
CARBOFURAN
First draft prepared by
E. Bosshard
Wetttswil, Switzerland1
Explanation
Evaluation for acceptable daily intake
Biochemical aspects: Absorption, distribution, and excretion
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal sensitization
Neurotoxicity
Inhibition of cholinesterase activity
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Carbofuran was evaluated for the first time by the JMPR in 1976
(Annex 1, reference 26), when a temporary ADI of 0-0.003 mg/kg bw was
established on the basis of a NOAEL of 10 ppm (equivalent to 0.5 mg/kg
bw per day) for reproductive parameters in a three-generation study in
rats conducted by Industrial Biotest Laboratories. The Meeting
expressed concern about the lack of appropriate data on reversible
cholinesterase depression in the dietary studies and the apparent
sensitivity of brain rather than erythrocyte or plasma cholinesterase.
A safety factor of 200 was used to establish the temporary ADI. The
1976 JMPR requested short-term studies in rodents in order to define
the dose that causes brain acetylcholinesterase depression in vivo.
Furthermore, comparative biochemical studies on cholinesterase
inhibition were considered to be desirable to compare the sensitivity
of juveniles and adults. The Meeting also considered that further
studies of reproductive toxicity would be desirable in order to define
the highest NOAEL. Carbofuran was re-evaluated by the JMPR in 1979
(Annex 1, reference 32), when the preliminary results of a long-term
study in rats were reviewed with respect to inhibition of brain
acetylcholinesterase activity. The Meeting was informed that several
toxicological studies were in progress; therefore, the temporary ADI
1 Until end 1995, at the Federal Office of Public Health
of 0-0.003 mg/kg bw was extended. The 1979 JMPR requested submission
of long-term feeding studies in appropriate species and further
studies of reproductive and developmental toxicity. The compound was
re-evaluated by the JMPR in 1980 (Annex 1, reference 34), which
established an ADI of 0-0.01 mg/kg bw on the basis of a NOAEL of
20 ppm (equivalent to 1 mg/kg bw per day) for growth reduction and
cholinesterase inhibition in a long-term study in rats. In 1982, the
JMPR noted (Annex 1, reference 38) that, although the 1976 evaluation
was based on data from the Industrial Biotest Laboratories, the 1979
and 1980 Joint Meetings had reviewed studies from other sources.
Therefore, the 1982 JMPR endorsed the conclusion of the 1980 JMPR and
supported the ADI of 0-0.01 mg/kg bw.
Carbofuran was evaluated by the present Meeting within the CCPR
periodic review programme. This monograph includes relevant data from
the previous monographs (Annex 1, references 27, 33, and 35) and
summarizes those that have become available in the meantime, from
sources other than Industrial Biotest Laboratories. The results of
Industrial Biotest Laboratories studies are included in a few cases in
which an independent audit validation report was available.
Evaluation for acceptable daily intake
1. Biochemical aspects: Absorption, distribution, and excretion
In a study first evaluated by the 1976 JMPR (Annex 1, reference
26), male Swiss white mice given an oral dose of 2 mg/kg bw of
3H-labelled carbofuran eliminated 37-67% of the dose in the urine
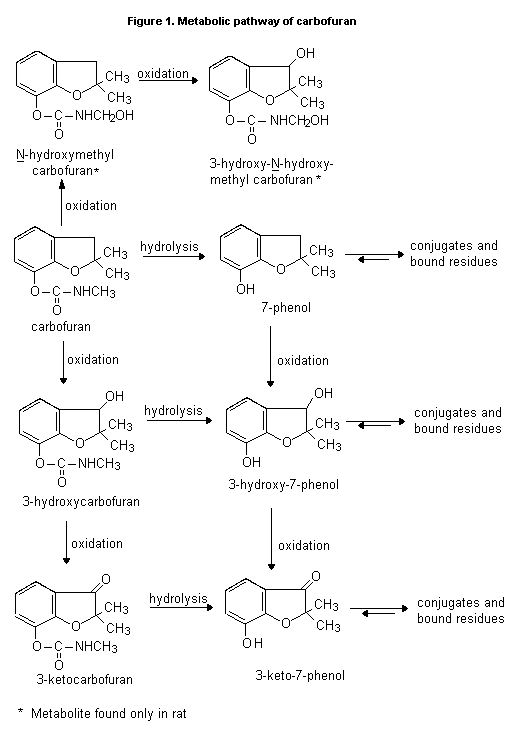
within the first 24 h. The main metabolic pathway starts with
hydroxylation, to give 3-hydroxycarbofuran, and continues with
oxidation, resulting in the formation of 3-ketocarbofuran. Breakage
of the carbamate ester linkage results in liberation of phenolic
derivatives and their corresponding conjugates, principally glycosides
(Metcalf et al., 1968; Fukuto & Metcalf, 1969).
In a study first evaluated by the 1976 JMPR (Annex 1, reference
26), rats were given either 0.4 mg/kg bw [14C-carbonyl]-carbofuran or
4 mg/kg bw [14C-phenyl ring]carbofuran. Measurement of 14C-carbon
dioxide expired by the rats treated with [14C-carbonyl]carbofuran
indicated that about 45% of the administered dose was hydrolysed
within 32 h; total urinary excretion amounted to 38% and faecal
excretion to about 4% of the dose. After administration of the
ring-labelled compound, total urinary excretion was about 92% and
faecal excretion was 3% of the dose. Most of the radiolabel was
eliminated within the first 24 h after treatment, indicating rapid
oxidative cleavage of the ester linkage and rapid elimination.
Analysis of metabolites in urine showed that 3-hydroxycarbofuran,
3-keto-carbofuran phenol, 3-hydroxy- N-hydroxymethylcarbofuran,
3-hydroxymethylcarbofuran, and carbofuran phenol were the major
metabolites. In vitro in rat liver homogenates, the main metabolites
were 3-hydroxycarbofuran and carbofuran phenol derivatives, indicating
that the metabolism in vivo follows the same general pattern
observed in vitro. In a further group of rats given a single oral
dose of 1 mg/kg bw, analysis of various tissues showed residues
ranging from 0.06 ppm in bone to < 1.4 ppm in liver. By 8 h after
treatment, all of the residue levels were < 0.8 ppm (Dorough, 1968).
In plants, carbofuran is metabolized by hydroxylation, hydrolysis,
and conjugation resulting in glycosides. After oral administration of
alfalfa residues of carbofuran to rats, the urinary metabolites were
glucuronides and sulfates, indicating cleavage of the conjugates and
reconjugation (Knaak et al., 1969).
Carbofuran is thus rapidly absorbed, metabolized, and eliminated,
primarily via the urine, in the species investigated. Hydroxylation,
hydrolysis, and conjugation reactions are the major metabolic steps.
The resulting metabolites are either esters or cleavage products of
the ester linkage (Dorough, 1968; Kuhr & Dorough, 1976).
A scheme of the proposed metabolic pathway is given in Figure 1.
 [14C-phenyl ring]Carbofuran was administered in capsules to
laying hens once a day for seven consecutive days at doses equivalent
to dietary concentrations of 0 and 25 ppm. Carbofuran was extensively
metabolized and rapidly eliminated, resulting in low residues in eggs
and tissues. Muscle tissue and fat contained < 0.01 ppm; kidney,
liver, and eggs contained concentrations of < 0.2 ppm carbofuran
equivalents. Conjugates formed after oxidative and hydrolytic
reactions were further conjugated to their corresponding sulfate
conjugates and appeared to bind with biomolecules (e.g. proteins) to
form nonextractable residues (Hoffman & Robinson, 1994a).
[14C-phenyl ring]Carbofuran was administered in gelatine
capsules to lactating goats once a day for seven consecutive days at
doses equivalent to dietary concentrations of 0 and 25 ppm. Carbofuran
was extensively metabolized and readily eliminated. Muscle and fat
contained < 0.01 ppm; kidney, liver, and milk contained 0.09-0.39
ppm. Metabolites occurred in nonconjugated and conjugated forms, the
latter being further conjugated to the sulfate conjugates, which bind
to biomolecules and result in nonextractable (bound) residues (Hoffman
& Robinson, 1994b).
2. Toxicological studies
(a) Acute toxicity
The results of numerous studies of the acute toxicity of
carbofuran in rats were evaluated previously (Annex I, reference 26)
and are summarized in Table 1. The oral LD50 values for rats were
6-18 mg/kg bw. The toxic signs observed were typical of cholinesterase
inhibition: salivation, cramp, trembling, and sedation were observed
within minutes after administration and lasted for up to three days.
Additional studies that have become available since the first
evaluation give oral LD50 values for technical-grade carbofuran in
rats of about 5 mg/kg bw in females and 14 mg/kg bw in males (Elleman,
1979; Sabol, 1979a, b; Norvell, 1983a,b). The dermal LD50 in rabbits
for carbofuran of a purity of 96.1% was > 2000 mg/kg bw (Mehta,
1981). In rats treated by inhalation with a carbofuran formulation
(Furadan 95), the LC50 value was 0.11 mg/litre for a 1-h exposure
(Signorin, 1995).
The results of studies on the acute toxicity of several
metabolites of carbofuran are summarized in Table 2.
Studies of combined treatment with carbofuran and thiofanox or
carbofuran and isofenphos revealed no potentiating effects. Additive
or even subadditive toxic effects were found after simultaneous
administration of the test compounds (Mihail, 1981; Flucke, 1986).
Table 1. Acute toxicity of carbofuran
Species Sex Route LD50 (mg/kg bw) Reference
or LC50 (mg/litre)
Mouse Oral 14.4 Kimmerle (1966)
Rat Male Oral 18.0 Kimmerle (1966)
Rat Male Oral 7.8 Grönning & Kimmerle (1974)
Rat Male Oral 11.9 Kohn (1967a)
Rat Female Oral 6.0 Grönning & Kimmerle (1974)
Rat Female Oral 13.8 Kimmerle (1966)
Rat Male and female Oral 14.1 Powers (1964)
Rat Male Intraperitoneal 8.2 Kimmerle (1966)
Rat Female Intraperitoneal 2.8 Kimmerle (1966)
Rat Male and female Intraperitoneal 1.4 Kohn (1967b)
Rat Male and female Dermal > 500 Kimmerle (1966)
Rat Male Inhalation (1 h) 0.108 Kimmerle (1966)
Rat Male Inhalation (1 h) 0.091 Grönning & Kimmerle (1974)
Rat Male Inhalation (4 h) 0.088 Kimmerle (1966)
Rat Female Inhalation (1 h) 0.080 Grönning & Kimmerle (1974)
Rat Female Inhalation (4 h) 0.075-0.11 Grönning & Kimmerle (1974)
Guinea-pig NR Oral 9.2 Kimmerle (1966)
Rabbit NR Oral 7.5 Kimmerle (1966)
Cat NR Oral 2.5-3.5 Kimmerle (1966)
Dog NR Oral 15-19 Kimmerle (1966)
Table 2. Acute oral toxicity of metabolites of carbofuran in rats
Metabolite LD50 Reference
(mg/kg bw)
3-Hydroxycarbofuran 21.9 (male) Freeman (1984a)
8.3 (female)
3-Ketocarbofuran 108 (male) Freeman (1984b)
93.1 (female)
3-Hydroxy-7-phenol 1916 (male) Freeman (1984c)
1654 (female)
3-Keto-7-phenol > 800 Freeman (1984d)
7-Phenol 2450 (male) Freeman (1984e)
1743 (female)
(b) Short-term toxicity
Rabbits
In a range-finding study, groups of rabbits were treated dermally
with carbofuran (technical-grade; purity, 96.9%) at doses of 0, 100,
300, or 1000 mg/kg bw per day over seven days; the contact time was
6 h/day. No clinical signs of toxicity and no local irritation
were observed. The treatment did not affect body weight or food
consumption. A 30% depression in plasma cholinesterase activity was
measured in males at 100 and 300 mg/kg bw per day and a 47% reduction
at 1000 mg/kg bw per day. Brain acetylcholinesterase activity was
reduced by 26% in males at 100 mg/kg bw per day, 49% in those at 300
mg/kg bw per day, and 54% in those at 1000 mg/kg bw per day. These
reductions were not statistically significant (Kedderis, 1985a).
New Zealand white rabbits received applications of carbofuran
(technical-grade; purity, 96.9%) for about 6 h/day at doses of 0, 10,
100, or 1000 mg/kg bw per day for 21 consecutive days. No signs
of toxicity and no effects on body weight, food consumption,
haematological or clinical chemical parameters, organ weights,
or gross or histopathological appearance were observed. Brain
acetylcholinesterase activity was reduced by 21% in males at 100 mg/kg
bw per day and 26% in those at 1000 mg/kg bw per day. The reductions
were not statistically significant. The NOAEL was 10 mg/kg bw per day
(Kedderis, 1985b).
Dogs
In a 14-day range-finding study, single beagle dogs were fed
diets designed to provide carbofuran (technical-grade; purity, 96.1%)
at concentrations of 0, 18, 32, 56, 100, 316, or 1000 ppm, equivalent
to 0, 0.45, 0.8, 1.4, 2.5, and 8 mg/kg bw per day. The animal that
initially received 18 ppm was changed to a dose of 1000 ppm on day 4
of the study. At this dose, body weight loss and depressed food intake
were observed, and clinical signs included muscle tremor, emesis, and
salivation. Although cholinesterase activity was measured in plasma
and erythrocytes, dose-related depression was found only in plasma, at
all doses; inhibition ranged from 16% (within the historical control
range) in animals at 18 ppm to < 92% at 1000 ppm (Burtner 1982).
In a 13-week feeding study, carbofuran (purity, 99.6%) was
administered in the diet to groups of four male and four female
beagle dogs to provide concentrations of 0, 10, 70, or 500/250 ppm,
equivalent to 0, 0.43, 3.1, and 10.6 mg/kg bw per day. Two additional
animals of each sex per group were assigned to recovery groups to
study the reversibility of the effects over a four-week period. The
feed concentration of 500 ppm was reduced to 250 ppm from treatment
day 6 onwards because of marked toxicity: one dog at this dose died on
day 5 of treatment due to a treatment-related invagination of the
jejunum. At concentrations > 10 ppm, hyperaemia (e.g. at ear pinnae
and oral mucous membranes) and increased salivation were observed.
Clinical signs at the highest dose consisted of muscular spasms,
ataxia (pronounced in males during the first week of dosing but
sporadic thereafter), decreased motility, tachypnoea and deep
respiration, and vomiting. At 500 ppm, food consumption was markedly
reduced and loss of body weight was observed; reduction of the dose to
250 ppm resulted in recovery of food consumption and body-weight gain.
The treatment had no effect on ophthalmoscopic, electrocardiographic,
haematological, or clinical chemical parameters but inhibited
cholinesterase activity and caused alterations in organ weights and in
gross and histopathological appearance. Dose-related inhibition of
plasma and erythrocyte cholinesterase activity was observed in all
treated groups. Maximal inhibition occurred on test day 1, when the
plasma activity was 70, 27, and 13% of the control activity at 10, 70,
and 500/250 ppm and the erythrocyte activity was 68, 29, and 11% of
the control, respectively. The plasma and erythrocyte cholinesterase
activities returned to normal during the four-week recovery period.
There was no inhibition of brain acetylcholinesterase activity by the
end of the treatment or recovery period. An NOAEL was not determined
because inhibition of erythrocyte acetylcholinesterase and increased
salivation were seen at the lowest dose tested (Bloch et al., 1987a).
This study was followed by a four-week feeding study in order to
establish a NOAEL with respect to clinical signs and cholinesterase
inhibition: Groups of four male beagle dogs were fed diets providing
concentrations of 0 or 5 ppm carbofuran (purity, 99.6%) for four
weeks. The treatment had no effects on clinical signs, mortality, body
weight, food consumption, or plasma or erythrocyte cholinesterase
activity. The NOAEL was 5 ppm, equal to 0.22 mg/kg bw per day (Bloch
et al., 1987b).
In an earlier one-year study, groups of six male and six female
beagle dogs were given diets providing concentrations of 0, 10, 20, or
500 ppm carbofuran (technical-grade; purity, 96.1%), equal to 0,
0.3, 0.6, and 13 mg/kg bw per day. At 500 ppm, body weight losses
associated with emesis were observed, particularly in males, and from
the fifth month these animals were fed a supplemented control diet.
One male at 500 ppm died. Muscle tremor and salivation occurred
sporadically at this dose, and all males showed marked depression of
the haematocrit, haemoglobin values, and erythrocyte count from five
months. Only transient, much less pronounced depressions of these
parameters were observed in females at the high dose. Plasma
cholinesterase activity was inhibited in most males at 10 and
20 ppm and markedly (77%) in all animals at 500 ppm. Erythrocyte
acetylcholinesterase activity was reduced by 21-27% in many males
at 500 ppm. At the end of the study, a 24% depression in brain
acetylcholinesterase activity was observed in males at 500 ppm,
whereas females at this dose showed a 44% increase. No relevant
inhibition of erythrocyte or brain acetylcholinesterase activity was
seen in animals at 10 or 20 ppm. The absolute brain and heart weights
of males at 500 ppm were depressed, but no macroscopic or microscopic
changes were found in these organs. Gross treatment-related lesions
observed in the animals at 500 ppm consisted of a marked loss of body
fat and alopecia. Histopathological examination revealed an increased
amount of hepatocellular endoplasmic reticulum in the treated dogs,
but no clear dose-response relationship was seen. Degeneration of the
seminiferous tubules, giant-cell formation, or aspermia was observed
in males at 500 ppm and in a single male at 20 ppm. Minimal-to-
moderate inflammatory changes of the lung were observed at a higher
incidence in the dogs at 500 ppm. The NOAEL was 10 ppm, equal to
0.3 mg/kg bw per day, on the basis of the histopathological changes
in the testes of one male at 20 ppm (Taylor, 1983).
(c) Long-term toxicity and carcinogenicity
Mice
In a two-year study first evaluated by the 1980 JMPR (Annex 1,
reference 34), groups of 100 male and 100 female Charles River CD-1
mice were fed diets providing concentrations of 0, 20, 125, or 500 ppm
carbofuran (technical-grade; purity, 95.6%), equal to 0, 2.8, 18,
and 70 mg/kg bw per day. The treatment had no effect on general
appearance, mortality, or urinary parameters. Body-weight gain was
slightly reduced in single males at 125 ppm and in most animals at
500 ppm during the first year of the study; however, a 20% reduction
in body-weight gain in comparison with the controls was observed in
females at 500 ppm at the end of the study. Food consumption was also
sporadically reduced in animals at the highest dose, particularly
during the first months of the study. Haematological and clinical
chemical examinations revealed no treatment-related changes, except
for inhibition of brain acetylcholinesterase activity; cholinesterase
activities were not measured in erythrocytes or plasma. A
statistically significant depression of brain acetylcholinesterase
activity was observed in animals at 125 and 500 ppm. Maximal
inhibition at 125 ppm was 31% of the activity in concurrent controls;
at 500 ppm, the maximal depression was 55%. Organ weights were changed
sporadically but were not related to treatment. No gross pathological
findings were observed that were indicative of a relationship with
treatment. Microscopic examination revealed no treatment-related
change and no evidence of a compound-related increase in tumour
incidences. The NOAEL was 20 ppm, equal to 2.8 mg/kg bw per day, on
the basis of inhibition of brain acetylcholinesterase activity at
125 ppm. The conclusions of the earlier evaluation were therefore
confirmed (Brown, 1980; Goldenthal, 1980, 1982a).
Rats
In a study reviewed by the JMPR in 1979 and 1980 (Annex 1,
references 32 and 34), carbofuran (technical-grade; purity, 95.6%) was
administered in the diet to groups of 90 male and 90 female Charles
River CD rats to provide concentrations of 0, 10, 20, or 100 ppm, for
two years. Groups of 10 animals of each sex per dose were killed at 6,
12, and 18 months, and blood and brain samples were collected for
determination of cholinesterase activity. The treatment did not affect
general appearance, mortality, food consumption, or ophthalmoscopic,
haematological, or urinary parameters. Body-weight gain was reduced in
animals of each sex at 100 ppm. Clinical chemical examinations
revealed statistically significant inhibition of cholinesterase
activity in plasma, erythrocytes, and brain in rats at 100 ppm. The
maximal reduction of cholinesterase activity in males was 37% in
plasma, 24% in erythrocytes, and 25% in brain; the corresponding
percentages in females at 100 ppm were 26% in plasma, 19% in
erythrocytes, and 43% in brain. No relevant inhibition of
acetylcholinesterase activity was seen in erythrocytes or brain of
animals at 10 or 20 ppm (Case & Wilson, 1979). Various changes in
organ weights were considered to be due to changes in body weight. No
treatment-related gross pathological or histopathological changes were
observed, and there was no evidence of tumorigenicity. The NOAEL was
20 ppm, equivalent to 1 mg/kg bw per day, on the basis of reduced body
weight gain and acetylcholinesterase inhibition in erythrocytes and
brain at the higher dose. The conclusions of the earlier evaluation
were thus confirmed (Goldenthal, 1979a; Rapp, 1980a; Goldenthal,
1982b).
(d) Reproductive toxicity
Rats
In a three-generation study first evaluated by the 1980 JMPR
(Annex 1, reference 34), groups of Charles River CD rats (10 males and
20 females per group for the first and second generations; 12 males
and 24 females per group for the third generation) were maintained on
a diet providing concentrations of 0, 20, or 100 ppm carbofuran
(purity, 95.6%), equal to 0, 1.2, and 6 mg/kg bw per day for males and
0, 1.9, and 9.7 mg/kg per day for females. Each generation was mated
twice to produce two litters. The treatment did not affect the general
behaviour, appearance, or survival of the parental rats. Parental
body-weight gain was lower at 100 ppm throughout the course of
treatment and was associated with lower food consumption, particularly
in males. Fertility indices were not adversely affected. The survival
of pups of the F1a, F2a, and F3a generations at 100 ppm was reduced
at lactation day 4 in comparison with the control. Dehydration was
seen in pups of some litters of the F3a and F3b generations at
100 ppm. The mean weights of pups at 100 ppm were lower than those of
the controls on day 21 post partum in all generations. No compound-
related gross pathological lesions were seen at necropsy in the F0,
F1, or F2 parental animals or the F2b or F3b litters. Various organ
weights of animals of the F2 parental and F3b pup generations at
20 and 100 ppm were statistically significantly changed. Because
clear dose-response relationships were not always seen and no
histopathological alterations were associated with these changes, the
findings were considered not to be of biological significance. The
NOAEL was 20 ppm, equal to 1.2 mg/kg bw per day, on the basis of
reductions in body-weight gain in the parental generation and
reductions in the growth and survival of pup generations at
100 ppm. Thus, the earlier evaluation of this study was confirmed
(Goldenthal, 1979b; Rapp, 1980b; Goldenthal, 1982c).
(e) Developmental toxicity
Rats
In a study first evaluated by the 1980 JMPR (Annex 1, reference
34), carbofuran (technical-grade; purity, 95.6%) was administered to
groups of 24 pregnant Charles River rats by intubation in 0.25%
carboxymethylcellulose at doses of 0, 0.1, 0.3, or 1 mg/kg bw per day
on days 6-15 of gestation. Transient, dose-dependent clinical signs
(chewing motions) were observed at doses > 0.1 mg/kg bw per day for
a short period after treatment. Overt signs of toxicity in animals at
0.3 mg/kg bw per day included rough coats and lethargy; at the high
dose, lacrimation, increased salivation, trembling, and convulsions
were also seen. One female at the high dose died. The treatment had no
effect on the body weights of dams or pups. Reproductive parameters
(e.g. numbers of corpora lutea, implantations, and resorptions and
litter size) were not adversely affected by treatment. Skeletal
examination revealed a higher incidence of some variations in
ossification of sternebrae in treated animals but with no clear
dose-response relationship. Similar alterations were prevalent in the
control group at an incidence of 47% of fetuses. There was no evidence
of teratogenicity. An NOAEL was not determined because transient
clinical signs were observed at the lowest dose (Barron et al., 1978).
Groups of 25 pregnant Charles River COBS CD rats were treated
with carbofuran (purity, 95.6%) by gavage in corn oil at daily doses
of 0, 0.25, 0.5, or 1.2 mg/kg bw on days 6-15 of gestation. The
treatment had no effect on the general appearance, behaviour,
mortality, or body weights of the dams. No differences between groups
were noted in reproductive parameters such as the numbers of corpora
lutea, implantations, and resorptions, litter size, or fetal body
weight. No evidence of teratogenicity was seen (Rodwell, 1980a).
In a pilot study, groups of 10 pregnant Charles River COBS rats
were fed carbofuran (purity, 95.6%) in the diet to give concentrations
of 0, 20, 60, 120, 160, or 200 ppm, equal to 0, 1.5, 4, 8, 11, and
13 mg/kg bw per day, on gestation days 6-19. Neither survival nor
behaviour was affected by the treatment. Hair loss, soft stools,
and scabbing were seen at higher incidences in the treated than
the control group. A dose-related loss in mean maternal body
weight occurred during the first two days of treatment with doses
> 60 ppm, resulting in reduced body-weight gains throughout the
treatment period in animals at 120, 160, and 200 ppm. A dose-related
decrease in food consumption was observed at doses > 60 ppm at the
beginning of the treatment period. Reproductive parameters (litter
size and the numbers of resorptions, implantations, and corpora lutea)
were not adversely affected. On the basis of these results, doses of
0, 20, 60, and 160 ppm were selected for the main study (Rodwell,
1980b, 1985).
The main study was conducted with groups of 40 pregnant Charles
River COBS rats fed diets giving concentrations of 0, 20, 60, or 160
ppm carbofuran (purity, 95.6%), equal to 0, 1.5, 4.4, and 11 mg/kg bw
per day, on gestation days 6-19. Treatment had no effect on appearance
or behaviour, except for a slight increase in hair loss, matting of
the ventral haircoat, and soft stools, particularly in females at 60
and 160 ppm. A dose-related reduction in body-weight gain was seen at
doses > 60 ppm during the treatment period, with single instances
of body-weight loss in animals at 60 and 160 ppm at the beginning of
treatment. Increases in body-weight gain of treated animals during the
subsequent lactation period resulted in very similar body weights in
all groups at the end of lactation. Food consumption was reduced
during the first few days of treatment in animals at 60 and 160 ppm.
No differences between groups were observed in reproductive parameters
(such as the numbers of corpora lutea, implantations, and resorptions
and litter size). The mean weights of pups at 160 ppm were decreased
throughout lactation. The incidence of malformations was not
increased. The NOAEL for maternal toxicity was 20 ppm, equal 1.5 mg/kg
bw per day, on the basis of reduced body-weight gain of the dams at
doses > 60 ppm. The NOAEL for pup toxicity was 60 ppm, equal to 4.4
mg/kg bw per day, on the basis of reduced weights of pups at 160 ppm
(Rodwell, 1981).
Groups of 24 mated female Sprague-Dawley (CD) rats were given
diets containing carbofuran (purity, 99.1%) to provide concentrations
of 0, 20, 75, or 300 ppm, equal to 0, 1.7, 5, and 20 mg/kg bw per day,
from gestation day 6 through lactation day 10. Physical observations
were made, and body weight and food consumption were measured for all
maternal animals at selected intervals throughout the gestation and
lactation periods. The numbers of live pups were recorded, and litter
parameters such as pinna detachment, incisor eruption, eye opening,
motor activity, auditory startle response, and brain weights were
evaluated. Neuropathological examinations were performed on six pups
of each sex per group that were killed on postnatal days 11 and 60.
Maternal animals were killed after weaning of the last litter. No
deaths occurred during the study. Reduced body-weight gain was seen in
dams at 75 and 300 ppm during gestation and also during the lactation
period for those at 300 ppm. Food consumption was reduced in dams at
these two doses at various periods during gestation. Treatment had no
effect on pregnancy rates, length of gestation, or litter size, and
there was no evidence of prolonged or difficult delivery. Treatment at
300 ppm resulted, however, in a greater number of dead pups at birth
than among the controls (not statistically significant). A dose-
dependent reduction in pup weight was observed in animals at 75 and
300 ppm throughout the lactation period, and the reductions persisted
after weaning. Pup survival was adversely affected by prenatal
administration of carbofuran at 75 or 300 ppm on lactation day 4 until
weaning at lactation day 21. Treatment at doses of 75 or 300 resulted
in developmental delays of three to four days in vaginal patency and
preputial separation; marginal delays were seen with respect to pinna
detachment, lower incisor eruption, and eye opening. No adverse effect
was noted on auditory startle response, motor activity, or swimming
development, but there was an angle reduction at doses > 75 ppm.
Brain weights were not affected by treatment. No treatment-related
macroscopic findings were observed in dams or pups. Microscopic
examinations of pups in the control and high-dose groups revealed no
compound-related abnormality. The NOAEL was 20 ppm, equal to 1.7 mg/kg
bw per day, on the basis of reduced body-weight gain of dams and pups,
reduced pup survival, and slight developmental delay at doses > 75 ppm
(Ponnock, 1994).
Rabbits
In a study first evaluated by the JMPR in 1980 (Annex 1,
reference 34), groups of 17 pregnant New Zealand white rabbits were
treated by intubation with carbofuran (purity, 95.6%) at doses of 0,
0.2, 0.6, or 2 mg/kg bw per day on gestation days 6-18. The deaths of
five females at 2 mg/kg bw per day were considered to be related to
treatment. Signs of maternal toxicity and behavioural changes in
animals at this dose included trembling, loss of muscle control,
salivation, sneezing, chewing motions, and reduced food and water
consumption. Body weight was not affected by the treatment. No
differences between groups were seen in reproductive parameters.
Macroscopic examination revealed no treatment-related tissue
alteration and no evidence of embryotoxicity or teratogenicity, even
at maternally toxic doses. The NOAEL was 0.6 mg/kg bw per day, on the
basis of maternal toxicity. The same conclusion was drawn by the 1980
JMPR (Rao, 1978 [cited incorrectly as Felton, 1978, in Annex 1
reference 35]).
Carbofuran (purity, 95.6%) was administered by gavage at doses of
0, 0.12, 0.5, or 2 mg/kg bw per day on gestation days 6-18 to groups
of 20 pregnant New Zealand white rabbits. One dam at 2 mg/kg bw per
day died of an unknown cause. Matting and/or staining of the haircoat
was seen at a somewhat higher frequency in the treated animals.
Although the mean maternal body weight was reduced during treatment,
no clear dose-response relationship was seen. A marked reduction in
body-weight gain was observed at 2 mg/kg bw per day early in the
treatment; no data were available on food consumption. Reproductive
parameters (numbers of corpora lutea, implantations, and resorption,
litter size, fetal body weight were not affected by treatment. A
slightly increased incidence of misaligned sternebrae was seen in
fetuses at 2 mg/kg bw per day. The NOAEL was 0.5 mg/kg bw per day, on
the basis of reduced body weight in dams at 2 mg/kg bw and the
increase in skeletal variations (Laveglia, 1981).
Carbofuran was given to CD rats by gastric intubation at doses of
0.05-5 mg/kg bw per day on days 7-19 of gestation and to CD-1 mice at
doses of 0.1-20 mg/kg bw per day on gestation days 6-16. At 1, 3, and
5 mg/kg bw per day, 40-55% of the rats died, and the numbers of
implantation sites and live fetuses were reduced. The frequency of
malformations was not increased. In mice at 10 or 20 mg/kg bw per day,
about 50% of dams died, and fetal body weight was reduced. A shift in
the rib profile (decreased incidence of 13 ribs, increased incidence
of 14 ribs) was observed at 10 and 20 mg/kg bw per day. There was no
evidence of teratogenicity in either rats or mice (Courtney et al.,
1985).
