
Propranolol
| 1. NAME |
| 1.1 Substance |
| 1.2 Group |
| 1.3 Synonyms |
| 1.4 Identification numbers |
| 1.4.1 CAS number |
| 1.4.2 Other numbers |
| 1.5 Main brand names, main trade names |
| 1.6 Manufacturers, Importers |
| 2. SUMMARY |
| 2.1 Main risks and target organs |
| 2.2 Summary of clinical effects |
| 2.3 Diagnosis |
| 2.4 First aid measures and management principles |
| 3. PHYSICO-CHEMICAL PROPERTIES |
| 3.1 Origin of the substance |
| 3.2 Chemical structure |
| 3.3 Physical properties |
| 3.3.1 Colour |
| 3.3.2 State/form |
| 3.3.3 Description |
| 3.4 Other characteristics |
| 3.4.1 Shelf-life of the substance |
| 3.4.2 Storage conditions |
| 4. USES |
| 4.1 Indications |
| 4.1.1 Indications |
| 4.1.2 Description |
| 4.2 Therapeutic dosage |
| 4.2.1 Adults |
| 4.2.2 Children |
| 4.3 Contraindications |
| 5. ROUTES OF ENTRY |
| 5.1 Oral |
| 5.2 Inhalation |
| 5.3 Dermal |
| 5.4 Eye |
| 5.5 Parenteral |
| 5.6 Other |
| 6. KINETICS |
| 6.1 Absorption by route of exposure |
| 6.2 Distribution by route of exposure |
| 6.3 Biological half-life by route of exposure |
| 6.4 Metabolism |
| 6.5 Elimination by route of exposure |
| 7. PHARMACOLOGY AND TOXICOLOGY |
| 7.1 Mode of action |
| 7.1.1 Toxicodynamics |
| 7.1.2 Pharmacodynamics |
| 7.2 Toxicity |
| 7.2.1 Human data |
| 7.2.1.1 Adults |
| 7.2.1.2 Children |
| 7.2.2 Relevant animal data |
| 7.2.3 Relevant in vitro data |
| 7.3 Carcinogenicity |
| 7.4 Teratogenicity |
| 7.5 Mutagenicity |
| 7.6 Interactions |
| 7.7 Main adverse effects |
| 8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS |
| 8.1 Material sampling plan |
| 8.1.1 Sampling and specimen collection |
| 8.1.1.1 Toxicological analyses |
| 8.1.1.2 Biomedical analyses |
| 8.1.1.3 Arterial blood gas analysis |
| 8.1.1.4 Haematological analyses |
| 8.1.1.5 Other (unspecified) analyses |
| 8.1.2 Storage of laboratory samples and specimens |
| 8.1.2.1 Toxicological analyses |
| 8.1.2.2 Biomedical analyses |
| 8.1.2.3 Arterial blood gas analysis |
| 8.1.2.4 Haematological analyses |
| 8.1.2.5 Other (unspecified) analyses |
| 8.1.3 Transport of laboratory samples and specimens |
| 8.1.3.1 Toxicological analyses |
| 8.1.3.2 Biomedical analyses |
| 8.1.3.3 Arterial blood gas analysis |
| 8.1.3.4 Haematological analyses |
| 8.1.3.5 Other (unspecified) analyses |
| 8.2 Toxicological analyses and their interpretation |
| 8.2.1 Tests on toxic ingredient(s) of material |
| 8.2.1.1 Simple qualitative test(s) |
| 8.2.1.2 Advanced qualitative confirmation test(s) |
| 8.2.1.3 Simple quantitative method(s) |
| 8.2.1.4 Advanced quantitative method(s) |
| 8.2.2 Tests for biological specimens |
| 8.2.2.1 Simple qualitative test(s) |
| 8.2.2.2 Advanced qualitative confirmation test(s) |
| 8.2.2.3 Simple quantitative method(s) |
| 8.2.2.4 Advanced quantitative method(s) |
| 8.2.2.5 Other dedicated method(s) |
| 8.2.3 Interpretation of toxicological analyses |
| 8.3 Biomedical investigations and their interpretation |
| 8.3.1 Biochemical analysis |
| 8.3.1.1 Blood, plasma or serum |
| 8.3.1.2 Urine |
| 8.3.1.3 Other fluids |
| 8.3.2 Arterial blood gas analyses |
| 8.3.3 Haematological analyses |
| 8.3.4 Interpretation of biomedical investigations |
| 8.4 Other biomedical (diagnostic) investigations and their interpretation |
| 8.5 Overall Interpretation of all toxicological analyses and toxicological investigations |
| 9. CLINICAL EFFECTS |
| 9.1 Acute poisoning |
| 9.1.1 Ingestion |
| 9.1.2 Inhalation |
| 9.1.3 Skin exposure |
| 9.1.4 Eye contact |
| 9.1.5 Parenteral exposure |
| 9.1.6 Other |
| 9.2 Chronic poisoning |
| 9.2.1 Ingestion |
| 9.2.2 Inhalation |
| 9.2.3 Skin exposure |
| 9.2.4 Eye contact |
| 9.2.5 Parenteral exposure |
| 9.2.6 Other |
| 9.3 Course, prognosis, cause of death |
| 9.4 Systematic description of clinical effects |
| 9.4.1 Cardiovascular |
| 9.4.2 Respiratory |
| 9.4.3 Neurological |
| 9.4.3.1 CNS |
| 9.4.3.2 Peripheral nervous system |
| 9.4.3.3 Autonomic nervous system |
| 9.4.3.4 keletal and smooth muscle |
| 9.4.4 Gastrointestinal |
| 9.4.5 Hepatic |
| 9.4.6 Urinary |
| 9.4.6.1 Renal |
| 9.4.6.2 Other |
| 9.4.7 Endocrine and reproductive systems |
| 9.4.8 Dermatological |
| 9.4.9 Eye, ear, nose, throat: local effects |
| 9.4.10 Haematological |
| 9.4.11 Immunological |
| 9.4.12 Metabolic |
| 9.4.12.1 Acid-base disturbances |
| 9.4.12.2 Fluid and electrolyte disturbances |
| 9.4.12.3 Others |
| 9.4.13 Allergic reactions |
| 9.4.14 Other clinical effects |
| 9.4.15 Special risks |
| 9.5 Other |
| 9.6 Summary |
| 10. MANAGEMENT |
| 10.1 General principles |
| 10.2 Life supportive procedures and symptomatic/specific treatment |
| 10.4 Decontamination |
| 10.5 Elimination |
| 10.6 Antidote treatment |
| 10.6.1 Adults |
| 10.6.2 Children |
| 10.7 Management discussion |
| 11. ILLUSTRATIVE CASES |
| 11.1 Case reports from literature |
| 12. Additional information |
| 12.1 Specific preventive measures |
| 12.2 Other |
| 13. REFERENCES |
| 14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE ADDRESS(ES) |
PROPRANOLOL
International Programme on Chemical Safety
Poisons Information Monograph 441
Pharmaceutical
1. NAME
1.1 Substance
Propranolol
1.2 Group
Beta-blocker
Beta-adrenergic receptor blocking agent
Class II antiarrhythmic drug
1.3 Synonyms
1-isopropylamine-3-(1-naphtylhoxy)-2 propanol;
1-isopropylamine-3-(alpha naphthoxy)-2 propanol-hydrochloride;
Propanolol;
1-isopropylamino-3-(1-naphthyloxy)propan-2-ol;
1-isopropylamino-3-(1-naphthyloxy)propan-2-olhydrochloride;
Dociton;
ICI 45520;
Inderal;
Inderal hydrochloride;
Inderol;
Propranolol hydrochloride;
Propranolon hydrochloride;
1.4 Identification numbers
1.4.1 CAS number
525-66-6
1.4.2 Other numbers
318-98-9
AY - 64043
ICI - 45520
NCS - 91523
RTECS: UB 7500000 propranolol base
RTECS: UB 7525000 propranolol hydrochloride
1.5 Main brand names, main trade names
Angilol (United Kingdom); Arcablock-Retard-Capsules
(Austria); Apsolol (United Kingdom); Avlocrdyl (France);
Bedranol (Switzerland - United Kingdom); Beprane (France);
Berkolol (United Kingdom); Beta-Neg (Italy); Betaryl
(France); Beta-tablinen (Germany); Blocardyl (Argentina);
Cardinol (Australia); Cadispare (South Africa); Caridolol
(Japan); Corotrend (Switzerland); Deralin (Australia,Israel);
Detensol (Canada); Dideral (Turkey); Dociton (Germany);
Efektolol (Germany); Elbrol (Germany); Euprovasin (Italy);
Frekven (Denmark; Norway; Sweden); Herzbase (Japan); Herzul
(Japan); Ikopal (Iceland); Indéral (Argentina; Australia;
Belgium; Canada; Denmark; Italy, Japan; Nether Lands; Norway;
South Africa; Sweden; Switzerland; United Kingdom; United
States of America); Inderalici (Italy); Indobloc (Germany);
Kemi (Japan); Nedis (Argentina); Noloten (Argentina);
Novopralol (Canada); Obsidan (Germany); Oposim (Argentina);
Pranolol (Norway); Prano-puren (Germany); Prolol (Australia);
Pronovan (Norway); Propabloc (Germany); Propalong
(Argentina); Propanur (Germany); Propra-ratiopharm (Germany);
Pur bloka (South Africa); Pylapron (Japan); Reducor (Turkey);
Rexigen (South Africa); Sagittol (Germany); Sawatal (Japan);
Sumial (Spain); Tensiflex (Argentina); Teenol (Japan); Tonum
(Italy).
1.6 Manufacturers, Importers
No data available.
2. SUMMARY
2.1 Main risks and target organs
Beta-blockers compete with endogenous and/or exogenous
beta-adrenergic agonists. Propranolol is not cardioselective
and it has no intrinsic sympathomimetic activity. It has
membrane stabilizing properties and is highly lipid soluble.
At toxic doses, propranolol has a pronounced negative
chronotropic and inotropic effect and also a quinidine-like
effect on the heart.
The cardiovascular system is the main target organ.
Propranolol decreases sinus rate, atrio-ventricular
conduction, intraventricular conduction and cardiac
contractility. Central nervous system toxicity (coma and
convulsions) may also occur because of its high
liposolubility.
2.2 Summary of clinical effects
Toxicity occurs within 1 to 2 hours following ingestion
but the delay in onset may vary according to the
formulation.
Symptoms may include:
* Cardiovascular disturbances: bradycardia, atrioventricular
block of varying degrees, intraventricular block,
hypotension, cardiogenic shock, pulmonary oedema
* Neurological symptoms: coma and convulsions
* Respiratory depression and apnoea.
* Cardiovascular collapse and apnoea may occur suddenly.
Patients with underlying cardiovascular disease are
predisposed to the adverse cardiac effects of propranolol.
Propranolol may induce bronchospasm in asthmatic
patients.
2.3 Diagnosis
Nausea, vomiting, bradycardia and hypotension are the
early features of propranolol poisoning. In severe cases
coma, convulsions, shock and respiratory depression may be
present.
Blood pressure monitoring and ECG are the relevant
investigations to detect cardiotoxicity. The ECG may show
bradycardia, AV block, widened QRS complex and bundle branch
block.
Measurement of propranolol concentration may be helpful for
diagnosis but is not useful for clinical management.
2.4 First aid measures and management principles
Patients with propranolol poisoning should be monitored
closely preferably in an intensive care unit.
Monitor vital signs: ECG, blood pressure, respiration
Treatment may include:
* gastric lavage, emesis only after immediate ingestion, oral
activated charcoal.
* supportive treatment of respiratory failure by artificial
ventilation.
* symptomatic treatment for convulsions with diazepam, and
shock with dopamine or norepinephrine.
* atropine in case of symptomatic bradycardia
* isoproterenol and/or glucagon act as antidote and should be
administered if atrioventricular block or shock are
present.
3. PHYSICO-CHEMICAL PROPERTIES
3.1 Origin of the substance
Synthetic substance.
3.2 Chemical structure
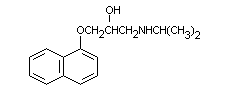 Propranolol: C16H21NO2
Molecular Weight = 259.35
Propranolol hydrochloride: C16H21NO2HCl
Molecular Weight = 295.84
3.3 Physical properties
3.3.1 Colour
White
3.3.2 State/form
3.3.3 Description
White, odourless crystalline powder. Soluble 1
in 20 of water or alcohol; slightly soluble in
chloroform; practically insoluble in ether.
Only stable at acidic pH; decomposes rapidly when
alkaline. Solutions are most stable at pH 3; in
aqueous solutions propranolol decomposes with
oxidation of the isopropylamine side-chain
(Martindale). Propranolol is a racemic mixture of
dextrorotary and levorotary forms.
3.4 Other characteristics
3.4.1 Shelf-life of the substance
No data available.
3.4.2 Storage conditions
Store in well-closed containers, protect from light.
4. USES
4.1 Indications
4.1.1 Indications
4.1.2 Description
Cardiovascular diseases
Propranolol, a non cardioselective beta-blocker, is
mostly used in the treatment of hypertension, angina,
for the prevention of re-infarction in patients who
have suffered from myocardial infarction. It is also
used to control symptoms of anxiety and in the
treatment of supraventricular tachycardia,
hypertrophic obstructive cardiomyopathy and tetralogy
of Fallot.
Endocrine disorders
In hyperthyroidism and thyrotoxic crisis; together
with alpha blocking agents in the preoperative
treatment of phaeochromocytoma.
