
Pesticide residues in food -- 1999
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
Toxicological evaluations
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Rome, 20-29 September 1999
PYRETHRUM EXTRACT (PYRETHRINS) (addendum)
First draft prepared by
Roland Solecki
Bundesinstitut für gesundheitlichen Verbraucherschutz und
Veterinärdmedizin,
Berlin, Germany
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies
Effects on the central nervous system
Effects on hepatic microsomal enzymes
Observations in humans
Comments
Toxicological evaluation
References
Appendix 1
Explanation
Extracts of flowers of the chrysanthemum (genus Chrysanthemus)
have been used as insecticides for a long time. The insecticidal
neurotoxic activity of these extracts is due to a mixture of three
naturally occurring, closely related insecticidal esters of
chrysanthemic acid (pyrethrins I) and three closely related esters of
pyrethric acid (pyrethrins II). Selection of varieties of
chrysanthemum rich in pyrethrins and extraction techniques have
improved over the years, and the currently available refined pyrethrum
extract contains 45-55% total pyrethrins and 23-25% other
phytochemical extracts containing triglyceride oils, terpenoids, and
carotinoid plant colours. Flavonoids, which have been associated with
skin allergies, are not found in the refined extracts. The extracts
usually also contain 20-25% light isoparaffins and 3-5% butylated
hydroxytoluene, which may be added during and after processing,
respectively, for extraction or as antioxidants. The pyrethrin product
used in the studies that were evaluated by the present Meeting was a
blend of refined pyrethrum extract from the four main growing areas,
with a total pyrethrin content of 57.6%. The ratio of pyrethrins I to
pyrethrins II in this sample was 1.85. In this document, the product
used in the studies is referred to as 'pyrethrins' in order to
differentiate it from the pyrethrum extract used earlier.
Pyrethrum, the active principle containing pyrethrin isomers, was
evaluated toxicologically by the 1965, 1966, 1970, and 1972 Joint
Meetings (Annex 1, references 3, 6, 14, and 18). An ADI of 0-0.04
mg/kg bw was allocated by the 1972 Meeting. The compound was reviewed
at the present meeting within the periodic review programme of the
Codex Committee on Pesticide Residues. This monograph addendum
summarizes data on pyrethrins that were not reviewed previously.
Evaluation for Acceptable Daily Intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Rats
One preliminary and three definitive experiments were conducted
with 14C-labelled pyrethrins I in rats to determine their
absorption, distribution, and excretion after oral administration of a
single or a repeated low dose of 10 mg/kg bw and a single high dose of
100 mg/kg bw in males and 50 mg/kg bw in females. This study was
conducted in compliance with guideline 85-1 of FIFRA and in accordance
with good laboratory practice (GLP). The concentration of radiolabel
in blood peaked between 5 and 8 h. More than 90% of the low dose was
absorbed, and < 10% of the parent compound was found in faeces. The
mean percentage of the administered radiolabel found in urine was
32-47% in males and 50-57% in females, whereas the mean percentage of
the administered radiolabel found in faeces with the various dosing
regimens was 55-71% for males and 50-52% for females. More of the
administered dose was found in the faeces of male rats given the
single high dose (71%) than in those given the single low dose (63%)
or repeated doses (55%). No such differences were seen in females. The
radiolabel was excreted faster by males and females given repeated low
doses than by those given the single dose. The half-lives of
pyrethrins I were calculated to be 5 h in males and 7 h in females.
The residues were widely distributed in the organs analysed, the
highest concentrations being found in fat in all groups. The
concentrations were similar in animals of each sex given the single
and repeated low dose, but female rats had an approximately two times
higher concentration of radiolabel in fat than the males (Selim,
1995).
(b) Biotransformation
Rats
In the phase of the study described above designed to investigate
metabolism, the major urinary metabolites were identified, and then
the metabolic profile was determined in urine and faeces with
quantification of the labelled residues. In the first step, additional
groups of five rats were given 14C-labelled pyrethrins I orally as a
single dose of 10 mg/kg bw (males and females), 100 mg/kg bw (males),
and 50 mg/kg bw (females). The dose administered to the females was
lower because it had been shown previously that pyrethrum extract is
more toxic to females than males. Chromatographic profiling indicated
a quantitatively similar metabolic profile in urine in males and
females at all doses and that all of the metabolites present in faeces
were also present in the urine. The urine from males at the high dose
was therefore used to isolate, purify, and identify the major
metabolites by repetitive injections of composited urine onto a
semi-preparative high-performance liquid chromatograph and collection
of radiolabelled metabolites. After isolation and purification, two
major and four minor metabolites were identified by chemical
manipulations and mass spectroscopy. The spectrum of metabolites
indicated that in rats pyrethrins I are metabolized through two major
metabolic pathways: oxidation of the double-bond on the cyclopentene
or the cyclopropane side of the molecule to form a diol, and/or
oxidation of the methyl groups on the side-chain of the cyclopropane
ring to form a carboxylic acid. A second pathway involves hydrolysis
of the ester bond to form the corresponding acid and alcohol.
After isolation and identification of the metabolites, the
distribution of metabolites was determined in urine and faeces. The
major metabolite in urine at all doses was chrysanthemum dicarboxylic
acid. In faeces, a significant amount of parent compound was present
(< 10% at the low dose), but another metabolite was the most
prevalent at all doses. The two metabolites represented over one-third
of the total excreted radiolabel. Male and female rats metabolized
pyrethrins I in a similar manner, regardless of the dose, and the
difference between males and females was quantitative rather than
qualitative (Selim, 1995).
The relative rates of microsomal oxidation are similar for the
four major pyrethrins, which are readily oxidized by cytochrome
P450-dependent oxidases. Multiple sites are involved on each of the
pyrethrum constituents. The toxicity of the pyrethrins is attributable
to a combination of the effects of the parent esters and the
metabolites they generate, and the metabolites might be of relatively
low toxicity (Casida & Quistad, 1995).
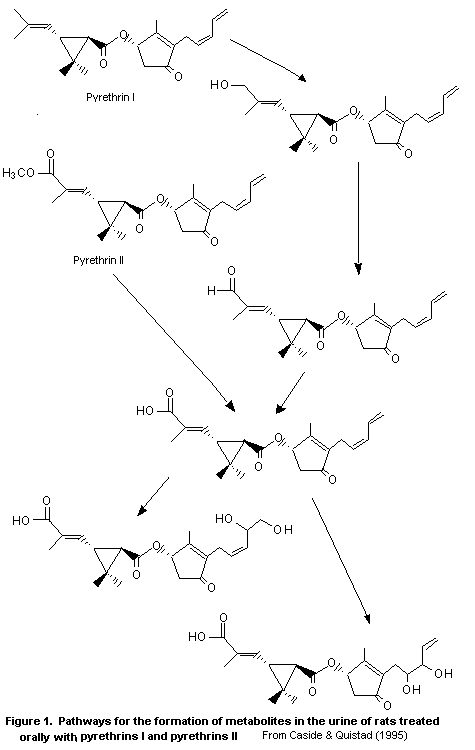
The principal metabolic pathway of pyrethrins is summarized in
Figure 1.
The metabolism of the six natural pyrethrins by mouse and rat
microsomes was studied in vitro, in a study which provided detailed
qualitative and some quantitative information on all six of the active
components present in pyrethrins. The metabolism of pyrethrins I was
shown to proceed principally through oxidative processes, while that
of pyrethrins II was shown to occur through a combination of
hydrolytic and oxidative processes. No metabolites were found that
would not have been expected on the basis of the chemistry of these
chemicals, nor were any of the metabolites found of apparent
toxicological concern (Class et al., 1989).
 2. Toxicological studies
(a) Acute toxicity
The results of studies on the acute toxicity of pyrethrum
extracts are summarized in Table 1. The methods used in these studies
complied with the corresponding FIFRA guidelines and with GLP.
Pyrethrins have little acute toxicity in rats treated orally, with
LD50 values of 2400 mg/kg bw for males and 1000 mg/kg bw for
females. The ratio of pyrethrins I and II (1.44 or 2.50) has no effect
on the toxicity of pyrethrins in male or female rats. The signs of
toxicity included ruffled appearance and tremors within 4-24 h after
dosing. Post-mortem examination of animals that died showed
haemorrhagic lungs, tan-to-yellow fluid in the lower gastrointestinal
tract and muzzle, and genital staining. The no-effect level for
clinical signs was 710 mg/kg bw in males and 320 mg/kg bw in females.
Dermal exposure to 2000 mg/kg bw was tolerated by rabbits, which
showed very slight to well-defined erythema, very slight oedema, and
stained test sites at 24 h. All animals appeared normal through the
14-day observation period.
Very slight acute toxicitywas found in rats exposed to aerosols
by inhalation. On the basis of the mean analytical concentration of
active ingredient and the resultant mortality, the LC50 was
calculated to be 3.4 mg/L for the two sexes. Tremors were seen during
exposure to the two higher concentrations. The significant findings
post mortem included discoloured and oedematous respiratory tissues.
Application of pyrethrins to the skin of albino rabbits produced
a minimally irritating skin irritation score of 0.42 (Romanelli,
1991a).
Instillations of the extracts into the conjunctival sac produced
irritation in the eyes of albino rabbits, but no irritation was
observed by 72 h. No corneal opacity or iritis was seen during the
observation period (Bielucke, 1991).
In a study of dermal sensitization in guinea-pigs with a modified
Buehler protocol, pyrethrins were not sensitizing (Romanelli, 1991b).
(b) Short-term studies of toxicity
Mice
In order to define a suitable maximum tolerated dose for a longer
study, doses of 5000 and 7000 ppm were evaluated in a 2-week study.
The method used complied to a certain extent with OECD guideline 407,
and the study was conducted in compliance with GLP. One mouse at
7000 ppm died. There was no effect on body weight, but food
consumption was slightly decreased at 7000 ppm. Statistically
significant increases in the absolute and relative weights of the
liver were seen at both doses. On the basis of the results of this
Table 1. Acute toxicity of pyrethrins
Species Strain Sex Route LD50 or LC50 Purity (%) or Reference
(mg/kg bw or mg/L air) ratio of pyrethrin I:II
Rat Sprague-Dawley M Oral 2400 58% Gabriel (1992)
Rat Sprague-Dawley F Oral 1000 58% Gabriel (1992)
Rat Sprague-Dawley M Oral 3900 1.4 Gabriel (1992)
Rat Sprague-Dawley F Oral 1300 1.4 Gabriel (1992)
Rat Sprague-Dawley M Oral 3900 2.5 Gabriel (1992)
Rat Sprague-Dawley F Oral 1200 2.5 Gabriel (1992)
Rabbit New Zealand M&F Dermal > 2000 58% Gabriel (1991)
Rat Sprague-Dawley M&F Inhalation 3.4 58% Hoffmann (1991)
M, male; F, female
study, 7000 ppm was selected as the high dose for the long-term study
in mice (Goldenthal, 1987).
Pyrethrins were offered to groups of 15 Charles River mice of
each sex in the diet at concentrations of 300, 1000, 3000, or 10 000
ppm for 13 weeks, equal to 47, 160, 460, and 1600 mg/kg bw per day for
males and 56, 200, 580, and 1800 mg/kg bw per day for females. The
method used complied to a certain extent with OECD guideline 408, and
the study was conducted in compliance with GLP. Four males and 10
females at the original high dose of 30 000 ppm died on day 2, and all
animals at this dose had died or were killed in extremis by day 10.
Four males and two females at 10 000 ppm also died on day 2, with
clinical signs that included tremors, pale exposed skin, dilated
pupils, altered activity, laboured breathing, cold to touch,
moribundity, and hunched posture. Tremors and increased activity were
seen in several animals at 10 000 ppm during the first 2 weeks of
study. No treatment-related clinical signs were observed in the
animals at 300, 1000, or 3000 ppm. The group mean body weights and
food consumption were similar for all groups with surviving animals.
The absolute weight of the liver and the liver:body weight and the
liver:brain weight ratios were all statistically significantly
increased in males and females at 3000 and 10 000 ppm, whereas the
absolute weights of the liver in females at 300 and 1000 ppm were
comparable to those of controls. A treatment-related increase in the
incidence and/or severity of congestion in the liver was observed in
surviving male and female mice at 10 000 ppm, and an increased
incidence but only mild severity was found in < 15% of investigated
animals at 3000 ppm. An increased incidence of hepatocellular
hypertrophy was present in surviving male and female mice at 3000 and
10 000 ppm; at 1000 ppm, only 2 of 15 mice showed mild congestion of
the liver on macroscopic observation. The NOAEL was 1000 ppm, equal to
160 mg/kg bw per day (Goldenthal, 1988a).
Rats
Pyrethrins were offered to groups of 15 Charles River rats of
each sex for 13 weeks in the diet at concentrations of 300, 1000,
3000, 10 000, or 20 000 ppm, equal to 17, 57, 170, 590, and 1200 mg/kg
bw per day for males and 22, 74, 220, 710, and 1400 mg/kg bw per day
for females. The method used in this study complied to a certain
extent with OECD guideline 408, and the study was conducted in
compliance with GLP. During the first week of the study (days 3-7),
one female at 10 000 ppm, one male at 20 000 ppm, and 12 females at
20 000 ppm died; all other animals survived to scheduled sacrifice.
The signs seen before death in all animals that died included
decreased defaecation, increased respiration rate, tremors, and
decreased or increased activity. Convulsions were seen in some
animals. The signs in most animals at 10 000 and 20 000 ppm that
survived until the end of the study were decreased defaecation,
tremors, increased respiration rate, and increased activity.
Convulsions occurred mainly in males at 20 000 ppm. Most of the signs
were seen only during the first two weeks of the study. Statistically
significant decreases in mean body weights were observed during most
or all of the study in males and females at 10 000 and 20 000 ppm.,
and statistically significant decreases in mean food consumption were
seen during most or all of the study in females at 10 000 ppm and
males and females at 20 000 ppm when compared with the respective
control groups. Statistically significantly decreased mean values for
haematocrit and haemoglobin were found in males at 20 000 ppm and for
erythrocytes, haematocrit, and haemoglobin in females at 10 000 and
20 000 ppm. Females at 3000 ppm also showed a decreased mean
haemoglobin value. The treatment-related macroscopic findings
consisted of enlargement and congestion of the liver in both males and
females, but primarily in males, at 10 000 and 20 000 ppm; however,
the macroscopic observation could not be confirmed microscopically.
The liver weight was statistically significantly increased in males at
10 000 and 20 000 ppm and in females at 3000, 10 000, and 20 000 ppm
The weight of the kidney was increased in males and females at 3000,
10 000, and 20 000 ppm The only microscopic finding that was possibly
realted to treatment was small focal or multifocal areas of tubular
degeneration and regeneration in the renal cortex in animals at 3000
and 10 000 ppm, but primarily in males. The NOAEL was 1000 ppm, equal
to 57 mg/kg bw per day (Goldenthal, 1988b).
In a study designed to assess the toxic effects of pyrethrins
administered by whole-body inhalation as a liquid aerosol, groups of
15 Charles River rats of each sex were exposed for 6 h/day, generally
5 days per week, a minimum of 65 times to target concentrations of the
active ingredient corresponding to 0.010, 0.030, 0.10, and 0.35 mg/L.
The mean analytical concentrations to which the groups were exposed
were 0 (control), 0.038, 0.068, 0.23, and 0.83 mg/L, respectively.
Determinations of particle size distribution showed an overall mass
median aerodynamic diameter of 2.7 µm.The controls were exposed only
to conditioned room air on the same schedule. The study was conducted
in compliance with FIFRA Guideline 824 and with GLP.
Two animals at the highest dose died, one animal on day 3.
Because this death was accidental and occurred very early in the
study, this animal was replaced with another animal from the same
shipment. A second animal died on day 15, having shown laboured
breathing similar to that of other animals in this group, and its
death was considered to be potentially related to exposure.
Dose-related increases in the frequency of clinical signs were
observed in animals at the three higher doses during observations in
the chamber and at detailed weekly examinations. These signs included
secretory signs such as nasal discharge and dried material in the
facial area in both males and females. Animals at the highest dose
showed laboured breathing, excess lachrymation, tremors, increased
activity, and matted coats. There were no ocular effects. The absolute
body weights and body-weight gains of both males and females at the
two higher doses were decreased, and after 13 weeks of exposure the
body-weight gains of males at these doses were about 9% lower than
those of controls. In females, the body-weight gain after 13 weeks of
exposure was lower than that of controls, by 13% at 0.10 mg/L and 17%
at 0.35 mg/L. Food consumption was also slightly decreased during the
first 1 or 2 weeks in males and females in these groups.
Nonregenerative anaemia was observed in males at the three higher
doses and in females at the highest dose, with significant decreases
in haemoglobin, haematocrit, and erythrocyte values. An increased
leukocyte count was also seen in the females at the highest dose.
Significant differences in clinical chemical parameters were seen
primarily at the highest dose, including decreased total protein and
globulin and an increased albumin:globulin ratio in males and
decreased glucose in females. Other changes occurred sporadically and
were considered to be unrelated to exposure. Several significant
increases in organ weights or ratios were observed. The increases in
liver weight were clearly related to treatment. Increases in the
organ:body weight ratios of the kidneys and lung might be a reflection
of a marginal increase in absolute organ weights and the decreased
body weights. Morphological abnormalities in the larynx,
nasoturbinates, nasopharynx, and lungs observed by light microscopy
were considered to be localized responses indicative of a
treatment-related effect. The NOAEL for systemic effects was 0.01 mg/L
(Newton, 1992).
Rabbits
Pyrethins were administered dermally to five male and five female
New Zealand white rabbits in the form of a 25% (w/v) mixture in
vegetable oil at doses of 0, 100, 300, or 1000 mg/kg bw once daily, 5
days per week for 3 weeks. Animals in the vehicle control group were
given vegetable oil on the same regimen and at the same volume as the
group receiving the high dose . This study was conducted in compliance
with OECD Guideline 410 and GLP. One rabbit at 1000 mg/kg bw was
sacrificed in extremis on day 10 after showing emaciation, decreased
activity, and decreased defaecation. Macroscopic examination of this
animal did not reveal the cause of death. A low incidence of
desquamation and/or red raised areas on the skin at the application
site was observed in all groups, including the vehicle controls.
Several animals in the treated groups showed very slight to
well-defined erythema of the skin at the application site, but no
clear pattern with regard to treatment was seen for any of these
findings. Microscopic evaluation revealed no evidence of systemic
toxicity. The microscopic lesions at the application site included
acanthosis, haemorrhage, hyperkeratosis, and chronic inflammation,
although haemorrhage was observed only in the group given the vehicle
alone. Thus, all of the dermal reactions appeared to be due to the
vegetable oil. The NOAEL for systemic effects was 1000 mg/kg bw, the
highest dose tested (Goldenthal, 1992).
This result is supported by that of a previous study performed to
assess dermal irritation with pyrethrins at concentrations of 25%,
50%, and 75% w/v in corn oil on rabbits (Myer, 1991).
Dogs
Pyrethins were incorporated into the basal diet of groups of two
pure-bred beagle dogs of each sex at concentrations of 600, 1000,
3000, or 6000 ppm for 8 weeks, equal to 18, 30, 86, and 170 mg/kg bw
per day for males and 19, 29, 94, and 200 mg/kg bw per day for
females. The method used complied to a certain extent with OECD
guideline 409, and the study was conducted in compliance with GLP. One
male and both females at 6000 ppm died or were killed in extremis
during the study. The treatment-related clinical signs observed in
animals at this dose included inappetence, thin appearance, ataxia,
trembling, oily coat, impaired limb function, shallow breathing,
moribundity, and death. With the exception of moribundity and death,
similar signs were observed at 3000 ppm. Males and females at 6000 ppm
lost weight during the study, but the body-weight gains of animals in
all other treated groups were comparable to those of controls. The
average food consumption was decreased for males at 3000 ppm and for
males and females at 6000 ppm when compared with controls. Decreased
haematocrit, haemoglobin, and erythrocyte values were seen at the end
of the study in males at 3000 and 6000 ppm, but there were no other
treatment-related haematological finding. One male and one female at
6000 ppm killed in extremis had increased leukocyte counts and
decreased haematocrit, haemoglobin, and erythrocyte values. Slightly
decreased glucose, calcium, phosphorus, and cholesterol values were
found at the end of the study in males at 6000 ppm, and males and
females at 3000 ppm had slightly decreased cholesterol concentrations.
The aspartate and alanine aminotransferase activities of males at 6000
ppm were slightly increased at the end of dosing, and the surviving
male at this dose had a very high creatinine phosphokinase value.
There were no other treatment-related biochemical findings at the end
of the study. Males and females at 6000 ppm killed in extremis
showed some variation in electrolyte levels, and the urea nitrogen
concentration was increased in both dogs. The female showed large
increases in aspartate and alanine aminotransferase and creatine
phosphokinase activities. The absolute weights of the liver in both
males and females at 1000 and 3000 ppm were increased in a
treatment-related fashion, and the absolute weight of the testis at
these doses appeared to decrease in a similar manner. There were no
macroscopic or microscopic lesions attributable to the administration
of pyrethrins. The NOAEL was 600 ppm, equal to 18 mg/kg bw per day
(Goldenthal, 1988c).
Pyrethrins were administered to groups of four beagle dogs of
each sex for 52 weeks in the diet at concentrations of 0, 100, 500, or
2500 ppm, equal to 2.6, 14, and 66 mg/kg bw per day for males and 2.8,
14, and 75 mg/kg bw per day for females. Four dogs of each sex were
evaluated at each dietary concentration. This study was conducted in
compliance with FIFRA Guideline 83-1 and with GLP. All animals
survived to the end of the study, and no remarkable clinical signs of
toxicity were found at any dose. Overall, the mean body weights of the
treated animals were similar to those of controls. The mean food
consumption of males at 2500 ppm and of females at 500 and 2500(c)
ppm was lower than that of controls during the first week of the
study, but consumption was similar for the remainder of the study,
except that males at 500 ppm consumed greater amounts of food than the
controls. Increased total leukocyte and segmented neutrophil counts
were found in females and decreased erythrocyte, haemoglobin, and
haematocrit values in males at 2500 ppm. Alanine aminotransferase
activity was statistically significantly increased in females at 2500
ppm at both the 6- and 12-month evaluations. The slight increase in
the activity of this enzyme in males was not statistically
significant. No treatment-related changes in urinary parameters were
seen at either interval. The relative and absolute weights of the
liver were significantly increased in males at 2500 ppm when compared
with controls, but no statistically significant differences in organ
weights were seen in females at this dose. No macroscopic or
microscopic treatment-related changes were observed in tissues. The
NOAEL was 500 ppm, equal to 14 mg/kg bw per day (Goldenthal, 1990a).
(c) Long-term tudies of toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female Charles River CD-1 mice received
diets containing pyrethrins at concentrations of 100, 2500, or 5000
ppm for 18 months, equal to doses of 14, 350, and 690 mg/kg bw per day
for males and 17, 410, and 830 mg/kg bw per day for females. This
study was conducted in compliance with FIFRA Guideline 83-2(b) and
with GLP. One male and one female at 5000 ppm were found dead during
the first week of the study, but no other treatment-related deaths
occurred, and survival was similar in the control and treated groups
throughout the study. All animals at 5000 ppm exhibited increased
activity when stimulated during the first week of the study but not
later. There were no other clinical signs seen that differentiated the
treated from the control groups. Statistically significant differences
in mean body weight and food consumption were seen between control and
treated groups sporadically throughout the study, but none of the
differences was considered to be related to treatment. No
treatment-related effect was observed in other parameters examined at
12 and 18 months of study. At necropsy, discoloured, dark livers were
more common in males at 5000 ppm and in females at 2500 and 5000 ppm,
and treatment-related increases in the absolute and relative weights
of the liver were seen in males and females at 2500 and 5000 ppm.
Microscopically, vacuolar fatty change was found in the livers of
males at these doses, and this change was considered to be related to
treatment. The dark discolouration seen macroscopically could not be
explained by the microscopic findings. The incidence of nodules and
masses in the lungs appeared to be slightly increased in animals at
5000 ppm. When the lungs were examined microscopically according the
original test protocol, the incidences of alveolar bronchiolar
adenomas were increased in females at 5000 ppm, and the increase was
statistically significant, exceeding the range in historical controls.
Treated males showed an apparent, not clearly dose-related increase in
the incidence of alveolar bronchiolar carcinomas which exceeded the
upper limit of 95% of the historical controls (Table 2). The sponsor
asked the testing laboratory to conduct serial sectioning of the
remaining lung tissue of female mice in the control and highest-dose
groups, which did not have diagnoses of lung tumours in the original
examination. As this was considered not to be an acceptable
toxicopathological practice, the results of the first evaluation were
taken into consideration for the risk assessment, and the increased
incidence of lung tumours was considered to be a treatment-related
effect. The NOAEL was 100 ppm, equal to 14 mg/kg bw per day
(Goldenthal, 1990b).
Table 2. Comparison of first and second evaluations of microscopic
neoplastic lesions in the lung of mice treated with
pyrethrins for 18 months
Sex Dose No. of lungs Alveolar bronchiolar neoplasm
(ppm) examined
Adenoma Carcinoma
Male 0 60 14 0a
0 60 16 0
100 60 15 1
2500 60 13 3b,c
5000 60 17 3b,c
Female 0 60 8 1
0 60 4 3
100 60 11 0
2500 60 5 2
5000 60 19c,d 2
a p < 0.05; Cochran trend test
b Significant differences in pair-wise comparison of the
high-dose group with controls at p < 0.05
c > 95% of of the upper range of historical controls
d p < 0.05; Fisher exact test
Rats
Groups of 60 Charles River CD rats of each sex received diets
containing pyrethrins at concentrations of 100, 1000, or 3000 ppm for
104 weeks, equal to 4, 43, and 130 mg/kg bw per day for males and 5,
56, and 170 mg/kg bw per day for females. This study was conducted in
compliance with FIFRA Guideline 83-5 and with GLP. Survival was
similar in the treated and control groups, and there were no clinical
findings attributable to treatment. Statistically significant
decreases in body weight which were considered to be related to
treatment were observed during the first 78 weeks of the study in both
male and female rats at 3000 ppm, with a difference from controls of
7% in males and 10% in females. A slight, treatment-related decrease
in food consumption was seen at the same time. No treatment-related
ophthalmological findings or organ weight changes were detected during
the study, and no haematological or urological changes were found. The
activities of serum transaminases were substantially increased at most
intervals of analysis in males at 3000 ppm, most of the values
reaching statistical significance. Increased incidences of benign
tumours of the liver, thyroid, and skin were also observed (Table 3),
and a statistically significantly higher incidence of hepatocellular
adenomas was described in females at the high dose. Follicular
adenomas and carcinomas were initially seen in the thyroid glands of
rats at the high dose, which appeared to be related to treatment, but
during a re-evaluation some of the carcinomas were reclassified as
adenomas and some adenomas were reclassified as hyperplasia. After the
re-evaluation, the incidence of hyperplasia was found to be enhanced
in males and females, and the incidence of follicular adenomas was
statistically significantly increased only in females at 3000 ppm.
Nevertheless, the tumour incidences in animals of each sex were higher
than the upper range seen in historical controls. The results of a
further, full histopathological peer review confirmed these increased
tumour incidences. Macroscopic examination of the skin showed a slight
increase in the incidence of cystic lesions in the skin and subcutis,
and the microscopic assessment showed an apparently higher incidence
of keratoacanthomas in males at the high dose, which was statistically
significant in comparison with both control groups. A peer review of
pathological lesions in all male rats resulted in removal of several
keratoacanthomas from the table of incidence in treated groups but
confirmed that the incidence of this lesion clearly exceeded the upper
limit of the range in historical controls (1.4-10%). The increased
incidences of liver and thyroid tumours and of keratoacanthomas of the
skin were considered to be treatment-related effects but to be
threshold phenomena of negligible relevance to the low doses to which
humans are exposed. The NOAEL was 100 ppm, equal to 4 mg/kg bw per day
(Goldenthal, 1990c).