(f) Genotoxicity
The results of tests for the genotoxicity of carbofuran are
summarized in Table 3. Carbofuran was included among other carbamate
insecticides tested in vitro in the porcine brain tubulin assembly
assay for the detection of action as a microtubule poison and
aneuploidy inducer (Stehrer & Wolf, 1995). Carbofuran induced a
dose-dependent reduction in the degree of polymerization of tubulin
and an 11% reduction in the maximal polymerization velocity. It was
active in S. typhimurium strains TA1538 and TA98 in the presence and
absence of an exogenous metabolic activation system (Moriya et al.,
1983), induced sister chromatid exchange in human peripheral
lymphocytes (Georgian et al., 1985), and induced gene mutation in
V79 hamster cells (Woijciechowski et al., 1982).
(g) Special studies
(i) Dermal sensitization
Guinea-pigs were given 10 intracutaneous injections of
technical-grade carbofuran as an inducer, followed by a final
challenge injection. Dinitrochlorobenzene was used as a positive
control. Carbofuran was not sensitizing (Schoenig, 1967; Ellison,
1980).
(ii) Neurotoxicity
Rats
In a 28-day range-finding study, groups of five male and five
female Sprague-Dawley CD rats were maintained on a diet providing
technical-grade carbofuran (purity, 98.6%) at concentrations of 0, 50,
200, 500, 1000, 3000, or 6000 ppm, equivalent to 0, 2.5, 10, 25, 50,
150, and 300 mg/kg bw per day. Two males receiving 6000 ppm died.
Dose-related clinical signs that were noted at doses > 200 ppm in
animals of each sex consisted of exophthalmia, splayed hindlimbs in
females at 200 ppm, tremors and staggered gait at 500 and 1000 ppm,
and loss of muscle control and ataxia at 3000 and 6000 ppm. Treatment-
related clinical signs seen in animals at doses > 500 ppm were
decreased locomotion, dehydration, lacrimation, and unthriftiness.
Body-weight gain was reduced at concentrations > 50 ppm among males
(marginal at 50 ppm) and at > 200 ppm among females (marginal at
200 ppm). Necropsy showed no treatment-related gross lesions (Freeman,
1993). The NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw per day.
Table 3. Results of tests for the genotoxicity of carbofuran
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 100-10 000 µg/plate 98.3 Negativea Haworth & Lawlor
TA98, TA100, Positiveb (1983a)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 98 Negativec Haworth & Lawlor
TA98, TA100, (1983b)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 80 Negativea Haworth & Lawlor
TA98, TA100, Positived (1983c)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 99 Negativee Haworth & Lawlor
TA98, TA100, (1983d)
TA1535, TA 1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 98 Negativef Haworth & Lawlor
TA98, TA100, (1983e)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 98 Negativef Haworth & Lawlor
TA98, TA100, (1983f)
TA1535, TA1537
TA1538
Table 3. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Reverse mutation S. typhimurium 100-10 000 µg/plate 97.6 Negativeg Haworth & Lawlor
TA98, TA100, (1983g)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 96 Negativeh Haworth & Lawlor
TA98, TA100, (1983h)
TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium 1-10 000 µg/plate 97.6 Negative DeGraff (1983a)
TA100
Reverse mutation S. typhimurium 61.7-5000 µg/plate 99 Negativea Farrow (1983a)
TA98 Positivei
Reverse mutation S. typhimurium 61.7-5000 µg/plate 97.6 Negativea DeGraff (1983a)
TA98, TA100, Positivej
TA1537, TA1538
Reverse mutation S. typhimurium 123-10 000 µg/plate 98.3 Negativea Farrow (1983c)
TA98, TA100, Positivek
TA1537
Reverse mutation S. typhimurium 1-5000 µg/plate NR Negative Simmon (1979)
TA98, TA100,
TA1535, TA1537,
TA1538
Reverse mutation E. coli WP2 uvrA 1-5000 µg/plate NR Negative Simmon (1979)
Mitotic recombination S. cerevisiae 1-50 mg/ml NR Negative Simmon (1979)
Unscheduled DNA synthesis Human fibroblasts 0.1-1000 µg/mll NR Negative Simmon (1979)
(WI-38)
Growth inhibition E. colim, B. subtilis 1-500 mg/ml NR Negativen Simmon (1979)
Unscheduled DNA synthesis Rat hepatocytes 1-100 µg/ml 97.6 Negativea Thilagar (1983a)
Table 3. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Sister chromatid exchange Chinese hamster 78.1-312.5 µg/ml with S9 97.6 Negativeo Thilagar (1983b)
ovary cells 12.5-100 µg/ml without S9 Negative
Sister chromatid exchange Chinese hamster 312.5-2500 µg/ml with S9 96 Positivep Thilagar (1983c)
ovary cells 12.5-100 µg/ml without S9 Positive
Chromosomal aberration Chinese hamster 312.5-2500 µg/ml with S9 96 Negative Thilagar (1983d)
ovary cells 50-1000 µg/ml without S9 Negative
Cell mutation tk locus Mouse lymphoma 134-1780 µg/ml with S9 97.6 Negativeq Kirby (1983a)
L5178 Y cells 16-211 µg/ml without S9 Positive
Cell mutation tk locus Mouse lymphoma 134-1780 µg/ml with S9 96 Positive Kirby (1983b)
L5178 Y cells 24-316 µg/ml without S9
In vivo
Sex-linked recessive Drosophila 10 ppm (feeding solution) NR Negative Valencia (1981)
lethal mutation melanogaster
Sex-linked recessive Drosophila 7.5 ppm (feeding solution) 97.6 Negative Valencia (1983)
lethal mutation melanogaster
Sex-linked recessive Drosophila 5 and 10 ppm (feeding 97.6 Negative DeGraff (1983b)
lethal mutation melanogaster solution)
Cytogenicity Male rat 0.6 or 2.6 mg/kg bw per 98 Negative Putman (1983a)
bone marrow day orally, 5 days
Cytogenicity Male rat 1, 6 or 10 mg/kg bw per 96 Negative Putman (1983b)
bone marrow day orally, 5 days
Table 3. (Cont'd)
NR not reported
S9 9000 × g supernatant of Aroclor-induced rat liver microsomes
a With metabolic activation
b Weak positive response (2.2-fold increase in revertants per plate) of strain TA1535 only in the absence of S9
c The 1.9-fold increase in TA1535 revertants per plate observed in the absence of S9 did not meet the criteria for a
positive response.
d Weak positive response (2.2-fold increase in revertants per plate) of strain TA1535 in the absence of S9
e The 1.7-fold increase in TA1535 revertants per plate in the absence of S9 did not meet the criteria for a positive
response.
f 1.9-fold increase in TA1535 revertants per plate in both tests (Haworth & Lawlor, 1983 e,f) in the absence of S9
g 1.8-fold increase in TA1535 revertants and 1.4-fold increase in TA100 revertants in the absence of S9
h 1.6-fold increase in TA1535 revertants and 1.5-fold increase in TA100 revertants in the absence of S9
i Twofold increase in TA1535 revertants at 5000 µg/plate in the absence of S9. Precipitation of the compound occurred
at this concentration.
j Dose-dependent increase in TA1535 revertants at all doses in the absence of S9
k About a threefold increase TA1535 revertants at 1048 and 3145 µg/plate
l Precipitation observed at 1000 µg/ml
m Relative toxicity assays (growth inhibition) in DNA repair-proficient and -deficient strains of E. coli and B. subtilis
n Statistically significant increase in sister chromatid exchange frequency in comparison with solvent control in the
presence of S9 at a concentration of 312.5 µg/ml and in the absence of S9 at 100 µg/ml. The increases did not meet the
criteria for a positive response.
o In the absence of S9
p Statistically significant increases in sister chromatid exchange frequency in comparison with solvent control at all
doses in the presence and absence of S9
q Three S9-activated cultures out of 20 had mutant frequencies that were more than twice the mean mutant frequency of
the solvent controls. The significance of the increase is questionable because growth was strongly inhibited (> 90%)
in these cultures.
In the main study, groups of 10 male and 10 female Charles River
Sprague-Dawley rats were maintained on a diet designed to provide
concentrations of 0, 50, 500, or 1000 ppm, equivalent to 0, 2.4, 27.3,
and 55.3 mg/kg bw per day in males and 0, 3.1, 35.3, and 64.4 mg/kg bw
per day in females. Clinical signs, mortality, body weight and food
consumption were recorded, and functional observational battery and
motor activity testing were conducted before treatment and at weeks
4, 8, and 13 of treatment. The nervous systems of five rats of each
sex at the high dose and in the control group were examined for
histopathological lesions. One male at 1000 ppm was found moribund
on day 83 and was killed, and single animals in all groups,
including controls, were killed during the study for humane reasons.
Treatment-related clinical signs observed at concentrations
> 500 ppm consisted of exophthalmia, splayed hindlimbs, loss of
muscle control (in females), staggered gait, and tremors. Animals at
all doses had a dose-related reduction in body-weight gain; the
differences attained statistical significance in males at all doses
but in females only at 1000 ppm. Sporadic reductions in food
consumption were seen in animals at 1000 ppm, particularly males;
females showed increased food consumption. Functional observational
battery testing revealed effects in animals receiving doses
> 500 ppm, including gait impairment, a reduction in hindlimb grip
strength, whole-body tremors, and abnormal posture. Motor activity was
significantly reduced among females receiving 1000 ppm. The single
male at 1000 ppm found moribund on day 83 had calcifications in the
urethra; no other macroscopic treatment-related lesions were found. No
treatment-related histopathological lesions were found in the central
or peripheral nervous system of animals at the high dose (Freeman,
1994). The NOAEL for neurotoxicity was thus 50 ppm, equal to 3 mg/kg
bw per day. There was no NOAEL for systemic toxicity.
(iii) Inhibition of cholinesterase activity
In a comparative study in newborn, weanling, and adult
Sprague-Dawley rats, first evaluated by JMPR in 1980 (Annex 1,
reference 34), the purpose was to determine the time of maximal
inhibition of cholinesterase activity in plasma, erythrocytes, and
brain and the time of recovery after the administration of a single
oral dose of carbofuran. The LD50 values were 8.1 mg/kg bw for
newborn and 7.3 for weanling rats. The baseline cholinesterase
activities in untreated animals were determined at the age of two to
three days for newborns, 27-29 days for weanlings, and 99-101 days for
adult rats. The baseline activities in erythrocytes were lower in
newborns and weanlings than in adults, and those in brain were lower
in newborns than in weanlings and adults. After administration of
single oral doses of carbofuran at 3.2 mg/kg bw for newborns, 2.2
mg/kg bw for weanlings, and 4 mg/kg bw for adults, maximal depression
of erythrocyte activity occurred within 1 h in all groups, and maximal
depression of brain activity occurred within 1 h in weanlings and
adults and after 4 h in neonates. Full recovery was attained within
24 h after dosing, independently of the age of the animals. There were
no differences in sensitivity to cholinesterase inhibition with age
(Becci, 1979).
In a study to investigate the relationship between carbofuran
metabolism in vivo and acetylcholinesterase inhibition, male
Sprague-Dawley rats were given single intravenous or oral doses of
14C carbofuran at 50 µg/kg bw, and urine, faeces, and expired air
were analysed for oxidative and hydrolytic metabolites; blood, plasma,
and various tissues were analysed for carbofuran and its main
oxidative metabolite, 3-hydroxycarbofuran. Erythrocyte acetyl-
cholinesterase activity in vitro was used as an index of toxicity.
From 41 to 47% of the administered dose was recovered as 14C-carbon
dioxide after 8 h, independently of the route of administration. About
15% of the dose was found in urine and < 1% in faeces. 3-Hydroxy-
carbofuran was formed rapidly and underwent enterohepatic circulation,
resulting in an elimination half-life of about 64 min for all tissues;
the elimination half-life of the parent compound was about 29 min.
Rapid recovery of the erythrocyte acetylcholinesterase activity
closely paralleled carbofuran metabolism, and the primary disposition
of 3-hydroxycarbofuran in vivo was by metabolic conjugation
(Ferguson et al., 1984).
3. Observations in humans
Carbofuran was reported to have induced sensitization in a patch
test in 30 farmers with contact dermatitis (Sharma & Kaur, 1990).
Poisoning was reported in three female farmworkers who threw
carbofuran granules onto a coffee plantation in Jamaica. The women did
not wear protective clothing. The signs of poisoning reported included
vomiting, lassitude, nausea, and hypersalivation. Cholinesterase
activity was not determined in these patients (Coleman et al., 1990).
Comments
Carbofuran is rapidly absorbed, metabolized, and eliminated,
mainly in the urine, after oral administration to mice and rats. After
oral administration of [phenyl-14C]carbofuran to rats, 92% of the
radiolabel was eliminated in the urine and 3% in faeces. Most of the
radiolabel was eliminated within 24 h after treatment. With a
[carbonyl-14C]-labelled compound, 45% was eliminated as 14C-carbon
dioxide. The metabolic pathway consists in hydroxylation, oxidation,
hydrolysis, and conjugation.
Carbofuran is highly toxic after acute oral administration. The
oral LD50 values in various species ranged from 3 to 19 mg/kg bw.
Carbofuran had no sensitizing potential in guinea-pigs, and no local
irritation was found in rabbits after repeated dermal applications
over 7 or 21 days. WHO has classified carbofuran as 'highly hazardous'
(WHO, 1996).
In a 13-week study in dogs fed diets providing 0, 10, 70, or
500/250 ppm (dose reduced because of marked toxicity), an NOAEL was
not identified because of inhibition of erythrocyte acetylcholin-
esterase activity and some clinical signs were observed at the lowest
dose. In a subsequent four-week study in dogs, the highest dose
administered was 5 ppm, equal to 0.22 mg/kg bw per day, which was the
NOAEL for clinical signs, mortality, effects on body weight and food
consumption, and cholinesterase activity in plasma and erythrocytes.
In a one-year study in dogs at dietary concentrations of 0, 10, 20, or
500 ppm, the NOAEL was 10 ppm, equal to 0.3 mg/kg bw per day, on the
basis of histopathological testicular changes in a single male at
20 ppm; similar changes were observed in animals at 500 ppm. There
was no inhibition of erythrocyte or brain acetylcholinesterase at
concentrations of 10 or 20 ppm. The overall NOAEL in these short-term
studies in dogs was 5 ppm, equal to 0.22 mg/kg bw per day.
In two-year studies of toxicity and carcinogenicity at dietary
concentrations of 0, 20, 125, or 500 ppm in mice and 0, 10, 20, or
100 ppm in rats, the NOAELs were 20 ppm, equal to 2.8 mg/kg bw per
day, in mice and 20 ppm, equivalent to 1 mg/kg bw per day, in rats, on
the basis of inhibition of erythrocyte and brain acetylcholinesterase
activity. There was no evidence of tumorigenicity.
In a three-generation study of reproductive toxicity in rats at
dietary concentrations of 0, 20, or 100 ppm, the NOAEL was 20 ppm,
equal to 1.6 mg/kg bw per day, on the basis of reduced body-weight
gain in parental animals and reduced pup growth and pup survival at
100 ppm.
In an early study of developmental toxicity, rats were given
carbofuran at doses of 0, 0.1, 0.3, or 1 mg/kg bw per day by gavage.
An NOAEL could not be identified in this study. Dose-dependent,
transient clinical signs (chewing motions) were observed in the dams.
In a later study in rats at oral doses of 0, 0.25, 0.5, or 1.2 mg/kg
bw per day, the NOAEL for maternal and fetal toxicity was 1.2 mg/kg bw
per day, the highest dose tested. In a further study of teratogenicity
in rats, with dietary administration of 0, 20, 60, or 160 ppm
carbofuran, the NOAEL for maternal toxicity was 20 ppm, equal to
1.5 mg/kg bw per day, on the basis of a reduction in body-weight gain
at 60 ppm. The NOAEL for pup toxicity, based on reduced pup weight,
was 60 ppm, equal to 4.4 mg/kg bw per day. None of the studies showed
teratogenic potential.
The results of an early study of teratogenicity in rabbits at
oral doses of 0, 0.2, 0.6, or 2 mg/kg bw per day showed an NOAEL of
0.6 mg/kg bw per day for maternal toxicity on the basis of clinical
signs and an NOAEL of 2 mg/kg bw per day for fetotoxicity and
teratogenicity. In a subsequent study in rabbits at doses of 0, 0.12,
0.5, or 2 mg/kg bw per day, the NOAEL was 0.5 mg/kg bw per day on the
basis of slightly reduced body-weight gain in dams and a slightly
increased incidence of skeletal variations in pups at 2 mg/kg bw per
day. These studies provide no evidence for teratogenicity.
In a 90-day study of neurotoxicity in rats at dietary
concentrations of 0, 50, 500, or 1000 ppm, systemic toxicity
(reduction in body-weight gain) was observed at all doses. Clinical
signs of neurotoxicity were observed at 500 and 1000 ppm. No
histopathological lesions were found in the nervous system.
In a study of developmental neurotoxicity, carbofuran was
administered in the diet of rats to provide concentrations of 0, 20,
75, or 300 ppm from gestation day 6 through lactation day 10.
Reductions in the body-weight gain of dams and pups and in pup
survival and some evidence of delayed pup development were found at
doses > 75 ppm. The NOAEL was 20 ppm, equal to 1.7 mg/kg bw per
day, on the basis of reduced body-weight gain in dams and signs of
fetotoxicity at higher doses.
Carbofuran has been tested for genotoxicity in a wide range of
tests in vivo and in vitro. The Meeting concluded that it is not
genotoxic.
An ADI of 0-0.002 mg/kg bw was allocated on the basis of the
NOAEL for erythrocyte acetylcholinesterase inhibition of 0.22 mg/kg bw
per day in a four-week study in the most sensitive species, the dog,
and using a 100-fold safety factor. The use of a short-term study to
set the ADI is justified because the effect observed was reversible
and acute.
Toxicological evaluation
Levels that cause no toxicological effect
Mouse: 20 ppm, equal to 2.8 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rat: 20 ppm, equivalent to 1 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
20 ppm, equal to 1.2 mg/kg bw per day (three-generation
study of reproductive toxicity)
1.2 mg/kg bw per day (highest dose tested in a study of
developmental toxicity)
20 ppm, equal to 1.5 mg/kg bw per day (study of
developmental toxicity)
20 ppm, equal to 1.7 mg/kg bw per day (study of
developmental neurotoxicity)
Rabbit: 0.6 mg/kg bw per day (study of developmental toxicity)
Dog: 5 ppm, equal to 0.22 mg/kg bw per day (four-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to carbofuran
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 6-14.4 mg/kg bw
Dermal, toxicity, rat LD50 > 500 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.088-0.1 mg/litre
Dermal, irritation, rabbit Not irritating
Ocular irritation, rabbit Not available
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated oral, 4 weeks, toxicity, dog NOAEL = 0.22 mg/kg bw per day
Repeated oral, reproductive toxicity, rat NOAEL = 1.6 mg/kg bw per day, parental
and pup toxicity
Repeated oral (gavage), developmental NOAEL = 1.2 mg/kg bw per day (highest
toxicity, rat dose tested). No evidence of teratogenicity
Repeated oral (feeding), developmental NOAEL = 1.5 mg/kg bw per day, maternal
toxicity, rat toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 0.6 mg/kg bw per day, maternal
toxicity. No evidence of teratogenicity
Repeated oral, developmental neurotoxicity, rat NOAEL = 1.7 mg/kg bw per day
Longterm (> 1 year) Repeated oral, 2 years, carcinogenicity, mouse NOAEL = 2.8 mg/kg bw per day,
cholinesterase inhibition. No evidence
of carcinogenicity
Repeated oral, 2 years, carcinogenicity, rat NOAEL = 1 mg/kg bw per day, reduced
body-weight gain and cholinesterase
inhibition. No evidence of carcinogenicity
References
Barron, P., Giesler, P. & Ras, G.N. (1978) Teratogenicity of
carbofuran in rats. Act-No. 184.33. Unpublished report prepared by
Warf Institute, Inc. Wisconsin, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Becci, G. (1979) Study of cholinesterase activity inhibition by
carbofuran in neonate, weanling and adult rats. Study-No. A 79-343.
Unpublished report prepared by Food and Drug Research Laboratories,
Inc. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Bloch, I., Frei, T.H., Madoerin, K., Luetkemeier, H., Vogel, W.,
Schlotke, B., Vogel, O. & Terrier, C. (1987a) 13-Week oral toxicity
feeding study with carbofuran (D1221) in the dog. RCC-No. 077837 (FMC
Study No. A95-4249). Unpublished report prepared by Research
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by FMC
Corp., Philadelphia, PA, USA.
Bloch, I. Frei, T., Luetkenmeier, H., Vogel, W. & Terrier, C. (1987b)
4-Week oral toxicity (feeding) study with carbofuran 'D 1221' in male
dogs. RCC No. 087963 (FMC Study No. A95-4248). Unpublished report
prepared by Research & Consulting Company AG, Itingen, Switzerland.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Brown, W.R. (1980) 2-Year dietary toxicity and carcinogenicity study
in mice with technical carbofuran. Act No. 150.52 Histopathology Part.
Unpublished report prepared by Research Pathology Services, Inc. PA,
USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Burtner, B.R. (1982) 14-Day oral toxicity (range finding) study in
beagle dogs of carbofuran. (FMC Study No. A81-604, Toxicogenics No.
410-0714). Unpublished report prepared by ToxiGenics, Inc., IL, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Case, R.S. & Wilson, N.H. (1979) Cholinesterase evaluation conducted
during a two year dietary toxicity/oncogenicity study with carbofuran
in rats. Study No. Tox-79-001. Unpublished report prepared for FMC.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Coleman, A.M.E., Smith, A. & Watson, D. (1990) Occupational carbamate
pesticide intoxication in three farm workers. West Indian Med. J.,
39, 109-113.
Courtney, K.D., Andrews, J.E., Springer, J. & Dalley, L. (1985)
Teratogenic evaluation of the pesticides Baygon, carbofuran,
dimethoate and EPN. J. Environ. Sci. Health B, 20, 373-406.
DeGraff, M.G. (1983a) Mutagenicity evaluation of FMC 10242 in the Ames
Salmonella/microsome plate test Study No. A 83-1037). Unpublished
report by Litton Bionetics, Inc., Kensington, MD, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
DeGraff, W.G. (1983b) Mutagenicity evaluation of MC 10242 for the
sex-linked recessive lethal test in Drosophila melanogaster. Study
No. A 83-1060. Unpublished report prepared by Litton Bionetics, Inc.
Kensington, MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Dorough, H.W. (1968) Metabolism of Furadan (NIA-10242) in rats and
houseflies. J. Agric. Food Chem., 16, 319-325.
Elleman, P.N. (1979) Acute oral toxicity (FIFRA-EPA) in rats with
carbofuran technical (FMC Study No. A 79-339, CSE-Study No. 0180).
Unpublished report prepared by Cosmopolitan Safety Evaluation (CSE)
Inc., NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Ellison, T. (1980) Audit validaton of the skin sensitization test on
NIA 10242 Technical. Unpublished report on Study No. A 5300. Submitted
to WHO by FMC Corp., Philadelphia, PA, USA.
Farrow, M.G. (1983a) Salmonella typhimurium/mammalian microsome
plate incorporation assay with compound FMC 10242 (FMC Study-No. A
83-905, HLA No. 331). Unpublished report prepared by Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Farrow, M.G. (1983b) Salmonella typhimurium/mammalian microsome
plate incorporation assay with compound FMC 10242 (FMC Study No. A
83-907, HLA No. 332). Unpublished report prepared by Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Farrow, M.G. (1983c) Salmonella typhimurium/mammalian microsome
plate incorporation assay with compound FMC 10242 (FMC Study No. A
83-909, HLA No. 333). Unpublished report prepared by Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Ferguson, P.W., Dey, M.S., Jewell, S.A. & Krieger, R.I. (1984)
Corbofuran metabolism and toxicity in the rat. Fundam. Appl.
Toxicol., 4, 14-21.
Freeman, C. (1984a) Acute oral toxicity of FMC 18209 technical
(3-hydroxy-carbofuran) in rats. FMC Study No. A 83-1136. Unpublished
report prepared by FMC Toxicology Laboratory, NJ, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Freeman, C. (1984b) Acute oral toxicity of FMC 17481 technical
(3-keto-carbofuran) in rats. FMC Study No. A 83-1137. Unpublished
report prepared by FMC Toxicology Laboratory, NJ, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Freeman, C. (1984c) Acute oral toxicity of FMC 16497 (3-hydroxy-
7-phenol) rats. FMC Study No. A83-1134. Unpublished report Pprepared
by FMC Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Freeman, C. (1984d) Acute oral toxicity of FMC 19841 (3-keto-7-phenol)
in rats. FMC Study No. A83-1135. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Freeman, C. (1984e) Acute oral toxicity of FMC 10272 (7-phenol) in
rats. FMC Study No. A83-1133. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Freeman, C. (1993) Twenty-eight day neurotoxicity range-finding study
in rats with carbofuran technical. FMC No. A 92-3704. Unpublished
report prepared by FMC Corporation, Toxicology Laboratory, NJ, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Freeman, C. (1994) Subchronic neurotoxicity screen in rats with
carbofuran technical. FMC Study No. 92-3705. Unpublished report
prepared by FMC Corporation Toxicology Laboratory, NJ, USA. Submitted
to WHO by FMC Corp., Philadelphia, PA, USA.
Flucke, W. (1986) SRA 12869 (isofenphos) and D 1221 (carbofuran):
Study for combination toxicity. Study No. 134337. Unpublished report
prepared by Bayer AG, Insititute of Toxicology. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Fukuto, T.R. & Metcalf, R.L. (1969) Metabolism of insecticides in
plants and animals. Biological effects of pesticides in mammalian
systems. Ann. NY Acad. Sci., 160, Art. 1.
Georgian, L.I., Moraru, I., Draghicescu, T. & Tarnavschi, R. (1985)
The effect of low concentrations of carbofuran, cholin salt of maleic
hydrazine, propham and chlorpropham on sister-chromatid exchange (SCE)
frequency in human lymphocytes in vitro. Mutat. Res., 147, 296.
Goldenthal, E.I. (1982a) 2-Year dietary toxicity and carcinogenicity
study in mice. FMC Study No. Act No. 150.52 (IRDC No. 167-116).
Unpublished report prepared by International Research and Development
Corporation, MI, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Goldenthal, E.I. (1982b) 2-Year dietary toxicity and carcinogenicity
study in rats. FMC Study No. Act No. 130.51 (IRDC No. 167-115).
Unpublished report prepared by International Research and Development
Corporation, MI, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Goldenthal, E.I. (1982c) Three generation reproduction study in rats.
FMC Study No. Act. No. 131.53 (IRDC No. 167-114). Unpublished report
prepared by International Research and Development Corporation, MI,
USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983a) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-868 (MBA Study No. 1921.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. 1983b) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-913 (MBA Study No. 1934.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983c) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
Technical. FMC Study No. A 83-948 (MBA Study No. 1938.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983d) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-949 (MBA Study No. 1936.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983e) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-950 (MBA Study No. 1935.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983f) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-951 (MBA Study No. 1937.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983g) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-973 (MBA Study No. 1982.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983h) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-1063. Unpublished report prepared by
Microbiological Associates, MD, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Hoffman, S.L. & Robinson, R.A. (1994a) Metabolism of C14-carbofuran
in laying hens. XBL Study No. XBL 92051; FMC Study No. 078 POU 92M1.
Unpublished report prepared by XenoBiotic Laboratories, Inc.,
Plainsboro, NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Hoffman, S.L. & Robinson, R.A. (1994b) Metabolism of C14-carbofuran
in lactating goats. XBL Study No. XBL 92051; FMC Study No. 078 GOA
92M1. Unpublished report prepared by XenoBiotic Laboratories, Inc.,
Plainsboro, NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Kedderis, L.B. (1985a) Seven-day repeated dose dermal toxicity
range-finding study in rabbits with FMC 10242 technical (carbofuran).
Study No. A 85-1645. Unpublished report prepared by FMC Corporation.
NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kedderis, L.B. (1985b) Twenty-one day repeated dose dermal toxicity
range-finding study in rabbits with FMC 10242 technical (carbofuran).
Study No. A 85-1678. Unpublished report prepared by FMC Corporation,
NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kirby, P.E. (1983a) L 5178Y TK+/- mouse lymphoma mutagenesis assay.
FMC Study No. A 83-962 and A 83-988 (MBA Study No. T1982.701).
Unpublished report prepared by Microbiological Associates, Bethesda,
MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kirby, P.E. (1983b) L5178Y TK+/- mouse lymphoma mutagenesis assay.
FMC Study No. A 83-1064 (MBA Study No. T2124.201). Unpublished report
prepared by Microbiological Associates, Bethesda, MD, USA. Submitted
to WHO by FMC Corp., Philadelphia, PA, USA.
Knaak, J.B., Munger, D.M. & McCarthy, J.F. (1969) The metabolism of
carbofuran alfalfa residues in the rat. Abstracts of Papers, 158th
National Meeting of the American Chemical Society, 7-12 September.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kuhr, R.J. & Dorough, H.W. (1976) Carbamate Insecticides: Chemistry,
Biochemistry and Toxicology, Cleveland, OH, CRC Press. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Laveglia, J. (1981) Teratology study in the rabbit with carbofuran.
FMC Study No. A 80-452 (IRDC No. 167-156). Unpublished report prepared
by International Research and Development Corporation, MI, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Mehta, C.S. (1981) Rabbit acute dermal toxicity. FMC Study No. A
81-564 (Stillmeadow No. 2142.81). Unpublished report prepared by
Stillmeadow, Inc., TX, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Metcalf, R.L., Fukuto, T.R., Collins, C., Borck, K., Abd El-Aziz, S.,
Munoz, R. & Cassil, C.C. (1968) Metabolism of 2,2-dimethyl-
2,3-dihydrobenzofuranyl-7 N-methylcarbamate (Furadan) in plants,
insects and mammals. J. Agric. Food Chem., 16, 300-311.
Mihail, F. (1981) D1221 (carbofuran) and Dacamox (thiofanox): Acute
combination toxicity study. Study No. 10280. Unpublished report
prepared by Bayer AG, Institute of Toxicology. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Moriya, M., Ohta, K., Watanabe, T., Miyazawa, K., Kato, K. & Shirasw
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Norwell, M.J. (1983a) Acute oral toxicity of FMC 10242, technical, in
rats. FMC Study No. A 83-1101. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Norwell, M.J. (1983b) Acute oral toxicity of FMC 10242, technical, in
rats. FMC Study No. A 83-1102. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Ponnock, K.S. (1994) A developmental neurotoxicity study of carbofuran
in the rat via dietary administration. Study No. 93-4506, FMC Study
No. A 93-3746. Unpublished report prepared by Pharmaco LSR, Inc.
Toxicology Services North America, NJ, USA. Submitted to WHO by FMC
Corp., Philadelphia, PA, USA.
Putman, D.L. (1983a) Activity of FMC 10242 in the in vivo cytogenetics
assay in Sprague-Dawley rats. FMC Study No. A 83-972 (MBA No.
T1982.102). Unpublished report prepared by Microbiological Associates,
Bethesda, MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Putman, D.L. (1983b) Activity of FMC 10242 in the subchronic in vivo
cytogenetics assay in male rats. Study No. A 83-1065 (MBA No.
T2124.102). Unpublished report prepared by Microbiological Associates,
Bethesda, MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Rao, G.N. (1978) Teratology study of carbofuran in rabbits. Study No.