Hepatic diseases
Prevention of haemorrhage in portal hypertension
Neurological disorders
Propranolol has also been used in the treatment of
extrapyramidal disorders and in the prophylaxis of
migraine headache.
Anxiety disorders
Propranolol may be used in acute stress reactions,
somatic anxiety and panic reactions, but its value is
questioned (Reynolds, 1993).
4.2 Therapeutic dosage
4.2.1 Adults
Propranolol dosing should be initiated at low
doses and gradually increased until the desired
therapeutic effect is achieved.
Oral administration
In cardiovascular diseases: the initial dose of 40 mg
twice daily, may be increased at weekly intervals to a
maintenance dose of 160 to 260 mg daily (some patients
require 320 mg daily).
In prevention of haemorrhage due to portal
hypertension: 20 mg twice daily.
In migraine headache: 40 to 120 mg daily
Intravenous administration
The optimal dose is 1 to 3 mg (inject slowly: 1
mg/minute maximum). The total dose should not exceed 5
to 10 mg.
4.2.2 Children
Oral administration
0.25 to 0.5 mg/kg/24 hours administered in divided
doses (3 or 4 times daily up to 1 mg/kg/24 hours)
Intravenous administration
0.025 to 0.05 mg/kg; 3 or 4 times daily under ECG
control
Caution: Propranolol should never be discontinued
abruptly in patients with coronary artery disease.
Gradually decrease the dosage.
4.3 Contraindications
Absolute: asthma, congestive cardiac failure,
atrio-ventricular block, bradycardia (below 50/minute),
treatment with amiodarone.
Relative: Raynaud's disease, diabetes mellitus.
5. ROUTES OF ENTRY
5.1 Oral
Ingestion is the most frequent cause of poisoning
5.2 Inhalation
No case has been reported. The effect of 10 mg
propanolol given by nasal route is rapid and equivalent to
the intravenous route (Hussain et al., 1980).
5.3 Dermal
No data available.
5.4 Eye
No data available.
5.5 Parenteral
No case of overdoses has been reported. Cardiovascular
symptoms have been reported after therapeutic
administration.
5.6 Other
No data available.
6. KINETICS
6.1 Absorption by route of exposure
After oral administration, propranolol is almost
completely and rapidly absorbed from the gastrointestinal
tract. However, because of the high first-pass metabolism and
hepatic tissue binding, the absolute bioavailability is only
about 30% and varies greatly between individuals. Peak plasma
concentration occurs one to two hours after administration
(Frishman et al., 1979; Goodman & Gilman, 1985).
After administration of the sustained release formulation,
the peak plasma concentration occurs 7 hours after
absorption.
6.2 Distribution by route of exposure
About 90 to 95 % of the drug is bound to plasma proteins.
The volume of distribution is 300 L/1.73 M2 or 3.9 L/kg
(approximately 200 L in an adult).
Propranolol is highly lipophilic: it crosses the blood-brain
barrier and the placenta: the ratio of concentration in the
blood between the foetus and the mother is 1.5.
6.3 Biological half-life by route of exposure
After oral administration, propranolol undergoes
saturable kinetics.
The plasma half-life is 3 to 6 hours and is about 12 hours
with the sustained release forms. The total body clearance is
800 mL/minute/1.73 m2.
After overdose, the plasma half-life is prolonged. Halloran
and Phillips (1981) reported a half-life of 16 hours. In two
cases reported by Tzen War Chen et al. (1986), the half-life
was 13.8 (ingestion of 3.12 g) and 8.3 hours (ingestion of
0.28 g). In five cases studied by Jaeger et al. (1990), the
mean plasma half-life was mean 10.5 hours (range: 5.1 to
17).
6.4 Metabolism
Propranolol is extensively metabolized by the liver. At
least one of the metabolites, the 4-hydroxypropranolol, is
biologically active. The hepatic metabolism is saturable and
bioavailability may be increased in overdoses.
6.5 Elimination by route of exposure
After a single oral dose, propranolol is completely
eliminated in 48 hours, mainly by hepatic metabolism. Less
than 0.5 % is excreted unchanged in urine. The renal
clearance is 12 mL/kg/minute. About 20% of the dose is
eliminated in urine mainly as glucuronide conjugates.
Propranolol is excreted in breast milk at a concentration of
50% that of blood.
Dialysis clearance is about 20 mL/minute with a blood flow of
250 mL/minute.
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
Propranolol is a non cardioselective
beta-blocker with no intrinsic sympathomimetic. It
has membrane stabilizing activity and is highly lipid
soluble. At toxic doses, propranolol has a pronounced
negative chronotropic and inotropic effect and a
quinidine-like effect on the heart: the result is a
reduction of the heart rate, a decrease of the
sino-atrial and atrioventricular conduction, a
prolongation of the intraventricular conduction and a
decrease of cardiac output. Blockade of beta-2
receptors may cause bronchospasm and hypoglycaemia.
Given its high lipid solubility, propranolol crosses
the blood-brain barrier and may cause coma and
convulsions (Langemeijer et al., 1986; de Wildt et
al., 1984).
7.1.2 Pharmacodynamics
Beta-blocking agents compete with endogenous
and/or exogenous beta-adrenergic agonists. Their
specific effects depend on their selectivity for
beta-1 receptors (located in the heart) or beta-2
receptors (located in bronchi, blood vessels, stomach,
gut, uterus). Beta-blockers are classified according
to their cardioselectivity, membrane stabilizing
effect, intrinsic sympathomimetic effect and lipid
solubility (Critchley & Ungar, 1989; Ellenhorn &
Barceloux, 1988; Frishman & Sivermon, 1979; Frishman,
1979; Goodman & Gilman, 1985; Weinstein, 1984).
At therapeutic doses, propranolol slightly decreases
heart rate (15%), supraventricular conduction and
cardiac output (15 to 20%) (Johnson et al., 1969).
Cardiac work and oxygen consumption are also
decreased. Propranolol decreases the secretion of
renin.
The pharmaceutical form of propranolol is a racemate:
the dextrorotary isomer accounts for most of the
beta-blocking effect, whereas the levorotary isomer
has a predominantly membrane stabilizing
effect.
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
Propranolol toxicity shows
individual variations which may be due to an
underlying cardiac disease, to the ingestion
of other cardiotoxic drugs and to variations
in first-pass metabolism.
The toxic dose is about 1 g. In 104 cases
reported in literature the mean toxic (but
non lethal) dose was 1.75 g (Gross 1991)
although survival has been reported after
ingestion of 5 to 8 g (Lagerfeldt & Matell,
1982; Tynan et al., 1981). Khan & Miller
(1985) reported survival of a 28-year-old man
following ingestion of 3 g.
The minimal lethal dose reported was 1.6 g in
a 57-year-old man (Auzepy et al., 1983). The
mean lethal dose in 17 cases reported in the
literature was 5.85 g although there is wide
interindividual variation (Gross,
1991).
7.2.1.2 Children
Ingestion of 70 mg by a 2-year-old
child produced drowsiness, 2nd degree
atrioventricular block and hypoglycaemia of
0.14 g/L (Hesse & Pedersen, 1973). Ingestion
of 100 mg by a 5-year-old child produced
drowsiness, delirium and hallucinations (Eibs
et al., 1982). An ingestion of 400 to 1,200
mg by a 3-year-old boy was uneventful after
early induced vomiting: the plasma level was
2.29 µg/L (Artman et al., 1982).
7.2.2 Relevant animal data
In mouse the LD50 are:
565 mg/kg after oral route
22 to 35 mg/kg after intravenous route
107 mg/kg after intraperitoneal route
(RETCS, 1985)
7.2.3 Relevant in vitro data
No data available.
7.3 Carcinogenicity
No data available.
7.4 Teratogenicity
No confirmed reports of teratogenic effect.
7.5 Mutagenicity
No data available.
7.6 Interactions
Decreased bioavailability
Antacids decrease the gastric absorption of propranolol.
Barbiturates, phenytoïn and rifampicin increase the
first-pass clearance of propranolol by hepatic enzyme
induction (Sotanieni et al., 1979).
Increased bioavailability
Plasma propranolol concentrations may be increased up to 50%
by histamine H2 antagonists and oral contraceptives, which
decrease hepatic metabolism by enzyme inhibition.
Diminished pharmacodynamic effects
Non-steroidal anti-inflammatory drugs decrease the
antihypertensive effect of propranolol. Nifedipine might
exacerbate the symptoms of beta-blocker withdrawal.
Enhanced pharmacodynamic effects
Digitalis, amiodarone, verapamil and diltiazem may increase
bradycardia due to propranolol. Verapamil, prenylamine,
flecainide and disopyramide enhance the negative inotropic
effect of propranolol.
7.7 Main adverse effects
Numerous adverse effects during propranolol treatment
have been reported (Reynolds, 1993; Drugdex, 1991; Dukes,
1984; Meyler et al., 1974; Goodman & Gillman, 1985).
* Cardiovascular: sinus bradycardia, atrioventricular block,
hypotension, increase of left ventricular failure,
cardiogenic shock, intermittent claudication.
* Respiratory: bronchospasm, exacerbation of asthmatic
symptoms in known asthmatics, pulmonary oedema.
* Central nervous system: depression, psychosis, convulsions,
hallucinations.
* Musculoskeletal: muscle weakness, aggravation of myasthenia
gravis, peripheral neuropathy.
* Gastrointestinal: vomiting, diarrhoea, dry mouth.
* Endocrine and metabolic: hypoglycaemia, hyperkalaemia,
hypothyroidism, sexual dysfunction (impotence).
* Dermatological: urticaria, exfoliative dermatitis.
* Haematological: agranulocytosis (immunologic reaction),
thrombocytopenia.
* Teratogenicity: a case of tracheoesophageal fistula in a
newborn of a mother treated with propranolol during the
pregnancy has been reported by Campbell, 1985. However, a
teratogenic effect of propranolol has not been
confirmed.
* Pregnancy: hypoglycaemia and lethargy have been reported in
newborn from mothers treated with propranolol before
delivery.
* Others: propranolol treatment may potentiate anaphylactic
shock.
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
Toxic ingredient: liquids, capsules,
scene residues
In case of ingestion:
Vomitus: total amount
Gastric aspirate: total amount (or gastric
lavage: first portion: 100 mL)
Whole blood without additives: 10 mL
Urine: random specimen: 50 mL
8.1.1.2 Biomedical analyses
Plasma (lithium heparin as
anticoagulant) or serum and urine for
standard biochemical analyses.
8.1.1.3 Arterial blood gas analysis
Heparinized arterial blood sample
(in severe cases).
8.1.1.4 Haematological analyses
Anticoagulated blood (e.g. EDTA) for
standard haematological analyses and
differential blood picture.
8.1.1.5 Other (unspecified) analyses
No further materials.
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
Some authors suggest the use of
antioxidants (ascorbic acid, sodium
metabisulphite) as stabilizers for
4-hydroxypropranolol, but loss appears to be
insignificant in samples stored at -30°C
without additives (Fu & Mason,
1989).
8.1.2.2 Biomedical analyses
No special requirements, but as
usually performed.
8.1.2.3 Arterial blood gas analysis
No special requirements, but as
usually performed.
8.1.2.4 Haematological analyses
No special requirements, but as
usually performed.
8.1.2.5 Other (unspecified) analyses
Serum, urine and other materials in
a refrigerator (4°C).
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
Some authors suggest the use of
antioxidants (ascorbic acid, sodium
metabisulphite) as stabilizers for
4-hydroxypropranolol, but loss appears to be
insignificant in samples stored at -30°C
without additives (Fu & Mason,
1989).
8.1.3.2 Biomedical analyses
No special requirements, but as
usually performed.
8.1.3.3 Arterial blood gas analysis
No special requirements, but as
usually performed.
8.1.3.4 Haematological analyses
No special requirements, but as
usually performed.
8.1.3.5 Other (unspecified) analyses
Not applicable.
8.2 Toxicological analyses and their interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple qualitative test(s)
8.2.1.2 Advanced qualitative confirmation test(s)
8.2.1.3 Simple quantitative method(s)
8.2.1.4 Advanced quantitative method(s)
8.2.2 Tests for biological specimens
8.2.2.1 Simple qualitative test(s)
8.2.2.2 Advanced qualitative confirmation test(s)
8.2.2.3 Simple quantitative method(s)
8.2.2.4 Advanced quantitative method(s)
8.2.2.5 Other dedicated method(s)
8.2.3 Interpretation of toxicological analyses
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
Sodium, potassium, chloride,
calcium, magnesium Glucose, amylase, creatine
kinase, creatinine (urea)
Optional: bicarbonate (or total CO2), uric
acid
8.3.1.2 Urine
8.3.1.3 Other fluids
No dedicated test.
8.3.2 Arterial blood gas analyses
8.3.3 Haematological analyses
8.3.4 Interpretation of biomedical investigations
8.4 Other biomedical (diagnostic) investigations and their
interpretation
Measurement of serum electrolytes, glucose, creatinine
and urea nitrogen, and arterial blood gases are useful to
optimize supportive care.
