
Fluoxetine
| 1. NAME |
| 1.1 Substance |
| 1.2 Group |
| 1.3 Synonyms |
| 1.4 Identification numbers |
| 1.4.1 CAS number |
| 1.4.2 Other numbers |
| 1.5 Main brand names, main trade names |
| 1.6 Main manufacturers, main importers |
| 2. SUMMARY |
| 2.1 Main risks and target organs |
| 2.2 Summary of clinical effects |
| 2.3 Diagnosis |
| 2.4 First aid measures and management principles |
| 3. PHYSICO-CHEMICAL PROPERTIES |
| 3.1 Origin of the substance |
| 3.2 Chemical structure |
| 3.3 Physical properties |
| 3.3.1 Properties |
| 3.3.3.1 Colour |
| 3.3.3.2 State/Form |
| 3.3.3.3 Description |
| 3.4 Other characteristics |
| 3.4.1 Shelf-life of the substance |
| 3.4.2 Storage conditions |
| 4. USES |
| 4.1 Indications |
| 4.1.1 Indications |
| 4.1.2 Description |
| 4.2 Therapeutic dosage |
| 4.2.1 Adults |
| 4.2.2 Children |
| 4.3 Contraindications |
| 5. ROUTES OF ENTRY |
| 5.1 Oral |
| 5.2 Inhalation |
| 5.3 Dermal |
| 5.4 Eye |
| 5.5 Parenteral |
| 5.6 Other |
| 6. KINETICS |
| 6.1 Absorption by route of exposure |
| 6.2 Distribution by route of exposure |
| 6.3 Biological half-life by route of exposure |
| 6.4 Metabolism |
| 6.5 Elimination and excretion |
| 7. PHARMACOLOGY AND TOXICOLOGY |
| 7.1 Mode of action |
| 7.1.1 Toxicodynamics |
| 7.1.2 Pharmacodynamics |
| 7.2 Toxicity |
| 7.2.1 Human data |
| 7.2.1.1 Adults |
| 7.2.1.2 Children |
| 7.2.2 Relevant animal data |
| 7.2.3 Relevant in vitro data |
| 7.3 Carcinogenicity |
| 7.4 Teratogenicity |
| 7.5 Mutagenicity |
| 7.6 Interactions |
| 7.7 Main adverse effects |
| 8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS |
| 8.1 Material sampling plan |
| 8.1.1 Sampling and specimen collection |
| 8.1.1.1 Toxicological analyses |
| 8.1.1.2 Biomedical analyses |
| 8.1.1.3 Arterial blood gas analyses |
| 8.1.1.4 Haematological analyses |
| 8.1.1.5 Other (unspecified) analyses |
| 8.1.2 Storage of laboratory samples and specimens |
| 8.1.2.1 Toxicological analyses |
| 8.1.2.2 Biomedical analyses |
| 8.1.2.3 Arterial blood gas analyses |
| 8.1.2.4 Haematological analyses |
| 8.1.2.5 Other (unspecified) analyses |
| 8.1.3 Transport of laboratory samples and specimens |
| 8.1.3.1 Toxicological analyses |
| 8.1.3.2 Biomedical analyses |
| 8.1.3.3 Arterial blood gas analyses |
| 8.1.3.4 Haematological analyses |
| 8.1.3.5 Other (unspecified) analyses |
| 8.2 Toxicological analysis and their interpretation |
| 8.2.1 Tests on toxic ingredient(s) of material |
| 8.2.1.1 Simple qualitative test(s) |
| 8.2.1.2 Advanced qualitative confirmation test(s) |
| 8.2.1.3 Simple quantitative method(s) |
| 8.2.1.4 Advanced quantitative method(s) |
| 8.2.2 Test for biological specimens |
| 8.2.2.1 Simple qualitative test(s) |
| 8.2.2.2 Advanced qualitative confirmation test(s) |
| 8.2.2.3 Simple quantitative method |
| 8.2.2.4 Advanced quantitative method(s) |
| 8.2.2.5 Other dedicated method(s) |
| 8.2.3 Interpretation of toxicological analysis |
| 8.3 Biomedical investigations and their interpretation |
| 8.3.1 Biochemical analysis |
| 8.3.1.1 Blood, plasma or serum |
| 8.3.1.2 Urine |
| 8.3.1.3 Other fluids |
| 8.3.2 Arterial blood gas analyses |
| 8.3.3 Haematological analyses |
| 8.3.4 Interpretation of biomedical investigations |
| 8.4 Other biomedical (diagnostic) investigations and their interpretation |
| 8.5 Overall interpretation of all toxicological analysis and toxicological investigations |
| 9. CLINICAL EFFECTS |
| 9.1 Acute poisoning |
| 9.1.1 Ingestion |
| 9.1.2 Inhalation |
| 9.1.3 Skin exposure |
| 9.1.4 Eye contact |
| 9.1.5 Parenteral exposure |
| 9.1.6 Other |
| 9.2 Chronic poisoning |
| 9.2.1 Ingestion |
| 9.2.2 Inhalation |
| 9.2.3 Skin exposure |
| 9.2.4 Eye contact |
| 9.2.5 Parenteral exposure |
| 9.2.6 Other |
| 9.3 Course, prognosis, cause of death |
| 9.4 Systematic description of clinical effects |
| 9.4.1 Cardiovascular |
| 9.4.2 Respiratory |
| 9.4.3 Neurological |
| 9.4.3.1 Central nervous system (CNS) |
| 9.4.3.2 Peripheral nervous system |
| 9.4.3.3 Autonomic nervous system |
| 9.4.3.4 Skeletal and smooth muscle |
| 9.4.4 Gastrointestinal |
| 9.4.5 Hepatic |
| 9.4.6 Urinary |
| 9.4.6.1 Renal |
| 9.4.6.2 Other |
| 9.4.7 Endocrine and reproductive systems |
| 9.4.8 Dermatological |
| 9.4.9 Eye, ear, nose, throat: local effects |
| 9.4.10 Haematological |
| 9.4.11 Immunological |
| 9.4.12 Metabolic |
| 9.4.12.1 Acid-base disturbances |
| 9.4.12.2 Fluid and electrolyte disturbances |
| 9.4.12.3 Others |
| 9.4.13 Allergic reactions |
| 9.4.14 Other clinical effects |
| 9.4.15 Special risks |
| 9.5 Other |
| 9.6 Summary |
| 10. MANAGEMENT |
| 10.1 General principles |
| 10.2 Life supportive procedures and symptomatic/specific treatment |
| 10.3 Decontamination |
| 10.4 Enhanced elimination |
| 10.5 Antidote treatment |
| 10.5.1 Adults |
| 10.6.2 Children |
| 10.6 Management discussion |
| 11. ILLUSTRATIVE CASES |
| 11.1 Case reports from literature |
| 12. ADDITIONAL INFORMATION |
| 12.1 Specific preventive measures |
| 12.2 Other |
| 13. REFERENCES |
| 14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE ADDRESS(ES) |
FLUOXETINE
International Programme on Chemical Safety
Poisons Information Monograph 651
Pharmaceutical
1. NAME
1.1 Substance
Fluoxetine
1.2 Group
ATC: Nervous system
Psychoanaleptic
Antidepressant
Selective serotonin reuptake inhibitor
ATC code: N06AB03
Straight chain phenylpropylamide structurally unrelated to
the tricyclic antidepressants.
(Dista Lab. Prod. Info., 1989).
1.3 Synonyms
LY-110140
1.4 Identification numbers
1.4.1 CAS number
54910-89-3
1.4.2 Other numbers
Fluoxetine hydrochloride CAS number: 59333-67-4
1.5 Main brand names, main trade names
Adofen (Spain);
Docutrix (Spain);
Fluctin (Germany);
Fluctine (Australia, Switzerland);
Flunirin;
Fluoxeren (Italy);
Fluoxetin;
Fluoxetina;
Fontex;
Fontisin;
Ladose;
Lovan;
Prozac (Australia, Belgium, Canada, France, Italy,
Netherlands, South Africa, Spain, U.K., U.S.A.);
Prozyn (South Africa);
Reneuron (Spain)
1.6 Main manufacturers, main importers
Dista (France)
Ferrer (Spain)
Hoechst (Germany)
Juste (Spain)
Lilly (U.S.A., Germany, Switzerland, UK)
Menarini (Italy)
2. SUMMARY
2.1 Main risks and target organs
Fluoxetine is safer in overdose than most other classes
of antidepressants. In overdosage, most patients experience
only mild neurological and gastroenterological symptoms;
significant cardiovascular toxicity is unusual.
The serotonergic effects of fluoxetine may be enhanced by
combination with other antidepressants, monoamine oxidase
inhibitors, carbamazepine or lithium and produce a
life-threatening serotoninergic syndrome comprising
hyperthermia, tremor and convulsions.
2.2 Summary of clinical effects
Drowsiness, tremor, headache, blurred vision, dizziness,
restlessness, and rarely, seizures and coma.
Nausea, vomiting, abdominal pain.
Bradycardia, mild hypertension or hypotension.
2.3 Diagnosis
Diagnosis of fluoxetine poisoning is clinical and based
on history of overdose and/or access to fluoxetine, and the
presence of minor neurological and/or gastroenterological
symptoms. Alternative diagnoses should be considered when
significant neurological or cardiovascular symptoms or signs
are present.
Diagnosis of the serotonergic syndrome should be considered
in the presence of three or more of the following symptoms:
behavioural change (confusion or hypomania), agitation,
myoclonus, hyperreflexia, sweating, shivering, tremor,
diarrhoea, motor incoordination, fever. The differential
diagnosis includes neuroleptic malignant syndrome, acute with
non-specific monoamine oxidase inhibitors or strychnine,
acute sepsis, or severe metabolic disturbances.
2.4 First aid measures and management principles
Management of isolated fluoxetine overdose consists
primarily of observation and basic supportive care until
symptoms resolve. Doses of 40 to 800 mg produce minimal, if
any, symptoms. Administration of activated charcoal is
recommended following ingestions of greater than 800 mg.
