
FENARIMOL
First draft prepared by
P.H. van Hoeven-Arentzen
National Institute of Public Health and Environmental Protection,
Bilthoven, Netherlands
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Other metabolic studies
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Mechanisms of effects on reproduction
Relevance to humans of adverse reproductive effects in
rodents
Comments
Toxicological evaluation
References
Explanation
Fenarimol is a pyrimidin-5-yl benzhydrol fungicide, which is used
on many crops. It was considered for the first time by the present
Meeting.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Several studies were performed (Hoffmann et al., 1985a,b,c) in
which groups of five male and five female Wistar rats were given a
single oral dose of 1 or 13 mg/kg bw 14C-fenarimol, as a mixture of
lots labelled at the carbinol carbon, the ortho-chlorophenyl ring,
and the para-chlorophenyl ring.
Fenarimol was rapidly absorbed, as measured in blood samples
taken periodically up to 24 h after the low dose and up to 48 h after
the high dose. The peak plasma levels occurred about 1 h after
treatment and were 0.08-0.21 µg/g after the low dose and 2.79-
3.62 µg/g after the high dose (in equivalents). The concentrations
then declined, with average half-lives of 11..9-16.8 h. Both the peak
plasma concentrations and the area under the plasma concentration-time
(0-24 h) curve (AUC) were dependent on sex and dose. The peak plasma
concentrations and the AUCs were higher in female than in male rats
given the low dose, but no difference was seen at 13 mg/kg bw. The
increase in the AUC in animals of each sex and the peak plasma
concentrations in males were much greater than the 13-fold difference
in dose, suggesting that the metabolism of fenarimol was saturated
after the high dose (Hoffman et al., 1985c).
The concentrations of radiolabel in the major tissues and organs
were measured 1 and 24 h and seven days after administration of the
two doses. After 1 h, the flushed-out intestines contained about 7.5%
of the administered dose after the low dose and about 3.2% after the
high dose. The liver contained 5.3-8.7% of the administered dose; all
other tissues contained < 0.7%. The highest concentrations in
tissues other than the intestine were in the liver (3 µg/g) at the low
dose and in fat (33-58 µg/g), liver, pancreas, and adrenal glands at
the high dose. The tissue concentrations decreased rapidly, and by
24 h liver and fat contained 19-47% and 7-18%, respectively, of the
1-h values. The major exception to this trend was the intestine, where
the amount of radiolabel increased between 1 and 24 h; this finding
is consistent with the observed biliary excretion of fenarimol
metabolites (see below). The amount of radiolabel recovered in
carcass (rinse excluded) and tissues after seven days was 1 and 2.2%,
respectively, of the low and high dose. At that time, all tissue
samples had residue concentrations < 1 µg/g (equivalents), except for
the ovaries and the adrenal, pituitary, and thyroid glands of females
at the high dose (1-3 µg/g). The radiocarbon levels in most tissues
were somewhat higher in females than in males (Hoffman et al.,
1985b,c).
14C-Fenarimol was rapidly and extensively eliminated, most
within 48 h and principally via the faeces. Within seven days, males
at either dose had excreted about 83% in faeces and 6.8% in urine;
females at the low dose had excreted 77% in faeces and 9% in urine,
and those at the high dose had excreted 66 and 13% in faeces and
urine, respectively. A preliminary experiment showed negligible
excretion in expired air. In a subsequent experiment with
bilecannulated rats, biliary excretion was shown to be a major route
of elimination, with no sex difference; by 24 h after treatment with
either dose, 57-78% had been eliminated in bile. Elimination in bile
plus urine by 24 h accounted for 78-79% and 65-83% of the administered
dose at 1 and 13 mg/kg bw, respectively. The amount of radiolabel in
the bile was proportional to the dose administered, indicating that
absorption was similar at the two doses (Hoffman et al., 1985a,c).
Groups of five male and five female Wistar rats received daily
oral doses of 1 mg/kg bw unlabelled fenarimol (purity, 97%) for 14
days and on day 15 they received the same dose of radiolabelled
compound. Urine and faeces were collected daily for seven days, and
then the animals were killed and organs and tissues were examined for
14C activity. Total urinary excretion accounted for 6.5% of the dose
in males and 9.2% in females, and total faecal excretion accounted for
76.2% in males and 78% in females. At 24 h, females excreted fenarimol
at a slightly lower rate than males. The levels of radiolabel in the
residual carcass were 1.2% of the dose in males and 1.5% in females.
The tissue levels ranged from < 0.005 to 0.04 µg/g (equivalents),
with the highest levels in liver in males and in adrenals in females
(Hoffman et al., 1985d).
A lactating goat was fed gelatin capsules containing fenarimol
labelled in the carbinol carbon twice daily for five days at a dose
equivalent to 10 ppm of its daily food consumption and was killed 16 h
later. A second lactating goat served as a control. Peak plasma
concentrations were observed about 4 h after the first dose. A
steady-state condition was reached between 56 and 96 h at a plasma
level of about 0.026 µg/ml equivalents. The major routes of
elimination were faeces (53%) and urine (28%); < 0.1% of the total
dose was excreted via milk, with a steady-state level of about
0.007 µg/ml equivalents reached after 56 h. The highest concentrations
of label were found in bile (2.97 µg/g), liver (0.42 µg/g), and kidney
(0.14 µg/g). The concentrations in fat, muscle, and carcass were
similar to the plasma concentration. High levels of radiolabel
remained in the gastrointestinal tract and contents at sacrifice (13%
of the total dose). Unchanged fenarimol represented about 21% of the
radiolabel in the liver; the principal metabolite in the liver, a
methyl sulfone derivative of fenarimol, represented about 33%.
Metabolites were not identified in bile, faeces, kidney, or urine
(Prout, 1994; Watson, 1994).
Dermal absorption of fenarimol was evaluated in two male and two
female rhesus monkeys by comparing the results of intravenous and
topical administration of 14C-fenarimol (mixture of three
radiotracers). Fenarimol dissolved in ethanol was administered by both
routes at a dose of 2 mg/kg bw. The topical dose was applied under an
occlusive bandage to 6 cm2 of shaved forearm for 24 h (the vehicle
was evaporated with a hairdryer) and then removed by washing. The
study was terminated 168 h after treatment, when 83% of the dermal
dose and 75% of the intravenous dose were recovered. The mean AUCs for
plasma radiolabel were 0.243 and 15.6 h x µg/ml after topical and
intravenous administration, respectively. Cumulative urinary plus
faecal excretion up to seven days after administration was 1.98 and
75.4%, respectively. The quantity of the applied dose that was
absorbed can be expressed either as the ratio of the topical to the
intravenous AUC or as the ratio of the percent excreted seven days
after topical versus intravenous administration. Low dermal absorption
values were obtained by the two routes: 1.56 and 2.63%, respectively.
Pharmacokinetic data derived after intravenous injection indicated
that 14C-fenarimol was partially bound to plasma protein and that
elimination from plasma was biphasic, with half-lives of 3.3 and
62.8 h (Hoffman et al., 1985e).
(b) Biotransformation
Wistar rats were given one oral dose of 1 or 13 mg/kg bw
14C-fenarimol, and urine and faeces were collected daily for seven
days. Samples were pooled by sex and dose and then analysed for the
presence of 14C-fenarimol and metabolites. Fenarimol was extensively
metabolized, less than 3% of the dose being excreted unchanged in the
faeces. More than 40 metabolites, each representing less than 10% of
the dose, were detected in urine and faeces, many of which appeared to
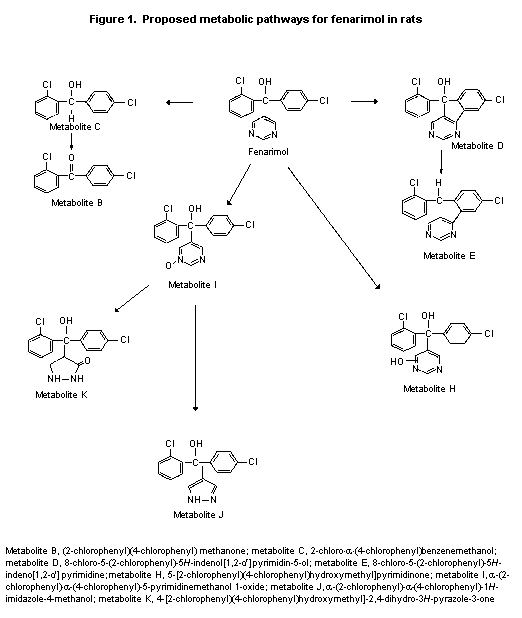
be common to urine and faeces. Some of these are shown in Figure 1.
Metabolites K, J, and F were the major metabolites, representing 6-9%,
3-4%, and 6-7% of the low dose, respectively. Lower percentages of
metabolites K and F and higher percentages of metabolite J were
observed after the 13 mg/kg bw dose, perhaps due to maturation of one
pathway. Given the large number of minor metabolites detected,
metabolism probably occurred at more than one site on the molecule and
involved several pathways. The proposed major metabolic pathways of
fenarimol are oxidation of the carbinol carbon atom, the chlorophenol
rings, and the pyrimidine ring. A proposed minor pathway involves
cyclization between a chlorophenyl ring an d the pyrimidine ring
(Figure 1) (Althaus, 1985a).
Only trace amounts of unchanged fenarimol were found in 24-h bile
samples from rats treated with 1 or 13 mg/kg bw 14C-fenarimol. The
biliary metabolites occurred predominantly as glucuronic acid
conjugates, and similar metabolic profiles were seen at the two doses.
After enzymatic hydrolysis, a complex mixture of metabolites was
 found, the main one being metabolite K. In contrast, most of the
radiolabel in the faeces was present as free compounds, indicating
that biliary metabolites undergo further metabolism or hydrolysis
before being excreted (Goebel, 1985).
The tissue distribution of fenarimol in Wistar rats was
determined 1 and 24 h after administration of 1 or 13 mg/kg bw
14C-fenarimol. The main radiolabelled constituents identified in
blood and kidney after 1 h were unchanged fenarimol and metabolite I.
The amount of fenarimol increased with dose, from 19 to 40% of the
total radioactivity in blood in males and from 50 to 73% in females.
Fenarimol also accounted for most of the radiolabel in the liver
(67-90%), whereas metabolite K represented 3-6%. Females had more
unchanged fenarimol residues than males, indicating more extensive
metabolism in males. Furthermore, metabolism was reduced at the higher
dose, suggesting saturation (Althaus, 1985b).
(c) Other metabolic studies
Some barrier to placental transport of fenarimol-derived
radiolabel was found in rats on days 14-17 of gestation but virtually
no barrier on day 18 (Hoffman & Hirsch, 1981a). Fenarimol-derived
radiolabel was excreted in milk and subsequently concentrated in the
hypothalamus of neonates. The concentration in milk up to 48 h after
treatment was three to five times greater than that in maternal plasma
(Hoffman & Hirsch, 1981b).
2. Toxicological data
(a) Acute toxicity
The results of studies of acute toxicity are summarized in
Table 1.
(b) Short-term toxicity
Mice
In a pilot study reported with few details, groups of four male
ICR mice were given fenarimol (purity unspecified) in the diet at 0
(10 animals), 25, 50, 100, 200, 400, 800, 1600, 3200, 6400, or
12 800 ppm for 14 days. No effects were observed on mortality or
clinical signs. terminal body weight was reduced at the highest dose.
In the liver (the only organ investigated), dose-related increases in
relative weight and in the rate of hepatic metabolism of para-
nitroanisole were seen at > 800 ppm. There were no effects on liver
pathology or histopathology (Hoffman et al., 1975b).
Table 1. Acute toxicity of fenarimol
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
ICR mouse Male Oral 4508a NR Hoffman et al. (1975a)
Female > 4000a
Wistar-derived Male Oral 2576a NR Hoffman et al. (1975a)
Harlan rat Female 2515a
Albino rabbit Male and Dermal > 2000b NR Hoffman et al. (1977a)
female
Fischer 344 rat Male and Inhalationc > 2.07 97% Hoffman et al. (1980a)
female (1-h)
Wistar-derived FemMe intraperitoneal 533 NR Hoffman et al. (1975a)
Harlan rat
Beagle dogc Male and Oral > 200 NR Hoffman et al. (1975a)
female
NR, not reported
a Signs of toxicity: hypoactivity, leg weakness, loss of righting reflex, ptosis (1-6 h after treatment);
loose stools; diuresis, diarrhoea, debilitation (24 h after treatment); recovery within 8-10 days
b Application on intact and abraded skin; one death, but no signs of toxicity or irritation
c Nose only; nominal concentration, 133 mg/litre; particle size, 12 µm
d Only one male and one female
In a poorly reported study, groups of 10 male and 10 female ICR
mice were fed diets containing 0, 365, 620, 1100, 2000, or 3300 ppm
fenarimol (purity unspecified) for three months, equivalent to 0, 52,
88, 157, 286, or 471 mg/kg bw per day. Food consumption was not
measured, and ophthalmological examinations were not performed. There
were no treatment-related effects on mortality, clinical signs of
toxicity, haematology, or body weight. A statistically significant
reduction in total bilirubin was observed in animals of each sex at
the two highest doses and in males at 1100 ppm. Hepatic para-
nitroanisole metabolism, determined in five animals of each sex in
each group, showed a significant dose-related increase in females at
> 1100 ppm and in males at > 2000 ppm. Relative liver weights
were increased in a dose-related manner in males at > 365 ppm (10%
increase) and in females at > 620 ppm (11% increase) and reached
statistical significance at 620 and 1100 ppm, respectively. Absolute
liver weights were increased at > 620 ppm. The main effects seen on
histopathological examination of tissues other than nervous tissue
were centrilobular hypertrophy and hepatomegaly in the livers of males
at 1100 ppm and 2000 ppm, respectively, and in females at the highest
dose. A shift from a diffuse distribution of fat droplets to a more
periportal distribution was observed in animals of each sex at
> 2000 ppm. In kidneys, a higher incidence of fat droplets was
observed in females at > 2000 ppm. The NOAEL was 365 ppm,
equivalent to 52 mg/kg bw per day (Hoffman et al., 1975c).
Rats
In a pilot study reported in little detail, four male and four
female male Wistar rats were fed diets containing fenarimol (purity
unspecified) at 0 (10 animals per group), 25, 50, 100, 200, 400, 800,
1600, 3200, 6400, or 12 800 ppm for 14 days. Three animals at the high
dose died. Signs of toxicity, including anorexia, piloerection,
ataxia, and tremors, and decreased body weight and food consumption
were seen at doses > 3200 ppm. In the liver, the only organ
investigated, there were dose-related increases in relative weight at
> 400 ppm and in the rate of hepatic metabolism of para-
nitroanisole at > 200 ppm. Histopathological examination of the
liver revealed centrilobular hypertrophy at > 400 ppm and focal
necrosis at > 6400 ppm; no effects were seen at < 100 ppm
(Hoffman et al., 1975d).
In a subsequent study in male Wistar rats, also poorly reported,
four animals per treatment group and 10 controls were fed diets
containing fenarimol (purity unspecified) at 0, 25, 50, 100, 200, 400,
800, 1600, 3200, or 6400 ppm for 14 days. Food consumption and body
weight were reduced at > 1600 ppm; increased absolute and relative
liver weights were seen at > 800 ppm and increased hepatic
microsomal protein content at > 400 ppm. A dose-related reduction
in glucose-6-phosphatase activity was seen at > 800 ppm. The rate
of hepatic metabolism of para-nitroanisole was increased in a
dose-related fashion at > 200 ppm. No effects on the liver were
seen at < 100 ppm (Hoffman et al., 1975e).
In another poorly reported study, groups of 20 male and 20 female
Wistar rats were fed diets containing 0 (25 animals per sex), 50, 200,
or 800 ppm fenarimol (purity unspecified) for three months, equivalent
to 0, 2.5, 10, or 40 mg/kg bw per day. Five animals of each sex in
each group were kept for a recovery period of two weeks. No
ophthalmological examinations were performed. There were no treatment
-related effects on mortality, clinical signs of toxicity, food
consumption, haematology, or clinical chemistry (only blood urea
nitrogen, glucose, and alanine aminotransferase measured). A slight
depression in body weight was observed in males at the high dose. A
dose-related increase in relative liver weight was observed in animals
of each sex, which was statistically significant at the high dose. The
relative weights of the kidneys of males and females and of the
thyroid of females were increased at the high dose. Hepatic para-
nitroanisole metabolism, determined in five animals of each sex in
each group, showed a significant, dose-related increase in females at
800 ppm and in males at > 200 ppm. The main effect seen on
histopathological examination of tissues other than nervous tissue was
centrilobular hypertrophy in males and a shift from slight to moderate
fatty metamorphosis in females at the high dose. At the end of the
recovery period, only increased liver weight was still observed at the
high dose. The NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw per day
(Hoffman et al., 1975f).
In a study with no recovery period and again with little detail
reported, groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 140, 200, 275, 365, or 500 ppm fenarimol (purity
unspecified) for three months, equivalent to 0, 7, 10, 14, 18, or
25 mg/kg bw per day. There were no treatment-related effects on
mortality, clinical signs of toxicity, body weight, food consumption,
haematology, clinical chemistry, or histopathology (nervous tissue was
not examined). A dose-related increase in relative liver weight was
observed in females at > 275 ppm and in males at 500 ppm. Hepatic
para-nitroanisole metabolism, determined in five animals of each sex
in each group, showed a dose-related increase, which was statistically
significant at > 275 ppm. The NOAEL was 200 ppm, equivalent to
10 mg/kg bw per day (Hoffman et al., 1975g).
Rabbits
A 21-day study of dermal toxicity was conducted in groups of five
male and five female New Zealand white rabbits, which received daily
applications of 0 or 1000 mg/kg bw technical-grade fenarimol (purity,
97%) or 500 or 1000 mg/kg bw of a wettable powder formulation
containing 50% fenarimol for 6 h/day. A further group received
1000 mg/kg bw of the formulation and was then held for a recovery
period of 14 days. Technical-grade fenarimol induced no treatment-
related effect on appearance, behaviour, body weight, food
consumption, ophthalmological parameters, dermal irritation,
haematological or clinical chemical parameters, organ weights, or
gross or histological appearance of tissues. The NOAEL was thus the
highest dose tested, 1000 mg/kg bw per day. The formulation induced no
treatment-related systemic toxicity, but dermal irritation was seen in
both groups, characterized by slight to severe erythema and slight
oedema during the treatment phase. The irritation subsided within nine
days after exposure (Hoffman et al., 1985f).
Dogs
Groups of four male and four female beagle dogs were fed gelatin
capsules containing fenarimol (purity unspecified) at doses of 0,
1.25, 5, or 20 mg/kg bw per day for three months. No treatment-related
effect was seen on appearance, behaviour, body weight, food
consumption, ophthalmological, haematological, or clinical chemical
parameters, urinalysis, organ weights, hepatic para-nitroanisole
metabolism, or gross or histological appearance of tissues (excluding
nervous tissue). The NOAEL was thus the highest dose tested, 20 mg/kg
bw per day (Hoffman et al., 1975h).
Groups of six male and six female beagle dogs were given gelatin
capsules containing fenarimol (purity, 96.7%) at doses of 0, 1.25,
12.5, or 125 mg/kg bw per day for one year. Two dogs of each sex in
each group were maintained for a recovery period of three months. No
treatment-related effect was seen on mortality, body weight, food
consumption, or ophthalmological, haematological, or urinary
parameters. One, five, seven, and 12 dogs in the four groups had
soft, runny, or mucoid stools on two, 10, 21, and 34 occasions,
respectively. Transient enlargement of the mammary glands of female
dogs was seen in all groups, and there was a slight treatment-related
increase in incidence and duration in four animals in the group at the
highest dose, lasting for up to 46 days. At the high dose, increased
alkaline phosphatase and liver para-nitroanisole O-demethylase
activities and increased absolute and relative liver weights were
observed in animals of each sex; a trend to increased relative ovarian
weight was also observed at this dose. These increases were still seen
after recovery. The only treatment-related histopathological finding
was mild hepatic bile stasis in one female in the treatment phase and
in two males after the recovery period. The NOAEL was 12.5 mg/kg bw
per day (Hoffman et al., 1985g).
(c) Long-term toxicity and carcinogenicity
In many of the studies described below, increased blood glucose
was found. Although apparent dose-dependent increases were observed in
several studies, the end-point (measured only at termination) was very
variable and inconsistent, usually with flat dose-response curves. No
clear explanation could be found for these apparent increases. The
effect was considered to be compound-related but toxicologically
insignificant and was not taken into account in establishing the NOAEL
for each study.
Mice
Groups of 20 male and 20 female ICR mice (30 of each sex in the
control group) were fed diets containing 0, 50, 170, or 600 ppm
fenarimol (purity, 97%), equivalent to 0, 7.1, 24.3, or 85.7 mg/kg bw
per day, for 12 months. Food consumption was not measured, urinalysis
was not performed, and haematology and clinical chemistry were
determined only at termination. There were no treatment-related
effects on mortality, clinical signs of toxicity, or haematology.
Body-weight gain of males at 600 ppm was slightly decreased. A
dose-related increase in blood glucose levels was observed at
> 50 ppm, which was statistically significant at 600 ppm in males
and at 170 ppm in females. A significant increase in serum creatinine
concentration was seen in females at 170 and 600 ppm. The activity of
alanine aminotransferase was increased in males at the high dose. The
absolute and relative liver and spleen weights were increased in males
at 600 ppm, and relative liver weight was increased in females at
600 ppm. Absolute and relative uterine weights were decreased at this
dose. A slight, dose-related increase in the incidence of very slight
or slight fatty change in the liver was observed, reaching 10-20% at
600 ppm (0% in controls). The NOAEL was 170 ppm, equivalent to
24.3 mg/kg bw per day (Hoffman et al., 1978a).
In two replicate studies, groups of 40 male and 40 female ICR
mice (60 animals of each sex in the control group) were fed diets
containing 0, 50, 170, or 600 ppm fenarimol (purity, 97.9%),
equivalent to 0, 7.1, 24.3, or 85.7 mg/kg bw per day, for two years.
Food consumption was not measured, and haematology and clinical
chemistry were carried out only at termination. Since the results of
the replicate studies were similar, they were reported as a single
study. There were no treatment-related effects on mortality, clinical
signs of toxicity, or haematological or clinical chemical parameters.
Body-weight gain of males at 600 ppm was slightly decreased. A
tendency towards increased relative liver weights and a slightly
increased incidence of hepatic fatty change (2.5-6%; 1-2% in controls)
were observed in animals of each sex at the high dose. The tumour
incidence was not enhanced. Although the laboratory considered that
there were no significant effects in these studies, the slight
reduction in body weight suggests minimal toxicity. The NOAEL was thus
170 ppm, equivalent to 24.3 mg/kg bw per day (Hoffman et al.,
1978b).
Rats
Wistar rats of the first parental generation in a multi-
generation study of reproduction {see Hoffman et al., 1977b) were
maintained on treated diets for another three months after the end of
the study and thereby received the diets for a total of one year.
Groups of 20 male and 20 female rats (30 of each sex in the control
group) received doses of 0, 50, 130, or 350 ppm fenarimol (purity,
97.9%), equivalent to 0, 2.5, 6.5, or 17.5 mg/kg bw per day.
Haematology and clinical chemistry were evaluated only at termination;
no urinalysis was performed, and nervous tissue was not examined at
histopathology. There were no treatment-related effects on mortality,
clinical signs of toxicity, or body weight. The limited data available
on food consumption (intake during weeks 1, 8, 42, and 54 only)
indicated no effects. Males at the high dose had increased erythrocyte
counts and and decreased leukocyte counts. Blood glucose levels were
elevated in males at all doses (not dose-related but highest at the
high dose) and in females at 130 and 350 ppm. A dose-related reduction
in blood urea nitrogen was observed in males at > 50 ppm. Absolute
and relative liver weights and relative kidney weight were increased
in females, and absolute and relative spleen weights were decreased in
males at the high dose. Slight atrophy of the acini of the pancreas
was observed more frequently at the high dose, predominantly in males.
The effects on clinical chemical parameters are not considered to be
adverse. The NOAEL was thus 130 ppm, equivalent to 6.5 mg/kg bw per
day (Hoffman et al., 1978c).
Groups of 20 male and 20 female Wistar rats (30 of each sex in
the control group) received diets containing 0, 50, 130, or 350 ppm
fenarimol (purity, 97.9%) for 18 months, equal to 0, 2.3, 6.0, or
16 mg/kg bw for males and 0, 3.6, 8.4, or 22.9 mg/kg bw per day for
females. Urinalysis and ophthalmological examinations were not
performed, and haematology and clinical chemistry were evaluated only
at termination. There were no treatment-related effects on mortality
(survival was a slightly higher at the highest dose), food
consumption, clinical signs of toxicity, or clinical chemistry. A
reduction in growth was seen consistently in all treated groups; on
several occasions, 10% reductions in body weight were seen at 130 and
350 ppm. At 350 ppm, a slight decrease in leukocyte count was observed
in males and in prothrombin time in females. A dose-related decrease
in serum prolactin levels was observed in females, but it was not
statistically significant, and the individual levels within the groups
were very variable. Relative liver weight was increased in all treated
groups, but the reduction was statistically significant only at
350 ppm. At this dose, absolute and relative ovarian weights were
increased, and absolute spleen weights were reduced in males. A slight
increase in the incidence of a fatty liver was seen in all treated
males (60-70%; 43% in controls), and an increase in the severity of
fatty changes in liver was observed in females at the high dose. The
NOAEL was 50 ppm, equal to 2.3 mg/kg bw for males and 3.6 mg/kg bw per
day for females (Hoffman et al., 1978d).
In two replicate studies, groups of 40 male and 40 female Wistar
rats (60 animals of each sex in the control group) were fed diets
containing 0, 50, 130, or 350 ppm fenarimol (purity, 97.9%), equal to
0, 2.0, 5.3, or 14.6 mg/kg bw for males and 0, 2.8, 7.6, or 21.5 mg/kg
bw for females, for 24 months. Haematology and clinical chemistry were
evaluated only at termination. Since the results of the two replicate
studies were similar, they were reported as a single study. There was
a dose-related increase in the rate of survival, increasing in males
from 27% (controls) to 44% (high dose) and in females from 38 to 54%;
however, the rate was not sufficient to meet the criteria for a valid
negative result in a study of carcinogenicity. There were no
treatment-related effects on clinical signs of toxicity or organ
weights. Body-weight gain of males was slightly reduced at 350 ppm
during the first nine months of the study. The leukocyte count was
decreased in animals of each sex at the high dose. Dose-related
increases in blood glucose levels were seen at all doses, which were
statistically significant in males at the high dose and in one study
at > 50 ppm in females. In one study, a dose-related reduction in
mean serum prolactin values was seen in females at > 50 ppm, which
reached statistical significance at 350 ppm; however, individual
values were very variable, with similar ranges in all groups. Males
also showed a statistically significant increase in prolactin levels
at 350 ppm, and females had increased levels of luteinizing hormone.
Although hormone levels were investigated to elucidate the effects of
fenarimol on reproduction, the laboratory considered the toxicological
significance of the observed changes to be unknown. An increased
incidence of fatty changes in the liver, which tended to be dose-
related, was seen especially in males; the overall incidences in males
and females in the two studies were 29, 40, 43, and 53% at 0, 50, 130,
and 350 ppm. An increase in severity was observed in animals of each
sex at the high dose. The incidence of hyperplastic nodules was
slightly increased: 0% in controls, 1.9% at 50 ppm, 1.9% at 130 ppm,
and 4.4% at 350 ppm, and reached statistical significance at the high
dose. In addition, hepatic-cell adenomas were seen in 0.6% of rats at
130 ppm and in 3.2% at 350 ppm (statistically significant). There was
no NOAEL, owing to the slight effects on the liver at the low dose
(Hoffman et al., 1978e).
A further two-year carcinogenicity study was conducted in order
to establish a no-effect level for the effects on the liver observed
in the previous study. Groups of 50 male and 50 female Wistar rats
were fed diets containing 0, 12.5, 25, or 50 ppm fenarimol (purity,
96.7%), equal to 0, 0.6, 1.2, or 2.5 mg/kg bw for males and 0, 0.7,
1.5, or 3.0 mg/kg bw per day for females, for 24 months. Haematology
and clinical chemistry were evaluated only at termination, and the
liver was the only organ examined at necropsy. The survival rate was
low due to an outbreak of chronic respiratory disease during the 16th
and 17th months: only 10-32% per sex per group survived for two years
There were no treatment-related effects on clinical signs of toxicity,
haematology, or liver weight. Reductions in body-weight gain, food
consumption, and food efficiency were observed in males at the high
dose during the first year of the study. The glucose content of blood
was increased in animals of each sex at 50 ppm. The incidence of fatty
liver was increased at the high dose, especially in males (60%; 26% in
controls). There was no evidence of an increase in tumour incidence,
but the survival rate was not sufficient to meet the criteria for a
valid negative result in a study of carcinogenicity. The NOAEL was
25 ppm, equal to 1.2 and 1.5 mg/kg bw per day for males and females,
respectively (Hoffman et al., 1982a).
Groups of 50 male and 50 female Wistar rats were fed fenarimol
(purity, 97%) at 0, 12.5, 25, or 50 ppm in their diet, equal to 0,
0.5, 1.0, or 2.0 mg/kg bw for males and 0, 0.6, 1.2, or 2.3 mg/kg bw
per day for females, for 24 months. Haematology and clinical chemistry
were evaluated only at termination, and the liver was the only organ
weighed. There was no treatment-related effect on survival rates
(29-44% for males and 56-74% for females), body-weight gain, food
consumption, food efficiency, haematology, hepatic para-nitroanisole
metabolism, liver weight, or histopathology of any organ. Slight
increases in the incidences of low body weight and pale eyes were
noted in all treated males. Blood glucose levels were increased at
25 ppm in males and at 50 ppm in animals of each sex. The tumour
incidence was not increased. The effects seen were considered not to
be adverse. The NOAEL was therefore 50 ppm, the highest dose tested,
equal to 2.0 and 2.3 mg/kg bw per day for males and females,
respectively (Hoffman & Pierce, 1985).
In a further analysis of the data on carcinogenicity from the
study conducted in 1986, the incidences of hepatocellular adenoma and
carcinoma at the high dose in the replicate study and at the low dose
in the last study were combined and analysed for trend. There was no
statistically significant evidence that fenarimol is oncogenic
(Rodricks et al., 1989).
In a study conducted to assess the initiating activity of
fenarimol, groups of six or 12 male Fischer 344 rats were fed 350 ppm
fenarimol (purity unspecified) in the diet for eight or 20 weeks;
one group fed fenarimol for eight weeks was subsequently fed
phenobarbital for 12 weeks. In order to investigate promotion
potential, hepatocellular foci were induced by exposure to N-2-
fluorenylacetamide for eight weeks, and then rats were fed 350 ppm
fenarimol for 12 weeks. Livers were examined after eight or 20 weeks
for the presence of altered hepatocellular foci that were iron-
excluding or contained gamma-glutamyl transpeptidase. Fenarimol had no
initiating or promoting activity in rat liver (Hoffman & Amundson,
1985).
(d) Reproductive toxicity
Mice
In two pilot studies, groups of 10 male and 10 female ICR mice
were fed fenarimol (purity, 97.9%) in the diet. In the first study,
treatment with doses of 50, 170, or 600 ppm was started one week
before mating and was continued up to day 21 of lactation. In the
second study, treatment with doses of 0, 170, 350, or 600 ppm was
started two weeks before mating and continued up to day 14 of
lactation. One female at 600 ppm died and one was killed with signs of
dystocia. No effect on body weight was detected. Fertility was clearly
reduced (and fewer females had copulatory plugs) at 350 and 600 ppm in
the second study only, and there was evidence of reduced fertility at
170 ppm. Liveborn litter size was reduced at 350 and 600 ppm,
apparently as a result of an increased incidence of stillbirths. A
lengthened gestation period was observed at 600 ppm No NOAEL could be
established owing to the limited study design (Markham et al.,
1978a).
In a three-generation study, groups of 20 male and 20 female ICR
mice (30 of each sex in the control group) were fed fenarimol (purity,
97.9%) at dietary levels of 0, 35, 70, or 140 ppm, equivalent to 0, 5,
10, or 20 mg/kg bw per day, starting 61-63 days before mating. Food
consumption was not measured, and the surviving parental animals were
not necropsied. No compound-related effects were observed in the
parents, on reproductive parameters, or on the pups. The NOAEL was
140 ppm, equivalent to 20 mg/kg bw per day (Markham et al., 1978b).
Rats
In a two-generation study, groups of 20 male and 20 female Wistar
rats (30 of each sex in the control group) were fed fenarimol (purity,
97.9%) in the diet at concentrations of 0, 50, 130, or 350 ppm,
equivalent to 0, 12.5, 6.5, or 17.5 mg/kg bw per day. Food consumption
was not measured. F0 rats were exposed for 55 days before the first
of three matings; after the third mating, the animals were maintained
on the test diets for another three months and then evaluated for
long-term toxicity (see Hoffman et al., 1978c). The F1b offspring
were destined to become F1 parents. The F1 animals were exposed to
the test diets for 58 days before the first mating. After weaning, the
F1 parents were placed on control diet for 63 days before a second
mating (reversibility test). For the third mating, a reversibility and
cross-over experiment was performed: the F1 animals continued to be
exposed to control diets, for a total of about five months by the time
of mating. F1 males were mated with untreated virgin females and
F1 females with untreated males. In each of the three F1
breedings, one female at 350 ppm died, perhaps due to dystocia. The
F1 males at 130 and 350 ppm had decreased body-weight gain. There
was a dose-related reduction in fertility (proportion of pregnant
females) at 130 and 350 ppm, and the effect was time-dependent, being
more pronounced after each successive F0 mating and even more
pronounced after the first F1 mating, in which fertility was 20 and
0%, respectively, at 130 and 350 ppm. There was some evidence of
reduced fertility at 50 ppm, but the effects were not statistically
significant. After the F1 parent animals had been fed the control
diet, fertility was 45 and 10%, respectively. Cross-over analysis
showed clearly that the reduced fertility was male-mediated, and there
was some, inconclusive evidence that it might also be female-mediated:
the fertility of treated females was lowered at all doses. The control
value for untreated females was, however, low, and age may have been a
factor since the females were over 10 months old A reduction in
liveborn litter size was seen at 350 ppm after the F0 matings,
probably due to an increase in the proportion of stillborn pups. In
all treated groups, but especially at 350 ppm, there were more females
with a lengthened gestation period (23-24 days) than among controls.
There was no consistent, substantive effect on the survival rate or
body weight of the progeny. Gross necropsy of weanlings revealed an
increased incidence (14.2% compared to 5.2% in controls; not
statistically significant) of hydronephrosis at 350 ppm after the
third mating of the F0 generation. No NOAEL could be established for
reproductive effects, because of the slight evidence of reduced
fertility at the lowest dose. The NOAEL for general systemic toxicity
in parental animals was 50 ppm, equivalent to 2.5 mg/kg bw per day
(Hoffman et al., 1977b).
In a further three-generation study, groups of 20 male and 20
female Wistar rats (30 of each sex in the control group) were fed
fenarimol (purity, 97.9%) in the diet at concentrations of 0, 12.5,
25, or 50 ppm, equivalent to 0, 0.625, 1.25, or 2.5 mg/kg bw per day.
Exposure started 56-71 days before the first mating, and three
generations of one, two, and one nests were bred. The surviving
parental animals were not necropsied. The death of one female during
parturition and signs of haemorrhage in another female during
parturition (signs of dystocia), both at 50 ppm, were attributed to
treatment. Body-weight gain was slightly reduced during the premating
period in F1 males at 50 ppm. After both F1 mating periods,
fertility was clearly reduced at 50 ppm, together with a reduction in
the proportion of females with copulatory plugs, and there was some
evidence of a reduction in fertility after the F2 mating at 50 ppm. A
statistically significant, dose-related reduction in liveborn litter
size (apparently due to an increase in stillbirths) at 25 and 50 ppm
after the second F1 mating was not considered to be related to
treatment by the laboratory; however, it occurred only after a long
exposure and has been seen to be a clear result of shorter exposure to
a higher concentration of fenarimol (see Hoffman et al., 1977b). The
NOAEL for reproductive effects was 12.5 ppm, equivalent to 0.625 mg/kg
bw per day. The NOAEL for general systemic toxicity was 25 ppm,
equivalent to 1.25 mg/kg bw per day (Markham et al., 1978c).
A single-generation study of cross-over design was conducted in
groups of 10 male and 10 female Wistar rats fed fenarimol (purity,
97.9%) by gavage at a dose of 0 or 35 mg/kg bw per day, starting one
month before mating and continuing up to one month thereafter for
males and up to day 20 post partum for females. In two groups,
control males were mated with control and treated females, and in the
third group treated males were mated with control females. The
fertility of treated males was reduced, together with a reduction in
the proportion of females with copulatory plugs, and the liveborn
litter size of treated females was reduced, apparently due to an
increased incidence of stillbirths. There was no NOAEL (Hoffman
et al., 1980b).
Groups of 15 male and 15 female Wistar and Sprague-Dawley rats
were compared with regard to their susceptibility to reproductive
effects under identical conditions in a cross-over study similar to
that described above (Hoffman et al., 1980e), except that the serum
levels of luteinizing hormone and prolactin were also investigated. In
both strains, body-weight loss and dystocia were observed during the
first week of treatment. Fertility was reduced in treated males, as
was the number of females with copulatory plugs. The mean liveborn
litter size was reduced in Sprague-Dawley rats only, due to an
increased incidence of stillbirths. Post-partum survival was reduced
in treated females of both strains. The serum levels of luteinizing
hormone were increased and those of prolactin decreased in treated
females of both strains. There was no NOAEL (Hoffman et al., 1980c).
In another single-generation study of cross-over design, groups
of 10 male and 10 female Wistar rats were fed fenarimol (purity,
97.9%) by gavage at 0 or 35 mg/kg bw per day, starting one month
before mating up to one month thereafter, encompassing gestation and
lactation. In two groups, control males were mated with control and
treated females, and in the third group treated males were mated with
control females. The serum levels of prolactin, luteinizing hormone,
and testosterone and para-nitroanisole- O-demethylase activity were
measured. Two treated females died due to dystocia. Fertility was
reduced in treated males, and the proportion of females with
copulatory plugs and vaginal sperm was reduced. In all cases in which
there was evidence of mating, it was not delayed, and the females
became pregnant. The liveborn litter size of treated females was
reduced, apparently due to an increased incidence of stillbirths; in
addition, gestation length was increased, and progeny survival through
day 7 post partum was slightly reduced. Absolute and relative liver
weights and liver enzyme activity were increased in treated males
and females; treated males also had hypertrophy of centrilobular
hepatocytes. There was no NOAEL (Hoffman et al., 1980d).
Guinea-pigs
In a two-generation study., groups of 15 male and 30 female
guinea-pigs of the F0 generation and 20 males and 20 females of the
F1 parent generation were fed fenarimol (purity, 98.5%) at dietary
levels of 0 or 400 ppm, starting four weeks before mating for the F0
parents and 70 days before mating for the F1 parents. There were
no treatment-related effects on mortality or food consumption.
Body-weight gain was reduced in males of both generations and in F0
females during the premating period. There was no effect on
reproductive parameters, except that the proportion of liveborn
progeny was marginally lower in treated animals of each generation.
The laboratory considered this to be a reflection of litter size, i.e.
lower survival in larger litters, which has previously been documented
in guinea-pigs. Although the data presented support this view for the
control groups, it does not do so for the treated groups. Therefore,
400 ppm, equal to 33 mg/kg bw per day for males and 35 mg/kg bw per
day for females, slightly affected gestational survival, although this
is not of clear toxicological importance (Hoffman et al., 1983a).
Rabbits
In a single-generation stud), of cross-over design, groups of
9-11 male and 9-11 female Dutch belted rabbits were fed fenarimol
(purity, 97.9%) by gavage at 0 or 35 mg/kg bw per day, starting one
month before mating and continuing up to one month thereafter for
males and up to day 6 post partum for females. In two groups,
control males were mated with control and treated females, and in the
third group treated males were mated with control females. Prolactin
and testosterone levels were determined, and premating semen volume,
sperm count, and sperm viability were measured. There was no evidence
of systemic toxicity or adverse reproductive effects. The. NOAEL was
the only dose tested, 35 mg/kg bw per day (Hoffman et al., 1980e).
(e) Developmental toxicity
Rats
Groups of 25 pregnant Wistar rats were fed fenarimol (purity,
97.9%) by gavage at 0, 5, 13, or 35 mg/kg bw per day during days 6-15
of gestation. None of the dams died, and there were no signs of
toxicity and no effects on food consumption or body weight. The only
finding in fetuses was an increased incidence of hydronephrosis at the
high dose (in 30% of fetuses and 62% of litters, in comparison with 9%
of fetuses and 25% of litters in controls). The NOAEL for maternal
toxicity was 35 mg/ kg bw per day and that for embryo- and
fetotoxicity, 13 mg/kg bw per day (Hoffman et al., 1980f).
A special study was conducted to investigate the reversibility of
fenarimol-induced hydronephrosis in neonatal rats. In three replicate
studies, pregnant Wistar rats were fed fenarimol (purity, 99.6%) by
gavage at 0 or 35 mg/kg bw per day during days 6-15 of gestation. Two
groups were sacrificed on day 20 or 21 of gestation, and the others
were allowed to litter and wean their progeny. The normal prenatal and
postnatal examinations were performed, and the kidneys and ureters of
the progeny were examined on days 20 and 21 of gestation and on days
1, 7, 21, 42, and 63 post partum. The total numbers of female rats
examined on gestation days 20 and 21 were 25 and 15, respectively;
postnatally, 15 in the control group and 20 in the test group were
examined. There were no treatment-related effects on dams with regard
to mortality, body weight, or food consumption, but sporadic cases of
dystocia were noted. Prenatal examination revealed a slight decrease
in the proportion of live fetuses due to an increase in early
resorptions. Fetal weights were slightly lower on day 20 but not day
21; on day 20, the number of runt fetuses was also increased.
Hydroureter and hydronephrosis were seen more often among exposed
fetuses on days 20 and 21, and a slightly increased incidence of
skeletal variants, such as cervical ribs and 14 thoracic fibs, was
observed, but only day-20 fetuses were examined. Fetal kidney weights
were depressed by treatment. Postnatal examination revealed a slight
increase in gestational length and reduced liveborn litter size.
Furthermore, neonatal mortality was increased during week 1 post
partum, pup body weights were increased, hypothermia was more
common, and the absolute kidney weights of the progeny were increased.
A reduction in the increased incidence of hydroureter, which was seen
prenatally, was observed with advancing neonatal age, but a
significant increase in hydronephrosis was seen in progeny on day 1
post partum (50% of treated pups and 23% of control pups). At
subsequent examinations, the incidence was still increased but was
substantially less than on day 1 post partum. The slight or moderate
microscopic alterations associated with the observed hydronephrosis
were limited to the renal pelvis and papilla and therefore did not
constitute true pathological hydronephrosis, which is associated with
severe damage, such as glomerular destruction, shortening and blunting
of renal tubules, and reduction or absence of the outer nephrogenic
layer. There was no effect on kidney function, including specific
gravity and osmolality, determined on day 63 post partum. As the
observed hydronephrosis was apparently not a significant pathological
condition and was to at least some degree reversible, together with
the fact that it is a common finding in the Wistar rat, its increased
incidence is considered to be due to delayed development and not a
teratogenic effect (Hoffman et al., 1983b).
