemistry parameters and the results of urinalysis, thyroid function
tests (protein-bound iodine) and gross pathological examination were
not affected by the treatment. At 2500 ppm, temporary convulsions
occurred; body-weight gain and food consumption were reduced,
particularly in males; and haematological parameters (haemoglobin
concentration, haematocrit, mean corpuscular volume and mean
corpuscular haemoglobin) were reduced in males. Histopathological
changes, confined to the liver, consisted of fatty accumulation in
three males at 2500 ppm (Bomhard et al., 1979).
(c) Embryotoxicity and teratogenicity
Groups of 25 pregnant Bor:WISW Wistar rats were treated orally
with 1,2,4-triazole (purity, 95.3%) on days 6-15 of gestation at
doses of 0, 10, 30 or 100 mg/kg bw per day. Treatment at 100 mg/kg
bw per day caused a reduction in body-weight gain of the dams,
reduced fetal weight and an increased number of runts. At the same
dose, the incidence of malformations, consisting mainly of eye
deformities such as anophthalmia and micro-phthalmia, was increased
over that in concurrent or historical controls (Renhof, 1988c).
In a similar study, groups of 25 pregnant Bor:WISW Wistar rats
were treated orally with 1,2,4-triazole at doses of 0, 100 or 200
mg/kg bw per day. The treatment impaired body-weight gain in both
treated groups but was statistically significant only at 200 mg/kg
bw per day. At this dose, an increased number of resorptions and a
reduction in fetal weight were observed. In both treated groups, a
dose-related increase in the incidence of runts was observed; the
incidence of malformations (mainly hydronephrosis and cleft palate)
was increased only at 200 mg/kg bw per day (Renhof, 1988d).
(d) Genotoxicity
The 1 H-1,2,4-triazole metabolite of tebuconazole (purity,
99.7%) was tested for its ability to induce reverse mutation in
Salmonella typhimurium strains TA98, TA100, TA1535 and TA1537.
Doses of 10-5000 µg/plate were ineffective, in either the presence
or the absence of metabolic activation (Poth, 1989).
4. Observations in humans
Employees working in the production of tebuconazole, who were
under continous medical supervision, did not show exposure-related
effects (Kollert, 1987).
Comments
After oral administration of tebuconazole to rats, 65-80% of
the dose was eliminated by the biliary and faecal route, whereas
elimination in urine amounted to about 16-35%. Males had a greater
biliary and faecal elimination than females. Biotransformation
proceeded by oxidation reactions, resulting in hydroxy, carboxy,
triol and ketoacid metabolites and conjugates as well as triazole.
Administration of acute oral doses to rats induced sedation,
abnormal gait and emaciation. Tebuconazole has low acute toxicity
and has been classified by WHO (1992) as unlikely to present an
acute hazard in normal use.
In a 13-week study, rats were fed tebuconazole at
concentrations of 0, 100, 400 or 1600 ppm. The NOAEL was 100 ppm,
equal to 9 mg/kg bw per day, on the basis of retardation of
body-weight gain and histopathological changes in the adrenal glands
at higher doses.
In two one-year studies in dogs fed diets containing 0, 40, 200
or 1000 ppm or 0, 100 or 150 ppm, an NOAEL of 100 ppm for the two
studies combined, equal to 3 mg/kg bw per day, was determined on the
basis of histopathological alterations in the adrenal glands at 150
ppm and above and cataract production at 200 ppm and above.
Two 21-month studies of toxicity and carcinogenicity were
conducted in mice, at dietary concentrations of 0, 20, 60 or 180 ppm
or 0, 500 or 1500 ppm. The NOAEL was 20 ppm, equal to 6 mg/kg bw per
day, on the basis of fatty changes in the liver. At 1500 ppm,
pronounced liver toxicity and an increased incidence of liver
tumours were observed. This tumorigenic potential is not considered
relevant to humans.
In a two-year study of toxicity and carcinogenicity in rats
treated at dietary concentrations of 0, 100, 300 or 1000 ppm, the
NOAEL was 100 ppm, equal to 5 mg/kg bw per day, on the basis of
reduced body-weight gain at higher doses. There was no evidence of
carcinogenicity.
