
TOLCLOFOS-METHYL
First draft prepared by
A. Moretto,
Institute of Occupational Medicine,
University of Padua, Padua, Italy
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Embryotoxicity and teratogenicity
Genotoxicity
Special studies
Skin and eye irritation and ski201
Delayed neuropathy
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Tolclofos-methyl is an organophosphorus fungicide that is
effective in controlling soil-borne diseases caused by infection of
Basidiomycetes fungi such as Rhizoctonia solani and Corticium
rolfsii. It was evaluated for the first time by the present JMPR.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution and excretion
Mice
Male and female ICR mice, eight weeks of age, were given an
oral dose of 5 mg/kg bw of [14C-4-methyl]-tolclofos-methyl
(radiochemical purity, 99%) dissolved in corn oil, and radiocarbon
was monitored in urine, faeces and expired air for seven days after
administration. Within 24 h, 69-76% of the administered radiolabel
was excreted in the urine, 4-6% in the faeces and less than 1% in
the expired air. Total radiocarbon residues in the whole body
represented less than 1% of the dose seven days after administration
(Mihara et al., 1981).
Rats
Six-week old Sprague-Dawley rats were given an oral dose of 5
mg/kg bw of [14C-4-methyl]-tolclofos-methyl (radiochemical purity,
99%) dissolved in corn oil, and radiocarbon was monitored in urine,
faeces and expired air for seven days after administration. Within
24 h, 62-67% of the administered dose was excreted in the urine,
16-21% in the faeces and less than 1% in the expired air. Total
radiocarbon residues in the whole body represented less than 1% of
the dose seven days after administration. Whole-body autoradiography
performed 1 and 6 h after treatment showed the highest accumulation
of radiolabel in stomach and intestines, followed by kidney and
liver (Mihara et al., 1981).
Male and female Sprague-Dawley rats received single oral doses
of 5 or 200 mg/kg bw of tolclofos-methyl labelled uniformly with
14C in the benzene ring (radiochemical purity, > 99%). Another
group of animals was treated orally for 14 consecutive days with
unlabelled tolclofos-methyl at 5 mg/kg bw per day and then with a
single oral dose of [14C-phenyl]-tolclofos-methyl at 5 mg/kg bw.
The administered radiocarbon was readily excreted, more than 95% of
the dose being eliminated in the urine and faeces within 48 h. The
amount excreted in urine after seven days was 85-91%; elimination in
faeces at that time was: consecutive dose group, 9.3% in males and
12% in females; low-dose group, 20% in males and 19% in females; and
high-dose group, 20% in males and 12% in females. Excretion as
14C-carbon dioxide accounted for < 0.1% of the dose in all
groups. The concentration of 14C reached a peak within 2 h in
almost all tissues. After administration of the low dose, the
highest concentrations were found in the kidney; expressed in
tolclofos-methyl equivalents, the levels were 4700 ng/g tissue in
males and 3450 ng/g tissue in females; the levels in plasma were
1140 ng/ml in males and 1270 ng/ml in females. Those in the liver
were 1240 ng/g tissue in males and 1220 ng/g tissue in females, and
those in blood were 736 ng/ml in males and 835 ng/ml in females. The
concentrations of 14C in various organs 72 h after administration
were < 5% of the respective peak concentrations. After seven
days, radioactive residues accounted for less than 1% of the
administered dose (Krautter et al., 1987, 1988; Esumi & Yokoshima,
1989).
In male and female rats with bile-duct cannulas, cumulative
excretion of 14C over 48 h was 5.8-12% of the dose in bile, 47-59%
in urine and 42-24% in faeces (Esumi & Yokoshima, 1989).
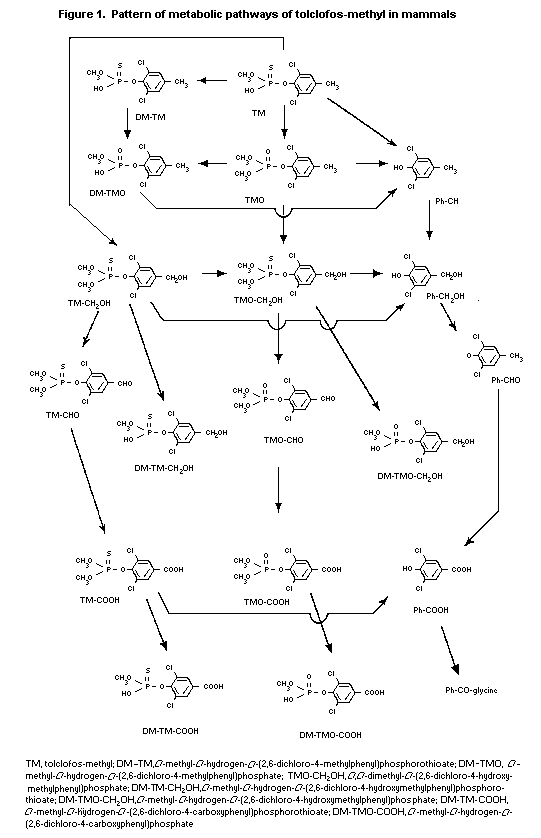
(b) Biotransformation
Male ICR mice were given 5 mg/kg bw [14C-4-methyl]-tolclofos-
methyl orally; metabolites were isolated from the faeces and urine
by chromatography and identified by co-chromatography with authentic
standards and/or spectroanalysis. The following major metabolites
were detected in the excreta: 2,6-dichloro-4-methylphenol (see
Figure 1; 9% of administered label), O,O-dimethyl- O-(2,6-
dichloro-4-carboxyphenyl)phosphate (11%), O-methyl- O-hydrogen-
O-(2,6-dichloro-4-carboxyphenyl)phosphate (12%), 3,5-dichloro-4-
hydroxybenzoic acid (12%) and 3,5- O-dichloro-4-hydroxybenzyl
glycine (13%). The major biotransformation reactions are oxidative
desulfuration to oxon and related derivatives, oxidation of the
4-methyl group to alcohols and acids, cleavage of P-O-aryl and
P-O-methyl linkages and conjugation of the resultant acid with
glycine. The metabolites found in mice are similar to those seen in
rats, except for 3,5- O-dichloro-4-hydroxybenzylglycine (Mihara et
al., 1981).
Male and female Sprague-Dawley rats were given an oral dose of
5 or 200 mg/kg bw tolclofos-methyl labelled with 14C either in the
4-methyl group or uniformly in the phenyl ring, with or without
pretreatment with unlabelled tolclofos-methyl at 5 mg/kg bw per day
for 14 consecutive days. Metabolites were isolated from the faeces,
urine, bile and major tissues by chromatography and identified by
co-chromatography with authentic standards and/or spectroanalysis.
More than 10 metabolites were detected in the excreta. No marked
differences were seen in relation to sex or dose. The major
metabolites detected in the excreta were O-methyl O-hydrogen-
O-(2,6-dichloro-4-methylphenyl)phosphate ((see Figure 1; 10-26% of
urinary 14C), O-methyl- O-hydrogen- O-(2,6-dichloro-4-
hydroxymethylphenyl) phosphorothioate (12-25%), O-methyl
O-hydrogen- O-(2,6-dichloro-4-carboxyphenyl)-phosphorothioate
(11-35%) and O-methyl- O-hydrogen- O-(2,6-dichloro-4-
methylphenyl)-phosphorothioate (12-44%). In rats with bile cannulas,
most of the radiolabel excreted into the bile within 24 h after
administration was associated with polar metabolites; the major
metabolites in the bile were O-methyl- O-hydrogen- O-(2,6-
dichloro-4-hydroxymethylphenyl)-phosphorothioate and 2,6-dichloro-4-
methylphenol glucuronides. Radiocarbon excreted into the faeces
within 24 h after administration was associated only with the parent
compound (Esumi & Yokoshima, 1989).
Two hours after oral administration, the major metabolites in
blood, liver and kidney were O,O-dimethyl- O-(2,6-dichloro-4-
carboxyphenyl) phosphorothioate ((see Figure 1), 3,5-dichloro-4-
hydroxybenzaldehyde, O-methyl- O-hydrogen O-(2,6-dichloro-4-
methylphenyl)-phosphorothioate and O-methyl- O-hydrogen- O-(2,6-
dichloro-4-hydroxymethylphenyl)-phosphorothioate. Only a small
amount of the parent compound was detected in the liver. The major
biotransformation reactions were oxidative desulfuration to oxon and
related derivatives, oxidation of the 4-methyl group to alcohols and
acids, cleavage of the P-O-aryl and P-O-methyl linkages and
conjugation of the resultant acids and phenols with glucoronic acids
(Mihara et al., 1981; Krautter et al., 1987, 1988; Esumi &
Yokoshima, 1989).
The general pattern of metabolism of tolclofos-methyl is shown
in Figure 1.
 2. Toxicological studies
(a) Acute toxicity
Data on the acute toxicity of tolclofos-methyl (technical-grade
and formulations) in laboratory animals are summarized in Table 1.
Animals showed decreased spontaneous motor activity, dyspnoea,
piloerection, urinary incontinence and ataxia. Recovery was complete
by day 10. Brain cholinesterase activity was lower 16 days after
treatment in dogs given 1000 mg/kg bw than in animals given lower
doses; however, as no control data were reported, conclusions cannot
be drawn. No treatment-related gross changes were seen at necropsy
in any species.
Table 1. Acute toxicity of technical-grade and formulated tolclofos-methyl in laboratory animals
Species Strain Sex Route Formulation LD50 Reference
(purity) (mg/kg bw)
Mouse dd M Oral Technical (97%) 3500 Segawa, 1978
F 3600
Mouse ICR M&F Oral 50% wettable > 5000 Segawa, 1981a
powder (98%)
Mouse ICR M&F Oral 10% dust (98.8%) > 5000 Segawa, 1981b
Mouse dd M&F Dermal Technical (97%) > 5000 Segawa, 1978
Mouse ICR M&F Dermal 50% wettable > 5000 Segawa, 1981c
powder (98%)
Mouse ICR M&F Dermal 10% dust (98.8%) > 5000 Segawa, 1981d
Mouse dd M Intraperitoneal Technical (97%) 1070 Segawa, 1978
F 1260
Mouse dd M&F Subcutaneous Technical (97%) > 5000 Segawa, 1978
Rat SD M&F Oral Technical (97%) approx. 5000 Segawa, 1978
Rat SD M&F Oral 50% wettable > 5000 Segawa, 1981e
powder (98%)
Rat SD M&F Oral 10% dust (98.8%) > 5000 Segawa, 1981f
Rat SD M&F Oral 50% flowable > 5000 Hiromori et al.,
1989a
Rat SD M&F Dermal Technical (97%) > 5000 Segawa, 1978
Rat SD M&F Dermal 50% wettable > 5000 Segawa, 1981g
powder (98%)
Rat SD M&F Dermal 10% dust (98.8%) > 5000 Segawa, 1981h
Rat NR M&F Dermal 50% flowable > 2000 Hiromori et al.,
1989b
Table 1 (contd)
Species Strain Sex Route Formulation LD50 Reference
(purity) (mg/kg bw)
Rat SD M Intraperitoneal Technical (97%) 5000 Segawa, 1978
F 4900
Rat SD M&F Subcutaneous Technical (97%) > 5000 Segawa, 1978
Rat Wistar M&F Inhalation Technical (97.7%) > 3.32 mg/la Hardy et al., 1986
Rat SD M&F Inhalation 50% wettable > 1.9 mg/l Eschbach &
powder (98%) Hogan, 1981a
Rat SD M&F Inhalation 10% dust (98.8%) > 1.8 mg/l Eschbach &
Hogan, 1981b
Rat SD M&F Inhalation 50% flowable > 1.07 mg/la Jackson et al., 1990
Dog NR M&F Oral Technical (98.7%) > 1000 Pence et al., 1978
SD, Sprague-Dawley; M, male; F, female; NR, not reported
a 4-h whole-body exposure
(b) Short-term toxicity
Mice
Groups of 15 male and 15 female ddY mice were fed diets
containing tolclofos-methyl (purity, 97%) at 0, 10, 30, 100 or 3000
ppm, equal to 1.2, 3.8, 12 or 510 mg/kg bw per day for males and
1.4, 4.1, 14 or 560 mg/kg bw per day for females, for nine months.
Three satellite groups of five males and five females each were
similarly treated and killed at 2, 4 and 13 weeks for determination
of cholinesterase activity. Animals were observed daily for
mortality and signs of toxicity; at the end of exposure, an
ophthalmological examination and clinical chemical analyses
(including plasma and erythrocyte cholinesterase) were undertaken,
and brain cholinesterase activity was measured. Most organs were
weighed and examined microscopically. There were no overt signs of
toxicity and no deaths. Body weights were reduced by about 20% and
body-weight gain by about 35% in animals of each sex at 3000 ppm,
but at this dose food consumption was increased in males and females
and water intake was increased in males. No treatment-related
ophthalmological changes were observed, and haematological
parameters were unaffected by treatment. The cholesterol level was
increased by 35% in females at 3000 ppm. A dose-related decrease in
plasma cholinesterase activity was found in animals of each sex,
with activities that were 63-94% of the control values in mice at 30
ppm, 30-56% at 100 ppm and 4-35% (61% in one case) at 3000 ppm. At
the high dose, the activity reached a plateau after four weeks.
Erythrocyte cholinesterase activity was decreased to 39-46% of the
control level in males at 3000 ppm at weeks 13 and 40 and to 57-75%
of the control level in females at this dose at all time points.
Brain cholinesterase activity was unaffected by treatment in females
but was reduced by about 24% in males at 3000 ppm at weeks 13 and
40. Organ weights in the 3000-ppm group were reduced, but neither
gross nor histopathological changes attributable to treatment were
observed. The NOAEL was 100 ppm, equal to 12 mg/kg per day, on the
basis of inhibition of brain cholinesterase activity and an effect
on body weight at the highest dose (Suzuki et al., 1978).
Rats
Groups of 10 male and 10 female Sprague-Dawley rats were fed
diets containing tolclofos-methyl (purity, 97.9%) at 0, 200, 1000,
5000 or 20 000 ppm for 32-34 days. Animals were observed twice daily
for mortality and clinical signs, and food consumption and body
weight were recorded weekly. Ophthalmoscopic examination, standard
haematological tests and clinical chemical analyses were performed
at week 4, and certain tests were repeated at termination. Plasma
and erythrocyte cholinesterase activities were determined in the
control and high-dose groups at week 4 and in all animals at
termination, when brain cholinesterase activity was also measured.
At terminal sacrifice, relevant organs were weighed and most organs
were examined microscopically. There were no treatment-related
deaths. Reductions in food consumption (-16% in males and -6% in
females) and body-weight gain (-45% in males and -37% in females)
were observed at 20 000 ppm. Body-weight gain was slightly reduced
in animals of each sex at 5000 ppm during week 1. Haematological
parameters were unaffected by treatment. Cholesterol levels were
increased (by 116-138%) in animals of each sex at 20 000 ppm, and
total protein and albumin levels were slightly higher in males in
this group. Other differences between treated and untreated animals
were detected, but the values were within the normal range. Plasma
cholinesterase activity was reduced by 14% in males and by 50% in
females at 20 000 ppm. Erythrocyte cholinesterase activity was
reduced by 9-19% in animals of each sex at 5000 and 20 000 ppm;
however, no clear dose-response relationship was seen. Brain
cholinesterase activity was lower (by 12-31%) than that in controls
in males in all treated groups and in females (by 20%) at 5000 and
20 000 ppm. Although the reduction was statistically significant in
all male groups, no clear dose-response relationship was found, and
the activity in controls was slightly higher than usual. There were
no ophthalmoscopic alterations or gross changes at necropsy. The
relative weights of livers were increased (by 12%) in females at
5000 ppm and in males and females at 20 000 ppm (by 27% in males and
39% in females). Hypertrophy of hepatocytes was observed in animals
of each sex at 20 000 ppm. The relative weights of the kidneys were
slightly increased in animals of each sex at 5000 and 20 000 ppm,
but no treatment-related histopathological changes were seen. The
NOAEL was 1000 ppm, equal to 79 mg/kg bw per day, on the basis of
increased relative kidney weights and brain cholinesterase activity
at higher doses (Colley et al., 1982).
Groups of 12 male and 12 female Sprague-Dawley rats were fed
diets containing tolclofos-methyl (96.6% pure) at 0, 100, 1000 or 10
000 ppm, equal to 6.5, 66 or 653 mg/kg bw per day for males and 7.1,
71 or 696 mg/kg bw per day for females, for 13 weeks. Animals were
observed daily for mortality and clinical signs, and body weight and
food and water consumption were determined weekly. Ophthalmological
analysis, urinalysis, standard haematological tests, clinical
chemical analyses and measurement of plasma, erythrocyte and brain
cholinesterase activities were undertaken at termination, when
relevant organs were weighed and most organs were examined
microscopically. No deaths and no significant abnormal clinical or
ophthalmological signs were noted during the study. Depression of
body-weight gain of males (-20%) and females (-15%) at 10 000 ppm
was seen throughout the treatment period and was associated with
similar decreases in food and water consumption. A slight decrease
in food consumption was noted in females at 1000 ppm during week 1.
Decreased activities of plasma (16-53%), brain (8-9%) and
erythrocyte (19-20%) cholinesterase were noted in animals of each
sex at 10 000 ppm; a minimal decrease in erythrocyte cholinesterase
activity (10%) was also seen in females at 1000 ppm. Liver weight
was increased in animals of each sex at 10 000 ppm, and this was
found at histopathological examination to be associated with
hypertrophy of hepatocytes. Changes in some clinical chemical
parameters were observed at 10 000 ppm, which included increased
levels of cholesterol (48% in males, 102% in females) and
phospholipids (37% in males, 64% in females) and slightly increased
alpha2- and ß-globulin levels in animals of each sex. Other
changes, although statistically significant, were minor and of
questionable biological significance. At 10 000 ppm, increased
relative kidney weights were seen in animals of each sex, increased
blood urea nitrogen levels were seen in males only and decreased
urinary pH was seen in males and females. At 1000 ppm, relative
kidney weights were slightly increased in females and relative liver
weights in males. The Meeting considered that the increased weight
ratios observed at 1000 ppm were not biologically relevant and that
the NOAEL was 1000 ppm, equal to 66 mg/kg bw per day (Kimura et
al., 1990).
Groups of 15 male and 15 female Sprague-Dawley rats were fed
diets containing tolclofos-methyl (purity, 97%) at 0, 300, 1000,
3000 or 10 000 ppm, equal to 16, 51, 164 or 540 mg/kg bw per day in
males and 18, 65, 184 or 623 mg/kg bw per day in females, for 28
weeks. Animals were observed daily for clinical signs, and body
weight and food consumption were measured weekly. Ophthalmologic
examinations were carried out after three months and at termination;
standard haematological tests and clinical chemical analyses were
performed at termination. Urinalysis was carried out in 12 animals
per group after three months and at termination. Plasma and
erythrocyte cholinesterase activities were determined after 2, 4 and
13 weeks, and at termination; brain cholinesterase was determined
only at termination, when relevant organs were weighed and several
were examined histologically.
There were no overt signs of toxicity and no treatment-related
deaths. Although the body weights of animals of each sex at 10 000
ppm were lower than those of controls throughout the study, their
initial body weights were also statistically significantly lower.
Body-weight gain was significantly reduced (by 18%) in females at 10
000 ppm. Food consumption was unaffected by treatment. Treatment did
not affect ophthalmologic, urinary, haematological or clinical
chemical parameters. Plasma cholinesterase activity was lower (by
23-41%) in females at 10 000 ppm than in controls at weeks 2, 4, 13
and 28. Erythrocyte cholinesterase activity was highly variable and
was reduced by 15-19% in comparison with controls in males at 10 000
ppm at weeks 2, 4 and 28. Brain cholinesterase activity was
unaffected by treatment. Kidney and liver weights were slightly
increased in animals of each sex at 10 000 ppm and in females at
1000 and 3000 ppm, but the biological significance of these findings
was questionable. No gross abnormalities were seen at necropsy.
Bile-duct proliferation was found in the livers of 3/15 females at
10 000 ppm and oval-cell proliferation was seen in 2/15 females at
3000 ppm and 3/15 at 10 000 ppm. No histopathological changes were
seen in the kidneys. The NOAEL was 1000 ppm, equal to 65 mg/kg bw
per day, on the basis of histopathological changes in the livers of
females at higher doses (Hiromori et al., 1978).
Rabbits
Groups of five male and five female New Zealand white rabbits
received tolclofos-methyl (purity, 97.7%) in acetone on the skin at
doses of 0, 30, 300 or 1000 mg/kg bw per day for 6 h per day, on
five days per week for 21 days. There were no deaths and no
treatment-related clinical signs; neither body weight nor food
consumption was affected by treatment. Dermal irritation (slight
erythema) was seen in some animals in all treated groups from day 6.
Eosinophil counts were increased in males at 1000 mg/kg bw per day,
and the level of inorganic phosphorus was decreased (by 14-20%) in
animals of each sex at this dose. Brain cholinesterase activity was
about 15% lower than that in controls in males and about 15% higher
in females at 300 and 1000 mg/kg bw per day. These changes were
considered not to be of biological significance. Erythrocyte
cholinesterase activity was lower than in controls in males at the
same two doses, but there was no dose-effect relationship. Plasma
cholinesterase activity was lower (by 22-29%) than in controls in
animals of each sex at 300 and 1000 mg/kg bw per day. The relative
weights of the kidneys were increased (by 20%) in females at 1000
mg/kg bw per day. Macroscopic findings consisted of accumulation of
the compound on treated skin; microscopic examination revealed
hyperkeratosis, acanthosis and subepidermal pleocellular
infiltration of the skin (Gargus, 1986).
Dogs
Groups of six male and six female beagle dogs were fed diets
containing tolclofos-methyl (purity, 98.7%) at 0, 200, 600 or 2000
ppm, equal to 7.4, 23 or 69 mg/kg bw per day in males and 4.1, 21 or
65 mg/kg bw per day in females, for 26 weeks. Animals were observed
daily for mortality and weekly for clinical signs, body weight and
food consumption. Ophthalmoscopic examinations were performed before
treatment and at termination of the study. Standard haematological
tests and clinical chemical analyses were performed at regular
intervals until termination. Erythrocyte and plasma cholinesterase
activities were determined at weeks 2, 4, 8, 13 and 26. Brain
cholinesterase was determined at termination, when relevant organs
were weighed and most were examined histologically. There were no
deaths and no overt signs of toxicity. Body-weight gain was reduced
by 54% in males and by 46% in females at 2000 ppm, although food
consumption was unaffected by treatment. There were no
treatment-related ocular changes. Although the haematocrit was
within the normal range, decreased values in comparison with
controls (-11% in males and -10% in females) were observed at 2000
ppm. The mean corpuscular volume and mean corpuscular haemoglobin
concentration were not affected by treatment. Alkaline phosphatase
activity was increased by 139-295% in animals of each sex at 2000
ppm throughout the study. Total bilirubin was not increased in
treated animals. At 2000 ppm, plasma cholinesterase activity was
decreased by 19-26% in females throughout the study, but no
significant decreases were seen in erythrocyte cholinesterase
activity in animals of each sex or in plasma cholinesterase activity
in males. Brain cholinesterase activity was unaffected by treatment.
Urinalysis gave unremarkable results, and there were no gross
changes at necropsy. Liver weights were increased (by 59% in males
and 43% in females) at 2000 ppm, but there were no concomitant
histological changes, and there were no treatment-related
histopathological changes. The NOAEL was 600 ppm, equal to 21 mg/kg
bw per day, on the basis of increased liver weights, reduced
body-weight gain and increased alkaline phosphatase activity at 2000
ppm (Pence et al., 1979a).
Groups of six male and six female beagle dogs were fed diets
containing tolclofos-methyl (purity, 96.7%) at 0, 80, 400 or 2000
ppm, for 52 weeks. Animals were observed daily for mortality and
weekly for clinical signs. Ophthalmoscopic examinations were
performed before treatment and at weeks 26 and 52. Standard
haematological tests, clinical chemical analyses and determinations
of plasma and erythrocyte cholinesterase activities were performed
one week before treatment and at weeks 13, 26 and 52. Brain
cholinesterase was determined at termination, when relevant organs
were weighed and most were examined histologically. No clinical
signs of toxicity were observed. Decreased food consumption (-9% in
males and -16% in females) and body-weight gain (-32% in males and
-54% in females), slightly lower erythrocyte counts (-8% in males
and -20% in females), haematocrit value (-7% in males and -17% in
females) and haemoglobin concentration (-8% in males and -7% in
females), slightly decreased albumin and total protein levels
(significantly at some time points) and elevated alkaline
phosphatase levels (> 200%) were found at 2000 ppm.
Plasma cholinesterase activity was slightly, but not
significantly lower than that in controls in females at the highest
dose; erythrocyte and brain cholinesterase activities were not
affected by the treatment. No treatment-related ophthalmoscopic
alterations were observed. Increases in mean liver weights, both
absolute (+32% in males and +61% in females) and relative (+52% in
males and +74% in females), were seen in dogs at 2000 ppm.
Microscopic examination of the livers revealed increased incidences
of hepatocytic hypertrophy and intracytoplasmic homogeneous material
and an increased amount of hepatocytic pigment in these animals. The
amount of this pigment was also increased at 400 ppm, and it was
present in control animals. Its nature has not been elucidated, but
it does not contain iron or bilirubin. These dogs also had decreased
absolute (-46%) and relative (-40%) weights of the prostate, and the
absolute and relative weights of the pancreas were increased by
16-27% and 28-49%, respectively. The NOAEL was 400 ppm, equal to 11
mg/kg bw per day, on the basis of reduced body-weight gain,
increased liver weights (with hepatic hypertrophy) and slight
anaemia at 2000 ppm (Cox et al., 1988; Moore, 1993).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 70 male and 70 female B6C3F1 mice were fed diets
containing tolclofos-methyl (purity, 94.3%) at 0, 10, 50, 250 or
1000 ppm, equal to 1.3, 6.5, 32 or 134 mg/kg bw per day in males and
1.3, 6.8, 34 or 137 mg/kg bw per day in females, for up to 104
weeks. Animals were observed twice daily for clinical symptoms and
mortality. Body weight was measured weekly during the first 13 weeks
and then once every four weeks. Food intake was measured weekly.
Standard urinalysis was performed in 10 animals from each group at
6, 12 and 18 months and in all survivors at termination of the
study. Standard haematological tests were performed in 10 animals
from each group at 12 months and in all survivors at termination.
Standard clinical chemical analyses and measurements of serum,
erythrocyte and brain cholinesterase activities were done in 10
animals from each group at 6 and 12 months and in all animals at
termination. Animals in the control and 1000-ppm groups were
subjected to ophthalmologic examination at 12 and 24 months. Ten
animals from each group were necropsied at 6 and at 12 months, at
termination or when found dead or moribund. Relevant organs were
weighed and examined microscopically.
There were no treatment-related effects on mortality and no
overt signs of toxicity, including ophthalmologic changes.
Body-weight gain was slightly reduced in females at 1000 ppm up to
week 52; food consumption was also slightly decreased in this group
after 52 and 104 weeks. Food conversion efficiency, water intake,
urinary parameters and the haematological profile were unaffected by
treatment. Serum cholinesterase activity was decreased in animals of
each sex at 250 ppm (by 25-52%) and at 1000 ppm (by 43-81%) at both
times, and erythrocyte cholinesterase activity was decreased by
11-28% at 250 ppm and by 13-47% at 1000 ppm; inhibition of both
enzymes was greater at termination. A dose-related decrease was
found in brain cholinesterase activity in females (by 7-24% at 250
ppm and 9-33% at 1000 ppm) and males (by 10-13% at 250 ppm and
17-26% at 1000 ppm). Serum and brain, but not erythrocyte,
cholinesterase activities were lower (by 12 and 18%, respectively)
in females at 50 ppm at week 28 than in controls, which had higher
levels than at week 52 and at termination. Glucose levels were
increased in males and were slightly increased in females at 1000
ppm at week 104. Absolute and/or relative kidney weights were
increased in animals of each sex at 1000 ppm and in males at 250 ppm
at week 52, and in females at 250 ppm at week 104. The weights of
the thymus were decreased in females at 1000 ppm at weeks 52 and
104, and pituitary weights were increased in females at this dose at
week 104. No treatment-related changes were seen at necropsy or on
histopathological examination at any time. Tolclofos-methyl was not
found to be carcinogenic. The NOAEL was 50 ppm, equal to 6.5 mg/kg
bw per day, on the basis of reduced brain cholinesterase activity
and increased absolute and relative kidney weights at higher doses
(Satoh et al., 1983).
