
Pesticide residues in food -- 1999
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
Toxicological evaluations
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Rome, 20-29 September 1999
GLUFOSINATE-AMMONIUM (addendum)
First draft prepared by
I. Dewhurst
Pesticides Safety Directorate, Ministry of Agriculture, Fisheries and
Food,
Mallard House, Kings Pool, York, United Kingdom
Explanation
Evaluation for acceptable daily intake
Biological data
Absorption, distribution and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Significance of glutamine synthetase inhibition to
humans
Toxicological studies
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Studies on metabolites
N-Acetylglufosinate
3-Methylphosphinicopropionic acid
Observations in humans
Medical surveillance of manufacturing plant
personnel
Poisoning incidents
Comments
Toxicological evaluation
References
Explanation
Glufosinate-ammonium was evaluated toxicologically by the 1991
Joint Meeting (Annex 1, reference 62), when an ADI of 0-0.02 mg/kg
bw was established on the basis of the NOAEL in a long-term studyof
toxicity in rats and a 100-fold safety factor. The 1991 Meeting also
requested additional observations in humans and information on the
biological significance of the increased renal glutamine synthetase
activity observed in rats.
The (-) isomer of N-acetylglufosinate is a major metabolite
when glufosinate-ammonium is applied to glufosinate-tolerant crops.
The 1998 JMPR considered residues issues arising from applications of
glufosinate-ammonium to tolerant crops and proposed that the residue
be defined as the 'sum of glufosinate-ammonium,
3-[hydroxy(methyl)phosphinoyl]propionic acid and
N-acetyl-glufosinate' (Annex 1, reference 83), but it could not
adopt this definition until N-acetyl-glufosinate had been evaluated
toxicologically. The present Meeting reviewed additional data on human
exposure, glutamine synthetase activity, the toxicity of repeated
doses, and the toxicokinetics of glufosinate-ammonium, together with
an extensive dossier on N-acetyl-glufosinate and a summary of the
results of studies on 3-[hydroxy(methyl)phosphinoyl]propionic acid,
another metabolite of glufosinate-ammonium.
Evaluation for Acceptable Daily Intake
1. Biological data
All of the studies contained statements of compliance with good
laboratory practice (GLP) and claimed to have been performed to
applicable guidelines of the OECD, European Union, or US Environmental
Protection Agency.
(a) Absorption, distribution, and excretion
(i) Oral administration
Rats
The absorption, distribution, and excretion of
[3,4-14C]-glufosinate-ammonium was studied after administration by
oral gavage in aqueous vehicles at doses of 2, 20, or 500 mg/kg bw
(Stumpf, 1993a; Lauck-Birkel, 1995a, 1996; Lauck-Birkel & Strunk,
1999a; Maas & Braun, 1999a). Samples of urine and faeces were
collected daily for up to 4 days, tissue, organ and excreta samples
were processed, and total radiolabel was determined by liquid
scintillation counting. The studies had much in common, and
representative findings are summarized in Table 1. Less than 10% of an
oral dose was absorbed, with rapid excretion, mainly as parent
compound, in the faeces within 24 h. The concentrations of residues
were markedly higher in kidney and liver than in plasma, while those
in brain were even lower, indicating limited penetration of the
blood-brain barrier. There were no marked differences between the
sexes. Administration at 500 mg/kg bw resulted in a more prolonged
absorption and excretion phase than after 20 or 2 mg/kg bw.
Goats
A lactating goat weighing about 50 kg was given
[3,4-14C]glufosinate-ammonium (99% radiochemical purity; specific
activity, 26 mCi/g) in capsules twice daily for 4 days at a dose of
~ 3 mg/kg bw per day. Over 80% of the administered dose was excreted
in faeces and approximately 3% in urine; only small amounts were found
in the tissues (< 0.1%) and milk (0.02%). The concentration of
radiolabel in milk reached a plateau by day 2 (0.02 µg/g). The
concentrations were higher in kidney (0.6 µg/g) and liver (0.4 µg/g)
than muscle and fat (< 0.01 µg/g) (Huang & Smith, 1995a).
Table 1. Radiolabel in excreta and tissues of Wistar rats given
[3,4-14C]glufosinate ammonium at 2, 20, or 500 mg/kg bw
Sample Radiolabel in excreta (% of dose) or tissues (µg/g)
500 mg/kg bwa 20 mg/kg bwb 2 mg/kg bwc
Males Females Males Males Females
Urine
24 h 3 3 5 8 8
Total 8 5 5 10 9
Faeces
24 h 38-71 35-51 92 88 91
Total 75 89 92 91 95
Liver, 6 h 15 10 0.7 NA NA
Kidney, 6 h 53 49 2.9 NA NA
Brain, 6 h 0.6 1 < 0.1 NA NA
Plasma, 6 h 1.3 1.4 < 0.1 NA N
Spleen, 6 h 9 19 NA NA NA
NA, not analysed
a Results from Lauck-Birkel (1995a); Maas & Braun (1995a)
b Results from Lauck-Birkel & Strunk (1999a); Maas & Braun (1999a)
c Results from Stumpf (1993a); Lauck-Birkel (1996)
Chickens
Laying hens were given [3,4-14C]glufosinate-ammonium (99%
radiochemical purity; specific activity, 26 mCi/g) in capsules twice
daily for 14 days at a dose of ~ 2 mg/kg bw per day. The excreta
contained over 90% of the administered dose, and < 0.02% of the
administered radiolabel was present in edible tissues and 0.07% in
eggs. The peak concentrations were 0.1 µg/g in liver, 0.07 µg/g in egg
white, and 0.02 µg/g in egg yolk (Huang & Smith, 1995b).
(ii) Intravenous administration
Rats
[3,4-14C]Glufosinate-ammonium (99% radiochemical purity;
specific activity, 5200 MBq/g) was administered in saline to groups of
five male Wistar rats at a dose of 2.3 mg/kg bw into the tail vein.
Groups of animals were sacrificed 2 or 24 h after dosing, and samples
of blood (for plasma), brain, kidney, and liver were processed and
assayed for total radiolabel by liquid scintillation counting. Urine
and faecal samples were obtained over 24 h. Excretion was rapid, 78%
of the administered dose being found in the 24-h urine sample and 2%
in faeces. The highest tissue concentrations were in the kidney
(15 µg/g at 2 h; equivalent to 5.5% of the administered dose) and
liver (1 µg/g; equivalent to 1.6% of the administered dose), with much
lower concentrations in brain (0.06 µg/g) and plasma (0.1 µg/g). The
half-times could not be determined owing to the limited number of
samples (Lauck-Birkel & Strunk, 1999b; Maas & Braun, 1999b).
(b) Biotransformation
(i) Oral administration
Rats
In two similar studies, [3,4-14C]-glufosinate-ammonium (> 93%
radiochemical purity; specific activity ~ 2400 or 7800 MBq/g) was
administered to groups of three or five male and female Wistar rats at
a dose of 2 mg/kg bw by gavage in saline. Urine and faecal samples
were obtained every 24 h for 2 or 4 days, pooled, and analysed for
total radiolabel and metabolites by liquid scintillation counting,
high-performance liquid chromatography (HPLC), and thin-layer
chromatography with comparison to standards. Less than 10% of the
administered dose was absorbed. Excretion was rapid (95% within the
first 24 h) and occurred predominantly as the parent compound in
faeces (> 70% of the administered dose). The main urinary metabolites
were 3-[hydroxy(methyl) phosphinoyl]propionic acid and
4-methylphosphinico-butanoic acid, N-acetylglufosinate being the
primary faecal metabolite with hydroxy-4-methylphosphinicobutanoic
acid. There were no marked differences between males and females. The
results are presented in Table 2 (Stumpf, 1993a; Lauck-Birkel, 1996).
[3,4-14C]Glufosinate-ammonium (> 98% radiochemical purity;
specific activity, 18 MBq/g) was administered to groups of one or five
male and female Wistar rats at a dose of 500 mg/kg bw by gavage in
saline. Urine and faecal samples were obtained every 24 h for 4 days,
pooled, and analysed for total radiolabel and metabolites by liquid
scintillation counting, HPLC, and thin-layer chromatography with
comparison to standards. Samples of liver, spleen, kidney, brain, and
blood were obtained from animals killed at 2, 6, 24, or 96 h, but
metabolites were not determined. Less than 10% of the administered
dose was absorbed. Excretion occurred predominantly as parent compound
in faeces (> 70% of the administered dose). The primary urinary
metabolite was 3-[hydroxy(methyl) phosphinoyl]propionic acid, and that
in faeces was N-acetylglufosinate. There were no marked differences
between males and females. The results are presented in Table 3
(Lauck-Birkel, 1995b).
[3,4-14C]Glufosinate-ammonium (99% radiochemical purity;
specific activity, ~ 1200 MBq/g) was administered to groups of five
male Wistar rats at a dose of 20 mg/kg bw by gavage in saline. Groups
of animals were killed 1, 6, or 24 h after dosing, and samples of
blood (for plasma), brain, kidney, and liver were pooled, processed,
and assayed for total radiolabel (liquid scintillation counting) and
metabolites (HPLC with comparison to standards). Urine and faecal
Table 2. Glufosinate ammonium and metabolites in pooled samples from Wistar rats given
[3,4-14C]-labelled compound at 2 mg/kg bw by gavage
Compound % of administered dose
Malesa Femalesa Malesb
Urine Faeces Urine Faeces Urine Faeces
0-96 h 0-96 h 0-96 h 0-96 h 0-48 h 0-48 h
Total radiolabel 10 90 9 95 6 94
Glufosinate ammonium 5.1 75 4.5 69 4.3 77
4-Methylphosphinicobutanoic acid 1.6 < LD 1.8 < LD 0.2 0.4
Hydroxy-4-methylphosphinicobutanoic acid < LD 3 < LD 3.5 0.1 4.3
3-Methylphosphinicopropionic acid 1.9 1 0.7 1 0.8 1.3
N-Acetylglufosinate < LD 7.4 < LD 9.2 0.1 7.5
LD, limit of determination, < 0.001% of the administered dose
a From Stumpf (1993a)
b From Lauck-Birkel (1996)
Table 3. Glufosinate ammonium and metabolites in pooled 96-h samples from Wistar
rats given [3,4-14C]-labelled compound at 500 mg/kg bw by gavage
Compound % of administered dose
Males Females
Urine Faeces Urine Faeces
Total radiolabel 7.7 75 5.2 89
Glufosinate ammonium 5.9 72 4.3 84
4-Methylphosphinicobutanoic acid 0.2 0.3 0.2 0.1
Hydroxy-4-methylphosphinicobutanoic acid < LD 0.3 < LD 0.3
3-Methylphosphinicopropionic acid 1.2 0.6 0.5 0.5
N-Acetylglufosinate 0.04 1.2 0.02 1.7
LD, limit of determination, < 0.001% of the administered dose
a From Stumpf (1993a)
samples were obtained over 24 h. Metabolites were not determined in
plasma or brain owing to insufficient total radiolabel. Less than 10%
of the administered dose was absorbed. Excretion was rapid (90% within
24 h) and occurred predominantly as parent compound in faeces (85% of
the administered dose). The primary urinary and tissue metabolite was
3-[hydroxy(methyl) phosphinoyl]propionic acid, and
N-acetylglufosinate was the primary faecal metabolite. The results
are presented in Table 4 (Lauck-Birkel & Strunk, 1999b).
Goats
A lactating goat weighing about 50 kg was given
[3,4-14C]glufosinate-ammonium (99% radiochemical purity; specific
activity, 26 mCi/g) in capsules, twice daily for 4 days at a dose of
about 3 mg/kg bw per day. Over 80% of the administered dose was
excreted in faeces and approximately 3% in urine; only small amounts
of the administered radiolabel were found in tissues (< 0.1%) and
milk (0.02%). The concentrations of radiolabel in milk reached a
plateau by day 2 (0.02 µg/g). The concentrations were higher in kidney
(0.6 µg/g) and liver (0.4 µg/g) than in muscle and fat (< 0.01 µg/g).
Glufosinate and 3-[hydroxy(methyl) phosphinoyl]propionic acid
comprised approximately 50% and 30%, respectively, of the radiolabel
in both kidney and liver. In milk, only 63% or the radiolabel was
identified, and glufosinate represented about 50% of the total. In
urine and faeces, glufosinate comprised > 75% and 3-[hydroxy(methyl)
phosphinoyl]propionic acid > 10% of the total radiolabel. In faeces,
N-acetylglufosinate represented 8% of the total residue (Huang &
Smith, 1995a).
Table 4. Glufosinate ammonium and metabolites in pooled samples from male Wistar rats given
[3,4-14C]-labelled compound at 20 mg/kg bw by gavage
Compound % of administered dose µg/g equivalent
Urine Faeces Kidney Liver
0-24 h 0-24 h 1 h 6 h 24 h 1 h 6 h 24 h
Total radiolabel 4.7 92 3.5 2.9 1.4 0.3 0.7 0.6
Glufosinate ammonium 3.3 87 3.1 2.5 1.3 0.2 0.3 0.4
4-Methylphosphinicobutanoic acid 0.2 < LD 0.1 0.2 0.02 0.02 0.06 0.03
3-Methylphosphinicopropionic acid 0.8 1 0.3 0.2 0.07 0.1 0.3 0.2
N-Acetylglufosinate < LD 3.5 < LD < LD < LD < LD < LD < LD
LD, limit of determination, < 0.001% of the administered dose
Chickens
Laying hens were given [3,4-14C]glufosinate-ammonium (99%
radiochemical purity; specific activity, 26 mCi/g) in capsules twice
daily for 14 days at a dose of about 2 mg/kg bw per day. The excreta
contained > 90% of the administered dose, and < 0.02% was present in
edible tissues and 0.07% in eggs. The peak concentrations were 0.1
µg/g in liver, 0.07 µg/g in egg white, and 0.02 µg/g in egg yolk.
Glufosinate was the main residue in eggs, and 3-[hydroxy(methyl)
phosphinoyl]propionic acid the main residue in liver (Huang & Smith,
1995b.
(ii) Intravenous administration
Rats
[3,4-14C]Glufosinate-ammonium (99% radiochemical purity;
specific activity, 5200 MBq/g) was administered in saline to groups of
five male Wistar rats at a dose of 2.3 mg/kg bw into the tail vein.
Groups of animals were sacrificed at 2 or 24 h after dosing and
samples of blood (for plasma), brain, kidney, and liver were pooled,
processed, and assayed for total radiolabel by liquid scintillation
counting and for metabolites by HPLC with comparison to standards.
Urine and faecal samples were obtained over 24 h. Metabolites were not
determined in plasma owing to insufficient total radiolabel. The
radiolabel was eliminated rapidly, primarily as the parent in urine.
The main metabolite, 3-[hydroxy(methyl) phosphinoyl]propionic acid,
which was excreted in urine, was formed by deamination at C-2. The
results are presented in Table 5 (Lauck-Birkel & Strunk, 1999b).
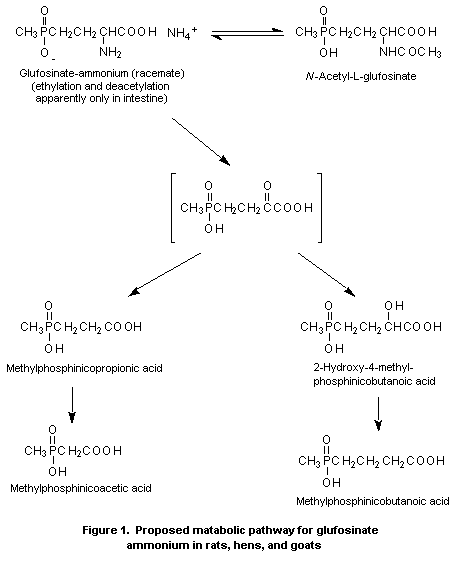
A metabolic pathway for glufosinate-ammonium in various species
is shown in Figure 1.
(c) Effects on enzymes and other biochemical parameters
The main biological property of glufosinate-ammonium is
inhibition of the enzyme glutamine synthetase. N-Acetylglufosinate
also inhibits this enzyme in a range of tissues, but the
interpretation of these results is confounded by the presence of
glufosinate-ammonium in the samples of N-acetylglufosinate tested.
In an attempt to determine the degree to which N-acetylglufosinate
inhibits glutamine synthetase, comparative studies were performed
in vitro and in vivo (Lutkemeier, 1999; Schmid et al., 1999).
The inhibition of glutamine synthetase by N-acetylglufosinate
and glufosinate-ammonium was investigated in vitro in tissues from
11-week-old Wistar rats. Samples of liver, kidney, and brain
(neocortex, medulla oblongata, and hypothallamic region) were removed
from 10 animals, rapidly cooled, and kept at -20°C before preparation.
Samples were pooled and homogenates prepared. Liver and kidney were
assayed as homogenates, and brain tissues were assayed as a 1500 × g
supernatant of a homogenate. The assay for glutamine synthetase is
based on the formation of gamma-glutamyl hydroxamate and ammonia from
Table 5. Glufosinate ammonium and metabolites in pooled samples from male Wistar rats given
[3,4-14C]glufosinate ammonium at 2.3 mg/kg bw intravenously
Compound % of administered dose µg/g equivalent
Urine Faeces Kidney Liver
0-24 h 0-24 h 1 h 6 h 24 h 1 h 6 h 24 h
Total radiolabel 78 2.3 0.06 0.04 15 1 1 0.5
Glufosinate ammonium 68 2 0.04 0.04 14 1 1 0.5
3-Methylphosphinicopropionic acid 10 0.05 < 0.01 0.01 1.4 0.1 0.1 0.1
N-Acetylglufosinate < LD 0.2 < LD < LD < LD < LD < LD < LD
LD, limit of determination, < 0.001% of the administered dose
 (-)-glutamine and hydroxylamine in the presence of arsenate,
manganese, and ADP, followed by spectrophotometric measurement of an
iron compound. A preincubation period of 10 min was used in the main
assays, as it had been shown that inhibition was not changed by
extending this period to 60 or 120 min. The samples were incubated for
20 min in the presence of N-acetylglufosinate (a 33.8% solution
containing 0.06% w/w glufosinate-ammonium) at 0-10 000 µg/ml or
glufosinate-ammonium (as a 50.2% solution) at 0-500 µg/ml.
Glufosinate-ammonium induced significant, concentration-related
inhibition of glutamine synthetase in all tissues at doses > 0.77
mmol/L (Table 6), the inhibition profile varying with tissue.
N-Acetylglufosinate induced only marginal inhibition at 13 mmol/L,
some of which can be attributed directly to the glufosinate-ammonium
content of the sample. The report did not provide results corrected
for protein content, and not all of the assays were performed in
duplicate; however, for the purposes of this comparative exercise, the
results are considered to be acceptable (Lutkemeier, 1999).
A comparative study was performed of the inhibition of glutamine
synthetase in tissues from groups of 10 male Wistar rats given diets
containing N-acetylglufosinate (with 0.06% w/w glufosinate-ammonium)
at 1000 or 10 000 ppm or glufosinate-ammonium at 0, 100, or 1000 ppm
(Schmid et al., 1999). The animals were exposed for 6, 13, 20, or 90
days with 91 days plus 31 days for recovery. In addition to standard
observations and gross necropsy, samples of liver, brain, and kidney
were removed, rapidly cooled, and stored at -70°C prior to processing
and assaying for glutamine synthetase activity, as described above.
There were no deaths or treatment-related clinical signs. The
absolute and relative weights of the kidney were increased by 8-23% in
all treated groups during the first 20 days of the study, but with no
associated pathological findings. Statistically significant inhibition
of glutamine synthetase activity was seen in liver and kidney samples
by day 6, and, except in liver from animals exposed to 1000 ppm
N-acetylglufosinate, did not increase markedly up to day 90 (Table
7). Significant recovery of glutamine synthetase activity occurred
during the 31-day recovery period. The results indicate that orally
administered glufosinate-ammonium is approximately 10 times more
potent at inhibiting glutamine synthetase than is
N-acetylglufosinate. The extent to which this inhibition is due
directly to de-acetylation of N-acetylglufosinate to
glufosinate-ammonium is uncertain. The activity of glutamine
synthetase in brain samples was not reduced markedly in animals
exposed to either N-acetylglufosinate or glufosinate-ammonium at
1000 ppm.
(d) Significance of glutamine synthetase inhibition to humans
Glutamine synthetase (E.C.6.3.1.2) is a key enzyme in the
metabolism of nitrogen and glutamate, catalysing the multi-step
reaction of
(-)-glutamate + ATP + NH3 <=> (-)-glutamine + ADP + P
Table 6. Inhibition of glutamine synthetase activity in vitro in tissue samples from rats, in the
presence of N-acetylglufosinate and glufosinate ammonium; in square brackets, absolute activity
expressed as mg gamma-glutamylhydroxamate formed per g tissue per 20 min
Compound Dose Liver Kidney Neocortex Medulla Hypothalamus
(mmol/L)
Glufosinate ammonium 0 0 [28] 0 [18] 0 [27] 0 [23] 0 [20]
0.003 1 0 0 1
0.008 2 1 0 0 0
0.026 4 1 3 1 0
0.077 14 3 5 6 3
0.26 36 5 13 20 11
0.77 60 13 32 42 29
1.3 72 17 45 53 41
N-Acetylglufosinate 0.13 1 0 0 0 0
0.38 1 0 0 0 0
0.63 2 0 0 0 0
1.3 2 0 0 0 0
6.3 9 1 2 2 1
13a 15 2 4 7 5
a Contains approximately 0.03 mmol/L glufosinate ammonium
Table 7. Activity of glutamine synthetase in samples from 10 male Wistar rats that
received N-acetylglufosinate or glufosinate ammonium in the diet or
control diet
Day Tissue Glutamine synthetase activity (mean % of control value)
Controla Glufosinate ammonium N-acetylglufosinate
100 ppm 1000 ppm 1000 ppm 10 000 ppm
6 Liver 24 55 36 96 46
Brain 28 101 89 94 93
Kidney 17 60 58 61 54
13 Liver 30 51 30 74 40
Brain 25 101 91 102 104
Kidney 17 61 58 59 54
90 Liver 24 60 40 58 54
Brain 22 104 82 99 98
Kidney 14 67 46 55 53
91 + 31 Liver 24 97 85 83 94
Brain 14 98 88 97 97
Kidney 22 90 97 95 87
a Absolute activity, expressed as mg gamma-glutamylhydroxamate formed per
g tissue per 20 min
In plants, glutamine synthetase is the main enzyme involved in the
control of ammonia concentrations, and its inhibition is the mechanism
of action of glufosinate-ammonium in plants. In mammals, other
pathways exist for the homeostatic control of ammonia, such as reverse
reaction of amino acid dehydrogenases and the carbamoyl phosphate
synthetase-urea cycle. Glutamate and glutamine can, however, play
significant roles in other biochemical and physiological processes in
mammals, such as neurotransmission (glutamate and gamma-aminobutyric
acid (GABA)). The activity of glutamine synthetase varies between
tissues and species (see below), but the amino acid sequence is
reported to be well conserved (LieVenema et al., 1998; Purich, 1998;
Ernst & Leist, 1999a).
The liver has two distinct systems for dealing with ammonia. A
high-capacity, low-affinity system exists in the periportal
hepatocytes which is based on carbamoyl phosphate synthetase and the
urea cycle. In central vein hepatocytes, a low-capacity, high-affinity
system exists which is based on glutamine synthetase and ornithine
aminotransferase. Hack et al. (1994) showed that doses of
glufosinate-ammonium did not increase ammonia concentrations in liver
at a dose (5000 ppm) that inhibited glutamine synthetase activity by
50%. While a 60% reduction in liver glutamine was seen at day 1, the
concentration had returned to normal by day 4, indicating the
induction of alternative pathways. Inhibition of liver glutamine
synthetase by up to 50% is therefore not considered to be adverse in
isolation.
The activity of this enzyme in kidney varies considerably between
species (LieVenema et al., 1998; see below), with relatively high
activity in rodents but negligible activity in dogs and humans.
Inhibition of kidney glutamine synthetase in the absence of
pathological findings is not considered to be relevant to human risk
assessment.
In the brain and central nervous system, ammonia homeostasis is
controlled by a number of enzymes including glutamine synthetase and
glutamate dehydrogenase. Under normal conditions (~ 100 µmol/L of
ammonium and 3 mmol/L of glutamate), the flux through glutamine
synthetase in brain is 2-10% of its theoretical capacity and that of
glutamate dehydrogenase is approximately 0.1% of its capacity
(Lie-Venema et al., 1998). With such excess capacity, inhibition of
brain glutamine synthetase is unlikely to result in significant
increases in brain ammonia concentrations. This conclusion is
supported by the finding of Hack et al. (1994) that brain ammonia
concentrations were not increased at doses of glufosinate-ammonium
that produced a 40% reduction in brain glutamine synthetase activity
in rats. However, the glutamine-glutamate shunt between GABA and
glutamate in neurons and glutamine in astrocytes plays a role in both
excitatory and inhibitory neurotransmission. The results of Hack et
al. (1994), although somewhat inconsistent, indicate that significant
changes in a range of biogenic amines in regions of the dog brain are
associated with changes of > 8% in glutamine synthetase activity
after administration of glufosinate-ammonium at 8 mg/kg bw for 28
days, a dose that produced 'increased gait activity'. It is thus
proposed that any statistically significant, > 10% inhibition of
glutamine synthetase activity in brain is a marker of potentially
adverse effects on brain biochemistry and behaviour.
2. Toxicological studies
All of the studies described below were included statements of
compliance with GLP and met the basic requirements of the OECD test
guidelines applicable at the time of study initiation, unless
otherwise stated.
(a) Short-term studies of toxicity
Mice
In response to concern that the doses used in the study of
carcinogenicity with glufosinate-ammonium in mice evaluated previously
had not been appropriately high (160 ppm in males, 320 ppm in
females), a 90-day study was performed in which groups of 10 NMRI mice
of each sex received diets containing glufosinate-ammonium (purity,
95.5%) at concentrations of 0, 1750, 3500, or 7000 ppm. The
homogeneity and achieved concentrations were acceptable, and the
intakes of animals were 561 and 644 mg/kg bw per day for males and
females at the intermediate dose and 274 and 356 mg/kg bw per day for
animals at the low dose, respectively. The investigations included
clinical signs, body weight, food consumption, haematology, clinical
chemistry, organ weights, and gross and microscopic pathology. All
animals at the high dose had died by day 8, 50% of those at 3500 ppm
had died by day 11, and one female at the low dose died. Clinical
signs (ruffled fur, sedation, and emaciation), reduced food
consumption, and initial body-weight loss were seen at all doses,
although the body-weight gain during the latter part of the study was
similar in surviving animals. There were no consistent clinical
chemical or haematological findings and no changes in organ weights or
on gross examination. Congestion in multiple organs was seen at
microscopic examination of many animals that died during the study,
but the cause of death was not determined. No NOAEL could be
identified, but the design was not optimal for this purpose. The
lowest dose tested (1750 ppm, equal to 270 mg/kg bw per day) was
approximately the maximum tolerated dose for a 90-day study, resulting
in a single death and marked initial effects on body weight. The
maximum tolerated dose for a 2-year study in mice would be about 600
ppm if a factor of 3 is used to extrapolate from the approximate dose
in the 90-day study (Dotti et al., 1994). The results of this study
indicate that the previous bioassay was performed within a factor of 2
of the estimated maximum tolerated dose and need not be repeated.
Rats
The toxicity of glufosinate-ammonium (purity, 95.5%) was
investigated in groups of 10 Wistar rats of each sex given the
compound in the diet at concentrations of 0, 7500, 10 000, or 20 000
ppm for 90 days. Routine observations and measurements were made, with
ophthalmoscopy before treatment and at termination and a basic
functional observation battery, which was administered before
treatment and at weeks 1, 2, 3, 4, 8, and 13 and involved observations
in the home cage, an external area, and in the hand, but no forced
physical activity such as grip strength or swimming. Blood samples for
haematological and clinical chemical analyses were taken from fasted
animals at week 13. At termination, five animals of each sex per group
were perfused to preserve nervous tissue. Major organs from all
animals at the highest dose and controls were examined histologically,
as was nervous tissue from all perfused animals and all gross lesions.
Organ weights were not determined. The homogeneity, stability, and
achieved concentrations in the diet were satisfactory, with intakes
equal to 0, 520, 690, and 1400 mg/kg bw per day in males and 0, 570,
740, and 1400 mg/kg bw per day in females.
Two females at the high dose died within the first 8 days of
dosing, but there were no other unscheduled deaths. Animals at the
high dose showed a range of signs during the first 2 weeks of
treatment, including sedation, dyspnoea, emaciation, and diarrhoea.
Food consumption was reduced by > 20% in all groups during the first
two weeks. Body-weight loss was seen in animals at the high dose, and
reductions in body-weight gain were seen in other groups during the
first week of dosing. All groups had similar body-weight gains during
the last weeks of the study. No abnormal ophthalmoscopic findings were
reported. The haematological findings were similar in test and control
groups, and an apparent reduction in erythrocyte count in males
appeared to be related to a high control value. A consistent pattern
of changes in serum lactate dehydrogenase and creatine kinase activity
was seen in animals of each sex, with 20% reductions at the low and
intermediate doses and an increase at the high dose. Small (< 10%)
but statistically significant ( p < 0.05) increases in serum calcium
and inorganic phosphorus concentrations were seen in animals at the
high dose. The functional observational battery identified a similar
pattern of changes in males and females that included miosis, apathy,
reduced alertness and grooming, increased body tone, and vocalization.
These changes were present in all treated groups, with no clear
pattern over time, but were more prevalent at the high dose. There
were no significant macroscopic findings, and the only microscopic
finding of significance was an increased incidence of renal pelvis
dilatation in males at the high dose; there were no treatment-related
effects on nervous tissues.
No NOAEL could be identified owing to alterations in calcium and
inorganic phosphorus and in the functional observational battery at
all doses, although there was no indication of irreversible
neurotoxicity. The effects on food consumption and body weight may
have been secondary to palatability, as they were mainly transient
(Dotti et al., 1993).
(b) Long-term studies of toxicity and carcinogenicity
Rats
A study was initiated in 1994 (Schmid et al., 1998) to include
doses above the maximum of 500 ppm used in the first study. Groups of
60 Wistar rats of each sex received diets containing
glufosinate-ammonium (purity, 96%) at concentrations of 0, 1000, 5000,
or 10 000 ppm for 104 weeks. These doses were based on increased
mortality seen at 20 000 ppm in the 90-day study (Dotti et al., 1993).
The animals were observed for survival, clinical signs, body-weight
gain, food consumption, and the presence of nodules or masses. Blood
smears were prepared from controls and animals at the high dose in
weeks 52, 78, and 104. At termination, over 30 tissues were removed,
nine were weighed, and gross and histopathological examination was
performed on all tissues from all animals. The stability, achieved
levels, and homogeneity of glufosinate-ammonium in the diets were
satisfactory, giving intakes equal to 0, 45, 230, and 470 mg/kg bw in
males and 0, 57, 280, and 580 mg/kg bw per day in females.
Survival was similar in all groups, being over 70% at 104 weeks.
There were no treatment-related changes in clinical signs, food use
efficiency, the occurrence of nodules or masses, or the appearance of
blood smears. All treated groups had reduced food consumption and
body-weight gain over the first month of dosing but these parameters
were subsequently within 10% of those of controls. The weight of the
kidney was increased by 15-30% in relation to dose in all treated
groups, but there were no histological correlates. Macroscopic
examination showed a decreased incidence of pituitary nodules in all
treated males but an increased incidence of adrenal gland foci in
males at the high dose. Microscopic examination revealed a
statistically significant ( p < 0.05) increase in the incidence of
retinal atrophy in males and females at 10 000 ppm and in females at
5000 ppm. This effect was considered to be related to treatment, as a
dose-response relationship was evident in females and it occurred in
animals of each sex, even though the incidence was within the range of
historical controls (males, 20%; females, 38%; Table 8). The incidence
of a rare skin tumour (trichofolliculoma) was increased in males at
the high dose, but it was not statistically significant and was not
seen in females or in males receiving half of the high dose; the
finding was therefore considered not to provide clear evidence of
carcinogenic potential. The total number of malignant and benign
tumours was similar in treated and control groups. The NOAEL was 1000
ppm, equal to 45 mg/kg bw per day, on the basis of the increased
incidence of retinal atrophy (Schmid et al., 1998).
Table 8. Incidences of lesions in Wistar rats receiving
glufosinate ammonium in the diet for 104 weeks
Dose (ppm) Incidence (%)
Retinal atrophy Trichofolliculoma
(males)
Males Females
0 4 3 0
1 000 3 2 0
5 000 4 19 0
10 000 12 29 4
(c) Studies on metabolites
(i) N-Acetylglufosinate
The (-) isomer of N-acetylglufosinate is a major metabolite of
glufosinate-ammonium after its application to glufosinate-tolerant
crops. In 1998, the Committee proposed that residues arising from
applications of glufosinate-ammonium to tolerant crops be defined as
the 'sum of glufosinate-ammonium, 3-[hydroxy(methyl)phosphinoyl]
propionic acid, and Nacetylglufosinate' but could not adopt this
definition until N-acetylglufosinate had been evaluated
toxicologically. Extensive toxicokinetic and toxicological studies on
N-acetylglufosinate have since been submitted. The preparation
tested was an aqueous solution of the disodium salt, and all of the
doses cited below have been corrected for the content of the
technical-grade (-)-isomer. The three batches of N-acetylglufosinate
used varied in purity from 74.5% to 99% and in glufosinate-ammonium
content from 0.06 to 4.5% (Weller, 1994). All of the studies conformed
to GLP and were claimed to have been performed according to guideline
85-1 of November 1984 of the US Environmental Protection Agency or to
meet the basic requirements of the OECD test guidelines applicable at
the time the study was initiated, unless otherwise stated.
Absorption, distribution, and excretion: The toxicokinetics of
N-acetylglufosinate was examined in several studies at doses of
3 mg/kg bw (Stumpf, 1993b; Kellner et al., 1993) or 1000 mg/kg bw
(Lauck-Birkel, 1995b; Maas & Braun, 1995b). Groups of five Wistar rats
of each sex received [3,4-14C] N-acetylglufosinate (purity, > 98%)
by gavage in saline. Urine and faeces were collected over 24-h periods
up to 96 h, and up to 12 tissue samples were taken from animals given
1000 mg/kg bw and killed at 2 and 6 h (two of each sex), 24 h (five of
each sex), and 96 h (five of each sex). Radiolabel in the
gastrointestinal tract was determined in one male each killed at 4 or
24 h after receiving 3 mg/kg bw, and one male at this dose killed at
96 h was studied by autoradiography (Kellner et al., 1993). Radiolabel
in tissues and excreta was determined by liquid scintillation counting
after appropriate processing. The samples were also prepared for
metabolic investigations (see below).
The doses administered differed somewhat among animals, but this
was considered not to have affected the results. Most of each
administered dose was excreted in the faeces within 48 h (Table 9),
although excretion was more rapid at the lower dose. The
concentrations in tissue peaked at 6 h and represented < 1% of the
administered radiolabel; the highest concentrations were detected in
kidney, and those in liver and kidney were greater than in plasma. In
animals at 3 mg/kg bw, the tissue concentration represented < 0.1% of
the administered dose at day 4, with the highest concentrations in
kidney (0.06 µg/g), a finding confirmed by autoradiography. Analysis
of the gastrointestinal tract 4 and 24 h after administration of
3 mg/kg bw showed that 3% and < 0.01% of the dose was in the stomach
and 91% and 3.5% in the intestine, respectively. Sporadic differences
by sex were seen but were not consistent between studies or individual
animals. The tissue concentrations were higher in female than male
rats given 1000 mg/kg bw, as was the urinary excretion after the dose
of 3 mg/kg bw. The results of the two studies with 1000 mg/kg bw
presented a similar profile, although Maas & Braun (1995b) found lower
tissue concentrations and higher urinary excretion in females (up to
20%, including cage washes); however, there was a threefold variation
between individual animals, and the possibility of contamination by
faeces cannot be dismissed.
Table 9. Radiolabel in excreta and tissues of rats given
[3,4-14C] N-acetylglufosinate orally at 3 or 1000 mg/kg bw
Sample Radiolabel in excreta (% of dose) or tissues (µg/g)
1000 mg/kg bwa 3 mg/kg bwb
Males Females Males Females
Urine
24 h 5 4 5 8
48 h 7 6 5 9
Faeces
24 h 58 63 97 93
48 h 82 83 100 96
Liver, 6 h 17 46 NA NA
Kidney, 6 h 44 672c NA NA
Brain, 6 h 1 1 NA NA
Plasma, 6 h 3 10 NA NA
NA, not analysed
a Results from Lauck-Birkel (1995b)
b Results from Kellner et al. (1993); Stumpf (1993b)
c Possible outlier
Groups of three Wistar rats of each sex were given
[3,4-14C] N-acetylglufosinate (purity, 98%; 1400 MBq/g) at 3 mg/kg
bw in saline, and blood samples were taken from the retro-orbital
plexus at 15, 30, and 60 min and 2, 4, 6, 8, 24, 48, 72, and 96 h. The
samples were absorbed onto filter paper and combusted, and radiolabel
was determined by liquid scintillation counting. The peak
concentration (0.05 µg/g) was seen at 60 min, although significant
amounts were detected at 15 min, showing rapid initial absorption. The
concentration in blood declined in a biphasic manner, in an initial
phase with a half-time of 0.8 h and a second phase with a half-time of
7 h. The concentration was at the limit of detection by 24 h. The
integrated area under the curve of concentration-time was ~ 0.2 µg
h/g. Comparison with the results of an identical study in which the
substance was administered intravenously showed that absorption after
oral administration represented approximately 5% of the dose over
24 h. There was no significant difference between the sexes (Kellner &
Braun, 1993a,b).
A lactating goat weighing 36 kg was dosed orally twice a day for
3 consecutive days with capsules containing
[3,4-14C] N-acetylglufosinate, equivalent to a dose of 3.0 mg/kg bw
per day. The feed intake was 1.4 kg/day. The animal was milked twice
daily and was slaughtered 16 h after the final dose. Most of the
administered radiolabel was excreted in the faeces (68%), with 7.3% in
urine and 19% in the gastronintestinal tract and its contents. Only
0.2% of the administered dose was found in the tissues and blood and
< 0.1% in milk. The concentrations in kidney were higher than in
other tissues. Those in milk reached a plateau by day 2 (Huang &
Smith, 1995c).
Six laying hens weighing 1.3-1.6 kg were dosed orally twice a day
for 14 consecutive days with capsules containing
[3,4-14C] N-acetylglufosinate, equivalent to a dose of 2.2 mg/kg bw
per day. The mean feed intake was 120 g/day. Eggs were collected twice
daily, and the birds were slaughtered 15 h after the final dose. Most
of the administered dose was excreted (86%), with 1.0% remaining in
the gastrointestinal tract; < 0.1% of the administered dose was
present in edible tissues and blood. The concentrations of radiolabel
associated with N-acetyl-L-glufosinate disodium salt were 0.076
mg/kg in liver, 0.013 mg/kg in muscle, and 0.011 mg/kg in fat; that in
egg white was only slightly above the level of quantification
(< 0.009 mg/kg) throughout the study, reaching a peak of 0.014 mg/kg.
The concentrations in egg yolk increased slowly throughout the 14
days, with a peak at necropsy of 0.056 mg/kg (Huang & Smith, 1995d).
Groups of three Wistar rats received an intravenous injection of
[3,4-14C] N-acetylglufosinate (purity, 98%; 1400 MBq/g) into the
tail vein at a dose of 3 mg/kg bw as a solution in saline. Blood
samples were taken from the retro-orbital plexus at 5, 15, 30, and 60
min and 2, 4, 6, 8, 24, 48, 72, and 96 h. The samples were absorbed
onto filter paper and combusted, and the radiolabel was determined by
liquid scintillation counting. The peak concentration (6-7.5 µg/g) was
seen at 5 min, with an initial decline of 4 h, a half-time of 0.3 h,
and a second phase with a half-time of 14 h. The concentration of
radiolabel in blood was at the limit of detection at 24 h. The
integrated area under the curve of concentration-time was ~ 3.8 µg
h/g. There as no significant difference between the sexes (Kellner &
Braun, 1993a,b).
The toxicokinetics of N-acetylglufosinate was investigated in
groups of five Wistar rats of each sex which received
[3,4-14C] N-acetylglufosinate (purity, 98%; 1400 MBq/g) in saline
intravenously at a dose of 3 mg/kg bw. Urine and faeces were collected
over 0-4, 4-8, 8-24, 24-48, 48-72, and 72-96 h. Tissue samples were
taken at 96 h. Autoradiography was performed on one male killed at 96
h. The radiolabel in tissues and excreta was determined by liquid
scintillation counting after appropriate processing. Excretion was
rapid, with > 85% of the radiolabel appearing in the 0-4-h urine
sample. By 96 h, approximately 95% of the dose had been excreted in
urine; faecal excretion accounted for 4% in females and 2% in males.
The excretory half-times were slightly longer in males than in
females. By 96 h, tissue radiolabel accounted for < 0.3% of the dose;
the highest concentrations were found in kidney, with 0.2 µg/g in
males and 0.07 µg/g in females (Kellner et al., 1993)
Biotransformation: Urine and faeces from Wistar rats given
[3,4-14C] N-acetylglufosinate at 3 or 1000 mg/kg bw by gavage in
the studies of Stumpf (1993b) and Lauck-Birkel (1995b), described
above, were extracted and analysed for metabolites by HPLC or
thin-layer chromatography with comparison to standards. The tissue
samples contained insufficient radiolabel for investigation of
metabolites. The extent of metabolism was greater at 3 mg/kg bw,
indicating the presence of a saturable reaction pathway. The main
compound in urine was N-acetylglufosinate, with low concentrations
of 3-[hydroxy(methyl) phosphinoyl]propionic acid and
4-methylphosphinico-butanoic acid. The faeces of animals given the low
dose contained a significant amount of glufosinate-ammonium, which was
not seen in those given the high dose. The study of Kellner et al.
(1993) showed that glufosinate-ammonium is formed in the intestine,
but the extent to which it is systemically available is not clear. The
results for male rats are presented in Table 10; female animals showed
a similar profile.
Table 10. N-Acetylglufosinate and metabolites in 96-h samples from male Wistar
rats given [3,4-14C]-labelled compound at 3 or 1000 mg/kg bw by gavage
Compound Percent administered dose
3 mg/kg bwa 1000 mg/kg bwb
Urine Faeces Urine Faeces
Total radiolabel 5.3 83 7.6 89
N-Acetylglufosinate 4.0 70 7.4 85
Glufodinate ammonium < LD 11 < LD 0.9
3-Methylphosphinicopropionic acid 0.7 0.6 0.1 0.4
4-Methylphosphinicobutanoic acid or 0.6 1.2 0.1 0.1
hydroxy-4-methylphosphinicobutanoic acid
a From Stumpf (1993b)
b From Lauck-Birkel (1995b)
[3,4-14C] N-Acetylglufosinate (98% radiochemical purity;
specific activity, 830 MBq/g) was dissolved in physiological saline
and administered to groups of five male Wistar rats at a dose of 30
mg/kg bw by gavage. Groups of animals were sacrificed 1, 6, or 24 h
after dosing, and samples of blood (for plasma), brain, kidney, and
liver were pooled, processed, and assayed for total radioactivity
(liquid scintillation counting) and metabolites (HPLC). Urine and
faeces were collected over 24 h. Metabolites were not determined in
plasma or brain owing to insufficient total radiolabel. There was
limited metabolism and rapid excretion; > 80% of the recovered
radiolabel in the faeces was N-acetylglufosinate. The concentration
of glufosinate-ammonium in kidney increased with time (Table 11;
Lauck-Birkel & Strunk, 1999d). A similar pattern of absorption,
distribution, and excretion was reported by Maas & Braun (1999c).
Two male Wistar rats received [3,4-14C] N-acetylglufosinate
(purity, > 98%) by gavage in saline at a dose of 3 mg/kg bw. The
animals were killed 4 or 24 h later, and the gastrointestinal tract
was examined for total radiolabel and metabolites. Significant
deacetylation of N-acetylglufosinate was found in the intestine,
giving rise to glufosinate-ammonium (Table 12; Kellner et al., 1993).
[3,4-14C] N-Acetylglufosinate (98% radiochemical purity;
specific activity, 7200 MBq/g) was dissolved in physiological saline
and administered intravenously into the tail vein of groups of five
male Wistar rats at a dose of 3 mg/kg bw. Groups of animals were
sacrificed 2 or 24 h after dosing, and samples of blood (for plasma),
brain, kidney, and liver were pooled, processed, and assayed for total
radiolabel (liquid scintillation counting) and metabolites (HPLC).
Urine and faeces were obtained over 24 h. Metabolites were not
determined in plasma or brain owing to insufficient total radiolabel.
There was limited metabolism and rapid excretion, and over 95% of the
radiolabel recovered in urine was N-acetylglufosinate. At 24 h,
glufosinate-ammonium was present at higher concentrations than
N-acetylglufosinate in kidney (Table 13; Lauck-Birkel & Strunk,
1999c). A similar pattern of distribution and excretion was reported
by Maas & Braun (1999d).
Samples from the study of Huang & Smith (1995c) on goats were
investigated for metabolites. N-Acetylglufosinate and glufosinate
accounted for 52% and 34% of the radiolabel in faeces. respectively.
Glufosinate was the main residue in kidney, liver, and milk, although
N-acetylglufosinate (the administered material) and
3-[hydroxy(methyl) phosphinoyl]propionic acid formed a substantial
proportion of the residue in kidney and liver. The concentration of
glufosinate-ammonium in the kidney (0.7 ppm) was four times that in
the liver (0.095 ppm). This study showed that de-acetylation of
N-acetylglufosinate to glufosinate-ammonium makes a significant
contribution to tissue residues.
Samples from the study of Huang & Smith (1995d) on hens were
investigated for metabolites. N-Acetyl-L-glufosinate disodium salt
comprised 73% of the radiolabel in faeces, with glufosinate and
3-[hydroxy(methyl) phosphinoyl]propionic acid comprising 13% and 8.6%,
respectively. N-Acetylglufosinate (the administered material) was
the main residue identified in liver and egg yolk, and glufosinate and
3-[hydroxy(methyl) phosphinoyl]propionic acid were also substantial
components of the liver residue. Glufosinate was the main residue in
egg white. This study showed that deacetylation of
N-acetylglufosinate to glufosinate-ammonium makes a significant
contribution to tissue residues.
Table 11. N-Acetylglufosinate and metabolites in pooled samples from male Wistar rats given
[3,4-14C]-labelled compound at 30 mg/kg bw by gavage
Compound % of administered dose µg/g equivalent
Urine Faeces Kidney Liver
0-24 h 0-24 h
6 h 24 h 6 h 24 h
Total radiolabel 2.1 88 1.1 0.7 0.5 0.2
N-Acetylglufosinate 1.7 82 0.6 0.04 0.08 0.05
Glufosinate ammonium 0.02 5 0.08 0.73 < LD < LD
3-Methylphosphinicopropionic acid 0.15 < LD 0.19 0.03 0.3 0.02
4-Methylphosphinicobutanoic acid (1% in dose) 0.2 1.4 0.09 < LD 0.03 0.01
LD, limit of determination, < 0.001% of the administered dose
Table 12. Residues in stomach and intestine after oral administration
of 3 mg/kg bw [3,4-14C] N-acetylglufosinate to two male rats
Compound Percent of radiolabel
Stomach Intestine
4 h 24 h 4 h 24 h
Total radiolabel 3.6 91 < 0.01 3.5
Glufosinate ammonium 0.0 2.6 ND 29
4-Methylphosphinicobutanoic acid 0.0 0.8 ND 0.0
3-Methylphosphinicopropionic acid 0.0 0.5 ND 4.5
N-Acetylglufosinate 99.8 96 ND 66
ND, not determined
Effects on enzymes and other biochemical parameters: The main
biological property of glufosinate-ammonium is inhibition of the
enzyme glutamine synthetase, and toxicological studies with
N-acetylglufosinate have also shown inhibition of this enzyme in a
range of tissues. The interpretation of these results is confounded by
the presence of glufosinate-ammonium in the samples of
N-acetylglufosinate tested. In an attempt to determine the degree to
which N-acetylglufosinate inhibits glutamine synthetase, comparative
studies were performed in vitro and in vivo (Lutkemeier, 1999;
Schmid et al., 1999). The studies are summarized in Tables 6 and 7.
In vitro, N-acetylglufosinate produced only marginal inhibition at
13 mmol/L, some of which can be attributed directly to the
glufosinate-ammonium in the sample. When glufosinate-ammonium is
administered orally, it is approximately 10 times more potent as an
inhibitor of glutamine synthetase than is N-acetylglufosinate. The
amount of this inhibition that is due directly to de-acetylation of
N-acetylglufosinate to glufosinate-ammonium is uncertain; however,
the work of Lauck-Birkel & Strunk (1999a,b; Table 11) and the results
in vitro (Table 6) indicate that most of the inhibition in kidney is
due to biotransformation of N-acetylglufosinate to
glufosinate-ammonium.
Acute toxicity: N-Acetylglufosinate has little toxicity when
given as a single oral dose (Table 14). When it was given by
intraperitoneal injection, deaths occurred at the lowest doses tested
(580 mg/kg bw) in both rats and mice, but there was no clear
dose-response relationship in mice. The compound was not tested by
other routes, as it is formed as a metabolite only in plants, and
exposure through the skin or by inhalation is unlikely. The clinical
signs of toxicity seen after oral exposure to N-acetylglufosinate
were reduced respiratory rate, reduced activity, contracted flanks,
and squatting during the first 24 h. The decreased respiratory rate
Table 13. N-Acetylglufosinate and metabolites in pooled samples from male Wistar rats given
[3,4-14C]-labelled compound at 3 mg/kg bw intravenously
Compound Percent of administered dose
Urine Faeces Liver Kidney
(24 h) (24 h)
2 h 24 h 2 h 24 h
Total radiolabel 86 1.8 0.5 0.1 0.9 0.1
N-Acetylglufosinate 85 1.7 0.4 0.1 0.8 0.01
Glufosinate ammonium < LD 0.1 0.01 0.01 0.05 0.06
3-Methylphosphinicopropionic acid < LD < LD 0.04 0.01 < LD 0.001
4-Methylphosphinicobutanoic acid (1% in dose) 1.1 0.02 0.01 < LD 0.01 < LD
LD, limit of determination, < 0.001% of the administered dose
Table 14. Acute toxicity of N-acetylglufosinate (purity, 79.4%; containing
4.5% glufosinate ammonium)
Species (strain) Route LD50 Reference
(mg/kg bw)
Rat (Wistar) Oral, gavage 290 Schollmeier & Leist (1989a)
Mouse (NMRI) Oral, gavage 290 Schollmeier & Leist (1989b)
Rat (Wistar) Intraperitoneal > 1200 Schollmeier & Leist (1989c)
Mouse (NMRI) Intraperitoneal > 2000 Schollmeier & Leist (1989d)
persisted for the duration of the study in mice. Gross examination
showed no abnormalities.
In a Magnusson and Kligman maximization protocol, groups of 20
female Pirbright guinea-pigs, 10 weeks of age, received an intradermal
induction with N-acetylglufosinate (purity, 79.4%; supplied as a
57.9% solution) at 5% in saline (equal to 2.9% N-acetylglufosinate)
and a 1:1 preparation of Freund's adjuvant. For topical induction and
challenge, undiluted test material was applied under an occlusive
dressing. There were no signs of erythema or oedema. Satisfactory,
contemporary data for positive controls were presented (Hofmann &
Jung, 1988). N-Acetylglufosinate was not a skin sensitizer in this
study (Schollmeier & Leist, 1989e).
Short-term studies of toxicity: Groups of five NMRI mice of
each sex were given diets containing N-acetylglufosinate (purity,
79.4%; 4.5% glufosinate-ammonium) at concentrations of 0, 120, 580,
2900, or 5800 ppm for 28 days. The content of N-acetylglufosinate in
the diets was routinely below the nominal value, sometimes by as much
as 30%. The stability and homogeneity of the diets were satisfactory,
providing intakes equivalent to 0, 19, 100, 520, and 1000 mg/kg bw per
day. All animals were examined routinely for a range of observations
and measurements, including basic neurological tests. Samples were
taken for clinical chemical and haematological examinations from all
mice (not fasted) at termination. Limited analysis was performed on
urine samples collected overnight on day 21-22 of the study from
fasted animals. Heart, lung, liver, kidney, spleen, brain, testis, and
ovaries were weighed and examined histologically, as was any tissue
with macroscopic abnormalities. Glutamine synthetase activity was
measured in brain and liver samples that had been cooled rapidly after
removal and kept frozen until assay.
There were no deaths or clinical signs of toxicity and no effects
on body-weight gain or food or water consumption, although the latter
two were very variable. Haematology and clinical chemistry showed no
treatment-related effects, and no substance-related macroscopic or
microscopic changes were seen. Glutamine synthetase activity in liver
and brain was significantly inhibited in animals of each sex at
5800 ppm, with significant inhibition in brain samples from females
and liver samples from males receiving 2900 ppm (Table 15). The NOAEL
was 580 ppm, equivalent to 100 mg/kg bw per day, on the basis of the
statistically significant, > 10% decrease in glutamine synthetase
activity in brains of females receiving 2900 ppm (Ebert, 1991a).
Table 15. Mean glutamine synthetase activity in liver and brain
from mice receiving N-acetylglufosinate in the diet for
28 days
Dose (ppm) Glutamine synthetase activity (nmol/s per mg protein)
Male Female
Liver Brain Liver Brain
0 0.43 1.2 0.38 1.5
120 0.38 1.4 0.5 1.4
580 0.38 1.5 0.4 1.3
2900 0.34* 1.1 0.42 0.92*
5800 0.24* 0.71* 0.22* 0.89*
* Statistically significant at p < 0.05
Groups of 20 NMRI mice of each sex received diets containing
N-acetylglufosinate (purity, 74.7; 0.5% glufosinate-ammonium) at
concentrations of 0, 500, 2000, or 8000 ppm for 13 weeks. The content
of test substance and the homogeneity and stability of the diet were
satisfactory. The achieved intakes were 0, 82, 320, and 1300 mg/kg bw
per day for males and 0, 110, 440, and 1700 mg/kg bw per day for
females at the control, low, intermediate, and high doses,
respectively. The animals were observed routinely for deaths, general
condition, clinical signs, behaviour, food consumption, and body
weight. Blood samples were taken from groups of 10 fasting mice per
sex per group at the end of the study for haematological and clinical
chemical investigations. After sacrifice, all animals were examined
macroscopically, and 10 organs were weighed and more than 30 tissues
from control and high-dose animals were examined histopathologically.
Limited histological examinations were performed on nine tissues from
animals at the low and intermediate doses. Liver, kidney, and brain
samples (pooled samples from two animals for the last two tissues)
were rapidly placed in liquid nitrogen before assay for glutamine
synthetase activity.