8.5 Overall Interpretation of all toxicological analyses and
toxicological investigations
Analysis of Materials
Propranolol can be detected and identified in materials by a
number of simple techniques. Marquis and Mandelin's test
both give a green-blue reaction (Moffat et al., 1986). The
spectrum in methanol gives deltamax at 290 nm (A| = 240), 306
nm (A| = 143), and 319 nm (A| = 86); the pattern in dilute
acid is similar. Thin layer chromatography is useful for
propranolol analysis, and the drug can be visualized as a
green-blue spot with Mandelin's reagent. The Rf is 0.50 in
both methanol / ammonia (100:1.5) and in ethyl acetate /
methanol / ammonia (85:15:5) (Moffat et al., 1986). Gas
chromatography is also useful, and the retention index for
propranolol is 2150 on OV1, SE30, SE54, HP5 or similar; FID
gives sufficient sensitivity for residues, but
nitrogen-phosphorus detection gives improved sensitivity and
specificity. GCMS may also be used for underivatized
propranolol, although the spectrum is not characteristic.
Analysis of Biological Specimens
Analysis in body fluids is fairly straightforward, although
quantitative analysis is only rarely indicated. There are no
commercially-available immunoassay kits which respond to
propranolol. For detection in urine or plasma an extraction
into a polar solvent is required (e.g. methyl tert-butyl
ether, dichloromethane:iso-propanol or ethyl acetate) from
basic buffer, and recovery from urine may be improved by
salting out. Urinalysis is best performed by thin layer
chromatography. The Rf of propranolol is 0.50 in both
methanol / ammonia (100:1.5) and in ethyl acetate / methanol
/ ammonia (85:15:5) with a positive iodoplatinate and a
green-blue Mandelin's response. The 4-hydroxy metabolite is
at Rf 0.50 in methanol / ammonia (100:1.5), and 0.40 in ethyl
acetate / methanol / ammonia (85:10:5), and has a
characteristic pale blue response with iodoplatinate, and a
red response to Marquis reagent (personal experience). Gas
chromatography of urine generally shows only propranolol
(retention index 2150 on OV1, SE30, SE54, HP5 or similar) and
sometimes the 4-hydroxy-metabolite at retention index 2650
(see below under advanced methods). Nitrogen-phosphorus
detection is required for low therapeutic amounts, or mass
spectrometry methods are suitable.
Quantitative analysis of toxic concentrations in serum may be
performed by direct fluorimetry. Drug is extracted from
alkalinized plasma (potassium carbonate) into toluene and
re-extracted into acetic acid (0.005M) in ethylene glycol (50
g/L). Excitation is at 290 nm, and emission measured at 358
nm with a sensitivity of 0.003 mg/L from 1 mL sample (Rao et
al., 1978; Baselt, 1987). Other authors prefer scanning the
emission pattern to identify potential interferences from
quinidine and other fluorescing compounds (Kraml & Robinson,
1978). The method can also be used to quantitate
4-hydroxypropanolol at 320 nm excitation and measuring
emission at 510 nm with a sensitivity of 0.015 mg/L (Rao et
al., 1978; Baselt, 1987). Most frequently quantitation in
plasma is performed by HPLC with fluorescence detection which
gives excellent sensitivity (sub-therapeutic concentrations
on less than 0.1 mL); excitation at 205 to 215 nm, emission
measured at 340 nm. Methylpropranolol, benzimidazole or
flecainide can be used as internal standard. HPLC of
propranolol can best be achieved with silica columns with
organic mobile phases containing modifiers such as
camphorsulfonic acid or ammonium sulphate (Bhamra et al.,
1985; Rognerud & Ou, 1989). For simultaneous measurement of
propranolol and 4-hydroxypropanolol, reverse-phase systems
are required and chromatography is slower. Sensitivity is
reduced (0.01 mg/L) due to the difference in emission
wavelength for the two compounds (Sood et al., 1988; Fu &
Mason, 1989). Some authors suggest the use of antioxidants
(ascorbic acid, sodium metabisulphite) as stabilizers for
4-hydroxypropranolol, but loss appears to be insignificant in
samples stored at -30°C without additives, although loss may
occur during the extraction procedure (Fu & Mason, 1989).
Gas chromatographic methods are less often used, but are
described electron capture after derivatization with
pentafluoropropionic anhydride / pyridine (1 minute at 70°C)
(Kates & Jones, 1977) or trifluoroacetic anhydride / benzene
/ trimethylamine for 5 min at 50°C (Walle, 1974) or TFAA in
toluene for 60 minutes at room temperature (Jack & Laugher,
1985). Isothermal analysis may be performed at 200 to 250°C
depending on the column and conditions. Detection limits are
0.001 to 0.005 mg/L. None of the GC methods report data for
4-hydroxypropranolol.
Advanced Methods of Analysis
Several more complex methods describe the screening urine for
a range of ß-blockers using GCMS of TMS derivatives (Dunasia
& Houghton, 1991), or for simultaneous analysis of
propranolol and its full pattern of metabolites by GLC (Walle
& Gaffney, 1972) or HPLC (Harrison et al., 1985). Methods
are currently available for separation of the R- and
S- enantiomers of propranolol, all of which use HPLC with
fluorescence detection. Separation is achieved either on
chiral columns (Herring & Johnson, 1993), or on conventional
columns after diastereoisomerization with a chiral reagent
(Pham-Huy et al., 1994; Toyo'oka et al., 1997), or after
addition of a chiral selector to the mobile phase (Karlsson &
Pettersson, 1989). Some of these methods also measure the
4-hydroxy metabolite.
Toxicity is only poorly correlated with serum propranolol
concentrations, and measurement of vital signs gives a more
reliable index of intoxication. Survival has been reported
in a patient with a plasma concentration of 4.8 mg/L (Gross,
1991). However, the following table of typical
concentrations is given for guidance:
mg/L µmol/L
Peak (2 hours) after single oral
dose (1.2 mg/kg) 0.03 to 0.05 0.12 to 0.19
Steady-state in therapy 0.03 to 0.15 0.12 to 0.58
Toxicity apparent > 0.5 > 1.93
Life threatening toxicity > 2.0 > 7.72
4-hydroxypropranolol 10% of propranolol
propranolol glucuronide 600% of propranolol
naphthoxylacetic acid 1000% of propranolol
Measurement of serum electrolytes, glucose, creatinine and
urea nitrogen, and arterial blood gases are useful to
optimize supportive care.
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
The severity of propranolol poisoning is due to
its cardiotoxicity and depends on the dose ingested,
the presence of underlying cardiac disease and
concomitant ingestion of other cardiotoxic drugs.
Symptoms and signs appear within one to two hours and
may include the following:
* Cardiovascular effects: bradycardia, hypotension,
cardiogenic shock. The ECG may show nodal rhythm,
atrioventricular block and QRS widening
* CNS effects: lethargy, coma and convulsions,
mydriasis
* Hypoventilation resulting from severe shock
9.1.2 Inhalation
No data available.
9.1.3 Skin exposure
No data available.
9.1.4 Eye contact
No data available.
9.1.5 Parenteral exposure
Cardiovascular effects: bradycardia,
hypotension, cardiogenic shock. The ECG may show nodal
rhythm, atrioventricular block and QRS widening CNS
effects: lethargy, coma and convulsions, mydriasis
Hypoventilation resulting from severe shock.
9.1.6 Other
No data available.
9.2 Chronic poisoning
9.2.1 Ingestion
No data available.
9.2.2 Inhalation
No data available.
9.2.3 Skin exposure
No data available.
9.2.4 Eye contact
No data available.
9.2.5 Parenteral exposure
No data available.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
Patients who survive 48 hours after acute poisoning or
who have not developed cardiac arrest before admission are
likely to recover. Death may occur from cardiac asystole
which is favoured by hypoxaemia.
The prognosis depends on the dose ingested and is worse in
patients with an underlying cardiac disease and in those who
have ingested other cardiotoxic drugs (Critchley & Ungar,
1989; Ellenhorn & Barceloux, 1988; Weinstein, 1984).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Acute:
Cardiovascular symptoms are the major features of
propranolol poisoning.
Bradycardia is the commonest symptom (present in 60 to
90% of cases) and occurs soon after ingestion.
Hypotension is observed in about 50 to 70% of the
cases. Hypotension and shock are due to decreased
cardiac output and vasodilatation (Amundson & Brodine,
1988).
Cardiac arrest may occur within 1 to 2 hours of
ingestion. Cardiac arrest has been reported to occur
in 45 minutes following the ingestion of 9.4 g of
propranolol by a 60-year-old man (Jones et al.,
1982).
ECG changes are always present in symptomatic
poisoning: sinus or nodal bradycardia,
atrioventricular block (1st to 3rd degree) are the
most common. Widening of the QRS interval, bundle
branch block or increased QT interval are less
frequently observed.
Chronic: No data available.
9.4.2 Respiratory
Acute:
Respiratory depression and apnoea is mostly associated
with severe shock and is due to cerebral hypoxia (Hong
et al., 1983).
Pulmonary oedema may occur, especially in patients
with a previous compromised cardiac function.
Bronchospasm may occur in susceptible patients.
Chronic: No data available.
9.4.3 Neurological
9.4.3.1 CNS
Acute:
Lethargy, drowsiness, agitation, delirium,
hallucinations and mydriasis may be
observed.
Coma is usually only seen in patients with
cardiovascular collapse.
Convulsions have been reported after
ingestion of large doses (Das & Ferris, 1988;
Frishman et al., 1979; Tynan et al., 1981).
Convulsions may be due to hypotension or to a
direct effect of propranolol (membrane
stabilizing effect).
Chronic:
Fatigue, CNS depression, hallucinations and
psychosis have been reported.
9.4.3.2 Peripheral nervous system
No data available.
9.4.3.3 Autonomic nervous system
Acute: Effects of beta-receptor blockade.
Chronic: Effects of beta-receptor blockade.
9.4.3.4 keletal and smooth muscle
Acute: No data available.
Chronic: Muscular fatigue may be observed.
9.4.4 Gastrointestinal
Acute: vomiting, nausea may be seen; spasm of
the lower oesophageal sphincter has been reported in 2
cases (Panos et al., 1986; Laake et al., 1981). A
case of mesenteric ischaemia following propranolol
overdose has been reported (Pettei et al., 1990).
Chronic: No data available.
9.4.5 Hepatic
No data available.
9.4.6 Urinary
9.4.6.1 Renal
No data available.
9.4.6.2 Other
No data available.
9.4.7 Endocrine and reproductive systems
No data available.
9.4.8 Dermatological
No data available.
9.4.9 Eye, ear, nose, throat: local effects
Acute: Mydriasis and diplopia may be noted.
Chronic: No data available.
9.4.10 Haematological
No data available.
9.4.11 Immunological
No data available.
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Metabolic acidosis may occur in
severe poisoning with shock.
9.4.12.2 Fluid and electrolyte disturbances
Hypokalaemia (Peterson et al.,
1984) or a hyperkalaemia (Delacour et al.,
1986) have been reported rarely.
9.4.12.3 Others
Hypoglycaemia (0.14 and 0.5 g/L)
was reported in two cases of poisoning in
children (Hesse & Pedersen, 1973).
9.4.13 Allergic reactions
No data available.
9.4.14 Other clinical effects
No data available.
9.4.15 Special risks
Pregnancy: No data available.
Breast-feeding: No data available.
Enzyme deficiencies: No data available.
9.5 Other
No data available.
9.6 Summary
10. MANAGEMENT
10.1 General principles
Patients with propranolol poisoning should be monitored
closely, preferably in an intensive care unit.
Make a proper assessment of airway, breathing, circulation
and neurological status of the patient.
Monitor ECG, blood pressure and respiration.
Treatment may include:
* gastric lavage, oral activated charcoal, emesis, only after
immediate ingestion
* supportive treatment for respiratory failure by artificial
ventilation; symptomatic treatment for convulsions with
diazepam, and shock with dopamine or norepinephrine
* atropine in case of symptomatic bradycardia
* isoproterenol and/or glucagon if atrioventricular block or
shock are present
10.2 Life supportive procedures and symptomatic/specific treatment
Sinus bradycardia may be treated with intravenous
atropine 1 to 2 mg in adults (10 to 30 µg/kg in
children).
Isoproterenol is the drug of choice conduction disturbances.
Administer as a continuous infusion, beginning at a dose of
0.3 µg/kg/minute and progressing in 0.2 µg/kg/minute
increments as needed.
Glucagon may also be used but it is usually less efficient
than isoproterenol.
Transvenous cardiac pacing may be indicated if severe
bradycardia does not respond to isoproterenol or
glucagon.
Hypertonic sodium solutions (molar sodium lactate or
bicarbonate) may be indicated if there is a widening of the
QRS interval (due to the membrane stabilizing effect).
If hypotension and shock does not respond to isoproterenol
and glucagon, dopamine or norepinephrine are indicated
(Halloran & Phillips, 1981; Weinstein, 1984). If shock does
not improve, haemodynamic study by a Swan-Ganz catheter
should be performed in order to assess the treatment.
An intra-aortic balloon pump has been used in a 17-year-old
woman who had ingested 8 g propranolol and developed shock
which did not respond to isoproterenol, adrenaline and
glucagon (Lane et al., 1987).
If cardiac arrest perform external cardiac massage and
mechanical ventilation with oxygen. Adrenaline is indicated:
1 to 4 mg intravenous in an adult, 0.25 to 0.5 mg by
intratracheal route in a child.
Respiratory depression should be treated by artificial
ventilation. Bronchospasm may require treatment with beta-2
agonists or aminophylline.
If convulsions persist despite the correction of hypotension,
they may be treated by diazepam or clonazepam, neither of
which have a negative inotropic effect.
Metabolic acidosis should be treated with sodium
bicarbonate.
Correct hypo- or hyperkalaemia.
Give intravenous glucose if hypoglycaemia is present.