185-33. Unpublished report prepared by Warf Institute, Inc. WI, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Rapp, W.R. (1980a) 24 Month dietary toxicity/carcinogenicity study of
carbofuran in rats. Act No. 130.51. Pathology report. Unpublished
report prepared by Wm. R. Rapp & Co. Inc., Allied Services for
Research, NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Rapp, W.R. (1980b) A three generation reproduction study of carbofuran
in rats. FMC Act No. 131.531. Pathology report. Unpublished report
prepared by Wm. R. Rapp & Co. Inc., Allied Services for Research, NJ,
USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Rodwell, D.E. (1980a) Teratology study in the rat with carbofuran.
(FMC Study No. A 80-453, IRDC No. 167-155). Unpublished report
prepared by International Research and Development Corp., MI, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Rodwell, D.E. (1980b; amended 1985) Pilot teratology study in the rat
with carbofuran in the diet (FMC Study No. A 80-443, IRDC No.
167-153). Unpublished report prepared by International Research and
Development Corp., MI, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Rodwell, D.E. (1981; with amendment) Teratology and postnatal study in
the rat with carbofuran (FMC Study No. A 80-444, IRDC No. 167-154).
Unpublished report prepared by International Research and Development
Corp., MI, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Sabol, R.J. (1979a) Rat acute oral toxicity with carbofuran technical.
Study No. 1083-79; FMC Study No. ACT 267.01-01. Unpublished report
prepared by Stillmeadow, Inc., TX, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Sabol, R.J. (1979b) Rat acute oral toxicity with furadan insecticide,
carbofuran 96.6%. Study No. 11231-79; FMC Study No. ACT 267.01-02
(01). Unpublished report prepared by Stillmeadow, Inc., TX, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Schoenig, G. (1967) Skin sensitization test on NIA 10242 technical.
IBT Study No. A 5300. FMC Study No. NCT 176.36. Unpublished report
prepared by Industrial Bio Test Laboratory, Inc, IL, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Sharma, V.K. & Kaur, S. (1990) Contact sensitization by pesticides in
farmers. Contact Derm., 23, 77-80.
Signorin, J. (1995) DOT acute inhalation toxicity study in rats. FMC
Study No. A 94-4097. Unpublished report prepared by FMC Corp.,
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Simmon, U.F. (1979) In vitro microbiological mutagenicity and
unscheduled DNA synthesis studies of eighteen pesticides. Study No.
68-01-2458 (FMC Study No. A81-509, EPA 600/1-79-041). Report prepared
by SRI International, Menlo Park, California, for Health Effects
Research Laboratory Office of Research and Development. US
Environmental Protection Agency, Research Triangle Park, NC, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Stehrer-Schmid, P. & Wolf, H.U. (1995) Effects of benzofuran and seven
benzofuran derivatives including four carbamate insecticides in the in
vitro porcine brain tubulin assembly assay and description of a new
approach for the evaluation of the test data. Mutat. Res., 339,
61-72.
Taylor, G.D. (1983) One-year chronic oral toxicity study in beagle
dogs with carbofuran. FMC study No. A 81-605 (Toxicogenics No.
410-0715). Unpublished report prepared by ToxiGenics, Inc., IL, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Thilagar, A. (1983a) Unscheduled DNA synthesis in rat primary
hepatocytes. FMC Study No. A 83-969 (MBA No. T1982.380). Unpublished
report prepared by Microbiological Associates, Bethesda, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Thilagar, A. (1983b) Sister chromatid exchange assay in Chinese
hamster ovary (CHO) cells. FMC Study No. A 83-1095 (MBA No.
1982.334001). Unpublished report prepared by Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Thilagar, A. (1983c) Sister chromatid exchange assay in Chinese
hamster ovary (CHO) cells. FMC Study No. A 83-1097 (MBA No.
2124.334001). Unpublished report prepared by Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Thilagar, A. (1983d) Chromosome aberrations in Chinese hamster ovary
(CHO) cells. FMC Study No. A 83-1096. Unpublished report prepared by
Microbiological Associates, Bethesda, MD, USA. Submitted to WHO by FMC
Corp., Philadelphia, PA, USA.
Valencia, R. (1981) Mutagenesis screening of pesticides using
Drosophila. FMC Study No. A 83-1042 (EPA 600/1-81-017). Report
prepared by Warf Institute Inc., Madison, WI, for Health Effects
Research Laboratory. Office of Research and Development, US
Environmental Protection Agency, Research Triangle Park, NC, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Valencia, R. (1983) Drosophila sex-linked recessive lethal assay
of 2,3-dihydro-2,2-dimethyl-7-benzofuranyl- N-methyl carbamate
(carbofuran). FMC Study No. A 83-1019 (MBA No. T1047.160). Unpublished
report prepared by Microbiological Associates, Bethesda, MD and the
University of Wisconsin, Zoology Department, USA. Submitted to WHO by
FMC Corp., Philadelphia, PA, USA.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
Woijcieckowski, J.P., Kaur, P. & Sabharwal, P.S. (1982) Induction of
ouabain resistance in V-79 cells by four carbamate pesticides.
Environ. Res., 29, 48-53.
[14C-phenyl ring]Carbofuran was administered in capsules to
laying hens once a day for seven consecutive days at doses equivalent
to dietary concentrations of 0 and 25 ppm. Carbofuran was extensively
metabolized and rapidly eliminated, resulting in low residues in eggs
and tissues. Muscle tissue and fat contained < 0.01 ppm; kidney,
liver, and eggs contained concentrations of < 0.2 ppm carbofuran
equivalents. Conjugates formed after oxidative and hydrolytic
reactions were further conjugated to their corresponding sulfate
conjugates and appeared to bind with biomolecules (e.g. proteins) to
form nonextractable residues (Hoffman & Robinson, 1994a).
[14C-phenyl ring]Carbofuran was administered in gelatine
capsules to lactating goats once a day for seven consecutive days at
doses equivalent to dietary concentrations of 0 and 25 ppm. Carbofuran
was extensively metabolized and readily eliminated. Muscle and fat
contained < 0.01 ppm; kidney, liver, and milk contained 0.09-0.39
ppm. Metabolites occurred in nonconjugated and conjugated forms, the
latter being further conjugated to the sulfate conjugates, which bind
to biomolecules and result in nonextractable (bound) residues (Hoffman
& Robinson, 1994b).
2. Toxicological studies
(a) Acute toxicity
The results of numerous studies of the acute toxicity of
carbofuran in rats were evaluated previously (Annex I, reference 26)
and are summarized in Table 1. The oral LD50 values for rats were
6-18 mg/kg bw. The toxic signs observed were typical of cholinesterase
inhibition: salivation, cramp, trembling, and sedation were observed
within minutes after administration and lasted for up to three days.
Additional studies that have become available since the first
evaluation give oral LD50 values for technical-grade carbofuran in
rats of about 5 mg/kg bw in females and 14 mg/kg bw in males (Elleman,
1979; Sabol, 1979a, b; Norvell, 1983a,b). The dermal LD50 in rabbits
for carbofuran of a purity of 96.1% was > 2000 mg/kg bw (Mehta,
1981). In rats treated by inhalation with a carbofuran formulation
(Furadan 95), the LC50 value was 0.11 mg/litre for a 1-h exposure
(Signorin, 1995).
The results of studies on the acute toxicity of several
metabolites of carbofuran are summarized in Table 2.
Studies of combined treatment with carbofuran and thiofanox or
carbofuran and isofenphos revealed no potentiating effects. Additive
or even subadditive toxic effects were found after simultaneous
administration of the test compounds (Mihail, 1981; Flucke, 1986).
Table 1. Acute toxicity of carbofuran
Species Sex Route LD50 (mg/kg bw) Reference
or LC50 (mg/litre)
Mouse Oral 14.4 Kimmerle (1966)
Rat Male Oral 18.0 Kimmerle (1966)
Rat Male Oral 7.8 Grönning & Kimmerle (1974)
Rat Male Oral 11.9 Kohn (1967a)
Rat Female Oral 6.0 Grönning & Kimmerle (1974)
Rat Female Oral 13.8 Kimmerle (1966)
Rat Male and female Oral 14.1 Powers (1964)
Rat Male Intraperitoneal 8.2 Kimmerle (1966)
Rat Female Intraperitoneal 2.8 Kimmerle (1966)
Rat Male and female Intraperitoneal 1.4 Kohn (1967b)
Rat Male and female Dermal > 500 Kimmerle (1966)
Rat Male Inhalation (1 h) 0.108 Kimmerle (1966)
Rat Male Inhalation (1 h) 0.091 Grönning & Kimmerle (1974)
Rat Male Inhalation (4 h) 0.088 Kimmerle (1966)
Rat Female Inhalation (1 h) 0.080 Grönning & Kimmerle (1974)
Rat Female Inhalation (4 h) 0.075-0.11 Grönning & Kimmerle (1974)
Guinea-pig NR Oral 9.2 Kimmerle (1966)
Rabbit NR Oral 7.5 Kimmerle (1966)
Cat NR Oral 2.5-3.5 Kimmerle (1966)
Dog NR Oral 15-19 Kimmerle (1966)
Table 2. Acute oral toxicity of metabolites of carbofuran in rats
Metabolite LD50 Reference
(mg/kg bw)
3-Hydroxycarbofuran 21.9 (male) Freeman (1984a)
8.3 (female)
3-Ketocarbofuran 108 (male) Freeman (1984b)
93.1 (female)
3-Hydroxy-7-phenol 1916 (male) Freeman (1984c)
1654 (female)
3-Keto-7-phenol > 800 Freeman (1984d)
7-Phenol 2450 (male) Freeman (1984e)
1743 (female)
(b) Short-term toxicity
Rabbits
In a range-finding study, groups of rabbits were treated dermally
with carbofuran (technical-grade; purity, 96.9%) at doses of 0, 100,
300, or 1000 mg/kg bw per day over seven days; the contact time was
6 h/day. No clinical signs of toxicity and no local irritation
were observed. The treatment did not affect body weight or food
consumption. A 30% depression in plasma cholinesterase activity was
measured in males at 100 and 300 mg/kg bw per day and a 47% reduction
at 1000 mg/kg bw per day. Brain acetylcholinesterase activity was
reduced by 26% in males at 100 mg/kg bw per day, 49% in those at 300
mg/kg bw per day, and 54% in those at 1000 mg/kg bw per day. These
reductions were not statistically significant (Kedderis, 1985a).
New Zealand white rabbits received applications of carbofuran
(technical-grade; purity, 96.9%) for about 6 h/day at doses of 0, 10,
100, or 1000 mg/kg bw per day for 21 consecutive days. No signs
of toxicity and no effects on body weight, food consumption,
haematological or clinical chemical parameters, organ weights,
or gross or histopathological appearance were observed. Brain
acetylcholinesterase activity was reduced by 21% in males at 100 mg/kg
bw per day and 26% in those at 1000 mg/kg bw per day. The reductions
were not statistically significant. The NOAEL was 10 mg/kg bw per day
(Kedderis, 1985b).
Dogs
In a 14-day range-finding study, single beagle dogs were fed
diets designed to provide carbofuran (technical-grade; purity, 96.1%)
at concentrations of 0, 18, 32, 56, 100, 316, or 1000 ppm, equivalent
to 0, 0.45, 0.8, 1.4, 2.5, and 8 mg/kg bw per day. The animal that
initially received 18 ppm was changed to a dose of 1000 ppm on day 4
of the study. At this dose, body weight loss and depressed food intake
were observed, and clinical signs included muscle tremor, emesis, and
salivation. Although cholinesterase activity was measured in plasma
and erythrocytes, dose-related depression was found only in plasma, at
all doses; inhibition ranged from 16% (within the historical control
range) in animals at 18 ppm to < 92% at 1000 ppm (Burtner 1982).
In a 13-week feeding study, carbofuran (purity, 99.6%) was
administered in the diet to groups of four male and four female
beagle dogs to provide concentrations of 0, 10, 70, or 500/250 ppm,
equivalent to 0, 0.43, 3.1, and 10.6 mg/kg bw per day. Two additional
animals of each sex per group were assigned to recovery groups to
study the reversibility of the effects over a four-week period. The
feed concentration of 500 ppm was reduced to 250 ppm from treatment
day 6 onwards because of marked toxicity: one dog at this dose died on
day 5 of treatment due to a treatment-related invagination of the
jejunum. At concentrations > 10 ppm, hyperaemia (e.g. at ear pinnae
and oral mucous membranes) and increased salivation were observed.
Clinical signs at the highest dose consisted of muscular spasms,
ataxia (pronounced in males during the first week of dosing but
sporadic thereafter), decreased motility, tachypnoea and deep
respiration, and vomiting. At 500 ppm, food consumption was markedly
reduced and loss of body weight was observed; reduction of the dose to
250 ppm resulted in recovery of food consumption and body-weight gain.
The treatment had no effect on ophthalmoscopic, electrocardiographic,
haematological, or clinical chemical parameters but inhibited
cholinesterase activity and caused alterations in organ weights and in
gross and histopathological appearance. Dose-related inhibition of
plasma and erythrocyte cholinesterase activity was observed in all
treated groups. Maximal inhibition occurred on test day 1, when the
plasma activity was 70, 27, and 13% of the control activity at 10, 70,
and 500/250 ppm and the erythrocyte activity was 68, 29, and 11% of
the control, respectively. The plasma and erythrocyte cholinesterase
activities returned to normal during the four-week recovery period.
There was no inhibition of brain acetylcholinesterase activity by the
end of the treatment or recovery period. An NOAEL was not determined
because inhibition of erythrocyte acetylcholinesterase and increased
salivation were seen at the lowest dose tested (Bloch et al., 1987a).
This study was followed by a four-week feeding study in order to
establish a NOAEL with respect to clinical signs and cholinesterase
inhibition: Groups of four male beagle dogs were fed diets providing
concentrations of 0 or 5 ppm carbofuran (purity, 99.6%) for four
weeks. The treatment had no effects on clinical signs, mortality, body
weight, food consumption, or plasma or erythrocyte cholinesterase
activity. The NOAEL was 5 ppm, equal to 0.22 mg/kg bw per day (Bloch
et al., 1987b).
In an earlier one-year study, groups of six male and six female
beagle dogs were given diets providing concentrations of 0, 10, 20, or
500 ppm carbofuran (technical-grade; purity, 96.1%), equal to 0,
0.3, 0.6, and 13 mg/kg bw per day. At 500 ppm, body weight losses
associated with emesis were observed, particularly in males, and from
the fifth month these animals were fed a supplemented control diet.
One male at 500 ppm died. Muscle tremor and salivation occurred
sporadically at this dose, and all males showed marked depression of
the haematocrit, haemoglobin values, and erythrocyte count from five
months. Only transient, much less pronounced depressions of these
parameters were observed in females at the high dose. Plasma
cholinesterase activity was inhibited in most males at 10 and
20 ppm and markedly (77%) in all animals at 500 ppm. Erythrocyte
acetylcholinesterase activity was reduced by 21-27% in many males
at 500 ppm. At the end of the study, a 24% depression in brain
acetylcholinesterase activity was observed in males at 500 ppm,
whereas females at this dose showed a 44% increase. No relevant
inhibition of erythrocyte or brain acetylcholinesterase activity was
seen in animals at 10 or 20 ppm. The absolute brain and heart weights
of males at 500 ppm were depressed, but no macroscopic or microscopic
changes were found in these organs. Gross treatment-related lesions
observed in the animals at 500 ppm consisted of a marked loss of body
fat and alopecia. Histopathological examination revealed an increased
amount of hepatocellular endoplasmic reticulum in the treated dogs,
but no clear dose-response relationship was seen. Degeneration of the
seminiferous tubules, giant-cell formation, or aspermia was observed
in males at 500 ppm and in a single male at 20 ppm. Minimal-to-
moderate inflammatory changes of the lung were observed at a higher
incidence in the dogs at 500 ppm. The NOAEL was 10 ppm, equal to
0.3 mg/kg bw per day, on the basis of the histopathological changes
in the testes of one male at 20 ppm (Taylor, 1983).
(c) Long-term toxicity and carcinogenicity
Mice
In a two-year study first evaluated by the 1980 JMPR (Annex 1,
reference 34), groups of 100 male and 100 female Charles River CD-1
mice were fed diets providing concentrations of 0, 20, 125, or 500 ppm
carbofuran (technical-grade; purity, 95.6%), equal to 0, 2.8, 18,
and 70 mg/kg bw per day. The treatment had no effect on general
appearance, mortality, or urinary parameters. Body-weight gain was
slightly reduced in single males at 125 ppm and in most animals at
500 ppm during the first year of the study; however, a 20% reduction
in body-weight gain in comparison with the controls was observed in
females at 500 ppm at the end of the study. Food consumption was also
sporadically reduced in animals at the highest dose, particularly
during the first months of the study. Haematological and clinical
chemical examinations revealed no treatment-related changes, except
for inhibition of brain acetylcholinesterase activity; cholinesterase
activities were not measured in erythrocytes or plasma. A
statistically significant depression of brain acetylcholinesterase
activity was observed in animals at 125 and 500 ppm. Maximal
inhibition at 125 ppm was 31% of the activity in concurrent controls;
at 500 ppm, the maximal depression was 55%. Organ weights were changed
sporadically but were not related to treatment. No gross pathological
findings were observed that were indicative of a relationship with
treatment. Microscopic examination revealed no treatment-related
change and no evidence of a compound-related increase in tumour
incidences. The NOAEL was 20 ppm, equal to 2.8 mg/kg bw per day, on
the basis of inhibition of brain acetylcholinesterase activity at
125 ppm. The conclusions of the earlier evaluation were therefore
confirmed (Brown, 1980; Goldenthal, 1980, 1982a).
Rats
In a study reviewed by the JMPR in 1979 and 1980 (Annex 1,
references 32 and 34), carbofuran (technical-grade; purity, 95.6%) was
administered in the diet to groups of 90 male and 90 female Charles
River CD rats to provide concentrations of 0, 10, 20, or 100 ppm, for
two years. Groups of 10 animals of each sex per dose were killed at 6,
12, and 18 months, and blood and brain samples were collected for
determination of cholinesterase activity. The treatment did not affect
general appearance, mortality, food consumption, or ophthalmoscopic,
haematological, or urinary parameters. Body-weight gain was reduced in
animals of each sex at 100 ppm. Clinical chemical examinations
revealed statistically significant inhibition of cholinesterase
activity in plasma, erythrocytes, and brain in rats at 100 ppm. The
maximal reduction of cholinesterase activity in males was 37% in
plasma, 24% in erythrocytes, and 25% in brain; the corresponding
percentages in females at 100 ppm were 26% in plasma, 19% in
erythrocytes, and 43% in brain. No relevant inhibition of
acetylcholinesterase activity was seen in erythrocytes or brain of
animals at 10 or 20 ppm (Case & Wilson, 1979). Various changes in
organ weights were considered to be due to changes in body weight. No
treatment-related gross pathological or histopathological changes were
observed, and there was no evidence of tumorigenicity. The NOAEL was
20 ppm, equivalent to 1 mg/kg bw per day, on the basis of reduced body
weight gain and acetylcholinesterase inhibition in erythrocytes and
brain at the higher dose. The conclusions of the earlier evaluation
were thus confirmed (Goldenthal, 1979a; Rapp, 1980a; Goldenthal,
1982b).
(d) Reproductive toxicity
Rats
In a three-generation study first evaluated by the 1980 JMPR
(Annex 1, reference 34), groups of Charles River CD rats (10 males and
20 females per group for the first and second generations; 12 males
and 24 females per group for the third generation) were maintained on
a diet providing concentrations of 0, 20, or 100 ppm carbofuran
(purity, 95.6%), equal to 0, 1.2, and 6 mg/kg bw per day for males and
0, 1.9, and 9.7 mg/kg per day for females. Each generation was mated
twice to produce two litters. The treatment did not affect the general
behaviour, appearance, or survival of the parental rats. Parental
body-weight gain was lower at 100 ppm throughout the course of
treatment and was associated with lower food consumption, particularly
in males. Fertility indices were not adversely affected. The survival
of pups of the F1a, F2a, and F3a generations at 100 ppm was reduced
at lactation day 4 in comparison with the control. Dehydration was
seen in pups of some litters of the F3a and F3b generations at
100 ppm. The mean weights of pups at 100 ppm were lower than those of
the controls on day 21 post partum in all generations. No compound-
related gross pathological lesions were seen at necropsy in the F0,
F1, or F2 parental animals or the F2b or F3b litters. Various organ
weights of animals of the F2 parental and F3b pup generations at
20 and 100 ppm were statistically significantly changed. Because
clear dose-response relationships were not always seen and no
histopathological alterations were associated with these changes, the
findings were considered not to be of biological significance. The
NOAEL was 20 ppm, equal to 1.2 mg/kg bw per day, on the basis of
reductions in body-weight gain in the parental generation and
reductions in the growth and survival of pup generations at
100 ppm. Thus, the earlier evaluation of this study was confirmed
(Goldenthal, 1979b; Rapp, 1980b; Goldenthal, 1982c).
(e) Developmental toxicity
Rats
In a study first evaluated by the 1980 JMPR (Annex 1, reference
34), carbofuran (technical-grade; purity, 95.6%) was administered to
groups of 24 pregnant Charles River rats by intubation in 0.25%
carboxymethylcellulose at doses of 0, 0.1, 0.3, or 1 mg/kg bw per day
on days 6-15 of gestation. Transient, dose-dependent clinical signs
(chewing motions) were observed at doses > 0.1 mg/kg bw per day for
a short period after treatment. Overt signs of toxicity in animals at
0.3 mg/kg bw per day included rough coats and lethargy; at the high
dose, lacrimation, increased salivation, trembling, and convulsions
were also seen. One female at the high dose died. The treatment had no
effect on the body weights of dams or pups. Reproductive parameters
(e.g. numbers of corpora lutea, implantations, and resorptions and
litter size) were not adversely affected by treatment. Skeletal
examination revealed a higher incidence of some variations in
ossification of sternebrae in treated animals but with no clear
dose-response relationship. Similar alterations were prevalent in the
control group at an incidence of 47% of fetuses. There was no evidence
of teratogenicity. An NOAEL was not determined because transient
clinical signs were observed at the lowest dose (Barron et al., 1978).
Groups of 25 pregnant Charles River COBS CD rats were treated
with carbofuran (purity, 95.6%) by gavage in corn oil at daily doses
of 0, 0.25, 0.5, or 1.2 mg/kg bw on days 6-15 of gestation. The
treatment had no effect on the general appearance, behaviour,
mortality, or body weights of the dams. No differences between groups
were noted in reproductive parameters such as the numbers of corpora
lutea, implantations, and resorptions, litter size, or fetal body
weight. No evidence of teratogenicity was seen (Rodwell, 1980a).
In a pilot study, groups of 10 pregnant Charles River COBS rats
were fed carbofuran (purity, 95.6%) in the diet to give concentrations
of 0, 20, 60, 120, 160, or 200 ppm, equal to 0, 1.5, 4, 8, 11, and
13 mg/kg bw per day, on gestation days 6-19. Neither survival nor
behaviour was affected by the treatment. Hair loss, soft stools,
and scabbing were seen at higher incidences in the treated than
the control group. A dose-related loss in mean maternal body
weight occurred during the first two days of treatment with doses
> 60 ppm, resulting in reduced body-weight gains throughout the
treatment period in animals at 120, 160, and 200 ppm. A dose-related
decrease in food consumption was observed at doses > 60 ppm at the
beginning of the treatment period. Reproductive parameters (litter
size and the numbers of resorptions, implantations, and corpora lutea)
were not adversely affected. On the basis of these results, doses of
0, 20, 60, and 160 ppm were selected for the main study (Rodwell,
1980b, 1985).
The main study was conducted with groups of 40 pregnant Charles
River COBS rats fed diets giving concentrations of 0, 20, 60, or 160
ppm carbofuran (purity, 95.6%), equal to 0, 1.5, 4.4, and 11 mg/kg bw
per day, on gestation days 6-19. Treatment had no effect on appearance
or behaviour, except for a slight increase in hair loss, matting of
the ventral haircoat, and soft stools, particularly in females at 60
and 160 ppm. A dose-related reduction in body-weight gain was seen at
doses > 60 ppm during the treatment period, with single instances
of body-weight loss in animals at 60 and 160 ppm at the beginning of
treatment. Increases in body-weight gain of treated animals during the
subsequent lactation period resulted in very similar body weights in
all groups at the end of lactation. Food consumption was reduced
during the first few days of treatment in animals at 60 and 160 ppm.
No differences between groups were observed in reproductive parameters
(such as the numbers of corpora lutea, implantations, and resorptions
and litter size). The mean weights of pups at 160 ppm were decreased
throughout lactation. The incidence of malformations was not
increased. The NOAEL for maternal toxicity was 20 ppm, equal 1.5 mg/kg
bw per day, on the basis of reduced body-weight gain of the dams at
doses > 60 ppm. The NOAEL for pup toxicity was 60 ppm, equal to 4.4
mg/kg bw per day, on the basis of reduced weights of pups at 160 ppm
(Rodwell, 1981).
Groups of 24 mated female Sprague-Dawley (CD) rats were given
diets containing carbofuran (purity, 99.1%) to provide concentrations
of 0, 20, 75, or 300 ppm, equal to 0, 1.7, 5, and 20 mg/kg bw per day,
from gestation day 6 through lactation day 10. Physical observations
were made, and body weight and food consumption were measured for all
maternal animals at selected intervals throughout the gestation and
lactation periods. The numbers of live pups were recorded, and litter
parameters such as pinna detachment, incisor eruption, eye opening,
motor activity, auditory startle response, and brain weights were
evaluated. Neuropathological examinations were performed on six pups
of each sex per group that were killed on postnatal days 11 and 60.
Maternal animals were killed after weaning of the last litter. No
deaths occurred during the study. Reduced body-weight gain was seen in
dams at 75 and 300 ppm during gestation and also during the lactation
period for those at 300 ppm. Food consumption was reduced in dams at
these two doses at various periods during gestation. Treatment had no
effect on pregnancy rates, length of gestation, or litter size, and
there was no evidence of prolonged or difficult delivery. Treatment at
300 ppm resulted, however, in a greater number of dead pups at birth
than among the controls (not statistically significant). A dose-
dependent reduction in pup weight was observed in animals at 75 and
300 ppm throughout the lactation period, and the reductions persisted
after weaning. Pup survival was adversely affected by prenatal
administration of carbofuran at 75 or 300 ppm on lactation day 4 until
weaning at lactation day 21. Treatment at doses of 75 or 300 resulted
in developmental delays of three to four days in vaginal patency and
preputial separation; marginal delays were seen with respect to pinna
detachment, lower incisor eruption, and eye opening. No adverse effect
was noted on auditory startle response, motor activity, or swimming
development, but there was an angle reduction at doses > 75 ppm.
Brain weights were not affected by treatment. No treatment-related
macroscopic findings were observed in dams or pups. Microscopic
examinations of pups in the control and high-dose groups revealed no
compound-related abnormality. The NOAEL was 20 ppm, equal to 1.7 mg/kg
bw per day, on the basis of reduced body-weight gain of dams and pups,
reduced pup survival, and slight developmental delay at doses > 75 ppm
(Ponnock, 1994).
Rabbits
In a study first evaluated by the JMPR in 1980 (Annex 1,
reference 34), groups of 17 pregnant New Zealand white rabbits were
treated by intubation with carbofuran (purity, 95.6%) at doses of 0,
0.2, 0.6, or 2 mg/kg bw per day on gestation days 6-18. The deaths of
five females at 2 mg/kg bw per day were considered to be related to
treatment. Signs of maternal toxicity and behavioural changes in
animals at this dose included trembling, loss of muscle control,
salivation, sneezing, chewing motions, and reduced food and water
consumption. Body weight was not affected by the treatment. No
differences between groups were seen in reproductive parameters.
Macroscopic examination revealed no treatment-related tissue
alteration and no evidence of embryotoxicity or teratogenicity, even
at maternally toxic doses. The NOAEL was 0.6 mg/kg bw per day, on the
basis of maternal toxicity. The same conclusion was drawn by the 1980
JMPR (Rao, 1978 [cited incorrectly as Felton, 1978, in Annex 1
reference 35]).
Carbofuran (purity, 95.6%) was administered by gavage at doses of
0, 0.12, 0.5, or 2 mg/kg bw per day on gestation days 6-18 to groups
of 20 pregnant New Zealand white rabbits. One dam at 2 mg/kg bw per
day died of an unknown cause. Matting and/or staining of the haircoat
was seen at a somewhat higher frequency in the treated animals.
Although the mean maternal body weight was reduced during treatment,
no clear dose-response relationship was seen. A marked reduction in
body-weight gain was observed at 2 mg/kg bw per day early in the
treatment; no data were available on food consumption. Reproductive
parameters (numbers of corpora lutea, implantations, and resorption,
litter size, fetal body weight were not affected by treatment. A
slightly increased incidence of misaligned sternebrae was seen in
fetuses at 2 mg/kg bw per day. The NOAEL was 0.5 mg/kg bw per day, on
the basis of reduced body weight in dams at 2 mg/kg bw and the
increase in skeletal variations (Laveglia, 1981).
Carbofuran was given to CD rats by gastric intubation at doses of
0.05-5 mg/kg bw per day on days 7-19 of gestation and to CD-1 mice at
doses of 0.1-20 mg/kg bw per day on gestation days 6-16. At 1, 3, and
5 mg/kg bw per day, 40-55% of the rats died, and the numbers of
implantation sites and live fetuses were reduced. The frequency of
malformations was not increased. In mice at 10 or 20 mg/kg bw per day,
about 50% of dams died, and fetal body weight was reduced. A shift in
the rib profile (decreased incidence of 13 ribs, increased incidence
of 14 ribs) was observed at 10 and 20 mg/kg bw per day. There was no
evidence of teratogenicity in either rats or mice (Courtney et al.,
1985).
(f) Genotoxicity
The results of tests for the genotoxicity of carbofuran are
summarized in Table 3. Carbofuran was included among other carbamate
insecticides tested in vitro in the porcine brain tubulin assembly
assay for the detection of action as a microtubule poison and
aneuploidy inducer (Stehrer & Wolf, 1995). Carbofuran induced a
dose-dependent reduction in the degree of polymerization of tubulin
and an 11% reduction in the maximal polymerization velocity. It was
active in S. typhimurium strains TA1538 and TA98 in the presence and
absence of an exogenous metabolic activation system (Moriya et al.,
1983), induced sister chromatid exchange in human peripheral
lymphocytes (Georgian et al., 1985), and induced gene mutation in
V79 hamster cells (Woijciechowski et al., 1982).
(g) Special studies
(i) Dermal sensitization
Guinea-pigs were given 10 intracutaneous injections of
technical-grade carbofuran as an inducer, followed by a final
challenge injection. Dinitrochlorobenzene was used as a positive
control. Carbofuran was not sensitizing (Schoenig, 1967; Ellison,
1980).
(ii) Neurotoxicity
Rats
In a 28-day range-finding study, groups of five male and five
female Sprague-Dawley CD rats were maintained on a diet providing
technical-grade carbofuran (purity, 98.6%) at concentrations of 0, 50,
200, 500, 1000, 3000, or 6000 ppm, equivalent to 0, 2.5, 10, 25, 50,
150, and 300 mg/kg bw per day. Two males receiving 6000 ppm died.
Dose-related clinical signs that were noted at doses > 200 ppm in
animals of each sex consisted of exophthalmia, splayed hindlimbs in
females at 200 ppm, tremors and staggered gait at 500 and 1000 ppm,
and loss of muscle control and ataxia at 3000 and 6000 ppm. Treatment-
related clinical signs seen in animals at doses > 500 ppm were
decreased locomotion, dehydration, lacrimation, and unthriftiness.
