
TEBUFENOZIDE
First draft prepared by
D. Grant & S. Ma
Pesticide Evaluation Division, Health Evaluation Division,
Health Canada, Tunney's Pasture, Ottawa, Ontario, Canada
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Neurotoxicity
Studies on metabolites
Comments
Toxicological evaluation
References
Explanation
Tebufenozide, 4-ethylbenzoic acid- N'-tert-butyl- N'-(3,5-di-
methylbenzoyl)hydrazide, is a fat-soluble insecticide used to control
Lepidoptera pests in fruits, vegetables, and other crops. It has a
novel mode of action, in that it mimics the action of the insect
moulting hormone, ecdysome. Lepidoptera larvae cease to feed within
hours of exposure to tebufenozide and then undergo a lethal
unsuccessful moult.
Tebufenozide was evaluated for the first time by the present
Meeting.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Three groups of four male and four female Crl:CD BR rats were
fasted overnight and then given a single dose by gavage of 250 mg/kg
bw of either [14C- tert-butyl]tebufenozide, [14C-A-ring]-tebufenozide,
or [14C-B-ring]tebufenozide. The profile of excretion of radiolabel
by males and females was similar. Absorption and excretion of 14C
label were rapid, with > 70% of the administered dose eliminated
within 48 h. The mean total excreted over seven days was > 82%. Faeces
was the major route of excretion, representing at least 98% of the
total radiolabel excreted; only minor amounts (1-2% of dose) were
excreted in the urine and trace amounts (< 0.05% of dose) in expired
air as 14C-carbon dioxide or volatile organic compounds. Little
radiolabel was retained in organs or tissues by seven days after
dosing: < 0.1% of the [14C- tert-butyl]-labelled material, < 0.03%
[14C-A-ring]tebufenozide, and < 0.01% [14C-B-ring]-tebufenozide.
The highest tissue concentrations were found in the liver, blood,
spleen, and fat (0.5-1.3 µg/g 14C-tebufenozide equivalents) of
animals given [14C- tert-butyl]- labelled material and in the
fat (< 1 µg/g) of animals given [14C-A-ring]-tebufenozide. The
concentrations of radiolabel in the tissues of animals given
[14C-B-ring]tebufenozide were near or below the limit of detection
(0.4 ppm) (LeVan, 1991).
Four male and four female Crl:CD BR rats received a bile-duct
cannula, were fasted overnight, and were then given a single dose of
3 mg [14C- tert-butyl]tebufenozide/kg bw by gavage. The absorption
and elimination of radiolabel were followed up to 72 h. No significant
difference in the excretion profile was seen between males and females.
The absorption and excretion of radiolabel were rapid, and about 100%
of the administered dose was recovered in excreta within 24 h. Most of
the radiolabel (67-70%) was unabsorbed and was eliminated directly in
the faeces. Systemic absorption was calculated to be 35-39% of the
total administered dose; 30-34% was excreted in the bile and approx. 5%
in the urine. Tissue retention of radiolabel was very low, with a mean
of 0.3-0.4% remaining in the carcass 72 h after dosing (Struble &
Hazelton, 1992a).
Groups of three to six male and female Crl:CD BR Sprague-Dawley
rats were given a single dose of either [14C- tert-butyl]-tebufenozide,
[14C-A-ring]tebufenozide, or [14C-B-ring]-tebufenozide by gavage
at nominal doses of 3 or 250 mg/kg bw. One group of animals was fed
a diet containing 30 ppm nonradioactive tebufenozide for two weeks
before receiving a single oral dose of 3 mg/kg bw [14C- tert-butyl]-
tebufenozide. The absorption and excretion of the radiolabel were
very rapid. The excretion profiles were similar, regardless of the
position of the 14C label, the dose, the sex of the rat, or whether
the animals were pretreated with tebufenozide. A mean total of 87-104%
of the administered dose was excreted within 48 h of dosing, primarily
via the faeces, which accounted for > 90% of the radiolabel excreted;
only minor amounts (< 1-8% of the dose) were excreted in the urine.
Trace amounts of radiolabel (< 0.1-0.4% of the dose) were recovered
as carbon dioxide and volatile organic compounds in the expired air
of rats dosed with [14C- tert-butyl]tebufenozide but not from
rats dosed with the A-ring or B-ring label. Maximal levels of
radiolabel were measured in the blood 0.5-12 h after dosing. Clearance
of the A-ring and B-ring labels from the circulation was very rapid,
such that no 14C was detected in blood 24 h after dosing. In
contrast, disappearance of radiolabel from the blood of animals given
the tert-butyl label was relatively slow, low levels being detected
10 days after dosing. The peak concentration of radiolabel in blood
was not proportional to the dose administered, suggesting that the
pharmacokinetics of 14C-tebufenozide are not linear between the low
(3 mg/kg bw) and high (250 mg/kg bw) doses. Tissue retention of
radiolabel was very low; by 168 h after treatment, the mean totals of
the administered dose retained were < 1, < 0.2, and < 0.01% of the
tert-butyl, A-ring, and B-ring labels, respectively, at 3 mg/kg bw
and < 0.01% of any of the three labels at 250 mg/kg bw. The highest
concentrations of radiolabel were consistently found in the liver,
fat, and kidneys; all other tissues contained < 0.01 ppm or
undetectable levels, regardless of the position of the label, the
dose, or the sex of the rat. The distribution in the tissues was
consistent with the pharmacokinetic data and indicated that the 14C
associated with the A-ring and B-ring labels was cleared more rapidly
from the tissues than that associated with the tert-butyl label
(Struble & Hazelton, 1992b).
(b) Biotransformation
Groups of Crl:CD BR Sprague-Dawley rats were given [14C-
tert-butyl]-, [14C-A-ring]-, or [14C-B-ring]tebufenozide by gavage
at a dose of 3 mg/kg bw or [14C- tert-butyl]- or [14C-B-ring]-
tebufenozide at 250 mg/kg bw. Faeces and urine were collected from
five rats of each sex per group and analysed for tebufenozide
metabolites. Parent tebufenozide was the major component present in the
faeces, accounting for > 90% of the administered dose at the high dose
and > 35% at the low dose. Eleven metabolites were identified in the
faeces of animals at the high dose and 14 in faeces from those at the
low dose; 10 of the metabolites were common to the two groups. No
qualitative difference in metabolite profile was seen with the
differently labelled versions of tebufenozide. No parent tebufenozide
was found in the urine, but many of the metabolites were the same as
those found in the faeces. An additional two or three unknown
metabolites, representing 3-3.5% of the total dose, were found in
acidified fractions of urine from animals given A- or B-ring labelled
material. These were probably partially fragmented metabolites of
tebufenozide in which the tert-butyl group had been metabolically
cleaved. The faecal and urinary analyses thus indicate a total of 15
metabolites (all except one present at < 1% of the dose) in excreta
from animals at the high dose and 14 metabolites (two present at > 10%
and nine at > 1% of the dose) in excreta from animals at the low dose.
The extent of metabolism of tebufenozide was highly dependent on the
amount of test material administered. Thus, only about 4% of the high
dose was metabolized, producing about 10 mg/kg bw of metabolites,
whereas about 46% of the low dose was metabolized, producing about
1.4 mg/kg bw of metabolites. The major route of metabolism of
tebufenozide appeared to be oxidation of benzylic carbons (A or B
ring) to provide a number of oxidized metabolites with various
combinations of oxidation states at the 3-carbon centres. One
exception was RH-2703 (see Figure 1), which was produced by oxidation
of the non-benzylic terminal carbon on the A-ring ethyl group. A
metabolic pathway for tebufenozide in rats was proposed (Figure 1)
(Hawkins et al., 1992).
The metabolism of tebufenozide was studied in Crl:CD BR
Sprague-Dawley rats given a single dose of 3 mg/kg bw of [14C- tert-
butyl]tebufenozide by gavage. One group was fed 30 ppm of non-
radioactive tebufenozide in the diet for two weeks before receiving
the radiolabelled material. Faeces and urine from five males and five
females were collected and analysed for tebufenozide metabolites. The
parent tebufenozide was a major component of faeces, accounting for
26-39% of the administered radiolabel, and 13 metabolites were
identified. Significant differences between males and females in the
levels of several metabolites were noted: the major faecal metabolites
were RH-0282, RH-120898, and RH-0126 in animals of each sex and
RH-122777 in females only. No parent tebufenozide was found in the
urine, but many of the 13 metabolites found were the same as those in
faeces. No significant qualitative differences in the metabolism of
14C-tebufenozide were found between rats pretreated with 30 ppm
dietary tebufenozide and those receiving the single 14C-radiolabelled
dose without pretreatment. Quantitatively, slightly less parent
tebufenozide was found in the excreta of pulse-dosed than those of
single-dosed animals, suggesting that slightly more tebufenozide
metabolism occurred in the former, which also had generally higher
levels of the more highly oxidized metabolites in their excreta
(Hawkins et al., 1993).
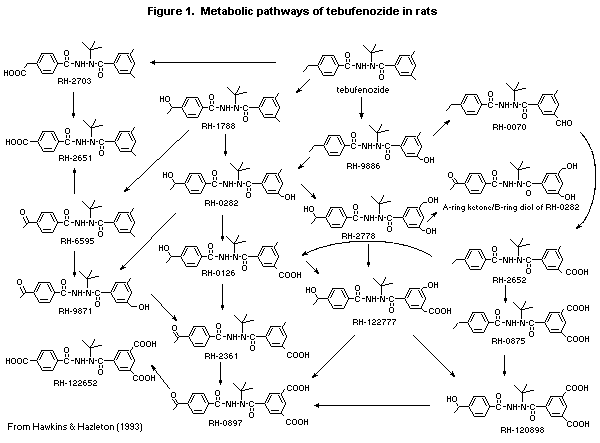 Groups of Crl:CD BR Sprague-Dawley rats received a bile-duct
cannula and then a single oral dose of 3 mg/kg bw [14C- tert-butyl]-
tebufenozide. Bile samples collected from one male and one female rat
during 0-6 h after treatment, representing about 70% of total biliary
excretion of radiolabel over 72 h, were analysed for tebufenozide
metabolites. No parent compound was found; 13 biliary metabolites were
identified, and five unknown metabolites were isolated. In general,
the biliary metabolites were identical to those in the faeces and
urine, but three new metabolites were observed: [A-ring]-ketone-
[B-ring]-diol, RH-122652, and RH-2652. The last two were also
identified by mass spectroscopy in the faeces but at levels too low
for quantification. The unknown metabolites in bile appeared to be
high-molecular-mass amino-acid conjugates of acidic tebufenozide
metabolites. It was postulated that these conjugates are metabolized
in rats before excretion in order to recover the amino acids; hence,
their absence in faeces (Hawkins & Hazelton, 1993).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of tebufenozide are
summarized in Table 1. Tebufenozide was of low toxicity to mice and
rats when given orally, dermally, or by inhalation. No deaths or
clinical signs of systemic toxicity were observed at doses < 5.0 g/kg
bw. Dermal administration resulted only in transient, mild local
erythema at the site of application. After inhalation, accumulation
of red-brown material was seen around the nose, mouth, and eyes,
sometimes with a purulent anogenital discharge which lasted for one
to three days.
(b) Short-term toxicity
Mice
Six groups of eight male albino Crl:CD-1 ICR BR mice were fed
diets containing technical-grade tebufenozide (purity, 94%) to provide
doses of 0, 60, 200, 600, 2000, or 6000 ppm, equal to 0, 12, 39, 97,
350, or 1100 mg/kg bw per day, for two weeks. At the highest dose, the
absolute and relative liver weights were significantly increased,
but, in the absence of histopathological data, the toxicological
significance of this change could not be fully evaluated. The NOAEL
was 600 ppm, equal to 97 mg/kg bw per day, on the basis of the
increased relative liver weights (Kyle, 1992).
Table 1. Acute toxicity of tebufenozide in experimental animals
Species Sex Route LD50 (mg/kg bw) Reference
or LC50 (mg/litre)
Mouse Male, female Oral > 5000 Morrison & Hamilton (1991a)
Rat Male, female Oral > 5000 Morrison & Hamilton (1991b)
Rat Male, female Dermal > 5000 Morrison & Hamilton (1991c)
Rat Male Inhalation > 4.3 Ulrich (1992)
Female > 4.5
Groups of 10 male and 10 female Crl:CD-1 ICR BR VAF/Plus mice
were fed diets containing technical-grade tebufenozide (purity, 98.6%)
providing levels of 0, 20, 200, 2000, or 20 000 ppm, equal to 0, 3.4,
35, 340, and 3300 mg/kg bw per day for males and 0, 4.3, 45, 430, and
4200 mg/kg bw per day for females, for 13 weeks. At 200 ppm, slightly
reduced mean body-weight gain (but not overall body weight) was seen
in males and a marginally higher incidence of increased extramedullary
haematopoiesis in the spleen and pigment accumulation in kidney
tubules (with no concomitant changes in haematological parameters) in
females; these changes were considered not to be toxicologically
significant. At 2000 and 20 000 ppm, significant haemolytic changes
and a reduced mean myeloid:erythroid ratio in bone marrow were seen,
with dose-related increases in the absolute and relative (to body
and to brain) weights of the spleen and liver. Histopathological
examination revealed an increased incidence and/or severity of pigment
deposition in the liver, spleen, and kidney tubules and increased
extramedullary haematopoiesis in the spleen. The pigment was
characterized as bile in the liver and haemosiderin in the liver,
spleen and kidney. The primary target of tebufenozide was the
erythrocyte, causing increased erythrocyte turnover and compensatory
responses from the haematopoietic tissues. The NOAEL was 200 ppm,
equal to 35 mg/kg bw per day (Osheroff, 1991a).
Rat
Groups of six male and six female Crl:CD BR rats were given
technical-grade tebufenozide (purity, 94%) in the diet to provide
levels of 0, 50, 250, 1000, 2500, or 10 000 ppm, equal to 0, 3.8, 19,
71, 180, and 700 mg/kg bw per day for males and 0, 4.5, 21, 85, 210
and 780 mg/kg bw per day for females, for two weeks. The relative
weight of the liver was increased in males and the absolute and
relative weights in females at 1000 ppm; however, in the absence of
histopathological data, the toxicological significance of these
changes could not be fully evaluated. At 10 000 ppm, slight reductions
in body-weight gain, food consumption, and erythrocyte parameters were
seen, which were within the normal range of biological variations and
were considered to be of minimal toxicological significance. At this
dose, increased absolute and relative spleen weights were observed in
males and females; however, in the absence of histopathological data,
the significance of these changes could not be evaluated. The NOAEL
was 250 ppm, equal to 19 mg/kg bw per day, on the basis of the changes
in liver weight (Kyle & Quinn, 1992).
In a range-finding study, groups of 10 male and 10 female Crl:CD
BR Sprague-Dawley rats were given diets containing technical-grade
tebufenozide (purity, 96.4%) to provide levels of 0 or 20 000 ppm,
equal to 0 and 1500 mg/kg bw per day in males and 0 and 1600 mg/kg bw
per day in females, for four weeks. Decreases in body weight (< 9%),
body-weight gain, and food consumption (< 12%) were observed, but the
mean efficiency of food use was not affected, suggesting reduced
palatability of the 20 000-ppm test diet. Slight reductions in
erythrocyte count, haemoglobin, and haematocrit were seen. The
absolute and relative liver weights of animals of each sex, the
absolute and relative spleen weights of males, and the relative kidney
weights of females were higher in the treated rats, but, in the
absence of histopathological data, the toxicological significance of
these changes could not be fully assessed. It was concluded that the
high dietary level of 20 000 ppm could be used in studies of short-
term toxicity in rats (Osheroff, 1991b).
Groups of 10 male and 10 female Crl:CD BR rats were fed diets
containing technical-grade tebufenozide (purity, 96.4 or 98.6%) in the
diet to provide levels of 0, 20, 200, 2000, or 20 000 ppm, equal to 0,
1.3, 13, 130, and 1300 mg/kg bw per day for males and 0, 1.6, 16, 160,
and 1600 mg/kg bw per day for females, for 13 weeks. The dose of
2000 ppm induced significant decreases in overall body-weight gain and
mean food consumption during the first four weeks of dosing and an
increase in the relative (to brain) weight of the liver in females.
Slight haemolytic anaemia, increased bone-marrow erythropoiesis
(decreased mean myeloid:erythroid ratio), and increased deposition of
pigment in the spleen were observed. At the highest dose, additional
treatment-related effects included overt haemolytic changes, slightly
elevated absolute and relative (to brain) spleen weights in animals of
each sex, elevated absolute liver weight in females, and tubular
nephrosis of the kidney in four males. The NOAEL was 200 ppm, equal to
13 mg/kg bw per day (Osheroff, 1991c).
Groups of six male and six female Crl:CD BR rats received
applications of technical-grade tebufenozide (purity, 97.2%) moistened
with 0.9% saline (1:6 w/v) at a dose of 1000 mg/kg bw per day on the
shaved, intact skin of the back under semi-occlusive bandages for
6 h per day, five days per week for four weeks (a total of 21
applications). No signs of treatment-related systemic toxicity or
dermal irritation were observed. The NOAEL was thus 1000 mg/kg bw per
day (Morrison et al., 1993).
Dogs
Five groups of four male beagle dogs received diets containing
technical-grade tebufenozide (purity, 96.8%) at 0, 150, 600, 2400,
or 9600 ppm, equal to 0, 5, 19, 77, and 290 mg/kg bw per day, for
two weeks. A significant increase in the mean spleen weight was
seen in animals at 600 ppm. At 9600 ppm, mild haemolytic anaemia
(significantly reduced erythrocyte count, haemoglobin, and
haematocrit) was observed. The NOAEL was 150 ppm, equal to 5 mg/kg bw
per day (Kehoe, 1990).
Two groups of four male beagle dogs were fed diets containing
technical-grade tebufenozide (purity, 97.5%) at 0 or 1500 ppm,
equal to 42 mg/kg bw per day, for six weeks. All animals were then
maintained on the basal diet for a four-week recovery period, after
which the study was terminated. Haematological examinations were
performed on all dogs before treatment and at 6, 8, and 10 weeks.
Administration of 1500 ppm resulted in mild regenerative anaemia, but
total recovery had occurred four weeks after cessation of treatment
(Yoshida, 1992).
Groups of four male and four female beagle dogs received diets
containing technical-grade tebufenozide (purity, 96.1%) at 0, 50,
500, or 5000 ppm, equal to 0, 2.1, 20, and 200 mg/kg bw per day for
males and 0, 2, 21, and 200 mg/kg bw per day for females, for 90
days. Animals of each sex at 500 ppm had an increased incidence of
Heinz bodies, and females at this dose had an elevated mean total
bilirubin level and increased absolute and relative spleen weights.
Histopathological examination revealed an increased incidence of
pigment deposition (haemosiderin) in the Kupffer cells of the liver
and increased haematopoiesis and sinusoidal engorgement of the spleen.
At the highest dose, significant haemolytic changes and increased
bone-marrow erythropoiesis (reduced mean myeloid:erythroid ratio) were
seen at weeks 6 and 13 of treatment. The mean total plasma bilirubin
level was elevated in animals of each sex, and bilirubin was present
in the urine of three males. Increased absolute and relative spleen
weights and slightly higher relative liver weights were observed.
Increased incidences of pigment deposition (haemosiderin) and the
presence of erythrocytes in some Kupffer cells of the liver suggested
active erythrophagocytosis. Increased splenic haematopoiesis, splenic
sinusoidal engorgement, and bone-marrow hyperplasia were also noted.
The primary target of tebufenozide in the dog was the erythrocytes,
leading to mild haemolytic anaemia and compensatory responses from the
haematopoietic tissues. The NOAEL was 50 ppm, equal to 2 mg/kg bw per
day (Clay, 1992).
Groups of four male and four female beagle dogs were fed diets
containing technical-grade tebufenozide (purity, 97.5%) providing
dietary levels of 0, 15, 50, 250, or 1500 ppm, equal to 0, 0.6, 1.8,
8.7, or 53 mg/kg bw per day for males and 0, 0.6, 1.9, 8.9, and
56 mg/kg bw per day for females, for 52 weeks. In animals at 250 ppm,
slight but consistent haemolytic changes and a slightly elevated total
plasma bilirubin level were seen, the latter especially in females,
over the 52-week period. The mean absolute and relative spleen weights
of females and the mean relative liver weight of males were increased,
and an increased incidence of pigment deposition in the Kupffer
cells of the liver, increased splenic haematopoiesis and sinusoidal
engorgement, and bone-marrow hyperplasia were also observed. Similar
treatment-related effects of increased magnitude and severity were
observed at the highest dose. The primary target of tebufenozide was
thus erythrocytes, leading to mild peripheral haemolytic anaemia and
compensatory responses from the haematopoietic tissues. The NOAEL was
50 ppm, equal to 1.8 mg/kg bw per day (Richards, 1992).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female Crl:CD-1(ICR)BR mice received
diets containing technical-grade tebufenozide (purity, 96.1%) at 0, 5,
50, 500, or 1000 ppm, equal to 0, 0.8, 7.8, 78, and 160 mg/kg bw per
day for males and 0, 0.9, 9.4, 94, and 190 mg/kg bw per day for
females, for 18 months. Each group included a satellite group of 10
mice of each sex that were killed at interim sacrifice after 52 weeks
of treatment. In animals at 500 ppm, a slight reduction in the
survival rate of males and increased pigment deposition in the spleens
of males and females were observed at both interim and terminal
sacrifices. At the highest dose, the survival rates of both males
and females were reduced, and signs of mild haemolytic anaemia (a
small but significant increase in the blood level of methaemoglobin
and increased incidences of polychromasia and echinocytosis in
erythrocytes) and further increases in splenic pigment deposition were
observed at interim and/or terminal sacrifice. At terminal sacrifice,
males had increased relative spleen weights, and females had an
increased incidence of extramedullary haematopoiesis in the spleen.
There was no evidence of an oncogenic effect at any dose. The NOAEL
for general toxicity was 50 ppm, equal to 7.8 mg/kg bw per day
(Trotter, 1992a).
Rats
Groups of 60 male and 60 female Crl:CD BR rats received diets
containing technical-grade tebufenozide (purity, 96.1%) providing
levels of 0, 10, 100, 1000, or 2000 ppm, equal to 0, 0.5, 4.8, 48, and
97 mg/kg bw per day for males and 0, 0.6, 6.1, 61, and 120 mg/kg bw
per day for females, for two years. Each group included a satellite
group of 10 rats of each sex that were killed at interim sacrifice
during week 53; two sentinel groups of five rats of each sex per group
were maintained to screen general health before and at the end of the
treatment period. Animals at 1000 or 2000 ppm showed significant
decreases in mean body weight and body-weight gain, the latter more
pronounced in females, and females had a reduced mean food consumption
throughout the study. Signs of mild haemolytic anaemia were seen only
during the first 52 weeks of treatment, suggesting that the effects
on the haematopoietic system were transient and reversible. Slight
increases in the incidence and/or severity of pigment deposition
(haemosiderin) were seen in the spleens of animals of each sex at
interim and/or terminal sacrifices, suggesting active splenic
erythrophagocytosis. In addition, females at 1000 and 2000 ppm had an
increased frequency of swelling of body areas (principally the mammary
gland regions) during the first 70 weeks of treatment; however, in the
absence of any supporting histopathological findings in the mammary
gland tissue or skin, the toxicological significance of these
transient swellings could not be ascertained. No treatment-related
neoplastic lesions were noted in any tissue or organ of any treated
rat at any dose. The NOAEL for systemic toxicity was 100 ppm, equal to
4.8 mg/kg bw per day (Trutter, 1992b).