Rabbits
Groups of 15 pregnant Dutch-belted rabbits were fed fenarimol
(purity, 97%) by gavage at 0, 3, 10, or 35 mg/kg bw per day on
gestation days 6-18 and were sacrificed on gestation day 28. There
were no treatment-related effects on mortality, body weight, or food
consumption. 4 slight reduction in litter size was observed at 10 and
35 mg/kg bw, but this was not considered to be related to treatment
since the number of implantations and the implantation index were also
reduced in these groups. The occurrence of oedema and various limb and
skeletal defects in five fetuses from one anorectic female at 35 mg/kg
bw was not clearly a direct response to treatment. There was no
evidence of irreversible structural changes. In the absence of clear
evidence of maternal or developmental toxicity, the NOAEL was 35 mg/kg
bw per day, the highest dose tested (Hoffman et al., 1977c).
Groups of 20 female New Zealand white rabbits were fed fenarimol
(purity, 96.6%) by gavage at 0, 15, 50, or 150 mg/kg bw per day on
gestation days 6-18 and were sacrificed on gestation day 28. There
were no treatment-related effects on mortality. Body weight and food
consumption were reduced at 150 mg/kg bw, and four rabbits at the
highest dose lost weight, became anorectic, and then aborted. The
number of live fetuses per litter was reduced, and there was an
increase in the number of early resorptions (neither statistically
significant). The only finding in fetuses was an increased incidence
of extrathoracic ribs (84% of fetuses, compared with 61% in controls).
There was no evidence of irreversible structural changes. The NOAEL
for maternal and embryo- and fetotoxicity was 50 mg/kg bw per day
(Hoffman & Russel, 1990).
(f) Genotoxicity
A number of tests for genotoxicity have been carried out with
fenarimol. The results are summarized in Table 2.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
A single, 4-h, semi-occluded application of 0.5 g technical-grade
fenarimol (purity stated to be 100%) onto the intact skin of three
male New Zealand white rabbits produced no skin irritation (Jones,
1994a).
After instillation of 0.1 ml (equivalent to 56 mg) of technical-
grade fenarimol (purity stated to be 100%) into the conjunctival sac
of the eyes of one male and two female New Zealand white rabbits,
slight conjunctival irritation was observed up to 24 h. Iridial
inflammation was noted in one treated eye 1 h after treatment. The
treated eyes appeared to be normal after 24-48 h (Jones, 1994b).
Fenarimol (purity, 97%) was tested in a Magnusson-Kligman
maximization test in 10 Hartley guinea-pigs; the control group
consisted of five animals. Barely perceptible erythema were seen in
two treated animals and in one of four surviving controls (Hoffman &
Arthur, 1980).
Table 2. Results of tests for the genotoxicity of fenarimol
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutationa S. typhimurium TA100, 125-2000 µg/plateb; 97 Negatived Hoffman et al.
TA98, TA1535, TA1537, 62.5-1000 µg/platec; (1988a)
E. coli WP2uvrA- toxicity and precipitation
at highest concentration;
vehicle, dimethyl sulfoxide
Chromosomal aberrationa Human lymphocytes 1.75-25 µg/mlb for 25 h 97 Negatived Murli (1988)
59.9-160 µg/mlc for 1 he;
vehicle, dimethyl sulfoxide
Gene mutationa Mouse lymphoma 1-50 µg/ml for 4 h; 97 Negatived Hoffman et al.
L5178Y tk+/-cells cytotoxic at > 35 µg/ml; (1988a)
vehicle, dimethyl sulfoxide
Unscheduled DNA Rat hepatocytes 0.05-100 nmol/ml for 5 h; 97 Negativea Probst (1979)
synthesisa,f cytotoxic at > 50 nmol/ml;
vehicle, dimethyl sulfoxide
In vivo
Chromosomal aberration 10 male Chinese hamsters; Oral; 250 mg/kg bw per day 98 Negatived Siou & Lerond-
bone-marrow cells for 2 daysg; sacrifice at 24, Conan (1982)
48, and 72 h; vehicle, peanut oil
Micronucleus formation Groups of 10 male Swiss Oral; 1000 mg/kg bw per day > 98 Weakly Siou et al. (1982)
mice; hone-marrow cells for 2 daysh; sacrifice at 24, positivei
48, and 72 h; vehicle, peanut oil
Micronucleus formation Groups of five male and Oral; 125, 625, or 1250 mg/kg 97 Negatived Ivett (1988)
five female Sprague-Dawley bwj; sacrifice at 24, 48, and
rats; bone-marrow cells 72h; vehicle, corn oil
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
In vivo (con't)
Dominant lethal mutation Groups of 10 male Wistar- Oral; 350 mg/kg bw; vehicle, 97 Inconclusivek Hoffman et al.
Harlan rats 5% acacia solution (1977d)
a No confirmatory experiment
b Without metabolic activation
c With metabolic activation
d Positive controls yielded positive result(s)
e As cell cycle delay was observed with metabolic activation in a preliminary test, incubation was for 45.3 h after treatment.
No cell cycle delay without activation; toxicity at highest doses tested
f Only one culture per concentration; only 20 cells per concentration analysed
g No mention of cytotoxicity; dose based on preliminary test in which all five animals died at 1000 mg/kg bw per day lot two days
h No mention of cytotoxicity; dose based on preliminary test in which all five animals died at 4000 mg/kg bw per day lot two days.
In the main test, one animal treated for 72 h died.
i Slight but statistically significant increase in micronuclei at 24 h sacrifice only; i.e. micronucleus frequencies per
1000 polychromatic erythrocytes; at 24 h: control, 0.19 ± 0.03; treated, 0.33 ± 0.06
j Toxic signs (lethargy, unsteady gait) at > 125 mg/g bw; one animal died at 1250 mg/kg bw.
k Treated males bred with untreated females weekly for eight weeks. Dose > 1/10 of the LD50. No sign of toxicity observed.
Only 7-10 pregnant females per mating interval per dose group. No reference to positive controls. No treatment-related effect on
fertility rate or pre- or post-implantation losses.
(ii) Mechanisms of effects on reproduction
Groups of six adult male Sprague-Dawley rats were given 35 mg/kg
bw per day fenarimol (purity unspecified) by gavage or subcutaneously
for 30 days; eight males served as controls. After 15 days of
treatment, each male was mated with two untreated females. No effect
was seen on male body weight, male fertility, the histopathology of
the testis or ventral prostate, or serum levels of testosterone,
follicle-stimulating hormone, or luteinizing hormone. Epididymal
weight was reduced after oral administration, but no histopathological
effects were observed. Serum prolactin levels were reduced after
systemic exposure, and prostatic prolactin binding was reduced after
exposure by either route. In a second experiment, groups of 15 males
were fed 0 or 35 mg/kg bw per day by gavage for 70 days and were bred
30 and 56 days after exposure. The parameters measured were the same
as in the first experiment except for follicle-stimulating hormone.
Serum prolactin levels and prostatic prolactin binding were reduced,
and the serum testosterone level was slightly elevated. There were no
other effects (Hanlin, 1981a).
Groups of 10 adult female Sprague-Dawley rats received fenarimol
(purity, 97%) at 0 or 35 mg/kg bw per day by gavage for about 60 days
before mating with untreated males, and then throughout pregnancy and
lactation. There were no effects on body weight or fertility. There
was some suggestion of an increase in proestrous prolactin level, but
the serum levels of prolactin were variable both before estrus and
during lactation and those of luteinizing hormone were variable before
estrus. Overall, there was no clear compound-related effect. There was
some evidence of a slight increase in estrous cycle length (Smalstig,
1981a).
Groups of 30 male Wistar rats were fed a diet containing 0 or
350 ppm fenarimol (purity, 97.7%) from 25 to 82 days of age. Twenty in
each group were mated and 10 were unmated; unmated males were killed
at the end of treatment, and the remaining animals were fed control
diet until day 117. Mating trials were conducted weekly with untreated
females on days 40-110. There were no significant effects on food
consumption or body-weight gain. A delay in the onset of reproductive
behaviour (percent mating and inseminating) was observed, but the
effect was reversible, since 100% fertility was attained at day 110.
The only effect on male reproductive organs was a reduction in
epididymal weight at the end of the treatment phase, with recovery
after cessation of treatment. No tissues were examined microscopically
(Hoffman & Hirsch, 1981c).
In a study of the possible involvement of uterine prostaglandins
in parturition, female Sprague-Dawley and Charles River CD rats
received 35 mg/kg bw per day fenarimol (purity, 97%) or the corn oil
vehicle orally from day 12 of pregnancy until parturition, or day 23
of pregnancy if no parturition occurred. The prostaglandin F2µ,
content, its release in and from the uterus, and serum progesterone
levels were measured some days before and at parturition. Sprague-
Dawley females showed delayed and/or difficult parturition and a
slight increase in the frequency of fetal and pup death, which
appeared to be related to the occurrence of difficult parturition.
There was no effect on prostaglandin F2µ, content; its release at
parturition was increased and not decreased, which would have been
expected with delayed parturition. Serum progesterone levels appeared
to be higher in treated females, and particularly in those that did
not deliver. The authors interpreted this finding as indicating that
progesterone did not fall to the low levels that facilitate or
initiate parturition in the rat (Smalstig, 1981b).
Progesterone concentrations in plasma were determined in late
pregnancy in groups of 12 Sprague-Dawley rats treated with 0 or
350 ppm fenarimol (purity unspecified) during pregnancy. Most of the
treated animals experienced delayed parturition associated with
dystocia and usually resulting in maternal death. High plasma
progesterone levels (66% of the peak value seen on day 19, in
comparison with 13% in controls) were seen in treated animals
immediately before normal parturition. The authors interpreted this
finding as indicating that luteolysis had not occurred, thus delaying
normal parturition (Tinsley, 1982).
A simulated rat ovarian microsomal system was used to measure the
effect of fenarimol on aromatase activity in vitro. Fenarimol
(purity unspecified) at molar concentrations 5-50 times the substrate
concentration inhibited aromatase activity by 55-90%. In a comparison
with aromatase inhibitors used clinically, fenarimol was as potent as
testolactone but less potent than aminoglutethimide and was therefore
considered to be a moderately weak inhibitor (Hoffman et al.,
1982b).
The urogenital sinuses were removed from 15-17-day-old male and
female Charles River rat embryos and implanted under the kidney
capsules of intact syngeneic male hosts (10 implants per animal). One
implanted male was then treated with 35 mg/kg bw per day fenarimol
(purity unspecified) for 21 days, one received the corn oil vehicle,
and one was treated with the positive control compound, cyproterone
acetate, which has anti-androgenic effects. Treatment with fenarimol
or corn oil had no effect on the differentiation or function of
transplanted urogenital sinuses in adult male rats. In contrast,
cyproterone acetate retarded prostatic development, and secretions
were absent (Neubauer et al., 1982).
Concentrations of 10-9 to 5 × 10-5 mol/litre fenarimol (purity
unspecified) were tested for androgen-binding capacity in vitro.
Fenarimol did not show affinity for binding to prostatic androgen
receptors, although 17% inhibition was seen at the highest
concentration. In an assay to determine whether fenarimol inhibits the
uptake of 3H-testosterone into the ventral prostate or the median
basal hypothalamus and preoptic areas of the brain, groups of 8-10
male Sprague-Dawley rats were given 0 or 35 mg/kg bw fenarimol
subcutaneously 20 and 3 h before sacrifice. Testosterone uptake was
not inhibited; in fact, uptake into the ventral prostate was
increased, whereas a positive control substance inhibited uptake into
the ventral prostate. In an assay to determine whether fenarimol
inhibits androgen-stimulated growth of seminal vesicles and ventral
prostate after castration, groups of six to eight immature rats were
given a daily subcutaneous injection of 0.25 mg/kg bw testosterone
propionate plus a daily oral or subcutaneous dose of 0, 5, 15, or
35 mg/kg bw fenarimol three days after castration for seven
consecutive days. A dose-related reduction in seminal vesicular weight
was seen with oral doses > 15 mg/kg bw, but the effect was not
statistically significant. The authors of the study concluded that
there was no inhibition (Hanlin, 1981b).
In a study to investigate whether fenarimol interferes with the
binding of androgens to the androgen receptor, groups of nine
castrated adult Wistar rats were fed fenarimol (purity unspecified) at
0 or 350 ppm in the diet for seven days. Cytosol from the hypothalamic
preoptic area, the frontal and parietal cerebral cortex, the
pituitary, and the ventral prostate was then incubated with
radiolabelled methyltrienolone (R1881; a potent synthetic androgen).
No reduction in binding to the cytoplasmic androgen receptors was
seen. In a second experiment, groups of one to two male rats were
treated similarly and then with radiolabelled R1881 before sacrifice.
There was no effect on binding to nuclear androgen receptors in the
tissues examined (the hypothalamic preoptic area, the pituitary, the
amygdala, the cerebral cortex, the prostate, the diaphragm, or the
liver), and the concentrations of conjugated and unconjugated labelled
R1881 were similar in all tissues. In a third experiment, pregnant
rats were fed 0 or 350 ppm fenarimol in the &let for most of the
period of gestation. Four female pups from each group were given a
subcutaneous injection of 3H-testosterone at the age of three or
four days and were killed 2 h later. There was no effect on the
binding of testosterone to the nuclear receptors in the hypothalamic
preoptic area amygdala (Hoffman et al., 1981a).
In a study of the estrogenic and anti-estrogenic potential of
fenarimol, groups of six immature female Holtzman rats received either
35 mg/kg bw fenarimol (purity unspecified) by gavage plus a
subcutaneous dose of estradiol, 35 mg/kg bw fenarimol by gavage, or a
subcutaneous dose of estradiol daily for three days. One dose of
fenarimol with several doses of estradiol, several doses of fenarimol
(8.75, 17.5, and 35 mg/kg bw) with one dose of estradiol, and
fenarimol in different vehicles were tested. There was no evidence for
agonistic or antagonistic effects on estrogen, as indicated by changes
in uterine weight. Fenarimol at concentrations of 1-1000 nmol/litre
did not inhibit the binding of estradiol to estrogen receptors in the
cytosol of immature rat uteri in vitro (Black, 1981a).
In a study of the effect of fenarimol on the binding of estrogen
to its receptor and on circulating estrogen levels at parturition,
fenarimol (purity unspecified) had no significant effect on the
nuclear uptake of estrogens by the hypothalamic preoptic area amygdala
or pituitary in groups of six adult ovariectomized rats fed 350 ppm in
the diet for seven days. Treatment of groups of four to six female
Wistar rats on days 0-21 of gestation at this dose did not affect the
concentration of estrone or estradiol in the plasma on day 21 (Hoffman
et al., 1981b).
Groups of three immature Dutch belted female rabbits were given
subcutaneous rejections of 0.5 µg oestradiol for six days and then
received either daily subcutaneous injections of 100 µg progesterone,
daily gavage doses of 60 mg fenarimol (purity unspecified), or the two
treatments combined, for five days. There was no evidence for a
progestational or anti-progestational effect in uteri examined
microscopically 24 h after the last dose (Black, 1981b).
Groups of seven pregnant Wistar rats were fed a diet containing
350 ppm fenarimol (purity, 97.7%) on gestation days 0 to 14-18. On the
last day of treatment, maternal blood and two fetuses per litter were
sampled 2 and 6 h after a gavage dose of radiolabelled fenarimol.
Placental transport was limited (the ratio of 14C in fetus to that
in maternal plasma was < 1) during days 14-17, the period associated
with genital tract morphogenesis, but increased steadily from day 15
onwards, to reach a ratio of 1 by day 18, when the organization of
sexual behaviour in the fetal brain begins (Hoffman & Hirsch, 1981a).
Female Wistar rats were fed 0 or 350 ppm fenarimol (purity,
97.7%) in the diet on days 0-5 post partum, and dams and pups
received an oral dose of radiolabelled fenarimol on day 5. One group
of 18 dams was used to study excretion into milk; the pups of seven
other dams were culled to three males and three females per litter.
The concentration of radiolabel in milk was three to five times higher
than that in maternal plasma up to 48 h after treatment. Radiolabel in
milk was rapidly taken up by the pups; the concentrations in the
hypothalamus increased more rapidly, attained higher maximal levels
(up to seven times higher 1 h after administration), and decreased
more slowly than in the remainder of the neonatal brain. In the
absence of previous exposure to fenarimol, an alteration was seen in
the rate but not the extent to which radiolabel was taken up by either
the hypothalamus or the brain. The major difference between pups
treated directly with 14C-fenarimol and those exposed via the milk
was in the rate of elimination, which was considerably faster after
direct treatment (Hoffman & Hirsch, 1981b).
In a study of the effects of fenarimol on the concentration of
estrogen receptors in the hypothalamus and on the conversion of
testosterone to estrogen, as an indirect measure of central aromatase
activity, pregnant Wistar rats received dietary concentrations of 0 or
350 ppm fenarimol (purity unspecified) from gestation day 3-4 to day
21. The concentration of nuclear estrogen receptors was measured in
the hypothalamic preoptic area amygdala of day-21 fetuses and of
neonates on day 3-4. The mean concentration was reduced by 44-46% in
fetuses of each sex and by 56% in neonates, especially in males.
Sex-specific differences in the concentration of receptors in the
exposed neonates were abolished, i.e. males no longer had higher
concentrations. In a second experiment, male Wistar rats that were
castrated but had testosterone implants and intact males received 0 or
350 ppm fenarimol in the diet for seven days. The concentration of
nuclear estrogen receptors was reduced in both groups. The circulating
levels of testosterone indicated that the effect on receptor
concentration could not be attributed to testosterone levels. In a
third experiment, pregnant Wistar rats received 0 or 350 ppm fenarimol
in the diet from about gestation day 18 to day 3-4 post partum, and
groups of 12 female neonates (used to eliminate endogenous
testosterone production as a variable) were then dosed with
radiolabelled testosterone. Significantly lower concentrations of
estrone and estradiol were observed in homogenates of hypothalamic
preoptic area amygdala from exposed pups, and, contrary to controls,
no labelled estradiol was detected in the nuclei. Fenarimol did not
affect the concentrations of testosterone or dihydrotestosterone in
the nuclear extracts of the hypothalamic preoptic area amygdala. Since
testosterone is metabolized to dihydrotestosterone by the enzyme
5µ-reductase and to estradiol by aromatase, this observation suggests
that the reduction in receptors is associated with inhibition of
aromatase (Hoffman et al., 1981c).
(iii) Relevance to humans of adverse reproductive effects in rodents
The adverse effect of fenarimol on the fertility of male mice
and rats appears to be due to an effect on the organization
(differentiation) and expression of male sexual behaviour, which is
known to be controlled within the central nervous system (Naftolin
et al., 1991). The mechanism is dependent on aromatization of
testosterone to estradiol-17ß. Evidence is available from several
studies that the expression of human male sexual behaviour has a
different hormonal basis from that in rats, i.e. depends on both a
direct action of testosterone and conversion of testosterone to
dihydrotestosterone, but not on the conversion of testosterone to
estradiol-17ß. Mantzoros et al. (1995) showed that the frequency of
human male sexual behaviour is correlated with the serum level of
dihydrotestosterone but not of estradiol-17ß. Gooren (1985)
administered an aromatase inhibitor (testolactone) or an estrogen
receptor antagonist (tamoxifen) to men and also concluded that
estradiol-17ß is not important in the control of male sexual
behaviour. Bagatell et al. (1994) showed that there was no change in
male sexual function after a treatment regimen involving testolactone,
testosterone, and a gonadotropin RH antagonist, which resulted in a
specific decrease in estradiol-17ß levels while keeping testosterone
and dihydrotestosterone at normal levels. A single study indicates a
role for estradiol-17ß in the expression of human male sexual
behaviour (Luisi & Franchi, 1980). The results showed that an
aromatizable form of testosterone is more effective in stimulating
libido than the non-aromatizable androgen mesterolone; however, this
result is difficult to interpret as it is not known whether
mesterolone can cross the blood-brain barrier.
Men with a genetic defect resulting in impaired androgen
receptor-mediated activity, but with no effect on estradiol-17ß
receptor-mediated activity or on aromatase, did not show normal male
sexual behaviour (Breedlove, 1994). A further study of genetic defects
(Smith et al., 1994) supports this conclusion, since an XY man
lacking functional estrogen receptors remained masculine, indicating
that estradiol-17ß does not control sexual differentiation in human
males; however, sexual activity was not assessed. Furthermore,
aromatase is confined mainly to the gonads and brain of rats and mice,
while it is also expressed in placenta, adipose tissue, and liver in
higher primates, including humans. Thus, the proportion of target
enzyme to inhibitor would be greater in humans than in rats, requiring
more inhibitor to achieve the same degree of inhibition at a given
target site.
Fenarimol would appear to have an adverse effect on reproductive
parameters in female mice and rats, as gestation and parturition are
extended, resulting in dystocia. This effect is also a direct effect
of the inhibition of aromatase, since circulating progesterone is
sustained at a high level, preventing timely luteolysis and resulting
in continued functioning of the corpora lutea and thus prolonging
gestation. In a review, Chwalisz (1994) outlined the mechanisms of
pregnancy and parturition in female rats. In order to maintain
pregnancy, rats depend on the corpus luteum as a source of
progesterone. Parturition requires an abrupt decrease in the
progesterone level, an increase in that of estrogen, and consequently
a decreased ratio of progesterone:estrogen. In contrast, humans (like
guinea-pigs) use the placenta as a source of progesterone during
pregnancy, and an abrupt decrease in progesterone secretion is not
required for parturition. These differences in endocrine control of
parturition, particularly the lesser role of estrogens in stimulating
labour in women, indicate that rats are probably not a relevant model
for predicting the effects of aromatase inhibition on human
parturition and that guinea-pigs are a better model.
Comments
Fenarimol given orally to rats was rapidly absorbed, distributed
and excreted. Elimination occurred principally in the faeces (76-83%),
mainly as a result of biliary excretion, and to a lesser extent in the
urine (6.5-9.2%). Residue levels in tissues were relatively low.
Dermal absorption of fenarimol by monkeys was low (about 2.5%). There
was some barrier to placental transport of fenarimol-derived
radiolabel in rats during part of the gestation period. Although the
levels of the radiolabel in the milk of lactating rats were three to
five times those in the plasma, only a very small proportion of the
dose (< 0.1%) was eliminated in the milk of a lactating goat.
Fenarimol is extensively metabolized to many metabolites. The
major pathways are oxidation of the carbinol-carbon atom, the
chlorophenol rings, and the pyrimidine ring. Repeated oral
administration to rats had no effect on the elimination of fenarimol
or its metabolites.
Fenarimol has low acute oral and dermal toxicity, the LD50
values in rats and rabbits being 2550 and > 2000 mg/kg bw
respectively. After inhalation, the LC50 was > 2 mg/litre (1-h
exposure). Fenarimol did not irritate skin or eyes and was not a skin
sensitizer in guinea-pigs in a maximization test. The WHO has
classified fenarimol as unlikely to present an acute hazard in normal
use.
In a 14-day pilot study in mice given dietary concentrations of
25-12 800 ppm, liver toxicity was observed at 800 ppm and above. In a
three-month study in mice given dietary concentrations of 0, 360, 620,
1100, 2000, or 3300 ppm, toxicity was observed mainly in the liver, as
increased liver weight, hepatic enzyme induction, centrilobular
hypertrophy, and hepatomegaly. The NOAEL was 360 ppm, equivalent to
52 mg/kg bw per day, on the basis of an increase in liver weight at
620 ppm.
In two 14-day pilot studies in rats given dietary concentrations
of 25-12 800 ppm, liver toxicity was observed at 200 ppm and above.
Dietary administration of fenarimol to rats for three months at 0, 50,
200, or 800 ppm also resulted in liver toxicity at and above
200 ppm, seen as increased liver weight, hepatic enzyme induction,
centrilobular hypertrophy, and fatty changes in the liver. The NOAEL
was 50 ppm, equivalent to 2.5 mg/kg bw per day, on the basis of
induction of liver enzymes. In another three-month study in rats, with
doses of 0, 140, 200, 280, 360, and 500 ppm, the NOAEL was 200 ppm,
equivalent to 10 mg/kg bw per day, on the basis of an increase in
liver weight and induction of hepatic enzymes at 280 ppm and above.
In a three-month study in dogs, oral administration of fenarimol
in gelatin capsules at doses of 0, 1.2, 5, or 20 mg/kg bw per day
resulted in no systemic toxicity. In a one-year study in which dogs
were given 0, 1.2, 12, or 120 mg/kg bw per day, toxicity was observed
at the highest dose. This included liver toxicity and, in female dogs,
a transient enlargement of the mammary gland and increased ovarian
weight; an increase in the occurrence of soft stools was observed at
all doses. The NOAEL was 12 mg/kg bw per day.
In a study of chronic toxicity, mice were administered fenarimol
at dietary levels of 0, 50, 170, or 600 ppm for 12 months. The NOAEL
was 170 ppm, equivalent to 24 mg/kg bw per day, on the basis of
decreased body-weight gain and effects on the liver at 600 ppm. In a
two-year study of carcinogenicity, mice were given diets containing 0,
50, 170, or 600 ppm fenarimol. The NOAEL was 370 ppm, equivalent to
24 mg/kg bw per day, on the basis of a slight decrease in body-weight
gain in males at 600 ppm. There was no evidence of carcinogenicity.
Two studies of chronic toxicity were performed in which rats were
administered dietary concentrations of fenarimol at 0, 50, 130 or
350 ppm for 12 or 18 months. In both studies, the main effects at the
highest dose were a decrease in the leukocyte count, increased liver
weight, and decreased spleen weight. In the 12-month study, the NOAEL
was 130 ppm, equal to 6 mg/kg bw per day. In the 18-month study, the
NOAEL was 50 ppm, equal to 2.3 mg/kg bw per day, on the basis of a
reduction in body weight.
Three carcinogenicity studies were performed in rats. In the
first, rats were administered diets containing 0, 50, 130, or 350 ppm
fenarimol. A small increase in the incidence of hepatocellular adenoma
was seen at the highest dose. There was no NOAEL, owing to a slight
increase in fatty changes and hyperplastic nodules in the liver at all
doses. No clear treatment-related effect on tumour incidence was seen.
Two further studies were performed at lower doses (O, 12, 25, or
50 ppm). In the first study, the survival was very low, owing to an
outbreak of chronic respiratory disease. There was evidence of reduced
body-weight gain and an increased incidence of fatty changes in the
liver at 50 ppm. No adverse effect was observed in the second study.
The overall NOAEL for all three studies was 25 ppm, equal to 1.2 mg/kg
bw per day. There was no evidence of carcinogenicity.
A three-generation study of reproductive toxicity in mice given
dietary levels of 0, 35, 70, or 140 ppm fenarimol revealed no adverse
effects on parental mice or offspring. In earlier pilot studies,
however, doses of 170 ppm and above reduced fertility and live-born
litter size and lengthened the gestation period. The NOAEL was
140 ppm, equivalent to 20 mg/kg bw per day.
In a two-generation study of reproductive toxicity in which
guinea-pigs were given 0 or 400 ppm fenarimol in the diet (equal to
33 mg/kg bw per day), parental toxicity was seen and there was
equivocal evidence of a slight effect on the proportion of live-born
progeny. In a single-generation study of reproductive toxicity with a
cross-over design, rabbits were treated orally with fenarimol at a
level of 0 or 35 mg/kg bw per day. There was no evidence of general
systemic toxicity or of effects on reproduction.
Two multigeneration studies of reproductive toxicity have been
performed in rats. In a two-generation study, rats were exposed to
dietary concentrations of 0, 50, 130, or 350 ppm, and in a three-
generation study to 0, 12, 25, or 50 ppm. Fenarimol reduced fertility,
caused dystocia, reduced live-born litter size and lengthened the
gestation period. In the first study, cross-over data showed that the
reduction in fertility was clearly mediated through the male and was
only slightly reversible. The overall NOAEL for general systemic
toxicity was 25 ppm, equivalent to 1.2 mg/kg bw per day, on the basis
of reduced body-weight gain in F1 males at 50 ppm and above. The
overall NOAEL for reproductive toxicity was 12 ppm, equivalent to
0.62 mg/kg bw per day, on the basis of a reduction in live-born litter
size at 25 ppm and above.
Three single-generation studies of reproductive toxicity with a
cross-over design have been conducted in rats, which were exposed
orally to fenarimol at doses 0 or 35 mg/kg bw per day. These studies
showed that the effects on reproduction were not strain-dependent, the
effects on fertility were clearly male-mediated, and the reduction in
live-born litter size (probably due to an increased incidence of
stillbirths) was female-mediated. No correlation was found between
fertility and the levels of prolactin, luteinizing hormone, or
testosterone, organ weights, histopathological findings, or sperm
morphology.
The single- and multigeneration studies of reproductive toxicity
show that fenarimol has adverse effects on reproduction in rats and
(at higher doses) in mice, but not in guinea-pigs or rabbits. A number
of studies were performed to investigate the mechanism of the adverse
effects on reproduction, and particularly those on fertility. The
results of these studies and of others in the open literature show
that fenarimol affects male sexual differentiation and subsequent
behaviour indirectly by inhibiting the aromatase-catalysed conversion
of testosterone to estradiol-17ß within the hypothalamus. Data from
the literature strongly indicate that while estradiol-17ß is the
central regulator of sexual differentiation in rats, human male sexual
behaviour is controlled by dihydrotestosterone, which is formed from
testosterone by the enzyme 5µ-reductase. There is evidence that the
effect of fenarimol in female rats (delayed parturition) is also due
to inhibition of aromatase, as this inhibition results in a sustained
level of circulating progesterone and prolonged corpus luteal
function, thus leading to delayed parturition. In contrast to rats, an
abrupt decrease in progesterone secretion is not required for
parturition in guinea-pigs or humans. The Meeting concluded that,
although not clear-cut, there is sufficient evidence that the adverse
effects on the organization and expression of sexual behaviour in male
rats and on delayed parturition in female rats are not relevant for
humans.
In a study of developmental toxicity, pregnant rats were given
fenarimol by gavage at doses of 0, 5, 13, or 35 mg/kg bw per day.
There was evidence of developmental toxicity (hydronephrosis) but no
signs of maternal toxicity at the high dose. In a study in which some
females were also allowed to litter and wean their progeny, pregnant
rats were exposed to 0 or 35 mg/kg bw per day during days 6-15 of
gestation. There were adverse effects on reproductive performance
(increased length of gestation, dystocia) and developmental toxicity
(increased hydronephrosis) but no evidence of general systemic
maternal toxicity. The increased incidence of hydronephrosis appeared
to be due to delayed development and was not considered a teratogenic
effect. Overall, the NOAEL for maternal toxicity was 35 mg/kg bw per
day, and that for embryo- and fetotoxicity was 13 mg/kg bw per day.
In a study of developmental toxicity in which rabbits were
administered fenarimol by gavage at doses of 0, 3, 10, or 35 mg/kg bw
per day, no signs of toxicity were observed in dams or fetuses. In a
second study, rabbits were exposed orally to doses of 0, 15, 50, or
150 mg/kg bw per day. The NOAEL for maternal toxicity was 50 mg/kg bw
per day, on the basis of reductions in body weight and food
consumption and an increased incidence of abortions. The NOAEL for
embryo- and fetotoxicity was also 50 mg/kg bw per day, on the basis of
a reduced number of live fetuses and an increased incidence of extra
ribs. There was no evidence of teratogenic potential.
Fenarimol has been adequately tested for genotoxicity in a series
of assays in vitro and in vivo. The Meeting concluded that
fenarimol is not genotoxic.
The Meeting concluded that the effects of fenarimol in male rats
(reduced fertility) and female rats (delayed parturition) are due to
inhibition of aromatase, an enzyme that is not involved in these
aspects of human reproduction. It therefore decided that it would be
inappropriate to use the NOAEL seen in studies of reproductive
toxicity in determining the ADI. An ADI of 0-0.01 mg/kg bw was
established on the basis of an overall NOAEL of 1.2 mg/kg bw per day,
seen in several studies of carcinogenicity in rats, and a safety
factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 24 mg/kg bw per day (12-month study of chronic toxicity)
20 mg/kg bw per day (three-generation study of reproductive
toxicity)
Rat: 1.2 mg/kg bw per day (three studies of carcinogenicity)
0.62 mg/kg bw per day (multigeneration study of reproductive
toxicity)*
13 mg/kg bw per day (embryo- and fetotoxicity in study of
developmental toxicity)
Dog: 12 mg/kg bw per day (one-year study of toxicity)
Rabbit: 50 mg/kg bw per day (maternal and embryo- or fetotoxicity in
study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information valuable for the continued
evaluation of the compound
Observations in humans
* Data considered irrelevant for evaluation with respect to human
health.
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenarimol
Exposure Relevant route, study type, species Result, remarks
Short-term (1-7 days) Skin, irritation, rabbit Not irritating
Eye, irritation, rabbit Not irritating
Skin, sensitization, guinea-pig Not sensitizing
Oral, toxicity, rat LD50 = 2550 mg/kg bw
Dermal, toxicity, rabbit LD50 > 2000 mg/kg bw
Inhalation, toxicity, rat, 1 h LC50 > 2 mg/litre
Medium-term (1-26 weeks) Dermal, toxicity, 21 -day, rabbit No systemic effect; no irritation at
1000 mg/kg bw
Dietary, toxicity, three months, rat NOAEL = 2.5 mg/kg bw; hepaticenzyme
induction
Dietary, three-generation, reproductive NOAEL = 0.625 mg/kg bw; reduced
toxicity, rat live-born litter size
Gavage, developmental toxicity, rat NOAEL = 13 mg/kg bw;
hydronephrosis; no maternal toxicity
Long-term (> one year) Dietary, toxicity, two years, rat NOAEL = 1.2 mg/kg bw; fatty changes
in liver
References
Althaus, W.A. (1985a) Characterization and identification of
radioactivity in urine and feces of Wistar rats given single oral
doses mixed-labeled 14C fenarimol. Unpublished report No.
ABC-0310 dated December 1985 from Lilly Research Laboratories,
USA. Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Althaus, W.A. (1985b) Characterization and identification of
radioactivity in blood, liver and kidney of Wistar rats given
single oral doses of mixed-labelled 14C fenarimol. Unpublished
report No. ABC-0321 dated December 1985 from Lily Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Bagatell, C.J., Heiman, J.R., Rivier, J.E. & Bremmer, W.J. (1994)
Effects of endogenous testosterone and oestradiol on sexual
behaviour in normal young men. J. Clin. Endocrinol. Metab., 78,
711-716.
Black, L.J. (1981a) Evaluation of the estrogenic and antiestrogenic
potential of EL-222 in the immature rat uterus. Unpublished
report dated September 1981 from Lilly Research Laboratories,
USA. Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Black, L.J. (1981b) Evaluation of the progestational or
antiprogestational potential of EL-222 in immature rabbits.
Unpublished report dated September 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Breedlove, S.M. (1994) Sexual differentiation of the human nervous
system. Ann. Rev. Psychol., 45, 389-418.
Chwalisz, K. (1994) The use of progesterone antagonists for cervical
ripening and as an adjunct to labour and delivery. Hum,
Reprod., 9 (Suppl. 1), 131-161.
Goebel, G.V. (1985) Characterization of radioactivity in bile from
rats dosed orally with 14C fenarimol. Unpublished report No.
ABC-0312 dated May 1985 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Gooren, L.J.G. (1985). Human male sexual functions do not require
aromatisation of testosterone: A study using tamoxifen,
testolactone, and dihydrotesterone. Arch. Sex. Behav., 14,
539-548.
Hanlin, M.L. (1981a) Fertility studies with EL-222 in the adult male
rat. Unpublished report dated September 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hanlin, M.L. (1981b) Anti-androgenic studies with EL-222 in the male
rat. Unpublished report dated September 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom
Hoffman, D.G. & Amundson, M. (1985) Initiation and promotion study of
fenarimol on rat liver carcinogenesis. Unpublished report dated
November 1985 from Naylor Dana Institute for Disease Prevention,
Valhalla, New York, USA Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G. & Arthur, B.H. (1980) Guinea pig sensitization study
with Lilly compound 56722 (fenarimol). Unpublished report No.
GP-9538 dated January 1980 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G. & Hirsch, K.S. (1981a) Placental transport of EL-222 in
the Wistar rat. Unpublished report No. R10480 dated October 1981
from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G. & Hirsch, K.S. (1981b) Exposure of the neonatal rat to
EL-222 as a component of milk: Evidence for the presence of
EL-222 in the central nervous system during the organization of
adult sexual behaviour. Unpublished report No R01781 dated
October 1981 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G. & Hirsch, K.S. (1981c) A special fertility study with
EL-222 (fenarimol) in the Wistar rat. Unpublished report No
R07380 dated October 1981 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D G. & Pierce, E.C. (1985) A chronic toxicity/oncogenicity
study in Wistar rats maintained on diets containing low
concentrations of fenarimol for two years. Unpublished report No.
R07781 dated May 1985 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G. & Russel, D.R. (1990) A developmental toxicity study of
fenarimol (EL-222, compound 056722) administered orally to New
Zealand white rabbits. Unpublished report No. B00290 dated
October 1990 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Broddle, W.D., Gibson, W.R. & Morton, D.M. (1975a) The
acute toxicity of EL-222 in mice, rats, dogs, ducks mid quail.
Unpublished report dated August 1975 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Bernhard, N.R., Gibson, W.R., Owen, N.V. & Morton, D.M.
(1975b) A pilot 2-week subacute toxicity study of EL-222 in mice.
Unpublished report No. M-9074, M-9084 dated August 1975 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975c)
Three-month subacute oral toxicity studies on EL-222 in mice.
Unpublished report No. M-9015 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Bernhard, N.R., Gibson, W.R., Harris, P.N. & Morton,
D.M. (1975d) A pilot 2-week subacute toxicity study of EL-222 in
rats. Unpublished report No. R-713, R-723 dated August 1975 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Bernhard, N.R. & Morton, D.M. (1975e) A 2-week subacute
study of the effects of EL-222 on hepatic p-nitroanisole
metabolism in rats. Unpublished report No. R-244, R-254 dated
August 1975 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975f)
Three-month subacute oral toxicity studies on EL-222 in rats.
Unpublished report No. R-1013 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975g)
Three-month subacute oral toxicity studies on EL-222 in rats.
Unpublished report No. R-1164 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975h)
Three-month subacute oral toxicity studies on EL-222 in dogs.
Unpublished report No. D-4183 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Arthur, B.H., Gibson, W.R. & Morton, D.M. (1977a) The
acute dermal and ocular toxicity of technical EL-222 in rabbits.
Unpublished report dated January 1977 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Adams, E.R., Markham, J.K., Owen, N.V., Gibson, W.R. &
Morton, D.M. (1977b) A multi-generation reproduction study with
EL-222 in the rat. Unpublished report No. R-715, R-1345, R-956,
R-966 dated November 1977 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Markham, J.K., Adams, E.R., Owen, N.V & Morton, D.M.
(1977c) A teratology study on compound 56722 (EL-222) in the
rabbit. Unpublished report No. B-7125 dated January 1977 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Markham, J.K., Owen, N.V. & Morton, D.M. (1977d) A
dominant lethal study of compound 56722 (EL-222) in the rat.
Unpublished report dated January 1977 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G. et al. (1978a) Twelve-month chronic oral toxicity of
EL-222 (56722) in mice. Unpublished report No. M-9155 dated April
1978 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C., Harris, P.N. & Morton, D.M.
(1978b) Twenty-four month chronic oral toxicity of EL-222 (56722)
in mice. Unpublished report No. M-9135, M-9145, dated August 1978
from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G. et al. (1978c) A one-year toxicity study with EL-222
in the rat. Unpublished report No. R-715 dated June 1978 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C. & Morton, D.M. (1978d)
Eighteen-month chronic oral toxicity of EL-222 (56722) in rats.
Unpublished report No. R-435 dated May 1978 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C. & Morton, D.M. (1978e)
Twenty-four month chronic oral toxicity of EL-222 (56722) in
rats. Unpublished report No. R-405, R-415, dated April 1978 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gomez, S.R. & Dorato, M.A. (1980a) The acute inhalation
toxicity of Lilly compound 56772, fenarimol, in the Fischer 344
rat. Unpublished report No. R-H-99-80 dated December 1980 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V. & Markham, J.K. (1980b) A cross-over
reproduction study with EL-222 (Lilly compound 56722) in the rat.
Unpublished report No. R-286 dated April 1980 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V. & Markham, J.K. (1980c) Additional
cross-over reproduction studies with EL-222 (Lilly compound
56722) in the Wistar and the Sprague-Dawley rat. Unpublished
report No. R-57, R-67 dated April 1980 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V & Adams, E.R. (1980d) A cross-over fertility
study with EL-222 in the Wistar rat. Unpublished report No.
R08379 dated June 1980 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Adams, E.R. & Owen, N.V. (1980e) A cross-over fertility
study with EL-222 in the Dutch-belted rabbit. Unpublished report
No. B7229, dated June 1980 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Owen, N.V. & Adams, E.R. (1980f) A teratology study
with EL-222 in the rat. Unpublished report No. R06279 dated
January 1980 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Hirsch, K.S. & MacLusky, N.J. (1981a) Effects of EL-222
on the binding of androgens to cytoplasmic and nuclear androgen
receptors. Unpublished report dated October 1981 from Yale
University Medical School Connecticut, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Hirsch, K.S. & MacLusky, N.J. (1981b) Effects of EL-222
on circulating estrogens and the binding of estrogens to estrogen
receptors in the rat central nervous system. Unpublished report
dated October 1981 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Hirsch, K.S. & MacLusky, N.J. (1981c) Effects of EL-222
on the concentration of estrogen receptors and the conversion of
testosterone to estrogens in the hypothalamus. Unpublished report
dated October 1981 from Yale University Medical School,
Connecticut, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C. & Morton, D.M. (1982a) A
low-dose chronic toxicity/oncogenicity study in Wistar rats
maintained on diets containing fenarimol for two years.
Unpublished report No. R06479 dated November 1982 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Brodie, A.M.H. & Hirsch, K.S. (1982b) An in vitro
measure of aromatase inhibition by EL-222. Unpublished report
dated January 1982 from the University of Maryland, Baltimore,
Maryland, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Markham, J.K & Miller, B.J. (1983a) A two-generation
reproduction study with fenarimol (EL-222, compound 56722) in
guinea pigs. Unpublished report No. G00682, G00483 dated December
1983 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V & Byrd, R.A. (1983b) The effect of prenatal
fenarimol (EL-222, compound 56722) exposure on kidney development
and maturation in the rat. Unpublished report No. R10682, R10782,
R10882 dated November 1983 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Masten, J.L. & Hanasono, G.K (1985a) The biliary
excretion of radioactivity by male and female Wistar rats given
single oral doses of 14C-fenarimol (compound 56722, EL-222).