Treatment of the serotonergic syndrome requires more
aggressive supportive care including diazepam, mechanical
ventilation and, if necessary, curarization. Although several
deaths are reported, the symptoms usually resolve within 1 to
2 days with supportive treatment.
3. PHYSICO-CHEMICAL PROPERTIES
3.1 Origin of the substance
Obtained by synthesis.
3.2 Chemical structure
Structural formula:
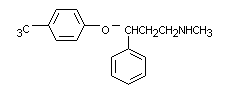 Molecular formula: C17H18F3NO, HCl
Molecular weight: 345.8 (Base: 302.3)
Structural name: (dl)-N-Methyl-3-phenyl-3-
(alpha,alpha,alpha-trifluoro-p-
tolyloxy)propylamine
hydrochloride.
3.3 Physical properties
3.3.1 Properties
3.3.3.1 Colour
White
3.3.3.2 State/Form
Crystals. Crystalline powder.
3.3.3.3 Description
Melting point:157.5 to 158.7°C
Solubility in: water: 50 mg/mL at 25°C
methanol: 250 mg/mL
chloroform: 125 mg/mL
Insoluble in hexane, ethyl acetate and
benzene.
pH 4.35 (aqueous saturated solution).
3.4 Other characteristics
3.4.1 Shelf-life of the substance
5 years at 15 to 25°C.
3.4.2 Storage conditions
Stable at 40°C and 75% humidity in polyethylene
bottles or foil packs for at least five years.
4. USES
4.1 Indications
4.1.1 Indications
4.1.2 Description
Accepted: Major mental depression
Obsessive compulsive disorder
Investigational: Pain syndromes
Panic disorder
Sleep disorders
Eating disorders
4.2 Therapeutic dosage
4.2.1 Adults
Usual dose: 20 mg daily; doses of up to 80 mg
daily in divided doses may be employed if necessary
(Dista Lab. Prod. Info., 1989; Reynolds, 1995; Vidal,
1996).
Recommended maximum dose for elderly patients: 60 mg
daily.
Lower doses and/or alternate-day dosing have been
recommended in patients with significant hepatic
impairment (Reynolds, 1995).
4.2.2 Children
Fluoxetine is presently regarded as
contraindicated for use in children. However, some
studies suggest it may have a role in the management
of major depression, anxiety and obsessive-compulsive
disorders in children (Geller et al., 1995).
4.3 Contraindications
Absolute: Hypersensitivity to fluoxetine
Children under 15 years old
Coadministration of sumatriptan, non-specific
monoamine oxidase inhibitors and B-specific
monoamine oxidase inhibitors.
Relative: Combination therapy with A-specific monoamine
oxidase inhibitors or other
antidepressants
Pregnancy
Lactation
5. ROUTES OF ENTRY
5.1 Oral
Fluoxetine is administered orally.
5.2 Inhalation
Not relevant.
5.3 Dermal
Not relevant.
5.4 Eye
Not relevant
5.5 Parenteral
Not relevant
5.6 Other
No data available.
6. KINETICS
6.1 Absorption by route of exposure
Fluoxetine hydrochloride is readily absorbed from the
gastrointestinal tract with peak plasma concentrations
appearing from 6 to 8 hours after oral administration.
Peak levels for 30 mg, 60 mg, and 75 mg doses were 30.1
ng/mL, 93.0 ng/mL and 134.6 ng/mL respectively (Saletu &
Grunberger, 1985).
The systemic bioavailability is greater than 85 % and does
not appear to be affected by food (Dista lab. Prod. Inf.,
1989).
6.2 Distribution by route of exposure
Fluoxetine is widely distributed throughout the body.
Plasma protein binding is 94 %.
The volume of distribution is highly variable, ranging from
11 to 88 L/kg.
6.3 Biological half-life by route of exposure
Fluoxetine has a relatively long and highly variable
half-life ranging from 1 to 4 days after a single dose and
averaging nearly 70 hours; patients receiving high doses over
long periods of time may exhibit prolonged elimination
half-lives.
The half-life of its active metabolite, norfluoxetine, is
about 7 to 9 days.
6.4 Metabolism
Fluoxetine is extensively metabolized in the liver to a
desmethyl metabolite, norfluoxetine, which has activity
similar to fluoxetine.
Peak plasma concentrations of the active metabolite,
norfluoxetine, occur around 76 hours after ingestion.
6.5 Elimination and excretion
The primary route of elimination appears to be further
hepatic metabolism to inactive metabolites which are
conjugated and then excreted in the urine.
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
Fluoxetine is a potent inhibitor of serotonin
re-uptake by Central Nervous System neurones and may
interact with other drugs or circumstances which cause
serotonin release. The enhancement of the serotonergic
effects may produce a life-threatening serotonin
syndrome.
7.1.2 Pharmacodynamics
Fluoxetine specifically inhibits neuronal
re-uptake of serotonin, thus increasing the
concentration of the serotonin at the synapse and
reinforcing of serotonergic neuronal transmission.
Fluoxetine has little effect on other
neurotransmitters.
Fluoxetine has no direct effect on the heart (Dista
lab. Prod. Inf., 1989).
Fluoxetine inhibits liver drug-metabolising enzymes
including CYP IID6, CYP IA2 and CYP IIIA4 (Lane et
al., 1995).
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
Estimated minimum lethal exposure:
1200 to 2000 mg (Kincaid et al., 1990).
Maximum tolerated exposure: 300 to 400 mg
with no co-ingestant.
7.2.1.2 Children
A 12-year-old boy ingested 1888 mg
(26 mg/kg) and experienced seizures and
minimal ECG changes (Riddle et al.,
1989).
A 4-year-old child ingested 43 mg/kg and
experienced unresponsiveness, sinus
tachycardia, moderate psychomotor agitation
and dyskinesia (Feierabend, 1995).
The highest dose at which no toxicity was
observed is 3.6 mg/kg (Borys et al.,
1990).
7.2.2 Relevant animal data
Lethal seizures occurred in 50% of various
animals given 200 to 700 mg/kg. The exact dose needed
is species dependent (Dista lab. Prod. Inf.,
1989).
7.2.3 Relevant in vitro data
No data available.
7.3 Carcinogenicity
Human studies: there is no evidence of carcinogenicity
in patients taking fluoxetine.
Animal studies: fluoxetine was not carcinogenic in rats and
mice at doses ten times the recommended daily dose for 24
months (Dista lab. Prod. Info., 1989).
7.4 Teratogenicity
Animal studies: in rats, fluoxetine and norfluoxetine
cross the placenta and distribute within the fetus during the
periods of organogenesis and postorganogenesis (Pohland et
al., 1989). Levels in fetal tissue are approximately half the
corresponding maternal concentrations.
Fluoxetine does not impair the fetal growth in rats or
rabbits at doses nine and eleven times the maximum daily
human dose respectively (Dista lab. Prod. Info., 1989).
Human studies: a study of 128 women exposed to fluoxetine
during the first trimester showed no increase in major fetal
malformations. It is not known, however, if the drug is a
human teratogen (Pastuszak et al., 1993).
A neonate whose mother had been taking fluoxetine during most
of her pregnancy sufferred tachypnoea, emesis, continuous
crying, irritability, tremor and increased muscle tone; the
symptoms resolved within 96 hours (Spencer, 1993).
Fluoxetine and norfluoxetine are excreted in breast milk
(Burch & Wells, 1992); the effects on the infant are
uncertain. Caution should be exercised when fluoxetine is
administered to a nursing mother (Dista lab. Prod.Info.,
1989).
7.5 Mutagenicity
In vitro: fluoxetine and norfluoxetine did not show
mutagenicity in the Ames test. There was no induction of
sister-chromatid exchange in the bone marrow of the chinese
hamster (Dista lab. Pro.Info., 1989).
7.6 Interactions
Drug interactions with fluoxetine have been reported
with L-tryptophan, L-dopa; monoamine oxidase inhibitors:
selegiline, tranylcypromine; tricyclic antidepressants;
selective serotonin re-uptake inhibitors: trazodone,
zimeldine; benzodiazepines: alprazolam, diazepam; buspirone,
lithium, anticonvulsants: carbamazepine, phenytoin,
valproate; pentazocine, dextromethorphan, fenfluramine,
calcium channel blockers, benztropine, cyproheptadine,
clarithromycin (Pollak et al., 1995), and substances of
abuse: cannabis, ethanol, LSD (Jackson & Hornfeldt, 1991;
Messiha, 1993).
At least 14 days should elapse between discontinuing a
MAO-inhibiting antidepressant and introducing fluoxetine.
Conversely, because of the long half-life of fluoxetine and
its metabolite, norfluoxetine, it is recommended that at
least 5 weeks should elapse between discontinuation of
fluoxetine and the introduction of a MAO inhibitor (Dista
lab. Prod. Info., 1989).
7.7 Main adverse effects
The major adverse effects reported with therapeutic
doses of fluoxetine are primarily those of headache,
insomnia, nausea, and nervousness, with a prevalence of 15 to
23 %. Less common adverse effects include tremors, sweating,
dry mouth, anxiety, drowsiness, and diarrhoea, with a
prevalence of 10 to 14 % (Messiha, 1993).
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
Toxic ingredient: liquids, capsules,
scene residues
In case of ingestion:
Vomitus: total amount
Gastric aspirate: total amount
(or gastric lavage: first
portion: 100 mL)
Whole blood without additives: 10 mL
Urine: random specimen: 50 mL
8.1.1.2 Biomedical analyses
Plasma (lithium heparin as
anticoagulant) or serum and urine for
standard biochemical analyses.
8.1.1.3 Arterial blood gas analyses
Heparinized arterial blood sample
(in severe cases).
8.1.1.4 Haematological analyses
Anticoagulated blood (e.g. EDTA) for
standard haematological analyses and
differential blood picture.