(d) Reproductive toxicity
Rats
In a two-generation study, with one litter per generation, groups
of 25 male and 25 female weanling Crl:CD BR rats were fed diets
containing technical-grade tebufenozide (purity, 97.5%) providing
dietary levels of 0, 10, 150, or 2000 ppm, equal to 0, 0.8, 12, and
150 mg/kg bw per day for males and 0, 0.9, 13, and 170 mg/kg bw per
day for females. The diets were fed for 70 (F0) or 105 days (F1)
before mating. No treatment-related effects were observed in either
generation at the low dose. At 150 ppm, an increased severity of
pigment deposition in the spleen of F0 and F1 females was reported.
At the highest dose, the body weight and feed consumption of F0 and
F1 males were decreased at some intervals before mating. Increased
splenic pigment deposition and extramedullary haematopoiesis were seen
in parental animals of each sex in both generations. The mean number
of implantation sites in F1 females (not measured in F0) was
decreased and the length of gestation in F1 (but not F0) dams
increased. There was also a small increase in the number of pregnant
females in both generations that had total resorptions and in the
number of F1 females that died during delivery. The NOAEL was 10 ppm,
equal to 0.8 mg/kg bw per day, for general toxicity and 150 ppm, equal
to 12 mg/kg bw per day, for reproductive toxicity (Danberry et al.,
1993).
In a similar study, groups of 24 male and 24 female weanling
Crj:CD(SD) rats were given diets containing technical-grade
tebufenozide (purity, 97.2%) providing levels of 0, 25, 200, or
2000 ppm, equal to 0, 1.6, 13, and 130 mg/kg bw per day for males and
0, 1.8, 15, and 140 mg/kg bw per day for females, for 10 weeks before
mating of the F0 and F1 parents to produce the respective F1 and F2
offspring. The study was terminated after weaning of the F2 litters.
An increased incidence of splenic lesions, seen as blackish changes
and/or congestion of the spleen, increased splenic extramedullary
haematopoiesis, and haemosiderin-laden cells, occurred in F0 and F1
parental animals at 200 and 2000 ppm. At 2000 ppm, slight decreases in
body-weight gain were seen in animals of each sex, and decreases in
uterine and ovarian weights were reported; the changes were small and
were considered not to be toxicologically significant. A significant
reduction in mean body-weight gain was seen in F1 and F2 pups at
2000 ppm between lactation days 14 and 21. The study revealed no
adverse effects on the reproductive ability of male or female rats.
The NOAEL was 25 ppm, equal to 1.6 mg/kg bw per day, for systemic
toxicity and 200 ppm, equal to 13 mg/kg bw per day, for reproductive
toxicity (Aso, 1995).
In both of the above studies, a consistent increase in the
incidence of gross and histopathological findings in the spleen,
including congestion, pigment, and extramedullary haematopoiesis, was
seen in F0 and F1 parental animals. Although tebufenozide at the
doses tested had no effect on the reproductive ability of male or
female rats, the minor reproductive effects observed at 2000 ppm
on parental females in the first study and on lactating pups in
the second study could not be discounted. The overall NOAEL for
reproductive toxicity was thus 200 ppm, equal to 13 mg/kg bw per day.
(e) Developmental toxicity
Rats
In a range-finding study, groups of nine mated female Crl:CD BR
rats were given technical-grade tebufenozide (purity, 96.8%) by gavage
at doses of 0 (vehicle control), 25, 75, 200, or 400 mg/kg bw per day
on days 6-15 of gestation. On day 20, all surviving dams were killed,
and their fetuses were removed from the uterus and necropsied.
No maternal, embryo, or fetal toxicity and no external gross
malformations or anomalies in the fetuses were seen at any dose. The
NOAEL for maternal, embryonic, and fetal toxicity and teratogenicity
was thus 400 mg/kg bw per day, the highest dose tested. In a
supplementary range-finding study, groups of six non-pregnant female
Crl:CD BR rats were given technical-grade tebufenozide (purity, 96.8%)
by gavage at doses of 0, 400, or 1000 mg/kg bw per day for 10 days. At
1000 mg/kg bw per day, a slight increase in liver weight was observed;
however, in the absence of histopathological data, the toxicological
significance of this finding could not be fully evaluated. On the
basis of the very limited data provided, the NOAEL was 400 mg/kg bw
per day (Solomon & Romanello, 1992).
In the main study, groups of 25 mated Crl:CD BR VAF/Plus
Sprague-Dawley rats were given technical-grade tebufenozide (purity,
96.1%) by gavage at doses of 0, 50, 250, or 1000 mg/kg bw per day on
days 6-15 of presumed gestation (day 0 taken as the day spermatozoa
were detected in a vaginal lavage or a copulatory plug was observed
in situ). On gestation day 20, all surviving dams were killed, and
their fetuses were removed and necropsied. At 1000 mg/kg bw per day,
initial slight, transient reductions in mean body-weight gain and food
consumption were seen during dosing, but the mean values over the
study were not affected; the changes were considered not to be
toxicologically significant. All of the fetuses showed normal
development, and no signs of fetotoxicity and no treatment-related
malformations were observed at any dose tested. The NOAEL was
1000 mg/kg bw per day for maternal toxicity and 1000 mg/kg bw per
day, the highest dose tested, for embryo- and fetotoxicity and
teratogenicity (Hoberman, 1991).
Rabbits
In a range-finding study, groups of six mated female Hra:(NZW)SPF
rabbits were given technical-grade tebufenozide (purity, 96.4%) by
gavage at doses of 0, 100, 300, or 1000 mg/kg bw per day on days 7-19
of presumed gestation (the day of mating confirmed by the presence of
seminal fluid in the vulva). All surviving does were sacrificed, and
all fetuses were removed and examined on day 29 of gestation. The
single death due to an unknown cause among animals at 1000 mg/kg bw
per day was unlikely to have been due to treatment, as no deaths
occurred in the main study. No other signs of maternal toxicity or
treatment-related disturbance of intrauterine development of the
conceptuses were observed. All fetuses showed normal development, and
no signs of fetal toxicity or treatment-related malformations were
seen at any dose. The NOAEL for embryo- and fetotoxicity and
teratogenicity was 1000 mg/kg bw per day, the highest dose tested
(Lemen, 1991).
In the main study, groups of 20 mated female New Zealand white
rabbits were given technical-grade tebufenozide (purity, 97.5%) by
gavage at doses of 0, 50, 250, or 1000 mg/kg bw per day on days 7-19
of presumed gestation (the day of mating confirmed by the presence of
seminal fluid in the vulva). All surviving does were sacrificed, and
their fetuses were removed and examined on day 29 of gestation. No
treatment-related deaths, clinical signs of maternal toxicity, or
disturbances of intrauterine development of the conceptuses were
observed at any dose. All of the fetuses showed normal development,
and no signs of fetotoxicity or treatment-related malformations were
observed at any dose. The NOAEL for maternal toxicity, embryo- and
fetotoxicity and teratogenicity was 1000 mg/kg bw per day, the highest
dose tested (Swenson & Solomon, 1992).
(f) Genotoxicity
A battery of tests for mutagenicity was conducted to assess the
potential of technical-grade tebufenozide (purity, > 95-97.5%) to
induce gene mutation, chromosomal aberration, or unscheduled DNA
synthesis. The results, summarized in Table 2, were all negative.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade tebufenozide (purity, 96-97%) was not irritating
to the skin and was minimally irritating to the eyes of male New
Zealand white rabbits, according to Draize scale scoring (Krajewski
et al., 1988a,b; Lutz & Parno, 1993).
Table 2. Results of tests for the genotoxicity of technical-grade tebufenozide
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 0, 50-5000a µg/plate > 95 Negativeb Black (1986)
TA98, TA100,
TA1535, TA1537
Reverse mutation S. typhimurium 0, 50-5000a µg/plate 96.1 Negativeb Sames & Streelman
TA98, TA100, (1991)
TA1535, TA1537
Reverse mutation S. typhimurium 0, 50-5000a µg/plate 96.1 Negativeb Sames & Elia (1993)
TA98, TA100, 0, 160-1600a µg/plate Negativeb
TA1535, TA1537
Reverse mutation S. typhimurium 0, 50-5000a µg/plate 96.1 Negativeb Sames & Elia (1994)
TA98, TA100, 0, 30-300a µg/plate Negativeb
TA1535, TA1537
Reverse mutation E. coli WP2 uvr A 0, 200-5000a µg/plate 96.1 Negativeb Watanabe (1992)
Forward mutation Chinese hamster 0, 10-60 µg/ml 96.4 Negativeb Thilagar (1990a)
ovary cells
(hprt locus)
Chromosomal aberration Chinese hamster 0, 5-30 µg/ml 96.8 Negativeb Thilagar (1987)
ovary cells
Unscheduled DNA Rat (SD) primary 0, 10-100a µg/ml 96.4 Negative Thilagar (1990b)
synthesis hepatocytes 0, 10-60 µg/ml Negative
In vivo
Chromosomal aberration Rat (CD, 5-7 of each 0, 0.5-5.0 g/kg bw 97.5 Negative Gudi (1992)
sex per dose) bone by single gavage
marrow
a Precipitation observed in all cultures
b Conducted with and without exogenous metabolic activation
Technical-grade tebufenozide (purity, 96%) did not sensitize the
skin of guinea-pigs assessed in the maximization test (males) and the
Buehler test (females) (Matsumoto, 1989; Glaza, 1993).
(ii) Neurotoxicity
Groups of 10 male and 10 female Crl:CD R BR rats were given
single doses of technical-grade tebufenozide (purity, 96.1%) in
0.5% w/v aqueous methylcellulose by gavage at 0, 500, 1000, or
2000 mg/kg bw. All rats were subjected to functional observational
battery testing and motor activity assessment before treatment and
1-3 h and 7 and 14 days after dosing, and a detailed neuropathological
examination was carried out at the end of the 14-day observation
period. No remarkable treatment-related neurological changes were
found at any dose. Treatment-related responses were elicited in
similar acute neurotoxicity studies conducted at the same testing
laboratory with substances known to affect functional observational
battery and motor activity testing, thus validating the competence of
the laboratory in this type of testing. The NOAEL for the neurotoxic
and neuropathological effects of tebufenozide was thus 2000 mg/kg bw,
the highest dose tested (Swenson et al., 1994).
(iii) Studies of metabolites
Five metabolites of tebufenozide were tested for acute toxicity
in Crj: CD-1 or Crl:CD-1 BR ICR mice. Metabolites RH-111788, RH-96595,
RH-120970, RH-089886, and RH-112651 (see Figure 1) given orally
induced no deaths and no clinical signs of systemic toxicity at doses
up to 5000 mg/kg bw. The LD50 values for these metabolites in male
and female mice was thus > 5000 mg/kg bw (Hazelton & Quinn, 1993).
Four tebufenozide metabolites, RH-111788, RH-96595, RH-120970,
and RH-089886, were tested for their potential to induce reverse
mutation in Salmonella typhimurium strains TA98, TA100, TA1535, and
TA1537 and in Escherichia coli strain WP2 uvr A at concentrations up
to the limit of solubility (1250-2500 µg/plate), with and without
exogenous metabolic activation. The results, summarized in Table 3,
were negative (Hazelton & Quinn, 1993).
Table 3. Results of tests for the genotoxicity of metabolites
of tebufenozide
Metabolite Concentration Result
(µg/plate)
RH-89886 (in rats, rice) 0, 313-5000a Negative
RH-111788 (in rats, rice) 0, 313-5000a Negative
RH-96595 (in rats, rice, soil) 0, 313-5000a Negative
RH-120970 (in rats, rice) 0, 313-5000a Negative
Potential for induction of reverse mutation in vitro in S.
typhimurium TA98, TA100, TA1535, and TA1537 and in E. coli
WP2 uvr A, with and without exogenous metabolic activation by
a microsomal fraction from rat liver
a Precipitation observed on all plates
Comments
Oral administration to rats of single doses of 3 or 250 mg/kg
bw of 14C-labelled tebufenozide resulted in rapid absorption and
excretion in urine and faeces, only trace amounts of 14C being
recovered in expired air. The excretion profiles were similar,
regardless of the position of the 14C label, the dose, the sex, or
whether the rats had been pretreated with 30 ppm of unlabelled
tebufenozide in the diet for two weeks. A mean total of 87-104% of the
administered radiolabel was eliminated within 48 h, primarily via the
faeces, which accounted for 90% of the 14C that was excreted; only
minor amounts (1-8%) were excreted in urine and trace amounts
(0.1-0.4%) in expired air. In animals at 3 mg/kg bw, absorption
accounted for 35-39 % of the administered radiolabel; 30-34% was
excreted in the bile and about 5% in the urine. In rats at 250 mg/kg
bw, only about 4% of the administered dose was absorbed and
metabolized. The highest levels of 14C in the blood were measured
0.5-12 h after dosing, and clearance of the radiolabel from the
circulation was rapid. Tissue retention of 14C was low, suggesting
that there is little or no bioaccumulation of tebufenozide in the
body.
Most of the 14C excreted in the faeces was in the form of
unabsorbed (parent) tebufenozide, which accounted for about 60 and 90%
of an administered dose of 3 and 250 mg/kg bw per day, respectively;
no unchanged tebufenozide was detected in the urine. The absorbed
14C-tebufenozide was extensively metabolized in rats. There were no
significant qualitative differences in the metabolic profiles
associated with the position of the 14C label, the dose, sex, or
whether rats were pretreated with unlabelled tebufenozide. In general,
the 13-15 metabolites identified in the urine, faeces, and bile were
identical. The main route of metabolism of tebufenozide appeared to be
oxidation of benzylic carbons (A- or B-ring), resulting in a number of
metabolites with various combinations of oxidation state at the three
oxidized carbon centres and one metabolite produced by oxidation of a
nonbenzylic, terminal carbon on the A-ring ethyl group.
Tebufenozide was of low acute toxicity after administration to
mice orally and to rats by the oral, dermal, or inhalation route. The
oral LD50 in mice and rats was > 5000 mg/kg bw; the dermal LD50 in
rats was > 5000 mg/kg bw; and the inhalation LC50 in rats was
> 4.3 mg/litre. The metabolites were also of low acute toxicity to
mice after oral administration. Tebufenozide was not irritating to
the skin and was minimally irritating to the eyes of male rabbits;
it was not a skin sensitizer in guinea-pigs. WHO has not classified
tebufenozide for acute toxicity.
Repeated short-term oral administration of tebufenozide to mice
(2 and 13 weeks), rats (2, 4, and 13 weeks), and dogs (2, 6, 13, and
52 weeks) resulted primarily in haematotoxic effects (regenerative
haemolytic anaemia and compensatory responses from the haematopoietic
tissues). The NOAEL for these effects was 200 ppm, equal to 35 mg/kg
bw per day, in mice in a 13-week study (0, 20, 200, 2000, and 20 000
ppm tested); 200 ppm, equal to 13.1 mg/kg bw per day, in rats in a
13-week study (0, 20, 200, 2000, and 20 000 ppm tested); 50 ppm, equal
to 2 mg/kg bw per day, in dogs in a 13-week study (0, 50, 500, and
5000 ppm tested); and 50 ppm, equal to 1.8 mg/kg bw per day, in a
one-year study in dogs (0, 15, 50, 250, and 1500 ppm tested). Repeated
dermal applications of tebufenozide to rats for four weeks caused
no systemic toxicity at doses < 1000 mg/kg bw per day. The dog
appeared to be the most sensitive species for both short-term and
long-term toxicity.
In an 18-month study of toxicity and carcinogenicity in mice
given tebufenozide in the diet at concentrations of 0, 5, 50, 500,
or 1000 ppm, the NOAEL for systemic toxicity was 50 ppm, equal to
7.8 mg/kg bw per day, on the basis of a slightly reduced survival
rate and mild regenerative haemolytic anaemia at higher doses. In
a two-year study of toxicity and carcinogenicity in rats given
tebufenozide in the diet at 0, 10, 100, 1000, or 2000 ppm, the NOAEL
was 100 ppm, equal to 4.8 mg/kg bw per day, on the basis of decreased
body weight and food consumption and mild regenerative haemolytic
anaemia at higher doses. Tebufenozide was not carcinogenic to mice or
rats under the conditions of the studies.
Tebufenozide and its metabolites have been adequately tested for
genotoxicity in a range of assays both in vitro and in vivo. The
Meeting concluded that neither tebufenozide nor its metabolites are
genotoxic.
In two two-generation studies of reproductive toxicity in rats,
with one litter per generation, doses of 0, 10, 150, or 2000 ppm and
0, 25, 200, or 2000 ppm were administered. The NOAEL for systemic
(parental) toxicity was 25 ppm, equal to 1.6 mg/kg bw per day, on
the basis of a consistent increase in the incidence of gross and
histopathological lesions in the spleens (congestion, pigment, and
extra-medullary haematopoiesis) of F0 and F1 parental animals at
higher doses (200 and 2000 ppm). The NOAEL for reproductive toxicity
was 13 mg/kg bw per day, on the basis of minor reproductive effects
(decreased mean number of implantation sites, prolonged gestation, a
slightly greater frequency of total resorptions, and a small increase
in the number of dams that died during delivery) at the high dose of
2000 ppm in dams in the first study and in lactating pups (decreased
mean weight gain on lactation days 14 and 21) in the second study.
In studies of developmental toxicity in rats and rabbits, doses
of 0, 50, 250, or 1000 mg/kg bw per day were administered. There was
no evidence of teratogenic potential. The NOAEL for maternal, embryo-,
and fetotoxicity and teratogenicity was 1000 mg/kg bw per day, the
highest dose tested, in both species.
In a study of acute neurotoxicity in rats, no treatment-related
effects were seen when single doses of 0, 500, 1000, or 2000 mg/kg bw
were administered. The NOAEL for acute neurotoxicity and neuro-
pathological effects was 2000 mg/kg bw, the highest dose tested.
In summary, exposure to tebufenozide by the oral route results
primarily in haematotoxicity. The main target of its action is the
peripheral haematopoietic system; the pivotal toxicological end-point
of concern, which is seen consistently across all species tested, is
mild regenerative haemolytic anaemia with compensatory responses from
the haematopoietic tissues.
An ADI of 0-0.02 mg/kg bw was established for tebufenozide on the
basis of the NOAELs for haematotoxicity of 1.8 mg/kg bw per day in the
one-year study in dogs and 1.6 mg/kg bw per day in a two-generation
study of reproductive toxicity in rats, using a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 200 ppm, equal to 35 mg/kg bw per day (13-week study of
toxicity)
50 ppm, equal to 7.8 mg/kg bw per day (haematotoxicity
in an 18-month study of toxicity and carcinogenicity)
Rat: 200 ppm, equal to 13 mg/kg bw per day (13-week study of
toxicity)
100 ppm, equal to 4.8 mg/kg bw per day (haematotoxicity
in a two-year study of toxicity and carcinogenicity)
25 ppm, equal to 1.6 mg/kg bw per day (maternal
haematotoxicity in a two-generation study of
reproductive toxicity)
200 ppm, equal to 13 mg/kg bw per day (reproductive
toxicity in a two-generation study)
1000 mg/kg bw per day, the highest dose tested
(maternal, embryo-, and fetotoxicity and teratogenicity
in a study of developmental toxicity)
Rabbit: 1000 mg/kg bw per day, the highest dose tested
(maternal, embryo-, and fetotoxicity and teratogenicity
in a study of developmental toxicity)
Dog: 50 ppm, equal to 1.8 mg/kg bw per day (haemotoxicity in
a one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Observations in humans
2. Studies on the mechanism of haematotoxicity
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to tebufenozide
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 > 5000 mg/kg bw
Dermal, toxicity, rat LD50 > 5000 mg/kg bw
Inhalation, 4 h, toxicity, rat LC50 > 4.3 mg/litre
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Minimally irritating
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated dietary, 90 days, toxicity, dog NOAEL = 2 mg/kg bw per day, primarily
haematotoxicity
Repeated dermal, 28 days, toxicity, rat NOAEL = 1000 mg/kg bw per day, highest dose
tested
Repeated dietary, reproductive toxicity, rat NOAEL = 13 mg/kg bw per day, minor reproductive
effects
Repeated gavage, developmental toxicity, NOAEL = 1000 mg/kg bw per day, highest dose
rat and rabbit tested; maternal, embryo-, and fetal toxicity
and teratogenicity
Long-term (> 1 year) Repeated dietary, 1 year, toxicity, dog NOAEL = 1.8 mg/kg bw per day, primarily
haematotoxicity
References
Aso, S. (1995) Two-generation reproduction study of RH-5992 in rats.
Unpublished report No. 93RC-101 from Hita Research Laboratories, Oita,
Japan. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Black, C.A. (1986) RH-75,992: Microbial mutagenicity assay (Ames
test). Unpublished report No. 86R-0013 from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Clay, H. (1992). RH-5992: 90-day oral (dietary) administration
toxicity study in the beagle. Unpublished report No. 90RC-062 from
Hazleton UK, North Yorkshire, United Kingdom. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Danberry, T.L., Romanello, A.S., Donofrio, K.F. & O'Hara, G.P. (1993)
RH-5992: Two-generation reproduction study in rats. Unpublished report
No. 9OR-202A from Rohm & Haas Co., Toxicology Department, Philadelphia,
PA, USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Glaza, S.M. (1993) Dermal sensitization study of RH-5992 technical in
guinea pigs - maximization test. Unpublished report No. 92RC-101 from
Hazleton Wisconsin, Inc., WI, USA. Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Gudi, R (1992) RH-5992 technical: Acute test for chemical induction of
chromosome aberration in rat bone marrow cells in vivo. Unpublished
report No. 9ORC-203 from Sitek Research Laboratories, MD, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hawkins, D.R. & Hazelton, G.A. (1993) 14C-RH-5992: Biliary excretion
study in rats, supplemental report A: Metabolism of RH-5992 in rats.
Unpublished report No. 9ORC-058A from Rohm & Haas Co., Agricultural
Products Development Department, PA, USA. Submitted to WHO by Rohm &
Haas Co., Philadelphia, PA, USA.