Unpublished report No. R02485 dated April 1985 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Masten, J.L & Hanasono, G.K. (1985b) Tissue
distribution of radioactivity in Wistar rats determined one or
twenty-four hours after single oral doses of 14C-fenarimol
(EL-222, compound 56722). Unpublished report No. R16684 dated
April 1985 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Masten, J.L., Walker, C.G. & Hanasono, G.K. (1985c)
Radiocarbon disposition studies on Wistar rats given single
oral doses of 14C-fenarimol (EL-222, compound 56722):
Pharmacokinetics, and excretion/tissue distribution seven days
after dosing. Unpublished report No. R06484, R08584 dated March
1985 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Weaver, D.E. & Van Lier, R.B.L. (1985d) Distribution of
radioactivity into tissues and organs from Wistar rats given oral
doses of unlabeled fenarimol daily for two weeks followed by a
single dose of 14C-fenarimol (EL-222, compound 56722).
Unpublished report No. R01285 dated March 1985 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Van Lier, R.B.L. & Weaver, D.E. (1985e) The
percutaneous absorption of 14C fenarimol dissolved in ethanol
in rhesus monkeys. Unpublished report No. P04085, P04585 dated
October 1985 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Pierce, E.C., Brown, G.E. & Negliski, S. (1985f)
Subchronic (21-day) dermal toxicity study in rabbits with
technical fenarimol (EL-222, compound 56722) and Rubigan 50W, a
wettable powder formulation (FN-0742) containing 50% fenarimol.
Unpublished report No. B02183 dated May 1985 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Gries, C.L., Pierce, E.C., Means, J.R. & White, J.F.
(1985g) A one year chronic oral toxicity study of fenarimol in
dogs with a three month recovery period. Unpublished report No.
D02683 dated May 1985 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Richardson, K.A. & Kokkino, A.J. (1988a) The effect of
fenarimol (EL-222, compound 56722) on the induction of reverse
mutations in Salmonella typhimurium and Escherichia coli using
the Ames test. Unpublished report No. 880215AMT4, 880222AMS4,
dated August 1988 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Oberly, T.J. & Richardson, K.A. (1988b) The effect of
fenarimol on the induction of forward mutation at the thymidine
kinase locus of L51787 mouse lymphoma cells. Unpublished report
No. 880106MLT4, 880113MLA4, dated August 1988 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Ivett, J.L. (1988) Mutagenicity test on 56722 (EL-222) in the in vivo
rat micronucleus assay. Unpublished report No. 10348-0-455 dated
October 1988 from Hazleton, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Jones, J.R. (1994a) Fenarimol technical: Acute dermal irritation test
in the rabbit. Unpublished report No. 291/50 dated February 1994
from Safepharm Laboratories, United Kingdom. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Jones, J.R. (1994b) Fenarimol technical: Acute eye irritation test in
the rabbit. Unpublished report No. 291/51 dated February 1994
from Safepharm Laboratories, United Kingdom. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Luisi, M. & Franchi, R. (1980) Double blind group comparative study of
testosterone undecanoate and mesterolone in hypogonadal male
patients. J. Endocrinol. Invest., 3, 305-308.
Mantzoros, C.S., Georgiadis, E.I. & Trichopoulos, D. (1995)
Contribution of dihydrotestosterone to male sexual behaviour.
Br. Med. J., 310, 1289-1291.
Markham, J.K., Hoffman, D.G., Adams, E.R., Owen, N.V. & Morton, D.M.
(1978a) Pilot reproduction studies with EL-222 (Lilly compound
56722) in the mouse. Unpublished report No. M-9165, M-9215 dated
July 1978 from Lilly Research Laboratories, USA. Submitted to WHO
by DowElanco Europe, Wantage, Oxon, United Kingdom.
Markham, J.K., Hoffman, D.G., Broddle, W.D., Owen, N.V. & Morton, D.M.
(1978b) A multi-generation study with EL-222 (Lilly compound
56722) in the mouse. Unpublished report No. M-9086, M-9296,
M-9326 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Markham, J.K., Hoffman, D.G., Owen, N.V. & Morton, D.M. (1978c) A
second multi-generation reproduction study with EL-222 (Lilly
compound 56722) in the rat. Unpublished report No. R-636, R-1076,
R-217 dated July 1978 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Murli, H. (1988) Mutagenicity test on 56722 in an in vitro cytogenetic
assay measuring chromosomal aberration frequencies in cultured
purified human lymphocytes. Unpublished report No. 10348-0-449
dated August 1988 from Hazelton, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Naftolin, F., Keefe, D., Apa, R., Palumbo, A. & Garcia-Segura, L.M.
(1991) The apparent paradox of sexual differentiation of the
brain. Contrib. Gynecol. Obstet., 18, 24-32.
Neubauer, B.L. et al. (1982) Effect of EL-222 (compound 56722,
fenarimol) on the developing reproductive tract. Unpublished
report dated January 1982 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Probst, G.S. (1979) The effect of Lilly compound 56722 (EL-222) on the
induction of DNA repair synthesis in primary cultures of adult
rat hepatocytes. Unpublished report No. 790502-1 dated June 1979
from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Prout, M. (1994) The disposition of 14C fenarimol in the lactating
goat. Unpublished report No. IRI 154147 dated October 1994 from
Inveresk Research International, Musselburgh, United Kingdom.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Rodricks, J.V. et al. (1989) Response to USSR concerns regarding the
potential oncogenicity of fenarimol. Unpublished report dated
February 1989 from Environ Corp., Washington DC, USA. Submitted
to WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Simpson, E.R., Mahendroo, M.S., Means, G.D., Kilgore, M.W.,
Hinshelwood, M.M., Graham-Lorence, S., Amarneh, B., Ito, Y.,
Fisher, C.R., Dodson, M.M., Mendelson, C.R & Bulun, S. (1994)
Aromatase cytochrome P450, the enzyme responsible for oestrogen
biosynthesis. Endocr. Rev., 15, 342-355.
Siou, G. & Lerond-Conan, L. (1982) Test for mutagenic potential of
technical-grade fenarimol by examination of chromosomal damage in
the Chinese hamster. Unpublished report No. 658 dated June 1982
from C.E.R.T.I., Versailles, France. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Siou, G., Lerond-Conan, L. et al (1982) Test for mutagenicity of
technical-grade fenarimol using a micronucleus technique in the
mouse. Unpublished report No. 650 dated May 1982 from C.E.R.T.I.,
Versailles, France. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Smalstig, E.B. (1981a) Study of the possible influence of compound
EL-222 on fertility in the adult female rat. Unpublished report
dated September 1981 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Smalstig, E.B. (1981b) Studies of the influence of EL-222 on uterine
prostaglandin F2alpha in the female rat at parturition.
Unpublished report dated November 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Smith, E.P., Boyd, J., Frank, G., Takahashi, H., Cohen, R.M., Specker,
B., Williams, T.C., Luhbahn, D.B. & Korach, K.S. (1994) Oestrogen
resistance caused by a mutation in the oestrogen-receptor gene in
a man. New Engl. J. Med., 331, 1056-1061.
Tinsley, F.C. (1982) Preparturiton progesterone levels in fenarimol
(EL-222) treated rats. Unpublished report dated August 1982 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Watson, D. (1994) Accurate measurement of an isolated metabolite of
fenarimol. Unpublished report No. DWC/719 dated October 1994 from
Huntingdon Research Centre, United Kingdom. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
found, the main one being metabolite K. In contrast, most of the
radiolabel in the faeces was present as free compounds, indicating
that biliary metabolites undergo further metabolism or hydrolysis
before being excreted (Goebel, 1985).
The tissue distribution of fenarimol in Wistar rats was
determined 1 and 24 h after administration of 1 or 13 mg/kg bw
14C-fenarimol. The main radiolabelled constituents identified in
blood and kidney after 1 h were unchanged fenarimol and metabolite I.
The amount of fenarimol increased with dose, from 19 to 40% of the
total radioactivity in blood in males and from 50 to 73% in females.
Fenarimol also accounted for most of the radiolabel in the liver
(67-90%), whereas metabolite K represented 3-6%. Females had more
unchanged fenarimol residues than males, indicating more extensive
metabolism in males. Furthermore, metabolism was reduced at the higher
dose, suggesting saturation (Althaus, 1985b).
(c) Other metabolic studies
Some barrier to placental transport of fenarimol-derived
radiolabel was found in rats on days 14-17 of gestation but virtually
no barrier on day 18 (Hoffman & Hirsch, 1981a). Fenarimol-derived
radiolabel was excreted in milk and subsequently concentrated in the
hypothalamus of neonates. The concentration in milk up to 48 h after
treatment was three to five times greater than that in maternal plasma
(Hoffman & Hirsch, 1981b).
2. Toxicological data
(a) Acute toxicity
The results of studies of acute toxicity are summarized in
Table 1.
(b) Short-term toxicity
Mice
In a pilot study reported with few details, groups of four male
ICR mice were given fenarimol (purity unspecified) in the diet at 0
(10 animals), 25, 50, 100, 200, 400, 800, 1600, 3200, 6400, or
12 800 ppm for 14 days. No effects were observed on mortality or
clinical signs. terminal body weight was reduced at the highest dose.
In the liver (the only organ investigated), dose-related increases in
relative weight and in the rate of hepatic metabolism of para-
nitroanisole were seen at > 800 ppm. There were no effects on liver
pathology or histopathology (Hoffman et al., 1975b).
Table 1. Acute toxicity of fenarimol
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
ICR mouse Male Oral 4508a NR Hoffman et al. (1975a)
Female > 4000a
Wistar-derived Male Oral 2576a NR Hoffman et al. (1975a)
Harlan rat Female 2515a
Albino rabbit Male and Dermal > 2000b NR Hoffman et al. (1977a)
female
Fischer 344 rat Male and Inhalationc > 2.07 97% Hoffman et al. (1980a)
female (1-h)
Wistar-derived FemMe intraperitoneal 533 NR Hoffman et al. (1975a)
Harlan rat
Beagle dogc Male and Oral > 200 NR Hoffman et al. (1975a)
female
NR, not reported
a Signs of toxicity: hypoactivity, leg weakness, loss of righting reflex, ptosis (1-6 h after treatment);
loose stools; diuresis, diarrhoea, debilitation (24 h after treatment); recovery within 8-10 days
b Application on intact and abraded skin; one death, but no signs of toxicity or irritation
c Nose only; nominal concentration, 133 mg/litre; particle size, 12 µm
d Only one male and one female
In a poorly reported study, groups of 10 male and 10 female ICR
mice were fed diets containing 0, 365, 620, 1100, 2000, or 3300 ppm
fenarimol (purity unspecified) for three months, equivalent to 0, 52,
88, 157, 286, or 471 mg/kg bw per day. Food consumption was not
measured, and ophthalmological examinations were not performed. There
were no treatment-related effects on mortality, clinical signs of
toxicity, haematology, or body weight. A statistically significant
reduction in total bilirubin was observed in animals of each sex at
the two highest doses and in males at 1100 ppm. Hepatic para-
nitroanisole metabolism, determined in five animals of each sex in
each group, showed a significant dose-related increase in females at
> 1100 ppm and in males at > 2000 ppm. Relative liver weights
were increased in a dose-related manner in males at > 365 ppm (10%
increase) and in females at > 620 ppm (11% increase) and reached
statistical significance at 620 and 1100 ppm, respectively. Absolute
liver weights were increased at > 620 ppm. The main effects seen on
histopathological examination of tissues other than nervous tissue
were centrilobular hypertrophy and hepatomegaly in the livers of males
at 1100 ppm and 2000 ppm, respectively, and in females at the highest
dose. A shift from a diffuse distribution of fat droplets to a more
periportal distribution was observed in animals of each sex at
> 2000 ppm. In kidneys, a higher incidence of fat droplets was
observed in females at > 2000 ppm. The NOAEL was 365 ppm,
equivalent to 52 mg/kg bw per day (Hoffman et al., 1975c).
Rats
In a pilot study reported in little detail, four male and four
female male Wistar rats were fed diets containing fenarimol (purity
unspecified) at 0 (10 animals per group), 25, 50, 100, 200, 400, 800,
1600, 3200, 6400, or 12 800 ppm for 14 days. Three animals at the high
dose died. Signs of toxicity, including anorexia, piloerection,
ataxia, and tremors, and decreased body weight and food consumption
were seen at doses > 3200 ppm. In the liver, the only organ
investigated, there were dose-related increases in relative weight at
> 400 ppm and in the rate of hepatic metabolism of para-
nitroanisole at > 200 ppm. Histopathological examination of the
liver revealed centrilobular hypertrophy at > 400 ppm and focal
necrosis at > 6400 ppm; no effects were seen at < 100 ppm
(Hoffman et al., 1975d).
In a subsequent study in male Wistar rats, also poorly reported,
four animals per treatment group and 10 controls were fed diets
containing fenarimol (purity unspecified) at 0, 25, 50, 100, 200, 400,
800, 1600, 3200, or 6400 ppm for 14 days. Food consumption and body
weight were reduced at > 1600 ppm; increased absolute and relative
liver weights were seen at > 800 ppm and increased hepatic
microsomal protein content at > 400 ppm. A dose-related reduction
in glucose-6-phosphatase activity was seen at > 800 ppm. The rate
of hepatic metabolism of para-nitroanisole was increased in a
dose-related fashion at > 200 ppm. No effects on the liver were
seen at < 100 ppm (Hoffman et al., 1975e).
In another poorly reported study, groups of 20 male and 20 female
Wistar rats were fed diets containing 0 (25 animals per sex), 50, 200,
or 800 ppm fenarimol (purity unspecified) for three months, equivalent
to 0, 2.5, 10, or 40 mg/kg bw per day. Five animals of each sex in
each group were kept for a recovery period of two weeks. No
ophthalmological examinations were performed. There were no treatment
-related effects on mortality, clinical signs of toxicity, food
consumption, haematology, or clinical chemistry (only blood urea
nitrogen, glucose, and alanine aminotransferase measured). A slight
depression in body weight was observed in males at the high dose. A
dose-related increase in relative liver weight was observed in animals
of each sex, which was statistically significant at the high dose. The
relative weights of the kidneys of males and females and of the
thyroid of females were increased at the high dose. Hepatic para-
nitroanisole metabolism, determined in five animals of each sex in
each group, showed a significant, dose-related increase in females at
800 ppm and in males at > 200 ppm. The main effect seen on
histopathological examination of tissues other than nervous tissue was
centrilobular hypertrophy in males and a shift from slight to moderate
fatty metamorphosis in females at the high dose. At the end of the
recovery period, only increased liver weight was still observed at the
high dose. The NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw per day
(Hoffman et al., 1975f).
In a study with no recovery period and again with little detail
reported, groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 140, 200, 275, 365, or 500 ppm fenarimol (purity
unspecified) for three months, equivalent to 0, 7, 10, 14, 18, or
25 mg/kg bw per day. There were no treatment-related effects on
mortality, clinical signs of toxicity, body weight, food consumption,
haematology, clinical chemistry, or histopathology (nervous tissue was
not examined). A dose-related increase in relative liver weight was
observed in females at > 275 ppm and in males at 500 ppm. Hepatic
para-nitroanisole metabolism, determined in five animals of each sex
in each group, showed a dose-related increase, which was statistically
significant at > 275 ppm. The NOAEL was 200 ppm, equivalent to
10 mg/kg bw per day (Hoffman et al., 1975g).
Rabbits
A 21-day study of dermal toxicity was conducted in groups of five
male and five female New Zealand white rabbits, which received daily
applications of 0 or 1000 mg/kg bw technical-grade fenarimol (purity,
97%) or 500 or 1000 mg/kg bw of a wettable powder formulation
containing 50% fenarimol for 6 h/day. A further group received
1000 mg/kg bw of the formulation and was then held for a recovery
period of 14 days. Technical-grade fenarimol induced no treatment-
related effect on appearance, behaviour, body weight, food
consumption, ophthalmological parameters, dermal irritation,
haematological or clinical chemical parameters, organ weights, or
gross or histological appearance of tissues. The NOAEL was thus the
highest dose tested, 1000 mg/kg bw per day. The formulation induced no
treatment-related systemic toxicity, but dermal irritation was seen in
both groups, characterized by slight to severe erythema and slight
oedema during the treatment phase. The irritation subsided within nine
days after exposure (Hoffman et al., 1985f).
Dogs
Groups of four male and four female beagle dogs were fed gelatin
capsules containing fenarimol (purity unspecified) at doses of 0,
1.25, 5, or 20 mg/kg bw per day for three months. No treatment-related
effect was seen on appearance, behaviour, body weight, food
consumption, ophthalmological, haematological, or clinical chemical
parameters, urinalysis, organ weights, hepatic para-nitroanisole
metabolism, or gross or histological appearance of tissues (excluding
nervous tissue). The NOAEL was thus the highest dose tested, 20 mg/kg
bw per day (Hoffman et al., 1975h).
Groups of six male and six female beagle dogs were given gelatin
capsules containing fenarimol (purity, 96.7%) at doses of 0, 1.25,
12.5, or 125 mg/kg bw per day for one year. Two dogs of each sex in
each group were maintained for a recovery period of three months. No
treatment-related effect was seen on mortality, body weight, food
consumption, or ophthalmological, haematological, or urinary
parameters. One, five, seven, and 12 dogs in the four groups had
soft, runny, or mucoid stools on two, 10, 21, and 34 occasions,
respectively. Transient enlargement of the mammary glands of female
dogs was seen in all groups, and there was a slight treatment-related
increase in incidence and duration in four animals in the group at the
highest dose, lasting for up to 46 days. At the high dose, increased
alkaline phosphatase and liver para-nitroanisole O-demethylase
activities and increased absolute and relative liver weights were
observed in animals of each sex; a trend to increased relative ovarian
weight was also observed at this dose. These increases were still seen
after recovery. The only treatment-related histopathological finding
was mild hepatic bile stasis in one female in the treatment phase and
in two males after the recovery period. The NOAEL was 12.5 mg/kg bw
per day (Hoffman et al., 1985g).
(c) Long-term toxicity and carcinogenicity
In many of the studies described below, increased blood glucose
was found. Although apparent dose-dependent increases were observed in
several studies, the end-point (measured only at termination) was very
variable and inconsistent, usually with flat dose-response curves. No
clear explanation could be found for these apparent increases. The
effect was considered to be compound-related but toxicologically
insignificant and was not taken into account in establishing the NOAEL
for each study.
Mice
Groups of 20 male and 20 female ICR mice (30 of each sex in the
control group) were fed diets containing 0, 50, 170, or 600 ppm
fenarimol (purity, 97%), equivalent to 0, 7.1, 24.3, or 85.7 mg/kg bw
per day, for 12 months. Food consumption was not measured, urinalysis
was not performed, and haematology and clinical chemistry were
determined only at termination. There were no treatment-related
effects on mortality, clinical signs of toxicity, or haematology.
Body-weight gain of males at 600 ppm was slightly decreased. A
dose-related increase in blood glucose levels was observed at
> 50 ppm, which was statistically significant at 600 ppm in males
and at 170 ppm in females. A significant increase in serum creatinine
concentration was seen in females at 170 and 600 ppm. The activity of
alanine aminotransferase was increased in males at the high dose. The
absolute and relative liver and spleen weights were increased in males
at 600 ppm, and relative liver weight was increased in females at
600 ppm. Absolute and relative uterine weights were decreased at this
dose. A slight, dose-related increase in the incidence of very slight
or slight fatty change in the liver was observed, reaching 10-20% at
600 ppm (0% in controls). The NOAEL was 170 ppm, equivalent to
24.3 mg/kg bw per day (Hoffman et al., 1978a).
In two replicate studies, groups of 40 male and 40 female ICR
mice (60 animals of each sex in the control group) were fed diets
containing 0, 50, 170, or 600 ppm fenarimol (purity, 97.9%),
equivalent to 0, 7.1, 24.3, or 85.7 mg/kg bw per day, for two years.
Food consumption was not measured, and haematology and clinical
chemistry were carried out only at termination. Since the results of
the replicate studies were similar, they were reported as a single
study. There were no treatment-related effects on mortality, clinical
signs of toxicity, or haematological or clinical chemical parameters.
Body-weight gain of males at 600 ppm was slightly decreased. A
tendency towards increased relative liver weights and a slightly
increased incidence of hepatic fatty change (2.5-6%; 1-2% in controls)
were observed in animals of each sex at the high dose. The tumour
incidence was not enhanced. Although the laboratory considered that
there were no significant effects in these studies, the slight
reduction in body weight suggests minimal toxicity. The NOAEL was thus
170 ppm, equivalent to 24.3 mg/kg bw per day (Hoffman et al.,
1978b).
Rats
Wistar rats of the first parental generation in a multi-
generation study of reproduction {see Hoffman et al., 1977b) were
maintained on treated diets for another three months after the end of
the study and thereby received the diets for a total of one year.
Groups of 20 male and 20 female rats (30 of each sex in the control
group) received doses of 0, 50, 130, or 350 ppm fenarimol (purity,
97.9%), equivalent to 0, 2.5, 6.5, or 17.5 mg/kg bw per day.
Haematology and clinical chemistry were evaluated only at termination;
no urinalysis was performed, and nervous tissue was not examined at
histopathology. There were no treatment-related effects on mortality,
clinical signs of toxicity, or body weight. The limited data available
on food consumption (intake during weeks 1, 8, 42, and 54 only)
indicated no effects. Males at the high dose had increased erythrocyte
counts and and decreased leukocyte counts. Blood glucose levels were
elevated in males at all doses (not dose-related but highest at the
high dose) and in females at 130 and 350 ppm. A dose-related reduction
in blood urea nitrogen was observed in males at > 50 ppm. Absolute
and relative liver weights and relative kidney weight were increased
in females, and absolute and relative spleen weights were decreased in
males at the high dose. Slight atrophy of the acini of the pancreas
was observed more frequently at the high dose, predominantly in males.
The effects on clinical chemical parameters are not considered to be
adverse. The NOAEL was thus 130 ppm, equivalent to 6.5 mg/kg bw per
day (Hoffman et al., 1978c).
Groups of 20 male and 20 female Wistar rats (30 of each sex in
the control group) received diets containing 0, 50, 130, or 350 ppm
fenarimol (purity, 97.9%) for 18 months, equal to 0, 2.3, 6.0, or
16 mg/kg bw for males and 0, 3.6, 8.4, or 22.9 mg/kg bw per day for
females. Urinalysis and ophthalmological examinations were not
performed, and haematology and clinical chemistry were evaluated only
at termination. There were no treatment-related effects on mortality
(survival was a slightly higher at the highest dose), food
consumption, clinical signs of toxicity, or clinical chemistry. A
reduction in growth was seen consistently in all treated groups; on
several occasions, 10% reductions in body weight were seen at 130 and
350 ppm. At 350 ppm, a slight decrease in leukocyte count was observed
in males and in prothrombin time in females. A dose-related decrease
in serum prolactin levels was observed in females, but it was not
statistically significant, and the individual levels within the groups
were very variable. Relative liver weight was increased in all treated
groups, but the reduction was statistically significant only at
350 ppm. At this dose, absolute and relative ovarian weights were
increased, and absolute spleen weights were reduced in males. A slight
increase in the incidence of a fatty liver was seen in all treated
males (60-70%; 43% in controls), and an increase in the severity of
fatty changes in liver was observed in females at the high dose. The
NOAEL was 50 ppm, equal to 2.3 mg/kg bw for males and 3.6 mg/kg bw per
day for females (Hoffman et al., 1978d).
In two replicate studies, groups of 40 male and 40 female Wistar
rats (60 animals of each sex in the control group) were fed diets
containing 0, 50, 130, or 350 ppm fenarimol (purity, 97.9%), equal to
0, 2.0, 5.3, or 14.6 mg/kg bw for males and 0, 2.8, 7.6, or 21.5 mg/kg
bw for females, for 24 months. Haematology and clinical chemistry were
evaluated only at termination. Since the results of the two replicate
studies were similar, they were reported as a single study. There was
a dose-related increase in the rate of survival, increasing in males
from 27% (controls) to 44% (high dose) and in females from 38 to 54%;
however, the rate was not sufficient to meet the criteria for a valid
negative result in a study of carcinogenicity. There were no
treatment-related effects on clinical signs of toxicity or organ
weights. Body-weight gain of males was slightly reduced at 350 ppm
during the first nine months of the study. The leukocyte count was
decreased in animals of each sex at the high dose. Dose-related
increases in blood glucose levels were seen at all doses, which were
statistically significant in males at the high dose and in one study
at > 50 ppm in females. In one study, a dose-related reduction in
mean serum prolactin values was seen in females at > 50 ppm, which
reached statistical significance at 350 ppm; however, individual
values were very variable, with similar ranges in all groups. Males
also showed a statistically significant increase in prolactin levels
at 350 ppm, and females had increased levels of luteinizing hormone.
Although hormone levels were investigated to elucidate the effects of
fenarimol on reproduction, the laboratory considered the toxicological
significance of the observed changes to be unknown. An increased
incidence of fatty changes in the liver, which tended to be dose-
related, was seen especially in males; the overall incidences in males
and females in the two studies were 29, 40, 43, and 53% at 0, 50, 130,
and 350 ppm. An increase in severity was observed in animals of each
sex at the high dose. The incidence of hyperplastic nodules was
slightly increased: 0% in controls, 1.9% at 50 ppm, 1.9% at 130 ppm,
and 4.4% at 350 ppm, and reached statistical significance at the high
dose. In addition, hepatic-cell adenomas were seen in 0.6% of rats at
130 ppm and in 3.2% at 350 ppm (statistically significant). There was
no NOAEL, owing to the slight effects on the liver at the low dose
(Hoffman et al., 1978e).
A further two-year carcinogenicity study was conducted in order
to establish a no-effect level for the effects on the liver observed
in the previous study. Groups of 50 male and 50 female Wistar rats
were fed diets containing 0, 12.5, 25, or 50 ppm fenarimol (purity,
96.7%), equal to 0, 0.6, 1.2, or 2.5 mg/kg bw for males and 0, 0.7,
1.5, or 3.0 mg/kg bw per day for females, for 24 months. Haematology
and clinical chemistry were evaluated only at termination, and the
liver was the only organ examined at necropsy. The survival rate was
low due to an outbreak of chronic respiratory disease during the 16th
and 17th months: only 10-32% per sex per group survived for two years
There were no treatment-related effects on clinical signs of toxicity,
haematology, or liver weight. Reductions in body-weight gain, food
consumption, and food efficiency were observed in males at the high
dose during the first year of the study. The glucose content of blood
was increased in animals of each sex at 50 ppm. The incidence of fatty
liver was increased at the high dose, especially in males (60%; 26% in
controls). There was no evidence of an increase in tumour incidence,
but the survival rate was not sufficient to meet the criteria for a
valid negative result in a study of carcinogenicity. The NOAEL was
25 ppm, equal to 1.2 and 1.5 mg/kg bw per day for males and females,
respectively (Hoffman et al., 1982a).
Groups of 50 male and 50 female Wistar rats were fed fenarimol
(purity, 97%) at 0, 12.5, 25, or 50 ppm in their diet, equal to 0,
0.5, 1.0, or 2.0 mg/kg bw for males and 0, 0.6, 1.2, or 2.3 mg/kg bw
per day for females, for 24 months. Haematology and clinical chemistry
were evaluated only at termination, and the liver was the only organ
weighed. There was no treatment-related effect on survival rates
(29-44% for males and 56-74% for females), body-weight gain, food
consumption, food efficiency, haematology, hepatic para-nitroanisole
metabolism, liver weight, or histopathology of any organ. Slight
increases in the incidences of low body weight and pale eyes were
noted in all treated males. Blood glucose levels were increased at
25 ppm in males and at 50 ppm in animals of each sex. The tumour
incidence was not increased. The effects seen were considered not to
be adverse. The NOAEL was therefore 50 ppm, the highest dose tested,
equal to 2.0 and 2.3 mg/kg bw per day for males and females,
respectively (Hoffman & Pierce, 1985).
In a further analysis of the data on carcinogenicity from the
study conducted in 1986, the incidences of hepatocellular adenoma and
carcinoma at the high dose in the replicate study and at the low dose
in the last study were combined and analysed for trend. There was no
statistically significant evidence that fenarimol is oncogenic
(Rodricks et al., 1989).
In a study conducted to assess the initiating activity of
fenarimol, groups of six or 12 male Fischer 344 rats were fed 350 ppm
fenarimol (purity unspecified) in the diet for eight or 20 weeks;
one group fed fenarimol for eight weeks was subsequently fed
phenobarbital for 12 weeks. In order to investigate promotion
potential, hepatocellular foci were induced by exposure to N-2-
fluorenylacetamide for eight weeks, and then rats were fed 350 ppm
fenarimol for 12 weeks. Livers were examined after eight or 20 weeks
for the presence of altered hepatocellular foci that were iron-
excluding or contained gamma-glutamyl transpeptidase. Fenarimol had no
initiating or promoting activity in rat liver (Hoffman & Amundson,
1985).
(d) Reproductive toxicity
Mice
In two pilot studies, groups of 10 male and 10 female ICR mice
were fed fenarimol (purity, 97.9%) in the diet. In the first study,
treatment with doses of 50, 170, or 600 ppm was started one week
before mating and was continued up to day 21 of lactation. In the
second study, treatment with doses of 0, 170, 350, or 600 ppm was
started two weeks before mating and continued up to day 14 of
lactation. One female at 600 ppm died and one was killed with signs of
dystocia. No effect on body weight was detected. Fertility was clearly
reduced (and fewer females had copulatory plugs) at 350 and 600 ppm in
the second study only, and there was evidence of reduced fertility at
170 ppm. Liveborn litter size was reduced at 350 and 600 ppm,
apparently as a result of an increased incidence of stillbirths. A
lengthened gestation period was observed at 600 ppm No NOAEL could be
established owing to the limited study design (Markham et al.,
1978a).
In a three-generation study, groups of 20 male and 20 female ICR
mice (30 of each sex in the control group) were fed fenarimol (purity,
97.9%) at dietary levels of 0, 35, 70, or 140 ppm, equivalent to 0, 5,
10, or 20 mg/kg bw per day, starting 61-63 days before mating. Food
consumption was not measured, and the surviving parental animals were
not necropsied. No compound-related effects were observed in the
parents, on reproductive parameters, or on the pups. The NOAEL was
140 ppm, equivalent to 20 mg/kg bw per day (Markham et al., 1978b).
Rats
In a two-generation study, groups of 20 male and 20 female Wistar
rats (30 of each sex in the control group) were fed fenarimol (purity,
97.9%) in the diet at concentrations of 0, 50, 130, or 350 ppm,
equivalent to 0, 12.5, 6.5, or 17.5 mg/kg bw per day. Food consumption
was not measured. F0 rats were exposed for 55 days before the first
of three matings; after the third mating, the animals were maintained
on the test diets for another three months and then evaluated for
long-term toxicity (see Hoffman et al., 1978c). The F1b offspring
were destined to become F1 parents. The F1 animals were exposed to
the test diets for 58 days before the first mating. After weaning, the
F1 parents were placed on control diet for 63 days before a second
mating (reversibility test). For the third mating, a reversibility and
cross-over experiment was performed: the F1 animals continued to be
exposed to control diets, for a total of about five months by the time
of mating. F1 males were mated with untreated virgin females and
F1 females with untreated males. In each of the three F1
breedings, one female at 350 ppm died, perhaps due to dystocia. The
F1 males at 130 and 350 ppm had decreased body-weight gain. There
was a dose-related reduction in fertility (proportion of pregnant
females) at 130 and 350 ppm, and the effect was time-dependent, being
more pronounced after each successive F0 mating and even more
pronounced after the first F1 mating, in which fertility was 20 and
0%, respectively, at 130 and 350 ppm. There was some evidence of
reduced fertility at 50 ppm, but the effects were not statistically
significant. After the F1 parent animals had been fed the control
diet, fertility was 45 and 10%, respectively. Cross-over analysis
showed clearly that the reduced fertility was male-mediated, and there
was some, inconclusive evidence that it might also be female-mediated:
the fertility of treated females was lowered at all doses. The control
value for untreated females was, however, low, and age may have been a
factor since the females were over 10 months old A reduction in
liveborn litter size was seen at 350 ppm after the F0 matings,
probably due to an increase in the proportion of stillborn pups. In
all treated groups, but especially at 350 ppm, there were more females
with a lengthened gestation period (23-24 days) than among controls.
There was no consistent, substantive effect on the survival rate or
body weight of the progeny. Gross necropsy of weanlings revealed an
increased incidence (14.2% compared to 5.2% in controls; not
statistically significant) of hydronephrosis at 350 ppm after the
third mating of the F0 generation. No NOAEL could be established for
reproductive effects, because of the slight evidence of reduced
fertility at the lowest dose. The NOAEL for general systemic toxicity
in parental animals was 50 ppm, equivalent to 2.5 mg/kg bw per day
(Hoffman et al., 1977b).
In a further three-generation study, groups of 20 male and 20
female Wistar rats (30 of each sex in the control group) were fed
fenarimol (purity, 97.9%) in the diet at concentrations of 0, 12.5,
25, or 50 ppm, equivalent to 0, 0.625, 1.25, or 2.5 mg/kg bw per day.
Exposure started 56-71 days before the first mating, and three
generations of one, two, and one nests were bred. The surviving
parental animals were not necropsied. The death of one female during
parturition and signs of haemorrhage in another female during
parturition (signs of dystocia), both at 50 ppm, were attributed to
treatment. Body-weight gain was slightly reduced during the premating
period in F1 males at 50 ppm. After both F1 mating periods,
fertility was clearly reduced at 50 ppm, together with a reduction in
the proportion of females with copulatory plugs, and there was some
evidence of a reduction in fertility after the F2 mating at 50 ppm. A
statistically significant, dose-related reduction in liveborn litter
size (apparently due to an increase in stillbirths) at 25 and 50 ppm
after the second F1 mating was not considered to be related to
treatment by the laboratory; however, it occurred only after a long
exposure and has been seen to be a clear result of shorter exposure to
a higher concentration of fenarimol (see Hoffman et al., 1977b). The
NOAEL for reproductive effects was 12.5 ppm, equivalent to 0.625 mg/kg
bw per day. The NOAEL for general systemic toxicity was 25 ppm,
equivalent to 1.25 mg/kg bw per day (Markham et al., 1978c).
A single-generation study of cross-over design was conducted in
groups of 10 male and 10 female Wistar rats fed fenarimol (purity,
97.9%) by gavage at a dose of 0 or 35 mg/kg bw per day, starting one
month before mating and continuing up to one month thereafter for
males and up to day 20 post partum for females. In two groups,
control males were mated with control and treated females, and in the
third group treated males were mated with control females. The
fertility of treated males was reduced, together with a reduction in
the proportion of females with copulatory plugs, and the liveborn
litter size of treated females was reduced, apparently due to an
increased incidence of stillbirths. There was no NOAEL (Hoffman
et al., 1980b).
Groups of 15 male and 15 female Wistar and Sprague-Dawley rats
were compared with regard to their susceptibility to reproductive
effects under identical conditions in a cross-over study similar to
that described above (Hoffman et al., 1980e), except that the serum
levels of luteinizing hormone and prolactin were also investigated. In
both strains, body-weight loss and dystocia were observed during the
first week of treatment. Fertility was reduced in treated males, as
was the number of females with copulatory plugs. The mean liveborn
litter size was reduced in Sprague-Dawley rats only, due to an
increased incidence of stillbirths. Post-partum survival was reduced
in treated females of both strains. The serum levels of luteinizing
hormone were increased and those of prolactin decreased in treated
females of both strains. There was no NOAEL (Hoffman et al., 1980c).
In another single-generation study of cross-over design, groups
of 10 male and 10 female Wistar rats were fed fenarimol (purity,
97.9%) by gavage at 0 or 35 mg/kg bw per day, starting one month
before mating up to one month thereafter, encompassing gestation and
lactation. In two groups, control males were mated with control and
treated females, and in the third group treated males were mated with
control females. The serum levels of prolactin, luteinizing hormone,
and testosterone and para-nitroanisole- O-demethylase activity were
measured. Two treated females died due to dystocia. Fertility was
reduced in treated males, and the proportion of females with
copulatory plugs and vaginal sperm was reduced. In all cases in which
there was evidence of mating, it was not delayed, and the females
became pregnant. The liveborn litter size of treated females was
reduced, apparently due to an increased incidence of stillbirths; in
addition, gestation length was increased, and progeny survival through
day 7 post partum was slightly reduced. Absolute and relative liver
weights and liver enzyme activity were increased in treated males
and females; treated males also had hypertrophy of centrilobular
hepatocytes. There was no NOAEL (Hoffman et al., 1980d).
Guinea-pigs
In a two-generation study., groups of 15 male and 30 female
guinea-pigs of the F0 generation and 20 males and 20 females of the
F1 parent generation were fed fenarimol (purity, 98.5%) at dietary
levels of 0 or 400 ppm, starting four weeks before mating for the F0
parents and 70 days before mating for the F1 parents. There were
no treatment-related effects on mortality or food consumption.
Body-weight gain was reduced in males of both generations and in F0
females during the premating period. There was no effect on
reproductive parameters, except that the proportion of liveborn
progeny was marginally lower in treated animals of each generation.
The laboratory considered this to be a reflection of litter size, i.e.
lower survival in larger litters, which has previously been documented
in guinea-pigs. Although the data presented support this view for the
control groups, it does not do so for the treated groups. Therefore,
400 ppm, equal to 33 mg/kg bw per day for males and 35 mg/kg bw per
day for females, slightly affected gestational survival, although this
is not of clear toxicological importance (Hoffman et al., 1983a).
Rabbits
In a single-generation stud), of cross-over design, groups of
9-11 male and 9-11 female Dutch belted rabbits were fed fenarimol
(purity, 97.9%) by gavage at 0 or 35 mg/kg bw per day, starting one
month before mating and continuing up to one month thereafter for
males and up to day 6 post partum for females. In two groups,
control males were mated with control and treated females, and in the
third group treated males were mated with control females. Prolactin
and testosterone levels were determined, and premating semen volume,
sperm count, and sperm viability were measured. There was no evidence
of systemic toxicity or adverse reproductive effects. The. NOAEL was
the only dose tested, 35 mg/kg bw per day (Hoffman et al., 1980e).
(e) Developmental toxicity
Rats
Groups of 25 pregnant Wistar rats were fed fenarimol (purity,
97.9%) by gavage at 0, 5, 13, or 35 mg/kg bw per day during days 6-15
of gestation. None of the dams died, and there were no signs of
toxicity and no effects on food consumption or body weight. The only
finding in fetuses was an increased incidence of hydronephrosis at the
high dose (in 30% of fetuses and 62% of litters, in comparison with 9%
of fetuses and 25% of litters in controls). The NOAEL for maternal
toxicity was 35 mg/ kg bw per day and that for embryo- and
fetotoxicity, 13 mg/kg bw per day (Hoffman et al., 1980f).
A special study was conducted to investigate the reversibility of
fenarimol-induced hydronephrosis in neonatal rats. In three replicate
studies, pregnant Wistar rats were fed fenarimol (purity, 99.6%) by
gavage at 0 or 35 mg/kg bw per day during days 6-15 of gestation. Two
groups were sacrificed on day 20 or 21 of gestation, and the others
were allowed to litter and wean their progeny. The normal prenatal and
postnatal examinations were performed, and the kidneys and ureters of
the progeny were examined on days 20 and 21 of gestation and on days
1, 7, 21, 42, and 63 post partum. The total numbers of female rats
examined on gestation days 20 and 21 were 25 and 15, respectively;
postnatally, 15 in the control group and 20 in the test group were
examined. There were no treatment-related effects on dams with regard
to mortality, body weight, or food consumption, but sporadic cases of
dystocia were noted. Prenatal examination revealed a slight decrease
in the proportion of live fetuses due to an increase in early
resorptions. Fetal weights were slightly lower on day 20 but not day
21; on day 20, the number of runt fetuses was also increased.
Hydroureter and hydronephrosis were seen more often among exposed
fetuses on days 20 and 21, and a slightly increased incidence of
skeletal variants, such as cervical ribs and 14 thoracic fibs, was
observed, but only day-20 fetuses were examined. Fetal kidney weights
were depressed by treatment. Postnatal examination revealed a slight
increase in gestational length and reduced liveborn litter size.
Furthermore, neonatal mortality was increased during week 1 post
partum, pup body weights were increased, hypothermia was more
common, and the absolute kidney weights of the progeny were increased.
A reduction in the increased incidence of hydroureter, which was seen
prenatally, was observed with advancing neonatal age, but a
significant increase in hydronephrosis was seen in progeny on day 1
post partum (50% of treated pups and 23% of control pups). At
subsequent examinations, the incidence was still increased but was
substantially less than on day 1 post partum. The slight or moderate
microscopic alterations associated with the observed hydronephrosis
were limited to the renal pelvis and papilla and therefore did not
constitute true pathological hydronephrosis, which is associated with
severe damage, such as glomerular destruction, shortening and blunting
of renal tubules, and reduction or absence of the outer nephrogenic
layer. There was no effect on kidney function, including specific
gravity and osmolality, determined on day 63 post partum. As the
observed hydronephrosis was apparently not a significant pathological
condition and was to at least some degree reversible, together with
the fact that it is a common finding in the Wistar rat, its increased
incidence is considered to be due to delayed development and not a
teratogenic effect (Hoffman et al., 1983b).
Rabbits
Groups of 15 pregnant Dutch-belted rabbits were fed fenarimol
(purity, 97%) by gavage at 0, 3, 10, or 35 mg/kg bw per day on
gestation days 6-18 and were sacrificed on gestation day 28. There
were no treatment-related effects on mortality, body weight, or food
consumption. 4 slight reduction in litter size was observed at 10 and
35 mg/kg bw, but this was not considered to be related to treatment
since the number of implantations and the implantation index were also
reduced in these groups. The occurrence of oedema and various limb and
skeletal defects in five fetuses from one anorectic female at 35 mg/kg
bw was not clearly a direct response to treatment. There was no
evidence of irreversible structural changes. In the absence of clear
evidence of maternal or developmental toxicity, the NOAEL was 35 mg/kg
bw per day, the highest dose tested (Hoffman et al., 1977c).
Groups of 20 female New Zealand white rabbits were fed fenarimol
(purity, 96.6%) by gavage at 0, 15, 50, or 150 mg/kg bw per day on
gestation days 6-18 and were sacrificed on gestation day 28. There
were no treatment-related effects on mortality. Body weight and food
consumption were reduced at 150 mg/kg bw, and four rabbits at the
highest dose lost weight, became anorectic, and then aborted. The
number of live fetuses per litter was reduced, and there was an
increase in the number of early resorptions (neither statistically
significant). The only finding in fetuses was an increased incidence
of extrathoracic ribs (84% of fetuses, compared with 61% in controls).
There was no evidence of irreversible structural changes. The NOAEL
for maternal and embryo- and fetotoxicity was 50 mg/kg bw per day
(Hoffman & Russel, 1990).
(f) Genotoxicity
A number of tests for genotoxicity have been carried out with
fenarimol. The results are summarized in Table 2.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
A single, 4-h, semi-occluded application of 0.5 g technical-grade
fenarimol (purity stated to be 100%) onto the intact skin of three
male New Zealand white rabbits produced no skin irritation (Jones,
1994a).
After instillation of 0.1 ml (equivalent to 56 mg) of technical-
grade fenarimol (purity stated to be 100%) into the conjunctival sac
of the eyes of one male and two female New Zealand white rabbits,
slight conjunctival irritation was observed up to 24 h. Iridial
inflammation was noted in one treated eye 1 h after treatment. The
treated eyes appeared to be normal after 24-48 h (Jones, 1994b).
Fenarimol (purity, 97%) was tested in a Magnusson-Kligman
maximization test in 10 Hartley guinea-pigs; the control group
consisted of five animals. Barely perceptible erythema were seen in
two treated animals and in one of four surviving controls (Hoffman &
Arthur, 1980).
