
Pesticide residues in food -- 1999
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
Toxicological evaluations
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Rome, 20-29 September 1999
PYRIPROXYFEN
First draft prepared by
K. Fujimori
National Institute of Health Sciences, Tokyo, Japan
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Studies on metabolites
Acute toxicity
Genotoxicity
Comments
Toxicological evaluation
References
Explanation
Pyriproxyfen, 4-phenoxyphenyl (RS)-2-(2-pyridyloxy)propyl
ether, is a 2-phenoxy phenoxy oxime used as an insecticide which acts
as an insect growth regulator. It is intended for use as a spray in
the control of arthropods, including cockroaches and fleas, on crops,
dumps and indoors. Pyriproxyfen was evaluated for the first time by
the Meeting.
Evaluation for Acceptable Daily Intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
In a study carried in compliance with good laboratory practice
(GLP), groups of five male and five female Sprague-Dawley rats were
given pyriproxyfen labelled uniformly with 14C on the phenoxyphenyl
ring (radiochemical purity, > 99%) as a solution in corn oil orally
by gavage at doses of 2 or 1000 mg/kg bw, with or without pretreatment
with unlabelled pyriproxyfen (2 mg/kg bw). Radiolabel was excreted
predominantly in the faeces (90%) at both doses, urinary excretion
representing 4-8% of the administered dose over 48 h. The total
recovery of radiolabel in the excreta over 168 h was 96-98% of the
dose, and the residual radiolabel in the tissues and carcass
represented no more than 0.3% of the dose. The tissue with the highest
concentration of radiolabel was fat, which contained 0.01 µg/g as
equivalent. Pretreatment with unlabelled pyriproxyfen before
administration of 2 mg/kg bw per day for 14 days increased the
residual radiolabel in the tissues slightly but did not change the
excretion pattern. There was no significant sex- or dose-related
difference in excretion rates or in the tissue distribution of
radiolabel.
In a further study, groups of three male and three female
Sprague-Dawley rats with cannulated bile ducts were given
[14C-phenoxyphenyl]pyriproxyfen orally at a single dose of 2 mg/kg
bw. Biliary excretion of the radioactive material represented 34-37%
of the dose within 48 h of administration. Urinary excretion
represented 2-3% of the dose and faecal excretion 38-51% (Isobe et
al., 1988a; GLP: Matsunaga et al. 1995). These experiments suggest
that as much as 50% of an oral dose of pyriproxyfen is not absorbed.
Groups of five male and five female Sprague-Dawley (Crl-CD) rats
were given pyriproxyfen labelled with 14C in the pyridyl ring at a
dose of 2 or 1000 mg/kg bw in a study that complied with GLP.
Radiolabel was excreted predominantly in the faeces, representing
about 90% of the dose, and urinary excretion comprised 5-11% of the
dose over 48 h. Total recovery of radiolabel in the excreta after
168 h represented 92-99% of the dose. Expired air contained < 0.5% of
the dose over 48 h. The residual radiolabel in the tissues and carcass
represented no more than 0.3% of the dose 168 h after administration.
The tissue with the highest concentration of radiolabel was fat, with
0.01-0.02 µg/g as equivalent. No significant differences associated
with the position of the label, sex or dose were seen in the excretion
rates or tissue distribution (Yoshino, 1993a; Matsunaga et al., 1995)
In another study that complied with GLP, groups of three male and
three female Sprague-Dawley rats were given
[14C-phenoxyphenyl]pyriproxyfen orally at a single dose of 2 mg/kg
bw, and radiolabel in tissues was determined 2, 4, 8, 12, 24, 48, and
72 h after dosing. The peak concentration of radiolabel in blood was
observed 8 h after dosing. The concentrations in blood were four times
higher in males than in females, with a terminal half-time of 10-14 h.
The time to peak concentration in most tissues was 2-8 h after dosing,
while that in fat was 12-24 h. At the respective peak time, the
concentration in the liver was the highest (2.1-2.4 mg/g at 8 h as
equivalent) of the tissues examined; however, 72 h after dosing, the
highest concentration was found in fat (0.08-0.09 mg/g tissue as
equivalent). The concentration in tissues other than liver (0.02-0.03
mg/g tissue as equivalent) was < 0.01 mg/g as equivalent 72 h after
dosing. The half-time of radiolabelled material in the tissues was
8-35 h (Isobe et al., 1988b; Matsunaga et al., 1995).
Groups of three male and three female Sprague-Dawley rats were
given [14C-phenoxyphenyl]-pyriproxyfen orally at a single dose of
1000 mg/kg bw, and radiolabel in tissues was determined 2, 4, 8, 12,
24, 48, and 72 h after dosing in a study that complied with GLP. The
peak concentration in blood was achieved after 8 h. The concentrations
of radiolabel in blood in males were six times higher than in females.
The time to peak concentration in all tissues except fat was 4 h in
males and 8 h in females. At the respective peak time, the highest
concentration was found in liver (160-320 mg/g as equivalent at 8 h,
decreasing to 8-12 mg/g at 72 h). The highest residual concentration
72 h after dosing was in fat (45-46 mg/g tissue as equivalent). The
concentration of radiolabel in fat peaked 12-24 h after dosing and
decreased with a half-time of 23 h in males and 35 h in females. The
residual concentrations in other tissues were < 10 mg/g tissue 72 h
after dosing, and the half-time was 5-17 h. There was no dose-related
difference in the tissue distribution of pyriproxyfen (Yoshino, 1993b;
Matsunaga et al., 1995).
(b) Biotransformation
Mice
Groups of three male and three female mice were given
[14C-pyridyl]pyriproxyfen orally at a dose of 2 or 1000 mg/kg bw.
Faecal excretion represented 78-90% of the low dose and 64-65% of the
high dose, and urinary excretion 10-27% and 35-37%, respectively, over
7 days. Complete recovery of radiolabel (100-105% of the dose) was
observed over that time. Twelve metabolites were identified by
thin-layer chromatography (TLC) and high-performance liquid
chromatography (HPLC). The major metabolite in faeces was
4'-hydroxypyriproxyfen (36-38% of the low dose and 13-15% of the high
dose); minor metabolites were 4-hydroxyphenyl (RS)-2-(2-pyridyloxy)
propyl ether (3%) and (RS)-2hydroxypropyl 4-phenoxyphenyl ether
(1-3%). The urinary metabolites were 4'-hydroxypyriproxyfen (0-5%),
4'-hydroxypyriproxyfen glucuronide (3-13% of the low dose, 18-28% of
the high dose), and (RS)-2-hydroxypropyl 4-phenoxyphenyl ether
sulfate (3-6% of dose). The percent of the dose represented by
glucuronide and sulfate conjugates in urine was higher in mice than in
rats, but no other species difference in the metabolic pathways of
pyriproxyfen were seen. There was no difference in 4'-hydroxylation by
sex (Yoshino et al., 1995).
Rats
The biotransformation of [14C-phenoxyphenyl]pyriproxyfen in
rats was investigated in samples from the study of Isobe et al.
(1988a,b), described above. After oral administration, more than 26
metabolites were detected in faeces and urine by TLC. The major
metabolite was 4'-hydroxypyriproxyfen (25-48% of the dose). Total
recovery of radiolabel in faeces and urine represented 93-96% of the
dose, and 31-37% was detected as parent compound in the faeces 48 h
after the low or high dose, while no parent compound was detected in
urine. Ten metabolites, including conjugates, were identified by TLC
in excreta, all 10 occurring in faeces at the high dose and two in
urine. The major metabolite identified in feces was
4'-hydroxypyriproxyfen, formed by oxidative metabolism of the phenyl
ring, and the others were oxidative products (2-hydroxypyriproxyfen
and 4',5''-hydroxypyriproxyfen), the products of ether cleavage
( (RS)-2-hydroxypropyl 4-phenoxyphenyl ether, 4-phenoxyphenol, and
their hydroxylated metabolites), and their conjugates (sulfates). The
metabolites identified in urine were sulfate conjugates of
4'hydroxypyriproxyfen (0.4-1.0% of the low dose, 0.5-1.0% of the high
dose) and 4'-hydroxy-4-phenoxyphenol (0.5-3.0% of the low dose,
0.3-1.6% of the high dose). Less parent compound was found in faeces
after repeated oral dosing, but repeated treatment with the vehicle,
corn oil, caused a similar decrease. The percentage of the dose
recovered as 4'-hydroxypyriproxyfen in faeces was higher in females
than in males. The major metabolites identified in fat, liver, kidney,
and blood collected 2, 4, 8, 12, 24, 48, and 72 h after administration
of 2 mg/kg bw of radiolabelled pyriproxyfen were
4',5''-hydroxypyriproxyfen sulfate in blood and 4-hydroxy- and
4',5''-hydroxypyriproxyfen in kidney and liver. At the time of peak
concentration, the parent compound represented 0% in males and 9% in
females of the total radiolabel present. A greater percentage of the
dose was recovered as 4'-hydroxypyriproxyfen in the liver of females
than males. In the kidney, sulfate conjugates represented a larger
percentage of the dose in males than in females. Unmetabolized
pyriproxyfen represented 89-93% of the radiolabel in the extractable
fraction of fat (90% was extracted) (Isobe et al., 1988a,b).
The biotransformation of [14C-pyridyl]pyriproxyfen in rats was
also investigated in samples from the study of Yoshino (1993a),
described above. More than 13 metabolites were detected in faeces and
urine by TLC and HPLC, with nine, including conjugates, in faeces and
four in urine. The major metabolite in faeces was
4'-hydroxypyriproxyfen (23-47% of the dose), and the other metabolites
were oxidative products (2-hydroxy- and 4',5''-hydroxypyriproxyfen),
the products of ether cleavage (4-hydroxyphenyl
(RS)-2-(2pyridyloxy)propyl ether and
(RS)-2-(2-pyridyloxy)-propionic acid), and their sulfate or
glucuronide conjugates. The metabolites identified in urine were
4'-hydroxypyriproxyfen (0% of the low dose, 1.0-5.6% of the high dose)
and its sulfate conjugate (0.3-0.4% of the low dose, 0% of the high
dose), the sulfate conjugate of 4',5''-hydroxypyriproxyfen (0% of the
low dose, 0.1-0.2% of the high dose), and (RS)-2-(2-pyridyloxy)
propionic acid (1-1.7% of the low dose, 0% of the high dose) (Yoshino,
1993a)
These studies indicate that the major route of metabolism of
pyriproxyfen is hydroxylation, with cleavage of the ether bonds and
conjugation as minor routes. Hydroxylation occurs primarily at the 4'
position of the phenyl ring (phenoxy group) and subsequently at the
5'' position of the pyridyl group. Conjugation produces mainly the
respective sulfates of the oxidative metabolites. There was no
evidence of induction of metabolism by pretreatment with pyriproxyfen.
The pattern of metabolites in the excreta and tissues of males and
females suggests a considerable sex difference in metabolic activity.
There were no significant differences in the metabolic pathway by dose
or frequency of dosing. The proposed metabolic pathway for
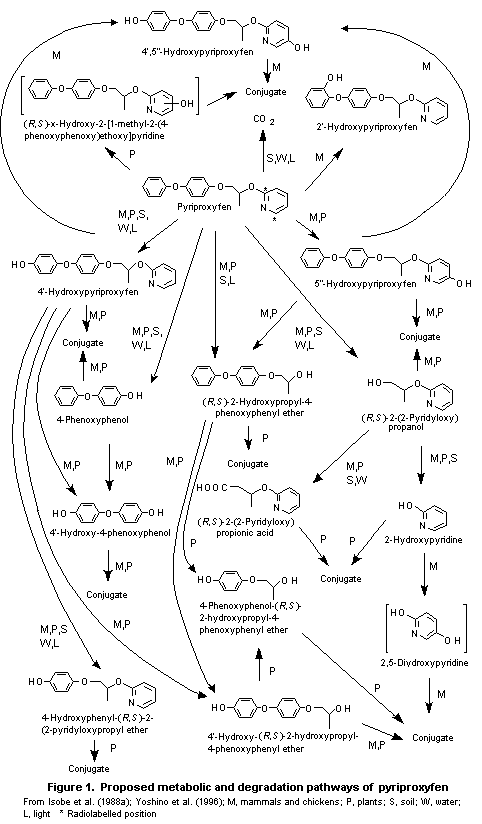
pyriproxyfen in various species is shown in Figure 1.
 Lactating goats
Lactating goats (Capra hircus, weighing 51-57 kg) were given
[14C-phenoxyphenyl]-pyriproxyfen (purity, 99.5%) in gelatin capsules
at a dose of 1.8-2.0 or 20 mg/animal per day for 5 consecutive days,
for a total dose of 100 mg. The animals were killed within 6 h of the
last dose. Faeces, urine, and milk were collected twice daily and
analysed. Metabolites were purified from extracts of tissues or
excreta by HPLC and identified by TLC and/or mass spectral analysis.
The study was carried out according to GLP. Elimination reached a
plateau by the third day. The percentages of total radiolabel
recovered 1 day after the last dose were 17-18% in urine, 58% in
faeces, and 0.3-0.8% in milk. The metabolites identified in milk
extracts were 4'-hydroxypyriproxy-fen sulfate (51% of the radiolabel
present), 4'-hydroxypyriproxyfen (2%), 4-phenoxyphenol sulfate (10%),
4'-hydroxy-4-phenoxyphenol sulfate (8%),
4'-hydroxy- (RS)-2-hydroxypropyl 4-phenoxyphenyl ether sulfate (3%),
and 4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen
(8%). Metabolites found in both liver and kidney were
4'-hydroxypyriproxyfen sulfate, 5''-hydroxypyriproxyfen-sulfate,
(RS)-2-hydroxypropyl 4-phenoxyphenyl ether,
4'-hydroxy (RS)-2-hydroxypropyl 4-phenoxyphenyl ether, and
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen. In
addition, 4'-hydroxypyriproxyfen and 5''-hydroxypyri-proxyfen were
identified in liver, and 4-phenoxyphenol sulfate and
4'-hydroxy-4-phenoxyphenol in kidney. The metabolites identified in
the faecal samples were 4'-hydroxypyriproxyfen (39% of the radiolabel
present), 5''-hydroxypyriproxyfen (6%),
4'-hydroxy- (RS)-2-hydroxypropyl 4-phenoxyphenyl ether (5%),
(RS)-2-hydroxypropyl 4phenoxyphenyl ether, and 4-hydroxyphenyl
(RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen. Parent compound
accounted for 11-13% of the dose in faeces, The major metabolites in
urine were 4-phenoxyphenol (36% of the radiolabel present),
4-phenoxyphenol sulfate (15%), 4'-hydroxy-4-phenoxyphenol (14%), and
4'-hydroxy-4-phenoxyphenol sulfate (17%) (Panthani et al., 1996a)
In another study that conformed to GLP, lactating goats
( Capra hircus, weighing 39-46 kg) were given
[14C-pyridyl]pyriproxyfen (purity, 97.6% ) in gelatin capsules at a
dose of 1.8-1.9 or 20 mg/animal per day for 5 consecutive days. The
animals were killed within 6 h of the last dose. Faeces, urine, and
milk were collected twice daily and analysed by HPLC and TLC and/or
mass spectrometry. The total radiolabel recovered represented 17-18%
of the dose in urine, 58% in faeces, and 0.4-0.8% in milk. The major
metabolites identified in milk extracts were 4'-hydroxypyriproxyfen
sulfate (35% of the radiolabel present) and 2,5-dihydroxypyridine
conjugate (29%), with smaller quantities of 4'-hydroxypyriproxyfen and
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen. The
major metabolites identified in liver and kidney were
4'-hydroxypyriproxyfen sulfate, 2,5-dihydroxypyridine conjugate, and
(RS)-2-(2-pyridyoxy) propanol conjugates. The major metabolites
identified in the urine were 4'-hydroxypyriproxyfen sulfate,
(RS)-2-(2-pyridyloxy) propionic acid, and 2,5-dihydroxypyridine
conjugate. The metabolites identified in the faecal samples were
4'-hydroxypyriproxyfen (43%), 5''-hydroxypyriproxyfen (5%), and
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen
(5%). These two studies indicate that the primary routes of metabolism
in goats are hydroxylation of the 4' position of the phenoxyphenyl
ring and the 5'' position of the pyridyl ring, cleavage of the ether
bonds, oxidation of the methylene moiety, and sulfate conjugation of
the 4'-hydroxyphenoxyphenyl moiety. There is thus no difference
between rats and goats in the metabolic pattern of pyriproxyfen
(Panthani et al., 1996b)
Chickens
Laying Leghorn hens (Gallus domesticus) were given
[14C-phenoxyphenyl]pyriproxyfen (purity, 99.1%) by gelatin capsule
at a concentration equivalent to 10 ppm in the feed for 8 consecutive
days and were killed within 4 h of the last dose. Excreta were
collected once daily and analysed by HPLC and TLC and/or mass
spectrometry. The study complied with GLP. The metabolites identified
in liver and kidney were free 4'-hydroxypyriproxyfen and its sulfate
conjugate, free 4'-hydroxy-4-phenoxyphenol and its sulfate conjugate,
free 4'-hydroxy- (RS)-2-hydroxypropyl-4-phenoxyphenyl ether and its
sulfate conjugate, 4-phenoxyphenol sulfate,
4-hydroxyphenyl- (RS)-2-(2-pyridyloxy)propyl ether pyriproxyfen, and
(RS)-2-hydroxypropyl-4-phenoxyphenyl ether. Metabolites identified
in excreta samples were 4'-hydroxypyriproxyfen, free and conjugated
4'-hydroxy-4-phenoxyphenol, free and conjugated
4'-hydroxy- (RS)-2-hydroxy-propyl 4-phenoxyphenyl ether,
4-hydroxyphenyl (RS)-2-(2pyridyloxy)propyl ether pyriproxyfen,
5''-hydroxypyriproxyfen, (RS)-2-hydroxypropyl-4-phenoxyphenyl ether,
and 4-phenoxyphenol (Panthani et al., 1996c)
Laying Leghorn hens (Gallus domesticus) were given
[14C-pyridyl]pyriproxyfen (purity, 98.2%) by gelatin capsule at a
concentration equivalent to 10 ppm in the feed for 8 consecutive days
and were killed within 4 h of the last dose. Excreta were collected
once daily and analysed by HPLC and TLC and/or mass spectrometry. The
study complied with GLP. The metabolites identified in liver and
kidney were free and conjugated 4'-hydroxypyriproxyfen,
2-hydroxypyridine, free and conjugated 5''-hydroxypyriproxyfen,
4-hydroxyphenyl- (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen, and
(RS)-2-(2-pyridyloxy)propionic acid. The metabolites identified in
excreta were (RS)-2-(2-pyridyloxy) propionic acid,
4'-hydroxypyriproxyfen, 4-hydroxyphenyl (RS)-2-(2-pyridyloxy)propyl
ether pyriproxyfen, 5''-hydroxypyriproxyfen, and 2-hydroxypyridine.
These two studies indicate that the routes of metabolism in laying
hens are hydroxylation at the 4' position of the phenoxyphenyl ring
and at the 5'' position of the pyridyl ring, cleavage of the ether
linkages, oxidation of the methylene moiety of the side-chain, and
sulfation of the 4'-hydroxyphenoxyphenyl moiety (Panthani et al.,
1996d)
In vitro
The metabolism of pyriproxyfen in rats and mice was investigated
in vitro in samples of 10 000 × g supernatant (S10) prepared from
kidney, lungs, and small intestine of 7-week-old Sprague-Dawley rats
and ICR mice. Hepatic microsomal and cytosolic fractions were prepared
by an established method (centrifugation of S10 at 105 000 × g), and
microsomal, S10, or cytosolic fractions were incubated with
[14C-phenoxyphenyl]pyriproxyfen at a concentration of 0, 0.05, 0.1,
0.5, or 1.0 mmol/L with b-NADPH as a cofactor. The reaction mixtures
were analysed by TLC. Pyriproxyfen was not metabolized by any of the
S10 preparations; it was almost completely metabolized by microsomal
fractions from liver but only very slightly by hepatic cytosol. Most
of the major metabolites identified in rats in vivo were observed
in vitro. There was no species difference in the major metabolic
reactions. The intrinsic clearance calculated from Lineweaver-Burk
plots revealed sex-related differences in metabolic reactions in rats
but not in mice, and 5''-hydroxylation was observed only in male rats
and not in mice of either sex. The intrinsic clearance for microsomal
4'-hydroxylation in female rats was twice that in males, but was not
different in male and female mice. Incubation of a hepatic microsomal
fraction from male rats with antisera against male-specific forms of
cytochrome P450 (CYP2C11 or CYP2C13) revealed that members of the
CYP2C family, the expression of which is sex-dependent in rats, are
involved in the major hydroxylation reactions of pyriproxyfen.
Antiserum against CYP2C11 inhibited all of the major metabolic
reactions of the hepatic microsomal fraction from male rats (> 88%
inhibition). The antiserum against CYP2C13 also inhibited all
reactions (25-64% inhibition) except 4'-hydroxylation (Yoshino et al.,
1996).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of pyriproxyfen are
summarized in Table 1. Pyriproxyfen dissolved in corn oil was
administered orally in a volume of 10 ml/kg bw to ICR (Crj:CD-1) mice
at doses of 1000, 2000, or 5000 mg/kg bw and to Sprague-Dawley
(Crj:CD) rats at 1000, 2500, or 5000 mg/kg bw. In mice, pyriproxyfen
reduced spontaneous motor activity and caused ataxia, abnormal
respiration, and death in males at 2000 mg/kg bw and in animals of
each sex at 5000 mg/kg bw. Transiently decreased body weight was
observed in males at 5000 mg/kg bw. Deaths occurred in two males at
2000 mg/kg bw and two at 5000 mg/kg bw in males and in one female at
5000 mg/kg bw. In rats, pyriproxyfen caused a decrease in body-weight
gain, decreased spontaneous activity, soft stools, and diarrhoea in
males at 2500 mg/kg bw and in animals of each sex at 5000 mg/kg bw,
but no deaths. Necropsy revealed no abnormal changes in the organs of
mice or rats. In dogs, oral administration of pyriproxyfen in capsules
caused no deaths at doses up to 5000 mg/kg bw. The only clinical sign,
occasional vomiting for the first 24 h, was observed at the highest
dose. Dermal application of 2000 mg/kg bw of pyriproxyfen dissolved in
corn oil (5 or 10 ml/kg bw) caused no deaths or signs of clinical
toxicity in ICR mice or Sprague-Dawley rats. Exposure of ICR mice or
Sprague-Dawley rats to a mist aerosol of pyriproxyfen dissolved in
corn oil at concentrations of 0.6 or 1.3 mg/L for 4 h caused no deaths
or pathological changes. The mass median aerodynamic diameter of the
particles was 0.8-0.9 µm. At the high concentration, salivation and
urinary incontinence were observed in rats 4 h after the start of
inhalation, and irregular respiration was observed in mice. These
clinical signs disappeared within 1 h of cessation of exposure.
(b) Short-term studies of toxicity
Mice
Groups of 10 male and 10 female ICR (Crj:CD-1) mice were given
diets containing technical-grade pyriproxyfen (purity, 95.3%) at
concentrations of 0, 200, 1000, 5000, or 10 000 ppm, equal to 0, 28,
150, 840, and 2000 mg/kg bw per day for males and 0, 38, 200, 960, and
2300 mg/kg bw per day for females, for 13 weeks. The observations
included clinical signs, mortality, food and water consumption, body
weight, clinical chemical parameters including serum enzymes, urinary
and haematological parameters, organ weights, and gross and
histopathological appearance. The serum enzymes assayed were alanine
aminotransferase), aspartate aminotransferase, and gamma-glutamyl
transpeptidase. Blood samples were collected shortly before
termination of the study. The study conformed to GLP.
Death occurred in two males at 5000 ppm and in seven males and
nine females at 10 000 ppm (one of the deaths in males was not
treatment-related). The clinical signs observed in the animals that
died prematurely were emaciation, hunched appearance, and few or no
faeces. There were no treatment-related clinical signs in the mice
that survived. Terminal body weights were significantly reduced in
males at 5000 ppm (89% of control) and at 10 000 ppm (69% of control);
the body weights of females were reduced at 5000 ppm during the first
half of the study but were comparable to control values (97% of
control) by the end. The water consumption of males at the two higher
doses was significantly increased, but food consumption was not
affected by treatment. There were significant decreases in erythrocyte
parameters, including cell count, haemoglobin concentration,
haematocrit, mean cell volume, and mean cell haemoglobin value, in
animals at 5000 and 10 000 ppm. Platelet counts were significantly
increased in animals of each sex at 5000 ppm and in males at 10 000
ppm. Significant increases in total cholesterol concentration were
observed in females at doses > 1000 ppm: 130% at 1000 ppm, 210% at
5000 ppm, and 140% of control values at 10 000 ppm. In males, the
activities of aspartate and alanine aminotransferases were increased
by up to twofold but reached significance only at 5000 ppm.
gamma-Glutamyl transpeptidase activity showed slight, nonsignificant
changes in some groups. The absolute weight of the liver was
significantly increased in females at 5000 ppm (120% at 5000 ppm and
160% of control values at 10 000 ppm) but not in males at any dose.
Microscopic examination revealed histomorphological alterations of the
Table 1. Acute toxicity of pyriproxyfen
Species Strain Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/L air)
Mouse ICR M&F Oral > 5000 97.2 Suzuki et al. (1987a)
Rat Sprague-Dawley M&F Oral > 5000 97.2 Suzuki et al. (1987b)
Dog Beagle M&F Oral > 5000 97.2 Nakano et al. (1986)
Mouse ICR M&F Dermal > 2000 97.2 Suzuki et al. (1987c)
Rat Sprague-Dawley M&F Dermal > 2000 97.2 Suzuki et al. (1987d)
Mouse ICR M&F Inhalation > 1.3 97.0 Suzuki et al. (1987e)
Rat Sprague-Dawley M&F Inhalation > 1.3 97.0 Kawaguchi et al. (1987)
These studies were conducted according to good laboratory practice.
kidney comprising tubular nephrosis with microcytosis and dilatation
of the renal tubules and focal mineralization and dilatation of the
renal pelvis in males at 5000 ppm and in animals of each sex at 10 000
ppm. There were no treatment-related morphological changes in the
liver. The NOAEL was 1000 ppm, equal to 150 mg/kg bw per day, on the
basis of effects on erythrocyte parameters, deaths, decreased body
weight, histomorphological alterations in the kidney, and increased
absolute liver weight at higher doses (Cox et al., 1990).
Rats
Groups of 10 male and 10 female Sprague-Dawley (Crl:CD) rats
received diets containing technical-grade pyriproxyfen (purity, 95.3%)
at concentrations of 0, 400, 2000, 5000, or 10 000 ppm, equal to 0,
23, 120, 310, and 640 mg/kg bw per day for males and 0, 28, 140, 360,
and 780 mg/kg bw per day for females, for 13 weeks. The observations
included clinical signs, deaths, body weight, food and water
consumption, ophthalmological, clinical chemical, haematological, and
urinary parameters, organ weights, and gross and histopathological
appearance. The clinical chemical examinations included assays for the
serum enzymes alkaline phosphatase, aspartate and alanine
aminotransferases, and gamma-glutamyl transpeptidase. Blood samples
were collected at the end of the study.
There were no treatment-related deaths, toxic signs, or
ophthalmological changes at any dose, and no changes in food or water
consumption. The body weights of animals of each sex were
significantly decreased at doses > 5000 ppm (91% of control at 5000
ppm and 88% at 10 000 ppm at termination). Erythrocyte parameters
including cell count, haemoglobin concentration, and haematocrit were
significantly decreased in animals at doses > 5000 ppm and in males
also at 2000 ppm. The mean cell volume was reduced in females at 2000
and 10 000 ppm by < 10%. There was no significant effect on platelet
count. The total cholesterol concentration was dose-dependently,
significantly increased in males at doses > 2000 ppm (150% at 2000
ppm, 200% at 5000 ppm, and 210% of control values at 10 000 ppm) and
in females at doses > 5000 ppm (130% at 5000 ppm and 180% of
control values at 10 000 ppm). The serum phospholipid concentration
was also significantly increased in males at doses > 2000 ppm (130%
at 2000 ppm, 180% at 5000 ppm, and 180% of control values at 10 000
ppm) and in females at 10 000 ppm (160% of control value).
Significantly increased gamma-glutamyl transpeptidase activity was
observed in animals at 10 000 ppm, but the activities of the other
serum enzymes were not significantly affected. Significant increases
in the absolute weight of the liver were observed in animals at doses
> 5000 ppm (110% at 2000 ppm, 130% at 5000 ppm, and 140% of control
values at 10 000 ppm in males and 100% at 2000 ppm, 120% at 5000 ppm,
and 140% of control values at 10 000 ppm in females), and the relative
weight of the liver was significantly increased in males at 2000 ppm
and in animals of each sex at doses > 5000 ppm (120% at 2000 ppm,
140% at 5000 ppm, and 170% of control values at 10 000 ppm in males
and 130% at 5000 ppm and 160% of control values at 10 000 ppm in
females). Significant increases in the relative weight of the kidney
were observed in animals at 10 000 ppm, but the absolute weights were
comparable to those of controls at all doses. Histopathological
examination revealed dose-dependent increases in cytoplasmic changes
in the liver in all treated groups (1/10 at 0 ppm, 2/10 at 400 ppm,
6/10 at 2000 ppm, 10/10 at 5000 ppm, and 9/10 at 10 000 ppm in males
and 1/10 at 0 ppm, 2/10 at 400 ppm, 6/9 at 2000 ppm, 7/10 at 5000 ppm,
and 9/10 at 10 000 ppm in females). The cytoplasmic changes consisted
of slight, often equivocal increases in cytoplasmic content reflected
in a visibly reduced nucleus:cytoplasm ratio and diminution of
sinusoidal spaces. The NOAEL was 400 ppm, equal to 23 mg/kg bw per
day, on the basis of mild anaemia, increased incidences of minimal
hepatic abnormalities, relative liver weights, and serum
concentrations of total cholesterol and phospholipids, indicating
effects on lipid metabolism, at higher doses (Cox et al., 1989).
Groups of 21 male and 21 female Sprague-Dawley rats were given
diets containing pyriproxyfen (purity, 97.2%) at concentrations of 0,
80, 400, 2000, or 10 000 ppm, equal to 0, 4.8, 24, 120, and 680 mg/kg
bw per day for males and 0, 5.4, 28, 140, and 690 mg/kg bw per day for
females, for 26 weeks. The observations included clinical signs,
deaths, food and water consumption, body weight, ophthalmological,
clinical chemical, haematological, and urinary parameters, organ
weights, and gross and histological appearance. The serum activities
of aspartate and alanine aminotransferases, alkaline phosphatase,
lactate dehydrogenase, leucine aminopeptidase, creatine phosphokinase,
and gamma-glutamyl transpeptidase were measured. Blood samples were
taken at the end of the study.
There were no deaths, and the only signs of toxicity were
increased incidences of alopecia around the neck and soft stools
during the early stage of the study in animals at 10 000 ppm. The body
weights of animals at 10 000 ppm were significantly decreased
throughout the study, by 86% in males and 87% in females at the end of
study. Marked decreases in body-weight gain were observed at this
dose. There were no treatment-related changes in food or water
consumption. Proteinuria and increased urinary excretion of potassium
ion were observed in animals at 10 000 ppm. Slight but significant
decreases in erythrocyte count and haematocrit were observed in males
at 2000 and 10 000 ppm and in females at 10 000 ppm. The haemoglobin
concentration was also slightly but significantly decreased at this
dose. No increase in platelet count was observed. Slight but
significant increases in total protein, albumin, and blood urea
nitrogen were observed in animals at 10 000 ppm. The albumin and
a2u-globulin fractions were slightly but significantly increased in
males at 10 000 ppm. Total cholesterol and phospholipid concentrations
were significantly increased in males at 2000 and 10 000 ppm and in
females at 10 000 ppm. Of the serum enzyme activities studied, only
that of gamma-glutamyl transpeptidase was significantly increased in
males at 10 000 ppm. Significantly increased absolute weights of the
liver were observed in animals at 10 000 ppm (130% of control value in
males and in females), and significant increases in the relative
weight were observed in males at doses > 2000 (110% at 2000 ppm and
160% of control values at 10 000 ppm) and in females at 10 000 ppm
(100% at 2000 ppm and 150% of control values at 10 000 ppm).
Significantly increased relative kidney weights were observed in
animals at 10 000 ppm, but the absolute weights were not significantly
increased. Histopathological examination showed slight hypertrophy of
the liver in all animals at 10 000 ppm. The NOAEL was 400 ppm, equal
to 24 mg/kg bw per day, on the basis of increased relative liver
weight, increased total cholesterol and phospholipid concentrations
indicating effects on lipid metabolism, and mild anaemia at higher
doses (Koyama et al., 1989).
Groups of five male and five female Sprague-Dawley (Crl:CD) rats
received dermal applications of pyriproxyfen (purity, 97.2%) dissolved
in corn oil at doses of 0, 100, 300, or 1000 mg/kg bw per day under a
semi-occlusive dressing for 6 h/day for 21 days. The observations
included clinical signs, deaths, food consumption, body weight, and
clinical chemical, haematological, and and histological examinations.
Serum was assayed for alanine and aspartate aminotransferases. Blood
samples were collected at termination. The study complied with GLP.
There were no treatment-related effects on the mortality rate,
clinical signs, or haematological or clinical chemical parameters,
including serum enzymes. There were no significant changes in body
weight or food consumption in the treated group, and no effect of
treatment on organ weights was observed. Histopathological examination
revealed no treatment-related alterations in the liver or any other
tissues examined. The NOAEL was 1000 mg/kg bw per day, the highest
dose tested (Moore et al., 1993).
Groups of 10 male and 10 female Sprague-Dawley (Jcl:CD) rats were
exposed to a mist aerosol of pyriproxyfen dissolved in corn oil at
concentrations of 270, 480, or 1000 mg/m3 for 4 h/day for 28 days.
The mass median aerodynamic diameter of the particles was 0.71-0.88
µm. The observations included clinical signs, deaths, food and water
consumption, body weight, and urinary, ophthalmological, clinical
chemical, haematological, and histological examinations. Serum was
assayed for leucine aminopeptidase, cholinesterase, lactate
dehydrogenase, creatine phosphokinase, alanine and aspartate
aminotransferase, and alkaline phosphatase activity. Blood samples
were collected at termination after a 16-h fast. The study complied
with GLP.
There were no deaths. Salivation was observed early in the study
in rats at the highest concentration, and the body-weight gain of
animals at this dose was sporadically, slightly but significantly
lower, although it was normal at the end of the study. There were no
treatment-related changes in food consumption or in haematological,
urinary, or ophthalmologic parameters. Slightly but significantly
increased lactate dehydrogenase activity was observed in males at the
highest dose, but the activities of the other serum enzymes showed no
treatment-related change. A slight but significant increase (9%) was
observed in the relative weight of the liver at 1000 mg/m3, but the
absolute weight was comparable to that of controls. Histopathological
examination revealed no treatment-related morphological changes in the
organs of exposed rats. The NOAEL was 480 mg/m3 on the basis of
salivation, sporadically reduced body-weight gain, and increased
lactate dehydrogenase activity at 1000 mg/m3 (Kawaguchi et al.,
1988).
Guinea-pigs
The skin sensitizing potential of pyriproxyfen (purity, 97.2%)
was tested in a GLP-compliant study in male Hartley guinea-pigs by the
maximization method. A volume of 0.05 ml of 1% pyriproxyfen in
Freund's complete adjuvant mixed with water or a 0.5% solution of
pyriproxyfen in corn oil was injected intradermally for initial
sensitization. As a challenge, 0.2 g of pyriproxyfen in 25% petrolatum
or 0.2 ml of a positive control was applied dermally in patches to
test animals for 24 h, 6 days after the first sensitization. No dermal
reaction was observed (Suzuki et al., 1987f).
Rabbits
New Zealand white rabbits of each sex received a single dose of
0.5 g of pyriproxyfen (purity, 97.2%) as a fine powder moistened with
physiological saline by dermal application for 4 h. The potential to
induce primary skin irritation was examined 4.5, 24, 48, and 72 h
after application. The study complied with GLP. No oedema or erythema
was observed (Suzuki et al., 1987g).
A single dose of 100 mg of pyriproxyfen (purity, 97.2%) in a
volume of 0.1 ml was applied to the right eye of three male and three
female New Zealand white rabbits in a study that complied with GLP.
The potential to induce primary eye irritation was examined 1, 24, 48,
and 72 h after application in unwashed eyes. Slight conjunctival
redness (grade 1) and chemosis (grade 1-2) were observed in all
treated animals 1 h after application. Conjunctival redness (grade 1),
chemosis (grade 1), and discharge (grade 2) were still apparent in one
or two animals after 24 h, but these changes had disappeared by 48 h
after application. Pyriproxyfen was considered to be a minimal ocular
irritant (Suzuki et al., 1987g).
Dogs
Groups of four male and four female beagle dogs, 6 months old,
received gelatine capsules containing pyriproxyfen (purity, 97.2%) at
doses of 0, 100, 300, or 1000 mg/kg bw per day for 3 months. The
observations included clinical signs, deaths, food consumption, body
weight, and ophthalmic, electrocardiographic, clinical chemical,
haematological, urinary, and histological parameters. The activities
of alkaline phosphatase, aspartate and alanine aminotransferases,
gamma-glutamyl transpeptidase, creatine phosphokinase, and lactate
dehydrogenase were measured in plasma. Ophthalmological examinations
were performed during weeks 0, 5, and 12 of treatment, and an
electrocardiograph was obtained at the same times. Blood samples were
collected during weeks 0, 4, 8, and 12 of treatment; hepatic function
was assessed by bromsulphalein retention during weeks 0, 6, and 13 of
treatment, and renal function was assessed by retention of
para-aminohippuric acid during weeks 0, 5, and 11 of treatment.
No deaths were observed. Female dogs at 1000 mg/kg bw per day had
a slightly increased incidence of soft stools, but no other
treatment-related toxic signs were observed. There were no changes in
body weight, body-weight gain, food consumption, or ophthalmological
parameters throughout the study, and no treatment-related changes in
the electrocardiograph were observed at any dose. Haematological
parameters were not significantly changed at any dose, although
slight, nonsignificant alterations in the number of platelets (by
< 39%) and total cholesterol concentration (by < 67%) were observed,
with no obvious dose-dependence. The phospholipid concentration was
significantly increased in females at 1000 mg/kg bw per day. No
significant changes was found in the activities of the serum enzymes
studied, although there were trends to increased alkaline phosphatase
activity in males at the high dose, lactate dehydrogenase activity in
males at all doses and in females at the high dose, and creatine
phosphokinase activity in males in all doses, with no dose-dependence.
Aspartate and alanine aminotransferase activities were unaltered.
Hepatic function was not significantly affected at any dose. The
absolute weights of the liver were significantly increased in males at
300 and 1000 mg/kg bw per day, by 30% and 26%, respectively, and
significant increases in relative liver weights were observed in males
at 300 mg/kg bw per day, by 24%. Increased incidences of
hepatocellular hypertrophy were observed in females at 300 mg/kg bw
per day and in animals of each sex at 1000 mg/kg bw (0/4 in controls,
0/4 at 100 mg/kg bw per day, 0/4 at 300 mg/kg bw per day, and 4/4 at
1000 mg/kg bw per day in males, and 0/4, 0/4, 3/4, and 4/4 in females,
respectively). At 1000 mg/kg bw per day, increased incidences of
eosinophilic bodies in the liver were observed (0/4 in controls, 0/4
at 100 mg/kg bw per day, 0/4 at 300 mg/kg bw per day, and 2/4 at 1000
mg/kg bw per day in males, and 0/4, 0/4, 0/4, and 2/4 in females,
respectively). Electron microscopic examination revealed a minimal to
slight increase in smooth endoplasmic reticulum with slight dilatation
in the livers of all animals at 1000 mg/kg bw per day. These changes
are consistent with adaptation of the liver to exposure to the
compound through enzyme induction. A slight but significantly
prolonged retention ratio in the test for renal function was observed
in males at 300 and 1000 mg/kg bw per day after 6 weeks of treatment,
but the retention ratio was normal by the end of the study, and no
treatment related histopathological changes were observed in the
kidney. The NOAEL was 100 mg/kg bw per day on the basis of increased
absolute and relative liver weights and an increased incidence of
hepatocellular hypertrophy at higher doses (Nakano et al., 1988).
Groups of four male and four female beagle dogs (23-27 weeks old)
were given gelatine capsules containing pyriproxyfen (purity, 95.3%)
at doses of 0, 30, 100, 300, or 1000 mg/kg bw per day for 52 weeks.
The observations included clinical signs, deaths, food consumption,
body weight, and ophthalmoscopic, clinical chemical, haematological,
urinary, and histological examinations. The activities of alanine and
aspartate aminotransferases, alkaline phosphatase, and creatine
phosphokinase were measured in plasma. Blood samples were collected 1
week before and on weeks 12, 24, 37, and 50 of treatment. The study
conformed to GLP.
Two males at 1000 mg/kg bw per day were killed in extremis with
acute weight loss and, in one case, liver failure, at weeks 17 and 31
of treatment. Slightly increased frequencies of salivation and
diarrhoea were observed in animals at 1000 mg/kg bw per day, and
emaciation was observed in males at doses > 300 mg/kg bw per day.
There were no treatment-related ophthalmological abnormalities. A
dose-dependent but nonsignificant reduction in body weight was seen
throughout the study, and body-weight gains were significantly reduced
in animals at doses > 300 mg/kg bw per day. Food consumption was
not reduced at any dose. Significant changes were seen in several
haematological parameters, including 10-20% decreases in haemoglobin
and erythrocyte counts and a slightly but significantly increased mean
corpuscular volume in males at doses > 300 mg/kg bw per day and in
females at doses > 100 mg/kg bw per day and a significantly
decreased packed cell volume in the latter group. The change in
haemoglobin in males at 1000 mg/kg bw per day was not significant.
These haematological changes might indicate slight anaemia. There was
no abnormality of cellularity or cell composition in the bone marrow.
Statistically significant decreases in the number of lymphocytes were
observed in all treated females at weeks 12 and 37, which were
attributed to transiently high values for the control group. The
platelet count was significantly increased in males at doses > 100
mg/kg bw per day and females at 1000 mg/kg bw per day throughout the
study. Slightly but significantly prolonged prothrombin times were
observed in males at 300 mg/kg bw per day and in animals of each sex
at 1000 mg/kg bw per day. Significantly increased plasma enzyme
activities were seen, including those of alkaline phosphatase in
animals at doses > 300 mg/kg bw per day throughout the study,
alanine aminotransferase in animals at 1000 mg/kg bw per day
throughout the study, and aspartate aminotransferase in males at 1000
mg/kg bw per day in weeks 12, 24, and 37 of treatment. The total
cholesterol concentration in plasma was significantly increased in
animals at doses > 30 mg/kg bw per day (by 24-58% at 30 mg/kg bw
per day, 64-160% at 100 mg/kg bw per day, 100-140% at 300 mg/kg bw per
day, and 76-100% at 1000 mg/kg bw per day) throughout the study, and
the plasma concentrations of triglycerides were significantly
increased in animals at doses > 100 mg/kg bw per day. These
increases were dose-dependent except at the highest dose in males, but
in this group there were only two survivors. No reduction in plasma
protein fractions was observed in the treated groups.
A slightly reduced pH and increased volume of urine were observed
in males at 1000 mg/kg bw per day throughout the study. The absolute
weights of the liver were dose-dependently increased in all treated
groups and significantly increased in males at doses > 100 mg/kg bw
per day (by 130% at 30 mg/kg bw per day, 150% at 100 mg/kg bw per day,
170% at 300 mg/kg bw per day, and 190% of control values at 1000 mg/kg
bw per day) and in females at doses > 300 mg/kg bw per day (110%,
120%, 140%, and 140%, respectively). The relative weights of the liver
were significantly increased in males at doses > 30 mg/kg bw per
day (130% of control value) and in females at doses > 300 mg/kg bw
per day. The absolute weights of the thyroid were significantly
increased in females at doses > 300 mg/kg bw per day, and the
relative weights were significantly increased in females at doses
> 100 mg/kg bw per day. Significantly increased relative renal
weights were observed in males at 300 mg/kg bw per day and in females
at doses > 300 mg/kg bw per day; the absolute weights were
dose-dependently but not significantly increased.
Macroscopic examination showed enlarged livers and hepatic damage
in the two dogs at 1000 mg/kg bw per day which died. Histopathological
examination revealed treatment-related hepatic damage in animals of
each sex at 1000 mg/kg bw per day, which was characterized by
centriacinar fibrosis in 2/2 males and 3/4 females and bile-duct
hyperplasia in 2/2 males and 3/4 females; foci of cystic degeneration
in 1/2 males and 1/4 females; active chronic inflammation in 2/2 males
and 2/4 females; and nodular hyperplasia in 2/2 males and 0/4 females.
Submucosal fibrosis in the gall-bladder was observed in all male
animals, including those that had died, and in 3/4 females at 1000
mg/kg bw per day, in association with bile-duct hyperplasia. One of
four males at 30 mg/kg bw per day had focal bile-duct hyperplasia and
focal subcapsular fibrosis, but these effects were not observed at 100
or 300 mg/kg bw per day. There were no preneoplastic or neoplastic
alterations. Although no morphological alterations were seen in the
liver, the increase in cholesterol concentration and relative liver
weight at low doses might be related to treatment. No NOAEL could be
identified, as increased total cholesterol concentrations indicating
effects on lipid metabolism and increased relative liver weights with
a trend towards increased absolute liver weights were seen at all
doses (Chapman et al., 1991).
In a complementary study which complied with GLP, groups of four
male and four female beagle dogs (19-24 weeks old) were given gelatine
capsules containing pyriproxyfen (purity, 95.3%) at doses of 0, 3, or
10 mg/kg bw per day for 52 weeks. The observations included clinical
signs, deaths, food consumption, body weight, and ophthalmic, clinical
chemical, haematological, urinary, and histological examinations. The
activities of alanine and aspartate aminotransferases, alkaline
phosphatase, and creatine phosphokinase were assayed in serum. Blood
samples were collected after 12, 24, 36, and 50 weeks of treatment.
There were no deaths, signs of clinical toxicity, or changes in
body weight, body-weight gain, or food consumption. Significantly
increased platelet counts were observed in males at 3 mg/kg bw per day
in weeks 24, 36, and 50 of treatment and in those at 10 mg/kg bw per
day in week 36, but with no clear dose-dependence. Prothrombin time
was not prolonged in males at any dose. Females at 10 mg/kg bw per day
also showed significantly increased platelet counts in weeks 36 and 50
(by 8% at 3 mg/kg bw per day and 10% at 10 mg/kg bw per day), and
prothrombin time was slightly but significantly prolonged at these
doses at the end of study. There were no other treatment-related
changes in haematological parameters. The total cholesterol
concentration was unchanged; slight but significant increases in total
triglyceride concentrations in males at 10 mg/kg bw per day were seen
in weeks 12 and 36 of treatment. There were no treatment-related
changes in urinary parameters. The absolute weight of the liver was
slightly increased in females at 10 mg/kg bw per day (110% of
control), but this was not significant. No histopathological changes
were found in any organ, including the liver and kidney. The range of
mean total platelet counts in controls in other studies in this
laboratory was 273-357 in males and 305-357 in females, whereas those
in the present study were 341-367 in controls, 414-462 at the low
dose, and 415-456 at the high dose in males, and 321-384 in controls
and 430-478 at the high dose in females. The increased numbers of
platelets and the prolonged prothrombin time were therefore
treatment-related changes although no significant increase in platelet
counts was observed in animals at 30 mg/kg bw per day in the previous
study (Mitchel et al., 1993).
The NOAEL for the two 52-week studies in dogs was 10 mg/kg bw per
day, on the basis of the absence of treatment-related toxicity at 10
mg/kg bw per day in the second study and changes in lipid metabolism
and increased liver weight at 30 mg/kg bw per day in the first study.
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female ICR(Crj-CD-1) mice were given
diets containing pyriproxyfen (purity, 95.3%; 97.6-98.7% dietary
concentration) at concentrations of 0, 120, 600, or 3000 ppm for
78 weeks, providing doses equal to 0, 16, 81, and 420 mg/kg bw per day
for males and 0, 21, 110, and 530 mg/kg bw per day for females. The
observations included clinical signs, deaths, food consumption, body
weight, organ weights, and ophthalmological, haematological, and gross
and histological examinations. Ten mice from each group were killed
during week 52 for interim examination, and the surviving mice were
killed during week 78. Blood samples were taken from 10 rats per group
during weeks 52 and 78 of treatment. No clinical chemical tests were
conducted. The study complied with GLP.