Hawkins, D.R., Spencer, W.O. & Hazelton, G.A. (1992) 14C-RH-5992
pharmacokinetic study in rats, supplemental report A: Metabolism of
RH-5992 in rats. Unpublished report No. 91RC-004A from Rohm & Haas
Co., Agricultural Products Development Department, PA, USA. Submitted
to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hawkins, D.R., Spencer W.O. & Hazelton, G.A. (1993) 14C-RH-5992:
Pharmacokinetic study in rats, supplemental report B: Metabolism of
RH-5992 in rats. Unpublished report No. 91RC-004B from Rohm & Haas
Co., Agricultural Products Development Department, PA, USA. Submitted
to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A. & Quinn, D.L. (1993) Hazard evaluation of RH-5992
technical in human and domestic animals. Part I: Hazard identification
and evaluation. Unpublished report No. 93R-1039 from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Hazelton, G.A. & Quinn, D.L. (1995) Evaluation of rat reproduction
studies conducted with RH-5992. Unpublished report No. 95R-1068 from
Rohm & Haas Co., Toxicology Department, Philadelphia, PA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hoberman, A.M. (1991) RH-5992: Oral developmental toxicity (embryo-
fetal toxicity and teratogenic potential) study in rats. Unpublished
report No. 9ORC-059 from Argus Research Laboratories, Inc., PA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Kehoe, D.F. (1990) RH-5992: Two-week dietary range-finding study in
male dogs. Unpublished report No. 87RC-034 from Hazleton Laboratories
America, Inc., WI, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988a) RH-75,992:
Skin irritation study in rabbits. Unpublished report No. 87R-088 from
Rohm & Haas Co., Toxicology Department, Philadelphia, PA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988b) RH-75,992: Eye
irritation study in rabbits. Unpublished report No. 87R-084 from Rohm
& Haas Co., Toxicology Department, Philadelphia, PA, USA. Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Kyle, M.E. (1992) RH-5992: Two-week dietary range-finding study in
male mice. Unpublished report No. 86R-228A from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Kyle, M.E. & Quinn, D.L. (1992) RH-5992: Two-week dietary range-
finding study in rats. Unpublished report No. 86R-226A from Rohm &
Haas Co., Toxicology Department, Philadelphia, PA, USA. Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lemen, J.K. (1991) RH-5992: Range-finding rabbit developmental
toxicity study. Unpublished report No. 89RC-100 from Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
LeVan, L.W. (1991) 14C-RH-5992: Distribution of radioactivity in rats
following a single oral dose. Unpublished report No. 89RC-099 from
Hazleton Laboratories America, Inc., WI, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Lutz, M.F. & Parno, J.R. (1993) RH-75,992: Eye irritation study
in rabbits. Unpublished report No. 93R-237 from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Matsumoto, R. (1989) Skin sensitization study of RH-5992 (technical)
in the guinea pig. Unpublished report No. 9ORC-1002A from Bozo
Research Centre, Inc., Tokyo, Japan. Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Morrison, R.D. & Hamilton, J.D. (1991a) RH-75,992 technical: Acute
oral toxicity study in male and female mice. Unpublished report No.
91R-003 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D. & Hamilton, J.D. (1991b) RH-75,992 technical: Acute
oral toxicity study in male and female rats. Unpublished report No.
9OR-199 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D. & Hamilton, J.D. (1991c) RH-75,992 technical: Acute
dermal toxicity study in male and female rats. Unpublished report No.
9OR-200 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D., Carbone, J.P., Parno, J.R. & Gillette, D.M. (1993)
RH-75,992 technical and RH-75,992 2F formulation: Four-week dermal
toxicity study in rats. Unpublished report No. 92R-150 from Rohm &
Haas Co., Toxicology Department, Philadelphia, PA, USA. Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Osheroff, M.R. (1991a) RH-5992: 13-Week dietary toxicity study in
mice. Unpublished report No. 89RC-102 from Hazleton Laboratories
America, Inc., VA, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Osheroff, M.R. (1991b) RH-5992: Four-week range-finding dietary
toxicity study in rats. Unpublished report No. 89RC-101A from Hazleton
Laboratories America, Inc., MD, USA. Submitted to WHO by Rohm & Haas
Co., PA, USA.
Osheroff, M.R. (1991c) RH-5992: 13-week dietary toxicity study in
rats. Unpublished report No. 89RC-101 from Hazleton Laboratories
America, Inc., VA, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Richards, J.F. (1992) RH-5992: 52-week oral (dietary administration)
chronic toxicity study in the beagle. Unpublished report No. 90RC-206
from Hazleton UK, North Yorkshire, United Kingdom. Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Elia, M.C. (1993) RH-75,992 technical: Salmonella
typhimurium gene mutation assay (Ames test). Unpublished report No.
93R-094 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Elia, M.C. (1994) RH-75,992 technical: Salmonella
typhimurium gene mutation assay (Ames test). Unpublished report No.
93R-236 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Streelman, D.R. (1991) RH-75,992 technical: Salmonella
typhimurium gene mutation assay (Ames test). Unpublished report No.
9IR-167 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Romanello, A.S. (1992) RH-5992: Range-finding (gavage)
developmental toxicology study in rats. Unpublished report No.
87R-105A from Rohm & Haas Co., Toxicology Department, Philadelphia,
PA, USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Struble, C.B. & Hazelton, G.A. (1992a) 14C-RH-5992: Biliary excretion
study in rats. Unpublished report No. 90RC-058 from Hazleton
Wisconsin, Inc., WI, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Struble, C.B. & Hazelton, G.A. (1992b) 14C-RH-5992: Pharmacokinetic
study in rats. Unpublished report No. 91RC-004 from Hazleton
Wisconsin, Inc., WI, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Swenson, R.E. & Solomon, H.M. (1992) RH-5992: Oral (gavage)
developmental toxicity study in rabbits. Unpublished report No.
90R-201 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Swenson, R.E., Gillette, D.M. & Parno, J.R. (1994) RH-75,992: Acute
oral (gavage) neurotoxicity study in rats. Unpublished report No.
93R-093 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1987) RH-75,992: Test for chemical induction of
chromosome aberration using monolayer cultures of Chinese hamster
ovary (CHO) cells with and without metabolic activation. Unpublished
report No. 88RC-011 from Sitek Research Laboratories, MD, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1990a) RH-5992: Test for chemical induction of gene
mutation at the HGPRT locus in cultured Chinese hamster ovary (CHO)
cells with and without metabolic activation. Unpublished report No.
89RC-097 from Sitek Research Laboratories, MD, USA. Submitted to WHO
by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1990b) RH-5992: Test for chemical induction of
unscheduled DNA synthesis in rat primary hepatocyte cultures by
autoradiography. Unpublished report No. 89RC-098 from Sitek Research
Laboratories, MD, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Trutter, J.A. (1992a) RH-5992: 18-Month dietary oncogenicity study in
mice. Unpublished report No. 90RC-061 and 90RC-061A from Hazleton
Washington, Inc., VA, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Trutter, J.A. (1992b) RH-5992: 24-Month combined dietary chronic
toxicity and oncogenicity study in rats. Unpublished report No.
90RC-060 and 90RC-060A from Hazleton Washington, Inc., VA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Ulrich, C.E. (1992) RH-5992 (technical): Acute inhalation toxicity
evaluation in rats. Unpublished report No. 90RC-057 from International
Research and Development Corporation, MI, USA. Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Watanabe, K. (1992) RH-5992: Reverse mutation assay with E. coli
(WP2 uvr strain). Unpublished report No. 92RN-1010 from The Institute
of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Rohm &
Haas Co., Philadelphia, PA, USA.
Yoshida, A. (1992) RH-5992: Blood recovery study in dogs. Unpublished
report No. 92RC-1040 from The Institute of Environmental Toxicology,
Tokyo, Japan. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA,
USA.
Groups of Crl:CD BR Sprague-Dawley rats received a bile-duct
cannula and then a single oral dose of 3 mg/kg bw [14C- tert-butyl]-
tebufenozide. Bile samples collected from one male and one female rat
during 0-6 h after treatment, representing about 70% of total biliary
excretion of radiolabel over 72 h, were analysed for tebufenozide
metabolites. No parent compound was found; 13 biliary metabolites were
identified, and five unknown metabolites were isolated. In general,
the biliary metabolites were identical to those in the faeces and
urine, but three new metabolites were observed: [A-ring]-ketone-
[B-ring]-diol, RH-122652, and RH-2652. The last two were also
identified by mass spectroscopy in the faeces but at levels too low
for quantification. The unknown metabolites in bile appeared to be
high-molecular-mass amino-acid conjugates of acidic tebufenozide
metabolites. It was postulated that these conjugates are metabolized
in rats before excretion in order to recover the amino acids; hence,
their absence in faeces (Hawkins & Hazelton, 1993).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of tebufenozide are
summarized in Table 1. Tebufenozide was of low toxicity to mice and
rats when given orally, dermally, or by inhalation. No deaths or
clinical signs of systemic toxicity were observed at doses < 5.0 g/kg
bw. Dermal administration resulted only in transient, mild local
erythema at the site of application. After inhalation, accumulation
of red-brown material was seen around the nose, mouth, and eyes,
sometimes with a purulent anogenital discharge which lasted for one
to three days.
(b) Short-term toxicity
Mice
Six groups of eight male albino Crl:CD-1 ICR BR mice were fed
diets containing technical-grade tebufenozide (purity, 94%) to provide
doses of 0, 60, 200, 600, 2000, or 6000 ppm, equal to 0, 12, 39, 97,
350, or 1100 mg/kg bw per day, for two weeks. At the highest dose, the
absolute and relative liver weights were significantly increased,
but, in the absence of histopathological data, the toxicological
significance of this change could not be fully evaluated. The NOAEL
was 600 ppm, equal to 97 mg/kg bw per day, on the basis of the
increased relative liver weights (Kyle, 1992).
Table 1. Acute toxicity of tebufenozide in experimental animals
Species Sex Route LD50 (mg/kg bw) Reference
or LC50 (mg/litre)
Mouse Male, female Oral > 5000 Morrison & Hamilton (1991a)
Rat Male, female Oral > 5000 Morrison & Hamilton (1991b)
Rat Male, female Dermal > 5000 Morrison & Hamilton (1991c)
Rat Male Inhalation > 4.3 Ulrich (1992)
Female > 4.5
Groups of 10 male and 10 female Crl:CD-1 ICR BR VAF/Plus mice
were fed diets containing technical-grade tebufenozide (purity, 98.6%)
providing levels of 0, 20, 200, 2000, or 20 000 ppm, equal to 0, 3.4,
35, 340, and 3300 mg/kg bw per day for males and 0, 4.3, 45, 430, and
4200 mg/kg bw per day for females, for 13 weeks. At 200 ppm, slightly
reduced mean body-weight gain (but not overall body weight) was seen
in males and a marginally higher incidence of increased extramedullary
haematopoiesis in the spleen and pigment accumulation in kidney
tubules (with no concomitant changes in haematological parameters) in
females; these changes were considered not to be toxicologically
significant. At 2000 and 20 000 ppm, significant haemolytic changes
and a reduced mean myeloid:erythroid ratio in bone marrow were seen,
with dose-related increases in the absolute and relative (to body
and to brain) weights of the spleen and liver. Histopathological
examination revealed an increased incidence and/or severity of pigment
deposition in the liver, spleen, and kidney tubules and increased
extramedullary haematopoiesis in the spleen. The pigment was
characterized as bile in the liver and haemosiderin in the liver,
spleen and kidney. The primary target of tebufenozide was the
erythrocyte, causing increased erythrocyte turnover and compensatory
responses from the haematopoietic tissues. The NOAEL was 200 ppm,
equal to 35 mg/kg bw per day (Osheroff, 1991a).
Rat
Groups of six male and six female Crl:CD BR rats were given
technical-grade tebufenozide (purity, 94%) in the diet to provide
levels of 0, 50, 250, 1000, 2500, or 10 000 ppm, equal to 0, 3.8, 19,
71, 180, and 700 mg/kg bw per day for males and 0, 4.5, 21, 85, 210
and 780 mg/kg bw per day for females, for two weeks. The relative
weight of the liver was increased in males and the absolute and
relative weights in females at 1000 ppm; however, in the absence of
histopathological data, the toxicological significance of these
changes could not be fully evaluated. At 10 000 ppm, slight reductions
in body-weight gain, food consumption, and erythrocyte parameters were
seen, which were within the normal range of biological variations and
were considered to be of minimal toxicological significance. At this
dose, increased absolute and relative spleen weights were observed in
males and females; however, in the absence of histopathological data,
the significance of these changes could not be evaluated. The NOAEL
was 250 ppm, equal to 19 mg/kg bw per day, on the basis of the changes
in liver weight (Kyle & Quinn, 1992).
In a range-finding study, groups of 10 male and 10 female Crl:CD
BR Sprague-Dawley rats were given diets containing technical-grade
tebufenozide (purity, 96.4%) to provide levels of 0 or 20 000 ppm,
equal to 0 and 1500 mg/kg bw per day in males and 0 and 1600 mg/kg bw
per day in females, for four weeks. Decreases in body weight (< 9%),
body-weight gain, and food consumption (< 12%) were observed, but the
mean efficiency of food use was not affected, suggesting reduced
palatability of the 20 000-ppm test diet. Slight reductions in
erythrocyte count, haemoglobin, and haematocrit were seen. The
absolute and relative liver weights of animals of each sex, the
absolute and relative spleen weights of males, and the relative kidney
weights of females were higher in the treated rats, but, in the
absence of histopathological data, the toxicological significance of
these changes could not be fully assessed. It was concluded that the
high dietary level of 20 000 ppm could be used in studies of short-
term toxicity in rats (Osheroff, 1991b).
Groups of 10 male and 10 female Crl:CD BR rats were fed diets
containing technical-grade tebufenozide (purity, 96.4 or 98.6%) in the
diet to provide levels of 0, 20, 200, 2000, or 20 000 ppm, equal to 0,
1.3, 13, 130, and 1300 mg/kg bw per day for males and 0, 1.6, 16, 160,
and 1600 mg/kg bw per day for females, for 13 weeks. The dose of
2000 ppm induced significant decreases in overall body-weight gain and
mean food consumption during the first four weeks of dosing and an
increase in the relative (to brain) weight of the liver in females.
Slight haemolytic anaemia, increased bone-marrow erythropoiesis
(decreased mean myeloid:erythroid ratio), and increased deposition of
pigment in the spleen were observed. At the highest dose, additional
treatment-related effects included overt haemolytic changes, slightly
elevated absolute and relative (to brain) spleen weights in animals of
each sex, elevated absolute liver weight in females, and tubular
nephrosis of the kidney in four males. The NOAEL was 200 ppm, equal to
13 mg/kg bw per day (Osheroff, 1991c).
Groups of six male and six female Crl:CD BR rats received
applications of technical-grade tebufenozide (purity, 97.2%) moistened
with 0.9% saline (1:6 w/v) at a dose of 1000 mg/kg bw per day on the
shaved, intact skin of the back under semi-occlusive bandages for
6 h per day, five days per week for four weeks (a total of 21
applications). No signs of treatment-related systemic toxicity or
dermal irritation were observed. The NOAEL was thus 1000 mg/kg bw per
day (Morrison et al., 1993).
Dogs
Five groups of four male beagle dogs received diets containing
technical-grade tebufenozide (purity, 96.8%) at 0, 150, 600, 2400,
or 9600 ppm, equal to 0, 5, 19, 77, and 290 mg/kg bw per day, for
two weeks. A significant increase in the mean spleen weight was
seen in animals at 600 ppm. At 9600 ppm, mild haemolytic anaemia
(significantly reduced erythrocyte count, haemoglobin, and
haematocrit) was observed. The NOAEL was 150 ppm, equal to 5 mg/kg bw
per day (Kehoe, 1990).
Two groups of four male beagle dogs were fed diets containing
technical-grade tebufenozide (purity, 97.5%) at 0 or 1500 ppm,
equal to 42 mg/kg bw per day, for six weeks. All animals were then
maintained on the basal diet for a four-week recovery period, after
which the study was terminated. Haematological examinations were
performed on all dogs before treatment and at 6, 8, and 10 weeks.
Administration of 1500 ppm resulted in mild regenerative anaemia, but
total recovery had occurred four weeks after cessation of treatment
(Yoshida, 1992).
Groups of four male and four female beagle dogs received diets
containing technical-grade tebufenozide (purity, 96.1%) at 0, 50,
500, or 5000 ppm, equal to 0, 2.1, 20, and 200 mg/kg bw per day for
males and 0, 2, 21, and 200 mg/kg bw per day for females, for 90
days. Animals of each sex at 500 ppm had an increased incidence of
Heinz bodies, and females at this dose had an elevated mean total
bilirubin level and increased absolute and relative spleen weights.
Histopathological examination revealed an increased incidence of
pigment deposition (haemosiderin) in the Kupffer cells of the liver
and increased haematopoiesis and sinusoidal engorgement of the spleen.
At the highest dose, significant haemolytic changes and increased
bone-marrow erythropoiesis (reduced mean myeloid:erythroid ratio) were
seen at weeks 6 and 13 of treatment. The mean total plasma bilirubin
level was elevated in animals of each sex, and bilirubin was present
in the urine of three males. Increased absolute and relative spleen
weights and slightly higher relative liver weights were observed.
Increased incidences of pigment deposition (haemosiderin) and the
presence of erythrocytes in some Kupffer cells of the liver suggested
active erythrophagocytosis. Increased splenic haematopoiesis, splenic
sinusoidal engorgement, and bone-marrow hyperplasia were also noted.
The primary target of tebufenozide in the dog was the erythrocytes,
leading to mild haemolytic anaemia and compensatory responses from the
haematopoietic tissues. The NOAEL was 50 ppm, equal to 2 mg/kg bw per
day (Clay, 1992).
Groups of four male and four female beagle dogs were fed diets
containing technical-grade tebufenozide (purity, 97.5%) providing
dietary levels of 0, 15, 50, 250, or 1500 ppm, equal to 0, 0.6, 1.8,
8.7, or 53 mg/kg bw per day for males and 0, 0.6, 1.9, 8.9, and
56 mg/kg bw per day for females, for 52 weeks. In animals at 250 ppm,
slight but consistent haemolytic changes and a slightly elevated total
plasma bilirubin level were seen, the latter especially in females,
over the 52-week period. The mean absolute and relative spleen weights
of females and the mean relative liver weight of males were increased,
and an increased incidence of pigment deposition in the Kupffer
cells of the liver, increased splenic haematopoiesis and sinusoidal
engorgement, and bone-marrow hyperplasia were also observed. Similar
treatment-related effects of increased magnitude and severity were
observed at the highest dose. The primary target of tebufenozide was
thus erythrocytes, leading to mild peripheral haemolytic anaemia and
compensatory responses from the haematopoietic tissues. The NOAEL was
50 ppm, equal to 1.8 mg/kg bw per day (Richards, 1992).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female Crl:CD-1(ICR)BR mice received
diets containing technical-grade tebufenozide (purity, 96.1%) at 0, 5,
50, 500, or 1000 ppm, equal to 0, 0.8, 7.8, 78, and 160 mg/kg bw per
day for males and 0, 0.9, 9.4, 94, and 190 mg/kg bw per day for
females, for 18 months. Each group included a satellite group of 10
mice of each sex that were killed at interim sacrifice after 52 weeks
of treatment. In animals at 500 ppm, a slight reduction in the
survival rate of males and increased pigment deposition in the spleens
of males and females were observed at both interim and terminal
sacrifices. At the highest dose, the survival rates of both males
and females were reduced, and signs of mild haemolytic anaemia (a
small but significant increase in the blood level of methaemoglobin
and increased incidences of polychromasia and echinocytosis in
erythrocytes) and further increases in splenic pigment deposition were
observed at interim and/or terminal sacrifice. At terminal sacrifice,
males had increased relative spleen weights, and females had an
increased incidence of extramedullary haematopoiesis in the spleen.
There was no evidence of an oncogenic effect at any dose. The NOAEL
for general toxicity was 50 ppm, equal to 7.8 mg/kg bw per day
(Trotter, 1992a).
Rats
Groups of 60 male and 60 female Crl:CD BR rats received diets
containing technical-grade tebufenozide (purity, 96.1%) providing
levels of 0, 10, 100, 1000, or 2000 ppm, equal to 0, 0.5, 4.8, 48, and
97 mg/kg bw per day for males and 0, 0.6, 6.1, 61, and 120 mg/kg bw
per day for females, for two years. Each group included a satellite
group of 10 rats of each sex that were killed at interim sacrifice
during week 53; two sentinel groups of five rats of each sex per group
were maintained to screen general health before and at the end of the
treatment period. Animals at 1000 or 2000 ppm showed significant
decreases in mean body weight and body-weight gain, the latter more
pronounced in females, and females had a reduced mean food consumption
throughout the study. Signs of mild haemolytic anaemia were seen only
during the first 52 weeks of treatment, suggesting that the effects
on the haematopoietic system were transient and reversible. Slight
increases in the incidence and/or severity of pigment deposition
(haemosiderin) were seen in the spleens of animals of each sex at
interim and/or terminal sacrifices, suggesting active splenic
erythrophagocytosis. In addition, females at 1000 and 2000 ppm had an
increased frequency of swelling of body areas (principally the mammary
gland regions) during the first 70 weeks of treatment; however, in the
absence of any supporting histopathological findings in the mammary
gland tissue or skin, the toxicological significance of these
transient swellings could not be ascertained. No treatment-related
neoplastic lesions were noted in any tissue or organ of any treated
rat at any dose. The NOAEL for systemic toxicity was 100 ppm, equal to
4.8 mg/kg bw per day (Trutter, 1992b).
(d) Reproductive toxicity
Rats
In a two-generation study, with one litter per generation, groups
of 25 male and 25 female weanling Crl:CD BR rats were fed diets
containing technical-grade tebufenozide (purity, 97.5%) providing
dietary levels of 0, 10, 150, or 2000 ppm, equal to 0, 0.8, 12, and
150 mg/kg bw per day for males and 0, 0.9, 13, and 170 mg/kg bw per
day for females. The diets were fed for 70 (F0) or 105 days (F1)
before mating. No treatment-related effects were observed in either
generation at the low dose. At 150 ppm, an increased severity of
pigment deposition in the spleen of F0 and F1 females was reported.
At the highest dose, the body weight and feed consumption of F0 and
F1 males were decreased at some intervals before mating. Increased
splenic pigment deposition and extramedullary haematopoiesis were seen
in parental animals of each sex in both generations. The mean number
of implantation sites in F1 females (not measured in F0) was
decreased and the length of gestation in F1 (but not F0) dams
increased. There was also a small increase in the number of pregnant
females in both generations that had total resorptions and in the
number of F1 females that died during delivery. The NOAEL was 10 ppm,
equal to 0.8 mg/kg bw per day, for general toxicity and 150 ppm, equal
to 12 mg/kg bw per day, for reproductive toxicity (Danberry et al.,
1993).
In a similar study, groups of 24 male and 24 female weanling
Crj:CD(SD) rats were given diets containing technical-grade
tebufenozide (purity, 97.2%) providing levels of 0, 25, 200, or
2000 ppm, equal to 0, 1.6, 13, and 130 mg/kg bw per day for males and
0, 1.8, 15, and 140 mg/kg bw per day for females, for 10 weeks before
mating of the F0 and F1 parents to produce the respective F1 and F2
offspring. The study was terminated after weaning of the F2 litters.
An increased incidence of splenic lesions, seen as blackish changes
and/or congestion of the spleen, increased splenic extramedullary
haematopoiesis, and haemosiderin-laden cells, occurred in F0 and F1
parental animals at 200 and 2000 ppm. At 2000 ppm, slight decreases in
body-weight gain were seen in animals of each sex, and decreases in
uterine and ovarian weights were reported; the changes were small and
were considered not to be toxicologically significant. A significant
reduction in mean body-weight gain was seen in F1 and F2 pups at
2000 ppm between lactation days 14 and 21. The study revealed no
adverse effects on the reproductive ability of male or female rats.
