
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 98
CHLOROTHALONIL
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1995
This is a companion volume to Environmental Health Criteria 183:
Chlorothalonil
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data
Health and safety guide for Chlorothalonil
(Health and safety guide ; no. 98)
1.Nitriles - toxicity 2.Fungicides, Industrial
3.Environmental exposure 4. I.Series
ISBN 92 4 151098 6 (NLM Classification: QD 305.N7)
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1995
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY
1.1. Identity
1.2. Physical and chemical properties
2. SUMMARY AND EVALUATION
2.1. Summary
2.1.1. Identity, physical and chemical properties, and
analytical methods
2.1.2. Sources of human and environmental exposure
2.1.3. Environmental transport, distribution, and
transformation
2.1.4. Environmental levels and human exposure
2.1.5. Kinetics and metabolism in laboratory animals
2.1.6. Effects on experimental mammals, and in vitro test
systems
2.1.7. Effects on humans
2.1.8. Effects on non-target organisms in the laboratory
and field
2.2. Evaluation
2.2.1. Toxicological assessment
2.2.2. Environmental assessment
2.2.2.1 Transport, distribution, and
transformation
2.2.2.2 Aquatic organisms
2.2.2.3 Terrestrial organisms
2.2.3. Toxicological criteria for setting guideline values
3. CONCLUSIONS AND RECOMMENDATIONS
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and protection,
first aid
4.1.1. Prevention and protection
4.1.2. First aid
4.2. Advice to physicians
4.3. Explosion and fire hazards
4.4. Storage and transport
4.5. Spillage and disposal
4.5.1. Spillage
4.5.2. Waste disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
6.1. Previous evaluations by international bodies
6.2. Exposure limit values
6.3. Specific restrictions
6.4. Labelling, packaging, and transport
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) monographs produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is a Summary of Chemical Safety
Information which should be readily available, and should be clearly
explained, to all who could come into contact with the chemical. The
section on regulatory information has been extracted from the legal
file of the International Register of Potentially Toxic Chemicals
(IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Director
International Programme on Chemical Safety
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Chemical structure
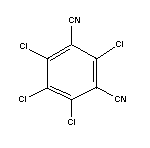 Molecular formula: C8Cl4N2
Relative molecular mass: 265.9
CAS chemical name: 2,4,5,6,-tetrachloro-1,3-
benzenedicarbonitrile
CAS registry number: 1897-45-6
RTECS registry number: NT2600000
Common name: chlorothalonil
IUPAC name: tetrachloroisophthalonitrile
Synonyms: m-TCPN;
2,4,5,6-tetrachloro-3-cyanobenzonitrile
Trade names: Bravo (ISK Biotech) (manufacturers &
suppliers); Daconil (ISK Biotech); Faber
(Tripart Farm Chemicals); Repulse (ICI);
Exotherm (Alto Elite); Nopocide (a
preservative in paints and adhesives)
Technical product purity: >97%
Technical product
impurities (%): Tetrachlorophthalonitrile (<0.1);
Tetrachloroterephthalonitrile (0.1-1.6);
Pentachlorobenzonitrile (0.5-2.5);
Partially chlorinated dicyanobenzenes
(0.2-1.0); Unchlorinated dicyanobenzenes
(0.1-1.6); Insoluble in xylene (0.1-1.0)
HCB (0.03); xylene insolubles (0.35)
1.2 Physical and Chemical Properties
The physical properties of chlorothalonil are listed in Table 1.
Table 1. Physical properties of chlorothalonil
Physical state crystalline solid
Colour colourless
Odour odourless
Melting point (°C) 250-251
Boiling point (°C) 350 (760 mmHg)
Vapour pressure at 25°C 5.72 × 10-7
Relative density 1.8
Octanol-water partition 2.88-3.86
coefficient (Log Kow)
Solubility in water (mg/litre) 0.6-1.2
at 25°C
Solubility in organic acetone 20, dimethylformamide 30,
solvents (g/litre) dimethylsulfoxide 20, xylene 80,
readily soluble in benzene
Chlorothalonil is non-flammable and non-explosive. It is thermally
stable under normal storage conditions and to UV radiation and is
chemically stable in neutral or acidic aqueous solutions. It breaks
down at pH 9, the rate following first-order kinetics at 1.8% per day
at 25°C. It has been shown that chlorothalonil is unstable to light
when dissolved in benzene and that 2,3,5-trichloro-4,6-dicyanobiphenyl
is a condensation product. Chlorothalonil is not corrosive.
2. SUMMARY AND EVALUATION
2.1 Summary
2.1.1 Identity, physical and chemical properties, and analytical
methods
Chlorothalonil is a colourless, odourless, crystalline solid with a
melting point of 250°C and a vapour pressure of 7.63 × 10-5 Pa (5.72 ×
10-7 mmHg) at 25°C. It is poorly soluble in water (0.6-1.2 mg/litre at
25°C) with an octanol/water partition coefficient (log Kow) of
2.882. It is slowly hydrolysed in water at pH 9, but is stable at pH 7
or below (at 25°C).
The most prevalent analytical method, after sample extraction and
clean-up, is gas-liquid chromatography using an electron-capture
detector.
2.1.2 Sources of human and environmental exposure
Chlorothalonil has been produced commercially since 1969 by the
chlorination of isophthalonitrile or by treatment of
tetrachloroisophthaloyl amide with phosphorus oxychloride. It is a
fungicide with a broad spectrum of activity, used mainly in
agriculture but also on turf, lawns, and ornamental plants. Crops
protected include pome and stone fruit, citrus, currants, berries,
bananas, tomatoes, green vegetables, coffee, peanuts, potatoes,
onions, and cereals. In addition, it is used in wood preservatives and
in paints.
The three main formulations are a suspension concentrate, a water
dispersible granule, and a wettable powder. They are diluted readily
with water and applied by ground spray systems or by air. Typical
active ingredient rates are 1.2-2.5 kg/ha for crops, such as beans,
celery, and onions. The main sources of human exposure will be during
the preparation and application of the products, and from ingestion of
crop residues in foodstuffs (see section 2.1.4).
2.1.3 Environmental transport, distribution, and transformation
Chlorothalonil is removed from aqueous media by strong adsorption on
suspended matter. Modelled data suggest little or no partition to
bottom sediment. Biodegradation involving enzyme processes may occur
in natural waters. Chlorothalonil is rapidly degraded in soil and
degradation may occur in water with the production of the 4-hydroxy
metabolite, 4-hydroxy-2,5,6-trichloroisophthalonitrile. Half-lives for
dissipation of the 4-hydroxy metabolite in soils range between 6 and
43 days.
Chlorothalonil does not translocate from the site of application to
other parts of a plant. It is metabolized only to a limited extent on
plants and the 4-hydroxy metabolite is usually <5% of the residue.
Chlorothalonil is metabolized in fish via glutathione conjugation to
give more polar excretory products. The enzyme glutathione- S-
transferase is involved in this conversion. High concentrations of
radiolabel found in the gall bladder and bile, after exposure of
rainbow trout to 14C-chlorothalonil, are consistent with the
excretion of the compound as glutathione conjugates. The
concentrations of radiolabel accumulating in the gall bladder and
other organs fell rapidly when the fish were placed in clean water.
Chlorothalonil does not bioaccumulate in aquatic organisms.
2.1.4 Environmental levels and human exposure
In a potato crop study, a small stream was oversprayed with
chlorothalonil. Subsequent sampling/analysis of downstream water
demonstrated rapid disappearance of chlorothalonil (i.e., 450 µg/litre
30 min after spraying to 2-6 µg/litre 12 h after spraying). The
routine spraying of irrigated field crops, such as potatoes and
barley, gave rise to low concentrations of chlorothalonil (0.04-
3.6 µg/litre) in tile drain water on a small number of sampling
occasions.
Crop residues are composed mainly of chlorothalonil itself. Residue
levels depend on the applied rate, the time interval between the last
application and harvest, and the type of crop. Residue levels at
harvest can be derived from the numerous supervised trials that have
taken place on many crops throughout the world and have been reported
to FAO/WHO. Residues of chlorothalonil in dairy products are expected
to be undetectable or very low. Dairy cows, given high concentrations
(up to 250 mg/kg) of chlorothalonil in their feed for 30 days, showed
no detectable residues in milk and only very low levels in tissues.
Total diet and individual food analyses in several countries have
shown low or undetectable concentrations of chlorothalonil in sampling
surveys. Residue levels on foodstuffs are further reduced by
preparation processes, such as washing, peeling, and cooking.
2.1.5 Kinetics and metabolism in laboratory animals
About 30% of an oral dose of chlorothalonil was absorbed after 48 h in
rats administered doses of up to 50 mg/kg body weight. At higher
doses, absorption was lower, indicating a saturation process. When
14C-chlorothalonil was given orally the radioactivity was distributed
into blood and tissues within 2 h. The greatest concentration was
found in the kidney, followed by the liver and blood. The kidneys
contained 0.3% of a dose of 5 mg/kg body weight after 24 h.