One male at 8000 ppm died after blood sampling. There were no
treatment-related effects on food consumption, body weight, clinical
signs, organ weights, macroscopic or macroscopic appearance, or
haematological parameters. The activity of serum lactate dehydrogenase
was increased by 180% over controls in males at the two higher doses,
but the results were within the normal range. Apparent increases in
the activities of a number of serum enzyme in females at the high dose
were due to a high value in a single animal and are considered to be
unrelated to treatment. The main finding was a dose-related decrease
in glutamine synthetase activity in liver, kidney, and brain (Table
16). Inhibition of liver and kidney glutamine synthetase activity in
isolation is not relevant to human risk assessment; however, there was
> 10% inhibition of glutamine synthetase activity in brain at doses
> 2000 ppm, and the mean values for animals of each sex were
outside the control range. The NOAEL was 500 ppm, equal to 82 mg/kg bw
per day (Tennekes et al., 1992a).
Table 16. Glutamine synthetase activity in groups of 10 mice receiving
N-acetylglufosinate in the diet for 90 days
Sex Dose (ppm) Glutamine synthetase activitya
Kidney Liver Brain
Mean Range Mean Range Mean Range
Male 0 1.4 1.2-1.7 4.0 3.2-4.8 3.6 3.4-3.8
500 1.0* 0.7-1.2 3.7 2.7-4.4 3.3* 3.1-3.6
2000 0.83* 0.6-1.1 3.3* 2.5-4.6 3.2* 2.9-3.4
8000 0.71* 0.4-0.9 2.9* 2.4-4.2 2.6* 2.4-2.9
Females 0 1.7 1.5-2.1 5.0 3.9-5.5 3.4 2.9-3.6
500 1.3* 1.1-1.5 4.9 4.2-5.4 3.3 3.0-3.5
2000 1.2* 0.9-1.4 4.6 3.8-5.3 2.9* 2.7-3.2
8000 1.1* 0.7-1.3 4.0* 3.2-5.5 2.2* 1.9-2.5
* p < 0.01
a µmol gamma-glutamyl hydroxamate formed per ml reaction mixture in 20 min at 37°C
Groups of five Wistar rats received diets containing
N-acetylglufosinate (purity, 79.4%; 4.5% glufosinate-ammonium) at
concentrations of 0, 120, 580, 2900, or 5800 ppm for 28 days. The
overall content, stability, and homogeneity of the test diets were
stated to be acceptable. The achieved intakes (assuming 100% analysis)
were 0, 12, 59, 310, and 590 mg/kg bw per day for males and 0, 11, 55,
280, and 560 mg/kg bw per day for females. All animals were examined
routinely for a range of observations and measurements, and control
and high-dose animals underwent a basic functional observation
battery. Samples were taken for extensive clinical chemical and
haematological examinations from all rats (not fasted) at the end of
the study. Limited analysis was performed on urine samples collected
overnight on day 21-22 of the study from fasted animals. Heart, lung,
liver, kidney, spleen, brain, testis, ovaries, and adrenal, pituitary,
and thyroid glands were weighed and examined histologically, as was
any tissue with macroscopic abnormalities. Glutamine synthetase
activity was measured in brain and liver samples that had been cooled
rapidly after removal and kept frozen until assay.
There were no deaths or clinical signs of toxicity and no effects
on body-weight gain or food or water consumption. 'Thrombin time' was
mildly increased (< 13%) in females at doses > 2900 ppm and in
males at 120, 580, and 5800 ppm; other measures of coagulation were
unaltered. A statistically significant decrease in lactate
dehydrogenase activity was found in females at doses > 2900 ppm,
which may have been related to treatment as there was evidence of a
dose-response relationship. Slight decreases in the absolute and
relative weights of the heart in males at 5800 ppm was of no clear
toxicological significance in the absence of a histological correlate.
No treatment-related macroscopic or microscopic changes were seen.
Glutamine synthetase activity in the liver was significantly inhibited
at doses > 580 ppm in animals of each sex, and was reduced in the
brains of all treated animals, although there was no clear
dose-response relationship (Table 17). Decreased activity of glutamine
synthetase in liver was considered not to be adverse, and the
alterations in glutamine synthetase activity in brain were not
consistent. There were no biologically significant changes in any
other parameter. The NOAEL was 5800 ppm, equal to 560 mg/kg bw per
day, the highest dose tested (Ebert, 1991b).
Table 17. Mean glutamine synthetase activity in liver and brain
samples from rats receiving N-acetylglufosinate in the
diet for 28 days
Dose (ppm) Glutamine synthetase activity (nmol/s per mg protein)
Male Female
Liver Brain Liver Brain
0 0.27 1.5 0.31 1.3
120 0.28 1.1* 0.33 1.1
580 0.17* 0.9* 0.27 1.1*
2900 0.17 1.0* 0.23 1.0*
5800 0.14* 1.3 0.15* 1.1*
* Statistically significant at p < 0.05
Groups of 20 Wistar rats of each sex received diets containing
N-acetylglufosinate (purity, 74.7%; 0.5% glufosinate-ammonium) at
concentrations of 0, 2000, or 10 000 ppm for 13 weeks; a group of 10
animals of each sex received 400 ppm. The content, homogeneity, and
stability of the test compound in the diet were satisfactory, and the
achieved intakes were 0, 29, 150, and 740 mg/kg bw per day for males
and 0, 31, 160, and 800 mg/kg bw per day for females. At 13 weeks, 10
males and 10 females in each group were killed, and the remainder were
allowed to recover for 4 weeks. The animals were observed regularly
for deaths, clinical signs, body weight, food consumption, and tissue
masses. Ophthalmoscopic examinations were performed before treatment
and at 11 and 16 weeks. Blood and urine samples were taken from fasted
animals at weeks 13 and 17 for clinical chemical and haematological
investigations. At necropsy, 10 organs were weighed and examined
macroscopically. An extensive range of tissues from control and
high-dose animals was examined histologically, as were major organs
from animals at the intermediate and low doses. Glutamine synthetase
activity was measured in brain, kidney, and liver samples that had
been rapidly cooled after removal and kept frozen until assay.
There were no deaths, clinical signs of toxicity, or effects on
body weight, the eyes, or urine. Food consumption was reduced during
the first week of treatment but not subsequently. A number of clinical
chemical parameters showed variations, but these were generally due to
values for individual animals or were within normal ranges. A decrease
in serum sodium concentration in animals at the high dose at 13 weeks
appeared to be related to treatment. Glutamine synthetase activity was
inhibited in liver, kidney, and brain (Table 18), and the effect was
significantly but not completely reversed after 4 weeks. A reversible
increase in kidney weight (< 15%) was seen in males at all doses, but
this finding was considered not to be adverse because there was no
clear dose-response relationship and no associated histological
change. There were no treatment-related macroscopic or microscopic
findings. The NOAEL was 2000 ppm, equal to 150 mg/kg bw per day, on
the basis of inhibition of glutamine synthetase activity in the brain
(Tennekes et al., 1992b).
Groups of four beagle dogs of each sex, aged 5-7 months, received
diets containing N-acetylglufosinate (purity, 74.7%; 0.5%
glufosinate-ammonium) at concentrations of 0, 500, 2000, or 8000 ppm
in 400 g of diet daily for 13 weeks. Additional groups of two animals
of each sex received the same diets and were then allowed to recover
for 4 weeks. The content, homogeneity, and stability of the test diet
were satisfactory. The achieved intakes were 0, 19, 72, and 290 mg/kg
bw per day for males and 0, 21, 79, and 300 mg/kg bw per day for
females. The animals were observed routinely for deaths, general
condition, clinical signs, behaviour, food consumption, and body
weight. Ophthalmoscopic examinations were performed on all animals
before treatment, at weeks 4 and 13, and at termination in the group
allowed to recover. Blood and urine samples were collected from fasted
animals before treatment, at weeks 4 and 13, and at the end of the
recovery period. At termination, all dogs were examined
macroscopically; a range of tissues were weighed, and > 30 tissues
Table 18. Glutamine synthetase activity in groups of 10 rats receiving N-acetylglufosinate in
the diet for 90 days and after a 4-week recovery period
Sex Period Dose Glutamine synthetase activitya
(days) (ppm)
Liver Kidney Brain
Mean Range Mean Range Mean Range
Male 90 0 3.8 3.3-4.3 2.1 1.6-2.3 3.2 3.1-3.3
400 2.8* 2.0-3.4 1.7* 1.5-1.9 3.3 3.1-3.6
2000 2.2* 1.9-2.4 1.5* 1.3-1.8 3.0 2.9-3.3
10 000 1.8* 1.3-2.2 1.6* 1.4-1.9 2.8* 2.6-3.1
90 + recovery 0 3.2 2.5-3.8 2.1 1.9-2.3 3.1 2.9-3.4
2000 3.5 3.2-3.9 2.1 1.6-2.4 3.0 2.6-3.4
10 000 3.4 2.9-4.1 1.9 1.2-2.5 2.9* 2.7-3.1
Female 90 0 3.7 2.8-4.3 1.2 1.0-1.3 3.1 2.8-3.3
400 3.1 2.2-3.5 1.1 1.0-1.2 3.1 2.9-3.2
2000 2.6* 1.8-3.2 1.2 1.1-1.5 3.0 2.9-3.2
10 000 2.4* 1.9-2.8 1.5* 1.4-1.7 2.8* 2.6-2.9
90 + recovery 0 3.7 3.2-4.1 1.3 1.2-1.4 3.1 3.0-3.3
2000 3.5 3.1-4.0 1.3 1.1-1.5 3.1 2.9-3.4
10 000 3.3 2.6-3.9 1.3 1-1.5 2.9 2.7-3.1
* p < 0.01
a µmol gamma-glutamyl hydroxamate formed per ml reaction mixture in 20 min at 37°C
were preserved and examined histopathologically. Liver, kidney, and
brain samples were immediately placed in liquid nitrogen and kept
frozen at -80°C before assay for glutamine synthetase activity;
samples of these tissues were also retained for future analysis.
There were no deaths or treatment-related clinical signs or
effects on food consumption, body weight, or ophthalmoscopic or
haematological parameters. A statistically significant, dose-dependent
decrease in glutamine synthetase activity was found in liver and brain
after 13 weeks of treatment (Table 19), which tended to return to
normal during the recovery period although it was not complete at the
end of the 4 weeks. The changes in glutamine synthetase activity were
not consistent in different tissues from the same animal. Activity in
the brain stem and cerebellum appear to be more sensitive to
inhibition than that in the cortex. Decreased creatine kinase activity
was seen at the high dose in males at 13 weeks (17%) and in females at
weeks 4 and 13 (30%). Lactate dehydrogenase activity was decreased by
30% in females at this dose at week 13 but in neither males nor
females after recovery. Exacerbation of the low pretreatment specific
gravity and osmolality of the urine of females was seen at at 8000 ppm
in weeks 4 and 13 of treatment and at the end of the recovery period.
The significance of this finding is unclear as it was not seen in a
1-year study in dogs. It is of note, however, that the kidney is a
target organ in rats.
A dose-related decrease in prostate gland weight was seen which
achieved statistical significance at 8000 ppm (40%) at 13 weeks, but
there was no histological correlate. The prostate weights were similar
to those of controls after 4 weeks' recovery from the dose of
2000 ppm, but not after administration of 8000 ppm. Although the
authors noted that slight differences in the rate of maturity of dogs
of this age can affect prostate size, the dose-response relationship
and evidence of recovery at 2000 ppm but not at 8000 ppm indicate an
association with treatment. No treatment-related effects were seen on
macroscopic examination. The only histopathological finding of note
was an increased incidence of pituitary cysts in animals receiving
8000 ppm for 13 weeks: 3/4 in animals of each sex and 1/4 in male
controls and 0/4 in female controls. The NOAEL was 500 ppm, equal to
19 mg/kg bw per day, on the basis of > 10% reductions in glutamine
synthetase activity in a number of areas of the brain. The effects on
prostate weight, urinary parameters, and the pituitary indicate that
8000 ppm was an effect level (Corney et al., 1992)
Groups of six beagle dogs of each sex received diets containing
N-acetylglufosinate (purity, 92.4%; 0.1% glufosinate-ammonium) at
concentrations of 0, 100, 1000, or 8000 ppm. Analyses of the diets
for homogeneity and content were satisfactory, and the overall intakes
of N-acetylglufosinate were 0, 4, 44, and 320 mg/kg bw per day for
males and 0, 4.4, 43, and 350 mg/kg bw per day for females. Two
animals of each sex per group were killed at 26 weeks and the
remainder at 52 weeks. The animals were observed routinely for
clinical signs, deaths, body weight, and food consumption.
Table 19. Glutamine synthetase activity in dogs receiving N-acetylglufosinate in the diet for 90 days with or without a 4-week recovery
period
Sex Period No. Dose Glutamine synthetase activitya
(ppm)
Liver Kidney Mid-brain Cerebellum Brain stem Brain cortex
Mean Range Mean Range Mean Range Mean Range Mean Range Mean Range
Male 13 weeks 4 0 2.7 2.6-2.9 0.02 0.01-0.04 2.3 1.8-2.7 1.6 1.5-1.8 1.4 1.2-1.5 3.2 2.8-3.5
500 1.9 1.4-2.2 0.04 0.02-0.07 2.6 1.9-2.9 1.4 1.2-1.5 1.3 1.2-1.4 3.4 3.3-3.4
2000 1.2 1.0-1.3 0.06 0.03-0.10 2.6 1.8-2.9 1.3 1.0-1.4 0.90 0.8-1.1 2.8 2.3-2.9
8000 0.58 0.5-0.7 0.05 0.01-0.14 1.4 1.7-2.6 0.85 0.7-1.0 0.47 0.4-0.5 2.3 1.8-2.8
Recovery 2 0 2.6 0.07 2.1 1.4 1.3 3.0
2000 2.4 0.05 2.3 1.2 0.95 2.4
8000 2.0 0.06 2.1 1.2 0.93 2.5
Females 13 weeks 4 0 1.8 1.6-2.1 0.04 0.02-0.06 2.7 2.2-3.0 1.6 1.5-1.7 1.2 1.1-1.3 2.9 2.7-3.3
500 1.6 1.2-2.4 0.10 0.05-0.17 2.2 1.9-2.5 1.5 1.4-1.5 1.2 1.0-1.3 3.2 2.9-3.3
2000 1.0 0.8-1.5 0.06 0.03-0.09 2.4 1.8-2.9 1.3 1.2-1.4 0.91 0.7-1.0 2.7 2.4-3.3
8000 0.68 0.5-0.9 0.09 0.01-0.15 2.0 1.7-2.6 0.87 0.8-0.9 0.73 0.6-0.8 2.5 1.9-2.8
Recovery 2 0 2.1 0.05 2.1 1.5 1.4 3.1
2000 1.7 0.08 2.1 1.3 1.2 2.7
8000 1.3 0.07 1.8 1.1 0.83 2.4
a µmol gamma-glutamyl hydroxamate formed per ml reaction mixture in 20 min at 37°C
Ophthalmoscopic examinations were performed before treatment and at
weeks 12, 25, and 51. Samples were taken from fasted animals for
urinary analysis, haematology, and clinical chemistry before treatment
and at weeks 13, 26, and 52. Post-mortem examinations were performed
on all animals; nine organs were weighed and > 30 tissues examined
histopathologically. Glutamine synthetase activity was not analysed.
The only death occurred in a male at the intermediate dose; the
cause was not found. The incidence of soft faeces was increased in
animals at the high dose and particularly in males. Reduced
body-weight gain was seen in animals at this dose, by 17% in males and
30% in females, during the first half of the study, and the effect
persisted in females until termination. There was no evidence of
treatment-related effects in ophthalmoscopic, haematological, or
urinary examinations and no effects on organ weights or gross or
histopathological appearance at either the interim or terminal
sacrifice. Reduced serum lactate dehydrogenase activity was seen
consistently in animals at the high dose (Table 20). Although there
was some overlap between the ranges for control and treated animals
and variation in control values over time, the consistency of the
effect in males indicates that it is possibly related to treatment.
The effects at 8000 ppm are not significant in isolation, but the
combination of findings in males indicates that this dose is an effect
level. The NOAEL was 1000 ppm, equal to 44 mg/kg bw per day, on the
basis of reduced body-weight gain, reduced lactate dehydrogenase
activity, and an increased incidence of soft faeces (Bernier, 1996).
Table 20. Lactate dehydrogenase activity in dogs fed diets containing
N-acetylglufosinate at 8000 ppm
Group No. Week Serum lactate dehydrogenase activity (U/L)
Males Females
Mean Range Mean Range
Controls 6 0 75 47-100 160 120-230
6 13 100 66-160 160 52-260
6 26 200 87-430 220 99-470
4 52 90 66-100 320 160-460
Treated 6 0 97 57-140 74 32-130
6 13 53 43-68 44 31-65
6 26 99 39-180 88 48-110
4 52 70 50-88 150 110-180
Long-term studies of toxicity and carcinogenicity: Groups of 90
Swiss Crl:CD-1 mice received diets containing N-acetylglufosinate
(purity, 92.4%; 0.1% glufosinate-ammonium) at concentrations of 100,
1000, or 8000 ppm. Groups of 20 animals were killed after 1 year and
the remainder after 2 years. The mice were housed singly, observed
daily, and monitored routinely for body weight and food consumption.
The homogeneity, content, and stability of the diet were acceptable,
and the achieved intakes were 0, 15, 150, and 1200 mg/kg bw per day
for males and 0, 19 190, and 1500 mg/kg bw per day for females.
Samples for clinical chemistry and haematology were taken from fasted
animals at interim sacrifice (all animals) and at termination (10 per
sex per group). Blood was taken from the tail vein at week 78. At
autopsy, the animals were examined macroscopially, nine organs were
weighed, and more than 30 tissues were preserved. Histological
examinations were conducted on tissues from all controls, animals at
the high dose, and those that died during the study and on the liver,
lung, kidney, and adrenals from animals at the low and intermediate
doses killed after 1 or 2 years.
Although the report stated that no treatment-related signs were
observed, data on individual animals were not presented, as this was
primarily a study of carcinogenicity. Survival was similar in all
groups (> 60% at week 90). Fluctuations in food consumption and body
weight showed no consistent pattern related to dose. There were no
effects on clinical chemical or haematological parameters or on organ
weights, any variation in group means being attributable to individual
values. Macroscopic examination detected no treatment-related effects.
Histopathological examination showed an increased incidence of
amyloidosis in various organs in males and females at the high dose
and testicular necrosis in males, although the only statistically
significant finding ( p = 0.028) was amyloidosis in the salivary
gland of females (Table 21). A high incidence of kidney lesions was
seen in both control and treated animals at 1 and 2 years, with no
significant differences between groups. Increased incidences of
uterine adenocarcinoma and thyroid follicular-cell adenoma were seen
in animals at the high dose; the incidences were not statistically
significantly increased when compared with concurrent controls but
were slightly greater than those of historical controls. The overall
incidences of benign and malignant tumours were similar in treated and
control groups. As the tumours that occurred at increased incidences
occur spontaneously in this strain of mouse, the findings were
considered not to indicate a carcinogenic potential of
N-acetylglufosinate. The NOAEL was 1000 ppm, equal to 150 mg/kg bw
per day, on the basis of an increased incidence of amyloidosis
(Farrell, 1997; Ernst & Stumpf, 1999a).
Groups of 70 Sprague-Dawley-derived rats of each sex received
diets containing N-acetylglufosinate (purity, 92.4%; 0.1%
glufosinate-ammonium) at concentrations of 0, 200, 2000, or 20 000 ppm
for 2 years; additional groups of 10 animals of each sex were killed
at 1 year and 20 at 2 years. The animals were housed singly, observed
daily, and monitored routinely for body weight and food consumption.
The homogeneity, content, and stability of the test diet were
Table 21. Incidences of lesions in mice receiving N-acetylglufosinate in the diet for 2 years, and ranges for
historical controls
Sex Lesion Incidence
0 100 ppm 1000 ppm 8000 ppm Historical controls
Male Testicular degeneration and necrosis 0/70 1/55 2/56 4/70 6-39% (for atrophy)
Testicular atrophy 25/70 28/55 30/56 26/70 6-39% (for atrophy)
Thyroid follicular-cell adenoma 0/70 0/69 1/69 3/70 0-2%
Female Salivary gland amyloidosis 1/70 1/45 3/43 7/70 No data
Liver haemangiosarcoma 0/70 1/70 1/70 2/70 0-4%
Uterine adenocarcinoma 0/70 2/69 1/69 3/70 0-2%
acceptable, and the achieved intakes were 0, 9, 91, and 1000 mg/kg bw
per day for males and 0, 11, 110, and 1200 mg/kg bw per day for
females. Samples for clinical chemistry and haematology were taken
from fasted rats in the interim sacrifice and satellite groups at 25,
51, 78, and 102 weeks. At necropsy, the animals were examined
macroscopically, nine organs from interim sacrifice animals were
weighed, and > 30 tissues were preserved. Full histopathological
examinations were performed on all controls, animals at the high dose,
and animals that died during the study; only liver, lung, kidney, and
adrenals from animals at the low and intermediate doses were examined
histopathologically.
Survival was similar in all groups (> 50% at week 96). Soft
faeces were more prevalent in animals at the high dose and especially
in males from week 8 onwards. Body-weight gain was reduced (~ 5%) in
males at this dose from week 16 onwards and in females (~10%) from
week 24, even though the food consumption of these groups increased by
5-10%. A range of changes in clinical chemistry and haematology were
seen repeatedly in animals of each sex at the high dose, including
reduced prothrombin time, potassium concentration, and lactate
dehydrogenase and creatine kinase activity, and increased sodium,
calcium, inorganic phosphorus, and glucose concentrations; blood urea
nitrogen was increased in males from week 25 onwards. In animals
killed after 1 year, the absolute and relative weights of the kidney
were increased in males receiving 20 000 ppm and in females receiving
2000 or 20 000 ppm. Macroscopic examination showed increased
incidences of a range of abnormalities of the kidney in treated
animals that were confirmed at histological examination. Renal lesions
are common in aged rats, and the incidences in treated animals in this
study were not statistically significant and were within the range
seen in historical controls; furthermore, they may have been
exacerbated by the high sodium content induced by
N-acetylglufosinate. They were therefore considered irrelevant to
the assessment of the study. Alterations were also seen in the spleen,
parathyroid, heart, blood vessels and adrenals at the high dose and in
some animals at the intermediate dose animals (Table 22). The
incidence of tumours was not statistically significantly increased,
although low incidences of uncommon tumours of the brain, liver,
adrenals, skin, and pancreas were seen in animals at the high dose
(Table 23). Although these incidences are outside the range in
historical controls, the sporadic nature of the findings and the clear
NOAEL for tumours at 2000 ppm indicate that N-acetylglufosinate does
not have significant carcinogenic potential. The overall incidences of
benign and malignant tumours were similar in all groups. The NOAEL was
200 ppm, equal to 9 mg/kg bw per day, on the basis of increased
incidences of polyarteritis nodosa, adrenal cortical hyperplasia, and
necrosis and extramedullary haematopoiesis of the spleen (Bernier,
1997; Ernst & Stumpf, 1999b).
Table 22. Incidences of non-neoplastic lesions in rats receiving N-acetylglufosinate in the diet for up to 2 years,
and ranges for historical controls
Sex Lesion Incidence
0 200 ppm 2000 ppm 20 000 ppm Historical
controls
Male Parathyroid hyperplasia 13/62 8/37 16/32 20/64 14-42%
Adrenal focal cortical hyperplasia 17/70 18/70 29/70 27/70 0-20%a
Adrenal necrosis 0/70 0/70 2/70 3/70 0-4%
Polyarteritis nodosa (blood vessels) 4/7 5/7 4/9 11/18 No data
Polyarteritis nodosa (testis; 2 years; main group) 15/70 13/53 13/50 23/70 16-31%
Polyarteritis nodosa (testis; 2 years; satellite group) 3/20 4/16 8/17 10/20 16-31%
Female Parathyroid hyperplasia 1/52 0/26 0/31 6/61 1-12%
Extramedullary splenic haematopoiesis 7/70 4/34 16/41 20/70 15-36%
Cardiomyopathy 12/70 5/30 6/41 22/70 1-70%
a Current study, all > 24%
Table 23. Incidences of neoplastic lesions in groups of 70 rats receiving N-acetylglufosinate in the diet
for 2 years, and ranges for historical controls
Sex Lesion Incidence
0 200 ppm 2000 ppm 20 000 ppm Historical controls
Male Adrenals: malignant phaeochromocytoma 0 1 1 3 0-3%
Brain: meningioma 0 0 0 1 0-1%
Brain: astrocytoma 0 1 0 1 0-2% (malignant
glioma)
Skin: keratocanthoma 4 5 1 9 (7)* 0-8%
Female Brain: oligodendroglioma 0 0 0 1 No data
Liver: cholangiocarcinoma 0 0 0 1 No data
Lung: alveolar/bronchiolar carcinoma 0 0 0 1 No data
Pancreas: islet-cell carcinoma 1 2 1 4 0-2%
* Re-evaluation of data indicates that 7 is the correct value.
Genotoxicity: An extensive range of assays for genotoxicity
have been performed with N-acetyl-glufosinate both in vitro and
in vivo (Table 24). Some of the protocols were less than optimal
with respect to length of exposure (e.g. for unscheduled DNA
synthesis) or the highest concentration tested, but these limitations
are considered not to affect the overall assessment of genotoxic
potential significantly. The Committee concluded that
N-acetylglufosinate is not genotoxic.
Reproductive toxicity: In a single-generation, range-finding
study in groups of 10 Sprague-Dawley rats of each sex that received
N-acetylglufosinate (purity, 92.4%; 0.1% glufosinate-ammonium), no
adverse effects were seen on fertility, reproduction, or development
at doses of 200, 2000, or 10 000 ppm (equivalent to 14, 140, or 700
mg/kg bw per day). Exposure was continuous from 21 days before mating
to day 21 post partum (Beyrouty, 1996a).
Groups of 30 Sprague-Dawley-derived rats received diets
containing N-acetylglufosinate (purity, 92.4%; 0.1%
glufosinate-ammonium) at concentrations of 0, 200, 2000, or 10 000 ppm
for two generations. The F0 generation was exposed from 70 days
before mating until the end of lactation, and F1 pups were exposed
from weaning (10-12 weeks before mating) until the end of lactation of
F2 pups. The stability and homogeneity of the diet were
satisfactory, and the mean achieved intakes were 0, 13, 140, and 700
mg/kg bw per day for males and 0, 18, 170, and 890 mg/kg bw per day
for females. Parental animals were observed routinely for clinical
signs, estrus cycling, body weight, and food consumption, and the
frequency of observation was increased during gestation and weaning.
Parturition was observed when possible. Pups were examined for
malformations, sex, viability, and body weight, and the litters were
culled to four pups of each sex on day 4. All parental animals, one
pup of each sex per litter, and any pups that died or were killed when
moribund were examined post mortem. The major organs and
reproductive tissues from all adult animals were examined
macroscopically, and these tissues from control and high-dose animals
were examined histologically. Sperm motility, count, and morphology,
and the number of spermatids were determined for all adult males.
One male at the intermediate dose and two at the high dose died
from unknown causes, and males of both generations at the high dose
had an increased incidence of soft faeces and brown staining of the
scrotum. There were no effects on body weight, although the food
consumption of animals at the high dose was slightly increased during
the first few weeks of treatment. No effects were seen on the weight
or histological appearance of organs from F0 animals or on mating
indices, length of gestation, estrus cycling, sperm parameters,
macroscopic appearance, litter size, pup survival, or pup weight in
either generation. A dose-related increase in the weight of the
seminal vesicles was seen in F1 adults, which reached statistical
significance ( p < 0.05; 14%) in males at the high dose. As there
were no associated abnormal histological findings and no functional
deficit, the effect was considered not to be adverse. An increased
Table 24. Results of studies of the genotoxicity of N-acetylglufosinate
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium TA98, 0, 2, 12, 59, 291, 1455, 79.4 Negative +S9 Muller (1989c)
TA100, TA1535, 2910, 5820 µg/plate Negative - S9
TA1537, TA1538;
E. coli WP2 uvrA
Gene mutation V79 Chinese hamster 0, 582, 873, 1164, 79.4 Negative +S9 Muller (1989a)
lung cells, Hprt locus 1554 µg/ml Negative - S9
Gene mutation V79 Chinese hamster 0, 444, 666, 888, 74.7 Negative +S9 Muller (1991b)
lung cells, Hprt locus 1186 µg/ml Negative - S9
Unscheduled DNA Human cell line A549 0, 1, 4, 13, 44, 133, 74.7 Negative +S9 Muller (1991c)
synthesis 444, 1332 µg/ml Negative - S9
Unscheduled DNA Human cell line A549 0, 0.6, 1.8, 5.8, 17, 58, 79.4 Negative +S9 Muller (1989d)
synthesis 175, 582 µg/ml, 3-h Negative - S9
exposure
Chromosomal Human lymphocytes 0, 0.6, 3.0, 5.0 mg/ml 74.7 Negative +S9 Heidemann &
aberrations at 24 h Voelkner (1992)
0, 5.0 mg/ml at 48 h Negative - S9
Chromosomal V79 Chinese hamster 0, 154, 773, 1546 µg/ml 79.4 Negative +S9 Muller (1989b)
aberrations lung cells for 18 h
0, 1546 µg/ml for 7 or Negative - S9
28 h
Table 24. (continued)
End-point Test object Concentration Purity Results Reference
(%)
In vivo
Micronucleus NMRI mouse bone 0, 222, 1111, 2222 74.7 Negative Muller (1991a)
formation marrow mg/kg bw by gavage for
24, 48 or 72 h
incidence of extramedullary haematopoiesis in the livers of F1 males
(8/30 versus 2/30 in controls) was also considered not to be adverse
as it is a common finding in rats. There were no effects on
reproduction. These minor effects on seminal vesicle weight and liver
extramedullary haematopoiesis in F1 males at 10 000 ppm combined
with the increased incidence of soft stools indicated that this is a
minimal effect level. The NOAEL was 2000 ppm, equal to 140 mg/kg bw
per day (Beyrouty, 1996b).
Developmental toxicity: Twenty mated female Wistar rats
received N-acetylglufosinate (purity, 74.7%) by gavage in distilled
water at the limit dose of 1000 mg/kg bw per day on days 7-16 of
gestation, whereas 21 controls received starch mucilage in distilled
water. The dose was based on the results of a preliminary study. The
dams were observed routinely for clinical signs, body weight, and food
consumption. At sacrifice on day 21, they were examined
macroscopically, and the uterus was opened to determine the numbers of
live and dead fetuses and resorptions, fetal weights, the sex ratio,
crown-rump lengths, and placental weights. All fetuses were examined
for external malformations; half were investigated by Wilson
sectioning for visceral abnormalities and the remainder stained with
Alizarin red S for visualization of skeletal defects.
There were no deaths or treatment-related clinical signs among
the pregnant dams, but a non-pregnant control was killed during the
study. The food consumption and body-weight gain of treated animals
were slightly reduced (~ 5%) on days 7-14 of gestation but were
similar to those of controls after cessation of dosing. There was no
effect on pregnancy rate, but a slight increase in pre- and
post-implantation losses resulted in a marginally reduced litter size
(13.6 in controls versus 12.7 in treated animals); the value was
within the typical range. No malformations were recorded, and there
was no increase in the incidence of skeletal or external
abnormalities. The incidence of blood in the pericardium/abdomen was
increased in the treated group (5/132 fetuses, 4/20 litters) when
compared with concurrent controls (2/140 fetuses) and historical
controls (0-1.5%). Fetal weight, crown-rump length, placental weight,
and sex ratio showed no treatment-related effects. The NOAEL for
maternal and fetotoxicity was 1000 mg/kg bw per day, the only dose
tested (Horstmann & Baeder, 1992).
Groups of 15 mated, 8-10-month-old, female Himalayan rabbits
received N-acetylglufosinate (purity, 92.4%) by gavage in distilled
water on days 6-18 of gestation at doses of 0, 64, 160, or 400 mg/kg
bw per day, on the basis of the results of a study that found maternal
and fetal toxicity at 500 mg/kg bw per day. The animals were housed
under standard conditions and observed daily. Body weights were
determined on days 0, 6, 13, 19, and 29 of gestation, and the dams
were killed on day 29 and examined macroscopically. The uteri were
opened, and the numbers of live and dead fetuses and resorptions were
determined. Live fetuses were removed to an incubator and their
survival was monitored for 24 h. Fetal body weight, crown-rump length,
and sex were determined, and external visceral and skeletal
examinations were performed by Wilson sectioning and Alizarin red S
staining. The combined incidences of some lesions were presented only
in summary tables, but this did not compromise the overall integrity
of the study.
There were no deaths during the study, and no consistent clinical
signs in dams. Abortions by one dam at the low dose and one at the
intermediate dose were considered not to be related to treatment. Food
consumption was reduced in relation to dose, by 12% in the groups at
the low dose, 23% at the intermediate dose, and 37% at the high dose,
but there were no consistent effects on body weight. Treatment had no
effect on corpora lutea, pregnancy rate, implantation, resorptions,
gravid uterine weight, or fetal survival. The pup weights were reduced
by 7% at 400 mg/kg bw per day. Morphological examination of fetuses
revealed an increased incidence of a supernumerary thoracic ribs
(2/90, 0/82, 8/73, and 11/93 in control, low-, intermediate-, and
high-dose groups, respectively, while the range among historical
controls was 0-12%). The only malformation reported was a case of
hydrocephalus in a fetus at the high dose; the incidence in historical
controls was 0-9%. The NOAEL for maternal toxicity and fetotoxicity
was 64 mg/kg bw per day on the basis of reduced food consumption in
dams and extra ribs in fetuses (Baeder & Hofmann, 1994; Ernst & Leist,
1999b).
Special studies on neurotoxicity: No overt neurotoxicity was
seen in basic functional observation batteries of tests with
N-acetylglufosinate included in short-term studies of toxicity in
mice and rats, nor does this substance belong to a class of compounds
with neurotoxic potential.
(ii) 3-[Hydroxy(methyl) phosphinoyl]propionic acid
3-[Hydroxy(methyl) phosphinoyl]propionic acid is a metabolite of
glufosinate-ammonium in rats and represents ~ 30% of the residue in
liver (Table 4). It may also represent a significant proportion of the
residue arising from administration of glufosinate-ammonium to plants,
goats, and hens (Annex 1, reference 83). In 1991, the Meeting
reviewed a 28-day study in rats and a study of the toxicokinetics of
3-[hydroxy(methyl) phosphinoyl]propionic acid, which showed that it is
rapidly and extensively absorbed and excreted, has little toxicity
(NOAEL, 280 mg/kg bw per day), and does not inhibit hepatic glutamine
synthetase activity. The 1998 Joint Meeting considered that
3-[hydroxy(methyl) phosphinoyl]propionic acid should be included in
the residue definition (Annex 1, reference 83).
A summary of various additional studies on 3-[hydroxy(methyl)
phosphinoyl]propionic acid has been submitted (Bremmer & Leist, 1998),
although the original reports were not made available. The summary
indicates that the acute toxicity of the substance is low, it is not a
skin sensitizer, and had no significant toxicity in studies in rats
(13 weeks; NOAEL, 560 mg/kg bw per day), mice (13 weeks; NOAEL, 1400
mg/kg bw per day), or dogs (15 weeks; NOAEL, 110 mg/kg bw per day)
given repeated doses. An extensive range of tests for genotoxicity was
reported to show that 3-[hydroxy(methyl) phosphinoyl]propionic acid
does not induce gene mutation in vitro, chromosomal aberrations
in vitro or in vivo, micronuclei in vivo, or DNA damage
in vitro or in vivo. Studies of developmental toxicity in rats and
rabbits showed evidence of maternal toxicity and mild fetotoxicity but
no teratogenicity, with cited NOAELs of 300 mg/kg bw per day in rats
and 50 mg/kg bw per day in rabbits.
3. Observations in humans
(a) Medical surveillance of personnel in manufacturing plants
No specific health problems were reported in workers during 18
years of production of glufosinate-ammonium. The exposure of the
workers was stated to be low, but no details of the extent of
investigations were available (Kaleja, 1999).
(b) Poisoning incidents
A pesticide formulation containing glufosinate-ammonium was
reported to have been involved in over 200 cases of attempted suicide
in Japan (Ernst & Leist, 1999c). The formulation contained 18% w/w of
glufosinate-ammonium, 30% surfactant, and a glycol ether solvent, and
it was not clear whether the effects seen were attributable to
glufosinate-ammonium. The initial signs were vomiting, diarrhoea, and
nausea. The main clinical concerns were the delayed (hours to 2 days)
neurological effects, including convulsions, impaired consciousness,
tremor, and coma, and extensive oedema. Treatment included gastric
lavage, diuretics, intravenous fluids, sedatives, haemoperfusion, and
artificial ventilation. After apparent recovery from the physical
signs, long-lasting amnesia, both retrograde and anterograde was
reported, although this may have been linked to the use of high-dose
benzodiazepine therapy (Koyama et al., 1994; Watanabe & Sano, 1998;
Ernst & Leist, 1999c).
Comments
Glufosinate-ammonium
Orally administered [14C]glufosinate-ammonium is rapidly but
sparingly (~ 10%) absorbed. Excretion of the absorbed dose was rapid.
The kidney and liver contained the highest concentrations of residue,
which were significantly higher than those in plasma. The
concentrations in brain were lower than those in plasma, indicating
limited penetration of the blood-brain barrier. There were no marked
differences between the sexes. Administration of 500 mg/kg bw resulted
in more prolonged absorption and excretion than with 20 or 2 mg/kg bw.
The metabolism of glufosinate-ammonium was limited (~ 30% of the
absorbed dose), and the main urinary and tissue residues were
3-[hydroxy(methyl)phosphinoyl]propionic acid,
methylphosphinicobutanoic acid, and
2-hydroxy-4-methylphosphinicobutanoic acid. In faeces, significant
concentrations (up to 10%) of N-acetylglufosinate were detected,
indicating that acetylation was performed by the gut microflora.
Metabolites were also found in tissues.
Glutamine synthetase (E.C.6.3.1.2) is a key enzyme involved in
the metabolism of nitrogen and glutamate. Inhibition of glutamine
synthetase, resulting in high levels of ammonia, is the mechanism of
action of glufosinate-ammonium in plants. The activity of glutamine
synthetase varies among tissues and species. The Meeting considered
reports on the relevance of glutamine synthetase activity in the liver
and kidney of experimental animals and humans, including data reviewed
by the 1991 JMPR. Because of the presence of alternative pathways for
the homeostatic control of ammonia, < 50% inhibition of glutamine
synthetase in rat liver was not associated with increased ammonia
concentrations and was not considered to be adverse. Glutamine
synthetase activity in the kidney shows considerable variation between
species, with relatively high activity in rodents and negligible
activity in dogs and humans. Inhibition of kidney glutamine synthetase
in the absence of pathological findings was considered not to be
relevant to human risk assessment.
In the central nervous system, ammonia homeostasis is maintained
by a number of enzymes, including glutamine synthetase and glutamate
dehydrogenase. Under normal conditions, the flux through glutamine
synthetase in brain is reported to be approximately 2-10% of its
theoretical capacity, and for glutamate dehydrogenase it is
approximately 0.1% of its capacity. With such excess capacity,
inhibition of brain glutamine synthetase will not necessarily result
in significant increases in brain ammonia concentrations; this
conclusion is confirmed by data showing that animals with decreased
glutamine synthetase activity do not have increased brain ammonia
levels. However, the 'glutamine-glutamate shunt', between GABA and
glutamate in neurons and glutamine in astrocytes, plays a role in both
excitatory and inhibitory neurotransmission. The results of studies
considered by the 1991 Meeting indicate that significant changes in a
range of biogenic amines in regions of the brain in dogs are
associated with > 8% changes in glutamine synthetase activity after
administration of glufosinate-ammonium at 8 mg/kg bw for 28 days, a
dose that produced 'increased gait activity'. Thus, it has been
proposed that any statistically significant inhibition of glutamine
synthetase activity in brain by > 10% be considered a marker of
potentially adverse effects on brain biochemistry and behaviour.
Studies in vitro and in vivo showed that glufosinate-ammonium
inhibits glutamine synthetase in the brain, kidney, and liver of rats.
With 100 ppm glufosinate-ammonium in the diet (equivalent to 10 mg/kg
bw per day), glutamine synthetase activity was inhibited in liver and
kidney but not in brain, and the Meeting concluded that the NOAEL was
10 mg/kg bw per day. The inhibition in liver and kidney was evident by
day 6, did not increase markedly up to day 90, and showed significant
reversal during a 31-day recovery period. The finding of increased
renal glutamine synthetase activity in a previous long-term study in
rats was considered to be a rebound response to continued inhibition
and to be of no relevance to human risk assessment.
New studies in which rats and mice received repeated, high doses
(270-1400 mg/kg bw per day) in the diet for 90 days were designed to
determine the maximum tolerated doses rather than NOAELs. There was no
evidence of specific toxicity or of irreversible neurobehavioural
effects in the rats. The LOAEL in rats was 7500 ppm, equal to 560
mg/kg bw per day. A new carcinogenicity study in rats showed that
glufosinate-ammonium had no significant carcinogenic potential at
doses up to 10 000 ppm, equal to 470 mg/kg bw per day. A significant
increase in retinal atrophy was seen in this study in females at doses
> 5000 ppm (equal to 280 mg/kg bw per day) and in males at 10 000 ppm
(equal to 470 mg/kg bw per day) but not in either sex at 1000 ppm
(equal to 45 mg/kg bw per day), the NOAEL. The results of these three
studies were consistent with those of previous investigations and
supported the overall NOAEL of 40 ppm (2 mg/kg bw per day) identified
previously in a long-term study in rats that included a more extensive
range of investigations.
No adverse findings were reported in workers in
glufosinate-ammonium production plants, but their exposure was stated
to be low. A number of cases of attempted suicide in Japan have
involved a glufosinate-ammonium-based formulation, but it was not
clear whether the effects reported were due to glufosinate-ammonium or
other constituents. The most significant effect was delayed
neurological symptoms. The available evidence indicates that exposure
to glufosinate-ammonium under normal conditions of use does not
present a significant risk to humans.
N-Acetylglufosinate
Oral doses of [14C] N-acetylglufosinate are absorbed to a
limited extent (5-10%), but the absorption is rapid, with peak plasma
concentrations found 1 h after a dose of 3 mg/kg bw. Excretion of an
absorbed dose is also rapid and occurs predominantly in the urine as
the parent compound. The excretory half-time is < 1 h for the initial
phase and approximately 7 h for the second phase. The residue
concentrations in the liver and particularly kidneys 4 days after
dosing were significantly greater than those in the plasma. Absorbed
N-acetylglufosinate undergoes limited biotransformation, but a
significant proportion (11%) of a low oral dose (3 mg/kg bw) was
de-acetylated to glufosinate-ammonium in the intestine. It is not
clear what proportion of the glufosinate-ammonium present in the
tissues after oral administration of N-acetylglufosinate is absorbed
from the intestine as glufosinate-ammonium. The toxicokinetics of
N-acetylglufosinate after repeated administration has not been
investigated.
N-Acetylglufosinate is of low acute toxicity after oral
administration to mice and rats (LD50 values > 2000 mg/kg bw) and
is not a skin sensitizer. Because N-acetylglufosinate is a plant
metabolite and is not present in pesticide formulations, it has not
been studied for acute toxicity by dermal or inhalation administration
or for ocular or dermal irritancy.
N-Acetylglufosinate has not been classified for toxicity by
WHO.
N-Acetylglufosinate is of low toxicity after repeated oral
administration to mice, rats, or dogs. Some and possibly all of the
inhibition of glutamine synthetase activity seen in all three species
was attributable to glufosinate-ammonium. The NOAEL for inhibition of
glutamine synthetase in the brain in the most sensitive species was
500 ppm (equal to 19 mg/kg bw per day) in a 90-day study in dogs
(glutamine synthetase activity was not measured in a 1-year study in
dogs). In a 2-year study in rats, there was evidence of chronic
progressive nephropathy and urolithiasis. The Meeting noted the
absence of pathological changes in the 90-day study, the lack of a
dose-response relationship for the renal lesions, the high sodium
concentrations associated with administration of
N-acetylglufosinate, and the high prevalence of renal lesions in
aged rats, and concluded that the renal lesions seen in the long-term
study in rats were not relevant to human risk assessment. Increased
incidences of adrenal cortical hyperplasia, adrenal necrosis, and
polyarteritis nodosa were seen in males receiving doses > 2000 ppm
(equal to > 91 mg/kg bw per day), and an increased incidence of
extramedullary haematopoiesis of the spleen was seen in females at
those doses. The NOAEL for pathological findings in rats, the most
sensitive species, was 200 ppm, equal to 9 mg/kg bw per day.
The Meeting concluded that N-acetylglufosinate is not
carcinogenic at the highest doses tested (equal to 1200 mg/kg bw per
day in mice and 1000 mg/kg bw per day in rats).
N-Acetylglufosinate has been studied in an adequate range of
tests for genotoxicity. The Meeting concluded, on the basis of the
results, that N-acetylglufosinate is not genotoxic.
Reproductive performance and outcome in a two-generation study of
reproductive toxicity in rats were not affected by administration of
N-acetylglufosinate at doses up to 700 mg/kg bw per day. The
compound was not teratogenic to either rats or rabbits and was not
fetotoxic to rats. An increased incidence of supernumerary thoracic
ribs was found in fetuses from rabbits exposed to
N-acetylglufosinate at > 160 mg/kg bw per day, a finding that may
be secondary to the maternal toxicity seen at such doses. The NOAEL
for fetotoxicity and maternal toxicity was 64 mg/kg bw per day.
3-[Hydroxy(methyl) phosphinoyl]propionic acid
Summaries of a range of studies on the genotoxicity, acute
toxicity, short-term toxicity, and teratogenicity of the
glufosinate-ammonium metabolite 3-[hydroxy(methyl)phosphinoyl]
propionic acid were available. The lowest NOAEL seen in these studies
(50 mg/kg bw per day) is 25-fold higher than the NOAEL used to derive
the ADI for glufosinate-ammonium.
Overall evaluation
The present Meeting compared the toxicity of
N-acetylglufosinate and 3-[hydroxy(methyl) phosphinoyl]propionic
acid with that of glufosinate-ammonium and concluded that the toxicity
of the metabolites was comparable to or less than that of the parent
compound. The Meeting established a group ADI of 0-0.02 mg/kg bw for
glufosinate-ammonium, N-acetylglufosinate, and 3-[hydroxy(methyl)
phosphinoyl]propionic acid (alone or in combination). This is the same
value as that of the ADI established for glufosinate-ammonium by the
1991 JMPR on the basis of the NOAEL in the long-term study in rats
given technical-grade glufosinate-ammonium, and applying a 100-fold
safety factor.
The present Meeting concluded that it was unnecessary to
establish an acute reference dose because glufosinate-ammonium,
N-acetylglufosinate, and 3-[hydroxy(methyl) phosphinoyl]propionic
acid are of low acute toxicity.
Toxicological evaluation
Levels that cause no toxic effect
Glufosinate-ammonium from 1991 JMPR)
Mouse: 80 ppm, equal to 11 mg/kg bw per day (toxicity in a 2-year
study of toxicity and carcinogenicity)
Rat: 40 ppm, equal to 2.1 mg/kg bw per day (toxicity in a 2-year
study of toxicity and carcinogenicity)
120 ppm equal to 6 mg/kg bw per day (toxicity in a study of
reproductive toxicity)
10 mg/kg bw per day (developmental effects, highest dose
tested in a study of developmental toxicity)
2.2 mg/kg bw per day (maternal and fetotoxicity in a study
of developmental toxicity)
Rabbit: 6.3 mg/kg bw per day (maternal and fetotoxicity in a study
of developmental toxicity)
20 mg/kg bw per day (developmental effects, highest dose
tested in a study of developmental toxicity)
Dog: 4.5 mg/kg bw per day (toxicity in a 1-year study)
N- Acetylglufosinate
Mouse: 1000 ppm, equal to 150 mg/kg bw per day (toxicity in a
2-year study of toxicity and carcinogenicity)
Rat: 200 ppm, equal to 9 mg/kg bw per day (toxicity in a 2-year
study of toxicity and carcinogenicity)
2000 ppm, equal to 140 mg/kg bw per day (parental toxicity
in a study of reproductive toxicity)
10 000 ppm equal to 700 mg/kg bw per day (reproductive
effects, highest dose tested in a study of reproductive
toxicity)
1000 mg/kg bw per day (highest dose tested in a study of
developmental toxicity)
Rabbit: 400 mg/kg bw per day (developmental effects, highest dose
tested in a study of developmental toxicity)
64 mg/kg bw per day (maternal and fetotoxicity in a study of
developmental toxicity)
Dog: 500 ppm, equal to 19 mg/kg bw per day (toxicity in a 90-day
study)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw (for glufosinate-ammonium, N-acetylglufosinate,
and 3-[hydroxy(methyl) phosphinoyl]propionic acid, alone or in
combination)
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological end-points relevant for estimating guidance values for dietary and non-dietary exposure to glufosinate-ammonium
Glufosinate-ammonium N-Acetylglufosinate (1991 JMPR)
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid but limited (5-10%) Rapid but limited (5-10%)
Distribution Extensive. Higher concentration in Extensive. Higher concentration in
kidney and liver kidney and liver
Potential for accumulation Minimal Minimal
Rate and extent of excretion Rats, rapid, > 92% of 30 mg/kg bw Rats, rapid, >95% of 3 mg/kg bw within
within 24 h, primarily in faeces 24 h, primarily in faeces
Metabolism in animals Main metabolite is 3-[hydroxy-(methyl) Limited. Some de-acetylation
phosphinoyl] propionic to glufosinate-ammonium (rat,
acid (rat, goat, hen) goat, hen)
Toxicologically significant compounds Parent Parent and glufosinate-ammonium
Acute toxicity
Rat, LD50, oral 1700 mg/kg bw > 3000 mg/kg bw
Rat, LD50, intraperitoneal ~ 100 mg/kg bw > 1200 mg/kg bw
Mouse, LD50, oral 420 mg/kg bw > 3000 mg/kg bw
Skin sensitization, guinea-pigs Negative (Buehler test) Negative (Magnusson & Kigman test)
Short-term toxicity
Target/critical effect Glutamine synthetase inhibition; Glutamine synthetase inhibition in brain
behaviour in dogs; kidney weights of dogs, mice, rats
and urinary parameters in rats
Lowest relevant oral NOAEL 5 mg/kg bw per day in dogs 19 mg/kg bw per day in dogs
Genotoxicity Not genotoxic Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Glutamine synthetase inhibition; Adrenal necrosis and hyperplasia;
increased kidney weight in rats spleen haematopoiesis in rats
Lowest relevant NOAEL 2 mg/kg bw per day in rats 9 mg/kg bw per day
Carcinogenicity Not carcinogenic Not carcinogenic
Reproductive toxicity
Reproductive target/critical effect Reduced litter size, rats None
Developmental target/critical effect General maternal and fetotoxicity Extra ribs/maternal toxicity in rabbits
Lowest relevant NOAEL for 12 mg/kg bw per day in rats 137 mg/kg bw per day for reproductive
general toxicity in rats toxicity
Lowest relevant NOAEL for 2 mg/kg bw per day in rats 64 mg/kg bw per day in rabbits
developmental toxicity
Neurotoxicity Possibly behavioural, but no No evidence of specific effectspathological findings
Medical data Suicidal poisonings producing coma, No data, not produced commercially
delayed neurological effects, death;
no findings in work force
Summary Value Study Safety factor
Group ADI for glufosinate-ammonium 0-0.02 mg/kg bw 2 years, rat 100
and N-acetylglufosinate
Acute reference dose Unnecessary
References
Note: Amendments have been issued to a number of studies; these are
not referenced separately.
Baeder, C. & Hofmann, T. (1994) Embryotoxicity after oral
administration in Himalayan rabbits. Hoe 099730 substance,
technical. Hoechst AG, Pharma Development, Corporate Toxicology.
Report No. 94.0392. A52948. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Bernier, L. (1996) 52-week dietary toxicity study in the beagle dog of
Hoe 099730 substance, technical. Bio-Research Laboratories.
Hoechst Schering AgrEvo GmbH. Report No. 85438. A55742.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Bernier, L. (1997) Dietary chronic toxicity and carcinogenicity study
in the Sprague-Dawley rat of Hoe 099730 substance, technical.
ClinTrials BioResearch Ltd, Senneville, Quebec, Canada. Report
No. 85440. A59280. Unpublished report submitted ubmitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Beyrouty, P. (1996a) Dietary range-finding reproduction study in the
Sprague-Dawley rat of Hoe 099730 substance, technical. Biological
Research Laboratory Ltd.AgrEvo Canada Inc. Hoechst Schering
AgrEvo GmbH. Report No. 95447. A55746. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Beyrouty, P. (1996b) Dietary 2-generation (1 Litter) reproduction
study in the SpragueDawley rat of Hoe 099730 substance,
technical. Bio-Research Laboratories. Report No. 95448. A55747.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Bremmer, J.N. & Leist, K.-H. (1998) Toxicology and metabolism studies
(summary and evaluation) 3-methyl phosphinico-propionic acid
(MPP) AE F061517 substance, technical. Hoechst Schering AgrEvo
GmbH. Safety Evaluation Frankfurt. Report No. TOX98/011. A59706.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Corney, S.J., Braunhofer, P. & Luetkemeier, H. (1992) 13-week oral
toxicity (feeding) study in the dog with Hoe 099730 substance,
technical. Research & Consulting Company Ltd, Switzerland. Report
No. 291104. A49064. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Dotti, A., Luetkemeier, H. & Powell, J. (1993) 13-week oral toxicity
(feeding) study in the rat with Hoe 039866 substance, technical.
Research & Consulting Company Ltd, Switzerland. Report No.
338400. A51836. Unpublished. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Dotti, A., Luetkemeier, H. & Biedermann, K. (1994) 13-week oral
toxicity (feeding) study in the mouse with Hoe 039866 substance,
technical. Research & Consulting Company Ltd, Switzerland. Report
No. 338411. A52107. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Ebert, E. (1991a) Repeated-dose oral toxicity (4-week feeding study)
in the NMRI mouse Hoe 099730 substance, technical. Hoechst AG,
Pharma Development, Corporate Toxicology. Report No. 91.0728.
A46543. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Ebert, E. (1991b) Repeated-dose oral toxicity (4-week feeding study)
in the Wistar rat Hoe 099730 substance, technical. Hoechst AG,
Pharma Development, Corporate Toxicology. Report No. 91.0594.
A46604. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Ernst, S.W. & Leist, K.-H. (1999a) Critical review of organ specific
glutamine synthetase activities. AgrEvo, Toxicology Frankfurt,
Germany. Report No. TOX98/057. C001918. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Ernst, S. & Leist, K.-H. (1999b) Hoe 099730 substance technical. Code
Hoe 099730 00 ZC92 001. Testing for embryotoxicity after oral
administration in Himalayan rabbits, Addendum to Document A52948,
Determination of the concentration of test substance during the
study. Toxicology, Frankfurt. Report No. TOX99/026. C004479.