Table 2. Results of tests for the genotoxicity of fenarimol
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutationa S. typhimurium TA100, 125-2000 µg/plateb; 97 Negatived Hoffman et al.
TA98, TA1535, TA1537, 62.5-1000 µg/platec; (1988a)
E. coli WP2uvrA- toxicity and precipitation
at highest concentration;
vehicle, dimethyl sulfoxide
Chromosomal aberrationa Human lymphocytes 1.75-25 µg/mlb for 25 h 97 Negatived Murli (1988)
59.9-160 µg/mlc for 1 he;
vehicle, dimethyl sulfoxide
Gene mutationa Mouse lymphoma 1-50 µg/ml for 4 h; 97 Negatived Hoffman et al.
L5178Y tk+/-cells cytotoxic at > 35 µg/ml; (1988a)
vehicle, dimethyl sulfoxide
Unscheduled DNA Rat hepatocytes 0.05-100 nmol/ml for 5 h; 97 Negativea Probst (1979)
synthesisa,f cytotoxic at > 50 nmol/ml;
vehicle, dimethyl sulfoxide
In vivo
Chromosomal aberration 10 male Chinese hamsters; Oral; 250 mg/kg bw per day 98 Negatived Siou & Lerond-
bone-marrow cells for 2 daysg; sacrifice at 24, Conan (1982)
48, and 72 h; vehicle, peanut oil
Micronucleus formation Groups of 10 male Swiss Oral; 1000 mg/kg bw per day > 98 Weakly Siou et al. (1982)
mice; hone-marrow cells for 2 daysh; sacrifice at 24, positivei
48, and 72 h; vehicle, peanut oil
Micronucleus formation Groups of five male and Oral; 125, 625, or 1250 mg/kg 97 Negatived Ivett (1988)
five female Sprague-Dawley bwj; sacrifice at 24, 48, and
rats; bone-marrow cells 72h; vehicle, corn oil
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
In vivo (con't)
Dominant lethal mutation Groups of 10 male Wistar- Oral; 350 mg/kg bw; vehicle, 97 Inconclusivek Hoffman et al.
Harlan rats 5% acacia solution (1977d)
a No confirmatory experiment
b Without metabolic activation
c With metabolic activation
d Positive controls yielded positive result(s)
e As cell cycle delay was observed with metabolic activation in a preliminary test, incubation was for 45.3 h after treatment.
No cell cycle delay without activation; toxicity at highest doses tested
f Only one culture per concentration; only 20 cells per concentration analysed
g No mention of cytotoxicity; dose based on preliminary test in which all five animals died at 1000 mg/kg bw per day lot two days
h No mention of cytotoxicity; dose based on preliminary test in which all five animals died at 4000 mg/kg bw per day lot two days.
In the main test, one animal treated for 72 h died.
i Slight but statistically significant increase in micronuclei at 24 h sacrifice only; i.e. micronucleus frequencies per
1000 polychromatic erythrocytes; at 24 h: control, 0.19 ± 0.03; treated, 0.33 ± 0.06
j Toxic signs (lethargy, unsteady gait) at > 125 mg/g bw; one animal died at 1250 mg/kg bw.
k Treated males bred with untreated females weekly for eight weeks. Dose > 1/10 of the LD50. No sign of toxicity observed.
Only 7-10 pregnant females per mating interval per dose group. No reference to positive controls. No treatment-related effect on
fertility rate or pre- or post-implantation losses.
(ii) Mechanisms of effects on reproduction
Groups of six adult male Sprague-Dawley rats were given 35 mg/kg
bw per day fenarimol (purity unspecified) by gavage or subcutaneously
for 30 days; eight males served as controls. After 15 days of
treatment, each male was mated with two untreated females. No effect
was seen on male body weight, male fertility, the histopathology of
the testis or ventral prostate, or serum levels of testosterone,
follicle-stimulating hormone, or luteinizing hormone. Epididymal
weight was reduced after oral administration, but no histopathological
effects were observed. Serum prolactin levels were reduced after
systemic exposure, and prostatic prolactin binding was reduced after
exposure by either route. In a second experiment, groups of 15 males
were fed 0 or 35 mg/kg bw per day by gavage for 70 days and were bred
30 and 56 days after exposure. The parameters measured were the same
as in the first experiment except for follicle-stimulating hormone.
Serum prolactin levels and prostatic prolactin binding were reduced,
and the serum testosterone level was slightly elevated. There were no
other effects (Hanlin, 1981a).
Groups of 10 adult female Sprague-Dawley rats received fenarimol
(purity, 97%) at 0 or 35 mg/kg bw per day by gavage for about 60 days
before mating with untreated males, and then throughout pregnancy and
lactation. There were no effects on body weight or fertility. There
was some suggestion of an increase in proestrous prolactin level, but
the serum levels of prolactin were variable both before estrus and
during lactation and those of luteinizing hormone were variable before
estrus. Overall, there was no clear compound-related effect. There was
some evidence of a slight increase in estrous cycle length (Smalstig,
1981a).
Groups of 30 male Wistar rats were fed a diet containing 0 or
350 ppm fenarimol (purity, 97.7%) from 25 to 82 days of age. Twenty in
each group were mated and 10 were unmated; unmated males were killed
at the end of treatment, and the remaining animals were fed control
diet until day 117. Mating trials were conducted weekly with untreated
females on days 40-110. There were no significant effects on food
consumption or body-weight gain. A delay in the onset of reproductive
behaviour (percent mating and inseminating) was observed, but the
effect was reversible, since 100% fertility was attained at day 110.
The only effect on male reproductive organs was a reduction in
epididymal weight at the end of the treatment phase, with recovery
after cessation of treatment. No tissues were examined microscopically
(Hoffman & Hirsch, 1981c).
In a study of the possible involvement of uterine prostaglandins
in parturition, female Sprague-Dawley and Charles River CD rats
received 35 mg/kg bw per day fenarimol (purity, 97%) or the corn oil
vehicle orally from day 12 of pregnancy until parturition, or day 23
of pregnancy if no parturition occurred. The prostaglandin F2µ,
content, its release in and from the uterus, and serum progesterone
levels were measured some days before and at parturition. Sprague-
Dawley females showed delayed and/or difficult parturition and a
slight increase in the frequency of fetal and pup death, which
appeared to be related to the occurrence of difficult parturition.
There was no effect on prostaglandin F2µ, content; its release at
parturition was increased and not decreased, which would have been
expected with delayed parturition. Serum progesterone levels appeared
to be higher in treated females, and particularly in those that did
not deliver. The authors interpreted this finding as indicating that
progesterone did not fall to the low levels that facilitate or
initiate parturition in the rat (Smalstig, 1981b).
Progesterone concentrations in plasma were determined in late
pregnancy in groups of 12 Sprague-Dawley rats treated with 0 or
350 ppm fenarimol (purity unspecified) during pregnancy. Most of the
treated animals experienced delayed parturition associated with
dystocia and usually resulting in maternal death. High plasma
progesterone levels (66% of the peak value seen on day 19, in
comparison with 13% in controls) were seen in treated animals
immediately before normal parturition. The authors interpreted this
finding as indicating that luteolysis had not occurred, thus delaying
normal parturition (Tinsley, 1982).
A simulated rat ovarian microsomal system was used to measure the
effect of fenarimol on aromatase activity in vitro. Fenarimol
(purity unspecified) at molar concentrations 5-50 times the substrate
concentration inhibited aromatase activity by 55-90%. In a comparison
with aromatase inhibitors used clinically, fenarimol was as potent as
testolactone but less potent than aminoglutethimide and was therefore
considered to be a moderately weak inhibitor (Hoffman et al.,
1982b).
The urogenital sinuses were removed from 15-17-day-old male and
female Charles River rat embryos and implanted under the kidney
capsules of intact syngeneic male hosts (10 implants per animal). One
implanted male was then treated with 35 mg/kg bw per day fenarimol
(purity unspecified) for 21 days, one received the corn oil vehicle,
and one was treated with the positive control compound, cyproterone
acetate, which has anti-androgenic effects. Treatment with fenarimol
or corn oil had no effect on the differentiation or function of
transplanted urogenital sinuses in adult male rats. In contrast,
cyproterone acetate retarded prostatic development, and secretions
were absent (Neubauer et al., 1982).
Concentrations of 10-9 to 5 × 10-5 mol/litre fenarimol (purity
unspecified) were tested for androgen-binding capacity in vitro.
Fenarimol did not show affinity for binding to prostatic androgen
receptors, although 17% inhibition was seen at the highest
concentration. In an assay to determine whether fenarimol inhibits the
uptake of 3H-testosterone into the ventral prostate or the median
basal hypothalamus and preoptic areas of the brain, groups of 8-10
male Sprague-Dawley rats were given 0 or 35 mg/kg bw fenarimol
subcutaneously 20 and 3 h before sacrifice. Testosterone uptake was
not inhibited; in fact, uptake into the ventral prostate was
increased, whereas a positive control substance inhibited uptake into
the ventral prostate. In an assay to determine whether fenarimol
inhibits androgen-stimulated growth of seminal vesicles and ventral
prostate after castration, groups of six to eight immature rats were
given a daily subcutaneous injection of 0.25 mg/kg bw testosterone
propionate plus a daily oral or subcutaneous dose of 0, 5, 15, or
35 mg/kg bw fenarimol three days after castration for seven
consecutive days. A dose-related reduction in seminal vesicular weight
was seen with oral doses > 15 mg/kg bw, but the effect was not
statistically significant. The authors of the study concluded that
there was no inhibition (Hanlin, 1981b).
In a study to investigate whether fenarimol interferes with the
binding of androgens to the androgen receptor, groups of nine
castrated adult Wistar rats were fed fenarimol (purity unspecified) at
0 or 350 ppm in the diet for seven days. Cytosol from the hypothalamic
preoptic area, the frontal and parietal cerebral cortex, the
pituitary, and the ventral prostate was then incubated with
radiolabelled methyltrienolone (R1881; a potent synthetic androgen).
No reduction in binding to the cytoplasmic androgen receptors was
seen. In a second experiment, groups of one to two male rats were
treated similarly and then with radiolabelled R1881 before sacrifice.
There was no effect on binding to nuclear androgen receptors in the
tissues examined (the hypothalamic preoptic area, the pituitary, the
amygdala, the cerebral cortex, the prostate, the diaphragm, or the
liver), and the concentrations of conjugated and unconjugated labelled
R1881 were similar in all tissues. In a third experiment, pregnant
rats were fed 0 or 350 ppm fenarimol in the &let for most of the
period of gestation. Four female pups from each group were given a
subcutaneous injection of 3H-testosterone at the age of three or
four days and were killed 2 h later. There was no effect on the
binding of testosterone to the nuclear receptors in the hypothalamic
preoptic area amygdala (Hoffman et al., 1981a).
In a study of the estrogenic and anti-estrogenic potential of
fenarimol, groups of six immature female Holtzman rats received either
35 mg/kg bw fenarimol (purity unspecified) by gavage plus a
subcutaneous dose of estradiol, 35 mg/kg bw fenarimol by gavage, or a
subcutaneous dose of estradiol daily for three days. One dose of
fenarimol with several doses of estradiol, several doses of fenarimol
(8.75, 17.5, and 35 mg/kg bw) with one dose of estradiol, and
fenarimol in different vehicles were tested. There was no evidence for
agonistic or antagonistic effects on estrogen, as indicated by changes
in uterine weight. Fenarimol at concentrations of 1-1000 nmol/litre
did not inhibit the binding of estradiol to estrogen receptors in the
cytosol of immature rat uteri in vitro (Black, 1981a).
In a study of the effect of fenarimol on the binding of estrogen
to its receptor and on circulating estrogen levels at parturition,
fenarimol (purity unspecified) had no significant effect on the
nuclear uptake of estrogens by the hypothalamic preoptic area amygdala
or pituitary in groups of six adult ovariectomized rats fed 350 ppm in
the diet for seven days. Treatment of groups of four to six female
Wistar rats on days 0-21 of gestation at this dose did not affect the
concentration of estrone or estradiol in the plasma on day 21 (Hoffman
et al., 1981b).
Groups of three immature Dutch belted female rabbits were given
subcutaneous rejections of 0.5 µg oestradiol for six days and then
received either daily subcutaneous injections of 100 µg progesterone,
daily gavage doses of 60 mg fenarimol (purity unspecified), or the two
treatments combined, for five days. There was no evidence for a
progestational or anti-progestational effect in uteri examined
microscopically 24 h after the last dose (Black, 1981b).
Groups of seven pregnant Wistar rats were fed a diet containing
350 ppm fenarimol (purity, 97.7%) on gestation days 0 to 14-18. On the
last day of treatment, maternal blood and two fetuses per litter were
sampled 2 and 6 h after a gavage dose of radiolabelled fenarimol.
Placental transport was limited (the ratio of 14C in fetus to that
in maternal plasma was < 1) during days 14-17, the period associated
with genital tract morphogenesis, but increased steadily from day 15
onwards, to reach a ratio of 1 by day 18, when the organization of
sexual behaviour in the fetal brain begins (Hoffman & Hirsch, 1981a).
Female Wistar rats were fed 0 or 350 ppm fenarimol (purity,
97.7%) in the diet on days 0-5 post partum, and dams and pups
received an oral dose of radiolabelled fenarimol on day 5. One group
of 18 dams was used to study excretion into milk; the pups of seven
other dams were culled to three males and three females per litter.
The concentration of radiolabel in milk was three to five times higher
than that in maternal plasma up to 48 h after treatment. Radiolabel in
milk was rapidly taken up by the pups; the concentrations in the
hypothalamus increased more rapidly, attained higher maximal levels
(up to seven times higher 1 h after administration), and decreased
more slowly than in the remainder of the neonatal brain. In the
absence of previous exposure to fenarimol, an alteration was seen in
the rate but not the extent to which radiolabel was taken up by either
the hypothalamus or the brain. The major difference between pups
treated directly with 14C-fenarimol and those exposed via the milk
was in the rate of elimination, which was considerably faster after
direct treatment (Hoffman & Hirsch, 1981b).
In a study of the effects of fenarimol on the concentration of
estrogen receptors in the hypothalamus and on the conversion of
testosterone to estrogen, as an indirect measure of central aromatase
activity, pregnant Wistar rats received dietary concentrations of 0 or
350 ppm fenarimol (purity unspecified) from gestation day 3-4 to day
21. The concentration of nuclear estrogen receptors was measured in
the hypothalamic preoptic area amygdala of day-21 fetuses and of
neonates on day 3-4. The mean concentration was reduced by 44-46% in
fetuses of each sex and by 56% in neonates, especially in males.
Sex-specific differences in the concentration of receptors in the
exposed neonates were abolished, i.e. males no longer had higher
concentrations. In a second experiment, male Wistar rats that were
castrated but had testosterone implants and intact males received 0 or
350 ppm fenarimol in the diet for seven days. The concentration of
nuclear estrogen receptors was reduced in both groups. The circulating
levels of testosterone indicated that the effect on receptor
concentration could not be attributed to testosterone levels. In a
third experiment, pregnant Wistar rats received 0 or 350 ppm fenarimol
in the diet from about gestation day 18 to day 3-4 post partum, and
groups of 12 female neonates (used to eliminate endogenous
testosterone production as a variable) were then dosed with
radiolabelled testosterone. Significantly lower concentrations of
estrone and estradiol were observed in homogenates of hypothalamic
preoptic area amygdala from exposed pups, and, contrary to controls,
no labelled estradiol was detected in the nuclei. Fenarimol did not
affect the concentrations of testosterone or dihydrotestosterone in
the nuclear extracts of the hypothalamic preoptic area amygdala. Since
testosterone is metabolized to dihydrotestosterone by the enzyme
5µ-reductase and to estradiol by aromatase, this observation suggests
that the reduction in receptors is associated with inhibition of
aromatase (Hoffman et al., 1981c).
(iii) Relevance to humans of adverse reproductive effects in rodents
The adverse effect of fenarimol on the fertility of male mice
and rats appears to be due to an effect on the organization
(differentiation) and expression of male sexual behaviour, which is
known to be controlled within the central nervous system (Naftolin
et al., 1991). The mechanism is dependent on aromatization of
testosterone to estradiol-17ß. Evidence is available from several
studies that the expression of human male sexual behaviour has a
different hormonal basis from that in rats, i.e. depends on both a
direct action of testosterone and conversion of testosterone to
dihydrotestosterone, but not on the conversion of testosterone to
estradiol-17ß. Mantzoros et al. (1995) showed that the frequency of
human male sexual behaviour is correlated with the serum level of
dihydrotestosterone but not of estradiol-17ß. Gooren (1985)
administered an aromatase inhibitor (testolactone) or an estrogen
receptor antagonist (tamoxifen) to men and also concluded that
estradiol-17ß is not important in the control of male sexual
behaviour. Bagatell et al. (1994) showed that there was no change in
male sexual function after a treatment regimen involving testolactone,
testosterone, and a gonadotropin RH antagonist, which resulted in a
specific decrease in estradiol-17ß levels while keeping testosterone
and dihydrotestosterone at normal levels. A single study indicates a
role for estradiol-17ß in the expression of human male sexual
behaviour (Luisi & Franchi, 1980). The results showed that an
aromatizable form of testosterone is more effective in stimulating
libido than the non-aromatizable androgen mesterolone; however, this
result is difficult to interpret as it is not known whether
mesterolone can cross the blood-brain barrier.
Men with a genetic defect resulting in impaired androgen
receptor-mediated activity, but with no effect on estradiol-17ß
receptor-mediated activity or on aromatase, did not show normal male
sexual behaviour (Breedlove, 1994). A further study of genetic defects
(Smith et al., 1994) supports this conclusion, since an XY man
lacking functional estrogen receptors remained masculine, indicating
that estradiol-17ß does not control sexual differentiation in human
males; however, sexual activity was not assessed. Furthermore,
aromatase is confined mainly to the gonads and brain of rats and mice,
while it is also expressed in placenta, adipose tissue, and liver in
higher primates, including humans. Thus, the proportion of target
enzyme to inhibitor would be greater in humans than in rats, requiring
more inhibitor to achieve the same degree of inhibition at a given
target site.
Fenarimol would appear to have an adverse effect on reproductive
parameters in female mice and rats, as gestation and parturition are
extended, resulting in dystocia. This effect is also a direct effect
of the inhibition of aromatase, since circulating progesterone is
sustained at a high level, preventing timely luteolysis and resulting
in continued functioning of the corpora lutea and thus prolonging
gestation. In a review, Chwalisz (1994) outlined the mechanisms of
pregnancy and parturition in female rats. In order to maintain
pregnancy, rats depend on the corpus luteum as a source of
progesterone. Parturition requires an abrupt decrease in the
progesterone level, an increase in that of estrogen, and consequently
a decreased ratio of progesterone:estrogen. In contrast, humans (like
guinea-pigs) use the placenta as a source of progesterone during
pregnancy, and an abrupt decrease in progesterone secretion is not
required for parturition. These differences in endocrine control of
parturition, particularly the lesser role of estrogens in stimulating
labour in women, indicate that rats are probably not a relevant model
for predicting the effects of aromatase inhibition on human
parturition and that guinea-pigs are a better model.
Comments
Fenarimol given orally to rats was rapidly absorbed, distributed
and excreted. Elimination occurred principally in the faeces (76-83%),
mainly as a result of biliary excretion, and to a lesser extent in the
urine (6.5-9.2%). Residue levels in tissues were relatively low.
Dermal absorption of fenarimol by monkeys was low (about 2.5%). There
was some barrier to placental transport of fenarimol-derived
radiolabel in rats during part of the gestation period. Although the
levels of the radiolabel in the milk of lactating rats were three to
five times those in the plasma, only a very small proportion of the
dose (< 0.1%) was eliminated in the milk of a lactating goat.
Fenarimol is extensively metabolized to many metabolites. The
major pathways are oxidation of the carbinol-carbon atom, the
chlorophenol rings, and the pyrimidine ring. Repeated oral
administration to rats had no effect on the elimination of fenarimol
or its metabolites.
Fenarimol has low acute oral and dermal toxicity, the LD50
values in rats and rabbits being 2550 and > 2000 mg/kg bw
respectively. After inhalation, the LC50 was > 2 mg/litre (1-h
exposure). Fenarimol did not irritate skin or eyes and was not a skin
sensitizer in guinea-pigs in a maximization test. The WHO has
classified fenarimol as unlikely to present an acute hazard in normal
use.
In a 14-day pilot study in mice given dietary concentrations of
25-12 800 ppm, liver toxicity was observed at 800 ppm and above. In a
three-month study in mice given dietary concentrations of 0, 360, 620,
1100, 2000, or 3300 ppm, toxicity was observed mainly in the liver, as
increased liver weight, hepatic enzyme induction, centrilobular
hypertrophy, and hepatomegaly. The NOAEL was 360 ppm, equivalent to
52 mg/kg bw per day, on the basis of an increase in liver weight at
620 ppm.
In two 14-day pilot studies in rats given dietary concentrations
of 25-12 800 ppm, liver toxicity was observed at 200 ppm and above.
Dietary administration of fenarimol to rats for three months at 0, 50,
200, or 800 ppm also resulted in liver toxicity at and above
200 ppm, seen as increased liver weight, hepatic enzyme induction,
centrilobular hypertrophy, and fatty changes in the liver. The NOAEL
was 50 ppm, equivalent to 2.5 mg/kg bw per day, on the basis of
induction of liver enzymes. In another three-month study in rats, with
doses of 0, 140, 200, 280, 360, and 500 ppm, the NOAEL was 200 ppm,
equivalent to 10 mg/kg bw per day, on the basis of an increase in
liver weight and induction of hepatic enzymes at 280 ppm and above.
In a three-month study in dogs, oral administration of fenarimol
in gelatin capsules at doses of 0, 1.2, 5, or 20 mg/kg bw per day
resulted in no systemic toxicity. In a one-year study in which dogs
were given 0, 1.2, 12, or 120 mg/kg bw per day, toxicity was observed
at the highest dose. This included liver toxicity and, in female dogs,
a transient enlargement of the mammary gland and increased ovarian
weight; an increase in the occurrence of soft stools was observed at
all doses. The NOAEL was 12 mg/kg bw per day.
In a study of chronic toxicity, mice were administered fenarimol
at dietary levels of 0, 50, 170, or 600 ppm for 12 months. The NOAEL
was 170 ppm, equivalent to 24 mg/kg bw per day, on the basis of
decreased body-weight gain and effects on the liver at 600 ppm. In a
two-year study of carcinogenicity, mice were given diets containing 0,
50, 170, or 600 ppm fenarimol. The NOAEL was 370 ppm, equivalent to
24 mg/kg bw per day, on the basis of a slight decrease in body-weight
gain in males at 600 ppm. There was no evidence of carcinogenicity.
Two studies of chronic toxicity were performed in which rats were
administered dietary concentrations of fenarimol at 0, 50, 130 or
350 ppm for 12 or 18 months. In both studies, the main effects at the
highest dose were a decrease in the leukocyte count, increased liver
weight, and decreased spleen weight. In the 12-month study, the NOAEL
was 130 ppm, equal to 6 mg/kg bw per day. In the 18-month study, the
NOAEL was 50 ppm, equal to 2.3 mg/kg bw per day, on the basis of a
reduction in body weight.
Three carcinogenicity studies were performed in rats. In the
first, rats were administered diets containing 0, 50, 130, or 350 ppm
fenarimol. A small increase in the incidence of hepatocellular adenoma
was seen at the highest dose. There was no NOAEL, owing to a slight
increase in fatty changes and hyperplastic nodules in the liver at all
doses. No clear treatment-related effect on tumour incidence was seen.
Two further studies were performed at lower doses (O, 12, 25, or
50 ppm). In the first study, the survival was very low, owing to an
outbreak of chronic respiratory disease. There was evidence of reduced
body-weight gain and an increased incidence of fatty changes in the
liver at 50 ppm. No adverse effect was observed in the second study.
The overall NOAEL for all three studies was 25 ppm, equal to 1.2 mg/kg
bw per day. There was no evidence of carcinogenicity.
A three-generation study of reproductive toxicity in mice given
dietary levels of 0, 35, 70, or 140 ppm fenarimol revealed no adverse
effects on parental mice or offspring. In earlier pilot studies,
however, doses of 170 ppm and above reduced fertility and live-born
litter size and lengthened the gestation period. The NOAEL was
140 ppm, equivalent to 20 mg/kg bw per day.
In a two-generation study of reproductive toxicity in which
guinea-pigs were given 0 or 400 ppm fenarimol in the diet (equal to
33 mg/kg bw per day), parental toxicity was seen and there was
equivocal evidence of a slight effect on the proportion of live-born
progeny. In a single-generation study of reproductive toxicity with a
cross-over design, rabbits were treated orally with fenarimol at a
level of 0 or 35 mg/kg bw per day. There was no evidence of general
systemic toxicity or of effects on reproduction.
Two multigeneration studies of reproductive toxicity have been
performed in rats. In a two-generation study, rats were exposed to
dietary concentrations of 0, 50, 130, or 350 ppm, and in a three-
generation study to 0, 12, 25, or 50 ppm. Fenarimol reduced fertility,
caused dystocia, reduced live-born litter size and lengthened the
gestation period. In the first study, cross-over data showed that the
reduction in fertility was clearly mediated through the male and was
only slightly reversible. The overall NOAEL for general systemic
toxicity was 25 ppm, equivalent to 1.2 mg/kg bw per day, on the basis
of reduced body-weight gain in F1 males at 50 ppm and above. The
overall NOAEL for reproductive toxicity was 12 ppm, equivalent to
0.62 mg/kg bw per day, on the basis of a reduction in live-born litter
size at 25 ppm and above.
Three single-generation studies of reproductive toxicity with a
cross-over design have been conducted in rats, which were exposed
orally to fenarimol at doses 0 or 35 mg/kg bw per day. These studies
showed that the effects on reproduction were not strain-dependent, the
effects on fertility were clearly male-mediated, and the reduction in
live-born litter size (probably due to an increased incidence of
stillbirths) was female-mediated. No correlation was found between
fertility and the levels of prolactin, luteinizing hormone, or
testosterone, organ weights, histopathological findings, or sperm
morphology.
The single- and multigeneration studies of reproductive toxicity
show that fenarimol has adverse effects on reproduction in rats and
(at higher doses) in mice, but not in guinea-pigs or rabbits. A number
of studies were performed to investigate the mechanism of the adverse
effects on reproduction, and particularly those on fertility. The
results of these studies and of others in the open literature show
that fenarimol affects male sexual differentiation and subsequent
behaviour indirectly by inhibiting the aromatase-catalysed conversion
of testosterone to estradiol-17ß within the hypothalamus. Data from
the literature strongly indicate that while estradiol-17ß is the
central regulator of sexual differentiation in rats, human male sexual
behaviour is controlled by dihydrotestosterone, which is formed from
testosterone by the enzyme 5µ-reductase. There is evidence that the
effect of fenarimol in female rats (delayed parturition) is also due
to inhibition of aromatase, as this inhibition results in a sustained
level of circulating progesterone and prolonged corpus luteal
function, thus leading to delayed parturition. In contrast to rats, an
abrupt decrease in progesterone secretion is not required for
parturition in guinea-pigs or humans. The Meeting concluded that,
although not clear-cut, there is sufficient evidence that the adverse
effects on the organization and expression of sexual behaviour in male
rats and on delayed parturition in female rats are not relevant for
humans.
In a study of developmental toxicity, pregnant rats were given
fenarimol by gavage at doses of 0, 5, 13, or 35 mg/kg bw per day.
There was evidence of developmental toxicity (hydronephrosis) but no
signs of maternal toxicity at the high dose. In a study in which some
females were also allowed to litter and wean their progeny, pregnant
rats were exposed to 0 or 35 mg/kg bw per day during days 6-15 of
gestation. There were adverse effects on reproductive performance
(increased length of gestation, dystocia) and developmental toxicity
(increased hydronephrosis) but no evidence of general systemic
maternal toxicity. The increased incidence of hydronephrosis appeared
to be due to delayed development and was not considered a teratogenic
effect. Overall, the NOAEL for maternal toxicity was 35 mg/kg bw per
day, and that for embryo- and fetotoxicity was 13 mg/kg bw per day.
In a study of developmental toxicity in which rabbits were
administered fenarimol by gavage at doses of 0, 3, 10, or 35 mg/kg bw
per day, no signs of toxicity were observed in dams or fetuses. In a
second study, rabbits were exposed orally to doses of 0, 15, 50, or
150 mg/kg bw per day. The NOAEL for maternal toxicity was 50 mg/kg bw
per day, on the basis of reductions in body weight and food
consumption and an increased incidence of abortions. The NOAEL for
embryo- and fetotoxicity was also 50 mg/kg bw per day, on the basis of
a reduced number of live fetuses and an increased incidence of extra
ribs. There was no evidence of teratogenic potential.
Fenarimol has been adequately tested for genotoxicity in a series
of assays in vitro and in vivo. The Meeting concluded that
fenarimol is not genotoxic.
The Meeting concluded that the effects of fenarimol in male rats
(reduced fertility) and female rats (delayed parturition) are due to
inhibition of aromatase, an enzyme that is not involved in these
aspects of human reproduction. It therefore decided that it would be
inappropriate to use the NOAEL seen in studies of reproductive
toxicity in determining the ADI. An ADI of 0-0.01 mg/kg bw was
established on the basis of an overall NOAEL of 1.2 mg/kg bw per day,
seen in several studies of carcinogenicity in rats, and a safety
factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 24 mg/kg bw per day (12-month study of chronic toxicity)
20 mg/kg bw per day (three-generation study of reproductive
toxicity)
Rat: 1.2 mg/kg bw per day (three studies of carcinogenicity)
0.62 mg/kg bw per day (multigeneration study of reproductive
toxicity)*
13 mg/kg bw per day (embryo- and fetotoxicity in study of
developmental toxicity)
Dog: 12 mg/kg bw per day (one-year study of toxicity)
Rabbit: 50 mg/kg bw per day (maternal and embryo- or fetotoxicity in
study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information valuable for the continued
evaluation of the compound
Observations in humans
* Data considered irrelevant for evaluation with respect to human
health.
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenarimol
Exposure Relevant route, study type, species Result, remarks
Short-term (1-7 days) Skin, irritation, rabbit Not irritating
Eye, irritation, rabbit Not irritating
Skin, sensitization, guinea-pig Not sensitizing
Oral, toxicity, rat LD50 = 2550 mg/kg bw
Dermal, toxicity, rabbit LD50 > 2000 mg/kg bw
Inhalation, toxicity, rat, 1 h LC50 > 2 mg/litre
Medium-term (1-26 weeks) Dermal, toxicity, 21 -day, rabbit No systemic effect; no irritation at
1000 mg/kg bw
Dietary, toxicity, three months, rat NOAEL = 2.5 mg/kg bw; hepaticenzyme
induction
Dietary, three-generation, reproductive NOAEL = 0.625 mg/kg bw; reduced
toxicity, rat live-born litter size
Gavage, developmental toxicity, rat NOAEL = 13 mg/kg bw;
hydronephrosis; no maternal toxicity
Long-term (> one year) Dietary, toxicity, two years, rat NOAEL = 1.2 mg/kg bw; fatty changes
in liver
References
Althaus, W.A. (1985a) Characterization and identification of
radioactivity in urine and feces of Wistar rats given single oral
doses mixed-labeled 14C fenarimol. Unpublished report No.
ABC-0310 dated December 1985 from Lilly Research Laboratories,
USA. Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Althaus, W.A. (1985b) Characterization and identification of
radioactivity in blood, liver and kidney of Wistar rats given
single oral doses of mixed-labelled 14C fenarimol. Unpublished
report No. ABC-0321 dated December 1985 from Lily Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Bagatell, C.J., Heiman, J.R., Rivier, J.E. & Bremmer, W.J. (1994)
Effects of endogenous testosterone and oestradiol on sexual
behaviour in normal young men. J. Clin. Endocrinol. Metab., 78,
711-716.
Black, L.J. (1981a) Evaluation of the estrogenic and antiestrogenic
potential of EL-222 in the immature rat uterus. Unpublished
report dated September 1981 from Lilly Research Laboratories,
USA. Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Black, L.J. (1981b) Evaluation of the progestational or
antiprogestational potential of EL-222 in immature rabbits.
Unpublished report dated September 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Breedlove, S.M. (1994) Sexual differentiation of the human nervous
system. Ann. Rev. Psychol., 45, 389-418.
Chwalisz, K. (1994) The use of progesterone antagonists for cervical
ripening and as an adjunct to labour and delivery. Hum,
Reprod., 9 (Suppl. 1), 131-161.
Goebel, G.V. (1985) Characterization of radioactivity in bile from
rats dosed orally with 14C fenarimol. Unpublished report No.
ABC-0312 dated May 1985 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Gooren, L.J.G. (1985). Human male sexual functions do not require
aromatisation of testosterone: A study using tamoxifen,
testolactone, and dihydrotesterone. Arch. Sex. Behav., 14,
539-548.
Hanlin, M.L. (1981a) Fertility studies with EL-222 in the adult male
rat. Unpublished report dated September 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hanlin, M.L. (1981b) Anti-androgenic studies with EL-222 in the male
rat. Unpublished report dated September 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom
Hoffman, D.G. & Amundson, M. (1985) Initiation and promotion study of
fenarimol on rat liver carcinogenesis. Unpublished report dated
November 1985 from Naylor Dana Institute for Disease Prevention,
Valhalla, New York, USA Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G. & Arthur, B.H. (1980) Guinea pig sensitization study
with Lilly compound 56722 (fenarimol). Unpublished report No.
GP-9538 dated January 1980 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G. & Hirsch, K.S. (1981a) Placental transport of EL-222 in
the Wistar rat. Unpublished report No. R10480 dated October 1981
from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G. & Hirsch, K.S. (1981b) Exposure of the neonatal rat to
EL-222 as a component of milk: Evidence for the presence of
EL-222 in the central nervous system during the organization of
adult sexual behaviour. Unpublished report No R01781 dated
October 1981 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G. & Hirsch, K.S. (1981c) A special fertility study with
EL-222 (fenarimol) in the Wistar rat. Unpublished report No
R07380 dated October 1981 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D G. & Pierce, E.C. (1985) A chronic toxicity/oncogenicity
study in Wistar rats maintained on diets containing low
concentrations of fenarimol for two years. Unpublished report No.
R07781 dated May 1985 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G. & Russel, D.R. (1990) A developmental toxicity study of
fenarimol (EL-222, compound 056722) administered orally to New
Zealand white rabbits. Unpublished report No. B00290 dated
October 1990 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Broddle, W.D., Gibson, W.R. & Morton, D.M. (1975a) The
acute toxicity of EL-222 in mice, rats, dogs, ducks mid quail.
Unpublished report dated August 1975 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Bernhard, N.R., Gibson, W.R., Owen, N.V. & Morton, D.M.
(1975b) A pilot 2-week subacute toxicity study of EL-222 in mice.
Unpublished report No. M-9074, M-9084 dated August 1975 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975c)
Three-month subacute oral toxicity studies on EL-222 in mice.
Unpublished report No. M-9015 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Bernhard, N.R., Gibson, W.R., Harris, P.N. & Morton,
D.M. (1975d) A pilot 2-week subacute toxicity study of EL-222 in
rats. Unpublished report No. R-713, R-723 dated August 1975 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Bernhard, N.R. & Morton, D.M. (1975e) A 2-week subacute
study of the effects of EL-222 on hepatic p-nitroanisole
metabolism in rats. Unpublished report No. R-244, R-254 dated
August 1975 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975f)
Three-month subacute oral toxicity studies on EL-222 in rats.
Unpublished report No. R-1013 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975g)
Three-month subacute oral toxicity studies on EL-222 in rats.
Unpublished report No. R-1164 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975h)
Three-month subacute oral toxicity studies on EL-222 in dogs.
Unpublished report No. D-4183 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Arthur, B.H., Gibson, W.R. & Morton, D.M. (1977a) The
acute dermal and ocular toxicity of technical EL-222 in rabbits.
Unpublished report dated January 1977 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Adams, E.R., Markham, J.K., Owen, N.V., Gibson, W.R. &
Morton, D.M. (1977b) A multi-generation reproduction study with
EL-222 in the rat. Unpublished report No. R-715, R-1345, R-956,
R-966 dated November 1977 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Markham, J.K., Adams, E.R., Owen, N.V & Morton, D.M.
(1977c) A teratology study on compound 56722 (EL-222) in the
rabbit. Unpublished report No. B-7125 dated January 1977 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Markham, J.K., Owen, N.V. & Morton, D.M. (1977d) A
dominant lethal study of compound 56722 (EL-222) in the rat.
Unpublished report dated January 1977 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G. et al. (1978a) Twelve-month chronic oral toxicity of
EL-222 (56722) in mice. Unpublished report No. M-9155 dated April
1978 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C., Harris, P.N. & Morton, D.M.
(1978b) Twenty-four month chronic oral toxicity of EL-222 (56722)
in mice. Unpublished report No. M-9135, M-9145, dated August 1978
from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G. et al. (1978c) A one-year toxicity study with EL-222
in the rat. Unpublished report No. R-715 dated June 1978 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C. & Morton, D.M. (1978d)
Eighteen-month chronic oral toxicity of EL-222 (56722) in rats.
Unpublished report No. R-435 dated May 1978 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C. & Morton, D.M. (1978e)
Twenty-four month chronic oral toxicity of EL-222 (56722) in
rats. Unpublished report No. R-405, R-415, dated April 1978 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gomez, S.R. & Dorato, M.A. (1980a) The acute inhalation
toxicity of Lilly compound 56772, fenarimol, in the Fischer 344
rat. Unpublished report No. R-H-99-80 dated December 1980 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V. & Markham, J.K. (1980b) A cross-over
reproduction study with EL-222 (Lilly compound 56722) in the rat.
Unpublished report No. R-286 dated April 1980 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V. & Markham, J.K. (1980c) Additional
cross-over reproduction studies with EL-222 (Lilly compound
56722) in the Wistar and the Sprague-Dawley rat. Unpublished
report No. R-57, R-67 dated April 1980 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V & Adams, E.R. (1980d) A cross-over fertility
study with EL-222 in the Wistar rat. Unpublished report No.
R08379 dated June 1980 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Adams, E.R. & Owen, N.V. (1980e) A cross-over fertility
study with EL-222 in the Dutch-belted rabbit. Unpublished report
No. B7229, dated June 1980 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Owen, N.V. & Adams, E.R. (1980f) A teratology study
with EL-222 in the rat. Unpublished report No. R06279 dated
January 1980 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Hirsch, K.S. & MacLusky, N.J. (1981a) Effects of EL-222
on the binding of androgens to cytoplasmic and nuclear androgen
receptors. Unpublished report dated October 1981 from Yale
University Medical School Connecticut, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Hirsch, K.S. & MacLusky, N.J. (1981b) Effects of EL-222
on circulating estrogens and the binding of estrogens to estrogen
receptors in the rat central nervous system. Unpublished report
dated October 1981 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Hirsch, K.S. & MacLusky, N.J. (1981c) Effects of EL-222
on the concentration of estrogen receptors and the conversion of
testosterone to estrogens in the hypothalamus. Unpublished report
dated October 1981 from Yale University Medical School,
Connecticut, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C. & Morton, D.M. (1982a) A
low-dose chronic toxicity/oncogenicity study in Wistar rats
maintained on diets containing fenarimol for two years.
Unpublished report No. R06479 dated November 1982 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Brodie, A.M.H. & Hirsch, K.S. (1982b) An in vitro
measure of aromatase inhibition by EL-222. Unpublished report
dated January 1982 from the University of Maryland, Baltimore,
Maryland, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Markham, J.K & Miller, B.J. (1983a) A two-generation
reproduction study with fenarimol (EL-222, compound 56722) in
guinea pigs. Unpublished report No. G00682, G00483 dated December
1983 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V & Byrd, R.A. (1983b) The effect of prenatal
fenarimol (EL-222, compound 56722) exposure on kidney development
and maturation in the rat. Unpublished report No. R10682, R10782,
R10882 dated November 1983 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Masten, J.L. & Hanasono, G.K (1985a) The biliary
excretion of radioactivity by male and female Wistar rats given
single oral doses of 14C-fenarimol (compound 56722, EL-222).
Unpublished report No. R02485 dated April 1985 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Masten, J.L & Hanasono, G.K. (1985b) Tissue
distribution of radioactivity in Wistar rats determined one or
twenty-four hours after single oral doses of 14C-fenarimol
(EL-222, compound 56722). Unpublished report No. R16684 dated
April 1985 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Masten, J.L., Walker, C.G. & Hanasono, G.K. (1985c)
Radiocarbon disposition studies on Wistar rats given single
oral doses of 14C-fenarimol (EL-222, compound 56722):
Pharmacokinetics, and excretion/tissue distribution seven days
after dosing. Unpublished report No. R06484, R08584 dated March
1985 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Weaver, D.E. & Van Lier, R.B.L. (1985d) Distribution of
radioactivity into tissues and organs from Wistar rats given oral
doses of unlabeled fenarimol daily for two weeks followed by a
single dose of 14C-fenarimol (EL-222, compound 56722).
Unpublished report No. R01285 dated March 1985 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Van Lier, R.B.L. & Weaver, D.E. (1985e) The
percutaneous absorption of 14C fenarimol dissolved in ethanol
in rhesus monkeys. Unpublished report No. P04085, P04585 dated
October 1985 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Pierce, E.C., Brown, G.E. & Negliski, S. (1985f)
Subchronic (21-day) dermal toxicity study in rabbits with
technical fenarimol (EL-222, compound 56722) and Rubigan 50W, a
wettable powder formulation (FN-0742) containing 50% fenarimol.
Unpublished report No. B02183 dated May 1985 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Gries, C.L., Pierce, E.C., Means, J.R. & White, J.F.
(1985g) A one year chronic oral toxicity study of fenarimol in
dogs with a three month recovery period. Unpublished report No.
D02683 dated May 1985 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Richardson, K.A. & Kokkino, A.J. (1988a) The effect of
fenarimol (EL-222, compound 56722) on the induction of reverse
mutations in Salmonella typhimurium and Escherichia coli using
the Ames test. Unpublished report No. 880215AMT4, 880222AMS4,
dated August 1988 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Oberly, T.J. & Richardson, K.A. (1988b) The effect of
fenarimol on the induction of forward mutation at the thymidine
kinase locus of L51787 mouse lymphoma cells. Unpublished report
No. 880106MLT4, 880113MLA4, dated August 1988 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Ivett, J.L. (1988) Mutagenicity test on 56722 (EL-222) in the in vivo
rat micronucleus assay. Unpublished report No. 10348-0-455 dated
October 1988 from Hazleton, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Jones, J.R. (1994a) Fenarimol technical: Acute dermal irritation test
in the rabbit. Unpublished report No. 291/50 dated February 1994
from Safepharm Laboratories, United Kingdom. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Jones, J.R. (1994b) Fenarimol technical: Acute eye irritation test in
the rabbit. Unpublished report No. 291/51 dated February 1994
from Safepharm Laboratories, United Kingdom. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Luisi, M. & Franchi, R. (1980) Double blind group comparative study of
testosterone undecanoate and mesterolone in hypogonadal male
patients. J. Endocrinol. Invest., 3, 305-308.