Body-weight gain was reduced at concentrations > 50 ppm among males
(marginal at 50 ppm) and at > 200 ppm among females (marginal at
200 ppm). Necropsy showed no treatment-related gross lesions (Freeman,
1993). The NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw per day.
Table 3. Results of tests for the genotoxicity of carbofuran
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 100-10 000 µg/plate 98.3 Negativea Haworth & Lawlor
TA98, TA100, Positiveb (1983a)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 98 Negativec Haworth & Lawlor
TA98, TA100, (1983b)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 80 Negativea Haworth & Lawlor
TA98, TA100, Positived (1983c)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 99 Negativee Haworth & Lawlor
TA98, TA100, (1983d)
TA1535, TA 1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 98 Negativef Haworth & Lawlor
TA98, TA100, (1983e)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 98 Negativef Haworth & Lawlor
TA98, TA100, (1983f)
TA1535, TA1537
TA1538
Table 3. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Reverse mutation S. typhimurium 100-10 000 µg/plate 97.6 Negativeg Haworth & Lawlor
TA98, TA100, (1983g)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 96 Negativeh Haworth & Lawlor
TA98, TA100, (1983h)
TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium 1-10 000 µg/plate 97.6 Negative DeGraff (1983a)
TA100
Reverse mutation S. typhimurium 61.7-5000 µg/plate 99 Negativea Farrow (1983a)
TA98 Positivei
Reverse mutation S. typhimurium 61.7-5000 µg/plate 97.6 Negativea DeGraff (1983a)
TA98, TA100, Positivej
TA1537, TA1538
Reverse mutation S. typhimurium 123-10 000 µg/plate 98.3 Negativea Farrow (1983c)
TA98, TA100, Positivek
TA1537
Reverse mutation S. typhimurium 1-5000 µg/plate NR Negative Simmon (1979)
TA98, TA100,
TA1535, TA1537,
TA1538
Reverse mutation E. coli WP2 uvrA 1-5000 µg/plate NR Negative Simmon (1979)
Mitotic recombination S. cerevisiae 1-50 mg/ml NR Negative Simmon (1979)
Unscheduled DNA synthesis Human fibroblasts 0.1-1000 µg/mll NR Negative Simmon (1979)
(WI-38)
Growth inhibition E. colim, B. subtilis 1-500 mg/ml NR Negativen Simmon (1979)
Unscheduled DNA synthesis Rat hepatocytes 1-100 µg/ml 97.6 Negativea Thilagar (1983a)
Table 3. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Sister chromatid exchange Chinese hamster 78.1-312.5 µg/ml with S9 97.6 Negativeo Thilagar (1983b)
ovary cells 12.5-100 µg/ml without S9 Negative
Sister chromatid exchange Chinese hamster 312.5-2500 µg/ml with S9 96 Positivep Thilagar (1983c)
ovary cells 12.5-100 µg/ml without S9 Positive
Chromosomal aberration Chinese hamster 312.5-2500 µg/ml with S9 96 Negative Thilagar (1983d)
ovary cells 50-1000 µg/ml without S9 Negative
Cell mutation tk locus Mouse lymphoma 134-1780 µg/ml with S9 97.6 Negativeq Kirby (1983a)
L5178 Y cells 16-211 µg/ml without S9 Positive
Cell mutation tk locus Mouse lymphoma 134-1780 µg/ml with S9 96 Positive Kirby (1983b)
L5178 Y cells 24-316 µg/ml without S9
In vivo
Sex-linked recessive Drosophila 10 ppm (feeding solution) NR Negative Valencia (1981)
lethal mutation melanogaster
Sex-linked recessive Drosophila 7.5 ppm (feeding solution) 97.6 Negative Valencia (1983)
lethal mutation melanogaster
Sex-linked recessive Drosophila 5 and 10 ppm (feeding 97.6 Negative DeGraff (1983b)
lethal mutation melanogaster solution)
Cytogenicity Male rat 0.6 or 2.6 mg/kg bw per 98 Negative Putman (1983a)
bone marrow day orally, 5 days
Cytogenicity Male rat 1, 6 or 10 mg/kg bw per 96 Negative Putman (1983b)
bone marrow day orally, 5 days
Table 3. (Cont'd)
NR not reported
S9 9000 × g supernatant of Aroclor-induced rat liver microsomes
a With metabolic activation
b Weak positive response (2.2-fold increase in revertants per plate) of strain TA1535 only in the absence of S9
c The 1.9-fold increase in TA1535 revertants per plate observed in the absence of S9 did not meet the criteria for a
positive response.
d Weak positive response (2.2-fold increase in revertants per plate) of strain TA1535 in the absence of S9
e The 1.7-fold increase in TA1535 revertants per plate in the absence of S9 did not meet the criteria for a positive
response.
f 1.9-fold increase in TA1535 revertants per plate in both tests (Haworth & Lawlor, 1983 e,f) in the absence of S9
g 1.8-fold increase in TA1535 revertants and 1.4-fold increase in TA100 revertants in the absence of S9
h 1.6-fold increase in TA1535 revertants and 1.5-fold increase in TA100 revertants in the absence of S9
i Twofold increase in TA1535 revertants at 5000 µg/plate in the absence of S9. Precipitation of the compound occurred
at this concentration.
j Dose-dependent increase in TA1535 revertants at all doses in the absence of S9
k About a threefold increase TA1535 revertants at 1048 and 3145 µg/plate
l Precipitation observed at 1000 µg/ml
m Relative toxicity assays (growth inhibition) in DNA repair-proficient and -deficient strains of E. coli and B. subtilis
n Statistically significant increase in sister chromatid exchange frequency in comparison with solvent control in the
presence of S9 at a concentration of 312.5 µg/ml and in the absence of S9 at 100 µg/ml. The increases did not meet the
criteria for a positive response.
o In the absence of S9
p Statistically significant increases in sister chromatid exchange frequency in comparison with solvent control at all
doses in the presence and absence of S9
q Three S9-activated cultures out of 20 had mutant frequencies that were more than twice the mean mutant frequency of
the solvent controls. The significance of the increase is questionable because growth was strongly inhibited (> 90%)
in these cultures.
In the main study, groups of 10 male and 10 female Charles River
Sprague-Dawley rats were maintained on a diet designed to provide
concentrations of 0, 50, 500, or 1000 ppm, equivalent to 0, 2.4, 27.3,
and 55.3 mg/kg bw per day in males and 0, 3.1, 35.3, and 64.4 mg/kg bw
per day in females. Clinical signs, mortality, body weight and food
consumption were recorded, and functional observational battery and
motor activity testing were conducted before treatment and at weeks
4, 8, and 13 of treatment. The nervous systems of five rats of each
sex at the high dose and in the control group were examined for
histopathological lesions. One male at 1000 ppm was found moribund
on day 83 and was killed, and single animals in all groups,
including controls, were killed during the study for humane reasons.
Treatment-related clinical signs observed at concentrations
> 500 ppm consisted of exophthalmia, splayed hindlimbs, loss of
muscle control (in females), staggered gait, and tremors. Animals at
all doses had a dose-related reduction in body-weight gain; the
differences attained statistical significance in males at all doses
but in females only at 1000 ppm. Sporadic reductions in food
consumption were seen in animals at 1000 ppm, particularly males;
females showed increased food consumption. Functional observational
battery testing revealed effects in animals receiving doses
> 500 ppm, including gait impairment, a reduction in hindlimb grip
strength, whole-body tremors, and abnormal posture. Motor activity was
significantly reduced among females receiving 1000 ppm. The single
male at 1000 ppm found moribund on day 83 had calcifications in the
urethra; no other macroscopic treatment-related lesions were found. No
treatment-related histopathological lesions were found in the central
or peripheral nervous system of animals at the high dose (Freeman,
1994). The NOAEL for neurotoxicity was thus 50 ppm, equal to 3 mg/kg
bw per day. There was no NOAEL for systemic toxicity.
(iii) Inhibition of cholinesterase activity
In a comparative study in newborn, weanling, and adult
Sprague-Dawley rats, first evaluated by JMPR in 1980 (Annex 1,
reference 34), the purpose was to determine the time of maximal
inhibition of cholinesterase activity in plasma, erythrocytes, and
brain and the time of recovery after the administration of a single
oral dose of carbofuran. The LD50 values were 8.1 mg/kg bw for
newborn and 7.3 for weanling rats. The baseline cholinesterase
activities in untreated animals were determined at the age of two to
three days for newborns, 27-29 days for weanlings, and 99-101 days for
adult rats. The baseline activities in erythrocytes were lower in
newborns and weanlings than in adults, and those in brain were lower
in newborns than in weanlings and adults. After administration of
single oral doses of carbofuran at 3.2 mg/kg bw for newborns, 2.2
mg/kg bw for weanlings, and 4 mg/kg bw for adults, maximal depression
of erythrocyte activity occurred within 1 h in all groups, and maximal
depression of brain activity occurred within 1 h in weanlings and
adults and after 4 h in neonates. Full recovery was attained within
24 h after dosing, independently of the age of the animals. There were
no differences in sensitivity to cholinesterase inhibition with age
(Becci, 1979).
In a study to investigate the relationship between carbofuran
metabolism in vivo and acetylcholinesterase inhibition, male
Sprague-Dawley rats were given single intravenous or oral doses of
14C carbofuran at 50 µg/kg bw, and urine, faeces, and expired air
were analysed for oxidative and hydrolytic metabolites; blood, plasma,
and various tissues were analysed for carbofuran and its main
oxidative metabolite, 3-hydroxycarbofuran. Erythrocyte acetyl-
cholinesterase activity in vitro was used as an index of toxicity.
From 41 to 47% of the administered dose was recovered as 14C-carbon
dioxide after 8 h, independently of the route of administration. About
15% of the dose was found in urine and < 1% in faeces. 3-Hydroxy-
carbofuran was formed rapidly and underwent enterohepatic circulation,
resulting in an elimination half-life of about 64 min for all tissues;
the elimination half-life of the parent compound was about 29 min.
Rapid recovery of the erythrocyte acetylcholinesterase activity
closely paralleled carbofuran metabolism, and the primary disposition
of 3-hydroxycarbofuran in vivo was by metabolic conjugation
(Ferguson et al., 1984).
3. Observations in humans
Carbofuran was reported to have induced sensitization in a patch
test in 30 farmers with contact dermatitis (Sharma & Kaur, 1990).
Poisoning was reported in three female farmworkers who threw
carbofuran granules onto a coffee plantation in Jamaica. The women did
not wear protective clothing. The signs of poisoning reported included
vomiting, lassitude, nausea, and hypersalivation. Cholinesterase
activity was not determined in these patients (Coleman et al., 1990).
Comments
Carbofuran is rapidly absorbed, metabolized, and eliminated,
mainly in the urine, after oral administration to mice and rats. After
oral administration of [phenyl-14C]carbofuran to rats, 92% of the
radiolabel was eliminated in the urine and 3% in faeces. Most of the
radiolabel was eliminated within 24 h after treatment. With a
[carbonyl-14C]-labelled compound, 45% was eliminated as 14C-carbon
dioxide. The metabolic pathway consists in hydroxylation, oxidation,
hydrolysis, and conjugation.
Carbofuran is highly toxic after acute oral administration. The
oral LD50 values in various species ranged from 3 to 19 mg/kg bw.
Carbofuran had no sensitizing potential in guinea-pigs, and no local
irritation was found in rabbits after repeated dermal applications
over 7 or 21 days. WHO has classified carbofuran as 'highly hazardous'
(WHO, 1996).
In a 13-week study in dogs fed diets providing 0, 10, 70, or
500/250 ppm (dose reduced because of marked toxicity), an NOAEL was
not identified because of inhibition of erythrocyte acetylcholin-
esterase activity and some clinical signs were observed at the lowest
dose. In a subsequent four-week study in dogs, the highest dose
administered was 5 ppm, equal to 0.22 mg/kg bw per day, which was the
NOAEL for clinical signs, mortality, effects on body weight and food
consumption, and cholinesterase activity in plasma and erythrocytes.
In a one-year study in dogs at dietary concentrations of 0, 10, 20, or
500 ppm, the NOAEL was 10 ppm, equal to 0.3 mg/kg bw per day, on the
basis of histopathological testicular changes in a single male at
20 ppm; similar changes were observed in animals at 500 ppm. There
was no inhibition of erythrocyte or brain acetylcholinesterase at
concentrations of 10 or 20 ppm. The overall NOAEL in these short-term
studies in dogs was 5 ppm, equal to 0.22 mg/kg bw per day.
In two-year studies of toxicity and carcinogenicity at dietary
concentrations of 0, 20, 125, or 500 ppm in mice and 0, 10, 20, or
100 ppm in rats, the NOAELs were 20 ppm, equal to 2.8 mg/kg bw per
day, in mice and 20 ppm, equivalent to 1 mg/kg bw per day, in rats, on
the basis of inhibition of erythrocyte and brain acetylcholinesterase
activity. There was no evidence of tumorigenicity.
In a three-generation study of reproductive toxicity in rats at
dietary concentrations of 0, 20, or 100 ppm, the NOAEL was 20 ppm,
equal to 1.6 mg/kg bw per day, on the basis of reduced body-weight
gain in parental animals and reduced pup growth and pup survival at
100 ppm.
In an early study of developmental toxicity, rats were given
carbofuran at doses of 0, 0.1, 0.3, or 1 mg/kg bw per day by gavage.
An NOAEL could not be identified in this study. Dose-dependent,
transient clinical signs (chewing motions) were observed in the dams.
In a later study in rats at oral doses of 0, 0.25, 0.5, or 1.2 mg/kg
bw per day, the NOAEL for maternal and fetal toxicity was 1.2 mg/kg bw
per day, the highest dose tested. In a further study of teratogenicity
in rats, with dietary administration of 0, 20, 60, or 160 ppm
carbofuran, the NOAEL for maternal toxicity was 20 ppm, equal to
1.5 mg/kg bw per day, on the basis of a reduction in body-weight gain
at 60 ppm. The NOAEL for pup toxicity, based on reduced pup weight,
was 60 ppm, equal to 4.4 mg/kg bw per day. None of the studies showed
teratogenic potential.
The results of an early study of teratogenicity in rabbits at
oral doses of 0, 0.2, 0.6, or 2 mg/kg bw per day showed an NOAEL of
0.6 mg/kg bw per day for maternal toxicity on the basis of clinical
signs and an NOAEL of 2 mg/kg bw per day for fetotoxicity and
teratogenicity. In a subsequent study in rabbits at doses of 0, 0.12,
0.5, or 2 mg/kg bw per day, the NOAEL was 0.5 mg/kg bw per day on the
basis of slightly reduced body-weight gain in dams and a slightly
increased incidence of skeletal variations in pups at 2 mg/kg bw per
day. These studies provide no evidence for teratogenicity.
In a 90-day study of neurotoxicity in rats at dietary
concentrations of 0, 50, 500, or 1000 ppm, systemic toxicity
(reduction in body-weight gain) was observed at all doses. Clinical
signs of neurotoxicity were observed at 500 and 1000 ppm. No
histopathological lesions were found in the nervous system.
In a study of developmental neurotoxicity, carbofuran was
administered in the diet of rats to provide concentrations of 0, 20,
75, or 300 ppm from gestation day 6 through lactation day 10.
Reductions in the body-weight gain of dams and pups and in pup
survival and some evidence of delayed pup development were found at
doses > 75 ppm. The NOAEL was 20 ppm, equal to 1.7 mg/kg bw per
day, on the basis of reduced body-weight gain in dams and signs of
fetotoxicity at higher doses.
Carbofuran has been tested for genotoxicity in a wide range of
tests in vivo and in vitro. The Meeting concluded that it is not
genotoxic.
An ADI of 0-0.002 mg/kg bw was allocated on the basis of the
NOAEL for erythrocyte acetylcholinesterase inhibition of 0.22 mg/kg bw
per day in a four-week study in the most sensitive species, the dog,
and using a 100-fold safety factor. The use of a short-term study to
set the ADI is justified because the effect observed was reversible
and acute.
Toxicological evaluation
Levels that cause no toxicological effect
Mouse: 20 ppm, equal to 2.8 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rat: 20 ppm, equivalent to 1 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
20 ppm, equal to 1.2 mg/kg bw per day (three-generation
study of reproductive toxicity)
1.2 mg/kg bw per day (highest dose tested in a study of
developmental toxicity)
20 ppm, equal to 1.5 mg/kg bw per day (study of
developmental toxicity)
20 ppm, equal to 1.7 mg/kg bw per day (study of
developmental neurotoxicity)
Rabbit: 0.6 mg/kg bw per day (study of developmental toxicity)
Dog: 5 ppm, equal to 0.22 mg/kg bw per day (four-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to carbofuran
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 6-14.4 mg/kg bw
Dermal, toxicity, rat LD50 > 500 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.088-0.1 mg/litre
Dermal, irritation, rabbit Not irritating
Ocular irritation, rabbit Not available
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated oral, 4 weeks, toxicity, dog NOAEL = 0.22 mg/kg bw per day
Repeated oral, reproductive toxicity, rat NOAEL = 1.6 mg/kg bw per day, parental
and pup toxicity
Repeated oral (gavage), developmental NOAEL = 1.2 mg/kg bw per day (highest
toxicity, rat dose tested). No evidence of teratogenicity
Repeated oral (feeding), developmental NOAEL = 1.5 mg/kg bw per day, maternal
toxicity, rat toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 0.6 mg/kg bw per day, maternal
toxicity. No evidence of teratogenicity
Repeated oral, developmental neurotoxicity, rat NOAEL = 1.7 mg/kg bw per day
Longterm (> 1 year) Repeated oral, 2 years, carcinogenicity, mouse NOAEL = 2.8 mg/kg bw per day,
cholinesterase inhibition. No evidence
of carcinogenicity
Repeated oral, 2 years, carcinogenicity, rat NOAEL = 1 mg/kg bw per day, reduced
body-weight gain and cholinesterase
inhibition. No evidence of carcinogenicity
References
Barron, P., Giesler, P. & Ras, G.N. (1978) Teratogenicity of
carbofuran in rats. Act-No. 184.33. Unpublished report prepared by
Warf Institute, Inc. Wisconsin, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Becci, G. (1979) Study of cholinesterase activity inhibition by
carbofuran in neonate, weanling and adult rats. Study-No. A 79-343.
Unpublished report prepared by Food and Drug Research Laboratories,
Inc. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Bloch, I., Frei, T.H., Madoerin, K., Luetkemeier, H., Vogel, W.,
Schlotke, B., Vogel, O. & Terrier, C. (1987a) 13-Week oral toxicity
feeding study with carbofuran (D1221) in the dog. RCC-No. 077837 (FMC
Study No. A95-4249). Unpublished report prepared by Research
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by FMC
Corp., Philadelphia, PA, USA.
Bloch, I. Frei, T., Luetkenmeier, H., Vogel, W. & Terrier, C. (1987b)
4-Week oral toxicity (feeding) study with carbofuran 'D 1221' in male
dogs. RCC No. 087963 (FMC Study No. A95-4248). Unpublished report
prepared by Research & Consulting Company AG, Itingen, Switzerland.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Brown, W.R. (1980) 2-Year dietary toxicity and carcinogenicity study
in mice with technical carbofuran. Act No. 150.52 Histopathology Part.
Unpublished report prepared by Research Pathology Services, Inc. PA,
USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Burtner, B.R. (1982) 14-Day oral toxicity (range finding) study in
beagle dogs of carbofuran. (FMC Study No. A81-604, Toxicogenics No.
410-0714). Unpublished report prepared by ToxiGenics, Inc., IL, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Case, R.S. & Wilson, N.H. (1979) Cholinesterase evaluation conducted
during a two year dietary toxicity/oncogenicity study with carbofuran
in rats. Study No. Tox-79-001. Unpublished report prepared for FMC.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Coleman, A.M.E., Smith, A. & Watson, D. (1990) Occupational carbamate
pesticide intoxication in three farm workers. West Indian Med. J.,
39, 109-113.
Courtney, K.D., Andrews, J.E., Springer, J. & Dalley, L. (1985)
Teratogenic evaluation of the pesticides Baygon, carbofuran,
dimethoate and EPN. J. Environ. Sci. Health B, 20, 373-406.
DeGraff, M.G. (1983a) Mutagenicity evaluation of FMC 10242 in the Ames
Salmonella/microsome plate test Study No. A 83-1037). Unpublished
report by Litton Bionetics, Inc., Kensington, MD, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
DeGraff, W.G. (1983b) Mutagenicity evaluation of MC 10242 for the
sex-linked recessive lethal test in Drosophila melanogaster. Study
No. A 83-1060. Unpublished report prepared by Litton Bionetics, Inc.
Kensington, MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Dorough, H.W. (1968) Metabolism of Furadan (NIA-10242) in rats and
houseflies. J. Agric. Food Chem., 16, 319-325.
Elleman, P.N. (1979) Acute oral toxicity (FIFRA-EPA) in rats with
carbofuran technical (FMC Study No. A 79-339, CSE-Study No. 0180).
Unpublished report prepared by Cosmopolitan Safety Evaluation (CSE)
Inc., NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Ellison, T. (1980) Audit validaton of the skin sensitization test on
NIA 10242 Technical. Unpublished report on Study No. A 5300. Submitted
to WHO by FMC Corp., Philadelphia, PA, USA.
Farrow, M.G. (1983a) Salmonella typhimurium/mammalian microsome
plate incorporation assay with compound FMC 10242 (FMC Study-No. A
83-905, HLA No. 331). Unpublished report prepared by Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Farrow, M.G. (1983b) Salmonella typhimurium/mammalian microsome
plate incorporation assay with compound FMC 10242 (FMC Study No. A
83-907, HLA No. 332). Unpublished report prepared by Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Farrow, M.G. (1983c) Salmonella typhimurium/mammalian microsome
plate incorporation assay with compound FMC 10242 (FMC Study No. A
83-909, HLA No. 333). Unpublished report prepared by Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Ferguson, P.W., Dey, M.S., Jewell, S.A. & Krieger, R.I. (1984)
Corbofuran metabolism and toxicity in the rat. Fundam. Appl.
Toxicol., 4, 14-21.
Freeman, C. (1984a) Acute oral toxicity of FMC 18209 technical
(3-hydroxy-carbofuran) in rats. FMC Study No. A 83-1136. Unpublished
report prepared by FMC Toxicology Laboratory, NJ, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Freeman, C. (1984b) Acute oral toxicity of FMC 17481 technical
(3-keto-carbofuran) in rats. FMC Study No. A 83-1137. Unpublished
report prepared by FMC Toxicology Laboratory, NJ, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Freeman, C. (1984c) Acute oral toxicity of FMC 16497 (3-hydroxy-
7-phenol) rats. FMC Study No. A83-1134. Unpublished report Pprepared
by FMC Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Freeman, C. (1984d) Acute oral toxicity of FMC 19841 (3-keto-7-phenol)
in rats. FMC Study No. A83-1135. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Freeman, C. (1984e) Acute oral toxicity of FMC 10272 (7-phenol) in
rats. FMC Study No. A83-1133. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Freeman, C. (1993) Twenty-eight day neurotoxicity range-finding study
in rats with carbofuran technical. FMC No. A 92-3704. Unpublished
report prepared by FMC Corporation, Toxicology Laboratory, NJ, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Freeman, C. (1994) Subchronic neurotoxicity screen in rats with
carbofuran technical. FMC Study No. 92-3705. Unpublished report
prepared by FMC Corporation Toxicology Laboratory, NJ, USA. Submitted
to WHO by FMC Corp., Philadelphia, PA, USA.
Flucke, W. (1986) SRA 12869 (isofenphos) and D 1221 (carbofuran):
Study for combination toxicity. Study No. 134337. Unpublished report
prepared by Bayer AG, Insititute of Toxicology. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Fukuto, T.R. & Metcalf, R.L. (1969) Metabolism of insecticides in
plants and animals. Biological effects of pesticides in mammalian
systems. Ann. NY Acad. Sci., 160, Art. 1.
Georgian, L.I., Moraru, I., Draghicescu, T. & Tarnavschi, R. (1985)
The effect of low concentrations of carbofuran, cholin salt of maleic
hydrazine, propham and chlorpropham on sister-chromatid exchange (SCE)
frequency in human lymphocytes in vitro. Mutat. Res., 147, 296.
Goldenthal, E.I. (1982a) 2-Year dietary toxicity and carcinogenicity
study in mice. FMC Study No. Act No. 150.52 (IRDC No. 167-116).
Unpublished report prepared by International Research and Development
Corporation, MI, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Goldenthal, E.I. (1982b) 2-Year dietary toxicity and carcinogenicity
study in rats. FMC Study No. Act No. 130.51 (IRDC No. 167-115).
Unpublished report prepared by International Research and Development
Corporation, MI, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Goldenthal, E.I. (1982c) Three generation reproduction study in rats.
FMC Study No. Act. No. 131.53 (IRDC No. 167-114). Unpublished report
prepared by International Research and Development Corporation, MI,
USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983a) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-868 (MBA Study No. 1921.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. 1983b) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-913 (MBA Study No. 1934.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983c) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
Technical. FMC Study No. A 83-948 (MBA Study No. 1938.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983d) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-949 (MBA Study No. 1936.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983e) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-950 (MBA Study No. 1935.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983f) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-951 (MBA Study No. 1937.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983g) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-973 (MBA Study No. 1982.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983h) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-1063. Unpublished report prepared by
Microbiological Associates, MD, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Hoffman, S.L. & Robinson, R.A. (1994a) Metabolism of C14-carbofuran
in laying hens. XBL Study No. XBL 92051; FMC Study No. 078 POU 92M1.
Unpublished report prepared by XenoBiotic Laboratories, Inc.,
Plainsboro, NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Hoffman, S.L. & Robinson, R.A. (1994b) Metabolism of C14-carbofuran
in lactating goats. XBL Study No. XBL 92051; FMC Study No. 078 GOA
92M1. Unpublished report prepared by XenoBiotic Laboratories, Inc.,
Plainsboro, NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Kedderis, L.B. (1985a) Seven-day repeated dose dermal toxicity
range-finding study in rabbits with FMC 10242 technical (carbofuran).
Study No. A 85-1645. Unpublished report prepared by FMC Corporation.
NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kedderis, L.B. (1985b) Twenty-one day repeated dose dermal toxicity
range-finding study in rabbits with FMC 10242 technical (carbofuran).
Study No. A 85-1678. Unpublished report prepared by FMC Corporation,
NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kirby, P.E. (1983a) L 5178Y TK+/- mouse lymphoma mutagenesis assay.
FMC Study No. A 83-962 and A 83-988 (MBA Study No. T1982.701).
Unpublished report prepared by Microbiological Associates, Bethesda,
MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kirby, P.E. (1983b) L5178Y TK+/- mouse lymphoma mutagenesis assay.
FMC Study No. A 83-1064 (MBA Study No. T2124.201). Unpublished report
prepared by Microbiological Associates, Bethesda, MD, USA. Submitted
to WHO by FMC Corp., Philadelphia, PA, USA.
Knaak, J.B., Munger, D.M. & McCarthy, J.F. (1969) The metabolism of
carbofuran alfalfa residues in the rat. Abstracts of Papers, 158th
National Meeting of the American Chemical Society, 7-12 September.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kuhr, R.J. & Dorough, H.W. (1976) Carbamate Insecticides: Chemistry,
Biochemistry and Toxicology, Cleveland, OH, CRC Press. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Laveglia, J. (1981) Teratology study in the rabbit with carbofuran.
FMC Study No. A 80-452 (IRDC No. 167-156). Unpublished report prepared
by International Research and Development Corporation, MI, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Mehta, C.S. (1981) Rabbit acute dermal toxicity. FMC Study No. A
81-564 (Stillmeadow No. 2142.81). Unpublished report prepared by
Stillmeadow, Inc., TX, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Metcalf, R.L., Fukuto, T.R., Collins, C., Borck, K., Abd El-Aziz, S.,
Munoz, R. & Cassil, C.C. (1968) Metabolism of 2,2-dimethyl-
2,3-dihydrobenzofuranyl-7 N-methylcarbamate (Furadan) in plants,
insects and mammals. J. Agric. Food Chem., 16, 300-311.
Mihail, F. (1981) D1221 (carbofuran) and Dacamox (thiofanox): Acute
combination toxicity study. Study No. 10280. Unpublished report
prepared by Bayer AG, Institute of Toxicology. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Moriya, M., Ohta, K., Watanabe, T., Miyazawa, K., Kato, K. & Shirasw
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Norwell, M.J. (1983a) Acute oral toxicity of FMC 10242, technical, in
rats. FMC Study No. A 83-1101. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Norwell, M.J. (1983b) Acute oral toxicity of FMC 10242, technical, in
rats. FMC Study No. A 83-1102. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Ponnock, K.S. (1994) A developmental neurotoxicity study of carbofuran
in the rat via dietary administration. Study No. 93-4506, FMC Study
No. A 93-3746. Unpublished report prepared by Pharmaco LSR, Inc.
Toxicology Services North America, NJ, USA. Submitted to WHO by FMC
Corp., Philadelphia, PA, USA.
Putman, D.L. (1983a) Activity of FMC 10242 in the in vivo cytogenetics
assay in Sprague-Dawley rats. FMC Study No. A 83-972 (MBA No.
T1982.102). Unpublished report prepared by Microbiological Associates,
Bethesda, MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Putman, D.L. (1983b) Activity of FMC 10242 in the subchronic in vivo
cytogenetics assay in male rats. Study No. A 83-1065 (MBA No.
T2124.102). Unpublished report prepared by Microbiological Associates,
Bethesda, MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Rao, G.N. (1978) Teratology study of carbofuran in rabbits. Study No.
185-33. Unpublished report prepared by Warf Institute, Inc. WI, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Rapp, W.R. (1980a) 24 Month dietary toxicity/carcinogenicity study of
carbofuran in rats. Act No. 130.51. Pathology report. Unpublished
report prepared by Wm. R. Rapp & Co. Inc., Allied Services for
Research, NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Rapp, W.R. (1980b) A three generation reproduction study of carbofuran
in rats. FMC Act No. 131.531. Pathology report. Unpublished report
prepared by Wm. R. Rapp & Co. Inc., Allied Services for Research, NJ,
USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Rodwell, D.E. (1980a) Teratology study in the rat with carbofuran.
(FMC Study No. A 80-453, IRDC No. 167-155). Unpublished report
prepared by International Research and Development Corp., MI, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Rodwell, D.E. (1980b; amended 1985) Pilot teratology study in the rat
with carbofuran in the diet (FMC Study No. A 80-443, IRDC No.
167-153). Unpublished report prepared by International Research and
Development Corp., MI, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Rodwell, D.E. (1981; with amendment) Teratology and postnatal study in
the rat with carbofuran (FMC Study No. A 80-444, IRDC No. 167-154).
Unpublished report prepared by International Research and Development
Corp., MI, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Sabol, R.J. (1979a) Rat acute oral toxicity with carbofuran technical.
Study No. 1083-79; FMC Study No. ACT 267.01-01. Unpublished report
prepared by Stillmeadow, Inc., TX, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Sabol, R.J. (1979b) Rat acute oral toxicity with furadan insecticide,
carbofuran 96.6%. Study No. 11231-79; FMC Study No. ACT 267.01-02
(01). Unpublished report prepared by Stillmeadow, Inc., TX, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Schoenig, G. (1967) Skin sensitization test on NIA 10242 technical.
IBT Study No. A 5300. FMC Study No. NCT 176.36. Unpublished report
prepared by Industrial Bio Test Laboratory, Inc, IL, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Sharma, V.K. & Kaur, S. (1990) Contact sensitization by pesticides in
farmers. Contact Derm., 23, 77-80.