The mortality rate was dose-dependent and significantly increased
in males at 600 and 3000 ppm (43% at 0 ppm, 55% at 120 ppm, 72% at 600
ppm, and 82% at 3000 ppm) and in females at 3000 ppm (39% at 0 ppm,
44% at 120 ppm, 55% at 600 ppm, and 64% at 3000 ppm). There were
slight, nonsignificant increases in the incidence of clinical signs,
including reduced motor activity and hunched position, in animals at
3000 ppm. Statistically significant decreases in body weights,
body-weight gains, and/or food consumption were observed in males at
3000 ppm during the study. The absolute and relative weights of the
liver were significantly increased in females at 3000 ppm during week
52 of treatment. The haematological parameters showed no
treatment-related change. Histopathological examination of animals
that died revealed a significantly increased incidence of systemic
amyloidosis in the glandular stomach of males at 600 and 3000 ppm and
the adrenal, thyroid, heart, liver, kidney, glandular stomach, and
duodenum of females at 3000 ppm, and a dose-related relationship was
found between the generalized amyloidosis and the mortality rate.
Statistical analysis of the incidence of graded amyloidosis revealed a
significant positive trend in renal amyloidosis in females and a
significant positive trend in hepatic amyloidosis in animals of each
sex at 3000 ppm. Females at this dose had a significant increase in
the incidence of lymphocytic infiltration in the liver (22/59 at 0 and
34/60 at 3000 ppm) and of tubular mineralization (3/59 at 0 and 46/60
at 3000 ppm). Deposition of amyloid in the kidney causes numerous
pathological changes including tubular mineralization and papillary
necrosis. In this study, however, the incidences of tubular
mineralization and segmental cortical atrophy were increased
independently of amyloidosis in female animals at 3000 ppm, suggesting
that the chronic nephrosis was directly related to treatment.
Histopathological examination revealed no increase in the incidence of
neoplastic lesions at any dose. The NOAEL was 120 ppm, equal to 16
mg/kg bw per day, on the basis of increased mortality at higher doses
(Osheroff et al., 1991a; Cardy et al., 1994).
Rats
Groups of 50 male and 50 female Sprague-Dawley (Crl:CD) rats were
given diets containing pyriproxyfen (purity, 95.3%) at concentrations
of 0, 120, 600, or 3000 ppm, equal to 0, 5.4, 27, and 140 mg/kg bw per
day for males and 0, 7.0, 35, and 180 mg/kg bw per day for females,
for 104 weeks. Satellite groups of 30 males and 30 females were also
treated orally. The observations included clinical signs, deaths, food
and water consumption, body weight, organ weights, and
ophthalmoscopic, clinical chemical, haematological, urinary, and gross
and histopathological examination. Assays were performed for the serum
enzymes aspartate and alanine aminotransferase, alkaline phosphatase,
creatine kinase, and gamma-glutamyl transpeptidase. Blood samples were
collected from satellite groups of rats on week 13, 26, 52, 78, and
104 of treatment. The study complied with GLP.
Treatment did not affect mortality (34-46% in males and 32-58% in
females), clinical signs, or ophthalmoscopic end-points. The body
weights of males were significantly reduced in weeks 13, 26, and 50 of
treatment (by 5-7%) and those of females in weeks 13, 26, 50, and 78
of treatment (by 12-14%) at 3000 ppm, but they had returned to the
control level by the end of study. The mean body-weight gain was
significantly reduced in females at 600 ppm and in animals of each sex
at 3000 ppm throughout the study. No treatment-related changes in food
consumption were observed. The only alteration in haematological
parameters was a transient increase in eosinophils. Alkaline
phosphatase activity was significantly increased in males at doses
> 120 ppm in weeks 26, 52, and 78 of treatment, but the activity
(87-104 U/L) remained within the range of historical controls (45-114
U/L), and the changes were not clearly dose-dependent. gamma-Glutamyl
transpeptidase activity was significantly increased in males at 3000
ppm in week 104 of treatment and in females at 600 and 3000 ppm in
weeks 26 and 52. The activities of other serum enzymes were not
significantly affected. Significantly increased total cholesterol
concentrations were observed in males at 3000 ppm in weeks 26 and 52
(149% and 147% of control, respectively). Slight, inconsistent,
nonsignificant increases in urinary protein concentration were
observed in females at 3000 ppm in week 26. At interim necroscopy, the
absolute weights of the liver were found to be nonsignificantly
increased at week 52 of treatment with 3000 ppm (by 15% in males and
13% in females). A significant increase in relative liver weight was
observed only in females at 3000 ppm (120% of control). The only
significant or treatment-related increases in the incidence of
morphological alterations at 104 weeks seen on gross and
histopathological examination were hyperkeratosis of the skin of males
at 3000 ppm, which was considered not to be biologically significant,
and a significant increase in the incidence of liver necrosis in males
at 3000 ppm that died during the study; however, no liver necrosis was
observed in the surviving males at 3000 ppm at the end of the study,
indicating nthat it was not related to treatment. Histopathological
examination revealed no evidence of neoplastic alterations. The NOAEL
was 600 ppm, equal to 27 mg/kg bw per day, on the basis of reductions
in body weight and mean body-weight gain and increased absolute and
relative liver weights at higher doses (Osheroff et al., 1994a,b).
(d) Genotoxicity
The results of tests for the genotoxicity of pyriproxyfen are
summarized in Table 2. All of the positive controls used in the assays
produced the expected positive responses. In assays for reverse
mutation, no induction of revertant colonies was observed at six doses
with or without an exogenous metabolic activation system (S9). In
tests for DNA repair, pyriproxyfen was inactive at six doses with or
without S9. In tests for gene mutation in mammalian cells, no
mutations were observed at four doses ranging from 10 to 300 ppm
without S9 or 10 to 100 ppm with S9. In assays for unscheduled DNA
repair synthesis, pyriproxyfen was cytotoxic and/or inhibited normal
DNA synthesis but it did not induce unscheduled DNA synthesis. In
tests for cytogeneticity, Chinese hamster ovary cells (CHOK1) were
exposed to pyriproxyfen at a concentration of 10, 30, or 100 µg/ml for
2 h in the presence of S9 and cultured for a further 16 or 22 h or
cultured with pyriproxyfen for 18 or 24 h in the absence of S9.
Although marked cytotoxicty, characterized by decreased mitotic index
and cell cycle delay, were observed at doses > 30 mg/ml without S9,
and at 100 mg/ml with S9, no increase in the total number of
structural aberrations or the frequency of cells with aberrations was
observed at any concentration. In the test for micronucleus formation
in mice in vivo, a single dose of pyriproxyfen at 5000 mg/kg bw
slightly but not statistically significantly increased the incidence
of micronucleated polychromatic erythrocytes.The Meeting concluded
that pyriproxyfen is not genotoxic in vivo or in vitro.
Table 2. Results of tests for the genotoxicity of pyriproxyfen
End-point Test system Concentration Purity Result Reference
(%)
In vitro
Reverse mutationa S. typhimurium 10-5000 µg/plate 97.2 Negative ± S9 Kogiso et al. (1988a)
TA98, TA100,
TA1537, TA1538,
E, coli WP2 uvrA
DNA repairb B.subtills M45, 673-21 500 µg/disc 95.3 Negative ± S9 Kogiso et al. (1992)
H17 in DMSO
Gene mutationc Chinese hamster 3-300 µg/ ml 95.3 Negative ± S9 Kogiso et al. (1990)
V79 cells, hprt locus
Unscheduled Human HeLa S3 0.1-205 µg/ ml 95.3 Negative ± S9 Henderson &
DNA synthesisd epithelioid cells Proudlock (1989)
Chromosomal Chinese hamster 10-300 µg/ml 97.2 Negative ± S9 Kogiso et al. (1989)
aberrationse ovary cells
Chromosomal Chinese hamster 9.6-321 µg/ml -S9 97.2 Negative ± S9 Kogiso et al. (1988)
aberrationsf ovary cells 50-200 µg/ml +S9
In vivo
Micronucleus CD-I mice, bone Single intraperitoneal 95.3 Negative Proudlock et al.
formationg marrow injections of 5000 mg/kg (1991)
bw at 24, 48, and 72 h
Table 2. (continued)
All studies were conducted according to good laboratory practice. DMSO, dimethyl sulfoxide
a Positive controls were methylmethanesulfonate for TA100, 2-nitrofluorene for TA98 and TA1538, sodium azide for TA1535,
ICR-191 for TA1537, N-ethyl- N'-nitro- N-nitrosoguanidine for WP2 uvrA, benzo[ a]pyrene for TA100, TA98, TA1537, and TA1538
and 2-aminoanthracene for TA1535 and WP2 uvrA.
b Positive controls were mitomycin C for the direct assay and sterigmatocystin for the activation assay. The negative
control was kanamycin in both assays.
c Positive controls were ethylmethane sulfonate for the direct assay and 9,10-dimethyl-1,2-benzanthracene for the activation
assay.
d Positive controls were 2-acetylaminofluorene for the activation assay and 4-nitroquinoline-1-oxide for the direct assay.
e Positive controls were mitomycin C for the direct assay and cyclophosphamide for the activation assay.
f Positive controls were mitomycin C for the direct assay and benzo[ a]pyrene for the activation assay.
g Positive control was mitomycin C.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 26 male and 26 female Sprague-Dawley (Crj) rats were
given diets containing technical-grade pyriproxyfen (purity, 95.3%) at
concentrations of 0, 200, 1000, or 5000 ppm. The F0 animals were
treated for 70 days before mating and then for 6 subsequent weeks for
males and during 3 weeks of gestation and 3 weeks of the lactation
period for females. The F1 generation were treated for 18 weeks from
the day of their weaning to the day of weaning of the F2 generation,
including 77-90 days before mating and the mating, gestation, and
lactation periods. The mean daily intakes of pyriproxyfen were 14, 68,
and 340 mg/kg bw per day in males and 20, 98, and 500 mg/kg bw per day
in females (18, 87, and 440 mg/kg bw per day before mating, 15, 77,
and 390 mg/kg bw per day during gestation, and 32, 160, and 830 mg/kg
bw per day during lactation) in the F0 generation, and 17, 83, and
440 mg/kg bw per day in males and 21, 110, and 560 mg/kg bw per day in
females (21, 100, and 550 mg/kg bw per day before mating, 14, 72, and
380 mg/kg bw per day during gestation, and 28, 160, and 810 mg/kg bw
per day during lactation) in the F1 generation.
The observations in parental rats included clinical signs,
deaths, food consumption, body weight, estrus cycle, and
histopathological and reproductive parameters which included mating,
fertility, gestation, and live birth indices. All parental animals
were killed at the end of weaning, and the reproductive organs, brain,
pituitary, liver, and kidney were examined histopathologically. The
organs from the F1 parental animals were weighed. Estrus cycles were
examined by a smear assay during the 10 days before mating. The
observations in the F1 and F2 pups included viability, body
weight, and lactation indices. Developmental indices were not
examined. Groups of 10 male and 10 female pups were selected randomly
from 10 litters for necroscopy. One male and one female were selected
randomly from each F1 litter to provide 26 pairs at each dose to
serve as parents for the F2 generation. Pups were weighed by sex.
The study complied with GLP.
The F0 parent animals showed no treatment-related changes in
clinical signs, mortality rate, reproductive parameters, or estrus
cycle. Body weight and body-weight gain were significantly reduced in
F0 animals at 5000 ppm during the periods of premating, gestation,
and lactation, and food consumption was significantly reduced in F0
females at this dose during gestation. No gross or histopathological
alterations were seen that were related to treatment. The NOAEL for
F0 parental toxicity was 1000 ppm, equal to 68 mg/kg bw per day, on
the basis of reductions in body weight and body-weight gain at 5000
ppm. The NOAEL for reproductive toxicity was 5000 ppm, equal to 340
mg/kg bw per day, the highest dose tested.
The F1 parent animals showed no treatment-related changes in
clinical signs, mortality rate, reproductive parameters, or estrus
cycles. The body weights of F1 males and F1 females at 5000 ppm
were significantly reduced, and the terminal body weights of animals
at this dose were significantly decreased (to 98% at 1000 ppm and 88%
at 5000 ppm in males and 100% at 1000 ppm and 95% at 5000 ppm in
females). Body-weight gain was also significantly reduced in F1
males at 5000 ppm during the premating period. Food consumption was
significantly reduced at this dose in F1 males during treatment and
in F1 females during gestation. The absolute weights of the liver
were significantly increased in F1 adults at 5000 ppm (110% at 1000
ppm and 110% at 5000 ppm in males and 120% at 5000 ppm in females),
and the relative weights were significantly increased in males at 1000
ppm (110% of control) and in animals of each sex at 5000 ppm (130% in
males and 130% in females). Histopathological examination showed an
increased incidence of focal clear cells in the liver in males at 5000
ppm, but this effect is commonly observed in male rats and was
considered to be unrelated to treatment. Significant increases in
relative kidney weights were observed in males at 1000 ppm (110% of
control values) and 5000 ppm (110%), but the absolute weights were not
significantly increased at any dose. Histopathological examination
showed an increased incidence of chronic interstitial nephrosis (7/26
at 0 ppm, 3/26 at 200 ppm, 7/26 at 1000 ppm, and 15/26 at 5000 ppm) in
males at 5000 ppm and in the incidence of hydronephrosis (1/26 at 0
ppm and 4/26 at 5000 ppm) in females at this dose; however, these
increases did not reach statistical significance. No other
treatment-related morphological lesions were observed. The NOAEL for
F1 parental toxicity was 1000 ppm, equal to 83 mg/kg bw per day, on
the basis of decreased body weight, decreased food consumption, and
increased absolute and relative liver weights at 5000 ppm. No evidence
of reproductive toxicity was observed. The NOAEL for reproductive
toxicity was 5000 ppm, equal to 340 mg/kg bw per day, the highest dose
tested.
In F1 pups, there was no treatment-related change in clinical
signs, sex ratio, viability index, or lactation index and no
significant difference between treated and control groups in body
weight at birth. Significant reductions in body weight were observed
in male F1 pups on day 21 and in female F1 pups on days 14 and 21
post partum at 5000 ppm. The total litter weight was also
significantly decreased on days 14 and 21 post partum at this dose.
No treatment-related effects were seen on gross examination. F2 pups
also showed no treatment-related change in clinical signs, sex ratio,
viability index, or lactation index. The mean pup weights were
significantly decreased on days 14 and 21 post partum at 5000 ppm.
No gross pathological alteration related to treatment was apparent at
any dose. The NOAEL for developmental toxicity was 1000 ppm, equal to
98 mg/kg bw per day, on the basis of reduced body weight in F1 and
F2 pups at 5000 ppm (Robinson et al., 1991).
In a study of treatment before and during the early stages of
gestation (segment 1) conducted according to GLP, groups of 24 male
and 24 female Sprague-Dawley rats were given pyriproxyfen (purity,
97.2%) dissolved in corn oil by gavage at doses of 0, 100, 300, 500,
or 1000 mg/kg bw per day for 12 weeks comprising 9 weeks before mating
and 3 weeks of mating, in males or for at least 3 weeks including 2
weeks before mating and the mating period and on days 0-7 of gestation
in females. The observations in parental rats included clinical signs,
deaths, food consumption, body weight, organ weights, gross
appearance, and reproductive performance including copulation and
fertility indices. Clinical examinations were performed twice a day.
Males were killed at the end of mating, and female animals on day 21
of gestation.
Treatment-related deaths occurred in two females at the highest
dose. Increased incidences of diarrhoea and erythema and swelling of
the anal region were observed in females at 300 mg/kg bw and in
animals of each sex at doses > 500 mg/kg bw per day. Dose-dependent
increases in the incidence of salivation were frequently observed in
all treated groups, with incidences of 0/24 in controls and 18/24,
21/24, 24/24, and 24/24 at the four doses respectively, in males, and
0/24, 1/24, 5/24, 7/24, and 8/24, respectively, in females. Body
weight was significantly reduced in females at 100 mg/kg bw per day
during gestation and in animals of each sex at doses > 300 mg/kg bw
per day throughout the study. Body-weight gain and food consumption
were also significantly decreased in males at doses > 300 mg/kg bw
per day throughout the study, but significant decreases were observed
only during treatment in females. Water consumption was significantly
increased in males at doses > 100 mg/kg bw per day and in females
at doses > 300 mg/kg bw per day. The absolute weights of the liver,
kidney, and adrenal glands were significantly increased and the
absolute weight of the thymus was significantly decreased in males at
doses > 300 mg/kg bw per day. In females, the absolute weights of
the kidney and adrenals were significantly increased at 1000 mg/kg bw
per day. Enlarged, dark-red livers, pitted, enlarged kidneys, enlarged
adrenals, and thymus atrophy were observed in males at doses > 300
mg/kg bw per day, and congested, enlarged livers, enlarged adrenals,
and atrophy of the spleen and thymus were observed in females at
1000 mg/kg bw per day. There was no treatment-related change in
reproductive performance. No NOAEL could be identified for maternal
toxicity, as reduced body weight during gestation was seen at all
doses. The NOAEL for reproductive toxicity was 1000 mg/kg bw per day,
the highest dose tested.
Slightly but significantly reduced numbers of corpora lutea (90%
of control) and of live fetuses (88% of control) were observed at 1000
mg/kg bw per day, but the values were within the range in historical
controls. Slight increases in placental weight and in fetal body
weight were observed at 1000 mg/kg bw per day, but the effects were
not dose-dependent and were within the range in historical controls. A
slight but significant increase in the number of phalanges of the
proximal forepaw was observed, but such increases are known to be
associated with an altered growth rate. The incidences of external,
visceral, and skeletal anomalies were not increased. The NOAEL for
developmental toxicity was 1000 mg/kg bw per day, the highest dose
tested (Saegusa et al., 1988a).
In a study of treatment during the perinatal and lactation
periods (segment 3) conducted according to GLP, groups of 23-24
pregnant Sprague-Dawley rats were given pyriproxyfen (purity, 97.2%)
dissolved in corn oil by gavage at doses of 0, 30, 100, 300, or 500
mg/kg bw per day from day 17 of gestation to day 20 post partum. The
dams were allowed to deliver naturally. On day 4 post partum, the
offspring were culled to adjust the litter size to eight (four males
and four females when possible) for tests of development (F1I), and
the remaining pups (F1II) were killed for skeletal examination on
the day of culling. The dams (F0) were killed at termination of
weaning. The observations in maternal rats included clinical signs,
deaths, body weight, food consumption, organ weights, gross
appearance, and reproductive indices including delivery rate, birth
rate, and body weight of live newborns. The F1I offspring were
weighed on days 0, 4, 7, 14, and 21 post partum during lactation;
after weaning, one male and one female per litter were weighed once a
week. Clinical signs were observed twice a day. The indices of
development during lactation included separation of the auricle,
emergence of abdominal hair, eruption of incisors, separation of
eyelids, and descent of testes or opening of the vagina. On day 20,
all F1 offspring were examined for sensory function in tests for
visual placing, righting and mid-air righting reflexes, and response
to pain. On day 21 post partum, two male and two female offspring
from each litter (F1Ia) were killed for visceral and skeletal
examination. After weaning, another male and female from each litter
(F1Ib) were examined for emotionality (behaviour in an open field)
at 4 weeks of age, for motor coordination (rotarod performance) at 5
weeks of age, and for learning ability (in a water-filled multiple T
maze) at 6 weeks of age. The F1Ib offspring were killed for necropsy
on day 56 post partum. Another male and female from each litter
(F1Ic) were tested only for reproductive performance after being
paired for mating within the same group (avoiding sibling matings) at
11 weeks of age. The fetuses were removed surgically from mated
females on day 21 of gestation. Reproductive performance was assessed
from indices of mating, fertility, gestation, and litters. The fetuses
(F2) were weighed and examined for external anomalies.
In the F0 maternal animals, treatment-related deaths were
observed at 500 mg/kg bw per day, an increased incidence of diarrhoea
at doses > 300 mg/kg bw per day, and dosedependent increases in the
incidence of salivation at doses > 100 mg/kg bw per day (0/23 in
controls and 0/23, 1/23, 2/24, and 4/24 at the four doses,
respectively). During gestation, significantly reduced body weights
were observed at 500 mg/kg bw per day, and significantly reduced
body-weight gain and food consumption were seen frequently during the
study at doses > 300 mg/kg bw per day. The absolute and relative
weights of the liver were significantly increased at doses > 300
mg/kg bw per day. Atrophy of the thymus, congestion of the liver and
kidneys, enlargement of the adrenals, and atrophy of the spleen were
observed at 500 mg/kg bw per day, and an increased number of
stillborns was seen at this dose. The body weight of live newborn pups
was significantly decreased at doses > 300 mg/kg bw per day. The
NOAEL for maternal toxicity was 100 mg/kg bw per day, on the basis of
clinical signs, reductions in body weight, body-weight gain, and food
consumption, and decreased body weight of delivered newborn at 300
mg/kg bw per day.
At 500 mg/kg bw per day, the survival rate of F1 offspring was
significantly decreased on days 0-4 post partum, and the weaning
rate was decreased on days 4-21 post partum. No decrease in the
viability of F1I offspring was observed on days 21-77. The body
weights of pups of each sex at doses > 300 mg/kg bw per day were
significantly reduced on days 0-49 or 56 post partum. All of the
physical developmental indices were significantly retarded and slight
retardation of sexual differentiation was observed at doses > 300
mg/kg bw per day. No treatment-related change in sensory functions was
observed in F1I offspring. Visceral examination of the F1Ia
offspring on day 21 post partum revealed a significant increase in
the number with anomalies at 30, 300, and 500 mg/kg bw per day (0% in
controls and 10%, 1.2%, 17.2%, and 29% at the four doses,
respectively).The anomalies consisted mainly of dilatation of the
renal pelvis and hyperaemia and/or inflammatory cell infiltration. The
incidence of dilatation of the renal pelvis was significantly
increased at doses > 300 mg/kg bw per day (0% in controls and 3.8%,
1.2%, 14%, and 18% at the four doses, respectively, with 0-3.2% in
historical controls). The incidence of hyperaemia and/or inflammatory
cell infiltration was significantly increased at 500 mg/kg bw per day
(0% in controls and 3.8%, 0%, 5.8%, and 12% at the four doses,
respectively). Although developmental insufficiency of the papilla or
stenosis and obstruction of the urethra can cause dilatation of the
renal pelvis, as can developmental retardation, the range in
historical controls of this strain was 0-2.0% in fetuses and 0-3.2% in
21-day-old offspring, and recovery from dilatation of the renal pelvis
usually occurs after birth. No skeletal anomalies were found that were
related to treatment. The absolute weights of all organs of F1
offspring killed on day 21 post partum were slightly but
significantly decreased, but no treatment-related change was found in
the absolute weights of the organs of F1Ib offspring killed on day
56 post partum. The changes in organ weights might have been due to
retarded physical development. Ambulation was also slightly but
significantly increased at doses > 100 mg/kg bw per day (by 31% at
100 mg/kg bw per day, 50% at 300 mg/kg bw per day, and 37% at 500
mg/kg bw per day). Motor coordination was comparable to that of
controls, and no treatment-related changes in learning ability were
observed. All the changes observed in the F1 offspring, including
increased ambulation, could have been due to retarded physical
development. After maturation, the reproductive perfomance of F1Ic
offspring was comparable to that of controls. The body weight and
body-weight gain of pregnant F1 animals were comparable to those of
controls during gestation. The NOAEL for developmental toxicity was
100 mg/kg bw per day, on the basis of decreased body weight,
retardation of physical development, and visceral anomalies associated
with the retarded growth rate at 300 mg/kg bw per day.
Significantly reduced numbers of implantations (12 in controls
and 10, 12, 11, and 9.2 at the four doses, respectively) and mean
numbers of live F2 fetuses (12 in controls and 9.3, 11, 10, and 8.5
at the four doses, respectively) were observed at 500 mg/kg bw per
day. Significantly reduced numbers of corpora lutea, implantations,
and live fetuses were found at 30 mg/kg bw per day but not at 100
mg/kg bw per day. These reductions were not dose-dependent. There were
no treatment-related differences in fetal body weight or in the
incidence of external anomalies between treated and control groups.
The NOAEL for F1 reproductive toxicity was 300 mg/kg bw per day, on
the basis of a reduction in the number of implantations and in the
mean number of live fetuses at 500 mg/kg bw per day (Saegusa et al.,
1988b).
(ii) Developmental toxicity
Rats
In a study that complied with GLP, groups of 36 female
Sprague-Dawley rats were given pyriproxyfen (purity, 97.2%) dissolved
in corn oil by gavage at doses of 0, 100, 300, or 1000 (42 animals)
mg/kg bw per day on days 7-17 of gestation. The fetuses (F1I) were
removed surgically from 23 pregnant dams (F0I) at 0, 100, or 300
mg/kg bw per day and from 20 at 1000 mg/kg bw per day on day 21 of
gestation and examined for developmental toxicity. The remaining 10-13
dams (F0II) were allowed to deliver normally. On day 4 post
partum, the offspring were culled to adjust the litter size to eight
(four males and four females when possible) for functional testing on
day 20 post partum (F1IIa). The remaining pups (F1IIb) were
killed at 21 days of age and examined for skeletal anomalies. During
lactation, developmental indices were examined. After weaning, one
male and one female from each litter (F1IIa1) allocated for
functional testing were examined for emotionality at 4 weeks of age,
for motor coordination at 5 weeks of age, and for learning ability at
6 weeks of age; these offspring were killed for necropsy at 8 weeks of
age. Another male and female from each litter (F1IIa2) were tested
for reproductive performance after being paired for mating within the
same group (avoiding sibling mating) at 11 weeks of age, and the
fetuses were removed surgically from the mated females on day 21 of
gestation. The remaining offspring (F1IIa3) were killed for
necroscopy at 21 days of age, after weaning.
The observations in parental rats included clinical signs,
deaths, food and water consumption, body weight, estrus cycle, and
organ weights. The postnatal developmental indices included separation
of the auricle, emergence of abdominal hair, eruption of incisors,
separation of eyelids, and descent of testes or opening of the vagina.
The sensory function tests included visual placing, righting and
mid-air righting reflexes, and response to pain. Emotionality was
evaluated by observation of behaviour in an open field, motor
coordination was examined in a rotarod performance test, and learning
ability was examined by behaviour in a water-filled multiple T maze
test. Reproductive performance included indices of mating, fertility,
gestation, and litters.
Of the F0 dams, 12 of 42 at 1000 mg/kg bw per day died between
day 11 and day 16 of gestation. No deaths occurred in the other
groups. Signs of toxicity were observed at doses > 300 mg/kg bw per
day. At the highest dose, these included diarrhoea, erythema and
swelling of the anal region, hypoactivity, wasting, hypothermia,
lachrymation, and piloelection. Signs of toxicity were also observed
at 300 mg/kg bw per day but in only one animal. Body weight and
body-weight gain were significantly reduced at 300 and 1000 mg/kg bw
per day during gestation and at 100 mg/kg bw per day during treatment.
Food consumption was significantly reduced at doses > 100 mg/kg bw
per day during treatment, and water consumption was dose-dependently
and significantly increased at doses > 300 mg/kg bw per day. Thymic
atrophy and enlarged adrenals were observed in the dams that died and
those killed on day 21 of gestation (F0I) at 1000 mg/kg bw per day.
In the F0I dams, from which fetuses were removed surgically on
day 21 of gestation, slight but significant increases were found in
the relative weights of the liver and kidney at 300 mg/kg bw per day,
but no significant increase in the absolute weights was observed at
this dose. At 1000 mg/kg bw per day, the absolute weights of the
kidney and adrenal were significantly increased and the absolute
weight of the thymus was significantly decreased. The relative weight
of the liver was significantly increased, but the absolute weight was
not significantly affected at 1000 mg/kg bw per day. Higher ratios of
resorbed or dead fetuses were observed at 1000 mg/kg bw per day (4.7%
in controls, 7.7% at 300 mg/kg bw per day, and 15% at 1000 mg/kg bw
per day). None of these differences achieved statistical significance
or was outside the historical control range (5.7%; 1.5-20%). There was
no difference in the mean numbers of corpora lutea or implantations or
the implantation rate. In the F0II dams, body weight and body-weight
gain were significantly reduced at 300 and 1000 mg/kg bw per day
during lactation. There were no treatment-related changes in the
number of live newborn, the length of gestation, or the delivery rate.
No NOAEL could be identified for F0 maternal toxicity since
decreased body-weight gain was seen at all doses. The NOAEL for
reproductive toxicity was 1000 mg/kg bw per day, the highest dose
tested.
No significant change in F1I fetal sex ratio, placental weight,
or fetal body weight was observed, but the ratio of resorbed or dead
fetuses was increased (5% in controls and 15% at 1000 mg/kg bw per
day) and the number of live fetuses was decreased at the highest dose,
but these changes were not statistically significant. Cyclopia and
polydactyly were each observed in one fetus at 300 mg/kg bw per day
(0.7%), but not in any other group, and these anomalies were
considered to be unrelated to treatment. There were no
treatment-related increases in the incidences of external, visceral,
or skeletal anomalies. A significant increase in the number of fetuses
with a skeletal variation consisting of opening of the foramen
transversarium of the seventh cervical vertebra was seen at 300 and
1000 mg/kg bw per day (0% in controls and 1.5%, 5.0%, and 14% at the
three doses, respectively). An increased number of fetuses with an
extra lumbar rib was observed at 1000 mg/kg bw per day, but this was
not significant. The NOAEL for developmental toxicity was 300 mg/kg bw
per day, on the basis of increased skeletal variations at 1000 mg/kg
bw per day.
The F1IIa offspring showed no-treatment related effect on
survival or weaning rates. The body weights of the treated groups were
comparable to those of controls during lactation and before mating.
There were no treatment-related effects on postnatal physical
development, sexual differentiation, sensory function, emotional
behaviour, or motor coordination. A significant retardation in the
time taken to reach the goal in the water-filled multiple T maze was
observed in females at 1000 mg/kg bw per day, but this slight
reduction in learning behaviour was observed only in the second of
three consecutive daily trials. There was no impairment of
reproductive performance of F1IIa2 offspring at any dose. No
external anomalies were observed in F2 fetuses. The NOAEL for
developmental toxicity was 1000 mg/kg bw per day, if the slight
evidence of behavioural teratogenicity in F1 offspring is ignored.
In the offspring killed at 21 days of age (F1IIb), an increased
incidence of skeletal variations was observed at 1000 mg/kg bw per day
(9% in controls and 18% at 1000 mg/kg bw per day), but this was not
statistically significant. No external or skeletal anomalies were
observed. The incidence of visceral anomalies was increased
significantly in F1IIaI offspring at 1000 mg/kg bw per day killed at
56 days of age (0/46 in controls and 7/39 at 1000 mg/kg bw per day).
F1IIb and F1IIaI offspring at 1000 mg/kg bw per day also showed an
increased incidence of dilatation of the renal pelvis. The total
incidence of dilatation of the renal pelvis observed in F1I
offspring (killed at 21 and 56 days of age and at the end of the
fertility test) was increased dose-dependently (0/98 in controls and
1/95, 3/85, and 9/79 at the three doses, respectively). The incidences
in controls from 15 previous studies were 0-2.0% in fetuses, 0-3.2% in
offspring 21 days old, 0-4.3% at 56 days of age, and 0-4.5% at the end
of the fertility test. The incidence in the controls in the present
study was 0% at all times. The incidence of protrusion or partial
adhesion of the liver parenchyma on the diaphragmatic side was also
increased at 1000 mg/kg bw per day (1/98 in controls and 0/95, 2/85,
and 6/79 at the three doses, respectively). These anomalies were not
observed in fetuses removed surgically. The overall NOAEL for
developmental toxicity was 300 mg/kg bw per day, on the basis of an
increased incidence of skeletal variations in fetuses and an increased
incidence of visceral anomalies in offspring at 1000 mg/kg bw per day
(Saegusa et al., 1988c).
Rabbits
Groups of 15-18 female JW-NIBS rabbits were treated by gavage
with pyriproxyfen (purity, 97.2%) at doses of 0, 100, 300, or 1000
mg/kg bw per day on days 6-18 of gestation and were killed on day 28
of gestation. The study complied with GLP.
In the maternal animals, abortion or premature delivery occurred
at doses > 300 mg/kg bw per day, in one control, none at 100 mg/kg
bw per day, three at 300 mg/kg bw per day, and six at 1000 mg/kg bw
per day. Dead and moribund animals were found at 1000 mg/kg bw per day
(none at 0, 100, or 300 mg/kg bw per day and three at 1000 mg/kg bw
per day). Several signs of toxicity, including soft stools,
emaciation, decreased spontaneous activity, and bradypnoea were
observed in aborted, prematurely delivered, prematurely dying, and
moribund dams at doses > 300 mg/kg bw per day. Body weight,
body-weight gain, and food consumption were significantly reduced in
dams at 1000 mg/kg bw per day. There were no significant effects on
the mean number of corpora lutea or implantations or the number, sex
ratio, or body weight of live fetuses.
The live fetuses showed no treatment-related external anomalies.
Skeletal or visceral malformations were observed in fetuses at 300
mg/kg bw per day, comprising a defect of the third distal phalanx of
the hind leg in one; cystic lung, ventricular septal defect,
hypoplasia of the left atrial auricle, and persistent truncus
arteriosus in one; and a defect of the gall-bladder in one. None was
observed in the other groups. External malformations were observed in
three fetuses, comprising local oedema, a visceral malformation, and
microphthalmia in one fetus at 300 mg/kg bw per day; persistent
truncus arteriosus in one fetus at 1000 mg/kg bw per day; and a
ventricular septal defect in another at this high dose. The total
incidence of malformations was 1% in controls, 1% at 100 mg/kg bw per
day, 6% at 300 mg/kg bw per day, and 2% at 1000 mg/kg bw per day. The
authors concluded that pyriproxyfen did not cause treatment-related
changes in the incidences of skeletal anomalies, skeletal variations,
ossification, visceral anomalies, or visceral variations. The NOAEL
for reproductive toxicity was 100 mg/kg bw per day on the basis of
abortion or premature delivery and a number of signs of toxicity at
300 mg/kg bw per day. The NOAEL for developmental toxicity was 1000
mg/kg bw per day, the highest dose tested (Hirohashi et al., 1988).
(f) Studies on metabolites
(i) Acute toxicity
The metabolites of pyriproxyfen were administered orally to mice
in a 0.5% solution of methylcellulose at 1000 or 2000 mg/kg bw. One
out of five males given 5''-hydroxypyriproxyfen (purity, 97.5%) at
2000 mg/kg bw died, but no deaths occurred with the other metabolites.
4'-Hydroxypyriproxyfen (purity, 98.3%) caused no abnormal clinical
signs; 5''-hydroxypyriproxyfen caused decreased spontaneous activity
in animals at both doses and ataxic gait at 2000 mg/kg bw;
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen
(purity, 90.1%) produced decreased spontaneous activity, ataxic gait,
and prone position in animals at 2000 mg/kg bw; (RS)-2-hydroxypropyl
4-phenoxyphenyl ether (purity, 99.0%) produced decreased spontaneous
activity, ataxic gait, prone position, lateral position, and irregular
respiration in animals at 2000 mg/kg bw; and (RS)-2-(2-pyridyloxy)
propionic acid (purity, 100%) produced decreased spontaneous activity
at 2000 mg/kg bw (Misaki, 1993a).
(ii) Genotoxicity
4'-Hydroxypyriproxyfen (purity, 98.3%), 5''-hydroxypyriproxyfen
(purity, 97.5%), 4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether
pyriproxyfen (purity, 90.2%), (RS)-2-hydroxypropyl 4-phenoxyphenyl
ether (purity, 99.0%), and (RS)-2-(2-pyridyloxy) propionic acid
(purity, 100%) were tested for mutagenicity in Salmonella
typhimurium TA98, TA100, TA1535, and TA1537 and in Escherichia
coli WP2 uvrA, with and without exogenous metabolic activation at
concentrations of 15-5000 µg/plate. None of the metabolites caused
reverse mutation, whereas the positive controls used in these assays
produced the anticipated responses (Hara et al., 1993).
No data were available on the effects of the metabolites of
pyriproxyfen on chromosomal integrity, but as these metabolites are
formed in vivo and pyriproxyfen had no effect on this end-point,
this is not considered a major gap in the data.
Comments
After oral administration to rats, [14C]pyriproxyfen is slowly
(time to peak concentration in plasma, 8 h) and incompletely (< 50%
of the dose) absorbed but is then rapidly eliminated, predominantly in
the faeces (90%), with only 4-11% in the urine, after 48 h. Absorbed
pyriproxyfen is excreted mainly via the bile (34-37% of the
administered dose in 48 h). The metabolism of pyriproxyfen is
qualitatively similar in rats, mice, lactating goats, and laying hens.
A large number of metabolites have been detected, the main route of
biotransformation being 4'-hydroxylation. Other pathways include
hydroxylation of the pyridyl ring, ether cleavage and conjugation.
Mice conjugate a much greater proportion of the dose than rats. The
concentration of pyriproxyfen in tissues other than fat was very low
(generally < 0.01 µg equivalent per g after 72 h; fat < 0.1µg
equivalent per g). The half-times of the radiolabel in tissues,
including blood and fat, were 8-36 h. The dermal absorption of
pyriproxyfen has not been studied.
The acute oral toxicity of pyriproxyfen is low, with LD50
values > 5000 mg/kg bw in mice, rats, and dogs. The acute dermal
toxicity is also low, with LD50 values > 2000 mg/kg bw in mice and
rats, and after exposure by inhalation, with an LC50 value > 1.3
mg/l air in mice and rats. WHO (1999) has classified pyriproxyfen as
'unlikely to present acute hazard in normal use'. Pyriproxyfen was
mildly irritating to the eye but not to the skin of rabbits. It did
not sensitize the skin of Hartley guinea-pigs in a maximization test.
In short- and long-term studies of the effects of pyriproxyfen in
mice, rats, and dogs, the liver was the main toxicological target,
with increases in liver weight and changes in plasma lipid
concentrations, particularly cholesterol, at doses of 120 mg/kg bw per
day and above in rats. There was some evidence that the compound might
cause modest anaemia in mice, rats, and dogs at high doses. In mice
treated with pyriproxyfen in the diet for three months, additional
effects seen included increased mortality rates, histopathological
changes in the kidney, and decreased body weight. The NOAEL was 150
mg/kg bw per day in mice, 23 mg/kg bw per day (two studies) in rats,
and 100 mg/kg bw per day in dogs fed pyriproxyfen in the diet for 3
months. In long-term studies of toxicity in mice, pyriproxyfen also
caused a dose-dependent increase in the occurrence of systemic
amyloidosis, which was associated with increased mortality rates. The
NOAEL was 120 ppm, equal to 16 mg/kg bw per day. In rats, the only
additional effect was reduced body-weight gain, and the NOAEL was 600
ppm, equal to 27 mg/kg bw per day. In two 1-year studies in dogs,
pyriproxyfen was administered in capsules. The overall NOAEL was 10
mg/kg bw per day on the basis of increased relative liver weight and
increased total plasma cholesterol concentration in males. There was
some evidence that pyriproxyfen can act as a hepatic enzyme inducer,
at least in dogs. Pyriproxyfen was not toxic when administered
dermally to rats for 21 days at doses of up to 1000 mg/kg bw per day.
Inhalation of pyriproxyfen for 4 h per day for 28 days caused only
minor effects in rats (initial salivation, sporadically reduced
body-weight gain, slightly increased serum lactate dehydrogenase
activity) at 10 000 mg/m3. The NOAEL was 480 mg/m3.
Pyriproxyfen was not carcinogenic when given in the diet at doses
up to 420 mg/kg bw per day in a study in mice or at doses up to 140
mg/kg bw per day in rats. Pyriproxyfen showed no evidence of
carcinogenicity in a 1-year study in dogs at doses up to 1000 mg/kg bw
per day. The Meeting concluded that pyriproxyfen does not pose a
carcinogenic risk to humans.
Pyriproxyfen was not genotoxic in an adequate range of tests for
mutagenicity and cytogenicity in vitro and in vivo. The Meeting
concluded that pyriproxyfen is not genotoxic.
The reproductive toxicity of pyriproxyfen in rats has been
investigated in a two-generation study of reproductive toxicity, a
study involving treatment of males and females before and in the early
stages of gestation (segment 1), and a study of treatment during the
prenatal and lactation periods (segment 3). The NOAEL for maternal
toxicity was 1000 ppm, equal to 98 mg/kg bw per day, in the
two-generation study and 100 mg/kg bw per day in the segment 3 study.
Reproductive toxicity was observed only in the segment 3 study, in
which there was an increased number of stillbirths in the F0
generation and a reduction in the number of implantations and in the
mean number of live fetuses in the F1 generation at 500 mg/kg bw per
day. The NOAEL for reproductive toxicity was 300 mg/kg bw per day. No
reproductive toxicity was observed in the two-generation study, the
NOAEL being 5000 ppm, equal to 340 mg/kg bw per day, the highest dose
tested, or in the segment 1 study, the NOAEL being 1000 mg/kg bw per
day, the highest dose tested.
The developmental toxicity of pyriproxyfen has been studied in
rats and rabbits. In rats, a NOAEL for maternal toxicity was not
identified, as decreased body-weight gain was observed at 100 mg/kg bw
per day, the lowest dose tested. Pyriproxyfen caused little
developmental toxicity and was not teratogenic. In a segment 3 study,
the F1 offspring were subjected to a series of developmental tests
for possible neurotoxicity, including physical indices, tests of
behaviour, motor and sensory function, and learning ability. Although
there were some effects on growth at doses > 300 mg/kg bw per day,
there was no developmental neurotoxicity at 500 mg/kg bw per day, the
highest dose tested. Visceral anomalies (dilatation of the renal
pelvis) were found at doses > 300 mg/kg bw per day. The NOAEL for
developmental toxicity was 100 mg/kg bw per day, on the basis of
retarded physical development and visceral anomalies at higher doses.
In a more conventional study of developmental toxicity in rats, no
evidence of growth retardation or of developmental neurotoxicity was
found at doses up to and including 1000 mg/kg bw per day, the highest
dose tested. There was an increased frequency of skeletal variations
(opening of the foramen transversalium of the seventh cervical
vertebra) in fetuses at 300 mg/kg bw per day. The frequency of
visceral anomalies was significantly increased in F1 offspring some
weeks after birth. The NOAEL for developmental toxicity was 300 mg/kg
bw per day, on the basis of an increased frequency of skeletal
variations with visceral anomalies in F1 offspring at 1000 mg/kg bw
per day. In a study of developmental toxicity in rabbits, signs of
maternal toxicity (abortion and premature delivery) were evident at
doses > 300 mg/kg bw per day (NOAEL, 100 mg/kg bw per day). No
developmental toxicity was observed, the NOAEL being 1000 mg/kg bw per
day, the highest dose tested.
The Meeting established an ADI of 0-0.1 mg/kg bw on the basis of
the NOAEL of 10 mg/kg bw per day in 1-year studies of toxicity in dogs
and a safety factor of 100.
The Meeting concluded that it was not necessary to establish an
acute reference dose because of the low acute toxicity of
pyriproxyfen.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 120 ppm, equal to 16 mg/kg bw per day (18-month study of
carcinogenicity)
Rat: 600 ppm, equal to 27 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
5000 ppm, equal to 345 mg/kg bw per day (reproductive
toxicity, two-generation study of reproductive toxicity,
highest dose tested)
100 mg/kg bw per day (developmental toxicity in a segment 3
study of developmental toxicity)
Rabbit: 100 mg/kg bw per day (maternal and reproductive toxicity in
a study of developmental toxicity)
1000 mg/kg bw per day (developmental toxicity in a study of
developmental toxicity, highest dose tested)
Dog: 10 mg/kg bw per day (1-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information valuable for continued
evaluation of the compound
Observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to
pyriproxyfen
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Slow, incomplete absorption (< 50%), rat
Dermal absorption No data (no systemic toxicity up to 1000 mg/kg bw per day by
dermal route, rat)
Distribution of total residues Highest concentrations of radiolabel in fat and, to lesser
extent, liver, rat
Potential for accumulation Possible limited accumulation in fat, rat
Rate and extent of excretion Rapid, complete, 88-96% within 48 h, primarily in faeces;
4-11% in urine, rat
Metabolism in animals Extensive. No parent compound detectable in urine; numerous
metabolites: main pathway is 4'-hydroxylation; also hydroxylation
of the pyridyl ring, ether cleavage, conjugation, mouse, rat,
goat, hen
Toxicologically significant compounds Pyriproxyfen
(animals, plants and environment)
Acute toxicity
LD50, oral > 5000 mg/kg bw, mouse, rat
LD50, dermal > 2000 mg/kg bw, mouse, rat
LC50, inhalation > 1.3 mg/L, mouse, rat
Dermal irritation Not irritating, rabbit
Ocular irritation Mildly irritating, rabbit
Dermal sensitization Not a sensitizer, guinea-pig
Short-term toxicity
Target/critical effect Mouse, rat, dog: liver, increased relative liver weight, mild
anaemia, altered lipid metabolism (increased serum cholesterol)
Lowest relevant oral NOAEL 13 weeks, rat, 24 mg/kg bw per day
Lowest relevant dermal NOAEL 21 days, rat, > 1000 mg/kg bw per day
Lowest relevant inhalation NOAEL 28-day, rat, > 1.3 mg/L
Long-term toxicity and carcinogenicity
Target/critical effect: Mouse, rat, dog: liver, increased liver weight, decreased body
weight, altered lipid metabolism (increased plasma cholesterol)
(rat, dog)
Lowest relevant NOAEL 1 year, dog, 10 mg/kg bw per day (diet)
Carcinogenicity Not carcinogenic, mouse, rat
Genotoxicity Not genotoxic
Reproductive toxicity
Reproductive target/critical effect Reduction in number of implantations and live F2 fetuses at F1
developmentally toxic dose, rat
Lowest relevant reproductive NOAEL 345 mg/kg bw per day, rat
Developmental target/critical effect Retardation of physical development in F1, rat
Lowest relevant developmental NOAEL 100 mg/kg bw per day, rat
Neurotoxicity/Delayed neurotoxicity No evidence of developmental neurobehavioural toxicity in rat. No
evidence of neurotoxicty or neuropathology in medium- or long-term
studies in mouse, rat, dog or during development in rat, rabbit
Other toxicological studies Possible enzyme inducer, at least in dogs
Medical data No data
Summary Value Study Safety factor
ADI 0-0.1 mg/kg bw 1-year, dog, toxicity 100
Acute reference dose Unnecessary
References
Cardy, R., Moore, M., Murphy, B.S., Thakur, A., Tellone, C., Ito, S.,
Lang, P., Ginevan, M., Driver, J., Stewart, R. & Wilkinson, C.
(1994) Supplemental data and review of oncogenicity study with
S-31183 (Sumilarv) in mice (MRID No. 421783-10). Unpublished
study from Technology Sciences Group Inc. Reference No.
NNT-41-0116. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Chapman, E.A, Lee, P., Virgo, D.M. & Sparrow, S. (1991) S-31183:
Toxicity study by oral (capsule) administration to beagle dogs
for 52 weeks. Unpublished study from Life Science Research Ltd.
Reference No. NNT-11-0081. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Cox, R.H., Zoetis, T., Cardy, R.H., Alsaker, R.D., Kuhlman, S.M.,
Lewis, S.A., Thakur, A.K. & Phipps, N.G. (1989) Subchronic
toxicity Sstudy with S-31183 in rat. Unpublished study from
Hazleton Laboratories America, Inc. Reference No. NNT-910045.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Cox, R.H., Zoetis, T., Voelker, R.W., Alsaker, R.D., Kuhlman, S.M.,
Lewis, S.A., Thakur, A.K. & Phipps, N.G. (1990) Subchronic
toxicity study in mice. Unpublished study from Hazleton
Laboratories America, Inc. Reference No. NNT-01-0066. Submitted
to WHO by Sumitomo Chemical Co., Ltd.
Hara, M., Katoh, H. & Yoshitake, A. (1993a) Reverse mutation test of
metabolites of pyriproxyfen, 4'-OH-Pyr, 5"-OH-Pyr, DPH-Pyr, POPA
and PYPAC, in bacterial systems. Unpublished study reference No.
NNT-30-0104. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Henderson, L.M. & Proudlock, R.J. (1989) Assessment of unscheduled DNA
repair synthesis in mammalian cells after exposure to S-31183.
Unpublished study from Huntingdon Research Centre Ltd. Reference
No. NNT-91-0053. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Hirohashi, A., Kannan, N., Kato, T. & Yamada, H. (1988) Study of
S-31183 by oral administration during the period of fetal
organogenesis in rabbits. Unpublished study reference No.
NNT-80-0033. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Isobe, N., Matsunaga, H., Kimura, K., Yoshitake, A. & Yamada, H.
(1988a) Metabolism of S31183 in rats. Unpublished study reference
No. NNM-80-0001. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Isobe, N., Matsunaga, H., Kimura, K., Yoshitake, A. & Yamada, H.
(1988b) Metabolism of S-31183 in rat (tissue distribution study).