Rats
Groups of 65 male and 65 female Fischer 344 rats were fed diets
containing tolclofos-methyl (purity, 94.9-98.7%) at 0, 100, 300 or
1000 ppm for 122 weeks (males) or 129 weeks (females). At week 52,
10 males and 10 females from each group were killed. Animals were
observed twice a day for mortality and signs of morbidity. Body
weight and food consumption were recorded weekly (weeks 0-26), every
two weeks (weeks 27-52) or every four weeks until termination.
Ophthalmoscopic examinations were performed every 26 weeks and at
termination. Standard haematological tests, clinical chemical
analyses and measurements of erythrocyte and plasma cholinesterase
activities were conducted in 10 animals of each sex in each group
one week before treatment, at weeks 4, 13, 26, 52, 78 and 104 and at
termination. Brain cholinesterase activity was determined in 10
animals of each sex per group at week 52 and at termination. All
animals killed or found dead were necropsied, and relevant organs
were weighed; most organs were then examined histologically.
There were no treatment-related effects on mortality and no
overt signs of toxicity. A slight decrease in body-weight gain was
observed in males at 1000 ppm. Food consumption and food conversion
efficiency were unaffected by treatment, as was the haematological
profile. There was a dose-related trend to decreased alkaline
phosphatase activity (by 9-46%) in all treated groups: The activity
was significantly decreased in males at 1000 ppm throughout the
study, in males at 300 ppm from week 13, and occasionally in the
other treated groups. Brain cholinesterase activity was decreased in
all treated groups at the time of the interim sacrifice, but there
was no dose-response relationship. At the time of terminal
sacrifice, brain cholinesterase activity was reduced in males at the
middle dose (by 52%) and in females at the low (by 57%) and middle
doses (by 49%), but not in animals at the high dose. Erythrocyte and
plasma cholinesterase activities were also inconsistently lower in
treated groups, although no dose-response relationship was seen.
Urinalysis revealed no remarkable findings, organ weights were
unaffected by treatment, and there were no treatment-related gross
changes at necropsy. Toclofos-methyl was not carcinogenic in this
study. No NOAEL could be identified, given the variability of the
data on cholinesterase activity (Pence et al., 1982).
Groups of 30 male and 30 female Fischer 344 rats were fed diets
containing tolclofos-methyl (purity, 98.3-97.3%) at 0, 100, 300 or
1000 ppm for 104 weeks, and brain, erythrocyte and plasma
cholinesterase activities were assessed. Ten males and 10 females
from each group were sacrificed at week 52. Animals were observed
daily for mortality, and they were examined for body weight, food
consumption and gross signs of toxicity weekly until week 26, every
two weeks during weeks 26-52 and every four weeks during weeks
52-104. Blood samples were taken for determination of plasma and
erythrocyte cholinesterase activities at initiation of the study, at
weeks 5, 14, 27, 53, 79 and at termination. Brain cholinesterase
activity was determined at week 52 and at termination.
There were no treatment-related effects on survival, and the
incidences of clinical signs and palpable tissue masses were
comparable among the groups. Mean plasma cholinesterase activities
were slightly decreased (by < 25%) in males when compared with
controls at 300 and 1000 ppm at weeks 27 (statistically
significant), 53, 79 and 105. Mean erythrocyte and brain
cholinesterase activities were comparable among the groups
throughout the study. Plasma cholinesterase activity was reduced (by
< 20%) at certain times in treated animals but no dose-response
relationship was found. Gross pathological findings were unrelated
to treatment. Doses < 1000 ppm did not depress erythrocyte or
brain cholinesterase activity or induce gross pathological effects
(Miyamoto, 1985; Pence et al., 1985a).
The combined NOAEL in these two studies was 1000 ppm, equal to
41 mg/kg bw per day, on the basis of the absence of any significant
findings.
(d) Reproductive toxicity
Rats
A three-generation study of reproductive toxicity was performed
in Sprague-Dawley rats fed diets containing tolclofos-methyl
(purity, 97.9-98.7% pure) at 0, 100, 300 or 1000 ppm, equivalent to
10, 30 or 100 mg/kg bw per day, with two litters per generation. The
F0 generation consisted of 30 rats of each sex per group. The
F1a, F2a and F3a generations were sacrificed at weaning and
underwent gross necropsy. Five rats of each sex in each group were
selected at weaning from the F1b, F2b and Fn3b litters for
histopathological examination, and 25 rats of each sex from each
group were selected from the F1b and F2b litters to breed the
following generation. A 15-week growth period was allowed before
mating of the 'a' litters, and a minimal 10-day rest period was
allowed between weaning of the 'a' litters and mating to produce the
'b' litters. After birth of the 'b' litter, all parental animals
were necropsied.
There were no treatment-related deaths or overt signs of
toxicity. Pregnancy rates were low at times in all groups, but the
pattern was not related to dose. The lowest pregnancy rate (52%) was
observed in the F1b generation at the middle dose. Pregnancy rates
were below 70% in three of the six generations in the controls, in
two generations at the low dose, in four generations at the middle
dose and in one generation at the high dose. The body weights of
F2a and F2b pups at 300 and 1000 ppm were lower than those of
controls at the start of the growth period; however, these
reductions were not dose-related, and the growth rates and body
weights in other groups were comparable throughout the study. There
were no treatment-related gross changes at necropsy in any group.
The weights of the ovaries were increased in non-pregnant adult
F2b females at 1000 ppm, but this was not associated with
histomorphological alterations. There was an increased incidence
(not statistically significant) of fine cytoplasmic vacuolation of
the adrenal cortex in adult F2b females at 1000 ppm that became
pregnant at both matings. The etiology and toxicological
significance of this finding, given its occurrence in the controls,
were unknown. There was no effect on reproductive parameters or on
offspring survival and development in any litter, and there were no
treatment-related gross or histopathological findings in the
litters. The NOAEL for reproductive toxicity was 1000 ppm
(equivalent to 100 mg/kg bw per day) on the basis of the absence of
significant findings at any dose level (Pence et al., 1985b).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 21-26 pregnant Fischer 344 rats were administered 0,
5, 15 or 50 mg/kg bw per day tolclofos-methyl (purity, 94.9%; dose
adjusted to 100%) in 0.5% w/v methylcellulose by gavage on days 6-15
of gestation. There were no overt signs of toxicity, and body-weight
gain and food consumption were unaffected by treatment. There were
no treatment-related gross lesions in the dams at necropsy.
Pregnancy rates were slightly higher in all treated groups (77-87%)
than in controls (70%). There were no treatment-related changes in
the number of fetal deaths, fetal viability or size or the
incidences of visceral or skeletal anomalies or variations.
Tolclofos-methyl was not embryotoxic, fetotoxic or teratogenic in
rats at doses up to and including 50 mg/kg bw per day. It should be
noted, however, that the highest dose was not maternally toxic
(Pence et al., 1979b).
Groups of 23 pregnant Sprague-Dawley rats were administered 0,
100, 300 or 1000 mg/kg bw per day tolclofos-methyl (purity, 96.7%)
in 0.5% methyl cellulose orally on days 6-15 of gestation. On day 20
of gestation, all animals were sacrificed and caesarean section was
performed. Rats were observed daily for mortality and clinical signs
and weighed. At termination, dams were observed for gross visceral
abnormalities, and their uteri were weighed and examined for
implantations and resorptions. Live fetuses were weighed, sexed and
examined for external, visceral and skeletal abnormalities. All dams
survived to the day of scheduled sacrifice with no clinical signs of
toxicity. Mean body-weight gain in animals at 100 and 1000 mg/kg bw
per day was significantly less than in controls on days 6-11. Both
mean body-weight gain (days 6-16) and mean net body-weight gain
(days 0-20) in rats at 1000 mg/kg bw per day were lower (by 10 and
14%, respectively) than the corresponding control value. In
addition, mean food consumption in animals at 1000 mg/kg bw per day
on days 6-16 and 16-20 was slightly below the control value. There
were no treatment-related differences in implantation efficiencies,
and mean fetal viability, sex ratio and fetal body weight were
similar in all groups. The number of fetuses with unossified fifth
and/or sixth sternebrae was significantly greater in the 1000-mg/kg
bw per day group than in the control group; however, the incidence
of total fetal skeletal variations was similar in all groups. Other
variations in development were not related to dose. Two to three
malformed fetuses were found in each group, but neither the type nor
the frequency of malformations indicated a teratogenic or
embryotoxic response. Tolclofos-methyl was found to be neither
teratogenic nor embryotoxic in this study at doses up to and
including 1000 mg/kg bw per day. This dose was slightly toxic to the
dams, as indicated by the lower body-weight gain. The NOAEL for
maternal toxicity was 300 mg/kg bw per day (Morseth et al., 1987).
Rabbits
Groups of 13-17 pregnant New Zealand white rabbits were
administered 0, 300, 1000 or 3000 mg/kg bw per day tolclofos-methyl
(purity, 98.7% pure) in 5% carboxymethylcellulose orally on days
6-18 of gestation. The animals were observed daily for mortality and
clinical signs and were weighed. At termination, dams were observed
for gross visceral abnormalities, and their uteri were weighed and
examined for implantations and resorptions. Live fetuses were
weighed, sexed and examined for external, visceral and skeletal
abnormalities. One rabbit at 3000 mg/kg bw per day died on day 14 of
gestation of an undetermined cause. There were no overt signs of
toxicity in any group. Spontaneous abortion occurred in one dam at
1000 mg/kg bw per day and in two at 3000 mg/kg bw per day on or
after day 21 of gestation. The mean body weights of animals in the
treated groups were significantly lower than the control values
throughout the study (including day 0). Body-weight gain was reduced
by 76% in animals at 3000 mg/kg bw per day; and at termination,
weight gain (days 0-29) was 19% lower than in controls. At 1000
mg/kg bw per day, body-weight gain was reduced by 56% during
treatment, but it was similar to that of controls at termination.
Food consumption was decreased by up to 38% in animals at 1000 and
3000 mg/kg bw per day during treatment. There were no
treatment-related changes in maternal organ weights. One animal at
3000 mg/kg bw per day resorbed her entire litter. There were no
other treatment-related changes in implantation efficiency, mean
fetal viability, size, sex ratio, fetal body weight or external,
visceral or skeletal development. Tolclofos-methyl was not
teratogenic in this study at doses up to and including 3000 mg/kg bw
per day, which was toxic to dams. The NOAEL for maternal toxicity
was 300 mg/kg bw per day (Motoyama et al., 1991).
(f) Genotoxicity
The results of tests for the genotoxicity of tolclofos-methyl
are summarized in Table 2.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Six male albino Japanese rabbits received 500 mg of
tolclofos-methyl (purity, 97%) on clipped intact or abraded dorsal
skin for 4 h under an occlusive dressing. No signs of irritation
were seen at any of the application sites seven days after treatment
(Matsubara et al., 1978).
Table 2. Results of tests for the genotoxicity of tolclofos-methyl
End-point Test system Concentration Purity Results Reference
of tolclofos-methyl (%)
In vitro
Reverse S. typhimurium TA98, 10, 100, 500, 1000, 2000 97.0 Negativea,b Suzuki & Miyamoto,
mutation 100, 1535, 1537, 1538 µg/plate
Reverse S. typhimurium TA98, 10, 50, 100, 500, 1000, 98.7 Negativea,c Moriya et al., 1981
mutation 100, 1535, 1537, 1538 5000 µg/plate
E. coli WP2uvrA
Reverse B. subtilis H17 rec+, 1, 10, 100, 1000 µg/disc 97.0 Negatived Suzuki & Miyamoto,
mutation M45 rec- 1978
Reverse B. subtilis 20, 50, 100, 200, 500, 98.7 Negativee Moriya et al., 1981
mutation H17 rec+, M45 rec- 1000, 2000, 5000 µg/disc
Host-mediated S. typhimurium in 870, 1750 mg/kg 97.0 Negativef Suzuki & Miyamoto,
assay male ICR mice 1978
Chromosomal Chinese hamster 10, 20, 40 µg/ml 96.6 Negativea,g Kogiso et al., 1990
aberration K1 ovary cells 37.5, 75, 150 µg/ml
Unscheduled Male Sprague-Dawley 0.3, 1, 3, 10, 20, 40 96.6 Negativea,i Hara et al., 1990
DNA synthesis rat hepatocytes µg/mlh
Unscheduled Human carcinoma 0.3, 3, 30, 300 µg/ml NR Negativej Monaco & Nunziata,
DNA synthesis cells (HeLa) 1981
Gene mutation Chinese hamster 1.5, 15, 150, 1500 µg/ml NR Negativea,k Monaco & Nunziata,
V79 lung cells 1981
Table 2 (contd)
End-point Test system Concentration Purity Results Reference
of tolclofos-methyl (%)
In vivo
Chromosomal Male ICR mice 500, 1000, 2000, 99.8 Negativei Hara & Suzuki, 1981
aberration 4000 mg/kg i.p.h
Dominant Male Sprague- 62.5, 208.3, 625.0 NR Negativej Brusick, 1981
lethal Dawley rats mg/kg orally
mutation
NR, not reported
a With and without metabolic activation.
b Positive controls ( N-methyl- N'-nitro- N-nitrosoguanidine and 2-acetylaminofluorene) yielded expected positive
results.
c Several positive controls yielded expected positive results.
d Positive control ( N-methyl- N'-nitro- N-nitrosoguanidine) yielded expected positive results.
e Positive (mitomycin C) and negative (Kanamycin) controls yielded expected results.
f Positive control ( N-nitrosodimethylamine) yielded expected positive results.
g Positive controls (mitomycin C and cyclophosphamide) yielded expected positive results.
h At 40 mg/ml, cell viability was less than 20%.
i Positive control (2-acetylaminofluorene) yielded expected positive results.
j Positive controls (methyl methanesulfonate and urethane) yield expected positive results.
k Positive controls (methyl methanesulfonate and N-nitrosodimethylamine) yielded expected positive results.
l At 2000 and 4000 mg/kg bw there was > 50% mortality at 48 h.
m Positive control (cyclophosphamide) yielded expected positive results.
n Positive control (triethylenemelamine) yielded expected positive results.
Groups of six male New Zealand white rabbits received 500 mg of
either a '50% wettable power' or '10% dust' moistened with saline on
1-inch2 (6.5-cm2) sites on the clipped dorsal intact or abraded
skin for 24 h under an occlusive dressing. No irritation, such as
erythema and oedema, was observed (Hara et al., 1981a,b).
Two male and one female New Zealand white rabbits received 0.5
ml of '50% flowable' tolclofos-methyl on 1 inch2 (6.5 cm2) of
clipped dorsal intact or abraded skin for 4 h under an occlusive
dressing. No irritation was observed (Nakanishi et al., 1989).
Each of eight male albino Japanese rabbits received 50 mg of
tolclofos-methyl (purity, 97%) in one eye. Five minutes after the
application, the treated eyes of five animals were flushed with 300
ml saline for 2 min. The treated eyes of the remaining animals were
similarly flushed 24 h after treatment. There were no corneal,
conjunctival or iridal effects up to seven days after treatment
(Matsubara et al., 1978).
Groups of nine male New Zealand white rabbits received 100 mg
of either a '50% wettable power' or '10% dust' in one eye; 30 s
after the application, the treated eyes of three animals per group
were flushed with 300 ml lukewarm water for 1 min. Slight congestion
of the iris was observed 24 h after application. Slight to moderate
hyperaemia and slight chemosis and/or discharge in conjunctiva were
also observed 1-48 h after application of the '50% wettable power'
to unwashed eyes. These changes had disappeared by 72 h after
application in all animals. No ocular lesions were found in the
washed eyes. The irritation potency of this formulation was judged
to be mild. Slight conjunctival hyperaemia and/or chemosis were
observed in animals with unwashed eyes and in one with washed eyes
1-24 h after application of the '10% dust'. There were no other
signs of irritation at any time. The formulation was classified as
minimally irritating to eyes (Hara et al., 1981a,b).
One male and two female New Zealand white rabbits received 0.1
ml of '50% flowable' tolclofos-methyl in one eye. Slight redness was
observed in conjunctiva after application, which disappeared within
24 h (Nakanishi et al., 1989).
The skin-sensitizing potential of tolclofos-methyl (purity,
97%) was assessed in guinea-pigs by the Landsteiner-Draize method.
Groups of 10 male Hartley guinea-pigs were given 10 intradermal
injections of 1 or 5% tolclofos-methyl (0.05 or 0.1 ml) in corn oil
at intervals of two to three days. Two weeks after the final
induction, the animals were challenged at a fresh site with 0.05-ml
intradermal injections of tolclofos-methyl at the same
concentrations used for induction. Negative control animals for both
dose groups were given the challenge injection only. Positive
control animals were treated with 0.05% 2,4-dinitrochlorobenzene
three times before challenge. Slight erythema and swelling were
observed in two animals after challenge treatment with 1%
tolclofos-methyl, in three after challenge with 5%, and in one
animal in each negative control group. Moderate erythema and/or
swelling was observed in the animals treated with
2,4-dinitrochlorobenzene. It was concluded that tolclofos-methyl did
not sensitize skin in this study (Matsubara et al., 1980).
The skin sensitizing potential of a '50% wettable powder' and a
'10% dust' of tolchlofos-methyl was tested in guinea-pigs by the
Buehler method. Groups of 10 male Hartley guinea-pigs received 500
mg of one formulation, slightly moistened with water, on clipped
dorsal skin under an occlusive dressing for 24 h. Induction was
performed 10 times at two- to three-day intervals.Positive control
animals were similarly treated with 0.5% 2,4-dinitro-chlorobenzene.
Negative controls were not subjected to the induction treatment. Two
weeks after the final induction, the test animals and positive
controls were challenged as in the sensitizing treatment. No skin
reactions were observed in the negative control or treated animals.
Slight to severe erythema and swelling were observed in the positive
controls. The formulations were considered not to sensitize skin
(Hara et al., 1981c,d).
The skin sensitizing potential of a '50% flowable formulation'
was tested in guinea-pigs by the Buehler method. Ten male Hartley
guinea-pigs received 0.5 ml of the formulation for 6 h once a week
for three weeks on the clipped dorsal skin under an occlusive
dressing. Positive control animals were similarly treated with 0.5%
2,4-dinitrochlorobenzene. Negative control animals were not
subjected to the induction treatment. Two weeks after the final
induction, animals were challenged as in the sensitizing treatment.
No skin reactions were observed in the negative control or treated
animals. Slight to severe erythema and swelling were observed in the
positive controls. The formulation was considered not to sensitize
skin (Nakanishi et al., 1990).
(ii) Delayed neuropathy
Groups of 10 Leghorn hens were administered 0 or 8000 mg/kg bw
tolclofos-methyl (purity, 97%) or 500 mg/kg bw tri- ortho-cresyl
phosphate orally in corn oil. After a 21-day observation period, a
second dose of vehicle or tolclofos-methyl was administered. Animals
were sacrificed 21 days after the second dose. There were no deaths
in the groups given the vehicle or tolclofos-methyl. Plasma
cholinesterase activity in the group treated with tolclofos-methyl
was decreased by nearly 50% eight days after the first dose but had
recovered to the pre-treatment level 21 days after dosing. Hens
treated with tolclofos-methyl had no signs of leg weakness or
paralysis and no histopathological changes in the nervous tissues.
Birds treated with tri- ortho-cresyl phosphate had the typical
clinical and histopathological signs of delayed polyneuropathy
(Okuno et al., 1982).
3. Observations in humans
The medical records of 20 workers in Japan were reviewed. All
workers had been engaged continuously in packaging operations since
manufacture of technical-grade tolclofos-methyl began in 1988, for
an average of 4 h/day. No occupation-related problems were observed
or reported. Plasma and erythrocyte cholinesterase activities were
not measured (Murayama, 1991).
Comments
Tolclofos-methyl is excreted rapidly in rats and mice,
predominantly in the urine; less than 1% of the dose was retained in
tissues after seven days. In both species, metabolism occurred
mainly by oxidation of P=S to P=O, oxidation of the 4-methyl group
and cleavage of the P-O-aryl and P-O-methyl linkages. There are four
main metabolites in mice, one of which is a glycine conjugate, and
four in rats, which are excreted as glucuronides.
Tolclofos-methyl had low acute toxicity when administered by
the oral, dermal, subcutaneous or intraperitoneal route. The overt
signs of acute toxicity are not typical of an anticholinesterase, as
no chromodaccryorrhoea, lachrymation or fasciculation was seen,
although some inhibition of plasma, erythrocyte and brain
cholinesterase activities was observed. WHO (1992) has classified
tolclofos-methyl as unlikely to present an acute hazard in normal
use.
In a nine-month study of toxicity in which mice were fed
tolclofos-methyl in the diet at 0, 10, 30, 100 or 3000 ppm, the
NOAEL was 100 ppm, equal to 12 mg/kg bw per day, on the basis of
inhibition of brain cholinesterase activity and effects on body
weight at 3000 ppm.
In a 32-34-day study of toxicity in which rats were fed diets
containing 0, 200, 1000, 5000 or 20 000 ppm, the NOAEL was 1000 ppm,
equal to 79 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity and increased relative kidney weight at 5000
ppm. In a 13-week study of toxicity in which rats were fed diets
containing 0, 100, 1000 or 10 000 ppm, the NOAEL was again 1000 ppm,
equal to 66 mg/kg bw per day, on the basis of effects on body, liver
and kidney weights at 10 000 ppm. In a 28-week study of toxicity in
which rats were fed dietary levels of 0, 300, 1000, 3000 or 10 000
ppm, the NOAEL was also 1000 ppm, equal to 65 mg/kg bw per day, on
the basis of histopathological changes in the livers of females at
3000 ppm.
In a 26-week dietary study in dogs fed levels of 0, 200, 600 or
2000 ppm, the NOAEL was 600 ppm, equal to 21 mg/kg bw per day, on
the basis of reduced body-weight gain, an increased serum level of
alkaline phosphatase and increased liver weight at 2000 ppm.
In a 52-week study of toxicity in dogs given dietary
concentrations of 0, 80, 400 or 2000 ppm, the NOAEL was 400 ppm,
equal to 11 mg/kg bw per day, on the basis of increased liver weight
(with hepatocytic hypertrophy), reduced body-weight gain and slight
anaemia at 2000 ppm.
In a 104-week study of toxicity and carcinogenicity in which
mice were given dietary concentrations of 0, 10, 50, 250 or 1000
ppm, the NOAEL was 50 ppm, equal to 6.5 mg/kg bw per day, on the
basis of reduced brain cholinesterase activity and increased
absolute and relative kidney weights at higher levels. There was no
evidence of carcinogenicity.
A 122-129-week study of toxicity and carcinogenicity was
performed in which rats were given dietary concentrations of 0, 100,
300 or 1000 ppm. The NOAEL was 1000 ppm, equal to 41 mg/kg bw per
day, on the basis of the absence of any significant finding. There
was no evidence of carcinogenicity.
In a three-generation study (two litters per generation) in
rats, tolclofos-methyl was given at dietary levels of 0, 100, 300 or
1000 ppm. The NOAEL was > 1000 ppm, equivalent to 100 mg/kg bw per
day, on the basis of the absence of any significant findings.
In a study of teratogenicity in which rats were given 0, 5, 15
or 50 mg/kg bw per day of tolclofos-methyl by gavage, the NOAEL was
50 mg/kg bw per day on the basis of the absence of any significant
findings. The study was not considered to be fully adequate because
the highest dose tested was not maternally toxic. A similar study
was conducted at levels of 0, 100, 300 or 1000 mg/kg bw per day. The
NOAEL was 300 mg/kg bw per day on the basis of reduced body-weight
gain in dams at 1000 mg/kg bw per day. There was no evidence of
teratogenicity.
A study of teratogenicity was conducted in rabbits given 0,
300, 1000 or 3000 mg/kg bw per day orally. The NOAEL for maternal
toxicity was 300 mg/kg bw per day on the basis of reduced
body-weight gain in dams at 1000 mg/kg bw per day. There was no
evidence of teratogenicity.
Tolclofos-methyl was studied in a wide range of tests for
genotoxicity in vivo and in vitro. The Meeting concluded that
the compound is not genotoxic.
Tolclofos-methyl did not cause delayed neuropathy in chickens.
The available observations in humans were considered by the
Meeting but did not directly contribute to an estimation of an ADI.
An ADI was established on the basis of a NOAEL of 50 ppm, equal
to 6.5 mg/kg bw per day, in the 104-week study of toxicity and
carcinogenicity study in mice, and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equal to 6.5 mg/kg bw per day (104-week study
of toxicity and carcinogenicity)
Rat: 1000 ppm, equal to 41 mg/kg bw per day (122/129-week
study of toxicity and carcinogenicity)
Rabbit: 300 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Dog: 400 ppm, equal to 11 mg/kg bw per day (52-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.07 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Brusick, D.J. (1981) Mutagenicity evaluation of S-3349 T.G. Lot No.
4, rat dominant lethal assay. Litton Bionetics Inc. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Colley, J., Welch, P.J., Heywood, R., Prentice, D.E., Cherry, C.P.,
Mullins, P.A., Gibson, W.A. & Almond, R.H. (1982) S-3349, toxicity
to rats by dietary administration for 4 weeks. Huntington Research
Centre Ltd. Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd, Osaka, Japan.
Cox, R.H. & Brusick, D.J. (1988) Chronic toxicity study in dogs with
S-3349. Hazleton Laboratories America Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Eschbach, J.C. & Hogan, G.K. (1981a) An acute inhalation toxicity
study of S-3349 50WP in the rat. Bio/dynamics Inc. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Eschbach, J.C. & Hogan, G.K. (1981b) An acute inhalation toxicity
study of S-3349 10d in the rat. Bio/dynamics Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Esumi, Y. & Yokoshima, T. (1989) Study on metabolism of
tolclofos-methyl in rats. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Gargus, J.L. (1986) 21-Day dermal toxicity study, in rabbits with
Rizolex, technical 97.7%. Hazleton Laboratories America Inc.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Hara, M. & Suzuki, H. (1981) In vivo chromosomal aberration test of
S-3349 on bone marrow cells of mice. Sumitomo Chemical Co., Ltd.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981a) Primary eye and skin
irritation tests of S3349 50% water-dispersible power in rabbits.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981b) Primary eye and skin
irritation tests of S-3349 10% dust formulation in rabbits. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981c) Skin sensitization test
of S3349 50% water-dispersible powder in guinea pigs. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981d) Skin sensitization test
of S3349 10% dust formulation in guinea pigs. Sumitomo Chemical Co.,
Ltd. Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd, Osaka, Japan.
Hara, M., Ota, M., Kogiso, S., Yoshitake, A. & Yamada, H. (1990) In
vitro unscheduled DNA synthesis (UDS) assay of Rizolex in rat
hepatocytes. Sumitomo Chemical Co., Ltd. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hardy, C.J., Jackson, G.C., Lewis, D.J. & Gopinath, C. (1986)
Technical Rezolex, acute inhalation toxicity in rats, 4-hour
exposure. Huntington Research Centre Ltd. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hiromori, T., Suzuki, T., Okuno, Y., Ito, S., Murakami, M., Kadota,
T. & Miyamoto, J. (1978) Six-month oral toxicity study of S-3349 in
rats. Sumitomo Chemical Co., Ltd. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hiromori, T., Misaki, Y., Ito, S., Kohda, A., Kato, T. & Yamada, H.
(1989a) Acute oral toxicity study of Rizolex 50 FL in rats. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Hiromori, T., Misaki, Y., Ito, S., Kohda, A., Kato, T. & Yamada, H.