8.1.1.5 Other (unspecified) analyses
No further materials.
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
Serum, urine and other materials in
a refrigerator (4°C).
8.1.2.2 Biomedical analyses
No special requirements, but as
usually performed.
8.1.2.3 Arterial blood gas analyses
No special requirements, but as
usually performed.
8.1.2.4 Haematological analyses
No special requirements, but as
usually performed.
8.1.2.5 Other (unspecified) analyses
Not applicable.
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
No special requirements, but as
usually performed.
8.1.3.2 Biomedical analyses
No special requirements, but as
usually performed.
8.1.3.3 Arterial blood gas analyses
No special requirements, but as
usually performed.
8.1.3.4 Haematological analyses
No special requirements, but as
usually performed.
8.1.3.5 Other (unspecified) analyses
Not applicable.
8.2 Toxicological analysis and their interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple qualitative test(s)
8.2.1.2 Advanced qualitative confirmation test(s)
8.2.1.3 Simple quantitative method(s)
8.2.1.4 Advanced quantitative method(s)
8.2.2 Test for biological specimens
8.2.2.1 Simple qualitative test(s)
8.2.2.2 Advanced qualitative confirmation test(s)
8.2.2.3 Simple quantitative method
8.2.2.4 Advanced quantitative method(s)
8.2.2.5 Other dedicated method(s)
8.2.3 Interpretation of toxicological analysis
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
Sodium, potassium, chloride,
calcium, magnesium Glucose, amylase, creatine
kinase, creatinine (urea) Optional:
bicarbonate (or total CO2), uric
acid
8.3.1.2 Urine
8.3.1.3 Other fluids
No dedicated test.
8.3.2 Arterial blood gas analyses
pH, pCO2, pO2, base excess, actual HCO3-,
and O2-saturation.
8.3.3 Haematological analyses
Complete blood count, including platelets,
haematocrit.
Differential blood picture.
8.3.4 Interpretation of biomedical investigations
8.4 Other biomedical (diagnostic) investigations and their
interpretation
Serum electrolytes, urea and creatinine, liver function
tests, amylase, creatine kinase and arterial blood gases are
useful to optimize supportive care.
8.5 Overall interpretation of all toxicological analysis and
toxicological investigations
Analysis of Materials
Fluoxetine is not easily detected in materials by simple
physicochemical tests. The UV spectrum in methanol gives
deltamax at 227 nm; A| = 372 (Risley & Bopp, 1990). Using
thin layer chromatography, the drug can be visualized with
Mandelin's reagent (pale grey). In methanol ammonia (100:1.5)
the Rf is 0.24, and in ethyl acetate / methanol / ammonia
(85:15:5) the Rf is 0.50 (personal experience). Gas
chromatography is also useful, and the retention index for
fluoxetine is 1950 on SE54, SE30, OV1 or HP5 or similar.
Flame ionization detection has sufficient sensitivity for
residues; electron capture or nitrogen-phosphorus detection
give improved selectivity.
Analysis of Biological Specimens
Confirmation of the presence of fluoxetine may be useful in
some instances to confirm the diagnosis of intoxication, or
to explain the occurrence of serotonergic syndrome.
Measurement of serum concentrations is not usual clinical
practice. There are no commonly-available immunoassay kits
for drug testing (including those for tricyclic
antidepressants) which respond to fluoxetine. Extraction of
fluoxetine and norfluoxetine from biological specimens is
easily achieved by pH adjustment to 8 to 12, and addition of
an organic solvent (ethyl acetate, hexane / iso-amyl alcohol
etc.). Cleaning by back-extraction into dilute sulphuric
acid may be necessary for low concentrations. Thin layer
chromatography is applicable to the analysis of gastric
contents and urine. In methanol / ammonia (100:1.5) the Rf of
fluoxetine is 0.24, and 0.30 for norfluoxetine; in ethyl
acetate / methanol / ammonia (85:15:5) the Rf is 0.50 for
both compounds (personal experience). Both compounds can be
visualized with Mandelin's reagent (pale grey), and give a
positive iodoplatinate reaction.
Advanced Methods of Analysis
Gas chromatography can be performed without derivatization by
many methods which are suitable for analysis of other
antidepressant drugs, from which both fluoxetine and
norfluoxetine are well separated (run earlier than
amitriptyline). Care should be taken to ensure separation
from caffeine, especially on nitrogen-phosphorus detection.
Isothermal analysis on OV1 or HP1 gives good separation at
180°C within 5 minutes (Rohrig & Prouty, 1989). DB5 and DB17
are also useful. FID is adequate for overdose concentrations
(0.02 mg/L), but nitrogen-phosphorus (Kincaid et al., 1990;
Fontanille et al., 1997) and electron capture detection
(Dixit et al., 1991) both give improved sensitivity (0.001 to
0.005 mg/L from 1 mL sample). Derivatization of both
compounds with butyric anhydride gives more characteristic
spectra on GCMS, particularly for fluoxetine (Kincaid et al.,
1990).
HPLC is commonly used for quantitative serum measurements.
Good chromatography over 8 minutes was obtained using C8
column with a mobile phase of triethylamine acetate (10 mL/L,
pH to 5.5 with glacial acetic acid) / acetonitrile (65:35)
mobile phase (Nichols et al., 1994), or with 0.067M potassium
dihydrogenphosphate buffer / acetonitrile (70:30) (Thomare et
al., 1992). Both methods use UV detection at 228 nm and give
sensitivity of 0.01 mg/L and 0.002 mg/L respectively.
Alternatively, excellent separation was seen over 12 minutes
on a C18 column with high sensitivity (<0.005 mg/L) by
pre-column derivatization with dansyl chloride and
fluorescence detection (Suckow et al., 1992).
Chiral analysis of fluoxetine and norfluoxetine is possible
after derivatization with the chiral reagent,
(S)-(-)-N-trifluoroacetylprolyl chloride followed by gas
chromatography on DB5 with electron capture detection
(Torok-Both et al., 1992).
Toxicity is not well correlated with fluoxetine
concentration, and toxicity can often be attributed to other
causative agents. Serotonergic syndromes may occur with
relatively low fluoxetine concentrations (<0.1 mg/L). As a
guide, typical concentrations of fluoxetine in serum are:
mg/L µmol/L
Peak after single oral dose
(30 - 75 mg)
6 - 8 hours 0.03 - 0.13 0.1- 0.43
Steady-state in therapy
fluoxetine <0.3 <1.0
norfluoxetine <0.7 <2.33
Serious toxicity possible >0.5 >1.67
Serum concentrations of 1 to 2 mg/L have been reported
without serious toxicity.
Serum electrolytes, urea and creatinine and arterial blood
gases are useful to optimize supportive care.
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Blurred vision, nausea, vomiting, abdominal
pain, diarrhoea, lethargy, dizziness, insomnia,
tremors, and rarely, seizures and coma.
Significant cardiovascular toxicity is unusual.
Effects have included mild hypo- or hypertension,
tachycardia, and ventricular dysrhythmia.
Co-ingestion of serotonin-reuptake inhibitors can
result in aggressive behaviour, confusion, tremor,
restlessness, shivering, diaphoresis, hyperthermia,
diarrhoea, and myoclonus.
9.1.2 Inhalation
Not relevant.
9.1.3 Skin exposure
Not relevant.
9.1.4 Eye contact
Unknown.
9.1.5 Parenteral exposure
No data available.
9.1.6 Other
No data available.
9.2 Chronic poisoning
9.2.1 Ingestion
No data available.
9.2.2 Inhalation
Not relevant.
9.2.3 Skin exposure
Not relevant.
9.2.4 Eye contact
No data available.
9.2.5 Parenteral exposure
No data available.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
The clinical course of fluoxetine poisoning is generally
very mild in nature and of short duration. Complete and rapid
recovery is the rule. A few deaths have been reported in the
forensic literature, seemingly after ingestion of very high
doses. Most involved co-ingestants and all are poorly
documented (Kincaid et al., 1990).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Sinus tachycardia, bradycardia (Feder, 1991),
hypotension, mild hypertension, ECG changes especially
QT interval prolongation and ventricular dysrhythmias.
Patients with preexisting cardiovascular disease or
taking cardiac medications may be at greater risk of
developing fluoxetine cardiotoxicity (Ellison et al.,
1990).
9.4.2 Respiratory
Dyspnea, cough and flu-like symptoms have been
reported (Kim & Pentel, 1989).
Induction of dyspnoea and coughing in a female smoker
has been attributed to a fluoxetine-nicotine
interaction. Dyspnoea, cough and flu-like symptoms
have been reported (Bass & Colebatch, 1992).
9.4.3 Neurological
9.4.3.1 Central nervous system (CNS)
Drowsiness, headache, restlessness,
delirium, insomnia, ataxia, extrapyramidal
syndrome (Baldwin et al., 1991; Eisenhauer &
Jermain, 1993), coma and seizures.
Psychiatric: manic and hypomanic states. It
has also been suggested that fluoxetine may
induce suicidal ideation in a small subset of
patients (Crundwell, 1993; Tueth,
1994).
9.4.3.2 Peripheral nervous system
Blurred vision.
9.4.3.3 Autonomic nervous system
Bradycardia or tachycardia.
9.4.3.4 Skeletal and smooth muscle
Tremor, myoclonus, dystonia.
9.4.4 Gastrointestinal
Abdominal pain, nausea, vomiting, diarrhoea.
Anorexia leading to weight loss.
9.4.5 Hepatic
Mild liver enzyme disturbances are reported
(Bobichon et al., 1993; Castiella & Arenas,
1994).
9.4.6 Urinary
9.4.6.1 Renal
No data available.
9.4.6.2 Other
No data available.
9.4.7 Endocrine and reproductive systems
Fluoxetine may alter glycaemic control in
diabetic subjects.
Fluoxetine is associated with the syndrome of
inappropriate secretion of antidiuretic hormone
(Staab, 1990).