Unpublished study reference No. NNM-800002. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Kawaguchi, S., Watanabe, T., Suzuki T., Kato, T. & Yamada, H. (1987)
Acute inhalation toxicity of S-31183 in rats. Unpublished study
reference No. NNT-70-0022. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Kawaguchi, S., Yoshioka, K., Ito, S., Suzuki, T., Kato, T. & Yamada,
H. (1988) Subacute inhalation toxicity of S-31183 in rats.
Unpublished study reference No. NNT-80-0031. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Kogiso, S., Hara, M., Yoshitake, A. & Yamada, H. (1988a) Reverse
mutation test with S31183 in bacteria systems. Unpublished study
reference No. NNT-80-0034. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Kogiso, S., Hara, M., Iwawaki, H. & Yamada, H. (1988b) In vitro
chromosomal aberration test of pyriproxyfen in Chinese hamster
ovary cells (CHO-K1). Unpublished study reference No.
NNT-80-0028J. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Kogiso, S., Yamamoto, K., Hara, M., Yoshitake, A. & Yamada, H. (1989)
In vitro chromosomal aberration test of pyriproxyfen in Chinese
hamster ovary cells (CHO-K1). Unpublished study reference No.
NNT-90-0054. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Kogiso, S., Yamada, T., Hara, M., Yoshitake, A. & Yamada, H. (1990) In
vitro gene mutation test of S-31183 in V79 Chinese hamster cells.
Unpublished study reference No. NNT-000067. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Kogiso, S. Kato, H. & Nakattuka, S. (1992) Reverse mutation test with
S-31183 in bacteria systems. Unpublished study reference No.
NNT-80-0088J. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Koyama, Y., Kimura, J., Yoshioka, K., Watanabe, T., Seki, T.,
Hosokawa, S., Yamada, H. & Hagiwara, H. (1989) A six-month
chronic dietary toxicity study of pyriproxyfen in rats.
J. Toxicol. Sci., 14, 43-64.
Matsunaga, H., Yoshino, H., Isobe, N., Kaneko, H., Nakatuka, I. &
Yamada, H. (1995) Metabolism of pyriproxyfen in rats. 1.
Absorption, disposition, excretion, and biotransformation studies
with [phenoxyphenyl-14C] pyriproxyfen. J. Agric. Food Chem.,
43, 235-240.
Misaki, Y. & Nakatuka, I. (1993) Acute oral toxicity study of
pyriproxyphen metabolites, 4'-OH-Pyr, 5"-OH-Pyr, DPH-Pyr, POPA
and PYPAC in mice. Unpublished study reference No. NNT-30-0107.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Mitchell, D.J., Virgo, D.M., Broadmeadow, A., Chase, K.R., Lee, P. &
Sparrow, S. (1993) S31183: Toxicity study by oral (capsule)
administration to beagle dogs for 52 weeks (additional
investigation). Unpublished study from Life Science Research Ltd.
Reference No. NNT-31-0102. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Moore, M.R., Zoetis, T., Doyle, D., Cardy, R.H., Pearson, R.C.,
Walker, M.D., Lewis, S.A., Thomas, D.L., Thakur, A.K., Burlew,
P.L., Hatcher, C.F. & Vegarra, M. (1993) 21-day dermal toxicity
study in rats with S-31183. Unpublished study from Hazleton
Washington, Inc. Reference No. NNT-31-0094. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Nakano, M., Yamamoto, T., Kato, T. & Miyamoto, J. (1986) Acute oral
toxicity study of S31183 in dogs. Unpublished study reference No.
NNT-60-0012. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Nakano, M., Kohda, A., Kato, T. & Yamada, H. (1988) Three-month oral
toxicity study of S31183 in dogs. Unpublished study reference No.
NNT-80-0037. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Osheroff, M.R., Ziegler, K.A., Cardy, R.H., Alsaker, R.D., Kuhlman,
S.M., Lewis, S.A., Thakur, A.K., Burlew, P.L. & Nasca, A.J.
(1991a) Oncogenicity study in mice with S31183. Unpublished study
from Hazleton Laboratories America, Inc. Reference No.
NNT-11-0084. Submitted to WHO by Sumitomo Chemical Co.
Osheroff, M.R., Ziegler, K.A., Machotka, S., Alsaker, R.D., Kuhlman,
S.M., Lewis, S.A., Thakur, A.K., Burlew, P.L., Devis, P.J. &
Graham, R. (1991b) Combined chronic toxicity and oncogenicity
study in rats with S-31183. Unpublished study from Hazleton
Washington, Inc. Reference No. NNT-11-0085. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Panthani, A.M., Walsh, K.J. & Turck, P. (1996a) Metabolism of
[phenoxyphenyl-14C]S71639 (pyriproxyfen) in lactating goats.
Unpublished study from Ricerca, Inc. Reference No. NNM-0043.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Panthani, A.M., Walsh, K.J. & Turck, P. (1996b) Metabolism of
[pyridyl-14C]S-7 1639 (pyriproxyfen) in lactating goats.
Unpublished study from Ricerca, Inc. Reference No. NNM-0046.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Panthani, A.M., DiFrancesco, P. & Savides, M.C. (1996c) Metabolism of
[phenoxyphenyl14C]S-71639 (pyriproxyfen) in laying hens.
Unpublished study from Ricerca, Inc. Reference No. NNM-0045.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Panthani, A.M., DiFrancesco, P. & Savides, M.C. (1996d) Metabolism of
[pyridyl-14C]S71639 (pyriproxyfen) in laying hens. Unpublished
study from Ricerca, Inc. Reference No. NNM-0044. Submitted to WHO
by Sumitomo Chemical Co., Ltd.
Proudlock, R.J., Haynes, P. & Goodenough, A.J. (1991) Mouse
micronucleus test on S31183. Unpublished study from Huntingdon
Research Centre Ltd. Reference No. NNT11-0082. Submitted to WHO
by Sumitomo Chemical Co., Ltd.
Robinson, K., Washer, G. & Noveroske, J. (1991) A dietary 2-generation
(1 litter) reproduction study of S-31183 in the rat. Unpublished
study from Bio-Research Laboratories Ltd. Reference No.
NNT-11-0087. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Saegusa, T., Kitajima, S. & Narama, I. (1988a) Study on administration
of a test substance prior to and in the early stages of pregnancy
in rats. Unpublished study from Hamamatsu, Seigiken Research Co.,
Ltd. Reference No. NNT-80-0036. Submitted to WHO by Sumitomo
Chemical Co., Ltd.
Saegusa, T., Kitajima, S. & Narama, I. (1988b) Study on administration
of S-31183 during the perinatal and lactation periods in rats.
Unpublished study from Hamamatsu, Seigiken Research Co., Ltd.
Reference No. NNT-80-0030. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Saegusa, T., Kitajima, S. & Narama, I. (1988c) Study by administration
of S-31183 during the period of fetal organogenesis in rats.
Unpublished study from Hamamatsu, Seigiken Research Co., Ltd.
Reference No. NNT-80-0029. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Sako, H., Okuno, Y., Kato, T. & Yamada, H. (1987a) Acute
oral toxicity of S31183 in mice. Unpublished study reference No.
NNT-70-0014. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Suzuki, T., Misaki, Y., Okuno, Y., Kato, T. & Miyamoto, J. (1987b)
Acute oral toxicity of S31183 in rats, Unpublished study
reference No. NNT-70-0005. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Sako, H., Okuno, Y., Kato, T. & Yamada, H. (1987c) Acute
dermal toxicity of S31183 in mice. Unpublished study reference
No. NNT-70-0015. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Suzuki, T., Misaki, Y., Okuno, Y., Kato., T. & Miyamoto, J. (1987d)
Acute dermal toxicity of S-31183 in rats. Unpublished study
reference No. NNT-70-0006. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Kawaguchi, S., Watanabe, T., Kato, T. & Yamada, H. (1987e)
Acute inhalation toxicity of S-31183 in mice Unpublished study
reference No. NNT-70-0023. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Nakanishi, T., Kato, T. & Miyamoto, J. (1987f) Skin
sensitization test with S31183 in guinea pigs. Unpublished study
reference No. NNT-70-0003. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Nakanishi, T., Kato, T. & Miyamoto, J. (1987g) Primary eye
and skin irritation tests with S-31183 in rabbits. Unpublished
study reference No. NNT-70-000. Submitted to WHO by Sumitomo
Chemical Co., Ltd.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Yoshino, H. (1993a) Metabolism of pyriproxyfen in rat (high-dose,
14C-concentrations in tissues). Unpublished study reference No.
NNM-30-0028. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Yoshino, H. (1993b) Metabolism study of
(pyridyl-2,6-14C)pyriproxyfen in rat (pyridyl14C-labeled test
compound, single oral administration at low- and high-doses).
Unpublished study reference No. NNM-30-0025. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Yoshino, H., Kaneko, H., Nakatsuka, I. & Yamada, H. (1995) Metabolism
of pyriproxyfen. 2. Comparison of in vivo metabolism between rats
and mice. J. Agric. Food Chem., 43, 2681-2686.
Yoshino, H., Kaneko, H., Nakatsuka, I. & Yamada, H. (1996) Metabolism
of pyriproxyfen. 3. In vitro metabolism in rats and mice.
J. Agric. Food Chem., 44, 1578-1581.
Lactating goats
Lactating goats (Capra hircus, weighing 51-57 kg) were given
[14C-phenoxyphenyl]-pyriproxyfen (purity, 99.5%) in gelatin capsules
at a dose of 1.8-2.0 or 20 mg/animal per day for 5 consecutive days,
for a total dose of 100 mg. The animals were killed within 6 h of the
last dose. Faeces, urine, and milk were collected twice daily and
analysed. Metabolites were purified from extracts of tissues or
excreta by HPLC and identified by TLC and/or mass spectral analysis.
The study was carried out according to GLP. Elimination reached a
plateau by the third day. The percentages of total radiolabel
recovered 1 day after the last dose were 17-18% in urine, 58% in
faeces, and 0.3-0.8% in milk. The metabolites identified in milk
extracts were 4'-hydroxypyriproxy-fen sulfate (51% of the radiolabel
present), 4'-hydroxypyriproxyfen (2%), 4-phenoxyphenol sulfate (10%),
4'-hydroxy-4-phenoxyphenol sulfate (8%),
4'-hydroxy- (RS)-2-hydroxypropyl 4-phenoxyphenyl ether sulfate (3%),
and 4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen
(8%). Metabolites found in both liver and kidney were
4'-hydroxypyriproxyfen sulfate, 5''-hydroxypyriproxyfen-sulfate,
(RS)-2-hydroxypropyl 4-phenoxyphenyl ether,
4'-hydroxy (RS)-2-hydroxypropyl 4-phenoxyphenyl ether, and
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen. In
addition, 4'-hydroxypyriproxyfen and 5''-hydroxypyri-proxyfen were
identified in liver, and 4-phenoxyphenol sulfate and
4'-hydroxy-4-phenoxyphenol in kidney. The metabolites identified in
the faecal samples were 4'-hydroxypyriproxyfen (39% of the radiolabel
present), 5''-hydroxypyriproxyfen (6%),
4'-hydroxy- (RS)-2-hydroxypropyl 4-phenoxyphenyl ether (5%),
(RS)-2-hydroxypropyl 4phenoxyphenyl ether, and 4-hydroxyphenyl
(RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen. Parent compound
accounted for 11-13% of the dose in faeces, The major metabolites in
urine were 4-phenoxyphenol (36% of the radiolabel present),
4-phenoxyphenol sulfate (15%), 4'-hydroxy-4-phenoxyphenol (14%), and
4'-hydroxy-4-phenoxyphenol sulfate (17%) (Panthani et al., 1996a)
In another study that conformed to GLP, lactating goats
( Capra hircus, weighing 39-46 kg) were given
[14C-pyridyl]pyriproxyfen (purity, 97.6% ) in gelatin capsules at a
dose of 1.8-1.9 or 20 mg/animal per day for 5 consecutive days. The
animals were killed within 6 h of the last dose. Faeces, urine, and
milk were collected twice daily and analysed by HPLC and TLC and/or
mass spectrometry. The total radiolabel recovered represented 17-18%
of the dose in urine, 58% in faeces, and 0.4-0.8% in milk. The major
metabolites identified in milk extracts were 4'-hydroxypyriproxyfen
sulfate (35% of the radiolabel present) and 2,5-dihydroxypyridine
conjugate (29%), with smaller quantities of 4'-hydroxypyriproxyfen and
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen. The
major metabolites identified in liver and kidney were
4'-hydroxypyriproxyfen sulfate, 2,5-dihydroxypyridine conjugate, and
(RS)-2-(2-pyridyoxy) propanol conjugates. The major metabolites
identified in the urine were 4'-hydroxypyriproxyfen sulfate,
(RS)-2-(2-pyridyloxy) propionic acid, and 2,5-dihydroxypyridine
conjugate. The metabolites identified in the faecal samples were
4'-hydroxypyriproxyfen (43%), 5''-hydroxypyriproxyfen (5%), and
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen
(5%). These two studies indicate that the primary routes of metabolism
in goats are hydroxylation of the 4' position of the phenoxyphenyl
ring and the 5'' position of the pyridyl ring, cleavage of the ether
bonds, oxidation of the methylene moiety, and sulfate conjugation of
the 4'-hydroxyphenoxyphenyl moiety. There is thus no difference
between rats and goats in the metabolic pattern of pyriproxyfen
(Panthani et al., 1996b)
Chickens
Laying Leghorn hens (Gallus domesticus) were given
[14C-phenoxyphenyl]pyriproxyfen (purity, 99.1%) by gelatin capsule
at a concentration equivalent to 10 ppm in the feed for 8 consecutive
days and were killed within 4 h of the last dose. Excreta were
collected once daily and analysed by HPLC and TLC and/or mass
spectrometry. The study complied with GLP. The metabolites identified
in liver and kidney were free 4'-hydroxypyriproxyfen and its sulfate
conjugate, free 4'-hydroxy-4-phenoxyphenol and its sulfate conjugate,
free 4'-hydroxy- (RS)-2-hydroxypropyl-4-phenoxyphenyl ether and its
sulfate conjugate, 4-phenoxyphenol sulfate,
4-hydroxyphenyl- (RS)-2-(2-pyridyloxy)propyl ether pyriproxyfen, and
(RS)-2-hydroxypropyl-4-phenoxyphenyl ether. Metabolites identified
in excreta samples were 4'-hydroxypyriproxyfen, free and conjugated
4'-hydroxy-4-phenoxyphenol, free and conjugated
4'-hydroxy- (RS)-2-hydroxy-propyl 4-phenoxyphenyl ether,
4-hydroxyphenyl (RS)-2-(2pyridyloxy)propyl ether pyriproxyfen,
5''-hydroxypyriproxyfen, (RS)-2-hydroxypropyl-4-phenoxyphenyl ether,
and 4-phenoxyphenol (Panthani et al., 1996c)
Laying Leghorn hens (Gallus domesticus) were given
[14C-pyridyl]pyriproxyfen (purity, 98.2%) by gelatin capsule at a
concentration equivalent to 10 ppm in the feed for 8 consecutive days
and were killed within 4 h of the last dose. Excreta were collected
once daily and analysed by HPLC and TLC and/or mass spectrometry. The
study complied with GLP. The metabolites identified in liver and
kidney were free and conjugated 4'-hydroxypyriproxyfen,
2-hydroxypyridine, free and conjugated 5''-hydroxypyriproxyfen,
4-hydroxyphenyl- (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen, and
(RS)-2-(2-pyridyloxy)propionic acid. The metabolites identified in
excreta were (RS)-2-(2-pyridyloxy) propionic acid,
4'-hydroxypyriproxyfen, 4-hydroxyphenyl (RS)-2-(2-pyridyloxy)propyl
ether pyriproxyfen, 5''-hydroxypyriproxyfen, and 2-hydroxypyridine.
These two studies indicate that the routes of metabolism in laying
hens are hydroxylation at the 4' position of the phenoxyphenyl ring
and at the 5'' position of the pyridyl ring, cleavage of the ether
linkages, oxidation of the methylene moiety of the side-chain, and
sulfation of the 4'-hydroxyphenoxyphenyl moiety (Panthani et al.,
1996d)
In vitro
The metabolism of pyriproxyfen in rats and mice was investigated
in vitro in samples of 10 000 × g supernatant (S10) prepared from
kidney, lungs, and small intestine of 7-week-old Sprague-Dawley rats
and ICR mice. Hepatic microsomal and cytosolic fractions were prepared
by an established method (centrifugation of S10 at 105 000 × g), and
microsomal, S10, or cytosolic fractions were incubated with
[14C-phenoxyphenyl]pyriproxyfen at a concentration of 0, 0.05, 0.1,
0.5, or 1.0 mmol/L with b-NADPH as a cofactor. The reaction mixtures
were analysed by TLC. Pyriproxyfen was not metabolized by any of the
S10 preparations; it was almost completely metabolized by microsomal
fractions from liver but only very slightly by hepatic cytosol. Most
of the major metabolites identified in rats in vivo were observed
in vitro. There was no species difference in the major metabolic
reactions. The intrinsic clearance calculated from Lineweaver-Burk
plots revealed sex-related differences in metabolic reactions in rats
but not in mice, and 5''-hydroxylation was observed only in male rats
and not in mice of either sex. The intrinsic clearance for microsomal
4'-hydroxylation in female rats was twice that in males, but was not
different in male and female mice. Incubation of a hepatic microsomal
fraction from male rats with antisera against male-specific forms of
cytochrome P450 (CYP2C11 or CYP2C13) revealed that members of the
CYP2C family, the expression of which is sex-dependent in rats, are
involved in the major hydroxylation reactions of pyriproxyfen.
Antiserum against CYP2C11 inhibited all of the major metabolic
reactions of the hepatic microsomal fraction from male rats (> 88%
inhibition). The antiserum against CYP2C13 also inhibited all
reactions (25-64% inhibition) except 4'-hydroxylation (Yoshino et al.,
1996).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of pyriproxyfen are
summarized in Table 1. Pyriproxyfen dissolved in corn oil was
administered orally in a volume of 10 ml/kg bw to ICR (Crj:CD-1) mice
at doses of 1000, 2000, or 5000 mg/kg bw and to Sprague-Dawley
(Crj:CD) rats at 1000, 2500, or 5000 mg/kg bw. In mice, pyriproxyfen
reduced spontaneous motor activity and caused ataxia, abnormal
respiration, and death in males at 2000 mg/kg bw and in animals of
each sex at 5000 mg/kg bw. Transiently decreased body weight was
observed in males at 5000 mg/kg bw. Deaths occurred in two males at
2000 mg/kg bw and two at 5000 mg/kg bw in males and in one female at
5000 mg/kg bw. In rats, pyriproxyfen caused a decrease in body-weight
gain, decreased spontaneous activity, soft stools, and diarrhoea in
males at 2500 mg/kg bw and in animals of each sex at 5000 mg/kg bw,
but no deaths. Necropsy revealed no abnormal changes in the organs of
mice or rats. In dogs, oral administration of pyriproxyfen in capsules
caused no deaths at doses up to 5000 mg/kg bw. The only clinical sign,
occasional vomiting for the first 24 h, was observed at the highest
dose. Dermal application of 2000 mg/kg bw of pyriproxyfen dissolved in
corn oil (5 or 10 ml/kg bw) caused no deaths or signs of clinical
toxicity in ICR mice or Sprague-Dawley rats. Exposure of ICR mice or
Sprague-Dawley rats to a mist aerosol of pyriproxyfen dissolved in
corn oil at concentrations of 0.6 or 1.3 mg/L for 4 h caused no deaths
or pathological changes. The mass median aerodynamic diameter of the
particles was 0.8-0.9 µm. At the high concentration, salivation and
urinary incontinence were observed in rats 4 h after the start of
inhalation, and irregular respiration was observed in mice. These
clinical signs disappeared within 1 h of cessation of exposure.
(b) Short-term studies of toxicity
Mice
Groups of 10 male and 10 female ICR (Crj:CD-1) mice were given
diets containing technical-grade pyriproxyfen (purity, 95.3%) at
concentrations of 0, 200, 1000, 5000, or 10 000 ppm, equal to 0, 28,
150, 840, and 2000 mg/kg bw per day for males and 0, 38, 200, 960, and
2300 mg/kg bw per day for females, for 13 weeks. The observations
included clinical signs, mortality, food and water consumption, body
weight, clinical chemical parameters including serum enzymes, urinary
and haematological parameters, organ weights, and gross and
histopathological appearance. The serum enzymes assayed were alanine
aminotransferase), aspartate aminotransferase, and gamma-glutamyl
transpeptidase. Blood samples were collected shortly before
termination of the study. The study conformed to GLP.
Death occurred in two males at 5000 ppm and in seven males and
nine females at 10 000 ppm (one of the deaths in males was not
treatment-related). The clinical signs observed in the animals that
died prematurely were emaciation, hunched appearance, and few or no
faeces. There were no treatment-related clinical signs in the mice
that survived. Terminal body weights were significantly reduced in
males at 5000 ppm (89% of control) and at 10 000 ppm (69% of control);
the body weights of females were reduced at 5000 ppm during the first
half of the study but were comparable to control values (97% of
control) by the end. The water consumption of males at the two higher
doses was significantly increased, but food consumption was not
affected by treatment. There were significant decreases in erythrocyte
parameters, including cell count, haemoglobin concentration,
haematocrit, mean cell volume, and mean cell haemoglobin value, in
animals at 5000 and 10 000 ppm. Platelet counts were significantly
increased in animals of each sex at 5000 ppm and in males at 10 000
ppm. Significant increases in total cholesterol concentration were
observed in females at doses > 1000 ppm: 130% at 1000 ppm, 210% at
5000 ppm, and 140% of control values at 10 000 ppm. In males, the
activities of aspartate and alanine aminotransferases were increased
by up to twofold but reached significance only at 5000 ppm.
gamma-Glutamyl transpeptidase activity showed slight, nonsignificant
changes in some groups. The absolute weight of the liver was
significantly increased in females at 5000 ppm (120% at 5000 ppm and
160% of control values at 10 000 ppm) but not in males at any dose.
Microscopic examination revealed histomorphological alterations of the
Table 1. Acute toxicity of pyriproxyfen
Species Strain Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/L air)
Mouse ICR M&F Oral > 5000 97.2 Suzuki et al. (1987a)
Rat Sprague-Dawley M&F Oral > 5000 97.2 Suzuki et al. (1987b)
Dog Beagle M&F Oral > 5000 97.2 Nakano et al. (1986)
Mouse ICR M&F Dermal > 2000 97.2 Suzuki et al. (1987c)
Rat Sprague-Dawley M&F Dermal > 2000 97.2 Suzuki et al. (1987d)
Mouse ICR M&F Inhalation > 1.3 97.0 Suzuki et al. (1987e)
Rat Sprague-Dawley M&F Inhalation > 1.3 97.0 Kawaguchi et al. (1987)
These studies were conducted according to good laboratory practice.
kidney comprising tubular nephrosis with microcytosis and dilatation
of the renal tubules and focal mineralization and dilatation of the
renal pelvis in males at 5000 ppm and in animals of each sex at 10 000
ppm. There were no treatment-related morphological changes in the
liver. The NOAEL was 1000 ppm, equal to 150 mg/kg bw per day, on the
basis of effects on erythrocyte parameters, deaths, decreased body
weight, histomorphological alterations in the kidney, and increased
absolute liver weight at higher doses (Cox et al., 1990).
Rats
Groups of 10 male and 10 female Sprague-Dawley (Crl:CD) rats
received diets containing technical-grade pyriproxyfen (purity, 95.3%)
at concentrations of 0, 400, 2000, 5000, or 10 000 ppm, equal to 0,
23, 120, 310, and 640 mg/kg bw per day for males and 0, 28, 140, 360,
and 780 mg/kg bw per day for females, for 13 weeks. The observations
included clinical signs, deaths, body weight, food and water
consumption, ophthalmological, clinical chemical, haematological, and
urinary parameters, organ weights, and gross and histopathological
appearance. The clinical chemical examinations included assays for the
serum enzymes alkaline phosphatase, aspartate and alanine
aminotransferases, and gamma-glutamyl transpeptidase. Blood samples
were collected at the end of the study.
There were no treatment-related deaths, toxic signs, or
ophthalmological changes at any dose, and no changes in food or water
consumption. The body weights of animals of each sex were
significantly decreased at doses > 5000 ppm (91% of control at 5000
ppm and 88% at 10 000 ppm at termination). Erythrocyte parameters
including cell count, haemoglobin concentration, and haematocrit were
significantly decreased in animals at doses > 5000 ppm and in males
also at 2000 ppm. The mean cell volume was reduced in females at 2000
and 10 000 ppm by < 10%. There was no significant effect on platelet
count. The total cholesterol concentration was dose-dependently,
significantly increased in males at doses > 2000 ppm (150% at 2000
ppm, 200% at 5000 ppm, and 210% of control values at 10 000 ppm) and
in females at doses > 5000 ppm (130% at 5000 ppm and 180% of
control values at 10 000 ppm). The serum phospholipid concentration
was also significantly increased in males at doses > 2000 ppm (130%
at 2000 ppm, 180% at 5000 ppm, and 180% of control values at 10 000
ppm) and in females at 10 000 ppm (160% of control value).
Significantly increased gamma-glutamyl transpeptidase activity was
observed in animals at 10 000 ppm, but the activities of the other
serum enzymes were not significantly affected. Significant increases
in the absolute weight of the liver were observed in animals at doses
> 5000 ppm (110% at 2000 ppm, 130% at 5000 ppm, and 140% of control
values at 10 000 ppm in males and 100% at 2000 ppm, 120% at 5000 ppm,
and 140% of control values at 10 000 ppm in females), and the relative
weight of the liver was significantly increased in males at 2000 ppm
and in animals of each sex at doses > 5000 ppm (120% at 2000 ppm,
140% at 5000 ppm, and 170% of control values at 10 000 ppm in males
and 130% at 5000 ppm and 160% of control values at 10 000 ppm in
females). Significant increases in the relative weight of the kidney
were observed in animals at 10 000 ppm, but the absolute weights were
comparable to those of controls at all doses. Histopathological
examination revealed dose-dependent increases in cytoplasmic changes
in the liver in all treated groups (1/10 at 0 ppm, 2/10 at 400 ppm,
6/10 at 2000 ppm, 10/10 at 5000 ppm, and 9/10 at 10 000 ppm in males
and 1/10 at 0 ppm, 2/10 at 400 ppm, 6/9 at 2000 ppm, 7/10 at 5000 ppm,
and 9/10 at 10 000 ppm in females). The cytoplasmic changes consisted
of slight, often equivocal increases in cytoplasmic content reflected
in a visibly reduced nucleus:cytoplasm ratio and diminution of
sinusoidal spaces. The NOAEL was 400 ppm, equal to 23 mg/kg bw per
day, on the basis of mild anaemia, increased incidences of minimal
hepatic abnormalities, relative liver weights, and serum
concentrations of total cholesterol and phospholipids, indicating
effects on lipid metabolism, at higher doses (Cox et al., 1989).
Groups of 21 male and 21 female Sprague-Dawley rats were given
diets containing pyriproxyfen (purity, 97.2%) at concentrations of 0,
80, 400, 2000, or 10 000 ppm, equal to 0, 4.8, 24, 120, and 680 mg/kg
bw per day for males and 0, 5.4, 28, 140, and 690 mg/kg bw per day for
females, for 26 weeks. The observations included clinical signs,
deaths, food and water consumption, body weight, ophthalmological,
clinical chemical, haematological, and urinary parameters, organ
weights, and gross and histological appearance. The serum activities
of aspartate and alanine aminotransferases, alkaline phosphatase,
lactate dehydrogenase, leucine aminopeptidase, creatine phosphokinase,
and gamma-glutamyl transpeptidase were measured. Blood samples were
taken at the end of the study.
There were no deaths, and the only signs of toxicity were
increased incidences of alopecia around the neck and soft stools
during the early stage of the study in animals at 10 000 ppm. The body
weights of animals at 10 000 ppm were significantly decreased
throughout the study, by 86% in males and 87% in females at the end of
study. Marked decreases in body-weight gain were observed at this
dose. There were no treatment-related changes in food or water
consumption. Proteinuria and increased urinary excretion of potassium
ion were observed in animals at 10 000 ppm. Slight but significant
decreases in erythrocyte count and haematocrit were observed in males
at 2000 and 10 000 ppm and in females at 10 000 ppm. The haemoglobin
concentration was also slightly but significantly decreased at this
dose. No increase in platelet count was observed. Slight but
significant increases in total protein, albumin, and blood urea
nitrogen were observed in animals at 10 000 ppm. The albumin and
a2u-globulin fractions were slightly but significantly increased in
males at 10 000 ppm. Total cholesterol and phospholipid concentrations
were significantly increased in males at 2000 and 10 000 ppm and in
females at 10 000 ppm. Of the serum enzyme activities studied, only
that of gamma-glutamyl transpeptidase was significantly increased in
males at 10 000 ppm. Significantly increased absolute weights of the
liver were observed in animals at 10 000 ppm (130% of control value in
males and in females), and significant increases in the relative
weight were observed in males at doses > 2000 (110% at 2000 ppm and
160% of control values at 10 000 ppm) and in females at 10 000 ppm
(100% at 2000 ppm and 150% of control values at 10 000 ppm).
Significantly increased relative kidney weights were observed in
animals at 10 000 ppm, but the absolute weights were not significantly
increased. Histopathological examination showed slight hypertrophy of
the liver in all animals at 10 000 ppm. The NOAEL was 400 ppm, equal
to 24 mg/kg bw per day, on the basis of increased relative liver
weight, increased total cholesterol and phospholipid concentrations
indicating effects on lipid metabolism, and mild anaemia at higher
doses (Koyama et al., 1989).
Groups of five male and five female Sprague-Dawley (Crl:CD) rats
received dermal applications of pyriproxyfen (purity, 97.2%) dissolved
in corn oil at doses of 0, 100, 300, or 1000 mg/kg bw per day under a
semi-occlusive dressing for 6 h/day for 21 days. The observations
included clinical signs, deaths, food consumption, body weight, and
clinical chemical, haematological, and and histological examinations.
Serum was assayed for alanine and aspartate aminotransferases. Blood
samples were collected at termination. The study complied with GLP.
There were no treatment-related effects on the mortality rate,
clinical signs, or haematological or clinical chemical parameters,
including serum enzymes. There were no significant changes in body
weight or food consumption in the treated group, and no effect of
treatment on organ weights was observed. Histopathological examination
revealed no treatment-related alterations in the liver or any other
tissues examined. The NOAEL was 1000 mg/kg bw per day, the highest
dose tested (Moore et al., 1993).
Groups of 10 male and 10 female Sprague-Dawley (Jcl:CD) rats were
exposed to a mist aerosol of pyriproxyfen dissolved in corn oil at
concentrations of 270, 480, or 1000 mg/m3 for 4 h/day for 28 days.
The mass median aerodynamic diameter of the particles was 0.71-0.88
µm. The observations included clinical signs, deaths, food and water
consumption, body weight, and urinary, ophthalmological, clinical
chemical, haematological, and histological examinations. Serum was
assayed for leucine aminopeptidase, cholinesterase, lactate
dehydrogenase, creatine phosphokinase, alanine and aspartate
aminotransferase, and alkaline phosphatase activity. Blood samples
were collected at termination after a 16-h fast. The study complied
with GLP.
There were no deaths. Salivation was observed early in the study
in rats at the highest concentration, and the body-weight gain of
animals at this dose was sporadically, slightly but significantly
lower, although it was normal at the end of the study. There were no
treatment-related changes in food consumption or in haematological,
urinary, or ophthalmologic parameters. Slightly but significantly
increased lactate dehydrogenase activity was observed in males at the
highest dose, but the activities of the other serum enzymes showed no
treatment-related change. A slight but significant increase (9%) was
observed in the relative weight of the liver at 1000 mg/m3, but the
absolute weight was comparable to that of controls. Histopathological
examination revealed no treatment-related morphological changes in the
organs of exposed rats. The NOAEL was 480 mg/m3 on the basis of
salivation, sporadically reduced body-weight gain, and increased
lactate dehydrogenase activity at 1000 mg/m3 (Kawaguchi et al.,
1988).
Guinea-pigs
The skin sensitizing potential of pyriproxyfen (purity, 97.2%)
was tested in a GLP-compliant study in male Hartley guinea-pigs by the
maximization method. A volume of 0.05 ml of 1% pyriproxyfen in
Freund's complete adjuvant mixed with water or a 0.5% solution of
pyriproxyfen in corn oil was injected intradermally for initial
sensitization. As a challenge, 0.2 g of pyriproxyfen in 25% petrolatum
or 0.2 ml of a positive control was applied dermally in patches to
test animals for 24 h, 6 days after the first sensitization. No dermal
reaction was observed (Suzuki et al., 1987f).
Rabbits
New Zealand white rabbits of each sex received a single dose of
0.5 g of pyriproxyfen (purity, 97.2%) as a fine powder moistened with
physiological saline by dermal application for 4 h. The potential to
induce primary skin irritation was examined 4.5, 24, 48, and 72 h
after application. The study complied with GLP. No oedema or erythema
was observed (Suzuki et al., 1987g).
A single dose of 100 mg of pyriproxyfen (purity, 97.2%) in a
volume of 0.1 ml was applied to the right eye of three male and three
female New Zealand white rabbits in a study that complied with GLP.
The potential to induce primary eye irritation was examined 1, 24, 48,
and 72 h after application in unwashed eyes. Slight conjunctival
redness (grade 1) and chemosis (grade 1-2) were observed in all
treated animals 1 h after application. Conjunctival redness (grade 1),
chemosis (grade 1), and discharge (grade 2) were still apparent in one
or two animals after 24 h, but these changes had disappeared by 48 h
after application. Pyriproxyfen was considered to be a minimal ocular
irritant (Suzuki et al., 1987g).
Dogs
Groups of four male and four female beagle dogs, 6 months old,
received gelatine capsules containing pyriproxyfen (purity, 97.2%) at
doses of 0, 100, 300, or 1000 mg/kg bw per day for 3 months. The
observations included clinical signs, deaths, food consumption, body
weight, and ophthalmic, electrocardiographic, clinical chemical,
haematological, urinary, and histological parameters. The activities
of alkaline phosphatase, aspartate and alanine aminotransferases,
gamma-glutamyl transpeptidase, creatine phosphokinase, and lactate
dehydrogenase were measured in plasma. Ophthalmological examinations
were performed during weeks 0, 5, and 12 of treatment, and an
electrocardiograph was obtained at the same times. Blood samples were
collected during weeks 0, 4, 8, and 12 of treatment; hepatic function
was assessed by bromsulphalein retention during weeks 0, 6, and 13 of
treatment, and renal function was assessed by retention of
para-aminohippuric acid during weeks 0, 5, and 11 of treatment.
No deaths were observed. Female dogs at 1000 mg/kg bw per day had
a slightly increased incidence of soft stools, but no other
treatment-related toxic signs were observed. There were no changes in
body weight, body-weight gain, food consumption, or ophthalmological
parameters throughout the study, and no treatment-related changes in
the electrocardiograph were observed at any dose. Haematological
parameters were not significantly changed at any dose, although
slight, nonsignificant alterations in the number of platelets (by
< 39%) and total cholesterol concentration (by < 67%) were observed,
with no obvious dose-dependence. The phospholipid concentration was
significantly increased in females at 1000 mg/kg bw per day. No
significant changes was found in the activities of the serum enzymes
studied, although there were trends to increased alkaline phosphatase
activity in males at the high dose, lactate dehydrogenase activity in
males at all doses and in females at the high dose, and creatine
phosphokinase activity in males in all doses, with no dose-dependence.
Aspartate and alanine aminotransferase activities were unaltered.
Hepatic function was not significantly affected at any dose. The
absolute weights of the liver were significantly increased in males at
300 and 1000 mg/kg bw per day, by 30% and 26%, respectively, and
significant increases in relative liver weights were observed in males
at 300 mg/kg bw per day, by 24%. Increased incidences of
hepatocellular hypertrophy were observed in females at 300 mg/kg bw
per day and in animals of each sex at 1000 mg/kg bw (0/4 in controls,
0/4 at 100 mg/kg bw per day, 0/4 at 300 mg/kg bw per day, and 4/4 at
1000 mg/kg bw per day in males, and 0/4, 0/4, 3/4, and 4/4 in females,
respectively). At 1000 mg/kg bw per day, increased incidences of
eosinophilic bodies in the liver were observed (0/4 in controls, 0/4
at 100 mg/kg bw per day, 0/4 at 300 mg/kg bw per day, and 2/4 at 1000
mg/kg bw per day in males, and 0/4, 0/4, 0/4, and 2/4 in females,
respectively). Electron microscopic examination revealed a minimal to
slight increase in smooth endoplasmic reticulum with slight dilatation
in the livers of all animals at 1000 mg/kg bw per day. These changes
are consistent with adaptation of the liver to exposure to the
compound through enzyme induction. A slight but significantly
prolonged retention ratio in the test for renal function was observed
in males at 300 and 1000 mg/kg bw per day after 6 weeks of treatment,
but the retention ratio was normal by the end of the study, and no
treatment related histopathological changes were observed in the
kidney. The NOAEL was 100 mg/kg bw per day on the basis of increased
absolute and relative liver weights and an increased incidence of
hepatocellular hypertrophy at higher doses (Nakano et al., 1988).
Groups of four male and four female beagle dogs (23-27 weeks old)
were given gelatine capsules containing pyriproxyfen (purity, 95.3%)
at doses of 0, 30, 100, 300, or 1000 mg/kg bw per day for 52 weeks.
The observations included clinical signs, deaths, food consumption,
body weight, and ophthalmoscopic, clinical chemical, haematological,
urinary, and histological examinations. The activities of alanine and
aspartate aminotransferases, alkaline phosphatase, and creatine
phosphokinase were measured in plasma. Blood samples were collected 1
week before and on weeks 12, 24, 37, and 50 of treatment. The study
conformed to GLP.
Two males at 1000 mg/kg bw per day were killed in extremis with
acute weight loss and, in one case, liver failure, at weeks 17 and 31
of treatment. Slightly increased frequencies of salivation and
diarrhoea were observed in animals at 1000 mg/kg bw per day, and
emaciation was observed in males at doses > 300 mg/kg bw per day.
There were no treatment-related ophthalmological abnormalities. A
dose-dependent but nonsignificant reduction in body weight was seen
throughout the study, and body-weight gains were significantly reduced
in animals at doses > 300 mg/kg bw per day. Food consumption was
not reduced at any dose. Significant changes were seen in several
haematological parameters, including 10-20% decreases in haemoglobin
and erythrocyte counts and a slightly but significantly increased mean
corpuscular volume in males at doses > 300 mg/kg bw per day and in
females at doses > 100 mg/kg bw per day and a significantly
decreased packed cell volume in the latter group. The change in
haemoglobin in males at 1000 mg/kg bw per day was not significant.
These haematological changes might indicate slight anaemia. There was
no abnormality of cellularity or cell composition in the bone marrow.
Statistically significant decreases in the number of lymphocytes were
observed in all treated females at weeks 12 and 37, which were
attributed to transiently high values for the control group. The
platelet count was significantly increased in males at doses > 100
mg/kg bw per day and females at 1000 mg/kg bw per day throughout the
study. Slightly but significantly prolonged prothrombin times were
observed in males at 300 mg/kg bw per day and in animals of each sex
at 1000 mg/kg bw per day. Significantly increased plasma enzyme
activities were seen, including those of alkaline phosphatase in
animals at doses > 300 mg/kg bw per day throughout the study,
alanine aminotransferase in animals at 1000 mg/kg bw per day
throughout the study, and aspartate aminotransferase in males at 1000
mg/kg bw per day in weeks 12, 24, and 37 of treatment. The total
cholesterol concentration in plasma was significantly increased in
animals at doses > 30 mg/kg bw per day (by 24-58% at 30 mg/kg bw
per day, 64-160% at 100 mg/kg bw per day, 100-140% at 300 mg/kg bw per
day, and 76-100% at 1000 mg/kg bw per day) throughout the study, and
the plasma concentrations of triglycerides were significantly
increased in animals at doses > 100 mg/kg bw per day. These
increases were dose-dependent except at the highest dose in males, but
in this group there were only two survivors. No reduction in plasma
protein fractions was observed in the treated groups.
A slightly reduced pH and increased volume of urine were observed
in males at 1000 mg/kg bw per day throughout the study. The absolute
weights of the liver were dose-dependently increased in all treated
groups and significantly increased in males at doses > 100 mg/kg bw
per day (by 130% at 30 mg/kg bw per day, 150% at 100 mg/kg bw per day,
170% at 300 mg/kg bw per day, and 190% of control values at 1000 mg/kg
bw per day) and in females at doses > 300 mg/kg bw per day (110%,
120%, 140%, and 140%, respectively). The relative weights of the liver
were significantly increased in males at doses > 30 mg/kg bw per
day (130% of control value) and in females at doses > 300 mg/kg bw
per day. The absolute weights of the thyroid were significantly
increased in females at doses > 300 mg/kg bw per day, and the
relative weights were significantly increased in females at doses
> 100 mg/kg bw per day. Significantly increased relative renal
weights were observed in males at 300 mg/kg bw per day and in females
at doses > 300 mg/kg bw per day; the absolute weights were
dose-dependently but not significantly increased.
Macroscopic examination showed enlarged livers and hepatic damage
in the two dogs at 1000 mg/kg bw per day which died. Histopathological
examination revealed treatment-related hepatic damage in animals of
each sex at 1000 mg/kg bw per day, which was characterized by
centriacinar fibrosis in 2/2 males and 3/4 females and bile-duct
hyperplasia in 2/2 males and 3/4 females; foci of cystic degeneration
in 1/2 males and 1/4 females; active chronic inflammation in 2/2 males
and 2/4 females; and nodular hyperplasia in 2/2 males and 0/4 females.
Submucosal fibrosis in the gall-bladder was observed in all male
animals, including those that had died, and in 3/4 females at 1000
mg/kg bw per day, in association with bile-duct hyperplasia. One of
four males at 30 mg/kg bw per day had focal bile-duct hyperplasia and
focal subcapsular fibrosis, but these effects were not observed at 100
or 300 mg/kg bw per day. There were no preneoplastic or neoplastic
alterations. Although no morphological alterations were seen in the
liver, the increase in cholesterol concentration and relative liver
weight at low doses might be related to treatment. No NOAEL could be
identified, as increased total cholesterol concentrations indicating
effects on lipid metabolism and increased relative liver weights with
a trend towards increased absolute liver weights were seen at all
doses (Chapman et al., 1991).
In a complementary study which complied with GLP, groups of four
male and four female beagle dogs (19-24 weeks old) were given gelatine
capsules containing pyriproxyfen (purity, 95.3%) at doses of 0, 3, or
10 mg/kg bw per day for 52 weeks. The observations included clinical
signs, deaths, food consumption, body weight, and ophthalmic, clinical
chemical, haematological, urinary, and histological examinations. The
activities of alanine and aspartate aminotransferases, alkaline
phosphatase, and creatine phosphokinase were assayed in serum. Blood
samples were collected after 12, 24, 36, and 50 weeks of treatment.
There were no deaths, signs of clinical toxicity, or changes in
body weight, body-weight gain, or food consumption. Significantly
increased platelet counts were observed in males at 3 mg/kg bw per day
in weeks 24, 36, and 50 of treatment and in those at 10 mg/kg bw per
day in week 36, but with no clear dose-dependence. Prothrombin time
was not prolonged in males at any dose. Females at 10 mg/kg bw per day
also showed significantly increased platelet counts in weeks 36 and 50
(by 8% at 3 mg/kg bw per day and 10% at 10 mg/kg bw per day), and
prothrombin time was slightly but significantly prolonged at these
doses at the end of study. There were no other treatment-related
changes in haematological parameters. The total cholesterol
concentration was unchanged; slight but significant increases in total
triglyceride concentrations in males at 10 mg/kg bw per day were seen
in weeks 12 and 36 of treatment. There were no treatment-related
changes in urinary parameters. The absolute weight of the liver was
slightly increased in females at 10 mg/kg bw per day (110% of
control), but this was not significant. No histopathological changes
were found in any organ, including the liver and kidney. The range of
mean total platelet counts in controls in other studies in this
laboratory was 273-357 in males and 305-357 in females, whereas those
in the present study were 341-367 in controls, 414-462 at the low
dose, and 415-456 at the high dose in males, and 321-384 in controls
and 430-478 at the high dose in females. The increased numbers of
platelets and the prolonged prothrombin time were therefore
treatment-related changes although no significant increase in platelet
counts was observed in animals at 30 mg/kg bw per day in the previous
study (Mitchel et al., 1993).
The NOAEL for the two 52-week studies in dogs was 10 mg/kg bw per
day, on the basis of the absence of treatment-related toxicity at 10
mg/kg bw per day in the second study and changes in lipid metabolism
and increased liver weight at 30 mg/kg bw per day in the first study.
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female ICR(Crj-CD-1) mice were given
diets containing pyriproxyfen (purity, 95.3%; 97.6-98.7% dietary
concentration) at concentrations of 0, 120, 600, or 3000 ppm for
78 weeks, providing doses equal to 0, 16, 81, and 420 mg/kg bw per day
for males and 0, 21, 110, and 530 mg/kg bw per day for females. The
observations included clinical signs, deaths, food consumption, body
weight, organ weights, and ophthalmological, haematological, and gross
and histological examinations. Ten mice from each group were killed
during week 52 for interim examination, and the surviving mice were
killed during week 78. Blood samples were taken from 10 rats per group
during weeks 52 and 78 of treatment. No clinical chemical tests were
conducted. The study complied with GLP.
The mortality rate was dose-dependent and significantly increased
in males at 600 and 3000 ppm (43% at 0 ppm, 55% at 120 ppm, 72% at 600
ppm, and 82% at 3000 ppm) and in females at 3000 ppm (39% at 0 ppm,
44% at 120 ppm, 55% at 600 ppm, and 64% at 3000 ppm). There were
slight, nonsignificant increases in the incidence of clinical signs,
including reduced motor activity and hunched position, in animals at
3000 ppm. Statistically significant decreases in body weights,
body-weight gains, and/or food consumption were observed in males at
3000 ppm during the study. The absolute and relative weights of the
liver were significantly increased in females at 3000 ppm during week
52 of treatment. The haematological parameters showed no
treatment-related change. Histopathological examination of animals
that died revealed a significantly increased incidence of systemic
amyloidosis in the glandular stomach of males at 600 and 3000 ppm and
the adrenal, thyroid, heart, liver, kidney, glandular stomach, and
duodenum of females at 3000 ppm, and a dose-related relationship was
found between the generalized amyloidosis and the mortality rate.
Statistical analysis of the incidence of graded amyloidosis revealed a
significant positive trend in renal amyloidosis in females and a
significant positive trend in hepatic amyloidosis in animals of each
sex at 3000 ppm. Females at this dose had a significant increase in
the incidence of lymphocytic infiltration in the liver (22/59 at 0 and
34/60 at 3000 ppm) and of tubular mineralization (3/59 at 0 and 46/60
at 3000 ppm). Deposition of amyloid in the kidney causes numerous
pathological changes including tubular mineralization and papillary
necrosis. In this study, however, the incidences of tubular
mineralization and segmental cortical atrophy were increased
independently of amyloidosis in female animals at 3000 ppm, suggesting
that the chronic nephrosis was directly related to treatment.
Histopathological examination revealed no increase in the incidence of
neoplastic lesions at any dose. The NOAEL was 120 ppm, equal to 16
mg/kg bw per day, on the basis of increased mortality at higher doses
(Osheroff et al., 1991a; Cardy et al., 1994).
Rats
Groups of 50 male and 50 female Sprague-Dawley (Crl:CD) rats were
given diets containing pyriproxyfen (purity, 95.3%) at concentrations
of 0, 120, 600, or 3000 ppm, equal to 0, 5.4, 27, and 140 mg/kg bw per
day for males and 0, 7.0, 35, and 180 mg/kg bw per day for females,
for 104 weeks. Satellite groups of 30 males and 30 females were also
treated orally. The observations included clinical signs, deaths, food
and water consumption, body weight, organ weights, and
ophthalmoscopic, clinical chemical, haematological, urinary, and gross
and histopathological examination. Assays were performed for the serum
enzymes aspartate and alanine aminotransferase, alkaline phosphatase,
creatine kinase, and gamma-glutamyl transpeptidase. Blood samples were
collected from satellite groups of rats on week 13, 26, 52, 78, and
104 of treatment. The study complied with GLP.
