
WHO Pesticide Residues Series, No. 1
1971 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
The evaluations contained in these monographs were prepared by the
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues that met
in Geneva from 22 to 29 November 1971.1
World Health Organization
Geneva
1972
1 Pesticide Residues in Food: Report of the 1971 Joint Meeting of
the FAO Working Party of Experts on Pesticide Residues and the WHO
Expert Committee on Pesticide Residues, Wld Hlth Org. techn. Rep.
Ser., No. 502; FAO Agricultural Studies, 1972, No. 88.
These monographs are also issued by the Food and Agriculture
Organization of the United Nations, Rome, as document AGP-1971/M/9/1.
FAO and WHO 1972
CHLORDIMEFORM
IDENTITY
Chemical name
N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine
Synonyms
Chlorphenamidine, C-8514, Schering 36,268, SN 36 268.
Galecron(R), Acaron(R), Fundal(R), Spike(R).
Structural formula
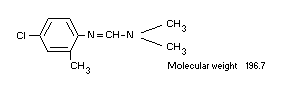 Other information on identity and properties
Chlordimeform is used as a free base or as its hydrochloride salt.
Physical properties of both base and hydrochloride salt are given
below.
Chlordimeform Chlordimeform
base hydrochloride
Melting point 32°C 225-227°C
Vapour pressure (20°) 3.5 × 10-4 Torr 2.2 × 10-7 Torr
Solubility in water 250 ppm >50%
chloroform >20% 1-2%
hexane >20% 0.1%
Chlordimeform base is applied as an emulsifiable solution while the
hydrochloride is used as a water-soluble powder.
Technical chlordimeform hydrochloride has a purity of a least 96%. The
major impurities are 2, methyl-4-chlorformamidine,
4-chloro-o-toluidine-hydrochloride and sodium chloride.
Chlordimeform is rather stable in strong acids. It is readily
hydrolyzed, however, in weakly-acid to weakly alkaline solutions. Its
half-life in water containing 5% of methanol was determined to be 42
hours at pH 7 (30°C) and five hours at pH 9 (30°C) respectively.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Table I shows the potential metabolites for chlordimeform.
Four 120 g male rats treated orally with 270 µg of phenyl
H3-labelled (tritiated) chlordimeform secreted 19.74 to 23.03% of
the dose in bile over a 24-hour period. Groups of four 130 g female
rats similarly treated eliminated 52.8% (range 41.8-59.6%) and 2.5%
(range 0.13-5.30%) in urine and faeces respectively in 24 hours. A
pair of rats observed for 14 hours eliminated 66.2, and 64.9%
administered H3 label in urine, and 11.4 and 14.9% administered H3
label in faeces. Twenty-four hour elimination following intravenous
injection of 270 µg phenyl H3-labelled chlordimeform in rat
comprised 53.7% (range 52.0-55.6%) and 1.42% (range 1.19-1.84%)
administered H3, in urine and faeces respectively (Gerhards and
Kolb, 1966).
The urine from a male rat collected over 72 hours subsequent to oral
administration of 1.1 mg H3-labelled chlordimeform contained 49% of
the administered H3 label. Free extractables comprised 22% of the
H3 label, 10% was in the water phase and 17% was reactive to form
extractable glucuronides. The free extractable H3 label comprised
chlordimeform, 4-chloro-o-toluidine (IV),
N-formyl-4-chloro-o-toluidine (III),
N'-(4-chloro-o-tolyl)-N-methyl-formamidine (II). Glucuronides were
based on the same compounds found as free extractables. The author
notes that identified compounds may be formed after secretion of the
urine (Gerhards, 1967).
Single oral dosing of groups of three 120 g male rats with 270 µg
phenyl H3-labelled chlordimeform resulted in residues of
administered H3 label (expressed as percentage of dose/g of tissue)
in liver (0.29%), kidney (0.22%) and lymph nodes (0.13%) after eight
hours. After 24 hours, 0.35% and 0.13% of administered H3 were
present per g of tissue in gastrointestinal tract (and content) and
liver respectively. All other tissues contained less than 0.1% H3
label/g at eight hours, and less than 0.06% H3 label at 24 hours.
Twenty-four hour urine and faeces contained 57.5 and 3.9% of the total
administered H3 label respectively compared with 46.4% and 4.3% from
rats sacrificed at eight hours (Gerhards and Kolb, 1966).
Three 120 g male rats were intubated with 270 µg phenyl H3-labelled
chlordimeform for seven consecutive days. Fifty-nine per cent. and 10%
of the administered H3 label were voided in urine and faeces
respectively during the dosing period. Tissue residues at the
termination of dosing were less than 0.03% of the administered H3
label (Gerhards and Kolb, 1966).
TABLE I. POTENTIAL TRANSFORMATION PRODUCTS OF CHLORDIMEFORM
Other information on identity and properties
Chlordimeform is used as a free base or as its hydrochloride salt.
Physical properties of both base and hydrochloride salt are given
below.
Chlordimeform Chlordimeform
base hydrochloride
Melting point 32°C 225-227°C
Vapour pressure (20°) 3.5 × 10-4 Torr 2.2 × 10-7 Torr
Solubility in water 250 ppm >50%
chloroform >20% 1-2%
hexane >20% 0.1%
Chlordimeform base is applied as an emulsifiable solution while the
hydrochloride is used as a water-soluble powder.
Technical chlordimeform hydrochloride has a purity of a least 96%. The
major impurities are 2, methyl-4-chlorformamidine,
4-chloro-o-toluidine-hydrochloride and sodium chloride.
Chlordimeform is rather stable in strong acids. It is readily
hydrolyzed, however, in weakly-acid to weakly alkaline solutions. Its
half-life in water containing 5% of methanol was determined to be 42
hours at pH 7 (30°C) and five hours at pH 9 (30°C) respectively.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Table I shows the potential metabolites for chlordimeform.
Four 120 g male rats treated orally with 270 µg of phenyl
H3-labelled (tritiated) chlordimeform secreted 19.74 to 23.03% of
the dose in bile over a 24-hour period. Groups of four 130 g female
rats similarly treated eliminated 52.8% (range 41.8-59.6%) and 2.5%
(range 0.13-5.30%) in urine and faeces respectively in 24 hours. A
pair of rats observed for 14 hours eliminated 66.2, and 64.9%
administered H3 label in urine, and 11.4 and 14.9% administered H3
label in faeces. Twenty-four hour elimination following intravenous
injection of 270 µg phenyl H3-labelled chlordimeform in rat
comprised 53.7% (range 52.0-55.6%) and 1.42% (range 1.19-1.84%)
administered H3, in urine and faeces respectively (Gerhards and
Kolb, 1966).
The urine from a male rat collected over 72 hours subsequent to oral
administration of 1.1 mg H3-labelled chlordimeform contained 49% of
the administered H3 label. Free extractables comprised 22% of the
H3 label, 10% was in the water phase and 17% was reactive to form
extractable glucuronides. The free extractable H3 label comprised
chlordimeform, 4-chloro-o-toluidine (IV),
N-formyl-4-chloro-o-toluidine (III),
N'-(4-chloro-o-tolyl)-N-methyl-formamidine (II). Glucuronides were
based on the same compounds found as free extractables. The author
notes that identified compounds may be formed after secretion of the
urine (Gerhards, 1967).
Single oral dosing of groups of three 120 g male rats with 270 µg
phenyl H3-labelled chlordimeform resulted in residues of
administered H3 label (expressed as percentage of dose/g of tissue)
in liver (0.29%), kidney (0.22%) and lymph nodes (0.13%) after eight
hours. After 24 hours, 0.35% and 0.13% of administered H3 were
present per g of tissue in gastrointestinal tract (and content) and
liver respectively. All other tissues contained less than 0.1% H3
label/g at eight hours, and less than 0.06% H3 label at 24 hours.
Twenty-four hour urine and faeces contained 57.5 and 3.9% of the total
administered H3 label respectively compared with 46.4% and 4.3% from
rats sacrificed at eight hours (Gerhards and Kolb, 1966).
Three 120 g male rats were intubated with 270 µg phenyl H3-labelled
chlordimeform for seven consecutive days. Fifty-nine per cent. and 10%
of the administered H3 label were voided in urine and faeces
respectively during the dosing period. Tissue residues at the
termination of dosing were less than 0.03% of the administered H3
label (Gerhards and Kolb, 1966).
TABLE I. POTENTIAL TRANSFORMATION PRODUCTS OF CHLORDIMEFORM
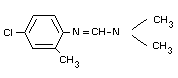 (I) N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine
(chlordimeform)
(I) N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine
(chlordimeform)
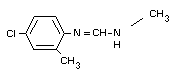 (II) N'-(4-chloro-o-tolyl)-N-methyl formamidine desmethyl
chlordimeform
(II) N'-(4-chloro-o-tolyl)-N-methyl formamidine desmethyl
chlordimeform
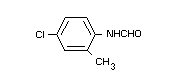 (III) N-formyl-4-chloro-o-toluidine
(III) N-formyl-4-chloro-o-toluidine
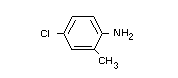 (IV) 4-chloro-o-toluidine
(IV) 4-chloro-o-toluidine
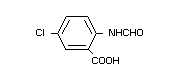 (V) N-formyl-5-chloroanthranilic acid
(V) N-formyl-5-chloroanthranilic acid
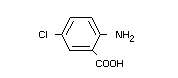 (VI) 5-chloroanthranilic acid
(VI) 5-chloroanthranilic acid
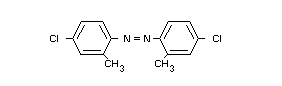 (VII) 2,2'-dimethyl-4,4'-dichloro-azobenzene
Pairs of male and female rats were treated orally with 3 µCi of
C14-toly, labelled chlordimeform. A similar group received C14
methyl labelled 4-chloro-o-toluidine (IV). Urine and faeces were
collected at 3, 12, 24, 48 and 72 hours after dosing. Urinary and
faecal elimination of C14 label after 72 hours comprised 88% and
7.5% of the administered dose of chlordimeform-C14, and 71% and
24.5% of the administered 4-chloro-o-toluidine-C14. Chloroform
extraction removed 30% of the radioactivity from the urine of
chlordimeform-C14 treated rats, the extract containing
chlordimeform, N'-(4-chloro-o-tolyl)-N-methylformamidine (II),
N-formyl-4-chloro-o-toluidine (III), and 4-chloro-o-toluidine
(IV), in addition to three unidentified metabolites. A considerable
amount of radioactivity remained at the point of origin of the
chromatograph, the amount remaining increasing with time, (30% at
three hours and 75% at 72 hours). At three hours, the four identified
compounds were present in approximately equal amounts. By 12 hours the
level of N'-(4-chloro-o-tolyl)-N-methylformamidine (II) had
decreased to approximately 25% of the level of any of the other three
compounds. By 48 hours, chlordimeform levels were half those of the
other two compounds, and by 72 hours, N-formyl-4-chloro-o-toluidine
(III) was present in the greatest proportion. At sacrifice (72 hours),
tissue levels based upon C14 levels were 0.21 ppm in liver, 0.15 ppm
in muscle, 0.11 ppm in fat and less than 0.1 ppm in other tissues.
Metabolites in ethyl acetate-extracted urine from rats given
C14-labelled 4-chloro-o-toluidine (IV) comprised
5-chloroanthranilic acid (VI) and N-formyl-5-chloroanthranilic acid
(V). The proportion of unmetabolized 4-chloro-o-toluidine (IV)
decreased with time whilst that of N-formyl-5-chloroanthranilic acid
(V) increased. The level of 5-chloroanthranilic (VI) acid remained
constant. A large amount (20-50%) of the radioactivity remained at the
origin of the chromatograph. Five unidentified compounds were noted.
Tissue levels based upon C14 levels at 72 hours after dosing were
0.33 ppm in fat. 0,26 ppm in liver, 0.2 ppm in kidney and oviduct,
0.1 ppm in brain, and less than 0.1 ppm in other tissues (Knowles and
Sen Gupta, 1970).
Two female dogs (18 and 20 kg) were given a single oral dose of 10 µCi
chlordimeform C14, and a single male dog (12 kg) which had undergone
cannulation of the gall-bladder and ligation of the bile duct was
given 20 µCi chlordimeform C14 orally. Urine was collected (by
catheterization) at 1, 3, 6, 12, 24, 48 and 72 hours. Faeces were
collected at similar time intervals. Of the administered C14 85% was
recovered in urine, 0.6% in faeces, and 5% in the bile by 72 hours.
Chloroform extraction of the urine removed 10% of the radioactivity.
Thin-layer chromatography of the extract revealed chlordimeform,
N'-(4-chloro-o-tolyl)-N-methylformamidine (II) and
4-chloro-o-toluidine (IV) in about equal quantities, but about four
times as much N-formyl-4-chloro-o-toluidine (III) at one hour after
treatment. The level of unchanged chlordimeform and
N'-(4-chloro-o-tolyl)-N-methylformamidine (II) decreased steadily
with time, whereas 4-chloro-o-toluidine (IV) and
N-formyl-4-chloro-o-toluidine (III) rose to maximum levels between
six and 12 hours prior to tapering off. Three unidentified metabolites
were present. In addition a lot of the radioactivity remained at the
origin of the chromatograph. Re-runs of this material in polar
solvents showed 5-chloroanthranilic acid (VI),
N-formyl-5-chloroanthranilic acid (V) and three unidentified compounds
were present. Some radioactivity still remained at the origin. The
urinary C14 not extracted by chloroform was treated with enzymes
(ß-glucuronidase, ß-glucuronidase-aryl sulfatase) to form "aglycones".
About 75% of the remaining C14 was extracted in this manner
(hydrochloric acid released 62%), and thin-layer chromatography showed
the same compounds as found in the chloroform extract, the major
metabolite being N-formyl-4-chloro-o-toluidine (III). In addition,
more of one of the unidentified metabolites was present. Again
re-chromatography of the 45% radioactivity remaining at the origin
with more polar solvents revealed 5-chloroanthranilic acid (VI) to be
the major product. In the bile, peak concentration of radioactivity
occurred at eight hours. About 10% of this activity could be
partitioned into ether, and thin-layer chromatography of the extract
indicated the same four compounds seen in urine chloroform extract.
N'-(4-chloro-o-tolyl)-N-methyl-formamidine (II),
N-formyl-4-chloro-o-toluidine (III) and an unidentified compound
accounted for most of the activity at two hours. By six hours, 75% of
the activity was due to N-formyl-4-chloro-o-toluidine (III).
Incubation of extracted bile with enzyme or acid gave the same
"aglycone" compounds as found in urine. Tissue residues of C14 at 72
hours ranged from 72 ppb in liver through kidney (30 ppb), lung (13.5
ppb), spleen and brain (11.9 ppb), heart and fat to pancreas at 5 ppb
(Sen Gupta and Knowles, 1970).
Incubation of chlordimeform (H3 labelled) for 120 minutes with rat
liver homogenate resulted in 24% unchanged chlordimeform, 45%
4-chloro-o-toluidine, and 11% unidentified metabolites being formed.
Rabbit liver homogenate yielded 53%, 40% and 7% respectively (Gerhards
& Kolb, 1966). The rat liver homogenate studies were confirmed using
C14 labelled chlordimeform. In addition, chlordimeform degradation
was shown to require the presence of nicotinamide. Three unidentified
metabolites were also observed. No azo-derivatives were detected.
Spleen homogenates were inactive with regard to chlordimeform
degradation (Rose, 1969a).
Further in vitro studies have shown that plant peroxidases can
result in the production of symmetrical azo-derivatives from
4-chloro-o-toluidine. Animal catalases do not result in formation of
azo-derivatives (see below) (Rose, 1969b).
Incubation of 60 µg H3-labelled chlordimeform (30 µCi) with 5 ml
human plasma yielded N-formyl-4-chloro-o-toluidine only. Conversion
was 25% in five hours, and 50% in 20 hours (Gerhards and Kolb, 1966).
A number of experiments were conducted by Ciba Ltd (1969c, d) to
verify the presence or absence of azobenzene formation from
chlordimeform or 4-chloro-o-toluidine in mammalian tissues. In the
first series it was demonstrated that peroxidase activity was
negligible in rat liver and spleen. Furthermore, catalase, which was
abundant in the same tissues, and which, like peroxidase, catalyzes
reactions between hydroxyperoxides and many oxidizable compounds, was
shown to be unable to form symmetrical azo-derivatives from
4-chloro-o-toluidine. In the second series of experiments it was
demonstrated that rat liver and spleen homogenates, which were
fortified with nicotinamide, and which degraded chlordimeform to
N-desmethyl-chlordimeform (II) and small quantities of
N-formyl-4-chloro-o-toluidine (III) and 4-chloro-o-toluidine (IV)
respectively, did not form any azobenzene derivatives. These compounds
therefore do not represent metabolites of chlordimeform or its
aromatic amine degradation products in animal tissues.
Chlordimeform degradation has been shown to proceed according to the
following pathways (Knowles, 1970):
(VII) 2,2'-dimethyl-4,4'-dichloro-azobenzene
Pairs of male and female rats were treated orally with 3 µCi of
C14-toly, labelled chlordimeform. A similar group received C14
methyl labelled 4-chloro-o-toluidine (IV). Urine and faeces were
collected at 3, 12, 24, 48 and 72 hours after dosing. Urinary and
faecal elimination of C14 label after 72 hours comprised 88% and
7.5% of the administered dose of chlordimeform-C14, and 71% and
24.5% of the administered 4-chloro-o-toluidine-C14. Chloroform
extraction removed 30% of the radioactivity from the urine of
chlordimeform-C14 treated rats, the extract containing
chlordimeform, N'-(4-chloro-o-tolyl)-N-methylformamidine (II),
N-formyl-4-chloro-o-toluidine (III), and 4-chloro-o-toluidine
(IV), in addition to three unidentified metabolites. A considerable
amount of radioactivity remained at the point of origin of the
chromatograph, the amount remaining increasing with time, (30% at
three hours and 75% at 72 hours). At three hours, the four identified
compounds were present in approximately equal amounts. By 12 hours the
level of N'-(4-chloro-o-tolyl)-N-methylformamidine (II) had
decreased to approximately 25% of the level of any of the other three
compounds. By 48 hours, chlordimeform levels were half those of the
other two compounds, and by 72 hours, N-formyl-4-chloro-o-toluidine
(III) was present in the greatest proportion. At sacrifice (72 hours),
tissue levels based upon C14 levels were 0.21 ppm in liver, 0.15 ppm
in muscle, 0.11 ppm in fat and less than 0.1 ppm in other tissues.
Metabolites in ethyl acetate-extracted urine from rats given
C14-labelled 4-chloro-o-toluidine (IV) comprised
5-chloroanthranilic acid (VI) and N-formyl-5-chloroanthranilic acid
(V). The proportion of unmetabolized 4-chloro-o-toluidine (IV)
decreased with time whilst that of N-formyl-5-chloroanthranilic acid
(V) increased. The level of 5-chloroanthranilic (VI) acid remained
constant. A large amount (20-50%) of the radioactivity remained at the
origin of the chromatograph. Five unidentified compounds were noted.
Tissue levels based upon C14 levels at 72 hours after dosing were
0.33 ppm in fat. 0,26 ppm in liver, 0.2 ppm in kidney and oviduct,
0.1 ppm in brain, and less than 0.1 ppm in other tissues (Knowles and
Sen Gupta, 1970).
Two female dogs (18 and 20 kg) were given a single oral dose of 10 µCi
chlordimeform C14, and a single male dog (12 kg) which had undergone
cannulation of the gall-bladder and ligation of the bile duct was
given 20 µCi chlordimeform C14 orally. Urine was collected (by
catheterization) at 1, 3, 6, 12, 24, 48 and 72 hours. Faeces were
collected at similar time intervals. Of the administered C14 85% was
recovered in urine, 0.6% in faeces, and 5% in the bile by 72 hours.
Chloroform extraction of the urine removed 10% of the radioactivity.
Thin-layer chromatography of the extract revealed chlordimeform,
N'-(4-chloro-o-tolyl)-N-methylformamidine (II) and
4-chloro-o-toluidine (IV) in about equal quantities, but about four
times as much N-formyl-4-chloro-o-toluidine (III) at one hour after
treatment. The level of unchanged chlordimeform and
N'-(4-chloro-o-tolyl)-N-methylformamidine (II) decreased steadily
with time, whereas 4-chloro-o-toluidine (IV) and
N-formyl-4-chloro-o-toluidine (III) rose to maximum levels between
six and 12 hours prior to tapering off. Three unidentified metabolites
were present. In addition a lot of the radioactivity remained at the
origin of the chromatograph. Re-runs of this material in polar
solvents showed 5-chloroanthranilic acid (VI),
N-formyl-5-chloroanthranilic acid (V) and three unidentified compounds
were present. Some radioactivity still remained at the origin. The
urinary C14 not extracted by chloroform was treated with enzymes
(ß-glucuronidase, ß-glucuronidase-aryl sulfatase) to form "aglycones".
About 75% of the remaining C14 was extracted in this manner
(hydrochloric acid released 62%), and thin-layer chromatography showed
the same compounds as found in the chloroform extract, the major
metabolite being N-formyl-4-chloro-o-toluidine (III). In addition,
more of one of the unidentified metabolites was present. Again
re-chromatography of the 45% radioactivity remaining at the origin
with more polar solvents revealed 5-chloroanthranilic acid (VI) to be
the major product. In the bile, peak concentration of radioactivity
occurred at eight hours. About 10% of this activity could be
partitioned into ether, and thin-layer chromatography of the extract
indicated the same four compounds seen in urine chloroform extract.
N'-(4-chloro-o-tolyl)-N-methyl-formamidine (II),
N-formyl-4-chloro-o-toluidine (III) and an unidentified compound
accounted for most of the activity at two hours. By six hours, 75% of
the activity was due to N-formyl-4-chloro-o-toluidine (III).
Incubation of extracted bile with enzyme or acid gave the same
"aglycone" compounds as found in urine. Tissue residues of C14 at 72
hours ranged from 72 ppb in liver through kidney (30 ppb), lung (13.5
ppb), spleen and brain (11.9 ppb), heart and fat to pancreas at 5 ppb
(Sen Gupta and Knowles, 1970).
Incubation of chlordimeform (H3 labelled) for 120 minutes with rat
liver homogenate resulted in 24% unchanged chlordimeform, 45%
4-chloro-o-toluidine, and 11% unidentified metabolites being formed.
Rabbit liver homogenate yielded 53%, 40% and 7% respectively (Gerhards
& Kolb, 1966). The rat liver homogenate studies were confirmed using
C14 labelled chlordimeform. In addition, chlordimeform degradation
was shown to require the presence of nicotinamide. Three unidentified
metabolites were also observed. No azo-derivatives were detected.
Spleen homogenates were inactive with regard to chlordimeform
degradation (Rose, 1969a).
Further in vitro studies have shown that plant peroxidases can
result in the production of symmetrical azo-derivatives from
4-chloro-o-toluidine. Animal catalases do not result in formation of
azo-derivatives (see below) (Rose, 1969b).
Incubation of 60 µg H3-labelled chlordimeform (30 µCi) with 5 ml
human plasma yielded N-formyl-4-chloro-o-toluidine only. Conversion
was 25% in five hours, and 50% in 20 hours (Gerhards and Kolb, 1966).
A number of experiments were conducted by Ciba Ltd (1969c, d) to
verify the presence or absence of azobenzene formation from
chlordimeform or 4-chloro-o-toluidine in mammalian tissues. In the
first series it was demonstrated that peroxidase activity was
negligible in rat liver and spleen. Furthermore, catalase, which was
abundant in the same tissues, and which, like peroxidase, catalyzes
reactions between hydroxyperoxides and many oxidizable compounds, was
shown to be unable to form symmetrical azo-derivatives from
4-chloro-o-toluidine. In the second series of experiments it was
demonstrated that rat liver and spleen homogenates, which were
fortified with nicotinamide, and which degraded chlordimeform to
N-desmethyl-chlordimeform (II) and small quantities of
N-formyl-4-chloro-o-toluidine (III) and 4-chloro-o-toluidine (IV)
respectively, did not form any azobenzene derivatives. These compounds
therefore do not represent metabolites of chlordimeform or its
aromatic amine degradation products in animal tissues.
Chlordimeform degradation has been shown to proceed according to the
following pathways (Knowles, 1970):
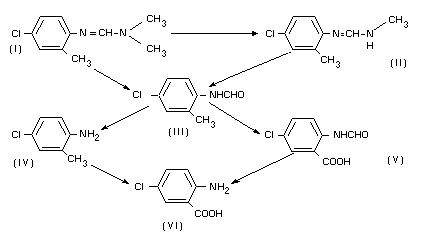 See also "Fate of Residues. In Animals".
TOXICOLOGICAL STUDIES
Special Studies
(a) Pharmacological studies
Chlordimeform hydrochloride was administered at graded doses of 0.01,
0.1, 1.0, 10 and 100 mg to the isolated perfused rabbit heart, the
organ being challenged with norepinephrine before and after dosing.
Contractile force was substantially decreased by 1.0 mg. Effect on
coronary flow and cardiac rate was less marked. Higher doses
temporarily stopped heart contractions. Guinea-pig isolated ileum was
exposed to graded doses of 1.0, 3.2, 10, 32, 100 and 320 µg/ml of
chlordimeform hydrochloride and evaluated for its effect on
acetylcholine, serotonin, histamine and barium induced contractions.
Bath concentrations of 3.2 µg/ml inhibited histamine contractions by
about 50% concentrations of 320 µg/ml were required to induce a
similar effect on acetylcholine, serotonin, and barium induced
contractions. In the intact dog, effect of graded doses of 1, 3, 10,
30 or 100 mg/kg chlordimeform hydrochloride on blood pressure, cardiac
rate, respiration, vasomotor response to epinephrine, acetylcholine,
histamine, tyramine, DMPP, carotid occlusion and peripheral vagal
stimulation were evaluated. Chlordimeform hydrochloride exerted a
hypotensive effect and caused increased respiration. A dose of 100
mg/kg was lethal. Vasomotor response to tyramine was enhanced, whereas
the pressor response to carotid occlusion was blocked (Teeters and
Blackmore, 1968).
(b) Reproduction studies
Rat. Four groups of 10 male and 20 female rats were fed 0, 100, 250
and 500 ppm chlordimeform in corn oil via the diet during three
parental, and three two-litter filial generations. Parental
body-weight prior to mating tended to be reduced in all test groups,
especially at 500 ppm. The same tendency was apparent with regard to
food consumption. Fertility index, gestation index, live birth index,
sex ratio, mean litter size, and birth weight of pups were comparable
to controls in all generations. At 500 ppm, lactation index was
reduced in F1a, F1b and F3a litters. Weaning weight of offspring was
depressed in all 500 ppm litters. Gross pathological examinations on
parents and pups dying during the study, and on 10 male and 10 female
weanlings of the F3b generation revealed no compound related effects
(Blackmore, 1969a).
Rabbit. Three groups of 10 impregnated female New Zealand white
rabbits (the day of impregnation being considered as day 0 of
gestation) were intubated on days 8 through 16 of gestation with 0,
7.5 or 30 mg chlordimeform/kg/day. Five rabbits per group were
sacrificed on day 28 of gestation. Parental mortality, abortion rate,
corpora lutea to implantation ratio, litter size, incidence of
resorptions, stillbirths, foetal weight, foetal length, and incidence
of skeletal, and tissue abnormalities were unaffected by the test
compound. In rabbits littering normally, gestation length, litter size
and litter weights were normal (Blackmore, 1969b).
(c) Studies on metabolites
Oral LD50 determinations in male and female rats.
Duration LD50
of (mg/kg)
Compound observation (days) References
N'-(4-chloro-o-tolyl)-N-methylformamidine 14 150 Sachsse and Bathe, 1971a
N-formyl-4-chloro-o-toluidine 14 2900 " " " 1970a
4-chloro-o-toluidine
(base) 7 ca1000 " " " 1971c
4-chloro-o-toluidine -HCl 14 860 " " " 1970d
Phenamidine (base) 7 ca1500 " " " 1971d
Phenamidine - HCl 14 860 " " " 1970f
o-chlordimeform (base) 7 300-400 " " " 1971f
o-chlordimeform - HCl 14 540 " " " 1970h
Dichlordimeform (base) 7 ca900 " " " 1971h
Dichlordimeform - HCl 14 260 " " " 1971j
Dermal LD50 determinations in male and female rats (24 hours occluded exposure).
Duration LD50
of (mg/kg)
Compound observation (days) References
N'-(4-chloro-o-tolyl)-N-methylformamidine 14 >2150 Sachsse and Bathe,1971b
N-formyl-4-chloro-o-toluidine 14 <2150 " " " 1970b
4-chloro-o-toluidine
(base) 7 ca1800 " " " 1971c
4-chloro-o-toluidine -HCl 14 <2150 " " " 1970e
Phenamidine (base) 7 ca1800 " " " 1971e
Phenamidine - HCl 14 <2150 " " " 1970g
o-chlordimeform (base) 7 ca300 " " " 1971g
o-chlordimeform - HCl 14 800 " " " 1970i
Dichlordimeform (base) 7 ca950 " " " 1971i
Dichlordimeform - HCl 14 <2150 " " " 1970k
Acute toxicity
ACUTE TOXICITY OF CHLORDIMEFORM BASE
LD50
Animal Route Sex (mg/kg) References
Mouse i.p. Mixed 110 Sachsse and Ullman, 1970
Rat Oral Male 178 Weir, 1968
Rat Oral Female 460 Weir, 1968
Rat Oral Male 220 Mastri et al., 1969
Rat Oral Female 170 Mastri et al., 1969
Dog Oral Female ca100 Weir, 1967
Dog Oral Male ca150 Hurni and Sachsse, 1969a
Dog Oral Female ca400 Hurni and Sachsse, 1969a
Rat Dermal Mixed 640 Sachsse and Bathe, 19701
Rat Inhalation* Mixed 17 400 Sachsse and Ullman, 1971
mg/m3
* Inhalation exposure was for one hour.
Hypoactivity, dyspnoea, muscular weakness, tremours, straub tail,
spasms and convulsions preceded death following oral administration.
Dyspnoea, exophthalmus, prostration, spasms and convulsions preceded
death following dermal application. No local skin irritation occurred.
No pathological changes were noted in rat following oral treatment,
although pale or blotchy livers, pale kidneys, and haemorrhagic
intestinal contents were observed after dermal treatment. In the dog,
oral administration resulted in congestion of liver, kidneys, and
lungs (the lungs also being oedomatous and haemorrhagic).
ACUTE TOXICITY OF CHLORDIMEFORM HYDROCHLORIDE
LD50
Animal Route Sex (mg/kg) References
Mouse Oral Mixed 220 Gunzel and Richter, 1967
Rat i.v. Male 95 Tripod, 1967
Rat s.c. Male 130 Tripod, 1967
Rat Oral Mixed 265 Gunzel and Richter, 1965
Rat Oral Mixed 355 Gunzel and Richter, 1965
Rat Oral Male 305 Tripod, 1967
Rat Oral Male 325 Mastri et al., 1969
Rat Oral Female 330 Mastri et al., 1969
Rat Dermal ca4000 Gunzel and Richter, 1966a
Rabbit Dermal >4000 Gunzel and Richter, 1966b
Rat Inhalation* >5.8 Sachsse and Ullman, undated
g/m3
* Inhalation exposure was for one hour.
Symptoms were similar to those for the base.
ACUTE TOXICITY OF CHLORDIMEFORM FORMULATIONS
LD50
Animal Route Sex Formulation (mg/kg) References
Mouse Oral Male EC 50 320 Aohi and Meda, 1966
Mouse Oral Female 50 s.p. 752 Shionogi C, undated
Rat Oral Mixed EC 50 610 Hurni and Sachsse, 1969b
Rat Dermal Mixed EC 50 2100 Sachsse and Bathe, 1971j
Rat Dermal Mixed 50 s.p. >3000 Hurni and Sachsse, 1969c
Rat Oral Mixed 50 s.p. 1100 Gunzel and Richter, 1969
Dog Oral Mixed 50 s.p. 400 Gunzel and Richter, 1968
A suicide victim ingested 30 ml of Galecron(R) 50 formulation. Upon
admission to hospital, an unknown time after drinking chlordimeform
solution, the patient was comatosed; and respiration and heartbeat had
ceased. The latter was restored by massage and adrenaline injection. A
respirator was used but death occurred within 24 hours. No autopsy was
performed (Oda, 1969).
Short-term studies
Rat. Four groups of 10 male and 10 female rats were intubated six
times weekly for one month with 0.5 ml/100 g body-weight of 2% CMC,
containing chlordimeform base at concentrations such as to give dose
levels of 0, 25, 50 or 100 mg/kg/dose. Body-weight was markedly
reduced in both sexes at 100 mg/kg/dose. Hyperexcitability was
observed in all test animals. At 100 mg/kg this was apparent 20-30
minutes after dosing, and was followed two to three hours after dosing
by decreased activity and apathy. Recovery was complete at four hours.
Similar effects were observed at 50, and 25 mg/kg/dose, but on a dose
related decreased scale, and with inconsistent frequency (Surber and
Cerioli, 1966).
Dog. Four groups of beagle dogs were fed 0 (10 male and 10 female)
250 (eight male and eight female), 500 (eight male and eight female)
or 1000 (10 male and 10 female) ppm of chlordimeform in a dry diet for
two years. Two male and two female dogs were sacrificed from each
group at 26, and 52 weeks. Body-weight was reduced at 1000 ppm, the
effect being slightly more pronounced in the females, Total leucocyte
counts were sporadically elevated in both sexes at 1000 ppm and in
females at 500 ppm. Haematocrit, haemoglobin, and erythrocyte counts
tended to be depressed terminally in both sexes at 1000 ppm. Sporadic
slight decreases in serum albumin were observed, more frequently in
males, at 1000 ppm. Terminal spleen to body-weight ratio was elevated
in males at 500 and 1000 ppm, and in females at 1000 ppm. Kidney to
body-weight ratio was elevated in females at 1000 ppm.
Histopathological examinations revealed bile duct hyperplasia,
pericholangitis and nodular hepatocytic hyperplasia at 500 and 1000
ppm in both sexes, and nodular hepatocytic hypertrophy at 1000 ppm in
both sexes in the liver. Kidneys showed an increased amount of
pigmentation at 500 and 1000 ppm in both sexes (Blackmore, 1969c).
Long-term studies
Rat. Five groups of 35 randomized male and 35 randomized female rats
(except at 100 ppm where 34 males and 36 females were used) were fed
0, 100, 250, 500 or 1000 ppm in the diet. The 100 ppm group commenced
treatment seven weeks after the other groups. This group was
originally part of the control group. Animals at that time were of
similar weight to those which had already been on test. The 1000 ppm
group was discontinued at three months due to severe growth
inhibition. Growth inhibition was observed in the males at 500 and
1000 ppm. In the females weight gain was reduced at 250 ppm and above.
In addition, female body-weight was reduced at 100 ppm between weeks
20 and 48. (This reduction may possibly be due to reduced food intake
(Weatherholtz, 1970) although this may not be the complete explanation
(Lyon, 1970).) Food intake was significantly reduced at 500 and 1000
ppm in both sexes. Dose related decreases in haematocrit, haemoglobin,
and erythrocyte counts, and a dose related increase in the leucocyte
count occurred in females at 250 and 500 ppm up to one year. During
the second year, haematocrit only was consistently depressed in
females at 500 ppm. Histo-pathological changes in the liver (nodules,
and foci of hyperplasia of hepatocytes) occurred in all groups, but
the incidence was greater at 250 and 500 ppm, and was more severe at
500 ppm. Some females at 500 ppm showed slight hypertrophy and
vacuolation of focal groups of cells in the adrenal cortex. Terminal
organ to body-weight ratios were increased in the liver (females at
250 and 500 ppm and males at 100 and 250 ppm), kidney (females at 250
and 500 ppm), thyroid (females at 250 and 500 ppm), heart (males at
250 ppm and females at 500 ppm), adrenals (males at 100 and 250 ppm)
and testes (100 and 500 ppm) (Blackmore, 1969d).
Comments
Chlordimeform appears to be fairly rapidly excreted in animals and
does not appear to be stored in body tissue. Information on metabolism
in man would however be of interest. A long-term feeding study is
available in the rat and a two-year study in the dog from which a
no-effect level was established. The Meeting expressed particular
concern about effect on organ to body-weight ratios in the rat and on
the histopathology of the liver and bile ducts of both the rat and the
dog. In these species nodular changes were observed in the liver but
histopathological information is insufficient to determine their
toxicological significance. Also of some concern were effects on the
total leucocyte counts of the dog and the pharmacological effects on
the heart and circulation together with the fact that the compound
appears to potentiate the effect of tyramine.
Because of the concern expressed above only a temporary acceptable
daily intake was established for this compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat - 100 ppm in the diet equivalent to 5 mg/kg body-weight per day
Dog - 250 ppm in the diet equivalent to 6.25 mg/kg body-weight per day
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Treatment of plants
Chlordimeform is a broad spectrum acaricide/insecticide applied to the
foliage of fruit, vegetables and other field crops acting through
contact and feeding and through vapour action on all stages of insects
and mites, including eggs of mites.
Pre-harvest treatments
Chlordimeform products have been registered in many countries for use
on a wide variety of crops of which typical examples are given in
Table 1.
Post-harvest treatment
No post-harvest treatments with chlordimeform are known.
Treatment of animals
In Australia chlordimeform is registered for use in cattle dips for
the control of cattle ticks (Boophilus micropus), the application
rate being 0.01-0.02% solution applied each 21 days by dipping often
in combination with organo-phosphorus acaricides such as dioxathion,
ethion, coumaphos, Dursban, bromophos-ethyl. Experimental work
designed to evaluate chlordimeform for use alone at a concentration of
0.1% is at an advanced stage.
Residues resulting from supervised trials
Fruit and vegetable crops
Detailed residue data are available from supervised trials in many
countries on many important crops and reports have been deposited with
FAO. The residue results are remarkably consistent between different
trials and different countries.
The most important and consistent feature of the residue trial data is
that the residue level remains remarkably constant irrespective of the
pre-harvest interval. On apples, grapes, citrus, peaches, pears, plums
and tomatoes the residue levels from sprays applied 30 days
pre-harvest are usually no more than half the level found when similar
rates were applied one to two days prior to harvest.
This rather unusual pattern is partly due to the analytical method
which determines all 4-chloro-o-toluidine containing metabolites
and/or conjugates as well as the unchanged parent compound.
Table II gives a representative sample of the results of supervised
residue trials carried out using registered label recommendations, use
patterns and rates of application.
Ciba and Schering (1971) have reported more than 50 trials designed to
determine the proportion of metabolites and parent compound in the
residues remaining on fruits at various intervals after application.
The separation of the components in the residue was performed by
thin-layer chromatography and by gas-chromatography.