Unpublished report submitted 05.07.99 to WHO by Hoechst Schering
AgrEvo GmbH, Germany
Ernst, S.W. & Leist, K.-H. (1999c) Glufosinate-ammonium -- Human
experience with herbicides containing glufosinate-ammonium.
Toxicology, Frankfurt. Report No. TOX99/021. C003758. Unpublished
report submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Ernst, S.W. & Stumpf, K. (1999a) A dietary chronic toxicity and
carcinogenicity study of Hoe 099730; substance technical in the
Swiss CRL:CD(R) mouse. Addendum 1 to Agrevo report A57755.
Historical control data of histopathological investigations
conducted at ClinTrials BioResearch Ltd, Senneville, Quebec,
Canada. Hoechst Schering AgrEvo GmbH, Toxicology Frankfurt,
Germany. Report No. TOX99/033. C004765. Unpublished report
submitted 27.07.99 to WHO by Hoechst Schering AgrEvo GmbH,
Germany.
Ernst, S. & Stumpf, K. (1999b) A dietary chronic toxicity and
carcinogenicity study of Hoe 099730; substance technical in the
Sprague-Dawley rat. Addendum 1 to Agrevo report A59280.
Historical control data of histopathological investigations
conducted at ClinTrials BioResearch Ltd, Senneville, Quebec,
Canada. Hoechst Schering AgrEvo GmbH, Toxicology Frankfurt,
Germany. Report No. TOX99/034. C004764. Unpublished report
submitted 27.07.99 to WHO by Hoechst Schering AgrEvo GmbH,
Germany.
Farrell, D.J. (1997) Dietary carcinogenicity study in the Swiss CRL:
CD(R) mouse of Hoe 099730 substance, technical. ClinTrials
BioResearch Ltd, Senneville, Quebec, Canada. AgrEvo Canada Inc.
Report No. 97.0451. A57755. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Hack, R., Ebert, E. & Ehling, G. (1994) Glufosinate-ammonium -- Some
aspects of mode of action in mammals. Food Chem. Toxicol., 32,
461-470.
Heidemann, A. & Voelkner, W. (1992) Chromosome aberration assay in
human lymphocytes in vitro with Hoe 099730, substance, technical.
Cytotest Cell Research. Report No. 275602. A49392. Unpublished
report submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Hofmann, T. & Jung, R. (1988) Nickel(II)-sulphate. Testing for
sensitising properties in the Pirbright-white guinea pig in a
maximisation test. Pharma Research Technology, Hoechst AG,
Frankfurt. Report No. 91.0792. A46366. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Horstmann, G. & Baeder, C. (1992) Embryotoxicity in the Wistar rat
after oral administration (limit test) Hoe 099730 substance,
technical. Hoechst AG, Pharma Development, Corporate Toxicology.
Report No. 92.0394. A48852. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995a) Metabolism of (14C)-glufosinate in
a lactating goat. AgrEvo USA Co., Pikeville, Hazleton Lab., USA.
Report No. U012A/A524R. A54158. A55808. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995b) Metabolism of (14C)-glufosinate in
laying hens. AgrEvo USA Co., Pikeville, USA, Hazleton Lab., USA.
Report No. U012A/A525R. A54159. A55808. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995c) Metabolism of (14C)-N-acetyl
glufosinate in a lactating goat. AgrEvo USA Co. Pikeville, PTRL
East Inc., USA. Report No. 502BK U012A/A524. A54155. A55808.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995d) Metabolism of (14C)-N-acetyl
glufosinate in laying hens. AgrEvo USA Co. Pikeville, USA. PTRL
East Inc., USA. Report No. 503BK,U012A/A525. A54157. A55808.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Kaleja, R. (1999) Statement on handling glufosinate-ammonium.
Occupational Health Center, InfraServ GmbH & Co. Höechst KG.
Document No. C003747. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Kellner, H.-M. & Braun, R. (1993a) Hoe 099730-14C blood levels in
rats following single oral and intravenous administration of 3
mg/kg body weight. Hoechst RCL, DEU. Report No. 01-L42-0671-93.
A49979. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Kellner, H.-M. & Braun, R. (1993b) Hoe 099730-14C blood levels in
rats following single oral and intravenous administration of 3
mg/kg body weight. Hoechst Radiochem. Laboratorium, Germany.
Report No. 01-L42-0671-93. A53276. Unpublished report submitted
to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Kellner, H.-M., Stumpf, K. & Braun, R. (1993) Hoe 099730-14C
pharmacokinetics in rats following single oral and intravenous
administration of 3 mg/kg body. Hoechst RCL, Germany. Report No.
01-L42-0670-93. A49978. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Koyama, K., Andou, Y., Saruki, K. & Matsuo, H. (1994) Delayed and
severe toxicities of a herbicide containing glufosinate and a
surfactant. Vet. Hum. Toxicol., 36, 17-18.
Lauck-Birkel, S. (1995a) Hoe 039866-14C (glufosinate-ammonium).
Metabolism in male and female rats following single oral
administration of test substance at a dose level of 500 mg/kg
body weight. AgrEvo Entwicklung Umweltforschung Oekochemie,
Germany. Report No. CM93/071. A54334. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Lauck-Birkel S. (1995b) Hoe 099730-14C Metabolism in male and female
rats following a single oral administration of test substance at
a dose level of 1000 mg/kg body weight. AgrEvo Entwicklung
Umweltforschung Oekochemie, Germany. Report No. CM93/070. A54333.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Lauck-Birkel, S. (1996) Hoe 039866-14C (glufosinate-ammonium)
Metabolism in male rats following single oral administration of
test substance at a dose level of 2 mg/kg body weight. Hoechst
Schering AgrEvo GmbH, Environmental Chemistry Frankfurt. Report
No. CM95/013. A54336. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B. (1999a) Glufosinate-ammonium. Rat
metabolism-Single oral intermediate dose (20 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/130. C001924. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B. (1999b) Glufosinate-ammonium. Rat
metabolism-Single intravenous low dose (2 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/131. C001922. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B.(1999c) N-Acetyl-glufosinate. Rat
metabolism-Single intravenous low dose (3 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/096. C001920. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B. (1999d). N-Acetyl-glufosinate. Rat
metabolism-Single oral intermediate dose (30 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/097. C001921. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany
Lie-Venema, H., Hakvoort, T.B.M., Moorman, A.F.M. & Lamers, W.H.
(1998) Regulation of the spatiotemporal pattern of expression of
the glutamine synthetase gene. Department of Anatomy and
Embryology. University of Amsterdam, Academic Medical Center,
Netherlands.
Luetkemeier, H. (1999) N-Acetyl-glufosinate and glufosinate. In vitro
investigations. Comparison of the inhibition of glutamine
synthetase. Report No. RCC 718795. C003753. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1995a) Hoe 039866-14C. Sampling of blood,
excrements, organs and tissues for metabolism studies in male and
female rats following single oral administration of approx. 500
mg/kg body weight (First addendum to report CM93/071 (A54334)).
Hoechst AG, Bereich Pharma, Kinetics/Metabolism, Germany. Report
No. 01-L420813-95. A54450. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1995b) Hoe 099730-14C. Pharmacokinetics in
rats following single oral administration of the test substance
at a dose level of 1000 mg/kg body weight. Hoechst AG, Bereich
Pharma, Kinetics/Metabolism, Germany. Report No. 01-L42-0815-95.
A54335. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1999a) Glufosinate-ammonium. Rat. Absorption,
distribution and elimination, single oral intermediate dose (20
mg/kg body weight). HMR, DI & A; Lead Optimization, Germany.
Report No. TEP57/16. C001926. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1999b) Glufosinate-ammonium. Rat. Absorption,
distribution and elimination, single intravenous low dose (2
mg/kg body weight). HMR, DI & A; Lead Optimization, Germany.
Report No. TEP57/15. C001923. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1999c) N-Acetyl-glufosinate. Rat. Absorption,
distribution and elimination, single oral intermediate dose (30
mg/kg body weight), HMR Preclinical Development,
Pharmacokinetics, Germany. Report No. TEP170/8. C001925.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Maas, J. & Braun, R. (1999d) N-Acetyl-glufosinate. Rat. Absorption,
distribution and elimination, single intravenous low dose (3
mg/kg body weight). HMR Preclinical Development,
Pharmacokinetics, Germany. Report No. TEP170/7. C001919.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Muller, W. (1989a) Mutagenic potential in strains of Salmonella
typhimurium (Ames test) and , Hoe 099730 substance, technical.
Hoechst AG. Pharma Development, Corporate. Report No. 89.0084.
A40532. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Muller, W. (1989b) Detection of gene mutations in somatic cells in
culture HGPRT-test with V79 cells Hoe 099730 substance,
technical. Hoechst AG. Pharma Development, Corporate Toxicology.
Report No. 89.1010. A41375. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Muller, W. (1989c) Unscheduled DNA synthesis test in mammalian cells
in vitro of Hoe 099730 substance, technical. Hoechst AG. Pharma
Development, Corporate Toxicology. Report No. 89.0024. A40926. U
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Muller, W. (1989d) Chromosome aberrations in vitro in V79 Chinese
hamster cells Hoe 099730 substance, technical. Hoechst AG. Pharma
Development, Corporate Toxicology. Report No. 89.1063. A41851.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany
Muller, W. (1991a) Detection of gene mutations in somatic cells in
culture. HGPRT-test with V79 cells Hoe 099730 substance,
technical. Hoechst AG. Pharma Development, Corporate Toxicology.
Report No. 91.0356. A45876. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany..
Muller, W. (1991b) Unscheduled DNA synthesis test in mammalian cells
in vitro of Hoe 099730 substance, technical. Hoechst AG. Pharma
Development, Corporate Toxicology. Report No. 91.0286. A46129.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Muller, W. (1991c) Micronucleus test in male and female NMRI mice
after oral administration Hoe 099730 substance, technical.
Hoechst AG. Pharma Development, Corporate Toxicology. Report No.
91.0438. A46128. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Purich, D.L. (1998) Advances in the enzymology of glutamine synthesis.
Adv. Enzymol. Relat. Areas Mol. Biol., 72, 9-42.
Schmid, H., Keller, B., Luetkemeier, H., Westen, H. & Biedermann, K.
(1998) Oncogenicity study in rats. Glufosinate-ammonium substance
technical. RCC Umweltchemie AG, Itingen, Switzerland. Hoechst
Schering AgrEvo GmbH. Safety Evaluation Frankfurt. Report No.
98.0256. A67303. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Schmid, H., Keller, B., Biedermann, K. & Luetkemeier, H. (1999)
N-Acetyl-L-glufosinate disodium and glufosinate-ammonium. 13-week
oral feeding study in the rat; Comparison of the potential to
inhibit glutamine synthetase. Report No. RCC711663. C004512.
Unpublished report submitted 08.07.99 to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989a) Acute oral toxicity in the male
and female Wistar rat Hoe 099730 substance, technical. Hoechst
AG. Pharma Development, Corporate Toxicology. Report No. 89.0879.
A41793. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989b) Acute oral toxicity in the male
and female NMRI mouse Hoe 099730 substance, technical. Hoechst
AG. Pharma Development, Corporate Toxicology. Report No. 89.0877.
A41791. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989c) Acute intraperitoneal toxicity
in the male and female Wistar rat Hoe 099730 substance,
technical. Hoechst AG. Pharma Development, Corporate Toxicology.
Report No. 89.0883. A41795. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989d) Acute intraperitoneal toxicity
in the male and female NMRI mouse Hoe 099730 substance,
technical. Pharma Development, Corporate Toxicology. Report No.
89.0882. A41789. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989e) Sensitising properties in the
Pirbright-White guinea pig in a maximisation test Hoe 099730
substance, technical. Hoechst AG, Pharma Development, Corporate
Toxicology. Report No. 89.0880. A41612. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Stumpf, K. (1993a) Hoe 039866-14C. Metabolism in male and female
rats following single oral administration of test substance at a
dose level of 2 mg/kg body weight. Hoechst AG, Bereich
Landwirtschaft, Produktentwicklung Oekologie 1, Germany. Report
No. CM90/028. A49981. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Stumpf, K. (1993b) Hoe 099730-14C. Metabolism in male and female
rats following single oral administration of test substance at a
dose level of 3 mg/kg body weight. Hoechst AG, Bereich
Landwirtschaft, Produktentwicklung Oekologie 1, Germany. Report
No. CM90/026. A49980. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Tennekes, H., Schmid, H. & Probst, D. (1992a) Sub-chronic oral
toxicity 13-week feeding study in mice Hoe 099730 substance,
technical. Research & Consulting Company Ltd, Switzerland. Report
No. RCC291025. A48186. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Tennekes, H., Probst, D. & Luetkemeier, H. (1992b) Sub-chronic oral
toxicity 13-week feeding study in rats Hoe 099730 substance,
technical. Research & Consulting Co. Ltd, Switzerland. Report No.
291093. A48187. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Watanabe, T. & Sano, T. (1998) Neurological effects of glufosinate
poisoning with a brief review. Hum. Exp. Toxicol., 17, 35-39.
Weller, O. (1994) Statement on certificates of analysis used for
different tox.-studies Hoe 099730 technical grade aqueous
solution. Hoechst Schering AgrEvo GmbH. Product Analysis
Frankfurt. Report No. OE94/053. A53577. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
(-)-glutamine and hydroxylamine in the presence of arsenate,
manganese, and ADP, followed by spectrophotometric measurement of an
iron compound. A preincubation period of 10 min was used in the main
assays, as it had been shown that inhibition was not changed by
extending this period to 60 or 120 min. The samples were incubated for
20 min in the presence of N-acetylglufosinate (a 33.8% solution
containing 0.06% w/w glufosinate-ammonium) at 0-10 000 µg/ml or
glufosinate-ammonium (as a 50.2% solution) at 0-500 µg/ml.
Glufosinate-ammonium induced significant, concentration-related
inhibition of glutamine synthetase in all tissues at doses > 0.77
mmol/L (Table 6), the inhibition profile varying with tissue.
N-Acetylglufosinate induced only marginal inhibition at 13 mmol/L,
some of which can be attributed directly to the glufosinate-ammonium
content of the sample. The report did not provide results corrected
for protein content, and not all of the assays were performed in
duplicate; however, for the purposes of this comparative exercise, the
results are considered to be acceptable (Lutkemeier, 1999).
A comparative study was performed of the inhibition of glutamine
synthetase in tissues from groups of 10 male Wistar rats given diets
containing N-acetylglufosinate (with 0.06% w/w glufosinate-ammonium)
at 1000 or 10 000 ppm or glufosinate-ammonium at 0, 100, or 1000 ppm
(Schmid et al., 1999). The animals were exposed for 6, 13, 20, or 90
days with 91 days plus 31 days for recovery. In addition to standard
observations and gross necropsy, samples of liver, brain, and kidney
were removed, rapidly cooled, and stored at -70°C prior to processing
and assaying for glutamine synthetase activity, as described above.
There were no deaths or treatment-related clinical signs. The
absolute and relative weights of the kidney were increased by 8-23% in
all treated groups during the first 20 days of the study, but with no
associated pathological findings. Statistically significant inhibition
of glutamine synthetase activity was seen in liver and kidney samples
by day 6, and, except in liver from animals exposed to 1000 ppm
N-acetylglufosinate, did not increase markedly up to day 90 (Table
7). Significant recovery of glutamine synthetase activity occurred
during the 31-day recovery period. The results indicate that orally
administered glufosinate-ammonium is approximately 10 times more
potent at inhibiting glutamine synthetase than is
N-acetylglufosinate. The extent to which this inhibition is due
directly to de-acetylation of N-acetylglufosinate to
glufosinate-ammonium is uncertain. The activity of glutamine
synthetase in brain samples was not reduced markedly in animals
exposed to either N-acetylglufosinate or glufosinate-ammonium at
1000 ppm.
(d) Significance of glutamine synthetase inhibition to humans
Glutamine synthetase (E.C.6.3.1.2) is a key enzyme in the
metabolism of nitrogen and glutamate, catalysing the multi-step
reaction of
(-)-glutamate + ATP + NH3 <=> (-)-glutamine + ADP + P
Table 6. Inhibition of glutamine synthetase activity in vitro in tissue samples from rats, in the
presence of N-acetylglufosinate and glufosinate ammonium; in square brackets, absolute activity
expressed as mg gamma-glutamylhydroxamate formed per g tissue per 20 min
Compound Dose Liver Kidney Neocortex Medulla Hypothalamus
(mmol/L)
Glufosinate ammonium 0 0 [28] 0 [18] 0 [27] 0 [23] 0 [20]
0.003 1 0 0 1
0.008 2 1 0 0 0
0.026 4 1 3 1 0
0.077 14 3 5 6 3
0.26 36 5 13 20 11
0.77 60 13 32 42 29
1.3 72 17 45 53 41
N-Acetylglufosinate 0.13 1 0 0 0 0
0.38 1 0 0 0 0
0.63 2 0 0 0 0
1.3 2 0 0 0 0
6.3 9 1 2 2 1
13a 15 2 4 7 5
a Contains approximately 0.03 mmol/L glufosinate ammonium
Table 7. Activity of glutamine synthetase in samples from 10 male Wistar rats that
received N-acetylglufosinate or glufosinate ammonium in the diet or
control diet
Day Tissue Glutamine synthetase activity (mean % of control value)
Controla Glufosinate ammonium N-acetylglufosinate
100 ppm 1000 ppm 1000 ppm 10 000 ppm
6 Liver 24 55 36 96 46
Brain 28 101 89 94 93
Kidney 17 60 58 61 54
13 Liver 30 51 30 74 40
Brain 25 101 91 102 104
Kidney 17 61 58 59 54
90 Liver 24 60 40 58 54
Brain 22 104 82 99 98
Kidney 14 67 46 55 53
91 + 31 Liver 24 97 85 83 94
Brain 14 98 88 97 97
Kidney 22 90 97 95 87
a Absolute activity, expressed as mg gamma-glutamylhydroxamate formed per
g tissue per 20 min
In plants, glutamine synthetase is the main enzyme involved in the
control of ammonia concentrations, and its inhibition is the mechanism
of action of glufosinate-ammonium in plants. In mammals, other
pathways exist for the homeostatic control of ammonia, such as reverse
reaction of amino acid dehydrogenases and the carbamoyl phosphate
synthetase-urea cycle. Glutamate and glutamine can, however, play
significant roles in other biochemical and physiological processes in
mammals, such as neurotransmission (glutamate and gamma-aminobutyric
acid (GABA)). The activity of glutamine synthetase varies between
tissues and species (see below), but the amino acid sequence is
reported to be well conserved (LieVenema et al., 1998; Purich, 1998;
Ernst & Leist, 1999a).
The liver has two distinct systems for dealing with ammonia. A
high-capacity, low-affinity system exists in the periportal
hepatocytes which is based on carbamoyl phosphate synthetase and the
urea cycle. In central vein hepatocytes, a low-capacity, high-affinity
system exists which is based on glutamine synthetase and ornithine
aminotransferase. Hack et al. (1994) showed that doses of
glufosinate-ammonium did not increase ammonia concentrations in liver
at a dose (5000 ppm) that inhibited glutamine synthetase activity by
50%. While a 60% reduction in liver glutamine was seen at day 1, the
concentration had returned to normal by day 4, indicating the
induction of alternative pathways. Inhibition of liver glutamine
synthetase by up to 50% is therefore not considered to be adverse in
isolation.
The activity of this enzyme in kidney varies considerably between
species (LieVenema et al., 1998; see below), with relatively high
activity in rodents but negligible activity in dogs and humans.
Inhibition of kidney glutamine synthetase in the absence of
pathological findings is not considered to be relevant to human risk
assessment.
In the brain and central nervous system, ammonia homeostasis is
controlled by a number of enzymes including glutamine synthetase and
glutamate dehydrogenase. Under normal conditions (~ 100 µmol/L of
ammonium and 3 mmol/L of glutamate), the flux through glutamine
synthetase in brain is 2-10% of its theoretical capacity and that of
glutamate dehydrogenase is approximately 0.1% of its capacity
(Lie-Venema et al., 1998). With such excess capacity, inhibition of
brain glutamine synthetase is unlikely to result in significant
increases in brain ammonia concentrations. This conclusion is
supported by the finding of Hack et al. (1994) that brain ammonia
concentrations were not increased at doses of glufosinate-ammonium
that produced a 40% reduction in brain glutamine synthetase activity
in rats. However, the glutamine-glutamate shunt between GABA and
glutamate in neurons and glutamine in astrocytes plays a role in both
excitatory and inhibitory neurotransmission. The results of Hack et
al. (1994), although somewhat inconsistent, indicate that significant
changes in a range of biogenic amines in regions of the dog brain are
associated with changes of > 8% in glutamine synthetase activity
after administration of glufosinate-ammonium at 8 mg/kg bw for 28
days, a dose that produced 'increased gait activity'. It is thus
proposed that any statistically significant, > 10% inhibition of
glutamine synthetase activity in brain is a marker of potentially
adverse effects on brain biochemistry and behaviour.
2. Toxicological studies
All of the studies described below were included statements of
compliance with GLP and met the basic requirements of the OECD test
guidelines applicable at the time of study initiation, unless
otherwise stated.
(a) Short-term studies of toxicity
Mice
In response to concern that the doses used in the study of
carcinogenicity with glufosinate-ammonium in mice evaluated previously
had not been appropriately high (160 ppm in males, 320 ppm in
females), a 90-day study was performed in which groups of 10 NMRI mice
of each sex received diets containing glufosinate-ammonium (purity,
95.5%) at concentrations of 0, 1750, 3500, or 7000 ppm. The
homogeneity and achieved concentrations were acceptable, and the
intakes of animals were 561 and 644 mg/kg bw per day for males and
females at the intermediate dose and 274 and 356 mg/kg bw per day for
animals at the low dose, respectively. The investigations included
clinical signs, body weight, food consumption, haematology, clinical
chemistry, organ weights, and gross and microscopic pathology. All
animals at the high dose had died by day 8, 50% of those at 3500 ppm
had died by day 11, and one female at the low dose died. Clinical
signs (ruffled fur, sedation, and emaciation), reduced food
consumption, and initial body-weight loss were seen at all doses,
although the body-weight gain during the latter part of the study was
similar in surviving animals. There were no consistent clinical
chemical or haematological findings and no changes in organ weights or
on gross examination. Congestion in multiple organs was seen at
microscopic examination of many animals that died during the study,
but the cause of death was not determined. No NOAEL could be
identified, but the design was not optimal for this purpose. The
lowest dose tested (1750 ppm, equal to 270 mg/kg bw per day) was
approximately the maximum tolerated dose for a 90-day study, resulting
in a single death and marked initial effects on body weight. The
maximum tolerated dose for a 2-year study in mice would be about 600
ppm if a factor of 3 is used to extrapolate from the approximate dose
in the 90-day study (Dotti et al., 1994). The results of this study
indicate that the previous bioassay was performed within a factor of 2
of the estimated maximum tolerated dose and need not be repeated.
Rats
The toxicity of glufosinate-ammonium (purity, 95.5%) was
investigated in groups of 10 Wistar rats of each sex given the
compound in the diet at concentrations of 0, 7500, 10 000, or 20 000
ppm for 90 days. Routine observations and measurements were made, with
ophthalmoscopy before treatment and at termination and a basic
functional observation battery, which was administered before
treatment and at weeks 1, 2, 3, 4, 8, and 13 and involved observations
in the home cage, an external area, and in the hand, but no forced
physical activity such as grip strength or swimming. Blood samples for
haematological and clinical chemical analyses were taken from fasted
animals at week 13. At termination, five animals of each sex per group
were perfused to preserve nervous tissue. Major organs from all
animals at the highest dose and controls were examined histologically,
as was nervous tissue from all perfused animals and all gross lesions.
Organ weights were not determined. The homogeneity, stability, and
achieved concentrations in the diet were satisfactory, with intakes
equal to 0, 520, 690, and 1400 mg/kg bw per day in males and 0, 570,
740, and 1400 mg/kg bw per day in females.
Two females at the high dose died within the first 8 days of
dosing, but there were no other unscheduled deaths. Animals at the
high dose showed a range of signs during the first 2 weeks of
treatment, including sedation, dyspnoea, emaciation, and diarrhoea.
Food consumption was reduced by > 20% in all groups during the first
two weeks. Body-weight loss was seen in animals at the high dose, and
reductions in body-weight gain were seen in other groups during the
first week of dosing. All groups had similar body-weight gains during
the last weeks of the study. No abnormal ophthalmoscopic findings were
reported. The haematological findings were similar in test and control
groups, and an apparent reduction in erythrocyte count in males
appeared to be related to a high control value. A consistent pattern
of changes in serum lactate dehydrogenase and creatine kinase activity
was seen in animals of each sex, with 20% reductions at the low and
intermediate doses and an increase at the high dose. Small (< 10%)
but statistically significant ( p < 0.05) increases in serum calcium
and inorganic phosphorus concentrations were seen in animals at the
high dose. The functional observational battery identified a similar
pattern of changes in males and females that included miosis, apathy,
reduced alertness and grooming, increased body tone, and vocalization.
These changes were present in all treated groups, with no clear
pattern over time, but were more prevalent at the high dose. There
were no significant macroscopic findings, and the only microscopic
finding of significance was an increased incidence of renal pelvis
dilatation in males at the high dose; there were no treatment-related
effects on nervous tissues.
No NOAEL could be identified owing to alterations in calcium and
inorganic phosphorus and in the functional observational battery at
all doses, although there was no indication of irreversible
neurotoxicity. The effects on food consumption and body weight may
have been secondary to palatability, as they were mainly transient
(Dotti et al., 1993).
(b) Long-term studies of toxicity and carcinogenicity
Rats
A study was initiated in 1994 (Schmid et al., 1998) to include
doses above the maximum of 500 ppm used in the first study. Groups of
60 Wistar rats of each sex received diets containing
glufosinate-ammonium (purity, 96%) at concentrations of 0, 1000, 5000,
or 10 000 ppm for 104 weeks. These doses were based on increased
mortality seen at 20 000 ppm in the 90-day study (Dotti et al., 1993).
The animals were observed for survival, clinical signs, body-weight
gain, food consumption, and the presence of nodules or masses. Blood
smears were prepared from controls and animals at the high dose in
weeks 52, 78, and 104. At termination, over 30 tissues were removed,
nine were weighed, and gross and histopathological examination was
performed on all tissues from all animals. The stability, achieved
levels, and homogeneity of glufosinate-ammonium in the diets were
satisfactory, giving intakes equal to 0, 45, 230, and 470 mg/kg bw in
males and 0, 57, 280, and 580 mg/kg bw per day in females.
Survival was similar in all groups, being over 70% at 104 weeks.
There were no treatment-related changes in clinical signs, food use
efficiency, the occurrence of nodules or masses, or the appearance of
blood smears. All treated groups had reduced food consumption and
body-weight gain over the first month of dosing but these parameters
were subsequently within 10% of those of controls. The weight of the
kidney was increased by 15-30% in relation to dose in all treated
groups, but there were no histological correlates. Macroscopic
examination showed a decreased incidence of pituitary nodules in all
treated males but an increased incidence of adrenal gland foci in
males at the high dose. Microscopic examination revealed a
statistically significant ( p < 0.05) increase in the incidence of
retinal atrophy in males and females at 10 000 ppm and in females at
5000 ppm. This effect was considered to be related to treatment, as a
dose-response relationship was evident in females and it occurred in
animals of each sex, even though the incidence was within the range of
historical controls (males, 20%; females, 38%; Table 8). The incidence
of a rare skin tumour (trichofolliculoma) was increased in males at
the high dose, but it was not statistically significant and was not
seen in females or in males receiving half of the high dose; the
finding was therefore considered not to provide clear evidence of
carcinogenic potential. The total number of malignant and benign
tumours was similar in treated and control groups. The NOAEL was 1000
ppm, equal to 45 mg/kg bw per day, on the basis of the increased
incidence of retinal atrophy (Schmid et al., 1998).
Table 8. Incidences of lesions in Wistar rats receiving
glufosinate ammonium in the diet for 104 weeks
Dose (ppm) Incidence (%)
Retinal atrophy Trichofolliculoma
(males)
Males Females
0 4 3 0
1 000 3 2 0
5 000 4 19 0
10 000 12 29 4
(c) Studies on metabolites
(i) N-Acetylglufosinate
The (-) isomer of N-acetylglufosinate is a major metabolite of
glufosinate-ammonium after its application to glufosinate-tolerant
crops. In 1998, the Committee proposed that residues arising from
applications of glufosinate-ammonium to tolerant crops be defined as
the 'sum of glufosinate-ammonium, 3-[hydroxy(methyl)phosphinoyl]
propionic acid, and Nacetylglufosinate' but could not adopt this
definition until N-acetylglufosinate had been evaluated
toxicologically. Extensive toxicokinetic and toxicological studies on
N-acetylglufosinate have since been submitted. The preparation
tested was an aqueous solution of the disodium salt, and all of the
doses cited below have been corrected for the content of the
technical-grade (-)-isomer. The three batches of N-acetylglufosinate
used varied in purity from 74.5% to 99% and in glufosinate-ammonium
content from 0.06 to 4.5% (Weller, 1994). All of the studies conformed
to GLP and were claimed to have been performed according to guideline
85-1 of November 1984 of the US Environmental Protection Agency or to
meet the basic requirements of the OECD test guidelines applicable at
the time the study was initiated, unless otherwise stated.
Absorption, distribution, and excretion: The toxicokinetics of
N-acetylglufosinate was examined in several studies at doses of
3 mg/kg bw (Stumpf, 1993b; Kellner et al., 1993) or 1000 mg/kg bw
(Lauck-Birkel, 1995b; Maas & Braun, 1995b). Groups of five Wistar rats
of each sex received [3,4-14C] N-acetylglufosinate (purity, > 98%)
by gavage in saline. Urine and faeces were collected over 24-h periods
up to 96 h, and up to 12 tissue samples were taken from animals given
1000 mg/kg bw and killed at 2 and 6 h (two of each sex), 24 h (five of
each sex), and 96 h (five of each sex). Radiolabel in the
gastrointestinal tract was determined in one male each killed at 4 or
24 h after receiving 3 mg/kg bw, and one male at this dose killed at
96 h was studied by autoradiography (Kellner et al., 1993). Radiolabel
in tissues and excreta was determined by liquid scintillation counting
after appropriate processing. The samples were also prepared for
metabolic investigations (see below).
The doses administered differed somewhat among animals, but this
was considered not to have affected the results. Most of each
administered dose was excreted in the faeces within 48 h (Table 9),
although excretion was more rapid at the lower dose. The
concentrations in tissue peaked at 6 h and represented < 1% of the
administered radiolabel; the highest concentrations were detected in
kidney, and those in liver and kidney were greater than in plasma. In
animals at 3 mg/kg bw, the tissue concentration represented < 0.1% of
the administered dose at day 4, with the highest concentrations in
kidney (0.06 µg/g), a finding confirmed by autoradiography. Analysis
of the gastrointestinal tract 4 and 24 h after administration of
3 mg/kg bw showed that 3% and < 0.01% of the dose was in the stomach
and 91% and 3.5% in the intestine, respectively. Sporadic differences
by sex were seen but were not consistent between studies or individual
animals. The tissue concentrations were higher in female than male
rats given 1000 mg/kg bw, as was the urinary excretion after the dose
of 3 mg/kg bw. The results of the two studies with 1000 mg/kg bw
presented a similar profile, although Maas & Braun (1995b) found lower
tissue concentrations and higher urinary excretion in females (up to
20%, including cage washes); however, there was a threefold variation
between individual animals, and the possibility of contamination by
faeces cannot be dismissed.
Table 9. Radiolabel in excreta and tissues of rats given
[3,4-14C] N-acetylglufosinate orally at 3 or 1000 mg/kg bw
Sample Radiolabel in excreta (% of dose) or tissues (µg/g)
1000 mg/kg bwa 3 mg/kg bwb
Males Females Males Females
Urine
24 h 5 4 5 8
48 h 7 6 5 9
Faeces
24 h 58 63 97 93
48 h 82 83 100 96
Liver, 6 h 17 46 NA NA
Kidney, 6 h 44 672c NA NA
Brain, 6 h 1 1 NA NA
Plasma, 6 h 3 10 NA NA
NA, not analysed
a Results from Lauck-Birkel (1995b)
b Results from Kellner et al. (1993); Stumpf (1993b)
c Possible outlier
Groups of three Wistar rats of each sex were given
[3,4-14C] N-acetylglufosinate (purity, 98%; 1400 MBq/g) at 3 mg/kg
bw in saline, and blood samples were taken from the retro-orbital
plexus at 15, 30, and 60 min and 2, 4, 6, 8, 24, 48, 72, and 96 h. The
samples were absorbed onto filter paper and combusted, and radiolabel
was determined by liquid scintillation counting. The peak
concentration (0.05 µg/g) was seen at 60 min, although significant
amounts were detected at 15 min, showing rapid initial absorption. The
concentration in blood declined in a biphasic manner, in an initial
phase with a half-time of 0.8 h and a second phase with a half-time of
7 h. The concentration was at the limit of detection by 24 h. The
integrated area under the curve of concentration-time was ~ 0.2 µg
h/g. Comparison with the results of an identical study in which the
substance was administered intravenously showed that absorption after
oral administration represented approximately 5% of the dose over
24 h. There was no significant difference between the sexes (Kellner &
Braun, 1993a,b).
A lactating goat weighing 36 kg was dosed orally twice a day for
3 consecutive days with capsules containing
[3,4-14C] N-acetylglufosinate, equivalent to a dose of 3.0 mg/kg bw
per day. The feed intake was 1.4 kg/day. The animal was milked twice
daily and was slaughtered 16 h after the final dose. Most of the
administered radiolabel was excreted in the faeces (68%), with 7.3% in
urine and 19% in the gastronintestinal tract and its contents. Only
0.2% of the administered dose was found in the tissues and blood and
< 0.1% in milk. The concentrations in kidney were higher than in
other tissues. Those in milk reached a plateau by day 2 (Huang &
Smith, 1995c).
Six laying hens weighing 1.3-1.6 kg were dosed orally twice a day
for 14 consecutive days with capsules containing
[3,4-14C] N-acetylglufosinate, equivalent to a dose of 2.2 mg/kg bw
per day. The mean feed intake was 120 g/day. Eggs were collected twice
daily, and the birds were slaughtered 15 h after the final dose. Most
of the administered dose was excreted (86%), with 1.0% remaining in
the gastrointestinal tract; < 0.1% of the administered dose was
present in edible tissues and blood. The concentrations of radiolabel
associated with N-acetyl-L-glufosinate disodium salt were 0.076
mg/kg in liver, 0.013 mg/kg in muscle, and 0.011 mg/kg in fat; that in
egg white was only slightly above the level of quantification
(< 0.009 mg/kg) throughout the study, reaching a peak of 0.014 mg/kg.
The concentrations in egg yolk increased slowly throughout the 14
days, with a peak at necropsy of 0.056 mg/kg (Huang & Smith, 1995d).
Groups of three Wistar rats received an intravenous injection of
[3,4-14C] N-acetylglufosinate (purity, 98%; 1400 MBq/g) into the
tail vein at a dose of 3 mg/kg bw as a solution in saline. Blood
samples were taken from the retro-orbital plexus at 5, 15, 30, and 60
min and 2, 4, 6, 8, 24, 48, 72, and 96 h. The samples were absorbed
onto filter paper and combusted, and the radiolabel was determined by
liquid scintillation counting. The peak concentration (6-7.5 µg/g) was
seen at 5 min, with an initial decline of 4 h, a half-time of 0.3 h,
and a second phase with a half-time of 14 h. The concentration of
radiolabel in blood was at the limit of detection at 24 h. The
integrated area under the curve of concentration-time was ~ 3.8 µg
h/g. There as no significant difference between the sexes (Kellner &
Braun, 1993a,b).
The toxicokinetics of N-acetylglufosinate was investigated in
groups of five Wistar rats of each sex which received
[3,4-14C] N-acetylglufosinate (purity, 98%; 1400 MBq/g) in saline
intravenously at a dose of 3 mg/kg bw. Urine and faeces were collected
over 0-4, 4-8, 8-24, 24-48, 48-72, and 72-96 h. Tissue samples were
taken at 96 h. Autoradiography was performed on one male killed at 96
h. The radiolabel in tissues and excreta was determined by liquid
scintillation counting after appropriate processing. Excretion was
rapid, with > 85% of the radiolabel appearing in the 0-4-h urine
sample. By 96 h, approximately 95% of the dose had been excreted in
urine; faecal excretion accounted for 4% in females and 2% in males.
The excretory half-times were slightly longer in males than in
females. By 96 h, tissue radiolabel accounted for < 0.3% of the dose;
the highest concentrations were found in kidney, with 0.2 µg/g in
males and 0.07 µg/g in females (Kellner et al., 1993)
Biotransformation: Urine and faeces from Wistar rats given
[3,4-14C] N-acetylglufosinate at 3 or 1000 mg/kg bw by gavage in
the studies of Stumpf (1993b) and Lauck-Birkel (1995b), described
above, were extracted and analysed for metabolites by HPLC or
thin-layer chromatography with comparison to standards. The tissue
samples contained insufficient radiolabel for investigation of
metabolites. The extent of metabolism was greater at 3 mg/kg bw,
indicating the presence of a saturable reaction pathway. The main
compound in urine was N-acetylglufosinate, with low concentrations
of 3-[hydroxy(methyl) phosphinoyl]propionic acid and
4-methylphosphinico-butanoic acid. The faeces of animals given the low
dose contained a significant amount of glufosinate-ammonium, which was
not seen in those given the high dose. The study of Kellner et al.
(1993) showed that glufosinate-ammonium is formed in the intestine,
but the extent to which it is systemically available is not clear. The
results for male rats are presented in Table 10; female animals showed
a similar profile.
Table 10. N-Acetylglufosinate and metabolites in 96-h samples from male Wistar
rats given [3,4-14C]-labelled compound at 3 or 1000 mg/kg bw by gavage
Compound Percent administered dose
3 mg/kg bwa 1000 mg/kg bwb
Urine Faeces Urine Faeces
Total radiolabel 5.3 83 7.6 89
N-Acetylglufosinate 4.0 70 7.4 85
Glufodinate ammonium < LD 11 < LD 0.9
3-Methylphosphinicopropionic acid 0.7 0.6 0.1 0.4
4-Methylphosphinicobutanoic acid or 0.6 1.2 0.1 0.1
hydroxy-4-methylphosphinicobutanoic acid
a From Stumpf (1993b)
b From Lauck-Birkel (1995b)
[3,4-14C] N-Acetylglufosinate (98% radiochemical purity;
specific activity, 830 MBq/g) was dissolved in physiological saline
and administered to groups of five male Wistar rats at a dose of 30
mg/kg bw by gavage. Groups of animals were sacrificed 1, 6, or 24 h
after dosing, and samples of blood (for plasma), brain, kidney, and
liver were pooled, processed, and assayed for total radioactivity
(liquid scintillation counting) and metabolites (HPLC). Urine and
faeces were collected over 24 h. Metabolites were not determined in
plasma or brain owing to insufficient total radiolabel. There was
limited metabolism and rapid excretion; > 80% of the recovered
radiolabel in the faeces was N-acetylglufosinate. The concentration
of glufosinate-ammonium in kidney increased with time (Table 11;
Lauck-Birkel & Strunk, 1999d). A similar pattern of absorption,
distribution, and excretion was reported by Maas & Braun (1999c).
Two male Wistar rats received [3,4-14C] N-acetylglufosinate
(purity, > 98%) by gavage in saline at a dose of 3 mg/kg bw. The
animals were killed 4 or 24 h later, and the gastrointestinal tract
was examined for total radiolabel and metabolites. Significant
deacetylation of N-acetylglufosinate was found in the intestine,
giving rise to glufosinate-ammonium (Table 12; Kellner et al., 1993).
[3,4-14C] N-Acetylglufosinate (98% radiochemical purity;
specific activity, 7200 MBq/g) was dissolved in physiological saline
and administered intravenously into the tail vein of groups of five
male Wistar rats at a dose of 3 mg/kg bw. Groups of animals were
sacrificed 2 or 24 h after dosing, and samples of blood (for plasma),
brain, kidney, and liver were pooled, processed, and assayed for total
radiolabel (liquid scintillation counting) and metabolites (HPLC).
Urine and faeces were obtained over 24 h. Metabolites were not
determined in plasma or brain owing to insufficient total radiolabel.
There was limited metabolism and rapid excretion, and over 95% of the
radiolabel recovered in urine was N-acetylglufosinate. At 24 h,
glufosinate-ammonium was present at higher concentrations than
N-acetylglufosinate in kidney (Table 13; Lauck-Birkel & Strunk,
1999c). A similar pattern of distribution and excretion was reported
by Maas & Braun (1999d).
Samples from the study of Huang & Smith (1995c) on goats were
investigated for metabolites. N-Acetylglufosinate and glufosinate
accounted for 52% and 34% of the radiolabel in faeces. respectively.
Glufosinate was the main residue in kidney, liver, and milk, although
N-acetylglufosinate (the administered material) and
3-[hydroxy(methyl) phosphinoyl]propionic acid formed a substantial
proportion of the residue in kidney and liver. The concentration of
glufosinate-ammonium in the kidney (0.7 ppm) was four times that in
the liver (0.095 ppm). This study showed that de-acetylation of
N-acetylglufosinate to glufosinate-ammonium makes a significant
contribution to tissue residues.
Samples from the study of Huang & Smith (1995d) on hens were
investigated for metabolites. N-Acetyl-L-glufosinate disodium salt
comprised 73% of the radiolabel in faeces, with glufosinate and
3-[hydroxy(methyl) phosphinoyl]propionic acid comprising 13% and 8.6%,
respectively. N-Acetylglufosinate (the administered material) was
the main residue identified in liver and egg yolk, and glufosinate and
3-[hydroxy(methyl) phosphinoyl]propionic acid were also substantial
components of the liver residue. Glufosinate was the main residue in
egg white. This study showed that deacetylation of
N-acetylglufosinate to glufosinate-ammonium makes a significant
contribution to tissue residues.
Table 11. N-Acetylglufosinate and metabolites in pooled samples from male Wistar rats given
[3,4-14C]-labelled compound at 30 mg/kg bw by gavage
Compound % of administered dose µg/g equivalent
Urine Faeces Kidney Liver
0-24 h 0-24 h
6 h 24 h 6 h 24 h
Total radiolabel 2.1 88 1.1 0.7 0.5 0.2
N-Acetylglufosinate 1.7 82 0.6 0.04 0.08 0.05
Glufosinate ammonium 0.02 5 0.08 0.73 < LD < LD
3-Methylphosphinicopropionic acid 0.15 < LD 0.19 0.03 0.3 0.02
4-Methylphosphinicobutanoic acid (1% in dose) 0.2 1.4 0.09 < LD 0.03 0.01
LD, limit of determination, < 0.001% of the administered dose
Table 12. Residues in stomach and intestine after oral administration
of 3 mg/kg bw [3,4-14C] N-acetylglufosinate to two male rats
Compound Percent of radiolabel
Stomach Intestine
4 h 24 h 4 h 24 h
Total radiolabel 3.6 91 < 0.01 3.5
Glufosinate ammonium 0.0 2.6 ND 29
4-Methylphosphinicobutanoic acid 0.0 0.8 ND 0.0
3-Methylphosphinicopropionic acid 0.0 0.5 ND 4.5
N-Acetylglufosinate 99.8 96 ND 66
ND, not determined
Effects on enzymes and other biochemical parameters: The main
biological property of glufosinate-ammonium is inhibition of the
enzyme glutamine synthetase, and toxicological studies with
N-acetylglufosinate have also shown inhibition of this enzyme in a
range of tissues. The interpretation of these results is confounded by
the presence of glufosinate-ammonium in the samples of
N-acetylglufosinate tested. In an attempt to determine the degree to
which N-acetylglufosinate inhibits glutamine synthetase, comparative
studies were performed in vitro and in vivo (Lutkemeier, 1999;
Schmid et al., 1999). The studies are summarized in Tables 6 and 7.
In vitro, N-acetylglufosinate produced only marginal inhibition at
13 mmol/L, some of which can be attributed directly to the
glufosinate-ammonium in the sample. When glufosinate-ammonium is
administered orally, it is approximately 10 times more potent as an
inhibitor of glutamine synthetase than is N-acetylglufosinate. The
amount of this inhibition that is due directly to de-acetylation of
N-acetylglufosinate to glufosinate-ammonium is uncertain; however,
the work of Lauck-Birkel & Strunk (1999a,b; Table 11) and the results
in vitro (Table 6) indicate that most of the inhibition in kidney is
due to biotransformation of N-acetylglufosinate to
glufosinate-ammonium.
Acute toxicity: N-Acetylglufosinate has little toxicity when
given as a single oral dose (Table 14). When it was given by
intraperitoneal injection, deaths occurred at the lowest doses tested
(580 mg/kg bw) in both rats and mice, but there was no clear
dose-response relationship in mice. The compound was not tested by
other routes, as it is formed as a metabolite only in plants, and
exposure through the skin or by inhalation is unlikely. The clinical
signs of toxicity seen after oral exposure to N-acetylglufosinate
were reduced respiratory rate, reduced activity, contracted flanks,
and squatting during the first 24 h. The decreased respiratory rate
Table 13. N-Acetylglufosinate and metabolites in pooled samples from male Wistar rats given
[3,4-14C]-labelled compound at 3 mg/kg bw intravenously
Compound Percent of administered dose
Urine Faeces Liver Kidney
(24 h) (24 h)
2 h 24 h 2 h 24 h
Total radiolabel 86 1.8 0.5 0.1 0.9 0.1
N-Acetylglufosinate 85 1.7 0.4 0.1 0.8 0.01
Glufosinate ammonium < LD 0.1 0.01 0.01 0.05 0.06
3-Methylphosphinicopropionic acid < LD < LD 0.04 0.01 < LD 0.001
4-Methylphosphinicobutanoic acid (1% in dose) 1.1 0.02 0.01 < LD 0.01 < LD
LD, limit of determination, < 0.001% of the administered dose
Table 14. Acute toxicity of N-acetylglufosinate (purity, 79.4%; containing
4.5% glufosinate ammonium)
Species (strain) Route LD50 Reference
(mg/kg bw)
Rat (Wistar) Oral, gavage 290 Schollmeier & Leist (1989a)
Mouse (NMRI) Oral, gavage 290 Schollmeier & Leist (1989b)
Rat (Wistar) Intraperitoneal > 1200 Schollmeier & Leist (1989c)
Mouse (NMRI) Intraperitoneal > 2000 Schollmeier & Leist (1989d)
persisted for the duration of the study in mice. Gross examination
showed no abnormalities.
In a Magnusson and Kligman maximization protocol, groups of 20
female Pirbright guinea-pigs, 10 weeks of age, received an intradermal
induction with N-acetylglufosinate (purity, 79.4%; supplied as a
57.9% solution) at 5% in saline (equal to 2.9% N-acetylglufosinate)
and a 1:1 preparation of Freund's adjuvant. For topical induction and
challenge, undiluted test material was applied under an occlusive
dressing. There were no signs of erythema or oedema. Satisfactory,
contemporary data for positive controls were presented (Hofmann &
Jung, 1988). N-Acetylglufosinate was not a skin sensitizer in this
study (Schollmeier & Leist, 1989e).
Short-term studies of toxicity: Groups of five NMRI mice of
each sex were given diets containing N-acetylglufosinate (purity,
79.4%; 4.5% glufosinate-ammonium) at concentrations of 0, 120, 580,
2900, or 5800 ppm for 28 days. The content of N-acetylglufosinate in
the diets was routinely below the nominal value, sometimes by as much
as 30%. The stability and homogeneity of the diets were satisfactory,
providing intakes equivalent to 0, 19, 100, 520, and 1000 mg/kg bw per
day. All animals were examined routinely for a range of observations
and measurements, including basic neurological tests. Samples were
taken for clinical chemical and haematological examinations from all
mice (not fasted) at termination. Limited analysis was performed on
urine samples collected overnight on day 21-22 of the study from
fasted animals. Heart, lung, liver, kidney, spleen, brain, testis, and
ovaries were weighed and examined histologically, as was any tissue
with macroscopic abnormalities. Glutamine synthetase activity was
measured in brain and liver samples that had been cooled rapidly after
removal and kept frozen until assay.
There were no deaths or clinical signs of toxicity and no effects
on body-weight gain or food or water consumption, although the latter
two were very variable. Haematology and clinical chemistry showed no
treatment-related effects, and no substance-related macroscopic or
microscopic changes were seen. Glutamine synthetase activity in liver
and brain was significantly inhibited in animals of each sex at
5800 ppm, with significant inhibition in brain samples from females
and liver samples from males receiving 2900 ppm (Table 15). The NOAEL
was 580 ppm, equivalent to 100 mg/kg bw per day, on the basis of the
statistically significant, > 10% decrease in glutamine synthetase
activity in brains of females receiving 2900 ppm (Ebert, 1991a).
Table 15. Mean glutamine synthetase activity in liver and brain
from mice receiving N-acetylglufosinate in the diet for
28 days
Dose (ppm) Glutamine synthetase activity (nmol/s per mg protein)
Male Female
Liver Brain Liver Brain
0 0.43 1.2 0.38 1.5
120 0.38 1.4 0.5 1.4
580 0.38 1.5 0.4 1.3
2900 0.34* 1.1 0.42 0.92*
5800 0.24* 0.71* 0.22* 0.89*
* Statistically significant at p < 0.05
Groups of 20 NMRI mice of each sex received diets containing
N-acetylglufosinate (purity, 74.7; 0.5% glufosinate-ammonium) at
concentrations of 0, 500, 2000, or 8000 ppm for 13 weeks. The content
of test substance and the homogeneity and stability of the diet were
satisfactory. The achieved intakes were 0, 82, 320, and 1300 mg/kg bw
per day for males and 0, 110, 440, and 1700 mg/kg bw per day for
females at the control, low, intermediate, and high doses,
respectively. The animals were observed routinely for deaths, general
condition, clinical signs, behaviour, food consumption, and body
weight. Blood samples were taken from groups of 10 fasting mice per
sex per group at the end of the study for haematological and clinical
chemical investigations. After sacrifice, all animals were examined
macroscopically, and 10 organs were weighed and more than 30 tissues
from control and high-dose animals were examined histopathologically.
Limited histological examinations were performed on nine tissues from
animals at the low and intermediate doses. Liver, kidney, and brain
samples (pooled samples from two animals for the last two tissues)
were rapidly placed in liquid nitrogen before assay for glutamine
synthetase activity.
One male at 8000 ppm died after blood sampling. There were no
treatment-related effects on food consumption, body weight, clinical
signs, organ weights, macroscopic or macroscopic appearance, or
haematological parameters. The activity of serum lactate dehydrogenase
was increased by 180% over controls in males at the two higher doses,
but the results were within the normal range. Apparent increases in
the activities of a number of serum enzyme in females at the high dose
were due to a high value in a single animal and are considered to be
unrelated to treatment. The main finding was a dose-related decrease
in glutamine synthetase activity in liver, kidney, and brain (Table
16). Inhibition of liver and kidney glutamine synthetase activity in
isolation is not relevant to human risk assessment; however, there was
> 10% inhibition of glutamine synthetase activity in brain at doses
> 2000 ppm, and the mean values for animals of each sex were
outside the control range. The NOAEL was 500 ppm, equal to 82 mg/kg bw
per day (Tennekes et al., 1992a).
Table 16. Glutamine synthetase activity in groups of 10 mice receiving
N-acetylglufosinate in the diet for 90 days
Sex Dose (ppm) Glutamine synthetase activitya
Kidney Liver Brain
Mean Range Mean Range Mean Range
Male 0 1.4 1.2-1.7 4.0 3.2-4.8 3.6 3.4-3.8
500 1.0* 0.7-1.2 3.7 2.7-4.4 3.3* 3.1-3.6
2000 0.83* 0.6-1.1 3.3* 2.5-4.6 3.2* 2.9-3.4
8000 0.71* 0.4-0.9 2.9* 2.4-4.2 2.6* 2.4-2.9
Females 0 1.7 1.5-2.1 5.0 3.9-5.5 3.4 2.9-3.6
500 1.3* 1.1-1.5 4.9 4.2-5.4 3.3 3.0-3.5
2000 1.2* 0.9-1.4 4.6 3.8-5.3 2.9* 2.7-3.2
8000 1.1* 0.7-1.3 4.0* 3.2-5.5 2.2* 1.9-2.5
* p < 0.01
a µmol gamma-glutamyl hydroxamate formed per ml reaction mixture in 20 min at 37°C
Groups of five Wistar rats received diets containing
N-acetylglufosinate (purity, 79.4%; 4.5% glufosinate-ammonium) at
concentrations of 0, 120, 580, 2900, or 5800 ppm for 28 days. The
overall content, stability, and homogeneity of the test diets were
stated to be acceptable. The achieved intakes (assuming 100% analysis)
were 0, 12, 59, 310, and 590 mg/kg bw per day for males and 0, 11, 55,
280, and 560 mg/kg bw per day for females. All animals were examined
routinely for a range of observations and measurements, and control
and high-dose animals underwent a basic functional observation
battery. Samples were taken for extensive clinical chemical and
haematological examinations from all rats (not fasted) at the end of
the study. Limited analysis was performed on urine samples collected
overnight on day 21-22 of the study from fasted animals. Heart, lung,
liver, kidney, spleen, brain, testis, ovaries, and adrenal, pituitary,
and thyroid glands were weighed and examined histologically, as was
any tissue with macroscopic abnormalities. Glutamine synthetase
activity was measured in brain and liver samples that had been cooled
rapidly after removal and kept frozen until assay.
There were no deaths or clinical signs of toxicity and no effects
on body-weight gain or food or water consumption. 'Thrombin time' was
mildly increased (< 13%) in females at doses > 2900 ppm and in
males at 120, 580, and 5800 ppm; other measures of coagulation were
unaltered. A statistically significant decrease in lactate
dehydrogenase activity was found in females at doses > 2900 ppm,
which may have been related to treatment as there was evidence of a
dose-response relationship. Slight decreases in the absolute and
relative weights of the heart in males at 5800 ppm was of no clear
toxicological significance in the absence of a histological correlate.
No treatment-related macroscopic or microscopic changes were seen.
Glutamine synthetase activity in the liver was significantly inhibited
at doses > 580 ppm in animals of each sex, and was reduced in the
brains of all treated animals, although there was no clear
dose-response relationship (Table 17). Decreased activity of glutamine
synthetase in liver was considered not to be adverse, and the
alterations in glutamine synthetase activity in brain were not
consistent. There were no biologically significant changes in any
other parameter. The NOAEL was 5800 ppm, equal to 560 mg/kg bw per
day, the highest dose tested (Ebert, 1991b).