Treatment did not affect mortality (34-46% in males and 32-58% in
females), clinical signs, or ophthalmoscopic end-points. The body
weights of males were significantly reduced in weeks 13, 26, and 50 of
treatment (by 5-7%) and those of females in weeks 13, 26, 50, and 78
of treatment (by 12-14%) at 3000 ppm, but they had returned to the
control level by the end of study. The mean body-weight gain was
significantly reduced in females at 600 ppm and in animals of each sex
at 3000 ppm throughout the study. No treatment-related changes in food
consumption were observed. The only alteration in haematological
parameters was a transient increase in eosinophils. Alkaline
phosphatase activity was significantly increased in males at doses
> 120 ppm in weeks 26, 52, and 78 of treatment, but the activity
(87-104 U/L) remained within the range of historical controls (45-114
U/L), and the changes were not clearly dose-dependent. gamma-Glutamyl
transpeptidase activity was significantly increased in males at 3000
ppm in week 104 of treatment and in females at 600 and 3000 ppm in
weeks 26 and 52. The activities of other serum enzymes were not
significantly affected. Significantly increased total cholesterol
concentrations were observed in males at 3000 ppm in weeks 26 and 52
(149% and 147% of control, respectively). Slight, inconsistent,
nonsignificant increases in urinary protein concentration were
observed in females at 3000 ppm in week 26. At interim necroscopy, the
absolute weights of the liver were found to be nonsignificantly
increased at week 52 of treatment with 3000 ppm (by 15% in males and
13% in females). A significant increase in relative liver weight was
observed only in females at 3000 ppm (120% of control). The only
significant or treatment-related increases in the incidence of
morphological alterations at 104 weeks seen on gross and
histopathological examination were hyperkeratosis of the skin of males
at 3000 ppm, which was considered not to be biologically significant,
and a significant increase in the incidence of liver necrosis in males
at 3000 ppm that died during the study; however, no liver necrosis was
observed in the surviving males at 3000 ppm at the end of the study,
indicating nthat it was not related to treatment. Histopathological
examination revealed no evidence of neoplastic alterations. The NOAEL
was 600 ppm, equal to 27 mg/kg bw per day, on the basis of reductions
in body weight and mean body-weight gain and increased absolute and
relative liver weights at higher doses (Osheroff et al., 1994a,b).
(d) Genotoxicity
The results of tests for the genotoxicity of pyriproxyfen are
summarized in Table 2. All of the positive controls used in the assays
produced the expected positive responses. In assays for reverse
mutation, no induction of revertant colonies was observed at six doses
with or without an exogenous metabolic activation system (S9). In
tests for DNA repair, pyriproxyfen was inactive at six doses with or
without S9. In tests for gene mutation in mammalian cells, no
mutations were observed at four doses ranging from 10 to 300 ppm
without S9 or 10 to 100 ppm with S9. In assays for unscheduled DNA
repair synthesis, pyriproxyfen was cytotoxic and/or inhibited normal
DNA synthesis but it did not induce unscheduled DNA synthesis. In
tests for cytogeneticity, Chinese hamster ovary cells (CHOK1) were
exposed to pyriproxyfen at a concentration of 10, 30, or 100 µg/ml for
2 h in the presence of S9 and cultured for a further 16 or 22 h or
cultured with pyriproxyfen for 18 or 24 h in the absence of S9.
Although marked cytotoxicty, characterized by decreased mitotic index
and cell cycle delay, were observed at doses > 30 mg/ml without S9,
and at 100 mg/ml with S9, no increase in the total number of
structural aberrations or the frequency of cells with aberrations was
observed at any concentration. In the test for micronucleus formation
in mice in vivo, a single dose of pyriproxyfen at 5000 mg/kg bw
slightly but not statistically significantly increased the incidence
of micronucleated polychromatic erythrocytes.The Meeting concluded
that pyriproxyfen is not genotoxic in vivo or in vitro.
Table 2. Results of tests for the genotoxicity of pyriproxyfen
End-point Test system Concentration Purity Result Reference
(%)
In vitro
Reverse mutationa S. typhimurium 10-5000 µg/plate 97.2 Negative ± S9 Kogiso et al. (1988a)
TA98, TA100,
TA1537, TA1538,
E, coli WP2 uvrA
DNA repairb B.subtills M45, 673-21 500 µg/disc 95.3 Negative ± S9 Kogiso et al. (1992)
H17 in DMSO
Gene mutationc Chinese hamster 3-300 µg/ ml 95.3 Negative ± S9 Kogiso et al. (1990)
V79 cells, hprt locus
Unscheduled Human HeLa S3 0.1-205 µg/ ml 95.3 Negative ± S9 Henderson &
DNA synthesisd epithelioid cells Proudlock (1989)
Chromosomal Chinese hamster 10-300 µg/ml 97.2 Negative ± S9 Kogiso et al. (1989)
aberrationse ovary cells
Chromosomal Chinese hamster 9.6-321 µg/ml -S9 97.2 Negative ± S9 Kogiso et al. (1988)
aberrationsf ovary cells 50-200 µg/ml +S9
In vivo
Micronucleus CD-I mice, bone Single intraperitoneal 95.3 Negative Proudlock et al.
formationg marrow injections of 5000 mg/kg (1991)
bw at 24, 48, and 72 h
Table 2. (continued)
All studies were conducted according to good laboratory practice. DMSO, dimethyl sulfoxide
a Positive controls were methylmethanesulfonate for TA100, 2-nitrofluorene for TA98 and TA1538, sodium azide for TA1535,
ICR-191 for TA1537, N-ethyl- N'-nitro- N-nitrosoguanidine for WP2 uvrA, benzo[ a]pyrene for TA100, TA98, TA1537, and TA1538
and 2-aminoanthracene for TA1535 and WP2 uvrA.
b Positive controls were mitomycin C for the direct assay and sterigmatocystin for the activation assay. The negative
control was kanamycin in both assays.
c Positive controls were ethylmethane sulfonate for the direct assay and 9,10-dimethyl-1,2-benzanthracene for the activation
assay.
d Positive controls were 2-acetylaminofluorene for the activation assay and 4-nitroquinoline-1-oxide for the direct assay.
e Positive controls were mitomycin C for the direct assay and cyclophosphamide for the activation assay.
f Positive controls were mitomycin C for the direct assay and benzo[ a]pyrene for the activation assay.
g Positive control was mitomycin C.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 26 male and 26 female Sprague-Dawley (Crj) rats were
given diets containing technical-grade pyriproxyfen (purity, 95.3%) at
concentrations of 0, 200, 1000, or 5000 ppm. The F0 animals were
treated for 70 days before mating and then for 6 subsequent weeks for
males and during 3 weeks of gestation and 3 weeks of the lactation
period for females. The F1 generation were treated for 18 weeks from
the day of their weaning to the day of weaning of the F2 generation,
including 77-90 days before mating and the mating, gestation, and
lactation periods. The mean daily intakes of pyriproxyfen were 14, 68,
and 340 mg/kg bw per day in males and 20, 98, and 500 mg/kg bw per day
in females (18, 87, and 440 mg/kg bw per day before mating, 15, 77,
and 390 mg/kg bw per day during gestation, and 32, 160, and 830 mg/kg
bw per day during lactation) in the F0 generation, and 17, 83, and
440 mg/kg bw per day in males and 21, 110, and 560 mg/kg bw per day in
females (21, 100, and 550 mg/kg bw per day before mating, 14, 72, and
380 mg/kg bw per day during gestation, and 28, 160, and 810 mg/kg bw
per day during lactation) in the F1 generation.
The observations in parental rats included clinical signs,
deaths, food consumption, body weight, estrus cycle, and
histopathological and reproductive parameters which included mating,
fertility, gestation, and live birth indices. All parental animals
were killed at the end of weaning, and the reproductive organs, brain,
pituitary, liver, and kidney were examined histopathologically. The
organs from the F1 parental animals were weighed. Estrus cycles were
examined by a smear assay during the 10 days before mating. The
observations in the F1 and F2 pups included viability, body
weight, and lactation indices. Developmental indices were not
examined. Groups of 10 male and 10 female pups were selected randomly
from 10 litters for necroscopy. One male and one female were selected
randomly from each F1 litter to provide 26 pairs at each dose to
serve as parents for the F2 generation. Pups were weighed by sex.
The study complied with GLP.
The F0 parent animals showed no treatment-related changes in
clinical signs, mortality rate, reproductive parameters, or estrus
cycle. Body weight and body-weight gain were significantly reduced in
F0 animals at 5000 ppm during the periods of premating, gestation,
and lactation, and food consumption was significantly reduced in F0
females at this dose during gestation. No gross or histopathological
alterations were seen that were related to treatment. The NOAEL for
F0 parental toxicity was 1000 ppm, equal to 68 mg/kg bw per day, on
the basis of reductions in body weight and body-weight gain at 5000
ppm. The NOAEL for reproductive toxicity was 5000 ppm, equal to 340
mg/kg bw per day, the highest dose tested.
The F1 parent animals showed no treatment-related changes in
clinical signs, mortality rate, reproductive parameters, or estrus
cycles. The body weights of F1 males and F1 females at 5000 ppm
were significantly reduced, and the terminal body weights of animals
at this dose were significantly decreased (to 98% at 1000 ppm and 88%
at 5000 ppm in males and 100% at 1000 ppm and 95% at 5000 ppm in
females). Body-weight gain was also significantly reduced in F1
males at 5000 ppm during the premating period. Food consumption was
significantly reduced at this dose in F1 males during treatment and
in F1 females during gestation. The absolute weights of the liver
were significantly increased in F1 adults at 5000 ppm (110% at 1000
ppm and 110% at 5000 ppm in males and 120% at 5000 ppm in females),
and the relative weights were significantly increased in males at 1000
ppm (110% of control) and in animals of each sex at 5000 ppm (130% in
males and 130% in females). Histopathological examination showed an
increased incidence of focal clear cells in the liver in males at 5000
ppm, but this effect is commonly observed in male rats and was
considered to be unrelated to treatment. Significant increases in
relative kidney weights were observed in males at 1000 ppm (110% of
control values) and 5000 ppm (110%), but the absolute weights were not
significantly increased at any dose. Histopathological examination
showed an increased incidence of chronic interstitial nephrosis (7/26
at 0 ppm, 3/26 at 200 ppm, 7/26 at 1000 ppm, and 15/26 at 5000 ppm) in
males at 5000 ppm and in the incidence of hydronephrosis (1/26 at 0
ppm and 4/26 at 5000 ppm) in females at this dose; however, these
increases did not reach statistical significance. No other
treatment-related morphological lesions were observed. The NOAEL for
F1 parental toxicity was 1000 ppm, equal to 83 mg/kg bw per day, on
the basis of decreased body weight, decreased food consumption, and
increased absolute and relative liver weights at 5000 ppm. No evidence
of reproductive toxicity was observed. The NOAEL for reproductive
toxicity was 5000 ppm, equal to 340 mg/kg bw per day, the highest dose
tested.
In F1 pups, there was no treatment-related change in clinical
signs, sex ratio, viability index, or lactation index and no
significant difference between treated and control groups in body
weight at birth. Significant reductions in body weight were observed
in male F1 pups on day 21 and in female F1 pups on days 14 and 21
post partum at 5000 ppm. The total litter weight was also
significantly decreased on days 14 and 21 post partum at this dose.
No treatment-related effects were seen on gross examination. F2 pups
also showed no treatment-related change in clinical signs, sex ratio,
viability index, or lactation index. The mean pup weights were
significantly decreased on days 14 and 21 post partum at 5000 ppm.
No gross pathological alteration related to treatment was apparent at
any dose. The NOAEL for developmental toxicity was 1000 ppm, equal to
98 mg/kg bw per day, on the basis of reduced body weight in F1 and
F2 pups at 5000 ppm (Robinson et al., 1991).
In a study of treatment before and during the early stages of
gestation (segment 1) conducted according to GLP, groups of 24 male
and 24 female Sprague-Dawley rats were given pyriproxyfen (purity,
97.2%) dissolved in corn oil by gavage at doses of 0, 100, 300, 500,
or 1000 mg/kg bw per day for 12 weeks comprising 9 weeks before mating
and 3 weeks of mating, in males or for at least 3 weeks including 2
weeks before mating and the mating period and on days 0-7 of gestation
in females. The observations in parental rats included clinical signs,
deaths, food consumption, body weight, organ weights, gross
appearance, and reproductive performance including copulation and
fertility indices. Clinical examinations were performed twice a day.
Males were killed at the end of mating, and female animals on day 21
of gestation.
Treatment-related deaths occurred in two females at the highest
dose. Increased incidences of diarrhoea and erythema and swelling of
the anal region were observed in females at 300 mg/kg bw and in
animals of each sex at doses > 500 mg/kg bw per day. Dose-dependent
increases in the incidence of salivation were frequently observed in
all treated groups, with incidences of 0/24 in controls and 18/24,
21/24, 24/24, and 24/24 at the four doses respectively, in males, and
0/24, 1/24, 5/24, 7/24, and 8/24, respectively, in females. Body
weight was significantly reduced in females at 100 mg/kg bw per day
during gestation and in animals of each sex at doses > 300 mg/kg bw
per day throughout the study. Body-weight gain and food consumption
were also significantly decreased in males at doses > 300 mg/kg bw
per day throughout the study, but significant decreases were observed
only during treatment in females. Water consumption was significantly
increased in males at doses > 100 mg/kg bw per day and in females
at doses > 300 mg/kg bw per day. The absolute weights of the liver,
kidney, and adrenal glands were significantly increased and the
absolute weight of the thymus was significantly decreased in males at
doses > 300 mg/kg bw per day. In females, the absolute weights of
the kidney and adrenals were significantly increased at 1000 mg/kg bw
per day. Enlarged, dark-red livers, pitted, enlarged kidneys, enlarged
adrenals, and thymus atrophy were observed in males at doses > 300
mg/kg bw per day, and congested, enlarged livers, enlarged adrenals,
and atrophy of the spleen and thymus were observed in females at
1000 mg/kg bw per day. There was no treatment-related change in
reproductive performance. No NOAEL could be identified for maternal
toxicity, as reduced body weight during gestation was seen at all
doses. The NOAEL for reproductive toxicity was 1000 mg/kg bw per day,
the highest dose tested.
Slightly but significantly reduced numbers of corpora lutea (90%
of control) and of live fetuses (88% of control) were observed at 1000
mg/kg bw per day, but the values were within the range in historical
controls. Slight increases in placental weight and in fetal body
weight were observed at 1000 mg/kg bw per day, but the effects were
not dose-dependent and were within the range in historical controls. A
slight but significant increase in the number of phalanges of the
proximal forepaw was observed, but such increases are known to be
associated with an altered growth rate. The incidences of external,
visceral, and skeletal anomalies were not increased. The NOAEL for
developmental toxicity was 1000 mg/kg bw per day, the highest dose
tested (Saegusa et al., 1988a).
In a study of treatment during the perinatal and lactation
periods (segment 3) conducted according to GLP, groups of 23-24
pregnant Sprague-Dawley rats were given pyriproxyfen (purity, 97.2%)
dissolved in corn oil by gavage at doses of 0, 30, 100, 300, or 500
mg/kg bw per day from day 17 of gestation to day 20 post partum. The
dams were allowed to deliver naturally. On day 4 post partum, the
offspring were culled to adjust the litter size to eight (four males
and four females when possible) for tests of development (F1I), and
the remaining pups (F1II) were killed for skeletal examination on
the day of culling. The dams (F0) were killed at termination of
weaning. The observations in maternal rats included clinical signs,
deaths, body weight, food consumption, organ weights, gross
appearance, and reproductive indices including delivery rate, birth
rate, and body weight of live newborns. The F1I offspring were
weighed on days 0, 4, 7, 14, and 21 post partum during lactation;
after weaning, one male and one female per litter were weighed once a
week. Clinical signs were observed twice a day. The indices of
development during lactation included separation of the auricle,
emergence of abdominal hair, eruption of incisors, separation of
eyelids, and descent of testes or opening of the vagina. On day 20,
all F1 offspring were examined for sensory function in tests for
visual placing, righting and mid-air righting reflexes, and response
to pain. On day 21 post partum, two male and two female offspring
from each litter (F1Ia) were killed for visceral and skeletal
examination. After weaning, another male and female from each litter
(F1Ib) were examined for emotionality (behaviour in an open field)
at 4 weeks of age, for motor coordination (rotarod performance) at 5
weeks of age, and for learning ability (in a water-filled multiple T
maze) at 6 weeks of age. The F1Ib offspring were killed for necropsy
on day 56 post partum. Another male and female from each litter
(F1Ic) were tested only for reproductive performance after being
paired for mating within the same group (avoiding sibling matings) at
11 weeks of age. The fetuses were removed surgically from mated
females on day 21 of gestation. Reproductive performance was assessed
from indices of mating, fertility, gestation, and litters. The fetuses
(F2) were weighed and examined for external anomalies.
In the F0 maternal animals, treatment-related deaths were
observed at 500 mg/kg bw per day, an increased incidence of diarrhoea
at doses > 300 mg/kg bw per day, and dosedependent increases in the
incidence of salivation at doses > 100 mg/kg bw per day (0/23 in
controls and 0/23, 1/23, 2/24, and 4/24 at the four doses,
respectively). During gestation, significantly reduced body weights
were observed at 500 mg/kg bw per day, and significantly reduced
body-weight gain and food consumption were seen frequently during the
study at doses > 300 mg/kg bw per day. The absolute and relative
weights of the liver were significantly increased at doses > 300
mg/kg bw per day. Atrophy of the thymus, congestion of the liver and
kidneys, enlargement of the adrenals, and atrophy of the spleen were
observed at 500 mg/kg bw per day, and an increased number of
stillborns was seen at this dose. The body weight of live newborn pups
was significantly decreased at doses > 300 mg/kg bw per day. The
NOAEL for maternal toxicity was 100 mg/kg bw per day, on the basis of
clinical signs, reductions in body weight, body-weight gain, and food
consumption, and decreased body weight of delivered newborn at 300
mg/kg bw per day.
At 500 mg/kg bw per day, the survival rate of F1 offspring was
significantly decreased on days 0-4 post partum, and the weaning
rate was decreased on days 4-21 post partum. No decrease in the
viability of F1I offspring was observed on days 21-77. The body
weights of pups of each sex at doses > 300 mg/kg bw per day were
significantly reduced on days 0-49 or 56 post partum. All of the
physical developmental indices were significantly retarded and slight
retardation of sexual differentiation was observed at doses > 300
mg/kg bw per day. No treatment-related change in sensory functions was
observed in F1I offspring. Visceral examination of the F1Ia
offspring on day 21 post partum revealed a significant increase in
the number with anomalies at 30, 300, and 500 mg/kg bw per day (0% in
controls and 10%, 1.2%, 17.2%, and 29% at the four doses,
respectively).The anomalies consisted mainly of dilatation of the
renal pelvis and hyperaemia and/or inflammatory cell infiltration. The
incidence of dilatation of the renal pelvis was significantly
increased at doses > 300 mg/kg bw per day (0% in controls and 3.8%,
1.2%, 14%, and 18% at the four doses, respectively, with 0-3.2% in
historical controls). The incidence of hyperaemia and/or inflammatory
cell infiltration was significantly increased at 500 mg/kg bw per day
(0% in controls and 3.8%, 0%, 5.8%, and 12% at the four doses,
respectively). Although developmental insufficiency of the papilla or
stenosis and obstruction of the urethra can cause dilatation of the
renal pelvis, as can developmental retardation, the range in
historical controls of this strain was 0-2.0% in fetuses and 0-3.2% in
21-day-old offspring, and recovery from dilatation of the renal pelvis
usually occurs after birth. No skeletal anomalies were found that were
related to treatment. The absolute weights of all organs of F1
offspring killed on day 21 post partum were slightly but
significantly decreased, but no treatment-related change was found in
the absolute weights of the organs of F1Ib offspring killed on day
56 post partum. The changes in organ weights might have been due to
retarded physical development. Ambulation was also slightly but
significantly increased at doses > 100 mg/kg bw per day (by 31% at
100 mg/kg bw per day, 50% at 300 mg/kg bw per day, and 37% at 500
mg/kg bw per day). Motor coordination was comparable to that of
controls, and no treatment-related changes in learning ability were
observed. All the changes observed in the F1 offspring, including
increased ambulation, could have been due to retarded physical
development. After maturation, the reproductive perfomance of F1Ic
offspring was comparable to that of controls. The body weight and
body-weight gain of pregnant F1 animals were comparable to those of
controls during gestation. The NOAEL for developmental toxicity was
100 mg/kg bw per day, on the basis of decreased body weight,
retardation of physical development, and visceral anomalies associated
with the retarded growth rate at 300 mg/kg bw per day.
Significantly reduced numbers of implantations (12 in controls
and 10, 12, 11, and 9.2 at the four doses, respectively) and mean
numbers of live F2 fetuses (12 in controls and 9.3, 11, 10, and 8.5
at the four doses, respectively) were observed at 500 mg/kg bw per
day. Significantly reduced numbers of corpora lutea, implantations,
and live fetuses were found at 30 mg/kg bw per day but not at 100
mg/kg bw per day. These reductions were not dose-dependent. There were
no treatment-related differences in fetal body weight or in the
incidence of external anomalies between treated and control groups.
The NOAEL for F1 reproductive toxicity was 300 mg/kg bw per day, on
the basis of a reduction in the number of implantations and in the
mean number of live fetuses at 500 mg/kg bw per day (Saegusa et al.,
1988b).
(ii) Developmental toxicity
Rats
In a study that complied with GLP, groups of 36 female
Sprague-Dawley rats were given pyriproxyfen (purity, 97.2%) dissolved
in corn oil by gavage at doses of 0, 100, 300, or 1000 (42 animals)
mg/kg bw per day on days 7-17 of gestation. The fetuses (F1I) were
removed surgically from 23 pregnant dams (F0I) at 0, 100, or 300
mg/kg bw per day and from 20 at 1000 mg/kg bw per day on day 21 of
gestation and examined for developmental toxicity. The remaining 10-13
dams (F0II) were allowed to deliver normally. On day 4 post
partum, the offspring were culled to adjust the litter size to eight
(four males and four females when possible) for functional testing on
day 20 post partum (F1IIa). The remaining pups (F1IIb) were
killed at 21 days of age and examined for skeletal anomalies. During
lactation, developmental indices were examined. After weaning, one
male and one female from each litter (F1IIa1) allocated for
functional testing were examined for emotionality at 4 weeks of age,
for motor coordination at 5 weeks of age, and for learning ability at
6 weeks of age; these offspring were killed for necropsy at 8 weeks of
age. Another male and female from each litter (F1IIa2) were tested
for reproductive performance after being paired for mating within the
same group (avoiding sibling mating) at 11 weeks of age, and the
fetuses were removed surgically from the mated females on day 21 of
gestation. The remaining offspring (F1IIa3) were killed for
necroscopy at 21 days of age, after weaning.
The observations in parental rats included clinical signs,
deaths, food and water consumption, body weight, estrus cycle, and
organ weights. The postnatal developmental indices included separation
of the auricle, emergence of abdominal hair, eruption of incisors,
separation of eyelids, and descent of testes or opening of the vagina.
The sensory function tests included visual placing, righting and
mid-air righting reflexes, and response to pain. Emotionality was
evaluated by observation of behaviour in an open field, motor
coordination was examined in a rotarod performance test, and learning
ability was examined by behaviour in a water-filled multiple T maze
test. Reproductive performance included indices of mating, fertility,
gestation, and litters.
Of the F0 dams, 12 of 42 at 1000 mg/kg bw per day died between
day 11 and day 16 of gestation. No deaths occurred in the other
groups. Signs of toxicity were observed at doses > 300 mg/kg bw per
day. At the highest dose, these included diarrhoea, erythema and
swelling of the anal region, hypoactivity, wasting, hypothermia,
lachrymation, and piloelection. Signs of toxicity were also observed
at 300 mg/kg bw per day but in only one animal. Body weight and
body-weight gain were significantly reduced at 300 and 1000 mg/kg bw
per day during gestation and at 100 mg/kg bw per day during treatment.
Food consumption was significantly reduced at doses > 100 mg/kg bw
per day during treatment, and water consumption was dose-dependently
and significantly increased at doses > 300 mg/kg bw per day. Thymic
atrophy and enlarged adrenals were observed in the dams that died and
those killed on day 21 of gestation (F0I) at 1000 mg/kg bw per day.
In the F0I dams, from which fetuses were removed surgically on
day 21 of gestation, slight but significant increases were found in
the relative weights of the liver and kidney at 300 mg/kg bw per day,
but no significant increase in the absolute weights was observed at
this dose. At 1000 mg/kg bw per day, the absolute weights of the
kidney and adrenal were significantly increased and the absolute
weight of the thymus was significantly decreased. The relative weight
of the liver was significantly increased, but the absolute weight was
not significantly affected at 1000 mg/kg bw per day. Higher ratios of
resorbed or dead fetuses were observed at 1000 mg/kg bw per day (4.7%
in controls, 7.7% at 300 mg/kg bw per day, and 15% at 1000 mg/kg bw
per day). None of these differences achieved statistical significance
or was outside the historical control range (5.7%; 1.5-20%). There was
no difference in the mean numbers of corpora lutea or implantations or
the implantation rate. In the F0II dams, body weight and body-weight
gain were significantly reduced at 300 and 1000 mg/kg bw per day
during lactation. There were no treatment-related changes in the
number of live newborn, the length of gestation, or the delivery rate.
No NOAEL could be identified for F0 maternal toxicity since
decreased body-weight gain was seen at all doses. The NOAEL for
reproductive toxicity was 1000 mg/kg bw per day, the highest dose
tested.
No significant change in F1I fetal sex ratio, placental weight,
or fetal body weight was observed, but the ratio of resorbed or dead
fetuses was increased (5% in controls and 15% at 1000 mg/kg bw per
day) and the number of live fetuses was decreased at the highest dose,
but these changes were not statistically significant. Cyclopia and
polydactyly were each observed in one fetus at 300 mg/kg bw per day
(0.7%), but not in any other group, and these anomalies were
considered to be unrelated to treatment. There were no
treatment-related increases in the incidences of external, visceral,
or skeletal anomalies. A significant increase in the number of fetuses
with a skeletal variation consisting of opening of the foramen
transversarium of the seventh cervical vertebra was seen at 300 and
1000 mg/kg bw per day (0% in controls and 1.5%, 5.0%, and 14% at the
three doses, respectively). An increased number of fetuses with an
extra lumbar rib was observed at 1000 mg/kg bw per day, but this was
not significant. The NOAEL for developmental toxicity was 300 mg/kg bw
per day, on the basis of increased skeletal variations at 1000 mg/kg
bw per day.
The F1IIa offspring showed no-treatment related effect on
survival or weaning rates. The body weights of the treated groups were
comparable to those of controls during lactation and before mating.
There were no treatment-related effects on postnatal physical
development, sexual differentiation, sensory function, emotional
behaviour, or motor coordination. A significant retardation in the
time taken to reach the goal in the water-filled multiple T maze was
observed in females at 1000 mg/kg bw per day, but this slight
reduction in learning behaviour was observed only in the second of
three consecutive daily trials. There was no impairment of
reproductive performance of F1IIa2 offspring at any dose. No
external anomalies were observed in F2 fetuses. The NOAEL for
developmental toxicity was 1000 mg/kg bw per day, if the slight
evidence of behavioural teratogenicity in F1 offspring is ignored.
In the offspring killed at 21 days of age (F1IIb), an increased
incidence of skeletal variations was observed at 1000 mg/kg bw per day
(9% in controls and 18% at 1000 mg/kg bw per day), but this was not
statistically significant. No external or skeletal anomalies were
observed. The incidence of visceral anomalies was increased
significantly in F1IIaI offspring at 1000 mg/kg bw per day killed at
56 days of age (0/46 in controls and 7/39 at 1000 mg/kg bw per day).
F1IIb and F1IIaI offspring at 1000 mg/kg bw per day also showed an
increased incidence of dilatation of the renal pelvis. The total
incidence of dilatation of the renal pelvis observed in F1I
offspring (killed at 21 and 56 days of age and at the end of the
fertility test) was increased dose-dependently (0/98 in controls and
1/95, 3/85, and 9/79 at the three doses, respectively). The incidences
in controls from 15 previous studies were 0-2.0% in fetuses, 0-3.2% in
offspring 21 days old, 0-4.3% at 56 days of age, and 0-4.5% at the end
of the fertility test. The incidence in the controls in the present
study was 0% at all times. The incidence of protrusion or partial
adhesion of the liver parenchyma on the diaphragmatic side was also
increased at 1000 mg/kg bw per day (1/98 in controls and 0/95, 2/85,
and 6/79 at the three doses, respectively). These anomalies were not
observed in fetuses removed surgically. The overall NOAEL for
developmental toxicity was 300 mg/kg bw per day, on the basis of an
increased incidence of skeletal variations in fetuses and an increased
incidence of visceral anomalies in offspring at 1000 mg/kg bw per day
(Saegusa et al., 1988c).
Rabbits
Groups of 15-18 female JW-NIBS rabbits were treated by gavage
with pyriproxyfen (purity, 97.2%) at doses of 0, 100, 300, or 1000
mg/kg bw per day on days 6-18 of gestation and were killed on day 28
of gestation. The study complied with GLP.
In the maternal animals, abortion or premature delivery occurred
at doses > 300 mg/kg bw per day, in one control, none at 100 mg/kg
bw per day, three at 300 mg/kg bw per day, and six at 1000 mg/kg bw
per day. Dead and moribund animals were found at 1000 mg/kg bw per day
(none at 0, 100, or 300 mg/kg bw per day and three at 1000 mg/kg bw
per day). Several signs of toxicity, including soft stools,
emaciation, decreased spontaneous activity, and bradypnoea were
observed in aborted, prematurely delivered, prematurely dying, and
moribund dams at doses > 300 mg/kg bw per day. Body weight,
body-weight gain, and food consumption were significantly reduced in
dams at 1000 mg/kg bw per day. There were no significant effects on
the mean number of corpora lutea or implantations or the number, sex
ratio, or body weight of live fetuses.
The live fetuses showed no treatment-related external anomalies.
Skeletal or visceral malformations were observed in fetuses at 300
mg/kg bw per day, comprising a defect of the third distal phalanx of
the hind leg in one; cystic lung, ventricular septal defect,
hypoplasia of the left atrial auricle, and persistent truncus
arteriosus in one; and a defect of the gall-bladder in one. None was
observed in the other groups. External malformations were observed in
three fetuses, comprising local oedema, a visceral malformation, and
microphthalmia in one fetus at 300 mg/kg bw per day; persistent
truncus arteriosus in one fetus at 1000 mg/kg bw per day; and a
ventricular septal defect in another at this high dose. The total
incidence of malformations was 1% in controls, 1% at 100 mg/kg bw per
day, 6% at 300 mg/kg bw per day, and 2% at 1000 mg/kg bw per day. The
authors concluded that pyriproxyfen did not cause treatment-related
changes in the incidences of skeletal anomalies, skeletal variations,
ossification, visceral anomalies, or visceral variations. The NOAEL
for reproductive toxicity was 100 mg/kg bw per day on the basis of
abortion or premature delivery and a number of signs of toxicity at
300 mg/kg bw per day. The NOAEL for developmental toxicity was 1000
mg/kg bw per day, the highest dose tested (Hirohashi et al., 1988).
(f) Studies on metabolites
(i) Acute toxicity
The metabolites of pyriproxyfen were administered orally to mice
in a 0.5% solution of methylcellulose at 1000 or 2000 mg/kg bw. One
out of five males given 5''-hydroxypyriproxyfen (purity, 97.5%) at
2000 mg/kg bw died, but no deaths occurred with the other metabolites.
4'-Hydroxypyriproxyfen (purity, 98.3%) caused no abnormal clinical
signs; 5''-hydroxypyriproxyfen caused decreased spontaneous activity
in animals at both doses and ataxic gait at 2000 mg/kg bw;
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen
(purity, 90.1%) produced decreased spontaneous activity, ataxic gait,
and prone position in animals at 2000 mg/kg bw; (RS)-2-hydroxypropyl
4-phenoxyphenyl ether (purity, 99.0%) produced decreased spontaneous
activity, ataxic gait, prone position, lateral position, and irregular
respiration in animals at 2000 mg/kg bw; and (RS)-2-(2-pyridyloxy)
propionic acid (purity, 100%) produced decreased spontaneous activity
at 2000 mg/kg bw (Misaki, 1993a).
(ii) Genotoxicity
4'-Hydroxypyriproxyfen (purity, 98.3%), 5''-hydroxypyriproxyfen
(purity, 97.5%), 4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether
pyriproxyfen (purity, 90.2%), (RS)-2-hydroxypropyl 4-phenoxyphenyl
ether (purity, 99.0%), and (RS)-2-(2-pyridyloxy) propionic acid
(purity, 100%) were tested for mutagenicity in Salmonella
typhimurium TA98, TA100, TA1535, and TA1537 and in Escherichia
coli WP2 uvrA, with and without exogenous metabolic activation at
concentrations of 15-5000 µg/plate. None of the metabolites caused
reverse mutation, whereas the positive controls used in these assays
produced the anticipated responses (Hara et al., 1993).
No data were available on the effects of the metabolites of
pyriproxyfen on chromosomal integrity, but as these metabolites are
formed in vivo and pyriproxyfen had no effect on this end-point,
this is not considered a major gap in the data.
Comments
After oral administration to rats, [14C]pyriproxyfen is slowly
(time to peak concentration in plasma, 8 h) and incompletely (< 50%
of the dose) absorbed but is then rapidly eliminated, predominantly in
the faeces (90%), with only 4-11% in the urine, after 48 h. Absorbed
pyriproxyfen is excreted mainly via the bile (34-37% of the
administered dose in 48 h). The metabolism of pyriproxyfen is
qualitatively similar in rats, mice, lactating goats, and laying hens.
A large number of metabolites have been detected, the main route of
biotransformation being 4'-hydroxylation. Other pathways include
hydroxylation of the pyridyl ring, ether cleavage and conjugation.
Mice conjugate a much greater proportion of the dose than rats. The
concentration of pyriproxyfen in tissues other than fat was very low
(generally < 0.01 µg equivalent per g after 72 h; fat < 0.1µg
equivalent per g). The half-times of the radiolabel in tissues,
including blood and fat, were 8-36 h. The dermal absorption of
pyriproxyfen has not been studied.
The acute oral toxicity of pyriproxyfen is low, with LD50
values > 5000 mg/kg bw in mice, rats, and dogs. The acute dermal
toxicity is also low, with LD50 values > 2000 mg/kg bw in mice and
rats, and after exposure by inhalation, with an LC50 value > 1.3
mg/l air in mice and rats. WHO (1999) has classified pyriproxyfen as
'unlikely to present acute hazard in normal use'. Pyriproxyfen was
mildly irritating to the eye but not to the skin of rabbits. It did
not sensitize the skin of Hartley guinea-pigs in a maximization test.
In short- and long-term studies of the effects of pyriproxyfen in
mice, rats, and dogs, the liver was the main toxicological target,
with increases in liver weight and changes in plasma lipid
concentrations, particularly cholesterol, at doses of 120 mg/kg bw per
day and above in rats. There was some evidence that the compound might
cause modest anaemia in mice, rats, and dogs at high doses. In mice
treated with pyriproxyfen in the diet for three months, additional
effects seen included increased mortality rates, histopathological
changes in the kidney, and decreased body weight. The NOAEL was 150
mg/kg bw per day in mice, 23 mg/kg bw per day (two studies) in rats,
and 100 mg/kg bw per day in dogs fed pyriproxyfen in the diet for 3
months. In long-term studies of toxicity in mice, pyriproxyfen also
caused a dose-dependent increase in the occurrence of systemic
amyloidosis, which was associated with increased mortality rates. The
NOAEL was 120 ppm, equal to 16 mg/kg bw per day. In rats, the only
additional effect was reduced body-weight gain, and the NOAEL was 600
ppm, equal to 27 mg/kg bw per day. In two 1-year studies in dogs,
pyriproxyfen was administered in capsules. The overall NOAEL was 10
mg/kg bw per day on the basis of increased relative liver weight and
increased total plasma cholesterol concentration in males. There was
some evidence that pyriproxyfen can act as a hepatic enzyme inducer,
at least in dogs. Pyriproxyfen was not toxic when administered
dermally to rats for 21 days at doses of up to 1000 mg/kg bw per day.
Inhalation of pyriproxyfen for 4 h per day for 28 days caused only
minor effects in rats (initial salivation, sporadically reduced
body-weight gain, slightly increased serum lactate dehydrogenase
activity) at 10 000 mg/m3. The NOAEL was 480 mg/m3.
Pyriproxyfen was not carcinogenic when given in the diet at doses
up to 420 mg/kg bw per day in a study in mice or at doses up to 140
mg/kg bw per day in rats. Pyriproxyfen showed no evidence of
carcinogenicity in a 1-year study in dogs at doses up to 1000 mg/kg bw
per day. The Meeting concluded that pyriproxyfen does not pose a
carcinogenic risk to humans.
Pyriproxyfen was not genotoxic in an adequate range of tests for
mutagenicity and cytogenicity in vitro and in vivo. The Meeting
concluded that pyriproxyfen is not genotoxic.
The reproductive toxicity of pyriproxyfen in rats has been
investigated in a two-generation study of reproductive toxicity, a
study involving treatment of males and females before and in the early
stages of gestation (segment 1), and a study of treatment during the
prenatal and lactation periods (segment 3). The NOAEL for maternal
toxicity was 1000 ppm, equal to 98 mg/kg bw per day, in the
two-generation study and 100 mg/kg bw per day in the segment 3 study.
Reproductive toxicity was observed only in the segment 3 study, in
which there was an increased number of stillbirths in the F0
generation and a reduction in the number of implantations and in the
mean number of live fetuses in the F1 generation at 500 mg/kg bw per
day. The NOAEL for reproductive toxicity was 300 mg/kg bw per day. No
reproductive toxicity was observed in the two-generation study, the
NOAEL being 5000 ppm, equal to 340 mg/kg bw per day, the highest dose
tested, or in the segment 1 study, the NOAEL being 1000 mg/kg bw per
day, the highest dose tested.
The developmental toxicity of pyriproxyfen has been studied in
rats and rabbits. In rats, a NOAEL for maternal toxicity was not
identified, as decreased body-weight gain was observed at 100 mg/kg bw
per day, the lowest dose tested. Pyriproxyfen caused little
developmental toxicity and was not teratogenic. In a segment 3 study,
the F1 offspring were subjected to a series of developmental tests
for possible neurotoxicity, including physical indices, tests of
behaviour, motor and sensory function, and learning ability. Although
there were some effects on growth at doses > 300 mg/kg bw per day,
there was no developmental neurotoxicity at 500 mg/kg bw per day, the
highest dose tested. Visceral anomalies (dilatation of the renal
pelvis) were found at doses > 300 mg/kg bw per day. The NOAEL for
developmental toxicity was 100 mg/kg bw per day, on the basis of
retarded physical development and visceral anomalies at higher doses.
In a more conventional study of developmental toxicity in rats, no
evidence of growth retardation or of developmental neurotoxicity was
found at doses up to and including 1000 mg/kg bw per day, the highest
dose tested. There was an increased frequency of skeletal variations
(opening of the foramen transversalium of the seventh cervical
vertebra) in fetuses at 300 mg/kg bw per day. The frequency of
visceral anomalies was significantly increased in F1 offspring some
weeks after birth. The NOAEL for developmental toxicity was 300 mg/kg
bw per day, on the basis of an increased frequency of skeletal
variations with visceral anomalies in F1 offspring at 1000 mg/kg bw
per day. In a study of developmental toxicity in rabbits, signs of
maternal toxicity (abortion and premature delivery) were evident at
doses > 300 mg/kg bw per day (NOAEL, 100 mg/kg bw per day). No
developmental toxicity was observed, the NOAEL being 1000 mg/kg bw per
day, the highest dose tested.
The Meeting established an ADI of 0-0.1 mg/kg bw on the basis of
the NOAEL of 10 mg/kg bw per day in 1-year studies of toxicity in dogs
and a safety factor of 100.
The Meeting concluded that it was not necessary to establish an
acute reference dose because of the low acute toxicity of
pyriproxyfen.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 120 ppm, equal to 16 mg/kg bw per day (18-month study of
carcinogenicity)
Rat: 600 ppm, equal to 27 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
5000 ppm, equal to 345 mg/kg bw per day (reproductive
toxicity, two-generation study of reproductive toxicity,
highest dose tested)
100 mg/kg bw per day (developmental toxicity in a segment 3
study of developmental toxicity)
Rabbit: 100 mg/kg bw per day (maternal and reproductive toxicity in
a study of developmental toxicity)
1000 mg/kg bw per day (developmental toxicity in a study of
developmental toxicity, highest dose tested)
Dog: 10 mg/kg bw per day (1-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information valuable for continued
evaluation of the compound
Observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to
pyriproxyfen
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Slow, incomplete absorption (< 50%), rat
Dermal absorption No data (no systemic toxicity up to 1000 mg/kg bw per day by
dermal route, rat)
Distribution of total residues Highest concentrations of radiolabel in fat and, to lesser
extent, liver, rat
Potential for accumulation Possible limited accumulation in fat, rat
Rate and extent of excretion Rapid, complete, 88-96% within 48 h, primarily in faeces;
4-11% in urine, rat
Metabolism in animals Extensive. No parent compound detectable in urine; numerous
metabolites: main pathway is 4'-hydroxylation; also hydroxylation
of the pyridyl ring, ether cleavage, conjugation, mouse, rat,
goat, hen
Toxicologically significant compounds Pyriproxyfen
(animals, plants and environment)
Acute toxicity
LD50, oral > 5000 mg/kg bw, mouse, rat
LD50, dermal > 2000 mg/kg bw, mouse, rat
LC50, inhalation > 1.3 mg/L, mouse, rat
Dermal irritation Not irritating, rabbit
Ocular irritation Mildly irritating, rabbit
Dermal sensitization Not a sensitizer, guinea-pig
Short-term toxicity
Target/critical effect Mouse, rat, dog: liver, increased relative liver weight, mild
anaemia, altered lipid metabolism (increased serum cholesterol)
Lowest relevant oral NOAEL 13 weeks, rat, 24 mg/kg bw per day
Lowest relevant dermal NOAEL 21 days, rat, > 1000 mg/kg bw per day
Lowest relevant inhalation NOAEL 28-day, rat, > 1.3 mg/L
Long-term toxicity and carcinogenicity
Target/critical effect: Mouse, rat, dog: liver, increased liver weight, decreased body
weight, altered lipid metabolism (increased plasma cholesterol)
(rat, dog)
Lowest relevant NOAEL 1 year, dog, 10 mg/kg bw per day (diet)
Carcinogenicity Not carcinogenic, mouse, rat
Genotoxicity Not genotoxic
Reproductive toxicity
Reproductive target/critical effect Reduction in number of implantations and live F2 fetuses at F1
developmentally toxic dose, rat
Lowest relevant reproductive NOAEL 345 mg/kg bw per day, rat
Developmental target/critical effect Retardation of physical development in F1, rat
Lowest relevant developmental NOAEL 100 mg/kg bw per day, rat
Neurotoxicity/Delayed neurotoxicity No evidence of developmental neurobehavioural toxicity in rat. No
evidence of neurotoxicty or neuropathology in medium- or long-term
studies in mouse, rat, dog or during development in rat, rabbit
Other toxicological studies Possible enzyme inducer, at least in dogs
Medical data No data
Summary Value Study Safety factor
ADI 0-0.1 mg/kg bw 1-year, dog, toxicity 100
Acute reference dose Unnecessary
References
Cardy, R., Moore, M., Murphy, B.S., Thakur, A., Tellone, C., Ito, S.,
Lang, P., Ginevan, M., Driver, J., Stewart, R. & Wilkinson, C.
(1994) Supplemental data and review of oncogenicity study with
S-31183 (Sumilarv) in mice (MRID No. 421783-10). Unpublished
study from Technology Sciences Group Inc. Reference No.
NNT-41-0116. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Chapman, E.A, Lee, P., Virgo, D.M. & Sparrow, S. (1991) S-31183:
Toxicity study by oral (capsule) administration to beagle dogs
for 52 weeks. Unpublished study from Life Science Research Ltd.
Reference No. NNT-11-0081. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Cox, R.H., Zoetis, T., Cardy, R.H., Alsaker, R.D., Kuhlman, S.M.,
Lewis, S.A., Thakur, A.K. & Phipps, N.G. (1989) Subchronic
toxicity Sstudy with S-31183 in rat. Unpublished study from
Hazleton Laboratories America, Inc. Reference No. NNT-910045.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Cox, R.H., Zoetis, T., Voelker, R.W., Alsaker, R.D., Kuhlman, S.M.,
Lewis, S.A., Thakur, A.K. & Phipps, N.G. (1990) Subchronic
toxicity study in mice. Unpublished study from Hazleton
Laboratories America, Inc. Reference No. NNT-01-0066. Submitted
to WHO by Sumitomo Chemical Co., Ltd.
Hara, M., Katoh, H. & Yoshitake, A. (1993a) Reverse mutation test of
metabolites of pyriproxyfen, 4'-OH-Pyr, 5"-OH-Pyr, DPH-Pyr, POPA
and PYPAC, in bacterial systems. Unpublished study reference No.
NNT-30-0104. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Henderson, L.M. & Proudlock, R.J. (1989) Assessment of unscheduled DNA
repair synthesis in mammalian cells after exposure to S-31183.
Unpublished study from Huntingdon Research Centre Ltd. Reference
No. NNT-91-0053. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Hirohashi, A., Kannan, N., Kato, T. & Yamada, H. (1988) Study of
S-31183 by oral administration during the period of fetal
organogenesis in rabbits. Unpublished study reference No.
NNT-80-0033. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Isobe, N., Matsunaga, H., Kimura, K., Yoshitake, A. & Yamada, H.
(1988a) Metabolism of S31183 in rats. Unpublished study reference
No. NNM-80-0001. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Isobe, N., Matsunaga, H., Kimura, K., Yoshitake, A. & Yamada, H.
(1988b) Metabolism of S-31183 in rat (tissue distribution study).
Unpublished study reference No. NNM-800002. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Kawaguchi, S., Watanabe, T., Suzuki T., Kato, T. & Yamada, H. (1987)
Acute inhalation toxicity of S-31183 in rats. Unpublished study
reference No. NNT-70-0022. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Kawaguchi, S., Yoshioka, K., Ito, S., Suzuki, T., Kato, T. & Yamada,
H. (1988) Subacute inhalation toxicity of S-31183 in rats.
Unpublished study reference No. NNT-80-0031. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Kogiso, S., Hara, M., Yoshitake, A. & Yamada, H. (1988a) Reverse
mutation test with S31183 in bacteria systems. Unpublished study
reference No. NNT-80-0034. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Kogiso, S., Hara, M., Iwawaki, H. & Yamada, H. (1988b) In vitro
chromosomal aberration test of pyriproxyfen in Chinese hamster
ovary cells (CHO-K1). Unpublished study reference No.
NNT-80-0028J. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Kogiso, S., Yamamoto, K., Hara, M., Yoshitake, A. & Yamada, H. (1989)
In vitro chromosomal aberration test of pyriproxyfen in Chinese
hamster ovary cells (CHO-K1). Unpublished study reference No.
NNT-90-0054. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Kogiso, S., Yamada, T., Hara, M., Yoshitake, A. & Yamada, H. (1990) In
vitro gene mutation test of S-31183 in V79 Chinese hamster cells.
Unpublished study reference No. NNT-000067. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Kogiso, S. Kato, H. & Nakattuka, S. (1992) Reverse mutation test with
S-31183 in bacteria systems. Unpublished study reference No.
NNT-80-0088J. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Koyama, Y., Kimura, J., Yoshioka, K., Watanabe, T., Seki, T.,
Hosokawa, S., Yamada, H. & Hagiwara, H. (1989) A six-month
chronic dietary toxicity study of pyriproxyfen in rats.
J. Toxicol. Sci., 14, 43-64.
Matsunaga, H., Yoshino, H., Isobe, N., Kaneko, H., Nakatuka, I. &
Yamada, H. (1995) Metabolism of pyriproxyfen in rats. 1.
Absorption, disposition, excretion, and biotransformation studies
with [phenoxyphenyl-14C] pyriproxyfen. J. Agric. Food Chem.,
43, 235-240.
Misaki, Y. & Nakatuka, I. (1993) Acute oral toxicity study of
pyriproxyphen metabolites, 4'-OH-Pyr, 5"-OH-Pyr, DPH-Pyr, POPA
and PYPAC in mice. Unpublished study reference No. NNT-30-0107.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Mitchell, D.J., Virgo, D.M., Broadmeadow, A., Chase, K.R., Lee, P. &
Sparrow, S. (1993) S31183: Toxicity study by oral (capsule)
administration to beagle dogs for 52 weeks (additional
investigation). Unpublished study from Life Science Research Ltd.
Reference No. NNT-31-0102. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Moore, M.R., Zoetis, T., Doyle, D., Cardy, R.H., Pearson, R.C.,
Walker, M.D., Lewis, S.A., Thomas, D.L., Thakur, A.K., Burlew,
P.L., Hatcher, C.F. & Vegarra, M. (1993) 21-day dermal toxicity
study in rats with S-31183. Unpublished study from Hazleton
Washington, Inc. Reference No. NNT-31-0094. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Nakano, M., Yamamoto, T., Kato, T. & Miyamoto, J. (1986) Acute oral
toxicity study of S31183 in dogs. Unpublished study reference No.