Signorin, J. (1995) DOT acute inhalation toxicity study in rats. FMC
Study No. A 94-4097. Unpublished report prepared by FMC Corp.,
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Simmon, U.F. (1979) In vitro microbiological mutagenicity and
unscheduled DNA synthesis studies of eighteen pesticides. Study No.
68-01-2458 (FMC Study No. A81-509, EPA 600/1-79-041). Report prepared
by SRI International, Menlo Park, California, for Health Effects
Research Laboratory Office of Research and Development. US
Environmental Protection Agency, Research Triangle Park, NC, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Stehrer-Schmid, P. & Wolf, H.U. (1995) Effects of benzofuran and seven
benzofuran derivatives including four carbamate insecticides in the in
vitro porcine brain tubulin assembly assay and description of a new
approach for the evaluation of the test data. Mutat. Res., 339,
61-72.
Taylor, G.D. (1983) One-year chronic oral toxicity study in beagle
dogs with carbofuran. FMC study No. A 81-605 (Toxicogenics No.
410-0715). Unpublished report prepared by ToxiGenics, Inc., IL, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Thilagar, A. (1983a) Unscheduled DNA synthesis in rat primary
hepatocytes. FMC Study No. A 83-969 (MBA No. T1982.380). Unpublished
report prepared by Microbiological Associates, Bethesda, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Thilagar, A. (1983b) Sister chromatid exchange assay in Chinese
hamster ovary (CHO) cells. FMC Study No. A 83-1095 (MBA No.
1982.334001). Unpublished report prepared by Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Thilagar, A. (1983c) Sister chromatid exchange assay in Chinese
hamster ovary (CHO) cells. FMC Study No. A 83-1097 (MBA No.
2124.334001). Unpublished report prepared by Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Thilagar, A. (1983d) Chromosome aberrations in Chinese hamster ovary
(CHO) cells. FMC Study No. A 83-1096. Unpublished report prepared by
Microbiological Associates, Bethesda, MD, USA. Submitted to WHO by FMC
Corp., Philadelphia, PA, USA.
Valencia, R. (1981) Mutagenesis screening of pesticides using
Drosophila. FMC Study No. A 83-1042 (EPA 600/1-81-017). Report
prepared by Warf Institute Inc., Madison, WI, for Health Effects
Research Laboratory. Office of Research and Development, US
Environmental Protection Agency, Research Triangle Park, NC, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Valencia, R. (1983) Drosophila sex-linked recessive lethal assay
of 2,3-dihydro-2,2-dimethyl-7-benzofuranyl- N-methyl carbamate
(carbofuran). FMC Study No. A 83-1019 (MBA No. T1047.160). Unpublished
report prepared by Microbiological Associates, Bethesda, MD and the
University of Wisconsin, Zoology Department, USA. Submitted to WHO by
FMC Corp., Philadelphia, PA, USA.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
Woijcieckowski, J.P., Kaur, P. & Sabharwal, P.S. (1982) Induction of
ouabain resistance in V-79 cells by four carbamate pesticides.
Environ. Res., 29, 48-53.
 [14C-phenyl ring]Carbofuran was administered in capsules to
laying hens once a day for seven consecutive days at doses equivalent
to dietary concentrations of 0 and 25 ppm. Carbofuran was extensively
metabolized and rapidly eliminated, resulting in low residues in eggs
and tissues. Muscle tissue and fat contained < 0.01 ppm; kidney,
liver, and eggs contained concentrations of < 0.2 ppm carbofuran
equivalents. Conjugates formed after oxidative and hydrolytic
reactions were further conjugated to their corresponding sulfate
conjugates and appeared to bind with biomolecules (e.g. proteins) to
form nonextractable residues (Hoffman & Robinson, 1994a).
[14C-phenyl ring]Carbofuran was administered in gelatine
capsules to lactating goats once a day for seven consecutive days at
doses equivalent to dietary concentrations of 0 and 25 ppm. Carbofuran
was extensively metabolized and readily eliminated. Muscle and fat
contained < 0.01 ppm; kidney, liver, and milk contained 0.09-0.39
ppm. Metabolites occurred in nonconjugated and conjugated forms, the
latter being further conjugated to the sulfate conjugates, which bind
to biomolecules and result in nonextractable (bound) residues (Hoffman
& Robinson, 1994b).
2. Toxicological studies
(a) Acute toxicity
The results of numerous studies of the acute toxicity of
carbofuran in rats were evaluated previously (Annex I, reference 26)
and are summarized in Table 1. The oral LD50 values for rats were
6-18 mg/kg bw. The toxic signs observed were typical of cholinesterase
inhibition: salivation, cramp, trembling, and sedation were observed
within minutes after administration and lasted for up to three days.
Additional studies that have become available since the first
evaluation give oral LD50 values for technical-grade carbofuran in
rats of about 5 mg/kg bw in females and 14 mg/kg bw in males (Elleman,
1979; Sabol, 1979a, b; Norvell, 1983a,b). The dermal LD50 in rabbits
for carbofuran of a purity of 96.1% was > 2000 mg/kg bw (Mehta,
1981). In rats treated by inhalation with a carbofuran formulation
(Furadan 95), the LC50 value was 0.11 mg/litre for a 1-h exposure
(Signorin, 1995).
The results of studies on the acute toxicity of several
metabolites of carbofuran are summarized in Table 2.
Studies of combined treatment with carbofuran and thiofanox or
carbofuran and isofenphos revealed no potentiating effects. Additive
or even subadditive toxic effects were found after simultaneous
administration of the test compounds (Mihail, 1981; Flucke, 1986).
Table 1. Acute toxicity of carbofuran
Species Sex Route LD50 (mg/kg bw) Reference
or LC50 (mg/litre)
Mouse Oral 14.4 Kimmerle (1966)
Rat Male Oral 18.0 Kimmerle (1966)
Rat Male Oral 7.8 Grönning & Kimmerle (1974)
Rat Male Oral 11.9 Kohn (1967a)
Rat Female Oral 6.0 Grönning & Kimmerle (1974)
Rat Female Oral 13.8 Kimmerle (1966)
Rat Male and female Oral 14.1 Powers (1964)
Rat Male Intraperitoneal 8.2 Kimmerle (1966)
Rat Female Intraperitoneal 2.8 Kimmerle (1966)
Rat Male and female Intraperitoneal 1.4 Kohn (1967b)
Rat Male and female Dermal > 500 Kimmerle (1966)
Rat Male Inhalation (1 h) 0.108 Kimmerle (1966)
Rat Male Inhalation (1 h) 0.091 Grönning & Kimmerle (1974)
Rat Male Inhalation (4 h) 0.088 Kimmerle (1966)
Rat Female Inhalation (1 h) 0.080 Grönning & Kimmerle (1974)
Rat Female Inhalation (4 h) 0.075-0.11 Grönning & Kimmerle (1974)
Guinea-pig NR Oral 9.2 Kimmerle (1966)
Rabbit NR Oral 7.5 Kimmerle (1966)
Cat NR Oral 2.5-3.5 Kimmerle (1966)
Dog NR Oral 15-19 Kimmerle (1966)
Table 2. Acute oral toxicity of metabolites of carbofuran in rats
Metabolite LD50 Reference
(mg/kg bw)
3-Hydroxycarbofuran 21.9 (male) Freeman (1984a)
8.3 (female)
3-Ketocarbofuran 108 (male) Freeman (1984b)
93.1 (female)
3-Hydroxy-7-phenol 1916 (male) Freeman (1984c)
1654 (female)
3-Keto-7-phenol > 800 Freeman (1984d)
7-Phenol 2450 (male) Freeman (1984e)
1743 (female)
(b) Short-term toxicity
Rabbits
In a range-finding study, groups of rabbits were treated dermally
with carbofuran (technical-grade; purity, 96.9%) at doses of 0, 100,
300, or 1000 mg/kg bw per day over seven days; the contact time was
6 h/day. No clinical signs of toxicity and no local irritation
were observed. The treatment did not affect body weight or food
consumption. A 30% depression in plasma cholinesterase activity was
measured in males at 100 and 300 mg/kg bw per day and a 47% reduction
at 1000 mg/kg bw per day. Brain acetylcholinesterase activity was
reduced by 26% in males at 100 mg/kg bw per day, 49% in those at 300
mg/kg bw per day, and 54% in those at 1000 mg/kg bw per day. These
reductions were not statistically significant (Kedderis, 1985a).
New Zealand white rabbits received applications of carbofuran
(technical-grade; purity, 96.9%) for about 6 h/day at doses of 0, 10,
100, or 1000 mg/kg bw per day for 21 consecutive days. No signs
of toxicity and no effects on body weight, food consumption,
haematological or clinical chemical parameters, organ weights,
or gross or histopathological appearance were observed. Brain
acetylcholinesterase activity was reduced by 21% in males at 100 mg/kg
bw per day and 26% in those at 1000 mg/kg bw per day. The reductions
were not statistically significant. The NOAEL was 10 mg/kg bw per day
(Kedderis, 1985b).
Dogs
In a 14-day range-finding study, single beagle dogs were fed
diets designed to provide carbofuran (technical-grade; purity, 96.1%)
at concentrations of 0, 18, 32, 56, 100, 316, or 1000 ppm, equivalent
to 0, 0.45, 0.8, 1.4, 2.5, and 8 mg/kg bw per day. The animal that
initially received 18 ppm was changed to a dose of 1000 ppm on day 4
of the study. At this dose, body weight loss and depressed food intake
were observed, and clinical signs included muscle tremor, emesis, and
salivation. Although cholinesterase activity was measured in plasma
and erythrocytes, dose-related depression was found only in plasma, at
all doses; inhibition ranged from 16% (within the historical control
range) in animals at 18 ppm to < 92% at 1000 ppm (Burtner 1982).
In a 13-week feeding study, carbofuran (purity, 99.6%) was
administered in the diet to groups of four male and four female
beagle dogs to provide concentrations of 0, 10, 70, or 500/250 ppm,
equivalent to 0, 0.43, 3.1, and 10.6 mg/kg bw per day. Two additional
animals of each sex per group were assigned to recovery groups to
study the reversibility of the effects over a four-week period. The
feed concentration of 500 ppm was reduced to 250 ppm from treatment
day 6 onwards because of marked toxicity: one dog at this dose died on
day 5 of treatment due to a treatment-related invagination of the
jejunum. At concentrations > 10 ppm, hyperaemia (e.g. at ear pinnae
and oral mucous membranes) and increased salivation were observed.
Clinical signs at the highest dose consisted of muscular spasms,
ataxia (pronounced in males during the first week of dosing but
sporadic thereafter), decreased motility, tachypnoea and deep
respiration, and vomiting. At 500 ppm, food consumption was markedly
reduced and loss of body weight was observed; reduction of the dose to
250 ppm resulted in recovery of food consumption and body-weight gain.
The treatment had no effect on ophthalmoscopic, electrocardiographic,
haematological, or clinical chemical parameters but inhibited
cholinesterase activity and caused alterations in organ weights and in
gross and histopathological appearance. Dose-related inhibition of
plasma and erythrocyte cholinesterase activity was observed in all
treated groups. Maximal inhibition occurred on test day 1, when the
plasma activity was 70, 27, and 13% of the control activity at 10, 70,
and 500/250 ppm and the erythrocyte activity was 68, 29, and 11% of
the control, respectively. The plasma and erythrocyte cholinesterase
activities returned to normal during the four-week recovery period.
There was no inhibition of brain acetylcholinesterase activity by the
end of the treatment or recovery period. An NOAEL was not determined
because inhibition of erythrocyte acetylcholinesterase and increased
salivation were seen at the lowest dose tested (Bloch et al., 1987a).
This study was followed by a four-week feeding study in order to
establish a NOAEL with respect to clinical signs and cholinesterase
inhibition: Groups of four male beagle dogs were fed diets providing
concentrations of 0 or 5 ppm carbofuran (purity, 99.6%) for four
weeks. The treatment had no effects on clinical signs, mortality, body
weight, food consumption, or plasma or erythrocyte cholinesterase
activity. The NOAEL was 5 ppm, equal to 0.22 mg/kg bw per day (Bloch
et al., 1987b).
In an earlier one-year study, groups of six male and six female
beagle dogs were given diets providing concentrations of 0, 10, 20, or
500 ppm carbofuran (technical-grade; purity, 96.1%), equal to 0,
0.3, 0.6, and 13 mg/kg bw per day. At 500 ppm, body weight losses
associated with emesis were observed, particularly in males, and from
the fifth month these animals were fed a supplemented control diet.
One male at 500 ppm died. Muscle tremor and salivation occurred
sporadically at this dose, and all males showed marked depression of
the haematocrit, haemoglobin values, and erythrocyte count from five
months. Only transient, much less pronounced depressions of these
parameters were observed in females at the high dose. Plasma
cholinesterase activity was inhibited in most males at 10 and
20 ppm and markedly (77%) in all animals at 500 ppm. Erythrocyte
acetylcholinesterase activity was reduced by 21-27% in many males
at 500 ppm. At the end of the study, a 24% depression in brain
acetylcholinesterase activity was observed in males at 500 ppm,
whereas females at this dose showed a 44% increase. No relevant
inhibition of erythrocyte or brain acetylcholinesterase activity was
seen in animals at 10 or 20 ppm. The absolute brain and heart weights
of males at 500 ppm were depressed, but no macroscopic or microscopic
changes were found in these organs. Gross treatment-related lesions
observed in the animals at 500 ppm consisted of a marked loss of body
fat and alopecia. Histopathological examination revealed an increased
amount of hepatocellular endoplasmic reticulum in the treated dogs,
but no clear dose-response relationship was seen. Degeneration of the
seminiferous tubules, giant-cell formation, or aspermia was observed
in males at 500 ppm and in a single male at 20 ppm. Minimal-to-
moderate inflammatory changes of the lung were observed at a higher
incidence in the dogs at 500 ppm. The NOAEL was 10 ppm, equal to
0.3 mg/kg bw per day, on the basis of the histopathological changes
in the testes of one male at 20 ppm (Taylor, 1983).
(c) Long-term toxicity and carcinogenicity
Mice
In a two-year study first evaluated by the 1980 JMPR (Annex 1,
reference 34), groups of 100 male and 100 female Charles River CD-1
mice were fed diets providing concentrations of 0, 20, 125, or 500 ppm
carbofuran (technical-grade; purity, 95.6%), equal to 0, 2.8, 18,
and 70 mg/kg bw per day. The treatment had no effect on general
appearance, mortality, or urinary parameters. Body-weight gain was
slightly reduced in single males at 125 ppm and in most animals at
500 ppm during the first year of the study; however, a 20% reduction
in body-weight gain in comparison with the controls was observed in
females at 500 ppm at the end of the study. Food consumption was also
sporadically reduced in animals at the highest dose, particularly
during the first months of the study. Haematological and clinical
chemical examinations revealed no treatment-related changes, except
for inhibition of brain acetylcholinesterase activity; cholinesterase
activities were not measured in erythrocytes or plasma. A
statistically significant depression of brain acetylcholinesterase
activity was observed in animals at 125 and 500 ppm. Maximal
inhibition at 125 ppm was 31% of the activity in concurrent controls;
at 500 ppm, the maximal depression was 55%. Organ weights were changed
sporadically but were not related to treatment. No gross pathological
findings were observed that were indicative of a relationship with
treatment. Microscopic examination revealed no treatment-related
change and no evidence of a compound-related increase in tumour
incidences. The NOAEL was 20 ppm, equal to 2.8 mg/kg bw per day, on
the basis of inhibition of brain acetylcholinesterase activity at
125 ppm. The conclusions of the earlier evaluation were therefore
confirmed (Brown, 1980; Goldenthal, 1980, 1982a).
Rats
In a study reviewed by the JMPR in 1979 and 1980 (Annex 1,
references 32 and 34), carbofuran (technical-grade; purity, 95.6%) was
administered in the diet to groups of 90 male and 90 female Charles
River CD rats to provide concentrations of 0, 10, 20, or 100 ppm, for
two years. Groups of 10 animals of each sex per dose were killed at 6,
12, and 18 months, and blood and brain samples were collected for
determination of cholinesterase activity. The treatment did not affect
general appearance, mortality, food consumption, or ophthalmoscopic,
haematological, or urinary parameters. Body-weight gain was reduced in
animals of each sex at 100 ppm. Clinical chemical examinations
revealed statistically significant inhibition of cholinesterase
activity in plasma, erythrocytes, and brain in rats at 100 ppm. The
maximal reduction of cholinesterase activity in males was 37% in
plasma, 24% in erythrocytes, and 25% in brain; the corresponding
percentages in females at 100 ppm were 26% in plasma, 19% in
erythrocytes, and 43% in brain. No relevant inhibition of
acetylcholinesterase activity was seen in erythrocytes or brain of
animals at 10 or 20 ppm (Case & Wilson, 1979). Various changes in
organ weights were considered to be due to changes in body weight. No
treatment-related gross pathological or histopathological changes were
observed, and there was no evidence of tumorigenicity. The NOAEL was
20 ppm, equivalent to 1 mg/kg bw per day, on the basis of reduced body
weight gain and acetylcholinesterase inhibition in erythrocytes and
brain at the higher dose. The conclusions of the earlier evaluation
were thus confirmed (Goldenthal, 1979a; Rapp, 1980a; Goldenthal,
1982b).
(d) Reproductive toxicity
Rats
In a three-generation study first evaluated by the 1980 JMPR
(Annex 1, reference 34), groups of Charles River CD rats (10 males and
20 females per group for the first and second generations; 12 males
and 24 females per group for the third generation) were maintained on
a diet providing concentrations of 0, 20, or 100 ppm carbofuran
(purity, 95.6%), equal to 0, 1.2, and 6 mg/kg bw per day for males and
0, 1.9, and 9.7 mg/kg per day for females. Each generation was mated
twice to produce two litters. The treatment did not affect the general
behaviour, appearance, or survival of the parental rats. Parental
body-weight gain was lower at 100 ppm throughout the course of
treatment and was associated with lower food consumption, particularly
in males. Fertility indices were not adversely affected. The survival
of pups of the F1a, F2a, and F3a generations at 100 ppm was reduced
at lactation day 4 in comparison with the control. Dehydration was
seen in pups of some litters of the F3a and F3b generations at
100 ppm. The mean weights of pups at 100 ppm were lower than those of
the controls on day 21 post partum in all generations. No compound-
related gross pathological lesions were seen at necropsy in the F0,
F1, or F2 parental animals or the F2b or F3b litters. Various organ
weights of animals of the F2 parental and F3b pup generations at
20 and 100 ppm were statistically significantly changed. Because
clear dose-response relationships were not always seen and no
histopathological alterations were associated with these changes, the
findings were considered not to be of biological significance. The
NOAEL was 20 ppm, equal to 1.2 mg/kg bw per day, on the basis of
reductions in body-weight gain in the parental generation and
reductions in the growth and survival of pup generations at
100 ppm. Thus, the earlier evaluation of this study was confirmed
(Goldenthal, 1979b; Rapp, 1980b; Goldenthal, 1982c).
(e) Developmental toxicity
Rats
In a study first evaluated by the 1980 JMPR (Annex 1, reference
34), carbofuran (technical-grade; purity, 95.6%) was administered to
groups of 24 pregnant Charles River rats by intubation in 0.25%
carboxymethylcellulose at doses of 0, 0.1, 0.3, or 1 mg/kg bw per day
on days 6-15 of gestation. Transient, dose-dependent clinical signs
(chewing motions) were observed at doses > 0.1 mg/kg bw per day for
a short period after treatment. Overt signs of toxicity in animals at
0.3 mg/kg bw per day included rough coats and lethargy; at the high
dose, lacrimation, increased salivation, trembling, and convulsions
were also seen. One female at the high dose died. The treatment had no
effect on the body weights of dams or pups. Reproductive parameters
(e.g. numbers of corpora lutea, implantations, and resorptions and
litter size) were not adversely affected by treatment. Skeletal
examination revealed a higher incidence of some variations in
ossification of sternebrae in treated animals but with no clear
dose-response relationship. Similar alterations were prevalent in the
control group at an incidence of 47% of fetuses. There was no evidence
of teratogenicity. An NOAEL was not determined because transient
clinical signs were observed at the lowest dose (Barron et al., 1978).
Groups of 25 pregnant Charles River COBS CD rats were treated
with carbofuran (purity, 95.6%) by gavage in corn oil at daily doses
of 0, 0.25, 0.5, or 1.2 mg/kg bw on days 6-15 of gestation. The
treatment had no effect on the general appearance, behaviour,
mortality, or body weights of the dams. No differences between groups
were noted in reproductive parameters such as the numbers of corpora
lutea, implantations, and resorptions, litter size, or fetal body
weight. No evidence of teratogenicity was seen (Rodwell, 1980a).
In a pilot study, groups of 10 pregnant Charles River COBS rats
were fed carbofuran (purity, 95.6%) in the diet to give concentrations
of 0, 20, 60, 120, 160, or 200 ppm, equal to 0, 1.5, 4, 8, 11, and
13 mg/kg bw per day, on gestation days 6-19. Neither survival nor
behaviour was affected by the treatment. Hair loss, soft stools,
and scabbing were seen at higher incidences in the treated than
the control group. A dose-related loss in mean maternal body
weight occurred during the first two days of treatment with doses
> 60 ppm, resulting in reduced body-weight gains throughout the
treatment period in animals at 120, 160, and 200 ppm. A dose-related
decrease in food consumption was observed at doses > 60 ppm at the
beginning of the treatment period. Reproductive parameters (litter
size and the numbers of resorptions, implantations, and corpora lutea)
were not adversely affected. On the basis of these results, doses of
0, 20, 60, and 160 ppm were selected for the main study (Rodwell,
1980b, 1985).
The main study was conducted with groups of 40 pregnant Charles
River COBS rats fed diets giving concentrations of 0, 20, 60, or 160
ppm carbofuran (purity, 95.6%), equal to 0, 1.5, 4.4, and 11 mg/kg bw
per day, on gestation days 6-19. Treatment had no effect on appearance
or behaviour, except for a slight increase in hair loss, matting of
the ventral haircoat, and soft stools, particularly in females at 60
and 160 ppm. A dose-related reduction in body-weight gain was seen at
doses > 60 ppm during the treatment period, with single instances
of body-weight loss in animals at 60 and 160 ppm at the beginning of
treatment. Increases in body-weight gain of treated animals during the
subsequent lactation period resulted in very similar body weights in
all groups at the end of lactation. Food consumption was reduced
during the first few days of treatment in animals at 60 and 160 ppm.
No differences between groups were observed in reproductive parameters
(such as the numbers of corpora lutea, implantations, and resorptions
and litter size). The mean weights of pups at 160 ppm were decreased
throughout lactation. The incidence of malformations was not
increased. The NOAEL for maternal toxicity was 20 ppm, equal 1.5 mg/kg
bw per day, on the basis of reduced body-weight gain of the dams at
doses > 60 ppm. The NOAEL for pup toxicity was 60 ppm, equal to 4.4
mg/kg bw per day, on the basis of reduced weights of pups at 160 ppm
(Rodwell, 1981).
Groups of 24 mated female Sprague-Dawley (CD) rats were given
diets containing carbofuran (purity, 99.1%) to provide concentrations
of 0, 20, 75, or 300 ppm, equal to 0, 1.7, 5, and 20 mg/kg bw per day,
from gestation day 6 through lactation day 10. Physical observations
were made, and body weight and food consumption were measured for all
maternal animals at selected intervals throughout the gestation and
lactation periods. The numbers of live pups were recorded, and litter
parameters such as pinna detachment, incisor eruption, eye opening,
motor activity, auditory startle response, and brain weights were
evaluated. Neuropathological examinations were performed on six pups
of each sex per group that were killed on postnatal days 11 and 60.
Maternal animals were killed after weaning of the last litter. No
deaths occurred during the study. Reduced body-weight gain was seen in
dams at 75 and 300 ppm during gestation and also during the lactation
period for those at 300 ppm. Food consumption was reduced in dams at
these two doses at various periods during gestation. Treatment had no
effect on pregnancy rates, length of gestation, or litter size, and
there was no evidence of prolonged or difficult delivery. Treatment at
300 ppm resulted, however, in a greater number of dead pups at birth
than among the controls (not statistically significant). A dose-
dependent reduction in pup weight was observed in animals at 75 and
300 ppm throughout the lactation period, and the reductions persisted
after weaning. Pup survival was adversely affected by prenatal
administration of carbofuran at 75 or 300 ppm on lactation day 4 until
weaning at lactation day 21. Treatment at doses of 75 or 300 resulted
in developmental delays of three to four days in vaginal patency and
preputial separation; marginal delays were seen with respect to pinna
detachment, lower incisor eruption, and eye opening. No adverse effect
was noted on auditory startle response, motor activity, or swimming
development, but there was an angle reduction at doses > 75 ppm.
Brain weights were not affected by treatment. No treatment-related
macroscopic findings were observed in dams or pups. Microscopic
examinations of pups in the control and high-dose groups revealed no
compound-related abnormality. The NOAEL was 20 ppm, equal to 1.7 mg/kg
bw per day, on the basis of reduced body-weight gain of dams and pups,
reduced pup survival, and slight developmental delay at doses > 75 ppm
(Ponnock, 1994).
Rabbits
In a study first evaluated by the JMPR in 1980 (Annex 1,
reference 34), groups of 17 pregnant New Zealand white rabbits were
treated by intubation with carbofuran (purity, 95.6%) at doses of 0,
0.2, 0.6, or 2 mg/kg bw per day on gestation days 6-18. The deaths of
five females at 2 mg/kg bw per day were considered to be related to
treatment. Signs of maternal toxicity and behavioural changes in
animals at this dose included trembling, loss of muscle control,
salivation, sneezing, chewing motions, and reduced food and water
consumption. Body weight was not affected by the treatment. No
differences between groups were seen in reproductive parameters.
Macroscopic examination revealed no treatment-related tissue
alteration and no evidence of embryotoxicity or teratogenicity, even
at maternally toxic doses. The NOAEL was 0.6 mg/kg bw per day, on the
basis of maternal toxicity. The same conclusion was drawn by the 1980
JMPR (Rao, 1978 [cited incorrectly as Felton, 1978, in Annex 1
reference 35]).
Carbofuran (purity, 95.6%) was administered by gavage at doses of
0, 0.12, 0.5, or 2 mg/kg bw per day on gestation days 6-18 to groups
of 20 pregnant New Zealand white rabbits. One dam at 2 mg/kg bw per
day died of an unknown cause. Matting and/or staining of the haircoat
was seen at a somewhat higher frequency in the treated animals.
Although the mean maternal body weight was reduced during treatment,
no clear dose-response relationship was seen. A marked reduction in
body-weight gain was observed at 2 mg/kg bw per day early in the
treatment; no data were available on food consumption. Reproductive
parameters (numbers of corpora lutea, implantations, and resorption,
litter size, fetal body weight were not affected by treatment. A
slightly increased incidence of misaligned sternebrae was seen in
fetuses at 2 mg/kg bw per day. The NOAEL was 0.5 mg/kg bw per day, on
the basis of reduced body weight in dams at 2 mg/kg bw and the
increase in skeletal variations (Laveglia, 1981).
Carbofuran was given to CD rats by gastric intubation at doses of
0.05-5 mg/kg bw per day on days 7-19 of gestation and to CD-1 mice at
doses of 0.1-20 mg/kg bw per day on gestation days 6-16. At 1, 3, and
5 mg/kg bw per day, 40-55% of the rats died, and the numbers of
implantation sites and live fetuses were reduced. The frequency of
malformations was not increased. In mice at 10 or 20 mg/kg bw per day,
about 50% of dams died, and fetal body weight was reduced. A shift in
the rib profile (decreased incidence of 13 ribs, increased incidence
of 14 ribs) was observed at 10 and 20 mg/kg bw per day. There was no
evidence of teratogenicity in either rats or mice (Courtney et al.,
1985).
(f) Genotoxicity
The results of tests for the genotoxicity of carbofuran are
summarized in Table 3. Carbofuran was included among other carbamate
insecticides tested in vitro in the porcine brain tubulin assembly
assay for the detection of action as a microtubule poison and
aneuploidy inducer (Stehrer & Wolf, 1995). Carbofuran induced a
dose-dependent reduction in the degree of polymerization of tubulin
and an 11% reduction in the maximal polymerization velocity. It was
active in S. typhimurium strains TA1538 and TA98 in the presence and
absence of an exogenous metabolic activation system (Moriya et al.,
1983), induced sister chromatid exchange in human peripheral
lymphocytes (Georgian et al., 1985), and induced gene mutation in
V79 hamster cells (Woijciechowski et al., 1982).
(g) Special studies
(i) Dermal sensitization
Guinea-pigs were given 10 intracutaneous injections of
technical-grade carbofuran as an inducer, followed by a final
challenge injection. Dinitrochlorobenzene was used as a positive
control. Carbofuran was not sensitizing (Schoenig, 1967; Ellison,
1980).
(ii) Neurotoxicity
Rats
In a 28-day range-finding study, groups of five male and five
female Sprague-Dawley CD rats were maintained on a diet providing
technical-grade carbofuran (purity, 98.6%) at concentrations of 0, 50,
200, 500, 1000, 3000, or 6000 ppm, equivalent to 0, 2.5, 10, 25, 50,
150, and 300 mg/kg bw per day. Two males receiving 6000 ppm died.
Dose-related clinical signs that were noted at doses > 200 ppm in
animals of each sex consisted of exophthalmia, splayed hindlimbs in
females at 200 ppm, tremors and staggered gait at 500 and 1000 ppm,
and loss of muscle control and ataxia at 3000 and 6000 ppm. Treatment-
related clinical signs seen in animals at doses > 500 ppm were
decreased locomotion, dehydration, lacrimation, and unthriftiness.
Body-weight gain was reduced at concentrations > 50 ppm among males
(marginal at 50 ppm) and at > 200 ppm among females (marginal at
200 ppm). Necropsy showed no treatment-related gross lesions (Freeman,
1993). The NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw per day.