Mantzoros, C.S., Georgiadis, E.I. & Trichopoulos, D. (1995)
Contribution of dihydrotestosterone to male sexual behaviour.
Br. Med. J., 310, 1289-1291.
Markham, J.K., Hoffman, D.G., Adams, E.R., Owen, N.V. & Morton, D.M.
(1978a) Pilot reproduction studies with EL-222 (Lilly compound
56722) in the mouse. Unpublished report No. M-9165, M-9215 dated
July 1978 from Lilly Research Laboratories, USA. Submitted to WHO
by DowElanco Europe, Wantage, Oxon, United Kingdom.
Markham, J.K., Hoffman, D.G., Broddle, W.D., Owen, N.V. & Morton, D.M.
(1978b) A multi-generation study with EL-222 (Lilly compound
56722) in the mouse. Unpublished report No. M-9086, M-9296,
M-9326 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Markham, J.K., Hoffman, D.G., Owen, N.V. & Morton, D.M. (1978c) A
second multi-generation reproduction study with EL-222 (Lilly
compound 56722) in the rat. Unpublished report No. R-636, R-1076,
R-217 dated July 1978 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Murli, H. (1988) Mutagenicity test on 56722 in an in vitro cytogenetic
assay measuring chromosomal aberration frequencies in cultured
purified human lymphocytes. Unpublished report No. 10348-0-449
dated August 1988 from Hazelton, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Naftolin, F., Keefe, D., Apa, R., Palumbo, A. & Garcia-Segura, L.M.
(1991) The apparent paradox of sexual differentiation of the
brain. Contrib. Gynecol. Obstet., 18, 24-32.
Neubauer, B.L. et al. (1982) Effect of EL-222 (compound 56722,
fenarimol) on the developing reproductive tract. Unpublished
report dated January 1982 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Probst, G.S. (1979) The effect of Lilly compound 56722 (EL-222) on the
induction of DNA repair synthesis in primary cultures of adult
rat hepatocytes. Unpublished report No. 790502-1 dated June 1979
from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Prout, M. (1994) The disposition of 14C fenarimol in the lactating
goat. Unpublished report No. IRI 154147 dated October 1994 from
Inveresk Research International, Musselburgh, United Kingdom.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Rodricks, J.V. et al. (1989) Response to USSR concerns regarding the
potential oncogenicity of fenarimol. Unpublished report dated
February 1989 from Environ Corp., Washington DC, USA. Submitted
to WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Simpson, E.R., Mahendroo, M.S., Means, G.D., Kilgore, M.W.,
Hinshelwood, M.M., Graham-Lorence, S., Amarneh, B., Ito, Y.,
Fisher, C.R., Dodson, M.M., Mendelson, C.R & Bulun, S. (1994)
Aromatase cytochrome P450, the enzyme responsible for oestrogen
biosynthesis. Endocr. Rev., 15, 342-355.
Siou, G. & Lerond-Conan, L. (1982) Test for mutagenic potential of
technical-grade fenarimol by examination of chromosomal damage in
the Chinese hamster. Unpublished report No. 658 dated June 1982
from C.E.R.T.I., Versailles, France. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Siou, G., Lerond-Conan, L. et al (1982) Test for mutagenicity of
technical-grade fenarimol using a micronucleus technique in the
mouse. Unpublished report No. 650 dated May 1982 from C.E.R.T.I.,
Versailles, France. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Smalstig, E.B. (1981a) Study of the possible influence of compound
EL-222 on fertility in the adult female rat. Unpublished report
dated September 1981 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Smalstig, E.B. (1981b) Studies of the influence of EL-222 on uterine
prostaglandin F2alpha in the female rat at parturition.
Unpublished report dated November 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Smith, E.P., Boyd, J., Frank, G., Takahashi, H., Cohen, R.M., Specker,
B., Williams, T.C., Luhbahn, D.B. & Korach, K.S. (1994) Oestrogen
resistance caused by a mutation in the oestrogen-receptor gene in
a man. New Engl. J. Med., 331, 1056-1061.
Tinsley, F.C. (1982) Preparturiton progesterone levels in fenarimol
(EL-222) treated rats. Unpublished report dated August 1982 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Watson, D. (1994) Accurate measurement of an isolated metabolite of
fenarimol. Unpublished report No. DWC/719 dated October 1994 from
Huntingdon Research Centre, United Kingdom. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
 found, the main one being metabolite K. In contrast, most of the
radiolabel in the faeces was present as free compounds, indicating
that biliary metabolites undergo further metabolism or hydrolysis
before being excreted (Goebel, 1985).
The tissue distribution of fenarimol in Wistar rats was
determined 1 and 24 h after administration of 1 or 13 mg/kg bw
14C-fenarimol. The main radiolabelled constituents identified in
blood and kidney after 1 h were unchanged fenarimol and metabolite I.
The amount of fenarimol increased with dose, from 19 to 40% of the
total radioactivity in blood in males and from 50 to 73% in females.
Fenarimol also accounted for most of the radiolabel in the liver
(67-90%), whereas metabolite K represented 3-6%. Females had more
unchanged fenarimol residues than males, indicating more extensive
metabolism in males. Furthermore, metabolism was reduced at the higher
dose, suggesting saturation (Althaus, 1985b).
(c) Other metabolic studies
Some barrier to placental transport of fenarimol-derived
radiolabel was found in rats on days 14-17 of gestation but virtually
no barrier on day 18 (Hoffman & Hirsch, 1981a). Fenarimol-derived
radiolabel was excreted in milk and subsequently concentrated in the
hypothalamus of neonates. The concentration in milk up to 48 h after
treatment was three to five times greater than that in maternal plasma
(Hoffman & Hirsch, 1981b).
2. Toxicological data
(a) Acute toxicity
The results of studies of acute toxicity are summarized in
Table 1.
(b) Short-term toxicity
Mice
In a pilot study reported with few details, groups of four male
ICR mice were given fenarimol (purity unspecified) in the diet at 0
(10 animals), 25, 50, 100, 200, 400, 800, 1600, 3200, 6400, or
12 800 ppm for 14 days. No effects were observed on mortality or
clinical signs. terminal body weight was reduced at the highest dose.
In the liver (the only organ investigated), dose-related increases in
relative weight and in the rate of hepatic metabolism of para-
nitroanisole were seen at > 800 ppm. There were no effects on liver
pathology or histopathology (Hoffman et al., 1975b).
Table 1. Acute toxicity of fenarimol
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
ICR mouse Male Oral 4508a NR Hoffman et al. (1975a)
Female > 4000a
Wistar-derived Male Oral 2576a NR Hoffman et al. (1975a)
Harlan rat Female 2515a
Albino rabbit Male and Dermal > 2000b NR Hoffman et al. (1977a)
female
Fischer 344 rat Male and Inhalationc > 2.07 97% Hoffman et al. (1980a)
female (1-h)
Wistar-derived FemMe intraperitoneal 533 NR Hoffman et al. (1975a)
Harlan rat
Beagle dogc Male and Oral > 200 NR Hoffman et al. (1975a)
female
NR, not reported
a Signs of toxicity: hypoactivity, leg weakness, loss of righting reflex, ptosis (1-6 h after treatment);
loose stools; diuresis, diarrhoea, debilitation (24 h after treatment); recovery within 8-10 days
b Application on intact and abraded skin; one death, but no signs of toxicity or irritation
c Nose only; nominal concentration, 133 mg/litre; particle size, 12 µm
d Only one male and one female
In a poorly reported study, groups of 10 male and 10 female ICR
mice were fed diets containing 0, 365, 620, 1100, 2000, or 3300 ppm
fenarimol (purity unspecified) for three months, equivalent to 0, 52,
88, 157, 286, or 471 mg/kg bw per day. Food consumption was not
measured, and ophthalmological examinations were not performed. There
were no treatment-related effects on mortality, clinical signs of
toxicity, haematology, or body weight. A statistically significant
reduction in total bilirubin was observed in animals of each sex at
the two highest doses and in males at 1100 ppm. Hepatic para-
nitroanisole metabolism, determined in five animals of each sex in
each group, showed a significant dose-related increase in females at
> 1100 ppm and in males at > 2000 ppm. Relative liver weights
were increased in a dose-related manner in males at > 365 ppm (10%
increase) and in females at > 620 ppm (11% increase) and reached
statistical significance at 620 and 1100 ppm, respectively. Absolute
liver weights were increased at > 620 ppm. The main effects seen on
histopathological examination of tissues other than nervous tissue
were centrilobular hypertrophy and hepatomegaly in the livers of males
at 1100 ppm and 2000 ppm, respectively, and in females at the highest
dose. A shift from a diffuse distribution of fat droplets to a more
periportal distribution was observed in animals of each sex at
> 2000 ppm. In kidneys, a higher incidence of fat droplets was
observed in females at > 2000 ppm. The NOAEL was 365 ppm,
equivalent to 52 mg/kg bw per day (Hoffman et al., 1975c).
Rats
In a pilot study reported in little detail, four male and four
female male Wistar rats were fed diets containing fenarimol (purity
unspecified) at 0 (10 animals per group), 25, 50, 100, 200, 400, 800,
1600, 3200, 6400, or 12 800 ppm for 14 days. Three animals at the high
dose died. Signs of toxicity, including anorexia, piloerection,
ataxia, and tremors, and decreased body weight and food consumption
were seen at doses > 3200 ppm. In the liver, the only organ
investigated, there were dose-related increases in relative weight at
> 400 ppm and in the rate of hepatic metabolism of para-
nitroanisole at > 200 ppm. Histopathological examination of the
liver revealed centrilobular hypertrophy at > 400 ppm and focal
necrosis at > 6400 ppm; no effects were seen at < 100 ppm
(Hoffman et al., 1975d).
In a subsequent study in male Wistar rats, also poorly reported,
four animals per treatment group and 10 controls were fed diets
containing fenarimol (purity unspecified) at 0, 25, 50, 100, 200, 400,
800, 1600, 3200, or 6400 ppm for 14 days. Food consumption and body
weight were reduced at > 1600 ppm; increased absolute and relative
liver weights were seen at > 800 ppm and increased hepatic
microsomal protein content at > 400 ppm. A dose-related reduction
in glucose-6-phosphatase activity was seen at > 800 ppm. The rate
of hepatic metabolism of para-nitroanisole was increased in a
dose-related fashion at > 200 ppm. No effects on the liver were
seen at < 100 ppm (Hoffman et al., 1975e).
In another poorly reported study, groups of 20 male and 20 female
Wistar rats were fed diets containing 0 (25 animals per sex), 50, 200,
or 800 ppm fenarimol (purity unspecified) for three months, equivalent
to 0, 2.5, 10, or 40 mg/kg bw per day. Five animals of each sex in
each group were kept for a recovery period of two weeks. No
ophthalmological examinations were performed. There were no treatment
-related effects on mortality, clinical signs of toxicity, food
consumption, haematology, or clinical chemistry (only blood urea
nitrogen, glucose, and alanine aminotransferase measured). A slight
depression in body weight was observed in males at the high dose. A
dose-related increase in relative liver weight was observed in animals
of each sex, which was statistically significant at the high dose. The
relative weights of the kidneys of males and females and of the
thyroid of females were increased at the high dose. Hepatic para-
nitroanisole metabolism, determined in five animals of each sex in
each group, showed a significant, dose-related increase in females at
800 ppm and in males at > 200 ppm. The main effect seen on
histopathological examination of tissues other than nervous tissue was
centrilobular hypertrophy in males and a shift from slight to moderate
fatty metamorphosis in females at the high dose. At the end of the
recovery period, only increased liver weight was still observed at the
high dose. The NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw per day
(Hoffman et al., 1975f).
In a study with no recovery period and again with little detail
reported, groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 140, 200, 275, 365, or 500 ppm fenarimol (purity
unspecified) for three months, equivalent to 0, 7, 10, 14, 18, or
25 mg/kg bw per day. There were no treatment-related effects on
mortality, clinical signs of toxicity, body weight, food consumption,
haematology, clinical chemistry, or histopathology (nervous tissue was
not examined). A dose-related increase in relative liver weight was
observed in females at > 275 ppm and in males at 500 ppm. Hepatic
para-nitroanisole metabolism, determined in five animals of each sex
in each group, showed a dose-related increase, which was statistically
significant at > 275 ppm. The NOAEL was 200 ppm, equivalent to
10 mg/kg bw per day (Hoffman et al., 1975g).
Rabbits
A 21-day study of dermal toxicity was conducted in groups of five
male and five female New Zealand white rabbits, which received daily
applications of 0 or 1000 mg/kg bw technical-grade fenarimol (purity,
97%) or 500 or 1000 mg/kg bw of a wettable powder formulation
containing 50% fenarimol for 6 h/day. A further group received
1000 mg/kg bw of the formulation and was then held for a recovery
period of 14 days. Technical-grade fenarimol induced no treatment-
related effect on appearance, behaviour, body weight, food
consumption, ophthalmological parameters, dermal irritation,
haematological or clinical chemical parameters, organ weights, or
gross or histological appearance of tissues. The NOAEL was thus the
highest dose tested, 1000 mg/kg bw per day. The formulation induced no
treatment-related systemic toxicity, but dermal irritation was seen in
both groups, characterized by slight to severe erythema and slight
oedema during the treatment phase. The irritation subsided within nine
days after exposure (Hoffman et al., 1985f).
Dogs
Groups of four male and four female beagle dogs were fed gelatin
capsules containing fenarimol (purity unspecified) at doses of 0,
1.25, 5, or 20 mg/kg bw per day for three months. No treatment-related
effect was seen on appearance, behaviour, body weight, food
consumption, ophthalmological, haematological, or clinical chemical
parameters, urinalysis, organ weights, hepatic para-nitroanisole
metabolism, or gross or histological appearance of tissues (excluding
nervous tissue). The NOAEL was thus the highest dose tested, 20 mg/kg
bw per day (Hoffman et al., 1975h).
Groups of six male and six female beagle dogs were given gelatin
capsules containing fenarimol (purity, 96.7%) at doses of 0, 1.25,
12.5, or 125 mg/kg bw per day for one year. Two dogs of each sex in
each group were maintained for a recovery period of three months. No
treatment-related effect was seen on mortality, body weight, food
consumption, or ophthalmological, haematological, or urinary
parameters. One, five, seven, and 12 dogs in the four groups had
soft, runny, or mucoid stools on two, 10, 21, and 34 occasions,
respectively. Transient enlargement of the mammary glands of female
dogs was seen in all groups, and there was a slight treatment-related
increase in incidence and duration in four animals in the group at the
highest dose, lasting for up to 46 days. At the high dose, increased
alkaline phosphatase and liver para-nitroanisole O-demethylase
activities and increased absolute and relative liver weights were
observed in animals of each sex; a trend to increased relative ovarian
weight was also observed at this dose. These increases were still seen
after recovery. The only treatment-related histopathological finding
was mild hepatic bile stasis in one female in the treatment phase and
in two males after the recovery period. The NOAEL was 12.5 mg/kg bw
per day (Hoffman et al., 1985g).
(c) Long-term toxicity and carcinogenicity
In many of the studies described below, increased blood glucose
was found. Although apparent dose-dependent increases were observed in
several studies, the end-point (measured only at termination) was very
variable and inconsistent, usually with flat dose-response curves. No
clear explanation could be found for these apparent increases. The
effect was considered to be compound-related but toxicologically
insignificant and was not taken into account in establishing the NOAEL
for each study.
Mice
Groups of 20 male and 20 female ICR mice (30 of each sex in the
control group) were fed diets containing 0, 50, 170, or 600 ppm
fenarimol (purity, 97%), equivalent to 0, 7.1, 24.3, or 85.7 mg/kg bw
per day, for 12 months. Food consumption was not measured, urinalysis
was not performed, and haematology and clinical chemistry were
determined only at termination. There were no treatment-related
effects on mortality, clinical signs of toxicity, or haematology.
Body-weight gain of males at 600 ppm was slightly decreased. A
dose-related increase in blood glucose levels was observed at
> 50 ppm, which was statistically significant at 600 ppm in males
and at 170 ppm in females. A significant increase in serum creatinine
concentration was seen in females at 170 and 600 ppm. The activity of
alanine aminotransferase was increased in males at the high dose. The
absolute and relative liver and spleen weights were increased in males
at 600 ppm, and relative liver weight was increased in females at
600 ppm. Absolute and relative uterine weights were decreased at this
dose. A slight, dose-related increase in the incidence of very slight
or slight fatty change in the liver was observed, reaching 10-20% at
600 ppm (0% in controls). The NOAEL was 170 ppm, equivalent to
24.3 mg/kg bw per day (Hoffman et al., 1978a).
In two replicate studies, groups of 40 male and 40 female ICR
mice (60 animals of each sex in the control group) were fed diets
containing 0, 50, 170, or 600 ppm fenarimol (purity, 97.9%),
equivalent to 0, 7.1, 24.3, or 85.7 mg/kg bw per day, for two years.
Food consumption was not measured, and haematology and clinical
chemistry were carried out only at termination. Since the results of
the replicate studies were similar, they were reported as a single
study. There were no treatment-related effects on mortality, clinical
signs of toxicity, or haematological or clinical chemical parameters.
Body-weight gain of males at 600 ppm was slightly decreased. A
tendency towards increased relative liver weights and a slightly
increased incidence of hepatic fatty change (2.5-6%; 1-2% in controls)
were observed in animals of each sex at the high dose. The tumour
incidence was not enhanced. Although the laboratory considered that
there were no significant effects in these studies, the slight
reduction in body weight suggests minimal toxicity. The NOAEL was thus
170 ppm, equivalent to 24.3 mg/kg bw per day (Hoffman et al.,
1978b).
Rats
Wistar rats of the first parental generation in a multi-
generation study of reproduction {see Hoffman et al., 1977b) were
maintained on treated diets for another three months after the end of
the study and thereby received the diets for a total of one year.
Groups of 20 male and 20 female rats (30 of each sex in the control
group) received doses of 0, 50, 130, or 350 ppm fenarimol (purity,
97.9%), equivalent to 0, 2.5, 6.5, or 17.5 mg/kg bw per day.
Haematology and clinical chemistry were evaluated only at termination;
no urinalysis was performed, and nervous tissue was not examined at
histopathology. There were no treatment-related effects on mortality,
clinical signs of toxicity, or body weight. The limited data available
on food consumption (intake during weeks 1, 8, 42, and 54 only)
indicated no effects. Males at the high dose had increased erythrocyte
counts and and decreased leukocyte counts. Blood glucose levels were
elevated in males at all doses (not dose-related but highest at the
high dose) and in females at 130 and 350 ppm. A dose-related reduction
in blood urea nitrogen was observed in males at > 50 ppm. Absolute
and relative liver weights and relative kidney weight were increased
in females, and absolute and relative spleen weights were decreased in
males at the high dose. Slight atrophy of the acini of the pancreas
was observed more frequently at the high dose, predominantly in males.
The effects on clinical chemical parameters are not considered to be
adverse. The NOAEL was thus 130 ppm, equivalent to 6.5 mg/kg bw per
day (Hoffman et al., 1978c).
Groups of 20 male and 20 female Wistar rats (30 of each sex in
the control group) received diets containing 0, 50, 130, or 350 ppm
fenarimol (purity, 97.9%) for 18 months, equal to 0, 2.3, 6.0, or
16 mg/kg bw for males and 0, 3.6, 8.4, or 22.9 mg/kg bw per day for
females. Urinalysis and ophthalmological examinations were not
performed, and haematology and clinical chemistry were evaluated only
at termination. There were no treatment-related effects on mortality
(survival was a slightly higher at the highest dose), food
consumption, clinical signs of toxicity, or clinical chemistry. A
reduction in growth was seen consistently in all treated groups; on
several occasions, 10% reductions in body weight were seen at 130 and
350 ppm. At 350 ppm, a slight decrease in leukocyte count was observed
in males and in prothrombin time in females. A dose-related decrease
in serum prolactin levels was observed in females, but it was not
statistically significant, and the individual levels within the groups
were very variable. Relative liver weight was increased in all treated
groups, but the reduction was statistically significant only at
350 ppm. At this dose, absolute and relative ovarian weights were
increased, and absolute spleen weights were reduced in males. A slight
increase in the incidence of a fatty liver was seen in all treated
males (60-70%; 43% in controls), and an increase in the severity of
fatty changes in liver was observed in females at the high dose. The
NOAEL was 50 ppm, equal to 2.3 mg/kg bw for males and 3.6 mg/kg bw per
day for females (Hoffman et al., 1978d).
In two replicate studies, groups of 40 male and 40 female Wistar
rats (60 animals of each sex in the control group) were fed diets
containing 0, 50, 130, or 350 ppm fenarimol (purity, 97.9%), equal to
0, 2.0, 5.3, or 14.6 mg/kg bw for males and 0, 2.8, 7.6, or 21.5 mg/kg
bw for females, for 24 months. Haematology and clinical chemistry were
evaluated only at termination. Since the results of the two replicate
studies were similar, they were reported as a single study. There was
a dose-related increase in the rate of survival, increasing in males
from 27% (controls) to 44% (high dose) and in females from 38 to 54%;
however, the rate was not sufficient to meet the criteria for a valid
negative result in a study of carcinogenicity. There were no
treatment-related effects on clinical signs of toxicity or organ
weights. Body-weight gain of males was slightly reduced at 350 ppm
during the first nine months of the study. The leukocyte count was
decreased in animals of each sex at the high dose. Dose-related
increases in blood glucose levels were seen at all doses, which were
statistically significant in males at the high dose and in one study
at > 50 ppm in females. In one study, a dose-related reduction in
mean serum prolactin values was seen in females at > 50 ppm, which
reached statistical significance at 350 ppm; however, individual
values were very variable, with similar ranges in all groups. Males
also showed a statistically significant increase in prolactin levels
at 350 ppm, and females had increased levels of luteinizing hormone.
Although hormone levels were investigated to elucidate the effects of
fenarimol on reproduction, the laboratory considered the toxicological
significance of the observed changes to be unknown. An increased
incidence of fatty changes in the liver, which tended to be dose-
related, was seen especially in males; the overall incidences in males
and females in the two studies were 29, 40, 43, and 53% at 0, 50, 130,
and 350 ppm. An increase in severity was observed in animals of each
sex at the high dose. The incidence of hyperplastic nodules was
slightly increased: 0% in controls, 1.9% at 50 ppm, 1.9% at 130 ppm,
and 4.4% at 350 ppm, and reached statistical significance at the high
dose. In addition, hepatic-cell adenomas were seen in 0.6% of rats at
130 ppm and in 3.2% at 350 ppm (statistically significant). There was
no NOAEL, owing to the slight effects on the liver at the low dose
(Hoffman et al., 1978e).
A further two-year carcinogenicity study was conducted in order
to establish a no-effect level for the effects on the liver observed
in the previous study. Groups of 50 male and 50 female Wistar rats
were fed diets containing 0, 12.5, 25, or 50 ppm fenarimol (purity,
96.7%), equal to 0, 0.6, 1.2, or 2.5 mg/kg bw for males and 0, 0.7,
1.5, or 3.0 mg/kg bw per day for females, for 24 months. Haematology
and clinical chemistry were evaluated only at termination, and the
liver was the only organ examined at necropsy. The survival rate was
low due to an outbreak of chronic respiratory disease during the 16th
and 17th months: only 10-32% per sex per group survived for two years
There were no treatment-related effects on clinical signs of toxicity,
haematology, or liver weight. Reductions in body-weight gain, food
consumption, and food efficiency were observed in males at the high
dose during the first year of the study. The glucose content of blood
was increased in animals of each sex at 50 ppm. The incidence of fatty
liver was increased at the high dose, especially in males (60%; 26% in
controls). There was no evidence of an increase in tumour incidence,
but the survival rate was not sufficient to meet the criteria for a
valid negative result in a study of carcinogenicity. The NOAEL was
25 ppm, equal to 1.2 and 1.5 mg/kg bw per day for males and females,
respectively (Hoffman et al., 1982a).
Groups of 50 male and 50 female Wistar rats were fed fenarimol
(purity, 97%) at 0, 12.5, 25, or 50 ppm in their diet, equal to 0,
0.5, 1.0, or 2.0 mg/kg bw for males and 0, 0.6, 1.2, or 2.3 mg/kg bw
per day for females, for 24 months. Haematology and clinical chemistry
were evaluated only at termination, and the liver was the only organ
weighed. There was no treatment-related effect on survival rates
(29-44% for males and 56-74% for females), body-weight gain, food
consumption, food efficiency, haematology, hepatic para-nitroanisole
metabolism, liver weight, or histopathology of any organ. Slight
increases in the incidences of low body weight and pale eyes were
noted in all treated males. Blood glucose levels were increased at
25 ppm in males and at 50 ppm in animals of each sex. The tumour
incidence was not increased. The effects seen were considered not to
be adverse. The NOAEL was therefore 50 ppm, the highest dose tested,
equal to 2.0 and 2.3 mg/kg bw per day for males and females,
respectively (Hoffman & Pierce, 1985).
In a further analysis of the data on carcinogenicity from the
study conducted in 1986, the incidences of hepatocellular adenoma and
carcinoma at the high dose in the replicate study and at the low dose
in the last study were combined and analysed for trend. There was no
statistically significant evidence that fenarimol is oncogenic
(Rodricks et al., 1989).
In a study conducted to assess the initiating activity of
fenarimol, groups of six or 12 male Fischer 344 rats were fed 350 ppm
fenarimol (purity unspecified) in the diet for eight or 20 weeks;
one group fed fenarimol for eight weeks was subsequently fed
phenobarbital for 12 weeks. In order to investigate promotion
potential, hepatocellular foci were induced by exposure to N-2-
fluorenylacetamide for eight weeks, and then rats were fed 350 ppm
fenarimol for 12 weeks. Livers were examined after eight or 20 weeks
for the presence of altered hepatocellular foci that were iron-
excluding or contained gamma-glutamyl transpeptidase. Fenarimol had no
initiating or promoting activity in rat liver (Hoffman & Amundson,
1985).
(d) Reproductive toxicity
Mice
In two pilot studies, groups of 10 male and 10 female ICR mice
were fed fenarimol (purity, 97.9%) in the diet. In the first study,
treatment with doses of 50, 170, or 600 ppm was started one week
before mating and was continued up to day 21 of lactation. In the
second study, treatment with doses of 0, 170, 350, or 600 ppm was
started two weeks before mating and continued up to day 14 of
lactation. One female at 600 ppm died and one was killed with signs of
dystocia. No effect on body weight was detected. Fertility was clearly
reduced (and fewer females had copulatory plugs) at 350 and 600 ppm in
the second study only, and there was evidence of reduced fertility at
170 ppm. Liveborn litter size was reduced at 350 and 600 ppm,
apparently as a result of an increased incidence of stillbirths. A
lengthened gestation period was observed at 600 ppm No NOAEL could be
established owing to the limited study design (Markham et al.,
1978a).
In a three-generation study, groups of 20 male and 20 female ICR
mice (30 of each sex in the control group) were fed fenarimol (purity,
97.9%) at dietary levels of 0, 35, 70, or 140 ppm, equivalent to 0, 5,
10, or 20 mg/kg bw per day, starting 61-63 days before mating. Food
consumption was not measured, and the surviving parental animals were
not necropsied. No compound-related effects were observed in the
parents, on reproductive parameters, or on the pups. The NOAEL was
140 ppm, equivalent to 20 mg/kg bw per day (Markham et al., 1978b).
Rats
In a two-generation study, groups of 20 male and 20 female Wistar
rats (30 of each sex in the control group) were fed fenarimol (purity,
97.9%) in the diet at concentrations of 0, 50, 130, or 350 ppm,
equivalent to 0, 12.5, 6.5, or 17.5 mg/kg bw per day. Food consumption
was not measured. F0 rats were exposed for 55 days before the first
of three matings; after the third mating, the animals were maintained
on the test diets for another three months and then evaluated for
long-term toxicity (see Hoffman et al., 1978c). The F1b offspring
were destined to become F1 parents. The F1 animals were exposed to
the test diets for 58 days before the first mating. After weaning, the
F1 parents were placed on control diet for 63 days before a second
mating (reversibility test). For the third mating, a reversibility and
cross-over experiment was performed: the F1 animals continued to be
exposed to control diets, for a total of about five months by the time
of mating. F1 males were mated with untreated virgin females and
F1 females with untreated males. In each of the three F1
breedings, one female at 350 ppm died, perhaps due to dystocia. The
F1 males at 130 and 350 ppm had decreased body-weight gain. There
was a dose-related reduction in fertility (proportion of pregnant
females) at 130 and 350 ppm, and the effect was time-dependent, being
more pronounced after each successive F0 mating and even more
pronounced after the first F1 mating, in which fertility was 20 and
0%, respectively, at 130 and 350 ppm. There was some evidence of
reduced fertility at 50 ppm, but the effects were not statistically
significant. After the F1 parent animals had been fed the control
diet, fertility was 45 and 10%, respectively. Cross-over analysis
showed clearly that the reduced fertility was male-mediated, and there
was some, inconclusive evidence that it might also be female-mediated:
the fertility of treated females was lowered at all doses. The control
value for untreated females was, however, low, and age may have been a
factor since the females were over 10 months old A reduction in
liveborn litter size was seen at 350 ppm after the F0 matings,
probably due to an increase in the proportion of stillborn pups. In
all treated groups, but especially at 350 ppm, there were more females
with a lengthened gestation period (23-24 days) than among controls.
There was no consistent, substantive effect on the survival rate or
body weight of the progeny. Gross necropsy of weanlings revealed an
increased incidence (14.2% compared to 5.2% in controls; not
statistically significant) of hydronephrosis at 350 ppm after the
third mating of the F0 generation. No NOAEL could be established for
reproductive effects, because of the slight evidence of reduced
fertility at the lowest dose. The NOAEL for general systemic toxicity
in parental animals was 50 ppm, equivalent to 2.5 mg/kg bw per day
(Hoffman et al., 1977b).
In a further three-generation study, groups of 20 male and 20
female Wistar rats (30 of each sex in the control group) were fed
fenarimol (purity, 97.9%) in the diet at concentrations of 0, 12.5,
25, or 50 ppm, equivalent to 0, 0.625, 1.25, or 2.5 mg/kg bw per day.
Exposure started 56-71 days before the first mating, and three
generations of one, two, and one nests were bred. The surviving
parental animals were not necropsied. The death of one female during
parturition and signs of haemorrhage in another female during
parturition (signs of dystocia), both at 50 ppm, were attributed to
treatment. Body-weight gain was slightly reduced during the premating
period in F1 males at 50 ppm. After both F1 mating periods,
fertility was clearly reduced at 50 ppm, together with a reduction in
the proportion of females with copulatory plugs, and there was some
evidence of a reduction in fertility after the F2 mating at 50 ppm. A
statistically significant, dose-related reduction in liveborn litter
size (apparently due to an increase in stillbirths) at 25 and 50 ppm
after the second F1 mating was not considered to be related to
treatment by the laboratory; however, it occurred only after a long
exposure and has been seen to be a clear result of shorter exposure to
a higher concentration of fenarimol (see Hoffman et al., 1977b). The
NOAEL for reproductive effects was 12.5 ppm, equivalent to 0.625 mg/kg
bw per day. The NOAEL for general systemic toxicity was 25 ppm,
equivalent to 1.25 mg/kg bw per day (Markham et al., 1978c).
A single-generation study of cross-over design was conducted in
groups of 10 male and 10 female Wistar rats fed fenarimol (purity,
97.9%) by gavage at a dose of 0 or 35 mg/kg bw per day, starting one
month before mating and continuing up to one month thereafter for
males and up to day 20 post partum for females. In two groups,
control males were mated with control and treated females, and in the
third group treated males were mated with control females. The
fertility of treated males was reduced, together with a reduction in
the proportion of females with copulatory plugs, and the liveborn
litter size of treated females was reduced, apparently due to an
increased incidence of stillbirths. There was no NOAEL (Hoffman
et al., 1980b).
Groups of 15 male and 15 female Wistar and Sprague-Dawley rats
were compared with regard to their susceptibility to reproductive
effects under identical conditions in a cross-over study similar to
that described above (Hoffman et al., 1980e), except that the serum
levels of luteinizing hormone and prolactin were also investigated. In
both strains, body-weight loss and dystocia were observed during the
first week of treatment. Fertility was reduced in treated males, as
was the number of females with copulatory plugs. The mean liveborn
litter size was reduced in Sprague-Dawley rats only, due to an
increased incidence of stillbirths. Post-partum survival was reduced
in treated females of both strains. The serum levels of luteinizing
hormone were increased and those of prolactin decreased in treated
females of both strains. There was no NOAEL (Hoffman et al., 1980c).
In another single-generation study of cross-over design, groups
of 10 male and 10 female Wistar rats were fed fenarimol (purity,
97.9%) by gavage at 0 or 35 mg/kg bw per day, starting one month
before mating up to one month thereafter, encompassing gestation and
lactation. In two groups, control males were mated with control and
treated females, and in the third group treated males were mated with
control females. The serum levels of prolactin, luteinizing hormone,
and testosterone and para-nitroanisole- O-demethylase activity were
measured. Two treated females died due to dystocia. Fertility was
reduced in treated males, and the proportion of females with
copulatory plugs and vaginal sperm was reduced. In all cases in which
there was evidence of mating, it was not delayed, and the females
became pregnant. The liveborn litter size of treated females was
reduced, apparently due to an increased incidence of stillbirths; in
addition, gestation length was increased, and progeny survival through
day 7 post partum was slightly reduced. Absolute and relative liver
weights and liver enzyme activity were increased in treated males
and females; treated males also had hypertrophy of centrilobular
hepatocytes. There was no NOAEL (Hoffman et al., 1980d).
Guinea-pigs
In a two-generation study., groups of 15 male and 30 female
guinea-pigs of the F0 generation and 20 males and 20 females of the
F1 parent generation were fed fenarimol (purity, 98.5%) at dietary
levels of 0 or 400 ppm, starting four weeks before mating for the F0
parents and 70 days before mating for the F1 parents. There were
no treatment-related effects on mortality or food consumption.
Body-weight gain was reduced in males of both generations and in F0
females during the premating period. There was no effect on
reproductive parameters, except that the proportion of liveborn
progeny was marginally lower in treated animals of each generation.
The laboratory considered this to be a reflection of litter size, i.e.
lower survival in larger litters, which has previously been documented
in guinea-pigs. Although the data presented support this view for the
control groups, it does not do so for the treated groups. Therefore,
400 ppm, equal to 33 mg/kg bw per day for males and 35 mg/kg bw per
day for females, slightly affected gestational survival, although this
is not of clear toxicological importance (Hoffman et al., 1983a).
Rabbits
In a single-generation stud), of cross-over design, groups of
9-11 male and 9-11 female Dutch belted rabbits were fed fenarimol
(purity, 97.9%) by gavage at 0 or 35 mg/kg bw per day, starting one
month before mating and continuing up to one month thereafter for
males and up to day 6 post partum for females. In two groups,
control males were mated with control and treated females, and in the
third group treated males were mated with control females. Prolactin
and testosterone levels were determined, and premating semen volume,
sperm count, and sperm viability were measured. There was no evidence
of systemic toxicity or adverse reproductive effects. The. NOAEL was
the only dose tested, 35 mg/kg bw per day (Hoffman et al., 1980e).
(e) Developmental toxicity
Rats
Groups of 25 pregnant Wistar rats were fed fenarimol (purity,
97.9%) by gavage at 0, 5, 13, or 35 mg/kg bw per day during days 6-15
of gestation. None of the dams died, and there were no signs of
toxicity and no effects on food consumption or body weight. The only
finding in fetuses was an increased incidence of hydronephrosis at the
high dose (in 30% of fetuses and 62% of litters, in comparison with 9%
of fetuses and 25% of litters in controls). The NOAEL for maternal
toxicity was 35 mg/ kg bw per day and that for embryo- and
fetotoxicity, 13 mg/kg bw per day (Hoffman et al., 1980f).
A special study was conducted to investigate the reversibility of
fenarimol-induced hydronephrosis in neonatal rats. In three replicate
studies, pregnant Wistar rats were fed fenarimol (purity, 99.6%) by
gavage at 0 or 35 mg/kg bw per day during days 6-15 of gestation. Two
groups were sacrificed on day 20 or 21 of gestation, and the others
were allowed to litter and wean their progeny. The normal prenatal and
postnatal examinations were performed, and the kidneys and ureters of
the progeny were examined on days 20 and 21 of gestation and on days
1, 7, 21, 42, and 63 post partum. The total numbers of female rats
examined on gestation days 20 and 21 were 25 and 15, respectively;
postnatally, 15 in the control group and 20 in the test group were
examined. There were no treatment-related effects on dams with regard
to mortality, body weight, or food consumption, but sporadic cases of
dystocia were noted. Prenatal examination revealed a slight decrease
in the proportion of live fetuses due to an increase in early
resorptions. Fetal weights were slightly lower on day 20 but not day
21; on day 20, the number of runt fetuses was also increased.
Hydroureter and hydronephrosis were seen more often among exposed
fetuses on days 20 and 21, and a slightly increased incidence of
skeletal variants, such as cervical ribs and 14 thoracic fibs, was
observed, but only day-20 fetuses were examined. Fetal kidney weights
were depressed by treatment. Postnatal examination revealed a slight
increase in gestational length and reduced liveborn litter size.
Furthermore, neonatal mortality was increased during week 1 post
partum, pup body weights were increased, hypothermia was more
common, and the absolute kidney weights of the progeny were increased.
A reduction in the increased incidence of hydroureter, which was seen
prenatally, was observed with advancing neonatal age, but a
significant increase in hydronephrosis was seen in progeny on day 1
post partum (50% of treated pups and 23% of control pups). At
subsequent examinations, the incidence was still increased but was
substantially less than on day 1 post partum. The slight or moderate
microscopic alterations associated with the observed hydronephrosis
were limited to the renal pelvis and papilla and therefore did not
constitute true pathological hydronephrosis, which is associated with
severe damage, such as glomerular destruction, shortening and blunting
of renal tubules, and reduction or absence of the outer nephrogenic
layer. There was no effect on kidney function, including specific
gravity and osmolality, determined on day 63 post partum. As the
observed hydronephrosis was apparently not a significant pathological
condition and was to at least some degree reversible, together with
the fact that it is a common finding in the Wistar rat, its increased
incidence is considered to be due to delayed development and not a
teratogenic effect (Hoffman et al., 1983b).
Rabbits
Groups of 15 pregnant Dutch-belted rabbits were fed fenarimol
(purity, 97%) by gavage at 0, 3, 10, or 35 mg/kg bw per day on
gestation days 6-18 and were sacrificed on gestation day 28. There
were no treatment-related effects on mortality, body weight, or food
consumption. 4 slight reduction in litter size was observed at 10 and
35 mg/kg bw, but this was not considered to be related to treatment
since the number of implantations and the implantation index were also
reduced in these groups. The occurrence of oedema and various limb and
skeletal defects in five fetuses from one anorectic female at 35 mg/kg
bw was not clearly a direct response to treatment. There was no
evidence of irreversible structural changes. In the absence of clear
evidence of maternal or developmental toxicity, the NOAEL was 35 mg/kg
bw per day, the highest dose tested (Hoffman et al., 1977c).
Groups of 20 female New Zealand white rabbits were fed fenarimol
(purity, 96.6%) by gavage at 0, 15, 50, or 150 mg/kg bw per day on
gestation days 6-18 and were sacrificed on gestation day 28. There
were no treatment-related effects on mortality. Body weight and food
consumption were reduced at 150 mg/kg bw, and four rabbits at the
highest dose lost weight, became anorectic, and then aborted. The
number of live fetuses per litter was reduced, and there was an
increase in the number of early resorptions (neither statistically
significant). The only finding in fetuses was an increased incidence
of extrathoracic ribs (84% of fetuses, compared with 61% in controls).
There was no evidence of irreversible structural changes. The NOAEL
for maternal and embryo- and fetotoxicity was 50 mg/kg bw per day
(Hoffman & Russel, 1990).
(f) Genotoxicity
A number of tests for genotoxicity have been carried out with
fenarimol. The results are summarized in Table 2.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
A single, 4-h, semi-occluded application of 0.5 g technical-grade
fenarimol (purity stated to be 100%) onto the intact skin of three
male New Zealand white rabbits produced no skin irritation (Jones,
1994a).
After instillation of 0.1 ml (equivalent to 56 mg) of technical-
grade fenarimol (purity stated to be 100%) into the conjunctival sac
of the eyes of one male and two female New Zealand white rabbits,
slight conjunctival irritation was observed up to 24 h. Iridial
inflammation was noted in one treated eye 1 h after treatment. The
treated eyes appeared to be normal after 24-48 h (Jones, 1994b).
Fenarimol (purity, 97%) was tested in a Magnusson-Kligman
maximization test in 10 Hartley guinea-pigs; the control group
consisted of five animals. Barely perceptible erythema were seen in
two treated animals and in one of four surviving controls (Hoffman &
Arthur, 1980).
Table 2. Results of tests for the genotoxicity of fenarimol
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutationa S. typhimurium TA100, 125-2000 µg/plateb; 97 Negatived Hoffman et al.
TA98, TA1535, TA1537, 62.5-1000 µg/platec; (1988a)
E. coli WP2uvrA- toxicity and precipitation
at highest concentration;
vehicle, dimethyl sulfoxide
Chromosomal aberrationa Human lymphocytes 1.75-25 µg/mlb for 25 h 97 Negatived Murli (1988)
59.9-160 µg/mlc for 1 he;
vehicle, dimethyl sulfoxide
Gene mutationa Mouse lymphoma 1-50 µg/ml for 4 h; 97 Negatived Hoffman et al.
L5178Y tk+/-cells cytotoxic at > 35 µg/ml; (1988a)
vehicle, dimethyl sulfoxide
Unscheduled DNA Rat hepatocytes 0.05-100 nmol/ml for 5 h; 97 Negativea Probst (1979)
synthesisa,f cytotoxic at > 50 nmol/ml;
vehicle, dimethyl sulfoxide
In vivo
Chromosomal aberration 10 male Chinese hamsters; Oral; 250 mg/kg bw per day 98 Negatived Siou & Lerond-
bone-marrow cells for 2 daysg; sacrifice at 24, Conan (1982)
48, and 72 h; vehicle, peanut oil
Micronucleus formation Groups of 10 male Swiss Oral; 1000 mg/kg bw per day > 98 Weakly Siou et al. (1982)
mice; hone-marrow cells for 2 daysh; sacrifice at 24, positivei
48, and 72 h; vehicle, peanut oil
Micronucleus formation Groups of five male and Oral; 125, 625, or 1250 mg/kg 97 Negatived Ivett (1988)
five female Sprague-Dawley bwj; sacrifice at 24, 48, and
rats; bone-marrow cells 72h; vehicle, corn oil
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
In vivo (con't)
Dominant lethal mutation Groups of 10 male Wistar- Oral; 350 mg/kg bw; vehicle, 97 Inconclusivek Hoffman et al.
Harlan rats 5% acacia solution (1977d)
a No confirmatory experiment
b Without metabolic activation
c With metabolic activation
d Positive controls yielded positive result(s)
e As cell cycle delay was observed with metabolic activation in a preliminary test, incubation was for 45.3 h after treatment.
No cell cycle delay without activation; toxicity at highest doses tested
f Only one culture per concentration; only 20 cells per concentration analysed
g No mention of cytotoxicity; dose based on preliminary test in which all five animals died at 1000 mg/kg bw per day lot two days
h No mention of cytotoxicity; dose based on preliminary test in which all five animals died at 4000 mg/kg bw per day lot two days.