The NOAEL was 25 ppm, equal to 1.6 mg/kg bw per day, for systemic
toxicity and 200 ppm, equal to 13 mg/kg bw per day, for reproductive
toxicity (Aso, 1995).
In both of the above studies, a consistent increase in the
incidence of gross and histopathological findings in the spleen,
including congestion, pigment, and extramedullary haematopoiesis, was
seen in F0 and F1 parental animals. Although tebufenozide at the
doses tested had no effect on the reproductive ability of male or
female rats, the minor reproductive effects observed at 2000 ppm
on parental females in the first study and on lactating pups in
the second study could not be discounted. The overall NOAEL for
reproductive toxicity was thus 200 ppm, equal to 13 mg/kg bw per day.
(e) Developmental toxicity
Rats
In a range-finding study, groups of nine mated female Crl:CD BR
rats were given technical-grade tebufenozide (purity, 96.8%) by gavage
at doses of 0 (vehicle control), 25, 75, 200, or 400 mg/kg bw per day
on days 6-15 of gestation. On day 20, all surviving dams were killed,
and their fetuses were removed from the uterus and necropsied.
No maternal, embryo, or fetal toxicity and no external gross
malformations or anomalies in the fetuses were seen at any dose. The
NOAEL for maternal, embryonic, and fetal toxicity and teratogenicity
was thus 400 mg/kg bw per day, the highest dose tested. In a
supplementary range-finding study, groups of six non-pregnant female
Crl:CD BR rats were given technical-grade tebufenozide (purity, 96.8%)
by gavage at doses of 0, 400, or 1000 mg/kg bw per day for 10 days. At
1000 mg/kg bw per day, a slight increase in liver weight was observed;
however, in the absence of histopathological data, the toxicological
significance of this finding could not be fully evaluated. On the
basis of the very limited data provided, the NOAEL was 400 mg/kg bw
per day (Solomon & Romanello, 1992).
In the main study, groups of 25 mated Crl:CD BR VAF/Plus
Sprague-Dawley rats were given technical-grade tebufenozide (purity,
96.1%) by gavage at doses of 0, 50, 250, or 1000 mg/kg bw per day on
days 6-15 of presumed gestation (day 0 taken as the day spermatozoa
were detected in a vaginal lavage or a copulatory plug was observed
in situ). On gestation day 20, all surviving dams were killed, and
their fetuses were removed and necropsied. At 1000 mg/kg bw per day,
initial slight, transient reductions in mean body-weight gain and food
consumption were seen during dosing, but the mean values over the
study were not affected; the changes were considered not to be
toxicologically significant. All of the fetuses showed normal
development, and no signs of fetotoxicity and no treatment-related
malformations were observed at any dose tested. The NOAEL was
1000 mg/kg bw per day for maternal toxicity and 1000 mg/kg bw per
day, the highest dose tested, for embryo- and fetotoxicity and
teratogenicity (Hoberman, 1991).
Rabbits
In a range-finding study, groups of six mated female Hra:(NZW)SPF
rabbits were given technical-grade tebufenozide (purity, 96.4%) by
gavage at doses of 0, 100, 300, or 1000 mg/kg bw per day on days 7-19
of presumed gestation (the day of mating confirmed by the presence of
seminal fluid in the vulva). All surviving does were sacrificed, and
all fetuses were removed and examined on day 29 of gestation. The
single death due to an unknown cause among animals at 1000 mg/kg bw
per day was unlikely to have been due to treatment, as no deaths
occurred in the main study. No other signs of maternal toxicity or
treatment-related disturbance of intrauterine development of the
conceptuses were observed. All fetuses showed normal development, and
no signs of fetal toxicity or treatment-related malformations were
seen at any dose. The NOAEL for embryo- and fetotoxicity and
teratogenicity was 1000 mg/kg bw per day, the highest dose tested
(Lemen, 1991).
In the main study, groups of 20 mated female New Zealand white
rabbits were given technical-grade tebufenozide (purity, 97.5%) by
gavage at doses of 0, 50, 250, or 1000 mg/kg bw per day on days 7-19
of presumed gestation (the day of mating confirmed by the presence of
seminal fluid in the vulva). All surviving does were sacrificed, and
their fetuses were removed and examined on day 29 of gestation. No
treatment-related deaths, clinical signs of maternal toxicity, or
disturbances of intrauterine development of the conceptuses were
observed at any dose. All of the fetuses showed normal development,
and no signs of fetotoxicity or treatment-related malformations were
observed at any dose. The NOAEL for maternal toxicity, embryo- and
fetotoxicity and teratogenicity was 1000 mg/kg bw per day, the highest
dose tested (Swenson & Solomon, 1992).
(f) Genotoxicity
A battery of tests for mutagenicity was conducted to assess the
potential of technical-grade tebufenozide (purity, > 95-97.5%) to
induce gene mutation, chromosomal aberration, or unscheduled DNA
synthesis. The results, summarized in Table 2, were all negative.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade tebufenozide (purity, 96-97%) was not irritating
to the skin and was minimally irritating to the eyes of male New
Zealand white rabbits, according to Draize scale scoring (Krajewski
et al., 1988a,b; Lutz & Parno, 1993).
Table 2. Results of tests for the genotoxicity of technical-grade tebufenozide
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 0, 50-5000a µg/plate > 95 Negativeb Black (1986)
TA98, TA100,
TA1535, TA1537
Reverse mutation S. typhimurium 0, 50-5000a µg/plate 96.1 Negativeb Sames & Streelman
TA98, TA100, (1991)
TA1535, TA1537
Reverse mutation S. typhimurium 0, 50-5000a µg/plate 96.1 Negativeb Sames & Elia (1993)
TA98, TA100, 0, 160-1600a µg/plate Negativeb
TA1535, TA1537
Reverse mutation S. typhimurium 0, 50-5000a µg/plate 96.1 Negativeb Sames & Elia (1994)
TA98, TA100, 0, 30-300a µg/plate Negativeb
TA1535, TA1537
Reverse mutation E. coli WP2 uvr A 0, 200-5000a µg/plate 96.1 Negativeb Watanabe (1992)
Forward mutation Chinese hamster 0, 10-60 µg/ml 96.4 Negativeb Thilagar (1990a)
ovary cells
(hprt locus)
Chromosomal aberration Chinese hamster 0, 5-30 µg/ml 96.8 Negativeb Thilagar (1987)
ovary cells
Unscheduled DNA Rat (SD) primary 0, 10-100a µg/ml 96.4 Negative Thilagar (1990b)
synthesis hepatocytes 0, 10-60 µg/ml Negative
In vivo
Chromosomal aberration Rat (CD, 5-7 of each 0, 0.5-5.0 g/kg bw 97.5 Negative Gudi (1992)
sex per dose) bone by single gavage
marrow
a Precipitation observed in all cultures
b Conducted with and without exogenous metabolic activation
Technical-grade tebufenozide (purity, 96%) did not sensitize the
skin of guinea-pigs assessed in the maximization test (males) and the
Buehler test (females) (Matsumoto, 1989; Glaza, 1993).
(ii) Neurotoxicity
Groups of 10 male and 10 female Crl:CD R BR rats were given
single doses of technical-grade tebufenozide (purity, 96.1%) in
0.5% w/v aqueous methylcellulose by gavage at 0, 500, 1000, or
2000 mg/kg bw. All rats were subjected to functional observational
battery testing and motor activity assessment before treatment and
1-3 h and 7 and 14 days after dosing, and a detailed neuropathological
examination was carried out at the end of the 14-day observation
period. No remarkable treatment-related neurological changes were
found at any dose. Treatment-related responses were elicited in
similar acute neurotoxicity studies conducted at the same testing
laboratory with substances known to affect functional observational
battery and motor activity testing, thus validating the competence of
the laboratory in this type of testing. The NOAEL for the neurotoxic
and neuropathological effects of tebufenozide was thus 2000 mg/kg bw,
the highest dose tested (Swenson et al., 1994).
(iii) Studies of metabolites
Five metabolites of tebufenozide were tested for acute toxicity
in Crj: CD-1 or Crl:CD-1 BR ICR mice. Metabolites RH-111788, RH-96595,
RH-120970, RH-089886, and RH-112651 (see Figure 1) given orally
induced no deaths and no clinical signs of systemic toxicity at doses
up to 5000 mg/kg bw. The LD50 values for these metabolites in male
and female mice was thus > 5000 mg/kg bw (Hazelton & Quinn, 1993).
Four tebufenozide metabolites, RH-111788, RH-96595, RH-120970,
and RH-089886, were tested for their potential to induce reverse
mutation in Salmonella typhimurium strains TA98, TA100, TA1535, and
TA1537 and in Escherichia coli strain WP2 uvr A at concentrations up
to the limit of solubility (1250-2500 µg/plate), with and without
exogenous metabolic activation. The results, summarized in Table 3,
were negative (Hazelton & Quinn, 1993).
Table 3. Results of tests for the genotoxicity of metabolites
of tebufenozide
Metabolite Concentration Result
(µg/plate)
RH-89886 (in rats, rice) 0, 313-5000a Negative
RH-111788 (in rats, rice) 0, 313-5000a Negative
RH-96595 (in rats, rice, soil) 0, 313-5000a Negative
RH-120970 (in rats, rice) 0, 313-5000a Negative
Potential for induction of reverse mutation in vitro in S.
typhimurium TA98, TA100, TA1535, and TA1537 and in E. coli
WP2 uvr A, with and without exogenous metabolic activation by
a microsomal fraction from rat liver
a Precipitation observed on all plates
Comments
Oral administration to rats of single doses of 3 or 250 mg/kg
bw of 14C-labelled tebufenozide resulted in rapid absorption and
excretion in urine and faeces, only trace amounts of 14C being
recovered in expired air. The excretion profiles were similar,
regardless of the position of the 14C label, the dose, the sex, or
whether the rats had been pretreated with 30 ppm of unlabelled
tebufenozide in the diet for two weeks. A mean total of 87-104% of the
administered radiolabel was eliminated within 48 h, primarily via the
faeces, which accounted for 90% of the 14C that was excreted; only
minor amounts (1-8%) were excreted in urine and trace amounts
(0.1-0.4%) in expired air. In animals at 3 mg/kg bw, absorption
accounted for 35-39 % of the administered radiolabel; 30-34% was
excreted in the bile and about 5% in the urine. In rats at 250 mg/kg
bw, only about 4% of the administered dose was absorbed and
metabolized. The highest levels of 14C in the blood were measured
0.5-12 h after dosing, and clearance of the radiolabel from the
circulation was rapid. Tissue retention of 14C was low, suggesting
that there is little or no bioaccumulation of tebufenozide in the
body.
Most of the 14C excreted in the faeces was in the form of
unabsorbed (parent) tebufenozide, which accounted for about 60 and 90%
of an administered dose of 3 and 250 mg/kg bw per day, respectively;
no unchanged tebufenozide was detected in the urine. The absorbed
14C-tebufenozide was extensively metabolized in rats. There were no
significant qualitative differences in the metabolic profiles
associated with the position of the 14C label, the dose, sex, or
whether rats were pretreated with unlabelled tebufenozide. In general,
the 13-15 metabolites identified in the urine, faeces, and bile were
identical. The main route of metabolism of tebufenozide appeared to be
oxidation of benzylic carbons (A- or B-ring), resulting in a number of
metabolites with various combinations of oxidation state at the three
oxidized carbon centres and one metabolite produced by oxidation of a
nonbenzylic, terminal carbon on the A-ring ethyl group.
Tebufenozide was of low acute toxicity after administration to
mice orally and to rats by the oral, dermal, or inhalation route. The
oral LD50 in mice and rats was > 5000 mg/kg bw; the dermal LD50 in
rats was > 5000 mg/kg bw; and the inhalation LC50 in rats was
> 4.3 mg/litre. The metabolites were also of low acute toxicity to
mice after oral administration. Tebufenozide was not irritating to
the skin and was minimally irritating to the eyes of male rabbits;
it was not a skin sensitizer in guinea-pigs. WHO has not classified
tebufenozide for acute toxicity.
Repeated short-term oral administration of tebufenozide to mice
(2 and 13 weeks), rats (2, 4, and 13 weeks), and dogs (2, 6, 13, and
52 weeks) resulted primarily in haematotoxic effects (regenerative
haemolytic anaemia and compensatory responses from the haematopoietic
tissues). The NOAEL for these effects was 200 ppm, equal to 35 mg/kg
bw per day, in mice in a 13-week study (0, 20, 200, 2000, and 20 000
ppm tested); 200 ppm, equal to 13.1 mg/kg bw per day, in rats in a
13-week study (0, 20, 200, 2000, and 20 000 ppm tested); 50 ppm, equal
to 2 mg/kg bw per day, in dogs in a 13-week study (0, 50, 500, and
5000 ppm tested); and 50 ppm, equal to 1.8 mg/kg bw per day, in a
one-year study in dogs (0, 15, 50, 250, and 1500 ppm tested). Repeated
dermal applications of tebufenozide to rats for four weeks caused
no systemic toxicity at doses < 1000 mg/kg bw per day. The dog
appeared to be the most sensitive species for both short-term and
long-term toxicity.
In an 18-month study of toxicity and carcinogenicity in mice
given tebufenozide in the diet at concentrations of 0, 5, 50, 500,
or 1000 ppm, the NOAEL for systemic toxicity was 50 ppm, equal to
7.8 mg/kg bw per day, on the basis of a slightly reduced survival
rate and mild regenerative haemolytic anaemia at higher doses. In
a two-year study of toxicity and carcinogenicity in rats given
tebufenozide in the diet at 0, 10, 100, 1000, or 2000 ppm, the NOAEL
was 100 ppm, equal to 4.8 mg/kg bw per day, on the basis of decreased
body weight and food consumption and mild regenerative haemolytic
anaemia at higher doses. Tebufenozide was not carcinogenic to mice or
rats under the conditions of the studies.
Tebufenozide and its metabolites have been adequately tested for
genotoxicity in a range of assays both in vitro and in vivo. The
Meeting concluded that neither tebufenozide nor its metabolites are
genotoxic.
In two two-generation studies of reproductive toxicity in rats,
with one litter per generation, doses of 0, 10, 150, or 2000 ppm and
0, 25, 200, or 2000 ppm were administered. The NOAEL for systemic
(parental) toxicity was 25 ppm, equal to 1.6 mg/kg bw per day, on
the basis of a consistent increase in the incidence of gross and
histopathological lesions in the spleens (congestion, pigment, and
extra-medullary haematopoiesis) of F0 and F1 parental animals at
higher doses (200 and 2000 ppm). The NOAEL for reproductive toxicity
was 13 mg/kg bw per day, on the basis of minor reproductive effects
(decreased mean number of implantation sites, prolonged gestation, a
slightly greater frequency of total resorptions, and a small increase
in the number of dams that died during delivery) at the high dose of
2000 ppm in dams in the first study and in lactating pups (decreased
mean weight gain on lactation days 14 and 21) in the second study.
In studies of developmental toxicity in rats and rabbits, doses
of 0, 50, 250, or 1000 mg/kg bw per day were administered. There was
no evidence of teratogenic potential. The NOAEL for maternal, embryo-,
and fetotoxicity and teratogenicity was 1000 mg/kg bw per day, the
highest dose tested, in both species.
In a study of acute neurotoxicity in rats, no treatment-related
effects were seen when single doses of 0, 500, 1000, or 2000 mg/kg bw
were administered. The NOAEL for acute neurotoxicity and neuro-
pathological effects was 2000 mg/kg bw, the highest dose tested.
In summary, exposure to tebufenozide by the oral route results
primarily in haematotoxicity. The main target of its action is the
peripheral haematopoietic system; the pivotal toxicological end-point
of concern, which is seen consistently across all species tested, is
mild regenerative haemolytic anaemia with compensatory responses from
the haematopoietic tissues.
An ADI of 0-0.02 mg/kg bw was established for tebufenozide on the
basis of the NOAELs for haematotoxicity of 1.8 mg/kg bw per day in the
one-year study in dogs and 1.6 mg/kg bw per day in a two-generation
study of reproductive toxicity in rats, using a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 200 ppm, equal to 35 mg/kg bw per day (13-week study of
toxicity)
50 ppm, equal to 7.8 mg/kg bw per day (haematotoxicity
in an 18-month study of toxicity and carcinogenicity)
Rat: 200 ppm, equal to 13 mg/kg bw per day (13-week study of
toxicity)
100 ppm, equal to 4.8 mg/kg bw per day (haematotoxicity
in a two-year study of toxicity and carcinogenicity)
25 ppm, equal to 1.6 mg/kg bw per day (maternal
haematotoxicity in a two-generation study of
reproductive toxicity)
200 ppm, equal to 13 mg/kg bw per day (reproductive
toxicity in a two-generation study)
1000 mg/kg bw per day, the highest dose tested
(maternal, embryo-, and fetotoxicity and teratogenicity
in a study of developmental toxicity)
Rabbit: 1000 mg/kg bw per day, the highest dose tested
(maternal, embryo-, and fetotoxicity and teratogenicity
in a study of developmental toxicity)
Dog: 50 ppm, equal to 1.8 mg/kg bw per day (haemotoxicity in
a one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Observations in humans
2. Studies on the mechanism of haematotoxicity
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to tebufenozide
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 > 5000 mg/kg bw
Dermal, toxicity, rat LD50 > 5000 mg/kg bw
Inhalation, 4 h, toxicity, rat LC50 > 4.3 mg/litre
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Minimally irritating
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated dietary, 90 days, toxicity, dog NOAEL = 2 mg/kg bw per day, primarily
haematotoxicity
Repeated dermal, 28 days, toxicity, rat NOAEL = 1000 mg/kg bw per day, highest dose
tested
Repeated dietary, reproductive toxicity, rat NOAEL = 13 mg/kg bw per day, minor reproductive
effects
Repeated gavage, developmental toxicity, NOAEL = 1000 mg/kg bw per day, highest dose
rat and rabbit tested; maternal, embryo-, and fetal toxicity
and teratogenicity
Long-term (> 1 year) Repeated dietary, 1 year, toxicity, dog NOAEL = 1.8 mg/kg bw per day, primarily
haematotoxicity
References
Aso, S. (1995) Two-generation reproduction study of RH-5992 in rats.
Unpublished report No. 93RC-101 from Hita Research Laboratories, Oita,
Japan. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Black, C.A. (1986) RH-75,992: Microbial mutagenicity assay (Ames
test). Unpublished report No. 86R-0013 from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Clay, H. (1992). RH-5992: 90-day oral (dietary) administration
toxicity study in the beagle. Unpublished report No. 90RC-062 from
Hazleton UK, North Yorkshire, United Kingdom. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Danberry, T.L., Romanello, A.S., Donofrio, K.F. & O'Hara, G.P. (1993)
RH-5992: Two-generation reproduction study in rats. Unpublished report
No. 9OR-202A from Rohm & Haas Co., Toxicology Department, Philadelphia,
PA, USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Glaza, S.M. (1993) Dermal sensitization study of RH-5992 technical in
guinea pigs - maximization test. Unpublished report No. 92RC-101 from
Hazleton Wisconsin, Inc., WI, USA. Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Gudi, R (1992) RH-5992 technical: Acute test for chemical induction of
chromosome aberration in rat bone marrow cells in vivo. Unpublished
report No. 9ORC-203 from Sitek Research Laboratories, MD, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hawkins, D.R. & Hazelton, G.A. (1993) 14C-RH-5992: Biliary excretion
study in rats, supplemental report A: Metabolism of RH-5992 in rats.
Unpublished report No. 9ORC-058A from Rohm & Haas Co., Agricultural
Products Development Department, PA, USA. Submitted to WHO by Rohm &
Haas Co., Philadelphia, PA, USA.
Hawkins, D.R., Spencer, W.O. & Hazelton, G.A. (1992) 14C-RH-5992
pharmacokinetic study in rats, supplemental report A: Metabolism of
RH-5992 in rats. Unpublished report No. 91RC-004A from Rohm & Haas
Co., Agricultural Products Development Department, PA, USA. Submitted
to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hawkins, D.R., Spencer W.O. & Hazelton, G.A. (1993) 14C-RH-5992:
Pharmacokinetic study in rats, supplemental report B: Metabolism of
RH-5992 in rats. Unpublished report No. 91RC-004B from Rohm & Haas
Co., Agricultural Products Development Department, PA, USA. Submitted
to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A. & Quinn, D.L. (1993) Hazard evaluation of RH-5992
technical in human and domestic animals. Part I: Hazard identification
and evaluation. Unpublished report No. 93R-1039 from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Hazelton, G.A. & Quinn, D.L. (1995) Evaluation of rat reproduction
studies conducted with RH-5992. Unpublished report No. 95R-1068 from
Rohm & Haas Co., Toxicology Department, Philadelphia, PA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hoberman, A.M. (1991) RH-5992: Oral developmental toxicity (embryo-
fetal toxicity and teratogenic potential) study in rats. Unpublished
report No. 9ORC-059 from Argus Research Laboratories, Inc., PA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Kehoe, D.F. (1990) RH-5992: Two-week dietary range-finding study in
male dogs. Unpublished report No. 87RC-034 from Hazleton Laboratories
America, Inc., WI, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988a) RH-75,992:
Skin irritation study in rabbits. Unpublished report No. 87R-088 from
Rohm & Haas Co., Toxicology Department, Philadelphia, PA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988b) RH-75,992: Eye
irritation study in rabbits. Unpublished report No. 87R-084 from Rohm
& Haas Co., Toxicology Department, Philadelphia, PA, USA. Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Kyle, M.E. (1992) RH-5992: Two-week dietary range-finding study in
male mice. Unpublished report No. 86R-228A from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Kyle, M.E. & Quinn, D.L. (1992) RH-5992: Two-week dietary range-
finding study in rats. Unpublished report No. 86R-226A from Rohm &
Haas Co., Toxicology Department, Philadelphia, PA, USA. Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lemen, J.K. (1991) RH-5992: Range-finding rabbit developmental
toxicity study. Unpublished report No. 89RC-100 from Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
LeVan, L.W. (1991) 14C-RH-5992: Distribution of radioactivity in rats
following a single oral dose. Unpublished report No. 89RC-099 from
Hazleton Laboratories America, Inc., WI, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Lutz, M.F. & Parno, J.R. (1993) RH-75,992: Eye irritation study
in rabbits. Unpublished report No. 93R-237 from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Matsumoto, R. (1989) Skin sensitization study of RH-5992 (technical)
in the guinea pig. Unpublished report No. 9ORC-1002A from Bozo
Research Centre, Inc., Tokyo, Japan. Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Morrison, R.D. & Hamilton, J.D. (1991a) RH-75,992 technical: Acute
oral toxicity study in male and female mice. Unpublished report No.
91R-003 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D. & Hamilton, J.D. (1991b) RH-75,992 technical: Acute
oral toxicity study in male and female rats. Unpublished report No.
9OR-199 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D. & Hamilton, J.D. (1991c) RH-75,992 technical: Acute
dermal toxicity study in male and female rats. Unpublished report No.