TABLE II. REGISTERED USE PATTERNS FOR CHLORDIMEFORM
Pre-harvest
Country Crop Rate interval
Argentina pome fruits 0.06-0.09% 28 days
Australia cattle 0.02% 1 day
cotton 0.1%
pome & stone fruits 0.1% 7 days
strawberries 0.05% 7 days
Austria fruit trees 0.05% -
Brazil horticulture 0.05%
Canada fruit trees 0.06% 14-28 days
cole crops 0.05% 28 days
TABLE II. (cont'd)
Pre-harvest
Country Crop Rate interval
Denmark fruit trees 0.05% -
France pome fruits 0.05% 15 days
grapes 0.05% 15 days
vegetables 0.05% 15 days
Germany fruit trees 0.05% -
hops, grapes 0.05% -
Greece horticulture 0.05% -
Israel deciduous fruit - -
Italy fruit trees 0.05% -
citrus, grapes 0.05% -
Japan apples 0.05-0.025% -
citrus 0.05-0.035% -
pears 0.05-0.035% -
Yugoslavia fruit trees 0.05-0.15% -
vegetables 0.05-0.15% -
New Zealand pome fruits 0.06% 14 days
peaches 0.06% 14 days
strawberries 0.06% 7 days
Peru cotton 1 kg/ha 15 days
South Africa citrus 0.05% 14 days
tomatoes 0.075% 30 days
Switzerland pome fruits 0.05% 6 weeks
grapes 0.05-0.075% 6 weeks
Turkey apples 0.05% -
citrus, cherries 0.05% -
cotton -
USA apples 4 kg/ha 14 days
citrus 4-6 kg/ha -
pears 4 kg/ha 28 days
Venezuela cotton, citrus 0.3-0.5 kg/ha -
horticulture 0.3-0.5 kg/ha -
TABLE III
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Apples USA 1967 0.06 3 s.p. 3.3 1.3 1.3 1.4
USA 1969 0.06 2 s.p. 8.3 1.9 1.4 1.2 0.6
USA 1969 0.12 2 s.p. 8.6 4.5 2.8 1.2 1.7
USA 1969 0.12 3 e.c. 2.9 2.0 1.0 1.2
Canada 1969 0.06 2 s.p. 1.6
Australia 1969 0.04 3 s.p. 1.2 1.8 2.5 0.5 0.9 0.8
New Zealand 1970 0.05 1 s.p. 1.5 1.0 0.7 0.6
New Zealand 1970 0.1 1 s.p. 2.2 1.2 0.9 0.8
South Africa 1969 0.05 1 s.p. 0.6 0.6 0.5 0.5
England 1967 0.08 1 e.c. 3.3 2.6 2.7 1.2 1.2
Germany 1966 0.06 1 s.p. 1.8 1.9 1.6 0.8 0.5
Germany 1969 0.034 1 s.p. 1.5 1.0 0.6 0.5
Germany 1969 0.07 1 s.p. 2.5 1.8 1.5 0.7
Switzerland 1965 0.05 1 s.p. 1.1 1.0 1.2 0.6 0.5 0.5
Switzerland 1967 0.05 1 e.c. 2.3 1.3 1.0 0.5
Cherries USA 1968 0.06 1 e.c. 1.9 0.5 0.3 0.2
USA 1968 0.12 1 e.c. 3.7 1.3 1.1 0.7
Grapes USA 1969 0.06 2 s.p. 1.6 0.8 0.5 0.5
USA 1969 0.12 2 s.o. 2.9 2.8 1.6 1.8
Germany 1970 0.034 2 s.p. 2.0 1.3 1.3 1.0
Germany 1970 0.034 2 s.p. 2.8 2.3 2.3 1.8
Italy 1968 0.034 1 s.p. 2.6 1.2 1.2 0.6
Italy 1968 0.045 1 s.p. 1.8 1.5 1.2 1.2
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Oranges
Pulp Italy 1967 0.1 1 w.p. 0.2 0.3 0.2 0.3
Peel Italy 1967 0.1 1 w.p. 3.8 3.6 2.3 2.2
Whole Italy 1967 0.1 1 w.p. 1.1 1.1 0.8 0.7
Pulp Italy 1968 0.05 1 s.p. 0.2 0.3 0.1 0.2 0.08 0.1
Peel Italy 1968 0.05 1 s.p. 4.6 3.8 1.7 3.7 2.2 2.3
Whole Italy 1968 0.05 1 s.p. 1.5 1.2 0.5 1.1 0.7 0.7
Pulp Spain 1968 0.08 1 s.p. 0.4 0.4 0.4
Peel Spain 1968 0.08 1 s.p. 12.1 10.9 6.0
Whole Spain 1968 0.08 1 s.p. 3.5 3.5 2.0
Peaches USA 1969 0.06 1 e.c. 6.6 5.0 4.4 2.3
USA 1969 0.06 1 s.p. 5.9 3.4 2.8 1.9
Australia 1970 0.04 1 s.p. 1.0 1.2 0.4 1.7 0.6 0.4
Australia 1970 0.08 1 s.p. 2.0 2.0 1.1 3.1 1.4 1.1
France 1969 0.05 1 s.p. 1.5 1.1 0.5 0.5
France 1969 0.1 1 s.p. 1.5 0.9 1.0
USA 1970 0.06 2 e.c. 7.5 6.6 4.1 3.3
USA 1970 0.06 2 s.p. 3.7 3.2 2.4 1.5
Pears USA 1969 0.06 2 s.p. 7.8 6.1 4.4 3.5 2.8
USA 1969 0.06 2 e.c. 6.4 5.5 4.1 2.8 1.6
Australia 1970 0.04 1 s.p. 1.5 1.1 0.7 1.7 0.6 0.7
Australia 1970 0.08 1 s.p. 3.0 2.4 1.6 2.2 1.6 1.3
Plums USA 1970 0.06 2 e.c. 1.7 1.3 1.9 1.7
USA 1970 0.06 2 s.p. 0.5 0.5 0.5 0.4
Germany 1969 0.07 1 s.p. 0.7 0.5 0.4 0.3
Germany 1969 0.07 1 s.p. 1.1 1.2 0.6 0.5
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Strawberries USA 1968 0.06 1 e.c. 5.7 4.0 3.6 2.6 0.04
USA 1968 0.12 2 s.p. 1.8
England 1967 0.1 1 e.c. 1.8
England 1967 0.1 1 s.p. 2.1
Tomangos
Whole South Africa 1970 0.2 1 s.p. 2.7 2.6 2.9
Pulp South Africa 1970 0.2 1 s.p. 1.0 0.9 1.3
Peel South Africa 1970 0.2 1 s.p. 7.0 6.5 7.1
Walnuts
Meat USA 1968 0.06 1 s.p. <0.05
Meat USA 1970 0.06 2 s.p. <0.05
Beans
Pod Germany 1969 0.034 1 s.p. 0.5 0.2 0.1
Pod Germany 1969 0.07 1 s.p. 0.5 0.1 0.1
Broccoli
Head trimmed USA 1970 0.05 5 s.p. 1.7 0.8 0.4 0.2
Head trimmed USA 1970 0.1 5 s.p. 4.4 1.2 0.7 0.2
Head trimmed Canada 1970 0.05 2 s.p. 2.4 0.8
Head trimmed Canada 1970 0.05 6 s.p. 3.7 1.7 1.3
Brussels sprouts USA 1969 0.06 9 e.c. 2.8 2.1 1.9
Canada 1970 0.05 6 s.p. 3.6 3.5 3.1
Canada 1970 0.05 8 s.p. 5.5 3.8 2.8
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Cabbage
Head trimmed USA 1968 0.2 6 e.c. 4.3 0.6 0.2
Head trimmed USA 1969 1 e.c. 0.2
Head trimmed USA 1969 11 s.p. 1.5 1.2 1.4
4 outer leaves Canada 1969 4 s.p. 0.5
Inner head Canada 1969 4 s.p. <0.05
Cauliflower
Head trimmed USA 1969 6 s.p. 2.6 2.6 0.5
Head trimmed USA 1970 4 s.p. 3.6 2.5 1.9
Head trimmed USA 1970 4 s.p. 14.9 8.4 3.7
Cotton
Seed USA 1970 0.5 9 e.c. 0.22
Lint cotton USA 1970 0.5 9 e.c. 0.37
Delinted
cotton-seed USA 1970 0.5 9 e.c. 0.19
Linters USA 1970 0.5 9 e.c. 0.47
Cottonseed hulls USA 1970 0.5 9 e.c. 0.20
Solvent extract
meal USA 1970 0.5 9 e.c. 0.15
Screwpress
extracted meal USA 1970 0.5 9 e.c. 0.09
Crude solvent
extracted oil USA 1970 0.5 9 e.c. 0.11
Refined solvent
extracted oil USA 1970 0.5 9 e.c. 0.09
Crude screwpress
oil USA 1970 0.5 9 e.c. 0.17
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Refined
screwpress oil USA 1970 0.5 9 e.c. 0.14
Seed USA 1970 0.2 6 e.c. 1.8 1.6 2.3
Seed USA 1970 6 s.p. 2.0 1.8 2.4
Oil crude USA 1968 10 s.p. 1.2
Oil refined USA 1968 10 s.p. 1.0
Oil crude USA 1968 10 s.p. 4.0
Oil refined USA 1968 10 s.p. 3.6
Oil crude USA 1970 9 e.c. 0.1
Oil refined USA 1970 9 e.c. 0.1
Sugar beet
Roots Germany 1968 1 s.p. <0.02
(60-120)
Roots Germany 1969 1 s.p. <0.02
<0.02
(60-120)
Tops Germany 1969 s.p. 0.1
<0.02
(60-120)
Tomatoes
Fruit Canada 1970 6 s.p. 0.6 0.2 0.2
Fruit USA 1970 7 e.c. 0.4 0.3 0.5 0.3
Table IV gives some typical examples of the results obtained in these
trials which involved commercial formulations applied at
concentrations normally used in practice.
TABLE IV
Fruit Location Interval Total Proportion of each
before residue metabolite%
harvest ppm
A B C D
Apples USA 30 0.92 87 <4 <4 <4
USA 90 0.42 55 <9 27 <9
Australia 2 1.03 75 <4 <15 <4
Australia 30 0.63 63 <10 <25 <10
Pears USA 30 1.78 79 <2 17 <2
USA 60 0.88 68 <3 23 <3
Italy 1 0.89 90 <3 <5 <3
Italy 21 0.62 80 <4 <8 8
Peaches Italy 1 2.32 95 <1 <5 <1
Italy 47 0.37 54 <5 35 <5
Prunes Switzerland 9 1.36 81 <1 15 <3
Switzerland 30 1.06 75 <2 20 <4
A = chlordimeform
B = N-(4-chloro-o-tolyl)-N-methylformamidine
C = N-formyl-4-chloro-o-toluidine
D = 4-chloro-o-toluidine
Where the sign < appears it is to be recognized that the limit of
detection, e.g. 0.02 or 0.04 ppm, means that the proportion may well
be much less than the figure shown.
A number of trials reported by Ciba and Schering (1971) show that
there is no significant difference in the level of chlordimeform
residues on the various fractions of cotton seed separated during
commercial processing. One series of results is shown in Table III
(see page 17). The fairly uniform distribution of residues between oil
and cotton seed meal may reflect the distribution of parent compound
and metabolite but possibly is also brought about by the analytical
procedure which does not distinguish metabolites from parent compound.
Animals
Ciba-Geigy (1971) report results of trials conducted in Australia to
determine the residue in cattle treated with chlordimeform to control
cattle tick. A calf weighing 200 kg was sprayed with 15 litres of
0.05% chlordimeform. The animal was slaughtered three days later and
residues of chlordimeform and metabolite hydrolysable to
4-chloro-o-toluidine were determined in tissues and fat with the
following results:
muscle 0.21 ppm omental fat 0.12 ppm
kidney 0.23 ppm subcutaneous fat 0.30 ppm
liver 0.62 ppm perirenal fat 0.21 ppm
In further trials cattle weighing 210 to 230 kg were dipped in 0.013%
chlordimeform and were slaughtered one or three days later. The
residues determined as above were as follows:
Interval
since Liver Fat Muscle Kidney
treatment
1 day 0.28±0.04 0.15±0.06 0.07±0.02 0.29±0.03
3 days 0.15±0.03 0.05±0.02 0.03±0.01 0.06±0.03
Buffering
As explained in some detail under "Fate of residues", chlordimeform
stability is extremely dependent on pH. Acceptable stability in cattle
dips can be achieved only if pH is held below 5.8 and preferably in
the range 5.0-5.5. In Australia this has been achieved by buffering
the dipping fluid with fertilizer grade superphosphate. Unbuffered
fluids must be used within a day of preparation.
At pH 5.2, some 99% of chlordimeform is present as the acid salt. In
this form it has adequate stability but its activity is reduced below
that of a similar concentration at a less acid pH value containing
proportionately more chlordimeform base.
Buffered dips leave only about half the hair deposits left by
unbuffered dips of equal concentration so that higher use levels are
required to produce the same acaricidal effect. Fortunately absorption
is also reduced and residue levels are not augmented by the increased
concentration required in dips.
Residues in milk and milk products
A Ciba-Geigy report (1971) describes milk and butter residues
following the treatment of lactating dairy cattle with 0.05%
chlordimeform, about 15 litres being applied to each animal. Three
animals were treated with a solution buffered to pH 5.6 and three with
an unbuffered solution of pH 9.4.
Time of 0.05% unbuffered 0.05% buffered
sampling Milk Butter Milk Butter
Pre-treatment 0.03 ppm 0.06 ppm <0.03 ppm 0.05 ppm
6 hours 0.22 ppm 1.52 ppm 0.06 ppm 0.52 ppm
24 hours 0.05 ppm 0.71 ppm 0.05 ppm 0.19 ppm
32 hours 0.08 ppm 0.18 ppm <0.03 ppm 0.14 ppm
48 hours 0.05 ppm 0.22 ppm <0.03 ppm 0.11 ppm
56 hours 0.16 ppm 0.10 ppm
72 hours 0.09 ppm 0.06 ppm
Each figure in the table is the mean of three individual samples.
The analytical technique employed determines the sum of chlordimeform
and all metabolites hydrolysable to 4 chloro-o-toluidine. The ratio
of milk residues to butter residues while somewhat variable, falls
consistently short of the level which would be expected if these
residues were partitioned exclusively in the fat phase.
The possibility that a proportion of the residues may be held in the
aqueous phase should be taken into consideration in the recommendation
of tolerances.
These results indicate the rapid rate of elimination which occurs
following spraying or dipping.
The level of residues is related to the concentration of the spray
solution or dip. Official trials in Australia showed that when the
concentration of the spray is increased from 0.03 to 0.3% the residue
levels in fat, kidney and liver of young calves increase in
approximately the same ratio. Residue levels in muscle do not
materially change in spite of the increase in concentrations. Residue
determinations were made 24 hours after spraying:
Adult cattle appear to metabolize the residue more rapidly because
animals weighing 450 kg dipped three times at 17-day intervals in a
bath containing 0.01% chlordimeform showed no residues in excess of
0.1 ppm when slaughtered three days after the third treatment. Adult
cattle weighing 300-520 kg sprayed with chlordimeform showed, as did
calves, that the residue level 24 hours after treatment increases
sharply with increasing concentration of spray. The residue level in
perirenal and omental fat increased from 0.1 ppm to 7.2 ppm when the
spray concentration was increased from 0.01 to 0.1%.
Concentration Liver Fat Muscle Kidney
of spray
0.03% <0.1 ppm 1.0 ppm <0.1 ppm 0.2 ppm
0.3% 1.2 ppm 10.0 ppm <0.1 ppm 0.5 ppm
Repeat treatments, as is normal in controlling cattle tick, does not
result in an accumulation of residues in animal tissues. Calves
receiving 10 dippings within eight weeks in a bath containing 0.01%
chlordimeform were slaughtered three days after the tenth treatment.
The results of residue analysis were as follows:
No. of Liver Fat Muscle
treatments
1 2.0 0.29 0.12
7 1.3 0.64 0.07
10 4.17 0.37 0.12
Fate of residues
General comments
Chlordimeform (I) is readily hydrolysed in weakly-acid to
weakly-alkaline solutions but is rather stable under strongly acid
conditions. In an aqueous buffer solution of pH 7.0 containing 5% of
methanol, the base showed a half-life of 42 hours at 30°C. At pH 9,
under otherwise equivalent conditions, a half-life of five hours was
observed (Kossmann et al., 1971). Hydrolysis of the parent compound
yields N-formyl-4-chloro-o-toluidine (III) and ultimately
4-chloro-o-toluidine (IV).
Structural alterations are observed when chlordimeform in solution or
on chromatoplates is exposed to irradiation by U.V. or natural
sunlight (Knowles and Sen Gupta, 1969). Although a number of
additional minor degradation products do occur,
N-formyl-4-chloro-o-toluidine (III) is the major transformation
product recovered.
A complete list of substances which appear to be potential metabolites
of chlordimeform is given in Table 1.
In animals
To supplement the studies described under "Biochemical aspects" on the
behaviour of chlordimeform in mammals, Ciba-Geigy Ltd (Ciba-Geigy,
1971) conducted experiments on the fate of the acaricide upon
continuous feeding to ruminants. Eight Brown Swiss cows were fed daily
rations containing from 4-24 ppm chlordimeform for periods of up to 42
days. Milk and tissue and organ samples were collected at regular
intervals and analysed by the total residue method (Geissbühler at
al., 1971) which accounts for all 4-chloro-o-toluidine containing
metabolites and/or conjugates of chlordimeform. At 21 days, residues
in liver were 0.09, and <O.03 ppm and in kidney were 0.38, and 0.07
ppm in animals fed 120 and 40 ppm respectively. Further cows fed 240
ppm in the diet showed liver levels of 0.45, 0.38, 0.50 and 0.44 ppm
at 7, 14, 21 and 42 days. Corresponding kidney residues were 0.05,
0.13, 0.13, and 0.09 ppm. Residues in milk, muscle and fat were below
0.03 ppm (Voss and Burkhard, 1971).
One male (36 kg) and one lactating female (39 kg) goat were given 10
µCi chlordimeform orally, urine and faeces being collected at 1, 3, 6,
12, 24, 48 and 72 hours post treatment. After 72 hours, the male had
voided 87%, and 1.8% of the administered radioactivity in the urine
and faeces respectively, and the female 68% and 1.8%. Only 0.3% of the
radioactivity appeared in the milk.
Chloroform extractable compounds identified by thin-layer
chromatography comprised chlordimeform,
N'-(4-chloro-o-tolyl)-N-methylformamidine, 4-chloro-o-toluidine,
and N-formyl-4-chloro-o-toluidine, the latter predominating. Of the
radioactivity 85-97% remained at the point of origin.
Re-chromatography with polar solvents yielded 5-chloroanthranilic
acid, and N-formyl-5-chloroanthranilic acid.
The 90% unextractable radioactivity, when incubated with
ß-glucuronidase, ß-glucuronidase-aryl sulphonase or hydrochloric acid
yielded chlordimeform, N'-14-chloro-o-tolyl-N-methylformamidine,
4-chloro-o-toluidine, N-formyl-4-chloro-o-toluidine and two
unidentified metabolites. N'-(4-chloro-o-tolyl)-N-ethylformamidine
was present in very small amounts, and the others in about equal
amounts. Re-chromatography of the 20% radioactivity which remained at
the origin yielded anthranilic acids, the 5-chloroanthranilic acid
predominating (Sen Gupta and Knowles, 1970).
In plants
A series of preliminary experiments carried out by Ciba (1965a, b) and
Schering (1966b) demonstrated that chlordimeform was quite rapidly
degraded in plant tissues with high inherent metabolic activity (i.e.
bean leaves) but was only slowly transformed in ripe fruits (Table V).
Green fruits, such as young grapes, and stems were observed to take an
intermediate position with regard to their ability to degrade the
acaricide.
TABLE V. RESIDUES OF CHLORDIMEFORM AND ITS DEGRADATION PRODUCTS IN AUSTRALIAN APPLES AFTER SPRAY APPLICATION OF THE
ACARICIDE (SPRAYING CONCENTRATION 0.05% a.i.) FROM CIBA (1965b)
Day Chlordimeform N-formyl-4-chloro-o-toluidine Desmethyl 4-chloro-o-toluidine
after ppm ppm chlordimeform ppm
spraying ppm
Apples
0 0.2 0.15 - -
1 0.6 <0.15 - -
2 0.8 <0.15 <0.04 <0.04
6 0.3 <0.15 - -
14 0.2 <0.05 <0.04 <0.04
21 0.4 <0.05 - -
30 0.4 <0.05 <0.04 <0,04
42 <0.05 <0.05 - -
Pears
0 0.50 0.25 - -
1 0.75 0.20 - -
2 0.45 0.10 <0.04 <0.04
6 0.75 0.10 - -
14 0.70 0.05 <0.04 <0.04
21 0.65 0.05 - -
30 0.60 <0.05 <0.04 <0.04
42 0.40 <0.05 - -
Tentative identification of the observed transformation products
indicated that in leaves both
N'-(4-chloro-o-tolyl)-N-methylformamidine
(desmethyl-chlorphenamidine, 11) and N-formyl-4-chloro-o-toluidine
(111) were prominent metabolites. On the other hand, in ripe apple and
pear fruits, only the formyl-derivative (III) was detected in
measurable quantities, whereas desmethyl-chlordimeform (II) was
normally absent (Table V). In all tissues analysed the free
4-chloro-o-toluidine (IV) was not detected at all or was present
only in small quantities. The lack of formation of significant residue
quantities of the free toluidine in edible plant parts, such as
fruits, was further examined by analysing plums which had been exposed
to a six times overdose treatment of chlordimeform (Ciba, 1967b). Even
after this application, which left a total residue of 4 to 6 ppm, the
amounts of free 4-chloro-o-toluidine were scarcely above the limit
of detection (0.05 ppm) of the colorimetric method applied.
Since chlordimeform was known to be quite volatile, the possibility
and extent of its evaporation from plant surfaces was investigated
(Ciba, 1968). Although the free base of the acaricide was observed to
readily evaporate from glass plates, disappearance from the surface of
bean leaves was considerably less pronounced. Losses by evaporation,
which occurred mainly during the first few hours after application,
were found to be of the order of 30 to 40% in terms of the original
dose applied. Evaporation was of the same order of magnitude when bean
leaves were treated with the hydro-chloride salt of chlordimeform.
This result suggested that, owing to the buffering capacity of plant
exudates on certain leaf surfaces, an equilibrium between free base
and salts was established, no matter which form of the acaricide was
applied. The behaviour of chlordimeform on leaf surfaces, as
described, was essentially confirmed by Sen Gupta and Knowles (1969)
when applying the acaricide to leaves of apple seedlings and by
Ehrhardt and Knowles (1970) when treating leaves of grapefruit
seedlings with both the free base and the hydrochloride salt.
Experiments carried out by Sen Gupta and Knowles (1969) on leaves of
apple seedlings and by Ehrhardt and Knowles (1970) on leaves of
grapefruit seedlings confirmed the limited ability of chlordimeform to
penetrate cuticular layers.
In the apple experiments (Sen Gupta and Knowles, 1969), 3H-as well
as 14C-labelled chlordimeform was applied to seedlings by either
leaf treatment or stem injection. The treated plants were cultured for
20 days and periodically analysed for the metabolites already
mentioned above. Sufficient quantities of the main transformation
products were collected to allow characterization by infra-red
analysis, melting point determinations, dye formation and
co-chromatography on thin-layer plates.
After both types of application dissipation/degradation of the
acaricide was observed to proceed at an intermediate rate, the
half-life of the compound being of the order of 12 to 16 days. Upon
termination of the experiment about 40% of the radioactivity applied
was still accounted for by the unchanged acaricide. Organosoluble
degradation products identified were desmethyl-chlordimeform (II),
N-formyl-4-chloro-o-toluidine (III) and free 4-chloro-o-toluidine.
The quantities of these metabolites relative to chlordimeform, however
were quite small and except for desmethyl-chlordimeform, never
exceeded 1% in terms of the radioactivity applied.
No so-called "non-extractable" radioactivity was evident after leaf
application of chlordimeform but was observed to be present in
increasing amounts (up to 30% of the quantity applied) after stem
injection of the acaricide. However, this non-extractable
radioactivity was confined to the stem section and could not be
observed in leaves to which part of the radioactivity had been
translocated. The authors suggested that non-extractable materials
represented chlordimeform degradation products that were complexed
with polymeric cell constituents.
The experiments with apple leaves also confirmed the limited ability
of chlordimeform to penetrate through cuticular plant tissues since
most of the radioactivity remaining on leaves could subsequently be
removed by rinsing them with organic solvents. No more and usually
less than 15% of the dose applied remained in the leaf tissues after
the rinsing process.
In the grapefruit experiments (Ehrhardt and Knowles, 1970) both the
free base and the hydrochloride salt of
14C-(tolyl-labelled)-chlordimeform were applied to the leaf surface
of growing seedlings. In these studies, dissipation of total
radioactivity was more pronounced than in the apple experiments, since
only 10 to 207 of the dose applied was recovered at the end of the
20-day observation period. It therefore appeared that a higher
percentage of chlordimeform evaporated from grapefruit than from apple
leaves. In addition, chlordimeform itself was degraded at a faster
rate by the citrus than by the apple tissue, since, after 20 days of
culturing, no more than 1% of the radioactivity applied was recovered
as the unchanged acaricide from the former tissue.
The pattern of metabolites on and in citrus leaves was essentially the
same as that reported for apple seedlings. Desmethyl-chlordimeform,
the formyl derivative and free 4-chloro-o-toluidine were the
principal metabolites, whose quantities, however, were small and never
exceeded 2% in terms of the radioactivity applied. Several unknown
minor radioactive metabolites were observed on thin-layer plates,
however, none of them represented more than 1% and normally did not
exceed 0.1% in terms of the radioactivity applied.
In several recent publications (Knowles et al., 1969a, b; Rosen et
al., 1970) which deal with in vitro chemical and biochemical model
systems, chlordimeform and its minor metabolite 4-chloro-o-toluidine
have been implicated or shown to be possible candidates for the
catalytic formation of azobenzene derivatives, such as
2,2'-dimethyl-4,4'-dichloroazobenzene (VII, Table I). Although a more
detailed discussion on the potential formation of azobenzene compounds
from chlordimeform is presented in the section on animal metabolism
("Biochemical aspects") results on the presence or absence of such
transformation products in plants are briefly discussed. In examining
field-treated fruits and vegetables for the presence of azobenzene
compounds, a sensitive gas-chromatographic residue method which
permitted the detection of 0.01 ppm of
2,2'-dimethyl-4,4'-dichloroazobenzene was used (Geissbühler et al.,
1971). This method was applied to apple fruits and leaves which had
been treated with a four times overdose of chlordimeform and which
were harvested 20, 30 and 40 days after application (Ciba, 1969b).
Although chlordimeform residues were found to be excessively high
during the whole observation period (10 to 4 ppm in fruits; 400 to 300
ppm in leaves), residues of the azobenzene compound were either not
detectable (<0.01 ppm in fruits) or were so small (0.04 ppm in
leaves) that they represented less than 0.02% in terms of the Parent
residue. Therefore, with normal concentrations of chlordimeform, no
detectable azobenzene residues are to be expected on edible crops.
From the extensive plant behaviour and metabolism data presented, the
pathways of chlordimeform transformation in plants are summarized as
follows (Sen Gupta and Knowles, 1969):
See also "Fate of Residues. In Animals".
TOXICOLOGICAL STUDIES
Special Studies
(a) Pharmacological studies
Chlordimeform hydrochloride was administered at graded doses of 0.01,
0.1, 1.0, 10 and 100 mg to the isolated perfused rabbit heart, the
organ being challenged with norepinephrine before and after dosing.
Contractile force was substantially decreased by 1.0 mg. Effect on
coronary flow and cardiac rate was less marked. Higher doses
temporarily stopped heart contractions. Guinea-pig isolated ileum was
exposed to graded doses of 1.0, 3.2, 10, 32, 100 and 320 µg/ml of
chlordimeform hydrochloride and evaluated for its effect on
acetylcholine, serotonin, histamine and barium induced contractions.
Bath concentrations of 3.2 µg/ml inhibited histamine contractions by
about 50% concentrations of 320 µg/ml were required to induce a
similar effect on acetylcholine, serotonin, and barium induced
contractions. In the intact dog, effect of graded doses of 1, 3, 10,
30 or 100 mg/kg chlordimeform hydrochloride on blood pressure, cardiac
rate, respiration, vasomotor response to epinephrine, acetylcholine,
histamine, tyramine, DMPP, carotid occlusion and peripheral vagal
stimulation were evaluated. Chlordimeform hydrochloride exerted a
hypotensive effect and caused increased respiration. A dose of 100
mg/kg was lethal. Vasomotor response to tyramine was enhanced, whereas
the pressor response to carotid occlusion was blocked (Teeters and
Blackmore, 1968).
(b) Reproduction studies
Rat. Four groups of 10 male and 20 female rats were fed 0, 100, 250
and 500 ppm chlordimeform in corn oil via the diet during three
parental, and three two-litter filial generations. Parental
body-weight prior to mating tended to be reduced in all test groups,
especially at 500 ppm. The same tendency was apparent with regard to
food consumption. Fertility index, gestation index, live birth index,
sex ratio, mean litter size, and birth weight of pups were comparable
to controls in all generations. At 500 ppm, lactation index was
reduced in F1a, F1b and F3a litters. Weaning weight of offspring was
depressed in all 500 ppm litters. Gross pathological examinations on
parents and pups dying during the study, and on 10 male and 10 female
weanlings of the F3b generation revealed no compound related effects
(Blackmore, 1969a).
Rabbit. Three groups of 10 impregnated female New Zealand white
rabbits (the day of impregnation being considered as day 0 of
gestation) were intubated on days 8 through 16 of gestation with 0,
7.5 or 30 mg chlordimeform/kg/day. Five rabbits per group were
sacrificed on day 28 of gestation. Parental mortality, abortion rate,
corpora lutea to implantation ratio, litter size, incidence of
resorptions, stillbirths, foetal weight, foetal length, and incidence
of skeletal, and tissue abnormalities were unaffected by the test
compound. In rabbits littering normally, gestation length, litter size
and litter weights were normal (Blackmore, 1969b).
(c) Studies on metabolites
Oral LD50 determinations in male and female rats.
Duration LD50
of (mg/kg)
Compound observation (days) References
N'-(4-chloro-o-tolyl)-N-methylformamidine 14 150 Sachsse and Bathe, 1971a
N-formyl-4-chloro-o-toluidine 14 2900 " " " 1970a
4-chloro-o-toluidine
(base) 7 ca1000 " " " 1971c
4-chloro-o-toluidine -HCl 14 860 " " " 1970d
Phenamidine (base) 7 ca1500 " " " 1971d
Phenamidine - HCl 14 860 " " " 1970f
o-chlordimeform (base) 7 300-400 " " " 1971f
o-chlordimeform - HCl 14 540 " " " 1970h
Dichlordimeform (base) 7 ca900 " " " 1971h
Dichlordimeform - HCl 14 260 " " " 1971j
Dermal LD50 determinations in male and female rats (24 hours occluded exposure).
Duration LD50
of (mg/kg)
Compound observation (days) References
N'-(4-chloro-o-tolyl)-N-methylformamidine 14 >2150 Sachsse and Bathe,1971b
N-formyl-4-chloro-o-toluidine 14 <2150 " " " 1970b
4-chloro-o-toluidine
(base) 7 ca1800 " " " 1971c
4-chloro-o-toluidine -HCl 14 <2150 " " " 1970e
Phenamidine (base) 7 ca1800 " " " 1971e
Phenamidine - HCl 14 <2150 " " " 1970g
o-chlordimeform (base) 7 ca300 " " " 1971g
o-chlordimeform - HCl 14 800 " " " 1970i
Dichlordimeform (base) 7 ca950 " " " 1971i
Dichlordimeform - HCl 14 <2150 " " " 1970k
Acute toxicity
ACUTE TOXICITY OF CHLORDIMEFORM BASE
LD50
Animal Route Sex (mg/kg) References
Mouse i.p. Mixed 110 Sachsse and Ullman, 1970
Rat Oral Male 178 Weir, 1968
Rat Oral Female 460 Weir, 1968
Rat Oral Male 220 Mastri et al., 1969
Rat Oral Female 170 Mastri et al., 1969
Dog Oral Female ca100 Weir, 1967
Dog Oral Male ca150 Hurni and Sachsse, 1969a
Dog Oral Female ca400 Hurni and Sachsse, 1969a
Rat Dermal Mixed 640 Sachsse and Bathe, 19701
Rat Inhalation* Mixed 17 400 Sachsse and Ullman, 1971
mg/m3
* Inhalation exposure was for one hour.
Hypoactivity, dyspnoea, muscular weakness, tremours, straub tail,
spasms and convulsions preceded death following oral administration.
Dyspnoea, exophthalmus, prostration, spasms and convulsions preceded
death following dermal application. No local skin irritation occurred.
No pathological changes were noted in rat following oral treatment,
although pale or blotchy livers, pale kidneys, and haemorrhagic
intestinal contents were observed after dermal treatment. In the dog,
oral administration resulted in congestion of liver, kidneys, and
lungs (the lungs also being oedomatous and haemorrhagic).
ACUTE TOXICITY OF CHLORDIMEFORM HYDROCHLORIDE
LD50
Animal Route Sex (mg/kg) References
Mouse Oral Mixed 220 Gunzel and Richter, 1967
Rat i.v. Male 95 Tripod, 1967
Rat s.c. Male 130 Tripod, 1967
Rat Oral Mixed 265 Gunzel and Richter, 1965
Rat Oral Mixed 355 Gunzel and Richter, 1965
Rat Oral Male 305 Tripod, 1967
Rat Oral Male 325 Mastri et al., 1969
Rat Oral Female 330 Mastri et al., 1969
Rat Dermal ca4000 Gunzel and Richter, 1966a
Rabbit Dermal >4000 Gunzel and Richter, 1966b
Rat Inhalation* >5.8 Sachsse and Ullman, undated
g/m3
* Inhalation exposure was for one hour.
Symptoms were similar to those for the base.
ACUTE TOXICITY OF CHLORDIMEFORM FORMULATIONS
LD50
Animal Route Sex Formulation (mg/kg) References
Mouse Oral Male EC 50 320 Aohi and Meda, 1966
Mouse Oral Female 50 s.p. 752 Shionogi C, undated
Rat Oral Mixed EC 50 610 Hurni and Sachsse, 1969b
Rat Dermal Mixed EC 50 2100 Sachsse and Bathe, 1971j
Rat Dermal Mixed 50 s.p. >3000 Hurni and Sachsse, 1969c
Rat Oral Mixed 50 s.p. 1100 Gunzel and Richter, 1969
Dog Oral Mixed 50 s.p. 400 Gunzel and Richter, 1968
A suicide victim ingested 30 ml of Galecron(R) 50 formulation. Upon
admission to hospital, an unknown time after drinking chlordimeform
solution, the patient was comatosed; and respiration and heartbeat had
ceased. The latter was restored by massage and adrenaline injection. A
respirator was used but death occurred within 24 hours. No autopsy was
performed (Oda, 1969).
Short-term studies
Rat. Four groups of 10 male and 10 female rats were intubated six
times weekly for one month with 0.5 ml/100 g body-weight of 2% CMC,
containing chlordimeform base at concentrations such as to give dose
levels of 0, 25, 50 or 100 mg/kg/dose. Body-weight was markedly
reduced in both sexes at 100 mg/kg/dose. Hyperexcitability was
observed in all test animals. At 100 mg/kg this was apparent 20-30
minutes after dosing, and was followed two to three hours after dosing
by decreased activity and apathy. Recovery was complete at four hours.
Similar effects were observed at 50, and 25 mg/kg/dose, but on a dose
related decreased scale, and with inconsistent frequency (Surber and
Cerioli, 1966).
Dog. Four groups of beagle dogs were fed 0 (10 male and 10 female)
250 (eight male and eight female), 500 (eight male and eight female)
or 1000 (10 male and 10 female) ppm of chlordimeform in a dry diet for
two years. Two male and two female dogs were sacrificed from each
group at 26, and 52 weeks. Body-weight was reduced at 1000 ppm, the
effect being slightly more pronounced in the females, Total leucocyte
counts were sporadically elevated in both sexes at 1000 ppm and in
females at 500 ppm. Haematocrit, haemoglobin, and erythrocyte counts
tended to be depressed terminally in both sexes at 1000 ppm. Sporadic
slight decreases in serum albumin were observed, more frequently in
males, at 1000 ppm. Terminal spleen to body-weight ratio was elevated
in males at 500 and 1000 ppm, and in females at 1000 ppm. Kidney to
body-weight ratio was elevated in females at 1000 ppm.
Histopathological examinations revealed bile duct hyperplasia,
pericholangitis and nodular hepatocytic hyperplasia at 500 and 1000
ppm in both sexes, and nodular hepatocytic hypertrophy at 1000 ppm in
both sexes in the liver. Kidneys showed an increased amount of
pigmentation at 500 and 1000 ppm in both sexes (Blackmore, 1969c).
Long-term studies
Rat. Five groups of 35 randomized male and 35 randomized female rats
(except at 100 ppm where 34 males and 36 females were used) were fed
0, 100, 250, 500 or 1000 ppm in the diet. The 100 ppm group commenced
treatment seven weeks after the other groups. This group was
originally part of the control group. Animals at that time were of
similar weight to those which had already been on test. The 1000 ppm
group was discontinued at three months due to severe growth
inhibition. Growth inhibition was observed in the males at 500 and
1000 ppm. In the females weight gain was reduced at 250 ppm and above.
In addition, female body-weight was reduced at 100 ppm between weeks
20 and 48. (This reduction may possibly be due to reduced food intake
(Weatherholtz, 1970) although this may not be the complete explanation
(Lyon, 1970).) Food intake was significantly reduced at 500 and 1000
ppm in both sexes. Dose related decreases in haematocrit, haemoglobin,
and erythrocyte counts, and a dose related increase in the leucocyte
count occurred in females at 250 and 500 ppm up to one year. During
the second year, haematocrit only was consistently depressed in
females at 500 ppm. Histo-pathological changes in the liver (nodules,
and foci of hyperplasia of hepatocytes) occurred in all groups, but
the incidence was greater at 250 and 500 ppm, and was more severe at
500 ppm. Some females at 500 ppm showed slight hypertrophy and
vacuolation of focal groups of cells in the adrenal cortex. Terminal
organ to body-weight ratios were increased in the liver (females at
250 and 500 ppm and males at 100 and 250 ppm), kidney (females at 250
and 500 ppm), thyroid (females at 250 and 500 ppm), heart (males at
250 ppm and females at 500 ppm), adrenals (males at 100 and 250 ppm)
and testes (100 and 500 ppm) (Blackmore, 1969d).
Comments
Chlordimeform appears to be fairly rapidly excreted in animals and
does not appear to be stored in body tissue. Information on metabolism
in man would however be of interest. A long-term feeding study is
available in the rat and a two-year study in the dog from which a
no-effect level was established. The Meeting expressed particular
concern about effect on organ to body-weight ratios in the rat and on
the histopathology of the liver and bile ducts of both the rat and the
dog. In these species nodular changes were observed in the liver but
histopathological information is insufficient to determine their
toxicological significance. Also of some concern were effects on the
total leucocyte counts of the dog and the pharmacological effects on
the heart and circulation together with the fact that the compound
appears to potentiate the effect of tyramine.
Because of the concern expressed above only a temporary acceptable
daily intake was established for this compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat - 100 ppm in the diet equivalent to 5 mg/kg body-weight per day
Dog - 250 ppm in the diet equivalent to 6.25 mg/kg body-weight per day
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Treatment of plants
Chlordimeform is a broad spectrum acaricide/insecticide applied to the
foliage of fruit, vegetables and other field crops acting through
contact and feeding and through vapour action on all stages of insects
and mites, including eggs of mites.
Pre-harvest treatments
Chlordimeform products have been registered in many countries for use
on a wide variety of crops of which typical examples are given in
Table 1.
Post-harvest treatment
No post-harvest treatments with chlordimeform are known.
Treatment of animals
In Australia chlordimeform is registered for use in cattle dips for
the control of cattle ticks (Boophilus micropus), the application
rate being 0.01-0.02% solution applied each 21 days by dipping often
in combination with organo-phosphorus acaricides such as dioxathion,
ethion, coumaphos, Dursban, bromophos-ethyl. Experimental work
designed to evaluate chlordimeform for use alone at a concentration of
0.1% is at an advanced stage.
Residues resulting from supervised trials
Fruit and vegetable crops
Detailed residue data are available from supervised trials in many
countries on many important crops and reports have been deposited with
FAO. The residue results are remarkably consistent between different
trials and different countries.
The most important and consistent feature of the residue trial data is
that the residue level remains remarkably constant irrespective of the
pre-harvest interval. On apples, grapes, citrus, peaches, pears, plums
and tomatoes the residue levels from sprays applied 30 days
pre-harvest are usually no more than half the level found when similar
rates were applied one to two days prior to harvest.
This rather unusual pattern is partly due to the analytical method
which determines all 4-chloro-o-toluidine containing metabolites
and/or conjugates as well as the unchanged parent compound.
Table II gives a representative sample of the results of supervised
residue trials carried out using registered label recommendations, use
patterns and rates of application.
Ciba and Schering (1971) have reported more than 50 trials designed to
determine the proportion of metabolites and parent compound in the
residues remaining on fruits at various intervals after application.
The separation of the components in the residue was performed by
thin-layer chromatography and by gas-chromatography.