(1989b) Acute dermal toxicity study of Rizolex 50 FL in rats.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Jackson, G.C., Hardy, C.J., Morrow, J. & Gopinath, C. (1990) Rizolex
50 FL, acute inhalation toxicity study in rats, 4-hour exposure.
Huntington Research Centre Ltd. Unpublished report submitted to WHO
by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kimura, J., Yosioka, K., Ozaki, K., Adachi, H., Seki, T., Matsuo, M
&, Yamada, H. (1990) 90-Day oral toxicity study of S-3349 in rats.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kogiso, S., Yamamoto, K., Hara, M., Yositake, A. & Yamada, H. (1990)
In vitro chromosomal aberration test of Rizolex in Chinese hamster
ovary cells (CHO-K1). Sumitomo Chemical Co., Ltd. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Krautter, G.R., Downs, J., Hoglan, N., Marsh, J.D. & Lawrence, L.J.
(1987) Metabolism of tolclofos-methyl in the rat. Pharmacology &
Toxicology Research Laboratory. Unpublished report submitted to WHO
by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Krautter, G.R., Downs, J., Hoglan, N., Marsh, J.D. & Lawrence, L.J.
(1988) Metabolism of tolclofos-methyl in the rat. Final report
amendment. Pharmacology & Toxicology Research Laboratory.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Matsubara, T., Hara, S. & Kadota, T. (1978) Eye and skin irritation
test of S-3349 in rabbits. Sumitomo Chemical Co., Ltd. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Matsubara, T., Hara, S., Suzuki, T., Kadota, T. & Miyamoto, J.
(1980) Skin sensitization test of S-3349 in guinea pigs. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Mihara, K., Ohkawa, H. & Miyamoto, J. (1981) Metabolism of
tolclofos-methyl in rats and mice. J. Pestic. Sci., 6, 65-74.
Miyamoto, J. (1985) Comments on toxic effects and minimum effect
level of S-3349 in chronic oral toxicity studies in rats. Sumitomo
Chemical Co., Ltd., Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Monaco, M. & Nunziata, A. (1981) Report of mutagenicity experiment
performed on the test substance S-3349, Sumitomo Chemical Co., Ltd.
Unpublished report from Centro Ricerca Farmaceutica, Pomezia, Italy.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Moore, M.R. (1993) Comments on the toxicological significance of
hepatocytic pigment and on the no-adverse-effect-level (NOAEL) in a
chronic toxicity study in dogs treated with S-3349 (Hazleton project
No. 343-176). Hazleton Washington Inc. Unpublished report submitted
to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Moriya, M., Ohta, T. & Shirasu, Y. (1981) S-3349, microbial
mutagenicity study. Institute of Environmental Toxicology.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Morseth, S.L., Burlew, J.A., Vargas, K.J., Lewis, S.A., Thakur, A.K.
& Phipps, N.G. (1987) Teratology study of S-3349 T.G. in rats.
Hazleton Laboratories America Inc. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Motoyama, M., Kashima, M. & Takahashi, M. (1991) Teratology study of
S-3349 in rabbits. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Murayama, F. (1991) A review on medical examination of factory
workers exposed to tolclofos-methyl. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Nakanishi, T., Kohda, A., Kato, T. & Yamada, H. (1989) Primary eye
and skin irritation tests with Rizolex 50 FL in rabbits. Sumitomo
Chemical Co., Ltd. Unpublished study, submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Nakanishi, T., Kohda, A., Kato, T. & Yamada, H. (1990) Skin
sensitization test of Rizolex 50 FL in guinea pigs. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Okuno, Y., Yamada, T., Hosokawa, S. & Miyamoto, J. (1982) Acute
delayed neurotoxicity study of S-3349 in hens. Sumitomo Chemical
Co., Ltd. Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd, Osaka, Japan.
Pence, D.H., Lemen, J.K. & Weatherholtz, W.M. (1978) Acute oral
toxicity study in male and female dogs, S-3349. Hazleton
Laboratories America Inc. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Weatherholtz, W.M., Kundzins, W., Alsaker, R.D., Brown,
H.R. & Greenspun, K.S. (1979a) Subacute dietary administration in
dogs, S-3349. Hazleton Laboratories America Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Pence, L.A., Durloo, R. & Twigg, C.J. (1979b)
Teratology study in rats, S-3349. Hazleton Laboratories America Inc.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Pence, D.H., Serota, D., Alsaker, R.D., Koka, M., Banas, D.A.,
Dawkins, B.G., Kundzins, W. & Hepner, K.E. (1982) Chronic toxicity
study in rat, S-3349. Hazleton Laboratories America Inc. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Phipps, N., Alsaker, R.D., Hepner, K.E. & Spicer, K.M.
(1985a) 104-Week cholinesterase activity study in male and female
rats, S-3349. Hazleton Laboratories America Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Hepner, K.E., Wolfe, G.W., Voelker, R.W. & Ulland, B.N.
(1985b) Three-generation reproduction study in rats, S-3349.
Hazleton Laboratories America Inc. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Satoh, R., Kashima, M., Satoh, H., Motoyama, M. & Ishikawa, A.
(1983) Twenty-four-month chronic toxicity study of S-3349 in
pulverized diet in mice. Nippon Experimental Medical Research
Institute. Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd, Osaka, Japan.
Segawa, T. (1978) Acute toxicity study of S-3349 in rats and mice.
Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981a) Acute oral toxicity study of S-3349 50% wettable
powder in mice. Hiroshima University School of Medicine. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981b) Acute oral toxicity study of S-3349 10% dust in
mice. Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981c) Acute dermal toxicity study of S-3349 50%
wettable powder in mice. Hiroshima University School of Medicine.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Segawa, T., (1981d) Acute dermal toxicity study of S-3349 10% dust
in mice. Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981e) Acute oral toxicity study of S-3349 50% wettable
powder in rats. Hiroshima University School of Medicine. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981f) Acute oral toxicity study of S-3349 10% dust in
rats. Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981g) Acute dermal toxicity study of S-3349 50%
wettable powder in rats. Hiroshima University School of Medicine.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Segawa, T. (1981h) Acute dermal toxicity study of S-3349 50%
wettable powder in rats. Hiroshima University School of Medicine.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Suzuki, H. & Miyamoto, J. (1978) Studies on mutagenicity of S-3349
with bacterial systems. Sumitomo Chemical Co., Ltd. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Suzuki, T., Okuno, Y., Hiromori, T., Ito, S., Murakami, M., Kadota,
T & Miyamoto, Y. (1978) Nine-month feeding study of S-3349 in mice.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
2. Toxicological studies
(a) Acute toxicity
Data on the acute toxicity of tolclofos-methyl (technical-grade
and formulations) in laboratory animals are summarized in Table 1.
Animals showed decreased spontaneous motor activity, dyspnoea,
piloerection, urinary incontinence and ataxia. Recovery was complete
by day 10. Brain cholinesterase activity was lower 16 days after
treatment in dogs given 1000 mg/kg bw than in animals given lower
doses; however, as no control data were reported, conclusions cannot
be drawn. No treatment-related gross changes were seen at necropsy
in any species.
Table 1. Acute toxicity of technical-grade and formulated tolclofos-methyl in laboratory animals
Species Strain Sex Route Formulation LD50 Reference
(purity) (mg/kg bw)
Mouse dd M Oral Technical (97%) 3500 Segawa, 1978
F 3600
Mouse ICR M&F Oral 50% wettable > 5000 Segawa, 1981a
powder (98%)
Mouse ICR M&F Oral 10% dust (98.8%) > 5000 Segawa, 1981b
Mouse dd M&F Dermal Technical (97%) > 5000 Segawa, 1978
Mouse ICR M&F Dermal 50% wettable > 5000 Segawa, 1981c
powder (98%)
Mouse ICR M&F Dermal 10% dust (98.8%) > 5000 Segawa, 1981d
Mouse dd M Intraperitoneal Technical (97%) 1070 Segawa, 1978
F 1260
Mouse dd M&F Subcutaneous Technical (97%) > 5000 Segawa, 1978
Rat SD M&F Oral Technical (97%) approx. 5000 Segawa, 1978
Rat SD M&F Oral 50% wettable > 5000 Segawa, 1981e
powder (98%)
Rat SD M&F Oral 10% dust (98.8%) > 5000 Segawa, 1981f
Rat SD M&F Oral 50% flowable > 5000 Hiromori et al.,
1989a
Rat SD M&F Dermal Technical (97%) > 5000 Segawa, 1978
Rat SD M&F Dermal 50% wettable > 5000 Segawa, 1981g
powder (98%)
Rat SD M&F Dermal 10% dust (98.8%) > 5000 Segawa, 1981h
Rat NR M&F Dermal 50% flowable > 2000 Hiromori et al.,
1989b
Table 1 (contd)
Species Strain Sex Route Formulation LD50 Reference
(purity) (mg/kg bw)
Rat SD M Intraperitoneal Technical (97%) 5000 Segawa, 1978
F 4900
Rat SD M&F Subcutaneous Technical (97%) > 5000 Segawa, 1978
Rat Wistar M&F Inhalation Technical (97.7%) > 3.32 mg/la Hardy et al., 1986
Rat SD M&F Inhalation 50% wettable > 1.9 mg/l Eschbach &
powder (98%) Hogan, 1981a
Rat SD M&F Inhalation 10% dust (98.8%) > 1.8 mg/l Eschbach &
Hogan, 1981b
Rat SD M&F Inhalation 50% flowable > 1.07 mg/la Jackson et al., 1990
Dog NR M&F Oral Technical (98.7%) > 1000 Pence et al., 1978
SD, Sprague-Dawley; M, male; F, female; NR, not reported
a 4-h whole-body exposure
(b) Short-term toxicity
Mice
Groups of 15 male and 15 female ddY mice were fed diets
containing tolclofos-methyl (purity, 97%) at 0, 10, 30, 100 or 3000
ppm, equal to 1.2, 3.8, 12 or 510 mg/kg bw per day for males and
1.4, 4.1, 14 or 560 mg/kg bw per day for females, for nine months.
Three satellite groups of five males and five females each were
similarly treated and killed at 2, 4 and 13 weeks for determination
of cholinesterase activity. Animals were observed daily for
mortality and signs of toxicity; at the end of exposure, an
ophthalmological examination and clinical chemical analyses
(including plasma and erythrocyte cholinesterase) were undertaken,
and brain cholinesterase activity was measured. Most organs were
weighed and examined microscopically. There were no overt signs of
toxicity and no deaths. Body weights were reduced by about 20% and
body-weight gain by about 35% in animals of each sex at 3000 ppm,
but at this dose food consumption was increased in males and females
and water intake was increased in males. No treatment-related
ophthalmological changes were observed, and haematological
parameters were unaffected by treatment. The cholesterol level was
increased by 35% in females at 3000 ppm. A dose-related decrease in
plasma cholinesterase activity was found in animals of each sex,
with activities that were 63-94% of the control values in mice at 30
ppm, 30-56% at 100 ppm and 4-35% (61% in one case) at 3000 ppm. At
the high dose, the activity reached a plateau after four weeks.
Erythrocyte cholinesterase activity was decreased to 39-46% of the
control level in males at 3000 ppm at weeks 13 and 40 and to 57-75%
of the control level in females at this dose at all time points.
Brain cholinesterase activity was unaffected by treatment in females
but was reduced by about 24% in males at 3000 ppm at weeks 13 and
40. Organ weights in the 3000-ppm group were reduced, but neither
gross nor histopathological changes attributable to treatment were
observed. The NOAEL was 100 ppm, equal to 12 mg/kg per day, on the
basis of inhibition of brain cholinesterase activity and an effect
on body weight at the highest dose (Suzuki et al., 1978).
Rats
Groups of 10 male and 10 female Sprague-Dawley rats were fed
diets containing tolclofos-methyl (purity, 97.9%) at 0, 200, 1000,
5000 or 20 000 ppm for 32-34 days. Animals were observed twice daily
for mortality and clinical signs, and food consumption and body
weight were recorded weekly. Ophthalmoscopic examination, standard
haematological tests and clinical chemical analyses were performed
at week 4, and certain tests were repeated at termination. Plasma
and erythrocyte cholinesterase activities were determined in the
control and high-dose groups at week 4 and in all animals at
termination, when brain cholinesterase activity was also measured.
At terminal sacrifice, relevant organs were weighed and most organs
were examined microscopically. There were no treatment-related
deaths. Reductions in food consumption (-16% in males and -6% in
females) and body-weight gain (-45% in males and -37% in females)
were observed at 20 000 ppm. Body-weight gain was slightly reduced
in animals of each sex at 5000 ppm during week 1. Haematological
parameters were unaffected by treatment. Cholesterol levels were
increased (by 116-138%) in animals of each sex at 20 000 ppm, and
total protein and albumin levels were slightly higher in males in
this group. Other differences between treated and untreated animals
were detected, but the values were within the normal range. Plasma
cholinesterase activity was reduced by 14% in males and by 50% in
females at 20 000 ppm. Erythrocyte cholinesterase activity was
reduced by 9-19% in animals of each sex at 5000 and 20 000 ppm;
however, no clear dose-response relationship was seen. Brain
cholinesterase activity was lower (by 12-31%) than that in controls
in males in all treated groups and in females (by 20%) at 5000 and
20 000 ppm. Although the reduction was statistically significant in
all male groups, no clear dose-response relationship was found, and
the activity in controls was slightly higher than usual. There were
no ophthalmoscopic alterations or gross changes at necropsy. The
relative weights of livers were increased (by 12%) in females at
5000 ppm and in males and females at 20 000 ppm (by 27% in males and
39% in females). Hypertrophy of hepatocytes was observed in animals
of each sex at 20 000 ppm. The relative weights of the kidneys were
slightly increased in animals of each sex at 5000 and 20 000 ppm,
but no treatment-related histopathological changes were seen. The
NOAEL was 1000 ppm, equal to 79 mg/kg bw per day, on the basis of
increased relative kidney weights and brain cholinesterase activity
at higher doses (Colley et al., 1982).
Groups of 12 male and 12 female Sprague-Dawley rats were fed
diets containing tolclofos-methyl (96.6% pure) at 0, 100, 1000 or 10
000 ppm, equal to 6.5, 66 or 653 mg/kg bw per day for males and 7.1,
71 or 696 mg/kg bw per day for females, for 13 weeks. Animals were
observed daily for mortality and clinical signs, and body weight and
food and water consumption were determined weekly. Ophthalmological
analysis, urinalysis, standard haematological tests, clinical
chemical analyses and measurement of plasma, erythrocyte and brain
cholinesterase activities were undertaken at termination, when
relevant organs were weighed and most organs were examined
microscopically. No deaths and no significant abnormal clinical or
ophthalmological signs were noted during the study. Depression of
body-weight gain of males (-20%) and females (-15%) at 10 000 ppm
was seen throughout the treatment period and was associated with
similar decreases in food and water consumption. A slight decrease
in food consumption was noted in females at 1000 ppm during week 1.
Decreased activities of plasma (16-53%), brain (8-9%) and
erythrocyte (19-20%) cholinesterase were noted in animals of each
sex at 10 000 ppm; a minimal decrease in erythrocyte cholinesterase
activity (10%) was also seen in females at 1000 ppm. Liver weight
was increased in animals of each sex at 10 000 ppm, and this was
found at histopathological examination to be associated with
hypertrophy of hepatocytes. Changes in some clinical chemical
parameters were observed at 10 000 ppm, which included increased
levels of cholesterol (48% in males, 102% in females) and
phospholipids (37% in males, 64% in females) and slightly increased
alpha2- and ß-globulin levels in animals of each sex. Other
changes, although statistically significant, were minor and of
questionable biological significance. At 10 000 ppm, increased
relative kidney weights were seen in animals of each sex, increased
blood urea nitrogen levels were seen in males only and decreased
urinary pH was seen in males and females. At 1000 ppm, relative
kidney weights were slightly increased in females and relative liver
weights in males. The Meeting considered that the increased weight
ratios observed at 1000 ppm were not biologically relevant and that
the NOAEL was 1000 ppm, equal to 66 mg/kg bw per day (Kimura et
al., 1990).
Groups of 15 male and 15 female Sprague-Dawley rats were fed
diets containing tolclofos-methyl (purity, 97%) at 0, 300, 1000,
3000 or 10 000 ppm, equal to 16, 51, 164 or 540 mg/kg bw per day in
males and 18, 65, 184 or 623 mg/kg bw per day in females, for 28
weeks. Animals were observed daily for clinical signs, and body
weight and food consumption were measured weekly. Ophthalmologic
examinations were carried out after three months and at termination;
standard haematological tests and clinical chemical analyses were
performed at termination. Urinalysis was carried out in 12 animals
per group after three months and at termination. Plasma and
erythrocyte cholinesterase activities were determined after 2, 4 and
13 weeks, and at termination; brain cholinesterase was determined
only at termination, when relevant organs were weighed and several
were examined histologically.
There were no overt signs of toxicity and no treatment-related
deaths. Although the body weights of animals of each sex at 10 000
ppm were lower than those of controls throughout the study, their
initial body weights were also statistically significantly lower.
Body-weight gain was significantly reduced (by 18%) in females at 10
000 ppm. Food consumption was unaffected by treatment. Treatment did
not affect ophthalmologic, urinary, haematological or clinical
chemical parameters. Plasma cholinesterase activity was lower (by
23-41%) in females at 10 000 ppm than in controls at weeks 2, 4, 13
and 28. Erythrocyte cholinesterase activity was highly variable and
was reduced by 15-19% in comparison with controls in males at 10 000
ppm at weeks 2, 4 and 28. Brain cholinesterase activity was
unaffected by treatment. Kidney and liver weights were slightly
increased in animals of each sex at 10 000 ppm and in females at
1000 and 3000 ppm, but the biological significance of these findings
was questionable. No gross abnormalities were seen at necropsy.
Bile-duct proliferation was found in the livers of 3/15 females at
10 000 ppm and oval-cell proliferation was seen in 2/15 females at
3000 ppm and 3/15 at 10 000 ppm. No histopathological changes were
seen in the kidneys. The NOAEL was 1000 ppm, equal to 65 mg/kg bw
per day, on the basis of histopathological changes in the livers of
females at higher doses (Hiromori et al., 1978).
Rabbits
Groups of five male and five female New Zealand white rabbits
received tolclofos-methyl (purity, 97.7%) in acetone on the skin at
doses of 0, 30, 300 or 1000 mg/kg bw per day for 6 h per day, on
five days per week for 21 days. There were no deaths and no
treatment-related clinical signs; neither body weight nor food
consumption was affected by treatment. Dermal irritation (slight
erythema) was seen in some animals in all treated groups from day 6.
Eosinophil counts were increased in males at 1000 mg/kg bw per day,
and the level of inorganic phosphorus was decreased (by 14-20%) in
animals of each sex at this dose. Brain cholinesterase activity was
about 15% lower than that in controls in males and about 15% higher
in females at 300 and 1000 mg/kg bw per day. These changes were
considered not to be of biological significance. Erythrocyte
cholinesterase activity was lower than in controls in males at the
same two doses, but there was no dose-effect relationship. Plasma
cholinesterase activity was lower (by 22-29%) than in controls in
animals of each sex at 300 and 1000 mg/kg bw per day. The relative
weights of the kidneys were increased (by 20%) in females at 1000
mg/kg bw per day. Macroscopic findings consisted of accumulation of
the compound on treated skin; microscopic examination revealed
hyperkeratosis, acanthosis and subepidermal pleocellular
infiltration of the skin (Gargus, 1986).
Dogs
Groups of six male and six female beagle dogs were fed diets
containing tolclofos-methyl (purity, 98.7%) at 0, 200, 600 or 2000
ppm, equal to 7.4, 23 or 69 mg/kg bw per day in males and 4.1, 21 or
65 mg/kg bw per day in females, for 26 weeks. Animals were observed
daily for mortality and weekly for clinical signs, body weight and
food consumption. Ophthalmoscopic examinations were performed before
treatment and at termination of the study. Standard haematological
tests and clinical chemical analyses were performed at regular
intervals until termination. Erythrocyte and plasma cholinesterase
activities were determined at weeks 2, 4, 8, 13 and 26. Brain
cholinesterase was determined at termination, when relevant organs
were weighed and most were examined histologically. There were no
deaths and no overt signs of toxicity. Body-weight gain was reduced
by 54% in males and by 46% in females at 2000 ppm, although food
consumption was unaffected by treatment. There were no
treatment-related ocular changes. Although the haematocrit was
within the normal range, decreased values in comparison with
controls (-11% in males and -10% in females) were observed at 2000
ppm. The mean corpuscular volume and mean corpuscular haemoglobin
concentration were not affected by treatment. Alkaline phosphatase
activity was increased by 139-295% in animals of each sex at 2000
ppm throughout the study. Total bilirubin was not increased in
treated animals. At 2000 ppm, plasma cholinesterase activity was
decreased by 19-26% in females throughout the study, but no
significant decreases were seen in erythrocyte cholinesterase
activity in animals of each sex or in plasma cholinesterase activity
in males. Brain cholinesterase activity was unaffected by treatment.
Urinalysis gave unremarkable results, and there were no gross
changes at necropsy. Liver weights were increased (by 59% in males
and 43% in females) at 2000 ppm, but there were no concomitant
histological changes, and there were no treatment-related
histopathological changes. The NOAEL was 600 ppm, equal to 21 mg/kg
bw per day, on the basis of increased liver weights, reduced
body-weight gain and increased alkaline phosphatase activity at 2000
ppm (Pence et al., 1979a).
Groups of six male and six female beagle dogs were fed diets
containing tolclofos-methyl (purity, 96.7%) at 0, 80, 400 or 2000
ppm, for 52 weeks. Animals were observed daily for mortality and
weekly for clinical signs. Ophthalmoscopic examinations were
performed before treatment and at weeks 26 and 52. Standard
haematological tests, clinical chemical analyses and determinations
of plasma and erythrocyte cholinesterase activities were performed
one week before treatment and at weeks 13, 26 and 52. Brain
cholinesterase was determined at termination, when relevant organs
were weighed and most were examined histologically. No clinical
signs of toxicity were observed. Decreased food consumption (-9% in
males and -16% in females) and body-weight gain (-32% in males and
-54% in females), slightly lower erythrocyte counts (-8% in males
and -20% in females), haematocrit value (-7% in males and -17% in
females) and haemoglobin concentration (-8% in males and -7% in
females), slightly decreased albumin and total protein levels
(significantly at some time points) and elevated alkaline
phosphatase levels (> 200%) were found at 2000 ppm.
Plasma cholinesterase activity was slightly, but not
significantly lower than that in controls in females at the highest
dose; erythrocyte and brain cholinesterase activities were not
affected by the treatment. No treatment-related ophthalmoscopic
alterations were observed. Increases in mean liver weights, both
absolute (+32% in males and +61% in females) and relative (+52% in
males and +74% in females), were seen in dogs at 2000 ppm.
Microscopic examination of the livers revealed increased incidences
of hepatocytic hypertrophy and intracytoplasmic homogeneous material
and an increased amount of hepatocytic pigment in these animals. The
amount of this pigment was also increased at 400 ppm, and it was
present in control animals. Its nature has not been elucidated, but
it does not contain iron or bilirubin. These dogs also had decreased
absolute (-46%) and relative (-40%) weights of the prostate, and the
absolute and relative weights of the pancreas were increased by
16-27% and 28-49%, respectively. The NOAEL was 400 ppm, equal to 11
mg/kg bw per day, on the basis of reduced body-weight gain,
increased liver weights (with hepatic hypertrophy) and slight
anaemia at 2000 ppm (Cox et al., 1988; Moore, 1993).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 70 male and 70 female B6C3F1 mice were fed diets
containing tolclofos-methyl (purity, 94.3%) at 0, 10, 50, 250 or
1000 ppm, equal to 1.3, 6.5, 32 or 134 mg/kg bw per day in males and
1.3, 6.8, 34 or 137 mg/kg bw per day in females, for up to 104
weeks. Animals were observed twice daily for clinical symptoms and
mortality. Body weight was measured weekly during the first 13 weeks
and then once every four weeks. Food intake was measured weekly.
Standard urinalysis was performed in 10 animals from each group at
6, 12 and 18 months and in all survivors at termination of the
study. Standard haematological tests were performed in 10 animals
from each group at 12 months and in all survivors at termination.
Standard clinical chemical analyses and measurements of serum,
erythrocyte and brain cholinesterase activities were done in 10
animals from each group at 6 and 12 months and in all animals at
termination. Animals in the control and 1000-ppm groups were
subjected to ophthalmologic examination at 12 and 24 months. Ten
animals from each group were necropsied at 6 and at 12 months, at
termination or when found dead or moribund. Relevant organs were
weighed and examined microscopically.
There were no treatment-related effects on mortality and no
overt signs of toxicity, including ophthalmologic changes.
Body-weight gain was slightly reduced in females at 1000 ppm up to
week 52; food consumption was also slightly decreased in this group
after 52 and 104 weeks. Food conversion efficiency, water intake,
urinary parameters and the haematological profile were unaffected by
treatment. Serum cholinesterase activity was decreased in animals of
each sex at 250 ppm (by 25-52%) and at 1000 ppm (by 43-81%) at both
times, and erythrocyte cholinesterase activity was decreased by
11-28% at 250 ppm and by 13-47% at 1000 ppm; inhibition of both
enzymes was greater at termination. A dose-related decrease was
found in brain cholinesterase activity in females (by 7-24% at 250
ppm and 9-33% at 1000 ppm) and males (by 10-13% at 250 ppm and
17-26% at 1000 ppm). Serum and brain, but not erythrocyte,
cholinesterase activities were lower (by 12 and 18%, respectively)
in females at 50 ppm at week 28 than in controls, which had higher
levels than at week 52 and at termination. Glucose levels were
increased in males and were slightly increased in females at 1000
ppm at week 104. Absolute and/or relative kidney weights were
increased in animals of each sex at 1000 ppm and in males at 250 ppm
at week 52, and in females at 250 ppm at week 104. The weights of
the thymus were decreased in females at 1000 ppm at weeks 52 and
104, and pituitary weights were increased in females at this dose at
week 104. No treatment-related changes were seen at necropsy or on
histopathological examination at any time. Tolclofos-methyl was not
found to be carcinogenic. The NOAEL was 50 ppm, equal to 6.5 mg/kg
bw per day, on the basis of reduced brain cholinesterase activity
and increased absolute and relative kidney weights at higher doses
(Satoh et al., 1983).
Rats
Groups of 65 male and 65 female Fischer 344 rats were fed diets
containing tolclofos-methyl (purity, 94.9-98.7%) at 0, 100, 300 or
1000 ppm for 122 weeks (males) or 129 weeks (females). At week 52,
10 males and 10 females from each group were killed. Animals were
observed twice a day for mortality and signs of morbidity. Body
weight and food consumption were recorded weekly (weeks 0-26), every
two weeks (weeks 27-52) or every four weeks until termination.
Ophthalmoscopic examinations were performed every 26 weeks and at
termination. Standard haematological tests, clinical chemical
analyses and measurements of erythrocyte and plasma cholinesterase
activities were conducted in 10 animals of each sex in each group
one week before treatment, at weeks 4, 13, 26, 52, 78 and 104 and at
termination. Brain cholinesterase activity was determined in 10
animals of each sex per group at week 52 and at termination. All
animals killed or found dead were necropsied, and relevant organs
were weighed; most organs were then examined histologically.
There were no treatment-related effects on mortality and no
overt signs of toxicity. A slight decrease in body-weight gain was
observed in males at 1000 ppm. Food consumption and food conversion
efficiency were unaffected by treatment, as was the haematological
profile. There was a dose-related trend to decreased alkaline
phosphatase activity (by 9-46%) in all treated groups: The activity
was significantly decreased in males at 1000 ppm throughout the
study, in males at 300 ppm from week 13, and occasionally in the
other treated groups. Brain cholinesterase activity was decreased in
all treated groups at the time of the interim sacrifice, but there
was no dose-response relationship. At the time of terminal
sacrifice, brain cholinesterase activity was reduced in males at the
middle dose (by 52%) and in females at the low (by 57%) and middle
doses (by 49%), but not in animals at the high dose. Erythrocyte and
plasma cholinesterase activities were also inconsistently lower in
treated groups, although no dose-response relationship was seen.