9OR-200 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D., Carbone, J.P., Parno, J.R. & Gillette, D.M. (1993)
RH-75,992 technical and RH-75,992 2F formulation: Four-week dermal
toxicity study in rats. Unpublished report No. 92R-150 from Rohm &
Haas Co., Toxicology Department, Philadelphia, PA, USA. Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Osheroff, M.R. (1991a) RH-5992: 13-Week dietary toxicity study in
mice. Unpublished report No. 89RC-102 from Hazleton Laboratories
America, Inc., VA, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Osheroff, M.R. (1991b) RH-5992: Four-week range-finding dietary
toxicity study in rats. Unpublished report No. 89RC-101A from Hazleton
Laboratories America, Inc., MD, USA. Submitted to WHO by Rohm & Haas
Co., PA, USA.
Osheroff, M.R. (1991c) RH-5992: 13-week dietary toxicity study in
rats. Unpublished report No. 89RC-101 from Hazleton Laboratories
America, Inc., VA, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Richards, J.F. (1992) RH-5992: 52-week oral (dietary administration)
chronic toxicity study in the beagle. Unpublished report No. 90RC-206
from Hazleton UK, North Yorkshire, United Kingdom. Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Elia, M.C. (1993) RH-75,992 technical: Salmonella
typhimurium gene mutation assay (Ames test). Unpublished report No.
93R-094 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Elia, M.C. (1994) RH-75,992 technical: Salmonella
typhimurium gene mutation assay (Ames test). Unpublished report No.
93R-236 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Streelman, D.R. (1991) RH-75,992 technical: Salmonella
typhimurium gene mutation assay (Ames test). Unpublished report No.
9IR-167 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Romanello, A.S. (1992) RH-5992: Range-finding (gavage)
developmental toxicology study in rats. Unpublished report No.
87R-105A from Rohm & Haas Co., Toxicology Department, Philadelphia,
PA, USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Struble, C.B. & Hazelton, G.A. (1992a) 14C-RH-5992: Biliary excretion
study in rats. Unpublished report No. 90RC-058 from Hazleton
Wisconsin, Inc., WI, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Struble, C.B. & Hazelton, G.A. (1992b) 14C-RH-5992: Pharmacokinetic
study in rats. Unpublished report No. 91RC-004 from Hazleton
Wisconsin, Inc., WI, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Swenson, R.E. & Solomon, H.M. (1992) RH-5992: Oral (gavage)
developmental toxicity study in rabbits. Unpublished report No.
90R-201 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Swenson, R.E., Gillette, D.M. & Parno, J.R. (1994) RH-75,992: Acute
oral (gavage) neurotoxicity study in rats. Unpublished report No.
93R-093 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1987) RH-75,992: Test for chemical induction of
chromosome aberration using monolayer cultures of Chinese hamster
ovary (CHO) cells with and without metabolic activation. Unpublished
report No. 88RC-011 from Sitek Research Laboratories, MD, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1990a) RH-5992: Test for chemical induction of gene
mutation at the HGPRT locus in cultured Chinese hamster ovary (CHO)
cells with and without metabolic activation. Unpublished report No.
89RC-097 from Sitek Research Laboratories, MD, USA. Submitted to WHO
by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1990b) RH-5992: Test for chemical induction of
unscheduled DNA synthesis in rat primary hepatocyte cultures by
autoradiography. Unpublished report No. 89RC-098 from Sitek Research
Laboratories, MD, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Trutter, J.A. (1992a) RH-5992: 18-Month dietary oncogenicity study in
mice. Unpublished report No. 90RC-061 and 90RC-061A from Hazleton
Washington, Inc., VA, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Trutter, J.A. (1992b) RH-5992: 24-Month combined dietary chronic
toxicity and oncogenicity study in rats. Unpublished report No.
90RC-060 and 90RC-060A from Hazleton Washington, Inc., VA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Ulrich, C.E. (1992) RH-5992 (technical): Acute inhalation toxicity
evaluation in rats. Unpublished report No. 90RC-057 from International
Research and Development Corporation, MI, USA. Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Watanabe, K. (1992) RH-5992: Reverse mutation assay with E. coli
(WP2 uvr strain). Unpublished report No. 92RN-1010 from The Institute
of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Rohm &
Haas Co., Philadelphia, PA, USA.
Yoshida, A. (1992) RH-5992: Blood recovery study in dogs. Unpublished
report No. 92RC-1040 from The Institute of Environmental Toxicology,
Tokyo, Japan. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA,
USA.
 Groups of Crl:CD BR Sprague-Dawley rats received a bile-duct
cannula and then a single oral dose of 3 mg/kg bw [14C- tert-butyl]-
tebufenozide. Bile samples collected from one male and one female rat
during 0-6 h after treatment, representing about 70% of total biliary
excretion of radiolabel over 72 h, were analysed for tebufenozide
metabolites. No parent compound was found; 13 biliary metabolites were
identified, and five unknown metabolites were isolated. In general,
the biliary metabolites were identical to those in the faeces and
urine, but three new metabolites were observed: [A-ring]-ketone-
[B-ring]-diol, RH-122652, and RH-2652. The last two were also
identified by mass spectroscopy in the faeces but at levels too low
for quantification. The unknown metabolites in bile appeared to be
high-molecular-mass amino-acid conjugates of acidic tebufenozide
metabolites. It was postulated that these conjugates are metabolized
in rats before excretion in order to recover the amino acids; hence,
their absence in faeces (Hawkins & Hazelton, 1993).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of tebufenozide are
summarized in Table 1. Tebufenozide was of low toxicity to mice and
rats when given orally, dermally, or by inhalation. No deaths or
clinical signs of systemic toxicity were observed at doses < 5.0 g/kg
bw. Dermal administration resulted only in transient, mild local
erythema at the site of application. After inhalation, accumulation
of red-brown material was seen around the nose, mouth, and eyes,
sometimes with a purulent anogenital discharge which lasted for one
to three days.
(b) Short-term toxicity
Mice
Six groups of eight male albino Crl:CD-1 ICR BR mice were fed
diets containing technical-grade tebufenozide (purity, 94%) to provide
doses of 0, 60, 200, 600, 2000, or 6000 ppm, equal to 0, 12, 39, 97,
350, or 1100 mg/kg bw per day, for two weeks. At the highest dose, the
absolute and relative liver weights were significantly increased,
but, in the absence of histopathological data, the toxicological
significance of this change could not be fully evaluated. The NOAEL
was 600 ppm, equal to 97 mg/kg bw per day, on the basis of the
increased relative liver weights (Kyle, 1992).
Table 1. Acute toxicity of tebufenozide in experimental animals
Species Sex Route LD50 (mg/kg bw) Reference
or LC50 (mg/litre)
Mouse Male, female Oral > 5000 Morrison & Hamilton (1991a)
Rat Male, female Oral > 5000 Morrison & Hamilton (1991b)
Rat Male, female Dermal > 5000 Morrison & Hamilton (1991c)
Rat Male Inhalation > 4.3 Ulrich (1992)
Female > 4.5
Groups of 10 male and 10 female Crl:CD-1 ICR BR VAF/Plus mice
were fed diets containing technical-grade tebufenozide (purity, 98.6%)
providing levels of 0, 20, 200, 2000, or 20 000 ppm, equal to 0, 3.4,
35, 340, and 3300 mg/kg bw per day for males and 0, 4.3, 45, 430, and
4200 mg/kg bw per day for females, for 13 weeks. At 200 ppm, slightly
reduced mean body-weight gain (but not overall body weight) was seen
in males and a marginally higher incidence of increased extramedullary
haematopoiesis in the spleen and pigment accumulation in kidney
tubules (with no concomitant changes in haematological parameters) in
females; these changes were considered not to be toxicologically
significant. At 2000 and 20 000 ppm, significant haemolytic changes
and a reduced mean myeloid:erythroid ratio in bone marrow were seen,
with dose-related increases in the absolute and relative (to body
and to brain) weights of the spleen and liver. Histopathological
examination revealed an increased incidence and/or severity of pigment
deposition in the liver, spleen, and kidney tubules and increased
extramedullary haematopoiesis in the spleen. The pigment was
characterized as bile in the liver and haemosiderin in the liver,
spleen and kidney. The primary target of tebufenozide was the
erythrocyte, causing increased erythrocyte turnover and compensatory
responses from the haematopoietic tissues. The NOAEL was 200 ppm,
equal to 35 mg/kg bw per day (Osheroff, 1991a).
Rat
Groups of six male and six female Crl:CD BR rats were given
technical-grade tebufenozide (purity, 94%) in the diet to provide
levels of 0, 50, 250, 1000, 2500, or 10 000 ppm, equal to 0, 3.8, 19,
71, 180, and 700 mg/kg bw per day for males and 0, 4.5, 21, 85, 210
and 780 mg/kg bw per day for females, for two weeks. The relative
weight of the liver was increased in males and the absolute and
relative weights in females at 1000 ppm; however, in the absence of
histopathological data, the toxicological significance of these
changes could not be fully evaluated. At 10 000 ppm, slight reductions
in body-weight gain, food consumption, and erythrocyte parameters were
seen, which were within the normal range of biological variations and
were considered to be of minimal toxicological significance. At this
dose, increased absolute and relative spleen weights were observed in
males and females; however, in the absence of histopathological data,
the significance of these changes could not be evaluated. The NOAEL
was 250 ppm, equal to 19 mg/kg bw per day, on the basis of the changes
in liver weight (Kyle & Quinn, 1992).
In a range-finding study, groups of 10 male and 10 female Crl:CD
BR Sprague-Dawley rats were given diets containing technical-grade
tebufenozide (purity, 96.4%) to provide levels of 0 or 20 000 ppm,
equal to 0 and 1500 mg/kg bw per day in males and 0 and 1600 mg/kg bw
per day in females, for four weeks. Decreases in body weight (< 9%),
body-weight gain, and food consumption (< 12%) were observed, but the
mean efficiency of food use was not affected, suggesting reduced
palatability of the 20 000-ppm test diet. Slight reductions in
erythrocyte count, haemoglobin, and haematocrit were seen. The
absolute and relative liver weights of animals of each sex, the
absolute and relative spleen weights of males, and the relative kidney
weights of females were higher in the treated rats, but, in the
absence of histopathological data, the toxicological significance of
these changes could not be fully assessed. It was concluded that the
high dietary level of 20 000 ppm could be used in studies of short-
term toxicity in rats (Osheroff, 1991b).
Groups of 10 male and 10 female Crl:CD BR rats were fed diets
containing technical-grade tebufenozide (purity, 96.4 or 98.6%) in the
diet to provide levels of 0, 20, 200, 2000, or 20 000 ppm, equal to 0,
1.3, 13, 130, and 1300 mg/kg bw per day for males and 0, 1.6, 16, 160,
and 1600 mg/kg bw per day for females, for 13 weeks. The dose of
2000 ppm induced significant decreases in overall body-weight gain and
mean food consumption during the first four weeks of dosing and an
increase in the relative (to brain) weight of the liver in females.
Slight haemolytic anaemia, increased bone-marrow erythropoiesis
(decreased mean myeloid:erythroid ratio), and increased deposition of
pigment in the spleen were observed. At the highest dose, additional
treatment-related effects included overt haemolytic changes, slightly
elevated absolute and relative (to brain) spleen weights in animals of
each sex, elevated absolute liver weight in females, and tubular
nephrosis of the kidney in four males. The NOAEL was 200 ppm, equal to
13 mg/kg bw per day (Osheroff, 1991c).
Groups of six male and six female Crl:CD BR rats received
applications of technical-grade tebufenozide (purity, 97.2%) moistened
with 0.9% saline (1:6 w/v) at a dose of 1000 mg/kg bw per day on the
shaved, intact skin of the back under semi-occlusive bandages for
6 h per day, five days per week for four weeks (a total of 21
applications). No signs of treatment-related systemic toxicity or
dermal irritation were observed. The NOAEL was thus 1000 mg/kg bw per
day (Morrison et al., 1993).
Dogs
Five groups of four male beagle dogs received diets containing
technical-grade tebufenozide (purity, 96.8%) at 0, 150, 600, 2400,
or 9600 ppm, equal to 0, 5, 19, 77, and 290 mg/kg bw per day, for
two weeks. A significant increase in the mean spleen weight was
seen in animals at 600 ppm. At 9600 ppm, mild haemolytic anaemia
(significantly reduced erythrocyte count, haemoglobin, and
haematocrit) was observed. The NOAEL was 150 ppm, equal to 5 mg/kg bw
per day (Kehoe, 1990).
Two groups of four male beagle dogs were fed diets containing
technical-grade tebufenozide (purity, 97.5%) at 0 or 1500 ppm,
equal to 42 mg/kg bw per day, for six weeks. All animals were then
maintained on the basal diet for a four-week recovery period, after
which the study was terminated. Haematological examinations were
performed on all dogs before treatment and at 6, 8, and 10 weeks.
Administration of 1500 ppm resulted in mild regenerative anaemia, but
total recovery had occurred four weeks after cessation of treatment
(Yoshida, 1992).
Groups of four male and four female beagle dogs received diets
containing technical-grade tebufenozide (purity, 96.1%) at 0, 50,
500, or 5000 ppm, equal to 0, 2.1, 20, and 200 mg/kg bw per day for
males and 0, 2, 21, and 200 mg/kg bw per day for females, for 90
days. Animals of each sex at 500 ppm had an increased incidence of
Heinz bodies, and females at this dose had an elevated mean total
bilirubin level and increased absolute and relative spleen weights.
Histopathological examination revealed an increased incidence of
pigment deposition (haemosiderin) in the Kupffer cells of the liver
and increased haematopoiesis and sinusoidal engorgement of the spleen.
At the highest dose, significant haemolytic changes and increased
bone-marrow erythropoiesis (reduced mean myeloid:erythroid ratio) were
seen at weeks 6 and 13 of treatment. The mean total plasma bilirubin
level was elevated in animals of each sex, and bilirubin was present
in the urine of three males. Increased absolute and relative spleen
weights and slightly higher relative liver weights were observed.
Increased incidences of pigment deposition (haemosiderin) and the
presence of erythrocytes in some Kupffer cells of the liver suggested
active erythrophagocytosis. Increased splenic haematopoiesis, splenic
sinusoidal engorgement, and bone-marrow hyperplasia were also noted.
The primary target of tebufenozide in the dog was the erythrocytes,
leading to mild haemolytic anaemia and compensatory responses from the
haematopoietic tissues. The NOAEL was 50 ppm, equal to 2 mg/kg bw per
day (Clay, 1992).
Groups of four male and four female beagle dogs were fed diets
containing technical-grade tebufenozide (purity, 97.5%) providing
dietary levels of 0, 15, 50, 250, or 1500 ppm, equal to 0, 0.6, 1.8,
8.7, or 53 mg/kg bw per day for males and 0, 0.6, 1.9, 8.9, and
56 mg/kg bw per day for females, for 52 weeks. In animals at 250 ppm,
slight but consistent haemolytic changes and a slightly elevated total
plasma bilirubin level were seen, the latter especially in females,
over the 52-week period. The mean absolute and relative spleen weights
of females and the mean relative liver weight of males were increased,
and an increased incidence of pigment deposition in the Kupffer
cells of the liver, increased splenic haematopoiesis and sinusoidal
engorgement, and bone-marrow hyperplasia were also observed. Similar
treatment-related effects of increased magnitude and severity were
observed at the highest dose. The primary target of tebufenozide was
thus erythrocytes, leading to mild peripheral haemolytic anaemia and
compensatory responses from the haematopoietic tissues. The NOAEL was
50 ppm, equal to 1.8 mg/kg bw per day (Richards, 1992).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female Crl:CD-1(ICR)BR mice received
diets containing technical-grade tebufenozide (purity, 96.1%) at 0, 5,
50, 500, or 1000 ppm, equal to 0, 0.8, 7.8, 78, and 160 mg/kg bw per
day for males and 0, 0.9, 9.4, 94, and 190 mg/kg bw per day for
females, for 18 months. Each group included a satellite group of 10
mice of each sex that were killed at interim sacrifice after 52 weeks
of treatment. In animals at 500 ppm, a slight reduction in the
survival rate of males and increased pigment deposition in the spleens
of males and females were observed at both interim and terminal
sacrifices. At the highest dose, the survival rates of both males
and females were reduced, and signs of mild haemolytic anaemia (a
small but significant increase in the blood level of methaemoglobin
and increased incidences of polychromasia and echinocytosis in
erythrocytes) and further increases in splenic pigment deposition were
observed at interim and/or terminal sacrifice. At terminal sacrifice,
males had increased relative spleen weights, and females had an
increased incidence of extramedullary haematopoiesis in the spleen.
There was no evidence of an oncogenic effect at any dose. The NOAEL
for general toxicity was 50 ppm, equal to 7.8 mg/kg bw per day
(Trotter, 1992a).
Rats
Groups of 60 male and 60 female Crl:CD BR rats received diets
containing technical-grade tebufenozide (purity, 96.1%) providing
levels of 0, 10, 100, 1000, or 2000 ppm, equal to 0, 0.5, 4.8, 48, and
97 mg/kg bw per day for males and 0, 0.6, 6.1, 61, and 120 mg/kg bw
per day for females, for two years. Each group included a satellite
group of 10 rats of each sex that were killed at interim sacrifice
during week 53; two sentinel groups of five rats of each sex per group
were maintained to screen general health before and at the end of the
treatment period. Animals at 1000 or 2000 ppm showed significant
decreases in mean body weight and body-weight gain, the latter more
pronounced in females, and females had a reduced mean food consumption
throughout the study. Signs of mild haemolytic anaemia were seen only
during the first 52 weeks of treatment, suggesting that the effects
on the haematopoietic system were transient and reversible. Slight
increases in the incidence and/or severity of pigment deposition
(haemosiderin) were seen in the spleens of animals of each sex at
interim and/or terminal sacrifices, suggesting active splenic
erythrophagocytosis. In addition, females at 1000 and 2000 ppm had an
increased frequency of swelling of body areas (principally the mammary
gland regions) during the first 70 weeks of treatment; however, in the
absence of any supporting histopathological findings in the mammary
gland tissue or skin, the toxicological significance of these
transient swellings could not be ascertained. No treatment-related
neoplastic lesions were noted in any tissue or organ of any treated
rat at any dose. The NOAEL for systemic toxicity was 100 ppm, equal to
4.8 mg/kg bw per day (Trutter, 1992b).
(d) Reproductive toxicity
Rats
In a two-generation study, with one litter per generation, groups
of 25 male and 25 female weanling Crl:CD BR rats were fed diets
containing technical-grade tebufenozide (purity, 97.5%) providing
dietary levels of 0, 10, 150, or 2000 ppm, equal to 0, 0.8, 12, and
150 mg/kg bw per day for males and 0, 0.9, 13, and 170 mg/kg bw per
day for females. The diets were fed for 70 (F0) or 105 days (F1)
before mating. No treatment-related effects were observed in either
generation at the low dose. At 150 ppm, an increased severity of
pigment deposition in the spleen of F0 and F1 females was reported.
At the highest dose, the body weight and feed consumption of F0 and
F1 males were decreased at some intervals before mating. Increased
splenic pigment deposition and extramedullary haematopoiesis were seen
in parental animals of each sex in both generations. The mean number
of implantation sites in F1 females (not measured in F0) was
decreased and the length of gestation in F1 (but not F0) dams
increased. There was also a small increase in the number of pregnant
females in both generations that had total resorptions and in the
number of F1 females that died during delivery. The NOAEL was 10 ppm,
equal to 0.8 mg/kg bw per day, for general toxicity and 150 ppm, equal
to 12 mg/kg bw per day, for reproductive toxicity (Danberry et al.,
1993).
In a similar study, groups of 24 male and 24 female weanling
Crj:CD(SD) rats were given diets containing technical-grade
tebufenozide (purity, 97.2%) providing levels of 0, 25, 200, or
2000 ppm, equal to 0, 1.6, 13, and 130 mg/kg bw per day for males and
0, 1.8, 15, and 140 mg/kg bw per day for females, for 10 weeks before
mating of the F0 and F1 parents to produce the respective F1 and F2
offspring. The study was terminated after weaning of the F2 litters.
An increased incidence of splenic lesions, seen as blackish changes
and/or congestion of the spleen, increased splenic extramedullary
haematopoiesis, and haemosiderin-laden cells, occurred in F0 and F1
parental animals at 200 and 2000 ppm. At 2000 ppm, slight decreases in
body-weight gain were seen in animals of each sex, and decreases in
uterine and ovarian weights were reported; the changes were small and
were considered not to be toxicologically significant. A significant
reduction in mean body-weight gain was seen in F1 and F2 pups at
2000 ppm between lactation days 14 and 21. The study revealed no
adverse effects on the reproductive ability of male or female rats.
The NOAEL was 25 ppm, equal to 1.6 mg/kg bw per day, for systemic
toxicity and 200 ppm, equal to 13 mg/kg bw per day, for reproductive
toxicity (Aso, 1995).
In both of the above studies, a consistent increase in the
incidence of gross and histopathological findings in the spleen,
including congestion, pigment, and extramedullary haematopoiesis, was
seen in F0 and F1 parental animals. Although tebufenozide at the
doses tested had no effect on the reproductive ability of male or
female rats, the minor reproductive effects observed at 2000 ppm
on parental females in the first study and on lactating pups in
the second study could not be discounted. The overall NOAEL for
reproductive toxicity was thus 200 ppm, equal to 13 mg/kg bw per day.
(e) Developmental toxicity
Rats
In a range-finding study, groups of nine mated female Crl:CD BR
rats were given technical-grade tebufenozide (purity, 96.8%) by gavage
at doses of 0 (vehicle control), 25, 75, 200, or 400 mg/kg bw per day
on days 6-15 of gestation. On day 20, all surviving dams were killed,
and their fetuses were removed from the uterus and necropsied.
No maternal, embryo, or fetal toxicity and no external gross
malformations or anomalies in the fetuses were seen at any dose. The
NOAEL for maternal, embryonic, and fetal toxicity and teratogenicity
was thus 400 mg/kg bw per day, the highest dose tested. In a
supplementary range-finding study, groups of six non-pregnant female
Crl:CD BR rats were given technical-grade tebufenozide (purity, 96.8%)
by gavage at doses of 0, 400, or 1000 mg/kg bw per day for 10 days. At
1000 mg/kg bw per day, a slight increase in liver weight was observed;
however, in the absence of histopathological data, the toxicological
significance of this finding could not be fully evaluated. On the
basis of the very limited data provided, the NOAEL was 400 mg/kg bw
per day (Solomon & Romanello, 1992).
In the main study, groups of 25 mated Crl:CD BR VAF/Plus
Sprague-Dawley rats were given technical-grade tebufenozide (purity,
96.1%) by gavage at doses of 0, 50, 250, or 1000 mg/kg bw per day on
days 6-15 of presumed gestation (day 0 taken as the day spermatozoa
were detected in a vaginal lavage or a copulatory plug was observed
in situ). On gestation day 20, all surviving dams were killed, and
their fetuses were removed and necropsied. At 1000 mg/kg bw per day,
initial slight, transient reductions in mean body-weight gain and food
consumption were seen during dosing, but the mean values over the
study were not affected; the changes were considered not to be
toxicologically significant. All of the fetuses showed normal
development, and no signs of fetotoxicity and no treatment-related
malformations were observed at any dose tested. The NOAEL was
1000 mg/kg bw per day for maternal toxicity and 1000 mg/kg bw per
day, the highest dose tested, for embryo- and fetotoxicity and
teratogenicity (Hoberman, 1991).
