
PARATHION-METHYL (addendum)
First draft prepared by
M.S. Morrow,
US Environmental Protection Agency
Washington DC, USA
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive and developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Neurotoxicity
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Parathion-methyl was last evaluated toxicologically by the Joint
Meeting in 1984., when an ADI of 0-0.02 mg/kg bw was allocated
(Annex I, reference 42). Since the last evaluation, additional studies
have been conducted on this chemical, which are summarized in this
monograph addendum. Parathion-methyl was re-evaluated at the present
Meeting within the periodic review programme of the CCPR, with
particular attention to the recent WHO Environmental Health Criteria
monograph on parathion-methyl (EHC 145).
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Parathion-methyl can be absorbed through the skin and the
digestive and respiratory tracts. Absorption from the gastrointestinal
tract is rapid, and detection in the bloodstream has been reported
immediately after oral administration. Differences between the oral
and intravenous toxicity of parathion-methyl that have been reported
are believed to occur during the first pass through the liver.
Activation reactions by liver microsomal oxidases result in the
formation of the toxic substance, methyl paraoxon. Detoxification of
parathion-methyl and methyl paraoxon are accomplished by conjugation
with glutathione.
When parathion-methyl was administered intravenously to mongrel
dogs at doses of 1, 3, 10, or 30 mg/kg bw, a rapid decrease in serum
parathion-methyl concentrations was reported during the first few
hours, due primarily to distribution and elimination of the lower
concentrations. At the two higher doses, a slower decrease in serum
concentrations was reported and was associated with linear deep-
compartment kinetics (Braeckman et al., 1980).
Administration of labelled parathion-methyl to male and female
rats as a single oral dose or as a single oral dose preceded by 14
oral doses of nonradioactive test material resulted in rapid
absorption of the material from the gastrointestinal tract, with
62-94% of the radiolabel excreted within 8 h. By 48 h, 76-99% of the
label had been excreted, urinary excretion being identified as the
major route. Negligible amounts of label were found in the blood,
tissues, organs, and expired air (Van Dijk, 1988).
(b) Biotransformation
Conversion of parathion-methyl to its toxic metabolite,
methyl paraoxon, occurs within minutes of oral administration
(Yamamoto et al., 1983). The liver is the primary organ for
detoxification by reactions involving either O-demethylation or
hydrolysis and resulting in the formation of demethyl compounds or
dimethyl phosphoric acids. The main metabolites recovered from the
urine of humans after administration of parathion-methyl were
para-nitrophenol and dimethyl phosphate.
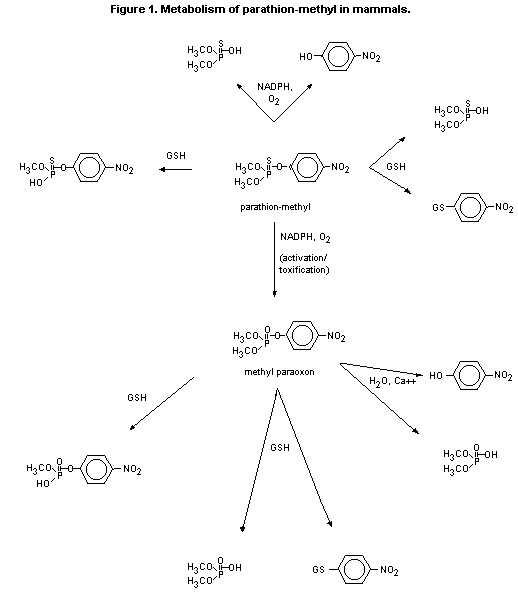
The metabolism of parathion-methyl in mammals is shown in
Figure 1.
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of parathion-methyl in rats is shown in
Table 1.
Table 1. Acute toxicity of parathion-methyl in rats
Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Male Oral 25 80 Cuthbert & Carr (1986)
Female 62
Male Dermal 483 80 Cuthbert & Carr (1986)
Female 481
Male Inhalation 0.135 80 Greenough & McDonald (1986)
(b) Short-term toxicity
Mice
Groups of 15 CD-1 mice of each sex were fed diets containing
parathion-methyl (purity, 93.65%) at levels of 0,10, 30, or 60 ppm.
The diets were analysed periodically to determine the content of
parathion-methyl. The mean body weights of males at 30 ppm were 3-7%
lower than those of controls, but only during the first five weeks of
the study. At the highest dose, the body weights of both males and
females were 4-20% lower than those of controls. No effects were
reported on mortality, clinical signs, or histopathological
appearance. A decrease in absolute and relative testicular weights was
reported in all males, but no histological abnormalities were seen.
Decreased ovarian weights were seen in females at the middle and high
doses. Cholinesterase inhibition was not measured. The NOAEL was
10 ppm, equivalent to 1.5 mg/kg bw per day (Daly & Rhinehart, 1980a).
 Rats
Groups of 20 Sprague-Dawley rats of each sex received diets
containing parathion-methyl (purity, 93.65%) at doses of 0, 2.5, 25,
or 75 ppm for 91-94 consecutive days. Females at the highest dose had
increased mortality during the first four weeks of the study, and
animals of each sex showed tremors, emaciation, anogenital staining,
and decreased body weight, although food consumption was greater than
that of controls. Erythrocyte parameters and the levels of glucose,
total protein, albumin, and globulin were decreased at one or more
intervals in animals at the high dose, and increases were reported in
serum aspartate aminotransferase and alkaline phosphatase activities
and in blood urea nitrogen. The haematological and serum chemistry
values were, however, within biologically normal ranges. Gross and
histopathological changes were reported in animals at the high dose,
including discoloured and abraded foci in the stomach, which were
correlated histologically with ulcerative gastritis, but these were
considered secondary to the stress that resulted from administration
of the test material. Additional histological findings included
lymphoid necrosis and necrosis of the submaxillary salivary glands. At
the medium dose, erythrocyte and plasma cholinesterase activities were
decreased in animals of each sex, whereas brain acetylcholinesterase
activity was inhibited only in females. Erythrocyte acetylcholine-
sterase activity was decreased in males at the low dose but not to a
biologically significant level. The NOAEL was 2.5 ppm, equivalent to
0.125 mg/kg bw per day, and was based on the decrease in brain
acetylcholinesterase activity in females (Daly & Rhinehart, 1980b).
Rabbits
Groups of five male and five female New Zealand white rabbits
received parathion-methyl (purity, 93.1%) topically at doses of 0, 1,
5, 10, or 100 mg/kg bw per day for 21 days. The compound was applied
as a 1% solution in carboxymethylcellulose to a depilated surface on
the body of each animal and was held in contact with the skin for 6 h
on each day of exposure. No dermal effects were reported in males, but
in females at 10 and 100 mg/kg bw per day inhibition of erythrocyte
acetylcholinesterase activity was seen. The LOAEL was 10 mg/kg bw per
day, and the NOAEL was 5 mg/kg bw per day (Goad, 1992).
Groups of six New Zealand white rabbits of each sex received
parathion-methyl (purity, 96.3%) topically at doses of 0,10, 50, or
250 mg/kg bw per day. One-half of the animals in each group were
tested on abraded skin. At > 50 mg/kg bw per day, brain and
erythrocyte acetylcholinesterase activities were inhibited. The NOAEL
was 10 mg/kg bw per day (Mihail & Vogel, 1984).
Dogs
Groups of two beagle dogs of each sex were given parathion-methyl
(purity, 94.3%) in the diet at levels of 0, 2.5, 5 or 10 mg/kg bw per
day for 14 days. Decreases in body-weight gain and/or body weight were
reported in males and females at the middle and high doses, and feed
consumption was significantly lower in all animals at the high dose
and in females at the middle dose. Cholinesterase inhibition was not
measured. The NOEL was 2.5 mg/kg bw per day, based on the effects on
body weight and food consumption at 5 mg/kg bw per day (Tegeris &
Underwood, 1977).
Groups of four beagle dogs of each sex were fed diets containing
parathion-methyl (purity, 94.32%) at levels of 0, 0.3,1, or 3 mg/kg bw
per day for 13 weeks (90 days). There were no effects on body weight,
food consumption, or mortality, and no clinical signs of toxicity;
however, significant decreases in brain, erythrocyte, and plasma
cholinesterase activities were seen in males and females at the high
dose at weeks 6 and 13. At the middle dose, plasma cholinesterase
activity was significantly decreased in males at week 13 and
erythrocyte cholinesterase was significantly decreased in animals of
each sex. The NOAEL was 1 mg/kg bw per day (Tegeris & Underwood,
1978).
Groups of eight beagle dogs of each sex received parathion-methyl
(purity, 94.9%) at doses of 0, 0.03, 0.3, or 3 mg/kg bw per day for 13
weeks, followed by a recovery period of up to seven weeks. Animals
were observed for clinical signs of toxicity; extensive
ophthalmological examinations were conducted, including fundic
observations, intraocular pressure, electroretinogram, and slit-lamp
examination. Brain cholinesterase was measured at terminal sacrifice.
Significant decreases (> 20% of control levels) were seen in plasma
and erythrocyte cholinesterase activities at weeks 6 and 13 in animals
at the highest dose, and at terminal sacrifice the brain choline-
sterase activity was 48% of that of controls. No functional or
morphological ocular impairment was observed. The NOAEL was 0.3 mg/kg
bw per day and the LOAEL was 3 mg/kg bw per day, based on the
decreases in brain cholinesterase activity (Daly, 1989).
(c) Long-term toxicity and carcinogenicity
Mice
Parathion-methyl (purity, 95.5%) was fed to male and female
B6C3F1 mice at dietary levels of 0, 1, 7, or 50 ppm for two years. No
carcinogenicity was seen. Clinical signs of toxicity were infrequent
in treated animals, and none of the gross or histopathological
findings were associated with treatment. Erythrocyte, plasma, and
brain cholinesterase activities were significantly inhibited in
animals receiving 50 ppm. Erythrocyte acetylcholinesterase activity
was also significantly inhibited (by 40-57% of the control value) in
animals at 7 ppm. The NOAEL was 7 ppm, equal to 1.6 mg/kg bw per day
(Eiben, 1991).
Rats
Groups of 70 Sprague-Dawley CD rats received diets containing
parathion-methyl (purity, 94.6%) at levels of 0, 0.5, 2.5, 12.5, or
50 ppm, equivalent to average intakes of 0.02, 0.107, 0.5, and
2.2 mg/kg bw per day, for one year. Fifty animals of each sex per
group were evaluated at the end of the study; two groups of five
animals per sex per group were evaluated for ocular toxicity at six
and 12 months; and two groups of five animals per sex per group were
evaluated for changes in peripheral nerves at six and 12 months. No
effects were seen on mortality, on the eye by ophthalmoscopic
examination, or on the optic nerve or retina microscopically. Clinical
signs of aggressiveness and hyperactivity were reported in animals
receiving 50 ppm; reduced mean body weights were also reported at this
dose. Plasma and erythrocyte cholinesterase levels were decreased by
> 20% of control levels in animals of each sex at the two highest
doses, and brain acetylcholinesterase activity was inhibited in
animals of each sex at the highest dose and in females at 12.5 ppm.
Peripheral neuropathy was seen in distal and proximal segments of the
sciatic nerve in animals at the two highest doses, which was
characterized by myelin bubbles and Schwann cell proliferation. An
increased percentage of large diameter fibres was seen in the tibial
nerves and an increased incidence of myelin ovoids in the sural nerves
of males receiving 12.5 ppm. Myelin bubbles and Schwann cell
proliferation were also seen in two of 10 rats at 2.5 ppm, but these
changes were not statistically significant nor of the same degree of
severity as those in rats receiving 12.5 ppm. The NOAEL was 2.5 ppm,
equal to 0.11 mg/kg bw per day (Daly, 1991).
Groups of 50 male and 50 female Wistar TNO/W74 rats received
diets containing parathion-methyl (purity, 94.8%) at levels of 2, 10,
or 50 ppm for two years. The control group comprised 100 rats of each
sex. No carcinogenic effects were seen. Most of the toxic effects were
observed at 50 ppm and included decreased body-weight gain, increased
food consumption, tremors, slightly increased mortality, and decreased
haemoglobin and haematocrit values in females at the high dose at
termination. Alkaline phosphatase activity was increased and plasma
protein levels were significantly lowered throughout the study,
perhaps correlated with an increase in urinary protein. The other
clinical findings were considered to be incidental. Plasma and
erythrocyte cholinesterase activities were slightly inhibited in rats
receiving 10 ppm of parathion-methyl, and brain cholinesterase
activity was only 78% of the control value in males at this dose.
Inhibition of plasma and erythrocyte cholinesterase activities was
reported in animals of each sex at 50 ppm, and the brain
cholinesterase activity was 50% of that reported for controls in males
and 37% in females. The NOAEL was 2 ppm, equivalent to 0.1 mg/kg bw
per day, and the LOAEL was 10 ppm, equivalent to 0.5 mg/kg bw per day,
based on a 22% reduction in brain acetylcholinesterase activity
(Bomhard et al., 1981).
Groups of 60 Sprague-Dawley rats of each sex received
parathion-methyl (purity, 93.65%) at dietary levels of 0, 0.5, 5, or
50 ppm for two years. No carcinogenic effects were seen. Toxic effects
were observed in animals at 50 ppm and included tremors, anogenital
staining, reduced body weight, retinal degeneration, decreases in
erythrocyte parameters, and sciatic nerve degeneration. The plasma,
erythrocyte, and brain cholinesterase activities of males at the high
dose at study termination were significantly lower than those of
controls (82, 9, and 76%, respectively, of the control value). In
females, brain cholinesterase activity was reduced by 79%. At 5 ppm,
an abnormal gait was reported in one female, and haemoglobin and
haematocrit and erythrocyte counts were slightly reduced in other
animals. No effects on cholinesterase activity were reported. The
NOAEL was 5 ppm, equivalent to 0.25 mg/kg bw per day (Daly, 1983).
Dogs
Groups of eight beagle dogs received parathion-methyl (purity,
93.7%) at levels of 0, 0.03, 0.1, or 0.3 mg/kg bw per day for one
year. Animals were observed daily, clinical chemistry and haematology
were assessed at designated intervals, and erythrocyte and plasma
cholinesterase activities were monitored monthly. Brain acetylcholine-
sterase activity was determined at study termination. No clinical
signs and no significant effects on body weight or food consumption
were reported, and clinical pathology, gross pathology, and
histopathology showed no compound-related changes. The NOAEL was
0.3 mg/kg bw per day, the highest dose tested (Ahmed & Saguartz,
1981).
(d) Reproductive and developmental toxicity
Rats
Groups of Wistar HAN rats were given parathion-methyl (purity,
97%) at doses of 0, 0.3, 1.0, or 3.0 mg/kg bw per day by gavage on
days 6-15 of gestation. Parental animals were monitored for mortality,
clinical signs of toxicity, body weights, food consumption, and
cholinesterase activity. Litters were evaluated for abnormalities,
sex, and weight. Five deaths and clinical signs of toxicity, including
somnolence, ataxia, and dyspnoea, were seen in maternal animals at the
high dose throughout the study. Increased post-implantation losses and
decreases in food consumption, body weight, and body-weight gain were
also reported, and plasma and erythrocyte cholinesterase activities
were decreased. Fetuses from dams receiving 3.0 mg/kg bw showed an
increased frequency of delayed ossification and reduced body weight.
The maternal and developmental NOAEL was 1 mg/kg bw per day
(Becker et al., 1987)
Inseminated female rats received parathion-methyl (purity, 94.4%)
by gavage on days 6-15 of gestation at doses of 0, 0.1, 0.3, or
1 mg/kg bw per day. At 1 mg/kg bw per day, maternal toxicity was seen,
manifested as significantly decreased body-weight gain during
treatment and gestation. Fetal weight was also significantly lower,
and the frequency of stunted fetuses was greater. The NOAEL for
maternal and developmental effects was 0.3 mg/kg bw per day
(Machemer, 1977).
In a multigeneration study of reproductive toxicity,
parathion-methyl (purity, 95%) was administered in the diets of 10
male and 20 female Wistar rats at levels of 0, 2, 10, or 50 ppm. Each
of the F0, F1, and F2 generations was mated twice. At 50 ppm,
convulsions were observed in F1b parents, and decreased growth was
reported among parental animals of all generations. At this dose,
adverse effects were reported on birth weight, litter size, and the
viability and growth of pups. At 10 ppm, fertility was decreased and
pup survival was slightly decreased in both the F1 and F2
generations. The NOAEL for reproductive toxicity was 2 ppm, equivalent
to 0.1 mg/kg bw per day (Löser & Eiben, 1982).
In a two-generation study of reproductive toxicity, groups of 15
male and 30 female Sprague-Dawley rats received parathion-methyl
(purity, 93.7%) at dietary levels of 0, 0.5, 5, or 25 ppm for 14 weeks
before mating of F0 parents and for 18 weeks before mating of F1
parents. Treatment was continued through gestation and weaning.
Selected F1 adults and five weanlings of each sex in each group per
generation were examined histologically. The body weights of females
at the high dose in both generations were reduced during the lactation
period, but the litters of both generations appeared to be unaffected
by treatment. No abnormalities were seen in reproductive performance,
as indicated by mating, pregnancy, and fertility rates, pup viability
and survival, and gross and microscopic lesions, nor were there
significant differences in the body weights of offspring. The NOAEL
was 5 ppm, equivalent to 0.25 mg/kg bw per day, for parental toxicity
and 25 ppm, equivalent to 1.25 mg/kg bw per day, for reproductive
toxicity (Daly 1982).
Rabbits
Groups of 15 inseminated Himalayan rabbits received parathion-
methyl (purity, 95.7%) at doses of 0, 0.3, 1, or 3 mg/kg bw per day by
gavage on gestation days 6-18. Fetuses were removed by caesarian
section on day 29. No effects were reported on litter parameters. In
maternal animals at 3 mg/kg bw, erythrocyte and plasma cholinesterase
activities were inhibited, but no other effects were reported. The
NOAEL was 1 mg/kg bw per day for maternal effects and
3 mg/kg bw per day for developmental effects (Renhof, 1984).