Urinalysis revealed no remarkable findings, organ weights were
unaffected by treatment, and there were no treatment-related gross
changes at necropsy. Toclofos-methyl was not carcinogenic in this
study. No NOAEL could be identified, given the variability of the
data on cholinesterase activity (Pence et al., 1982).
Groups of 30 male and 30 female Fischer 344 rats were fed diets
containing tolclofos-methyl (purity, 98.3-97.3%) at 0, 100, 300 or
1000 ppm for 104 weeks, and brain, erythrocyte and plasma
cholinesterase activities were assessed. Ten males and 10 females
from each group were sacrificed at week 52. Animals were observed
daily for mortality, and they were examined for body weight, food
consumption and gross signs of toxicity weekly until week 26, every
two weeks during weeks 26-52 and every four weeks during weeks
52-104. Blood samples were taken for determination of plasma and
erythrocyte cholinesterase activities at initiation of the study, at
weeks 5, 14, 27, 53, 79 and at termination. Brain cholinesterase
activity was determined at week 52 and at termination.
There were no treatment-related effects on survival, and the
incidences of clinical signs and palpable tissue masses were
comparable among the groups. Mean plasma cholinesterase activities
were slightly decreased (by < 25%) in males when compared with
controls at 300 and 1000 ppm at weeks 27 (statistically
significant), 53, 79 and 105. Mean erythrocyte and brain
cholinesterase activities were comparable among the groups
throughout the study. Plasma cholinesterase activity was reduced (by
< 20%) at certain times in treated animals but no dose-response
relationship was found. Gross pathological findings were unrelated
to treatment. Doses < 1000 ppm did not depress erythrocyte or
brain cholinesterase activity or induce gross pathological effects
(Miyamoto, 1985; Pence et al., 1985a).
The combined NOAEL in these two studies was 1000 ppm, equal to
41 mg/kg bw per day, on the basis of the absence of any significant
findings.
(d) Reproductive toxicity
Rats
A three-generation study of reproductive toxicity was performed
in Sprague-Dawley rats fed diets containing tolclofos-methyl
(purity, 97.9-98.7% pure) at 0, 100, 300 or 1000 ppm, equivalent to
10, 30 or 100 mg/kg bw per day, with two litters per generation. The
F0 generation consisted of 30 rats of each sex per group. The
F1a, F2a and F3a generations were sacrificed at weaning and
underwent gross necropsy. Five rats of each sex in each group were
selected at weaning from the F1b, F2b and Fn3b litters for
histopathological examination, and 25 rats of each sex from each
group were selected from the F1b and F2b litters to breed the
following generation. A 15-week growth period was allowed before
mating of the 'a' litters, and a minimal 10-day rest period was
allowed between weaning of the 'a' litters and mating to produce the
'b' litters. After birth of the 'b' litter, all parental animals
were necropsied.
There were no treatment-related deaths or overt signs of
toxicity. Pregnancy rates were low at times in all groups, but the
pattern was not related to dose. The lowest pregnancy rate (52%) was
observed in the F1b generation at the middle dose. Pregnancy rates
were below 70% in three of the six generations in the controls, in
two generations at the low dose, in four generations at the middle
dose and in one generation at the high dose. The body weights of
F2a and F2b pups at 300 and 1000 ppm were lower than those of
controls at the start of the growth period; however, these
reductions were not dose-related, and the growth rates and body
weights in other groups were comparable throughout the study. There
were no treatment-related gross changes at necropsy in any group.
The weights of the ovaries were increased in non-pregnant adult
F2b females at 1000 ppm, but this was not associated with
histomorphological alterations. There was an increased incidence
(not statistically significant) of fine cytoplasmic vacuolation of
the adrenal cortex in adult F2b females at 1000 ppm that became
pregnant at both matings. The etiology and toxicological
significance of this finding, given its occurrence in the controls,
were unknown. There was no effect on reproductive parameters or on
offspring survival and development in any litter, and there were no
treatment-related gross or histopathological findings in the
litters. The NOAEL for reproductive toxicity was 1000 ppm
(equivalent to 100 mg/kg bw per day) on the basis of the absence of
significant findings at any dose level (Pence et al., 1985b).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 21-26 pregnant Fischer 344 rats were administered 0,
5, 15 or 50 mg/kg bw per day tolclofos-methyl (purity, 94.9%; dose
adjusted to 100%) in 0.5% w/v methylcellulose by gavage on days 6-15
of gestation. There were no overt signs of toxicity, and body-weight
gain and food consumption were unaffected by treatment. There were
no treatment-related gross lesions in the dams at necropsy.
Pregnancy rates were slightly higher in all treated groups (77-87%)
than in controls (70%). There were no treatment-related changes in
the number of fetal deaths, fetal viability or size or the
incidences of visceral or skeletal anomalies or variations.
Tolclofos-methyl was not embryotoxic, fetotoxic or teratogenic in
rats at doses up to and including 50 mg/kg bw per day. It should be
noted, however, that the highest dose was not maternally toxic
(Pence et al., 1979b).
Groups of 23 pregnant Sprague-Dawley rats were administered 0,
100, 300 or 1000 mg/kg bw per day tolclofos-methyl (purity, 96.7%)
in 0.5% methyl cellulose orally on days 6-15 of gestation. On day 20
of gestation, all animals were sacrificed and caesarean section was
performed. Rats were observed daily for mortality and clinical signs
and weighed. At termination, dams were observed for gross visceral
abnormalities, and their uteri were weighed and examined for
implantations and resorptions. Live fetuses were weighed, sexed and
examined for external, visceral and skeletal abnormalities. All dams
survived to the day of scheduled sacrifice with no clinical signs of
toxicity. Mean body-weight gain in animals at 100 and 1000 mg/kg bw
per day was significantly less than in controls on days 6-11. Both
mean body-weight gain (days 6-16) and mean net body-weight gain
(days 0-20) in rats at 1000 mg/kg bw per day were lower (by 10 and
14%, respectively) than the corresponding control value. In
addition, mean food consumption in animals at 1000 mg/kg bw per day
on days 6-16 and 16-20 was slightly below the control value. There
were no treatment-related differences in implantation efficiencies,
and mean fetal viability, sex ratio and fetal body weight were
similar in all groups. The number of fetuses with unossified fifth
and/or sixth sternebrae was significantly greater in the 1000-mg/kg
bw per day group than in the control group; however, the incidence
of total fetal skeletal variations was similar in all groups. Other
variations in development were not related to dose. Two to three
malformed fetuses were found in each group, but neither the type nor
the frequency of malformations indicated a teratogenic or
embryotoxic response. Tolclofos-methyl was found to be neither
teratogenic nor embryotoxic in this study at doses up to and
including 1000 mg/kg bw per day. This dose was slightly toxic to the
dams, as indicated by the lower body-weight gain. The NOAEL for
maternal toxicity was 300 mg/kg bw per day (Morseth et al., 1987).
Rabbits
Groups of 13-17 pregnant New Zealand white rabbits were
administered 0, 300, 1000 or 3000 mg/kg bw per day tolclofos-methyl
(purity, 98.7% pure) in 5% carboxymethylcellulose orally on days
6-18 of gestation. The animals were observed daily for mortality and
clinical signs and were weighed. At termination, dams were observed
for gross visceral abnormalities, and their uteri were weighed and
examined for implantations and resorptions. Live fetuses were
weighed, sexed and examined for external, visceral and skeletal
abnormalities. One rabbit at 3000 mg/kg bw per day died on day 14 of
gestation of an undetermined cause. There were no overt signs of
toxicity in any group. Spontaneous abortion occurred in one dam at
1000 mg/kg bw per day and in two at 3000 mg/kg bw per day on or
after day 21 of gestation. The mean body weights of animals in the
treated groups were significantly lower than the control values
throughout the study (including day 0). Body-weight gain was reduced
by 76% in animals at 3000 mg/kg bw per day; and at termination,
weight gain (days 0-29) was 19% lower than in controls. At 1000
mg/kg bw per day, body-weight gain was reduced by 56% during
treatment, but it was similar to that of controls at termination.
Food consumption was decreased by up to 38% in animals at 1000 and
3000 mg/kg bw per day during treatment. There were no
treatment-related changes in maternal organ weights. One animal at
3000 mg/kg bw per day resorbed her entire litter. There were no
other treatment-related changes in implantation efficiency, mean
fetal viability, size, sex ratio, fetal body weight or external,
visceral or skeletal development. Tolclofos-methyl was not
teratogenic in this study at doses up to and including 3000 mg/kg bw
per day, which was toxic to dams. The NOAEL for maternal toxicity
was 300 mg/kg bw per day (Motoyama et al., 1991).
(f) Genotoxicity
The results of tests for the genotoxicity of tolclofos-methyl
are summarized in Table 2.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Six male albino Japanese rabbits received 500 mg of
tolclofos-methyl (purity, 97%) on clipped intact or abraded dorsal
skin for 4 h under an occlusive dressing. No signs of irritation
were seen at any of the application sites seven days after treatment
(Matsubara et al., 1978).
Table 2. Results of tests for the genotoxicity of tolclofos-methyl
End-point Test system Concentration Purity Results Reference
of tolclofos-methyl (%)
In vitro
Reverse S. typhimurium TA98, 10, 100, 500, 1000, 2000 97.0 Negativea,b Suzuki & Miyamoto,
mutation 100, 1535, 1537, 1538 µg/plate
Reverse S. typhimurium TA98, 10, 50, 100, 500, 1000, 98.7 Negativea,c Moriya et al., 1981
mutation 100, 1535, 1537, 1538 5000 µg/plate
E. coli WP2uvrA
Reverse B. subtilis H17 rec+, 1, 10, 100, 1000 µg/disc 97.0 Negatived Suzuki & Miyamoto,
mutation M45 rec- 1978
Reverse B. subtilis 20, 50, 100, 200, 500, 98.7 Negativee Moriya et al., 1981
mutation H17 rec+, M45 rec- 1000, 2000, 5000 µg/disc
Host-mediated S. typhimurium in 870, 1750 mg/kg 97.0 Negativef Suzuki & Miyamoto,
assay male ICR mice 1978
Chromosomal Chinese hamster 10, 20, 40 µg/ml 96.6 Negativea,g Kogiso et al., 1990
aberration K1 ovary cells 37.5, 75, 150 µg/ml
Unscheduled Male Sprague-Dawley 0.3, 1, 3, 10, 20, 40 96.6 Negativea,i Hara et al., 1990
DNA synthesis rat hepatocytes µg/mlh
Unscheduled Human carcinoma 0.3, 3, 30, 300 µg/ml NR Negativej Monaco & Nunziata,
DNA synthesis cells (HeLa) 1981
Gene mutation Chinese hamster 1.5, 15, 150, 1500 µg/ml NR Negativea,k Monaco & Nunziata,
V79 lung cells 1981
Table 2 (contd)
End-point Test system Concentration Purity Results Reference
of tolclofos-methyl (%)
In vivo
Chromosomal Male ICR mice 500, 1000, 2000, 99.8 Negativei Hara & Suzuki, 1981
aberration 4000 mg/kg i.p.h
Dominant Male Sprague- 62.5, 208.3, 625.0 NR Negativej Brusick, 1981
lethal Dawley rats mg/kg orally
mutation
NR, not reported
a With and without metabolic activation.
b Positive controls ( N-methyl- N'-nitro- N-nitrosoguanidine and 2-acetylaminofluorene) yielded expected positive
results.
c Several positive controls yielded expected positive results.
d Positive control ( N-methyl- N'-nitro- N-nitrosoguanidine) yielded expected positive results.
e Positive (mitomycin C) and negative (Kanamycin) controls yielded expected results.
f Positive control ( N-nitrosodimethylamine) yielded expected positive results.
g Positive controls (mitomycin C and cyclophosphamide) yielded expected positive results.
h At 40 mg/ml, cell viability was less than 20%.
i Positive control (2-acetylaminofluorene) yielded expected positive results.
j Positive controls (methyl methanesulfonate and urethane) yield expected positive results.
k Positive controls (methyl methanesulfonate and N-nitrosodimethylamine) yielded expected positive results.
l At 2000 and 4000 mg/kg bw there was > 50% mortality at 48 h.
m Positive control (cyclophosphamide) yielded expected positive results.
n Positive control (triethylenemelamine) yielded expected positive results.
Groups of six male New Zealand white rabbits received 500 mg of
either a '50% wettable power' or '10% dust' moistened with saline on
1-inch2 (6.5-cm2) sites on the clipped dorsal intact or abraded
skin for 24 h under an occlusive dressing. No irritation, such as
erythema and oedema, was observed (Hara et al., 1981a,b).
Two male and one female New Zealand white rabbits received 0.5
ml of '50% flowable' tolclofos-methyl on 1 inch2 (6.5 cm2) of
clipped dorsal intact or abraded skin for 4 h under an occlusive
dressing. No irritation was observed (Nakanishi et al., 1989).
Each of eight male albino Japanese rabbits received 50 mg of
tolclofos-methyl (purity, 97%) in one eye. Five minutes after the
application, the treated eyes of five animals were flushed with 300
ml saline for 2 min. The treated eyes of the remaining animals were
similarly flushed 24 h after treatment. There were no corneal,
conjunctival or iridal effects up to seven days after treatment
(Matsubara et al., 1978).
Groups of nine male New Zealand white rabbits received 100 mg
of either a '50% wettable power' or '10% dust' in one eye; 30 s
after the application, the treated eyes of three animals per group
were flushed with 300 ml lukewarm water for 1 min. Slight congestion
of the iris was observed 24 h after application. Slight to moderate
hyperaemia and slight chemosis and/or discharge in conjunctiva were
also observed 1-48 h after application of the '50% wettable power'
to unwashed eyes. These changes had disappeared by 72 h after
application in all animals. No ocular lesions were found in the
washed eyes. The irritation potency of this formulation was judged
to be mild. Slight conjunctival hyperaemia and/or chemosis were
observed in animals with unwashed eyes and in one with washed eyes
1-24 h after application of the '10% dust'. There were no other
signs of irritation at any time. The formulation was classified as
minimally irritating to eyes (Hara et al., 1981a,b).
One male and two female New Zealand white rabbits received 0.1
ml of '50% flowable' tolclofos-methyl in one eye. Slight redness was
observed in conjunctiva after application, which disappeared within
24 h (Nakanishi et al., 1989).
The skin-sensitizing potential of tolclofos-methyl (purity,
97%) was assessed in guinea-pigs by the Landsteiner-Draize method.
Groups of 10 male Hartley guinea-pigs were given 10 intradermal
injections of 1 or 5% tolclofos-methyl (0.05 or 0.1 ml) in corn oil
at intervals of two to three days. Two weeks after the final
induction, the animals were challenged at a fresh site with 0.05-ml
intradermal injections of tolclofos-methyl at the same
concentrations used for induction. Negative control animals for both
dose groups were given the challenge injection only. Positive
control animals were treated with 0.05% 2,4-dinitrochlorobenzene
three times before challenge. Slight erythema and swelling were
observed in two animals after challenge treatment with 1%
tolclofos-methyl, in three after challenge with 5%, and in one
animal in each negative control group. Moderate erythema and/or
swelling was observed in the animals treated with
2,4-dinitrochlorobenzene. It was concluded that tolclofos-methyl did
not sensitize skin in this study (Matsubara et al., 1980).
The skin sensitizing potential of a '50% wettable powder' and a
'10% dust' of tolchlofos-methyl was tested in guinea-pigs by the
Buehler method. Groups of 10 male Hartley guinea-pigs received 500
mg of one formulation, slightly moistened with water, on clipped
dorsal skin under an occlusive dressing for 24 h. Induction was
performed 10 times at two- to three-day intervals.Positive control
animals were similarly treated with 0.5% 2,4-dinitro-chlorobenzene.
Negative controls were not subjected to the induction treatment. Two
weeks after the final induction, the test animals and positive
controls were challenged as in the sensitizing treatment. No skin
reactions were observed in the negative control or treated animals.
Slight to severe erythema and swelling were observed in the positive
controls. The formulations were considered not to sensitize skin
(Hara et al., 1981c,d).
The skin sensitizing potential of a '50% flowable formulation'
was tested in guinea-pigs by the Buehler method. Ten male Hartley
guinea-pigs received 0.5 ml of the formulation for 6 h once a week
for three weeks on the clipped dorsal skin under an occlusive
dressing. Positive control animals were similarly treated with 0.5%
2,4-dinitrochlorobenzene. Negative control animals were not
subjected to the induction treatment. Two weeks after the final
induction, animals were challenged as in the sensitizing treatment.
No skin reactions were observed in the negative control or treated
animals. Slight to severe erythema and swelling were observed in the
positive controls. The formulation was considered not to sensitize
skin (Nakanishi et al., 1990).
(ii) Delayed neuropathy
Groups of 10 Leghorn hens were administered 0 or 8000 mg/kg bw
tolclofos-methyl (purity, 97%) or 500 mg/kg bw tri- ortho-cresyl
phosphate orally in corn oil. After a 21-day observation period, a
second dose of vehicle or tolclofos-methyl was administered. Animals
were sacrificed 21 days after the second dose. There were no deaths
in the groups given the vehicle or tolclofos-methyl. Plasma
cholinesterase activity in the group treated with tolclofos-methyl
was decreased by nearly 50% eight days after the first dose but had
recovered to the pre-treatment level 21 days after dosing. Hens
treated with tolclofos-methyl had no signs of leg weakness or
paralysis and no histopathological changes in the nervous tissues.
Birds treated with tri- ortho-cresyl phosphate had the typical
clinical and histopathological signs of delayed polyneuropathy
(Okuno et al., 1982).
3. Observations in humans
The medical records of 20 workers in Japan were reviewed. All
workers had been engaged continuously in packaging operations since
manufacture of technical-grade tolclofos-methyl began in 1988, for
an average of 4 h/day. No occupation-related problems were observed
or reported. Plasma and erythrocyte cholinesterase activities were
not measured (Murayama, 1991).
Comments
Tolclofos-methyl is excreted rapidly in rats and mice,
predominantly in the urine; less than 1% of the dose was retained in
tissues after seven days. In both species, metabolism occurred
mainly by oxidation of P=S to P=O, oxidation of the 4-methyl group
and cleavage of the P-O-aryl and P-O-methyl linkages. There are four
main metabolites in mice, one of which is a glycine conjugate, and
four in rats, which are excreted as glucuronides.
Tolclofos-methyl had low acute toxicity when administered by
the oral, dermal, subcutaneous or intraperitoneal route. The overt
signs of acute toxicity are not typical of an anticholinesterase, as
no chromodaccryorrhoea, lachrymation or fasciculation was seen,
although some inhibition of plasma, erythrocyte and brain
cholinesterase activities was observed. WHO (1992) has classified
tolclofos-methyl as unlikely to present an acute hazard in normal
use.
In a nine-month study of toxicity in which mice were fed
tolclofos-methyl in the diet at 0, 10, 30, 100 or 3000 ppm, the
NOAEL was 100 ppm, equal to 12 mg/kg bw per day, on the basis of
inhibition of brain cholinesterase activity and effects on body
weight at 3000 ppm.
In a 32-34-day study of toxicity in which rats were fed diets
containing 0, 200, 1000, 5000 or 20 000 ppm, the NOAEL was 1000 ppm,
equal to 79 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity and increased relative kidney weight at 5000
ppm. In a 13-week study of toxicity in which rats were fed diets
containing 0, 100, 1000 or 10 000 ppm, the NOAEL was again 1000 ppm,
equal to 66 mg/kg bw per day, on the basis of effects on body, liver
and kidney weights at 10 000 ppm. In a 28-week study of toxicity in
which rats were fed dietary levels of 0, 300, 1000, 3000 or 10 000
ppm, the NOAEL was also 1000 ppm, equal to 65 mg/kg bw per day, on
the basis of histopathological changes in the livers of females at
3000 ppm.
In a 26-week dietary study in dogs fed levels of 0, 200, 600 or
2000 ppm, the NOAEL was 600 ppm, equal to 21 mg/kg bw per day, on
the basis of reduced body-weight gain, an increased serum level of
alkaline phosphatase and increased liver weight at 2000 ppm.
In a 52-week study of toxicity in dogs given dietary
concentrations of 0, 80, 400 or 2000 ppm, the NOAEL was 400 ppm,
equal to 11 mg/kg bw per day, on the basis of increased liver weight
(with hepatocytic hypertrophy), reduced body-weight gain and slight
anaemia at 2000 ppm.
In a 104-week study of toxicity and carcinogenicity in which
mice were given dietary concentrations of 0, 10, 50, 250 or 1000
ppm, the NOAEL was 50 ppm, equal to 6.5 mg/kg bw per day, on the
basis of reduced brain cholinesterase activity and increased
absolute and relative kidney weights at higher levels. There was no
evidence of carcinogenicity.
A 122-129-week study of toxicity and carcinogenicity was
performed in which rats were given dietary concentrations of 0, 100,
300 or 1000 ppm. The NOAEL was 1000 ppm, equal to 41 mg/kg bw per
day, on the basis of the absence of any significant finding. There
was no evidence of carcinogenicity.
In a three-generation study (two litters per generation) in
rats, tolclofos-methyl was given at dietary levels of 0, 100, 300 or
1000 ppm. The NOAEL was > 1000 ppm, equivalent to 100 mg/kg bw per
day, on the basis of the absence of any significant findings.
In a study of teratogenicity in which rats were given 0, 5, 15
or 50 mg/kg bw per day of tolclofos-methyl by gavage, the NOAEL was
50 mg/kg bw per day on the basis of the absence of any significant
findings. The study was not considered to be fully adequate because
the highest dose tested was not maternally toxic. A similar study
was conducted at levels of 0, 100, 300 or 1000 mg/kg bw per day. The
NOAEL was 300 mg/kg bw per day on the basis of reduced body-weight
gain in dams at 1000 mg/kg bw per day. There was no evidence of
teratogenicity.
A study of teratogenicity was conducted in rabbits given 0,
300, 1000 or 3000 mg/kg bw per day orally. The NOAEL for maternal
toxicity was 300 mg/kg bw per day on the basis of reduced
body-weight gain in dams at 1000 mg/kg bw per day. There was no
evidence of teratogenicity.
Tolclofos-methyl was studied in a wide range of tests for
genotoxicity in vivo and in vitro. The Meeting concluded that
the compound is not genotoxic.
Tolclofos-methyl did not cause delayed neuropathy in chickens.
The available observations in humans were considered by the
Meeting but did not directly contribute to an estimation of an ADI.
An ADI was established on the basis of a NOAEL of 50 ppm, equal
to 6.5 mg/kg bw per day, in the 104-week study of toxicity and
carcinogenicity study in mice, and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equal to 6.5 mg/kg bw per day (104-week study
of toxicity and carcinogenicity)
Rat: 1000 ppm, equal to 41 mg/kg bw per day (122/129-week
study of toxicity and carcinogenicity)
Rabbit: 300 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Dog: 400 ppm, equal to 11 mg/kg bw per day (52-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.07 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Brusick, D.J. (1981) Mutagenicity evaluation of S-3349 T.G. Lot No.
4, rat dominant lethal assay. Litton Bionetics Inc. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Colley, J., Welch, P.J., Heywood, R., Prentice, D.E., Cherry, C.P.,
Mullins, P.A., Gibson, W.A. & Almond, R.H. (1982) S-3349, toxicity
to rats by dietary administration for 4 weeks. Huntington Research
Centre Ltd. Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd, Osaka, Japan.
Cox, R.H. & Brusick, D.J. (1988) Chronic toxicity study in dogs with
S-3349. Hazleton Laboratories America Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Eschbach, J.C. & Hogan, G.K. (1981a) An acute inhalation toxicity
study of S-3349 50WP in the rat. Bio/dynamics Inc. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Eschbach, J.C. & Hogan, G.K. (1981b) An acute inhalation toxicity
study of S-3349 10d in the rat. Bio/dynamics Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Esumi, Y. & Yokoshima, T. (1989) Study on metabolism of
tolclofos-methyl in rats. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Gargus, J.L. (1986) 21-Day dermal toxicity study, in rabbits with
Rizolex, technical 97.7%. Hazleton Laboratories America Inc.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Hara, M. & Suzuki, H. (1981) In vivo chromosomal aberration test of
S-3349 on bone marrow cells of mice. Sumitomo Chemical Co., Ltd.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981a) Primary eye and skin
irritation tests of S3349 50% water-dispersible power in rabbits.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981b) Primary eye and skin
irritation tests of S-3349 10% dust formulation in rabbits. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981c) Skin sensitization test
of S3349 50% water-dispersible powder in guinea pigs. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981d) Skin sensitization test
of S3349 10% dust formulation in guinea pigs. Sumitomo Chemical Co.,
Ltd. Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd, Osaka, Japan.
Hara, M., Ota, M., Kogiso, S., Yoshitake, A. & Yamada, H. (1990) In
vitro unscheduled DNA synthesis (UDS) assay of Rizolex in rat
hepatocytes. Sumitomo Chemical Co., Ltd. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hardy, C.J., Jackson, G.C., Lewis, D.J. & Gopinath, C. (1986)
Technical Rezolex, acute inhalation toxicity in rats, 4-hour
exposure. Huntington Research Centre Ltd. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hiromori, T., Suzuki, T., Okuno, Y., Ito, S., Murakami, M., Kadota,
T. & Miyamoto, J. (1978) Six-month oral toxicity study of S-3349 in
rats. Sumitomo Chemical Co., Ltd. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hiromori, T., Misaki, Y., Ito, S., Kohda, A., Kato, T. & Yamada, H.
(1989a) Acute oral toxicity study of Rizolex 50 FL in rats. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Hiromori, T., Misaki, Y., Ito, S., Kohda, A., Kato, T. & Yamada, H.
(1989b) Acute dermal toxicity study of Rizolex 50 FL in rats.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Jackson, G.C., Hardy, C.J., Morrow, J. & Gopinath, C. (1990) Rizolex
50 FL, acute inhalation toxicity study in rats, 4-hour exposure.
Huntington Research Centre Ltd. Unpublished report submitted to WHO
by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kimura, J., Yosioka, K., Ozaki, K., Adachi, H., Seki, T., Matsuo, M
&, Yamada, H. (1990) 90-Day oral toxicity study of S-3349 in rats.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kogiso, S., Yamamoto, K., Hara, M., Yositake, A. & Yamada, H. (1990)
In vitro chromosomal aberration test of Rizolex in Chinese hamster
ovary cells (CHO-K1). Sumitomo Chemical Co., Ltd. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Krautter, G.R., Downs, J., Hoglan, N., Marsh, J.D. & Lawrence, L.J.
(1987) Metabolism of tolclofos-methyl in the rat. Pharmacology &
Toxicology Research Laboratory. Unpublished report submitted to WHO
by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Krautter, G.R., Downs, J., Hoglan, N., Marsh, J.D. & Lawrence, L.J.
(1988) Metabolism of tolclofos-methyl in the rat. Final report
amendment. Pharmacology & Toxicology Research Laboratory.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Matsubara, T., Hara, S. & Kadota, T. (1978) Eye and skin irritation
test of S-3349 in rabbits. Sumitomo Chemical Co., Ltd. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Matsubara, T., Hara, S., Suzuki, T., Kadota, T. & Miyamoto, J.
(1980) Skin sensitization test of S-3349 in guinea pigs. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Mihara, K., Ohkawa, H. & Miyamoto, J. (1981) Metabolism of
tolclofos-methyl in rats and mice. J. Pestic. Sci., 6, 65-74.
Miyamoto, J. (1985) Comments on toxic effects and minimum effect
level of S-3349 in chronic oral toxicity studies in rats. Sumitomo
Chemical Co., Ltd., Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Monaco, M. & Nunziata, A. (1981) Report of mutagenicity experiment
performed on the test substance S-3349, Sumitomo Chemical Co., Ltd.
Unpublished report from Centro Ricerca Farmaceutica, Pomezia, Italy.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Moore, M.R. (1993) Comments on the toxicological significance of
hepatocytic pigment and on the no-adverse-effect-level (NOAEL) in a
chronic toxicity study in dogs treated with S-3349 (Hazleton project
No. 343-176). Hazleton Washington Inc. Unpublished report submitted
to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Moriya, M., Ohta, T. & Shirasu, Y. (1981) S-3349, microbial
mutagenicity study. Institute of Environmental Toxicology.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Morseth, S.L., Burlew, J.A., Vargas, K.J., Lewis, S.A., Thakur, A.K.