10.4 Decontamination
Emesis
Emesis may only be indicated in cases of recent ingestion.
However, it has been reported that induced emesis has
precipitated severe bradycardia and hypotension because it
increases the vagal tone (Soni et al., 1983; Smith et al.,
1986). Because cardiotoxic symptoms may appear rapidly and
suddenly, emesis induced by syrup of ipecac is not
recommended.
Gastric lavage
Gastric lavage may also precipitate bradycardia and
hypotension. Therefore it should be performed under strict
ECG and blood pressure monitoring. Because of the rapid
absorption of propranolol, gastric lavage should be performed
within 4 hours of ingestion.
Oral activated charcoal
Activated charcoal should be administered following gastric
lavage. Repeated doses may interfere with the enterohepatic
recirculation of propranolol. The efficiency of oral
activated charcoal has been documented for atenolol, nadolol,
pindolol and sotalol but not for propranolol (Neuvonen &
Olkkola, 1988).
10.5 Elimination
Forced diuresis
Forced diuresis is not recommended because only a very small
amount of propranolol is excreted in urine.
Haemodialysis, Haemoperfusion
Considering the pharmacokinetics of propranolol, enhanced
elimination by haemodialysis or haemoperfusion is useless and
not recommended (see section 6).
10.6 Antidote treatment
10.6.1 Adults
Atropine
Although atropine has been frequently used in the
cases reported in literature, it is the least
effective treatment (Weinstein, 1984). Atropine may
only abolish the increased vagal stimulation and is
ineffective in severe bradycardia.
Atropine may be used in sinus bradycardia: administer
1 to 2 mg intravenous in adults. If bradycardia does
not respond give isoproterenol.
Isoproterenol
Isoproterenol is a beta agonist which will
competitively antagonize the effects of the beta
blocker. It is indicated for the treatment of
conduction disturbances and shock. Administer
isoproterenol in continuous infusion. Begin at a dose
of 0.3 µg/kg/minute and progress in 0.2 µg/kg/minute
increments as needed. In several reports isoproterenol
was ineffective, but this was certainly due to
inadequate dosage (Critchley & Ungar, 1989). High
doses may be administered until the beta-blocking
effect is reversed (Rang & Dale, 1987). In the cases
reported by Halloran & Phillips (1981) and Lagerfeldt
& Matell (1976), doses of 25 to 74 µg/minute were
given. Massive propranolol ingestions reportedly
require doses of isoproterenol up to 200 µg/minute
(Agura et al., 1986) and even 800 µg/minute (Tynan et
al., 1981) for effective treatment.
Prenalterol
Prenalterol (a beta-agonist) was used successfully for
refractory hypotension unresponsive to glucagon and
vasopressor in a case of massive propranolol overdose
(Kulling et al., 1983). The recommended dose is 5 to
10 mg followed by an infusion of 5 mg/hour. Doses up
to 50 and 100 mg may be required.
Glucagon
Glucagon has positive inotropic and chronotropic
effects which are thought to be mediated by the
activation of the adenylcyclase system independently
of beta-adrenoreceptors (Kosinski & Malindzak, 1973).
It has been reported that glucagon increases
myocardial contractility in patients refractive to
isoproterenol. The dose required for a significant
myocardial action is much higher than that used to
stimulate gluconeogenesis. This may result in side
effects like nausea and vomiting. The initial dose is
50 to 150 µg/kg (5 to 10 mg) intravenously over one
minute, followed by an infusion of 1 to 5 mg/hour.
(Illingworth, 1979; Kosinski & Malindzak, 1973;
Parmley, 1971; Robson, 1980; Ward & Jones,
1976).
10.6.2 Children
Doses should be adapted according to the body
weight. No particular dosage regimen for
isoproterenol, prenalterol or glucagon have been
recommended for propranolol poisoning in
children.
10.7 Management discussion
All patients with an history of acute ingestion of
propranolol should have an ECG and be monitored for at least
6 hours.
Patients can be managed outside intensive care unit if there
is a history of recent ingestion and no cardiotoxic effects
are present.
If symptoms of cardiotoxicity are present transfer the
patient to an intensive care unit and monitor vital signs and
biochemical parameters.
Keep the patient in the intensive care unit until cardiotoxic
symptoms have disappeared.
A patient who presented with cardiotoxic effects and seizures
after propranolol overdose did not respond to adrenaline,
atropine, fluids and bicarbonate. Intravenous calcium
chloride produced a dramatic restoration of blood pressure
and a narrowing of the QRS complexes. After a bolus of 1 g
intravenous, calcium chloride was given by an infusion 0.08 g
per minute, which was reduced to 0.005 g per minute according
to the ECG pattern and the blood pressure level. The authors
believe that calcium may have a role to play in the treatment
of propranolol overdose (Brimacombe et al., 1991).
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
After poisoning with 800 mg propranolol, a 65-year-old
man was admitted two hours after ingestion. He was comatose
and had bradycardia (50/minute) and shock. The patient was
treated by artificial ventilation, atropine (ineffective),
isoproterenol (40 µg/hour) and recovered after 12 hours. Peak
serum propranolol concentration was 1536 µg/l and serum
half-life was 6.6 hours (Ducret et al., 1973).
Poisoned with 8 g propranolol, a 14-year-old girl was
admitted 35 minutes following ingestion. Her blood pressure
was 130/70 mm Hg and pulse was 34/minute. Ten minutes after
induced emesis by ipecac the patient developed sudden
hypotension and convulsions. ECG showed a second degree
atrioventricular block and a right bundle-branch block.
Treatment consisted of artificial ventilation, external
cardiac massage, atropine 1.2 mg intravenous, isoproterenol
0.4 mg intravenous and calcium gluconate 1 g intravenous.
Blood pressure increased to 110/70 mmHg with isoproterenol at
a rate of 0.8 mg/minute. Isoproterenol was discontinued seven
hours following admission and the patient was extubated
(Tynan et al., 1981).
Agura et al. reported (1986) acute poisoning in a 40-year-old
woman. The cardiogenic shock did not improve with atropine
(1 mg), adrenaline (1 mg), isoproterenol (16 to 20
µg/minute), dopamine (15 to 20 µg/minute) and noradrenaline
(10 to 12 µg/minute). Blood pressure and heart rate were only
restored by glucagon (10 mg intravenous then 5 mg/hour) and
high doses of isoproterenol (160 to 200 µg/minute).
Elkharrat & Bismuth (1982) reported 25 cases of propranolol
poisoning (1 to 2.5 g). Symptoms were present in 40 % of the
cases: sinus bradycardia in 25%, atrioventricular block in
20%, hypotension in 10% and shock in 5%. No mortality or
sequelae were noted.
Refractory hypotension following propranolol overdose was
treated with, in addition to isoprenaline and glucagon,
extracorporeal circulatory support using femoral vein
- femoral artery bypass (McVey & Corke, 1991).
12. Additional information
12.1 Specific preventive measures
No data available.
12.2 Other
No data available.
13. REFERENCES
Agura ED, Wexler LF, Witzburg RA (1986). Massive propranolol
overdose: successful treatment with high dose isoproterenol and
glucagon. Am J Med 80: 755-757.
Amundson DE, Brodine SK (1988). A fatal case of propranolol
poisoning. Drug Intell Clin Pharm 22: 781-782.
Artman M, Grayson M, Boerth R (1982). Propranolol in children:
safety/toxicity. Pediatrics 70: 30-31.
Auzepy Ph, Boukara N, Richard Ch, Giudicelli JF (1983).
Intoxications aiguës par les bêta-bloquants chez l'adulte (à
propos de sept cas). Ann Cardiol Angeiol 32: 253-258.
Baselt RC (1987) Analytical Procedures for Therapeutic Dug
Monitoring and Emergency Toxicology. 2nd ed. California,
Biomedical Publications, pp 248-249.
Bhamra RK, Flanagan RJ & Holt DW (1985) Propranolol using solvent
extraction and high-performance liquid chromatography. In:
Sunshine I ed. Methodology for Analytical Toxicology Vol III.
Boca Raton, Florida, CRC Press, pp165-168.
Brimacombe JR, Scully M, Swainston R (1991). Propranolol overdose
- a dramatic response to calcium chloride. Med J Aust 155:
267 - 268.
Campbell JW (1985). A possible teratogenic effect of propranolol.
New Engl J Med 313: 518.
Critchley JAJH, Ungar A (1989). The management of acute poisoning
due to beta-adrenoceptor antagonists. Med Toxicol 4: 32-45.
Das G, Ferris JC (1988). Generalized convulsions in a patient
receiving ultrashort-acting beta-blocker infusion. Drug Intell
Clin Pharm 22: 484-485.
Delacour JL, Blanc PL, Wagschal G, Daoudal P (1986). Hyperkaliémie
au cours d'une intoxication par a-bloqueur. Presse Med 15: 1377.
De Wildt D, Sangster B, Langemeijer J, De Groot G (1984).
Different toxicological profiles for various beta-blocking agents
on cardiac function in isolated rat hearts. Clin Toxicol 22:
115-132.
Drugdex (1991) Micromedex Inc.
Ducret F, Zech P, Pernet D, Moskovtchenko JM, Traeger J (1978).
Intoxication volontaire par le Propranolol. Nouv Presse Med 71:
27-28.
Dukes MNG (1984). Side effects of drugs. Annual 8. Elsevier Ed.,
Amsterdam.
Dunasia MC & Houghton E (1991) Screening and confirmatory
analysis of ß-agonists, ß-antagonists and their metabolites in
horse urine by capillary gas chromatography-mass spectrometry. J
Chromatogr, 564: 503-513.
Eibs HG, Oberdisse U, Brambach U (1982). Vergiftung mit
Betarezeptorenblockern bei Kindern und Jugendlichen. Monatschr
Kinderheildk, 130: 292-5
Elkharrat D, Bismuth Ch (1982). Acute intoxication by
beta-blocking agents: no mortality in 40 cases. Int J Clin Pharm
Res 2: 207-210.
Ellenhorn MJ, Barceloux DG (1988). Medical toxicology. Diagnosis
and treatment of human poisoning. Elsevier Ed., New York.
Frishman W, Jacob H, Eisenberg E (1979). Clinical pharmacology of
the new beta-adrenergic blocking drugs. Part. 8. Self-poisoning
with beta-adrenoceptor blocking agents: recognition and
management. Am Heart J 98: 798-811.
Frishman W, Silverman R (1979). Clinical pharmacology of the new
B-adrenergic blocking drugs (part 4 - adverse effects). Choosing a
B-adrenergic blocker. Am Heart J 98: 256.
Fu CW & Mason WD (1989) Determination of propranolol and
4-hydroxypropranolol in human plasma by high-performance liquid
chromatography. Analyst, 114: 1219-1223.
Goodman L.S, Gilman A (1985). The Pharmacological basis of
therapeutics. Fourth Edition, MacMillan Company, New York.
Halloran TJ, Phillips CE (1981). Propranolol intoxication. A
severe case responding to Norepinephrine therapy. Arch Intern Med
141: 810-811.
Harrison PM, Tonkin AM, Cahill CM & McLean AJ (1985) Rapid and
simultaneous extraction of propranolol, its neutral and basic
metabolites from plasma, and assay by high-performance liquid
chromatography. J Chromatogr, 343: 349-358.
Herring VL & Johnson JA (1993) Direct high-performance liquid
chromatographic determination in urine of the enantiomers of
propranolol and its major basic metabolite 4-hydroypropranolol. J
Chromatogr, 612: 215-221.
Hesse B, Pedersen JT (1973). Hypoglycaemia after Propranolol in
children Acta Med Scand 193: 551-552.
Hong CY, Yang WC, Chiang BN (1983). Importance of membrane
stabilizing effect in massive overdose of propranolol: plasma
level study in a fatal case Hum Toxicol 3: 511-517.
Hussain A, Forster T, Hirai T (1980). Nasal absorption of
propranolol in humans. J Pharma Sci 69: 1240-1242.
Illingworth RN (1979). Glucagon for beta-blocker poisoning.
Practitioner 223: 683-685.
Jack DB & Laugher SJ (1985) Metoprolol using electron capture
gas-liquid chromatography. In: Sunshine I ed. Methodology for
Analytical Toxicology Vol III. Boca Raton, Florida, CRC Press, pp
117-120.
Jaeger A, Tritsch L, Mangin P, Sauder Ph, Kopferschmitt J, Dahlet
M (1990) Toxicokinetics of beta-blockers. Proceedings of the
International Congress on Clinical Toxicology. Poison Control and
Analytical Toxicology. Lux Tox '90 p. 524.
Johnson G, De Guzman M, Bergman H, Sannerstedt R (1969). The
haemodynamic effects of Alprenolol and Propranolol at rest and
during exercise in hypertensive patients. Pharmacol Clin 2:
34-39.
Jones JW, Clark MA, Mullen BL (1982). Suicide by ingestion of
propranolol. J Forensic Sci 27: 213-216.
Karlsson A & Pettersson C (1989) Determination of (R)- and
(S)-propranolol in plasma by high-performance liquid
chromatography using N-benzoxycarbonylglycyl-L-proline as chiral
selector in the mobile phase. J Chromatogr, 494:157-171.
Kates RE & Jones CL (1977) Rapid GLC determination of propranolol
in human plasma samples. J Pharm Sci, 66: 1490-1492.
Khan MI, Miller MT (1985). Beta-blocker toxicity: the role of
Glucagon. Report of two cases. S Afr Med J 67:1062-1063.
Kosinski EJ, Malindzak GS (1973). Glucagon and Isoproterenol in
reversing propranolol toxicity. Arch Intern Med 132: 840-843.
Kraml M & Robinson WT (1978) Fluorimetry of propranolol and its
glucuronide: applicability, specificity, and limitations. Clin
Chem, 24: 169-171.
Kulling P, Eleborg L, Persson H (1983). Beta-adrenoceptor blocker
intoxication: Epidemiological data. Prenalterol as an alternative
in the treatment of cardiac dysfunction. Human Toxicol, 2:
175-181.
Laake K, Kittang D, Fefstad SO (1981). Convulsions and possible
spasm of the lower oesophageal sphincter in a fatal case of
propranolol intoxication. Acta Med Scand 210: 137-138.
Lagerfeldt J, Matell G (1976). Attempted suicide with 5.1 g of
propranolol: a case report. Acta Med Scand 199: 517-518.
Lane A, Woodward A, Goldman M (1987). Massive propranolol
overdose, poorly responsive to pharmacologic therapy: use of the
intra-aortic balloon pump. Ann Emerg Med 16: 1381-1383.
Langemeijer J, De Wildt D, De Groot G, Sangster B (1986). Calcium
interferes with the cardiodepressive effects of beta-blocker
overdose in isolated rat hearts. Clin Toxicol 24: 111-133.
McVey FK & Corke CF (1991). Extracorporeal circulation in the
management of massive propranolol overdose. Anaesthesia 46:
744-746.
Moffat AC, Jackson JV, Moss MS & Widdop B eds (1986) Clarke's
Isolation and Identification of Drugs, 2nd Edition. The
Pharmaceutical Press, London.
Neuvonen PJ, Olkkola KT (1988). Oral activated charcoal in the
treatment of intoxications. Role of single and repeated doses.
Med Toxicol 3:33-58.
Panos RJ, Tso E, Barish RA, Browne BJ (1986). Esophageal spasm
following propranolol overdose, relieved by glucagon. Am J Emerg
Med 4: 227-228.
Parmley WW (1971). The role of glucagon in cardiac therapy. New
Eng J Med 285: 801-802.
Peterson Ch D, Leeder JS, Sterner S. Glucagon therapy for B
blocker overdose (1984). Drug Intell Clin Pharm 18: 394-398.
Pettei MJ, Levy J, Abramson S. (1990) Nonocclusive mesenteric
ischaemia associated with propranolol overdose: implications
regarding splanchnic circulation. J. Pediatr. Gastroenterol. Nutr.
10: 544 - 547.
Pham-Huy C, Sahui-Gnassi A, Saada V, Grammond JP, Galons H,
Ellouk-Achard S, Levresse V, Fompeydie D & Claude JR (1994)
Microassay of propranolol enantiomers and conjugates in human
plasma and urine by high-performance liquid chromatography after
chiral derivatization for pharmacokinetic study. J Pharm Biomed
Anal, 12: 1189-1198.
Reynolds JEF (1993) Martindale, The Extra Pharmacopoeia. The
Pharmaceutical Press. London.
Rao PS, Quesada LC & Mueller HS (1978) A simple micromethod for
simultaneous determination of plasma propranolol and
4-hydroxypropranolol. Clin Chim Acta, 88: 355-361
Robson RH (1980) Glucagon for beta-blocker poisoning (letter).
Lancet 1: 1357-1358.
Rognerud CL & Ou C-N (1989) Concurrent measurement of flecainide
acetate and propranolol by normal-phase high-performance liquid
chromatography. Clin Chem 35: 857-860.
Smith RC, Wilkinson J, Hull RL (1986). Traitement du surdosage en
propranolol par le glucagon. J Am Med Assoc 255: 2025-6.
Sood SP, Green VI, Mason RP (1988) Routine methods in toxicology
and therapeutic drug monitoring by high-performance liquid
chromatography. IV. A rapid micro-scale method for determination
of propranolol and 4-hydroxypropranolol in plasma. Ther Drug
Monit, 10: 224-230.
Soni N, Barnier D, Pearson IY (1983). Cardiovascular collapse and
propranolol overdose. Med J Aust 2: 629-630.
Toyo'oka T, Toriumi M & Ishii Y (1997) Enantioseparation of
beta-blockers labelled with a chiral fluorescent reagent,
R(-)-DBD-PyNCS, by reversed-phase liquid chromatography. J Pharm
Biomed Anal, 15: 1467-1476.
Tynan F, Fischer MMc D, Ibels L (1981). Self poisoning with
propranolol. Med J Austr 1: 82-83.
Walle T (1974) GLC determination of propranolol, other ß-blocking
drugs, and metabolites in biological fluids and tissues. J Pharm
Sci, 63: 1885-1891.
Walle T & Gaffney TE (1972) Propranolol metabolism in man and
dog: mass spectrophotometric identification of six new
metabolites. J Pharmacol Expt Therapeutics, 182: 83-92.
Ward DE, Jones B (1976). Glucagon and Beta-blocker toxicity. Br
Med J 2: 151.
Weinstein RS (1984). Recognition and management of poisoning with
beta-adrenergic blocking agents. Ann Emerg Med 13:
1123-1131.
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE
ADDRESS(ES)
Authors: L. Tritsch, M. Dahlet, Ph, Sauder,
J. Kopferschmitt, F. Flesch, A. Jaeger.
Service de Réanimation Médicale et Centre
Anti Poisons
CHU, Pavillon Pasteur
67091 Strasbourg Cedex
France
Tel: 33-88161144
Fax: 33-88161330
Date: 27 March 1991
Peer
Review: Adelaide, Australia, April 1991
Finalized
IPCS: May 1994
Author
Section 8: Dr S. Dawling
Center for Clinical Toxicology
Vanderbilt University Medical Center
501 Oxford House
1161 21st Avenue South
Nashville, TN 37232-4632
United States of America
Tel: 1-615-9360760
Fax: 1-615-9360756
E-mail: sheila.dawling@mcmail.vanderbilt.edu
Date: March 1998
Editor: Mrs J. Duménil
International Programme on Chemical Safety
Date: May 1999
Propranolol: C16H21NO2
Molecular Weight = 259.35
Propranolol hydrochloride: C16H21NO2HCl
Molecular Weight = 295.84
3.3 Physical properties
3.3.1 Colour
White
3.3.2 State/form
3.3.3 Description
White, odourless crystalline powder. Soluble 1
in 20 of water or alcohol; slightly soluble in
chloroform; practically insoluble in ether.
Only stable at acidic pH; decomposes rapidly when
alkaline. Solutions are most stable at pH 3; in
aqueous solutions propranolol decomposes with
oxidation of the isopropylamine side-chain
(Martindale). Propranolol is a racemic mixture of
dextrorotary and levorotary forms.
3.4 Other characteristics
3.4.1 Shelf-life of the substance
No data available.
3.4.2 Storage conditions
Store in well-closed containers, protect from light.
4. USES
4.1 Indications
4.1.1 Indications
4.1.2 Description
Cardiovascular diseases
Propranolol, a non cardioselective beta-blocker, is
mostly used in the treatment of hypertension, angina,
for the prevention of re-infarction in patients who
have suffered from myocardial infarction. It is also
used to control symptoms of anxiety and in the
treatment of supraventricular tachycardia,
hypertrophic obstructive cardiomyopathy and tetralogy
of Fallot.
Endocrine disorders
In hyperthyroidism and thyrotoxic crisis; together
with alpha blocking agents in the preoperative
treatment of phaeochromocytoma.
Hepatic diseases
Prevention of haemorrhage in portal hypertension
Neurological disorders
Propranolol has also been used in the treatment of
extrapyramidal disorders and in the prophylaxis of
migraine headache.
Anxiety disorders
Propranolol may be used in acute stress reactions,
somatic anxiety and panic reactions, but its value is
questioned (Reynolds, 1993).
4.2 Therapeutic dosage
4.2.1 Adults
Propranolol dosing should be initiated at low
doses and gradually increased until the desired
therapeutic effect is achieved.
Oral administration
In cardiovascular diseases: the initial dose of 40 mg
twice daily, may be increased at weekly intervals to a
maintenance dose of 160 to 260 mg daily (some patients
require 320 mg daily).
In prevention of haemorrhage due to portal
hypertension: 20 mg twice daily.
In migraine headache: 40 to 120 mg daily
Intravenous administration
The optimal dose is 1 to 3 mg (inject slowly: 1
mg/minute maximum). The total dose should not exceed 5
to 10 mg.
4.2.2 Children
Oral administration
0.25 to 0.5 mg/kg/24 hours administered in divided
doses (3 or 4 times daily up to 1 mg/kg/24 hours)
Intravenous administration
0.025 to 0.05 mg/kg; 3 or 4 times daily under ECG
control
Caution: Propranolol should never be discontinued
abruptly in patients with coronary artery disease.
Gradually decrease the dosage.
4.3 Contraindications
Absolute: asthma, congestive cardiac failure,
atrio-ventricular block, bradycardia (below 50/minute),
treatment with amiodarone.
Relative: Raynaud's disease, diabetes mellitus.
5. ROUTES OF ENTRY
5.1 Oral
Ingestion is the most frequent cause of poisoning
5.2 Inhalation
No case has been reported. The effect of 10 mg
propanolol given by nasal route is rapid and equivalent to
the intravenous route (Hussain et al., 1980).
5.3 Dermal
No data available.
5.4 Eye
No data available.
5.5 Parenteral
No case of overdoses has been reported. Cardiovascular
symptoms have been reported after therapeutic
administration.
5.6 Other
No data available.
6. KINETICS
6.1 Absorption by route of exposure
After oral administration, propranolol is almost
completely and rapidly absorbed from the gastrointestinal
tract. However, because of the high first-pass metabolism and
hepatic tissue binding, the absolute bioavailability is only
about 30% and varies greatly between individuals. Peak plasma
concentration occurs one to two hours after administration
(Frishman et al., 1979; Goodman & Gilman, 1985).
After administration of the sustained release formulation,
the peak plasma concentration occurs 7 hours after
absorption.
6.2 Distribution by route of exposure
About 90 to 95 % of the drug is bound to plasma proteins.
The volume of distribution is 300 L/1.73 M2 or 3.9 L/kg
(approximately 200 L in an adult).
Propranolol is highly lipophilic: it crosses the blood-brain
barrier and the placenta: the ratio of concentration in the
blood between the foetus and the mother is 1.5.
6.3 Biological half-life by route of exposure
After oral administration, propranolol undergoes
saturable kinetics.
The plasma half-life is 3 to 6 hours and is about 12 hours
with the sustained release forms. The total body clearance is
800 mL/minute/1.73 m2.
After overdose, the plasma half-life is prolonged. Halloran
and Phillips (1981) reported a half-life of 16 hours. In two
cases reported by Tzen War Chen et al. (1986), the half-life
was 13.8 (ingestion of 3.12 g) and 8.3 hours (ingestion of
0.28 g). In five cases studied by Jaeger et al. (1990), the
mean plasma half-life was mean 10.5 hours (range: 5.1 to
17).
6.4 Metabolism
Propranolol is extensively metabolized by the liver. At
least one of the metabolites, the 4-hydroxypropranolol, is
biologically active. The hepatic metabolism is saturable and
bioavailability may be increased in overdoses.
6.5 Elimination by route of exposure
After a single oral dose, propranolol is completely
eliminated in 48 hours, mainly by hepatic metabolism. Less
than 0.5 % is excreted unchanged in urine. The renal
clearance is 12 mL/kg/minute. About 20% of the dose is
eliminated in urine mainly as glucuronide conjugates.
Propranolol is excreted in breast milk at a concentration of
50% that of blood.
Dialysis clearance is about 20 mL/minute with a blood flow of
250 mL/minute.
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
Propranolol is a non cardioselective
beta-blocker with no intrinsic sympathomimetic. It
has membrane stabilizing activity and is highly lipid
soluble. At toxic doses, propranolol has a pronounced
negative chronotropic and inotropic effect and a
quinidine-like effect on the heart: the result is a
reduction of the heart rate, a decrease of the
sino-atrial and atrioventricular conduction, a
prolongation of the intraventricular conduction and a
decrease of cardiac output. Blockade of beta-2
receptors may cause bronchospasm and hypoglycaemia.
Given its high lipid solubility, propranolol crosses
the blood-brain barrier and may cause coma and
convulsions (Langemeijer et al., 1986; de Wildt et
al., 1984).
7.1.2 Pharmacodynamics
Beta-blocking agents compete with endogenous
and/or exogenous beta-adrenergic agonists. Their
specific effects depend on their selectivity for
beta-1 receptors (located in the heart) or beta-2
receptors (located in bronchi, blood vessels, stomach,
gut, uterus). Beta-blockers are classified according
to their cardioselectivity, membrane stabilizing
effect, intrinsic sympathomimetic effect and lipid
solubility (Critchley & Ungar, 1989; Ellenhorn &
Barceloux, 1988; Frishman & Sivermon, 1979; Frishman,
1979; Goodman & Gilman, 1985; Weinstein, 1984).
At therapeutic doses, propranolol slightly decreases
heart rate (15%), supraventricular conduction and
cardiac output (15 to 20%) (Johnson et al., 1969).
Cardiac work and oxygen consumption are also
decreased. Propranolol decreases the secretion of
renin.
The pharmaceutical form of propranolol is a racemate:
the dextrorotary isomer accounts for most of the
beta-blocking effect, whereas the levorotary isomer
has a predominantly membrane stabilizing
effect.
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
Propranolol toxicity shows
individual variations which may be due to an
underlying cardiac disease, to the ingestion
of other cardiotoxic drugs and to variations
in first-pass metabolism.
The toxic dose is about 1 g. In 104 cases
reported in literature the mean toxic (but
non lethal) dose was 1.75 g (Gross 1991)
although survival has been reported after
ingestion of 5 to 8 g (Lagerfeldt & Matell,
1982; Tynan et al., 1981). Khan & Miller
(1985) reported survival of a 28-year-old man
following ingestion of 3 g.
The minimal lethal dose reported was 1.6 g in
a 57-year-old man (Auzepy et al., 1983). The
mean lethal dose in 17 cases reported in the
literature was 5.85 g although there is wide
interindividual variation (Gross,
1991).
7.2.1.2 Children
Ingestion of 70 mg by a 2-year-old
child produced drowsiness, 2nd degree
atrioventricular block and hypoglycaemia of
0.14 g/L (Hesse & Pedersen, 1973). Ingestion
of 100 mg by a 5-year-old child produced
drowsiness, delirium and hallucinations (Eibs
et al., 1982). An ingestion of 400 to 1,200
mg by a 3-year-old boy was uneventful after
early induced vomiting: the plasma level was
2.29 µg/L (Artman et al., 1982).
7.2.2 Relevant animal data
In mouse the LD50 are:
565 mg/kg after oral route
22 to 35 mg/kg after intravenous route
107 mg/kg after intraperitoneal route
(RETCS, 1985)
7.2.3 Relevant in vitro data
No data available.
7.3 Carcinogenicity
No data available.
7.4 Teratogenicity
No confirmed reports of teratogenic effect.
7.5 Mutagenicity
No data available.
7.6 Interactions
Decreased bioavailability
Antacids decrease the gastric absorption of propranolol.
Barbiturates, phenytoïn and rifampicin increase the
first-pass clearance of propranolol by hepatic enzyme
induction (Sotanieni et al., 1979).
Increased bioavailability
Plasma propranolol concentrations may be increased up to 50%
by histamine H2 antagonists and oral contraceptives, which
decrease hepatic metabolism by enzyme inhibition.
Diminished pharmacodynamic effects
Non-steroidal anti-inflammatory drugs decrease the
antihypertensive effect of propranolol. Nifedipine might
exacerbate the symptoms of beta-blocker withdrawal.
Enhanced pharmacodynamic effects
Digitalis, amiodarone, verapamil and diltiazem may increase
bradycardia due to propranolol. Verapamil, prenylamine,
flecainide and disopyramide enhance the negative inotropic
effect of propranolol.
7.7 Main adverse effects
Numerous adverse effects during propranolol treatment
have been reported (Reynolds, 1993; Drugdex, 1991; Dukes,
1984; Meyler et al., 1974; Goodman & Gillman, 1985).
* Cardiovascular: sinus bradycardia, atrioventricular block,
hypotension, increase of left ventricular failure,
cardiogenic shock, intermittent claudication.
* Respiratory: bronchospasm, exacerbation of asthmatic
symptoms in known asthmatics, pulmonary oedema.
* Central nervous system: depression, psychosis, convulsions,
hallucinations.
* Musculoskeletal: muscle weakness, aggravation of myasthenia
gravis, peripheral neuropathy.
* Gastrointestinal: vomiting, diarrhoea, dry mouth.
* Endocrine and metabolic: hypoglycaemia, hyperkalaemia,
hypothyroidism, sexual dysfunction (impotence).
* Dermatological: urticaria, exfoliative dermatitis.
* Haematological: agranulocytosis (immunologic reaction),
thrombocytopenia.
* Teratogenicity: a case of tracheoesophageal fistula in a
newborn of a mother treated with propranolol during the
pregnancy has been reported by Campbell, 1985. However, a
teratogenic effect of propranolol has not been
confirmed.
* Pregnancy: hypoglycaemia and lethargy have been reported in
newborn from mothers treated with propranolol before
delivery.
* Others: propranolol treatment may potentiate anaphylactic
shock.
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
Toxic ingredient: liquids, capsules,
scene residues
In case of ingestion:
Vomitus: total amount
Gastric aspirate: total amount (or gastric
lavage: first portion: 100 mL)
Whole blood without additives: 10 mL
Urine: random specimen: 50 mL
8.1.1.2 Biomedical analyses
Plasma (lithium heparin as
anticoagulant) or serum and urine for
standard biochemical analyses.
8.1.1.3 Arterial blood gas analysis
Heparinized arterial blood sample
(in severe cases).
8.1.1.4 Haematological analyses
Anticoagulated blood (e.g. EDTA) for
standard haematological analyses and
differential blood picture.
8.1.1.5 Other (unspecified) analyses
No further materials.
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
Some authors suggest the use of
antioxidants (ascorbic acid, sodium
metabisulphite) as stabilizers for
4-hydroxypropranolol, but loss appears to be
insignificant in samples stored at -30°C
without additives (Fu & Mason,
1989).
8.1.2.2 Biomedical analyses
No special requirements, but as
usually performed.
8.1.2.3 Arterial blood gas analysis
No special requirements, but as
usually performed.
8.1.2.4 Haematological analyses
No special requirements, but as
usually performed.
8.1.2.5 Other (unspecified) analyses
Serum, urine and other materials in
a refrigerator (4°C).
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
Some authors suggest the use of
antioxidants (ascorbic acid, sodium
metabisulphite) as stabilizers for
4-hydroxypropranolol, but loss appears to be
insignificant in samples stored at -30°C
without additives (Fu & Mason,
1989).
8.1.3.2 Biomedical analyses
No special requirements, but as
usually performed.
8.1.3.3 Arterial blood gas analysis
No special requirements, but as
usually performed.
8.1.3.4 Haematological analyses
No special requirements, but as
usually performed.
8.1.3.5 Other (unspecified) analyses
Not applicable.
8.2 Toxicological analyses and their interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple qualitative test(s)
8.2.1.2 Advanced qualitative confirmation test(s)
8.2.1.3 Simple quantitative method(s)
8.2.1.4 Advanced quantitative method(s)
8.2.2 Tests for biological specimens
8.2.2.1 Simple qualitative test(s)
8.2.2.2 Advanced qualitative confirmation test(s)
8.2.2.3 Simple quantitative method(s)
8.2.2.4 Advanced quantitative method(s)
8.2.2.5 Other dedicated method(s)
8.2.3 Interpretation of toxicological analyses
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
Sodium, potassium, chloride,
calcium, magnesium Glucose, amylase, creatine
kinase, creatinine (urea)
Optional: bicarbonate (or total CO2), uric
acid
8.3.1.2 Urine
8.3.1.3 Other fluids
No dedicated test.
8.3.2 Arterial blood gas analyses
8.3.3 Haematological analyses
8.3.4 Interpretation of biomedical investigations
8.4 Other biomedical (diagnostic) investigations and their
interpretation
Measurement of serum electrolytes, glucose, creatinine
and urea nitrogen, and arterial blood gases are useful to
optimize supportive care.
8.5 Overall Interpretation of all toxicological analyses and
toxicological investigations
Analysis of Materials
Propranolol can be detected and identified in materials by a
number of simple techniques. Marquis and Mandelin's test
both give a green-blue reaction (Moffat et al., 1986). The
spectrum in methanol gives deltamax at 290 nm (A| = 240), 306
nm (A| = 143), and 319 nm (A| = 86); the pattern in dilute
acid is similar. Thin layer chromatography is useful for
propranolol analysis, and the drug can be visualized as a
green-blue spot with Mandelin's reagent. The Rf is 0.50 in
both methanol / ammonia (100:1.5) and in ethyl acetate /
methanol / ammonia (85:15:5) (Moffat et al., 1986). Gas
chromatography is also useful, and the retention index for
propranolol is 2150 on OV1, SE30, SE54, HP5 or similar; FID
gives sufficient sensitivity for residues, but
nitrogen-phosphorus detection gives improved sensitivity and
specificity. GCMS may also be used for underivatized
propranolol, although the spectrum is not characteristic.
Analysis of Biological Specimens
Analysis in body fluids is fairly straightforward, although
quantitative analysis is only rarely indicated. There are no
commercially-available immunoassay kits which respond to
propranolol. For detection in urine or plasma an extraction
into a polar solvent is required (e.g. methyl tert-butyl
ether, dichloromethane:iso-propanol or ethyl acetate) from
basic buffer, and recovery from urine may be improved by
salting out. Urinalysis is best performed by thin layer
chromatography. The Rf of propranolol is 0.50 in both
methanol / ammonia (100:1.5) and in ethyl acetate / methanol
/ ammonia (85:15:5) with a positive iodoplatinate and a
green-blue Mandelin's response. The 4-hydroxy metabolite is
at Rf 0.50 in methanol / ammonia (100:1.5), and 0.40 in ethyl
acetate / methanol / ammonia (85:10:5), and has a
characteristic pale blue response with iodoplatinate, and a
red response to Marquis reagent (personal experience). Gas
chromatography of urine generally shows only propranolol
(retention index 2150 on OV1, SE30, SE54, HP5 or similar) and
sometimes the 4-hydroxy-metabolite at retention index 2650
(see below under advanced methods). Nitrogen-phosphorus
detection is required for low therapeutic amounts, or mass
spectrometry methods are suitable.
Quantitative analysis of toxic concentrations in serum may be
performed by direct fluorimetry. Drug is extracted from
alkalinized plasma (potassium carbonate) into toluene and
re-extracted into acetic acid (0.005M) in ethylene glycol (50
g/L). Excitation is at 290 nm, and emission measured at 358
nm with a sensitivity of 0.003 mg/L from 1 mL sample (Rao et
al., 1978; Baselt, 1987). Other authors prefer scanning the
emission pattern to identify potential interferences from
quinidine and other fluorescing compounds (Kraml & Robinson,
1978). The method can also be used to quantitate
4-hydroxypropanolol at 320 nm excitation and measuring
emission at 510 nm with a sensitivity of 0.015 mg/L (Rao et
al., 1978; Baselt, 1987). Most frequently quantitation in
plasma is performed by HPLC with fluorescence detection which
gives excellent sensitivity (sub-therapeutic concentrations
on less than 0.1 mL); excitation at 205 to 215 nm, emission
measured at 340 nm. Methylpropranolol, benzimidazole or
flecainide can be used as internal standard. HPLC of
propranolol can best be achieved with silica columns with
organic mobile phases containing modifiers such as
camphorsulfonic acid or ammonium sulphate (Bhamra et al.,
1985; Rognerud & Ou, 1989). For simultaneous measurement of
propranolol and 4-hydroxypropanolol, reverse-phase systems
are required and chromatography is slower. Sensitivity is
reduced (0.01 mg/L) due to the difference in emission
wavelength for the two compounds (Sood et al., 1988; Fu &
Mason, 1989). Some authors suggest the use of antioxidants
(ascorbic acid, sodium metabisulphite) as stabilizers for
4-hydroxypropranolol, but loss appears to be insignificant in
samples stored at -30°C without additives, although loss may
occur during the extraction procedure (Fu & Mason, 1989).
Gas chromatographic methods are less often used, but are
described electron capture after derivatization with
pentafluoropropionic anhydride / pyridine (1 minute at 70°C)
(Kates & Jones, 1977) or trifluoroacetic anhydride / benzene
/ trimethylamine for 5 min at 50°C (Walle, 1974) or TFAA in
toluene for 60 minutes at room temperature (Jack & Laugher,
1985). Isothermal analysis may be performed at 200 to 250°C
depending on the column and conditions. Detection limits are
0.001 to 0.005 mg/L. None of the GC methods report data for
4-hydroxypropranolol.
Advanced Methods of Analysis
Several more complex methods describe the screening urine for
a range of ß-blockers using GCMS of TMS derivatives (Dunasia
& Houghton, 1991), or for simultaneous analysis of
propranolol and its full pattern of metabolites by GLC (Walle
& Gaffney, 1972) or HPLC (Harrison et al., 1985). Methods
are currently available for separation of the R- and
S- enantiomers of propranolol, all of which use HPLC with
fluorescence detection. Separation is achieved either on
chiral columns (Herring & Johnson, 1993), or on conventional
columns after diastereoisomerization with a chiral reagent
(Pham-Huy et al., 1994; Toyo'oka et al., 1997), or after
addition of a chiral selector to the mobile phase (Karlsson &
Pettersson, 1989). Some of these methods also measure the
4-hydroxy metabolite.
Toxicity is only poorly correlated with serum propranolol
concentrations, and measurement of vital signs gives a more
reliable index of intoxication. Survival has been reported
in a patient with a plasma concentration of 4.8 mg/L (Gross,
1991). However, the following table of typical
concentrations is given for guidance:
mg/L µmol/L
Peak (2 hours) after single oral
dose (1.2 mg/kg) 0.03 to 0.05 0.12 to 0.19
Steady-state in therapy 0.03 to 0.15 0.12 to 0.58
Toxicity apparent > 0.5 > 1.93
Life threatening toxicity > 2.0 > 7.72
4-hydroxypropranolol 10% of propranolol
propranolol glucuronide 600% of propranolol
naphthoxylacetic acid 1000% of propranolol
Measurement of serum electrolytes, glucose, creatinine and
urea nitrogen, and arterial blood gases are useful to
optimize supportive care.
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
The severity of propranolol poisoning is due to
its cardiotoxicity and depends on the dose ingested,
the presence of underlying cardiac disease and
concomitant ingestion of other cardiotoxic drugs.