(e) Genotoxicity
The genotoxic effects of parathion-methyl are summarized in Table
2.
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Three male and three female New Zealand white rabbits received
0.1 ml of parathion-methyl (purity, 80%) by instillation into the
right eye. Slight redness was observed in the eyes of all rabbits 1 h
after instillation; all had returned to normal by 48 h.
Topical application of parathion-methyl (purity, 80%) to New
Zealand white rabbits was associated with very slight to moderate
erythema 1 and 24 h after removal of gauze patches. Slight oedema was
also observed in one of six rabbits. The skin of all animals was
normal by 48 h.
In the Magnusson-Kligman test, female guinea-pigs received six
intradermal doses of parathion-methyl (purity, 80%) in 5% paraffin oil
or emulsified with 0.05 ml of Freund's complete adjuvant. A challenge
concentration was applied two weeks after induction, and reactions
were assessed 24 and 48 h after the patch was removed. Parathion-
methyl was not considered to be a sensitizing agent in guinea-pigs
(Cuthbert & Carr, 1986).
(ii) Neurotoxicity
Groups of 10 male and 10 female Sprague-Dawley rats received
parathion-methyl (purity, 93.1%) by gavage as single doses of 0,
0.025, 7.5, 10 (males only), or 15 (females only) mg/kg bw.
Neurobehavioural evaluations, consisting of a battery of tests for
motor activity and functional observation, were conducted at
designated intervals during the 14-day observation period, and plasma
and erythrocyte cholinesterase activities were determined at several
intervals during the study. Acetylcholinesterase activity was measured
in six regions of the brain at the time of the peak effect and at day
Table 2. Results of tests for the genotoxicity of parathion-methyl
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 2-12 500 µg/plate approx. Positive in Herbold (1986a)
TA100, TA1535, TA1537 96% TA100 at
> 900 µg/plate
Reverse mutation S. typhimurium 2-12 500 µg/plate 94 Positive Herbold (1986b)
Unscheduled DNA Rat hepatocytes 3 × 10-5-3 × 10-2 µl/ml NR Negative Curren (1989)
synthesis
In vivo
Dominant lethal mutation Mouse 10 mg/kg bw 95.7 Negative Herbold (1984)
Micronucleus formation Bor:NMRI mouse 2 × 5, 2 × 10 mg/kg bw 95.6 Negative Herbold (1982)
NR, not reported
14. At the middle and high doses, parathion-methyl had transient
effects on motor activity and function, inducing lacrimation,
salivation, tremors, muscle fasciculations, ataxia, muscle weakness,
and miosis; and plasma, erythrocyte, and brain cholinesterase
activities were inhibited by 67, 56, and 76% at the time of the peak
effect, respectively. in males at the high dose, body-weight gain was
significantly lower than that of controls; similar findings were not
made in females, and the mean body weights were not affected. In males
at this dose, the number of sites at which focal demyelination was
present was increased in comparison with controls, with demyelination
in the cervical (3/6) and lumbar (5/6) dorsal root fibres, in the
cervical (2/6) and lumbar (4/6) ventral root fibres, and in the lumbar
spinal (1/6), tibial (3/6), and sural nerves (2/6). Similar findings
were not found in females. The NOAEL for acute neurotoxicity was
0.025 mg/kg bw (Minnema, 1994).
In the study of long-term toxicity by Daly (1991) in
Sprague-Dawley CD rats, ophthalmoscopic examination, electro-
retinograms, light microscopy, and electron microscopy revealed no
ocular toxicity. Microscopic and morphometric studies of various
preparations of the sciatic nerve and its extensions revealed several
lesions in animals at 12.5 and 50 ppm, which are consistent with
demyelination. These included myelin bubbles, myelin ovoids, Schwann
cell proliferation, and the presence of phagocytic cells. In addition,
increased lengths of demyelination were observed in teased nerve
preparations. The NOAEL for neurotoxicity was 2.5 ppm, equivalent to
0.11 mg/kg bw per day (Daly, 1992).
Hens received parathion-methyl (purity, 95.8%) by intubation at
doses of 0 or 250 mg/kg bw; the test dose thus exceeded the LD50 of
215 mg/kg bw. Positive control animals received 600 mg/kg bw of
tri- ortho-cresyl phosphate. A second dose was administered 21 days
after the first. No clinical signs were seen, and there were no
histopathological lesions in the brain, spinal cord, or nervous tissue
that were indicative of delayed neurotoxicity. One-half of the treated
hens died; surviving birds showed clinical signs of toxicity including
lethargy, depression, wing droop, salivation, shallow and rapid
respiration, and cyanotic comb. The hens recovered within about seven
days (Beaver et al., 1990).
3. Observations in humans
Five male volunteers received daily doses of 3 mg parathion-
methyl for 28 days, followed by 3.5 mg per day for the next 28 days
and then by 4 mg per day for 43 days. No signs of toxicity were
reported, and no effect on plasma or erythrocyte cholinesterase
activity was observed (Moeller & Rider, 1961).
Three groups of five volunteers were given 4.5 mg parathion-
methyl for 30 days, followed by 5 mg per day for 29 days or 5.5 mg per
day for 24 days. These doses were followed by 6 mg per day for 29 days
or by 6.5 mg/day for 35 days, and finally by 7 mg per day for 24 days.
No significant inhibition of plasma or erythrocyte cholinesterase
activity was reported (Moeller & Rider, 1962).
After oral doses of up to 19 mg to male volunteers for 30 days,
no evidence of toxicity was reported (Rider et al., 1969); however,
when doses of 24 mg were given daily for four weeks, plasma and
erythrocyte cholinesterase activities were inhibited, by 23-24% and
27-55%, respectively (Rider et al., 1970). In another study
(Rider et al., 1971), doses of parathion-methyl that were increased
from 14 to 20 mg over a 30-day period had no effect on cholinesterase
activity. The NOAEL was thus 0.3 mg/kg bw per day.
Comments
Parathion-methyl is absorbed through the skin and from the
respiratory and digestive tracts. Observed differences between its
oral and intravenous toxicity are believed to be associated with
first-pass effects in the liver. The compound is rapidly excreted;
negligible amounts of the labelled dose were present in the blood,
tissues, and organs at 48 h. Conversion of parathion-methyl to
paraoxon-methyl has been shown to occur within minutes after oral
administration to rats. Detoxification is achieved by O-demethylation
or hydrolysis to para-nitrophenol. In humans the primary urinary
metabolites were para-nitrophenol and dimethyl phosphate.
Parathion-methyl is acutely toxic at low doses when administered
either orally (LD50 = 4 mg/kg bw) or by inhalation (LC50 =
0.13 mg/litre). The compound is slightly irritating to the skin and
the eyes but has not been shown to be a sensitizing agent. WHO has
classified parathion-methyl as 'extremely hazardous'.
In a 90-day study in mice at dietary levels of 0, 10, 30, or
60 ppm, the NOAEL was 10 ppm (equivalent to 1.5 mg/kg bw per day) on
the basis of significant decreases in absolute and relative testicular
weights. Cholinesterase activity was not measured in this study. In a
90-day study in rats, the NOAEL was 2.5 ppm (equivalent to 0.12 mg/kg
bw per day) on the basis of significant decreases in plasma,
erythrocyte, and brain cholinesterase activities at 25 ppm (equivalent
to 1.2 mg/kg bw per day).
Two 13-week studies were conducted in dogs, in which parathion-
methyl was administered at dietary levels of 0, 0.3, 1, or 3 mg/kg bw
per day in one study and 0, 0.03, 0.3, or 3 mg/kg bw per day in the
other. In both studies, the LOAEL was 3 mg/kg bw per day, on the basis
of decreases in erythrocyte, plasma, and brain cholinesterase activity.
The NOAELs were 1 and 0.3 mg/kg bw per day, respectively. In a one-year
study in dogs, the NOAEL was also 0.3 mg/kg bw per day, the highest
dose tested.
In a one-year study in rats to determine the ocular and
neurotoxic effects of parathion-methyl, dietary levels of 0, 0.5, 2.5,
12, or 50 ppm were administered. Ocular toxicity was not observed.
Degenerative changes of the sciatic nerve and its extensions
consistent with demyelination were observed at the two highest doses.
The LOAEL was 12 ppm, equal to 0.5 mg/kg bw per day. The NOAEL was
2.5 ppm, equal to 0.1 mg/kg bw per day.
In a two-year study in mice, parathion-methyl was not carcino-
genic at dietary levels up to 50 ppm (equal to 9.2 mg/kg bw per day).
The NOAEL was 7 ppm, equal to 1.6 mg/kg bw per day, on the basis of
significant decreases in erythrocyte, plasma, and brain cholinesterase
activities. In a study of toxicity and carcinogenicity in rats fed
parathion-methyl at dietary levels of 0, 2, 10, or 50 ppm, there was
no evidence of carcinogenicity. The NOAEL was 2 ppm (equivalent to
0.1 mg/kg bw per day), and the LOAEL was 10 ppm (equivalent to
0.5 mg/kg bw per day), on the basis of a reduction in brain
acetylcholinesterase activity. In another two-year study In rats,
parathion-methyl did not induce carcinogenic effects. The NOAEL was
5 ppm (equivalent to 0.25 mg/kg bw per day) on the basis of tremors,
anogenital staining, reduced body weight, retinal degeneration,
sciatic nerve degeneration, decreased packed cell volume and
haemoglobin and erythrocyte counts, and decreased brain cholinesterase
activity in males and females at 50 ppm (equivalent to 2.5 mg/kg bw
per day).
Two studies of developmental toxicity were conducted in rats. In
one study, animals received parathion-methyl by gavage at doses of
0, 0.3,1, or 3 mg/kg bw per day. The NOAEL for maternal and
developmental toxicity was 1 mg/kg bw per day on the basis of
increased numbers of deaths, ataxia, and dyspnoea in dams and delayed
ossification in fetuses. In another study, the NOAEL for maternal and
developmental toxicity was 0.3 mg/kg bw per day on the basis of
significant decreases in maternal body-weight gain during treatment
and gestation and an increased incidence of stunted fetuses at 1 mg/kg
bw per day. In studies of developmental toxicity in rabbits, decreased
erythrocyte and plasma cholinesterase activities were reported in dams
receiving 3 mg/kg bw per day by gavage. No developmental effects were
reported that could be attributed to the administration of parathion-
methyl. The NOAEL for maternal toxicity was 1 mg/kg bw, and that for
developmental toxicity was 3 mg/kg bw.
In a multigeneration study in rats, dietary levels of 0, 2, 10,
or 50 ppm were administered. Slight effects on pup survival were
reported in animals receiving 10 ppm, equivalent to 0.5 mg/kg bw per
day. The NOAEL was 2 ppm, equivalent to 0.1 mg/kg bw per day. In
another study, in which dietary levels of 0, 0.5, 5, or 25 ppm were
administered, decreases in maternal body weights during the lactation
period were observed at 25 ppm. The NOAEL was 5 ppm, equivalent to
0.25 mg/kg bw per day. The overall NOAEL in the two studies of
reproductive toxicity was 5 ppm, equivalent to 0.25 mg/kg bw per day.
Parathion-methyl was mutagenic in bacteria, but there was no
evidence of genotoxicity in a limited range of studies in mammalian
systems.
The NOAEL derived from the combined results of several studies
conducted in humans, based on the depression of erythrocyte and plasma
cholinesterase activities, was 0.3 mg/kg bw per day.
An ADI of 0-0.003 mg/kg bw was established on the basis of the
NOAEL of 5 ppm, equivalent to 0.25 mg/kg bw per day, in the two-year
study in rats for retinal degeneration, sciatic nerve demyelination,
reduced body weight, anaemia, and decreased brain acetylcholinesterase
activity. A safety factor of 100 was used. Since the toxicological
end-points seen in animals were other than acetylcholinesterase
inhibition, a safety factor of 10 could not be applied to the NOAEL in
humans.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 7 ppm, equal to 1.6 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
Rat: 5 ppm, equivalent to 0.25 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
5 ppm, equivalent to 0.25 mg/kg bw per day (study of
reproductive toxicity)
0.3 mg/kg bw per day (maternal, embryo-, and fetotoxicity
and teratogenicity in study of developmental toxicity)
Rabbit: 1 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
3 mg/kg bw per day (no embryo- or fetotoxicity or
teratogenicity in study of developmental toxicity)
Dog: 0.3 mg/kg bw per day (one-year study of toxicity and
carcinogenicity)
Human: 0.3 mg/kg bw per day
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Estimate of acute reference dose
An acute reference dose of 0.03 mg/kg bw was derived by applying
the usual 10-fold safety factor to an NOAEL of 19 mg/kg bw (highest
oral dose), corresponding to about 0.3 mg/kg bw per day, in humans,
based on the absence of inhibition of erythrocyte acetylcholinesterase
activity.
Studies that would provide information useful for continued evaluation
of the compound
Observations in humans, particularly reports of poisoning
incidents and/or evaluation of potential long-term neurological and
behavioural effects.
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to parathion-methyl
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Skin, sensitization, guinea-pig Not sensitizing
Eye, sensitization, rabbit Slightly irritating
Skin, irritation, rabbit Slightly irritating
Inhalation 4-h, toxicity, rat LC50 = 0.13 mg/litre
Oral, toxicity, rat LD50 = 4-62 mg/kg bw
Dermal, toxicity, rat LD50 = 480 mg/kg bw
Acute neurotoxicity, one dose, rat NOAEL = 0.025 mg/kg bw per day
Medium-term (1-26 weeks) Repeated dermal, 21-day, toxicity, rabbit NOAEL = 10 mg/kg bw per day; decreases
in brain acetylcholinesterase activity
Repeated oral, 90-day, toxicity, rat NOAEL = 0.125 mg/kg bw per day
Oral, developmental toxicity, rat NOAEL = 0.3 mg/kg bw per day; decreased
maternal body weights, decreased fetal
weights, stunted growth
Repeated oral, reproductive toxicity, rat NOAEL = 0.25 mg/kg bw per day; slight
effects on pup survival
Long-term (> one year) Repeated oral, two-year toxicity and NOAEL = 0.25 mg/kg bw per day
carcinogenicity, rat
References
Ahmed, E & Saguartz, J.W. (1981) Methyl parathion: One year dog study.
Unpublished report from Pharmacopathics Research Laboratories,
Inc., USA. Submitted to WHO by Cheminova Agro SA,
Lemvig, Denmark.
Beaver, J.B., Foster, J., Cockrell, B.Y. & Jabar, M. (1990) Methyl
parathion: An acute delayed neurotoxicity in the laying hen
(Gallus gallus domesticus). Unpublished report No. USAE1200
10590 from Wildlife International Ltd, USA. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Becker, H., Frei, D., Luetkemeier, H., Vogel, W. & Terrier, C. (1987)
Embryotoxicity including teratogenicity study with E-120
technical in the rat. Unpublished report provided by Cheminova
Agro SA, Lemvig, Denmark.
Bomhard, E., Loser, E. & Schilde, B. (1981) E-605 methyl
(parathion-methyl) chronic toxicological study on rats (feeding
experiment over two years). Unpublished report No. 9889 from
Bayer AG. Submitted to WHO by Cheminova Agro SA, Lemvig, Denmark.
Braeckman, R.A., Godefroot, M.G., Blondeel, G.M. & Belpaire, F.M.
(1980) Kinetic analysis of the fate of methyl parathion in the
dog. Arch. Toxicol., 43, 263-271.
Curren, R.D. (1989) Methyl parathion-Unscheduled DNA synthesis in rat
primary hepatocytes. Unpublished report No. USAE 120220689 from
Microbiological Associates, Inc., USA. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Cuthbert, J.A. & Carr, S.M.A. (1986) Methyl parathion 80% technical:
Acute toxicity tests. Unpublished report No. IRI Project
No. 234117 from Inveresk Research International, Musselburgh,
United Kingdom. Submitted to WHO by Cheminova Agro SA,
Lemvig, Denmark.
Daly, I.W. (1982) A two generation reproduction study of methyl
parathion in rats. Unpublished report from Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
SA, Lemvig, Denmark.
Daly, I.W. (1983) A two-year chronic feeding study of methyl parathion
in rats. Unpublished report No. 77-2060(BD-78019) from
Biodynamics Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Daly, I.W. (1989) A 13-week subchronic toxicity study of: methyl
parathion in dogs via the diet followed by a one month recovery.
Unpublished report No. MOB1158 from Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
SA, Lemvig, Denmark.
Daly, I.W. (1991) A twelve month oral toxicity study of methyl
parathion (E120) in the rat via dietary admixture with special
focus on ocular an d sciatic nerve effects. Unpublished report
No. MOB5072 from Biodynamics Inc., East Millstone, New Jersey,
USA. Submitted to WHO by Cheminova Agro SA, Lemvig, Denmark.
Daly, I.W & Rhinehart, W.E. (1980a) A three month feeding study of
methyl parathion in mice. Unpublished report No. 77-2057 from
Biodynamics, Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Daly, I.W & Rhinehart, W.E. (1980b) A three month feeding study of
methyl parathion in rats. Unpublished report No. 77-2059 from
Biodynamics, Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Cheminova AgroSA, Lemvig, Denmark.
Eiben, R. (1991) E120 (methyl parathion) study for chronic toxicity
and carcinogenicity in B6C3F1 mice (administration in the
diet over a period of 24 months). Unpublished report No. 20258
from Bayer AG. Snbmitted to WHO by Cheminova AgroSA,
Lemvig, Denmark.
Goad, M.E.P. (1992) Twenty-one day subchronic dermal toxicity study
with methyl parathion in rabbits. Unpublished report No. 67376
from Arthur D. Little. Submitted to WHO by Cheminova AgroSA,
Lemvig, Denmark.
Greenough, R.J. & McDonald, P. (1986) Methyl parathion 80%
technical/acute inhalation toxicity study in rats. IRI Project
No. 632295 from Inveresk Research International, Musselburgh,
Scotland. Submitted to WHO by Cheminova AgroSA, Lemvig, Denmark.