Sexual disorders: anorgasmia, penile anaesthesia,
sexual arousal (Modell, 1989; Morris, 1991; Measom,
1992).
9.4.8 Dermatological
Skin rash, pruritus, urticaria, psoriasis,
photosensitivity reactions, alopecia (Hemlock et al.,
1992; Gupta et al., 1993; Ogilvie, 1993; Gaufberg &
Ellison, 1995; O'Brien, 1995; Roger et al.,
1995).
9.4.9 Eye, ear, nose, throat: local effects
Voice disorder (Murray, 1995).
9.4.10 Haematological
Abnormal platelet aggregation, prolongation of
the bleeding time (Humphries et al., 1990; Alderman et
al., 1992; Aranth & Lindberg, 1992).
9.4.11 Immunological
Skin rashes (Gupta et al., 1993), angioedema,
serum sickness and anaphylactoid reactions have been
reported (Roger et al., 1995).
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Acidosis is expected in association
with coma and/or convulsions.
9.4.12.2 Fluid and electrolyte disturbances
Several cases of hyponatraemia,
hypo-osmolarity and syndrome of inappropriate
secretion of antidiuretic hormone have been
reported (Staab, 1990).
9.4.12.3 Others
No data available.
9.4.13 Allergic reactions
Skin rashes.
9.4.14 Other clinical effects
Stuttering (Guthrie & Grunhaus, 1990).
9.4.15 Special risks
The safety of fluoxetine during pregnancy and
lactation is not established (see section
7.4).
9.5 Other
Several cases of fluoxetine withdrawal syndrome have
been described, occurring from one to seven days after
discontinuation of the medication. Symptoms included
vertigo, dizziness, light-headedness, irritability, anxiety,
headache, sweating, and nausea (Einbinder, 1995; Berlin,
1996).
9.6 Summary
10. MANAGEMENT
10.1 General principles
The primary management of isolated fluoxetine overdose
consists of institution of appropriate observation and
supportive care. In most cases, this consists of no more
than careful observation of vital signs and neurological
status until signs and symptoms resolve. Intravenous access
should be established as soon as practicable. In more severe
intoxications or where there is a co-ingestion, more
aggressive measures such as establishment of an airway,
ventilation, administration of intravenous fluids, control of
seizures, and control of hyperthermia may be
necessary.
10.2 Life supportive procedures and symptomatic/specific
treatment
Intensive supportive care is rarely required. Measures
that may occcasionally be required are: endotracheal
intubation and/or artificial ventilation if coma is present,
intravenous fluid resuscitation if hypotension is present,
control of seizures, cooling if hyperthermia is present
(refer to appropriate treatment guides for more detailed
management guidance).
10.3 Decontamination
Gastrointestinal decontamination by administration of a
single dose of oral activated charcoal may be indicated
following ingestion of more than 800 mg of fluoxetine by an
adult presenting within two hours, or where there has been a
significant co-ingestion.
10.4 Enhanced elimination
There are no effective methods known to enhance the
elimination of fluoxetine.
10.5 Antidote treatment
10.5.1 Adults
No data available.
10.6.2 Children
No data available.
10.6 Management discussion
Although of theoretical benefit, the role of dantrolene
in the management of the serotonergic syndrome has yet to be
evaluated.
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
A 15-year-old female ingested forty five 20 mg
fluoxetine tablets. Ten hours later, she was found jerking,
frothing at the mouth and with a staring gaze. On arrival at
hospital thirty minutes later, she had a normal level of
consciousness. She denied the ingestion of any other
medications and had no previous history of seizures or
trauma. Physical examination and all routine laboratory
studies were unremarkable. Comprehensive drug screening did
not detect the presence of any other substances. Serum
fluoxetine and norfluoxetine concentrations were 1956 ng/mL
and 416 ng/mL respectively (Braitberg & Curry, 1995).
A 4-year-old child ingested 43 mg/kg of fluoxetine. Toxic
effects consisted of a brief period of unresponsiveness,
sinus tachycardia and moderate psychomotor agitation and
dyskinesia. The child recovered completely with supportive
care (Feierabend, 1995).
A 40-year-old female developed a classical serotoninergic
syndrome with severe hyperthermia (43°C) eight hours after
ingesting 340 mg fluoxetine, 6000 mg moclobemide, 300 mg
clomipramine and 12 mg clonazepam. She died from the
subsequent complications of fulminant disseminated
intravascular coagulation and multiple organ failure (Power
et al., 1995).
A patient being treated with carbamazepine 200 mg/day for an
affective disorder, developed a serotonergic syndrome 14 days
following institution of fluoxetine therapy at a dose of 20
mg/day. In addition to features of the serotonergic
syndrome, she reported dizziness, experienced an oculogyric
crisis, and developed leucopenia and thrombocytopenia. She
made a complete recovery within 72 hours of the
discontinuation of fluoxetine (Dursun et al., 1993).
12. ADDITIONAL INFORMATION
12.1 Specific preventive measures
12.2 Other
13. REFERENCES
Alderman CP, Moritz CK, & Ben-Tovim DI (1992) Abnormal
platelet aggregation associated with fluoxetine therapy. Ann
Pharmacotherapy, 26: 1517-1519.
Aranth J & Lindberg C (1992) Bleeding as a side effect of
fluoxetine. Am J Psychiatry, 149: 412.
Baldwin D, Fineberg N, & Montgomery S (1991) Fluoxetine,
fluvoxamine and extrapyramidal tract disorders. Int Clin
Psychopharmacol, 6: 51-58.
Bass SP & Colebatch HJH (1992) Fluoxetine-induced lung damage. Med
J Austral, 156: 364.
Berlin C (1996) Fluoxetine withdrawal symptoms. J Clin Psychiatry,
57, (2): 93-94.
Bobichon R, Bernard G, & Mion F (1993) [Acute hepatitis during
treatment with fluoxetine]. Gastroenterologie Clinique et
Biologique, 17 (5): 406-7 (in French).
Borys D, Setzer S, Ling L, Reisdorf J, Day L, & Krenzelok E (1990)
The effects of fluoxetine in the overdose patient. Clin Toxicol,
28 (3): 331-340.
Braitberg G & Curry SC (1995) Seizure after isolated fluoxetine
overdose. Ann Emerg Med, Aug; 26 (2): 234-7.
Burch KJ & Wells BG (1992) Fluoxetine/norfluoxetine concentrations
in human milk. Pediatrics, 98: 676-677.
Castiella A & Arenas J (1994) Fluoxetine hepatotoxicity. Am J
Gastroenterology, 89 (3): 458-9.
Crundwell JK (1993) Fluoxetine and suicidal ideation-a review of
the literature. [review] Int J Neurosci, 68 (1-2): 73-84.
Dista Laboratoires (1989) Prozac 20 mg. Manufacturer information.
92213 Saint Cloud, France.
Dixit V, Nguyen H & Dixit YM (1991) Solid-phase extraction of
fluoxetine and norfluoxetine from serum with gas
chromatography-electron capture detection. J Chromatogr, 563:
379-384.
Dursun S, Mathew V, & Reveley M (1993) Toxic serotonin syndrome
after fluoxetine plus carbamazepine. Lancet, 342: 442-3.
Einbinder E (1995) Fluoxetine withdrawal. Amer J Psychiatry, 152,
(8): 1235.
Eisenhauer G & Jermain DM (1993) Fluoxetine and tics in an
adolescent. Annals of Pharmacotherapy, 27 (6): 725-6.
Ellison J, Milofsky J, & Ely E (1990) Fluoxetine-induced
bradycardia and syncope in two patients. J Clin Psychiatry, 51:
385-386.
Feder R (1991) Bradycardia and syncope induced by fluoxetine. J
Clin Psychiatry, 52: 139.
Feierabend R (1995) Benign course in a child with a massive
fluoxetine overdose. J Fam Pract, 41 (3): 289-291.
Fontanille P, Jourdil N, Villier C & Bessard G (1997) Direct
analysis of fluoxetine and norfluoxetine in plasma by gas
chromatography with nitrogen-phosphorus detection. J Chromatogr,
692: 337-343.
Gaufberg E & Ellison JM (1995) Photosensitivity reaction to
fluoxetine. J Clin Psychiatry, 56, (10): 486.
Geller DA, Biederman J, Reed ED, Spencer T, & Wilens TE (1995)
Similarities in response to fluoxetine in the treatment of
children and adolescents with obsessive-compulsive disorder. J
Amer Acad Child Adol. Psychiatry, 34 (1): 36-44.
Gupta R, Parker G, Norman T, Judd F, & Burrows G (1993)
Fluoxetine-delayed half-life and an adverse event. Med J
Australia, 158: 722-723.
Guthrie S & Grunhaus L (1990) Fluoxetine-induced stuttering. J
Clin Psychiatry, 51: 85.
Hemlock C, Rosenthal J, & Winston A (1992) Fluoxetine-induced
psoriasis. Ann Pharmacother, 26: 211-212.
Humphries J, Wheby M, & Vanden Berg S (1990) Fluoxetine and the
bleeding time. Arch Path Lab Med, 114: 727-728.
Jackson TW & Hornfeldt C (1991) Seizure activity following
recreational LSD use in patients treated with lithium and
fluoxetine (abstract). Vet Hum Toxicol, 33: 387.
Kim SW & Pentel P (1989) Flu-like symptoms associated with
fluoxetine overdose: a case report. J Toxicol Clin Toxicol, 27:
389-393.
Kincaid R, Mc Mullin M, Crookham S, & Reiders F (1990) Report of a
fluoxetine fatality. J Anal Toxicol, 14: 327-329.
Lane R, Baldwin D, & Preskorn S (1995) The SSRIs: Advantages,
disadvantages and differences. J Psychopharmacol, 9/2 suppl,
163-178.
Measom M (1992) Penile anesthesia and fluoxetine. Am J Psychiatry,
149: 709.