Rabbits
In a range-finding study, groups of six mated female Hra:(NZW)SPF
rabbits were given technical-grade tebufenozide (purity, 96.4%) by
gavage at doses of 0, 100, 300, or 1000 mg/kg bw per day on days 7-19
of presumed gestation (the day of mating confirmed by the presence of
seminal fluid in the vulva). All surviving does were sacrificed, and
all fetuses were removed and examined on day 29 of gestation. The
single death due to an unknown cause among animals at 1000 mg/kg bw
per day was unlikely to have been due to treatment, as no deaths
occurred in the main study. No other signs of maternal toxicity or
treatment-related disturbance of intrauterine development of the
conceptuses were observed. All fetuses showed normal development, and
no signs of fetal toxicity or treatment-related malformations were
seen at any dose. The NOAEL for embryo- and fetotoxicity and
teratogenicity was 1000 mg/kg bw per day, the highest dose tested
(Lemen, 1991).
In the main study, groups of 20 mated female New Zealand white
rabbits were given technical-grade tebufenozide (purity, 97.5%) by
gavage at doses of 0, 50, 250, or 1000 mg/kg bw per day on days 7-19
of presumed gestation (the day of mating confirmed by the presence of
seminal fluid in the vulva). All surviving does were sacrificed, and
their fetuses were removed and examined on day 29 of gestation. No
treatment-related deaths, clinical signs of maternal toxicity, or
disturbances of intrauterine development of the conceptuses were
observed at any dose. All of the fetuses showed normal development,
and no signs of fetotoxicity or treatment-related malformations were
observed at any dose. The NOAEL for maternal toxicity, embryo- and
fetotoxicity and teratogenicity was 1000 mg/kg bw per day, the highest
dose tested (Swenson & Solomon, 1992).
(f) Genotoxicity
A battery of tests for mutagenicity was conducted to assess the
potential of technical-grade tebufenozide (purity, > 95-97.5%) to
induce gene mutation, chromosomal aberration, or unscheduled DNA
synthesis. The results, summarized in Table 2, were all negative.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade tebufenozide (purity, 96-97%) was not irritating
to the skin and was minimally irritating to the eyes of male New
Zealand white rabbits, according to Draize scale scoring (Krajewski
et al., 1988a,b; Lutz & Parno, 1993).
Table 2. Results of tests for the genotoxicity of technical-grade tebufenozide
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 0, 50-5000a µg/plate > 95 Negativeb Black (1986)
TA98, TA100,
TA1535, TA1537
Reverse mutation S. typhimurium 0, 50-5000a µg/plate 96.1 Negativeb Sames & Streelman
TA98, TA100, (1991)
TA1535, TA1537
Reverse mutation S. typhimurium 0, 50-5000a µg/plate 96.1 Negativeb Sames & Elia (1993)
TA98, TA100, 0, 160-1600a µg/plate Negativeb
TA1535, TA1537
Reverse mutation S. typhimurium 0, 50-5000a µg/plate 96.1 Negativeb Sames & Elia (1994)
TA98, TA100, 0, 30-300a µg/plate Negativeb
TA1535, TA1537
Reverse mutation E. coli WP2 uvr A 0, 200-5000a µg/plate 96.1 Negativeb Watanabe (1992)
Forward mutation Chinese hamster 0, 10-60 µg/ml 96.4 Negativeb Thilagar (1990a)
ovary cells
(hprt locus)
Chromosomal aberration Chinese hamster 0, 5-30 µg/ml 96.8 Negativeb Thilagar (1987)
ovary cells
Unscheduled DNA Rat (SD) primary 0, 10-100a µg/ml 96.4 Negative Thilagar (1990b)
synthesis hepatocytes 0, 10-60 µg/ml Negative
In vivo
Chromosomal aberration Rat (CD, 5-7 of each 0, 0.5-5.0 g/kg bw 97.5 Negative Gudi (1992)
sex per dose) bone by single gavage
marrow
a Precipitation observed in all cultures
b Conducted with and without exogenous metabolic activation
Technical-grade tebufenozide (purity, 96%) did not sensitize the
skin of guinea-pigs assessed in the maximization test (males) and the
Buehler test (females) (Matsumoto, 1989; Glaza, 1993).
(ii) Neurotoxicity
Groups of 10 male and 10 female Crl:CD R BR rats were given
single doses of technical-grade tebufenozide (purity, 96.1%) in
0.5% w/v aqueous methylcellulose by gavage at 0, 500, 1000, or
2000 mg/kg bw. All rats were subjected to functional observational
battery testing and motor activity assessment before treatment and
1-3 h and 7 and 14 days after dosing, and a detailed neuropathological
examination was carried out at the end of the 14-day observation
period. No remarkable treatment-related neurological changes were
found at any dose. Treatment-related responses were elicited in
similar acute neurotoxicity studies conducted at the same testing
laboratory with substances known to affect functional observational
battery and motor activity testing, thus validating the competence of
the laboratory in this type of testing. The NOAEL for the neurotoxic
and neuropathological effects of tebufenozide was thus 2000 mg/kg bw,
the highest dose tested (Swenson et al., 1994).
(iii) Studies of metabolites
Five metabolites of tebufenozide were tested for acute toxicity
in Crj: CD-1 or Crl:CD-1 BR ICR mice. Metabolites RH-111788, RH-96595,
RH-120970, RH-089886, and RH-112651 (see Figure 1) given orally
induced no deaths and no clinical signs of systemic toxicity at doses
up to 5000 mg/kg bw. The LD50 values for these metabolites in male
and female mice was thus > 5000 mg/kg bw (Hazelton & Quinn, 1993).
Four tebufenozide metabolites, RH-111788, RH-96595, RH-120970,
and RH-089886, were tested for their potential to induce reverse
mutation in Salmonella typhimurium strains TA98, TA100, TA1535, and
TA1537 and in Escherichia coli strain WP2 uvr A at concentrations up
to the limit of solubility (1250-2500 µg/plate), with and without
exogenous metabolic activation. The results, summarized in Table 3,
were negative (Hazelton & Quinn, 1993).
Table 3. Results of tests for the genotoxicity of metabolites
of tebufenozide
Metabolite Concentration Result
(µg/plate)
RH-89886 (in rats, rice) 0, 313-5000a Negative
RH-111788 (in rats, rice) 0, 313-5000a Negative
RH-96595 (in rats, rice, soil) 0, 313-5000a Negative
RH-120970 (in rats, rice) 0, 313-5000a Negative
Potential for induction of reverse mutation in vitro in S.
typhimurium TA98, TA100, TA1535, and TA1537 and in E. coli
WP2 uvr A, with and without exogenous metabolic activation by
a microsomal fraction from rat liver
a Precipitation observed on all plates
Comments
Oral administration to rats of single doses of 3 or 250 mg/kg
bw of 14C-labelled tebufenozide resulted in rapid absorption and
excretion in urine and faeces, only trace amounts of 14C being
recovered in expired air. The excretion profiles were similar,
regardless of the position of the 14C label, the dose, the sex, or
whether the rats had been pretreated with 30 ppm of unlabelled
tebufenozide in the diet for two weeks. A mean total of 87-104% of the
administered radiolabel was eliminated within 48 h, primarily via the
faeces, which accounted for 90% of the 14C that was excreted; only
minor amounts (1-8%) were excreted in urine and trace amounts
(0.1-0.4%) in expired air. In animals at 3 mg/kg bw, absorption
accounted for 35-39 % of the administered radiolabel; 30-34% was
excreted in the bile and about 5% in the urine. In rats at 250 mg/kg
bw, only about 4% of the administered dose was absorbed and
metabolized. The highest levels of 14C in the blood were measured
0.5-12 h after dosing, and clearance of the radiolabel from the
circulation was rapid. Tissue retention of 14C was low, suggesting
that there is little or no bioaccumulation of tebufenozide in the
body.
Most of the 14C excreted in the faeces was in the form of
unabsorbed (parent) tebufenozide, which accounted for about 60 and 90%
of an administered dose of 3 and 250 mg/kg bw per day, respectively;
no unchanged tebufenozide was detected in the urine. The absorbed
14C-tebufenozide was extensively metabolized in rats. There were no
significant qualitative differences in the metabolic profiles
associated with the position of the 14C label, the dose, sex, or
whether rats were pretreated with unlabelled tebufenozide. In general,
the 13-15 metabolites identified in the urine, faeces, and bile were
identical. The main route of metabolism of tebufenozide appeared to be
oxidation of benzylic carbons (A- or B-ring), resulting in a number of
metabolites with various combinations of oxidation state at the three
oxidized carbon centres and one metabolite produced by oxidation of a
nonbenzylic, terminal carbon on the A-ring ethyl group.
Tebufenozide was of low acute toxicity after administration to
mice orally and to rats by the oral, dermal, or inhalation route. The
oral LD50 in mice and rats was > 5000 mg/kg bw; the dermal LD50 in
rats was > 5000 mg/kg bw; and the inhalation LC50 in rats was
> 4.3 mg/litre. The metabolites were also of low acute toxicity to
mice after oral administration. Tebufenozide was not irritating to
the skin and was minimally irritating to the eyes of male rabbits;
it was not a skin sensitizer in guinea-pigs. WHO has not classified
tebufenozide for acute toxicity.
Repeated short-term oral administration of tebufenozide to mice
(2 and 13 weeks), rats (2, 4, and 13 weeks), and dogs (2, 6, 13, and
52 weeks) resulted primarily in haematotoxic effects (regenerative
haemolytic anaemia and compensatory responses from the haematopoietic
tissues). The NOAEL for these effects was 200 ppm, equal to 35 mg/kg
bw per day, in mice in a 13-week study (0, 20, 200, 2000, and 20 000
ppm tested); 200 ppm, equal to 13.1 mg/kg bw per day, in rats in a
13-week study (0, 20, 200, 2000, and 20 000 ppm tested); 50 ppm, equal
to 2 mg/kg bw per day, in dogs in a 13-week study (0, 50, 500, and
5000 ppm tested); and 50 ppm, equal to 1.8 mg/kg bw per day, in a
one-year study in dogs (0, 15, 50, 250, and 1500 ppm tested). Repeated
dermal applications of tebufenozide to rats for four weeks caused
no systemic toxicity at doses < 1000 mg/kg bw per day. The dog
appeared to be the most sensitive species for both short-term and
long-term toxicity.
In an 18-month study of toxicity and carcinogenicity in mice
given tebufenozide in the diet at concentrations of 0, 5, 50, 500,
or 1000 ppm, the NOAEL for systemic toxicity was 50 ppm, equal to
7.8 mg/kg bw per day, on the basis of a slightly reduced survival
rate and mild regenerative haemolytic anaemia at higher doses. In
a two-year study of toxicity and carcinogenicity in rats given
tebufenozide in the diet at 0, 10, 100, 1000, or 2000 ppm, the NOAEL
was 100 ppm, equal to 4.8 mg/kg bw per day, on the basis of decreased
body weight and food consumption and mild regenerative haemolytic
anaemia at higher doses. Tebufenozide was not carcinogenic to mice or
rats under the conditions of the studies.
Tebufenozide and its metabolites have been adequately tested for
genotoxicity in a range of assays both in vitro and in vivo. The
Meeting concluded that neither tebufenozide nor its metabolites are
genotoxic.
In two two-generation studies of reproductive toxicity in rats,
with one litter per generation, doses of 0, 10, 150, or 2000 ppm and
0, 25, 200, or 2000 ppm were administered. The NOAEL for systemic
(parental) toxicity was 25 ppm, equal to 1.6 mg/kg bw per day, on
the basis of a consistent increase in the incidence of gross and
histopathological lesions in the spleens (congestion, pigment, and
extra-medullary haematopoiesis) of F0 and F1 parental animals at
higher doses (200 and 2000 ppm). The NOAEL for reproductive toxicity
was 13 mg/kg bw per day, on the basis of minor reproductive effects
(decreased mean number of implantation sites, prolonged gestation, a
slightly greater frequency of total resorptions, and a small increase
in the number of dams that died during delivery) at the high dose of
2000 ppm in dams in the first study and in lactating pups (decreased
mean weight gain on lactation days 14 and 21) in the second study.
In studies of developmental toxicity in rats and rabbits, doses
of 0, 50, 250, or 1000 mg/kg bw per day were administered. There was
no evidence of teratogenic potential. The NOAEL for maternal, embryo-,
and fetotoxicity and teratogenicity was 1000 mg/kg bw per day, the
highest dose tested, in both species.
In a study of acute neurotoxicity in rats, no treatment-related
effects were seen when single doses of 0, 500, 1000, or 2000 mg/kg bw
were administered. The NOAEL for acute neurotoxicity and neuro-
pathological effects was 2000 mg/kg bw, the highest dose tested.
In summary, exposure to tebufenozide by the oral route results
primarily in haematotoxicity. The main target of its action is the
peripheral haematopoietic system; the pivotal toxicological end-point
of concern, which is seen consistently across all species tested, is
mild regenerative haemolytic anaemia with compensatory responses from
the haematopoietic tissues.
An ADI of 0-0.02 mg/kg bw was established for tebufenozide on the
basis of the NOAELs for haematotoxicity of 1.8 mg/kg bw per day in the
one-year study in dogs and 1.6 mg/kg bw per day in a two-generation
study of reproductive toxicity in rats, using a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 200 ppm, equal to 35 mg/kg bw per day (13-week study of
toxicity)
50 ppm, equal to 7.8 mg/kg bw per day (haematotoxicity
in an 18-month study of toxicity and carcinogenicity)
Rat: 200 ppm, equal to 13 mg/kg bw per day (13-week study of
toxicity)
100 ppm, equal to 4.8 mg/kg bw per day (haematotoxicity
in a two-year study of toxicity and carcinogenicity)
25 ppm, equal to 1.6 mg/kg bw per day (maternal
haematotoxicity in a two-generation study of
reproductive toxicity)
200 ppm, equal to 13 mg/kg bw per day (reproductive
toxicity in a two-generation study)
1000 mg/kg bw per day, the highest dose tested
(maternal, embryo-, and fetotoxicity and teratogenicity
in a study of developmental toxicity)
Rabbit: 1000 mg/kg bw per day, the highest dose tested
(maternal, embryo-, and fetotoxicity and teratogenicity
in a study of developmental toxicity)
Dog: 50 ppm, equal to 1.8 mg/kg bw per day (haemotoxicity in
a one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Observations in humans
2. Studies on the mechanism of haematotoxicity
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to tebufenozide
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 > 5000 mg/kg bw
Dermal, toxicity, rat LD50 > 5000 mg/kg bw
Inhalation, 4 h, toxicity, rat LC50 > 4.3 mg/litre
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Minimally irritating
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated dietary, 90 days, toxicity, dog NOAEL = 2 mg/kg bw per day, primarily
haematotoxicity
Repeated dermal, 28 days, toxicity, rat NOAEL = 1000 mg/kg bw per day, highest dose
tested
Repeated dietary, reproductive toxicity, rat NOAEL = 13 mg/kg bw per day, minor reproductive
effects
Repeated gavage, developmental toxicity, NOAEL = 1000 mg/kg bw per day, highest dose
rat and rabbit tested; maternal, embryo-, and fetal toxicity
and teratogenicity
Long-term (> 1 year) Repeated dietary, 1 year, toxicity, dog NOAEL = 1.8 mg/kg bw per day, primarily
haematotoxicity
References
Aso, S. (1995) Two-generation reproduction study of RH-5992 in rats.
Unpublished report No. 93RC-101 from Hita Research Laboratories, Oita,
Japan. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Black, C.A. (1986) RH-75,992: Microbial mutagenicity assay (Ames
test). Unpublished report No. 86R-0013 from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Clay, H. (1992). RH-5992: 90-day oral (dietary) administration
toxicity study in the beagle. Unpublished report No. 90RC-062 from
Hazleton UK, North Yorkshire, United Kingdom. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Danberry, T.L., Romanello, A.S., Donofrio, K.F. & O'Hara, G.P. (1993)
RH-5992: Two-generation reproduction study in rats. Unpublished report
No. 9OR-202A from Rohm & Haas Co., Toxicology Department, Philadelphia,
PA, USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Glaza, S.M. (1993) Dermal sensitization study of RH-5992 technical in
guinea pigs - maximization test. Unpublished report No. 92RC-101 from
Hazleton Wisconsin, Inc., WI, USA. Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Gudi, R (1992) RH-5992 technical: Acute test for chemical induction of
chromosome aberration in rat bone marrow cells in vivo. Unpublished
report No. 9ORC-203 from Sitek Research Laboratories, MD, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hawkins, D.R. & Hazelton, G.A. (1993) 14C-RH-5992: Biliary excretion
study in rats, supplemental report A: Metabolism of RH-5992 in rats.
Unpublished report No. 9ORC-058A from Rohm & Haas Co., Agricultural
Products Development Department, PA, USA. Submitted to WHO by Rohm &
Haas Co., Philadelphia, PA, USA.
Hawkins, D.R., Spencer, W.O. & Hazelton, G.A. (1992) 14C-RH-5992
pharmacokinetic study in rats, supplemental report A: Metabolism of
RH-5992 in rats. Unpublished report No. 91RC-004A from Rohm & Haas
Co., Agricultural Products Development Department, PA, USA. Submitted
to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hawkins, D.R., Spencer W.O. & Hazelton, G.A. (1993) 14C-RH-5992:
Pharmacokinetic study in rats, supplemental report B: Metabolism of
RH-5992 in rats. Unpublished report No. 91RC-004B from Rohm & Haas
Co., Agricultural Products Development Department, PA, USA. Submitted
to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A. & Quinn, D.L. (1993) Hazard evaluation of RH-5992
technical in human and domestic animals. Part I: Hazard identification
and evaluation. Unpublished report No. 93R-1039 from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Hazelton, G.A. & Quinn, D.L. (1995) Evaluation of rat reproduction
studies conducted with RH-5992. Unpublished report No. 95R-1068 from
Rohm & Haas Co., Toxicology Department, Philadelphia, PA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hoberman, A.M. (1991) RH-5992: Oral developmental toxicity (embryo-
fetal toxicity and teratogenic potential) study in rats. Unpublished
report No. 9ORC-059 from Argus Research Laboratories, Inc., PA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Kehoe, D.F. (1990) RH-5992: Two-week dietary range-finding study in
male dogs. Unpublished report No. 87RC-034 from Hazleton Laboratories
America, Inc., WI, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988a) RH-75,992:
Skin irritation study in rabbits. Unpublished report No. 87R-088 from
Rohm & Haas Co., Toxicology Department, Philadelphia, PA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988b) RH-75,992: Eye
irritation study in rabbits. Unpublished report No. 87R-084 from Rohm
& Haas Co., Toxicology Department, Philadelphia, PA, USA. Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Kyle, M.E. (1992) RH-5992: Two-week dietary range-finding study in
male mice. Unpublished report No. 86R-228A from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Kyle, M.E. & Quinn, D.L. (1992) RH-5992: Two-week dietary range-
finding study in rats. Unpublished report No. 86R-226A from Rohm &
Haas Co., Toxicology Department, Philadelphia, PA, USA. Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lemen, J.K. (1991) RH-5992: Range-finding rabbit developmental
toxicity study. Unpublished report No. 89RC-100 from Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
LeVan, L.W. (1991) 14C-RH-5992: Distribution of radioactivity in rats
following a single oral dose. Unpublished report No. 89RC-099 from
Hazleton Laboratories America, Inc., WI, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Lutz, M.F. & Parno, J.R. (1993) RH-75,992: Eye irritation study
in rabbits. Unpublished report No. 93R-237 from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Matsumoto, R. (1989) Skin sensitization study of RH-5992 (technical)
in the guinea pig. Unpublished report No. 9ORC-1002A from Bozo
Research Centre, Inc., Tokyo, Japan. Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Morrison, R.D. & Hamilton, J.D. (1991a) RH-75,992 technical: Acute
oral toxicity study in male and female mice. Unpublished report No.
91R-003 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D. & Hamilton, J.D. (1991b) RH-75,992 technical: Acute
oral toxicity study in male and female rats. Unpublished report No.
9OR-199 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D. & Hamilton, J.D. (1991c) RH-75,992 technical: Acute
dermal toxicity study in male and female rats. Unpublished report No.
9OR-200 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D., Carbone, J.P., Parno, J.R. & Gillette, D.M. (1993)
RH-75,992 technical and RH-75,992 2F formulation: Four-week dermal
toxicity study in rats. Unpublished report No. 92R-150 from Rohm &
Haas Co., Toxicology Department, Philadelphia, PA, USA. Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Osheroff, M.R. (1991a) RH-5992: 13-Week dietary toxicity study in
mice. Unpublished report No. 89RC-102 from Hazleton Laboratories
America, Inc., VA, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Osheroff, M.R. (1991b) RH-5992: Four-week range-finding dietary
toxicity study in rats. Unpublished report No. 89RC-101A from Hazleton
Laboratories America, Inc., MD, USA. Submitted to WHO by Rohm & Haas
Co., PA, USA.
Osheroff, M.R. (1991c) RH-5992: 13-week dietary toxicity study in
rats. Unpublished report No. 89RC-101 from Hazleton Laboratories
America, Inc., VA, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Richards, J.F. (1992) RH-5992: 52-week oral (dietary administration)
chronic toxicity study in the beagle. Unpublished report No. 90RC-206
from Hazleton UK, North Yorkshire, United Kingdom. Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Elia, M.C. (1993) RH-75,992 technical: Salmonella
typhimurium gene mutation assay (Ames test). Unpublished report No.
93R-094 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Elia, M.C. (1994) RH-75,992 technical: Salmonella
typhimurium gene mutation assay (Ames test). Unpublished report No.
93R-236 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Streelman, D.R. (1991) RH-75,992 technical: Salmonella
typhimurium gene mutation assay (Ames test). Unpublished report No.
9IR-167 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Romanello, A.S. (1992) RH-5992: Range-finding (gavage)
developmental toxicology study in rats. Unpublished report No.
87R-105A from Rohm & Haas Co., Toxicology Department, Philadelphia,
PA, USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Struble, C.B. & Hazelton, G.A. (1992a) 14C-RH-5992: Biliary excretion
study in rats. Unpublished report No. 90RC-058 from Hazleton
Wisconsin, Inc., WI, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Struble, C.B. & Hazelton, G.A. (1992b) 14C-RH-5992: Pharmacokinetic
study in rats. Unpublished report No. 91RC-004 from Hazleton
Wisconsin, Inc., WI, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Swenson, R.E. & Solomon, H.M. (1992) RH-5992: Oral (gavage)
developmental toxicity study in rabbits. Unpublished report No.
90R-201 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Swenson, R.E., Gillette, D.M. & Parno, J.R. (1994) RH-75,992: Acute
oral (gavage) neurotoxicity study in rats. Unpublished report No.