In a two-generation study in rats fed dietary concentrations of
0, 100, 300 or 1000 ppm, the NOAEL was 300 ppm, equal to 22 mg/kg bw
per day, on the basis of reduced body-weight gain in the parental
generation and adverse effects on litters at higher dose.
Teratogenicity was investigated in mice, rats and rabbits. In
mice, doses of 0, 10, 20, 30 or 100 mg/kg bw per day were
administered. Increased enzyme activities in liver were observed at
all doses but were not dose-related, so that there was no clear
NOAEL for maternal toxicity. In mice fed doses of 0, 10, 30 or 100
mg/kg bw per day, a higher incidence of runts was seen at 30 mg/kg
bw per day and a higher incidence of malformations (mainly cleft
palate) at 100 mg/kg bw per day. The NOAEL for embryotoxicity and
teratogenicity was thus 10 mg/kg bw per day.
In rats treated with doses of 0, 10, 30 or 100 mg/kg bw per day
or 0, 30, 60 or 120 mg/kg bw per day, the NOAEL was 10 mg/kg bw per
day for maternal toxicity on the basis of reduced body-weight gain
at higher doses. Embryotoxicity and an increased incidence of
malformations (mainly microphthalmia) and visceral and skeletal
variations were found at 100 mg/kg bw per day and above, giving an
NOAEL for embryotoxicity and teratogenicity of 60 mg/kg bw per day.
In rabbits treated at doses of 0, 3, 10 or 30 mg/kg bw per day
or 0, 10, 30 or 100 mg/kg bw per day, the NOAELs were 10 mg/kg bw
per day for maternal toxicity and 30 mg/kg bw per day for
embryotoxicity and teratogenicity. At 100 mg/kg bw per day,
embryotoxicity and an increased incidence of external malformations
(mainly peromelia) were observed.
Tebuconazole has been studied in a range of tests for
genotoxicity in vivo and in vitro. The Meeting concluded that
there was no evidence of genotoxicity.
An ADI was established on the basis of an NOAEL of 100 ppm in a
one-year dietary study in dogs and a 100-fold safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 20 ppm, equal to 6 mg/kg bw per day (21-month study
of toxicity and carcinogenicity)
< 10 mg/kg bw per day (maternal toxicity in a study
of teratogenicity)
10 mg/kg bw per day (embryotoxicity in a study of
teratogenicity)
Rat: 100 ppm, equal to 5 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
10 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
60 mg/kg bw per day (embryotoxicity in a study of
teratogenicity)
Rabbit: 10 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
30 mg/kg bw per day (embryotoxicity in a study of
teratogenicity)
Dog: 100 ppm, equal to 3 mg/kg bw per day (one-year study)
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Astroff, A.B. & Hagen, L.L. (1992) Acute toxicity study with HWG
2443 (a metabolite of Tebuconazole Folicur(TM)) in female rats.
Unpublished report Ref. No. 92-012-PB, prepared by Miles Inc.,
Agricultural Division, Toxicology, Stillwell, KS, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Becker, H., Vogel, W. & Terrier, C. (1988a) Embryotoxicity study
(including teratogenicity) with HWG 1608 technical in the rat.
Unpublished report Ref. No. R 4451, prepared by Research &
Consulting Co. AG (RCC), Itingen, Switzerland. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Becker, H., Vogel, W. & Terrier, C. (1988b) Embryotoxicity study
(including teratogenicity) with HWG 1608 technical in the rabbit.
Unpublished report Ref. No. R 4323, prepared by Research &
Consulting Co. AG (RCC), Itingen, Switzerland. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Becker, H., Vogel, O., Terrier, C., Biedermann, K. & Luetkemeier, H.
(1990) Embryotoxicity study (including teratogenicity) with HWG 1608
technical in the mouse (dermal application). Unpublished report Ref.
No. R 5116, prepared by Research & Consulting Co. AG (RCC), Itingen,
Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomhard, E. (1991) HWG 1608--Toxic dose range carcinogenicity study
in NMRI mice (supplement to study T 601 89 53 with administration in
diet over a 21-month period). Unpublished report Ref. No. 16376 A,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Bomhard, E. & Ramm, W. (1988a) HWG 1608--Study for cancerogenicity
in NMRI mice (administration in diet for up to twenty-one months).