Most of an oral dose of chlorothalonil in rats was found in the faeces
(>82% within 48-72 h, regardless of dose). Biliary excretion was
rapid, peaking within 2 h after an oral dose of 5 mg/kg body weight,
and was saturated at doses of 50 mg/kg body weight and above. Urinary
excretion accounted for 5-10% of the dose in rats.
Faecal elimination is the main route in dogs and monkeys, but urinary
excretion (<4%) is less than in rats.
Metabolic studies on rats indicate that chlorothalonil is conjugated
with glutathione in the liver as well as in the gastrointestinal
tract. Some of the glutathione conjugates may be absorbed from the
intestine and transported to the kidneys, where they are converted by
cytosolic ß-lyase to thiol analogues that are excreted in the urine.
When germ-free rats were dosed with chlorothalonil, the thiol
metabolites appeared in the urine in much smaller amounts than in
normal rats, indicating the involvement of intestinal microflora in
the metabolism of chlorothalonil. Dogs or monkeys dosed orally with
chlorothalonil excreted little, or no, thiol derivatives in the urine.
When 14C-chlorothalonil was applied to rat skin, approximately 28%
of the dose was absorbed within 120 h. About 18% of the dose was found
in the faeces and 6% in the urine within 120 h.
2.1.6 Effects on laboratory mammals, and in vitro test systems
The acute oral and dermal toxicities of chlorothalonil in rats and
rabbits are low (acute oral and dermal LD50s of >10 000 mg/kg body
weight). Hammer-milled technical chlorothalonil (MMAD 5-8 µm)
exhibited high toxicity in rats in an inhalation study with a 4-h
LC50 of 0.1 mg/litre.
Chlorothalonil was a skin and eye irritant in the rabbit. Skin
sensitization studies in the guinea-pig were inconclusive.
The main effects of repeated oral dosing in rats were on the stomach
and kidney. Groups of 25 rats/sex per group were fed chlorothalonil at
0, 1.5, 3, 10, or 40 mg/kg body weight per day in the diet for 13
weeks, followed by a 13-week recovery period. Increased incidences of
hyperplasia and hyperkeratosis of the forestomach occurred at 10 and
40 mg/kg; these reversed when treatment ceased. At 40 mg/kg, there was
an increased incidence of hyperplasia of kidney proximal tubular
epithelium in males at 13 weeks and after the recovery period. The
NOEL was 3 mg/kg body weight per day, based on lack of forestomach
lesions. The onset of the forestomach and kidney changes was shown to
be rapid, with the lesions developing within 4-7 days in male rats at
a dietary level of 175 mg/kg body weight per day.
In a 13-week study on mice (0, 7.5, 15, 50, 275, or 750 mg/kg diet),
increased incidences of hyperplasia and hyperkeratosis of the squamous
epithelial cells of the forestomach occurred in males and females at
50 mg/kg diet and above. On the basis of these changes, the NOEL was
15 mg chlorothalonil/kg diet, equivalent to 3 mg/kg body weight per
day.
A 16-week study on dogs receiving dietary levels of chlorothalonil of
0, 250, 500, or 750 mg/kg showed no treatment-related changes.
The forestomach and kidney lesions were investigated further in 2-year
studies on rats, mice, and dogs. In a study on rats (0, 1.8, 3.8, 15,
or 175 mg/kg body weight per day), the effects of chlorothalonil were
characterized histologically as an increase in both the incidence and
severity of hyperplasia, hyperkeratosis and ulcers and erosions of the
squamous mucosa of the forestomach, and epithelial hyperplasia of the
kidney proximal convoluted tubules at doses of 3.8 mg/kg and above.
The NOEL for non-neoplastic effects was, therefore, 1.8 mg/kg. The
incidence of renal tumours (adenomas and carcinomas) and forestomach
tumours (papillomas and carcinomas) was markedly increased at
175 mg/kg. There was evidence for increased incidence in kidney
tumours in males at 15 mg/kg and for stomach tumours at 3.8 and
15 mg/kg in both males and females. The NOEL for neoplastic effects
was, therefore, 1.8 mg/kg body weight per day, on the basis of changes
in forestomach tumour incidence. Supporting evidence for the
carcinogenic potential of chlorothalonil in the kidney and forestomach
of rats was provided by the results from other 2-year studies at
higher dose levels.
In a study on mice (0, 15, 40, 175, or 750 mg chlorothalonil/kg diet),
an increased incidence of renal tubular hyperplasia occurred at doses
of 175 mg/kg and above and of hyperplasia and hyperkeratosis of the
forestomach at 40 mg/kg and above. The incidence of squamous tumours
of the forestomach was slightly increased at 750 mg/kg. The NOELs for
neoplastic and non-neoplastic changes were, therefore, 175 and
15 mg/kg in the diet (equivalent to 17.5 and 1.6 mg/kg body weight per
day, respectively). Supporting evidence for these effects in the mouse
was provided in another study at higher dose levels, but a study on
B6C3F1 mice did not show any evidence for carcinogenic potential at
high dose levels.
In a 2-year study on dogs (60 and 120 mg/kg diet), no effects
attributable to chlorothalonil were found. The NOEL was, therefore,
120 mg/kg diet (equivalent to 3 mg/kg body weight per day).
Chlorothalonil was not mutagenic in several in vitro and in vivo
tests, though it was positive in a small number of assays.
The monothio, dithio, trithio, dicysteine, tricysteine, and
monoglutathione derivatives of chlorothalonil, which are potential
nephrotoxicants, were shown to be negative in the Ames assay.
Chlorothalonil was not teratogenic in rats or rabbits at doses of up
to 400 and 50 mg/kg body weight per day, respectively. Reproductive
parameters, such as mating, fertility, and gestation length were not
affected by chlorothalonil at levels of up to 1500 mg/kg diet in a
two-generation study on rats.
The acute oral toxicity of the 4-hydroxy metabolite is greater than
that of chlorothalonil itself (acute oral LD50 of 332 mg/kg body
weight versus >10 000 mg/kg body weight). Several studies have been
undertaken to characterize the toxicological profile of this
metabolite and to establish NOELs.
2.1.7 Effects on humans
Contact dermatitis has been reported for personnel working in
chlorothalonil manufacturing and in farmers and horticultural workers.
Workers in the manufacture of wood products have also developed
contact dermatitis on the hands and face, when wood preservatives
containing chlorothalonil were used.
2.1.8 Effects on non-target organisms in the laboratory and field
Chlorothalonil was highly toxic for fish and aquatic invertebrates in
laboratory studies, with similar LC50s below 0.5 mg/litre. The
maximum acceptable toxicant concentration (MATC) in a two-generation
reproduction study on Daphnia magna was 35 µg/litre.
With minor exceptions, chlorothalonil is not phytotoxic.
The LC50 of a suspension concentrate formulation (500 g
chlorothalonil/litre) in artificial soil for earthworms was
>1000 mg/kg soil (14 days). Earwigs suffered increased mortality when
in contact with chlorothalonil residues on peanut foliage or ingesting
it as a food source in laboratory tests; there was no other indication
of insecticidal action.
The toxicity of chlorothalonil for birds is low, with a reported acute
oral LD50 of 4640 mg/kg diet in the mallard duck. No significant
reproductive effects were reported.
The results of a field study on aquatic organisms, exposed following
chlorothalonil application, suggest that the toxicity is less than
that predicted from laboratory studies; this is again consistent with
the physical and chemical properties of the compound. While deaths
were seen in some species exposed experimentally in the field, there
have been no reported incidents of kills in the environment. Although
the residence time of chlorothalonil in environmental media is short,
kills would be expected to occur. However, linking kills to the
compound would be difficult because residues would not persist long
enough for chlorothalonil to be identified.
2.2 Evaluation
2.2.1 Toxicological assessment
A review of the toxicological data for chlorothalonil revealed that
the most important studies for human risk estimation were the
long-term studies on rodents and dogs.
In the rodent studies, chlorothalonil caused lesions in the
forestomach and kidney. The lesions in the forestomach were
characterized as hyperplasia and hyperkeratosis of the squamous
epithelial cells. These occurred soon after dosing and were shown to
be reversible after dosing ceased. Long-term administration led to the
formation of tumours (papilloma and carcinoma). The renal lesions in
rodents were of rapid onset and characterized as hyperplasia of the
proximal tubular epithelium. On longer-term administration, renal
tumours (adenoma and carcinoma) occurred in the rat and in one study
on mice.
In order to interpret the significance of these findings, the results
of the mutagenic studies were taken into account. Chlorothalonil gave
negative results in in vitro and in vivo mutagenic assays in which
a variety of end-points were studied. Thiol derivatives of
chlorothalonil were negative in the Ames test, and 14C-chlorothalonil
did not bind to rat kidney DNA in vivo. The compound does not appear
to have genotoxic potential on this basis, indicating that it probably
exerts its carcinogenic effect in rodents via a non-genotoxic
mechanism. The initial forestomach lesions in rodents were attributed
to the irritant action of chlorothalonil and, where this does not
occur, a NOEL can be attained. The irritant action on rodent
forestomach in conjunction with the relatively long residence time of
the compound in this organ were seen to be factors presenting the
opportunity for the initiation of the lesions and leading to
carcinogenic action on prolonged administration. It was concluded
that, since humans do not possess a comparable organ, rodents are
probably not representatives of the action of this compound in man in
this respect. This reasoning is also supported by the fact that
another animal species, the dog, is not affected by the compound at
similar or higher doses.