Messiha F (1993) Fluoxetine: adverse effects and
drug-drug-interactions. Clin Toxicol, 31 (4): 603-630.
Modell JG (1989) Repeated observations of yawning, clitoral
engorgement, and orgasm associated with fluoxetine administration.
J Clin Psychopharmacol, 9: 63-65.
Morris PLP (1991) Fluoxetine and orgasmic sexual experiences. Int
J Psychiatry Med, 21: 379-389.
Murray V (1995) Laryngeal dystonia. Br J Psychiatry, 167 (5):
698-9.
Nichols JH, Charlson JR & Lawson GM (1994) Automated HPLC assay
of fluoxetine and norfluoxetine in serum. Clin Chem, 40:
1312-1316.
O'Brien T (1995) Phototherapy burns and fluoxetine. Australasian J
Dermatology, 36 (2): 103.
Ogilvie A (1993) Hair loss during fluoxetine treatment. Lancet,
342: 1423.
Pastuszak A, Schick-Boschetto B, Zuber C, Feldkamp M, Pinelli M,
et al. (1993) Pregnancy outcome following first trimester exposure
to fluoxetine (Prozac). JAMA, 269: 2246-2248.
Pohland R, Byrd T, Hamilton M, & Koons J (1989) Placental transfer
and fetal distribution of fluoxetine in the rat. Toxicol Appl
Pharmacol, 98: 198-205.
Pollak P, Sketris I, Mc Kenzie S, & Hewlett T (1995) Delirium
probably induced by clarithromycin in a patient receiving
fluoxetine. Ann Pharmacother, 29 (5): 486-8.
Power B, Pinder M, Hackett L, & Ilett K (1995) Fatal serotonin
syndrome following a combined overdose of moclobemide,
clomipramine and fluoxetine. Anaesth Intensive Care, 23 (4):
499-502.
Reynolds JEF ed. (1995) Martindale: The Extra Pharmacopoeia, 31th
ed. London, The Pharmaceutical Press. pp 312-314.
Riddle MA & al. (1989) Fluoxetine overdose in an adolescent. J Am
Acad Child Adolesc Psychiatr, 28: 587-588.
Risley DS & Bopp RJ (1990) Fluoxetine. In: Florey K ed
Analytical Profiles of Drug Substances, New York, Academic press,
19: pp 193-219.
Roger D. Rolle F, Mausset J, Lavignac C, & Bonnetblanc J (1995)
Urticarial vasculitis induced by fluoxetine. Dermatology, 191 (2):
164.
Rohrig T & Prouty RW (1989) Fluoxetine overdose: a case report. J
Anal Toxicol, 13: 305-307.
Saletu B & Grunberger T (1985) Classification and determination of
cerebral bioavailability of fluoxetine: pharmacokinetic,
pharmaco-EEG, and psychometric analysis. J Clin Psychiatry, 46:
45-52.
Spencer M (1993) Fluoxetine hydrochloride (Prozac) toxicity in a
neonate. Pediatrics, 92 (5): 721-722.
Staab J (1990) Transient SIADH associated with fluoxetine. Am J
Psychiatry, 147:1569-1570.
Suckow RF, Zhang MF, Cooper TB (1992) Sensitive and selective
liquid-chromatographic assay of fluoxetine and norfluoxetine in
plasma with fluorescent detection after precolumn derivatization.
Clin Chem, 38: 1756-1761.
Thomare P, Wang K, Van Der Meersch-Mougeot V & Diquet B (1992)
Sensitive micro-method for column liquid chromatographic
determination of fluoxetine and norfluoxetine in human plasma. J
Chromatogr, 583: 217-221.
TIAFT, The Bulletin of the International Association of Forensic
Toxicologists, 1995, 26 (1) suppl.
Torok-Both GA, Baker GB, Coutts RT, McKenna KF & Aspeslet LJ
(1992) Simultaneous determination of fluoxetine and norfluoxetine
enantiomers in biological samples by gas chromatography with
electron capture detection. J Chromatogr, 579: 99-106.
Tueth MJ (1994) Revisiting fluoxetine (Prozac) and suicidal
preoccupations. J Emerg Med, 12 (5): 685-687.
Vidal (1996), 72th ed. Paris.
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE
ADDRESS(ES)
Author: M.O. Rambourg Schepens,
Centre Anti-Poisons,
Hôpital Maison Blanche,
45 Rue Cognacq-Jay
F-51092 REIMS Cedex, France,
Tel: 33-326862686
Fax: 33-326865548
E-mail: marie-odile.rambourg@wanadoo.fr
Date: August 1996.
Reviewer: L.M. Murray,
Department of Emergency Medicine,
Prince of Wales Hospital,
Randwick, NSW, Australia.
Peer
review: Cardiff, United Kingdom, September 1996
(A. Borges, R. Ferner, M. Hanafy, M. Kowalczyk, L.
Murray, M.O. Rambourg Schepens)
Author
Section 8: Dr S. Dawling
Center for Clinical Toxicology
Vanderbilt University Medical Center
501 Oxford House
1161 21st Avenue South
Nashville, TN 37232-4632
United States of America
Tel: 1-615-9360760
Fax: 1-615-9360756
E-mail: sheila.dawling@mcmail.vanderbilt.edu
Date: March 1998
Editors: Dr M. Ruse (April 1997); Mrs J. Duménil (May 1999)
Molecular formula: C17H18F3NO, HCl
Molecular weight: 345.8 (Base: 302.3)
Structural name: (dl)-N-Methyl-3-phenyl-3-
(alpha,alpha,alpha-trifluoro-p-
tolyloxy)propylamine
hydrochloride.
3.3 Physical properties
3.3.1 Properties
3.3.3.1 Colour
White
3.3.3.2 State/Form
Crystals. Crystalline powder.
3.3.3.3 Description
Melting point:157.5 to 158.7°C
Solubility in: water: 50 mg/mL at 25°C
methanol: 250 mg/mL
chloroform: 125 mg/mL
Insoluble in hexane, ethyl acetate and
benzene.
pH 4.35 (aqueous saturated solution).
3.4 Other characteristics
3.4.1 Shelf-life of the substance
5 years at 15 to 25°C.
3.4.2 Storage conditions
Stable at 40°C and 75% humidity in polyethylene
bottles or foil packs for at least five years.
4. USES
4.1 Indications
4.1.1 Indications
4.1.2 Description
Accepted: Major mental depression
Obsessive compulsive disorder
Investigational: Pain syndromes
Panic disorder
Sleep disorders
Eating disorders
4.2 Therapeutic dosage
4.2.1 Adults
Usual dose: 20 mg daily; doses of up to 80 mg
daily in divided doses may be employed if necessary
(Dista Lab. Prod. Info., 1989; Reynolds, 1995; Vidal,
1996).
Recommended maximum dose for elderly patients: 60 mg
daily.
Lower doses and/or alternate-day dosing have been
recommended in patients with significant hepatic
impairment (Reynolds, 1995).
4.2.2 Children
Fluoxetine is presently regarded as
contraindicated for use in children. However, some
studies suggest it may have a role in the management
of major depression, anxiety and obsessive-compulsive
disorders in children (Geller et al., 1995).
4.3 Contraindications
Absolute: Hypersensitivity to fluoxetine
Children under 15 years old
Coadministration of sumatriptan, non-specific
monoamine oxidase inhibitors and B-specific
monoamine oxidase inhibitors.
Relative: Combination therapy with A-specific monoamine
oxidase inhibitors or other
antidepressants
Pregnancy
Lactation
5. ROUTES OF ENTRY
5.1 Oral
Fluoxetine is administered orally.
5.2 Inhalation
Not relevant.
5.3 Dermal
Not relevant.
5.4 Eye
Not relevant
5.5 Parenteral
Not relevant
5.6 Other
No data available.
6. KINETICS
6.1 Absorption by route of exposure
Fluoxetine hydrochloride is readily absorbed from the
gastrointestinal tract with peak plasma concentrations
appearing from 6 to 8 hours after oral administration.
Peak levels for 30 mg, 60 mg, and 75 mg doses were 30.1
ng/mL, 93.0 ng/mL and 134.6 ng/mL respectively (Saletu &
Grunberger, 1985).
The systemic bioavailability is greater than 85 % and does
not appear to be affected by food (Dista lab. Prod. Inf.,
1989).
6.2 Distribution by route of exposure
Fluoxetine is widely distributed throughout the body.
Plasma protein binding is 94 %.
The volume of distribution is highly variable, ranging from
11 to 88 L/kg.
6.3 Biological half-life by route of exposure
Fluoxetine has a relatively long and highly variable
half-life ranging from 1 to 4 days after a single dose and
averaging nearly 70 hours; patients receiving high doses over
long periods of time may exhibit prolonged elimination
half-lives.
The half-life of its active metabolite, norfluoxetine, is
about 7 to 9 days.
6.4 Metabolism
Fluoxetine is extensively metabolized in the liver to a
desmethyl metabolite, norfluoxetine, which has activity
similar to fluoxetine.
Peak plasma concentrations of the active metabolite,
norfluoxetine, occur around 76 hours after ingestion.
6.5 Elimination and excretion
The primary route of elimination appears to be further
hepatic metabolism to inactive metabolites which are
conjugated and then excreted in the urine.
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
Fluoxetine is a potent inhibitor of serotonin
re-uptake by Central Nervous System neurones and may
interact with other drugs or circumstances which cause
serotonin release. The enhancement of the serotonergic
effects may produce a life-threatening serotonin
syndrome.
7.1.2 Pharmacodynamics
Fluoxetine specifically inhibits neuronal
re-uptake of serotonin, thus increasing the
concentration of the serotonin at the synapse and
reinforcing of serotonergic neuronal transmission.
Fluoxetine has little effect on other
neurotransmitters.
Fluoxetine has no direct effect on the heart (Dista
lab. Prod. Inf., 1989).