Table 17. Mean glutamine synthetase activity in liver and brain
samples from rats receiving N-acetylglufosinate in the
diet for 28 days
Dose (ppm) Glutamine synthetase activity (nmol/s per mg protein)
Male Female
Liver Brain Liver Brain
0 0.27 1.5 0.31 1.3
120 0.28 1.1* 0.33 1.1
580 0.17* 0.9* 0.27 1.1*
2900 0.17 1.0* 0.23 1.0*
5800 0.14* 1.3 0.15* 1.1*
* Statistically significant at p < 0.05
Groups of 20 Wistar rats of each sex received diets containing
N-acetylglufosinate (purity, 74.7%; 0.5% glufosinate-ammonium) at
concentrations of 0, 2000, or 10 000 ppm for 13 weeks; a group of 10
animals of each sex received 400 ppm. The content, homogeneity, and
stability of the test compound in the diet were satisfactory, and the
achieved intakes were 0, 29, 150, and 740 mg/kg bw per day for males
and 0, 31, 160, and 800 mg/kg bw per day for females. At 13 weeks, 10
males and 10 females in each group were killed, and the remainder were
allowed to recover for 4 weeks. The animals were observed regularly
for deaths, clinical signs, body weight, food consumption, and tissue
masses. Ophthalmoscopic examinations were performed before treatment
and at 11 and 16 weeks. Blood and urine samples were taken from fasted
animals at weeks 13 and 17 for clinical chemical and haematological
investigations. At necropsy, 10 organs were weighed and examined
macroscopically. An extensive range of tissues from control and
high-dose animals was examined histologically, as were major organs
from animals at the intermediate and low doses. Glutamine synthetase
activity was measured in brain, kidney, and liver samples that had
been rapidly cooled after removal and kept frozen until assay.
There were no deaths, clinical signs of toxicity, or effects on
body weight, the eyes, or urine. Food consumption was reduced during
the first week of treatment but not subsequently. A number of clinical
chemical parameters showed variations, but these were generally due to
values for individual animals or were within normal ranges. A decrease
in serum sodium concentration in animals at the high dose at 13 weeks
appeared to be related to treatment. Glutamine synthetase activity was
inhibited in liver, kidney, and brain (Table 18), and the effect was
significantly but not completely reversed after 4 weeks. A reversible
increase in kidney weight (< 15%) was seen in males at all doses, but
this finding was considered not to be adverse because there was no
clear dose-response relationship and no associated histological
change. There were no treatment-related macroscopic or microscopic
findings. The NOAEL was 2000 ppm, equal to 150 mg/kg bw per day, on
the basis of inhibition of glutamine synthetase activity in the brain
(Tennekes et al., 1992b).
Groups of four beagle dogs of each sex, aged 5-7 months, received
diets containing N-acetylglufosinate (purity, 74.7%; 0.5%
glufosinate-ammonium) at concentrations of 0, 500, 2000, or 8000 ppm
in 400 g of diet daily for 13 weeks. Additional groups of two animals
of each sex received the same diets and were then allowed to recover
for 4 weeks. The content, homogeneity, and stability of the test diet
were satisfactory. The achieved intakes were 0, 19, 72, and 290 mg/kg
bw per day for males and 0, 21, 79, and 300 mg/kg bw per day for
females. The animals were observed routinely for deaths, general
condition, clinical signs, behaviour, food consumption, and body
weight. Ophthalmoscopic examinations were performed on all animals
before treatment, at weeks 4 and 13, and at termination in the group
allowed to recover. Blood and urine samples were collected from fasted
animals before treatment, at weeks 4 and 13, and at the end of the
recovery period. At termination, all dogs were examined
macroscopically; a range of tissues were weighed, and > 30 tissues
Table 18. Glutamine synthetase activity in groups of 10 rats receiving N-acetylglufosinate in
the diet for 90 days and after a 4-week recovery period
Sex Period Dose Glutamine synthetase activitya
(days) (ppm)
Liver Kidney Brain
Mean Range Mean Range Mean Range
Male 90 0 3.8 3.3-4.3 2.1 1.6-2.3 3.2 3.1-3.3
400 2.8* 2.0-3.4 1.7* 1.5-1.9 3.3 3.1-3.6
2000 2.2* 1.9-2.4 1.5* 1.3-1.8 3.0 2.9-3.3
10 000 1.8* 1.3-2.2 1.6* 1.4-1.9 2.8* 2.6-3.1
90 + recovery 0 3.2 2.5-3.8 2.1 1.9-2.3 3.1 2.9-3.4
2000 3.5 3.2-3.9 2.1 1.6-2.4 3.0 2.6-3.4
10 000 3.4 2.9-4.1 1.9 1.2-2.5 2.9* 2.7-3.1
Female 90 0 3.7 2.8-4.3 1.2 1.0-1.3 3.1 2.8-3.3
400 3.1 2.2-3.5 1.1 1.0-1.2 3.1 2.9-3.2
2000 2.6* 1.8-3.2 1.2 1.1-1.5 3.0 2.9-3.2
10 000 2.4* 1.9-2.8 1.5* 1.4-1.7 2.8* 2.6-2.9
90 + recovery 0 3.7 3.2-4.1 1.3 1.2-1.4 3.1 3.0-3.3
2000 3.5 3.1-4.0 1.3 1.1-1.5 3.1 2.9-3.4
10 000 3.3 2.6-3.9 1.3 1-1.5 2.9 2.7-3.1
* p < 0.01
a µmol gamma-glutamyl hydroxamate formed per ml reaction mixture in 20 min at 37°C
were preserved and examined histopathologically. Liver, kidney, and
brain samples were immediately placed in liquid nitrogen and kept
frozen at -80°C before assay for glutamine synthetase activity;
samples of these tissues were also retained for future analysis.
There were no deaths or treatment-related clinical signs or
effects on food consumption, body weight, or ophthalmoscopic or
haematological parameters. A statistically significant, dose-dependent
decrease in glutamine synthetase activity was found in liver and brain
after 13 weeks of treatment (Table 19), which tended to return to
normal during the recovery period although it was not complete at the
end of the 4 weeks. The changes in glutamine synthetase activity were
not consistent in different tissues from the same animal. Activity in
the brain stem and cerebellum appear to be more sensitive to
inhibition than that in the cortex. Decreased creatine kinase activity
was seen at the high dose in males at 13 weeks (17%) and in females at
weeks 4 and 13 (30%). Lactate dehydrogenase activity was decreased by
30% in females at this dose at week 13 but in neither males nor
females after recovery. Exacerbation of the low pretreatment specific
gravity and osmolality of the urine of females was seen at at 8000 ppm
in weeks 4 and 13 of treatment and at the end of the recovery period.
The significance of this finding is unclear as it was not seen in a
1-year study in dogs. It is of note, however, that the kidney is a
target organ in rats.
A dose-related decrease in prostate gland weight was seen which
achieved statistical significance at 8000 ppm (40%) at 13 weeks, but
there was no histological correlate. The prostate weights were similar
to those of controls after 4 weeks' recovery from the dose of
2000 ppm, but not after administration of 8000 ppm. Although the
authors noted that slight differences in the rate of maturity of dogs
of this age can affect prostate size, the dose-response relationship
and evidence of recovery at 2000 ppm but not at 8000 ppm indicate an
association with treatment. No treatment-related effects were seen on
macroscopic examination. The only histopathological finding of note
was an increased incidence of pituitary cysts in animals receiving
8000 ppm for 13 weeks: 3/4 in animals of each sex and 1/4 in male
controls and 0/4 in female controls. The NOAEL was 500 ppm, equal to
19 mg/kg bw per day, on the basis of > 10% reductions in glutamine
synthetase activity in a number of areas of the brain. The effects on
prostate weight, urinary parameters, and the pituitary indicate that
8000 ppm was an effect level (Corney et al., 1992)
Groups of six beagle dogs of each sex received diets containing
N-acetylglufosinate (purity, 92.4%; 0.1% glufosinate-ammonium) at
concentrations of 0, 100, 1000, or 8000 ppm. Analyses of the diets
for homogeneity and content were satisfactory, and the overall intakes
of N-acetylglufosinate were 0, 4, 44, and 320 mg/kg bw per day for
males and 0, 4.4, 43, and 350 mg/kg bw per day for females. Two
animals of each sex per group were killed at 26 weeks and the
remainder at 52 weeks. The animals were observed routinely for
clinical signs, deaths, body weight, and food consumption.
Table 19. Glutamine synthetase activity in dogs receiving N-acetylglufosinate in the diet for 90 days with or without a 4-week recovery
period
Sex Period No. Dose Glutamine synthetase activitya
(ppm)
Liver Kidney Mid-brain Cerebellum Brain stem Brain cortex
Mean Range Mean Range Mean Range Mean Range Mean Range Mean Range
Male 13 weeks 4 0 2.7 2.6-2.9 0.02 0.01-0.04 2.3 1.8-2.7 1.6 1.5-1.8 1.4 1.2-1.5 3.2 2.8-3.5
500 1.9 1.4-2.2 0.04 0.02-0.07 2.6 1.9-2.9 1.4 1.2-1.5 1.3 1.2-1.4 3.4 3.3-3.4
2000 1.2 1.0-1.3 0.06 0.03-0.10 2.6 1.8-2.9 1.3 1.0-1.4 0.90 0.8-1.1 2.8 2.3-2.9
8000 0.58 0.5-0.7 0.05 0.01-0.14 1.4 1.7-2.6 0.85 0.7-1.0 0.47 0.4-0.5 2.3 1.8-2.8
Recovery 2 0 2.6 0.07 2.1 1.4 1.3 3.0
2000 2.4 0.05 2.3 1.2 0.95 2.4
8000 2.0 0.06 2.1 1.2 0.93 2.5
Females 13 weeks 4 0 1.8 1.6-2.1 0.04 0.02-0.06 2.7 2.2-3.0 1.6 1.5-1.7 1.2 1.1-1.3 2.9 2.7-3.3
500 1.6 1.2-2.4 0.10 0.05-0.17 2.2 1.9-2.5 1.5 1.4-1.5 1.2 1.0-1.3 3.2 2.9-3.3
2000 1.0 0.8-1.5 0.06 0.03-0.09 2.4 1.8-2.9 1.3 1.2-1.4 0.91 0.7-1.0 2.7 2.4-3.3
8000 0.68 0.5-0.9 0.09 0.01-0.15 2.0 1.7-2.6 0.87 0.8-0.9 0.73 0.6-0.8 2.5 1.9-2.8
Recovery 2 0 2.1 0.05 2.1 1.5 1.4 3.1
2000 1.7 0.08 2.1 1.3 1.2 2.7
8000 1.3 0.07 1.8 1.1 0.83 2.4
a µmol gamma-glutamyl hydroxamate formed per ml reaction mixture in 20 min at 37°C
Ophthalmoscopic examinations were performed before treatment and at
weeks 12, 25, and 51. Samples were taken from fasted animals for
urinary analysis, haematology, and clinical chemistry before treatment
and at weeks 13, 26, and 52. Post-mortem examinations were performed
on all animals; nine organs were weighed and > 30 tissues examined
histopathologically. Glutamine synthetase activity was not analysed.
The only death occurred in a male at the intermediate dose; the
cause was not found. The incidence of soft faeces was increased in
animals at the high dose and particularly in males. Reduced
body-weight gain was seen in animals at this dose, by 17% in males and
30% in females, during the first half of the study, and the effect
persisted in females until termination. There was no evidence of
treatment-related effects in ophthalmoscopic, haematological, or
urinary examinations and no effects on organ weights or gross or
histopathological appearance at either the interim or terminal
sacrifice. Reduced serum lactate dehydrogenase activity was seen
consistently in animals at the high dose (Table 20). Although there
was some overlap between the ranges for control and treated animals
and variation in control values over time, the consistency of the
effect in males indicates that it is possibly related to treatment.
The effects at 8000 ppm are not significant in isolation, but the
combination of findings in males indicates that this dose is an effect
level. The NOAEL was 1000 ppm, equal to 44 mg/kg bw per day, on the
basis of reduced body-weight gain, reduced lactate dehydrogenase
activity, and an increased incidence of soft faeces (Bernier, 1996).
Table 20. Lactate dehydrogenase activity in dogs fed diets containing
N-acetylglufosinate at 8000 ppm
Group No. Week Serum lactate dehydrogenase activity (U/L)
Males Females
Mean Range Mean Range
Controls 6 0 75 47-100 160 120-230
6 13 100 66-160 160 52-260
6 26 200 87-430 220 99-470
4 52 90 66-100 320 160-460
Treated 6 0 97 57-140 74 32-130
6 13 53 43-68 44 31-65
6 26 99 39-180 88 48-110
4 52 70 50-88 150 110-180
Long-term studies of toxicity and carcinogenicity: Groups of 90
Swiss Crl:CD-1 mice received diets containing N-acetylglufosinate
(purity, 92.4%; 0.1% glufosinate-ammonium) at concentrations of 100,
1000, or 8000 ppm. Groups of 20 animals were killed after 1 year and
the remainder after 2 years. The mice were housed singly, observed
daily, and monitored routinely for body weight and food consumption.
The homogeneity, content, and stability of the diet were acceptable,
and the achieved intakes were 0, 15, 150, and 1200 mg/kg bw per day
for males and 0, 19 190, and 1500 mg/kg bw per day for females.
Samples for clinical chemistry and haematology were taken from fasted
animals at interim sacrifice (all animals) and at termination (10 per
sex per group). Blood was taken from the tail vein at week 78. At
autopsy, the animals were examined macroscopially, nine organs were
weighed, and more than 30 tissues were preserved. Histological
examinations were conducted on tissues from all controls, animals at
the high dose, and those that died during the study and on the liver,
lung, kidney, and adrenals from animals at the low and intermediate
doses killed after 1 or 2 years.
Although the report stated that no treatment-related signs were
observed, data on individual animals were not presented, as this was
primarily a study of carcinogenicity. Survival was similar in all
groups (> 60% at week 90). Fluctuations in food consumption and body
weight showed no consistent pattern related to dose. There were no
effects on clinical chemical or haematological parameters or on organ
weights, any variation in group means being attributable to individual
values. Macroscopic examination detected no treatment-related effects.
Histopathological examination showed an increased incidence of
amyloidosis in various organs in males and females at the high dose
and testicular necrosis in males, although the only statistically
significant finding ( p = 0.028) was amyloidosis in the salivary
gland of females (Table 21). A high incidence of kidney lesions was
seen in both control and treated animals at 1 and 2 years, with no
significant differences between groups. Increased incidences of
uterine adenocarcinoma and thyroid follicular-cell adenoma were seen
in animals at the high dose; the incidences were not statistically
significantly increased when compared with concurrent controls but
were slightly greater than those of historical controls. The overall
incidences of benign and malignant tumours were similar in treated and
control groups. As the tumours that occurred at increased incidences
occur spontaneously in this strain of mouse, the findings were
considered not to indicate a carcinogenic potential of
N-acetylglufosinate. The NOAEL was 1000 ppm, equal to 150 mg/kg bw
per day, on the basis of an increased incidence of amyloidosis
(Farrell, 1997; Ernst & Stumpf, 1999a).
Groups of 70 Sprague-Dawley-derived rats of each sex received
diets containing N-acetylglufosinate (purity, 92.4%; 0.1%
glufosinate-ammonium) at concentrations of 0, 200, 2000, or 20 000 ppm
for 2 years; additional groups of 10 animals of each sex were killed
at 1 year and 20 at 2 years. The animals were housed singly, observed
daily, and monitored routinely for body weight and food consumption.
The homogeneity, content, and stability of the test diet were
Table 21. Incidences of lesions in mice receiving N-acetylglufosinate in the diet for 2 years, and ranges for
historical controls
Sex Lesion Incidence
0 100 ppm 1000 ppm 8000 ppm Historical controls
Male Testicular degeneration and necrosis 0/70 1/55 2/56 4/70 6-39% (for atrophy)
Testicular atrophy 25/70 28/55 30/56 26/70 6-39% (for atrophy)
Thyroid follicular-cell adenoma 0/70 0/69 1/69 3/70 0-2%
Female Salivary gland amyloidosis 1/70 1/45 3/43 7/70 No data
Liver haemangiosarcoma 0/70 1/70 1/70 2/70 0-4%
Uterine adenocarcinoma 0/70 2/69 1/69 3/70 0-2%
acceptable, and the achieved intakes were 0, 9, 91, and 1000 mg/kg bw
per day for males and 0, 11, 110, and 1200 mg/kg bw per day for
females. Samples for clinical chemistry and haematology were taken
from fasted rats in the interim sacrifice and satellite groups at 25,
51, 78, and 102 weeks. At necropsy, the animals were examined
macroscopically, nine organs from interim sacrifice animals were
weighed, and > 30 tissues were preserved. Full histopathological
examinations were performed on all controls, animals at the high dose,
and animals that died during the study; only liver, lung, kidney, and
adrenals from animals at the low and intermediate doses were examined
histopathologically.
Survival was similar in all groups (> 50% at week 96). Soft
faeces were more prevalent in animals at the high dose and especially
in males from week 8 onwards. Body-weight gain was reduced (~ 5%) in
males at this dose from week 16 onwards and in females (~10%) from
week 24, even though the food consumption of these groups increased by
5-10%. A range of changes in clinical chemistry and haematology were
seen repeatedly in animals of each sex at the high dose, including
reduced prothrombin time, potassium concentration, and lactate
dehydrogenase and creatine kinase activity, and increased sodium,
calcium, inorganic phosphorus, and glucose concentrations; blood urea
nitrogen was increased in males from week 25 onwards. In animals
killed after 1 year, the absolute and relative weights of the kidney
were increased in males receiving 20 000 ppm and in females receiving
2000 or 20 000 ppm. Macroscopic examination showed increased
incidences of a range of abnormalities of the kidney in treated
animals that were confirmed at histological examination. Renal lesions
are common in aged rats, and the incidences in treated animals in this
study were not statistically significant and were within the range
seen in historical controls; furthermore, they may have been
exacerbated by the high sodium content induced by
N-acetylglufosinate. They were therefore considered irrelevant to
the assessment of the study. Alterations were also seen in the spleen,
parathyroid, heart, blood vessels and adrenals at the high dose and in
some animals at the intermediate dose animals (Table 22). The
incidence of tumours was not statistically significantly increased,
although low incidences of uncommon tumours of the brain, liver,
adrenals, skin, and pancreas were seen in animals at the high dose
(Table 23). Although these incidences are outside the range in
historical controls, the sporadic nature of the findings and the clear
NOAEL for tumours at 2000 ppm indicate that N-acetylglufosinate does
not have significant carcinogenic potential. The overall incidences of
benign and malignant tumours were similar in all groups. The NOAEL was
200 ppm, equal to 9 mg/kg bw per day, on the basis of increased
incidences of polyarteritis nodosa, adrenal cortical hyperplasia, and
necrosis and extramedullary haematopoiesis of the spleen (Bernier,
1997; Ernst & Stumpf, 1999b).
Table 22. Incidences of non-neoplastic lesions in rats receiving N-acetylglufosinate in the diet for up to 2 years,
and ranges for historical controls
Sex Lesion Incidence
0 200 ppm 2000 ppm 20 000 ppm Historical
controls
Male Parathyroid hyperplasia 13/62 8/37 16/32 20/64 14-42%
Adrenal focal cortical hyperplasia 17/70 18/70 29/70 27/70 0-20%a
Adrenal necrosis 0/70 0/70 2/70 3/70 0-4%
Polyarteritis nodosa (blood vessels) 4/7 5/7 4/9 11/18 No data
Polyarteritis nodosa (testis; 2 years; main group) 15/70 13/53 13/50 23/70 16-31%
Polyarteritis nodosa (testis; 2 years; satellite group) 3/20 4/16 8/17 10/20 16-31%
Female Parathyroid hyperplasia 1/52 0/26 0/31 6/61 1-12%
Extramedullary splenic haematopoiesis 7/70 4/34 16/41 20/70 15-36%
Cardiomyopathy 12/70 5/30 6/41 22/70 1-70%
a Current study, all > 24%
Table 23. Incidences of neoplastic lesions in groups of 70 rats receiving N-acetylglufosinate in the diet
for 2 years, and ranges for historical controls
Sex Lesion Incidence
0 200 ppm 2000 ppm 20 000 ppm Historical controls
Male Adrenals: malignant phaeochromocytoma 0 1 1 3 0-3%
Brain: meningioma 0 0 0 1 0-1%
Brain: astrocytoma 0 1 0 1 0-2% (malignant
glioma)
Skin: keratocanthoma 4 5 1 9 (7)* 0-8%
Female Brain: oligodendroglioma 0 0 0 1 No data
Liver: cholangiocarcinoma 0 0 0 1 No data
Lung: alveolar/bronchiolar carcinoma 0 0 0 1 No data
Pancreas: islet-cell carcinoma 1 2 1 4 0-2%
* Re-evaluation of data indicates that 7 is the correct value.
Genotoxicity: An extensive range of assays for genotoxicity
have been performed with N-acetyl-glufosinate both in vitro and
in vivo (Table 24). Some of the protocols were less than optimal
with respect to length of exposure (e.g. for unscheduled DNA
synthesis) or the highest concentration tested, but these limitations
are considered not to affect the overall assessment of genotoxic
potential significantly. The Committee concluded that
N-acetylglufosinate is not genotoxic.
Reproductive toxicity: In a single-generation, range-finding
study in groups of 10 Sprague-Dawley rats of each sex that received
N-acetylglufosinate (purity, 92.4%; 0.1% glufosinate-ammonium), no
adverse effects were seen on fertility, reproduction, or development
at doses of 200, 2000, or 10 000 ppm (equivalent to 14, 140, or 700
mg/kg bw per day). Exposure was continuous from 21 days before mating
to day 21 post partum (Beyrouty, 1996a).
Groups of 30 Sprague-Dawley-derived rats received diets
containing N-acetylglufosinate (purity, 92.4%; 0.1%
glufosinate-ammonium) at concentrations of 0, 200, 2000, or 10 000 ppm
for two generations. The F0 generation was exposed from 70 days
before mating until the end of lactation, and F1 pups were exposed
from weaning (10-12 weeks before mating) until the end of lactation of
F2 pups. The stability and homogeneity of the diet were
satisfactory, and the mean achieved intakes were 0, 13, 140, and 700
mg/kg bw per day for males and 0, 18, 170, and 890 mg/kg bw per day
for females. Parental animals were observed routinely for clinical
signs, estrus cycling, body weight, and food consumption, and the
frequency of observation was increased during gestation and weaning.
Parturition was observed when possible. Pups were examined for
malformations, sex, viability, and body weight, and the litters were
culled to four pups of each sex on day 4. All parental animals, one
pup of each sex per litter, and any pups that died or were killed when
moribund were examined post mortem. The major organs and
reproductive tissues from all adult animals were examined
macroscopically, and these tissues from control and high-dose animals
were examined histologically. Sperm motility, count, and morphology,
and the number of spermatids were determined for all adult males.
One male at the intermediate dose and two at the high dose died
from unknown causes, and males of both generations at the high dose
had an increased incidence of soft faeces and brown staining of the
scrotum. There were no effects on body weight, although the food
consumption of animals at the high dose was slightly increased during
the first few weeks of treatment. No effects were seen on the weight
or histological appearance of organs from F0 animals or on mating
indices, length of gestation, estrus cycling, sperm parameters,
macroscopic appearance, litter size, pup survival, or pup weight in
either generation. A dose-related increase in the weight of the
seminal vesicles was seen in F1 adults, which reached statistical
significance ( p < 0.05; 14%) in males at the high dose. As there
were no associated abnormal histological findings and no functional
deficit, the effect was considered not to be adverse. An increased
Table 24. Results of studies of the genotoxicity of N-acetylglufosinate
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium TA98, 0, 2, 12, 59, 291, 1455, 79.4 Negative +S9 Muller (1989c)
TA100, TA1535, 2910, 5820 µg/plate Negative - S9
TA1537, TA1538;
E. coli WP2 uvrA
Gene mutation V79 Chinese hamster 0, 582, 873, 1164, 79.4 Negative +S9 Muller (1989a)
lung cells, Hprt locus 1554 µg/ml Negative - S9
Gene mutation V79 Chinese hamster 0, 444, 666, 888, 74.7 Negative +S9 Muller (1991b)
lung cells, Hprt locus 1186 µg/ml Negative - S9
Unscheduled DNA Human cell line A549 0, 1, 4, 13, 44, 133, 74.7 Negative +S9 Muller (1991c)
synthesis 444, 1332 µg/ml Negative - S9
Unscheduled DNA Human cell line A549 0, 0.6, 1.8, 5.8, 17, 58, 79.4 Negative +S9 Muller (1989d)
synthesis 175, 582 µg/ml, 3-h Negative - S9
exposure
Chromosomal Human lymphocytes 0, 0.6, 3.0, 5.0 mg/ml 74.7 Negative +S9 Heidemann &
aberrations at 24 h Voelkner (1992)
0, 5.0 mg/ml at 48 h Negative - S9
Chromosomal V79 Chinese hamster 0, 154, 773, 1546 µg/ml 79.4 Negative +S9 Muller (1989b)
aberrations lung cells for 18 h
0, 1546 µg/ml for 7 or Negative - S9
28 h
Table 24. (continued)
End-point Test object Concentration Purity Results Reference
(%)
In vivo
Micronucleus NMRI mouse bone 0, 222, 1111, 2222 74.7 Negative Muller (1991a)
formation marrow mg/kg bw by gavage for
24, 48 or 72 h
incidence of extramedullary haematopoiesis in the livers of F1 males
(8/30 versus 2/30 in controls) was also considered not to be adverse
as it is a common finding in rats. There were no effects on
reproduction. These minor effects on seminal vesicle weight and liver
extramedullary haematopoiesis in F1 males at 10 000 ppm combined
with the increased incidence of soft stools indicated that this is a
minimal effect level. The NOAEL was 2000 ppm, equal to 140 mg/kg bw
per day (Beyrouty, 1996b).
Developmental toxicity: Twenty mated female Wistar rats
received N-acetylglufosinate (purity, 74.7%) by gavage in distilled
water at the limit dose of 1000 mg/kg bw per day on days 7-16 of
gestation, whereas 21 controls received starch mucilage in distilled
water. The dose was based on the results of a preliminary study. The
dams were observed routinely for clinical signs, body weight, and food
consumption. At sacrifice on day 21, they were examined
macroscopically, and the uterus was opened to determine the numbers of
live and dead fetuses and resorptions, fetal weights, the sex ratio,
crown-rump lengths, and placental weights. All fetuses were examined
for external malformations; half were investigated by Wilson
sectioning for visceral abnormalities and the remainder stained with
Alizarin red S for visualization of skeletal defects.
There were no deaths or treatment-related clinical signs among
the pregnant dams, but a non-pregnant control was killed during the
study. The food consumption and body-weight gain of treated animals
were slightly reduced (~ 5%) on days 7-14 of gestation but were
similar to those of controls after cessation of dosing. There was no
effect on pregnancy rate, but a slight increase in pre- and
post-implantation losses resulted in a marginally reduced litter size
(13.6 in controls versus 12.7 in treated animals); the value was
within the typical range. No malformations were recorded, and there
was no increase in the incidence of skeletal or external
abnormalities. The incidence of blood in the pericardium/abdomen was
increased in the treated group (5/132 fetuses, 4/20 litters) when
compared with concurrent controls (2/140 fetuses) and historical
controls (0-1.5%). Fetal weight, crown-rump length, placental weight,
and sex ratio showed no treatment-related effects. The NOAEL for
maternal and fetotoxicity was 1000 mg/kg bw per day, the only dose
tested (Horstmann & Baeder, 1992).
Groups of 15 mated, 8-10-month-old, female Himalayan rabbits
received N-acetylglufosinate (purity, 92.4%) by gavage in distilled
water on days 6-18 of gestation at doses of 0, 64, 160, or 400 mg/kg
bw per day, on the basis of the results of a study that found maternal
and fetal toxicity at 500 mg/kg bw per day. The animals were housed
under standard conditions and observed daily. Body weights were
determined on days 0, 6, 13, 19, and 29 of gestation, and the dams
were killed on day 29 and examined macroscopically. The uteri were
opened, and the numbers of live and dead fetuses and resorptions were
determined. Live fetuses were removed to an incubator and their
survival was monitored for 24 h. Fetal body weight, crown-rump length,
and sex were determined, and external visceral and skeletal
examinations were performed by Wilson sectioning and Alizarin red S
staining. The combined incidences of some lesions were presented only
in summary tables, but this did not compromise the overall integrity
of the study.
There were no deaths during the study, and no consistent clinical
signs in dams. Abortions by one dam at the low dose and one at the
intermediate dose were considered not to be related to treatment. Food
consumption was reduced in relation to dose, by 12% in the groups at
the low dose, 23% at the intermediate dose, and 37% at the high dose,
but there were no consistent effects on body weight. Treatment had no
effect on corpora lutea, pregnancy rate, implantation, resorptions,
gravid uterine weight, or fetal survival. The pup weights were reduced
by 7% at 400 mg/kg bw per day. Morphological examination of fetuses
revealed an increased incidence of a supernumerary thoracic ribs
(2/90, 0/82, 8/73, and 11/93 in control, low-, intermediate-, and
high-dose groups, respectively, while the range among historical
controls was 0-12%). The only malformation reported was a case of
hydrocephalus in a fetus at the high dose; the incidence in historical
controls was 0-9%. The NOAEL for maternal toxicity and fetotoxicity
was 64 mg/kg bw per day on the basis of reduced food consumption in
dams and extra ribs in fetuses (Baeder & Hofmann, 1994; Ernst & Leist,
1999b).
Special studies on neurotoxicity: No overt neurotoxicity was
seen in basic functional observation batteries of tests with
N-acetylglufosinate included in short-term studies of toxicity in
mice and rats, nor does this substance belong to a class of compounds
with neurotoxic potential.
(ii) 3-[Hydroxy(methyl) phosphinoyl]propionic acid
3-[Hydroxy(methyl) phosphinoyl]propionic acid is a metabolite of
glufosinate-ammonium in rats and represents ~ 30% of the residue in
liver (Table 4). It may also represent a significant proportion of the
residue arising from administration of glufosinate-ammonium to plants,
goats, and hens (Annex 1, reference 83). In 1991, the Meeting
reviewed a 28-day study in rats and a study of the toxicokinetics of
3-[hydroxy(methyl) phosphinoyl]propionic acid, which showed that it is
rapidly and extensively absorbed and excreted, has little toxicity
(NOAEL, 280 mg/kg bw per day), and does not inhibit hepatic glutamine
synthetase activity. The 1998 Joint Meeting considered that
3-[hydroxy(methyl) phosphinoyl]propionic acid should be included in
the residue definition (Annex 1, reference 83).
A summary of various additional studies on 3-[hydroxy(methyl)
phosphinoyl]propionic acid has been submitted (Bremmer & Leist, 1998),
although the original reports were not made available. The summary
indicates that the acute toxicity of the substance is low, it is not a
skin sensitizer, and had no significant toxicity in studies in rats
(13 weeks; NOAEL, 560 mg/kg bw per day), mice (13 weeks; NOAEL, 1400
mg/kg bw per day), or dogs (15 weeks; NOAEL, 110 mg/kg bw per day)
given repeated doses. An extensive range of tests for genotoxicity was
reported to show that 3-[hydroxy(methyl) phosphinoyl]propionic acid
does not induce gene mutation in vitro, chromosomal aberrations
in vitro or in vivo, micronuclei in vivo, or DNA damage
in vitro or in vivo. Studies of developmental toxicity in rats and
rabbits showed evidence of maternal toxicity and mild fetotoxicity but
no teratogenicity, with cited NOAELs of 300 mg/kg bw per day in rats
and 50 mg/kg bw per day in rabbits.
3. Observations in humans
(a) Medical surveillance of personnel in manufacturing plants
No specific health problems were reported in workers during 18
years of production of glufosinate-ammonium. The exposure of the
workers was stated to be low, but no details of the extent of
investigations were available (Kaleja, 1999).
(b) Poisoning incidents
A pesticide formulation containing glufosinate-ammonium was
reported to have been involved in over 200 cases of attempted suicide
in Japan (Ernst & Leist, 1999c). The formulation contained 18% w/w of
glufosinate-ammonium, 30% surfactant, and a glycol ether solvent, and
it was not clear whether the effects seen were attributable to
glufosinate-ammonium. The initial signs were vomiting, diarrhoea, and
nausea. The main clinical concerns were the delayed (hours to 2 days)
neurological effects, including convulsions, impaired consciousness,
tremor, and coma, and extensive oedema. Treatment included gastric
lavage, diuretics, intravenous fluids, sedatives, haemoperfusion, and
artificial ventilation. After apparent recovery from the physical
signs, long-lasting amnesia, both retrograde and anterograde was
reported, although this may have been linked to the use of high-dose
benzodiazepine therapy (Koyama et al., 1994; Watanabe & Sano, 1998;
Ernst & Leist, 1999c).
Comments
Glufosinate-ammonium
Orally administered [14C]glufosinate-ammonium is rapidly but
sparingly (~ 10%) absorbed. Excretion of the absorbed dose was rapid.
The kidney and liver contained the highest concentrations of residue,
which were significantly higher than those in plasma. The
concentrations in brain were lower than those in plasma, indicating
limited penetration of the blood-brain barrier. There were no marked
differences between the sexes. Administration of 500 mg/kg bw resulted
in more prolonged absorption and excretion than with 20 or 2 mg/kg bw.
The metabolism of glufosinate-ammonium was limited (~ 30% of the
absorbed dose), and the main urinary and tissue residues were
3-[hydroxy(methyl)phosphinoyl]propionic acid,
methylphosphinicobutanoic acid, and
2-hydroxy-4-methylphosphinicobutanoic acid. In faeces, significant
concentrations (up to 10%) of N-acetylglufosinate were detected,
indicating that acetylation was performed by the gut microflora.
Metabolites were also found in tissues.
Glutamine synthetase (E.C.6.3.1.2) is a key enzyme involved in
the metabolism of nitrogen and glutamate. Inhibition of glutamine
synthetase, resulting in high levels of ammonia, is the mechanism of
action of glufosinate-ammonium in plants. The activity of glutamine
synthetase varies among tissues and species. The Meeting considered
reports on the relevance of glutamine synthetase activity in the liver
and kidney of experimental animals and humans, including data reviewed
by the 1991 JMPR. Because of the presence of alternative pathways for
the homeostatic control of ammonia, < 50% inhibition of glutamine
synthetase in rat liver was not associated with increased ammonia
concentrations and was not considered to be adverse. Glutamine
synthetase activity in the kidney shows considerable variation between
species, with relatively high activity in rodents and negligible
activity in dogs and humans. Inhibition of kidney glutamine synthetase
in the absence of pathological findings was considered not to be
relevant to human risk assessment.
In the central nervous system, ammonia homeostasis is maintained
by a number of enzymes, including glutamine synthetase and glutamate
dehydrogenase. Under normal conditions, the flux through glutamine
synthetase in brain is reported to be approximately 2-10% of its
theoretical capacity, and for glutamate dehydrogenase it is
approximately 0.1% of its capacity. With such excess capacity,
inhibition of brain glutamine synthetase will not necessarily result
in significant increases in brain ammonia concentrations; this
conclusion is confirmed by data showing that animals with decreased
glutamine synthetase activity do not have increased brain ammonia
levels. However, the 'glutamine-glutamate shunt', between GABA and
glutamate in neurons and glutamine in astrocytes, plays a role in both
excitatory and inhibitory neurotransmission. The results of studies
considered by the 1991 Meeting indicate that significant changes in a
range of biogenic amines in regions of the brain in dogs are
associated with > 8% changes in glutamine synthetase activity after
administration of glufosinate-ammonium at 8 mg/kg bw for 28 days, a
dose that produced 'increased gait activity'. Thus, it has been
proposed that any statistically significant inhibition of glutamine
synthetase activity in brain by > 10% be considered a marker of
potentially adverse effects on brain biochemistry and behaviour.
Studies in vitro and in vivo showed that glufosinate-ammonium
inhibits glutamine synthetase in the brain, kidney, and liver of rats.
With 100 ppm glufosinate-ammonium in the diet (equivalent to 10 mg/kg
bw per day), glutamine synthetase activity was inhibited in liver and
kidney but not in brain, and the Meeting concluded that the NOAEL was
10 mg/kg bw per day. The inhibition in liver and kidney was evident by
day 6, did not increase markedly up to day 90, and showed significant
reversal during a 31-day recovery period. The finding of increased
renal glutamine synthetase activity in a previous long-term study in
rats was considered to be a rebound response to continued inhibition
and to be of no relevance to human risk assessment.
New studies in which rats and mice received repeated, high doses
(270-1400 mg/kg bw per day) in the diet for 90 days were designed to
determine the maximum tolerated doses rather than NOAELs. There was no
evidence of specific toxicity or of irreversible neurobehavioural
effects in the rats. The LOAEL in rats was 7500 ppm, equal to 560
mg/kg bw per day. A new carcinogenicity study in rats showed that
glufosinate-ammonium had no significant carcinogenic potential at
doses up to 10 000 ppm, equal to 470 mg/kg bw per day. A significant
increase in retinal atrophy was seen in this study in females at doses
> 5000 ppm (equal to 280 mg/kg bw per day) and in males at 10 000 ppm
(equal to 470 mg/kg bw per day) but not in either sex at 1000 ppm
(equal to 45 mg/kg bw per day), the NOAEL. The results of these three
studies were consistent with those of previous investigations and
supported the overall NOAEL of 40 ppm (2 mg/kg bw per day) identified
previously in a long-term study in rats that included a more extensive
range of investigations.
No adverse findings were reported in workers in
glufosinate-ammonium production plants, but their exposure was stated
to be low. A number of cases of attempted suicide in Japan have
involved a glufosinate-ammonium-based formulation, but it was not
clear whether the effects reported were due to glufosinate-ammonium or
other constituents. The most significant effect was delayed
neurological symptoms. The available evidence indicates that exposure
to glufosinate-ammonium under normal conditions of use does not
present a significant risk to humans.
N-Acetylglufosinate
Oral doses of [14C] N-acetylglufosinate are absorbed to a
limited extent (5-10%), but the absorption is rapid, with peak plasma
concentrations found 1 h after a dose of 3 mg/kg bw. Excretion of an
absorbed dose is also rapid and occurs predominantly in the urine as
the parent compound. The excretory half-time is < 1 h for the initial
phase and approximately 7 h for the second phase. The residue
concentrations in the liver and particularly kidneys 4 days after
dosing were significantly greater than those in the plasma. Absorbed
N-acetylglufosinate undergoes limited biotransformation, but a
significant proportion (11%) of a low oral dose (3 mg/kg bw) was
de-acetylated to glufosinate-ammonium in the intestine. It is not
clear what proportion of the glufosinate-ammonium present in the
tissues after oral administration of N-acetylglufosinate is absorbed
from the intestine as glufosinate-ammonium. The toxicokinetics of
N-acetylglufosinate after repeated administration has not been
investigated.
N-Acetylglufosinate is of low acute toxicity after oral
administration to mice and rats (LD50 values > 2000 mg/kg bw) and
is not a skin sensitizer. Because N-acetylglufosinate is a plant
metabolite and is not present in pesticide formulations, it has not
been studied for acute toxicity by dermal or inhalation administration
or for ocular or dermal irritancy.
N-Acetylglufosinate has not been classified for toxicity by
WHO.
N-Acetylglufosinate is of low toxicity after repeated oral
administration to mice, rats, or dogs. Some and possibly all of the
inhibition of glutamine synthetase activity seen in all three species
was attributable to glufosinate-ammonium. The NOAEL for inhibition of
glutamine synthetase in the brain in the most sensitive species was
500 ppm (equal to 19 mg/kg bw per day) in a 90-day study in dogs
(glutamine synthetase activity was not measured in a 1-year study in
dogs). In a 2-year study in rats, there was evidence of chronic
progressive nephropathy and urolithiasis. The Meeting noted the
absence of pathological changes in the 90-day study, the lack of a
dose-response relationship for the renal lesions, the high sodium
concentrations associated with administration of
N-acetylglufosinate, and the high prevalence of renal lesions in
aged rats, and concluded that the renal lesions seen in the long-term
study in rats were not relevant to human risk assessment. Increased
incidences of adrenal cortical hyperplasia, adrenal necrosis, and
polyarteritis nodosa were seen in males receiving doses > 2000 ppm
(equal to > 91 mg/kg bw per day), and an increased incidence of
extramedullary haematopoiesis of the spleen was seen in females at
those doses. The NOAEL for pathological findings in rats, the most
sensitive species, was 200 ppm, equal to 9 mg/kg bw per day.
The Meeting concluded that N-acetylglufosinate is not
carcinogenic at the highest doses tested (equal to 1200 mg/kg bw per
day in mice and 1000 mg/kg bw per day in rats).
N-Acetylglufosinate has been studied in an adequate range of
tests for genotoxicity. The Meeting concluded, on the basis of the
results, that N-acetylglufosinate is not genotoxic.
Reproductive performance and outcome in a two-generation study of
reproductive toxicity in rats were not affected by administration of
N-acetylglufosinate at doses up to 700 mg/kg bw per day. The
compound was not teratogenic to either rats or rabbits and was not
fetotoxic to rats. An increased incidence of supernumerary thoracic
ribs was found in fetuses from rabbits exposed to
N-acetylglufosinate at > 160 mg/kg bw per day, a finding that may
be secondary to the maternal toxicity seen at such doses. The NOAEL
for fetotoxicity and maternal toxicity was 64 mg/kg bw per day.
3-[Hydroxy(methyl) phosphinoyl]propionic acid
Summaries of a range of studies on the genotoxicity, acute
toxicity, short-term toxicity, and teratogenicity of the
glufosinate-ammonium metabolite 3-[hydroxy(methyl)phosphinoyl]
propionic acid were available. The lowest NOAEL seen in these studies
(50 mg/kg bw per day) is 25-fold higher than the NOAEL used to derive
the ADI for glufosinate-ammonium.
Overall evaluation
The present Meeting compared the toxicity of
N-acetylglufosinate and 3-[hydroxy(methyl) phosphinoyl]propionic
acid with that of glufosinate-ammonium and concluded that the toxicity
of the metabolites was comparable to or less than that of the parent
compound. The Meeting established a group ADI of 0-0.02 mg/kg bw for
glufosinate-ammonium, N-acetylglufosinate, and 3-[hydroxy(methyl)
phosphinoyl]propionic acid (alone or in combination). This is the same
value as that of the ADI established for glufosinate-ammonium by the
1991 JMPR on the basis of the NOAEL in the long-term study in rats
given technical-grade glufosinate-ammonium, and applying a 100-fold
safety factor.
The present Meeting concluded that it was unnecessary to
establish an acute reference dose because glufosinate-ammonium,
N-acetylglufosinate, and 3-[hydroxy(methyl) phosphinoyl]propionic
acid are of low acute toxicity.
Toxicological evaluation
Levels that cause no toxic effect
Glufosinate-ammonium from 1991 JMPR)
Mouse: 80 ppm, equal to 11 mg/kg bw per day (toxicity in a 2-year
study of toxicity and carcinogenicity)
Rat: 40 ppm, equal to 2.1 mg/kg bw per day (toxicity in a 2-year
study of toxicity and carcinogenicity)
120 ppm equal to 6 mg/kg bw per day (toxicity in a study of
reproductive toxicity)
10 mg/kg bw per day (developmental effects, highest dose
tested in a study of developmental toxicity)
2.2 mg/kg bw per day (maternal and fetotoxicity in a study
of developmental toxicity)
Rabbit: 6.3 mg/kg bw per day (maternal and fetotoxicity in a study
of developmental toxicity)
20 mg/kg bw per day (developmental effects, highest dose
tested in a study of developmental toxicity)
Dog: 4.5 mg/kg bw per day (toxicity in a 1-year study)
N- Acetylglufosinate
Mouse: 1000 ppm, equal to 150 mg/kg bw per day (toxicity in a
2-year study of toxicity and carcinogenicity)
Rat: 200 ppm, equal to 9 mg/kg bw per day (toxicity in a 2-year
study of toxicity and carcinogenicity)
2000 ppm, equal to 140 mg/kg bw per day (parental toxicity
in a study of reproductive toxicity)
10 000 ppm equal to 700 mg/kg bw per day (reproductive
effects, highest dose tested in a study of reproductive
toxicity)
1000 mg/kg bw per day (highest dose tested in a study of
developmental toxicity)
Rabbit: 400 mg/kg bw per day (developmental effects, highest dose
tested in a study of developmental toxicity)
64 mg/kg bw per day (maternal and fetotoxicity in a study of
developmental toxicity)
Dog: 500 ppm, equal to 19 mg/kg bw per day (toxicity in a 90-day
study)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw (for glufosinate-ammonium, N-acetylglufosinate,
and 3-[hydroxy(methyl) phosphinoyl]propionic acid, alone or in
combination)
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological end-points relevant for estimating guidance values for dietary and non-dietary exposure to glufosinate-ammonium
Glufosinate-ammonium N-Acetylglufosinate (1991 JMPR)
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid but limited (5-10%) Rapid but limited (5-10%)
Distribution Extensive. Higher concentration in Extensive. Higher concentration in
kidney and liver kidney and liver
Potential for accumulation Minimal Minimal
Rate and extent of excretion Rats, rapid, > 92% of 30 mg/kg bw Rats, rapid, >95% of 3 mg/kg bw within
within 24 h, primarily in faeces 24 h, primarily in faeces
Metabolism in animals Main metabolite is 3-[hydroxy-(methyl) Limited. Some de-acetylation
phosphinoyl] propionic to glufosinate-ammonium (rat,
acid (rat, goat, hen) goat, hen)
Toxicologically significant compounds Parent Parent and glufosinate-ammonium
Acute toxicity
Rat, LD50, oral 1700 mg/kg bw > 3000 mg/kg bw
Rat, LD50, intraperitoneal ~ 100 mg/kg bw > 1200 mg/kg bw
Mouse, LD50, oral 420 mg/kg bw > 3000 mg/kg bw
Skin sensitization, guinea-pigs Negative (Buehler test) Negative (Magnusson & Kigman test)
Short-term toxicity
Target/critical effect Glutamine synthetase inhibition; Glutamine synthetase inhibition in brain
behaviour in dogs; kidney weights of dogs, mice, rats
and urinary parameters in rats
Lowest relevant oral NOAEL 5 mg/kg bw per day in dogs 19 mg/kg bw per day in dogs
Genotoxicity Not genotoxic Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Glutamine synthetase inhibition; Adrenal necrosis and hyperplasia;
increased kidney weight in rats spleen haematopoiesis in rats
Lowest relevant NOAEL 2 mg/kg bw per day in rats 9 mg/kg bw per day
Carcinogenicity Not carcinogenic Not carcinogenic
Reproductive toxicity
Reproductive target/critical effect Reduced litter size, rats None
Developmental target/critical effect General maternal and fetotoxicity Extra ribs/maternal toxicity in rabbits
Lowest relevant NOAEL for 12 mg/kg bw per day in rats 137 mg/kg bw per day for reproductive
general toxicity in rats toxicity
Lowest relevant NOAEL for 2 mg/kg bw per day in rats 64 mg/kg bw per day in rabbits
developmental toxicity
Neurotoxicity Possibly behavioural, but no No evidence of specific effectspathological findings
Medical data Suicidal poisonings producing coma, No data, not produced commercially
delayed neurological effects, death;
no findings in work force
Summary Value Study Safety factor
Group ADI for glufosinate-ammonium 0-0.02 mg/kg bw 2 years, rat 100
and N-acetylglufosinate
Acute reference dose Unnecessary
References
Note: Amendments have been issued to a number of studies; these are
not referenced separately.
Baeder, C. & Hofmann, T. (1994) Embryotoxicity after oral
administration in Himalayan rabbits. Hoe 099730 substance,
technical. Hoechst AG, Pharma Development, Corporate Toxicology.
Report No. 94.0392. A52948. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Bernier, L. (1996) 52-week dietary toxicity study in the beagle dog of
Hoe 099730 substance, technical. Bio-Research Laboratories.
Hoechst Schering AgrEvo GmbH. Report No. 85438. A55742.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Bernier, L. (1997) Dietary chronic toxicity and carcinogenicity study
in the Sprague-Dawley rat of Hoe 099730 substance, technical.
ClinTrials BioResearch Ltd, Senneville, Quebec, Canada. Report
No. 85440. A59280. Unpublished report submitted ubmitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Beyrouty, P. (1996a) Dietary range-finding reproduction study in the
Sprague-Dawley rat of Hoe 099730 substance, technical. Biological
Research Laboratory Ltd.AgrEvo Canada Inc. Hoechst Schering
AgrEvo GmbH. Report No. 95447. A55746. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Beyrouty, P. (1996b) Dietary 2-generation (1 Litter) reproduction
study in the SpragueDawley rat of Hoe 099730 substance,
technical. Bio-Research Laboratories. Report No. 95448. A55747.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Bremmer, J.N. & Leist, K.-H. (1998) Toxicology and metabolism studies
(summary and evaluation) 3-methyl phosphinico-propionic acid
(MPP) AE F061517 substance, technical. Hoechst Schering AgrEvo
GmbH. Safety Evaluation Frankfurt. Report No. TOX98/011. A59706.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Corney, S.J., Braunhofer, P. & Luetkemeier, H. (1992) 13-week oral
toxicity (feeding) study in the dog with Hoe 099730 substance,
technical. Research & Consulting Company Ltd, Switzerland. Report
No. 291104. A49064. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Dotti, A., Luetkemeier, H. & Powell, J. (1993) 13-week oral toxicity
(feeding) study in the rat with Hoe 039866 substance, technical.
Research & Consulting Company Ltd, Switzerland. Report No.
338400. A51836. Unpublished. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Dotti, A., Luetkemeier, H. & Biedermann, K. (1994) 13-week oral
toxicity (feeding) study in the mouse with Hoe 039866 substance,
technical. Research & Consulting Company Ltd, Switzerland. Report
No. 338411. A52107. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Ebert, E. (1991a) Repeated-dose oral toxicity (4-week feeding study)
in the NMRI mouse Hoe 099730 substance, technical. Hoechst AG,
Pharma Development, Corporate Toxicology. Report No. 91.0728.
A46543. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Ebert, E. (1991b) Repeated-dose oral toxicity (4-week feeding study)
in the Wistar rat Hoe 099730 substance, technical. Hoechst AG,
Pharma Development, Corporate Toxicology. Report No. 91.0594.
A46604. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Ernst, S.W. & Leist, K.-H. (1999a) Critical review of organ specific
glutamine synthetase activities. AgrEvo, Toxicology Frankfurt,
Germany. Report No. TOX98/057. C001918. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Ernst, S. & Leist, K.-H. (1999b) Hoe 099730 substance technical. Code
Hoe 099730 00 ZC92 001. Testing for embryotoxicity after oral
administration in Himalayan rabbits, Addendum to Document A52948,
Determination of the concentration of test substance during the
study. Toxicology, Frankfurt. Report No. TOX99/026. C004479.
Unpublished report submitted 05.07.99 to WHO by Hoechst Schering
AgrEvo GmbH, Germany
Ernst, S.W. & Leist, K.-H. (1999c) Glufosinate-ammonium -- Human
experience with herbicides containing glufosinate-ammonium.
Toxicology, Frankfurt. Report No. TOX99/021. C003758. Unpublished
report submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Ernst, S.W. & Stumpf, K. (1999a) A dietary chronic toxicity and
carcinogenicity study of Hoe 099730; substance technical in the
Swiss CRL:CD(R) mouse. Addendum 1 to Agrevo report A57755.
Historical control data of histopathological investigations
conducted at ClinTrials BioResearch Ltd, Senneville, Quebec,
Canada. Hoechst Schering AgrEvo GmbH, Toxicology Frankfurt,
Germany. Report No. TOX99/033. C004765. Unpublished report
submitted 27.07.99 to WHO by Hoechst Schering AgrEvo GmbH,
Germany.
Ernst, S. & Stumpf, K. (1999b) A dietary chronic toxicity and
carcinogenicity study of Hoe 099730; substance technical in the
Sprague-Dawley rat. Addendum 1 to Agrevo report A59280.
Historical control data of histopathological investigations
conducted at ClinTrials BioResearch Ltd, Senneville, Quebec,
Canada. Hoechst Schering AgrEvo GmbH, Toxicology Frankfurt,
Germany. Report No. TOX99/034. C004764. Unpublished report
submitted 27.07.99 to WHO by Hoechst Schering AgrEvo GmbH,
Germany.
Farrell, D.J. (1997) Dietary carcinogenicity study in the Swiss CRL:
CD(R) mouse of Hoe 099730 substance, technical. ClinTrials
BioResearch Ltd, Senneville, Quebec, Canada. AgrEvo Canada Inc.
Report No. 97.0451. A57755. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Hack, R., Ebert, E. & Ehling, G. (1994) Glufosinate-ammonium -- Some
aspects of mode of action in mammals. Food Chem. Toxicol., 32,
461-470.
Heidemann, A. & Voelkner, W. (1992) Chromosome aberration assay in
human lymphocytes in vitro with Hoe 099730, substance, technical.
Cytotest Cell Research. Report No. 275602. A49392. Unpublished
report submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Hofmann, T. & Jung, R. (1988) Nickel(II)-sulphate. Testing for
sensitising properties in the Pirbright-white guinea pig in a
maximisation test. Pharma Research Technology, Hoechst AG,
Frankfurt. Report No. 91.0792. A46366. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Horstmann, G. & Baeder, C. (1992) Embryotoxicity in the Wistar rat
after oral administration (limit test) Hoe 099730 substance,
technical. Hoechst AG, Pharma Development, Corporate Toxicology.
Report No. 92.0394. A48852. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995a) Metabolism of (14C)-glufosinate in
a lactating goat. AgrEvo USA Co., Pikeville, Hazleton Lab., USA.
Report No. U012A/A524R. A54158. A55808. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995b) Metabolism of (14C)-glufosinate in
laying hens. AgrEvo USA Co., Pikeville, USA, Hazleton Lab., USA.
Report No. U012A/A525R. A54159. A55808. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995c) Metabolism of (14C)-N-acetyl
glufosinate in a lactating goat. AgrEvo USA Co. Pikeville, PTRL
East Inc., USA. Report No. 502BK U012A/A524. A54155. A55808.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995d) Metabolism of (14C)-N-acetyl
glufosinate in laying hens. AgrEvo USA Co. Pikeville, USA. PTRL
East Inc., USA. Report No. 503BK,U012A/A525. A54157. A55808.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Kaleja, R. (1999) Statement on handling glufosinate-ammonium.
Occupational Health Center, InfraServ GmbH & Co. Höechst KG.
Document No. C003747. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Kellner, H.-M. & Braun, R. (1993a) Hoe 099730-14C blood levels in
rats following single oral and intravenous administration of 3
mg/kg body weight. Hoechst RCL, DEU. Report No. 01-L42-0671-93.
A49979. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Kellner, H.-M. & Braun, R. (1993b) Hoe 099730-14C blood levels in
rats following single oral and intravenous administration of 3
mg/kg body weight. Hoechst Radiochem. Laboratorium, Germany.
Report No. 01-L42-0671-93. A53276. Unpublished report submitted
to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Kellner, H.-M., Stumpf, K. & Braun, R. (1993) Hoe 099730-14C
pharmacokinetics in rats following single oral and intravenous
administration of 3 mg/kg body. Hoechst RCL, Germany. Report No.
01-L42-0670-93. A49978. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Koyama, K., Andou, Y., Saruki, K. & Matsuo, H. (1994) Delayed and
severe toxicities of a herbicide containing glufosinate and a
surfactant. Vet. Hum. Toxicol., 36, 17-18.