In the assessment of the relevance of the rodent renal lesions, the
metabolic conversion of chlorothalonil to metabolites that act
directly upon the kidney was seen to be a major factor. In the kidney,
glutathione conjugates are converted by ß-lyase to chlorothalonil
thiol derivatives. Chlorothalonil is thought to be conjugated with
glutathione (GSH) mainly in the gastrointestinal tract prior to
absorption, though there is evidence of glutathione conjugation at
other sites. After absorption, the conjugates pass to the kidney where
they are converted to chlorothalonil thiol derivatives following the
action of ß-lyase. It has been shown in vitro that the di- and trithiol
metabolites inhibit the function of renal cortical mitochondria.
Therefore, a cycle of cell death and regenerative renal hyperplasia
may be initiated.
In adducing the relevance of these findings for humans, the species
differences in the metabolic pathway for chlorothalonil was taken into
account. It was noted that the formation of the thiol metabolites, as
determined by urinary excretion, was considerably diminished when
chlorothalonil was fed to germ-free rats. This indicates that the type
and/or quantity of gut microflora has a determining role in the
production of the thiol derivatives. In studies on dogs and monkeys,
the excretion of the thiol derivatives was barely detectable after
oral administration of chlorothalonil. This suggests that the rat is
rather different from other species in this respect. Furthermore,
there is some evidence that ß-lyase activity in the kidney varies
among species, being an order of magnitude lower in humans than in
rats.
For all the reasons stated above, it was concluded that the rodent was
not the most relevant species for evaluating the long-term effects of
chlorothalonil in humans and that the dog was a more representative
species for this purpose. The NOEL of 120 mg/kg diet in the 2-year
study on dogs, equivalent to 3 mg/kg body weight per day, should,
therefore, be used for the purposes of human risk estimation.
2.2.2 Environmental assessment
Chlorothalonil is algicidal for a number of algal species. The
fungicide does not inhibit bacterial growth, except at very high
concentrations in laboratory culture. Field and laboratory evidence
shows no effects on nitrogen fixation or nitrification at recommended
application rates and minimal effects at higher application rates in
temperate soils. There was insufficient information to assess effects
on the nitrogen cycle in tropical soils.
Acute toxicity tests in the laboratory showed chlorothalonil to be
very highly toxic for many aquatic animals including fish and Daphnia,
but that molluscs appeared to be insensitive. The LC50 concentrations
for a range of fish and invertebrates were similar and were below
0.5 mg/litre.
A single study indicated reproductive effects in fish following
continuous exposure to chlorothalonil for 35 days. Since the compound
both adsorbs on suspended material and is degraded rapidly, the
significance of this finding was considered to be questionable.
The results of a field study on aquatic organisms exposed following
chlorothalonil application suggest that the toxicity is less than that
predicted from laboratory studies; this is again consistent with the
physical and chemical properties of the compound. Deaths were seen in
some species exposed experimentally in the field, but there have been
no reported incidents of kills in the environment. Despite the short
residence time of chlorothalonil in environmental media, kills would
be expected to occur immediately after application. However, linking
kills to the compound would be difficult as residues would not persist
long enough for chlorothalonil to be identified.
With minor exceptions, chlorothalonil is not phytotoxic.
Several studies have shown that chlorothalonil at recommended
application rates was not toxic for earthworms. At an exposure of five
times the maximum recommended rate, the compound severely reduced worm
reproduction.
Chlorothalonil is classified as "relatively non-toxic" for honey-bees.
Earwigs exposed to residues topically and via food showed some
mortality (20-55%), but there was no other evidence of insecticidal
action.
In acute or dietary tests the toxicity of chlorothalonil for birds was
low. The low acute toxicity of chlorothalonil for laboratory mammals
tempered with its short persistence in the environment suggests
minimal hazards for wild mammal species.
2.2.2.1 Transport, distribution, and transformation
Chlorothalonil adsorbs strongly on organic matter in soil and
suspended material in water. It is not, therefore, leached from soil
to groundwater. It is removed rapidly from surface water on to
suspended material and to a lesser extent on to bottom sediment.
Chlorothalonil is not translocated in plants from the site of
application.
Abiotic degradation of chlorothalonil in water through photolysis does
not occur. Some hydrolysis does take place at higher pH.
Microbial degradation is the major cause of dissipation in soil and
may take place, to some extent, in water; this involves several
parallel processes, one of which leads to formation of the 4-hydroxy
metabolite. Half-lives for the dissipation of this metabolite from
non-sterile soils ranged between 6 and 43 days. Biodegradation on
plants is limited and the 4-hydroxy metabolite comprises less than 5%
of the total residues.
During exposure, fish bioconcentrate chlorothalonil, but almost total
degradation occurs within 2 weeks following termination of exposure.
Chlorothalonil is metabolized in fish through glutathione conjugation
and the conjugates are excreted through the bile.
2.2.2.2 Aquatic organisms
The results were available of a single field study in which
concentrations of chlorothalonil in water were measured following
overspraying of the water; corresponding data on concentrations of
chlorothalonil in suspended and bottom sediment were unreliable.
Output from the EXAMS II fate model using the same application
scenario produced estimated water concentrations that closely
corresponded to the measured ones. Little or no chlorothalonil was
predicted in bottom sediment.
On the basis of this combination of measured and modelled data, the
ratio between a "toxic" concentration (the rainbow trout LC50) and
expected concentration is less than 1 for up to 5 h after overspray
and increases rapidly thereafter. Similar results were obtained for
daphnids. Therefore, despite its rapid removal from water and
degradation, the high toxicity of chlorothalonil is expected to cause
deaths of aquatic organisms in the period immediately after spraying.
This is the worst case situation of direct water overspray. There were
no data to extend this quantitative evaluation to other field
situations or climates.
2.2.2.3 Terrestrial organisms
A calculated maximum soil concentration, based on application of
chlorothalonil at 2.5 kg a.i./ha and complete bioavailability, is 3
orders of magnitude higher than the lowest estimate of LC50 for
earthworms.
For grazing birds (ducks and geese), total daily intake is at least a
factor of 100 below the NOEL for oral toxicity. For rabbits, total
daily intake is also at least 2 orders of magnitude lower than the
reported NOEL. This is based on a maximum recommended application rate
of 2.5 kg a.i./ha, an estimated worst case value for residues on
grass, no degradation of the compound, consumption of the total daily
intake at a single time and no choice but to eat contaminated food.
Table 2 contains a summary of risk quotients for avian and fish risk
categories.
Table 2. Toxicity-exposure ratios for birds and fish based on application rates
of 2.5 kg a.i./ha of chlorothalonil to soybeans (worst case)
Risk category LC50 as Estimated exposurea,b Toxicity/exposure
mg/litre or as mg/litre ratio (TER)c
mg/kg diet or mg/kg diet
Acute bird 4640 73.7-535.7 63.0-8.7
Acute fish (stream) 0.01 0.009-0.04 1.1-0.25
Acute fish (pond) 0.01 0.01 1.0
Acute aquatic,
invertebrate (stream) 0.07 0.009-0.04 7.8-1.8
Acute aquatic,
invertebrate (pond) 0.07 0.01 7.0
a Estimated environmental concentration in the terrestrial environment (for bird
exposure) is based on the stated application rate and the assumption of deposition
on short grass using the US EPA nomogram.
b Aquatic exposure concentrations were taken from the STEAM model based on a single
application and estimated runoff into water; no direct overspray is included.
c TER is the toxicity (as LC50) divided by the exposure; values at, or below,
1.0 indicate likely exposure to toxic concentrations by organisms in the different
risk categories.
2.2.3 Toxicological criteria for setting guideline values
The toxicological studies on chlorothalonil of relevance for setting
guideline values are displayed in Table 3. The study results and their
significance are described briefly, and gaps in test requirements are
indicated.