In the main test, one animal treated for 72 h died.
i Slight but statistically significant increase in micronuclei at 24 h sacrifice only; i.e. micronucleus frequencies per
1000 polychromatic erythrocytes; at 24 h: control, 0.19 ± 0.03; treated, 0.33 ± 0.06
j Toxic signs (lethargy, unsteady gait) at > 125 mg/g bw; one animal died at 1250 mg/kg bw.
k Treated males bred with untreated females weekly for eight weeks. Dose > 1/10 of the LD50. No sign of toxicity observed.
Only 7-10 pregnant females per mating interval per dose group. No reference to positive controls. No treatment-related effect on
fertility rate or pre- or post-implantation losses.
(ii) Mechanisms of effects on reproduction
Groups of six adult male Sprague-Dawley rats were given 35 mg/kg
bw per day fenarimol (purity unspecified) by gavage or subcutaneously
for 30 days; eight males served as controls. After 15 days of
treatment, each male was mated with two untreated females. No effect
was seen on male body weight, male fertility, the histopathology of
the testis or ventral prostate, or serum levels of testosterone,
follicle-stimulating hormone, or luteinizing hormone. Epididymal
weight was reduced after oral administration, but no histopathological
effects were observed. Serum prolactin levels were reduced after
systemic exposure, and prostatic prolactin binding was reduced after
exposure by either route. In a second experiment, groups of 15 males
were fed 0 or 35 mg/kg bw per day by gavage for 70 days and were bred
30 and 56 days after exposure. The parameters measured were the same
as in the first experiment except for follicle-stimulating hormone.
Serum prolactin levels and prostatic prolactin binding were reduced,
and the serum testosterone level was slightly elevated. There were no
other effects (Hanlin, 1981a).
Groups of 10 adult female Sprague-Dawley rats received fenarimol
(purity, 97%) at 0 or 35 mg/kg bw per day by gavage for about 60 days
before mating with untreated males, and then throughout pregnancy and
lactation. There were no effects on body weight or fertility. There
was some suggestion of an increase in proestrous prolactin level, but
the serum levels of prolactin were variable both before estrus and
during lactation and those of luteinizing hormone were variable before
estrus. Overall, there was no clear compound-related effect. There was
some evidence of a slight increase in estrous cycle length (Smalstig,
1981a).
Groups of 30 male Wistar rats were fed a diet containing 0 or
350 ppm fenarimol (purity, 97.7%) from 25 to 82 days of age. Twenty in
each group were mated and 10 were unmated; unmated males were killed
at the end of treatment, and the remaining animals were fed control
diet until day 117. Mating trials were conducted weekly with untreated
females on days 40-110. There were no significant effects on food
consumption or body-weight gain. A delay in the onset of reproductive
behaviour (percent mating and inseminating) was observed, but the
effect was reversible, since 100% fertility was attained at day 110.
The only effect on male reproductive organs was a reduction in
epididymal weight at the end of the treatment phase, with recovery
after cessation of treatment. No tissues were examined microscopically
(Hoffman & Hirsch, 1981c).
In a study of the possible involvement of uterine prostaglandins
in parturition, female Sprague-Dawley and Charles River CD rats
received 35 mg/kg bw per day fenarimol (purity, 97%) or the corn oil
vehicle orally from day 12 of pregnancy until parturition, or day 23
of pregnancy if no parturition occurred. The prostaglandin F2µ,
content, its release in and from the uterus, and serum progesterone
levels were measured some days before and at parturition. Sprague-
Dawley females showed delayed and/or difficult parturition and a
slight increase in the frequency of fetal and pup death, which
appeared to be related to the occurrence of difficult parturition.
There was no effect on prostaglandin F2µ, content; its release at
parturition was increased and not decreased, which would have been
expected with delayed parturition. Serum progesterone levels appeared
to be higher in treated females, and particularly in those that did
not deliver. The authors interpreted this finding as indicating that
progesterone did not fall to the low levels that facilitate or
initiate parturition in the rat (Smalstig, 1981b).
Progesterone concentrations in plasma were determined in late
pregnancy in groups of 12 Sprague-Dawley rats treated with 0 or
350 ppm fenarimol (purity unspecified) during pregnancy. Most of the
treated animals experienced delayed parturition associated with
dystocia and usually resulting in maternal death. High plasma
progesterone levels (66% of the peak value seen on day 19, in
comparison with 13% in controls) were seen in treated animals
immediately before normal parturition. The authors interpreted this
finding as indicating that luteolysis had not occurred, thus delaying
normal parturition (Tinsley, 1982).
A simulated rat ovarian microsomal system was used to measure the
effect of fenarimol on aromatase activity in vitro. Fenarimol
(purity unspecified) at molar concentrations 5-50 times the substrate
concentration inhibited aromatase activity by 55-90%. In a comparison
with aromatase inhibitors used clinically, fenarimol was as potent as
testolactone but less potent than aminoglutethimide and was therefore
considered to be a moderately weak inhibitor (Hoffman et al.,
1982b).
The urogenital sinuses were removed from 15-17-day-old male and
female Charles River rat embryos and implanted under the kidney
capsules of intact syngeneic male hosts (10 implants per animal). One
implanted male was then treated with 35 mg/kg bw per day fenarimol
(purity unspecified) for 21 days, one received the corn oil vehicle,
and one was treated with the positive control compound, cyproterone
acetate, which has anti-androgenic effects. Treatment with fenarimol
or corn oil had no effect on the differentiation or function of
transplanted urogenital sinuses in adult male rats. In contrast,
cyproterone acetate retarded prostatic development, and secretions
were absent (Neubauer et al., 1982).
Concentrations of 10-9 to 5 × 10-5 mol/litre fenarimol (purity
unspecified) were tested for androgen-binding capacity in vitro.
Fenarimol did not show affinity for binding to prostatic androgen
receptors, although 17% inhibition was seen at the highest
concentration. In an assay to determine whether fenarimol inhibits the
uptake of 3H-testosterone into the ventral prostate or the median
basal hypothalamus and preoptic areas of the brain, groups of 8-10
male Sprague-Dawley rats were given 0 or 35 mg/kg bw fenarimol
subcutaneously 20 and 3 h before sacrifice. Testosterone uptake was
not inhibited; in fact, uptake into the ventral prostate was
increased, whereas a positive control substance inhibited uptake into
the ventral prostate. In an assay to determine whether fenarimol
inhibits androgen-stimulated growth of seminal vesicles and ventral
prostate after castration, groups of six to eight immature rats were
given a daily subcutaneous injection of 0.25 mg/kg bw testosterone
propionate plus a daily oral or subcutaneous dose of 0, 5, 15, or
35 mg/kg bw fenarimol three days after castration for seven
consecutive days. A dose-related reduction in seminal vesicular weight
was seen with oral doses > 15 mg/kg bw, but the effect was not
statistically significant. The authors of the study concluded that
there was no inhibition (Hanlin, 1981b).
In a study to investigate whether fenarimol interferes with the
binding of androgens to the androgen receptor, groups of nine
castrated adult Wistar rats were fed fenarimol (purity unspecified) at
0 or 350 ppm in the diet for seven days. Cytosol from the hypothalamic
preoptic area, the frontal and parietal cerebral cortex, the
pituitary, and the ventral prostate was then incubated with
radiolabelled methyltrienolone (R1881; a potent synthetic androgen).
No reduction in binding to the cytoplasmic androgen receptors was
seen. In a second experiment, groups of one to two male rats were
treated similarly and then with radiolabelled R1881 before sacrifice.
There was no effect on binding to nuclear androgen receptors in the
tissues examined (the hypothalamic preoptic area, the pituitary, the
amygdala, the cerebral cortex, the prostate, the diaphragm, or the
liver), and the concentrations of conjugated and unconjugated labelled
R1881 were similar in all tissues. In a third experiment, pregnant
rats were fed 0 or 350 ppm fenarimol in the &let for most of the
period of gestation. Four female pups from each group were given a
subcutaneous injection of 3H-testosterone at the age of three or
four days and were killed 2 h later. There was no effect on the
binding of testosterone to the nuclear receptors in the hypothalamic
preoptic area amygdala (Hoffman et al., 1981a).
In a study of the estrogenic and anti-estrogenic potential of
fenarimol, groups of six immature female Holtzman rats received either
35 mg/kg bw fenarimol (purity unspecified) by gavage plus a
subcutaneous dose of estradiol, 35 mg/kg bw fenarimol by gavage, or a
subcutaneous dose of estradiol daily for three days. One dose of
fenarimol with several doses of estradiol, several doses of fenarimol
(8.75, 17.5, and 35 mg/kg bw) with one dose of estradiol, and
fenarimol in different vehicles were tested. There was no evidence for
agonistic or antagonistic effects on estrogen, as indicated by changes
in uterine weight. Fenarimol at concentrations of 1-1000 nmol/litre
did not inhibit the binding of estradiol to estrogen receptors in the
cytosol of immature rat uteri in vitro (Black, 1981a).
In a study of the effect of fenarimol on the binding of estrogen
to its receptor and on circulating estrogen levels at parturition,
fenarimol (purity unspecified) had no significant effect on the
nuclear uptake of estrogens by the hypothalamic preoptic area amygdala
or pituitary in groups of six adult ovariectomized rats fed 350 ppm in
the diet for seven days. Treatment of groups of four to six female
Wistar rats on days 0-21 of gestation at this dose did not affect the
concentration of estrone or estradiol in the plasma on day 21 (Hoffman
et al., 1981b).
Groups of three immature Dutch belted female rabbits were given
subcutaneous rejections of 0.5 µg oestradiol for six days and then
received either daily subcutaneous injections of 100 µg progesterone,
daily gavage doses of 60 mg fenarimol (purity unspecified), or the two
treatments combined, for five days. There was no evidence for a
progestational or anti-progestational effect in uteri examined
microscopically 24 h after the last dose (Black, 1981b).
Groups of seven pregnant Wistar rats were fed a diet containing
350 ppm fenarimol (purity, 97.7%) on gestation days 0 to 14-18. On the
last day of treatment, maternal blood and two fetuses per litter were
sampled 2 and 6 h after a gavage dose of radiolabelled fenarimol.
Placental transport was limited (the ratio of 14C in fetus to that
in maternal plasma was < 1) during days 14-17, the period associated
with genital tract morphogenesis, but increased steadily from day 15
onwards, to reach a ratio of 1 by day 18, when the organization of
sexual behaviour in the fetal brain begins (Hoffman & Hirsch, 1981a).
Female Wistar rats were fed 0 or 350 ppm fenarimol (purity,
97.7%) in the diet on days 0-5 post partum, and dams and pups
received an oral dose of radiolabelled fenarimol on day 5. One group
of 18 dams was used to study excretion into milk; the pups of seven
other dams were culled to three males and three females per litter.
The concentration of radiolabel in milk was three to five times higher
than that in maternal plasma up to 48 h after treatment. Radiolabel in
milk was rapidly taken up by the pups; the concentrations in the
hypothalamus increased more rapidly, attained higher maximal levels
(up to seven times higher 1 h after administration), and decreased
more slowly than in the remainder of the neonatal brain. In the
absence of previous exposure to fenarimol, an alteration was seen in
the rate but not the extent to which radiolabel was taken up by either
the hypothalamus or the brain. The major difference between pups
treated directly with 14C-fenarimol and those exposed via the milk
was in the rate of elimination, which was considerably faster after
direct treatment (Hoffman & Hirsch, 1981b).
In a study of the effects of fenarimol on the concentration of
estrogen receptors in the hypothalamus and on the conversion of
testosterone to estrogen, as an indirect measure of central aromatase
activity, pregnant Wistar rats received dietary concentrations of 0 or
350 ppm fenarimol (purity unspecified) from gestation day 3-4 to day
21. The concentration of nuclear estrogen receptors was measured in
the hypothalamic preoptic area amygdala of day-21 fetuses and of
neonates on day 3-4. The mean concentration was reduced by 44-46% in
fetuses of each sex and by 56% in neonates, especially in males.
Sex-specific differences in the concentration of receptors in the
exposed neonates were abolished, i.e. males no longer had higher
concentrations. In a second experiment, male Wistar rats that were
castrated but had testosterone implants and intact males received 0 or
350 ppm fenarimol in the diet for seven days. The concentration of
nuclear estrogen receptors was reduced in both groups. The circulating
levels of testosterone indicated that the effect on receptor
concentration could not be attributed to testosterone levels. In a
third experiment, pregnant Wistar rats received 0 or 350 ppm fenarimol
in the diet from about gestation day 18 to day 3-4 post partum, and
groups of 12 female neonates (used to eliminate endogenous
testosterone production as a variable) were then dosed with
radiolabelled testosterone. Significantly lower concentrations of
estrone and estradiol were observed in homogenates of hypothalamic
preoptic area amygdala from exposed pups, and, contrary to controls,
no labelled estradiol was detected in the nuclei. Fenarimol did not
affect the concentrations of testosterone or dihydrotestosterone in
the nuclear extracts of the hypothalamic preoptic area amygdala. Since
testosterone is metabolized to dihydrotestosterone by the enzyme
5µ-reductase and to estradiol by aromatase, this observation suggests
that the reduction in receptors is associated with inhibition of
aromatase (Hoffman et al., 1981c).
(iii) Relevance to humans of adverse reproductive effects in rodents
The adverse effect of fenarimol on the fertility of male mice
and rats appears to be due to an effect on the organization
(differentiation) and expression of male sexual behaviour, which is
known to be controlled within the central nervous system (Naftolin
et al., 1991). The mechanism is dependent on aromatization of
testosterone to estradiol-17ß. Evidence is available from several
studies that the expression of human male sexual behaviour has a
different hormonal basis from that in rats, i.e. depends on both a
direct action of testosterone and conversion of testosterone to
dihydrotestosterone, but not on the conversion of testosterone to
estradiol-17ß. Mantzoros et al. (1995) showed that the frequency of
human male sexual behaviour is correlated with the serum level of
dihydrotestosterone but not of estradiol-17ß. Gooren (1985)
administered an aromatase inhibitor (testolactone) or an estrogen
receptor antagonist (tamoxifen) to men and also concluded that
estradiol-17ß is not important in the control of male sexual
behaviour. Bagatell et al. (1994) showed that there was no change in
male sexual function after a treatment regimen involving testolactone,
testosterone, and a gonadotropin RH antagonist, which resulted in a
specific decrease in estradiol-17ß levels while keeping testosterone
and dihydrotestosterone at normal levels. A single study indicates a
role for estradiol-17ß in the expression of human male sexual
behaviour (Luisi & Franchi, 1980). The results showed that an
aromatizable form of testosterone is more effective in stimulating
libido than the non-aromatizable androgen mesterolone; however, this
result is difficult to interpret as it is not known whether
mesterolone can cross the blood-brain barrier.
Men with a genetic defect resulting in impaired androgen
receptor-mediated activity, but with no effect on estradiol-17ß
receptor-mediated activity or on aromatase, did not show normal male
sexual behaviour (Breedlove, 1994). A further study of genetic defects
(Smith et al., 1994) supports this conclusion, since an XY man
lacking functional estrogen receptors remained masculine, indicating
that estradiol-17ß does not control sexual differentiation in human
males; however, sexual activity was not assessed. Furthermore,
aromatase is confined mainly to the gonads and brain of rats and mice,
while it is also expressed in placenta, adipose tissue, and liver in
higher primates, including humans. Thus, the proportion of target
enzyme to inhibitor would be greater in humans than in rats, requiring
more inhibitor to achieve the same degree of inhibition at a given
target site.
Fenarimol would appear to have an adverse effect on reproductive
parameters in female mice and rats, as gestation and parturition are
extended, resulting in dystocia. This effect is also a direct effect
of the inhibition of aromatase, since circulating progesterone is
sustained at a high level, preventing timely luteolysis and resulting
in continued functioning of the corpora lutea and thus prolonging
gestation. In a review, Chwalisz (1994) outlined the mechanisms of
pregnancy and parturition in female rats. In order to maintain
pregnancy, rats depend on the corpus luteum as a source of
progesterone. Parturition requires an abrupt decrease in the
progesterone level, an increase in that of estrogen, and consequently
a decreased ratio of progesterone:estrogen. In contrast, humans (like
guinea-pigs) use the placenta as a source of progesterone during
pregnancy, and an abrupt decrease in progesterone secretion is not
required for parturition. These differences in endocrine control of
parturition, particularly the lesser role of estrogens in stimulating
labour in women, indicate that rats are probably not a relevant model
for predicting the effects of aromatase inhibition on human
parturition and that guinea-pigs are a better model.
Comments
Fenarimol given orally to rats was rapidly absorbed, distributed
and excreted. Elimination occurred principally in the faeces (76-83%),
mainly as a result of biliary excretion, and to a lesser extent in the
urine (6.5-9.2%). Residue levels in tissues were relatively low.
Dermal absorption of fenarimol by monkeys was low (about 2.5%). There
was some barrier to placental transport of fenarimol-derived
radiolabel in rats during part of the gestation period. Although the
levels of the radiolabel in the milk of lactating rats were three to
five times those in the plasma, only a very small proportion of the
dose (< 0.1%) was eliminated in the milk of a lactating goat.
Fenarimol is extensively metabolized to many metabolites. The
major pathways are oxidation of the carbinol-carbon atom, the
chlorophenol rings, and the pyrimidine ring. Repeated oral
administration to rats had no effect on the elimination of fenarimol
or its metabolites.
Fenarimol has low acute oral and dermal toxicity, the LD50
values in rats and rabbits being 2550 and > 2000 mg/kg bw
respectively. After inhalation, the LC50 was > 2 mg/litre (1-h
exposure). Fenarimol did not irritate skin or eyes and was not a skin
sensitizer in guinea-pigs in a maximization test. The WHO has
classified fenarimol as unlikely to present an acute hazard in normal
use.
In a 14-day pilot study in mice given dietary concentrations of
25-12 800 ppm, liver toxicity was observed at 800 ppm and above. In a
three-month study in mice given dietary concentrations of 0, 360, 620,
1100, 2000, or 3300 ppm, toxicity was observed mainly in the liver, as
increased liver weight, hepatic enzyme induction, centrilobular
hypertrophy, and hepatomegaly. The NOAEL was 360 ppm, equivalent to
52 mg/kg bw per day, on the basis of an increase in liver weight at
620 ppm.
In two 14-day pilot studies in rats given dietary concentrations
of 25-12 800 ppm, liver toxicity was observed at 200 ppm and above.
Dietary administration of fenarimol to rats for three months at 0, 50,
200, or 800 ppm also resulted in liver toxicity at and above
200 ppm, seen as increased liver weight, hepatic enzyme induction,
centrilobular hypertrophy, and fatty changes in the liver. The NOAEL
was 50 ppm, equivalent to 2.5 mg/kg bw per day, on the basis of
induction of liver enzymes. In another three-month study in rats, with
doses of 0, 140, 200, 280, 360, and 500 ppm, the NOAEL was 200 ppm,
equivalent to 10 mg/kg bw per day, on the basis of an increase in
liver weight and induction of hepatic enzymes at 280 ppm and above.
In a three-month study in dogs, oral administration of fenarimol
in gelatin capsules at doses of 0, 1.2, 5, or 20 mg/kg bw per day
resulted in no systemic toxicity. In a one-year study in which dogs
were given 0, 1.2, 12, or 120 mg/kg bw per day, toxicity was observed
at the highest dose. This included liver toxicity and, in female dogs,
a transient enlargement of the mammary gland and increased ovarian
weight; an increase in the occurrence of soft stools was observed at
all doses. The NOAEL was 12 mg/kg bw per day.
In a study of chronic toxicity, mice were administered fenarimol
at dietary levels of 0, 50, 170, or 600 ppm for 12 months. The NOAEL
was 170 ppm, equivalent to 24 mg/kg bw per day, on the basis of
decreased body-weight gain and effects on the liver at 600 ppm. In a
two-year study of carcinogenicity, mice were given diets containing 0,
50, 170, or 600 ppm fenarimol. The NOAEL was 370 ppm, equivalent to
24 mg/kg bw per day, on the basis of a slight decrease in body-weight
gain in males at 600 ppm. There was no evidence of carcinogenicity.
Two studies of chronic toxicity were performed in which rats were
administered dietary concentrations of fenarimol at 0, 50, 130 or
350 ppm for 12 or 18 months. In both studies, the main effects at the
highest dose were a decrease in the leukocyte count, increased liver
weight, and decreased spleen weight. In the 12-month study, the NOAEL
was 130 ppm, equal to 6 mg/kg bw per day. In the 18-month study, the
NOAEL was 50 ppm, equal to 2.3 mg/kg bw per day, on the basis of a
reduction in body weight.
Three carcinogenicity studies were performed in rats. In the
first, rats were administered diets containing 0, 50, 130, or 350 ppm
fenarimol. A small increase in the incidence of hepatocellular adenoma
was seen at the highest dose. There was no NOAEL, owing to a slight
increase in fatty changes and hyperplastic nodules in the liver at all
doses. No clear treatment-related effect on tumour incidence was seen.
Two further studies were performed at lower doses (O, 12, 25, or
50 ppm). In the first study, the survival was very low, owing to an
outbreak of chronic respiratory disease. There was evidence of reduced
body-weight gain and an increased incidence of fatty changes in the
liver at 50 ppm. No adverse effect was observed in the second study.
The overall NOAEL for all three studies was 25 ppm, equal to 1.2 mg/kg
bw per day. There was no evidence of carcinogenicity.
A three-generation study of reproductive toxicity in mice given
dietary levels of 0, 35, 70, or 140 ppm fenarimol revealed no adverse
effects on parental mice or offspring. In earlier pilot studies,
however, doses of 170 ppm and above reduced fertility and live-born
litter size and lengthened the gestation period. The NOAEL was
140 ppm, equivalent to 20 mg/kg bw per day.
In a two-generation study of reproductive toxicity in which
guinea-pigs were given 0 or 400 ppm fenarimol in the diet (equal to
33 mg/kg bw per day), parental toxicity was seen and there was
equivocal evidence of a slight effect on the proportion of live-born
progeny. In a single-generation study of reproductive toxicity with a
cross-over design, rabbits were treated orally with fenarimol at a
level of 0 or 35 mg/kg bw per day. There was no evidence of general
systemic toxicity or of effects on reproduction.
Two multigeneration studies of reproductive toxicity have been
performed in rats. In a two-generation study, rats were exposed to
dietary concentrations of 0, 50, 130, or 350 ppm, and in a three-
generation study to 0, 12, 25, or 50 ppm. Fenarimol reduced fertility,
caused dystocia, reduced live-born litter size and lengthened the
gestation period. In the first study, cross-over data showed that the
reduction in fertility was clearly mediated through the male and was
only slightly reversible. The overall NOAEL for general systemic
toxicity was 25 ppm, equivalent to 1.2 mg/kg bw per day, on the basis
of reduced body-weight gain in F1 males at 50 ppm and above. The
overall NOAEL for reproductive toxicity was 12 ppm, equivalent to
0.62 mg/kg bw per day, on the basis of a reduction in live-born litter
size at 25 ppm and above.
Three single-generation studies of reproductive toxicity with a
cross-over design have been conducted in rats, which were exposed
orally to fenarimol at doses 0 or 35 mg/kg bw per day. These studies
showed that the effects on reproduction were not strain-dependent, the
effects on fertility were clearly male-mediated, and the reduction in
live-born litter size (probably due to an increased incidence of
stillbirths) was female-mediated. No correlation was found between
fertility and the levels of prolactin, luteinizing hormone, or
testosterone, organ weights, histopathological findings, or sperm
morphology.
The single- and multigeneration studies of reproductive toxicity
show that fenarimol has adverse effects on reproduction in rats and
(at higher doses) in mice, but not in guinea-pigs or rabbits. A number
of studies were performed to investigate the mechanism of the adverse
effects on reproduction, and particularly those on fertility. The
results of these studies and of others in the open literature show
that fenarimol affects male sexual differentiation and subsequent
behaviour indirectly by inhibiting the aromatase-catalysed conversion
of testosterone to estradiol-17ß within the hypothalamus. Data from
the literature strongly indicate that while estradiol-17ß is the
central regulator of sexual differentiation in rats, human male sexual
behaviour is controlled by dihydrotestosterone, which is formed from
testosterone by the enzyme 5µ-reductase. There is evidence that the
effect of fenarimol in female rats (delayed parturition) is also due
to inhibition of aromatase, as this inhibition results in a sustained
level of circulating progesterone and prolonged corpus luteal
function, thus leading to delayed parturition. In contrast to rats, an
abrupt decrease in progesterone secretion is not required for
parturition in guinea-pigs or humans. The Meeting concluded that,
although not clear-cut, there is sufficient evidence that the adverse
effects on the organization and expression of sexual behaviour in male
rats and on delayed parturition in female rats are not relevant for
humans.
In a study of developmental toxicity, pregnant rats were given
fenarimol by gavage at doses of 0, 5, 13, or 35 mg/kg bw per day.
There was evidence of developmental toxicity (hydronephrosis) but no
signs of maternal toxicity at the high dose. In a study in which some
females were also allowed to litter and wean their progeny, pregnant
rats were exposed to 0 or 35 mg/kg bw per day during days 6-15 of
gestation. There were adverse effects on reproductive performance
(increased length of gestation, dystocia) and developmental toxicity
(increased hydronephrosis) but no evidence of general systemic
maternal toxicity. The increased incidence of hydronephrosis appeared
to be due to delayed development and was not considered a teratogenic
effect. Overall, the NOAEL for maternal toxicity was 35 mg/kg bw per
day, and that for embryo- and fetotoxicity was 13 mg/kg bw per day.
In a study of developmental toxicity in which rabbits were
administered fenarimol by gavage at doses of 0, 3, 10, or 35 mg/kg bw
per day, no signs of toxicity were observed in dams or fetuses. In a
second study, rabbits were exposed orally to doses of 0, 15, 50, or
150 mg/kg bw per day. The NOAEL for maternal toxicity was 50 mg/kg bw
per day, on the basis of reductions in body weight and food
consumption and an increased incidence of abortions. The NOAEL for
embryo- and fetotoxicity was also 50 mg/kg bw per day, on the basis of
a reduced number of live fetuses and an increased incidence of extra
ribs. There was no evidence of teratogenic potential.
Fenarimol has been adequately tested for genotoxicity in a series
of assays in vitro and in vivo. The Meeting concluded that
fenarimol is not genotoxic.
The Meeting concluded that the effects of fenarimol in male rats
(reduced fertility) and female rats (delayed parturition) are due to
inhibition of aromatase, an enzyme that is not involved in these
aspects of human reproduction. It therefore decided that it would be
inappropriate to use the NOAEL seen in studies of reproductive
toxicity in determining the ADI. An ADI of 0-0.01 mg/kg bw was
established on the basis of an overall NOAEL of 1.2 mg/kg bw per day,
seen in several studies of carcinogenicity in rats, and a safety
factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 24 mg/kg bw per day (12-month study of chronic toxicity)
20 mg/kg bw per day (three-generation study of reproductive
toxicity)
Rat: 1.2 mg/kg bw per day (three studies of carcinogenicity)
0.62 mg/kg bw per day (multigeneration study of reproductive
toxicity)*
13 mg/kg bw per day (embryo- and fetotoxicity in study of
developmental toxicity)
Dog: 12 mg/kg bw per day (one-year study of toxicity)
Rabbit: 50 mg/kg bw per day (maternal and embryo- or fetotoxicity in
study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information valuable for the continued
evaluation of the compound
Observations in humans
* Data considered irrelevant for evaluation with respect to human
health.
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenarimol
Exposure Relevant route, study type, species Result, remarks
Short-term (1-7 days) Skin, irritation, rabbit Not irritating
Eye, irritation, rabbit Not irritating
Skin, sensitization, guinea-pig Not sensitizing
Oral, toxicity, rat LD50 = 2550 mg/kg bw
Dermal, toxicity, rabbit LD50 > 2000 mg/kg bw
Inhalation, toxicity, rat, 1 h LC50 > 2 mg/litre
Medium-term (1-26 weeks) Dermal, toxicity, 21 -day, rabbit No systemic effect; no irritation at
1000 mg/kg bw
Dietary, toxicity, three months, rat NOAEL = 2.5 mg/kg bw; hepaticenzyme
induction
Dietary, three-generation, reproductive NOAEL = 0.625 mg/kg bw; reduced
toxicity, rat live-born litter size
Gavage, developmental toxicity, rat NOAEL = 13 mg/kg bw;
hydronephrosis; no maternal toxicity
Long-term (> one year) Dietary, toxicity, two years, rat NOAEL = 1.2 mg/kg bw; fatty changes
in liver
References
Althaus, W.A. (1985a) Characterization and identification of
radioactivity in urine and feces of Wistar rats given single oral
doses mixed-labeled 14C fenarimol. Unpublished report No.
ABC-0310 dated December 1985 from Lilly Research Laboratories,
USA. Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Althaus, W.A. (1985b) Characterization and identification of
radioactivity in blood, liver and kidney of Wistar rats given
single oral doses of mixed-labelled 14C fenarimol. Unpublished
report No. ABC-0321 dated December 1985 from Lily Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Bagatell, C.J., Heiman, J.R., Rivier, J.E. & Bremmer, W.J. (1994)
Effects of endogenous testosterone and oestradiol on sexual
behaviour in normal young men. J. Clin. Endocrinol. Metab., 78,
711-716.
Black, L.J. (1981a) Evaluation of the estrogenic and antiestrogenic
potential of EL-222 in the immature rat uterus. Unpublished
report dated September 1981 from Lilly Research Laboratories,
USA. Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Black, L.J. (1981b) Evaluation of the progestational or
antiprogestational potential of EL-222 in immature rabbits.
Unpublished report dated September 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Breedlove, S.M. (1994) Sexual differentiation of the human nervous
system. Ann. Rev. Psychol., 45, 389-418.
Chwalisz, K. (1994) The use of progesterone antagonists for cervical
ripening and as an adjunct to labour and delivery. Hum,
Reprod., 9 (Suppl. 1), 131-161.
Goebel, G.V. (1985) Characterization of radioactivity in bile from
rats dosed orally with 14C fenarimol. Unpublished report No.
ABC-0312 dated May 1985 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Gooren, L.J.G. (1985). Human male sexual functions do not require
aromatisation of testosterone: A study using tamoxifen,
testolactone, and dihydrotesterone. Arch. Sex. Behav., 14,
539-548.
Hanlin, M.L. (1981a) Fertility studies with EL-222 in the adult male
rat. Unpublished report dated September 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hanlin, M.L. (1981b) Anti-androgenic studies with EL-222 in the male
rat. Unpublished report dated September 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom
Hoffman, D.G. & Amundson, M. (1985) Initiation and promotion study of
fenarimol on rat liver carcinogenesis. Unpublished report dated
November 1985 from Naylor Dana Institute for Disease Prevention,
Valhalla, New York, USA Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G. & Arthur, B.H. (1980) Guinea pig sensitization study
with Lilly compound 56722 (fenarimol). Unpublished report No.
GP-9538 dated January 1980 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G. & Hirsch, K.S. (1981a) Placental transport of EL-222 in
the Wistar rat. Unpublished report No. R10480 dated October 1981
from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G. & Hirsch, K.S. (1981b) Exposure of the neonatal rat to
EL-222 as a component of milk: Evidence for the presence of
EL-222 in the central nervous system during the organization of
adult sexual behaviour. Unpublished report No R01781 dated
October 1981 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G. & Hirsch, K.S. (1981c) A special fertility study with
EL-222 (fenarimol) in the Wistar rat. Unpublished report No
R07380 dated October 1981 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D G. & Pierce, E.C. (1985) A chronic toxicity/oncogenicity
study in Wistar rats maintained on diets containing low
concentrations of fenarimol for two years. Unpublished report No.
R07781 dated May 1985 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G. & Russel, D.R. (1990) A developmental toxicity study of
fenarimol (EL-222, compound 056722) administered orally to New
Zealand white rabbits. Unpublished report No. B00290 dated
October 1990 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Broddle, W.D., Gibson, W.R. & Morton, D.M. (1975a) The
acute toxicity of EL-222 in mice, rats, dogs, ducks mid quail.
Unpublished report dated August 1975 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Bernhard, N.R., Gibson, W.R., Owen, N.V. & Morton, D.M.
(1975b) A pilot 2-week subacute toxicity study of EL-222 in mice.
Unpublished report No. M-9074, M-9084 dated August 1975 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975c)
Three-month subacute oral toxicity studies on EL-222 in mice.
Unpublished report No. M-9015 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Bernhard, N.R., Gibson, W.R., Harris, P.N. & Morton,
D.M. (1975d) A pilot 2-week subacute toxicity study of EL-222 in
rats. Unpublished report No. R-713, R-723 dated August 1975 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Bernhard, N.R. & Morton, D.M. (1975e) A 2-week subacute
study of the effects of EL-222 on hepatic p-nitroanisole
metabolism in rats. Unpublished report No. R-244, R-254 dated
August 1975 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975f)
Three-month subacute oral toxicity studies on EL-222 in rats.
Unpublished report No. R-1013 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975g)
Three-month subacute oral toxicity studies on EL-222 in rats.
Unpublished report No. R-1164 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975h)
Three-month subacute oral toxicity studies on EL-222 in dogs.
Unpublished report No. D-4183 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Arthur, B.H., Gibson, W.R. & Morton, D.M. (1977a) The
acute dermal and ocular toxicity of technical EL-222 in rabbits.
Unpublished report dated January 1977 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Adams, E.R., Markham, J.K., Owen, N.V., Gibson, W.R. &
Morton, D.M. (1977b) A multi-generation reproduction study with
EL-222 in the rat. Unpublished report No. R-715, R-1345, R-956,
R-966 dated November 1977 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Markham, J.K., Adams, E.R., Owen, N.V & Morton, D.M.
(1977c) A teratology study on compound 56722 (EL-222) in the
rabbit. Unpublished report No. B-7125 dated January 1977 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Markham, J.K., Owen, N.V. & Morton, D.M. (1977d) A
dominant lethal study of compound 56722 (EL-222) in the rat.
Unpublished report dated January 1977 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G. et al. (1978a) Twelve-month chronic oral toxicity of
EL-222 (56722) in mice. Unpublished report No. M-9155 dated April
1978 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C., Harris, P.N. & Morton, D.M.
(1978b) Twenty-four month chronic oral toxicity of EL-222 (56722)
in mice. Unpublished report No. M-9135, M-9145, dated August 1978
from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G. et al. (1978c) A one-year toxicity study with EL-222
in the rat. Unpublished report No. R-715 dated June 1978 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C. & Morton, D.M. (1978d)
Eighteen-month chronic oral toxicity of EL-222 (56722) in rats.
Unpublished report No. R-435 dated May 1978 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C. & Morton, D.M. (1978e)
Twenty-four month chronic oral toxicity of EL-222 (56722) in
rats. Unpublished report No. R-405, R-415, dated April 1978 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gomez, S.R. & Dorato, M.A. (1980a) The acute inhalation
toxicity of Lilly compound 56772, fenarimol, in the Fischer 344
rat. Unpublished report No. R-H-99-80 dated December 1980 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V. & Markham, J.K. (1980b) A cross-over
reproduction study with EL-222 (Lilly compound 56722) in the rat.
Unpublished report No. R-286 dated April 1980 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V. & Markham, J.K. (1980c) Additional
cross-over reproduction studies with EL-222 (Lilly compound
56722) in the Wistar and the Sprague-Dawley rat. Unpublished
report No. R-57, R-67 dated April 1980 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V & Adams, E.R. (1980d) A cross-over fertility
study with EL-222 in the Wistar rat. Unpublished report No.
R08379 dated June 1980 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Adams, E.R. & Owen, N.V. (1980e) A cross-over fertility
study with EL-222 in the Dutch-belted rabbit. Unpublished report
No. B7229, dated June 1980 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Owen, N.V. & Adams, E.R. (1980f) A teratology study
with EL-222 in the rat. Unpublished report No. R06279 dated
January 1980 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Hirsch, K.S. & MacLusky, N.J. (1981a) Effects of EL-222
on the binding of androgens to cytoplasmic and nuclear androgen
receptors. Unpublished report dated October 1981 from Yale
University Medical School Connecticut, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Hirsch, K.S. & MacLusky, N.J. (1981b) Effects of EL-222
on circulating estrogens and the binding of estrogens to estrogen
receptors in the rat central nervous system. Unpublished report
dated October 1981 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Hirsch, K.S. & MacLusky, N.J. (1981c) Effects of EL-222
on the concentration of estrogen receptors and the conversion of
testosterone to estrogens in the hypothalamus. Unpublished report
dated October 1981 from Yale University Medical School,
Connecticut, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C. & Morton, D.M. (1982a) A
low-dose chronic toxicity/oncogenicity study in Wistar rats
maintained on diets containing fenarimol for two years.
Unpublished report No. R06479 dated November 1982 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Brodie, A.M.H. & Hirsch, K.S. (1982b) An in vitro
measure of aromatase inhibition by EL-222. Unpublished report
dated January 1982 from the University of Maryland, Baltimore,
Maryland, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Markham, J.K & Miller, B.J. (1983a) A two-generation
reproduction study with fenarimol (EL-222, compound 56722) in
guinea pigs. Unpublished report No. G00682, G00483 dated December
1983 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V & Byrd, R.A. (1983b) The effect of prenatal
fenarimol (EL-222, compound 56722) exposure on kidney development
and maturation in the rat. Unpublished report No. R10682, R10782,
R10882 dated November 1983 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Masten, J.L. & Hanasono, G.K (1985a) The biliary
excretion of radioactivity by male and female Wistar rats given
single oral doses of 14C-fenarimol (compound 56722, EL-222).
Unpublished report No. R02485 dated April 1985 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Masten, J.L & Hanasono, G.K. (1985b) Tissue
distribution of radioactivity in Wistar rats determined one or
twenty-four hours after single oral doses of 14C-fenarimol
(EL-222, compound 56722). Unpublished report No. R16684 dated
April 1985 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Masten, J.L., Walker, C.G. & Hanasono, G.K. (1985c)
Radiocarbon disposition studies on Wistar rats given single
oral doses of 14C-fenarimol (EL-222, compound 56722):
Pharmacokinetics, and excretion/tissue distribution seven days
after dosing. Unpublished report No. R06484, R08584 dated March
1985 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Weaver, D.E. & Van Lier, R.B.L. (1985d) Distribution of
radioactivity into tissues and organs from Wistar rats given oral
doses of unlabeled fenarimol daily for two weeks followed by a
single dose of 14C-fenarimol (EL-222, compound 56722).
Unpublished report No. R01285 dated March 1985 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Van Lier, R.B.L. & Weaver, D.E. (1985e) The
percutaneous absorption of 14C fenarimol dissolved in ethanol
in rhesus monkeys. Unpublished report No. P04085, P04585 dated
October 1985 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Pierce, E.C., Brown, G.E. & Negliski, S. (1985f)
Subchronic (21-day) dermal toxicity study in rabbits with
technical fenarimol (EL-222, compound 56722) and Rubigan 50W, a
wettable powder formulation (FN-0742) containing 50% fenarimol.
Unpublished report No. B02183 dated May 1985 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Gries, C.L., Pierce, E.C., Means, J.R. & White, J.F.
(1985g) A one year chronic oral toxicity study of fenarimol in
dogs with a three month recovery period. Unpublished report No.
D02683 dated May 1985 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Richardson, K.A. & Kokkino, A.J. (1988a) The effect of
fenarimol (EL-222, compound 56722) on the induction of reverse
mutations in Salmonella typhimurium and Escherichia coli using
the Ames test. Unpublished report No. 880215AMT4, 880222AMS4,
dated August 1988 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Oberly, T.J. & Richardson, K.A. (1988b) The effect of
fenarimol on the induction of forward mutation at the thymidine
kinase locus of L51787 mouse lymphoma cells. Unpublished report
No. 880106MLT4, 880113MLA4, dated August 1988 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Ivett, J.L. (1988) Mutagenicity test on 56722 (EL-222) in the in vivo
rat micronucleus assay. Unpublished report No. 10348-0-455 dated
October 1988 from Hazleton, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Jones, J.R. (1994a) Fenarimol technical: Acute dermal irritation test
in the rabbit. Unpublished report No. 291/50 dated February 1994
from Safepharm Laboratories, United Kingdom. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Jones, J.R. (1994b) Fenarimol technical: Acute eye irritation test in
the rabbit. Unpublished report No. 291/51 dated February 1994
from Safepharm Laboratories, United Kingdom. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Luisi, M. & Franchi, R. (1980) Double blind group comparative study of
testosterone undecanoate and mesterolone in hypogonadal male
patients. J. Endocrinol. Invest., 3, 305-308.
Mantzoros, C.S., Georgiadis, E.I. & Trichopoulos, D. (1995)
Contribution of dihydrotestosterone to male sexual behaviour.
Br. Med. J., 310, 1289-1291.
Markham, J.K., Hoffman, D.G., Adams, E.R., Owen, N.V. & Morton, D.M.
(1978a) Pilot reproduction studies with EL-222 (Lilly compound
56722) in the mouse. Unpublished report No. M-9165, M-9215 dated
July 1978 from Lilly Research Laboratories, USA. Submitted to WHO
by DowElanco Europe, Wantage, Oxon, United Kingdom.
Markham, J.K., Hoffman, D.G., Broddle, W.D., Owen, N.V. & Morton, D.M.
(1978b) A multi-generation study with EL-222 (Lilly compound
56722) in the mouse. Unpublished report No. M-9086, M-9296,
M-9326 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Markham, J.K., Hoffman, D.G., Owen, N.V. & Morton, D.M. (1978c) A
second multi-generation reproduction study with EL-222 (Lilly
compound 56722) in the rat. Unpublished report No. R-636, R-1076,
R-217 dated July 1978 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Murli, H. (1988) Mutagenicity test on 56722 in an in vitro cytogenetic
assay measuring chromosomal aberration frequencies in cultured
purified human lymphocytes. Unpublished report No. 10348-0-449
dated August 1988 from Hazelton, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Naftolin, F., Keefe, D., Apa, R., Palumbo, A. & Garcia-Segura, L.M.
(1991) The apparent paradox of sexual differentiation of the
brain. Contrib. Gynecol. Obstet., 18, 24-32.
Neubauer, B.L. et al. (1982) Effect of EL-222 (compound 56722,
fenarimol) on the developing reproductive tract. Unpublished
report dated January 1982 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Probst, G.S. (1979) The effect of Lilly compound 56722 (EL-222) on the
induction of DNA repair synthesis in primary cultures of adult
rat hepatocytes. Unpublished report No. 790502-1 dated June 1979
from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Prout, M. (1994) The disposition of 14C fenarimol in the lactating
goat. Unpublished report No. IRI 154147 dated October 1994 from
Inveresk Research International, Musselburgh, United Kingdom.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Rodricks, J.V. et al. (1989) Response to USSR concerns regarding the
potential oncogenicity of fenarimol. Unpublished report dated
February 1989 from Environ Corp., Washington DC, USA. Submitted
to WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Simpson, E.R., Mahendroo, M.S., Means, G.D., Kilgore, M.W.,
Hinshelwood, M.M., Graham-Lorence, S., Amarneh, B., Ito, Y.,
Fisher, C.R., Dodson, M.M., Mendelson, C.R & Bulun, S. (1994)
Aromatase cytochrome P450, the enzyme responsible for oestrogen
biosynthesis. Endocr. Rev., 15, 342-355.