TABLE II. REGISTERED USE PATTERNS FOR CHLORDIMEFORM
Pre-harvest
Country Crop Rate interval
Argentina pome fruits 0.06-0.09% 28 days
Australia cattle 0.02% 1 day
cotton 0.1%
pome & stone fruits 0.1% 7 days
strawberries 0.05% 7 days
Austria fruit trees 0.05% -
Brazil horticulture 0.05%
Canada fruit trees 0.06% 14-28 days
cole crops 0.05% 28 days
TABLE II. (cont'd)
Pre-harvest
Country Crop Rate interval
Denmark fruit trees 0.05% -
France pome fruits 0.05% 15 days
grapes 0.05% 15 days
vegetables 0.05% 15 days
Germany fruit trees 0.05% -
hops, grapes 0.05% -
Greece horticulture 0.05% -
Israel deciduous fruit - -
Italy fruit trees 0.05% -
citrus, grapes 0.05% -
Japan apples 0.05-0.025% -
citrus 0.05-0.035% -
pears 0.05-0.035% -
Yugoslavia fruit trees 0.05-0.15% -
vegetables 0.05-0.15% -
New Zealand pome fruits 0.06% 14 days
peaches 0.06% 14 days
strawberries 0.06% 7 days
Peru cotton 1 kg/ha 15 days
South Africa citrus 0.05% 14 days
tomatoes 0.075% 30 days
Switzerland pome fruits 0.05% 6 weeks
grapes 0.05-0.075% 6 weeks
Turkey apples 0.05% -
citrus, cherries 0.05% -
cotton -
USA apples 4 kg/ha 14 days
citrus 4-6 kg/ha -
pears 4 kg/ha 28 days
Venezuela cotton, citrus 0.3-0.5 kg/ha -
horticulture 0.3-0.5 kg/ha -
TABLE III
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Apples USA 1967 0.06 3 s.p. 3.3 1.3 1.3 1.4
USA 1969 0.06 2 s.p. 8.3 1.9 1.4 1.2 0.6
USA 1969 0.12 2 s.p. 8.6 4.5 2.8 1.2 1.7
USA 1969 0.12 3 e.c. 2.9 2.0 1.0 1.2
Canada 1969 0.06 2 s.p. 1.6
Australia 1969 0.04 3 s.p. 1.2 1.8 2.5 0.5 0.9 0.8
New Zealand 1970 0.05 1 s.p. 1.5 1.0 0.7 0.6
New Zealand 1970 0.1 1 s.p. 2.2 1.2 0.9 0.8
South Africa 1969 0.05 1 s.p. 0.6 0.6 0.5 0.5
England 1967 0.08 1 e.c. 3.3 2.6 2.7 1.2 1.2
Germany 1966 0.06 1 s.p. 1.8 1.9 1.6 0.8 0.5
Germany 1969 0.034 1 s.p. 1.5 1.0 0.6 0.5
Germany 1969 0.07 1 s.p. 2.5 1.8 1.5 0.7
Switzerland 1965 0.05 1 s.p. 1.1 1.0 1.2 0.6 0.5 0.5
Switzerland 1967 0.05 1 e.c. 2.3 1.3 1.0 0.5
Cherries USA 1968 0.06 1 e.c. 1.9 0.5 0.3 0.2
USA 1968 0.12 1 e.c. 3.7 1.3 1.1 0.7
Grapes USA 1969 0.06 2 s.p. 1.6 0.8 0.5 0.5
USA 1969 0.12 2 s.o. 2.9 2.8 1.6 1.8
Germany 1970 0.034 2 s.p. 2.0 1.3 1.3 1.0
Germany 1970 0.034 2 s.p. 2.8 2.3 2.3 1.8
Italy 1968 0.034 1 s.p. 2.6 1.2 1.2 0.6
Italy 1968 0.045 1 s.p. 1.8 1.5 1.2 1.2
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Oranges
Pulp Italy 1967 0.1 1 w.p. 0.2 0.3 0.2 0.3
Peel Italy 1967 0.1 1 w.p. 3.8 3.6 2.3 2.2
Whole Italy 1967 0.1 1 w.p. 1.1 1.1 0.8 0.7
Pulp Italy 1968 0.05 1 s.p. 0.2 0.3 0.1 0.2 0.08 0.1
Peel Italy 1968 0.05 1 s.p. 4.6 3.8 1.7 3.7 2.2 2.3
Whole Italy 1968 0.05 1 s.p. 1.5 1.2 0.5 1.1 0.7 0.7
Pulp Spain 1968 0.08 1 s.p. 0.4 0.4 0.4
Peel Spain 1968 0.08 1 s.p. 12.1 10.9 6.0
Whole Spain 1968 0.08 1 s.p. 3.5 3.5 2.0
Peaches USA 1969 0.06 1 e.c. 6.6 5.0 4.4 2.3
USA 1969 0.06 1 s.p. 5.9 3.4 2.8 1.9
Australia 1970 0.04 1 s.p. 1.0 1.2 0.4 1.7 0.6 0.4
Australia 1970 0.08 1 s.p. 2.0 2.0 1.1 3.1 1.4 1.1
France 1969 0.05 1 s.p. 1.5 1.1 0.5 0.5
France 1969 0.1 1 s.p. 1.5 0.9 1.0
USA 1970 0.06 2 e.c. 7.5 6.6 4.1 3.3
USA 1970 0.06 2 s.p. 3.7 3.2 2.4 1.5
Pears USA 1969 0.06 2 s.p. 7.8 6.1 4.4 3.5 2.8
USA 1969 0.06 2 e.c. 6.4 5.5 4.1 2.8 1.6
Australia 1970 0.04 1 s.p. 1.5 1.1 0.7 1.7 0.6 0.7
Australia 1970 0.08 1 s.p. 3.0 2.4 1.6 2.2 1.6 1.3
Plums USA 1970 0.06 2 e.c. 1.7 1.3 1.9 1.7
USA 1970 0.06 2 s.p. 0.5 0.5 0.5 0.4
Germany 1969 0.07 1 s.p. 0.7 0.5 0.4 0.3
Germany 1969 0.07 1 s.p. 1.1 1.2 0.6 0.5
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Strawberries USA 1968 0.06 1 e.c. 5.7 4.0 3.6 2.6 0.04
USA 1968 0.12 2 s.p. 1.8
England 1967 0.1 1 e.c. 1.8
England 1967 0.1 1 s.p. 2.1
Tomangos
Whole South Africa 1970 0.2 1 s.p. 2.7 2.6 2.9
Pulp South Africa 1970 0.2 1 s.p. 1.0 0.9 1.3
Peel South Africa 1970 0.2 1 s.p. 7.0 6.5 7.1
Walnuts
Meat USA 1968 0.06 1 s.p. <0.05
Meat USA 1970 0.06 2 s.p. <0.05
Beans
Pod Germany 1969 0.034 1 s.p. 0.5 0.2 0.1
Pod Germany 1969 0.07 1 s.p. 0.5 0.1 0.1
Broccoli
Head trimmed USA 1970 0.05 5 s.p. 1.7 0.8 0.4 0.2
Head trimmed USA 1970 0.1 5 s.p. 4.4 1.2 0.7 0.2
Head trimmed Canada 1970 0.05 2 s.p. 2.4 0.8
Head trimmed Canada 1970 0.05 6 s.p. 3.7 1.7 1.3
Brussels sprouts USA 1969 0.06 9 e.c. 2.8 2.1 1.9
Canada 1970 0.05 6 s.p. 3.6 3.5 3.1
Canada 1970 0.05 8 s.p. 5.5 3.8 2.8
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Cabbage
Head trimmed USA 1968 0.2 6 e.c. 4.3 0.6 0.2
Head trimmed USA 1969 1 e.c. 0.2
Head trimmed USA 1969 11 s.p. 1.5 1.2 1.4
4 outer leaves Canada 1969 4 s.p. 0.5
Inner head Canada 1969 4 s.p. <0.05
Cauliflower
Head trimmed USA 1969 6 s.p. 2.6 2.6 0.5
Head trimmed USA 1970 4 s.p. 3.6 2.5 1.9
Head trimmed USA 1970 4 s.p. 14.9 8.4 3.7
Cotton
Seed USA 1970 0.5 9 e.c. 0.22
Lint cotton USA 1970 0.5 9 e.c. 0.37
Delinted
cotton-seed USA 1970 0.5 9 e.c. 0.19
Linters USA 1970 0.5 9 e.c. 0.47
Cottonseed hulls USA 1970 0.5 9 e.c. 0.20
Solvent extract
meal USA 1970 0.5 9 e.c. 0.15
Screwpress
extracted meal USA 1970 0.5 9 e.c. 0.09
Crude solvent
extracted oil USA 1970 0.5 9 e.c. 0.11
Refined solvent
extracted oil USA 1970 0.5 9 e.c. 0.09
Crude screwpress
oil USA 1970 0.5 9 e.c. 0.17
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Refined
screwpress oil USA 1970 0.5 9 e.c. 0.14
Seed USA 1970 0.2 6 e.c. 1.8 1.6 2.3
Seed USA 1970 6 s.p. 2.0 1.8 2.4
Oil crude USA 1968 10 s.p. 1.2
Oil refined USA 1968 10 s.p. 1.0
Oil crude USA 1968 10 s.p. 4.0
Oil refined USA 1968 10 s.p. 3.6
Oil crude USA 1970 9 e.c. 0.1
Oil refined USA 1970 9 e.c. 0.1
Sugar beet
Roots Germany 1968 1 s.p. <0.02
(60-120)
Roots Germany 1969 1 s.p. <0.02
<0.02
(60-120)
Tops Germany 1969 s.p. 0.1
<0.02
(60-120)
Tomatoes
Fruit Canada 1970 6 s.p. 0.6 0.2 0.2
Fruit USA 1970 7 e.c. 0.4 0.3 0.5 0.3
Table IV gives some typical examples of the results obtained in these
trials which involved commercial formulations applied at
concentrations normally used in practice.
TABLE IV
Fruit Location Interval Total Proportion of each
before residue metabolite%
harvest ppm
A B C D
Apples USA 30 0.92 87 <4 <4 <4
USA 90 0.42 55 <9 27 <9
Australia 2 1.03 75 <4 <15 <4
Australia 30 0.63 63 <10 <25 <10
Pears USA 30 1.78 79 <2 17 <2
USA 60 0.88 68 <3 23 <3
Italy 1 0.89 90 <3 <5 <3
Italy 21 0.62 80 <4 <8 8
Peaches Italy 1 2.32 95 <1 <5 <1
Italy 47 0.37 54 <5 35 <5
Prunes Switzerland 9 1.36 81 <1 15 <3
Switzerland 30 1.06 75 <2 20 <4
A = chlordimeform
B = N-(4-chloro-o-tolyl)-N-methylformamidine
C = N-formyl-4-chloro-o-toluidine
D = 4-chloro-o-toluidine
Where the sign < appears it is to be recognized that the limit of
detection, e.g. 0.02 or 0.04 ppm, means that the proportion may well
be much less than the figure shown.
A number of trials reported by Ciba and Schering (1971) show that
there is no significant difference in the level of chlordimeform
residues on the various fractions of cotton seed separated during
commercial processing. One series of results is shown in Table III
(see page 17). The fairly uniform distribution of residues between oil
and cotton seed meal may reflect the distribution of parent compound
and metabolite but possibly is also brought about by the analytical
procedure which does not distinguish metabolites from parent compound.
Animals
Ciba-Geigy (1971) report results of trials conducted in Australia to
determine the residue in cattle treated with chlordimeform to control
cattle tick. A calf weighing 200 kg was sprayed with 15 litres of
0.05% chlordimeform. The animal was slaughtered three days later and
residues of chlordimeform and metabolite hydrolysable to
4-chloro-o-toluidine were determined in tissues and fat with the
following results:
muscle 0.21 ppm omental fat 0.12 ppm
kidney 0.23 ppm subcutaneous fat 0.30 ppm
liver 0.62 ppm perirenal fat 0.21 ppm
In further trials cattle weighing 210 to 230 kg were dipped in 0.013%
chlordimeform and were slaughtered one or three days later. The
residues determined as above were as follows:
Interval
since Liver Fat Muscle Kidney
treatment
1 day 0.28±0.04 0.15±0.06 0.07±0.02 0.29±0.03
3 days 0.15±0.03 0.05±0.02 0.03±0.01 0.06±0.03
Buffering
As explained in some detail under "Fate of residues", chlordimeform
stability is extremely dependent on pH. Acceptable stability in cattle
dips can be achieved only if pH is held below 5.8 and preferably in
the range 5.0-5.5. In Australia this has been achieved by buffering
the dipping fluid with fertilizer grade superphosphate. Unbuffered
fluids must be used within a day of preparation.
At pH 5.2, some 99% of chlordimeform is present as the acid salt. In
this form it has adequate stability but its activity is reduced below
that of a similar concentration at a less acid pH value containing
proportionately more chlordimeform base.
Buffered dips leave only about half the hair deposits left by
unbuffered dips of equal concentration so that higher use levels are
required to produce the same acaricidal effect. Fortunately absorption
is also reduced and residue levels are not augmented by the increased
concentration required in dips.
Residues in milk and milk products
A Ciba-Geigy report (1971) describes milk and butter residues
following the treatment of lactating dairy cattle with 0.05%
chlordimeform, about 15 litres being applied to each animal. Three
animals were treated with a solution buffered to pH 5.6 and three with
an unbuffered solution of pH 9.4.
Time of 0.05% unbuffered 0.05% buffered
sampling Milk Butter Milk Butter
Pre-treatment 0.03 ppm 0.06 ppm <0.03 ppm 0.05 ppm
6 hours 0.22 ppm 1.52 ppm 0.06 ppm 0.52 ppm
24 hours 0.05 ppm 0.71 ppm 0.05 ppm 0.19 ppm
32 hours 0.08 ppm 0.18 ppm <0.03 ppm 0.14 ppm
48 hours 0.05 ppm 0.22 ppm <0.03 ppm 0.11 ppm
56 hours 0.16 ppm 0.10 ppm
72 hours 0.09 ppm 0.06 ppm
Each figure in the table is the mean of three individual samples.
The analytical technique employed determines the sum of chlordimeform
and all metabolites hydrolysable to 4 chloro-o-toluidine. The ratio
of milk residues to butter residues while somewhat variable, falls
consistently short of the level which would be expected if these
residues were partitioned exclusively in the fat phase.
The possibility that a proportion of the residues may be held in the
aqueous phase should be taken into consideration in the recommendation
of tolerances.
These results indicate the rapid rate of elimination which occurs
following spraying or dipping.
The level of residues is related to the concentration of the spray
solution or dip. Official trials in Australia showed that when the
concentration of the spray is increased from 0.03 to 0.3% the residue
levels in fat, kidney and liver of young calves increase in
approximately the same ratio. Residue levels in muscle do not
materially change in spite of the increase in concentrations. Residue
determinations were made 24 hours after spraying:
Adult cattle appear to metabolize the residue more rapidly because
animals weighing 450 kg dipped three times at 17-day intervals in a
bath containing 0.01% chlordimeform showed no residues in excess of
0.1 ppm when slaughtered three days after the third treatment. Adult
cattle weighing 300-520 kg sprayed with chlordimeform showed, as did
calves, that the residue level 24 hours after treatment increases
sharply with increasing concentration of spray. The residue level in
perirenal and omental fat increased from 0.1 ppm to 7.2 ppm when the
spray concentration was increased from 0.01 to 0.1%.
Concentration Liver Fat Muscle Kidney
of spray
0.03% <0.1 ppm 1.0 ppm <0.1 ppm 0.2 ppm
0.3% 1.2 ppm 10.0 ppm <0.1 ppm 0.5 ppm
Repeat treatments, as is normal in controlling cattle tick, does not
result in an accumulation of residues in animal tissues. Calves
receiving 10 dippings within eight weeks in a bath containing 0.01%
chlordimeform were slaughtered three days after the tenth treatment.
The results of residue analysis were as follows:
No. of Liver Fat Muscle
treatments
1 2.0 0.29 0.12
7 1.3 0.64 0.07
10 4.17 0.37 0.12
Fate of residues
General comments
Chlordimeform (I) is readily hydrolysed in weakly-acid to
weakly-alkaline solutions but is rather stable under strongly acid
conditions. In an aqueous buffer solution of pH 7.0 containing 5% of
methanol, the base showed a half-life of 42 hours at 30°C. At pH 9,
under otherwise equivalent conditions, a half-life of five hours was
observed (Kossmann et al., 1971). Hydrolysis of the parent compound
yields N-formyl-4-chloro-o-toluidine (III) and ultimately
4-chloro-o-toluidine (IV).
Structural alterations are observed when chlordimeform in solution or
on chromatoplates is exposed to irradiation by U.V. or natural
sunlight (Knowles and Sen Gupta, 1969). Although a number of
additional minor degradation products do occur,
N-formyl-4-chloro-o-toluidine (III) is the major transformation
product recovered.
A complete list of substances which appear to be potential metabolites
of chlordimeform is given in Table 1.
In animals
To supplement the studies described under "Biochemical aspects" on the
behaviour of chlordimeform in mammals, Ciba-Geigy Ltd (Ciba-Geigy,
1971) conducted experiments on the fate of the acaricide upon
continuous feeding to ruminants. Eight Brown Swiss cows were fed daily
rations containing from 4-24 ppm chlordimeform for periods of up to 42
days. Milk and tissue and organ samples were collected at regular
intervals and analysed by the total residue method (Geissbühler at
al., 1971) which accounts for all 4-chloro-o-toluidine containing
metabolites and/or conjugates of chlordimeform. At 21 days, residues
in liver were 0.09, and <O.03 ppm and in kidney were 0.38, and 0.07
ppm in animals fed 120 and 40 ppm respectively. Further cows fed 240
ppm in the diet showed liver levels of 0.45, 0.38, 0.50 and 0.44 ppm
at 7, 14, 21 and 42 days. Corresponding kidney residues were 0.05,
0.13, 0.13, and 0.09 ppm. Residues in milk, muscle and fat were below
0.03 ppm (Voss and Burkhard, 1971).
One male (36 kg) and one lactating female (39 kg) goat were given 10
µCi chlordimeform orally, urine and faeces being collected at 1, 3, 6,
12, 24, 48 and 72 hours post treatment. After 72 hours, the male had
voided 87%, and 1.8% of the administered radioactivity in the urine
and faeces respectively, and the female 68% and 1.8%. Only 0.3% of the
radioactivity appeared in the milk.
Chloroform extractable compounds identified by thin-layer
chromatography comprised chlordimeform,
N'-(4-chloro-o-tolyl)-N-methylformamidine, 4-chloro-o-toluidine,
and N-formyl-4-chloro-o-toluidine, the latter predominating. Of the
radioactivity 85-97% remained at the point of origin.
Re-chromatography with polar solvents yielded 5-chloroanthranilic
acid, and N-formyl-5-chloroanthranilic acid.
The 90% unextractable radioactivity, when incubated with
ß-glucuronidase, ß-glucuronidase-aryl sulphonase or hydrochloric acid
yielded chlordimeform, N'-14-chloro-o-tolyl-N-methylformamidine,
4-chloro-o-toluidine, N-formyl-4-chloro-o-toluidine and two
unidentified metabolites. N'-(4-chloro-o-tolyl)-N-ethylformamidine
was present in very small amounts, and the others in about equal
amounts. Re-chromatography of the 20% radioactivity which remained at
the origin yielded anthranilic acids, the 5-chloroanthranilic acid
predominating (Sen Gupta and Knowles, 1970).
In plants
A series of preliminary experiments carried out by Ciba (1965a, b) and
Schering (1966b) demonstrated that chlordimeform was quite rapidly
degraded in plant tissues with high inherent metabolic activity (i.e.
bean leaves) but was only slowly transformed in ripe fruits (Table V).
Green fruits, such as young grapes, and stems were observed to take an
intermediate position with regard to their ability to degrade the
acaricide.
TABLE V. RESIDUES OF CHLORDIMEFORM AND ITS DEGRADATION PRODUCTS IN AUSTRALIAN APPLES AFTER SPRAY APPLICATION OF THE
ACARICIDE (SPRAYING CONCENTRATION 0.05% a.i.) FROM CIBA (1965b)
Day Chlordimeform N-formyl-4-chloro-o-toluidine Desmethyl 4-chloro-o-toluidine
after ppm ppm chlordimeform ppm
spraying ppm
Apples
0 0.2 0.15 - -
1 0.6 <0.15 - -
2 0.8 <0.15 <0.04 <0.04
6 0.3 <0.15 - -
14 0.2 <0.05 <0.04 <0.04
21 0.4 <0.05 - -
30 0.4 <0.05 <0.04 <0,04
42 <0.05 <0.05 - -
Pears
0 0.50 0.25 - -
1 0.75 0.20 - -
2 0.45 0.10 <0.04 <0.04
6 0.75 0.10 - -
14 0.70 0.05 <0.04 <0.04
21 0.65 0.05 - -
30 0.60 <0.05 <0.04 <0.04
42 0.40 <0.05 - -
Tentative identification of the observed transformation products
indicated that in leaves both
N'-(4-chloro-o-tolyl)-N-methylformamidine
(desmethyl-chlorphenamidine, 11) and N-formyl-4-chloro-o-toluidine
(111) were prominent metabolites. On the other hand, in ripe apple and
pear fruits, only the formyl-derivative (III) was detected in
measurable quantities, whereas desmethyl-chlordimeform (II) was
normally absent (Table V). In all tissues analysed the free
4-chloro-o-toluidine (IV) was not detected at all or was present
only in small quantities. The lack of formation of significant residue
quantities of the free toluidine in edible plant parts, such as
fruits, was further examined by analysing plums which had been exposed
to a six times overdose treatment of chlordimeform (Ciba, 1967b). Even
after this application, which left a total residue of 4 to 6 ppm, the
amounts of free 4-chloro-o-toluidine were scarcely above the limit
of detection (0.05 ppm) of the colorimetric method applied.
Since chlordimeform was known to be quite volatile, the possibility
and extent of its evaporation from plant surfaces was investigated
(Ciba, 1968). Although the free base of the acaricide was observed to
readily evaporate from glass plates, disappearance from the surface of
bean leaves was considerably less pronounced. Losses by evaporation,
which occurred mainly during the first few hours after application,
were found to be of the order of 30 to 40% in terms of the original
dose applied. Evaporation was of the same order of magnitude when bean
leaves were treated with the hydro-chloride salt of chlordimeform.
This result suggested that, owing to the buffering capacity of plant
exudates on certain leaf surfaces, an equilibrium between free base
and salts was established, no matter which form of the acaricide was
applied. The behaviour of chlordimeform on leaf surfaces, as
described, was essentially confirmed by Sen Gupta and Knowles (1969)
when applying the acaricide to leaves of apple seedlings and by
Ehrhardt and Knowles (1970) when treating leaves of grapefruit
seedlings with both the free base and the hydrochloride salt.
Experiments carried out by Sen Gupta and Knowles (1969) on leaves of
apple seedlings and by Ehrhardt and Knowles (1970) on leaves of
grapefruit seedlings confirmed the limited ability of chlordimeform to
penetrate cuticular layers.
In the apple experiments (Sen Gupta and Knowles, 1969), 3H-as well
as 14C-labelled chlordimeform was applied to seedlings by either
leaf treatment or stem injection. The treated plants were cultured for
20 days and periodically analysed for the metabolites already
mentioned above. Sufficient quantities of the main transformation
products were collected to allow characterization by infra-red
analysis, melting point determinations, dye formation and
co-chromatography on thin-layer plates.
After both types of application dissipation/degradation of the
acaricide was observed to proceed at an intermediate rate, the
half-life of the compound being of the order of 12 to 16 days. Upon
termination of the experiment about 40% of the radioactivity applied
was still accounted for by the unchanged acaricide. Organosoluble
degradation products identified were desmethyl-chlordimeform (II),
N-formyl-4-chloro-o-toluidine (III) and free 4-chloro-o-toluidine.
The quantities of these metabolites relative to chlordimeform, however
were quite small and except for desmethyl-chlordimeform, never
exceeded 1% in terms of the radioactivity applied.
No so-called "non-extractable" radioactivity was evident after leaf
application of chlordimeform but was observed to be present in
increasing amounts (up to 30% of the quantity applied) after stem
injection of the acaricide. However, this non-extractable
radioactivity was confined to the stem section and could not be
observed in leaves to which part of the radioactivity had been
translocated. The authors suggested that non-extractable materials
represented chlordimeform degradation products that were complexed
with polymeric cell constituents.
The experiments with apple leaves also confirmed the limited ability
of chlordimeform to penetrate through cuticular plant tissues since
most of the radioactivity remaining on leaves could subsequently be
removed by rinsing them with organic solvents. No more and usually
less than 15% of the dose applied remained in the leaf tissues after
the rinsing process.
In the grapefruit experiments (Ehrhardt and Knowles, 1970) both the
free base and the hydrochloride salt of
14C-(tolyl-labelled)-chlordimeform were applied to the leaf surface
of growing seedlings. In these studies, dissipation of total
radioactivity was more pronounced than in the apple experiments, since
only 10 to 207 of the dose applied was recovered at the end of the
20-day observation period. It therefore appeared that a higher
percentage of chlordimeform evaporated from grapefruit than from apple
leaves. In addition, chlordimeform itself was degraded at a faster
rate by the citrus than by the apple tissue, since, after 20 days of
culturing, no more than 1% of the radioactivity applied was recovered
as the unchanged acaricide from the former tissue.
The pattern of metabolites on and in citrus leaves was essentially the
same as that reported for apple seedlings. Desmethyl-chlordimeform,
the formyl derivative and free 4-chloro-o-toluidine were the
principal metabolites, whose quantities, however, were small and never
exceeded 2% in terms of the radioactivity applied. Several unknown
minor radioactive metabolites were observed on thin-layer plates,
however, none of them represented more than 1% and normally did not
exceed 0.1% in terms of the radioactivity applied.
In several recent publications (Knowles et al., 1969a, b; Rosen et
al., 1970) which deal with in vitro chemical and biochemical model
systems, chlordimeform and its minor metabolite 4-chloro-o-toluidine
have been implicated or shown to be possible candidates for the
catalytic formation of azobenzene derivatives, such as
2,2'-dimethyl-4,4'-dichloroazobenzene (VII, Table I). Although a more
detailed discussion on the potential formation of azobenzene compounds
from chlordimeform is presented in the section on animal metabolism
("Biochemical aspects") results on the presence or absence of such
transformation products in plants are briefly discussed. In examining
field-treated fruits and vegetables for the presence of azobenzene
compounds, a sensitive gas-chromatographic residue method which
permitted the detection of 0.01 ppm of
2,2'-dimethyl-4,4'-dichloroazobenzene was used (Geissbühler et al.,
1971). This method was applied to apple fruits and leaves which had
been treated with a four times overdose of chlordimeform and which
were harvested 20, 30 and 40 days after application (Ciba, 1969b).
Although chlordimeform residues were found to be excessively high
during the whole observation period (10 to 4 ppm in fruits; 400 to 300
ppm in leaves), residues of the azobenzene compound were either not
detectable (<0.01 ppm in fruits) or were so small (0.04 ppm in
leaves) that they represented less than 0.02% in terms of the Parent
residue. Therefore, with normal concentrations of chlordimeform, no
detectable azobenzene residues are to be expected on edible crops.
From the extensive plant behaviour and metabolism data presented, the
pathways of chlordimeform transformation in plants are summarized as
follows (Sen Gupta and Knowles, 1969):
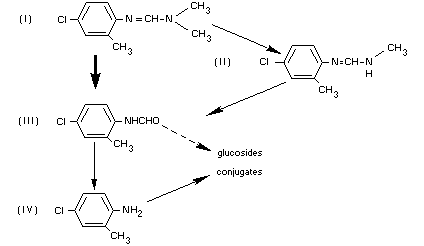 Whereas in leafy tissues, stems or green fruits, the whole pathway of
degradation is operative to a small extent, in ripe fruits only
N-formyl-4-chloro-o-toluidine has consistently been observed as a
metabolite. The residue methods designed account for all metabolites
and/or conjugates (Geissbühler et al., 1971; Kossman et al., 1971).
In the course of an extensive study of the fate and persistence of
chlordimeform on six different crops Witkonton (1969) found that the
total amount of residue is directly related to the amount of chemical
applied, an inverse function of the number of days the fruit was
sampled after the last application of the chemical and influenced by
the nature of the fruit surface.
Fruit was analysed for chlordimeform and three possible major
degradation products. Only the parent compound and one degradation
product, N-formyl-2-methyl-4-chloroaniline, could be detected.
N-formyl-2-methyl-4-chloroaniline was found only in small amounts in
apples, pears and cherries; but greater amounts were found in plums
and strawberries. The results of this investigation did not show any
real correlation between the amounts of the parent compound and
N-formyl-2-ethyl-4-chloroaniline to such variables as amount of
chemical applied or sampling date. The type of fruit, and especially
the nature of the fruit surface, and such factors as sunlight and
rainfall appear to be predominant in affecting the degradation of
chlordimeform.
Witkonton (1969) also reported that chlordimeform appears to adhere to
the outer surface of fruit and does not appear to translocate readily
to the fleshy parts, The chief factors which seem to account for the
decrease of chlordimeform residues in fruit appear to be
volatilization, weathering and growth dilution.
In soil
Chlordimeform deposited inadvertently on soil surfaces after spray
application may be expected to be dissipated by the following
processes: volatilization, chemical hydrolysis, photodecomposition
(Knowles and Sen Gupta, 1969) and microbial degradation. Although no
special volatilization experiments with soil samples were conducted,
the contribution of this process is likely to be of the same order of
magnitude as or slightly higher than that observed for leaf surfaces.
Hydrolysis of chlordimeform to N-formyl-4-chloro-o-toluidine (III)
shown to be substantial under the slightly acid or alkaline conditions
which normally prevail in soils (Kossmann et al., 1971).
As regards microbial degradation of the acaricide, Johnson and Knowles
(1970) demonstrated that several bacterial, actinomycete and fungus
species are capable of extensively degrading chlordimeform. The
principal metabolite in the culture media of most species was
N-formyl-4-chloro-o-toluidine, except for the actinomycete
Streptomyces griseus which formed mainly 4-chloro-o-toluidine.
None of the microbes formed symmetrical azo-compounds.
Under field conditions, chlordimeform and its 4-chloro-o-toluidine
containing metabolites were dissipated according to first order
reactions with half-lives ranging from 20 to 40 days (Schering, 1969;
Ciba, 1969e). From the present experiments it way be concluded that
chlordimeform is not accumulated in the soil.
Evidence of residues in food in commerce or at consumption
No data were available to indicate the incidence or level of
chlordimeform residues in food commodities moving in commerce.
A number of detailed studies have been conducted to identify terminal
residues after application of chlordimeform. Results indicate that
breakdown of the parent compound takes place particularly in tissues
which are metabolically active, such as young bean leaves, while
fruits like apples and prunes show practically no metabolic breakdown
of the acaricide. The hydrolytic pathway of degradation leading to
small amounts of N-formyl-4-chloro-o-toluidine has been demonstrated
to occur to a certain extent also on fruit crops. Hydrolysis of
chlordimeform, however, apparently takes place independent of plant
tissue activities due to the chemical instability of the parent
compound (Kossmann et al., 1971),
Furthermore it has been confirmed that
2,2'-dimethyl-4,4'-dichloroazobenzene is not expected to be a
potential transformation product of chlordimeform in apple fruit.
All chlordimeform metabolites identified so far in various plant
materials are determined by the described total residue methods.
A number of special investigations reveal that chlordimeform residues
are located in the outer parts of crops such as fruit peels or outer
leaves of cole crops. Excessive residues therefore might be removed by
peeling of fruit (apples, citrus) or by trimming the outer leaves of
cole crops.
Washing of fruit will remove only a small part of the total residue.
External residues will probably dissipate by volatilization, rainfall,
or penetration into the cuticular wax layers,
Schering (1971) reports on the effect of cooking on chlordimeform
residues in apples, grapes, tomatoes, cauliflower, beans and sugar
beet foliage and shows that the behaviour of chlordimeform residues
during cooking depends on the pH value of the aqueous suspension as
well as on the duration of the cooking period.
This report shows that the rate of loss of chlordimeform is a function
of pH value, the loss being much more rapid in a neutral medium such
as green beans (pH 5) or cauliflower (pH 6) than in apples (pH 2.5) or
tomatoes (pH 3). The hydrolysis proceeds according to the scheme
outlined in page 30 and all metabolites are determined by the
analytical method for chlordimeform parent compound. The loss of total
4-chloro-o-toluidine moiety is not significant showing that
volatilization in steam is not an important contributing factor.
Methods of residue analysis
(a) Methods for the detection of unchanged chlordimeform and its
breakdown products (terminal residues)
Identification of terminal residues in plant materials and specific
determination of unchanged chlordimeform has been carried out by
thin-layer and/or gas chromatography procedures. A detailed
description of the separation and detection of
N'-(4-chloro-o-tolyl)-N-methylformamidine,
N-formyl-4-chloro-o-toluidine, and 4-chloro-o-toluidine as well as
the parent compound including quantitation by colorimetry or flame
ionization gas chromatography has been given by Kossmann et al.
(1971).
Investigations for the presence of
2,2'-dimethyl-4,4'-dichloroazobenzene have been performed by a gas
chromatographic procedure following reductive cleavage of the azo
compound and subsequent Sandmeyer iodination of the resulting
4-chloro-o-toluidine (Geissbühler et al., 1971).
Selective determination of unchanged chlordimeform may be needed after
usage of chlordimeform/formetanate mixtures. Both active substances
have the structure of formamidine derivatives. For this combination
product a thin-layer chromatographic procedure has been established
(Schering AG, 1969b) which includes direct quantitation on the
chromatoplate by reflectance measurement in the UV range. Gas
chromatographic procedures described above allow specific
determination of chlordimeform in presence of formetanate as well and
account then for the total chlordimeform residue including
metabolites.
(b) Total chlordimeform residue methods
For regulatory purposes and routine residue analysis methods were
developed that account in a single procedure for chlordimeform and for
all its 4-chloro-o-toluidine containing breakdown products and/or
conjugates. Principle of determination of "total chlordimeform" is
based on a two step hydrolysis by successive treatments with acetic
acid and sodium hydroxide respectively. The joint hydrolysis product,
4-chloro-o-toluidine, is steam distilled and extracted into
iso-octane. Quantitation of 4-chloro-o-toluidine may be carried out
by colorimetry (specificity limited) or gas chromatography (highly
specific).
Colorimetry is based upon diazotization reaction of
4-chloro-o-toluidine and coupling with N-ethyl-1-naphthylamine
(Geissbühler et al., 1971). A number of investigations has been made
by using 1-naphthylethylenediamine as a coupling agent (Schering AG,
1968). Both procedures are sensitive to 0.05 ppm of chlordimeform as
calculated for a 50-gram crop sample. Recovery values obtained from
various crops fortified within the range of 0.05 to 5 ppm
chlordimeform in general exceeded 85% (Geissbühler et al., 1971;
Schering AG, 1969a).
An interlaboratory study demonstrated the sufficient reproducibility
of the total residue procedures established. Since specificity of
colorimetric evaluation is limited, additional identification of
chlordimeform residues may be done by confirmatory thin-layer
chromatography of the resulting azo dye on cellulose plates
(Geissbühler et al., 1971).
Gas chromatography has also been effectively used for quantitative
determination of 4-chloro-o-toluidine moiety following
transformation into halogenated derivatives of extremely high electron
affinity. Sandmeyer iodination reaction of 4-chloro-o-toluidine
results in the 5-chloro-2-iodo-toluene structure and a 0.05 ppm
detection limit for chlordimeform by using a tritium foil equipped
electron capture detector. Recovery figures correspond very well to
those reported for colorimetric measurement (Geissbühler et al.,
1971).
A lower detection limit may be attainable by bromination of
4-chloro-o-toluidine in aqueous solution yielding
6-bromo-4-chloro-o-toluidine and subsequent gas chromatographic
determination using a Ni63 electron capture detector (Kossmann,
1971),
Direct gas chromatographic measurement of 4-chloro-o-toluidine by a
halogen microcoulometric titration cell has been reported by Del Monte
Corporation (Del Monte, 1969) for the determination of chlordimeform
residues in crops.
All the gas chromatographic procedures mentioned are specific for
chlordimeform residues. Up to date no other pesticide with the
4-chloro-o-toluidine nucleus is known to be used in agricultural
practice.
Examples of national tolerances
The following are examples of national tolerances and withholding
periods that have been established:
Country Crop Tolerance Withholding
ppm period days
Australia pome fruit 2 7
stone fruit 2 7
strawberries 1 7
fat of meat of cattle 0.5 1
Country Crop Tolerance Withholding
ppm period days
Canada pears 5 28
peaches, plums, prunes 4 14
apples 3 14
cauliflower, brussels
sprouts 3 28
broccoli 2 28
cabbage 0.5 28
turnip roots <0.05 28
Germany (GFR) grapes, stone fruit
(excl. cherries) 3 14
pome fruit 2
Italy fruit, citrus, grapes 1 20
New Zealand pome fruit 1 14
strawberries 7
Switzerland pome fruit, grapes 1 42
South Africa apples, pears, citrus 1 14
USA pears 5 28
apples 3 14
cabbage, cauliflower,
broccoli, brussels
sprouts 2 28
Venezuela fruit, citrus 2 14
Appraisal
Chlordimeform is a new insecticide/acaricide primarily effective
against eggs and larvae of spider mites with adequate activity also
against adult mites. It is effective against mites resistant to
organo-phosphorus insecticides. It kills eggs, larvae and adults not
only by contact but also in the vapour phase. Penetration and slight
systemic effect have been demonstrated. It is also effective in
control of some lepidopterous insects including codling moth but the
major field of use so far developed is in the control of mites on
deciduous fruit. Chlordimeform is also being used commercially in
Australia for the control of cattle tick by spraying and dipping
because of its ability to control ticks which have developed strains
resistant to all other currently available acaricides.
Chlordimeform is registered for use on fruit trees, grapes and
vegetables in many countries and to a lesser extent on cotton and
hops. The concentration applied ranges from 0.01 to 0.1%.
Chlordimeform is used both as the base and as the hydrochloride. The
base is formulated as an emulsifiable solution while the hydrochloride
is used as a water soluble powder.
The residue data available to the meeting were obtained from
supervised field trials in Australia, Canada, England, France,
Germany, Italy, New Zealand, South Africa, Spain, Switzerland and the
United States of America. Whilst there is a sharp drop in the residue
level between the day of application and the second or third day
post-treatment, thereafter the rate of decline is remarkably slow with
a half-life on apples, grapes, pears and tomatoes exceeding 21 days.
The rate of decline is somewhat faster on cruciferous vegetables but
even so the interval between application and harvest does not have a
pronounced bearing on the residues level following approved
treatments.
Loss of residue is initially due to volatilization but the portion of
the deposit absorbed or dissolved into surface tissues of plants
appears to remain at a relatively constant level. The residue data
reflect this durability but they are influenced by the fact that the
analytical procedure determines both parent compound and all
metabolites containing the toluidine moiety.
Extensive evidence is available on the fate of residues in plants and
animals as well as on the route and rate of degradation. The initial
pathway of metabolism in animals and plants is substantially similar.
leading to N-formyl-4-chloro-o-toluidine and 4-chloro-o-toluidine
in both organisms but proceeding further to
N-formyl-5-chloroanthranilic acid and 5-chloroanthranilic acid
respectively in animals.
Some data were available on the effect of processing and cooking as
the fate of chlordimeform residues. The chlordimeform residue
generally decreases with cooking, the decline being dependent upon
length of cooking time. The rate of decrease is a function of the pH
value. In a more neutral medium caused by green beans (pH 5) or
cauliflower (pH 6) loss of chlordimeform is much more rapid than in an
acid medium such as apples (pH 2.5) or tomatoes (pH 3). Studies
revealed no detectable change in the residue level or composition when
apples and pears were held in cold storage. Evidence was presented to
show that substantially all the residue occurs in the skin of apples
and citrus; in the outer leaves of cruciferous vegetables and in the
tops of root vegetables. Chlordimeform, applied to cotton, appears as
a residue in cotton seed cake as well as in cotton seed oil and the
residue level is not significantly reduced by refining.
Analytical methods based on the determination of
4-chloro-o-toluidine formed by successive treatments with acetic
acid and sodium hydroxide are specific for chlordimeform and its
metabolites and are capable of determining levels down to 0.05 ppm.
Quantitation may be made either by colorimetry based upon
diazotization and coupling with N-ethyl-1-naphthylamine or by gas
chromatography. Though direct measurement by gas chromatography is
possible, the accuracy and sensitivity is improved by transformation
of the residue into halogenated derivatives of extremely high electron
affinity.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Temporary tolerances (effective to June 1975)
Pears, peaches, prunes 5 ppm
Apples, grapes, plums, strawberries 3 ppm
Brassicas, cherries, citrus fruit,
cotton seed oil (crude and refined),
cotton seed 2 ppm
Beans 0.5 ppm
Fat, meat and meat products of cattle 0.5 ppm
milk (whole) 0.05 ppm
Butter 0.5 ppm
Further work or information
Required (before 30 June 1975)
1. A further long-term feeding study in rats to obtain precise
information on the incidence and nature of the histopathological
changes in the liver and bile ducts of rats exposed to levels
above and below 100 ppm in the diet. An effort should be made to
explain the changes in organ to body-weight ratios noted in the
previous long-term rat study.
2. Further investigation of the nature of the hepatic lesions
observed in the dog.
3. Metabolic studies in several animal species, preferably including
man.
4. Further data on the nature and levels of residues in animal
tissues after use in cattle sprays and dips.
5. Further data on residues in milk after use in cattle sprays and
dips, especially information on the nature of the residues and
their distribution between aqueous and lipid phases.
6. Data on the residue levels in commercial butter and cheese.
Desirable
1. Further studies on the haematological effects.
2. Elucidation of the pharmacodynamic activity on the heart,
including the potentiation of the effects of pressor amines.
3. Further data on the disappearance of residues during storage,
processing, and cooking.
4. Data on residue levels in raw agricultural commodities moving in
commerce.
REFERENCES
Aoki, H. and Ueda, K. (1966) Unpublished report of the Tokyo Dental
College submitted by Ciba-Geigy
Bertha, R. and Framer, D. (1967) Pesticide transformation to aniline
and azo compounds in soil. Science, 156: 1617
Blackmore, R. H. (1969a) Three generation reproduction study - rats.
Unpublished report of Hazelton Laboratories, submitted by Ciba-Geigy
Blackmore, R. H. (1969b) Teratology study - rabbits. Unpublished
report of Hazelton Laboratories submitted by Ciba-Geigy
Blackmore, H. H. (1969c) Two year repeated feeding - dogs. Schering
361 268 (Chlorphenamidine). Unpublished report from Hazelton
Laboratories, submitted by Ciba-Geigy
Blackmore, R. H. (1969d) Two year repeated feeding - rats. Schering
361 268 (Chlorphenamidine). Unpublished report from Hazelton
Laboratories, submitted by Ciba-Geigy
Ciba Ltd. (1965a) C-8514. Rückstände in den Blättern der Bohne. Report
of 20 May 1965
Ciba Ltd. (1965b) C-8514. Residues in Australian apples and pears.
Report of 25 May 1965
Ciba Ltd. (1967a) Modell einer Synthese von radioaktivem Galecron.