Table 3. Toxicological criteria for setting guideline values for chlorothalonil
Exposure Relevant route/effect/ Result/remarks
scenario species
Short-term Skin, irritation, rabbit Irritant
(1-7 days) Eye, irritation, rabbit Irritant
Skin, sensitization, Tests were inconclusive;
guinea-pig evidence in humans of contact
dermatitis
Inhalation, lethality, rat High toxicity in 4-h study with
hammer-milled technical
chlorothalonil (MMAD 5-8 µm);
not relevant for most human
exposure situations
Medium-term Repeat dermal, rabbit 21-day study; irritant at
(1-26 weeks) 2.5 mg/kg body weight per day
and above; no systemic effects
at 50 mg/kg body weight per day
Repeat oral, mice and rats 13-22 week studies; NOEL=
3 mg/kg body weight per day
in rats and mice
Maternal, oral, rabbit Teratology study; maternal
toxicity NOEL = 10 mg/kg body
weight per day by gavage; no
fetotoxic or teratogenic effect
Long-term Repeat oral, dog 2-year study; NOEL = 3 mg/kg
body weight per day
3. CONCLUSIONS AND RECOMMENDATIONS
Considering the toxicological characteristics of chlorothalonil, both
qualitatively and quantitatively, the CAG concluded, using the NOEL of
3 mg/kg body weight per day in the 2-year study on dogs and applying a
100-fold uncertainty factor, that 0.03 mg/kg body weight per day will
probably not cause adverse effects in humans, by any route of
exposure.
A study to assess the skin irritation potential is needed.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Human Health Hazards, Prevention and Protection, First Aid
The acute oral toxicity of technical chlorothalonil for human beings
is low. The compound may cause irritation of the respiratory tract,
the skin, and the eyes. It may act as a sensitizer.
In view of its irritating properties, exposure of humans beings should
be kept to a minimum.
4.1.1 Prevention and protection
The following precautions should be observed during the handling and
use of chlorothalonil, in order to reduce the risk of accidental
contamination.
* Avoid contact with the skin and eyes by using protective clothing
and goggles or a face-shield.
* Do not smoke, drink, or eat in the workplace. Wash hands and any
exposed skin before eating, drinking, or smoking, and after work.
* Avoid raising a dust cloud when handling wettable powder
formulations.
* Avoid breathing the dust from powder products.
* When unloading and handling containers, wear protective PVC or
neoprene gloves.
* When handling leaking containers, or when dealing with leaks and
spills, wear overalls, PVC or neoprene gloves, boots, and
eye/face protection. Avoid creating a dust. If overalls become
contaminated, change and wash them thoroughly before re-use.
* Store products in closed original containers, out of reach of
children and unauthorized persons, and away from food, drink, and
animal feed.
4.1.2 First aid
Acute poisoning by chlorothalonil is unlikely, unless large amounts
are ingested. In cases of over-exposure, apply routine first aid
measures. If the compound has been spilled on the skin, immediately
remove the victim from the source of contamination, remove all
contaminated clothing, and wash affected areas with soap and running
water. If the material is in the eyes, flush with clean water for at
least 15 min. In case of ingestion of significant quantities, if the
victim is conscious, give several glasses of water or a slurry of
activated charcoal in water. Do not induce vomiting. In serious cases,
medical attention should be sought.
4.2 Advice to Physicians
The acute oral toxicity of chlorothalonil for human beings is low.
There is no specific antidote. Treat symptomatically, paying special
attention to respiratory and dermal symptoms when necessary. In cases
of ingestion of large amounts, gastric lavage may be indicated.
4.3 Explosion and Fire Hazards
Chlorothalonil is not flammable but, on heating, may produce toxic
fumes, such as nitrogen oxides, hydrochloric acid, and phosgene.
Extinguish small fires with carbon dioxide, dry powder, or
alcohol-resistant foam. Water spray can be used for larger fires and
for the cooling of unaffected stock, but avoid the accumulation of
polluted run-off from the site. Fire service personnel should be
advised that self-contained breathing apparatus may be required,
because of the generation of noxious fumes.
4.4 Storage and Transport
All products should be stored in secure buildings, out of reach of
children and animals, and local regulations should be complied with.
Containers should be sound and adequately labelled.
4.5 Spillage and Disposal
4.5.1 Spillage
Avoid contact with the solid or dust. Keep spectators away from any
leakage. This pesticide is highly toxic for fish and other aquatic
organisms. Prevent contamination of other goods or cargo, and of
nearby vegetation and waterways.
Absorb spilled liquid products with earth or sand. If available,
sawdust, peat, moss, or straw are also suitable absorbents; sweep up
and place in a separate container. Empty any product remaining in
damaged or leaking containers into a clean, empty container, which
should be suitably labelled. Sweep up any spilled powder with damp
sawdust, taking care not to raise a dust cloud (use a vacuum cleaner).
Remove trapped material with suction hoses. Place in a separate
container for subsequent disposal. Use mechanical dredges or lifts to
remove immobilized masses of pollutants and precipitates.
4.5.2 Waste disposal
Chlorothalonil can be incinerated in units operating at 850°C fitted
with effluent-gas scrubbing equipment.
The disposal methods for waste pesticides and containers advocated by
FAO and GIFAP should be applied to unused chlorothalonil products and
their empty packages (FAO, 1985b; GIFAP, 1987). However,
chlorothalonil is most difficult to remove, even by 3 rinsings with
water (Braun et al., 1983).
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Chlorothalonil is readily degraded in soil and adsorbed on suspended
matter in water. It may bioaccumulate. It is toxic for aquatic
organisms. It is moderately toxic for honey-bees, and its toxicity for
birds is low.
Avoid contamination of soil, water, and the atmosphere by proper
methods of use, storage, transport, handling, and waste disposal. In
case of spillage, use the methods advised in section 4.5.1.
6. CURRENT REGULATIONS, GUIDELINES AND STANDARDS
The information in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. It is a representative but non-exhaustive
overview of current regulations, guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
6.1 Previous Evaluations by International Bodies
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) discussed and
evaluated chlorothalonil at its meetings in 1974, 1977, 1978, 1979,
1981, 1983, 1985, 1987, and 1990. In 1990, an acceptable daily intake
(ADI) of 0-0.03 mg/kg body weight was established. This ADI was
confirmed in 1992, on the basis of the no-observed-adverse-effect
level (NOAEL) of 3 mg/kg body weight per day, established in the
two-year dog study.
Temporary maximum residue limits (MRLs) have been recommended for
various crops at the above evaluations (see Table 4).
WHO has classified chlorothalonil as a technical product unlikely to
present an acute hazard in normal use (WHO, 1992).
On the basis of data available at the time, IARC evaluated
chlorothalonil as showing limited evidence of carcinogenicity in
animal studies and categorized it as an agent not classifiable as to
its carcinogenicity to humans (IARC, 1987).
6.2 Exposure Limit Values
Some exposure limit values are given in Table 4.
For preharvest intervals, check with the competent National Authority.
6.3 Specific Restrictions
Chlorothalonil is approved as a pesticide in many countries. Specific
uses, limitations, and precautions are listed in national regulatory
documents.
6.4 Labelling, Packaging, and Transport
Neither the United Nations Committee of Experts on the Transportation
of Dangerous Goods nor the European Economic Community Legislation
give specific labelling requirements for chlorothalonil.
Table 4. Codex MRLs for chlorothalonila
Commodity MRL in mg/kg product
Peanuts (kernels), potatoes 0.1
Lima beans (without pod), peanuts (whole) 0.5
Carrots, sugar beets, sweet corn 1
Beans (green in the pod), broccoli,
brussel sprouts, cabbage, cauliflower,
citrus fruit, cranberries, cucumbers, melons,
onions, pumpkins, squash, tomatoes 5
Blackberries, cherries, chicory sprouts,
collards, endive, kale, lettuce (head),
peppers, raspberries 10
Celery 15
Currants (red, black, white), peaches 25
a From: FAO/WHO (1986).
BIBLIOGRAPHY
BRAUN, H.E., MORROW, D.C., RIPLEY, B.D., & FRANK, R. (1983) Efficiency
of water rinsing for the decontamination of used pesticide containers.
Arch. Environ. Contam. Toxicol., 12: 257-264.
CEC (1987) Legislation on dangerous substances - Classification and
labelling in the European Communities. Vol. 1 & 2, Commission of the
European Communities, London, Graham & Trotman, Ltd.
CEC/IPCS (1991) International chemical safety card (ICSC) No. 0134 on
Chlorothalonil. Luxembourg, Grand Duchy of Luxembourg, Commission of
the European Communities (CEC); Geneva, Switzerland, International
Programme on Chemical Safety (IPCS), World Health Organization, 2 pp.
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1964-present) Evaluations of pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1986) Codex Maximum Limits for pesticide residues, Codex
Alimentarius Commission. CAC/Vol. XIII, Supplement 1 & 2, 3rd ed.
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues. 4th ed. Rome, Codex Committee on Pesticide Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packaging, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticides
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
HAYES, W.J., Jr & LAWS, E.R., Jr (1991) Handbook of pesticide
toxicology. 3 Vol., New York, Academic Press.
IARC (1972-present) IARC monographs on the evaluation of carcinogenic
risk of chemicals to man. Lyon, International Agency for Research on
Cancer.
ILO (1991) Safety and health in the use of agro-chemicals - a guide.
Geneva, International Labour Office.
IPCS (in preparation) Environmental Health Criteria 183:
Chlorothalonil. Geneva, World Health Organization.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization (Report
No. VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, van Nostrand Reinhold Company, Inc.