Symptoms and signs appear within one to two hours and
may include the following:
* Cardiovascular effects: bradycardia, hypotension,
cardiogenic shock. The ECG may show nodal rhythm,
atrioventricular block and QRS widening
* CNS effects: lethargy, coma and convulsions,
mydriasis
* Hypoventilation resulting from severe shock
9.1.2 Inhalation
No data available.
9.1.3 Skin exposure
No data available.
9.1.4 Eye contact
No data available.
9.1.5 Parenteral exposure
Cardiovascular effects: bradycardia,
hypotension, cardiogenic shock. The ECG may show nodal
rhythm, atrioventricular block and QRS widening CNS
effects: lethargy, coma and convulsions, mydriasis
Hypoventilation resulting from severe shock.
9.1.6 Other
No data available.
9.2 Chronic poisoning
9.2.1 Ingestion
No data available.
9.2.2 Inhalation
No data available.
9.2.3 Skin exposure
No data available.
9.2.4 Eye contact
No data available.
9.2.5 Parenteral exposure
No data available.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
Patients who survive 48 hours after acute poisoning or
who have not developed cardiac arrest before admission are
likely to recover. Death may occur from cardiac asystole
which is favoured by hypoxaemia.
The prognosis depends on the dose ingested and is worse in
patients with an underlying cardiac disease and in those who
have ingested other cardiotoxic drugs (Critchley & Ungar,
1989; Ellenhorn & Barceloux, 1988; Weinstein, 1984).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Acute:
Cardiovascular symptoms are the major features of
propranolol poisoning.
Bradycardia is the commonest symptom (present in 60 to
90% of cases) and occurs soon after ingestion.
Hypotension is observed in about 50 to 70% of the
cases. Hypotension and shock are due to decreased
cardiac output and vasodilatation (Amundson & Brodine,
1988).
Cardiac arrest may occur within 1 to 2 hours of
ingestion. Cardiac arrest has been reported to occur
in 45 minutes following the ingestion of 9.4 g of
propranolol by a 60-year-old man (Jones et al.,
1982).
ECG changes are always present in symptomatic
poisoning: sinus or nodal bradycardia,
atrioventricular block (1st to 3rd degree) are the
most common. Widening of the QRS interval, bundle
branch block or increased QT interval are less
frequently observed.
Chronic: No data available.
9.4.2 Respiratory
Acute:
Respiratory depression and apnoea is mostly associated
with severe shock and is due to cerebral hypoxia (Hong
et al., 1983).
Pulmonary oedema may occur, especially in patients
with a previous compromised cardiac function.
Bronchospasm may occur in susceptible patients.
Chronic: No data available.
9.4.3 Neurological
9.4.3.1 CNS
Acute:
Lethargy, drowsiness, agitation, delirium,
hallucinations and mydriasis may be
observed.
Coma is usually only seen in patients with
cardiovascular collapse.
Convulsions have been reported after
ingestion of large doses (Das & Ferris, 1988;
Frishman et al., 1979; Tynan et al., 1981).
Convulsions may be due to hypotension or to a
direct effect of propranolol (membrane
stabilizing effect).
Chronic:
Fatigue, CNS depression, hallucinations and
psychosis have been reported.
9.4.3.2 Peripheral nervous system
No data available.
9.4.3.3 Autonomic nervous system
Acute: Effects of beta-receptor blockade.
Chronic: Effects of beta-receptor blockade.
9.4.3.4 keletal and smooth muscle
Acute: No data available.
Chronic: Muscular fatigue may be observed.
9.4.4 Gastrointestinal
Acute: vomiting, nausea may be seen; spasm of
the lower oesophageal sphincter has been reported in 2
cases (Panos et al., 1986; Laake et al., 1981). A
case of mesenteric ischaemia following propranolol
overdose has been reported (Pettei et al., 1990).
Chronic: No data available.
9.4.5 Hepatic
No data available.
9.4.6 Urinary
9.4.6.1 Renal
No data available.
9.4.6.2 Other
No data available.
9.4.7 Endocrine and reproductive systems
No data available.
9.4.8 Dermatological
No data available.
9.4.9 Eye, ear, nose, throat: local effects
Acute: Mydriasis and diplopia may be noted.
Chronic: No data available.
9.4.10 Haematological
No data available.
9.4.11 Immunological
No data available.
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Metabolic acidosis may occur in
severe poisoning with shock.
9.4.12.2 Fluid and electrolyte disturbances
Hypokalaemia (Peterson et al.,
1984) or a hyperkalaemia (Delacour et al.,
1986) have been reported rarely.
9.4.12.3 Others
Hypoglycaemia (0.14 and 0.5 g/L)
was reported in two cases of poisoning in
children (Hesse & Pedersen, 1973).
9.4.13 Allergic reactions
No data available.
9.4.14 Other clinical effects
No data available.
9.4.15 Special risks
Pregnancy: No data available.
Breast-feeding: No data available.
Enzyme deficiencies: No data available.
9.5 Other
No data available.
9.6 Summary
10. MANAGEMENT
10.1 General principles
Patients with propranolol poisoning should be monitored
closely, preferably in an intensive care unit.
Make a proper assessment of airway, breathing, circulation
and neurological status of the patient.
Monitor ECG, blood pressure and respiration.
Treatment may include:
* gastric lavage, oral activated charcoal, emesis, only after
immediate ingestion
* supportive treatment for respiratory failure by artificial
ventilation; symptomatic treatment for convulsions with
diazepam, and shock with dopamine or norepinephrine
* atropine in case of symptomatic bradycardia
* isoproterenol and/or glucagon if atrioventricular block or
shock are present
10.2 Life supportive procedures and symptomatic/specific treatment
Sinus bradycardia may be treated with intravenous
atropine 1 to 2 mg in adults (10 to 30 µg/kg in
children).
Isoproterenol is the drug of choice conduction disturbances.
Administer as a continuous infusion, beginning at a dose of
0.3 µg/kg/minute and progressing in 0.2 µg/kg/minute
increments as needed.
Glucagon may also be used but it is usually less efficient
than isoproterenol.
Transvenous cardiac pacing may be indicated if severe
bradycardia does not respond to isoproterenol or
glucagon.
Hypertonic sodium solutions (molar sodium lactate or
bicarbonate) may be indicated if there is a widening of the
QRS interval (due to the membrane stabilizing effect).
If hypotension and shock does not respond to isoproterenol
and glucagon, dopamine or norepinephrine are indicated
(Halloran & Phillips, 1981; Weinstein, 1984). If shock does
not improve, haemodynamic study by a Swan-Ganz catheter
should be performed in order to assess the treatment.
An intra-aortic balloon pump has been used in a 17-year-old
woman who had ingested 8 g propranolol and developed shock
which did not respond to isoproterenol, adrenaline and
glucagon (Lane et al., 1987).
If cardiac arrest perform external cardiac massage and
mechanical ventilation with oxygen. Adrenaline is indicated:
1 to 4 mg intravenous in an adult, 0.25 to 0.5 mg by
intratracheal route in a child.
Respiratory depression should be treated by artificial
ventilation. Bronchospasm may require treatment with beta-2
agonists or aminophylline.
If convulsions persist despite the correction of hypotension,
they may be treated by diazepam or clonazepam, neither of
which have a negative inotropic effect.
Metabolic acidosis should be treated with sodium
bicarbonate.
Correct hypo- or hyperkalaemia.
Give intravenous glucose if hypoglycaemia is present.
10.4 Decontamination
Emesis
Emesis may only be indicated in cases of recent ingestion.
However, it has been reported that induced emesis has
precipitated severe bradycardia and hypotension because it
increases the vagal tone (Soni et al., 1983; Smith et al.,
1986). Because cardiotoxic symptoms may appear rapidly and
suddenly, emesis induced by syrup of ipecac is not
recommended.
Gastric lavage
Gastric lavage may also precipitate bradycardia and
hypotension. Therefore it should be performed under strict
ECG and blood pressure monitoring. Because of the rapid
absorption of propranolol, gastric lavage should be performed
within 4 hours of ingestion.
Oral activated charcoal
Activated charcoal should be administered following gastric
lavage. Repeated doses may interfere with the enterohepatic
recirculation of propranolol. The efficiency of oral
activated charcoal has been documented for atenolol, nadolol,
pindolol and sotalol but not for propranolol (Neuvonen &
Olkkola, 1988).
10.5 Elimination
Forced diuresis
Forced diuresis is not recommended because only a very small
amount of propranolol is excreted in urine.
Haemodialysis, Haemoperfusion
Considering the pharmacokinetics of propranolol, enhanced
elimination by haemodialysis or haemoperfusion is useless and
not recommended (see section 6).
10.6 Antidote treatment
10.6.1 Adults
Atropine
Although atropine has been frequently used in the
cases reported in literature, it is the least
effective treatment (Weinstein, 1984). Atropine may
only abolish the increased vagal stimulation and is
ineffective in severe bradycardia.
Atropine may be used in sinus bradycardia: administer
1 to 2 mg intravenous in adults. If bradycardia does
not respond give isoproterenol.
Isoproterenol
Isoproterenol is a beta agonist which will
competitively antagonize the effects of the beta
blocker. It is indicated for the treatment of
conduction disturbances and shock. Administer
isoproterenol in continuous infusion. Begin at a dose
of 0.3 µg/kg/minute and progress in 0.2 µg/kg/minute
increments as needed. In several reports isoproterenol
was ineffective, but this was certainly due to
inadequate dosage (Critchley & Ungar, 1989). High
doses may be administered until the beta-blocking
effect is reversed (Rang & Dale, 1987). In the cases
reported by Halloran & Phillips (1981) and Lagerfeldt
& Matell (1976), doses of 25 to 74 µg/minute were
given. Massive propranolol ingestions reportedly
require doses of isoproterenol up to 200 µg/minute
(Agura et al., 1986) and even 800 µg/minute (Tynan et
al., 1981) for effective treatment.
Prenalterol
Prenalterol (a beta-agonist) was used successfully for
refractory hypotension unresponsive to glucagon and
vasopressor in a case of massive propranolol overdose
(Kulling et al., 1983). The recommended dose is 5 to
10 mg followed by an infusion of 5 mg/hour. Doses up
to 50 and 100 mg may be required.
Glucagon
Glucagon has positive inotropic and chronotropic
effects which are thought to be mediated by the
activation of the adenylcyclase system independently
of beta-adrenoreceptors (Kosinski & Malindzak, 1973).
It has been reported that glucagon increases
myocardial contractility in patients refractive to
isoproterenol. The dose required for a significant
myocardial action is much higher than that used to
stimulate gluconeogenesis. This may result in side
effects like nausea and vomiting. The initial dose is
50 to 150 µg/kg (5 to 10 mg) intravenously over one
minute, followed by an infusion of 1 to 5 mg/hour.
(Illingworth, 1979; Kosinski & Malindzak, 1973;
Parmley, 1971; Robson, 1980; Ward & Jones,
1976).
10.6.2 Children
Doses should be adapted according to the body
weight. No particular dosage regimen for
isoproterenol, prenalterol or glucagon have been
recommended for propranolol poisoning in
children.
10.7 Management discussion
All patients with an history of acute ingestion of
propranolol should have an ECG and be monitored for at least
6 hours.
Patients can be managed outside intensive care unit if there
is a history of recent ingestion and no cardiotoxic effects
are present.
If symptoms of cardiotoxicity are present transfer the
patient to an intensive care unit and monitor vital signs and
biochemical parameters.
Keep the patient in the intensive care unit until cardiotoxic
symptoms have disappeared.
A patient who presented with cardiotoxic effects and seizures
after propranolol overdose did not respond to adrenaline,
atropine, fluids and bicarbonate. Intravenous calcium
chloride produced a dramatic restoration of blood pressure
and a narrowing of the QRS complexes. After a bolus of 1 g
intravenous, calcium chloride was given by an infusion 0.08 g
per minute, which was reduced to 0.005 g per minute according
to the ECG pattern and the blood pressure level. The authors
believe that calcium may have a role to play in the treatment
of propranolol overdose (Brimacombe et al., 1991).
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
After poisoning with 800 mg propranolol, a 65-year-old
man was admitted two hours after ingestion. He was comatose
and had bradycardia (50/minute) and shock. The patient was
treated by artificial ventilation, atropine (ineffective),
isoproterenol (40 µg/hour) and recovered after 12 hours. Peak
serum propranolol concentration was 1536 µg/l and serum
half-life was 6.6 hours (Ducret et al., 1973).
Poisoned with 8 g propranolol, a 14-year-old girl was
admitted 35 minutes following ingestion. Her blood pressure
was 130/70 mm Hg and pulse was 34/minute. Ten minutes after
induced emesis by ipecac the patient developed sudden
hypotension and convulsions. ECG showed a second degree
atrioventricular block and a right bundle-branch block.
Treatment consisted of artificial ventilation, external
cardiac massage, atropine 1.2 mg intravenous, isoproterenol
0.4 mg intravenous and calcium gluconate 1 g intravenous.
Blood pressure increased to 110/70 mmHg with isoproterenol at
a rate of 0.8 mg/minute. Isoproterenol was discontinued seven
hours following admission and the patient was extubated
(Tynan et al., 1981).