Report of 17 August 1967
Ciba Ltd. (1967b) Galecron. Absence of 4-chloro-o-toluidine as a
metabolite in prunes after overdose spraying. Report of 31 January
1967
Ciba Ltd. (1968) Galecron. Behaviour of different formulations on
citrus leaves. Report of 14 May 1968
Ciba Ltd. (1969a) Galecron. Residues in Italian oranges. Report of 2
March 1969
Ciba Ltd. (1969b) Galecron. Analysis of Swiss apples for residues of
2,2'-dimethyl-4,4'-dichloroazobenzene after overdose spraying. Report
of 20 November 1969.
Ciba Ltd. (1969c) Galecron. Formation of a symmetrical azo derivative
from 4-chloro-o-toluidine by coupled peroxidatic reactions. Report
of 12 September 1969
Ciba Ltd. (1969d) Galecron. Degradation of chlorphenamidine by rat
liver and spleen homogenates, with particular reference to the
possible formation of azo derivatives. Report of 10 September 1969
Ciba Ltd. (1969e) Galecron. Dissipation in a Swiss soil as determined
by chemical analysis. Report of 16 September 1969
Ciba-Geigy Ltd. (1971) Galecron. Residues in milk, meat, fat, liver
and kidney of Swiss cows. Report of 19 March 1971
Ciba-Geigy Ltd. (1971) Determination of chlorphenamidine residues in
milk and butter following treatment with "Spike" cattle dip at 0.05%
a.i. w/v. Report of 6 August 1971
Del Monte Corporation Research Center. (1969) Determination of total
chlorphenamidine residues in plant material, 10.6.1969. Unpublished
Dittrich, V. (1971) Biological action of chlordimeform. Report
prepared for FAO/WHO
Ehrhardt, D. A. and Knowles, C. O. (1970) Metabolism and translocation
of N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine (chlorphenamidine)
and its hydrochloride salt in grapefruit seedlings. J. Econ. Entomol.,
63: 1306
Geissbühler, H. (1969) The substituted ureas. In: Degradation of
herbicides (P. C. Kearney and D. D. Kaufman, ed.), p. 79. New York,
Marcel Dekker Inc.
Geissbühler, H., Kossmann, K., Baunok, I. and Boyd, V. F. (1971)
Determination of total residues of chlorphenamidine
[(N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine] in plant and soil
material by colorimetry and thin-layer and electron capture gas
chromatography. J. Agr. Food Chem., 19: 365
Gerhards, E. and Kolb. (1966) Unpublished report of Schering A.G.
submitted by Ciba-Geigy
Gerhards, E. (1967) Unpublished report of Schering A.G. submitted by
Ciba-Geigy
Gunzel, P. and Richter. (1965) Systemische Vertraglichkeitsprufung an
Ratten bei einmaliger p.o. Verabreichung (DL50). Unpublished report
from Schering A.G. submitted by Ciba-Geigy
Gunzel, P. and Richter. (1966a) Systemische Vertraglichkeitsprufung an
Ratten bei einmaliger dermaler. Verabreichung (DL50). Unpublished
report of Schering AG, submitted by Ciba-Geigy
Gunzel, P. and Richter. (1966b) Systemische Vertraglichkeitsprufung an
Kaninchen bei einmaliger dermaler Verabreichung (DL50). Unpublished
report of Schering A.G., submitted by Ciba-Geigy
Gunzel, P. and Richter. (1968) Systemische Vertraglichkeitsprufung an
Hunden bei einmaliger p.o. Verabreichung (DL50). Unpublished report
from Schering AG., submitted by Ciba-Geigy
Gunzel, P. and Richter. (1969) Systemische Vertraglichkeitsprufung an
Ratten bei einmaliger p.o. Verabreichung (DL50), Unpublished report
of Schering AG., submitted by Ciba-Geigy
Herbert, R. A. (1969) Methyl and phenylcarbamates. In: Degradation of
herbicides (P. C. Kearney and D. D. Kaufman, ed.), p. 113, New York,
Marcel Dekker Inc.
Hurni, H. and Sachsse, K. (1969a) Report on the determination of the
acute oral LD50 to the dog of chlorphenamidine technical.
Unpublished report of Tierfarm AG, submitted by Ciba-Geigy
Hurni, H. and Sachsse, K. (1969b) Report on the determination of the
acute oral LD50 to the rat of Galecron 50 EC. Unpublished report of
Tierfarm AG, submitted by Ciba-Geigy
Hurni, H. and Sachsse, K. (1969c) Report on the determination of the
acute dermal LD50 to the rat of Galecron 50 SP. Unpublished report
of Tierfarm AG, submitted by Ciba-Geigy
Johnson. B, T. and Knowles, C. O. (1970) Microbial degradation of the
acaricide N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine. Bull.
Environ. Contam. Toxicol., 5: 158
Knowles, C. O. (1970) Metabolism of two acaricidal chemicals,
N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine (chlorphenamidine) and
m-{[dimethylamino methylene] amino} phenyl methyl-carbamate
hydrochloride (formetanate). J. Agr. Food Chem., 18: 1038
Knowles, C. O. (1970) Microbial degradation of chlordimeform. Bull
Envir. Contam. Toxicol., 5 (2): 158
Knowles, C. O. and Sen Gupta, A. K. (1969) Photodecomposition of the
acaricide N'-(4-chloro-o-tolyl) N,N-dimethylformamidine. J. econ.
Entomol., 62: 344
Knowles, C. O. and Sen Gupta, A. K. (1970)
N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine-14C (Galecron) and
4-chloro-o-toluidine metabolism in the white rat. J. econ. Entomol.
63: 856
Knowles, C. O., Sen Gupta, A. K. and Hassan, T. K. (1969a) Formation
of symmetrical azo derivatives of monochlorotoluidines by peroxidatic
catalysis. J. econ. Entomol., 62; 411
Knowles, C. O. and Sen Gupta, A. K. (1969b) Formation of symmetrical
azo derivative of 4-chloro-o-toluidine in a ferrous iron-hydrogen
peroxide system. J. econ. Entomol., 62: 1229
Kossmann, K. (1971) New aspects upon electron capture gas
chromatography of acylanilide, phenylcarbamate, and phenylurea type
herbicides. Proc. 2nd Intern. Congr. Pesticide Chemistry, Tel Aviv,
1971
Kossmann, K., Geissbühler, H. and Boyd, V. F. (1971) Specific
determination of chlorphenamidine
[N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine] and of some
potential metabolites in plant material by thin-layer and flame
ionisation gas chromatography. J. Agr. Food Chem., 19: 360
Lyon, D. A. (1970) Chlorphenamidine on weight gain of female rats.
Internal unpublished memorandum. Food and Drug Directorate, Canada
Mastri, C., Keplinger, M. L. and Francher, O. E. (1969) Acute oral
toxicity study on two samples of chlorphenamidine in male and female
albino rats. Unpublished report from Industrial Biotest Labs. Inc.
submitted by Ciba-Geigy
Oda. (1969) Suicide by drinking Galecron. Unpublished report from
Chikugo Municipal Hospital, made available by Nor-Rh Chemicals
Rose, J. A. (1969a) Degradation of chlorphenamidine by rat liver and
spleen homogenatis with particular reference to the possible formation
of azo-derivatives. Unpublished report submitted by Ciba-Geigy
Rose, J. A. (1969b) Formation of a symmetrical azo-derivative from
4-chloro-o-toluidine by coupled peroxidatic reactions. Unpublished
report submitted by Ciba-Geigy
Rosen, J. D., Siewierski, M. and Winnett, G. (1970) FMN-sensitized
photolyses of chloroanilines. J, Agr. Food Chem., 18: 494
Sachsse, K. and Bathe, R. (1970a) Report on the determination of the
acute oral LD50 of formylchlorotholuidine to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Giegy
Sachsse, K, and Bathe, R. (1970b) Report an the determination of the
dermal LD50 of formylchlorotoluidine to the rat. Unpublished report
of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970c) Report on the determination of the
acute oral LD50 of 5-chloro-2-amino-toluene (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1970d) Report on the determination of the
acute oral LD50 of 5-chloro-2-amino toluene-HCl to the rat.
Unpublished report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970e) Report on the determination of the
acute dermal LD50 of 5-chloro-2-amino-toluene-HCl to the rat.
Unpublished report of Tierfarm A.G. submitted by Ciba Geigy
Sachsse, K. and Bathe, R. (1970f) Report on the determination of the
acute oral LD50 of phenamidine-HCl to the rat. Unpublished report of
Tierfarm AG submitted by Ciba-Geigy
Sachsse. K. and Bathe, R. (1970g) Report on the determination of the
acute dermal LD50 of phenamidine-HCl to the rat. Unpublished report
of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970h) Report on the determination of the
acute oral LD50 of o-chlorphenamidine-HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970i) Report on the determination of the
acute dermal LD50 of o-chlorphenamidine-HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970j) Report on the determination of the
acute oral LD50 of dichlorphenamidine-HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970k) Report on the determination of the
acute dermal LD50 of dichlorphenamidine HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970l) Report on the determination of the
acute dermal LD50 of technical chlorphenamidine (base) to the rat.
Unpublished report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1971a) Report on the determination of the
acute oral LD50 of technical C8520 to the rat. (Dimethyl
chlordimeform). Unpublished report of Tierfarm AG submitted by
Ciba-Geigy
Sachsse, K. and Bathe, R. (1971b) Report on the determination of the
acute dermal LD50 of technical C8520 to the rat. Unpublished report
of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1971c) Report on the determination of the
acute dermal LD50 of 5-chloro-2-amino toluene (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe. R. (1971d) Report on the determination of the
acute oral LD50of phenamidine (base) to the rat. Unpublished report
of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971e) Report on the determination of the
acute dermal LD50 of phenamidine (base) to the rat. Unpublished
report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971f) Report on the determination of the
acute oral LD50 of o-chlorphenamidine (base) to the rat. Unpublished
report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971g) Report on the determination of the
acute dermal LD50 of o-chlorphenamidine (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971h) Report on the determination of the
acute oral LD50 of dichlorphenamidine (base) to the rat. Unpublished
report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971i) Report on the determination of the
acute dermal LD50 of dichlorphenamidine (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971j) Report on the determination of the
acute dermal LD50 of Galecron 50EC to the rat. Unpublished report of
Ciba-Geigy
Schering AG. (1965) Versuche zur Kenntnis des Eindringvermögens von
36268 in Aepfel
Schering AG. (1966a) Chlorphenamidin. Stoffwechselversuche Stand Dez.
1966
Schering AG. (1966b) Rückstandsuntersuchungen Schering 36268. 1.
Mitteilung 1966. Erntegut: Weintrauben, Aepfel, Birnen, Pfirsich
Schering AG. (1967) Chlorphenamidine. Stoffwechselversuche Stand Dez.
1967
Schering AG. (1968) Rückstandsbestimung von Chlorphenamidin (Schering
3628) in Pflanzenmaterial, 36 268/6, 13.9.1968 (unpublished)
Schering AG. (1969) Chlorphenamidin 1/69. Abbaugeschwindigkeit von
Chlorphenamidin-Hydrochlorid im Boden
Schering AG. (1969a) Chlorphenamidin III/69, Verfahrensausbeuten nach
36 268/6, 17.3.1969 (unpublished)
Schering AG. (1969b) Method for the determination of Fundal(R) forte
residues in plant material using thin-layer chromatography,
36 056 - 36 268/1, 18.11.1969
Sen Gupta, A. K. and Knowles, C. O. (1969) Metabolism of
N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine by apple seedlings.
J. Agr. Food Chem., 17: 595
Sen Gupta, A. K. and Knowles, C. O. (1970) Galecron-14C
[N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine] metabolism in the
dog and goat. J. Econ. Entomol., 63: 951
Shionogi and Co. (Undated) Unpublished report of the Aburahi Labs. of
Shionogi and Co. Ltd,
Surber, W. and Cerioli, A. (1966) Prolonged toxicity study on rats of
Ciba's preparation, 39, 12. (C8514). Unpublished report from Battelle
Institute submitted by Ciba-Geigy
Teeters, W. R. and Blackmore, R. H. (1968) Pharmacological study of
chlorphenamidine in the perfused rabbit heart, isolated guinea-pig
ileum, and dog pharmacodynamic preparation. Hazelton Laboratories
report C5230, made available by Nor-Am. Chemicals
Tripod, J. (1967) Unpublished report from Ciba Ltd., made available by
Nor-Am Chemicals
Voss, G. and Burkhard, N. (1971) Unpublished report submitted by
Ciba-Geigy
Weatherholtz, W. M. (1970) Statistical analysis of body weight data
and food consumption data, chlorphenamidine. Unpublished report from
Hazelton Labs. Inc. made available by Nor-Am. Agricultural Products
Ltd.
Weir, R. J. (1967) Unpublished report from Hazelton Labs. made
available by Nor-Am. Chemicals
Weir, R. J. (1968) Acute oral administration - rats. Unpublished
report from Hazelton Labs., made available by Nor-Am. Chemicals
Williams, C. T. (1959) Detoxification mechanisms. London, Chapman and
Hall
Witkonton, Sujit. (1969) The fate and persistence of chlorphenamidine
on six different crops. Thesis for degree of Master of Science,
Department of Entomology, Pennsylvania State University, September
1969 (53 pp)
Whereas in leafy tissues, stems or green fruits, the whole pathway of
degradation is operative to a small extent, in ripe fruits only
N-formyl-4-chloro-o-toluidine has consistently been observed as a
metabolite. The residue methods designed account for all metabolites
and/or conjugates (Geissbühler et al., 1971; Kossman et al., 1971).
In the course of an extensive study of the fate and persistence of
chlordimeform on six different crops Witkonton (1969) found that the
total amount of residue is directly related to the amount of chemical
applied, an inverse function of the number of days the fruit was
sampled after the last application of the chemical and influenced by
the nature of the fruit surface.
Fruit was analysed for chlordimeform and three possible major
degradation products. Only the parent compound and one degradation
product, N-formyl-2-methyl-4-chloroaniline, could be detected.
N-formyl-2-methyl-4-chloroaniline was found only in small amounts in
apples, pears and cherries; but greater amounts were found in plums
and strawberries. The results of this investigation did not show any
real correlation between the amounts of the parent compound and
N-formyl-2-ethyl-4-chloroaniline to such variables as amount of
chemical applied or sampling date. The type of fruit, and especially
the nature of the fruit surface, and such factors as sunlight and
rainfall appear to be predominant in affecting the degradation of
chlordimeform.
Witkonton (1969) also reported that chlordimeform appears to adhere to
the outer surface of fruit and does not appear to translocate readily
to the fleshy parts, The chief factors which seem to account for the
decrease of chlordimeform residues in fruit appear to be
volatilization, weathering and growth dilution.
In soil
Chlordimeform deposited inadvertently on soil surfaces after spray
application may be expected to be dissipated by the following
processes: volatilization, chemical hydrolysis, photodecomposition
(Knowles and Sen Gupta, 1969) and microbial degradation. Although no
special volatilization experiments with soil samples were conducted,
the contribution of this process is likely to be of the same order of
magnitude as or slightly higher than that observed for leaf surfaces.
Hydrolysis of chlordimeform to N-formyl-4-chloro-o-toluidine (III)
shown to be substantial under the slightly acid or alkaline conditions
which normally prevail in soils (Kossmann et al., 1971).
As regards microbial degradation of the acaricide, Johnson and Knowles
(1970) demonstrated that several bacterial, actinomycete and fungus
species are capable of extensively degrading chlordimeform. The
principal metabolite in the culture media of most species was
N-formyl-4-chloro-o-toluidine, except for the actinomycete
Streptomyces griseus which formed mainly 4-chloro-o-toluidine.
None of the microbes formed symmetrical azo-compounds.
Under field conditions, chlordimeform and its 4-chloro-o-toluidine
containing metabolites were dissipated according to first order
reactions with half-lives ranging from 20 to 40 days (Schering, 1969;
Ciba, 1969e). From the present experiments it way be concluded that
chlordimeform is not accumulated in the soil.
Evidence of residues in food in commerce or at consumption
No data were available to indicate the incidence or level of
chlordimeform residues in food commodities moving in commerce.
A number of detailed studies have been conducted to identify terminal
residues after application of chlordimeform. Results indicate that
breakdown of the parent compound takes place particularly in tissues
which are metabolically active, such as young bean leaves, while
fruits like apples and prunes show practically no metabolic breakdown
of the acaricide. The hydrolytic pathway of degradation leading to
small amounts of N-formyl-4-chloro-o-toluidine has been demonstrated
to occur to a certain extent also on fruit crops. Hydrolysis of
chlordimeform, however, apparently takes place independent of plant
tissue activities due to the chemical instability of the parent
compound (Kossmann et al., 1971),
Furthermore it has been confirmed that
2,2'-dimethyl-4,4'-dichloroazobenzene is not expected to be a
potential transformation product of chlordimeform in apple fruit.
All chlordimeform metabolites identified so far in various plant
materials are determined by the described total residue methods.
A number of special investigations reveal that chlordimeform residues
are located in the outer parts of crops such as fruit peels or outer
leaves of cole crops. Excessive residues therefore might be removed by
peeling of fruit (apples, citrus) or by trimming the outer leaves of
cole crops.
Washing of fruit will remove only a small part of the total residue.
External residues will probably dissipate by volatilization, rainfall,
or penetration into the cuticular wax layers,
Schering (1971) reports on the effect of cooking on chlordimeform
residues in apples, grapes, tomatoes, cauliflower, beans and sugar
beet foliage and shows that the behaviour of chlordimeform residues
during cooking depends on the pH value of the aqueous suspension as
well as on the duration of the cooking period.
This report shows that the rate of loss of chlordimeform is a function
of pH value, the loss being much more rapid in a neutral medium such
as green beans (pH 5) or cauliflower (pH 6) than in apples (pH 2.5) or
tomatoes (pH 3). The hydrolysis proceeds according to the scheme
outlined in page 30 and all metabolites are determined by the
analytical method for chlordimeform parent compound. The loss of total
4-chloro-o-toluidine moiety is not significant showing that
volatilization in steam is not an important contributing factor.
Methods of residue analysis
(a) Methods for the detection of unchanged chlordimeform and its
breakdown products (terminal residues)
Identification of terminal residues in plant materials and specific
determination of unchanged chlordimeform has been carried out by
thin-layer and/or gas chromatography procedures. A detailed
description of the separation and detection of
N'-(4-chloro-o-tolyl)-N-methylformamidine,
N-formyl-4-chloro-o-toluidine, and 4-chloro-o-toluidine as well as
the parent compound including quantitation by colorimetry or flame
ionization gas chromatography has been given by Kossmann et al.
(1971).
Investigations for the presence of
2,2'-dimethyl-4,4'-dichloroazobenzene have been performed by a gas
chromatographic procedure following reductive cleavage of the azo
compound and subsequent Sandmeyer iodination of the resulting
4-chloro-o-toluidine (Geissbühler et al., 1971).
Selective determination of unchanged chlordimeform may be needed after
usage of chlordimeform/formetanate mixtures. Both active substances
have the structure of formamidine derivatives. For this combination
product a thin-layer chromatographic procedure has been established
(Schering AG, 1969b) which includes direct quantitation on the
chromatoplate by reflectance measurement in the UV range. Gas
chromatographic procedures described above allow specific
determination of chlordimeform in presence of formetanate as well and
account then for the total chlordimeform residue including
metabolites.
(b) Total chlordimeform residue methods
For regulatory purposes and routine residue analysis methods were
developed that account in a single procedure for chlordimeform and for
all its 4-chloro-o-toluidine containing breakdown products and/or
conjugates. Principle of determination of "total chlordimeform" is
based on a two step hydrolysis by successive treatments with acetic
acid and sodium hydroxide respectively. The joint hydrolysis product,
4-chloro-o-toluidine, is steam distilled and extracted into
iso-octane. Quantitation of 4-chloro-o-toluidine may be carried out
by colorimetry (specificity limited) or gas chromatography (highly
specific).
Colorimetry is based upon diazotization reaction of
4-chloro-o-toluidine and coupling with N-ethyl-1-naphthylamine
(Geissbühler et al., 1971). A number of investigations has been made
by using 1-naphthylethylenediamine as a coupling agent (Schering AG,
1968). Both procedures are sensitive to 0.05 ppm of chlordimeform as
calculated for a 50-gram crop sample. Recovery values obtained from
various crops fortified within the range of 0.05 to 5 ppm
chlordimeform in general exceeded 85% (Geissbühler et al., 1971;
Schering AG, 1969a).
An interlaboratory study demonstrated the sufficient reproducibility
of the total residue procedures established. Since specificity of
colorimetric evaluation is limited, additional identification of
chlordimeform residues may be done by confirmatory thin-layer
chromatography of the resulting azo dye on cellulose plates
(Geissbühler et al., 1971).
Gas chromatography has also been effectively used for quantitative
determination of 4-chloro-o-toluidine moiety following
transformation into halogenated derivatives of extremely high electron
affinity. Sandmeyer iodination reaction of 4-chloro-o-toluidine
results in the 5-chloro-2-iodo-toluene structure and a 0.05 ppm
detection limit for chlordimeform by using a tritium foil equipped
electron capture detector. Recovery figures correspond very well to
those reported for colorimetric measurement (Geissbühler et al.,
1971).
A lower detection limit may be attainable by bromination of
4-chloro-o-toluidine in aqueous solution yielding
6-bromo-4-chloro-o-toluidine and subsequent gas chromatographic
determination using a Ni63 electron capture detector (Kossmann,
1971),
Direct gas chromatographic measurement of 4-chloro-o-toluidine by a
halogen microcoulometric titration cell has been reported by Del Monte
Corporation (Del Monte, 1969) for the determination of chlordimeform
residues in crops.
All the gas chromatographic procedures mentioned are specific for
chlordimeform residues. Up to date no other pesticide with the
4-chloro-o-toluidine nucleus is known to be used in agricultural
practice.
Examples of national tolerances
The following are examples of national tolerances and withholding
periods that have been established:
Country Crop Tolerance Withholding
ppm period days
Australia pome fruit 2 7
stone fruit 2 7
strawberries 1 7
fat of meat of cattle 0.5 1
Country Crop Tolerance Withholding
ppm period days
Canada pears 5 28
peaches, plums, prunes 4 14
apples 3 14
cauliflower, brussels
sprouts 3 28
broccoli 2 28
cabbage 0.5 28
turnip roots <0.05 28
Germany (GFR) grapes, stone fruit
(excl. cherries) 3 14
pome fruit 2
Italy fruit, citrus, grapes 1 20
New Zealand pome fruit 1 14
strawberries 7
Switzerland pome fruit, grapes 1 42
South Africa apples, pears, citrus 1 14
USA pears 5 28
apples 3 14
cabbage, cauliflower,
broccoli, brussels
sprouts 2 28
Venezuela fruit, citrus 2 14
Appraisal
Chlordimeform is a new insecticide/acaricide primarily effective
against eggs and larvae of spider mites with adequate activity also
against adult mites. It is effective against mites resistant to
organo-phosphorus insecticides. It kills eggs, larvae and adults not
only by contact but also in the vapour phase. Penetration and slight
systemic effect have been demonstrated. It is also effective in
control of some lepidopterous insects including codling moth but the
major field of use so far developed is in the control of mites on
deciduous fruit. Chlordimeform is also being used commercially in
Australia for the control of cattle tick by spraying and dipping
because of its ability to control ticks which have developed strains
resistant to all other currently available acaricides.
Chlordimeform is registered for use on fruit trees, grapes and
vegetables in many countries and to a lesser extent on cotton and
hops. The concentration applied ranges from 0.01 to 0.1%.
Chlordimeform is used both as the base and as the hydrochloride. The
base is formulated as an emulsifiable solution while the hydrochloride
is used as a water soluble powder.
The residue data available to the meeting were obtained from
supervised field trials in Australia, Canada, England, France,
Germany, Italy, New Zealand, South Africa, Spain, Switzerland and the
United States of America. Whilst there is a sharp drop in the residue
level between the day of application and the second or third day
post-treatment, thereafter the rate of decline is remarkably slow with
a half-life on apples, grapes, pears and tomatoes exceeding 21 days.
The rate of decline is somewhat faster on cruciferous vegetables but
even so the interval between application and harvest does not have a
pronounced bearing on the residues level following approved
treatments.
Loss of residue is initially due to volatilization but the portion of
the deposit absorbed or dissolved into surface tissues of plants
appears to remain at a relatively constant level. The residue data
reflect this durability but they are influenced by the fact that the
analytical procedure determines both parent compound and all
metabolites containing the toluidine moiety.
Extensive evidence is available on the fate of residues in plants and
animals as well as on the route and rate of degradation. The initial
pathway of metabolism in animals and plants is substantially similar.
leading to N-formyl-4-chloro-o-toluidine and 4-chloro-o-toluidine
in both organisms but proceeding further to
N-formyl-5-chloroanthranilic acid and 5-chloroanthranilic acid
respectively in animals.
Some data were available on the effect of processing and cooking as
the fate of chlordimeform residues. The chlordimeform residue
generally decreases with cooking, the decline being dependent upon
length of cooking time. The rate of decrease is a function of the pH
value. In a more neutral medium caused by green beans (pH 5) or
cauliflower (pH 6) loss of chlordimeform is much more rapid than in an
acid medium such as apples (pH 2.5) or tomatoes (pH 3). Studies
revealed no detectable change in the residue level or composition when
apples and pears were held in cold storage. Evidence was presented to
show that substantially all the residue occurs in the skin of apples
and citrus; in the outer leaves of cruciferous vegetables and in the
tops of root vegetables. Chlordimeform, applied to cotton, appears as
a residue in cotton seed cake as well as in cotton seed oil and the
residue level is not significantly reduced by refining.
Analytical methods based on the determination of
4-chloro-o-toluidine formed by successive treatments with acetic
acid and sodium hydroxide are specific for chlordimeform and its
metabolites and are capable of determining levels down to 0.05 ppm.
Quantitation may be made either by colorimetry based upon
diazotization and coupling with N-ethyl-1-naphthylamine or by gas
chromatography. Though direct measurement by gas chromatography is
possible, the accuracy and sensitivity is improved by transformation
of the residue into halogenated derivatives of extremely high electron
affinity.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Temporary tolerances (effective to June 1975)
Pears, peaches, prunes 5 ppm
Apples, grapes, plums, strawberries 3 ppm
Brassicas, cherries, citrus fruit,
cotton seed oil (crude and refined),
cotton seed 2 ppm
Beans 0.5 ppm
Fat, meat and meat products of cattle 0.5 ppm
milk (whole) 0.05 ppm
Butter 0.5 ppm
Further work or information
Required (before 30 June 1975)
1. A further long-term feeding study in rats to obtain precise
information on the incidence and nature of the histopathological
changes in the liver and bile ducts of rats exposed to levels
above and below 100 ppm in the diet. An effort should be made to
explain the changes in organ to body-weight ratios noted in the
previous long-term rat study.
2. Further investigation of the nature of the hepatic lesions
observed in the dog.
3. Metabolic studies in several animal species, preferably including
man.
4. Further data on the nature and levels of residues in animal
tissues after use in cattle sprays and dips.
5. Further data on residues in milk after use in cattle sprays and
dips, especially information on the nature of the residues and
their distribution between aqueous and lipid phases.
6. Data on the residue levels in commercial butter and cheese.
Desirable
1. Further studies on the haematological effects.
2. Elucidation of the pharmacodynamic activity on the heart,
including the potentiation of the effects of pressor amines.
3. Further data on the disappearance of residues during storage,
processing, and cooking.
4. Data on residue levels in raw agricultural commodities moving in
commerce.
REFERENCES
Aoki, H. and Ueda, K. (1966) Unpublished report of the Tokyo Dental
College submitted by Ciba-Geigy
Bertha, R. and Framer, D. (1967) Pesticide transformation to aniline
and azo compounds in soil. Science, 156: 1617
Blackmore, R. H. (1969a) Three generation reproduction study - rats.
Unpublished report of Hazelton Laboratories, submitted by Ciba-Geigy
Blackmore, R. H. (1969b) Teratology study - rabbits. Unpublished
report of Hazelton Laboratories submitted by Ciba-Geigy
Blackmore, H. H. (1969c) Two year repeated feeding - dogs. Schering
361 268 (Chlorphenamidine). Unpublished report from Hazelton
Laboratories, submitted by Ciba-Geigy
Blackmore, R. H. (1969d) Two year repeated feeding - rats. Schering
361 268 (Chlorphenamidine). Unpublished report from Hazelton
Laboratories, submitted by Ciba-Geigy
Ciba Ltd. (1965a) C-8514. Rückstände in den Blättern der Bohne. Report
of 20 May 1965
Ciba Ltd. (1965b) C-8514. Residues in Australian apples and pears.
Report of 25 May 1965
Ciba Ltd. (1967a) Modell einer Synthese von radioaktivem Galecron.
Report of 17 August 1967
Ciba Ltd. (1967b) Galecron. Absence of 4-chloro-o-toluidine as a
metabolite in prunes after overdose spraying. Report of 31 January
1967
Ciba Ltd. (1968) Galecron. Behaviour of different formulations on
citrus leaves. Report of 14 May 1968
Ciba Ltd. (1969a) Galecron. Residues in Italian oranges. Report of 2
March 1969
Ciba Ltd. (1969b) Galecron. Analysis of Swiss apples for residues of
2,2'-dimethyl-4,4'-dichloroazobenzene after overdose spraying. Report
of 20 November 1969.
Ciba Ltd. (1969c) Galecron. Formation of a symmetrical azo derivative
from 4-chloro-o-toluidine by coupled peroxidatic reactions. Report
of 12 September 1969
Ciba Ltd. (1969d) Galecron. Degradation of chlorphenamidine by rat
liver and spleen homogenates, with particular reference to the
possible formation of azo derivatives. Report of 10 September 1969
Ciba Ltd. (1969e) Galecron. Dissipation in a Swiss soil as determined
by chemical analysis. Report of 16 September 1969
Ciba-Geigy Ltd. (1971) Galecron. Residues in milk, meat, fat, liver
and kidney of Swiss cows. Report of 19 March 1971
Ciba-Geigy Ltd. (1971) Determination of chlorphenamidine residues in
milk and butter following treatment with "Spike" cattle dip at 0.05%
a.i. w/v. Report of 6 August 1971
Del Monte Corporation Research Center. (1969) Determination of total
chlorphenamidine residues in plant material, 10.6.1969. Unpublished
Dittrich, V. (1971) Biological action of chlordimeform. Report
prepared for FAO/WHO
Ehrhardt, D. A. and Knowles, C. O. (1970) Metabolism and translocation
of N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine (chlorphenamidine)
and its hydrochloride salt in grapefruit seedlings. J. Econ. Entomol.,
63: 1306
Geissbühler, H. (1969) The substituted ureas. In: Degradation of
herbicides (P. C. Kearney and D. D. Kaufman, ed.), p. 79. New York,
Marcel Dekker Inc.
Geissbühler, H., Kossmann, K., Baunok, I. and Boyd, V. F. (1971)
Determination of total residues of chlorphenamidine
[(N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine] in plant and soil
material by colorimetry and thin-layer and electron capture gas
chromatography. J. Agr. Food Chem., 19: 365
Gerhards, E. and Kolb. (1966) Unpublished report of Schering A.G.
submitted by Ciba-Geigy
Gerhards, E. (1967) Unpublished report of Schering A.G. submitted by
Ciba-Geigy
Gunzel, P. and Richter. (1965) Systemische Vertraglichkeitsprufung an
Ratten bei einmaliger p.o. Verabreichung (DL50). Unpublished report
from Schering A.G. submitted by Ciba-Geigy
Gunzel, P. and Richter. (1966a) Systemische Vertraglichkeitsprufung an
Ratten bei einmaliger dermaler. Verabreichung (DL50). Unpublished
report of Schering AG, submitted by Ciba-Geigy
Gunzel, P. and Richter. (1966b) Systemische Vertraglichkeitsprufung an
Kaninchen bei einmaliger dermaler Verabreichung (DL50). Unpublished
report of Schering A.G., submitted by Ciba-Geigy
Gunzel, P. and Richter. (1968) Systemische Vertraglichkeitsprufung an
Hunden bei einmaliger p.o. Verabreichung (DL50). Unpublished report
from Schering AG., submitted by Ciba-Geigy
Gunzel, P. and Richter. (1969) Systemische Vertraglichkeitsprufung an
Ratten bei einmaliger p.o. Verabreichung (DL50), Unpublished report
of Schering AG., submitted by Ciba-Geigy
Herbert, R. A. (1969) Methyl and phenylcarbamates. In: Degradation of
herbicides (P. C. Kearney and D. D. Kaufman, ed.), p. 113, New York,
Marcel Dekker Inc.
Hurni, H. and Sachsse, K. (1969a) Report on the determination of the
acute oral LD50 to the dog of chlorphenamidine technical.
Unpublished report of Tierfarm AG, submitted by Ciba-Geigy
Hurni, H. and Sachsse, K. (1969b) Report on the determination of the
acute oral LD50 to the rat of Galecron 50 EC. Unpublished report of
Tierfarm AG, submitted by Ciba-Geigy
Hurni, H. and Sachsse, K. (1969c) Report on the determination of the
acute dermal LD50 to the rat of Galecron 50 SP. Unpublished report
of Tierfarm AG, submitted by Ciba-Geigy
Johnson. B, T. and Knowles, C. O. (1970) Microbial degradation of the
acaricide N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine. Bull.
Environ. Contam. Toxicol., 5: 158
Knowles, C. O. (1970) Metabolism of two acaricidal chemicals,
N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine (chlorphenamidine) and
m-{[dimethylamino methylene] amino} phenyl methyl-carbamate
hydrochloride (formetanate). J. Agr. Food Chem., 18: 1038
Knowles, C. O. (1970) Microbial degradation of chlordimeform. Bull
Envir. Contam. Toxicol., 5 (2): 158
Knowles, C. O. and Sen Gupta, A. K. (1969) Photodecomposition of the
acaricide N'-(4-chloro-o-tolyl) N,N-dimethylformamidine. J. econ.
Entomol., 62: 344
Knowles, C. O. and Sen Gupta, A. K. (1970)
N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine-14C (Galecron) and
4-chloro-o-toluidine metabolism in the white rat. J. econ. Entomol.
63: 856
Knowles, C. O., Sen Gupta, A. K. and Hassan, T. K. (1969a) Formation
of symmetrical azo derivatives of monochlorotoluidines by peroxidatic
catalysis. J. econ. Entomol., 62; 411
Knowles, C. O. and Sen Gupta, A. K. (1969b) Formation of symmetrical
azo derivative of 4-chloro-o-toluidine in a ferrous iron-hydrogen
peroxide system. J. econ. Entomol., 62: 1229
Kossmann, K. (1971) New aspects upon electron capture gas
chromatography of acylanilide, phenylcarbamate, and phenylurea type
herbicides. Proc. 2nd Intern. Congr. Pesticide Chemistry, Tel Aviv,
1971
Kossmann, K., Geissbühler, H. and Boyd, V. F. (1971) Specific
determination of chlorphenamidine
[N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine] and of some
potential metabolites in plant material by thin-layer and flame
ionisation gas chromatography. J. Agr. Food Chem., 19: 360
Lyon, D. A. (1970) Chlorphenamidine on weight gain of female rats.
Internal unpublished memorandum. Food and Drug Directorate, Canada
Mastri, C., Keplinger, M. L. and Francher, O. E. (1969) Acute oral
toxicity study on two samples of chlorphenamidine in male and female
albino rats. Unpublished report from Industrial Biotest Labs. Inc.
submitted by Ciba-Geigy
Oda. (1969) Suicide by drinking Galecron. Unpublished report from
Chikugo Municipal Hospital, made available by Nor-Rh Chemicals
Rose, J. A. (1969a) Degradation of chlorphenamidine by rat liver and
spleen homogenatis with particular reference to the possible formation
of azo-derivatives. Unpublished report submitted by Ciba-Geigy
Rose, J. A. (1969b) Formation of a symmetrical azo-derivative from
4-chloro-o-toluidine by coupled peroxidatic reactions. Unpublished
report submitted by Ciba-Geigy
Rosen, J. D., Siewierski, M. and Winnett, G. (1970) FMN-sensitized
photolyses of chloroanilines. J, Agr. Food Chem., 18: 494
Sachsse, K. and Bathe, R. (1970a) Report on the determination of the
acute oral LD50 of formylchlorotholuidine to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Giegy
Sachsse, K, and Bathe, R. (1970b) Report an the determination of the
dermal LD50 of formylchlorotoluidine to the rat. Unpublished report
of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970c) Report on the determination of the
acute oral LD50 of 5-chloro-2-amino-toluene (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1970d) Report on the determination of the
acute oral LD50 of 5-chloro-2-amino toluene-HCl to the rat.
Unpublished report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970e) Report on the determination of the
acute dermal LD50 of 5-chloro-2-amino-toluene-HCl to the rat.
Unpublished report of Tierfarm A.G. submitted by Ciba Geigy
Sachsse, K. and Bathe, R. (1970f) Report on the determination of the
acute oral LD50 of phenamidine-HCl to the rat. Unpublished report of
Tierfarm AG submitted by Ciba-Geigy
Sachsse. K. and Bathe, R. (1970g) Report on the determination of the
acute dermal LD50 of phenamidine-HCl to the rat. Unpublished report
of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970h) Report on the determination of the
acute oral LD50 of o-chlorphenamidine-HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970i) Report on the determination of the
acute dermal LD50 of o-chlorphenamidine-HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970j) Report on the determination of the
acute oral LD50 of dichlorphenamidine-HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970k) Report on the determination of the
acute dermal LD50 of dichlorphenamidine HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970l) Report on the determination of the
acute dermal LD50 of technical chlorphenamidine (base) to the rat.
Unpublished report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1971a) Report on the determination of the
acute oral LD50 of technical C8520 to the rat. (Dimethyl
chlordimeform). Unpublished report of Tierfarm AG submitted by
Ciba-Geigy
Sachsse, K. and Bathe, R. (1971b) Report on the determination of the
acute dermal LD50 of technical C8520 to the rat. Unpublished report
of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1971c) Report on the determination of the
acute dermal LD50 of 5-chloro-2-amino toluene (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe. R. (1971d) Report on the determination of the
acute oral LD50of phenamidine (base) to the rat. Unpublished report
of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971e) Report on the determination of the
acute dermal LD50 of phenamidine (base) to the rat. Unpublished
report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971f) Report on the determination of the
acute oral LD50 of o-chlorphenamidine (base) to the rat. Unpublished
report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971g) Report on the determination of the
acute dermal LD50 of o-chlorphenamidine (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971h) Report on the determination of the
acute oral LD50 of dichlorphenamidine (base) to the rat. Unpublished
report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971i) Report on the determination of the
acute dermal LD50 of dichlorphenamidine (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971j) Report on the determination of the
acute dermal LD50 of Galecron 50EC to the rat. Unpublished report of
Ciba-Geigy
Schering AG. (1965) Versuche zur Kenntnis des Eindringvermögens von
36268 in Aepfel
Schering AG. (1966a) Chlorphenamidin. Stoffwechselversuche Stand Dez.
1966
Schering AG. (1966b) Rückstandsuntersuchungen Schering 36268. 1.
Mitteilung 1966. Erntegut: Weintrauben, Aepfel, Birnen, Pfirsich
Schering AG. (1967) Chlorphenamidine. Stoffwechselversuche Stand Dez.
1967
Schering AG. (1968) Rückstandsbestimung von Chlorphenamidin (Schering
3628) in Pflanzenmaterial, 36 268/6, 13.9.1968 (unpublished)
Schering AG. (1969) Chlorphenamidin 1/69. Abbaugeschwindigkeit von
Chlorphenamidin-Hydrochlorid im Boden
Schering AG. (1969a) Chlorphenamidin III/69, Verfahrensausbeuten nach
36 268/6, 17.3.1969 (unpublished)
Schering AG. (1969b) Method for the determination of Fundal(R) forte
residues in plant material using thin-layer chromatography,
36 056 - 36 268/1, 18.11.1969
Sen Gupta, A. K. and Knowles, C. O. (1969) Metabolism of
N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine by apple seedlings.
J. Agr. Food Chem., 17: 595
Sen Gupta, A. K. and Knowles, C. O. (1970) Galecron-14C
[N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine] metabolism in the
dog and goat. J. Econ. Entomol., 63: 951
Shionogi and Co. (Undated) Unpublished report of the Aburahi Labs. of
Shionogi and Co. Ltd,
Surber, W. and Cerioli, A. (1966) Prolonged toxicity study on rats of
Ciba's preparation, 39, 12. (C8514). Unpublished report from Battelle
Institute submitted by Ciba-Geigy
Teeters, W. R. and Blackmore, R. H. (1968) Pharmacological study of
chlorphenamidine in the perfused rabbit heart, isolated guinea-pig
ileum, and dog pharmacodynamic preparation. Hazelton Laboratories
report C5230, made available by Nor-Am. Chemicals
Tripod, J. (1967) Unpublished report from Ciba Ltd., made available by
Nor-Am Chemicals
Voss, G. and Burkhard, N. (1971) Unpublished report submitted by
Ciba-Geigy
Weatherholtz, W. M. (1970) Statistical analysis of body weight data
and food consumption data, chlorphenamidine. Unpublished report from
Hazelton Labs. Inc. made available by Nor-Am. Agricultural Products
Ltd.