UNEP/IEO (1990) Storage of hazardous materials: a technical guide for
safe warehousing of hazardous materials. Paris, United Nations
Environment Programme - Industry and Environment Office, 80 p.
UNITED NATIONS (1989) Consolidated list of products whose consumption
and/or sale have been banned, withdrawn, severely restricted or not
approved by Governments. 2nd ed. New York, United Nations.
UNITED NATIONS (1989) Recommendations on the transport of dangerous
goods. 6th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (1992) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1992-93. Geneva, World Health
Organization (unpublished document WHO/PCS/92.14).
WORTHING, C.R. & HANCE, R.J. (1991) The pesticide manual, 9th ed.,
Farnham, United Kingdom, British Crop Protection Council.
Molecular formula: C8Cl4N2
Relative molecular mass: 265.9
CAS chemical name: 2,4,5,6,-tetrachloro-1,3-
benzenedicarbonitrile
CAS registry number: 1897-45-6
RTECS registry number: NT2600000
Common name: chlorothalonil
IUPAC name: tetrachloroisophthalonitrile
Synonyms: m-TCPN;
2,4,5,6-tetrachloro-3-cyanobenzonitrile
Trade names: Bravo (ISK Biotech) (manufacturers &
suppliers); Daconil (ISK Biotech); Faber
(Tripart Farm Chemicals); Repulse (ICI);
Exotherm (Alto Elite); Nopocide (a
preservative in paints and adhesives)
Technical product purity: >97%
Technical product
impurities (%): Tetrachlorophthalonitrile (<0.1);
Tetrachloroterephthalonitrile (0.1-1.6);
Pentachlorobenzonitrile (0.5-2.5);
Partially chlorinated dicyanobenzenes
(0.2-1.0); Unchlorinated dicyanobenzenes
(0.1-1.6); Insoluble in xylene (0.1-1.0)
HCB (0.03); xylene insolubles (0.35)
1.2 Physical and Chemical Properties
The physical properties of chlorothalonil are listed in Table 1.
Table 1. Physical properties of chlorothalonil
Physical state crystalline solid
Colour colourless
Odour odourless
Melting point (°C) 250-251
Boiling point (°C) 350 (760 mmHg)
Vapour pressure at 25°C 5.72 × 10-7
Relative density 1.8
Octanol-water partition 2.88-3.86
coefficient (Log Kow)
Solubility in water (mg/litre) 0.6-1.2
at 25°C
Solubility in organic acetone 20, dimethylformamide 30,
solvents (g/litre) dimethylsulfoxide 20, xylene 80,
readily soluble in benzene
Chlorothalonil is non-flammable and non-explosive. It is thermally
stable under normal storage conditions and to UV radiation and is
chemically stable in neutral or acidic aqueous solutions. It breaks
down at pH 9, the rate following first-order kinetics at 1.8% per day
at 25°C. It has been shown that chlorothalonil is unstable to light
when dissolved in benzene and that 2,3,5-trichloro-4,6-dicyanobiphenyl
is a condensation product. Chlorothalonil is not corrosive.
2. SUMMARY AND EVALUATION
2.1 Summary
2.1.1 Identity, physical and chemical properties, and analytical
methods
Chlorothalonil is a colourless, odourless, crystalline solid with a
melting point of 250°C and a vapour pressure of 7.63 × 10-5 Pa (5.72 ×
10-7 mmHg) at 25°C. It is poorly soluble in water (0.6-1.2 mg/litre at
25°C) with an octanol/water partition coefficient (log Kow) of
2.882. It is slowly hydrolysed in water at pH 9, but is stable at pH 7
or below (at 25°C).
The most prevalent analytical method, after sample extraction and
clean-up, is gas-liquid chromatography using an electron-capture
detector.
2.1.2 Sources of human and environmental exposure
Chlorothalonil has been produced commercially since 1969 by the
chlorination of isophthalonitrile or by treatment of
tetrachloroisophthaloyl amide with phosphorus oxychloride. It is a
fungicide with a broad spectrum of activity, used mainly in
agriculture but also on turf, lawns, and ornamental plants. Crops
protected include pome and stone fruit, citrus, currants, berries,
bananas, tomatoes, green vegetables, coffee, peanuts, potatoes,
onions, and cereals. In addition, it is used in wood preservatives and
in paints.
The three main formulations are a suspension concentrate, a water
dispersible granule, and a wettable powder. They are diluted readily
with water and applied by ground spray systems or by air. Typical
active ingredient rates are 1.2-2.5 kg/ha for crops, such as beans,
celery, and onions. The main sources of human exposure will be during
the preparation and application of the products, and from ingestion of
crop residues in foodstuffs (see section 2.1.4).
2.1.3 Environmental transport, distribution, and transformation
Chlorothalonil is removed from aqueous media by strong adsorption on
suspended matter. Modelled data suggest little or no partition to
bottom sediment. Biodegradation involving enzyme processes may occur
in natural waters. Chlorothalonil is rapidly degraded in soil and
degradation may occur in water with the production of the 4-hydroxy
metabolite, 4-hydroxy-2,5,6-trichloroisophthalonitrile. Half-lives for
dissipation of the 4-hydroxy metabolite in soils range between 6 and
43 days.
Chlorothalonil does not translocate from the site of application to
other parts of a plant. It is metabolized only to a limited extent on
plants and the 4-hydroxy metabolite is usually <5% of the residue.
Chlorothalonil is metabolized in fish via glutathione conjugation to
give more polar excretory products. The enzyme glutathione- S-
transferase is involved in this conversion. High concentrations of
radiolabel found in the gall bladder and bile, after exposure of
rainbow trout to 14C-chlorothalonil, are consistent with the
excretion of the compound as glutathione conjugates. The
concentrations of radiolabel accumulating in the gall bladder and
other organs fell rapidly when the fish were placed in clean water.
Chlorothalonil does not bioaccumulate in aquatic organisms.
2.1.4 Environmental levels and human exposure
In a potato crop study, a small stream was oversprayed with
chlorothalonil. Subsequent sampling/analysis of downstream water
demonstrated rapid disappearance of chlorothalonil (i.e., 450 µg/litre
30 min after spraying to 2-6 µg/litre 12 h after spraying). The
routine spraying of irrigated field crops, such as potatoes and
barley, gave rise to low concentrations of chlorothalonil (0.04-
3.6 µg/litre) in tile drain water on a small number of sampling
occasions.
Crop residues are composed mainly of chlorothalonil itself. Residue
levels depend on the applied rate, the time interval between the last
application and harvest, and the type of crop. Residue levels at
harvest can be derived from the numerous supervised trials that have
taken place on many crops throughout the world and have been reported
to FAO/WHO. Residues of chlorothalonil in dairy products are expected
to be undetectable or very low. Dairy cows, given high concentrations
(up to 250 mg/kg) of chlorothalonil in their feed for 30 days, showed
no detectable residues in milk and only very low levels in tissues.
Total diet and individual food analyses in several countries have
shown low or undetectable concentrations of chlorothalonil in sampling
surveys. Residue levels on foodstuffs are further reduced by
preparation processes, such as washing, peeling, and cooking.
2.1.5 Kinetics and metabolism in laboratory animals
About 30% of an oral dose of chlorothalonil was absorbed after 48 h in
rats administered doses of up to 50 mg/kg body weight. At higher
doses, absorption was lower, indicating a saturation process. When
14C-chlorothalonil was given orally the radioactivity was distributed
into blood and tissues within 2 h. The greatest concentration was
found in the kidney, followed by the liver and blood. The kidneys
contained 0.3% of a dose of 5 mg/kg body weight after 24 h.
Most of an oral dose of chlorothalonil in rats was found in the faeces
(>82% within 48-72 h, regardless of dose). Biliary excretion was
rapid, peaking within 2 h after an oral dose of 5 mg/kg body weight,
and was saturated at doses of 50 mg/kg body weight and above. Urinary
excretion accounted for 5-10% of the dose in rats.
Faecal elimination is the main route in dogs and monkeys, but urinary
excretion (<4%) is less than in rats.
Metabolic studies on rats indicate that chlorothalonil is conjugated
with glutathione in the liver as well as in the gastrointestinal
tract. Some of the glutathione conjugates may be absorbed from the
intestine and transported to the kidneys, where they are converted by
cytosolic ß-lyase to thiol analogues that are excreted in the urine.
When germ-free rats were dosed with chlorothalonil, the thiol
metabolites appeared in the urine in much smaller amounts than in
normal rats, indicating the involvement of intestinal microflora in
the metabolism of chlorothalonil. Dogs or monkeys dosed orally with
chlorothalonil excreted little, or no, thiol derivatives in the urine.
When 14C-chlorothalonil was applied to rat skin, approximately 28%
of the dose was absorbed within 120 h. About 18% of the dose was found
in the faeces and 6% in the urine within 120 h.
2.1.6 Effects on laboratory mammals, and in vitro test systems
The acute oral and dermal toxicities of chlorothalonil in rats and
rabbits are low (acute oral and dermal LD50s of >10 000 mg/kg body
weight). Hammer-milled technical chlorothalonil (MMAD 5-8 µm)
exhibited high toxicity in rats in an inhalation study with a 4-h
LC50 of 0.1 mg/litre.