Fluoxetine inhibits liver drug-metabolising enzymes
including CYP IID6, CYP IA2 and CYP IIIA4 (Lane et
al., 1995).
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
Estimated minimum lethal exposure:
1200 to 2000 mg (Kincaid et al., 1990).
Maximum tolerated exposure: 300 to 400 mg
with no co-ingestant.
7.2.1.2 Children
A 12-year-old boy ingested 1888 mg
(26 mg/kg) and experienced seizures and
minimal ECG changes (Riddle et al.,
1989).
A 4-year-old child ingested 43 mg/kg and
experienced unresponsiveness, sinus
tachycardia, moderate psychomotor agitation
and dyskinesia (Feierabend, 1995).
The highest dose at which no toxicity was
observed is 3.6 mg/kg (Borys et al.,
1990).
7.2.2 Relevant animal data
Lethal seizures occurred in 50% of various
animals given 200 to 700 mg/kg. The exact dose needed
is species dependent (Dista lab. Prod. Inf.,
1989).
7.2.3 Relevant in vitro data
No data available.
7.3 Carcinogenicity
Human studies: there is no evidence of carcinogenicity
in patients taking fluoxetine.
Animal studies: fluoxetine was not carcinogenic in rats and
mice at doses ten times the recommended daily dose for 24
months (Dista lab. Prod. Info., 1989).
7.4 Teratogenicity
Animal studies: in rats, fluoxetine and norfluoxetine
cross the placenta and distribute within the fetus during the
periods of organogenesis and postorganogenesis (Pohland et
al., 1989). Levels in fetal tissue are approximately half the
corresponding maternal concentrations.
Fluoxetine does not impair the fetal growth in rats or
rabbits at doses nine and eleven times the maximum daily
human dose respectively (Dista lab. Prod. Info., 1989).
Human studies: a study of 128 women exposed to fluoxetine
during the first trimester showed no increase in major fetal
malformations. It is not known, however, if the drug is a
human teratogen (Pastuszak et al., 1993).
A neonate whose mother had been taking fluoxetine during most
of her pregnancy sufferred tachypnoea, emesis, continuous
crying, irritability, tremor and increased muscle tone; the
symptoms resolved within 96 hours (Spencer, 1993).
Fluoxetine and norfluoxetine are excreted in breast milk
(Burch & Wells, 1992); the effects on the infant are
uncertain. Caution should be exercised when fluoxetine is
administered to a nursing mother (Dista lab. Prod.Info.,
1989).
7.5 Mutagenicity
In vitro: fluoxetine and norfluoxetine did not show
mutagenicity in the Ames test. There was no induction of
sister-chromatid exchange in the bone marrow of the chinese
hamster (Dista lab. Pro.Info., 1989).
7.6 Interactions
Drug interactions with fluoxetine have been reported
with L-tryptophan, L-dopa; monoamine oxidase inhibitors:
selegiline, tranylcypromine; tricyclic antidepressants;
selective serotonin re-uptake inhibitors: trazodone,
zimeldine; benzodiazepines: alprazolam, diazepam; buspirone,
lithium, anticonvulsants: carbamazepine, phenytoin,
valproate; pentazocine, dextromethorphan, fenfluramine,
calcium channel blockers, benztropine, cyproheptadine,
clarithromycin (Pollak et al., 1995), and substances of
abuse: cannabis, ethanol, LSD (Jackson & Hornfeldt, 1991;
Messiha, 1993).
At least 14 days should elapse between discontinuing a
MAO-inhibiting antidepressant and introducing fluoxetine.
Conversely, because of the long half-life of fluoxetine and
its metabolite, norfluoxetine, it is recommended that at
least 5 weeks should elapse between discontinuation of
fluoxetine and the introduction of a MAO inhibitor (Dista
lab. Prod. Info., 1989).
7.7 Main adverse effects
The major adverse effects reported with therapeutic
doses of fluoxetine are primarily those of headache,
insomnia, nausea, and nervousness, with a prevalence of 15 to
23 %. Less common adverse effects include tremors, sweating,
dry mouth, anxiety, drowsiness, and diarrhoea, with a
prevalence of 10 to 14 % (Messiha, 1993).
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
Toxic ingredient: liquids, capsules,
scene residues
In case of ingestion:
Vomitus: total amount
Gastric aspirate: total amount
(or gastric lavage: first
portion: 100 mL)
Whole blood without additives: 10 mL
Urine: random specimen: 50 mL
8.1.1.2 Biomedical analyses
Plasma (lithium heparin as
anticoagulant) or serum and urine for
standard biochemical analyses.
8.1.1.3 Arterial blood gas analyses
Heparinized arterial blood sample
(in severe cases).
8.1.1.4 Haematological analyses
Anticoagulated blood (e.g. EDTA) for
standard haematological analyses and
differential blood picture.
8.1.1.5 Other (unspecified) analyses
No further materials.
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
Serum, urine and other materials in
a refrigerator (4°C).
8.1.2.2 Biomedical analyses
No special requirements, but as
usually performed.
8.1.2.3 Arterial blood gas analyses
No special requirements, but as
usually performed.
8.1.2.4 Haematological analyses
No special requirements, but as
usually performed.
8.1.2.5 Other (unspecified) analyses
Not applicable.
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
No special requirements, but as
usually performed.
8.1.3.2 Biomedical analyses
No special requirements, but as
usually performed.
8.1.3.3 Arterial blood gas analyses
No special requirements, but as
usually performed.
8.1.3.4 Haematological analyses
No special requirements, but as
usually performed.
8.1.3.5 Other (unspecified) analyses
Not applicable.
8.2 Toxicological analysis and their interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple qualitative test(s)
8.2.1.2 Advanced qualitative confirmation test(s)
8.2.1.3 Simple quantitative method(s)
8.2.1.4 Advanced quantitative method(s)
8.2.2 Test for biological specimens
8.2.2.1 Simple qualitative test(s)
8.2.2.2 Advanced qualitative confirmation test(s)
8.2.2.3 Simple quantitative method
8.2.2.4 Advanced quantitative method(s)
8.2.2.5 Other dedicated method(s)
8.2.3 Interpretation of toxicological analysis
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
Sodium, potassium, chloride,
calcium, magnesium Glucose, amylase, creatine
kinase, creatinine (urea) Optional:
bicarbonate (or total CO2), uric
acid
8.3.1.2 Urine
8.3.1.3 Other fluids
No dedicated test.
8.3.2 Arterial blood gas analyses
pH, pCO2, pO2, base excess, actual HCO3-,
and O2-saturation.
8.3.3 Haematological analyses
Complete blood count, including platelets,
haematocrit.
Differential blood picture.
8.3.4 Interpretation of biomedical investigations
8.4 Other biomedical (diagnostic) investigations and their
interpretation
Serum electrolytes, urea and creatinine, liver function
tests, amylase, creatine kinase and arterial blood gases are
useful to optimize supportive care.
8.5 Overall interpretation of all toxicological analysis and
toxicological investigations
Analysis of Materials
Fluoxetine is not easily detected in materials by simple
physicochemical tests. The UV spectrum in methanol gives
deltamax at 227 nm; A| = 372 (Risley & Bopp, 1990). Using
thin layer chromatography, the drug can be visualized with
Mandelin's reagent (pale grey). In methanol ammonia (100:1.5)
the Rf is 0.24, and in ethyl acetate / methanol / ammonia
(85:15:5) the Rf is 0.50 (personal experience). Gas
chromatography is also useful, and the retention index for
fluoxetine is 1950 on SE54, SE30, OV1 or HP5 or similar.
Flame ionization detection has sufficient sensitivity for
residues; electron capture or nitrogen-phosphorus detection
give improved selectivity.
Analysis of Biological Specimens
Confirmation of the presence of fluoxetine may be useful in
some instances to confirm the diagnosis of intoxication, or
to explain the occurrence of serotonergic syndrome.
Measurement of serum concentrations is not usual clinical
practice. There are no commonly-available immunoassay kits
for drug testing (including those for tricyclic
antidepressants) which respond to fluoxetine. Extraction of
fluoxetine and norfluoxetine from biological specimens is
easily achieved by pH adjustment to 8 to 12, and addition of
an organic solvent (ethyl acetate, hexane / iso-amyl alcohol
etc.). Cleaning by back-extraction into dilute sulphuric
acid may be necessary for low concentrations. Thin layer
chromatography is applicable to the analysis of gastric
contents and urine. In methanol / ammonia (100:1.5) the Rf of
fluoxetine is 0.24, and 0.30 for norfluoxetine; in ethyl
acetate / methanol / ammonia (85:15:5) the Rf is 0.50 for
both compounds (personal experience). Both compounds can be
visualized with Mandelin's reagent (pale grey), and give a
positive iodoplatinate reaction.
Advanced Methods of Analysis
Gas chromatography can be performed without derivatization by
many methods which are suitable for analysis of other
antidepressant drugs, from which both fluoxetine and
norfluoxetine are well separated (run earlier than
amitriptyline). Care should be taken to ensure separation
from caffeine, especially on nitrogen-phosphorus detection.
Isothermal analysis on OV1 or HP1 gives good separation at
180°C within 5 minutes (Rohrig & Prouty, 1989). DB5 and DB17
are also useful. FID is adequate for overdose concentrations
(0.02 mg/L), but nitrogen-phosphorus (Kincaid et al., 1990;
Fontanille et al., 1997) and electron capture detection
(Dixit et al., 1991) both give improved sensitivity (0.001 to
0.005 mg/L from 1 mL sample). Derivatization of both
compounds with butyric anhydride gives more characteristic
spectra on GCMS, particularly for fluoxetine (Kincaid et al.,
1990).
HPLC is commonly used for quantitative serum measurements.
Good chromatography over 8 minutes was obtained using C8
column with a mobile phase of triethylamine acetate (10 mL/L,
pH to 5.5 with glacial acetic acid) / acetonitrile (65:35)
mobile phase (Nichols et al., 1994), or with 0.067M potassium
dihydrogenphosphate buffer / acetonitrile (70:30) (Thomare et
al., 1992). Both methods use UV detection at 228 nm and give
sensitivity of 0.01 mg/L and 0.002 mg/L respectively.