& Phipps, N.G. (1987) Teratology study of S-3349 T.G. in rats.
Hazleton Laboratories America Inc. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Motoyama, M., Kashima, M. & Takahashi, M. (1991) Teratology study of
S-3349 in rabbits. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Murayama, F. (1991) A review on medical examination of factory
workers exposed to tolclofos-methyl. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Nakanishi, T., Kohda, A., Kato, T. & Yamada, H. (1989) Primary eye
and skin irritation tests with Rizolex 50 FL in rabbits. Sumitomo
Chemical Co., Ltd. Unpublished study, submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Nakanishi, T., Kohda, A., Kato, T. & Yamada, H. (1990) Skin
sensitization test of Rizolex 50 FL in guinea pigs. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Okuno, Y., Yamada, T., Hosokawa, S. & Miyamoto, J. (1982) Acute
delayed neurotoxicity study of S-3349 in hens. Sumitomo Chemical
Co., Ltd. Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd, Osaka, Japan.
Pence, D.H., Lemen, J.K. & Weatherholtz, W.M. (1978) Acute oral
toxicity study in male and female dogs, S-3349. Hazleton
Laboratories America Inc. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Weatherholtz, W.M., Kundzins, W., Alsaker, R.D., Brown,
H.R. & Greenspun, K.S. (1979a) Subacute dietary administration in
dogs, S-3349. Hazleton Laboratories America Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Pence, L.A., Durloo, R. & Twigg, C.J. (1979b)
Teratology study in rats, S-3349. Hazleton Laboratories America Inc.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Pence, D.H., Serota, D., Alsaker, R.D., Koka, M., Banas, D.A.,
Dawkins, B.G., Kundzins, W. & Hepner, K.E. (1982) Chronic toxicity
study in rat, S-3349. Hazleton Laboratories America Inc. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Phipps, N., Alsaker, R.D., Hepner, K.E. & Spicer, K.M.
(1985a) 104-Week cholinesterase activity study in male and female
rats, S-3349. Hazleton Laboratories America Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Hepner, K.E., Wolfe, G.W., Voelker, R.W. & Ulland, B.N.
(1985b) Three-generation reproduction study in rats, S-3349.
Hazleton Laboratories America Inc. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Satoh, R., Kashima, M., Satoh, H., Motoyama, M. & Ishikawa, A.
(1983) Twenty-four-month chronic toxicity study of S-3349 in
pulverized diet in mice. Nippon Experimental Medical Research
Institute. Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd, Osaka, Japan.
Segawa, T. (1978) Acute toxicity study of S-3349 in rats and mice.
Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981a) Acute oral toxicity study of S-3349 50% wettable
powder in mice. Hiroshima University School of Medicine. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981b) Acute oral toxicity study of S-3349 10% dust in
mice. Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981c) Acute dermal toxicity study of S-3349 50%
wettable powder in mice. Hiroshima University School of Medicine.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Segawa, T., (1981d) Acute dermal toxicity study of S-3349 10% dust
in mice. Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981e) Acute oral toxicity study of S-3349 50% wettable
powder in rats. Hiroshima University School of Medicine. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981f) Acute oral toxicity study of S-3349 10% dust in
rats. Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981g) Acute dermal toxicity study of S-3349 50%
wettable powder in rats. Hiroshima University School of Medicine.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Segawa, T. (1981h) Acute dermal toxicity study of S-3349 50%
wettable powder in rats. Hiroshima University School of Medicine.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Suzuki, H. & Miyamoto, J. (1978) Studies on mutagenicity of S-3349
with bacterial systems. Sumitomo Chemical Co., Ltd. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Suzuki, T., Okuno, Y., Hiromori, T., Ito, S., Murakami, M., Kadota,
T & Miyamoto, Y. (1978) Nine-month feeding study of S-3349 in mice.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
 2. Toxicological studies
(a) Acute toxicity
Data on the acute toxicity of tolclofos-methyl (technical-grade
and formulations) in laboratory animals are summarized in Table 1.
Animals showed decreased spontaneous motor activity, dyspnoea,
piloerection, urinary incontinence and ataxia. Recovery was complete
by day 10. Brain cholinesterase activity was lower 16 days after
treatment in dogs given 1000 mg/kg bw than in animals given lower
doses; however, as no control data were reported, conclusions cannot
be drawn. No treatment-related gross changes were seen at necropsy
in any species.
Table 1. Acute toxicity of technical-grade and formulated tolclofos-methyl in laboratory animals
Species Strain Sex Route Formulation LD50 Reference
(purity) (mg/kg bw)
Mouse dd M Oral Technical (97%) 3500 Segawa, 1978
F 3600
Mouse ICR M&F Oral 50% wettable > 5000 Segawa, 1981a
powder (98%)
Mouse ICR M&F Oral 10% dust (98.8%) > 5000 Segawa, 1981b
Mouse dd M&F Dermal Technical (97%) > 5000 Segawa, 1978
Mouse ICR M&F Dermal 50% wettable > 5000 Segawa, 1981c
powder (98%)
Mouse ICR M&F Dermal 10% dust (98.8%) > 5000 Segawa, 1981d
Mouse dd M Intraperitoneal Technical (97%) 1070 Segawa, 1978
F 1260
Mouse dd M&F Subcutaneous Technical (97%) > 5000 Segawa, 1978
Rat SD M&F Oral Technical (97%) approx. 5000 Segawa, 1978
Rat SD M&F Oral 50% wettable > 5000 Segawa, 1981e
powder (98%)
Rat SD M&F Oral 10% dust (98.8%) > 5000 Segawa, 1981f
Rat SD M&F Oral 50% flowable > 5000 Hiromori et al.,
1989a
Rat SD M&F Dermal Technical (97%) > 5000 Segawa, 1978
Rat SD M&F Dermal 50% wettable > 5000 Segawa, 1981g
powder (98%)
Rat SD M&F Dermal 10% dust (98.8%) > 5000 Segawa, 1981h
Rat NR M&F Dermal 50% flowable > 2000 Hiromori et al.,
1989b
Table 1 (contd)
Species Strain Sex Route Formulation LD50 Reference
(purity) (mg/kg bw)
Rat SD M Intraperitoneal Technical (97%) 5000 Segawa, 1978
F 4900
Rat SD M&F Subcutaneous Technical (97%) > 5000 Segawa, 1978
Rat Wistar M&F Inhalation Technical (97.7%) > 3.32 mg/la Hardy et al., 1986
Rat SD M&F Inhalation 50% wettable > 1.9 mg/l Eschbach &
powder (98%) Hogan, 1981a
Rat SD M&F Inhalation 10% dust (98.8%) > 1.8 mg/l Eschbach &
Hogan, 1981b
Rat SD M&F Inhalation 50% flowable > 1.07 mg/la Jackson et al., 1990
Dog NR M&F Oral Technical (98.7%) > 1000 Pence et al., 1978
SD, Sprague-Dawley; M, male; F, female; NR, not reported
a 4-h whole-body exposure
(b) Short-term toxicity
Mice
Groups of 15 male and 15 female ddY mice were fed diets
containing tolclofos-methyl (purity, 97%) at 0, 10, 30, 100 or 3000
ppm, equal to 1.2, 3.8, 12 or 510 mg/kg bw per day for males and
1.4, 4.1, 14 or 560 mg/kg bw per day for females, for nine months.
Three satellite groups of five males and five females each were
similarly treated and killed at 2, 4 and 13 weeks for determination
of cholinesterase activity. Animals were observed daily for
mortality and signs of toxicity; at the end of exposure, an
ophthalmological examination and clinical chemical analyses
(including plasma and erythrocyte cholinesterase) were undertaken,
and brain cholinesterase activity was measured. Most organs were
weighed and examined microscopically. There were no overt signs of
toxicity and no deaths. Body weights were reduced by about 20% and
body-weight gain by about 35% in animals of each sex at 3000 ppm,
but at this dose food consumption was increased in males and females
and water intake was increased in males. No treatment-related
ophthalmological changes were observed, and haematological
parameters were unaffected by treatment. The cholesterol level was
increased by 35% in females at 3000 ppm. A dose-related decrease in
plasma cholinesterase activity was found in animals of each sex,
with activities that were 63-94% of the control values in mice at 30
ppm, 30-56% at 100 ppm and 4-35% (61% in one case) at 3000 ppm. At
the high dose, the activity reached a plateau after four weeks.
Erythrocyte cholinesterase activity was decreased to 39-46% of the
control level in males at 3000 ppm at weeks 13 and 40 and to 57-75%
of the control level in females at this dose at all time points.
Brain cholinesterase activity was unaffected by treatment in females
but was reduced by about 24% in males at 3000 ppm at weeks 13 and
40. Organ weights in the 3000-ppm group were reduced, but neither
gross nor histopathological changes attributable to treatment were
observed. The NOAEL was 100 ppm, equal to 12 mg/kg per day, on the
basis of inhibition of brain cholinesterase activity and an effect
on body weight at the highest dose (Suzuki et al., 1978).
Rats
Groups of 10 male and 10 female Sprague-Dawley rats were fed
diets containing tolclofos-methyl (purity, 97.9%) at 0, 200, 1000,
5000 or 20 000 ppm for 32-34 days. Animals were observed twice daily
for mortality and clinical signs, and food consumption and body
weight were recorded weekly. Ophthalmoscopic examination, standard
haematological tests and clinical chemical analyses were performed
at week 4, and certain tests were repeated at termination. Plasma
and erythrocyte cholinesterase activities were determined in the
control and high-dose groups at week 4 and in all animals at
termination, when brain cholinesterase activity was also measured.
At terminal sacrifice, relevant organs were weighed and most organs
were examined microscopically. There were no treatment-related
deaths. Reductions in food consumption (-16% in males and -6% in
females) and body-weight gain (-45% in males and -37% in females)
were observed at 20 000 ppm. Body-weight gain was slightly reduced
in animals of each sex at 5000 ppm during week 1. Haematological
parameters were unaffected by treatment. Cholesterol levels were
increased (by 116-138%) in animals of each sex at 20 000 ppm, and
total protein and albumin levels were slightly higher in males in
this group. Other differences between treated and untreated animals
were detected, but the values were within the normal range. Plasma
cholinesterase activity was reduced by 14% in males and by 50% in
females at 20 000 ppm. Erythrocyte cholinesterase activity was
reduced by 9-19% in animals of each sex at 5000 and 20 000 ppm;
however, no clear dose-response relationship was seen. Brain
cholinesterase activity was lower (by 12-31%) than that in controls
in males in all treated groups and in females (by 20%) at 5000 and
20 000 ppm. Although the reduction was statistically significant in
all male groups, no clear dose-response relationship was found, and
the activity in controls was slightly higher than usual. There were
no ophthalmoscopic alterations or gross changes at necropsy. The
relative weights of livers were increased (by 12%) in females at
5000 ppm and in males and females at 20 000 ppm (by 27% in males and
39% in females). Hypertrophy of hepatocytes was observed in animals
of each sex at 20 000 ppm. The relative weights of the kidneys were
slightly increased in animals of each sex at 5000 and 20 000 ppm,
but no treatment-related histopathological changes were seen. The
NOAEL was 1000 ppm, equal to 79 mg/kg bw per day, on the basis of
increased relative kidney weights and brain cholinesterase activity
at higher doses (Colley et al., 1982).
Groups of 12 male and 12 female Sprague-Dawley rats were fed
diets containing tolclofos-methyl (96.6% pure) at 0, 100, 1000 or 10
000 ppm, equal to 6.5, 66 or 653 mg/kg bw per day for males and 7.1,
71 or 696 mg/kg bw per day for females, for 13 weeks. Animals were
observed daily for mortality and clinical signs, and body weight and
food and water consumption were determined weekly. Ophthalmological
analysis, urinalysis, standard haematological tests, clinical
chemical analyses and measurement of plasma, erythrocyte and brain
cholinesterase activities were undertaken at termination, when
relevant organs were weighed and most organs were examined
microscopically. No deaths and no significant abnormal clinical or
ophthalmological signs were noted during the study. Depression of
body-weight gain of males (-20%) and females (-15%) at 10 000 ppm
was seen throughout the treatment period and was associated with
similar decreases in food and water consumption. A slight decrease
in food consumption was noted in females at 1000 ppm during week 1.
Decreased activities of plasma (16-53%), brain (8-9%) and
erythrocyte (19-20%) cholinesterase were noted in animals of each
sex at 10 000 ppm; a minimal decrease in erythrocyte cholinesterase
activity (10%) was also seen in females at 1000 ppm. Liver weight
was increased in animals of each sex at 10 000 ppm, and this was
found at histopathological examination to be associated with
hypertrophy of hepatocytes. Changes in some clinical chemical
parameters were observed at 10 000 ppm, which included increased
levels of cholesterol (48% in males, 102% in females) and
phospholipids (37% in males, 64% in females) and slightly increased
alpha2- and ß-globulin levels in animals of each sex. Other
changes, although statistically significant, were minor and of
questionable biological significance. At 10 000 ppm, increased
relative kidney weights were seen in animals of each sex, increased
blood urea nitrogen levels were seen in males only and decreased
urinary pH was seen in males and females. At 1000 ppm, relative
kidney weights were slightly increased in females and relative liver
weights in males. The Meeting considered that the increased weight
ratios observed at 1000 ppm were not biologically relevant and that
the NOAEL was 1000 ppm, equal to 66 mg/kg bw per day (Kimura et
al., 1990).
Groups of 15 male and 15 female Sprague-Dawley rats were fed
diets containing tolclofos-methyl (purity, 97%) at 0, 300, 1000,
3000 or 10 000 ppm, equal to 16, 51, 164 or 540 mg/kg bw per day in
males and 18, 65, 184 or 623 mg/kg bw per day in females, for 28
weeks. Animals were observed daily for clinical signs, and body
weight and food consumption were measured weekly. Ophthalmologic
examinations were carried out after three months and at termination;
standard haematological tests and clinical chemical analyses were
performed at termination. Urinalysis was carried out in 12 animals
per group after three months and at termination. Plasma and
erythrocyte cholinesterase activities were determined after 2, 4 and
13 weeks, and at termination; brain cholinesterase was determined
only at termination, when relevant organs were weighed and several
were examined histologically.
There were no overt signs of toxicity and no treatment-related
deaths. Although the body weights of animals of each sex at 10 000
ppm were lower than those of controls throughout the study, their
initial body weights were also statistically significantly lower.
Body-weight gain was significantly reduced (by 18%) in females at 10
000 ppm. Food consumption was unaffected by treatment. Treatment did
not affect ophthalmologic, urinary, haematological or clinical
chemical parameters. Plasma cholinesterase activity was lower (by
23-41%) in females at 10 000 ppm than in controls at weeks 2, 4, 13
and 28. Erythrocyte cholinesterase activity was highly variable and
was reduced by 15-19% in comparison with controls in males at 10 000
ppm at weeks 2, 4 and 28. Brain cholinesterase activity was
unaffected by treatment. Kidney and liver weights were slightly
increased in animals of each sex at 10 000 ppm and in females at
1000 and 3000 ppm, but the biological significance of these findings
was questionable. No gross abnormalities were seen at necropsy.
Bile-duct proliferation was found in the livers of 3/15 females at
10 000 ppm and oval-cell proliferation was seen in 2/15 females at
3000 ppm and 3/15 at 10 000 ppm. No histopathological changes were
seen in the kidneys. The NOAEL was 1000 ppm, equal to 65 mg/kg bw
per day, on the basis of histopathological changes in the livers of
females at higher doses (Hiromori et al., 1978).
Rabbits
Groups of five male and five female New Zealand white rabbits
received tolclofos-methyl (purity, 97.7%) in acetone on the skin at
doses of 0, 30, 300 or 1000 mg/kg bw per day for 6 h per day, on
five days per week for 21 days. There were no deaths and no
treatment-related clinical signs; neither body weight nor food
consumption was affected by treatment. Dermal irritation (slight
erythema) was seen in some animals in all treated groups from day 6.
Eosinophil counts were increased in males at 1000 mg/kg bw per day,
and the level of inorganic phosphorus was decreased (by 14-20%) in
animals of each sex at this dose. Brain cholinesterase activity was
about 15% lower than that in controls in males and about 15% higher
in females at 300 and 1000 mg/kg bw per day. These changes were
considered not to be of biological significance. Erythrocyte
cholinesterase activity was lower than in controls in males at the
same two doses, but there was no dose-effect relationship. Plasma
cholinesterase activity was lower (by 22-29%) than in controls in
animals of each sex at 300 and 1000 mg/kg bw per day. The relative
weights of the kidneys were increased (by 20%) in females at 1000
mg/kg bw per day. Macroscopic findings consisted of accumulation of
the compound on treated skin; microscopic examination revealed
hyperkeratosis, acanthosis and subepidermal pleocellular
infiltration of the skin (Gargus, 1986).
Dogs
Groups of six male and six female beagle dogs were fed diets
containing tolclofos-methyl (purity, 98.7%) at 0, 200, 600 or 2000
ppm, equal to 7.4, 23 or 69 mg/kg bw per day in males and 4.1, 21 or
65 mg/kg bw per day in females, for 26 weeks. Animals were observed
daily for mortality and weekly for clinical signs, body weight and
food consumption. Ophthalmoscopic examinations were performed before
treatment and at termination of the study. Standard haematological
tests and clinical chemical analyses were performed at regular
intervals until termination. Erythrocyte and plasma cholinesterase
activities were determined at weeks 2, 4, 8, 13 and 26. Brain
cholinesterase was determined at termination, when relevant organs
were weighed and most were examined histologically. There were no
deaths and no overt signs of toxicity. Body-weight gain was reduced
by 54% in males and by 46% in females at 2000 ppm, although food
consumption was unaffected by treatment. There were no
treatment-related ocular changes. Although the haematocrit was
within the normal range, decreased values in comparison with
controls (-11% in males and -10% in females) were observed at 2000
ppm. The mean corpuscular volume and mean corpuscular haemoglobin
concentration were not affected by treatment. Alkaline phosphatase
activity was increased by 139-295% in animals of each sex at 2000
ppm throughout the study. Total bilirubin was not increased in
treated animals. At 2000 ppm, plasma cholinesterase activity was
decreased by 19-26% in females throughout the study, but no
significant decreases were seen in erythrocyte cholinesterase
activity in animals of each sex or in plasma cholinesterase activity
in males. Brain cholinesterase activity was unaffected by treatment.
Urinalysis gave unremarkable results, and there were no gross
changes at necropsy. Liver weights were increased (by 59% in males
and 43% in females) at 2000 ppm, but there were no concomitant
histological changes, and there were no treatment-related
histopathological changes. The NOAEL was 600 ppm, equal to 21 mg/kg
bw per day, on the basis of increased liver weights, reduced
body-weight gain and increased alkaline phosphatase activity at 2000
ppm (Pence et al., 1979a).
Groups of six male and six female beagle dogs were fed diets
containing tolclofos-methyl (purity, 96.7%) at 0, 80, 400 or 2000
ppm, for 52 weeks. Animals were observed daily for mortality and
weekly for clinical signs. Ophthalmoscopic examinations were
performed before treatment and at weeks 26 and 52. Standard
haematological tests, clinical chemical analyses and determinations
of plasma and erythrocyte cholinesterase activities were performed
one week before treatment and at weeks 13, 26 and 52. Brain
cholinesterase was determined at termination, when relevant organs
were weighed and most were examined histologically. No clinical
signs of toxicity were observed. Decreased food consumption (-9% in
males and -16% in females) and body-weight gain (-32% in males and
-54% in females), slightly lower erythrocyte counts (-8% in males
and -20% in females), haematocrit value (-7% in males and -17% in
females) and haemoglobin concentration (-8% in males and -7% in
females), slightly decreased albumin and total protein levels
(significantly at some time points) and elevated alkaline
phosphatase levels (> 200%) were found at 2000 ppm.
Plasma cholinesterase activity was slightly, but not
significantly lower than that in controls in females at the highest
dose; erythrocyte and brain cholinesterase activities were not
affected by the treatment. No treatment-related ophthalmoscopic
alterations were observed. Increases in mean liver weights, both
absolute (+32% in males and +61% in females) and relative (+52% in
males and +74% in females), were seen in dogs at 2000 ppm.
Microscopic examination of the livers revealed increased incidences
of hepatocytic hypertrophy and intracytoplasmic homogeneous material
and an increased amount of hepatocytic pigment in these animals. The
amount of this pigment was also increased at 400 ppm, and it was
present in control animals. Its nature has not been elucidated, but
it does not contain iron or bilirubin. These dogs also had decreased
absolute (-46%) and relative (-40%) weights of the prostate, and the
absolute and relative weights of the pancreas were increased by
16-27% and 28-49%, respectively. The NOAEL was 400 ppm, equal to 11
mg/kg bw per day, on the basis of reduced body-weight gain,
increased liver weights (with hepatic hypertrophy) and slight
anaemia at 2000 ppm (Cox et al., 1988; Moore, 1993).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 70 male and 70 female B6C3F1 mice were fed diets
containing tolclofos-methyl (purity, 94.3%) at 0, 10, 50, 250 or
1000 ppm, equal to 1.3, 6.5, 32 or 134 mg/kg bw per day in males and
1.3, 6.8, 34 or 137 mg/kg bw per day in females, for up to 104
weeks. Animals were observed twice daily for clinical symptoms and
mortality. Body weight was measured weekly during the first 13 weeks
and then once every four weeks. Food intake was measured weekly.
Standard urinalysis was performed in 10 animals from each group at
6, 12 and 18 months and in all survivors at termination of the
study. Standard haematological tests were performed in 10 animals
from each group at 12 months and in all survivors at termination.
Standard clinical chemical analyses and measurements of serum,
erythrocyte and brain cholinesterase activities were done in 10
animals from each group at 6 and 12 months and in all animals at
termination. Animals in the control and 1000-ppm groups were
subjected to ophthalmologic examination at 12 and 24 months. Ten
animals from each group were necropsied at 6 and at 12 months, at
termination or when found dead or moribund. Relevant organs were
weighed and examined microscopically.
There were no treatment-related effects on mortality and no
overt signs of toxicity, including ophthalmologic changes.
Body-weight gain was slightly reduced in females at 1000 ppm up to
week 52; food consumption was also slightly decreased in this group
after 52 and 104 weeks. Food conversion efficiency, water intake,
urinary parameters and the haematological profile were unaffected by
treatment. Serum cholinesterase activity was decreased in animals of
each sex at 250 ppm (by 25-52%) and at 1000 ppm (by 43-81%) at both
times, and erythrocyte cholinesterase activity was decreased by
11-28% at 250 ppm and by 13-47% at 1000 ppm; inhibition of both
enzymes was greater at termination. A dose-related decrease was
found in brain cholinesterase activity in females (by 7-24% at 250
ppm and 9-33% at 1000 ppm) and males (by 10-13% at 250 ppm and
17-26% at 1000 ppm). Serum and brain, but not erythrocyte,
cholinesterase activities were lower (by 12 and 18%, respectively)
in females at 50 ppm at week 28 than in controls, which had higher
levels than at week 52 and at termination. Glucose levels were
increased in males and were slightly increased in females at 1000
ppm at week 104. Absolute and/or relative kidney weights were
increased in animals of each sex at 1000 ppm and in males at 250 ppm
at week 52, and in females at 250 ppm at week 104. The weights of
the thymus were decreased in females at 1000 ppm at weeks 52 and
104, and pituitary weights were increased in females at this dose at
week 104. No treatment-related changes were seen at necropsy or on
histopathological examination at any time. Tolclofos-methyl was not
found to be carcinogenic. The NOAEL was 50 ppm, equal to 6.5 mg/kg
bw per day, on the basis of reduced brain cholinesterase activity
and increased absolute and relative kidney weights at higher doses
(Satoh et al., 1983).
Rats
Groups of 65 male and 65 female Fischer 344 rats were fed diets
containing tolclofos-methyl (purity, 94.9-98.7%) at 0, 100, 300 or
1000 ppm for 122 weeks (males) or 129 weeks (females). At week 52,
10 males and 10 females from each group were killed. Animals were
observed twice a day for mortality and signs of morbidity. Body
weight and food consumption were recorded weekly (weeks 0-26), every
two weeks (weeks 27-52) or every four weeks until termination.
Ophthalmoscopic examinations were performed every 26 weeks and at
termination. Standard haematological tests, clinical chemical
analyses and measurements of erythrocyte and plasma cholinesterase
activities were conducted in 10 animals of each sex in each group
one week before treatment, at weeks 4, 13, 26, 52, 78 and 104 and at
termination. Brain cholinesterase activity was determined in 10
animals of each sex per group at week 52 and at termination. All
animals killed or found dead were necropsied, and relevant organs
were weighed; most organs were then examined histologically.
There were no treatment-related effects on mortality and no
overt signs of toxicity. A slight decrease in body-weight gain was
observed in males at 1000 ppm. Food consumption and food conversion
efficiency were unaffected by treatment, as was the haematological
profile. There was a dose-related trend to decreased alkaline
phosphatase activity (by 9-46%) in all treated groups: The activity
was significantly decreased in males at 1000 ppm throughout the
study, in males at 300 ppm from week 13, and occasionally in the
other treated groups. Brain cholinesterase activity was decreased in
all treated groups at the time of the interim sacrifice, but there
was no dose-response relationship. At the time of terminal
sacrifice, brain cholinesterase activity was reduced in males at the
middle dose (by 52%) and in females at the low (by 57%) and middle
doses (by 49%), but not in animals at the high dose. Erythrocyte and
plasma cholinesterase activities were also inconsistently lower in
treated groups, although no dose-response relationship was seen.
Urinalysis revealed no remarkable findings, organ weights were
unaffected by treatment, and there were no treatment-related gross
changes at necropsy. Toclofos-methyl was not carcinogenic in this
study. No NOAEL could be identified, given the variability of the
data on cholinesterase activity (Pence et al., 1982).
Groups of 30 male and 30 female Fischer 344 rats were fed diets
containing tolclofos-methyl (purity, 98.3-97.3%) at 0, 100, 300 or
1000 ppm for 104 weeks, and brain, erythrocyte and plasma
cholinesterase activities were assessed. Ten males and 10 females
from each group were sacrificed at week 52. Animals were observed
daily for mortality, and they were examined for body weight, food
consumption and gross signs of toxicity weekly until week 26, every
two weeks during weeks 26-52 and every four weeks during weeks
52-104. Blood samples were taken for determination of plasma and
erythrocyte cholinesterase activities at initiation of the study, at
weeks 5, 14, 27, 53, 79 and at termination. Brain cholinesterase
activity was determined at week 52 and at termination.
There were no treatment-related effects on survival, and the
incidences of clinical signs and palpable tissue masses were
comparable among the groups. Mean plasma cholinesterase activities
were slightly decreased (by < 25%) in males when compared with
controls at 300 and 1000 ppm at weeks 27 (statistically
significant), 53, 79 and 105. Mean erythrocyte and brain
cholinesterase activities were comparable among the groups
throughout the study. Plasma cholinesterase activity was reduced (by
< 20%) at certain times in treated animals but no dose-response
relationship was found. Gross pathological findings were unrelated
to treatment. Doses < 1000 ppm did not depress erythrocyte or
brain cholinesterase activity or induce gross pathological effects
(Miyamoto, 1985; Pence et al., 1985a).
The combined NOAEL in these two studies was 1000 ppm, equal to
41 mg/kg bw per day, on the basis of the absence of any significant
findings.
(d) Reproductive toxicity
Rats
A three-generation study of reproductive toxicity was performed
in Sprague-Dawley rats fed diets containing tolclofos-methyl
(purity, 97.9-98.7% pure) at 0, 100, 300 or 1000 ppm, equivalent to
10, 30 or 100 mg/kg bw per day, with two litters per generation. The
F0 generation consisted of 30 rats of each sex per group. The
F1a, F2a and F3a generations were sacrificed at weaning and
underwent gross necropsy. Five rats of each sex in each group were
selected at weaning from the F1b, F2b and Fn3b litters for
histopathological examination, and 25 rats of each sex from each
group were selected from the F1b and F2b litters to breed the
following generation. A 15-week growth period was allowed before
mating of the 'a' litters, and a minimal 10-day rest period was
allowed between weaning of the 'a' litters and mating to produce the
'b' litters. After birth of the 'b' litter, all parental animals
were necropsied.