Herbold, B. (1982) E120-parathion-methyl-folidal M active ingredient
-- Micronucleus test on the mouse to evaluate for mutagenic
effect. Unpublished report No. 10769 from Bayer AG. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Herbold, B. (1984) E120-parathion methyl -- Dominant lethal test on
the male mouse to evaluate for mutagenic effect. Unpublished
report No. 12731 from Bayer AG. Submitted to WHO by Cheminova
Agro SA, Lemvig, Denmark.
Herbold, B. (1986a) E120 (c.n. parathion methyl) -- Salmonella/
microsome test to evaluate for point mutagenic effect.
Unpublished report No. 15306 from Bayer AG. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Herbold, B. (1986b) E120 (c.n. parathion methyl) -- Salmonella/
microsome test to evaluate for point mutagenic effect.
Unpublished report No. 15230 from Bayer AG. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Löser, E. & Eiben, R. (1982) E-605 methyl-Multigeneration studies on
rats. Unpublished report No. 6812 from Bayer AG. Submitted to WHO
by Cheminova Agro SA, Lemvig, Denmark.
Machemer, L. (1977) Parathion-methyl -- Evaluation for embryotoxic and
teratogenic effects on rats following oral administration.
Unpublished report submitted to WHO by Cheminova AgroSA,
Lemvig, Denmark.
Mihail, F. & Vogel, O. (1984) Subacute dermal toxicity studies with
rabbits. Unpublished report No. 12484 from Bayer AG. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Minnema, D.J. (1994) Acute neurotoxicity study of methyl parathion in
rats. Unpublished report No. HWA2688-102 from Hazelton. Submitted
to WHO by Cheminova Agro SA, Lemvig, Denmark.
Moeller, H.C. & Rider, J.A. (1961) Fed. Proc., 20, 434.
Moeller, H.C. & Rider, J.A. (1962) Fed. Proc., 21, 451.
Renhof, M. (1984) Parathion-methyl (Folidol M active ingredient) study
for embryotoxic effects on rabbits after oral administration.
Unpublished report No. 12907 from Bayer AG. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Rider, J.A., Moeller, H.C., Puletti, E.J. & Swader, J.I. (1969)
Toxicity of parathion, systox, octamethyl pyrophosphoramide and
methyl parathion in men. Toxicol. Appl. Pharmacol.,
14, 603-611.
Rider, J.A., Swader, J.I. & Puletti, E.J. (1970) Methyl parathion and
guthion anticholinesterase effects in human subjects (Abstract
No. 588). Fed. Proc., 29, 349.
Rider, J.A., Swader, J.I. & Puletti, E.J. (1971) Anticholinesterase
toxicity studies with methyl parathion, guthion and phosdrin in
human subjects. Fed. Proc., 30, 1382.
Tegeris, A.S. & Underwood, P.C. (1977) A 14 day study of methyl
parathion in the dog. Unpublished report No. 7754 from
Pharmacopathics Research Laboratories, Inc., USA. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Tegeris, A.S. & Underwood, P.C. (1978) Methyl parathion: Ninety day
feeding to dogs. Unpublished report No. 7754 from Pharmacopathics
Research Laboratories, Inc., USA. Submitted to WHO by Cheminova
Agro SA, Lemvig, Denmark.
Van Dijk, H. (1988) C14-parathion methyl: Absorption, distribution,
excretion and metabolism after single and repeated oral
administration to rats. Unpublished report No. 090876 from RCC
Umweltchemie AG. Submitted to WHO by Cheminova Agro SA,
Lemvig, Denmark.
Yamamoto, T., Egashira, T., Yoshida, T. & Kurwin, Y. (1983)
Comparative metabolism of fenitrothion and methylparathion in
male rats. Acta Pharmacol Toxicol., 53, 96-102.
Rats
Groups of 20 Sprague-Dawley rats of each sex received diets
containing parathion-methyl (purity, 93.65%) at doses of 0, 2.5, 25,
or 75 ppm for 91-94 consecutive days. Females at the highest dose had
increased mortality during the first four weeks of the study, and
animals of each sex showed tremors, emaciation, anogenital staining,
and decreased body weight, although food consumption was greater than
that of controls. Erythrocyte parameters and the levels of glucose,
total protein, albumin, and globulin were decreased at one or more
intervals in animals at the high dose, and increases were reported in
serum aspartate aminotransferase and alkaline phosphatase activities
and in blood urea nitrogen. The haematological and serum chemistry
values were, however, within biologically normal ranges. Gross and
histopathological changes were reported in animals at the high dose,
including discoloured and abraded foci in the stomach, which were
correlated histologically with ulcerative gastritis, but these were
considered secondary to the stress that resulted from administration
of the test material. Additional histological findings included
lymphoid necrosis and necrosis of the submaxillary salivary glands. At
the medium dose, erythrocyte and plasma cholinesterase activities were
decreased in animals of each sex, whereas brain acetylcholinesterase
activity was inhibited only in females. Erythrocyte acetylcholine-
sterase activity was decreased in males at the low dose but not to a
biologically significant level. The NOAEL was 2.5 ppm, equivalent to
0.125 mg/kg bw per day, and was based on the decrease in brain
acetylcholinesterase activity in females (Daly & Rhinehart, 1980b).
Rabbits
Groups of five male and five female New Zealand white rabbits
received parathion-methyl (purity, 93.1%) topically at doses of 0, 1,
5, 10, or 100 mg/kg bw per day for 21 days. The compound was applied
as a 1% solution in carboxymethylcellulose to a depilated surface on
the body of each animal and was held in contact with the skin for 6 h
on each day of exposure. No dermal effects were reported in males, but
in females at 10 and 100 mg/kg bw per day inhibition of erythrocyte
acetylcholinesterase activity was seen. The LOAEL was 10 mg/kg bw per
day, and the NOAEL was 5 mg/kg bw per day (Goad, 1992).
Groups of six New Zealand white rabbits of each sex received
parathion-methyl (purity, 96.3%) topically at doses of 0,10, 50, or
250 mg/kg bw per day. One-half of the animals in each group were
tested on abraded skin. At > 50 mg/kg bw per day, brain and
erythrocyte acetylcholinesterase activities were inhibited. The NOAEL
was 10 mg/kg bw per day (Mihail & Vogel, 1984).
Dogs
Groups of two beagle dogs of each sex were given parathion-methyl
(purity, 94.3%) in the diet at levels of 0, 2.5, 5 or 10 mg/kg bw per
day for 14 days. Decreases in body-weight gain and/or body weight were
reported in males and females at the middle and high doses, and feed
consumption was significantly lower in all animals at the high dose
and in females at the middle dose. Cholinesterase inhibition was not
measured. The NOEL was 2.5 mg/kg bw per day, based on the effects on
body weight and food consumption at 5 mg/kg bw per day (Tegeris &
Underwood, 1977).
Groups of four beagle dogs of each sex were fed diets containing
parathion-methyl (purity, 94.32%) at levels of 0, 0.3,1, or 3 mg/kg bw
per day for 13 weeks (90 days). There were no effects on body weight,
food consumption, or mortality, and no clinical signs of toxicity;
however, significant decreases in brain, erythrocyte, and plasma
cholinesterase activities were seen in males and females at the high
dose at weeks 6 and 13. At the middle dose, plasma cholinesterase
activity was significantly decreased in males at week 13 and
erythrocyte cholinesterase was significantly decreased in animals of
each sex. The NOAEL was 1 mg/kg bw per day (Tegeris & Underwood,
1978).
Groups of eight beagle dogs of each sex received parathion-methyl
(purity, 94.9%) at doses of 0, 0.03, 0.3, or 3 mg/kg bw per day for 13
weeks, followed by a recovery period of up to seven weeks. Animals
were observed for clinical signs of toxicity; extensive
ophthalmological examinations were conducted, including fundic
observations, intraocular pressure, electroretinogram, and slit-lamp
examination. Brain cholinesterase was measured at terminal sacrifice.
Significant decreases (> 20% of control levels) were seen in plasma
and erythrocyte cholinesterase activities at weeks 6 and 13 in animals
at the highest dose, and at terminal sacrifice the brain choline-
sterase activity was 48% of that of controls. No functional or
morphological ocular impairment was observed. The NOAEL was 0.3 mg/kg
bw per day and the LOAEL was 3 mg/kg bw per day, based on the
decreases in brain cholinesterase activity (Daly, 1989).
(c) Long-term toxicity and carcinogenicity
Mice
Parathion-methyl (purity, 95.5%) was fed to male and female
B6C3F1 mice at dietary levels of 0, 1, 7, or 50 ppm for two years. No
carcinogenicity was seen. Clinical signs of toxicity were infrequent
in treated animals, and none of the gross or histopathological
findings were associated with treatment. Erythrocyte, plasma, and
brain cholinesterase activities were significantly inhibited in
animals receiving 50 ppm. Erythrocyte acetylcholinesterase activity
was also significantly inhibited (by 40-57% of the control value) in
animals at 7 ppm. The NOAEL was 7 ppm, equal to 1.6 mg/kg bw per day
(Eiben, 1991).
Rats
Groups of 70 Sprague-Dawley CD rats received diets containing
parathion-methyl (purity, 94.6%) at levels of 0, 0.5, 2.5, 12.5, or
50 ppm, equivalent to average intakes of 0.02, 0.107, 0.5, and
2.2 mg/kg bw per day, for one year. Fifty animals of each sex per
group were evaluated at the end of the study; two groups of five
animals per sex per group were evaluated for ocular toxicity at six
and 12 months; and two groups of five animals per sex per group were
evaluated for changes in peripheral nerves at six and 12 months. No
effects were seen on mortality, on the eye by ophthalmoscopic
examination, or on the optic nerve or retina microscopically. Clinical
signs of aggressiveness and hyperactivity were reported in animals
receiving 50 ppm; reduced mean body weights were also reported at this
dose. Plasma and erythrocyte cholinesterase levels were decreased by
> 20% of control levels in animals of each sex at the two highest
doses, and brain acetylcholinesterase activity was inhibited in
animals of each sex at the highest dose and in females at 12.5 ppm.
Peripheral neuropathy was seen in distal and proximal segments of the
sciatic nerve in animals at the two highest doses, which was
characterized by myelin bubbles and Schwann cell proliferation. An
increased percentage of large diameter fibres was seen in the tibial
nerves and an increased incidence of myelin ovoids in the sural nerves
of males receiving 12.5 ppm. Myelin bubbles and Schwann cell
proliferation were also seen in two of 10 rats at 2.5 ppm, but these
changes were not statistically significant nor of the same degree of
severity as those in rats receiving 12.5 ppm. The NOAEL was 2.5 ppm,
equal to 0.11 mg/kg bw per day (Daly, 1991).
Groups of 50 male and 50 female Wistar TNO/W74 rats received
diets containing parathion-methyl (purity, 94.8%) at levels of 2, 10,
or 50 ppm for two years. The control group comprised 100 rats of each
sex. No carcinogenic effects were seen. Most of the toxic effects were
observed at 50 ppm and included decreased body-weight gain, increased
food consumption, tremors, slightly increased mortality, and decreased
haemoglobin and haematocrit values in females at the high dose at
termination. Alkaline phosphatase activity was increased and plasma
protein levels were significantly lowered throughout the study,
perhaps correlated with an increase in urinary protein. The other
clinical findings were considered to be incidental. Plasma and
erythrocyte cholinesterase activities were slightly inhibited in rats
receiving 10 ppm of parathion-methyl, and brain cholinesterase
activity was only 78% of the control value in males at this dose.
Inhibition of plasma and erythrocyte cholinesterase activities was
reported in animals of each sex at 50 ppm, and the brain
cholinesterase activity was 50% of that reported for controls in males
and 37% in females. The NOAEL was 2 ppm, equivalent to 0.1 mg/kg bw
per day, and the LOAEL was 10 ppm, equivalent to 0.5 mg/kg bw per day,
based on a 22% reduction in brain acetylcholinesterase activity
(Bomhard et al., 1981).
Groups of 60 Sprague-Dawley rats of each sex received
parathion-methyl (purity, 93.65%) at dietary levels of 0, 0.5, 5, or
50 ppm for two years. No carcinogenic effects were seen. Toxic effects
were observed in animals at 50 ppm and included tremors, anogenital
staining, reduced body weight, retinal degeneration, decreases in
erythrocyte parameters, and sciatic nerve degeneration. The plasma,
erythrocyte, and brain cholinesterase activities of males at the high
dose at study termination were significantly lower than those of
controls (82, 9, and 76%, respectively, of the control value). In
females, brain cholinesterase activity was reduced by 79%. At 5 ppm,
an abnormal gait was reported in one female, and haemoglobin and
haematocrit and erythrocyte counts were slightly reduced in other
animals. No effects on cholinesterase activity were reported. The
NOAEL was 5 ppm, equivalent to 0.25 mg/kg bw per day (Daly, 1983).
Dogs
Groups of eight beagle dogs received parathion-methyl (purity,
93.7%) at levels of 0, 0.03, 0.1, or 0.3 mg/kg bw per day for one
year. Animals were observed daily, clinical chemistry and haematology
were assessed at designated intervals, and erythrocyte and plasma
cholinesterase activities were monitored monthly. Brain acetylcholine-
sterase activity was determined at study termination. No clinical
signs and no significant effects on body weight or food consumption
were reported, and clinical pathology, gross pathology, and
histopathology showed no compound-related changes. The NOAEL was
0.3 mg/kg bw per day, the highest dose tested (Ahmed & Saguartz,
1981).
(d) Reproductive and developmental toxicity
Rats
Groups of Wistar HAN rats were given parathion-methyl (purity,
97%) at doses of 0, 0.3, 1.0, or 3.0 mg/kg bw per day by gavage on
days 6-15 of gestation. Parental animals were monitored for mortality,
clinical signs of toxicity, body weights, food consumption, and
cholinesterase activity. Litters were evaluated for abnormalities,
sex, and weight. Five deaths and clinical signs of toxicity, including
somnolence, ataxia, and dyspnoea, were seen in maternal animals at the
high dose throughout the study. Increased post-implantation losses and
decreases in food consumption, body weight, and body-weight gain were
also reported, and plasma and erythrocyte cholinesterase activities
were decreased. Fetuses from dams receiving 3.0 mg/kg bw showed an
increased frequency of delayed ossification and reduced body weight.
The maternal and developmental NOAEL was 1 mg/kg bw per day
(Becker et al., 1987)
Inseminated female rats received parathion-methyl (purity, 94.4%)
by gavage on days 6-15 of gestation at doses of 0, 0.1, 0.3, or
1 mg/kg bw per day. At 1 mg/kg bw per day, maternal toxicity was seen,
manifested as significantly decreased body-weight gain during
treatment and gestation. Fetal weight was also significantly lower,
and the frequency of stunted fetuses was greater. The NOAEL for
maternal and developmental effects was 0.3 mg/kg bw per day
(Machemer, 1977).
In a multigeneration study of reproductive toxicity,
parathion-methyl (purity, 95%) was administered in the diets of 10
male and 20 female Wistar rats at levels of 0, 2, 10, or 50 ppm. Each
of the F0, F1, and F2 generations was mated twice. At 50 ppm,
convulsions were observed in F1b parents, and decreased growth was
reported among parental animals of all generations. At this dose,
adverse effects were reported on birth weight, litter size, and the
viability and growth of pups. At 10 ppm, fertility was decreased and
pup survival was slightly decreased in both the F1 and F2
generations. The NOAEL for reproductive toxicity was 2 ppm, equivalent
to 0.1 mg/kg bw per day (Löser & Eiben, 1982).
In a two-generation study of reproductive toxicity, groups of 15
male and 30 female Sprague-Dawley rats received parathion-methyl
(purity, 93.7%) at dietary levels of 0, 0.5, 5, or 25 ppm for 14 weeks
before mating of F0 parents and for 18 weeks before mating of F1
parents. Treatment was continued through gestation and weaning.
Selected F1 adults and five weanlings of each sex in each group per
generation were examined histologically. The body weights of females
at the high dose in both generations were reduced during the lactation
period, but the litters of both generations appeared to be unaffected
by treatment. No abnormalities were seen in reproductive performance,
as indicated by mating, pregnancy, and fertility rates, pup viability
and survival, and gross and microscopic lesions, nor were there
significant differences in the body weights of offspring. The NOAEL
was 5 ppm, equivalent to 0.25 mg/kg bw per day, for parental toxicity
and 25 ppm, equivalent to 1.25 mg/kg bw per day, for reproductive
toxicity (Daly 1982).
Rabbits
Groups of 15 inseminated Himalayan rabbits received parathion-
methyl (purity, 95.7%) at doses of 0, 0.3, 1, or 3 mg/kg bw per day by
gavage on gestation days 6-18. Fetuses were removed by caesarian
section on day 29. No effects were reported on litter parameters. In
maternal animals at 3 mg/kg bw, erythrocyte and plasma cholinesterase
activities were inhibited, but no other effects were reported. The
NOAEL was 1 mg/kg bw per day for maternal effects and
3 mg/kg bw per day for developmental effects (Renhof, 1984).
(e) Genotoxicity
The genotoxic effects of parathion-methyl are summarized in Table
2.
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Three male and three female New Zealand white rabbits received
0.1 ml of parathion-methyl (purity, 80%) by instillation into the
right eye. Slight redness was observed in the eyes of all rabbits 1 h
after instillation; all had returned to normal by 48 h.
Topical application of parathion-methyl (purity, 80%) to New
Zealand white rabbits was associated with very slight to moderate
erythema 1 and 24 h after removal of gauze patches. Slight oedema was
also observed in one of six rabbits. The skin of all animals was
normal by 48 h.
In the Magnusson-Kligman test, female guinea-pigs received six
intradermal doses of parathion-methyl (purity, 80%) in 5% paraffin oil
or emulsified with 0.05 ml of Freund's complete adjuvant. A challenge
concentration was applied two weeks after induction, and reactions
were assessed 24 and 48 h after the patch was removed. Parathion-
methyl was not considered to be a sensitizing agent in guinea-pigs
(Cuthbert & Carr, 1986).
(ii) Neurotoxicity
Groups of 10 male and 10 female Sprague-Dawley rats received
parathion-methyl (purity, 93.1%) by gavage as single doses of 0,
0.025, 7.5, 10 (males only), or 15 (females only) mg/kg bw.