NNT-60-0012. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Nakano, M., Kohda, A., Kato, T. & Yamada, H. (1988) Three-month oral
toxicity study of S31183 in dogs. Unpublished study reference No.
NNT-80-0037. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Osheroff, M.R., Ziegler, K.A., Cardy, R.H., Alsaker, R.D., Kuhlman,
S.M., Lewis, S.A., Thakur, A.K., Burlew, P.L. & Nasca, A.J.
(1991a) Oncogenicity study in mice with S31183. Unpublished study
from Hazleton Laboratories America, Inc. Reference No.
NNT-11-0084. Submitted to WHO by Sumitomo Chemical Co.
Osheroff, M.R., Ziegler, K.A., Machotka, S., Alsaker, R.D., Kuhlman,
S.M., Lewis, S.A., Thakur, A.K., Burlew, P.L., Devis, P.J. &
Graham, R. (1991b) Combined chronic toxicity and oncogenicity
study in rats with S-31183. Unpublished study from Hazleton
Washington, Inc. Reference No. NNT-11-0085. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Panthani, A.M., Walsh, K.J. & Turck, P. (1996a) Metabolism of
[phenoxyphenyl-14C]S71639 (pyriproxyfen) in lactating goats.
Unpublished study from Ricerca, Inc. Reference No. NNM-0043.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Panthani, A.M., Walsh, K.J. & Turck, P. (1996b) Metabolism of
[pyridyl-14C]S-7 1639 (pyriproxyfen) in lactating goats.
Unpublished study from Ricerca, Inc. Reference No. NNM-0046.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Panthani, A.M., DiFrancesco, P. & Savides, M.C. (1996c) Metabolism of
[phenoxyphenyl14C]S-71639 (pyriproxyfen) in laying hens.
Unpublished study from Ricerca, Inc. Reference No. NNM-0045.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Panthani, A.M., DiFrancesco, P. & Savides, M.C. (1996d) Metabolism of
[pyridyl-14C]S71639 (pyriproxyfen) in laying hens. Unpublished
study from Ricerca, Inc. Reference No. NNM-0044. Submitted to WHO
by Sumitomo Chemical Co., Ltd.
Proudlock, R.J., Haynes, P. & Goodenough, A.J. (1991) Mouse
micronucleus test on S31183. Unpublished study from Huntingdon
Research Centre Ltd. Reference No. NNT11-0082. Submitted to WHO
by Sumitomo Chemical Co., Ltd.
Robinson, K., Washer, G. & Noveroske, J. (1991) A dietary 2-generation
(1 litter) reproduction study of S-31183 in the rat. Unpublished
study from Bio-Research Laboratories Ltd. Reference No.
NNT-11-0087. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Saegusa, T., Kitajima, S. & Narama, I. (1988a) Study on administration
of a test substance prior to and in the early stages of pregnancy
in rats. Unpublished study from Hamamatsu, Seigiken Research Co.,
Ltd. Reference No. NNT-80-0036. Submitted to WHO by Sumitomo
Chemical Co., Ltd.
Saegusa, T., Kitajima, S. & Narama, I. (1988b) Study on administration
of S-31183 during the perinatal and lactation periods in rats.
Unpublished study from Hamamatsu, Seigiken Research Co., Ltd.
Reference No. NNT-80-0030. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Saegusa, T., Kitajima, S. & Narama, I. (1988c) Study by administration
of S-31183 during the period of fetal organogenesis in rats.
Unpublished study from Hamamatsu, Seigiken Research Co., Ltd.
Reference No. NNT-80-0029. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Sako, H., Okuno, Y., Kato, T. & Yamada, H. (1987a) Acute
oral toxicity of S31183 in mice. Unpublished study reference No.
NNT-70-0014. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Suzuki, T., Misaki, Y., Okuno, Y., Kato, T. & Miyamoto, J. (1987b)
Acute oral toxicity of S31183 in rats, Unpublished study
reference No. NNT-70-0005. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Sako, H., Okuno, Y., Kato, T. & Yamada, H. (1987c) Acute
dermal toxicity of S31183 in mice. Unpublished study reference
No. NNT-70-0015. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Suzuki, T., Misaki, Y., Okuno, Y., Kato., T. & Miyamoto, J. (1987d)
Acute dermal toxicity of S-31183 in rats. Unpublished study
reference No. NNT-70-0006. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Kawaguchi, S., Watanabe, T., Kato, T. & Yamada, H. (1987e)
Acute inhalation toxicity of S-31183 in mice Unpublished study
reference No. NNT-70-0023. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Nakanishi, T., Kato, T. & Miyamoto, J. (1987f) Skin
sensitization test with S31183 in guinea pigs. Unpublished study
reference No. NNT-70-0003. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Nakanishi, T., Kato, T. & Miyamoto, J. (1987g) Primary eye
and skin irritation tests with S-31183 in rabbits. Unpublished
study reference No. NNT-70-000. Submitted to WHO by Sumitomo
Chemical Co., Ltd.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Yoshino, H. (1993a) Metabolism of pyriproxyfen in rat (high-dose,
14C-concentrations in tissues). Unpublished study reference No.
NNM-30-0028. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Yoshino, H. (1993b) Metabolism study of
(pyridyl-2,6-14C)pyriproxyfen in rat (pyridyl14C-labeled test
compound, single oral administration at low- and high-doses).
Unpublished study reference No. NNM-30-0025. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Yoshino, H., Kaneko, H., Nakatsuka, I. & Yamada, H. (1995) Metabolism
of pyriproxyfen. 2. Comparison of in vivo metabolism between rats
and mice. J. Agric. Food Chem., 43, 2681-2686.
Yoshino, H., Kaneko, H., Nakatsuka, I. & Yamada, H. (1996) Metabolism
of pyriproxyfen. 3. In vitro metabolism in rats and mice.
J. Agric. Food Chem., 44, 1578-1581.
 Lactating goats
Lactating goats (Capra hircus, weighing 51-57 kg) were given
[14C-phenoxyphenyl]-pyriproxyfen (purity, 99.5%) in gelatin capsules
at a dose of 1.8-2.0 or 20 mg/animal per day for 5 consecutive days,
for a total dose of 100 mg. The animals were killed within 6 h of the
last dose. Faeces, urine, and milk were collected twice daily and
analysed. Metabolites were purified from extracts of tissues or
excreta by HPLC and identified by TLC and/or mass spectral analysis.
The study was carried out according to GLP. Elimination reached a
plateau by the third day. The percentages of total radiolabel
recovered 1 day after the last dose were 17-18% in urine, 58% in
faeces, and 0.3-0.8% in milk. The metabolites identified in milk
extracts were 4'-hydroxypyriproxy-fen sulfate (51% of the radiolabel
present), 4'-hydroxypyriproxyfen (2%), 4-phenoxyphenol sulfate (10%),
4'-hydroxy-4-phenoxyphenol sulfate (8%),
4'-hydroxy- (RS)-2-hydroxypropyl 4-phenoxyphenyl ether sulfate (3%),
and 4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen
(8%). Metabolites found in both liver and kidney were
4'-hydroxypyriproxyfen sulfate, 5''-hydroxypyriproxyfen-sulfate,
(RS)-2-hydroxypropyl 4-phenoxyphenyl ether,
4'-hydroxy (RS)-2-hydroxypropyl 4-phenoxyphenyl ether, and
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen. In
addition, 4'-hydroxypyriproxyfen and 5''-hydroxypyri-proxyfen were
identified in liver, and 4-phenoxyphenol sulfate and
4'-hydroxy-4-phenoxyphenol in kidney. The metabolites identified in
the faecal samples were 4'-hydroxypyriproxyfen (39% of the radiolabel
present), 5''-hydroxypyriproxyfen (6%),
4'-hydroxy- (RS)-2-hydroxypropyl 4-phenoxyphenyl ether (5%),
(RS)-2-hydroxypropyl 4phenoxyphenyl ether, and 4-hydroxyphenyl
(RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen. Parent compound
accounted for 11-13% of the dose in faeces, The major metabolites in
urine were 4-phenoxyphenol (36% of the radiolabel present),
4-phenoxyphenol sulfate (15%), 4'-hydroxy-4-phenoxyphenol (14%), and
4'-hydroxy-4-phenoxyphenol sulfate (17%) (Panthani et al., 1996a)
In another study that conformed to GLP, lactating goats
( Capra hircus, weighing 39-46 kg) were given
[14C-pyridyl]pyriproxyfen (purity, 97.6% ) in gelatin capsules at a
dose of 1.8-1.9 or 20 mg/animal per day for 5 consecutive days. The
animals were killed within 6 h of the last dose. Faeces, urine, and
milk were collected twice daily and analysed by HPLC and TLC and/or
mass spectrometry. The total radiolabel recovered represented 17-18%
of the dose in urine, 58% in faeces, and 0.4-0.8% in milk. The major
metabolites identified in milk extracts were 4'-hydroxypyriproxyfen
sulfate (35% of the radiolabel present) and 2,5-dihydroxypyridine
conjugate (29%), with smaller quantities of 4'-hydroxypyriproxyfen and
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen. The
major metabolites identified in liver and kidney were
4'-hydroxypyriproxyfen sulfate, 2,5-dihydroxypyridine conjugate, and
(RS)-2-(2-pyridyoxy) propanol conjugates. The major metabolites
identified in the urine were 4'-hydroxypyriproxyfen sulfate,
(RS)-2-(2-pyridyloxy) propionic acid, and 2,5-dihydroxypyridine
conjugate. The metabolites identified in the faecal samples were
4'-hydroxypyriproxyfen (43%), 5''-hydroxypyriproxyfen (5%), and
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen
(5%). These two studies indicate that the primary routes of metabolism
in goats are hydroxylation of the 4' position of the phenoxyphenyl
ring and the 5'' position of the pyridyl ring, cleavage of the ether
bonds, oxidation of the methylene moiety, and sulfate conjugation of
the 4'-hydroxyphenoxyphenyl moiety. There is thus no difference
between rats and goats in the metabolic pattern of pyriproxyfen
(Panthani et al., 1996b)
Chickens
Laying Leghorn hens (Gallus domesticus) were given
[14C-phenoxyphenyl]pyriproxyfen (purity, 99.1%) by gelatin capsule
at a concentration equivalent to 10 ppm in the feed for 8 consecutive
days and were killed within 4 h of the last dose. Excreta were
collected once daily and analysed by HPLC and TLC and/or mass
spectrometry. The study complied with GLP. The metabolites identified
in liver and kidney were free 4'-hydroxypyriproxyfen and its sulfate
conjugate, free 4'-hydroxy-4-phenoxyphenol and its sulfate conjugate,
free 4'-hydroxy- (RS)-2-hydroxypropyl-4-phenoxyphenyl ether and its
sulfate conjugate, 4-phenoxyphenol sulfate,
4-hydroxyphenyl- (RS)-2-(2-pyridyloxy)propyl ether pyriproxyfen, and
(RS)-2-hydroxypropyl-4-phenoxyphenyl ether. Metabolites identified
in excreta samples were 4'-hydroxypyriproxyfen, free and conjugated
4'-hydroxy-4-phenoxyphenol, free and conjugated
4'-hydroxy- (RS)-2-hydroxy-propyl 4-phenoxyphenyl ether,
4-hydroxyphenyl (RS)-2-(2pyridyloxy)propyl ether pyriproxyfen,
5''-hydroxypyriproxyfen, (RS)-2-hydroxypropyl-4-phenoxyphenyl ether,
and 4-phenoxyphenol (Panthani et al., 1996c)
Laying Leghorn hens (Gallus domesticus) were given
[14C-pyridyl]pyriproxyfen (purity, 98.2%) by gelatin capsule at a
concentration equivalent to 10 ppm in the feed for 8 consecutive days
and were killed within 4 h of the last dose. Excreta were collected
once daily and analysed by HPLC and TLC and/or mass spectrometry. The
study complied with GLP. The metabolites identified in liver and
kidney were free and conjugated 4'-hydroxypyriproxyfen,
2-hydroxypyridine, free and conjugated 5''-hydroxypyriproxyfen,
4-hydroxyphenyl- (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen, and
(RS)-2-(2-pyridyloxy)propionic acid. The metabolites identified in
excreta were (RS)-2-(2-pyridyloxy) propionic acid,
4'-hydroxypyriproxyfen, 4-hydroxyphenyl (RS)-2-(2-pyridyloxy)propyl
ether pyriproxyfen, 5''-hydroxypyriproxyfen, and 2-hydroxypyridine.
These two studies indicate that the routes of metabolism in laying
hens are hydroxylation at the 4' position of the phenoxyphenyl ring
and at the 5'' position of the pyridyl ring, cleavage of the ether
linkages, oxidation of the methylene moiety of the side-chain, and
sulfation of the 4'-hydroxyphenoxyphenyl moiety (Panthani et al.,
1996d)
In vitro
The metabolism of pyriproxyfen in rats and mice was investigated
in vitro in samples of 10 000 × g supernatant (S10) prepared from
kidney, lungs, and small intestine of 7-week-old Sprague-Dawley rats
and ICR mice. Hepatic microsomal and cytosolic fractions were prepared
by an established method (centrifugation of S10 at 105 000 × g), and
microsomal, S10, or cytosolic fractions were incubated with
[14C-phenoxyphenyl]pyriproxyfen at a concentration of 0, 0.05, 0.1,
0.5, or 1.0 mmol/L with b-NADPH as a cofactor. The reaction mixtures
were analysed by TLC. Pyriproxyfen was not metabolized by any of the
S10 preparations; it was almost completely metabolized by microsomal
fractions from liver but only very slightly by hepatic cytosol. Most
of the major metabolites identified in rats in vivo were observed
in vitro. There was no species difference in the major metabolic
reactions. The intrinsic clearance calculated from Lineweaver-Burk
plots revealed sex-related differences in metabolic reactions in rats
but not in mice, and 5''-hydroxylation was observed only in male rats
and not in mice of either sex. The intrinsic clearance for microsomal
4'-hydroxylation in female rats was twice that in males, but was not
different in male and female mice. Incubation of a hepatic microsomal
fraction from male rats with antisera against male-specific forms of
cytochrome P450 (CYP2C11 or CYP2C13) revealed that members of the
CYP2C family, the expression of which is sex-dependent in rats, are
involved in the major hydroxylation reactions of pyriproxyfen.
Antiserum against CYP2C11 inhibited all of the major metabolic
reactions of the hepatic microsomal fraction from male rats (> 88%
inhibition). The antiserum against CYP2C13 also inhibited all
reactions (25-64% inhibition) except 4'-hydroxylation (Yoshino et al.,
1996).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of pyriproxyfen are
summarized in Table 1. Pyriproxyfen dissolved in corn oil was
administered orally in a volume of 10 ml/kg bw to ICR (Crj:CD-1) mice
at doses of 1000, 2000, or 5000 mg/kg bw and to Sprague-Dawley
(Crj:CD) rats at 1000, 2500, or 5000 mg/kg bw. In mice, pyriproxyfen
reduced spontaneous motor activity and caused ataxia, abnormal
respiration, and death in males at 2000 mg/kg bw and in animals of
each sex at 5000 mg/kg bw. Transiently decreased body weight was
observed in males at 5000 mg/kg bw. Deaths occurred in two males at
2000 mg/kg bw and two at 5000 mg/kg bw in males and in one female at
5000 mg/kg bw. In rats, pyriproxyfen caused a decrease in body-weight
gain, decreased spontaneous activity, soft stools, and diarrhoea in
males at 2500 mg/kg bw and in animals of each sex at 5000 mg/kg bw,
but no deaths. Necropsy revealed no abnormal changes in the organs of
mice or rats. In dogs, oral administration of pyriproxyfen in capsules
caused no deaths at doses up to 5000 mg/kg bw. The only clinical sign,
occasional vomiting for the first 24 h, was observed at the highest
dose. Dermal application of 2000 mg/kg bw of pyriproxyfen dissolved in
corn oil (5 or 10 ml/kg bw) caused no deaths or signs of clinical
toxicity in ICR mice or Sprague-Dawley rats. Exposure of ICR mice or
Sprague-Dawley rats to a mist aerosol of pyriproxyfen dissolved in
corn oil at concentrations of 0.6 or 1.3 mg/L for 4 h caused no deaths
or pathological changes. The mass median aerodynamic diameter of the
particles was 0.8-0.9 µm. At the high concentration, salivation and
urinary incontinence were observed in rats 4 h after the start of
inhalation, and irregular respiration was observed in mice. These
clinical signs disappeared within 1 h of cessation of exposure.
(b) Short-term studies of toxicity
Mice
Groups of 10 male and 10 female ICR (Crj:CD-1) mice were given
diets containing technical-grade pyriproxyfen (purity, 95.3%) at
concentrations of 0, 200, 1000, 5000, or 10 000 ppm, equal to 0, 28,
150, 840, and 2000 mg/kg bw per day for males and 0, 38, 200, 960, and
2300 mg/kg bw per day for females, for 13 weeks. The observations
included clinical signs, mortality, food and water consumption, body
weight, clinical chemical parameters including serum enzymes, urinary
and haematological parameters, organ weights, and gross and
histopathological appearance. The serum enzymes assayed were alanine
aminotransferase), aspartate aminotransferase, and gamma-glutamyl
transpeptidase. Blood samples were collected shortly before
termination of the study. The study conformed to GLP.
Death occurred in two males at 5000 ppm and in seven males and
nine females at 10 000 ppm (one of the deaths in males was not
treatment-related). The clinical signs observed in the animals that
died prematurely were emaciation, hunched appearance, and few or no
faeces. There were no treatment-related clinical signs in the mice
that survived. Terminal body weights were significantly reduced in
males at 5000 ppm (89% of control) and at 10 000 ppm (69% of control);
the body weights of females were reduced at 5000 ppm during the first
half of the study but were comparable to control values (97% of
control) by the end. The water consumption of males at the two higher
doses was significantly increased, but food consumption was not
affected by treatment. There were significant decreases in erythrocyte
parameters, including cell count, haemoglobin concentration,
haematocrit, mean cell volume, and mean cell haemoglobin value, in
animals at 5000 and 10 000 ppm. Platelet counts were significantly
increased in animals of each sex at 5000 ppm and in males at 10 000
ppm. Significant increases in total cholesterol concentration were
observed in females at doses > 1000 ppm: 130% at 1000 ppm, 210% at
5000 ppm, and 140% of control values at 10 000 ppm. In males, the
activities of aspartate and alanine aminotransferases were increased
by up to twofold but reached significance only at 5000 ppm.
gamma-Glutamyl transpeptidase activity showed slight, nonsignificant
changes in some groups. The absolute weight of the liver was
significantly increased in females at 5000 ppm (120% at 5000 ppm and
160% of control values at 10 000 ppm) but not in males at any dose.
Microscopic examination revealed histomorphological alterations of the
Table 1. Acute toxicity of pyriproxyfen
Species Strain Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/L air)
Mouse ICR M&F Oral > 5000 97.2 Suzuki et al. (1987a)
Rat Sprague-Dawley M&F Oral > 5000 97.2 Suzuki et al. (1987b)
Dog Beagle M&F Oral > 5000 97.2 Nakano et al. (1986)
Mouse ICR M&F Dermal > 2000 97.2 Suzuki et al. (1987c)
Rat Sprague-Dawley M&F Dermal > 2000 97.2 Suzuki et al. (1987d)
Mouse ICR M&F Inhalation > 1.3 97.0 Suzuki et al. (1987e)
Rat Sprague-Dawley M&F Inhalation > 1.3 97.0 Kawaguchi et al. (1987)
These studies were conducted according to good laboratory practice.
kidney comprising tubular nephrosis with microcytosis and dilatation
of the renal tubules and focal mineralization and dilatation of the
renal pelvis in males at 5000 ppm and in animals of each sex at 10 000
ppm. There were no treatment-related morphological changes in the
liver. The NOAEL was 1000 ppm, equal to 150 mg/kg bw per day, on the
basis of effects on erythrocyte parameters, deaths, decreased body
weight, histomorphological alterations in the kidney, and increased
absolute liver weight at higher doses (Cox et al., 1990).
Rats
Groups of 10 male and 10 female Sprague-Dawley (Crl:CD) rats
received diets containing technical-grade pyriproxyfen (purity, 95.3%)
at concentrations of 0, 400, 2000, 5000, or 10 000 ppm, equal to 0,
23, 120, 310, and 640 mg/kg bw per day for males and 0, 28, 140, 360,
and 780 mg/kg bw per day for females, for 13 weeks. The observations
included clinical signs, deaths, body weight, food and water
consumption, ophthalmological, clinical chemical, haematological, and
urinary parameters, organ weights, and gross and histopathological
appearance. The clinical chemical examinations included assays for the
serum enzymes alkaline phosphatase, aspartate and alanine
aminotransferases, and gamma-glutamyl transpeptidase. Blood samples
were collected at the end of the study.
There were no treatment-related deaths, toxic signs, or
ophthalmological changes at any dose, and no changes in food or water
consumption. The body weights of animals of each sex were
significantly decreased at doses > 5000 ppm (91% of control at 5000
ppm and 88% at 10 000 ppm at termination). Erythrocyte parameters
including cell count, haemoglobin concentration, and haematocrit were
significantly decreased in animals at doses > 5000 ppm and in males
also at 2000 ppm. The mean cell volume was reduced in females at 2000
and 10 000 ppm by < 10%. There was no significant effect on platelet
count. The total cholesterol concentration was dose-dependently,
significantly increased in males at doses > 2000 ppm (150% at 2000
ppm, 200% at 5000 ppm, and 210% of control values at 10 000 ppm) and
in females at doses > 5000 ppm (130% at 5000 ppm and 180% of
control values at 10 000 ppm). The serum phospholipid concentration
was also significantly increased in males at doses > 2000 ppm (130%
at 2000 ppm, 180% at 5000 ppm, and 180% of control values at 10 000
ppm) and in females at 10 000 ppm (160% of control value).
Significantly increased gamma-glutamyl transpeptidase activity was
observed in animals at 10 000 ppm, but the activities of the other
serum enzymes were not significantly affected. Significant increases
in the absolute weight of the liver were observed in animals at doses
> 5000 ppm (110% at 2000 ppm, 130% at 5000 ppm, and 140% of control
values at 10 000 ppm in males and 100% at 2000 ppm, 120% at 5000 ppm,
and 140% of control values at 10 000 ppm in females), and the relative
weight of the liver was significantly increased in males at 2000 ppm
and in animals of each sex at doses > 5000 ppm (120% at 2000 ppm,
140% at 5000 ppm, and 170% of control values at 10 000 ppm in males
and 130% at 5000 ppm and 160% of control values at 10 000 ppm in
females). Significant increases in the relative weight of the kidney
were observed in animals at 10 000 ppm, but the absolute weights were
comparable to those of controls at all doses. Histopathological
examination revealed dose-dependent increases in cytoplasmic changes
in the liver in all treated groups (1/10 at 0 ppm, 2/10 at 400 ppm,
6/10 at 2000 ppm, 10/10 at 5000 ppm, and 9/10 at 10 000 ppm in males
and 1/10 at 0 ppm, 2/10 at 400 ppm, 6/9 at 2000 ppm, 7/10 at 5000 ppm,
and 9/10 at 10 000 ppm in females). The cytoplasmic changes consisted
of slight, often equivocal increases in cytoplasmic content reflected
in a visibly reduced nucleus:cytoplasm ratio and diminution of
sinusoidal spaces. The NOAEL was 400 ppm, equal to 23 mg/kg bw per
day, on the basis of mild anaemia, increased incidences of minimal
hepatic abnormalities, relative liver weights, and serum
concentrations of total cholesterol and phospholipids, indicating
effects on lipid metabolism, at higher doses (Cox et al., 1989).
Groups of 21 male and 21 female Sprague-Dawley rats were given
diets containing pyriproxyfen (purity, 97.2%) at concentrations of 0,
80, 400, 2000, or 10 000 ppm, equal to 0, 4.8, 24, 120, and 680 mg/kg
bw per day for males and 0, 5.4, 28, 140, and 690 mg/kg bw per day for
females, for 26 weeks. The observations included clinical signs,
deaths, food and water consumption, body weight, ophthalmological,
clinical chemical, haematological, and urinary parameters, organ
weights, and gross and histological appearance. The serum activities
of aspartate and alanine aminotransferases, alkaline phosphatase,
lactate dehydrogenase, leucine aminopeptidase, creatine phosphokinase,
and gamma-glutamyl transpeptidase were measured. Blood samples were
taken at the end of the study.
There were no deaths, and the only signs of toxicity were
increased incidences of alopecia around the neck and soft stools
during the early stage of the study in animals at 10 000 ppm. The body
weights of animals at 10 000 ppm were significantly decreased
throughout the study, by 86% in males and 87% in females at the end of
study. Marked decreases in body-weight gain were observed at this
dose. There were no treatment-related changes in food or water
consumption. Proteinuria and increased urinary excretion of potassium
ion were observed in animals at 10 000 ppm. Slight but significant
decreases in erythrocyte count and haematocrit were observed in males
at 2000 and 10 000 ppm and in females at 10 000 ppm. The haemoglobin
concentration was also slightly but significantly decreased at this
dose. No increase in platelet count was observed. Slight but
significant increases in total protein, albumin, and blood urea
nitrogen were observed in animals at 10 000 ppm. The albumin and
a2u-globulin fractions were slightly but significantly increased in
males at 10 000 ppm. Total cholesterol and phospholipid concentrations
were significantly increased in males at 2000 and 10 000 ppm and in
females at 10 000 ppm. Of the serum enzyme activities studied, only
that of gamma-glutamyl transpeptidase was significantly increased in
males at 10 000 ppm. Significantly increased absolute weights of the
liver were observed in animals at 10 000 ppm (130% of control value in
males and in females), and significant increases in the relative
weight were observed in males at doses > 2000 (110% at 2000 ppm and
160% of control values at 10 000 ppm) and in females at 10 000 ppm
(100% at 2000 ppm and 150% of control values at 10 000 ppm).
Significantly increased relative kidney weights were observed in
animals at 10 000 ppm, but the absolute weights were not significantly
increased. Histopathological examination showed slight hypertrophy of
the liver in all animals at 10 000 ppm. The NOAEL was 400 ppm, equal
to 24 mg/kg bw per day, on the basis of increased relative liver
weight, increased total cholesterol and phospholipid concentrations
indicating effects on lipid metabolism, and mild anaemia at higher
doses (Koyama et al., 1989).
Groups of five male and five female Sprague-Dawley (Crl:CD) rats
received dermal applications of pyriproxyfen (purity, 97.2%) dissolved
in corn oil at doses of 0, 100, 300, or 1000 mg/kg bw per day under a
semi-occlusive dressing for 6 h/day for 21 days. The observations
included clinical signs, deaths, food consumption, body weight, and
clinical chemical, haematological, and and histological examinations.
Serum was assayed for alanine and aspartate aminotransferases. Blood
samples were collected at termination. The study complied with GLP.
There were no treatment-related effects on the mortality rate,
clinical signs, or haematological or clinical chemical parameters,
including serum enzymes. There were no significant changes in body
weight or food consumption in the treated group, and no effect of
treatment on organ weights was observed. Histopathological examination
revealed no treatment-related alterations in the liver or any other
tissues examined. The NOAEL was 1000 mg/kg bw per day, the highest
dose tested (Moore et al., 1993).
Groups of 10 male and 10 female Sprague-Dawley (Jcl:CD) rats were
exposed to a mist aerosol of pyriproxyfen dissolved in corn oil at
concentrations of 270, 480, or 1000 mg/m3 for 4 h/day for 28 days.
The mass median aerodynamic diameter of the particles was 0.71-0.88
µm. The observations included clinical signs, deaths, food and water
consumption, body weight, and urinary, ophthalmological, clinical
chemical, haematological, and histological examinations. Serum was
assayed for leucine aminopeptidase, cholinesterase, lactate
dehydrogenase, creatine phosphokinase, alanine and aspartate
aminotransferase, and alkaline phosphatase activity. Blood samples
were collected at termination after a 16-h fast. The study complied
with GLP.
There were no deaths. Salivation was observed early in the study
in rats at the highest concentration, and the body-weight gain of
animals at this dose was sporadically, slightly but significantly
lower, although it was normal at the end of the study. There were no
treatment-related changes in food consumption or in haematological,
urinary, or ophthalmologic parameters. Slightly but significantly
increased lactate dehydrogenase activity was observed in males at the
highest dose, but the activities of the other serum enzymes showed no
treatment-related change. A slight but significant increase (9%) was
observed in the relative weight of the liver at 1000 mg/m3, but the
absolute weight was comparable to that of controls. Histopathological
examination revealed no treatment-related morphological changes in the
organs of exposed rats. The NOAEL was 480 mg/m3 on the basis of
salivation, sporadically reduced body-weight gain, and increased
lactate dehydrogenase activity at 1000 mg/m3 (Kawaguchi et al.,
1988).
Guinea-pigs
The skin sensitizing potential of pyriproxyfen (purity, 97.2%)
was tested in a GLP-compliant study in male Hartley guinea-pigs by the
maximization method. A volume of 0.05 ml of 1% pyriproxyfen in
Freund's complete adjuvant mixed with water or a 0.5% solution of
pyriproxyfen in corn oil was injected intradermally for initial
sensitization. As a challenge, 0.2 g of pyriproxyfen in 25% petrolatum
or 0.2 ml of a positive control was applied dermally in patches to
test animals for 24 h, 6 days after the first sensitization. No dermal
reaction was observed (Suzuki et al., 1987f).
Rabbits
New Zealand white rabbits of each sex received a single dose of
0.5 g of pyriproxyfen (purity, 97.2%) as a fine powder moistened with
physiological saline by dermal application for 4 h. The potential to
induce primary skin irritation was examined 4.5, 24, 48, and 72 h
after application. The study complied with GLP. No oedema or erythema
was observed (Suzuki et al., 1987g).
A single dose of 100 mg of pyriproxyfen (purity, 97.2%) in a
volume of 0.1 ml was applied to the right eye of three male and three
female New Zealand white rabbits in a study that complied with GLP.
The potential to induce primary eye irritation was examined 1, 24, 48,
and 72 h after application in unwashed eyes. Slight conjunctival
redness (grade 1) and chemosis (grade 1-2) were observed in all
treated animals 1 h after application. Conjunctival redness (grade 1),
chemosis (grade 1), and discharge (grade 2) were still apparent in one
or two animals after 24 h, but these changes had disappeared by 48 h
after application. Pyriproxyfen was considered to be a minimal ocular
irritant (Suzuki et al., 1987g).
Dogs
Groups of four male and four female beagle dogs, 6 months old,
received gelatine capsules containing pyriproxyfen (purity, 97.2%) at
doses of 0, 100, 300, or 1000 mg/kg bw per day for 3 months. The
observations included clinical signs, deaths, food consumption, body
weight, and ophthalmic, electrocardiographic, clinical chemical,
haematological, urinary, and histological parameters. The activities
of alkaline phosphatase, aspartate and alanine aminotransferases,
gamma-glutamyl transpeptidase, creatine phosphokinase, and lactate
dehydrogenase were measured in plasma. Ophthalmological examinations
were performed during weeks 0, 5, and 12 of treatment, and an
electrocardiograph was obtained at the same times. Blood samples were
collected during weeks 0, 4, 8, and 12 of treatment; hepatic function
was assessed by bromsulphalein retention during weeks 0, 6, and 13 of
treatment, and renal function was assessed by retention of
para-aminohippuric acid during weeks 0, 5, and 11 of treatment.
No deaths were observed. Female dogs at 1000 mg/kg bw per day had
a slightly increased incidence of soft stools, but no other
treatment-related toxic signs were observed. There were no changes in
body weight, body-weight gain, food consumption, or ophthalmological
parameters throughout the study, and no treatment-related changes in
the electrocardiograph were observed at any dose. Haematological
parameters were not significantly changed at any dose, although
slight, nonsignificant alterations in the number of platelets (by
< 39%) and total cholesterol concentration (by < 67%) were observed,
with no obvious dose-dependence. The phospholipid concentration was
significantly increased in females at 1000 mg/kg bw per day. No
significant changes was found in the activities of the serum enzymes
studied, although there were trends to increased alkaline phosphatase
activity in males at the high dose, lactate dehydrogenase activity in
males at all doses and in females at the high dose, and creatine
phosphokinase activity in males in all doses, with no dose-dependence.
Aspartate and alanine aminotransferase activities were unaltered.
Hepatic function was not significantly affected at any dose. The
absolute weights of the liver were significantly increased in males at
300 and 1000 mg/kg bw per day, by 30% and 26%, respectively, and
significant increases in relative liver weights were observed in males
at 300 mg/kg bw per day, by 24%. Increased incidences of
hepatocellular hypertrophy were observed in females at 300 mg/kg bw
per day and in animals of each sex at 1000 mg/kg bw (0/4 in controls,
0/4 at 100 mg/kg bw per day, 0/4 at 300 mg/kg bw per day, and 4/4 at
1000 mg/kg bw per day in males, and 0/4, 0/4, 3/4, and 4/4 in females,
respectively). At 1000 mg/kg bw per day, increased incidences of
eosinophilic bodies in the liver were observed (0/4 in controls, 0/4
at 100 mg/kg bw per day, 0/4 at 300 mg/kg bw per day, and 2/4 at 1000
mg/kg bw per day in males, and 0/4, 0/4, 0/4, and 2/4 in females,
respectively). Electron microscopic examination revealed a minimal to
slight increase in smooth endoplasmic reticulum with slight dilatation
in the livers of all animals at 1000 mg/kg bw per day. These changes
are consistent with adaptation of the liver to exposure to the
compound through enzyme induction. A slight but significantly
prolonged retention ratio in the test for renal function was observed
in males at 300 and 1000 mg/kg bw per day after 6 weeks of treatment,
but the retention ratio was normal by the end of the study, and no
treatment related histopathological changes were observed in the
kidney. The NOAEL was 100 mg/kg bw per day on the basis of increased
absolute and relative liver weights and an increased incidence of
hepatocellular hypertrophy at higher doses (Nakano et al., 1988).
Groups of four male and four female beagle dogs (23-27 weeks old)
were given gelatine capsules containing pyriproxyfen (purity, 95.3%)
at doses of 0, 30, 100, 300, or 1000 mg/kg bw per day for 52 weeks.
The observations included clinical signs, deaths, food consumption,
body weight, and ophthalmoscopic, clinical chemical, haematological,
urinary, and histological examinations. The activities of alanine and
aspartate aminotransferases, alkaline phosphatase, and creatine
phosphokinase were measured in plasma. Blood samples were collected 1
week before and on weeks 12, 24, 37, and 50 of treatment. The study
conformed to GLP.
Two males at 1000 mg/kg bw per day were killed in extremis with
acute weight loss and, in one case, liver failure, at weeks 17 and 31
of treatment. Slightly increased frequencies of salivation and
diarrhoea were observed in animals at 1000 mg/kg bw per day, and
emaciation was observed in males at doses > 300 mg/kg bw per day.
There were no treatment-related ophthalmological abnormalities. A
dose-dependent but nonsignificant reduction in body weight was seen
throughout the study, and body-weight gains were significantly reduced
in animals at doses > 300 mg/kg bw per day. Food consumption was
not reduced at any dose. Significant changes were seen in several
haematological parameters, including 10-20% decreases in haemoglobin
and erythrocyte counts and a slightly but significantly increased mean
corpuscular volume in males at doses > 300 mg/kg bw per day and in
females at doses > 100 mg/kg bw per day and a significantly
decreased packed cell volume in the latter group. The change in
haemoglobin in males at 1000 mg/kg bw per day was not significant.
These haematological changes might indicate slight anaemia. There was
no abnormality of cellularity or cell composition in the bone marrow.
Statistically significant decreases in the number of lymphocytes were
observed in all treated females at weeks 12 and 37, which were
attributed to transiently high values for the control group. The
platelet count was significantly increased in males at doses > 100
mg/kg bw per day and females at 1000 mg/kg bw per day throughout the
study. Slightly but significantly prolonged prothrombin times were
observed in males at 300 mg/kg bw per day and in animals of each sex
at 1000 mg/kg bw per day. Significantly increased plasma enzyme
activities were seen, including those of alkaline phosphatase in
animals at doses > 300 mg/kg bw per day throughout the study,
alanine aminotransferase in animals at 1000 mg/kg bw per day
throughout the study, and aspartate aminotransferase in males at 1000
mg/kg bw per day in weeks 12, 24, and 37 of treatment. The total
cholesterol concentration in plasma was significantly increased in
animals at doses > 30 mg/kg bw per day (by 24-58% at 30 mg/kg bw
per day, 64-160% at 100 mg/kg bw per day, 100-140% at 300 mg/kg bw per
day, and 76-100% at 1000 mg/kg bw per day) throughout the study, and
the plasma concentrations of triglycerides were significantly
increased in animals at doses > 100 mg/kg bw per day. These
increases were dose-dependent except at the highest dose in males, but
in this group there were only two survivors. No reduction in plasma
protein fractions was observed in the treated groups.
A slightly reduced pH and increased volume of urine were observed
in males at 1000 mg/kg bw per day throughout the study. The absolute
weights of the liver were dose-dependently increased in all treated
groups and significantly increased in males at doses > 100 mg/kg bw
per day (by 130% at 30 mg/kg bw per day, 150% at 100 mg/kg bw per day,
170% at 300 mg/kg bw per day, and 190% of control values at 1000 mg/kg
bw per day) and in females at doses > 300 mg/kg bw per day (110%,
120%, 140%, and 140%, respectively). The relative weights of the liver
were significantly increased in males at doses > 30 mg/kg bw per
day (130% of control value) and in females at doses > 300 mg/kg bw
per day. The absolute weights of the thyroid were significantly
increased in females at doses > 300 mg/kg bw per day, and the
relative weights were significantly increased in females at doses
> 100 mg/kg bw per day. Significantly increased relative renal
weights were observed in males at 300 mg/kg bw per day and in females
at doses > 300 mg/kg bw per day; the absolute weights were
dose-dependently but not significantly increased.
Macroscopic examination showed enlarged livers and hepatic damage
in the two dogs at 1000 mg/kg bw per day which died. Histopathological
examination revealed treatment-related hepatic damage in animals of
each sex at 1000 mg/kg bw per day, which was characterized by
centriacinar fibrosis in 2/2 males and 3/4 females and bile-duct
hyperplasia in 2/2 males and 3/4 females; foci of cystic degeneration
in 1/2 males and 1/4 females; active chronic inflammation in 2/2 males
and 2/4 females; and nodular hyperplasia in 2/2 males and 0/4 females.
Submucosal fibrosis in the gall-bladder was observed in all male
animals, including those that had died, and in 3/4 females at 1000
mg/kg bw per day, in association with bile-duct hyperplasia. One of
four males at 30 mg/kg bw per day had focal bile-duct hyperplasia and
focal subcapsular fibrosis, but these effects were not observed at 100
or 300 mg/kg bw per day. There were no preneoplastic or neoplastic
alterations. Although no morphological alterations were seen in the
liver, the increase in cholesterol concentration and relative liver
weight at low doses might be related to treatment. No NOAEL could be
identified, as increased total cholesterol concentrations indicating
effects on lipid metabolism and increased relative liver weights with
a trend towards increased absolute liver weights were seen at all
doses (Chapman et al., 1991).
In a complementary study which complied with GLP, groups of four
male and four female beagle dogs (19-24 weeks old) were given gelatine
capsules containing pyriproxyfen (purity, 95.3%) at doses of 0, 3, or
10 mg/kg bw per day for 52 weeks. The observations included clinical
signs, deaths, food consumption, body weight, and ophthalmic, clinical
chemical, haematological, urinary, and histological examinations. The
activities of alanine and aspartate aminotransferases, alkaline
phosphatase, and creatine phosphokinase were assayed in serum. Blood
samples were collected after 12, 24, 36, and 50 weeks of treatment.
There were no deaths, signs of clinical toxicity, or changes in
body weight, body-weight gain, or food consumption. Significantly
increased platelet counts were observed in males at 3 mg/kg bw per day
in weeks 24, 36, and 50 of treatment and in those at 10 mg/kg bw per
day in week 36, but with no clear dose-dependence. Prothrombin time
was not prolonged in males at any dose. Females at 10 mg/kg bw per day
also showed significantly increased platelet counts in weeks 36 and 50
(by 8% at 3 mg/kg bw per day and 10% at 10 mg/kg bw per day), and
prothrombin time was slightly but significantly prolonged at these
doses at the end of study. There were no other treatment-related
changes in haematological parameters. The total cholesterol
concentration was unchanged; slight but significant increases in total
triglyceride concentrations in males at 10 mg/kg bw per day were seen
in weeks 12 and 36 of treatment. There were no treatment-related
changes in urinary parameters. The absolute weight of the liver was
slightly increased in females at 10 mg/kg bw per day (110% of
control), but this was not significant. No histopathological changes
were found in any organ, including the liver and kidney. The range of
mean total platelet counts in controls in other studies in this
laboratory was 273-357 in males and 305-357 in females, whereas those
in the present study were 341-367 in controls, 414-462 at the low
dose, and 415-456 at the high dose in males, and 321-384 in controls
and 430-478 at the high dose in females. The increased numbers of
platelets and the prolonged prothrombin time were therefore
treatment-related changes although no significant increase in platelet
counts was observed in animals at 30 mg/kg bw per day in the previous
study (Mitchel et al., 1993).
The NOAEL for the two 52-week studies in dogs was 10 mg/kg bw per
day, on the basis of the absence of treatment-related toxicity at 10
mg/kg bw per day in the second study and changes in lipid metabolism
and increased liver weight at 30 mg/kg bw per day in the first study.
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female ICR(Crj-CD-1) mice were given
diets containing pyriproxyfen (purity, 95.3%; 97.6-98.7% dietary
concentration) at concentrations of 0, 120, 600, or 3000 ppm for
78 weeks, providing doses equal to 0, 16, 81, and 420 mg/kg bw per day
for males and 0, 21, 110, and 530 mg/kg bw per day for females. The
observations included clinical signs, deaths, food consumption, body
weight, organ weights, and ophthalmological, haematological, and gross
and histological examinations. Ten mice from each group were killed
during week 52 for interim examination, and the surviving mice were
killed during week 78. Blood samples were taken from 10 rats per group
during weeks 52 and 78 of treatment. No clinical chemical tests were
conducted. The study complied with GLP.
The mortality rate was dose-dependent and significantly increased
in males at 600 and 3000 ppm (43% at 0 ppm, 55% at 120 ppm, 72% at 600
ppm, and 82% at 3000 ppm) and in females at 3000 ppm (39% at 0 ppm,
44% at 120 ppm, 55% at 600 ppm, and 64% at 3000 ppm). There were
slight, nonsignificant increases in the incidence of clinical signs,
including reduced motor activity and hunched position, in animals at
3000 ppm. Statistically significant decreases in body weights,
body-weight gains, and/or food consumption were observed in males at
3000 ppm during the study. The absolute and relative weights of the
liver were significantly increased in females at 3000 ppm during week
52 of treatment. The haematological parameters showed no
treatment-related change. Histopathological examination of animals
that died revealed a significantly increased incidence of systemic
amyloidosis in the glandular stomach of males at 600 and 3000 ppm and
the adrenal, thyroid, heart, liver, kidney, glandular stomach, and
duodenum of females at 3000 ppm, and a dose-related relationship was
found between the generalized amyloidosis and the mortality rate.
Statistical analysis of the incidence of graded amyloidosis revealed a
significant positive trend in renal amyloidosis in females and a
significant positive trend in hepatic amyloidosis in animals of each
sex at 3000 ppm. Females at this dose had a significant increase in
the incidence of lymphocytic infiltration in the liver (22/59 at 0 and
34/60 at 3000 ppm) and of tubular mineralization (3/59 at 0 and 46/60
at 3000 ppm). Deposition of amyloid in the kidney causes numerous
pathological changes including tubular mineralization and papillary
necrosis. In this study, however, the incidences of tubular
mineralization and segmental cortical atrophy were increased
independently of amyloidosis in female animals at 3000 ppm, suggesting
that the chronic nephrosis was directly related to treatment.
Histopathological examination revealed no increase in the incidence of
neoplastic lesions at any dose. The NOAEL was 120 ppm, equal to 16
mg/kg bw per day, on the basis of increased mortality at higher doses
(Osheroff et al., 1991a; Cardy et al., 1994).
Rats
Groups of 50 male and 50 female Sprague-Dawley (Crl:CD) rats were
given diets containing pyriproxyfen (purity, 95.3%) at concentrations
of 0, 120, 600, or 3000 ppm, equal to 0, 5.4, 27, and 140 mg/kg bw per
day for males and 0, 7.0, 35, and 180 mg/kg bw per day for females,
for 104 weeks. Satellite groups of 30 males and 30 females were also
treated orally. The observations included clinical signs, deaths, food
and water consumption, body weight, organ weights, and
ophthalmoscopic, clinical chemical, haematological, urinary, and gross
and histopathological examination. Assays were performed for the serum
enzymes aspartate and alanine aminotransferase, alkaline phosphatase,
creatine kinase, and gamma-glutamyl transpeptidase. Blood samples were
collected from satellite groups of rats on week 13, 26, 52, 78, and
104 of treatment. The study complied with GLP.
Treatment did not affect mortality (34-46% in males and 32-58% in
females), clinical signs, or ophthalmoscopic end-points. The body
weights of males were significantly reduced in weeks 13, 26, and 50 of
treatment (by 5-7%) and those of females in weeks 13, 26, 50, and 78
of treatment (by 12-14%) at 3000 ppm, but they had returned to the
control level by the end of study. The mean body-weight gain was
significantly reduced in females at 600 ppm and in animals of each sex
at 3000 ppm throughout the study. No treatment-related changes in food
consumption were observed. The only alteration in haematological
parameters was a transient increase in eosinophils. Alkaline
phosphatase activity was significantly increased in males at doses
> 120 ppm in weeks 26, 52, and 78 of treatment, but the activity
(87-104 U/L) remained within the range of historical controls (45-114
U/L), and the changes were not clearly dose-dependent. gamma-Glutamyl
transpeptidase activity was significantly increased in males at 3000
ppm in week 104 of treatment and in females at 600 and 3000 ppm in
weeks 26 and 52. The activities of other serum enzymes were not
significantly affected. Significantly increased total cholesterol
concentrations were observed in males at 3000 ppm in weeks 26 and 52
(149% and 147% of control, respectively). Slight, inconsistent,
nonsignificant increases in urinary protein concentration were
observed in females at 3000 ppm in week 26. At interim necroscopy, the
absolute weights of the liver were found to be nonsignificantly
increased at week 52 of treatment with 3000 ppm (by 15% in males and
13% in females). A significant increase in relative liver weight was
observed only in females at 3000 ppm (120% of control). The only
significant or treatment-related increases in the incidence of
morphological alterations at 104 weeks seen on gross and
histopathological examination were hyperkeratosis of the skin of males
at 3000 ppm, which was considered not to be biologically significant,
and a significant increase in the incidence of liver necrosis in males
at 3000 ppm that died during the study; however, no liver necrosis was
observed in the surviving males at 3000 ppm at the end of the study,
indicating nthat it was not related to treatment. Histopathological
examination revealed no evidence of neoplastic alterations. The NOAEL
was 600 ppm, equal to 27 mg/kg bw per day, on the basis of reductions
in body weight and mean body-weight gain and increased absolute and
relative liver weights at higher doses (Osheroff et al., 1994a,b).
(d) Genotoxicity
The results of tests for the genotoxicity of pyriproxyfen are
summarized in Table 2. All of the positive controls used in the assays
produced the expected positive responses. In assays for reverse
mutation, no induction of revertant colonies was observed at six doses
with or without an exogenous metabolic activation system (S9). In
tests for DNA repair, pyriproxyfen was inactive at six doses with or
without S9. In tests for gene mutation in mammalian cells, no
mutations were observed at four doses ranging from 10 to 300 ppm
without S9 or 10 to 100 ppm with S9. In assays for unscheduled DNA
repair synthesis, pyriproxyfen was cytotoxic and/or inhibited normal
DNA synthesis but it did not induce unscheduled DNA synthesis. In
tests for cytogeneticity, Chinese hamster ovary cells (CHOK1) were
exposed to pyriproxyfen at a concentration of 10, 30, or 100 µg/ml for
2 h in the presence of S9 and cultured for a further 16 or 22 h or
cultured with pyriproxyfen for 18 or 24 h in the absence of S9.
Although marked cytotoxicty, characterized by decreased mitotic index
and cell cycle delay, were observed at doses > 30 mg/ml without S9,
and at 100 mg/ml with S9, no increase in the total number of
structural aberrations or the frequency of cells with aberrations was
observed at any concentration. In the test for micronucleus formation
in mice in vivo, a single dose of pyriproxyfen at 5000 mg/kg bw
slightly but not statistically significantly increased the incidence
of micronucleated polychromatic erythrocytes.The Meeting concluded
that pyriproxyfen is not genotoxic in vivo or in vitro.