Chlorothalonil was a skin and eye irritant in the rabbit. Skin
sensitization studies in the guinea-pig were inconclusive.
The main effects of repeated oral dosing in rats were on the stomach
and kidney. Groups of 25 rats/sex per group were fed chlorothalonil at
0, 1.5, 3, 10, or 40 mg/kg body weight per day in the diet for 13
weeks, followed by a 13-week recovery period. Increased incidences of
hyperplasia and hyperkeratosis of the forestomach occurred at 10 and
40 mg/kg; these reversed when treatment ceased. At 40 mg/kg, there was
an increased incidence of hyperplasia of kidney proximal tubular
epithelium in males at 13 weeks and after the recovery period. The
NOEL was 3 mg/kg body weight per day, based on lack of forestomach
lesions. The onset of the forestomach and kidney changes was shown to
be rapid, with the lesions developing within 4-7 days in male rats at
a dietary level of 175 mg/kg body weight per day.
In a 13-week study on mice (0, 7.5, 15, 50, 275, or 750 mg/kg diet),
increased incidences of hyperplasia and hyperkeratosis of the squamous
epithelial cells of the forestomach occurred in males and females at
50 mg/kg diet and above. On the basis of these changes, the NOEL was
15 mg chlorothalonil/kg diet, equivalent to 3 mg/kg body weight per
day.
A 16-week study on dogs receiving dietary levels of chlorothalonil of
0, 250, 500, or 750 mg/kg showed no treatment-related changes.
The forestomach and kidney lesions were investigated further in 2-year
studies on rats, mice, and dogs. In a study on rats (0, 1.8, 3.8, 15,
or 175 mg/kg body weight per day), the effects of chlorothalonil were
characterized histologically as an increase in both the incidence and
severity of hyperplasia, hyperkeratosis and ulcers and erosions of the
squamous mucosa of the forestomach, and epithelial hyperplasia of the
kidney proximal convoluted tubules at doses of 3.8 mg/kg and above.
The NOEL for non-neoplastic effects was, therefore, 1.8 mg/kg. The
incidence of renal tumours (adenomas and carcinomas) and forestomach
tumours (papillomas and carcinomas) was markedly increased at
175 mg/kg. There was evidence for increased incidence in kidney
tumours in males at 15 mg/kg and for stomach tumours at 3.8 and
15 mg/kg in both males and females. The NOEL for neoplastic effects
was, therefore, 1.8 mg/kg body weight per day, on the basis of changes
in forestomach tumour incidence. Supporting evidence for the
carcinogenic potential of chlorothalonil in the kidney and forestomach
of rats was provided by the results from other 2-year studies at
higher dose levels.
In a study on mice (0, 15, 40, 175, or 750 mg chlorothalonil/kg diet),
an increased incidence of renal tubular hyperplasia occurred at doses
of 175 mg/kg and above and of hyperplasia and hyperkeratosis of the
forestomach at 40 mg/kg and above. The incidence of squamous tumours
of the forestomach was slightly increased at 750 mg/kg. The NOELs for
neoplastic and non-neoplastic changes were, therefore, 175 and
15 mg/kg in the diet (equivalent to 17.5 and 1.6 mg/kg body weight per
day, respectively). Supporting evidence for these effects in the mouse
was provided in another study at higher dose levels, but a study on
B6C3F1 mice did not show any evidence for carcinogenic potential at
high dose levels.
In a 2-year study on dogs (60 and 120 mg/kg diet), no effects
attributable to chlorothalonil were found. The NOEL was, therefore,
120 mg/kg diet (equivalent to 3 mg/kg body weight per day).
Chlorothalonil was not mutagenic in several in vitro and in vivo
tests, though it was positive in a small number of assays.
The monothio, dithio, trithio, dicysteine, tricysteine, and
monoglutathione derivatives of chlorothalonil, which are potential
nephrotoxicants, were shown to be negative in the Ames assay.
Chlorothalonil was not teratogenic in rats or rabbits at doses of up
to 400 and 50 mg/kg body weight per day, respectively. Reproductive
parameters, such as mating, fertility, and gestation length were not
affected by chlorothalonil at levels of up to 1500 mg/kg diet in a
two-generation study on rats.
The acute oral toxicity of the 4-hydroxy metabolite is greater than
that of chlorothalonil itself (acute oral LD50 of 332 mg/kg body
weight versus >10 000 mg/kg body weight). Several studies have been
undertaken to characterize the toxicological profile of this
metabolite and to establish NOELs.
2.1.7 Effects on humans
Contact dermatitis has been reported for personnel working in
chlorothalonil manufacturing and in farmers and horticultural workers.
Workers in the manufacture of wood products have also developed
contact dermatitis on the hands and face, when wood preservatives
containing chlorothalonil were used.
2.1.8 Effects on non-target organisms in the laboratory and field
Chlorothalonil was highly toxic for fish and aquatic invertebrates in
laboratory studies, with similar LC50s below 0.5 mg/litre. The
maximum acceptable toxicant concentration (MATC) in a two-generation
reproduction study on Daphnia magna was 35 µg/litre.
With minor exceptions, chlorothalonil is not phytotoxic.
The LC50 of a suspension concentrate formulation (500 g
chlorothalonil/litre) in artificial soil for earthworms was
>1000 mg/kg soil (14 days). Earwigs suffered increased mortality when
in contact with chlorothalonil residues on peanut foliage or ingesting
it as a food source in laboratory tests; there was no other indication
of insecticidal action.
The toxicity of chlorothalonil for birds is low, with a reported acute
oral LD50 of 4640 mg/kg diet in the mallard duck. No significant
reproductive effects were reported.
The results of a field study on aquatic organisms, exposed following
chlorothalonil application, suggest that the toxicity is less than
that predicted from laboratory studies; this is again consistent with
the physical and chemical properties of the compound. While deaths
were seen in some species exposed experimentally in the field, there
have been no reported incidents of kills in the environment. Although
the residence time of chlorothalonil in environmental media is short,
kills would be expected to occur. However, linking kills to the
compound would be difficult because residues would not persist long
enough for chlorothalonil to be identified.
2.2 Evaluation
2.2.1 Toxicological assessment
A review of the toxicological data for chlorothalonil revealed that
the most important studies for human risk estimation were the
long-term studies on rodents and dogs.
In the rodent studies, chlorothalonil caused lesions in the
forestomach and kidney. The lesions in the forestomach were
characterized as hyperplasia and hyperkeratosis of the squamous
epithelial cells. These occurred soon after dosing and were shown to
be reversible after dosing ceased. Long-term administration led to the
formation of tumours (papilloma and carcinoma). The renal lesions in
rodents were of rapid onset and characterized as hyperplasia of the
proximal tubular epithelium. On longer-term administration, renal
tumours (adenoma and carcinoma) occurred in the rat and in one study
on mice.
In order to interpret the significance of these findings, the results
of the mutagenic studies were taken into account. Chlorothalonil gave
negative results in in vitro and in vivo mutagenic assays in which
a variety of end-points were studied. Thiol derivatives of
chlorothalonil were negative in the Ames test, and 14C-chlorothalonil
did not bind to rat kidney DNA in vivo. The compound does not appear
to have genotoxic potential on this basis, indicating that it probably
exerts its carcinogenic effect in rodents via a non-genotoxic
mechanism. The initial forestomach lesions in rodents were attributed
to the irritant action of chlorothalonil and, where this does not
occur, a NOEL can be attained. The irritant action on rodent
forestomach in conjunction with the relatively long residence time of
the compound in this organ were seen to be factors presenting the
opportunity for the initiation of the lesions and leading to
carcinogenic action on prolonged administration. It was concluded
that, since humans do not possess a comparable organ, rodents are
probably not representatives of the action of this compound in man in
this respect. This reasoning is also supported by the fact that
another animal species, the dog, is not affected by the compound at
similar or higher doses.
In the assessment of the relevance of the rodent renal lesions, the
metabolic conversion of chlorothalonil to metabolites that act
directly upon the kidney was seen to be a major factor. In the kidney,
glutathione conjugates are converted by ß-lyase to chlorothalonil
thiol derivatives. Chlorothalonil is thought to be conjugated with
glutathione (GSH) mainly in the gastrointestinal tract prior to
absorption, though there is evidence of glutathione conjugation at
other sites. After absorption, the conjugates pass to the kidney where
they are converted to chlorothalonil thiol derivatives following the
action of ß-lyase. It has been shown in vitro that the di- and trithiol
metabolites inhibit the function of renal cortical mitochondria.
Therefore, a cycle of cell death and regenerative renal hyperplasia
may be initiated.