Neurobehavioural evaluations, consisting of a battery of tests for
motor activity and functional observation, were conducted at
designated intervals during the 14-day observation period, and plasma
and erythrocyte cholinesterase activities were determined at several
intervals during the study. Acetylcholinesterase activity was measured
in six regions of the brain at the time of the peak effect and at day
Table 2. Results of tests for the genotoxicity of parathion-methyl
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 2-12 500 µg/plate approx. Positive in Herbold (1986a)
TA100, TA1535, TA1537 96% TA100 at
> 900 µg/plate
Reverse mutation S. typhimurium 2-12 500 µg/plate 94 Positive Herbold (1986b)
Unscheduled DNA Rat hepatocytes 3 × 10-5-3 × 10-2 µl/ml NR Negative Curren (1989)
synthesis
In vivo
Dominant lethal mutation Mouse 10 mg/kg bw 95.7 Negative Herbold (1984)
Micronucleus formation Bor:NMRI mouse 2 × 5, 2 × 10 mg/kg bw 95.6 Negative Herbold (1982)
NR, not reported
14. At the middle and high doses, parathion-methyl had transient
effects on motor activity and function, inducing lacrimation,
salivation, tremors, muscle fasciculations, ataxia, muscle weakness,
and miosis; and plasma, erythrocyte, and brain cholinesterase
activities were inhibited by 67, 56, and 76% at the time of the peak
effect, respectively. in males at the high dose, body-weight gain was
significantly lower than that of controls; similar findings were not
made in females, and the mean body weights were not affected. In males
at this dose, the number of sites at which focal demyelination was
present was increased in comparison with controls, with demyelination
in the cervical (3/6) and lumbar (5/6) dorsal root fibres, in the
cervical (2/6) and lumbar (4/6) ventral root fibres, and in the lumbar
spinal (1/6), tibial (3/6), and sural nerves (2/6). Similar findings
were not found in females. The NOAEL for acute neurotoxicity was
0.025 mg/kg bw (Minnema, 1994).
In the study of long-term toxicity by Daly (1991) in
Sprague-Dawley CD rats, ophthalmoscopic examination, electro-
retinograms, light microscopy, and electron microscopy revealed no
ocular toxicity. Microscopic and morphometric studies of various
preparations of the sciatic nerve and its extensions revealed several
lesions in animals at 12.5 and 50 ppm, which are consistent with
demyelination. These included myelin bubbles, myelin ovoids, Schwann
cell proliferation, and the presence of phagocytic cells. In addition,
increased lengths of demyelination were observed in teased nerve
preparations. The NOAEL for neurotoxicity was 2.5 ppm, equivalent to
0.11 mg/kg bw per day (Daly, 1992).
Hens received parathion-methyl (purity, 95.8%) by intubation at
doses of 0 or 250 mg/kg bw; the test dose thus exceeded the LD50 of
215 mg/kg bw. Positive control animals received 600 mg/kg bw of
tri- ortho-cresyl phosphate. A second dose was administered 21 days
after the first. No clinical signs were seen, and there were no
histopathological lesions in the brain, spinal cord, or nervous tissue
that were indicative of delayed neurotoxicity. One-half of the treated
hens died; surviving birds showed clinical signs of toxicity including
lethargy, depression, wing droop, salivation, shallow and rapid
respiration, and cyanotic comb. The hens recovered within about seven
days (Beaver et al., 1990).
3. Observations in humans
Five male volunteers received daily doses of 3 mg parathion-
methyl for 28 days, followed by 3.5 mg per day for the next 28 days
and then by 4 mg per day for 43 days. No signs of toxicity were
reported, and no effect on plasma or erythrocyte cholinesterase
activity was observed (Moeller & Rider, 1961).
Three groups of five volunteers were given 4.5 mg parathion-
methyl for 30 days, followed by 5 mg per day for 29 days or 5.5 mg per
day for 24 days. These doses were followed by 6 mg per day for 29 days
or by 6.5 mg/day for 35 days, and finally by 7 mg per day for 24 days.
No significant inhibition of plasma or erythrocyte cholinesterase
activity was reported (Moeller & Rider, 1962).
After oral doses of up to 19 mg to male volunteers for 30 days,
no evidence of toxicity was reported (Rider et al., 1969); however,
when doses of 24 mg were given daily for four weeks, plasma and
erythrocyte cholinesterase activities were inhibited, by 23-24% and
27-55%, respectively (Rider et al., 1970). In another study
(Rider et al., 1971), doses of parathion-methyl that were increased
from 14 to 20 mg over a 30-day period had no effect on cholinesterase
activity. The NOAEL was thus 0.3 mg/kg bw per day.
Comments
Parathion-methyl is absorbed through the skin and from the
respiratory and digestive tracts. Observed differences between its
oral and intravenous toxicity are believed to be associated with
first-pass effects in the liver. The compound is rapidly excreted;
negligible amounts of the labelled dose were present in the blood,
tissues, and organs at 48 h. Conversion of parathion-methyl to
paraoxon-methyl has been shown to occur within minutes after oral
administration to rats. Detoxification is achieved by O-demethylation
or hydrolysis to para-nitrophenol. In humans the primary urinary
metabolites were para-nitrophenol and dimethyl phosphate.
Parathion-methyl is acutely toxic at low doses when administered
either orally (LD50 = 4 mg/kg bw) or by inhalation (LC50 =
0.13 mg/litre). The compound is slightly irritating to the skin and
the eyes but has not been shown to be a sensitizing agent. WHO has
classified parathion-methyl as 'extremely hazardous'.
In a 90-day study in mice at dietary levels of 0, 10, 30, or
60 ppm, the NOAEL was 10 ppm (equivalent to 1.5 mg/kg bw per day) on
the basis of significant decreases in absolute and relative testicular
weights. Cholinesterase activity was not measured in this study. In a
90-day study in rats, the NOAEL was 2.5 ppm (equivalent to 0.12 mg/kg
bw per day) on the basis of significant decreases in plasma,
erythrocyte, and brain cholinesterase activities at 25 ppm (equivalent
to 1.2 mg/kg bw per day).
Two 13-week studies were conducted in dogs, in which parathion-
methyl was administered at dietary levels of 0, 0.3, 1, or 3 mg/kg bw
per day in one study and 0, 0.03, 0.3, or 3 mg/kg bw per day in the
other. In both studies, the LOAEL was 3 mg/kg bw per day, on the basis
of decreases in erythrocyte, plasma, and brain cholinesterase activity.
The NOAELs were 1 and 0.3 mg/kg bw per day, respectively. In a one-year
study in dogs, the NOAEL was also 0.3 mg/kg bw per day, the highest
dose tested.
In a one-year study in rats to determine the ocular and
neurotoxic effects of parathion-methyl, dietary levels of 0, 0.5, 2.5,
12, or 50 ppm were administered. Ocular toxicity was not observed.
Degenerative changes of the sciatic nerve and its extensions
consistent with demyelination were observed at the two highest doses.
The LOAEL was 12 ppm, equal to 0.5 mg/kg bw per day. The NOAEL was
2.5 ppm, equal to 0.1 mg/kg bw per day.
In a two-year study in mice, parathion-methyl was not carcino-
genic at dietary levels up to 50 ppm (equal to 9.2 mg/kg bw per day).
The NOAEL was 7 ppm, equal to 1.6 mg/kg bw per day, on the basis of
significant decreases in erythrocyte, plasma, and brain cholinesterase
activities. In a study of toxicity and carcinogenicity in rats fed
parathion-methyl at dietary levels of 0, 2, 10, or 50 ppm, there was
no evidence of carcinogenicity. The NOAEL was 2 ppm (equivalent to
0.1 mg/kg bw per day), and the LOAEL was 10 ppm (equivalent to
0.5 mg/kg bw per day), on the basis of a reduction in brain
acetylcholinesterase activity. In another two-year study In rats,
parathion-methyl did not induce carcinogenic effects. The NOAEL was
5 ppm (equivalent to 0.25 mg/kg bw per day) on the basis of tremors,
anogenital staining, reduced body weight, retinal degeneration,
sciatic nerve degeneration, decreased packed cell volume and
haemoglobin and erythrocyte counts, and decreased brain cholinesterase
activity in males and females at 50 ppm (equivalent to 2.5 mg/kg bw
per day).
Two studies of developmental toxicity were conducted in rats. In
one study, animals received parathion-methyl by gavage at doses of
0, 0.3,1, or 3 mg/kg bw per day. The NOAEL for maternal and
developmental toxicity was 1 mg/kg bw per day on the basis of
increased numbers of deaths, ataxia, and dyspnoea in dams and delayed
ossification in fetuses. In another study, the NOAEL for maternal and
developmental toxicity was 0.3 mg/kg bw per day on the basis of
significant decreases in maternal body-weight gain during treatment
and gestation and an increased incidence of stunted fetuses at 1 mg/kg
bw per day. In studies of developmental toxicity in rabbits, decreased
erythrocyte and plasma cholinesterase activities were reported in dams
receiving 3 mg/kg bw per day by gavage. No developmental effects were
reported that could be attributed to the administration of parathion-
methyl. The NOAEL for maternal toxicity was 1 mg/kg bw, and that for
developmental toxicity was 3 mg/kg bw.
In a multigeneration study in rats, dietary levels of 0, 2, 10,
or 50 ppm were administered. Slight effects on pup survival were
reported in animals receiving 10 ppm, equivalent to 0.5 mg/kg bw per
day. The NOAEL was 2 ppm, equivalent to 0.1 mg/kg bw per day. In
another study, in which dietary levels of 0, 0.5, 5, or 25 ppm were
administered, decreases in maternal body weights during the lactation
period were observed at 25 ppm. The NOAEL was 5 ppm, equivalent to
0.25 mg/kg bw per day. The overall NOAEL in the two studies of
reproductive toxicity was 5 ppm, equivalent to 0.25 mg/kg bw per day.
Parathion-methyl was mutagenic in bacteria, but there was no
evidence of genotoxicity in a limited range of studies in mammalian
systems.
The NOAEL derived from the combined results of several studies
conducted in humans, based on the depression of erythrocyte and plasma
cholinesterase activities, was 0.3 mg/kg bw per day.
An ADI of 0-0.003 mg/kg bw was established on the basis of the
NOAEL of 5 ppm, equivalent to 0.25 mg/kg bw per day, in the two-year
study in rats for retinal degeneration, sciatic nerve demyelination,
reduced body weight, anaemia, and decreased brain acetylcholinesterase
activity. A safety factor of 100 was used. Since the toxicological
end-points seen in animals were other than acetylcholinesterase
inhibition, a safety factor of 10 could not be applied to the NOAEL in
humans.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 7 ppm, equal to 1.6 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
Rat: 5 ppm, equivalent to 0.25 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
5 ppm, equivalent to 0.25 mg/kg bw per day (study of
reproductive toxicity)
0.3 mg/kg bw per day (maternal, embryo-, and fetotoxicity
and teratogenicity in study of developmental toxicity)
Rabbit: 1 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
3 mg/kg bw per day (no embryo- or fetotoxicity or
teratogenicity in study of developmental toxicity)
Dog: 0.3 mg/kg bw per day (one-year study of toxicity and
carcinogenicity)
Human: 0.3 mg/kg bw per day
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Estimate of acute reference dose
An acute reference dose of 0.03 mg/kg bw was derived by applying
the usual 10-fold safety factor to an NOAEL of 19 mg/kg bw (highest
oral dose), corresponding to about 0.3 mg/kg bw per day, in humans,
based on the absence of inhibition of erythrocyte acetylcholinesterase
activity.
Studies that would provide information useful for continued evaluation
of the compound
Observations in humans, particularly reports of poisoning
incidents and/or evaluation of potential long-term neurological and
behavioural effects.
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to parathion-methyl
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Skin, sensitization, guinea-pig Not sensitizing
Eye, sensitization, rabbit Slightly irritating
Skin, irritation, rabbit Slightly irritating
Inhalation 4-h, toxicity, rat LC50 = 0.13 mg/litre
Oral, toxicity, rat LD50 = 4-62 mg/kg bw
Dermal, toxicity, rat LD50 = 480 mg/kg bw
Acute neurotoxicity, one dose, rat NOAEL = 0.025 mg/kg bw per day
Medium-term (1-26 weeks) Repeated dermal, 21-day, toxicity, rabbit NOAEL = 10 mg/kg bw per day; decreases
in brain acetylcholinesterase activity
Repeated oral, 90-day, toxicity, rat NOAEL = 0.125 mg/kg bw per day
Oral, developmental toxicity, rat NOAEL = 0.3 mg/kg bw per day; decreased
maternal body weights, decreased fetal
weights, stunted growth
Repeated oral, reproductive toxicity, rat NOAEL = 0.25 mg/kg bw per day; slight
effects on pup survival
Long-term (> one year) Repeated oral, two-year toxicity and NOAEL = 0.25 mg/kg bw per day
carcinogenicity, rat
References
Ahmed, E & Saguartz, J.W. (1981) Methyl parathion: One year dog study.
Unpublished report from Pharmacopathics Research Laboratories,
Inc., USA. Submitted to WHO by Cheminova Agro SA,
Lemvig, Denmark.
Beaver, J.B., Foster, J., Cockrell, B.Y. & Jabar, M. (1990) Methyl
parathion: An acute delayed neurotoxicity in the laying hen
(Gallus gallus domesticus). Unpublished report No. USAE1200
10590 from Wildlife International Ltd, USA. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Becker, H., Frei, D., Luetkemeier, H., Vogel, W. & Terrier, C. (1987)
Embryotoxicity including teratogenicity study with E-120
technical in the rat. Unpublished report provided by Cheminova
Agro SA, Lemvig, Denmark.
Bomhard, E., Loser, E. & Schilde, B. (1981) E-605 methyl
(parathion-methyl) chronic toxicological study on rats (feeding
experiment over two years). Unpublished report No. 9889 from
Bayer AG. Submitted to WHO by Cheminova Agro SA, Lemvig, Denmark.
Braeckman, R.A., Godefroot, M.G., Blondeel, G.M. & Belpaire, F.M.
(1980) Kinetic analysis of the fate of methyl parathion in the
dog. Arch. Toxicol., 43, 263-271.
Curren, R.D. (1989) Methyl parathion-Unscheduled DNA synthesis in rat
primary hepatocytes. Unpublished report No. USAE 120220689 from
Microbiological Associates, Inc., USA. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Cuthbert, J.A. & Carr, S.M.A. (1986) Methyl parathion 80% technical:
Acute toxicity tests. Unpublished report No. IRI Project
No. 234117 from Inveresk Research International, Musselburgh,
United Kingdom. Submitted to WHO by Cheminova Agro SA,
Lemvig, Denmark.
Daly, I.W. (1982) A two generation reproduction study of methyl
parathion in rats. Unpublished report from Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
SA, Lemvig, Denmark.
Daly, I.W. (1983) A two-year chronic feeding study of methyl parathion
in rats. Unpublished report No. 77-2060(BD-78019) from
Biodynamics Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Daly, I.W. (1989) A 13-week subchronic toxicity study of: methyl
parathion in dogs via the diet followed by a one month recovery.
Unpublished report No. MOB1158 from Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
SA, Lemvig, Denmark.
Daly, I.W. (1991) A twelve month oral toxicity study of methyl
parathion (E120) in the rat via dietary admixture with special
focus on ocular an d sciatic nerve effects. Unpublished report
No. MOB5072 from Biodynamics Inc., East Millstone, New Jersey,
USA. Submitted to WHO by Cheminova Agro SA, Lemvig, Denmark.
Daly, I.W & Rhinehart, W.E. (1980a) A three month feeding study of
methyl parathion in mice. Unpublished report No. 77-2057 from
Biodynamics, Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Daly, I.W & Rhinehart, W.E. (1980b) A three month feeding study of
methyl parathion in rats. Unpublished report No. 77-2059 from
Biodynamics, Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Cheminova AgroSA, Lemvig, Denmark.
Eiben, R. (1991) E120 (methyl parathion) study for chronic toxicity
and carcinogenicity in B6C3F1 mice (administration in the
diet over a period of 24 months). Unpublished report No. 20258
from Bayer AG. Snbmitted to WHO by Cheminova AgroSA,
Lemvig, Denmark.
Goad, M.E.P. (1992) Twenty-one day subchronic dermal toxicity study
with methyl parathion in rabbits. Unpublished report No. 67376
from Arthur D. Little. Submitted to WHO by Cheminova AgroSA,
Lemvig, Denmark.
Greenough, R.J. & McDonald, P. (1986) Methyl parathion 80%
technical/acute inhalation toxicity study in rats. IRI Project
No. 632295 from Inveresk Research International, Musselburgh,
Scotland. Submitted to WHO by Cheminova AgroSA, Lemvig, Denmark.
Herbold, B. (1982) E120-parathion-methyl-folidal M active ingredient
-- Micronucleus test on the mouse to evaluate for mutagenic
effect. Unpublished report No. 10769 from Bayer AG. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Herbold, B. (1984) E120-parathion methyl -- Dominant lethal test on
the male mouse to evaluate for mutagenic effect. Unpublished
report No. 12731 from Bayer AG. Submitted to WHO by Cheminova
Agro SA, Lemvig, Denmark.
Herbold, B. (1986a) E120 (c.n. parathion methyl) -- Salmonella/
microsome test to evaluate for point mutagenic effect.
Unpublished report No. 15306 from Bayer AG. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Herbold, B. (1986b) E120 (c.n. parathion methyl) -- Salmonella/
microsome test to evaluate for point mutagenic effect.
Unpublished report No. 15230 from Bayer AG. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Löser, E. & Eiben, R. (1982) E-605 methyl-Multigeneration studies on
rats. Unpublished report No. 6812 from Bayer AG. Submitted to WHO
by Cheminova Agro SA, Lemvig, Denmark.
Machemer, L. (1977) Parathion-methyl -- Evaluation for embryotoxic and
teratogenic effects on rats following oral administration.
Unpublished report submitted to WHO by Cheminova AgroSA,
Lemvig, Denmark.