Table 2. Results of tests for the genotoxicity of pyriproxyfen
End-point Test system Concentration Purity Result Reference
(%)
In vitro
Reverse mutationa S. typhimurium 10-5000 µg/plate 97.2 Negative ± S9 Kogiso et al. (1988a)
TA98, TA100,
TA1537, TA1538,
E, coli WP2 uvrA
DNA repairb B.subtills M45, 673-21 500 µg/disc 95.3 Negative ± S9 Kogiso et al. (1992)
H17 in DMSO
Gene mutationc Chinese hamster 3-300 µg/ ml 95.3 Negative ± S9 Kogiso et al. (1990)
V79 cells, hprt locus
Unscheduled Human HeLa S3 0.1-205 µg/ ml 95.3 Negative ± S9 Henderson &
DNA synthesisd epithelioid cells Proudlock (1989)
Chromosomal Chinese hamster 10-300 µg/ml 97.2 Negative ± S9 Kogiso et al. (1989)
aberrationse ovary cells
Chromosomal Chinese hamster 9.6-321 µg/ml -S9 97.2 Negative ± S9 Kogiso et al. (1988)
aberrationsf ovary cells 50-200 µg/ml +S9
In vivo
Micronucleus CD-I mice, bone Single intraperitoneal 95.3 Negative Proudlock et al.
formationg marrow injections of 5000 mg/kg (1991)
bw at 24, 48, and 72 h
Table 2. (continued)
All studies were conducted according to good laboratory practice. DMSO, dimethyl sulfoxide
a Positive controls were methylmethanesulfonate for TA100, 2-nitrofluorene for TA98 and TA1538, sodium azide for TA1535,
ICR-191 for TA1537, N-ethyl- N'-nitro- N-nitrosoguanidine for WP2 uvrA, benzo[ a]pyrene for TA100, TA98, TA1537, and TA1538
and 2-aminoanthracene for TA1535 and WP2 uvrA.
b Positive controls were mitomycin C for the direct assay and sterigmatocystin for the activation assay. The negative
control was kanamycin in both assays.
c Positive controls were ethylmethane sulfonate for the direct assay and 9,10-dimethyl-1,2-benzanthracene for the activation
assay.
d Positive controls were 2-acetylaminofluorene for the activation assay and 4-nitroquinoline-1-oxide for the direct assay.
e Positive controls were mitomycin C for the direct assay and cyclophosphamide for the activation assay.
f Positive controls were mitomycin C for the direct assay and benzo[ a]pyrene for the activation assay.
g Positive control was mitomycin C.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 26 male and 26 female Sprague-Dawley (Crj) rats were
given diets containing technical-grade pyriproxyfen (purity, 95.3%) at
concentrations of 0, 200, 1000, or 5000 ppm. The F0 animals were
treated for 70 days before mating and then for 6 subsequent weeks for
males and during 3 weeks of gestation and 3 weeks of the lactation
period for females. The F1 generation were treated for 18 weeks from
the day of their weaning to the day of weaning of the F2 generation,
including 77-90 days before mating and the mating, gestation, and
lactation periods. The mean daily intakes of pyriproxyfen were 14, 68,
and 340 mg/kg bw per day in males and 20, 98, and 500 mg/kg bw per day
in females (18, 87, and 440 mg/kg bw per day before mating, 15, 77,
and 390 mg/kg bw per day during gestation, and 32, 160, and 830 mg/kg
bw per day during lactation) in the F0 generation, and 17, 83, and
440 mg/kg bw per day in males and 21, 110, and 560 mg/kg bw per day in
females (21, 100, and 550 mg/kg bw per day before mating, 14, 72, and
380 mg/kg bw per day during gestation, and 28, 160, and 810 mg/kg bw
per day during lactation) in the F1 generation.
The observations in parental rats included clinical signs,
deaths, food consumption, body weight, estrus cycle, and
histopathological and reproductive parameters which included mating,
fertility, gestation, and live birth indices. All parental animals
were killed at the end of weaning, and the reproductive organs, brain,
pituitary, liver, and kidney were examined histopathologically. The
organs from the F1 parental animals were weighed. Estrus cycles were
examined by a smear assay during the 10 days before mating. The
observations in the F1 and F2 pups included viability, body
weight, and lactation indices. Developmental indices were not
examined. Groups of 10 male and 10 female pups were selected randomly
from 10 litters for necroscopy. One male and one female were selected
randomly from each F1 litter to provide 26 pairs at each dose to
serve as parents for the F2 generation. Pups were weighed by sex.
The study complied with GLP.
The F0 parent animals showed no treatment-related changes in
clinical signs, mortality rate, reproductive parameters, or estrus
cycle. Body weight and body-weight gain were significantly reduced in
F0 animals at 5000 ppm during the periods of premating, gestation,
and lactation, and food consumption was significantly reduced in F0
females at this dose during gestation. No gross or histopathological
alterations were seen that were related to treatment. The NOAEL for
F0 parental toxicity was 1000 ppm, equal to 68 mg/kg bw per day, on
the basis of reductions in body weight and body-weight gain at 5000
ppm. The NOAEL for reproductive toxicity was 5000 ppm, equal to 340
mg/kg bw per day, the highest dose tested.
The F1 parent animals showed no treatment-related changes in
clinical signs, mortality rate, reproductive parameters, or estrus
cycles. The body weights of F1 males and F1 females at 5000 ppm
were significantly reduced, and the terminal body weights of animals
at this dose were significantly decreased (to 98% at 1000 ppm and 88%
at 5000 ppm in males and 100% at 1000 ppm and 95% at 5000 ppm in
females). Body-weight gain was also significantly reduced in F1
males at 5000 ppm during the premating period. Food consumption was
significantly reduced at this dose in F1 males during treatment and
in F1 females during gestation. The absolute weights of the liver
were significantly increased in F1 adults at 5000 ppm (110% at 1000
ppm and 110% at 5000 ppm in males and 120% at 5000 ppm in females),
and the relative weights were significantly increased in males at 1000
ppm (110% of control) and in animals of each sex at 5000 ppm (130% in
males and 130% in females). Histopathological examination showed an
increased incidence of focal clear cells in the liver in males at 5000
ppm, but this effect is commonly observed in male rats and was
considered to be unrelated to treatment. Significant increases in
relative kidney weights were observed in males at 1000 ppm (110% of
control values) and 5000 ppm (110%), but the absolute weights were not
significantly increased at any dose. Histopathological examination
showed an increased incidence of chronic interstitial nephrosis (7/26
at 0 ppm, 3/26 at 200 ppm, 7/26 at 1000 ppm, and 15/26 at 5000 ppm) in
males at 5000 ppm and in the incidence of hydronephrosis (1/26 at 0
ppm and 4/26 at 5000 ppm) in females at this dose; however, these
increases did not reach statistical significance. No other
treatment-related morphological lesions were observed. The NOAEL for
F1 parental toxicity was 1000 ppm, equal to 83 mg/kg bw per day, on
the basis of decreased body weight, decreased food consumption, and
increased absolute and relative liver weights at 5000 ppm. No evidence
of reproductive toxicity was observed. The NOAEL for reproductive
toxicity was 5000 ppm, equal to 340 mg/kg bw per day, the highest dose
tested.
In F1 pups, there was no treatment-related change in clinical
signs, sex ratio, viability index, or lactation index and no
significant difference between treated and control groups in body
weight at birth. Significant reductions in body weight were observed
in male F1 pups on day 21 and in female F1 pups on days 14 and 21
post partum at 5000 ppm. The total litter weight was also
significantly decreased on days 14 and 21 post partum at this dose.
No treatment-related effects were seen on gross examination. F2 pups
also showed no treatment-related change in clinical signs, sex ratio,
viability index, or lactation index. The mean pup weights were
significantly decreased on days 14 and 21 post partum at 5000 ppm.
No gross pathological alteration related to treatment was apparent at
any dose. The NOAEL for developmental toxicity was 1000 ppm, equal to
98 mg/kg bw per day, on the basis of reduced body weight in F1 and
F2 pups at 5000 ppm (Robinson et al., 1991).
In a study of treatment before and during the early stages of
gestation (segment 1) conducted according to GLP, groups of 24 male
and 24 female Sprague-Dawley rats were given pyriproxyfen (purity,
97.2%) dissolved in corn oil by gavage at doses of 0, 100, 300, 500,
or 1000 mg/kg bw per day for 12 weeks comprising 9 weeks before mating
and 3 weeks of mating, in males or for at least 3 weeks including 2
weeks before mating and the mating period and on days 0-7 of gestation
in females. The observations in parental rats included clinical signs,
deaths, food consumption, body weight, organ weights, gross
appearance, and reproductive performance including copulation and
fertility indices. Clinical examinations were performed twice a day.
Males were killed at the end of mating, and female animals on day 21
of gestation.
Treatment-related deaths occurred in two females at the highest
dose. Increased incidences of diarrhoea and erythema and swelling of
the anal region were observed in females at 300 mg/kg bw and in
animals of each sex at doses > 500 mg/kg bw per day. Dose-dependent
increases in the incidence of salivation were frequently observed in
all treated groups, with incidences of 0/24 in controls and 18/24,
21/24, 24/24, and 24/24 at the four doses respectively, in males, and
0/24, 1/24, 5/24, 7/24, and 8/24, respectively, in females. Body
weight was significantly reduced in females at 100 mg/kg bw per day
during gestation and in animals of each sex at doses > 300 mg/kg bw
per day throughout the study. Body-weight gain and food consumption
were also significantly decreased in males at doses > 300 mg/kg bw
per day throughout the study, but significant decreases were observed
only during treatment in females. Water consumption was significantly
increased in males at doses > 100 mg/kg bw per day and in females
at doses > 300 mg/kg bw per day. The absolute weights of the liver,
kidney, and adrenal glands were significantly increased and the
absolute weight of the thymus was significantly decreased in males at
doses > 300 mg/kg bw per day. In females, the absolute weights of
the kidney and adrenals were significantly increased at 1000 mg/kg bw
per day. Enlarged, dark-red livers, pitted, enlarged kidneys, enlarged
adrenals, and thymus atrophy were observed in males at doses > 300
mg/kg bw per day, and congested, enlarged livers, enlarged adrenals,
and atrophy of the spleen and thymus were observed in females at
1000 mg/kg bw per day. There was no treatment-related change in
reproductive performance. No NOAEL could be identified for maternal
toxicity, as reduced body weight during gestation was seen at all
doses. The NOAEL for reproductive toxicity was 1000 mg/kg bw per day,
the highest dose tested.
Slightly but significantly reduced numbers of corpora lutea (90%
of control) and of live fetuses (88% of control) were observed at 1000
mg/kg bw per day, but the values were within the range in historical
controls. Slight increases in placental weight and in fetal body
weight were observed at 1000 mg/kg bw per day, but the effects were
not dose-dependent and were within the range in historical controls. A
slight but significant increase in the number of phalanges of the
proximal forepaw was observed, but such increases are known to be
associated with an altered growth rate. The incidences of external,
visceral, and skeletal anomalies were not increased. The NOAEL for
developmental toxicity was 1000 mg/kg bw per day, the highest dose
tested (Saegusa et al., 1988a).
In a study of treatment during the perinatal and lactation
periods (segment 3) conducted according to GLP, groups of 23-24
pregnant Sprague-Dawley rats were given pyriproxyfen (purity, 97.2%)
dissolved in corn oil by gavage at doses of 0, 30, 100, 300, or 500
mg/kg bw per day from day 17 of gestation to day 20 post partum. The
dams were allowed to deliver naturally. On day 4 post partum, the
offspring were culled to adjust the litter size to eight (four males
and four females when possible) for tests of development (F1I), and
the remaining pups (F1II) were killed for skeletal examination on
the day of culling. The dams (F0) were killed at termination of
weaning. The observations in maternal rats included clinical signs,
deaths, body weight, food consumption, organ weights, gross
appearance, and reproductive indices including delivery rate, birth
rate, and body weight of live newborns. The F1I offspring were
weighed on days 0, 4, 7, 14, and 21 post partum during lactation;
after weaning, one male and one female per litter were weighed once a
week. Clinical signs were observed twice a day. The indices of
development during lactation included separation of the auricle,
emergence of abdominal hair, eruption of incisors, separation of
eyelids, and descent of testes or opening of the vagina. On day 20,
all F1 offspring were examined for sensory function in tests for
visual placing, righting and mid-air righting reflexes, and response
to pain. On day 21 post partum, two male and two female offspring
from each litter (F1Ia) were killed for visceral and skeletal
examination. After weaning, another male and female from each litter
(F1Ib) were examined for emotionality (behaviour in an open field)
at 4 weeks of age, for motor coordination (rotarod performance) at 5
weeks of age, and for learning ability (in a water-filled multiple T
maze) at 6 weeks of age. The F1Ib offspring were killed for necropsy
on day 56 post partum. Another male and female from each litter
(F1Ic) were tested only for reproductive performance after being
paired for mating within the same group (avoiding sibling matings) at
11 weeks of age. The fetuses were removed surgically from mated
females on day 21 of gestation. Reproductive performance was assessed
from indices of mating, fertility, gestation, and litters. The fetuses
(F2) were weighed and examined for external anomalies.
In the F0 maternal animals, treatment-related deaths were
observed at 500 mg/kg bw per day, an increased incidence of diarrhoea
at doses > 300 mg/kg bw per day, and dosedependent increases in the
incidence of salivation at doses > 100 mg/kg bw per day (0/23 in
controls and 0/23, 1/23, 2/24, and 4/24 at the four doses,
respectively). During gestation, significantly reduced body weights
were observed at 500 mg/kg bw per day, and significantly reduced
body-weight gain and food consumption were seen frequently during the
study at doses > 300 mg/kg bw per day. The absolute and relative
weights of the liver were significantly increased at doses > 300
mg/kg bw per day. Atrophy of the thymus, congestion of the liver and
kidneys, enlargement of the adrenals, and atrophy of the spleen were
observed at 500 mg/kg bw per day, and an increased number of
stillborns was seen at this dose. The body weight of live newborn pups
was significantly decreased at doses > 300 mg/kg bw per day. The
NOAEL for maternal toxicity was 100 mg/kg bw per day, on the basis of
clinical signs, reductions in body weight, body-weight gain, and food
consumption, and decreased body weight of delivered newborn at 300
mg/kg bw per day.
At 500 mg/kg bw per day, the survival rate of F1 offspring was
significantly decreased on days 0-4 post partum, and the weaning
rate was decreased on days 4-21 post partum. No decrease in the
viability of F1I offspring was observed on days 21-77. The body
weights of pups of each sex at doses > 300 mg/kg bw per day were
significantly reduced on days 0-49 or 56 post partum. All of the
physical developmental indices were significantly retarded and slight
retardation of sexual differentiation was observed at doses > 300
mg/kg bw per day. No treatment-related change in sensory functions was
observed in F1I offspring. Visceral examination of the F1Ia
offspring on day 21 post partum revealed a significant increase in
the number with anomalies at 30, 300, and 500 mg/kg bw per day (0% in
controls and 10%, 1.2%, 17.2%, and 29% at the four doses,
respectively).The anomalies consisted mainly of dilatation of the
renal pelvis and hyperaemia and/or inflammatory cell infiltration. The
incidence of dilatation of the renal pelvis was significantly
increased at doses > 300 mg/kg bw per day (0% in controls and 3.8%,
1.2%, 14%, and 18% at the four doses, respectively, with 0-3.2% in
historical controls). The incidence of hyperaemia and/or inflammatory
cell infiltration was significantly increased at 500 mg/kg bw per day
(0% in controls and 3.8%, 0%, 5.8%, and 12% at the four doses,
respectively). Although developmental insufficiency of the papilla or
stenosis and obstruction of the urethra can cause dilatation of the
renal pelvis, as can developmental retardation, the range in
historical controls of this strain was 0-2.0% in fetuses and 0-3.2% in
21-day-old offspring, and recovery from dilatation of the renal pelvis
usually occurs after birth. No skeletal anomalies were found that were
related to treatment. The absolute weights of all organs of F1
offspring killed on day 21 post partum were slightly but
significantly decreased, but no treatment-related change was found in
the absolute weights of the organs of F1Ib offspring killed on day
56 post partum. The changes in organ weights might have been due to
retarded physical development. Ambulation was also slightly but
significantly increased at doses > 100 mg/kg bw per day (by 31% at
100 mg/kg bw per day, 50% at 300 mg/kg bw per day, and 37% at 500
mg/kg bw per day). Motor coordination was comparable to that of
controls, and no treatment-related changes in learning ability were
observed. All the changes observed in the F1 offspring, including
increased ambulation, could have been due to retarded physical
development. After maturation, the reproductive perfomance of F1Ic
offspring was comparable to that of controls. The body weight and
body-weight gain of pregnant F1 animals were comparable to those of
controls during gestation. The NOAEL for developmental toxicity was
100 mg/kg bw per day, on the basis of decreased body weight,
retardation of physical development, and visceral anomalies associated
with the retarded growth rate at 300 mg/kg bw per day.
Significantly reduced numbers of implantations (12 in controls
and 10, 12, 11, and 9.2 at the four doses, respectively) and mean
numbers of live F2 fetuses (12 in controls and 9.3, 11, 10, and 8.5
at the four doses, respectively) were observed at 500 mg/kg bw per
day. Significantly reduced numbers of corpora lutea, implantations,
and live fetuses were found at 30 mg/kg bw per day but not at 100
mg/kg bw per day. These reductions were not dose-dependent. There were
no treatment-related differences in fetal body weight or in the
incidence of external anomalies between treated and control groups.
The NOAEL for F1 reproductive toxicity was 300 mg/kg bw per day, on
the basis of a reduction in the number of implantations and in the
mean number of live fetuses at 500 mg/kg bw per day (Saegusa et al.,
1988b).
(ii) Developmental toxicity
Rats
In a study that complied with GLP, groups of 36 female
Sprague-Dawley rats were given pyriproxyfen (purity, 97.2%) dissolved
in corn oil by gavage at doses of 0, 100, 300, or 1000 (42 animals)
mg/kg bw per day on days 7-17 of gestation. The fetuses (F1I) were
removed surgically from 23 pregnant dams (F0I) at 0, 100, or 300
mg/kg bw per day and from 20 at 1000 mg/kg bw per day on day 21 of
gestation and examined for developmental toxicity. The remaining 10-13
dams (F0II) were allowed to deliver normally. On day 4 post
partum, the offspring were culled to adjust the litter size to eight
(four males and four females when possible) for functional testing on
day 20 post partum (F1IIa). The remaining pups (F1IIb) were
killed at 21 days of age and examined for skeletal anomalies. During
lactation, developmental indices were examined. After weaning, one
male and one female from each litter (F1IIa1) allocated for
functional testing were examined for emotionality at 4 weeks of age,
for motor coordination at 5 weeks of age, and for learning ability at
6 weeks of age; these offspring were killed for necropsy at 8 weeks of
age. Another male and female from each litter (F1IIa2) were tested
for reproductive performance after being paired for mating within the
same group (avoiding sibling mating) at 11 weeks of age, and the
fetuses were removed surgically from the mated females on day 21 of
gestation. The remaining offspring (F1IIa3) were killed for
necroscopy at 21 days of age, after weaning.
The observations in parental rats included clinical signs,
deaths, food and water consumption, body weight, estrus cycle, and
organ weights. The postnatal developmental indices included separation
of the auricle, emergence of abdominal hair, eruption of incisors,
separation of eyelids, and descent of testes or opening of the vagina.
The sensory function tests included visual placing, righting and
mid-air righting reflexes, and response to pain. Emotionality was
evaluated by observation of behaviour in an open field, motor
coordination was examined in a rotarod performance test, and learning
ability was examined by behaviour in a water-filled multiple T maze
test. Reproductive performance included indices of mating, fertility,
gestation, and litters.
Of the F0 dams, 12 of 42 at 1000 mg/kg bw per day died between
day 11 and day 16 of gestation. No deaths occurred in the other
groups. Signs of toxicity were observed at doses > 300 mg/kg bw per
day. At the highest dose, these included diarrhoea, erythema and
swelling of the anal region, hypoactivity, wasting, hypothermia,
lachrymation, and piloelection. Signs of toxicity were also observed
at 300 mg/kg bw per day but in only one animal. Body weight and
body-weight gain were significantly reduced at 300 and 1000 mg/kg bw
per day during gestation and at 100 mg/kg bw per day during treatment.
Food consumption was significantly reduced at doses > 100 mg/kg bw
per day during treatment, and water consumption was dose-dependently
and significantly increased at doses > 300 mg/kg bw per day. Thymic
atrophy and enlarged adrenals were observed in the dams that died and
those killed on day 21 of gestation (F0I) at 1000 mg/kg bw per day.
In the F0I dams, from which fetuses were removed surgically on
day 21 of gestation, slight but significant increases were found in
the relative weights of the liver and kidney at 300 mg/kg bw per day,
but no significant increase in the absolute weights was observed at
this dose. At 1000 mg/kg bw per day, the absolute weights of the
kidney and adrenal were significantly increased and the absolute
weight of the thymus was significantly decreased. The relative weight
of the liver was significantly increased, but the absolute weight was
not significantly affected at 1000 mg/kg bw per day. Higher ratios of
resorbed or dead fetuses were observed at 1000 mg/kg bw per day (4.7%
in controls, 7.7% at 300 mg/kg bw per day, and 15% at 1000 mg/kg bw
per day). None of these differences achieved statistical significance
or was outside the historical control range (5.7%; 1.5-20%). There was
no difference in the mean numbers of corpora lutea or implantations or
the implantation rate. In the F0II dams, body weight and body-weight
gain were significantly reduced at 300 and 1000 mg/kg bw per day
during lactation. There were no treatment-related changes in the
number of live newborn, the length of gestation, or the delivery rate.
No NOAEL could be identified for F0 maternal toxicity since
decreased body-weight gain was seen at all doses. The NOAEL for
reproductive toxicity was 1000 mg/kg bw per day, the highest dose
tested.
No significant change in F1I fetal sex ratio, placental weight,
or fetal body weight was observed, but the ratio of resorbed or dead
fetuses was increased (5% in controls and 15% at 1000 mg/kg bw per
day) and the number of live fetuses was decreased at the highest dose,
but these changes were not statistically significant. Cyclopia and
polydactyly were each observed in one fetus at 300 mg/kg bw per day
(0.7%), but not in any other group, and these anomalies were
considered to be unrelated to treatment. There were no
treatment-related increases in the incidences of external, visceral,
or skeletal anomalies. A significant increase in the number of fetuses
with a skeletal variation consisting of opening of the foramen
transversarium of the seventh cervical vertebra was seen at 300 and
1000 mg/kg bw per day (0% in controls and 1.5%, 5.0%, and 14% at the
three doses, respectively). An increased number of fetuses with an
extra lumbar rib was observed at 1000 mg/kg bw per day, but this was
not significant. The NOAEL for developmental toxicity was 300 mg/kg bw
per day, on the basis of increased skeletal variations at 1000 mg/kg
bw per day.
The F1IIa offspring showed no-treatment related effect on
survival or weaning rates. The body weights of the treated groups were
comparable to those of controls during lactation and before mating.
There were no treatment-related effects on postnatal physical
development, sexual differentiation, sensory function, emotional
behaviour, or motor coordination. A significant retardation in the
time taken to reach the goal in the water-filled multiple T maze was
observed in females at 1000 mg/kg bw per day, but this slight
reduction in learning behaviour was observed only in the second of
three consecutive daily trials. There was no impairment of
reproductive performance of F1IIa2 offspring at any dose. No
external anomalies were observed in F2 fetuses. The NOAEL for
developmental toxicity was 1000 mg/kg bw per day, if the slight
evidence of behavioural teratogenicity in F1 offspring is ignored.
In the offspring killed at 21 days of age (F1IIb), an increased
incidence of skeletal variations was observed at 1000 mg/kg bw per day
(9% in controls and 18% at 1000 mg/kg bw per day), but this was not
statistically significant. No external or skeletal anomalies were
observed. The incidence of visceral anomalies was increased
significantly in F1IIaI offspring at 1000 mg/kg bw per day killed at
56 days of age (0/46 in controls and 7/39 at 1000 mg/kg bw per day).
F1IIb and F1IIaI offspring at 1000 mg/kg bw per day also showed an
increased incidence of dilatation of the renal pelvis. The total
incidence of dilatation of the renal pelvis observed in F1I
offspring (killed at 21 and 56 days of age and at the end of the
fertility test) was increased dose-dependently (0/98 in controls and
1/95, 3/85, and 9/79 at the three doses, respectively). The incidences
in controls from 15 previous studies were 0-2.0% in fetuses, 0-3.2% in
offspring 21 days old, 0-4.3% at 56 days of age, and 0-4.5% at the end
of the fertility test. The incidence in the controls in the present
study was 0% at all times. The incidence of protrusion or partial
adhesion of the liver parenchyma on the diaphragmatic side was also
increased at 1000 mg/kg bw per day (1/98 in controls and 0/95, 2/85,
and 6/79 at the three doses, respectively). These anomalies were not
observed in fetuses removed surgically. The overall NOAEL for
developmental toxicity was 300 mg/kg bw per day, on the basis of an
increased incidence of skeletal variations in fetuses and an increased
incidence of visceral anomalies in offspring at 1000 mg/kg bw per day
(Saegusa et al., 1988c).
Rabbits
Groups of 15-18 female JW-NIBS rabbits were treated by gavage
with pyriproxyfen (purity, 97.2%) at doses of 0, 100, 300, or 1000
mg/kg bw per day on days 6-18 of gestation and were killed on day 28
of gestation. The study complied with GLP.
In the maternal animals, abortion or premature delivery occurred
at doses > 300 mg/kg bw per day, in one control, none at 100 mg/kg
bw per day, three at 300 mg/kg bw per day, and six at 1000 mg/kg bw
per day. Dead and moribund animals were found at 1000 mg/kg bw per day
(none at 0, 100, or 300 mg/kg bw per day and three at 1000 mg/kg bw
per day). Several signs of toxicity, including soft stools,
emaciation, decreased spontaneous activity, and bradypnoea were
observed in aborted, prematurely delivered, prematurely dying, and
moribund dams at doses > 300 mg/kg bw per day. Body weight,
body-weight gain, and food consumption were significantly reduced in
dams at 1000 mg/kg bw per day. There were no significant effects on
the mean number of corpora lutea or implantations or the number, sex
ratio, or body weight of live fetuses.
The live fetuses showed no treatment-related external anomalies.
Skeletal or visceral malformations were observed in fetuses at 300
mg/kg bw per day, comprising a defect of the third distal phalanx of
the hind leg in one; cystic lung, ventricular septal defect,
hypoplasia of the left atrial auricle, and persistent truncus
arteriosus in one; and a defect of the gall-bladder in one. None was
observed in the other groups. External malformations were observed in
three fetuses, comprising local oedema, a visceral malformation, and
microphthalmia in one fetus at 300 mg/kg bw per day; persistent
truncus arteriosus in one fetus at 1000 mg/kg bw per day; and a
ventricular septal defect in another at this high dose. The total
incidence of malformations was 1% in controls, 1% at 100 mg/kg bw per
day, 6% at 300 mg/kg bw per day, and 2% at 1000 mg/kg bw per day. The
authors concluded that pyriproxyfen did not cause treatment-related
changes in the incidences of skeletal anomalies, skeletal variations,
ossification, visceral anomalies, or visceral variations. The NOAEL
for reproductive toxicity was 100 mg/kg bw per day on the basis of
abortion or premature delivery and a number of signs of toxicity at
300 mg/kg bw per day. The NOAEL for developmental toxicity was 1000
mg/kg bw per day, the highest dose tested (Hirohashi et al., 1988).
(f) Studies on metabolites
(i) Acute toxicity
The metabolites of pyriproxyfen were administered orally to mice
in a 0.5% solution of methylcellulose at 1000 or 2000 mg/kg bw. One
out of five males given 5''-hydroxypyriproxyfen (purity, 97.5%) at
2000 mg/kg bw died, but no deaths occurred with the other metabolites.
4'-Hydroxypyriproxyfen (purity, 98.3%) caused no abnormal clinical
signs; 5''-hydroxypyriproxyfen caused decreased spontaneous activity
in animals at both doses and ataxic gait at 2000 mg/kg bw;
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen
(purity, 90.1%) produced decreased spontaneous activity, ataxic gait,
and prone position in animals at 2000 mg/kg bw; (RS)-2-hydroxypropyl
4-phenoxyphenyl ether (purity, 99.0%) produced decreased spontaneous
activity, ataxic gait, prone position, lateral position, and irregular
respiration in animals at 2000 mg/kg bw; and (RS)-2-(2-pyridyloxy)
propionic acid (purity, 100%) produced decreased spontaneous activity
at 2000 mg/kg bw (Misaki, 1993a).
(ii) Genotoxicity
4'-Hydroxypyriproxyfen (purity, 98.3%), 5''-hydroxypyriproxyfen
(purity, 97.5%), 4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether
pyriproxyfen (purity, 90.2%), (RS)-2-hydroxypropyl 4-phenoxyphenyl
ether (purity, 99.0%), and (RS)-2-(2-pyridyloxy) propionic acid
(purity, 100%) were tested for mutagenicity in Salmonella
typhimurium TA98, TA100, TA1535, and TA1537 and in Escherichia
coli WP2 uvrA, with and without exogenous metabolic activation at
concentrations of 15-5000 µg/plate. None of the metabolites caused
reverse mutation, whereas the positive controls used in these assays
produced the anticipated responses (Hara et al., 1993).
No data were available on the effects of the metabolites of
pyriproxyfen on chromosomal integrity, but as these metabolites are
formed in vivo and pyriproxyfen had no effect on this end-point,
this is not considered a major gap in the data.
Comments
After oral administration to rats, [14C]pyriproxyfen is slowly
(time to peak concentration in plasma, 8 h) and incompletely (< 50%
of the dose) absorbed but is then rapidly eliminated, predominantly in
the faeces (90%), with only 4-11% in the urine, after 48 h. Absorbed
pyriproxyfen is excreted mainly via the bile (34-37% of the
administered dose in 48 h). The metabolism of pyriproxyfen is
qualitatively similar in rats, mice, lactating goats, and laying hens.
A large number of metabolites have been detected, the main route of
biotransformation being 4'-hydroxylation. Other pathways include
hydroxylation of the pyridyl ring, ether cleavage and conjugation.
Mice conjugate a much greater proportion of the dose than rats. The
concentration of pyriproxyfen in tissues other than fat was very low
(generally < 0.01 µg equivalent per g after 72 h; fat < 0.1µg
equivalent per g). The half-times of the radiolabel in tissues,
including blood and fat, were 8-36 h. The dermal absorption of
pyriproxyfen has not been studied.
The acute oral toxicity of pyriproxyfen is low, with LD50
values > 5000 mg/kg bw in mice, rats, and dogs. The acute dermal
toxicity is also low, with LD50 values > 2000 mg/kg bw in mice and
rats, and after exposure by inhalation, with an LC50 value > 1.3
mg/l air in mice and rats. WHO (1999) has classified pyriproxyfen as
'unlikely to present acute hazard in normal use'. Pyriproxyfen was
mildly irritating to the eye but not to the skin of rabbits. It did
not sensitize the skin of Hartley guinea-pigs in a maximization test.
In short- and long-term studies of the effects of pyriproxyfen in
mice, rats, and dogs, the liver was the main toxicological target,
with increases in liver weight and changes in plasma lipid
concentrations, particularly cholesterol, at doses of 120 mg/kg bw per
day and above in rats. There was some evidence that the compound might
cause modest anaemia in mice, rats, and dogs at high doses. In mice
treated with pyriproxyfen in the diet for three months, additional
effects seen included increased mortality rates, histopathological
changes in the kidney, and decreased body weight. The NOAEL was 150
mg/kg bw per day in mice, 23 mg/kg bw per day (two studies) in rats,
and 100 mg/kg bw per day in dogs fed pyriproxyfen in the diet for 3
months. In long-term studies of toxicity in mice, pyriproxyfen also
caused a dose-dependent increase in the occurrence of systemic
amyloidosis, which was associated with increased mortality rates. The
NOAEL was 120 ppm, equal to 16 mg/kg bw per day. In rats, the only
additional effect was reduced body-weight gain, and the NOAEL was 600
ppm, equal to 27 mg/kg bw per day. In two 1-year studies in dogs,
pyriproxyfen was administered in capsules. The overall NOAEL was 10
mg/kg bw per day on the basis of increased relative liver weight and
increased total plasma cholesterol concentration in males. There was
some evidence that pyriproxyfen can act as a hepatic enzyme inducer,
at least in dogs. Pyriproxyfen was not toxic when administered
dermally to rats for 21 days at doses of up to 1000 mg/kg bw per day.
Inhalation of pyriproxyfen for 4 h per day for 28 days caused only
minor effects in rats (initial salivation, sporadically reduced
body-weight gain, slightly increased serum lactate dehydrogenase
activity) at 10 000 mg/m3. The NOAEL was 480 mg/m3.
Pyriproxyfen was not carcinogenic when given in the diet at doses
up to 420 mg/kg bw per day in a study in mice or at doses up to 140
mg/kg bw per day in rats. Pyriproxyfen showed no evidence of
carcinogenicity in a 1-year study in dogs at doses up to 1000 mg/kg bw
per day. The Meeting concluded that pyriproxyfen does not pose a
carcinogenic risk to humans.
Pyriproxyfen was not genotoxic in an adequate range of tests for
mutagenicity and cytogenicity in vitro and in vivo. The Meeting
concluded that pyriproxyfen is not genotoxic.
The reproductive toxicity of pyriproxyfen in rats has been
investigated in a two-generation study of reproductive toxicity, a
study involving treatment of males and females before and in the early
stages of gestation (segment 1), and a study of treatment during the
prenatal and lactation periods (segment 3). The NOAEL for maternal
toxicity was 1000 ppm, equal to 98 mg/kg bw per day, in the
two-generation study and 100 mg/kg bw per day in the segment 3 study.
Reproductive toxicity was observed only in the segment 3 study, in
which there was an increased number of stillbirths in the F0
generation and a reduction in the number of implantations and in the
mean number of live fetuses in the F1 generation at 500 mg/kg bw per
day. The NOAEL for reproductive toxicity was 300 mg/kg bw per day. No
reproductive toxicity was observed in the two-generation study, the
NOAEL being 5000 ppm, equal to 340 mg/kg bw per day, the highest dose
tested, or in the segment 1 study, the NOAEL being 1000 mg/kg bw per
day, the highest dose tested.
The developmental toxicity of pyriproxyfen has been studied in
rats and rabbits. In rats, a NOAEL for maternal toxicity was not
identified, as decreased body-weight gain was observed at 100 mg/kg bw
per day, the lowest dose tested. Pyriproxyfen caused little
developmental toxicity and was not teratogenic. In a segment 3 study,
the F1 offspring were subjected to a series of developmental tests
for possible neurotoxicity, including physical indices, tests of
behaviour, motor and sensory function, and learning ability. Although
there were some effects on growth at doses > 300 mg/kg bw per day,
there was no developmental neurotoxicity at 500 mg/kg bw per day, the
highest dose tested. Visceral anomalies (dilatation of the renal
pelvis) were found at doses > 300 mg/kg bw per day. The NOAEL for
developmental toxicity was 100 mg/kg bw per day, on the basis of
retarded physical development and visceral anomalies at higher doses.
In a more conventional study of developmental toxicity in rats, no
evidence of growth retardation or of developmental neurotoxicity was
found at doses up to and including 1000 mg/kg bw per day, the highest
dose tested. There was an increased frequency of skeletal variations
(opening of the foramen transversalium of the seventh cervical
vertebra) in fetuses at 300 mg/kg bw per day. The frequency of
visceral anomalies was significantly increased in F1 offspring some
weeks after birth. The NOAEL for developmental toxicity was 300 mg/kg
bw per day, on the basis of an increased frequency of skeletal
variations with visceral anomalies in F1 offspring at 1000 mg/kg bw
per day. In a study of developmental toxicity in rabbits, signs of
maternal toxicity (abortion and premature delivery) were evident at
doses > 300 mg/kg bw per day (NOAEL, 100 mg/kg bw per day). No
developmental toxicity was observed, the NOAEL being 1000 mg/kg bw per
day, the highest dose tested.
The Meeting established an ADI of 0-0.1 mg/kg bw on the basis of
the NOAEL of 10 mg/kg bw per day in 1-year studies of toxicity in dogs
and a safety factor of 100.
The Meeting concluded that it was not necessary to establish an
acute reference dose because of the low acute toxicity of
pyriproxyfen.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 120 ppm, equal to 16 mg/kg bw per day (18-month study of
carcinogenicity)
Rat: 600 ppm, equal to 27 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
5000 ppm, equal to 345 mg/kg bw per day (reproductive
toxicity, two-generation study of reproductive toxicity,
highest dose tested)
100 mg/kg bw per day (developmental toxicity in a segment 3
study of developmental toxicity)
Rabbit: 100 mg/kg bw per day (maternal and reproductive toxicity in
a study of developmental toxicity)
1000 mg/kg bw per day (developmental toxicity in a study of
developmental toxicity, highest dose tested)
Dog: 10 mg/kg bw per day (1-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information valuable for continued
evaluation of the compound
Observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to
pyriproxyfen
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Slow, incomplete absorption (< 50%), rat
Dermal absorption No data (no systemic toxicity up to 1000 mg/kg bw per day by
dermal route, rat)
Distribution of total residues Highest concentrations of radiolabel in fat and, to lesser
extent, liver, rat
Potential for accumulation Possible limited accumulation in fat, rat
Rate and extent of excretion Rapid, complete, 88-96% within 48 h, primarily in faeces;
4-11% in urine, rat
Metabolism in animals Extensive. No parent compound detectable in urine; numerous
metabolites: main pathway is 4'-hydroxylation; also hydroxylation
of the pyridyl ring, ether cleavage, conjugation, mouse, rat,
goat, hen
Toxicologically significant compounds Pyriproxyfen
(animals, plants and environment)
Acute toxicity
LD50, oral > 5000 mg/kg bw, mouse, rat
LD50, dermal > 2000 mg/kg bw, mouse, rat
LC50, inhalation > 1.3 mg/L, mouse, rat
Dermal irritation Not irritating, rabbit
Ocular irritation Mildly irritating, rabbit
Dermal sensitization Not a sensitizer, guinea-pig
Short-term toxicity
Target/critical effect Mouse, rat, dog: liver, increased relative liver weight, mild
anaemia, altered lipid metabolism (increased serum cholesterol)
Lowest relevant oral NOAEL 13 weeks, rat, 24 mg/kg bw per day
Lowest relevant dermal NOAEL 21 days, rat, > 1000 mg/kg bw per day
Lowest relevant inhalation NOAEL 28-day, rat, > 1.3 mg/L
Long-term toxicity and carcinogenicity
Target/critical effect: Mouse, rat, dog: liver, increased liver weight, decreased body
weight, altered lipid metabolism (increased plasma cholesterol)
(rat, dog)
Lowest relevant NOAEL 1 year, dog, 10 mg/kg bw per day (diet)
Carcinogenicity Not carcinogenic, mouse, rat
Genotoxicity Not genotoxic
Reproductive toxicity
Reproductive target/critical effect Reduction in number of implantations and live F2 fetuses at F1
developmentally toxic dose, rat
Lowest relevant reproductive NOAEL 345 mg/kg bw per day, rat
Developmental target/critical effect Retardation of physical development in F1, rat
Lowest relevant developmental NOAEL 100 mg/kg bw per day, rat
Neurotoxicity/Delayed neurotoxicity No evidence of developmental neurobehavioural toxicity in rat. No
evidence of neurotoxicty or neuropathology in medium- or long-term
studies in mouse, rat, dog or during development in rat, rabbit
Other toxicological studies Possible enzyme inducer, at least in dogs
Medical data No data
Summary Value Study Safety factor
ADI 0-0.1 mg/kg bw 1-year, dog, toxicity 100
Acute reference dose Unnecessary
References
Cardy, R., Moore, M., Murphy, B.S., Thakur, A., Tellone, C., Ito, S.,
Lang, P., Ginevan, M., Driver, J., Stewart, R. & Wilkinson, C.
(1994) Supplemental data and review of oncogenicity study with
S-31183 (Sumilarv) in mice (MRID No. 421783-10). Unpublished
study from Technology Sciences Group Inc. Reference No.
NNT-41-0116. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Chapman, E.A, Lee, P., Virgo, D.M. & Sparrow, S. (1991) S-31183:
Toxicity study by oral (capsule) administration to beagle dogs
for 52 weeks. Unpublished study from Life Science Research Ltd.
Reference No. NNT-11-0081. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Cox, R.H., Zoetis, T., Cardy, R.H., Alsaker, R.D., Kuhlman, S.M.,
Lewis, S.A., Thakur, A.K. & Phipps, N.G. (1989) Subchronic
toxicity Sstudy with S-31183 in rat. Unpublished study from
Hazleton Laboratories America, Inc. Reference No. NNT-910045.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Cox, R.H., Zoetis, T., Voelker, R.W., Alsaker, R.D., Kuhlman, S.M.,
Lewis, S.A., Thakur, A.K. & Phipps, N.G. (1990) Subchronic
toxicity study in mice. Unpublished study from Hazleton
Laboratories America, Inc. Reference No. NNT-01-0066. Submitted
to WHO by Sumitomo Chemical Co., Ltd.
Hara, M., Katoh, H. & Yoshitake, A. (1993a) Reverse mutation test of
metabolites of pyriproxyfen, 4'-OH-Pyr, 5"-OH-Pyr, DPH-Pyr, POPA
and PYPAC, in bacterial systems. Unpublished study reference No.
NNT-30-0104. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Henderson, L.M. & Proudlock, R.J. (1989) Assessment of unscheduled DNA
repair synthesis in mammalian cells after exposure to S-31183.
Unpublished study from Huntingdon Research Centre Ltd. Reference
No. NNT-91-0053. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Hirohashi, A., Kannan, N., Kato, T. & Yamada, H. (1988) Study of
S-31183 by oral administration during the period of fetal
organogenesis in rabbits. Unpublished study reference No.
NNT-80-0033. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Isobe, N., Matsunaga, H., Kimura, K., Yoshitake, A. & Yamada, H.
(1988a) Metabolism of S31183 in rats. Unpublished study reference
No. NNM-80-0001. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Isobe, N., Matsunaga, H., Kimura, K., Yoshitake, A. & Yamada, H.
(1988b) Metabolism of S-31183 in rat (tissue distribution study).
Unpublished study reference No. NNM-800002. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Kawaguchi, S., Watanabe, T., Suzuki T., Kato, T. & Yamada, H. (1987)
Acute inhalation toxicity of S-31183 in rats. Unpublished study
reference No. NNT-70-0022. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Kawaguchi, S., Yoshioka, K., Ito, S., Suzuki, T., Kato, T. & Yamada,
H. (1988) Subacute inhalation toxicity of S-31183 in rats.
Unpublished study reference No. NNT-80-0031. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Kogiso, S., Hara, M., Yoshitake, A. & Yamada, H. (1988a) Reverse
mutation test with S31183 in bacteria systems. Unpublished study
reference No. NNT-80-0034. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Kogiso, S., Hara, M., Iwawaki, H. & Yamada, H. (1988b) In vitro
chromosomal aberration test of pyriproxyfen in Chinese hamster
ovary cells (CHO-K1). Unpublished study reference No.
NNT-80-0028J. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Kogiso, S., Yamamoto, K., Hara, M., Yoshitake, A. & Yamada, H. (1989)
In vitro chromosomal aberration test of pyriproxyfen in Chinese
hamster ovary cells (CHO-K1). Unpublished study reference No.
NNT-90-0054. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Kogiso, S., Yamada, T., Hara, M., Yoshitake, A. & Yamada, H. (1990) In
vitro gene mutation test of S-31183 in V79 Chinese hamster cells.
Unpublished study reference No. NNT-000067. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Kogiso, S. Kato, H. & Nakattuka, S. (1992) Reverse mutation test with
S-31183 in bacteria systems. Unpublished study reference No.
NNT-80-0088J. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Koyama, Y., Kimura, J., Yoshioka, K., Watanabe, T., Seki, T.,
Hosokawa, S., Yamada, H. & Hagiwara, H. (1989) A six-month
chronic dietary toxicity study of pyriproxyfen in rats.
J. Toxicol. Sci., 14, 43-64.
Matsunaga, H., Yoshino, H., Isobe, N., Kaneko, H., Nakatuka, I. &
Yamada, H. (1995) Metabolism of pyriproxyfen in rats. 1.
Absorption, disposition, excretion, and biotransformation studies
with [phenoxyphenyl-14C] pyriproxyfen. J. Agric. Food Chem.,
43, 235-240.
Misaki, Y. & Nakatuka, I. (1993) Acute oral toxicity study of
pyriproxyphen metabolites, 4'-OH-Pyr, 5"-OH-Pyr, DPH-Pyr, POPA
and PYPAC in mice. Unpublished study reference No. NNT-30-0107.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Mitchell, D.J., Virgo, D.M., Broadmeadow, A., Chase, K.R., Lee, P. &
Sparrow, S. (1993) S31183: Toxicity study by oral (capsule)
administration to beagle dogs for 52 weeks (additional
investigation). Unpublished study from Life Science Research Ltd.
Reference No. NNT-31-0102. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Moore, M.R., Zoetis, T., Doyle, D., Cardy, R.H., Pearson, R.C.,
Walker, M.D., Lewis, S.A., Thomas, D.L., Thakur, A.K., Burlew,
P.L., Hatcher, C.F. & Vegarra, M. (1993) 21-day dermal toxicity
study in rats with S-31183. Unpublished study from Hazleton
Washington, Inc. Reference No. NNT-31-0094. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Nakano, M., Yamamoto, T., Kato, T. & Miyamoto, J. (1986) Acute oral
toxicity study of S31183 in dogs. Unpublished study reference No.
NNT-60-0012. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Nakano, M., Kohda, A., Kato, T. & Yamada, H. (1988) Three-month oral
toxicity study of S31183 in dogs. Unpublished study reference No.
NNT-80-0037. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Osheroff, M.R., Ziegler, K.A., Cardy, R.H., Alsaker, R.D., Kuhlman,
S.M., Lewis, S.A., Thakur, A.K., Burlew, P.L. & Nasca, A.J.
(1991a) Oncogenicity study in mice with S31183. Unpublished study
from Hazleton Laboratories America, Inc. Reference No.
NNT-11-0084. Submitted to WHO by Sumitomo Chemical Co.
Osheroff, M.R., Ziegler, K.A., Machotka, S., Alsaker, R.D., Kuhlman,
S.M., Lewis, S.A., Thakur, A.K., Burlew, P.L., Devis, P.J. &
Graham, R. (1991b) Combined chronic toxicity and oncogenicity
study in rats with S-31183. Unpublished study from Hazleton
Washington, Inc. Reference No. NNT-11-0085. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Panthani, A.M., Walsh, K.J. & Turck, P. (1996a) Metabolism of
[phenoxyphenyl-14C]S71639 (pyriproxyfen) in lactating goats.
Unpublished study from Ricerca, Inc. Reference No. NNM-0043.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Panthani, A.M., Walsh, K.J. & Turck, P. (1996b) Metabolism of
[pyridyl-14C]S-7 1639 (pyriproxyfen) in lactating goats.
Unpublished study from Ricerca, Inc. Reference No. NNM-0046.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Panthani, A.M., DiFrancesco, P. & Savides, M.C. (1996c) Metabolism of
[phenoxyphenyl14C]S-71639 (pyriproxyfen) in laying hens.
Unpublished study from Ricerca, Inc. Reference No. NNM-0045.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Panthani, A.M., DiFrancesco, P. & Savides, M.C. (1996d) Metabolism of
[pyridyl-14C]S71639 (pyriproxyfen) in laying hens. Unpublished
study from Ricerca, Inc. Reference No. NNM-0044. Submitted to WHO
by Sumitomo Chemical Co., Ltd.
Proudlock, R.J., Haynes, P. & Goodenough, A.J. (1991) Mouse
micronucleus test on S31183. Unpublished study from Huntingdon
Research Centre Ltd. Reference No. NNT11-0082. Submitted to WHO
by Sumitomo Chemical Co., Ltd.
Robinson, K., Washer, G. & Noveroske, J. (1991) A dietary 2-generation
(1 litter) reproduction study of S-31183 in the rat. Unpublished
study from Bio-Research Laboratories Ltd. Reference No.
NNT-11-0087. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Saegusa, T., Kitajima, S. & Narama, I. (1988a) Study on administration
of a test substance prior to and in the early stages of pregnancy
in rats. Unpublished study from Hamamatsu, Seigiken Research Co.,
Ltd. Reference No. NNT-80-0036. Submitted to WHO by Sumitomo
Chemical Co., Ltd.