In adducing the relevance of these findings for humans, the species
differences in the metabolic pathway for chlorothalonil was taken into
account. It was noted that the formation of the thiol metabolites, as
determined by urinary excretion, was considerably diminished when
chlorothalonil was fed to germ-free rats. This indicates that the type
and/or quantity of gut microflora has a determining role in the
production of the thiol derivatives. In studies on dogs and monkeys,
the excretion of the thiol derivatives was barely detectable after
oral administration of chlorothalonil. This suggests that the rat is
rather different from other species in this respect. Furthermore,
there is some evidence that ß-lyase activity in the kidney varies
among species, being an order of magnitude lower in humans than in
rats.
For all the reasons stated above, it was concluded that the rodent was
not the most relevant species for evaluating the long-term effects of
chlorothalonil in humans and that the dog was a more representative
species for this purpose. The NOEL of 120 mg/kg diet in the 2-year
study on dogs, equivalent to 3 mg/kg body weight per day, should,
therefore, be used for the purposes of human risk estimation.
2.2.2 Environmental assessment
Chlorothalonil is algicidal for a number of algal species. The
fungicide does not inhibit bacterial growth, except at very high
concentrations in laboratory culture. Field and laboratory evidence
shows no effects on nitrogen fixation or nitrification at recommended
application rates and minimal effects at higher application rates in
temperate soils. There was insufficient information to assess effects
on the nitrogen cycle in tropical soils.
Acute toxicity tests in the laboratory showed chlorothalonil to be
very highly toxic for many aquatic animals including fish and Daphnia,
but that molluscs appeared to be insensitive. The LC50 concentrations
for a range of fish and invertebrates were similar and were below
0.5 mg/litre.
A single study indicated reproductive effects in fish following
continuous exposure to chlorothalonil for 35 days. Since the compound
both adsorbs on suspended material and is degraded rapidly, the
significance of this finding was considered to be questionable.
The results of a field study on aquatic organisms exposed following
chlorothalonil application suggest that the toxicity is less than that
predicted from laboratory studies; this is again consistent with the
physical and chemical properties of the compound. Deaths were seen in
some species exposed experimentally in the field, but there have been
no reported incidents of kills in the environment. Despite the short
residence time of chlorothalonil in environmental media, kills would
be expected to occur immediately after application. However, linking
kills to the compound would be difficult as residues would not persist
long enough for chlorothalonil to be identified.
With minor exceptions, chlorothalonil is not phytotoxic.
Several studies have shown that chlorothalonil at recommended
application rates was not toxic for earthworms. At an exposure of five
times the maximum recommended rate, the compound severely reduced worm
reproduction.
Chlorothalonil is classified as "relatively non-toxic" for honey-bees.
Earwigs exposed to residues topically and via food showed some
mortality (20-55%), but there was no other evidence of insecticidal
action.
In acute or dietary tests the toxicity of chlorothalonil for birds was
low. The low acute toxicity of chlorothalonil for laboratory mammals
tempered with its short persistence in the environment suggests
minimal hazards for wild mammal species.
2.2.2.1 Transport, distribution, and transformation
Chlorothalonil adsorbs strongly on organic matter in soil and
suspended material in water. It is not, therefore, leached from soil
to groundwater. It is removed rapidly from surface water on to
suspended material and to a lesser extent on to bottom sediment.
Chlorothalonil is not translocated in plants from the site of
application.
Abiotic degradation of chlorothalonil in water through photolysis does
not occur. Some hydrolysis does take place at higher pH.
Microbial degradation is the major cause of dissipation in soil and
may take place, to some extent, in water; this involves several
parallel processes, one of which leads to formation of the 4-hydroxy
metabolite. Half-lives for the dissipation of this metabolite from
non-sterile soils ranged between 6 and 43 days. Biodegradation on
plants is limited and the 4-hydroxy metabolite comprises less than 5%
of the total residues.
During exposure, fish bioconcentrate chlorothalonil, but almost total
degradation occurs within 2 weeks following termination of exposure.
Chlorothalonil is metabolized in fish through glutathione conjugation
and the conjugates are excreted through the bile.
2.2.2.2 Aquatic organisms
The results were available of a single field study in which
concentrations of chlorothalonil in water were measured following
overspraying of the water; corresponding data on concentrations of
chlorothalonil in suspended and bottom sediment were unreliable.
Output from the EXAMS II fate model using the same application
scenario produced estimated water concentrations that closely
corresponded to the measured ones. Little or no chlorothalonil was
predicted in bottom sediment.
On the basis of this combination of measured and modelled data, the
ratio between a "toxic" concentration (the rainbow trout LC50) and
expected concentration is less than 1 for up to 5 h after overspray
and increases rapidly thereafter. Similar results were obtained for
daphnids. Therefore, despite its rapid removal from water and
degradation, the high toxicity of chlorothalonil is expected to cause
deaths of aquatic organisms in the period immediately after spraying.
This is the worst case situation of direct water overspray. There were
no data to extend this quantitative evaluation to other field
situations or climates.
2.2.2.3 Terrestrial organisms
A calculated maximum soil concentration, based on application of
chlorothalonil at 2.5 kg a.i./ha and complete bioavailability, is 3
orders of magnitude higher than the lowest estimate of LC50 for
earthworms.
For grazing birds (ducks and geese), total daily intake is at least a
factor of 100 below the NOEL for oral toxicity. For rabbits, total
daily intake is also at least 2 orders of magnitude lower than the
reported NOEL. This is based on a maximum recommended application rate
of 2.5 kg a.i./ha, an estimated worst case value for residues on
grass, no degradation of the compound, consumption of the total daily
intake at a single time and no choice but to eat contaminated food.
Table 2 contains a summary of risk quotients for avian and fish risk
categories.
Table 2. Toxicity-exposure ratios for birds and fish based on application rates
of 2.5 kg a.i./ha of chlorothalonil to soybeans (worst case)
Risk category LC50 as Estimated exposurea,b Toxicity/exposure
mg/litre or as mg/litre ratio (TER)c
mg/kg diet or mg/kg diet
Acute bird 4640 73.7-535.7 63.0-8.7
Acute fish (stream) 0.01 0.009-0.04 1.1-0.25
Acute fish (pond) 0.01 0.01 1.0
Acute aquatic,
invertebrate (stream) 0.07 0.009-0.04 7.8-1.8
Acute aquatic,
invertebrate (pond) 0.07 0.01 7.0
a Estimated environmental concentration in the terrestrial environment (for bird
exposure) is based on the stated application rate and the assumption of deposition
on short grass using the US EPA nomogram.
b Aquatic exposure concentrations were taken from the STEAM model based on a single
application and estimated runoff into water; no direct overspray is included.
c TER is the toxicity (as LC50) divided by the exposure; values at, or below,
1.0 indicate likely exposure to toxic concentrations by organisms in the different
risk categories.
2.2.3 Toxicological criteria for setting guideline values
The toxicological studies on chlorothalonil of relevance for setting
guideline values are displayed in Table 3. The study results and their
significance are described briefly, and gaps in test requirements are
indicated.
Table 3. Toxicological criteria for setting guideline values for chlorothalonil
Exposure Relevant route/effect/ Result/remarks
scenario species
Short-term Skin, irritation, rabbit Irritant
(1-7 days) Eye, irritation, rabbit Irritant
Skin, sensitization, Tests were inconclusive;
guinea-pig evidence in humans of contact
dermatitis
Inhalation, lethality, rat High toxicity in 4-h study with
hammer-milled technical
chlorothalonil (MMAD 5-8 µm);
not relevant for most human
exposure situations
Medium-term Repeat dermal, rabbit 21-day study; irritant at
(1-26 weeks) 2.5 mg/kg body weight per day
and above; no systemic effects
at 50 mg/kg body weight per day
Repeat oral, mice and rats 13-22 week studies; NOEL=
3 mg/kg body weight per day
in rats and mice
Maternal, oral, rabbit Teratology study; maternal
toxicity NOEL = 10 mg/kg body
weight per day by gavage; no
fetotoxic or teratogenic effect
Long-term Repeat oral, dog 2-year study; NOEL = 3 mg/kg
body weight per day
3. CONCLUSIONS AND RECOMMENDATIONS
Considering the toxicological characteristics of chlorothalonil, both
qualitatively and quantitatively, the CAG concluded, using the NOEL of
3 mg/kg body weight per day in the 2-year study on dogs and applying a
100-fold uncertainty factor, that 0.03 mg/kg body weight per day will
probably not cause adverse effects in humans, by any route of
exposure.
A study to assess the skin irritation potential is needed.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Human Health Hazards, Prevention and Protection, First Aid
The acute oral toxicity of technical chlorothalonil for human beings
is low. The compound may cause irritation of the respiratory tract,
the skin, and the eyes. It may act as a sensitizer.
In view of its irritating properties, exposure of humans beings should
be kept to a minimum.
4.1.1 Prevention and protection
The following precautions should be observed during the handling and
use of chlorothalonil, in order to reduce the risk of accidental
contamination.
* Avoid contact with the skin and eyes by using protective clothing
and goggles or a face-shield.
* Do not smoke, drink, or eat in the workplace. Wash hands and any
exposed skin before eating, drinking, or smoking, and after work.