Alternatively, excellent separation was seen over 12 minutes
on a C18 column with high sensitivity (<0.005 mg/L) by
pre-column derivatization with dansyl chloride and
fluorescence detection (Suckow et al., 1992).
Chiral analysis of fluoxetine and norfluoxetine is possible
after derivatization with the chiral reagent,
(S)-(-)-N-trifluoroacetylprolyl chloride followed by gas
chromatography on DB5 with electron capture detection
(Torok-Both et al., 1992).
Toxicity is not well correlated with fluoxetine
concentration, and toxicity can often be attributed to other
causative agents. Serotonergic syndromes may occur with
relatively low fluoxetine concentrations (<0.1 mg/L). As a
guide, typical concentrations of fluoxetine in serum are:
mg/L µmol/L
Peak after single oral dose
(30 - 75 mg)
6 - 8 hours 0.03 - 0.13 0.1- 0.43
Steady-state in therapy
fluoxetine <0.3 <1.0
norfluoxetine <0.7 <2.33
Serious toxicity possible >0.5 >1.67
Serum concentrations of 1 to 2 mg/L have been reported
without serious toxicity.
Serum electrolytes, urea and creatinine and arterial blood
gases are useful to optimize supportive care.
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Blurred vision, nausea, vomiting, abdominal
pain, diarrhoea, lethargy, dizziness, insomnia,
tremors, and rarely, seizures and coma.
Significant cardiovascular toxicity is unusual.
Effects have included mild hypo- or hypertension,
tachycardia, and ventricular dysrhythmia.
Co-ingestion of serotonin-reuptake inhibitors can
result in aggressive behaviour, confusion, tremor,
restlessness, shivering, diaphoresis, hyperthermia,
diarrhoea, and myoclonus.
9.1.2 Inhalation
Not relevant.
9.1.3 Skin exposure
Not relevant.
9.1.4 Eye contact
Unknown.
9.1.5 Parenteral exposure
No data available.
9.1.6 Other
No data available.
9.2 Chronic poisoning
9.2.1 Ingestion
No data available.
9.2.2 Inhalation
Not relevant.
9.2.3 Skin exposure
Not relevant.
9.2.4 Eye contact
No data available.
9.2.5 Parenteral exposure
No data available.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
The clinical course of fluoxetine poisoning is generally
very mild in nature and of short duration. Complete and rapid
recovery is the rule. A few deaths have been reported in the
forensic literature, seemingly after ingestion of very high
doses. Most involved co-ingestants and all are poorly
documented (Kincaid et al., 1990).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Sinus tachycardia, bradycardia (Feder, 1991),
hypotension, mild hypertension, ECG changes especially
QT interval prolongation and ventricular dysrhythmias.
Patients with preexisting cardiovascular disease or
taking cardiac medications may be at greater risk of
developing fluoxetine cardiotoxicity (Ellison et al.,
1990).
9.4.2 Respiratory
Dyspnea, cough and flu-like symptoms have been
reported (Kim & Pentel, 1989).
Induction of dyspnoea and coughing in a female smoker
has been attributed to a fluoxetine-nicotine
interaction. Dyspnoea, cough and flu-like symptoms
have been reported (Bass & Colebatch, 1992).
9.4.3 Neurological
9.4.3.1 Central nervous system (CNS)
Drowsiness, headache, restlessness,
delirium, insomnia, ataxia, extrapyramidal
syndrome (Baldwin et al., 1991; Eisenhauer &
Jermain, 1993), coma and seizures.
Psychiatric: manic and hypomanic states. It
has also been suggested that fluoxetine may
induce suicidal ideation in a small subset of
patients (Crundwell, 1993; Tueth,
1994).
9.4.3.2 Peripheral nervous system
Blurred vision.
9.4.3.3 Autonomic nervous system
Bradycardia or tachycardia.
9.4.3.4 Skeletal and smooth muscle
Tremor, myoclonus, dystonia.
9.4.4 Gastrointestinal
Abdominal pain, nausea, vomiting, diarrhoea.
Anorexia leading to weight loss.
9.4.5 Hepatic
Mild liver enzyme disturbances are reported
(Bobichon et al., 1993; Castiella & Arenas,
1994).
9.4.6 Urinary
9.4.6.1 Renal
No data available.
9.4.6.2 Other
No data available.
9.4.7 Endocrine and reproductive systems
Fluoxetine may alter glycaemic control in
diabetic subjects.
Fluoxetine is associated with the syndrome of
inappropriate secretion of antidiuretic hormone
(Staab, 1990).
Sexual disorders: anorgasmia, penile anaesthesia,
sexual arousal (Modell, 1989; Morris, 1991; Measom,
1992).
9.4.8 Dermatological
Skin rash, pruritus, urticaria, psoriasis,
photosensitivity reactions, alopecia (Hemlock et al.,
1992; Gupta et al., 1993; Ogilvie, 1993; Gaufberg &
Ellison, 1995; O'Brien, 1995; Roger et al.,
1995).
9.4.9 Eye, ear, nose, throat: local effects
Voice disorder (Murray, 1995).
9.4.10 Haematological
Abnormal platelet aggregation, prolongation of
the bleeding time (Humphries et al., 1990; Alderman et
al., 1992; Aranth & Lindberg, 1992).
9.4.11 Immunological
Skin rashes (Gupta et al., 1993), angioedema,
serum sickness and anaphylactoid reactions have been
reported (Roger et al., 1995).
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Acidosis is expected in association
with coma and/or convulsions.
9.4.12.2 Fluid and electrolyte disturbances
Several cases of hyponatraemia,
hypo-osmolarity and syndrome of inappropriate
secretion of antidiuretic hormone have been
reported (Staab, 1990).
9.4.12.3 Others
No data available.
9.4.13 Allergic reactions
Skin rashes.
9.4.14 Other clinical effects
Stuttering (Guthrie & Grunhaus, 1990).
9.4.15 Special risks
The safety of fluoxetine during pregnancy and
lactation is not established (see section
7.4).
9.5 Other
Several cases of fluoxetine withdrawal syndrome have
been described, occurring from one to seven days after
discontinuation of the medication. Symptoms included
vertigo, dizziness, light-headedness, irritability, anxiety,
headache, sweating, and nausea (Einbinder, 1995; Berlin,
1996).
9.6 Summary
10. MANAGEMENT
10.1 General principles
The primary management of isolated fluoxetine overdose
consists of institution of appropriate observation and
supportive care. In most cases, this consists of no more
than careful observation of vital signs and neurological
status until signs and symptoms resolve. Intravenous access
should be established as soon as practicable. In more severe
intoxications or where there is a co-ingestion, more
aggressive measures such as establishment of an airway,
ventilation, administration of intravenous fluids, control of
seizures, and control of hyperthermia may be
necessary.
10.2 Life supportive procedures and symptomatic/specific
treatment
Intensive supportive care is rarely required. Measures
that may occcasionally be required are: endotracheal
intubation and/or artificial ventilation if coma is present,
intravenous fluid resuscitation if hypotension is present,
control of seizures, cooling if hyperthermia is present
(refer to appropriate treatment guides for more detailed
management guidance).
10.3 Decontamination
Gastrointestinal decontamination by administration of a
single dose of oral activated charcoal may be indicated
following ingestion of more than 800 mg of fluoxetine by an
adult presenting within two hours, or where there has been a
significant co-ingestion.
10.4 Enhanced elimination
There are no effective methods known to enhance the
elimination of fluoxetine.
10.5 Antidote treatment
10.5.1 Adults
No data available.
10.6.2 Children
No data available.
10.6 Management discussion
Although of theoretical benefit, the role of dantrolene
in the management of the serotonergic syndrome has yet to be
evaluated.
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
A 15-year-old female ingested forty five 20 mg
fluoxetine tablets. Ten hours later, she was found jerking,
frothing at the mouth and with a staring gaze. On arrival at
hospital thirty minutes later, she had a normal level of
consciousness. She denied the ingestion of any other
medications and had no previous history of seizures or
trauma. Physical examination and all routine laboratory
studies were unremarkable. Comprehensive drug screening did
not detect the presence of any other substances. Serum
fluoxetine and norfluoxetine concentrations were 1956 ng/mL
and 416 ng/mL respectively (Braitberg & Curry, 1995).
A 4-year-old child ingested 43 mg/kg of fluoxetine. Toxic
effects consisted of a brief period of unresponsiveness,
sinus tachycardia and moderate psychomotor agitation and
dyskinesia. The child recovered completely with supportive
care (Feierabend, 1995).
A 40-year-old female developed a classical serotoninergic
syndrome with severe hyperthermia (43°C) eight hours after
ingesting 340 mg fluoxetine, 6000 mg moclobemide, 300 mg
clomipramine and 12 mg clonazepam. She died from the
subsequent complications of fulminant disseminated
intravascular coagulation and multiple organ failure (Power
et al., 1995).
A patient being treated with carbamazepine 200 mg/day for an
affective disorder, developed a serotonergic syndrome 14 days
following institution of fluoxetine therapy at a dose of 20
mg/day. In addition to features of the serotonergic
syndrome, she reported dizziness, experienced an oculogyric
crisis, and developed leucopenia and thrombocytopenia. She
made a complete recovery within 72 hours of the
discontinuation of fluoxetine (Dursun et al., 1993).
12. ADDITIONAL INFORMATION
12.1 Specific preventive measures
12.2 Other
13. REFERENCES
Alderman CP, Moritz CK, & Ben-Tovim DI (1992) Abnormal
platelet aggregation associated with fluoxetine therapy. Ann
Pharmacotherapy, 26: 1517-1519.
Aranth J & Lindberg C (1992) Bleeding as a side effect of
fluoxetine. Am J Psychiatry, 149: 412.