(d) Genotoxicity
The results of tests for the genotoxicity of pyrethrins in
vitro are summarized in Table 4.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a two-generation study, groups of 28 male and 28 female
Charles River rats received diets containing pyrethrins at
concentrations of 100, 1000, or 3000 ppm, equivalent to 10, 100, and
300 mg/kg bw per day. A control group received the basal laboratory
diet on an identical regimen. The F0 parental generation were
treated for a minimum of 77 days before the first of two matings. The
same numbers of weanlings from the F1b litters were selected
randomly to become parents of the F1 generation and were treated for
a minimum of 95 days before being mated twice to produce the F2a and
F2b litters. This study was conducted in compliance with FIFRA
Guideline 83-4 and with GLP. No treatment-related effects were noted
with respect to clinical signs, body weights, or food consumption in
Table 3. Incidences of microscopic neoplastic lesions in rats fed diets containing pyrethrins for 104 weeks
Sex Dose Liver Thyroid Skin
(ppm)
Total Hepatocellular tumours Total Hyperplasia Follicular tumours Total Cystic Keratoacanthomasb
examined examined examined lesions
Adenoma Carcinoma Adenomaa Carcinomaa
Male 0 60 6 1 60 2 2c 0 60 5 4
0 60 1 0 60 0 1 1 60 2 5
100 60 0 0 60 2 4 1 24 1 4
1000 60 3 0 59 5 5d 2 14 5 4
3000 60 3 1 60 7 5d 2 60 9 11d,e
Female 0 60 0 1 60 0 0 1 60 0 0
0 60 1 0 60 2 1 1 59 3 0
100 60 0 0 60 1 2 0 8 2 1
1000 60 1 0 60 1 3d 0 8 2 1
3000 60 5d.f 0 60 5 5d 1 60 0 0
a Results of the histopathological peer review
b Results of the re-analysis only in males
c p < 0.05; Cochran trend test
d Greater that the upper limit of historical control data
e Significant difference in the pair-wise comparison of the high-dose group with the controls at p < 0.05
f p < 0.05; Fisher exact test
Table 4. Results of assays for the genotoxicity of pyrethrins in vitro
Test system Test object Concentration Purity Results Reference
(%)
Reverse mutationa S. typhimurium TA98, 8.8-8772 µg/plate in 58 Negative ± S9 San & Springfield
TA100, TA1535, TA1537, acetone (1989)
TA1538
Chromosomal Chinese hamster ovary 0.02-0.32 Fl/ml + S9 58 Negative ± S9 Putman & Morris
aberrationa cells 0.005-0.08 Fl/ml - S9 (1989)
in DMSO
Unscheduled DNA Rat primary hepatocytes 0.0-1.0 Fl/ml in 58 Negative Curren (1989)
synthesis acetone
a Study conducted in compliance with GLP and to a certain extent with OECD guidelines
the parental rats of the F0 generation, but the body weights and
food consumption of the parental rats of the F1 generation were
significantly reduced at 3000 ppm and sporadically reduced at 1000
ppm. These reductions were considered to be treatment-related.
Treatment at 3000 ppm resulted in significantly reduced body weights
at birth for F1a males and F2a pups of each sex and during
lactation for the male and female offspring of both matings of both
generations. The mean body weights of pups at 1000 ppm were also lower
than those of controls for male F2a pups at birth and for F1b and
F2a pups during lactation. Reproductive performance and other litter
parameters were not affected by the treated diet at any dose. The
NOAEL for parental and reproductive toxicity was 100 ppm, equivalent
to 10 mg/kg bw per day (Schardein, 1989).
(ii) Developmental toxicity
Rats
Groups of five mated female Charles River rats were used in a
range-finding study to determine the doses of pyrethrin to be used in
a later study. Doses of 37.5, 75, 150, 300, or 600 mg/kg bw per day
were administered orally by gavage on days 6-15 of gestation at a
volume of 3 ml/kg. The control group received only the vehicle, 0.5%
methylcellulose, on a comparable regimen. Uterine examinations were
performed on all surviving females on day 20 of gestation. The method
used in this study complied to a certain extent with OECD Guideline
414, and the study was conducted in compliance with GLP.
Treatment-related maternal toxicity, observed as deaths, convulsions,
and/or tremors, occurred at 150, 300, and 600 mg/kg bw per day. No
treatment-related maternal deaths occurred at 75 mg/kg bw per day,
although tremors were observed in this group. No treatment-related
clinical signs were observed at 37.5 mg/kg bw per day. On the basis of
these results, doses of 5, 25, and 75 mg/kg bw per day were selected
for use in the following study (Schardein, 1987a).
Groups of 25 mated female Charles River rats were given
pyrethrins suspended in 0.5% methylcellulose orally by gavage at doses
of 5, 25, or 75 mg/kg bw per day on days 6-15 of gestation at a volume
of 3 ml/kg. The control group received the vehicle only on a
comparable regimen. On day 20 of gestation, the fetuses were removed
surgically for evaluation. The method used in this study complied to a
certain extent with OECD Guideline 414, and the study was conducted in
compliance with GLP. No animals died or were killed in extremis
during the study, and no treatment-related clinical signs were
observed. The body-weight gains of the treated groups were comparable
to those of the controls during treatment. No evidence of fetotoxicity
was found, and morphological examination revealed no teratogenic
effects at any dose tested. The NOAEL for maternal toxicity was 75
mg/kg bw per day, and that for developmental toxicity was 75 mg/kg bw
per day, the highest dose tested (Schardein, 1987b).
Rabbits
Groups of five inseminated female New Zealand white SPF rabbits
were used in a range-finding study to determine the doses of
pyrethrins for use in a later study. Doses of 37.5, 75, 150, 300, or
600 mg/kg bw per day were administered orally by gavage on days 7-19
of gestation at a volume of 3 ml/kg. The control group received only
the vehicle, 0.5% methylcellulose, on a comparable regimen. Uterine
examinations were performed on all surviving females on day 29 of
gestation. The method used complied to a certain extent with OECD
Guideline 414, and the study was conducted in compliance with GLP.
Treatment-related maternal toxicity, seen as deaths, tremors,
convulsions, and weight loss, and fetal toxicity, seen as high
postimplantation loss, were found at 600 mg/kg bw per day. Maternal
toxicity, in terms of weight loss during treatment and tremors was
seen at 300 mg/kg bw per day. No clear treatment-related effects were
observed at 37.5, 75, or 150 mg/kg bw per day. On the basis of these
results, doses of 25, 100, and 250 mg/kg bw per day were selected for
use in the following study (Schardein, 1987c).
Groups of 16 inseminated female New Zealand white SPF rabbits
were randomly assigned to receive pyrethrins at doses of 25, 100, or
250 mg/kg bw per day orally by gavage on days 7-19 of gestation at a
volume of 3 ml/kg. The control group received only the vehicle, 0.5%
methylcellulose, on a comparable regimen. On day 29 of gestation, the
fetuses were removed surgically for evaluation. The study was
conducted in compliance with OECD Guideline 414 and with GLP. All
animals survived to the end of treatment. One doe at the high dose
aborted near term, on day 28 of gestation, after showing decreased or
absent defaecation on several days previously. No gross lesions were
present at necropsy. Excessive salivation, arched head, and/or
laboured breathing were observed in a few females at the high dose on
days 18-19 of gestation. One female at the intermediate dose had
excessive salivation and arched head on gestation day 19. No apparent
treatment-related clinical signs were seen at the low dose. There were
no treatment-related gross pathological changes in any of the animals
at the end of the study. Body-weight loss was seen in does at the high
dose throughout treatment, and slightly reduced body-weight gain
relative to the control value was observed in animals at the
intermediate dose. The body-weight gains of treated groups were
comparable to that of the control group throughout gestation (days
0-29). When the body weights on day 29 were adjusted by subtracting
the uterine weights to reflect only maternal weight change, mean
weight losses were evident in all groups, including the control, over
the entire gestation period. There were no biologically meaningful or
statistically significant differences in the mean numbers of viable
fetuses, postimplantation losses, total implantations, or corpora
lutea and in fetal body weight or fetal sex distribution in the
treated groups in comparison with the control values. One doe at the
high dose resorbed its litter, but it is not clear if this finding was
related to treatment. There was no treatment-related or statistically
significant difference in the incidence of fetal malformations or
variations. The NOAEL for maternal toxicity was 25 mg/kg bw per day
and that for developmental toxicity was 250 mg/kg bw per day, the
highest dose tested (Schardein, 1987d).
(f) Special studies
(i) Effects on the central nervous system
Rats
Male Sprague-Dawley rats were treated once by oral gavage with a
10% or 25% solution of pyrethrins in corn oil at doses of 0, 40, 100,
200, 400, 800, 1400, or 2000 mg/kg bw, and females received a 2.5, 5,
or 10% solution of pyrethrins in corn oil at doses of 0, 25, 50, 100,
150, 200, 400, or 800 mg/kg bw. This study was conducted in compliance
with FIFRA Guideline 81-8 and with GLP. The clinical signs were
characterized by mild-to-severe tremors. On the basis of the
occurrence, severity, and onset of these reactions, a solution of 10%
pyrethrins in corn oil and doses of 40, 125, and 400 mg/kg bw were
selected for the study of acute neurotoxicity in males and a solution
of 5% pyrethrins in corn oil and doses of 20, 63, and 200 mg/kg bw in
females (Hermansky & Hurley, 1993a).
In the main study, groups of 15 male Sprague-Dawley rats received
by gavage a 10% w/v solution of pyrethrins in corn oil at doses of 0,
40, 125, or 400 mg/kg bw, and the same numbers of females received a
5% w/v solution of pyrethrins in corn oil at doses of 0, 20, 63, or
200 mg/kg bw. This study was conducted in compliance with FIFRA
Guideline 81-8 and with GLP. Five males and two females at the high
dose died on the day of treatment, and a variety of acute neurological
signs were observed in the other animals at this dose, including
tremors, urogenital area wetness, salivation, perinasal encrustation,
exaggerated startle response, decreased grip strength, hind leg splay,
and increased body temperature. Tremors were also observed in three
females at the intermediate dose. Measurements of motor activity on
the day of treatment indicated increased fine movement and decreased
rearing and ambulation in animals of each sex at the high dose and
decreased fine movement, rearing, and ambulation in males at the
intermediate dose. In addition, slight, statistically nonsignificant
decreases in body weight were seen in males at the high dose on days 7
and 14. There was no evidence of tany gross, treatment-related lesion.
The microscopic changes were limited mainly to sections of the sciatic
nerve and its branches. The histomorphological changes within the
peripheral nerve sections indicated the presence of scattered
degenerating nerve fibres or myelin sheaths. These changes were seen
in only a few animals, were graded as minimal, and were not
dose-related. The NOAEL was 20 mg/kg bw (Hermansky & Hurley, 1993b).
(ii) Effects on hepatic microsomal enzymes
Rats
Oral administration of pyrethrins to male rats at 85, 200, or 500
mg/kg bw per day for 3 weeks resulted in liver enlargement and
decreased hepatic DNA concentrations. Significantly decreased
hexobarbital-induced hypnosis without concomitant changes in
barbital-induced hypnosis suggested an alteration in hepatic drug
metabolism. The activities of hepatic microsomal enzymes responsible
for detoxification of
O-ethyl- O-(4-nitrophenyl)phenyl-phosphonothioate,
para-nitroanisole demethylation, and hexobarbital oxidation were
increased at 200 mg/kg bw per day to 150, 173, and 241% of the control
values, respectively. Increased liver weight, the detoxification of
pyrethrins, and demethylation of para-nitroanisole were found to be
dose-related. Small increases in enzyme activities were observed when
the lowest dose was given for 15 days. At 500 mg/kg bw per day, the
liver weight and enzyme activities were increased up to 17 days of
treatment but returned to the control level within 7 days after
cessation of treatment. NADPH cytochrome c reductase activity and the
cytochrome P450 concentration were also increased. It was suggested
that pyrethrins caused induction of microsomal enzymes. The LOAEL was
85 mg/kg bw per day (Springfield et al., 1973).
3. Observations in humans
The main adverse effects seen after exposure to pyrethrum
extracts in older studies were those manifesting as either skin or
respiratory reactions. Much of the research performed to date has
focused on the dermatological effects of pyrethrins, although mention
has been made of the respiratory reactions that have often accompanied
those of the skin. Studies by Ramirez (1930) and Feinberg (1934), in
which the association between sensitivity to ragweed and to pyrethrins
was delineated, contributed much to the understanding of allergic
responses to pyrethrins. Several investigations have since been
undertaken to isolate and characterize the allergen responsible for
the dermal reactions. Mitchell et al. (1972) isolated a sesquiterpene
lactone, pyrethrosin, from pyrethrins which induced positive dermal
responses in humans given a patch test. None of the other fractions
induced reactions, with the exception of pyrethrins II, which elicited
a weak response. Zucker (1965) demonstrated that a dermal reaction to
unrefined pyrethrum extract does not result in allergy to refined
pyrethrins. The previous evaluation by the Joint Meeting in 1970 cited
an unpublished report in which 200 people were given patch tests with
a 1% water dispersion of pyrethrins, and no evidence of primary
irritation or of sensitization was found. Rickett et al. (1972)
concluded that the refined extracts that have been marketed since 1957
do not induce skin allergies when tested on sensitive subjects and
that the dermal effects reported in the early literature are not
relevant to an assessment of refined pyrethrins.
Case reports of adverse respiratory effects, such as
anaphylactoid reactions and asthma, attributed to pyrethrins indicate
that these responses often occur in individuals with a history of
asthma. Although investigations of the dermal effects of pyrethrum
extracts suggests that pyrethrins are not the causative agent, no
thorough investigation of the agent(s) responsible for the adverse
respiratory responses has been conducted.
Comments
Absorption, distribution, and excretion in rats were investigated
only for pyrethrins I. After oral administration, more than 90% of a
low dose of pyrethrins I was absorbed, and the concentration of
radiolabel in blood peaked between 5 and 8 h. The radiolabelled
residues were widely distributed in the organs analysed, with the
highest concentrations in fat in females. The elimination half-time of
pyrethrins I in males and females was approximately 6 h. The mean
percentage of administered radiolabel found in the urine ranged from
32 to 47% in males and from 50 to 57% in females, the remainder being
excreted in faeces.
The substance is extensively metabolized, the residues of the
parent compound in faeces and urine representing only 10%. Six
metabolites were identified and two major metabolic pathways were
suggested, the first involving oxidation of the double-bond and/or the
methyl groups and the second involving hydrolysis of the ester bond.
Pyrethrins I are metabolized mainly through oxidative processes, while
pyrethrins II are metabolized through a combination of hydrolytic and
oxidative processes.
Pyrethrins show little acute toxicity, with an oral LD50 in
rats of > 1200 mg/kg bw and NOAELs for clinical signs of 710 mg/kg bw
for males and 320 mg/kg bw for females, a dermal LD50 in rabbits of
> 2000 mg/kg bw, and an inhalation LC50 in rats of 3.4 mg/L. The
compounds are minimally irritating to the skin and eye and show no
potential for skin sensitization. Pyrethrum extracts have not been
classified by WHO for acute toxicity.
In short-term tests for toxicity in mice, rats, and dogs, the
lowest relevant NOAELs after oral administration were 1000, 1000, and
600 ppm, equal to 160, 57, and 18 mg/kg bw per day, respectively, for
the three species. Statistically significant decreases in mean body
weight or body-weight gain were observed at the high doses throughout
most or all of the studies.
The liver is the main target organ in mice, rats, and dogs, and
an increased liver weight was frequently accompanied by changes in
serum transaminase activity. In mice, increased liver weights were
associated with a higher incidence of hepatocellular hypertrophy. In
the livers of rats and dogs, generally unremarkable histopathological
changes were observed. At doses of 85 mg/kg bw per day and above, a
pyrethrum extract containing 20% pyrethrins induced microsomal enzymes
in rats. Furthermore, anaemia was observed in rats and dogs at doses
of 3000 ppm and above. The kidney was another target, but only in
rats. In a 13-week study, rats at doses greater than 1000 ppm had
increased kidney weights associated with tubular degeneration and
regeneration in the renal cortex.
In a 13-week study in rats exposed by inhalation, the NOAEL for
systemic toxicity was 0.011 mg/L. The increases in liver weight were
clearly related to exposure and were accompanied by changes in serum
transaminase activity. Nonregenerative anaemia was also observed. The
weights of the kidney and lung were increased in relation to body
weight. The morphological abnormalities observed in the larynx,
nasoturbinates, nasopharynx and lungs by light microscopy were
considered to be localized responses indicative of a treatment-related
effect.
Dermal administration of pyrethrins at doses up to 1000 mg/kg bw
per day for 21 days caused no systemic toxicity in rabbits.
In a two-year study of toxicity and carcinogenicity in rats and
an 18-month study of carcinogenicity in mice, the NOAEL was 100 ppm in
both species, equal to 14 and 4 mg/kg bw per day in mice and rats,
respectively. The liver was the main target. A treatment-related
effect on the incidence of lung tumours was seen in mice and increased
incidences of benign tumours of the skin, liver, and thyroid were
observed in rats. The increased incidences of hepatocellular adenomas
were associated with persistent induction of cytochrome P450 enzymes
and hepatocellular hypertrophy, suggesting that pyrethrins are
rodent-specific hepatoproliferative carcinogens. Enzyme induction
leading to increased clearance of thyroid hormones would also be
consistent with the higher incidence of follicular hyperplasia and
follicular adenomas. However, additional studies on the mechanism of
formation of the liver and thyroid tumours are required. The Meeting
concluded that the increased tumour incidences caused by pyrethrins
are threshold phenomena of negligible relevance to the low doses to
which humans are exposed (see Appendix 1 to this monograph addendum).
Pyrethrins did not induce reverse mutagenicity in Salmonella
typhimurium with metabolic activation, did not induce chromosomal
aberration in Chinese hamster ovary cells, and did not induce
unscheduled DNA synthesis in rat primary hepatocytes. The Meeting
concluded that pyrethrins have no genotoxic or mutagenic potential,
but a test for gene mutation in mammalian cells is lacking.
Pyrethrins did not show developmental toxicity in rats or rabbits
at the highest maternally toxic doses tested, which were 75 and 250
mg/kg bw per day, respectively. The only effects on the offspring,
observed in a two-generation study of reproductive toxicity in rats,
were reduced body weights at the parentally toxic doses of 1000 and
3000 ppm, with a NOAEL of 100 ppm, equivalent to 10 mg/kg bw per day.
In a study of neurotoxicity in rats given single oral doses,
acute neurological disorders (tremors, wetness of the urogenital area,
salivation, perinasal encrustation, exaggerated startle response,
decreased grip strength, and hind-leg splay) and behavioural effects
(increased motor activity and decreased rearing and ambulation) were
noted, with a NOAEL of 20 mg/kg bw.
The available data on humans did not show a causal relationship
between exposure to modern pyrethrin-containing products and
significant adverse health effects.
An ADI of 0-0.04 mg/kg bw was established for the tested blend of
refined pyrethrum extract, which was based on the NOAEL of 100 ppm,
equal to 4 mg/kg bw per day, observed in the long-term study of
toxicity and carcinogenicity in rats and a safety factor of 100. This
figure is identical to the ADI derived by the 1972 Meeting, which was
based on a NOAEL of 200 ppm, equivalent to 10 mg/kg bw per day, in a
long-term study in rats and a safety factor of 250.
The acute and long-term toxicity of the pyrethrins differ
significantly. The acute toxicity of orally administered pyrethrins is
expressed as neurotoxic effects. The longer-term toxicity is based
principally on effects on the liver. Therefore, an acute reference
dose of 0.2 mg/kg bw was allocated for the tested blend of refined
pyrethrum, which was based on the NOAEL of 20 mg/kg bw for acute
neurotoxicity in rats and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effects
Mouse: 100 ppm, equal to 14 mg/kg bw per day (18-month study of
carcinogenicity)
Rat: 20 mg/kg (study of acute neurotoxicity)
100 ppm, equal to 4 mg/kg bw per day (2-year study of
carcinogenicity)
100 ppm, equivalent to 10 mg/kg bw per day (parental and
reproductive toxicity in a two-generation study of
reproductive toxicity)
75 mg/kg bw per day (maternal toxicity in two studies of
teratogenicity, no developmental toxicity in a study of
teratogenicity at the highest dose tested)
Rabbit: 25 mg/kg bw per day (maternal toxicity in a study of
teratogenicity, no developmental toxicity in a study of
teratogenicity at the highest dose tested)
Dog: 500 ppm, equal to 14 mg/kg bw per day (toxicity in a 1-year
study)
Estimate of acceptable daily intake for humans
0-0.04 mg/kg bw
Estimate of acute reference dose
0.2 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Gene mutation test in mammalian cells (required for submission to
WHO by 2001)
2. Mechanistic study on liver and thyroid tumorigenesis (see
Appendix 1; required for submission to WHO by 2001)
3. Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to pyrethrins
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Immediately (peak between 5 and 8 h) and nearly complete (> 90%)
in rats
Distribution Widely distributed in rats, highest concentrations in fat
Potential for accumulation None
Rate and extent of excretion Nearly complete excretion in urine (32-47% and 50-57% in male and
female rats) and in faeces
Metabolism in animals Extensively metabolized in rats, six metabolites identified; two
major metabolic pathways.
Toxicologically significant compounds Parent compound and metabolites
(animals, plants and environment)
Acute toxicity
Rat, LD50, oral > 1200 mg/kg bw
Rabbit, LD50, dermal > 2000 mg/kg bw
Rat, LC50, inhalation > 3.4 mg/L (4 h)
Dermal irritation Non, rabbits
Ocular irritation None, rabbits
Dermal sensitization Not a sensitizer (Buehler test in guinea-pigs)
Short-term toxicity
Target/critical effect Liver (mice, rat, dog), erythrocytes (rat, dog), kidney (rat)
Lowest relevant oral NOAEL 90 days, dog: 600 ppm (18 mg/kg bw per day)
Lowest relevant dermal NOAEL 3 weeks, rabbit: > 1000 mg/kg bw per day
Lowest relevant inhalation NOAEL 3 months, rat: 0.01 mg/L
Long-term toxicity and carcinogenicity
Target/critical effect Liver
Lowest relevant NOAEL/NOEL 2 years, rat: 100 ppm (4 mg/kg bw per day)
Carcinogenicity Increased tumour incidences in liver, thyroid, skin (rats) and
lungs (mice)
Genotoxicity In an incomplete range of studies, no genotoxic or mutagenic
potential identified
Reproductive toxicity
Reproductive target/critical effect Reproductive effects (reduced pup body weights) at parentally
toxic doses
Lowest relevant reproductive NOAEL Rat: 100 ppm (10 mg/kg bw per day)
Developmental target/critical effect No developmental effects at maternally toxic doses
Lowest relevant developmental NOAEL Rat: 75 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Acute clinical disorders and behavioural effects
Acute neurotoxic NOAEL Rat: 20 mg/kg bw
Other toxicological studies Induction of hepatic microsomal activity
Medical data Available human data do not show causal relationships between
exposure to modern pyrethrin-containing products and significant
adverse health effects.
Summary Value Study Safety factor
ADI 0-0.04 mg/kg bw Long-term toxicity, rats 100
Acute reference dose 0.2 mg/kg bw Acute neurotoxicity, rats 100
References
Bielucke, J. (1991) Primary eye irritation in rabbits (New Zealand
white). Unpublished Report, Project No. 91-7316A, MRID #41964802
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Casida, J.E. & Quistad, G.B. (1995) Metabolism and synergism of
pyrethrins. In: Casida, J.E. & Quistad, G.B., eds, Pyrethrum
Flowers: Production, Chemistry, Toxicology and Uses, Oxford:
Oxford University Press, pp. 258-277.
Class, T.J., Ando, T. & Casida, J.E. (1989) Pyrethroid metabolism;
microsomal oxidase metabolites of (S)-Bioallethrin and the six
natural pyrethrins. Unpublished report MRID #41248801 from
Pesticide Chemistry & Toxicology Laboratory. Submitted to WHO by
Kenya Pyrethrum Information Centre, Oberalm, Austria.
Curren, R.D. (1989) Unscheduled DNA synthesis assay in rat primary
hepatocytes with a confirmatory assay. Unpublished report,
laboratory study No. T8729.380009, MRID #41344501 from
Microbiological Associates, Inc. Submitted to WHO by Kenya
Pyrethrum Information Centre, Oberalm, Austria.
Feinberg, S.M. (1934) Pyrethrum sensitization. J. Am. Med. Assoc.,
102, 1557-1558.
Gabriel, D. (1991) Acute dermal toxicity in rabbits (New Zealand
white). Unpublished report, project No. 91-7316A, MRID #41964801
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Gabriel, D. (1992) Summary of results of acute oral toxicity study
Unpublished report, project No. 92-7529A, from Biosearch Inc.,
Philadelphia, Pennsylvania, USA. Submitted to WHO by Kenya
Pyrethrum Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1987) Evaluation of pyrethrum extract in a 2-week
dietary toxicity study in mice. Unpublished report, laboratory
project ID: 556-114 from International Research & Development
Corp. Submitted to WHO by Kenya Pyrethrum Information Centre,
Oberalm, Austria.
Goldenthal, E.I. (1988a) Evaluation of pyrethrum extract in a 13-week
dose range-finding study in mice. Unpublished report, laboratory
project ID: 556-008, MRID #433585201 from International Research
& Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1988b) Evaluation of pyrethrum extract in a 13-week
dose range-finding study in rats. Unpublished report, laboratory
project ID: 556-010 from International Research & Development
Corporation. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Goldenthal, E.I. (1988c) Toxicity study in dogs. Unpublished report,
laboratory project ID: 556-006 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Goldenthal, E.I. (1990a) Evaluation of pyrethrum extract in a 1-year
chronic toxicity study in dogs. Unpublished report, laboratory
project ID: 556-007, MRID #41496502 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Goldenthal, E.I. (1990b) Evaluation of pyrethrum extract in an
18-month dietary oncogenic study in mice. Unpublished report,
laboratory project ID: 556-013, MRID #41559401 from International
Research & Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1990c) Evaluation of pyrethrum extract in a two-year
dietary toxicity and oncogenicity study in rats. Unpublished
report, laboratory project ID: 556-011, MRID #41559501 from
International Research & Development Corp. Submitted to WHO by
Kenya Pyrethrum Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1992) 21-day repeated dose dermal toxicity study
with pyrethrum extract in rabbits. Laboratory project ID:
556-018, MRID #42212601 from International Research & Development
Corp. Submitted to WHO by Kenya Pyrethrum Information Centre,
Oberalm, Austria.
Hermansky, S.J. & Hurley, J.M. (1993a) Peroral (gavage) neurotoxicity
probe study with pyrethrins in rats (Sprague Dawley). Unpublished
report, laboratory project ID: 91N0122, MRID #42930401 from Bushy
Run Research Center. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Hermansky, S.J. & Hurley, J.M. (1993b) Acute oral neurotoxicity study
with pyrethrins in rats (Sprague Dawley). Unpublished report,
laboratory project ID: 92N1036, MRID #42925801 from Bushy Run
Research Center. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Hoffmann, G.M. (1991) Acute inhalation toxicity in rats (Sprague
Dawley). Unpublished report, project No. 91-8331, MRID #42008001
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Mitchell, J.C., Dupuis, G. & Towers, G.H.N. (1972) Allergic contact
dermatitis from pyrethrum (Chrysanthemum sp.). Br. J.
Dermatol., 86, 568-573.
Myer, J.R. (1991) Evaluation of pyrethrum extract in a 5-day toxicity
study in New Zealand white rabbits. Unpublished report,
laboratory project ID: 556-017 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Newton, P.E. (1992) 90-day inhalation toxicity study of pyrethrum
extract in the rat via whole body exposure. Unpublished report,
project No. 91-8335, MRID #42478201 from Bio/dynamics, Inc.
Submitted to WHO by Kenya Pyrethrum Information Centre, Oberalm,
Austria.
Putman, D.L. & Morris, M.J. (1989) Chromosome aberrations in Chinese
hamster ovary (CHO) cells. Unpublished report, laboratory study
No. T8729.337, MRID #41344601 from Microbiological Associates
Inc. Submitted to WHO by Kenya Pyrethrum Information Centre,
Oberalm, Austria.
Ramirez, M.A. (1930) Pyrethrum: An etiologic factor in vasomotor
rhinitis and asthma. J. Allergy, 1, 149-155.
Rickett, F.E., Tyszkiewicz, K. & Brown, N.C. (1972) Pyrethrum
dermatitis. Part I: The allergenic properties of various extracts
of pyrethrum flowers. Pyrethrum Post, 11, 85.
Romanelli, P. (1991a) Primary skin irritation in rabbits (New Zealand
white). Unpublished report, project No. 91-7316A, MRID #41964803
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Romanelli, P. (1991b) Sensitization study in guinea pig (Hartley
strain). Unpublished report, project No. 91-7316A, MRID #
41964804 from Biosearch Inc., Philadelphia, Pennsylvania, USA.
Submitted to WHO by Kenya Pyrethrum Information Centre, Oberalm,
Austria.
San, R.H.C. & Springfield, K.A. (1989) Salmonella/
mammalian-microsome plate incorporation mutagenicity assay (Ames
test) with a confirmatory assay. Unpublished report, laboratory
study No. T8729.501014, MRID #41344701 from Microbiological
Associates, Inc. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Schardein, J.L. (1987a) Evaluation of pyrethrum extract in a dose
range-finding teratology study in rats. Unpublished report,
laboratory project ID: 556-001, MRID #40603701 form International
Research & Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Schardein, J.L. (1987b) Evaluation of pyrethrum extract in definitive
rat teratology study. Unpublished report, laboratory project ID:
IRDC 556-002, MRID #40288202 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Schardein, J.L. (1987c) Evaluation of pyrethrum extract in a dose
range-finding teratology study in rabbits. Unpublished report.
laboratory project ID: 556-003, MRID #40603702 from International
Research & Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Schardein, J.L. (1987d) Evaluation of pyrethrum extract in a
definitive rabbit teratology study. Unpublished report,
laboratory project ID: IRDC 556-004, MRID #40288203 from
International Research & Development Corp. Submitted to WHO by
Kenya Pyrethrum Information Centre, Oberalm, Austria.