Mihail, F. & Vogel, O. (1984) Subacute dermal toxicity studies with
rabbits. Unpublished report No. 12484 from Bayer AG. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Minnema, D.J. (1994) Acute neurotoxicity study of methyl parathion in
rats. Unpublished report No. HWA2688-102 from Hazelton. Submitted
to WHO by Cheminova Agro SA, Lemvig, Denmark.
Moeller, H.C. & Rider, J.A. (1961) Fed. Proc., 20, 434.
Moeller, H.C. & Rider, J.A. (1962) Fed. Proc., 21, 451.
Renhof, M. (1984) Parathion-methyl (Folidol M active ingredient) study
for embryotoxic effects on rabbits after oral administration.
Unpublished report No. 12907 from Bayer AG. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Rider, J.A., Moeller, H.C., Puletti, E.J. & Swader, J.I. (1969)
Toxicity of parathion, systox, octamethyl pyrophosphoramide and
methyl parathion in men. Toxicol. Appl. Pharmacol.,
14, 603-611.
Rider, J.A., Swader, J.I. & Puletti, E.J. (1970) Methyl parathion and
guthion anticholinesterase effects in human subjects (Abstract
No. 588). Fed. Proc., 29, 349.
Rider, J.A., Swader, J.I. & Puletti, E.J. (1971) Anticholinesterase
toxicity studies with methyl parathion, guthion and phosdrin in
human subjects. Fed. Proc., 30, 1382.
Tegeris, A.S. & Underwood, P.C. (1977) A 14 day study of methyl
parathion in the dog. Unpublished report No. 7754 from
Pharmacopathics Research Laboratories, Inc., USA. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Tegeris, A.S. & Underwood, P.C. (1978) Methyl parathion: Ninety day
feeding to dogs. Unpublished report No. 7754 from Pharmacopathics
Research Laboratories, Inc., USA. Submitted to WHO by Cheminova
Agro SA, Lemvig, Denmark.
Van Dijk, H. (1988) C14-parathion methyl: Absorption, distribution,
excretion and metabolism after single and repeated oral
administration to rats. Unpublished report No. 090876 from RCC
Umweltchemie AG. Submitted to WHO by Cheminova Agro SA,
Lemvig, Denmark.
Yamamoto, T., Egashira, T., Yoshida, T. & Kurwin, Y. (1983)
Comparative metabolism of fenitrothion and methylparathion in
male rats. Acta Pharmacol Toxicol., 53, 96-102.
 Rats
Groups of 20 Sprague-Dawley rats of each sex received diets
containing parathion-methyl (purity, 93.65%) at doses of 0, 2.5, 25,
or 75 ppm for 91-94 consecutive days. Females at the highest dose had
increased mortality during the first four weeks of the study, and
animals of each sex showed tremors, emaciation, anogenital staining,
and decreased body weight, although food consumption was greater than
that of controls. Erythrocyte parameters and the levels of glucose,
total protein, albumin, and globulin were decreased at one or more
intervals in animals at the high dose, and increases were reported in
serum aspartate aminotransferase and alkaline phosphatase activities
and in blood urea nitrogen. The haematological and serum chemistry
values were, however, within biologically normal ranges. Gross and
histopathological changes were reported in animals at the high dose,
including discoloured and abraded foci in the stomach, which were
correlated histologically with ulcerative gastritis, but these were
considered secondary to the stress that resulted from administration
of the test material. Additional histological findings included
lymphoid necrosis and necrosis of the submaxillary salivary glands. At
the medium dose, erythrocyte and plasma cholinesterase activities were
decreased in animals of each sex, whereas brain acetylcholinesterase
activity was inhibited only in females. Erythrocyte acetylcholine-
sterase activity was decreased in males at the low dose but not to a
biologically significant level. The NOAEL was 2.5 ppm, equivalent to
0.125 mg/kg bw per day, and was based on the decrease in brain
acetylcholinesterase activity in females (Daly & Rhinehart, 1980b).
Rabbits
Groups of five male and five female New Zealand white rabbits
received parathion-methyl (purity, 93.1%) topically at doses of 0, 1,
5, 10, or 100 mg/kg bw per day for 21 days. The compound was applied
as a 1% solution in carboxymethylcellulose to a depilated surface on
the body of each animal and was held in contact with the skin for 6 h
on each day of exposure. No dermal effects were reported in males, but
in females at 10 and 100 mg/kg bw per day inhibition of erythrocyte
acetylcholinesterase activity was seen. The LOAEL was 10 mg/kg bw per
day, and the NOAEL was 5 mg/kg bw per day (Goad, 1992).
Groups of six New Zealand white rabbits of each sex received
parathion-methyl (purity, 96.3%) topically at doses of 0,10, 50, or
250 mg/kg bw per day. One-half of the animals in each group were
tested on abraded skin. At > 50 mg/kg bw per day, brain and
erythrocyte acetylcholinesterase activities were inhibited. The NOAEL
was 10 mg/kg bw per day (Mihail & Vogel, 1984).
Dogs
Groups of two beagle dogs of each sex were given parathion-methyl
(purity, 94.3%) in the diet at levels of 0, 2.5, 5 or 10 mg/kg bw per
day for 14 days. Decreases in body-weight gain and/or body weight were
reported in males and females at the middle and high doses, and feed
consumption was significantly lower in all animals at the high dose
and in females at the middle dose. Cholinesterase inhibition was not
measured. The NOEL was 2.5 mg/kg bw per day, based on the effects on
body weight and food consumption at 5 mg/kg bw per day (Tegeris &
Underwood, 1977).
Groups of four beagle dogs of each sex were fed diets containing
parathion-methyl (purity, 94.32%) at levels of 0, 0.3,1, or 3 mg/kg bw
per day for 13 weeks (90 days). There were no effects on body weight,
food consumption, or mortality, and no clinical signs of toxicity;
however, significant decreases in brain, erythrocyte, and plasma
cholinesterase activities were seen in males and females at the high
dose at weeks 6 and 13. At the middle dose, plasma cholinesterase
activity was significantly decreased in males at week 13 and
erythrocyte cholinesterase was significantly decreased in animals of
each sex. The NOAEL was 1 mg/kg bw per day (Tegeris & Underwood,
1978).
Groups of eight beagle dogs of each sex received parathion-methyl
(purity, 94.9%) at doses of 0, 0.03, 0.3, or 3 mg/kg bw per day for 13
weeks, followed by a recovery period of up to seven weeks. Animals
were observed for clinical signs of toxicity; extensive
ophthalmological examinations were conducted, including fundic
observations, intraocular pressure, electroretinogram, and slit-lamp
examination. Brain cholinesterase was measured at terminal sacrifice.
Significant decreases (> 20% of control levels) were seen in plasma
and erythrocyte cholinesterase activities at weeks 6 and 13 in animals
at the highest dose, and at terminal sacrifice the brain choline-
sterase activity was 48% of that of controls. No functional or
morphological ocular impairment was observed. The NOAEL was 0.3 mg/kg
bw per day and the LOAEL was 3 mg/kg bw per day, based on the
decreases in brain cholinesterase activity (Daly, 1989).
(c) Long-term toxicity and carcinogenicity
Mice
Parathion-methyl (purity, 95.5%) was fed to male and female
B6C3F1 mice at dietary levels of 0, 1, 7, or 50 ppm for two years. No
carcinogenicity was seen. Clinical signs of toxicity were infrequent
in treated animals, and none of the gross or histopathological
findings were associated with treatment. Erythrocyte, plasma, and
brain cholinesterase activities were significantly inhibited in
animals receiving 50 ppm. Erythrocyte acetylcholinesterase activity
was also significantly inhibited (by 40-57% of the control value) in
animals at 7 ppm. The NOAEL was 7 ppm, equal to 1.6 mg/kg bw per day
(Eiben, 1991).
Rats
Groups of 70 Sprague-Dawley CD rats received diets containing
parathion-methyl (purity, 94.6%) at levels of 0, 0.5, 2.5, 12.5, or
50 ppm, equivalent to average intakes of 0.02, 0.107, 0.5, and
2.2 mg/kg bw per day, for one year. Fifty animals of each sex per
group were evaluated at the end of the study; two groups of five
animals per sex per group were evaluated for ocular toxicity at six
and 12 months; and two groups of five animals per sex per group were
evaluated for changes in peripheral nerves at six and 12 months. No
effects were seen on mortality, on the eye by ophthalmoscopic
examination, or on the optic nerve or retina microscopically. Clinical
signs of aggressiveness and hyperactivity were reported in animals
receiving 50 ppm; reduced mean body weights were also reported at this
dose. Plasma and erythrocyte cholinesterase levels were decreased by
> 20% of control levels in animals of each sex at the two highest
doses, and brain acetylcholinesterase activity was inhibited in
animals of each sex at the highest dose and in females at 12.5 ppm.
Peripheral neuropathy was seen in distal and proximal segments of the
sciatic nerve in animals at the two highest doses, which was
characterized by myelin bubbles and Schwann cell proliferation. An
increased percentage of large diameter fibres was seen in the tibial
nerves and an increased incidence of myelin ovoids in the sural nerves
of males receiving 12.5 ppm. Myelin bubbles and Schwann cell
proliferation were also seen in two of 10 rats at 2.5 ppm, but these
changes were not statistically significant nor of the same degree of
severity as those in rats receiving 12.5 ppm. The NOAEL was 2.5 ppm,
equal to 0.11 mg/kg bw per day (Daly, 1991).
Groups of 50 male and 50 female Wistar TNO/W74 rats received
diets containing parathion-methyl (purity, 94.8%) at levels of 2, 10,
or 50 ppm for two years. The control group comprised 100 rats of each
sex. No carcinogenic effects were seen. Most of the toxic effects were
observed at 50 ppm and included decreased body-weight gain, increased
food consumption, tremors, slightly increased mortality, and decreased
haemoglobin and haematocrit values in females at the high dose at
termination. Alkaline phosphatase activity was increased and plasma
protein levels were significantly lowered throughout the study,
perhaps correlated with an increase in urinary protein. The other
clinical findings were considered to be incidental. Plasma and
erythrocyte cholinesterase activities were slightly inhibited in rats
receiving 10 ppm of parathion-methyl, and brain cholinesterase
activity was only 78% of the control value in males at this dose.
Inhibition of plasma and erythrocyte cholinesterase activities was
reported in animals of each sex at 50 ppm, and the brain
cholinesterase activity was 50% of that reported for controls in males
and 37% in females. The NOAEL was 2 ppm, equivalent to 0.1 mg/kg bw
per day, and the LOAEL was 10 ppm, equivalent to 0.5 mg/kg bw per day,
based on a 22% reduction in brain acetylcholinesterase activity
(Bomhard et al., 1981).
Groups of 60 Sprague-Dawley rats of each sex received
parathion-methyl (purity, 93.65%) at dietary levels of 0, 0.5, 5, or
50 ppm for two years. No carcinogenic effects were seen. Toxic effects
were observed in animals at 50 ppm and included tremors, anogenital
staining, reduced body weight, retinal degeneration, decreases in
erythrocyte parameters, and sciatic nerve degeneration. The plasma,
erythrocyte, and brain cholinesterase activities of males at the high
dose at study termination were significantly lower than those of
controls (82, 9, and 76%, respectively, of the control value). In
females, brain cholinesterase activity was reduced by 79%. At 5 ppm,
an abnormal gait was reported in one female, and haemoglobin and
haematocrit and erythrocyte counts were slightly reduced in other
animals. No effects on cholinesterase activity were reported. The
NOAEL was 5 ppm, equivalent to 0.25 mg/kg bw per day (Daly, 1983).
Dogs
Groups of eight beagle dogs received parathion-methyl (purity,
93.7%) at levels of 0, 0.03, 0.1, or 0.3 mg/kg bw per day for one
year. Animals were observed daily, clinical chemistry and haematology
were assessed at designated intervals, and erythrocyte and plasma
cholinesterase activities were monitored monthly. Brain acetylcholine-
sterase activity was determined at study termination. No clinical
signs and no significant effects on body weight or food consumption
were reported, and clinical pathology, gross pathology, and
histopathology showed no compound-related changes. The NOAEL was
0.3 mg/kg bw per day, the highest dose tested (Ahmed & Saguartz,
1981).
(d) Reproductive and developmental toxicity
Rats
Groups of Wistar HAN rats were given parathion-methyl (purity,
97%) at doses of 0, 0.3, 1.0, or 3.0 mg/kg bw per day by gavage on
days 6-15 of gestation. Parental animals were monitored for mortality,
clinical signs of toxicity, body weights, food consumption, and
cholinesterase activity. Litters were evaluated for abnormalities,
sex, and weight. Five deaths and clinical signs of toxicity, including
somnolence, ataxia, and dyspnoea, were seen in maternal animals at the
high dose throughout the study. Increased post-implantation losses and
decreases in food consumption, body weight, and body-weight gain were
also reported, and plasma and erythrocyte cholinesterase activities
were decreased. Fetuses from dams receiving 3.0 mg/kg bw showed an
increased frequency of delayed ossification and reduced body weight.
The maternal and developmental NOAEL was 1 mg/kg bw per day
(Becker et al., 1987)
Inseminated female rats received parathion-methyl (purity, 94.4%)
by gavage on days 6-15 of gestation at doses of 0, 0.1, 0.3, or
1 mg/kg bw per day. At 1 mg/kg bw per day, maternal toxicity was seen,
manifested as significantly decreased body-weight gain during
treatment and gestation. Fetal weight was also significantly lower,
and the frequency of stunted fetuses was greater. The NOAEL for
maternal and developmental effects was 0.3 mg/kg bw per day
(Machemer, 1977).
In a multigeneration study of reproductive toxicity,
parathion-methyl (purity, 95%) was administered in the diets of 10
male and 20 female Wistar rats at levels of 0, 2, 10, or 50 ppm. Each
of the F0, F1, and F2 generations was mated twice. At 50 ppm,
convulsions were observed in F1b parents, and decreased growth was
reported among parental animals of all generations. At this dose,
adverse effects were reported on birth weight, litter size, and the
viability and growth of pups. At 10 ppm, fertility was decreased and
pup survival was slightly decreased in both the F1 and F2
generations. The NOAEL for reproductive toxicity was 2 ppm, equivalent
to 0.1 mg/kg bw per day (Löser & Eiben, 1982).
In a two-generation study of reproductive toxicity, groups of 15
male and 30 female Sprague-Dawley rats received parathion-methyl
(purity, 93.7%) at dietary levels of 0, 0.5, 5, or 25 ppm for 14 weeks
before mating of F0 parents and for 18 weeks before mating of F1
parents. Treatment was continued through gestation and weaning.
Selected F1 adults and five weanlings of each sex in each group per
generation were examined histologically. The body weights of females
at the high dose in both generations were reduced during the lactation
period, but the litters of both generations appeared to be unaffected
by treatment. No abnormalities were seen in reproductive performance,
as indicated by mating, pregnancy, and fertility rates, pup viability
and survival, and gross and microscopic lesions, nor were there
significant differences in the body weights of offspring. The NOAEL
was 5 ppm, equivalent to 0.25 mg/kg bw per day, for parental toxicity
and 25 ppm, equivalent to 1.25 mg/kg bw per day, for reproductive
toxicity (Daly 1982).
Rabbits
Groups of 15 inseminated Himalayan rabbits received parathion-
methyl (purity, 95.7%) at doses of 0, 0.3, 1, or 3 mg/kg bw per day by
gavage on gestation days 6-18. Fetuses were removed by caesarian
section on day 29. No effects were reported on litter parameters. In
maternal animals at 3 mg/kg bw, erythrocyte and plasma cholinesterase
activities were inhibited, but no other effects were reported. The
NOAEL was 1 mg/kg bw per day for maternal effects and
3 mg/kg bw per day for developmental effects (Renhof, 1984).
(e) Genotoxicity
The genotoxic effects of parathion-methyl are summarized in Table
2.
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Three male and three female New Zealand white rabbits received
0.1 ml of parathion-methyl (purity, 80%) by instillation into the
right eye. Slight redness was observed in the eyes of all rabbits 1 h
after instillation; all had returned to normal by 48 h.
Topical application of parathion-methyl (purity, 80%) to New
Zealand white rabbits was associated with very slight to moderate
erythema 1 and 24 h after removal of gauze patches. Slight oedema was
also observed in one of six rabbits. The skin of all animals was
normal by 48 h.
In the Magnusson-Kligman test, female guinea-pigs received six
intradermal doses of parathion-methyl (purity, 80%) in 5% paraffin oil
or emulsified with 0.05 ml of Freund's complete adjuvant. A challenge
concentration was applied two weeks after induction, and reactions
were assessed 24 and 48 h after the patch was removed. Parathion-
methyl was not considered to be a sensitizing agent in guinea-pigs
(Cuthbert & Carr, 1986).
(ii) Neurotoxicity
Groups of 10 male and 10 female Sprague-Dawley rats received
parathion-methyl (purity, 93.1%) by gavage as single doses of 0,
0.025, 7.5, 10 (males only), or 15 (females only) mg/kg bw.
Neurobehavioural evaluations, consisting of a battery of tests for
motor activity and functional observation, were conducted at
designated intervals during the 14-day observation period, and plasma
and erythrocyte cholinesterase activities were determined at several
intervals during the study. Acetylcholinesterase activity was measured
in six regions of the brain at the time of the peak effect and at day
Table 2. Results of tests for the genotoxicity of parathion-methyl
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 2-12 500 µg/plate approx. Positive in Herbold (1986a)
TA100, TA1535, TA1537 96% TA100 at
> 900 µg/plate
Reverse mutation S. typhimurium 2-12 500 µg/plate 94 Positive Herbold (1986b)
Unscheduled DNA Rat hepatocytes 3 × 10-5-3 × 10-2 µl/ml NR Negative Curren (1989)
synthesis
In vivo
Dominant lethal mutation Mouse 10 mg/kg bw 95.7 Negative Herbold (1984)
Micronucleus formation Bor:NMRI mouse 2 × 5, 2 × 10 mg/kg bw 95.6 Negative Herbold (1982)
NR, not reported
14. At the middle and high doses, parathion-methyl had transient
effects on motor activity and function, inducing lacrimation,
salivation, tremors, muscle fasciculations, ataxia, muscle weakness,
and miosis; and plasma, erythrocyte, and brain cholinesterase
activities were inhibited by 67, 56, and 76% at the time of the peak
effect, respectively. in males at the high dose, body-weight gain was
significantly lower than that of controls; similar findings were not
made in females, and the mean body weights were not affected. In males
at this dose, the number of sites at which focal demyelination was
present was increased in comparison with controls, with demyelination
in the cervical (3/6) and lumbar (5/6) dorsal root fibres, in the
cervical (2/6) and lumbar (4/6) ventral root fibres, and in the lumbar
spinal (1/6), tibial (3/6), and sural nerves (2/6). Similar findings
were not found in females. The NOAEL for acute neurotoxicity was
0.025 mg/kg bw (Minnema, 1994).