Agura et al. reported (1986) acute poisoning in a 40-year-old
woman. The cardiogenic shock did not improve with atropine
(1 mg), adrenaline (1 mg), isoproterenol (16 to 20
µg/minute), dopamine (15 to 20 µg/minute) and noradrenaline
(10 to 12 µg/minute). Blood pressure and heart rate were only
restored by glucagon (10 mg intravenous then 5 mg/hour) and
high doses of isoproterenol (160 to 200 µg/minute).
Elkharrat & Bismuth (1982) reported 25 cases of propranolol
poisoning (1 to 2.5 g). Symptoms were present in 40 % of the
cases: sinus bradycardia in 25%, atrioventricular block in
20%, hypotension in 10% and shock in 5%. No mortality or
sequelae were noted.
Refractory hypotension following propranolol overdose was
treated with, in addition to isoprenaline and glucagon,
extracorporeal circulatory support using femoral vein
- femoral artery bypass (McVey & Corke, 1991).
12. Additional information
12.1 Specific preventive measures
No data available.
12.2 Other
No data available.
13. REFERENCES
Agura ED, Wexler LF, Witzburg RA (1986). Massive propranolol
overdose: successful treatment with high dose isoproterenol and
glucagon. Am J Med 80: 755-757.
Amundson DE, Brodine SK (1988). A fatal case of propranolol
poisoning. Drug Intell Clin Pharm 22: 781-782.
Artman M, Grayson M, Boerth R (1982). Propranolol in children:
safety/toxicity. Pediatrics 70: 30-31.
Auzepy Ph, Boukara N, Richard Ch, Giudicelli JF (1983).
Intoxications aiguës par les bêta-bloquants chez l'adulte (à
propos de sept cas). Ann Cardiol Angeiol 32: 253-258.
Baselt RC (1987) Analytical Procedures for Therapeutic Dug
Monitoring and Emergency Toxicology. 2nd ed. California,
Biomedical Publications, pp 248-249.
Bhamra RK, Flanagan RJ & Holt DW (1985) Propranolol using solvent
extraction and high-performance liquid chromatography. In:
Sunshine I ed. Methodology for Analytical Toxicology Vol III.
Boca Raton, Florida, CRC Press, pp165-168.
Brimacombe JR, Scully M, Swainston R (1991). Propranolol overdose
- a dramatic response to calcium chloride. Med J Aust 155:
267 - 268.
Campbell JW (1985). A possible teratogenic effect of propranolol.
New Engl J Med 313: 518.
Critchley JAJH, Ungar A (1989). The management of acute poisoning
due to beta-adrenoceptor antagonists. Med Toxicol 4: 32-45.
Das G, Ferris JC (1988). Generalized convulsions in a patient
receiving ultrashort-acting beta-blocker infusion. Drug Intell
Clin Pharm 22: 484-485.
Delacour JL, Blanc PL, Wagschal G, Daoudal P (1986). Hyperkaliémie
au cours d'une intoxication par a-bloqueur. Presse Med 15: 1377.
De Wildt D, Sangster B, Langemeijer J, De Groot G (1984).
Different toxicological profiles for various beta-blocking agents
on cardiac function in isolated rat hearts. Clin Toxicol 22:
115-132.
Drugdex (1991) Micromedex Inc.
Ducret F, Zech P, Pernet D, Moskovtchenko JM, Traeger J (1978).
Intoxication volontaire par le Propranolol. Nouv Presse Med 71:
27-28.
Dukes MNG (1984). Side effects of drugs. Annual 8. Elsevier Ed.,
Amsterdam.
Dunasia MC & Houghton E (1991) Screening and confirmatory
analysis of ß-agonists, ß-antagonists and their metabolites in
horse urine by capillary gas chromatography-mass spectrometry. J
Chromatogr, 564: 503-513.
Eibs HG, Oberdisse U, Brambach U (1982). Vergiftung mit
Betarezeptorenblockern bei Kindern und Jugendlichen. Monatschr
Kinderheildk, 130: 292-5
Elkharrat D, Bismuth Ch (1982). Acute intoxication by
beta-blocking agents: no mortality in 40 cases. Int J Clin Pharm
Res 2: 207-210.
Ellenhorn MJ, Barceloux DG (1988). Medical toxicology. Diagnosis
and treatment of human poisoning. Elsevier Ed., New York.
Frishman W, Jacob H, Eisenberg E (1979). Clinical pharmacology of
the new beta-adrenergic blocking drugs. Part. 8. Self-poisoning
with beta-adrenoceptor blocking agents: recognition and
management. Am Heart J 98: 798-811.
Frishman W, Silverman R (1979). Clinical pharmacology of the new
B-adrenergic blocking drugs (part 4 - adverse effects). Choosing a
B-adrenergic blocker. Am Heart J 98: 256.
Fu CW & Mason WD (1989) Determination of propranolol and
4-hydroxypropranolol in human plasma by high-performance liquid
chromatography. Analyst, 114: 1219-1223.
Goodman L.S, Gilman A (1985). The Pharmacological basis of
therapeutics. Fourth Edition, MacMillan Company, New York.
Halloran TJ, Phillips CE (1981). Propranolol intoxication. A
severe case responding to Norepinephrine therapy. Arch Intern Med
141: 810-811.
Harrison PM, Tonkin AM, Cahill CM & McLean AJ (1985) Rapid and
simultaneous extraction of propranolol, its neutral and basic
metabolites from plasma, and assay by high-performance liquid
chromatography. J Chromatogr, 343: 349-358.
Herring VL & Johnson JA (1993) Direct high-performance liquid
chromatographic determination in urine of the enantiomers of
propranolol and its major basic metabolite 4-hydroypropranolol. J
Chromatogr, 612: 215-221.
Hesse B, Pedersen JT (1973). Hypoglycaemia after Propranolol in
children Acta Med Scand 193: 551-552.
Hong CY, Yang WC, Chiang BN (1983). Importance of membrane
stabilizing effect in massive overdose of propranolol: plasma
level study in a fatal case Hum Toxicol 3: 511-517.
Hussain A, Forster T, Hirai T (1980). Nasal absorption of
propranolol in humans. J Pharma Sci 69: 1240-1242.
Illingworth RN (1979). Glucagon for beta-blocker poisoning.
Practitioner 223: 683-685.
Jack DB & Laugher SJ (1985) Metoprolol using electron capture
gas-liquid chromatography. In: Sunshine I ed. Methodology for
Analytical Toxicology Vol III. Boca Raton, Florida, CRC Press, pp
117-120.
Jaeger A, Tritsch L, Mangin P, Sauder Ph, Kopferschmitt J, Dahlet
M (1990) Toxicokinetics of beta-blockers. Proceedings of the
International Congress on Clinical Toxicology. Poison Control and
Analytical Toxicology. Lux Tox '90 p. 524.
Johnson G, De Guzman M, Bergman H, Sannerstedt R (1969). The
haemodynamic effects of Alprenolol and Propranolol at rest and
during exercise in hypertensive patients. Pharmacol Clin 2:
34-39.
Jones JW, Clark MA, Mullen BL (1982). Suicide by ingestion of
propranolol. J Forensic Sci 27: 213-216.
Karlsson A & Pettersson C (1989) Determination of (R)- and
(S)-propranolol in plasma by high-performance liquid
chromatography using N-benzoxycarbonylglycyl-L-proline as chiral
selector in the mobile phase. J Chromatogr, 494:157-171.
Kates RE & Jones CL (1977) Rapid GLC determination of propranolol
in human plasma samples. J Pharm Sci, 66: 1490-1492.
Khan MI, Miller MT (1985). Beta-blocker toxicity: the role of
Glucagon. Report of two cases. S Afr Med J 67:1062-1063.
Kosinski EJ, Malindzak GS (1973). Glucagon and Isoproterenol in
reversing propranolol toxicity. Arch Intern Med 132: 840-843.
Kraml M & Robinson WT (1978) Fluorimetry of propranolol and its
glucuronide: applicability, specificity, and limitations. Clin
Chem, 24: 169-171.
Kulling P, Eleborg L, Persson H (1983). Beta-adrenoceptor blocker
intoxication: Epidemiological data. Prenalterol as an alternative
in the treatment of cardiac dysfunction. Human Toxicol, 2:
175-181.
Laake K, Kittang D, Fefstad SO (1981). Convulsions and possible
spasm of the lower oesophageal sphincter in a fatal case of
propranolol intoxication. Acta Med Scand 210: 137-138.
Lagerfeldt J, Matell G (1976). Attempted suicide with 5.1 g of
propranolol: a case report. Acta Med Scand 199: 517-518.
Lane A, Woodward A, Goldman M (1987). Massive propranolol
overdose, poorly responsive to pharmacologic therapy: use of the
intra-aortic balloon pump. Ann Emerg Med 16: 1381-1383.
Langemeijer J, De Wildt D, De Groot G, Sangster B (1986). Calcium
interferes with the cardiodepressive effects of beta-blocker
overdose in isolated rat hearts. Clin Toxicol 24: 111-133.
McVey FK & Corke CF (1991). Extracorporeal circulation in the
management of massive propranolol overdose. Anaesthesia 46:
744-746.
Moffat AC, Jackson JV, Moss MS & Widdop B eds (1986) Clarke's
Isolation and Identification of Drugs, 2nd Edition. The
Pharmaceutical Press, London.
Neuvonen PJ, Olkkola KT (1988). Oral activated charcoal in the
treatment of intoxications. Role of single and repeated doses.
Med Toxicol 3:33-58.
Panos RJ, Tso E, Barish RA, Browne BJ (1986). Esophageal spasm
following propranolol overdose, relieved by glucagon. Am J Emerg
Med 4: 227-228.
Parmley WW (1971). The role of glucagon in cardiac therapy. New
Eng J Med 285: 801-802.
Peterson Ch D, Leeder JS, Sterner S. Glucagon therapy for B
blocker overdose (1984). Drug Intell Clin Pharm 18: 394-398.
Pettei MJ, Levy J, Abramson S. (1990) Nonocclusive mesenteric
ischaemia associated with propranolol overdose: implications
regarding splanchnic circulation. J. Pediatr. Gastroenterol. Nutr.
10: 544 - 547.
Pham-Huy C, Sahui-Gnassi A, Saada V, Grammond JP, Galons H,
Ellouk-Achard S, Levresse V, Fompeydie D & Claude JR (1994)
Microassay of propranolol enantiomers and conjugates in human
plasma and urine by high-performance liquid chromatography after
chiral derivatization for pharmacokinetic study. J Pharm Biomed
Anal, 12: 1189-1198.
Reynolds JEF (1993) Martindale, The Extra Pharmacopoeia. The
Pharmaceutical Press. London.
Rao PS, Quesada LC & Mueller HS (1978) A simple micromethod for
simultaneous determination of plasma propranolol and
4-hydroxypropranolol. Clin Chim Acta, 88: 355-361
Robson RH (1980) Glucagon for beta-blocker poisoning (letter).
Lancet 1: 1357-1358.
Rognerud CL & Ou C-N (1989) Concurrent measurement of flecainide
acetate and propranolol by normal-phase high-performance liquid
chromatography. Clin Chem 35: 857-860.
Smith RC, Wilkinson J, Hull RL (1986). Traitement du surdosage en
propranolol par le glucagon. J Am Med Assoc 255: 2025-6.
Sood SP, Green VI, Mason RP (1988) Routine methods in toxicology
and therapeutic drug monitoring by high-performance liquid
chromatography. IV. A rapid micro-scale method for determination
of propranolol and 4-hydroxypropranolol in plasma. Ther Drug
Monit, 10: 224-230.
Soni N, Barnier D, Pearson IY (1983). Cardiovascular collapse and
propranolol overdose. Med J Aust 2: 629-630.
Toyo'oka T, Toriumi M & Ishii Y (1997) Enantioseparation of
beta-blockers labelled with a chiral fluorescent reagent,
R(-)-DBD-PyNCS, by reversed-phase liquid chromatography. J Pharm
Biomed Anal, 15: 1467-1476.
Tynan F, Fischer MMc D, Ibels L (1981). Self poisoning with
propranolol. Med J Austr 1: 82-83.
Walle T (1974) GLC determination of propranolol, other ß-blocking
drugs, and metabolites in biological fluids and tissues. J Pharm
Sci, 63: 1885-1891.
Walle T & Gaffney TE (1972) Propranolol metabolism in man and
dog: mass spectrophotometric identification of six new
metabolites. J Pharmacol Expt Therapeutics, 182: 83-92.
Ward DE, Jones B (1976). Glucagon and Beta-blocker toxicity. Br
Med J 2: 151.
Weinstein RS (1984). Recognition and management of poisoning with
beta-adrenergic blocking agents. Ann Emerg Med 13:
1123-1131.
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE
ADDRESS(ES)
Authors: L. Tritsch, M. Dahlet, Ph, Sauder,
J. Kopferschmitt, F. Flesch, A. Jaeger.
Service de Réanimation Médicale et Centre
Anti Poisons
CHU, Pavillon Pasteur
67091 Strasbourg Cedex
France
Tel: 33-88161144
Fax: 33-88161330
Date: 27 March 1991
Peer
Review: Adelaide, Australia, April 1991
Finalized
IPCS: May 1994
Author
Section 8: Dr S. Dawling
Center for Clinical Toxicology
Vanderbilt University Medical Center
501 Oxford House
1161 21st Avenue South
Nashville, TN 37232-4632
United States of America
Tel: 1-615-9360760
Fax: 1-615-9360756
E-mail: sheila.dawling@mcmail.vanderbilt.edu
Date: March 1998
Editor: Mrs J. Duménil
International Programme on Chemical Safety
Date: May 1999