Schardein, J.L. (1989) Two generation reproduction study in rats with
pyrethrum extract. Unpublished report, laboratory project ID:
IRDC 556-005, MRID #41327501 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Selim, S. (1995) Pharmacokinetics and metabolism of 14C pyrethrin I
in the rat. Unpublished report, study No. P1092006, MRID
#43554304 from Biological Test Center. Submitted to WHO by Kenya
Pyrethrum Information Centre, Oberalm, Austria.
Springfield, A.C., Carlson, G.P. & DeFeo, J.J. (1973) Liver
enlargement and modification of hepatic microsomal drug
metabolism in rats by pyrethrum. Toxicol. Appl. Pharmacol., 24,
298-308.
Zucker, A. (1965) Investigation of purified pyrethrum extracts.
Ann. Allergy, 23, 335-339.
Appendix 1: Application of the Conceptual Framework for Cancer Risk
Assessment
(Revised on the basis of discussions at the IPCS Workshop on
Developing a Conceptual Framework for Cancer Risk Assessment, 16-18
February 1999, Lyon, France)
This framework, developed by an IPCS working group, provides a
generic approach to the principles commonly used in evaluating a
postulated mode of action for tumour induction by a chemical. Thus,
the framework was used by the 1999 JMPR to provide a structured
approach to the assessment of the overall weight-of-evidence for the
postulated mechanism of the increased incidences of benign tumours of
the liver, thyroid, and skin in rats and a treatment-related effect on
the incidence of lung tumours in mice observed after long-term
administration of pyrethrins.
The framework guidelines suggested 10 section headings. The
introduction (see monograph section 2(c), 'Long-term studies of
toxicity and carcinogenicity') describes the cancer end-points that
have been observed. Three of these (rat liver and thyroid neoplasms,
rat keratoacanthomas, and mouse lung tumours) are addressed separately
in the analysis. An approriate mode of action is postulated and the
key events identified; the observed dose-response relationships and
temporal relationships are discussed. The strength, consistency, and
specificity of the association of tumour response with key events and
the biological plausibility are analysed. Alternative modes of action
are identified and found not to be supported. The three postulated
modes of action are discussed below, with some uncertainties about
both the biology of tumour development and the database on the
compound and any inconsistencies in the method that were identified.
* The increased incidences of hepatocellular adenomas were associated
with persistent induction of cytochrome P450 enzymes and
hepatocellular hypertrophy, suggesting that pyrethrins are
rodent-specific hepatoproliferative carcinogens. Enzyme induction
leading to increased clearance of thyroid hormones would also be
consistent with the increased incidence of follicular hyperplasia
and follicular adenomas seen. The level of confidence in this
postulated mode of action is moderate, because some uncertainties
and inconsistencies were introduced by the method. Therefore,
additional studies to identify more key events in the liver and
thyroid (e.g. measurements of enzyme induction with purified
pyrethrins, estimation of thyroid hormone changes, and estimation
of thyroid and pituitary weights) are required.
* The higher incidence of keratoacanthomas in male rats at the high
dose, which exceeds the upper limit of the range in historical
controls, was considered to be related to treatment. The slight
increase in the incidence of cystic lesions in the skin and
subcutis in males observed macroscopically provided some support
that the increased incidence of neoplastic lesions is a result of
irritating chronic injury of the skin. There were some
uncertainties introduced by the method, and differing opinions on
the biology of these tumours were found in the literature. The
level of confidence in this postulated mode of action is very low,
and no further studies were identified to support it.
* The increased incidence of lung tumours in mice might be a result
of proliferative processes following chronic injury of the
respiratory epithelium or activation of microsomal mixed-function
enzymes, especially in Clara cells, which contain high
concentrations of P450 enzymes. Such chronic injury might be
followed by cell proliferation with a dose-response relationship.
The level of confidence in this postulated mode of action is,
however, very low.
There was also little confidence in other possible modes of action.
Uncertainties in the database on pyrethrins were identified.
Improvement of the method used in the study of carcinogenicity (e.g.
detailed histological sectioning of each lung lobe cut at the level of
bronchi and additional microscopic examination of step-sections of the
remaining lung tissue beginning at the level of the bronchi in all
animals of each sex, followed by a re-evaluation of all histological
slides in the absence of knowledge of their origin; reporting of the
number and size of neoplastic and preneoplastic lesions in each
animal) could serve to increase confidence that these tumours have no
relevance at the low concentrations to which humans and animals are
exposed.
The Meeting concluded that the increased tumour incidences
associated with exposure to pyrethrins are threshold phenomena of
negligible relevance to the low concentrations to which humans are
exposed and that pyrethrins have no genotoxic or mutagenic potential.
Therefore, no classification of cancer risk is necessary. However,
additional studies are required.
The discussion of the postulated mode of action of tumour induction
by pyrethrins was helpful in the overall process of hazard
characterization and risk assessment and contributed to consideration
of the relevance of the findings in animals to the human situation.
Application of the framework also promoted confidence in the
conclusions reached, as it represents use of a defined procedure which
mandates consistent documentation of the facts and reasoning that
includes consideration of inconsistencies and uncertainties. The
Meeting concluded that the framework could be developed for use both
by regulators and by researchers in identifying research needs on the
basis of clear delineation of data gaps and inconsistencies.
2. Toxicological studies
(a) Acute toxicity
The results of studies on the acute toxicity of pyrethrum
extracts are summarized in Table 1. The methods used in these studies
complied with the corresponding FIFRA guidelines and with GLP.
Pyrethrins have little acute toxicity in rats treated orally, with
LD50 values of 2400 mg/kg bw for males and 1000 mg/kg bw for
females. The ratio of pyrethrins I and II (1.44 or 2.50) has no effect
on the toxicity of pyrethrins in male or female rats. The signs of
toxicity included ruffled appearance and tremors within 4-24 h after
dosing. Post-mortem examination of animals that died showed
haemorrhagic lungs, tan-to-yellow fluid in the lower gastrointestinal
tract and muzzle, and genital staining. The no-effect level for
clinical signs was 710 mg/kg bw in males and 320 mg/kg bw in females.
Dermal exposure to 2000 mg/kg bw was tolerated by rabbits, which
showed very slight to well-defined erythema, very slight oedema, and
stained test sites at 24 h. All animals appeared normal through the
14-day observation period.
Very slight acute toxicitywas found in rats exposed to aerosols
by inhalation. On the basis of the mean analytical concentration of
active ingredient and the resultant mortality, the LC50 was
calculated to be 3.4 mg/L for the two sexes. Tremors were seen during
exposure to the two higher concentrations. The significant findings
post mortem included discoloured and oedematous respiratory tissues.
Application of pyrethrins to the skin of albino rabbits produced
a minimally irritating skin irritation score of 0.42 (Romanelli,
1991a).
Instillations of the extracts into the conjunctival sac produced
irritation in the eyes of albino rabbits, but no irritation was
observed by 72 h. No corneal opacity or iritis was seen during the
observation period (Bielucke, 1991).
In a study of dermal sensitization in guinea-pigs with a modified
Buehler protocol, pyrethrins were not sensitizing (Romanelli, 1991b).
(b) Short-term studies of toxicity
Mice
In order to define a suitable maximum tolerated dose for a longer
study, doses of 5000 and 7000 ppm were evaluated in a 2-week study.
The method used complied to a certain extent with OECD guideline 407,
and the study was conducted in compliance with GLP. One mouse at
7000 ppm died. There was no effect on body weight, but food
consumption was slightly decreased at 7000 ppm. Statistically
significant increases in the absolute and relative weights of the
liver were seen at both doses. On the basis of the results of this
Table 1. Acute toxicity of pyrethrins
Species Strain Sex Route LD50 or LC50 Purity (%) or Reference
(mg/kg bw or mg/L air) ratio of pyrethrin I:II
Rat Sprague-Dawley M Oral 2400 58% Gabriel (1992)
Rat Sprague-Dawley F Oral 1000 58% Gabriel (1992)
Rat Sprague-Dawley M Oral 3900 1.4 Gabriel (1992)
Rat Sprague-Dawley F Oral 1300 1.4 Gabriel (1992)
Rat Sprague-Dawley M Oral 3900 2.5 Gabriel (1992)
Rat Sprague-Dawley F Oral 1200 2.5 Gabriel (1992)
Rabbit New Zealand M&F Dermal > 2000 58% Gabriel (1991)
Rat Sprague-Dawley M&F Inhalation 3.4 58% Hoffmann (1991)
M, male; F, female
study, 7000 ppm was selected as the high dose for the long-term study
in mice (Goldenthal, 1987).
Pyrethrins were offered to groups of 15 Charles River mice of
each sex in the diet at concentrations of 300, 1000, 3000, or 10 000
ppm for 13 weeks, equal to 47, 160, 460, and 1600 mg/kg bw per day for
males and 56, 200, 580, and 1800 mg/kg bw per day for females. The
method used complied to a certain extent with OECD guideline 408, and
the study was conducted in compliance with GLP. Four males and 10
females at the original high dose of 30 000 ppm died on day 2, and all
animals at this dose had died or were killed in extremis by day 10.
Four males and two females at 10 000 ppm also died on day 2, with
clinical signs that included tremors, pale exposed skin, dilated
pupils, altered activity, laboured breathing, cold to touch,
moribundity, and hunched posture. Tremors and increased activity were
seen in several animals at 10 000 ppm during the first 2 weeks of
study. No treatment-related clinical signs were observed in the
animals at 300, 1000, or 3000 ppm. The group mean body weights and
food consumption were similar for all groups with surviving animals.
The absolute weight of the liver and the liver:body weight and the
liver:brain weight ratios were all statistically significantly
increased in males and females at 3000 and 10 000 ppm, whereas the
absolute weights of the liver in females at 300 and 1000 ppm were
comparable to those of controls. A treatment-related increase in the
incidence and/or severity of congestion in the liver was observed in
surviving male and female mice at 10 000 ppm, and an increased
incidence but only mild severity was found in < 15% of investigated
animals at 3000 ppm. An increased incidence of hepatocellular
hypertrophy was present in surviving male and female mice at 3000 and
10 000 ppm; at 1000 ppm, only 2 of 15 mice showed mild congestion of
the liver on macroscopic observation. The NOAEL was 1000 ppm, equal to
160 mg/kg bw per day (Goldenthal, 1988a).
Rats
Pyrethrins were offered to groups of 15 Charles River rats of
each sex for 13 weeks in the diet at concentrations of 300, 1000,
3000, 10 000, or 20 000 ppm, equal to 17, 57, 170, 590, and 1200 mg/kg
bw per day for males and 22, 74, 220, 710, and 1400 mg/kg bw per day
for females. The method used in this study complied to a certain
extent with OECD guideline 408, and the study was conducted in
compliance with GLP. During the first week of the study (days 3-7),
one female at 10 000 ppm, one male at 20 000 ppm, and 12 females at
20 000 ppm died; all other animals survived to scheduled sacrifice.
The signs seen before death in all animals that died included
decreased defaecation, increased respiration rate, tremors, and
decreased or increased activity. Convulsions were seen in some
animals. The signs in most animals at 10 000 and 20 000 ppm that
survived until the end of the study were decreased defaecation,
tremors, increased respiration rate, and increased activity.
Convulsions occurred mainly in males at 20 000 ppm. Most of the signs
were seen only during the first two weeks of the study. Statistically
significant decreases in mean body weights were observed during most
or all of the study in males and females at 10 000 and 20 000 ppm.,
and statistically significant decreases in mean food consumption were
seen during most or all of the study in females at 10 000 ppm and
males and females at 20 000 ppm when compared with the respective
control groups. Statistically significantly decreased mean values for
haematocrit and haemoglobin were found in males at 20 000 ppm and for
erythrocytes, haematocrit, and haemoglobin in females at 10 000 and
20 000 ppm. Females at 3000 ppm also showed a decreased mean
haemoglobin value. The treatment-related macroscopic findings
consisted of enlargement and congestion of the liver in both males and
females, but primarily in males, at 10 000 and 20 000 ppm; however,
the macroscopic observation could not be confirmed microscopically.
The liver weight was statistically significantly increased in males at
10 000 and 20 000 ppm and in females at 3000, 10 000, and 20 000 ppm
The weight of the kidney was increased in males and females at 3000,
10 000, and 20 000 ppm The only microscopic finding that was possibly
realted to treatment was small focal or multifocal areas of tubular
degeneration and regeneration in the renal cortex in animals at 3000
and 10 000 ppm, but primarily in males. The NOAEL was 1000 ppm, equal
to 57 mg/kg bw per day (Goldenthal, 1988b).
In a study designed to assess the toxic effects of pyrethrins
administered by whole-body inhalation as a liquid aerosol, groups of
15 Charles River rats of each sex were exposed for 6 h/day, generally
5 days per week, a minimum of 65 times to target concentrations of the
active ingredient corresponding to 0.010, 0.030, 0.10, and 0.35 mg/L.
The mean analytical concentrations to which the groups were exposed
were 0 (control), 0.038, 0.068, 0.23, and 0.83 mg/L, respectively.
Determinations of particle size distribution showed an overall mass
median aerodynamic diameter of 2.7 µm.The controls were exposed only
to conditioned room air on the same schedule. The study was conducted
in compliance with FIFRA Guideline 824 and with GLP.
Two animals at the highest dose died, one animal on day 3.
Because this death was accidental and occurred very early in the
study, this animal was replaced with another animal from the same
shipment. A second animal died on day 15, having shown laboured
breathing similar to that of other animals in this group, and its
death was considered to be potentially related to exposure.
Dose-related increases in the frequency of clinical signs were
observed in animals at the three higher doses during observations in
the chamber and at detailed weekly examinations. These signs included
secretory signs such as nasal discharge and dried material in the
facial area in both males and females. Animals at the highest dose
showed laboured breathing, excess lachrymation, tremors, increased
activity, and matted coats. There were no ocular effects. The absolute
body weights and body-weight gains of both males and females at the
two higher doses were decreased, and after 13 weeks of exposure the
body-weight gains of males at these doses were about 9% lower than
those of controls. In females, the body-weight gain after 13 weeks of
exposure was lower than that of controls, by 13% at 0.10 mg/L and 17%
at 0.35 mg/L. Food consumption was also slightly decreased during the
first 1 or 2 weeks in males and females in these groups.
Nonregenerative anaemia was observed in males at the three higher
doses and in females at the highest dose, with significant decreases
in haemoglobin, haematocrit, and erythrocyte values. An increased
leukocyte count was also seen in the females at the highest dose.
Significant differences in clinical chemical parameters were seen
primarily at the highest dose, including decreased total protein and
globulin and an increased albumin:globulin ratio in males and
decreased glucose in females. Other changes occurred sporadically and
were considered to be unrelated to exposure. Several significant
increases in organ weights or ratios were observed. The increases in
liver weight were clearly related to treatment. Increases in the
organ:body weight ratios of the kidneys and lung might be a reflection
of a marginal increase in absolute organ weights and the decreased
body weights. Morphological abnormalities in the larynx,
nasoturbinates, nasopharynx, and lungs observed by light microscopy
were considered to be localized responses indicative of a
treatment-related effect. The NOAEL for systemic effects was 0.01 mg/L
(Newton, 1992).
Rabbits
Pyrethins were administered dermally to five male and five female
New Zealand white rabbits in the form of a 25% (w/v) mixture in
vegetable oil at doses of 0, 100, 300, or 1000 mg/kg bw once daily, 5
days per week for 3 weeks. Animals in the vehicle control group were
given vegetable oil on the same regimen and at the same volume as the
group receiving the high dose . This study was conducted in compliance
with OECD Guideline 410 and GLP. One rabbit at 1000 mg/kg bw was
sacrificed in extremis on day 10 after showing emaciation, decreased
activity, and decreased defaecation. Macroscopic examination of this
animal did not reveal the cause of death. A low incidence of
desquamation and/or red raised areas on the skin at the application
site was observed in all groups, including the vehicle controls.
Several animals in the treated groups showed very slight to
well-defined erythema of the skin at the application site, but no
clear pattern with regard to treatment was seen for any of these
findings. Microscopic evaluation revealed no evidence of systemic
toxicity. The microscopic lesions at the application site included
acanthosis, haemorrhage, hyperkeratosis, and chronic inflammation,
although haemorrhage was observed only in the group given the vehicle
alone. Thus, all of the dermal reactions appeared to be due to the
vegetable oil. The NOAEL for systemic effects was 1000 mg/kg bw, the
highest dose tested (Goldenthal, 1992).
This result is supported by that of a previous study performed to
assess dermal irritation with pyrethrins at concentrations of 25%,
50%, and 75% w/v in corn oil on rabbits (Myer, 1991).
Dogs
Pyrethins were incorporated into the basal diet of groups of two
pure-bred beagle dogs of each sex at concentrations of 600, 1000,
3000, or 6000 ppm for 8 weeks, equal to 18, 30, 86, and 170 mg/kg bw
per day for males and 19, 29, 94, and 200 mg/kg bw per day for
females. The method used complied to a certain extent with OECD
guideline 409, and the study was conducted in compliance with GLP. One
male and both females at 6000 ppm died or were killed in extremis
during the study. The treatment-related clinical signs observed in
animals at this dose included inappetence, thin appearance, ataxia,
trembling, oily coat, impaired limb function, shallow breathing,
moribundity, and death. With the exception of moribundity and death,
similar signs were observed at 3000 ppm. Males and females at 6000 ppm
lost weight during the study, but the body-weight gains of animals in
all other treated groups were comparable to those of controls. The
average food consumption was decreased for males at 3000 ppm and for
males and females at 6000 ppm when compared with controls. Decreased
haematocrit, haemoglobin, and erythrocyte values were seen at the end
of the study in males at 3000 and 6000 ppm, but there were no other
treatment-related haematological finding. One male and one female at
6000 ppm killed in extremis had increased leukocyte counts and
decreased haematocrit, haemoglobin, and erythrocyte values. Slightly
decreased glucose, calcium, phosphorus, and cholesterol values were
found at the end of the study in males at 6000 ppm, and males and
females at 3000 ppm had slightly decreased cholesterol concentrations.
The aspartate and alanine aminotransferase activities of males at 6000
ppm were slightly increased at the end of dosing, and the surviving
male at this dose had a very high creatinine phosphokinase value.
There were no other treatment-related biochemical findings at the end
of the study. Males and females at 6000 ppm killed in extremis
showed some variation in electrolyte levels, and the urea nitrogen
concentration was increased in both dogs. The female showed large
increases in aspartate and alanine aminotransferase and creatine
phosphokinase activities. The absolute weights of the liver in both
males and females at 1000 and 3000 ppm were increased in a
treatment-related fashion, and the absolute weight of the testis at
these doses appeared to decrease in a similar manner. There were no
macroscopic or microscopic lesions attributable to the administration
of pyrethrins. The NOAEL was 600 ppm, equal to 18 mg/kg bw per day
(Goldenthal, 1988c).
Pyrethrins were administered to groups of four beagle dogs of
each sex for 52 weeks in the diet at concentrations of 0, 100, 500, or
2500 ppm, equal to 2.6, 14, and 66 mg/kg bw per day for males and 2.8,
14, and 75 mg/kg bw per day for females. Four dogs of each sex were
evaluated at each dietary concentration. This study was conducted in
compliance with FIFRA Guideline 83-1 and with GLP. All animals
survived to the end of the study, and no remarkable clinical signs of
toxicity were found at any dose. Overall, the mean body weights of the
treated animals were similar to those of controls. The mean food
consumption of males at 2500 ppm and of females at 500 and 2500(c)
ppm was lower than that of controls during the first week of the
study, but consumption was similar for the remainder of the study,
except that males at 500 ppm consumed greater amounts of food than the
controls. Increased total leukocyte and segmented neutrophil counts
were found in females and decreased erythrocyte, haemoglobin, and
haematocrit values in males at 2500 ppm. Alanine aminotransferase
activity was statistically significantly increased in females at 2500
ppm at both the 6- and 12-month evaluations. The slight increase in
the activity of this enzyme in males was not statistically
significant. No treatment-related changes in urinary parameters were
seen at either interval. The relative and absolute weights of the
liver were significantly increased in males at 2500 ppm when compared
with controls, but no statistically significant differences in organ
weights were seen in females at this dose. No macroscopic or
microscopic treatment-related changes were observed in tissues. The
NOAEL was 500 ppm, equal to 14 mg/kg bw per day (Goldenthal, 1990a).
(c) Long-term tudies of toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female Charles River CD-1 mice received
diets containing pyrethrins at concentrations of 100, 2500, or 5000
ppm for 18 months, equal to doses of 14, 350, and 690 mg/kg bw per day
for males and 17, 410, and 830 mg/kg bw per day for females. This
study was conducted in compliance with FIFRA Guideline 83-2(b) and
with GLP. One male and one female at 5000 ppm were found dead during
the first week of the study, but no other treatment-related deaths
occurred, and survival was similar in the control and treated groups
throughout the study. All animals at 5000 ppm exhibited increased
activity when stimulated during the first week of the study but not
later. There were no other clinical signs seen that differentiated the
treated from the control groups. Statistically significant differences
in mean body weight and food consumption were seen between control and
treated groups sporadically throughout the study, but none of the
differences was considered to be related to treatment. No
treatment-related effect was observed in other parameters examined at
12 and 18 months of study. At necropsy, discoloured, dark livers were
more common in males at 5000 ppm and in females at 2500 and 5000 ppm,
and treatment-related increases in the absolute and relative weights
of the liver were seen in males and females at 2500 and 5000 ppm.
Microscopically, vacuolar fatty change was found in the livers of
males at these doses, and this change was considered to be related to
treatment. The dark discolouration seen macroscopically could not be
explained by the microscopic findings. The incidence of nodules and
masses in the lungs appeared to be slightly increased in animals at
5000 ppm. When the lungs were examined microscopically according the
original test protocol, the incidences of alveolar bronchiolar
adenomas were increased in females at 5000 ppm, and the increase was
statistically significant, exceeding the range in historical controls.
Treated males showed an apparent, not clearly dose-related increase in
the incidence of alveolar bronchiolar carcinomas which exceeded the
upper limit of 95% of the historical controls (Table 2). The sponsor
asked the testing laboratory to conduct serial sectioning of the
remaining lung tissue of female mice in the control and highest-dose
groups, which did not have diagnoses of lung tumours in the original
examination. As this was considered not to be an acceptable
toxicopathological practice, the results of the first evaluation were
taken into consideration for the risk assessment, and the increased
incidence of lung tumours was considered to be a treatment-related
effect. The NOAEL was 100 ppm, equal to 14 mg/kg bw per day
(Goldenthal, 1990b).
Table 2. Comparison of first and second evaluations of microscopic
neoplastic lesions in the lung of mice treated with
pyrethrins for 18 months
Sex Dose No. of lungs Alveolar bronchiolar neoplasm
(ppm) examined
Adenoma Carcinoma
Male 0 60 14 0a
0 60 16 0
100 60 15 1
2500 60 13 3b,c
5000 60 17 3b,c
Female 0 60 8 1
0 60 4 3
100 60 11 0
2500 60 5 2
5000 60 19c,d 2
a p < 0.05; Cochran trend test
b Significant differences in pair-wise comparison of the
high-dose group with controls at p < 0.05
c > 95% of of the upper range of historical controls
d p < 0.05; Fisher exact test
Rats
Groups of 60 Charles River CD rats of each sex received diets
containing pyrethrins at concentrations of 100, 1000, or 3000 ppm for
104 weeks, equal to 4, 43, and 130 mg/kg bw per day for males and 5,
56, and 170 mg/kg bw per day for females. This study was conducted in
compliance with FIFRA Guideline 83-5 and with GLP. Survival was
similar in the treated and control groups, and there were no clinical
findings attributable to treatment. Statistically significant
decreases in body weight which were considered to be related to
treatment were observed during the first 78 weeks of the study in both
male and female rats at 3000 ppm, with a difference from controls of
7% in males and 10% in females. A slight, treatment-related decrease
in food consumption was seen at the same time. No treatment-related
ophthalmological findings or organ weight changes were detected during
the study, and no haematological or urological changes were found. The
activities of serum transaminases were substantially increased at most
intervals of analysis in males at 3000 ppm, most of the values
reaching statistical significance. Increased incidences of benign
tumours of the liver, thyroid, and skin were also observed (Table 3),
and a statistically significantly higher incidence of hepatocellular
adenomas was described in females at the high dose. Follicular
adenomas and carcinomas were initially seen in the thyroid glands of
rats at the high dose, which appeared to be related to treatment, but
during a re-evaluation some of the carcinomas were reclassified as
adenomas and some adenomas were reclassified as hyperplasia. After the
re-evaluation, the incidence of hyperplasia was found to be enhanced
in males and females, and the incidence of follicular adenomas was
statistically significantly increased only in females at 3000 ppm.
Nevertheless, the tumour incidences in animals of each sex were higher
than the upper range seen in historical controls. The results of a
further, full histopathological peer review confirmed these increased
tumour incidences. Macroscopic examination of the skin showed a slight
increase in the incidence of cystic lesions in the skin and subcutis,
and the microscopic assessment showed an apparently higher incidence
of keratoacanthomas in males at the high dose, which was statistically
significant in comparison with both control groups. A peer review of
pathological lesions in all male rats resulted in removal of several
keratoacanthomas from the table of incidence in treated groups but
confirmed that the incidence of this lesion clearly exceeded the upper
limit of the range in historical controls (1.4-10%). The increased
incidences of liver and thyroid tumours and of keratoacanthomas of the
skin were considered to be treatment-related effects but to be
threshold phenomena of negligible relevance to the low doses to which
humans are exposed. The NOAEL was 100 ppm, equal to 4 mg/kg bw per day
(Goldenthal, 1990c).
(d) Genotoxicity
The results of tests for the genotoxicity of pyrethrins in
vitro are summarized in Table 4.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a two-generation study, groups of 28 male and 28 female
Charles River rats received diets containing pyrethrins at
concentrations of 100, 1000, or 3000 ppm, equivalent to 10, 100, and
300 mg/kg bw per day. A control group received the basal laboratory
diet on an identical regimen. The F0 parental generation were
treated for a minimum of 77 days before the first of two matings. The
same numbers of weanlings from the F1b litters were selected
randomly to become parents of the F1 generation and were treated for
a minimum of 95 days before being mated twice to produce the F2a and
F2b litters. This study was conducted in compliance with FIFRA
Guideline 83-4 and with GLP. No treatment-related effects were noted
with respect to clinical signs, body weights, or food consumption in
Table 3. Incidences of microscopic neoplastic lesions in rats fed diets containing pyrethrins for 104 weeks
Sex Dose Liver Thyroid Skin
(ppm)
Total Hepatocellular tumours Total Hyperplasia Follicular tumours Total Cystic Keratoacanthomasb
examined examined examined lesions
Adenoma Carcinoma Adenomaa Carcinomaa
Male 0 60 6 1 60 2 2c 0 60 5 4
0 60 1 0 60 0 1 1 60 2 5
100 60 0 0 60 2 4 1 24 1 4
1000 60 3 0 59 5 5d 2 14 5 4
3000 60 3 1 60 7 5d 2 60 9 11d,e
Female 0 60 0 1 60 0 0 1 60 0 0
0 60 1 0 60 2 1 1 59 3 0
100 60 0 0 60 1 2 0 8 2 1
1000 60 1 0 60 1 3d 0 8 2 1
3000 60 5d.f 0 60 5 5d 1 60 0 0
a Results of the histopathological peer review
b Results of the re-analysis only in males
c p < 0.05; Cochran trend test
d Greater that the upper limit of historical control data
e Significant difference in the pair-wise comparison of the high-dose group with the controls at p < 0.05
f p < 0.05; Fisher exact test
Table 4. Results of assays for the genotoxicity of pyrethrins in vitro
Test system Test object Concentration Purity Results Reference
(%)
Reverse mutationa S. typhimurium TA98, 8.8-8772 µg/plate in 58 Negative ± S9 San & Springfield
TA100, TA1535, TA1537, acetone (1989)
TA1538
Chromosomal Chinese hamster ovary 0.02-0.32 Fl/ml + S9 58 Negative ± S9 Putman & Morris
aberrationa cells 0.005-0.08 Fl/ml - S9 (1989)
in DMSO
Unscheduled DNA Rat primary hepatocytes 0.0-1.0 Fl/ml in 58 Negative Curren (1989)
synthesis acetone
a Study conducted in compliance with GLP and to a certain extent with OECD guidelines
the parental rats of the F0 generation, but the body weights and
food consumption of the parental rats of the F1 generation were
significantly reduced at 3000 ppm and sporadically reduced at 1000
ppm. These reductions were considered to be treatment-related.
Treatment at 3000 ppm resulted in significantly reduced body weights
at birth for F1a males and F2a pups of each sex and during
lactation for the male and female offspring of both matings of both
generations. The mean body weights of pups at 1000 ppm were also lower
than those of controls for male F2a pups at birth and for F1b and
F2a pups during lactation. Reproductive performance and other litter
parameters were not affected by the treated diet at any dose. The
NOAEL for parental and reproductive toxicity was 100 ppm, equivalent
to 10 mg/kg bw per day (Schardein, 1989).
(ii) Developmental toxicity
Rats
Groups of five mated female Charles River rats were used in a
range-finding study to determine the doses of pyrethrin to be used in
a later study. Doses of 37.5, 75, 150, 300, or 600 mg/kg bw per day
were administered orally by gavage on days 6-15 of gestation at a
volume of 3 ml/kg. The control group received only the vehicle, 0.5%
methylcellulose, on a comparable regimen. Uterine examinations were
performed on all surviving females on day 20 of gestation. The method
used in this study complied to a certain extent with OECD Guideline
414, and the study was conducted in compliance with GLP.
Treatment-related maternal toxicity, observed as deaths, convulsions,
and/or tremors, occurred at 150, 300, and 600 mg/kg bw per day. No
treatment-related maternal deaths occurred at 75 mg/kg bw per day,
although tremors were observed in this group. No treatment-related
clinical signs were observed at 37.5 mg/kg bw per day. On the basis of
these results, doses of 5, 25, and 75 mg/kg bw per day were selected
for use in the following study (Schardein, 1987a).
Groups of 25 mated female Charles River rats were given
pyrethrins suspended in 0.5% methylcellulose orally by gavage at doses
of 5, 25, or 75 mg/kg bw per day on days 6-15 of gestation at a volume
of 3 ml/kg. The control group received the vehicle only on a
comparable regimen. On day 20 of gestation, the fetuses were removed
surgically for evaluation. The method used in this study complied to a
certain extent with OECD Guideline 414, and the study was conducted in
compliance with GLP. No animals died or were killed in extremis
during the study, and no treatment-related clinical signs were
observed. The body-weight gains of the treated groups were comparable
to those of the controls during treatment. No evidence of fetotoxicity
was found, and morphological examination revealed no teratogenic
effects at any dose tested. The NOAEL for maternal toxicity was 75
mg/kg bw per day, and that for developmental toxicity was 75 mg/kg bw
per day, the highest dose tested (Schardein, 1987b).
Rabbits
Groups of five inseminated female New Zealand white SPF rabbits
were used in a range-finding study to determine the doses of
pyrethrins for use in a later study. Doses of 37.5, 75, 150, 300, or
600 mg/kg bw per day were administered orally by gavage on days 7-19
of gestation at a volume of 3 ml/kg. The control group received only
the vehicle, 0.5% methylcellulose, on a comparable regimen. Uterine
examinations were performed on all surviving females on day 29 of
gestation. The method used complied to a certain extent with OECD
Guideline 414, and the study was conducted in compliance with GLP.
Treatment-related maternal toxicity, seen as deaths, tremors,
convulsions, and weight loss, and fetal toxicity, seen as high
postimplantation loss, were found at 600 mg/kg bw per day. Maternal
toxicity, in terms of weight loss during treatment and tremors was
seen at 300 mg/kg bw per day. No clear treatment-related effects were
observed at 37.5, 75, or 150 mg/kg bw per day. On the basis of these
results, doses of 25, 100, and 250 mg/kg bw per day were selected for
use in the following study (Schardein, 1987c).
Groups of 16 inseminated female New Zealand white SPF rabbits
were randomly assigned to receive pyrethrins at doses of 25, 100, or
250 mg/kg bw per day orally by gavage on days 7-19 of gestation at a
volume of 3 ml/kg. The control group received only the vehicle, 0.5%
methylcellulose, on a comparable regimen. On day 29 of gestation, the
fetuses were removed surgically for evaluation. The study was
conducted in compliance with OECD Guideline 414 and with GLP. All
animals survived to the end of treatment. One doe at the high dose
aborted near term, on day 28 of gestation, after showing decreased or
absent defaecation on several days previously. No gross lesions were
present at necropsy. Excessive salivation, arched head, and/or
laboured breathing were observed in a few females at the high dose on
days 18-19 of gestation. One female at the intermediate dose had
excessive salivation and arched head on gestation day 19. No apparent
treatment-related clinical signs were seen at the low dose. There were
no treatment-related gross pathological changes in any of the animals
at the end of the study. Body-weight loss was seen in does at the high
dose throughout treatment, and slightly reduced body-weight gain
relative to the control value was observed in animals at the
intermediate dose. The body-weight gains of treated groups were
comparable to that of the control group throughout gestation (days
0-29). When the body weights on day 29 were adjusted by subtracting
the uterine weights to reflect only maternal weight change, mean
weight losses were evident in all groups, including the control, over
the entire gestation period. There were no biologically meaningful or
statistically significant differences in the mean numbers of viable
fetuses, postimplantation losses, total implantations, or corpora
lutea and in fetal body weight or fetal sex distribution in the
treated groups in comparison with the control values. One doe at the
high dose resorbed its litter, but it is not clear if this finding was
related to treatment. There was no treatment-related or statistically
significant difference in the incidence of fetal malformations or
variations. The NOAEL for maternal toxicity was 25 mg/kg bw per day
and that for developmental toxicity was 250 mg/kg bw per day, the
highest dose tested (Schardein, 1987d).
(f) Special studies
(i) Effects on the central nervous system
Rats
Male Sprague-Dawley rats were treated once by oral gavage with a
10% or 25% solution of pyrethrins in corn oil at doses of 0, 40, 100,
200, 400, 800, 1400, or 2000 mg/kg bw, and females received a 2.5, 5,
or 10% solution of pyrethrins in corn oil at doses of 0, 25, 50, 100,
150, 200, 400, or 800 mg/kg bw. This study was conducted in compliance
with FIFRA Guideline 81-8 and with GLP. The clinical signs were
characterized by mild-to-severe tremors. On the basis of the
occurrence, severity, and onset of these reactions, a solution of 10%
pyrethrins in corn oil and doses of 40, 125, and 400 mg/kg bw were
selected for the study of acute neurotoxicity in males and a solution
of 5% pyrethrins in corn oil and doses of 20, 63, and 200 mg/kg bw in
females (Hermansky & Hurley, 1993a).
In the main study, groups of 15 male Sprague-Dawley rats received
by gavage a 10% w/v solution of pyrethrins in corn oil at doses of 0,
40, 125, or 400 mg/kg bw, and the same numbers of females received a
5% w/v solution of pyrethrins in corn oil at doses of 0, 20, 63, or
200 mg/kg bw. This study was conducted in compliance with FIFRA
Guideline 81-8 and with GLP. Five males and two females at the high
dose died on the day of treatment, and a variety of acute neurological
signs were observed in the other animals at this dose, including
tremors, urogenital area wetness, salivation, perinasal encrustation,
exaggerated startle response, decreased grip strength, hind leg splay,
and increased body temperature. Tremors were also observed in three
females at the intermediate dose. Measurements of motor activity on
the day of treatment indicated increased fine movement and decreased
rearing and ambulation in animals of each sex at the high dose and
decreased fine movement, rearing, and ambulation in males at the
intermediate dose. In addition, slight, statistically nonsignificant
decreases in body weight were seen in males at the high dose on days 7
and 14. There was no evidence of tany gross, treatment-related lesion.
The microscopic changes were limited mainly to sections of the sciatic
nerve and its branches. The histomorphological changes within the
peripheral nerve sections indicated the presence of scattered
degenerating nerve fibres or myelin sheaths. These changes were seen
in only a few animals, were graded as minimal, and were not
dose-related. The NOAEL was 20 mg/kg bw (Hermansky & Hurley, 1993b).
(ii) Effects on hepatic microsomal enzymes
Rats
Oral administration of pyrethrins to male rats at 85, 200, or 500
mg/kg bw per day for 3 weeks resulted in liver enlargement and
decreased hepatic DNA concentrations. Significantly decreased
hexobarbital-induced hypnosis without concomitant changes in
barbital-induced hypnosis suggested an alteration in hepatic drug
metabolism. The activities of hepatic microsomal enzymes responsible
for detoxification of
O-ethyl- O-(4-nitrophenyl)phenyl-phosphonothioate,
para-nitroanisole demethylation, and hexobarbital oxidation were
increased at 200 mg/kg bw per day to 150, 173, and 241% of the control
values, respectively. Increased liver weight, the detoxification of
pyrethrins, and demethylation of para-nitroanisole were found to be
dose-related. Small increases in enzyme activities were observed when
the lowest dose was given for 15 days. At 500 mg/kg bw per day, the
liver weight and enzyme activities were increased up to 17 days of
treatment but returned to the control level within 7 days after
cessation of treatment. NADPH cytochrome c reductase activity and the
cytochrome P450 concentration were also increased. It was suggested
that pyrethrins caused induction of microsomal enzymes. The LOAEL was
85 mg/kg bw per day (Springfield et al., 1973).
3. Observations in humans
The main adverse effects seen after exposure to pyrethrum
extracts in older studies were those manifesting as either skin or
respiratory reactions. Much of the research performed to date has
focused on the dermatological effects of pyrethrins, although mention
has been made of the respiratory reactions that have often accompanied
those of the skin. Studies by Ramirez (1930) and Feinberg (1934), in
which the association between sensitivity to ragweed and to pyrethrins
was delineated, contributed much to the understanding of allergic
responses to pyrethrins. Several investigations have since been
undertaken to isolate and characterize the allergen responsible for
the dermal reactions. Mitchell et al. (1972) isolated a sesquiterpene
lactone, pyrethrosin, from pyrethrins which induced positive dermal
responses in humans given a patch test. None of the other fractions
induced reactions, with the exception of pyrethrins II, which elicited
a weak response. Zucker (1965) demonstrated that a dermal reaction to
unrefined pyrethrum extract does not result in allergy to refined
pyrethrins. The previous evaluation by the Joint Meeting in 1970 cited
an unpublished report in which 200 people were given patch tests with
a 1% water dispersion of pyrethrins, and no evidence of primary
irritation or of sensitization was found. Rickett et al. (1972)
concluded that the refined extracts that have been marketed since 1957
do not induce skin allergies when tested on sensitive subjects and
that the dermal effects reported in the early literature are not
relevant to an assessment of refined pyrethrins.
Case reports of adverse respiratory effects, such as
anaphylactoid reactions and asthma, attributed to pyrethrins indicate
that these responses often occur in individuals with a history of
asthma. Although investigations of the dermal effects of pyrethrum
extracts suggests that pyrethrins are not the causative agent, no
thorough investigation of the agent(s) responsible for the adverse
respiratory responses has been conducted.
Comments
Absorption, distribution, and excretion in rats were investigated
only for pyrethrins I. After oral administration, more than 90% of a
low dose of pyrethrins I was absorbed, and the concentration of
radiolabel in blood peaked between 5 and 8 h. The radiolabelled
residues were widely distributed in the organs analysed, with the
highest concentrations in fat in females. The elimination half-time of
pyrethrins I in males and females was approximately 6 h. The mean
percentage of administered radiolabel found in the urine ranged from
32 to 47% in males and from 50 to 57% in females, the remainder being
excreted in faeces.
The substance is extensively metabolized, the residues of the
parent compound in faeces and urine representing only 10%. Six
metabolites were identified and two major metabolic pathways were
suggested, the first involving oxidation of the double-bond and/or the
methyl groups and the second involving hydrolysis of the ester bond.
Pyrethrins I are metabolized mainly through oxidative processes, while
pyrethrins II are metabolized through a combination of hydrolytic and
oxidative processes.
Pyrethrins show little acute toxicity, with an oral LD50 in
rats of > 1200 mg/kg bw and NOAELs for clinical signs of 710 mg/kg bw
for males and 320 mg/kg bw for females, a dermal LD50 in rabbits of
> 2000 mg/kg bw, and an inhalation LC50 in rats of 3.4 mg/L. The
compounds are minimally irritating to the skin and eye and show no
potential for skin sensitization. Pyrethrum extracts have not been
classified by WHO for acute toxicity.
In short-term tests for toxicity in mice, rats, and dogs, the
lowest relevant NOAELs after oral administration were 1000, 1000, and
600 ppm, equal to 160, 57, and 18 mg/kg bw per day, respectively, for
the three species. Statistically significant decreases in mean body
weight or body-weight gain were observed at the high doses throughout
most or all of the studies.
The liver is the main target organ in mice, rats, and dogs, and
an increased liver weight was frequently accompanied by changes in
serum transaminase activity. In mice, increased liver weights were
associated with a higher incidence of hepatocellular hypertrophy. In
the livers of rats and dogs, generally unremarkable histopathological
changes were observed. At doses of 85 mg/kg bw per day and above, a
pyrethrum extract containing 20% pyrethrins induced microsomal enzymes
in rats. Furthermore, anaemia was observed in rats and dogs at doses
of 3000 ppm and above. The kidney was another target, but only in
rats. In a 13-week study, rats at doses greater than 1000 ppm had
increased kidney weights associated with tubular degeneration and
regeneration in the renal cortex.
In a 13-week study in rats exposed by inhalation, the NOAEL for
systemic toxicity was 0.011 mg/L. The increases in liver weight were
clearly related to exposure and were accompanied by changes in serum
transaminase activity. Nonregenerative anaemia was also observed. The
weights of the kidney and lung were increased in relation to body
weight. The morphological abnormalities observed in the larynx,
nasoturbinates, nasopharynx and lungs by light microscopy were
considered to be localized responses indicative of a treatment-related
effect.
Dermal administration of pyrethrins at doses up to 1000 mg/kg bw
per day for 21 days caused no systemic toxicity in rabbits.
In a two-year study of toxicity and carcinogenicity in rats and
an 18-month study of carcinogenicity in mice, the NOAEL was 100 ppm in
both species, equal to 14 and 4 mg/kg bw per day in mice and rats,
respectively. The liver was the main target. A treatment-related
effect on the incidence of lung tumours was seen in mice and increased
incidences of benign tumours of the skin, liver, and thyroid were
observed in rats. The increased incidences of hepatocellular adenomas
were associated with persistent induction of cytochrome P450 enzymes
and hepatocellular hypertrophy, suggesting that pyrethrins are
rodent-specific hepatoproliferative carcinogens. Enzyme induction
leading to increased clearance of thyroid hormones would also be
consistent with the higher incidence of follicular hyperplasia and
follicular adenomas. However, additional studies on the mechanism of
formation of the liver and thyroid tumours are required. The Meeting
concluded that the increased tumour incidences caused by pyrethrins
are threshold phenomena of negligible relevance to the low doses to
which humans are exposed (see Appendix 1 to this monograph addendum).
Pyrethrins did not induce reverse mutagenicity in Salmonella
typhimurium with metabolic activation, did not induce chromosomal
aberration in Chinese hamster ovary cells, and did not induce
unscheduled DNA synthesis in rat primary hepatocytes. The Meeting
concluded that pyrethrins have no genotoxic or mutagenic potential,
but a test for gene mutation in mammalian cells is lacking.
Pyrethrins did not show developmental toxicity in rats or rabbits
at the highest maternally toxic doses tested, which were 75 and 250
mg/kg bw per day, respectively. The only effects on the offspring,
observed in a two-generation study of reproductive toxicity in rats,
were reduced body weights at the parentally toxic doses of 1000 and
3000 ppm, with a NOAEL of 100 ppm, equivalent to 10 mg/kg bw per day.
In a study of neurotoxicity in rats given single oral doses,
acute neurological disorders (tremors, wetness of the urogenital area,
salivation, perinasal encrustation, exaggerated startle response,
decreased grip strength, and hind-leg splay) and behavioural effects
(increased motor activity and decreased rearing and ambulation) were
noted, with a NOAEL of 20 mg/kg bw.
The available data on humans did not show a causal relationship
between exposure to modern pyrethrin-containing products and
significant adverse health effects.
An ADI of 0-0.04 mg/kg bw was established for the tested blend of
refined pyrethrum extract, which was based on the NOAEL of 100 ppm,
equal to 4 mg/kg bw per day, observed in the long-term study of
toxicity and carcinogenicity in rats and a safety factor of 100. This
figure is identical to the ADI derived by the 1972 Meeting, which was
based on a NOAEL of 200 ppm, equivalent to 10 mg/kg bw per day, in a
long-term study in rats and a safety factor of 250.
The acute and long-term toxicity of the pyrethrins differ
significantly. The acute toxicity of orally administered pyrethrins is
expressed as neurotoxic effects. The longer-term toxicity is based
principally on effects on the liver. Therefore, an acute reference
dose of 0.2 mg/kg bw was allocated for the tested blend of refined
pyrethrum, which was based on the NOAEL of 20 mg/kg bw for acute
neurotoxicity in rats and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effects
Mouse: 100 ppm, equal to 14 mg/kg bw per day (18-month study of
carcinogenicity)
Rat: 20 mg/kg (study of acute neurotoxicity)
100 ppm, equal to 4 mg/kg bw per day (2-year study of
carcinogenicity)
100 ppm, equivalent to 10 mg/kg bw per day (parental and
reproductive toxicity in a two-generation study of
reproductive toxicity)
75 mg/kg bw per day (maternal toxicity in two studies of
teratogenicity, no developmental toxicity in a study of
teratogenicity at the highest dose tested)
Rabbit: 25 mg/kg bw per day (maternal toxicity in a study of
teratogenicity, no developmental toxicity in a study of
teratogenicity at the highest dose tested)
Dog: 500 ppm, equal to 14 mg/kg bw per day (toxicity in a 1-year
study)
Estimate of acceptable daily intake for humans
0-0.04 mg/kg bw
Estimate of acute reference dose
0.2 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Gene mutation test in mammalian cells (required for submission to
WHO by 2001)
2. Mechanistic study on liver and thyroid tumorigenesis (see
Appendix 1; required for submission to WHO by 2001)
3. Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to pyrethrins
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Immediately (peak between 5 and 8 h) and nearly complete (> 90%)
in rats
Distribution Widely distributed in rats, highest concentrations in fat
Potential for accumulation None
Rate and extent of excretion Nearly complete excretion in urine (32-47% and 50-57% in male and
female rats) and in faeces
Metabolism in animals Extensively metabolized in rats, six metabolites identified; two
major metabolic pathways.
Toxicologically significant compounds Parent compound and metabolites
(animals, plants and environment)
Acute toxicity
Rat, LD50, oral > 1200 mg/kg bw
Rabbit, LD50, dermal > 2000 mg/kg bw
Rat, LC50, inhalation > 3.4 mg/L (4 h)
Dermal irritation Non, rabbits
Ocular irritation None, rabbits
Dermal sensitization Not a sensitizer (Buehler test in guinea-pigs)
Short-term toxicity
Target/critical effect Liver (mice, rat, dog), erythrocytes (rat, dog), kidney (rat)
Lowest relevant oral NOAEL 90 days, dog: 600 ppm (18 mg/kg bw per day)
Lowest relevant dermal NOAEL 3 weeks, rabbit: > 1000 mg/kg bw per day
Lowest relevant inhalation NOAEL 3 months, rat: 0.01 mg/L
Long-term toxicity and carcinogenicity
Target/critical effect Liver
Lowest relevant NOAEL/NOEL 2 years, rat: 100 ppm (4 mg/kg bw per day)
Carcinogenicity Increased tumour incidences in liver, thyroid, skin (rats) and
lungs (mice)
Genotoxicity In an incomplete range of studies, no genotoxic or mutagenic
potential identified
Reproductive toxicity
Reproductive target/critical effect Reproductive effects (reduced pup body weights) at parentally
toxic doses
Lowest relevant reproductive NOAEL Rat: 100 ppm (10 mg/kg bw per day)
Developmental target/critical effect No developmental effects at maternally toxic doses
Lowest relevant developmental NOAEL Rat: 75 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Acute clinical disorders and behavioural effects
Acute neurotoxic NOAEL Rat: 20 mg/kg bw
Other toxicological studies Induction of hepatic microsomal activity
Medical data Available human data do not show causal relationships between
exposure to modern pyrethrin-containing products and significant
adverse health effects.
Summary Value Study Safety factor
ADI 0-0.04 mg/kg bw Long-term toxicity, rats 100
Acute reference dose 0.2 mg/kg bw Acute neurotoxicity, rats 100
References
Bielucke, J. (1991) Primary eye irritation in rabbits (New Zealand
white). Unpublished Report, Project No. 91-7316A, MRID #41964802
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Casida, J.E. & Quistad, G.B. (1995) Metabolism and synergism of
pyrethrins. In: Casida, J.E. & Quistad, G.B., eds, Pyrethrum
Flowers: Production, Chemistry, Toxicology and Uses, Oxford:
Oxford University Press, pp. 258-277.
Class, T.J., Ando, T. & Casida, J.E. (1989) Pyrethroid metabolism;
microsomal oxidase metabolites of (S)-Bioallethrin and the six
natural pyrethrins. Unpublished report MRID #41248801 from
Pesticide Chemistry & Toxicology Laboratory. Submitted to WHO by
Kenya Pyrethrum Information Centre, Oberalm, Austria.
Curren, R.D. (1989) Unscheduled DNA synthesis assay in rat primary
hepatocytes with a confirmatory assay. Unpublished report,
laboratory study No. T8729.380009, MRID #41344501 from
Microbiological Associates, Inc. Submitted to WHO by Kenya
Pyrethrum Information Centre, Oberalm, Austria.
Feinberg, S.M. (1934) Pyrethrum sensitization. J. Am. Med. Assoc.,
102, 1557-1558.
Gabriel, D. (1991) Acute dermal toxicity in rabbits (New Zealand
white). Unpublished report, project No. 91-7316A, MRID #41964801
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Gabriel, D. (1992) Summary of results of acute oral toxicity study
Unpublished report, project No. 92-7529A, from Biosearch Inc.,
Philadelphia, Pennsylvania, USA. Submitted to WHO by Kenya
Pyrethrum Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1987) Evaluation of pyrethrum extract in a 2-week
dietary toxicity study in mice. Unpublished report, laboratory
project ID: 556-114 from International Research & Development
Corp. Submitted to WHO by Kenya Pyrethrum Information Centre,
Oberalm, Austria.
Goldenthal, E.I. (1988a) Evaluation of pyrethrum extract in a 13-week
dose range-finding study in mice. Unpublished report, laboratory
project ID: 556-008, MRID #433585201 from International Research
& Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1988b) Evaluation of pyrethrum extract in a 13-week
dose range-finding study in rats. Unpublished report, laboratory
project ID: 556-010 from International Research & Development
Corporation. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Goldenthal, E.I. (1988c) Toxicity study in dogs. Unpublished report,
laboratory project ID: 556-006 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Goldenthal, E.I. (1990a) Evaluation of pyrethrum extract in a 1-year
chronic toxicity study in dogs. Unpublished report, laboratory
project ID: 556-007, MRID #41496502 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Goldenthal, E.I. (1990b) Evaluation of pyrethrum extract in an
18-month dietary oncogenic study in mice. Unpublished report,
laboratory project ID: 556-013, MRID #41559401 from International
Research & Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1990c) Evaluation of pyrethrum extract in a two-year
dietary toxicity and oncogenicity study in rats. Unpublished
report, laboratory project ID: 556-011, MRID #41559501 from
International Research & Development Corp. Submitted to WHO by
Kenya Pyrethrum Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1992) 21-day repeated dose dermal toxicity study
with pyrethrum extract in rabbits. Laboratory project ID:
556-018, MRID #42212601 from International Research & Development
Corp. Submitted to WHO by Kenya Pyrethrum Information Centre,
Oberalm, Austria.
Hermansky, S.J. & Hurley, J.M. (1993a) Peroral (gavage) neurotoxicity
probe study with pyrethrins in rats (Sprague Dawley). Unpublished
report, laboratory project ID: 91N0122, MRID #42930401 from Bushy
Run Research Center. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Hermansky, S.J. & Hurley, J.M. (1993b) Acute oral neurotoxicity study
with pyrethrins in rats (Sprague Dawley). Unpublished report,
laboratory project ID: 92N1036, MRID #42925801 from Bushy Run
Research Center. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Hoffmann, G.M. (1991) Acute inhalation toxicity in rats (Sprague
Dawley). Unpublished report, project No. 91-8331, MRID #42008001
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Mitchell, J.C., Dupuis, G. & Towers, G.H.N. (1972) Allergic contact
dermatitis from pyrethrum (Chrysanthemum sp.). Br. J.
Dermatol., 86, 568-573.
Myer, J.R. (1991) Evaluation of pyrethrum extract in a 5-day toxicity
study in New Zealand white rabbits. Unpublished report,
laboratory project ID: 556-017 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Newton, P.E. (1992) 90-day inhalation toxicity study of pyrethrum
extract in the rat via whole body exposure. Unpublished report,
project No. 91-8335, MRID #42478201 from Bio/dynamics, Inc.
Submitted to WHO by Kenya Pyrethrum Information Centre, Oberalm,
Austria.
Putman, D.L. & Morris, M.J. (1989) Chromosome aberrations in Chinese
hamster ovary (CHO) cells. Unpublished report, laboratory study
No. T8729.337, MRID #41344601 from Microbiological Associates
Inc. Submitted to WHO by Kenya Pyrethrum Information Centre,
Oberalm, Austria.
Ramirez, M.A. (1930) Pyrethrum: An etiologic factor in vasomotor
rhinitis and asthma. J. Allergy, 1, 149-155.
Rickett, F.E., Tyszkiewicz, K. & Brown, N.C. (1972) Pyrethrum
dermatitis. Part I: The allergenic properties of various extracts
of pyrethrum flowers. Pyrethrum Post, 11, 85.
Romanelli, P. (1991a) Primary skin irritation in rabbits (New Zealand
white). Unpublished report, project No. 91-7316A, MRID #41964803
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Romanelli, P. (1991b) Sensitization study in guinea pig (Hartley
strain). Unpublished report, project No. 91-7316A, MRID #
41964804 from Biosearch Inc., Philadelphia, Pennsylvania, USA.
Submitted to WHO by Kenya Pyrethrum Information Centre, Oberalm,
Austria.
San, R.H.C. & Springfield, K.A. (1989) Salmonella/
mammalian-microsome plate incorporation mutagenicity assay (Ames
test) with a confirmatory assay. Unpublished report, laboratory
study No. T8729.501014, MRID #41344701 from Microbiological
Associates, Inc. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Schardein, J.L. (1987a) Evaluation of pyrethrum extract in a dose
range-finding teratology study in rats. Unpublished report,
laboratory project ID: 556-001, MRID #40603701 form International
Research & Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Schardein, J.L. (1987b) Evaluation of pyrethrum extract in definitive
rat teratology study. Unpublished report, laboratory project ID:
IRDC 556-002, MRID #40288202 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Schardein, J.L. (1987c) Evaluation of pyrethrum extract in a dose
range-finding teratology study in rabbits. Unpublished report.
laboratory project ID: 556-003, MRID #40603702 from International
Research & Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Schardein, J.L. (1987d) Evaluation of pyrethrum extract in a
definitive rabbit teratology study. Unpublished report,
laboratory project ID: IRDC 556-004, MRID #40288203 from
International Research & Development Corp. Submitted to WHO by
Kenya Pyrethrum Information Centre, Oberalm, Austria.
Schardein, J.L. (1989) Two generation reproduction study in rats with
pyrethrum extract. Unpublished report, laboratory project ID:
IRDC 556-005, MRID #41327501 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Selim, S. (1995) Pharmacokinetics and metabolism of 14C pyrethrin I
in the rat. Unpublished report, study No. P1092006, MRID
#43554304 from Biological Test Center. Submitted to WHO by Kenya
Pyrethrum Information Centre, Oberalm, Austria.
Springfield, A.C., Carlson, G.P. & DeFeo, J.J. (1973) Liver
enlargement and modification of hepatic microsomal drug
metabolism in rats by pyrethrum. Toxicol. Appl. Pharmacol., 24,
298-308.
Zucker, A. (1965) Investigation of purified pyrethrum extracts.
Ann. Allergy, 23, 335-339.
Appendix 1: Application of the Conceptual Framework for Cancer Risk
Assessment
(Revised on the basis of discussions at the IPCS Workshop on
Developing a Conceptual Framework for Cancer Risk Assessment, 16-18
February 1999, Lyon, France)
This framework, developed by an IPCS working group, provides a
generic approach to the principles commonly used in evaluating a
postulated mode of action for tumour induction by a chemical. Thus,
the framework was used by the 1999 JMPR to provide a structured
approach to the assessment of the overall weight-of-evidence for the
postulated mechanism of the increased incidences of benign tumours of
the liver, thyroid, and skin in rats and a treatment-related effect on
the incidence of lung tumours in mice observed after long-term
administration of pyrethrins.