Saegusa, T., Kitajima, S. & Narama, I. (1988b) Study on administration
of S-31183 during the perinatal and lactation periods in rats.
Unpublished study from Hamamatsu, Seigiken Research Co., Ltd.
Reference No. NNT-80-0030. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Saegusa, T., Kitajima, S. & Narama, I. (1988c) Study by administration
of S-31183 during the period of fetal organogenesis in rats.
Unpublished study from Hamamatsu, Seigiken Research Co., Ltd.
Reference No. NNT-80-0029. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Sako, H., Okuno, Y., Kato, T. & Yamada, H. (1987a) Acute
oral toxicity of S31183 in mice. Unpublished study reference No.
NNT-70-0014. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Suzuki, T., Misaki, Y., Okuno, Y., Kato, T. & Miyamoto, J. (1987b)
Acute oral toxicity of S31183 in rats, Unpublished study
reference No. NNT-70-0005. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Sako, H., Okuno, Y., Kato, T. & Yamada, H. (1987c) Acute
dermal toxicity of S31183 in mice. Unpublished study reference
No. NNT-70-0015. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Suzuki, T., Misaki, Y., Okuno, Y., Kato., T. & Miyamoto, J. (1987d)
Acute dermal toxicity of S-31183 in rats. Unpublished study
reference No. NNT-70-0006. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Kawaguchi, S., Watanabe, T., Kato, T. & Yamada, H. (1987e)
Acute inhalation toxicity of S-31183 in mice Unpublished study
reference No. NNT-70-0023. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Nakanishi, T., Kato, T. & Miyamoto, J. (1987f) Skin
sensitization test with S31183 in guinea pigs. Unpublished study
reference No. NNT-70-0003. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Nakanishi, T., Kato, T. & Miyamoto, J. (1987g) Primary eye
and skin irritation tests with S-31183 in rabbits. Unpublished
study reference No. NNT-70-000. Submitted to WHO by Sumitomo
Chemical Co., Ltd.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Yoshino, H. (1993a) Metabolism of pyriproxyfen in rat (high-dose,
14C-concentrations in tissues). Unpublished study reference No.
NNM-30-0028. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Yoshino, H. (1993b) Metabolism study of
(pyridyl-2,6-14C)pyriproxyfen in rat (pyridyl14C-labeled test
compound, single oral administration at low- and high-doses).
Unpublished study reference No. NNM-30-0025. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Yoshino, H., Kaneko, H., Nakatsuka, I. & Yamada, H. (1995) Metabolism
of pyriproxyfen. 2. Comparison of in vivo metabolism between rats
and mice. J. Agric. Food Chem., 43, 2681-2686.
Yoshino, H., Kaneko, H., Nakatsuka, I. & Yamada, H. (1996) Metabolism
of pyriproxyfen. 3. In vitro metabolism in rats and mice.
J. Agric. Food Chem., 44, 1578-1581.
Lactating goats
Lactating goats (Capra hircus, weighing 51-57 kg) were given
[14C-phenoxyphenyl]-pyriproxyfen (purity, 99.5%) in gelatin capsules
at a dose of 1.8-2.0 or 20 mg/animal per day for 5 consecutive days,
for a total dose of 100 mg. The animals were killed within 6 h of the
last dose. Faeces, urine, and milk were collected twice daily and
analysed. Metabolites were purified from extracts of tissues or
excreta by HPLC and identified by TLC and/or mass spectral analysis.
The study was carried out according to GLP. Elimination reached a
plateau by the third day. The percentages of total radiolabel
recovered 1 day after the last dose were 17-18% in urine, 58% in
faeces, and 0.3-0.8% in milk. The metabolites identified in milk
extracts were 4'-hydroxypyriproxy-fen sulfate (51% of the radiolabel
present), 4'-hydroxypyriproxyfen (2%), 4-phenoxyphenol sulfate (10%),
4'-hydroxy-4-phenoxyphenol sulfate (8%),
4'-hydroxy- (RS)-2-hydroxypropyl 4-phenoxyphenyl ether sulfate (3%),
and 4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen
(8%). Metabolites found in both liver and kidney were
4'-hydroxypyriproxyfen sulfate, 5''-hydroxypyriproxyfen-sulfate,
(RS)-2-hydroxypropyl 4-phenoxyphenyl ether,
4'-hydroxy (RS)-2-hydroxypropyl 4-phenoxyphenyl ether, and
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen. In
addition, 4'-hydroxypyriproxyfen and 5''-hydroxypyri-proxyfen were
identified in liver, and 4-phenoxyphenol sulfate and
4'-hydroxy-4-phenoxyphenol in kidney. The metabolites identified in
the faecal samples were 4'-hydroxypyriproxyfen (39% of the radiolabel
present), 5''-hydroxypyriproxyfen (6%),
4'-hydroxy- (RS)-2-hydroxypropyl 4-phenoxyphenyl ether (5%),
(RS)-2-hydroxypropyl 4phenoxyphenyl ether, and 4-hydroxyphenyl
(RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen. Parent compound
accounted for 11-13% of the dose in faeces, The major metabolites in
urine were 4-phenoxyphenol (36% of the radiolabel present),
4-phenoxyphenol sulfate (15%), 4'-hydroxy-4-phenoxyphenol (14%), and
4'-hydroxy-4-phenoxyphenol sulfate (17%) (Panthani et al., 1996a)
In another study that conformed to GLP, lactating goats
( Capra hircus, weighing 39-46 kg) were given
[14C-pyridyl]pyriproxyfen (purity, 97.6% ) in gelatin capsules at a
dose of 1.8-1.9 or 20 mg/animal per day for 5 consecutive days. The
animals were killed within 6 h of the last dose. Faeces, urine, and
milk were collected twice daily and analysed by HPLC and TLC and/or
mass spectrometry. The total radiolabel recovered represented 17-18%
of the dose in urine, 58% in faeces, and 0.4-0.8% in milk. The major
metabolites identified in milk extracts were 4'-hydroxypyriproxyfen
sulfate (35% of the radiolabel present) and 2,5-dihydroxypyridine
conjugate (29%), with smaller quantities of 4'-hydroxypyriproxyfen and
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen. The
major metabolites identified in liver and kidney were
4'-hydroxypyriproxyfen sulfate, 2,5-dihydroxypyridine conjugate, and
(RS)-2-(2-pyridyoxy) propanol conjugates. The major metabolites
identified in the urine were 4'-hydroxypyriproxyfen sulfate,
(RS)-2-(2-pyridyloxy) propionic acid, and 2,5-dihydroxypyridine
conjugate. The metabolites identified in the faecal samples were
4'-hydroxypyriproxyfen (43%), 5''-hydroxypyriproxyfen (5%), and
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen
(5%). These two studies indicate that the primary routes of metabolism
in goats are hydroxylation of the 4' position of the phenoxyphenyl
ring and the 5'' position of the pyridyl ring, cleavage of the ether
bonds, oxidation of the methylene moiety, and sulfate conjugation of
the 4'-hydroxyphenoxyphenyl moiety. There is thus no difference
between rats and goats in the metabolic pattern of pyriproxyfen
(Panthani et al., 1996b)
Chickens
Laying Leghorn hens (Gallus domesticus) were given
[14C-phenoxyphenyl]pyriproxyfen (purity, 99.1%) by gelatin capsule
at a concentration equivalent to 10 ppm in the feed for 8 consecutive
days and were killed within 4 h of the last dose. Excreta were
collected once daily and analysed by HPLC and TLC and/or mass
spectrometry. The study complied with GLP. The metabolites identified
in liver and kidney were free 4'-hydroxypyriproxyfen and its sulfate
conjugate, free 4'-hydroxy-4-phenoxyphenol and its sulfate conjugate,
free 4'-hydroxy- (RS)-2-hydroxypropyl-4-phenoxyphenyl ether and its
sulfate conjugate, 4-phenoxyphenol sulfate,
4-hydroxyphenyl- (RS)-2-(2-pyridyloxy)propyl ether pyriproxyfen, and
(RS)-2-hydroxypropyl-4-phenoxyphenyl ether. Metabolites identified
in excreta samples were 4'-hydroxypyriproxyfen, free and conjugated
4'-hydroxy-4-phenoxyphenol, free and conjugated
4'-hydroxy- (RS)-2-hydroxy-propyl 4-phenoxyphenyl ether,
4-hydroxyphenyl (RS)-2-(2pyridyloxy)propyl ether pyriproxyfen,
5''-hydroxypyriproxyfen, (RS)-2-hydroxypropyl-4-phenoxyphenyl ether,
and 4-phenoxyphenol (Panthani et al., 1996c)
Laying Leghorn hens (Gallus domesticus) were given
[14C-pyridyl]pyriproxyfen (purity, 98.2%) by gelatin capsule at a
concentration equivalent to 10 ppm in the feed for 8 consecutive days
and were killed within 4 h of the last dose. Excreta were collected
once daily and analysed by HPLC and TLC and/or mass spectrometry. The
study complied with GLP. The metabolites identified in liver and
kidney were free and conjugated 4'-hydroxypyriproxyfen,
2-hydroxypyridine, free and conjugated 5''-hydroxypyriproxyfen,
4-hydroxyphenyl- (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen, and
(RS)-2-(2-pyridyloxy)propionic acid. The metabolites identified in
excreta were (RS)-2-(2-pyridyloxy) propionic acid,
4'-hydroxypyriproxyfen, 4-hydroxyphenyl (RS)-2-(2-pyridyloxy)propyl
ether pyriproxyfen, 5''-hydroxypyriproxyfen, and 2-hydroxypyridine.
These two studies indicate that the routes of metabolism in laying
hens are hydroxylation at the 4' position of the phenoxyphenyl ring
and at the 5'' position of the pyridyl ring, cleavage of the ether
linkages, oxidation of the methylene moiety of the side-chain, and
sulfation of the 4'-hydroxyphenoxyphenyl moiety (Panthani et al.,
1996d)
In vitro
The metabolism of pyriproxyfen in rats and mice was investigated
in vitro in samples of 10 000 × g supernatant (S10) prepared from
kidney, lungs, and small intestine of 7-week-old Sprague-Dawley rats
and ICR mice. Hepatic microsomal and cytosolic fractions were prepared
by an established method (centrifugation of S10 at 105 000 × g), and
microsomal, S10, or cytosolic fractions were incubated with
[14C-phenoxyphenyl]pyriproxyfen at a concentration of 0, 0.05, 0.1,
0.5, or 1.0 mmol/L with b-NADPH as a cofactor. The reaction mixtures
were analysed by TLC. Pyriproxyfen was not metabolized by any of the
S10 preparations; it was almost completely metabolized by microsomal
fractions from liver but only very slightly by hepatic cytosol. Most
of the major metabolites identified in rats in vivo were observed
in vitro. There was no species difference in the major metabolic
reactions. The intrinsic clearance calculated from Lineweaver-Burk
plots revealed sex-related differences in metabolic reactions in rats
but not in mice, and 5''-hydroxylation was observed only in male rats
and not in mice of either sex. The intrinsic clearance for microsomal
4'-hydroxylation in female rats was twice that in males, but was not
different in male and female mice. Incubation of a hepatic microsomal
fraction from male rats with antisera against male-specific forms of
cytochrome P450 (CYP2C11 or CYP2C13) revealed that members of the
CYP2C family, the expression of which is sex-dependent in rats, are
involved in the major hydroxylation reactions of pyriproxyfen.
Antiserum against CYP2C11 inhibited all of the major metabolic
reactions of the hepatic microsomal fraction from male rats (> 88%
inhibition). The antiserum against CYP2C13 also inhibited all
reactions (25-64% inhibition) except 4'-hydroxylation (Yoshino et al.,
1996).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of pyriproxyfen are
summarized in Table 1. Pyriproxyfen dissolved in corn oil was
administered orally in a volume of 10 ml/kg bw to ICR (Crj:CD-1) mice
at doses of 1000, 2000, or 5000 mg/kg bw and to Sprague-Dawley
(Crj:CD) rats at 1000, 2500, or 5000 mg/kg bw. In mice, pyriproxyfen
reduced spontaneous motor activity and caused ataxia, abnormal
respiration, and death in males at 2000 mg/kg bw and in animals of
each sex at 5000 mg/kg bw. Transiently decreased body weight was
observed in males at 5000 mg/kg bw. Deaths occurred in two males at
2000 mg/kg bw and two at 5000 mg/kg bw in males and in one female at
5000 mg/kg bw. In rats, pyriproxyfen caused a decrease in body-weight
gain, decreased spontaneous activity, soft stools, and diarrhoea in
males at 2500 mg/kg bw and in animals of each sex at 5000 mg/kg bw,
but no deaths. Necropsy revealed no abnormal changes in the organs of
mice or rats. In dogs, oral administration of pyriproxyfen in capsules
caused no deaths at doses up to 5000 mg/kg bw. The only clinical sign,
occasional vomiting for the first 24 h, was observed at the highest
dose. Dermal application of 2000 mg/kg bw of pyriproxyfen dissolved in
corn oil (5 or 10 ml/kg bw) caused no deaths or signs of clinical
toxicity in ICR mice or Sprague-Dawley rats. Exposure of ICR mice or
Sprague-Dawley rats to a mist aerosol of pyriproxyfen dissolved in
corn oil at concentrations of 0.6 or 1.3 mg/L for 4 h caused no deaths
or pathological changes. The mass median aerodynamic diameter of the
particles was 0.8-0.9 µm. At the high concentration, salivation and
urinary incontinence were observed in rats 4 h after the start of
inhalation, and irregular respiration was observed in mice. These
clinical signs disappeared within 1 h of cessation of exposure.
(b) Short-term studies of toxicity
Mice
Groups of 10 male and 10 female ICR (Crj:CD-1) mice were given
diets containing technical-grade pyriproxyfen (purity, 95.3%) at
concentrations of 0, 200, 1000, 5000, or 10 000 ppm, equal to 0, 28,
150, 840, and 2000 mg/kg bw per day for males and 0, 38, 200, 960, and
2300 mg/kg bw per day for females, for 13 weeks. The observations
included clinical signs, mortality, food and water consumption, body
weight, clinical chemical parameters including serum enzymes, urinary
and haematological parameters, organ weights, and gross and
histopathological appearance. The serum enzymes assayed were alanine
aminotransferase), aspartate aminotransferase, and gamma-glutamyl
transpeptidase. Blood samples were collected shortly before
termination of the study. The study conformed to GLP.
Death occurred in two males at 5000 ppm and in seven males and
nine females at 10 000 ppm (one of the deaths in males was not
treatment-related). The clinical signs observed in the animals that
died prematurely were emaciation, hunched appearance, and few or no
faeces. There were no treatment-related clinical signs in the mice
that survived. Terminal body weights were significantly reduced in
males at 5000 ppm (89% of control) and at 10 000 ppm (69% of control);
the body weights of females were reduced at 5000 ppm during the first
half of the study but were comparable to control values (97% of
control) by the end. The water consumption of males at the two higher
doses was significantly increased, but food consumption was not
affected by treatment. There were significant decreases in erythrocyte
parameters, including cell count, haemoglobin concentration,
haematocrit, mean cell volume, and mean cell haemoglobin value, in
animals at 5000 and 10 000 ppm. Platelet counts were significantly
increased in animals of each sex at 5000 ppm and in males at 10 000
ppm. Significant increases in total cholesterol concentration were
observed in females at doses > 1000 ppm: 130% at 1000 ppm, 210% at
5000 ppm, and 140% of control values at 10 000 ppm. In males, the
activities of aspartate and alanine aminotransferases were increased
by up to twofold but reached significance only at 5000 ppm.
gamma-Glutamyl transpeptidase activity showed slight, nonsignificant
changes in some groups. The absolute weight of the liver was
significantly increased in females at 5000 ppm (120% at 5000 ppm and
160% of control values at 10 000 ppm) but not in males at any dose.
Microscopic examination revealed histomorphological alterations of the
Table 1. Acute toxicity of pyriproxyfen
Species Strain Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/L air)
Mouse ICR M&F Oral > 5000 97.2 Suzuki et al. (1987a)
Rat Sprague-Dawley M&F Oral > 5000 97.2 Suzuki et al. (1987b)
Dog Beagle M&F Oral > 5000 97.2 Nakano et al. (1986)
Mouse ICR M&F Dermal > 2000 97.2 Suzuki et al. (1987c)
Rat Sprague-Dawley M&F Dermal > 2000 97.2 Suzuki et al. (1987d)
Mouse ICR M&F Inhalation > 1.3 97.0 Suzuki et al. (1987e)
Rat Sprague-Dawley M&F Inhalation > 1.3 97.0 Kawaguchi et al. (1987)
These studies were conducted according to good laboratory practice.
kidney comprising tubular nephrosis with microcytosis and dilatation
of the renal tubules and focal mineralization and dilatation of the
renal pelvis in males at 5000 ppm and in animals of each sex at 10 000
ppm. There were no treatment-related morphological changes in the
liver. The NOAEL was 1000 ppm, equal to 150 mg/kg bw per day, on the
basis of effects on erythrocyte parameters, deaths, decreased body
weight, histomorphological alterations in the kidney, and increased
absolute liver weight at higher doses (Cox et al., 1990).
Rats
Groups of 10 male and 10 female Sprague-Dawley (Crl:CD) rats
received diets containing technical-grade pyriproxyfen (purity, 95.3%)
at concentrations of 0, 400, 2000, 5000, or 10 000 ppm, equal to 0,
23, 120, 310, and 640 mg/kg bw per day for males and 0, 28, 140, 360,
and 780 mg/kg bw per day for females, for 13 weeks. The observations
included clinical signs, deaths, body weight, food and water
consumption, ophthalmological, clinical chemical, haematological, and
urinary parameters, organ weights, and gross and histopathological
appearance. The clinical chemical examinations included assays for the
serum enzymes alkaline phosphatase, aspartate and alanine
aminotransferases, and gamma-glutamyl transpeptidase. Blood samples
were collected at the end of the study.
There were no treatment-related deaths, toxic signs, or
ophthalmological changes at any dose, and no changes in food or water
consumption. The body weights of animals of each sex were
significantly decreased at doses > 5000 ppm (91% of control at 5000
ppm and 88% at 10 000 ppm at termination). Erythrocyte parameters
including cell count, haemoglobin concentration, and haematocrit were
significantly decreased in animals at doses > 5000 ppm and in males
also at 2000 ppm. The mean cell volume was reduced in females at 2000
and 10 000 ppm by < 10%. There was no significant effect on platelet
count. The total cholesterol concentration was dose-dependently,
significantly increased in males at doses > 2000 ppm (150% at 2000
ppm, 200% at 5000 ppm, and 210% of control values at 10 000 ppm) and
in females at doses > 5000 ppm (130% at 5000 ppm and 180% of
control values at 10 000 ppm). The serum phospholipid concentration
was also significantly increased in males at doses > 2000 ppm (130%
at 2000 ppm, 180% at 5000 ppm, and 180% of control values at 10 000
ppm) and in females at 10 000 ppm (160% of control value).
Significantly increased gamma-glutamyl transpeptidase activity was
observed in animals at 10 000 ppm, but the activities of the other
serum enzymes were not significantly affected. Significant increases
in the absolute weight of the liver were observed in animals at doses
> 5000 ppm (110% at 2000 ppm, 130% at 5000 ppm, and 140% of control
values at 10 000 ppm in males and 100% at 2000 ppm, 120% at 5000 ppm,
and 140% of control values at 10 000 ppm in females), and the relative
weight of the liver was significantly increased in males at 2000 ppm
and in animals of each sex at doses > 5000 ppm (120% at 2000 ppm,
140% at 5000 ppm, and 170% of control values at 10 000 ppm in males
and 130% at 5000 ppm and 160% of control values at 10 000 ppm in
females). Significant increases in the relative weight of the kidney
were observed in animals at 10 000 ppm, but the absolute weights were
comparable to those of controls at all doses. Histopathological
examination revealed dose-dependent increases in cytoplasmic changes
in the liver in all treated groups (1/10 at 0 ppm, 2/10 at 400 ppm,
6/10 at 2000 ppm, 10/10 at 5000 ppm, and 9/10 at 10 000 ppm in males
and 1/10 at 0 ppm, 2/10 at 400 ppm, 6/9 at 2000 ppm, 7/10 at 5000 ppm,
and 9/10 at 10 000 ppm in females). The cytoplasmic changes consisted
of slight, often equivocal increases in cytoplasmic content reflected
in a visibly reduced nucleus:cytoplasm ratio and diminution of
sinusoidal spaces. The NOAEL was 400 ppm, equal to 23 mg/kg bw per
day, on the basis of mild anaemia, increased incidences of minimal
hepatic abnormalities, relative liver weights, and serum
concentrations of total cholesterol and phospholipids, indicating
effects on lipid metabolism, at higher doses (Cox et al., 1989).
Groups of 21 male and 21 female Sprague-Dawley rats were given
diets containing pyriproxyfen (purity, 97.2%) at concentrations of 0,
80, 400, 2000, or 10 000 ppm, equal to 0, 4.8, 24, 120, and 680 mg/kg
bw per day for males and 0, 5.4, 28, 140, and 690 mg/kg bw per day for
females, for 26 weeks. The observations included clinical signs,
deaths, food and water consumption, body weight, ophthalmological,
clinical chemical, haematological, and urinary parameters, organ
weights, and gross and histological appearance. The serum activities
of aspartate and alanine aminotransferases, alkaline phosphatase,
lactate dehydrogenase, leucine aminopeptidase, creatine phosphokinase,
and gamma-glutamyl transpeptidase were measured. Blood samples were
taken at the end of the study.
There were no deaths, and the only signs of toxicity were
increased incidences of alopecia around the neck and soft stools
during the early stage of the study in animals at 10 000 ppm. The body
weights of animals at 10 000 ppm were significantly decreased
throughout the study, by 86% in males and 87% in females at the end of
study. Marked decreases in body-weight gain were observed at this
dose. There were no treatment-related changes in food or water
consumption. Proteinuria and increased urinary excretion of potassium
ion were observed in animals at 10 000 ppm. Slight but significant
decreases in erythrocyte count and haematocrit were observed in males
at 2000 and 10 000 ppm and in females at 10 000 ppm. The haemoglobin
concentration was also slightly but significantly decreased at this
dose. No increase in platelet count was observed. Slight but
significant increases in total protein, albumin, and blood urea
nitrogen were observed in animals at 10 000 ppm. The albumin and
a2u-globulin fractions were slightly but significantly increased in
males at 10 000 ppm. Total cholesterol and phospholipid concentrations
were significantly increased in males at 2000 and 10 000 ppm and in
females at 10 000 ppm. Of the serum enzyme activities studied, only
that of gamma-glutamyl transpeptidase was significantly increased in
males at 10 000 ppm. Significantly increased absolute weights of the
liver were observed in animals at 10 000 ppm (130% of control value in
males and in females), and significant increases in the relative
weight were observed in males at doses > 2000 (110% at 2000 ppm and
160% of control values at 10 000 ppm) and in females at 10 000 ppm
(100% at 2000 ppm and 150% of control values at 10 000 ppm).
Significantly increased relative kidney weights were observed in
animals at 10 000 ppm, but the absolute weights were not significantly
increased. Histopathological examination showed slight hypertrophy of
the liver in all animals at 10 000 ppm. The NOAEL was 400 ppm, equal
to 24 mg/kg bw per day, on the basis of increased relative liver
weight, increased total cholesterol and phospholipid concentrations
indicating effects on lipid metabolism, and mild anaemia at higher
doses (Koyama et al., 1989).
Groups of five male and five female Sprague-Dawley (Crl:CD) rats
received dermal applications of pyriproxyfen (purity, 97.2%) dissolved
in corn oil at doses of 0, 100, 300, or 1000 mg/kg bw per day under a
semi-occlusive dressing for 6 h/day for 21 days. The observations
included clinical signs, deaths, food consumption, body weight, and
clinical chemical, haematological, and and histological examinations.
Serum was assayed for alanine and aspartate aminotransferases. Blood
samples were collected at termination. The study complied with GLP.
There were no treatment-related effects on the mortality rate,
clinical signs, or haematological or clinical chemical parameters,
including serum enzymes. There were no significant changes in body
weight or food consumption in the treated group, and no effect of
treatment on organ weights was observed. Histopathological examination
revealed no treatment-related alterations in the liver or any other
tissues examined. The NOAEL was 1000 mg/kg bw per day, the highest
dose tested (Moore et al., 1993).
Groups of 10 male and 10 female Sprague-Dawley (Jcl:CD) rats were
exposed to a mist aerosol of pyriproxyfen dissolved in corn oil at
concentrations of 270, 480, or 1000 mg/m3 for 4 h/day for 28 days.
The mass median aerodynamic diameter of the particles was 0.71-0.88
µm. The observations included clinical signs, deaths, food and water
consumption, body weight, and urinary, ophthalmological, clinical
chemical, haematological, and histological examinations. Serum was
assayed for leucine aminopeptidase, cholinesterase, lactate
dehydrogenase, creatine phosphokinase, alanine and aspartate
aminotransferase, and alkaline phosphatase activity. Blood samples
were collected at termination after a 16-h fast. The study complied
with GLP.
There were no deaths. Salivation was observed early in the study
in rats at the highest concentration, and the body-weight gain of
animals at this dose was sporadically, slightly but significantly
lower, although it was normal at the end of the study. There were no
treatment-related changes in food consumption or in haematological,
urinary, or ophthalmologic parameters. Slightly but significantly
increased lactate dehydrogenase activity was observed in males at the
highest dose, but the activities of the other serum enzymes showed no
treatment-related change. A slight but significant increase (9%) was
observed in the relative weight of the liver at 1000 mg/m3, but the
absolute weight was comparable to that of controls. Histopathological
examination revealed no treatment-related morphological changes in the
organs of exposed rats. The NOAEL was 480 mg/m3 on the basis of
salivation, sporadically reduced body-weight gain, and increased
lactate dehydrogenase activity at 1000 mg/m3 (Kawaguchi et al.,
1988).
Guinea-pigs
The skin sensitizing potential of pyriproxyfen (purity, 97.2%)
was tested in a GLP-compliant study in male Hartley guinea-pigs by the
maximization method. A volume of 0.05 ml of 1% pyriproxyfen in
Freund's complete adjuvant mixed with water or a 0.5% solution of
pyriproxyfen in corn oil was injected intradermally for initial
sensitization. As a challenge, 0.2 g of pyriproxyfen in 25% petrolatum
or 0.2 ml of a positive control was applied dermally in patches to
test animals for 24 h, 6 days after the first sensitization. No dermal
reaction was observed (Suzuki et al., 1987f).
Rabbits
New Zealand white rabbits of each sex received a single dose of
0.5 g of pyriproxyfen (purity, 97.2%) as a fine powder moistened with
physiological saline by dermal application for 4 h. The potential to
induce primary skin irritation was examined 4.5, 24, 48, and 72 h
after application. The study complied with GLP. No oedema or erythema
was observed (Suzuki et al., 1987g).
A single dose of 100 mg of pyriproxyfen (purity, 97.2%) in a
volume of 0.1 ml was applied to the right eye of three male and three
female New Zealand white rabbits in a study that complied with GLP.
The potential to induce primary eye irritation was examined 1, 24, 48,
and 72 h after application in unwashed eyes. Slight conjunctival
redness (grade 1) and chemosis (grade 1-2) were observed in all
treated animals 1 h after application. Conjunctival redness (grade 1),
chemosis (grade 1), and discharge (grade 2) were still apparent in one
or two animals after 24 h, but these changes had disappeared by 48 h
after application. Pyriproxyfen was considered to be a minimal ocular
irritant (Suzuki et al., 1987g).
Dogs
Groups of four male and four female beagle dogs, 6 months old,
received gelatine capsules containing pyriproxyfen (purity, 97.2%) at
doses of 0, 100, 300, or 1000 mg/kg bw per day for 3 months. The
observations included clinical signs, deaths, food consumption, body
weight, and ophthalmic, electrocardiographic, clinical chemical,
haematological, urinary, and histological parameters. The activities
of alkaline phosphatase, aspartate and alanine aminotransferases,
gamma-glutamyl transpeptidase, creatine phosphokinase, and lactate
dehydrogenase were measured in plasma. Ophthalmological examinations
were performed during weeks 0, 5, and 12 of treatment, and an
electrocardiograph was obtained at the same times. Blood samples were
collected during weeks 0, 4, 8, and 12 of treatment; hepatic function
was assessed by bromsulphalein retention during weeks 0, 6, and 13 of
treatment, and renal function was assessed by retention of
para-aminohippuric acid during weeks 0, 5, and 11 of treatment.
No deaths were observed. Female dogs at 1000 mg/kg bw per day had
a slightly increased incidence of soft stools, but no other
treatment-related toxic signs were observed. There were no changes in
body weight, body-weight gain, food consumption, or ophthalmological
parameters throughout the study, and no treatment-related changes in
the electrocardiograph were observed at any dose. Haematological
parameters were not significantly changed at any dose, although
slight, nonsignificant alterations in the number of platelets (by
< 39%) and total cholesterol concentration (by < 67%) were observed,
with no obvious dose-dependence. The phospholipid concentration was
significantly increased in females at 1000 mg/kg bw per day. No
significant changes was found in the activities of the serum enzymes
studied, although there were trends to increased alkaline phosphatase
activity in males at the high dose, lactate dehydrogenase activity in
males at all doses and in females at the high dose, and creatine
phosphokinase activity in males in all doses, with no dose-dependence.
Aspartate and alanine aminotransferase activities were unaltered.
Hepatic function was not significantly affected at any dose. The
absolute weights of the liver were significantly increased in males at
300 and 1000 mg/kg bw per day, by 30% and 26%, respectively, and
significant increases in relative liver weights were observed in males
at 300 mg/kg bw per day, by 24%. Increased incidences of
hepatocellular hypertrophy were observed in females at 300 mg/kg bw
per day and in animals of each sex at 1000 mg/kg bw (0/4 in controls,
0/4 at 100 mg/kg bw per day, 0/4 at 300 mg/kg bw per day, and 4/4 at
1000 mg/kg bw per day in males, and 0/4, 0/4, 3/4, and 4/4 in females,
respectively). At 1000 mg/kg bw per day, increased incidences of
eosinophilic bodies in the liver were observed (0/4 in controls, 0/4
at 100 mg/kg bw per day, 0/4 at 300 mg/kg bw per day, and 2/4 at 1000
mg/kg bw per day in males, and 0/4, 0/4, 0/4, and 2/4 in females,
respectively). Electron microscopic examination revealed a minimal to
slight increase in smooth endoplasmic reticulum with slight dilatation
in the livers of all animals at 1000 mg/kg bw per day. These changes
are consistent with adaptation of the liver to exposure to the
compound through enzyme induction. A slight but significantly
prolonged retention ratio in the test for renal function was observed
in males at 300 and 1000 mg/kg bw per day after 6 weeks of treatment,
but the retention ratio was normal by the end of the study, and no
treatment related histopathological changes were observed in the
kidney. The NOAEL was 100 mg/kg bw per day on the basis of increased
absolute and relative liver weights and an increased incidence of
hepatocellular hypertrophy at higher doses (Nakano et al., 1988).
Groups of four male and four female beagle dogs (23-27 weeks old)
were given gelatine capsules containing pyriproxyfen (purity, 95.3%)
at doses of 0, 30, 100, 300, or 1000 mg/kg bw per day for 52 weeks.
The observations included clinical signs, deaths, food consumption,
body weight, and ophthalmoscopic, clinical chemical, haematological,
urinary, and histological examinations. The activities of alanine and
aspartate aminotransferases, alkaline phosphatase, and creatine
phosphokinase were measured in plasma. Blood samples were collected 1
week before and on weeks 12, 24, 37, and 50 of treatment. The study
conformed to GLP.
Two males at 1000 mg/kg bw per day were killed in extremis with
acute weight loss and, in one case, liver failure, at weeks 17 and 31
of treatment. Slightly increased frequencies of salivation and
diarrhoea were observed in animals at 1000 mg/kg bw per day, and
emaciation was observed in males at doses > 300 mg/kg bw per day.
There were no treatment-related ophthalmological abnormalities. A
dose-dependent but nonsignificant reduction in body weight was seen
throughout the study, and body-weight gains were significantly reduced
in animals at doses > 300 mg/kg bw per day. Food consumption was
not reduced at any dose. Significant changes were seen in several
haematological parameters, including 10-20% decreases in haemoglobin
and erythrocyte counts and a slightly but significantly increased mean
corpuscular volume in males at doses > 300 mg/kg bw per day and in
females at doses > 100 mg/kg bw per day and a significantly
decreased packed cell volume in the latter group. The change in
haemoglobin in males at 1000 mg/kg bw per day was not significant.
These haematological changes might indicate slight anaemia. There was
no abnormality of cellularity or cell composition in the bone marrow.
Statistically significant decreases in the number of lymphocytes were
observed in all treated females at weeks 12 and 37, which were
attributed to transiently high values for the control group. The
platelet count was significantly increased in males at doses > 100
mg/kg bw per day and females at 1000 mg/kg bw per day throughout the
study. Slightly but significantly prolonged prothrombin times were
observed in males at 300 mg/kg bw per day and in animals of each sex
at 1000 mg/kg bw per day. Significantly increased plasma enzyme
activities were seen, including those of alkaline phosphatase in
animals at doses > 300 mg/kg bw per day throughout the study,
alanine aminotransferase in animals at 1000 mg/kg bw per day
throughout the study, and aspartate aminotransferase in males at 1000
mg/kg bw per day in weeks 12, 24, and 37 of treatment. The total
cholesterol concentration in plasma was significantly increased in
animals at doses > 30 mg/kg bw per day (by 24-58% at 30 mg/kg bw
per day, 64-160% at 100 mg/kg bw per day, 100-140% at 300 mg/kg bw per
day, and 76-100% at 1000 mg/kg bw per day) throughout the study, and
the plasma concentrations of triglycerides were significantly
increased in animals at doses > 100 mg/kg bw per day. These
increases were dose-dependent except at the highest dose in males, but
in this group there were only two survivors. No reduction in plasma
protein fractions was observed in the treated groups.
A slightly reduced pH and increased volume of urine were observed
in males at 1000 mg/kg bw per day throughout the study. The absolute
weights of the liver were dose-dependently increased in all treated
groups and significantly increased in males at doses > 100 mg/kg bw
per day (by 130% at 30 mg/kg bw per day, 150% at 100 mg/kg bw per day,
170% at 300 mg/kg bw per day, and 190% of control values at 1000 mg/kg
bw per day) and in females at doses > 300 mg/kg bw per day (110%,
120%, 140%, and 140%, respectively). The relative weights of the liver
were significantly increased in males at doses > 30 mg/kg bw per
day (130% of control value) and in females at doses > 300 mg/kg bw
per day. The absolute weights of the thyroid were significantly
increased in females at doses > 300 mg/kg bw per day, and the
relative weights were significantly increased in females at doses
> 100 mg/kg bw per day. Significantly increased relative renal
weights were observed in males at 300 mg/kg bw per day and in females
at doses > 300 mg/kg bw per day; the absolute weights were
dose-dependently but not significantly increased.
Macroscopic examination showed enlarged livers and hepatic damage
in the two dogs at 1000 mg/kg bw per day which died. Histopathological
examination revealed treatment-related hepatic damage in animals of
each sex at 1000 mg/kg bw per day, which was characterized by
centriacinar fibrosis in 2/2 males and 3/4 females and bile-duct
hyperplasia in 2/2 males and 3/4 females; foci of cystic degeneration
in 1/2 males and 1/4 females; active chronic inflammation in 2/2 males
and 2/4 females; and nodular hyperplasia in 2/2 males and 0/4 females.
Submucosal fibrosis in the gall-bladder was observed in all male
animals, including those that had died, and in 3/4 females at 1000
mg/kg bw per day, in association with bile-duct hyperplasia. One of
four males at 30 mg/kg bw per day had focal bile-duct hyperplasia and
focal subcapsular fibrosis, but these effects were not observed at 100
or 300 mg/kg bw per day. There were no preneoplastic or neoplastic
alterations. Although no morphological alterations were seen in the
liver, the increase in cholesterol concentration and relative liver
weight at low doses might be related to treatment. No NOAEL could be
identified, as increased total cholesterol concentrations indicating
effects on lipid metabolism and increased relative liver weights with
a trend towards increased absolute liver weights were seen at all
doses (Chapman et al., 1991).
In a complementary study which complied with GLP, groups of four
male and four female beagle dogs (19-24 weeks old) were given gelatine
capsules containing pyriproxyfen (purity, 95.3%) at doses of 0, 3, or
10 mg/kg bw per day for 52 weeks. The observations included clinical
signs, deaths, food consumption, body weight, and ophthalmic, clinical
chemical, haematological, urinary, and histological examinations. The
activities of alanine and aspartate aminotransferases, alkaline
phosphatase, and creatine phosphokinase were assayed in serum. Blood
samples were collected after 12, 24, 36, and 50 weeks of treatment.
There were no deaths, signs of clinical toxicity, or changes in
body weight, body-weight gain, or food consumption. Significantly
increased platelet counts were observed in males at 3 mg/kg bw per day
in weeks 24, 36, and 50 of treatment and in those at 10 mg/kg bw per
day in week 36, but with no clear dose-dependence. Prothrombin time
was not prolonged in males at any dose. Females at 10 mg/kg bw per day
also showed significantly increased platelet counts in weeks 36 and 50
(by 8% at 3 mg/kg bw per day and 10% at 10 mg/kg bw per day), and
prothrombin time was slightly but significantly prolonged at these
doses at the end of study. There were no other treatment-related
changes in haematological parameters. The total cholesterol
concentration was unchanged; slight but significant increases in total
triglyceride concentrations in males at 10 mg/kg bw per day were seen
in weeks 12 and 36 of treatment. There were no treatment-related
changes in urinary parameters. The absolute weight of the liver was
slightly increased in females at 10 mg/kg bw per day (110% of
control), but this was not significant. No histopathological changes
were found in any organ, including the liver and kidney. The range of
mean total platelet counts in controls in other studies in this
laboratory was 273-357 in males and 305-357 in females, whereas those
in the present study were 341-367 in controls, 414-462 at the low
dose, and 415-456 at the high dose in males, and 321-384 in controls
and 430-478 at the high dose in females. The increased numbers of
platelets and the prolonged prothrombin time were therefore
treatment-related changes although no significant increase in platelet
counts was observed in animals at 30 mg/kg bw per day in the previous
study (Mitchel et al., 1993).
The NOAEL for the two 52-week studies in dogs was 10 mg/kg bw per
day, on the basis of the absence of treatment-related toxicity at 10
mg/kg bw per day in the second study and changes in lipid metabolism
and increased liver weight at 30 mg/kg bw per day in the first study.
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female ICR(Crj-CD-1) mice were given
diets containing pyriproxyfen (purity, 95.3%; 97.6-98.7% dietary
concentration) at concentrations of 0, 120, 600, or 3000 ppm for
78 weeks, providing doses equal to 0, 16, 81, and 420 mg/kg bw per day
for males and 0, 21, 110, and 530 mg/kg bw per day for females. The
observations included clinical signs, deaths, food consumption, body
weight, organ weights, and ophthalmological, haematological, and gross
and histological examinations. Ten mice from each group were killed
during week 52 for interim examination, and the surviving mice were
killed during week 78. Blood samples were taken from 10 rats per group
during weeks 52 and 78 of treatment. No clinical chemical tests were
conducted. The study complied with GLP.
The mortality rate was dose-dependent and significantly increased
in males at 600 and 3000 ppm (43% at 0 ppm, 55% at 120 ppm, 72% at 600
ppm, and 82% at 3000 ppm) and in females at 3000 ppm (39% at 0 ppm,
44% at 120 ppm, 55% at 600 ppm, and 64% at 3000 ppm). There were
slight, nonsignificant increases in the incidence of clinical signs,
including reduced motor activity and hunched position, in animals at
3000 ppm. Statistically significant decreases in body weights,
body-weight gains, and/or food consumption were observed in males at
3000 ppm during the study. The absolute and relative weights of the
liver were significantly increased in females at 3000 ppm during week
52 of treatment. The haematological parameters showed no
treatment-related change. Histopathological examination of animals
that died revealed a significantly increased incidence of systemic
amyloidosis in the glandular stomach of males at 600 and 3000 ppm and
the adrenal, thyroid, heart, liver, kidney, glandular stomach, and
duodenum of females at 3000 ppm, and a dose-related relationship was
found between the generalized amyloidosis and the mortality rate.
Statistical analysis of the incidence of graded amyloidosis revealed a
significant positive trend in renal amyloidosis in females and a
significant positive trend in hepatic amyloidosis in animals of each
sex at 3000 ppm. Females at this dose had a significant increase in
the incidence of lymphocytic infiltration in the liver (22/59 at 0 and
34/60 at 3000 ppm) and of tubular mineralization (3/59 at 0 and 46/60
at 3000 ppm). Deposition of amyloid in the kidney causes numerous
pathological changes including tubular mineralization and papillary
necrosis. In this study, however, the incidences of tubular
mineralization and segmental cortical atrophy were increased
independently of amyloidosis in female animals at 3000 ppm, suggesting
that the chronic nephrosis was directly related to treatment.
Histopathological examination revealed no increase in the incidence of
neoplastic lesions at any dose. The NOAEL was 120 ppm, equal to 16
mg/kg bw per day, on the basis of increased mortality at higher doses
(Osheroff et al., 1991a; Cardy et al., 1994).
Rats
Groups of 50 male and 50 female Sprague-Dawley (Crl:CD) rats were
given diets containing pyriproxyfen (purity, 95.3%) at concentrations
of 0, 120, 600, or 3000 ppm, equal to 0, 5.4, 27, and 140 mg/kg bw per
day for males and 0, 7.0, 35, and 180 mg/kg bw per day for females,
for 104 weeks. Satellite groups of 30 males and 30 females were also
treated orally. The observations included clinical signs, deaths, food
and water consumption, body weight, organ weights, and
ophthalmoscopic, clinical chemical, haematological, urinary, and gross
and histopathological examination. Assays were performed for the serum
enzymes aspartate and alanine aminotransferase, alkaline phosphatase,
creatine kinase, and gamma-glutamyl transpeptidase. Blood samples were
collected from satellite groups of rats on week 13, 26, 52, 78, and
104 of treatment. The study complied with GLP.
Treatment did not affect mortality (34-46% in males and 32-58% in
females), clinical signs, or ophthalmoscopic end-points. The body
weights of males were significantly reduced in weeks 13, 26, and 50 of
treatment (by 5-7%) and those of females in weeks 13, 26, 50, and 78
of treatment (by 12-14%) at 3000 ppm, but they had returned to the
control level by the end of study. The mean body-weight gain was
significantly reduced in females at 600 ppm and in animals of each sex
at 3000 ppm throughout the study. No treatment-related changes in food
consumption were observed. The only alteration in haematological
parameters was a transient increase in eosinophils. Alkaline
phosphatase activity was significantly increased in males at doses
> 120 ppm in weeks 26, 52, and 78 of treatment, but the activity
(87-104 U/L) remained within the range of historical controls (45-114
U/L), and the changes were not clearly dose-dependent. gamma-Glutamyl
transpeptidase activity was significantly increased in males at 3000
ppm in week 104 of treatment and in females at 600 and 3000 ppm in
weeks 26 and 52. The activities of other serum enzymes were not
significantly affected. Significantly increased total cholesterol
concentrations were observed in males at 3000 ppm in weeks 26 and 52
(149% and 147% of control, respectively). Slight, inconsistent,
nonsignificant increases in urinary protein concentration were
observed in females at 3000 ppm in week 26. At interim necroscopy, the
absolute weights of the liver were found to be nonsignificantly
increased at week 52 of treatment with 3000 ppm (by 15% in males and
13% in females). A significant increase in relative liver weight was
observed only in females at 3000 ppm (120% of control). The only
significant or treatment-related increases in the incidence of
morphological alterations at 104 weeks seen on gross and
histopathological examination were hyperkeratosis of the skin of males
at 3000 ppm, which was considered not to be biologically significant,
and a significant increase in the incidence of liver necrosis in males
at 3000 ppm that died during the study; however, no liver necrosis was
observed in the surviving males at 3000 ppm at the end of the study,
indicating nthat it was not related to treatment. Histopathological
examination revealed no evidence of neoplastic alterations. The NOAEL
was 600 ppm, equal to 27 mg/kg bw per day, on the basis of reductions
in body weight and mean body-weight gain and increased absolute and
relative liver weights at higher doses (Osheroff et al., 1994a,b).
(d) Genotoxicity
The results of tests for the genotoxicity of pyriproxyfen are
summarized in Table 2. All of the positive controls used in the assays
produced the expected positive responses. In assays for reverse
mutation, no induction of revertant colonies was observed at six doses
with or without an exogenous metabolic activation system (S9). In
tests for DNA repair, pyriproxyfen was inactive at six doses with or
without S9. In tests for gene mutation in mammalian cells, no
mutations were observed at four doses ranging from 10 to 300 ppm
without S9 or 10 to 100 ppm with S9. In assays for unscheduled DNA
repair synthesis, pyriproxyfen was cytotoxic and/or inhibited normal
DNA synthesis but it did not induce unscheduled DNA synthesis. In
tests for cytogeneticity, Chinese hamster ovary cells (CHOK1) were
exposed to pyriproxyfen at a concentration of 10, 30, or 100 µg/ml for
2 h in the presence of S9 and cultured for a further 16 or 22 h or
cultured with pyriproxyfen for 18 or 24 h in the absence of S9.
Although marked cytotoxicty, characterized by decreased mitotic index
and cell cycle delay, were observed at doses > 30 mg/ml without S9,
and at 100 mg/ml with S9, no increase in the total number of
structural aberrations or the frequency of cells with aberrations was
observed at any concentration. In the test for micronucleus formation
in mice in vivo, a single dose of pyriproxyfen at 5000 mg/kg bw
slightly but not statistically significantly increased the incidence
of micronucleated polychromatic erythrocytes.The Meeting concluded
that pyriproxyfen is not genotoxic in vivo or in vitro.
Table 2. Results of tests for the genotoxicity of pyriproxyfen
End-point Test system Concentration Purity Result Reference
(%)
In vitro
Reverse mutationa S. typhimurium 10-5000 µg/plate 97.2 Negative ± S9 Kogiso et al. (1988a)
TA98, TA100,
TA1537, TA1538,
E, coli WP2 uvrA
DNA repairb B.subtills M45, 673-21 500 µg/disc 95.3 Negative ± S9 Kogiso et al. (1992)
H17 in DMSO
Gene mutationc Chinese hamster 3-300 µg/ ml 95.3 Negative ± S9 Kogiso et al. (1990)
V79 cells, hprt locus
Unscheduled Human HeLa S3 0.1-205 µg/ ml 95.3 Negative ± S9 Henderson &
DNA synthesisd epithelioid cells Proudlock (1989)
Chromosomal Chinese hamster 10-300 µg/ml 97.2 Negative ± S9 Kogiso et al. (1989)
aberrationse ovary cells
Chromosomal Chinese hamster 9.6-321 µg/ml -S9 97.2 Negative ± S9 Kogiso et al. (1988)
aberrationsf ovary cells 50-200 µg/ml +S9
In vivo
Micronucleus CD-I mice, bone Single intraperitoneal 95.3 Negative Proudlock et al.
formationg marrow injections of 5000 mg/kg (1991)
bw at 24, 48, and 72 h
Table 2. (continued)
All studies were conducted according to good laboratory practice. DMSO, dimethyl sulfoxide
a Positive controls were methylmethanesulfonate for TA100, 2-nitrofluorene for TA98 and TA1538, sodium azide for TA1535,
ICR-191 for TA1537, N-ethyl- N'-nitro- N-nitrosoguanidine for WP2 uvrA, benzo[ a]pyrene for TA100, TA98, TA1537, and TA1538
and 2-aminoanthracene for TA1535 and WP2 uvrA.
b Positive controls were mitomycin C for the direct assay and sterigmatocystin for the activation assay. The negative
control was kanamycin in both assays.
c Positive controls were ethylmethane sulfonate for the direct assay and 9,10-dimethyl-1,2-benzanthracene for the activation
assay.
d Positive controls were 2-acetylaminofluorene for the activation assay and 4-nitroquinoline-1-oxide for the direct assay.
e Positive controls were mitomycin C for the direct assay and cyclophosphamide for the activation assay.
f Positive controls were mitomycin C for the direct assay and benzo[ a]pyrene for the activation assay.
g Positive control was mitomycin C.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 26 male and 26 female Sprague-Dawley (Crj) rats were
given diets containing technical-grade pyriproxyfen (purity, 95.3%) at
concentrations of 0, 200, 1000, or 5000 ppm. The F0 animals were
treated for 70 days before mating and then for 6 subsequent weeks for
males and during 3 weeks of gestation and 3 weeks of the lactation
period for females. The F1 generation were treated for 18 weeks from
the day of their weaning to the day of weaning of the F2 generation,
including 77-90 days before mating and the mating, gestation, and
lactation periods. The mean daily intakes of pyriproxyfen were 14, 68,
and 340 mg/kg bw per day in males and 20, 98, and 500 mg/kg bw per day
in females (18, 87, and 440 mg/kg bw per day before mating, 15, 77,
and 390 mg/kg bw per day during gestation, and 32, 160, and 830 mg/kg
bw per day during lactation) in the F0 generation, and 17, 83, and
440 mg/kg bw per day in males and 21, 110, and 560 mg/kg bw per day in
females (21, 100, and 550 mg/kg bw per day before mating, 14, 72, and
380 mg/kg bw per day during gestation, and 28, 160, and 810 mg/kg bw
per day during lactation) in the F1 generation.