Unpublished report Ref. No. 16376, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bomhard, E. & Ramm, W. (1988b) HWG 1608--Study for chronic toxicity
and cancerogenicity in Wistar rats (administration in diet for two
years). Unpublished report Ref. No. 16375, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E. & Schilde, B. (1986) HWG 1608--Subchronic toxicological
study with rats (feeding for thirteen weeks). Unpublished report
Ref. No. 15211, prepared by Bayer AG, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Bomhard, E., Löser, E. & Schilde, B. (1979) 1,2,4-Triazole:
Subchronic toxicological study with rats (feeding study over three
months). Unpublished report Ref. No. 8667, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Bomhard, E., Karbe, E. & Loeser, E. (1986) Spontaneous tumours of
2000 Wistar TNO/W. 70 rats in two-year carcinogenicity studies.
J.E.P.T.O. 7: 1/2, pp. 35-52.
Brain, K.R. (1991) In vitro skin permeability of tebuconazole.
Unpublished report Ref. No. M 7591, prepared by An-eX Analytical
Services Ltd., Cardiff, United Kingdom. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Chopade, H.M. (1992) Addendum I. [Phenyl-U-14C] HWG 1608: Study of
biokinetic behavior in the rat. Response to EPA requests and
inquiries. Unpublished report Ref. No. MR 97439-1 Metab I/2,
prepared by Miles Inc., Stilwell, KS, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Cifone, M.A. (1988) Mutagenicity test on HWG 1608 techn. in the rat
primary hepatocyte unscheduled DNA synthesis assay. Unpublished
report Ref. No. R 4111a, prepared by Hazelton Laboratories America
Inc., Kensington, MD, USA. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Ecker, W. & Weber, H. (1991) [Chlorophenyl-U-14C] tebuconazole
absorption, distribution, excretion and metabolism in laying hens.
Unpublished report Ref. No. PF 3587, prepared by Bayer AG,
Leverkusen, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Ecker, W., Brauner, A., Klein, O. & Weber, H. (1987) Folicur:
Metabolism part of the general metabolism study in the rat.
Unpublished report Ref. No. PF 2907 and MR 97438. Metab I/3,
prepared by Bayer AG, Leverkusen, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Eiben, R. (1987) HWG 1608--Two-generation study in rats. Unpublished
report Ref. No. 16223, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Eigenberg, D.A. (1988) Primary eye irritation of Folicur (HWG 1608)
technical in albino rabbits. Unpublished report Ref. No. 1003,
prepared by Mobay Corp., Stilwell, KS, USA. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Eigenberg, D.A. (199) Dermal absorption of 14C HWG 1608 technical
in rats. Unpublished report Ref. No. MR 97470. Metab I/5, prepared
by Mobay Corp., Stilwell, KS, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Flucke, W. (1978) Determination of LD 50--Compound: 1,2,4-Triazole.
Unpublished letter report by Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Flucke, W. (1987) HWG 1608 and KWG 0519 (c.n. Triadimenol)/HWG 1608
and KUE 13032c (c.n. Dichlofluanid) combination toxicity study.
Unpublished report Ref. No. 15747, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Heimann, K.G. (1983) HWG 1608--Study for skin-sensitising effect on
guinea pigs. Unpublished report Ref. No. 12024, prepared by Bayer
AG, Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K.G. (1987) HWG 1608 technical--Study of skin sensitization
effect on guinea pigs (Buehler patch test). Unpublished report Ref.
No. 16238, prepared by Bayer AG, Wuppertal, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Heimann, K.G. & Kaliner, G. (1984) HWG 1608--Study of the subacute
oral toxicity to rats. Unpublished report Ref. No. 13028, prepared
by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Heimann, K.G. & Pauluhn, J. (1983) HWG 1608 study for acute
toxicity. Unpublished report Ref. No. 12168, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Heimann, K.G. & Schilde, B. (1984) HWG 1608--Subacute study of
dermal toxicity to rabbits. Unpublished report Ref. No. 12669,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Heimann, K.G. & Schilde, B. (1988) HWG 1608 technical--Subacute
dermal study of toxicity to rabbits. Unpublished report Ref. No.
12669a (Addendum to report No. 12669), prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Herbold, B.A. (1983a) HWG 1608--Salmonella/microsome test to
evaluate for point mutagenic effect. Unpublished report Ref. No.