93R-093 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1987) RH-75,992: Test for chemical induction of
chromosome aberration using monolayer cultures of Chinese hamster
ovary (CHO) cells with and without metabolic activation. Unpublished
report No. 88RC-011 from Sitek Research Laboratories, MD, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1990a) RH-5992: Test for chemical induction of gene
mutation at the HGPRT locus in cultured Chinese hamster ovary (CHO)
cells with and without metabolic activation. Unpublished report No.
89RC-097 from Sitek Research Laboratories, MD, USA. Submitted to WHO
by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1990b) RH-5992: Test for chemical induction of
unscheduled DNA synthesis in rat primary hepatocyte cultures by
autoradiography. Unpublished report No. 89RC-098 from Sitek Research
Laboratories, MD, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Trutter, J.A. (1992a) RH-5992: 18-Month dietary oncogenicity study in
mice. Unpublished report No. 90RC-061 and 90RC-061A from Hazleton
Washington, Inc., VA, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Trutter, J.A. (1992b) RH-5992: 24-Month combined dietary chronic
toxicity and oncogenicity study in rats. Unpublished report No.
90RC-060 and 90RC-060A from Hazleton Washington, Inc., VA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Ulrich, C.E. (1992) RH-5992 (technical): Acute inhalation toxicity
evaluation in rats. Unpublished report No. 90RC-057 from International
Research and Development Corporation, MI, USA. Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Watanabe, K. (1992) RH-5992: Reverse mutation assay with E. coli
(WP2 uvr strain). Unpublished report No. 92RN-1010 from The Institute
of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Rohm &
Haas Co., Philadelphia, PA, USA.
Yoshida, A. (1992) RH-5992: Blood recovery study in dogs. Unpublished
report No. 92RC-1040 from The Institute of Environmental Toxicology,
Tokyo, Japan. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA,
USA.
Groups of Crl:CD BR Sprague-Dawley rats received a bile-duct
cannula and then a single oral dose of 3 mg/kg bw [14C- tert-butyl]-
tebufenozide. Bile samples collected from one male and one female rat
during 0-6 h after treatment, representing about 70% of total biliary
excretion of radiolabel over 72 h, were analysed for tebufenozide
metabolites. No parent compound was found; 13 biliary metabolites were
identified, and five unknown metabolites were isolated. In general,
the biliary metabolites were identical to those in the faeces and
urine, but three new metabolites were observed: [A-ring]-ketone-
[B-ring]-diol, RH-122652, and RH-2652. The last two were also
identified by mass spectroscopy in the faeces but at levels too low
for quantification. The unknown metabolites in bile appeared to be
high-molecular-mass amino-acid conjugates of acidic tebufenozide
metabolites. It was postulated that these conjugates are metabolized
in rats before excretion in order to recover the amino acids; hence,
their absence in faeces (Hawkins & Hazelton, 1993).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of tebufenozide are
summarized in Table 1. Tebufenozide was of low toxicity to mice and
rats when given orally, dermally, or by inhalation. No deaths or
clinical signs of systemic toxicity were observed at doses < 5.0 g/kg
bw. Dermal administration resulted only in transient, mild local
erythema at the site of application. After inhalation, accumulation
of red-brown material was seen around the nose, mouth, and eyes,
sometimes with a purulent anogenital discharge which lasted for one
to three days.
(b) Short-term toxicity
Mice
Six groups of eight male albino Crl:CD-1 ICR BR mice were fed
diets containing technical-grade tebufenozide (purity, 94%) to provide
doses of 0, 60, 200, 600, 2000, or 6000 ppm, equal to 0, 12, 39, 97,
350, or 1100 mg/kg bw per day, for two weeks. At the highest dose, the
absolute and relative liver weights were significantly increased,
but, in the absence of histopathological data, the toxicological
significance of this change could not be fully evaluated. The NOAEL
was 600 ppm, equal to 97 mg/kg bw per day, on the basis of the
increased relative liver weights (Kyle, 1992).
Table 1. Acute toxicity of tebufenozide in experimental animals
Species Sex Route LD50 (mg/kg bw) Reference
or LC50 (mg/litre)
Mouse Male, female Oral > 5000 Morrison & Hamilton (1991a)
Rat Male, female Oral > 5000 Morrison & Hamilton (1991b)
Rat Male, female Dermal > 5000 Morrison & Hamilton (1991c)
Rat Male Inhalation > 4.3 Ulrich (1992)
Female > 4.5
Groups of 10 male and 10 female Crl:CD-1 ICR BR VAF/Plus mice
were fed diets containing technical-grade tebufenozide (purity, 98.6%)
providing levels of 0, 20, 200, 2000, or 20 000 ppm, equal to 0, 3.4,
35, 340, and 3300 mg/kg bw per day for males and 0, 4.3, 45, 430, and
4200 mg/kg bw per day for females, for 13 weeks. At 200 ppm, slightly
reduced mean body-weight gain (but not overall body weight) was seen
in males and a marginally higher incidence of increased extramedullary
haematopoiesis in the spleen and pigment accumulation in kidney
tubules (with no concomitant changes in haematological parameters) in
females; these changes were considered not to be toxicologically
significant. At 2000 and 20 000 ppm, significant haemolytic changes
and a reduced mean myeloid:erythroid ratio in bone marrow were seen,
with dose-related increases in the absolute and relative (to body
and to brain) weights of the spleen and liver. Histopathological
examination revealed an increased incidence and/or severity of pigment
deposition in the liver, spleen, and kidney tubules and increased
extramedullary haematopoiesis in the spleen. The pigment was
characterized as bile in the liver and haemosiderin in the liver,
spleen and kidney. The primary target of tebufenozide was the
erythrocyte, causing increased erythrocyte turnover and compensatory
responses from the haematopoietic tissues. The NOAEL was 200 ppm,
equal to 35 mg/kg bw per day (Osheroff, 1991a).
Rat
Groups of six male and six female Crl:CD BR rats were given
technical-grade tebufenozide (purity, 94%) in the diet to provide
levels of 0, 50, 250, 1000, 2500, or 10 000 ppm, equal to 0, 3.8, 19,
71, 180, and 700 mg/kg bw per day for males and 0, 4.5, 21, 85, 210
and 780 mg/kg bw per day for females, for two weeks. The relative
weight of the liver was increased in males and the absolute and
relative weights in females at 1000 ppm; however, in the absence of
histopathological data, the toxicological significance of these
changes could not be fully evaluated. At 10 000 ppm, slight reductions
in body-weight gain, food consumption, and erythrocyte parameters were
seen, which were within the normal range of biological variations and
were considered to be of minimal toxicological significance. At this
dose, increased absolute and relative spleen weights were observed in
males and females; however, in the absence of histopathological data,
the significance of these changes could not be evaluated. The NOAEL
was 250 ppm, equal to 19 mg/kg bw per day, on the basis of the changes
in liver weight (Kyle & Quinn, 1992).
In a range-finding study, groups of 10 male and 10 female Crl:CD
BR Sprague-Dawley rats were given diets containing technical-grade
tebufenozide (purity, 96.4%) to provide levels of 0 or 20 000 ppm,
equal to 0 and 1500 mg/kg bw per day in males and 0 and 1600 mg/kg bw
per day in females, for four weeks. Decreases in body weight (< 9%),
body-weight gain, and food consumption (< 12%) were observed, but the
mean efficiency of food use was not affected, suggesting reduced
palatability of the 20 000-ppm test diet. Slight reductions in
erythrocyte count, haemoglobin, and haematocrit were seen. The
absolute and relative liver weights of animals of each sex, the
absolute and relative spleen weights of males, and the relative kidney
weights of females were higher in the treated rats, but, in the
absence of histopathological data, the toxicological significance of
these changes could not be fully assessed. It was concluded that the
high dietary level of 20 000 ppm could be used in studies of short-
term toxicity in rats (Osheroff, 1991b).
Groups of 10 male and 10 female Crl:CD BR rats were fed diets
containing technical-grade tebufenozide (purity, 96.4 or 98.6%) in the
diet to provide levels of 0, 20, 200, 2000, or 20 000 ppm, equal to 0,
1.3, 13, 130, and 1300 mg/kg bw per day for males and 0, 1.6, 16, 160,
and 1600 mg/kg bw per day for females, for 13 weeks. The dose of
2000 ppm induced significant decreases in overall body-weight gain and
mean food consumption during the first four weeks of dosing and an
increase in the relative (to brain) weight of the liver in females.
Slight haemolytic anaemia, increased bone-marrow erythropoiesis
(decreased mean myeloid:erythroid ratio), and increased deposition of
pigment in the spleen were observed. At the highest dose, additional
treatment-related effects included overt haemolytic changes, slightly
elevated absolute and relative (to brain) spleen weights in animals of
each sex, elevated absolute liver weight in females, and tubular
nephrosis of the kidney in four males. The NOAEL was 200 ppm, equal to
13 mg/kg bw per day (Osheroff, 1991c).
Groups of six male and six female Crl:CD BR rats received
applications of technical-grade tebufenozide (purity, 97.2%) moistened
with 0.9% saline (1:6 w/v) at a dose of 1000 mg/kg bw per day on the
shaved, intact skin of the back under semi-occlusive bandages for
6 h per day, five days per week for four weeks (a total of 21
applications). No signs of treatment-related systemic toxicity or
dermal irritation were observed. The NOAEL was thus 1000 mg/kg bw per
day (Morrison et al., 1993).
Dogs
Five groups of four male beagle dogs received diets containing
technical-grade tebufenozide (purity, 96.8%) at 0, 150, 600, 2400,
or 9600 ppm, equal to 0, 5, 19, 77, and 290 mg/kg bw per day, for
two weeks. A significant increase in the mean spleen weight was
seen in animals at 600 ppm. At 9600 ppm, mild haemolytic anaemia
(significantly reduced erythrocyte count, haemoglobin, and
haematocrit) was observed. The NOAEL was 150 ppm, equal to 5 mg/kg bw
per day (Kehoe, 1990).
Two groups of four male beagle dogs were fed diets containing
technical-grade tebufenozide (purity, 97.5%) at 0 or 1500 ppm,
equal to 42 mg/kg bw per day, for six weeks. All animals were then
maintained on the basal diet for a four-week recovery period, after
which the study was terminated. Haematological examinations were
performed on all dogs before treatment and at 6, 8, and 10 weeks.
Administration of 1500 ppm resulted in mild regenerative anaemia, but
total recovery had occurred four weeks after cessation of treatment
(Yoshida, 1992).
Groups of four male and four female beagle dogs received diets
containing technical-grade tebufenozide (purity, 96.1%) at 0, 50,
500, or 5000 ppm, equal to 0, 2.1, 20, and 200 mg/kg bw per day for
males and 0, 2, 21, and 200 mg/kg bw per day for females, for 90
days. Animals of each sex at 500 ppm had an increased incidence of
Heinz bodies, and females at this dose had an elevated mean total
bilirubin level and increased absolute and relative spleen weights.
Histopathological examination revealed an increased incidence of
pigment deposition (haemosiderin) in the Kupffer cells of the liver
and increased haematopoiesis and sinusoidal engorgement of the spleen.
At the highest dose, significant haemolytic changes and increased
bone-marrow erythropoiesis (reduced mean myeloid:erythroid ratio) were
seen at weeks 6 and 13 of treatment. The mean total plasma bilirubin
level was elevated in animals of each sex, and bilirubin was present
in the urine of three males. Increased absolute and relative spleen
weights and slightly higher relative liver weights were observed.
Increased incidences of pigment deposition (haemosiderin) and the
presence of erythrocytes in some Kupffer cells of the liver suggested
active erythrophagocytosis. Increased splenic haematopoiesis, splenic
sinusoidal engorgement, and bone-marrow hyperplasia were also noted.
The primary target of tebufenozide in the dog was the erythrocytes,
leading to mild haemolytic anaemia and compensatory responses from the
haematopoietic tissues. The NOAEL was 50 ppm, equal to 2 mg/kg bw per
day (Clay, 1992).
Groups of four male and four female beagle dogs were fed diets
containing technical-grade tebufenozide (purity, 97.5%) providing
dietary levels of 0, 15, 50, 250, or 1500 ppm, equal to 0, 0.6, 1.8,
8.7, or 53 mg/kg bw per day for males and 0, 0.6, 1.9, 8.9, and
56 mg/kg bw per day for females, for 52 weeks. In animals at 250 ppm,
slight but consistent haemolytic changes and a slightly elevated total
plasma bilirubin level were seen, the latter especially in females,
over the 52-week period. The mean absolute and relative spleen weights
of females and the mean relative liver weight of males were increased,
and an increased incidence of pigment deposition in the Kupffer
cells of the liver, increased splenic haematopoiesis and sinusoidal
engorgement, and bone-marrow hyperplasia were also observed. Similar
treatment-related effects of increased magnitude and severity were
observed at the highest dose. The primary target of tebufenozide was
thus erythrocytes, leading to mild peripheral haemolytic anaemia and
compensatory responses from the haematopoietic tissues. The NOAEL was
50 ppm, equal to 1.8 mg/kg bw per day (Richards, 1992).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female Crl:CD-1(ICR)BR mice received
diets containing technical-grade tebufenozide (purity, 96.1%) at 0, 5,
50, 500, or 1000 ppm, equal to 0, 0.8, 7.8, 78, and 160 mg/kg bw per
day for males and 0, 0.9, 9.4, 94, and 190 mg/kg bw per day for
females, for 18 months. Each group included a satellite group of 10
mice of each sex that were killed at interim sacrifice after 52 weeks
of treatment. In animals at 500 ppm, a slight reduction in the
survival rate of males and increased pigment deposition in the spleens
of males and females were observed at both interim and terminal
sacrifices. At the highest dose, the survival rates of both males
and females were reduced, and signs of mild haemolytic anaemia (a
small but significant increase in the blood level of methaemoglobin
and increased incidences of polychromasia and echinocytosis in
erythrocytes) and further increases in splenic pigment deposition were
observed at interim and/or terminal sacrifice. At terminal sacrifice,
males had increased relative spleen weights, and females had an
increased incidence of extramedullary haematopoiesis in the spleen.
There was no evidence of an oncogenic effect at any dose. The NOAEL
for general toxicity was 50 ppm, equal to 7.8 mg/kg bw per day
(Trotter, 1992a).
Rats
Groups of 60 male and 60 female Crl:CD BR rats received diets
containing technical-grade tebufenozide (purity, 96.1%) providing
levels of 0, 10, 100, 1000, or 2000 ppm, equal to 0, 0.5, 4.8, 48, and
97 mg/kg bw per day for males and 0, 0.6, 6.1, 61, and 120 mg/kg bw
per day for females, for two years. Each group included a satellite
group of 10 rats of each sex that were killed at interim sacrifice
during week 53; two sentinel groups of five rats of each sex per group
were maintained to screen general health before and at the end of the
treatment period. Animals at 1000 or 2000 ppm showed significant
decreases in mean body weight and body-weight gain, the latter more
pronounced in females, and females had a reduced mean food consumption
throughout the study. Signs of mild haemolytic anaemia were seen only
during the first 52 weeks of treatment, suggesting that the effects
on the haematopoietic system were transient and reversible. Slight
increases in the incidence and/or severity of pigment deposition
(haemosiderin) were seen in the spleens of animals of each sex at
interim and/or terminal sacrifices, suggesting active splenic
erythrophagocytosis. In addition, females at 1000 and 2000 ppm had an
increased frequency of swelling of body areas (principally the mammary
gland regions) during the first 70 weeks of treatment; however, in the
absence of any supporting histopathological findings in the mammary
gland tissue or skin, the toxicological significance of these
transient swellings could not be ascertained. No treatment-related
neoplastic lesions were noted in any tissue or organ of any treated
rat at any dose. The NOAEL for systemic toxicity was 100 ppm, equal to
4.8 mg/kg bw per day (Trutter, 1992b).
(d) Reproductive toxicity
Rats
In a two-generation study, with one litter per generation, groups
of 25 male and 25 female weanling Crl:CD BR rats were fed diets
containing technical-grade tebufenozide (purity, 97.5%) providing
dietary levels of 0, 10, 150, or 2000 ppm, equal to 0, 0.8, 12, and
150 mg/kg bw per day for males and 0, 0.9, 13, and 170 mg/kg bw per
day for females. The diets were fed for 70 (F0) or 105 days (F1)
before mating. No treatment-related effects were observed in either
generation at the low dose. At 150 ppm, an increased severity of
pigment deposition in the spleen of F0 and F1 females was reported.
At the highest dose, the body weight and feed consumption of F0 and
F1 males were decreased at some intervals before mating. Increased
splenic pigment deposition and extramedullary haematopoiesis were seen
in parental animals of each sex in both generations. The mean number
of implantation sites in F1 females (not measured in F0) was
decreased and the length of gestation in F1 (but not F0) dams
increased. There was also a small increase in the number of pregnant
females in both generations that had total resorptions and in the
number of F1 females that died during delivery. The NOAEL was 10 ppm,
equal to 0.8 mg/kg bw per day, for general toxicity and 150 ppm, equal
to 12 mg/kg bw per day, for reproductive toxicity (Danberry et al.,
1993).
In a similar study, groups of 24 male and 24 female weanling
Crj:CD(SD) rats were given diets containing technical-grade
tebufenozide (purity, 97.2%) providing levels of 0, 25, 200, or
2000 ppm, equal to 0, 1.6, 13, and 130 mg/kg bw per day for males and
0, 1.8, 15, and 140 mg/kg bw per day for females, for 10 weeks before
mating of the F0 and F1 parents to produce the respective F1 and F2
offspring. The study was terminated after weaning of the F2 litters.
An increased incidence of splenic lesions, seen as blackish changes
and/or congestion of the spleen, increased splenic extramedullary
haematopoiesis, and haemosiderin-laden cells, occurred in F0 and F1
parental animals at 200 and 2000 ppm. At 2000 ppm, slight decreases in
body-weight gain were seen in animals of each sex, and decreases in
uterine and ovarian weights were reported; the changes were small and
were considered not to be toxicologically significant. A significant
reduction in mean body-weight gain was seen in F1 and F2 pups at
2000 ppm between lactation days 14 and 21. The study revealed no
adverse effects on the reproductive ability of male or female rats.
The NOAEL was 25 ppm, equal to 1.6 mg/kg bw per day, for systemic
toxicity and 200 ppm, equal to 13 mg/kg bw per day, for reproductive
toxicity (Aso, 1995).
In both of the above studies, a consistent increase in the
incidence of gross and histopathological findings in the spleen,
including congestion, pigment, and extramedullary haematopoiesis, was
seen in F0 and F1 parental animals. Although tebufenozide at the
doses tested had no effect on the reproductive ability of male or
female rats, the minor reproductive effects observed at 2000 ppm
on parental females in the first study and on lactating pups in
the second study could not be discounted. The overall NOAEL for
reproductive toxicity was thus 200 ppm, equal to 13 mg/kg bw per day.
(e) Developmental toxicity
Rats
In a range-finding study, groups of nine mated female Crl:CD BR
rats were given technical-grade tebufenozide (purity, 96.8%) by gavage
at doses of 0 (vehicle control), 25, 75, 200, or 400 mg/kg bw per day
on days 6-15 of gestation. On day 20, all surviving dams were killed,
and their fetuses were removed from the uterus and necropsied.
No maternal, embryo, or fetal toxicity and no external gross
malformations or anomalies in the fetuses were seen at any dose. The
NOAEL for maternal, embryonic, and fetal toxicity and teratogenicity
was thus 400 mg/kg bw per day, the highest dose tested. In a
supplementary range-finding study, groups of six non-pregnant female
Crl:CD BR rats were given technical-grade tebufenozide (purity, 96.8%)
by gavage at doses of 0, 400, or 1000 mg/kg bw per day for 10 days. At
1000 mg/kg bw per day, a slight increase in liver weight was observed;
however, in the absence of histopathological data, the toxicological
significance of this finding could not be fully evaluated. On the
basis of the very limited data provided, the NOAEL was 400 mg/kg bw
per day (Solomon & Romanello, 1992).
In the main study, groups of 25 mated Crl:CD BR VAF/Plus
Sprague-Dawley rats were given technical-grade tebufenozide (purity,
96.1%) by gavage at doses of 0, 50, 250, or 1000 mg/kg bw per day on
days 6-15 of presumed gestation (day 0 taken as the day spermatozoa
were detected in a vaginal lavage or a copulatory plug was observed
in situ). On gestation day 20, all surviving dams were killed, and
their fetuses were removed and necropsied. At 1000 mg/kg bw per day,
initial slight, transient reductions in mean body-weight gain and food
consumption were seen during dosing, but the mean values over the
study were not affected; the changes were considered not to be
toxicologically significant. All of the fetuses showed normal
development, and no signs of fetotoxicity and no treatment-related
malformations were observed at any dose tested. The NOAEL was
1000 mg/kg bw per day for maternal toxicity and 1000 mg/kg bw per
day, the highest dose tested, for embryo- and fetotoxicity and
teratogenicity (Hoberman, 1991).
Rabbits
In a range-finding study, groups of six mated female Hra:(NZW)SPF
rabbits were given technical-grade tebufenozide (purity, 96.4%) by
gavage at doses of 0, 100, 300, or 1000 mg/kg bw per day on days 7-19
of presumed gestation (the day of mating confirmed by the presence of
seminal fluid in the vulva). All surviving does were sacrificed, and
all fetuses were removed and examined on day 29 of gestation. The
single death due to an unknown cause among animals at 1000 mg/kg bw
per day was unlikely to have been due to treatment, as no deaths
occurred in the main study. No other signs of maternal toxicity or
treatment-related disturbance of intrauterine development of the
conceptuses were observed. All fetuses showed normal development, and
no signs of fetal toxicity or treatment-related malformations were
seen at any dose. The NOAEL for embryo- and fetotoxicity and
teratogenicity was 1000 mg/kg bw per day, the highest dose tested
(Lemen, 1991).
In the main study, groups of 20 mated female New Zealand white
rabbits were given technical-grade tebufenozide (purity, 97.5%) by
gavage at doses of 0, 50, 250, or 1000 mg/kg bw per day on days 7-19
of presumed gestation (the day of mating confirmed by the presence of
seminal fluid in the vulva). All surviving does were sacrificed, and
their fetuses were removed and examined on day 29 of gestation. No
treatment-related deaths, clinical signs of maternal toxicity, or
disturbances of intrauterine development of the conceptuses were
observed at any dose. All of the fetuses showed normal development,
and no signs of fetotoxicity or treatment-related malformations were
observed at any dose. The NOAEL for maternal toxicity, embryo- and
fetotoxicity and teratogenicity was 1000 mg/kg bw per day, the highest
dose tested (Swenson & Solomon, 1992).
(f) Genotoxicity
A battery of tests for mutagenicity was conducted to assess the
potential of technical-grade tebufenozide (purity, > 95-97.5%) to
induce gene mutation, chromosomal aberration, or unscheduled DNA
synthesis. The results, summarized in Table 2, were all negative.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade tebufenozide (purity, 96-97%) was not irritating
to the skin and was minimally irritating to the eyes of male New
Zealand white rabbits, according to Draize scale scoring (Krajewski
et al., 1988a,b; Lutz & Parno, 1993).