* Avoid raising a dust cloud when handling wettable powder
formulations.
* Avoid breathing the dust from powder products.
* When unloading and handling containers, wear protective PVC or
neoprene gloves.
* When handling leaking containers, or when dealing with leaks and
spills, wear overalls, PVC or neoprene gloves, boots, and
eye/face protection. Avoid creating a dust. If overalls become
contaminated, change and wash them thoroughly before re-use.
* Store products in closed original containers, out of reach of
children and unauthorized persons, and away from food, drink, and
animal feed.
4.1.2 First aid
Acute poisoning by chlorothalonil is unlikely, unless large amounts
are ingested. In cases of over-exposure, apply routine first aid
measures. If the compound has been spilled on the skin, immediately
remove the victim from the source of contamination, remove all
contaminated clothing, and wash affected areas with soap and running
water. If the material is in the eyes, flush with clean water for at
least 15 min. In case of ingestion of significant quantities, if the
victim is conscious, give several glasses of water or a slurry of
activated charcoal in water. Do not induce vomiting. In serious cases,
medical attention should be sought.
4.2 Advice to Physicians
The acute oral toxicity of chlorothalonil for human beings is low.
There is no specific antidote. Treat symptomatically, paying special
attention to respiratory and dermal symptoms when necessary. In cases
of ingestion of large amounts, gastric lavage may be indicated.
4.3 Explosion and Fire Hazards
Chlorothalonil is not flammable but, on heating, may produce toxic
fumes, such as nitrogen oxides, hydrochloric acid, and phosgene.
Extinguish small fires with carbon dioxide, dry powder, or
alcohol-resistant foam. Water spray can be used for larger fires and
for the cooling of unaffected stock, but avoid the accumulation of
polluted run-off from the site. Fire service personnel should be
advised that self-contained breathing apparatus may be required,
because of the generation of noxious fumes.
4.4 Storage and Transport
All products should be stored in secure buildings, out of reach of
children and animals, and local regulations should be complied with.
Containers should be sound and adequately labelled.
4.5 Spillage and Disposal
4.5.1 Spillage
Avoid contact with the solid or dust. Keep spectators away from any
leakage. This pesticide is highly toxic for fish and other aquatic
organisms. Prevent contamination of other goods or cargo, and of
nearby vegetation and waterways.
Absorb spilled liquid products with earth or sand. If available,
sawdust, peat, moss, or straw are also suitable absorbents; sweep up
and place in a separate container. Empty any product remaining in
damaged or leaking containers into a clean, empty container, which
should be suitably labelled. Sweep up any spilled powder with damp
sawdust, taking care not to raise a dust cloud (use a vacuum cleaner).
Remove trapped material with suction hoses. Place in a separate
container for subsequent disposal. Use mechanical dredges or lifts to
remove immobilized masses of pollutants and precipitates.
4.5.2 Waste disposal
Chlorothalonil can be incinerated in units operating at 850°C fitted
with effluent-gas scrubbing equipment.
The disposal methods for waste pesticides and containers advocated by
FAO and GIFAP should be applied to unused chlorothalonil products and
their empty packages (FAO, 1985b; GIFAP, 1987). However,
chlorothalonil is most difficult to remove, even by 3 rinsings with
water (Braun et al., 1983).
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Chlorothalonil is readily degraded in soil and adsorbed on suspended
matter in water. It may bioaccumulate. It is toxic for aquatic
organisms. It is moderately toxic for honey-bees, and its toxicity for
birds is low.
Avoid contamination of soil, water, and the atmosphere by proper
methods of use, storage, transport, handling, and waste disposal. In
case of spillage, use the methods advised in section 4.5.1.
6. CURRENT REGULATIONS, GUIDELINES AND STANDARDS
The information in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. It is a representative but non-exhaustive
overview of current regulations, guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with the appropriate regulatory authorities
before application.
6.1 Previous Evaluations by International Bodies
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) discussed and
evaluated chlorothalonil at its meetings in 1974, 1977, 1978, 1979,
1981, 1983, 1985, 1987, and 1990. In 1990, an acceptable daily intake
(ADI) of 0-0.03 mg/kg body weight was established. This ADI was
confirmed in 1992, on the basis of the no-observed-adverse-effect
level (NOAEL) of 3 mg/kg body weight per day, established in the
two-year dog study.
Temporary maximum residue limits (MRLs) have been recommended for
various crops at the above evaluations (see Table 4).
WHO has classified chlorothalonil as a technical product unlikely to
present an acute hazard in normal use (WHO, 1992).
On the basis of data available at the time, IARC evaluated
chlorothalonil as showing limited evidence of carcinogenicity in
animal studies and categorized it as an agent not classifiable as to
its carcinogenicity to humans (IARC, 1987).
6.2 Exposure Limit Values
Some exposure limit values are given in Table 4.
For preharvest intervals, check with the competent National Authority.
6.3 Specific Restrictions
Chlorothalonil is approved as a pesticide in many countries. Specific
uses, limitations, and precautions are listed in national regulatory
documents.
6.4 Labelling, Packaging, and Transport
Neither the United Nations Committee of Experts on the Transportation
of Dangerous Goods nor the European Economic Community Legislation
give specific labelling requirements for chlorothalonil.
Table 4. Codex MRLs for chlorothalonila
Commodity MRL in mg/kg product
Peanuts (kernels), potatoes 0.1
Lima beans (without pod), peanuts (whole) 0.5
Carrots, sugar beets, sweet corn 1
Beans (green in the pod), broccoli,
brussel sprouts, cabbage, cauliflower,
citrus fruit, cranberries, cucumbers, melons,
onions, pumpkins, squash, tomatoes 5
Blackberries, cherries, chicory sprouts,
collards, endive, kale, lettuce (head),
peppers, raspberries 10
Celery 15
Currants (red, black, white), peaches 25
a From: FAO/WHO (1986).
BIBLIOGRAPHY
BRAUN, H.E., MORROW, D.C., RIPLEY, B.D., & FRANK, R. (1983) Efficiency
of water rinsing for the decontamination of used pesticide containers.
Arch. Environ. Contam. Toxicol., 12: 257-264.
CEC (1987) Legislation on dangerous substances - Classification and
labelling in the European Communities. Vol. 1 & 2, Commission of the
European Communities, London, Graham & Trotman, Ltd.
CEC/IPCS (1991) International chemical safety card (ICSC) No. 0134 on
Chlorothalonil. Luxembourg, Grand Duchy of Luxembourg, Commission of
the European Communities (CEC); Geneva, Switzerland, International
Programme on Chemical Safety (IPCS), World Health Organization, 2 pp.
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1964-present) Evaluations of pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1986) Codex Maximum Limits for pesticide residues, Codex
Alimentarius Commission. CAC/Vol. XIII, Supplement 1 & 2, 3rd ed.
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues. 4th ed. Rome, Codex Committee on Pesticide Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packaging, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticides
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
HAYES, W.J., Jr & LAWS, E.R., Jr (1991) Handbook of pesticide
toxicology. 3 Vol., New York, Academic Press.
IARC (1972-present) IARC monographs on the evaluation of carcinogenic
risk of chemicals to man. Lyon, International Agency for Research on
Cancer.
ILO (1991) Safety and health in the use of agro-chemicals - a guide.
Geneva, International Labour Office.
IPCS (in preparation) Environmental Health Criteria 183:
Chlorothalonil. Geneva, World Health Organization.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization (Report
No. VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, van Nostrand Reinhold Company, Inc.
UNEP/IEO (1990) Storage of hazardous materials: a technical guide for
safe warehousing of hazardous materials. Paris, United Nations
Environment Programme - Industry and Environment Office, 80 p.
UNITED NATIONS (1989) Consolidated list of products whose consumption
and/or sale have been banned, withdrawn, severely restricted or not
approved by Governments. 2nd ed. New York, United Nations.
UNITED NATIONS (1989) Recommendations on the transport of dangerous
goods. 6th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (1992) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1992-93. Geneva, World Health
Organization (unpublished document WHO/PCS/92.14).
WORTHING, C.R. & HANCE, R.J. (1991) The pesticide manual, 9th ed.,
Farnham, United Kingdom, British Crop Protection Council.
 Molecular formula: C8Cl4N2
Relative molecular mass: 265.9
CAS chemical name: 2,4,5,6,-tetrachloro-1,3-
benzenedicarbonitrile
CAS registry number:
Molecular formula: C8Cl4N2
Relative molecular mass: 265.9
CAS chemical name: 2,4,5,6,-tetrachloro-1,3-
benzenedicarbonitrile
CAS registry number: 
 Molecular formula: C8Cl4N2
Relative molecular mass: 265.9
CAS chemical name: 2,4,5,6,-tetrachloro-1,3-
benzenedicarbonitrile
CAS registry number:
Molecular formula: C8Cl4N2
Relative molecular mass: 265.9
CAS chemical name: 2,4,5,6,-tetrachloro-1,3-
benzenedicarbonitrile
CAS registry number: