
Concise International Chemical Assessment Document 13
TRIPHENYLTIN COMPOUNDS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Concise International Chemical Assessment Document 13
TRIPHENYLTIN COMPOUNDS
First draft prepared by Dr J. Sekizawa, National Institute of Health
Sciences, Tokyo, Japan
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1999
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Triphenyltin compounds.
(Concise international chemical assessment document ; 13)
1.Organotin compounds - adverse effects
2.Organotin compounds - toxicity
3.Environmental exposure 4.Maximum permissible exposure level
I.International Programme on Chemical Safety II.Series
ISBN 92 4 153013 8 (NLM classification: QV 290)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1999
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
8.8. Mode of action
9. EFFECTS ON HUMANS
9.1. Case reports
9.2. Epidemiological studies
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic environment
10.2. Terrestrial environment
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for triphenyltin
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
13.3. Health surveillance advice
13.4. Spillage and disposal
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 -- SOURCE DOCUMENTS
APPENDIX 2 -- CICAD PEER REVIEW
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994)
Assessing human health risks of chemicals: deriviation of guidance
values for health-based exposure limits. Geneva, World Health
Organization (Environmental Health Criteria 170).
Procedures
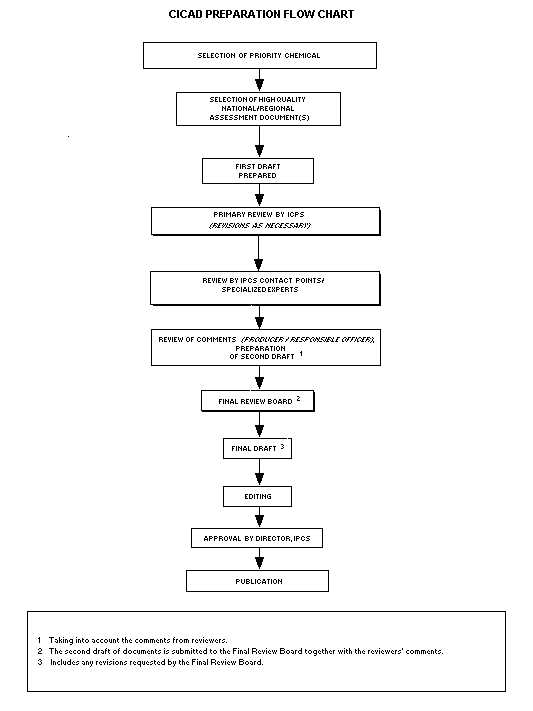
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
 Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on triphenyltin compounds was based on a review
prepared by the National Committee for Concise International Chemical
Assessment Documents of Japan (CICAD National Committee, 1997). Many
critical studies on health effects in this review were cited from
monographs on pesticide residues prepared by the Food and Agriculture
Organisation of the United Nations (FAO, 1991a,b) and the World Health
Organization (WHO, 1992). These monographs report summaries of the
many studies submitted to WHO by manufacturers for evaluation, in
addition to summaries of published papers. In the case of studies
submitted by manufacturers, original papers are proprietary and were
not available to authors of the review prepared by the CICAD National
Committee (1997), to authors of the CICAD draft, or to the CICAD Final
Review Board. Therefore, this CICAD inevitably relies on the
evaluations made by the Joint FAO/WHO Meeting on Pesticide Residues
(JMPR) for those studies cited from summaries of proprietary data.
Extensive information on environmental effects was obtained from
a review of the environmental effects of triorganotin compounds,
prepared by the Advisory Committee on Pesticides of the Health and
Safety Executive of the United Kingdom (HSE, 1992). Additional data
were obtained through a search of Medline and Toxline Plus databases
up to October 1997. Information on the nature of the review processes
and the availability of the source documents is presented in Appendix
1. Information on the peer review of this CICAD is presented in
Appendix 2. This CICAD was approved as an international assessment at
a meeting of the Final Review Board, held in Tokyo, Japan, on 30 June
- 2 July 1998. Participants at the Final Review Board meeting are
listed in Appendix 3. The International Chemical Safety Card (ICSC
1283) for triphenyltin hydroxide (TPTH), produced by the International
Programme on Chemical Safety (IPCS, 1996), has also been reproduced in
this document.
Triphenyltin compounds are triphenyl derivatives of tetravalent
tin. They are colourless solids with low vapour pressures. They are
lipophilic and have low solubility in water.
Triphenyltin and tributyltin compounds have been used extensively
as algicides and molluscicides in antifouling products since the
1960s. Use of triorganotins in antifouling paints has been restricted
in many countries because of their catastrophic effects on the oyster
industry and more general effects on the aquatic ecosystem.
Triphenyltin is used as a non-systemic fungicide with mainly
protective action.
Triphenyltin is strongly adsorbed to sediment and soil, and
little desorption occurs. Its half-life in water has been estimated to
be several days in June and 2-3 weeks in November. Although
triphenyltin compounds can be degraded by stepwise dephenylation and
excreted in conjugated forms, they bioaccumulate in fish and snails,
with bioconcentration factors (BCFs) ranging from several hundred to
32 500 (in the intestinal sac of Lymnaea stagnalis).
Environmental concentrations of triphenyltin compounds vary
depending upon how, when, and where the compounds were used.
Concentrations ranging from 0 ng/litre to nearly 200 ng/litre have
been detected in bay areas or marinas as a result of leaching from
ships treated with triphenyltin-based antifouling paints.
Environmental concentrations of triphenyltin compounds have decreased
in recent years as a result of tightening restrictions on their use in
antifouling paints.
Triphenyltin compounds given orally to rats are not readily
absorbed and are excreted primarily in faeces and partly in urine.
They are metabolized to diphenyltin, monophenyltin, and
non-extractable bound residues. Absorbed triphenyltin compounds
accumulate in kidney and liver to the greatest extent, with smaller
amounts in other organs. Triphenyltin compounds applied dermally can
penetrate through the skin in a time- and dose-dependent manner.
Triphenyltin exerts a variety of health effects in various animal
species, including effects on the immune system,
reproductive/developmental effects at levels near those that are
maternally toxic (most lowest-observed-adverse-effect levels, or
LOAELs, are in the several mg/kg range or lower), hyperplasia/adenomas
in endocrine organs, apoptosis in thymus cells, calcium release in
sarcoplasmic reticulum cells, and eye irritation. The underlying
mechanisms of these effects are under investigation; a common
mechanism may explain this toxicity profile.
Triphenyltin compounds are moderately acutely toxic to rats. They
are not carcinogenic, but some data show that they are co-clastogenic.
Reproductive and developmental effects include a decrease in the
number of implantations and live fetuses (at 1.0 mg triphenyltin
acetate [TPTA]/kg body weight per day in a rabbit gavage study),
reduction in litter size/pup weight and in relative thymus or spleen
weight in the weanlings (at 1.5 mg TPTH/kg body weight per day in the
diet in a two-generation reproduction study in rats;
no-observed-adverse-effect level, or NOAEL, 0.4 mg/kg body weight per
day), and abortion and reduction in fetal weight (at 0.9 mg TPTH/kg
body weight per day in a rabbit gavage study).
The lowest NOAEL detected in the toxicity tests was 0.1 mg
TPTH/kg body weight per day for maternal toxicity in a rabbit gavage
study, based on decreased food consumption and body weight gain at 0.3
mg/kg body weight per day. The same value was obtained in an early
2-year rat study in which a slight decrease in white blood cells was
seen at higher doses. In a 52-week dog study, the NOAEL was estimated
to be 0.21 mg TPTH/kg body weight per day based on a decrease in
relative liver weight in females at higher doses.
Triphenyltin compounds affect the immune system. A decrease in
immunoglobulin (Ig) concentrations (even at the lowest dose level,
i.e., 0.3 mg TPTH/kg body weight per day in a 2-year feeding study in
rats), lymphopenia (at 0.3 mg TPTH/kg body weight per day in another
2-year feeding study in rats or at 0.338 mg TPTH/m3 in a 13-week
inhalation study in rats), thymus atrophy (at 1.5 mg triphenyltin
chloride [TPTCl]/kg body weight per day in a 2-week feeding study with
weanling rats), and splenic atrophy (at 5 mg TPTH/kg body weight per
day in a 28-day feeding study in mice) have been observed. Females are
generally more susceptible than males.
Several end-points were taken into consideration by JMPR in
establishing the acceptable daily intake (ADI) of triphenyltin for
oral exposure (FAO, 1991b; WHO, 1992). First, a 200-fold uncertainty
factor was applied to the no-observed-effect level (NOEL) of 0.1 mg/kg
body weight per day (based on a finding of reduced white blood cell
counts at higher doses in a 2-year study in rats) to arrive at an ADI
of 0-0.5 µg/kg body weight. Secondly, a 500-fold uncertainty factor
was applied to a LOAEL of 0.3 mg/kg body weight per day in a 2-year
study in rats in which increased mortality and reduced serum
immunoglobulin levels were noted. Other NOAELs that were taken into
consideration together with the above effect levels are 0.4 mg/kg body
weight per day in a two-generation reproduction study with rats (a
dose-related decrease in spleen and thymus weights in F1 and F2 male
and female weanlings was observed at higher levels), 0.3 mg/kg body
weight per day in a short-term study in rats (reduction in white blood
cells, decrease in IgG, and increase in relative testes weight were
seen at higher levels), 0.21 mg/kg body weight per day in a short-term
dog study (increase in relative liver weight and decrease in serum
albumin/globulin ratio were seen at higher levels), and 0.1 mg/kg body
weight per day in a teratology study in rabbits (maternal toxicity was
seen at higher levels).
There are no data concerning occupational exposure to
triphenyltin compounds. A few poisoning case reports describe
neurotoxic effects, which appeared to persist. Exposure of the general
public to triphenyltin compounds occurs mostly from ingestion of
contaminated seafood, which in some cases has been found to contain
triphenyltin levels as high as 1 µg/g (in muscle of some fish
species). Triphenyltin intake from contaminated foods in Japan in 1997
was estimated to be around 11% of the ADI (i.e., 2.75 µg/day for a
50-kg person) established by JMPR.
Triphenyltin compounds exert deleterious effects on aquatic
organisms at very low concentrations. For example, imposex of rock
shells (Japanese gastropods) was seen at around 1 ng/litre
(no-observed-effect concentration, or NOEC, not determined), and
chronic toxicity to fathead minnow ( Pimephales promelas) larvae was
observed at 0.23 µg/litre (lowest-observed-effect concentration, or
LOEC). Triphenyltin is considered to be an endocrine disruptor,
because imposex, a phenomenon in which female gastropods develop male
sex organs, is probably caused by hormonal disturbance.
No NOEC for triphenyltin has been established for imposex in
molluscs. Experimentally, by injection, triphenyltin has a potency
similar to that of tributyltin in the genus Thais. Triphenyltin is
less potent than tributyltin in Nucella; however, triphenyltin shows
greater bioaccumulation than tributyltin. From this, it can be
estimated that the NOEC for triphenyltin will be a few ng/litre or
lower. The observed prevalence of imposex in Thais in the field with
ambient concentrations supports this estimate. Because residues of
triphenyltin and tributyltin occur together in the environment, their
relative contribution to observed imposex cannot be assessed for
Thais species. Use of either triphenyltin or tributyltin in
antifouling paint would lead to population declines of marine
invertebrates on this basis.
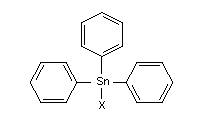
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Triphenyltin compounds are triphenyl derivatives of tetravalent
tin. They conform to the general formula (C6H5)3Sn-X, where X is an
anion or an anionic group, such as chloride, hydroxide, and acetate.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on triphenyltin compounds was based on a review
prepared by the National Committee for Concise International Chemical
Assessment Documents of Japan (CICAD National Committee, 1997). Many
critical studies on health effects in this review were cited from
monographs on pesticide residues prepared by the Food and Agriculture
Organisation of the United Nations (FAO, 1991a,b) and the World Health
Organization (WHO, 1992). These monographs report summaries of the
many studies submitted to WHO by manufacturers for evaluation, in
addition to summaries of published papers. In the case of studies
submitted by manufacturers, original papers are proprietary and were
not available to authors of the review prepared by the CICAD National
Committee (1997), to authors of the CICAD draft, or to the CICAD Final
Review Board. Therefore, this CICAD inevitably relies on the
evaluations made by the Joint FAO/WHO Meeting on Pesticide Residues
(JMPR) for those studies cited from summaries of proprietary data.
Extensive information on environmental effects was obtained from
a review of the environmental effects of triorganotin compounds,
prepared by the Advisory Committee on Pesticides of the Health and
Safety Executive of the United Kingdom (HSE, 1992). Additional data
were obtained through a search of Medline and Toxline Plus databases
up to October 1997. Information on the nature of the review processes
and the availability of the source documents is presented in Appendix
1. Information on the peer review of this CICAD is presented in
Appendix 2. This CICAD was approved as an international assessment at
a meeting of the Final Review Board, held in Tokyo, Japan, on 30 June
- 2 July 1998. Participants at the Final Review Board meeting are
listed in Appendix 3. The International Chemical Safety Card (ICSC
1283) for triphenyltin hydroxide (TPTH), produced by the International
Programme on Chemical Safety (IPCS, 1996), has also been reproduced in
this document.
Triphenyltin compounds are triphenyl derivatives of tetravalent
tin. They are colourless solids with low vapour pressures. They are
lipophilic and have low solubility in water.
Triphenyltin and tributyltin compounds have been used extensively
as algicides and molluscicides in antifouling products since the
1960s. Use of triorganotins in antifouling paints has been restricted
in many countries because of their catastrophic effects on the oyster
industry and more general effects on the aquatic ecosystem.
Triphenyltin is used as a non-systemic fungicide with mainly
protective action.
Triphenyltin is strongly adsorbed to sediment and soil, and
little desorption occurs. Its half-life in water has been estimated to
be several days in June and 2-3 weeks in November. Although
triphenyltin compounds can be degraded by stepwise dephenylation and
excreted in conjugated forms, they bioaccumulate in fish and snails,
with bioconcentration factors (BCFs) ranging from several hundred to
32 500 (in the intestinal sac of Lymnaea stagnalis).
Environmental concentrations of triphenyltin compounds vary
depending upon how, when, and where the compounds were used.
Concentrations ranging from 0 ng/litre to nearly 200 ng/litre have
been detected in bay areas or marinas as a result of leaching from
ships treated with triphenyltin-based antifouling paints.
Environmental concentrations of triphenyltin compounds have decreased
in recent years as a result of tightening restrictions on their use in
antifouling paints.
Triphenyltin compounds given orally to rats are not readily
absorbed and are excreted primarily in faeces and partly in urine.
They are metabolized to diphenyltin, monophenyltin, and
non-extractable bound residues. Absorbed triphenyltin compounds
accumulate in kidney and liver to the greatest extent, with smaller
amounts in other organs. Triphenyltin compounds applied dermally can
penetrate through the skin in a time- and dose-dependent manner.
Triphenyltin exerts a variety of health effects in various animal
species, including effects on the immune system,
reproductive/developmental effects at levels near those that are
maternally toxic (most lowest-observed-adverse-effect levels, or
LOAELs, are in the several mg/kg range or lower), hyperplasia/adenomas
in endocrine organs, apoptosis in thymus cells, calcium release in
sarcoplasmic reticulum cells, and eye irritation. The underlying
mechanisms of these effects are under investigation; a common
mechanism may explain this toxicity profile.
Triphenyltin compounds are moderately acutely toxic to rats. They
are not carcinogenic, but some data show that they are co-clastogenic.
Reproductive and developmental effects include a decrease in the
number of implantations and live fetuses (at 1.0 mg triphenyltin
acetate [TPTA]/kg body weight per day in a rabbit gavage study),
reduction in litter size/pup weight and in relative thymus or spleen
weight in the weanlings (at 1.5 mg TPTH/kg body weight per day in the
diet in a two-generation reproduction study in rats;
no-observed-adverse-effect level, or NOAEL, 0.4 mg/kg body weight per
day), and abortion and reduction in fetal weight (at 0.9 mg TPTH/kg
body weight per day in a rabbit gavage study).
The lowest NOAEL detected in the toxicity tests was 0.1 mg
TPTH/kg body weight per day for maternal toxicity in a rabbit gavage
study, based on decreased food consumption and body weight gain at 0.3
mg/kg body weight per day. The same value was obtained in an early
2-year rat study in which a slight decrease in white blood cells was
seen at higher doses. In a 52-week dog study, the NOAEL was estimated
to be 0.21 mg TPTH/kg body weight per day based on a decrease in
relative liver weight in females at higher doses.
Triphenyltin compounds affect the immune system. A decrease in
immunoglobulin (Ig) concentrations (even at the lowest dose level,
i.e., 0.3 mg TPTH/kg body weight per day in a 2-year feeding study in
rats), lymphopenia (at 0.3 mg TPTH/kg body weight per day in another
2-year feeding study in rats or at 0.338 mg TPTH/m3 in a 13-week
inhalation study in rats), thymus atrophy (at 1.5 mg triphenyltin
chloride [TPTCl]/kg body weight per day in a 2-week feeding study with
weanling rats), and splenic atrophy (at 5 mg TPTH/kg body weight per
day in a 28-day feeding study in mice) have been observed. Females are
generally more susceptible than males.
Several end-points were taken into consideration by JMPR in
establishing the acceptable daily intake (ADI) of triphenyltin for
oral exposure (FAO, 1991b; WHO, 1992). First, a 200-fold uncertainty
factor was applied to the no-observed-effect level (NOEL) of 0.1 mg/kg
body weight per day (based on a finding of reduced white blood cell
counts at higher doses in a 2-year study in rats) to arrive at an ADI
of 0-0.5 µg/kg body weight. Secondly, a 500-fold uncertainty factor
was applied to a LOAEL of 0.3 mg/kg body weight per day in a 2-year
study in rats in which increased mortality and reduced serum
immunoglobulin levels were noted. Other NOAELs that were taken into
consideration together with the above effect levels are 0.4 mg/kg body
weight per day in a two-generation reproduction study with rats (a
dose-related decrease in spleen and thymus weights in F1 and F2 male
and female weanlings was observed at higher levels), 0.3 mg/kg body
weight per day in a short-term study in rats (reduction in white blood
cells, decrease in IgG, and increase in relative testes weight were
seen at higher levels), 0.21 mg/kg body weight per day in a short-term
dog study (increase in relative liver weight and decrease in serum
albumin/globulin ratio were seen at higher levels), and 0.1 mg/kg body
weight per day in a teratology study in rabbits (maternal toxicity was
seen at higher levels).
There are no data concerning occupational exposure to
triphenyltin compounds. A few poisoning case reports describe
neurotoxic effects, which appeared to persist. Exposure of the general
public to triphenyltin compounds occurs mostly from ingestion of
contaminated seafood, which in some cases has been found to contain
triphenyltin levels as high as 1 µg/g (in muscle of some fish
species). Triphenyltin intake from contaminated foods in Japan in 1997
was estimated to be around 11% of the ADI (i.e., 2.75 µg/day for a
50-kg person) established by JMPR.
Triphenyltin compounds exert deleterious effects on aquatic
organisms at very low concentrations. For example, imposex of rock
shells (Japanese gastropods) was seen at around 1 ng/litre
(no-observed-effect concentration, or NOEC, not determined), and
chronic toxicity to fathead minnow ( Pimephales promelas) larvae was
observed at 0.23 µg/litre (lowest-observed-effect concentration, or
LOEC). Triphenyltin is considered to be an endocrine disruptor,
because imposex, a phenomenon in which female gastropods develop male
sex organs, is probably caused by hormonal disturbance.
No NOEC for triphenyltin has been established for imposex in
molluscs. Experimentally, by injection, triphenyltin has a potency
similar to that of tributyltin in the genus Thais. Triphenyltin is
less potent than tributyltin in Nucella; however, triphenyltin shows
greater bioaccumulation than tributyltin. From this, it can be
estimated that the NOEC for triphenyltin will be a few ng/litre or
lower. The observed prevalence of imposex in Thais in the field with
ambient concentrations supports this estimate. Because residues of
triphenyltin and tributyltin occur together in the environment, their
relative contribution to observed imposex cannot be assessed for
Thais species. Use of either triphenyltin or tributyltin in
antifouling paint would lead to population declines of marine
invertebrates on this basis.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Triphenyltin compounds are triphenyl derivatives of tetravalent
tin. They conform to the general formula (C6H5)3Sn-X, where X is an
anion or an anionic group, such as chloride, hydroxide, and acetate.
 Physical and chemical properties of triphenyltin compounds vary
depending upon the anion linked to tin. At ambient temperatures in the
pH range of 3-8, TPTA and TPTCl are hydrolysed to TPTH within 1 min;
as a consequence, the results of most studies with TPTA or TPTCl can
be applied to TPTH. Triphenyltin compounds are colourless solids with
low vapour pressures (<2 mPa at 50°C). The compounds are lipophilic
and have low water solubility (typically a few mg/litre at neutral
pH).
The identity and physical/chemical properties of TPTH, TPTA, and
TPTCl are given in Table 1. Additional properties of TPTH are
presented in the International Chemical Safety Card (ICSC 1283)
reproduced in this document.
Table 1: Identity and physical/chemical properties of some triphenyltin compounds.a
Triphenyltin hydroxide Triphenyltin acetate Triphenyltin chloride
Synonyms Fentin hydroxide; TPTH Fentin acetate; TPTA Fentin chloride; TPTCl
Chemical Abstracts 76-87-9 900-95-8 639-58-7
Service (CAS) Registry No.
Molecular formula C18H16OSn C20H18O2Sn C18H15ClSn
Molecular weight 367.0 409.1 385.5
Melting point 122-123.5°C 122-124°C 106°C
Solubility in water (20°C) 1 mg/litre at pH 7 9 mg/litre at pH 5 40 mg/litre
greater at lower pH (pH not given)
Solubility in other 10 g/litre (ethanol) 22 g/litre (ethanol) moderately soluble
solvents (20°C) 171 g/litre (dichloromethane) 82 g/litre (ethyl acetate) in organic solvents
28 g/litre (diethyl ether) 5 g/litre (hexane)
50 g/litre (acetone) 460 g/litre (dichloromethane)
89 g/litre (toluene)
Vapour pressure 0.047 mPa (50°C) 1.9 mPa (60°C) 0.021 mPa
Log Kow 3.43 3.43 -
a From Tomlin (1997); NLM (1998).
3. ANALYTICAL METHODS
Triphenyltin compounds and their degradation products can be
analysed in food commodities and in environmental or biological media
using several techniques, depending upon type of medium and
sensitivity required. The procedure usually starts with either liquid
extraction or adsorption onto a solid matrix, followed by
re-extraction and/or concentration. Quantification is then performed
using flame or flameless atomic absorption spectrometry, gas
chromatography with flame photometric or mass spectrometric detection,
or normal-phase high-performance liquid chromatography with
ultraviolet or fluorescence detection (Hattori et al., 1984; Ishizaka
et al., 1989; Fent & Hunn, 1991; Gomez-Ariza et al., 1992; Staeb et
al., 1992; Tsunoda, 1993; Kohri et al., 1995; Suzuki et al., 1996).
The detection limits of these techniques are in the range of
ng/litre for water and <1 µg/kg for sediments and biological samples.
Triphenyltin can also be separated from samples by capillary
supercritical fluid chromatography and measured by inductively coupled
plasma mass spectrometry. A detection limit of 12.0 pg was obtained
for triphenyltin using this method (Vela & Caruso, 1993).
Triphenyltin in water, sediment, and biological samples, as well
as inorganic tin that is excreted in urine following exposure to
triphenyltin, can be extracted with hydrogen chloride and
n-hexane/benzene (3:2 v/v) in the presence of tropolone, then
pentylated with a Grignard reagent prior to gas chromatography with
flame photometric detection. Quantification limits by this method were
found to be 3 ng/litre for water, 0.5 µg/kg for sediments and
biological samples, and 3 pg as tin for urine (Ohhira & Matsui, 1991,
1993; Harino et al., 1992).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Triphenyltin compounds have been used extensively as algicides
and molluscicides in antifouling products since the 1960s (HSE, 1992).
TPTA and TPTH are used mainly as fungicides with a preventive
action on potatoes, sugar beets, hops, and celery (FAO, 1991a).
Triphenyltin compounds are used on rice against fungal diseases,
algae, and molluscs.
Use of triorganotins in antifouling paints has been restricted in
many countries because of their catastrophic effects on the oyster
industry and more general effects on the aquatic ecosystem.
Information on amounts of triphenyltins used has been obtained
only from Japan. Use of triphenyltin compounds for antifouling paints
in Japan decreased from 4835 tonnes in 1983 to 346 tonnes (formulation
basis) in 1989 (Sugita, 1992). Their use for antifouling paints was 40
tonnes (active ingredient) in 1989 and stopped after 1990 in Japan
(MITI, 1998). About 120-140 tonnes (active ingredient) were produced
each year between 1994 and 1996 in Japan for export (MITI, 1998).
Between 1978 and 1990, 33-75 tonnes (active ingredient) of
phenyltin compounds were produced in Japan for use as a fungicide;
production ceased in 1990 (JPPA, 1982-1996).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Degradation of triphenyltin occurs through sequential
dephenylation resulting from cleavage of the tin-carbon bond through
biological, ultraviolet irradiation, chemical, or thermal mechanisms;
biological cleavage and cleavage by ultraviolet irradiation are
considered to be the most significant processes. Abiotic factors, such
as elevated temperatures, increased intensity of sunlight, and aerobic
conditions, seem to enhance triphenyltin degradation in the
environment (CICAD National Committee, 1997).
Hydrolysis of triphenyltin compounds in water leads to the
formation of principally TPTH and various hydrated oxides (Beurkle,
1985). It has been demonstrated that the presence of chloride from
seawater lowers the solubility of triphenyltin compounds by reaction
with the hydrated cation to form the covalent organotin chloride
(Ozcan & Good, 1980).
In plants, no translocation occurs from treated leaves (FAO,
1991a). TPTA and TPTCl are spontaneously hydrolysed to form TPTH.
Phenyl groups are split off from TPTH to form diphenyl and monophenyl
compounds. Both parent compound and metabolites conjugate to form
glycosides or glutathione conjugates.
The persistence of TPTA and TPTH depends on soil type and pH.
TPTH is strongly adsorbed to sediment and soil, and little desorption
occurs. Therefore, uptake into plants via roots may be expected to be
extremely low.
14C-labelled TPTA in soil degraded to inorganic tin with
evolution of 14C-labelled carbon dioxide. Similar experiments on
sterile soil showed insignificant evolution of labelled carbon
dioxide, which suggests that degradation can be attributed to
microorganisms (Barnes et al., 1971). Soil respiration was slightly
enhanced after treatment with TPTA, indicating that there were no
adverse effects on aerobic microorganisms (Suess & Eben, 1973).
A half-life of 1-3 months has been reported for TPTH in sandy and
silt loam soils and 126 days in flooded silt loam (US EPA, 1987). The
half-life of triphenyltin in water was estimated to be several days in
June and 2-3 weeks in November (Soderquist & Crosby, 1980).
Half-lives of triphenyltin in mussels ( Mytilus edulis) taken in
the summer of 1989 in Yokohama (a busy port, heavily contaminated with
triphenyltin) and Urayasu (a river mouth, about 10 times less polluted
than Yokohama) in Japan were estimated to be 139 and 127 days,
respectively (Shiraishi & Soma, 1992). Biological half-lives of
triphenyltin in short-necked clams ( Tapes [ Amygdala] japonica)
and guppy ( Poecilia reticulata) were estimated to be approximately
30 days and 48 days, respectively (Takeuchi et al., 1989; Tas et al.,
1990). The ecological half-life of triphenyltin in gastropods was
estimated to be 347 days (Mensink et al., 1996).
Temporal variations of phenyltin concentrations in zebra mussels
( Dreissena polymorpha) were studied at two locations near potato
fields during and after the triphenyltin fungicide spraying season in
the Netherlands (Staeb et al., 1995). Phenyltin concentrations in
zebra mussels were high in the period before and during harvesting but
not during the spraying season, which suggests that phenyltin
compounds in some foliage ended up in the water and were taken up by
the mussels. Although higher concentrations were detected in locations
near areas of spray operation, marinas, and harbours, the widespread
presence of triphenyltin residues in mussels collected in 56 locations
all over the Netherlands suggests the contribution of transport via
the air.
An extensive study on the presence of nine organotin compounds in
a freshwater food-web (zebra mussel, eel, roach bream, pike, perch,
pike perch, and cormorant; details and scientific names of species not
given in the report) revealed that phenyltin concentrations in benthic
species were higher than butyltin concentrations in lower trophic
levels (Staeb et al., 1996). This suggests that triphenyltin is to a
large extent taken up from the sediment by benthic organisms. At
higher trophic levels, net bioaccumulation of triphenyltin compounds
was greater than that of tributyltins, resulting in relatively higher
triphenyltin concentrations. With birds, the highest concentrations of
organotins were in liver and kidney and not in subcutaneous fat, which
shows that organotins accumulate via mechanisms different from those
of traditional lipophilic compounds.
BCFs in daphnids did not exceed 300 (Filenko & Isakova, 1979). In
fish, BCFs ranged from 257 to 4100. The highest value (4100) was
estimated for filefish ( Rudarius ercodes) cultivated in water
containing 148 ng triphenyltin/litre for 56 days (Yamada & Takayanagi,
1992). When Lymnaea stagnalis (a freshwater snail) was exposed to 2
µg TPTH/litre for 5 weeks, tin accumulated to the greatest extent in
the intestinal sac, to a level of 65.1 mg/kg (i.e., BCF of 32 500; Van
der Maas et al., 1972).
Tissue concentrations of triphenyltin in common carp ( Cyprinus
carpio) exposed to 5.6 µg TPTCl/litre for 10 days, which reached a
plateau after 7 days, were examined. The BCFs were highest in the
kidney (2090), followed by liver (912), muscle (269), and gall bladder
(257) (Tsuda et al., 1987).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Triphenyltin levels in ambient water, sediment, and organisms
were surveyed in about 30 locations (estuaries and bay) in Japan
between 1982 and 1995 (Japan Environment Agency, 1983, 1996).
Triphenyltin levels in water (detection limit 5 ng/litre) and sediment
(detection limit 1.0 ng/g) of bay and inshore areas decreased from
2.7-8.0 ng/litre and 3.3-7.8 ng/g in 1988-1991 to 2.5-3.0 ng/litre and
1.5-2.3 ng/g in 1992-1995, respectively.
Triphenyltin levels in ambient water and sediment in the Tokyo
bay area gradually decreased from peak levels (geometric means: 25.1
ng/litre in water, 4.3 ng/g in sediment) to 1.8 ng/litre (water) and
0.19 ng/g (sediment) in 1993 because of consecutive tightening of
regulations and voluntary withdrawal of use by coastal fishery
industries (Takeuchi et al., 1991).
Triphenyltin levels were measured in fish and shellfish obtained
from the Tokyo Central Fish Wholesale Market from April 1988 to March
1991 (Takeuchi et al., 1991; see section 6.2). The finding of
triphenyltin in coastal fish, as well as in open-ocean or pelagic
fish, is suggestive of biomagnification through the food-chain. High
levels in clams and oysters showed that direct uptake from water or
sediment also plays an important role for these species.
In the Netherlands, up to 920 ng triphenyltin (as tin)/g sediment
was found in the Westinder lake system in 1993, whereas no
triphenyltin was found in the water (detection limit 5 ng/litre)
(Staeb et al., 1996). In freshwater marinas in Switzerland, up to 191
ng triphenyltin/litre was detected in 1988-1990, whereas 107 ng/g dry
weight was the highest value measured in vertical sediment core
profiles, and concentrations up to 11 ng triphenyltin/litre were
measured in the river system (Fent & Hunn, 1991, 1995). In Dreissena
mussels of the same marinas, up to 3.88 µg triphenyltin/g wet weight
was detected (Fent & Hunn, 1991), whereas up to 0.31 µg triphenyltin
(as tin)/g dry weight in Mytilus mussels and up to 0.24 µg
triphenyltin/g in Thais snails were detected in a Spanish marina
area in 1995 (Morcillo et al., 1997).
Biological monitoring of triphenyltin concentrations in fish from
coastal areas of Japan showed that concentrations decreased between
1989 (detected in 40 out of 65 samples, maximum concentration 2.6 µg/g
wet weight in muscle, detection limit 20 ng/g) and 1995 (detected in
21 out of 70 samples, maximum concentration 0.25 µg/g) (Japan
Environment Agency, 1996). Similarly, triphenyltin levels in mussels
and birds decreased over the same period: triphenyl was detected in 17
out of 25 samples of mussels (maximum concentration 0.45 µg/g) and in
5 out of 10 samples of birds (maximum concentration 0.05 µg/g) in
1989, compared with 0 out of 35 samples of mussels and 0 out of 10
samples of birds in 1995 (detection limit 0.02 µg/g in both years)
(Japan Environment Agency, 1996).
Zebra mussels were used as a biomonitor to evaluate organotin
pollution in Dutch fresh waters (Staeb et al., 1995). High
concentrations (1700-3200 ng tin/g dry weight) were found near
locations where triphenyltin fungicide had been sprayed. Degradation
products (di- and monophenyltins) were also detected in nearly all
mussels.
In pecan orchards (Georgia, USA) where triphenyltin fungicides
were sprayed, triphenyltin concentrations in foliage and soils were
8.5-37 µg/g dry weight and 1.2-12 µg/g dry weight, respectively
(Kannan & Lee, 1996). Although triphenyltin was absent in surface soil
where the fungicide had been sprayed 8-10 times a year until 2 years
earlier, monophenyltin was detected at approximately the same
concentration as in recently sprayed orchards. Fish (bluegill
[ Lepomis macrochirus], largemouth bass [ Micropterus salmoides],
and channel catfish [ Ictalurus punctatus]) from a pond near a
recently sprayed orchard contained predominantly monophenyltin (with
the highest concentration of 22 µg/g wet weight in the liver of
catfish) in addition to smaller amounts of triphenyltin and
diphenyltin.
6.2 Human exposure
No data are available on occupational exposure to triphenyltin
compounds. There are also no data on levels of triphenyltin in indoor
or ambient air or in drinking-water.
The residue data available in support of registration of
triphenyltin compounds in the United Kingdom, obtained using various
colorimetric methods, ranged between 0.013 and 0.016 mg/kg in 3 out of
25 samples of potatoes provided by the Potato Marketing Board and
known to have been treated with a triphenyltin fungicide. The
remaining samples contained residues below 0.013 mg/kg, which is the
limit of detection (ACP, 1990). In supervised trials of triphenyltin
formulations (wettable powder; 54%; 216-324 g active ingredient/ha) on
potatoes in Germany, residues ranged from 0.3 mg/kg to less than the
detection limit (0.01 mg/kg) 7 days after application (FAO, 1991a).
Supervised trials of triphenyltin formulations (wettable powder; 50 or
54%; 216-324 g active ingredient/ha) in Germany on sugar beets showed
residues ranging from 0.1 to 1.9 mg/kg in leaves and less than the
detection limit (0.05 mg/kg) in beets 35 days after application. In
supervised trials of triphenyltin formulations (wettable powder) on
rice in the USA, residues ranged from less than the detection limit
(0.01 mg/kg) to 0.03 mg/kg 22-23 days after application (57.5%; 536 g
active ingredient/ha, twice) and from less than the detection limit
(0.01 mg/kg) in milled rice or bran 22-46 days after application
(47.5%; 250 or 500 g active ingredient/ha) (FAO, 1991a).
When 14C-labelled TPTH was administered orally to dairy cows
over a period of 60 days at doses of 1.13, 5.61, or 22.44 mg
triphenyltin/kg diet (dry matter), residues were 0.08, 0.31, and 0.9
g/kg in meat and 0.006, 0.026, and 0.41 mg/kg in milk, corresponding
to transfer factors of 0.038-0.068 in meat and 0.004-0.006 in milk
(Smith, 1981).
Triphenyltin levels were measured in fish, clams, and shrimps
obtained from the Tokyo Central Fish Wholesale Market from April 1988
to March 1991. Levels were higher in cultured fish and in fish from
coastal or bay areas than in pelagic fish (mean concentration 0.048
µg/g) (Takeuchi et al., 1991). Freshwater fish were relatively
uncontaminated. Fish obtained from bay or inshore areas were the most
contaminated; the highest concentration measured in 82 samples of four
fish species was >1.0 µg triphenyltin/g muscle (mean 0.317 µg/g).
Triphenyltin levels in clams and shrimps ranged from 0 to 0.83 µg/g
edible portion (mean 0.113 µg/g). Triphenyltin intake from pelagic
fish was estimated based on analyses of fish samples in 1988-1991 by a
market basket study in Tokyo as 3.15 µg (mean concentration of 0.048
µg/g times 65.6 g intake of pelagic fish by a Japanese person per
day). Although tributyltin was used more abundantly than triphenyltin
in antifouling paints, residue levels in fish and shellfish were
mostly comparable, with several differences among fish groups.
National market basket studies, including the above study, have
estimated daily intakes of triphenyltin per 50-kg person in Japan
(expressed as TPTCl) to be 4.3, 10.4, 2.7, 0.6, 1.2, 1.4, 0.7, and 2.7
µg in 1990, 1991, 1992, 1993, 1994, 1995, 1996, and 1997, respectively
(NIHS, 1998). Triphenyltin compounds were found mostly in seafood. As
about a twofold difference was observed between the above estimated
daily intakes (averages of 10 local laboratories, including the Shiga
Prefecture) and estimated intakes in the Shiga Prefecture (Tsuda et
al., 1995), this implies that differences in food intake patterns or
some other factor may influence estimates of daily intake. This fact
and coincidental contamination with tributyltin must be taken into
account in any risk estimation for exposure by the oral route.
Another report of a market basket survey estimated the intake of
triphenyltin from raw and processed seafood in Nagasaki Prefecture (a
southern part of Japan) in 1989-1991 to be 8.51 µg/day per person
(Baba et al., 1991). TPTCl concentrations in fish, shellfish, seaweed,
canned fish/shellfish, fish paste product, and salted/dried fish were
274, 80, 21, 12, 16, and 22 ng/g (averages), respectively. Cooking did
not reduce the triphenyltin content of fish and shellfish samples.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
Several studies have shown that TPTH orally administered to rats
is eliminated mainly via the faeces, with smaller amounts in the
urine. Metabolites found in faeces included di- and monophenyltin as
well as a significant portion of non-extractable bound residues (the
sulfate conjugates of hydroquinone, catechol, and phenol). In faeces,
the major substance present was unchanged parent compound.
TPTA was rapidly and completely hydrolysed to TPTH at pH 3-8 and
23-24°C (Beurkle, 1985).
Seven days after oral administration to rats, TPTH residues
(approximately 3% of the administered dose) were distributed mainly in
the kidneys, followed by liver, brain, and heart (Eckert et al., 1989;
Kellner & Eckert, 1989). Similar results were obtained after chronic
exposure for 104 weeks (Dorn & Werner, 1989; Tennekes et al., 1989a).
Species differences in the metabolism of triphenyltin were
investigated by Ohhira & Matsui (1996). Dearylation of triphenyltin
was slower in hamsters than in rats, and pancreatic accumulation of
triphenyltin was higher in hamsters. There was a good correlation
between tin concentrations in the pancreas and plasma glucose levels,
indicating that triphenyltin-induced hyperglycaemia depends upon the
amount of tin compounds absorbed into the pancreas. Most of the tin
compounds in the brains of both species were triphenyltin.
Percutaneously absorbed TPTA in guinea-pigs was distributed to
the highest extent in the liver, followed by the adrenal glands,
kidneys, brain, spinal cord, and pancreas (Nagamatsu et al., 1978).
Triphenyltin, diphenyltin, and monophenyltin were detected in faeces
in a ratio of 15:6:2. The biological half-life of triphenyltin was
estimated to be 9.4 days.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Because TPTA and TPTCl are hydrolysed rapidly to TPTH in aqueous
media, the results of oral toxicity studies with these triphenyltin
compounds can be applied to TPTH. Many critical studies described
below were cited from WHO (1992), which summarizes evaluations of the
original proprietary reports; as details of these original proprietary
reports were not available to the CICAD authors, we have relied on the
WHO evaluation for these studies.
8.1 Single exposure
After a single oral administration of triphenyltin, toxic signs
observed in various species include anorexia, emesis, tremor, and
diarrhoea, followed by drowsiness and ataxia (WHO, 1992). No further
details were provided. Clinical signs appeared a day after
administration and were exacerbated over the following 3 days or so
(CICAD National Committee, 1997). Oral LD50s for TPTH were
approximately 160 mg/kg body weight in rats and 100-245 mg/kg body
weight in mice; oral LD50s for TPTA were 140-298 mg/kg body weight in
rats and 81-93 mg/kg body weight in mice (Ueda & Iijima, 1961; Scholz
& Weigand, 1969; Hollander & Weigand, 1974; Ikeda, 1977; Leist &
Weigand, 1981a,b).
Dermal LD50s were 127 mg/kg body weight in rabbits and 1600
mg/kg body weight in rats for TPTH (Leist & Weigand, 1981c,d) and 350
mg/kg body weight in mice and >2000 mg/kg body weight in rats for
TPTA (Ueda & Iijima, 1961; Diehl & Leist, 1986a). Inhalation LC50s
were 44-69 mg/m3 in rats for TPTH and TPTA (Hollander & Weigand,
1981, 1986).
8.2 Irritation and sensitization
TPTA was not irritating to the skin of rabbits (Diehl & Leist,
1986b). However, severe ocular lesions developed in rabbits; these
were not reversible (Diehl & Leist, 1986c).
At concentrations that were irritating to skin, TPTH (purity
97.0%) showed no skin sensitization in guinea-pigs in the Buehler test
(Leist & Weigand, 1981e; Schollmeier & Leist, 1989) or in the
maximization test (Diehl & Leist, 1987). TPTA gave a positive response
when tested for skin sensitization in guinea-pigs in the Buehler test
(Diehl & Leist, 1986d). Further details were not given.
8.3 Short-term exposure
One unpublished dermal exposure study in rats submitted to WHO
(1992) is described in Table 2. A NOAEL of 10 mg/kg body weight was
identified in this study. Additional short-term studies that examined
effects on the immune system are discussed in section 8.7.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Several subchronic studies on the effects of TPTH on several
animal species exposed by various routes have been performed (WHO,
1992). Studies that show effects at the lowest doses in each animal
species are summarized in Table 2. Further studies and details are
available in WHO (1992) and CICAD National Committee (1997).
Dietary studies with rats, mice, and dogs showed a decrease in
immunoglobulin levels, body weight gain, and white blood cells and an
increase in liver weights and death. Decreases in immunoglobulin
levels and white blood cells were observed consistently at lowest
effect levels in all tests. NOAELs for dietary studies were identified
as 3.4-4.1 mg/kg body weight per day in mice (3-month exposure),
0.30-0.35 mg/kg body weight per day in rats (13-week exposure), and
0.21 mg/kg body weight per day in dogs (52-week exposure) (Table 2).
Several species were affected in a similar way, although mice appeared
to be least sensitive.
In an inhalation study in rats exposed to TPTH, macroscopic
lesions in the lungs were observed at 2.0 mg/m3 in most of the dead
rats (all males and one female died at this dose), and histopathology
revealed severe effects in lower air passages and in the lungs. The
NOAEL was 0.014 mg/m3.
8.4.2 Chronic exposure and carcinogenicity
Chronic toxicity/carcinogenicity studies in rats and mice exposed
to TPTH (WHO, 1992) are summarized in Table 3. There were indications
that the doses received by animals in the US National Toxicology
Program (NTP) studies may have been lower than intended owing to
instability of the test compound in the diet. The size of the control
group (small compared with test groups) in the NTP mouse study limits
interpretation of the results. Consistent findings in all studies are
a decrease in immunoglobulin concentrations at lowest effect levels
and a higher susceptibility of females, as seen in a higher mortality
rate and reduced body weight gain at low doses.
Effects on immunoglobulin levels were reported with both rats and
mice. In an 80-week feeding study in mice, a decrease in
immunoglobulin concentrations was seen at dose levels of 5, 20, and 80
ppm TPTH in the diet. The incidence of hepatocellular adenoma in both
sexes and the incidence of hepatocellular carcinoma in females only
were increased at the highest dose (80 ppm) (Tennekes et al., 1989a;
Table 3). The NOAEL in this study was 5 ppm, equivalent to 0.85 mg/kg
body weight per day for males and 1.36 mg/kg body weight per day for
females, based on decreased body weight gain in females.
Table 2: Short-term and subchronic studies on TPTH.
Study type, Dose Species Observed effects (mostly at lowest effect Reference
duration (purity; %) (strain, number/dose) level) and NOAEL values
Diet 0, 4, 20, 100 ppm Mouse At 100 ppm, haematological and biochemical Suter & Horst,
3 months (97.2%) (NMRI, 10/group) parameters were affected, including a reduction 1986a
in erythrocyte count and haemoglobin level,
an increase in platelet count, and a decrease
in IgG, IgA, and IgM (females only). Liver
weight was increased in both sexes, and
relative weights of ovaries, adrenals, kidneys,
heart, and brain were decreased in females at
this dose. NOAEL: 20 ppm (3.4 mg/kg body weight
per day for males, 4.1 mg/kg body weight per day
for females).
Diet 0, 4, 20, 100 ppm Rat Relative testis weight was significantly higher in Suter & Horst,
13 weeks, (97.2%) (Wistar, 15/group) high-dose males, whereas no effects on spleen and 1986b
4-week thymus weights were observed. In females, white blood
recovery cells decreased at 20 ppm (corresponding to 1.75 mg/kg
body weight per day) and 100 ppm. After the recovery
period, IgG decreased significantly in females at
all dose levels. NOAEL: 4 ppm (0.30 mg/kg body
weight per day for males, 0.35 mg/kg body weight per
day for females).
Diet 0, 2, 6, 18 ppm Dog At 18 ppm, relative liver weight was increased in Sachose et al., 1987
52 weeks (97.2%) (beagle, 10/group) females and serum albumin/globulin ratio was
decreased in males. NOAEL: 6 ppm (0.21 mg/kg body
weight per day).
Table 2 (continued)
Study type, Dose Species Observed effects (mostly at lowest effect Reference
duration (purity; %) (strain, number/dose) level) and NOAEL values
Dermal 0, 5, 10, 20 Rat Dose-related increase in erythema and scale Leist, 1988
21 applications mg/kg body (unknown, formation was seen. At 20 mg/kg body weight, four
in 29 days, weight each 6-12/group) rats died. Lymphocytes decreased in both sexes and
14-day recovery time (97.1%) monocytes increased in females at 20 mg/kg body
weight. NOAEL for systemic toxicity: 10 mg/kg body
weight.
Inhalation 0.014, 0.338, Rat All males and one female died at the highest dose. At Duchosal et
6 h/day, 1.997 mg/m3, (Wistar, 0.338 mg/m3, a decrease in white blood cells and al., 1989
5 days/week, nose-only 10/group) biochemical and haematological changes were seen in
13 weeks, exposurea (96.2%) females. IgM increase was seen in males at 0.338
4-week recovery mg/m3. Histopathology revealed degenerative and
inflammatory lesions in the anterior part of the nasal
cavity, in the trachea, and in the lungs in the
highest dose group of both sexes. NOAEL: 0.014 mg/m3.
a Mass median aerodynamic diameter: 3 µm.
In a 2-year rat feeding study with dietary concentrations of 0,
5, 20, and 80 ppm TPTH (Tennekes et al., 1989b), a decrease in
immunoglobulin concentrations was observed in all triphenyltin-dosed
groups. An increase in the incidence of pituitary adenoma in females
and an increase in testicular Leydig cell tumours at higher doses were
accompanied by non-neoplastic lesions in these organs. Low survival at
higher doses limits interpretation of the results in females. A NOAEL
could not be established at the lowest concentration of 5 ppm
(equivalent to 0.3 and 0.4 mg/kg body weight per day in males and
females, respectively) because of increased mortality in females and
reduced serum immunoglobulin levels at this dose.
Although some tumours were detected in the above studies, the WHO
expert group apparently evaluated those as being not significant (WHO,
1992). No detailed explanation of the reasons for this or results of
statistical analysis were provided in the report. Recently, Clegg et
al. (1997) critically examined the human relevance of Leydig cell
hyperplasia and adenoma formation in rodents after chronic exposure
and suggested that a hormonal mode of action, which may be of little
relevance to humans, either mechanistically or quantitatively, could
be operating. They also pointed out the very low incidence of Leydig
cell adenomas in humans (age-adjusted occurrence of 0.4 per million).
An early 2-year rat study showed a reduction in white blood cell
count at a TPTH dietary level of 5 ppm (corresponding to 0.3 mg/kg
body weight per day) (Til et al., 1970). The NOEL was chosen as 2 ppm
in the diet (equivalent to 0.1 mg/kg body weight per day) based on
this finding.
8.5 Genotoxicity and related end-points
Most in vitro and in vivo genotoxicity tests, such as the
Salmonella mutagenicity test, yeast forward mutation test, mitotic
gene conversion assay, mouse lymphoma forward mutation assay,
chromosomal aberration assay, unscheduled DNA synthesis, micronucleus
test in mice, cytogenetic assay in Chinese hamster, and dominant
lethal assay in rats, showed negative results at the maximum doses
tested, based on studies reviewed in WHO (1992).
There are no new data that impact on the conclusion in WHO (1992)
that triphenyltin is not genotoxic. Recent data indicate, however,
that triphenyltin potentiates the genotoxicity of other substances.
Triphenyltin showed potentiation of mitomycin C-induced breakage-type
chromosomal aberrations in cultured hamster cells when cells were
treated during the G2 phase (Sasaki et al., 1993). Similarly, the
frequency of micronuclei induction by mitomycin C (1 mg/kg
intraperitoneal injection) in mouse peripheral reticulocytes was
enhanced by treatment with TPTCl, although TPTCl itself did not induce
micronuclei (Yamada & Sasaki, 1993). Positive responses in these
assays may be related to the toxic effects of triphenyltin on
lymphocytes, because two in vivo studies for chromosomal aberrations
(a micronucleus test in mice and a cytogenetic test in Chinese
hamsters) were negative. These data support the conclusion of the WHO
(1992) group.
It is therefore concluded that triphenyltin does not present a
genotoxic hazard.
8.6 Reproductive and developmental toxicity
Triphenyltin appears to cause reproductive effects in rats and
developmental toxicity in rats, rabbits, and hamsters at low doses
(around 1 mg/kg body weight and higher) at which maternal toxicity is
observed. Studies that show effects at the lowest doses for various
end-points in experimental animals are summarized in Table 4 (WHO,
1992; CICAD National Committee, 1997). Decreases in number of
implantations, live fetuses, and mean fetal weight and increases in
resorption were the consistent findings observed at lowest effect
doses.
An increase in the number of dead F1 pups and a decrease in mean
litter size, pup weight, and relative spleen and thymus weights in the
weanlings were observed in a two-generation rat study at 18.5 ppm TPTH
in the diet (approximately 1.5 mg/kg body weight per day); at this
concentration, body weight gain and food consumption of the parents
were not affected (Young, 1986). The NOAEL in this study was 5 ppm
(equivalent to 0.4 mg/kg body weight per day).
A general paucity of mature sperm was seen in rats treated with
TPTA and TPTCl in the diet (20 mg/kg body weight per day) for 20 days,
and spermatogenic anomalies were observed in histological sections
(Snow & Hays, 1983). Reduced food intake and the resultant decrease in
weight gain were suspected as the cause, as malnutrition is known to
precede gonadal dysfunction and even atrophy. However, differences in
the distribution of spermatogenic phases in rats treated with TPTA and
TPTCl do not support this explanation.
TPTCl prevented implantation in rats in a dose-dependent manner
when administered at 0, 3.1, 4.7, or 6.3 mg/kg body weight per day on
days 0-3 and at 0, 6.3, 12.5, or 25.0 mg/kg body weight per day on
days 4-6. The compound caused larger implantation failures when
administered during earlier stages of blastogenesis (Ema et al.,
1997). Implantation failure was observed at 4.7 and 6.3 mg/kg body
weight per day on days 0-3 and at 12.5 and 25.0 mg/kg body weight per
day on days 4-6. The effects of TPTCl on uterine function, as a cause
of implantation failure, were determined using pseudopregnant rats
dosed at 0, 3.1, 4.7, or 6.3 mg/kg body weight per day on days 0-3
(Ema et al., 1998). A significant suppression of the uterine
decidualization and decrease in the serum progesterone levels were
Table 3: Chronic exposure and carcinogenicity studies on TPTH.
Duration Chemical dose Species Effects, NOAEL Reference
(purity; %) (strain,
number/dose)
78 weeks with 0, 37.5, 75 ppm Mouse No treatment-related effects were seen in NTP, 1978
26-week (not stated) (B6C3F1, clinical signs or body weight, although survival
observation After 1 week, 50/group) decreased in females with increased dose. No
period only 57.9% of tumour incidence was found histopathologically.
the initial dose The size of the control group (20 mice of
recovered each sex) limits interpretation of the results.
80 weeks 0, 5, 20, 80 ppm Mouse (KFM-Han, Body weight gain decreased at 80 ppm for males and Tennekes et
(97.2% prepared NMRI, 50/group) at 20 and 80 ppm for females. Decreases in al., 1989a
daily) immunoglobulin concentrations were observed at
various levels. An increased relative number of
lymphoid cells was detected in femoral bone
marrow myelogram for all treated groups.
Incidence of hepatocellular adenomas was 12.2,
20, 26, and 32% at 0, 5, 20, and 80 ppm,
respectively, for males and 0, 0, 0, and 18%
at 0, 5, 20, and 80 ppm, respectively, for
females. At 80 ppm, increased incidence of
hepatocellular carcinoma was seen in females
(6% compared with 0% at other doses). Based on
reduced body weight gain, NOAEL was 5 ppm
(corresponding to 0.85 mg/kg body weight per day
for males and 1.36 mg/kg body weight per day for
females).
Table 3 (continued)
Duration Chemical dose Species Effects, NOAEL Reference
(purity; %) (strain,
number/dose)
2 years 0, 0.5, 1, 2, 5, Rat (not stated, A slight decrease in white blood cells was seen Til et
10 ppm (not stated) 25/group) at the highest dose in the first year. This effect al., 1970
was less often seen at 5 ppm (corresponding to
0.3 mg/kg body weight per day), and only once at
2 ppm in the males. The relative thyroid weight
was slightly decreased at 10 ppm in the females
only. Average relative weights of other organs,
gross autopsy findings, and microscopic
examinations did not reveal significant differences
between treated and control groups. NOEL was
2 ppm in the diet, equivalent to 0.1 mg/kg body
weight per day.
78 weeks with 0, 37.5, 75 ppm Rat (Fischer 344, No effects were observed on clinical signs, NTP, 1978
26-week (not stated) 50/group) mortality, food consumption, macroscopy, or
observation After 1 week, only histopathology. No increase in tumour incidence.
period 57.9% of the initial
dose recovered
104 weeks 0, 5, 20, 80 ppm Rat (SPF KFM-Han In females, mortality was increased (survival Tennekes et
prepared twice Wistar, 70/group) was 75, 51, 36, and 23%, respectively, with al., 1989b
monthly from frozen increasing dose). Immunoglobulin decrease
stock (IgG1 and IgG2a for females, and IgG2c for
males) was observed at all doses. IgA levels
for males decreased, and IgM levels increased
for both sexes at 20 and 80 ppm. Leydig cell
tumours were 1.7, 8.5, 5.0, and 16.7% at 0, 5,
20, and 80 ppm, respectively. The incidence of
pituitary adenomas was increased in females at
20 and 80 ppm. These changes were accompanied by
non-neoplastic lesions in the pituitary and
testis. NOAEL was not established because of
observations of mortality increase in females and
Table 3 (continued)
Duration Chemical dose Species Effects, NOAEL Reference
(purity; %) (strain,
number/dose)
104 weeks serum immunoglobulin decrease at the lowest level,
(continued) 5 ppm (equal to 0.3 mg/kg body weight per day for
males and 0.4 mg/kg body weight per day for females).
Table 4. Reproductive and developmental toxicity studies on triphenyltin.
Species Study design Effects Reference
(strain,
number/sex/dose)
Rat In a two-generation reproduction study, rats The number of dead F1 pups was Young, 1986
(Wistar, were given TPTH in diet (0, 5, 18.5, or 50 increased and mean litter size
30/sex/group) ppm) during growth, mating, gestation, and decreased at 18.5 and 50 ppm. In
lactation for one litter per generation. Fo parents, the body weight gains
Clinical signs, body weight, food consumption, and food consumption of both sexes
mating performance, and reproductive parameters were lower at 50 ppm. At 50 ppm, the
were observed. Organ weights of parents relative weights of brain, testes,
and pups were recorded. Pups were ovaries, adrenals, kidneys, spleen,
sexed and examined for gross malformations and heart were increased in F0
and the number of stillborn and live pups. and/or in F1 adults and/or F1 and F2
weanlings. A dose-related decrease was
observed in spleen and thymus weight
in F1 and F2 weanlings at 50 and 18.5
ppm (equal to 1.5 mg/kg body weight
per day). The NOAEL was 5 ppm, equal to
0.4 mg/kg body weight per day.
Table 4 (continued)
Species Study design Effects Reference
(strain,
number/sex/dose)
Rat TPTA or TPTCl (0 or 20 mg/kg body weight per In rats sacrificed after 21 days, all Snow & Hays,
(Holtzman, day in diet) was dosed for 20 days. Four eight spermatogenic phases were seen, 1983
13 males/group) animals from each group were sacrificed on but there was a general paucity of mature
day 21, and the remaining animals were sperm, and the distribution showed some
sacrificed after 4 more days with test diets predominance of immature sperm. Recovery
and a recovery period (70 days). Distribution was seen after the 70-day control diet.
of the eight phases of spermatogenesis was Treated animals ate about two-thirds as
observed. much food as the controls.
Pregnant rat TPTCl was dosed by gavage at 0, 3.1, 4.7, or In successfully mated females, TPTCl Ema et al.,
(Wistar, 6.3 mg/kg body weight per day on day 0 to day prevented implantation in a dose-dependent 1997
10-13/group) 3 of gestation or at 0, 6.3, 12.5 or 25.0 mg/kg manner. The pregnancy rate was significantly
body weight per day on day 4 to day 6 of decreased after administration of TPTCl on
gestation. Dams were sacrificed on day 20 of days 0 to 3 at 4.7 and 6.3 mg/kg body weight
gestation. Numbers of live/dead fetuses and per day, and days 4 to 6 at 12.5 and 25.0
resorptions were counted. Live fetuses were mg/kg body weight per day. TPTCl caused larger
sexed, weighed, and inspected for malformations failures in implantations when administered
externally. during earlier stages of blastogenesis.
Pregnant rat TPTH was dosed at 0, 0.35, 1, 2.8, or 8 A dose-related decrease in body weight gain Rodwell,
(Sprague-Dawley, mg/kg body weight per day on day 6 through and food consumption was seen in the 2.8 and 1985
45/group) day 15 of gestation. On day 20 of gestation, 8 mg/kg body weight per day groups. At 8
all rats were sacrificed. Clinical signs, body mg/kg body weight per day, an abortion in one
weights, and food consumption were examined. dam, increase of number of non-gravid dams,
After sacrifice, the dams were observed for total litter resorptions, early resorptions,
number and location of viable and non-viable and significant decrease of number of viable
fetuses, early and late resorptions, and the fetuses and fetal weight were observed. The
number of implantation sites. The corpora lutea incidence of absent/delayed ossification was
were counted. Fetuses were weighted, sexed, and increased in high-dose litters. The percentage
examined for external, internal, and skeletal of fetuses with hydrocephaly was 0.4, 0, 0,
anomalies. 0.4, and 1%, and with omphalocele 0.2, 0.2, 0.2
0, and 0.5%, respectively, for the 0, 0.35, 1,
Table 4 (continued)
Species Study design Effects Reference
(strain,
number/sex/dose)
2.8, and 8 mg/kg body weight per day groups.
There was no evidence for TPTH-induced
irreversible structural effects. The NOAEL for
maternal toxicity was 1 mg/kg body weight per
day, and for embrytoxicity, 2.8 mg/kg body weight
per day.
Pregnant hamster TPTH was dosed by gavage (0, 2.25, 5.08, or The 12 mg/kg body weight per day group showed Carlton &
(Syrian, 12 mg/kg body weight per day) from day 5 to a decrease in mean body weight gain, food Howard, 1982
20-25/group) day 14. All dams were sacrificed on gestation consumption, pup weight, and death (4 animals).
day 15. The gravid uterus was weighed, and Two animals died in each of the 2.25 and 5.08
corpora lutea were counted. Fetuses and mg/kg body weight per day groups. The average
resorption sites were noted. Fetuses were number of minor anomalies of fetuses per litter
weighed and observed for external, visceral, and delayed ossifications were significantly
and skeletal malformations. greater among the 12 mg/kg body weight per day
group. Three cases of hydronephrosis were seen
at 5.08 mg/kg body weight per day and one case
of hydrocephalus was seen at 12 mg/kg body weight
per day.
Pregnant TPTA (0, 0.1, 0.32, or 1.0 mg/kg body weight In the 1.0 mg/kg body weight per day Baeder,
rabbit per day) was dosed by gavage from day 6 to group, one dam died, three dams aborted, 1987
(Himalayan, day 18 of gestation. On day 29 of gestation, one dam gave a premature delivery, and
15/group) the dams were sacrificed. Dams were observed two dams had intrauterine deaths. The
for clinical signs, body weight, food number of implantations and of live
consumption, number of resorptions, fetuses decreased at 1.0 mg/kg body
implantations, corpora lutea, viable and weight per day. Mean fetal weight,
non-viable tissues, organ weights, and crown/rump length, and placental weight
macroscopy. Fetuses were weighed and examined decreased in pups at 1.0 mg/kg body weight
for sex, length, and external, internal, and per day. At 1.0 mg/kg body weight per day,
skeletal anomalies. four pups showed omphalocele with protrusion
Table 4 (continued)
Species Study design Effects Reference
(strain,
number/sex/dose)
of intestinal coils or liver tissue. Slight
retardation of skeletal ossification was
detected at 1.0 mg/kg body weight per day.
An increase in the number of fetuses with
fewer ossified caudal vertebrae, weak
ossification of the hyoid bone, and non-/only
slight ossification of the os pubis in some
fetuses were shown. NOAEL for maternal and
embryo toxicity was 0.32 mg/kg body weight
per day.
Pregnant TPTH was dosed by gavage at 0, 0.1, 0.3, or Two rabbits from the 0.9 mg/kg body weight Rodwell,
rabbit 0.9 mg/kg body weight per day on day 6 to day per day group aborted. A dose-related decrease 1987
(New Zealand 18 of gestation. Dams were sacrificed on day 29 in mean body weight gain and food consumption
white, 22/group) of gestation. Corpora lutea, early/late was observed in the 0.3 and 0.9 mg/kg body
resorptions, and number of implantations were weight per day groups. Mean fetal weight was
counted. Fetuses were weighed, sexed, and lower in the 0.9 mg/kg body weight per day
examined for external, skeletal, and visceral group. The NOAEL for maternal toxicity was
anomalies and developmental variations. 0.1 mg/kg body weight per day, and the NOAEL
for embryotoxicity was 0.3 mg/kg body weight
per day.
found at 4.7 and 6.3 mg/kg body weight per day, at which doses
implantation failure was caused in pregnant rats. These findings
suggest that implantation failure due to TPTCl may be mediated via the
suppression of uterine decidualization correlated with the reduction
in serum progesterone levels.
In hamsters administered TPTH by gavage where death was observed
(2.25 mg/kg body weight per day and higher), anomalies such as
hydronephrosis, hydrocephalus, and delayed ossification were detected
in the pups at 5.08 mg/kg body weight per day and higher (Carlton &
Howard, 1982). Although delayed ossification was observed in rabbits,
which are the most sensitive species, when TPTA was dosed at 1.0 mg/kg
body weight per day by gavage during gestation days 6 through 18,
maternal effects were also detected at this dose level (Baeder, 1987).
The percentages of fetuses with hydrocephaly and omphalocele were not
significantly higher in rats dosed with TPTH (0-8 mg/kg body weight
per day on gestation days 6-15), and it was concluded that there was
no evidence for TPTH-induced irreversible structural effects in rats
(Rodwell, 1985).
The lowest NOAEL for maternal toxicity was seen in rabbits -- 0.1
mg/kg body weight per day, above which dose reductions in body weight
gain and food consumption were observed. The lowest NOAEL for
embryo-toxicity in rabbits was 0.3 mg/kg body weight per day, above
which abortion and a decrease in mean fetal weight were observed
(Rodwell, 1987).
8.7 Immunological and neurological effects
Effects on the immune system were observed in short-term as well
as long-term toxicity studies (WHO, 1992; CICAD National Committee,
1997). Effects of organotin compounds on lymphoid organs and lymphoid
functions were reviewed (Penninks et al., 1990). Like other organotin
compounds, triphenyltin showed immunosuppressive properties
(lymphopenia and a decrease in spleen and thymus weights), resulting
in altered humoral and cellular immunity in rats, mice, and
guinea-pigs, although effects were usually less severe than those
observed with tributyltin.
When weanling male SPF Wistar rats were fed diets containing
TPTCl at 0, 15, 50, or 150 ppm for 2 weeks, thymus weight was
decreased at 15 ppm (corresponding to 1.5 mg/kg body weight per day)
or above, and spleen weight was decreased dose dependently (Snoeij et
al., 1985). At 150 ppm, decreases in body weight and brain weight were
seen, and the liver was enlarged. The effects of TPTCl were similar to
those of tributyltin chloride or tripropyltin chloride in a parallel
test, but less severe.
Groups of mice were given 0, 1, 5, 25, 50, or 125 ppm TPTH in the
diet for 28 days. Twelve male and 12 female mice were killed on day
29, and the remaining mice were returned to control diets and killed
on day 57. A significantly decreased body weight gain was observed in
male and female mice at 125 ppm, from which they recovered after 28
days. Food consumption was significantly decreased at 50 and 125 ppm.
Relative liver weight was increased at 25 (females only), 50, and 125
ppm, relative spleen weight was clearly decreased in males at 50 and
125 ppm and in females at 25 ppm (corresponding to 5 mg/kg body weight
per day) and higher, and relative thymus weight was decreased at
125 ppm in males. At histopathology, lymphoid depletion in the thymus
and spleen was observed in mice at 125 ppm. A decrease in total white
blood cells, neutrophils, and lymphocytes at 50 and 125 ppm in males
and females was noted. At the highest dose, a decrease in total cells
in spleen and splenic B-cells was observed, and a decrease in total
cells in thymus and splenic T-cells was seen in males. IgM levels were
decreased in females at 25 ppm and higher, but the decrease was not
clearly dose related. All effects were reversible. The NOAEL was 5
ppm, equal to 1 mg/kg body weight per day for males and 1.15 mg/kg
body weight per day for females (MacCormick & Thomas, 1990).
When triphenyltin was injected intraperitoneally into mice at
doses of 0, 1, 3, or 10 mg/kg body weight per day for 14 days, it
inhibited the T-cell-dependent humoral (IgM and IgE production) and
cellular (induction of cytotoxic T-cell or induction of delayed
hypersensitivity) immune response at 3 mg/kg body weight per day and
above (Nishida et al., 1990).
In a long-term study with female guinea-pigs fed 15 ppm TPTA in
the diet (corresponding to approximately 1.5 mg/kg body weight per
day), decreases in thymus weight and in the number of plasma cells of
the spleen and lymph nodes were seen in guinea-pigs examined on days
47 and 77. Repeated dosing for 104 days inhibited the immunological
reaction against tetanus toxoids (Verschuuren et al., 1970). The dosed
group had a lower antibody count and fewer antitoxoid-producing cells
at the popliteal fossa than the controls when examined
immunohistologically.
Triphenyltin showed relatively slight neurotoxicological effects
at relatively high doses compared with other trialkyltin compounds
(i.e., triethyltin, trimethyltin, tributyltin, tripropyltin) (Bouldin
et al., 1981; Wada et al., 1982). In neonatal rats dosed orally with
30 mg TPTA/kg body weight per day from day 3 to day 30, no light
microscopic or electron microscopic changes were observed in the
hippocampus or pyriform cortex/lobe, which are susceptible to neuronal
necrosis with trimethyltin (Bouldin et al., 1981). In addition,
triphenyltin did not cause oedema in the myelin sheath, as was usually
induced by triethyltin (Bouldin et al., 1981).
In the maze learning test, rats orally given Tinestan (a product
containing 60% TPTA) at doses of 0.6 (corresponding to 0.36 mg TPTA/kg
body weight per day) or 6 mg/kg body weight per day, 6 days/week for 6
weeks, made many mistakes and showed slow reaction speed (Lehotzky et
al., 1982). In the conditioned avoidance response test, no difference
was observed between the dose groups and the control group; however,
extinction of behaviour was delayed in the high-dose group (6 mg/kg
body weight per day) after discontinuation of the stimulus. Resting
time during swimming tests was shortened by treatment with
amphetamine; in rats given 23 mg Tinestan/kg body weight per day for
20 days, however, amphetamine-induced hyperkinesis was antagonized on
the 20th day. Tin levels in the brain tissues increased in some of the
rats after administration of triphenyltin.
8.8 Mode of action
Treatment of rat thymocytes with immunotoxic organotins (TPTCl,
tributyltin, dibutyltin) at 5 µmol/litre, but not non-immunotoxic
organotins (trimethyltin, triethyltin), caused a rapid decrease in the
F-actin content, resulting in the depolymerization of thymocyte
F-actin (Chow & Orrenius, 1994). Immunotoxic effects of organotin
compounds may involve cytoskeletal modification in addition to the
perturbation of thymocyte calcium homeostasis.
Triphenyltin at concentrations of 0.5-10 µmol/litre induced
calcium overload in rat pheochromocytoma cells, which caused
internucleosomal DNA cleavage typical of apoptotic cell death (Viviani
et al., 1995). Triethyltin or trimethyltin, which did not modify cell
viability, did not enhance or showed little effect on calcium influx.
Triphenyltin induced calcium release in ruthenium red (a calcium
release channel blocker) sensitive and insensitive ways, with EC50
values of 75 and 270 µmol/litre, respectively. The Ca2+-ATPase
activity and calcium uptake of sarcoplasmic reticulum were also
inhibited by triphenyltin. The study suggested that the internal
calcium store of skeletal muscle could be depleted by triphenyltin
through the inhibition of calcium uptake and the induction of calcium
release by acting on the Ca2+-ATPase and calcium release channel.
Development of muscle weakness in organotin intoxication could be
partly explained by this peripheral myopathy-related finding (Kang et
al., 1997).
Oral administration of a single dose of TPTCl (60 mg/kg body
weight) induced diabetes with decreased insulin secretion in hamsters
after 2-3 days, without morphological changes in pancreatic islets.
Administration of TPTCl strongly inhibited a rise in cytoplasmic
calcium concentration induced by 27.8 mmol glucose/litre, 100 µmol
acetylcholine/litre in the presence of 5.5 mmol glucose/litre, and 100
nmol gastric inhibitory polypeptide/litre in the presence of 5.5 mmol
glucose/litre. TPTCl administration impaired the insulin secretion in
islet cells induced by 27.8 mmol glucose/litre, 100 nmol gastric
inhibitory polypeptide/litre in the presence of 5.5 mmol
glucose/litre, and 100 µmol acetylcholine/litre in the presence of
5.5 mmol glucose/litre. The pathology of triphenyltin-induced diabetes
in hamsters involves a defect in cellular calcium response due to a
reduced calcium influx through voltage-gated calcium channels (Miura
et al., 1997).