Table 2. Results of tests for the genotoxicity of technical-grade tebufenozide
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 0, 50-5000a µg/plate > 95 Negativeb Black (1986)
TA98, TA100,
TA1535, TA1537
Reverse mutation S. typhimurium 0, 50-5000a µg/plate 96.1 Negativeb Sames & Streelman
TA98, TA100, (1991)
TA1535, TA1537
Reverse mutation S. typhimurium 0, 50-5000a µg/plate 96.1 Negativeb Sames & Elia (1993)
TA98, TA100, 0, 160-1600a µg/plate Negativeb
TA1535, TA1537
Reverse mutation S. typhimurium 0, 50-5000a µg/plate 96.1 Negativeb Sames & Elia (1994)
TA98, TA100, 0, 30-300a µg/plate Negativeb
TA1535, TA1537
Reverse mutation E. coli WP2 uvr A 0, 200-5000a µg/plate 96.1 Negativeb Watanabe (1992)
Forward mutation Chinese hamster 0, 10-60 µg/ml 96.4 Negativeb Thilagar (1990a)
ovary cells
(hprt locus)
Chromosomal aberration Chinese hamster 0, 5-30 µg/ml 96.8 Negativeb Thilagar (1987)
ovary cells
Unscheduled DNA Rat (SD) primary 0, 10-100a µg/ml 96.4 Negative Thilagar (1990b)
synthesis hepatocytes 0, 10-60 µg/ml Negative
In vivo
Chromosomal aberration Rat (CD, 5-7 of each 0, 0.5-5.0 g/kg bw 97.5 Negative Gudi (1992)
sex per dose) bone by single gavage
marrow
a Precipitation observed in all cultures
b Conducted with and without exogenous metabolic activation
Technical-grade tebufenozide (purity, 96%) did not sensitize the
skin of guinea-pigs assessed in the maximization test (males) and the
Buehler test (females) (Matsumoto, 1989; Glaza, 1993).
(ii) Neurotoxicity
Groups of 10 male and 10 female Crl:CD R BR rats were given
single doses of technical-grade tebufenozide (purity, 96.1%) in
0.5% w/v aqueous methylcellulose by gavage at 0, 500, 1000, or
2000 mg/kg bw. All rats were subjected to functional observational
battery testing and motor activity assessment before treatment and
1-3 h and 7 and 14 days after dosing, and a detailed neuropathological
examination was carried out at the end of the 14-day observation
period. No remarkable treatment-related neurological changes were
found at any dose. Treatment-related responses were elicited in
similar acute neurotoxicity studies conducted at the same testing
laboratory with substances known to affect functional observational
battery and motor activity testing, thus validating the competence of
the laboratory in this type of testing. The NOAEL for the neurotoxic
and neuropathological effects of tebufenozide was thus 2000 mg/kg bw,
the highest dose tested (Swenson et al., 1994).
(iii) Studies of metabolites
Five metabolites of tebufenozide were tested for acute toxicity
in Crj: CD-1 or Crl:CD-1 BR ICR mice. Metabolites RH-111788, RH-96595,
RH-120970, RH-089886, and RH-112651 (see Figure 1) given orally
induced no deaths and no clinical signs of systemic toxicity at doses
up to 5000 mg/kg bw. The LD50 values for these metabolites in male
and female mice was thus > 5000 mg/kg bw (Hazelton & Quinn, 1993).
Four tebufenozide metabolites, RH-111788, RH-96595, RH-120970,
and RH-089886, were tested for their potential to induce reverse
mutation in Salmonella typhimurium strains TA98, TA100, TA1535, and
TA1537 and in Escherichia coli strain WP2 uvr A at concentrations up
to the limit of solubility (1250-2500 µg/plate), with and without
exogenous metabolic activation. The results, summarized in Table 3,
were negative (Hazelton & Quinn, 1993).
Table 3. Results of tests for the genotoxicity of metabolites
of tebufenozide
Metabolite Concentration Result
(µg/plate)
RH-89886 (in rats, rice) 0, 313-5000a Negative
RH-111788 (in rats, rice) 0, 313-5000a Negative
RH-96595 (in rats, rice, soil) 0, 313-5000a Negative
RH-120970 (in rats, rice) 0, 313-5000a Negative
Potential for induction of reverse mutation in vitro in S.
typhimurium TA98, TA100, TA1535, and TA1537 and in E. coli
WP2 uvr A, with and without exogenous metabolic activation by
a microsomal fraction from rat liver
a Precipitation observed on all plates
Comments
Oral administration to rats of single doses of 3 or 250 mg/kg
bw of 14C-labelled tebufenozide resulted in rapid absorption and
excretion in urine and faeces, only trace amounts of 14C being
recovered in expired air. The excretion profiles were similar,
regardless of the position of the 14C label, the dose, the sex, or
whether the rats had been pretreated with 30 ppm of unlabelled
tebufenozide in the diet for two weeks. A mean total of 87-104% of the
administered radiolabel was eliminated within 48 h, primarily via the
faeces, which accounted for 90% of the 14C that was excreted; only
minor amounts (1-8%) were excreted in urine and trace amounts
(0.1-0.4%) in expired air. In animals at 3 mg/kg bw, absorption
accounted for 35-39 % of the administered radiolabel; 30-34% was
excreted in the bile and about 5% in the urine. In rats at 250 mg/kg
bw, only about 4% of the administered dose was absorbed and
metabolized. The highest levels of 14C in the blood were measured
0.5-12 h after dosing, and clearance of the radiolabel from the
circulation was rapid. Tissue retention of 14C was low, suggesting
that there is little or no bioaccumulation of tebufenozide in the
body.
Most of the 14C excreted in the faeces was in the form of
unabsorbed (parent) tebufenozide, which accounted for about 60 and 90%
of an administered dose of 3 and 250 mg/kg bw per day, respectively;
no unchanged tebufenozide was detected in the urine. The absorbed
14C-tebufenozide was extensively metabolized in rats. There were no
significant qualitative differences in the metabolic profiles
associated with the position of the 14C label, the dose, sex, or
whether rats were pretreated with unlabelled tebufenozide. In general,
the 13-15 metabolites identified in the urine, faeces, and bile were
identical. The main route of metabolism of tebufenozide appeared to be
oxidation of benzylic carbons (A- or B-ring), resulting in a number of
metabolites with various combinations of oxidation state at the three
oxidized carbon centres and one metabolite produced by oxidation of a
nonbenzylic, terminal carbon on the A-ring ethyl group.
Tebufenozide was of low acute toxicity after administration to
mice orally and to rats by the oral, dermal, or inhalation route. The
oral LD50 in mice and rats was > 5000 mg/kg bw; the dermal LD50 in
rats was > 5000 mg/kg bw; and the inhalation LC50 in rats was
> 4.3 mg/litre. The metabolites were also of low acute toxicity to
mice after oral administration. Tebufenozide was not irritating to
the skin and was minimally irritating to the eyes of male rabbits;
it was not a skin sensitizer in guinea-pigs. WHO has not classified
tebufenozide for acute toxicity.
Repeated short-term oral administration of tebufenozide to mice
(2 and 13 weeks), rats (2, 4, and 13 weeks), and dogs (2, 6, 13, and
52 weeks) resulted primarily in haematotoxic effects (regenerative
haemolytic anaemia and compensatory responses from the haematopoietic
tissues). The NOAEL for these effects was 200 ppm, equal to 35 mg/kg
bw per day, in mice in a 13-week study (0, 20, 200, 2000, and 20 000
ppm tested); 200 ppm, equal to 13.1 mg/kg bw per day, in rats in a
13-week study (0, 20, 200, 2000, and 20 000 ppm tested); 50 ppm, equal
to 2 mg/kg bw per day, in dogs in a 13-week study (0, 50, 500, and
5000 ppm tested); and 50 ppm, equal to 1.8 mg/kg bw per day, in a
one-year study in dogs (0, 15, 50, 250, and 1500 ppm tested). Repeated
dermal applications of tebufenozide to rats for four weeks caused
no systemic toxicity at doses < 1000 mg/kg bw per day. The dog
appeared to be the most sensitive species for both short-term and
long-term toxicity.
In an 18-month study of toxicity and carcinogenicity in mice
given tebufenozide in the diet at concentrations of 0, 5, 50, 500,
or 1000 ppm, the NOAEL for systemic toxicity was 50 ppm, equal to
7.8 mg/kg bw per day, on the basis of a slightly reduced survival
rate and mild regenerative haemolytic anaemia at higher doses. In
a two-year study of toxicity and carcinogenicity in rats given
tebufenozide in the diet at 0, 10, 100, 1000, or 2000 ppm, the NOAEL
was 100 ppm, equal to 4.8 mg/kg bw per day, on the basis of decreased
body weight and food consumption and mild regenerative haemolytic
anaemia at higher doses. Tebufenozide was not carcinogenic to mice or
rats under the conditions of the studies.
Tebufenozide and its metabolites have been adequately tested for
genotoxicity in a range of assays both in vitro and in vivo. The
Meeting concluded that neither tebufenozide nor its metabolites are
genotoxic.
In two two-generation studies of reproductive toxicity in rats,
with one litter per generation, doses of 0, 10, 150, or 2000 ppm and
0, 25, 200, or 2000 ppm were administered. The NOAEL for systemic
(parental) toxicity was 25 ppm, equal to 1.6 mg/kg bw per day, on
the basis of a consistent increase in the incidence of gross and
histopathological lesions in the spleens (congestion, pigment, and
extra-medullary haematopoiesis) of F0 and F1 parental animals at
higher doses (200 and 2000 ppm). The NOAEL for reproductive toxicity
was 13 mg/kg bw per day, on the basis of minor reproductive effects
(decreased mean number of implantation sites, prolonged gestation, a
slightly greater frequency of total resorptions, and a small increase
in the number of dams that died during delivery) at the high dose of
2000 ppm in dams in the first study and in lactating pups (decreased
mean weight gain on lactation days 14 and 21) in the second study.
In studies of developmental toxicity in rats and rabbits, doses
of 0, 50, 250, or 1000 mg/kg bw per day were administered. There was
no evidence of teratogenic potential. The NOAEL for maternal, embryo-,
and fetotoxicity and teratogenicity was 1000 mg/kg bw per day, the
highest dose tested, in both species.
In a study of acute neurotoxicity in rats, no treatment-related
effects were seen when single doses of 0, 500, 1000, or 2000 mg/kg bw
were administered. The NOAEL for acute neurotoxicity and neuro-
pathological effects was 2000 mg/kg bw, the highest dose tested.
In summary, exposure to tebufenozide by the oral route results
primarily in haematotoxicity. The main target of its action is the
peripheral haematopoietic system; the pivotal toxicological end-point
of concern, which is seen consistently across all species tested, is
mild regenerative haemolytic anaemia with compensatory responses from
the haematopoietic tissues.
An ADI of 0-0.02 mg/kg bw was established for tebufenozide on the
basis of the NOAELs for haematotoxicity of 1.8 mg/kg bw per day in the
one-year study in dogs and 1.6 mg/kg bw per day in a two-generation
study of reproductive toxicity in rats, using a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 200 ppm, equal to 35 mg/kg bw per day (13-week study of
toxicity)
50 ppm, equal to 7.8 mg/kg bw per day (haematotoxicity
in an 18-month study of toxicity and carcinogenicity)
Rat: 200 ppm, equal to 13 mg/kg bw per day (13-week study of
toxicity)
100 ppm, equal to 4.8 mg/kg bw per day (haematotoxicity
in a two-year study of toxicity and carcinogenicity)
25 ppm, equal to 1.6 mg/kg bw per day (maternal
haematotoxicity in a two-generation study of
reproductive toxicity)
200 ppm, equal to 13 mg/kg bw per day (reproductive
toxicity in a two-generation study)
1000 mg/kg bw per day, the highest dose tested
(maternal, embryo-, and fetotoxicity and teratogenicity
in a study of developmental toxicity)
Rabbit: 1000 mg/kg bw per day, the highest dose tested
(maternal, embryo-, and fetotoxicity and teratogenicity
in a study of developmental toxicity)
Dog: 50 ppm, equal to 1.8 mg/kg bw per day (haemotoxicity in
a one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Observations in humans
2. Studies on the mechanism of haematotoxicity
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to tebufenozide
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 > 5000 mg/kg bw
Dermal, toxicity, rat LD50 > 5000 mg/kg bw
Inhalation, 4 h, toxicity, rat LC50 > 4.3 mg/litre
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Minimally irritating
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated dietary, 90 days, toxicity, dog NOAEL = 2 mg/kg bw per day, primarily
haematotoxicity
Repeated dermal, 28 days, toxicity, rat NOAEL = 1000 mg/kg bw per day, highest dose
tested
Repeated dietary, reproductive toxicity, rat NOAEL = 13 mg/kg bw per day, minor reproductive
effects
Repeated gavage, developmental toxicity, NOAEL = 1000 mg/kg bw per day, highest dose
rat and rabbit tested; maternal, embryo-, and fetal toxicity
and teratogenicity
Long-term (> 1 year) Repeated dietary, 1 year, toxicity, dog NOAEL = 1.8 mg/kg bw per day, primarily
haematotoxicity
References
Aso, S. (1995) Two-generation reproduction study of RH-5992 in rats.
Unpublished report No. 93RC-101 from Hita Research Laboratories, Oita,
Japan. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Black, C.A. (1986) RH-75,992: Microbial mutagenicity assay (Ames
test). Unpublished report No. 86R-0013 from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Clay, H. (1992). RH-5992: 90-day oral (dietary) administration
toxicity study in the beagle. Unpublished report No. 90RC-062 from
Hazleton UK, North Yorkshire, United Kingdom. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Danberry, T.L., Romanello, A.S., Donofrio, K.F. & O'Hara, G.P. (1993)
RH-5992: Two-generation reproduction study in rats. Unpublished report
No. 9OR-202A from Rohm & Haas Co., Toxicology Department, Philadelphia,
PA, USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Glaza, S.M. (1993) Dermal sensitization study of RH-5992 technical in
guinea pigs - maximization test. Unpublished report No. 92RC-101 from
Hazleton Wisconsin, Inc., WI, USA. Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Gudi, R (1992) RH-5992 technical: Acute test for chemical induction of
chromosome aberration in rat bone marrow cells in vivo. Unpublished
report No. 9ORC-203 from Sitek Research Laboratories, MD, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hawkins, D.R. & Hazelton, G.A. (1993) 14C-RH-5992: Biliary excretion
study in rats, supplemental report A: Metabolism of RH-5992 in rats.
Unpublished report No. 9ORC-058A from Rohm & Haas Co., Agricultural
Products Development Department, PA, USA. Submitted to WHO by Rohm &
Haas Co., Philadelphia, PA, USA.
Hawkins, D.R., Spencer, W.O. & Hazelton, G.A. (1992) 14C-RH-5992
pharmacokinetic study in rats, supplemental report A: Metabolism of
RH-5992 in rats. Unpublished report No. 91RC-004A from Rohm & Haas
Co., Agricultural Products Development Department, PA, USA. Submitted
to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hawkins, D.R., Spencer W.O. & Hazelton, G.A. (1993) 14C-RH-5992:
Pharmacokinetic study in rats, supplemental report B: Metabolism of
RH-5992 in rats. Unpublished report No. 91RC-004B from Rohm & Haas
Co., Agricultural Products Development Department, PA, USA. Submitted
to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hazelton, G.A. & Quinn, D.L. (1993) Hazard evaluation of RH-5992
technical in human and domestic animals. Part I: Hazard identification
and evaluation. Unpublished report No. 93R-1039 from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Hazelton, G.A. & Quinn, D.L. (1995) Evaluation of rat reproduction
studies conducted with RH-5992. Unpublished report No. 95R-1068 from
Rohm & Haas Co., Toxicology Department, Philadelphia, PA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Hoberman, A.M. (1991) RH-5992: Oral developmental toxicity (embryo-
fetal toxicity and teratogenic potential) study in rats. Unpublished
report No. 9ORC-059 from Argus Research Laboratories, Inc., PA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Kehoe, D.F. (1990) RH-5992: Two-week dietary range-finding study in
male dogs. Unpublished report No. 87RC-034 from Hazleton Laboratories
America, Inc., WI, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988a) RH-75,992:
Skin irritation study in rabbits. Unpublished report No. 87R-088 from
Rohm & Haas Co., Toxicology Department, Philadelphia, PA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1988b) RH-75,992: Eye
irritation study in rabbits. Unpublished report No. 87R-084 from Rohm
& Haas Co., Toxicology Department, Philadelphia, PA, USA. Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Kyle, M.E. (1992) RH-5992: Two-week dietary range-finding study in
male mice. Unpublished report No. 86R-228A from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Kyle, M.E. & Quinn, D.L. (1992) RH-5992: Two-week dietary range-
finding study in rats. Unpublished report No. 86R-226A from Rohm &
Haas Co., Toxicology Department, Philadelphia, PA, USA. Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Lemen, J.K. (1991) RH-5992: Range-finding rabbit developmental
toxicity study. Unpublished report No. 89RC-100 from Hazleton
Laboratories America, Inc., VA, USA. Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
LeVan, L.W. (1991) 14C-RH-5992: Distribution of radioactivity in rats
following a single oral dose. Unpublished report No. 89RC-099 from
Hazleton Laboratories America, Inc., WI, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Lutz, M.F. & Parno, J.R. (1993) RH-75,992: Eye irritation study
in rabbits. Unpublished report No. 93R-237 from Rohm & Haas Co.,
Toxicology Department, Philadelphia, PA, USA. Submitted to WHO by Rohm
& Haas Co., Philadelphia, PA, USA.
Matsumoto, R. (1989) Skin sensitization study of RH-5992 (technical)
in the guinea pig. Unpublished report No. 9ORC-1002A from Bozo
Research Centre, Inc., Tokyo, Japan. Submitted to WHO by Rohm & Haas
Co., Philadelphia, PA, USA.
Morrison, R.D. & Hamilton, J.D. (1991a) RH-75,992 technical: Acute
oral toxicity study in male and female mice. Unpublished report No.
91R-003 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D. & Hamilton, J.D. (1991b) RH-75,992 technical: Acute
oral toxicity study in male and female rats. Unpublished report No.
9OR-199 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D. & Hamilton, J.D. (1991c) RH-75,992 technical: Acute
dermal toxicity study in male and female rats. Unpublished report No.
9OR-200 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Morrison, R.D., Carbone, J.P., Parno, J.R. & Gillette, D.M. (1993)
RH-75,992 technical and RH-75,992 2F formulation: Four-week dermal
toxicity study in rats. Unpublished report No. 92R-150 from Rohm &
Haas Co., Toxicology Department, Philadelphia, PA, USA. Submitted to
WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Osheroff, M.R. (1991a) RH-5992: 13-Week dietary toxicity study in
mice. Unpublished report No. 89RC-102 from Hazleton Laboratories
America, Inc., VA, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Osheroff, M.R. (1991b) RH-5992: Four-week range-finding dietary
toxicity study in rats. Unpublished report No. 89RC-101A from Hazleton
Laboratories America, Inc., MD, USA. Submitted to WHO by Rohm & Haas
Co., PA, USA.
Osheroff, M.R. (1991c) RH-5992: 13-week dietary toxicity study in
rats. Unpublished report No. 89RC-101 from Hazleton Laboratories
America, Inc., VA, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Richards, J.F. (1992) RH-5992: 52-week oral (dietary administration)
chronic toxicity study in the beagle. Unpublished report No. 90RC-206
from Hazleton UK, North Yorkshire, United Kingdom. Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Elia, M.C. (1993) RH-75,992 technical: Salmonella
typhimurium gene mutation assay (Ames test). Unpublished report No.
93R-094 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Elia, M.C. (1994) RH-75,992 technical: Salmonella
typhimurium gene mutation assay (Ames test). Unpublished report No.
93R-236 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Sames, J.L. & Streelman, D.R. (1991) RH-75,992 technical: Salmonella
typhimurium gene mutation assay (Ames test). Unpublished report No.
9IR-167 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Solomon, H.M. & Romanello, A.S. (1992) RH-5992: Range-finding (gavage)
developmental toxicology study in rats. Unpublished report No.
87R-105A from Rohm & Haas Co., Toxicology Department, Philadelphia,
PA, USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Struble, C.B. & Hazelton, G.A. (1992a) 14C-RH-5992: Biliary excretion
study in rats. Unpublished report No. 90RC-058 from Hazleton
Wisconsin, Inc., WI, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Struble, C.B. & Hazelton, G.A. (1992b) 14C-RH-5992: Pharmacokinetic
study in rats. Unpublished report No. 91RC-004 from Hazleton
Wisconsin, Inc., WI, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Swenson, R.E. & Solomon, H.M. (1992) RH-5992: Oral (gavage)
developmental toxicity study in rabbits. Unpublished report No.
90R-201 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Swenson, R.E., Gillette, D.M. & Parno, J.R. (1994) RH-75,992: Acute
oral (gavage) neurotoxicity study in rats. Unpublished report No.
93R-093 from Rohm & Haas Co., Toxicology Department, Philadelphia, PA,
USA. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1987) RH-75,992: Test for chemical induction of
chromosome aberration using monolayer cultures of Chinese hamster
ovary (CHO) cells with and without metabolic activation. Unpublished
report No. 88RC-011 from Sitek Research Laboratories, MD, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1990a) RH-5992: Test for chemical induction of gene
mutation at the HGPRT locus in cultured Chinese hamster ovary (CHO)
cells with and without metabolic activation. Unpublished report No.
89RC-097 from Sitek Research Laboratories, MD, USA. Submitted to WHO
by Rohm & Haas Co., Philadelphia, PA, USA.
Thilagar, A. (1990b) RH-5992: Test for chemical induction of
unscheduled DNA synthesis in rat primary hepatocyte cultures by
autoradiography. Unpublished report No. 89RC-098 from Sitek Research
Laboratories, MD, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Trutter, J.A. (1992a) RH-5992: 18-Month dietary oncogenicity study in
mice. Unpublished report No. 90RC-061 and 90RC-061A from Hazleton
Washington, Inc., VA, USA. Submitted to WHO by Rohm & Haas Co.,
Philadelphia, PA, USA.
Trutter, J.A. (1992b) RH-5992: 24-Month combined dietary chronic
toxicity and oncogenicity study in rats. Unpublished report No.
90RC-060 and 90RC-060A from Hazleton Washington, Inc., VA, USA.
Submitted to WHO by Rohm & Haas Co., Philadelphia, PA, USA.
Ulrich, C.E. (1992) RH-5992 (technical): Acute inhalation toxicity
evaluation in rats. Unpublished report No. 90RC-057 from International
Research and Development Corporation, MI, USA. Submitted to WHO by
Rohm & Haas Co., Philadelphia, PA, USA.
Watanabe, K. (1992) RH-5992: Reverse mutation assay with E. coli
(WP2 uvr strain). Unpublished report No. 92RN-1010 from The Institute
of Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Rohm &
Haas Co., Philadelphia, PA, USA.
Yoshida, A. (1992) RH-5992: Blood recovery study in dogs. Unpublished
report No. 92RC-1040 from The Institute of Environmental Toxicology,
Tokyo, Japan. Submitted to WHO by Rohm & Haas Co., Philadelphia, PA,
USA.