12086, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1983b) HWG 1608--Pol test on E. coli to evaluate for
harmful effects on DNA. Unpublished report Ref. No. 11902, prepared
by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Herbold, B.A. (1985) HWG 1608--Micronucleus test on the mouse to
evaluate for mutagenic effect. Unpublished report Ref. No. 13159,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1986) HWG 1608--Dominant lethal test on the male
mouse to evaluate for mutagenic effect. Unpublished report Ref. No.
14985, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1988a) HWG 160--Salmonella/microsome test to evaluate
for point mutagenic effects. Unpublished report Ref. No. 16383,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1988b) HWG 1608--Salmonella/microsome test using TA
1538 to evaluate for point mutagenic effects (addendum to final
report Ref. No. 16383). Unpublished report Ref. No. 16383 A,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1988c) In vitro cytogenetic study with human
lymphocytes for the detection of induced clastogenic effects.
Unpublished report Ref. No. 16395, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1990) HWG 1608--Salmonella/microsome test for
point-mutagenic effects (appendix to Report 12086). Unpublished
report Ref. No. 12086A, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Hoffmann, K. (1983a) HWG 1608 acute toxicity to the dog after oral
administration. Unpublished report Ref. No. 11974, prepared by Bayer
AG, Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hoffmann, K. (1983b) HWG 1608 acute toxicity to the sheep after oral
administration. Unpublished report Ref. No. 11970, prepared by Bayer
AG, Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
von Keutz, E. & Schilde, B. (1987a) HWG 1608--Subchronic study of
toxicity to dogs with oral administration (thirteen-weeks feeding
study). Unpublished report Ref. No. 15763, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
von Keutz, E. & Schilde, B. (1987b) HWG 1608--Study of chronic
toxicity to dogs after oral administration (twelve-month feeding
study). Unpublished report Ref. No. 16211, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Kollert, W. (1987) Report dated 10 November 1987, HWE 1608--Internal
experiments, Bayer Medical Department, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Lee, S.G.K. & Wood, S.E. (1987) The metabolism of Folicur in dairy
goats. Unpublished report Ref. No. MR 94882, prepared by Mobay
Corporation, Kansas City, MO, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Lee, S.G.K., Hanna, L.A., Johnston, K., Wood, S.E. & Leimkuehler,
W.M. (1988) The metabolism of 14C-Folicur in chickens. Unpublished
report Ref. No. 87 156, prepared by Mobay Corp., Kansas City, MO,
USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Lehn, H. (1988) HWG 1608--Mutagenicity study for the detection of
induced forward mutations in the CHO-HGPRT assay in vitro.
Unpublished report Ref. No. 16749, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Märtins, T. (1990) HWG 1608--Study for skin and eye
irritation/corrosion in rabbits. Unpublished report Ref. No. 12168 B
(addendum report to report No. 12168), prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Märtins, T. (1991) HWG 1608 (tebuconazole)--Subacute inhalation
toxicity to dogs--Study for cataracts. Unpublished report Ref. No.
20884, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Märtins, T., Pauluhn, J. & Krötlinger, F. (1990) HWG 1608
(tebuconazole)--Subacute inhalation toxicity to cats--Study for
cataracts. Unpublished report Ref. No. 19644, prepared by Bayer AG,
Wuppertal, Germany. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Ohta, K. (1991a) HWG 1608 technical--Acute oral toxicity study on
rats. Study No. 91A016. Unpublished report prepared by Nihon Bayer
Agrochem K.K., Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Ohta, K. (1991b) HWG 1608 technical--Acute dermal toxicity study on
rats. Study No. 91A012. Unpublished report prepared by Nihon Bayer
Agrochem K.K. Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Ohta, K. (1991c) HWG 1608 technical--Acute oral toxicity study on
mice. Study No. 91A017. Unpublished report prepared by Nihon Bayer
Agrochem K.K., Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Ohta, K. (1991d) HWG 1608--Reverse mutation assay (Salmonella
typhimurium and Escherichia coli). Unpublished report Ref. No. RA
91036, prepared by Nikon Bayer Agrochem K.K., Tokyo, Japan.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Ohta, K. (1992) HWG 1608--Rec- assay with spores in the bacterial
system. Unpublished report Ref. No. RA 92007, prepared by Nihon
Bayer Agrochem K.K., Tokyo, Japan. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Pauluhn, J. (1985) HWG 1608--Study for subacute inhalation toxicity
to rat for three weeks (exposure 15 x 6 hours). Unpublished report
Ref. No. 13305, prepared by Bayer AG, Wuppertal, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1987) HWG 1608--Study for subacute inhalation toxicity
to rat for three weeks (exposure 15 x 6 hours). Histopathological
examinations. Unpublished report Ref. No. 13305A (addendum to Ref.