Weir, R. J. (1967) Unpublished report from Hazelton Labs. made
available by Nor-Am. Chemicals
Weir, R. J. (1968) Acute oral administration - rats. Unpublished
report from Hazelton Labs., made available by Nor-Am. Chemicals
Williams, C. T. (1959) Detoxification mechanisms. London, Chapman and
Hall
Witkonton, Sujit. (1969) The fate and persistence of chlorphenamidine
on six different crops. Thesis for degree of Master of Science,
Department of Entomology, Pennsylvania State University, September
1969 (53 pp)
See Also:
Toxicological Abbreviations
Chlordimeform (EHC 199, 1998)
Chlordimeform (ICSC)
Chlordimeform (WHO Pesticide Residues Series 5)
Chlordimeform (Pesticide residues in food: 1978 evaluations)
Chlordimeform (Pesticide residues in food: 1979 evaluations)
Chlordimeform (Pesticide residues in food: 1980 evaluations)
Chlordimeform (Pesticide residues in food: 1985 evaluations Part II Toxicology)
Chlordimeform (Pesticide residues in food: 1987 evaluations Part II Toxicology)
Chlordimeform (IARC Summary & Evaluation, Volume 30, 1983)
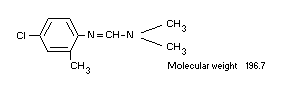 Other information on identity and properties
Chlordimeform is used as a free base or as its hydrochloride salt.
Physical properties of both base and hydrochloride salt are given
below.
Chlordimeform Chlordimeform
base hydrochloride
Melting point 32°C 225-227°C
Vapour pressure (20°) 3.5 × 10-4 Torr 2.2 × 10-7 Torr
Solubility in water 250 ppm >50%
chloroform >20% 1-2%
hexane >20% 0.1%
Chlordimeform base is applied as an emulsifiable solution while the
hydrochloride is used as a water-soluble powder.
Technical chlordimeform hydrochloride has a purity of a least 96%. The
major impurities are 2, methyl-4-chlorformamidine,
4-chloro-o-toluidine-hydrochloride and sodium chloride.
Chlordimeform is rather stable in strong acids. It is readily
hydrolyzed, however, in weakly-acid to weakly alkaline solutions. Its
half-life in water containing 5% of methanol was determined to be 42
hours at pH 7 (30°C) and five hours at pH 9 (30°C) respectively.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Table I shows the potential metabolites for chlordimeform.
Four 120 g male rats treated orally with 270 µg of phenyl
H3-labelled (tritiated) chlordimeform secreted 19.74 to 23.03% of
the dose in bile over a 24-hour period. Groups of four 130 g female
rats similarly treated eliminated 52.8% (range 41.8-59.6%) and 2.5%
(range 0.13-5.30%) in urine and faeces respectively in 24 hours. A
pair of rats observed for 14 hours eliminated 66.2, and 64.9%
administered H3 label in urine, and 11.4 and 14.9% administered H3
label in faeces. Twenty-four hour elimination following intravenous
injection of 270 µg phenyl H3-labelled chlordimeform in rat
comprised 53.7% (range 52.0-55.6%) and 1.42% (range 1.19-1.84%)
administered H3, in urine and faeces respectively (Gerhards and
Kolb, 1966).
The urine from a male rat collected over 72 hours subsequent to oral
administration of 1.1 mg H3-labelled chlordimeform contained 49% of
the administered H3 label. Free extractables comprised 22% of the
H3 label, 10% was in the water phase and 17% was reactive to form
extractable glucuronides. The free extractable H3 label comprised
chlordimeform, 4-chloro-o-toluidine (IV),
N-formyl-4-chloro-o-toluidine (III),
N'-(4-chloro-o-tolyl)-N-methyl-formamidine (II). Glucuronides were
based on the same compounds found as free extractables. The author
notes that identified compounds may be formed after secretion of the
urine (Gerhards, 1967).
Single oral dosing of groups of three 120 g male rats with 270 µg
phenyl H3-labelled chlordimeform resulted in residues of
administered H3 label (expressed as percentage of dose/g of tissue)
in liver (0.29%), kidney (0.22%) and lymph nodes (0.13%) after eight
hours. After 24 hours, 0.35% and 0.13% of administered H3 were
present per g of tissue in gastrointestinal tract (and content) and
liver respectively. All other tissues contained less than 0.1% H3
label/g at eight hours, and less than 0.06% H3 label at 24 hours.
Twenty-four hour urine and faeces contained 57.5 and 3.9% of the total
administered H3 label respectively compared with 46.4% and 4.3% from
rats sacrificed at eight hours (Gerhards and Kolb, 1966).
Three 120 g male rats were intubated with 270 µg phenyl H3-labelled
chlordimeform for seven consecutive days. Fifty-nine per cent. and 10%
of the administered H3 label were voided in urine and faeces
respectively during the dosing period. Tissue residues at the
termination of dosing were less than 0.03% of the administered H3
label (Gerhards and Kolb, 1966).
TABLE I. POTENTIAL TRANSFORMATION PRODUCTS OF CHLORDIMEFORM
Other information on identity and properties
Chlordimeform is used as a free base or as its hydrochloride salt.
Physical properties of both base and hydrochloride salt are given
below.
Chlordimeform Chlordimeform
base hydrochloride
Melting point 32°C 225-227°C
Vapour pressure (20°) 3.5 × 10-4 Torr 2.2 × 10-7 Torr
Solubility in water 250 ppm >50%
chloroform >20% 1-2%
hexane >20% 0.1%
Chlordimeform base is applied as an emulsifiable solution while the
hydrochloride is used as a water-soluble powder.
Technical chlordimeform hydrochloride has a purity of a least 96%. The
major impurities are 2, methyl-4-chlorformamidine,
4-chloro-o-toluidine-hydrochloride and sodium chloride.
Chlordimeform is rather stable in strong acids. It is readily
hydrolyzed, however, in weakly-acid to weakly alkaline solutions. Its
half-life in water containing 5% of methanol was determined to be 42
hours at pH 7 (30°C) and five hours at pH 9 (30°C) respectively.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Table I shows the potential metabolites for chlordimeform.
Four 120 g male rats treated orally with 270 µg of phenyl
H3-labelled (tritiated) chlordimeform secreted 19.74 to 23.03% of
the dose in bile over a 24-hour period. Groups of four 130 g female
rats similarly treated eliminated 52.8% (range 41.8-59.6%) and 2.5%
(range 0.13-5.30%) in urine and faeces respectively in 24 hours. A
pair of rats observed for 14 hours eliminated 66.2, and 64.9%
administered H3 label in urine, and 11.4 and 14.9% administered H3
label in faeces. Twenty-four hour elimination following intravenous
injection of 270 µg phenyl H3-labelled chlordimeform in rat
comprised 53.7% (range 52.0-55.6%) and 1.42% (range 1.19-1.84%)
administered H3, in urine and faeces respectively (Gerhards and
Kolb, 1966).
The urine from a male rat collected over 72 hours subsequent to oral
administration of 1.1 mg H3-labelled chlordimeform contained 49% of
the administered H3 label. Free extractables comprised 22% of the
H3 label, 10% was in the water phase and 17% was reactive to form
extractable glucuronides. The free extractable H3 label comprised
chlordimeform, 4-chloro-o-toluidine (IV),
N-formyl-4-chloro-o-toluidine (III),
N'-(4-chloro-o-tolyl)-N-methyl-formamidine (II). Glucuronides were
based on the same compounds found as free extractables. The author
notes that identified compounds may be formed after secretion of the
urine (Gerhards, 1967).
Single oral dosing of groups of three 120 g male rats with 270 µg
phenyl H3-labelled chlordimeform resulted in residues of
administered H3 label (expressed as percentage of dose/g of tissue)
in liver (0.29%), kidney (0.22%) and lymph nodes (0.13%) after eight
hours. After 24 hours, 0.35% and 0.13% of administered H3 were
present per g of tissue in gastrointestinal tract (and content) and
liver respectively. All other tissues contained less than 0.1% H3
label/g at eight hours, and less than 0.06% H3 label at 24 hours.
Twenty-four hour urine and faeces contained 57.5 and 3.9% of the total
administered H3 label respectively compared with 46.4% and 4.3% from
rats sacrificed at eight hours (Gerhards and Kolb, 1966).
Three 120 g male rats were intubated with 270 µg phenyl H3-labelled
chlordimeform for seven consecutive days. Fifty-nine per cent. and 10%
of the administered H3 label were voided in urine and faeces
respectively during the dosing period. Tissue residues at the
termination of dosing were less than 0.03% of the administered H3
label (Gerhards and Kolb, 1966).
TABLE I. POTENTIAL TRANSFORMATION PRODUCTS OF CHLORDIMEFORM
 (I) N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine
(chlordimeform)
(I) N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine
(chlordimeform)
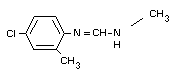 (II) N'-(4-chloro-o-tolyl)-N-methyl formamidine desmethyl
chlordimeform
(II) N'-(4-chloro-o-tolyl)-N-methyl formamidine desmethyl
chlordimeform
 (III) N-formyl-4-chloro-o-toluidine
(III) N-formyl-4-chloro-o-toluidine
 (IV) 4-chloro-o-toluidine
(IV) 4-chloro-o-toluidine
 (V) N-formyl-5-chloroanthranilic acid
(V) N-formyl-5-chloroanthranilic acid
 (VI) 5-chloroanthranilic acid
(VI) 5-chloroanthranilic acid
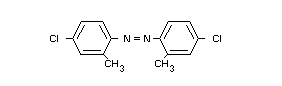 (VII) 2,2'-dimethyl-4,4'-dichloro-azobenzene
Pairs of male and female rats were treated orally with 3 µCi of
C14-toly, labelled chlordimeform. A similar group received C14
methyl labelled 4-chloro-o-toluidine (IV). Urine and faeces were
collected at 3, 12, 24, 48 and 72 hours after dosing. Urinary and
faecal elimination of C14 label after 72 hours comprised 88% and
7.5% of the administered dose of chlordimeform-C14, and 71% and
24.5% of the administered 4-chloro-o-toluidine-C14. Chloroform
extraction removed 30% of the radioactivity from the urine of
chlordimeform-C14 treated rats, the extract containing
chlordimeform, N'-(4-chloro-o-tolyl)-N-methylformamidine (II),
N-formyl-4-chloro-o-toluidine (III), and 4-chloro-o-toluidine
(IV), in addition to three unidentified metabolites. A considerable
amount of radioactivity remained at the point of origin of the
chromatograph, the amount remaining increasing with time, (30% at
three hours and 75% at 72 hours). At three hours, the four identified
compounds were present in approximately equal amounts. By 12 hours the
level of N'-(4-chloro-o-tolyl)-N-methylformamidine (II) had
decreased to approximately 25% of the level of any of the other three
compounds. By 48 hours, chlordimeform levels were half those of the
other two compounds, and by 72 hours, N-formyl-4-chloro-o-toluidine
(III) was present in the greatest proportion. At sacrifice (72 hours),
tissue levels based upon C14 levels were 0.21 ppm in liver, 0.15 ppm
in muscle, 0.11 ppm in fat and less than 0.1 ppm in other tissues.
Metabolites in ethyl acetate-extracted urine from rats given
C14-labelled 4-chloro-o-toluidine (IV) comprised
5-chloroanthranilic acid (VI) and N-formyl-5-chloroanthranilic acid
(V). The proportion of unmetabolized 4-chloro-o-toluidine (IV)
decreased with time whilst that of N-formyl-5-chloroanthranilic acid
(V) increased. The level of 5-chloroanthranilic (VI) acid remained
constant. A large amount (20-50%) of the radioactivity remained at the
origin of the chromatograph. Five unidentified compounds were noted.
Tissue levels based upon C14 levels at 72 hours after dosing were
0.33 ppm in fat. 0,26 ppm in liver, 0.2 ppm in kidney and oviduct,
0.1 ppm in brain, and less than 0.1 ppm in other tissues (Knowles and
Sen Gupta, 1970).
Two female dogs (18 and 20 kg) were given a single oral dose of 10 µCi
chlordimeform C14, and a single male dog (12 kg) which had undergone
cannulation of the gall-bladder and ligation of the bile duct was
given 20 µCi chlordimeform C14 orally. Urine was collected (by
catheterization) at 1, 3, 6, 12, 24, 48 and 72 hours. Faeces were
collected at similar time intervals. Of the administered C14 85% was
recovered in urine, 0.6% in faeces, and 5% in the bile by 72 hours.
Chloroform extraction of the urine removed 10% of the radioactivity.
Thin-layer chromatography of the extract revealed chlordimeform,
N'-(4-chloro-o-tolyl)-N-methylformamidine (II) and
4-chloro-o-toluidine (IV) in about equal quantities, but about four
times as much N-formyl-4-chloro-o-toluidine (III) at one hour after
treatment. The level of unchanged chlordimeform and
N'-(4-chloro-o-tolyl)-N-methylformamidine (II) decreased steadily
with time, whereas 4-chloro-o-toluidine (IV) and
N-formyl-4-chloro-o-toluidine (III) rose to maximum levels between
six and 12 hours prior to tapering off. Three unidentified metabolites
were present. In addition a lot of the radioactivity remained at the
origin of the chromatograph. Re-runs of this material in polar
solvents showed 5-chloroanthranilic acid (VI),
N-formyl-5-chloroanthranilic acid (V) and three unidentified compounds
were present. Some radioactivity still remained at the origin. The
urinary C14 not extracted by chloroform was treated with enzymes
(ß-glucuronidase, ß-glucuronidase-aryl sulfatase) to form "aglycones".
About 75% of the remaining C14 was extracted in this manner
(hydrochloric acid released 62%), and thin-layer chromatography showed
the same compounds as found in the chloroform extract, the major
metabolite being N-formyl-4-chloro-o-toluidine (III). In addition,
more of one of the unidentified metabolites was present. Again
re-chromatography of the 45% radioactivity remaining at the origin
with more polar solvents revealed 5-chloroanthranilic acid (VI) to be
the major product. In the bile, peak concentration of radioactivity
occurred at eight hours. About 10% of this activity could be
partitioned into ether, and thin-layer chromatography of the extract
indicated the same four compounds seen in urine chloroform extract.
N'-(4-chloro-o-tolyl)-N-methyl-formamidine (II),
N-formyl-4-chloro-o-toluidine (III) and an unidentified compound
accounted for most of the activity at two hours. By six hours, 75% of
the activity was due to N-formyl-4-chloro-o-toluidine (III).
Incubation of extracted bile with enzyme or acid gave the same
"aglycone" compounds as found in urine. Tissue residues of C14 at 72
hours ranged from 72 ppb in liver through kidney (30 ppb), lung (13.5
ppb), spleen and brain (11.9 ppb), heart and fat to pancreas at 5 ppb
(Sen Gupta and Knowles, 1970).
Incubation of chlordimeform (H3 labelled) for 120 minutes with rat
liver homogenate resulted in 24% unchanged chlordimeform, 45%
4-chloro-o-toluidine, and 11% unidentified metabolites being formed.
Rabbit liver homogenate yielded 53%, 40% and 7% respectively (Gerhards
& Kolb, 1966). The rat liver homogenate studies were confirmed using
C14 labelled chlordimeform. In addition, chlordimeform degradation
was shown to require the presence of nicotinamide. Three unidentified
metabolites were also observed. No azo-derivatives were detected.
Spleen homogenates were inactive with regard to chlordimeform
degradation (Rose, 1969a).
Further in vitro studies have shown that plant peroxidases can
result in the production of symmetrical azo-derivatives from
4-chloro-o-toluidine. Animal catalases do not result in formation of
azo-derivatives (see below) (Rose, 1969b).
Incubation of 60 µg H3-labelled chlordimeform (30 µCi) with 5 ml
human plasma yielded N-formyl-4-chloro-o-toluidine only. Conversion
was 25% in five hours, and 50% in 20 hours (Gerhards and Kolb, 1966).
A number of experiments were conducted by Ciba Ltd (1969c, d) to
verify the presence or absence of azobenzene formation from
chlordimeform or 4-chloro-o-toluidine in mammalian tissues. In the
first series it was demonstrated that peroxidase activity was
negligible in rat liver and spleen. Furthermore, catalase, which was
abundant in the same tissues, and which, like peroxidase, catalyzes
reactions between hydroxyperoxides and many oxidizable compounds, was
shown to be unable to form symmetrical azo-derivatives from
4-chloro-o-toluidine. In the second series of experiments it was
demonstrated that rat liver and spleen homogenates, which were
fortified with nicotinamide, and which degraded chlordimeform to
N-desmethyl-chlordimeform (II) and small quantities of
N-formyl-4-chloro-o-toluidine (III) and 4-chloro-o-toluidine (IV)
respectively, did not form any azobenzene derivatives. These compounds
therefore do not represent metabolites of chlordimeform or its
aromatic amine degradation products in animal tissues.
Chlordimeform degradation has been shown to proceed according to the
following pathways (Knowles, 1970):
(VII) 2,2'-dimethyl-4,4'-dichloro-azobenzene
Pairs of male and female rats were treated orally with 3 µCi of
C14-toly, labelled chlordimeform. A similar group received C14
methyl labelled 4-chloro-o-toluidine (IV). Urine and faeces were
collected at 3, 12, 24, 48 and 72 hours after dosing. Urinary and
faecal elimination of C14 label after 72 hours comprised 88% and
7.5% of the administered dose of chlordimeform-C14, and 71% and
24.5% of the administered 4-chloro-o-toluidine-C14. Chloroform
extraction removed 30% of the radioactivity from the urine of
chlordimeform-C14 treated rats, the extract containing
chlordimeform, N'-(4-chloro-o-tolyl)-N-methylformamidine (II),
N-formyl-4-chloro-o-toluidine (III), and 4-chloro-o-toluidine
(IV), in addition to three unidentified metabolites. A considerable
amount of radioactivity remained at the point of origin of the
chromatograph, the amount remaining increasing with time, (30% at
three hours and 75% at 72 hours). At three hours, the four identified
compounds were present in approximately equal amounts. By 12 hours the
level of N'-(4-chloro-o-tolyl)-N-methylformamidine (II) had
decreased to approximately 25% of the level of any of the other three
compounds. By 48 hours, chlordimeform levels were half those of the
other two compounds, and by 72 hours, N-formyl-4-chloro-o-toluidine
(III) was present in the greatest proportion. At sacrifice (72 hours),
tissue levels based upon C14 levels were 0.21 ppm in liver, 0.15 ppm
in muscle, 0.11 ppm in fat and less than 0.1 ppm in other tissues.
Metabolites in ethyl acetate-extracted urine from rats given
C14-labelled 4-chloro-o-toluidine (IV) comprised
5-chloroanthranilic acid (VI) and N-formyl-5-chloroanthranilic acid
(V). The proportion of unmetabolized 4-chloro-o-toluidine (IV)
decreased with time whilst that of N-formyl-5-chloroanthranilic acid
(V) increased. The level of 5-chloroanthranilic (VI) acid remained
constant. A large amount (20-50%) of the radioactivity remained at the
origin of the chromatograph. Five unidentified compounds were noted.
Tissue levels based upon C14 levels at 72 hours after dosing were
0.33 ppm in fat. 0,26 ppm in liver, 0.2 ppm in kidney and oviduct,
0.1 ppm in brain, and less than 0.1 ppm in other tissues (Knowles and
Sen Gupta, 1970).
Two female dogs (18 and 20 kg) were given a single oral dose of 10 µCi
chlordimeform C14, and a single male dog (12 kg) which had undergone
cannulation of the gall-bladder and ligation of the bile duct was
given 20 µCi chlordimeform C14 orally. Urine was collected (by
catheterization) at 1, 3, 6, 12, 24, 48 and 72 hours. Faeces were
collected at similar time intervals. Of the administered C14 85% was
recovered in urine, 0.6% in faeces, and 5% in the bile by 72 hours.
Chloroform extraction of the urine removed 10% of the radioactivity.
Thin-layer chromatography of the extract revealed chlordimeform,
N'-(4-chloro-o-tolyl)-N-methylformamidine (II) and
4-chloro-o-toluidine (IV) in about equal quantities, but about four
times as much N-formyl-4-chloro-o-toluidine (III) at one hour after
treatment. The level of unchanged chlordimeform and
N'-(4-chloro-o-tolyl)-N-methylformamidine (II) decreased steadily
with time, whereas 4-chloro-o-toluidine (IV) and
N-formyl-4-chloro-o-toluidine (III) rose to maximum levels between
six and 12 hours prior to tapering off. Three unidentified metabolites
were present. In addition a lot of the radioactivity remained at the
origin of the chromatograph. Re-runs of this material in polar
solvents showed 5-chloroanthranilic acid (VI),
N-formyl-5-chloroanthranilic acid (V) and three unidentified compounds
were present. Some radioactivity still remained at the origin. The
urinary C14 not extracted by chloroform was treated with enzymes
(ß-glucuronidase, ß-glucuronidase-aryl sulfatase) to form "aglycones".
About 75% of the remaining C14 was extracted in this manner
(hydrochloric acid released 62%), and thin-layer chromatography showed
the same compounds as found in the chloroform extract, the major
metabolite being N-formyl-4-chloro-o-toluidine (III). In addition,
more of one of the unidentified metabolites was present. Again
re-chromatography of the 45% radioactivity remaining at the origin
with more polar solvents revealed 5-chloroanthranilic acid (VI) to be
the major product. In the bile, peak concentration of radioactivity
occurred at eight hours. About 10% of this activity could be
partitioned into ether, and thin-layer chromatography of the extract
indicated the same four compounds seen in urine chloroform extract.
N'-(4-chloro-o-tolyl)-N-methyl-formamidine (II),
N-formyl-4-chloro-o-toluidine (III) and an unidentified compound
accounted for most of the activity at two hours. By six hours, 75% of
the activity was due to N-formyl-4-chloro-o-toluidine (III).
Incubation of extracted bile with enzyme or acid gave the same
"aglycone" compounds as found in urine. Tissue residues of C14 at 72
hours ranged from 72 ppb in liver through kidney (30 ppb), lung (13.5
ppb), spleen and brain (11.9 ppb), heart and fat to pancreas at 5 ppb
(Sen Gupta and Knowles, 1970).
Incubation of chlordimeform (H3 labelled) for 120 minutes with rat
liver homogenate resulted in 24% unchanged chlordimeform, 45%
4-chloro-o-toluidine, and 11% unidentified metabolites being formed.
Rabbit liver homogenate yielded 53%, 40% and 7% respectively (Gerhards
& Kolb, 1966). The rat liver homogenate studies were confirmed using
C14 labelled chlordimeform. In addition, chlordimeform degradation
was shown to require the presence of nicotinamide. Three unidentified
metabolites were also observed. No azo-derivatives were detected.
Spleen homogenates were inactive with regard to chlordimeform
degradation (Rose, 1969a).
Further in vitro studies have shown that plant peroxidases can
result in the production of symmetrical azo-derivatives from
4-chloro-o-toluidine. Animal catalases do not result in formation of
azo-derivatives (see below) (Rose, 1969b).
Incubation of 60 µg H3-labelled chlordimeform (30 µCi) with 5 ml
human plasma yielded N-formyl-4-chloro-o-toluidine only. Conversion
was 25% in five hours, and 50% in 20 hours (Gerhards and Kolb, 1966).
A number of experiments were conducted by Ciba Ltd (1969c, d) to
verify the presence or absence of azobenzene formation from
chlordimeform or 4-chloro-o-toluidine in mammalian tissues. In the
first series it was demonstrated that peroxidase activity was
negligible in rat liver and spleen. Furthermore, catalase, which was
abundant in the same tissues, and which, like peroxidase, catalyzes
reactions between hydroxyperoxides and many oxidizable compounds, was
shown to be unable to form symmetrical azo-derivatives from
4-chloro-o-toluidine. In the second series of experiments it was
demonstrated that rat liver and spleen homogenates, which were
fortified with nicotinamide, and which degraded chlordimeform to
N-desmethyl-chlordimeform (II) and small quantities of
N-formyl-4-chloro-o-toluidine (III) and 4-chloro-o-toluidine (IV)
respectively, did not form any azobenzene derivatives. These compounds
therefore do not represent metabolites of chlordimeform or its
aromatic amine degradation products in animal tissues.
Chlordimeform degradation has been shown to proceed according to the
following pathways (Knowles, 1970):
 See also "Fate of Residues. In Animals".
TOXICOLOGICAL STUDIES
Special Studies
(a) Pharmacological studies
Chlordimeform hydrochloride was administered at graded doses of 0.01,
0.1, 1.0, 10 and 100 mg to the isolated perfused rabbit heart, the
organ being challenged with norepinephrine before and after dosing.
Contractile force was substantially decreased by 1.0 mg. Effect on
coronary flow and cardiac rate was less marked. Higher doses
temporarily stopped heart contractions. Guinea-pig isolated ileum was
exposed to graded doses of 1.0, 3.2, 10, 32, 100 and 320 µg/ml of
chlordimeform hydrochloride and evaluated for its effect on
acetylcholine, serotonin, histamine and barium induced contractions.
Bath concentrations of 3.2 µg/ml inhibited histamine contractions by
about 50% concentrations of 320 µg/ml were required to induce a
similar effect on acetylcholine, serotonin, and barium induced
contractions. In the intact dog, effect of graded doses of 1, 3, 10,
30 or 100 mg/kg chlordimeform hydrochloride on blood pressure, cardiac
rate, respiration, vasomotor response to epinephrine, acetylcholine,
histamine, tyramine, DMPP, carotid occlusion and peripheral vagal
stimulation were evaluated. Chlordimeform hydrochloride exerted a
hypotensive effect and caused increased respiration. A dose of 100
mg/kg was lethal. Vasomotor response to tyramine was enhanced, whereas
the pressor response to carotid occlusion was blocked (Teeters and
Blackmore, 1968).
(b) Reproduction studies
Rat. Four groups of 10 male and 20 female rats were fed 0, 100, 250
and 500 ppm chlordimeform in corn oil via the diet during three
parental, and three two-litter filial generations. Parental
body-weight prior to mating tended to be reduced in all test groups,
especially at 500 ppm. The same tendency was apparent with regard to
food consumption. Fertility index, gestation index, live birth index,
sex ratio, mean litter size, and birth weight of pups were comparable
to controls in all generations. At 500 ppm, lactation index was
reduced in F1a, F1b and F3a litters. Weaning weight of offspring was
depressed in all 500 ppm litters. Gross pathological examinations on
parents and pups dying during the study, and on 10 male and 10 female
weanlings of the F3b generation revealed no compound related effects
(Blackmore, 1969a).
Rabbit. Three groups of 10 impregnated female New Zealand white
rabbits (the day of impregnation being considered as day 0 of
gestation) were intubated on days 8 through 16 of gestation with 0,
7.5 or 30 mg chlordimeform/kg/day. Five rabbits per group were
sacrificed on day 28 of gestation. Parental mortality, abortion rate,
corpora lutea to implantation ratio, litter size, incidence of
resorptions, stillbirths, foetal weight, foetal length, and incidence
of skeletal, and tissue abnormalities were unaffected by the test
compound. In rabbits littering normally, gestation length, litter size
and litter weights were normal (Blackmore, 1969b).
(c) Studies on metabolites
Oral LD50 determinations in male and female rats.
Duration LD50
of (mg/kg)
Compound observation (days) References
N'-(4-chloro-o-tolyl)-N-methylformamidine 14 150 Sachsse and Bathe, 1971a
N-formyl-4-chloro-o-toluidine 14 2900 " " " 1970a
4-chloro-o-toluidine
(base) 7 ca1000 " " " 1971c
4-chloro-o-toluidine -HCl 14 860 " " " 1970d
Phenamidine (base) 7 ca1500 " " " 1971d
Phenamidine - HCl 14 860 " " " 1970f
o-chlordimeform (base) 7 300-400 " " " 1971f
o-chlordimeform - HCl 14 540 " " " 1970h
Dichlordimeform (base) 7 ca900 " " " 1971h
Dichlordimeform - HCl 14 260 " " " 1971j
Dermal LD50 determinations in male and female rats (24 hours occluded exposure).
Duration LD50
of (mg/kg)
Compound observation (days) References
N'-(4-chloro-o-tolyl)-N-methylformamidine 14 >2150 Sachsse and Bathe,1971b
N-formyl-4-chloro-o-toluidine 14 <2150 " " " 1970b
4-chloro-o-toluidine
(base) 7 ca1800 " " " 1971c
4-chloro-o-toluidine -HCl 14 <2150 " " " 1970e
Phenamidine (base) 7 ca1800 " " " 1971e
Phenamidine - HCl 14 <2150 " " " 1970g
o-chlordimeform (base) 7 ca300 " " " 1971g
o-chlordimeform - HCl 14 800 " " " 1970i
Dichlordimeform (base) 7 ca950 " " " 1971i
Dichlordimeform - HCl 14 <2150 " " " 1970k
Acute toxicity
ACUTE TOXICITY OF CHLORDIMEFORM BASE
LD50
Animal Route Sex (mg/kg) References
Mouse i.p. Mixed 110 Sachsse and Ullman, 1970
Rat Oral Male 178 Weir, 1968
Rat Oral Female 460 Weir, 1968
Rat Oral Male 220 Mastri et al., 1969
Rat Oral Female 170 Mastri et al., 1969
Dog Oral Female ca100 Weir, 1967
Dog Oral Male ca150 Hurni and Sachsse, 1969a
Dog Oral Female ca400 Hurni and Sachsse, 1969a
Rat Dermal Mixed 640 Sachsse and Bathe, 19701
Rat Inhalation* Mixed 17 400 Sachsse and Ullman, 1971
mg/m3
* Inhalation exposure was for one hour.
Hypoactivity, dyspnoea, muscular weakness, tremours, straub tail,
spasms and convulsions preceded death following oral administration.
Dyspnoea, exophthalmus, prostration, spasms and convulsions preceded
death following dermal application. No local skin irritation occurred.
No pathological changes were noted in rat following oral treatment,
although pale or blotchy livers, pale kidneys, and haemorrhagic
intestinal contents were observed after dermal treatment. In the dog,
oral administration resulted in congestion of liver, kidneys, and
lungs (the lungs also being oedomatous and haemorrhagic).
ACUTE TOXICITY OF CHLORDIMEFORM HYDROCHLORIDE
LD50
Animal Route Sex (mg/kg) References
Mouse Oral Mixed 220 Gunzel and Richter, 1967
Rat i.v. Male 95 Tripod, 1967
Rat s.c. Male 130 Tripod, 1967
Rat Oral Mixed 265 Gunzel and Richter, 1965
Rat Oral Mixed 355 Gunzel and Richter, 1965
Rat Oral Male 305 Tripod, 1967
Rat Oral Male 325 Mastri et al., 1969
Rat Oral Female 330 Mastri et al., 1969
Rat Dermal ca4000 Gunzel and Richter, 1966a
Rabbit Dermal >4000 Gunzel and Richter, 1966b
Rat Inhalation* >5.8 Sachsse and Ullman, undated
g/m3
* Inhalation exposure was for one hour.
Symptoms were similar to those for the base.
ACUTE TOXICITY OF CHLORDIMEFORM FORMULATIONS
LD50
Animal Route Sex Formulation (mg/kg) References
Mouse Oral Male EC 50 320 Aohi and Meda, 1966
Mouse Oral Female 50 s.p. 752 Shionogi C, undated
Rat Oral Mixed EC 50 610 Hurni and Sachsse, 1969b
Rat Dermal Mixed EC 50 2100 Sachsse and Bathe, 1971j
Rat Dermal Mixed 50 s.p. >3000 Hurni and Sachsse, 1969c
Rat Oral Mixed 50 s.p. 1100 Gunzel and Richter, 1969
Dog Oral Mixed 50 s.p. 400 Gunzel and Richter, 1968
A suicide victim ingested 30 ml of Galecron(R) 50 formulation. Upon
admission to hospital, an unknown time after drinking chlordimeform
solution, the patient was comatosed; and respiration and heartbeat had
ceased. The latter was restored by massage and adrenaline injection. A
respirator was used but death occurred within 24 hours. No autopsy was
performed (Oda, 1969).
Short-term studies
Rat. Four groups of 10 male and 10 female rats were intubated six
times weekly for one month with 0.5 ml/100 g body-weight of 2% CMC,
containing chlordimeform base at concentrations such as to give dose
levels of 0, 25, 50 or 100 mg/kg/dose. Body-weight was markedly
reduced in both sexes at 100 mg/kg/dose. Hyperexcitability was
observed in all test animals. At 100 mg/kg this was apparent 20-30
minutes after dosing, and was followed two to three hours after dosing
by decreased activity and apathy. Recovery was complete at four hours.
Similar effects were observed at 50, and 25 mg/kg/dose, but on a dose
related decreased scale, and with inconsistent frequency (Surber and
Cerioli, 1966).
Dog. Four groups of beagle dogs were fed 0 (10 male and 10 female)
250 (eight male and eight female), 500 (eight male and eight female)
or 1000 (10 male and 10 female) ppm of chlordimeform in a dry diet for
two years. Two male and two female dogs were sacrificed from each
group at 26, and 52 weeks. Body-weight was reduced at 1000 ppm, the
effect being slightly more pronounced in the females, Total leucocyte
counts were sporadically elevated in both sexes at 1000 ppm and in
females at 500 ppm. Haematocrit, haemoglobin, and erythrocyte counts
tended to be depressed terminally in both sexes at 1000 ppm. Sporadic
slight decreases in serum albumin were observed, more frequently in
males, at 1000 ppm. Terminal spleen to body-weight ratio was elevated
in males at 500 and 1000 ppm, and in females at 1000 ppm. Kidney to
body-weight ratio was elevated in females at 1000 ppm.
Histopathological examinations revealed bile duct hyperplasia,
pericholangitis and nodular hepatocytic hyperplasia at 500 and 1000
ppm in both sexes, and nodular hepatocytic hypertrophy at 1000 ppm in
both sexes in the liver. Kidneys showed an increased amount of
pigmentation at 500 and 1000 ppm in both sexes (Blackmore, 1969c).
Long-term studies
Rat. Five groups of 35 randomized male and 35 randomized female rats
(except at 100 ppm where 34 males and 36 females were used) were fed
0, 100, 250, 500 or 1000 ppm in the diet. The 100 ppm group commenced
treatment seven weeks after the other groups. This group was
originally part of the control group. Animals at that time were of
similar weight to those which had already been on test. The 1000 ppm
group was discontinued at three months due to severe growth
inhibition. Growth inhibition was observed in the males at 500 and
1000 ppm. In the females weight gain was reduced at 250 ppm and above.
In addition, female body-weight was reduced at 100 ppm between weeks
20 and 48. (This reduction may possibly be due to reduced food intake
(Weatherholtz, 1970) although this may not be the complete explanation
(Lyon, 1970).) Food intake was significantly reduced at 500 and 1000
ppm in both sexes. Dose related decreases in haematocrit, haemoglobin,
and erythrocyte counts, and a dose related increase in the leucocyte
count occurred in females at 250 and 500 ppm up to one year. During
the second year, haematocrit only was consistently depressed in
females at 500 ppm. Histo-pathological changes in the liver (nodules,
and foci of hyperplasia of hepatocytes) occurred in all groups, but
the incidence was greater at 250 and 500 ppm, and was more severe at
500 ppm. Some females at 500 ppm showed slight hypertrophy and
vacuolation of focal groups of cells in the adrenal cortex. Terminal
organ to body-weight ratios were increased in the liver (females at
250 and 500 ppm and males at 100 and 250 ppm), kidney (females at 250
and 500 ppm), thyroid (females at 250 and 500 ppm), heart (males at
250 ppm and females at 500 ppm), adrenals (males at 100 and 250 ppm)
and testes (100 and 500 ppm) (Blackmore, 1969d).
Comments
Chlordimeform appears to be fairly rapidly excreted in animals and
does not appear to be stored in body tissue. Information on metabolism
in man would however be of interest. A long-term feeding study is
available in the rat and a two-year study in the dog from which a
no-effect level was established. The Meeting expressed particular
concern about effect on organ to body-weight ratios in the rat and on
the histopathology of the liver and bile ducts of both the rat and the
dog. In these species nodular changes were observed in the liver but
histopathological information is insufficient to determine their
toxicological significance. Also of some concern were effects on the
total leucocyte counts of the dog and the pharmacological effects on
the heart and circulation together with the fact that the compound
appears to potentiate the effect of tyramine.
Because of the concern expressed above only a temporary acceptable
daily intake was established for this compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat - 100 ppm in the diet equivalent to 5 mg/kg body-weight per day
Dog - 250 ppm in the diet equivalent to 6.25 mg/kg body-weight per day
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Treatment of plants
Chlordimeform is a broad spectrum acaricide/insecticide applied to the
foliage of fruit, vegetables and other field crops acting through
contact and feeding and through vapour action on all stages of insects
and mites, including eggs of mites.
Pre-harvest treatments
Chlordimeform products have been registered in many countries for use
on a wide variety of crops of which typical examples are given in
Table 1.
Post-harvest treatment
No post-harvest treatments with chlordimeform are known.
Treatment of animals
In Australia chlordimeform is registered for use in cattle dips for
the control of cattle ticks (Boophilus micropus), the application
rate being 0.01-0.02% solution applied each 21 days by dipping often
in combination with organo-phosphorus acaricides such as dioxathion,
ethion, coumaphos, Dursban, bromophos-ethyl. Experimental work
designed to evaluate chlordimeform for use alone at a concentration of
0.1% is at an advanced stage.
Residues resulting from supervised trials
Fruit and vegetable crops
Detailed residue data are available from supervised trials in many
countries on many important crops and reports have been deposited with
FAO. The residue results are remarkably consistent between different
trials and different countries.
The most important and consistent feature of the residue trial data is
that the residue level remains remarkably constant irrespective of the
pre-harvest interval. On apples, grapes, citrus, peaches, pears, plums
and tomatoes the residue levels from sprays applied 30 days
pre-harvest are usually no more than half the level found when similar
rates were applied one to two days prior to harvest.
This rather unusual pattern is partly due to the analytical method
which determines all 4-chloro-o-toluidine containing metabolites
and/or conjugates as well as the unchanged parent compound.
Table II gives a representative sample of the results of supervised
residue trials carried out using registered label recommendations, use
patterns and rates of application.
Ciba and Schering (1971) have reported more than 50 trials designed to
determine the proportion of metabolites and parent compound in the
residues remaining on fruits at various intervals after application.
The separation of the components in the residue was performed by
thin-layer chromatography and by gas-chromatography.