Siou, G. & Lerond-Conan, L. (1982) Test for mutagenic potential of
technical-grade fenarimol by examination of chromosomal damage in
the Chinese hamster. Unpublished report No. 658 dated June 1982
from C.E.R.T.I., Versailles, France. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Siou, G., Lerond-Conan, L. et al (1982) Test for mutagenicity of
technical-grade fenarimol using a micronucleus technique in the
mouse. Unpublished report No. 650 dated May 1982 from C.E.R.T.I.,
Versailles, France. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Smalstig, E.B. (1981a) Study of the possible influence of compound
EL-222 on fertility in the adult female rat. Unpublished report
dated September 1981 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Smalstig, E.B. (1981b) Studies of the influence of EL-222 on uterine
prostaglandin F2alpha in the female rat at parturition.
Unpublished report dated November 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Smith, E.P., Boyd, J., Frank, G., Takahashi, H., Cohen, R.M., Specker,
B., Williams, T.C., Luhbahn, D.B. & Korach, K.S. (1994) Oestrogen
resistance caused by a mutation in the oestrogen-receptor gene in
a man. New Engl. J. Med., 331, 1056-1061.
Tinsley, F.C. (1982) Preparturiton progesterone levels in fenarimol
(EL-222) treated rats. Unpublished report dated August 1982 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Watson, D. (1994) Accurate measurement of an isolated metabolite of
fenarimol. Unpublished report No. DWC/719 dated October 1994 from
Huntingdon Research Centre, United Kingdom. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
found, the main one being metabolite K. In contrast, most of the
radiolabel in the faeces was present as free compounds, indicating
that biliary metabolites undergo further metabolism or hydrolysis
before being excreted (Goebel, 1985).
The tissue distribution of fenarimol in Wistar rats was
determined 1 and 24 h after administration of 1 or 13 mg/kg bw
14C-fenarimol. The main radiolabelled constituents identified in
blood and kidney after 1 h were unchanged fenarimol and metabolite I.
The amount of fenarimol increased with dose, from 19 to 40% of the
total radioactivity in blood in males and from 50 to 73% in females.
Fenarimol also accounted for most of the radiolabel in the liver
(67-90%), whereas metabolite K represented 3-6%. Females had more
unchanged fenarimol residues than males, indicating more extensive
metabolism in males. Furthermore, metabolism was reduced at the higher
dose, suggesting saturation (Althaus, 1985b).
(c) Other metabolic studies
Some barrier to placental transport of fenarimol-derived
radiolabel was found in rats on days 14-17 of gestation but virtually
no barrier on day 18 (Hoffman & Hirsch, 1981a). Fenarimol-derived
radiolabel was excreted in milk and subsequently concentrated in the
hypothalamus of neonates. The concentration in milk up to 48 h after
treatment was three to five times greater than that in maternal plasma
(Hoffman & Hirsch, 1981b).
2. Toxicological data
(a) Acute toxicity
The results of studies of acute toxicity are summarized in
Table 1.
(b) Short-term toxicity
Mice
In a pilot study reported with few details, groups of four male
ICR mice were given fenarimol (purity unspecified) in the diet at 0
(10 animals), 25, 50, 100, 200, 400, 800, 1600, 3200, 6400, or
12 800 ppm for 14 days. No effects were observed on mortality or
clinical signs. terminal body weight was reduced at the highest dose.
In the liver (the only organ investigated), dose-related increases in
relative weight and in the rate of hepatic metabolism of para-
nitroanisole were seen at > 800 ppm. There were no effects on liver
pathology or histopathology (Hoffman et al., 1975b).
Table 1. Acute toxicity of fenarimol
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
ICR mouse Male Oral 4508a NR Hoffman et al. (1975a)
Female > 4000a
Wistar-derived Male Oral 2576a NR Hoffman et al. (1975a)
Harlan rat Female 2515a
Albino rabbit Male and Dermal > 2000b NR Hoffman et al. (1977a)
female
Fischer 344 rat Male and Inhalationc > 2.07 97% Hoffman et al. (1980a)
female (1-h)
Wistar-derived FemMe intraperitoneal 533 NR Hoffman et al. (1975a)
Harlan rat
Beagle dogc Male and Oral > 200 NR Hoffman et al. (1975a)
female
NR, not reported
a Signs of toxicity: hypoactivity, leg weakness, loss of righting reflex, ptosis (1-6 h after treatment);
loose stools; diuresis, diarrhoea, debilitation (24 h after treatment); recovery within 8-10 days
b Application on intact and abraded skin; one death, but no signs of toxicity or irritation
c Nose only; nominal concentration, 133 mg/litre; particle size, 12 µm
d Only one male and one female
In a poorly reported study, groups of 10 male and 10 female ICR
mice were fed diets containing 0, 365, 620, 1100, 2000, or 3300 ppm
fenarimol (purity unspecified) for three months, equivalent to 0, 52,
88, 157, 286, or 471 mg/kg bw per day. Food consumption was not
measured, and ophthalmological examinations were not performed. There
were no treatment-related effects on mortality, clinical signs of
toxicity, haematology, or body weight. A statistically significant
reduction in total bilirubin was observed in animals of each sex at
the two highest doses and in males at 1100 ppm. Hepatic para-
nitroanisole metabolism, determined in five animals of each sex in
each group, showed a significant dose-related increase in females at
> 1100 ppm and in males at > 2000 ppm. Relative liver weights
were increased in a dose-related manner in males at > 365 ppm (10%
increase) and in females at > 620 ppm (11% increase) and reached
statistical significance at 620 and 1100 ppm, respectively. Absolute
liver weights were increased at > 620 ppm. The main effects seen on
histopathological examination of tissues other than nervous tissue
were centrilobular hypertrophy and hepatomegaly in the livers of males
at 1100 ppm and 2000 ppm, respectively, and in females at the highest
dose. A shift from a diffuse distribution of fat droplets to a more
periportal distribution was observed in animals of each sex at
> 2000 ppm. In kidneys, a higher incidence of fat droplets was
observed in females at > 2000 ppm. The NOAEL was 365 ppm,
equivalent to 52 mg/kg bw per day (Hoffman et al., 1975c).
Rats
In a pilot study reported in little detail, four male and four
female male Wistar rats were fed diets containing fenarimol (purity
unspecified) at 0 (10 animals per group), 25, 50, 100, 200, 400, 800,
1600, 3200, 6400, or 12 800 ppm for 14 days. Three animals at the high
dose died. Signs of toxicity, including anorexia, piloerection,
ataxia, and tremors, and decreased body weight and food consumption
were seen at doses > 3200 ppm. In the liver, the only organ
investigated, there were dose-related increases in relative weight at
> 400 ppm and in the rate of hepatic metabolism of para-
nitroanisole at > 200 ppm. Histopathological examination of the
liver revealed centrilobular hypertrophy at > 400 ppm and focal
necrosis at > 6400 ppm; no effects were seen at < 100 ppm
(Hoffman et al., 1975d).
In a subsequent study in male Wistar rats, also poorly reported,
four animals per treatment group and 10 controls were fed diets
containing fenarimol (purity unspecified) at 0, 25, 50, 100, 200, 400,
800, 1600, 3200, or 6400 ppm for 14 days. Food consumption and body
weight were reduced at > 1600 ppm; increased absolute and relative
liver weights were seen at > 800 ppm and increased hepatic
microsomal protein content at > 400 ppm. A dose-related reduction
in glucose-6-phosphatase activity was seen at > 800 ppm. The rate
of hepatic metabolism of para-nitroanisole was increased in a
dose-related fashion at > 200 ppm. No effects on the liver were
seen at < 100 ppm (Hoffman et al., 1975e).
In another poorly reported study, groups of 20 male and 20 female
Wistar rats were fed diets containing 0 (25 animals per sex), 50, 200,
or 800 ppm fenarimol (purity unspecified) for three months, equivalent
to 0, 2.5, 10, or 40 mg/kg bw per day. Five animals of each sex in
each group were kept for a recovery period of two weeks. No
ophthalmological examinations were performed. There were no treatment
-related effects on mortality, clinical signs of toxicity, food
consumption, haematology, or clinical chemistry (only blood urea
nitrogen, glucose, and alanine aminotransferase measured). A slight
depression in body weight was observed in males at the high dose. A
dose-related increase in relative liver weight was observed in animals
of each sex, which was statistically significant at the high dose. The
relative weights of the kidneys of males and females and of the
thyroid of females were increased at the high dose. Hepatic para-
nitroanisole metabolism, determined in five animals of each sex in
each group, showed a significant, dose-related increase in females at
800 ppm and in males at > 200 ppm. The main effect seen on
histopathological examination of tissues other than nervous tissue was
centrilobular hypertrophy in males and a shift from slight to moderate
fatty metamorphosis in females at the high dose. At the end of the
recovery period, only increased liver weight was still observed at the
high dose. The NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw per day
(Hoffman et al., 1975f).
In a study with no recovery period and again with little detail
reported, groups of 10 male and 10 female Wistar rats were fed diets
containing 0, 140, 200, 275, 365, or 500 ppm fenarimol (purity
unspecified) for three months, equivalent to 0, 7, 10, 14, 18, or
25 mg/kg bw per day. There were no treatment-related effects on
mortality, clinical signs of toxicity, body weight, food consumption,
haematology, clinical chemistry, or histopathology (nervous tissue was
not examined). A dose-related increase in relative liver weight was
observed in females at > 275 ppm and in males at 500 ppm. Hepatic
para-nitroanisole metabolism, determined in five animals of each sex
in each group, showed a dose-related increase, which was statistically
significant at > 275 ppm. The NOAEL was 200 ppm, equivalent to
10 mg/kg bw per day (Hoffman et al., 1975g).
Rabbits
A 21-day study of dermal toxicity was conducted in groups of five
male and five female New Zealand white rabbits, which received daily
applications of 0 or 1000 mg/kg bw technical-grade fenarimol (purity,
97%) or 500 or 1000 mg/kg bw of a wettable powder formulation
containing 50% fenarimol for 6 h/day. A further group received
1000 mg/kg bw of the formulation and was then held for a recovery
period of 14 days. Technical-grade fenarimol induced no treatment-
related effect on appearance, behaviour, body weight, food
consumption, ophthalmological parameters, dermal irritation,
haematological or clinical chemical parameters, organ weights, or
gross or histological appearance of tissues. The NOAEL was thus the
highest dose tested, 1000 mg/kg bw per day. The formulation induced no
treatment-related systemic toxicity, but dermal irritation was seen in
both groups, characterized by slight to severe erythema and slight
oedema during the treatment phase. The irritation subsided within nine
days after exposure (Hoffman et al., 1985f).
Dogs
Groups of four male and four female beagle dogs were fed gelatin
capsules containing fenarimol (purity unspecified) at doses of 0,
1.25, 5, or 20 mg/kg bw per day for three months. No treatment-related
effect was seen on appearance, behaviour, body weight, food
consumption, ophthalmological, haematological, or clinical chemical
parameters, urinalysis, organ weights, hepatic para-nitroanisole
metabolism, or gross or histological appearance of tissues (excluding
nervous tissue). The NOAEL was thus the highest dose tested, 20 mg/kg
bw per day (Hoffman et al., 1975h).
Groups of six male and six female beagle dogs were given gelatin
capsules containing fenarimol (purity, 96.7%) at doses of 0, 1.25,
12.5, or 125 mg/kg bw per day for one year. Two dogs of each sex in
each group were maintained for a recovery period of three months. No
treatment-related effect was seen on mortality, body weight, food
consumption, or ophthalmological, haematological, or urinary
parameters. One, five, seven, and 12 dogs in the four groups had
soft, runny, or mucoid stools on two, 10, 21, and 34 occasions,
respectively. Transient enlargement of the mammary glands of female
dogs was seen in all groups, and there was a slight treatment-related
increase in incidence and duration in four animals in the group at the
highest dose, lasting for up to 46 days. At the high dose, increased
alkaline phosphatase and liver para-nitroanisole O-demethylase
activities and increased absolute and relative liver weights were
observed in animals of each sex; a trend to increased relative ovarian
weight was also observed at this dose. These increases were still seen
after recovery. The only treatment-related histopathological finding
was mild hepatic bile stasis in one female in the treatment phase and
in two males after the recovery period. The NOAEL was 12.5 mg/kg bw
per day (Hoffman et al., 1985g).
(c) Long-term toxicity and carcinogenicity
In many of the studies described below, increased blood glucose
was found. Although apparent dose-dependent increases were observed in
several studies, the end-point (measured only at termination) was very
variable and inconsistent, usually with flat dose-response curves. No
clear explanation could be found for these apparent increases. The
effect was considered to be compound-related but toxicologically
insignificant and was not taken into account in establishing the NOAEL
for each study.
Mice
Groups of 20 male and 20 female ICR mice (30 of each sex in the
control group) were fed diets containing 0, 50, 170, or 600 ppm
fenarimol (purity, 97%), equivalent to 0, 7.1, 24.3, or 85.7 mg/kg bw
per day, for 12 months. Food consumption was not measured, urinalysis
was not performed, and haematology and clinical chemistry were
determined only at termination. There were no treatment-related
effects on mortality, clinical signs of toxicity, or haematology.
Body-weight gain of males at 600 ppm was slightly decreased. A
dose-related increase in blood glucose levels was observed at
> 50 ppm, which was statistically significant at 600 ppm in males
and at 170 ppm in females. A significant increase in serum creatinine
concentration was seen in females at 170 and 600 ppm. The activity of
alanine aminotransferase was increased in males at the high dose. The
absolute and relative liver and spleen weights were increased in males
at 600 ppm, and relative liver weight was increased in females at
600 ppm. Absolute and relative uterine weights were decreased at this
dose. A slight, dose-related increase in the incidence of very slight
or slight fatty change in the liver was observed, reaching 10-20% at
600 ppm (0% in controls). The NOAEL was 170 ppm, equivalent to
24.3 mg/kg bw per day (Hoffman et al., 1978a).
In two replicate studies, groups of 40 male and 40 female ICR
mice (60 animals of each sex in the control group) were fed diets
containing 0, 50, 170, or 600 ppm fenarimol (purity, 97.9%),
equivalent to 0, 7.1, 24.3, or 85.7 mg/kg bw per day, for two years.
Food consumption was not measured, and haematology and clinical
chemistry were carried out only at termination. Since the results of
the replicate studies were similar, they were reported as a single
study. There were no treatment-related effects on mortality, clinical
signs of toxicity, or haematological or clinical chemical parameters.
Body-weight gain of males at 600 ppm was slightly decreased. A
tendency towards increased relative liver weights and a slightly
increased incidence of hepatic fatty change (2.5-6%; 1-2% in controls)
were observed in animals of each sex at the high dose. The tumour
incidence was not enhanced. Although the laboratory considered that
there were no significant effects in these studies, the slight
reduction in body weight suggests minimal toxicity. The NOAEL was thus
170 ppm, equivalent to 24.3 mg/kg bw per day (Hoffman et al.,
1978b).
Rats
Wistar rats of the first parental generation in a multi-
generation study of reproduction {see Hoffman et al., 1977b) were
maintained on treated diets for another three months after the end of
the study and thereby received the diets for a total of one year.
Groups of 20 male and 20 female rats (30 of each sex in the control
group) received doses of 0, 50, 130, or 350 ppm fenarimol (purity,
97.9%), equivalent to 0, 2.5, 6.5, or 17.5 mg/kg bw per day.
Haematology and clinical chemistry were evaluated only at termination;
no urinalysis was performed, and nervous tissue was not examined at
histopathology. There were no treatment-related effects on mortality,
clinical signs of toxicity, or body weight. The limited data available
on food consumption (intake during weeks 1, 8, 42, and 54 only)
indicated no effects. Males at the high dose had increased erythrocyte
counts and and decreased leukocyte counts. Blood glucose levels were
elevated in males at all doses (not dose-related but highest at the
high dose) and in females at 130 and 350 ppm. A dose-related reduction
in blood urea nitrogen was observed in males at > 50 ppm. Absolute
and relative liver weights and relative kidney weight were increased
in females, and absolute and relative spleen weights were decreased in
males at the high dose. Slight atrophy of the acini of the pancreas
was observed more frequently at the high dose, predominantly in males.
The effects on clinical chemical parameters are not considered to be
adverse. The NOAEL was thus 130 ppm, equivalent to 6.5 mg/kg bw per
day (Hoffman et al., 1978c).
Groups of 20 male and 20 female Wistar rats (30 of each sex in
the control group) received diets containing 0, 50, 130, or 350 ppm
fenarimol (purity, 97.9%) for 18 months, equal to 0, 2.3, 6.0, or
16 mg/kg bw for males and 0, 3.6, 8.4, or 22.9 mg/kg bw per day for
females. Urinalysis and ophthalmological examinations were not
performed, and haematology and clinical chemistry were evaluated only
at termination. There were no treatment-related effects on mortality
(survival was a slightly higher at the highest dose), food
consumption, clinical signs of toxicity, or clinical chemistry. A
reduction in growth was seen consistently in all treated groups; on
several occasions, 10% reductions in body weight were seen at 130 and
350 ppm. At 350 ppm, a slight decrease in leukocyte count was observed
in males and in prothrombin time in females. A dose-related decrease
in serum prolactin levels was observed in females, but it was not
statistically significant, and the individual levels within the groups
were very variable. Relative liver weight was increased in all treated
groups, but the reduction was statistically significant only at
350 ppm. At this dose, absolute and relative ovarian weights were
increased, and absolute spleen weights were reduced in males. A slight
increase in the incidence of a fatty liver was seen in all treated
males (60-70%; 43% in controls), and an increase in the severity of
fatty changes in liver was observed in females at the high dose. The
NOAEL was 50 ppm, equal to 2.3 mg/kg bw for males and 3.6 mg/kg bw per
day for females (Hoffman et al., 1978d).
In two replicate studies, groups of 40 male and 40 female Wistar
rats (60 animals of each sex in the control group) were fed diets
containing 0, 50, 130, or 350 ppm fenarimol (purity, 97.9%), equal to
0, 2.0, 5.3, or 14.6 mg/kg bw for males and 0, 2.8, 7.6, or 21.5 mg/kg
bw for females, for 24 months. Haematology and clinical chemistry were
evaluated only at termination. Since the results of the two replicate
studies were similar, they were reported as a single study. There was
a dose-related increase in the rate of survival, increasing in males
from 27% (controls) to 44% (high dose) and in females from 38 to 54%;
however, the rate was not sufficient to meet the criteria for a valid
negative result in a study of carcinogenicity. There were no
treatment-related effects on clinical signs of toxicity or organ
weights. Body-weight gain of males was slightly reduced at 350 ppm
during the first nine months of the study. The leukocyte count was
decreased in animals of each sex at the high dose. Dose-related
increases in blood glucose levels were seen at all doses, which were
statistically significant in males at the high dose and in one study
at > 50 ppm in females. In one study, a dose-related reduction in
mean serum prolactin values was seen in females at > 50 ppm, which
reached statistical significance at 350 ppm; however, individual
values were very variable, with similar ranges in all groups. Males
also showed a statistically significant increase in prolactin levels
at 350 ppm, and females had increased levels of luteinizing hormone.
Although hormone levels were investigated to elucidate the effects of
fenarimol on reproduction, the laboratory considered the toxicological
significance of the observed changes to be unknown. An increased
incidence of fatty changes in the liver, which tended to be dose-
related, was seen especially in males; the overall incidences in males
and females in the two studies were 29, 40, 43, and 53% at 0, 50, 130,
and 350 ppm. An increase in severity was observed in animals of each
sex at the high dose. The incidence of hyperplastic nodules was
slightly increased: 0% in controls, 1.9% at 50 ppm, 1.9% at 130 ppm,
and 4.4% at 350 ppm, and reached statistical significance at the high
dose. In addition, hepatic-cell adenomas were seen in 0.6% of rats at
130 ppm and in 3.2% at 350 ppm (statistically significant). There was
no NOAEL, owing to the slight effects on the liver at the low dose
(Hoffman et al., 1978e).
A further two-year carcinogenicity study was conducted in order
to establish a no-effect level for the effects on the liver observed
in the previous study. Groups of 50 male and 50 female Wistar rats
were fed diets containing 0, 12.5, 25, or 50 ppm fenarimol (purity,
96.7%), equal to 0, 0.6, 1.2, or 2.5 mg/kg bw for males and 0, 0.7,
1.5, or 3.0 mg/kg bw per day for females, for 24 months. Haematology
and clinical chemistry were evaluated only at termination, and the
liver was the only organ examined at necropsy. The survival rate was
low due to an outbreak of chronic respiratory disease during the 16th
and 17th months: only 10-32% per sex per group survived for two years
There were no treatment-related effects on clinical signs of toxicity,
haematology, or liver weight. Reductions in body-weight gain, food
consumption, and food efficiency were observed in males at the high
dose during the first year of the study. The glucose content of blood
was increased in animals of each sex at 50 ppm. The incidence of fatty
liver was increased at the high dose, especially in males (60%; 26% in
controls). There was no evidence of an increase in tumour incidence,
but the survival rate was not sufficient to meet the criteria for a
valid negative result in a study of carcinogenicity. The NOAEL was
25 ppm, equal to 1.2 and 1.5 mg/kg bw per day for males and females,
respectively (Hoffman et al., 1982a).
Groups of 50 male and 50 female Wistar rats were fed fenarimol
(purity, 97%) at 0, 12.5, 25, or 50 ppm in their diet, equal to 0,
0.5, 1.0, or 2.0 mg/kg bw for males and 0, 0.6, 1.2, or 2.3 mg/kg bw
per day for females, for 24 months. Haematology and clinical chemistry
were evaluated only at termination, and the liver was the only organ
weighed. There was no treatment-related effect on survival rates
(29-44% for males and 56-74% for females), body-weight gain, food
consumption, food efficiency, haematology, hepatic para-nitroanisole
metabolism, liver weight, or histopathology of any organ. Slight
increases in the incidences of low body weight and pale eyes were
noted in all treated males. Blood glucose levels were increased at
25 ppm in males and at 50 ppm in animals of each sex. The tumour
incidence was not increased. The effects seen were considered not to
be adverse. The NOAEL was therefore 50 ppm, the highest dose tested,
equal to 2.0 and 2.3 mg/kg bw per day for males and females,
respectively (Hoffman & Pierce, 1985).
In a further analysis of the data on carcinogenicity from the
study conducted in 1986, the incidences of hepatocellular adenoma and
carcinoma at the high dose in the replicate study and at the low dose
in the last study were combined and analysed for trend. There was no
statistically significant evidence that fenarimol is oncogenic
(Rodricks et al., 1989).
In a study conducted to assess the initiating activity of
fenarimol, groups of six or 12 male Fischer 344 rats were fed 350 ppm
fenarimol (purity unspecified) in the diet for eight or 20 weeks;
one group fed fenarimol for eight weeks was subsequently fed
phenobarbital for 12 weeks. In order to investigate promotion
potential, hepatocellular foci were induced by exposure to N-2-
fluorenylacetamide for eight weeks, and then rats were fed 350 ppm
fenarimol for 12 weeks. Livers were examined after eight or 20 weeks
for the presence of altered hepatocellular foci that were iron-
excluding or contained gamma-glutamyl transpeptidase. Fenarimol had no
initiating or promoting activity in rat liver (Hoffman & Amundson,
1985).
(d) Reproductive toxicity
Mice
In two pilot studies, groups of 10 male and 10 female ICR mice
were fed fenarimol (purity, 97.9%) in the diet. In the first study,
treatment with doses of 50, 170, or 600 ppm was started one week
before mating and was continued up to day 21 of lactation. In the
second study, treatment with doses of 0, 170, 350, or 600 ppm was
started two weeks before mating and continued up to day 14 of
lactation. One female at 600 ppm died and one was killed with signs of
dystocia. No effect on body weight was detected. Fertility was clearly
reduced (and fewer females had copulatory plugs) at 350 and 600 ppm in
the second study only, and there was evidence of reduced fertility at
170 ppm. Liveborn litter size was reduced at 350 and 600 ppm,
apparently as a result of an increased incidence of stillbirths. A
lengthened gestation period was observed at 600 ppm No NOAEL could be
established owing to the limited study design (Markham et al.,
1978a).
In a three-generation study, groups of 20 male and 20 female ICR
mice (30 of each sex in the control group) were fed fenarimol (purity,
97.9%) at dietary levels of 0, 35, 70, or 140 ppm, equivalent to 0, 5,
10, or 20 mg/kg bw per day, starting 61-63 days before mating. Food
consumption was not measured, and the surviving parental animals were
not necropsied. No compound-related effects were observed in the
parents, on reproductive parameters, or on the pups. The NOAEL was
140 ppm, equivalent to 20 mg/kg bw per day (Markham et al., 1978b).
Rats
In a two-generation study, groups of 20 male and 20 female Wistar
rats (30 of each sex in the control group) were fed fenarimol (purity,
97.9%) in the diet at concentrations of 0, 50, 130, or 350 ppm,
equivalent to 0, 12.5, 6.5, or 17.5 mg/kg bw per day. Food consumption
was not measured. F0 rats were exposed for 55 days before the first
of three matings; after the third mating, the animals were maintained
on the test diets for another three months and then evaluated for
long-term toxicity (see Hoffman et al., 1978c). The F1b offspring
were destined to become F1 parents. The F1 animals were exposed to
the test diets for 58 days before the first mating. After weaning, the
F1 parents were placed on control diet for 63 days before a second
mating (reversibility test). For the third mating, a reversibility and
cross-over experiment was performed: the F1 animals continued to be
exposed to control diets, for a total of about five months by the time
of mating. F1 males were mated with untreated virgin females and
F1 females with untreated males. In each of the three F1
breedings, one female at 350 ppm died, perhaps due to dystocia. The
F1 males at 130 and 350 ppm had decreased body-weight gain. There
was a dose-related reduction in fertility (proportion of pregnant
females) at 130 and 350 ppm, and the effect was time-dependent, being
more pronounced after each successive F0 mating and even more
pronounced after the first F1 mating, in which fertility was 20 and
0%, respectively, at 130 and 350 ppm. There was some evidence of
reduced fertility at 50 ppm, but the effects were not statistically
significant. After the F1 parent animals had been fed the control
diet, fertility was 45 and 10%, respectively. Cross-over analysis
showed clearly that the reduced fertility was male-mediated, and there
was some, inconclusive evidence that it might also be female-mediated:
the fertility of treated females was lowered at all doses. The control
value for untreated females was, however, low, and age may have been a
factor since the females were over 10 months old A reduction in
liveborn litter size was seen at 350 ppm after the F0 matings,
probably due to an increase in the proportion of stillborn pups. In
all treated groups, but especially at 350 ppm, there were more females
with a lengthened gestation period (23-24 days) than among controls.
There was no consistent, substantive effect on the survival rate or
body weight of the progeny. Gross necropsy of weanlings revealed an
increased incidence (14.2% compared to 5.2% in controls; not
statistically significant) of hydronephrosis at 350 ppm after the
third mating of the F0 generation. No NOAEL could be established for
reproductive effects, because of the slight evidence of reduced
fertility at the lowest dose. The NOAEL for general systemic toxicity
in parental animals was 50 ppm, equivalent to 2.5 mg/kg bw per day
(Hoffman et al., 1977b).
In a further three-generation study, groups of 20 male and 20
female Wistar rats (30 of each sex in the control group) were fed
fenarimol (purity, 97.9%) in the diet at concentrations of 0, 12.5,
25, or 50 ppm, equivalent to 0, 0.625, 1.25, or 2.5 mg/kg bw per day.
Exposure started 56-71 days before the first mating, and three
generations of one, two, and one nests were bred. The surviving
parental animals were not necropsied. The death of one female during
parturition and signs of haemorrhage in another female during
parturition (signs of dystocia), both at 50 ppm, were attributed to
treatment. Body-weight gain was slightly reduced during the premating
period in F1 males at 50 ppm. After both F1 mating periods,
fertility was clearly reduced at 50 ppm, together with a reduction in
the proportion of females with copulatory plugs, and there was some
evidence of a reduction in fertility after the F2 mating at 50 ppm. A
statistically significant, dose-related reduction in liveborn litter
size (apparently due to an increase in stillbirths) at 25 and 50 ppm
after the second F1 mating was not considered to be related to
treatment by the laboratory; however, it occurred only after a long
exposure and has been seen to be a clear result of shorter exposure to
a higher concentration of fenarimol (see Hoffman et al., 1977b). The
NOAEL for reproductive effects was 12.5 ppm, equivalent to 0.625 mg/kg
bw per day. The NOAEL for general systemic toxicity was 25 ppm,
equivalent to 1.25 mg/kg bw per day (Markham et al., 1978c).
A single-generation study of cross-over design was conducted in
groups of 10 male and 10 female Wistar rats fed fenarimol (purity,
97.9%) by gavage at a dose of 0 or 35 mg/kg bw per day, starting one
month before mating and continuing up to one month thereafter for
males and up to day 20 post partum for females. In two groups,
control males were mated with control and treated females, and in the
third group treated males were mated with control females. The
fertility of treated males was reduced, together with a reduction in
the proportion of females with copulatory plugs, and the liveborn
litter size of treated females was reduced, apparently due to an
increased incidence of stillbirths. There was no NOAEL (Hoffman
et al., 1980b).
Groups of 15 male and 15 female Wistar and Sprague-Dawley rats
were compared with regard to their susceptibility to reproductive
effects under identical conditions in a cross-over study similar to
that described above (Hoffman et al., 1980e), except that the serum
levels of luteinizing hormone and prolactin were also investigated. In
both strains, body-weight loss and dystocia were observed during the
first week of treatment. Fertility was reduced in treated males, as
was the number of females with copulatory plugs. The mean liveborn
litter size was reduced in Sprague-Dawley rats only, due to an
increased incidence of stillbirths. Post-partum survival was reduced
in treated females of both strains. The serum levels of luteinizing
hormone were increased and those of prolactin decreased in treated
females of both strains. There was no NOAEL (Hoffman et al., 1980c).
In another single-generation study of cross-over design, groups
of 10 male and 10 female Wistar rats were fed fenarimol (purity,
97.9%) by gavage at 0 or 35 mg/kg bw per day, starting one month
before mating up to one month thereafter, encompassing gestation and
lactation. In two groups, control males were mated with control and
treated females, and in the third group treated males were mated with
control females. The serum levels of prolactin, luteinizing hormone,
and testosterone and para-nitroanisole- O-demethylase activity were
measured. Two treated females died due to dystocia. Fertility was
reduced in treated males, and the proportion of females with
copulatory plugs and vaginal sperm was reduced. In all cases in which
there was evidence of mating, it was not delayed, and the females
became pregnant. The liveborn litter size of treated females was
reduced, apparently due to an increased incidence of stillbirths; in
addition, gestation length was increased, and progeny survival through
day 7 post partum was slightly reduced. Absolute and relative liver
weights and liver enzyme activity were increased in treated males
and females; treated males also had hypertrophy of centrilobular
hepatocytes. There was no NOAEL (Hoffman et al., 1980d).
Guinea-pigs
In a two-generation study., groups of 15 male and 30 female
guinea-pigs of the F0 generation and 20 males and 20 females of the
F1 parent generation were fed fenarimol (purity, 98.5%) at dietary
levels of 0 or 400 ppm, starting four weeks before mating for the F0
parents and 70 days before mating for the F1 parents. There were
no treatment-related effects on mortality or food consumption.
Body-weight gain was reduced in males of both generations and in F0
females during the premating period. There was no effect on
reproductive parameters, except that the proportion of liveborn
progeny was marginally lower in treated animals of each generation.
The laboratory considered this to be a reflection of litter size, i.e.
lower survival in larger litters, which has previously been documented
in guinea-pigs. Although the data presented support this view for the
control groups, it does not do so for the treated groups. Therefore,
400 ppm, equal to 33 mg/kg bw per day for males and 35 mg/kg bw per
day for females, slightly affected gestational survival, although this
is not of clear toxicological importance (Hoffman et al., 1983a).
Rabbits
In a single-generation stud), of cross-over design, groups of
9-11 male and 9-11 female Dutch belted rabbits were fed fenarimol
(purity, 97.9%) by gavage at 0 or 35 mg/kg bw per day, starting one
month before mating and continuing up to one month thereafter for
males and up to day 6 post partum for females. In two groups,
control males were mated with control and treated females, and in the
third group treated males were mated with control females. Prolactin
and testosterone levels were determined, and premating semen volume,
sperm count, and sperm viability were measured. There was no evidence
of systemic toxicity or adverse reproductive effects. The. NOAEL was
the only dose tested, 35 mg/kg bw per day (Hoffman et al., 1980e).
(e) Developmental toxicity
Rats
Groups of 25 pregnant Wistar rats were fed fenarimol (purity,
97.9%) by gavage at 0, 5, 13, or 35 mg/kg bw per day during days 6-15
of gestation. None of the dams died, and there were no signs of
toxicity and no effects on food consumption or body weight. The only
finding in fetuses was an increased incidence of hydronephrosis at the
high dose (in 30% of fetuses and 62% of litters, in comparison with 9%
of fetuses and 25% of litters in controls). The NOAEL for maternal
toxicity was 35 mg/ kg bw per day and that for embryo- and
fetotoxicity, 13 mg/kg bw per day (Hoffman et al., 1980f).
A special study was conducted to investigate the reversibility of
fenarimol-induced hydronephrosis in neonatal rats. In three replicate
studies, pregnant Wistar rats were fed fenarimol (purity, 99.6%) by
gavage at 0 or 35 mg/kg bw per day during days 6-15 of gestation. Two
groups were sacrificed on day 20 or 21 of gestation, and the others
were allowed to litter and wean their progeny. The normal prenatal and
postnatal examinations were performed, and the kidneys and ureters of
the progeny were examined on days 20 and 21 of gestation and on days
1, 7, 21, 42, and 63 post partum. The total numbers of female rats
examined on gestation days 20 and 21 were 25 and 15, respectively;
postnatally, 15 in the control group and 20 in the test group were
examined. There were no treatment-related effects on dams with regard
to mortality, body weight, or food consumption, but sporadic cases of
dystocia were noted. Prenatal examination revealed a slight decrease
in the proportion of live fetuses due to an increase in early
resorptions. Fetal weights were slightly lower on day 20 but not day
21; on day 20, the number of runt fetuses was also increased.
Hydroureter and hydronephrosis were seen more often among exposed
fetuses on days 20 and 21, and a slightly increased incidence of
skeletal variants, such as cervical ribs and 14 thoracic fibs, was
observed, but only day-20 fetuses were examined. Fetal kidney weights
were depressed by treatment. Postnatal examination revealed a slight
increase in gestational length and reduced liveborn litter size.
Furthermore, neonatal mortality was increased during week 1 post
partum, pup body weights were increased, hypothermia was more
common, and the absolute kidney weights of the progeny were increased.
A reduction in the increased incidence of hydroureter, which was seen
prenatally, was observed with advancing neonatal age, but a
significant increase in hydronephrosis was seen in progeny on day 1
post partum (50% of treated pups and 23% of control pups). At
subsequent examinations, the incidence was still increased but was
substantially less than on day 1 post partum. The slight or moderate
microscopic alterations associated with the observed hydronephrosis
were limited to the renal pelvis and papilla and therefore did not
constitute true pathological hydronephrosis, which is associated with
severe damage, such as glomerular destruction, shortening and blunting
of renal tubules, and reduction or absence of the outer nephrogenic
layer. There was no effect on kidney function, including specific
gravity and osmolality, determined on day 63 post partum. As the
observed hydronephrosis was apparently not a significant pathological
condition and was to at least some degree reversible, together with
the fact that it is a common finding in the Wistar rat, its increased
incidence is considered to be due to delayed development and not a
teratogenic effect (Hoffman et al., 1983b).
Rabbits
Groups of 15 pregnant Dutch-belted rabbits were fed fenarimol
(purity, 97%) by gavage at 0, 3, 10, or 35 mg/kg bw per day on
gestation days 6-18 and were sacrificed on gestation day 28. There
were no treatment-related effects on mortality, body weight, or food
consumption. 4 slight reduction in litter size was observed at 10 and
35 mg/kg bw, but this was not considered to be related to treatment
since the number of implantations and the implantation index were also
reduced in these groups. The occurrence of oedema and various limb and
skeletal defects in five fetuses from one anorectic female at 35 mg/kg
bw was not clearly a direct response to treatment. There was no
evidence of irreversible structural changes. In the absence of clear
evidence of maternal or developmental toxicity, the NOAEL was 35 mg/kg
bw per day, the highest dose tested (Hoffman et al., 1977c).
Groups of 20 female New Zealand white rabbits were fed fenarimol
(purity, 96.6%) by gavage at 0, 15, 50, or 150 mg/kg bw per day on
gestation days 6-18 and were sacrificed on gestation day 28. There
were no treatment-related effects on mortality. Body weight and food
consumption were reduced at 150 mg/kg bw, and four rabbits at the
highest dose lost weight, became anorectic, and then aborted. The
number of live fetuses per litter was reduced, and there was an
increase in the number of early resorptions (neither statistically
significant). The only finding in fetuses was an increased incidence
of extrathoracic ribs (84% of fetuses, compared with 61% in controls).
There was no evidence of irreversible structural changes. The NOAEL
for maternal and embryo- and fetotoxicity was 50 mg/kg bw per day
(Hoffman & Russel, 1990).
(f) Genotoxicity
A number of tests for genotoxicity have been carried out with
fenarimol. The results are summarized in Table 2.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
A single, 4-h, semi-occluded application of 0.5 g technical-grade
fenarimol (purity stated to be 100%) onto the intact skin of three
male New Zealand white rabbits produced no skin irritation (Jones,
1994a).
After instillation of 0.1 ml (equivalent to 56 mg) of technical-
grade fenarimol (purity stated to be 100%) into the conjunctival sac
of the eyes of one male and two female New Zealand white rabbits,
slight conjunctival irritation was observed up to 24 h. Iridial
inflammation was noted in one treated eye 1 h after treatment. The
treated eyes appeared to be normal after 24-48 h (Jones, 1994b).
Fenarimol (purity, 97%) was tested in a Magnusson-Kligman
maximization test in 10 Hartley guinea-pigs; the control group
consisted of five animals. Barely perceptible erythema were seen in
two treated animals and in one of four surviving controls (Hoffman &
Arthur, 1980).
Table 2. Results of tests for the genotoxicity of fenarimol
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutationa S. typhimurium TA100, 125-2000 µg/plateb; 97 Negatived Hoffman et al.
TA98, TA1535, TA1537, 62.5-1000 µg/platec; (1988a)
E. coli WP2uvrA- toxicity and precipitation
at highest concentration;
vehicle, dimethyl sulfoxide
Chromosomal aberrationa Human lymphocytes 1.75-25 µg/mlb for 25 h 97 Negatived Murli (1988)
59.9-160 µg/mlc for 1 he;
vehicle, dimethyl sulfoxide
Gene mutationa Mouse lymphoma 1-50 µg/ml for 4 h; 97 Negatived Hoffman et al.
L5178Y tk+/-cells cytotoxic at > 35 µg/ml; (1988a)
vehicle, dimethyl sulfoxide
Unscheduled DNA Rat hepatocytes 0.05-100 nmol/ml for 5 h; 97 Negativea Probst (1979)
synthesisa,f cytotoxic at > 50 nmol/ml;
vehicle, dimethyl sulfoxide
In vivo
Chromosomal aberration 10 male Chinese hamsters; Oral; 250 mg/kg bw per day 98 Negatived Siou & Lerond-
bone-marrow cells for 2 daysg; sacrifice at 24, Conan (1982)
48, and 72 h; vehicle, peanut oil
Micronucleus formation Groups of 10 male Swiss Oral; 1000 mg/kg bw per day > 98 Weakly Siou et al. (1982)
mice; hone-marrow cells for 2 daysh; sacrifice at 24, positivei
48, and 72 h; vehicle, peanut oil
Micronucleus formation Groups of five male and Oral; 125, 625, or 1250 mg/kg 97 Negatived Ivett (1988)
five female Sprague-Dawley bwj; sacrifice at 24, 48, and
rats; bone-marrow cells 72h; vehicle, corn oil
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
In vivo (con't)
Dominant lethal mutation Groups of 10 male Wistar- Oral; 350 mg/kg bw; vehicle, 97 Inconclusivek Hoffman et al.
Harlan rats 5% acacia solution (1977d)
a No confirmatory experiment
b Without metabolic activation
c With metabolic activation
d Positive controls yielded positive result(s)
e As cell cycle delay was observed with metabolic activation in a preliminary test, incubation was for 45.3 h after treatment.
No cell cycle delay without activation; toxicity at highest doses tested
f Only one culture per concentration; only 20 cells per concentration analysed
g No mention of cytotoxicity; dose based on preliminary test in which all five animals died at 1000 mg/kg bw per day lot two days
h No mention of cytotoxicity; dose based on preliminary test in which all five animals died at 4000 mg/kg bw per day lot two days.
In the main test, one animal treated for 72 h died.
i Slight but statistically significant increase in micronuclei at 24 h sacrifice only; i.e. micronucleus frequencies per
1000 polychromatic erythrocytes; at 24 h: control, 0.19 ± 0.03; treated, 0.33 ± 0.06
j Toxic signs (lethargy, unsteady gait) at > 125 mg/g bw; one animal died at 1250 mg/kg bw.
k Treated males bred with untreated females weekly for eight weeks. Dose > 1/10 of the LD50. No sign of toxicity observed.
Only 7-10 pregnant females per mating interval per dose group. No reference to positive controls. No treatment-related effect on
fertility rate or pre- or post-implantation losses.
(ii) Mechanisms of effects on reproduction
Groups of six adult male Sprague-Dawley rats were given 35 mg/kg
bw per day fenarimol (purity unspecified) by gavage or subcutaneously
for 30 days; eight males served as controls. After 15 days of
treatment, each male was mated with two untreated females. No effect
was seen on male body weight, male fertility, the histopathology of
the testis or ventral prostate, or serum levels of testosterone,
follicle-stimulating hormone, or luteinizing hormone. Epididymal
weight was reduced after oral administration, but no histopathological
effects were observed. Serum prolactin levels were reduced after
systemic exposure, and prostatic prolactin binding was reduced after
exposure by either route. In a second experiment, groups of 15 males
were fed 0 or 35 mg/kg bw per day by gavage for 70 days and were bred
30 and 56 days after exposure. The parameters measured were the same
as in the first experiment except for follicle-stimulating hormone.
Serum prolactin levels and prostatic prolactin binding were reduced,
and the serum testosterone level was slightly elevated. There were no
other effects (Hanlin, 1981a).
Groups of 10 adult female Sprague-Dawley rats received fenarimol
(purity, 97%) at 0 or 35 mg/kg bw per day by gavage for about 60 days
before mating with untreated males, and then throughout pregnancy and
lactation. There were no effects on body weight or fertility. There
was some suggestion of an increase in proestrous prolactin level, but
the serum levels of prolactin were variable both before estrus and
during lactation and those of luteinizing hormone were variable before
estrus. Overall, there was no clear compound-related effect. There was
some evidence of a slight increase in estrous cycle length (Smalstig,
1981a).