In the study of long-term toxicity by Daly (1991) in
Sprague-Dawley CD rats, ophthalmoscopic examination, electro-
retinograms, light microscopy, and electron microscopy revealed no
ocular toxicity. Microscopic and morphometric studies of various
preparations of the sciatic nerve and its extensions revealed several
lesions in animals at 12.5 and 50 ppm, which are consistent with
demyelination. These included myelin bubbles, myelin ovoids, Schwann
cell proliferation, and the presence of phagocytic cells. In addition,
increased lengths of demyelination were observed in teased nerve
preparations. The NOAEL for neurotoxicity was 2.5 ppm, equivalent to
0.11 mg/kg bw per day (Daly, 1992).
Hens received parathion-methyl (purity, 95.8%) by intubation at
doses of 0 or 250 mg/kg bw; the test dose thus exceeded the LD50 of
215 mg/kg bw. Positive control animals received 600 mg/kg bw of
tri- ortho-cresyl phosphate. A second dose was administered 21 days
after the first. No clinical signs were seen, and there were no
histopathological lesions in the brain, spinal cord, or nervous tissue
that were indicative of delayed neurotoxicity. One-half of the treated
hens died; surviving birds showed clinical signs of toxicity including
lethargy, depression, wing droop, salivation, shallow and rapid
respiration, and cyanotic comb. The hens recovered within about seven
days (Beaver et al., 1990).
3. Observations in humans
Five male volunteers received daily doses of 3 mg parathion-
methyl for 28 days, followed by 3.5 mg per day for the next 28 days
and then by 4 mg per day for 43 days. No signs of toxicity were
reported, and no effect on plasma or erythrocyte cholinesterase
activity was observed (Moeller & Rider, 1961).
Three groups of five volunteers were given 4.5 mg parathion-
methyl for 30 days, followed by 5 mg per day for 29 days or 5.5 mg per
day for 24 days. These doses were followed by 6 mg per day for 29 days
or by 6.5 mg/day for 35 days, and finally by 7 mg per day for 24 days.
No significant inhibition of plasma or erythrocyte cholinesterase
activity was reported (Moeller & Rider, 1962).
After oral doses of up to 19 mg to male volunteers for 30 days,
no evidence of toxicity was reported (Rider et al., 1969); however,
when doses of 24 mg were given daily for four weeks, plasma and
erythrocyte cholinesterase activities were inhibited, by 23-24% and
27-55%, respectively (Rider et al., 1970). In another study
(Rider et al., 1971), doses of parathion-methyl that were increased
from 14 to 20 mg over a 30-day period had no effect on cholinesterase
activity. The NOAEL was thus 0.3 mg/kg bw per day.
Comments
Parathion-methyl is absorbed through the skin and from the
respiratory and digestive tracts. Observed differences between its
oral and intravenous toxicity are believed to be associated with
first-pass effects in the liver. The compound is rapidly excreted;
negligible amounts of the labelled dose were present in the blood,
tissues, and organs at 48 h. Conversion of parathion-methyl to
paraoxon-methyl has been shown to occur within minutes after oral
administration to rats. Detoxification is achieved by O-demethylation
or hydrolysis to para-nitrophenol. In humans the primary urinary
metabolites were para-nitrophenol and dimethyl phosphate.
Parathion-methyl is acutely toxic at low doses when administered
either orally (LD50 = 4 mg/kg bw) or by inhalation (LC50 =
0.13 mg/litre). The compound is slightly irritating to the skin and
the eyes but has not been shown to be a sensitizing agent. WHO has
classified parathion-methyl as 'extremely hazardous'.
In a 90-day study in mice at dietary levels of 0, 10, 30, or
60 ppm, the NOAEL was 10 ppm (equivalent to 1.5 mg/kg bw per day) on
the basis of significant decreases in absolute and relative testicular
weights. Cholinesterase activity was not measured in this study. In a
90-day study in rats, the NOAEL was 2.5 ppm (equivalent to 0.12 mg/kg
bw per day) on the basis of significant decreases in plasma,
erythrocyte, and brain cholinesterase activities at 25 ppm (equivalent
to 1.2 mg/kg bw per day).
Two 13-week studies were conducted in dogs, in which parathion-
methyl was administered at dietary levels of 0, 0.3, 1, or 3 mg/kg bw
per day in one study and 0, 0.03, 0.3, or 3 mg/kg bw per day in the
other. In both studies, the LOAEL was 3 mg/kg bw per day, on the basis
of decreases in erythrocyte, plasma, and brain cholinesterase activity.
The NOAELs were 1 and 0.3 mg/kg bw per day, respectively. In a one-year
study in dogs, the NOAEL was also 0.3 mg/kg bw per day, the highest
dose tested.
In a one-year study in rats to determine the ocular and
neurotoxic effects of parathion-methyl, dietary levels of 0, 0.5, 2.5,
12, or 50 ppm were administered. Ocular toxicity was not observed.
Degenerative changes of the sciatic nerve and its extensions
consistent with demyelination were observed at the two highest doses.
The LOAEL was 12 ppm, equal to 0.5 mg/kg bw per day. The NOAEL was
2.5 ppm, equal to 0.1 mg/kg bw per day.
In a two-year study in mice, parathion-methyl was not carcino-
genic at dietary levels up to 50 ppm (equal to 9.2 mg/kg bw per day).
The NOAEL was 7 ppm, equal to 1.6 mg/kg bw per day, on the basis of
significant decreases in erythrocyte, plasma, and brain cholinesterase
activities. In a study of toxicity and carcinogenicity in rats fed
parathion-methyl at dietary levels of 0, 2, 10, or 50 ppm, there was
no evidence of carcinogenicity. The NOAEL was 2 ppm (equivalent to
0.1 mg/kg bw per day), and the LOAEL was 10 ppm (equivalent to
0.5 mg/kg bw per day), on the basis of a reduction in brain
acetylcholinesterase activity. In another two-year study In rats,
parathion-methyl did not induce carcinogenic effects. The NOAEL was
5 ppm (equivalent to 0.25 mg/kg bw per day) on the basis of tremors,
anogenital staining, reduced body weight, retinal degeneration,
sciatic nerve degeneration, decreased packed cell volume and
haemoglobin and erythrocyte counts, and decreased brain cholinesterase
activity in males and females at 50 ppm (equivalent to 2.5 mg/kg bw
per day).
Two studies of developmental toxicity were conducted in rats. In
one study, animals received parathion-methyl by gavage at doses of
0, 0.3,1, or 3 mg/kg bw per day. The NOAEL for maternal and
developmental toxicity was 1 mg/kg bw per day on the basis of
increased numbers of deaths, ataxia, and dyspnoea in dams and delayed
ossification in fetuses. In another study, the NOAEL for maternal and
developmental toxicity was 0.3 mg/kg bw per day on the basis of
significant decreases in maternal body-weight gain during treatment
and gestation and an increased incidence of stunted fetuses at 1 mg/kg
bw per day. In studies of developmental toxicity in rabbits, decreased
erythrocyte and plasma cholinesterase activities were reported in dams
receiving 3 mg/kg bw per day by gavage. No developmental effects were
reported that could be attributed to the administration of parathion-
methyl. The NOAEL for maternal toxicity was 1 mg/kg bw, and that for
developmental toxicity was 3 mg/kg bw.
In a multigeneration study in rats, dietary levels of 0, 2, 10,
or 50 ppm were administered. Slight effects on pup survival were
reported in animals receiving 10 ppm, equivalent to 0.5 mg/kg bw per
day. The NOAEL was 2 ppm, equivalent to 0.1 mg/kg bw per day. In
another study, in which dietary levels of 0, 0.5, 5, or 25 ppm were
administered, decreases in maternal body weights during the lactation
period were observed at 25 ppm. The NOAEL was 5 ppm, equivalent to
0.25 mg/kg bw per day. The overall NOAEL in the two studies of
reproductive toxicity was 5 ppm, equivalent to 0.25 mg/kg bw per day.
Parathion-methyl was mutagenic in bacteria, but there was no
evidence of genotoxicity in a limited range of studies in mammalian
systems.
The NOAEL derived from the combined results of several studies
conducted in humans, based on the depression of erythrocyte and plasma
cholinesterase activities, was 0.3 mg/kg bw per day.
An ADI of 0-0.003 mg/kg bw was established on the basis of the
NOAEL of 5 ppm, equivalent to 0.25 mg/kg bw per day, in the two-year
study in rats for retinal degeneration, sciatic nerve demyelination,
reduced body weight, anaemia, and decreased brain acetylcholinesterase
activity. A safety factor of 100 was used. Since the toxicological
end-points seen in animals were other than acetylcholinesterase
inhibition, a safety factor of 10 could not be applied to the NOAEL in
humans.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 7 ppm, equal to 1.6 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
Rat: 5 ppm, equivalent to 0.25 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
5 ppm, equivalent to 0.25 mg/kg bw per day (study of
reproductive toxicity)
0.3 mg/kg bw per day (maternal, embryo-, and fetotoxicity
and teratogenicity in study of developmental toxicity)
Rabbit: 1 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
3 mg/kg bw per day (no embryo- or fetotoxicity or
teratogenicity in study of developmental toxicity)
Dog: 0.3 mg/kg bw per day (one-year study of toxicity and
carcinogenicity)
Human: 0.3 mg/kg bw per day
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Estimate of acute reference dose
An acute reference dose of 0.03 mg/kg bw was derived by applying
the usual 10-fold safety factor to an NOAEL of 19 mg/kg bw (highest
oral dose), corresponding to about 0.3 mg/kg bw per day, in humans,
based on the absence of inhibition of erythrocyte acetylcholinesterase
activity.
Studies that would provide information useful for continued evaluation
of the compound
Observations in humans, particularly reports of poisoning
incidents and/or evaluation of potential long-term neurological and
behavioural effects.
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to parathion-methyl
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Skin, sensitization, guinea-pig Not sensitizing
Eye, sensitization, rabbit Slightly irritating
Skin, irritation, rabbit Slightly irritating
Inhalation 4-h, toxicity, rat LC50 = 0.13 mg/litre
Oral, toxicity, rat LD50 = 4-62 mg/kg bw
Dermal, toxicity, rat LD50 = 480 mg/kg bw
Acute neurotoxicity, one dose, rat NOAEL = 0.025 mg/kg bw per day
Medium-term (1-26 weeks) Repeated dermal, 21-day, toxicity, rabbit NOAEL = 10 mg/kg bw per day; decreases
in brain acetylcholinesterase activity
Repeated oral, 90-day, toxicity, rat NOAEL = 0.125 mg/kg bw per day
Oral, developmental toxicity, rat NOAEL = 0.3 mg/kg bw per day; decreased
maternal body weights, decreased fetal
weights, stunted growth
Repeated oral, reproductive toxicity, rat NOAEL = 0.25 mg/kg bw per day; slight
effects on pup survival
Long-term (> one year) Repeated oral, two-year toxicity and NOAEL = 0.25 mg/kg bw per day
carcinogenicity, rat
References
Ahmed, E & Saguartz, J.W. (1981) Methyl parathion: One year dog study.
Unpublished report from Pharmacopathics Research Laboratories,
Inc., USA. Submitted to WHO by Cheminova Agro SA,
Lemvig, Denmark.
Beaver, J.B., Foster, J., Cockrell, B.Y. & Jabar, M. (1990) Methyl
parathion: An acute delayed neurotoxicity in the laying hen
(Gallus gallus domesticus). Unpublished report No. USAE1200
10590 from Wildlife International Ltd, USA. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Becker, H., Frei, D., Luetkemeier, H., Vogel, W. & Terrier, C. (1987)
Embryotoxicity including teratogenicity study with E-120
technical in the rat. Unpublished report provided by Cheminova
Agro SA, Lemvig, Denmark.
Bomhard, E., Loser, E. & Schilde, B. (1981) E-605 methyl
(parathion-methyl) chronic toxicological study on rats (feeding
experiment over two years). Unpublished report No. 9889 from
Bayer AG. Submitted to WHO by Cheminova Agro SA, Lemvig, Denmark.
Braeckman, R.A., Godefroot, M.G., Blondeel, G.M. & Belpaire, F.M.
(1980) Kinetic analysis of the fate of methyl parathion in the
dog. Arch. Toxicol., 43, 263-271.
Curren, R.D. (1989) Methyl parathion-Unscheduled DNA synthesis in rat
primary hepatocytes. Unpublished report No. USAE 120220689 from
Microbiological Associates, Inc., USA. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Cuthbert, J.A. & Carr, S.M.A. (1986) Methyl parathion 80% technical:
Acute toxicity tests. Unpublished report No. IRI Project
No. 234117 from Inveresk Research International, Musselburgh,
United Kingdom. Submitted to WHO by Cheminova Agro SA,
Lemvig, Denmark.
Daly, I.W. (1982) A two generation reproduction study of methyl
parathion in rats. Unpublished report from Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
SA, Lemvig, Denmark.
Daly, I.W. (1983) A two-year chronic feeding study of methyl parathion
in rats. Unpublished report No. 77-2060(BD-78019) from
Biodynamics Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Daly, I.W. (1989) A 13-week subchronic toxicity study of: methyl
parathion in dogs via the diet followed by a one month recovery.
Unpublished report No. MOB1158 from Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
SA, Lemvig, Denmark.
Daly, I.W. (1991) A twelve month oral toxicity study of methyl
parathion (E120) in the rat via dietary admixture with special
focus on ocular an d sciatic nerve effects. Unpublished report
No. MOB5072 from Biodynamics Inc., East Millstone, New Jersey,
USA. Submitted to WHO by Cheminova Agro SA, Lemvig, Denmark.
Daly, I.W & Rhinehart, W.E. (1980a) A three month feeding study of
methyl parathion in mice. Unpublished report No. 77-2057 from
Biodynamics, Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Daly, I.W & Rhinehart, W.E. (1980b) A three month feeding study of
methyl parathion in rats. Unpublished report No. 77-2059 from
Biodynamics, Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Cheminova AgroSA, Lemvig, Denmark.
Eiben, R. (1991) E120 (methyl parathion) study for chronic toxicity
and carcinogenicity in B6C3F1 mice (administration in the
diet over a period of 24 months). Unpublished report No. 20258
from Bayer AG. Snbmitted to WHO by Cheminova AgroSA,
Lemvig, Denmark.
Goad, M.E.P. (1992) Twenty-one day subchronic dermal toxicity study
with methyl parathion in rabbits. Unpublished report No. 67376
from Arthur D. Little. Submitted to WHO by Cheminova AgroSA,
Lemvig, Denmark.
Greenough, R.J. & McDonald, P. (1986) Methyl parathion 80%
technical/acute inhalation toxicity study in rats. IRI Project
No. 632295 from Inveresk Research International, Musselburgh,
Scotland. Submitted to WHO by Cheminova AgroSA, Lemvig, Denmark.
Herbold, B. (1982) E120-parathion-methyl-folidal M active ingredient
-- Micronucleus test on the mouse to evaluate for mutagenic
effect. Unpublished report No. 10769 from Bayer AG. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Herbold, B. (1984) E120-parathion methyl -- Dominant lethal test on
the male mouse to evaluate for mutagenic effect. Unpublished
report No. 12731 from Bayer AG. Submitted to WHO by Cheminova
Agro SA, Lemvig, Denmark.
Herbold, B. (1986a) E120 (c.n. parathion methyl) -- Salmonella/
microsome test to evaluate for point mutagenic effect.
Unpublished report No. 15306 from Bayer AG. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Herbold, B. (1986b) E120 (c.n. parathion methyl) -- Salmonella/
microsome test to evaluate for point mutagenic effect.
Unpublished report No. 15230 from Bayer AG. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Löser, E. & Eiben, R. (1982) E-605 methyl-Multigeneration studies on
rats. Unpublished report No. 6812 from Bayer AG. Submitted to WHO
by Cheminova Agro SA, Lemvig, Denmark.
Machemer, L. (1977) Parathion-methyl -- Evaluation for embryotoxic and
teratogenic effects on rats following oral administration.
Unpublished report submitted to WHO by Cheminova AgroSA,
Lemvig, Denmark.
Mihail, F. & Vogel, O. (1984) Subacute dermal toxicity studies with
rabbits. Unpublished report No. 12484 from Bayer AG. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Minnema, D.J. (1994) Acute neurotoxicity study of methyl parathion in
rats. Unpublished report No. HWA2688-102 from Hazelton. Submitted
to WHO by Cheminova Agro SA, Lemvig, Denmark.
Moeller, H.C. & Rider, J.A. (1961) Fed. Proc., 20, 434.
Moeller, H.C. & Rider, J.A. (1962) Fed. Proc., 21, 451.
Renhof, M. (1984) Parathion-methyl (Folidol M active ingredient) study
for embryotoxic effects on rabbits after oral administration.
Unpublished report No. 12907 from Bayer AG. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Rider, J.A., Moeller, H.C., Puletti, E.J. & Swader, J.I. (1969)
Toxicity of parathion, systox, octamethyl pyrophosphoramide and
methyl parathion in men. Toxicol. Appl. Pharmacol.,
14, 603-611.
Rider, J.A., Swader, J.I. & Puletti, E.J. (1970) Methyl parathion and
guthion anticholinesterase effects in human subjects (Abstract
No. 588). Fed. Proc., 29, 349.
Rider, J.A., Swader, J.I. & Puletti, E.J. (1971) Anticholinesterase
toxicity studies with methyl parathion, guthion and phosdrin in
human subjects. Fed. Proc., 30, 1382.