The framework guidelines suggested 10 section headings. The
introduction (see monograph section 2(c), 'Long-term studies of
toxicity and carcinogenicity') describes the cancer end-points that
have been observed. Three of these (rat liver and thyroid neoplasms,
rat keratoacanthomas, and mouse lung tumours) are addressed separately
in the analysis. An approriate mode of action is postulated and the
key events identified; the observed dose-response relationships and
temporal relationships are discussed. The strength, consistency, and
specificity of the association of tumour response with key events and
the biological plausibility are analysed. Alternative modes of action
are identified and found not to be supported. The three postulated
modes of action are discussed below, with some uncertainties about
both the biology of tumour development and the database on the
compound and any inconsistencies in the method that were identified.
* The increased incidences of hepatocellular adenomas were associated
with persistent induction of cytochrome P450 enzymes and
hepatocellular hypertrophy, suggesting that pyrethrins are
rodent-specific hepatoproliferative carcinogens. Enzyme induction
leading to increased clearance of thyroid hormones would also be
consistent with the increased incidence of follicular hyperplasia
and follicular adenomas seen. The level of confidence in this
postulated mode of action is moderate, because some uncertainties
and inconsistencies were introduced by the method. Therefore,
additional studies to identify more key events in the liver and
thyroid (e.g. measurements of enzyme induction with purified
pyrethrins, estimation of thyroid hormone changes, and estimation
of thyroid and pituitary weights) are required.
* The higher incidence of keratoacanthomas in male rats at the high
dose, which exceeds the upper limit of the range in historical
controls, was considered to be related to treatment. The slight
increase in the incidence of cystic lesions in the skin and
subcutis in males observed macroscopically provided some support
that the increased incidence of neoplastic lesions is a result of
irritating chronic injury of the skin. There were some
uncertainties introduced by the method, and differing opinions on
the biology of these tumours were found in the literature. The
level of confidence in this postulated mode of action is very low,
and no further studies were identified to support it.
* The increased incidence of lung tumours in mice might be a result
of proliferative processes following chronic injury of the
respiratory epithelium or activation of microsomal mixed-function
enzymes, especially in Clara cells, which contain high
concentrations of P450 enzymes. Such chronic injury might be
followed by cell proliferation with a dose-response relationship.
The level of confidence in this postulated mode of action is,
however, very low.
There was also little confidence in other possible modes of action.
Uncertainties in the database on pyrethrins were identified.
Improvement of the method used in the study of carcinogenicity (e.g.
detailed histological sectioning of each lung lobe cut at the level of
bronchi and additional microscopic examination of step-sections of the
remaining lung tissue beginning at the level of the bronchi in all
animals of each sex, followed by a re-evaluation of all histological
slides in the absence of knowledge of their origin; reporting of the
number and size of neoplastic and preneoplastic lesions in each
animal) could serve to increase confidence that these tumours have no
relevance at the low concentrations to which humans and animals are
exposed.
The Meeting concluded that the increased tumour incidences
associated with exposure to pyrethrins are threshold phenomena of
negligible relevance to the low concentrations to which humans are
exposed and that pyrethrins have no genotoxic or mutagenic potential.
Therefore, no classification of cancer risk is necessary. However,
additional studies are required.
The discussion of the postulated mode of action of tumour induction
by pyrethrins was helpful in the overall process of hazard
characterization and risk assessment and contributed to consideration
of the relevance of the findings in animals to the human situation.
Application of the framework also promoted confidence in the
conclusions reached, as it represents use of a defined procedure which
mandates consistent documentation of the facts and reasoning that
includes consideration of inconsistencies and uncertainties. The
Meeting concluded that the framework could be developed for use both
by regulators and by researchers in identifying research needs on the
basis of clear delineation of data gaps and inconsistencies.
 2. Toxicological studies
(a) Acute toxicity
The results of studies on the acute toxicity of pyrethrum
extracts are summarized in Table 1. The methods used in these studies
complied with the corresponding FIFRA guidelines and with GLP.
Pyrethrins have little acute toxicity in rats treated orally, with
LD50 values of 2400 mg/kg bw for males and 1000 mg/kg bw for
females. The ratio of pyrethrins I and II (1.44 or 2.50) has no effect
on the toxicity of pyrethrins in male or female rats. The signs of
toxicity included ruffled appearance and tremors within 4-24 h after
dosing. Post-mortem examination of animals that died showed
haemorrhagic lungs, tan-to-yellow fluid in the lower gastrointestinal
tract and muzzle, and genital staining. The no-effect level for
clinical signs was 710 mg/kg bw in males and 320 mg/kg bw in females.
Dermal exposure to 2000 mg/kg bw was tolerated by rabbits, which
showed very slight to well-defined erythema, very slight oedema, and
stained test sites at 24 h. All animals appeared normal through the
14-day observation period.
Very slight acute toxicitywas found in rats exposed to aerosols
by inhalation. On the basis of the mean analytical concentration of
active ingredient and the resultant mortality, the LC50 was
calculated to be 3.4 mg/L for the two sexes. Tremors were seen during
exposure to the two higher concentrations. The significant findings
post mortem included discoloured and oedematous respiratory tissues.
Application of pyrethrins to the skin of albino rabbits produced
a minimally irritating skin irritation score of 0.42 (Romanelli,
1991a).
Instillations of the extracts into the conjunctival sac produced
irritation in the eyes of albino rabbits, but no irritation was
observed by 72 h. No corneal opacity or iritis was seen during the
observation period (Bielucke, 1991).
In a study of dermal sensitization in guinea-pigs with a modified
Buehler protocol, pyrethrins were not sensitizing (Romanelli, 1991b).
(b) Short-term studies of toxicity
Mice
In order to define a suitable maximum tolerated dose for a longer
study, doses of 5000 and 7000 ppm were evaluated in a 2-week study.
The method used complied to a certain extent with OECD guideline 407,
and the study was conducted in compliance with GLP. One mouse at
7000 ppm died. There was no effect on body weight, but food
consumption was slightly decreased at 7000 ppm. Statistically
significant increases in the absolute and relative weights of the
liver were seen at both doses. On the basis of the results of this
Table 1. Acute toxicity of pyrethrins
Species Strain Sex Route LD50 or LC50 Purity (%) or Reference
(mg/kg bw or mg/L air) ratio of pyrethrin I:II
Rat Sprague-Dawley M Oral 2400 58% Gabriel (1992)
Rat Sprague-Dawley F Oral 1000 58% Gabriel (1992)
Rat Sprague-Dawley M Oral 3900 1.4 Gabriel (1992)
Rat Sprague-Dawley F Oral 1300 1.4 Gabriel (1992)
Rat Sprague-Dawley M Oral 3900 2.5 Gabriel (1992)
Rat Sprague-Dawley F Oral 1200 2.5 Gabriel (1992)
Rabbit New Zealand M&F Dermal > 2000 58% Gabriel (1991)
Rat Sprague-Dawley M&F Inhalation 3.4 58% Hoffmann (1991)
M, male; F, female
study, 7000 ppm was selected as the high dose for the long-term study
in mice (Goldenthal, 1987).
Pyrethrins were offered to groups of 15 Charles River mice of
each sex in the diet at concentrations of 300, 1000, 3000, or 10 000
ppm for 13 weeks, equal to 47, 160, 460, and 1600 mg/kg bw per day for
males and 56, 200, 580, and 1800 mg/kg bw per day for females. The
method used complied to a certain extent with OECD guideline 408, and
the study was conducted in compliance with GLP. Four males and 10
females at the original high dose of 30 000 ppm died on day 2, and all
animals at this dose had died or were killed in extremis by day 10.
Four males and two females at 10 000 ppm also died on day 2, with
clinical signs that included tremors, pale exposed skin, dilated
pupils, altered activity, laboured breathing, cold to touch,
moribundity, and hunched posture. Tremors and increased activity were
seen in several animals at 10 000 ppm during the first 2 weeks of
study. No treatment-related clinical signs were observed in the
animals at 300, 1000, or 3000 ppm. The group mean body weights and
food consumption were similar for all groups with surviving animals.
The absolute weight of the liver and the liver:body weight and the
liver:brain weight ratios were all statistically significantly
increased in males and females at 3000 and 10 000 ppm, whereas the
absolute weights of the liver in females at 300 and 1000 ppm were
comparable to those of controls. A treatment-related increase in the
incidence and/or severity of congestion in the liver was observed in
surviving male and female mice at 10 000 ppm, and an increased
incidence but only mild severity was found in < 15% of investigated
animals at 3000 ppm. An increased incidence of hepatocellular
hypertrophy was present in surviving male and female mice at 3000 and
10 000 ppm; at 1000 ppm, only 2 of 15 mice showed mild congestion of
the liver on macroscopic observation. The NOAEL was 1000 ppm, equal to
160 mg/kg bw per day (Goldenthal, 1988a).
Rats
Pyrethrins were offered to groups of 15 Charles River rats of
each sex for 13 weeks in the diet at concentrations of 300, 1000,
3000, 10 000, or 20 000 ppm, equal to 17, 57, 170, 590, and 1200 mg/kg
bw per day for males and 22, 74, 220, 710, and 1400 mg/kg bw per day
for females. The method used in this study complied to a certain
extent with OECD guideline 408, and the study was conducted in
compliance with GLP. During the first week of the study (days 3-7),
one female at 10 000 ppm, one male at 20 000 ppm, and 12 females at
20 000 ppm died; all other animals survived to scheduled sacrifice.
The signs seen before death in all animals that died included
decreased defaecation, increased respiration rate, tremors, and
decreased or increased activity. Convulsions were seen in some
animals. The signs in most animals at 10 000 and 20 000 ppm that
survived until the end of the study were decreased defaecation,
tremors, increased respiration rate, and increased activity.
Convulsions occurred mainly in males at 20 000 ppm. Most of the signs
were seen only during the first two weeks of the study. Statistically
significant decreases in mean body weights were observed during most
or all of the study in males and females at 10 000 and 20 000 ppm.,
and statistically significant decreases in mean food consumption were
seen during most or all of the study in females at 10 000 ppm and
males and females at 20 000 ppm when compared with the respective
control groups. Statistically significantly decreased mean values for
haematocrit and haemoglobin were found in males at 20 000 ppm and for
erythrocytes, haematocrit, and haemoglobin in females at 10 000 and
20 000 ppm. Females at 3000 ppm also showed a decreased mean
haemoglobin value. The treatment-related macroscopic findings
consisted of enlargement and congestion of the liver in both males and
females, but primarily in males, at 10 000 and 20 000 ppm; however,
the macroscopic observation could not be confirmed microscopically.
The liver weight was statistically significantly increased in males at
10 000 and 20 000 ppm and in females at 3000, 10 000, and 20 000 ppm
The weight of the kidney was increased in males and females at 3000,
10 000, and 20 000 ppm The only microscopic finding that was possibly
realted to treatment was small focal or multifocal areas of tubular
degeneration and regeneration in the renal cortex in animals at 3000
and 10 000 ppm, but primarily in males. The NOAEL was 1000 ppm, equal
to 57 mg/kg bw per day (Goldenthal, 1988b).
In a study designed to assess the toxic effects of pyrethrins
administered by whole-body inhalation as a liquid aerosol, groups of
15 Charles River rats of each sex were exposed for 6 h/day, generally
5 days per week, a minimum of 65 times to target concentrations of the
active ingredient corresponding to 0.010, 0.030, 0.10, and 0.35 mg/L.
The mean analytical concentrations to which the groups were exposed
were 0 (control), 0.038, 0.068, 0.23, and 0.83 mg/L, respectively.
Determinations of particle size distribution showed an overall mass
median aerodynamic diameter of 2.7 µm.The controls were exposed only
to conditioned room air on the same schedule. The study was conducted
in compliance with FIFRA Guideline 824 and with GLP.
Two animals at the highest dose died, one animal on day 3.
Because this death was accidental and occurred very early in the
study, this animal was replaced with another animal from the same
shipment. A second animal died on day 15, having shown laboured
breathing similar to that of other animals in this group, and its
death was considered to be potentially related to exposure.
Dose-related increases in the frequency of clinical signs were
observed in animals at the three higher doses during observations in
the chamber and at detailed weekly examinations. These signs included
secretory signs such as nasal discharge and dried material in the
facial area in both males and females. Animals at the highest dose
showed laboured breathing, excess lachrymation, tremors, increased
activity, and matted coats. There were no ocular effects. The absolute
body weights and body-weight gains of both males and females at the
two higher doses were decreased, and after 13 weeks of exposure the
body-weight gains of males at these doses were about 9% lower than
those of controls. In females, the body-weight gain after 13 weeks of
exposure was lower than that of controls, by 13% at 0.10 mg/L and 17%
at 0.35 mg/L. Food consumption was also slightly decreased during the
first 1 or 2 weeks in males and females in these groups.
Nonregenerative anaemia was observed in males at the three higher
doses and in females at the highest dose, with significant decreases
in haemoglobin, haematocrit, and erythrocyte values. An increased
leukocyte count was also seen in the females at the highest dose.
Significant differences in clinical chemical parameters were seen
primarily at the highest dose, including decreased total protein and
globulin and an increased albumin:globulin ratio in males and
decreased glucose in females. Other changes occurred sporadically and
were considered to be unrelated to exposure. Several significant
increases in organ weights or ratios were observed. The increases in
liver weight were clearly related to treatment. Increases in the
organ:body weight ratios of the kidneys and lung might be a reflection
of a marginal increase in absolute organ weights and the decreased
body weights. Morphological abnormalities in the larynx,
nasoturbinates, nasopharynx, and lungs observed by light microscopy
were considered to be localized responses indicative of a
treatment-related effect. The NOAEL for systemic effects was 0.01 mg/L
(Newton, 1992).
Rabbits
Pyrethins were administered dermally to five male and five female
New Zealand white rabbits in the form of a 25% (w/v) mixture in
vegetable oil at doses of 0, 100, 300, or 1000 mg/kg bw once daily, 5
days per week for 3 weeks. Animals in the vehicle control group were
given vegetable oil on the same regimen and at the same volume as the
group receiving the high dose . This study was conducted in compliance
with OECD Guideline 410 and GLP. One rabbit at 1000 mg/kg bw was
sacrificed in extremis on day 10 after showing emaciation, decreased
activity, and decreased defaecation. Macroscopic examination of this
animal did not reveal the cause of death. A low incidence of
desquamation and/or red raised areas on the skin at the application
site was observed in all groups, including the vehicle controls.
Several animals in the treated groups showed very slight to
well-defined erythema of the skin at the application site, but no
clear pattern with regard to treatment was seen for any of these
findings. Microscopic evaluation revealed no evidence of systemic
toxicity. The microscopic lesions at the application site included
acanthosis, haemorrhage, hyperkeratosis, and chronic inflammation,
although haemorrhage was observed only in the group given the vehicle
alone. Thus, all of the dermal reactions appeared to be due to the
vegetable oil. The NOAEL for systemic effects was 1000 mg/kg bw, the
highest dose tested (Goldenthal, 1992).
This result is supported by that of a previous study performed to
assess dermal irritation with pyrethrins at concentrations of 25%,
50%, and 75% w/v in corn oil on rabbits (Myer, 1991).
Dogs
Pyrethins were incorporated into the basal diet of groups of two
pure-bred beagle dogs of each sex at concentrations of 600, 1000,
3000, or 6000 ppm for 8 weeks, equal to 18, 30, 86, and 170 mg/kg bw
per day for males and 19, 29, 94, and 200 mg/kg bw per day for
females. The method used complied to a certain extent with OECD
guideline 409, and the study was conducted in compliance with GLP. One
male and both females at 6000 ppm died or were killed in extremis
during the study. The treatment-related clinical signs observed in
animals at this dose included inappetence, thin appearance, ataxia,
trembling, oily coat, impaired limb function, shallow breathing,
moribundity, and death. With the exception of moribundity and death,
similar signs were observed at 3000 ppm. Males and females at 6000 ppm
lost weight during the study, but the body-weight gains of animals in
all other treated groups were comparable to those of controls. The
average food consumption was decreased for males at 3000 ppm and for
males and females at 6000 ppm when compared with controls. Decreased
haematocrit, haemoglobin, and erythrocyte values were seen at the end
of the study in males at 3000 and 6000 ppm, but there were no other
treatment-related haematological finding. One male and one female at
6000 ppm killed in extremis had increased leukocyte counts and
decreased haematocrit, haemoglobin, and erythrocyte values. Slightly
decreased glucose, calcium, phosphorus, and cholesterol values were
found at the end of the study in males at 6000 ppm, and males and
females at 3000 ppm had slightly decreased cholesterol concentrations.
The aspartate and alanine aminotransferase activities of males at 6000
ppm were slightly increased at the end of dosing, and the surviving
male at this dose had a very high creatinine phosphokinase value.
There were no other treatment-related biochemical findings at the end
of the study. Males and females at 6000 ppm killed in extremis
showed some variation in electrolyte levels, and the urea nitrogen
concentration was increased in both dogs. The female showed large
increases in aspartate and alanine aminotransferase and creatine
phosphokinase activities. The absolute weights of the liver in both
males and females at 1000 and 3000 ppm were increased in a
treatment-related fashion, and the absolute weight of the testis at
these doses appeared to decrease in a similar manner. There were no
macroscopic or microscopic lesions attributable to the administration
of pyrethrins. The NOAEL was 600 ppm, equal to 18 mg/kg bw per day
(Goldenthal, 1988c).
Pyrethrins were administered to groups of four beagle dogs of
each sex for 52 weeks in the diet at concentrations of 0, 100, 500, or
2500 ppm, equal to 2.6, 14, and 66 mg/kg bw per day for males and 2.8,
14, and 75 mg/kg bw per day for females. Four dogs of each sex were
evaluated at each dietary concentration. This study was conducted in
compliance with FIFRA Guideline 83-1 and with GLP. All animals
survived to the end of the study, and no remarkable clinical signs of
toxicity were found at any dose. Overall, the mean body weights of the
treated animals were similar to those of controls. The mean food
consumption of males at 2500 ppm and of females at 500 and 2500(c)
ppm was lower than that of controls during the first week of the
study, but consumption was similar for the remainder of the study,
except that males at 500 ppm consumed greater amounts of food than the
controls. Increased total leukocyte and segmented neutrophil counts
were found in females and decreased erythrocyte, haemoglobin, and
haematocrit values in males at 2500 ppm. Alanine aminotransferase
activity was statistically significantly increased in females at 2500
ppm at both the 6- and 12-month evaluations. The slight increase in
the activity of this enzyme in males was not statistically
significant. No treatment-related changes in urinary parameters were
seen at either interval. The relative and absolute weights of the
liver were significantly increased in males at 2500 ppm when compared
with controls, but no statistically significant differences in organ
weights were seen in females at this dose. No macroscopic or
microscopic treatment-related changes were observed in tissues. The
NOAEL was 500 ppm, equal to 14 mg/kg bw per day (Goldenthal, 1990a).
(c) Long-term tudies of toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female Charles River CD-1 mice received
diets containing pyrethrins at concentrations of 100, 2500, or 5000
ppm for 18 months, equal to doses of 14, 350, and 690 mg/kg bw per day
for males and 17, 410, and 830 mg/kg bw per day for females. This
study was conducted in compliance with FIFRA Guideline 83-2(b) and
with GLP. One male and one female at 5000 ppm were found dead during
the first week of the study, but no other treatment-related deaths
occurred, and survival was similar in the control and treated groups
throughout the study. All animals at 5000 ppm exhibited increased
activity when stimulated during the first week of the study but not
later. There were no other clinical signs seen that differentiated the
treated from the control groups. Statistically significant differences
in mean body weight and food consumption were seen between control and
treated groups sporadically throughout the study, but none of the
differences was considered to be related to treatment. No
treatment-related effect was observed in other parameters examined at
12 and 18 months of study. At necropsy, discoloured, dark livers were
more common in males at 5000 ppm and in females at 2500 and 5000 ppm,
and treatment-related increases in the absolute and relative weights
of the liver were seen in males and females at 2500 and 5000 ppm.
Microscopically, vacuolar fatty change was found in the livers of
males at these doses, and this change was considered to be related to
treatment. The dark discolouration seen macroscopically could not be
explained by the microscopic findings. The incidence of nodules and
masses in the lungs appeared to be slightly increased in animals at
5000 ppm. When the lungs were examined microscopically according the
original test protocol, the incidences of alveolar bronchiolar
adenomas were increased in females at 5000 ppm, and the increase was
statistically significant, exceeding the range in historical controls.
Treated males showed an apparent, not clearly dose-related increase in
the incidence of alveolar bronchiolar carcinomas which exceeded the
upper limit of 95% of the historical controls (Table 2). The sponsor
asked the testing laboratory to conduct serial sectioning of the
remaining lung tissue of female mice in the control and highest-dose
groups, which did not have diagnoses of lung tumours in the original
examination. As this was considered not to be an acceptable
toxicopathological practice, the results of the first evaluation were
taken into consideration for the risk assessment, and the increased
incidence of lung tumours was considered to be a treatment-related
effect. The NOAEL was 100 ppm, equal to 14 mg/kg bw per day
(Goldenthal, 1990b).
Table 2. Comparison of first and second evaluations of microscopic
neoplastic lesions in the lung of mice treated with
pyrethrins for 18 months
Sex Dose No. of lungs Alveolar bronchiolar neoplasm
(ppm) examined
Adenoma Carcinoma
Male 0 60 14 0a
0 60 16 0
100 60 15 1
2500 60 13 3b,c
5000 60 17 3b,c
Female 0 60 8 1
0 60 4 3
100 60 11 0
2500 60 5 2
5000 60 19c,d 2
a p < 0.05; Cochran trend test
b Significant differences in pair-wise comparison of the
high-dose group with controls at p < 0.05
c > 95% of of the upper range of historical controls
d p < 0.05; Fisher exact test
Rats
Groups of 60 Charles River CD rats of each sex received diets
containing pyrethrins at concentrations of 100, 1000, or 3000 ppm for
104 weeks, equal to 4, 43, and 130 mg/kg bw per day for males and 5,
56, and 170 mg/kg bw per day for females. This study was conducted in
compliance with FIFRA Guideline 83-5 and with GLP. Survival was
similar in the treated and control groups, and there were no clinical
findings attributable to treatment. Statistically significant
decreases in body weight which were considered to be related to
treatment were observed during the first 78 weeks of the study in both
male and female rats at 3000 ppm, with a difference from controls of
7% in males and 10% in females. A slight, treatment-related decrease
in food consumption was seen at the same time. No treatment-related
ophthalmological findings or organ weight changes were detected during
the study, and no haematological or urological changes were found. The
activities of serum transaminases were substantially increased at most
intervals of analysis in males at 3000 ppm, most of the values
reaching statistical significance. Increased incidences of benign
tumours of the liver, thyroid, and skin were also observed (Table 3),
and a statistically significantly higher incidence of hepatocellular
adenomas was described in females at the high dose. Follicular
adenomas and carcinomas were initially seen in the thyroid glands of
rats at the high dose, which appeared to be related to treatment, but
during a re-evaluation some of the carcinomas were reclassified as
adenomas and some adenomas were reclassified as hyperplasia. After the
re-evaluation, the incidence of hyperplasia was found to be enhanced
in males and females, and the incidence of follicular adenomas was
statistically significantly increased only in females at 3000 ppm.
Nevertheless, the tumour incidences in animals of each sex were higher
than the upper range seen in historical controls. The results of a
further, full histopathological peer review confirmed these increased
tumour incidences. Macroscopic examination of the skin showed a slight
increase in the incidence of cystic lesions in the skin and subcutis,
and the microscopic assessment showed an apparently higher incidence
of keratoacanthomas in males at the high dose, which was statistically
significant in comparison with both control groups. A peer review of
pathological lesions in all male rats resulted in removal of several
keratoacanthomas from the table of incidence in treated groups but
confirmed that the incidence of this lesion clearly exceeded the upper
limit of the range in historical controls (1.4-10%). The increased
incidences of liver and thyroid tumours and of keratoacanthomas of the
skin were considered to be treatment-related effects but to be
threshold phenomena of negligible relevance to the low doses to which
humans are exposed. The NOAEL was 100 ppm, equal to 4 mg/kg bw per day
(Goldenthal, 1990c).
(d) Genotoxicity
The results of tests for the genotoxicity of pyrethrins in
vitro are summarized in Table 4.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a two-generation study, groups of 28 male and 28 female
Charles River rats received diets containing pyrethrins at
concentrations of 100, 1000, or 3000 ppm, equivalent to 10, 100, and
300 mg/kg bw per day. A control group received the basal laboratory
diet on an identical regimen. The F0 parental generation were
treated for a minimum of 77 days before the first of two matings. The
same numbers of weanlings from the F1b litters were selected
randomly to become parents of the F1 generation and were treated for
a minimum of 95 days before being mated twice to produce the F2a and
F2b litters. This study was conducted in compliance with FIFRA
Guideline 83-4 and with GLP. No treatment-related effects were noted
with respect to clinical signs, body weights, or food consumption in
Table 3. Incidences of microscopic neoplastic lesions in rats fed diets containing pyrethrins for 104 weeks
Sex Dose Liver Thyroid Skin
(ppm)
Total Hepatocellular tumours Total Hyperplasia Follicular tumours Total Cystic Keratoacanthomasb
examined examined examined lesions
Adenoma Carcinoma Adenomaa Carcinomaa
Male 0 60 6 1 60 2 2c 0 60 5 4
0 60 1 0 60 0 1 1 60 2 5
100 60 0 0 60 2 4 1 24 1 4
1000 60 3 0 59 5 5d 2 14 5 4
3000 60 3 1 60 7 5d 2 60 9 11d,e
Female 0 60 0 1 60 0 0 1 60 0 0
0 60 1 0 60 2 1 1 59 3 0
100 60 0 0 60 1 2 0 8 2 1
1000 60 1 0 60 1 3d 0 8 2 1
3000 60 5d.f 0 60 5 5d 1 60 0 0
a Results of the histopathological peer review
b Results of the re-analysis only in males
c p < 0.05; Cochran trend test
d Greater that the upper limit of historical control data
e Significant difference in the pair-wise comparison of the high-dose group with the controls at p < 0.05
f p < 0.05; Fisher exact test
Table 4. Results of assays for the genotoxicity of pyrethrins in vitro
Test system Test object Concentration Purity Results Reference
(%)
Reverse mutationa S. typhimurium TA98, 8.8-8772 µg/plate in 58 Negative ± S9 San & Springfield
TA100, TA1535, TA1537, acetone (1989)
TA1538
Chromosomal Chinese hamster ovary 0.02-0.32 Fl/ml + S9 58 Negative ± S9 Putman & Morris
aberrationa cells 0.005-0.08 Fl/ml - S9 (1989)
in DMSO
Unscheduled DNA Rat primary hepatocytes 0.0-1.0 Fl/ml in 58 Negative Curren (1989)
synthesis acetone
a Study conducted in compliance with GLP and to a certain extent with OECD guidelines
the parental rats of the F0 generation, but the body weights and
food consumption of the parental rats of the F1 generation were
significantly reduced at 3000 ppm and sporadically reduced at 1000
ppm. These reductions were considered to be treatment-related.
Treatment at 3000 ppm resulted in significantly reduced body weights
at birth for F1a males and F2a pups of each sex and during
lactation for the male and female offspring of both matings of both
generations. The mean body weights of pups at 1000 ppm were also lower
than those of controls for male F2a pups at birth and for F1b and
F2a pups during lactation. Reproductive performance and other litter
parameters were not affected by the treated diet at any dose. The
NOAEL for parental and reproductive toxicity was 100 ppm, equivalent
to 10 mg/kg bw per day (Schardein, 1989).
(ii) Developmental toxicity
Rats
Groups of five mated female Charles River rats were used in a
range-finding study to determine the doses of pyrethrin to be used in
a later study. Doses of 37.5, 75, 150, 300, or 600 mg/kg bw per day
were administered orally by gavage on days 6-15 of gestation at a
volume of 3 ml/kg. The control group received only the vehicle, 0.5%
methylcellulose, on a comparable regimen. Uterine examinations were
performed on all surviving females on day 20 of gestation. The method
used in this study complied to a certain extent with OECD Guideline
414, and the study was conducted in compliance with GLP.
Treatment-related maternal toxicity, observed as deaths, convulsions,
and/or tremors, occurred at 150, 300, and 600 mg/kg bw per day. No
treatment-related maternal deaths occurred at 75 mg/kg bw per day,
although tremors were observed in this group. No treatment-related
clinical signs were observed at 37.5 mg/kg bw per day. On the basis of
these results, doses of 5, 25, and 75 mg/kg bw per day were selected
for use in the following study (Schardein, 1987a).