Groups of 30 male Wistar rats were fed a diet containing 0 or
350 ppm fenarimol (purity, 97.7%) from 25 to 82 days of age. Twenty in
each group were mated and 10 were unmated; unmated males were killed
at the end of treatment, and the remaining animals were fed control
diet until day 117. Mating trials were conducted weekly with untreated
females on days 40-110. There were no significant effects on food
consumption or body-weight gain. A delay in the onset of reproductive
behaviour (percent mating and inseminating) was observed, but the
effect was reversible, since 100% fertility was attained at day 110.
The only effect on male reproductive organs was a reduction in
epididymal weight at the end of the treatment phase, with recovery
after cessation of treatment. No tissues were examined microscopically
(Hoffman & Hirsch, 1981c).
In a study of the possible involvement of uterine prostaglandins
in parturition, female Sprague-Dawley and Charles River CD rats
received 35 mg/kg bw per day fenarimol (purity, 97%) or the corn oil
vehicle orally from day 12 of pregnancy until parturition, or day 23
of pregnancy if no parturition occurred. The prostaglandin F2µ,
content, its release in and from the uterus, and serum progesterone
levels were measured some days before and at parturition. Sprague-
Dawley females showed delayed and/or difficult parturition and a
slight increase in the frequency of fetal and pup death, which
appeared to be related to the occurrence of difficult parturition.
There was no effect on prostaglandin F2µ, content; its release at
parturition was increased and not decreased, which would have been
expected with delayed parturition. Serum progesterone levels appeared
to be higher in treated females, and particularly in those that did
not deliver. The authors interpreted this finding as indicating that
progesterone did not fall to the low levels that facilitate or
initiate parturition in the rat (Smalstig, 1981b).
Progesterone concentrations in plasma were determined in late
pregnancy in groups of 12 Sprague-Dawley rats treated with 0 or
350 ppm fenarimol (purity unspecified) during pregnancy. Most of the
treated animals experienced delayed parturition associated with
dystocia and usually resulting in maternal death. High plasma
progesterone levels (66% of the peak value seen on day 19, in
comparison with 13% in controls) were seen in treated animals
immediately before normal parturition. The authors interpreted this
finding as indicating that luteolysis had not occurred, thus delaying
normal parturition (Tinsley, 1982).
A simulated rat ovarian microsomal system was used to measure the
effect of fenarimol on aromatase activity in vitro. Fenarimol
(purity unspecified) at molar concentrations 5-50 times the substrate
concentration inhibited aromatase activity by 55-90%. In a comparison
with aromatase inhibitors used clinically, fenarimol was as potent as
testolactone but less potent than aminoglutethimide and was therefore
considered to be a moderately weak inhibitor (Hoffman et al.,
1982b).
The urogenital sinuses were removed from 15-17-day-old male and
female Charles River rat embryos and implanted under the kidney
capsules of intact syngeneic male hosts (10 implants per animal). One
implanted male was then treated with 35 mg/kg bw per day fenarimol
(purity unspecified) for 21 days, one received the corn oil vehicle,
and one was treated with the positive control compound, cyproterone
acetate, which has anti-androgenic effects. Treatment with fenarimol
or corn oil had no effect on the differentiation or function of
transplanted urogenital sinuses in adult male rats. In contrast,
cyproterone acetate retarded prostatic development, and secretions
were absent (Neubauer et al., 1982).
Concentrations of 10-9 to 5 × 10-5 mol/litre fenarimol (purity
unspecified) were tested for androgen-binding capacity in vitro.
Fenarimol did not show affinity for binding to prostatic androgen
receptors, although 17% inhibition was seen at the highest
concentration. In an assay to determine whether fenarimol inhibits the
uptake of 3H-testosterone into the ventral prostate or the median
basal hypothalamus and preoptic areas of the brain, groups of 8-10
male Sprague-Dawley rats were given 0 or 35 mg/kg bw fenarimol
subcutaneously 20 and 3 h before sacrifice. Testosterone uptake was
not inhibited; in fact, uptake into the ventral prostate was
increased, whereas a positive control substance inhibited uptake into
the ventral prostate. In an assay to determine whether fenarimol
inhibits androgen-stimulated growth of seminal vesicles and ventral
prostate after castration, groups of six to eight immature rats were
given a daily subcutaneous injection of 0.25 mg/kg bw testosterone
propionate plus a daily oral or subcutaneous dose of 0, 5, 15, or
35 mg/kg bw fenarimol three days after castration for seven
consecutive days. A dose-related reduction in seminal vesicular weight
was seen with oral doses > 15 mg/kg bw, but the effect was not
statistically significant. The authors of the study concluded that
there was no inhibition (Hanlin, 1981b).
In a study to investigate whether fenarimol interferes with the
binding of androgens to the androgen receptor, groups of nine
castrated adult Wistar rats were fed fenarimol (purity unspecified) at
0 or 350 ppm in the diet for seven days. Cytosol from the hypothalamic
preoptic area, the frontal and parietal cerebral cortex, the
pituitary, and the ventral prostate was then incubated with
radiolabelled methyltrienolone (R1881; a potent synthetic androgen).
No reduction in binding to the cytoplasmic androgen receptors was
seen. In a second experiment, groups of one to two male rats were
treated similarly and then with radiolabelled R1881 before sacrifice.
There was no effect on binding to nuclear androgen receptors in the
tissues examined (the hypothalamic preoptic area, the pituitary, the
amygdala, the cerebral cortex, the prostate, the diaphragm, or the
liver), and the concentrations of conjugated and unconjugated labelled
R1881 were similar in all tissues. In a third experiment, pregnant
rats were fed 0 or 350 ppm fenarimol in the &let for most of the
period of gestation. Four female pups from each group were given a
subcutaneous injection of 3H-testosterone at the age of three or
four days and were killed 2 h later. There was no effect on the
binding of testosterone to the nuclear receptors in the hypothalamic
preoptic area amygdala (Hoffman et al., 1981a).
In a study of the estrogenic and anti-estrogenic potential of
fenarimol, groups of six immature female Holtzman rats received either
35 mg/kg bw fenarimol (purity unspecified) by gavage plus a
subcutaneous dose of estradiol, 35 mg/kg bw fenarimol by gavage, or a
subcutaneous dose of estradiol daily for three days. One dose of
fenarimol with several doses of estradiol, several doses of fenarimol
(8.75, 17.5, and 35 mg/kg bw) with one dose of estradiol, and
fenarimol in different vehicles were tested. There was no evidence for
agonistic or antagonistic effects on estrogen, as indicated by changes
in uterine weight. Fenarimol at concentrations of 1-1000 nmol/litre
did not inhibit the binding of estradiol to estrogen receptors in the
cytosol of immature rat uteri in vitro (Black, 1981a).
In a study of the effect of fenarimol on the binding of estrogen
to its receptor and on circulating estrogen levels at parturition,
fenarimol (purity unspecified) had no significant effect on the
nuclear uptake of estrogens by the hypothalamic preoptic area amygdala
or pituitary in groups of six adult ovariectomized rats fed 350 ppm in
the diet for seven days. Treatment of groups of four to six female
Wistar rats on days 0-21 of gestation at this dose did not affect the
concentration of estrone or estradiol in the plasma on day 21 (Hoffman
et al., 1981b).
Groups of three immature Dutch belted female rabbits were given
subcutaneous rejections of 0.5 µg oestradiol for six days and then
received either daily subcutaneous injections of 100 µg progesterone,
daily gavage doses of 60 mg fenarimol (purity unspecified), or the two
treatments combined, for five days. There was no evidence for a
progestational or anti-progestational effect in uteri examined
microscopically 24 h after the last dose (Black, 1981b).
Groups of seven pregnant Wistar rats were fed a diet containing
350 ppm fenarimol (purity, 97.7%) on gestation days 0 to 14-18. On the
last day of treatment, maternal blood and two fetuses per litter were
sampled 2 and 6 h after a gavage dose of radiolabelled fenarimol.
Placental transport was limited (the ratio of 14C in fetus to that
in maternal plasma was < 1) during days 14-17, the period associated
with genital tract morphogenesis, but increased steadily from day 15
onwards, to reach a ratio of 1 by day 18, when the organization of
sexual behaviour in the fetal brain begins (Hoffman & Hirsch, 1981a).
Female Wistar rats were fed 0 or 350 ppm fenarimol (purity,
97.7%) in the diet on days 0-5 post partum, and dams and pups
received an oral dose of radiolabelled fenarimol on day 5. One group
of 18 dams was used to study excretion into milk; the pups of seven
other dams were culled to three males and three females per litter.
The concentration of radiolabel in milk was three to five times higher
than that in maternal plasma up to 48 h after treatment. Radiolabel in
milk was rapidly taken up by the pups; the concentrations in the
hypothalamus increased more rapidly, attained higher maximal levels
(up to seven times higher 1 h after administration), and decreased
more slowly than in the remainder of the neonatal brain. In the
absence of previous exposure to fenarimol, an alteration was seen in
the rate but not the extent to which radiolabel was taken up by either
the hypothalamus or the brain. The major difference between pups
treated directly with 14C-fenarimol and those exposed via the milk
was in the rate of elimination, which was considerably faster after
direct treatment (Hoffman & Hirsch, 1981b).
In a study of the effects of fenarimol on the concentration of
estrogen receptors in the hypothalamus and on the conversion of
testosterone to estrogen, as an indirect measure of central aromatase
activity, pregnant Wistar rats received dietary concentrations of 0 or
350 ppm fenarimol (purity unspecified) from gestation day 3-4 to day
21. The concentration of nuclear estrogen receptors was measured in
the hypothalamic preoptic area amygdala of day-21 fetuses and of
neonates on day 3-4. The mean concentration was reduced by 44-46% in
fetuses of each sex and by 56% in neonates, especially in males.
Sex-specific differences in the concentration of receptors in the
exposed neonates were abolished, i.e. males no longer had higher
concentrations. In a second experiment, male Wistar rats that were
castrated but had testosterone implants and intact males received 0 or
350 ppm fenarimol in the diet for seven days. The concentration of
nuclear estrogen receptors was reduced in both groups. The circulating
levels of testosterone indicated that the effect on receptor
concentration could not be attributed to testosterone levels. In a
third experiment, pregnant Wistar rats received 0 or 350 ppm fenarimol
in the diet from about gestation day 18 to day 3-4 post partum, and
groups of 12 female neonates (used to eliminate endogenous
testosterone production as a variable) were then dosed with
radiolabelled testosterone. Significantly lower concentrations of
estrone and estradiol were observed in homogenates of hypothalamic
preoptic area amygdala from exposed pups, and, contrary to controls,
no labelled estradiol was detected in the nuclei. Fenarimol did not
affect the concentrations of testosterone or dihydrotestosterone in
the nuclear extracts of the hypothalamic preoptic area amygdala. Since
testosterone is metabolized to dihydrotestosterone by the enzyme
5µ-reductase and to estradiol by aromatase, this observation suggests
that the reduction in receptors is associated with inhibition of
aromatase (Hoffman et al., 1981c).
(iii) Relevance to humans of adverse reproductive effects in rodents
The adverse effect of fenarimol on the fertility of male mice
and rats appears to be due to an effect on the organization
(differentiation) and expression of male sexual behaviour, which is
known to be controlled within the central nervous system (Naftolin
et al., 1991). The mechanism is dependent on aromatization of
testosterone to estradiol-17ß. Evidence is available from several
studies that the expression of human male sexual behaviour has a
different hormonal basis from that in rats, i.e. depends on both a
direct action of testosterone and conversion of testosterone to
dihydrotestosterone, but not on the conversion of testosterone to
estradiol-17ß. Mantzoros et al. (1995) showed that the frequency of
human male sexual behaviour is correlated with the serum level of
dihydrotestosterone but not of estradiol-17ß. Gooren (1985)
administered an aromatase inhibitor (testolactone) or an estrogen
receptor antagonist (tamoxifen) to men and also concluded that
estradiol-17ß is not important in the control of male sexual
behaviour. Bagatell et al. (1994) showed that there was no change in
male sexual function after a treatment regimen involving testolactone,
testosterone, and a gonadotropin RH antagonist, which resulted in a
specific decrease in estradiol-17ß levels while keeping testosterone
and dihydrotestosterone at normal levels. A single study indicates a
role for estradiol-17ß in the expression of human male sexual
behaviour (Luisi & Franchi, 1980). The results showed that an
aromatizable form of testosterone is more effective in stimulating
libido than the non-aromatizable androgen mesterolone; however, this
result is difficult to interpret as it is not known whether
mesterolone can cross the blood-brain barrier.
Men with a genetic defect resulting in impaired androgen
receptor-mediated activity, but with no effect on estradiol-17ß
receptor-mediated activity or on aromatase, did not show normal male
sexual behaviour (Breedlove, 1994). A further study of genetic defects
(Smith et al., 1994) supports this conclusion, since an XY man
lacking functional estrogen receptors remained masculine, indicating
that estradiol-17ß does not control sexual differentiation in human
males; however, sexual activity was not assessed. Furthermore,
aromatase is confined mainly to the gonads and brain of rats and mice,
while it is also expressed in placenta, adipose tissue, and liver in
higher primates, including humans. Thus, the proportion of target
enzyme to inhibitor would be greater in humans than in rats, requiring
more inhibitor to achieve the same degree of inhibition at a given
target site.
Fenarimol would appear to have an adverse effect on reproductive
parameters in female mice and rats, as gestation and parturition are
extended, resulting in dystocia. This effect is also a direct effect
of the inhibition of aromatase, since circulating progesterone is
sustained at a high level, preventing timely luteolysis and resulting
in continued functioning of the corpora lutea and thus prolonging
gestation. In a review, Chwalisz (1994) outlined the mechanisms of
pregnancy and parturition in female rats. In order to maintain
pregnancy, rats depend on the corpus luteum as a source of
progesterone. Parturition requires an abrupt decrease in the
progesterone level, an increase in that of estrogen, and consequently
a decreased ratio of progesterone:estrogen. In contrast, humans (like
guinea-pigs) use the placenta as a source of progesterone during
pregnancy, and an abrupt decrease in progesterone secretion is not
required for parturition. These differences in endocrine control of
parturition, particularly the lesser role of estrogens in stimulating
labour in women, indicate that rats are probably not a relevant model
for predicting the effects of aromatase inhibition on human
parturition and that guinea-pigs are a better model.
Comments
Fenarimol given orally to rats was rapidly absorbed, distributed
and excreted. Elimination occurred principally in the faeces (76-83%),
mainly as a result of biliary excretion, and to a lesser extent in the
urine (6.5-9.2%). Residue levels in tissues were relatively low.
Dermal absorption of fenarimol by monkeys was low (about 2.5%). There
was some barrier to placental transport of fenarimol-derived
radiolabel in rats during part of the gestation period. Although the
levels of the radiolabel in the milk of lactating rats were three to
five times those in the plasma, only a very small proportion of the
dose (< 0.1%) was eliminated in the milk of a lactating goat.
Fenarimol is extensively metabolized to many metabolites. The
major pathways are oxidation of the carbinol-carbon atom, the
chlorophenol rings, and the pyrimidine ring. Repeated oral
administration to rats had no effect on the elimination of fenarimol
or its metabolites.
Fenarimol has low acute oral and dermal toxicity, the LD50
values in rats and rabbits being 2550 and > 2000 mg/kg bw
respectively. After inhalation, the LC50 was > 2 mg/litre (1-h
exposure). Fenarimol did not irritate skin or eyes and was not a skin
sensitizer in guinea-pigs in a maximization test. The WHO has
classified fenarimol as unlikely to present an acute hazard in normal
use.
In a 14-day pilot study in mice given dietary concentrations of
25-12 800 ppm, liver toxicity was observed at 800 ppm and above. In a
three-month study in mice given dietary concentrations of 0, 360, 620,
1100, 2000, or 3300 ppm, toxicity was observed mainly in the liver, as
increased liver weight, hepatic enzyme induction, centrilobular
hypertrophy, and hepatomegaly. The NOAEL was 360 ppm, equivalent to
52 mg/kg bw per day, on the basis of an increase in liver weight at
620 ppm.
In two 14-day pilot studies in rats given dietary concentrations
of 25-12 800 ppm, liver toxicity was observed at 200 ppm and above.
Dietary administration of fenarimol to rats for three months at 0, 50,
200, or 800 ppm also resulted in liver toxicity at and above
200 ppm, seen as increased liver weight, hepatic enzyme induction,
centrilobular hypertrophy, and fatty changes in the liver. The NOAEL
was 50 ppm, equivalent to 2.5 mg/kg bw per day, on the basis of
induction of liver enzymes. In another three-month study in rats, with
doses of 0, 140, 200, 280, 360, and 500 ppm, the NOAEL was 200 ppm,
equivalent to 10 mg/kg bw per day, on the basis of an increase in
liver weight and induction of hepatic enzymes at 280 ppm and above.
In a three-month study in dogs, oral administration of fenarimol
in gelatin capsules at doses of 0, 1.2, 5, or 20 mg/kg bw per day
resulted in no systemic toxicity. In a one-year study in which dogs
were given 0, 1.2, 12, or 120 mg/kg bw per day, toxicity was observed
at the highest dose. This included liver toxicity and, in female dogs,
a transient enlargement of the mammary gland and increased ovarian
weight; an increase in the occurrence of soft stools was observed at
all doses. The NOAEL was 12 mg/kg bw per day.
In a study of chronic toxicity, mice were administered fenarimol
at dietary levels of 0, 50, 170, or 600 ppm for 12 months. The NOAEL
was 170 ppm, equivalent to 24 mg/kg bw per day, on the basis of
decreased body-weight gain and effects on the liver at 600 ppm. In a
two-year study of carcinogenicity, mice were given diets containing 0,
50, 170, or 600 ppm fenarimol. The NOAEL was 370 ppm, equivalent to
24 mg/kg bw per day, on the basis of a slight decrease in body-weight
gain in males at 600 ppm. There was no evidence of carcinogenicity.
Two studies of chronic toxicity were performed in which rats were
administered dietary concentrations of fenarimol at 0, 50, 130 or
350 ppm for 12 or 18 months. In both studies, the main effects at the
highest dose were a decrease in the leukocyte count, increased liver
weight, and decreased spleen weight. In the 12-month study, the NOAEL
was 130 ppm, equal to 6 mg/kg bw per day. In the 18-month study, the
NOAEL was 50 ppm, equal to 2.3 mg/kg bw per day, on the basis of a
reduction in body weight.
Three carcinogenicity studies were performed in rats. In the
first, rats were administered diets containing 0, 50, 130, or 350 ppm
fenarimol. A small increase in the incidence of hepatocellular adenoma
was seen at the highest dose. There was no NOAEL, owing to a slight
increase in fatty changes and hyperplastic nodules in the liver at all
doses. No clear treatment-related effect on tumour incidence was seen.
Two further studies were performed at lower doses (O, 12, 25, or
50 ppm). In the first study, the survival was very low, owing to an
outbreak of chronic respiratory disease. There was evidence of reduced
body-weight gain and an increased incidence of fatty changes in the
liver at 50 ppm. No adverse effect was observed in the second study.
The overall NOAEL for all three studies was 25 ppm, equal to 1.2 mg/kg
bw per day. There was no evidence of carcinogenicity.
A three-generation study of reproductive toxicity in mice given
dietary levels of 0, 35, 70, or 140 ppm fenarimol revealed no adverse
effects on parental mice or offspring. In earlier pilot studies,
however, doses of 170 ppm and above reduced fertility and live-born
litter size and lengthened the gestation period. The NOAEL was
140 ppm, equivalent to 20 mg/kg bw per day.
In a two-generation study of reproductive toxicity in which
guinea-pigs were given 0 or 400 ppm fenarimol in the diet (equal to
33 mg/kg bw per day), parental toxicity was seen and there was
equivocal evidence of a slight effect on the proportion of live-born
progeny. In a single-generation study of reproductive toxicity with a
cross-over design, rabbits were treated orally with fenarimol at a
level of 0 or 35 mg/kg bw per day. There was no evidence of general
systemic toxicity or of effects on reproduction.
Two multigeneration studies of reproductive toxicity have been
performed in rats. In a two-generation study, rats were exposed to
dietary concentrations of 0, 50, 130, or 350 ppm, and in a three-
generation study to 0, 12, 25, or 50 ppm. Fenarimol reduced fertility,
caused dystocia, reduced live-born litter size and lengthened the
gestation period. In the first study, cross-over data showed that the
reduction in fertility was clearly mediated through the male and was
only slightly reversible. The overall NOAEL for general systemic
toxicity was 25 ppm, equivalent to 1.2 mg/kg bw per day, on the basis
of reduced body-weight gain in F1 males at 50 ppm and above. The
overall NOAEL for reproductive toxicity was 12 ppm, equivalent to
0.62 mg/kg bw per day, on the basis of a reduction in live-born litter
size at 25 ppm and above.
Three single-generation studies of reproductive toxicity with a
cross-over design have been conducted in rats, which were exposed
orally to fenarimol at doses 0 or 35 mg/kg bw per day. These studies
showed that the effects on reproduction were not strain-dependent, the
effects on fertility were clearly male-mediated, and the reduction in
live-born litter size (probably due to an increased incidence of
stillbirths) was female-mediated. No correlation was found between
fertility and the levels of prolactin, luteinizing hormone, or
testosterone, organ weights, histopathological findings, or sperm
morphology.
The single- and multigeneration studies of reproductive toxicity
show that fenarimol has adverse effects on reproduction in rats and
(at higher doses) in mice, but not in guinea-pigs or rabbits. A number
of studies were performed to investigate the mechanism of the adverse
effects on reproduction, and particularly those on fertility. The
results of these studies and of others in the open literature show
that fenarimol affects male sexual differentiation and subsequent
behaviour indirectly by inhibiting the aromatase-catalysed conversion
of testosterone to estradiol-17ß within the hypothalamus. Data from
the literature strongly indicate that while estradiol-17ß is the
central regulator of sexual differentiation in rats, human male sexual
behaviour is controlled by dihydrotestosterone, which is formed from
testosterone by the enzyme 5µ-reductase. There is evidence that the
effect of fenarimol in female rats (delayed parturition) is also due
to inhibition of aromatase, as this inhibition results in a sustained
level of circulating progesterone and prolonged corpus luteal
function, thus leading to delayed parturition. In contrast to rats, an
abrupt decrease in progesterone secretion is not required for
parturition in guinea-pigs or humans. The Meeting concluded that,
although not clear-cut, there is sufficient evidence that the adverse
effects on the organization and expression of sexual behaviour in male
rats and on delayed parturition in female rats are not relevant for
humans.
In a study of developmental toxicity, pregnant rats were given
fenarimol by gavage at doses of 0, 5, 13, or 35 mg/kg bw per day.
There was evidence of developmental toxicity (hydronephrosis) but no
signs of maternal toxicity at the high dose. In a study in which some
females were also allowed to litter and wean their progeny, pregnant
rats were exposed to 0 or 35 mg/kg bw per day during days 6-15 of
gestation. There were adverse effects on reproductive performance
(increased length of gestation, dystocia) and developmental toxicity
(increased hydronephrosis) but no evidence of general systemic
maternal toxicity. The increased incidence of hydronephrosis appeared
to be due to delayed development and was not considered a teratogenic
effect. Overall, the NOAEL for maternal toxicity was 35 mg/kg bw per
day, and that for embryo- and fetotoxicity was 13 mg/kg bw per day.
In a study of developmental toxicity in which rabbits were
administered fenarimol by gavage at doses of 0, 3, 10, or 35 mg/kg bw
per day, no signs of toxicity were observed in dams or fetuses. In a
second study, rabbits were exposed orally to doses of 0, 15, 50, or
150 mg/kg bw per day. The NOAEL for maternal toxicity was 50 mg/kg bw
per day, on the basis of reductions in body weight and food
consumption and an increased incidence of abortions. The NOAEL for
embryo- and fetotoxicity was also 50 mg/kg bw per day, on the basis of
a reduced number of live fetuses and an increased incidence of extra
ribs. There was no evidence of teratogenic potential.
Fenarimol has been adequately tested for genotoxicity in a series
of assays in vitro and in vivo. The Meeting concluded that
fenarimol is not genotoxic.
The Meeting concluded that the effects of fenarimol in male rats
(reduced fertility) and female rats (delayed parturition) are due to
inhibition of aromatase, an enzyme that is not involved in these
aspects of human reproduction. It therefore decided that it would be
inappropriate to use the NOAEL seen in studies of reproductive
toxicity in determining the ADI. An ADI of 0-0.01 mg/kg bw was
established on the basis of an overall NOAEL of 1.2 mg/kg bw per day,
seen in several studies of carcinogenicity in rats, and a safety
factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 24 mg/kg bw per day (12-month study of chronic toxicity)
20 mg/kg bw per day (three-generation study of reproductive
toxicity)
Rat: 1.2 mg/kg bw per day (three studies of carcinogenicity)
0.62 mg/kg bw per day (multigeneration study of reproductive
toxicity)*
13 mg/kg bw per day (embryo- and fetotoxicity in study of
developmental toxicity)
Dog: 12 mg/kg bw per day (one-year study of toxicity)
Rabbit: 50 mg/kg bw per day (maternal and embryo- or fetotoxicity in
study of developmental toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information valuable for the continued
evaluation of the compound
Observations in humans
* Data considered irrelevant for evaluation with respect to human
health.
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenarimol
Exposure Relevant route, study type, species Result, remarks
Short-term (1-7 days) Skin, irritation, rabbit Not irritating
Eye, irritation, rabbit Not irritating
Skin, sensitization, guinea-pig Not sensitizing
Oral, toxicity, rat LD50 = 2550 mg/kg bw
Dermal, toxicity, rabbit LD50 > 2000 mg/kg bw
Inhalation, toxicity, rat, 1 h LC50 > 2 mg/litre
Medium-term (1-26 weeks) Dermal, toxicity, 21 -day, rabbit No systemic effect; no irritation at
1000 mg/kg bw
Dietary, toxicity, three months, rat NOAEL = 2.5 mg/kg bw; hepaticenzyme
induction
Dietary, three-generation, reproductive NOAEL = 0.625 mg/kg bw; reduced
toxicity, rat live-born litter size
Gavage, developmental toxicity, rat NOAEL = 13 mg/kg bw;
hydronephrosis; no maternal toxicity
Long-term (> one year) Dietary, toxicity, two years, rat NOAEL = 1.2 mg/kg bw; fatty changes
in liver
References
Althaus, W.A. (1985a) Characterization and identification of
radioactivity in urine and feces of Wistar rats given single oral
doses mixed-labeled 14C fenarimol. Unpublished report No.
ABC-0310 dated December 1985 from Lilly Research Laboratories,
USA. Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Althaus, W.A. (1985b) Characterization and identification of
radioactivity in blood, liver and kidney of Wistar rats given
single oral doses of mixed-labelled 14C fenarimol. Unpublished
report No. ABC-0321 dated December 1985 from Lily Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Bagatell, C.J., Heiman, J.R., Rivier, J.E. & Bremmer, W.J. (1994)
Effects of endogenous testosterone and oestradiol on sexual
behaviour in normal young men. J. Clin. Endocrinol. Metab., 78,
711-716.
Black, L.J. (1981a) Evaluation of the estrogenic and antiestrogenic
potential of EL-222 in the immature rat uterus. Unpublished
report dated September 1981 from Lilly Research Laboratories,
USA. Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Black, L.J. (1981b) Evaluation of the progestational or
antiprogestational potential of EL-222 in immature rabbits.
Unpublished report dated September 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Breedlove, S.M. (1994) Sexual differentiation of the human nervous
system. Ann. Rev. Psychol., 45, 389-418.
Chwalisz, K. (1994) The use of progesterone antagonists for cervical
ripening and as an adjunct to labour and delivery. Hum,
Reprod., 9 (Suppl. 1), 131-161.
Goebel, G.V. (1985) Characterization of radioactivity in bile from
rats dosed orally with 14C fenarimol. Unpublished report No.
ABC-0312 dated May 1985 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Gooren, L.J.G. (1985). Human male sexual functions do not require
aromatisation of testosterone: A study using tamoxifen,
testolactone, and dihydrotesterone. Arch. Sex. Behav., 14,
539-548.
Hanlin, M.L. (1981a) Fertility studies with EL-222 in the adult male
rat. Unpublished report dated September 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hanlin, M.L. (1981b) Anti-androgenic studies with EL-222 in the male
rat. Unpublished report dated September 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom
Hoffman, D.G. & Amundson, M. (1985) Initiation and promotion study of
fenarimol on rat liver carcinogenesis. Unpublished report dated
November 1985 from Naylor Dana Institute for Disease Prevention,
Valhalla, New York, USA Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G. & Arthur, B.H. (1980) Guinea pig sensitization study
with Lilly compound 56722 (fenarimol). Unpublished report No.
GP-9538 dated January 1980 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G. & Hirsch, K.S. (1981a) Placental transport of EL-222 in
the Wistar rat. Unpublished report No. R10480 dated October 1981
from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G. & Hirsch, K.S. (1981b) Exposure of the neonatal rat to
EL-222 as a component of milk: Evidence for the presence of
EL-222 in the central nervous system during the organization of
adult sexual behaviour. Unpublished report No R01781 dated
October 1981 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G. & Hirsch, K.S. (1981c) A special fertility study with
EL-222 (fenarimol) in the Wistar rat. Unpublished report No
R07380 dated October 1981 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D G. & Pierce, E.C. (1985) A chronic toxicity/oncogenicity
study in Wistar rats maintained on diets containing low
concentrations of fenarimol for two years. Unpublished report No.
R07781 dated May 1985 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G. & Russel, D.R. (1990) A developmental toxicity study of
fenarimol (EL-222, compound 056722) administered orally to New
Zealand white rabbits. Unpublished report No. B00290 dated
October 1990 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Broddle, W.D., Gibson, W.R. & Morton, D.M. (1975a) The
acute toxicity of EL-222 in mice, rats, dogs, ducks mid quail.
Unpublished report dated August 1975 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Bernhard, N.R., Gibson, W.R., Owen, N.V. & Morton, D.M.
(1975b) A pilot 2-week subacute toxicity study of EL-222 in mice.
Unpublished report No. M-9074, M-9084 dated August 1975 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975c)
Three-month subacute oral toxicity studies on EL-222 in mice.
Unpublished report No. M-9015 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Bernhard, N.R., Gibson, W.R., Harris, P.N. & Morton,
D.M. (1975d) A pilot 2-week subacute toxicity study of EL-222 in
rats. Unpublished report No. R-713, R-723 dated August 1975 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Bernhard, N.R. & Morton, D.M. (1975e) A 2-week subacute
study of the effects of EL-222 on hepatic p-nitroanisole
metabolism in rats. Unpublished report No. R-244, R-254 dated
August 1975 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975f)
Three-month subacute oral toxicity studies on EL-222 in rats.
Unpublished report No. R-1013 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975g)
Three-month subacute oral toxicity studies on EL-222 in rats.
Unpublished report No. R-1164 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Harris, P.N. & Morton, D.M. (1975h)
Three-month subacute oral toxicity studies on EL-222 in dogs.
Unpublished report No. D-4183 dated August 1975 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Arthur, B.H., Gibson, W.R. & Morton, D.M. (1977a) The
acute dermal and ocular toxicity of technical EL-222 in rabbits.
Unpublished report dated January 1977 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Adams, E.R., Markham, J.K., Owen, N.V., Gibson, W.R. &
Morton, D.M. (1977b) A multi-generation reproduction study with
EL-222 in the rat. Unpublished report No. R-715, R-1345, R-956,
R-966 dated November 1977 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Markham, J.K., Adams, E.R., Owen, N.V & Morton, D.M.
(1977c) A teratology study on compound 56722 (EL-222) in the
rabbit. Unpublished report No. B-7125 dated January 1977 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Markham, J.K., Owen, N.V. & Morton, D.M. (1977d) A
dominant lethal study of compound 56722 (EL-222) in the rat.
Unpublished report dated January 1977 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G. et al. (1978a) Twelve-month chronic oral toxicity of
EL-222 (56722) in mice. Unpublished report No. M-9155 dated April
1978 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C., Harris, P.N. & Morton, D.M.
(1978b) Twenty-four month chronic oral toxicity of EL-222 (56722)
in mice. Unpublished report No. M-9135, M-9145, dated August 1978
from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G. et al. (1978c) A one-year toxicity study with EL-222
in the rat. Unpublished report No. R-715 dated June 1978 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C. & Morton, D.M. (1978d)
Eighteen-month chronic oral toxicity of EL-222 (56722) in rats.
Unpublished report No. R-435 dated May 1978 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C. & Morton, D.M. (1978e)
Twenty-four month chronic oral toxicity of EL-222 (56722) in
rats. Unpublished report No. R-405, R-415, dated April 1978 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Gomez, S.R. & Dorato, M.A. (1980a) The acute inhalation
toxicity of Lilly compound 56772, fenarimol, in the Fischer 344
rat. Unpublished report No. R-H-99-80 dated December 1980 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V. & Markham, J.K. (1980b) A cross-over
reproduction study with EL-222 (Lilly compound 56722) in the rat.
Unpublished report No. R-286 dated April 1980 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V. & Markham, J.K. (1980c) Additional
cross-over reproduction studies with EL-222 (Lilly compound
56722) in the Wistar and the Sprague-Dawley rat. Unpublished
report No. R-57, R-67 dated April 1980 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V & Adams, E.R. (1980d) A cross-over fertility
study with EL-222 in the Wistar rat. Unpublished report No.
R08379 dated June 1980 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Adams, E.R. & Owen, N.V. (1980e) A cross-over fertility
study with EL-222 in the Dutch-belted rabbit. Unpublished report
No. B7229, dated June 1980 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Owen, N.V. & Adams, E.R. (1980f) A teratology study
with EL-222 in the rat. Unpublished report No. R06279 dated
January 1980 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Hirsch, K.S. & MacLusky, N.J. (1981a) Effects of EL-222
on the binding of androgens to cytoplasmic and nuclear androgen
receptors. Unpublished report dated October 1981 from Yale
University Medical School Connecticut, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Hirsch, K.S. & MacLusky, N.J. (1981b) Effects of EL-222
on circulating estrogens and the binding of estrogens to estrogen
receptors in the rat central nervous system. Unpublished report
dated October 1981 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Hirsch, K.S. & MacLusky, N.J. (1981c) Effects of EL-222
on the concentration of estrogen receptors and the conversion of
testosterone to estrogens in the hypothalamus. Unpublished report
dated October 1981 from Yale University Medical School,
Connecticut, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Gibson, W.R., Pierce, E.C. & Morton, D.M. (1982a) A
low-dose chronic toxicity/oncogenicity study in Wistar rats
maintained on diets containing fenarimol for two years.
Unpublished report No. R06479 dated November 1982 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Brodie, A.M.H. & Hirsch, K.S. (1982b) An in vitro
measure of aromatase inhibition by EL-222. Unpublished report
dated January 1982 from the University of Maryland, Baltimore,
Maryland, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Markham, J.K & Miller, B.J. (1983a) A two-generation
reproduction study with fenarimol (EL-222, compound 56722) in
guinea pigs. Unpublished report No. G00682, G00483 dated December
1983 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Owen, N.V & Byrd, R.A. (1983b) The effect of prenatal
fenarimol (EL-222, compound 56722) exposure on kidney development
and maturation in the rat. Unpublished report No. R10682, R10782,
R10882 dated November 1983 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Masten, J.L. & Hanasono, G.K (1985a) The biliary
excretion of radioactivity by male and female Wistar rats given
single oral doses of 14C-fenarimol (compound 56722, EL-222).
Unpublished report No. R02485 dated April 1985 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Masten, J.L & Hanasono, G.K. (1985b) Tissue
distribution of radioactivity in Wistar rats determined one or
twenty-four hours after single oral doses of 14C-fenarimol
(EL-222, compound 56722). Unpublished report No. R16684 dated
April 1985 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Masten, J.L., Walker, C.G. & Hanasono, G.K. (1985c)
Radiocarbon disposition studies on Wistar rats given single
oral doses of 14C-fenarimol (EL-222, compound 56722):
Pharmacokinetics, and excretion/tissue distribution seven days
after dosing. Unpublished report No. R06484, R08584 dated March
1985 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Weaver, D.E. & Van Lier, R.B.L. (1985d) Distribution of
radioactivity into tissues and organs from Wistar rats given oral
doses of unlabeled fenarimol daily for two weeks followed by a
single dose of 14C-fenarimol (EL-222, compound 56722).
Unpublished report No. R01285 dated March 1985 from Lilly
Research Laboratories, USA. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Hoffman, D.G., Van Lier, R.B.L. & Weaver, D.E. (1985e) The
percutaneous absorption of 14C fenarimol dissolved in ethanol
in rhesus monkeys. Unpublished report No. P04085, P04585 dated
October 1985 from Lilly Research Laboratories, USA. Submitted to
WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Hoffman, D.G., Pierce, E.C., Brown, G.E. & Negliski, S. (1985f)
Subchronic (21-day) dermal toxicity study in rabbits with
technical fenarimol (EL-222, compound 56722) and Rubigan 50W, a
wettable powder formulation (FN-0742) containing 50% fenarimol.
Unpublished report No. B02183 dated May 1985 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Hoffman, D.G., Gries, C.L., Pierce, E.C., Means, J.R. & White, J.F.
(1985g) A one year chronic oral toxicity study of fenarimol in
dogs with a three month recovery period. Unpublished report No.
D02683 dated May 1985 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Richardson, K.A. & Kokkino, A.J. (1988a) The effect of
fenarimol (EL-222, compound 56722) on the induction of reverse
mutations in Salmonella typhimurium and Escherichia coli using
the Ames test. Unpublished report No. 880215AMT4, 880222AMS4,
dated August 1988 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Hoffman, D.G., Oberly, T.J. & Richardson, K.A. (1988b) The effect of
fenarimol on the induction of forward mutation at the thymidine
kinase locus of L51787 mouse lymphoma cells. Unpublished report
No. 880106MLT4, 880113MLA4, dated August 1988 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Ivett, J.L. (1988) Mutagenicity test on 56722 (EL-222) in the in vivo
rat micronucleus assay. Unpublished report No. 10348-0-455 dated
October 1988 from Hazleton, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Jones, J.R. (1994a) Fenarimol technical: Acute dermal irritation test
in the rabbit. Unpublished report No. 291/50 dated February 1994
from Safepharm Laboratories, United Kingdom. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Jones, J.R. (1994b) Fenarimol technical: Acute eye irritation test in
the rabbit. Unpublished report No. 291/51 dated February 1994
from Safepharm Laboratories, United Kingdom. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Luisi, M. & Franchi, R. (1980) Double blind group comparative study of
testosterone undecanoate and mesterolone in hypogonadal male
patients. J. Endocrinol. Invest., 3, 305-308.
Mantzoros, C.S., Georgiadis, E.I. & Trichopoulos, D. (1995)
Contribution of dihydrotestosterone to male sexual behaviour.
Br. Med. J., 310, 1289-1291.
Markham, J.K., Hoffman, D.G., Adams, E.R., Owen, N.V. & Morton, D.M.
(1978a) Pilot reproduction studies with EL-222 (Lilly compound
56722) in the mouse. Unpublished report No. M-9165, M-9215 dated
July 1978 from Lilly Research Laboratories, USA. Submitted to WHO
by DowElanco Europe, Wantage, Oxon, United Kingdom.
Markham, J.K., Hoffman, D.G., Broddle, W.D., Owen, N.V. & Morton, D.M.
(1978b) A multi-generation study with EL-222 (Lilly compound
56722) in the mouse. Unpublished report No. M-9086, M-9296,
M-9326 from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Markham, J.K., Hoffman, D.G., Owen, N.V. & Morton, D.M. (1978c) A
second multi-generation reproduction study with EL-222 (Lilly
compound 56722) in the rat. Unpublished report No. R-636, R-1076,
R-217 dated July 1978 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Murli, H. (1988) Mutagenicity test on 56722 in an in vitro cytogenetic
assay measuring chromosomal aberration frequencies in cultured
purified human lymphocytes. Unpublished report No. 10348-0-449
dated August 1988 from Hazelton, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Naftolin, F., Keefe, D., Apa, R., Palumbo, A. & Garcia-Segura, L.M.
(1991) The apparent paradox of sexual differentiation of the
brain. Contrib. Gynecol. Obstet., 18, 24-32.
Neubauer, B.L. et al. (1982) Effect of EL-222 (compound 56722,
fenarimol) on the developing reproductive tract. Unpublished
report dated January 1982 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Probst, G.S. (1979) The effect of Lilly compound 56722 (EL-222) on the
induction of DNA repair synthesis in primary cultures of adult
rat hepatocytes. Unpublished report No. 790502-1 dated June 1979
from Lilly Research Laboratories, USA. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Prout, M. (1994) The disposition of 14C fenarimol in the lactating
goat. Unpublished report No. IRI 154147 dated October 1994 from
Inveresk Research International, Musselburgh, United Kingdom.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Rodricks, J.V. et al. (1989) Response to USSR concerns regarding the
potential oncogenicity of fenarimol. Unpublished report dated
February 1989 from Environ Corp., Washington DC, USA. Submitted
to WHO by DowElanco Europe, Wantage, Oxon, United Kingdom.
Simpson, E.R., Mahendroo, M.S., Means, G.D., Kilgore, M.W.,
Hinshelwood, M.M., Graham-Lorence, S., Amarneh, B., Ito, Y.,
Fisher, C.R., Dodson, M.M., Mendelson, C.R & Bulun, S. (1994)
Aromatase cytochrome P450, the enzyme responsible for oestrogen
biosynthesis. Endocr. Rev., 15, 342-355.
Siou, G. & Lerond-Conan, L. (1982) Test for mutagenic potential of
technical-grade fenarimol by examination of chromosomal damage in
the Chinese hamster. Unpublished report No. 658 dated June 1982
from C.E.R.T.I., Versailles, France. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
Siou, G., Lerond-Conan, L. et al (1982) Test for mutagenicity of
technical-grade fenarimol using a micronucleus technique in the
mouse. Unpublished report No. 650 dated May 1982 from C.E.R.T.I.,
Versailles, France. Submitted to WHO by DowElanco Europe,
Wantage, Oxon, United Kingdom.
Smalstig, E.B. (1981a) Study of the possible influence of compound
EL-222 on fertility in the adult female rat. Unpublished report
dated September 1981 from Lilly Research Laboratories, USA.
Submitted to WHO by DowElanco Europe, Wantage, Oxon, United
Kingdom.
Smalstig, E.B. (1981b) Studies of the influence of EL-222 on uterine
prostaglandin F2alpha in the female rat at parturition.
Unpublished report dated November 1981 from Lilly Research
Laboratories, USA. Submitted to WHO by DowElanco Europe, Wantage,
Oxon, United Kingdom.
Smith, E.P., Boyd, J., Frank, G., Takahashi, H., Cohen, R.M., Specker,
B., Williams, T.C., Luhbahn, D.B. & Korach, K.S. (1994) Oestrogen
resistance caused by a mutation in the oestrogen-receptor gene in
a man. New Engl. J. Med., 331, 1056-1061.
Tinsley, F.C. (1982) Preparturiton progesterone levels in fenarimol
(EL-222) treated rats. Unpublished report dated August 1982 from
Lilly Research Laboratories, USA. Submitted to WHO by DowElanco
Europe, Wantage, Oxon, United Kingdom.
Watson, D. (1994) Accurate measurement of an isolated metabolite of
fenarimol. Unpublished report No. DWC/719 dated October 1994 from
Huntingdon Research Centre, United Kingdom. Submitted to WHO by
DowElanco Europe, Wantage, Oxon, United Kingdom.