TABLE II. REGISTERED USE PATTERNS FOR CHLORDIMEFORM
Pre-harvest
Country Crop Rate interval
Argentina pome fruits 0.06-0.09% 28 days
Australia cattle 0.02% 1 day
cotton 0.1%
pome & stone fruits 0.1% 7 days
strawberries 0.05% 7 days
Austria fruit trees 0.05% -
Brazil horticulture 0.05%
Canada fruit trees 0.06% 14-28 days
cole crops 0.05% 28 days
TABLE II. (cont'd)
Pre-harvest
Country Crop Rate interval
Denmark fruit trees 0.05% -
France pome fruits 0.05% 15 days
grapes 0.05% 15 days
vegetables 0.05% 15 days
Germany fruit trees 0.05% -
hops, grapes 0.05% -
Greece horticulture 0.05% -
Israel deciduous fruit - -
Italy fruit trees 0.05% -
citrus, grapes 0.05% -
Japan apples 0.05-0.025% -
citrus 0.05-0.035% -
pears 0.05-0.035% -
Yugoslavia fruit trees 0.05-0.15% -
vegetables 0.05-0.15% -
New Zealand pome fruits 0.06% 14 days
peaches 0.06% 14 days
strawberries 0.06% 7 days
Peru cotton 1 kg/ha 15 days
South Africa citrus 0.05% 14 days
tomatoes 0.075% 30 days
Switzerland pome fruits 0.05% 6 weeks
grapes 0.05-0.075% 6 weeks
Turkey apples 0.05% -
citrus, cherries 0.05% -
cotton -
USA apples 4 kg/ha 14 days
citrus 4-6 kg/ha -
pears 4 kg/ha 28 days
Venezuela cotton, citrus 0.3-0.5 kg/ha -
horticulture 0.3-0.5 kg/ha -
TABLE III
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Apples USA 1967 0.06 3 s.p. 3.3 1.3 1.3 1.4
USA 1969 0.06 2 s.p. 8.3 1.9 1.4 1.2 0.6
USA 1969 0.12 2 s.p. 8.6 4.5 2.8 1.2 1.7
USA 1969 0.12 3 e.c. 2.9 2.0 1.0 1.2
Canada 1969 0.06 2 s.p. 1.6
Australia 1969 0.04 3 s.p. 1.2 1.8 2.5 0.5 0.9 0.8
New Zealand 1970 0.05 1 s.p. 1.5 1.0 0.7 0.6
New Zealand 1970 0.1 1 s.p. 2.2 1.2 0.9 0.8
South Africa 1969 0.05 1 s.p. 0.6 0.6 0.5 0.5
England 1967 0.08 1 e.c. 3.3 2.6 2.7 1.2 1.2
Germany 1966 0.06 1 s.p. 1.8 1.9 1.6 0.8 0.5
Germany 1969 0.034 1 s.p. 1.5 1.0 0.6 0.5
Germany 1969 0.07 1 s.p. 2.5 1.8 1.5 0.7
Switzerland 1965 0.05 1 s.p. 1.1 1.0 1.2 0.6 0.5 0.5
Switzerland 1967 0.05 1 e.c. 2.3 1.3 1.0 0.5
Cherries USA 1968 0.06 1 e.c. 1.9 0.5 0.3 0.2
USA 1968 0.12 1 e.c. 3.7 1.3 1.1 0.7
Grapes USA 1969 0.06 2 s.p. 1.6 0.8 0.5 0.5
USA 1969 0.12 2 s.o. 2.9 2.8 1.6 1.8
Germany 1970 0.034 2 s.p. 2.0 1.3 1.3 1.0
Germany 1970 0.034 2 s.p. 2.8 2.3 2.3 1.8
Italy 1968 0.034 1 s.p. 2.6 1.2 1.2 0.6
Italy 1968 0.045 1 s.p. 1.8 1.5 1.2 1.2
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Oranges
Pulp Italy 1967 0.1 1 w.p. 0.2 0.3 0.2 0.3
Peel Italy 1967 0.1 1 w.p. 3.8 3.6 2.3 2.2
Whole Italy 1967 0.1 1 w.p. 1.1 1.1 0.8 0.7
Pulp Italy 1968 0.05 1 s.p. 0.2 0.3 0.1 0.2 0.08 0.1
Peel Italy 1968 0.05 1 s.p. 4.6 3.8 1.7 3.7 2.2 2.3
Whole Italy 1968 0.05 1 s.p. 1.5 1.2 0.5 1.1 0.7 0.7
Pulp Spain 1968 0.08 1 s.p. 0.4 0.4 0.4
Peel Spain 1968 0.08 1 s.p. 12.1 10.9 6.0
Whole Spain 1968 0.08 1 s.p. 3.5 3.5 2.0
Peaches USA 1969 0.06 1 e.c. 6.6 5.0 4.4 2.3
USA 1969 0.06 1 s.p. 5.9 3.4 2.8 1.9
Australia 1970 0.04 1 s.p. 1.0 1.2 0.4 1.7 0.6 0.4
Australia 1970 0.08 1 s.p. 2.0 2.0 1.1 3.1 1.4 1.1
France 1969 0.05 1 s.p. 1.5 1.1 0.5 0.5
France 1969 0.1 1 s.p. 1.5 0.9 1.0
USA 1970 0.06 2 e.c. 7.5 6.6 4.1 3.3
USA 1970 0.06 2 s.p. 3.7 3.2 2.4 1.5
Pears USA 1969 0.06 2 s.p. 7.8 6.1 4.4 3.5 2.8
USA 1969 0.06 2 e.c. 6.4 5.5 4.1 2.8 1.6
Australia 1970 0.04 1 s.p. 1.5 1.1 0.7 1.7 0.6 0.7
Australia 1970 0.08 1 s.p. 3.0 2.4 1.6 2.2 1.6 1.3
Plums USA 1970 0.06 2 e.c. 1.7 1.3 1.9 1.7
USA 1970 0.06 2 s.p. 0.5 0.5 0.5 0.4
Germany 1969 0.07 1 s.p. 0.7 0.5 0.4 0.3
Germany 1969 0.07 1 s.p. 1.1 1.2 0.6 0.5
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Strawberries USA 1968 0.06 1 e.c. 5.7 4.0 3.6 2.6 0.04
USA 1968 0.12 2 s.p. 1.8
England 1967 0.1 1 e.c. 1.8
England 1967 0.1 1 s.p. 2.1
Tomangos
Whole South Africa 1970 0.2 1 s.p. 2.7 2.6 2.9
Pulp South Africa 1970 0.2 1 s.p. 1.0 0.9 1.3
Peel South Africa 1970 0.2 1 s.p. 7.0 6.5 7.1
Walnuts
Meat USA 1968 0.06 1 s.p. <0.05
Meat USA 1970 0.06 2 s.p. <0.05
Beans
Pod Germany 1969 0.034 1 s.p. 0.5 0.2 0.1
Pod Germany 1969 0.07 1 s.p. 0.5 0.1 0.1
Broccoli
Head trimmed USA 1970 0.05 5 s.p. 1.7 0.8 0.4 0.2
Head trimmed USA 1970 0.1 5 s.p. 4.4 1.2 0.7 0.2
Head trimmed Canada 1970 0.05 2 s.p. 2.4 0.8
Head trimmed Canada 1970 0.05 6 s.p. 3.7 1.7 1.3
Brussels sprouts USA 1969 0.06 9 e.c. 2.8 2.1 1.9
Canada 1970 0.05 6 s.p. 3.6 3.5 3.1
Canada 1970 0.05 8 s.p. 5.5 3.8 2.8
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Cabbage
Head trimmed USA 1968 0.2 6 e.c. 4.3 0.6 0.2
Head trimmed USA 1969 1 e.c. 0.2
Head trimmed USA 1969 11 s.p. 1.5 1.2 1.4
4 outer leaves Canada 1969 4 s.p. 0.5
Inner head Canada 1969 4 s.p. <0.05
Cauliflower
Head trimmed USA 1969 6 s.p. 2.6 2.6 0.5
Head trimmed USA 1970 4 s.p. 3.6 2.5 1.9
Head trimmed USA 1970 4 s.p. 14.9 8.4 3.7
Cotton
Seed USA 1970 0.5 9 e.c. 0.22
Lint cotton USA 1970 0.5 9 e.c. 0.37
Delinted
cotton-seed USA 1970 0.5 9 e.c. 0.19
Linters USA 1970 0.5 9 e.c. 0.47
Cottonseed hulls USA 1970 0.5 9 e.c. 0.20
Solvent extract
meal USA 1970 0.5 9 e.c. 0.15
Screwpress
extracted meal USA 1970 0.5 9 e.c. 0.09
Crude solvent
extracted oil USA 1970 0.5 9 e.c. 0.11
Refined solvent
extracted oil USA 1970 0.5 9 e.c. 0.09
Crude screwpress
oil USA 1970 0.5 9 e.c. 0.17
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Refined
screwpress oil USA 1970 0.5 9 e.c. 0.14
Seed USA 1970 0.2 6 e.c. 1.8 1.6 2.3
Seed USA 1970 6 s.p. 2.0 1.8 2.4
Oil crude USA 1968 10 s.p. 1.2
Oil refined USA 1968 10 s.p. 1.0
Oil crude USA 1968 10 s.p. 4.0
Oil refined USA 1968 10 s.p. 3.6
Oil crude USA 1970 9 e.c. 0.1
Oil refined USA 1970 9 e.c. 0.1
Sugar beet
Roots Germany 1968 1 s.p. <0.02
(60-120)
Roots Germany 1969 1 s.p. <0.02
<0.02
(60-120)
Tops Germany 1969 s.p. 0.1
<0.02
(60-120)
Tomatoes
Fruit Canada 1970 6 s.p. 0.6 0.2 0.2
Fruit USA 1970 7 e.c. 0.4 0.3 0.5 0.3
Table IV gives some typical examples of the results obtained in these
trials which involved commercial formulations applied at
concentrations normally used in practice.
TABLE IV
Fruit Location Interval Total Proportion of each
before residue metabolite%
harvest ppm
A B C D
Apples USA 30 0.92 87 <4 <4 <4
USA 90 0.42 55 <9 27 <9
Australia 2 1.03 75 <4 <15 <4
Australia 30 0.63 63 <10 <25 <10
Pears USA 30 1.78 79 <2 17 <2
USA 60 0.88 68 <3 23 <3
Italy 1 0.89 90 <3 <5 <3
Italy 21 0.62 80 <4 <8 8
Peaches Italy 1 2.32 95 <1 <5 <1
Italy 47 0.37 54 <5 35 <5
Prunes Switzerland 9 1.36 81 <1 15 <3
Switzerland 30 1.06 75 <2 20 <4
A = chlordimeform
B = N-(4-chloro-o-tolyl)-N-methylformamidine
C = N-formyl-4-chloro-o-toluidine
D = 4-chloro-o-toluidine
Where the sign < appears it is to be recognized that the limit of
detection, e.g. 0.02 or 0.04 ppm, means that the proportion may well
be much less than the figure shown.
A number of trials reported by Ciba and Schering (1971) show that
there is no significant difference in the level of chlordimeform
residues on the various fractions of cotton seed separated during
commercial processing. One series of results is shown in Table III
(see page 17). The fairly uniform distribution of residues between oil
and cotton seed meal may reflect the distribution of parent compound
and metabolite but possibly is also brought about by the analytical
procedure which does not distinguish metabolites from parent compound.
Animals
Ciba-Geigy (1971) report results of trials conducted in Australia to
determine the residue in cattle treated with chlordimeform to control
cattle tick. A calf weighing 200 kg was sprayed with 15 litres of
0.05% chlordimeform. The animal was slaughtered three days later and
residues of chlordimeform and metabolite hydrolysable to
4-chloro-o-toluidine were determined in tissues and fat with the
following results:
muscle 0.21 ppm omental fat 0.12 ppm
kidney 0.23 ppm subcutaneous fat 0.30 ppm
liver 0.62 ppm perirenal fat 0.21 ppm
In further trials cattle weighing 210 to 230 kg were dipped in 0.013%
chlordimeform and were slaughtered one or three days later. The
residues determined as above were as follows:
Interval
since Liver Fat Muscle Kidney
treatment
1 day 0.28±0.04 0.15±0.06 0.07±0.02 0.29±0.03
3 days 0.15±0.03 0.05±0.02 0.03±0.01 0.06±0.03
Buffering
As explained in some detail under "Fate of residues", chlordimeform
stability is extremely dependent on pH. Acceptable stability in cattle
dips can be achieved only if pH is held below 5.8 and preferably in
the range 5.0-5.5. In Australia this has been achieved by buffering
the dipping fluid with fertilizer grade superphosphate. Unbuffered
fluids must be used within a day of preparation.
At pH 5.2, some 99% of chlordimeform is present as the acid salt. In
this form it has adequate stability but its activity is reduced below
that of a similar concentration at a less acid pH value containing
proportionately more chlordimeform base.
Buffered dips leave only about half the hair deposits left by
unbuffered dips of equal concentration so that higher use levels are
required to produce the same acaricidal effect. Fortunately absorption
is also reduced and residue levels are not augmented by the increased
concentration required in dips.
Residues in milk and milk products
A Ciba-Geigy report (1971) describes milk and butter residues
following the treatment of lactating dairy cattle with 0.05%
chlordimeform, about 15 litres being applied to each animal. Three
animals were treated with a solution buffered to pH 5.6 and three with
an unbuffered solution of pH 9.4.
Time of 0.05% unbuffered 0.05% buffered
sampling Milk Butter Milk Butter
Pre-treatment 0.03 ppm 0.06 ppm <0.03 ppm 0.05 ppm
6 hours 0.22 ppm 1.52 ppm 0.06 ppm 0.52 ppm
24 hours 0.05 ppm 0.71 ppm 0.05 ppm 0.19 ppm
32 hours 0.08 ppm 0.18 ppm <0.03 ppm 0.14 ppm
48 hours 0.05 ppm 0.22 ppm <0.03 ppm 0.11 ppm
56 hours 0.16 ppm 0.10 ppm
72 hours 0.09 ppm 0.06 ppm
Each figure in the table is the mean of three individual samples.
The analytical technique employed determines the sum of chlordimeform
and all metabolites hydrolysable to 4 chloro-o-toluidine. The ratio
of milk residues to butter residues while somewhat variable, falls
consistently short of the level which would be expected if these
residues were partitioned exclusively in the fat phase.
The possibility that a proportion of the residues may be held in the
aqueous phase should be taken into consideration in the recommendation
of tolerances.
These results indicate the rapid rate of elimination which occurs
following spraying or dipping.
The level of residues is related to the concentration of the spray
solution or dip. Official trials in Australia showed that when the
concentration of the spray is increased from 0.03 to 0.3% the residue
levels in fat, kidney and liver of young calves increase in
approximately the same ratio. Residue levels in muscle do not
materially change in spite of the increase in concentrations. Residue
determinations were made 24 hours after spraying:
Adult cattle appear to metabolize the residue more rapidly because
animals weighing 450 kg dipped three times at 17-day intervals in a
bath containing 0.01% chlordimeform showed no residues in excess of
0.1 ppm when slaughtered three days after the third treatment. Adult
cattle weighing 300-520 kg sprayed with chlordimeform showed, as did
calves, that the residue level 24 hours after treatment increases
sharply with increasing concentration of spray. The residue level in
perirenal and omental fat increased from 0.1 ppm to 7.2 ppm when the
spray concentration was increased from 0.01 to 0.1%.
Concentration Liver Fat Muscle Kidney
of spray
0.03% <0.1 ppm 1.0 ppm <0.1 ppm 0.2 ppm
0.3% 1.2 ppm 10.0 ppm <0.1 ppm 0.5 ppm
Repeat treatments, as is normal in controlling cattle tick, does not
result in an accumulation of residues in animal tissues. Calves
receiving 10 dippings within eight weeks in a bath containing 0.01%
chlordimeform were slaughtered three days after the tenth treatment.
The results of residue analysis were as follows:
No. of Liver Fat Muscle
treatments
1 2.0 0.29 0.12
7 1.3 0.64 0.07
10 4.17 0.37 0.12
Fate of residues
General comments
Chlordimeform (I) is readily hydrolysed in weakly-acid to
weakly-alkaline solutions but is rather stable under strongly acid
conditions. In an aqueous buffer solution of pH 7.0 containing 5% of
methanol, the base showed a half-life of 42 hours at 30°C. At pH 9,
under otherwise equivalent conditions, a half-life of five hours was
observed (Kossmann et al., 1971). Hydrolysis of the parent compound
yields N-formyl-4-chloro-o-toluidine (III) and ultimately
4-chloro-o-toluidine (IV).
Structural alterations are observed when chlordimeform in solution or
on chromatoplates is exposed to irradiation by U.V. or natural
sunlight (Knowles and Sen Gupta, 1969). Although a number of
additional minor degradation products do occur,
N-formyl-4-chloro-o-toluidine (III) is the major transformation
product recovered.
A complete list of substances which appear to be potential metabolites
of chlordimeform is given in Table 1.
In animals
To supplement the studies described under "Biochemical aspects" on the
behaviour of chlordimeform in mammals, Ciba-Geigy Ltd (Ciba-Geigy,
1971) conducted experiments on the fate of the acaricide upon
continuous feeding to ruminants. Eight Brown Swiss cows were fed daily
rations containing from 4-24 ppm chlordimeform for periods of up to 42
days. Milk and tissue and organ samples were collected at regular
intervals and analysed by the total residue method (Geissbühler at
al., 1971) which accounts for all 4-chloro-o-toluidine containing
metabolites and/or conjugates of chlordimeform. At 21 days, residues
in liver were 0.09, and <O.03 ppm and in kidney were 0.38, and 0.07
ppm in animals fed 120 and 40 ppm respectively. Further cows fed 240
ppm in the diet showed liver levels of 0.45, 0.38, 0.50 and 0.44 ppm
at 7, 14, 21 and 42 days. Corresponding kidney residues were 0.05,
0.13, 0.13, and 0.09 ppm. Residues in milk, muscle and fat were below
0.03 ppm (Voss and Burkhard, 1971).
One male (36 kg) and one lactating female (39 kg) goat were given 10
µCi chlordimeform orally, urine and faeces being collected at 1, 3, 6,
12, 24, 48 and 72 hours post treatment. After 72 hours, the male had
voided 87%, and 1.8% of the administered radioactivity in the urine
and faeces respectively, and the female 68% and 1.8%. Only 0.3% of the
radioactivity appeared in the milk.
Chloroform extractable compounds identified by thin-layer
chromatography comprised chlordimeform,
N'-(4-chloro-o-tolyl)-N-methylformamidine, 4-chloro-o-toluidine,
and N-formyl-4-chloro-o-toluidine, the latter predominating. Of the
radioactivity 85-97% remained at the point of origin.
Re-chromatography with polar solvents yielded 5-chloroanthranilic
acid, and N-formyl-5-chloroanthranilic acid.
The 90% unextractable radioactivity, when incubated with
ß-glucuronidase, ß-glucuronidase-aryl sulphonase or hydrochloric acid
yielded chlordimeform, N'-14-chloro-o-tolyl-N-methylformamidine,
4-chloro-o-toluidine, N-formyl-4-chloro-o-toluidine and two
unidentified metabolites. N'-(4-chloro-o-tolyl)-N-ethylformamidine
was present in very small amounts, and the others in about equal
amounts. Re-chromatography of the 20% radioactivity which remained at
the origin yielded anthranilic acids, the 5-chloroanthranilic acid
predominating (Sen Gupta and Knowles, 1970).
In plants
A series of preliminary experiments carried out by Ciba (1965a, b) and
Schering (1966b) demonstrated that chlordimeform was quite rapidly
degraded in plant tissues with high inherent metabolic activity (i.e.
bean leaves) but was only slowly transformed in ripe fruits (Table V).
Green fruits, such as young grapes, and stems were observed to take an
intermediate position with regard to their ability to degrade the
acaricide.
TABLE V. RESIDUES OF CHLORDIMEFORM AND ITS DEGRADATION PRODUCTS IN AUSTRALIAN APPLES AFTER SPRAY APPLICATION OF THE
ACARICIDE (SPRAYING CONCENTRATION 0.05% a.i.) FROM CIBA (1965b)
Day Chlordimeform N-formyl-4-chloro-o-toluidine Desmethyl 4-chloro-o-toluidine
after ppm ppm chlordimeform ppm
spraying ppm
Apples
0 0.2 0.15 - -
1 0.6 <0.15 - -
2 0.8 <0.15 <0.04 <0.04
6 0.3 <0.15 - -
14 0.2 <0.05 <0.04 <0.04
21 0.4 <0.05 - -
30 0.4 <0.05 <0.04 <0,04
42 <0.05 <0.05 - -
Pears
0 0.50 0.25 - -
1 0.75 0.20 - -
2 0.45 0.10 <0.04 <0.04
6 0.75 0.10 - -
14 0.70 0.05 <0.04 <0.04
21 0.65 0.05 - -
30 0.60 <0.05 <0.04 <0.04
42 0.40 <0.05 - -
Tentative identification of the observed transformation products
indicated that in leaves both
N'-(4-chloro-o-tolyl)-N-methylformamidine
(desmethyl-chlorphenamidine, 11) and N-formyl-4-chloro-o-toluidine
(111) were prominent metabolites. On the other hand, in ripe apple and
pear fruits, only the formyl-derivative (III) was detected in
measurable quantities, whereas desmethyl-chlordimeform (II) was
normally absent (Table V). In all tissues analysed the free
4-chloro-o-toluidine (IV) was not detected at all or was present
only in small quantities. The lack of formation of significant residue
quantities of the free toluidine in edible plant parts, such as
fruits, was further examined by analysing plums which had been exposed
to a six times overdose treatment of chlordimeform (Ciba, 1967b). Even
after this application, which left a total residue of 4 to 6 ppm, the
amounts of free 4-chloro-o-toluidine were scarcely above the limit
of detection (0.05 ppm) of the colorimetric method applied.
Since chlordimeform was known to be quite volatile, the possibility
and extent of its evaporation from plant surfaces was investigated
(Ciba, 1968). Although the free base of the acaricide was observed to
readily evaporate from glass plates, disappearance from the surface of
bean leaves was considerably less pronounced. Losses by evaporation,
which occurred mainly during the first few hours after application,
were found to be of the order of 30 to 40% in terms of the original
dose applied. Evaporation was of the same order of magnitude when bean
leaves were treated with the hydro-chloride salt of chlordimeform.
This result suggested that, owing to the buffering capacity of plant
exudates on certain leaf surfaces, an equilibrium between free base
and salts was established, no matter which form of the acaricide was
applied. The behaviour of chlordimeform on leaf surfaces, as
described, was essentially confirmed by Sen Gupta and Knowles (1969)
when applying the acaricide to leaves of apple seedlings and by
Ehrhardt and Knowles (1970) when treating leaves of grapefruit
seedlings with both the free base and the hydrochloride salt.
Experiments carried out by Sen Gupta and Knowles (1969) on leaves of
apple seedlings and by Ehrhardt and Knowles (1970) on leaves of
grapefruit seedlings confirmed the limited ability of chlordimeform to
penetrate cuticular layers.
In the apple experiments (Sen Gupta and Knowles, 1969), 3H-as well
as 14C-labelled chlordimeform was applied to seedlings by either
leaf treatment or stem injection. The treated plants were cultured for
20 days and periodically analysed for the metabolites already
mentioned above. Sufficient quantities of the main transformation
products were collected to allow characterization by infra-red
analysis, melting point determinations, dye formation and
co-chromatography on thin-layer plates.
After both types of application dissipation/degradation of the
acaricide was observed to proceed at an intermediate rate, the
half-life of the compound being of the order of 12 to 16 days. Upon
termination of the experiment about 40% of the radioactivity applied
was still accounted for by the unchanged acaricide. Organosoluble
degradation products identified were desmethyl-chlordimeform (II),
N-formyl-4-chloro-o-toluidine (III) and free 4-chloro-o-toluidine.
The quantities of these metabolites relative to chlordimeform, however
were quite small and except for desmethyl-chlordimeform, never
exceeded 1% in terms of the radioactivity applied.
No so-called "non-extractable" radioactivity was evident after leaf
application of chlordimeform but was observed to be present in
increasing amounts (up to 30% of the quantity applied) after stem
injection of the acaricide. However, this non-extractable
radioactivity was confined to the stem section and could not be
observed in leaves to which part of the radioactivity had been
translocated. The authors suggested that non-extractable materials
represented chlordimeform degradation products that were complexed
with polymeric cell constituents.
The experiments with apple leaves also confirmed the limited ability
of chlordimeform to penetrate through cuticular plant tissues since
most of the radioactivity remaining on leaves could subsequently be
removed by rinsing them with organic solvents. No more and usually
less than 15% of the dose applied remained in the leaf tissues after
the rinsing process.
In the grapefruit experiments (Ehrhardt and Knowles, 1970) both the
free base and the hydrochloride salt of
14C-(tolyl-labelled)-chlordimeform were applied to the leaf surface
of growing seedlings. In these studies, dissipation of total
radioactivity was more pronounced than in the apple experiments, since
only 10 to 207 of the dose applied was recovered at the end of the
20-day observation period. It therefore appeared that a higher
percentage of chlordimeform evaporated from grapefruit than from apple
leaves. In addition, chlordimeform itself was degraded at a faster
rate by the citrus than by the apple tissue, since, after 20 days of
culturing, no more than 1% of the radioactivity applied was recovered
as the unchanged acaricide from the former tissue.
The pattern of metabolites on and in citrus leaves was essentially the
same as that reported for apple seedlings. Desmethyl-chlordimeform,
the formyl derivative and free 4-chloro-o-toluidine were the
principal metabolites, whose quantities, however, were small and never
exceeded 2% in terms of the radioactivity applied. Several unknown
minor radioactive metabolites were observed on thin-layer plates,
however, none of them represented more than 1% and normally did not
exceed 0.1% in terms of the radioactivity applied.
In several recent publications (Knowles et al., 1969a, b; Rosen et
al., 1970) which deal with in vitro chemical and biochemical model
systems, chlordimeform and its minor metabolite 4-chloro-o-toluidine
have been implicated or shown to be possible candidates for the
catalytic formation of azobenzene derivatives, such as
2,2'-dimethyl-4,4'-dichloroazobenzene (VII, Table I). Although a more
detailed discussion on the potential formation of azobenzene compounds
from chlordimeform is presented in the section on animal metabolism
("Biochemical aspects") results on the presence or absence of such
transformation products in plants are briefly discussed. In examining
field-treated fruits and vegetables for the presence of azobenzene
compounds, a sensitive gas-chromatographic residue method which
permitted the detection of 0.01 ppm of
2,2'-dimethyl-4,4'-dichloroazobenzene was used (Geissbühler et al.,
1971). This method was applied to apple fruits and leaves which had
been treated with a four times overdose of chlordimeform and which
were harvested 20, 30 and 40 days after application (Ciba, 1969b).
Although chlordimeform residues were found to be excessively high
during the whole observation period (10 to 4 ppm in fruits; 400 to 300
ppm in leaves), residues of the azobenzene compound were either not
detectable (<0.01 ppm in fruits) or were so small (0.04 ppm in
leaves) that they represented less than 0.02% in terms of the Parent
residue. Therefore, with normal concentrations of chlordimeform, no
detectable azobenzene residues are to be expected on edible crops.
From the extensive plant behaviour and metabolism data presented, the
pathways of chlordimeform transformation in plants are summarized as
follows (Sen Gupta and Knowles, 1969):
See also "Fate of Residues. In Animals".
TOXICOLOGICAL STUDIES
Special Studies
(a) Pharmacological studies
Chlordimeform hydrochloride was administered at graded doses of 0.01,
0.1, 1.0, 10 and 100 mg to the isolated perfused rabbit heart, the
organ being challenged with norepinephrine before and after dosing.
Contractile force was substantially decreased by 1.0 mg. Effect on
coronary flow and cardiac rate was less marked. Higher doses
temporarily stopped heart contractions. Guinea-pig isolated ileum was
exposed to graded doses of 1.0, 3.2, 10, 32, 100 and 320 µg/ml of
chlordimeform hydrochloride and evaluated for its effect on
acetylcholine, serotonin, histamine and barium induced contractions.
Bath concentrations of 3.2 µg/ml inhibited histamine contractions by
about 50% concentrations of 320 µg/ml were required to induce a
similar effect on acetylcholine, serotonin, and barium induced
contractions. In the intact dog, effect of graded doses of 1, 3, 10,
30 or 100 mg/kg chlordimeform hydrochloride on blood pressure, cardiac
rate, respiration, vasomotor response to epinephrine, acetylcholine,
histamine, tyramine, DMPP, carotid occlusion and peripheral vagal
stimulation were evaluated. Chlordimeform hydrochloride exerted a
hypotensive effect and caused increased respiration. A dose of 100
mg/kg was lethal. Vasomotor response to tyramine was enhanced, whereas
the pressor response to carotid occlusion was blocked (Teeters and
Blackmore, 1968).
(b) Reproduction studies
Rat. Four groups of 10 male and 20 female rats were fed 0, 100, 250
and 500 ppm chlordimeform in corn oil via the diet during three
parental, and three two-litter filial generations. Parental
body-weight prior to mating tended to be reduced in all test groups,
especially at 500 ppm. The same tendency was apparent with regard to
food consumption. Fertility index, gestation index, live birth index,
sex ratio, mean litter size, and birth weight of pups were comparable
to controls in all generations. At 500 ppm, lactation index was
reduced in F1a, F1b and F3a litters. Weaning weight of offspring was
depressed in all 500 ppm litters. Gross pathological examinations on
parents and pups dying during the study, and on 10 male and 10 female
weanlings of the F3b generation revealed no compound related effects
(Blackmore, 1969a).
Rabbit. Three groups of 10 impregnated female New Zealand white
rabbits (the day of impregnation being considered as day 0 of
gestation) were intubated on days 8 through 16 of gestation with 0,
7.5 or 30 mg chlordimeform/kg/day. Five rabbits per group were
sacrificed on day 28 of gestation. Parental mortality, abortion rate,
corpora lutea to implantation ratio, litter size, incidence of
resorptions, stillbirths, foetal weight, foetal length, and incidence
of skeletal, and tissue abnormalities were unaffected by the test
compound. In rabbits littering normally, gestation length, litter size
and litter weights were normal (Blackmore, 1969b).
(c) Studies on metabolites
Oral LD50 determinations in male and female rats.
Duration LD50
of (mg/kg)
Compound observation (days) References
N'-(4-chloro-o-tolyl)-N-methylformamidine 14 150 Sachsse and Bathe, 1971a
N-formyl-4-chloro-o-toluidine 14 2900 " " " 1970a
4-chloro-o-toluidine
(base) 7 ca1000 " " " 1971c
4-chloro-o-toluidine -HCl 14 860 " " " 1970d
Phenamidine (base) 7 ca1500 " " " 1971d
Phenamidine - HCl 14 860 " " " 1970f
o-chlordimeform (base) 7 300-400 " " " 1971f
o-chlordimeform - HCl 14 540 " " " 1970h
Dichlordimeform (base) 7 ca900 " " " 1971h
Dichlordimeform - HCl 14 260 " " " 1971j
Dermal LD50 determinations in male and female rats (24 hours occluded exposure).
Duration LD50
of (mg/kg)
Compound observation (days) References
N'-(4-chloro-o-tolyl)-N-methylformamidine 14 >2150 Sachsse and Bathe,1971b
N-formyl-4-chloro-o-toluidine 14 <2150 " " " 1970b
4-chloro-o-toluidine
(base) 7 ca1800 " " " 1971c
4-chloro-o-toluidine -HCl 14 <2150 " " " 1970e
Phenamidine (base) 7 ca1800 " " " 1971e
Phenamidine - HCl 14 <2150 " " " 1970g
o-chlordimeform (base) 7 ca300 " " " 1971g
o-chlordimeform - HCl 14 800 " " " 1970i
Dichlordimeform (base) 7 ca950 " " " 1971i
Dichlordimeform - HCl 14 <2150 " " " 1970k
Acute toxicity
ACUTE TOXICITY OF CHLORDIMEFORM BASE
LD50
Animal Route Sex (mg/kg) References
Mouse i.p. Mixed 110 Sachsse and Ullman, 1970
Rat Oral Male 178 Weir, 1968
Rat Oral Female 460 Weir, 1968
Rat Oral Male 220 Mastri et al., 1969
Rat Oral Female 170 Mastri et al., 1969
Dog Oral Female ca100 Weir, 1967
Dog Oral Male ca150 Hurni and Sachsse, 1969a
Dog Oral Female ca400 Hurni and Sachsse, 1969a
Rat Dermal Mixed 640 Sachsse and Bathe, 19701
Rat Inhalation* Mixed 17 400 Sachsse and Ullman, 1971
mg/m3
* Inhalation exposure was for one hour.
Hypoactivity, dyspnoea, muscular weakness, tremours, straub tail,
spasms and convulsions preceded death following oral administration.
Dyspnoea, exophthalmus, prostration, spasms and convulsions preceded
death following dermal application. No local skin irritation occurred.
No pathological changes were noted in rat following oral treatment,
although pale or blotchy livers, pale kidneys, and haemorrhagic
intestinal contents were observed after dermal treatment. In the dog,
oral administration resulted in congestion of liver, kidneys, and
lungs (the lungs also being oedomatous and haemorrhagic).
ACUTE TOXICITY OF CHLORDIMEFORM HYDROCHLORIDE
LD50
Animal Route Sex (mg/kg) References
Mouse Oral Mixed 220 Gunzel and Richter, 1967
Rat i.v. Male 95 Tripod, 1967
Rat s.c. Male 130 Tripod, 1967
Rat Oral Mixed 265 Gunzel and Richter, 1965
Rat Oral Mixed 355 Gunzel and Richter, 1965
Rat Oral Male 305 Tripod, 1967
Rat Oral Male 325 Mastri et al., 1969
Rat Oral Female 330 Mastri et al., 1969
Rat Dermal ca4000 Gunzel and Richter, 1966a
Rabbit Dermal >4000 Gunzel and Richter, 1966b
Rat Inhalation* >5.8 Sachsse and Ullman, undated
g/m3
* Inhalation exposure was for one hour.
Symptoms were similar to those for the base.
ACUTE TOXICITY OF CHLORDIMEFORM FORMULATIONS
LD50
Animal Route Sex Formulation (mg/kg) References
Mouse Oral Male EC 50 320 Aohi and Meda, 1966
Mouse Oral Female 50 s.p. 752 Shionogi C, undated
Rat Oral Mixed EC 50 610 Hurni and Sachsse, 1969b
Rat Dermal Mixed EC 50 2100 Sachsse and Bathe, 1971j
Rat Dermal Mixed 50 s.p. >3000 Hurni and Sachsse, 1969c
Rat Oral Mixed 50 s.p. 1100 Gunzel and Richter, 1969
Dog Oral Mixed 50 s.p. 400 Gunzel and Richter, 1968
A suicide victim ingested 30 ml of Galecron(R) 50 formulation. Upon
admission to hospital, an unknown time after drinking chlordimeform
solution, the patient was comatosed; and respiration and heartbeat had
ceased. The latter was restored by massage and adrenaline injection. A
respirator was used but death occurred within 24 hours. No autopsy was
performed (Oda, 1969).
Short-term studies
Rat. Four groups of 10 male and 10 female rats were intubated six
times weekly for one month with 0.5 ml/100 g body-weight of 2% CMC,
containing chlordimeform base at concentrations such as to give dose
levels of 0, 25, 50 or 100 mg/kg/dose. Body-weight was markedly
reduced in both sexes at 100 mg/kg/dose. Hyperexcitability was
observed in all test animals. At 100 mg/kg this was apparent 20-30
minutes after dosing, and was followed two to three hours after dosing
by decreased activity and apathy. Recovery was complete at four hours.
Similar effects were observed at 50, and 25 mg/kg/dose, but on a dose
related decreased scale, and with inconsistent frequency (Surber and
Cerioli, 1966).
Dog. Four groups of beagle dogs were fed 0 (10 male and 10 female)
250 (eight male and eight female), 500 (eight male and eight female)
or 1000 (10 male and 10 female) ppm of chlordimeform in a dry diet for
two years. Two male and two female dogs were sacrificed from each
group at 26, and 52 weeks. Body-weight was reduced at 1000 ppm, the
effect being slightly more pronounced in the females, Total leucocyte
counts were sporadically elevated in both sexes at 1000 ppm and in
females at 500 ppm. Haematocrit, haemoglobin, and erythrocyte counts
tended to be depressed terminally in both sexes at 1000 ppm. Sporadic
slight decreases in serum albumin were observed, more frequently in
males, at 1000 ppm. Terminal spleen to body-weight ratio was elevated
in males at 500 and 1000 ppm, and in females at 1000 ppm. Kidney to
body-weight ratio was elevated in females at 1000 ppm.
Histopathological examinations revealed bile duct hyperplasia,
pericholangitis and nodular hepatocytic hyperplasia at 500 and 1000
ppm in both sexes, and nodular hepatocytic hypertrophy at 1000 ppm in
both sexes in the liver. Kidneys showed an increased amount of
pigmentation at 500 and 1000 ppm in both sexes (Blackmore, 1969c).
Long-term studies
Rat. Five groups of 35 randomized male and 35 randomized female rats
(except at 100 ppm where 34 males and 36 females were used) were fed
0, 100, 250, 500 or 1000 ppm in the diet. The 100 ppm group commenced
treatment seven weeks after the other groups. This group was
originally part of the control group. Animals at that time were of
similar weight to those which had already been on test. The 1000 ppm
group was discontinued at three months due to severe growth
inhibition. Growth inhibition was observed in the males at 500 and
1000 ppm. In the females weight gain was reduced at 250 ppm and above.
In addition, female body-weight was reduced at 100 ppm between weeks
20 and 48. (This reduction may possibly be due to reduced food intake
(Weatherholtz, 1970) although this may not be the complete explanation
(Lyon, 1970).) Food intake was significantly reduced at 500 and 1000
ppm in both sexes. Dose related decreases in haematocrit, haemoglobin,
and erythrocyte counts, and a dose related increase in the leucocyte
count occurred in females at 250 and 500 ppm up to one year. During
the second year, haematocrit only was consistently depressed in
females at 500 ppm. Histo-pathological changes in the liver (nodules,
and foci of hyperplasia of hepatocytes) occurred in all groups, but
the incidence was greater at 250 and 500 ppm, and was more severe at
500 ppm. Some females at 500 ppm showed slight hypertrophy and
vacuolation of focal groups of cells in the adrenal cortex. Terminal
organ to body-weight ratios were increased in the liver (females at
250 and 500 ppm and males at 100 and 250 ppm), kidney (females at 250
and 500 ppm), thyroid (females at 250 and 500 ppm), heart (males at
250 ppm and females at 500 ppm), adrenals (males at 100 and 250 ppm)
and testes (100 and 500 ppm) (Blackmore, 1969d).
Comments
Chlordimeform appears to be fairly rapidly excreted in animals and
does not appear to be stored in body tissue. Information on metabolism
in man would however be of interest. A long-term feeding study is
available in the rat and a two-year study in the dog from which a
no-effect level was established. The Meeting expressed particular
concern about effect on organ to body-weight ratios in the rat and on
the histopathology of the liver and bile ducts of both the rat and the
dog. In these species nodular changes were observed in the liver but
histopathological information is insufficient to determine their
toxicological significance. Also of some concern were effects on the
total leucocyte counts of the dog and the pharmacological effects on
the heart and circulation together with the fact that the compound
appears to potentiate the effect of tyramine.
Because of the concern expressed above only a temporary acceptable
daily intake was established for this compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat - 100 ppm in the diet equivalent to 5 mg/kg body-weight per day
Dog - 250 ppm in the diet equivalent to 6.25 mg/kg body-weight per day
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Treatment of plants
Chlordimeform is a broad spectrum acaricide/insecticide applied to the
foliage of fruit, vegetables and other field crops acting through
contact and feeding and through vapour action on all stages of insects
and mites, including eggs of mites.
Pre-harvest treatments
Chlordimeform products have been registered in many countries for use
on a wide variety of crops of which typical examples are given in
Table 1.
Post-harvest treatment
No post-harvest treatments with chlordimeform are known.
Treatment of animals
In Australia chlordimeform is registered for use in cattle dips for
the control of cattle ticks (Boophilus micropus), the application
rate being 0.01-0.02% solution applied each 21 days by dipping often
in combination with organo-phosphorus acaricides such as dioxathion,
ethion, coumaphos, Dursban, bromophos-ethyl. Experimental work
designed to evaluate chlordimeform for use alone at a concentration of
0.1% is at an advanced stage.
Residues resulting from supervised trials
Fruit and vegetable crops
Detailed residue data are available from supervised trials in many
countries on many important crops and reports have been deposited with
FAO. The residue results are remarkably consistent between different
trials and different countries.
The most important and consistent feature of the residue trial data is
that the residue level remains remarkably constant irrespective of the
pre-harvest interval. On apples, grapes, citrus, peaches, pears, plums
and tomatoes the residue levels from sprays applied 30 days
pre-harvest are usually no more than half the level found when similar
rates were applied one to two days prior to harvest.
This rather unusual pattern is partly due to the analytical method
which determines all 4-chloro-o-toluidine containing metabolites
and/or conjugates as well as the unchanged parent compound.
Table II gives a representative sample of the results of supervised
residue trials carried out using registered label recommendations, use
patterns and rates of application.
Ciba and Schering (1971) have reported more than 50 trials designed to
determine the proportion of metabolites and parent compound in the
residues remaining on fruits at various intervals after application.
The separation of the components in the residue was performed by
thin-layer chromatography and by gas-chromatography.