Groups of 25 mated female Charles River rats were given
pyrethrins suspended in 0.5% methylcellulose orally by gavage at doses
of 5, 25, or 75 mg/kg bw per day on days 6-15 of gestation at a volume
of 3 ml/kg. The control group received the vehicle only on a
comparable regimen. On day 20 of gestation, the fetuses were removed
surgically for evaluation. The method used in this study complied to a
certain extent with OECD Guideline 414, and the study was conducted in
compliance with GLP. No animals died or were killed in extremis
during the study, and no treatment-related clinical signs were
observed. The body-weight gains of the treated groups were comparable
to those of the controls during treatment. No evidence of fetotoxicity
was found, and morphological examination revealed no teratogenic
effects at any dose tested. The NOAEL for maternal toxicity was 75
mg/kg bw per day, and that for developmental toxicity was 75 mg/kg bw
per day, the highest dose tested (Schardein, 1987b).
Rabbits
Groups of five inseminated female New Zealand white SPF rabbits
were used in a range-finding study to determine the doses of
pyrethrins for use in a later study. Doses of 37.5, 75, 150, 300, or
600 mg/kg bw per day were administered orally by gavage on days 7-19
of gestation at a volume of 3 ml/kg. The control group received only
the vehicle, 0.5% methylcellulose, on a comparable regimen. Uterine
examinations were performed on all surviving females on day 29 of
gestation. The method used complied to a certain extent with OECD
Guideline 414, and the study was conducted in compliance with GLP.
Treatment-related maternal toxicity, seen as deaths, tremors,
convulsions, and weight loss, and fetal toxicity, seen as high
postimplantation loss, were found at 600 mg/kg bw per day. Maternal
toxicity, in terms of weight loss during treatment and tremors was
seen at 300 mg/kg bw per day. No clear treatment-related effects were
observed at 37.5, 75, or 150 mg/kg bw per day. On the basis of these
results, doses of 25, 100, and 250 mg/kg bw per day were selected for
use in the following study (Schardein, 1987c).
Groups of 16 inseminated female New Zealand white SPF rabbits
were randomly assigned to receive pyrethrins at doses of 25, 100, or
250 mg/kg bw per day orally by gavage on days 7-19 of gestation at a
volume of 3 ml/kg. The control group received only the vehicle, 0.5%
methylcellulose, on a comparable regimen. On day 29 of gestation, the
fetuses were removed surgically for evaluation. The study was
conducted in compliance with OECD Guideline 414 and with GLP. All
animals survived to the end of treatment. One doe at the high dose
aborted near term, on day 28 of gestation, after showing decreased or
absent defaecation on several days previously. No gross lesions were
present at necropsy. Excessive salivation, arched head, and/or
laboured breathing were observed in a few females at the high dose on
days 18-19 of gestation. One female at the intermediate dose had
excessive salivation and arched head on gestation day 19. No apparent
treatment-related clinical signs were seen at the low dose. There were
no treatment-related gross pathological changes in any of the animals
at the end of the study. Body-weight loss was seen in does at the high
dose throughout treatment, and slightly reduced body-weight gain
relative to the control value was observed in animals at the
intermediate dose. The body-weight gains of treated groups were
comparable to that of the control group throughout gestation (days
0-29). When the body weights on day 29 were adjusted by subtracting
the uterine weights to reflect only maternal weight change, mean
weight losses were evident in all groups, including the control, over
the entire gestation period. There were no biologically meaningful or
statistically significant differences in the mean numbers of viable
fetuses, postimplantation losses, total implantations, or corpora
lutea and in fetal body weight or fetal sex distribution in the
treated groups in comparison with the control values. One doe at the
high dose resorbed its litter, but it is not clear if this finding was
related to treatment. There was no treatment-related or statistically
significant difference in the incidence of fetal malformations or
variations. The NOAEL for maternal toxicity was 25 mg/kg bw per day
and that for developmental toxicity was 250 mg/kg bw per day, the
highest dose tested (Schardein, 1987d).
(f) Special studies
(i) Effects on the central nervous system
Rats
Male Sprague-Dawley rats were treated once by oral gavage with a
10% or 25% solution of pyrethrins in corn oil at doses of 0, 40, 100,
200, 400, 800, 1400, or 2000 mg/kg bw, and females received a 2.5, 5,
or 10% solution of pyrethrins in corn oil at doses of 0, 25, 50, 100,
150, 200, 400, or 800 mg/kg bw. This study was conducted in compliance
with FIFRA Guideline 81-8 and with GLP. The clinical signs were
characterized by mild-to-severe tremors. On the basis of the
occurrence, severity, and onset of these reactions, a solution of 10%
pyrethrins in corn oil and doses of 40, 125, and 400 mg/kg bw were
selected for the study of acute neurotoxicity in males and a solution
of 5% pyrethrins in corn oil and doses of 20, 63, and 200 mg/kg bw in
females (Hermansky & Hurley, 1993a).
In the main study, groups of 15 male Sprague-Dawley rats received
by gavage a 10% w/v solution of pyrethrins in corn oil at doses of 0,
40, 125, or 400 mg/kg bw, and the same numbers of females received a
5% w/v solution of pyrethrins in corn oil at doses of 0, 20, 63, or
200 mg/kg bw. This study was conducted in compliance with FIFRA
Guideline 81-8 and with GLP. Five males and two females at the high
dose died on the day of treatment, and a variety of acute neurological
signs were observed in the other animals at this dose, including
tremors, urogenital area wetness, salivation, perinasal encrustation,
exaggerated startle response, decreased grip strength, hind leg splay,
and increased body temperature. Tremors were also observed in three
females at the intermediate dose. Measurements of motor activity on
the day of treatment indicated increased fine movement and decreased
rearing and ambulation in animals of each sex at the high dose and
decreased fine movement, rearing, and ambulation in males at the
intermediate dose. In addition, slight, statistically nonsignificant
decreases in body weight were seen in males at the high dose on days 7
and 14. There was no evidence of tany gross, treatment-related lesion.
The microscopic changes were limited mainly to sections of the sciatic
nerve and its branches. The histomorphological changes within the
peripheral nerve sections indicated the presence of scattered
degenerating nerve fibres or myelin sheaths. These changes were seen
in only a few animals, were graded as minimal, and were not
dose-related. The NOAEL was 20 mg/kg bw (Hermansky & Hurley, 1993b).
(ii) Effects on hepatic microsomal enzymes
Rats
Oral administration of pyrethrins to male rats at 85, 200, or 500
mg/kg bw per day for 3 weeks resulted in liver enlargement and
decreased hepatic DNA concentrations. Significantly decreased
hexobarbital-induced hypnosis without concomitant changes in
barbital-induced hypnosis suggested an alteration in hepatic drug
metabolism. The activities of hepatic microsomal enzymes responsible
for detoxification of
O-ethyl- O-(4-nitrophenyl)phenyl-phosphonothioate,
para-nitroanisole demethylation, and hexobarbital oxidation were
increased at 200 mg/kg bw per day to 150, 173, and 241% of the control
values, respectively. Increased liver weight, the detoxification of
pyrethrins, and demethylation of para-nitroanisole were found to be
dose-related. Small increases in enzyme activities were observed when
the lowest dose was given for 15 days. At 500 mg/kg bw per day, the
liver weight and enzyme activities were increased up to 17 days of
treatment but returned to the control level within 7 days after
cessation of treatment. NADPH cytochrome c reductase activity and the
cytochrome P450 concentration were also increased. It was suggested
that pyrethrins caused induction of microsomal enzymes. The LOAEL was
85 mg/kg bw per day (Springfield et al., 1973).
3. Observations in humans
The main adverse effects seen after exposure to pyrethrum
extracts in older studies were those manifesting as either skin or
respiratory reactions. Much of the research performed to date has
focused on the dermatological effects of pyrethrins, although mention
has been made of the respiratory reactions that have often accompanied
those of the skin. Studies by Ramirez (1930) and Feinberg (1934), in
which the association between sensitivity to ragweed and to pyrethrins
was delineated, contributed much to the understanding of allergic
responses to pyrethrins. Several investigations have since been
undertaken to isolate and characterize the allergen responsible for
the dermal reactions. Mitchell et al. (1972) isolated a sesquiterpene
lactone, pyrethrosin, from pyrethrins which induced positive dermal
responses in humans given a patch test. None of the other fractions
induced reactions, with the exception of pyrethrins II, which elicited
a weak response. Zucker (1965) demonstrated that a dermal reaction to
unrefined pyrethrum extract does not result in allergy to refined
pyrethrins. The previous evaluation by the Joint Meeting in 1970 cited
an unpublished report in which 200 people were given patch tests with
a 1% water dispersion of pyrethrins, and no evidence of primary
irritation or of sensitization was found. Rickett et al. (1972)
concluded that the refined extracts that have been marketed since 1957
do not induce skin allergies when tested on sensitive subjects and
that the dermal effects reported in the early literature are not
relevant to an assessment of refined pyrethrins.
Case reports of adverse respiratory effects, such as
anaphylactoid reactions and asthma, attributed to pyrethrins indicate
that these responses often occur in individuals with a history of
asthma. Although investigations of the dermal effects of pyrethrum
extracts suggests that pyrethrins are not the causative agent, no
thorough investigation of the agent(s) responsible for the adverse
respiratory responses has been conducted.
Comments
Absorption, distribution, and excretion in rats were investigated
only for pyrethrins I. After oral administration, more than 90% of a
low dose of pyrethrins I was absorbed, and the concentration of
radiolabel in blood peaked between 5 and 8 h. The radiolabelled
residues were widely distributed in the organs analysed, with the
highest concentrations in fat in females. The elimination half-time of
pyrethrins I in males and females was approximately 6 h. The mean
percentage of administered radiolabel found in the urine ranged from
32 to 47% in males and from 50 to 57% in females, the remainder being
excreted in faeces.
The substance is extensively metabolized, the residues of the
parent compound in faeces and urine representing only 10%. Six
metabolites were identified and two major metabolic pathways were
suggested, the first involving oxidation of the double-bond and/or the
methyl groups and the second involving hydrolysis of the ester bond.
Pyrethrins I are metabolized mainly through oxidative processes, while
pyrethrins II are metabolized through a combination of hydrolytic and
oxidative processes.
Pyrethrins show little acute toxicity, with an oral LD50 in
rats of > 1200 mg/kg bw and NOAELs for clinical signs of 710 mg/kg bw
for males and 320 mg/kg bw for females, a dermal LD50 in rabbits of
> 2000 mg/kg bw, and an inhalation LC50 in rats of 3.4 mg/L. The
compounds are minimally irritating to the skin and eye and show no
potential for skin sensitization. Pyrethrum extracts have not been
classified by WHO for acute toxicity.
In short-term tests for toxicity in mice, rats, and dogs, the
lowest relevant NOAELs after oral administration were 1000, 1000, and
600 ppm, equal to 160, 57, and 18 mg/kg bw per day, respectively, for
the three species. Statistically significant decreases in mean body
weight or body-weight gain were observed at the high doses throughout
most or all of the studies.
The liver is the main target organ in mice, rats, and dogs, and
an increased liver weight was frequently accompanied by changes in
serum transaminase activity. In mice, increased liver weights were
associated with a higher incidence of hepatocellular hypertrophy. In
the livers of rats and dogs, generally unremarkable histopathological
changes were observed. At doses of 85 mg/kg bw per day and above, a
pyrethrum extract containing 20% pyrethrins induced microsomal enzymes
in rats. Furthermore, anaemia was observed in rats and dogs at doses
of 3000 ppm and above. The kidney was another target, but only in
rats. In a 13-week study, rats at doses greater than 1000 ppm had
increased kidney weights associated with tubular degeneration and
regeneration in the renal cortex.
In a 13-week study in rats exposed by inhalation, the NOAEL for
systemic toxicity was 0.011 mg/L. The increases in liver weight were
clearly related to exposure and were accompanied by changes in serum
transaminase activity. Nonregenerative anaemia was also observed. The
weights of the kidney and lung were increased in relation to body
weight. The morphological abnormalities observed in the larynx,
nasoturbinates, nasopharynx and lungs by light microscopy were
considered to be localized responses indicative of a treatment-related
effect.
Dermal administration of pyrethrins at doses up to 1000 mg/kg bw
per day for 21 days caused no systemic toxicity in rabbits.
In a two-year study of toxicity and carcinogenicity in rats and
an 18-month study of carcinogenicity in mice, the NOAEL was 100 ppm in
both species, equal to 14 and 4 mg/kg bw per day in mice and rats,
respectively. The liver was the main target. A treatment-related
effect on the incidence of lung tumours was seen in mice and increased
incidences of benign tumours of the skin, liver, and thyroid were
observed in rats. The increased incidences of hepatocellular adenomas
were associated with persistent induction of cytochrome P450 enzymes
and hepatocellular hypertrophy, suggesting that pyrethrins are
rodent-specific hepatoproliferative carcinogens. Enzyme induction
leading to increased clearance of thyroid hormones would also be
consistent with the higher incidence of follicular hyperplasia and
follicular adenomas. However, additional studies on the mechanism of
formation of the liver and thyroid tumours are required. The Meeting
concluded that the increased tumour incidences caused by pyrethrins
are threshold phenomena of negligible relevance to the low doses to
which humans are exposed (see Appendix 1 to this monograph addendum).
Pyrethrins did not induce reverse mutagenicity in Salmonella
typhimurium with metabolic activation, did not induce chromosomal
aberration in Chinese hamster ovary cells, and did not induce
unscheduled DNA synthesis in rat primary hepatocytes. The Meeting
concluded that pyrethrins have no genotoxic or mutagenic potential,
but a test for gene mutation in mammalian cells is lacking.
Pyrethrins did not show developmental toxicity in rats or rabbits
at the highest maternally toxic doses tested, which were 75 and 250
mg/kg bw per day, respectively. The only effects on the offspring,
observed in a two-generation study of reproductive toxicity in rats,
were reduced body weights at the parentally toxic doses of 1000 and
3000 ppm, with a NOAEL of 100 ppm, equivalent to 10 mg/kg bw per day.
In a study of neurotoxicity in rats given single oral doses,
acute neurological disorders (tremors, wetness of the urogenital area,
salivation, perinasal encrustation, exaggerated startle response,
decreased grip strength, and hind-leg splay) and behavioural effects
(increased motor activity and decreased rearing and ambulation) were
noted, with a NOAEL of 20 mg/kg bw.
The available data on humans did not show a causal relationship
between exposure to modern pyrethrin-containing products and
significant adverse health effects.
An ADI of 0-0.04 mg/kg bw was established for the tested blend of
refined pyrethrum extract, which was based on the NOAEL of 100 ppm,
equal to 4 mg/kg bw per day, observed in the long-term study of
toxicity and carcinogenicity in rats and a safety factor of 100. This
figure is identical to the ADI derived by the 1972 Meeting, which was
based on a NOAEL of 200 ppm, equivalent to 10 mg/kg bw per day, in a
long-term study in rats and a safety factor of 250.
The acute and long-term toxicity of the pyrethrins differ
significantly. The acute toxicity of orally administered pyrethrins is
expressed as neurotoxic effects. The longer-term toxicity is based
principally on effects on the liver. Therefore, an acute reference
dose of 0.2 mg/kg bw was allocated for the tested blend of refined
pyrethrum, which was based on the NOAEL of 20 mg/kg bw for acute
neurotoxicity in rats and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effects
Mouse: 100 ppm, equal to 14 mg/kg bw per day (18-month study of
carcinogenicity)
Rat: 20 mg/kg (study of acute neurotoxicity)
100 ppm, equal to 4 mg/kg bw per day (2-year study of
carcinogenicity)
100 ppm, equivalent to 10 mg/kg bw per day (parental and
reproductive toxicity in a two-generation study of
reproductive toxicity)
75 mg/kg bw per day (maternal toxicity in two studies of
teratogenicity, no developmental toxicity in a study of
teratogenicity at the highest dose tested)
Rabbit: 25 mg/kg bw per day (maternal toxicity in a study of
teratogenicity, no developmental toxicity in a study of
teratogenicity at the highest dose tested)
Dog: 500 ppm, equal to 14 mg/kg bw per day (toxicity in a 1-year
study)
Estimate of acceptable daily intake for humans
0-0.04 mg/kg bw
Estimate of acute reference dose
0.2 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Gene mutation test in mammalian cells (required for submission to
WHO by 2001)
2. Mechanistic study on liver and thyroid tumorigenesis (see
Appendix 1; required for submission to WHO by 2001)
3. Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to pyrethrins
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Immediately (peak between 5 and 8 h) and nearly complete (> 90%)
in rats
Distribution Widely distributed in rats, highest concentrations in fat
Potential for accumulation None
Rate and extent of excretion Nearly complete excretion in urine (32-47% and 50-57% in male and
female rats) and in faeces
Metabolism in animals Extensively metabolized in rats, six metabolites identified; two
major metabolic pathways.
Toxicologically significant compounds Parent compound and metabolites
(animals, plants and environment)
Acute toxicity
Rat, LD50, oral > 1200 mg/kg bw
Rabbit, LD50, dermal > 2000 mg/kg bw
Rat, LC50, inhalation > 3.4 mg/L (4 h)
Dermal irritation Non, rabbits
Ocular irritation None, rabbits
Dermal sensitization Not a sensitizer (Buehler test in guinea-pigs)
Short-term toxicity
Target/critical effect Liver (mice, rat, dog), erythrocytes (rat, dog), kidney (rat)
Lowest relevant oral NOAEL 90 days, dog: 600 ppm (18 mg/kg bw per day)
Lowest relevant dermal NOAEL 3 weeks, rabbit: > 1000 mg/kg bw per day
Lowest relevant inhalation NOAEL 3 months, rat: 0.01 mg/L
Long-term toxicity and carcinogenicity
Target/critical effect Liver
Lowest relevant NOAEL/NOEL 2 years, rat: 100 ppm (4 mg/kg bw per day)
Carcinogenicity Increased tumour incidences in liver, thyroid, skin (rats) and
lungs (mice)
Genotoxicity In an incomplete range of studies, no genotoxic or mutagenic
potential identified
Reproductive toxicity
Reproductive target/critical effect Reproductive effects (reduced pup body weights) at parentally
toxic doses
Lowest relevant reproductive NOAEL Rat: 100 ppm (10 mg/kg bw per day)
Developmental target/critical effect No developmental effects at maternally toxic doses
Lowest relevant developmental NOAEL Rat: 75 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Acute clinical disorders and behavioural effects
Acute neurotoxic NOAEL Rat: 20 mg/kg bw
Other toxicological studies Induction of hepatic microsomal activity
Medical data Available human data do not show causal relationships between
exposure to modern pyrethrin-containing products and significant
adverse health effects.
Summary Value Study Safety factor
ADI 0-0.04 mg/kg bw Long-term toxicity, rats 100
Acute reference dose 0.2 mg/kg bw Acute neurotoxicity, rats 100
References
Bielucke, J. (1991) Primary eye irritation in rabbits (New Zealand
white). Unpublished Report, Project No. 91-7316A, MRID #41964802
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Casida, J.E. & Quistad, G.B. (1995) Metabolism and synergism of
pyrethrins. In: Casida, J.E. & Quistad, G.B., eds, Pyrethrum
Flowers: Production, Chemistry, Toxicology and Uses, Oxford:
Oxford University Press, pp. 258-277.
Class, T.J., Ando, T. & Casida, J.E. (1989) Pyrethroid metabolism;
microsomal oxidase metabolites of (S)-Bioallethrin and the six
natural pyrethrins. Unpublished report MRID #41248801 from
Pesticide Chemistry & Toxicology Laboratory. Submitted to WHO by
Kenya Pyrethrum Information Centre, Oberalm, Austria.
Curren, R.D. (1989) Unscheduled DNA synthesis assay in rat primary
hepatocytes with a confirmatory assay. Unpublished report,
laboratory study No. T8729.380009, MRID #41344501 from
Microbiological Associates, Inc. Submitted to WHO by Kenya
Pyrethrum Information Centre, Oberalm, Austria.
Feinberg, S.M. (1934) Pyrethrum sensitization. J. Am. Med. Assoc.,
102, 1557-1558.
Gabriel, D. (1991) Acute dermal toxicity in rabbits (New Zealand
white). Unpublished report, project No. 91-7316A, MRID #41964801
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Gabriel, D. (1992) Summary of results of acute oral toxicity study
Unpublished report, project No. 92-7529A, from Biosearch Inc.,
Philadelphia, Pennsylvania, USA. Submitted to WHO by Kenya
Pyrethrum Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1987) Evaluation of pyrethrum extract in a 2-week
dietary toxicity study in mice. Unpublished report, laboratory
project ID: 556-114 from International Research & Development
Corp. Submitted to WHO by Kenya Pyrethrum Information Centre,
Oberalm, Austria.
Goldenthal, E.I. (1988a) Evaluation of pyrethrum extract in a 13-week
dose range-finding study in mice. Unpublished report, laboratory
project ID: 556-008, MRID #433585201 from International Research
& Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1988b) Evaluation of pyrethrum extract in a 13-week
dose range-finding study in rats. Unpublished report, laboratory
project ID: 556-010 from International Research & Development
Corporation. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Goldenthal, E.I. (1988c) Toxicity study in dogs. Unpublished report,
laboratory project ID: 556-006 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Goldenthal, E.I. (1990a) Evaluation of pyrethrum extract in a 1-year
chronic toxicity study in dogs. Unpublished report, laboratory
project ID: 556-007, MRID #41496502 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Goldenthal, E.I. (1990b) Evaluation of pyrethrum extract in an
18-month dietary oncogenic study in mice. Unpublished report,
laboratory project ID: 556-013, MRID #41559401 from International
Research & Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1990c) Evaluation of pyrethrum extract in a two-year
dietary toxicity and oncogenicity study in rats. Unpublished
report, laboratory project ID: 556-011, MRID #41559501 from
International Research & Development Corp. Submitted to WHO by
Kenya Pyrethrum Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1992) 21-day repeated dose dermal toxicity study
with pyrethrum extract in rabbits. Laboratory project ID:
556-018, MRID #42212601 from International Research & Development
Corp. Submitted to WHO by Kenya Pyrethrum Information Centre,
Oberalm, Austria.
Hermansky, S.J. & Hurley, J.M. (1993a) Peroral (gavage) neurotoxicity
probe study with pyrethrins in rats (Sprague Dawley). Unpublished
report, laboratory project ID: 91N0122, MRID #42930401 from Bushy
Run Research Center. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Hermansky, S.J. & Hurley, J.M. (1993b) Acute oral neurotoxicity study
with pyrethrins in rats (Sprague Dawley). Unpublished report,
laboratory project ID: 92N1036, MRID #42925801 from Bushy Run
Research Center. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Hoffmann, G.M. (1991) Acute inhalation toxicity in rats (Sprague
Dawley). Unpublished report, project No. 91-8331, MRID #42008001
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Mitchell, J.C., Dupuis, G. & Towers, G.H.N. (1972) Allergic contact
dermatitis from pyrethrum (Chrysanthemum sp.). Br. J.
Dermatol., 86, 568-573.
Myer, J.R. (1991) Evaluation of pyrethrum extract in a 5-day toxicity
study in New Zealand white rabbits. Unpublished report,
laboratory project ID: 556-017 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Newton, P.E. (1992) 90-day inhalation toxicity study of pyrethrum
extract in the rat via whole body exposure. Unpublished report,
project No. 91-8335, MRID #42478201 from Bio/dynamics, Inc.
Submitted to WHO by Kenya Pyrethrum Information Centre, Oberalm,
Austria.
Putman, D.L. & Morris, M.J. (1989) Chromosome aberrations in Chinese
hamster ovary (CHO) cells. Unpublished report, laboratory study
No. T8729.337, MRID #41344601 from Microbiological Associates
Inc. Submitted to WHO by Kenya Pyrethrum Information Centre,
Oberalm, Austria.
Ramirez, M.A. (1930) Pyrethrum: An etiologic factor in vasomotor
rhinitis and asthma. J. Allergy, 1, 149-155.
Rickett, F.E., Tyszkiewicz, K. & Brown, N.C. (1972) Pyrethrum
dermatitis. Part I: The allergenic properties of various extracts
of pyrethrum flowers. Pyrethrum Post, 11, 85.
Romanelli, P. (1991a) Primary skin irritation in rabbits (New Zealand
white). Unpublished report, project No. 91-7316A, MRID #41964803
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Romanelli, P. (1991b) Sensitization study in guinea pig (Hartley
strain). Unpublished report, project No. 91-7316A, MRID #
41964804 from Biosearch Inc., Philadelphia, Pennsylvania, USA.
Submitted to WHO by Kenya Pyrethrum Information Centre, Oberalm,
Austria.
San, R.H.C. & Springfield, K.A. (1989) Salmonella/
mammalian-microsome plate incorporation mutagenicity assay (Ames
test) with a confirmatory assay. Unpublished report, laboratory
study No. T8729.501014, MRID #41344701 from Microbiological
Associates, Inc. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Schardein, J.L. (1987a) Evaluation of pyrethrum extract in a dose
range-finding teratology study in rats. Unpublished report,
laboratory project ID: 556-001, MRID #40603701 form International
Research & Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Schardein, J.L. (1987b) Evaluation of pyrethrum extract in definitive
rat teratology study. Unpublished report, laboratory project ID:
IRDC 556-002, MRID #40288202 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Schardein, J.L. (1987c) Evaluation of pyrethrum extract in a dose
range-finding teratology study in rabbits. Unpublished report.
laboratory project ID: 556-003, MRID #40603702 from International
Research & Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Schardein, J.L. (1987d) Evaluation of pyrethrum extract in a
definitive rabbit teratology study. Unpublished report,
laboratory project ID: IRDC 556-004, MRID #40288203 from
International Research & Development Corp. Submitted to WHO by
Kenya Pyrethrum Information Centre, Oberalm, Austria.
Schardein, J.L. (1989) Two generation reproduction study in rats with
pyrethrum extract. Unpublished report, laboratory project ID:
IRDC 556-005, MRID #41327501 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Selim, S. (1995) Pharmacokinetics and metabolism of 14C pyrethrin I
in the rat. Unpublished report, study No. P1092006, MRID
#43554304 from Biological Test Center. Submitted to WHO by Kenya
Pyrethrum Information Centre, Oberalm, Austria.
Springfield, A.C., Carlson, G.P. & DeFeo, J.J. (1973) Liver
enlargement and modification of hepatic microsomal drug
metabolism in rats by pyrethrum. Toxicol. Appl. Pharmacol., 24,
298-308.
Zucker, A. (1965) Investigation of purified pyrethrum extracts.
Ann. Allergy, 23, 335-339.
Appendix 1: Application of the Conceptual Framework for Cancer Risk
Assessment
(Revised on the basis of discussions at the IPCS Workshop on
Developing a Conceptual Framework for Cancer Risk Assessment, 16-18
February 1999, Lyon, France)
This framework, developed by an IPCS working group, provides a
generic approach to the principles commonly used in evaluating a
postulated mode of action for tumour induction by a chemical. Thus,
the framework was used by the 1999 JMPR to provide a structured
approach to the assessment of the overall weight-of-evidence for the
postulated mechanism of the increased incidences of benign tumours of
the liver, thyroid, and skin in rats and a treatment-related effect on
the incidence of lung tumours in mice observed after long-term
administration of pyrethrins.
The framework guidelines suggested 10 section headings. The
introduction (see monograph section 2(c), 'Long-term studies of
toxicity and carcinogenicity') describes the cancer end-points that
have been observed. Three of these (rat liver and thyroid neoplasms,
rat keratoacanthomas, and mouse lung tumours) are addressed separately
in the analysis. An approriate mode of action is postulated and the
key events identified; the observed dose-response relationships and
temporal relationships are discussed. The strength, consistency, and
specificity of the association of tumour response with key events and
the biological plausibility are analysed. Alternative modes of action
are identified and found not to be supported. The three postulated
modes of action are discussed below, with some uncertainties about
both the biology of tumour development and the database on the
compound and any inconsistencies in the method that were identified.
* The increased incidences of hepatocellular adenomas were associated
with persistent induction of cytochrome P450 enzymes and
hepatocellular hypertrophy, suggesting that pyrethrins are
rodent-specific hepatoproliferative carcinogens. Enzyme induction
leading to increased clearance of thyroid hormones would also be
consistent with the increased incidence of follicular hyperplasia
and follicular adenomas seen. The level of confidence in this
postulated mode of action is moderate, because some uncertainties
and inconsistencies were introduced by the method. Therefore,
additional studies to identify more key events in the liver and
thyroid (e.g. measurements of enzyme induction with purified
pyrethrins, estimation of thyroid hormone changes, and estimation
of thyroid and pituitary weights) are required.
* The higher incidence of keratoacanthomas in male rats at the high
dose, which exceeds the upper limit of the range in historical
controls, was considered to be related to treatment. The slight
increase in the incidence of cystic lesions in the skin and
subcutis in males observed macroscopically provided some support
that the increased incidence of neoplastic lesions is a result of
irritating chronic injury of the skin. There were some
uncertainties introduced by the method, and differing opinions on
the biology of these tumours were found in the literature. The
level of confidence in this postulated mode of action is very low,
and no further studies were identified to support it.
* The increased incidence of lung tumours in mice might be a result
of proliferative processes following chronic injury of the
respiratory epithelium or activation of microsomal mixed-function
enzymes, especially in Clara cells, which contain high
concentrations of P450 enzymes. Such chronic injury might be
followed by cell proliferation with a dose-response relationship.
The level of confidence in this postulated mode of action is,
however, very low.
There was also little confidence in other possible modes of action.
Uncertainties in the database on pyrethrins were identified.
Improvement of the method used in the study of carcinogenicity (e.g.
detailed histological sectioning of each lung lobe cut at the level of
bronchi and additional microscopic examination of step-sections of the
remaining lung tissue beginning at the level of the bronchi in all
animals of each sex, followed by a re-evaluation of all histological
slides in the absence of knowledge of their origin; reporting of the
number and size of neoplastic and preneoplastic lesions in each
animal) could serve to increase confidence that these tumours have no
relevance at the low concentrations to which humans and animals are
exposed.
The Meeting concluded that the increased tumour incidences
associated with exposure to pyrethrins are threshold phenomena of
negligible relevance to the low concentrations to which humans are
exposed and that pyrethrins have no genotoxic or mutagenic potential.
Therefore, no classification of cancer risk is necessary. However,
additional studies are required.
The discussion of the postulated mode of action of tumour induction
by pyrethrins was helpful in the overall process of hazard
characterization and risk assessment and contributed to consideration
of the relevance of the findings in animals to the human situation.
Application of the framework also promoted confidence in the
conclusions reached, as it represents use of a defined procedure which
mandates consistent documentation of the facts and reasoning that
includes consideration of inconsistencies and uncertainties. The
Meeting concluded that the framework could be developed for use both
by regulators and by researchers in identifying research needs on the
basis of clear delineation of data gaps and inconsistencies.
2. Toxicological studies
(a) Acute toxicity
The results of studies on the acute toxicity of pyrethrum
extracts are summarized in Table 1. The methods used in these studies
complied with the corresponding FIFRA guidelines and with GLP.
Pyrethrins have little acute toxicity in rats treated orally, with
LD50 values of 2400 mg/kg bw for males and 1000 mg/kg bw for
females. The ratio of pyrethrins I and II (1.44 or 2.50) has no effect
on the toxicity of pyrethrins in male or female rats. The signs of
toxicity included ruffled appearance and tremors within 4-24 h after
dosing. Post-mortem examination of animals that died showed
haemorrhagic lungs, tan-to-yellow fluid in the lower gastrointestinal
tract and muzzle, and genital staining. The no-effect level for
clinical signs was 710 mg/kg bw in males and 320 mg/kg bw in females.
Dermal exposure to 2000 mg/kg bw was tolerated by rabbits, which
showed very slight to well-defined erythema, very slight oedema, and
stained test sites at 24 h. All animals appeared normal through the
14-day observation period.
Very slight acute toxicitywas found in rats exposed to aerosols
by inhalation. On the basis of the mean analytical concentration of
active ingredient and the resultant mortality, the LC50 was
calculated to be 3.4 mg/L for the two sexes. Tremors were seen during
exposure to the two higher concentrations. The significant findings
post mortem included discoloured and oedematous respiratory tissues.
Application of pyrethrins to the skin of albino rabbits produced
a minimally irritating skin irritation score of 0.42 (Romanelli,
1991a).
Instillations of the extracts into the conjunctival sac produced
irritation in the eyes of albino rabbits, but no irritation was
observed by 72 h. No corneal opacity or iritis was seen during the
observation period (Bielucke, 1991).
In a study of dermal sensitization in guinea-pigs with a modified
Buehler protocol, pyrethrins were not sensitizing (Romanelli, 1991b).
(b) Short-term studies of toxicity
Mice
In order to define a suitable maximum tolerated dose for a longer
study, doses of 5000 and 7000 ppm were evaluated in a 2-week study.
The method used complied to a certain extent with OECD guideline 407,
and the study was conducted in compliance with GLP. One mouse at
7000 ppm died. There was no effect on body weight, but food
consumption was slightly decreased at 7000 ppm. Statistically
significant increases in the absolute and relative weights of the
liver were seen at both doses. On the basis of the results of this
Table 1. Acute toxicity of pyrethrins
Species Strain Sex Route LD50 or LC50 Purity (%) or Reference
(mg/kg bw or mg/L air) ratio of pyrethrin I:II
Rat Sprague-Dawley M Oral 2400 58% Gabriel (1992)
Rat Sprague-Dawley F Oral 1000 58% Gabriel (1992)
Rat Sprague-Dawley M Oral 3900 1.4 Gabriel (1992)
Rat Sprague-Dawley F Oral 1300 1.4 Gabriel (1992)
Rat Sprague-Dawley M Oral 3900 2.5 Gabriel (1992)
Rat Sprague-Dawley F Oral 1200 2.5 Gabriel (1992)
Rabbit New Zealand M&F Dermal > 2000 58% Gabriel (1991)
Rat Sprague-Dawley M&F Inhalation 3.4 58% Hoffmann (1991)
M, male; F, female
study, 7000 ppm was selected as the high dose for the long-term study
in mice (Goldenthal, 1987).
Pyrethrins were offered to groups of 15 Charles River mice of
each sex in the diet at concentrations of 300, 1000, 3000, or 10 000
ppm for 13 weeks, equal to 47, 160, 460, and 1600 mg/kg bw per day for
males and 56, 200, 580, and 1800 mg/kg bw per day for females. The
method used complied to a certain extent with OECD guideline 408, and
the study was conducted in compliance with GLP. Four males and 10
females at the original high dose of 30 000 ppm died on day 2, and all
animals at this dose had died or were killed in extremis by day 10.
Four males and two females at 10 000 ppm also died on day 2, with
clinical signs that included tremors, pale exposed skin, dilated
pupils, altered activity, laboured breathing, cold to touch,
moribundity, and hunched posture. Tremors and increased activity were
seen in several animals at 10 000 ppm during the first 2 weeks of
study. No treatment-related clinical signs were observed in the
animals at 300, 1000, or 3000 ppm. The group mean body weights and
food consumption were similar for all groups with surviving animals.
The absolute weight of the liver and the liver:body weight and the
liver:brain weight ratios were all statistically significantly
increased in males and females at 3000 and 10 000 ppm, whereas the
absolute weights of the liver in females at 300 and 1000 ppm were
comparable to those of controls. A treatment-related increase in the
incidence and/or severity of congestion in the liver was observed in
surviving male and female mice at 10 000 ppm, and an increased
incidence but only mild severity was found in < 15% of investigated
animals at 3000 ppm. An increased incidence of hepatocellular
hypertrophy was present in surviving male and female mice at 3000 and
10 000 ppm; at 1000 ppm, only 2 of 15 mice showed mild congestion of
the liver on macroscopic observation. The NOAEL was 1000 ppm, equal to
160 mg/kg bw per day (Goldenthal, 1988a).
Rats
Pyrethrins were offered to groups of 15 Charles River rats of
each sex for 13 weeks in the diet at concentrations of 300, 1000,
3000, 10 000, or 20 000 ppm, equal to 17, 57, 170, 590, and 1200 mg/kg
bw per day for males and 22, 74, 220, 710, and 1400 mg/kg bw per day
for females. The method used in this study complied to a certain
extent with OECD guideline 408, and the study was conducted in
compliance with GLP. During the first week of the study (days 3-7),
one female at 10 000 ppm, one male at 20 000 ppm, and 12 females at
20 000 ppm died; all other animals survived to scheduled sacrifice.
The signs seen before death in all animals that died included
decreased defaecation, increased respiration rate, tremors, and
decreased or increased activity. Convulsions were seen in some
animals. The signs in most animals at 10 000 and 20 000 ppm that
survived until the end of the study were decreased defaecation,
tremors, increased respiration rate, and increased activity.
Convulsions occurred mainly in males at 20 000 ppm. Most of the signs
were seen only during the first two weeks of the study. Statistically
significant decreases in mean body weights were observed during most
or all of the study in males and females at 10 000 and 20 000 ppm.,
and statistically significant decreases in mean food consumption were
seen during most or all of the study in females at 10 000 ppm and
males and females at 20 000 ppm when compared with the respective
control groups. Statistically significantly decreased mean values for
haematocrit and haemoglobin were found in males at 20 000 ppm and for
erythrocytes, haematocrit, and haemoglobin in females at 10 000 and
20 000 ppm. Females at 3000 ppm also showed a decreased mean
haemoglobin value. The treatment-related macroscopic findings
consisted of enlargement and congestion of the liver in both males and
females, but primarily in males, at 10 000 and 20 000 ppm; however,
the macroscopic observation could not be confirmed microscopically.
The liver weight was statistically significantly increased in males at
10 000 and 20 000 ppm and in females at 3000, 10 000, and 20 000 ppm
The weight of the kidney was increased in males and females at 3000,
10 000, and 20 000 ppm The only microscopic finding that was possibly
realted to treatment was small focal or multifocal areas of tubular
degeneration and regeneration in the renal cortex in animals at 3000
and 10 000 ppm, but primarily in males. The NOAEL was 1000 ppm, equal
to 57 mg/kg bw per day (Goldenthal, 1988b).
In a study designed to assess the toxic effects of pyrethrins
administered by whole-body inhalation as a liquid aerosol, groups of
15 Charles River rats of each sex were exposed for 6 h/day, generally
5 days per week, a minimum of 65 times to target concentrations of the
active ingredient corresponding to 0.010, 0.030, 0.10, and 0.35 mg/L.
The mean analytical concentrations to which the groups were exposed
were 0 (control), 0.038, 0.068, 0.23, and 0.83 mg/L, respectively.
Determinations of particle size distribution showed an overall mass
median aerodynamic diameter of 2.7 µm.The controls were exposed only
to conditioned room air on the same schedule. The study was conducted
in compliance with FIFRA Guideline 824 and with GLP.
Two animals at the highest dose died, one animal on day 3.
Because this death was accidental and occurred very early in the
study, this animal was replaced with another animal from the same
shipment. A second animal died on day 15, having shown laboured
breathing similar to that of other animals in this group, and its
death was considered to be potentially related to exposure.
Dose-related increases in the frequency of clinical signs were
observed in animals at the three higher doses during observations in
the chamber and at detailed weekly examinations. These signs included
secretory signs such as nasal discharge and dried material in the
facial area in both males and females. Animals at the highest dose
showed laboured breathing, excess lachrymation, tremors, increased
activity, and matted coats. There were no ocular effects. The absolute
body weights and body-weight gains of both males and females at the
two higher doses were decreased, and after 13 weeks of exposure the
body-weight gains of males at these doses were about 9% lower than
those of controls. In females, the body-weight gain after 13 weeks of
exposure was lower than that of controls, by 13% at 0.10 mg/L and 17%
at 0.35 mg/L. Food consumption was also slightly decreased during the
first 1 or 2 weeks in males and females in these groups.
Nonregenerative anaemia was observed in males at the three higher
doses and in females at the highest dose, with significant decreases
in haemoglobin, haematocrit, and erythrocyte values. An increased
leukocyte count was also seen in the females at the highest dose.
Significant differences in clinical chemical parameters were seen
primarily at the highest dose, including decreased total protein and
globulin and an increased albumin:globulin ratio in males and
decreased glucose in females. Other changes occurred sporadically and
were considered to be unrelated to exposure. Several significant
increases in organ weights or ratios were observed. The increases in
liver weight were clearly related to treatment. Increases in the
organ:body weight ratios of the kidneys and lung might be a reflection
of a marginal increase in absolute organ weights and the decreased
body weights. Morphological abnormalities in the larynx,
nasoturbinates, nasopharynx, and lungs observed by light microscopy
were considered to be localized responses indicative of a
treatment-related effect. The NOAEL for systemic effects was 0.01 mg/L
(Newton, 1992).
Rabbits
Pyrethins were administered dermally to five male and five female
New Zealand white rabbits in the form of a 25% (w/v) mixture in
vegetable oil at doses of 0, 100, 300, or 1000 mg/kg bw once daily, 5
days per week for 3 weeks. Animals in the vehicle control group were
given vegetable oil on the same regimen and at the same volume as the
group receiving the high dose . This study was conducted in compliance
with OECD Guideline 410 and GLP. One rabbit at 1000 mg/kg bw was
sacrificed in extremis on day 10 after showing emaciation, decreased
activity, and decreased defaecation. Macroscopic examination of this
animal did not reveal the cause of death. A low incidence of
desquamation and/or red raised areas on the skin at the application
site was observed in all groups, including the vehicle controls.
Several animals in the treated groups showed very slight to
well-defined erythema of the skin at the application site, but no
clear pattern with regard to treatment was seen for any of these
findings. Microscopic evaluation revealed no evidence of systemic
toxicity. The microscopic lesions at the application site included
acanthosis, haemorrhage, hyperkeratosis, and chronic inflammation,
although haemorrhage was observed only in the group given the vehicle
alone. Thus, all of the dermal reactions appeared to be due to the
vegetable oil. The NOAEL for systemic effects was 1000 mg/kg bw, the
highest dose tested (Goldenthal, 1992).
This result is supported by that of a previous study performed to
assess dermal irritation with pyrethrins at concentrations of 25%,
50%, and 75% w/v in corn oil on rabbits (Myer, 1991).
Dogs
Pyrethins were incorporated into the basal diet of groups of two
pure-bred beagle dogs of each sex at concentrations of 600, 1000,
3000, or 6000 ppm for 8 weeks, equal to 18, 30, 86, and 170 mg/kg bw
per day for males and 19, 29, 94, and 200 mg/kg bw per day for
females. The method used complied to a certain extent with OECD
guideline 409, and the study was conducted in compliance with GLP. One
male and both females at 6000 ppm died or were killed in extremis
during the study. The treatment-related clinical signs observed in
animals at this dose included inappetence, thin appearance, ataxia,
trembling, oily coat, impaired limb function, shallow breathing,
moribundity, and death. With the exception of moribundity and death,
similar signs were observed at 3000 ppm. Males and females at 6000 ppm
lost weight during the study, but the body-weight gains of animals in
all other treated groups were comparable to those of controls. The
average food consumption was decreased for males at 3000 ppm and for
males and females at 6000 ppm when compared with controls. Decreased
haematocrit, haemoglobin, and erythrocyte values were seen at the end
of the study in males at 3000 and 6000 ppm, but there were no other
treatment-related haematological finding. One male and one female at
6000 ppm killed in extremis had increased leukocyte counts and
decreased haematocrit, haemoglobin, and erythrocyte values. Slightly
decreased glucose, calcium, phosphorus, and cholesterol values were
found at the end of the study in males at 6000 ppm, and males and
females at 3000 ppm had slightly decreased cholesterol concentrations.
The aspartate and alanine aminotransferase activities of males at 6000
ppm were slightly increased at the end of dosing, and the surviving
male at this dose had a very high creatinine phosphokinase value.
There were no other treatment-related biochemical findings at the end
of the study. Males and females at 6000 ppm killed in extremis
showed some variation in electrolyte levels, and the urea nitrogen
concentration was increased in both dogs. The female showed large
increases in aspartate and alanine aminotransferase and creatine
phosphokinase activities. The absolute weights of the liver in both
males and females at 1000 and 3000 ppm were increased in a
treatment-related fashion, and the absolute weight of the testis at
these doses appeared to decrease in a similar manner. There were no
macroscopic or microscopic lesions attributable to the administration
of pyrethrins. The NOAEL was 600 ppm, equal to 18 mg/kg bw per day
(Goldenthal, 1988c).
Pyrethrins were administered to groups of four beagle dogs of
each sex for 52 weeks in the diet at concentrations of 0, 100, 500, or
2500 ppm, equal to 2.6, 14, and 66 mg/kg bw per day for males and 2.8,
14, and 75 mg/kg bw per day for females. Four dogs of each sex were
evaluated at each dietary concentration. This study was conducted in
compliance with FIFRA Guideline 83-1 and with GLP. All animals
survived to the end of the study, and no remarkable clinical signs of
toxicity were found at any dose. Overall, the mean body weights of the
treated animals were similar to those of controls. The mean food
consumption of males at 2500 ppm and of females at 500 and 2500(c)
ppm was lower than that of controls during the first week of the
study, but consumption was similar for the remainder of the study,
except that males at 500 ppm consumed greater amounts of food than the
controls. Increased total leukocyte and segmented neutrophil counts
were found in females and decreased erythrocyte, haemoglobin, and
haematocrit values in males at 2500 ppm. Alanine aminotransferase
activity was statistically significantly increased in females at 2500
ppm at both the 6- and 12-month evaluations. The slight increase in
the activity of this enzyme in males was not statistically
significant. No treatment-related changes in urinary parameters were
seen at either interval. The relative and absolute weights of the
liver were significantly increased in males at 2500 ppm when compared
with controls, but no statistically significant differences in organ
weights were seen in females at this dose. No macroscopic or
microscopic treatment-related changes were observed in tissues. The
NOAEL was 500 ppm, equal to 14 mg/kg bw per day (Goldenthal, 1990a).
(c) Long-term tudies of toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female Charles River CD-1 mice received
diets containing pyrethrins at concentrations of 100, 2500, or 5000
ppm for 18 months, equal to doses of 14, 350, and 690 mg/kg bw per day
for males and 17, 410, and 830 mg/kg bw per day for females. This
study was conducted in compliance with FIFRA Guideline 83-2(b) and
with GLP. One male and one female at 5000 ppm were found dead during
the first week of the study, but no other treatment-related deaths
occurred, and survival was similar in the control and treated groups
throughout the study. All animals at 5000 ppm exhibited increased
activity when stimulated during the first week of the study but not
later. There were no other clinical signs seen that differentiated the
treated from the control groups. Statistically significant differences
in mean body weight and food consumption were seen between control and
treated groups sporadically throughout the study, but none of the
differences was considered to be related to treatment. No
treatment-related effect was observed in other parameters examined at
12 and 18 months of study. At necropsy, discoloured, dark livers were
more common in males at 5000 ppm and in females at 2500 and 5000 ppm,
and treatment-related increases in the absolute and relative weights
of the liver were seen in males and females at 2500 and 5000 ppm.
Microscopically, vacuolar fatty change was found in the livers of
males at these doses, and this change was considered to be related to
treatment. The dark discolouration seen macroscopically could not be
explained by the microscopic findings. The incidence of nodules and
masses in the lungs appeared to be slightly increased in animals at
5000 ppm. When the lungs were examined microscopically according the
original test protocol, the incidences of alveolar bronchiolar
adenomas were increased in females at 5000 ppm, and the increase was
statistically significant, exceeding the range in historical controls.
Treated males showed an apparent, not clearly dose-related increase in
the incidence of alveolar bronchiolar carcinomas which exceeded the
upper limit of 95% of the historical controls (Table 2). The sponsor
asked the testing laboratory to conduct serial sectioning of the
remaining lung tissue of female mice in the control and highest-dose
groups, which did not have diagnoses of lung tumours in the original
examination. As this was considered not to be an acceptable
toxicopathological practice, the results of the first evaluation were
taken into consideration for the risk assessment, and the increased
incidence of lung tumours was considered to be a treatment-related
effect. The NOAEL was 100 ppm, equal to 14 mg/kg bw per day
(Goldenthal, 1990b).
Table 2. Comparison of first and second evaluations of microscopic
neoplastic lesions in the lung of mice treated with
pyrethrins for 18 months
Sex Dose No. of lungs Alveolar bronchiolar neoplasm
(ppm) examined
Adenoma Carcinoma
Male 0 60 14 0a
0 60 16 0
100 60 15 1
2500 60 13 3b,c
5000 60 17 3b,c
Female 0 60 8 1
0 60 4 3
100 60 11 0
2500 60 5 2
5000 60 19c,d 2
a p < 0.05; Cochran trend test
b Significant differences in pair-wise comparison of the
high-dose group with controls at p < 0.05
c > 95% of of the upper range of historical controls
d p < 0.05; Fisher exact test
Rats
Groups of 60 Charles River CD rats of each sex received diets
containing pyrethrins at concentrations of 100, 1000, or 3000 ppm for
104 weeks, equal to 4, 43, and 130 mg/kg bw per day for males and 5,
56, and 170 mg/kg bw per day for females. This study was conducted in
compliance with FIFRA Guideline 83-5 and with GLP. Survival was
similar in the treated and control groups, and there were no clinical
findings attributable to treatment. Statistically significant
decreases in body weight which were considered to be related to
treatment were observed during the first 78 weeks of the study in both
male and female rats at 3000 ppm, with a difference from controls of
7% in males and 10% in females. A slight, treatment-related decrease
in food consumption was seen at the same time. No treatment-related
ophthalmological findings or organ weight changes were detected during
the study, and no haematological or urological changes were found. The
activities of serum transaminases were substantially increased at most
intervals of analysis in males at 3000 ppm, most of the values
reaching statistical significance. Increased incidences of benign
tumours of the liver, thyroid, and skin were also observed (Table 3),
and a statistically significantly higher incidence of hepatocellular
adenomas was described in females at the high dose. Follicular
adenomas and carcinomas were initially seen in the thyroid glands of
rats at the high dose, which appeared to be related to treatment, but
during a re-evaluation some of the carcinomas were reclassified as
adenomas and some adenomas were reclassified as hyperplasia. After the
re-evaluation, the incidence of hyperplasia was found to be enhanced
in males and females, and the incidence of follicular adenomas was
statistically significantly increased only in females at 3000 ppm.
Nevertheless, the tumour incidences in animals of each sex were higher
than the upper range seen in historical controls. The results of a
further, full histopathological peer review confirmed these increased
tumour incidences. Macroscopic examination of the skin showed a slight
increase in the incidence of cystic lesions in the skin and subcutis,
and the microscopic assessment showed an apparently higher incidence
of keratoacanthomas in males at the high dose, which was statistically
significant in comparison with both control groups. A peer review of
pathological lesions in all male rats resulted in removal of several
keratoacanthomas from the table of incidence in treated groups but
confirmed that the incidence of this lesion clearly exceeded the upper
limit of the range in historical controls (1.4-10%). The increased
incidences of liver and thyroid tumours and of keratoacanthomas of the
skin were considered to be treatment-related effects but to be
threshold phenomena of negligible relevance to the low doses to which
humans are exposed. The NOAEL was 100 ppm, equal to 4 mg/kg bw per day
(Goldenthal, 1990c).
(d) Genotoxicity
The results of tests for the genotoxicity of pyrethrins in
vitro are summarized in Table 4.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a two-generation study, groups of 28 male and 28 female
Charles River rats received diets containing pyrethrins at
concentrations of 100, 1000, or 3000 ppm, equivalent to 10, 100, and
300 mg/kg bw per day. A control group received the basal laboratory
diet on an identical regimen. The F0 parental generation were
treated for a minimum of 77 days before the first of two matings. The
same numbers of weanlings from the F1b litters were selected
randomly to become parents of the F1 generation and were treated for
a minimum of 95 days before being mated twice to produce the F2a and
F2b litters. This study was conducted in compliance with FIFRA
Guideline 83-4 and with GLP. No treatment-related effects were noted
with respect to clinical signs, body weights, or food consumption in
Table 3. Incidences of microscopic neoplastic lesions in rats fed diets containing pyrethrins for 104 weeks
Sex Dose Liver Thyroid Skin
(ppm)
Total Hepatocellular tumours Total Hyperplasia Follicular tumours Total Cystic Keratoacanthomasb
examined examined examined lesions
Adenoma Carcinoma Adenomaa Carcinomaa
Male 0 60 6 1 60 2 2c 0 60 5 4
0 60 1 0 60 0 1 1 60 2 5
100 60 0 0 60 2 4 1 24 1 4
1000 60 3 0 59 5 5d 2 14 5 4
3000 60 3 1 60 7 5d 2 60 9 11d,e
Female 0 60 0 1 60 0 0 1 60 0 0
0 60 1 0 60 2 1 1 59 3 0
100 60 0 0 60 1 2 0 8 2 1
1000 60 1 0 60 1 3d 0 8 2 1
3000 60 5d.f 0 60 5 5d 1 60 0 0
a Results of the histopathological peer review
b Results of the re-analysis only in males
c p < 0.05; Cochran trend test
d Greater that the upper limit of historical control data
e Significant difference in the pair-wise comparison of the high-dose group with the controls at p < 0.05
f p < 0.05; Fisher exact test
Table 4. Results of assays for the genotoxicity of pyrethrins in vitro
Test system Test object Concentration Purity Results Reference
(%)
Reverse mutationa S. typhimurium TA98, 8.8-8772 µg/plate in 58 Negative ± S9 San & Springfield
TA100, TA1535, TA1537, acetone (1989)
TA1538
Chromosomal Chinese hamster ovary 0.02-0.32 Fl/ml + S9 58 Negative ± S9 Putman & Morris
aberrationa cells 0.005-0.08 Fl/ml - S9 (1989)
in DMSO
Unscheduled DNA Rat primary hepatocytes 0.0-1.0 Fl/ml in 58 Negative Curren (1989)
synthesis acetone
a Study conducted in compliance with GLP and to a certain extent with OECD guidelines
the parental rats of the F0 generation, but the body weights and
food consumption of the parental rats of the F1 generation were
significantly reduced at 3000 ppm and sporadically reduced at 1000
ppm. These reductions were considered to be treatment-related.
Treatment at 3000 ppm resulted in significantly reduced body weights
at birth for F1a males and F2a pups of each sex and during
lactation for the male and female offspring of both matings of both
generations. The mean body weights of pups at 1000 ppm were also lower
than those of controls for male F2a pups at birth and for F1b and
F2a pups during lactation. Reproductive performance and other litter
parameters were not affected by the treated diet at any dose. The
NOAEL for parental and reproductive toxicity was 100 ppm, equivalent
to 10 mg/kg bw per day (Schardein, 1989).
(ii) Developmental toxicity
Rats
Groups of five mated female Charles River rats were used in a
range-finding study to determine the doses of pyrethrin to be used in
a later study. Doses of 37.5, 75, 150, 300, or 600 mg/kg bw per day
were administered orally by gavage on days 6-15 of gestation at a
volume of 3 ml/kg. The control group received only the vehicle, 0.5%
methylcellulose, on a comparable regimen. Uterine examinations were
performed on all surviving females on day 20 of gestation. The method
used in this study complied to a certain extent with OECD Guideline
414, and the study was conducted in compliance with GLP.
Treatment-related maternal toxicity, observed as deaths, convulsions,
and/or tremors, occurred at 150, 300, and 600 mg/kg bw per day. No
treatment-related maternal deaths occurred at 75 mg/kg bw per day,
although tremors were observed in this group. No treatment-related
clinical signs were observed at 37.5 mg/kg bw per day. On the basis of
these results, doses of 5, 25, and 75 mg/kg bw per day were selected
for use in the following study (Schardein, 1987a).
Groups of 25 mated female Charles River rats were given
pyrethrins suspended in 0.5% methylcellulose orally by gavage at doses
of 5, 25, or 75 mg/kg bw per day on days 6-15 of gestation at a volume
of 3 ml/kg. The control group received the vehicle only on a
comparable regimen. On day 20 of gestation, the fetuses were removed
surgically for evaluation. The method used in this study complied to a
certain extent with OECD Guideline 414, and the study was conducted in
compliance with GLP. No animals died or were killed in extremis
during the study, and no treatment-related clinical signs were
observed. The body-weight gains of the treated groups were comparable
to those of the controls during treatment. No evidence of fetotoxicity
was found, and morphological examination revealed no teratogenic
effects at any dose tested. The NOAEL for maternal toxicity was 75
mg/kg bw per day, and that for developmental toxicity was 75 mg/kg bw
per day, the highest dose tested (Schardein, 1987b).
Rabbits
Groups of five inseminated female New Zealand white SPF rabbits
were used in a range-finding study to determine the doses of
pyrethrins for use in a later study. Doses of 37.5, 75, 150, 300, or
600 mg/kg bw per day were administered orally by gavage on days 7-19
of gestation at a volume of 3 ml/kg. The control group received only
the vehicle, 0.5% methylcellulose, on a comparable regimen. Uterine
examinations were performed on all surviving females on day 29 of
gestation. The method used complied to a certain extent with OECD
Guideline 414, and the study was conducted in compliance with GLP.
Treatment-related maternal toxicity, seen as deaths, tremors,
convulsions, and weight loss, and fetal toxicity, seen as high
postimplantation loss, were found at 600 mg/kg bw per day. Maternal
toxicity, in terms of weight loss during treatment and tremors was
seen at 300 mg/kg bw per day. No clear treatment-related effects were
observed at 37.5, 75, or 150 mg/kg bw per day. On the basis of these
results, doses of 25, 100, and 250 mg/kg bw per day were selected for
use in the following study (Schardein, 1987c).
Groups of 16 inseminated female New Zealand white SPF rabbits
were randomly assigned to receive pyrethrins at doses of 25, 100, or
250 mg/kg bw per day orally by gavage on days 7-19 of gestation at a
volume of 3 ml/kg. The control group received only the vehicle, 0.5%
methylcellulose, on a comparable regimen. On day 29 of gestation, the
fetuses were removed surgically for evaluation. The study was
conducted in compliance with OECD Guideline 414 and with GLP. All
animals survived to the end of treatment. One doe at the high dose
aborted near term, on day 28 of gestation, after showing decreased or
absent defaecation on several days previously. No gross lesions were
present at necropsy. Excessive salivation, arched head, and/or
laboured breathing were observed in a few females at the high dose on
days 18-19 of gestation. One female at the intermediate dose had
excessive salivation and arched head on gestation day 19. No apparent
treatment-related clinical signs were seen at the low dose. There were
no treatment-related gross pathological changes in any of the animals
at the end of the study. Body-weight loss was seen in does at the high
dose throughout treatment, and slightly reduced body-weight gain
relative to the control value was observed in animals at the
intermediate dose. The body-weight gains of treated groups were
comparable to that of the control group throughout gestation (days
0-29). When the body weights on day 29 were adjusted by subtracting
the uterine weights to reflect only maternal weight change, mean
weight losses were evident in all groups, including the control, over
the entire gestation period. There were no biologically meaningful or
statistically significant differences in the mean numbers of viable
fetuses, postimplantation losses, total implantations, or corpora
lutea and in fetal body weight or fetal sex distribution in the
treated groups in comparison with the control values. One doe at the
high dose resorbed its litter, but it is not clear if this finding was
related to treatment. There was no treatment-related or statistically
significant difference in the incidence of fetal malformations or
variations. The NOAEL for maternal toxicity was 25 mg/kg bw per day
and that for developmental toxicity was 250 mg/kg bw per day, the
highest dose tested (Schardein, 1987d).
(f) Special studies
(i) Effects on the central nervous system
Rats
Male Sprague-Dawley rats were treated once by oral gavage with a
10% or 25% solution of pyrethrins in corn oil at doses of 0, 40, 100,
200, 400, 800, 1400, or 2000 mg/kg bw, and females received a 2.5, 5,
or 10% solution of pyrethrins in corn oil at doses of 0, 25, 50, 100,
150, 200, 400, or 800 mg/kg bw. This study was conducted in compliance
with FIFRA Guideline 81-8 and with GLP. The clinical signs were
characterized by mild-to-severe tremors. On the basis of the
occurrence, severity, and onset of these reactions, a solution of 10%
pyrethrins in corn oil and doses of 40, 125, and 400 mg/kg bw were
selected for the study of acute neurotoxicity in males and a solution
of 5% pyrethrins in corn oil and doses of 20, 63, and 200 mg/kg bw in
females (Hermansky & Hurley, 1993a).
In the main study, groups of 15 male Sprague-Dawley rats received
by gavage a 10% w/v solution of pyrethrins in corn oil at doses of 0,
40, 125, or 400 mg/kg bw, and the same numbers of females received a
5% w/v solution of pyrethrins in corn oil at doses of 0, 20, 63, or
200 mg/kg bw. This study was conducted in compliance with FIFRA
Guideline 81-8 and with GLP. Five males and two females at the high
dose died on the day of treatment, and a variety of acute neurological
signs were observed in the other animals at this dose, including
tremors, urogenital area wetness, salivation, perinasal encrustation,
exaggerated startle response, decreased grip strength, hind leg splay,
and increased body temperature. Tremors were also observed in three
females at the intermediate dose. Measurements of motor activity on
the day of treatment indicated increased fine movement and decreased
rearing and ambulation in animals of each sex at the high dose and
decreased fine movement, rearing, and ambulation in males at the
intermediate dose. In addition, slight, statistically nonsignificant
decreases in body weight were seen in males at the high dose on days 7
and 14. There was no evidence of tany gross, treatment-related lesion.
The microscopic changes were limited mainly to sections of the sciatic
nerve and its branches. The histomorphological changes within the
peripheral nerve sections indicated the presence of scattered
degenerating nerve fibres or myelin sheaths. These changes were seen
in only a few animals, were graded as minimal, and were not
dose-related. The NOAEL was 20 mg/kg bw (Hermansky & Hurley, 1993b).
(ii) Effects on hepatic microsomal enzymes
Rats
Oral administration of pyrethrins to male rats at 85, 200, or 500
mg/kg bw per day for 3 weeks resulted in liver enlargement and
decreased hepatic DNA concentrations. Significantly decreased
hexobarbital-induced hypnosis without concomitant changes in
barbital-induced hypnosis suggested an alteration in hepatic drug
metabolism. The activities of hepatic microsomal enzymes responsible
for detoxification of
O-ethyl- O-(4-nitrophenyl)phenyl-phosphonothioate,
para-nitroanisole demethylation, and hexobarbital oxidation were
increased at 200 mg/kg bw per day to 150, 173, and 241% of the control
values, respectively. Increased liver weight, the detoxification of
pyrethrins, and demethylation of para-nitroanisole were found to be
dose-related. Small increases in enzyme activities were observed when
the lowest dose was given for 15 days. At 500 mg/kg bw per day, the
liver weight and enzyme activities were increased up to 17 days of
treatment but returned to the control level within 7 days after
cessation of treatment. NADPH cytochrome c reductase activity and the
cytochrome P450 concentration were also increased. It was suggested
that pyrethrins caused induction of microsomal enzymes. The LOAEL was
85 mg/kg bw per day (Springfield et al., 1973).
3. Observations in humans
The main adverse effects seen after exposure to pyrethrum
extracts in older studies were those manifesting as either skin or
respiratory reactions. Much of the research performed to date has
focused on the dermatological effects of pyrethrins, although mention
has been made of the respiratory reactions that have often accompanied
those of the skin. Studies by Ramirez (1930) and Feinberg (1934), in
which the association between sensitivity to ragweed and to pyrethrins
was delineated, contributed much to the understanding of allergic
responses to pyrethrins. Several investigations have since been
undertaken to isolate and characterize the allergen responsible for
the dermal reactions. Mitchell et al. (1972) isolated a sesquiterpene
lactone, pyrethrosin, from pyrethrins which induced positive dermal
responses in humans given a patch test. None of the other fractions
induced reactions, with the exception of pyrethrins II, which elicited
a weak response. Zucker (1965) demonstrated that a dermal reaction to
unrefined pyrethrum extract does not result in allergy to refined
pyrethrins. The previous evaluation by the Joint Meeting in 1970 cited
an unpublished report in which 200 people were given patch tests with
a 1% water dispersion of pyrethrins, and no evidence of primary
irritation or of sensitization was found. Rickett et al. (1972)
concluded that the refined extracts that have been marketed since 1957
do not induce skin allergies when tested on sensitive subjects and
that the dermal effects reported in the early literature are not
relevant to an assessment of refined pyrethrins.
Case reports of adverse respiratory effects, such as
anaphylactoid reactions and asthma, attributed to pyrethrins indicate
that these responses often occur in individuals with a history of
asthma. Although investigations of the dermal effects of pyrethrum
extracts suggests that pyrethrins are not the causative agent, no
thorough investigation of the agent(s) responsible for the adverse
respiratory responses has been conducted.
Comments
Absorption, distribution, and excretion in rats were investigated
only for pyrethrins I. After oral administration, more than 90% of a
low dose of pyrethrins I was absorbed, and the concentration of
radiolabel in blood peaked between 5 and 8 h. The radiolabelled
residues were widely distributed in the organs analysed, with the
highest concentrations in fat in females. The elimination half-time of
pyrethrins I in males and females was approximately 6 h. The mean
percentage of administered radiolabel found in the urine ranged from
32 to 47% in males and from 50 to 57% in females, the remainder being
excreted in faeces.
The substance is extensively metabolized, the residues of the
parent compound in faeces and urine representing only 10%. Six
metabolites were identified and two major metabolic pathways were
suggested, the first involving oxidation of the double-bond and/or the
methyl groups and the second involving hydrolysis of the ester bond.
Pyrethrins I are metabolized mainly through oxidative processes, while
pyrethrins II are metabolized through a combination of hydrolytic and
oxidative processes.
Pyrethrins show little acute toxicity, with an oral LD50 in
rats of > 1200 mg/kg bw and NOAELs for clinical signs of 710 mg/kg bw
for males and 320 mg/kg bw for females, a dermal LD50 in rabbits of
> 2000 mg/kg bw, and an inhalation LC50 in rats of 3.4 mg/L. The
compounds are minimally irritating to the skin and eye and show no
potential for skin sensitization. Pyrethrum extracts have not been
classified by WHO for acute toxicity.
In short-term tests for toxicity in mice, rats, and dogs, the
lowest relevant NOAELs after oral administration were 1000, 1000, and
600 ppm, equal to 160, 57, and 18 mg/kg bw per day, respectively, for
the three species. Statistically significant decreases in mean body
weight or body-weight gain were observed at the high doses throughout
most or all of the studies.
The liver is the main target organ in mice, rats, and dogs, and
an increased liver weight was frequently accompanied by changes in
serum transaminase activity. In mice, increased liver weights were
associated with a higher incidence of hepatocellular hypertrophy. In
the livers of rats and dogs, generally unremarkable histopathological
changes were observed. At doses of 85 mg/kg bw per day and above, a
pyrethrum extract containing 20% pyrethrins induced microsomal enzymes
in rats. Furthermore, anaemia was observed in rats and dogs at doses
of 3000 ppm and above. The kidney was another target, but only in
rats. In a 13-week study, rats at doses greater than 1000 ppm had
increased kidney weights associated with tubular degeneration and
regeneration in the renal cortex.
In a 13-week study in rats exposed by inhalation, the NOAEL for
systemic toxicity was 0.011 mg/L. The increases in liver weight were
clearly related to exposure and were accompanied by changes in serum
transaminase activity. Nonregenerative anaemia was also observed. The
weights of the kidney and lung were increased in relation to body
weight. The morphological abnormalities observed in the larynx,
nasoturbinates, nasopharynx and lungs by light microscopy were
considered to be localized responses indicative of a treatment-related
effect.
Dermal administration of pyrethrins at doses up to 1000 mg/kg bw
per day for 21 days caused no systemic toxicity in rabbits.
In a two-year study of toxicity and carcinogenicity in rats and
an 18-month study of carcinogenicity in mice, the NOAEL was 100 ppm in
both species, equal to 14 and 4 mg/kg bw per day in mice and rats,
respectively. The liver was the main target. A treatment-related
effect on the incidence of lung tumours was seen in mice and increased
incidences of benign tumours of the skin, liver, and thyroid were
observed in rats. The increased incidences of hepatocellular adenomas
were associated with persistent induction of cytochrome P450 enzymes
and hepatocellular hypertrophy, suggesting that pyrethrins are
rodent-specific hepatoproliferative carcinogens. Enzyme induction
leading to increased clearance of thyroid hormones would also be
consistent with the higher incidence of follicular hyperplasia and
follicular adenomas. However, additional studies on the mechanism of
formation of the liver and thyroid tumours are required. The Meeting
concluded that the increased tumour incidences caused by pyrethrins
are threshold phenomena of negligible relevance to the low doses to
which humans are exposed (see Appendix 1 to this monograph addendum).
Pyrethrins did not induce reverse mutagenicity in Salmonella
typhimurium with metabolic activation, did not induce chromosomal
aberration in Chinese hamster ovary cells, and did not induce
unscheduled DNA synthesis in rat primary hepatocytes. The Meeting
concluded that pyrethrins have no genotoxic or mutagenic potential,
but a test for gene mutation in mammalian cells is lacking.
Pyrethrins did not show developmental toxicity in rats or rabbits
at the highest maternally toxic doses tested, which were 75 and 250
mg/kg bw per day, respectively. The only effects on the offspring,
observed in a two-generation study of reproductive toxicity in rats,
were reduced body weights at the parentally toxic doses of 1000 and
3000 ppm, with a NOAEL of 100 ppm, equivalent to 10 mg/kg bw per day.
In a study of neurotoxicity in rats given single oral doses,
acute neurological disorders (tremors, wetness of the urogenital area,
salivation, perinasal encrustation, exaggerated startle response,
decreased grip strength, and hind-leg splay) and behavioural effects
(increased motor activity and decreased rearing and ambulation) were
noted, with a NOAEL of 20 mg/kg bw.
The available data on humans did not show a causal relationship
between exposure to modern pyrethrin-containing products and
significant adverse health effects.
An ADI of 0-0.04 mg/kg bw was established for the tested blend of
refined pyrethrum extract, which was based on the NOAEL of 100 ppm,
equal to 4 mg/kg bw per day, observed in the long-term study of
toxicity and carcinogenicity in rats and a safety factor of 100. This
figure is identical to the ADI derived by the 1972 Meeting, which was
based on a NOAEL of 200 ppm, equivalent to 10 mg/kg bw per day, in a
long-term study in rats and a safety factor of 250.
The acute and long-term toxicity of the pyrethrins differ
significantly. The acute toxicity of orally administered pyrethrins is
expressed as neurotoxic effects. The longer-term toxicity is based
principally on effects on the liver. Therefore, an acute reference
dose of 0.2 mg/kg bw was allocated for the tested blend of refined
pyrethrum, which was based on the NOAEL of 20 mg/kg bw for acute
neurotoxicity in rats and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effects
Mouse: 100 ppm, equal to 14 mg/kg bw per day (18-month study of
carcinogenicity)
Rat: 20 mg/kg (study of acute neurotoxicity)
100 ppm, equal to 4 mg/kg bw per day (2-year study of
carcinogenicity)
100 ppm, equivalent to 10 mg/kg bw per day (parental and
reproductive toxicity in a two-generation study of
reproductive toxicity)
75 mg/kg bw per day (maternal toxicity in two studies of
teratogenicity, no developmental toxicity in a study of
teratogenicity at the highest dose tested)
Rabbit: 25 mg/kg bw per day (maternal toxicity in a study of
teratogenicity, no developmental toxicity in a study of
teratogenicity at the highest dose tested)
Dog: 500 ppm, equal to 14 mg/kg bw per day (toxicity in a 1-year
study)
Estimate of acceptable daily intake for humans
0-0.04 mg/kg bw
Estimate of acute reference dose
0.2 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Gene mutation test in mammalian cells (required for submission to
WHO by 2001)
2. Mechanistic study on liver and thyroid tumorigenesis (see
Appendix 1; required for submission to WHO by 2001)
3. Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to pyrethrins
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Immediately (peak between 5 and 8 h) and nearly complete (> 90%)
in rats
Distribution Widely distributed in rats, highest concentrations in fat
Potential for accumulation None
Rate and extent of excretion Nearly complete excretion in urine (32-47% and 50-57% in male and
female rats) and in faeces
Metabolism in animals Extensively metabolized in rats, six metabolites identified; two
major metabolic pathways.
Toxicologically significant compounds Parent compound and metabolites
(animals, plants and environment)
Acute toxicity
Rat, LD50, oral > 1200 mg/kg bw
Rabbit, LD50, dermal > 2000 mg/kg bw
Rat, LC50, inhalation > 3.4 mg/L (4 h)
Dermal irritation Non, rabbits
Ocular irritation None, rabbits
Dermal sensitization Not a sensitizer (Buehler test in guinea-pigs)
Short-term toxicity
Target/critical effect Liver (mice, rat, dog), erythrocytes (rat, dog), kidney (rat)
Lowest relevant oral NOAEL 90 days, dog: 600 ppm (18 mg/kg bw per day)
Lowest relevant dermal NOAEL 3 weeks, rabbit: > 1000 mg/kg bw per day
Lowest relevant inhalation NOAEL 3 months, rat: 0.01 mg/L
Long-term toxicity and carcinogenicity
Target/critical effect Liver
Lowest relevant NOAEL/NOEL 2 years, rat: 100 ppm (4 mg/kg bw per day)
Carcinogenicity Increased tumour incidences in liver, thyroid, skin (rats) and
lungs (mice)
Genotoxicity In an incomplete range of studies, no genotoxic or mutagenic
potential identified
Reproductive toxicity
Reproductive target/critical effect Reproductive effects (reduced pup body weights) at parentally
toxic doses
Lowest relevant reproductive NOAEL Rat: 100 ppm (10 mg/kg bw per day)
Developmental target/critical effect No developmental effects at maternally toxic doses
Lowest relevant developmental NOAEL Rat: 75 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Acute clinical disorders and behavioural effects
Acute neurotoxic NOAEL Rat: 20 mg/kg bw
Other toxicological studies Induction of hepatic microsomal activity
Medical data Available human data do not show causal relationships between
exposure to modern pyrethrin-containing products and significant
adverse health effects.
Summary Value Study Safety factor
ADI 0-0.04 mg/kg bw Long-term toxicity, rats 100
Acute reference dose 0.2 mg/kg bw Acute neurotoxicity, rats 100
References
Bielucke, J. (1991) Primary eye irritation in rabbits (New Zealand
white). Unpublished Report, Project No. 91-7316A, MRID #41964802
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Casida, J.E. & Quistad, G.B. (1995) Metabolism and synergism of
pyrethrins. In: Casida, J.E. & Quistad, G.B., eds, Pyrethrum
Flowers: Production, Chemistry, Toxicology and Uses, Oxford:
Oxford University Press, pp. 258-277.
Class, T.J., Ando, T. & Casida, J.E. (1989) Pyrethroid metabolism;
microsomal oxidase metabolites of (S)-Bioallethrin and the six
natural pyrethrins. Unpublished report MRID #41248801 from
Pesticide Chemistry & Toxicology Laboratory. Submitted to WHO by
Kenya Pyrethrum Information Centre, Oberalm, Austria.
Curren, R.D. (1989) Unscheduled DNA synthesis assay in rat primary
hepatocytes with a confirmatory assay. Unpublished report,
laboratory study No. T8729.380009, MRID #41344501 from
Microbiological Associates, Inc. Submitted to WHO by Kenya
Pyrethrum Information Centre, Oberalm, Austria.
Feinberg, S.M. (1934) Pyrethrum sensitization. J. Am. Med. Assoc.,
102, 1557-1558.
Gabriel, D. (1991) Acute dermal toxicity in rabbits (New Zealand
white). Unpublished report, project No. 91-7316A, MRID #41964801
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Gabriel, D. (1992) Summary of results of acute oral toxicity study
Unpublished report, project No. 92-7529A, from Biosearch Inc.,
Philadelphia, Pennsylvania, USA. Submitted to WHO by Kenya
Pyrethrum Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1987) Evaluation of pyrethrum extract in a 2-week
dietary toxicity study in mice. Unpublished report, laboratory
project ID: 556-114 from International Research & Development
Corp. Submitted to WHO by Kenya Pyrethrum Information Centre,
Oberalm, Austria.
Goldenthal, E.I. (1988a) Evaluation of pyrethrum extract in a 13-week
dose range-finding study in mice. Unpublished report, laboratory
project ID: 556-008, MRID #433585201 from International Research
& Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1988b) Evaluation of pyrethrum extract in a 13-week
dose range-finding study in rats. Unpublished report, laboratory
project ID: 556-010 from International Research & Development
Corporation. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Goldenthal, E.I. (1988c) Toxicity study in dogs. Unpublished report,
laboratory project ID: 556-006 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Goldenthal, E.I. (1990a) Evaluation of pyrethrum extract in a 1-year
chronic toxicity study in dogs. Unpublished report, laboratory
project ID: 556-007, MRID #41496502 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Goldenthal, E.I. (1990b) Evaluation of pyrethrum extract in an
18-month dietary oncogenic study in mice. Unpublished report,
laboratory project ID: 556-013, MRID #41559401 from International
Research & Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1990c) Evaluation of pyrethrum extract in a two-year
dietary toxicity and oncogenicity study in rats. Unpublished
report, laboratory project ID: 556-011, MRID #41559501 from
International Research & Development Corp. Submitted to WHO by
Kenya Pyrethrum Information Centre, Oberalm, Austria.
Goldenthal, E.I. (1992) 21-day repeated dose dermal toxicity study
with pyrethrum extract in rabbits. Laboratory project ID:
556-018, MRID #42212601 from International Research & Development
Corp. Submitted to WHO by Kenya Pyrethrum Information Centre,
Oberalm, Austria.
Hermansky, S.J. & Hurley, J.M. (1993a) Peroral (gavage) neurotoxicity
probe study with pyrethrins in rats (Sprague Dawley). Unpublished
report, laboratory project ID: 91N0122, MRID #42930401 from Bushy
Run Research Center. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Hermansky, S.J. & Hurley, J.M. (1993b) Acute oral neurotoxicity study
with pyrethrins in rats (Sprague Dawley). Unpublished report,
laboratory project ID: 92N1036, MRID #42925801 from Bushy Run
Research Center. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Hoffmann, G.M. (1991) Acute inhalation toxicity in rats (Sprague
Dawley). Unpublished report, project No. 91-8331, MRID #42008001
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Mitchell, J.C., Dupuis, G. & Towers, G.H.N. (1972) Allergic contact
dermatitis from pyrethrum (Chrysanthemum sp.). Br. J.
Dermatol., 86, 568-573.
Myer, J.R. (1991) Evaluation of pyrethrum extract in a 5-day toxicity
study in New Zealand white rabbits. Unpublished report,
laboratory project ID: 556-017 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Newton, P.E. (1992) 90-day inhalation toxicity study of pyrethrum
extract in the rat via whole body exposure. Unpublished report,
project No. 91-8335, MRID #42478201 from Bio/dynamics, Inc.
Submitted to WHO by Kenya Pyrethrum Information Centre, Oberalm,
Austria.
Putman, D.L. & Morris, M.J. (1989) Chromosome aberrations in Chinese
hamster ovary (CHO) cells. Unpublished report, laboratory study
No. T8729.337, MRID #41344601 from Microbiological Associates
Inc. Submitted to WHO by Kenya Pyrethrum Information Centre,
Oberalm, Austria.
Ramirez, M.A. (1930) Pyrethrum: An etiologic factor in vasomotor
rhinitis and asthma. J. Allergy, 1, 149-155.
Rickett, F.E., Tyszkiewicz, K. & Brown, N.C. (1972) Pyrethrum
dermatitis. Part I: The allergenic properties of various extracts
of pyrethrum flowers. Pyrethrum Post, 11, 85.
Romanelli, P. (1991a) Primary skin irritation in rabbits (New Zealand
white). Unpublished report, project No. 91-7316A, MRID #41964803
from Biosearch Inc., Philadelphia, Pennsylvania, USA. Submitted
to WHO by Kenya Pyrethrum Information Centre, Oberalm, Austria.
Romanelli, P. (1991b) Sensitization study in guinea pig (Hartley
strain). Unpublished report, project No. 91-7316A, MRID #
41964804 from Biosearch Inc., Philadelphia, Pennsylvania, USA.
Submitted to WHO by Kenya Pyrethrum Information Centre, Oberalm,
Austria.
San, R.H.C. & Springfield, K.A. (1989) Salmonella/
mammalian-microsome plate incorporation mutagenicity assay (Ames
test) with a confirmatory assay. Unpublished report, laboratory
study No. T8729.501014, MRID #41344701 from Microbiological
Associates, Inc. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Schardein, J.L. (1987a) Evaluation of pyrethrum extract in a dose
range-finding teratology study in rats. Unpublished report,
laboratory project ID: 556-001, MRID #40603701 form International
Research & Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Schardein, J.L. (1987b) Evaluation of pyrethrum extract in definitive
rat teratology study. Unpublished report, laboratory project ID:
IRDC 556-002, MRID #40288202 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Schardein, J.L. (1987c) Evaluation of pyrethrum extract in a dose
range-finding teratology study in rabbits. Unpublished report.
laboratory project ID: 556-003, MRID #40603702 from International
Research & Development Corp. Submitted to WHO by Kenya Pyrethrum
Information Centre, Oberalm, Austria.
Schardein, J.L. (1987d) Evaluation of pyrethrum extract in a
definitive rabbit teratology study. Unpublished report,
laboratory project ID: IRDC 556-004, MRID #40288203 from
International Research & Development Corp. Submitted to WHO by
Kenya Pyrethrum Information Centre, Oberalm, Austria.
Schardein, J.L. (1989) Two generation reproduction study in rats with
pyrethrum extract. Unpublished report, laboratory project ID:
IRDC 556-005, MRID #41327501 from International Research &
Development Corp. Submitted to WHO by Kenya Pyrethrum Information
Centre, Oberalm, Austria.
Selim, S. (1995) Pharmacokinetics and metabolism of 14C pyrethrin I
in the rat. Unpublished report, study No. P1092006, MRID
#43554304 from Biological Test Center. Submitted to WHO by Kenya
Pyrethrum Information Centre, Oberalm, Austria.
Springfield, A.C., Carlson, G.P. & DeFeo, J.J. (1973) Liver
enlargement and modification of hepatic microsomal drug
metabolism in rats by pyrethrum. Toxicol. Appl. Pharmacol., 24,
298-308.
Zucker, A. (1965) Investigation of purified pyrethrum extracts.
Ann. Allergy, 23, 335-339.
Appendix 1: Application of the Conceptual Framework for Cancer Risk
Assessment
(Revised on the basis of discussions at the IPCS Workshop on
Developing a Conceptual Framework for Cancer Risk Assessment, 16-18
February 1999, Lyon, France)
This framework, developed by an IPCS working group, provides a
generic approach to the principles commonly used in evaluating a
postulated mode of action for tumour induction by a chemical. Thus,
the framework was used by the 1999 JMPR to provide a structured
approach to the assessment of the overall weight-of-evidence for the
postulated mechanism of the increased incidences of benign tumours of
the liver, thyroid, and skin in rats and a treatment-related effect on
the incidence of lung tumours in mice observed after long-term
administration of pyrethrins.
The framework guidelines suggested 10 section headings. The
introduction (see monograph section 2(c), 'Long-term studies of
toxicity and carcinogenicity') describes the cancer end-points that
have been observed. Three of these (rat liver and thyroid neoplasms,
rat keratoacanthomas, and mouse lung tumours) are addressed separately
in the analysis. An approriate mode of action is postulated and the
key events identified; the observed dose-response relationships and
temporal relationships are discussed. The strength, consistency, and
specificity of the association of tumour response with key events and
the biological plausibility are analysed. Alternative modes of action
are identified and found not to be supported. The three postulated
modes of action are discussed below, with some uncertainties about
both the biology of tumour development and the database on the
compound and any inconsistencies in the method that were identified.
* The increased incidences of hepatocellular adenomas were associated
with persistent induction of cytochrome P450 enzymes and
hepatocellular hypertrophy, suggesting that pyrethrins are
rodent-specific hepatoproliferative carcinogens. Enzyme induction
leading to increased clearance of thyroid hormones would also be
consistent with the increased incidence of follicular hyperplasia
and follicular adenomas seen. The level of confidence in this
postulated mode of action is moderate, because some uncertainties
and inconsistencies were introduced by the method. Therefore,
additional studies to identify more key events in the liver and
thyroid (e.g. measurements of enzyme induction with purified
pyrethrins, estimation of thyroid hormone changes, and estimation
of thyroid and pituitary weights) are required.
* The higher incidence of keratoacanthomas in male rats at the high
dose, which exceeds the upper limit of the range in historical
controls, was considered to be related to treatment. The slight
increase in the incidence of cystic lesions in the skin and
subcutis in males observed macroscopically provided some support
that the increased incidence of neoplastic lesions is a result of
irritating chronic injury of the skin. There were some
uncertainties introduced by the method, and differing opinions on
the biology of these tumours were found in the literature. The
level of confidence in this postulated mode of action is very low,
and no further studies were identified to support it.
* The increased incidence of lung tumours in mice might be a result
of proliferative processes following chronic injury of the
respiratory epithelium or activation of microsomal mixed-function
enzymes, especially in Clara cells, which contain high
concentrations of P450 enzymes. Such chronic injury might be
followed by cell proliferation with a dose-response relationship.
The level of confidence in this postulated mode of action is,
however, very low.
There was also little confidence in other possible modes of action.
Uncertainties in the database on pyrethrins were identified.
Improvement of the method used in the study of carcinogenicity (e.g.
detailed histological sectioning of each lung lobe cut at the level of
bronchi and additional microscopic examination of step-sections of the
remaining lung tissue beginning at the level of the bronchi in all
animals of each sex, followed by a re-evaluation of all histological
slides in the absence of knowledge of their origin; reporting of the
number and size of neoplastic and preneoplastic lesions in each
animal) could serve to increase confidence that these tumours have no
relevance at the low concentrations to which humans and animals are
exposed.
The Meeting concluded that the increased tumour incidences
associated with exposure to pyrethrins are threshold phenomena of
negligible relevance to the low concentrations to which humans are
exposed and that pyrethrins have no genotoxic or mutagenic potential.
Therefore, no classification of cancer risk is necessary. However,
additional studies are required.
The discussion of the postulated mode of action of tumour induction
by pyrethrins was helpful in the overall process of hazard
characterization and risk assessment and contributed to consideration
of the relevance of the findings in animals to the human situation.
Application of the framework also promoted confidence in the
conclusions reached, as it represents use of a defined procedure which
mandates consistent documentation of the facts and reasoning that
includes consideration of inconsistencies and uncertainties. The
Meeting concluded that the framework could be developed for use both
by regulators and by researchers in identifying research needs on the
basis of clear delineation of data gaps and inconsistencies.