9. EFFECTS ON HUMANS
Major complaints concerning toxic effects experienced during the
spraying of TPTA formulations involved the central nervous system,
including headache, nausea, vomiting, and photophobia, and were
exacerbated 1 day after exposure.
9.1 Case reports
Two cases of poisoning by TPTA were reported (Manzo et al.,
1981). A patient who inhaled, 5 days before hospitalization, a certain
amount of fungicide powder containing 60% TPTA (Brestan(R)) complained
about dizziness, nausea, and photophobia. He had an episode of sudden
malaise with dizziness and temporary loss of consciousness 1 day
before his visit. He soon recovered; however, he experienced a brief
loss of consciousness, nausea, and vomiting. On admission, general
appearance and physical examination showed no abnormality except for a
mild impairment of body balance. In spite of treatment with various
antiemetics, nausea and photophobia persisted until the 4th day.
Complete recovery was seen 10 days after hospitalization. Another
patient inhaled an unknown amount of Brestan(R) in an aqueous solution
3 h before his visit while spraying that solution onto a rice field.
He noted general malaise, weakness, and dryness of the mouth. At the
time of admission, the subjective symptoms had totally disappeared.
There were no abnormal neurological findings. Severe headache,
weakness, and photophobia appeared on the day following
hospitalization. All these symptoms disappeared on the 4th day after
admission. The mean concentrations of tin in the blood and urine
collected in 24 h during his hospital stay were 48 + 29 ng/ml
(normal value 2 ng/ml or less) and 113 + 20.6 ng/ml (normal range
10-65 ng/ml), respectively.
9.2 Epidemiological studies
Hypersensitivity reaction to a series of 36
triphenyltin-containing pesticide formulations was surveyed among 652
subjects in Italy (Lisi et al., 1987). Among them, 180 were
agricultural and 43 were ex-agricultural workers. Of the 652 subjects,
274 had contact dermatitis, mostly on the hands, and the other 378
were hospitalized for non-allergic skin disorders. Patch tests were
performed on the upper back, and irritant and allergic reactions were
evaluated. Irritant and allergic reactions were seen in 45 of 350
subjects and in 1 out of 350 subjects, respectively, with a patch of
1% TPTH. At 0.5% TPTH, irritant reactions were seen in 5 of 109
subjects, whereas no allergic reactions were seen in any of the 109
subjects. The report showed that TPTH is a moderately strong irritant
among the fungicides used in Italy.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Extensive data exist on the toxicity of triphenyltin compounds to
organisms in the environment (HSE, 1992; CICAD National Committee,
1997). Data that show the most severe effects on typical species are
listed in Tables 5 and 6. Available data show that triphenyltin is
extremely toxic to various species of aquatic organisms, although the
concentrations of triphenyltin that produce toxic effects vary
according to species.
Growth inhibition of yeasts and fungi with TPTCl occurs at 5
µg/litre and above (Hallas & Cooney, 1981).
Reproduction of freshwater algae was inhibited more than 50% at
2-5 µg/litre (Wong et al., 1982). Indigenous algae were more sensitive
than pure cultures. EC50s for inhibition of germination or carbon
fixation of marine and estuarine algae were 0.92-2 µg/litre (Walsh et
al., 1985).
The LC50 in a 96-h exposure for a copepod was 8 µg/litre (Linden
et al., 1979). LC50s in a 48-h exposure for Daphnia magna were
10-200 µg/litre (FAO, 1991a). The NOEC for reproduction in the same
species in a 21-day exposure was 0.1 µg/litre (FAO, 1991a).
One of the most sensitive effects of triphenyltin on organisms in
the environment is imposex (the development of male sex organs in
female gastropods) in rock shells (Japanese gastropods,
Thais clavigera and T. bronni), which supposedly occurs at levels
(1 ng/litre) similar to those that are seen with tributyltin compounds
(Horiguchi et al., 1994). When triphenyltin was injected into rock
shells, it was approximately as strong as tributyltin in promoting
imposex (Horiguchi et al., 1997), although it was less potent than
tributyltin for inducing imposex in Nucella. As imposex is probably
caused by hormonal disturbance, triphenyltin is considered to be an
endocrine disruptor.
Testosterone (500 ng/litre) induces faster and more intensive
imposex development in Nucella lapillus than that induced by
tributyltin. Simultaneous exposure to tributyltin and to the
antiandrogen cyproterone acetate, which suppresses imposex development
completely in N. lapillus and reduces imposex development in Hinia
reticulatus, proves that the imposex-inducing effects of tributyltin
are mediated by an increasing androgen level and are not caused
directly by the organotin compound itself. Furthermore,
tributyltin-induced imposex development can be suppressed in both
snails by adding estrogens to the aqueous medium. These observations
suggest that tributyltin causes an inhibition of the cytochrome
P-450-dependent aromatase system, which catalyses the aromatization of
androgens to estrogens. Artificial inhibition of the cytochrome
P-450-dependent aromatase system using SH 489
(1-methyl-1,4-androstadiene-3,17-dione) as a steroidal aromatase
inhibitor and flavone as a non-steroidal aromatase inhibitor induces
development of imposex in both snails. The same mechanism may apply to
triphenyltin (Bettin et al., 1996).
Field surveys in 1990-1992 and in 1993-1995 in Japan showed 100%
occurrence of imposex in T. clavigera. In surveys from 1992 to 1995,
the incidence of imposex in the common whelk ( Buccinum undatum) was
always greater than 90% (Mensink et al., 1996). Concentrations of
phenyltin compounds (up to 625 ng tin/g dry weight in the organism)
were much higher than those of butyltin compounds.
No NOEC was established for the effects of triphenyltin on
imposex; however, from the above observations, the NOEC can be assumed
to be around 1 ng/litre or lower.
Another sensitive effect of triphenyltin was the inhibition of
arm regeneration in brittle star ( Ophioderma brevispina) at 0.01
µg/litre. Neurotoxicological action of triphenyltin was suggested as
the cause (Walsh et al., 1986).
The 96-h LC50s of triphenyltin in fish were 7.1 µg/litre
(fathead minnow) and higher (Jarvinen et al., 1988). A subchronic
toxicity study with fathead minnow larvae showed the strong toxicity
of triphenyltin, with a 30-day LC50 of 1.5 µg/litre and a 30-day NOEC
of 0.15 µg/litre (LOEC 0.23 µg/litre). The need for studies of
cumulative effects in a full life cycle at lower concentrations was
suggested.
Beginning with yolk sac fry, rainbow trout ( Oncorhynchus
mykiss) was continuously exposed for 110 days to TPTCl at
concentrations of 0.12-15 nmol/litre or to diphenyltin chloride at
160-4000 nmol/litre. Diphenyltin chloride was about 3 orders of
magnitude less toxic than TPTCl. A NOEC of 160 nmol/litre
(corresponding to 60 µg/litre) was established for diphenyltin
chloride, and a NOEC of 0.12 nmol/litre (corresponding to 50 ng/litre)
was established for TPTCl. Histopathological examination revealed
depletion of glycogen in liver cells of both di- and
triphenyltin-exposed fish. At the end of the exposure period,
resistance to infection was examined by an intraperitoneal challenge
with Aeromonas hydrophila, a secondary pathogenic bacterium in fish.
Resistance to bacterial challenge was found to be decreased even at
the lowest-effect concentration of both di- and triphenyltin compounds
(de Vries et al., 1991).
Because thymus reduction, decrease in numbers of lymphocytes, and
inhibition of gonad development in fish species exposed to tributyltin
have been reported, triphenyltin may have similar effects on the
immune and reproductive systems of fish (Shimizu & Kimura, 1992).
Table 5: Acute toxicity to aquatic organisms.
Compound Organism Criterion Levels/remarks Reference
TPTCl Debaryomyces hansenii Minimum inhibitory 5 µg/ml Hallas & Cooney, 1981
(yeast) concentration
TPTCl Ankistrodesmus 4-h IC50 for primary 10 µg/litre, Wong et al., 1982
(freshwater alga) productivity static, 20 °C
TPTCl Skeletonema costatum, EC50 for 0.92 µg/litre Walsh et al., 1985
a major component of carbon fixation 13.8 µg/litre
fouling slimea LC50
TPTH Daphnia magna (water 48-h LC50 10 µg/litre FAO, 1991a
flea)
TPTFb Nitrocra spinipes 96-h LC50 8 µg/litre Linden et al., 1979
(harpacticoid copepod)
TPTH Eight fish species 96-h LC50 Pimephales promelas Javienen et al., 1988
(fathead minnow)
was the most
sensitive species,
7.1 µg/litre
TPTCl Pagrus major 48-h LC50 12.6 µg/litre Yamada & Takayanagi, 1992
(red sea bream)a
a Marine and estuarine species.
b Triphenyltin fluoride.
Table 6: Chronic/subchronic toxicity to aquatic organisms.
Compound Organism Criterion Levels/remarks Reference
TPTCl Natural community of 50% reduction of 2 µg/litre, Wong et al., 1982
freshwater algae reproduction and indigenous algae
primary production more sensitive than
pure cultures
Ankistrodesmus falcatus 85% inhibition of 5 µg/litre
reproduction
TPTH Daphnia magna 21-day NOEC 0.1 µg/litre FAO, 1991a
TPTH Lymnaea stagnalis: 9-day LC100, or 10 µg/litre for Van der Maas et
a freshwater sludge deficiencies in LC100, 2 µg/litre al., 1972
snail growth, mobility, for deficiencies
and embryo development
after 5 weeks of
exposure
TPTCl Thais clavigera Relative penis length Relative penis length Horiguchi et al., 1997
(Japanese rock shell)a in female significantly increased
with injection of 0.1 µg
triphenyltin/g wet
tissue and culture for
30 days
TPTH Pimephales promelas 30-day LC50, NOEC, 1.5, 0.15, and 0.23 Jarvinen et al., 1988
(fathead minnow) larvae and LOEC µg/litre, respectively
a Marine and estuarine species.
10.2 Terrestrial environment
Triphenyltin compounds applied to crops at the recommended dosage
rate did not harm wild animals, birds, or non-target insects (HSE,
1992). The EC50 for honey bees ( Apis mellifera) was many times
higher than that for a range of common pesticides (Eisler, 1989).
LD50s for triphenyltin compounds were 46.5-114 mg/kg body weight
in Japanese quail ( Coturnix japonica) and bobwhite quail ( Colinus
virginianus) and 285-378 mg/kg body weight in mallards ( Anas
platyrhynchos) (Booth et al., 1980; Ebert & Weigand, 1982; Ebert &
Leist, 1987, 1988).
Gavage administration of 2 mg TPTCl/kg body weight to chickens
( Gallus domesticus) from the 19th day after hatching for 10 days
resulted in atrophy of the thymus and the bursa of Fabricius
(Guta-Socaciu et al., 1986).
Female Peking ducks ( Anas platyrhynchos v. domestica)
(30 weeks old) administered 25 mg TPTH/kg body weight per day by
gavage for 4 weeks showed a decrease in body weight, a gradual
decrease in the number of eggs or total lack of egg production, mild
anaemia, enlargement of the spleen, liver, and kidneys, and atrophy
of the reproductive organs (Masoud et al., 1985). Changes to the
spleen, liver, and kidneys reversed within 4 weeks after the end of
the exposure, but the uterine tube and ovaries did not completely
return to normal.
11. EFFECTS EVALUATION
As tributyltin compounds have been used more abundantly and more
extensively than triphenyltin compounds in many locations, and as
tributyltin and triphenyltin compounds have similar effects on humans
and organisms in the environment, risk from exposure to triphenyltin
must be considered together with risk from exposure to tributyltin
(IPCS, 1990; Sekizawa, 1998). There are many uncertainties in the
potential risk posed by triphenyltin and its metabolites and in the
mechanism underlying the immunotoxicological and reproductive effects
caused by these compounds, and further studies on these aspects are
necessary to improve the risk assessment on triphenyltin.
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
No quantitative data on humans are available. In two poisoning
case reports of inhalation exposure to TPTA formulations, neurotoxic
effects appeared to persist for a few days. A moderate level of
irritant action was detected in a patch test study.
Triphenyltin compounds given orally to rats are not readily
absorbed and are excreted primarily in faeces and to a lesser extent
in urine. Triphenyltin compounds are metabolized to diphenyltin,
monophenyltin, and non-extractable bound residues. Absorbed
triphenyltin compounds accumulate to the greatest extent in kidney and
liver and to a smaller degree in other organs. Triphenyltin compounds
applied dermally can penetrate through the skin in a time- and
dose-dependent manner.
Triphenyltin exerts a variety of effects on several animal
species, including effects on the immune system,
reproductive/developmental effects at levels near those that are
maternally toxic (most LOAELs are in the several mg/kg range or
lower), hyperplasia/adenomas in endocrine organs, apoptosis in thymus
cells, calcium release in sarcoplasmic reticulum cells, and eye
irritation. The underlying mechanism of these effects is under
investigation; a common mechanism may explain this toxicity profile.
Health effects observed in laboratory animals and toxicological
criteria for setting guidance values are summarized in Table 7.
Triphenyltin compounds are moderately toxic in acute tests, and the
lowest NOAELs for the oral, dermal, and inhalation routes in
short-term and subchronic studies were 0.21 mg/kg body weight per day
in dog (52-week exposure), 10 mg/kg body weight per day in rat (29-day
exposure), and 0.014 mg/m3 in rat (4-week exposure), respectively.
Triphenyltin is not carcinogenic or genotoxic.
Table 7: Toxicological criteria for setting guidance values for dietary and non-dietary exposure to
triphenyltin compounds.
Type of test Organisms (route of exposure, Results/remarks
duration of test)
Single exposure Rat LD50: 160 mg TPTH/kg body weight
Short-term Dog (oral, 52 weeks), rat NOAEL for oral, dog: 0.21 mg TPTH/kg body
(dermal, 29 days), rat weight per day, based on relative liver
(inhalation, 4 weeks) weight decrease at effect levels; NOAEL
for dermal, rat: 10 mg TPTH/kg body weight
per day, based on erythema, mortality,
lymphocyte decrease at effect levels; NOAEL
for inhalation, rat: 0.014 mg TPTH/m3,
based on IgM increase at effect levels
Long-term Mouse (80 weeks), rat NOAEL for mouse: 0.85-1.36 mg TPTH/kg body
(104 weeks) weight per day, based on decreased body weight
at effect levels; NOAEL for rat: 0.1 mg TPTH/kg
body weight per day, based on reduction in white
blood cell counts at effect levels
Genotoxicity In vivo/in vitro Mostly negative
Reproduction Rat NOAEL: 0.4 mg TPTH/kg body weight per day, based
on decreased litter size, pup weight, relative
spleen/thymus weight in weanlings at effect levels
Teratogenicity Rabbit NOAEL for maternal toxicity: 0.1 mg TPTH/kg body
weight per day, based on decreased body weight
gain at effect levels
Table 7 (continued)
Type of test Organisms (route of exposure, Results/remarks
duration of test)
Immunotoxicity Mouse/rat/guinea-pig Immunosuppressive; LOAEL: 0.3 mg TPTH/kg body
weight per day in rat
Neurotoxicity Rat (6 weeks) Toxic at 0.36 mg TPTA/kg body weight per day in
maze learning test
Reproductive and developmental effects include a decrease in the
number of implantations and live fetuses (at 1.0 mg TPTA/kg body
weight per day in a rabbit gavage study), a reduction in litter
size/pup weight and in relative thymus or spleen weight in the
weanlings (at 1.5 mg TPTH/kg body weight per day in diet in a
two-generation reproduction study in rats; NOAEL 0.4 mg/kg body weight
per day), and abortion and a reduction in fetal weight (at 0.9 mg
TPTH/kg body weight per day in a rabbit gavage study).
Triphenyltin compounds show effects on the immune system, such as
a decrease in immunoglobulin concentrations (even at the lowest dose
level, i.e., 0.3 mg TPTH/kg body weight per day in a 2-year rat
feeding study), lymphopenia (at 1.75 mg TPTH/kg body weight per day in
a 13-week dietary study in rats or at 0.338 mg/m3 in a 13-week
inhalation study in rats), and thymus or splenic atrophy (at 1.5 mg
TPTCl/kg body weight per day in a 2-week feeding study with weanling
rats or at 5 mg TPTH/kg body weight per day in a 28-day feeding study
in mice, respectively). Females are generally more susceptible than
males with respect to these effects.
The lowest NOAEL detected in the toxicity tests was 0.1 mg/kg
body weight per day for maternal toxicity in a rabbit gavage study,
based on decreased food consumption and body weight gain at 0.3 mg/kg
body weight per day; the same NOAEL was obtained in an early 2-year
rat study in which a slight decrease in white blood cells was seen at
higher doses.
11.1.2 Criteria for setting guidance values for triphenyltin
No data are available on occupational exposure to triphenyltin.
Considering its irritant action, neurotoxic symptoms in poisoning, and
effects on the immune and reproductive systems, care must be taken to
prevent dermal or inhalation exposure to triphenyltin as much as
possible.
Although no data are available on concentrations of triphenyltin
in air or drinking-water, it is unlikely that triphenyltin would be
present as a contaminant in these media at detectable levels
considering its physical/chemical properties and levels of
triphenyltin that have been detected in ambient water.
The major exposure route for the general public is through intake
of foods contaminated with triphenyltin. Estimation of exposure from
residue data in supervised trials or maximum residue limits in foods
will lead to overestimates of intake, because not all crops are
treated with triphenyltin, and residues will not always be at the
maximum residue limits. Exposure to triphenyltin from treated crops
and dairy products is considered to be very low to negligible, as long
as Good Agricultural Practice in the use of pesticides, as defined by
WHO (1976), is observed. Therefore, the major route of exposure for
the general public is probably from the ingestion of fish and
shellfish contaminated with triphenyltin used in antifouling paints.
Triphenyltin levels found in pelagic fish suggest that pollution from
offshore boats is not negligible and that triphenyltins are persistent
in the organisms, probably accumulated through the food-web.
Several end-points were taken into consideration in establishing
the ADI for oral exposure by JMPR (FAO, 1991b; WHO, 1992). First, a
200-fold safety factor (uncertainty factor) was applied to the NOEL of
0.1 mg/kg body weight per day (based on a finding of reduced white
blood cell count at higher doses in a 2-year rat study) to arrive at
an ADI of 0-0.5 µg/kg body weight. Secondly, a 500-fold uncertainty
factor was applied to a LOAEL of 0.3 mg/kg body weight per day in a
2-year study in rats in which increased mortality and reduced serum
immunoglobulins were noted, to derive the same ADI. Other NOAELs taken
into account are 0.4 mg/kg body weight per day in a two-generation
reproduction study with rats (a dose-related decrease in spleen and
thymus weight in F1 and F2 male and female weanlings was observed at
higher levels), 0.3 mg/kg body weight per day in a 13-week study in
rats (reduction in white blood cells, IgG decrease, and relative
testes weight increase seen at higher levels), 0.21 mg/kg body weight
per day in dogs (relative liver weight increase and serum
albumin/globulin ratio decrease seen at higher levels), and 0.1 mg/kg
body weight per day in a teratology study in rabbits (maternal
toxicity seen at higher levels). No additional information regarding
derivation of the above two uncertainty factors is available in the
WHO monograph.
11.1.3 Sample risk characterization
Owing to wide variation in the consumption of fish and shellfish
and local differences in residue levels, only illustrative estimates
relating to effects and exposure can be made. It should be emphasized
that local measurements of residues, local estimates of seafood
consumption, and local decisions on acceptable safety margins must be
made to assess potential risk. Some examples of risk assessments
follow.
Intake of triphenyltin estimated from a market basket survey in
Japan in 1997 was 2.7 µg/day per person; values fluctuated between 0.6
and 2.7 µg/day per person over the 1992-1997 period. There was about a
twofold difference between average daily intake estimates from 10
local laboratories and intakes estimated by one local government.
There are people who eat more seafood than the average person. All
these uncertainties and variations must be taken into account in an
exposure assessment.
Triphenyltin intakes can be compared with the high end of the ADI
of JMPR (0.5 µg/kg body weight per day), which corresponds to 25
µg/day for a 50-kg Japanese person; intakes are calculated to be 2.4%
or 10.8% of the ADI for market basket surveys in different periods.
These data suggest that if actions had not been taken,
contamination of seafood with triphenyltin may have posed some health
risks to Japanese consumers. Similar estimates of intake through
market basket studies in Tokyo reported in 1991 support the above
estimation.
The above risk estimation was performed using data on
triphenyltin compounds alone. Coincidental contamination with
tributyltin must be taken into account in risk estimation from oral
exposure. The risk from exposure to triphenyltin compounds will be
better characterized when combined with risks from other organotin
compounds that exert similar effects (IPCS, 1990; CICAD National
Committee, 1997).
11.2 Evaluation of environmental effects
Triphenyltins enter the environment through their use in
antifouling paints for boats and fishnets and as fungicides for
certain crops.
Strong adsorption of triphenyltin to soil suggests that organisms
in treated soil may not be widely affected. The fact that soil
respiration was not affected significantly suggests that there were no
adverse effects on aerobic microorganisms.
Triphenyltins are very toxic to various species in the
environment at extremely low concentrations. The most sensitive
effects of triphenyltin are imposex in rock shells (Japanese
gastropods), supposed to occur at 1 ng/litre, and inhibition of arm
regeneration in brittle star, at 0.01 µg/litre. The NOEC for
reproduction (21-day exposure) in Daphnia magna and the NOEC (30-day
exposure) for fathead minnow were 0.1 µg/litre and 0.15 µg/litre,
respectively. The EC50s for carbon fixation, reproduction, and
primary production in both marine and freshwater algae were in the
range of 1-2 µg/litre. The LC50 (30-day exposure) for fathead minnow
was also at a similar level. Acute effects (IC50 for primary
productivity in algae, 48-h LC50 for daphnid, and 96-h LC50 for fish)
were seen at 1-10 µg/litre.
For sensitive invertebrates, critical concentrations are 0.01-0.1
µg/litre and lower. Sensitive algae and fish species may be
susceptible at levels below 1 µg/litre.
Ambient surveys in Japan showed that triphenyltin levels in bay
and inshore area water and in sediment were 2.5-3.0 ng/litre and
1.5-2.3 ng/g, respectively, in 1992-1995. Exposure of organisms in the
environment varies widely depending on where and when the triphenyltin
compounds were used or discharged.
No NOEC has been established for triphenyltin-induced imposex in
molluscs. Experimentally, by injection, triphenyltin has a similar
potency to tributyltin in the genus Thais. Triphenyltin is less
potent than tributyltin in Nucella; however, triphenyltin shows
greater bio-accumulation than tributyltin. From this, it can be
assumed that the NOEC for triphenyltin will be a few ng/litre or
lower. The observed prevalence of imposex in Thais in the wild with
ambient concentrations in this range supports this assumption. Because
residues of triphenyltin and tributyltin occur together in the
environment, their relative contribution to observed imposex cannot be
assessed for Thais species. Use of either triphenyltin or
tributyltin in antifouling paint would lead to population declines of
marine invertebrates on this basis.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Triphenyltin was evaluated by JMPR in 1963, 1965, 1970, and 1991.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 1283) reproduced in this
document.
13.1 Human health hazards
Triphenyltin compounds may affect the immune system, resulting in
impaired function. They have also been found to cause reproductive
effects and developmental toxicity in animal studies.
13.2 Advice to physicians
In case of poisoning, treatment is supportive. Special attention
should be given to pregnant women exposed to triphenyltin compounds.
13.3 Health surveillance advice
Periodic medical examination of the immune system should be
included in a health surveillance programme.
13.4 Spillage and disposal
Triphenyltin compounds are absorbed through the skin. In case of
spillage, emergency crew should wear proper equipment, including eye
protection in combination with breathing protection. The compounds
should not be allowed to enter drains or watercourses.
Triphenyltin compounds may be disposed of in sealed containers.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
TRIPHENYLTIN HYDROXIDE ICSC: 1283
November 1998
CAS # 76-87-9 Hydroxytriphenylstannane
RTECS # WH8575000 Hydroxytriphenylstannate
UN # 2786 Fentin hydroxide
EC # 050-004-00-1 C18H16OSn
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/
EXPOSURE SYMPTOMS FIRE FIGHTING
FIRE Combustible. Liquid NO open flames. Powder, water spray,
formulations containing foam, carbon dioxide.
organic solvents may be
flammable.
EXPLOSION In case of fire: keep
drums, etc., cool by
spraying with water.
EXPOSURE PREVENT DISPERSION OF DUST!
STRICT HYGIENE! AVOID
EXPOSURE OF (PREGNANT)
WOMEN!
Inhalation Cough. Sore throat. Ventilation, local exhaust, Fresh air, rest. Refer
or breathing protection. for medical attention.
Skin MAY BE ABSORBED! Redness. Protective gloves. Remove contaminated
Pain. Protective clothing. clothes. Rinse and
then wash skin with
water and soap. Refer
for medical attention.
Eyes Redness. Pain. Blurred Safety spectacles, face First rinse with
vision. shield, or eye protection plenty of water for several
in combination with minutes (remove contact
breathing protection. lenses if easily
possible), then take
to a doctor.
Ingestion Do not eat, drink, or smoke Give plenty of water to
during work. drink. Refer for medical
attention.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Do NOT wash away into sewer. Carefully Do not transport with food and feedstuffs.
collect remainder, then remove to safe Severe marine pollutant.
place. (Extra personal protection: P3 Symbol: T+, N
filter respirator for toxic particles). R: 24/25-26-36/38-50/53
Use face shield. Chemical protection suit. S: (1/2-)36/37-45-60-61
UN Classification
UN Hazard Class: 6.1
UN Pack Group: II
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC(R)-61G41b Provision to contain effluent from
fire extinguishing. Separated from food
and feedstuffs.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
WHITE CRYSTALLINE POWDER The substance can be absorbed into
the body by inhalation, through the
skin and by ingestion.
OCCUPATIONAL EXPOSURE LIMITS: INHALATION RISK:
TLV (as organic compounds (tin)): ppm 0.1 Evaporation at 20°C is negligible;
mg/m3 (skin) (STEL) (ACGIH 1998). a harmful concentration of airborne particles
MAK as tin: ppm, 0.1 mg/m3; skin (D) (1995) can, however, be reached quickly when dispersed.
EFFECTS OF SHORT-TERM EXPOSURE:
The substance irritates the eyes severely, the
skin and the respiratory tract. The substance
may cause effects on the immune system,
resulting in impaired functions
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
Animal tests show that this substance possibly
causes malformations in human babies.
PHYSICAL PROPERTIES
Decomposes below melting point at 80°C.
Solubility in water, g/100 ml: 0.008
Flash point: 400°C
ENVIRONMENTAL DATA
The substance is very toxic to aquatic organisms. In the food chain important to humans,
bioaccumulation takes place, specifically in molluscs. Avoid release to the
environment in circumstances different to normal use.
NOTES
Carrier solvents used in commercial formulations may change physical and toxicological
properties. Do NOT take working clothes home.
ADDITIONAL INFORMATION
LEGAL NOTICE
Neither the CEC nor the IPCS nor any person acting on behalf of the CEC or the IPCS
is responsible for the use which might be made of this information.
REFERENCES
ACP (1990) Evaluation on fentin hydroxide. In: Food and
Environmental Protection Act, 1985, Part III. Evaluation of Fully
Approved or Provisionally Approved Products. York, Ministry of
Agriculture, Fisheries and Food, Pesticide Safety Directorate,
Advisory Committee on Pesticides, 18 pp.
Baba T, Rikioka Y, Toyomura K (1991) Content of tributyltin (TBT) and
triphenyltin (TPT) compounds in marine products and its processed
products in Nagasaki prefecture. Annual report of Nagasaki
Prefectural Institute of Public Health and Environmental Sciences
(Nagasakiken Eisei Kogai Kenkyujo Nenpo), 34:98-102 (in Japanese).
Baeder C (1987) Fentin acetate-substance, technical (Code: HOE
002782 OF ZD97 0002). Testing for embryotoxicity in the Himalayan
rabbit after oral administration. Unpublished report 89.1559
(A42324) of Hoechst Pharma Research Toxicology and Pathology.
Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO,
1992].
Barnes RD, Bull AT, Poller RC (1971) Behaviour of triphenyltin acetate
in soil. Chemistry and industry, 8:204.
Bettin C, Oehlmann J, Stroben E (1996) TBT-induced imposex in marine
neogastropods is mediated by an increasing androgen level.
Helgolander Meeresuntersuchungen, 50:299-317.
Beurkle WL (1985) HOE 002782-14-C (triphenyltin acetate), analysis
of hydrolysis by-products. Unpublished report CM009/85 (A31534) from
Analytisches Laboratorium, Hoechst. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Booth GM, Jackson C, Warren S (1980) Acute oral toxicity of
technical triphenyltin hydroxide and Du-ter to mallard ducks. Provo,
UT, Brigham Young University, Department of Zoology.
Bouldin TW, Goines ND, Bagnell CR, Krigman MR (1981) Pathogenesis of
trimethyltin neuronal toxicity. Ultrastructural and cytochemical
observations. American journal of pathology, 104:237-249.
Carlton BD, Howard M (1982) The evaluation of the teratogenicity of
triphenyltin hydroxide (TPTH) in the Syrian hamster. Unpublished
report 723-0100 (A22884) of Batelle Columbus Laboratories, Columbus,
OH. Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO,
1992].
Chow SC, Orrenius S (1994) Rapid cytoskeleton modification in
thymocytes induced by the immunotoxicant tributyltin. Toxicology and
applied pharmacology, 127:19-26.
CICAD National Committee (1997) A critical review on triphenyltin
compounds (provisional), August 1997. Tokyo, National Institute of
Health Sciences, National Committee for Concise International Chemical
Assessment Documents, 76 pp.
Clegg ED, Cook JC, Chapin RE, Foster PMD, Daston GP (1997) Leydig cell
hyperplasia and adenoma formation: Mechanisms and relevance to humans.
Reproductive toxicology, 11:107-121.
De Vries H, Penninks AH, Snoeij NJ, Seinen W (1991) Comparative
toxicity of organotin compounds to rainbow trout ( Oncorhynchus
mykiss) yolk sac fry. The science of the total environment,
103:229-243.
Diehl KH, Leist KH (1986a) Fentin acetate-active ingredient
technical. Testing for acute dermal toxicity in the male and female
Wistar rat. Unpublished report 86.0816 (A37384) of Hoechst Pharma
Research Toxicology and Pathology. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Diehl KH, Leist KH (1986b) Fentin acetate-active ingredient
technical. Testing for primary dermal irritation in the rabbit.
Unpublished report 86.0619 (A37383) of Hoechst Pharma Research
Toxicology and Pathology. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Diehl KH, Leist KH (1986c) Fentin acetate-active ingredient
technical. Testing for primary eye irritation in the rabbit.
Unpublished report 86.0620 (A37382) of Hoechst Pharma Research
Toxicology and Pathology. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Diehl KH, Leist KH (1986d) Testing for sensitising properties in the
Pirbright-White guinea pig according to the technique of Buehler.
Unpublished report 86.0803 (A36975) of Hoechst Pharma Research
Toxicology and Pathology. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Diehl KH, Leist KH (1987) Fentin-hydroxide-active ingredient
technical (Code: HOE 029664 OF ZD97 0004). Testing for sensitising
properties in the Pirbright-White guinea pig in a maximisation test.
Unpublished report 87.0201 (A35628) of Hoechst Pharma Research
Toxicology and Pathology. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Dorn E, Werner HJ (1989) Triphenyltin hydroxide (HOE 029664 OF ZD 97
0004). Determination of residues in organs, tissues and blood
of rats after chronic feeding (104 weeks). Unpublished report
CR077/88 (A41974) of Analytisches Laboratorium Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO, 1992].
Duchosal F, Thevenaz P, Luetkemeier H, Vogel O, Pappritz G, Mladenovic
P, Terrier C (1989) Fentin hydroxide (TPTH) technical grade.
Subchronic (90-days) repeated dose inhalation toxicity study in
rats. Unpublished report 202353 (A40323) of Research and Consulting
Company AG, Carouge-Geneva [cited in WHO, 1992].
Ebert E, Leist KH (1987) Fentin-Acetat-Substanz, technisch (Code:
HOE 002782 OF ZD97 0002). Pruefung der akuten oralen Toxizitaet an
maennlichen und weiblichen Virginischen Baumwacteln (Colinus
virginianus). Hoechst Pharma Forschung Toxikologie, Report 87.0291.
Submitted to FAO by Hoechst AG, Frankfurt-am-Main [cited in FAO,
1991a].
Ebert E, Leist KH (1988) Fentin acetate active ingredient technical
(Code: HOE 002782 OF ZD97 0002). Testing for acute oral toxicity in
the male and female mallard duck. Hoechst Pharma Forschung
Toxikologie, Report 88.0544. Submitted to FAO by Hoechst AG,
Frankfurt-am-Main [cited in FAO, 1991a].
Ebert E, Weigand W (1982) Acute oral toxicity (LD50) of HOE
29664-active ingredient (Code: HOE 29664 OF AT205) in Japanese quail
(Coturnix coturnix). Submitted to FAO by Hoechst AG,
Frankfurt-am-Main [cited in FAO, 1991a].
Eckert HG, Kellner HM, Beurkle WL (1989) Accumulation study,
kinetics and metabolism in the rat after single and repeated oral
administration, of 2 mg/kg body weight. Unpublished report
01-L42-0565-89 (A41407) of Hoechst Pharma Forschung GB-L
Radiochemisches Laboratorium and Produktentwicklung GB-L, Oekologie.
Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO,
1992].
Eisler R (1989) Tin hazards to fish, wildlife and invertebrates:
a synoptic review. US Fish and Wildlife Service Biological Report
85(1.15), p. 83 [cited in HSE, 1992].
Ema M, Miyawaki E, Harazono A, Ogawa Y (1997) Effect of triphenyltin
chloride on implantation and pregnancy in rats. Reproductive
toxicology, 11:201-206.
Ema M, Miyawaki E, Kawasaki K (1998) Inhibition of decidual cell
response as a cause of implantation failure induced by triphenyltin
chloride (TPTCl) in rats. Toxicologist, 42:261.
FAO (1991a) Pesticide residues in food -- 1991. Evaluations, Part I
-- Residues. Rome, Food and Agriculture Organization of the United
Nations, pp. 337-371 (FAO Plant Production and Protection Paper
113/1).
FAO (1991b) Pesticide residues in food -- 1991 report. Rome, Food
and Agriculture Organization of the United Nations, pp. 57-62 (FAO
Plant Production and Protection Paper 111).
Fent K, Hunn J (1991) Phenyltins in water, sediment, and biota of
freshwater marinas. Environmental science and technology,
25:956-963.
Fent K, Hunn J (1995) Organotins in fresh water harbours and rivers:
temporal distribution, annual trends and fate. Environmental
toxicology and chemistry, 14:1123-1132.
Filenko OF, Isakova EF (1979) Dynamics of triphenyltin chloride
accumulation by Daphnia. Biologicheskie Nauki, 3:46-49 (in
Russian).
Gomez-Ariza JL, Morales E, Ruiz-Benitez M (1992) GC FPD method for the
simultaneous speciation of butyltin and phenyltin compounds in waters.
Applied organometallic chemistry, 6(3):279-286.
Guta-Socaciu C, Giurgea R, Rosioru C (1986) Thymo-bursal and adrenal
modifications induced by triphenyltin compounds in chickens.
Archives of experimental veterinary medicine, 40:307-311.
Hallas LE, Cooney JJ (1981) Effects of stannic chloride and organotin
on estuarine micro-organisms. Developments in industrial
microbiology, 22:529-535.
Harino H, Fukushima M, Tanaka M (1992) Simultaneous determination of
butyltin and phenyltin compounds in the aquatic environment by gas
chromatography. Analytica Chimica Acta, 264(1):91-96.
Hattori Y, Kobayashi A, Takemoto S, Takami K, Kuge Y, Sugimae A,
Nakamoto M (1984) Determination of trialkyltin, dialkyltin, and
triphenyltin compounds in environmental water and sediments. Journal
of chromatography, 315:341-349.
Hollander H, Weigand W (1974) Triphenyltin acetate. Acute oral
toxicity (LD50) in female albino-rats. Unpublished report 16454
(A16454) of Hoechst Pharma Forschung Toxikologie. Submitted to WHO by
Hoechst AG, Frankfurt-am-Main [cited in WHO, 1992].
Hollander H, Weigand W (1981) Dust inhalation of HOE 29664-active
ingredient in the male and female SPF-Wistar rat. 4h-LD50.
Unpublished report 335/81 (A22293) of Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited
in WHO, 1992].
Hollander H, Weigand W (1986) Fentin acetate-active ingredient
technical. Testing for acute dust inhalation toxicity in the male
and female SPF rat. 4-hour LC50. Unpublished report 86.1497
(A37523) of Hoechst Pharma Research Toxicology. Submitted to WHO by
Hoechst AG, Frankfurt-am-Main [cited in WHO, 1992].
Horiguchi T, Shiraishi H, Shimizu M, Morita M (1994) Imposex and
organotin compounds in Thais clavigera and T. bronni in Japan.
Journal of the Marine Biological Association of the United Kingdom,
74:651-669.
Horiguchi T, Shiraishi H, Shimizu M, Morita M (1997) Effects of
triphenyltin chloride and five other organotin compounds on the
development of imposex in the rock shell, Thais clavigera.
Environmental pollution, 95(1):85-91.
HSE (1992) A review of the environmental effects of triorganotin
compounds. Merseyside, Health and Safety Executive, Pesticide
Registration Section, Advisory Committee on Pesticides, 142 pp.
(Report No. 111).
Ikeda M, ed. (1977) Industrial poisoning handbook, 2nd ed. Tokyo,
Ishiyaku Shuppan, 303 pp. (in Japanese).
IPCS (1990) Tributyltin compounds. Geneva, World Health
Organization, International Programme on Chemical Safety
(Environmental Health Criteria 116).
IPCS (1996) International Chemical Safety Card -- Triphenyltin
hydroxide. Geneva, World Health Organization, International
Programme on Chemical Safety (ICSC 1283).
Ishizaka T, Nemoto S, Sasaki K, Suzuki T, Saito Y (1989) Simultaneous
determination of tri- n-butyltin, di- n-butyltin, and triphenyltin
compounds in marine products. Journal of agricultural and food
chemistry, 37:1523-1526.
Japan Environment Agency (1983) Chemicals in the environment. Tokyo,
Environmental Health Department, Office of Health Studies.
Japan Environment Agency (1996) Chemicals in the environment. Tokyo,
Environmental Health Department, Office of Health Studies.
Jarvinen AW, Tanner DK, Kline ER, Knuth ML (1988) Acute and chronic
toxicity of triphenyltin hydroxide to fathead minnows ( Pimephales
promelas) following brief or continuous exposure. Environmental
pollution, 52:289-301.
JPPA (1982-1996) Summary data on pesticides. Tokyo, Japan Plant
Protection Association (in Japanese).
Kang J, Chen I, Cheng Y (1997) Induction of calcium release from
isolated sarcoplasmic reticulum by triphenyl tin. Journal of
bio-chemistry, 122:173-177.
Kannan K, Lee RF (1996) Triphenyltin and its degradation products in
foliage and soils from sprayed pecan orchards in fish from adjacent
ponds. Environmental toxicology and chemistry, 15(9):1492-1499.
Kellner HM, Eckert HG (1989) Addendum to report 01-L42-0565-89. HOE
029664 (TPTH)-113Sn. Accumulation and depletion study in rats after
single and repeated oral administration of 2 mg/kg body weight.
Unpublished report 01-L42-582-90(A43434) of Hoechst Pharma Forschung
GB-L Radiochemisches Laboratorium. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Kohri M, Sato K, Inoue Y, Ide K, Okochi H (1995) Batch operation of
solid phase extraction for separation and concentration of organotin
compounds in seawater. Bunseki Kagaku, 44(7):537-542.
Lehotzky K, Szeberenyi JM, Horkay F, Kiss A (1982) The neurotoxicity
of organotin: behavioural changes in rats. Acta Biologica Academiae
Scientiarum Hungaricae, 33:15-22.
Leist KH (1988) Fentin acetate-substance technical (Code: HOE 002782
OF ZD97 0002). Repeated dose dermal toxicity study (21 applications
in 29 days) with a 14 day withdrawal period. Unpublished report
(A39231) 3104 TSR of Hoechst Pharma Forschung Toxikologie und
Pathologie. Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited
in WHO, 1992].
Leist KH, Weigand W (1981a) Acute oral toxicity of HOE 29664-active
ingredient (Code: HOE 29664 OF AT201) to the male rat. Unpublished
report (A22115) 182/81 of Hoechst Pharma Forschung Toxikologie.
Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO,
1992].
Leist KH, Weigand W (1981b) Acute oral toxicity of HOE 29664-active
ingredient (Code: HOE 19664 OF AT201) to the female rat. Unpublished
report (A22114) 183/81 of Hoechst Pharma Forschung Toxikologie.
Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO,
1992].
Leist KH, Weigand W (1981c) Acute percutaneous toxicity of HOE
29664-active ingredient (Code: HOE 29664 OF AT201) to the female
rat. Unpublished report (A22292) 184/81 of Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited
in WHO, 1992].
Leist KH, Weigand W (1981d) Acute percutaneous toxicity of HOE
29664-active ingredient (Code: HOE 29664 OF AT201) to the male
rabbit. Unpublished report (A22112) 229/81 of Hoechst Pharma
Forschung Toxikologie. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Leist KH, Weigand W (1981e) Test for sensitising properties of
fentin hydroxide-technical (Code: HOE 29664 OF AT201) in guinea-pig
(according to Buehler). Unpublished report (A22433) 724/81 of
Hoechst Pharma Forschung Toxikologie. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Linden E, Bengtsson BE, Svanberg O, Sundstrom G (1979) The acute
toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms: the bleak ( Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 11:843.
Lisi P, Carraffini S, Assalve D (1987) Irritation and sensitisation
potential of pesticides. Contact dermatitis, 17:212-218.
MacCormick DL, Thomas PT (1990) 28-day oral diet immunotoxicity
study of triphenyltin hydroxide (TPTH),substance technical (Code HOE
029664 OFZD97 0004) in mice. Unpublished report L08252SN02(A44054)
of Life Science Research. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Manzo L, Richelmi P, Sabbioni E, Pietra R, Bono F, Guardia L (1981)
Poisoning by triphenyltin acetate. Report of two cases and
determination of tin in blood and urine by neutron activation
analysis. Clinical toxicology, 18:1343-1353.
Masoud AH, El-Habbak MME, Hegazi AG, Soufy H, Samy MM (1985) The
influence of triphenyltin hydroxide on some biological aspects in
mature female peking ducks. Mededelingen van de Faculteit
Landbouwwetenschappen, Rijksuniversiteit Gent, 50:925-937.
Mensink BP, Ten-Hallers-Tjabbes CC, Kralt J, Freriks IL, Boon JP
(1996) Assessment of imposex in the common whelk, Buccinum undatum
(L.) from the Eastern Scheldt, The Netherlands. Marine
environmental research, 41(4):315-325.
MITI (1998) Industrial production statistics. Chemical Safety Policy
Office, Basic Industries Bureau, Ministry of International Trade and
Industry, Japan.
Miura Y, Kato M, Ogino K, Matsui H (1997) Impaired cytosolic Ca2+
response to glucose and gastric inhibitory polypeptide in pancreatic
ß-cells from triphenyltin-induced diabetic hamster. Endocrinology,
138:2769-2775.
Morcillo Y, Borghi V, Porte C (1997) Survey of organotin compounds in
the Western Mediterranean using molluscs and fish as sentinel
organisms. Archives of environmental contamination and toxicology,
32:35-46.
Nagamatsu K, Kido Y, Urakubo G, Aida Y, Ikeda Y, Suzuki Y (1978)
Absorption, distribution and excretion of triphenyltin acetate and
stannous chloride in the guinea pig. Japanese journal of hygiene,
33:486-496.
NIHS (1998) A report of surveys on daily intake of contaminants in
food. Unpublished report. Tokyo, National Institute of Health
Sciences (in Japanese).
Nishida H, Matsui H, Sugiura H, Kitagi K, Fuchigami M, Inagaki N,
Nagai H, Koda A (1990) The immunotoxicity of triphenyltin chloride in
mice. Journal of 'Pharmacobio-Dynamics', 13(9):543-548.
NLM (1998) Hazardous Substances Data Bank. Bethesda, MD, US National
Library of Medicine. CHEM-BANK CD-ROM, distributed by SilverPlatter
International, NV.
NTP (1978) Bioassay of triphenyltin hydroxide for possible
carcinogenicity. CAS No. 76-87-9 (A199994). Research Triangle Park,
NC, US Department of Health, Education and Welfare, National
Institutes of Health, National Toxicology Program (Technical Report
Series No.139).
Ohhira S, Matsui H (1991) Comparison of sulfur-mode and tin-mode flame
photometric detectors for the gas chromatographic determination of
organotin compounds. Chromatography and biomedical applications,
566(1):207-214.
Ohhira S, Matsui H (1993) Gas chromatographic determination of
inorganic tin in rat urine after a single oral administration of
stannous chloride and mono-, di-, and triphenyltin chloride. Journal
of chromatography and biomedical applications, 622(2):173-178.
Ohhira S, Matsui H (1996) Comparative study of the metabolism of
triphenyltin in hamsters and rats after a single oral treatment with
triphenyltin chloride. Toxicology letters, 85:3-8.
Ozcan M, Good ML (1980) Abstract in Proceedings of the American
Chemical Society. Division of Environmental Chemistry, Houston, TX
[cited in HSE, 1992].
Penninks AH, Snoeij RJ, Pieters RHH, Seinen W (1990) Effect of
organotin compounds on lymphoid organs and lymphoid functions: an
overview. In: Dayan AD, Hertel RF, Heseltin E, Kazantzis G, Smith EM,
Van der Venne MT, eds. Immunotoxicity of metals and
immunotoxicology. New York, NY, Plenum Press, pp. 191-207.
Rodwell DE (1985) A teratology study in rats with triphenyltin
hydroxide (Code 029664 OF ZD97 0001 technical substance).
Unpublished report WIL-39011 (A31079) of WIL Research Laboratories
Inc., Ashland. Submitted to WHO by Hoechst AG, Frankfurt-am-Main
[cited in WHO, 1992].
Rodwell DE (1987) An embryotoxicity study in rabbits with
triphenyltin hydroxide (Code: HOE 209664 OF ZD97 0004). Unpublished
report WIL-39012 (A35220) of WIL Research Laboratories Inc., Ashland.
Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO,
1992].
Sachose K, Frei T, Luetkemeier H, Vogel W, Pappritz G, Terrier C
(1987) TPTH-substance technical (HOE 029664 OF ZD 97 0004). Chronic
oral toxicity 52-week feeding study in beagle dogs. Unpublished
report 047013/047024 (A35733) of RCC Research and Consulting Company
AG, Itingen. Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited
in WHO, 1992].
Sasaki YF, Yamada H, Sugiyama C, Kinae N (1993) Increasing effect of
tri- n-butyltins and triphenyltins on the frequency of chemically
induced chromosome aberrations in the cultured Chinese hamster cells.
Mutation research, 300:5-14.
Schollmeier U, Leist KH (1989) Triphenyltin hydroxide-active
ingredient technical (Code: HOE 029664 OF ZD97 0004). Testing for
sensitising properties in the Pirbright-White guinea pig according to
the technique of Buehler. Unpublished report 89.1006 (A42667) of
Hoechst Pharma Research Toxicology and Pathology. Submitted to WHO by
Hoechst AG, Frankfurt-am-Main [cited in WHO, 1992].
Scholz D, Weigand W (1969) Triphenyltin acetate study No. 2116.
Unpublished data A37396 of Laboratory for Industrial and Drug
Toxicology. Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited
in WHO, 1992].
Sekizawa J (1998) Health and environmental risk assessment of
organotin pollution in Japan. Bulletin of the National Institute of
Health Sciences, 116:126-131 (in Japanese).
Shimizu A, Kimura S (1992) Long-term effects of bis( n-tributyltin)
oxide (TBTO) on salt-water goby Chasmichotys dolichognathus.
Nippon Suisan Gakkaishi, 58:1595-1602 (in Japanese).
Shiraishi H, Soma M (1992) Triphenyltin compounds in mussels in Tokyo
Bay after restriction of use in Japan. Chemosphere, 24(8):1103-1109.
Smith KS (1981) 14-C-TPTH residue levels in milk and tissues of
lactating dairy cows. Cannon Laboratories, USA. Report Project OF
7489 [cited in FAO, 1991a].
Snoeij NJ, Van Iersel AAJ, Penninks AH, Seinen W (1985) Toxicity of
triorganotin compounds: Comparative in vivo studies with a series of
trialkyltin compounds and triphenyltin chloride in male rats.
Toxicology and applied pharmacology, 81:274-286.
Snow RL, Hays RL (1983) Phasic distribution of seminiferous tubules in
rats treated with triphenyltin compounds. Bulletin of environmental
contamination and toxicology, 31:658-665.
Soderquist C, Crosby DG (1980) Degradation of triphenyltin hydroxide
in water. Journal of agricultural and food chemistry, 28:111-117.
Staeb JA, Rozing MJM, Van-Hattum B, Cofino WP, Brinkman UAT (1992)
Normal-phase high-performance liquid chromatography with UV
irradiation, morin complexation and fluorescence detection for the
determination of organotin pesticides. Journal of chromatography,
609(1-2):195-203.
Staeb JA, Frenay M, Freriks IL, Brinkman UAT, Cofino WP (1995) Survey
of nine organotin compounds in the Netherlands using the zebra mussel
( Dreissena polymorpha) as biomonitor. Environmental toxicology and
chemistry, 14(12):2023-2032.
Staeb JA, Traas TP, Stroomberg G, van-Kesteren J, Leonards P,
van-Hattum B, Brinkman UA, Cofino WP (1996) Determination of organotin
compounds in the foodweb of a shallow freshwater lake in the
Netherlands. Archives of environmental contamination and toxicology,
31(3):319-328.
Suess A, Eben C (1973) Das Verhalten von Triphenyltzinnacetat (TPZA)
im Boden und die Aufnahme durch Pflanzen aus behandelten Boeden.
Zeitschrift fuer Pflanzenkrankheiten und Pflanzenschutz, 80
(H. 5):288-294 (in German).
Sugita A (1992) Historical background of organotin pollution. In:
Satomi Y, Shimizu M, eds. Organotin pollution and effects on aquatic
organisms. Tokyo, Koseisha Koseikaku, pp. 9-19 (in Japanese).
Suter P, Horst K (1986a) 13-week oral toxicity (feeding) study with
TPTH-technical (Code: HOE 029664 OF ZD97 0004) in the mouse.
Unpublished report project 046991 (A32420) of RCC Research and
Consulting Company AG, Itingen. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Suter P, Horst K (1986b) 13-week oral toxicity (feeding) study with
TPTH-technical (Code: HOE 029664 OF ZD97 0004) in the rat.
Unpublished report project 046978 (A32419) of RCC Research and
Consulting Company AG, Itingen. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Suzuki T, Yamada H, Yamamoto I, Nishimura K, Kondo K, Murayama M,
Uchiyama M (1996) Chemical species of organotin compounds in seawater
and their seasonal variations. Agricultural and food chemistry,
4(12):3989-3995.
Takeuchi M, Mizuishi K, Yamanobe H, Watanabe Y, Doguchi M (1989)
Hygienic chemical studies on organotin compounds (VI). A consideration
on the pollution of Tokyo Bay with tributyltin and triphenyltin
compounds. Annual report of the Tokyo Metropolitan Research
Laboratory for Public Health, 40:127-132 (in Japanese).
Takeuchi M, Mizuishi K, Yamanobe H, Nakamura H (1991) Hygienic
chemical studies on organotin compounds (VII). Content of tributyltin
and triphenyltin compounds in fish and shellfish (1988-1990). Annual
report of the Tokyo Metropolitan Research Laboratory for Public
Health, 42:77-85.
Tas JW, Oppe Vizen A, Seinen W (1990) Uptake and elimination kinetics
of triphenyltin hydroxide by two fish species. Toxicology and
environmental chemistry, 28:129-141.
Tennekes H, Horst K, Luetkemeier H, Vogel W, Vogel O, Armstrong J,
Ehlers HA, Muller E, Terrier C (1989a) TPTH-technical (Code: HOE
029664 OF ZD97 0004) oncogenicity 80-week feeding study in mice.
Unpublished report 047002 (A40467) of RCC Research and Consulting
Company AG, Itingen. Submitted to WHO by Hoechst AG, Frankfurt-am-Main
[cited in WHO, 1992].
Tennekes H, Horst K, Luetkemeier H, Vogel W, Schlotke B, Vogel O,
Ehlers HA, Muller E, Terrier C (1989b) TPTH-technical (Code: HOE
029664 OF ZD97 0007) chronic toxicity/oncogenicity 104-week feeding
study in rats. Unpublished report 046980 (A40468) of RCC Research
and Consulting Company AG, Itingen. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Til HP, Feron VJ, de Groot AP (1970) Chronic toxicity study with
triphenyltin hydroxide in rats for two years. Unpublished TNO
(Netherlands Organization for Applied Scientific Research) report
R-3138, submitted to WHO by Phillips-Duphar [cited in WHO/FAO, 1971].
Tomlin C, ed. (1997) The pesticide manual, 11th ed. Surrey, British
Crop Protection Council; Cambridge, The Royal Society of Chemistry,
1606 pp.
Tsuda T, Nakanishi H, Aoki S, Takebayashi J (1987) Bioconcentration
and metabolism of phenyltin chlorides in carp. Water research,
21(8):949-953.
Tsuda T, Inoue T, Kojima M, Aoki S (1995) Daily intakes of tributyltin
and triphenyltin compounds from meals. Journal of the Association of
Official Analytical Chemists International, 78(4):941-943.
Tsunoda M (1993) Simultaneous determination of organotin compounds in
fish and shellfish by gas chromatography with a flame photometric
detector. Tohoku journal of experimental medicine, 169(2):167-178.
Ueda K, Iijima K (1961) Acute toxicity of Brestan technical
(triphenyltin acetate) for mice. Unpublished report A15046 of
Laboratory of Hygienics, Tokyo Dental College, Tokyo. Submitted to WHO
by Hoechst AG, Frankfurt-am-Main [cited in WHO, 1992].
US EPA (1987) Tributyltin support document. Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances, Office of Pesticide Programs.
Van der Maas HL, Van Deyl L, Kroon JJ (1972) The acute and subacute
toxicity of the fungicide triphenyltin hydroxide to the common pond
snail, Lymnaea stagnalis L. Mededelingen van de Faculteit
Landbouwwetenschappen, Rijksuniversiteit Gent, 37(2):850-858.
Vela NP, Caruso JA (1993) Comparison of flame ionisation and
inductively coupled plasma mass spectrometry for the detection of
organometallics separated by capillary supercritical fluid
chromatography. Journal of chromatography, 641(2):337-345.
Verschuuren HG, Ruitenberg EJ, Peetoom F, Helleman PW, van Esch GJ
(1970) Influence of triphenyltin acetate on lymphatic tissue and
immune responses in guinea pigs. Toxicology and applied
pharmacology, 16:400-410.
Viviani B, Rossi AD, Chow SC, Nicotera P (1995) Organotin compounds
induce calcium overload and apoptosis in PC12 cells.
Neurotoxicology, 16(1):19-25.
Wada O, Manabe S, Iwai H, Arakawa Y (1982) Recent progress in the
study of analytical methods, toxicity, metabolism and health effects
of organotin compounds. Japanese journal of industrial health,
24:24-54 (in Japanese).
Walsh GE, McLaughlin LL, Lores EM, Louie MK, Deans CH (1985) Effects
of organotins on growth and survival of two marine diatoms
Skeletonema costatus and Thalassiosira pseudonana. Chemosphere,
14(3-4):383-392.
Walsh GE, McLaughlin LL, Louie MK, Deans CH, Lores EM (1986)
Inhibition of arm regeneration by Ophioderma brevispina
(Echinodermata, Ophiuroidea) by tributyltin and triphenyltin oxide.
Ecotoxicology and environmental safety, 12:95-100.
WHO (1976) Report of the 1975 Joint Meeting of the FAO Working Party
of Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues. Geneva, World Health Organization, 45 pp. (WHO
Technical Report Series No. 592).
WHO (1992) Pesticide residues in food -- 1991, Evaluations 1991 Part
II -- Toxicology. Geneva, World Health Organization, pp. 173-208.
WHO/FAO (1971) 1970 evaluation of some pesticide residues in food.
Rome, World Health Organization/Food and Agriculture Organization of
the United Nations, pp. 327-366.
Wong PTS, Chau YK, Kramar O, Bengert GA (1982) Structure toxicity
relationship of tin compounds on algae. Canadian journal of
fisheries and aquatic science, 39:483-488.
Yamada H, Sasaki YF (1993) Organotins are co-clastogens in a whole
mammalian system. Mutation research, 301:195-200.
Yamada H, Takayanagi K (1992) Bioconcentration and elimination of
bis(tributyltin) oxide (TBTO) and triphenyltin chloride (TPTC) in
several marine fish species. Water research, 26(12):1589-1595.
Young DL (1986) A dietary two-generation reproduction study in rats
with triphenyltin hydroxide (Code: HOE 0.29664 OF ZD97 0004
technical substance). Final report project WIl-39022 (A35378) of WIL
Research Laboratories, Inc., Ashland. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
APPENDIX 1 -- SOURCE DOCUMENTS
CICAD National Committee (1997): A critical review on triphenyltin
compounds
The 1997 review on triphenyltin compounds (provisional) was
developed by the National Committee for Concise International Chemical
Assessment Documents of Japan (CICAD National Committee, 1997). Much
important information on health effects was obtained from monographs
on pesticide residues prepared by the FAO and WHO (FAO, 1991a,b; WHO,
1992); these monographs describe summary evaluations of data,
including proprietary information. Extensive information on
environmental effects was obtained from a 1992 review of the
environmental effects of triorganotin compounds, prepared by the
Advisory Committee on Pesticides, Health and Safety Executive, United
Kingdom (HSE, 1992).
The CICAD National Committee of Japan is composed of the members
and observers listed below. Members are experts in the areas of
toxicology, chemistry, environmental science, occupational safety,
chemical management, or information science. Observers represent
divisions related to chemical safety or international activities in
various ministries and agencies. This committee is independent of
industry. Its activities are communicated on the homepage of the
National Institute of Health Sciences.
The draft review on triphenyltin compounds was prepared by Dr Jun
Sekizawa and was circulated for comments among members and observers,
then revised. This review is available by request from Dr Jun
Sekizawa, National Institute of Health Sciences.
Members
Dr S. Hatakeyama, Ecological Hazard Evaluation Team, National
Institute of Environmental Studies
Dr T. Kaminuma, Division of Chem-Bio Informatics, National Institute
of Health Sciences
Dr J. Kato, Yokohama Laboratory, Mitsubishi-kasei Institute of
Toxicological and Environmental Sciences
Dr Y. Kurokawa, Biological Safety Research Centre, National Institute
of Health Sciences ( Chairperson)
Dr K. Matsumoto, Department of Chemistry, Waseda University
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences
Mr T. Oshima, Japan Chemical Safety Institute
Dr H. Sakurai, National Institute for Industrial Health
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences
Mr Y. Shiraishi, Chemical Safety Management Centre, Japan Chemical
Industry Association
Dr I. Uchiyama, National Institute of Public Health
Dr M. Yasuno, Department of Environmental Science, Shiga Prefectural
University
Observers1
Division of Chemical Products Safety, Ministry of International Trade
and Industry
Division of Chemical Substance Investigation, Ministry of Labour
Division of Environmental Chemicals Safety, Ministry of Health and
Welfare
Division of Environmental Health and Safety, Environment Agency
Division of Food Chemicals, Ministry of Health and Welfare
Division of Foreign Affairs, Ministry of Health and Welfare
Division of Standards on Drinking Water, Ministry of Health and
Welfare
WHO (1992): Pesticide residues in food -- 1991, Evaluations
1991 Part II -- Toxicology
The Joint FAO/WHO Meeting on Pesticide Residues, known as JMPR,
has been evaluating pesticides that are used on food crops and that
may leave residues on them since 1963. JMPR comprises two groups of
scientists: namely, the FAO panel, which has responsibility for
reviewing pesticide residue data and for recommending maximum residue
limits, and the WHO group, which has responsibility for reviewing
toxicological data and for recommending acceptable daily intakes
(ADIs). The principles used by the WHO group for the toxicological
assessment of pesticide residues in food have been publicized through
its monographs and reports and in the Environmental Health Criteria of
the IPCS. Although many of the data come from manufacturers that
carried out major toxicological studies, the independent nature of the
JMPR evaluation has been secured through various mechanisms.
1 Representatives of the listed divisions in ministries and
agencies.
The toxicity of triphenyltin compounds was reviewed by JMPR in
1963, 1965, 1970, and 1991 (WHO, 1992). At the 1991 meeting, the
following scientists participated in the WHO group, and 21 other
experts from various international organizations and countries joined
in the evaluation (WHO, 1992).
Members of WHO Expert Group on Pesticide Residues in the 1991 JMPR
Professor U.G. Ahlborg, Institute of Environmental Medicine, Sweden
Dr A.L. Black, Department of Health, Housing and Community Services,
Australia
Dr J.F. Borzelleca, Medical College of Virginia, Virginia Commonwealth
University, USA
Mr D.J. Clegg, Health Protection Branch, Health and Welfare Canada,
Canada
Professor M. Lotti, Universita di Padova, Istituto di Medicina del
Lavoro, Italy
Dr F.R. Puga, Instituto Biologico, Brazil
Dr P. Yao, Institute of Occupational Medicine, Chinese Academy of
Preventive Medicine, Ministry of Public Health, People's Republic of
China
HSE (1992): A review of the environmental effects of
triorganotin compounds
The Health and Safety Executive of the United Kingdom developed
and published the report entitled A review of the environmental
effects of triorganotin compounds (HSE Report No. 111). This review
was prepared by the Advisory Committee on Pesticides, a tripartite
committee with representatives from industry, trade unions, and
academia who give their advice and approve the use under the Control
of Pesticides Regulations 1986.
The Health and Safety Executive is responsible for human health
aspects and pesticide efficacy. The Ministry of Agriculture, Fisheries
and Food (Pesticides Safety Directorate) is responsible for the
evaluation of non-human aspects.
Public access to raw data underlying this publication can be
arranged by contacting the Pesticide Registration Section, Health and
Safety Executive, Magdalen House, Stanley Precinct, Bootle,
Merseyside, United Kingdom L20 3QZ.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on triphenyltin compounds was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
International Council on Metals and the Environment, Ottawa, Canada
Karolinska Institute, Stockholm, Sweden
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Institute for Occupational Safety and Health, Cincinnati, USA
National Institute for Working Life, Solna, Sweden
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences, Research Triangle Park,
USA)
United States Environmental Protection Agency, Denver, USA
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Tokyo, Japan, 30 June - 2 July 1998
Members
Dr R. Benson, Drinking Water Program, United States Environmental
Protection Agency, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Mr R. Cary, Health Directorate, Health and Safety Executive,
Merseyside, United Kingdom
Dr C. DeRosa, Agency for Toxic Substances and Disease Registry, Center
for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire, United
Kingdom
Dr H. Gibb, National Center for Environmental Assessment, United
States Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada ( Chairperson)
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan ( Vice-Chairperson)
Professor S.A. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia ( Rapporteur)
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute of
Occupational Medicine, Beijing, People's Republic of China
Observers
Professor F.M.C. Carpanini,1 Secretary-General, ECETOC (European
Centre for Ecotoxicology and Toxicology of Chemicals), Brussels,
Belgium
Dr M. Ema, Division of Biological Evaluation, National Institute of
Health Sciences, Osakai, Japan
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission,
Ispra, Italy
Mr T. Jacob,1 Dupont, Washington, DC, USA
Dr H. Koeter, Organisation for Economic Co-operation and Development,
Paris, France
Mr H. Kondo, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Ms J. Matsui, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Mr R. Montaigne,1 European Chemical Industry Council (CEFIC),
Brussels, Belgium
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr H. Nishimura, Environmental Health Science Laboratory, National
Institute of Health Sciences, Osaka, Japan
Ms C. Ohtake, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr T. Suzuki, Division of Food, National Institute of Health Sciences,
Tokyo, Japan
Dr K. Takeda, Mitsubishikagaku Institute of Toxicological and
Environmental Sciences, Yokohama, Japan
Dr K. Tasaka, Department of Chemistry, International Christian
University, Tokyo, Japan
Dr H. Yamada, Environment Conservation Division, National Research
Institute of Fisheries Science, Kanagawa, Japan
1 Invited but unable to attend.
Dr M. Yamamoto, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr M. Yasuno, School of Environmental Science, The University of Shiga
Prefecture, Hikone, Japan
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD sur les dérivés du triphénylétain a été préparé à partir
d'une évaluation effectuée par la Commission nationale japonaise des
CICAD (CICAD National Committee, 1997). De nombreuses études critiques
sur les effets sanitaires qui sont citées dans cette évaluation sont
tirées de monographies préparées par l'Organisation des Nations Unies
pour l'Alimentation et l'Agriculture (FAO, 1991a,b) et par
l'Organisation mondiale de la Santé (WHO, 1992). Ces monographies
récapitulent les nombreuses études communiquées à l'OMS par les
producteurs en vue d'une évaluation, en plus des résumés des articles
publiés. Dans le cas des études communiquées par les producteurs, les
documents originaux sont la propriété de leurs auteurs et n'ont pas
été mis à la disposition des auteurs de l'évaluation préparée par la
Commission nationale des CICAD (CICAD National Committee, 1997), des
rédacteurs du projet de CICAD et du Comité d'évaluation finale. Pour
ce qui concerne les données figurant dans ces documents originaux, les
rédacteurs du présent CICAD n'ont donc eu d'autre solution que de
s'appuyer sur les évaluations effectuées lors de la réunion conjointe
FAO/OMS sur les résidus de pesticides (JMPR).
De nombreux renseignements concernant les effets environnementaux
de ce composé ont été tirés d'une étude consacrée aux effets des
organostanniques sur l'environnement, publiée par l'Advisory Committee
on Pesticides of the Health and Safety Executive du Royaume-Uni (HSE,
1992). D'autres informations ont été obtenues en interrogeant les
bases de données Medline et Toxline Plus jusqu'à octobre 1997.On
trouvera à l'appendice 1 des indications sur les sources documentaires
utilisées ainsi que sur leur mode de dépouillement. Les renseignements
concernant l'examen du CICAD par des pairs font l'objet de l'appendice
2. Ce CICAD a été approuvé en tant qu'évaluation internationale lors
d'une réunion du Comité d'évaluation finale qui s'est tenue à Tokyo
(Japon) du 30 juin au 2 juillet 1998. La liste des participants à
cette réunion figure à l'appendice 3. La fiche d'information
internationale sur la sécurité chimique (ICSC No 1283) établie pour
l'hydroxyde de triphénylétain par le Programme international sur la
sécurité chimique (IPCS, 1996) est également reproduite dans ce
document.
Les composés du triphénylétain sont des dérivés triphénylés de
l'étain IV. Ce sont des solides incolores à faible tension de vapeur.
Ils sont lipophiles et peu solubles dans l'eau.
Depuis les années 1960, on fait grand usage des dérivés du
triphénylétain et du tributylétain comme algicides et molluscicides
dans les produits antisalissures. L'incorporation de composés
triorganostanniques dans les peintures marines antisalissures est
limitée dans de nombreux pays du fait de leurs effets catastrophiques
sur l'ostréiculture et sur les écosystèmes aquatiques en général. Le
triphénylétain est utilisé comme fongicide non endothérapique à action
essentiellement protectrice.
Le triphénylétain est fortement adsorbé par les solides en
suspension et le sol et ne se désorbe plus guère ensuite. On estime sa
demi-vie dans l'eau à quelques jours durant le mois de juin et à 2 ou
3 semaine en novembre. Bien que susceptibles de se décomposer par
déphénylation progressive et d'être excrétés sous la forme de
conjugués, les dérivés du triphénylétain s'accumulent dans l'organisme
des poissons et des gastéropodes, avec un facteur de bioconcentration
dont la valeur va de quelques centaines à 32 500 (dans le sac
intestinal de Lymnaea stagnalis).
La concentration des dérivés du triphénylétain dans
l'environnement dépend de leur mode, de leur moment et de leur lieu
d'utilisation. Dans des baies et des marinas, on a trouvé des
concentrations allant de 0 à près de 200 ng/litre, ces dernières
valeurs par suite du lessivage des peintures à base de triphénylétain
utilisées pour protéger la coque des bateaux contre les salissures. La
concentration des composés du triphénylétain dans l'environnement
diminue depuis quelques années en raison des restrictions sévères
imposées à leur utilisation dans les peintures antisalissures.
Administrés per os à des rats, les dérivés du triphénylétain ne
sont pas aisément résorbés et ils sont excrétés principalement dans
les matières fécales et en partie également dans les urines. Par
métabolisation, ils sont transformés en diphénylétain, en
monophénylétain et en résidus liés non extractibles. Une fois
absorbés, ils s'accumulent en majeure partie au niveau des reins et du
foie et en plus faible quantité dans d'autres organes. Appliqués sur
l'épiderme, ils peuvent traverser la peau selon un processus qui est
fonction du temps et de la dose.
Le triphénylétain exerce des effets variés sur l'organisme des
diverses espèces animales, notamment sur le système immunitaire ou sur
la reproduction et le développement à des des doses proches de celles
qui sont toxiques pour les mères (la plupart des valeurs de la dose la
plus faible sans effet observable ou LOAEL, sont de l'ordre de
quelques mg par kg de poids corporel ou moins). On observe également
des hyperplasies et des adénomes des glandes endocrines, l'apoptose
des cellules thymiques, la libération de calcium au niveau des
cellules du réticulum sarcoplasmique et une irritation oculaire. Les
mécanismes qui sont à l'origine de ces effets font encore l'objet
d'investigations et il est possible que ce profil toxicologique
s'explique par un mécanisme général.
Les dérivés du triphénylétain sont modérément toxiques pour le
rat. Ils ne sont pas cancérogènes, mais certaines données montrent
qu'ils ont une action coclastogène.
Parmi les effets sur la reproduction et le développement, on peut
citer la réduction du nombre des nidations et du nombre de foetus
vivants (à la dose journalière de 1,0 mg/kg p.c. d'acétate de
triphénylétain, ou TPTA, administré par gavage à des lapins), la
diminution de la taille des portées et du poids des lapereaux avec
également une réduction du poids relatif du thymus ou de la rate chez
des ratons juste sevrés (dans une étude sur deux générations des rats
recevant une alimentation contenant l'équivalent de 1,5 mg d'hydroxyde
de triphénylétain - TPTH - par kg de poids corporel et par jour; dans
cette étude, la dose sans effet nocif observable ou NOAEL, était de
0,4 mg/kg p.c. par jour) ainsi que des avortements et une diminution
du poids des foetus (dans une étude sur des lapins recevant
quotidiennement par gavage une dose de 0,9 mg de TPTH par kg de poids
corporel).
La valeur la plus faible de la NOAEL qui ait été obtenue dans une
étude toxicologique est de 0,1 mg de TPTH par kg p.c. par jour pour la
toxicité maternelle, les critères de toxicité retenus étant la
réduction de la consommation de nourriture et du gain de poids à la
dose quotidienne de 0,3 mg par kg de poids corporel. Une valeur
identique a été obtenue au début d'une étude de 2 ans sur des rats, au
cours de laquelle on a observé une légère diminution du nombre de
leucocytes aux doses élevées. Lors d'une étude de 52 semaines sur des
chiens, on a trouvé une NOAEL égale à 0,21 mg de TPTH par kg de poids
corporel par jour, le critère retenu étant la réduction du poids
relatif du foie chez les femelles soumises à des doses élevées.
Les dérivés du triphénylétain peuvent affecter le système
immunitaire. On a observé une diminution de la concentration des
immunoglobulines (Ig) (même à la dose la plus faible, soit 0,3 mg de
TPTH par kg p.c. par jour lors d'une étude d'alimentation de 2 ans sur
des rats), une lymphopénie (à la dose de 0,3 mg par kg p.c. par jour
dans une autre étude d'alimentation de 2 ans portant également sur des
rats et à la dose de 0,338 mg/m3 dans une étude d'inhalation sur des
rats), une atrophie du thymus (à la dose de 1,5 mg de chlorure de
triphénylétain - TPTCl - par kg p.c. par jour dans une étude
d'alimentation sur des rats juste sevrés) et enfin une atrophie de la
rate (à la dose de 5 mg de TPTH par kg p.c. par jour lors d'une étude
d'alimentation de 28 jours sur des souris). Les femelles sont
généralement plus sensibles que les mâles.
Pour établir la dose journalière admissible (DJA) de
triphénylétain en cas d'exposition per os, le JMPR a pris en
considération plusieurs points d'aboutissement des effets
toxicologiques (FAO, 1991b; WHO, 1992). En premier lieu, on a appliqué
un coefficient d'incertitude de 200 à la dose sans effet observable
(NOEL) de 0,1 mg/kg p.c. par jour (basée sur l'observation d'une
diminution du nombre de leucocytes à dose élevée lors d'une étude de 2
ans sur des rats) pour obtenir une DJA de 0-0,5 µg/kg de poids
corporel. On a ensuite appliqué un coefficient d'incertitude de 500 à
la LOAEL de 0,3 mg/kg p.c. par jour tirée d'une étude de 2 ans sur des
rats, au cours de laquelle on avait noté une augmentation de la
mortalité et une diminution des taux d'immunoglobulines sériques. Les
autres valeurs de la NOAEL qui ont été prises en considération
parallèlement aux résultats précités sont les suivantes : 0,4 mg/kg
p.c. par jour dans le cas d'une étude sur la reproduction portant sur
deux générations de rats (cette étude a révélé une réduction du poids
de la rate et du thymus chez les ratons mâles et femelles juste sevrés
des générations F1 et F2 aux doses les plus élevées); 0,3 mg/kg p.c.
par jour à l'occasion d'une étude à court terme sur des rats
(réduction du nombre de leucocytes, diminution du taux des IgG et
augmentation du poids relatif des testicules aux doses élevées); 0,21
mg/kg p.c. par jour lors d'une étude à court terme sur des chiens
(augmentation du poids relatif du foie et diminution du rapport
albumine sérique / globulines aux doses élevées); enfin, 0,1 mg/kg
p.c. lors d'une étude tératologique sur des lapins (toxicité
maternelle constatée aux doses élevées).
On ne possède pas de données sur l'exposition professionnelle aux
dérivés du triphénylétain. Il existe toutefois un certain nombre de
rapports sur des cas d'intoxication où sont décrits des effets
neurotoxiques apparemment durables. L'exposition de la population
générale à ces composés provient essentiellement de l'ingestion de
produits de la mer contaminés. En effet, on a trouvé des
concentrations de triphénylétain pouvant atteindre 1 µg/g dans les
muscles de certaines espèces de poissons. Au Japon, on estime qu'en
1997 l'absorption de triphénylétain par suite de la consommation
d'aliments contaminés se situait aux alentours de 11 % de la DJA
(c'est-à-dire 2,75 µg/jour pour un sujet de 50 kg) établie par le
JMPR.
Les dérivés du triphénylétain exercent des effets délétères sur
les organismes aquatiques à très faible concentration. Par exemple, on
observé l'apparition d'organes mâles chez des femelles de gastéropodes
japonais à la concentration d'environ 1 ng/litre (concentration sans
effet ou NOEC, non déterminée) et des effets toxiques ont été observés
chez les larves d'une sorte de vairon, Pimephales promelas, à la
concentration de 0,23 µg/litre (concentration la plus faible
produisant un effet ou LOEC). On estime que le triphénylétain perturbe
les fonctions endocriniennes; en effet, l'apparition d'organes sexuels
mâles chez les gastéropodes femelles est probablement due à un trouble
hormonal.
On n'a pas établi de NOEC relative au changement de sexe chez les
mollusques par suite d'une exposition au triphénylétain. On a constaté
expérimentalement, en procédant à des injections, que le
triphénylétain avait une activité du même ordre que celle du
tributylétain vis-à-vis du genre Thais. Chez les mollusques du
genre Nucella il est moins actif que le tributylétain, mais sa
bioaccumulation est supérieure. De ces expérimentations, on peut
conclure que la NOEC du triphénylétain doit être de quelques ng/litre
tout au plus. Cette estimation est corroborée par la fréquence de
l'appartion, en situation réelle, d'organes mâles chez des mollusques
femelles du genre Thais exposés aux concentrations ambiantes. Dans
l'environnement, les résidus de triphénylétain accompagnent ceux de
tributylétain, aussi ne peut-on évaluer leur contribution respective
au phénomène d'apparition d'organes mâles chez les gastéropodes
femelles du genre Thais. Dans ces conditions, on peut conclure que
l'utilisation de l'un ou l'autre de ces organostanniques dans les
peintures antisalissures conduit de tout manière à la décimation des
invertébrés marins.
RESUMEN DE ORIENTACION
Este CICAD sobre los compuestos del trifenilestaño se basa en el
examen preparado por el Comité Nacional para los Documentos
Internacionales Concisos sobre Evaluación de Sustancias Químicas
(Comité Nacional para los CICAD, 1997). En el presente examen se citan
numerosos estudios críticos de los efectos en la salud procedentes de
monografías sobre residuos de los plaguicidas preparadas por la
Organización de las Naciones Unidas para la Agricultura y la
Alimentación (FAO, 1991a,b) y la Organización Mundial de la Salud
(OMS, 1992). Estas monografías contienen los resúmenes de los
numerosos estudios que los fabricantes presentaron a la OMS para su
evaluación, además de resúmenes de los documentos publicados. En el
caso de los estudios presentados por los fabricantes, los documentos
originales son privados y los autores del examen preparado por el
Comité Nacional para los CICAD (1977), los autores del proyecto del
CICAD o la Junta de Evaluación Final del CICAD no pudieron disponer de
ellos. Por consiguiente, este CICAD se basa inevitablemente en las
evaluaciones realizadas en la reunión conjunta FAO/OMS sobre residuos
de plaguicidas (JMPR) para los estudios citados de los resúmenes de
datos privados.
Se obtuvo amplia información sobre los efectos en el medio
ambiente de un examen acerca de los efectos ecológicos de los
compuestos de estaño con tres grupos orgánicos, preparado por el
Comité Consultivo sobre Plaguicidas de la Dirección de Salud y
Seguridad del Reino Unido (HSE, 1992). Otros datos proceden de una
búsqueda en las bases de datos Medline y Toxline Plus hasta octubre de
1997. La información relativa al carácter de los procesos de examen y
la disponibilidad de los documentos originales figura en el apéndice
1. La información acerca del examen colegiado de este CICAD se
presenta en el apéndice 2. Su aprobación tuvo lugar como evaluación
internacional en una reunión de la Junta de Evaluación Final,
celebrada en Tokio, Japón, del 30 de junio al 2 de julio de 1998. La
lista de participantes en esta reunión aparece en el apéndice 3. La
Ficha internacional de seguridad química (ICSC 1283) para el hidróxido
de trifenilestaño (TPTH), preparada por el Programa Internacional de
Seguridad de las Sustancias Químicas (IPCS, 1996) también se reproduce
en el presente documento.
Los compuestos de trifenilestaño son derivados trifenílicos del
estaño tetravalente. Son sólidos incoloros con presiones de vapor
bajas. Son lipófilos y su solubilidad en agua es escasa.
Los compuestos de trifenilestaño y tributilestaño se han
utilizado ampliamente desde los años sesenta como alguicidas y
molusquicidas en los productos antiincrustantes. Se ha restringido el
empleo de los compuestos de estaño con tres grupos orgánicos en las
pinturas antiincrustantes debido a sus efectos devastadores en la
industria de las ostras y a los más generales en el ecosistema
acuático. El trifenilestaño se utiliza como fungicida no sistémico de
acción fundamentalmente protectora.
El trifenilestaño se adsorbe fuertemente al sedimento y al suelo
y la desorción es escasa. Su semivida en agua se ha estimado en varios
días en junio y en 2-3 semanas en noviembre. Si bien los compuestos de
trifenilestaño se pueden degradar mediante defenilación escalonada y
excretarse en forma conjugada, se ha observado bioacumulación en los
peces y los caracoles, con factores de bioconcentración que oscilan
entre varios cientos y 32 500 (en el saco intestinal de Lymnaea
stagnalis).
Las concentraciones de los compuestos de trifenilestaño en el
medio ambiente varían en función de la manera, el momento y el lugar
de utilización de esos compuestos. Se han detectado concentraciones
que oscilan entre 0 ng/litro y casi 200 ng/litro en zonas de bahías o
puertos deportivos debido a la lixiviación a partir de las
embarcaciones tratadas con pinturas antiincrustantes que contienen
trifenilestaño. Las concentraciones de compuestos de trifenilestaño en
el medio ambiente se han reducido en los últimos años como
consecuencia del endurecimiento de las restricciones sobre su uso en
las pinturas antiincrustantes.
Administrados a ratas por vía oral, los compuestos de
trifenilestaño no se absorben con facilidad y se excretan
fundamentalmente en las heces y parcialmente en la orina. Se
metabolizan a difenilestaño, monofenilestaño y residuos ligados no
extraíbles. Los compuestos de trifenilestaño absorbidos se acumulan
sobre todo en el riñón y el hígado y en menor cantidad en otros
órganos. Tras su aplicación cutánea pueden penetrar a través de la
piel, de forma dependiente del tiempo y la concentración.
El trifenilestaño tiene diversos efectos en la salud de las
distintas especies animales, en particular en el sistema inmunitario,
efectos en la reproducción/desarrollo, con niveles próximos a los de
toxicidad materna (las concentraciones más bajas con efectos adversos
observados o LOAEL son en general del orden de mg/kg o inferiores),
hiperplasia/adenomas en los órganos endocrinos, apoptosis en las
células del timo, liberación de calcio en las células del retículo
sarcoplásmico e irritación ocular. Se están investigando los
mecanismos que provocan esos efectos; este perfil de toxicidad se
puede explicar por un mecanismo común.
Los compuestos de trifenilestaño tienen una toxicidad
moderadamente aguda en las ratas. No son carcinogénicos, pero algunos
datos ponen de manifiesto una acción coclastogénica.
Los efectos reproductivos y en el desarrollo son un aumento en el
número de implantaciones y fetos vivos (con 1,0 mg de acetato de
trifenilestaño (TPTA)/kg de peso corporal al día en un estudio de
administración con sonda realizado en conejos), reducción del tamaño
de la camada/peso de las crías y del peso relativo del timo o el bazo
en las crías destetadas (con 1,5 mg de TPTH/kg de peso corporal al día
en los alimentos en un estudio de reproducción en dos generaciones
realizado en ratas; la concentración sin efectos adversos observados o
NOAEL es de 0,4 mg/kg de peso corporal al día) y aborto y reducción
del peso fetal (con 0,9 mg de TPTH/kg de peso corporal al día en un
estudio de administración con sonda realizado en conejos).
La NOAEL más baja detectada en las pruebas de toxicidad fue de
0,1 mg de TPTH/kg de peso corporal al día para la toxicidad materna en
un estudio de administración con sonda realizado en conejos, basado en
la disminución del consumo de alimentos y del aumento del peso
corporal con 0,3 mg/kg de peso corporal al día. Se obtuvo el mismo
valor en un estudio inicial de dos años realizado con ratas, en el
cual se observó que con las dosis más altas se producía una ligera
disminución de la concentración de leucocitos. En un estudio de 52
semanas realizado con perros se estimó una NOAEL de 0,21 mg de TPTH/kg
de peso corporal al día, tomando como base una disminución del peso
relativo del hígado en las hembras con las dosis más altas.
Los compuestos de tributilestaño afectan al sistema inmunitario.
Se ha observado una disminución de la concentración de
inmunoglobulinas (incluso con la dosis más baja, es decir, 0,3 mg de
TPTH/kg de peso corporal al día, en un estudio de alimentación de dos
años realizado con ratas), linfopenia (con 0,3 mg de TPTH/kg de peso
corporal al día en otro estudio de alimentación de dos años realizado
con ratas o de 0,338 mg/m3 en un estudio de inhalación de 13 semanas
con ratas), atrofia del timo (con 1,5 mg de cloruro de trifenilestaño
(TPTCl)/kg de peso corporal al día en un estudio de alimentación de
dos semanas realizado con ratas destetadas) y atrofia del bazo (con 5
mg de TPTH/kg de peso corporal al día en un estudio de alimentación de
28 días realizado con ratones). En general, las hembras son más
susceptibles que los machos.
La JMPR tuvo en cuenta varios efectos finales al establecer la
ingesta diaria admisible (IDA) de trifenilestaño para la exposición
oral (FAO, 1991b; OMS, 1992). En primer lugar, se aplicó un factor de
incertidumbre de 200 a la concentración sin efectos observados (NOEL)
de 0,1 mg/kg de peso corporal al día (basada en el resultado de la
disminución de la concentración de leucocitos con las dosis más altas
obtenido en un estudio de dos años realizado con ratas) para llegar a
una IDA de 0-0,5 µg/kg de peso corporal. En segundo lugar, se aplicó
un factor de incertidumbre de 500 a una LOAEL de 0,3 mg/kg de peso
corporal al día en un estudio de dos años realizado en ratas en el que
se observó un aumento de la mortalidad y una reducción de la
concentración de inmunoglobulina sérica. Otras LOAEL que se tuvieron
en cuenta, junto con los efectos citados más arriba, son 0,4 mg/kg de
peso corporal al día en un estudio de la reproducción en dos
generaciones realizado con ratas (con los niveles más altos se observó
una disminución dependiente de la dosis de los pesos relativos del
bazo y el timo en los machos y las hembras destetados de la F1 y la
F2), 0,3 mg/kg de peso corporal al día en un estudio de corta
duración realizado en ratas (con las dosis más altas se detectó una
reducción de la concentración de leucocitos y de la IgG y un aumento
del peso relativo de los testículos), 0,21 mg/kg de peso corporal al
día en un estudio de corta duración realizado en perros (con las dosis
más altas se observó un aumento del peso relativo del hígado y una
disminución de la razón albúmina sérica/globulina) y 0,1 mg/kg de peso
corporal al día en un estudio de teratología en conejos (con las dosis
más altas se detectó toxicidad materna).
No se dispone de datos relativos a la exposición ocupacional a
los compuestos de trifenilestaño. En un pequeño número de informes de
casos de intoxicación se describen efectos neurotóxicos, que parecían
persistir. La exposición del público general a estos compuestos se
produce fundamentalmente por la ingestión de alimentos marinos
contaminados, en los cuales se han encontrado a veces concentraciones
de hasta 1 µg/g (en el músculo de algunas especies de peces). En 1997
se estimó en el Japón una ingesta de trifenilestaño a partir de
alimentos contaminados de alrededor del 11% de la IDA (es decir, 2,75
µg/día para una persona de 50 kg) establecida por la JMPR.
Los compuestos de trifenilestaño tienen efectos nocivos en los
organismos acuáticos a concentraciones muy bajas. Por ejemplo, se
observó imposexo en especies del género Thais (gasterópodos del
Japón) con concentraciones de alrededor de 1 ng/litro (no se determinó
la concentración sin efectos observados o NOEC) y se observó toxicidad
crónica en las larvas de Pimephales promelas con 0,23 µg/litro
(concentración más baja con efectos observados o LOEC). Se considera
que el trifenilestaño es un perturbador del sistema endocrino a causa
del imposexo, fenómeno en el cual se forman órganos sexuales
masculinos en los gasterópodos hembra, probablemente debido a un
trastorno hormonal.
No se ha establecido la NOEC del trifenilestaño para el imposexo
de los moluscos. El trifenilestaño tiene experimentalmente, mediante
inyección, un efecto semejante al del tributilestaño en el género
Thais. El primero es menos potente que el segundo en Nucella; sin
embargo, su bioacumulación es mayor. A partir de esta información se
puede estimar que la NOEC para el trifenilestaño será de varios
ng/litro o más baja. La prevalencia de imposexo en Thais observada
sobre el terreno en presencia de determinadas concentraciones en el
medio ambiente apoya esta estimación. Habida cuenta de que los
residuos de trifenilestaño y tributilestaño aparecen juntos en el
medio ambiente, no se puede evaluar su contribución relativa al
imposexo de las especies de Thais. Conforme a esta información, el
uso de trifenilestaño o de tributilestaño en la pintura
antiincrustante produciría una disminución de la población de los
invertebrados marinos.
Physical and chemical properties of triphenyltin compounds vary
depending upon the anion linked to tin. At ambient temperatures in the
pH range of 3-8, TPTA and TPTCl are hydrolysed to TPTH within 1 min;
as a consequence, the results of most studies with TPTA or TPTCl can
be applied to TPTH. Triphenyltin compounds are colourless solids with
low vapour pressures (<2 mPa at 50°C). The compounds are lipophilic
and have low water solubility (typically a few mg/litre at neutral
pH).
The identity and physical/chemical properties of TPTH, TPTA, and
TPTCl are given in Table 1. Additional properties of TPTH are
presented in the International Chemical Safety Card (ICSC 1283)
reproduced in this document.
Table 1: Identity and physical/chemical properties of some triphenyltin compounds.a
Triphenyltin hydroxide Triphenyltin acetate Triphenyltin chloride
Synonyms Fentin hydroxide; TPTH Fentin acetate; TPTA Fentin chloride; TPTCl
Chemical Abstracts 76-87-9 900-95-8 639-58-7
Service (CAS) Registry No.
Molecular formula C18H16OSn C20H18O2Sn C18H15ClSn
Molecular weight 367.0 409.1 385.5
Melting point 122-123.5°C 122-124°C 106°C
Solubility in water (20°C) 1 mg/litre at pH 7 9 mg/litre at pH 5 40 mg/litre
greater at lower pH (pH not given)
Solubility in other 10 g/litre (ethanol) 22 g/litre (ethanol) moderately soluble
solvents (20°C) 171 g/litre (dichloromethane) 82 g/litre (ethyl acetate) in organic solvents
28 g/litre (diethyl ether) 5 g/litre (hexane)
50 g/litre (acetone) 460 g/litre (dichloromethane)
89 g/litre (toluene)
Vapour pressure 0.047 mPa (50°C) 1.9 mPa (60°C) 0.021 mPa
Log Kow 3.43 3.43 -
a From Tomlin (1997); NLM (1998).
3. ANALYTICAL METHODS
Triphenyltin compounds and their degradation products can be
analysed in food commodities and in environmental or biological media
using several techniques, depending upon type of medium and
sensitivity required. The procedure usually starts with either liquid
extraction or adsorption onto a solid matrix, followed by
re-extraction and/or concentration. Quantification is then performed
using flame or flameless atomic absorption spectrometry, gas
chromatography with flame photometric or mass spectrometric detection,
or normal-phase high-performance liquid chromatography with
ultraviolet or fluorescence detection (Hattori et al., 1984; Ishizaka
et al., 1989; Fent & Hunn, 1991; Gomez-Ariza et al., 1992; Staeb et
al., 1992; Tsunoda, 1993; Kohri et al., 1995; Suzuki et al., 1996).
The detection limits of these techniques are in the range of
ng/litre for water and <1 µg/kg for sediments and biological samples.
Triphenyltin can also be separated from samples by capillary
supercritical fluid chromatography and measured by inductively coupled
plasma mass spectrometry. A detection limit of 12.0 pg was obtained
for triphenyltin using this method (Vela & Caruso, 1993).
Triphenyltin in water, sediment, and biological samples, as well
as inorganic tin that is excreted in urine following exposure to
triphenyltin, can be extracted with hydrogen chloride and
n-hexane/benzene (3:2 v/v) in the presence of tropolone, then
pentylated with a Grignard reagent prior to gas chromatography with
flame photometric detection. Quantification limits by this method were
found to be 3 ng/litre for water, 0.5 µg/kg for sediments and
biological samples, and 3 pg as tin for urine (Ohhira & Matsui, 1991,
1993; Harino et al., 1992).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Triphenyltin compounds have been used extensively as algicides
and molluscicides in antifouling products since the 1960s (HSE, 1992).
TPTA and TPTH are used mainly as fungicides with a preventive
action on potatoes, sugar beets, hops, and celery (FAO, 1991a).
Triphenyltin compounds are used on rice against fungal diseases,
algae, and molluscs.
Use of triorganotins in antifouling paints has been restricted in
many countries because of their catastrophic effects on the oyster
industry and more general effects on the aquatic ecosystem.
Information on amounts of triphenyltins used has been obtained
only from Japan. Use of triphenyltin compounds for antifouling paints
in Japan decreased from 4835 tonnes in 1983 to 346 tonnes (formulation
basis) in 1989 (Sugita, 1992). Their use for antifouling paints was 40
tonnes (active ingredient) in 1989 and stopped after 1990 in Japan
(MITI, 1998). About 120-140 tonnes (active ingredient) were produced
each year between 1994 and 1996 in Japan for export (MITI, 1998).
Between 1978 and 1990, 33-75 tonnes (active ingredient) of
phenyltin compounds were produced in Japan for use as a fungicide;
production ceased in 1990 (JPPA, 1982-1996).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Degradation of triphenyltin occurs through sequential
dephenylation resulting from cleavage of the tin-carbon bond through
biological, ultraviolet irradiation, chemical, or thermal mechanisms;
biological cleavage and cleavage by ultraviolet irradiation are
considered to be the most significant processes. Abiotic factors, such
as elevated temperatures, increased intensity of sunlight, and aerobic
conditions, seem to enhance triphenyltin degradation in the
environment (CICAD National Committee, 1997).
Hydrolysis of triphenyltin compounds in water leads to the
formation of principally TPTH and various hydrated oxides (Beurkle,
1985). It has been demonstrated that the presence of chloride from
seawater lowers the solubility of triphenyltin compounds by reaction
with the hydrated cation to form the covalent organotin chloride
(Ozcan & Good, 1980).
In plants, no translocation occurs from treated leaves (FAO,
1991a). TPTA and TPTCl are spontaneously hydrolysed to form TPTH.
Phenyl groups are split off from TPTH to form diphenyl and monophenyl
compounds. Both parent compound and metabolites conjugate to form
glycosides or glutathione conjugates.
The persistence of TPTA and TPTH depends on soil type and pH.
TPTH is strongly adsorbed to sediment and soil, and little desorption
occurs. Therefore, uptake into plants via roots may be expected to be
extremely low.
14C-labelled TPTA in soil degraded to inorganic tin with
evolution of 14C-labelled carbon dioxide. Similar experiments on
sterile soil showed insignificant evolution of labelled carbon
dioxide, which suggests that degradation can be attributed to
microorganisms (Barnes et al., 1971). Soil respiration was slightly
enhanced after treatment with TPTA, indicating that there were no
adverse effects on aerobic microorganisms (Suess & Eben, 1973).
A half-life of 1-3 months has been reported for TPTH in sandy and
silt loam soils and 126 days in flooded silt loam (US EPA, 1987). The
half-life of triphenyltin in water was estimated to be several days in
June and 2-3 weeks in November (Soderquist & Crosby, 1980).
Half-lives of triphenyltin in mussels ( Mytilus edulis) taken in
the summer of 1989 in Yokohama (a busy port, heavily contaminated with
triphenyltin) and Urayasu (a river mouth, about 10 times less polluted
than Yokohama) in Japan were estimated to be 139 and 127 days,
respectively (Shiraishi & Soma, 1992). Biological half-lives of
triphenyltin in short-necked clams ( Tapes [ Amygdala] japonica)
and guppy ( Poecilia reticulata) were estimated to be approximately
30 days and 48 days, respectively (Takeuchi et al., 1989; Tas et al.,
1990). The ecological half-life of triphenyltin in gastropods was
estimated to be 347 days (Mensink et al., 1996).
Temporal variations of phenyltin concentrations in zebra mussels
( Dreissena polymorpha) were studied at two locations near potato
fields during and after the triphenyltin fungicide spraying season in
the Netherlands (Staeb et al., 1995). Phenyltin concentrations in
zebra mussels were high in the period before and during harvesting but
not during the spraying season, which suggests that phenyltin
compounds in some foliage ended up in the water and were taken up by
the mussels. Although higher concentrations were detected in locations
near areas of spray operation, marinas, and harbours, the widespread
presence of triphenyltin residues in mussels collected in 56 locations
all over the Netherlands suggests the contribution of transport via
the air.
An extensive study on the presence of nine organotin compounds in
a freshwater food-web (zebra mussel, eel, roach bream, pike, perch,
pike perch, and cormorant; details and scientific names of species not
given in the report) revealed that phenyltin concentrations in benthic
species were higher than butyltin concentrations in lower trophic
levels (Staeb et al., 1996). This suggests that triphenyltin is to a
large extent taken up from the sediment by benthic organisms. At
higher trophic levels, net bioaccumulation of triphenyltin compounds
was greater than that of tributyltins, resulting in relatively higher
triphenyltin concentrations. With birds, the highest concentrations of
organotins were in liver and kidney and not in subcutaneous fat, which
shows that organotins accumulate via mechanisms different from those
of traditional lipophilic compounds.
BCFs in daphnids did not exceed 300 (Filenko & Isakova, 1979). In
fish, BCFs ranged from 257 to 4100. The highest value (4100) was
estimated for filefish ( Rudarius ercodes) cultivated in water
containing 148 ng triphenyltin/litre for 56 days (Yamada & Takayanagi,
1992). When Lymnaea stagnalis (a freshwater snail) was exposed to 2
µg TPTH/litre for 5 weeks, tin accumulated to the greatest extent in
the intestinal sac, to a level of 65.1 mg/kg (i.e., BCF of 32 500; Van
der Maas et al., 1972).
Tissue concentrations of triphenyltin in common carp ( Cyprinus
carpio) exposed to 5.6 µg TPTCl/litre for 10 days, which reached a
plateau after 7 days, were examined. The BCFs were highest in the
kidney (2090), followed by liver (912), muscle (269), and gall bladder
(257) (Tsuda et al., 1987).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Triphenyltin levels in ambient water, sediment, and organisms
were surveyed in about 30 locations (estuaries and bay) in Japan
between 1982 and 1995 (Japan Environment Agency, 1983, 1996).
Triphenyltin levels in water (detection limit 5 ng/litre) and sediment
(detection limit 1.0 ng/g) of bay and inshore areas decreased from
2.7-8.0 ng/litre and 3.3-7.8 ng/g in 1988-1991 to 2.5-3.0 ng/litre and
1.5-2.3 ng/g in 1992-1995, respectively.
Triphenyltin levels in ambient water and sediment in the Tokyo
bay area gradually decreased from peak levels (geometric means: 25.1
ng/litre in water, 4.3 ng/g in sediment) to 1.8 ng/litre (water) and
0.19 ng/g (sediment) in 1993 because of consecutive tightening of
regulations and voluntary withdrawal of use by coastal fishery
industries (Takeuchi et al., 1991).
Triphenyltin levels were measured in fish and shellfish obtained
from the Tokyo Central Fish Wholesale Market from April 1988 to March
1991 (Takeuchi et al., 1991; see section 6.2). The finding of
triphenyltin in coastal fish, as well as in open-ocean or pelagic
fish, is suggestive of biomagnification through the food-chain. High
levels in clams and oysters showed that direct uptake from water or
sediment also plays an important role for these species.
In the Netherlands, up to 920 ng triphenyltin (as tin)/g sediment
was found in the Westinder lake system in 1993, whereas no
triphenyltin was found in the water (detection limit 5 ng/litre)
(Staeb et al., 1996). In freshwater marinas in Switzerland, up to 191
ng triphenyltin/litre was detected in 1988-1990, whereas 107 ng/g dry
weight was the highest value measured in vertical sediment core
profiles, and concentrations up to 11 ng triphenyltin/litre were
measured in the river system (Fent & Hunn, 1991, 1995). In Dreissena
mussels of the same marinas, up to 3.88 µg triphenyltin/g wet weight
was detected (Fent & Hunn, 1991), whereas up to 0.31 µg triphenyltin
(as tin)/g dry weight in Mytilus mussels and up to 0.24 µg
triphenyltin/g in Thais snails were detected in a Spanish marina
area in 1995 (Morcillo et al., 1997).
Biological monitoring of triphenyltin concentrations in fish from
coastal areas of Japan showed that concentrations decreased between
1989 (detected in 40 out of 65 samples, maximum concentration 2.6 µg/g
wet weight in muscle, detection limit 20 ng/g) and 1995 (detected in
21 out of 70 samples, maximum concentration 0.25 µg/g) (Japan
Environment Agency, 1996). Similarly, triphenyltin levels in mussels
and birds decreased over the same period: triphenyl was detected in 17
out of 25 samples of mussels (maximum concentration 0.45 µg/g) and in
5 out of 10 samples of birds (maximum concentration 0.05 µg/g) in
1989, compared with 0 out of 35 samples of mussels and 0 out of 10
samples of birds in 1995 (detection limit 0.02 µg/g in both years)
(Japan Environment Agency, 1996).
Zebra mussels were used as a biomonitor to evaluate organotin
pollution in Dutch fresh waters (Staeb et al., 1995). High
concentrations (1700-3200 ng tin/g dry weight) were found near
locations where triphenyltin fungicide had been sprayed. Degradation
products (di- and monophenyltins) were also detected in nearly all
mussels.
In pecan orchards (Georgia, USA) where triphenyltin fungicides
were sprayed, triphenyltin concentrations in foliage and soils were
8.5-37 µg/g dry weight and 1.2-12 µg/g dry weight, respectively
(Kannan & Lee, 1996). Although triphenyltin was absent in surface soil
where the fungicide had been sprayed 8-10 times a year until 2 years
earlier, monophenyltin was detected at approximately the same
concentration as in recently sprayed orchards. Fish (bluegill
[ Lepomis macrochirus], largemouth bass [ Micropterus salmoides],
and channel catfish [ Ictalurus punctatus]) from a pond near a
recently sprayed orchard contained predominantly monophenyltin (with
the highest concentration of 22 µg/g wet weight in the liver of
catfish) in addition to smaller amounts of triphenyltin and
diphenyltin.
6.2 Human exposure
No data are available on occupational exposure to triphenyltin
compounds. There are also no data on levels of triphenyltin in indoor
or ambient air or in drinking-water.
The residue data available in support of registration of
triphenyltin compounds in the United Kingdom, obtained using various
colorimetric methods, ranged between 0.013 and 0.016 mg/kg in 3 out of
25 samples of potatoes provided by the Potato Marketing Board and
known to have been treated with a triphenyltin fungicide. The
remaining samples contained residues below 0.013 mg/kg, which is the
limit of detection (ACP, 1990). In supervised trials of triphenyltin
formulations (wettable powder; 54%; 216-324 g active ingredient/ha) on
potatoes in Germany, residues ranged from 0.3 mg/kg to less than the
detection limit (0.01 mg/kg) 7 days after application (FAO, 1991a).
Supervised trials of triphenyltin formulations (wettable powder; 50 or
54%; 216-324 g active ingredient/ha) in Germany on sugar beets showed
residues ranging from 0.1 to 1.9 mg/kg in leaves and less than the
detection limit (0.05 mg/kg) in beets 35 days after application. In
supervised trials of triphenyltin formulations (wettable powder) on
rice in the USA, residues ranged from less than the detection limit
(0.01 mg/kg) to 0.03 mg/kg 22-23 days after application (57.5%; 536 g
active ingredient/ha, twice) and from less than the detection limit
(0.01 mg/kg) in milled rice or bran 22-46 days after application
(47.5%; 250 or 500 g active ingredient/ha) (FAO, 1991a).
When 14C-labelled TPTH was administered orally to dairy cows
over a period of 60 days at doses of 1.13, 5.61, or 22.44 mg
triphenyltin/kg diet (dry matter), residues were 0.08, 0.31, and 0.9
g/kg in meat and 0.006, 0.026, and 0.41 mg/kg in milk, corresponding
to transfer factors of 0.038-0.068 in meat and 0.004-0.006 in milk
(Smith, 1981).
Triphenyltin levels were measured in fish, clams, and shrimps
obtained from the Tokyo Central Fish Wholesale Market from April 1988
to March 1991. Levels were higher in cultured fish and in fish from
coastal or bay areas than in pelagic fish (mean concentration 0.048
µg/g) (Takeuchi et al., 1991). Freshwater fish were relatively
uncontaminated. Fish obtained from bay or inshore areas were the most
contaminated; the highest concentration measured in 82 samples of four
fish species was >1.0 µg triphenyltin/g muscle (mean 0.317 µg/g).
Triphenyltin levels in clams and shrimps ranged from 0 to 0.83 µg/g
edible portion (mean 0.113 µg/g). Triphenyltin intake from pelagic
fish was estimated based on analyses of fish samples in 1988-1991 by a
market basket study in Tokyo as 3.15 µg (mean concentration of 0.048
µg/g times 65.6 g intake of pelagic fish by a Japanese person per
day). Although tributyltin was used more abundantly than triphenyltin
in antifouling paints, residue levels in fish and shellfish were
mostly comparable, with several differences among fish groups.
National market basket studies, including the above study, have
estimated daily intakes of triphenyltin per 50-kg person in Japan
(expressed as TPTCl) to be 4.3, 10.4, 2.7, 0.6, 1.2, 1.4, 0.7, and 2.7
µg in 1990, 1991, 1992, 1993, 1994, 1995, 1996, and 1997, respectively
(NIHS, 1998). Triphenyltin compounds were found mostly in seafood. As
about a twofold difference was observed between the above estimated
daily intakes (averages of 10 local laboratories, including the Shiga
Prefecture) and estimated intakes in the Shiga Prefecture (Tsuda et
al., 1995), this implies that differences in food intake patterns or
some other factor may influence estimates of daily intake. This fact
and coincidental contamination with tributyltin must be taken into
account in any risk estimation for exposure by the oral route.
Another report of a market basket survey estimated the intake of
triphenyltin from raw and processed seafood in Nagasaki Prefecture (a
southern part of Japan) in 1989-1991 to be 8.51 µg/day per person
(Baba et al., 1991). TPTCl concentrations in fish, shellfish, seaweed,
canned fish/shellfish, fish paste product, and salted/dried fish were
274, 80, 21, 12, 16, and 22 ng/g (averages), respectively. Cooking did
not reduce the triphenyltin content of fish and shellfish samples.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
Several studies have shown that TPTH orally administered to rats
is eliminated mainly via the faeces, with smaller amounts in the
urine. Metabolites found in faeces included di- and monophenyltin as
well as a significant portion of non-extractable bound residues (the
sulfate conjugates of hydroquinone, catechol, and phenol). In faeces,
the major substance present was unchanged parent compound.
TPTA was rapidly and completely hydrolysed to TPTH at pH 3-8 and
23-24°C (Beurkle, 1985).
Seven days after oral administration to rats, TPTH residues
(approximately 3% of the administered dose) were distributed mainly in
the kidneys, followed by liver, brain, and heart (Eckert et al., 1989;
Kellner & Eckert, 1989). Similar results were obtained after chronic
exposure for 104 weeks (Dorn & Werner, 1989; Tennekes et al., 1989a).
Species differences in the metabolism of triphenyltin were
investigated by Ohhira & Matsui (1996). Dearylation of triphenyltin
was slower in hamsters than in rats, and pancreatic accumulation of
triphenyltin was higher in hamsters. There was a good correlation
between tin concentrations in the pancreas and plasma glucose levels,
indicating that triphenyltin-induced hyperglycaemia depends upon the
amount of tin compounds absorbed into the pancreas. Most of the tin
compounds in the brains of both species were triphenyltin.
Percutaneously absorbed TPTA in guinea-pigs was distributed to
the highest extent in the liver, followed by the adrenal glands,
kidneys, brain, spinal cord, and pancreas (Nagamatsu et al., 1978).
Triphenyltin, diphenyltin, and monophenyltin were detected in faeces
in a ratio of 15:6:2. The biological half-life of triphenyltin was
estimated to be 9.4 days.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Because TPTA and TPTCl are hydrolysed rapidly to TPTH in aqueous
media, the results of oral toxicity studies with these triphenyltin
compounds can be applied to TPTH. Many critical studies described
below were cited from WHO (1992), which summarizes evaluations of the
original proprietary reports; as details of these original proprietary
reports were not available to the CICAD authors, we have relied on the
WHO evaluation for these studies.
8.1 Single exposure
After a single oral administration of triphenyltin, toxic signs
observed in various species include anorexia, emesis, tremor, and
diarrhoea, followed by drowsiness and ataxia (WHO, 1992). No further
details were provided. Clinical signs appeared a day after
administration and were exacerbated over the following 3 days or so
(CICAD National Committee, 1997). Oral LD50s for TPTH were
approximately 160 mg/kg body weight in rats and 100-245 mg/kg body
weight in mice; oral LD50s for TPTA were 140-298 mg/kg body weight in
rats and 81-93 mg/kg body weight in mice (Ueda & Iijima, 1961; Scholz
& Weigand, 1969; Hollander & Weigand, 1974; Ikeda, 1977; Leist &
Weigand, 1981a,b).
Dermal LD50s were 127 mg/kg body weight in rabbits and 1600
mg/kg body weight in rats for TPTH (Leist & Weigand, 1981c,d) and 350
mg/kg body weight in mice and >2000 mg/kg body weight in rats for
TPTA (Ueda & Iijima, 1961; Diehl & Leist, 1986a). Inhalation LC50s
were 44-69 mg/m3 in rats for TPTH and TPTA (Hollander & Weigand,
1981, 1986).
8.2 Irritation and sensitization
TPTA was not irritating to the skin of rabbits (Diehl & Leist,
1986b). However, severe ocular lesions developed in rabbits; these
were not reversible (Diehl & Leist, 1986c).
At concentrations that were irritating to skin, TPTH (purity
97.0%) showed no skin sensitization in guinea-pigs in the Buehler test
(Leist & Weigand, 1981e; Schollmeier & Leist, 1989) or in the
maximization test (Diehl & Leist, 1987). TPTA gave a positive response
when tested for skin sensitization in guinea-pigs in the Buehler test
(Diehl & Leist, 1986d). Further details were not given.
8.3 Short-term exposure
One unpublished dermal exposure study in rats submitted to WHO
(1992) is described in Table 2. A NOAEL of 10 mg/kg body weight was
identified in this study. Additional short-term studies that examined
effects on the immune system are discussed in section 8.7.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Several subchronic studies on the effects of TPTH on several
animal species exposed by various routes have been performed (WHO,
1992). Studies that show effects at the lowest doses in each animal
species are summarized in Table 2. Further studies and details are
available in WHO (1992) and CICAD National Committee (1997).
Dietary studies with rats, mice, and dogs showed a decrease in
immunoglobulin levels, body weight gain, and white blood cells and an
increase in liver weights and death. Decreases in immunoglobulin
levels and white blood cells were observed consistently at lowest
effect levels in all tests. NOAELs for dietary studies were identified
as 3.4-4.1 mg/kg body weight per day in mice (3-month exposure),
0.30-0.35 mg/kg body weight per day in rats (13-week exposure), and
0.21 mg/kg body weight per day in dogs (52-week exposure) (Table 2).
Several species were affected in a similar way, although mice appeared
to be least sensitive.
In an inhalation study in rats exposed to TPTH, macroscopic
lesions in the lungs were observed at 2.0 mg/m3 in most of the dead
rats (all males and one female died at this dose), and histopathology
revealed severe effects in lower air passages and in the lungs. The
NOAEL was 0.014 mg/m3.
8.4.2 Chronic exposure and carcinogenicity
Chronic toxicity/carcinogenicity studies in rats and mice exposed
to TPTH (WHO, 1992) are summarized in Table 3. There were indications
that the doses received by animals in the US National Toxicology
Program (NTP) studies may have been lower than intended owing to
instability of the test compound in the diet. The size of the control
group (small compared with test groups) in the NTP mouse study limits
interpretation of the results. Consistent findings in all studies are
a decrease in immunoglobulin concentrations at lowest effect levels
and a higher susceptibility of females, as seen in a higher mortality
rate and reduced body weight gain at low doses.
Effects on immunoglobulin levels were reported with both rats and
mice. In an 80-week feeding study in mice, a decrease in
immunoglobulin concentrations was seen at dose levels of 5, 20, and 80
ppm TPTH in the diet. The incidence of hepatocellular adenoma in both
sexes and the incidence of hepatocellular carcinoma in females only
were increased at the highest dose (80 ppm) (Tennekes et al., 1989a;
Table 3). The NOAEL in this study was 5 ppm, equivalent to 0.85 mg/kg
body weight per day for males and 1.36 mg/kg body weight per day for
females, based on decreased body weight gain in females.
Table 2: Short-term and subchronic studies on TPTH.
Study type, Dose Species Observed effects (mostly at lowest effect Reference
duration (purity; %) (strain, number/dose) level) and NOAEL values
Diet 0, 4, 20, 100 ppm Mouse At 100 ppm, haematological and biochemical Suter & Horst,
3 months (97.2%) (NMRI, 10/group) parameters were affected, including a reduction 1986a
in erythrocyte count and haemoglobin level,
an increase in platelet count, and a decrease
in IgG, IgA, and IgM (females only). Liver
weight was increased in both sexes, and
relative weights of ovaries, adrenals, kidneys,
heart, and brain were decreased in females at
this dose. NOAEL: 20 ppm (3.4 mg/kg body weight
per day for males, 4.1 mg/kg body weight per day
for females).
Diet 0, 4, 20, 100 ppm Rat Relative testis weight was significantly higher in Suter & Horst,
13 weeks, (97.2%) (Wistar, 15/group) high-dose males, whereas no effects on spleen and 1986b
4-week thymus weights were observed. In females, white blood
recovery cells decreased at 20 ppm (corresponding to 1.75 mg/kg
body weight per day) and 100 ppm. After the recovery
period, IgG decreased significantly in females at
all dose levels. NOAEL: 4 ppm (0.30 mg/kg body
weight per day for males, 0.35 mg/kg body weight per
day for females).
Diet 0, 2, 6, 18 ppm Dog At 18 ppm, relative liver weight was increased in Sachose et al., 1987
52 weeks (97.2%) (beagle, 10/group) females and serum albumin/globulin ratio was
decreased in males. NOAEL: 6 ppm (0.21 mg/kg body
weight per day).
Table 2 (continued)
Study type, Dose Species Observed effects (mostly at lowest effect Reference
duration (purity; %) (strain, number/dose) level) and NOAEL values
Dermal 0, 5, 10, 20 Rat Dose-related increase in erythema and scale Leist, 1988
21 applications mg/kg body (unknown, formation was seen. At 20 mg/kg body weight, four
in 29 days, weight each 6-12/group) rats died. Lymphocytes decreased in both sexes and
14-day recovery time (97.1%) monocytes increased in females at 20 mg/kg body
weight. NOAEL for systemic toxicity: 10 mg/kg body
weight.
Inhalation 0.014, 0.338, Rat All males and one female died at the highest dose. At Duchosal et
6 h/day, 1.997 mg/m3, (Wistar, 0.338 mg/m3, a decrease in white blood cells and al., 1989
5 days/week, nose-only 10/group) biochemical and haematological changes were seen in
13 weeks, exposurea (96.2%) females. IgM increase was seen in males at 0.338
4-week recovery mg/m3. Histopathology revealed degenerative and
inflammatory lesions in the anterior part of the nasal
cavity, in the trachea, and in the lungs in the
highest dose group of both sexes. NOAEL: 0.014 mg/m3.
a Mass median aerodynamic diameter: 3 µm.
In a 2-year rat feeding study with dietary concentrations of 0,
5, 20, and 80 ppm TPTH (Tennekes et al., 1989b), a decrease in
immunoglobulin concentrations was observed in all triphenyltin-dosed
groups. An increase in the incidence of pituitary adenoma in females
and an increase in testicular Leydig cell tumours at higher doses were
accompanied by non-neoplastic lesions in these organs. Low survival at
higher doses limits interpretation of the results in females. A NOAEL
could not be established at the lowest concentration of 5 ppm
(equivalent to 0.3 and 0.4 mg/kg body weight per day in males and
females, respectively) because of increased mortality in females and
reduced serum immunoglobulin levels at this dose.
Although some tumours were detected in the above studies, the WHO
expert group apparently evaluated those as being not significant (WHO,
1992). No detailed explanation of the reasons for this or results of
statistical analysis were provided in the report. Recently, Clegg et
al. (1997) critically examined the human relevance of Leydig cell
hyperplasia and adenoma formation in rodents after chronic exposure
and suggested that a hormonal mode of action, which may be of little
relevance to humans, either mechanistically or quantitatively, could
be operating. They also pointed out the very low incidence of Leydig
cell adenomas in humans (age-adjusted occurrence of 0.4 per million).
An early 2-year rat study showed a reduction in white blood cell
count at a TPTH dietary level of 5 ppm (corresponding to 0.3 mg/kg
body weight per day) (Til et al., 1970). The NOEL was chosen as 2 ppm
in the diet (equivalent to 0.1 mg/kg body weight per day) based on
this finding.
8.5 Genotoxicity and related end-points
Most in vitro and in vivo genotoxicity tests, such as the
Salmonella mutagenicity test, yeast forward mutation test, mitotic
gene conversion assay, mouse lymphoma forward mutation assay,
chromosomal aberration assay, unscheduled DNA synthesis, micronucleus
test in mice, cytogenetic assay in Chinese hamster, and dominant
lethal assay in rats, showed negative results at the maximum doses
tested, based on studies reviewed in WHO (1992).
There are no new data that impact on the conclusion in WHO (1992)
that triphenyltin is not genotoxic. Recent data indicate, however,
that triphenyltin potentiates the genotoxicity of other substances.
Triphenyltin showed potentiation of mitomycin C-induced breakage-type
chromosomal aberrations in cultured hamster cells when cells were
treated during the G2 phase (Sasaki et al., 1993). Similarly, the
frequency of micronuclei induction by mitomycin C (1 mg/kg
intraperitoneal injection) in mouse peripheral reticulocytes was
enhanced by treatment with TPTCl, although TPTCl itself did not induce
micronuclei (Yamada & Sasaki, 1993). Positive responses in these
assays may be related to the toxic effects of triphenyltin on
lymphocytes, because two in vivo studies for chromosomal aberrations
(a micronucleus test in mice and a cytogenetic test in Chinese
hamsters) were negative. These data support the conclusion of the WHO
(1992) group.
It is therefore concluded that triphenyltin does not present a
genotoxic hazard.
8.6 Reproductive and developmental toxicity
Triphenyltin appears to cause reproductive effects in rats and
developmental toxicity in rats, rabbits, and hamsters at low doses
(around 1 mg/kg body weight and higher) at which maternal toxicity is
observed. Studies that show effects at the lowest doses for various
end-points in experimental animals are summarized in Table 4 (WHO,
1992; CICAD National Committee, 1997). Decreases in number of
implantations, live fetuses, and mean fetal weight and increases in
resorption were the consistent findings observed at lowest effect
doses.
An increase in the number of dead F1 pups and a decrease in mean
litter size, pup weight, and relative spleen and thymus weights in the
weanlings were observed in a two-generation rat study at 18.5 ppm TPTH
in the diet (approximately 1.5 mg/kg body weight per day); at this
concentration, body weight gain and food consumption of the parents
were not affected (Young, 1986). The NOAEL in this study was 5 ppm
(equivalent to 0.4 mg/kg body weight per day).
A general paucity of mature sperm was seen in rats treated with
TPTA and TPTCl in the diet (20 mg/kg body weight per day) for 20 days,
and spermatogenic anomalies were observed in histological sections
(Snow & Hays, 1983). Reduced food intake and the resultant decrease in
weight gain were suspected as the cause, as malnutrition is known to
precede gonadal dysfunction and even atrophy. However, differences in
the distribution of spermatogenic phases in rats treated with TPTA and
TPTCl do not support this explanation.
TPTCl prevented implantation in rats in a dose-dependent manner
when administered at 0, 3.1, 4.7, or 6.3 mg/kg body weight per day on
days 0-3 and at 0, 6.3, 12.5, or 25.0 mg/kg body weight per day on
days 4-6. The compound caused larger implantation failures when
administered during earlier stages of blastogenesis (Ema et al.,
1997). Implantation failure was observed at 4.7 and 6.3 mg/kg body
weight per day on days 0-3 and at 12.5 and 25.0 mg/kg body weight per
day on days 4-6. The effects of TPTCl on uterine function, as a cause
of implantation failure, were determined using pseudopregnant rats
dosed at 0, 3.1, 4.7, or 6.3 mg/kg body weight per day on days 0-3
(Ema et al., 1998). A significant suppression of the uterine
decidualization and decrease in the serum progesterone levels were
Table 3: Chronic exposure and carcinogenicity studies on TPTH.
Duration Chemical dose Species Effects, NOAEL Reference
(purity; %) (strain,
number/dose)
78 weeks with 0, 37.5, 75 ppm Mouse No treatment-related effects were seen in NTP, 1978
26-week (not stated) (B6C3F1, clinical signs or body weight, although survival
observation After 1 week, 50/group) decreased in females with increased dose. No
period only 57.9% of tumour incidence was found histopathologically.
the initial dose The size of the control group (20 mice of
recovered each sex) limits interpretation of the results.
80 weeks 0, 5, 20, 80 ppm Mouse (KFM-Han, Body weight gain decreased at 80 ppm for males and Tennekes et
(97.2% prepared NMRI, 50/group) at 20 and 80 ppm for females. Decreases in al., 1989a
daily) immunoglobulin concentrations were observed at
various levels. An increased relative number of
lymphoid cells was detected in femoral bone
marrow myelogram for all treated groups.
Incidence of hepatocellular adenomas was 12.2,
20, 26, and 32% at 0, 5, 20, and 80 ppm,
respectively, for males and 0, 0, 0, and 18%
at 0, 5, 20, and 80 ppm, respectively, for
females. At 80 ppm, increased incidence of
hepatocellular carcinoma was seen in females
(6% compared with 0% at other doses). Based on
reduced body weight gain, NOAEL was 5 ppm
(corresponding to 0.85 mg/kg body weight per day
for males and 1.36 mg/kg body weight per day for
females).
Table 3 (continued)
Duration Chemical dose Species Effects, NOAEL Reference
(purity; %) (strain,
number/dose)
2 years 0, 0.5, 1, 2, 5, Rat (not stated, A slight decrease in white blood cells was seen Til et
10 ppm (not stated) 25/group) at the highest dose in the first year. This effect al., 1970
was less often seen at 5 ppm (corresponding to
0.3 mg/kg body weight per day), and only once at
2 ppm in the males. The relative thyroid weight
was slightly decreased at 10 ppm in the females
only. Average relative weights of other organs,
gross autopsy findings, and microscopic
examinations did not reveal significant differences
between treated and control groups. NOEL was
2 ppm in the diet, equivalent to 0.1 mg/kg body
weight per day.
78 weeks with 0, 37.5, 75 ppm Rat (Fischer 344, No effects were observed on clinical signs, NTP, 1978
26-week (not stated) 50/group) mortality, food consumption, macroscopy, or
observation After 1 week, only histopathology. No increase in tumour incidence.
period 57.9% of the initial
dose recovered
104 weeks 0, 5, 20, 80 ppm Rat (SPF KFM-Han In females, mortality was increased (survival Tennekes et
prepared twice Wistar, 70/group) was 75, 51, 36, and 23%, respectively, with al., 1989b
monthly from frozen increasing dose). Immunoglobulin decrease
stock (IgG1 and IgG2a for females, and IgG2c for
males) was observed at all doses. IgA levels
for males decreased, and IgM levels increased
for both sexes at 20 and 80 ppm. Leydig cell
tumours were 1.7, 8.5, 5.0, and 16.7% at 0, 5,
20, and 80 ppm, respectively. The incidence of
pituitary adenomas was increased in females at
20 and 80 ppm. These changes were accompanied by
non-neoplastic lesions in the pituitary and
testis. NOAEL was not established because of
observations of mortality increase in females and
Table 3 (continued)
Duration Chemical dose Species Effects, NOAEL Reference
(purity; %) (strain,
number/dose)
104 weeks serum immunoglobulin decrease at the lowest level,
(continued) 5 ppm (equal to 0.3 mg/kg body weight per day for
males and 0.4 mg/kg body weight per day for females).
Table 4. Reproductive and developmental toxicity studies on triphenyltin.
Species Study design Effects Reference
(strain,
number/sex/dose)
Rat In a two-generation reproduction study, rats The number of dead F1 pups was Young, 1986
(Wistar, were given TPTH in diet (0, 5, 18.5, or 50 increased and mean litter size
30/sex/group) ppm) during growth, mating, gestation, and decreased at 18.5 and 50 ppm. In
lactation for one litter per generation. Fo parents, the body weight gains
Clinical signs, body weight, food consumption, and food consumption of both sexes
mating performance, and reproductive parameters were lower at 50 ppm. At 50 ppm, the
were observed. Organ weights of parents relative weights of brain, testes,
and pups were recorded. Pups were ovaries, adrenals, kidneys, spleen,
sexed and examined for gross malformations and heart were increased in F0
and the number of stillborn and live pups. and/or in F1 adults and/or F1 and F2
weanlings. A dose-related decrease was
observed in spleen and thymus weight
in F1 and F2 weanlings at 50 and 18.5
ppm (equal to 1.5 mg/kg body weight
per day). The NOAEL was 5 ppm, equal to
0.4 mg/kg body weight per day.
Table 4 (continued)
Species Study design Effects Reference
(strain,
number/sex/dose)
Rat TPTA or TPTCl (0 or 20 mg/kg body weight per In rats sacrificed after 21 days, all Snow & Hays,
(Holtzman, day in diet) was dosed for 20 days. Four eight spermatogenic phases were seen, 1983
13 males/group) animals from each group were sacrificed on but there was a general paucity of mature
day 21, and the remaining animals were sperm, and the distribution showed some
sacrificed after 4 more days with test diets predominance of immature sperm. Recovery
and a recovery period (70 days). Distribution was seen after the 70-day control diet.
of the eight phases of spermatogenesis was Treated animals ate about two-thirds as
observed. much food as the controls.
Pregnant rat TPTCl was dosed by gavage at 0, 3.1, 4.7, or In successfully mated females, TPTCl Ema et al.,
(Wistar, 6.3 mg/kg body weight per day on day 0 to day prevented implantation in a dose-dependent 1997
10-13/group) 3 of gestation or at 0, 6.3, 12.5 or 25.0 mg/kg manner. The pregnancy rate was significantly
body weight per day on day 4 to day 6 of decreased after administration of TPTCl on
gestation. Dams were sacrificed on day 20 of days 0 to 3 at 4.7 and 6.3 mg/kg body weight
gestation. Numbers of live/dead fetuses and per day, and days 4 to 6 at 12.5 and 25.0
resorptions were counted. Live fetuses were mg/kg body weight per day. TPTCl caused larger
sexed, weighed, and inspected for malformations failures in implantations when administered
externally. during earlier stages of blastogenesis.
Pregnant rat TPTH was dosed at 0, 0.35, 1, 2.8, or 8 A dose-related decrease in body weight gain Rodwell,
(Sprague-Dawley, mg/kg body weight per day on day 6 through and food consumption was seen in the 2.8 and 1985
45/group) day 15 of gestation. On day 20 of gestation, 8 mg/kg body weight per day groups. At 8
all rats were sacrificed. Clinical signs, body mg/kg body weight per day, an abortion in one
weights, and food consumption were examined. dam, increase of number of non-gravid dams,
After sacrifice, the dams were observed for total litter resorptions, early resorptions,
number and location of viable and non-viable and significant decrease of number of viable
fetuses, early and late resorptions, and the fetuses and fetal weight were observed. The
number of implantation sites. The corpora lutea incidence of absent/delayed ossification was
were counted. Fetuses were weighted, sexed, and increased in high-dose litters. The percentage
examined for external, internal, and skeletal of fetuses with hydrocephaly was 0.4, 0, 0,
anomalies. 0.4, and 1%, and with omphalocele 0.2, 0.2, 0.2
0, and 0.5%, respectively, for the 0, 0.35, 1,
Table 4 (continued)
Species Study design Effects Reference
(strain,
number/sex/dose)
2.8, and 8 mg/kg body weight per day groups.
There was no evidence for TPTH-induced
irreversible structural effects. The NOAEL for
maternal toxicity was 1 mg/kg body weight per
day, and for embrytoxicity, 2.8 mg/kg body weight
per day.
Pregnant hamster TPTH was dosed by gavage (0, 2.25, 5.08, or The 12 mg/kg body weight per day group showed Carlton &
(Syrian, 12 mg/kg body weight per day) from day 5 to a decrease in mean body weight gain, food Howard, 1982
20-25/group) day 14. All dams were sacrificed on gestation consumption, pup weight, and death (4 animals).
day 15. The gravid uterus was weighed, and Two animals died in each of the 2.25 and 5.08
corpora lutea were counted. Fetuses and mg/kg body weight per day groups. The average
resorption sites were noted. Fetuses were number of minor anomalies of fetuses per litter
weighed and observed for external, visceral, and delayed ossifications were significantly
and skeletal malformations. greater among the 12 mg/kg body weight per day
group. Three cases of hydronephrosis were seen
at 5.08 mg/kg body weight per day and one case
of hydrocephalus was seen at 12 mg/kg body weight
per day.
Pregnant TPTA (0, 0.1, 0.32, or 1.0 mg/kg body weight In the 1.0 mg/kg body weight per day Baeder,
rabbit per day) was dosed by gavage from day 6 to group, one dam died, three dams aborted, 1987
(Himalayan, day 18 of gestation. On day 29 of gestation, one dam gave a premature delivery, and
15/group) the dams were sacrificed. Dams were observed two dams had intrauterine deaths. The
for clinical signs, body weight, food number of implantations and of live
consumption, number of resorptions, fetuses decreased at 1.0 mg/kg body
implantations, corpora lutea, viable and weight per day. Mean fetal weight,
non-viable tissues, organ weights, and crown/rump length, and placental weight
macroscopy. Fetuses were weighed and examined decreased in pups at 1.0 mg/kg body weight
for sex, length, and external, internal, and per day. At 1.0 mg/kg body weight per day,
skeletal anomalies. four pups showed omphalocele with protrusion
Table 4 (continued)
Species Study design Effects Reference
(strain,
number/sex/dose)
of intestinal coils or liver tissue. Slight
retardation of skeletal ossification was
detected at 1.0 mg/kg body weight per day.
An increase in the number of fetuses with
fewer ossified caudal vertebrae, weak
ossification of the hyoid bone, and non-/only
slight ossification of the os pubis in some
fetuses were shown. NOAEL for maternal and
embryo toxicity was 0.32 mg/kg body weight
per day.
Pregnant TPTH was dosed by gavage at 0, 0.1, 0.3, or Two rabbits from the 0.9 mg/kg body weight Rodwell,
rabbit 0.9 mg/kg body weight per day on day 6 to day per day group aborted. A dose-related decrease 1987
(New Zealand 18 of gestation. Dams were sacrificed on day 29 in mean body weight gain and food consumption
white, 22/group) of gestation. Corpora lutea, early/late was observed in the 0.3 and 0.9 mg/kg body
resorptions, and number of implantations were weight per day groups. Mean fetal weight was
counted. Fetuses were weighed, sexed, and lower in the 0.9 mg/kg body weight per day
examined for external, skeletal, and visceral group. The NOAEL for maternal toxicity was
anomalies and developmental variations. 0.1 mg/kg body weight per day, and the NOAEL
for embryotoxicity was 0.3 mg/kg body weight
per day.
found at 4.7 and 6.3 mg/kg body weight per day, at which doses
implantation failure was caused in pregnant rats. These findings
suggest that implantation failure due to TPTCl may be mediated via the
suppression of uterine decidualization correlated with the reduction
in serum progesterone levels.
In hamsters administered TPTH by gavage where death was observed
(2.25 mg/kg body weight per day and higher), anomalies such as
hydronephrosis, hydrocephalus, and delayed ossification were detected
in the pups at 5.08 mg/kg body weight per day and higher (Carlton &
Howard, 1982). Although delayed ossification was observed in rabbits,
which are the most sensitive species, when TPTA was dosed at 1.0 mg/kg
body weight per day by gavage during gestation days 6 through 18,
maternal effects were also detected at this dose level (Baeder, 1987).
The percentages of fetuses with hydrocephaly and omphalocele were not
significantly higher in rats dosed with TPTH (0-8 mg/kg body weight
per day on gestation days 6-15), and it was concluded that there was
no evidence for TPTH-induced irreversible structural effects in rats
(Rodwell, 1985).
The lowest NOAEL for maternal toxicity was seen in rabbits -- 0.1
mg/kg body weight per day, above which dose reductions in body weight
gain and food consumption were observed. The lowest NOAEL for
embryo-toxicity in rabbits was 0.3 mg/kg body weight per day, above
which abortion and a decrease in mean fetal weight were observed
(Rodwell, 1987).
8.7 Immunological and neurological effects
Effects on the immune system were observed in short-term as well
as long-term toxicity studies (WHO, 1992; CICAD National Committee,
1997). Effects of organotin compounds on lymphoid organs and lymphoid
functions were reviewed (Penninks et al., 1990). Like other organotin
compounds, triphenyltin showed immunosuppressive properties
(lymphopenia and a decrease in spleen and thymus weights), resulting
in altered humoral and cellular immunity in rats, mice, and
guinea-pigs, although effects were usually less severe than those
observed with tributyltin.
When weanling male SPF Wistar rats were fed diets containing
TPTCl at 0, 15, 50, or 150 ppm for 2 weeks, thymus weight was
decreased at 15 ppm (corresponding to 1.5 mg/kg body weight per day)
or above, and spleen weight was decreased dose dependently (Snoeij et
al., 1985). At 150 ppm, decreases in body weight and brain weight were
seen, and the liver was enlarged. The effects of TPTCl were similar to
those of tributyltin chloride or tripropyltin chloride in a parallel
test, but less severe.
Groups of mice were given 0, 1, 5, 25, 50, or 125 ppm TPTH in the
diet for 28 days. Twelve male and 12 female mice were killed on day
29, and the remaining mice were returned to control diets and killed
on day 57. A significantly decreased body weight gain was observed in
male and female mice at 125 ppm, from which they recovered after 28
days. Food consumption was significantly decreased at 50 and 125 ppm.
Relative liver weight was increased at 25 (females only), 50, and 125
ppm, relative spleen weight was clearly decreased in males at 50 and
125 ppm and in females at 25 ppm (corresponding to 5 mg/kg body weight
per day) and higher, and relative thymus weight was decreased at
125 ppm in males. At histopathology, lymphoid depletion in the thymus
and spleen was observed in mice at 125 ppm. A decrease in total white
blood cells, neutrophils, and lymphocytes at 50 and 125 ppm in males
and females was noted. At the highest dose, a decrease in total cells
in spleen and splenic B-cells was observed, and a decrease in total
cells in thymus and splenic T-cells was seen in males. IgM levels were
decreased in females at 25 ppm and higher, but the decrease was not
clearly dose related. All effects were reversible. The NOAEL was 5
ppm, equal to 1 mg/kg body weight per day for males and 1.15 mg/kg
body weight per day for females (MacCormick & Thomas, 1990).
When triphenyltin was injected intraperitoneally into mice at
doses of 0, 1, 3, or 10 mg/kg body weight per day for 14 days, it
inhibited the T-cell-dependent humoral (IgM and IgE production) and
cellular (induction of cytotoxic T-cell or induction of delayed
hypersensitivity) immune response at 3 mg/kg body weight per day and
above (Nishida et al., 1990).
In a long-term study with female guinea-pigs fed 15 ppm TPTA in
the diet (corresponding to approximately 1.5 mg/kg body weight per
day), decreases in thymus weight and in the number of plasma cells of
the spleen and lymph nodes were seen in guinea-pigs examined on days
47 and 77. Repeated dosing for 104 days inhibited the immunological
reaction against tetanus toxoids (Verschuuren et al., 1970). The dosed
group had a lower antibody count and fewer antitoxoid-producing cells
at the popliteal fossa than the controls when examined
immunohistologically.
Triphenyltin showed relatively slight neurotoxicological effects
at relatively high doses compared with other trialkyltin compounds
(i.e., triethyltin, trimethyltin, tributyltin, tripropyltin) (Bouldin
et al., 1981; Wada et al., 1982). In neonatal rats dosed orally with
30 mg TPTA/kg body weight per day from day 3 to day 30, no light
microscopic or electron microscopic changes were observed in the
hippocampus or pyriform cortex/lobe, which are susceptible to neuronal
necrosis with trimethyltin (Bouldin et al., 1981). In addition,
triphenyltin did not cause oedema in the myelin sheath, as was usually
induced by triethyltin (Bouldin et al., 1981).
In the maze learning test, rats orally given Tinestan (a product
containing 60% TPTA) at doses of 0.6 (corresponding to 0.36 mg TPTA/kg
body weight per day) or 6 mg/kg body weight per day, 6 days/week for 6
weeks, made many mistakes and showed slow reaction speed (Lehotzky et
al., 1982). In the conditioned avoidance response test, no difference
was observed between the dose groups and the control group; however,
extinction of behaviour was delayed in the high-dose group (6 mg/kg
body weight per day) after discontinuation of the stimulus. Resting
time during swimming tests was shortened by treatment with
amphetamine; in rats given 23 mg Tinestan/kg body weight per day for
20 days, however, amphetamine-induced hyperkinesis was antagonized on
the 20th day. Tin levels in the brain tissues increased in some of the
rats after administration of triphenyltin.
8.8 Mode of action
Treatment of rat thymocytes with immunotoxic organotins (TPTCl,
tributyltin, dibutyltin) at 5 µmol/litre, but not non-immunotoxic
organotins (trimethyltin, triethyltin), caused a rapid decrease in the
F-actin content, resulting in the depolymerization of thymocyte
F-actin (Chow & Orrenius, 1994). Immunotoxic effects of organotin
compounds may involve cytoskeletal modification in addition to the
perturbation of thymocyte calcium homeostasis.
Triphenyltin at concentrations of 0.5-10 µmol/litre induced
calcium overload in rat pheochromocytoma cells, which caused
internucleosomal DNA cleavage typical of apoptotic cell death (Viviani
et al., 1995). Triethyltin or trimethyltin, which did not modify cell
viability, did not enhance or showed little effect on calcium influx.
Triphenyltin induced calcium release in ruthenium red (a calcium
release channel blocker) sensitive and insensitive ways, with EC50
values of 75 and 270 µmol/litre, respectively. The Ca2+-ATPase
activity and calcium uptake of sarcoplasmic reticulum were also
inhibited by triphenyltin. The study suggested that the internal
calcium store of skeletal muscle could be depleted by triphenyltin
through the inhibition of calcium uptake and the induction of calcium
release by acting on the Ca2+-ATPase and calcium release channel.
Development of muscle weakness in organotin intoxication could be
partly explained by this peripheral myopathy-related finding (Kang et
al., 1997).
Oral administration of a single dose of TPTCl (60 mg/kg body
weight) induced diabetes with decreased insulin secretion in hamsters
after 2-3 days, without morphological changes in pancreatic islets.
Administration of TPTCl strongly inhibited a rise in cytoplasmic
calcium concentration induced by 27.8 mmol glucose/litre, 100 µmol
acetylcholine/litre in the presence of 5.5 mmol glucose/litre, and 100
nmol gastric inhibitory polypeptide/litre in the presence of 5.5 mmol
glucose/litre. TPTCl administration impaired the insulin secretion in
islet cells induced by 27.8 mmol glucose/litre, 100 nmol gastric
inhibitory polypeptide/litre in the presence of 5.5 mmol
glucose/litre, and 100 µmol acetylcholine/litre in the presence of
5.5 mmol glucose/litre. The pathology of triphenyltin-induced diabetes
in hamsters involves a defect in cellular calcium response due to a
reduced calcium influx through voltage-gated calcium channels (Miura
et al., 1997).
9. EFFECTS ON HUMANS
Major complaints concerning toxic effects experienced during the
spraying of TPTA formulations involved the central nervous system,
including headache, nausea, vomiting, and photophobia, and were
exacerbated 1 day after exposure.
9.1 Case reports
Two cases of poisoning by TPTA were reported (Manzo et al.,
1981). A patient who inhaled, 5 days before hospitalization, a certain
amount of fungicide powder containing 60% TPTA (Brestan(R)) complained
about dizziness, nausea, and photophobia. He had an episode of sudden
malaise with dizziness and temporary loss of consciousness 1 day
before his visit. He soon recovered; however, he experienced a brief
loss of consciousness, nausea, and vomiting. On admission, general
appearance and physical examination showed no abnormality except for a
mild impairment of body balance. In spite of treatment with various
antiemetics, nausea and photophobia persisted until the 4th day.
Complete recovery was seen 10 days after hospitalization. Another
patient inhaled an unknown amount of Brestan(R) in an aqueous solution
3 h before his visit while spraying that solution onto a rice field.
He noted general malaise, weakness, and dryness of the mouth. At the
time of admission, the subjective symptoms had totally disappeared.
There were no abnormal neurological findings. Severe headache,
weakness, and photophobia appeared on the day following
hospitalization. All these symptoms disappeared on the 4th day after
admission. The mean concentrations of tin in the blood and urine
collected in 24 h during his hospital stay were 48 + 29 ng/ml
(normal value 2 ng/ml or less) and 113 + 20.6 ng/ml (normal range
10-65 ng/ml), respectively.
9.2 Epidemiological studies
Hypersensitivity reaction to a series of 36
triphenyltin-containing pesticide formulations was surveyed among 652
subjects in Italy (Lisi et al., 1987). Among them, 180 were
agricultural and 43 were ex-agricultural workers. Of the 652 subjects,
274 had contact dermatitis, mostly on the hands, and the other 378
were hospitalized for non-allergic skin disorders. Patch tests were
performed on the upper back, and irritant and allergic reactions were
evaluated. Irritant and allergic reactions were seen in 45 of 350
subjects and in 1 out of 350 subjects, respectively, with a patch of
1% TPTH. At 0.5% TPTH, irritant reactions were seen in 5 of 109
subjects, whereas no allergic reactions were seen in any of the 109
subjects. The report showed that TPTH is a moderately strong irritant
among the fungicides used in Italy.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Extensive data exist on the toxicity of triphenyltin compounds to
organisms in the environment (HSE, 1992; CICAD National Committee,
1997). Data that show the most severe effects on typical species are
listed in Tables 5 and 6. Available data show that triphenyltin is
extremely toxic to various species of aquatic organisms, although the
concentrations of triphenyltin that produce toxic effects vary
according to species.
Growth inhibition of yeasts and fungi with TPTCl occurs at 5
µg/litre and above (Hallas & Cooney, 1981).
Reproduction of freshwater algae was inhibited more than 50% at
2-5 µg/litre (Wong et al., 1982). Indigenous algae were more sensitive
than pure cultures. EC50s for inhibition of germination or carbon
fixation of marine and estuarine algae were 0.92-2 µg/litre (Walsh et
al., 1985).
The LC50 in a 96-h exposure for a copepod was 8 µg/litre (Linden
et al., 1979). LC50s in a 48-h exposure for Daphnia magna were
10-200 µg/litre (FAO, 1991a). The NOEC for reproduction in the same
species in a 21-day exposure was 0.1 µg/litre (FAO, 1991a).
One of the most sensitive effects of triphenyltin on organisms in
the environment is imposex (the development of male sex organs in
female gastropods) in rock shells (Japanese gastropods,
Thais clavigera and T. bronni), which supposedly occurs at levels
(1 ng/litre) similar to those that are seen with tributyltin compounds
(Horiguchi et al., 1994). When triphenyltin was injected into rock
shells, it was approximately as strong as tributyltin in promoting
imposex (Horiguchi et al., 1997), although it was less potent than
tributyltin for inducing imposex in Nucella. As imposex is probably
caused by hormonal disturbance, triphenyltin is considered to be an
endocrine disruptor.
Testosterone (500 ng/litre) induces faster and more intensive
imposex development in Nucella lapillus than that induced by
tributyltin. Simultaneous exposure to tributyltin and to the
antiandrogen cyproterone acetate, which suppresses imposex development
completely in N. lapillus and reduces imposex development in Hinia
reticulatus, proves that the imposex-inducing effects of tributyltin
are mediated by an increasing androgen level and are not caused
directly by the organotin compound itself. Furthermore,
tributyltin-induced imposex development can be suppressed in both
snails by adding estrogens to the aqueous medium. These observations
suggest that tributyltin causes an inhibition of the cytochrome
P-450-dependent aromatase system, which catalyses the aromatization of
androgens to estrogens. Artificial inhibition of the cytochrome
P-450-dependent aromatase system using SH 489
(1-methyl-1,4-androstadiene-3,17-dione) as a steroidal aromatase
inhibitor and flavone as a non-steroidal aromatase inhibitor induces
development of imposex in both snails. The same mechanism may apply to
triphenyltin (Bettin et al., 1996).
Field surveys in 1990-1992 and in 1993-1995 in Japan showed 100%
occurrence of imposex in T. clavigera. In surveys from 1992 to 1995,
the incidence of imposex in the common whelk ( Buccinum undatum) was
always greater than 90% (Mensink et al., 1996). Concentrations of
phenyltin compounds (up to 625 ng tin/g dry weight in the organism)
were much higher than those of butyltin compounds.
No NOEC was established for the effects of triphenyltin on
imposex; however, from the above observations, the NOEC can be assumed
to be around 1 ng/litre or lower.
Another sensitive effect of triphenyltin was the inhibition of
arm regeneration in brittle star ( Ophioderma brevispina) at 0.01
µg/litre. Neurotoxicological action of triphenyltin was suggested as
the cause (Walsh et al., 1986).
The 96-h LC50s of triphenyltin in fish were 7.1 µg/litre
(fathead minnow) and higher (Jarvinen et al., 1988). A subchronic
toxicity study with fathead minnow larvae showed the strong toxicity
of triphenyltin, with a 30-day LC50 of 1.5 µg/litre and a 30-day NOEC
of 0.15 µg/litre (LOEC 0.23 µg/litre). The need for studies of
cumulative effects in a full life cycle at lower concentrations was
suggested.
Beginning with yolk sac fry, rainbow trout ( Oncorhynchus
mykiss) was continuously exposed for 110 days to TPTCl at
concentrations of 0.12-15 nmol/litre or to diphenyltin chloride at
160-4000 nmol/litre. Diphenyltin chloride was about 3 orders of
magnitude less toxic than TPTCl. A NOEC of 160 nmol/litre
(corresponding to 60 µg/litre) was established for diphenyltin
chloride, and a NOEC of 0.12 nmol/litre (corresponding to 50 ng/litre)
was established for TPTCl. Histopathological examination revealed
depletion of glycogen in liver cells of both di- and
triphenyltin-exposed fish. At the end of the exposure period,
resistance to infection was examined by an intraperitoneal challenge
with Aeromonas hydrophila, a secondary pathogenic bacterium in fish.
Resistance to bacterial challenge was found to be decreased even at
the lowest-effect concentration of both di- and triphenyltin compounds
(de Vries et al., 1991).
Because thymus reduction, decrease in numbers of lymphocytes, and
inhibition of gonad development in fish species exposed to tributyltin
have been reported, triphenyltin may have similar effects on the
immune and reproductive systems of fish (Shimizu & Kimura, 1992).
Table 5: Acute toxicity to aquatic organisms.
Compound Organism Criterion Levels/remarks Reference
TPTCl Debaryomyces hansenii Minimum inhibitory 5 µg/ml Hallas & Cooney, 1981
(yeast) concentration
TPTCl Ankistrodesmus 4-h IC50 for primary 10 µg/litre, Wong et al., 1982
(freshwater alga) productivity static, 20 °C
TPTCl Skeletonema costatum, EC50 for 0.92 µg/litre Walsh et al., 1985
a major component of carbon fixation 13.8 µg/litre
fouling slimea LC50
TPTH Daphnia magna (water 48-h LC50 10 µg/litre FAO, 1991a
flea)
TPTFb Nitrocra spinipes 96-h LC50 8 µg/litre Linden et al., 1979
(harpacticoid copepod)
TPTH Eight fish species 96-h LC50 Pimephales promelas Javienen et al., 1988
(fathead minnow)
was the most
sensitive species,
7.1 µg/litre
TPTCl Pagrus major 48-h LC50 12.6 µg/litre Yamada & Takayanagi, 1992
(red sea bream)a
a Marine and estuarine species.
b Triphenyltin fluoride.
Table 6: Chronic/subchronic toxicity to aquatic organisms.
Compound Organism Criterion Levels/remarks Reference
TPTCl Natural community of 50% reduction of 2 µg/litre, Wong et al., 1982
freshwater algae reproduction and indigenous algae
primary production more sensitive than
pure cultures
Ankistrodesmus falcatus 85% inhibition of 5 µg/litre
reproduction
TPTH Daphnia magna 21-day NOEC 0.1 µg/litre FAO, 1991a
TPTH Lymnaea stagnalis: 9-day LC100, or 10 µg/litre for Van der Maas et
a freshwater sludge deficiencies in LC100, 2 µg/litre al., 1972
snail growth, mobility, for deficiencies
and embryo development
after 5 weeks of
exposure
TPTCl Thais clavigera Relative penis length Relative penis length Horiguchi et al., 1997
(Japanese rock shell)a in female significantly increased
with injection of 0.1 µg
triphenyltin/g wet
tissue and culture for
30 days
TPTH Pimephales promelas 30-day LC50, NOEC, 1.5, 0.15, and 0.23 Jarvinen et al., 1988
(fathead minnow) larvae and LOEC µg/litre, respectively
a Marine and estuarine species.
10.2 Terrestrial environment
Triphenyltin compounds applied to crops at the recommended dosage
rate did not harm wild animals, birds, or non-target insects (HSE,
1992). The EC50 for honey bees ( Apis mellifera) was many times
higher than that for a range of common pesticides (Eisler, 1989).
LD50s for triphenyltin compounds were 46.5-114 mg/kg body weight
in Japanese quail ( Coturnix japonica) and bobwhite quail ( Colinus
virginianus) and 285-378 mg/kg body weight in mallards ( Anas
platyrhynchos) (Booth et al., 1980; Ebert & Weigand, 1982; Ebert &
Leist, 1987, 1988).
Gavage administration of 2 mg TPTCl/kg body weight to chickens
( Gallus domesticus) from the 19th day after hatching for 10 days
resulted in atrophy of the thymus and the bursa of Fabricius
(Guta-Socaciu et al., 1986).
Female Peking ducks ( Anas platyrhynchos v. domestica)
(30 weeks old) administered 25 mg TPTH/kg body weight per day by
gavage for 4 weeks showed a decrease in body weight, a gradual
decrease in the number of eggs or total lack of egg production, mild
anaemia, enlargement of the spleen, liver, and kidneys, and atrophy
of the reproductive organs (Masoud et al., 1985). Changes to the
spleen, liver, and kidneys reversed within 4 weeks after the end of
the exposure, but the uterine tube and ovaries did not completely
return to normal.
11. EFFECTS EVALUATION
As tributyltin compounds have been used more abundantly and more
extensively than triphenyltin compounds in many locations, and as
tributyltin and triphenyltin compounds have similar effects on humans
and organisms in the environment, risk from exposure to triphenyltin
must be considered together with risk from exposure to tributyltin
(IPCS, 1990; Sekizawa, 1998). There are many uncertainties in the
potential risk posed by triphenyltin and its metabolites and in the
mechanism underlying the immunotoxicological and reproductive effects
caused by these compounds, and further studies on these aspects are
necessary to improve the risk assessment on triphenyltin.
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
No quantitative data on humans are available. In two poisoning
case reports of inhalation exposure to TPTA formulations, neurotoxic
effects appeared to persist for a few days. A moderate level of
irritant action was detected in a patch test study.
Triphenyltin compounds given orally to rats are not readily
absorbed and are excreted primarily in faeces and to a lesser extent
in urine. Triphenyltin compounds are metabolized to diphenyltin,
monophenyltin, and non-extractable bound residues. Absorbed
triphenyltin compounds accumulate to the greatest extent in kidney and
liver and to a smaller degree in other organs. Triphenyltin compounds
applied dermally can penetrate through the skin in a time- and
dose-dependent manner.
Triphenyltin exerts a variety of effects on several animal
species, including effects on the immune system,
reproductive/developmental effects at levels near those that are
maternally toxic (most LOAELs are in the several mg/kg range or
lower), hyperplasia/adenomas in endocrine organs, apoptosis in thymus
cells, calcium release in sarcoplasmic reticulum cells, and eye
irritation. The underlying mechanism of these effects is under
investigation; a common mechanism may explain this toxicity profile.
Health effects observed in laboratory animals and toxicological
criteria for setting guidance values are summarized in Table 7.
Triphenyltin compounds are moderately toxic in acute tests, and the
lowest NOAELs for the oral, dermal, and inhalation routes in
short-term and subchronic studies were 0.21 mg/kg body weight per day
in dog (52-week exposure), 10 mg/kg body weight per day in rat (29-day
exposure), and 0.014 mg/m3 in rat (4-week exposure), respectively.
Triphenyltin is not carcinogenic or genotoxic.
Table 7: Toxicological criteria for setting guidance values for dietary and non-dietary exposure to
triphenyltin compounds.
Type of test Organisms (route of exposure, Results/remarks
duration of test)
Single exposure Rat LD50: 160 mg TPTH/kg body weight
Short-term Dog (oral, 52 weeks), rat NOAEL for oral, dog: 0.21 mg TPTH/kg body
(dermal, 29 days), rat weight per day, based on relative liver
(inhalation, 4 weeks) weight decrease at effect levels; NOAEL
for dermal, rat: 10 mg TPTH/kg body weight
per day, based on erythema, mortality,
lymphocyte decrease at effect levels; NOAEL
for inhalation, rat: 0.014 mg TPTH/m3,
based on IgM increase at effect levels
Long-term Mouse (80 weeks), rat NOAEL for mouse: 0.85-1.36 mg TPTH/kg body
(104 weeks) weight per day, based on decreased body weight
at effect levels; NOAEL for rat: 0.1 mg TPTH/kg
body weight per day, based on reduction in white
blood cell counts at effect levels
Genotoxicity In vivo/in vitro Mostly negative
Reproduction Rat NOAEL: 0.4 mg TPTH/kg body weight per day, based
on decreased litter size, pup weight, relative
spleen/thymus weight in weanlings at effect levels
Teratogenicity Rabbit NOAEL for maternal toxicity: 0.1 mg TPTH/kg body
weight per day, based on decreased body weight
gain at effect levels
Table 7 (continued)
Type of test Organisms (route of exposure, Results/remarks
duration of test)
Immunotoxicity Mouse/rat/guinea-pig Immunosuppressive; LOAEL: 0.3 mg TPTH/kg body
weight per day in rat
Neurotoxicity Rat (6 weeks) Toxic at 0.36 mg TPTA/kg body weight per day in
maze learning test
Reproductive and developmental effects include a decrease in the
number of implantations and live fetuses (at 1.0 mg TPTA/kg body
weight per day in a rabbit gavage study), a reduction in litter
size/pup weight and in relative thymus or spleen weight in the
weanlings (at 1.5 mg TPTH/kg body weight per day in diet in a
two-generation reproduction study in rats; NOAEL 0.4 mg/kg body weight
per day), and abortion and a reduction in fetal weight (at 0.9 mg
TPTH/kg body weight per day in a rabbit gavage study).
Triphenyltin compounds show effects on the immune system, such as
a decrease in immunoglobulin concentrations (even at the lowest dose
level, i.e., 0.3 mg TPTH/kg body weight per day in a 2-year rat
feeding study), lymphopenia (at 1.75 mg TPTH/kg body weight per day in
a 13-week dietary study in rats or at 0.338 mg/m3 in a 13-week
inhalation study in rats), and thymus or splenic atrophy (at 1.5 mg
TPTCl/kg body weight per day in a 2-week feeding study with weanling
rats or at 5 mg TPTH/kg body weight per day in a 28-day feeding study
in mice, respectively). Females are generally more susceptible than
males with respect to these effects.
The lowest NOAEL detected in the toxicity tests was 0.1 mg/kg
body weight per day for maternal toxicity in a rabbit gavage study,
based on decreased food consumption and body weight gain at 0.3 mg/kg
body weight per day; the same NOAEL was obtained in an early 2-year
rat study in which a slight decrease in white blood cells was seen at
higher doses.
11.1.2 Criteria for setting guidance values for triphenyltin
No data are available on occupational exposure to triphenyltin.
Considering its irritant action, neurotoxic symptoms in poisoning, and
effects on the immune and reproductive systems, care must be taken to
prevent dermal or inhalation exposure to triphenyltin as much as
possible.
Although no data are available on concentrations of triphenyltin
in air or drinking-water, it is unlikely that triphenyltin would be
present as a contaminant in these media at detectable levels
considering its physical/chemical properties and levels of
triphenyltin that have been detected in ambient water.
The major exposure route for the general public is through intake
of foods contaminated with triphenyltin. Estimation of exposure from
residue data in supervised trials or maximum residue limits in foods
will lead to overestimates of intake, because not all crops are
treated with triphenyltin, and residues will not always be at the
maximum residue limits. Exposure to triphenyltin from treated crops
and dairy products is considered to be very low to negligible, as long
as Good Agricultural Practice in the use of pesticides, as defined by
WHO (1976), is observed. Therefore, the major route of exposure for
the general public is probably from the ingestion of fish and
shellfish contaminated with triphenyltin used in antifouling paints.
Triphenyltin levels found in pelagic fish suggest that pollution from
offshore boats is not negligible and that triphenyltins are persistent
in the organisms, probably accumulated through the food-web.
Several end-points were taken into consideration in establishing
the ADI for oral exposure by JMPR (FAO, 1991b; WHO, 1992). First, a
200-fold safety factor (uncertainty factor) was applied to the NOEL of
0.1 mg/kg body weight per day (based on a finding of reduced white
blood cell count at higher doses in a 2-year rat study) to arrive at
an ADI of 0-0.5 µg/kg body weight. Secondly, a 500-fold uncertainty
factor was applied to a LOAEL of 0.3 mg/kg body weight per day in a
2-year study in rats in which increased mortality and reduced serum
immunoglobulins were noted, to derive the same ADI. Other NOAELs taken
into account are 0.4 mg/kg body weight per day in a two-generation
reproduction study with rats (a dose-related decrease in spleen and
thymus weight in F1 and F2 male and female weanlings was observed at
higher levels), 0.3 mg/kg body weight per day in a 13-week study in
rats (reduction in white blood cells, IgG decrease, and relative
testes weight increase seen at higher levels), 0.21 mg/kg body weight
per day in dogs (relative liver weight increase and serum
albumin/globulin ratio decrease seen at higher levels), and 0.1 mg/kg
body weight per day in a teratology study in rabbits (maternal
toxicity seen at higher levels). No additional information regarding
derivation of the above two uncertainty factors is available in the
WHO monograph.
11.1.3 Sample risk characterization
Owing to wide variation in the consumption of fish and shellfish
and local differences in residue levels, only illustrative estimates
relating to effects and exposure can be made. It should be emphasized
that local measurements of residues, local estimates of seafood
consumption, and local decisions on acceptable safety margins must be
made to assess potential risk. Some examples of risk assessments
follow.
Intake of triphenyltin estimated from a market basket survey in
Japan in 1997 was 2.7 µg/day per person; values fluctuated between 0.6
and 2.7 µg/day per person over the 1992-1997 period. There was about a
twofold difference between average daily intake estimates from 10
local laboratories and intakes estimated by one local government.
There are people who eat more seafood than the average person. All
these uncertainties and variations must be taken into account in an
exposure assessment.
Triphenyltin intakes can be compared with the high end of the ADI
of JMPR (0.5 µg/kg body weight per day), which corresponds to 25
µg/day for a 50-kg Japanese person; intakes are calculated to be 2.4%
or 10.8% of the ADI for market basket surveys in different periods.
These data suggest that if actions had not been taken,
contamination of seafood with triphenyltin may have posed some health
risks to Japanese consumers. Similar estimates of intake through
market basket studies in Tokyo reported in 1991 support the above
estimation.
The above risk estimation was performed using data on
triphenyltin compounds alone. Coincidental contamination with
tributyltin must be taken into account in risk estimation from oral
exposure. The risk from exposure to triphenyltin compounds will be
better characterized when combined with risks from other organotin
compounds that exert similar effects (IPCS, 1990; CICAD National
Committee, 1997).
11.2 Evaluation of environmental effects
Triphenyltins enter the environment through their use in
antifouling paints for boats and fishnets and as fungicides for
certain crops.
Strong adsorption of triphenyltin to soil suggests that organisms
in treated soil may not be widely affected. The fact that soil
respiration was not affected significantly suggests that there were no
adverse effects on aerobic microorganisms.
Triphenyltins are very toxic to various species in the
environment at extremely low concentrations. The most sensitive
effects of triphenyltin are imposex in rock shells (Japanese
gastropods), supposed to occur at 1 ng/litre, and inhibition of arm
regeneration in brittle star, at 0.01 µg/litre. The NOEC for
reproduction (21-day exposure) in Daphnia magna and the NOEC (30-day
exposure) for fathead minnow were 0.1 µg/litre and 0.15 µg/litre,
respectively. The EC50s for carbon fixation, reproduction, and
primary production in both marine and freshwater algae were in the
range of 1-2 µg/litre. The LC50 (30-day exposure) for fathead minnow
was also at a similar level. Acute effects (IC50 for primary
productivity in algae, 48-h LC50 for daphnid, and 96-h LC50 for fish)
were seen at 1-10 µg/litre.
For sensitive invertebrates, critical concentrations are 0.01-0.1
µg/litre and lower. Sensitive algae and fish species may be
susceptible at levels below 1 µg/litre.
Ambient surveys in Japan showed that triphenyltin levels in bay
and inshore area water and in sediment were 2.5-3.0 ng/litre and
1.5-2.3 ng/g, respectively, in 1992-1995. Exposure of organisms in the
environment varies widely depending on where and when the triphenyltin
compounds were used or discharged.
No NOEC has been established for triphenyltin-induced imposex in
molluscs. Experimentally, by injection, triphenyltin has a similar
potency to tributyltin in the genus Thais. Triphenyltin is less
potent than tributyltin in Nucella; however, triphenyltin shows
greater bio-accumulation than tributyltin. From this, it can be
assumed that the NOEC for triphenyltin will be a few ng/litre or
lower. The observed prevalence of imposex in Thais in the wild with
ambient concentrations in this range supports this assumption. Because
residues of triphenyltin and tributyltin occur together in the
environment, their relative contribution to observed imposex cannot be
assessed for Thais species. Use of either triphenyltin or
tributyltin in antifouling paint would lead to population declines of
marine invertebrates on this basis.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Triphenyltin was evaluated by JMPR in 1963, 1965, 1970, and 1991.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 1283) reproduced in this
document.
13.1 Human health hazards
Triphenyltin compounds may affect the immune system, resulting in
impaired function. They have also been found to cause reproductive
effects and developmental toxicity in animal studies.
13.2 Advice to physicians
In case of poisoning, treatment is supportive. Special attention
should be given to pregnant women exposed to triphenyltin compounds.
13.3 Health surveillance advice
Periodic medical examination of the immune system should be
included in a health surveillance programme.
13.4 Spillage and disposal
Triphenyltin compounds are absorbed through the skin. In case of
spillage, emergency crew should wear proper equipment, including eye
protection in combination with breathing protection. The compounds
should not be allowed to enter drains or watercourses.
Triphenyltin compounds may be disposed of in sealed containers.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
TRIPHENYLTIN HYDROXIDE ICSC: 1283
November 1998
CAS # 76-87-9 Hydroxytriphenylstannane
RTECS # WH8575000 Hydroxytriphenylstannate
UN # 2786 Fentin hydroxide
EC # 050-004-00-1 C18H16OSn
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/
EXPOSURE SYMPTOMS FIRE FIGHTING
FIRE Combustible. Liquid NO open flames. Powder, water spray,
formulations containing foam, carbon dioxide.
organic solvents may be
flammable.
EXPLOSION In case of fire: keep
drums, etc., cool by
spraying with water.
EXPOSURE PREVENT DISPERSION OF DUST!
STRICT HYGIENE! AVOID
EXPOSURE OF (PREGNANT)
WOMEN!
Inhalation Cough. Sore throat. Ventilation, local exhaust, Fresh air, rest. Refer
or breathing protection. for medical attention.
Skin MAY BE ABSORBED! Redness. Protective gloves. Remove contaminated
Pain. Protective clothing. clothes. Rinse and
then wash skin with
water and soap. Refer
for medical attention.
Eyes Redness. Pain. Blurred Safety spectacles, face First rinse with
vision. shield, or eye protection plenty of water for several
in combination with minutes (remove contact
breathing protection. lenses if easily
possible), then take
to a doctor.
Ingestion Do not eat, drink, or smoke Give plenty of water to
during work. drink. Refer for medical
attention.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Do NOT wash away into sewer. Carefully Do not transport with food and feedstuffs.
collect remainder, then remove to safe Severe marine pollutant.
place. (Extra personal protection: P3 Symbol: T+, N
filter respirator for toxic particles). R: 24/25-26-36/38-50/53
Use face shield. Chemical protection suit. S: (1/2-)36/37-45-60-61
UN Classification
UN Hazard Class: 6.1
UN Pack Group: II
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC(R)-61G41b Provision to contain effluent from
fire extinguishing. Separated from food
and feedstuffs.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
WHITE CRYSTALLINE POWDER The substance can be absorbed into
the body by inhalation, through the
skin and by ingestion.
OCCUPATIONAL EXPOSURE LIMITS: INHALATION RISK:
TLV (as organic compounds (tin)): ppm 0.1 Evaporation at 20°C is negligible;
mg/m3 (skin) (STEL) (ACGIH 1998). a harmful concentration of airborne particles
MAK as tin: ppm, 0.1 mg/m3; skin (D) (1995) can, however, be reached quickly when dispersed.
EFFECTS OF SHORT-TERM EXPOSURE:
The substance irritates the eyes severely, the
skin and the respiratory tract. The substance
may cause effects on the immune system,
resulting in impaired functions
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
Animal tests show that this substance possibly
causes malformations in human babies.
PHYSICAL PROPERTIES
Decomposes below melting point at 80°C.
Solubility in water, g/100 ml: 0.008
Flash point: 400°C
ENVIRONMENTAL DATA
The substance is very toxic to aquatic organisms. In the food chain important to humans,
bioaccumulation takes place, specifically in molluscs. Avoid release to the
environment in circumstances different to normal use.
NOTES
Carrier solvents used in commercial formulations may change physical and toxicological
properties. Do NOT take working clothes home.
ADDITIONAL INFORMATION
LEGAL NOTICE
Neither the CEC nor the IPCS nor any person acting on behalf of the CEC or the IPCS
is responsible for the use which might be made of this information.
REFERENCES
ACP (1990) Evaluation on fentin hydroxide. In: Food and
Environmental Protection Act, 1985, Part III. Evaluation of Fully
Approved or Provisionally Approved Products. York, Ministry of
Agriculture, Fisheries and Food, Pesticide Safety Directorate,
Advisory Committee on Pesticides, 18 pp.
Baba T, Rikioka Y, Toyomura K (1991) Content of tributyltin (TBT) and
triphenyltin (TPT) compounds in marine products and its processed
products in Nagasaki prefecture. Annual report of Nagasaki
Prefectural Institute of Public Health and Environmental Sciences
(Nagasakiken Eisei Kogai Kenkyujo Nenpo), 34:98-102 (in Japanese).
Baeder C (1987) Fentin acetate-substance, technical (Code: HOE
002782 OF ZD97 0002). Testing for embryotoxicity in the Himalayan
rabbit after oral administration. Unpublished report 89.1559
(A42324) of Hoechst Pharma Research Toxicology and Pathology.
Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO,
1992].
Barnes RD, Bull AT, Poller RC (1971) Behaviour of triphenyltin acetate
in soil. Chemistry and industry, 8:204.
Bettin C, Oehlmann J, Stroben E (1996) TBT-induced imposex in marine
neogastropods is mediated by an increasing androgen level.
Helgolander Meeresuntersuchungen, 50:299-317.
Beurkle WL (1985) HOE 002782-14-C (triphenyltin acetate), analysis
of hydrolysis by-products. Unpublished report CM009/85 (A31534) from
Analytisches Laboratorium, Hoechst. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Booth GM, Jackson C, Warren S (1980) Acute oral toxicity of
technical triphenyltin hydroxide and Du-ter to mallard ducks. Provo,
UT, Brigham Young University, Department of Zoology.
Bouldin TW, Goines ND, Bagnell CR, Krigman MR (1981) Pathogenesis of
trimethyltin neuronal toxicity. Ultrastructural and cytochemical
observations. American journal of pathology, 104:237-249.
Carlton BD, Howard M (1982) The evaluation of the teratogenicity of
triphenyltin hydroxide (TPTH) in the Syrian hamster. Unpublished
report 723-0100 (A22884) of Batelle Columbus Laboratories, Columbus,
OH. Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO,
1992].
Chow SC, Orrenius S (1994) Rapid cytoskeleton modification in
thymocytes induced by the immunotoxicant tributyltin. Toxicology and
applied pharmacology, 127:19-26.
CICAD National Committee (1997) A critical review on triphenyltin
compounds (provisional), August 1997. Tokyo, National Institute of
Health Sciences, National Committee for Concise International Chemical
Assessment Documents, 76 pp.
Clegg ED, Cook JC, Chapin RE, Foster PMD, Daston GP (1997) Leydig cell
hyperplasia and adenoma formation: Mechanisms and relevance to humans.
Reproductive toxicology, 11:107-121.
De Vries H, Penninks AH, Snoeij NJ, Seinen W (1991) Comparative
toxicity of organotin compounds to rainbow trout ( Oncorhynchus
mykiss) yolk sac fry. The science of the total environment,
103:229-243.
Diehl KH, Leist KH (1986a) Fentin acetate-active ingredient
technical. Testing for acute dermal toxicity in the male and female
Wistar rat. Unpublished report 86.0816 (A37384) of Hoechst Pharma
Research Toxicology and Pathology. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Diehl KH, Leist KH (1986b) Fentin acetate-active ingredient
technical. Testing for primary dermal irritation in the rabbit.
Unpublished report 86.0619 (A37383) of Hoechst Pharma Research
Toxicology and Pathology. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Diehl KH, Leist KH (1986c) Fentin acetate-active ingredient
technical. Testing for primary eye irritation in the rabbit.
Unpublished report 86.0620 (A37382) of Hoechst Pharma Research
Toxicology and Pathology. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Diehl KH, Leist KH (1986d) Testing for sensitising properties in the
Pirbright-White guinea pig according to the technique of Buehler.
Unpublished report 86.0803 (A36975) of Hoechst Pharma Research
Toxicology and Pathology. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Diehl KH, Leist KH (1987) Fentin-hydroxide-active ingredient
technical (Code: HOE 029664 OF ZD97 0004). Testing for sensitising
properties in the Pirbright-White guinea pig in a maximisation test.
Unpublished report 87.0201 (A35628) of Hoechst Pharma Research
Toxicology and Pathology. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Dorn E, Werner HJ (1989) Triphenyltin hydroxide (HOE 029664 OF ZD 97
0004). Determination of residues in organs, tissues and blood
of rats after chronic feeding (104 weeks). Unpublished report
CR077/88 (A41974) of Analytisches Laboratorium Hoechst AG. Submitted
to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO, 1992].
Duchosal F, Thevenaz P, Luetkemeier H, Vogel O, Pappritz G, Mladenovic
P, Terrier C (1989) Fentin hydroxide (TPTH) technical grade.
Subchronic (90-days) repeated dose inhalation toxicity study in
rats. Unpublished report 202353 (A40323) of Research and Consulting
Company AG, Carouge-Geneva [cited in WHO, 1992].
Ebert E, Leist KH (1987) Fentin-Acetat-Substanz, technisch (Code:
HOE 002782 OF ZD97 0002). Pruefung der akuten oralen Toxizitaet an
maennlichen und weiblichen Virginischen Baumwacteln (Colinus
virginianus). Hoechst Pharma Forschung Toxikologie, Report 87.0291.
Submitted to FAO by Hoechst AG, Frankfurt-am-Main [cited in FAO,
1991a].
Ebert E, Leist KH (1988) Fentin acetate active ingredient technical
(Code: HOE 002782 OF ZD97 0002). Testing for acute oral toxicity in
the male and female mallard duck. Hoechst Pharma Forschung
Toxikologie, Report 88.0544. Submitted to FAO by Hoechst AG,
Frankfurt-am-Main [cited in FAO, 1991a].
Ebert E, Weigand W (1982) Acute oral toxicity (LD50) of HOE
29664-active ingredient (Code: HOE 29664 OF AT205) in Japanese quail
(Coturnix coturnix). Submitted to FAO by Hoechst AG,
Frankfurt-am-Main [cited in FAO, 1991a].
Eckert HG, Kellner HM, Beurkle WL (1989) Accumulation study,
kinetics and metabolism in the rat after single and repeated oral
administration, of 2 mg/kg body weight. Unpublished report
01-L42-0565-89 (A41407) of Hoechst Pharma Forschung GB-L
Radiochemisches Laboratorium and Produktentwicklung GB-L, Oekologie.
Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO,
1992].
Eisler R (1989) Tin hazards to fish, wildlife and invertebrates:
a synoptic review. US Fish and Wildlife Service Biological Report
85(1.15), p. 83 [cited in HSE, 1992].
Ema M, Miyawaki E, Harazono A, Ogawa Y (1997) Effect of triphenyltin
chloride on implantation and pregnancy in rats. Reproductive
toxicology, 11:201-206.
Ema M, Miyawaki E, Kawasaki K (1998) Inhibition of decidual cell
response as a cause of implantation failure induced by triphenyltin
chloride (TPTCl) in rats. Toxicologist, 42:261.
FAO (1991a) Pesticide residues in food -- 1991. Evaluations, Part I
-- Residues. Rome, Food and Agriculture Organization of the United
Nations, pp. 337-371 (FAO Plant Production and Protection Paper
113/1).
FAO (1991b) Pesticide residues in food -- 1991 report. Rome, Food
and Agriculture Organization of the United Nations, pp. 57-62 (FAO
Plant Production and Protection Paper 111).
Fent K, Hunn J (1991) Phenyltins in water, sediment, and biota of
freshwater marinas. Environmental science and technology,
25:956-963.
Fent K, Hunn J (1995) Organotins in fresh water harbours and rivers:
temporal distribution, annual trends and fate. Environmental
toxicology and chemistry, 14:1123-1132.
Filenko OF, Isakova EF (1979) Dynamics of triphenyltin chloride
accumulation by Daphnia. Biologicheskie Nauki, 3:46-49 (in
Russian).
Gomez-Ariza JL, Morales E, Ruiz-Benitez M (1992) GC FPD method for the
simultaneous speciation of butyltin and phenyltin compounds in waters.
Applied organometallic chemistry, 6(3):279-286.
Guta-Socaciu C, Giurgea R, Rosioru C (1986) Thymo-bursal and adrenal
modifications induced by triphenyltin compounds in chickens.
Archives of experimental veterinary medicine, 40:307-311.
Hallas LE, Cooney JJ (1981) Effects of stannic chloride and organotin
on estuarine micro-organisms. Developments in industrial
microbiology, 22:529-535.
Harino H, Fukushima M, Tanaka M (1992) Simultaneous determination of
butyltin and phenyltin compounds in the aquatic environment by gas
chromatography. Analytica Chimica Acta, 264(1):91-96.
Hattori Y, Kobayashi A, Takemoto S, Takami K, Kuge Y, Sugimae A,
Nakamoto M (1984) Determination of trialkyltin, dialkyltin, and
triphenyltin compounds in environmental water and sediments. Journal
of chromatography, 315:341-349.
Hollander H, Weigand W (1974) Triphenyltin acetate. Acute oral
toxicity (LD50) in female albino-rats. Unpublished report 16454
(A16454) of Hoechst Pharma Forschung Toxikologie. Submitted to WHO by
Hoechst AG, Frankfurt-am-Main [cited in WHO, 1992].
Hollander H, Weigand W (1981) Dust inhalation of HOE 29664-active
ingredient in the male and female SPF-Wistar rat. 4h-LD50.
Unpublished report 335/81 (A22293) of Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited
in WHO, 1992].
Hollander H, Weigand W (1986) Fentin acetate-active ingredient
technical. Testing for acute dust inhalation toxicity in the male
and female SPF rat. 4-hour LC50. Unpublished report 86.1497
(A37523) of Hoechst Pharma Research Toxicology. Submitted to WHO by
Hoechst AG, Frankfurt-am-Main [cited in WHO, 1992].
Horiguchi T, Shiraishi H, Shimizu M, Morita M (1994) Imposex and
organotin compounds in Thais clavigera and T. bronni in Japan.
Journal of the Marine Biological Association of the United Kingdom,
74:651-669.
Horiguchi T, Shiraishi H, Shimizu M, Morita M (1997) Effects of
triphenyltin chloride and five other organotin compounds on the
development of imposex in the rock shell, Thais clavigera.
Environmental pollution, 95(1):85-91.
HSE (1992) A review of the environmental effects of triorganotin
compounds. Merseyside, Health and Safety Executive, Pesticide
Registration Section, Advisory Committee on Pesticides, 142 pp.
(Report No. 111).
Ikeda M, ed. (1977) Industrial poisoning handbook, 2nd ed. Tokyo,
Ishiyaku Shuppan, 303 pp. (in Japanese).
IPCS (1990) Tributyltin compounds. Geneva, World Health
Organization, International Programme on Chemical Safety
(Environmental Health Criteria 116).
IPCS (1996) International Chemical Safety Card -- Triphenyltin
hydroxide. Geneva, World Health Organization, International
Programme on Chemical Safety (ICSC 1283).
Ishizaka T, Nemoto S, Sasaki K, Suzuki T, Saito Y (1989) Simultaneous
determination of tri- n-butyltin, di- n-butyltin, and triphenyltin
compounds in marine products. Journal of agricultural and food
chemistry, 37:1523-1526.
Japan Environment Agency (1983) Chemicals in the environment. Tokyo,
Environmental Health Department, Office of Health Studies.
Japan Environment Agency (1996) Chemicals in the environment. Tokyo,
Environmental Health Department, Office of Health Studies.
Jarvinen AW, Tanner DK, Kline ER, Knuth ML (1988) Acute and chronic
toxicity of triphenyltin hydroxide to fathead minnows ( Pimephales
promelas) following brief or continuous exposure. Environmental
pollution, 52:289-301.
JPPA (1982-1996) Summary data on pesticides. Tokyo, Japan Plant
Protection Association (in Japanese).
Kang J, Chen I, Cheng Y (1997) Induction of calcium release from
isolated sarcoplasmic reticulum by triphenyl tin. Journal of
bio-chemistry, 122:173-177.
Kannan K, Lee RF (1996) Triphenyltin and its degradation products in
foliage and soils from sprayed pecan orchards in fish from adjacent
ponds. Environmental toxicology and chemistry, 15(9):1492-1499.
Kellner HM, Eckert HG (1989) Addendum to report 01-L42-0565-89. HOE
029664 (TPTH)-113Sn. Accumulation and depletion study in rats after
single and repeated oral administration of 2 mg/kg body weight.