There were no treatment-related deaths or overt signs of
toxicity. Pregnancy rates were low at times in all groups, but the
pattern was not related to dose. The lowest pregnancy rate (52%) was
observed in the F1b generation at the middle dose. Pregnancy rates
were below 70% in three of the six generations in the controls, in
two generations at the low dose, in four generations at the middle
dose and in one generation at the high dose. The body weights of
F2a and F2b pups at 300 and 1000 ppm were lower than those of
controls at the start of the growth period; however, these
reductions were not dose-related, and the growth rates and body
weights in other groups were comparable throughout the study. There
were no treatment-related gross changes at necropsy in any group.
The weights of the ovaries were increased in non-pregnant adult
F2b females at 1000 ppm, but this was not associated with
histomorphological alterations. There was an increased incidence
(not statistically significant) of fine cytoplasmic vacuolation of
the adrenal cortex in adult F2b females at 1000 ppm that became
pregnant at both matings. The etiology and toxicological
significance of this finding, given its occurrence in the controls,
were unknown. There was no effect on reproductive parameters or on
offspring survival and development in any litter, and there were no
treatment-related gross or histopathological findings in the
litters. The NOAEL for reproductive toxicity was 1000 ppm
(equivalent to 100 mg/kg bw per day) on the basis of the absence of
significant findings at any dose level (Pence et al., 1985b).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 21-26 pregnant Fischer 344 rats were administered 0,
5, 15 or 50 mg/kg bw per day tolclofos-methyl (purity, 94.9%; dose
adjusted to 100%) in 0.5% w/v methylcellulose by gavage on days 6-15
of gestation. There were no overt signs of toxicity, and body-weight
gain and food consumption were unaffected by treatment. There were
no treatment-related gross lesions in the dams at necropsy.
Pregnancy rates were slightly higher in all treated groups (77-87%)
than in controls (70%). There were no treatment-related changes in
the number of fetal deaths, fetal viability or size or the
incidences of visceral or skeletal anomalies or variations.
Tolclofos-methyl was not embryotoxic, fetotoxic or teratogenic in
rats at doses up to and including 50 mg/kg bw per day. It should be
noted, however, that the highest dose was not maternally toxic
(Pence et al., 1979b).
Groups of 23 pregnant Sprague-Dawley rats were administered 0,
100, 300 or 1000 mg/kg bw per day tolclofos-methyl (purity, 96.7%)
in 0.5% methyl cellulose orally on days 6-15 of gestation. On day 20
of gestation, all animals were sacrificed and caesarean section was
performed. Rats were observed daily for mortality and clinical signs
and weighed. At termination, dams were observed for gross visceral
abnormalities, and their uteri were weighed and examined for
implantations and resorptions. Live fetuses were weighed, sexed and
examined for external, visceral and skeletal abnormalities. All dams
survived to the day of scheduled sacrifice with no clinical signs of
toxicity. Mean body-weight gain in animals at 100 and 1000 mg/kg bw
per day was significantly less than in controls on days 6-11. Both
mean body-weight gain (days 6-16) and mean net body-weight gain
(days 0-20) in rats at 1000 mg/kg bw per day were lower (by 10 and
14%, respectively) than the corresponding control value. In
addition, mean food consumption in animals at 1000 mg/kg bw per day
on days 6-16 and 16-20 was slightly below the control value. There
were no treatment-related differences in implantation efficiencies,
and mean fetal viability, sex ratio and fetal body weight were
similar in all groups. The number of fetuses with unossified fifth
and/or sixth sternebrae was significantly greater in the 1000-mg/kg
bw per day group than in the control group; however, the incidence
of total fetal skeletal variations was similar in all groups. Other
variations in development were not related to dose. Two to three
malformed fetuses were found in each group, but neither the type nor
the frequency of malformations indicated a teratogenic or
embryotoxic response. Tolclofos-methyl was found to be neither
teratogenic nor embryotoxic in this study at doses up to and
including 1000 mg/kg bw per day. This dose was slightly toxic to the
dams, as indicated by the lower body-weight gain. The NOAEL for
maternal toxicity was 300 mg/kg bw per day (Morseth et al., 1987).
Rabbits
Groups of 13-17 pregnant New Zealand white rabbits were
administered 0, 300, 1000 or 3000 mg/kg bw per day tolclofos-methyl
(purity, 98.7% pure) in 5% carboxymethylcellulose orally on days
6-18 of gestation. The animals were observed daily for mortality and
clinical signs and were weighed. At termination, dams were observed
for gross visceral abnormalities, and their uteri were weighed and
examined for implantations and resorptions. Live fetuses were
weighed, sexed and examined for external, visceral and skeletal
abnormalities. One rabbit at 3000 mg/kg bw per day died on day 14 of
gestation of an undetermined cause. There were no overt signs of
toxicity in any group. Spontaneous abortion occurred in one dam at
1000 mg/kg bw per day and in two at 3000 mg/kg bw per day on or
after day 21 of gestation. The mean body weights of animals in the
treated groups were significantly lower than the control values
throughout the study (including day 0). Body-weight gain was reduced
by 76% in animals at 3000 mg/kg bw per day; and at termination,
weight gain (days 0-29) was 19% lower than in controls. At 1000
mg/kg bw per day, body-weight gain was reduced by 56% during
treatment, but it was similar to that of controls at termination.
Food consumption was decreased by up to 38% in animals at 1000 and
3000 mg/kg bw per day during treatment. There were no
treatment-related changes in maternal organ weights. One animal at
3000 mg/kg bw per day resorbed her entire litter. There were no
other treatment-related changes in implantation efficiency, mean
fetal viability, size, sex ratio, fetal body weight or external,
visceral or skeletal development. Tolclofos-methyl was not
teratogenic in this study at doses up to and including 3000 mg/kg bw
per day, which was toxic to dams. The NOAEL for maternal toxicity
was 300 mg/kg bw per day (Motoyama et al., 1991).
(f) Genotoxicity
The results of tests for the genotoxicity of tolclofos-methyl
are summarized in Table 2.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Six male albino Japanese rabbits received 500 mg of
tolclofos-methyl (purity, 97%) on clipped intact or abraded dorsal
skin for 4 h under an occlusive dressing. No signs of irritation
were seen at any of the application sites seven days after treatment
(Matsubara et al., 1978).
Table 2. Results of tests for the genotoxicity of tolclofos-methyl
End-point Test system Concentration Purity Results Reference
of tolclofos-methyl (%)
In vitro
Reverse S. typhimurium TA98, 10, 100, 500, 1000, 2000 97.0 Negativea,b Suzuki & Miyamoto,
mutation 100, 1535, 1537, 1538 µg/plate
Reverse S. typhimurium TA98, 10, 50, 100, 500, 1000, 98.7 Negativea,c Moriya et al., 1981
mutation 100, 1535, 1537, 1538 5000 µg/plate
E. coli WP2uvrA
Reverse B. subtilis H17 rec+, 1, 10, 100, 1000 µg/disc 97.0 Negatived Suzuki & Miyamoto,
mutation M45 rec- 1978
Reverse B. subtilis 20, 50, 100, 200, 500, 98.7 Negativee Moriya et al., 1981
mutation H17 rec+, M45 rec- 1000, 2000, 5000 µg/disc
Host-mediated S. typhimurium in 870, 1750 mg/kg 97.0 Negativef Suzuki & Miyamoto,
assay male ICR mice 1978
Chromosomal Chinese hamster 10, 20, 40 µg/ml 96.6 Negativea,g Kogiso et al., 1990
aberration K1 ovary cells 37.5, 75, 150 µg/ml
Unscheduled Male Sprague-Dawley 0.3, 1, 3, 10, 20, 40 96.6 Negativea,i Hara et al., 1990
DNA synthesis rat hepatocytes µg/mlh
Unscheduled Human carcinoma 0.3, 3, 30, 300 µg/ml NR Negativej Monaco & Nunziata,
DNA synthesis cells (HeLa) 1981
Gene mutation Chinese hamster 1.5, 15, 150, 1500 µg/ml NR Negativea,k Monaco & Nunziata,
V79 lung cells 1981
Table 2 (contd)
End-point Test system Concentration Purity Results Reference
of tolclofos-methyl (%)
In vivo
Chromosomal Male ICR mice 500, 1000, 2000, 99.8 Negativei Hara & Suzuki, 1981
aberration 4000 mg/kg i.p.h
Dominant Male Sprague- 62.5, 208.3, 625.0 NR Negativej Brusick, 1981
lethal Dawley rats mg/kg orally
mutation
NR, not reported
a With and without metabolic activation.
b Positive controls ( N-methyl- N'-nitro- N-nitrosoguanidine and 2-acetylaminofluorene) yielded expected positive
results.
c Several positive controls yielded expected positive results.
d Positive control ( N-methyl- N'-nitro- N-nitrosoguanidine) yielded expected positive results.
e Positive (mitomycin C) and negative (Kanamycin) controls yielded expected results.
f Positive control ( N-nitrosodimethylamine) yielded expected positive results.
g Positive controls (mitomycin C and cyclophosphamide) yielded expected positive results.
h At 40 mg/ml, cell viability was less than 20%.
i Positive control (2-acetylaminofluorene) yielded expected positive results.
j Positive controls (methyl methanesulfonate and urethane) yield expected positive results.
k Positive controls (methyl methanesulfonate and N-nitrosodimethylamine) yielded expected positive results.
l At 2000 and 4000 mg/kg bw there was > 50% mortality at 48 h.
m Positive control (cyclophosphamide) yielded expected positive results.
n Positive control (triethylenemelamine) yielded expected positive results.
Groups of six male New Zealand white rabbits received 500 mg of
either a '50% wettable power' or '10% dust' moistened with saline on
1-inch2 (6.5-cm2) sites on the clipped dorsal intact or abraded
skin for 24 h under an occlusive dressing. No irritation, such as
erythema and oedema, was observed (Hara et al., 1981a,b).
Two male and one female New Zealand white rabbits received 0.5
ml of '50% flowable' tolclofos-methyl on 1 inch2 (6.5 cm2) of
clipped dorsal intact or abraded skin for 4 h under an occlusive
dressing. No irritation was observed (Nakanishi et al., 1989).
Each of eight male albino Japanese rabbits received 50 mg of
tolclofos-methyl (purity, 97%) in one eye. Five minutes after the
application, the treated eyes of five animals were flushed with 300
ml saline for 2 min. The treated eyes of the remaining animals were
similarly flushed 24 h after treatment. There were no corneal,
conjunctival or iridal effects up to seven days after treatment
(Matsubara et al., 1978).
Groups of nine male New Zealand white rabbits received 100 mg
of either a '50% wettable power' or '10% dust' in one eye; 30 s
after the application, the treated eyes of three animals per group
were flushed with 300 ml lukewarm water for 1 min. Slight congestion
of the iris was observed 24 h after application. Slight to moderate
hyperaemia and slight chemosis and/or discharge in conjunctiva were
also observed 1-48 h after application of the '50% wettable power'
to unwashed eyes. These changes had disappeared by 72 h after
application in all animals. No ocular lesions were found in the
washed eyes. The irritation potency of this formulation was judged
to be mild. Slight conjunctival hyperaemia and/or chemosis were
observed in animals with unwashed eyes and in one with washed eyes
1-24 h after application of the '10% dust'. There were no other
signs of irritation at any time. The formulation was classified as
minimally irritating to eyes (Hara et al., 1981a,b).
One male and two female New Zealand white rabbits received 0.1
ml of '50% flowable' tolclofos-methyl in one eye. Slight redness was
observed in conjunctiva after application, which disappeared within
24 h (Nakanishi et al., 1989).
The skin-sensitizing potential of tolclofos-methyl (purity,
97%) was assessed in guinea-pigs by the Landsteiner-Draize method.
Groups of 10 male Hartley guinea-pigs were given 10 intradermal
injections of 1 or 5% tolclofos-methyl (0.05 or 0.1 ml) in corn oil
at intervals of two to three days. Two weeks after the final
induction, the animals were challenged at a fresh site with 0.05-ml
intradermal injections of tolclofos-methyl at the same
concentrations used for induction. Negative control animals for both
dose groups were given the challenge injection only. Positive
control animals were treated with 0.05% 2,4-dinitrochlorobenzene
three times before challenge. Slight erythema and swelling were
observed in two animals after challenge treatment with 1%
tolclofos-methyl, in three after challenge with 5%, and in one
animal in each negative control group. Moderate erythema and/or
swelling was observed in the animals treated with
2,4-dinitrochlorobenzene. It was concluded that tolclofos-methyl did
not sensitize skin in this study (Matsubara et al., 1980).
The skin sensitizing potential of a '50% wettable powder' and a
'10% dust' of tolchlofos-methyl was tested in guinea-pigs by the
Buehler method. Groups of 10 male Hartley guinea-pigs received 500
mg of one formulation, slightly moistened with water, on clipped
dorsal skin under an occlusive dressing for 24 h. Induction was
performed 10 times at two- to three-day intervals.Positive control
animals were similarly treated with 0.5% 2,4-dinitro-chlorobenzene.
Negative controls were not subjected to the induction treatment. Two
weeks after the final induction, the test animals and positive
controls were challenged as in the sensitizing treatment. No skin
reactions were observed in the negative control or treated animals.
Slight to severe erythema and swelling were observed in the positive
controls. The formulations were considered not to sensitize skin
(Hara et al., 1981c,d).
The skin sensitizing potential of a '50% flowable formulation'
was tested in guinea-pigs by the Buehler method. Ten male Hartley
guinea-pigs received 0.5 ml of the formulation for 6 h once a week
for three weeks on the clipped dorsal skin under an occlusive
dressing. Positive control animals were similarly treated with 0.5%
2,4-dinitrochlorobenzene. Negative control animals were not
subjected to the induction treatment. Two weeks after the final
induction, animals were challenged as in the sensitizing treatment.
No skin reactions were observed in the negative control or treated
animals. Slight to severe erythema and swelling were observed in the
positive controls. The formulation was considered not to sensitize
skin (Nakanishi et al., 1990).
(ii) Delayed neuropathy
Groups of 10 Leghorn hens were administered 0 or 8000 mg/kg bw
tolclofos-methyl (purity, 97%) or 500 mg/kg bw tri- ortho-cresyl
phosphate orally in corn oil. After a 21-day observation period, a
second dose of vehicle or tolclofos-methyl was administered. Animals
were sacrificed 21 days after the second dose. There were no deaths
in the groups given the vehicle or tolclofos-methyl. Plasma
cholinesterase activity in the group treated with tolclofos-methyl
was decreased by nearly 50% eight days after the first dose but had
recovered to the pre-treatment level 21 days after dosing. Hens
treated with tolclofos-methyl had no signs of leg weakness or
paralysis and no histopathological changes in the nervous tissues.
Birds treated with tri- ortho-cresyl phosphate had the typical
clinical and histopathological signs of delayed polyneuropathy
(Okuno et al., 1982).
3. Observations in humans
The medical records of 20 workers in Japan were reviewed. All
workers had been engaged continuously in packaging operations since
manufacture of technical-grade tolclofos-methyl began in 1988, for
an average of 4 h/day. No occupation-related problems were observed
or reported. Plasma and erythrocyte cholinesterase activities were
not measured (Murayama, 1991).
Comments
Tolclofos-methyl is excreted rapidly in rats and mice,
predominantly in the urine; less than 1% of the dose was retained in
tissues after seven days. In both species, metabolism occurred
mainly by oxidation of P=S to P=O, oxidation of the 4-methyl group
and cleavage of the P-O-aryl and P-O-methyl linkages. There are four
main metabolites in mice, one of which is a glycine conjugate, and
four in rats, which are excreted as glucuronides.
Tolclofos-methyl had low acute toxicity when administered by
the oral, dermal, subcutaneous or intraperitoneal route. The overt
signs of acute toxicity are not typical of an anticholinesterase, as
no chromodaccryorrhoea, lachrymation or fasciculation was seen,
although some inhibition of plasma, erythrocyte and brain
cholinesterase activities was observed. WHO (1992) has classified
tolclofos-methyl as unlikely to present an acute hazard in normal
use.
In a nine-month study of toxicity in which mice were fed
tolclofos-methyl in the diet at 0, 10, 30, 100 or 3000 ppm, the
NOAEL was 100 ppm, equal to 12 mg/kg bw per day, on the basis of
inhibition of brain cholinesterase activity and effects on body
weight at 3000 ppm.
In a 32-34-day study of toxicity in which rats were fed diets
containing 0, 200, 1000, 5000 or 20 000 ppm, the NOAEL was 1000 ppm,
equal to 79 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity and increased relative kidney weight at 5000
ppm. In a 13-week study of toxicity in which rats were fed diets
containing 0, 100, 1000 or 10 000 ppm, the NOAEL was again 1000 ppm,
equal to 66 mg/kg bw per day, on the basis of effects on body, liver
and kidney weights at 10 000 ppm. In a 28-week study of toxicity in
which rats were fed dietary levels of 0, 300, 1000, 3000 or 10 000
ppm, the NOAEL was also 1000 ppm, equal to 65 mg/kg bw per day, on
the basis of histopathological changes in the livers of females at
3000 ppm.
In a 26-week dietary study in dogs fed levels of 0, 200, 600 or
2000 ppm, the NOAEL was 600 ppm, equal to 21 mg/kg bw per day, on
the basis of reduced body-weight gain, an increased serum level of
alkaline phosphatase and increased liver weight at 2000 ppm.
In a 52-week study of toxicity in dogs given dietary
concentrations of 0, 80, 400 or 2000 ppm, the NOAEL was 400 ppm,
equal to 11 mg/kg bw per day, on the basis of increased liver weight
(with hepatocytic hypertrophy), reduced body-weight gain and slight
anaemia at 2000 ppm.
In a 104-week study of toxicity and carcinogenicity in which
mice were given dietary concentrations of 0, 10, 50, 250 or 1000
ppm, the NOAEL was 50 ppm, equal to 6.5 mg/kg bw per day, on the
basis of reduced brain cholinesterase activity and increased
absolute and relative kidney weights at higher levels. There was no
evidence of carcinogenicity.
A 122-129-week study of toxicity and carcinogenicity was
performed in which rats were given dietary concentrations of 0, 100,
300 or 1000 ppm. The NOAEL was 1000 ppm, equal to 41 mg/kg bw per
day, on the basis of the absence of any significant finding. There
was no evidence of carcinogenicity.
In a three-generation study (two litters per generation) in
rats, tolclofos-methyl was given at dietary levels of 0, 100, 300 or
1000 ppm. The NOAEL was > 1000 ppm, equivalent to 100 mg/kg bw per
day, on the basis of the absence of any significant findings.
In a study of teratogenicity in which rats were given 0, 5, 15
or 50 mg/kg bw per day of tolclofos-methyl by gavage, the NOAEL was
50 mg/kg bw per day on the basis of the absence of any significant
findings. The study was not considered to be fully adequate because
the highest dose tested was not maternally toxic. A similar study
was conducted at levels of 0, 100, 300 or 1000 mg/kg bw per day. The
NOAEL was 300 mg/kg bw per day on the basis of reduced body-weight
gain in dams at 1000 mg/kg bw per day. There was no evidence of
teratogenicity.
A study of teratogenicity was conducted in rabbits given 0,
300, 1000 or 3000 mg/kg bw per day orally. The NOAEL for maternal
toxicity was 300 mg/kg bw per day on the basis of reduced
body-weight gain in dams at 1000 mg/kg bw per day. There was no
evidence of teratogenicity.
Tolclofos-methyl was studied in a wide range of tests for
genotoxicity in vivo and in vitro. The Meeting concluded that
the compound is not genotoxic.
Tolclofos-methyl did not cause delayed neuropathy in chickens.
The available observations in humans were considered by the
Meeting but did not directly contribute to an estimation of an ADI.
An ADI was established on the basis of a NOAEL of 50 ppm, equal
to 6.5 mg/kg bw per day, in the 104-week study of toxicity and
carcinogenicity study in mice, and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equal to 6.5 mg/kg bw per day (104-week study
of toxicity and carcinogenicity)
Rat: 1000 ppm, equal to 41 mg/kg bw per day (122/129-week
study of toxicity and carcinogenicity)
Rabbit: 300 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Dog: 400 ppm, equal to 11 mg/kg bw per day (52-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.07 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Brusick, D.J. (1981) Mutagenicity evaluation of S-3349 T.G. Lot No.
4, rat dominant lethal assay. Litton Bionetics Inc. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Colley, J., Welch, P.J., Heywood, R., Prentice, D.E., Cherry, C.P.,
Mullins, P.A., Gibson, W.A. & Almond, R.H. (1982) S-3349, toxicity
to rats by dietary administration for 4 weeks. Huntington Research
Centre Ltd. Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd, Osaka, Japan.
Cox, R.H. & Brusick, D.J. (1988) Chronic toxicity study in dogs with
S-3349. Hazleton Laboratories America Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Eschbach, J.C. & Hogan, G.K. (1981a) An acute inhalation toxicity
study of S-3349 50WP in the rat. Bio/dynamics Inc. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Eschbach, J.C. & Hogan, G.K. (1981b) An acute inhalation toxicity
study of S-3349 10d in the rat. Bio/dynamics Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Esumi, Y. & Yokoshima, T. (1989) Study on metabolism of
tolclofos-methyl in rats. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Gargus, J.L. (1986) 21-Day dermal toxicity study, in rabbits with
Rizolex, technical 97.7%. Hazleton Laboratories America Inc.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Hara, M. & Suzuki, H. (1981) In vivo chromosomal aberration test of
S-3349 on bone marrow cells of mice. Sumitomo Chemical Co., Ltd.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981a) Primary eye and skin
irritation tests of S3349 50% water-dispersible power in rabbits.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981b) Primary eye and skin
irritation tests of S-3349 10% dust formulation in rabbits. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981c) Skin sensitization test
of S3349 50% water-dispersible powder in guinea pigs. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981d) Skin sensitization test
of S3349 10% dust formulation in guinea pigs. Sumitomo Chemical Co.,
Ltd. Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd, Osaka, Japan.
Hara, M., Ota, M., Kogiso, S., Yoshitake, A. & Yamada, H. (1990) In
vitro unscheduled DNA synthesis (UDS) assay of Rizolex in rat
hepatocytes. Sumitomo Chemical Co., Ltd. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hardy, C.J., Jackson, G.C., Lewis, D.J. & Gopinath, C. (1986)
Technical Rezolex, acute inhalation toxicity in rats, 4-hour
exposure. Huntington Research Centre Ltd. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hiromori, T., Suzuki, T., Okuno, Y., Ito, S., Murakami, M., Kadota,
T. & Miyamoto, J. (1978) Six-month oral toxicity study of S-3349 in
rats. Sumitomo Chemical Co., Ltd. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hiromori, T., Misaki, Y., Ito, S., Kohda, A., Kato, T. & Yamada, H.
(1989a) Acute oral toxicity study of Rizolex 50 FL in rats. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Hiromori, T., Misaki, Y., Ito, S., Kohda, A., Kato, T. & Yamada, H.
(1989b) Acute dermal toxicity study of Rizolex 50 FL in rats.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Jackson, G.C., Hardy, C.J., Morrow, J. & Gopinath, C. (1990) Rizolex
50 FL, acute inhalation toxicity study in rats, 4-hour exposure.
Huntington Research Centre Ltd. Unpublished report submitted to WHO
by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kimura, J., Yosioka, K., Ozaki, K., Adachi, H., Seki, T., Matsuo, M
&, Yamada, H. (1990) 90-Day oral toxicity study of S-3349 in rats.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kogiso, S., Yamamoto, K., Hara, M., Yositake, A. & Yamada, H. (1990)
In vitro chromosomal aberration test of Rizolex in Chinese hamster
ovary cells (CHO-K1). Sumitomo Chemical Co., Ltd. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Krautter, G.R., Downs, J., Hoglan, N., Marsh, J.D. & Lawrence, L.J.
(1987) Metabolism of tolclofos-methyl in the rat. Pharmacology &
Toxicology Research Laboratory. Unpublished report submitted to WHO
by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Krautter, G.R., Downs, J., Hoglan, N., Marsh, J.D. & Lawrence, L.J.
(1988) Metabolism of tolclofos-methyl in the rat. Final report
amendment. Pharmacology & Toxicology Research Laboratory.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Matsubara, T., Hara, S. & Kadota, T. (1978) Eye and skin irritation
test of S-3349 in rabbits. Sumitomo Chemical Co., Ltd. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Matsubara, T., Hara, S., Suzuki, T., Kadota, T. & Miyamoto, J.
(1980) Skin sensitization test of S-3349 in guinea pigs. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Mihara, K., Ohkawa, H. & Miyamoto, J. (1981) Metabolism of
tolclofos-methyl in rats and mice. J. Pestic. Sci., 6, 65-74.
Miyamoto, J. (1985) Comments on toxic effects and minimum effect
level of S-3349 in chronic oral toxicity studies in rats. Sumitomo
Chemical Co., Ltd., Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Monaco, M. & Nunziata, A. (1981) Report of mutagenicity experiment
performed on the test substance S-3349, Sumitomo Chemical Co., Ltd.
Unpublished report from Centro Ricerca Farmaceutica, Pomezia, Italy.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Moore, M.R. (1993) Comments on the toxicological significance of
hepatocytic pigment and on the no-adverse-effect-level (NOAEL) in a
chronic toxicity study in dogs treated with S-3349 (Hazleton project
No. 343-176). Hazleton Washington Inc. Unpublished report submitted
to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Moriya, M., Ohta, T. & Shirasu, Y. (1981) S-3349, microbial
mutagenicity study. Institute of Environmental Toxicology.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Morseth, S.L., Burlew, J.A., Vargas, K.J., Lewis, S.A., Thakur, A.K.
& Phipps, N.G. (1987) Teratology study of S-3349 T.G. in rats.
Hazleton Laboratories America Inc. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Motoyama, M., Kashima, M. & Takahashi, M. (1991) Teratology study of
S-3349 in rabbits. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Murayama, F. (1991) A review on medical examination of factory
workers exposed to tolclofos-methyl. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Nakanishi, T., Kohda, A., Kato, T. & Yamada, H. (1989) Primary eye
and skin irritation tests with Rizolex 50 FL in rabbits. Sumitomo
Chemical Co., Ltd. Unpublished study, submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Nakanishi, T., Kohda, A., Kato, T. & Yamada, H. (1990) Skin
sensitization test of Rizolex 50 FL in guinea pigs. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Okuno, Y., Yamada, T., Hosokawa, S. & Miyamoto, J. (1982) Acute
delayed neurotoxicity study of S-3349 in hens. Sumitomo Chemical
Co., Ltd. Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd, Osaka, Japan.
Pence, D.H., Lemen, J.K. & Weatherholtz, W.M. (1978) Acute oral
toxicity study in male and female dogs, S-3349. Hazleton
Laboratories America Inc. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Weatherholtz, W.M., Kundzins, W., Alsaker, R.D., Brown,
H.R. & Greenspun, K.S. (1979a) Subacute dietary administration in
dogs, S-3349. Hazleton Laboratories America Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Pence, L.A., Durloo, R. & Twigg, C.J. (1979b)
Teratology study in rats, S-3349. Hazleton Laboratories America Inc.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Pence, D.H., Serota, D., Alsaker, R.D., Koka, M., Banas, D.A.,
Dawkins, B.G., Kundzins, W. & Hepner, K.E. (1982) Chronic toxicity
study in rat, S-3349. Hazleton Laboratories America Inc. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Phipps, N., Alsaker, R.D., Hepner, K.E. & Spicer, K.M.
(1985a) 104-Week cholinesterase activity study in male and female
rats, S-3349. Hazleton Laboratories America Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Hepner, K.E., Wolfe, G.W., Voelker, R.W. & Ulland, B.N.
(1985b) Three-generation reproduction study in rats, S-3349.