Lauck-Birkel, S. (1995a) Hoe 039866-14C (glufosinate-ammonium).
Metabolism in male and female rats following single oral
administration of test substance at a dose level of 500 mg/kg
body weight. AgrEvo Entwicklung Umweltforschung Oekochemie,
Germany. Report No. CM93/071. A54334. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Lauck-Birkel S. (1995b) Hoe 099730-14C Metabolism in male and female
rats following a single oral administration of test substance at
a dose level of 1000 mg/kg body weight. AgrEvo Entwicklung
Umweltforschung Oekochemie, Germany. Report No. CM93/070. A54333.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Lauck-Birkel, S. (1996) Hoe 039866-14C (glufosinate-ammonium)
Metabolism in male rats following single oral administration of
test substance at a dose level of 2 mg/kg body weight. Hoechst
Schering AgrEvo GmbH, Environmental Chemistry Frankfurt. Report
No. CM95/013. A54336. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B. (1999a) Glufosinate-ammonium. Rat
metabolism-Single oral intermediate dose (20 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/130. C001924. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B. (1999b) Glufosinate-ammonium. Rat
metabolism-Single intravenous low dose (2 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/131. C001922. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B.(1999c) N-Acetyl-glufosinate. Rat
metabolism-Single intravenous low dose (3 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/096. C001920. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B. (1999d). N-Acetyl-glufosinate. Rat
metabolism-Single oral intermediate dose (30 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/097. C001921. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany
Lie-Venema, H., Hakvoort, T.B.M., Moorman, A.F.M. & Lamers, W.H.
(1998) Regulation of the spatiotemporal pattern of expression of
the glutamine synthetase gene. Department of Anatomy and
Embryology. University of Amsterdam, Academic Medical Center,
Netherlands.
Luetkemeier, H. (1999) N-Acetyl-glufosinate and glufosinate. In vitro
investigations. Comparison of the inhibition of glutamine
synthetase. Report No. RCC 718795. C003753. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1995a) Hoe 039866-14C. Sampling of blood,
excrements, organs and tissues for metabolism studies in male and
female rats following single oral administration of approx. 500
mg/kg body weight (First addendum to report CM93/071 (A54334)).
Hoechst AG, Bereich Pharma, Kinetics/Metabolism, Germany. Report
No. 01-L420813-95. A54450. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1995b) Hoe 099730-14C. Pharmacokinetics in
rats following single oral administration of the test substance
at a dose level of 1000 mg/kg body weight. Hoechst AG, Bereich
Pharma, Kinetics/Metabolism, Germany. Report No. 01-L42-0815-95.
A54335. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1999a) Glufosinate-ammonium. Rat. Absorption,
distribution and elimination, single oral intermediate dose (20
mg/kg body weight). HMR, DI & A; Lead Optimization, Germany.
Report No. TEP57/16. C001926. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1999b) Glufosinate-ammonium. Rat. Absorption,
distribution and elimination, single intravenous low dose (2
mg/kg body weight). HMR, DI & A; Lead Optimization, Germany.
Report No. TEP57/15. C001923. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1999c) N-Acetyl-glufosinate. Rat. Absorption,
distribution and elimination, single oral intermediate dose (30
mg/kg body weight), HMR Preclinical Development,
Pharmacokinetics, Germany. Report No. TEP170/8. C001925.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Maas, J. & Braun, R. (1999d) N-Acetyl-glufosinate. Rat. Absorption,
distribution and elimination, single intravenous low dose (3
mg/kg body weight). HMR Preclinical Development,
Pharmacokinetics, Germany. Report No. TEP170/7. C001919.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Muller, W. (1989a) Mutagenic potential in strains of Salmonella
typhimurium (Ames test) and , Hoe 099730 substance, technical.
Hoechst AG. Pharma Development, Corporate. Report No. 89.0084.
A40532. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Muller, W. (1989b) Detection of gene mutations in somatic cells in
culture HGPRT-test with V79 cells Hoe 099730 substance,
technical. Hoechst AG. Pharma Development, Corporate Toxicology.
Report No. 89.1010. A41375. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Muller, W. (1989c) Unscheduled DNA synthesis test in mammalian cells
in vitro of Hoe 099730 substance, technical. Hoechst AG. Pharma
Development, Corporate Toxicology. Report No. 89.0024. A40926. U
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Muller, W. (1989d) Chromosome aberrations in vitro in V79 Chinese
hamster cells Hoe 099730 substance, technical. Hoechst AG. Pharma
Development, Corporate Toxicology. Report No. 89.1063. A41851.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany
Muller, W. (1991a) Detection of gene mutations in somatic cells in
culture. HGPRT-test with V79 cells Hoe 099730 substance,
technical. Hoechst AG. Pharma Development, Corporate Toxicology.
Report No. 91.0356. A45876. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany..
Muller, W. (1991b) Unscheduled DNA synthesis test in mammalian cells
in vitro of Hoe 099730 substance, technical. Hoechst AG. Pharma
Development, Corporate Toxicology. Report No. 91.0286. A46129.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Muller, W. (1991c) Micronucleus test in male and female NMRI mice
after oral administration Hoe 099730 substance, technical.
Hoechst AG. Pharma Development, Corporate Toxicology. Report No.
91.0438. A46128. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Purich, D.L. (1998) Advances in the enzymology of glutamine synthesis.
Adv. Enzymol. Relat. Areas Mol. Biol., 72, 9-42.
Schmid, H., Keller, B., Luetkemeier, H., Westen, H. & Biedermann, K.
(1998) Oncogenicity study in rats. Glufosinate-ammonium substance
technical. RCC Umweltchemie AG, Itingen, Switzerland. Hoechst
Schering AgrEvo GmbH. Safety Evaluation Frankfurt. Report No.
98.0256. A67303. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Schmid, H., Keller, B., Biedermann, K. & Luetkemeier, H. (1999)
N-Acetyl-L-glufosinate disodium and glufosinate-ammonium. 13-week
oral feeding study in the rat; Comparison of the potential to
inhibit glutamine synthetase. Report No. RCC711663. C004512.
Unpublished report submitted 08.07.99 to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989a) Acute oral toxicity in the male
and female Wistar rat Hoe 099730 substance, technical. Hoechst
AG. Pharma Development, Corporate Toxicology. Report No. 89.0879.
A41793. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989b) Acute oral toxicity in the male
and female NMRI mouse Hoe 099730 substance, technical. Hoechst
AG. Pharma Development, Corporate Toxicology. Report No. 89.0877.
A41791. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989c) Acute intraperitoneal toxicity
in the male and female Wistar rat Hoe 099730 substance,
technical. Hoechst AG. Pharma Development, Corporate Toxicology.
Report No. 89.0883. A41795. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989d) Acute intraperitoneal toxicity
in the male and female NMRI mouse Hoe 099730 substance,
technical. Pharma Development, Corporate Toxicology. Report No.
89.0882. A41789. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989e) Sensitising properties in the
Pirbright-White guinea pig in a maximisation test Hoe 099730
substance, technical. Hoechst AG, Pharma Development, Corporate
Toxicology. Report No. 89.0880. A41612. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Stumpf, K. (1993a) Hoe 039866-14C. Metabolism in male and female
rats following single oral administration of test substance at a
dose level of 2 mg/kg body weight. Hoechst AG, Bereich
Landwirtschaft, Produktentwicklung Oekologie 1, Germany. Report
No. CM90/028. A49981. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Stumpf, K. (1993b) Hoe 099730-14C. Metabolism in male and female
rats following single oral administration of test substance at a
dose level of 3 mg/kg body weight. Hoechst AG, Bereich
Landwirtschaft, Produktentwicklung Oekologie 1, Germany. Report
No. CM90/026. A49980. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Tennekes, H., Schmid, H. & Probst, D. (1992a) Sub-chronic oral
toxicity 13-week feeding study in mice Hoe 099730 substance,
technical. Research & Consulting Company Ltd, Switzerland. Report
No. RCC291025. A48186. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Tennekes, H., Probst, D. & Luetkemeier, H. (1992b) Sub-chronic oral
toxicity 13-week feeding study in rats Hoe 099730 substance,
technical. Research & Consulting Co. Ltd, Switzerland. Report No.
291093. A48187. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Watanabe, T. & Sano, T. (1998) Neurological effects of glufosinate
poisoning with a brief review. Hum. Exp. Toxicol., 17, 35-39.
Weller, O. (1994) Statement on certificates of analysis used for
different tox.-studies Hoe 099730 technical grade aqueous
solution. Hoechst Schering AgrEvo GmbH. Product Analysis
Frankfurt. Report No. OE94/053. A53577. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
 (-)-glutamine and hydroxylamine in the presence of arsenate,
manganese, and ADP, followed by spectrophotometric measurement of an
iron compound. A preincubation period of 10 min was used in the main
assays, as it had been shown that inhibition was not changed by
extending this period to 60 or 120 min. The samples were incubated for
20 min in the presence of N-acetylglufosinate (a 33.8% solution
containing 0.06% w/w glufosinate-ammonium) at 0-10 000 µg/ml or
glufosinate-ammonium (as a 50.2% solution) at 0-500 µg/ml.
Glufosinate-ammonium induced significant, concentration-related
inhibition of glutamine synthetase in all tissues at doses > 0.77
mmol/L (Table 6), the inhibition profile varying with tissue.
N-Acetylglufosinate induced only marginal inhibition at 13 mmol/L,
some of which can be attributed directly to the glufosinate-ammonium
content of the sample. The report did not provide results corrected
for protein content, and not all of the assays were performed in
duplicate; however, for the purposes of this comparative exercise, the
results are considered to be acceptable (Lutkemeier, 1999).
A comparative study was performed of the inhibition of glutamine
synthetase in tissues from groups of 10 male Wistar rats given diets
containing N-acetylglufosinate (with 0.06% w/w glufosinate-ammonium)
at 1000 or 10 000 ppm or glufosinate-ammonium at 0, 100, or 1000 ppm
(Schmid et al., 1999). The animals were exposed for 6, 13, 20, or 90
days with 91 days plus 31 days for recovery. In addition to standard
observations and gross necropsy, samples of liver, brain, and kidney
were removed, rapidly cooled, and stored at -70°C prior to processing
and assaying for glutamine synthetase activity, as described above.
There were no deaths or treatment-related clinical signs. The
absolute and relative weights of the kidney were increased by 8-23% in
all treated groups during the first 20 days of the study, but with no
associated pathological findings. Statistically significant inhibition
of glutamine synthetase activity was seen in liver and kidney samples
by day 6, and, except in liver from animals exposed to 1000 ppm
N-acetylglufosinate, did not increase markedly up to day 90 (Table
7). Significant recovery of glutamine synthetase activity occurred
during the 31-day recovery period. The results indicate that orally
administered glufosinate-ammonium is approximately 10 times more
potent at inhibiting glutamine synthetase than is
N-acetylglufosinate. The extent to which this inhibition is due
directly to de-acetylation of N-acetylglufosinate to
glufosinate-ammonium is uncertain. The activity of glutamine
synthetase in brain samples was not reduced markedly in animals
exposed to either N-acetylglufosinate or glufosinate-ammonium at
1000 ppm.
(d) Significance of glutamine synthetase inhibition to humans
Glutamine synthetase (E.C.6.3.1.2) is a key enzyme in the
metabolism of nitrogen and glutamate, catalysing the multi-step
reaction of
(-)-glutamate + ATP + NH3 <=> (-)-glutamine + ADP + P
Table 6. Inhibition of glutamine synthetase activity in vitro in tissue samples from rats, in the
presence of N-acetylglufosinate and glufosinate ammonium; in square brackets, absolute activity
expressed as mg gamma-glutamylhydroxamate formed per g tissue per 20 min
Compound Dose Liver Kidney Neocortex Medulla Hypothalamus
(mmol/L)
Glufosinate ammonium 0 0 [28] 0 [18] 0 [27] 0 [23] 0 [20]
0.003 1 0 0 1
0.008 2 1 0 0 0
0.026 4 1 3 1 0
0.077 14 3 5 6 3
0.26 36 5 13 20 11
0.77 60 13 32 42 29
1.3 72 17 45 53 41
N-Acetylglufosinate 0.13 1 0 0 0 0
0.38 1 0 0 0 0
0.63 2 0 0 0 0
1.3 2 0 0 0 0
6.3 9 1 2 2 1
13a 15 2 4 7 5
a Contains approximately 0.03 mmol/L glufosinate ammonium
Table 7. Activity of glutamine synthetase in samples from 10 male Wistar rats that
received N-acetylglufosinate or glufosinate ammonium in the diet or
control diet
Day Tissue Glutamine synthetase activity (mean % of control value)
Controla Glufosinate ammonium N-acetylglufosinate
100 ppm 1000 ppm 1000 ppm 10 000 ppm
6 Liver 24 55 36 96 46
Brain 28 101 89 94 93
Kidney 17 60 58 61 54
13 Liver 30 51 30 74 40
Brain 25 101 91 102 104
Kidney 17 61 58 59 54
90 Liver 24 60 40 58 54
Brain 22 104 82 99 98
Kidney 14 67 46 55 53
91 + 31 Liver 24 97 85 83 94
Brain 14 98 88 97 97
Kidney 22 90 97 95 87
a Absolute activity, expressed as mg gamma-glutamylhydroxamate formed per
g tissue per 20 min
In plants, glutamine synthetase is the main enzyme involved in the
control of ammonia concentrations, and its inhibition is the mechanism
of action of glufosinate-ammonium in plants. In mammals, other
pathways exist for the homeostatic control of ammonia, such as reverse
reaction of amino acid dehydrogenases and the carbamoyl phosphate
synthetase-urea cycle. Glutamate and glutamine can, however, play
significant roles in other biochemical and physiological processes in
mammals, such as neurotransmission (glutamate and gamma-aminobutyric
acid (GABA)). The activity of glutamine synthetase varies between
tissues and species (see below), but the amino acid sequence is
reported to be well conserved (LieVenema et al., 1998; Purich, 1998;
Ernst & Leist, 1999a).
The liver has two distinct systems for dealing with ammonia. A
high-capacity, low-affinity system exists in the periportal
hepatocytes which is based on carbamoyl phosphate synthetase and the
urea cycle. In central vein hepatocytes, a low-capacity, high-affinity
system exists which is based on glutamine synthetase and ornithine
aminotransferase. Hack et al. (1994) showed that doses of
glufosinate-ammonium did not increase ammonia concentrations in liver
at a dose (5000 ppm) that inhibited glutamine synthetase activity by
50%. While a 60% reduction in liver glutamine was seen at day 1, the
concentration had returned to normal by day 4, indicating the
induction of alternative pathways. Inhibition of liver glutamine
synthetase by up to 50% is therefore not considered to be adverse in
isolation.
The activity of this enzyme in kidney varies considerably between
species (LieVenema et al., 1998; see below), with relatively high
activity in rodents but negligible activity in dogs and humans.
Inhibition of kidney glutamine synthetase in the absence of
pathological findings is not considered to be relevant to human risk
assessment.
In the brain and central nervous system, ammonia homeostasis is
controlled by a number of enzymes including glutamine synthetase and
glutamate dehydrogenase. Under normal conditions (~ 100 µmol/L of
ammonium and 3 mmol/L of glutamate), the flux through glutamine
synthetase in brain is 2-10% of its theoretical capacity and that of
glutamate dehydrogenase is approximately 0.1% of its capacity
(Lie-Venema et al., 1998). With such excess capacity, inhibition of
brain glutamine synthetase is unlikely to result in significant
increases in brain ammonia concentrations. This conclusion is
supported by the finding of Hack et al. (1994) that brain ammonia
concentrations were not increased at doses of glufosinate-ammonium
that produced a 40% reduction in brain glutamine synthetase activity
in rats. However, the glutamine-glutamate shunt between GABA and
glutamate in neurons and glutamine in astrocytes plays a role in both
excitatory and inhibitory neurotransmission. The results of Hack et
al. (1994), although somewhat inconsistent, indicate that significant
changes in a range of biogenic amines in regions of the dog brain are
associated with changes of > 8% in glutamine synthetase activity
after administration of glufosinate-ammonium at 8 mg/kg bw for 28
days, a dose that produced 'increased gait activity'. It is thus
proposed that any statistically significant, > 10% inhibition of
glutamine synthetase activity in brain is a marker of potentially
adverse effects on brain biochemistry and behaviour.
2. Toxicological studies
All of the studies described below were included statements of
compliance with GLP and met the basic requirements of the OECD test
guidelines applicable at the time of study initiation, unless
otherwise stated.
(a) Short-term studies of toxicity
Mice
In response to concern that the doses used in the study of
carcinogenicity with glufosinate-ammonium in mice evaluated previously
had not been appropriately high (160 ppm in males, 320 ppm in
females), a 90-day study was performed in which groups of 10 NMRI mice
of each sex received diets containing glufosinate-ammonium (purity,
95.5%) at concentrations of 0, 1750, 3500, or 7000 ppm. The
homogeneity and achieved concentrations were acceptable, and the
intakes of animals were 561 and 644 mg/kg bw per day for males and
females at the intermediate dose and 274 and 356 mg/kg bw per day for
animals at the low dose, respectively. The investigations included
clinical signs, body weight, food consumption, haematology, clinical
chemistry, organ weights, and gross and microscopic pathology. All
animals at the high dose had died by day 8, 50% of those at 3500 ppm
had died by day 11, and one female at the low dose died. Clinical
signs (ruffled fur, sedation, and emaciation), reduced food
consumption, and initial body-weight loss were seen at all doses,
although the body-weight gain during the latter part of the study was
similar in surviving animals. There were no consistent clinical
chemical or haematological findings and no changes in organ weights or
on gross examination. Congestion in multiple organs was seen at
microscopic examination of many animals that died during the study,
but the cause of death was not determined. No NOAEL could be
identified, but the design was not optimal for this purpose. The
lowest dose tested (1750 ppm, equal to 270 mg/kg bw per day) was
approximately the maximum tolerated dose for a 90-day study, resulting
in a single death and marked initial effects on body weight. The
maximum tolerated dose for a 2-year study in mice would be about 600
ppm if a factor of 3 is used to extrapolate from the approximate dose
in the 90-day study (Dotti et al., 1994). The results of this study
indicate that the previous bioassay was performed within a factor of 2
of the estimated maximum tolerated dose and need not be repeated.
Rats
The toxicity of glufosinate-ammonium (purity, 95.5%) was
investigated in groups of 10 Wistar rats of each sex given the
compound in the diet at concentrations of 0, 7500, 10 000, or 20 000
ppm for 90 days. Routine observations and measurements were made, with
ophthalmoscopy before treatment and at termination and a basic
functional observation battery, which was administered before
treatment and at weeks 1, 2, 3, 4, 8, and 13 and involved observations
in the home cage, an external area, and in the hand, but no forced
physical activity such as grip strength or swimming. Blood samples for
haematological and clinical chemical analyses were taken from fasted
animals at week 13. At termination, five animals of each sex per group
were perfused to preserve nervous tissue. Major organs from all
animals at the highest dose and controls were examined histologically,
as was nervous tissue from all perfused animals and all gross lesions.
Organ weights were not determined. The homogeneity, stability, and
achieved concentrations in the diet were satisfactory, with intakes
equal to 0, 520, 690, and 1400 mg/kg bw per day in males and 0, 570,
740, and 1400 mg/kg bw per day in females.
Two females at the high dose died within the first 8 days of
dosing, but there were no other unscheduled deaths. Animals at the
high dose showed a range of signs during the first 2 weeks of
treatment, including sedation, dyspnoea, emaciation, and diarrhoea.
Food consumption was reduced by > 20% in all groups during the first
two weeks. Body-weight loss was seen in animals at the high dose, and
reductions in body-weight gain were seen in other groups during the
first week of dosing. All groups had similar body-weight gains during
the last weeks of the study. No abnormal ophthalmoscopic findings were
reported. The haematological findings were similar in test and control
groups, and an apparent reduction in erythrocyte count in males
appeared to be related to a high control value. A consistent pattern
of changes in serum lactate dehydrogenase and creatine kinase activity
was seen in animals of each sex, with 20% reductions at the low and
intermediate doses and an increase at the high dose. Small (< 10%)
but statistically significant ( p < 0.05) increases in serum calcium
and inorganic phosphorus concentrations were seen in animals at the
high dose. The functional observational battery identified a similar
pattern of changes in males and females that included miosis, apathy,
reduced alertness and grooming, increased body tone, and vocalization.
These changes were present in all treated groups, with no clear
pattern over time, but were more prevalent at the high dose. There
were no significant macroscopic findings, and the only microscopic
finding of significance was an increased incidence of renal pelvis
dilatation in males at the high dose; there were no treatment-related
effects on nervous tissues.
No NOAEL could be identified owing to alterations in calcium and
inorganic phosphorus and in the functional observational battery at
all doses, although there was no indication of irreversible
neurotoxicity. The effects on food consumption and body weight may
have been secondary to palatability, as they were mainly transient
(Dotti et al., 1993).
(b) Long-term studies of toxicity and carcinogenicity
Rats
A study was initiated in 1994 (Schmid et al., 1998) to include
doses above the maximum of 500 ppm used in the first study. Groups of
60 Wistar rats of each sex received diets containing
glufosinate-ammonium (purity, 96%) at concentrations of 0, 1000, 5000,
or 10 000 ppm for 104 weeks. These doses were based on increased
mortality seen at 20 000 ppm in the 90-day study (Dotti et al., 1993).
The animals were observed for survival, clinical signs, body-weight
gain, food consumption, and the presence of nodules or masses. Blood
smears were prepared from controls and animals at the high dose in
weeks 52, 78, and 104. At termination, over 30 tissues were removed,
nine were weighed, and gross and histopathological examination was
performed on all tissues from all animals. The stability, achieved
levels, and homogeneity of glufosinate-ammonium in the diets were
satisfactory, giving intakes equal to 0, 45, 230, and 470 mg/kg bw in
males and 0, 57, 280, and 580 mg/kg bw per day in females.
Survival was similar in all groups, being over 70% at 104 weeks.
There were no treatment-related changes in clinical signs, food use
efficiency, the occurrence of nodules or masses, or the appearance of
blood smears. All treated groups had reduced food consumption and
body-weight gain over the first month of dosing but these parameters
were subsequently within 10% of those of controls. The weight of the
kidney was increased by 15-30% in relation to dose in all treated
groups, but there were no histological correlates. Macroscopic
examination showed a decreased incidence of pituitary nodules in all
treated males but an increased incidence of adrenal gland foci in
males at the high dose. Microscopic examination revealed a
statistically significant ( p < 0.05) increase in the incidence of
retinal atrophy in males and females at 10 000 ppm and in females at
5000 ppm. This effect was considered to be related to treatment, as a
dose-response relationship was evident in females and it occurred in
animals of each sex, even though the incidence was within the range of
historical controls (males, 20%; females, 38%; Table 8). The incidence
of a rare skin tumour (trichofolliculoma) was increased in males at
the high dose, but it was not statistically significant and was not
seen in females or in males receiving half of the high dose; the
finding was therefore considered not to provide clear evidence of
carcinogenic potential. The total number of malignant and benign
tumours was similar in treated and control groups. The NOAEL was 1000
ppm, equal to 45 mg/kg bw per day, on the basis of the increased
incidence of retinal atrophy (Schmid et al., 1998).
Table 8. Incidences of lesions in Wistar rats receiving
glufosinate ammonium in the diet for 104 weeks
Dose (ppm) Incidence (%)
Retinal atrophy Trichofolliculoma
(males)
Males Females
0 4 3 0
1 000 3 2 0
5 000 4 19 0
10 000 12 29 4
(c) Studies on metabolites
(i) N-Acetylglufosinate
The (-) isomer of N-acetylglufosinate is a major metabolite of
glufosinate-ammonium after its application to glufosinate-tolerant
crops. In 1998, the Committee proposed that residues arising from
applications of glufosinate-ammonium to tolerant crops be defined as
the 'sum of glufosinate-ammonium, 3-[hydroxy(methyl)phosphinoyl]
propionic acid, and Nacetylglufosinate' but could not adopt this
definition until N-acetylglufosinate had been evaluated
toxicologically. Extensive toxicokinetic and toxicological studies on
N-acetylglufosinate have since been submitted. The preparation
tested was an aqueous solution of the disodium salt, and all of the
doses cited below have been corrected for the content of the
technical-grade (-)-isomer. The three batches of N-acetylglufosinate
used varied in purity from 74.5% to 99% and in glufosinate-ammonium
content from 0.06 to 4.5% (Weller, 1994). All of the studies conformed
to GLP and were claimed to have been performed according to guideline
85-1 of November 1984 of the US Environmental Protection Agency or to
meet the basic requirements of the OECD test guidelines applicable at
the time the study was initiated, unless otherwise stated.
Absorption, distribution, and excretion: The toxicokinetics of
N-acetylglufosinate was examined in several studies at doses of
3 mg/kg bw (Stumpf, 1993b; Kellner et al., 1993) or 1000 mg/kg bw
(Lauck-Birkel, 1995b; Maas & Braun, 1995b). Groups of five Wistar rats
of each sex received [3,4-14C] N-acetylglufosinate (purity, > 98%)
by gavage in saline. Urine and faeces were collected over 24-h periods
up to 96 h, and up to 12 tissue samples were taken from animals given
1000 mg/kg bw and killed at 2 and 6 h (two of each sex), 24 h (five of
each sex), and 96 h (five of each sex). Radiolabel in the
gastrointestinal tract was determined in one male each killed at 4 or
24 h after receiving 3 mg/kg bw, and one male at this dose killed at
96 h was studied by autoradiography (Kellner et al., 1993). Radiolabel
in tissues and excreta was determined by liquid scintillation counting
after appropriate processing. The samples were also prepared for
metabolic investigations (see below).
The doses administered differed somewhat among animals, but this
was considered not to have affected the results. Most of each
administered dose was excreted in the faeces within 48 h (Table 9),
although excretion was more rapid at the lower dose. The
concentrations in tissue peaked at 6 h and represented < 1% of the
administered radiolabel; the highest concentrations were detected in
kidney, and those in liver and kidney were greater than in plasma. In
animals at 3 mg/kg bw, the tissue concentration represented < 0.1% of
the administered dose at day 4, with the highest concentrations in
kidney (0.06 µg/g), a finding confirmed by autoradiography. Analysis
of the gastrointestinal tract 4 and 24 h after administration of
3 mg/kg bw showed that 3% and < 0.01% of the dose was in the stomach
and 91% and 3.5% in the intestine, respectively. Sporadic differences
by sex were seen but were not consistent between studies or individual
animals. The tissue concentrations were higher in female than male
rats given 1000 mg/kg bw, as was the urinary excretion after the dose
of 3 mg/kg bw. The results of the two studies with 1000 mg/kg bw
presented a similar profile, although Maas & Braun (1995b) found lower
tissue concentrations and higher urinary excretion in females (up to
20%, including cage washes); however, there was a threefold variation
between individual animals, and the possibility of contamination by
faeces cannot be dismissed.
Table 9. Radiolabel in excreta and tissues of rats given
[3,4-14C] N-acetylglufosinate orally at 3 or 1000 mg/kg bw
Sample Radiolabel in excreta (% of dose) or tissues (µg/g)
1000 mg/kg bwa 3 mg/kg bwb
Males Females Males Females
Urine
24 h 5 4 5 8
48 h 7 6 5 9
Faeces
24 h 58 63 97 93
48 h 82 83 100 96
Liver, 6 h 17 46 NA NA
Kidney, 6 h 44 672c NA NA
Brain, 6 h 1 1 NA NA
Plasma, 6 h 3 10 NA NA
NA, not analysed
a Results from Lauck-Birkel (1995b)
b Results from Kellner et al. (1993); Stumpf (1993b)
c Possible outlier
Groups of three Wistar rats of each sex were given
[3,4-14C] N-acetylglufosinate (purity, 98%; 1400 MBq/g) at 3 mg/kg
bw in saline, and blood samples were taken from the retro-orbital
plexus at 15, 30, and 60 min and 2, 4, 6, 8, 24, 48, 72, and 96 h. The
samples were absorbed onto filter paper and combusted, and radiolabel
was determined by liquid scintillation counting. The peak
concentration (0.05 µg/g) was seen at 60 min, although significant
amounts were detected at 15 min, showing rapid initial absorption. The
concentration in blood declined in a biphasic manner, in an initial
phase with a half-time of 0.8 h and a second phase with a half-time of
7 h. The concentration was at the limit of detection by 24 h. The
integrated area under the curve of concentration-time was ~ 0.2 µg
h/g. Comparison with the results of an identical study in which the
substance was administered intravenously showed that absorption after
oral administration represented approximately 5% of the dose over
24 h. There was no significant difference between the sexes (Kellner &
Braun, 1993a,b).
A lactating goat weighing 36 kg was dosed orally twice a day for
3 consecutive days with capsules containing
[3,4-14C] N-acetylglufosinate, equivalent to a dose of 3.0 mg/kg bw
per day. The feed intake was 1.4 kg/day. The animal was milked twice
daily and was slaughtered 16 h after the final dose. Most of the
administered radiolabel was excreted in the faeces (68%), with 7.3% in
urine and 19% in the gastronintestinal tract and its contents. Only
0.2% of the administered dose was found in the tissues and blood and
< 0.1% in milk. The concentrations in kidney were higher than in
other tissues. Those in milk reached a plateau by day 2 (Huang &
Smith, 1995c).
Six laying hens weighing 1.3-1.6 kg were dosed orally twice a day
for 14 consecutive days with capsules containing
[3,4-14C] N-acetylglufosinate, equivalent to a dose of 2.2 mg/kg bw
per day. The mean feed intake was 120 g/day. Eggs were collected twice
daily, and the birds were slaughtered 15 h after the final dose. Most
of the administered dose was excreted (86%), with 1.0% remaining in
the gastrointestinal tract; < 0.1% of the administered dose was
present in edible tissues and blood. The concentrations of radiolabel
associated with N-acetyl-L-glufosinate disodium salt were 0.076
mg/kg in liver, 0.013 mg/kg in muscle, and 0.011 mg/kg in fat; that in
egg white was only slightly above the level of quantification
(< 0.009 mg/kg) throughout the study, reaching a peak of 0.014 mg/kg.
The concentrations in egg yolk increased slowly throughout the 14
days, with a peak at necropsy of 0.056 mg/kg (Huang & Smith, 1995d).
Groups of three Wistar rats received an intravenous injection of
[3,4-14C] N-acetylglufosinate (purity, 98%; 1400 MBq/g) into the
tail vein at a dose of 3 mg/kg bw as a solution in saline. Blood
samples were taken from the retro-orbital plexus at 5, 15, 30, and 60
min and 2, 4, 6, 8, 24, 48, 72, and 96 h. The samples were absorbed
onto filter paper and combusted, and the radiolabel was determined by
liquid scintillation counting. The peak concentration (6-7.5 µg/g) was
seen at 5 min, with an initial decline of 4 h, a half-time of 0.3 h,
and a second phase with a half-time of 14 h. The concentration of
radiolabel in blood was at the limit of detection at 24 h. The
integrated area under the curve of concentration-time was ~ 3.8 µg
h/g. There as no significant difference between the sexes (Kellner &
Braun, 1993a,b).
The toxicokinetics of N-acetylglufosinate was investigated in
groups of five Wistar rats of each sex which received
[3,4-14C] N-acetylglufosinate (purity, 98%; 1400 MBq/g) in saline
intravenously at a dose of 3 mg/kg bw. Urine and faeces were collected
over 0-4, 4-8, 8-24, 24-48, 48-72, and 72-96 h. Tissue samples were
taken at 96 h. Autoradiography was performed on one male killed at 96
h. The radiolabel in tissues and excreta was determined by liquid
scintillation counting after appropriate processing. Excretion was
rapid, with > 85% of the radiolabel appearing in the 0-4-h urine
sample. By 96 h, approximately 95% of the dose had been excreted in
urine; faecal excretion accounted for 4% in females and 2% in males.
The excretory half-times were slightly longer in males than in
females. By 96 h, tissue radiolabel accounted for < 0.3% of the dose;
the highest concentrations were found in kidney, with 0.2 µg/g in
males and 0.07 µg/g in females (Kellner et al., 1993)
Biotransformation: Urine and faeces from Wistar rats given
[3,4-14C] N-acetylglufosinate at 3 or 1000 mg/kg bw by gavage in
the studies of Stumpf (1993b) and Lauck-Birkel (1995b), described
above, were extracted and analysed for metabolites by HPLC or
thin-layer chromatography with comparison to standards. The tissue
samples contained insufficient radiolabel for investigation of
metabolites. The extent of metabolism was greater at 3 mg/kg bw,
indicating the presence of a saturable reaction pathway. The main
compound in urine was N-acetylglufosinate, with low concentrations
of 3-[hydroxy(methyl) phosphinoyl]propionic acid and
4-methylphosphinico-butanoic acid. The faeces of animals given the low
dose contained a significant amount of glufosinate-ammonium, which was
not seen in those given the high dose. The study of Kellner et al.
(1993) showed that glufosinate-ammonium is formed in the intestine,
but the extent to which it is systemically available is not clear. The
results for male rats are presented in Table 10; female animals showed
a similar profile.
Table 10. N-Acetylglufosinate and metabolites in 96-h samples from male Wistar
rats given [3,4-14C]-labelled compound at 3 or 1000 mg/kg bw by gavage
Compound Percent administered dose
3 mg/kg bwa 1000 mg/kg bwb
Urine Faeces Urine Faeces
Total radiolabel 5.3 83 7.6 89
N-Acetylglufosinate 4.0 70 7.4 85
Glufodinate ammonium < LD 11 < LD 0.9
3-Methylphosphinicopropionic acid 0.7 0.6 0.1 0.4
4-Methylphosphinicobutanoic acid or 0.6 1.2 0.1 0.1
hydroxy-4-methylphosphinicobutanoic acid
a From Stumpf (1993b)
b From Lauck-Birkel (1995b)
[3,4-14C] N-Acetylglufosinate (98% radiochemical purity;
specific activity, 830 MBq/g) was dissolved in physiological saline
and administered to groups of five male Wistar rats at a dose of 30
mg/kg bw by gavage. Groups of animals were sacrificed 1, 6, or 24 h
after dosing, and samples of blood (for plasma), brain, kidney, and
liver were pooled, processed, and assayed for total radioactivity
(liquid scintillation counting) and metabolites (HPLC). Urine and
faeces were collected over 24 h. Metabolites were not determined in
plasma or brain owing to insufficient total radiolabel. There was
limited metabolism and rapid excretion; > 80% of the recovered
radiolabel in the faeces was N-acetylglufosinate. The concentration
of glufosinate-ammonium in kidney increased with time (Table 11;
Lauck-Birkel & Strunk, 1999d). A similar pattern of absorption,
distribution, and excretion was reported by Maas & Braun (1999c).
Two male Wistar rats received [3,4-14C] N-acetylglufosinate
(purity, > 98%) by gavage in saline at a dose of 3 mg/kg bw. The
animals were killed 4 or 24 h later, and the gastrointestinal tract
was examined for total radiolabel and metabolites. Significant
deacetylation of N-acetylglufosinate was found in the intestine,
giving rise to glufosinate-ammonium (Table 12; Kellner et al., 1993).
[3,4-14C] N-Acetylglufosinate (98% radiochemical purity;
specific activity, 7200 MBq/g) was dissolved in physiological saline
and administered intravenously into the tail vein of groups of five
male Wistar rats at a dose of 3 mg/kg bw. Groups of animals were
sacrificed 2 or 24 h after dosing, and samples of blood (for plasma),
brain, kidney, and liver were pooled, processed, and assayed for total
radiolabel (liquid scintillation counting) and metabolites (HPLC).
Urine and faeces were obtained over 24 h. Metabolites were not
determined in plasma or brain owing to insufficient total radiolabel.
There was limited metabolism and rapid excretion, and over 95% of the
radiolabel recovered in urine was N-acetylglufosinate. At 24 h,
glufosinate-ammonium was present at higher concentrations than
N-acetylglufosinate in kidney (Table 13; Lauck-Birkel & Strunk,
1999c). A similar pattern of distribution and excretion was reported
by Maas & Braun (1999d).
Samples from the study of Huang & Smith (1995c) on goats were
investigated for metabolites. N-Acetylglufosinate and glufosinate
accounted for 52% and 34% of the radiolabel in faeces. respectively.
Glufosinate was the main residue in kidney, liver, and milk, although
N-acetylglufosinate (the administered material) and
3-[hydroxy(methyl) phosphinoyl]propionic acid formed a substantial
proportion of the residue in kidney and liver. The concentration of
glufosinate-ammonium in the kidney (0.7 ppm) was four times that in
the liver (0.095 ppm). This study showed that de-acetylation of
N-acetylglufosinate to glufosinate-ammonium makes a significant
contribution to tissue residues.
Samples from the study of Huang & Smith (1995d) on hens were
investigated for metabolites. N-Acetyl-L-glufosinate disodium salt
comprised 73% of the radiolabel in faeces, with glufosinate and
3-[hydroxy(methyl) phosphinoyl]propionic acid comprising 13% and 8.6%,
respectively. N-Acetylglufosinate (the administered material) was
the main residue identified in liver and egg yolk, and glufosinate and
3-[hydroxy(methyl) phosphinoyl]propionic acid were also substantial
components of the liver residue. Glufosinate was the main residue in
egg white. This study showed that deacetylation of
N-acetylglufosinate to glufosinate-ammonium makes a significant
contribution to tissue residues.
Table 11. N-Acetylglufosinate and metabolites in pooled samples from male Wistar rats given
[3,4-14C]-labelled compound at 30 mg/kg bw by gavage
Compound % of administered dose µg/g equivalent
Urine Faeces Kidney Liver
0-24 h 0-24 h
6 h 24 h 6 h 24 h
Total radiolabel 2.1 88 1.1 0.7 0.5 0.2
N-Acetylglufosinate 1.7 82 0.6 0.04 0.08 0.05
Glufosinate ammonium 0.02 5 0.08 0.73 < LD < LD
3-Methylphosphinicopropionic acid 0.15 < LD 0.19 0.03 0.3 0.02
4-Methylphosphinicobutanoic acid (1% in dose) 0.2 1.4 0.09 < LD 0.03 0.01
LD, limit of determination, < 0.001% of the administered dose
Table 12. Residues in stomach and intestine after oral administration
of 3 mg/kg bw [3,4-14C] N-acetylglufosinate to two male rats
Compound Percent of radiolabel
Stomach Intestine
4 h 24 h 4 h 24 h
Total radiolabel 3.6 91 < 0.01 3.5
Glufosinate ammonium 0.0 2.6 ND 29
4-Methylphosphinicobutanoic acid 0.0 0.8 ND 0.0
3-Methylphosphinicopropionic acid 0.0 0.5 ND 4.5
N-Acetylglufosinate 99.8 96 ND 66
ND, not determined
Effects on enzymes and other biochemical parameters: The main
biological property of glufosinate-ammonium is inhibition of the
enzyme glutamine synthetase, and toxicological studies with
N-acetylglufosinate have also shown inhibition of this enzyme in a
range of tissues. The interpretation of these results is confounded by
the presence of glufosinate-ammonium in the samples of
N-acetylglufosinate tested. In an attempt to determine the degree to
which N-acetylglufosinate inhibits glutamine synthetase, comparative
studies were performed in vitro and in vivo (Lutkemeier, 1999;
Schmid et al., 1999). The studies are summarized in Tables 6 and 7.
In vitro, N-acetylglufosinate produced only marginal inhibition at
13 mmol/L, some of which can be attributed directly to the
glufosinate-ammonium in the sample. When glufosinate-ammonium is
administered orally, it is approximately 10 times more potent as an
inhibitor of glutamine synthetase than is N-acetylglufosinate. The
amount of this inhibition that is due directly to de-acetylation of
N-acetylglufosinate to glufosinate-ammonium is uncertain; however,
the work of Lauck-Birkel & Strunk (1999a,b; Table 11) and the results
in vitro (Table 6) indicate that most of the inhibition in kidney is
due to biotransformation of N-acetylglufosinate to
glufosinate-ammonium.
Acute toxicity: N-Acetylglufosinate has little toxicity when
given as a single oral dose (Table 14). When it was given by
intraperitoneal injection, deaths occurred at the lowest doses tested
(580 mg/kg bw) in both rats and mice, but there was no clear
dose-response relationship in mice. The compound was not tested by
other routes, as it is formed as a metabolite only in plants, and
exposure through the skin or by inhalation is unlikely. The clinical
signs of toxicity seen after oral exposure to N-acetylglufosinate
were reduced respiratory rate, reduced activity, contracted flanks,
and squatting during the first 24 h. The decreased respiratory rate
Table 13. N-Acetylglufosinate and metabolites in pooled samples from male Wistar rats given
[3,4-14C]-labelled compound at 3 mg/kg bw intravenously
Compound Percent of administered dose
Urine Faeces Liver Kidney
(24 h) (24 h)
2 h 24 h 2 h 24 h
Total radiolabel 86 1.8 0.5 0.1 0.9 0.1
N-Acetylglufosinate 85 1.7 0.4 0.1 0.8 0.01
Glufosinate ammonium < LD 0.1 0.01 0.01 0.05 0.06
3-Methylphosphinicopropionic acid < LD < LD 0.04 0.01 < LD 0.001
4-Methylphosphinicobutanoic acid (1% in dose) 1.1 0.02 0.01 < LD 0.01 < LD
LD, limit of determination, < 0.001% of the administered dose
Table 14. Acute toxicity of N-acetylglufosinate (purity, 79.4%; containing
4.5% glufosinate ammonium)
Species (strain) Route LD50 Reference
(mg/kg bw)
Rat (Wistar) Oral, gavage 290 Schollmeier & Leist (1989a)
Mouse (NMRI) Oral, gavage 290 Schollmeier & Leist (1989b)
Rat (Wistar) Intraperitoneal > 1200 Schollmeier & Leist (1989c)
Mouse (NMRI) Intraperitoneal > 2000 Schollmeier & Leist (1989d)
persisted for the duration of the study in mice. Gross examination
showed no abnormalities.
In a Magnusson and Kligman maximization protocol, groups of 20
female Pirbright guinea-pigs, 10 weeks of age, received an intradermal
induction with N-acetylglufosinate (purity, 79.4%; supplied as a
57.9% solution) at 5% in saline (equal to 2.9% N-acetylglufosinate)
and a 1:1 preparation of Freund's adjuvant. For topical induction and
challenge, undiluted test material was applied under an occlusive
dressing. There were no signs of erythema or oedema. Satisfactory,
contemporary data for positive controls were presented (Hofmann &
Jung, 1988). N-Acetylglufosinate was not a skin sensitizer in this
study (Schollmeier & Leist, 1989e).
Short-term studies of toxicity: Groups of five NMRI mice of
each sex were given diets containing N-acetylglufosinate (purity,
79.4%; 4.5% glufosinate-ammonium) at concentrations of 0, 120, 580,
2900, or 5800 ppm for 28 days. The content of N-acetylglufosinate in
the diets was routinely below the nominal value, sometimes by as much
as 30%. The stability and homogeneity of the diets were satisfactory,
providing intakes equivalent to 0, 19, 100, 520, and 1000 mg/kg bw per
day. All animals were examined routinely for a range of observations
and measurements, including basic neurological tests. Samples were
taken for clinical chemical and haematological examinations from all
mice (not fasted) at termination. Limited analysis was performed on
urine samples collected overnight on day 21-22 of the study from
fasted animals. Heart, lung, liver, kidney, spleen, brain, testis, and
ovaries were weighed and examined histologically, as was any tissue
with macroscopic abnormalities. Glutamine synthetase activity was
measured in brain and liver samples that had been cooled rapidly after
removal and kept frozen until assay.
There were no deaths or clinical signs of toxicity and no effects
on body-weight gain or food or water consumption, although the latter
two were very variable. Haematology and clinical chemistry showed no
treatment-related effects, and no substance-related macroscopic or
microscopic changes were seen. Glutamine synthetase activity in liver
and brain was significantly inhibited in animals of each sex at
5800 ppm, with significant inhibition in brain samples from females
and liver samples from males receiving 2900 ppm (Table 15). The NOAEL
was 580 ppm, equivalent to 100 mg/kg bw per day, on the basis of the
statistically significant, > 10% decrease in glutamine synthetase
activity in brains of females receiving 2900 ppm (Ebert, 1991a).
Table 15. Mean glutamine synthetase activity in liver and brain
from mice receiving N-acetylglufosinate in the diet for
28 days
Dose (ppm) Glutamine synthetase activity (nmol/s per mg protein)
Male Female
Liver Brain Liver Brain
0 0.43 1.2 0.38 1.5
120 0.38 1.4 0.5 1.4
580 0.38 1.5 0.4 1.3
2900 0.34* 1.1 0.42 0.92*
5800 0.24* 0.71* 0.22* 0.89*
* Statistically significant at p < 0.05
Groups of 20 NMRI mice of each sex received diets containing
N-acetylglufosinate (purity, 74.7; 0.5% glufosinate-ammonium) at
concentrations of 0, 500, 2000, or 8000 ppm for 13 weeks. The content
of test substance and the homogeneity and stability of the diet were
satisfactory. The achieved intakes were 0, 82, 320, and 1300 mg/kg bw
per day for males and 0, 110, 440, and 1700 mg/kg bw per day for
females at the control, low, intermediate, and high doses,
respectively. The animals were observed routinely for deaths, general
condition, clinical signs, behaviour, food consumption, and body
weight. Blood samples were taken from groups of 10 fasting mice per
sex per group at the end of the study for haematological and clinical
chemical investigations. After sacrifice, all animals were examined
macroscopically, and 10 organs were weighed and more than 30 tissues
from control and high-dose animals were examined histopathologically.
Limited histological examinations were performed on nine tissues from
animals at the low and intermediate doses. Liver, kidney, and brain
samples (pooled samples from two animals for the last two tissues)
were rapidly placed in liquid nitrogen before assay for glutamine
synthetase activity.
One male at 8000 ppm died after blood sampling. There were no
treatment-related effects on food consumption, body weight, clinical
signs, organ weights, macroscopic or macroscopic appearance, or
haematological parameters. The activity of serum lactate dehydrogenase
was increased by 180% over controls in males at the two higher doses,
but the results were within the normal range. Apparent increases in
the activities of a number of serum enzyme in females at the high dose
were due to a high value in a single animal and are considered to be
unrelated to treatment. The main finding was a dose-related decrease
in glutamine synthetase activity in liver, kidney, and brain (Table
16). Inhibition of liver and kidney glutamine synthetase activity in
isolation is not relevant to human risk assessment; however, there was
> 10% inhibition of glutamine synthetase activity in brain at doses
> 2000 ppm, and the mean values for animals of each sex were
outside the control range. The NOAEL was 500 ppm, equal to 82 mg/kg bw
per day (Tennekes et al., 1992a).
Table 16. Glutamine synthetase activity in groups of 10 mice receiving
N-acetylglufosinate in the diet for 90 days
Sex Dose (ppm) Glutamine synthetase activitya
Kidney Liver Brain
Mean Range Mean Range Mean Range
Male 0 1.4 1.2-1.7 4.0 3.2-4.8 3.6 3.4-3.8
500 1.0* 0.7-1.2 3.7 2.7-4.4 3.3* 3.1-3.6
2000 0.83* 0.6-1.1 3.3* 2.5-4.6 3.2* 2.9-3.4
8000 0.71* 0.4-0.9 2.9* 2.4-4.2 2.6* 2.4-2.9
Females 0 1.7 1.5-2.1 5.0 3.9-5.5 3.4 2.9-3.6
500 1.3* 1.1-1.5 4.9 4.2-5.4 3.3 3.0-3.5
2000 1.2* 0.9-1.4 4.6 3.8-5.3 2.9* 2.7-3.2
8000 1.1* 0.7-1.3 4.0* 3.2-5.5 2.2* 1.9-2.5
* p < 0.01
a µmol gamma-glutamyl hydroxamate formed per ml reaction mixture in 20 min at 37°C
Groups of five Wistar rats received diets containing
N-acetylglufosinate (purity, 79.4%; 4.5% glufosinate-ammonium) at
concentrations of 0, 120, 580, 2900, or 5800 ppm for 28 days. The
overall content, stability, and homogeneity of the test diets were
stated to be acceptable. The achieved intakes (assuming 100% analysis)
were 0, 12, 59, 310, and 590 mg/kg bw per day for males and 0, 11, 55,
280, and 560 mg/kg bw per day for females. All animals were examined
routinely for a range of observations and measurements, and control
and high-dose animals underwent a basic functional observation
battery. Samples were taken for extensive clinical chemical and
haematological examinations from all rats (not fasted) at the end of
the study. Limited analysis was performed on urine samples collected
overnight on day 21-22 of the study from fasted animals. Heart, lung,
liver, kidney, spleen, brain, testis, ovaries, and adrenal, pituitary,
and thyroid glands were weighed and examined histologically, as was
any tissue with macroscopic abnormalities. Glutamine synthetase
activity was measured in brain and liver samples that had been cooled
rapidly after removal and kept frozen until assay.
There were no deaths or clinical signs of toxicity and no effects
on body-weight gain or food or water consumption. 'Thrombin time' was
mildly increased (< 13%) in females at doses > 2900 ppm and in
males at 120, 580, and 5800 ppm; other measures of coagulation were
unaltered. A statistically significant decrease in lactate
dehydrogenase activity was found in females at doses > 2900 ppm,
which may have been related to treatment as there was evidence of a
dose-response relationship. Slight decreases in the absolute and
relative weights of the heart in males at 5800 ppm was of no clear
toxicological significance in the absence of a histological correlate.
No treatment-related macroscopic or microscopic changes were seen.
Glutamine synthetase activity in the liver was significantly inhibited
at doses > 580 ppm in animals of each sex, and was reduced in the
brains of all treated animals, although there was no clear
dose-response relationship (Table 17). Decreased activity of glutamine
synthetase in liver was considered not to be adverse, and the
alterations in glutamine synthetase activity in brain were not
consistent. There were no biologically significant changes in any
other parameter. The NOAEL was 5800 ppm, equal to 560 mg/kg bw per
day, the highest dose tested (Ebert, 1991b).
Table 17. Mean glutamine synthetase activity in liver and brain
samples from rats receiving N-acetylglufosinate in the
diet for 28 days
Dose (ppm) Glutamine synthetase activity (nmol/s per mg protein)
Male Female
Liver Brain Liver Brain
0 0.27 1.5 0.31 1.3
120 0.28 1.1* 0.33 1.1
580 0.17* 0.9* 0.27 1.1*
2900 0.17 1.0* 0.23 1.0*
5800 0.14* 1.3 0.15* 1.1*
* Statistically significant at p < 0.05
Groups of 20 Wistar rats of each sex received diets containing
N-acetylglufosinate (purity, 74.7%; 0.5% glufosinate-ammonium) at
concentrations of 0, 2000, or 10 000 ppm for 13 weeks; a group of 10
animals of each sex received 400 ppm. The content, homogeneity, and
stability of the test compound in the diet were satisfactory, and the
achieved intakes were 0, 29, 150, and 740 mg/kg bw per day for males
and 0, 31, 160, and 800 mg/kg bw per day for females. At 13 weeks, 10
males and 10 females in each group were killed, and the remainder were
allowed to recover for 4 weeks. The animals were observed regularly
for deaths, clinical signs, body weight, food consumption, and tissue
masses. Ophthalmoscopic examinations were performed before treatment
and at 11 and 16 weeks. Blood and urine samples were taken from fasted
animals at weeks 13 and 17 for clinical chemical and haematological
investigations. At necropsy, 10 organs were weighed and examined
macroscopically. An extensive range of tissues from control and
high-dose animals was examined histologically, as were major organs
from animals at the intermediate and low doses. Glutamine synthetase
activity was measured in brain, kidney, and liver samples that had
been rapidly cooled after removal and kept frozen until assay.
There were no deaths, clinical signs of toxicity, or effects on
body weight, the eyes, or urine. Food consumption was reduced during
the first week of treatment but not subsequently. A number of clinical
chemical parameters showed variations, but these were generally due to
values for individual animals or were within normal ranges. A decrease
in serum sodium concentration in animals at the high dose at 13 weeks
appeared to be related to treatment. Glutamine synthetase activity was
inhibited in liver, kidney, and brain (Table 18), and the effect was
significantly but not completely reversed after 4 weeks. A reversible
increase in kidney weight (< 15%) was seen in males at all doses, but
this finding was considered not to be adverse because there was no
clear dose-response relationship and no associated histological
change. There were no treatment-related macroscopic or microscopic
findings. The NOAEL was 2000 ppm, equal to 150 mg/kg bw per day, on
the basis of inhibition of glutamine synthetase activity in the brain
(Tennekes et al., 1992b).
Groups of four beagle dogs of each sex, aged 5-7 months, received
diets containing N-acetylglufosinate (purity, 74.7%; 0.5%
glufosinate-ammonium) at concentrations of 0, 500, 2000, or 8000 ppm
in 400 g of diet daily for 13 weeks. Additional groups of two animals
of each sex received the same diets and were then allowed to recover
for 4 weeks. The content, homogeneity, and stability of the test diet
were satisfactory. The achieved intakes were 0, 19, 72, and 290 mg/kg
bw per day for males and 0, 21, 79, and 300 mg/kg bw per day for
females. The animals were observed routinely for deaths, general
condition, clinical signs, behaviour, food consumption, and body
weight. Ophthalmoscopic examinations were performed on all animals
before treatment, at weeks 4 and 13, and at termination in the group
allowed to recover. Blood and urine samples were collected from fasted
animals before treatment, at weeks 4 and 13, and at the end of the
recovery period. At termination, all dogs were examined
macroscopically; a range of tissues were weighed, and > 30 tissues
Table 18. Glutamine synthetase activity in groups of 10 rats receiving N-acetylglufosinate in
the diet for 90 days and after a 4-week recovery period
Sex Period Dose Glutamine synthetase activitya
(days) (ppm)
Liver Kidney Brain
Mean Range Mean Range Mean Range
Male 90 0 3.8 3.3-4.3 2.1 1.6-2.3 3.2 3.1-3.3
400 2.8* 2.0-3.4 1.7* 1.5-1.9 3.3 3.1-3.6
2000 2.2* 1.9-2.4 1.5* 1.3-1.8 3.0 2.9-3.3
10 000 1.8* 1.3-2.2 1.6* 1.4-1.9 2.8* 2.6-3.1
90 + recovery 0 3.2 2.5-3.8 2.1 1.9-2.3 3.1 2.9-3.4
2000 3.5 3.2-3.9 2.1 1.6-2.4 3.0 2.6-3.4
10 000 3.4 2.9-4.1 1.9 1.2-2.5 2.9* 2.7-3.1
Female 90 0 3.7 2.8-4.3 1.2 1.0-1.3 3.1 2.8-3.3
400 3.1 2.2-3.5 1.1 1.0-1.2 3.1 2.9-3.2
2000 2.6* 1.8-3.2 1.2 1.1-1.5 3.0 2.9-3.2
10 000 2.4* 1.9-2.8 1.5* 1.4-1.7 2.8* 2.6-2.9
90 + recovery 0 3.7 3.2-4.1 1.3 1.2-1.4 3.1 3.0-3.3
2000 3.5 3.1-4.0 1.3 1.1-1.5 3.1 2.9-3.4
10 000 3.3 2.6-3.9 1.3 1-1.5 2.9 2.7-3.1
* p < 0.01
a µmol gamma-glutamyl hydroxamate formed per ml reaction mixture in 20 min at 37°C
were preserved and examined histopathologically. Liver, kidney, and
brain samples were immediately placed in liquid nitrogen and kept
frozen at -80°C before assay for glutamine synthetase activity;
samples of these tissues were also retained for future analysis.