Tegeris, A.S. & Underwood, P.C. (1977) A 14 day study of methyl
parathion in the dog. Unpublished report No. 7754 from
Pharmacopathics Research Laboratories, Inc., USA. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Tegeris, A.S. & Underwood, P.C. (1978) Methyl parathion: Ninety day
feeding to dogs. Unpublished report No. 7754 from Pharmacopathics
Research Laboratories, Inc., USA. Submitted to WHO by Cheminova
Agro SA, Lemvig, Denmark.
Van Dijk, H. (1988) C14-parathion methyl: Absorption, distribution,
excretion and metabolism after single and repeated oral
administration to rats. Unpublished report No. 090876 from RCC
Umweltchemie AG. Submitted to WHO by Cheminova Agro SA,
Lemvig, Denmark.
Yamamoto, T., Egashira, T., Yoshida, T. & Kurwin, Y. (1983)
Comparative metabolism of fenitrothion and methylparathion in
male rats. Acta Pharmacol Toxicol., 53, 96-102.
Rats
Groups of 20 Sprague-Dawley rats of each sex received diets
containing parathion-methyl (purity, 93.65%) at doses of 0, 2.5, 25,
or 75 ppm for 91-94 consecutive days. Females at the highest dose had
increased mortality during the first four weeks of the study, and
animals of each sex showed tremors, emaciation, anogenital staining,
and decreased body weight, although food consumption was greater than
that of controls. Erythrocyte parameters and the levels of glucose,
total protein, albumin, and globulin were decreased at one or more
intervals in animals at the high dose, and increases were reported in
serum aspartate aminotransferase and alkaline phosphatase activities
and in blood urea nitrogen. The haematological and serum chemistry
values were, however, within biologically normal ranges. Gross and
histopathological changes were reported in animals at the high dose,
including discoloured and abraded foci in the stomach, which were
correlated histologically with ulcerative gastritis, but these were
considered secondary to the stress that resulted from administration
of the test material. Additional histological findings included
lymphoid necrosis and necrosis of the submaxillary salivary glands. At
the medium dose, erythrocyte and plasma cholinesterase activities were
decreased in animals of each sex, whereas brain acetylcholinesterase
activity was inhibited only in females. Erythrocyte acetylcholine-
sterase activity was decreased in males at the low dose but not to a
biologically significant level. The NOAEL was 2.5 ppm, equivalent to
0.125 mg/kg bw per day, and was based on the decrease in brain
acetylcholinesterase activity in females (Daly & Rhinehart, 1980b).
Rabbits
Groups of five male and five female New Zealand white rabbits
received parathion-methyl (purity, 93.1%) topically at doses of 0, 1,
5, 10, or 100 mg/kg bw per day for 21 days. The compound was applied
as a 1% solution in carboxymethylcellulose to a depilated surface on
the body of each animal and was held in contact with the skin for 6 h
on each day of exposure. No dermal effects were reported in males, but
in females at 10 and 100 mg/kg bw per day inhibition of erythrocyte
acetylcholinesterase activity was seen. The LOAEL was 10 mg/kg bw per
day, and the NOAEL was 5 mg/kg bw per day (Goad, 1992).
Groups of six New Zealand white rabbits of each sex received
parathion-methyl (purity, 96.3%) topically at doses of 0,10, 50, or
250 mg/kg bw per day. One-half of the animals in each group were
tested on abraded skin. At > 50 mg/kg bw per day, brain and
erythrocyte acetylcholinesterase activities were inhibited. The NOAEL
was 10 mg/kg bw per day (Mihail & Vogel, 1984).
Dogs
Groups of two beagle dogs of each sex were given parathion-methyl
(purity, 94.3%) in the diet at levels of 0, 2.5, 5 or 10 mg/kg bw per
day for 14 days. Decreases in body-weight gain and/or body weight were
reported in males and females at the middle and high doses, and feed
consumption was significantly lower in all animals at the high dose
and in females at the middle dose. Cholinesterase inhibition was not
measured. The NOEL was 2.5 mg/kg bw per day, based on the effects on
body weight and food consumption at 5 mg/kg bw per day (Tegeris &
Underwood, 1977).
Groups of four beagle dogs of each sex were fed diets containing
parathion-methyl (purity, 94.32%) at levels of 0, 0.3,1, or 3 mg/kg bw
per day for 13 weeks (90 days). There were no effects on body weight,
food consumption, or mortality, and no clinical signs of toxicity;
however, significant decreases in brain, erythrocyte, and plasma
cholinesterase activities were seen in males and females at the high
dose at weeks 6 and 13. At the middle dose, plasma cholinesterase
activity was significantly decreased in males at week 13 and
erythrocyte cholinesterase was significantly decreased in animals of
each sex. The NOAEL was 1 mg/kg bw per day (Tegeris & Underwood,
1978).
Groups of eight beagle dogs of each sex received parathion-methyl
(purity, 94.9%) at doses of 0, 0.03, 0.3, or 3 mg/kg bw per day for 13
weeks, followed by a recovery period of up to seven weeks. Animals
were observed for clinical signs of toxicity; extensive
ophthalmological examinations were conducted, including fundic
observations, intraocular pressure, electroretinogram, and slit-lamp
examination. Brain cholinesterase was measured at terminal sacrifice.
Significant decreases (> 20% of control levels) were seen in plasma
and erythrocyte cholinesterase activities at weeks 6 and 13 in animals
at the highest dose, and at terminal sacrifice the brain choline-
sterase activity was 48% of that of controls. No functional or
morphological ocular impairment was observed. The NOAEL was 0.3 mg/kg
bw per day and the LOAEL was 3 mg/kg bw per day, based on the
decreases in brain cholinesterase activity (Daly, 1989).
(c) Long-term toxicity and carcinogenicity
Mice
Parathion-methyl (purity, 95.5%) was fed to male and female
B6C3F1 mice at dietary levels of 0, 1, 7, or 50 ppm for two years. No
carcinogenicity was seen. Clinical signs of toxicity were infrequent
in treated animals, and none of the gross or histopathological
findings were associated with treatment. Erythrocyte, plasma, and
brain cholinesterase activities were significantly inhibited in
animals receiving 50 ppm. Erythrocyte acetylcholinesterase activity
was also significantly inhibited (by 40-57% of the control value) in
animals at 7 ppm. The NOAEL was 7 ppm, equal to 1.6 mg/kg bw per day
(Eiben, 1991).
Rats
Groups of 70 Sprague-Dawley CD rats received diets containing
parathion-methyl (purity, 94.6%) at levels of 0, 0.5, 2.5, 12.5, or
50 ppm, equivalent to average intakes of 0.02, 0.107, 0.5, and
2.2 mg/kg bw per day, for one year. Fifty animals of each sex per
group were evaluated at the end of the study; two groups of five
animals per sex per group were evaluated for ocular toxicity at six
and 12 months; and two groups of five animals per sex per group were
evaluated for changes in peripheral nerves at six and 12 months. No
effects were seen on mortality, on the eye by ophthalmoscopic
examination, or on the optic nerve or retina microscopically. Clinical
signs of aggressiveness and hyperactivity were reported in animals
receiving 50 ppm; reduced mean body weights were also reported at this
dose. Plasma and erythrocyte cholinesterase levels were decreased by
> 20% of control levels in animals of each sex at the two highest
doses, and brain acetylcholinesterase activity was inhibited in
animals of each sex at the highest dose and in females at 12.5 ppm.
Peripheral neuropathy was seen in distal and proximal segments of the
sciatic nerve in animals at the two highest doses, which was
characterized by myelin bubbles and Schwann cell proliferation. An
increased percentage of large diameter fibres was seen in the tibial
nerves and an increased incidence of myelin ovoids in the sural nerves
of males receiving 12.5 ppm. Myelin bubbles and Schwann cell
proliferation were also seen in two of 10 rats at 2.5 ppm, but these
changes were not statistically significant nor of the same degree of
severity as those in rats receiving 12.5 ppm. The NOAEL was 2.5 ppm,
equal to 0.11 mg/kg bw per day (Daly, 1991).
Groups of 50 male and 50 female Wistar TNO/W74 rats received
diets containing parathion-methyl (purity, 94.8%) at levels of 2, 10,
or 50 ppm for two years. The control group comprised 100 rats of each
sex. No carcinogenic effects were seen. Most of the toxic effects were
observed at 50 ppm and included decreased body-weight gain, increased
food consumption, tremors, slightly increased mortality, and decreased
haemoglobin and haematocrit values in females at the high dose at
termination. Alkaline phosphatase activity was increased and plasma
protein levels were significantly lowered throughout the study,
perhaps correlated with an increase in urinary protein. The other
clinical findings were considered to be incidental. Plasma and
erythrocyte cholinesterase activities were slightly inhibited in rats
receiving 10 ppm of parathion-methyl, and brain cholinesterase
activity was only 78% of the control value in males at this dose.
Inhibition of plasma and erythrocyte cholinesterase activities was
reported in animals of each sex at 50 ppm, and the brain
cholinesterase activity was 50% of that reported for controls in males
and 37% in females. The NOAEL was 2 ppm, equivalent to 0.1 mg/kg bw
per day, and the LOAEL was 10 ppm, equivalent to 0.5 mg/kg bw per day,
based on a 22% reduction in brain acetylcholinesterase activity
(Bomhard et al., 1981).
Groups of 60 Sprague-Dawley rats of each sex received
parathion-methyl (purity, 93.65%) at dietary levels of 0, 0.5, 5, or
50 ppm for two years. No carcinogenic effects were seen. Toxic effects
were observed in animals at 50 ppm and included tremors, anogenital
staining, reduced body weight, retinal degeneration, decreases in
erythrocyte parameters, and sciatic nerve degeneration. The plasma,
erythrocyte, and brain cholinesterase activities of males at the high
dose at study termination were significantly lower than those of
controls (82, 9, and 76%, respectively, of the control value). In
females, brain cholinesterase activity was reduced by 79%. At 5 ppm,
an abnormal gait was reported in one female, and haemoglobin and
haematocrit and erythrocyte counts were slightly reduced in other
animals. No effects on cholinesterase activity were reported. The
NOAEL was 5 ppm, equivalent to 0.25 mg/kg bw per day (Daly, 1983).
Dogs
Groups of eight beagle dogs received parathion-methyl (purity,
93.7%) at levels of 0, 0.03, 0.1, or 0.3 mg/kg bw per day for one
year. Animals were observed daily, clinical chemistry and haematology
were assessed at designated intervals, and erythrocyte and plasma
cholinesterase activities were monitored monthly. Brain acetylcholine-
sterase activity was determined at study termination. No clinical
signs and no significant effects on body weight or food consumption
were reported, and clinical pathology, gross pathology, and
histopathology showed no compound-related changes. The NOAEL was
0.3 mg/kg bw per day, the highest dose tested (Ahmed & Saguartz,
1981).
(d) Reproductive and developmental toxicity
Rats
Groups of Wistar HAN rats were given parathion-methyl (purity,
97%) at doses of 0, 0.3, 1.0, or 3.0 mg/kg bw per day by gavage on
days 6-15 of gestation. Parental animals were monitored for mortality,
clinical signs of toxicity, body weights, food consumption, and
cholinesterase activity. Litters were evaluated for abnormalities,
sex, and weight. Five deaths and clinical signs of toxicity, including
somnolence, ataxia, and dyspnoea, were seen in maternal animals at the
high dose throughout the study. Increased post-implantation losses and
decreases in food consumption, body weight, and body-weight gain were
also reported, and plasma and erythrocyte cholinesterase activities
were decreased. Fetuses from dams receiving 3.0 mg/kg bw showed an
increased frequency of delayed ossification and reduced body weight.
The maternal and developmental NOAEL was 1 mg/kg bw per day
(Becker et al., 1987)
Inseminated female rats received parathion-methyl (purity, 94.4%)
by gavage on days 6-15 of gestation at doses of 0, 0.1, 0.3, or
1 mg/kg bw per day. At 1 mg/kg bw per day, maternal toxicity was seen,
manifested as significantly decreased body-weight gain during
treatment and gestation. Fetal weight was also significantly lower,
and the frequency of stunted fetuses was greater. The NOAEL for
maternal and developmental effects was 0.3 mg/kg bw per day
(Machemer, 1977).
In a multigeneration study of reproductive toxicity,
parathion-methyl (purity, 95%) was administered in the diets of 10
male and 20 female Wistar rats at levels of 0, 2, 10, or 50 ppm. Each
of the F0, F1, and F2 generations was mated twice. At 50 ppm,
convulsions were observed in F1b parents, and decreased growth was
reported among parental animals of all generations. At this dose,
adverse effects were reported on birth weight, litter size, and the
viability and growth of pups. At 10 ppm, fertility was decreased and
pup survival was slightly decreased in both the F1 and F2
generations. The NOAEL for reproductive toxicity was 2 ppm, equivalent
to 0.1 mg/kg bw per day (Löser & Eiben, 1982).
In a two-generation study of reproductive toxicity, groups of 15
male and 30 female Sprague-Dawley rats received parathion-methyl
(purity, 93.7%) at dietary levels of 0, 0.5, 5, or 25 ppm for 14 weeks
before mating of F0 parents and for 18 weeks before mating of F1
parents. Treatment was continued through gestation and weaning.
Selected F1 adults and five weanlings of each sex in each group per
generation were examined histologically. The body weights of females
at the high dose in both generations were reduced during the lactation
period, but the litters of both generations appeared to be unaffected
by treatment. No abnormalities were seen in reproductive performance,
as indicated by mating, pregnancy, and fertility rates, pup viability
and survival, and gross and microscopic lesions, nor were there
significant differences in the body weights of offspring. The NOAEL
was 5 ppm, equivalent to 0.25 mg/kg bw per day, for parental toxicity
and 25 ppm, equivalent to 1.25 mg/kg bw per day, for reproductive
toxicity (Daly 1982).
Rabbits
Groups of 15 inseminated Himalayan rabbits received parathion-
methyl (purity, 95.7%) at doses of 0, 0.3, 1, or 3 mg/kg bw per day by
gavage on gestation days 6-18. Fetuses were removed by caesarian
section on day 29. No effects were reported on litter parameters. In
maternal animals at 3 mg/kg bw, erythrocyte and plasma cholinesterase
activities were inhibited, but no other effects were reported. The
NOAEL was 1 mg/kg bw per day for maternal effects and
3 mg/kg bw per day for developmental effects (Renhof, 1984).
(e) Genotoxicity
The genotoxic effects of parathion-methyl are summarized in Table
2.
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Three male and three female New Zealand white rabbits received
0.1 ml of parathion-methyl (purity, 80%) by instillation into the
right eye. Slight redness was observed in the eyes of all rabbits 1 h
after instillation; all had returned to normal by 48 h.
Topical application of parathion-methyl (purity, 80%) to New
Zealand white rabbits was associated with very slight to moderate
erythema 1 and 24 h after removal of gauze patches. Slight oedema was
also observed in one of six rabbits. The skin of all animals was
normal by 48 h.
In the Magnusson-Kligman test, female guinea-pigs received six
intradermal doses of parathion-methyl (purity, 80%) in 5% paraffin oil
or emulsified with 0.05 ml of Freund's complete adjuvant. A challenge
concentration was applied two weeks after induction, and reactions
were assessed 24 and 48 h after the patch was removed. Parathion-
methyl was not considered to be a sensitizing agent in guinea-pigs
(Cuthbert & Carr, 1986).
(ii) Neurotoxicity
Groups of 10 male and 10 female Sprague-Dawley rats received
parathion-methyl (purity, 93.1%) by gavage as single doses of 0,
0.025, 7.5, 10 (males only), or 15 (females only) mg/kg bw.
Neurobehavioural evaluations, consisting of a battery of tests for
motor activity and functional observation, were conducted at
designated intervals during the 14-day observation period, and plasma
and erythrocyte cholinesterase activities were determined at several
intervals during the study. Acetylcholinesterase activity was measured
in six regions of the brain at the time of the peak effect and at day
Table 2. Results of tests for the genotoxicity of parathion-methyl
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 2-12 500 µg/plate approx. Positive in Herbold (1986a)
TA100, TA1535, TA1537 96% TA100 at
> 900 µg/plate
Reverse mutation S. typhimurium 2-12 500 µg/plate 94 Positive Herbold (1986b)
Unscheduled DNA Rat hepatocytes 3 × 10-5-3 × 10-2 µl/ml NR Negative Curren (1989)
synthesis
In vivo
Dominant lethal mutation Mouse 10 mg/kg bw 95.7 Negative Herbold (1984)
Micronucleus formation Bor:NMRI mouse 2 × 5, 2 × 10 mg/kg bw 95.6 Negative Herbold (1982)
NR, not reported
14. At the middle and high doses, parathion-methyl had transient
effects on motor activity and function, inducing lacrimation,
salivation, tremors, muscle fasciculations, ataxia, muscle weakness,
and miosis; and plasma, erythrocyte, and brain cholinesterase
activities were inhibited by 67, 56, and 76% at the time of the peak
effect, respectively. in males at the high dose, body-weight gain was
significantly lower than that of controls; similar findings were not
made in females, and the mean body weights were not affected. In males
at this dose, the number of sites at which focal demyelination was
present was increased in comparison with controls, with demyelination
in the cervical (3/6) and lumbar (5/6) dorsal root fibres, in the
cervical (2/6) and lumbar (4/6) ventral root fibres, and in the lumbar
spinal (1/6), tibial (3/6), and sural nerves (2/6). Similar findings
were not found in females. The NOAEL for acute neurotoxicity was
0.025 mg/kg bw (Minnema, 1994).
In the study of long-term toxicity by Daly (1991) in
Sprague-Dawley CD rats, ophthalmoscopic examination, electro-
retinograms, light microscopy, and electron microscopy revealed no
ocular toxicity. Microscopic and morphometric studies of various
preparations of the sciatic nerve and its extensions revealed several
lesions in animals at 12.5 and 50 ppm, which are consistent with
demyelination. These included myelin bubbles, myelin ovoids, Schwann
cell proliferation, and the presence of phagocytic cells. In addition,
increased lengths of demyelination were observed in teased nerve
preparations. The NOAEL for neurotoxicity was 2.5 ppm, equivalent to
0.11 mg/kg bw per day (Daly, 1992).