TABLE II. REGISTERED USE PATTERNS FOR CHLORDIMEFORM
Pre-harvest
Country Crop Rate interval
Argentina pome fruits 0.06-0.09% 28 days
Australia cattle 0.02% 1 day
cotton 0.1%
pome & stone fruits 0.1% 7 days
strawberries 0.05% 7 days
Austria fruit trees 0.05% -
Brazil horticulture 0.05%
Canada fruit trees 0.06% 14-28 days
cole crops 0.05% 28 days
TABLE II. (cont'd)
Pre-harvest
Country Crop Rate interval
Denmark fruit trees 0.05% -
France pome fruits 0.05% 15 days
grapes 0.05% 15 days
vegetables 0.05% 15 days
Germany fruit trees 0.05% -
hops, grapes 0.05% -
Greece horticulture 0.05% -
Israel deciduous fruit - -
Italy fruit trees 0.05% -
citrus, grapes 0.05% -
Japan apples 0.05-0.025% -
citrus 0.05-0.035% -
pears 0.05-0.035% -
Yugoslavia fruit trees 0.05-0.15% -
vegetables 0.05-0.15% -
New Zealand pome fruits 0.06% 14 days
peaches 0.06% 14 days
strawberries 0.06% 7 days
Peru cotton 1 kg/ha 15 days
South Africa citrus 0.05% 14 days
tomatoes 0.075% 30 days
Switzerland pome fruits 0.05% 6 weeks
grapes 0.05-0.075% 6 weeks
Turkey apples 0.05% -
citrus, cherries 0.05% -
cotton -
USA apples 4 kg/ha 14 days
citrus 4-6 kg/ha -
pears 4 kg/ha 28 days
Venezuela cotton, citrus 0.3-0.5 kg/ha -
horticulture 0.3-0.5 kg/ha -
TABLE III
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Apples USA 1967 0.06 3 s.p. 3.3 1.3 1.3 1.4
USA 1969 0.06 2 s.p. 8.3 1.9 1.4 1.2 0.6
USA 1969 0.12 2 s.p. 8.6 4.5 2.8 1.2 1.7
USA 1969 0.12 3 e.c. 2.9 2.0 1.0 1.2
Canada 1969 0.06 2 s.p. 1.6
Australia 1969 0.04 3 s.p. 1.2 1.8 2.5 0.5 0.9 0.8
New Zealand 1970 0.05 1 s.p. 1.5 1.0 0.7 0.6
New Zealand 1970 0.1 1 s.p. 2.2 1.2 0.9 0.8
South Africa 1969 0.05 1 s.p. 0.6 0.6 0.5 0.5
England 1967 0.08 1 e.c. 3.3 2.6 2.7 1.2 1.2
Germany 1966 0.06 1 s.p. 1.8 1.9 1.6 0.8 0.5
Germany 1969 0.034 1 s.p. 1.5 1.0 0.6 0.5
Germany 1969 0.07 1 s.p. 2.5 1.8 1.5 0.7
Switzerland 1965 0.05 1 s.p. 1.1 1.0 1.2 0.6 0.5 0.5
Switzerland 1967 0.05 1 e.c. 2.3 1.3 1.0 0.5
Cherries USA 1968 0.06 1 e.c. 1.9 0.5 0.3 0.2
USA 1968 0.12 1 e.c. 3.7 1.3 1.1 0.7
Grapes USA 1969 0.06 2 s.p. 1.6 0.8 0.5 0.5
USA 1969 0.12 2 s.o. 2.9 2.8 1.6 1.8
Germany 1970 0.034 2 s.p. 2.0 1.3 1.3 1.0
Germany 1970 0.034 2 s.p. 2.8 2.3 2.3 1.8
Italy 1968 0.034 1 s.p. 2.6 1.2 1.2 0.6
Italy 1968 0.045 1 s.p. 1.8 1.5 1.2 1.2
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Oranges
Pulp Italy 1967 0.1 1 w.p. 0.2 0.3 0.2 0.3
Peel Italy 1967 0.1 1 w.p. 3.8 3.6 2.3 2.2
Whole Italy 1967 0.1 1 w.p. 1.1 1.1 0.8 0.7
Pulp Italy 1968 0.05 1 s.p. 0.2 0.3 0.1 0.2 0.08 0.1
Peel Italy 1968 0.05 1 s.p. 4.6 3.8 1.7 3.7 2.2 2.3
Whole Italy 1968 0.05 1 s.p. 1.5 1.2 0.5 1.1 0.7 0.7
Pulp Spain 1968 0.08 1 s.p. 0.4 0.4 0.4
Peel Spain 1968 0.08 1 s.p. 12.1 10.9 6.0
Whole Spain 1968 0.08 1 s.p. 3.5 3.5 2.0
Peaches USA 1969 0.06 1 e.c. 6.6 5.0 4.4 2.3
USA 1969 0.06 1 s.p. 5.9 3.4 2.8 1.9
Australia 1970 0.04 1 s.p. 1.0 1.2 0.4 1.7 0.6 0.4
Australia 1970 0.08 1 s.p. 2.0 2.0 1.1 3.1 1.4 1.1
France 1969 0.05 1 s.p. 1.5 1.1 0.5 0.5
France 1969 0.1 1 s.p. 1.5 0.9 1.0
USA 1970 0.06 2 e.c. 7.5 6.6 4.1 3.3
USA 1970 0.06 2 s.p. 3.7 3.2 2.4 1.5
Pears USA 1969 0.06 2 s.p. 7.8 6.1 4.4 3.5 2.8
USA 1969 0.06 2 e.c. 6.4 5.5 4.1 2.8 1.6
Australia 1970 0.04 1 s.p. 1.5 1.1 0.7 1.7 0.6 0.7
Australia 1970 0.08 1 s.p. 3.0 2.4 1.6 2.2 1.6 1.3
Plums USA 1970 0.06 2 e.c. 1.7 1.3 1.9 1.7
USA 1970 0.06 2 s.p. 0.5 0.5 0.5 0.4
Germany 1969 0.07 1 s.p. 0.7 0.5 0.4 0.3
Germany 1969 0.07 1 s.p. 1.1 1.2 0.6 0.5
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Strawberries USA 1968 0.06 1 e.c. 5.7 4.0 3.6 2.6 0.04
USA 1968 0.12 2 s.p. 1.8
England 1967 0.1 1 e.c. 1.8
England 1967 0.1 1 s.p. 2.1
Tomangos
Whole South Africa 1970 0.2 1 s.p. 2.7 2.6 2.9
Pulp South Africa 1970 0.2 1 s.p. 1.0 0.9 1.3
Peel South Africa 1970 0.2 1 s.p. 7.0 6.5 7.1
Walnuts
Meat USA 1968 0.06 1 s.p. <0.05
Meat USA 1970 0.06 2 s.p. <0.05
Beans
Pod Germany 1969 0.034 1 s.p. 0.5 0.2 0.1
Pod Germany 1969 0.07 1 s.p. 0.5 0.1 0.1
Broccoli
Head trimmed USA 1970 0.05 5 s.p. 1.7 0.8 0.4 0.2
Head trimmed USA 1970 0.1 5 s.p. 4.4 1.2 0.7 0.2
Head trimmed Canada 1970 0.05 2 s.p. 2.4 0.8
Head trimmed Canada 1970 0.05 6 s.p. 3.7 1.7 1.3
Brussels sprouts USA 1969 0.06 9 e.c. 2.8 2.1 1.9
Canada 1970 0.05 6 s.p. 3.6 3.5 3.1
Canada 1970 0.05 8 s.p. 5.5 3.8 2.8
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Cabbage
Head trimmed USA 1968 0.2 6 e.c. 4.3 0.6 0.2
Head trimmed USA 1969 1 e.c. 0.2
Head trimmed USA 1969 11 s.p. 1.5 1.2 1.4
4 outer leaves Canada 1969 4 s.p. 0.5
Inner head Canada 1969 4 s.p. <0.05
Cauliflower
Head trimmed USA 1969 6 s.p. 2.6 2.6 0.5
Head trimmed USA 1970 4 s.p. 3.6 2.5 1.9
Head trimmed USA 1970 4 s.p. 14.9 8.4 3.7
Cotton
Seed USA 1970 0.5 9 e.c. 0.22
Lint cotton USA 1970 0.5 9 e.c. 0.37
Delinted
cotton-seed USA 1970 0.5 9 e.c. 0.19
Linters USA 1970 0.5 9 e.c. 0.47
Cottonseed hulls USA 1970 0.5 9 e.c. 0.20
Solvent extract
meal USA 1970 0.5 9 e.c. 0.15
Screwpress
extracted meal USA 1970 0.5 9 e.c. 0.09
Crude solvent
extracted oil USA 1970 0.5 9 e.c. 0.11
Refined solvent
extracted oil USA 1970 0.5 9 e.c. 0.09
Crude screwpress
oil USA 1970 0.5 9 e.c. 0.17
TABLE III (cont'd)
Time interval in days
Fruit
sample Origin Year Concentration No. Formulation 0-1 2-4 5-9 10-19 20-30 31-60
Refined
screwpress oil USA 1970 0.5 9 e.c. 0.14
Seed USA 1970 0.2 6 e.c. 1.8 1.6 2.3
Seed USA 1970 6 s.p. 2.0 1.8 2.4
Oil crude USA 1968 10 s.p. 1.2
Oil refined USA 1968 10 s.p. 1.0
Oil crude USA 1968 10 s.p. 4.0
Oil refined USA 1968 10 s.p. 3.6
Oil crude USA 1970 9 e.c. 0.1
Oil refined USA 1970 9 e.c. 0.1
Sugar beet
Roots Germany 1968 1 s.p. <0.02
(60-120)
Roots Germany 1969 1 s.p. <0.02
<0.02
(60-120)
Tops Germany 1969 s.p. 0.1
<0.02
(60-120)
Tomatoes
Fruit Canada 1970 6 s.p. 0.6 0.2 0.2
Fruit USA 1970 7 e.c. 0.4 0.3 0.5 0.3
Table IV gives some typical examples of the results obtained in these
trials which involved commercial formulations applied at
concentrations normally used in practice.
TABLE IV
Fruit Location Interval Total Proportion of each
before residue metabolite%
harvest ppm
A B C D
Apples USA 30 0.92 87 <4 <4 <4
USA 90 0.42 55 <9 27 <9
Australia 2 1.03 75 <4 <15 <4
Australia 30 0.63 63 <10 <25 <10
Pears USA 30 1.78 79 <2 17 <2
USA 60 0.88 68 <3 23 <3
Italy 1 0.89 90 <3 <5 <3
Italy 21 0.62 80 <4 <8 8
Peaches Italy 1 2.32 95 <1 <5 <1
Italy 47 0.37 54 <5 35 <5
Prunes Switzerland 9 1.36 81 <1 15 <3
Switzerland 30 1.06 75 <2 20 <4
A = chlordimeform
B = N-(4-chloro-o-tolyl)-N-methylformamidine
C = N-formyl-4-chloro-o-toluidine
D = 4-chloro-o-toluidine
Where the sign < appears it is to be recognized that the limit of
detection, e.g. 0.02 or 0.04 ppm, means that the proportion may well
be much less than the figure shown.
A number of trials reported by Ciba and Schering (1971) show that
there is no significant difference in the level of chlordimeform
residues on the various fractions of cotton seed separated during
commercial processing. One series of results is shown in Table III
(see page 17). The fairly uniform distribution of residues between oil
and cotton seed meal may reflect the distribution of parent compound
and metabolite but possibly is also brought about by the analytical
procedure which does not distinguish metabolites from parent compound.
Animals
Ciba-Geigy (1971) report results of trials conducted in Australia to
determine the residue in cattle treated with chlordimeform to control
cattle tick. A calf weighing 200 kg was sprayed with 15 litres of
0.05% chlordimeform. The animal was slaughtered three days later and
residues of chlordimeform and metabolite hydrolysable to
4-chloro-o-toluidine were determined in tissues and fat with the
following results:
muscle 0.21 ppm omental fat 0.12 ppm
kidney 0.23 ppm subcutaneous fat 0.30 ppm
liver 0.62 ppm perirenal fat 0.21 ppm
In further trials cattle weighing 210 to 230 kg were dipped in 0.013%
chlordimeform and were slaughtered one or three days later. The
residues determined as above were as follows:
Interval
since Liver Fat Muscle Kidney
treatment
1 day 0.28±0.04 0.15±0.06 0.07±0.02 0.29±0.03
3 days 0.15±0.03 0.05±0.02 0.03±0.01 0.06±0.03
Buffering
As explained in some detail under "Fate of residues", chlordimeform
stability is extremely dependent on pH. Acceptable stability in cattle
dips can be achieved only if pH is held below 5.8 and preferably in
the range 5.0-5.5. In Australia this has been achieved by buffering
the dipping fluid with fertilizer grade superphosphate. Unbuffered
fluids must be used within a day of preparation.
At pH 5.2, some 99% of chlordimeform is present as the acid salt. In
this form it has adequate stability but its activity is reduced below
that of a similar concentration at a less acid pH value containing
proportionately more chlordimeform base.
Buffered dips leave only about half the hair deposits left by
unbuffered dips of equal concentration so that higher use levels are
required to produce the same acaricidal effect. Fortunately absorption
is also reduced and residue levels are not augmented by the increased
concentration required in dips.
Residues in milk and milk products
A Ciba-Geigy report (1971) describes milk and butter residues
following the treatment of lactating dairy cattle with 0.05%
chlordimeform, about 15 litres being applied to each animal. Three
animals were treated with a solution buffered to pH 5.6 and three with
an unbuffered solution of pH 9.4.
Time of 0.05% unbuffered 0.05% buffered
sampling Milk Butter Milk Butter
Pre-treatment 0.03 ppm 0.06 ppm <0.03 ppm 0.05 ppm
6 hours 0.22 ppm 1.52 ppm 0.06 ppm 0.52 ppm
24 hours 0.05 ppm 0.71 ppm 0.05 ppm 0.19 ppm
32 hours 0.08 ppm 0.18 ppm <0.03 ppm 0.14 ppm
48 hours 0.05 ppm 0.22 ppm <0.03 ppm 0.11 ppm
56 hours 0.16 ppm 0.10 ppm
72 hours 0.09 ppm 0.06 ppm
Each figure in the table is the mean of three individual samples.
The analytical technique employed determines the sum of chlordimeform
and all metabolites hydrolysable to 4 chloro-o-toluidine. The ratio
of milk residues to butter residues while somewhat variable, falls
consistently short of the level which would be expected if these
residues were partitioned exclusively in the fat phase.
The possibility that a proportion of the residues may be held in the
aqueous phase should be taken into consideration in the recommendation
of tolerances.
These results indicate the rapid rate of elimination which occurs
following spraying or dipping.
The level of residues is related to the concentration of the spray
solution or dip. Official trials in Australia showed that when the
concentration of the spray is increased from 0.03 to 0.3% the residue
levels in fat, kidney and liver of young calves increase in
approximately the same ratio. Residue levels in muscle do not
materially change in spite of the increase in concentrations. Residue
determinations were made 24 hours after spraying:
Adult cattle appear to metabolize the residue more rapidly because
animals weighing 450 kg dipped three times at 17-day intervals in a
bath containing 0.01% chlordimeform showed no residues in excess of
0.1 ppm when slaughtered three days after the third treatment. Adult
cattle weighing 300-520 kg sprayed with chlordimeform showed, as did
calves, that the residue level 24 hours after treatment increases
sharply with increasing concentration of spray. The residue level in
perirenal and omental fat increased from 0.1 ppm to 7.2 ppm when the
spray concentration was increased from 0.01 to 0.1%.
Concentration Liver Fat Muscle Kidney
of spray
0.03% <0.1 ppm 1.0 ppm <0.1 ppm 0.2 ppm
0.3% 1.2 ppm 10.0 ppm <0.1 ppm 0.5 ppm
Repeat treatments, as is normal in controlling cattle tick, does not
result in an accumulation of residues in animal tissues. Calves
receiving 10 dippings within eight weeks in a bath containing 0.01%
chlordimeform were slaughtered three days after the tenth treatment.
The results of residue analysis were as follows:
No. of Liver Fat Muscle
treatments
1 2.0 0.29 0.12
7 1.3 0.64 0.07
10 4.17 0.37 0.12
Fate of residues
General comments
Chlordimeform (I) is readily hydrolysed in weakly-acid to
weakly-alkaline solutions but is rather stable under strongly acid
conditions. In an aqueous buffer solution of pH 7.0 containing 5% of
methanol, the base showed a half-life of 42 hours at 30°C. At pH 9,
under otherwise equivalent conditions, a half-life of five hours was
observed (Kossmann et al., 1971). Hydrolysis of the parent compound
yields N-formyl-4-chloro-o-toluidine (III) and ultimately
4-chloro-o-toluidine (IV).
Structural alterations are observed when chlordimeform in solution or
on chromatoplates is exposed to irradiation by U.V. or natural
sunlight (Knowles and Sen Gupta, 1969). Although a number of
additional minor degradation products do occur,
N-formyl-4-chloro-o-toluidine (III) is the major transformation
product recovered.
A complete list of substances which appear to be potential metabolites
of chlordimeform is given in Table 1.
In animals
To supplement the studies described under "Biochemical aspects" on the
behaviour of chlordimeform in mammals, Ciba-Geigy Ltd (Ciba-Geigy,
1971) conducted experiments on the fate of the acaricide upon
continuous feeding to ruminants. Eight Brown Swiss cows were fed daily
rations containing from 4-24 ppm chlordimeform for periods of up to 42
days. Milk and tissue and organ samples were collected at regular
intervals and analysed by the total residue method (Geissbühler at
al., 1971) which accounts for all 4-chloro-o-toluidine containing
metabolites and/or conjugates of chlordimeform. At 21 days, residues
in liver were 0.09, and <O.03 ppm and in kidney were 0.38, and 0.07
ppm in animals fed 120 and 40 ppm respectively. Further cows fed 240
ppm in the diet showed liver levels of 0.45, 0.38, 0.50 and 0.44 ppm
at 7, 14, 21 and 42 days. Corresponding kidney residues were 0.05,
0.13, 0.13, and 0.09 ppm. Residues in milk, muscle and fat were below
0.03 ppm (Voss and Burkhard, 1971).
One male (36 kg) and one lactating female (39 kg) goat were given 10
µCi chlordimeform orally, urine and faeces being collected at 1, 3, 6,
12, 24, 48 and 72 hours post treatment. After 72 hours, the male had
voided 87%, and 1.8% of the administered radioactivity in the urine
and faeces respectively, and the female 68% and 1.8%. Only 0.3% of the
radioactivity appeared in the milk.
Chloroform extractable compounds identified by thin-layer
chromatography comprised chlordimeform,
N'-(4-chloro-o-tolyl)-N-methylformamidine, 4-chloro-o-toluidine,
and N-formyl-4-chloro-o-toluidine, the latter predominating. Of the
radioactivity 85-97% remained at the point of origin.
Re-chromatography with polar solvents yielded 5-chloroanthranilic
acid, and N-formyl-5-chloroanthranilic acid.
The 90% unextractable radioactivity, when incubated with
ß-glucuronidase, ß-glucuronidase-aryl sulphonase or hydrochloric acid
yielded chlordimeform, N'-14-chloro-o-tolyl-N-methylformamidine,
4-chloro-o-toluidine, N-formyl-4-chloro-o-toluidine and two
unidentified metabolites. N'-(4-chloro-o-tolyl)-N-ethylformamidine
was present in very small amounts, and the others in about equal
amounts. Re-chromatography of the 20% radioactivity which remained at
the origin yielded anthranilic acids, the 5-chloroanthranilic acid
predominating (Sen Gupta and Knowles, 1970).
In plants
A series of preliminary experiments carried out by Ciba (1965a, b) and
Schering (1966b) demonstrated that chlordimeform was quite rapidly
degraded in plant tissues with high inherent metabolic activity (i.e.
bean leaves) but was only slowly transformed in ripe fruits (Table V).
Green fruits, such as young grapes, and stems were observed to take an
intermediate position with regard to their ability to degrade the
acaricide.
TABLE V. RESIDUES OF CHLORDIMEFORM AND ITS DEGRADATION PRODUCTS IN AUSTRALIAN APPLES AFTER SPRAY APPLICATION OF THE
ACARICIDE (SPRAYING CONCENTRATION 0.05% a.i.) FROM CIBA (1965b)
Day Chlordimeform N-formyl-4-chloro-o-toluidine Desmethyl 4-chloro-o-toluidine
after ppm ppm chlordimeform ppm
spraying ppm
Apples
0 0.2 0.15 - -
1 0.6 <0.15 - -
2 0.8 <0.15 <0.04 <0.04
6 0.3 <0.15 - -
14 0.2 <0.05 <0.04 <0.04
21 0.4 <0.05 - -
30 0.4 <0.05 <0.04 <0,04
42 <0.05 <0.05 - -
Pears
0 0.50 0.25 - -
1 0.75 0.20 - -
2 0.45 0.10 <0.04 <0.04
6 0.75 0.10 - -
14 0.70 0.05 <0.04 <0.04
21 0.65 0.05 - -
30 0.60 <0.05 <0.04 <0.04
42 0.40 <0.05 - -
Tentative identification of the observed transformation products
indicated that in leaves both
N'-(4-chloro-o-tolyl)-N-methylformamidine
(desmethyl-chlorphenamidine, 11) and N-formyl-4-chloro-o-toluidine
(111) were prominent metabolites. On the other hand, in ripe apple and
pear fruits, only the formyl-derivative (III) was detected in
measurable quantities, whereas desmethyl-chlordimeform (II) was
normally absent (Table V). In all tissues analysed the free
4-chloro-o-toluidine (IV) was not detected at all or was present
only in small quantities. The lack of formation of significant residue
quantities of the free toluidine in edible plant parts, such as
fruits, was further examined by analysing plums which had been exposed
to a six times overdose treatment of chlordimeform (Ciba, 1967b). Even
after this application, which left a total residue of 4 to 6 ppm, the
amounts of free 4-chloro-o-toluidine were scarcely above the limit
of detection (0.05 ppm) of the colorimetric method applied.
Since chlordimeform was known to be quite volatile, the possibility
and extent of its evaporation from plant surfaces was investigated
(Ciba, 1968). Although the free base of the acaricide was observed to
readily evaporate from glass plates, disappearance from the surface of
bean leaves was considerably less pronounced. Losses by evaporation,
which occurred mainly during the first few hours after application,
were found to be of the order of 30 to 40% in terms of the original
dose applied. Evaporation was of the same order of magnitude when bean
leaves were treated with the hydro-chloride salt of chlordimeform.
This result suggested that, owing to the buffering capacity of plant
exudates on certain leaf surfaces, an equilibrium between free base
and salts was established, no matter which form of the acaricide was
applied. The behaviour of chlordimeform on leaf surfaces, as
described, was essentially confirmed by Sen Gupta and Knowles (1969)
when applying the acaricide to leaves of apple seedlings and by
Ehrhardt and Knowles (1970) when treating leaves of grapefruit
seedlings with both the free base and the hydrochloride salt.
Experiments carried out by Sen Gupta and Knowles (1969) on leaves of
apple seedlings and by Ehrhardt and Knowles (1970) on leaves of
grapefruit seedlings confirmed the limited ability of chlordimeform to
penetrate cuticular layers.
In the apple experiments (Sen Gupta and Knowles, 1969), 3H-as well
as 14C-labelled chlordimeform was applied to seedlings by either
leaf treatment or stem injection. The treated plants were cultured for
20 days and periodically analysed for the metabolites already
mentioned above. Sufficient quantities of the main transformation
products were collected to allow characterization by infra-red
analysis, melting point determinations, dye formation and
co-chromatography on thin-layer plates.
After both types of application dissipation/degradation of the
acaricide was observed to proceed at an intermediate rate, the
half-life of the compound being of the order of 12 to 16 days. Upon
termination of the experiment about 40% of the radioactivity applied
was still accounted for by the unchanged acaricide. Organosoluble
degradation products identified were desmethyl-chlordimeform (II),
N-formyl-4-chloro-o-toluidine (III) and free 4-chloro-o-toluidine.
The quantities of these metabolites relative to chlordimeform, however
were quite small and except for desmethyl-chlordimeform, never
exceeded 1% in terms of the radioactivity applied.
No so-called "non-extractable" radioactivity was evident after leaf
application of chlordimeform but was observed to be present in
increasing amounts (up to 30% of the quantity applied) after stem
injection of the acaricide. However, this non-extractable
radioactivity was confined to the stem section and could not be
observed in leaves to which part of the radioactivity had been
translocated. The authors suggested that non-extractable materials
represented chlordimeform degradation products that were complexed
with polymeric cell constituents.
The experiments with apple leaves also confirmed the limited ability
of chlordimeform to penetrate through cuticular plant tissues since
most of the radioactivity remaining on leaves could subsequently be
removed by rinsing them with organic solvents. No more and usually
less than 15% of the dose applied remained in the leaf tissues after
the rinsing process.
In the grapefruit experiments (Ehrhardt and Knowles, 1970) both the
free base and the hydrochloride salt of
14C-(tolyl-labelled)-chlordimeform were applied to the leaf surface
of growing seedlings. In these studies, dissipation of total
radioactivity was more pronounced than in the apple experiments, since
only 10 to 207 of the dose applied was recovered at the end of the
20-day observation period. It therefore appeared that a higher
percentage of chlordimeform evaporated from grapefruit than from apple
leaves. In addition, chlordimeform itself was degraded at a faster
rate by the citrus than by the apple tissue, since, after 20 days of
culturing, no more than 1% of the radioactivity applied was recovered
as the unchanged acaricide from the former tissue.
The pattern of metabolites on and in citrus leaves was essentially the
same as that reported for apple seedlings. Desmethyl-chlordimeform,
the formyl derivative and free 4-chloro-o-toluidine were the
principal metabolites, whose quantities, however, were small and never
exceeded 2% in terms of the radioactivity applied. Several unknown
minor radioactive metabolites were observed on thin-layer plates,
however, none of them represented more than 1% and normally did not
exceed 0.1% in terms of the radioactivity applied.
In several recent publications (Knowles et al., 1969a, b; Rosen et
al., 1970) which deal with in vitro chemical and biochemical model
systems, chlordimeform and its minor metabolite 4-chloro-o-toluidine
have been implicated or shown to be possible candidates for the
catalytic formation of azobenzene derivatives, such as
2,2'-dimethyl-4,4'-dichloroazobenzene (VII, Table I). Although a more
detailed discussion on the potential formation of azobenzene compounds
from chlordimeform is presented in the section on animal metabolism
("Biochemical aspects") results on the presence or absence of such
transformation products in plants are briefly discussed. In examining
field-treated fruits and vegetables for the presence of azobenzene
compounds, a sensitive gas-chromatographic residue method which
permitted the detection of 0.01 ppm of
2,2'-dimethyl-4,4'-dichloroazobenzene was used (Geissbühler et al.,
1971). This method was applied to apple fruits and leaves which had
been treated with a four times overdose of chlordimeform and which
were harvested 20, 30 and 40 days after application (Ciba, 1969b).
Although chlordimeform residues were found to be excessively high
during the whole observation period (10 to 4 ppm in fruits; 400 to 300
ppm in leaves), residues of the azobenzene compound were either not
detectable (<0.01 ppm in fruits) or were so small (0.04 ppm in
leaves) that they represented less than 0.02% in terms of the Parent
residue. Therefore, with normal concentrations of chlordimeform, no
detectable azobenzene residues are to be expected on edible crops.
From the extensive plant behaviour and metabolism data presented, the
pathways of chlordimeform transformation in plants are summarized as
follows (Sen Gupta and Knowles, 1969):
 Whereas in leafy tissues, stems or green fruits, the whole pathway of
degradation is operative to a small extent, in ripe fruits only
N-formyl-4-chloro-o-toluidine has consistently been observed as a
metabolite. The residue methods designed account for all metabolites
and/or conjugates (Geissbühler et al., 1971; Kossman et al., 1971).
In the course of an extensive study of the fate and persistence of
chlordimeform on six different crops Witkonton (1969) found that the
total amount of residue is directly related to the amount of chemical
applied, an inverse function of the number of days the fruit was
sampled after the last application of the chemical and influenced by
the nature of the fruit surface.
Fruit was analysed for chlordimeform and three possible major
degradation products. Only the parent compound and one degradation
product, N-formyl-2-methyl-4-chloroaniline, could be detected.
N-formyl-2-methyl-4-chloroaniline was found only in small amounts in
apples, pears and cherries; but greater amounts were found in plums
and strawberries. The results of this investigation did not show any
real correlation between the amounts of the parent compound and
N-formyl-2-ethyl-4-chloroaniline to such variables as amount of
chemical applied or sampling date. The type of fruit, and especially
the nature of the fruit surface, and such factors as sunlight and
rainfall appear to be predominant in affecting the degradation of
chlordimeform.
Witkonton (1969) also reported that chlordimeform appears to adhere to
the outer surface of fruit and does not appear to translocate readily
to the fleshy parts, The chief factors which seem to account for the
decrease of chlordimeform residues in fruit appear to be
volatilization, weathering and growth dilution.
In soil
Chlordimeform deposited inadvertently on soil surfaces after spray
application may be expected to be dissipated by the following
processes: volatilization, chemical hydrolysis, photodecomposition
(Knowles and Sen Gupta, 1969) and microbial degradation. Although no
special volatilization experiments with soil samples were conducted,
the contribution of this process is likely to be of the same order of
magnitude as or slightly higher than that observed for leaf surfaces.
Hydrolysis of chlordimeform to N-formyl-4-chloro-o-toluidine (III)
shown to be substantial under the slightly acid or alkaline conditions
which normally prevail in soils (Kossmann et al., 1971).
As regards microbial degradation of the acaricide, Johnson and Knowles
(1970) demonstrated that several bacterial, actinomycete and fungus
species are capable of extensively degrading chlordimeform. The
principal metabolite in the culture media of most species was
N-formyl-4-chloro-o-toluidine, except for the actinomycete
Streptomyces griseus which formed mainly 4-chloro-o-toluidine.
None of the microbes formed symmetrical azo-compounds.
Under field conditions, chlordimeform and its 4-chloro-o-toluidine
containing metabolites were dissipated according to first order
reactions with half-lives ranging from 20 to 40 days (Schering, 1969;
Ciba, 1969e). From the present experiments it way be concluded that
chlordimeform is not accumulated in the soil.
Evidence of residues in food in commerce or at consumption
No data were available to indicate the incidence or level of
chlordimeform residues in food commodities moving in commerce.
A number of detailed studies have been conducted to identify terminal
residues after application of chlordimeform. Results indicate that
breakdown of the parent compound takes place particularly in tissues
which are metabolically active, such as young bean leaves, while
fruits like apples and prunes show practically no metabolic breakdown
of the acaricide. The hydrolytic pathway of degradation leading to
small amounts of N-formyl-4-chloro-o-toluidine has been demonstrated
to occur to a certain extent also on fruit crops. Hydrolysis of
chlordimeform, however, apparently takes place independent of plant
tissue activities due to the chemical instability of the parent
compound (Kossmann et al., 1971),
Furthermore it has been confirmed that
2,2'-dimethyl-4,4'-dichloroazobenzene is not expected to be a
potential transformation product of chlordimeform in apple fruit.
All chlordimeform metabolites identified so far in various plant
materials are determined by the described total residue methods.
A number of special investigations reveal that chlordimeform residues
are located in the outer parts of crops such as fruit peels or outer
leaves of cole crops. Excessive residues therefore might be removed by
peeling of fruit (apples, citrus) or by trimming the outer leaves of
cole crops.
Washing of fruit will remove only a small part of the total residue.
External residues will probably dissipate by volatilization, rainfall,
or penetration into the cuticular wax layers,
Schering (1971) reports on the effect of cooking on chlordimeform
residues in apples, grapes, tomatoes, cauliflower, beans and sugar
beet foliage and shows that the behaviour of chlordimeform residues
during cooking depends on the pH value of the aqueous suspension as
well as on the duration of the cooking period.
This report shows that the rate of loss of chlordimeform is a function
of pH value, the loss being much more rapid in a neutral medium such
as green beans (pH 5) or cauliflower (pH 6) than in apples (pH 2.5) or
tomatoes (pH 3). The hydrolysis proceeds according to the scheme
outlined in page 30 and all metabolites are determined by the
analytical method for chlordimeform parent compound. The loss of total
4-chloro-o-toluidine moiety is not significant showing that
volatilization in steam is not an important contributing factor.
Methods of residue analysis
(a) Methods for the detection of unchanged chlordimeform and its
breakdown products (terminal residues)
Identification of terminal residues in plant materials and specific
determination of unchanged chlordimeform has been carried out by
thin-layer and/or gas chromatography procedures. A detailed
description of the separation and detection of
N'-(4-chloro-o-tolyl)-N-methylformamidine,
N-formyl-4-chloro-o-toluidine, and 4-chloro-o-toluidine as well as
the parent compound including quantitation by colorimetry or flame
ionization gas chromatography has been given by Kossmann et al.
(1971).
Investigations for the presence of
2,2'-dimethyl-4,4'-dichloroazobenzene have been performed by a gas
chromatographic procedure following reductive cleavage of the azo
compound and subsequent Sandmeyer iodination of the resulting
4-chloro-o-toluidine (Geissbühler et al., 1971).
Selective determination of unchanged chlordimeform may be needed after
usage of chlordimeform/formetanate mixtures. Both active substances
have the structure of formamidine derivatives. For this combination
product a thin-layer chromatographic procedure has been established
(Schering AG, 1969b) which includes direct quantitation on the
chromatoplate by reflectance measurement in the UV range. Gas
chromatographic procedures described above allow specific
determination of chlordimeform in presence of formetanate as well and
account then for the total chlordimeform residue including
metabolites.
(b) Total chlordimeform residue methods
For regulatory purposes and routine residue analysis methods were
developed that account in a single procedure for chlordimeform and for
all its 4-chloro-o-toluidine containing breakdown products and/or
conjugates. Principle of determination of "total chlordimeform" is
based on a two step hydrolysis by successive treatments with acetic
acid and sodium hydroxide respectively. The joint hydrolysis product,
4-chloro-o-toluidine, is steam distilled and extracted into
iso-octane. Quantitation of 4-chloro-o-toluidine may be carried out
by colorimetry (specificity limited) or gas chromatography (highly
specific).
Colorimetry is based upon diazotization reaction of
4-chloro-o-toluidine and coupling with N-ethyl-1-naphthylamine
(Geissbühler et al., 1971). A number of investigations has been made
by using 1-naphthylethylenediamine as a coupling agent (Schering AG,
1968). Both procedures are sensitive to 0.05 ppm of chlordimeform as
calculated for a 50-gram crop sample. Recovery values obtained from
various crops fortified within the range of 0.05 to 5 ppm
chlordimeform in general exceeded 85% (Geissbühler et al., 1971;
Schering AG, 1969a).
An interlaboratory study demonstrated the sufficient reproducibility
of the total residue procedures established. Since specificity of
colorimetric evaluation is limited, additional identification of
chlordimeform residues may be done by confirmatory thin-layer
chromatography of the resulting azo dye on cellulose plates
(Geissbühler et al., 1971).
Gas chromatography has also been effectively used for quantitative
determination of 4-chloro-o-toluidine moiety following
transformation into halogenated derivatives of extremely high electron
affinity. Sandmeyer iodination reaction of 4-chloro-o-toluidine
results in the 5-chloro-2-iodo-toluene structure and a 0.05 ppm
detection limit for chlordimeform by using a tritium foil equipped
electron capture detector. Recovery figures correspond very well to
those reported for colorimetric measurement (Geissbühler et al.,
1971).
A lower detection limit may be attainable by bromination of
4-chloro-o-toluidine in aqueous solution yielding
6-bromo-4-chloro-o-toluidine and subsequent gas chromatographic
determination using a Ni63 electron capture detector (Kossmann,
1971),
Direct gas chromatographic measurement of 4-chloro-o-toluidine by a
halogen microcoulometric titration cell has been reported by Del Monte
Corporation (Del Monte, 1969) for the determination of chlordimeform
residues in crops.
All the gas chromatographic procedures mentioned are specific for
chlordimeform residues. Up to date no other pesticide with the
4-chloro-o-toluidine nucleus is known to be used in agricultural
practice.
Examples of national tolerances
The following are examples of national tolerances and withholding
periods that have been established:
Country Crop Tolerance Withholding
ppm period days
Australia pome fruit 2 7
stone fruit 2 7
strawberries 1 7
fat of meat of cattle 0.5 1
Country Crop Tolerance Withholding
ppm period days
Canada pears 5 28
peaches, plums, prunes 4 14
apples 3 14
cauliflower, brussels
sprouts 3 28
broccoli 2 28
cabbage 0.5 28
turnip roots <0.05 28
Germany (GFR) grapes, stone fruit
(excl. cherries) 3 14
pome fruit 2
Italy fruit, citrus, grapes 1 20
New Zealand pome fruit 1 14
strawberries 7
Switzerland pome fruit, grapes 1 42
South Africa apples, pears, citrus 1 14
USA pears 5 28
apples 3 14
cabbage, cauliflower,
broccoli, brussels
sprouts 2 28
Venezuela fruit, citrus 2 14
Appraisal
Chlordimeform is a new insecticide/acaricide primarily effective
against eggs and larvae of spider mites with adequate activity also
against adult mites. It is effective against mites resistant to
organo-phosphorus insecticides. It kills eggs, larvae and adults not
only by contact but also in the vapour phase. Penetration and slight
systemic effect have been demonstrated. It is also effective in
control of some lepidopterous insects including codling moth but the
major field of use so far developed is in the control of mites on
deciduous fruit. Chlordimeform is also being used commercially in
Australia for the control of cattle tick by spraying and dipping
because of its ability to control ticks which have developed strains
resistant to all other currently available acaricides.
Chlordimeform is registered for use on fruit trees, grapes and
vegetables in many countries and to a lesser extent on cotton and
hops. The concentration applied ranges from 0.01 to 0.1%.
Chlordimeform is used both as the base and as the hydrochloride. The
base is formulated as an emulsifiable solution while the hydrochloride
is used as a water soluble powder.
The residue data available to the meeting were obtained from
supervised field trials in Australia, Canada, England, France,
Germany, Italy, New Zealand, South Africa, Spain, Switzerland and the
United States of America. Whilst there is a sharp drop in the residue
level between the day of application and the second or third day
post-treatment, thereafter the rate of decline is remarkably slow with
a half-life on apples, grapes, pears and tomatoes exceeding 21 days.
The rate of decline is somewhat faster on cruciferous vegetables but
even so the interval between application and harvest does not have a
pronounced bearing on the residues level following approved
treatments.
Loss of residue is initially due to volatilization but the portion of
the deposit absorbed or dissolved into surface tissues of plants
appears to remain at a relatively constant level. The residue data
reflect this durability but they are influenced by the fact that the
analytical procedure determines both parent compound and all
metabolites containing the toluidine moiety.
Extensive evidence is available on the fate of residues in plants and
animals as well as on the route and rate of degradation. The initial
pathway of metabolism in animals and plants is substantially similar.
leading to N-formyl-4-chloro-o-toluidine and 4-chloro-o-toluidine
in both organisms but proceeding further to
N-formyl-5-chloroanthranilic acid and 5-chloroanthranilic acid
respectively in animals.
Some data were available on the effect of processing and cooking as
the fate of chlordimeform residues. The chlordimeform residue
generally decreases with cooking, the decline being dependent upon
length of cooking time. The rate of decrease is a function of the pH
value. In a more neutral medium caused by green beans (pH 5) or
cauliflower (pH 6) loss of chlordimeform is much more rapid than in an
acid medium such as apples (pH 2.5) or tomatoes (pH 3). Studies
revealed no detectable change in the residue level or composition when
apples and pears were held in cold storage. Evidence was presented to
show that substantially all the residue occurs in the skin of apples
and citrus; in the outer leaves of cruciferous vegetables and in the
tops of root vegetables. Chlordimeform, applied to cotton, appears as
a residue in cotton seed cake as well as in cotton seed oil and the
residue level is not significantly reduced by refining.
Analytical methods based on the determination of
4-chloro-o-toluidine formed by successive treatments with acetic
acid and sodium hydroxide are specific for chlordimeform and its
metabolites and are capable of determining levels down to 0.05 ppm.
Quantitation may be made either by colorimetry based upon
diazotization and coupling with N-ethyl-1-naphthylamine or by gas
chromatography. Though direct measurement by gas chromatography is
possible, the accuracy and sensitivity is improved by transformation
of the residue into halogenated derivatives of extremely high electron
affinity.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Temporary tolerances (effective to June 1975)
Pears, peaches, prunes 5 ppm
Apples, grapes, plums, strawberries 3 ppm
Brassicas, cherries, citrus fruit,
cotton seed oil (crude and refined),
cotton seed 2 ppm
Beans 0.5 ppm
Fat, meat and meat products of cattle 0.5 ppm
milk (whole) 0.05 ppm
Butter 0.5 ppm
Further work or information
Required (before 30 June 1975)
1. A further long-term feeding study in rats to obtain precise
information on the incidence and nature of the histopathological
changes in the liver and bile ducts of rats exposed to levels
above and below 100 ppm in the diet. An effort should be made to
explain the changes in organ to body-weight ratios noted in the
previous long-term rat study.
2. Further investigation of the nature of the hepatic lesions
observed in the dog.
3. Metabolic studies in several animal species, preferably including
man.
4. Further data on the nature and levels of residues in animal
tissues after use in cattle sprays and dips.
5. Further data on residues in milk after use in cattle sprays and
dips, especially information on the nature of the residues and
their distribution between aqueous and lipid phases.
6. Data on the residue levels in commercial butter and cheese.
Desirable
1. Further studies on the haematological effects.
2. Elucidation of the pharmacodynamic activity on the heart,
including the potentiation of the effects of pressor amines.
3. Further data on the disappearance of residues during storage,
processing, and cooking.
4. Data on residue levels in raw agricultural commodities moving in
commerce.
REFERENCES
Aoki, H. and Ueda, K. (1966) Unpublished report of the Tokyo Dental
College submitted by Ciba-Geigy
Bertha, R. and Framer, D. (1967) Pesticide transformation to aniline
and azo compounds in soil. Science, 156: 1617
Blackmore, R. H. (1969a) Three generation reproduction study - rats.
Unpublished report of Hazelton Laboratories, submitted by Ciba-Geigy
Blackmore, R. H. (1969b) Teratology study - rabbits. Unpublished
report of Hazelton Laboratories submitted by Ciba-Geigy
Blackmore, H. H. (1969c) Two year repeated feeding - dogs. Schering
361 268 (Chlorphenamidine). Unpublished report from Hazelton
Laboratories, submitted by Ciba-Geigy
Blackmore, R. H. (1969d) Two year repeated feeding - rats. Schering
361 268 (Chlorphenamidine). Unpublished report from Hazelton
Laboratories, submitted by Ciba-Geigy
Ciba Ltd. (1965a) C-8514. Rückstände in den Blättern der Bohne. Report
of 20 May 1965
Ciba Ltd. (1965b) C-8514. Residues in Australian apples and pears.
Report of 25 May 1965
Ciba Ltd. (1967a) Modell einer Synthese von radioaktivem Galecron.
Report of 17 August 1967
Ciba Ltd. (1967b) Galecron. Absence of 4-chloro-o-toluidine as a
metabolite in prunes after overdose spraying. Report of 31 January
1967
Ciba Ltd. (1968) Galecron. Behaviour of different formulations on
citrus leaves. Report of 14 May 1968
Ciba Ltd. (1969a) Galecron. Residues in Italian oranges. Report of 2
March 1969
Ciba Ltd. (1969b) Galecron. Analysis of Swiss apples for residues of
2,2'-dimethyl-4,4'-dichloroazobenzene after overdose spraying. Report
of 20 November 1969.