There were no deaths or treatment-related clinical signs or
effects on food consumption, body weight, or ophthalmoscopic or
haematological parameters. A statistically significant, dose-dependent
decrease in glutamine synthetase activity was found in liver and brain
after 13 weeks of treatment (Table 19), which tended to return to
normal during the recovery period although it was not complete at the
end of the 4 weeks. The changes in glutamine synthetase activity were
not consistent in different tissues from the same animal. Activity in
the brain stem and cerebellum appear to be more sensitive to
inhibition than that in the cortex. Decreased creatine kinase activity
was seen at the high dose in males at 13 weeks (17%) and in females at
weeks 4 and 13 (30%). Lactate dehydrogenase activity was decreased by
30% in females at this dose at week 13 but in neither males nor
females after recovery. Exacerbation of the low pretreatment specific
gravity and osmolality of the urine of females was seen at at 8000 ppm
in weeks 4 and 13 of treatment and at the end of the recovery period.
The significance of this finding is unclear as it was not seen in a
1-year study in dogs. It is of note, however, that the kidney is a
target organ in rats.
A dose-related decrease in prostate gland weight was seen which
achieved statistical significance at 8000 ppm (40%) at 13 weeks, but
there was no histological correlate. The prostate weights were similar
to those of controls after 4 weeks' recovery from the dose of
2000 ppm, but not after administration of 8000 ppm. Although the
authors noted that slight differences in the rate of maturity of dogs
of this age can affect prostate size, the dose-response relationship
and evidence of recovery at 2000 ppm but not at 8000 ppm indicate an
association with treatment. No treatment-related effects were seen on
macroscopic examination. The only histopathological finding of note
was an increased incidence of pituitary cysts in animals receiving
8000 ppm for 13 weeks: 3/4 in animals of each sex and 1/4 in male
controls and 0/4 in female controls. The NOAEL was 500 ppm, equal to
19 mg/kg bw per day, on the basis of > 10% reductions in glutamine
synthetase activity in a number of areas of the brain. The effects on
prostate weight, urinary parameters, and the pituitary indicate that
8000 ppm was an effect level (Corney et al., 1992)
Groups of six beagle dogs of each sex received diets containing
N-acetylglufosinate (purity, 92.4%; 0.1% glufosinate-ammonium) at
concentrations of 0, 100, 1000, or 8000 ppm. Analyses of the diets
for homogeneity and content were satisfactory, and the overall intakes
of N-acetylglufosinate were 0, 4, 44, and 320 mg/kg bw per day for
males and 0, 4.4, 43, and 350 mg/kg bw per day for females. Two
animals of each sex per group were killed at 26 weeks and the
remainder at 52 weeks. The animals were observed routinely for
clinical signs, deaths, body weight, and food consumption.
Table 19. Glutamine synthetase activity in dogs receiving N-acetylglufosinate in the diet for 90 days with or without a 4-week recovery
period
Sex Period No. Dose Glutamine synthetase activitya
(ppm)
Liver Kidney Mid-brain Cerebellum Brain stem Brain cortex
Mean Range Mean Range Mean Range Mean Range Mean Range Mean Range
Male 13 weeks 4 0 2.7 2.6-2.9 0.02 0.01-0.04 2.3 1.8-2.7 1.6 1.5-1.8 1.4 1.2-1.5 3.2 2.8-3.5
500 1.9 1.4-2.2 0.04 0.02-0.07 2.6 1.9-2.9 1.4 1.2-1.5 1.3 1.2-1.4 3.4 3.3-3.4
2000 1.2 1.0-1.3 0.06 0.03-0.10 2.6 1.8-2.9 1.3 1.0-1.4 0.90 0.8-1.1 2.8 2.3-2.9
8000 0.58 0.5-0.7 0.05 0.01-0.14 1.4 1.7-2.6 0.85 0.7-1.0 0.47 0.4-0.5 2.3 1.8-2.8
Recovery 2 0 2.6 0.07 2.1 1.4 1.3 3.0
2000 2.4 0.05 2.3 1.2 0.95 2.4
8000 2.0 0.06 2.1 1.2 0.93 2.5
Females 13 weeks 4 0 1.8 1.6-2.1 0.04 0.02-0.06 2.7 2.2-3.0 1.6 1.5-1.7 1.2 1.1-1.3 2.9 2.7-3.3
500 1.6 1.2-2.4 0.10 0.05-0.17 2.2 1.9-2.5 1.5 1.4-1.5 1.2 1.0-1.3 3.2 2.9-3.3
2000 1.0 0.8-1.5 0.06 0.03-0.09 2.4 1.8-2.9 1.3 1.2-1.4 0.91 0.7-1.0 2.7 2.4-3.3
8000 0.68 0.5-0.9 0.09 0.01-0.15 2.0 1.7-2.6 0.87 0.8-0.9 0.73 0.6-0.8 2.5 1.9-2.8
Recovery 2 0 2.1 0.05 2.1 1.5 1.4 3.1
2000 1.7 0.08 2.1 1.3 1.2 2.7
8000 1.3 0.07 1.8 1.1 0.83 2.4
a µmol gamma-glutamyl hydroxamate formed per ml reaction mixture in 20 min at 37°C
Ophthalmoscopic examinations were performed before treatment and at
weeks 12, 25, and 51. Samples were taken from fasted animals for
urinary analysis, haematology, and clinical chemistry before treatment
and at weeks 13, 26, and 52. Post-mortem examinations were performed
on all animals; nine organs were weighed and > 30 tissues examined
histopathologically. Glutamine synthetase activity was not analysed.
The only death occurred in a male at the intermediate dose; the
cause was not found. The incidence of soft faeces was increased in
animals at the high dose and particularly in males. Reduced
body-weight gain was seen in animals at this dose, by 17% in males and
30% in females, during the first half of the study, and the effect
persisted in females until termination. There was no evidence of
treatment-related effects in ophthalmoscopic, haematological, or
urinary examinations and no effects on organ weights or gross or
histopathological appearance at either the interim or terminal
sacrifice. Reduced serum lactate dehydrogenase activity was seen
consistently in animals at the high dose (Table 20). Although there
was some overlap between the ranges for control and treated animals
and variation in control values over time, the consistency of the
effect in males indicates that it is possibly related to treatment.
The effects at 8000 ppm are not significant in isolation, but the
combination of findings in males indicates that this dose is an effect
level. The NOAEL was 1000 ppm, equal to 44 mg/kg bw per day, on the
basis of reduced body-weight gain, reduced lactate dehydrogenase
activity, and an increased incidence of soft faeces (Bernier, 1996).
Table 20. Lactate dehydrogenase activity in dogs fed diets containing
N-acetylglufosinate at 8000 ppm
Group No. Week Serum lactate dehydrogenase activity (U/L)
Males Females
Mean Range Mean Range
Controls 6 0 75 47-100 160 120-230
6 13 100 66-160 160 52-260
6 26 200 87-430 220 99-470
4 52 90 66-100 320 160-460
Treated 6 0 97 57-140 74 32-130
6 13 53 43-68 44 31-65
6 26 99 39-180 88 48-110
4 52 70 50-88 150 110-180
Long-term studies of toxicity and carcinogenicity: Groups of 90
Swiss Crl:CD-1 mice received diets containing N-acetylglufosinate
(purity, 92.4%; 0.1% glufosinate-ammonium) at concentrations of 100,
1000, or 8000 ppm. Groups of 20 animals were killed after 1 year and
the remainder after 2 years. The mice were housed singly, observed
daily, and monitored routinely for body weight and food consumption.
The homogeneity, content, and stability of the diet were acceptable,
and the achieved intakes were 0, 15, 150, and 1200 mg/kg bw per day
for males and 0, 19 190, and 1500 mg/kg bw per day for females.
Samples for clinical chemistry and haematology were taken from fasted
animals at interim sacrifice (all animals) and at termination (10 per
sex per group). Blood was taken from the tail vein at week 78. At
autopsy, the animals were examined macroscopially, nine organs were
weighed, and more than 30 tissues were preserved. Histological
examinations were conducted on tissues from all controls, animals at
the high dose, and those that died during the study and on the liver,
lung, kidney, and adrenals from animals at the low and intermediate
doses killed after 1 or 2 years.
Although the report stated that no treatment-related signs were
observed, data on individual animals were not presented, as this was
primarily a study of carcinogenicity. Survival was similar in all
groups (> 60% at week 90). Fluctuations in food consumption and body
weight showed no consistent pattern related to dose. There were no
effects on clinical chemical or haematological parameters or on organ
weights, any variation in group means being attributable to individual
values. Macroscopic examination detected no treatment-related effects.
Histopathological examination showed an increased incidence of
amyloidosis in various organs in males and females at the high dose
and testicular necrosis in males, although the only statistically
significant finding ( p = 0.028) was amyloidosis in the salivary
gland of females (Table 21). A high incidence of kidney lesions was
seen in both control and treated animals at 1 and 2 years, with no
significant differences between groups. Increased incidences of
uterine adenocarcinoma and thyroid follicular-cell adenoma were seen
in animals at the high dose; the incidences were not statistically
significantly increased when compared with concurrent controls but
were slightly greater than those of historical controls. The overall
incidences of benign and malignant tumours were similar in treated and
control groups. As the tumours that occurred at increased incidences
occur spontaneously in this strain of mouse, the findings were
considered not to indicate a carcinogenic potential of
N-acetylglufosinate. The NOAEL was 1000 ppm, equal to 150 mg/kg bw
per day, on the basis of an increased incidence of amyloidosis
(Farrell, 1997; Ernst & Stumpf, 1999a).
Groups of 70 Sprague-Dawley-derived rats of each sex received
diets containing N-acetylglufosinate (purity, 92.4%; 0.1%
glufosinate-ammonium) at concentrations of 0, 200, 2000, or 20 000 ppm
for 2 years; additional groups of 10 animals of each sex were killed
at 1 year and 20 at 2 years. The animals were housed singly, observed
daily, and monitored routinely for body weight and food consumption.
The homogeneity, content, and stability of the test diet were
Table 21. Incidences of lesions in mice receiving N-acetylglufosinate in the diet for 2 years, and ranges for
historical controls
Sex Lesion Incidence
0 100 ppm 1000 ppm 8000 ppm Historical controls
Male Testicular degeneration and necrosis 0/70 1/55 2/56 4/70 6-39% (for atrophy)
Testicular atrophy 25/70 28/55 30/56 26/70 6-39% (for atrophy)
Thyroid follicular-cell adenoma 0/70 0/69 1/69 3/70 0-2%
Female Salivary gland amyloidosis 1/70 1/45 3/43 7/70 No data
Liver haemangiosarcoma 0/70 1/70 1/70 2/70 0-4%
Uterine adenocarcinoma 0/70 2/69 1/69 3/70 0-2%
acceptable, and the achieved intakes were 0, 9, 91, and 1000 mg/kg bw
per day for males and 0, 11, 110, and 1200 mg/kg bw per day for
females. Samples for clinical chemistry and haematology were taken
from fasted rats in the interim sacrifice and satellite groups at 25,
51, 78, and 102 weeks. At necropsy, the animals were examined
macroscopically, nine organs from interim sacrifice animals were
weighed, and > 30 tissues were preserved. Full histopathological
examinations were performed on all controls, animals at the high dose,
and animals that died during the study; only liver, lung, kidney, and
adrenals from animals at the low and intermediate doses were examined
histopathologically.
Survival was similar in all groups (> 50% at week 96). Soft
faeces were more prevalent in animals at the high dose and especially
in males from week 8 onwards. Body-weight gain was reduced (~ 5%) in
males at this dose from week 16 onwards and in females (~10%) from
week 24, even though the food consumption of these groups increased by
5-10%. A range of changes in clinical chemistry and haematology were
seen repeatedly in animals of each sex at the high dose, including
reduced prothrombin time, potassium concentration, and lactate
dehydrogenase and creatine kinase activity, and increased sodium,
calcium, inorganic phosphorus, and glucose concentrations; blood urea
nitrogen was increased in males from week 25 onwards. In animals
killed after 1 year, the absolute and relative weights of the kidney
were increased in males receiving 20 000 ppm and in females receiving
2000 or 20 000 ppm. Macroscopic examination showed increased
incidences of a range of abnormalities of the kidney in treated
animals that were confirmed at histological examination. Renal lesions
are common in aged rats, and the incidences in treated animals in this
study were not statistically significant and were within the range
seen in historical controls; furthermore, they may have been
exacerbated by the high sodium content induced by
N-acetylglufosinate. They were therefore considered irrelevant to
the assessment of the study. Alterations were also seen in the spleen,
parathyroid, heart, blood vessels and adrenals at the high dose and in
some animals at the intermediate dose animals (Table 22). The
incidence of tumours was not statistically significantly increased,
although low incidences of uncommon tumours of the brain, liver,
adrenals, skin, and pancreas were seen in animals at the high dose
(Table 23). Although these incidences are outside the range in
historical controls, the sporadic nature of the findings and the clear
NOAEL for tumours at 2000 ppm indicate that N-acetylglufosinate does
not have significant carcinogenic potential. The overall incidences of
benign and malignant tumours were similar in all groups. The NOAEL was
200 ppm, equal to 9 mg/kg bw per day, on the basis of increased
incidences of polyarteritis nodosa, adrenal cortical hyperplasia, and
necrosis and extramedullary haematopoiesis of the spleen (Bernier,
1997; Ernst & Stumpf, 1999b).
Table 22. Incidences of non-neoplastic lesions in rats receiving N-acetylglufosinate in the diet for up to 2 years,
and ranges for historical controls
Sex Lesion Incidence
0 200 ppm 2000 ppm 20 000 ppm Historical
controls
Male Parathyroid hyperplasia 13/62 8/37 16/32 20/64 14-42%
Adrenal focal cortical hyperplasia 17/70 18/70 29/70 27/70 0-20%a
Adrenal necrosis 0/70 0/70 2/70 3/70 0-4%
Polyarteritis nodosa (blood vessels) 4/7 5/7 4/9 11/18 No data
Polyarteritis nodosa (testis; 2 years; main group) 15/70 13/53 13/50 23/70 16-31%
Polyarteritis nodosa (testis; 2 years; satellite group) 3/20 4/16 8/17 10/20 16-31%
Female Parathyroid hyperplasia 1/52 0/26 0/31 6/61 1-12%
Extramedullary splenic haematopoiesis 7/70 4/34 16/41 20/70 15-36%
Cardiomyopathy 12/70 5/30 6/41 22/70 1-70%
a Current study, all > 24%
Table 23. Incidences of neoplastic lesions in groups of 70 rats receiving N-acetylglufosinate in the diet
for 2 years, and ranges for historical controls
Sex Lesion Incidence
0 200 ppm 2000 ppm 20 000 ppm Historical controls
Male Adrenals: malignant phaeochromocytoma 0 1 1 3 0-3%
Brain: meningioma 0 0 0 1 0-1%
Brain: astrocytoma 0 1 0 1 0-2% (malignant
glioma)
Skin: keratocanthoma 4 5 1 9 (7)* 0-8%
Female Brain: oligodendroglioma 0 0 0 1 No data
Liver: cholangiocarcinoma 0 0 0 1 No data
Lung: alveolar/bronchiolar carcinoma 0 0 0 1 No data
Pancreas: islet-cell carcinoma 1 2 1 4 0-2%
* Re-evaluation of data indicates that 7 is the correct value.
Genotoxicity: An extensive range of assays for genotoxicity
have been performed with N-acetyl-glufosinate both in vitro and
in vivo (Table 24). Some of the protocols were less than optimal
with respect to length of exposure (e.g. for unscheduled DNA
synthesis) or the highest concentration tested, but these limitations
are considered not to affect the overall assessment of genotoxic
potential significantly. The Committee concluded that
N-acetylglufosinate is not genotoxic.
Reproductive toxicity: In a single-generation, range-finding
study in groups of 10 Sprague-Dawley rats of each sex that received
N-acetylglufosinate (purity, 92.4%; 0.1% glufosinate-ammonium), no
adverse effects were seen on fertility, reproduction, or development
at doses of 200, 2000, or 10 000 ppm (equivalent to 14, 140, or 700
mg/kg bw per day). Exposure was continuous from 21 days before mating
to day 21 post partum (Beyrouty, 1996a).
Groups of 30 Sprague-Dawley-derived rats received diets
containing N-acetylglufosinate (purity, 92.4%; 0.1%
glufosinate-ammonium) at concentrations of 0, 200, 2000, or 10 000 ppm
for two generations. The F0 generation was exposed from 70 days
before mating until the end of lactation, and F1 pups were exposed
from weaning (10-12 weeks before mating) until the end of lactation of
F2 pups. The stability and homogeneity of the diet were
satisfactory, and the mean achieved intakes were 0, 13, 140, and 700
mg/kg bw per day for males and 0, 18, 170, and 890 mg/kg bw per day
for females. Parental animals were observed routinely for clinical
signs, estrus cycling, body weight, and food consumption, and the
frequency of observation was increased during gestation and weaning.
Parturition was observed when possible. Pups were examined for
malformations, sex, viability, and body weight, and the litters were
culled to four pups of each sex on day 4. All parental animals, one
pup of each sex per litter, and any pups that died or were killed when
moribund were examined post mortem. The major organs and
reproductive tissues from all adult animals were examined
macroscopically, and these tissues from control and high-dose animals
were examined histologically. Sperm motility, count, and morphology,
and the number of spermatids were determined for all adult males.
One male at the intermediate dose and two at the high dose died
from unknown causes, and males of both generations at the high dose
had an increased incidence of soft faeces and brown staining of the
scrotum. There were no effects on body weight, although the food
consumption of animals at the high dose was slightly increased during
the first few weeks of treatment. No effects were seen on the weight
or histological appearance of organs from F0 animals or on mating
indices, length of gestation, estrus cycling, sperm parameters,
macroscopic appearance, litter size, pup survival, or pup weight in
either generation. A dose-related increase in the weight of the
seminal vesicles was seen in F1 adults, which reached statistical
significance ( p < 0.05; 14%) in males at the high dose. As there
were no associated abnormal histological findings and no functional
deficit, the effect was considered not to be adverse. An increased
Table 24. Results of studies of the genotoxicity of N-acetylglufosinate
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium TA98, 0, 2, 12, 59, 291, 1455, 79.4 Negative +S9 Muller (1989c)
TA100, TA1535, 2910, 5820 µg/plate Negative - S9
TA1537, TA1538;
E. coli WP2 uvrA
Gene mutation V79 Chinese hamster 0, 582, 873, 1164, 79.4 Negative +S9 Muller (1989a)
lung cells, Hprt locus 1554 µg/ml Negative - S9
Gene mutation V79 Chinese hamster 0, 444, 666, 888, 74.7 Negative +S9 Muller (1991b)
lung cells, Hprt locus 1186 µg/ml Negative - S9
Unscheduled DNA Human cell line A549 0, 1, 4, 13, 44, 133, 74.7 Negative +S9 Muller (1991c)
synthesis 444, 1332 µg/ml Negative - S9
Unscheduled DNA Human cell line A549 0, 0.6, 1.8, 5.8, 17, 58, 79.4 Negative +S9 Muller (1989d)
synthesis 175, 582 µg/ml, 3-h Negative - S9
exposure
Chromosomal Human lymphocytes 0, 0.6, 3.0, 5.0 mg/ml 74.7 Negative +S9 Heidemann &
aberrations at 24 h Voelkner (1992)
0, 5.0 mg/ml at 48 h Negative - S9
Chromosomal V79 Chinese hamster 0, 154, 773, 1546 µg/ml 79.4 Negative +S9 Muller (1989b)
aberrations lung cells for 18 h
0, 1546 µg/ml for 7 or Negative - S9
28 h
Table 24. (continued)
End-point Test object Concentration Purity Results Reference
(%)
In vivo
Micronucleus NMRI mouse bone 0, 222, 1111, 2222 74.7 Negative Muller (1991a)
formation marrow mg/kg bw by gavage for
24, 48 or 72 h
incidence of extramedullary haematopoiesis in the livers of F1 males
(8/30 versus 2/30 in controls) was also considered not to be adverse
as it is a common finding in rats. There were no effects on
reproduction. These minor effects on seminal vesicle weight and liver
extramedullary haematopoiesis in F1 males at 10 000 ppm combined
with the increased incidence of soft stools indicated that this is a
minimal effect level. The NOAEL was 2000 ppm, equal to 140 mg/kg bw
per day (Beyrouty, 1996b).
Developmental toxicity: Twenty mated female Wistar rats
received N-acetylglufosinate (purity, 74.7%) by gavage in distilled
water at the limit dose of 1000 mg/kg bw per day on days 7-16 of
gestation, whereas 21 controls received starch mucilage in distilled
water. The dose was based on the results of a preliminary study. The
dams were observed routinely for clinical signs, body weight, and food
consumption. At sacrifice on day 21, they were examined
macroscopically, and the uterus was opened to determine the numbers of
live and dead fetuses and resorptions, fetal weights, the sex ratio,
crown-rump lengths, and placental weights. All fetuses were examined
for external malformations; half were investigated by Wilson
sectioning for visceral abnormalities and the remainder stained with
Alizarin red S for visualization of skeletal defects.
There were no deaths or treatment-related clinical signs among
the pregnant dams, but a non-pregnant control was killed during the
study. The food consumption and body-weight gain of treated animals
were slightly reduced (~ 5%) on days 7-14 of gestation but were
similar to those of controls after cessation of dosing. There was no
effect on pregnancy rate, but a slight increase in pre- and
post-implantation losses resulted in a marginally reduced litter size
(13.6 in controls versus 12.7 in treated animals); the value was
within the typical range. No malformations were recorded, and there
was no increase in the incidence of skeletal or external
abnormalities. The incidence of blood in the pericardium/abdomen was
increased in the treated group (5/132 fetuses, 4/20 litters) when
compared with concurrent controls (2/140 fetuses) and historical
controls (0-1.5%). Fetal weight, crown-rump length, placental weight,
and sex ratio showed no treatment-related effects. The NOAEL for
maternal and fetotoxicity was 1000 mg/kg bw per day, the only dose
tested (Horstmann & Baeder, 1992).
Groups of 15 mated, 8-10-month-old, female Himalayan rabbits
received N-acetylglufosinate (purity, 92.4%) by gavage in distilled
water on days 6-18 of gestation at doses of 0, 64, 160, or 400 mg/kg
bw per day, on the basis of the results of a study that found maternal
and fetal toxicity at 500 mg/kg bw per day. The animals were housed
under standard conditions and observed daily. Body weights were
determined on days 0, 6, 13, 19, and 29 of gestation, and the dams
were killed on day 29 and examined macroscopically. The uteri were
opened, and the numbers of live and dead fetuses and resorptions were
determined. Live fetuses were removed to an incubator and their
survival was monitored for 24 h. Fetal body weight, crown-rump length,
and sex were determined, and external visceral and skeletal
examinations were performed by Wilson sectioning and Alizarin red S
staining. The combined incidences of some lesions were presented only
in summary tables, but this did not compromise the overall integrity
of the study.
There were no deaths during the study, and no consistent clinical
signs in dams. Abortions by one dam at the low dose and one at the
intermediate dose were considered not to be related to treatment. Food
consumption was reduced in relation to dose, by 12% in the groups at
the low dose, 23% at the intermediate dose, and 37% at the high dose,
but there were no consistent effects on body weight. Treatment had no
effect on corpora lutea, pregnancy rate, implantation, resorptions,
gravid uterine weight, or fetal survival. The pup weights were reduced
by 7% at 400 mg/kg bw per day. Morphological examination of fetuses
revealed an increased incidence of a supernumerary thoracic ribs
(2/90, 0/82, 8/73, and 11/93 in control, low-, intermediate-, and
high-dose groups, respectively, while the range among historical
controls was 0-12%). The only malformation reported was a case of
hydrocephalus in a fetus at the high dose; the incidence in historical
controls was 0-9%. The NOAEL for maternal toxicity and fetotoxicity
was 64 mg/kg bw per day on the basis of reduced food consumption in
dams and extra ribs in fetuses (Baeder & Hofmann, 1994; Ernst & Leist,
1999b).
Special studies on neurotoxicity: No overt neurotoxicity was
seen in basic functional observation batteries of tests with
N-acetylglufosinate included in short-term studies of toxicity in
mice and rats, nor does this substance belong to a class of compounds
with neurotoxic potential.
(ii) 3-[Hydroxy(methyl) phosphinoyl]propionic acid
3-[Hydroxy(methyl) phosphinoyl]propionic acid is a metabolite of
glufosinate-ammonium in rats and represents ~ 30% of the residue in
liver (Table 4). It may also represent a significant proportion of the
residue arising from administration of glufosinate-ammonium to plants,
goats, and hens (Annex 1, reference 83). In 1991, the Meeting
reviewed a 28-day study in rats and a study of the toxicokinetics of
3-[hydroxy(methyl) phosphinoyl]propionic acid, which showed that it is
rapidly and extensively absorbed and excreted, has little toxicity
(NOAEL, 280 mg/kg bw per day), and does not inhibit hepatic glutamine
synthetase activity. The 1998 Joint Meeting considered that
3-[hydroxy(methyl) phosphinoyl]propionic acid should be included in
the residue definition (Annex 1, reference 83).
A summary of various additional studies on 3-[hydroxy(methyl)
phosphinoyl]propionic acid has been submitted (Bremmer & Leist, 1998),
although the original reports were not made available. The summary
indicates that the acute toxicity of the substance is low, it is not a
skin sensitizer, and had no significant toxicity in studies in rats
(13 weeks; NOAEL, 560 mg/kg bw per day), mice (13 weeks; NOAEL, 1400
mg/kg bw per day), or dogs (15 weeks; NOAEL, 110 mg/kg bw per day)
given repeated doses. An extensive range of tests for genotoxicity was
reported to show that 3-[hydroxy(methyl) phosphinoyl]propionic acid
does not induce gene mutation in vitro, chromosomal aberrations
in vitro or in vivo, micronuclei in vivo, or DNA damage
in vitro or in vivo. Studies of developmental toxicity in rats and
rabbits showed evidence of maternal toxicity and mild fetotoxicity but
no teratogenicity, with cited NOAELs of 300 mg/kg bw per day in rats
and 50 mg/kg bw per day in rabbits.
3. Observations in humans
(a) Medical surveillance of personnel in manufacturing plants
No specific health problems were reported in workers during 18
years of production of glufosinate-ammonium. The exposure of the
workers was stated to be low, but no details of the extent of
investigations were available (Kaleja, 1999).
(b) Poisoning incidents
A pesticide formulation containing glufosinate-ammonium was
reported to have been involved in over 200 cases of attempted suicide
in Japan (Ernst & Leist, 1999c). The formulation contained 18% w/w of
glufosinate-ammonium, 30% surfactant, and a glycol ether solvent, and
it was not clear whether the effects seen were attributable to
glufosinate-ammonium. The initial signs were vomiting, diarrhoea, and
nausea. The main clinical concerns were the delayed (hours to 2 days)
neurological effects, including convulsions, impaired consciousness,
tremor, and coma, and extensive oedema. Treatment included gastric
lavage, diuretics, intravenous fluids, sedatives, haemoperfusion, and
artificial ventilation. After apparent recovery from the physical
signs, long-lasting amnesia, both retrograde and anterograde was
reported, although this may have been linked to the use of high-dose
benzodiazepine therapy (Koyama et al., 1994; Watanabe & Sano, 1998;
Ernst & Leist, 1999c).
Comments
Glufosinate-ammonium
Orally administered [14C]glufosinate-ammonium is rapidly but
sparingly (~ 10%) absorbed. Excretion of the absorbed dose was rapid.
The kidney and liver contained the highest concentrations of residue,
which were significantly higher than those in plasma. The
concentrations in brain were lower than those in plasma, indicating
limited penetration of the blood-brain barrier. There were no marked
differences between the sexes. Administration of 500 mg/kg bw resulted
in more prolonged absorption and excretion than with 20 or 2 mg/kg bw.
The metabolism of glufosinate-ammonium was limited (~ 30% of the
absorbed dose), and the main urinary and tissue residues were
3-[hydroxy(methyl)phosphinoyl]propionic acid,
methylphosphinicobutanoic acid, and
2-hydroxy-4-methylphosphinicobutanoic acid. In faeces, significant
concentrations (up to 10%) of N-acetylglufosinate were detected,
indicating that acetylation was performed by the gut microflora.
Metabolites were also found in tissues.
Glutamine synthetase (E.C.6.3.1.2) is a key enzyme involved in
the metabolism of nitrogen and glutamate. Inhibition of glutamine
synthetase, resulting in high levels of ammonia, is the mechanism of
action of glufosinate-ammonium in plants. The activity of glutamine
synthetase varies among tissues and species. The Meeting considered
reports on the relevance of glutamine synthetase activity in the liver
and kidney of experimental animals and humans, including data reviewed
by the 1991 JMPR. Because of the presence of alternative pathways for
the homeostatic control of ammonia, < 50% inhibition of glutamine
synthetase in rat liver was not associated with increased ammonia
concentrations and was not considered to be adverse. Glutamine
synthetase activity in the kidney shows considerable variation between
species, with relatively high activity in rodents and negligible
activity in dogs and humans. Inhibition of kidney glutamine synthetase
in the absence of pathological findings was considered not to be
relevant to human risk assessment.
In the central nervous system, ammonia homeostasis is maintained
by a number of enzymes, including glutamine synthetase and glutamate
dehydrogenase. Under normal conditions, the flux through glutamine
synthetase in brain is reported to be approximately 2-10% of its
theoretical capacity, and for glutamate dehydrogenase it is
approximately 0.1% of its capacity. With such excess capacity,
inhibition of brain glutamine synthetase will not necessarily result
in significant increases in brain ammonia concentrations; this
conclusion is confirmed by data showing that animals with decreased
glutamine synthetase activity do not have increased brain ammonia
levels. However, the 'glutamine-glutamate shunt', between GABA and
glutamate in neurons and glutamine in astrocytes, plays a role in both
excitatory and inhibitory neurotransmission. The results of studies
considered by the 1991 Meeting indicate that significant changes in a
range of biogenic amines in regions of the brain in dogs are
associated with > 8% changes in glutamine synthetase activity after
administration of glufosinate-ammonium at 8 mg/kg bw for 28 days, a
dose that produced 'increased gait activity'. Thus, it has been
proposed that any statistically significant inhibition of glutamine
synthetase activity in brain by > 10% be considered a marker of
potentially adverse effects on brain biochemistry and behaviour.
Studies in vitro and in vivo showed that glufosinate-ammonium
inhibits glutamine synthetase in the brain, kidney, and liver of rats.
With 100 ppm glufosinate-ammonium in the diet (equivalent to 10 mg/kg
bw per day), glutamine synthetase activity was inhibited in liver and
kidney but not in brain, and the Meeting concluded that the NOAEL was
10 mg/kg bw per day. The inhibition in liver and kidney was evident by
day 6, did not increase markedly up to day 90, and showed significant
reversal during a 31-day recovery period. The finding of increased
renal glutamine synthetase activity in a previous long-term study in
rats was considered to be a rebound response to continued inhibition
and to be of no relevance to human risk assessment.
New studies in which rats and mice received repeated, high doses
(270-1400 mg/kg bw per day) in the diet for 90 days were designed to
determine the maximum tolerated doses rather than NOAELs. There was no
evidence of specific toxicity or of irreversible neurobehavioural
effects in the rats. The LOAEL in rats was 7500 ppm, equal to 560
mg/kg bw per day. A new carcinogenicity study in rats showed that
glufosinate-ammonium had no significant carcinogenic potential at
doses up to 10 000 ppm, equal to 470 mg/kg bw per day. A significant
increase in retinal atrophy was seen in this study in females at doses
> 5000 ppm (equal to 280 mg/kg bw per day) and in males at 10 000 ppm
(equal to 470 mg/kg bw per day) but not in either sex at 1000 ppm
(equal to 45 mg/kg bw per day), the NOAEL. The results of these three
studies were consistent with those of previous investigations and
supported the overall NOAEL of 40 ppm (2 mg/kg bw per day) identified
previously in a long-term study in rats that included a more extensive
range of investigations.
No adverse findings were reported in workers in
glufosinate-ammonium production plants, but their exposure was stated
to be low. A number of cases of attempted suicide in Japan have
involved a glufosinate-ammonium-based formulation, but it was not
clear whether the effects reported were due to glufosinate-ammonium or
other constituents. The most significant effect was delayed
neurological symptoms. The available evidence indicates that exposure
to glufosinate-ammonium under normal conditions of use does not
present a significant risk to humans.
N-Acetylglufosinate
Oral doses of [14C] N-acetylglufosinate are absorbed to a
limited extent (5-10%), but the absorption is rapid, with peak plasma
concentrations found 1 h after a dose of 3 mg/kg bw. Excretion of an
absorbed dose is also rapid and occurs predominantly in the urine as
the parent compound. The excretory half-time is < 1 h for the initial
phase and approximately 7 h for the second phase. The residue
concentrations in the liver and particularly kidneys 4 days after
dosing were significantly greater than those in the plasma. Absorbed
N-acetylglufosinate undergoes limited biotransformation, but a
significant proportion (11%) of a low oral dose (3 mg/kg bw) was
de-acetylated to glufosinate-ammonium in the intestine. It is not
clear what proportion of the glufosinate-ammonium present in the
tissues after oral administration of N-acetylglufosinate is absorbed
from the intestine as glufosinate-ammonium. The toxicokinetics of
N-acetylglufosinate after repeated administration has not been
investigated.
N-Acetylglufosinate is of low acute toxicity after oral
administration to mice and rats (LD50 values > 2000 mg/kg bw) and
is not a skin sensitizer. Because N-acetylglufosinate is a plant
metabolite and is not present in pesticide formulations, it has not
been studied for acute toxicity by dermal or inhalation administration
or for ocular or dermal irritancy.
N-Acetylglufosinate has not been classified for toxicity by
WHO.
N-Acetylglufosinate is of low toxicity after repeated oral
administration to mice, rats, or dogs. Some and possibly all of the
inhibition of glutamine synthetase activity seen in all three species
was attributable to glufosinate-ammonium. The NOAEL for inhibition of
glutamine synthetase in the brain in the most sensitive species was
500 ppm (equal to 19 mg/kg bw per day) in a 90-day study in dogs
(glutamine synthetase activity was not measured in a 1-year study in
dogs). In a 2-year study in rats, there was evidence of chronic
progressive nephropathy and urolithiasis. The Meeting noted the
absence of pathological changes in the 90-day study, the lack of a
dose-response relationship for the renal lesions, the high sodium
concentrations associated with administration of
N-acetylglufosinate, and the high prevalence of renal lesions in
aged rats, and concluded that the renal lesions seen in the long-term
study in rats were not relevant to human risk assessment. Increased
incidences of adrenal cortical hyperplasia, adrenal necrosis, and
polyarteritis nodosa were seen in males receiving doses > 2000 ppm
(equal to > 91 mg/kg bw per day), and an increased incidence of
extramedullary haematopoiesis of the spleen was seen in females at
those doses. The NOAEL for pathological findings in rats, the most
sensitive species, was 200 ppm, equal to 9 mg/kg bw per day.
The Meeting concluded that N-acetylglufosinate is not
carcinogenic at the highest doses tested (equal to 1200 mg/kg bw per
day in mice and 1000 mg/kg bw per day in rats).
N-Acetylglufosinate has been studied in an adequate range of
tests for genotoxicity. The Meeting concluded, on the basis of the
results, that N-acetylglufosinate is not genotoxic.
Reproductive performance and outcome in a two-generation study of
reproductive toxicity in rats were not affected by administration of
N-acetylglufosinate at doses up to 700 mg/kg bw per day. The
compound was not teratogenic to either rats or rabbits and was not
fetotoxic to rats. An increased incidence of supernumerary thoracic
ribs was found in fetuses from rabbits exposed to
N-acetylglufosinate at > 160 mg/kg bw per day, a finding that may
be secondary to the maternal toxicity seen at such doses. The NOAEL
for fetotoxicity and maternal toxicity was 64 mg/kg bw per day.
3-[Hydroxy(methyl) phosphinoyl]propionic acid
Summaries of a range of studies on the genotoxicity, acute
toxicity, short-term toxicity, and teratogenicity of the
glufosinate-ammonium metabolite 3-[hydroxy(methyl)phosphinoyl]
propionic acid were available. The lowest NOAEL seen in these studies
(50 mg/kg bw per day) is 25-fold higher than the NOAEL used to derive
the ADI for glufosinate-ammonium.
Overall evaluation
The present Meeting compared the toxicity of
N-acetylglufosinate and 3-[hydroxy(methyl) phosphinoyl]propionic
acid with that of glufosinate-ammonium and concluded that the toxicity
of the metabolites was comparable to or less than that of the parent
compound. The Meeting established a group ADI of 0-0.02 mg/kg bw for
glufosinate-ammonium, N-acetylglufosinate, and 3-[hydroxy(methyl)
phosphinoyl]propionic acid (alone or in combination). This is the same
value as that of the ADI established for glufosinate-ammonium by the
1991 JMPR on the basis of the NOAEL in the long-term study in rats
given technical-grade glufosinate-ammonium, and applying a 100-fold
safety factor.
The present Meeting concluded that it was unnecessary to
establish an acute reference dose because glufosinate-ammonium,
N-acetylglufosinate, and 3-[hydroxy(methyl) phosphinoyl]propionic
acid are of low acute toxicity.
Toxicological evaluation
Levels that cause no toxic effect
Glufosinate-ammonium from 1991 JMPR)
Mouse: 80 ppm, equal to 11 mg/kg bw per day (toxicity in a 2-year
study of toxicity and carcinogenicity)
Rat: 40 ppm, equal to 2.1 mg/kg bw per day (toxicity in a 2-year
study of toxicity and carcinogenicity)
120 ppm equal to 6 mg/kg bw per day (toxicity in a study of
reproductive toxicity)
10 mg/kg bw per day (developmental effects, highest dose
tested in a study of developmental toxicity)
2.2 mg/kg bw per day (maternal and fetotoxicity in a study
of developmental toxicity)
Rabbit: 6.3 mg/kg bw per day (maternal and fetotoxicity in a study
of developmental toxicity)
20 mg/kg bw per day (developmental effects, highest dose
tested in a study of developmental toxicity)
Dog: 4.5 mg/kg bw per day (toxicity in a 1-year study)
N- Acetylglufosinate
Mouse: 1000 ppm, equal to 150 mg/kg bw per day (toxicity in a
2-year study of toxicity and carcinogenicity)
Rat: 200 ppm, equal to 9 mg/kg bw per day (toxicity in a 2-year
study of toxicity and carcinogenicity)
2000 ppm, equal to 140 mg/kg bw per day (parental toxicity
in a study of reproductive toxicity)
10 000 ppm equal to 700 mg/kg bw per day (reproductive
effects, highest dose tested in a study of reproductive
toxicity)
1000 mg/kg bw per day (highest dose tested in a study of
developmental toxicity)
Rabbit: 400 mg/kg bw per day (developmental effects, highest dose
tested in a study of developmental toxicity)
64 mg/kg bw per day (maternal and fetotoxicity in a study of
developmental toxicity)
Dog: 500 ppm, equal to 19 mg/kg bw per day (toxicity in a 90-day
study)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw (for glufosinate-ammonium, N-acetylglufosinate,
and 3-[hydroxy(methyl) phosphinoyl]propionic acid, alone or in
combination)
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological end-points relevant for estimating guidance values for dietary and non-dietary exposure to glufosinate-ammonium
Glufosinate-ammonium N-Acetylglufosinate (1991 JMPR)
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid but limited (5-10%) Rapid but limited (5-10%)
Distribution Extensive. Higher concentration in Extensive. Higher concentration in
kidney and liver kidney and liver
Potential for accumulation Minimal Minimal
Rate and extent of excretion Rats, rapid, > 92% of 30 mg/kg bw Rats, rapid, >95% of 3 mg/kg bw within
within 24 h, primarily in faeces 24 h, primarily in faeces
Metabolism in animals Main metabolite is 3-[hydroxy-(methyl) Limited. Some de-acetylation
phosphinoyl] propionic to glufosinate-ammonium (rat,
acid (rat, goat, hen) goat, hen)
Toxicologically significant compounds Parent Parent and glufosinate-ammonium
Acute toxicity
Rat, LD50, oral 1700 mg/kg bw > 3000 mg/kg bw
Rat, LD50, intraperitoneal ~ 100 mg/kg bw > 1200 mg/kg bw
Mouse, LD50, oral 420 mg/kg bw > 3000 mg/kg bw
Skin sensitization, guinea-pigs Negative (Buehler test) Negative (Magnusson & Kigman test)
Short-term toxicity
Target/critical effect Glutamine synthetase inhibition; Glutamine synthetase inhibition in brain
behaviour in dogs; kidney weights of dogs, mice, rats
and urinary parameters in rats
Lowest relevant oral NOAEL 5 mg/kg bw per day in dogs 19 mg/kg bw per day in dogs
Genotoxicity Not genotoxic Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Glutamine synthetase inhibition; Adrenal necrosis and hyperplasia;
increased kidney weight in rats spleen haematopoiesis in rats
Lowest relevant NOAEL 2 mg/kg bw per day in rats 9 mg/kg bw per day
Carcinogenicity Not carcinogenic Not carcinogenic
Reproductive toxicity
Reproductive target/critical effect Reduced litter size, rats None
Developmental target/critical effect General maternal and fetotoxicity Extra ribs/maternal toxicity in rabbits
Lowest relevant NOAEL for 12 mg/kg bw per day in rats 137 mg/kg bw per day for reproductive
general toxicity in rats toxicity
Lowest relevant NOAEL for 2 mg/kg bw per day in rats 64 mg/kg bw per day in rabbits
developmental toxicity
Neurotoxicity Possibly behavioural, but no No evidence of specific effectspathological findings
Medical data Suicidal poisonings producing coma, No data, not produced commercially
delayed neurological effects, death;
no findings in work force
Summary Value Study Safety factor
Group ADI for glufosinate-ammonium 0-0.02 mg/kg bw 2 years, rat 100
and N-acetylglufosinate
Acute reference dose Unnecessary
References
Note: Amendments have been issued to a number of studies; these are
not referenced separately.
Baeder, C. & Hofmann, T. (1994) Embryotoxicity after oral
administration in Himalayan rabbits. Hoe 099730 substance,
technical. Hoechst AG, Pharma Development, Corporate Toxicology.
Report No. 94.0392. A52948. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Bernier, L. (1996) 52-week dietary toxicity study in the beagle dog of
Hoe 099730 substance, technical. Bio-Research Laboratories.
Hoechst Schering AgrEvo GmbH. Report No. 85438. A55742.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Bernier, L. (1997) Dietary chronic toxicity and carcinogenicity study
in the Sprague-Dawley rat of Hoe 099730 substance, technical.
ClinTrials BioResearch Ltd, Senneville, Quebec, Canada. Report
No. 85440. A59280. Unpublished report submitted ubmitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Beyrouty, P. (1996a) Dietary range-finding reproduction study in the
Sprague-Dawley rat of Hoe 099730 substance, technical. Biological
Research Laboratory Ltd.AgrEvo Canada Inc. Hoechst Schering
AgrEvo GmbH. Report No. 95447. A55746. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Beyrouty, P. (1996b) Dietary 2-generation (1 Litter) reproduction
study in the SpragueDawley rat of Hoe 099730 substance,
technical. Bio-Research Laboratories. Report No. 95448. A55747.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Bremmer, J.N. & Leist, K.-H. (1998) Toxicology and metabolism studies
(summary and evaluation) 3-methyl phosphinico-propionic acid
(MPP) AE F061517 substance, technical. Hoechst Schering AgrEvo
GmbH. Safety Evaluation Frankfurt. Report No. TOX98/011. A59706.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Corney, S.J., Braunhofer, P. & Luetkemeier, H. (1992) 13-week oral
toxicity (feeding) study in the dog with Hoe 099730 substance,
technical. Research & Consulting Company Ltd, Switzerland. Report
No. 291104. A49064. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Dotti, A., Luetkemeier, H. & Powell, J. (1993) 13-week oral toxicity
(feeding) study in the rat with Hoe 039866 substance, technical.
Research & Consulting Company Ltd, Switzerland. Report No.
338400. A51836. Unpublished. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Dotti, A., Luetkemeier, H. & Biedermann, K. (1994) 13-week oral
toxicity (feeding) study in the mouse with Hoe 039866 substance,
technical. Research & Consulting Company Ltd, Switzerland. Report
No. 338411. A52107. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Ebert, E. (1991a) Repeated-dose oral toxicity (4-week feeding study)
in the NMRI mouse Hoe 099730 substance, technical. Hoechst AG,
Pharma Development, Corporate Toxicology. Report No. 91.0728.
A46543. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Ebert, E. (1991b) Repeated-dose oral toxicity (4-week feeding study)
in the Wistar rat Hoe 099730 substance, technical. Hoechst AG,
Pharma Development, Corporate Toxicology. Report No. 91.0594.
A46604. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Ernst, S.W. & Leist, K.-H. (1999a) Critical review of organ specific
glutamine synthetase activities. AgrEvo, Toxicology Frankfurt,
Germany. Report No. TOX98/057. C001918. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Ernst, S. & Leist, K.-H. (1999b) Hoe 099730 substance technical. Code
Hoe 099730 00 ZC92 001. Testing for embryotoxicity after oral
administration in Himalayan rabbits, Addendum to Document A52948,
Determination of the concentration of test substance during the
study. Toxicology, Frankfurt. Report No. TOX99/026. C004479.
Unpublished report submitted 05.07.99 to WHO by Hoechst Schering
AgrEvo GmbH, Germany
Ernst, S.W. & Leist, K.-H. (1999c) Glufosinate-ammonium -- Human
experience with herbicides containing glufosinate-ammonium.
Toxicology, Frankfurt. Report No. TOX99/021. C003758. Unpublished
report submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Ernst, S.W. & Stumpf, K. (1999a) A dietary chronic toxicity and
carcinogenicity study of Hoe 099730; substance technical in the
Swiss CRL:CD(R) mouse. Addendum 1 to Agrevo report A57755.
Historical control data of histopathological investigations
conducted at ClinTrials BioResearch Ltd, Senneville, Quebec,
Canada. Hoechst Schering AgrEvo GmbH, Toxicology Frankfurt,
Germany. Report No. TOX99/033. C004765. Unpublished report
submitted 27.07.99 to WHO by Hoechst Schering AgrEvo GmbH,
Germany.
Ernst, S. & Stumpf, K. (1999b) A dietary chronic toxicity and
carcinogenicity study of Hoe 099730; substance technical in the
Sprague-Dawley rat. Addendum 1 to Agrevo report A59280.
Historical control data of histopathological investigations
conducted at ClinTrials BioResearch Ltd, Senneville, Quebec,
Canada. Hoechst Schering AgrEvo GmbH, Toxicology Frankfurt,
Germany. Report No. TOX99/034. C004764. Unpublished report
submitted 27.07.99 to WHO by Hoechst Schering AgrEvo GmbH,
Germany.
Farrell, D.J. (1997) Dietary carcinogenicity study in the Swiss CRL:
CD(R) mouse of Hoe 099730 substance, technical. ClinTrials
BioResearch Ltd, Senneville, Quebec, Canada. AgrEvo Canada Inc.
Report No. 97.0451. A57755. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Hack, R., Ebert, E. & Ehling, G. (1994) Glufosinate-ammonium -- Some
aspects of mode of action in mammals. Food Chem. Toxicol., 32,
461-470.
Heidemann, A. & Voelkner, W. (1992) Chromosome aberration assay in
human lymphocytes in vitro with Hoe 099730, substance, technical.
Cytotest Cell Research. Report No. 275602. A49392. Unpublished
report submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Hofmann, T. & Jung, R. (1988) Nickel(II)-sulphate. Testing for
sensitising properties in the Pirbright-white guinea pig in a
maximisation test. Pharma Research Technology, Hoechst AG,
Frankfurt. Report No. 91.0792. A46366. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Horstmann, G. & Baeder, C. (1992) Embryotoxicity in the Wistar rat
after oral administration (limit test) Hoe 099730 substance,
technical. Hoechst AG, Pharma Development, Corporate Toxicology.
Report No. 92.0394. A48852. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995a) Metabolism of (14C)-glufosinate in
a lactating goat. AgrEvo USA Co., Pikeville, Hazleton Lab., USA.
Report No. U012A/A524R. A54158. A55808. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995b) Metabolism of (14C)-glufosinate in
laying hens. AgrEvo USA Co., Pikeville, USA, Hazleton Lab., USA.
Report No. U012A/A525R. A54159. A55808. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995c) Metabolism of (14C)-N-acetyl
glufosinate in a lactating goat. AgrEvo USA Co. Pikeville, PTRL
East Inc., USA. Report No. 502BK U012A/A524. A54155. A55808.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995d) Metabolism of (14C)-N-acetyl
glufosinate in laying hens. AgrEvo USA Co. Pikeville, USA. PTRL
East Inc., USA. Report No. 503BK,U012A/A525. A54157. A55808.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Kaleja, R. (1999) Statement on handling glufosinate-ammonium.
Occupational Health Center, InfraServ GmbH & Co. Höechst KG.
Document No. C003747. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Kellner, H.-M. & Braun, R. (1993a) Hoe 099730-14C blood levels in
rats following single oral and intravenous administration of 3
mg/kg body weight. Hoechst RCL, DEU. Report No. 01-L42-0671-93.
A49979. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Kellner, H.-M. & Braun, R. (1993b) Hoe 099730-14C blood levels in
rats following single oral and intravenous administration of 3
mg/kg body weight. Hoechst Radiochem. Laboratorium, Germany.
Report No. 01-L42-0671-93. A53276. Unpublished report submitted
to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Kellner, H.-M., Stumpf, K. & Braun, R. (1993) Hoe 099730-14C
pharmacokinetics in rats following single oral and intravenous
administration of 3 mg/kg body. Hoechst RCL, Germany. Report No.
01-L42-0670-93. A49978. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Koyama, K., Andou, Y., Saruki, K. & Matsuo, H. (1994) Delayed and
severe toxicities of a herbicide containing glufosinate and a
surfactant. Vet. Hum. Toxicol., 36, 17-18.
Lauck-Birkel, S. (1995a) Hoe 039866-14C (glufosinate-ammonium).
Metabolism in male and female rats following single oral
administration of test substance at a dose level of 500 mg/kg
body weight. AgrEvo Entwicklung Umweltforschung Oekochemie,
Germany. Report No. CM93/071. A54334. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Lauck-Birkel S. (1995b) Hoe 099730-14C Metabolism in male and female
rats following a single oral administration of test substance at
a dose level of 1000 mg/kg body weight. AgrEvo Entwicklung
Umweltforschung Oekochemie, Germany. Report No. CM93/070. A54333.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Lauck-Birkel, S. (1996) Hoe 039866-14C (glufosinate-ammonium)
Metabolism in male rats following single oral administration of
test substance at a dose level of 2 mg/kg body weight. Hoechst
Schering AgrEvo GmbH, Environmental Chemistry Frankfurt. Report
No. CM95/013. A54336. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B. (1999a) Glufosinate-ammonium. Rat
metabolism-Single oral intermediate dose (20 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/130. C001924. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B. (1999b) Glufosinate-ammonium. Rat
metabolism-Single intravenous low dose (2 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/131. C001922. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B.(1999c) N-Acetyl-glufosinate. Rat
metabolism-Single intravenous low dose (3 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/096. C001920. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B. (1999d). N-Acetyl-glufosinate. Rat
metabolism-Single oral intermediate dose (30 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/097. C001921. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany
Lie-Venema, H., Hakvoort, T.B.M., Moorman, A.F.M. & Lamers, W.H.
(1998) Regulation of the spatiotemporal pattern of expression of
the glutamine synthetase gene. Department of Anatomy and
Embryology. University of Amsterdam, Academic Medical Center,
Netherlands.
Luetkemeier, H. (1999) N-Acetyl-glufosinate and glufosinate. In vitro
investigations. Comparison of the inhibition of glutamine
synthetase. Report No. RCC 718795. C003753. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1995a) Hoe 039866-14C. Sampling of blood,
excrements, organs and tissues for metabolism studies in male and
female rats following single oral administration of approx. 500
mg/kg body weight (First addendum to report CM93/071 (A54334)).
Hoechst AG, Bereich Pharma, Kinetics/Metabolism, Germany. Report
No. 01-L420813-95. A54450. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1995b) Hoe 099730-14C. Pharmacokinetics in
rats following single oral administration of the test substance
at a dose level of 1000 mg/kg body weight. Hoechst AG, Bereich
Pharma, Kinetics/Metabolism, Germany. Report No. 01-L42-0815-95.
A54335. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1999a) Glufosinate-ammonium. Rat. Absorption,
distribution and elimination, single oral intermediate dose (20
mg/kg body weight). HMR, DI & A; Lead Optimization, Germany.
Report No. TEP57/16. C001926. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1999b) Glufosinate-ammonium. Rat. Absorption,
distribution and elimination, single intravenous low dose (2
mg/kg body weight). HMR, DI & A; Lead Optimization, Germany.
Report No. TEP57/15. C001923. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1999c) N-Acetyl-glufosinate. Rat. Absorption,
distribution and elimination, single oral intermediate dose (30
mg/kg body weight), HMR Preclinical Development,
Pharmacokinetics, Germany. Report No. TEP170/8. C001925.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Maas, J. & Braun, R. (1999d) N-Acetyl-glufosinate. Rat. Absorption,
distribution and elimination, single intravenous low dose (3
mg/kg body weight). HMR Preclinical Development,
Pharmacokinetics, Germany. Report No. TEP170/7. C001919.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Muller, W. (1989a) Mutagenic potential in strains of Salmonella
typhimurium (Ames test) and , Hoe 099730 substance, technical.
Hoechst AG. Pharma Development, Corporate. Report No. 89.0084.
A40532. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Muller, W. (1989b) Detection of gene mutations in somatic cells in
culture HGPRT-test with V79 cells Hoe 099730 substance,
technical. Hoechst AG. Pharma Development, Corporate Toxicology.
Report No. 89.1010. A41375. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Muller, W. (1989c) Unscheduled DNA synthesis test in mammalian cells
in vitro of Hoe 099730 substance, technical. Hoechst AG. Pharma
Development, Corporate Toxicology. Report No. 89.0024. A40926. U
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Muller, W. (1989d) Chromosome aberrations in vitro in V79 Chinese
hamster cells Hoe 099730 substance, technical. Hoechst AG. Pharma
Development, Corporate Toxicology. Report No. 89.1063. A41851.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany
Muller, W. (1991a) Detection of gene mutations in somatic cells in
culture. HGPRT-test with V79 cells Hoe 099730 substance,
technical. Hoechst AG. Pharma Development, Corporate Toxicology.