Hens received parathion-methyl (purity, 95.8%) by intubation at
doses of 0 or 250 mg/kg bw; the test dose thus exceeded the LD50 of
215 mg/kg bw. Positive control animals received 600 mg/kg bw of
tri- ortho-cresyl phosphate. A second dose was administered 21 days
after the first. No clinical signs were seen, and there were no
histopathological lesions in the brain, spinal cord, or nervous tissue
that were indicative of delayed neurotoxicity. One-half of the treated
hens died; surviving birds showed clinical signs of toxicity including
lethargy, depression, wing droop, salivation, shallow and rapid
respiration, and cyanotic comb. The hens recovered within about seven
days (Beaver et al., 1990).
3. Observations in humans
Five male volunteers received daily doses of 3 mg parathion-
methyl for 28 days, followed by 3.5 mg per day for the next 28 days
and then by 4 mg per day for 43 days. No signs of toxicity were
reported, and no effect on plasma or erythrocyte cholinesterase
activity was observed (Moeller & Rider, 1961).
Three groups of five volunteers were given 4.5 mg parathion-
methyl for 30 days, followed by 5 mg per day for 29 days or 5.5 mg per
day for 24 days. These doses were followed by 6 mg per day for 29 days
or by 6.5 mg/day for 35 days, and finally by 7 mg per day for 24 days.
No significant inhibition of plasma or erythrocyte cholinesterase
activity was reported (Moeller & Rider, 1962).
After oral doses of up to 19 mg to male volunteers for 30 days,
no evidence of toxicity was reported (Rider et al., 1969); however,
when doses of 24 mg were given daily for four weeks, plasma and
erythrocyte cholinesterase activities were inhibited, by 23-24% and
27-55%, respectively (Rider et al., 1970). In another study
(Rider et al., 1971), doses of parathion-methyl that were increased
from 14 to 20 mg over a 30-day period had no effect on cholinesterase
activity. The NOAEL was thus 0.3 mg/kg bw per day.
Comments
Parathion-methyl is absorbed through the skin and from the
respiratory and digestive tracts. Observed differences between its
oral and intravenous toxicity are believed to be associated with
first-pass effects in the liver. The compound is rapidly excreted;
negligible amounts of the labelled dose were present in the blood,
tissues, and organs at 48 h. Conversion of parathion-methyl to
paraoxon-methyl has been shown to occur within minutes after oral
administration to rats. Detoxification is achieved by O-demethylation
or hydrolysis to para-nitrophenol. In humans the primary urinary
metabolites were para-nitrophenol and dimethyl phosphate.
Parathion-methyl is acutely toxic at low doses when administered
either orally (LD50 = 4 mg/kg bw) or by inhalation (LC50 =
0.13 mg/litre). The compound is slightly irritating to the skin and
the eyes but has not been shown to be a sensitizing agent. WHO has
classified parathion-methyl as 'extremely hazardous'.
In a 90-day study in mice at dietary levels of 0, 10, 30, or
60 ppm, the NOAEL was 10 ppm (equivalent to 1.5 mg/kg bw per day) on
the basis of significant decreases in absolute and relative testicular
weights. Cholinesterase activity was not measured in this study. In a
90-day study in rats, the NOAEL was 2.5 ppm (equivalent to 0.12 mg/kg
bw per day) on the basis of significant decreases in plasma,
erythrocyte, and brain cholinesterase activities at 25 ppm (equivalent
to 1.2 mg/kg bw per day).
Two 13-week studies were conducted in dogs, in which parathion-
methyl was administered at dietary levels of 0, 0.3, 1, or 3 mg/kg bw
per day in one study and 0, 0.03, 0.3, or 3 mg/kg bw per day in the
other. In both studies, the LOAEL was 3 mg/kg bw per day, on the basis
of decreases in erythrocyte, plasma, and brain cholinesterase activity.
The NOAELs were 1 and 0.3 mg/kg bw per day, respectively. In a one-year
study in dogs, the NOAEL was also 0.3 mg/kg bw per day, the highest
dose tested.
In a one-year study in rats to determine the ocular and
neurotoxic effects of parathion-methyl, dietary levels of 0, 0.5, 2.5,
12, or 50 ppm were administered. Ocular toxicity was not observed.
Degenerative changes of the sciatic nerve and its extensions
consistent with demyelination were observed at the two highest doses.
The LOAEL was 12 ppm, equal to 0.5 mg/kg bw per day. The NOAEL was
2.5 ppm, equal to 0.1 mg/kg bw per day.
In a two-year study in mice, parathion-methyl was not carcino-
genic at dietary levels up to 50 ppm (equal to 9.2 mg/kg bw per day).
The NOAEL was 7 ppm, equal to 1.6 mg/kg bw per day, on the basis of
significant decreases in erythrocyte, plasma, and brain cholinesterase
activities. In a study of toxicity and carcinogenicity in rats fed
parathion-methyl at dietary levels of 0, 2, 10, or 50 ppm, there was
no evidence of carcinogenicity. The NOAEL was 2 ppm (equivalent to
0.1 mg/kg bw per day), and the LOAEL was 10 ppm (equivalent to
0.5 mg/kg bw per day), on the basis of a reduction in brain
acetylcholinesterase activity. In another two-year study In rats,
parathion-methyl did not induce carcinogenic effects. The NOAEL was
5 ppm (equivalent to 0.25 mg/kg bw per day) on the basis of tremors,
anogenital staining, reduced body weight, retinal degeneration,
sciatic nerve degeneration, decreased packed cell volume and
haemoglobin and erythrocyte counts, and decreased brain cholinesterase
activity in males and females at 50 ppm (equivalent to 2.5 mg/kg bw
per day).
Two studies of developmental toxicity were conducted in rats. In
one study, animals received parathion-methyl by gavage at doses of
0, 0.3,1, or 3 mg/kg bw per day. The NOAEL for maternal and
developmental toxicity was 1 mg/kg bw per day on the basis of
increased numbers of deaths, ataxia, and dyspnoea in dams and delayed
ossification in fetuses. In another study, the NOAEL for maternal and
developmental toxicity was 0.3 mg/kg bw per day on the basis of
significant decreases in maternal body-weight gain during treatment
and gestation and an increased incidence of stunted fetuses at 1 mg/kg
bw per day. In studies of developmental toxicity in rabbits, decreased
erythrocyte and plasma cholinesterase activities were reported in dams
receiving 3 mg/kg bw per day by gavage. No developmental effects were
reported that could be attributed to the administration of parathion-
methyl. The NOAEL for maternal toxicity was 1 mg/kg bw, and that for
developmental toxicity was 3 mg/kg bw.
In a multigeneration study in rats, dietary levels of 0, 2, 10,
or 50 ppm were administered. Slight effects on pup survival were
reported in animals receiving 10 ppm, equivalent to 0.5 mg/kg bw per
day. The NOAEL was 2 ppm, equivalent to 0.1 mg/kg bw per day. In
another study, in which dietary levels of 0, 0.5, 5, or 25 ppm were
administered, decreases in maternal body weights during the lactation
period were observed at 25 ppm. The NOAEL was 5 ppm, equivalent to
0.25 mg/kg bw per day. The overall NOAEL in the two studies of
reproductive toxicity was 5 ppm, equivalent to 0.25 mg/kg bw per day.
Parathion-methyl was mutagenic in bacteria, but there was no
evidence of genotoxicity in a limited range of studies in mammalian
systems.
The NOAEL derived from the combined results of several studies
conducted in humans, based on the depression of erythrocyte and plasma
cholinesterase activities, was 0.3 mg/kg bw per day.
An ADI of 0-0.003 mg/kg bw was established on the basis of the
NOAEL of 5 ppm, equivalent to 0.25 mg/kg bw per day, in the two-year
study in rats for retinal degeneration, sciatic nerve demyelination,
reduced body weight, anaemia, and decreased brain acetylcholinesterase
activity. A safety factor of 100 was used. Since the toxicological
end-points seen in animals were other than acetylcholinesterase
inhibition, a safety factor of 10 could not be applied to the NOAEL in
humans.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 7 ppm, equal to 1.6 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
Rat: 5 ppm, equivalent to 0.25 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
5 ppm, equivalent to 0.25 mg/kg bw per day (study of
reproductive toxicity)
0.3 mg/kg bw per day (maternal, embryo-, and fetotoxicity
and teratogenicity in study of developmental toxicity)
Rabbit: 1 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
3 mg/kg bw per day (no embryo- or fetotoxicity or
teratogenicity in study of developmental toxicity)
Dog: 0.3 mg/kg bw per day (one-year study of toxicity and
carcinogenicity)
Human: 0.3 mg/kg bw per day
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Estimate of acute reference dose
An acute reference dose of 0.03 mg/kg bw was derived by applying
the usual 10-fold safety factor to an NOAEL of 19 mg/kg bw (highest
oral dose), corresponding to about 0.3 mg/kg bw per day, in humans,
based on the absence of inhibition of erythrocyte acetylcholinesterase
activity.
Studies that would provide information useful for continued evaluation
of the compound
Observations in humans, particularly reports of poisoning
incidents and/or evaluation of potential long-term neurological and
behavioural effects.
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to parathion-methyl
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Skin, sensitization, guinea-pig Not sensitizing
Eye, sensitization, rabbit Slightly irritating
Skin, irritation, rabbit Slightly irritating
Inhalation 4-h, toxicity, rat LC50 = 0.13 mg/litre
Oral, toxicity, rat LD50 = 4-62 mg/kg bw
Dermal, toxicity, rat LD50 = 480 mg/kg bw
Acute neurotoxicity, one dose, rat NOAEL = 0.025 mg/kg bw per day
Medium-term (1-26 weeks) Repeated dermal, 21-day, toxicity, rabbit NOAEL = 10 mg/kg bw per day; decreases
in brain acetylcholinesterase activity
Repeated oral, 90-day, toxicity, rat NOAEL = 0.125 mg/kg bw per day
Oral, developmental toxicity, rat NOAEL = 0.3 mg/kg bw per day; decreased
maternal body weights, decreased fetal
weights, stunted growth
Repeated oral, reproductive toxicity, rat NOAEL = 0.25 mg/kg bw per day; slight
effects on pup survival
Long-term (> one year) Repeated oral, two-year toxicity and NOAEL = 0.25 mg/kg bw per day
carcinogenicity, rat
References
Ahmed, E & Saguartz, J.W. (1981) Methyl parathion: One year dog study.
Unpublished report from Pharmacopathics Research Laboratories,
Inc., USA. Submitted to WHO by Cheminova Agro SA,
Lemvig, Denmark.
Beaver, J.B., Foster, J., Cockrell, B.Y. & Jabar, M. (1990) Methyl
parathion: An acute delayed neurotoxicity in the laying hen
(Gallus gallus domesticus). Unpublished report No. USAE1200
10590 from Wildlife International Ltd, USA. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Becker, H., Frei, D., Luetkemeier, H., Vogel, W. & Terrier, C. (1987)
Embryotoxicity including teratogenicity study with E-120
technical in the rat. Unpublished report provided by Cheminova
Agro SA, Lemvig, Denmark.
Bomhard, E., Loser, E. & Schilde, B. (1981) E-605 methyl
(parathion-methyl) chronic toxicological study on rats (feeding
experiment over two years). Unpublished report No. 9889 from
Bayer AG. Submitted to WHO by Cheminova Agro SA, Lemvig, Denmark.
Braeckman, R.A., Godefroot, M.G., Blondeel, G.M. & Belpaire, F.M.
(1980) Kinetic analysis of the fate of methyl parathion in the
dog. Arch. Toxicol., 43, 263-271.
Curren, R.D. (1989) Methyl parathion-Unscheduled DNA synthesis in rat
primary hepatocytes. Unpublished report No. USAE 120220689 from
Microbiological Associates, Inc., USA. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Cuthbert, J.A. & Carr, S.M.A. (1986) Methyl parathion 80% technical:
Acute toxicity tests. Unpublished report No. IRI Project
No. 234117 from Inveresk Research International, Musselburgh,
United Kingdom. Submitted to WHO by Cheminova Agro SA,
Lemvig, Denmark.
Daly, I.W. (1982) A two generation reproduction study of methyl
parathion in rats. Unpublished report from Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
SA, Lemvig, Denmark.
Daly, I.W. (1983) A two-year chronic feeding study of methyl parathion
in rats. Unpublished report No. 77-2060(BD-78019) from
Biodynamics Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Daly, I.W. (1989) A 13-week subchronic toxicity study of: methyl
parathion in dogs via the diet followed by a one month recovery.
Unpublished report No. MOB1158 from Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
SA, Lemvig, Denmark.
Daly, I.W. (1991) A twelve month oral toxicity study of methyl
parathion (E120) in the rat via dietary admixture with special
focus on ocular an d sciatic nerve effects. Unpublished report
No. MOB5072 from Biodynamics Inc., East Millstone, New Jersey,
USA. Submitted to WHO by Cheminova Agro SA, Lemvig, Denmark.
Daly, I.W & Rhinehart, W.E. (1980a) A three month feeding study of
methyl parathion in mice. Unpublished report No. 77-2057 from
Biodynamics, Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Daly, I.W & Rhinehart, W.E. (1980b) A three month feeding study of
methyl parathion in rats. Unpublished report No. 77-2059 from
Biodynamics, Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Cheminova AgroSA, Lemvig, Denmark.
Eiben, R. (1991) E120 (methyl parathion) study for chronic toxicity
and carcinogenicity in B6C3F1 mice (administration in the
diet over a period of 24 months). Unpublished report No. 20258
from Bayer AG. Snbmitted to WHO by Cheminova AgroSA,
Lemvig, Denmark.
Goad, M.E.P. (1992) Twenty-one day subchronic dermal toxicity study
with methyl parathion in rabbits. Unpublished report No. 67376
from Arthur D. Little. Submitted to WHO by Cheminova AgroSA,
Lemvig, Denmark.
Greenough, R.J. & McDonald, P. (1986) Methyl parathion 80%
technical/acute inhalation toxicity study in rats. IRI Project
No. 632295 from Inveresk Research International, Musselburgh,
Scotland. Submitted to WHO by Cheminova AgroSA, Lemvig, Denmark.
Herbold, B. (1982) E120-parathion-methyl-folidal M active ingredient
-- Micronucleus test on the mouse to evaluate for mutagenic
effect. Unpublished report No. 10769 from Bayer AG. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Herbold, B. (1984) E120-parathion methyl -- Dominant lethal test on
the male mouse to evaluate for mutagenic effect. Unpublished
report No. 12731 from Bayer AG. Submitted to WHO by Cheminova
Agro SA, Lemvig, Denmark.
Herbold, B. (1986a) E120 (c.n. parathion methyl) -- Salmonella/
microsome test to evaluate for point mutagenic effect.
Unpublished report No. 15306 from Bayer AG. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Herbold, B. (1986b) E120 (c.n. parathion methyl) -- Salmonella/
microsome test to evaluate for point mutagenic effect.
Unpublished report No. 15230 from Bayer AG. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Löser, E. & Eiben, R. (1982) E-605 methyl-Multigeneration studies on
rats. Unpublished report No. 6812 from Bayer AG. Submitted to WHO
by Cheminova Agro SA, Lemvig, Denmark.
Machemer, L. (1977) Parathion-methyl -- Evaluation for embryotoxic and
teratogenic effects on rats following oral administration.
Unpublished report submitted to WHO by Cheminova AgroSA,
Lemvig, Denmark.
Mihail, F. & Vogel, O. (1984) Subacute dermal toxicity studies with
rabbits. Unpublished report No. 12484 from Bayer AG. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Minnema, D.J. (1994) Acute neurotoxicity study of methyl parathion in
rats. Unpublished report No. HWA2688-102 from Hazelton. Submitted
to WHO by Cheminova Agro SA, Lemvig, Denmark.
Moeller, H.C. & Rider, J.A. (1961) Fed. Proc., 20, 434.
Moeller, H.C. & Rider, J.A. (1962) Fed. Proc., 21, 451.
Renhof, M. (1984) Parathion-methyl (Folidol M active ingredient) study
for embryotoxic effects on rabbits after oral administration.
Unpublished report No. 12907 from Bayer AG. Submitted to WHO by
Cheminova Agro SA, Lemvig, Denmark.
Rider, J.A., Moeller, H.C., Puletti, E.J. & Swader, J.I. (1969)
Toxicity of parathion, systox, octamethyl pyrophosphoramide and
methyl parathion in men. Toxicol. Appl. Pharmacol.,
14, 603-611.
Rider, J.A., Swader, J.I. & Puletti, E.J. (1970) Methyl parathion and
guthion anticholinesterase effects in human subjects (Abstract
No. 588). Fed. Proc., 29, 349.
Rider, J.A., Swader, J.I. & Puletti, E.J. (1971) Anticholinesterase
toxicity studies with methyl parathion, guthion and phosdrin in
human subjects. Fed. Proc., 30, 1382.
Tegeris, A.S. & Underwood, P.C. (1977) A 14 day study of methyl
parathion in the dog. Unpublished report No. 7754 from
Pharmacopathics Research Laboratories, Inc., USA. Submitted to
WHO by Cheminova Agro SA, Lemvig, Denmark.
Tegeris, A.S. & Underwood, P.C. (1978) Methyl parathion: Ninety day
feeding to dogs. Unpublished report No. 7754 from Pharmacopathics
Research Laboratories, Inc., USA. Submitted to WHO by Cheminova
Agro SA, Lemvig, Denmark.
Van Dijk, H. (1988) C14-parathion methyl: Absorption, distribution,
excretion and metabolism after single and repeated oral
administration to rats. Unpublished report No. 090876 from RCC
Umweltchemie AG. Submitted to WHO by Cheminova Agro SA,
Lemvig, Denmark.
Yamamoto, T., Egashira, T., Yoshida, T. & Kurwin, Y. (1983)
Comparative metabolism of fenitrothion and methylparathion in
male rats. Acta Pharmacol Toxicol., 53, 96-102.