Hazleton Laboratories America Inc. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Satoh, R., Kashima, M., Satoh, H., Motoyama, M. & Ishikawa, A.
(1983) Twenty-four-month chronic toxicity study of S-3349 in
pulverized diet in mice. Nippon Experimental Medical Research
Institute. Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd, Osaka, Japan.
Segawa, T. (1978) Acute toxicity study of S-3349 in rats and mice.
Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981a) Acute oral toxicity study of S-3349 50% wettable
powder in mice. Hiroshima University School of Medicine. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981b) Acute oral toxicity study of S-3349 10% dust in
mice. Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981c) Acute dermal toxicity study of S-3349 50%
wettable powder in mice. Hiroshima University School of Medicine.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Segawa, T., (1981d) Acute dermal toxicity study of S-3349 10% dust
in mice. Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981e) Acute oral toxicity study of S-3349 50% wettable
powder in rats. Hiroshima University School of Medicine. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981f) Acute oral toxicity study of S-3349 10% dust in
rats. Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981g) Acute dermal toxicity study of S-3349 50%
wettable powder in rats. Hiroshima University School of Medicine.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Segawa, T. (1981h) Acute dermal toxicity study of S-3349 50%
wettable powder in rats. Hiroshima University School of Medicine.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Suzuki, H. & Miyamoto, J. (1978) Studies on mutagenicity of S-3349
with bacterial systems. Sumitomo Chemical Co., Ltd. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Suzuki, T., Okuno, Y., Hiromori, T., Ito, S., Murakami, M., Kadota,
T & Miyamoto, Y. (1978) Nine-month feeding study of S-3349 in mice.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
2. Toxicological studies
(a) Acute toxicity
Data on the acute toxicity of tolclofos-methyl (technical-grade
and formulations) in laboratory animals are summarized in Table 1.
Animals showed decreased spontaneous motor activity, dyspnoea,
piloerection, urinary incontinence and ataxia. Recovery was complete
by day 10. Brain cholinesterase activity was lower 16 days after
treatment in dogs given 1000 mg/kg bw than in animals given lower
doses; however, as no control data were reported, conclusions cannot
be drawn. No treatment-related gross changes were seen at necropsy
in any species.
Table 1. Acute toxicity of technical-grade and formulated tolclofos-methyl in laboratory animals
Species Strain Sex Route Formulation LD50 Reference
(purity) (mg/kg bw)
Mouse dd M Oral Technical (97%) 3500 Segawa, 1978
F 3600
Mouse ICR M&F Oral 50% wettable > 5000 Segawa, 1981a
powder (98%)
Mouse ICR M&F Oral 10% dust (98.8%) > 5000 Segawa, 1981b
Mouse dd M&F Dermal Technical (97%) > 5000 Segawa, 1978
Mouse ICR M&F Dermal 50% wettable > 5000 Segawa, 1981c
powder (98%)
Mouse ICR M&F Dermal 10% dust (98.8%) > 5000 Segawa, 1981d
Mouse dd M Intraperitoneal Technical (97%) 1070 Segawa, 1978
F 1260
Mouse dd M&F Subcutaneous Technical (97%) > 5000 Segawa, 1978
Rat SD M&F Oral Technical (97%) approx. 5000 Segawa, 1978
Rat SD M&F Oral 50% wettable > 5000 Segawa, 1981e
powder (98%)
Rat SD M&F Oral 10% dust (98.8%) > 5000 Segawa, 1981f
Rat SD M&F Oral 50% flowable > 5000 Hiromori et al.,
1989a
Rat SD M&F Dermal Technical (97%) > 5000 Segawa, 1978
Rat SD M&F Dermal 50% wettable > 5000 Segawa, 1981g
powder (98%)
Rat SD M&F Dermal 10% dust (98.8%) > 5000 Segawa, 1981h
Rat NR M&F Dermal 50% flowable > 2000 Hiromori et al.,
1989b
Table 1 (contd)
Species Strain Sex Route Formulation LD50 Reference
(purity) (mg/kg bw)
Rat SD M Intraperitoneal Technical (97%) 5000 Segawa, 1978
F 4900
Rat SD M&F Subcutaneous Technical (97%) > 5000 Segawa, 1978
Rat Wistar M&F Inhalation Technical (97.7%) > 3.32 mg/la Hardy et al., 1986
Rat SD M&F Inhalation 50% wettable > 1.9 mg/l Eschbach &
powder (98%) Hogan, 1981a
Rat SD M&F Inhalation 10% dust (98.8%) > 1.8 mg/l Eschbach &
Hogan, 1981b
Rat SD M&F Inhalation 50% flowable > 1.07 mg/la Jackson et al., 1990
Dog NR M&F Oral Technical (98.7%) > 1000 Pence et al., 1978
SD, Sprague-Dawley; M, male; F, female; NR, not reported
a 4-h whole-body exposure
(b) Short-term toxicity
Mice
Groups of 15 male and 15 female ddY mice were fed diets
containing tolclofos-methyl (purity, 97%) at 0, 10, 30, 100 or 3000
ppm, equal to 1.2, 3.8, 12 or 510 mg/kg bw per day for males and
1.4, 4.1, 14 or 560 mg/kg bw per day for females, for nine months.
Three satellite groups of five males and five females each were
similarly treated and killed at 2, 4 and 13 weeks for determination
of cholinesterase activity. Animals were observed daily for
mortality and signs of toxicity; at the end of exposure, an
ophthalmological examination and clinical chemical analyses
(including plasma and erythrocyte cholinesterase) were undertaken,
and brain cholinesterase activity was measured. Most organs were
weighed and examined microscopically. There were no overt signs of
toxicity and no deaths. Body weights were reduced by about 20% and
body-weight gain by about 35% in animals of each sex at 3000 ppm,
but at this dose food consumption was increased in males and females
and water intake was increased in males. No treatment-related
ophthalmological changes were observed, and haematological
parameters were unaffected by treatment. The cholesterol level was
increased by 35% in females at 3000 ppm. A dose-related decrease in
plasma cholinesterase activity was found in animals of each sex,
with activities that were 63-94% of the control values in mice at 30
ppm, 30-56% at 100 ppm and 4-35% (61% in one case) at 3000 ppm. At
the high dose, the activity reached a plateau after four weeks.
Erythrocyte cholinesterase activity was decreased to 39-46% of the
control level in males at 3000 ppm at weeks 13 and 40 and to 57-75%
of the control level in females at this dose at all time points.
Brain cholinesterase activity was unaffected by treatment in females
but was reduced by about 24% in males at 3000 ppm at weeks 13 and
40. Organ weights in the 3000-ppm group were reduced, but neither
gross nor histopathological changes attributable to treatment were
observed. The NOAEL was 100 ppm, equal to 12 mg/kg per day, on the
basis of inhibition of brain cholinesterase activity and an effect
on body weight at the highest dose (Suzuki et al., 1978).
Rats
Groups of 10 male and 10 female Sprague-Dawley rats were fed
diets containing tolclofos-methyl (purity, 97.9%) at 0, 200, 1000,
5000 or 20 000 ppm for 32-34 days. Animals were observed twice daily
for mortality and clinical signs, and food consumption and body
weight were recorded weekly. Ophthalmoscopic examination, standard
haematological tests and clinical chemical analyses were performed
at week 4, and certain tests were repeated at termination. Plasma
and erythrocyte cholinesterase activities were determined in the
control and high-dose groups at week 4 and in all animals at
termination, when brain cholinesterase activity was also measured.
At terminal sacrifice, relevant organs were weighed and most organs
were examined microscopically. There were no treatment-related
deaths. Reductions in food consumption (-16% in males and -6% in
females) and body-weight gain (-45% in males and -37% in females)
were observed at 20 000 ppm. Body-weight gain was slightly reduced
in animals of each sex at 5000 ppm during week 1. Haematological
parameters were unaffected by treatment. Cholesterol levels were
increased (by 116-138%) in animals of each sex at 20 000 ppm, and
total protein and albumin levels were slightly higher in males in
this group. Other differences between treated and untreated animals
were detected, but the values were within the normal range. Plasma
cholinesterase activity was reduced by 14% in males and by 50% in
females at 20 000 ppm. Erythrocyte cholinesterase activity was
reduced by 9-19% in animals of each sex at 5000 and 20 000 ppm;
however, no clear dose-response relationship was seen. Brain
cholinesterase activity was lower (by 12-31%) than that in controls
in males in all treated groups and in females (by 20%) at 5000 and
20 000 ppm. Although the reduction was statistically significant in
all male groups, no clear dose-response relationship was found, and
the activity in controls was slightly higher than usual. There were
no ophthalmoscopic alterations or gross changes at necropsy. The
relative weights of livers were increased (by 12%) in females at
5000 ppm and in males and females at 20 000 ppm (by 27% in males and
39% in females). Hypertrophy of hepatocytes was observed in animals
of each sex at 20 000 ppm. The relative weights of the kidneys were
slightly increased in animals of each sex at 5000 and 20 000 ppm,
but no treatment-related histopathological changes were seen. The
NOAEL was 1000 ppm, equal to 79 mg/kg bw per day, on the basis of
increased relative kidney weights and brain cholinesterase activity
at higher doses (Colley et al., 1982).
Groups of 12 male and 12 female Sprague-Dawley rats were fed
diets containing tolclofos-methyl (96.6% pure) at 0, 100, 1000 or 10
000 ppm, equal to 6.5, 66 or 653 mg/kg bw per day for males and 7.1,
71 or 696 mg/kg bw per day for females, for 13 weeks. Animals were
observed daily for mortality and clinical signs, and body weight and
food and water consumption were determined weekly. Ophthalmological
analysis, urinalysis, standard haematological tests, clinical
chemical analyses and measurement of plasma, erythrocyte and brain
cholinesterase activities were undertaken at termination, when
relevant organs were weighed and most organs were examined
microscopically. No deaths and no significant abnormal clinical or
ophthalmological signs were noted during the study. Depression of
body-weight gain of males (-20%) and females (-15%) at 10 000 ppm
was seen throughout the treatment period and was associated with
similar decreases in food and water consumption. A slight decrease
in food consumption was noted in females at 1000 ppm during week 1.
Decreased activities of plasma (16-53%), brain (8-9%) and
erythrocyte (19-20%) cholinesterase were noted in animals of each
sex at 10 000 ppm; a minimal decrease in erythrocyte cholinesterase
activity (10%) was also seen in females at 1000 ppm. Liver weight
was increased in animals of each sex at 10 000 ppm, and this was
found at histopathological examination to be associated with
hypertrophy of hepatocytes. Changes in some clinical chemical
parameters were observed at 10 000 ppm, which included increased
levels of cholesterol (48% in males, 102% in females) and
phospholipids (37% in males, 64% in females) and slightly increased
alpha2- and ß-globulin levels in animals of each sex. Other
changes, although statistically significant, were minor and of
questionable biological significance. At 10 000 ppm, increased
relative kidney weights were seen in animals of each sex, increased
blood urea nitrogen levels were seen in males only and decreased
urinary pH was seen in males and females. At 1000 ppm, relative
kidney weights were slightly increased in females and relative liver
weights in males. The Meeting considered that the increased weight
ratios observed at 1000 ppm were not biologically relevant and that
the NOAEL was 1000 ppm, equal to 66 mg/kg bw per day (Kimura et
al., 1990).
Groups of 15 male and 15 female Sprague-Dawley rats were fed
diets containing tolclofos-methyl (purity, 97%) at 0, 300, 1000,
3000 or 10 000 ppm, equal to 16, 51, 164 or 540 mg/kg bw per day in
males and 18, 65, 184 or 623 mg/kg bw per day in females, for 28
weeks. Animals were observed daily for clinical signs, and body
weight and food consumption were measured weekly. Ophthalmologic
examinations were carried out after three months and at termination;
standard haematological tests and clinical chemical analyses were
performed at termination. Urinalysis was carried out in 12 animals
per group after three months and at termination. Plasma and
erythrocyte cholinesterase activities were determined after 2, 4 and
13 weeks, and at termination; brain cholinesterase was determined
only at termination, when relevant organs were weighed and several
were examined histologically.
There were no overt signs of toxicity and no treatment-related
deaths. Although the body weights of animals of each sex at 10 000
ppm were lower than those of controls throughout the study, their
initial body weights were also statistically significantly lower.
Body-weight gain was significantly reduced (by 18%) in females at 10
000 ppm. Food consumption was unaffected by treatment. Treatment did
not affect ophthalmologic, urinary, haematological or clinical
chemical parameters. Plasma cholinesterase activity was lower (by
23-41%) in females at 10 000 ppm than in controls at weeks 2, 4, 13
and 28. Erythrocyte cholinesterase activity was highly variable and
was reduced by 15-19% in comparison with controls in males at 10 000
ppm at weeks 2, 4 and 28. Brain cholinesterase activity was
unaffected by treatment. Kidney and liver weights were slightly
increased in animals of each sex at 10 000 ppm and in females at
1000 and 3000 ppm, but the biological significance of these findings
was questionable. No gross abnormalities were seen at necropsy.
Bile-duct proliferation was found in the livers of 3/15 females at
10 000 ppm and oval-cell proliferation was seen in 2/15 females at
3000 ppm and 3/15 at 10 000 ppm. No histopathological changes were
seen in the kidneys. The NOAEL was 1000 ppm, equal to 65 mg/kg bw
per day, on the basis of histopathological changes in the livers of
females at higher doses (Hiromori et al., 1978).
Rabbits
Groups of five male and five female New Zealand white rabbits
received tolclofos-methyl (purity, 97.7%) in acetone on the skin at
doses of 0, 30, 300 or 1000 mg/kg bw per day for 6 h per day, on
five days per week for 21 days. There were no deaths and no
treatment-related clinical signs; neither body weight nor food
consumption was affected by treatment. Dermal irritation (slight
erythema) was seen in some animals in all treated groups from day 6.
Eosinophil counts were increased in males at 1000 mg/kg bw per day,
and the level of inorganic phosphorus was decreased (by 14-20%) in
animals of each sex at this dose. Brain cholinesterase activity was
about 15% lower than that in controls in males and about 15% higher
in females at 300 and 1000 mg/kg bw per day. These changes were
considered not to be of biological significance. Erythrocyte
cholinesterase activity was lower than in controls in males at the
same two doses, but there was no dose-effect relationship. Plasma
cholinesterase activity was lower (by 22-29%) than in controls in
animals of each sex at 300 and 1000 mg/kg bw per day. The relative
weights of the kidneys were increased (by 20%) in females at 1000
mg/kg bw per day. Macroscopic findings consisted of accumulation of
the compound on treated skin; microscopic examination revealed
hyperkeratosis, acanthosis and subepidermal pleocellular
infiltration of the skin (Gargus, 1986).
Dogs
Groups of six male and six female beagle dogs were fed diets
containing tolclofos-methyl (purity, 98.7%) at 0, 200, 600 or 2000
ppm, equal to 7.4, 23 or 69 mg/kg bw per day in males and 4.1, 21 or
65 mg/kg bw per day in females, for 26 weeks. Animals were observed
daily for mortality and weekly for clinical signs, body weight and
food consumption. Ophthalmoscopic examinations were performed before
treatment and at termination of the study. Standard haematological
tests and clinical chemical analyses were performed at regular
intervals until termination. Erythrocyte and plasma cholinesterase
activities were determined at weeks 2, 4, 8, 13 and 26. Brain
cholinesterase was determined at termination, when relevant organs
were weighed and most were examined histologically. There were no
deaths and no overt signs of toxicity. Body-weight gain was reduced
by 54% in males and by 46% in females at 2000 ppm, although food
consumption was unaffected by treatment. There were no
treatment-related ocular changes. Although the haematocrit was
within the normal range, decreased values in comparison with
controls (-11% in males and -10% in females) were observed at 2000
ppm. The mean corpuscular volume and mean corpuscular haemoglobin
concentration were not affected by treatment. Alkaline phosphatase
activity was increased by 139-295% in animals of each sex at 2000
ppm throughout the study. Total bilirubin was not increased in
treated animals. At 2000 ppm, plasma cholinesterase activity was
decreased by 19-26% in females throughout the study, but no
significant decreases were seen in erythrocyte cholinesterase
activity in animals of each sex or in plasma cholinesterase activity
in males. Brain cholinesterase activity was unaffected by treatment.
Urinalysis gave unremarkable results, and there were no gross
changes at necropsy. Liver weights were increased (by 59% in males
and 43% in females) at 2000 ppm, but there were no concomitant
histological changes, and there were no treatment-related
histopathological changes. The NOAEL was 600 ppm, equal to 21 mg/kg
bw per day, on the basis of increased liver weights, reduced
body-weight gain and increased alkaline phosphatase activity at 2000
ppm (Pence et al., 1979a).
Groups of six male and six female beagle dogs were fed diets
containing tolclofos-methyl (purity, 96.7%) at 0, 80, 400 or 2000
ppm, for 52 weeks. Animals were observed daily for mortality and
weekly for clinical signs. Ophthalmoscopic examinations were
performed before treatment and at weeks 26 and 52. Standard
haematological tests, clinical chemical analyses and determinations
of plasma and erythrocyte cholinesterase activities were performed
one week before treatment and at weeks 13, 26 and 52. Brain
cholinesterase was determined at termination, when relevant organs
were weighed and most were examined histologically. No clinical
signs of toxicity were observed. Decreased food consumption (-9% in
males and -16% in females) and body-weight gain (-32% in males and
-54% in females), slightly lower erythrocyte counts (-8% in males
and -20% in females), haematocrit value (-7% in males and -17% in
females) and haemoglobin concentration (-8% in males and -7% in
females), slightly decreased albumin and total protein levels
(significantly at some time points) and elevated alkaline
phosphatase levels (> 200%) were found at 2000 ppm.
Plasma cholinesterase activity was slightly, but not
significantly lower than that in controls in females at the highest
dose; erythrocyte and brain cholinesterase activities were not
affected by the treatment. No treatment-related ophthalmoscopic
alterations were observed. Increases in mean liver weights, both
absolute (+32% in males and +61% in females) and relative (+52% in
males and +74% in females), were seen in dogs at 2000 ppm.
Microscopic examination of the livers revealed increased incidences
of hepatocytic hypertrophy and intracytoplasmic homogeneous material
and an increased amount of hepatocytic pigment in these animals. The
amount of this pigment was also increased at 400 ppm, and it was
present in control animals. Its nature has not been elucidated, but
it does not contain iron or bilirubin. These dogs also had decreased
absolute (-46%) and relative (-40%) weights of the prostate, and the
absolute and relative weights of the pancreas were increased by
16-27% and 28-49%, respectively. The NOAEL was 400 ppm, equal to 11
mg/kg bw per day, on the basis of reduced body-weight gain,
increased liver weights (with hepatic hypertrophy) and slight
anaemia at 2000 ppm (Cox et al., 1988; Moore, 1993).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 70 male and 70 female B6C3F1 mice were fed diets
containing tolclofos-methyl (purity, 94.3%) at 0, 10, 50, 250 or
1000 ppm, equal to 1.3, 6.5, 32 or 134 mg/kg bw per day in males and
1.3, 6.8, 34 or 137 mg/kg bw per day in females, for up to 104
weeks. Animals were observed twice daily for clinical symptoms and
mortality. Body weight was measured weekly during the first 13 weeks
and then once every four weeks. Food intake was measured weekly.
Standard urinalysis was performed in 10 animals from each group at
6, 12 and 18 months and in all survivors at termination of the
study. Standard haematological tests were performed in 10 animals
from each group at 12 months and in all survivors at termination.
Standard clinical chemical analyses and measurements of serum,
erythrocyte and brain cholinesterase activities were done in 10
animals from each group at 6 and 12 months and in all animals at
termination. Animals in the control and 1000-ppm groups were
subjected to ophthalmologic examination at 12 and 24 months. Ten
animals from each group were necropsied at 6 and at 12 months, at
termination or when found dead or moribund. Relevant organs were
weighed and examined microscopically.
There were no treatment-related effects on mortality and no
overt signs of toxicity, including ophthalmologic changes.
Body-weight gain was slightly reduced in females at 1000 ppm up to
week 52; food consumption was also slightly decreased in this group
after 52 and 104 weeks. Food conversion efficiency, water intake,
urinary parameters and the haematological profile were unaffected by
treatment. Serum cholinesterase activity was decreased in animals of
each sex at 250 ppm (by 25-52%) and at 1000 ppm (by 43-81%) at both
times, and erythrocyte cholinesterase activity was decreased by
11-28% at 250 ppm and by 13-47% at 1000 ppm; inhibition of both
enzymes was greater at termination. A dose-related decrease was
found in brain cholinesterase activity in females (by 7-24% at 250
ppm and 9-33% at 1000 ppm) and males (by 10-13% at 250 ppm and
17-26% at 1000 ppm). Serum and brain, but not erythrocyte,
cholinesterase activities were lower (by 12 and 18%, respectively)
in females at 50 ppm at week 28 than in controls, which had higher
levels than at week 52 and at termination. Glucose levels were
increased in males and were slightly increased in females at 1000
ppm at week 104. Absolute and/or relative kidney weights were
increased in animals of each sex at 1000 ppm and in males at 250 ppm
at week 52, and in females at 250 ppm at week 104. The weights of
the thymus were decreased in females at 1000 ppm at weeks 52 and
104, and pituitary weights were increased in females at this dose at
week 104. No treatment-related changes were seen at necropsy or on
histopathological examination at any time. Tolclofos-methyl was not
found to be carcinogenic. The NOAEL was 50 ppm, equal to 6.5 mg/kg
bw per day, on the basis of reduced brain cholinesterase activity
and increased absolute and relative kidney weights at higher doses
(Satoh et al., 1983).
Rats
Groups of 65 male and 65 female Fischer 344 rats were fed diets
containing tolclofos-methyl (purity, 94.9-98.7%) at 0, 100, 300 or
1000 ppm for 122 weeks (males) or 129 weeks (females). At week 52,
10 males and 10 females from each group were killed. Animals were
observed twice a day for mortality and signs of morbidity. Body
weight and food consumption were recorded weekly (weeks 0-26), every
two weeks (weeks 27-52) or every four weeks until termination.
Ophthalmoscopic examinations were performed every 26 weeks and at
termination. Standard haematological tests, clinical chemical
analyses and measurements of erythrocyte and plasma cholinesterase
activities were conducted in 10 animals of each sex in each group
one week before treatment, at weeks 4, 13, 26, 52, 78 and 104 and at
termination. Brain cholinesterase activity was determined in 10
animals of each sex per group at week 52 and at termination. All
animals killed or found dead were necropsied, and relevant organs
were weighed; most organs were then examined histologically.
There were no treatment-related effects on mortality and no
overt signs of toxicity. A slight decrease in body-weight gain was
observed in males at 1000 ppm. Food consumption and food conversion
efficiency were unaffected by treatment, as was the haematological
profile. There was a dose-related trend to decreased alkaline
phosphatase activity (by 9-46%) in all treated groups: The activity
was significantly decreased in males at 1000 ppm throughout the
study, in males at 300 ppm from week 13, and occasionally in the
other treated groups. Brain cholinesterase activity was decreased in
all treated groups at the time of the interim sacrifice, but there
was no dose-response relationship. At the time of terminal
sacrifice, brain cholinesterase activity was reduced in males at the
middle dose (by 52%) and in females at the low (by 57%) and middle
doses (by 49%), but not in animals at the high dose. Erythrocyte and
plasma cholinesterase activities were also inconsistently lower in
treated groups, although no dose-response relationship was seen.
Urinalysis revealed no remarkable findings, organ weights were
unaffected by treatment, and there were no treatment-related gross
changes at necropsy. Toclofos-methyl was not carcinogenic in this
study. No NOAEL could be identified, given the variability of the
data on cholinesterase activity (Pence et al., 1982).
Groups of 30 male and 30 female Fischer 344 rats were fed diets
containing tolclofos-methyl (purity, 98.3-97.3%) at 0, 100, 300 or
1000 ppm for 104 weeks, and brain, erythrocyte and plasma
cholinesterase activities were assessed. Ten males and 10 females
from each group were sacrificed at week 52. Animals were observed
daily for mortality, and they were examined for body weight, food
consumption and gross signs of toxicity weekly until week 26, every
two weeks during weeks 26-52 and every four weeks during weeks
52-104. Blood samples were taken for determination of plasma and
erythrocyte cholinesterase activities at initiation of the study, at
weeks 5, 14, 27, 53, 79 and at termination. Brain cholinesterase
activity was determined at week 52 and at termination.
There were no treatment-related effects on survival, and the
incidences of clinical signs and palpable tissue masses were
comparable among the groups. Mean plasma cholinesterase activities
were slightly decreased (by < 25%) in males when compared with
controls at 300 and 1000 ppm at weeks 27 (statistically
significant), 53, 79 and 105. Mean erythrocyte and brain
cholinesterase activities were comparable among the groups
throughout the study. Plasma cholinesterase activity was reduced (by
< 20%) at certain times in treated animals but no dose-response
relationship was found. Gross pathological findings were unrelated
to treatment. Doses < 1000 ppm did not depress erythrocyte or
brain cholinesterase activity or induce gross pathological effects
(Miyamoto, 1985; Pence et al., 1985a).
The combined NOAEL in these two studies was 1000 ppm, equal to
41 mg/kg bw per day, on the basis of the absence of any significant
findings.
(d) Reproductive toxicity
Rats
A three-generation study of reproductive toxicity was performed
in Sprague-Dawley rats fed diets containing tolclofos-methyl
(purity, 97.9-98.7% pure) at 0, 100, 300 or 1000 ppm, equivalent to
10, 30 or 100 mg/kg bw per day, with two litters per generation. The
F0 generation consisted of 30 rats of each sex per group. The
F1a, F2a and F3a generations were sacrificed at weaning and
underwent gross necropsy. Five rats of each sex in each group were
selected at weaning from the F1b, F2b and Fn3b litters for
histopathological examination, and 25 rats of each sex from each
group were selected from the F1b and F2b litters to breed the
following generation. A 15-week growth period was allowed before
mating of the 'a' litters, and a minimal 10-day rest period was
allowed between weaning of the 'a' litters and mating to produce the
'b' litters. After birth of the 'b' litter, all parental animals
were necropsied.
There were no treatment-related deaths or overt signs of
toxicity. Pregnancy rates were low at times in all groups, but the
pattern was not related to dose. The lowest pregnancy rate (52%) was
observed in the F1b generation at the middle dose. Pregnancy rates
were below 70% in three of the six generations in the controls, in
two generations at the low dose, in four generations at the middle
dose and in one generation at the high dose. The body weights of
F2a and F2b pups at 300 and 1000 ppm were lower than those of
controls at the start of the growth period; however, these
reductions were not dose-related, and the growth rates and body
weights in other groups were comparable throughout the study. There
were no treatment-related gross changes at necropsy in any group.
The weights of the ovaries were increased in non-pregnant adult
F2b females at 1000 ppm, but this was not associated with
histomorphological alterations. There was an increased incidence
(not statistically significant) of fine cytoplasmic vacuolation of
the adrenal cortex in adult F2b females at 1000 ppm that became
pregnant at both matings. The etiology and toxicological
significance of this finding, given its occurrence in the controls,
were unknown. There was no effect on reproductive parameters or on
offspring survival and development in any litter, and there were no
treatment-related gross or histopathological findings in the
litters. The NOAEL for reproductive toxicity was 1000 ppm
(equivalent to 100 mg/kg bw per day) on the basis of the absence of
significant findings at any dose level (Pence et al., 1985b).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 21-26 pregnant Fischer 344 rats were administered 0,
5, 15 or 50 mg/kg bw per day tolclofos-methyl (purity, 94.9%; dose
adjusted to 100%) in 0.5% w/v methylcellulose by gavage on days 6-15
of gestation. There were no overt signs of toxicity, and body-weight
gain and food consumption were unaffected by treatment. There were
no treatment-related gross lesions in the dams at necropsy.
Pregnancy rates were slightly higher in all treated groups (77-87%)
than in controls (70%). There were no treatment-related changes in
the number of fetal deaths, fetal viability or size or the
incidences of visceral or skeletal anomalies or variations.
Tolclofos-methyl was not embryotoxic, fetotoxic or teratogenic in
rats at doses up to and including 50 mg/kg bw per day. It should be
noted, however, that the highest dose was not maternally toxic
(Pence et al., 1979b).