Report No. 91.0356. A45876. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany..
Muller, W. (1991b) Unscheduled DNA synthesis test in mammalian cells
in vitro of Hoe 099730 substance, technical. Hoechst AG. Pharma
Development, Corporate Toxicology. Report No. 91.0286. A46129.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Muller, W. (1991c) Micronucleus test in male and female NMRI mice
after oral administration Hoe 099730 substance, technical.
Hoechst AG. Pharma Development, Corporate Toxicology. Report No.
91.0438. A46128. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Purich, D.L. (1998) Advances in the enzymology of glutamine synthesis.
Adv. Enzymol. Relat. Areas Mol. Biol., 72, 9-42.
Schmid, H., Keller, B., Luetkemeier, H., Westen, H. & Biedermann, K.
(1998) Oncogenicity study in rats. Glufosinate-ammonium substance
technical. RCC Umweltchemie AG, Itingen, Switzerland. Hoechst
Schering AgrEvo GmbH. Safety Evaluation Frankfurt. Report No.
98.0256. A67303. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Schmid, H., Keller, B., Biedermann, K. & Luetkemeier, H. (1999)
N-Acetyl-L-glufosinate disodium and glufosinate-ammonium. 13-week
oral feeding study in the rat; Comparison of the potential to
inhibit glutamine synthetase. Report No. RCC711663. C004512.
Unpublished report submitted 08.07.99 to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989a) Acute oral toxicity in the male
and female Wistar rat Hoe 099730 substance, technical. Hoechst
AG. Pharma Development, Corporate Toxicology. Report No. 89.0879.
A41793. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989b) Acute oral toxicity in the male
and female NMRI mouse Hoe 099730 substance, technical. Hoechst
AG. Pharma Development, Corporate Toxicology. Report No. 89.0877.
A41791. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989c) Acute intraperitoneal toxicity
in the male and female Wistar rat Hoe 099730 substance,
technical. Hoechst AG. Pharma Development, Corporate Toxicology.
Report No. 89.0883. A41795. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989d) Acute intraperitoneal toxicity
in the male and female NMRI mouse Hoe 099730 substance,
technical. Pharma Development, Corporate Toxicology. Report No.
89.0882. A41789. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989e) Sensitising properties in the
Pirbright-White guinea pig in a maximisation test Hoe 099730
substance, technical. Hoechst AG, Pharma Development, Corporate
Toxicology. Report No. 89.0880. A41612. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Stumpf, K. (1993a) Hoe 039866-14C. Metabolism in male and female
rats following single oral administration of test substance at a
dose level of 2 mg/kg body weight. Hoechst AG, Bereich
Landwirtschaft, Produktentwicklung Oekologie 1, Germany. Report
No. CM90/028. A49981. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Stumpf, K. (1993b) Hoe 099730-14C. Metabolism in male and female
rats following single oral administration of test substance at a
dose level of 3 mg/kg body weight. Hoechst AG, Bereich
Landwirtschaft, Produktentwicklung Oekologie 1, Germany. Report
No. CM90/026. A49980. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Tennekes, H., Schmid, H. & Probst, D. (1992a) Sub-chronic oral
toxicity 13-week feeding study in mice Hoe 099730 substance,
technical. Research & Consulting Company Ltd, Switzerland. Report
No. RCC291025. A48186. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Tennekes, H., Probst, D. & Luetkemeier, H. (1992b) Sub-chronic oral
toxicity 13-week feeding study in rats Hoe 099730 substance,
technical. Research & Consulting Co. Ltd, Switzerland. Report No.
291093. A48187. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Watanabe, T. & Sano, T. (1998) Neurological effects of glufosinate
poisoning with a brief review. Hum. Exp. Toxicol., 17, 35-39.
Weller, O. (1994) Statement on certificates of analysis used for
different tox.-studies Hoe 099730 technical grade aqueous
solution. Hoechst Schering AgrEvo GmbH. Product Analysis
Frankfurt. Report No. OE94/053. A53577. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
(-)-glutamine and hydroxylamine in the presence of arsenate,
manganese, and ADP, followed by spectrophotometric measurement of an
iron compound. A preincubation period of 10 min was used in the main
assays, as it had been shown that inhibition was not changed by
extending this period to 60 or 120 min. The samples were incubated for
20 min in the presence of N-acetylglufosinate (a 33.8% solution
containing 0.06% w/w glufosinate-ammonium) at 0-10 000 µg/ml or
glufosinate-ammonium (as a 50.2% solution) at 0-500 µg/ml.
Glufosinate-ammonium induced significant, concentration-related
inhibition of glutamine synthetase in all tissues at doses > 0.77
mmol/L (Table 6), the inhibition profile varying with tissue.
N-Acetylglufosinate induced only marginal inhibition at 13 mmol/L,
some of which can be attributed directly to the glufosinate-ammonium
content of the sample. The report did not provide results corrected
for protein content, and not all of the assays were performed in
duplicate; however, for the purposes of this comparative exercise, the
results are considered to be acceptable (Lutkemeier, 1999).
A comparative study was performed of the inhibition of glutamine
synthetase in tissues from groups of 10 male Wistar rats given diets
containing N-acetylglufosinate (with 0.06% w/w glufosinate-ammonium)
at 1000 or 10 000 ppm or glufosinate-ammonium at 0, 100, or 1000 ppm
(Schmid et al., 1999). The animals were exposed for 6, 13, 20, or 90
days with 91 days plus 31 days for recovery. In addition to standard
observations and gross necropsy, samples of liver, brain, and kidney
were removed, rapidly cooled, and stored at -70°C prior to processing
and assaying for glutamine synthetase activity, as described above.
There were no deaths or treatment-related clinical signs. The
absolute and relative weights of the kidney were increased by 8-23% in
all treated groups during the first 20 days of the study, but with no
associated pathological findings. Statistically significant inhibition
of glutamine synthetase activity was seen in liver and kidney samples
by day 6, and, except in liver from animals exposed to 1000 ppm
N-acetylglufosinate, did not increase markedly up to day 90 (Table
7). Significant recovery of glutamine synthetase activity occurred
during the 31-day recovery period. The results indicate that orally
administered glufosinate-ammonium is approximately 10 times more
potent at inhibiting glutamine synthetase than is
N-acetylglufosinate. The extent to which this inhibition is due
directly to de-acetylation of N-acetylglufosinate to
glufosinate-ammonium is uncertain. The activity of glutamine
synthetase in brain samples was not reduced markedly in animals
exposed to either N-acetylglufosinate or glufosinate-ammonium at
1000 ppm.
(d) Significance of glutamine synthetase inhibition to humans
Glutamine synthetase (E.C.6.3.1.2) is a key enzyme in the
metabolism of nitrogen and glutamate, catalysing the multi-step
reaction of
(-)-glutamate + ATP + NH3 <=> (-)-glutamine + ADP + P
Table 6. Inhibition of glutamine synthetase activity in vitro in tissue samples from rats, in the
presence of N-acetylglufosinate and glufosinate ammonium; in square brackets, absolute activity
expressed as mg gamma-glutamylhydroxamate formed per g tissue per 20 min
Compound Dose Liver Kidney Neocortex Medulla Hypothalamus
(mmol/L)
Glufosinate ammonium 0 0 [28] 0 [18] 0 [27] 0 [23] 0 [20]
0.003 1 0 0 1
0.008 2 1 0 0 0
0.026 4 1 3 1 0
0.077 14 3 5 6 3
0.26 36 5 13 20 11
0.77 60 13 32 42 29
1.3 72 17 45 53 41
N-Acetylglufosinate 0.13 1 0 0 0 0
0.38 1 0 0 0 0
0.63 2 0 0 0 0
1.3 2 0 0 0 0
6.3 9 1 2 2 1
13a 15 2 4 7 5
a Contains approximately 0.03 mmol/L glufosinate ammonium
Table 7. Activity of glutamine synthetase in samples from 10 male Wistar rats that
received N-acetylglufosinate or glufosinate ammonium in the diet or
control diet
Day Tissue Glutamine synthetase activity (mean % of control value)
Controla Glufosinate ammonium N-acetylglufosinate
100 ppm 1000 ppm 1000 ppm 10 000 ppm
6 Liver 24 55 36 96 46
Brain 28 101 89 94 93
Kidney 17 60 58 61 54
13 Liver 30 51 30 74 40
Brain 25 101 91 102 104
Kidney 17 61 58 59 54
90 Liver 24 60 40 58 54
Brain 22 104 82 99 98
Kidney 14 67 46 55 53
91 + 31 Liver 24 97 85 83 94
Brain 14 98 88 97 97
Kidney 22 90 97 95 87
a Absolute activity, expressed as mg gamma-glutamylhydroxamate formed per
g tissue per 20 min
In plants, glutamine synthetase is the main enzyme involved in the
control of ammonia concentrations, and its inhibition is the mechanism
of action of glufosinate-ammonium in plants. In mammals, other
pathways exist for the homeostatic control of ammonia, such as reverse
reaction of amino acid dehydrogenases and the carbamoyl phosphate
synthetase-urea cycle. Glutamate and glutamine can, however, play
significant roles in other biochemical and physiological processes in
mammals, such as neurotransmission (glutamate and gamma-aminobutyric
acid (GABA)). The activity of glutamine synthetase varies between
tissues and species (see below), but the amino acid sequence is
reported to be well conserved (LieVenema et al., 1998; Purich, 1998;
Ernst & Leist, 1999a).
The liver has two distinct systems for dealing with ammonia. A
high-capacity, low-affinity system exists in the periportal
hepatocytes which is based on carbamoyl phosphate synthetase and the
urea cycle. In central vein hepatocytes, a low-capacity, high-affinity
system exists which is based on glutamine synthetase and ornithine
aminotransferase. Hack et al. (1994) showed that doses of
glufosinate-ammonium did not increase ammonia concentrations in liver
at a dose (5000 ppm) that inhibited glutamine synthetase activity by
50%. While a 60% reduction in liver glutamine was seen at day 1, the
concentration had returned to normal by day 4, indicating the
induction of alternative pathways. Inhibition of liver glutamine
synthetase by up to 50% is therefore not considered to be adverse in
isolation.
The activity of this enzyme in kidney varies considerably between
species (LieVenema et al., 1998; see below), with relatively high
activity in rodents but negligible activity in dogs and humans.
Inhibition of kidney glutamine synthetase in the absence of
pathological findings is not considered to be relevant to human risk
assessment.
In the brain and central nervous system, ammonia homeostasis is
controlled by a number of enzymes including glutamine synthetase and
glutamate dehydrogenase. Under normal conditions (~ 100 µmol/L of
ammonium and 3 mmol/L of glutamate), the flux through glutamine
synthetase in brain is 2-10% of its theoretical capacity and that of
glutamate dehydrogenase is approximately 0.1% of its capacity
(Lie-Venema et al., 1998). With such excess capacity, inhibition of
brain glutamine synthetase is unlikely to result in significant
increases in brain ammonia concentrations. This conclusion is
supported by the finding of Hack et al. (1994) that brain ammonia
concentrations were not increased at doses of glufosinate-ammonium
that produced a 40% reduction in brain glutamine synthetase activity
in rats. However, the glutamine-glutamate shunt between GABA and
glutamate in neurons and glutamine in astrocytes plays a role in both
excitatory and inhibitory neurotransmission. The results of Hack et
al. (1994), although somewhat inconsistent, indicate that significant
changes in a range of biogenic amines in regions of the dog brain are
associated with changes of > 8% in glutamine synthetase activity
after administration of glufosinate-ammonium at 8 mg/kg bw for 28
days, a dose that produced 'increased gait activity'. It is thus
proposed that any statistically significant, > 10% inhibition of
glutamine synthetase activity in brain is a marker of potentially
adverse effects on brain biochemistry and behaviour.
2. Toxicological studies
All of the studies described below were included statements of
compliance with GLP and met the basic requirements of the OECD test
guidelines applicable at the time of study initiation, unless
otherwise stated.
(a) Short-term studies of toxicity
Mice
In response to concern that the doses used in the study of
carcinogenicity with glufosinate-ammonium in mice evaluated previously
had not been appropriately high (160 ppm in males, 320 ppm in
females), a 90-day study was performed in which groups of 10 NMRI mice
of each sex received diets containing glufosinate-ammonium (purity,
95.5%) at concentrations of 0, 1750, 3500, or 7000 ppm. The
homogeneity and achieved concentrations were acceptable, and the
intakes of animals were 561 and 644 mg/kg bw per day for males and
females at the intermediate dose and 274 and 356 mg/kg bw per day for
animals at the low dose, respectively. The investigations included
clinical signs, body weight, food consumption, haematology, clinical
chemistry, organ weights, and gross and microscopic pathology. All
animals at the high dose had died by day 8, 50% of those at 3500 ppm
had died by day 11, and one female at the low dose died. Clinical
signs (ruffled fur, sedation, and emaciation), reduced food
consumption, and initial body-weight loss were seen at all doses,
although the body-weight gain during the latter part of the study was
similar in surviving animals. There were no consistent clinical
chemical or haematological findings and no changes in organ weights or
on gross examination. Congestion in multiple organs was seen at
microscopic examination of many animals that died during the study,
but the cause of death was not determined. No NOAEL could be
identified, but the design was not optimal for this purpose. The
lowest dose tested (1750 ppm, equal to 270 mg/kg bw per day) was
approximately the maximum tolerated dose for a 90-day study, resulting
in a single death and marked initial effects on body weight. The
maximum tolerated dose for a 2-year study in mice would be about 600
ppm if a factor of 3 is used to extrapolate from the approximate dose
in the 90-day study (Dotti et al., 1994). The results of this study
indicate that the previous bioassay was performed within a factor of 2
of the estimated maximum tolerated dose and need not be repeated.
Rats
The toxicity of glufosinate-ammonium (purity, 95.5%) was
investigated in groups of 10 Wistar rats of each sex given the
compound in the diet at concentrations of 0, 7500, 10 000, or 20 000
ppm for 90 days. Routine observations and measurements were made, with
ophthalmoscopy before treatment and at termination and a basic
functional observation battery, which was administered before
treatment and at weeks 1, 2, 3, 4, 8, and 13 and involved observations
in the home cage, an external area, and in the hand, but no forced
physical activity such as grip strength or swimming. Blood samples for
haematological and clinical chemical analyses were taken from fasted
animals at week 13. At termination, five animals of each sex per group
were perfused to preserve nervous tissue. Major organs from all
animals at the highest dose and controls were examined histologically,
as was nervous tissue from all perfused animals and all gross lesions.
Organ weights were not determined. The homogeneity, stability, and
achieved concentrations in the diet were satisfactory, with intakes
equal to 0, 520, 690, and 1400 mg/kg bw per day in males and 0, 570,
740, and 1400 mg/kg bw per day in females.
Two females at the high dose died within the first 8 days of
dosing, but there were no other unscheduled deaths. Animals at the
high dose showed a range of signs during the first 2 weeks of
treatment, including sedation, dyspnoea, emaciation, and diarrhoea.
Food consumption was reduced by > 20% in all groups during the first
two weeks. Body-weight loss was seen in animals at the high dose, and
reductions in body-weight gain were seen in other groups during the
first week of dosing. All groups had similar body-weight gains during
the last weeks of the study. No abnormal ophthalmoscopic findings were
reported. The haematological findings were similar in test and control
groups, and an apparent reduction in erythrocyte count in males
appeared to be related to a high control value. A consistent pattern
of changes in serum lactate dehydrogenase and creatine kinase activity
was seen in animals of each sex, with 20% reductions at the low and
intermediate doses and an increase at the high dose. Small (< 10%)
but statistically significant ( p < 0.05) increases in serum calcium
and inorganic phosphorus concentrations were seen in animals at the
high dose. The functional observational battery identified a similar
pattern of changes in males and females that included miosis, apathy,
reduced alertness and grooming, increased body tone, and vocalization.
These changes were present in all treated groups, with no clear
pattern over time, but were more prevalent at the high dose. There
were no significant macroscopic findings, and the only microscopic
finding of significance was an increased incidence of renal pelvis
dilatation in males at the high dose; there were no treatment-related
effects on nervous tissues.
No NOAEL could be identified owing to alterations in calcium and
inorganic phosphorus and in the functional observational battery at
all doses, although there was no indication of irreversible
neurotoxicity. The effects on food consumption and body weight may
have been secondary to palatability, as they were mainly transient
(Dotti et al., 1993).
(b) Long-term studies of toxicity and carcinogenicity
Rats
A study was initiated in 1994 (Schmid et al., 1998) to include
doses above the maximum of 500 ppm used in the first study. Groups of
60 Wistar rats of each sex received diets containing
glufosinate-ammonium (purity, 96%) at concentrations of 0, 1000, 5000,
or 10 000 ppm for 104 weeks. These doses were based on increased
mortality seen at 20 000 ppm in the 90-day study (Dotti et al., 1993).
The animals were observed for survival, clinical signs, body-weight
gain, food consumption, and the presence of nodules or masses. Blood
smears were prepared from controls and animals at the high dose in
weeks 52, 78, and 104. At termination, over 30 tissues were removed,
nine were weighed, and gross and histopathological examination was
performed on all tissues from all animals. The stability, achieved
levels, and homogeneity of glufosinate-ammonium in the diets were
satisfactory, giving intakes equal to 0, 45, 230, and 470 mg/kg bw in
males and 0, 57, 280, and 580 mg/kg bw per day in females.
Survival was similar in all groups, being over 70% at 104 weeks.
There were no treatment-related changes in clinical signs, food use
efficiency, the occurrence of nodules or masses, or the appearance of
blood smears. All treated groups had reduced food consumption and
body-weight gain over the first month of dosing but these parameters
were subsequently within 10% of those of controls. The weight of the
kidney was increased by 15-30% in relation to dose in all treated
groups, but there were no histological correlates. Macroscopic
examination showed a decreased incidence of pituitary nodules in all
treated males but an increased incidence of adrenal gland foci in
males at the high dose. Microscopic examination revealed a
statistically significant ( p < 0.05) increase in the incidence of
retinal atrophy in males and females at 10 000 ppm and in females at
5000 ppm. This effect was considered to be related to treatment, as a
dose-response relationship was evident in females and it occurred in
animals of each sex, even though the incidence was within the range of
historical controls (males, 20%; females, 38%; Table 8). The incidence
of a rare skin tumour (trichofolliculoma) was increased in males at
the high dose, but it was not statistically significant and was not
seen in females or in males receiving half of the high dose; the
finding was therefore considered not to provide clear evidence of
carcinogenic potential. The total number of malignant and benign
tumours was similar in treated and control groups. The NOAEL was 1000
ppm, equal to 45 mg/kg bw per day, on the basis of the increased
incidence of retinal atrophy (Schmid et al., 1998).
Table 8. Incidences of lesions in Wistar rats receiving
glufosinate ammonium in the diet for 104 weeks
Dose (ppm) Incidence (%)
Retinal atrophy Trichofolliculoma
(males)
Males Females
0 4 3 0
1 000 3 2 0
5 000 4 19 0
10 000 12 29 4
(c) Studies on metabolites
(i) N-Acetylglufosinate
The (-) isomer of N-acetylglufosinate is a major metabolite of
glufosinate-ammonium after its application to glufosinate-tolerant
crops. In 1998, the Committee proposed that residues arising from
applications of glufosinate-ammonium to tolerant crops be defined as
the 'sum of glufosinate-ammonium, 3-[hydroxy(methyl)phosphinoyl]
propionic acid, and Nacetylglufosinate' but could not adopt this
definition until N-acetylglufosinate had been evaluated
toxicologically. Extensive toxicokinetic and toxicological studies on
N-acetylglufosinate have since been submitted. The preparation
tested was an aqueous solution of the disodium salt, and all of the
doses cited below have been corrected for the content of the
technical-grade (-)-isomer. The three batches of N-acetylglufosinate
used varied in purity from 74.5% to 99% and in glufosinate-ammonium
content from 0.06 to 4.5% (Weller, 1994). All of the studies conformed
to GLP and were claimed to have been performed according to guideline
85-1 of November 1984 of the US Environmental Protection Agency or to
meet the basic requirements of the OECD test guidelines applicable at
the time the study was initiated, unless otherwise stated.
Absorption, distribution, and excretion: The toxicokinetics of
N-acetylglufosinate was examined in several studies at doses of
3 mg/kg bw (Stumpf, 1993b; Kellner et al., 1993) or 1000 mg/kg bw
(Lauck-Birkel, 1995b; Maas & Braun, 1995b). Groups of five Wistar rats
of each sex received [3,4-14C] N-acetylglufosinate (purity, > 98%)
by gavage in saline. Urine and faeces were collected over 24-h periods
up to 96 h, and up to 12 tissue samples were taken from animals given
1000 mg/kg bw and killed at 2 and 6 h (two of each sex), 24 h (five of
each sex), and 96 h (five of each sex). Radiolabel in the
gastrointestinal tract was determined in one male each killed at 4 or
24 h after receiving 3 mg/kg bw, and one male at this dose killed at
96 h was studied by autoradiography (Kellner et al., 1993). Radiolabel
in tissues and excreta was determined by liquid scintillation counting
after appropriate processing. The samples were also prepared for
metabolic investigations (see below).
The doses administered differed somewhat among animals, but this
was considered not to have affected the results. Most of each
administered dose was excreted in the faeces within 48 h (Table 9),
although excretion was more rapid at the lower dose. The
concentrations in tissue peaked at 6 h and represented < 1% of the
administered radiolabel; the highest concentrations were detected in
kidney, and those in liver and kidney were greater than in plasma. In
animals at 3 mg/kg bw, the tissue concentration represented < 0.1% of
the administered dose at day 4, with the highest concentrations in
kidney (0.06 µg/g), a finding confirmed by autoradiography. Analysis
of the gastrointestinal tract 4 and 24 h after administration of
3 mg/kg bw showed that 3% and < 0.01% of the dose was in the stomach
and 91% and 3.5% in the intestine, respectively. Sporadic differences
by sex were seen but were not consistent between studies or individual
animals. The tissue concentrations were higher in female than male
rats given 1000 mg/kg bw, as was the urinary excretion after the dose
of 3 mg/kg bw. The results of the two studies with 1000 mg/kg bw
presented a similar profile, although Maas & Braun (1995b) found lower
tissue concentrations and higher urinary excretion in females (up to
20%, including cage washes); however, there was a threefold variation
between individual animals, and the possibility of contamination by
faeces cannot be dismissed.
Table 9. Radiolabel in excreta and tissues of rats given
[3,4-14C] N-acetylglufosinate orally at 3 or 1000 mg/kg bw
Sample Radiolabel in excreta (% of dose) or tissues (µg/g)
1000 mg/kg bwa 3 mg/kg bwb
Males Females Males Females
Urine
24 h 5 4 5 8
48 h 7 6 5 9
Faeces
24 h 58 63 97 93
48 h 82 83 100 96
Liver, 6 h 17 46 NA NA
Kidney, 6 h 44 672c NA NA
Brain, 6 h 1 1 NA NA
Plasma, 6 h 3 10 NA NA
NA, not analysed
a Results from Lauck-Birkel (1995b)
b Results from Kellner et al. (1993); Stumpf (1993b)
c Possible outlier
Groups of three Wistar rats of each sex were given
[3,4-14C] N-acetylglufosinate (purity, 98%; 1400 MBq/g) at 3 mg/kg
bw in saline, and blood samples were taken from the retro-orbital
plexus at 15, 30, and 60 min and 2, 4, 6, 8, 24, 48, 72, and 96 h. The
samples were absorbed onto filter paper and combusted, and radiolabel
was determined by liquid scintillation counting. The peak
concentration (0.05 µg/g) was seen at 60 min, although significant
amounts were detected at 15 min, showing rapid initial absorption. The
concentration in blood declined in a biphasic manner, in an initial
phase with a half-time of 0.8 h and a second phase with a half-time of
7 h. The concentration was at the limit of detection by 24 h. The
integrated area under the curve of concentration-time was ~ 0.2 µg
h/g. Comparison with the results of an identical study in which the
substance was administered intravenously showed that absorption after
oral administration represented approximately 5% of the dose over
24 h. There was no significant difference between the sexes (Kellner &
Braun, 1993a,b).
A lactating goat weighing 36 kg was dosed orally twice a day for
3 consecutive days with capsules containing
[3,4-14C] N-acetylglufosinate, equivalent to a dose of 3.0 mg/kg bw
per day. The feed intake was 1.4 kg/day. The animal was milked twice
daily and was slaughtered 16 h after the final dose. Most of the
administered radiolabel was excreted in the faeces (68%), with 7.3% in
urine and 19% in the gastronintestinal tract and its contents. Only
0.2% of the administered dose was found in the tissues and blood and
< 0.1% in milk. The concentrations in kidney were higher than in
other tissues. Those in milk reached a plateau by day 2 (Huang &
Smith, 1995c).
Six laying hens weighing 1.3-1.6 kg were dosed orally twice a day
for 14 consecutive days with capsules containing
[3,4-14C] N-acetylglufosinate, equivalent to a dose of 2.2 mg/kg bw
per day. The mean feed intake was 120 g/day. Eggs were collected twice
daily, and the birds were slaughtered 15 h after the final dose. Most
of the administered dose was excreted (86%), with 1.0% remaining in
the gastrointestinal tract; < 0.1% of the administered dose was
present in edible tissues and blood. The concentrations of radiolabel
associated with N-acetyl-L-glufosinate disodium salt were 0.076
mg/kg in liver, 0.013 mg/kg in muscle, and 0.011 mg/kg in fat; that in
egg white was only slightly above the level of quantification
(< 0.009 mg/kg) throughout the study, reaching a peak of 0.014 mg/kg.
The concentrations in egg yolk increased slowly throughout the 14
days, with a peak at necropsy of 0.056 mg/kg (Huang & Smith, 1995d).
Groups of three Wistar rats received an intravenous injection of
[3,4-14C] N-acetylglufosinate (purity, 98%; 1400 MBq/g) into the
tail vein at a dose of 3 mg/kg bw as a solution in saline. Blood
samples were taken from the retro-orbital plexus at 5, 15, 30, and 60
min and 2, 4, 6, 8, 24, 48, 72, and 96 h. The samples were absorbed
onto filter paper and combusted, and the radiolabel was determined by
liquid scintillation counting. The peak concentration (6-7.5 µg/g) was
seen at 5 min, with an initial decline of 4 h, a half-time of 0.3 h,
and a second phase with a half-time of 14 h. The concentration of
radiolabel in blood was at the limit of detection at 24 h. The
integrated area under the curve of concentration-time was ~ 3.8 µg
h/g. There as no significant difference between the sexes (Kellner &
Braun, 1993a,b).
The toxicokinetics of N-acetylglufosinate was investigated in
groups of five Wistar rats of each sex which received
[3,4-14C] N-acetylglufosinate (purity, 98%; 1400 MBq/g) in saline
intravenously at a dose of 3 mg/kg bw. Urine and faeces were collected
over 0-4, 4-8, 8-24, 24-48, 48-72, and 72-96 h. Tissue samples were
taken at 96 h. Autoradiography was performed on one male killed at 96
h. The radiolabel in tissues and excreta was determined by liquid
scintillation counting after appropriate processing. Excretion was
rapid, with > 85% of the radiolabel appearing in the 0-4-h urine
sample. By 96 h, approximately 95% of the dose had been excreted in
urine; faecal excretion accounted for 4% in females and 2% in males.
The excretory half-times were slightly longer in males than in
females. By 96 h, tissue radiolabel accounted for < 0.3% of the dose;
the highest concentrations were found in kidney, with 0.2 µg/g in
males and 0.07 µg/g in females (Kellner et al., 1993)
Biotransformation: Urine and faeces from Wistar rats given
[3,4-14C] N-acetylglufosinate at 3 or 1000 mg/kg bw by gavage in
the studies of Stumpf (1993b) and Lauck-Birkel (1995b), described
above, were extracted and analysed for metabolites by HPLC or
thin-layer chromatography with comparison to standards. The tissue
samples contained insufficient radiolabel for investigation of
metabolites. The extent of metabolism was greater at 3 mg/kg bw,
indicating the presence of a saturable reaction pathway. The main
compound in urine was N-acetylglufosinate, with low concentrations
of 3-[hydroxy(methyl) phosphinoyl]propionic acid and
4-methylphosphinico-butanoic acid. The faeces of animals given the low
dose contained a significant amount of glufosinate-ammonium, which was
not seen in those given the high dose. The study of Kellner et al.
(1993) showed that glufosinate-ammonium is formed in the intestine,
but the extent to which it is systemically available is not clear. The
results for male rats are presented in Table 10; female animals showed
a similar profile.
Table 10. N-Acetylglufosinate and metabolites in 96-h samples from male Wistar
rats given [3,4-14C]-labelled compound at 3 or 1000 mg/kg bw by gavage
Compound Percent administered dose
3 mg/kg bwa 1000 mg/kg bwb
Urine Faeces Urine Faeces
Total radiolabel 5.3 83 7.6 89
N-Acetylglufosinate 4.0 70 7.4 85
Glufodinate ammonium < LD 11 < LD 0.9
3-Methylphosphinicopropionic acid 0.7 0.6 0.1 0.4
4-Methylphosphinicobutanoic acid or 0.6 1.2 0.1 0.1
hydroxy-4-methylphosphinicobutanoic acid
a From Stumpf (1993b)
b From Lauck-Birkel (1995b)
[3,4-14C] N-Acetylglufosinate (98% radiochemical purity;
specific activity, 830 MBq/g) was dissolved in physiological saline
and administered to groups of five male Wistar rats at a dose of 30
mg/kg bw by gavage. Groups of animals were sacrificed 1, 6, or 24 h
after dosing, and samples of blood (for plasma), brain, kidney, and
liver were pooled, processed, and assayed for total radioactivity
(liquid scintillation counting) and metabolites (HPLC). Urine and
faeces were collected over 24 h. Metabolites were not determined in
plasma or brain owing to insufficient total radiolabel. There was
limited metabolism and rapid excretion; > 80% of the recovered
radiolabel in the faeces was N-acetylglufosinate. The concentration
of glufosinate-ammonium in kidney increased with time (Table 11;
Lauck-Birkel & Strunk, 1999d). A similar pattern of absorption,
distribution, and excretion was reported by Maas & Braun (1999c).
Two male Wistar rats received [3,4-14C] N-acetylglufosinate
(purity, > 98%) by gavage in saline at a dose of 3 mg/kg bw. The
animals were killed 4 or 24 h later, and the gastrointestinal tract
was examined for total radiolabel and metabolites. Significant
deacetylation of N-acetylglufosinate was found in the intestine,
giving rise to glufosinate-ammonium (Table 12; Kellner et al., 1993).
[3,4-14C] N-Acetylglufosinate (98% radiochemical purity;
specific activity, 7200 MBq/g) was dissolved in physiological saline
and administered intravenously into the tail vein of groups of five
male Wistar rats at a dose of 3 mg/kg bw. Groups of animals were
sacrificed 2 or 24 h after dosing, and samples of blood (for plasma),
brain, kidney, and liver were pooled, processed, and assayed for total
radiolabel (liquid scintillation counting) and metabolites (HPLC).
Urine and faeces were obtained over 24 h. Metabolites were not
determined in plasma or brain owing to insufficient total radiolabel.
There was limited metabolism and rapid excretion, and over 95% of the
radiolabel recovered in urine was N-acetylglufosinate. At 24 h,
glufosinate-ammonium was present at higher concentrations than
N-acetylglufosinate in kidney (Table 13; Lauck-Birkel & Strunk,
1999c). A similar pattern of distribution and excretion was reported
by Maas & Braun (1999d).
Samples from the study of Huang & Smith (1995c) on goats were
investigated for metabolites. N-Acetylglufosinate and glufosinate
accounted for 52% and 34% of the radiolabel in faeces. respectively.
Glufosinate was the main residue in kidney, liver, and milk, although
N-acetylglufosinate (the administered material) and
3-[hydroxy(methyl) phosphinoyl]propionic acid formed a substantial
proportion of the residue in kidney and liver. The concentration of
glufosinate-ammonium in the kidney (0.7 ppm) was four times that in
the liver (0.095 ppm). This study showed that de-acetylation of
N-acetylglufosinate to glufosinate-ammonium makes a significant
contribution to tissue residues.
Samples from the study of Huang & Smith (1995d) on hens were
investigated for metabolites. N-Acetyl-L-glufosinate disodium salt
comprised 73% of the radiolabel in faeces, with glufosinate and
3-[hydroxy(methyl) phosphinoyl]propionic acid comprising 13% and 8.6%,
respectively. N-Acetylglufosinate (the administered material) was
the main residue identified in liver and egg yolk, and glufosinate and
3-[hydroxy(methyl) phosphinoyl]propionic acid were also substantial
components of the liver residue. Glufosinate was the main residue in
egg white. This study showed that deacetylation of
N-acetylglufosinate to glufosinate-ammonium makes a significant
contribution to tissue residues.
Table 11. N-Acetylglufosinate and metabolites in pooled samples from male Wistar rats given
[3,4-14C]-labelled compound at 30 mg/kg bw by gavage
Compound % of administered dose µg/g equivalent
Urine Faeces Kidney Liver
0-24 h 0-24 h
6 h 24 h 6 h 24 h
Total radiolabel 2.1 88 1.1 0.7 0.5 0.2
N-Acetylglufosinate 1.7 82 0.6 0.04 0.08 0.05
Glufosinate ammonium 0.02 5 0.08 0.73 < LD < LD
3-Methylphosphinicopropionic acid 0.15 < LD 0.19 0.03 0.3 0.02
4-Methylphosphinicobutanoic acid (1% in dose) 0.2 1.4 0.09 < LD 0.03 0.01
LD, limit of determination, < 0.001% of the administered dose
Table 12. Residues in stomach and intestine after oral administration
of 3 mg/kg bw [3,4-14C] N-acetylglufosinate to two male rats
Compound Percent of radiolabel
Stomach Intestine
4 h 24 h 4 h 24 h
Total radiolabel 3.6 91 < 0.01 3.5
Glufosinate ammonium 0.0 2.6 ND 29
4-Methylphosphinicobutanoic acid 0.0 0.8 ND 0.0
3-Methylphosphinicopropionic acid 0.0 0.5 ND 4.5
N-Acetylglufosinate 99.8 96 ND 66
ND, not determined
Effects on enzymes and other biochemical parameters: The main
biological property of glufosinate-ammonium is inhibition of the
enzyme glutamine synthetase, and toxicological studies with
N-acetylglufosinate have also shown inhibition of this enzyme in a
range of tissues. The interpretation of these results is confounded by
the presence of glufosinate-ammonium in the samples of
N-acetylglufosinate tested. In an attempt to determine the degree to
which N-acetylglufosinate inhibits glutamine synthetase, comparative
studies were performed in vitro and in vivo (Lutkemeier, 1999;
Schmid et al., 1999). The studies are summarized in Tables 6 and 7.
In vitro, N-acetylglufosinate produced only marginal inhibition at
13 mmol/L, some of which can be attributed directly to the
glufosinate-ammonium in the sample. When glufosinate-ammonium is
administered orally, it is approximately 10 times more potent as an
inhibitor of glutamine synthetase than is N-acetylglufosinate. The
amount of this inhibition that is due directly to de-acetylation of
N-acetylglufosinate to glufosinate-ammonium is uncertain; however,
the work of Lauck-Birkel & Strunk (1999a,b; Table 11) and the results
in vitro (Table 6) indicate that most of the inhibition in kidney is
due to biotransformation of N-acetylglufosinate to
glufosinate-ammonium.
Acute toxicity: N-Acetylglufosinate has little toxicity when
given as a single oral dose (Table 14). When it was given by
intraperitoneal injection, deaths occurred at the lowest doses tested
(580 mg/kg bw) in both rats and mice, but there was no clear
dose-response relationship in mice. The compound was not tested by
other routes, as it is formed as a metabolite only in plants, and
exposure through the skin or by inhalation is unlikely. The clinical
signs of toxicity seen after oral exposure to N-acetylglufosinate
were reduced respiratory rate, reduced activity, contracted flanks,
and squatting during the first 24 h. The decreased respiratory rate
Table 13. N-Acetylglufosinate and metabolites in pooled samples from male Wistar rats given
[3,4-14C]-labelled compound at 3 mg/kg bw intravenously
Compound Percent of administered dose
Urine Faeces Liver Kidney
(24 h) (24 h)
2 h 24 h 2 h 24 h
Total radiolabel 86 1.8 0.5 0.1 0.9 0.1
N-Acetylglufosinate 85 1.7 0.4 0.1 0.8 0.01
Glufosinate ammonium < LD 0.1 0.01 0.01 0.05 0.06
3-Methylphosphinicopropionic acid < LD < LD 0.04 0.01 < LD 0.001
4-Methylphosphinicobutanoic acid (1% in dose) 1.1 0.02 0.01 < LD 0.01 < LD
LD, limit of determination, < 0.001% of the administered dose
Table 14. Acute toxicity of N-acetylglufosinate (purity, 79.4%; containing
4.5% glufosinate ammonium)
Species (strain) Route LD50 Reference
(mg/kg bw)
Rat (Wistar) Oral, gavage 290 Schollmeier & Leist (1989a)
Mouse (NMRI) Oral, gavage 290 Schollmeier & Leist (1989b)
Rat (Wistar) Intraperitoneal > 1200 Schollmeier & Leist (1989c)
Mouse (NMRI) Intraperitoneal > 2000 Schollmeier & Leist (1989d)
persisted for the duration of the study in mice. Gross examination
showed no abnormalities.
In a Magnusson and Kligman maximization protocol, groups of 20
female Pirbright guinea-pigs, 10 weeks of age, received an intradermal
induction with N-acetylglufosinate (purity, 79.4%; supplied as a
57.9% solution) at 5% in saline (equal to 2.9% N-acetylglufosinate)
and a 1:1 preparation of Freund's adjuvant. For topical induction and
challenge, undiluted test material was applied under an occlusive
dressing. There were no signs of erythema or oedema. Satisfactory,
contemporary data for positive controls were presented (Hofmann &
Jung, 1988). N-Acetylglufosinate was not a skin sensitizer in this
study (Schollmeier & Leist, 1989e).
Short-term studies of toxicity: Groups of five NMRI mice of
each sex were given diets containing N-acetylglufosinate (purity,
79.4%; 4.5% glufosinate-ammonium) at concentrations of 0, 120, 580,
2900, or 5800 ppm for 28 days. The content of N-acetylglufosinate in
the diets was routinely below the nominal value, sometimes by as much
as 30%. The stability and homogeneity of the diets were satisfactory,
providing intakes equivalent to 0, 19, 100, 520, and 1000 mg/kg bw per
day. All animals were examined routinely for a range of observations
and measurements, including basic neurological tests. Samples were
taken for clinical chemical and haematological examinations from all
mice (not fasted) at termination. Limited analysis was performed on
urine samples collected overnight on day 21-22 of the study from
fasted animals. Heart, lung, liver, kidney, spleen, brain, testis, and
ovaries were weighed and examined histologically, as was any tissue
with macroscopic abnormalities. Glutamine synthetase activity was
measured in brain and liver samples that had been cooled rapidly after
removal and kept frozen until assay.
There were no deaths or clinical signs of toxicity and no effects
on body-weight gain or food or water consumption, although the latter
two were very variable. Haematology and clinical chemistry showed no
treatment-related effects, and no substance-related macroscopic or
microscopic changes were seen. Glutamine synthetase activity in liver
and brain was significantly inhibited in animals of each sex at
5800 ppm, with significant inhibition in brain samples from females
and liver samples from males receiving 2900 ppm (Table 15). The NOAEL
was 580 ppm, equivalent to 100 mg/kg bw per day, on the basis of the
statistically significant, > 10% decrease in glutamine synthetase
activity in brains of females receiving 2900 ppm (Ebert, 1991a).
Table 15. Mean glutamine synthetase activity in liver and brain
from mice receiving N-acetylglufosinate in the diet for
28 days
Dose (ppm) Glutamine synthetase activity (nmol/s per mg protein)
Male Female
Liver Brain Liver Brain
0 0.43 1.2 0.38 1.5
120 0.38 1.4 0.5 1.4
580 0.38 1.5 0.4 1.3
2900 0.34* 1.1 0.42 0.92*
5800 0.24* 0.71* 0.22* 0.89*
* Statistically significant at p < 0.05
Groups of 20 NMRI mice of each sex received diets containing
N-acetylglufosinate (purity, 74.7; 0.5% glufosinate-ammonium) at
concentrations of 0, 500, 2000, or 8000 ppm for 13 weeks. The content
of test substance and the homogeneity and stability of the diet were
satisfactory. The achieved intakes were 0, 82, 320, and 1300 mg/kg bw
per day for males and 0, 110, 440, and 1700 mg/kg bw per day for
females at the control, low, intermediate, and high doses,
respectively. The animals were observed routinely for deaths, general
condition, clinical signs, behaviour, food consumption, and body
weight. Blood samples were taken from groups of 10 fasting mice per
sex per group at the end of the study for haematological and clinical
chemical investigations. After sacrifice, all animals were examined
macroscopically, and 10 organs were weighed and more than 30 tissues
from control and high-dose animals were examined histopathologically.
Limited histological examinations were performed on nine tissues from
animals at the low and intermediate doses. Liver, kidney, and brain
samples (pooled samples from two animals for the last two tissues)
were rapidly placed in liquid nitrogen before assay for glutamine
synthetase activity.
One male at 8000 ppm died after blood sampling. There were no
treatment-related effects on food consumption, body weight, clinical
signs, organ weights, macroscopic or macroscopic appearance, or
haematological parameters. The activity of serum lactate dehydrogenase
was increased by 180% over controls in males at the two higher doses,
but the results were within the normal range. Apparent increases in
the activities of a number of serum enzyme in females at the high dose
were due to a high value in a single animal and are considered to be
unrelated to treatment. The main finding was a dose-related decrease
in glutamine synthetase activity in liver, kidney, and brain (Table
16). Inhibition of liver and kidney glutamine synthetase activity in
isolation is not relevant to human risk assessment; however, there was
> 10% inhibition of glutamine synthetase activity in brain at doses
> 2000 ppm, and the mean values for animals of each sex were
outside the control range. The NOAEL was 500 ppm, equal to 82 mg/kg bw
per day (Tennekes et al., 1992a).
Table 16. Glutamine synthetase activity in groups of 10 mice receiving
N-acetylglufosinate in the diet for 90 days
Sex Dose (ppm) Glutamine synthetase activitya
Kidney Liver Brain
Mean Range Mean Range Mean Range
Male 0 1.4 1.2-1.7 4.0 3.2-4.8 3.6 3.4-3.8
500 1.0* 0.7-1.2 3.7 2.7-4.4 3.3* 3.1-3.6
2000 0.83* 0.6-1.1 3.3* 2.5-4.6 3.2* 2.9-3.4
8000 0.71* 0.4-0.9 2.9* 2.4-4.2 2.6* 2.4-2.9
Females 0 1.7 1.5-2.1 5.0 3.9-5.5 3.4 2.9-3.6
500 1.3* 1.1-1.5 4.9 4.2-5.4 3.3 3.0-3.5
2000 1.2* 0.9-1.4 4.6 3.8-5.3 2.9* 2.7-3.2
8000 1.1* 0.7-1.3 4.0* 3.2-5.5 2.2* 1.9-2.5
* p < 0.01
a µmol gamma-glutamyl hydroxamate formed per ml reaction mixture in 20 min at 37°C
Groups of five Wistar rats received diets containing
N-acetylglufosinate (purity, 79.4%; 4.5% glufosinate-ammonium) at
concentrations of 0, 120, 580, 2900, or 5800 ppm for 28 days. The
overall content, stability, and homogeneity of the test diets were
stated to be acceptable. The achieved intakes (assuming 100% analysis)
were 0, 12, 59, 310, and 590 mg/kg bw per day for males and 0, 11, 55,
280, and 560 mg/kg bw per day for females. All animals were examined
routinely for a range of observations and measurements, and control
and high-dose animals underwent a basic functional observation
battery. Samples were taken for extensive clinical chemical and
haematological examinations from all rats (not fasted) at the end of
the study. Limited analysis was performed on urine samples collected
overnight on day 21-22 of the study from fasted animals. Heart, lung,
liver, kidney, spleen, brain, testis, ovaries, and adrenal, pituitary,
and thyroid glands were weighed and examined histologically, as was
any tissue with macroscopic abnormalities. Glutamine synthetase
activity was measured in brain and liver samples that had been cooled
rapidly after removal and kept frozen until assay.
There were no deaths or clinical signs of toxicity and no effects
on body-weight gain or food or water consumption. 'Thrombin time' was
mildly increased (< 13%) in females at doses > 2900 ppm and in
males at 120, 580, and 5800 ppm; other measures of coagulation were
unaltered. A statistically significant decrease in lactate
dehydrogenase activity was found in females at doses > 2900 ppm,
which may have been related to treatment as there was evidence of a
dose-response relationship. Slight decreases in the absolute and
relative weights of the heart in males at 5800 ppm was of no clear
toxicological significance in the absence of a histological correlate.
No treatment-related macroscopic or microscopic changes were seen.
Glutamine synthetase activity in the liver was significantly inhibited
at doses > 580 ppm in animals of each sex, and was reduced in the
brains of all treated animals, although there was no clear
dose-response relationship (Table 17). Decreased activity of glutamine
synthetase in liver was considered not to be adverse, and the
alterations in glutamine synthetase activity in brain were not
consistent. There were no biologically significant changes in any
other parameter. The NOAEL was 5800 ppm, equal to 560 mg/kg bw per
day, the highest dose tested (Ebert, 1991b).
Table 17. Mean glutamine synthetase activity in liver and brain
samples from rats receiving N-acetylglufosinate in the
diet for 28 days
Dose (ppm) Glutamine synthetase activity (nmol/s per mg protein)
Male Female
Liver Brain Liver Brain
0 0.27 1.5 0.31 1.3
120 0.28 1.1* 0.33 1.1
580 0.17* 0.9* 0.27 1.1*
2900 0.17 1.0* 0.23 1.0*
5800 0.14* 1.3 0.15* 1.1*
* Statistically significant at p < 0.05
Groups of 20 Wistar rats of each sex received diets containing
N-acetylglufosinate (purity, 74.7%; 0.5% glufosinate-ammonium) at
concentrations of 0, 2000, or 10 000 ppm for 13 weeks; a group of 10
animals of each sex received 400 ppm. The content, homogeneity, and
stability of the test compound in the diet were satisfactory, and the
achieved intakes were 0, 29, 150, and 740 mg/kg bw per day for males
and 0, 31, 160, and 800 mg/kg bw per day for females. At 13 weeks, 10
males and 10 females in each group were killed, and the remainder were
allowed to recover for 4 weeks. The animals were observed regularly
for deaths, clinical signs, body weight, food consumption, and tissue
masses. Ophthalmoscopic examinations were performed before treatment
and at 11 and 16 weeks. Blood and urine samples were taken from fasted
animals at weeks 13 and 17 for clinical chemical and haematological
investigations. At necropsy, 10 organs were weighed and examined
macroscopically. An extensive range of tissues from control and
high-dose animals was examined histologically, as were major organs
from animals at the intermediate and low doses. Glutamine synthetase
activity was measured in brain, kidney, and liver samples that had
been rapidly cooled after removal and kept frozen until assay.
There were no deaths, clinical signs of toxicity, or effects on
body weight, the eyes, or urine. Food consumption was reduced during
the first week of treatment but not subsequently. A number of clinical
chemical parameters showed variations, but these were generally due to
values for individual animals or were within normal ranges. A decrease
in serum sodium concentration in animals at the high dose at 13 weeks
appeared to be related to treatment. Glutamine synthetase activity was
inhibited in liver, kidney, and brain (Table 18), and the effect was
significantly but not completely reversed after 4 weeks. A reversible
increase in kidney weight (< 15%) was seen in males at all doses, but
this finding was considered not to be adverse because there was no
clear dose-response relationship and no associated histological
change. There were no treatment-related macroscopic or microscopic
findings. The NOAEL was 2000 ppm, equal to 150 mg/kg bw per day, on
the basis of inhibition of glutamine synthetase activity in the brain
(Tennekes et al., 1992b).
Groups of four beagle dogs of each sex, aged 5-7 months, received
diets containing N-acetylglufosinate (purity, 74.7%; 0.5%
glufosinate-ammonium) at concentrations of 0, 500, 2000, or 8000 ppm
in 400 g of diet daily for 13 weeks. Additional groups of two animals
of each sex received the same diets and were then allowed to recover
for 4 weeks. The content, homogeneity, and stability of the test diet
were satisfactory. The achieved intakes were 0, 19, 72, and 290 mg/kg
bw per day for males and 0, 21, 79, and 300 mg/kg bw per day for
females. The animals were observed routinely for deaths, general
condition, clinical signs, behaviour, food consumption, and body
weight. Ophthalmoscopic examinations were performed on all animals
before treatment, at weeks 4 and 13, and at termination in the group
allowed to recover. Blood and urine samples were collected from fasted
animals before treatment, at weeks 4 and 13, and at the end of the
recovery period. At termination, all dogs were examined
macroscopically; a range of tissues were weighed, and > 30 tissues
Table 18. Glutamine synthetase activity in groups of 10 rats receiving N-acetylglufosinate in
the diet for 90 days and after a 4-week recovery period
Sex Period Dose Glutamine synthetase activitya
(days) (ppm)
Liver Kidney Brain
Mean Range Mean Range Mean Range
Male 90 0 3.8 3.3-4.3 2.1 1.6-2.3 3.2 3.1-3.3
400 2.8* 2.0-3.4 1.7* 1.5-1.9 3.3 3.1-3.6
2000 2.2* 1.9-2.4 1.5* 1.3-1.8 3.0 2.9-3.3
10 000 1.8* 1.3-2.2 1.6* 1.4-1.9 2.8* 2.6-3.1
90 + recovery 0 3.2 2.5-3.8 2.1 1.9-2.3 3.1 2.9-3.4
2000 3.5 3.2-3.9 2.1 1.6-2.4 3.0 2.6-3.4
10 000 3.4 2.9-4.1 1.9 1.2-2.5 2.9* 2.7-3.1
Female 90 0 3.7 2.8-4.3 1.2 1.0-1.3 3.1 2.8-3.3
400 3.1 2.2-3.5 1.1 1.0-1.2 3.1 2.9-3.2
2000 2.6* 1.8-3.2 1.2 1.1-1.5 3.0 2.9-3.2
10 000 2.4* 1.9-2.8 1.5* 1.4-1.7 2.8* 2.6-2.9
90 + recovery 0 3.7 3.2-4.1 1.3 1.2-1.4 3.1 3.0-3.3
2000 3.5 3.1-4.0 1.3 1.1-1.5 3.1 2.9-3.4
10 000 3.3 2.6-3.9 1.3 1-1.5 2.9 2.7-3.1
* p < 0.01
a µmol gamma-glutamyl hydroxamate formed per ml reaction mixture in 20 min at 37°C
were preserved and examined histopathologically. Liver, kidney, and
brain samples were immediately placed in liquid nitrogen and kept
frozen at -80°C before assay for glutamine synthetase activity;
samples of these tissues were also retained for future analysis.
There were no deaths or treatment-related clinical signs or
effects on food consumption, body weight, or ophthalmoscopic or
haematological parameters. A statistically significant, dose-dependent
decrease in glutamine synthetase activity was found in liver and brain
after 13 weeks of treatment (Table 19), which tended to return to
normal during the recovery period although it was not complete at the
end of the 4 weeks. The changes in glutamine synthetase activity were
not consistent in different tissues from the same animal. Activity in
the brain stem and cerebellum appear to be more sensitive to
inhibition than that in the cortex. Decreased creatine kinase activity
was seen at the high dose in males at 13 weeks (17%) and in females at
weeks 4 and 13 (30%). Lactate dehydrogenase activity was decreased by
30% in females at this dose at week 13 but in neither males nor
females after recovery. Exacerbation of the low pretreatment specific
gravity and osmolality of the urine of females was seen at at 8000 ppm
in weeks 4 and 13 of treatment and at the end of the recovery period.
The significance of this finding is unclear as it was not seen in a
1-year study in dogs. It is of note, however, that the kidney is a
target organ in rats.
A dose-related decrease in prostate gland weight was seen which
achieved statistical significance at 8000 ppm (40%) at 13 weeks, but
there was no histological correlate. The prostate weights were similar
to those of controls after 4 weeks' recovery from the dose of
2000 ppm, but not after administration of 8000 ppm. Although the
authors noted that slight differences in the rate of maturity of dogs
of this age can affect prostate size, the dose-response relationship
and evidence of recovery at 2000 ppm but not at 8000 ppm indicate an
association with treatment. No treatment-related effects were seen on
macroscopic examination. The only histopathological finding of note
was an increased incidence of pituitary cysts in animals receiving
8000 ppm for 13 weeks: 3/4 in animals of each sex and 1/4 in male
controls and 0/4 in female controls. The NOAEL was 500 ppm, equal to
19 mg/kg bw per day, on the basis of > 10% reductions in glutamine
synthetase activity in a number of areas of the brain. The effects on
prostate weight, urinary parameters, and the pituitary indicate that
8000 ppm was an effect level (Corney et al., 1992)
Groups of six beagle dogs of each sex received diets containing
N-acetylglufosinate (purity, 92.4%; 0.1% glufosinate-ammonium) at
concentrations of 0, 100, 1000, or 8000 ppm. Analyses of the diets
for homogeneity and content were satisfactory, and the overall intakes
of N-acetylglufosinate were 0, 4, 44, and 320 mg/kg bw per day for
males and 0, 4.4, 43, and 350 mg/kg bw per day for females. Two
animals of each sex per group were killed at 26 weeks and the
remainder at 52 weeks. The animals were observed routinely for
clinical signs, deaths, body weight, and food consumption.
Table 19. Glutamine synthetase activity in dogs receiving N-acetylglufosinate in the diet for 90 days with or without a 4-week recovery
period
Sex Period No. Dose Glutamine synthetase activitya
(ppm)
Liver Kidney Mid-brain Cerebellum Brain stem Brain cortex
Mean Range Mean Range Mean Range Mean Range Mean Range Mean Range
Male 13 weeks 4 0 2.7 2.6-2.9 0.02 0.01-0.04 2.3 1.8-2.7 1.6 1.5-1.8 1.4 1.2-1.5 3.2 2.8-3.5
500 1.9 1.4-2.2 0.04 0.02-0.07 2.6 1.9-2.9 1.4 1.2-1.5 1.3 1.2-1.4 3.4 3.3-3.4
2000 1.2 1.0-1.3 0.06 0.03-0.10 2.6 1.8-2.9 1.3 1.0-1.4 0.90 0.8-1.1 2.8 2.3-2.9
8000 0.58 0.5-0.7 0.05 0.01-0.14 1.4 1.7-2.6 0.85 0.7-1.0 0.47 0.4-0.5 2.3 1.8-2.8
Recovery 2 0 2.6 0.07 2.1 1.4 1.3 3.0
2000 2.4 0.05 2.3 1.2 0.95 2.4
8000 2.0 0.06 2.1 1.2 0.93 2.5
Females 13 weeks 4 0 1.8 1.6-2.1 0.04 0.02-0.06 2.7 2.2-3.0 1.6 1.5-1.7 1.2 1.1-1.3 2.9 2.7-3.3
500 1.6 1.2-2.4 0.10 0.05-0.17 2.2 1.9-2.5 1.5 1.4-1.5 1.2 1.0-1.3 3.2 2.9-3.3
2000 1.0 0.8-1.5 0.06 0.03-0.09 2.4 1.8-2.9 1.3 1.2-1.4 0.91 0.7-1.0 2.7 2.4-3.3
8000 0.68 0.5-0.9 0.09 0.01-0.15 2.0 1.7-2.6 0.87 0.8-0.9 0.73 0.6-0.8 2.5 1.9-2.8
Recovery 2 0 2.1 0.05 2.1 1.5 1.4 3.1
2000 1.7 0.08 2.1 1.3 1.2 2.7
8000 1.3 0.07 1.8 1.1 0.83 2.4
a µmol gamma-glutamyl hydroxamate formed per ml reaction mixture in 20 min at 37°C
Ophthalmoscopic examinations were performed before treatment and at
weeks 12, 25, and 51. Samples were taken from fasted animals for
urinary analysis, haematology, and clinical chemistry before treatment
and at weeks 13, 26, and 52. Post-mortem examinations were performed
on all animals; nine organs were weighed and > 30 tissues examined
histopathologically. Glutamine synthetase activity was not analysed.