The observations in parental rats included clinical signs,
deaths, food consumption, body weight, estrus cycle, and
histopathological and reproductive parameters which included mating,
fertility, gestation, and live birth indices. All parental animals
were killed at the end of weaning, and the reproductive organs, brain,
pituitary, liver, and kidney were examined histopathologically. The
organs from the F1 parental animals were weighed. Estrus cycles were
examined by a smear assay during the 10 days before mating. The
observations in the F1 and F2 pups included viability, body
weight, and lactation indices. Developmental indices were not
examined. Groups of 10 male and 10 female pups were selected randomly
from 10 litters for necroscopy. One male and one female were selected
randomly from each F1 litter to provide 26 pairs at each dose to
serve as parents for the F2 generation. Pups were weighed by sex.
The study complied with GLP.
The F0 parent animals showed no treatment-related changes in
clinical signs, mortality rate, reproductive parameters, or estrus
cycle. Body weight and body-weight gain were significantly reduced in
F0 animals at 5000 ppm during the periods of premating, gestation,
and lactation, and food consumption was significantly reduced in F0
females at this dose during gestation. No gross or histopathological
alterations were seen that were related to treatment. The NOAEL for
F0 parental toxicity was 1000 ppm, equal to 68 mg/kg bw per day, on
the basis of reductions in body weight and body-weight gain at 5000
ppm. The NOAEL for reproductive toxicity was 5000 ppm, equal to 340
mg/kg bw per day, the highest dose tested.
The F1 parent animals showed no treatment-related changes in
clinical signs, mortality rate, reproductive parameters, or estrus
cycles. The body weights of F1 males and F1 females at 5000 ppm
were significantly reduced, and the terminal body weights of animals
at this dose were significantly decreased (to 98% at 1000 ppm and 88%
at 5000 ppm in males and 100% at 1000 ppm and 95% at 5000 ppm in
females). Body-weight gain was also significantly reduced in F1
males at 5000 ppm during the premating period. Food consumption was
significantly reduced at this dose in F1 males during treatment and
in F1 females during gestation. The absolute weights of the liver
were significantly increased in F1 adults at 5000 ppm (110% at 1000
ppm and 110% at 5000 ppm in males and 120% at 5000 ppm in females),
and the relative weights were significantly increased in males at 1000
ppm (110% of control) and in animals of each sex at 5000 ppm (130% in
males and 130% in females). Histopathological examination showed an
increased incidence of focal clear cells in the liver in males at 5000
ppm, but this effect is commonly observed in male rats and was
considered to be unrelated to treatment. Significant increases in
relative kidney weights were observed in males at 1000 ppm (110% of
control values) and 5000 ppm (110%), but the absolute weights were not
significantly increased at any dose. Histopathological examination
showed an increased incidence of chronic interstitial nephrosis (7/26
at 0 ppm, 3/26 at 200 ppm, 7/26 at 1000 ppm, and 15/26 at 5000 ppm) in
males at 5000 ppm and in the incidence of hydronephrosis (1/26 at 0
ppm and 4/26 at 5000 ppm) in females at this dose; however, these
increases did not reach statistical significance. No other
treatment-related morphological lesions were observed. The NOAEL for
F1 parental toxicity was 1000 ppm, equal to 83 mg/kg bw per day, on
the basis of decreased body weight, decreased food consumption, and
increased absolute and relative liver weights at 5000 ppm. No evidence
of reproductive toxicity was observed. The NOAEL for reproductive
toxicity was 5000 ppm, equal to 340 mg/kg bw per day, the highest dose
tested.
In F1 pups, there was no treatment-related change in clinical
signs, sex ratio, viability index, or lactation index and no
significant difference between treated and control groups in body
weight at birth. Significant reductions in body weight were observed
in male F1 pups on day 21 and in female F1 pups on days 14 and 21
post partum at 5000 ppm. The total litter weight was also
significantly decreased on days 14 and 21 post partum at this dose.
No treatment-related effects were seen on gross examination. F2 pups
also showed no treatment-related change in clinical signs, sex ratio,
viability index, or lactation index. The mean pup weights were
significantly decreased on days 14 and 21 post partum at 5000 ppm.
No gross pathological alteration related to treatment was apparent at
any dose. The NOAEL for developmental toxicity was 1000 ppm, equal to
98 mg/kg bw per day, on the basis of reduced body weight in F1 and
F2 pups at 5000 ppm (Robinson et al., 1991).
In a study of treatment before and during the early stages of
gestation (segment 1) conducted according to GLP, groups of 24 male
and 24 female Sprague-Dawley rats were given pyriproxyfen (purity,
97.2%) dissolved in corn oil by gavage at doses of 0, 100, 300, 500,
or 1000 mg/kg bw per day for 12 weeks comprising 9 weeks before mating
and 3 weeks of mating, in males or for at least 3 weeks including 2
weeks before mating and the mating period and on days 0-7 of gestation
in females. The observations in parental rats included clinical signs,
deaths, food consumption, body weight, organ weights, gross
appearance, and reproductive performance including copulation and
fertility indices. Clinical examinations were performed twice a day.
Males were killed at the end of mating, and female animals on day 21
of gestation.
Treatment-related deaths occurred in two females at the highest
dose. Increased incidences of diarrhoea and erythema and swelling of
the anal region were observed in females at 300 mg/kg bw and in
animals of each sex at doses > 500 mg/kg bw per day. Dose-dependent
increases in the incidence of salivation were frequently observed in
all treated groups, with incidences of 0/24 in controls and 18/24,
21/24, 24/24, and 24/24 at the four doses respectively, in males, and
0/24, 1/24, 5/24, 7/24, and 8/24, respectively, in females. Body
weight was significantly reduced in females at 100 mg/kg bw per day
during gestation and in animals of each sex at doses > 300 mg/kg bw
per day throughout the study. Body-weight gain and food consumption
were also significantly decreased in males at doses > 300 mg/kg bw
per day throughout the study, but significant decreases were observed
only during treatment in females. Water consumption was significantly
increased in males at doses > 100 mg/kg bw per day and in females
at doses > 300 mg/kg bw per day. The absolute weights of the liver,
kidney, and adrenal glands were significantly increased and the
absolute weight of the thymus was significantly decreased in males at
doses > 300 mg/kg bw per day. In females, the absolute weights of
the kidney and adrenals were significantly increased at 1000 mg/kg bw
per day. Enlarged, dark-red livers, pitted, enlarged kidneys, enlarged
adrenals, and thymus atrophy were observed in males at doses > 300
mg/kg bw per day, and congested, enlarged livers, enlarged adrenals,
and atrophy of the spleen and thymus were observed in females at
1000 mg/kg bw per day. There was no treatment-related change in
reproductive performance. No NOAEL could be identified for maternal
toxicity, as reduced body weight during gestation was seen at all
doses. The NOAEL for reproductive toxicity was 1000 mg/kg bw per day,
the highest dose tested.
Slightly but significantly reduced numbers of corpora lutea (90%
of control) and of live fetuses (88% of control) were observed at 1000
mg/kg bw per day, but the values were within the range in historical
controls. Slight increases in placental weight and in fetal body
weight were observed at 1000 mg/kg bw per day, but the effects were
not dose-dependent and were within the range in historical controls. A
slight but significant increase in the number of phalanges of the
proximal forepaw was observed, but such increases are known to be
associated with an altered growth rate. The incidences of external,
visceral, and skeletal anomalies were not increased. The NOAEL for
developmental toxicity was 1000 mg/kg bw per day, the highest dose
tested (Saegusa et al., 1988a).
In a study of treatment during the perinatal and lactation
periods (segment 3) conducted according to GLP, groups of 23-24
pregnant Sprague-Dawley rats were given pyriproxyfen (purity, 97.2%)
dissolved in corn oil by gavage at doses of 0, 30, 100, 300, or 500
mg/kg bw per day from day 17 of gestation to day 20 post partum. The
dams were allowed to deliver naturally. On day 4 post partum, the
offspring were culled to adjust the litter size to eight (four males
and four females when possible) for tests of development (F1I), and
the remaining pups (F1II) were killed for skeletal examination on
the day of culling. The dams (F0) were killed at termination of
weaning. The observations in maternal rats included clinical signs,
deaths, body weight, food consumption, organ weights, gross
appearance, and reproductive indices including delivery rate, birth
rate, and body weight of live newborns. The F1I offspring were
weighed on days 0, 4, 7, 14, and 21 post partum during lactation;
after weaning, one male and one female per litter were weighed once a
week. Clinical signs were observed twice a day. The indices of
development during lactation included separation of the auricle,
emergence of abdominal hair, eruption of incisors, separation of
eyelids, and descent of testes or opening of the vagina. On day 20,
all F1 offspring were examined for sensory function in tests for
visual placing, righting and mid-air righting reflexes, and response
to pain. On day 21 post partum, two male and two female offspring
from each litter (F1Ia) were killed for visceral and skeletal
examination. After weaning, another male and female from each litter
(F1Ib) were examined for emotionality (behaviour in an open field)
at 4 weeks of age, for motor coordination (rotarod performance) at 5
weeks of age, and for learning ability (in a water-filled multiple T
maze) at 6 weeks of age. The F1Ib offspring were killed for necropsy
on day 56 post partum. Another male and female from each litter
(F1Ic) were tested only for reproductive performance after being
paired for mating within the same group (avoiding sibling matings) at
11 weeks of age. The fetuses were removed surgically from mated
females on day 21 of gestation. Reproductive performance was assessed
from indices of mating, fertility, gestation, and litters. The fetuses
(F2) were weighed and examined for external anomalies.
In the F0 maternal animals, treatment-related deaths were
observed at 500 mg/kg bw per day, an increased incidence of diarrhoea
at doses > 300 mg/kg bw per day, and dosedependent increases in the
incidence of salivation at doses > 100 mg/kg bw per day (0/23 in
controls and 0/23, 1/23, 2/24, and 4/24 at the four doses,
respectively). During gestation, significantly reduced body weights
were observed at 500 mg/kg bw per day, and significantly reduced
body-weight gain and food consumption were seen frequently during the
study at doses > 300 mg/kg bw per day. The absolute and relative
weights of the liver were significantly increased at doses > 300
mg/kg bw per day. Atrophy of the thymus, congestion of the liver and
kidneys, enlargement of the adrenals, and atrophy of the spleen were
observed at 500 mg/kg bw per day, and an increased number of
stillborns was seen at this dose. The body weight of live newborn pups
was significantly decreased at doses > 300 mg/kg bw per day. The
NOAEL for maternal toxicity was 100 mg/kg bw per day, on the basis of
clinical signs, reductions in body weight, body-weight gain, and food
consumption, and decreased body weight of delivered newborn at 300
mg/kg bw per day.
At 500 mg/kg bw per day, the survival rate of F1 offspring was
significantly decreased on days 0-4 post partum, and the weaning
rate was decreased on days 4-21 post partum. No decrease in the
viability of F1I offspring was observed on days 21-77. The body
weights of pups of each sex at doses > 300 mg/kg bw per day were
significantly reduced on days 0-49 or 56 post partum. All of the
physical developmental indices were significantly retarded and slight
retardation of sexual differentiation was observed at doses > 300
mg/kg bw per day. No treatment-related change in sensory functions was
observed in F1I offspring. Visceral examination of the F1Ia
offspring on day 21 post partum revealed a significant increase in
the number with anomalies at 30, 300, and 500 mg/kg bw per day (0% in
controls and 10%, 1.2%, 17.2%, and 29% at the four doses,
respectively).The anomalies consisted mainly of dilatation of the
renal pelvis and hyperaemia and/or inflammatory cell infiltration. The
incidence of dilatation of the renal pelvis was significantly
increased at doses > 300 mg/kg bw per day (0% in controls and 3.8%,
1.2%, 14%, and 18% at the four doses, respectively, with 0-3.2% in
historical controls). The incidence of hyperaemia and/or inflammatory
cell infiltration was significantly increased at 500 mg/kg bw per day
(0% in controls and 3.8%, 0%, 5.8%, and 12% at the four doses,
respectively). Although developmental insufficiency of the papilla or
stenosis and obstruction of the urethra can cause dilatation of the
renal pelvis, as can developmental retardation, the range in
historical controls of this strain was 0-2.0% in fetuses and 0-3.2% in
21-day-old offspring, and recovery from dilatation of the renal pelvis
usually occurs after birth. No skeletal anomalies were found that were
related to treatment. The absolute weights of all organs of F1
offspring killed on day 21 post partum were slightly but
significantly decreased, but no treatment-related change was found in
the absolute weights of the organs of F1Ib offspring killed on day
56 post partum. The changes in organ weights might have been due to
retarded physical development. Ambulation was also slightly but
significantly increased at doses > 100 mg/kg bw per day (by 31% at
100 mg/kg bw per day, 50% at 300 mg/kg bw per day, and 37% at 500
mg/kg bw per day). Motor coordination was comparable to that of
controls, and no treatment-related changes in learning ability were
observed. All the changes observed in the F1 offspring, including
increased ambulation, could have been due to retarded physical
development. After maturation, the reproductive perfomance of F1Ic
offspring was comparable to that of controls. The body weight and
body-weight gain of pregnant F1 animals were comparable to those of
controls during gestation. The NOAEL for developmental toxicity was
100 mg/kg bw per day, on the basis of decreased body weight,
retardation of physical development, and visceral anomalies associated
with the retarded growth rate at 300 mg/kg bw per day.
Significantly reduced numbers of implantations (12 in controls
and 10, 12, 11, and 9.2 at the four doses, respectively) and mean
numbers of live F2 fetuses (12 in controls and 9.3, 11, 10, and 8.5
at the four doses, respectively) were observed at 500 mg/kg bw per
day. Significantly reduced numbers of corpora lutea, implantations,
and live fetuses were found at 30 mg/kg bw per day but not at 100
mg/kg bw per day. These reductions were not dose-dependent. There were
no treatment-related differences in fetal body weight or in the
incidence of external anomalies between treated and control groups.
The NOAEL for F1 reproductive toxicity was 300 mg/kg bw per day, on
the basis of a reduction in the number of implantations and in the
mean number of live fetuses at 500 mg/kg bw per day (Saegusa et al.,
1988b).
(ii) Developmental toxicity
Rats
In a study that complied with GLP, groups of 36 female
Sprague-Dawley rats were given pyriproxyfen (purity, 97.2%) dissolved
in corn oil by gavage at doses of 0, 100, 300, or 1000 (42 animals)
mg/kg bw per day on days 7-17 of gestation. The fetuses (F1I) were
removed surgically from 23 pregnant dams (F0I) at 0, 100, or 300
mg/kg bw per day and from 20 at 1000 mg/kg bw per day on day 21 of
gestation and examined for developmental toxicity. The remaining 10-13
dams (F0II) were allowed to deliver normally. On day 4 post
partum, the offspring were culled to adjust the litter size to eight
(four males and four females when possible) for functional testing on
day 20 post partum (F1IIa). The remaining pups (F1IIb) were
killed at 21 days of age and examined for skeletal anomalies. During
lactation, developmental indices were examined. After weaning, one
male and one female from each litter (F1IIa1) allocated for
functional testing were examined for emotionality at 4 weeks of age,
for motor coordination at 5 weeks of age, and for learning ability at
6 weeks of age; these offspring were killed for necropsy at 8 weeks of
age. Another male and female from each litter (F1IIa2) were tested
for reproductive performance after being paired for mating within the
same group (avoiding sibling mating) at 11 weeks of age, and the
fetuses were removed surgically from the mated females on day 21 of
gestation. The remaining offspring (F1IIa3) were killed for
necroscopy at 21 days of age, after weaning.
The observations in parental rats included clinical signs,
deaths, food and water consumption, body weight, estrus cycle, and
organ weights. The postnatal developmental indices included separation
of the auricle, emergence of abdominal hair, eruption of incisors,
separation of eyelids, and descent of testes or opening of the vagina.
The sensory function tests included visual placing, righting and
mid-air righting reflexes, and response to pain. Emotionality was
evaluated by observation of behaviour in an open field, motor
coordination was examined in a rotarod performance test, and learning
ability was examined by behaviour in a water-filled multiple T maze
test. Reproductive performance included indices of mating, fertility,
gestation, and litters.
Of the F0 dams, 12 of 42 at 1000 mg/kg bw per day died between
day 11 and day 16 of gestation. No deaths occurred in the other
groups. Signs of toxicity were observed at doses > 300 mg/kg bw per
day. At the highest dose, these included diarrhoea, erythema and
swelling of the anal region, hypoactivity, wasting, hypothermia,
lachrymation, and piloelection. Signs of toxicity were also observed
at 300 mg/kg bw per day but in only one animal. Body weight and
body-weight gain were significantly reduced at 300 and 1000 mg/kg bw
per day during gestation and at 100 mg/kg bw per day during treatment.
Food consumption was significantly reduced at doses > 100 mg/kg bw
per day during treatment, and water consumption was dose-dependently
and significantly increased at doses > 300 mg/kg bw per day. Thymic
atrophy and enlarged adrenals were observed in the dams that died and
those killed on day 21 of gestation (F0I) at 1000 mg/kg bw per day.
In the F0I dams, from which fetuses were removed surgically on
day 21 of gestation, slight but significant increases were found in
the relative weights of the liver and kidney at 300 mg/kg bw per day,
but no significant increase in the absolute weights was observed at
this dose. At 1000 mg/kg bw per day, the absolute weights of the
kidney and adrenal were significantly increased and the absolute
weight of the thymus was significantly decreased. The relative weight
of the liver was significantly increased, but the absolute weight was
not significantly affected at 1000 mg/kg bw per day. Higher ratios of
resorbed or dead fetuses were observed at 1000 mg/kg bw per day (4.7%
in controls, 7.7% at 300 mg/kg bw per day, and 15% at 1000 mg/kg bw
per day). None of these differences achieved statistical significance
or was outside the historical control range (5.7%; 1.5-20%). There was
no difference in the mean numbers of corpora lutea or implantations or
the implantation rate. In the F0II dams, body weight and body-weight
gain were significantly reduced at 300 and 1000 mg/kg bw per day
during lactation. There were no treatment-related changes in the
number of live newborn, the length of gestation, or the delivery rate.
No NOAEL could be identified for F0 maternal toxicity since
decreased body-weight gain was seen at all doses. The NOAEL for
reproductive toxicity was 1000 mg/kg bw per day, the highest dose
tested.
No significant change in F1I fetal sex ratio, placental weight,
or fetal body weight was observed, but the ratio of resorbed or dead
fetuses was increased (5% in controls and 15% at 1000 mg/kg bw per
day) and the number of live fetuses was decreased at the highest dose,
but these changes were not statistically significant. Cyclopia and
polydactyly were each observed in one fetus at 300 mg/kg bw per day
(0.7%), but not in any other group, and these anomalies were
considered to be unrelated to treatment. There were no
treatment-related increases in the incidences of external, visceral,
or skeletal anomalies. A significant increase in the number of fetuses
with a skeletal variation consisting of opening of the foramen
transversarium of the seventh cervical vertebra was seen at 300 and
1000 mg/kg bw per day (0% in controls and 1.5%, 5.0%, and 14% at the
three doses, respectively). An increased number of fetuses with an
extra lumbar rib was observed at 1000 mg/kg bw per day, but this was
not significant. The NOAEL for developmental toxicity was 300 mg/kg bw
per day, on the basis of increased skeletal variations at 1000 mg/kg
bw per day.
The F1IIa offspring showed no-treatment related effect on
survival or weaning rates. The body weights of the treated groups were
comparable to those of controls during lactation and before mating.
There were no treatment-related effects on postnatal physical
development, sexual differentiation, sensory function, emotional
behaviour, or motor coordination. A significant retardation in the
time taken to reach the goal in the water-filled multiple T maze was
observed in females at 1000 mg/kg bw per day, but this slight
reduction in learning behaviour was observed only in the second of
three consecutive daily trials. There was no impairment of
reproductive performance of F1IIa2 offspring at any dose. No
external anomalies were observed in F2 fetuses. The NOAEL for
developmental toxicity was 1000 mg/kg bw per day, if the slight
evidence of behavioural teratogenicity in F1 offspring is ignored.
In the offspring killed at 21 days of age (F1IIb), an increased
incidence of skeletal variations was observed at 1000 mg/kg bw per day
(9% in controls and 18% at 1000 mg/kg bw per day), but this was not
statistically significant. No external or skeletal anomalies were
observed. The incidence of visceral anomalies was increased
significantly in F1IIaI offspring at 1000 mg/kg bw per day killed at
56 days of age (0/46 in controls and 7/39 at 1000 mg/kg bw per day).
F1IIb and F1IIaI offspring at 1000 mg/kg bw per day also showed an
increased incidence of dilatation of the renal pelvis. The total
incidence of dilatation of the renal pelvis observed in F1I
offspring (killed at 21 and 56 days of age and at the end of the
fertility test) was increased dose-dependently (0/98 in controls and
1/95, 3/85, and 9/79 at the three doses, respectively). The incidences
in controls from 15 previous studies were 0-2.0% in fetuses, 0-3.2% in
offspring 21 days old, 0-4.3% at 56 days of age, and 0-4.5% at the end
of the fertility test. The incidence in the controls in the present
study was 0% at all times. The incidence of protrusion or partial
adhesion of the liver parenchyma on the diaphragmatic side was also
increased at 1000 mg/kg bw per day (1/98 in controls and 0/95, 2/85,
and 6/79 at the three doses, respectively). These anomalies were not
observed in fetuses removed surgically. The overall NOAEL for
developmental toxicity was 300 mg/kg bw per day, on the basis of an
increased incidence of skeletal variations in fetuses and an increased
incidence of visceral anomalies in offspring at 1000 mg/kg bw per day
(Saegusa et al., 1988c).
Rabbits
Groups of 15-18 female JW-NIBS rabbits were treated by gavage
with pyriproxyfen (purity, 97.2%) at doses of 0, 100, 300, or 1000
mg/kg bw per day on days 6-18 of gestation and were killed on day 28
of gestation. The study complied with GLP.
In the maternal animals, abortion or premature delivery occurred
at doses > 300 mg/kg bw per day, in one control, none at 100 mg/kg
bw per day, three at 300 mg/kg bw per day, and six at 1000 mg/kg bw
per day. Dead and moribund animals were found at 1000 mg/kg bw per day
(none at 0, 100, or 300 mg/kg bw per day and three at 1000 mg/kg bw
per day). Several signs of toxicity, including soft stools,
emaciation, decreased spontaneous activity, and bradypnoea were
observed in aborted, prematurely delivered, prematurely dying, and
moribund dams at doses > 300 mg/kg bw per day. Body weight,
body-weight gain, and food consumption were significantly reduced in
dams at 1000 mg/kg bw per day. There were no significant effects on
the mean number of corpora lutea or implantations or the number, sex
ratio, or body weight of live fetuses.
The live fetuses showed no treatment-related external anomalies.
Skeletal or visceral malformations were observed in fetuses at 300
mg/kg bw per day, comprising a defect of the third distal phalanx of
the hind leg in one; cystic lung, ventricular septal defect,
hypoplasia of the left atrial auricle, and persistent truncus
arteriosus in one; and a defect of the gall-bladder in one. None was
observed in the other groups. External malformations were observed in
three fetuses, comprising local oedema, a visceral malformation, and
microphthalmia in one fetus at 300 mg/kg bw per day; persistent
truncus arteriosus in one fetus at 1000 mg/kg bw per day; and a
ventricular septal defect in another at this high dose. The total
incidence of malformations was 1% in controls, 1% at 100 mg/kg bw per
day, 6% at 300 mg/kg bw per day, and 2% at 1000 mg/kg bw per day. The
authors concluded that pyriproxyfen did not cause treatment-related
changes in the incidences of skeletal anomalies, skeletal variations,
ossification, visceral anomalies, or visceral variations. The NOAEL
for reproductive toxicity was 100 mg/kg bw per day on the basis of
abortion or premature delivery and a number of signs of toxicity at
300 mg/kg bw per day. The NOAEL for developmental toxicity was 1000
mg/kg bw per day, the highest dose tested (Hirohashi et al., 1988).
(f) Studies on metabolites
(i) Acute toxicity
The metabolites of pyriproxyfen were administered orally to mice
in a 0.5% solution of methylcellulose at 1000 or 2000 mg/kg bw. One
out of five males given 5''-hydroxypyriproxyfen (purity, 97.5%) at
2000 mg/kg bw died, but no deaths occurred with the other metabolites.
4'-Hydroxypyriproxyfen (purity, 98.3%) caused no abnormal clinical
signs; 5''-hydroxypyriproxyfen caused decreased spontaneous activity
in animals at both doses and ataxic gait at 2000 mg/kg bw;
4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether pyriproxyfen
(purity, 90.1%) produced decreased spontaneous activity, ataxic gait,
and prone position in animals at 2000 mg/kg bw; (RS)-2-hydroxypropyl
4-phenoxyphenyl ether (purity, 99.0%) produced decreased spontaneous
activity, ataxic gait, prone position, lateral position, and irregular
respiration in animals at 2000 mg/kg bw; and (RS)-2-(2-pyridyloxy)
propionic acid (purity, 100%) produced decreased spontaneous activity
at 2000 mg/kg bw (Misaki, 1993a).
(ii) Genotoxicity
4'-Hydroxypyriproxyfen (purity, 98.3%), 5''-hydroxypyriproxyfen
(purity, 97.5%), 4-hydroxyphenyl (RS)-2-(2-pyridyloxy) propyl ether
pyriproxyfen (purity, 90.2%), (RS)-2-hydroxypropyl 4-phenoxyphenyl
ether (purity, 99.0%), and (RS)-2-(2-pyridyloxy) propionic acid
(purity, 100%) were tested for mutagenicity in Salmonella
typhimurium TA98, TA100, TA1535, and TA1537 and in Escherichia
coli WP2 uvrA, with and without exogenous metabolic activation at
concentrations of 15-5000 µg/plate. None of the metabolites caused
reverse mutation, whereas the positive controls used in these assays
produced the anticipated responses (Hara et al., 1993).
No data were available on the effects of the metabolites of
pyriproxyfen on chromosomal integrity, but as these metabolites are
formed in vivo and pyriproxyfen had no effect on this end-point,
this is not considered a major gap in the data.
Comments
After oral administration to rats, [14C]pyriproxyfen is slowly
(time to peak concentration in plasma, 8 h) and incompletely (< 50%
of the dose) absorbed but is then rapidly eliminated, predominantly in
the faeces (90%), with only 4-11% in the urine, after 48 h. Absorbed
pyriproxyfen is excreted mainly via the bile (34-37% of the
administered dose in 48 h). The metabolism of pyriproxyfen is
qualitatively similar in rats, mice, lactating goats, and laying hens.
A large number of metabolites have been detected, the main route of
biotransformation being 4'-hydroxylation. Other pathways include
hydroxylation of the pyridyl ring, ether cleavage and conjugation.
Mice conjugate a much greater proportion of the dose than rats. The
concentration of pyriproxyfen in tissues other than fat was very low
(generally < 0.01 µg equivalent per g after 72 h; fat < 0.1µg
equivalent per g). The half-times of the radiolabel in tissues,
including blood and fat, were 8-36 h. The dermal absorption of
pyriproxyfen has not been studied.
The acute oral toxicity of pyriproxyfen is low, with LD50
values > 5000 mg/kg bw in mice, rats, and dogs. The acute dermal
toxicity is also low, with LD50 values > 2000 mg/kg bw in mice and
rats, and after exposure by inhalation, with an LC50 value > 1.3
mg/l air in mice and rats. WHO (1999) has classified pyriproxyfen as
'unlikely to present acute hazard in normal use'. Pyriproxyfen was
mildly irritating to the eye but not to the skin of rabbits. It did
not sensitize the skin of Hartley guinea-pigs in a maximization test.
In short- and long-term studies of the effects of pyriproxyfen in
mice, rats, and dogs, the liver was the main toxicological target,
with increases in liver weight and changes in plasma lipid
concentrations, particularly cholesterol, at doses of 120 mg/kg bw per
day and above in rats. There was some evidence that the compound might
cause modest anaemia in mice, rats, and dogs at high doses. In mice
treated with pyriproxyfen in the diet for three months, additional
effects seen included increased mortality rates, histopathological
changes in the kidney, and decreased body weight. The NOAEL was 150
mg/kg bw per day in mice, 23 mg/kg bw per day (two studies) in rats,
and 100 mg/kg bw per day in dogs fed pyriproxyfen in the diet for 3
months. In long-term studies of toxicity in mice, pyriproxyfen also
caused a dose-dependent increase in the occurrence of systemic
amyloidosis, which was associated with increased mortality rates. The
NOAEL was 120 ppm, equal to 16 mg/kg bw per day. In rats, the only
additional effect was reduced body-weight gain, and the NOAEL was 600
ppm, equal to 27 mg/kg bw per day. In two 1-year studies in dogs,
pyriproxyfen was administered in capsules. The overall NOAEL was 10
mg/kg bw per day on the basis of increased relative liver weight and
increased total plasma cholesterol concentration in males. There was
some evidence that pyriproxyfen can act as a hepatic enzyme inducer,
at least in dogs. Pyriproxyfen was not toxic when administered
dermally to rats for 21 days at doses of up to 1000 mg/kg bw per day.
Inhalation of pyriproxyfen for 4 h per day for 28 days caused only
minor effects in rats (initial salivation, sporadically reduced
body-weight gain, slightly increased serum lactate dehydrogenase
activity) at 10 000 mg/m3. The NOAEL was 480 mg/m3.
Pyriproxyfen was not carcinogenic when given in the diet at doses
up to 420 mg/kg bw per day in a study in mice or at doses up to 140
mg/kg bw per day in rats. Pyriproxyfen showed no evidence of
carcinogenicity in a 1-year study in dogs at doses up to 1000 mg/kg bw
per day. The Meeting concluded that pyriproxyfen does not pose a
carcinogenic risk to humans.
Pyriproxyfen was not genotoxic in an adequate range of tests for
mutagenicity and cytogenicity in vitro and in vivo. The Meeting
concluded that pyriproxyfen is not genotoxic.
The reproductive toxicity of pyriproxyfen in rats has been
investigated in a two-generation study of reproductive toxicity, a
study involving treatment of males and females before and in the early
stages of gestation (segment 1), and a study of treatment during the
prenatal and lactation periods (segment 3). The NOAEL for maternal
toxicity was 1000 ppm, equal to 98 mg/kg bw per day, in the
two-generation study and 100 mg/kg bw per day in the segment 3 study.
Reproductive toxicity was observed only in the segment 3 study, in
which there was an increased number of stillbirths in the F0
generation and a reduction in the number of implantations and in the
mean number of live fetuses in the F1 generation at 500 mg/kg bw per
day. The NOAEL for reproductive toxicity was 300 mg/kg bw per day. No
reproductive toxicity was observed in the two-generation study, the
NOAEL being 5000 ppm, equal to 340 mg/kg bw per day, the highest dose
tested, or in the segment 1 study, the NOAEL being 1000 mg/kg bw per
day, the highest dose tested.
The developmental toxicity of pyriproxyfen has been studied in
rats and rabbits. In rats, a NOAEL for maternal toxicity was not
identified, as decreased body-weight gain was observed at 100 mg/kg bw
per day, the lowest dose tested. Pyriproxyfen caused little
developmental toxicity and was not teratogenic. In a segment 3 study,
the F1 offspring were subjected to a series of developmental tests
for possible neurotoxicity, including physical indices, tests of
behaviour, motor and sensory function, and learning ability. Although
there were some effects on growth at doses > 300 mg/kg bw per day,
there was no developmental neurotoxicity at 500 mg/kg bw per day, the
highest dose tested. Visceral anomalies (dilatation of the renal
pelvis) were found at doses > 300 mg/kg bw per day. The NOAEL for
developmental toxicity was 100 mg/kg bw per day, on the basis of
retarded physical development and visceral anomalies at higher doses.
In a more conventional study of developmental toxicity in rats, no
evidence of growth retardation or of developmental neurotoxicity was
found at doses up to and including 1000 mg/kg bw per day, the highest
dose tested. There was an increased frequency of skeletal variations
(opening of the foramen transversalium of the seventh cervical
vertebra) in fetuses at 300 mg/kg bw per day. The frequency of
visceral anomalies was significantly increased in F1 offspring some
weeks after birth. The NOAEL for developmental toxicity was 300 mg/kg
bw per day, on the basis of an increased frequency of skeletal
variations with visceral anomalies in F1 offspring at 1000 mg/kg bw
per day. In a study of developmental toxicity in rabbits, signs of
maternal toxicity (abortion and premature delivery) were evident at
doses > 300 mg/kg bw per day (NOAEL, 100 mg/kg bw per day). No
developmental toxicity was observed, the NOAEL being 1000 mg/kg bw per
day, the highest dose tested.
The Meeting established an ADI of 0-0.1 mg/kg bw on the basis of
the NOAEL of 10 mg/kg bw per day in 1-year studies of toxicity in dogs
and a safety factor of 100.
The Meeting concluded that it was not necessary to establish an
acute reference dose because of the low acute toxicity of
pyriproxyfen.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 120 ppm, equal to 16 mg/kg bw per day (18-month study of
carcinogenicity)
Rat: 600 ppm, equal to 27 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
5000 ppm, equal to 345 mg/kg bw per day (reproductive
toxicity, two-generation study of reproductive toxicity,
highest dose tested)
100 mg/kg bw per day (developmental toxicity in a segment 3
study of developmental toxicity)
Rabbit: 100 mg/kg bw per day (maternal and reproductive toxicity in
a study of developmental toxicity)
1000 mg/kg bw per day (developmental toxicity in a study of
developmental toxicity, highest dose tested)
Dog: 10 mg/kg bw per day (1-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information valuable for continued
evaluation of the compound
Observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to
pyriproxyfen
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Slow, incomplete absorption (< 50%), rat
Dermal absorption No data (no systemic toxicity up to 1000 mg/kg bw per day by
dermal route, rat)
Distribution of total residues Highest concentrations of radiolabel in fat and, to lesser
extent, liver, rat
Potential for accumulation Possible limited accumulation in fat, rat
Rate and extent of excretion Rapid, complete, 88-96% within 48 h, primarily in faeces;
4-11% in urine, rat
Metabolism in animals Extensive. No parent compound detectable in urine; numerous
metabolites: main pathway is 4'-hydroxylation; also hydroxylation
of the pyridyl ring, ether cleavage, conjugation, mouse, rat,
goat, hen
Toxicologically significant compounds Pyriproxyfen
(animals, plants and environment)
Acute toxicity
LD50, oral > 5000 mg/kg bw, mouse, rat
LD50, dermal > 2000 mg/kg bw, mouse, rat
LC50, inhalation > 1.3 mg/L, mouse, rat
Dermal irritation Not irritating, rabbit
Ocular irritation Mildly irritating, rabbit
Dermal sensitization Not a sensitizer, guinea-pig
Short-term toxicity
Target/critical effect Mouse, rat, dog: liver, increased relative liver weight, mild
anaemia, altered lipid metabolism (increased serum cholesterol)
Lowest relevant oral NOAEL 13 weeks, rat, 24 mg/kg bw per day
Lowest relevant dermal NOAEL 21 days, rat, > 1000 mg/kg bw per day
Lowest relevant inhalation NOAEL 28-day, rat, > 1.3 mg/L
Long-term toxicity and carcinogenicity
Target/critical effect: Mouse, rat, dog: liver, increased liver weight, decreased body
weight, altered lipid metabolism (increased plasma cholesterol)
(rat, dog)
Lowest relevant NOAEL 1 year, dog, 10 mg/kg bw per day (diet)
Carcinogenicity Not carcinogenic, mouse, rat
Genotoxicity Not genotoxic
Reproductive toxicity
Reproductive target/critical effect Reduction in number of implantations and live F2 fetuses at F1
developmentally toxic dose, rat
Lowest relevant reproductive NOAEL 345 mg/kg bw per day, rat
Developmental target/critical effect Retardation of physical development in F1, rat
Lowest relevant developmental NOAEL 100 mg/kg bw per day, rat
Neurotoxicity/Delayed neurotoxicity No evidence of developmental neurobehavioural toxicity in rat. No
evidence of neurotoxicty or neuropathology in medium- or long-term
studies in mouse, rat, dog or during development in rat, rabbit
Other toxicological studies Possible enzyme inducer, at least in dogs
Medical data No data
Summary Value Study Safety factor
ADI 0-0.1 mg/kg bw 1-year, dog, toxicity 100
Acute reference dose Unnecessary
References
Cardy, R., Moore, M., Murphy, B.S., Thakur, A., Tellone, C., Ito, S.,
Lang, P., Ginevan, M., Driver, J., Stewart, R. & Wilkinson, C.
(1994) Supplemental data and review of oncogenicity study with
S-31183 (Sumilarv) in mice (MRID No. 421783-10). Unpublished
study from Technology Sciences Group Inc. Reference No.
NNT-41-0116. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Chapman, E.A, Lee, P., Virgo, D.M. & Sparrow, S. (1991) S-31183:
Toxicity study by oral (capsule) administration to beagle dogs
for 52 weeks. Unpublished study from Life Science Research Ltd.
Reference No. NNT-11-0081. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Cox, R.H., Zoetis, T., Cardy, R.H., Alsaker, R.D., Kuhlman, S.M.,
Lewis, S.A., Thakur, A.K. & Phipps, N.G. (1989) Subchronic
toxicity Sstudy with S-31183 in rat. Unpublished study from
Hazleton Laboratories America, Inc. Reference No. NNT-910045.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Cox, R.H., Zoetis, T., Voelker, R.W., Alsaker, R.D., Kuhlman, S.M.,
Lewis, S.A., Thakur, A.K. & Phipps, N.G. (1990) Subchronic
toxicity study in mice. Unpublished study from Hazleton
Laboratories America, Inc. Reference No. NNT-01-0066. Submitted
to WHO by Sumitomo Chemical Co., Ltd.
Hara, M., Katoh, H. & Yoshitake, A. (1993a) Reverse mutation test of
metabolites of pyriproxyfen, 4'-OH-Pyr, 5"-OH-Pyr, DPH-Pyr, POPA
and PYPAC, in bacterial systems. Unpublished study reference No.
NNT-30-0104. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Henderson, L.M. & Proudlock, R.J. (1989) Assessment of unscheduled DNA
repair synthesis in mammalian cells after exposure to S-31183.
Unpublished study from Huntingdon Research Centre Ltd. Reference
No. NNT-91-0053. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Hirohashi, A., Kannan, N., Kato, T. & Yamada, H. (1988) Study of
S-31183 by oral administration during the period of fetal
organogenesis in rabbits. Unpublished study reference No.
NNT-80-0033. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Isobe, N., Matsunaga, H., Kimura, K., Yoshitake, A. & Yamada, H.
(1988a) Metabolism of S31183 in rats. Unpublished study reference
No. NNM-80-0001. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Isobe, N., Matsunaga, H., Kimura, K., Yoshitake, A. & Yamada, H.
(1988b) Metabolism of S-31183 in rat (tissue distribution study).
Unpublished study reference No. NNM-800002. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Kawaguchi, S., Watanabe, T., Suzuki T., Kato, T. & Yamada, H. (1987)
Acute inhalation toxicity of S-31183 in rats. Unpublished study
reference No. NNT-70-0022. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Kawaguchi, S., Yoshioka, K., Ito, S., Suzuki, T., Kato, T. & Yamada,
H. (1988) Subacute inhalation toxicity of S-31183 in rats.
Unpublished study reference No. NNT-80-0031. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Kogiso, S., Hara, M., Yoshitake, A. & Yamada, H. (1988a) Reverse
mutation test with S31183 in bacteria systems. Unpublished study
reference No. NNT-80-0034. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Kogiso, S., Hara, M., Iwawaki, H. & Yamada, H. (1988b) In vitro
chromosomal aberration test of pyriproxyfen in Chinese hamster
ovary cells (CHO-K1). Unpublished study reference No.
NNT-80-0028J. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Kogiso, S., Yamamoto, K., Hara, M., Yoshitake, A. & Yamada, H. (1989)
In vitro chromosomal aberration test of pyriproxyfen in Chinese
hamster ovary cells (CHO-K1). Unpublished study reference No.
NNT-90-0054. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Kogiso, S., Yamada, T., Hara, M., Yoshitake, A. & Yamada, H. (1990) In
vitro gene mutation test of S-31183 in V79 Chinese hamster cells.
Unpublished study reference No. NNT-000067. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Kogiso, S. Kato, H. & Nakattuka, S. (1992) Reverse mutation test with
S-31183 in bacteria systems. Unpublished study reference No.
NNT-80-0088J. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Koyama, Y., Kimura, J., Yoshioka, K., Watanabe, T., Seki, T.,
Hosokawa, S., Yamada, H. & Hagiwara, H. (1989) A six-month
chronic dietary toxicity study of pyriproxyfen in rats.
J. Toxicol. Sci., 14, 43-64.
Matsunaga, H., Yoshino, H., Isobe, N., Kaneko, H., Nakatuka, I. &
Yamada, H. (1995) Metabolism of pyriproxyfen in rats. 1.
Absorption, disposition, excretion, and biotransformation studies
with [phenoxyphenyl-14C] pyriproxyfen. J. Agric. Food Chem.,
43, 235-240.
Misaki, Y. & Nakatuka, I. (1993) Acute oral toxicity study of
pyriproxyphen metabolites, 4'-OH-Pyr, 5"-OH-Pyr, DPH-Pyr, POPA
and PYPAC in mice. Unpublished study reference No. NNT-30-0107.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Mitchell, D.J., Virgo, D.M., Broadmeadow, A., Chase, K.R., Lee, P. &
Sparrow, S. (1993) S31183: Toxicity study by oral (capsule)
administration to beagle dogs for 52 weeks (additional
investigation). Unpublished study from Life Science Research Ltd.
Reference No. NNT-31-0102. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Moore, M.R., Zoetis, T., Doyle, D., Cardy, R.H., Pearson, R.C.,
Walker, M.D., Lewis, S.A., Thomas, D.L., Thakur, A.K., Burlew,
P.L., Hatcher, C.F. & Vegarra, M. (1993) 21-day dermal toxicity
study in rats with S-31183. Unpublished study from Hazleton
Washington, Inc. Reference No. NNT-31-0094. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Nakano, M., Yamamoto, T., Kato, T. & Miyamoto, J. (1986) Acute oral
toxicity study of S31183 in dogs. Unpublished study reference No.
NNT-60-0012. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Nakano, M., Kohda, A., Kato, T. & Yamada, H. (1988) Three-month oral
toxicity study of S31183 in dogs. Unpublished study reference No.
NNT-80-0037. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Osheroff, M.R., Ziegler, K.A., Cardy, R.H., Alsaker, R.D., Kuhlman,
S.M., Lewis, S.A., Thakur, A.K., Burlew, P.L. & Nasca, A.J.
(1991a) Oncogenicity study in mice with S31183. Unpublished study
from Hazleton Laboratories America, Inc. Reference No.
NNT-11-0084. Submitted to WHO by Sumitomo Chemical Co.
Osheroff, M.R., Ziegler, K.A., Machotka, S., Alsaker, R.D., Kuhlman,
S.M., Lewis, S.A., Thakur, A.K., Burlew, P.L., Devis, P.J. &
Graham, R. (1991b) Combined chronic toxicity and oncogenicity
study in rats with S-31183. Unpublished study from Hazleton
Washington, Inc. Reference No. NNT-11-0085. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Panthani, A.M., Walsh, K.J. & Turck, P. (1996a) Metabolism of
[phenoxyphenyl-14C]S71639 (pyriproxyfen) in lactating goats.
Unpublished study from Ricerca, Inc. Reference No. NNM-0043.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Panthani, A.M., Walsh, K.J. & Turck, P. (1996b) Metabolism of
[pyridyl-14C]S-7 1639 (pyriproxyfen) in lactating goats.
Unpublished study from Ricerca, Inc. Reference No. NNM-0046.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Panthani, A.M., DiFrancesco, P. & Savides, M.C. (1996c) Metabolism of
[phenoxyphenyl14C]S-71639 (pyriproxyfen) in laying hens.
Unpublished study from Ricerca, Inc. Reference No. NNM-0045.
Submitted to WHO by Sumitomo Chemical Co., Ltd.
Panthani, A.M., DiFrancesco, P. & Savides, M.C. (1996d) Metabolism of
[pyridyl-14C]S71639 (pyriproxyfen) in laying hens. Unpublished
study from Ricerca, Inc. Reference No. NNM-0044. Submitted to WHO
by Sumitomo Chemical Co., Ltd.
Proudlock, R.J., Haynes, P. & Goodenough, A.J. (1991) Mouse
micronucleus test on S31183. Unpublished study from Huntingdon
Research Centre Ltd. Reference No. NNT11-0082. Submitted to WHO
by Sumitomo Chemical Co., Ltd.
Robinson, K., Washer, G. & Noveroske, J. (1991) A dietary 2-generation
(1 litter) reproduction study of S-31183 in the rat. Unpublished
study from Bio-Research Laboratories Ltd. Reference No.
NNT-11-0087. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Saegusa, T., Kitajima, S. & Narama, I. (1988a) Study on administration
of a test substance prior to and in the early stages of pregnancy
in rats. Unpublished study from Hamamatsu, Seigiken Research Co.,
Ltd. Reference No. NNT-80-0036. Submitted to WHO by Sumitomo
Chemical Co., Ltd.
Saegusa, T., Kitajima, S. & Narama, I. (1988b) Study on administration
of S-31183 during the perinatal and lactation periods in rats.
Unpublished study from Hamamatsu, Seigiken Research Co., Ltd.
Reference No. NNT-80-0030. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Saegusa, T., Kitajima, S. & Narama, I. (1988c) Study by administration
of S-31183 during the period of fetal organogenesis in rats.
Unpublished study from Hamamatsu, Seigiken Research Co., Ltd.
Reference No. NNT-80-0029. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Sako, H., Okuno, Y., Kato, T. & Yamada, H. (1987a) Acute
oral toxicity of S31183 in mice. Unpublished study reference No.
NNT-70-0014. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Suzuki, T., Misaki, Y., Okuno, Y., Kato, T. & Miyamoto, J. (1987b)
Acute oral toxicity of S31183 in rats, Unpublished study
reference No. NNT-70-0005. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Sako, H., Okuno, Y., Kato, T. & Yamada, H. (1987c) Acute
dermal toxicity of S31183 in mice. Unpublished study reference
No. NNT-70-0015. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Suzuki, T., Misaki, Y., Okuno, Y., Kato., T. & Miyamoto, J. (1987d)
Acute dermal toxicity of S-31183 in rats. Unpublished study
reference No. NNT-70-0006. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Kawaguchi, S., Watanabe, T., Kato, T. & Yamada, H. (1987e)
Acute inhalation toxicity of S-31183 in mice Unpublished study
reference No. NNT-70-0023. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Nakanishi, T., Kato, T. & Miyamoto, J. (1987f) Skin
sensitization test with S31183 in guinea pigs. Unpublished study
reference No. NNT-70-0003. Submitted to WHO by Sumitomo Chemical
Co., Ltd.
Suzuki, T., Nakanishi, T., Kato, T. & Miyamoto, J. (1987g) Primary eye
and skin irritation tests with S-31183 in rabbits. Unpublished
study reference No. NNT-70-000. Submitted to WHO by Sumitomo
Chemical Co., Ltd.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Yoshino, H. (1993a) Metabolism of pyriproxyfen in rat (high-dose,
14C-concentrations in tissues). Unpublished study reference No.
NNM-30-0028. Submitted to WHO by Sumitomo Chemical Co., Ltd.
Yoshino, H. (1993b) Metabolism study of
(pyridyl-2,6-14C)pyriproxyfen in rat (pyridyl14C-labeled test
compound, single oral administration at low- and high-doses).
Unpublished study reference No. NNM-30-0025. Submitted to WHO by
Sumitomo Chemical Co., Ltd.
Yoshino, H., Kaneko, H., Nakatsuka, I. & Yamada, H. (1995) Metabolism
of pyriproxyfen. 2. Comparison of in vivo metabolism between rats
and mice. J. Agric. Food Chem., 43, 2681-2686.
Yoshino, H., Kaneko, H., Nakatsuka, I. & Yamada, H. (1996) Metabolism
of pyriproxyfen. 3. In vitro metabolism in rats and mice.
J. Agric. Food Chem., 44, 1578-1581.