Baldwin D, Fineberg N, & Montgomery S (1991) Fluoxetine,
fluvoxamine and extrapyramidal tract disorders. Int Clin
Psychopharmacol, 6: 51-58.
Bass SP & Colebatch HJH (1992) Fluoxetine-induced lung damage. Med
J Austral, 156: 364.
Berlin C (1996) Fluoxetine withdrawal symptoms. J Clin Psychiatry,
57, (2): 93-94.
Bobichon R, Bernard G, & Mion F (1993) [Acute hepatitis during
treatment with fluoxetine]. Gastroenterologie Clinique et
Biologique, 17 (5): 406-7 (in French).
Borys D, Setzer S, Ling L, Reisdorf J, Day L, & Krenzelok E (1990)
The effects of fluoxetine in the overdose patient. Clin Toxicol,
28 (3): 331-340.
Braitberg G & Curry SC (1995) Seizure after isolated fluoxetine
overdose. Ann Emerg Med, Aug; 26 (2): 234-7.
Burch KJ & Wells BG (1992) Fluoxetine/norfluoxetine concentrations
in human milk. Pediatrics, 98: 676-677.
Castiella A & Arenas J (1994) Fluoxetine hepatotoxicity. Am J
Gastroenterology, 89 (3): 458-9.
Crundwell JK (1993) Fluoxetine and suicidal ideation-a review of
the literature. [review] Int J Neurosci, 68 (1-2): 73-84.
Dista Laboratoires (1989) Prozac 20 mg. Manufacturer information.
92213 Saint Cloud, France.
Dixit V, Nguyen H & Dixit YM (1991) Solid-phase extraction of
fluoxetine and norfluoxetine from serum with gas
chromatography-electron capture detection. J Chromatogr, 563:
379-384.
Dursun S, Mathew V, & Reveley M (1993) Toxic serotonin syndrome
after fluoxetine plus carbamazepine. Lancet, 342: 442-3.
Einbinder E (1995) Fluoxetine withdrawal. Amer J Psychiatry, 152,
(8): 1235.
Eisenhauer G & Jermain DM (1993) Fluoxetine and tics in an
adolescent. Annals of Pharmacotherapy, 27 (6): 725-6.
Ellison J, Milofsky J, & Ely E (1990) Fluoxetine-induced
bradycardia and syncope in two patients. J Clin Psychiatry, 51:
385-386.
Feder R (1991) Bradycardia and syncope induced by fluoxetine. J
Clin Psychiatry, 52: 139.
Feierabend R (1995) Benign course in a child with a massive
fluoxetine overdose. J Fam Pract, 41 (3): 289-291.
Fontanille P, Jourdil N, Villier C & Bessard G (1997) Direct
analysis of fluoxetine and norfluoxetine in plasma by gas
chromatography with nitrogen-phosphorus detection. J Chromatogr,
692: 337-343.
Gaufberg E & Ellison JM (1995) Photosensitivity reaction to
fluoxetine. J Clin Psychiatry, 56, (10): 486.
Geller DA, Biederman J, Reed ED, Spencer T, & Wilens TE (1995)
Similarities in response to fluoxetine in the treatment of
children and adolescents with obsessive-compulsive disorder. J
Amer Acad Child Adol. Psychiatry, 34 (1): 36-44.
Gupta R, Parker G, Norman T, Judd F, & Burrows G (1993)
Fluoxetine-delayed half-life and an adverse event. Med J
Australia, 158: 722-723.
Guthrie S & Grunhaus L (1990) Fluoxetine-induced stuttering. J
Clin Psychiatry, 51: 85.
Hemlock C, Rosenthal J, & Winston A (1992) Fluoxetine-induced
psoriasis. Ann Pharmacother, 26: 211-212.
Humphries J, Wheby M, & Vanden Berg S (1990) Fluoxetine and the
bleeding time. Arch Path Lab Med, 114: 727-728.
Jackson TW & Hornfeldt C (1991) Seizure activity following
recreational LSD use in patients treated with lithium and
fluoxetine (abstract). Vet Hum Toxicol, 33: 387.
Kim SW & Pentel P (1989) Flu-like symptoms associated with
fluoxetine overdose: a case report. J Toxicol Clin Toxicol, 27:
389-393.
Kincaid R, Mc Mullin M, Crookham S, & Reiders F (1990) Report of a
fluoxetine fatality. J Anal Toxicol, 14: 327-329.
Lane R, Baldwin D, & Preskorn S (1995) The SSRIs: Advantages,
disadvantages and differences. J Psychopharmacol, 9/2 suppl,
163-178.
Measom M (1992) Penile anesthesia and fluoxetine. Am J Psychiatry,
149: 709.
Messiha F (1993) Fluoxetine: adverse effects and
drug-drug-interactions. Clin Toxicol, 31 (4): 603-630.
Modell JG (1989) Repeated observations of yawning, clitoral
engorgement, and orgasm associated with fluoxetine administration.
J Clin Psychopharmacol, 9: 63-65.
Morris PLP (1991) Fluoxetine and orgasmic sexual experiences. Int
J Psychiatry Med, 21: 379-389.
Murray V (1995) Laryngeal dystonia. Br J Psychiatry, 167 (5):
698-9.
Nichols JH, Charlson JR & Lawson GM (1994) Automated HPLC assay
of fluoxetine and norfluoxetine in serum. Clin Chem, 40:
1312-1316.
O'Brien T (1995) Phototherapy burns and fluoxetine. Australasian J
Dermatology, 36 (2): 103.
Ogilvie A (1993) Hair loss during fluoxetine treatment. Lancet,
342: 1423.
Pastuszak A, Schick-Boschetto B, Zuber C, Feldkamp M, Pinelli M,
et al. (1993) Pregnancy outcome following first trimester exposure
to fluoxetine (Prozac). JAMA, 269: 2246-2248.
Pohland R, Byrd T, Hamilton M, & Koons J (1989) Placental transfer
and fetal distribution of fluoxetine in the rat. Toxicol Appl
Pharmacol, 98: 198-205.
Pollak P, Sketris I, Mc Kenzie S, & Hewlett T (1995) Delirium
probably induced by clarithromycin in a patient receiving
fluoxetine. Ann Pharmacother, 29 (5): 486-8.
Power B, Pinder M, Hackett L, & Ilett K (1995) Fatal serotonin
syndrome following a combined overdose of moclobemide,
clomipramine and fluoxetine. Anaesth Intensive Care, 23 (4):
499-502.
Reynolds JEF ed. (1995) Martindale: The Extra Pharmacopoeia, 31th
ed. London, The Pharmaceutical Press. pp 312-314.
Riddle MA & al. (1989) Fluoxetine overdose in an adolescent. J Am
Acad Child Adolesc Psychiatr, 28: 587-588.
Risley DS & Bopp RJ (1990) Fluoxetine. In: Florey K ed
Analytical Profiles of Drug Substances, New York, Academic press,
19: pp 193-219.
Roger D. Rolle F, Mausset J, Lavignac C, & Bonnetblanc J (1995)
Urticarial vasculitis induced by fluoxetine. Dermatology, 191 (2):
164.
Rohrig T & Prouty RW (1989) Fluoxetine overdose: a case report. J
Anal Toxicol, 13: 305-307.
Saletu B & Grunberger T (1985) Classification and determination of
cerebral bioavailability of fluoxetine: pharmacokinetic,
pharmaco-EEG, and psychometric analysis. J Clin Psychiatry, 46:
45-52.
Spencer M (1993) Fluoxetine hydrochloride (Prozac) toxicity in a
neonate. Pediatrics, 92 (5): 721-722.
Staab J (1990) Transient SIADH associated with fluoxetine. Am J
Psychiatry, 147:1569-1570.
Suckow RF, Zhang MF, Cooper TB (1992) Sensitive and selective
liquid-chromatographic assay of fluoxetine and norfluoxetine in
plasma with fluorescent detection after precolumn derivatization.
Clin Chem, 38: 1756-1761.
Thomare P, Wang K, Van Der Meersch-Mougeot V & Diquet B (1992)
Sensitive micro-method for column liquid chromatographic
determination of fluoxetine and norfluoxetine in human plasma. J
Chromatogr, 583: 217-221.
TIAFT, The Bulletin of the International Association of Forensic
Toxicologists, 1995, 26 (1) suppl.
Torok-Both GA, Baker GB, Coutts RT, McKenna KF & Aspeslet LJ
(1992) Simultaneous determination of fluoxetine and norfluoxetine
enantiomers in biological samples by gas chromatography with
electron capture detection. J Chromatogr, 579: 99-106.
Tueth MJ (1994) Revisiting fluoxetine (Prozac) and suicidal
preoccupations. J Emerg Med, 12 (5): 685-687.
Vidal (1996), 72th ed. Paris.
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE
ADDRESS(ES)
Author: M.O. Rambourg Schepens,
Centre Anti-Poisons,
Hôpital Maison Blanche,
45 Rue Cognacq-Jay
F-51092 REIMS Cedex, France,
Tel: 33-326862686
Fax: 33-326865548
E-mail: marie-odile.rambourg@wanadoo.fr
Date: August 1996.
Reviewer: L.M. Murray,
Department of Emergency Medicine,
Prince of Wales Hospital,
Randwick, NSW, Australia.
Peer
review: Cardiff, United Kingdom, September 1996
(A. Borges, R. Ferner, M. Hanafy, M. Kowalczyk, L.
Murray, M.O. Rambourg Schepens)
Author
Section 8: Dr S. Dawling
Center for Clinical Toxicology
Vanderbilt University Medical Center
501 Oxford House
1161 21st Avenue South
Nashville, TN 37232-4632
United States of America
Tel: 1-615-9360760
Fax: 1-615-9360756
E-mail: sheila.dawling@mcmail.vanderbilt.edu
Date: March 1998
Editors: Dr M. Ruse (April 1997); Mrs J. Duménil (May 1999)