The only death occurred in a male at the intermediate dose; the
cause was not found. The incidence of soft faeces was increased in
animals at the high dose and particularly in males. Reduced
body-weight gain was seen in animals at this dose, by 17% in males and
30% in females, during the first half of the study, and the effect
persisted in females until termination. There was no evidence of
treatment-related effects in ophthalmoscopic, haematological, or
urinary examinations and no effects on organ weights or gross or
histopathological appearance at either the interim or terminal
sacrifice. Reduced serum lactate dehydrogenase activity was seen
consistently in animals at the high dose (Table 20). Although there
was some overlap between the ranges for control and treated animals
and variation in control values over time, the consistency of the
effect in males indicates that it is possibly related to treatment.
The effects at 8000 ppm are not significant in isolation, but the
combination of findings in males indicates that this dose is an effect
level. The NOAEL was 1000 ppm, equal to 44 mg/kg bw per day, on the
basis of reduced body-weight gain, reduced lactate dehydrogenase
activity, and an increased incidence of soft faeces (Bernier, 1996).
Table 20. Lactate dehydrogenase activity in dogs fed diets containing
N-acetylglufosinate at 8000 ppm
Group No. Week Serum lactate dehydrogenase activity (U/L)
Males Females
Mean Range Mean Range
Controls 6 0 75 47-100 160 120-230
6 13 100 66-160 160 52-260
6 26 200 87-430 220 99-470
4 52 90 66-100 320 160-460
Treated 6 0 97 57-140 74 32-130
6 13 53 43-68 44 31-65
6 26 99 39-180 88 48-110
4 52 70 50-88 150 110-180
Long-term studies of toxicity and carcinogenicity: Groups of 90
Swiss Crl:CD-1 mice received diets containing N-acetylglufosinate
(purity, 92.4%; 0.1% glufosinate-ammonium) at concentrations of 100,
1000, or 8000 ppm. Groups of 20 animals were killed after 1 year and
the remainder after 2 years. The mice were housed singly, observed
daily, and monitored routinely for body weight and food consumption.
The homogeneity, content, and stability of the diet were acceptable,
and the achieved intakes were 0, 15, 150, and 1200 mg/kg bw per day
for males and 0, 19 190, and 1500 mg/kg bw per day for females.
Samples for clinical chemistry and haematology were taken from fasted
animals at interim sacrifice (all animals) and at termination (10 per
sex per group). Blood was taken from the tail vein at week 78. At
autopsy, the animals were examined macroscopially, nine organs were
weighed, and more than 30 tissues were preserved. Histological
examinations were conducted on tissues from all controls, animals at
the high dose, and those that died during the study and on the liver,
lung, kidney, and adrenals from animals at the low and intermediate
doses killed after 1 or 2 years.
Although the report stated that no treatment-related signs were
observed, data on individual animals were not presented, as this was
primarily a study of carcinogenicity. Survival was similar in all
groups (> 60% at week 90). Fluctuations in food consumption and body
weight showed no consistent pattern related to dose. There were no
effects on clinical chemical or haematological parameters or on organ
weights, any variation in group means being attributable to individual
values. Macroscopic examination detected no treatment-related effects.
Histopathological examination showed an increased incidence of
amyloidosis in various organs in males and females at the high dose
and testicular necrosis in males, although the only statistically
significant finding ( p = 0.028) was amyloidosis in the salivary
gland of females (Table 21). A high incidence of kidney lesions was
seen in both control and treated animals at 1 and 2 years, with no
significant differences between groups. Increased incidences of
uterine adenocarcinoma and thyroid follicular-cell adenoma were seen
in animals at the high dose; the incidences were not statistically
significantly increased when compared with concurrent controls but
were slightly greater than those of historical controls. The overall
incidences of benign and malignant tumours were similar in treated and
control groups. As the tumours that occurred at increased incidences
occur spontaneously in this strain of mouse, the findings were
considered not to indicate a carcinogenic potential of
N-acetylglufosinate. The NOAEL was 1000 ppm, equal to 150 mg/kg bw
per day, on the basis of an increased incidence of amyloidosis
(Farrell, 1997; Ernst & Stumpf, 1999a).
Groups of 70 Sprague-Dawley-derived rats of each sex received
diets containing N-acetylglufosinate (purity, 92.4%; 0.1%
glufosinate-ammonium) at concentrations of 0, 200, 2000, or 20 000 ppm
for 2 years; additional groups of 10 animals of each sex were killed
at 1 year and 20 at 2 years. The animals were housed singly, observed
daily, and monitored routinely for body weight and food consumption.
The homogeneity, content, and stability of the test diet were
Table 21. Incidences of lesions in mice receiving N-acetylglufosinate in the diet for 2 years, and ranges for
historical controls
Sex Lesion Incidence
0 100 ppm 1000 ppm 8000 ppm Historical controls
Male Testicular degeneration and necrosis 0/70 1/55 2/56 4/70 6-39% (for atrophy)
Testicular atrophy 25/70 28/55 30/56 26/70 6-39% (for atrophy)
Thyroid follicular-cell adenoma 0/70 0/69 1/69 3/70 0-2%
Female Salivary gland amyloidosis 1/70 1/45 3/43 7/70 No data
Liver haemangiosarcoma 0/70 1/70 1/70 2/70 0-4%
Uterine adenocarcinoma 0/70 2/69 1/69 3/70 0-2%
acceptable, and the achieved intakes were 0, 9, 91, and 1000 mg/kg bw
per day for males and 0, 11, 110, and 1200 mg/kg bw per day for
females. Samples for clinical chemistry and haematology were taken
from fasted rats in the interim sacrifice and satellite groups at 25,
51, 78, and 102 weeks. At necropsy, the animals were examined
macroscopically, nine organs from interim sacrifice animals were
weighed, and > 30 tissues were preserved. Full histopathological
examinations were performed on all controls, animals at the high dose,
and animals that died during the study; only liver, lung, kidney, and
adrenals from animals at the low and intermediate doses were examined
histopathologically.
Survival was similar in all groups (> 50% at week 96). Soft
faeces were more prevalent in animals at the high dose and especially
in males from week 8 onwards. Body-weight gain was reduced (~ 5%) in
males at this dose from week 16 onwards and in females (~10%) from
week 24, even though the food consumption of these groups increased by
5-10%. A range of changes in clinical chemistry and haematology were
seen repeatedly in animals of each sex at the high dose, including
reduced prothrombin time, potassium concentration, and lactate
dehydrogenase and creatine kinase activity, and increased sodium,
calcium, inorganic phosphorus, and glucose concentrations; blood urea
nitrogen was increased in males from week 25 onwards. In animals
killed after 1 year, the absolute and relative weights of the kidney
were increased in males receiving 20 000 ppm and in females receiving
2000 or 20 000 ppm. Macroscopic examination showed increased
incidences of a range of abnormalities of the kidney in treated
animals that were confirmed at histological examination. Renal lesions
are common in aged rats, and the incidences in treated animals in this
study were not statistically significant and were within the range
seen in historical controls; furthermore, they may have been
exacerbated by the high sodium content induced by
N-acetylglufosinate. They were therefore considered irrelevant to
the assessment of the study. Alterations were also seen in the spleen,
parathyroid, heart, blood vessels and adrenals at the high dose and in
some animals at the intermediate dose animals (Table 22). The
incidence of tumours was not statistically significantly increased,
although low incidences of uncommon tumours of the brain, liver,
adrenals, skin, and pancreas were seen in animals at the high dose
(Table 23). Although these incidences are outside the range in
historical controls, the sporadic nature of the findings and the clear
NOAEL for tumours at 2000 ppm indicate that N-acetylglufosinate does
not have significant carcinogenic potential. The overall incidences of
benign and malignant tumours were similar in all groups. The NOAEL was
200 ppm, equal to 9 mg/kg bw per day, on the basis of increased
incidences of polyarteritis nodosa, adrenal cortical hyperplasia, and
necrosis and extramedullary haematopoiesis of the spleen (Bernier,
1997; Ernst & Stumpf, 1999b).
Table 22. Incidences of non-neoplastic lesions in rats receiving N-acetylglufosinate in the diet for up to 2 years,
and ranges for historical controls
Sex Lesion Incidence
0 200 ppm 2000 ppm 20 000 ppm Historical
controls
Male Parathyroid hyperplasia 13/62 8/37 16/32 20/64 14-42%
Adrenal focal cortical hyperplasia 17/70 18/70 29/70 27/70 0-20%a
Adrenal necrosis 0/70 0/70 2/70 3/70 0-4%
Polyarteritis nodosa (blood vessels) 4/7 5/7 4/9 11/18 No data
Polyarteritis nodosa (testis; 2 years; main group) 15/70 13/53 13/50 23/70 16-31%
Polyarteritis nodosa (testis; 2 years; satellite group) 3/20 4/16 8/17 10/20 16-31%
Female Parathyroid hyperplasia 1/52 0/26 0/31 6/61 1-12%
Extramedullary splenic haematopoiesis 7/70 4/34 16/41 20/70 15-36%
Cardiomyopathy 12/70 5/30 6/41 22/70 1-70%
a Current study, all > 24%
Table 23. Incidences of neoplastic lesions in groups of 70 rats receiving N-acetylglufosinate in the diet
for 2 years, and ranges for historical controls
Sex Lesion Incidence
0 200 ppm 2000 ppm 20 000 ppm Historical controls
Male Adrenals: malignant phaeochromocytoma 0 1 1 3 0-3%
Brain: meningioma 0 0 0 1 0-1%
Brain: astrocytoma 0 1 0 1 0-2% (malignant
glioma)
Skin: keratocanthoma 4 5 1 9 (7)* 0-8%
Female Brain: oligodendroglioma 0 0 0 1 No data
Liver: cholangiocarcinoma 0 0 0 1 No data
Lung: alveolar/bronchiolar carcinoma 0 0 0 1 No data
Pancreas: islet-cell carcinoma 1 2 1 4 0-2%
* Re-evaluation of data indicates that 7 is the correct value.
Genotoxicity: An extensive range of assays for genotoxicity
have been performed with N-acetyl-glufosinate both in vitro and
in vivo (Table 24). Some of the protocols were less than optimal
with respect to length of exposure (e.g. for unscheduled DNA
synthesis) or the highest concentration tested, but these limitations
are considered not to affect the overall assessment of genotoxic
potential significantly. The Committee concluded that
N-acetylglufosinate is not genotoxic.
Reproductive toxicity: In a single-generation, range-finding
study in groups of 10 Sprague-Dawley rats of each sex that received
N-acetylglufosinate (purity, 92.4%; 0.1% glufosinate-ammonium), no
adverse effects were seen on fertility, reproduction, or development
at doses of 200, 2000, or 10 000 ppm (equivalent to 14, 140, or 700
mg/kg bw per day). Exposure was continuous from 21 days before mating
to day 21 post partum (Beyrouty, 1996a).
Groups of 30 Sprague-Dawley-derived rats received diets
containing N-acetylglufosinate (purity, 92.4%; 0.1%
glufosinate-ammonium) at concentrations of 0, 200, 2000, or 10 000 ppm
for two generations. The F0 generation was exposed from 70 days
before mating until the end of lactation, and F1 pups were exposed
from weaning (10-12 weeks before mating) until the end of lactation of
F2 pups. The stability and homogeneity of the diet were
satisfactory, and the mean achieved intakes were 0, 13, 140, and 700
mg/kg bw per day for males and 0, 18, 170, and 890 mg/kg bw per day
for females. Parental animals were observed routinely for clinical
signs, estrus cycling, body weight, and food consumption, and the
frequency of observation was increased during gestation and weaning.
Parturition was observed when possible. Pups were examined for
malformations, sex, viability, and body weight, and the litters were
culled to four pups of each sex on day 4. All parental animals, one
pup of each sex per litter, and any pups that died or were killed when
moribund were examined post mortem. The major organs and
reproductive tissues from all adult animals were examined
macroscopically, and these tissues from control and high-dose animals
were examined histologically. Sperm motility, count, and morphology,
and the number of spermatids were determined for all adult males.
One male at the intermediate dose and two at the high dose died
from unknown causes, and males of both generations at the high dose
had an increased incidence of soft faeces and brown staining of the
scrotum. There were no effects on body weight, although the food
consumption of animals at the high dose was slightly increased during
the first few weeks of treatment. No effects were seen on the weight
or histological appearance of organs from F0 animals or on mating
indices, length of gestation, estrus cycling, sperm parameters,
macroscopic appearance, litter size, pup survival, or pup weight in
either generation. A dose-related increase in the weight of the
seminal vesicles was seen in F1 adults, which reached statistical
significance ( p < 0.05; 14%) in males at the high dose. As there
were no associated abnormal histological findings and no functional
deficit, the effect was considered not to be adverse. An increased
Table 24. Results of studies of the genotoxicity of N-acetylglufosinate
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium TA98, 0, 2, 12, 59, 291, 1455, 79.4 Negative +S9 Muller (1989c)
TA100, TA1535, 2910, 5820 µg/plate Negative - S9
TA1537, TA1538;
E. coli WP2 uvrA
Gene mutation V79 Chinese hamster 0, 582, 873, 1164, 79.4 Negative +S9 Muller (1989a)
lung cells, Hprt locus 1554 µg/ml Negative - S9
Gene mutation V79 Chinese hamster 0, 444, 666, 888, 74.7 Negative +S9 Muller (1991b)
lung cells, Hprt locus 1186 µg/ml Negative - S9
Unscheduled DNA Human cell line A549 0, 1, 4, 13, 44, 133, 74.7 Negative +S9 Muller (1991c)
synthesis 444, 1332 µg/ml Negative - S9
Unscheduled DNA Human cell line A549 0, 0.6, 1.8, 5.8, 17, 58, 79.4 Negative +S9 Muller (1989d)
synthesis 175, 582 µg/ml, 3-h Negative - S9
exposure
Chromosomal Human lymphocytes 0, 0.6, 3.0, 5.0 mg/ml 74.7 Negative +S9 Heidemann &
aberrations at 24 h Voelkner (1992)
0, 5.0 mg/ml at 48 h Negative - S9
Chromosomal V79 Chinese hamster 0, 154, 773, 1546 µg/ml 79.4 Negative +S9 Muller (1989b)
aberrations lung cells for 18 h
0, 1546 µg/ml for 7 or Negative - S9
28 h
Table 24. (continued)
End-point Test object Concentration Purity Results Reference
(%)
In vivo
Micronucleus NMRI mouse bone 0, 222, 1111, 2222 74.7 Negative Muller (1991a)
formation marrow mg/kg bw by gavage for
24, 48 or 72 h
incidence of extramedullary haematopoiesis in the livers of F1 males
(8/30 versus 2/30 in controls) was also considered not to be adverse
as it is a common finding in rats. There were no effects on
reproduction. These minor effects on seminal vesicle weight and liver
extramedullary haematopoiesis in F1 males at 10 000 ppm combined
with the increased incidence of soft stools indicated that this is a
minimal effect level. The NOAEL was 2000 ppm, equal to 140 mg/kg bw
per day (Beyrouty, 1996b).
Developmental toxicity: Twenty mated female Wistar rats
received N-acetylglufosinate (purity, 74.7%) by gavage in distilled
water at the limit dose of 1000 mg/kg bw per day on days 7-16 of
gestation, whereas 21 controls received starch mucilage in distilled
water. The dose was based on the results of a preliminary study. The
dams were observed routinely for clinical signs, body weight, and food
consumption. At sacrifice on day 21, they were examined
macroscopically, and the uterus was opened to determine the numbers of
live and dead fetuses and resorptions, fetal weights, the sex ratio,
crown-rump lengths, and placental weights. All fetuses were examined
for external malformations; half were investigated by Wilson
sectioning for visceral abnormalities and the remainder stained with
Alizarin red S for visualization of skeletal defects.
There were no deaths or treatment-related clinical signs among
the pregnant dams, but a non-pregnant control was killed during the
study. The food consumption and body-weight gain of treated animals
were slightly reduced (~ 5%) on days 7-14 of gestation but were
similar to those of controls after cessation of dosing. There was no
effect on pregnancy rate, but a slight increase in pre- and
post-implantation losses resulted in a marginally reduced litter size
(13.6 in controls versus 12.7 in treated animals); the value was
within the typical range. No malformations were recorded, and there
was no increase in the incidence of skeletal or external
abnormalities. The incidence of blood in the pericardium/abdomen was
increased in the treated group (5/132 fetuses, 4/20 litters) when
compared with concurrent controls (2/140 fetuses) and historical
controls (0-1.5%). Fetal weight, crown-rump length, placental weight,
and sex ratio showed no treatment-related effects. The NOAEL for
maternal and fetotoxicity was 1000 mg/kg bw per day, the only dose
tested (Horstmann & Baeder, 1992).
Groups of 15 mated, 8-10-month-old, female Himalayan rabbits
received N-acetylglufosinate (purity, 92.4%) by gavage in distilled
water on days 6-18 of gestation at doses of 0, 64, 160, or 400 mg/kg
bw per day, on the basis of the results of a study that found maternal
and fetal toxicity at 500 mg/kg bw per day. The animals were housed
under standard conditions and observed daily. Body weights were
determined on days 0, 6, 13, 19, and 29 of gestation, and the dams
were killed on day 29 and examined macroscopically. The uteri were
opened, and the numbers of live and dead fetuses and resorptions were
determined. Live fetuses were removed to an incubator and their
survival was monitored for 24 h. Fetal body weight, crown-rump length,
and sex were determined, and external visceral and skeletal
examinations were performed by Wilson sectioning and Alizarin red S
staining. The combined incidences of some lesions were presented only
in summary tables, but this did not compromise the overall integrity
of the study.
There were no deaths during the study, and no consistent clinical
signs in dams. Abortions by one dam at the low dose and one at the
intermediate dose were considered not to be related to treatment. Food
consumption was reduced in relation to dose, by 12% in the groups at
the low dose, 23% at the intermediate dose, and 37% at the high dose,
but there were no consistent effects on body weight. Treatment had no
effect on corpora lutea, pregnancy rate, implantation, resorptions,
gravid uterine weight, or fetal survival. The pup weights were reduced
by 7% at 400 mg/kg bw per day. Morphological examination of fetuses
revealed an increased incidence of a supernumerary thoracic ribs
(2/90, 0/82, 8/73, and 11/93 in control, low-, intermediate-, and
high-dose groups, respectively, while the range among historical
controls was 0-12%). The only malformation reported was a case of
hydrocephalus in a fetus at the high dose; the incidence in historical
controls was 0-9%. The NOAEL for maternal toxicity and fetotoxicity
was 64 mg/kg bw per day on the basis of reduced food consumption in
dams and extra ribs in fetuses (Baeder & Hofmann, 1994; Ernst & Leist,
1999b).
Special studies on neurotoxicity: No overt neurotoxicity was
seen in basic functional observation batteries of tests with
N-acetylglufosinate included in short-term studies of toxicity in
mice and rats, nor does this substance belong to a class of compounds
with neurotoxic potential.
(ii) 3-[Hydroxy(methyl) phosphinoyl]propionic acid
3-[Hydroxy(methyl) phosphinoyl]propionic acid is a metabolite of
glufosinate-ammonium in rats and represents ~ 30% of the residue in
liver (Table 4). It may also represent a significant proportion of the
residue arising from administration of glufosinate-ammonium to plants,
goats, and hens (Annex 1, reference 83). In 1991, the Meeting
reviewed a 28-day study in rats and a study of the toxicokinetics of
3-[hydroxy(methyl) phosphinoyl]propionic acid, which showed that it is
rapidly and extensively absorbed and excreted, has little toxicity
(NOAEL, 280 mg/kg bw per day), and does not inhibit hepatic glutamine
synthetase activity. The 1998 Joint Meeting considered that
3-[hydroxy(methyl) phosphinoyl]propionic acid should be included in
the residue definition (Annex 1, reference 83).
A summary of various additional studies on 3-[hydroxy(methyl)
phosphinoyl]propionic acid has been submitted (Bremmer & Leist, 1998),
although the original reports were not made available. The summary
indicates that the acute toxicity of the substance is low, it is not a
skin sensitizer, and had no significant toxicity in studies in rats
(13 weeks; NOAEL, 560 mg/kg bw per day), mice (13 weeks; NOAEL, 1400
mg/kg bw per day), or dogs (15 weeks; NOAEL, 110 mg/kg bw per day)
given repeated doses. An extensive range of tests for genotoxicity was
reported to show that 3-[hydroxy(methyl) phosphinoyl]propionic acid
does not induce gene mutation in vitro, chromosomal aberrations
in vitro or in vivo, micronuclei in vivo, or DNA damage
in vitro or in vivo. Studies of developmental toxicity in rats and
rabbits showed evidence of maternal toxicity and mild fetotoxicity but
no teratogenicity, with cited NOAELs of 300 mg/kg bw per day in rats
and 50 mg/kg bw per day in rabbits.
3. Observations in humans
(a) Medical surveillance of personnel in manufacturing plants
No specific health problems were reported in workers during 18
years of production of glufosinate-ammonium. The exposure of the
workers was stated to be low, but no details of the extent of
investigations were available (Kaleja, 1999).
(b) Poisoning incidents
A pesticide formulation containing glufosinate-ammonium was
reported to have been involved in over 200 cases of attempted suicide
in Japan (Ernst & Leist, 1999c). The formulation contained 18% w/w of
glufosinate-ammonium, 30% surfactant, and a glycol ether solvent, and
it was not clear whether the effects seen were attributable to
glufosinate-ammonium. The initial signs were vomiting, diarrhoea, and
nausea. The main clinical concerns were the delayed (hours to 2 days)
neurological effects, including convulsions, impaired consciousness,
tremor, and coma, and extensive oedema. Treatment included gastric
lavage, diuretics, intravenous fluids, sedatives, haemoperfusion, and
artificial ventilation. After apparent recovery from the physical
signs, long-lasting amnesia, both retrograde and anterograde was
reported, although this may have been linked to the use of high-dose
benzodiazepine therapy (Koyama et al., 1994; Watanabe & Sano, 1998;
Ernst & Leist, 1999c).
Comments
Glufosinate-ammonium
Orally administered [14C]glufosinate-ammonium is rapidly but
sparingly (~ 10%) absorbed. Excretion of the absorbed dose was rapid.
The kidney and liver contained the highest concentrations of residue,
which were significantly higher than those in plasma. The
concentrations in brain were lower than those in plasma, indicating
limited penetration of the blood-brain barrier. There were no marked
differences between the sexes. Administration of 500 mg/kg bw resulted
in more prolonged absorption and excretion than with 20 or 2 mg/kg bw.
The metabolism of glufosinate-ammonium was limited (~ 30% of the
absorbed dose), and the main urinary and tissue residues were
3-[hydroxy(methyl)phosphinoyl]propionic acid,
methylphosphinicobutanoic acid, and
2-hydroxy-4-methylphosphinicobutanoic acid. In faeces, significant
concentrations (up to 10%) of N-acetylglufosinate were detected,
indicating that acetylation was performed by the gut microflora.
Metabolites were also found in tissues.
Glutamine synthetase (E.C.6.3.1.2) is a key enzyme involved in
the metabolism of nitrogen and glutamate. Inhibition of glutamine
synthetase, resulting in high levels of ammonia, is the mechanism of
action of glufosinate-ammonium in plants. The activity of glutamine
synthetase varies among tissues and species. The Meeting considered
reports on the relevance of glutamine synthetase activity in the liver
and kidney of experimental animals and humans, including data reviewed
by the 1991 JMPR. Because of the presence of alternative pathways for
the homeostatic control of ammonia, < 50% inhibition of glutamine
synthetase in rat liver was not associated with increased ammonia
concentrations and was not considered to be adverse. Glutamine
synthetase activity in the kidney shows considerable variation between
species, with relatively high activity in rodents and negligible
activity in dogs and humans. Inhibition of kidney glutamine synthetase
in the absence of pathological findings was considered not to be
relevant to human risk assessment.
In the central nervous system, ammonia homeostasis is maintained
by a number of enzymes, including glutamine synthetase and glutamate
dehydrogenase. Under normal conditions, the flux through glutamine
synthetase in brain is reported to be approximately 2-10% of its
theoretical capacity, and for glutamate dehydrogenase it is
approximately 0.1% of its capacity. With such excess capacity,
inhibition of brain glutamine synthetase will not necessarily result
in significant increases in brain ammonia concentrations; this
conclusion is confirmed by data showing that animals with decreased
glutamine synthetase activity do not have increased brain ammonia
levels. However, the 'glutamine-glutamate shunt', between GABA and
glutamate in neurons and glutamine in astrocytes, plays a role in both
excitatory and inhibitory neurotransmission. The results of studies
considered by the 1991 Meeting indicate that significant changes in a
range of biogenic amines in regions of the brain in dogs are
associated with > 8% changes in glutamine synthetase activity after
administration of glufosinate-ammonium at 8 mg/kg bw for 28 days, a
dose that produced 'increased gait activity'. Thus, it has been
proposed that any statistically significant inhibition of glutamine
synthetase activity in brain by > 10% be considered a marker of
potentially adverse effects on brain biochemistry and behaviour.
Studies in vitro and in vivo showed that glufosinate-ammonium
inhibits glutamine synthetase in the brain, kidney, and liver of rats.
With 100 ppm glufosinate-ammonium in the diet (equivalent to 10 mg/kg
bw per day), glutamine synthetase activity was inhibited in liver and
kidney but not in brain, and the Meeting concluded that the NOAEL was
10 mg/kg bw per day. The inhibition in liver and kidney was evident by
day 6, did not increase markedly up to day 90, and showed significant
reversal during a 31-day recovery period. The finding of increased
renal glutamine synthetase activity in a previous long-term study in
rats was considered to be a rebound response to continued inhibition
and to be of no relevance to human risk assessment.
New studies in which rats and mice received repeated, high doses
(270-1400 mg/kg bw per day) in the diet for 90 days were designed to
determine the maximum tolerated doses rather than NOAELs. There was no
evidence of specific toxicity or of irreversible neurobehavioural
effects in the rats. The LOAEL in rats was 7500 ppm, equal to 560
mg/kg bw per day. A new carcinogenicity study in rats showed that
glufosinate-ammonium had no significant carcinogenic potential at
doses up to 10 000 ppm, equal to 470 mg/kg bw per day. A significant
increase in retinal atrophy was seen in this study in females at doses
> 5000 ppm (equal to 280 mg/kg bw per day) and in males at 10 000 ppm
(equal to 470 mg/kg bw per day) but not in either sex at 1000 ppm
(equal to 45 mg/kg bw per day), the NOAEL. The results of these three
studies were consistent with those of previous investigations and
supported the overall NOAEL of 40 ppm (2 mg/kg bw per day) identified
previously in a long-term study in rats that included a more extensive
range of investigations.
No adverse findings were reported in workers in
glufosinate-ammonium production plants, but their exposure was stated
to be low. A number of cases of attempted suicide in Japan have
involved a glufosinate-ammonium-based formulation, but it was not
clear whether the effects reported were due to glufosinate-ammonium or
other constituents. The most significant effect was delayed
neurological symptoms. The available evidence indicates that exposure
to glufosinate-ammonium under normal conditions of use does not
present a significant risk to humans.
N-Acetylglufosinate
Oral doses of [14C] N-acetylglufosinate are absorbed to a
limited extent (5-10%), but the absorption is rapid, with peak plasma
concentrations found 1 h after a dose of 3 mg/kg bw. Excretion of an
absorbed dose is also rapid and occurs predominantly in the urine as
the parent compound. The excretory half-time is < 1 h for the initial
phase and approximately 7 h for the second phase. The residue
concentrations in the liver and particularly kidneys 4 days after
dosing were significantly greater than those in the plasma. Absorbed
N-acetylglufosinate undergoes limited biotransformation, but a
significant proportion (11%) of a low oral dose (3 mg/kg bw) was
de-acetylated to glufosinate-ammonium in the intestine. It is not
clear what proportion of the glufosinate-ammonium present in the
tissues after oral administration of N-acetylglufosinate is absorbed
from the intestine as glufosinate-ammonium. The toxicokinetics of
N-acetylglufosinate after repeated administration has not been
investigated.
N-Acetylglufosinate is of low acute toxicity after oral
administration to mice and rats (LD50 values > 2000 mg/kg bw) and
is not a skin sensitizer. Because N-acetylglufosinate is a plant
metabolite and is not present in pesticide formulations, it has not
been studied for acute toxicity by dermal or inhalation administration
or for ocular or dermal irritancy.
N-Acetylglufosinate has not been classified for toxicity by
WHO.
N-Acetylglufosinate is of low toxicity after repeated oral
administration to mice, rats, or dogs. Some and possibly all of the
inhibition of glutamine synthetase activity seen in all three species
was attributable to glufosinate-ammonium. The NOAEL for inhibition of
glutamine synthetase in the brain in the most sensitive species was
500 ppm (equal to 19 mg/kg bw per day) in a 90-day study in dogs
(glutamine synthetase activity was not measured in a 1-year study in
dogs). In a 2-year study in rats, there was evidence of chronic
progressive nephropathy and urolithiasis. The Meeting noted the
absence of pathological changes in the 90-day study, the lack of a
dose-response relationship for the renal lesions, the high sodium
concentrations associated with administration of
N-acetylglufosinate, and the high prevalence of renal lesions in
aged rats, and concluded that the renal lesions seen in the long-term
study in rats were not relevant to human risk assessment. Increased
incidences of adrenal cortical hyperplasia, adrenal necrosis, and
polyarteritis nodosa were seen in males receiving doses > 2000 ppm
(equal to > 91 mg/kg bw per day), and an increased incidence of
extramedullary haematopoiesis of the spleen was seen in females at
those doses. The NOAEL for pathological findings in rats, the most
sensitive species, was 200 ppm, equal to 9 mg/kg bw per day.
The Meeting concluded that N-acetylglufosinate is not
carcinogenic at the highest doses tested (equal to 1200 mg/kg bw per
day in mice and 1000 mg/kg bw per day in rats).
N-Acetylglufosinate has been studied in an adequate range of
tests for genotoxicity. The Meeting concluded, on the basis of the
results, that N-acetylglufosinate is not genotoxic.
Reproductive performance and outcome in a two-generation study of
reproductive toxicity in rats were not affected by administration of
N-acetylglufosinate at doses up to 700 mg/kg bw per day. The
compound was not teratogenic to either rats or rabbits and was not
fetotoxic to rats. An increased incidence of supernumerary thoracic
ribs was found in fetuses from rabbits exposed to
N-acetylglufosinate at > 160 mg/kg bw per day, a finding that may
be secondary to the maternal toxicity seen at such doses. The NOAEL
for fetotoxicity and maternal toxicity was 64 mg/kg bw per day.
3-[Hydroxy(methyl) phosphinoyl]propionic acid
Summaries of a range of studies on the genotoxicity, acute
toxicity, short-term toxicity, and teratogenicity of the
glufosinate-ammonium metabolite 3-[hydroxy(methyl)phosphinoyl]
propionic acid were available. The lowest NOAEL seen in these studies
(50 mg/kg bw per day) is 25-fold higher than the NOAEL used to derive
the ADI for glufosinate-ammonium.
Overall evaluation
The present Meeting compared the toxicity of
N-acetylglufosinate and 3-[hydroxy(methyl) phosphinoyl]propionic
acid with that of glufosinate-ammonium and concluded that the toxicity
of the metabolites was comparable to or less than that of the parent
compound. The Meeting established a group ADI of 0-0.02 mg/kg bw for
glufosinate-ammonium, N-acetylglufosinate, and 3-[hydroxy(methyl)
phosphinoyl]propionic acid (alone or in combination). This is the same
value as that of the ADI established for glufosinate-ammonium by the
1991 JMPR on the basis of the NOAEL in the long-term study in rats
given technical-grade glufosinate-ammonium, and applying a 100-fold
safety factor.
The present Meeting concluded that it was unnecessary to
establish an acute reference dose because glufosinate-ammonium,
N-acetylglufosinate, and 3-[hydroxy(methyl) phosphinoyl]propionic
acid are of low acute toxicity.
Toxicological evaluation
Levels that cause no toxic effect
Glufosinate-ammonium from 1991 JMPR)
Mouse: 80 ppm, equal to 11 mg/kg bw per day (toxicity in a 2-year
study of toxicity and carcinogenicity)
Rat: 40 ppm, equal to 2.1 mg/kg bw per day (toxicity in a 2-year
study of toxicity and carcinogenicity)
120 ppm equal to 6 mg/kg bw per day (toxicity in a study of
reproductive toxicity)
10 mg/kg bw per day (developmental effects, highest dose
tested in a study of developmental toxicity)
2.2 mg/kg bw per day (maternal and fetotoxicity in a study
of developmental toxicity)
Rabbit: 6.3 mg/kg bw per day (maternal and fetotoxicity in a study
of developmental toxicity)
20 mg/kg bw per day (developmental effects, highest dose
tested in a study of developmental toxicity)
Dog: 4.5 mg/kg bw per day (toxicity in a 1-year study)
N- Acetylglufosinate
Mouse: 1000 ppm, equal to 150 mg/kg bw per day (toxicity in a
2-year study of toxicity and carcinogenicity)
Rat: 200 ppm, equal to 9 mg/kg bw per day (toxicity in a 2-year
study of toxicity and carcinogenicity)
2000 ppm, equal to 140 mg/kg bw per day (parental toxicity
in a study of reproductive toxicity)
10 000 ppm equal to 700 mg/kg bw per day (reproductive
effects, highest dose tested in a study of reproductive
toxicity)
1000 mg/kg bw per day (highest dose tested in a study of
developmental toxicity)
Rabbit: 400 mg/kg bw per day (developmental effects, highest dose
tested in a study of developmental toxicity)
64 mg/kg bw per day (maternal and fetotoxicity in a study of
developmental toxicity)
Dog: 500 ppm, equal to 19 mg/kg bw per day (toxicity in a 90-day
study)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw (for glufosinate-ammonium, N-acetylglufosinate,
and 3-[hydroxy(methyl) phosphinoyl]propionic acid, alone or in
combination)
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological end-points relevant for estimating guidance values for dietary and non-dietary exposure to glufosinate-ammonium
Glufosinate-ammonium N-Acetylglufosinate (1991 JMPR)
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption Rapid but limited (5-10%) Rapid but limited (5-10%)
Distribution Extensive. Higher concentration in Extensive. Higher concentration in
kidney and liver kidney and liver
Potential for accumulation Minimal Minimal
Rate and extent of excretion Rats, rapid, > 92% of 30 mg/kg bw Rats, rapid, >95% of 3 mg/kg bw within
within 24 h, primarily in faeces 24 h, primarily in faeces
Metabolism in animals Main metabolite is 3-[hydroxy-(methyl) Limited. Some de-acetylation
phosphinoyl] propionic to glufosinate-ammonium (rat,
acid (rat, goat, hen) goat, hen)
Toxicologically significant compounds Parent Parent and glufosinate-ammonium
Acute toxicity
Rat, LD50, oral 1700 mg/kg bw > 3000 mg/kg bw
Rat, LD50, intraperitoneal ~ 100 mg/kg bw > 1200 mg/kg bw
Mouse, LD50, oral 420 mg/kg bw > 3000 mg/kg bw
Skin sensitization, guinea-pigs Negative (Buehler test) Negative (Magnusson & Kigman test)
Short-term toxicity
Target/critical effect Glutamine synthetase inhibition; Glutamine synthetase inhibition in brain
behaviour in dogs; kidney weights of dogs, mice, rats
and urinary parameters in rats
Lowest relevant oral NOAEL 5 mg/kg bw per day in dogs 19 mg/kg bw per day in dogs
Genotoxicity Not genotoxic Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Glutamine synthetase inhibition; Adrenal necrosis and hyperplasia;
increased kidney weight in rats spleen haematopoiesis in rats
Lowest relevant NOAEL 2 mg/kg bw per day in rats 9 mg/kg bw per day
Carcinogenicity Not carcinogenic Not carcinogenic
Reproductive toxicity
Reproductive target/critical effect Reduced litter size, rats None
Developmental target/critical effect General maternal and fetotoxicity Extra ribs/maternal toxicity in rabbits
Lowest relevant NOAEL for 12 mg/kg bw per day in rats 137 mg/kg bw per day for reproductive
general toxicity in rats toxicity
Lowest relevant NOAEL for 2 mg/kg bw per day in rats 64 mg/kg bw per day in rabbits
developmental toxicity
Neurotoxicity Possibly behavioural, but no No evidence of specific effectspathological findings
Medical data Suicidal poisonings producing coma, No data, not produced commercially
delayed neurological effects, death;
no findings in work force
Summary Value Study Safety factor
Group ADI for glufosinate-ammonium 0-0.02 mg/kg bw 2 years, rat 100
and N-acetylglufosinate
Acute reference dose Unnecessary
References
Note: Amendments have been issued to a number of studies; these are
not referenced separately.
Baeder, C. & Hofmann, T. (1994) Embryotoxicity after oral
administration in Himalayan rabbits. Hoe 099730 substance,
technical. Hoechst AG, Pharma Development, Corporate Toxicology.
Report No. 94.0392. A52948. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Bernier, L. (1996) 52-week dietary toxicity study in the beagle dog of
Hoe 099730 substance, technical. Bio-Research Laboratories.
Hoechst Schering AgrEvo GmbH. Report No. 85438. A55742.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Bernier, L. (1997) Dietary chronic toxicity and carcinogenicity study
in the Sprague-Dawley rat of Hoe 099730 substance, technical.
ClinTrials BioResearch Ltd, Senneville, Quebec, Canada. Report
No. 85440. A59280. Unpublished report submitted ubmitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Beyrouty, P. (1996a) Dietary range-finding reproduction study in the
Sprague-Dawley rat of Hoe 099730 substance, technical. Biological
Research Laboratory Ltd.AgrEvo Canada Inc. Hoechst Schering
AgrEvo GmbH. Report No. 95447. A55746. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Beyrouty, P. (1996b) Dietary 2-generation (1 Litter) reproduction
study in the SpragueDawley rat of Hoe 099730 substance,
technical. Bio-Research Laboratories. Report No. 95448. A55747.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Bremmer, J.N. & Leist, K.-H. (1998) Toxicology and metabolism studies
(summary and evaluation) 3-methyl phosphinico-propionic acid
(MPP) AE F061517 substance, technical. Hoechst Schering AgrEvo
GmbH. Safety Evaluation Frankfurt. Report No. TOX98/011. A59706.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Corney, S.J., Braunhofer, P. & Luetkemeier, H. (1992) 13-week oral
toxicity (feeding) study in the dog with Hoe 099730 substance,
technical. Research & Consulting Company Ltd, Switzerland. Report
No. 291104. A49064. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Dotti, A., Luetkemeier, H. & Powell, J. (1993) 13-week oral toxicity
(feeding) study in the rat with Hoe 039866 substance, technical.
Research & Consulting Company Ltd, Switzerland. Report No.
338400. A51836. Unpublished. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Dotti, A., Luetkemeier, H. & Biedermann, K. (1994) 13-week oral
toxicity (feeding) study in the mouse with Hoe 039866 substance,
technical. Research & Consulting Company Ltd, Switzerland. Report
No. 338411. A52107. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Ebert, E. (1991a) Repeated-dose oral toxicity (4-week feeding study)
in the NMRI mouse Hoe 099730 substance, technical. Hoechst AG,
Pharma Development, Corporate Toxicology. Report No. 91.0728.
A46543. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Ebert, E. (1991b) Repeated-dose oral toxicity (4-week feeding study)
in the Wistar rat Hoe 099730 substance, technical. Hoechst AG,
Pharma Development, Corporate Toxicology. Report No. 91.0594.
A46604. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Ernst, S.W. & Leist, K.-H. (1999a) Critical review of organ specific
glutamine synthetase activities. AgrEvo, Toxicology Frankfurt,
Germany. Report No. TOX98/057. C001918. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Ernst, S. & Leist, K.-H. (1999b) Hoe 099730 substance technical. Code
Hoe 099730 00 ZC92 001. Testing for embryotoxicity after oral
administration in Himalayan rabbits, Addendum to Document A52948,
Determination of the concentration of test substance during the
study. Toxicology, Frankfurt. Report No. TOX99/026. C004479.
Unpublished report submitted 05.07.99 to WHO by Hoechst Schering
AgrEvo GmbH, Germany
Ernst, S.W. & Leist, K.-H. (1999c) Glufosinate-ammonium -- Human
experience with herbicides containing glufosinate-ammonium.
Toxicology, Frankfurt. Report No. TOX99/021. C003758. Unpublished
report submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Ernst, S.W. & Stumpf, K. (1999a) A dietary chronic toxicity and
carcinogenicity study of Hoe 099730; substance technical in the
Swiss CRL:CD(R) mouse. Addendum 1 to Agrevo report A57755.
Historical control data of histopathological investigations
conducted at ClinTrials BioResearch Ltd, Senneville, Quebec,
Canada. Hoechst Schering AgrEvo GmbH, Toxicology Frankfurt,
Germany. Report No. TOX99/033. C004765. Unpublished report
submitted 27.07.99 to WHO by Hoechst Schering AgrEvo GmbH,
Germany.
Ernst, S. & Stumpf, K. (1999b) A dietary chronic toxicity and
carcinogenicity study of Hoe 099730; substance technical in the
Sprague-Dawley rat. Addendum 1 to Agrevo report A59280.
Historical control data of histopathological investigations
conducted at ClinTrials BioResearch Ltd, Senneville, Quebec,
Canada. Hoechst Schering AgrEvo GmbH, Toxicology Frankfurt,
Germany. Report No. TOX99/034. C004764. Unpublished report
submitted 27.07.99 to WHO by Hoechst Schering AgrEvo GmbH,
Germany.
Farrell, D.J. (1997) Dietary carcinogenicity study in the Swiss CRL:
CD(R) mouse of Hoe 099730 substance, technical. ClinTrials
BioResearch Ltd, Senneville, Quebec, Canada. AgrEvo Canada Inc.
Report No. 97.0451. A57755. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Hack, R., Ebert, E. & Ehling, G. (1994) Glufosinate-ammonium -- Some
aspects of mode of action in mammals. Food Chem. Toxicol., 32,
461-470.
Heidemann, A. & Voelkner, W. (1992) Chromosome aberration assay in
human lymphocytes in vitro with Hoe 099730, substance, technical.
Cytotest Cell Research. Report No. 275602. A49392. Unpublished
report submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Hofmann, T. & Jung, R. (1988) Nickel(II)-sulphate. Testing for
sensitising properties in the Pirbright-white guinea pig in a
maximisation test. Pharma Research Technology, Hoechst AG,
Frankfurt. Report No. 91.0792. A46366. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Horstmann, G. & Baeder, C. (1992) Embryotoxicity in the Wistar rat
after oral administration (limit test) Hoe 099730 substance,
technical. Hoechst AG, Pharma Development, Corporate Toxicology.
Report No. 92.0394. A48852. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995a) Metabolism of (14C)-glufosinate in
a lactating goat. AgrEvo USA Co., Pikeville, Hazleton Lab., USA.
Report No. U012A/A524R. A54158. A55808. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995b) Metabolism of (14C)-glufosinate in
laying hens. AgrEvo USA Co., Pikeville, USA, Hazleton Lab., USA.
Report No. U012A/A525R. A54159. A55808. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995c) Metabolism of (14C)-N-acetyl
glufosinate in a lactating goat. AgrEvo USA Co. Pikeville, PTRL
East Inc., USA. Report No. 502BK U012A/A524. A54155. A55808.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Huang, M.N. & Smith, S.M. (1995d) Metabolism of (14C)-N-acetyl
glufosinate in laying hens. AgrEvo USA Co. Pikeville, USA. PTRL
East Inc., USA. Report No. 503BK,U012A/A525. A54157. A55808.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Kaleja, R. (1999) Statement on handling glufosinate-ammonium.
Occupational Health Center, InfraServ GmbH & Co. Höechst KG.
Document No. C003747. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Kellner, H.-M. & Braun, R. (1993a) Hoe 099730-14C blood levels in
rats following single oral and intravenous administration of 3
mg/kg body weight. Hoechst RCL, DEU. Report No. 01-L42-0671-93.
A49979. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Kellner, H.-M. & Braun, R. (1993b) Hoe 099730-14C blood levels in
rats following single oral and intravenous administration of 3
mg/kg body weight. Hoechst Radiochem. Laboratorium, Germany.
Report No. 01-L42-0671-93. A53276. Unpublished report submitted
to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Kellner, H.-M., Stumpf, K. & Braun, R. (1993) Hoe 099730-14C
pharmacokinetics in rats following single oral and intravenous
administration of 3 mg/kg body. Hoechst RCL, Germany. Report No.
01-L42-0670-93. A49978. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Koyama, K., Andou, Y., Saruki, K. & Matsuo, H. (1994) Delayed and
severe toxicities of a herbicide containing glufosinate and a
surfactant. Vet. Hum. Toxicol., 36, 17-18.
Lauck-Birkel, S. (1995a) Hoe 039866-14C (glufosinate-ammonium).
Metabolism in male and female rats following single oral
administration of test substance at a dose level of 500 mg/kg
body weight. AgrEvo Entwicklung Umweltforschung Oekochemie,
Germany. Report No. CM93/071. A54334. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Lauck-Birkel S. (1995b) Hoe 099730-14C Metabolism in male and female
rats following a single oral administration of test substance at
a dose level of 1000 mg/kg body weight. AgrEvo Entwicklung
Umweltforschung Oekochemie, Germany. Report No. CM93/070. A54333.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Lauck-Birkel, S. (1996) Hoe 039866-14C (glufosinate-ammonium)
Metabolism in male rats following single oral administration of
test substance at a dose level of 2 mg/kg body weight. Hoechst
Schering AgrEvo GmbH, Environmental Chemistry Frankfurt. Report
No. CM95/013. A54336. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B. (1999a) Glufosinate-ammonium. Rat
metabolism-Single oral intermediate dose (20 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/130. C001924. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B. (1999b) Glufosinate-ammonium. Rat
metabolism-Single intravenous low dose (2 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/131. C001922. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B.(1999c) N-Acetyl-glufosinate. Rat
metabolism-Single intravenous low dose (3 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/096. C001920. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Lauck-Birkel, S. & Strunk, B. (1999d). N-Acetyl-glufosinate. Rat
metabolism-Single oral intermediate dose (30 mg/kg body weight).
AgrEvo Entwicklung, Umweltforschung, Frankfurt. Report No.
CM98/097. C001921. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany
Lie-Venema, H., Hakvoort, T.B.M., Moorman, A.F.M. & Lamers, W.H.
(1998) Regulation of the spatiotemporal pattern of expression of
the glutamine synthetase gene. Department of Anatomy and
Embryology. University of Amsterdam, Academic Medical Center,
Netherlands.
Luetkemeier, H. (1999) N-Acetyl-glufosinate and glufosinate. In vitro
investigations. Comparison of the inhibition of glutamine
synthetase. Report No. RCC 718795. C003753. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1995a) Hoe 039866-14C. Sampling of blood,
excrements, organs and tissues for metabolism studies in male and
female rats following single oral administration of approx. 500
mg/kg body weight (First addendum to report CM93/071 (A54334)).
Hoechst AG, Bereich Pharma, Kinetics/Metabolism, Germany. Report
No. 01-L420813-95. A54450. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1995b) Hoe 099730-14C. Pharmacokinetics in
rats following single oral administration of the test substance
at a dose level of 1000 mg/kg body weight. Hoechst AG, Bereich
Pharma, Kinetics/Metabolism, Germany. Report No. 01-L42-0815-95.
A54335. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1999a) Glufosinate-ammonium. Rat. Absorption,
distribution and elimination, single oral intermediate dose (20
mg/kg body weight). HMR, DI & A; Lead Optimization, Germany.
Report No. TEP57/16. C001926. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1999b) Glufosinate-ammonium. Rat. Absorption,
distribution and elimination, single intravenous low dose (2
mg/kg body weight). HMR, DI & A; Lead Optimization, Germany.
Report No. TEP57/15. C001923. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Maas, J. & Braun, R. (1999c) N-Acetyl-glufosinate. Rat. Absorption,
distribution and elimination, single oral intermediate dose (30
mg/kg body weight), HMR Preclinical Development,
Pharmacokinetics, Germany. Report No. TEP170/8. C001925.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Maas, J. & Braun, R. (1999d) N-Acetyl-glufosinate. Rat. Absorption,
distribution and elimination, single intravenous low dose (3
mg/kg body weight). HMR Preclinical Development,
Pharmacokinetics, Germany. Report No. TEP170/7. C001919.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Muller, W. (1989a) Mutagenic potential in strains of Salmonella
typhimurium (Ames test) and , Hoe 099730 substance, technical.
Hoechst AG. Pharma Development, Corporate. Report No. 89.0084.
A40532. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Muller, W. (1989b) Detection of gene mutations in somatic cells in
culture HGPRT-test with V79 cells Hoe 099730 substance,
technical. Hoechst AG. Pharma Development, Corporate Toxicology.
Report No. 89.1010. A41375. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Muller, W. (1989c) Unscheduled DNA synthesis test in mammalian cells
in vitro of Hoe 099730 substance, technical. Hoechst AG. Pharma
Development, Corporate Toxicology. Report No. 89.0024. A40926. U
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Muller, W. (1989d) Chromosome aberrations in vitro in V79 Chinese
hamster cells Hoe 099730 substance, technical. Hoechst AG. Pharma
Development, Corporate Toxicology. Report No. 89.1063. A41851.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany
Muller, W. (1991a) Detection of gene mutations in somatic cells in
culture. HGPRT-test with V79 cells Hoe 099730 substance,
technical. Hoechst AG. Pharma Development, Corporate Toxicology.
Report No. 91.0356. A45876. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany..
Muller, W. (1991b) Unscheduled DNA synthesis test in mammalian cells
in vitro of Hoe 099730 substance, technical. Hoechst AG. Pharma
Development, Corporate Toxicology. Report No. 91.0286. A46129.
Unpublished report submitted to WHO by Hoechst Schering AgrEvo
GmbH, Germany.
Muller, W. (1991c) Micronucleus test in male and female NMRI mice
after oral administration Hoe 099730 substance, technical.
Hoechst AG. Pharma Development, Corporate Toxicology. Report No.
91.0438. A46128. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Purich, D.L. (1998) Advances in the enzymology of glutamine synthesis.
Adv. Enzymol. Relat. Areas Mol. Biol., 72, 9-42.
Schmid, H., Keller, B., Luetkemeier, H., Westen, H. & Biedermann, K.
(1998) Oncogenicity study in rats. Glufosinate-ammonium substance
technical. RCC Umweltchemie AG, Itingen, Switzerland. Hoechst
Schering AgrEvo GmbH. Safety Evaluation Frankfurt. Report No.
98.0256. A67303. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Schmid, H., Keller, B., Biedermann, K. & Luetkemeier, H. (1999)
N-Acetyl-L-glufosinate disodium and glufosinate-ammonium. 13-week
oral feeding study in the rat; Comparison of the potential to
inhibit glutamine synthetase. Report No. RCC711663. C004512.
Unpublished report submitted 08.07.99 to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989a) Acute oral toxicity in the male
and female Wistar rat Hoe 099730 substance, technical. Hoechst
AG. Pharma Development, Corporate Toxicology. Report No. 89.0879.
A41793. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989b) Acute oral toxicity in the male
and female NMRI mouse Hoe 099730 substance, technical. Hoechst
AG. Pharma Development, Corporate Toxicology. Report No. 89.0877.
A41791. Unpublished report submitted to WHO by Hoechst Schering
AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989c) Acute intraperitoneal toxicity
in the male and female Wistar rat Hoe 099730 substance,
technical. Hoechst AG. Pharma Development, Corporate Toxicology.
Report No. 89.0883. A41795. Unpublished report submitted to WHO
by Hoechst Schering AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989d) Acute intraperitoneal toxicity
in the male and female NMRI mouse Hoe 099730 substance,
technical. Pharma Development, Corporate Toxicology. Report No.
89.0882. A41789. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Schollmeier, U. & Leist, K.-H. (1989e) Sensitising properties in the
Pirbright-White guinea pig in a maximisation test Hoe 099730
substance, technical. Hoechst AG, Pharma Development, Corporate
Toxicology. Report No. 89.0880. A41612. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
Stumpf, K. (1993a) Hoe 039866-14C. Metabolism in male and female
rats following single oral administration of test substance at a
dose level of 2 mg/kg body weight. Hoechst AG, Bereich
Landwirtschaft, Produktentwicklung Oekologie 1, Germany. Report
No. CM90/028. A49981. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Stumpf, K. (1993b) Hoe 099730-14C. Metabolism in male and female
rats following single oral administration of test substance at a
dose level of 3 mg/kg body weight. Hoechst AG, Bereich
Landwirtschaft, Produktentwicklung Oekologie 1, Germany. Report
No. CM90/026. A49980. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Tennekes, H., Schmid, H. & Probst, D. (1992a) Sub-chronic oral
toxicity 13-week feeding study in mice Hoe 099730 substance,
technical. Research & Consulting Company Ltd, Switzerland. Report
No. RCC291025. A48186. Unpublished report submitted to WHO by
Hoechst Schering AgrEvo GmbH, Germany.
Tennekes, H., Probst, D. & Luetkemeier, H. (1992b) Sub-chronic oral
toxicity 13-week feeding study in rats Hoe 099730 substance,
technical. Research & Consulting Co. Ltd, Switzerland. Report No.
291093. A48187. Unpublished report submitted to WHO by Hoechst
Schering AgrEvo GmbH, Germany.
Watanabe, T. & Sano, T. (1998) Neurological effects of glufosinate
poisoning with a brief review. Hum. Exp. Toxicol., 17, 35-39.
Weller, O. (1994) Statement on certificates of analysis used for
different tox.-studies Hoe 099730 technical grade aqueous
solution. Hoechst Schering AgrEvo GmbH. Product Analysis
Frankfurt. Report No. OE94/053. A53577. Unpublished report
submitted to WHO by Hoechst Schering AgrEvo GmbH, Germany.