Groups of 23 pregnant Sprague-Dawley rats were administered 0,
100, 300 or 1000 mg/kg bw per day tolclofos-methyl (purity, 96.7%)
in 0.5% methyl cellulose orally on days 6-15 of gestation. On day 20
of gestation, all animals were sacrificed and caesarean section was
performed. Rats were observed daily for mortality and clinical signs
and weighed. At termination, dams were observed for gross visceral
abnormalities, and their uteri were weighed and examined for
implantations and resorptions. Live fetuses were weighed, sexed and
examined for external, visceral and skeletal abnormalities. All dams
survived to the day of scheduled sacrifice with no clinical signs of
toxicity. Mean body-weight gain in animals at 100 and 1000 mg/kg bw
per day was significantly less than in controls on days 6-11. Both
mean body-weight gain (days 6-16) and mean net body-weight gain
(days 0-20) in rats at 1000 mg/kg bw per day were lower (by 10 and
14%, respectively) than the corresponding control value. In
addition, mean food consumption in animals at 1000 mg/kg bw per day
on days 6-16 and 16-20 was slightly below the control value. There
were no treatment-related differences in implantation efficiencies,
and mean fetal viability, sex ratio and fetal body weight were
similar in all groups. The number of fetuses with unossified fifth
and/or sixth sternebrae was significantly greater in the 1000-mg/kg
bw per day group than in the control group; however, the incidence
of total fetal skeletal variations was similar in all groups. Other
variations in development were not related to dose. Two to three
malformed fetuses were found in each group, but neither the type nor
the frequency of malformations indicated a teratogenic or
embryotoxic response. Tolclofos-methyl was found to be neither
teratogenic nor embryotoxic in this study at doses up to and
including 1000 mg/kg bw per day. This dose was slightly toxic to the
dams, as indicated by the lower body-weight gain. The NOAEL for
maternal toxicity was 300 mg/kg bw per day (Morseth et al., 1987).
Rabbits
Groups of 13-17 pregnant New Zealand white rabbits were
administered 0, 300, 1000 or 3000 mg/kg bw per day tolclofos-methyl
(purity, 98.7% pure) in 5% carboxymethylcellulose orally on days
6-18 of gestation. The animals were observed daily for mortality and
clinical signs and were weighed. At termination, dams were observed
for gross visceral abnormalities, and their uteri were weighed and
examined for implantations and resorptions. Live fetuses were
weighed, sexed and examined for external, visceral and skeletal
abnormalities. One rabbit at 3000 mg/kg bw per day died on day 14 of
gestation of an undetermined cause. There were no overt signs of
toxicity in any group. Spontaneous abortion occurred in one dam at
1000 mg/kg bw per day and in two at 3000 mg/kg bw per day on or
after day 21 of gestation. The mean body weights of animals in the
treated groups were significantly lower than the control values
throughout the study (including day 0). Body-weight gain was reduced
by 76% in animals at 3000 mg/kg bw per day; and at termination,
weight gain (days 0-29) was 19% lower than in controls. At 1000
mg/kg bw per day, body-weight gain was reduced by 56% during
treatment, but it was similar to that of controls at termination.
Food consumption was decreased by up to 38% in animals at 1000 and
3000 mg/kg bw per day during treatment. There were no
treatment-related changes in maternal organ weights. One animal at
3000 mg/kg bw per day resorbed her entire litter. There were no
other treatment-related changes in implantation efficiency, mean
fetal viability, size, sex ratio, fetal body weight or external,
visceral or skeletal development. Tolclofos-methyl was not
teratogenic in this study at doses up to and including 3000 mg/kg bw
per day, which was toxic to dams. The NOAEL for maternal toxicity
was 300 mg/kg bw per day (Motoyama et al., 1991).
(f) Genotoxicity
The results of tests for the genotoxicity of tolclofos-methyl
are summarized in Table 2.
(g) Special studies
(i) Skin and eye irritation and skin sensitization
Six male albino Japanese rabbits received 500 mg of
tolclofos-methyl (purity, 97%) on clipped intact or abraded dorsal
skin for 4 h under an occlusive dressing. No signs of irritation
were seen at any of the application sites seven days after treatment
(Matsubara et al., 1978).
Table 2. Results of tests for the genotoxicity of tolclofos-methyl
End-point Test system Concentration Purity Results Reference
of tolclofos-methyl (%)
In vitro
Reverse S. typhimurium TA98, 10, 100, 500, 1000, 2000 97.0 Negativea,b Suzuki & Miyamoto,
mutation 100, 1535, 1537, 1538 µg/plate
Reverse S. typhimurium TA98, 10, 50, 100, 500, 1000, 98.7 Negativea,c Moriya et al., 1981
mutation 100, 1535, 1537, 1538 5000 µg/plate
E. coli WP2uvrA
Reverse B. subtilis H17 rec+, 1, 10, 100, 1000 µg/disc 97.0 Negatived Suzuki & Miyamoto,
mutation M45 rec- 1978
Reverse B. subtilis 20, 50, 100, 200, 500, 98.7 Negativee Moriya et al., 1981
mutation H17 rec+, M45 rec- 1000, 2000, 5000 µg/disc
Host-mediated S. typhimurium in 870, 1750 mg/kg 97.0 Negativef Suzuki & Miyamoto,
assay male ICR mice 1978
Chromosomal Chinese hamster 10, 20, 40 µg/ml 96.6 Negativea,g Kogiso et al., 1990
aberration K1 ovary cells 37.5, 75, 150 µg/ml
Unscheduled Male Sprague-Dawley 0.3, 1, 3, 10, 20, 40 96.6 Negativea,i Hara et al., 1990
DNA synthesis rat hepatocytes µg/mlh
Unscheduled Human carcinoma 0.3, 3, 30, 300 µg/ml NR Negativej Monaco & Nunziata,
DNA synthesis cells (HeLa) 1981
Gene mutation Chinese hamster 1.5, 15, 150, 1500 µg/ml NR Negativea,k Monaco & Nunziata,
V79 lung cells 1981
Table 2 (contd)
End-point Test system Concentration Purity Results Reference
of tolclofos-methyl (%)
In vivo
Chromosomal Male ICR mice 500, 1000, 2000, 99.8 Negativei Hara & Suzuki, 1981
aberration 4000 mg/kg i.p.h
Dominant Male Sprague- 62.5, 208.3, 625.0 NR Negativej Brusick, 1981
lethal Dawley rats mg/kg orally
mutation
NR, not reported
a With and without metabolic activation.
b Positive controls ( N-methyl- N'-nitro- N-nitrosoguanidine and 2-acetylaminofluorene) yielded expected positive
results.
c Several positive controls yielded expected positive results.
d Positive control ( N-methyl- N'-nitro- N-nitrosoguanidine) yielded expected positive results.
e Positive (mitomycin C) and negative (Kanamycin) controls yielded expected results.
f Positive control ( N-nitrosodimethylamine) yielded expected positive results.
g Positive controls (mitomycin C and cyclophosphamide) yielded expected positive results.
h At 40 mg/ml, cell viability was less than 20%.
i Positive control (2-acetylaminofluorene) yielded expected positive results.
j Positive controls (methyl methanesulfonate and urethane) yield expected positive results.
k Positive controls (methyl methanesulfonate and N-nitrosodimethylamine) yielded expected positive results.
l At 2000 and 4000 mg/kg bw there was > 50% mortality at 48 h.
m Positive control (cyclophosphamide) yielded expected positive results.
n Positive control (triethylenemelamine) yielded expected positive results.
Groups of six male New Zealand white rabbits received 500 mg of
either a '50% wettable power' or '10% dust' moistened with saline on
1-inch2 (6.5-cm2) sites on the clipped dorsal intact or abraded
skin for 24 h under an occlusive dressing. No irritation, such as
erythema and oedema, was observed (Hara et al., 1981a,b).
Two male and one female New Zealand white rabbits received 0.5
ml of '50% flowable' tolclofos-methyl on 1 inch2 (6.5 cm2) of
clipped dorsal intact or abraded skin for 4 h under an occlusive
dressing. No irritation was observed (Nakanishi et al., 1989).
Each of eight male albino Japanese rabbits received 50 mg of
tolclofos-methyl (purity, 97%) in one eye. Five minutes after the
application, the treated eyes of five animals were flushed with 300
ml saline for 2 min. The treated eyes of the remaining animals were
similarly flushed 24 h after treatment. There were no corneal,
conjunctival or iridal effects up to seven days after treatment
(Matsubara et al., 1978).
Groups of nine male New Zealand white rabbits received 100 mg
of either a '50% wettable power' or '10% dust' in one eye; 30 s
after the application, the treated eyes of three animals per group
were flushed with 300 ml lukewarm water for 1 min. Slight congestion
of the iris was observed 24 h after application. Slight to moderate
hyperaemia and slight chemosis and/or discharge in conjunctiva were
also observed 1-48 h after application of the '50% wettable power'
to unwashed eyes. These changes had disappeared by 72 h after
application in all animals. No ocular lesions were found in the
washed eyes. The irritation potency of this formulation was judged
to be mild. Slight conjunctival hyperaemia and/or chemosis were
observed in animals with unwashed eyes and in one with washed eyes
1-24 h after application of the '10% dust'. There were no other
signs of irritation at any time. The formulation was classified as
minimally irritating to eyes (Hara et al., 1981a,b).
One male and two female New Zealand white rabbits received 0.1
ml of '50% flowable' tolclofos-methyl in one eye. Slight redness was
observed in conjunctiva after application, which disappeared within
24 h (Nakanishi et al., 1989).
The skin-sensitizing potential of tolclofos-methyl (purity,
97%) was assessed in guinea-pigs by the Landsteiner-Draize method.
Groups of 10 male Hartley guinea-pigs were given 10 intradermal
injections of 1 or 5% tolclofos-methyl (0.05 or 0.1 ml) in corn oil
at intervals of two to three days. Two weeks after the final
induction, the animals were challenged at a fresh site with 0.05-ml
intradermal injections of tolclofos-methyl at the same
concentrations used for induction. Negative control animals for both
dose groups were given the challenge injection only. Positive
control animals were treated with 0.05% 2,4-dinitrochlorobenzene
three times before challenge. Slight erythema and swelling were
observed in two animals after challenge treatment with 1%
tolclofos-methyl, in three after challenge with 5%, and in one
animal in each negative control group. Moderate erythema and/or
swelling was observed in the animals treated with
2,4-dinitrochlorobenzene. It was concluded that tolclofos-methyl did
not sensitize skin in this study (Matsubara et al., 1980).
The skin sensitizing potential of a '50% wettable powder' and a
'10% dust' of tolchlofos-methyl was tested in guinea-pigs by the
Buehler method. Groups of 10 male Hartley guinea-pigs received 500
mg of one formulation, slightly moistened with water, on clipped
dorsal skin under an occlusive dressing for 24 h. Induction was
performed 10 times at two- to three-day intervals.Positive control
animals were similarly treated with 0.5% 2,4-dinitro-chlorobenzene.
Negative controls were not subjected to the induction treatment. Two
weeks after the final induction, the test animals and positive
controls were challenged as in the sensitizing treatment. No skin
reactions were observed in the negative control or treated animals.
Slight to severe erythema and swelling were observed in the positive
controls. The formulations were considered not to sensitize skin
(Hara et al., 1981c,d).
The skin sensitizing potential of a '50% flowable formulation'
was tested in guinea-pigs by the Buehler method. Ten male Hartley
guinea-pigs received 0.5 ml of the formulation for 6 h once a week
for three weeks on the clipped dorsal skin under an occlusive
dressing. Positive control animals were similarly treated with 0.5%
2,4-dinitrochlorobenzene. Negative control animals were not
subjected to the induction treatment. Two weeks after the final
induction, animals were challenged as in the sensitizing treatment.
No skin reactions were observed in the negative control or treated
animals. Slight to severe erythema and swelling were observed in the
positive controls. The formulation was considered not to sensitize
skin (Nakanishi et al., 1990).
(ii) Delayed neuropathy
Groups of 10 Leghorn hens were administered 0 or 8000 mg/kg bw
tolclofos-methyl (purity, 97%) or 500 mg/kg bw tri- ortho-cresyl
phosphate orally in corn oil. After a 21-day observation period, a
second dose of vehicle or tolclofos-methyl was administered. Animals
were sacrificed 21 days after the second dose. There were no deaths
in the groups given the vehicle or tolclofos-methyl. Plasma
cholinesterase activity in the group treated with tolclofos-methyl
was decreased by nearly 50% eight days after the first dose but had
recovered to the pre-treatment level 21 days after dosing. Hens
treated with tolclofos-methyl had no signs of leg weakness or
paralysis and no histopathological changes in the nervous tissues.
Birds treated with tri- ortho-cresyl phosphate had the typical
clinical and histopathological signs of delayed polyneuropathy
(Okuno et al., 1982).
3. Observations in humans
The medical records of 20 workers in Japan were reviewed. All
workers had been engaged continuously in packaging operations since
manufacture of technical-grade tolclofos-methyl began in 1988, for
an average of 4 h/day. No occupation-related problems were observed
or reported. Plasma and erythrocyte cholinesterase activities were
not measured (Murayama, 1991).
Comments
Tolclofos-methyl is excreted rapidly in rats and mice,
predominantly in the urine; less than 1% of the dose was retained in
tissues after seven days. In both species, metabolism occurred
mainly by oxidation of P=S to P=O, oxidation of the 4-methyl group
and cleavage of the P-O-aryl and P-O-methyl linkages. There are four
main metabolites in mice, one of which is a glycine conjugate, and
four in rats, which are excreted as glucuronides.
Tolclofos-methyl had low acute toxicity when administered by
the oral, dermal, subcutaneous or intraperitoneal route. The overt
signs of acute toxicity are not typical of an anticholinesterase, as
no chromodaccryorrhoea, lachrymation or fasciculation was seen,
although some inhibition of plasma, erythrocyte and brain
cholinesterase activities was observed. WHO (1992) has classified
tolclofos-methyl as unlikely to present an acute hazard in normal
use.
In a nine-month study of toxicity in which mice were fed
tolclofos-methyl in the diet at 0, 10, 30, 100 or 3000 ppm, the
NOAEL was 100 ppm, equal to 12 mg/kg bw per day, on the basis of
inhibition of brain cholinesterase activity and effects on body
weight at 3000 ppm.
In a 32-34-day study of toxicity in which rats were fed diets
containing 0, 200, 1000, 5000 or 20 000 ppm, the NOAEL was 1000 ppm,
equal to 79 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity and increased relative kidney weight at 5000
ppm. In a 13-week study of toxicity in which rats were fed diets
containing 0, 100, 1000 or 10 000 ppm, the NOAEL was again 1000 ppm,
equal to 66 mg/kg bw per day, on the basis of effects on body, liver
and kidney weights at 10 000 ppm. In a 28-week study of toxicity in
which rats were fed dietary levels of 0, 300, 1000, 3000 or 10 000
ppm, the NOAEL was also 1000 ppm, equal to 65 mg/kg bw per day, on
the basis of histopathological changes in the livers of females at
3000 ppm.
In a 26-week dietary study in dogs fed levels of 0, 200, 600 or
2000 ppm, the NOAEL was 600 ppm, equal to 21 mg/kg bw per day, on
the basis of reduced body-weight gain, an increased serum level of
alkaline phosphatase and increased liver weight at 2000 ppm.
In a 52-week study of toxicity in dogs given dietary
concentrations of 0, 80, 400 or 2000 ppm, the NOAEL was 400 ppm,
equal to 11 mg/kg bw per day, on the basis of increased liver weight
(with hepatocytic hypertrophy), reduced body-weight gain and slight
anaemia at 2000 ppm.
In a 104-week study of toxicity and carcinogenicity in which
mice were given dietary concentrations of 0, 10, 50, 250 or 1000
ppm, the NOAEL was 50 ppm, equal to 6.5 mg/kg bw per day, on the
basis of reduced brain cholinesterase activity and increased
absolute and relative kidney weights at higher levels. There was no
evidence of carcinogenicity.
A 122-129-week study of toxicity and carcinogenicity was
performed in which rats were given dietary concentrations of 0, 100,
300 or 1000 ppm. The NOAEL was 1000 ppm, equal to 41 mg/kg bw per
day, on the basis of the absence of any significant finding. There
was no evidence of carcinogenicity.
In a three-generation study (two litters per generation) in
rats, tolclofos-methyl was given at dietary levels of 0, 100, 300 or
1000 ppm. The NOAEL was > 1000 ppm, equivalent to 100 mg/kg bw per
day, on the basis of the absence of any significant findings.
In a study of teratogenicity in which rats were given 0, 5, 15
or 50 mg/kg bw per day of tolclofos-methyl by gavage, the NOAEL was
50 mg/kg bw per day on the basis of the absence of any significant
findings. The study was not considered to be fully adequate because
the highest dose tested was not maternally toxic. A similar study
was conducted at levels of 0, 100, 300 or 1000 mg/kg bw per day. The
NOAEL was 300 mg/kg bw per day on the basis of reduced body-weight
gain in dams at 1000 mg/kg bw per day. There was no evidence of
teratogenicity.
A study of teratogenicity was conducted in rabbits given 0,
300, 1000 or 3000 mg/kg bw per day orally. The NOAEL for maternal
toxicity was 300 mg/kg bw per day on the basis of reduced
body-weight gain in dams at 1000 mg/kg bw per day. There was no
evidence of teratogenicity.
Tolclofos-methyl was studied in a wide range of tests for
genotoxicity in vivo and in vitro. The Meeting concluded that
the compound is not genotoxic.
Tolclofos-methyl did not cause delayed neuropathy in chickens.
The available observations in humans were considered by the
Meeting but did not directly contribute to an estimation of an ADI.
An ADI was established on the basis of a NOAEL of 50 ppm, equal
to 6.5 mg/kg bw per day, in the 104-week study of toxicity and
carcinogenicity study in mice, and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equal to 6.5 mg/kg bw per day (104-week study
of toxicity and carcinogenicity)
Rat: 1000 ppm, equal to 41 mg/kg bw per day (122/129-week
study of toxicity and carcinogenicity)
Rabbit: 300 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Dog: 400 ppm, equal to 11 mg/kg bw per day (52-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.07 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Brusick, D.J. (1981) Mutagenicity evaluation of S-3349 T.G. Lot No.
4, rat dominant lethal assay. Litton Bionetics Inc. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Colley, J., Welch, P.J., Heywood, R., Prentice, D.E., Cherry, C.P.,
Mullins, P.A., Gibson, W.A. & Almond, R.H. (1982) S-3349, toxicity
to rats by dietary administration for 4 weeks. Huntington Research
Centre Ltd. Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd, Osaka, Japan.
Cox, R.H. & Brusick, D.J. (1988) Chronic toxicity study in dogs with
S-3349. Hazleton Laboratories America Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Eschbach, J.C. & Hogan, G.K. (1981a) An acute inhalation toxicity
study of S-3349 50WP in the rat. Bio/dynamics Inc. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Eschbach, J.C. & Hogan, G.K. (1981b) An acute inhalation toxicity
study of S-3349 10d in the rat. Bio/dynamics Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Esumi, Y. & Yokoshima, T. (1989) Study on metabolism of
tolclofos-methyl in rats. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Gargus, J.L. (1986) 21-Day dermal toxicity study, in rabbits with
Rizolex, technical 97.7%. Hazleton Laboratories America Inc.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Hara, M. & Suzuki, H. (1981) In vivo chromosomal aberration test of
S-3349 on bone marrow cells of mice. Sumitomo Chemical Co., Ltd.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981a) Primary eye and skin
irritation tests of S3349 50% water-dispersible power in rabbits.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981b) Primary eye and skin
irritation tests of S-3349 10% dust formulation in rabbits. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981c) Skin sensitization test
of S3349 50% water-dispersible powder in guinea pigs. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1981d) Skin sensitization test
of S3349 10% dust formulation in guinea pigs. Sumitomo Chemical Co.,
Ltd. Unpublished report submitted to WHO by Sumitomo Chemical Co.,
Ltd, Osaka, Japan.
Hara, M., Ota, M., Kogiso, S., Yoshitake, A. & Yamada, H. (1990) In
vitro unscheduled DNA synthesis (UDS) assay of Rizolex in rat
hepatocytes. Sumitomo Chemical Co., Ltd. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hardy, C.J., Jackson, G.C., Lewis, D.J. & Gopinath, C. (1986)
Technical Rezolex, acute inhalation toxicity in rats, 4-hour
exposure. Huntington Research Centre Ltd. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hiromori, T., Suzuki, T., Okuno, Y., Ito, S., Murakami, M., Kadota,
T. & Miyamoto, J. (1978) Six-month oral toxicity study of S-3349 in
rats. Sumitomo Chemical Co., Ltd. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Hiromori, T., Misaki, Y., Ito, S., Kohda, A., Kato, T. & Yamada, H.
(1989a) Acute oral toxicity study of Rizolex 50 FL in rats. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Hiromori, T., Misaki, Y., Ito, S., Kohda, A., Kato, T. & Yamada, H.
(1989b) Acute dermal toxicity study of Rizolex 50 FL in rats.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Jackson, G.C., Hardy, C.J., Morrow, J. & Gopinath, C. (1990) Rizolex
50 FL, acute inhalation toxicity study in rats, 4-hour exposure.
Huntington Research Centre Ltd. Unpublished report submitted to WHO
by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kimura, J., Yosioka, K., Ozaki, K., Adachi, H., Seki, T., Matsuo, M
&, Yamada, H. (1990) 90-Day oral toxicity study of S-3349 in rats.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Kogiso, S., Yamamoto, K., Hara, M., Yositake, A. & Yamada, H. (1990)
In vitro chromosomal aberration test of Rizolex in Chinese hamster
ovary cells (CHO-K1). Sumitomo Chemical Co., Ltd. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Krautter, G.R., Downs, J., Hoglan, N., Marsh, J.D. & Lawrence, L.J.
(1987) Metabolism of tolclofos-methyl in the rat. Pharmacology &
Toxicology Research Laboratory. Unpublished report submitted to WHO
by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Krautter, G.R., Downs, J., Hoglan, N., Marsh, J.D. & Lawrence, L.J.
(1988) Metabolism of tolclofos-methyl in the rat. Final report
amendment. Pharmacology & Toxicology Research Laboratory.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Matsubara, T., Hara, S. & Kadota, T. (1978) Eye and skin irritation
test of S-3349 in rabbits. Sumitomo Chemical Co., Ltd. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Matsubara, T., Hara, S., Suzuki, T., Kadota, T. & Miyamoto, J.
(1980) Skin sensitization test of S-3349 in guinea pigs. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Mihara, K., Ohkawa, H. & Miyamoto, J. (1981) Metabolism of
tolclofos-methyl in rats and mice. J. Pestic. Sci., 6, 65-74.
Miyamoto, J. (1985) Comments on toxic effects and minimum effect
level of S-3349 in chronic oral toxicity studies in rats. Sumitomo
Chemical Co., Ltd., Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Monaco, M. & Nunziata, A. (1981) Report of mutagenicity experiment
performed on the test substance S-3349, Sumitomo Chemical Co., Ltd.
Unpublished report from Centro Ricerca Farmaceutica, Pomezia, Italy.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Moore, M.R. (1993) Comments on the toxicological significance of
hepatocytic pigment and on the no-adverse-effect-level (NOAEL) in a
chronic toxicity study in dogs treated with S-3349 (Hazleton project
No. 343-176). Hazleton Washington Inc. Unpublished report submitted
to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Moriya, M., Ohta, T. & Shirasu, Y. (1981) S-3349, microbial
mutagenicity study. Institute of Environmental Toxicology.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Morseth, S.L., Burlew, J.A., Vargas, K.J., Lewis, S.A., Thakur, A.K.
& Phipps, N.G. (1987) Teratology study of S-3349 T.G. in rats.
Hazleton Laboratories America Inc. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Motoyama, M., Kashima, M. & Takahashi, M. (1991) Teratology study of
S-3349 in rabbits. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Murayama, F. (1991) A review on medical examination of factory
workers exposed to tolclofos-methyl. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Nakanishi, T., Kohda, A., Kato, T. & Yamada, H. (1989) Primary eye
and skin irritation tests with Rizolex 50 FL in rabbits. Sumitomo
Chemical Co., Ltd. Unpublished study, submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Nakanishi, T., Kohda, A., Kato, T. & Yamada, H. (1990) Skin
sensitization test of Rizolex 50 FL in guinea pigs. Sumitomo
Chemical Co., Ltd. Unpublished report submitted to WHO by Sumitomo
Chemical Co., Ltd, Osaka, Japan.
Okuno, Y., Yamada, T., Hosokawa, S. & Miyamoto, J. (1982) Acute
delayed neurotoxicity study of S-3349 in hens. Sumitomo Chemical
Co., Ltd. Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd, Osaka, Japan.
Pence, D.H., Lemen, J.K. & Weatherholtz, W.M. (1978) Acute oral
toxicity study in male and female dogs, S-3349. Hazleton
Laboratories America Inc. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Weatherholtz, W.M., Kundzins, W., Alsaker, R.D., Brown,
H.R. & Greenspun, K.S. (1979a) Subacute dietary administration in
dogs, S-3349. Hazleton Laboratories America Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Pence, L.A., Durloo, R. & Twigg, C.J. (1979b)
Teratology study in rats, S-3349. Hazleton Laboratories America Inc.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Pence, D.H., Serota, D., Alsaker, R.D., Koka, M., Banas, D.A.,
Dawkins, B.G., Kundzins, W. & Hepner, K.E. (1982) Chronic toxicity
study in rat, S-3349. Hazleton Laboratories America Inc. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Phipps, N., Alsaker, R.D., Hepner, K.E. & Spicer, K.M.
(1985a) 104-Week cholinesterase activity study in male and female
rats, S-3349. Hazleton Laboratories America Inc. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Pence, D.H., Hepner, K.E., Wolfe, G.W., Voelker, R.W. & Ulland, B.N.
(1985b) Three-generation reproduction study in rats, S-3349.
Hazleton Laboratories America Inc. Unpublished report submitted to
WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Satoh, R., Kashima, M., Satoh, H., Motoyama, M. & Ishikawa, A.
(1983) Twenty-four-month chronic toxicity study of S-3349 in
pulverized diet in mice. Nippon Experimental Medical Research
Institute. Unpublished report submitted to WHO by Sumitomo Chemical
Co., Ltd, Osaka, Japan.
Segawa, T. (1978) Acute toxicity study of S-3349 in rats and mice.
Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981a) Acute oral toxicity study of S-3349 50% wettable
powder in mice. Hiroshima University School of Medicine. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981b) Acute oral toxicity study of S-3349 10% dust in
mice. Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981c) Acute dermal toxicity study of S-3349 50%
wettable powder in mice. Hiroshima University School of Medicine.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Segawa, T., (1981d) Acute dermal toxicity study of S-3349 10% dust
in mice. Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981e) Acute oral toxicity study of S-3349 50% wettable
powder in rats. Hiroshima University School of Medicine. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981f) Acute oral toxicity study of S-3349 10% dust in
rats. Hiroshima University School of Medicine. Unpublished report
submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Segawa, T. (1981g) Acute dermal toxicity study of S-3349 50%
wettable powder in rats. Hiroshima University School of Medicine.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Segawa, T. (1981h) Acute dermal toxicity study of S-3349 50%
wettable powder in rats. Hiroshima University School of Medicine.
Unpublished report submitted to WHO by Sumitomo Chemical Co., Ltd,
Osaka, Japan.
Suzuki, H. & Miyamoto, J. (1978) Studies on mutagenicity of S-3349
with bacterial systems. Sumitomo Chemical Co., Ltd. Unpublished
report submitted to WHO by Sumitomo Chemical Co., Ltd, Osaka, Japan.
Suzuki, T., Okuno, Y., Hiromori, T., Ito, S., Murakami, M., Kadota,
T & Miyamoto, Y. (1978) Nine-month feeding study of S-3349 in mice.
Sumitomo Chemical Co., Ltd. Unpublished report submitted to WHO by
Sumitomo Chemical Co., Ltd, Osaka, Japan.