Ciba Ltd. (1969c) Galecron. Formation of a symmetrical azo derivative
from 4-chloro-o-toluidine by coupled peroxidatic reactions. Report
of 12 September 1969
Ciba Ltd. (1969d) Galecron. Degradation of chlorphenamidine by rat
liver and spleen homogenates, with particular reference to the
possible formation of azo derivatives. Report of 10 September 1969
Ciba Ltd. (1969e) Galecron. Dissipation in a Swiss soil as determined
by chemical analysis. Report of 16 September 1969
Ciba-Geigy Ltd. (1971) Galecron. Residues in milk, meat, fat, liver
and kidney of Swiss cows. Report of 19 March 1971
Ciba-Geigy Ltd. (1971) Determination of chlorphenamidine residues in
milk and butter following treatment with "Spike" cattle dip at 0.05%
a.i. w/v. Report of 6 August 1971
Del Monte Corporation Research Center. (1969) Determination of total
chlorphenamidine residues in plant material, 10.6.1969. Unpublished
Dittrich, V. (1971) Biological action of chlordimeform. Report
prepared for FAO/WHO
Ehrhardt, D. A. and Knowles, C. O. (1970) Metabolism and translocation
of N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine (chlorphenamidine)
and its hydrochloride salt in grapefruit seedlings. J. Econ. Entomol.,
63: 1306
Geissbühler, H. (1969) The substituted ureas. In: Degradation of
herbicides (P. C. Kearney and D. D. Kaufman, ed.), p. 79. New York,
Marcel Dekker Inc.
Geissbühler, H., Kossmann, K., Baunok, I. and Boyd, V. F. (1971)
Determination of total residues of chlorphenamidine
[(N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine] in plant and soil
material by colorimetry and thin-layer and electron capture gas
chromatography. J. Agr. Food Chem., 19: 365
Gerhards, E. and Kolb. (1966) Unpublished report of Schering A.G.
submitted by Ciba-Geigy
Gerhards, E. (1967) Unpublished report of Schering A.G. submitted by
Ciba-Geigy
Gunzel, P. and Richter. (1965) Systemische Vertraglichkeitsprufung an
Ratten bei einmaliger p.o. Verabreichung (DL50). Unpublished report
from Schering A.G. submitted by Ciba-Geigy
Gunzel, P. and Richter. (1966a) Systemische Vertraglichkeitsprufung an
Ratten bei einmaliger dermaler. Verabreichung (DL50). Unpublished
report of Schering AG, submitted by Ciba-Geigy
Gunzel, P. and Richter. (1966b) Systemische Vertraglichkeitsprufung an
Kaninchen bei einmaliger dermaler Verabreichung (DL50). Unpublished
report of Schering A.G., submitted by Ciba-Geigy
Gunzel, P. and Richter. (1968) Systemische Vertraglichkeitsprufung an
Hunden bei einmaliger p.o. Verabreichung (DL50). Unpublished report
from Schering AG., submitted by Ciba-Geigy
Gunzel, P. and Richter. (1969) Systemische Vertraglichkeitsprufung an
Ratten bei einmaliger p.o. Verabreichung (DL50), Unpublished report
of Schering AG., submitted by Ciba-Geigy
Herbert, R. A. (1969) Methyl and phenylcarbamates. In: Degradation of
herbicides (P. C. Kearney and D. D. Kaufman, ed.), p. 113, New York,
Marcel Dekker Inc.
Hurni, H. and Sachsse, K. (1969a) Report on the determination of the
acute oral LD50 to the dog of chlorphenamidine technical.
Unpublished report of Tierfarm AG, submitted by Ciba-Geigy
Hurni, H. and Sachsse, K. (1969b) Report on the determination of the
acute oral LD50 to the rat of Galecron 50 EC. Unpublished report of
Tierfarm AG, submitted by Ciba-Geigy
Hurni, H. and Sachsse, K. (1969c) Report on the determination of the
acute dermal LD50 to the rat of Galecron 50 SP. Unpublished report
of Tierfarm AG, submitted by Ciba-Geigy
Johnson. B, T. and Knowles, C. O. (1970) Microbial degradation of the
acaricide N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine. Bull.
Environ. Contam. Toxicol., 5: 158
Knowles, C. O. (1970) Metabolism of two acaricidal chemicals,
N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine (chlorphenamidine) and
m-{[dimethylamino methylene] amino} phenyl methyl-carbamate
hydrochloride (formetanate). J. Agr. Food Chem., 18: 1038
Knowles, C. O. (1970) Microbial degradation of chlordimeform. Bull
Envir. Contam. Toxicol., 5 (2): 158
Knowles, C. O. and Sen Gupta, A. K. (1969) Photodecomposition of the
acaricide N'-(4-chloro-o-tolyl) N,N-dimethylformamidine. J. econ.
Entomol., 62: 344
Knowles, C. O. and Sen Gupta, A. K. (1970)
N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine-14C (Galecron) and
4-chloro-o-toluidine metabolism in the white rat. J. econ. Entomol.
63: 856
Knowles, C. O., Sen Gupta, A. K. and Hassan, T. K. (1969a) Formation
of symmetrical azo derivatives of monochlorotoluidines by peroxidatic
catalysis. J. econ. Entomol., 62; 411
Knowles, C. O. and Sen Gupta, A. K. (1969b) Formation of symmetrical
azo derivative of 4-chloro-o-toluidine in a ferrous iron-hydrogen
peroxide system. J. econ. Entomol., 62: 1229
Kossmann, K. (1971) New aspects upon electron capture gas
chromatography of acylanilide, phenylcarbamate, and phenylurea type
herbicides. Proc. 2nd Intern. Congr. Pesticide Chemistry, Tel Aviv,
1971
Kossmann, K., Geissbühler, H. and Boyd, V. F. (1971) Specific
determination of chlorphenamidine
[N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine] and of some
potential metabolites in plant material by thin-layer and flame
ionisation gas chromatography. J. Agr. Food Chem., 19: 360
Lyon, D. A. (1970) Chlorphenamidine on weight gain of female rats.
Internal unpublished memorandum. Food and Drug Directorate, Canada
Mastri, C., Keplinger, M. L. and Francher, O. E. (1969) Acute oral
toxicity study on two samples of chlorphenamidine in male and female
albino rats. Unpublished report from Industrial Biotest Labs. Inc.
submitted by Ciba-Geigy
Oda. (1969) Suicide by drinking Galecron. Unpublished report from
Chikugo Municipal Hospital, made available by Nor-Rh Chemicals
Rose, J. A. (1969a) Degradation of chlorphenamidine by rat liver and
spleen homogenatis with particular reference to the possible formation
of azo-derivatives. Unpublished report submitted by Ciba-Geigy
Rose, J. A. (1969b) Formation of a symmetrical azo-derivative from
4-chloro-o-toluidine by coupled peroxidatic reactions. Unpublished
report submitted by Ciba-Geigy
Rosen, J. D., Siewierski, M. and Winnett, G. (1970) FMN-sensitized
photolyses of chloroanilines. J, Agr. Food Chem., 18: 494
Sachsse, K. and Bathe, R. (1970a) Report on the determination of the
acute oral LD50 of formylchlorotholuidine to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Giegy
Sachsse, K, and Bathe, R. (1970b) Report an the determination of the
dermal LD50 of formylchlorotoluidine to the rat. Unpublished report
of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970c) Report on the determination of the
acute oral LD50 of 5-chloro-2-amino-toluene (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1970d) Report on the determination of the
acute oral LD50 of 5-chloro-2-amino toluene-HCl to the rat.
Unpublished report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970e) Report on the determination of the
acute dermal LD50 of 5-chloro-2-amino-toluene-HCl to the rat.
Unpublished report of Tierfarm A.G. submitted by Ciba Geigy
Sachsse, K. and Bathe, R. (1970f) Report on the determination of the
acute oral LD50 of phenamidine-HCl to the rat. Unpublished report of
Tierfarm AG submitted by Ciba-Geigy
Sachsse. K. and Bathe, R. (1970g) Report on the determination of the
acute dermal LD50 of phenamidine-HCl to the rat. Unpublished report
of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970h) Report on the determination of the
acute oral LD50 of o-chlorphenamidine-HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970i) Report on the determination of the
acute dermal LD50 of o-chlorphenamidine-HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970j) Report on the determination of the
acute oral LD50 of dichlorphenamidine-HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970k) Report on the determination of the
acute dermal LD50 of dichlorphenamidine HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970l) Report on the determination of the
acute dermal LD50 of technical chlorphenamidine (base) to the rat.
Unpublished report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1971a) Report on the determination of the
acute oral LD50 of technical C8520 to the rat. (Dimethyl
chlordimeform). Unpublished report of Tierfarm AG submitted by
Ciba-Geigy
Sachsse, K. and Bathe, R. (1971b) Report on the determination of the
acute dermal LD50 of technical C8520 to the rat. Unpublished report
of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1971c) Report on the determination of the
acute dermal LD50 of 5-chloro-2-amino toluene (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe. R. (1971d) Report on the determination of the
acute oral LD50of phenamidine (base) to the rat. Unpublished report
of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971e) Report on the determination of the
acute dermal LD50 of phenamidine (base) to the rat. Unpublished
report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971f) Report on the determination of the
acute oral LD50 of o-chlorphenamidine (base) to the rat. Unpublished
report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971g) Report on the determination of the
acute dermal LD50 of o-chlorphenamidine (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971h) Report on the determination of the
acute oral LD50 of dichlorphenamidine (base) to the rat. Unpublished
report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971i) Report on the determination of the
acute dermal LD50 of dichlorphenamidine (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971j) Report on the determination of the
acute dermal LD50 of Galecron 50EC to the rat. Unpublished report of
Ciba-Geigy
Schering AG. (1965) Versuche zur Kenntnis des Eindringvermögens von
36268 in Aepfel
Schering AG. (1966a) Chlorphenamidin. Stoffwechselversuche Stand Dez.
1966
Schering AG. (1966b) Rückstandsuntersuchungen Schering 36268. 1.
Mitteilung 1966. Erntegut: Weintrauben, Aepfel, Birnen, Pfirsich
Schering AG. (1967) Chlorphenamidine. Stoffwechselversuche Stand Dez.
1967
Schering AG. (1968) Rückstandsbestimung von Chlorphenamidin (Schering
3628) in Pflanzenmaterial, 36 268/6, 13.9.1968 (unpublished)
Schering AG. (1969) Chlorphenamidin 1/69. Abbaugeschwindigkeit von
Chlorphenamidin-Hydrochlorid im Boden
Schering AG. (1969a) Chlorphenamidin III/69, Verfahrensausbeuten nach
36 268/6, 17.3.1969 (unpublished)
Schering AG. (1969b) Method for the determination of Fundal(R) forte
residues in plant material using thin-layer chromatography,
36 056 - 36 268/1, 18.11.1969
Sen Gupta, A. K. and Knowles, C. O. (1969) Metabolism of
N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine by apple seedlings.
J. Agr. Food Chem., 17: 595
Sen Gupta, A. K. and Knowles, C. O. (1970) Galecron-14C
[N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine] metabolism in the
dog and goat. J. Econ. Entomol., 63: 951
Shionogi and Co. (Undated) Unpublished report of the Aburahi Labs. of
Shionogi and Co. Ltd,
Surber, W. and Cerioli, A. (1966) Prolonged toxicity study on rats of
Ciba's preparation, 39, 12. (C8514). Unpublished report from Battelle
Institute submitted by Ciba-Geigy
Teeters, W. R. and Blackmore, R. H. (1968) Pharmacological study of
chlorphenamidine in the perfused rabbit heart, isolated guinea-pig
ileum, and dog pharmacodynamic preparation. Hazelton Laboratories
report C5230, made available by Nor-Am. Chemicals
Tripod, J. (1967) Unpublished report from Ciba Ltd., made available by
Nor-Am Chemicals
Voss, G. and Burkhard, N. (1971) Unpublished report submitted by
Ciba-Geigy
Weatherholtz, W. M. (1970) Statistical analysis of body weight data
and food consumption data, chlorphenamidine. Unpublished report from
Hazelton Labs. Inc. made available by Nor-Am. Agricultural Products
Ltd.
Weir, R. J. (1967) Unpublished report from Hazelton Labs. made
available by Nor-Am. Chemicals
Weir, R. J. (1968) Acute oral administration - rats. Unpublished
report from Hazelton Labs., made available by Nor-Am. Chemicals
Williams, C. T. (1959) Detoxification mechanisms. London, Chapman and
Hall
Witkonton, Sujit. (1969) The fate and persistence of chlorphenamidine
on six different crops. Thesis for degree of Master of Science,
Department of Entomology, Pennsylvania State University, September
1969 (53 pp)
Whereas in leafy tissues, stems or green fruits, the whole pathway of
degradation is operative to a small extent, in ripe fruits only
N-formyl-4-chloro-o-toluidine has consistently been observed as a
metabolite. The residue methods designed account for all metabolites
and/or conjugates (Geissbühler et al., 1971; Kossman et al., 1971).
In the course of an extensive study of the fate and persistence of
chlordimeform on six different crops Witkonton (1969) found that the
total amount of residue is directly related to the amount of chemical
applied, an inverse function of the number of days the fruit was
sampled after the last application of the chemical and influenced by
the nature of the fruit surface.
Fruit was analysed for chlordimeform and three possible major
degradation products. Only the parent compound and one degradation
product, N-formyl-2-methyl-4-chloroaniline, could be detected.
N-formyl-2-methyl-4-chloroaniline was found only in small amounts in
apples, pears and cherries; but greater amounts were found in plums
and strawberries. The results of this investigation did not show any
real correlation between the amounts of the parent compound and
N-formyl-2-ethyl-4-chloroaniline to such variables as amount of
chemical applied or sampling date. The type of fruit, and especially
the nature of the fruit surface, and such factors as sunlight and
rainfall appear to be predominant in affecting the degradation of
chlordimeform.
Witkonton (1969) also reported that chlordimeform appears to adhere to
the outer surface of fruit and does not appear to translocate readily
to the fleshy parts, The chief factors which seem to account for the
decrease of chlordimeform residues in fruit appear to be
volatilization, weathering and growth dilution.
In soil
Chlordimeform deposited inadvertently on soil surfaces after spray
application may be expected to be dissipated by the following
processes: volatilization, chemical hydrolysis, photodecomposition
(Knowles and Sen Gupta, 1969) and microbial degradation. Although no
special volatilization experiments with soil samples were conducted,
the contribution of this process is likely to be of the same order of
magnitude as or slightly higher than that observed for leaf surfaces.
Hydrolysis of chlordimeform to N-formyl-4-chloro-o-toluidine (III)
shown to be substantial under the slightly acid or alkaline conditions
which normally prevail in soils (Kossmann et al., 1971).
As regards microbial degradation of the acaricide, Johnson and Knowles
(1970) demonstrated that several bacterial, actinomycete and fungus
species are capable of extensively degrading chlordimeform. The
principal metabolite in the culture media of most species was
N-formyl-4-chloro-o-toluidine, except for the actinomycete
Streptomyces griseus which formed mainly 4-chloro-o-toluidine.
None of the microbes formed symmetrical azo-compounds.
Under field conditions, chlordimeform and its 4-chloro-o-toluidine
containing metabolites were dissipated according to first order
reactions with half-lives ranging from 20 to 40 days (Schering, 1969;
Ciba, 1969e). From the present experiments it way be concluded that
chlordimeform is not accumulated in the soil.
Evidence of residues in food in commerce or at consumption
No data were available to indicate the incidence or level of
chlordimeform residues in food commodities moving in commerce.
A number of detailed studies have been conducted to identify terminal
residues after application of chlordimeform. Results indicate that
breakdown of the parent compound takes place particularly in tissues
which are metabolically active, such as young bean leaves, while
fruits like apples and prunes show practically no metabolic breakdown
of the acaricide. The hydrolytic pathway of degradation leading to
small amounts of N-formyl-4-chloro-o-toluidine has been demonstrated
to occur to a certain extent also on fruit crops. Hydrolysis of
chlordimeform, however, apparently takes place independent of plant
tissue activities due to the chemical instability of the parent
compound (Kossmann et al., 1971),
Furthermore it has been confirmed that
2,2'-dimethyl-4,4'-dichloroazobenzene is not expected to be a
potential transformation product of chlordimeform in apple fruit.
All chlordimeform metabolites identified so far in various plant
materials are determined by the described total residue methods.
A number of special investigations reveal that chlordimeform residues
are located in the outer parts of crops such as fruit peels or outer
leaves of cole crops. Excessive residues therefore might be removed by
peeling of fruit (apples, citrus) or by trimming the outer leaves of
cole crops.
Washing of fruit will remove only a small part of the total residue.
External residues will probably dissipate by volatilization, rainfall,
or penetration into the cuticular wax layers,
Schering (1971) reports on the effect of cooking on chlordimeform
residues in apples, grapes, tomatoes, cauliflower, beans and sugar
beet foliage and shows that the behaviour of chlordimeform residues
during cooking depends on the pH value of the aqueous suspension as
well as on the duration of the cooking period.
This report shows that the rate of loss of chlordimeform is a function
of pH value, the loss being much more rapid in a neutral medium such
as green beans (pH 5) or cauliflower (pH 6) than in apples (pH 2.5) or
tomatoes (pH 3). The hydrolysis proceeds according to the scheme
outlined in page 30 and all metabolites are determined by the
analytical method for chlordimeform parent compound. The loss of total
4-chloro-o-toluidine moiety is not significant showing that
volatilization in steam is not an important contributing factor.
Methods of residue analysis
(a) Methods for the detection of unchanged chlordimeform and its
breakdown products (terminal residues)
Identification of terminal residues in plant materials and specific
determination of unchanged chlordimeform has been carried out by
thin-layer and/or gas chromatography procedures. A detailed
description of the separation and detection of
N'-(4-chloro-o-tolyl)-N-methylformamidine,
N-formyl-4-chloro-o-toluidine, and 4-chloro-o-toluidine as well as
the parent compound including quantitation by colorimetry or flame
ionization gas chromatography has been given by Kossmann et al.
(1971).
Investigations for the presence of
2,2'-dimethyl-4,4'-dichloroazobenzene have been performed by a gas
chromatographic procedure following reductive cleavage of the azo
compound and subsequent Sandmeyer iodination of the resulting
4-chloro-o-toluidine (Geissbühler et al., 1971).
Selective determination of unchanged chlordimeform may be needed after
usage of chlordimeform/formetanate mixtures. Both active substances
have the structure of formamidine derivatives. For this combination
product a thin-layer chromatographic procedure has been established
(Schering AG, 1969b) which includes direct quantitation on the
chromatoplate by reflectance measurement in the UV range. Gas
chromatographic procedures described above allow specific
determination of chlordimeform in presence of formetanate as well and
account then for the total chlordimeform residue including
metabolites.
(b) Total chlordimeform residue methods
For regulatory purposes and routine residue analysis methods were
developed that account in a single procedure for chlordimeform and for
all its 4-chloro-o-toluidine containing breakdown products and/or
conjugates. Principle of determination of "total chlordimeform" is
based on a two step hydrolysis by successive treatments with acetic
acid and sodium hydroxide respectively. The joint hydrolysis product,
4-chloro-o-toluidine, is steam distilled and extracted into
iso-octane. Quantitation of 4-chloro-o-toluidine may be carried out
by colorimetry (specificity limited) or gas chromatography (highly
specific).
Colorimetry is based upon diazotization reaction of
4-chloro-o-toluidine and coupling with N-ethyl-1-naphthylamine
(Geissbühler et al., 1971). A number of investigations has been made
by using 1-naphthylethylenediamine as a coupling agent (Schering AG,
1968). Both procedures are sensitive to 0.05 ppm of chlordimeform as
calculated for a 50-gram crop sample. Recovery values obtained from
various crops fortified within the range of 0.05 to 5 ppm
chlordimeform in general exceeded 85% (Geissbühler et al., 1971;
Schering AG, 1969a).
An interlaboratory study demonstrated the sufficient reproducibility
of the total residue procedures established. Since specificity of
colorimetric evaluation is limited, additional identification of
chlordimeform residues may be done by confirmatory thin-layer
chromatography of the resulting azo dye on cellulose plates
(Geissbühler et al., 1971).
Gas chromatography has also been effectively used for quantitative
determination of 4-chloro-o-toluidine moiety following
transformation into halogenated derivatives of extremely high electron
affinity. Sandmeyer iodination reaction of 4-chloro-o-toluidine
results in the 5-chloro-2-iodo-toluene structure and a 0.05 ppm
detection limit for chlordimeform by using a tritium foil equipped
electron capture detector. Recovery figures correspond very well to
those reported for colorimetric measurement (Geissbühler et al.,
1971).
A lower detection limit may be attainable by bromination of
4-chloro-o-toluidine in aqueous solution yielding
6-bromo-4-chloro-o-toluidine and subsequent gas chromatographic
determination using a Ni63 electron capture detector (Kossmann,
1971),
Direct gas chromatographic measurement of 4-chloro-o-toluidine by a
halogen microcoulometric titration cell has been reported by Del Monte
Corporation (Del Monte, 1969) for the determination of chlordimeform
residues in crops.
All the gas chromatographic procedures mentioned are specific for
chlordimeform residues. Up to date no other pesticide with the
4-chloro-o-toluidine nucleus is known to be used in agricultural
practice.
Examples of national tolerances
The following are examples of national tolerances and withholding
periods that have been established:
Country Crop Tolerance Withholding
ppm period days
Australia pome fruit 2 7
stone fruit 2 7
strawberries 1 7
fat of meat of cattle 0.5 1
Country Crop Tolerance Withholding
ppm period days
Canada pears 5 28
peaches, plums, prunes 4 14
apples 3 14
cauliflower, brussels
sprouts 3 28
broccoli 2 28
cabbage 0.5 28
turnip roots <0.05 28
Germany (GFR) grapes, stone fruit
(excl. cherries) 3 14
pome fruit 2
Italy fruit, citrus, grapes 1 20
New Zealand pome fruit 1 14
strawberries 7
Switzerland pome fruit, grapes 1 42
South Africa apples, pears, citrus 1 14
USA pears 5 28
apples 3 14
cabbage, cauliflower,
broccoli, brussels
sprouts 2 28
Venezuela fruit, citrus 2 14
Appraisal
Chlordimeform is a new insecticide/acaricide primarily effective
against eggs and larvae of spider mites with adequate activity also
against adult mites. It is effective against mites resistant to
organo-phosphorus insecticides. It kills eggs, larvae and adults not
only by contact but also in the vapour phase. Penetration and slight
systemic effect have been demonstrated. It is also effective in
control of some lepidopterous insects including codling moth but the
major field of use so far developed is in the control of mites on
deciduous fruit. Chlordimeform is also being used commercially in
Australia for the control of cattle tick by spraying and dipping
because of its ability to control ticks which have developed strains
resistant to all other currently available acaricides.
Chlordimeform is registered for use on fruit trees, grapes and
vegetables in many countries and to a lesser extent on cotton and
hops. The concentration applied ranges from 0.01 to 0.1%.
Chlordimeform is used both as the base and as the hydrochloride. The
base is formulated as an emulsifiable solution while the hydrochloride
is used as a water soluble powder.
The residue data available to the meeting were obtained from
supervised field trials in Australia, Canada, England, France,
Germany, Italy, New Zealand, South Africa, Spain, Switzerland and the
United States of America. Whilst there is a sharp drop in the residue
level between the day of application and the second or third day
post-treatment, thereafter the rate of decline is remarkably slow with
a half-life on apples, grapes, pears and tomatoes exceeding 21 days.
The rate of decline is somewhat faster on cruciferous vegetables but
even so the interval between application and harvest does not have a
pronounced bearing on the residues level following approved
treatments.
Loss of residue is initially due to volatilization but the portion of
the deposit absorbed or dissolved into surface tissues of plants
appears to remain at a relatively constant level. The residue data
reflect this durability but they are influenced by the fact that the
analytical procedure determines both parent compound and all
metabolites containing the toluidine moiety.
Extensive evidence is available on the fate of residues in plants and
animals as well as on the route and rate of degradation. The initial
pathway of metabolism in animals and plants is substantially similar.
leading to N-formyl-4-chloro-o-toluidine and 4-chloro-o-toluidine
in both organisms but proceeding further to
N-formyl-5-chloroanthranilic acid and 5-chloroanthranilic acid
respectively in animals.
Some data were available on the effect of processing and cooking as
the fate of chlordimeform residues. The chlordimeform residue
generally decreases with cooking, the decline being dependent upon
length of cooking time. The rate of decrease is a function of the pH
value. In a more neutral medium caused by green beans (pH 5) or
cauliflower (pH 6) loss of chlordimeform is much more rapid than in an
acid medium such as apples (pH 2.5) or tomatoes (pH 3). Studies
revealed no detectable change in the residue level or composition when
apples and pears were held in cold storage. Evidence was presented to
show that substantially all the residue occurs in the skin of apples
and citrus; in the outer leaves of cruciferous vegetables and in the
tops of root vegetables. Chlordimeform, applied to cotton, appears as
a residue in cotton seed cake as well as in cotton seed oil and the
residue level is not significantly reduced by refining.
Analytical methods based on the determination of
4-chloro-o-toluidine formed by successive treatments with acetic
acid and sodium hydroxide are specific for chlordimeform and its
metabolites and are capable of determining levels down to 0.05 ppm.
Quantitation may be made either by colorimetry based upon
diazotization and coupling with N-ethyl-1-naphthylamine or by gas
chromatography. Though direct measurement by gas chromatography is
possible, the accuracy and sensitivity is improved by transformation
of the residue into halogenated derivatives of extremely high electron
affinity.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Temporary tolerances (effective to June 1975)
Pears, peaches, prunes 5 ppm
Apples, grapes, plums, strawberries 3 ppm
Brassicas, cherries, citrus fruit,
cotton seed oil (crude and refined),
cotton seed 2 ppm
Beans 0.5 ppm
Fat, meat and meat products of cattle 0.5 ppm
milk (whole) 0.05 ppm
Butter 0.5 ppm
Further work or information
Required (before 30 June 1975)
1. A further long-term feeding study in rats to obtain precise
information on the incidence and nature of the histopathological
changes in the liver and bile ducts of rats exposed to levels
above and below 100 ppm in the diet. An effort should be made to
explain the changes in organ to body-weight ratios noted in the
previous long-term rat study.
2. Further investigation of the nature of the hepatic lesions
observed in the dog.
3. Metabolic studies in several animal species, preferably including
man.
4. Further data on the nature and levels of residues in animal
tissues after use in cattle sprays and dips.
5. Further data on residues in milk after use in cattle sprays and
dips, especially information on the nature of the residues and
their distribution between aqueous and lipid phases.
6. Data on the residue levels in commercial butter and cheese.
Desirable
1. Further studies on the haematological effects.
2. Elucidation of the pharmacodynamic activity on the heart,
including the potentiation of the effects of pressor amines.
3. Further data on the disappearance of residues during storage,
processing, and cooking.
4. Data on residue levels in raw agricultural commodities moving in
commerce.
REFERENCES
Aoki, H. and Ueda, K. (1966) Unpublished report of the Tokyo Dental
College submitted by Ciba-Geigy
Bertha, R. and Framer, D. (1967) Pesticide transformation to aniline
and azo compounds in soil. Science, 156: 1617
Blackmore, R. H. (1969a) Three generation reproduction study - rats.
Unpublished report of Hazelton Laboratories, submitted by Ciba-Geigy
Blackmore, R. H. (1969b) Teratology study - rabbits. Unpublished
report of Hazelton Laboratories submitted by Ciba-Geigy
Blackmore, H. H. (1969c) Two year repeated feeding - dogs. Schering
361 268 (Chlorphenamidine). Unpublished report from Hazelton
Laboratories, submitted by Ciba-Geigy
Blackmore, R. H. (1969d) Two year repeated feeding - rats. Schering
361 268 (Chlorphenamidine). Unpublished report from Hazelton
Laboratories, submitted by Ciba-Geigy
Ciba Ltd. (1965a) C-8514. Rückstände in den Blättern der Bohne. Report
of 20 May 1965
Ciba Ltd. (1965b) C-8514. Residues in Australian apples and pears.
Report of 25 May 1965
Ciba Ltd. (1967a) Modell einer Synthese von radioaktivem Galecron.
Report of 17 August 1967
Ciba Ltd. (1967b) Galecron. Absence of 4-chloro-o-toluidine as a
metabolite in prunes after overdose spraying. Report of 31 January
1967
Ciba Ltd. (1968) Galecron. Behaviour of different formulations on
citrus leaves. Report of 14 May 1968
Ciba Ltd. (1969a) Galecron. Residues in Italian oranges. Report of 2
March 1969
Ciba Ltd. (1969b) Galecron. Analysis of Swiss apples for residues of
2,2'-dimethyl-4,4'-dichloroazobenzene after overdose spraying. Report
of 20 November 1969.
Ciba Ltd. (1969c) Galecron. Formation of a symmetrical azo derivative
from 4-chloro-o-toluidine by coupled peroxidatic reactions. Report
of 12 September 1969
Ciba Ltd. (1969d) Galecron. Degradation of chlorphenamidine by rat
liver and spleen homogenates, with particular reference to the
possible formation of azo derivatives. Report of 10 September 1969
Ciba Ltd. (1969e) Galecron. Dissipation in a Swiss soil as determined
by chemical analysis. Report of 16 September 1969
Ciba-Geigy Ltd. (1971) Galecron. Residues in milk, meat, fat, liver
and kidney of Swiss cows. Report of 19 March 1971
Ciba-Geigy Ltd. (1971) Determination of chlorphenamidine residues in
milk and butter following treatment with "Spike" cattle dip at 0.05%
a.i. w/v. Report of 6 August 1971
Del Monte Corporation Research Center. (1969) Determination of total
chlorphenamidine residues in plant material, 10.6.1969. Unpublished
Dittrich, V. (1971) Biological action of chlordimeform. Report
prepared for FAO/WHO
Ehrhardt, D. A. and Knowles, C. O. (1970) Metabolism and translocation
of N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine (chlorphenamidine)
and its hydrochloride salt in grapefruit seedlings. J. Econ. Entomol.,
63: 1306
Geissbühler, H. (1969) The substituted ureas. In: Degradation of
herbicides (P. C. Kearney and D. D. Kaufman, ed.), p. 79. New York,
Marcel Dekker Inc.
Geissbühler, H., Kossmann, K., Baunok, I. and Boyd, V. F. (1971)
Determination of total residues of chlorphenamidine
[(N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine] in plant and soil
material by colorimetry and thin-layer and electron capture gas
chromatography. J. Agr. Food Chem., 19: 365
Gerhards, E. and Kolb. (1966) Unpublished report of Schering A.G.
submitted by Ciba-Geigy
Gerhards, E. (1967) Unpublished report of Schering A.G. submitted by
Ciba-Geigy
Gunzel, P. and Richter. (1965) Systemische Vertraglichkeitsprufung an
Ratten bei einmaliger p.o. Verabreichung (DL50). Unpublished report
from Schering A.G. submitted by Ciba-Geigy
Gunzel, P. and Richter. (1966a) Systemische Vertraglichkeitsprufung an
Ratten bei einmaliger dermaler. Verabreichung (DL50). Unpublished
report of Schering AG, submitted by Ciba-Geigy
Gunzel, P. and Richter. (1966b) Systemische Vertraglichkeitsprufung an
Kaninchen bei einmaliger dermaler Verabreichung (DL50). Unpublished
report of Schering A.G., submitted by Ciba-Geigy
Gunzel, P. and Richter. (1968) Systemische Vertraglichkeitsprufung an
Hunden bei einmaliger p.o. Verabreichung (DL50). Unpublished report
from Schering AG., submitted by Ciba-Geigy
Gunzel, P. and Richter. (1969) Systemische Vertraglichkeitsprufung an
Ratten bei einmaliger p.o. Verabreichung (DL50), Unpublished report
of Schering AG., submitted by Ciba-Geigy
Herbert, R. A. (1969) Methyl and phenylcarbamates. In: Degradation of
herbicides (P. C. Kearney and D. D. Kaufman, ed.), p. 113, New York,
Marcel Dekker Inc.
Hurni, H. and Sachsse, K. (1969a) Report on the determination of the
acute oral LD50 to the dog of chlorphenamidine technical.
Unpublished report of Tierfarm AG, submitted by Ciba-Geigy
Hurni, H. and Sachsse, K. (1969b) Report on the determination of the
acute oral LD50 to the rat of Galecron 50 EC. Unpublished report of
Tierfarm AG, submitted by Ciba-Geigy
Hurni, H. and Sachsse, K. (1969c) Report on the determination of the
acute dermal LD50 to the rat of Galecron 50 SP. Unpublished report
of Tierfarm AG, submitted by Ciba-Geigy
Johnson. B, T. and Knowles, C. O. (1970) Microbial degradation of the
acaricide N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine. Bull.
Environ. Contam. Toxicol., 5: 158
Knowles, C. O. (1970) Metabolism of two acaricidal chemicals,
N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine (chlorphenamidine) and
m-{[dimethylamino methylene] amino} phenyl methyl-carbamate
hydrochloride (formetanate). J. Agr. Food Chem., 18: 1038
Knowles, C. O. (1970) Microbial degradation of chlordimeform. Bull
Envir. Contam. Toxicol., 5 (2): 158
Knowles, C. O. and Sen Gupta, A. K. (1969) Photodecomposition of the
acaricide N'-(4-chloro-o-tolyl) N,N-dimethylformamidine. J. econ.
Entomol., 62: 344
Knowles, C. O. and Sen Gupta, A. K. (1970)
N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine-14C (Galecron) and
4-chloro-o-toluidine metabolism in the white rat. J. econ. Entomol.
63: 856
Knowles, C. O., Sen Gupta, A. K. and Hassan, T. K. (1969a) Formation
of symmetrical azo derivatives of monochlorotoluidines by peroxidatic
catalysis. J. econ. Entomol., 62; 411
Knowles, C. O. and Sen Gupta, A. K. (1969b) Formation of symmetrical
azo derivative of 4-chloro-o-toluidine in a ferrous iron-hydrogen
peroxide system. J. econ. Entomol., 62: 1229
Kossmann, K. (1971) New aspects upon electron capture gas
chromatography of acylanilide, phenylcarbamate, and phenylurea type
herbicides. Proc. 2nd Intern. Congr. Pesticide Chemistry, Tel Aviv,
1971
Kossmann, K., Geissbühler, H. and Boyd, V. F. (1971) Specific
determination of chlorphenamidine
[N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine] and of some
potential metabolites in plant material by thin-layer and flame
ionisation gas chromatography. J. Agr. Food Chem., 19: 360
Lyon, D. A. (1970) Chlorphenamidine on weight gain of female rats.
Internal unpublished memorandum. Food and Drug Directorate, Canada
Mastri, C., Keplinger, M. L. and Francher, O. E. (1969) Acute oral
toxicity study on two samples of chlorphenamidine in male and female
albino rats. Unpublished report from Industrial Biotest Labs. Inc.
submitted by Ciba-Geigy
Oda. (1969) Suicide by drinking Galecron. Unpublished report from
Chikugo Municipal Hospital, made available by Nor-Rh Chemicals
Rose, J. A. (1969a) Degradation of chlorphenamidine by rat liver and
spleen homogenatis with particular reference to the possible formation
of azo-derivatives. Unpublished report submitted by Ciba-Geigy
Rose, J. A. (1969b) Formation of a symmetrical azo-derivative from
4-chloro-o-toluidine by coupled peroxidatic reactions. Unpublished
report submitted by Ciba-Geigy
Rosen, J. D., Siewierski, M. and Winnett, G. (1970) FMN-sensitized
photolyses of chloroanilines. J, Agr. Food Chem., 18: 494
Sachsse, K. and Bathe, R. (1970a) Report on the determination of the
acute oral LD50 of formylchlorotholuidine to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Giegy
Sachsse, K, and Bathe, R. (1970b) Report an the determination of the
dermal LD50 of formylchlorotoluidine to the rat. Unpublished report
of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970c) Report on the determination of the
acute oral LD50 of 5-chloro-2-amino-toluene (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1970d) Report on the determination of the
acute oral LD50 of 5-chloro-2-amino toluene-HCl to the rat.
Unpublished report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970e) Report on the determination of the
acute dermal LD50 of 5-chloro-2-amino-toluene-HCl to the rat.
Unpublished report of Tierfarm A.G. submitted by Ciba Geigy
Sachsse, K. and Bathe, R. (1970f) Report on the determination of the
acute oral LD50 of phenamidine-HCl to the rat. Unpublished report of
Tierfarm AG submitted by Ciba-Geigy
Sachsse. K. and Bathe, R. (1970g) Report on the determination of the
acute dermal LD50 of phenamidine-HCl to the rat. Unpublished report
of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970h) Report on the determination of the
acute oral LD50 of o-chlorphenamidine-HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970i) Report on the determination of the
acute dermal LD50 of o-chlorphenamidine-HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970j) Report on the determination of the
acute oral LD50 of dichlorphenamidine-HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970k) Report on the determination of the
acute dermal LD50 of dichlorphenamidine HCl to the rat. Unpublished
report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1970l) Report on the determination of the
acute dermal LD50 of technical chlorphenamidine (base) to the rat.
Unpublished report of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1971a) Report on the determination of the
acute oral LD50 of technical C8520 to the rat. (Dimethyl
chlordimeform). Unpublished report of Tierfarm AG submitted by
Ciba-Geigy
Sachsse, K. and Bathe, R. (1971b) Report on the determination of the
acute dermal LD50 of technical C8520 to the rat. Unpublished report
of Tierfarm AG submitted by Ciba-Geigy
Sachsse, K. and Bathe, R. (1971c) Report on the determination of the
acute dermal LD50 of 5-chloro-2-amino toluene (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe. R. (1971d) Report on the determination of the
acute oral LD50of phenamidine (base) to the rat. Unpublished report
of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971e) Report on the determination of the
acute dermal LD50 of phenamidine (base) to the rat. Unpublished
report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971f) Report on the determination of the
acute oral LD50 of o-chlorphenamidine (base) to the rat. Unpublished
report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971g) Report on the determination of the
acute dermal LD50 of o-chlorphenamidine (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971h) Report on the determination of the
acute oral LD50 of dichlorphenamidine (base) to the rat. Unpublished
report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971i) Report on the determination of the
acute dermal LD50 of dichlorphenamidine (base) to the rat.
Unpublished report of Ciba-Geigy
Sachsse, K. and Bathe, R. (1971j) Report on the determination of the
acute dermal LD50 of Galecron 50EC to the rat. Unpublished report of
Ciba-Geigy
Schering AG. (1965) Versuche zur Kenntnis des Eindringvermögens von
36268 in Aepfel
Schering AG. (1966a) Chlorphenamidin. Stoffwechselversuche Stand Dez.
1966
Schering AG. (1966b) Rückstandsuntersuchungen Schering 36268. 1.
Mitteilung 1966. Erntegut: Weintrauben, Aepfel, Birnen, Pfirsich
Schering AG. (1967) Chlorphenamidine. Stoffwechselversuche Stand Dez.
1967
Schering AG. (1968) Rückstandsbestimung von Chlorphenamidin (Schering
3628) in Pflanzenmaterial, 36 268/6, 13.9.1968 (unpublished)
Schering AG. (1969) Chlorphenamidin 1/69. Abbaugeschwindigkeit von
Chlorphenamidin-Hydrochlorid im Boden
Schering AG. (1969a) Chlorphenamidin III/69, Verfahrensausbeuten nach
36 268/6, 17.3.1969 (unpublished)
Schering AG. (1969b) Method for the determination of Fundal(R) forte
residues in plant material using thin-layer chromatography,
36 056 - 36 268/1, 18.11.1969
Sen Gupta, A. K. and Knowles, C. O. (1969) Metabolism of
N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine by apple seedlings.
J. Agr. Food Chem., 17: 595
Sen Gupta, A. K. and Knowles, C. O. (1970) Galecron-14C
[N'-(4-chloro-o-tolyl)-N,N-dimethylformamidine] metabolism in the
dog and goat. J. Econ. Entomol., 63: 951
Shionogi and Co. (Undated) Unpublished report of the Aburahi Labs. of
Shionogi and Co. Ltd,
Surber, W. and Cerioli, A. (1966) Prolonged toxicity study on rats of
Ciba's preparation, 39, 12. (C8514). Unpublished report from Battelle
Institute submitted by Ciba-Geigy
Teeters, W. R. and Blackmore, R. H. (1968) Pharmacological study of
chlorphenamidine in the perfused rabbit heart, isolated guinea-pig
ileum, and dog pharmacodynamic preparation. Hazelton Laboratories
report C5230, made available by Nor-Am. Chemicals
Tripod, J. (1967) Unpublished report from Ciba Ltd., made available by
Nor-Am Chemicals
Voss, G. and Burkhard, N. (1971) Unpublished report submitted by
Ciba-Geigy
Weatherholtz, W. M. (1970) Statistical analysis of body weight data
and food consumption data, chlorphenamidine. Unpublished report from
Hazelton Labs. Inc. made available by Nor-Am. Agricultural Products
Ltd.
Weir, R. J. (1967) Unpublished report from Hazelton Labs. made
available by Nor-Am. Chemicals
Weir, R. J. (1968) Acute oral administration - rats. Unpublished
report from Hazelton Labs., made available by Nor-Am. Chemicals
Williams, C. T. (1959) Detoxification mechanisms. London, Chapman and
Hall
Witkonton, Sujit. (1969) The fate and persistence of chlorphenamidine
on six different crops. Thesis for degree of Master of Science,
Department of Entomology, Pennsylvania State University, September
1969 (53 pp)