Table 3. Results of tests for the genotoxicity of carbofuran
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 100-10 000 µg/plate 98.3 Negativea Haworth & Lawlor
TA98, TA100, Positiveb (1983a)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 98 Negativec Haworth & Lawlor
TA98, TA100, (1983b)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 80 Negativea Haworth & Lawlor
TA98, TA100, Positived (1983c)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 99 Negativee Haworth & Lawlor
TA98, TA100, (1983d)
TA1535, TA 1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 98 Negativef Haworth & Lawlor
TA98, TA100, (1983e)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 98 Negativef Haworth & Lawlor
TA98, TA100, (1983f)
TA1535, TA1537
TA1538
Table 3. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Reverse mutation S. typhimurium 100-10 000 µg/plate 97.6 Negativeg Haworth & Lawlor
TA98, TA100, (1983g)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 96 Negativeh Haworth & Lawlor
TA98, TA100, (1983h)
TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium 1-10 000 µg/plate 97.6 Negative DeGraff (1983a)
TA100
Reverse mutation S. typhimurium 61.7-5000 µg/plate 99 Negativea Farrow (1983a)
TA98 Positivei
Reverse mutation S. typhimurium 61.7-5000 µg/plate 97.6 Negativea DeGraff (1983a)
TA98, TA100, Positivej
TA1537, TA1538
Reverse mutation S. typhimurium 123-10 000 µg/plate 98.3 Negativea Farrow (1983c)
TA98, TA100, Positivek
TA1537
Reverse mutation S. typhimurium 1-5000 µg/plate NR Negative Simmon (1979)
TA98, TA100,
TA1535, TA1537,
TA1538
Reverse mutation E. coli WP2 uvrA 1-5000 µg/plate NR Negative Simmon (1979)
Mitotic recombination S. cerevisiae 1-50 mg/ml NR Negative Simmon (1979)
Unscheduled DNA synthesis Human fibroblasts 0.1-1000 µg/mll NR Negative Simmon (1979)
(WI-38)
Growth inhibition E. colim, B. subtilis 1-500 mg/ml NR Negativen Simmon (1979)
Unscheduled DNA synthesis Rat hepatocytes 1-100 µg/ml 97.6 Negativea Thilagar (1983a)
Table 3. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Sister chromatid exchange Chinese hamster 78.1-312.5 µg/ml with S9 97.6 Negativeo Thilagar (1983b)
ovary cells 12.5-100 µg/ml without S9 Negative
Sister chromatid exchange Chinese hamster 312.5-2500 µg/ml with S9 96 Positivep Thilagar (1983c)
ovary cells 12.5-100 µg/ml without S9 Positive
Chromosomal aberration Chinese hamster 312.5-2500 µg/ml with S9 96 Negative Thilagar (1983d)
ovary cells 50-1000 µg/ml without S9 Negative
Cell mutation tk locus Mouse lymphoma 134-1780 µg/ml with S9 97.6 Negativeq Kirby (1983a)
L5178 Y cells 16-211 µg/ml without S9 Positive
Cell mutation tk locus Mouse lymphoma 134-1780 µg/ml with S9 96 Positive Kirby (1983b)
L5178 Y cells 24-316 µg/ml without S9
In vivo
Sex-linked recessive Drosophila 10 ppm (feeding solution) NR Negative Valencia (1981)
lethal mutation melanogaster
Sex-linked recessive Drosophila 7.5 ppm (feeding solution) 97.6 Negative Valencia (1983)
lethal mutation melanogaster
Sex-linked recessive Drosophila 5 and 10 ppm (feeding 97.6 Negative DeGraff (1983b)
lethal mutation melanogaster solution)
Cytogenicity Male rat 0.6 or 2.6 mg/kg bw per 98 Negative Putman (1983a)
bone marrow day orally, 5 days
Cytogenicity Male rat 1, 6 or 10 mg/kg bw per 96 Negative Putman (1983b)
bone marrow day orally, 5 days
Table 3. (Cont'd)
NR not reported
S9 9000 × g supernatant of Aroclor-induced rat liver microsomes
a With metabolic activation
b Weak positive response (2.2-fold increase in revertants per plate) of strain TA1535 only in the absence of S9
c The 1.9-fold increase in TA1535 revertants per plate observed in the absence of S9 did not meet the criteria for a
positive response.
d Weak positive response (2.2-fold increase in revertants per plate) of strain TA1535 in the absence of S9
e The 1.7-fold increase in TA1535 revertants per plate in the absence of S9 did not meet the criteria for a positive
response.
f 1.9-fold increase in TA1535 revertants per plate in both tests (Haworth & Lawlor, 1983 e,f) in the absence of S9
g 1.8-fold increase in TA1535 revertants and 1.4-fold increase in TA100 revertants in the absence of S9
h 1.6-fold increase in TA1535 revertants and 1.5-fold increase in TA100 revertants in the absence of S9
i Twofold increase in TA1535 revertants at 5000 µg/plate in the absence of S9. Precipitation of the compound occurred
at this concentration.
j Dose-dependent increase in TA1535 revertants at all doses in the absence of S9
k About a threefold increase TA1535 revertants at 1048 and 3145 µg/plate
l Precipitation observed at 1000 µg/ml
m Relative toxicity assays (growth inhibition) in DNA repair-proficient and -deficient strains of E. coli and B. subtilis
n Statistically significant increase in sister chromatid exchange frequency in comparison with solvent control in the
presence of S9 at a concentration of 312.5 µg/ml and in the absence of S9 at 100 µg/ml. The increases did not meet the
criteria for a positive response.
o In the absence of S9
p Statistically significant increases in sister chromatid exchange frequency in comparison with solvent control at all
doses in the presence and absence of S9
q Three S9-activated cultures out of 20 had mutant frequencies that were more than twice the mean mutant frequency of
the solvent controls. The significance of the increase is questionable because growth was strongly inhibited (> 90%)
in these cultures.
In the main study, groups of 10 male and 10 female Charles River
Sprague-Dawley rats were maintained on a diet designed to provide
concentrations of 0, 50, 500, or 1000 ppm, equivalent to 0, 2.4, 27.3,
and 55.3 mg/kg bw per day in males and 0, 3.1, 35.3, and 64.4 mg/kg bw
per day in females. Clinical signs, mortality, body weight and food
consumption were recorded, and functional observational battery and
motor activity testing were conducted before treatment and at weeks
4, 8, and 13 of treatment. The nervous systems of five rats of each
sex at the high dose and in the control group were examined for
histopathological lesions. One male at 1000 ppm was found moribund
on day 83 and was killed, and single animals in all groups,
including controls, were killed during the study for humane reasons.
Treatment-related clinical signs observed at concentrations
> 500 ppm consisted of exophthalmia, splayed hindlimbs, loss of
muscle control (in females), staggered gait, and tremors. Animals at
all doses had a dose-related reduction in body-weight gain; the
differences attained statistical significance in males at all doses
but in females only at 1000 ppm. Sporadic reductions in food
consumption were seen in animals at 1000 ppm, particularly males;
females showed increased food consumption. Functional observational
battery testing revealed effects in animals receiving doses
> 500 ppm, including gait impairment, a reduction in hindlimb grip
strength, whole-body tremors, and abnormal posture. Motor activity was
significantly reduced among females receiving 1000 ppm. The single
male at 1000 ppm found moribund on day 83 had calcifications in the
urethra; no other macroscopic treatment-related lesions were found. No
treatment-related histopathological lesions were found in the central
or peripheral nervous system of animals at the high dose (Freeman,
1994). The NOAEL for neurotoxicity was thus 50 ppm, equal to 3 mg/kg
bw per day. There was no NOAEL for systemic toxicity.
(iii) Inhibition of cholinesterase activity
In a comparative study in newborn, weanling, and adult
Sprague-Dawley rats, first evaluated by JMPR in 1980 (Annex 1,
reference 34), the purpose was to determine the time of maximal
inhibition of cholinesterase activity in plasma, erythrocytes, and
brain and the time of recovery after the administration of a single
oral dose of carbofuran. The LD50 values were 8.1 mg/kg bw for
newborn and 7.3 for weanling rats. The baseline cholinesterase
activities in untreated animals were determined at the age of two to
three days for newborns, 27-29 days for weanlings, and 99-101 days for
adult rats. The baseline activities in erythrocytes were lower in
newborns and weanlings than in adults, and those in brain were lower
in newborns than in weanlings and adults. After administration of
single oral doses of carbofuran at 3.2 mg/kg bw for newborns, 2.2
mg/kg bw for weanlings, and 4 mg/kg bw for adults, maximal depression
of erythrocyte activity occurred within 1 h in all groups, and maximal
depression of brain activity occurred within 1 h in weanlings and
adults and after 4 h in neonates. Full recovery was attained within
24 h after dosing, independently of the age of the animals. There were
no differences in sensitivity to cholinesterase inhibition with age
(Becci, 1979).
In a study to investigate the relationship between carbofuran
metabolism in vivo and acetylcholinesterase inhibition, male
Sprague-Dawley rats were given single intravenous or oral doses of
14C carbofuran at 50 µg/kg bw, and urine, faeces, and expired air
were analysed for oxidative and hydrolytic metabolites; blood, plasma,
and various tissues were analysed for carbofuran and its main
oxidative metabolite, 3-hydroxycarbofuran. Erythrocyte acetyl-
cholinesterase activity in vitro was used as an index of toxicity.
From 41 to 47% of the administered dose was recovered as 14C-carbon
dioxide after 8 h, independently of the route of administration. About
15% of the dose was found in urine and < 1% in faeces. 3-Hydroxy-
carbofuran was formed rapidly and underwent enterohepatic circulation,
resulting in an elimination half-life of about 64 min for all tissues;
the elimination half-life of the parent compound was about 29 min.
Rapid recovery of the erythrocyte acetylcholinesterase activity
closely paralleled carbofuran metabolism, and the primary disposition
of 3-hydroxycarbofuran in vivo was by metabolic conjugation
(Ferguson et al., 1984).
3. Observations in humans
Carbofuran was reported to have induced sensitization in a patch
test in 30 farmers with contact dermatitis (Sharma & Kaur, 1990).
Poisoning was reported in three female farmworkers who threw
carbofuran granules onto a coffee plantation in Jamaica. The women did
not wear protective clothing. The signs of poisoning reported included
vomiting, lassitude, nausea, and hypersalivation. Cholinesterase
activity was not determined in these patients (Coleman et al., 1990).
Comments
Carbofuran is rapidly absorbed, metabolized, and eliminated,
mainly in the urine, after oral administration to mice and rats. After
oral administration of [phenyl-14C]carbofuran to rats, 92% of the
radiolabel was eliminated in the urine and 3% in faeces. Most of the
radiolabel was eliminated within 24 h after treatment. With a
[carbonyl-14C]-labelled compound, 45% was eliminated as 14C-carbon
dioxide. The metabolic pathway consists in hydroxylation, oxidation,
hydrolysis, and conjugation.
Carbofuran is highly toxic after acute oral administration. The
oral LD50 values in various species ranged from 3 to 19 mg/kg bw.
Carbofuran had no sensitizing potential in guinea-pigs, and no local
irritation was found in rabbits after repeated dermal applications
over 7 or 21 days. WHO has classified carbofuran as 'highly hazardous'
(WHO, 1996).
In a 13-week study in dogs fed diets providing 0, 10, 70, or
500/250 ppm (dose reduced because of marked toxicity), an NOAEL was
not identified because of inhibition of erythrocyte acetylcholin-
esterase activity and some clinical signs were observed at the lowest
dose. In a subsequent four-week study in dogs, the highest dose
administered was 5 ppm, equal to 0.22 mg/kg bw per day, which was the
NOAEL for clinical signs, mortality, effects on body weight and food
consumption, and cholinesterase activity in plasma and erythrocytes.
In a one-year study in dogs at dietary concentrations of 0, 10, 20, or
500 ppm, the NOAEL was 10 ppm, equal to 0.3 mg/kg bw per day, on the
basis of histopathological testicular changes in a single male at
20 ppm; similar changes were observed in animals at 500 ppm. There
was no inhibition of erythrocyte or brain acetylcholinesterase at
concentrations of 10 or 20 ppm. The overall NOAEL in these short-term
studies in dogs was 5 ppm, equal to 0.22 mg/kg bw per day.
In two-year studies of toxicity and carcinogenicity at dietary
concentrations of 0, 20, 125, or 500 ppm in mice and 0, 10, 20, or
100 ppm in rats, the NOAELs were 20 ppm, equal to 2.8 mg/kg bw per
day, in mice and 20 ppm, equivalent to 1 mg/kg bw per day, in rats, on
the basis of inhibition of erythrocyte and brain acetylcholinesterase
activity. There was no evidence of tumorigenicity.
In a three-generation study of reproductive toxicity in rats at
dietary concentrations of 0, 20, or 100 ppm, the NOAEL was 20 ppm,
equal to 1.6 mg/kg bw per day, on the basis of reduced body-weight
gain in parental animals and reduced pup growth and pup survival at
100 ppm.
In an early study of developmental toxicity, rats were given
carbofuran at doses of 0, 0.1, 0.3, or 1 mg/kg bw per day by gavage.
An NOAEL could not be identified in this study. Dose-dependent,
transient clinical signs (chewing motions) were observed in the dams.
In a later study in rats at oral doses of 0, 0.25, 0.5, or 1.2 mg/kg
bw per day, the NOAEL for maternal and fetal toxicity was 1.2 mg/kg bw
per day, the highest dose tested. In a further study of teratogenicity
in rats, with dietary administration of 0, 20, 60, or 160 ppm
carbofuran, the NOAEL for maternal toxicity was 20 ppm, equal to
1.5 mg/kg bw per day, on the basis of a reduction in body-weight gain
at 60 ppm. The NOAEL for pup toxicity, based on reduced pup weight,
was 60 ppm, equal to 4.4 mg/kg bw per day. None of the studies showed
teratogenic potential.
The results of an early study of teratogenicity in rabbits at
oral doses of 0, 0.2, 0.6, or 2 mg/kg bw per day showed an NOAEL of
0.6 mg/kg bw per day for maternal toxicity on the basis of clinical
signs and an NOAEL of 2 mg/kg bw per day for fetotoxicity and
teratogenicity. In a subsequent study in rabbits at doses of 0, 0.12,
0.5, or 2 mg/kg bw per day, the NOAEL was 0.5 mg/kg bw per day on the
basis of slightly reduced body-weight gain in dams and a slightly
increased incidence of skeletal variations in pups at 2 mg/kg bw per
day. These studies provide no evidence for teratogenicity.
In a 90-day study of neurotoxicity in rats at dietary
concentrations of 0, 50, 500, or 1000 ppm, systemic toxicity
(reduction in body-weight gain) was observed at all doses. Clinical
signs of neurotoxicity were observed at 500 and 1000 ppm. No
histopathological lesions were found in the nervous system.
In a study of developmental neurotoxicity, carbofuran was
administered in the diet of rats to provide concentrations of 0, 20,
75, or 300 ppm from gestation day 6 through lactation day 10.
Reductions in the body-weight gain of dams and pups and in pup
survival and some evidence of delayed pup development were found at
doses > 75 ppm. The NOAEL was 20 ppm, equal to 1.7 mg/kg bw per
day, on the basis of reduced body-weight gain in dams and signs of
fetotoxicity at higher doses.
Carbofuran has been tested for genotoxicity in a wide range of
tests in vivo and in vitro. The Meeting concluded that it is not
genotoxic.
An ADI of 0-0.002 mg/kg bw was allocated on the basis of the
NOAEL for erythrocyte acetylcholinesterase inhibition of 0.22 mg/kg bw
per day in a four-week study in the most sensitive species, the dog,
and using a 100-fold safety factor. The use of a short-term study to
set the ADI is justified because the effect observed was reversible
and acute.
Toxicological evaluation
Levels that cause no toxicological effect
Mouse: 20 ppm, equal to 2.8 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rat: 20 ppm, equivalent to 1 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
20 ppm, equal to 1.2 mg/kg bw per day (three-generation
study of reproductive toxicity)
1.2 mg/kg bw per day (highest dose tested in a study of
developmental toxicity)
20 ppm, equal to 1.5 mg/kg bw per day (study of
developmental toxicity)
20 ppm, equal to 1.7 mg/kg bw per day (study of
developmental neurotoxicity)
Rabbit: 0.6 mg/kg bw per day (study of developmental toxicity)
Dog: 5 ppm, equal to 0.22 mg/kg bw per day (four-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to carbofuran
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 6-14.4 mg/kg bw
Dermal, toxicity, rat LD50 > 500 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.088-0.1 mg/litre
Dermal, irritation, rabbit Not irritating
Ocular irritation, rabbit Not available
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated oral, 4 weeks, toxicity, dog NOAEL = 0.22 mg/kg bw per day
Repeated oral, reproductive toxicity, rat NOAEL = 1.6 mg/kg bw per day, parental
and pup toxicity
Repeated oral (gavage), developmental NOAEL = 1.2 mg/kg bw per day (highest
toxicity, rat dose tested). No evidence of teratogenicity
Repeated oral (feeding), developmental NOAEL = 1.5 mg/kg bw per day, maternal
toxicity, rat toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 0.6 mg/kg bw per day, maternal
toxicity. No evidence of teratogenicity
Repeated oral, developmental neurotoxicity, rat NOAEL = 1.7 mg/kg bw per day
Longterm (> 1 year) Repeated oral, 2 years, carcinogenicity, mouse NOAEL = 2.8 mg/kg bw per day,
cholinesterase inhibition. No evidence
of carcinogenicity
Repeated oral, 2 years, carcinogenicity, rat NOAEL = 1 mg/kg bw per day, reduced
body-weight gain and cholinesterase
inhibition. No evidence of carcinogenicity
References
Barron, P., Giesler, P. & Ras, G.N. (1978) Teratogenicity of
carbofuran in rats. Act-No. 184.33. Unpublished report prepared by
Warf Institute, Inc. Wisconsin, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Becci, G. (1979) Study of cholinesterase activity inhibition by
carbofuran in neonate, weanling and adult rats. Study-No. A 79-343.
Unpublished report prepared by Food and Drug Research Laboratories,
Inc. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Bloch, I., Frei, T.H., Madoerin, K., Luetkemeier, H., Vogel, W.,
Schlotke, B., Vogel, O. & Terrier, C. (1987a) 13-Week oral toxicity
feeding study with carbofuran (D1221) in the dog. RCC-No. 077837 (FMC
Study No. A95-4249). Unpublished report prepared by Research
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by FMC
Corp., Philadelphia, PA, USA.
Bloch, I. Frei, T., Luetkenmeier, H., Vogel, W. & Terrier, C. (1987b)
4-Week oral toxicity (feeding) study with carbofuran 'D 1221' in male
dogs. RCC No. 087963 (FMC Study No. A95-4248). Unpublished report
prepared by Research & Consulting Company AG, Itingen, Switzerland.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Brown, W.R. (1980) 2-Year dietary toxicity and carcinogenicity study
in mice with technical carbofuran. Act No. 150.52 Histopathology Part.
Unpublished report prepared by Research Pathology Services, Inc. PA,
USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Burtner, B.R. (1982) 14-Day oral toxicity (range finding) study in
beagle dogs of carbofuran. (FMC Study No. A81-604, Toxicogenics No.
410-0714). Unpublished report prepared by ToxiGenics, Inc., IL, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Case, R.S. & Wilson, N.H. (1979) Cholinesterase evaluation conducted
during a two year dietary toxicity/oncogenicity study with carbofuran
in rats. Study No. Tox-79-001. Unpublished report prepared for FMC.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Coleman, A.M.E., Smith, A. & Watson, D. (1990) Occupational carbamate
pesticide intoxication in three farm workers. West Indian Med. J.,
39, 109-113.
Courtney, K.D., Andrews, J.E., Springer, J. & Dalley, L. (1985)
Teratogenic evaluation of the pesticides Baygon, carbofuran,
dimethoate and EPN. J. Environ. Sci. Health B, 20, 373-406.
DeGraff, M.G. (1983a) Mutagenicity evaluation of FMC 10242 in the Ames
Salmonella/microsome plate test Study No. A 83-1037). Unpublished
report by Litton Bionetics, Inc., Kensington, MD, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
DeGraff, W.G. (1983b) Mutagenicity evaluation of MC 10242 for the
sex-linked recessive lethal test in Drosophila melanogaster. Study
No. A 83-1060. Unpublished report prepared by Litton Bionetics, Inc.
Kensington, MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Dorough, H.W. (1968) Metabolism of Furadan (NIA-10242) in rats and
houseflies. J. Agric. Food Chem., 16, 319-325.
Elleman, P.N. (1979) Acute oral toxicity (FIFRA-EPA) in rats with
carbofuran technical (FMC Study No. A 79-339, CSE-Study No. 0180).
Unpublished report prepared by Cosmopolitan Safety Evaluation (CSE)
Inc., NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Ellison, T. (1980) Audit validaton of the skin sensitization test on
NIA 10242 Technical. Unpublished report on Study No. A 5300. Submitted
to WHO by FMC Corp., Philadelphia, PA, USA.
Farrow, M.G. (1983a) Salmonella typhimurium/mammalian microsome
plate incorporation assay with compound FMC 10242 (FMC Study-No. A
83-905, HLA No. 331). Unpublished report prepared by Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Farrow, M.G. (1983b) Salmonella typhimurium/mammalian microsome
plate incorporation assay with compound FMC 10242 (FMC Study No. A
83-907, HLA No. 332). Unpublished report prepared by Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Farrow, M.G. (1983c) Salmonella typhimurium/mammalian microsome
plate incorporation assay with compound FMC 10242 (FMC Study No. A
83-909, HLA No. 333). Unpublished report prepared by Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Ferguson, P.W., Dey, M.S., Jewell, S.A. & Krieger, R.I. (1984)
Corbofuran metabolism and toxicity in the rat. Fundam. Appl.
Toxicol., 4, 14-21.
Freeman, C. (1984a) Acute oral toxicity of FMC 18209 technical
(3-hydroxy-carbofuran) in rats. FMC Study No. A 83-1136. Unpublished
report prepared by FMC Toxicology Laboratory, NJ, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Freeman, C. (1984b) Acute oral toxicity of FMC 17481 technical
(3-keto-carbofuran) in rats. FMC Study No. A 83-1137. Unpublished
report prepared by FMC Toxicology Laboratory, NJ, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Freeman, C. (1984c) Acute oral toxicity of FMC 16497 (3-hydroxy-
7-phenol) rats. FMC Study No. A83-1134. Unpublished report Pprepared
by FMC Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Freeman, C. (1984d) Acute oral toxicity of FMC 19841 (3-keto-7-phenol)
in rats. FMC Study No. A83-1135. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Freeman, C. (1984e) Acute oral toxicity of FMC 10272 (7-phenol) in
rats. FMC Study No. A83-1133. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Freeman, C. (1993) Twenty-eight day neurotoxicity range-finding study
in rats with carbofuran technical. FMC No. A 92-3704. Unpublished
report prepared by FMC Corporation, Toxicology Laboratory, NJ, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Freeman, C. (1994) Subchronic neurotoxicity screen in rats with
carbofuran technical. FMC Study No. 92-3705. Unpublished report
prepared by FMC Corporation Toxicology Laboratory, NJ, USA. Submitted
to WHO by FMC Corp., Philadelphia, PA, USA.
Flucke, W. (1986) SRA 12869 (isofenphos) and D 1221 (carbofuran):
Study for combination toxicity. Study No. 134337. Unpublished report
prepared by Bayer AG, Insititute of Toxicology. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Fukuto, T.R. & Metcalf, R.L. (1969) Metabolism of insecticides in
plants and animals. Biological effects of pesticides in mammalian
systems. Ann. NY Acad. Sci., 160, Art. 1.
Georgian, L.I., Moraru, I., Draghicescu, T. & Tarnavschi, R. (1985)
The effect of low concentrations of carbofuran, cholin salt of maleic
hydrazine, propham and chlorpropham on sister-chromatid exchange (SCE)
frequency in human lymphocytes in vitro. Mutat. Res., 147, 296.
Goldenthal, E.I. (1982a) 2-Year dietary toxicity and carcinogenicity
study in mice. FMC Study No. Act No. 150.52 (IRDC No. 167-116).
Unpublished report prepared by International Research and Development
Corporation, MI, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Goldenthal, E.I. (1982b) 2-Year dietary toxicity and carcinogenicity
study in rats. FMC Study No. Act No. 130.51 (IRDC No. 167-115).
Unpublished report prepared by International Research and Development
Corporation, MI, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Goldenthal, E.I. (1982c) Three generation reproduction study in rats.
FMC Study No. Act. No. 131.53 (IRDC No. 167-114). Unpublished report
prepared by International Research and Development Corporation, MI,
USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983a) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-868 (MBA Study No. 1921.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. 1983b) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-913 (MBA Study No. 1934.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983c) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
Technical. FMC Study No. A 83-948 (MBA Study No. 1938.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983d) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-949 (MBA Study No. 1936.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983e) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-950 (MBA Study No. 1935.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983f) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-951 (MBA Study No. 1937.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983g) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-973 (MBA Study No. 1982.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983h) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-1063. Unpublished report prepared by
Microbiological Associates, MD, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Hoffman, S.L. & Robinson, R.A. (1994a) Metabolism of C14-carbofuran
in laying hens. XBL Study No. XBL 92051; FMC Study No. 078 POU 92M1.
Unpublished report prepared by XenoBiotic Laboratories, Inc.,
Plainsboro, NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Hoffman, S.L. & Robinson, R.A. (1994b) Metabolism of C14-carbofuran
in lactating goats. XBL Study No. XBL 92051; FMC Study No. 078 GOA
92M1. Unpublished report prepared by XenoBiotic Laboratories, Inc.,
Plainsboro, NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Kedderis, L.B. (1985a) Seven-day repeated dose dermal toxicity
range-finding study in rabbits with FMC 10242 technical (carbofuran).
Study No. A 85-1645. Unpublished report prepared by FMC Corporation.
NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kedderis, L.B. (1985b) Twenty-one day repeated dose dermal toxicity
range-finding study in rabbits with FMC 10242 technical (carbofuran).
Study No. A 85-1678. Unpublished report prepared by FMC Corporation,
NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kirby, P.E. (1983a) L 5178Y TK+/- mouse lymphoma mutagenesis assay.
FMC Study No. A 83-962 and A 83-988 (MBA Study No. T1982.701).
Unpublished report prepared by Microbiological Associates, Bethesda,
MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kirby, P.E. (1983b) L5178Y TK+/- mouse lymphoma mutagenesis assay.
FMC Study No. A 83-1064 (MBA Study No. T2124.201). Unpublished report
prepared by Microbiological Associates, Bethesda, MD, USA. Submitted
to WHO by FMC Corp., Philadelphia, PA, USA.
Knaak, J.B., Munger, D.M. & McCarthy, J.F. (1969) The metabolism of
carbofuran alfalfa residues in the rat. Abstracts of Papers, 158th
National Meeting of the American Chemical Society, 7-12 September.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kuhr, R.J. & Dorough, H.W. (1976) Carbamate Insecticides: Chemistry,
Biochemistry and Toxicology, Cleveland, OH, CRC Press. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Laveglia, J. (1981) Teratology study in the rabbit with carbofuran.
FMC Study No. A 80-452 (IRDC No. 167-156). Unpublished report prepared
by International Research and Development Corporation, MI, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Mehta, C.S. (1981) Rabbit acute dermal toxicity. FMC Study No. A
81-564 (Stillmeadow No. 2142.81). Unpublished report prepared by
Stillmeadow, Inc., TX, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Metcalf, R.L., Fukuto, T.R., Collins, C., Borck, K., Abd El-Aziz, S.,
Munoz, R. & Cassil, C.C. (1968) Metabolism of 2,2-dimethyl-
2,3-dihydrobenzofuranyl-7 N-methylcarbamate (Furadan) in plants,
insects and mammals. J. Agric. Food Chem., 16, 300-311.
Mihail, F. (1981) D1221 (carbofuran) and Dacamox (thiofanox): Acute
combination toxicity study. Study No. 10280. Unpublished report
prepared by Bayer AG, Institute of Toxicology. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Moriya, M., Ohta, K., Watanabe, T., Miyazawa, K., Kato, K. & Shirasw
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Norwell, M.J. (1983a) Acute oral toxicity of FMC 10242, technical, in
rats. FMC Study No. A 83-1101. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Norwell, M.J. (1983b) Acute oral toxicity of FMC 10242, technical, in
rats. FMC Study No. A 83-1102. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Ponnock, K.S. (1994) A developmental neurotoxicity study of carbofuran
in the rat via dietary administration. Study No. 93-4506, FMC Study
No. A 93-3746. Unpublished report prepared by Pharmaco LSR, Inc.
Toxicology Services North America, NJ, USA. Submitted to WHO by FMC
Corp., Philadelphia, PA, USA.
Putman, D.L. (1983a) Activity of FMC 10242 in the in vivo cytogenetics
assay in Sprague-Dawley rats. FMC Study No. A 83-972 (MBA No.
T1982.102). Unpublished report prepared by Microbiological Associates,
Bethesda, MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Putman, D.L. (1983b) Activity of FMC 10242 in the subchronic in vivo
cytogenetics assay in male rats. Study No. A 83-1065 (MBA No.
T2124.102). Unpublished report prepared by Microbiological Associates,
Bethesda, MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Rao, G.N. (1978) Teratology study of carbofuran in rabbits. Study No.
185-33. Unpublished report prepared by Warf Institute, Inc. WI, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Rapp, W.R. (1980a) 24 Month dietary toxicity/carcinogenicity study of
carbofuran in rats. Act No. 130.51. Pathology report. Unpublished
report prepared by Wm. R. Rapp & Co. Inc., Allied Services for
Research, NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Rapp, W.R. (1980b) A three generation reproduction study of carbofuran
in rats. FMC Act No. 131.531. Pathology report. Unpublished report
prepared by Wm. R. Rapp & Co. Inc., Allied Services for Research, NJ,
USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Rodwell, D.E. (1980a) Teratology study in the rat with carbofuran.
(FMC Study No. A 80-453, IRDC No. 167-155). Unpublished report
prepared by International Research and Development Corp., MI, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Rodwell, D.E. (1980b; amended 1985) Pilot teratology study in the rat
with carbofuran in the diet (FMC Study No. A 80-443, IRDC No.
167-153). Unpublished report prepared by International Research and
Development Corp., MI, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Rodwell, D.E. (1981; with amendment) Teratology and postnatal study in
the rat with carbofuran (FMC Study No. A 80-444, IRDC No. 167-154).
Unpublished report prepared by International Research and Development
Corp., MI, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Sabol, R.J. (1979a) Rat acute oral toxicity with carbofuran technical.
Study No. 1083-79; FMC Study No. ACT 267.01-01. Unpublished report
prepared by Stillmeadow, Inc., TX, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Sabol, R.J. (1979b) Rat acute oral toxicity with furadan insecticide,
carbofuran 96.6%. Study No. 11231-79; FMC Study No. ACT 267.01-02
(01). Unpublished report prepared by Stillmeadow, Inc., TX, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Schoenig, G. (1967) Skin sensitization test on NIA 10242 technical.
IBT Study No. A 5300. FMC Study No. NCT 176.36. Unpublished report
prepared by Industrial Bio Test Laboratory, Inc, IL, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Sharma, V.K. & Kaur, S. (1990) Contact sensitization by pesticides in
farmers. Contact Derm., 23, 77-80.
Signorin, J. (1995) DOT acute inhalation toxicity study in rats. FMC
Study No. A 94-4097. Unpublished report prepared by FMC Corp.,
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Simmon, U.F. (1979) In vitro microbiological mutagenicity and
unscheduled DNA synthesis studies of eighteen pesticides. Study No.
68-01-2458 (FMC Study No. A81-509, EPA 600/1-79-041). Report prepared
by SRI International, Menlo Park, California, for Health Effects
Research Laboratory Office of Research and Development. US
Environmental Protection Agency, Research Triangle Park, NC, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Stehrer-Schmid, P. & Wolf, H.U. (1995) Effects of benzofuran and seven
benzofuran derivatives including four carbamate insecticides in the in
vitro porcine brain tubulin assembly assay and description of a new
approach for the evaluation of the test data. Mutat. Res., 339,
61-72.
Taylor, G.D. (1983) One-year chronic oral toxicity study in beagle
dogs with carbofuran. FMC study No. A 81-605 (Toxicogenics No.
410-0715). Unpublished report prepared by ToxiGenics, Inc., IL, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Thilagar, A. (1983a) Unscheduled DNA synthesis in rat primary
hepatocytes. FMC Study No. A 83-969 (MBA No. T1982.380). Unpublished
report prepared by Microbiological Associates, Bethesda, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Thilagar, A. (1983b) Sister chromatid exchange assay in Chinese
hamster ovary (CHO) cells. FMC Study No. A 83-1095 (MBA No.
1982.334001). Unpublished report prepared by Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Thilagar, A. (1983c) Sister chromatid exchange assay in Chinese
hamster ovary (CHO) cells. FMC Study No. A 83-1097 (MBA No.
2124.334001). Unpublished report prepared by Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Thilagar, A. (1983d) Chromosome aberrations in Chinese hamster ovary
(CHO) cells. FMC Study No. A 83-1096. Unpublished report prepared by
Microbiological Associates, Bethesda, MD, USA. Submitted to WHO by FMC
Corp., Philadelphia, PA, USA.
Valencia, R. (1981) Mutagenesis screening of pesticides using
Drosophila. FMC Study No. A 83-1042 (EPA 600/1-81-017). Report
prepared by Warf Institute Inc., Madison, WI, for Health Effects
Research Laboratory. Office of Research and Development, US
Environmental Protection Agency, Research Triangle Park, NC, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Valencia, R. (1983) Drosophila sex-linked recessive lethal assay
of 2,3-dihydro-2,2-dimethyl-7-benzofuranyl- N-methyl carbamate
(carbofuran). FMC Study No. A 83-1019 (MBA No. T1047.160). Unpublished
report prepared by Microbiological Associates, Bethesda, MD and the
University of Wisconsin, Zoology Department, USA. Submitted to WHO by
FMC Corp., Philadelphia, PA, USA.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
Woijcieckowski, J.P., Kaur, P. & Sabharwal, P.S. (1982) Induction of
ouabain resistance in V-79 cells by four carbamate pesticides.
Environ. Res., 29, 48-53.
[14C-phenyl ring]Carbofuran was administered in capsules to
laying hens once a day for seven consecutive days at doses equivalent
to dietary concentrations of 0 and 25 ppm. Carbofuran was extensively
metabolized and rapidly eliminated, resulting in low residues in eggs
and tissues. Muscle tissue and fat contained < 0.01 ppm; kidney,
liver, and eggs contained concentrations of < 0.2 ppm carbofuran
equivalents. Conjugates formed after oxidative and hydrolytic
reactions were further conjugated to their corresponding sulfate
conjugates and appeared to bind with biomolecules (e.g. proteins) to
form nonextractable residues (Hoffman & Robinson, 1994a).
[14C-phenyl ring]Carbofuran was administered in gelatine
capsules to lactating goats once a day for seven consecutive days at
doses equivalent to dietary concentrations of 0 and 25 ppm. Carbofuran
was extensively metabolized and readily eliminated. Muscle and fat
contained < 0.01 ppm; kidney, liver, and milk contained 0.09-0.39
ppm. Metabolites occurred in nonconjugated and conjugated forms, the
latter being further conjugated to the sulfate conjugates, which bind
to biomolecules and result in nonextractable (bound) residues (Hoffman
& Robinson, 1994b).
2. Toxicological studies
(a) Acute toxicity
The results of numerous studies of the acute toxicity of
carbofuran in rats were evaluated previously (Annex I, reference 26)
and are summarized in Table 1. The oral LD50 values for rats were
6-18 mg/kg bw. The toxic signs observed were typical of cholinesterase
inhibition: salivation, cramp, trembling, and sedation were observed
within minutes after administration and lasted for up to three days.
Additional studies that have become available since the first
evaluation give oral LD50 values for technical-grade carbofuran in
rats of about 5 mg/kg bw in females and 14 mg/kg bw in males (Elleman,
1979; Sabol, 1979a, b; Norvell, 1983a,b). The dermal LD50 in rabbits
for carbofuran of a purity of 96.1% was > 2000 mg/kg bw (Mehta,
1981). In rats treated by inhalation with a carbofuran formulation
(Furadan 95), the LC50 value was 0.11 mg/litre for a 1-h exposure
(Signorin, 1995).
The results of studies on the acute toxicity of several
metabolites of carbofuran are summarized in Table 2.
Studies of combined treatment with carbofuran and thiofanox or
carbofuran and isofenphos revealed no potentiating effects. Additive
or even subadditive toxic effects were found after simultaneous
administration of the test compounds (Mihail, 1981; Flucke, 1986).
Table 1. Acute toxicity of carbofuran
Species Sex Route LD50 (mg/kg bw) Reference
or LC50 (mg/litre)
Mouse Oral 14.4 Kimmerle (1966)
Rat Male Oral 18.0 Kimmerle (1966)
Rat Male Oral 7.8 Grönning & Kimmerle (1974)
Rat Male Oral 11.9 Kohn (1967a)
Rat Female Oral 6.0 Grönning & Kimmerle (1974)
Rat Female Oral 13.8 Kimmerle (1966)
Rat Male and female Oral 14.1 Powers (1964)
Rat Male Intraperitoneal 8.2 Kimmerle (1966)
Rat Female Intraperitoneal 2.8 Kimmerle (1966)
Rat Male and female Intraperitoneal 1.4 Kohn (1967b)
Rat Male and female Dermal > 500 Kimmerle (1966)
Rat Male Inhalation (1 h) 0.108 Kimmerle (1966)
Rat Male Inhalation (1 h) 0.091 Grönning & Kimmerle (1974)
Rat Male Inhalation (4 h) 0.088 Kimmerle (1966)
Rat Female Inhalation (1 h) 0.080 Grönning & Kimmerle (1974)
Rat Female Inhalation (4 h) 0.075-0.11 Grönning & Kimmerle (1974)
Guinea-pig NR Oral 9.2 Kimmerle (1966)
Rabbit NR Oral 7.5 Kimmerle (1966)
Cat NR Oral 2.5-3.5 Kimmerle (1966)
Dog NR Oral 15-19 Kimmerle (1966)
Table 2. Acute oral toxicity of metabolites of carbofuran in rats
Metabolite LD50 Reference
(mg/kg bw)
3-Hydroxycarbofuran 21.9 (male) Freeman (1984a)
8.3 (female)
3-Ketocarbofuran 108 (male) Freeman (1984b)
93.1 (female)
3-Hydroxy-7-phenol 1916 (male) Freeman (1984c)
1654 (female)
3-Keto-7-phenol > 800 Freeman (1984d)
7-Phenol 2450 (male) Freeman (1984e)
1743 (female)
(b) Short-term toxicity
Rabbits
In a range-finding study, groups of rabbits were treated dermally
with carbofuran (technical-grade; purity, 96.9%) at doses of 0, 100,
300, or 1000 mg/kg bw per day over seven days; the contact time was
6 h/day. No clinical signs of toxicity and no local irritation
were observed. The treatment did not affect body weight or food
consumption. A 30% depression in plasma cholinesterase activity was
measured in males at 100 and 300 mg/kg bw per day and a 47% reduction
at 1000 mg/kg bw per day. Brain acetylcholinesterase activity was
reduced by 26% in males at 100 mg/kg bw per day, 49% in those at 300
mg/kg bw per day, and 54% in those at 1000 mg/kg bw per day. These
reductions were not statistically significant (Kedderis, 1985a).
New Zealand white rabbits received applications of carbofuran
(technical-grade; purity, 96.9%) for about 6 h/day at doses of 0, 10,
100, or 1000 mg/kg bw per day for 21 consecutive days. No signs
of toxicity and no effects on body weight, food consumption,
haematological or clinical chemical parameters, organ weights,
or gross or histopathological appearance were observed. Brain
acetylcholinesterase activity was reduced by 21% in males at 100 mg/kg
bw per day and 26% in those at 1000 mg/kg bw per day. The reductions
were not statistically significant. The NOAEL was 10 mg/kg bw per day
(Kedderis, 1985b).
Dogs
In a 14-day range-finding study, single beagle dogs were fed
diets designed to provide carbofuran (technical-grade; purity, 96.1%)
at concentrations of 0, 18, 32, 56, 100, 316, or 1000 ppm, equivalent
to 0, 0.45, 0.8, 1.4, 2.5, and 8 mg/kg bw per day. The animal that
initially received 18 ppm was changed to a dose of 1000 ppm on day 4
of the study. At this dose, body weight loss and depressed food intake
were observed, and clinical signs included muscle tremor, emesis, and
salivation. Although cholinesterase activity was measured in plasma
and erythrocytes, dose-related depression was found only in plasma, at
all doses; inhibition ranged from 16% (within the historical control
range) in animals at 18 ppm to < 92% at 1000 ppm (Burtner 1982).
In a 13-week feeding study, carbofuran (purity, 99.6%) was
administered in the diet to groups of four male and four female
beagle dogs to provide concentrations of 0, 10, 70, or 500/250 ppm,
equivalent to 0, 0.43, 3.1, and 10.6 mg/kg bw per day. Two additional
animals of each sex per group were assigned to recovery groups to
study the reversibility of the effects over a four-week period. The
feed concentration of 500 ppm was reduced to 250 ppm from treatment
day 6 onwards because of marked toxicity: one dog at this dose died on
day 5 of treatment due to a treatment-related invagination of the
jejunum. At concentrations > 10 ppm, hyperaemia (e.g. at ear pinnae
and oral mucous membranes) and increased salivation were observed.
Clinical signs at the highest dose consisted of muscular spasms,
ataxia (pronounced in males during the first week of dosing but
sporadic thereafter), decreased motility, tachypnoea and deep
respiration, and vomiting. At 500 ppm, food consumption was markedly
reduced and loss of body weight was observed; reduction of the dose to
250 ppm resulted in recovery of food consumption and body-weight gain.
The treatment had no effect on ophthalmoscopic, electrocardiographic,
haematological, or clinical chemical parameters but inhibited
cholinesterase activity and caused alterations in organ weights and in
gross and histopathological appearance. Dose-related inhibition of
plasma and erythrocyte cholinesterase activity was observed in all
treated groups. Maximal inhibition occurred on test day 1, when the
plasma activity was 70, 27, and 13% of the control activity at 10, 70,
and 500/250 ppm and the erythrocyte activity was 68, 29, and 11% of
the control, respectively. The plasma and erythrocyte cholinesterase
activities returned to normal during the four-week recovery period.
There was no inhibition of brain acetylcholinesterase activity by the
end of the treatment or recovery period. An NOAEL was not determined
because inhibition of erythrocyte acetylcholinesterase and increased
salivation were seen at the lowest dose tested (Bloch et al., 1987a).
This study was followed by a four-week feeding study in order to
establish a NOAEL with respect to clinical signs and cholinesterase
inhibition: Groups of four male beagle dogs were fed diets providing
concentrations of 0 or 5 ppm carbofuran (purity, 99.6%) for four
weeks. The treatment had no effects on clinical signs, mortality, body
weight, food consumption, or plasma or erythrocyte cholinesterase
activity. The NOAEL was 5 ppm, equal to 0.22 mg/kg bw per day (Bloch
et al., 1987b).
In an earlier one-year study, groups of six male and six female
beagle dogs were given diets providing concentrations of 0, 10, 20, or
500 ppm carbofuran (technical-grade; purity, 96.1%), equal to 0,
0.3, 0.6, and 13 mg/kg bw per day. At 500 ppm, body weight losses
associated with emesis were observed, particularly in males, and from
the fifth month these animals were fed a supplemented control diet.
One male at 500 ppm died. Muscle tremor and salivation occurred
sporadically at this dose, and all males showed marked depression of
the haematocrit, haemoglobin values, and erythrocyte count from five
months. Only transient, much less pronounced depressions of these
parameters were observed in females at the high dose. Plasma
cholinesterase activity was inhibited in most males at 10 and
20 ppm and markedly (77%) in all animals at 500 ppm. Erythrocyte
acetylcholinesterase activity was reduced by 21-27% in many males
at 500 ppm. At the end of the study, a 24% depression in brain
acetylcholinesterase activity was observed in males at 500 ppm,
whereas females at this dose showed a 44% increase. No relevant
inhibition of erythrocyte or brain acetylcholinesterase activity was
seen in animals at 10 or 20 ppm. The absolute brain and heart weights
of males at 500 ppm were depressed, but no macroscopic or microscopic
changes were found in these organs. Gross treatment-related lesions
observed in the animals at 500 ppm consisted of a marked loss of body
fat and alopecia. Histopathological examination revealed an increased
amount of hepatocellular endoplasmic reticulum in the treated dogs,
but no clear dose-response relationship was seen. Degeneration of the
seminiferous tubules, giant-cell formation, or aspermia was observed
in males at 500 ppm and in a single male at 20 ppm. Minimal-to-
moderate inflammatory changes of the lung were observed at a higher
incidence in the dogs at 500 ppm. The NOAEL was 10 ppm, equal to
0.3 mg/kg bw per day, on the basis of the histopathological changes
in the testes of one male at 20 ppm (Taylor, 1983).
(c) Long-term toxicity and carcinogenicity
Mice
In a two-year study first evaluated by the 1980 JMPR (Annex 1,
reference 34), groups of 100 male and 100 female Charles River CD-1
mice were fed diets providing concentrations of 0, 20, 125, or 500 ppm
carbofuran (technical-grade; purity, 95.6%), equal to 0, 2.8, 18,
and 70 mg/kg bw per day. The treatment had no effect on general
appearance, mortality, or urinary parameters. Body-weight gain was
slightly reduced in single males at 125 ppm and in most animals at
500 ppm during the first year of the study; however, a 20% reduction
in body-weight gain in comparison with the controls was observed in
females at 500 ppm at the end of the study. Food consumption was also
sporadically reduced in animals at the highest dose, particularly
during the first months of the study. Haematological and clinical
chemical examinations revealed no treatment-related changes, except
for inhibition of brain acetylcholinesterase activity; cholinesterase
activities were not measured in erythrocytes or plasma. A
statistically significant depression of brain acetylcholinesterase
activity was observed in animals at 125 and 500 ppm. Maximal
inhibition at 125 ppm was 31% of the activity in concurrent controls;
at 500 ppm, the maximal depression was 55%. Organ weights were changed
sporadically but were not related to treatment. No gross pathological
findings were observed that were indicative of a relationship with
treatment. Microscopic examination revealed no treatment-related
change and no evidence of a compound-related increase in tumour
incidences. The NOAEL was 20 ppm, equal to 2.8 mg/kg bw per day, on
the basis of inhibition of brain acetylcholinesterase activity at
125 ppm. The conclusions of the earlier evaluation were therefore
confirmed (Brown, 1980; Goldenthal, 1980, 1982a).
Rats
In a study reviewed by the JMPR in 1979 and 1980 (Annex 1,
references 32 and 34), carbofuran (technical-grade; purity, 95.6%) was
administered in the diet to groups of 90 male and 90 female Charles
River CD rats to provide concentrations of 0, 10, 20, or 100 ppm, for
two years. Groups of 10 animals of each sex per dose were killed at 6,
12, and 18 months, and blood and brain samples were collected for
determination of cholinesterase activity. The treatment did not affect
general appearance, mortality, food consumption, or ophthalmoscopic,
haematological, or urinary parameters. Body-weight gain was reduced in
animals of each sex at 100 ppm. Clinical chemical examinations
revealed statistically significant inhibition of cholinesterase
activity in plasma, erythrocytes, and brain in rats at 100 ppm. The
maximal reduction of cholinesterase activity in males was 37% in
plasma, 24% in erythrocytes, and 25% in brain; the corresponding
percentages in females at 100 ppm were 26% in plasma, 19% in
erythrocytes, and 43% in brain. No relevant inhibition of
acetylcholinesterase activity was seen in erythrocytes or brain of
animals at 10 or 20 ppm (Case & Wilson, 1979). Various changes in
organ weights were considered to be due to changes in body weight. No
treatment-related gross pathological or histopathological changes were
observed, and there was no evidence of tumorigenicity. The NOAEL was
20 ppm, equivalent to 1 mg/kg bw per day, on the basis of reduced body
weight gain and acetylcholinesterase inhibition in erythrocytes and
brain at the higher dose. The conclusions of the earlier evaluation
were thus confirmed (Goldenthal, 1979a; Rapp, 1980a; Goldenthal,
1982b).
(d) Reproductive toxicity
Rats
In a three-generation study first evaluated by the 1980 JMPR
(Annex 1, reference 34), groups of Charles River CD rats (10 males and
20 females per group for the first and second generations; 12 males
and 24 females per group for the third generation) were maintained on
a diet providing concentrations of 0, 20, or 100 ppm carbofuran
(purity, 95.6%), equal to 0, 1.2, and 6 mg/kg bw per day for males and
0, 1.9, and 9.7 mg/kg per day for females. Each generation was mated
twice to produce two litters. The treatment did not affect the general
behaviour, appearance, or survival of the parental rats. Parental
body-weight gain was lower at 100 ppm throughout the course of
treatment and was associated with lower food consumption, particularly
in males. Fertility indices were not adversely affected. The survival
of pups of the F1a, F2a, and F3a generations at 100 ppm was reduced
at lactation day 4 in comparison with the control. Dehydration was
seen in pups of some litters of the F3a and F3b generations at
100 ppm. The mean weights of pups at 100 ppm were lower than those of
the controls on day 21 post partum in all generations. No compound-
related gross pathological lesions were seen at necropsy in the F0,
F1, or F2 parental animals or the F2b or F3b litters. Various organ
weights of animals of the F2 parental and F3b pup generations at
20 and 100 ppm were statistically significantly changed. Because
clear dose-response relationships were not always seen and no
histopathological alterations were associated with these changes, the
findings were considered not to be of biological significance. The
NOAEL was 20 ppm, equal to 1.2 mg/kg bw per day, on the basis of
reductions in body-weight gain in the parental generation and
reductions in the growth and survival of pup generations at
100 ppm. Thus, the earlier evaluation of this study was confirmed
(Goldenthal, 1979b; Rapp, 1980b; Goldenthal, 1982c).
(e) Developmental toxicity
Rats
In a study first evaluated by the 1980 JMPR (Annex 1, reference
34), carbofuran (technical-grade; purity, 95.6%) was administered to
groups of 24 pregnant Charles River rats by intubation in 0.25%
carboxymethylcellulose at doses of 0, 0.1, 0.3, or 1 mg/kg bw per day
on days 6-15 of gestation. Transient, dose-dependent clinical signs
(chewing motions) were observed at doses > 0.1 mg/kg bw per day for
a short period after treatment. Overt signs of toxicity in animals at
0.3 mg/kg bw per day included rough coats and lethargy; at the high
dose, lacrimation, increased salivation, trembling, and convulsions
were also seen. One female at the high dose died. The treatment had no
effect on the body weights of dams or pups. Reproductive parameters
(e.g. numbers of corpora lutea, implantations, and resorptions and
litter size) were not adversely affected by treatment. Skeletal
examination revealed a higher incidence of some variations in
ossification of sternebrae in treated animals but with no clear
dose-response relationship. Similar alterations were prevalent in the
control group at an incidence of 47% of fetuses. There was no evidence
of teratogenicity. An NOAEL was not determined because transient
clinical signs were observed at the lowest dose (Barron et al., 1978).
Groups of 25 pregnant Charles River COBS CD rats were treated
with carbofuran (purity, 95.6%) by gavage in corn oil at daily doses
of 0, 0.25, 0.5, or 1.2 mg/kg bw on days 6-15 of gestation. The
treatment had no effect on the general appearance, behaviour,
mortality, or body weights of the dams. No differences between groups
were noted in reproductive parameters such as the numbers of corpora
lutea, implantations, and resorptions, litter size, or fetal body
weight. No evidence of teratogenicity was seen (Rodwell, 1980a).
In a pilot study, groups of 10 pregnant Charles River COBS rats
were fed carbofuran (purity, 95.6%) in the diet to give concentrations
of 0, 20, 60, 120, 160, or 200 ppm, equal to 0, 1.5, 4, 8, 11, and
13 mg/kg bw per day, on gestation days 6-19. Neither survival nor
behaviour was affected by the treatment. Hair loss, soft stools,
and scabbing were seen at higher incidences in the treated than
the control group. A dose-related loss in mean maternal body
weight occurred during the first two days of treatment with doses
> 60 ppm, resulting in reduced body-weight gains throughout the
treatment period in animals at 120, 160, and 200 ppm. A dose-related
decrease in food consumption was observed at doses > 60 ppm at the
beginning of the treatment period. Reproductive parameters (litter
size and the numbers of resorptions, implantations, and corpora lutea)
were not adversely affected. On the basis of these results, doses of
0, 20, 60, and 160 ppm were selected for the main study (Rodwell,
1980b, 1985).
The main study was conducted with groups of 40 pregnant Charles
River COBS rats fed diets giving concentrations of 0, 20, 60, or 160
ppm carbofuran (purity, 95.6%), equal to 0, 1.5, 4.4, and 11 mg/kg bw
per day, on gestation days 6-19. Treatment had no effect on appearance
or behaviour, except for a slight increase in hair loss, matting of
the ventral haircoat, and soft stools, particularly in females at 60
and 160 ppm. A dose-related reduction in body-weight gain was seen at
doses > 60 ppm during the treatment period, with single instances
of body-weight loss in animals at 60 and 160 ppm at the beginning of
treatment. Increases in body-weight gain of treated animals during the
subsequent lactation period resulted in very similar body weights in
all groups at the end of lactation. Food consumption was reduced
during the first few days of treatment in animals at 60 and 160 ppm.
No differences between groups were observed in reproductive parameters
(such as the numbers of corpora lutea, implantations, and resorptions
and litter size). The mean weights of pups at 160 ppm were decreased
throughout lactation. The incidence of malformations was not
increased. The NOAEL for maternal toxicity was 20 ppm, equal 1.5 mg/kg
bw per day, on the basis of reduced body-weight gain of the dams at
doses > 60 ppm. The NOAEL for pup toxicity was 60 ppm, equal to 4.4
mg/kg bw per day, on the basis of reduced weights of pups at 160 ppm
(Rodwell, 1981).
Groups of 24 mated female Sprague-Dawley (CD) rats were given
diets containing carbofuran (purity, 99.1%) to provide concentrations
of 0, 20, 75, or 300 ppm, equal to 0, 1.7, 5, and 20 mg/kg bw per day,
from gestation day 6 through lactation day 10. Physical observations
were made, and body weight and food consumption were measured for all
maternal animals at selected intervals throughout the gestation and
lactation periods. The numbers of live pups were recorded, and litter
parameters such as pinna detachment, incisor eruption, eye opening,
motor activity, auditory startle response, and brain weights were
evaluated. Neuropathological examinations were performed on six pups
of each sex per group that were killed on postnatal days 11 and 60.
Maternal animals were killed after weaning of the last litter. No
deaths occurred during the study. Reduced body-weight gain was seen in
dams at 75 and 300 ppm during gestation and also during the lactation
period for those at 300 ppm. Food consumption was reduced in dams at
these two doses at various periods during gestation. Treatment had no
effect on pregnancy rates, length of gestation, or litter size, and
there was no evidence of prolonged or difficult delivery. Treatment at
300 ppm resulted, however, in a greater number of dead pups at birth
than among the controls (not statistically significant). A dose-
dependent reduction in pup weight was observed in animals at 75 and
300 ppm throughout the lactation period, and the reductions persisted
after weaning. Pup survival was adversely affected by prenatal
administration of carbofuran at 75 or 300 ppm on lactation day 4 until
weaning at lactation day 21. Treatment at doses of 75 or 300 resulted
in developmental delays of three to four days in vaginal patency and
preputial separation; marginal delays were seen with respect to pinna
detachment, lower incisor eruption, and eye opening. No adverse effect
was noted on auditory startle response, motor activity, or swimming
development, but there was an angle reduction at doses > 75 ppm.
Brain weights were not affected by treatment. No treatment-related
macroscopic findings were observed in dams or pups. Microscopic
examinations of pups in the control and high-dose groups revealed no
compound-related abnormality. The NOAEL was 20 ppm, equal to 1.7 mg/kg
bw per day, on the basis of reduced body-weight gain of dams and pups,
reduced pup survival, and slight developmental delay at doses > 75 ppm
(Ponnock, 1994).
Rabbits
In a study first evaluated by the JMPR in 1980 (Annex 1,
reference 34), groups of 17 pregnant New Zealand white rabbits were
treated by intubation with carbofuran (purity, 95.6%) at doses of 0,
0.2, 0.6, or 2 mg/kg bw per day on gestation days 6-18. The deaths of
five females at 2 mg/kg bw per day were considered to be related to
treatment. Signs of maternal toxicity and behavioural changes in
animals at this dose included trembling, loss of muscle control,
salivation, sneezing, chewing motions, and reduced food and water
consumption. Body weight was not affected by the treatment. No
differences between groups were seen in reproductive parameters.
Macroscopic examination revealed no treatment-related tissue
alteration and no evidence of embryotoxicity or teratogenicity, even
at maternally toxic doses. The NOAEL was 0.6 mg/kg bw per day, on the
basis of maternal toxicity. The same conclusion was drawn by the 1980
JMPR (Rao, 1978 [cited incorrectly as Felton, 1978, in Annex 1
reference 35]).
Carbofuran (purity, 95.6%) was administered by gavage at doses of
0, 0.12, 0.5, or 2 mg/kg bw per day on gestation days 6-18 to groups
of 20 pregnant New Zealand white rabbits. One dam at 2 mg/kg bw per
day died of an unknown cause. Matting and/or staining of the haircoat
was seen at a somewhat higher frequency in the treated animals.
Although the mean maternal body weight was reduced during treatment,
no clear dose-response relationship was seen. A marked reduction in
body-weight gain was observed at 2 mg/kg bw per day early in the
treatment; no data were available on food consumption. Reproductive
parameters (numbers of corpora lutea, implantations, and resorption,
litter size, fetal body weight were not affected by treatment. A
slightly increased incidence of misaligned sternebrae was seen in
fetuses at 2 mg/kg bw per day. The NOAEL was 0.5 mg/kg bw per day, on
the basis of reduced body weight in dams at 2 mg/kg bw and the
increase in skeletal variations (Laveglia, 1981).
Carbofuran was given to CD rats by gastric intubation at doses of
0.05-5 mg/kg bw per day on days 7-19 of gestation and to CD-1 mice at
doses of 0.1-20 mg/kg bw per day on gestation days 6-16. At 1, 3, and
5 mg/kg bw per day, 40-55% of the rats died, and the numbers of
implantation sites and live fetuses were reduced. The frequency of
malformations was not increased. In mice at 10 or 20 mg/kg bw per day,
about 50% of dams died, and fetal body weight was reduced. A shift in
the rib profile (decreased incidence of 13 ribs, increased incidence
of 14 ribs) was observed at 10 and 20 mg/kg bw per day. There was no
evidence of teratogenicity in either rats or mice (Courtney et al.,
1985).
(f) Genotoxicity
The results of tests for the genotoxicity of carbofuran are
summarized in Table 3. Carbofuran was included among other carbamate
insecticides tested in vitro in the porcine brain tubulin assembly
assay for the detection of action as a microtubule poison and
aneuploidy inducer (Stehrer & Wolf, 1995). Carbofuran induced a
dose-dependent reduction in the degree of polymerization of tubulin
and an 11% reduction in the maximal polymerization velocity. It was
active in S. typhimurium strains TA1538 and TA98 in the presence and
absence of an exogenous metabolic activation system (Moriya et al.,
1983), induced sister chromatid exchange in human peripheral
lymphocytes (Georgian et al., 1985), and induced gene mutation in
V79 hamster cells (Woijciechowski et al., 1982).
(g) Special studies
(i) Dermal sensitization
Guinea-pigs were given 10 intracutaneous injections of
technical-grade carbofuran as an inducer, followed by a final
challenge injection. Dinitrochlorobenzene was used as a positive
control. Carbofuran was not sensitizing (Schoenig, 1967; Ellison,
1980).
(ii) Neurotoxicity
Rats
In a 28-day range-finding study, groups of five male and five
female Sprague-Dawley CD rats were maintained on a diet providing
technical-grade carbofuran (purity, 98.6%) at concentrations of 0, 50,
200, 500, 1000, 3000, or 6000 ppm, equivalent to 0, 2.5, 10, 25, 50,
150, and 300 mg/kg bw per day. Two males receiving 6000 ppm died.
Dose-related clinical signs that were noted at doses > 200 ppm in
animals of each sex consisted of exophthalmia, splayed hindlimbs in
females at 200 ppm, tremors and staggered gait at 500 and 1000 ppm,
and loss of muscle control and ataxia at 3000 and 6000 ppm. Treatment-
related clinical signs seen in animals at doses > 500 ppm were
decreased locomotion, dehydration, lacrimation, and unthriftiness.
Body-weight gain was reduced at concentrations > 50 ppm among males
(marginal at 50 ppm) and at > 200 ppm among females (marginal at
200 ppm). Necropsy showed no treatment-related gross lesions (Freeman,
1993). The NOAEL was 50 ppm, equivalent to 2.5 mg/kg bw per day.
Table 3. Results of tests for the genotoxicity of carbofuran
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 100-10 000 µg/plate 98.3 Negativea Haworth & Lawlor
TA98, TA100, Positiveb (1983a)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 98 Negativec Haworth & Lawlor
TA98, TA100, (1983b)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 80 Negativea Haworth & Lawlor
TA98, TA100, Positived (1983c)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 99 Negativee Haworth & Lawlor
TA98, TA100, (1983d)
TA1535, TA 1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 98 Negativef Haworth & Lawlor
TA98, TA100, (1983e)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 98 Negativef Haworth & Lawlor
TA98, TA100, (1983f)
TA1535, TA1537
TA1538
Table 3. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Reverse mutation S. typhimurium 100-10 000 µg/plate 97.6 Negativeg Haworth & Lawlor
TA98, TA100, (1983g)
TA1535, TA1537
TA1538
Reverse mutation S. typhimurium 100-10 000 µg/plate 96 Negativeh Haworth & Lawlor
TA98, TA100, (1983h)
TA1535, TA1537,
TA1538
Reverse mutation S. typhimurium 1-10 000 µg/plate 97.6 Negative DeGraff (1983a)
TA100
Reverse mutation S. typhimurium 61.7-5000 µg/plate 99 Negativea Farrow (1983a)
TA98 Positivei
Reverse mutation S. typhimurium 61.7-5000 µg/plate 97.6 Negativea DeGraff (1983a)
TA98, TA100, Positivej
TA1537, TA1538
Reverse mutation S. typhimurium 123-10 000 µg/plate 98.3 Negativea Farrow (1983c)
TA98, TA100, Positivek
TA1537
Reverse mutation S. typhimurium 1-5000 µg/plate NR Negative Simmon (1979)
TA98, TA100,
TA1535, TA1537,
TA1538
Reverse mutation E. coli WP2 uvrA 1-5000 µg/plate NR Negative Simmon (1979)
Mitotic recombination S. cerevisiae 1-50 mg/ml NR Negative Simmon (1979)
Unscheduled DNA synthesis Human fibroblasts 0.1-1000 µg/mll NR Negative Simmon (1979)
(WI-38)
Growth inhibition E. colim, B. subtilis 1-500 mg/ml NR Negativen Simmon (1979)
Unscheduled DNA synthesis Rat hepatocytes 1-100 µg/ml 97.6 Negativea Thilagar (1983a)
Table 3. (Cont'd)
End-point Test system Concentration Purity Results Reference
(%)
Sister chromatid exchange Chinese hamster 78.1-312.5 µg/ml with S9 97.6 Negativeo Thilagar (1983b)
ovary cells 12.5-100 µg/ml without S9 Negative
Sister chromatid exchange Chinese hamster 312.5-2500 µg/ml with S9 96 Positivep Thilagar (1983c)
ovary cells 12.5-100 µg/ml without S9 Positive
Chromosomal aberration Chinese hamster 312.5-2500 µg/ml with S9 96 Negative Thilagar (1983d)
ovary cells 50-1000 µg/ml without S9 Negative
Cell mutation tk locus Mouse lymphoma 134-1780 µg/ml with S9 97.6 Negativeq Kirby (1983a)
L5178 Y cells 16-211 µg/ml without S9 Positive
Cell mutation tk locus Mouse lymphoma 134-1780 µg/ml with S9 96 Positive Kirby (1983b)
L5178 Y cells 24-316 µg/ml without S9
In vivo
Sex-linked recessive Drosophila 10 ppm (feeding solution) NR Negative Valencia (1981)
lethal mutation melanogaster
Sex-linked recessive Drosophila 7.5 ppm (feeding solution) 97.6 Negative Valencia (1983)
lethal mutation melanogaster
Sex-linked recessive Drosophila 5 and 10 ppm (feeding 97.6 Negative DeGraff (1983b)
lethal mutation melanogaster solution)
Cytogenicity Male rat 0.6 or 2.6 mg/kg bw per 98 Negative Putman (1983a)
bone marrow day orally, 5 days
Cytogenicity Male rat 1, 6 or 10 mg/kg bw per 96 Negative Putman (1983b)
bone marrow day orally, 5 days
Table 3. (Cont'd)
NR not reported
S9 9000 × g supernatant of Aroclor-induced rat liver microsomes
a With metabolic activation
b Weak positive response (2.2-fold increase in revertants per plate) of strain TA1535 only in the absence of S9
c The 1.9-fold increase in TA1535 revertants per plate observed in the absence of S9 did not meet the criteria for a
positive response.
d Weak positive response (2.2-fold increase in revertants per plate) of strain TA1535 in the absence of S9
e The 1.7-fold increase in TA1535 revertants per plate in the absence of S9 did not meet the criteria for a positive
response.
f 1.9-fold increase in TA1535 revertants per plate in both tests (Haworth & Lawlor, 1983 e,f) in the absence of S9
g 1.8-fold increase in TA1535 revertants and 1.4-fold increase in TA100 revertants in the absence of S9
h 1.6-fold increase in TA1535 revertants and 1.5-fold increase in TA100 revertants in the absence of S9
i Twofold increase in TA1535 revertants at 5000 µg/plate in the absence of S9. Precipitation of the compound occurred
at this concentration.
j Dose-dependent increase in TA1535 revertants at all doses in the absence of S9
k About a threefold increase TA1535 revertants at 1048 and 3145 µg/plate
l Precipitation observed at 1000 µg/ml
m Relative toxicity assays (growth inhibition) in DNA repair-proficient and -deficient strains of E. coli and B. subtilis
n Statistically significant increase in sister chromatid exchange frequency in comparison with solvent control in the
presence of S9 at a concentration of 312.5 µg/ml and in the absence of S9 at 100 µg/ml. The increases did not meet the
criteria for a positive response.
o In the absence of S9
p Statistically significant increases in sister chromatid exchange frequency in comparison with solvent control at all
doses in the presence and absence of S9
q Three S9-activated cultures out of 20 had mutant frequencies that were more than twice the mean mutant frequency of
the solvent controls. The significance of the increase is questionable because growth was strongly inhibited (> 90%)
in these cultures.