No. 13305), prepared by Bayer AG, Wuppertal, Germany. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Pauluhn, J. (1988) HWG 1608--Study for acute inhalation toxicity to
the rat to OECD-guideline No. 403. Unpublished report Ref. No.
16345, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Porter, M.C., Jasty, V., Troup, C.M. & Hartnagel, R.E. (1989) Safety
evaluation of HWG 1608: Chronic (1 year) feeding study in dogs.
Unpublished report Ref. No. R 4781, prepared by Toxicology
Department, Miles Inc., Elkhart, IN, USA. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Poth, A. (1989) Salmonella typhimurium reverse mutation assay with
1H-1,2,4-triazole. Unpublished report Ref. No. R 4859, prepared by
Cytotest Cell Research GMBH & Co. KG, Rossdorf, Germany. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Putman, D.L. (1987) HWG 1608--Sister chromatid exchange assay in
Chinese hamster ovary (CHO) cells. Unpublished report Ref. No. 953,
prepared by Microbiological Associates Inc., Bethesda, MD, USA.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1984) HWG 1608--Study for embryotoxic effects on rats
after oral administration. Unpublished report Ref. No. 12457,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1985a) HWG 1608--Study for embryotoxic effects on rats
after oral administration. Unpublished report Ref. No. 13273,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1985b) HWG 1608--Study for embryotoxic effects on
rabbits after oral administration. Unpublished report Ref. No.
13287, prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Renhof, M. (1988a) HWG 1608--Study for embryotoxic effects on mice
following oral administration. Unpublished report Ref. No. 16527,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1988b) HWG 1608--Study for embryotoxic effects on rats
after dermal administration. Unpublished report Ref. No. 17089,
prepared by Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Renhof, M. (1988c) Triazole--Investigations into embryotoxic effects
on rats after oral administration. Unpublished report No. 17401,
Ref. No. 50 19 339, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. (1988d) Triazole--Investigations into embryotoxic effects
on rats after oral administration. Unpublished report No. 17402,
Supplement to Ref. No. T 50 19 339, prepared by Bayer AG, Wuppertal,
Germany. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Renhof, M. & Karbe, E. (1988) HWG 1608--Supplementary study for
maternal toxicity on mice following oral administration. Unpublished
report Ref. No. 16511, prepared by Bayer AG, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Sheets, L.P. (1988) Primary dermal irritation of technical grade
Folicur in rabbits. Unpublished report Ref. No. 1066, prepared by
Mobay Corp., Stilwell, KS, USA. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Sheets, L.P. (1990) Dermal sensitization study with technical grade
tebuconazole (Folicur) in guinea pigs. Unpublished report Ref. No.
5052, prepared by Mobay Corp., Stilwell, KS, USA. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Sheets, L.P. & Phillips, S.D. (1992) Acute oral toxicity study with
HWG 2061 (a metabolite of tebuconazole Folicur(TM)) in female
rats. Unpublished report Ref. No. 6685, prepared by Miles Inc.,
Agricultural Division, Toxicology, Stilwell, KS, USA. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Thyssen, J. & Kimmerle, G. (1976) 1,2,4-Triazole--Occupational
toxicology study. Unpublished report Ref. No. 5926, prepared by
Bayer AG, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Weber, H. (1987) [Phenyl-U-14C] HWG 1608: Study of biokinetic
behaviour in the rat. Unpublished report Ref. No. PF 2859. Metab
II/15, prepared by Bayer AG, Leverkusen, Germany. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Weber, H. (1988) [Phenyl-U-14C] HWG 1608:
Ganzkörperautoradiographische Verteilung der Radioaktivität in der
Ratte. Unpublished report Ref. No. PF 2962. Metab II/16, prepared by
Bayer AG, Leverkusen, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