In the main study, groups of 10 male and 10 female Charles River
Sprague-Dawley rats were maintained on a diet designed to provide
concentrations of 0, 50, 500, or 1000 ppm, equivalent to 0, 2.4, 27.3,
and 55.3 mg/kg bw per day in males and 0, 3.1, 35.3, and 64.4 mg/kg bw
per day in females. Clinical signs, mortality, body weight and food
consumption were recorded, and functional observational battery and
motor activity testing were conducted before treatment and at weeks
4, 8, and 13 of treatment. The nervous systems of five rats of each
sex at the high dose and in the control group were examined for
histopathological lesions. One male at 1000 ppm was found moribund
on day 83 and was killed, and single animals in all groups,
including controls, were killed during the study for humane reasons.
Treatment-related clinical signs observed at concentrations
> 500 ppm consisted of exophthalmia, splayed hindlimbs, loss of
muscle control (in females), staggered gait, and tremors. Animals at
all doses had a dose-related reduction in body-weight gain; the
differences attained statistical significance in males at all doses
but in females only at 1000 ppm. Sporadic reductions in food
consumption were seen in animals at 1000 ppm, particularly males;
females showed increased food consumption. Functional observational
battery testing revealed effects in animals receiving doses
> 500 ppm, including gait impairment, a reduction in hindlimb grip
strength, whole-body tremors, and abnormal posture. Motor activity was
significantly reduced among females receiving 1000 ppm. The single
male at 1000 ppm found moribund on day 83 had calcifications in the
urethra; no other macroscopic treatment-related lesions were found. No
treatment-related histopathological lesions were found in the central
or peripheral nervous system of animals at the high dose (Freeman,
1994). The NOAEL for neurotoxicity was thus 50 ppm, equal to 3 mg/kg
bw per day. There was no NOAEL for systemic toxicity.
(iii) Inhibition of cholinesterase activity
In a comparative study in newborn, weanling, and adult
Sprague-Dawley rats, first evaluated by JMPR in 1980 (Annex 1,
reference 34), the purpose was to determine the time of maximal
inhibition of cholinesterase activity in plasma, erythrocytes, and
brain and the time of recovery after the administration of a single
oral dose of carbofuran. The LD50 values were 8.1 mg/kg bw for
newborn and 7.3 for weanling rats. The baseline cholinesterase
activities in untreated animals were determined at the age of two to
three days for newborns, 27-29 days for weanlings, and 99-101 days for
adult rats. The baseline activities in erythrocytes were lower in
newborns and weanlings than in adults, and those in brain were lower
in newborns than in weanlings and adults. After administration of
single oral doses of carbofuran at 3.2 mg/kg bw for newborns, 2.2
mg/kg bw for weanlings, and 4 mg/kg bw for adults, maximal depression
of erythrocyte activity occurred within 1 h in all groups, and maximal
depression of brain activity occurred within 1 h in weanlings and
adults and after 4 h in neonates. Full recovery was attained within
24 h after dosing, independently of the age of the animals. There were
no differences in sensitivity to cholinesterase inhibition with age
(Becci, 1979).
In a study to investigate the relationship between carbofuran
metabolism in vivo and acetylcholinesterase inhibition, male
Sprague-Dawley rats were given single intravenous or oral doses of
14C carbofuran at 50 µg/kg bw, and urine, faeces, and expired air
were analysed for oxidative and hydrolytic metabolites; blood, plasma,
and various tissues were analysed for carbofuran and its main
oxidative metabolite, 3-hydroxycarbofuran. Erythrocyte acetyl-
cholinesterase activity in vitro was used as an index of toxicity.
From 41 to 47% of the administered dose was recovered as 14C-carbon
dioxide after 8 h, independently of the route of administration. About
15% of the dose was found in urine and < 1% in faeces. 3-Hydroxy-
carbofuran was formed rapidly and underwent enterohepatic circulation,
resulting in an elimination half-life of about 64 min for all tissues;
the elimination half-life of the parent compound was about 29 min.
Rapid recovery of the erythrocyte acetylcholinesterase activity
closely paralleled carbofuran metabolism, and the primary disposition
of 3-hydroxycarbofuran in vivo was by metabolic conjugation
(Ferguson et al., 1984).
3. Observations in humans
Carbofuran was reported to have induced sensitization in a patch
test in 30 farmers with contact dermatitis (Sharma & Kaur, 1990).
Poisoning was reported in three female farmworkers who threw
carbofuran granules onto a coffee plantation in Jamaica. The women did
not wear protective clothing. The signs of poisoning reported included
vomiting, lassitude, nausea, and hypersalivation. Cholinesterase
activity was not determined in these patients (Coleman et al., 1990).
Comments
Carbofuran is rapidly absorbed, metabolized, and eliminated,
mainly in the urine, after oral administration to mice and rats. After
oral administration of [phenyl-14C]carbofuran to rats, 92% of the
radiolabel was eliminated in the urine and 3% in faeces. Most of the
radiolabel was eliminated within 24 h after treatment. With a
[carbonyl-14C]-labelled compound, 45% was eliminated as 14C-carbon
dioxide. The metabolic pathway consists in hydroxylation, oxidation,
hydrolysis, and conjugation.
Carbofuran is highly toxic after acute oral administration. The
oral LD50 values in various species ranged from 3 to 19 mg/kg bw.
Carbofuran had no sensitizing potential in guinea-pigs, and no local
irritation was found in rabbits after repeated dermal applications
over 7 or 21 days. WHO has classified carbofuran as 'highly hazardous'
(WHO, 1996).
In a 13-week study in dogs fed diets providing 0, 10, 70, or
500/250 ppm (dose reduced because of marked toxicity), an NOAEL was
not identified because of inhibition of erythrocyte acetylcholin-
esterase activity and some clinical signs were observed at the lowest
dose. In a subsequent four-week study in dogs, the highest dose
administered was 5 ppm, equal to 0.22 mg/kg bw per day, which was the
NOAEL for clinical signs, mortality, effects on body weight and food
consumption, and cholinesterase activity in plasma and erythrocytes.
In a one-year study in dogs at dietary concentrations of 0, 10, 20, or
500 ppm, the NOAEL was 10 ppm, equal to 0.3 mg/kg bw per day, on the
basis of histopathological testicular changes in a single male at
20 ppm; similar changes were observed in animals at 500 ppm. There
was no inhibition of erythrocyte or brain acetylcholinesterase at
concentrations of 10 or 20 ppm. The overall NOAEL in these short-term
studies in dogs was 5 ppm, equal to 0.22 mg/kg bw per day.
In two-year studies of toxicity and carcinogenicity at dietary
concentrations of 0, 20, 125, or 500 ppm in mice and 0, 10, 20, or
100 ppm in rats, the NOAELs were 20 ppm, equal to 2.8 mg/kg bw per
day, in mice and 20 ppm, equivalent to 1 mg/kg bw per day, in rats, on
the basis of inhibition of erythrocyte and brain acetylcholinesterase
activity. There was no evidence of tumorigenicity.
In a three-generation study of reproductive toxicity in rats at
dietary concentrations of 0, 20, or 100 ppm, the NOAEL was 20 ppm,
equal to 1.6 mg/kg bw per day, on the basis of reduced body-weight
gain in parental animals and reduced pup growth and pup survival at
100 ppm.
In an early study of developmental toxicity, rats were given
carbofuran at doses of 0, 0.1, 0.3, or 1 mg/kg bw per day by gavage.
An NOAEL could not be identified in this study. Dose-dependent,
transient clinical signs (chewing motions) were observed in the dams.
In a later study in rats at oral doses of 0, 0.25, 0.5, or 1.2 mg/kg
bw per day, the NOAEL for maternal and fetal toxicity was 1.2 mg/kg bw
per day, the highest dose tested. In a further study of teratogenicity
in rats, with dietary administration of 0, 20, 60, or 160 ppm
carbofuran, the NOAEL for maternal toxicity was 20 ppm, equal to
1.5 mg/kg bw per day, on the basis of a reduction in body-weight gain
at 60 ppm. The NOAEL for pup toxicity, based on reduced pup weight,
was 60 ppm, equal to 4.4 mg/kg bw per day. None of the studies showed
teratogenic potential.
The results of an early study of teratogenicity in rabbits at
oral doses of 0, 0.2, 0.6, or 2 mg/kg bw per day showed an NOAEL of
0.6 mg/kg bw per day for maternal toxicity on the basis of clinical
signs and an NOAEL of 2 mg/kg bw per day for fetotoxicity and
teratogenicity. In a subsequent study in rabbits at doses of 0, 0.12,
0.5, or 2 mg/kg bw per day, the NOAEL was 0.5 mg/kg bw per day on the
basis of slightly reduced body-weight gain in dams and a slightly
increased incidence of skeletal variations in pups at 2 mg/kg bw per
day. These studies provide no evidence for teratogenicity.
In a 90-day study of neurotoxicity in rats at dietary
concentrations of 0, 50, 500, or 1000 ppm, systemic toxicity
(reduction in body-weight gain) was observed at all doses. Clinical
signs of neurotoxicity were observed at 500 and 1000 ppm. No
histopathological lesions were found in the nervous system.
In a study of developmental neurotoxicity, carbofuran was
administered in the diet of rats to provide concentrations of 0, 20,
75, or 300 ppm from gestation day 6 through lactation day 10.
Reductions in the body-weight gain of dams and pups and in pup
survival and some evidence of delayed pup development were found at
doses > 75 ppm. The NOAEL was 20 ppm, equal to 1.7 mg/kg bw per
day, on the basis of reduced body-weight gain in dams and signs of
fetotoxicity at higher doses.
Carbofuran has been tested for genotoxicity in a wide range of
tests in vivo and in vitro. The Meeting concluded that it is not
genotoxic.
An ADI of 0-0.002 mg/kg bw was allocated on the basis of the
NOAEL for erythrocyte acetylcholinesterase inhibition of 0.22 mg/kg bw
per day in a four-week study in the most sensitive species, the dog,
and using a 100-fold safety factor. The use of a short-term study to
set the ADI is justified because the effect observed was reversible
and acute.
Toxicological evaluation
Levels that cause no toxicological effect
Mouse: 20 ppm, equal to 2.8 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rat: 20 ppm, equivalent to 1 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
20 ppm, equal to 1.2 mg/kg bw per day (three-generation
study of reproductive toxicity)
1.2 mg/kg bw per day (highest dose tested in a study of
developmental toxicity)
20 ppm, equal to 1.5 mg/kg bw per day (study of
developmental toxicity)
20 ppm, equal to 1.7 mg/kg bw per day (study of
developmental neurotoxicity)
Rabbit: 0.6 mg/kg bw per day (study of developmental toxicity)
Dog: 5 ppm, equal to 0.22 mg/kg bw per day (four-week study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to carbofuran
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 6-14.4 mg/kg bw
Dermal, toxicity, rat LD50 > 500 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.088-0.1 mg/litre
Dermal, irritation, rabbit Not irritating
Ocular irritation, rabbit Not available
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated oral, 4 weeks, toxicity, dog NOAEL = 0.22 mg/kg bw per day
Repeated oral, reproductive toxicity, rat NOAEL = 1.6 mg/kg bw per day, parental
and pup toxicity
Repeated oral (gavage), developmental NOAEL = 1.2 mg/kg bw per day (highest
toxicity, rat dose tested). No evidence of teratogenicity
Repeated oral (feeding), developmental NOAEL = 1.5 mg/kg bw per day, maternal
toxicity, rat toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 0.6 mg/kg bw per day, maternal
toxicity. No evidence of teratogenicity
Repeated oral, developmental neurotoxicity, rat NOAEL = 1.7 mg/kg bw per day
Longterm (> 1 year) Repeated oral, 2 years, carcinogenicity, mouse NOAEL = 2.8 mg/kg bw per day,
cholinesterase inhibition. No evidence
of carcinogenicity
Repeated oral, 2 years, carcinogenicity, rat NOAEL = 1 mg/kg bw per day, reduced
body-weight gain and cholinesterase
inhibition. No evidence of carcinogenicity
References
Barron, P., Giesler, P. & Ras, G.N. (1978) Teratogenicity of
carbofuran in rats. Act-No. 184.33. Unpublished report prepared by
Warf Institute, Inc. Wisconsin, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Becci, G. (1979) Study of cholinesterase activity inhibition by
carbofuran in neonate, weanling and adult rats. Study-No. A 79-343.
Unpublished report prepared by Food and Drug Research Laboratories,
Inc. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Bloch, I., Frei, T.H., Madoerin, K., Luetkemeier, H., Vogel, W.,
Schlotke, B., Vogel, O. & Terrier, C. (1987a) 13-Week oral toxicity
feeding study with carbofuran (D1221) in the dog. RCC-No. 077837 (FMC
Study No. A95-4249). Unpublished report prepared by Research
Consulting Company AG, Itingen, Switzerland. Submitted to WHO by FMC
Corp., Philadelphia, PA, USA.
Bloch, I. Frei, T., Luetkenmeier, H., Vogel, W. & Terrier, C. (1987b)
4-Week oral toxicity (feeding) study with carbofuran 'D 1221' in male
dogs. RCC No. 087963 (FMC Study No. A95-4248). Unpublished report
prepared by Research & Consulting Company AG, Itingen, Switzerland.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Brown, W.R. (1980) 2-Year dietary toxicity and carcinogenicity study
in mice with technical carbofuran. Act No. 150.52 Histopathology Part.
Unpublished report prepared by Research Pathology Services, Inc. PA,
USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Burtner, B.R. (1982) 14-Day oral toxicity (range finding) study in
beagle dogs of carbofuran. (FMC Study No. A81-604, Toxicogenics No.
410-0714). Unpublished report prepared by ToxiGenics, Inc., IL, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Case, R.S. & Wilson, N.H. (1979) Cholinesterase evaluation conducted
during a two year dietary toxicity/oncogenicity study with carbofuran
in rats. Study No. Tox-79-001. Unpublished report prepared for FMC.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Coleman, A.M.E., Smith, A. & Watson, D. (1990) Occupational carbamate
pesticide intoxication in three farm workers. West Indian Med. J.,
39, 109-113.
Courtney, K.D., Andrews, J.E., Springer, J. & Dalley, L. (1985)
Teratogenic evaluation of the pesticides Baygon, carbofuran,
dimethoate and EPN. J. Environ. Sci. Health B, 20, 373-406.
DeGraff, M.G. (1983a) Mutagenicity evaluation of FMC 10242 in the Ames
Salmonella/microsome plate test Study No. A 83-1037). Unpublished
report by Litton Bionetics, Inc., Kensington, MD, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
DeGraff, W.G. (1983b) Mutagenicity evaluation of MC 10242 for the
sex-linked recessive lethal test in Drosophila melanogaster. Study
No. A 83-1060. Unpublished report prepared by Litton Bionetics, Inc.
Kensington, MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Dorough, H.W. (1968) Metabolism of Furadan (NIA-10242) in rats and
houseflies. J. Agric. Food Chem., 16, 319-325.
Elleman, P.N. (1979) Acute oral toxicity (FIFRA-EPA) in rats with
carbofuran technical (FMC Study No. A 79-339, CSE-Study No. 0180).
Unpublished report prepared by Cosmopolitan Safety Evaluation (CSE)
Inc., NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Ellison, T. (1980) Audit validaton of the skin sensitization test on
NIA 10242 Technical. Unpublished report on Study No. A 5300. Submitted
to WHO by FMC Corp., Philadelphia, PA, USA.
Farrow, M.G. (1983a) Salmonella typhimurium/mammalian microsome
plate incorporation assay with compound FMC 10242 (FMC Study-No. A
83-905, HLA No. 331). Unpublished report prepared by Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Farrow, M.G. (1983b) Salmonella typhimurium/mammalian microsome
plate incorporation assay with compound FMC 10242 (FMC Study No. A
83-907, HLA No. 332). Unpublished report prepared by Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Farrow, M.G. (1983c) Salmonella typhimurium/mammalian microsome
plate incorporation assay with compound FMC 10242 (FMC Study No. A
83-909, HLA No. 333). Unpublished report prepared by Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Ferguson, P.W., Dey, M.S., Jewell, S.A. & Krieger, R.I. (1984)
Corbofuran metabolism and toxicity in the rat. Fundam. Appl.
Toxicol., 4, 14-21.
Freeman, C. (1984a) Acute oral toxicity of FMC 18209 technical
(3-hydroxy-carbofuran) in rats. FMC Study No. A 83-1136. Unpublished
report prepared by FMC Toxicology Laboratory, NJ, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Freeman, C. (1984b) Acute oral toxicity of FMC 17481 technical
(3-keto-carbofuran) in rats. FMC Study No. A 83-1137. Unpublished
report prepared by FMC Toxicology Laboratory, NJ, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Freeman, C. (1984c) Acute oral toxicity of FMC 16497 (3-hydroxy-
7-phenol) rats. FMC Study No. A83-1134. Unpublished report Pprepared
by FMC Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Freeman, C. (1984d) Acute oral toxicity of FMC 19841 (3-keto-7-phenol)
in rats. FMC Study No. A83-1135. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Freeman, C. (1984e) Acute oral toxicity of FMC 10272 (7-phenol) in
rats. FMC Study No. A83-1133. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Freeman, C. (1993) Twenty-eight day neurotoxicity range-finding study
in rats with carbofuran technical. FMC No. A 92-3704. Unpublished
report prepared by FMC Corporation, Toxicology Laboratory, NJ, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Freeman, C. (1994) Subchronic neurotoxicity screen in rats with
carbofuran technical. FMC Study No. 92-3705. Unpublished report
prepared by FMC Corporation Toxicology Laboratory, NJ, USA. Submitted
to WHO by FMC Corp., Philadelphia, PA, USA.
Flucke, W. (1986) SRA 12869 (isofenphos) and D 1221 (carbofuran):
Study for combination toxicity. Study No. 134337. Unpublished report
prepared by Bayer AG, Insititute of Toxicology. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Fukuto, T.R. & Metcalf, R.L. (1969) Metabolism of insecticides in
plants and animals. Biological effects of pesticides in mammalian
systems. Ann. NY Acad. Sci., 160, Art. 1.
Georgian, L.I., Moraru, I., Draghicescu, T. & Tarnavschi, R. (1985)
The effect of low concentrations of carbofuran, cholin salt of maleic
hydrazine, propham and chlorpropham on sister-chromatid exchange (SCE)
frequency in human lymphocytes in vitro. Mutat. Res., 147, 296.
Goldenthal, E.I. (1982a) 2-Year dietary toxicity and carcinogenicity
study in mice. FMC Study No. Act No. 150.52 (IRDC No. 167-116).
Unpublished report prepared by International Research and Development
Corporation, MI, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Goldenthal, E.I. (1982b) 2-Year dietary toxicity and carcinogenicity
study in rats. FMC Study No. Act No. 130.51 (IRDC No. 167-115).
Unpublished report prepared by International Research and Development
Corporation, MI, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Goldenthal, E.I. (1982c) Three generation reproduction study in rats.
FMC Study No. Act. No. 131.53 (IRDC No. 167-114). Unpublished report
prepared by International Research and Development Corporation, MI,
USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983a) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-868 (MBA Study No. 1921.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. 1983b) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-913 (MBA Study No. 1934.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983c) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
Technical. FMC Study No. A 83-948 (MBA Study No. 1938.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983d) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-949 (MBA Study No. 1936.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983e) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-950 (MBA Study No. 1935.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983f) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-951 (MBA Study No. 1937.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983g) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-973 (MBA Study No. 1982.501).
Unpublished report prepared by Microbiological Associates, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Haworth, S.R. & Lawlor, T.E. (1983h) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test). Carbofuran
technical. FMC Study No. A 83-1063. Unpublished report prepared by
Microbiological Associates, MD, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Hoffman, S.L. & Robinson, R.A. (1994a) Metabolism of C14-carbofuran
in laying hens. XBL Study No. XBL 92051; FMC Study No. 078 POU 92M1.
Unpublished report prepared by XenoBiotic Laboratories, Inc.,
Plainsboro, NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Hoffman, S.L. & Robinson, R.A. (1994b) Metabolism of C14-carbofuran
in lactating goats. XBL Study No. XBL 92051; FMC Study No. 078 GOA
92M1. Unpublished report prepared by XenoBiotic Laboratories, Inc.,
Plainsboro, NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Kedderis, L.B. (1985a) Seven-day repeated dose dermal toxicity
range-finding study in rabbits with FMC 10242 technical (carbofuran).
Study No. A 85-1645. Unpublished report prepared by FMC Corporation.
NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kedderis, L.B. (1985b) Twenty-one day repeated dose dermal toxicity
range-finding study in rabbits with FMC 10242 technical (carbofuran).
Study No. A 85-1678. Unpublished report prepared by FMC Corporation,
NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kirby, P.E. (1983a) L 5178Y TK+/- mouse lymphoma mutagenesis assay.
FMC Study No. A 83-962 and A 83-988 (MBA Study No. T1982.701).
Unpublished report prepared by Microbiological Associates, Bethesda,
MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kirby, P.E. (1983b) L5178Y TK+/- mouse lymphoma mutagenesis assay.
FMC Study No. A 83-1064 (MBA Study No. T2124.201). Unpublished report
prepared by Microbiological Associates, Bethesda, MD, USA. Submitted
to WHO by FMC Corp., Philadelphia, PA, USA.
Knaak, J.B., Munger, D.M. & McCarthy, J.F. (1969) The metabolism of
carbofuran alfalfa residues in the rat. Abstracts of Papers, 158th
National Meeting of the American Chemical Society, 7-12 September.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Kuhr, R.J. & Dorough, H.W. (1976) Carbamate Insecticides: Chemistry,
Biochemistry and Toxicology, Cleveland, OH, CRC Press. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Laveglia, J. (1981) Teratology study in the rabbit with carbofuran.
FMC Study No. A 80-452 (IRDC No. 167-156). Unpublished report prepared
by International Research and Development Corporation, MI, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Mehta, C.S. (1981) Rabbit acute dermal toxicity. FMC Study No. A
81-564 (Stillmeadow No. 2142.81). Unpublished report prepared by
Stillmeadow, Inc., TX, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Metcalf, R.L., Fukuto, T.R., Collins, C., Borck, K., Abd El-Aziz, S.,
Munoz, R. & Cassil, C.C. (1968) Metabolism of 2,2-dimethyl-
2,3-dihydrobenzofuranyl-7 N-methylcarbamate (Furadan) in plants,
insects and mammals. J. Agric. Food Chem., 16, 300-311.
Mihail, F. (1981) D1221 (carbofuran) and Dacamox (thiofanox): Acute
combination toxicity study. Study No. 10280. Unpublished report
prepared by Bayer AG, Institute of Toxicology. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Moriya, M., Ohta, K., Watanabe, T., Miyazawa, K., Kato, K. & Shirasw
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Norwell, M.J. (1983a) Acute oral toxicity of FMC 10242, technical, in
rats. FMC Study No. A 83-1101. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Norwell, M.J. (1983b) Acute oral toxicity of FMC 10242, technical, in
rats. FMC Study No. A 83-1102. Unpublished report prepared by FMC
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Ponnock, K.S. (1994) A developmental neurotoxicity study of carbofuran
in the rat via dietary administration. Study No. 93-4506, FMC Study
No. A 93-3746. Unpublished report prepared by Pharmaco LSR, Inc.
Toxicology Services North America, NJ, USA. Submitted to WHO by FMC
Corp., Philadelphia, PA, USA.
Putman, D.L. (1983a) Activity of FMC 10242 in the in vivo cytogenetics
assay in Sprague-Dawley rats. FMC Study No. A 83-972 (MBA No.
T1982.102). Unpublished report prepared by Microbiological Associates,
Bethesda, MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Putman, D.L. (1983b) Activity of FMC 10242 in the subchronic in vivo
cytogenetics assay in male rats. Study No. A 83-1065 (MBA No.
T2124.102). Unpublished report prepared by Microbiological Associates,
Bethesda, MD, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Rao, G.N. (1978) Teratology study of carbofuran in rabbits. Study No.
185-33. Unpublished report prepared by Warf Institute, Inc. WI, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Rapp, W.R. (1980a) 24 Month dietary toxicity/carcinogenicity study of
carbofuran in rats. Act No. 130.51. Pathology report. Unpublished
report prepared by Wm. R. Rapp & Co. Inc., Allied Services for
Research, NJ, USA. Submitted to WHO by FMC Corp., Philadelphia, PA,
USA.
Rapp, W.R. (1980b) A three generation reproduction study of carbofuran
in rats. FMC Act No. 131.531. Pathology report. Unpublished report
prepared by Wm. R. Rapp & Co. Inc., Allied Services for Research, NJ,
USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Rodwell, D.E. (1980a) Teratology study in the rat with carbofuran.
(FMC Study No. A 80-453, IRDC No. 167-155). Unpublished report
prepared by International Research and Development Corp., MI, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Rodwell, D.E. (1980b; amended 1985) Pilot teratology study in the rat
with carbofuran in the diet (FMC Study No. A 80-443, IRDC No.
167-153). Unpublished report prepared by International Research and
Development Corp., MI, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Rodwell, D.E. (1981; with amendment) Teratology and postnatal study in
the rat with carbofuran (FMC Study No. A 80-444, IRDC No. 167-154).
Unpublished report prepared by International Research and Development
Corp., MI, USA. Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Sabol, R.J. (1979a) Rat acute oral toxicity with carbofuran technical.
Study No. 1083-79; FMC Study No. ACT 267.01-01. Unpublished report
prepared by Stillmeadow, Inc., TX, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Sabol, R.J. (1979b) Rat acute oral toxicity with furadan insecticide,
carbofuran 96.6%. Study No. 11231-79; FMC Study No. ACT 267.01-02
(01). Unpublished report prepared by Stillmeadow, Inc., TX, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Schoenig, G. (1967) Skin sensitization test on NIA 10242 technical.
IBT Study No. A 5300. FMC Study No. NCT 176.36. Unpublished report
prepared by Industrial Bio Test Laboratory, Inc, IL, USA. Submitted to
WHO by FMC Corp., Philadelphia, PA, USA.
Sharma, V.K. & Kaur, S. (1990) Contact sensitization by pesticides in
farmers. Contact Derm., 23, 77-80.
Signorin, J. (1995) DOT acute inhalation toxicity study in rats. FMC
Study No. A 94-4097. Unpublished report prepared by FMC Corp.,
Toxicology Laboratory, NJ, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Simmon, U.F. (1979) In vitro microbiological mutagenicity and
unscheduled DNA synthesis studies of eighteen pesticides. Study No.
68-01-2458 (FMC Study No. A81-509, EPA 600/1-79-041). Report prepared
by SRI International, Menlo Park, California, for Health Effects
Research Laboratory Office of Research and Development. US
Environmental Protection Agency, Research Triangle Park, NC, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Stehrer-Schmid, P. & Wolf, H.U. (1995) Effects of benzofuran and seven
benzofuran derivatives including four carbamate insecticides in the in
vitro porcine brain tubulin assembly assay and description of a new
approach for the evaluation of the test data. Mutat. Res., 339,
61-72.
Taylor, G.D. (1983) One-year chronic oral toxicity study in beagle
dogs with carbofuran. FMC study No. A 81-605 (Toxicogenics No.
410-0715). Unpublished report prepared by ToxiGenics, Inc., IL, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Thilagar, A. (1983a) Unscheduled DNA synthesis in rat primary
hepatocytes. FMC Study No. A 83-969 (MBA No. T1982.380). Unpublished
report prepared by Microbiological Associates, Bethesda, MD, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Thilagar, A. (1983b) Sister chromatid exchange assay in Chinese
hamster ovary (CHO) cells. FMC Study No. A 83-1095 (MBA No.
1982.334001). Unpublished report prepared by Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Thilagar, A. (1983c) Sister chromatid exchange assay in Chinese
hamster ovary (CHO) cells. FMC Study No. A 83-1097 (MBA No.
2124.334001). Unpublished report prepared by Microbiological
Associates, Bethesda, MD, USA. Submitted to WHO by FMC Corp.,
Philadelphia, PA, USA.
Thilagar, A. (1983d) Chromosome aberrations in Chinese hamster ovary
(CHO) cells. FMC Study No. A 83-1096. Unpublished report prepared by
Microbiological Associates, Bethesda, MD, USA. Submitted to WHO by FMC
Corp., Philadelphia, PA, USA.
Valencia, R. (1981) Mutagenesis screening of pesticides using
Drosophila. FMC Study No. A 83-1042 (EPA 600/1-81-017). Report
prepared by Warf Institute Inc., Madison, WI, for Health Effects
Research Laboratory. Office of Research and Development, US
Environmental Protection Agency, Research Triangle Park, NC, USA.
Submitted to WHO by FMC Corp., Philadelphia, PA, USA.
Valencia, R. (1983) Drosophila sex-linked recessive lethal assay
of 2,3-dihydro-2,2-dimethyl-7-benzofuranyl- N-methyl carbamate
(carbofuran). FMC Study No. A 83-1019 (MBA No. T1047.160). Unpublished
report prepared by Microbiological Associates, Bethesda, MD and the
University of Wisconsin, Zoology Department, USA. Submitted to WHO by
FMC Corp., Philadelphia, PA, USA.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
Woijcieckowski, J.P., Kaur, P. & Sabharwal, P.S. (1982) Induction of
ouabain resistance in V-79 cells by four carbamate pesticides.
Environ. Res., 29, 48-53.