Unpublished report 01-L42-582-90(A43434) of Hoechst Pharma Forschung
GB-L Radiochemisches Laboratorium. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Kohri M, Sato K, Inoue Y, Ide K, Okochi H (1995) Batch operation of
solid phase extraction for separation and concentration of organotin
compounds in seawater. Bunseki Kagaku, 44(7):537-542.
Lehotzky K, Szeberenyi JM, Horkay F, Kiss A (1982) The neurotoxicity
of organotin: behavioural changes in rats. Acta Biologica Academiae
Scientiarum Hungaricae, 33:15-22.
Leist KH (1988) Fentin acetate-substance technical (Code: HOE 002782
OF ZD97 0002). Repeated dose dermal toxicity study (21 applications
in 29 days) with a 14 day withdrawal period. Unpublished report
(A39231) 3104 TSR of Hoechst Pharma Forschung Toxikologie und
Pathologie. Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited
in WHO, 1992].
Leist KH, Weigand W (1981a) Acute oral toxicity of HOE 29664-active
ingredient (Code: HOE 29664 OF AT201) to the male rat. Unpublished
report (A22115) 182/81 of Hoechst Pharma Forschung Toxikologie.
Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO,
1992].
Leist KH, Weigand W (1981b) Acute oral toxicity of HOE 29664-active
ingredient (Code: HOE 19664 OF AT201) to the female rat. Unpublished
report (A22114) 183/81 of Hoechst Pharma Forschung Toxikologie.
Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO,
1992].
Leist KH, Weigand W (1981c) Acute percutaneous toxicity of HOE
29664-active ingredient (Code: HOE 29664 OF AT201) to the female
rat. Unpublished report (A22292) 184/81 of Hoechst Pharma Forschung
Toxikologie. Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited
in WHO, 1992].
Leist KH, Weigand W (1981d) Acute percutaneous toxicity of HOE
29664-active ingredient (Code: HOE 29664 OF AT201) to the male
rabbit. Unpublished report (A22112) 229/81 of Hoechst Pharma
Forschung Toxikologie. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Leist KH, Weigand W (1981e) Test for sensitising properties of
fentin hydroxide-technical (Code: HOE 29664 OF AT201) in guinea-pig
(according to Buehler). Unpublished report (A22433) 724/81 of
Hoechst Pharma Forschung Toxikologie. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Linden E, Bengtsson BE, Svanberg O, Sundstrom G (1979) The acute
toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms: the bleak ( Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 11:843.
Lisi P, Carraffini S, Assalve D (1987) Irritation and sensitisation
potential of pesticides. Contact dermatitis, 17:212-218.
MacCormick DL, Thomas PT (1990) 28-day oral diet immunotoxicity
study of triphenyltin hydroxide (TPTH),substance technical (Code HOE
029664 OFZD97 0004) in mice. Unpublished report L08252SN02(A44054)
of Life Science Research. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Manzo L, Richelmi P, Sabbioni E, Pietra R, Bono F, Guardia L (1981)
Poisoning by triphenyltin acetate. Report of two cases and
determination of tin in blood and urine by neutron activation
analysis. Clinical toxicology, 18:1343-1353.
Masoud AH, El-Habbak MME, Hegazi AG, Soufy H, Samy MM (1985) The
influence of triphenyltin hydroxide on some biological aspects in
mature female peking ducks. Mededelingen van de Faculteit
Landbouwwetenschappen, Rijksuniversiteit Gent, 50:925-937.
Mensink BP, Ten-Hallers-Tjabbes CC, Kralt J, Freriks IL, Boon JP
(1996) Assessment of imposex in the common whelk, Buccinum undatum
(L.) from the Eastern Scheldt, The Netherlands. Marine
environmental research, 41(4):315-325.
MITI (1998) Industrial production statistics. Chemical Safety Policy
Office, Basic Industries Bureau, Ministry of International Trade and
Industry, Japan.
Miura Y, Kato M, Ogino K, Matsui H (1997) Impaired cytosolic Ca2+
response to glucose and gastric inhibitory polypeptide in pancreatic
ß-cells from triphenyltin-induced diabetic hamster. Endocrinology,
138:2769-2775.
Morcillo Y, Borghi V, Porte C (1997) Survey of organotin compounds in
the Western Mediterranean using molluscs and fish as sentinel
organisms. Archives of environmental contamination and toxicology,
32:35-46.
Nagamatsu K, Kido Y, Urakubo G, Aida Y, Ikeda Y, Suzuki Y (1978)
Absorption, distribution and excretion of triphenyltin acetate and
stannous chloride in the guinea pig. Japanese journal of hygiene,
33:486-496.
NIHS (1998) A report of surveys on daily intake of contaminants in
food. Unpublished report. Tokyo, National Institute of Health
Sciences (in Japanese).
Nishida H, Matsui H, Sugiura H, Kitagi K, Fuchigami M, Inagaki N,
Nagai H, Koda A (1990) The immunotoxicity of triphenyltin chloride in
mice. Journal of 'Pharmacobio-Dynamics', 13(9):543-548.
NLM (1998) Hazardous Substances Data Bank. Bethesda, MD, US National
Library of Medicine. CHEM-BANK CD-ROM, distributed by SilverPlatter
International, NV.
NTP (1978) Bioassay of triphenyltin hydroxide for possible
carcinogenicity. CAS No. 76-87-9 (A199994). Research Triangle Park,
NC, US Department of Health, Education and Welfare, National
Institutes of Health, National Toxicology Program (Technical Report
Series No.139).
Ohhira S, Matsui H (1991) Comparison of sulfur-mode and tin-mode flame
photometric detectors for the gas chromatographic determination of
organotin compounds. Chromatography and biomedical applications,
566(1):207-214.
Ohhira S, Matsui H (1993) Gas chromatographic determination of
inorganic tin in rat urine after a single oral administration of
stannous chloride and mono-, di-, and triphenyltin chloride. Journal
of chromatography and biomedical applications, 622(2):173-178.
Ohhira S, Matsui H (1996) Comparative study of the metabolism of
triphenyltin in hamsters and rats after a single oral treatment with
triphenyltin chloride. Toxicology letters, 85:3-8.
Ozcan M, Good ML (1980) Abstract in Proceedings of the American
Chemical Society. Division of Environmental Chemistry, Houston, TX
[cited in HSE, 1992].
Penninks AH, Snoeij RJ, Pieters RHH, Seinen W (1990) Effect of
organotin compounds on lymphoid organs and lymphoid functions: an
overview. In: Dayan AD, Hertel RF, Heseltin E, Kazantzis G, Smith EM,
Van der Venne MT, eds. Immunotoxicity of metals and
immunotoxicology. New York, NY, Plenum Press, pp. 191-207.
Rodwell DE (1985) A teratology study in rats with triphenyltin
hydroxide (Code 029664 OF ZD97 0001 technical substance).
Unpublished report WIL-39011 (A31079) of WIL Research Laboratories
Inc., Ashland. Submitted to WHO by Hoechst AG, Frankfurt-am-Main
[cited in WHO, 1992].
Rodwell DE (1987) An embryotoxicity study in rabbits with
triphenyltin hydroxide (Code: HOE 209664 OF ZD97 0004). Unpublished
report WIL-39012 (A35220) of WIL Research Laboratories Inc., Ashland.
Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited in WHO,
1992].
Sachose K, Frei T, Luetkemeier H, Vogel W, Pappritz G, Terrier C
(1987) TPTH-substance technical (HOE 029664 OF ZD 97 0004). Chronic
oral toxicity 52-week feeding study in beagle dogs. Unpublished
report 047013/047024 (A35733) of RCC Research and Consulting Company
AG, Itingen. Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited
in WHO, 1992].
Sasaki YF, Yamada H, Sugiyama C, Kinae N (1993) Increasing effect of
tri- n-butyltins and triphenyltins on the frequency of chemically
induced chromosome aberrations in the cultured Chinese hamster cells.
Mutation research, 300:5-14.
Schollmeier U, Leist KH (1989) Triphenyltin hydroxide-active
ingredient technical (Code: HOE 029664 OF ZD97 0004). Testing for
sensitising properties in the Pirbright-White guinea pig according to
the technique of Buehler. Unpublished report 89.1006 (A42667) of
Hoechst Pharma Research Toxicology and Pathology. Submitted to WHO by
Hoechst AG, Frankfurt-am-Main [cited in WHO, 1992].
Scholz D, Weigand W (1969) Triphenyltin acetate study No. 2116.
Unpublished data A37396 of Laboratory for Industrial and Drug
Toxicology. Submitted to WHO by Hoechst AG, Frankfurt-am-Main [cited
in WHO, 1992].
Sekizawa J (1998) Health and environmental risk assessment of
organotin pollution in Japan. Bulletin of the National Institute of
Health Sciences, 116:126-131 (in Japanese).
Shimizu A, Kimura S (1992) Long-term effects of bis( n-tributyltin)
oxide (TBTO) on salt-water goby Chasmichotys dolichognathus.
Nippon Suisan Gakkaishi, 58:1595-1602 (in Japanese).
Shiraishi H, Soma M (1992) Triphenyltin compounds in mussels in Tokyo
Bay after restriction of use in Japan. Chemosphere, 24(8):1103-1109.
Smith KS (1981) 14-C-TPTH residue levels in milk and tissues of
lactating dairy cows. Cannon Laboratories, USA. Report Project OF
7489 [cited in FAO, 1991a].
Snoeij NJ, Van Iersel AAJ, Penninks AH, Seinen W (1985) Toxicity of
triorganotin compounds: Comparative in vivo studies with a series of
trialkyltin compounds and triphenyltin chloride in male rats.
Toxicology and applied pharmacology, 81:274-286.
Snow RL, Hays RL (1983) Phasic distribution of seminiferous tubules in
rats treated with triphenyltin compounds. Bulletin of environmental
contamination and toxicology, 31:658-665.
Soderquist C, Crosby DG (1980) Degradation of triphenyltin hydroxide
in water. Journal of agricultural and food chemistry, 28:111-117.
Staeb JA, Rozing MJM, Van-Hattum B, Cofino WP, Brinkman UAT (1992)
Normal-phase high-performance liquid chromatography with UV
irradiation, morin complexation and fluorescence detection for the
determination of organotin pesticides. Journal of chromatography,
609(1-2):195-203.
Staeb JA, Frenay M, Freriks IL, Brinkman UAT, Cofino WP (1995) Survey
of nine organotin compounds in the Netherlands using the zebra mussel
( Dreissena polymorpha) as biomonitor. Environmental toxicology and
chemistry, 14(12):2023-2032.
Staeb JA, Traas TP, Stroomberg G, van-Kesteren J, Leonards P,
van-Hattum B, Brinkman UA, Cofino WP (1996) Determination of organotin
compounds in the foodweb of a shallow freshwater lake in the
Netherlands. Archives of environmental contamination and toxicology,
31(3):319-328.
Suess A, Eben C (1973) Das Verhalten von Triphenyltzinnacetat (TPZA)
im Boden und die Aufnahme durch Pflanzen aus behandelten Boeden.
Zeitschrift fuer Pflanzenkrankheiten und Pflanzenschutz, 80
(H. 5):288-294 (in German).
Sugita A (1992) Historical background of organotin pollution. In:
Satomi Y, Shimizu M, eds. Organotin pollution and effects on aquatic
organisms. Tokyo, Koseisha Koseikaku, pp. 9-19 (in Japanese).
Suter P, Horst K (1986a) 13-week oral toxicity (feeding) study with
TPTH-technical (Code: HOE 029664 OF ZD97 0004) in the mouse.
Unpublished report project 046991 (A32420) of RCC Research and
Consulting Company AG, Itingen. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Suter P, Horst K (1986b) 13-week oral toxicity (feeding) study with
TPTH-technical (Code: HOE 029664 OF ZD97 0004) in the rat.
Unpublished report project 046978 (A32419) of RCC Research and
Consulting Company AG, Itingen. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Suzuki T, Yamada H, Yamamoto I, Nishimura K, Kondo K, Murayama M,
Uchiyama M (1996) Chemical species of organotin compounds in seawater
and their seasonal variations. Agricultural and food chemistry,
4(12):3989-3995.
Takeuchi M, Mizuishi K, Yamanobe H, Watanabe Y, Doguchi M (1989)
Hygienic chemical studies on organotin compounds (VI). A consideration
on the pollution of Tokyo Bay with tributyltin and triphenyltin
compounds. Annual report of the Tokyo Metropolitan Research
Laboratory for Public Health, 40:127-132 (in Japanese).
Takeuchi M, Mizuishi K, Yamanobe H, Nakamura H (1991) Hygienic
chemical studies on organotin compounds (VII). Content of tributyltin
and triphenyltin compounds in fish and shellfish (1988-1990). Annual
report of the Tokyo Metropolitan Research Laboratory for Public
Health, 42:77-85.
Tas JW, Oppe Vizen A, Seinen W (1990) Uptake and elimination kinetics
of triphenyltin hydroxide by two fish species. Toxicology and
environmental chemistry, 28:129-141.
Tennekes H, Horst K, Luetkemeier H, Vogel W, Vogel O, Armstrong J,
Ehlers HA, Muller E, Terrier C (1989a) TPTH-technical (Code: HOE
029664 OF ZD97 0004) oncogenicity 80-week feeding study in mice.
Unpublished report 047002 (A40467) of RCC Research and Consulting
Company AG, Itingen. Submitted to WHO by Hoechst AG, Frankfurt-am-Main
[cited in WHO, 1992].
Tennekes H, Horst K, Luetkemeier H, Vogel W, Schlotke B, Vogel O,
Ehlers HA, Muller E, Terrier C (1989b) TPTH-technical (Code: HOE
029664 OF ZD97 0007) chronic toxicity/oncogenicity 104-week feeding
study in rats. Unpublished report 046980 (A40468) of RCC Research
and Consulting Company AG, Itingen. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
Til HP, Feron VJ, de Groot AP (1970) Chronic toxicity study with
triphenyltin hydroxide in rats for two years. Unpublished TNO
(Netherlands Organization for Applied Scientific Research) report
R-3138, submitted to WHO by Phillips-Duphar [cited in WHO/FAO, 1971].
Tomlin C, ed. (1997) The pesticide manual, 11th ed. Surrey, British
Crop Protection Council; Cambridge, The Royal Society of Chemistry,
1606 pp.
Tsuda T, Nakanishi H, Aoki S, Takebayashi J (1987) Bioconcentration
and metabolism of phenyltin chlorides in carp. Water research,
21(8):949-953.
Tsuda T, Inoue T, Kojima M, Aoki S (1995) Daily intakes of tributyltin
and triphenyltin compounds from meals. Journal of the Association of
Official Analytical Chemists International, 78(4):941-943.
Tsunoda M (1993) Simultaneous determination of organotin compounds in
fish and shellfish by gas chromatography with a flame photometric
detector. Tohoku journal of experimental medicine, 169(2):167-178.
Ueda K, Iijima K (1961) Acute toxicity of Brestan technical
(triphenyltin acetate) for mice. Unpublished report A15046 of
Laboratory of Hygienics, Tokyo Dental College, Tokyo. Submitted to WHO
by Hoechst AG, Frankfurt-am-Main [cited in WHO, 1992].
US EPA (1987) Tributyltin support document. Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances, Office of Pesticide Programs.
Van der Maas HL, Van Deyl L, Kroon JJ (1972) The acute and subacute
toxicity of the fungicide triphenyltin hydroxide to the common pond
snail, Lymnaea stagnalis L. Mededelingen van de Faculteit
Landbouwwetenschappen, Rijksuniversiteit Gent, 37(2):850-858.
Vela NP, Caruso JA (1993) Comparison of flame ionisation and
inductively coupled plasma mass spectrometry for the detection of
organometallics separated by capillary supercritical fluid
chromatography. Journal of chromatography, 641(2):337-345.
Verschuuren HG, Ruitenberg EJ, Peetoom F, Helleman PW, van Esch GJ
(1970) Influence of triphenyltin acetate on lymphatic tissue and
immune responses in guinea pigs. Toxicology and applied
pharmacology, 16:400-410.
Viviani B, Rossi AD, Chow SC, Nicotera P (1995) Organotin compounds
induce calcium overload and apoptosis in PC12 cells.
Neurotoxicology, 16(1):19-25.
Wada O, Manabe S, Iwai H, Arakawa Y (1982) Recent progress in the
study of analytical methods, toxicity, metabolism and health effects
of organotin compounds. Japanese journal of industrial health,
24:24-54 (in Japanese).
Walsh GE, McLaughlin LL, Lores EM, Louie MK, Deans CH (1985) Effects
of organotins on growth and survival of two marine diatoms
Skeletonema costatus and Thalassiosira pseudonana. Chemosphere,
14(3-4):383-392.
Walsh GE, McLaughlin LL, Louie MK, Deans CH, Lores EM (1986)
Inhibition of arm regeneration by Ophioderma brevispina
(Echinodermata, Ophiuroidea) by tributyltin and triphenyltin oxide.
Ecotoxicology and environmental safety, 12:95-100.
WHO (1976) Report of the 1975 Joint Meeting of the FAO Working Party
of Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues. Geneva, World Health Organization, 45 pp. (WHO
Technical Report Series No. 592).
WHO (1992) Pesticide residues in food -- 1991, Evaluations 1991 Part
II -- Toxicology. Geneva, World Health Organization, pp. 173-208.
WHO/FAO (1971) 1970 evaluation of some pesticide residues in food.
Rome, World Health Organization/Food and Agriculture Organization of
the United Nations, pp. 327-366.
Wong PTS, Chau YK, Kramar O, Bengert GA (1982) Structure toxicity
relationship of tin compounds on algae. Canadian journal of
fisheries and aquatic science, 39:483-488.
Yamada H, Sasaki YF (1993) Organotins are co-clastogens in a whole
mammalian system. Mutation research, 301:195-200.
Yamada H, Takayanagi K (1992) Bioconcentration and elimination of
bis(tributyltin) oxide (TBTO) and triphenyltin chloride (TPTC) in
several marine fish species. Water research, 26(12):1589-1595.
Young DL (1986) A dietary two-generation reproduction study in rats
with triphenyltin hydroxide (Code: HOE 0.29664 OF ZD97 0004
technical substance). Final report project WIl-39022 (A35378) of WIL
Research Laboratories, Inc., Ashland. Submitted to WHO by Hoechst AG,
Frankfurt-am-Main [cited in WHO, 1992].
APPENDIX 1 -- SOURCE DOCUMENTS
CICAD National Committee (1997): A critical review on triphenyltin
compounds
The 1997 review on triphenyltin compounds (provisional) was
developed by the National Committee for Concise International Chemical
Assessment Documents of Japan (CICAD National Committee, 1997). Much
important information on health effects was obtained from monographs
on pesticide residues prepared by the FAO and WHO (FAO, 1991a,b; WHO,
1992); these monographs describe summary evaluations of data,
including proprietary information. Extensive information on
environmental effects was obtained from a 1992 review of the
environmental effects of triorganotin compounds, prepared by the
Advisory Committee on Pesticides, Health and Safety Executive, United
Kingdom (HSE, 1992).
The CICAD National Committee of Japan is composed of the members
and observers listed below. Members are experts in the areas of
toxicology, chemistry, environmental science, occupational safety,
chemical management, or information science. Observers represent
divisions related to chemical safety or international activities in
various ministries and agencies. This committee is independent of
industry. Its activities are communicated on the homepage of the
National Institute of Health Sciences.
The draft review on triphenyltin compounds was prepared by Dr Jun
Sekizawa and was circulated for comments among members and observers,
then revised. This review is available by request from Dr Jun
Sekizawa, National Institute of Health Sciences.
Members
Dr S. Hatakeyama, Ecological Hazard Evaluation Team, National
Institute of Environmental Studies
Dr T. Kaminuma, Division of Chem-Bio Informatics, National Institute
of Health Sciences
Dr J. Kato, Yokohama Laboratory, Mitsubishi-kasei Institute of
Toxicological and Environmental Sciences
Dr Y. Kurokawa, Biological Safety Research Centre, National Institute
of Health Sciences ( Chairperson)
Dr K. Matsumoto, Department of Chemistry, Waseda University
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences
Mr T. Oshima, Japan Chemical Safety Institute
Dr H. Sakurai, National Institute for Industrial Health
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences
Mr Y. Shiraishi, Chemical Safety Management Centre, Japan Chemical
Industry Association
Dr I. Uchiyama, National Institute of Public Health
Dr M. Yasuno, Department of Environmental Science, Shiga Prefectural
University
Observers1
Division of Chemical Products Safety, Ministry of International Trade
and Industry
Division of Chemical Substance Investigation, Ministry of Labour
Division of Environmental Chemicals Safety, Ministry of Health and
Welfare
Division of Environmental Health and Safety, Environment Agency
Division of Food Chemicals, Ministry of Health and Welfare
Division of Foreign Affairs, Ministry of Health and Welfare
Division of Standards on Drinking Water, Ministry of Health and
Welfare
WHO (1992): Pesticide residues in food -- 1991, Evaluations
1991 Part II -- Toxicology
The Joint FAO/WHO Meeting on Pesticide Residues, known as JMPR,
has been evaluating pesticides that are used on food crops and that
may leave residues on them since 1963. JMPR comprises two groups of
scientists: namely, the FAO panel, which has responsibility for
reviewing pesticide residue data and for recommending maximum residue
limits, and the WHO group, which has responsibility for reviewing
toxicological data and for recommending acceptable daily intakes
(ADIs). The principles used by the WHO group for the toxicological
assessment of pesticide residues in food have been publicized through
its monographs and reports and in the Environmental Health Criteria of
the IPCS. Although many of the data come from manufacturers that
carried out major toxicological studies, the independent nature of the
JMPR evaluation has been secured through various mechanisms.
1 Representatives of the listed divisions in ministries and
agencies.
The toxicity of triphenyltin compounds was reviewed by JMPR in
1963, 1965, 1970, and 1991 (WHO, 1992). At the 1991 meeting, the
following scientists participated in the WHO group, and 21 other
experts from various international organizations and countries joined
in the evaluation (WHO, 1992).
Members of WHO Expert Group on Pesticide Residues in the 1991 JMPR
Professor U.G. Ahlborg, Institute of Environmental Medicine, Sweden
Dr A.L. Black, Department of Health, Housing and Community Services,
Australia
Dr J.F. Borzelleca, Medical College of Virginia, Virginia Commonwealth
University, USA
Mr D.J. Clegg, Health Protection Branch, Health and Welfare Canada,
Canada
Professor M. Lotti, Universita di Padova, Istituto di Medicina del
Lavoro, Italy
Dr F.R. Puga, Instituto Biologico, Brazil
Dr P. Yao, Institute of Occupational Medicine, Chinese Academy of
Preventive Medicine, Ministry of Public Health, People's Republic of
China
HSE (1992): A review of the environmental effects of
triorganotin compounds
The Health and Safety Executive of the United Kingdom developed
and published the report entitled A review of the environmental
effects of triorganotin compounds (HSE Report No. 111). This review
was prepared by the Advisory Committee on Pesticides, a tripartite
committee with representatives from industry, trade unions, and
academia who give their advice and approve the use under the Control
of Pesticides Regulations 1986.
The Health and Safety Executive is responsible for human health
aspects and pesticide efficacy. The Ministry of Agriculture, Fisheries
and Food (Pesticides Safety Directorate) is responsible for the
evaluation of non-human aspects.
Public access to raw data underlying this publication can be
arranged by contacting the Pesticide Registration Section, Health and
Safety Executive, Magdalen House, Stanley Precinct, Bootle,
Merseyside, United Kingdom L20 3QZ.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on triphenyltin compounds was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
International Council on Metals and the Environment, Ottawa, Canada
Karolinska Institute, Stockholm, Sweden
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Institute for Occupational Safety and Health, Cincinnati, USA
National Institute for Working Life, Solna, Sweden
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences, Research Triangle Park,
USA)
United States Environmental Protection Agency, Denver, USA
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Tokyo, Japan, 30 June - 2 July 1998
Members
Dr R. Benson, Drinking Water Program, United States Environmental
Protection Agency, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Mr R. Cary, Health Directorate, Health and Safety Executive,
Merseyside, United Kingdom
Dr C. DeRosa, Agency for Toxic Substances and Disease Registry, Center
for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire, United
Kingdom
Dr H. Gibb, National Center for Environmental Assessment, United
States Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada ( Chairperson)
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan ( Vice-Chairperson)
Professor S.A. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia ( Rapporteur)
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute of
Occupational Medicine, Beijing, People's Republic of China
Observers
Professor F.M.C. Carpanini,1 Secretary-General, ECETOC (European
Centre for Ecotoxicology and Toxicology of Chemicals), Brussels,
Belgium
Dr M. Ema, Division of Biological Evaluation, National Institute of
Health Sciences, Osakai, Japan
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission,
Ispra, Italy
Mr T. Jacob,1 Dupont, Washington, DC, USA
Dr H. Koeter, Organisation for Economic Co-operation and Development,
Paris, France
Mr H. Kondo, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Ms J. Matsui, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Mr R. Montaigne,1 European Chemical Industry Council (CEFIC),
Brussels, Belgium
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr H. Nishimura, Environmental Health Science Laboratory, National
Institute of Health Sciences, Osaka, Japan
Ms C. Ohtake, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr T. Suzuki, Division of Food, National Institute of Health Sciences,
Tokyo, Japan
Dr K. Takeda, Mitsubishikagaku Institute of Toxicological and
Environmental Sciences, Yokohama, Japan
Dr K. Tasaka, Department of Chemistry, International Christian
University, Tokyo, Japan
Dr H. Yamada, Environment Conservation Division, National Research
Institute of Fisheries Science, Kanagawa, Japan
1 Invited but unable to attend.
Dr M. Yamamoto, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr M. Yasuno, School of Environmental Science, The University of Shiga
Prefecture, Hikone, Japan
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD sur les dérivés du triphénylétain a été préparé à partir
d'une évaluation effectuée par la Commission nationale japonaise des
CICAD (CICAD National Committee, 1997). De nombreuses études critiques
sur les effets sanitaires qui sont citées dans cette évaluation sont
tirées de monographies préparées par l'Organisation des Nations Unies
pour l'Alimentation et l'Agriculture (FAO, 1991a,b) et par
l'Organisation mondiale de la Santé (WHO, 1992). Ces monographies
récapitulent les nombreuses études communiquées à l'OMS par les
producteurs en vue d'une évaluation, en plus des résumés des articles
publiés. Dans le cas des études communiquées par les producteurs, les
documents originaux sont la propriété de leurs auteurs et n'ont pas
été mis à la disposition des auteurs de l'évaluation préparée par la
Commission nationale des CICAD (CICAD National Committee, 1997), des
rédacteurs du projet de CICAD et du Comité d'évaluation finale. Pour
ce qui concerne les données figurant dans ces documents originaux, les
rédacteurs du présent CICAD n'ont donc eu d'autre solution que de
s'appuyer sur les évaluations effectuées lors de la réunion conjointe
FAO/OMS sur les résidus de pesticides (JMPR).
De nombreux renseignements concernant les effets environnementaux
de ce composé ont été tirés d'une étude consacrée aux effets des
organostanniques sur l'environnement, publiée par l'Advisory Committee
on Pesticides of the Health and Safety Executive du Royaume-Uni (HSE,
1992). D'autres informations ont été obtenues en interrogeant les
bases de données Medline et Toxline Plus jusqu'à octobre 1997.On
trouvera à l'appendice 1 des indications sur les sources documentaires
utilisées ainsi que sur leur mode de dépouillement. Les renseignements
concernant l'examen du CICAD par des pairs font l'objet de l'appendice
2. Ce CICAD a été approuvé en tant qu'évaluation internationale lors
d'une réunion du Comité d'évaluation finale qui s'est tenue à Tokyo
(Japon) du 30 juin au 2 juillet 1998. La liste des participants à
cette réunion figure à l'appendice 3. La fiche d'information
internationale sur la sécurité chimique (ICSC No 1283) établie pour
l'hydroxyde de triphénylétain par le Programme international sur la
sécurité chimique (IPCS, 1996) est également reproduite dans ce
document.
Les composés du triphénylétain sont des dérivés triphénylés de
l'étain IV. Ce sont des solides incolores à faible tension de vapeur.
Ils sont lipophiles et peu solubles dans l'eau.
Depuis les années 1960, on fait grand usage des dérivés du
triphénylétain et du tributylétain comme algicides et molluscicides
dans les produits antisalissures. L'incorporation de composés
triorganostanniques dans les peintures marines antisalissures est
limitée dans de nombreux pays du fait de leurs effets catastrophiques
sur l'ostréiculture et sur les écosystèmes aquatiques en général. Le
triphénylétain est utilisé comme fongicide non endothérapique à action
essentiellement protectrice.
Le triphénylétain est fortement adsorbé par les solides en
suspension et le sol et ne se désorbe plus guère ensuite. On estime sa
demi-vie dans l'eau à quelques jours durant le mois de juin et à 2 ou
3 semaine en novembre. Bien que susceptibles de se décomposer par
déphénylation progressive et d'être excrétés sous la forme de
conjugués, les dérivés du triphénylétain s'accumulent dans l'organisme
des poissons et des gastéropodes, avec un facteur de bioconcentration
dont la valeur va de quelques centaines à 32 500 (dans le sac
intestinal de Lymnaea stagnalis).
La concentration des dérivés du triphénylétain dans
l'environnement dépend de leur mode, de leur moment et de leur lieu
d'utilisation. Dans des baies et des marinas, on a trouvé des
concentrations allant de 0 à près de 200 ng/litre, ces dernières
valeurs par suite du lessivage des peintures à base de triphénylétain
utilisées pour protéger la coque des bateaux contre les salissures. La
concentration des composés du triphénylétain dans l'environnement
diminue depuis quelques années en raison des restrictions sévères
imposées à leur utilisation dans les peintures antisalissures.
Administrés per os à des rats, les dérivés du triphénylétain ne
sont pas aisément résorbés et ils sont excrétés principalement dans
les matières fécales et en partie également dans les urines. Par
métabolisation, ils sont transformés en diphénylétain, en
monophénylétain et en résidus liés non extractibles. Une fois
absorbés, ils s'accumulent en majeure partie au niveau des reins et du
foie et en plus faible quantité dans d'autres organes. Appliqués sur
l'épiderme, ils peuvent traverser la peau selon un processus qui est
fonction du temps et de la dose.
Le triphénylétain exerce des effets variés sur l'organisme des
diverses espèces animales, notamment sur le système immunitaire ou sur
la reproduction et le développement à des des doses proches de celles
qui sont toxiques pour les mères (la plupart des valeurs de la dose la
plus faible sans effet observable ou LOAEL, sont de l'ordre de
quelques mg par kg de poids corporel ou moins). On observe également
des hyperplasies et des adénomes des glandes endocrines, l'apoptose
des cellules thymiques, la libération de calcium au niveau des
cellules du réticulum sarcoplasmique et une irritation oculaire. Les
mécanismes qui sont à l'origine de ces effets font encore l'objet
d'investigations et il est possible que ce profil toxicologique
s'explique par un mécanisme général.
Les dérivés du triphénylétain sont modérément toxiques pour le
rat. Ils ne sont pas cancérogènes, mais certaines données montrent
qu'ils ont une action coclastogène.
Parmi les effets sur la reproduction et le développement, on peut
citer la réduction du nombre des nidations et du nombre de foetus
vivants (à la dose journalière de 1,0 mg/kg p.c. d'acétate de
triphénylétain, ou TPTA, administré par gavage à des lapins), la
diminution de la taille des portées et du poids des lapereaux avec
également une réduction du poids relatif du thymus ou de la rate chez
des ratons juste sevrés (dans une étude sur deux générations des rats
recevant une alimentation contenant l'équivalent de 1,5 mg d'hydroxyde
de triphénylétain - TPTH - par kg de poids corporel et par jour; dans
cette étude, la dose sans effet nocif observable ou NOAEL, était de
0,4 mg/kg p.c. par jour) ainsi que des avortements et une diminution
du poids des foetus (dans une étude sur des lapins recevant
quotidiennement par gavage une dose de 0,9 mg de TPTH par kg de poids
corporel).
La valeur la plus faible de la NOAEL qui ait été obtenue dans une
étude toxicologique est de 0,1 mg de TPTH par kg p.c. par jour pour la
toxicité maternelle, les critères de toxicité retenus étant la
réduction de la consommation de nourriture et du gain de poids à la
dose quotidienne de 0,3 mg par kg de poids corporel. Une valeur
identique a été obtenue au début d'une étude de 2 ans sur des rats, au
cours de laquelle on a observé une légère diminution du nombre de
leucocytes aux doses élevées. Lors d'une étude de 52 semaines sur des
chiens, on a trouvé une NOAEL égale à 0,21 mg de TPTH par kg de poids
corporel par jour, le critère retenu étant la réduction du poids
relatif du foie chez les femelles soumises à des doses élevées.
Les dérivés du triphénylétain peuvent affecter le système
immunitaire. On a observé une diminution de la concentration des
immunoglobulines (Ig) (même à la dose la plus faible, soit 0,3 mg de
TPTH par kg p.c. par jour lors d'une étude d'alimentation de 2 ans sur
des rats), une lymphopénie (à la dose de 0,3 mg par kg p.c. par jour
dans une autre étude d'alimentation de 2 ans portant également sur des
rats et à la dose de 0,338 mg/m3 dans une étude d'inhalation sur des
rats), une atrophie du thymus (à la dose de 1,5 mg de chlorure de
triphénylétain - TPTCl - par kg p.c. par jour dans une étude
d'alimentation sur des rats juste sevrés) et enfin une atrophie de la
rate (à la dose de 5 mg de TPTH par kg p.c. par jour lors d'une étude
d'alimentation de 28 jours sur des souris). Les femelles sont
généralement plus sensibles que les mâles.
Pour établir la dose journalière admissible (DJA) de
triphénylétain en cas d'exposition per os, le JMPR a pris en
considération plusieurs points d'aboutissement des effets
toxicologiques (FAO, 1991b; WHO, 1992). En premier lieu, on a appliqué
un coefficient d'incertitude de 200 à la dose sans effet observable
(NOEL) de 0,1 mg/kg p.c. par jour (basée sur l'observation d'une
diminution du nombre de leucocytes à dose élevée lors d'une étude de 2
ans sur des rats) pour obtenir une DJA de 0-0,5 µg/kg de poids
corporel. On a ensuite appliqué un coefficient d'incertitude de 500 à
la LOAEL de 0,3 mg/kg p.c. par jour tirée d'une étude de 2 ans sur des
rats, au cours de laquelle on avait noté une augmentation de la
mortalité et une diminution des taux d'immunoglobulines sériques. Les
autres valeurs de la NOAEL qui ont été prises en considération
parallèlement aux résultats précités sont les suivantes : 0,4 mg/kg
p.c. par jour dans le cas d'une étude sur la reproduction portant sur
deux générations de rats (cette étude a révélé une réduction du poids
de la rate et du thymus chez les ratons mâles et femelles juste sevrés
des générations F1 et F2 aux doses les plus élevées); 0,3 mg/kg p.c.
par jour à l'occasion d'une étude à court terme sur des rats
(réduction du nombre de leucocytes, diminution du taux des IgG et
augmentation du poids relatif des testicules aux doses élevées); 0,21
mg/kg p.c. par jour lors d'une étude à court terme sur des chiens
(augmentation du poids relatif du foie et diminution du rapport
albumine sérique / globulines aux doses élevées); enfin, 0,1 mg/kg
p.c. lors d'une étude tératologique sur des lapins (toxicité
maternelle constatée aux doses élevées).
On ne possède pas de données sur l'exposition professionnelle aux
dérivés du triphénylétain. Il existe toutefois un certain nombre de
rapports sur des cas d'intoxication où sont décrits des effets
neurotoxiques apparemment durables. L'exposition de la population
générale à ces composés provient essentiellement de l'ingestion de
produits de la mer contaminés. En effet, on a trouvé des
concentrations de triphénylétain pouvant atteindre 1 µg/g dans les
muscles de certaines espèces de poissons. Au Japon, on estime qu'en
1997 l'absorption de triphénylétain par suite de la consommation
d'aliments contaminés se situait aux alentours de 11 % de la DJA
(c'est-à-dire 2,75 µg/jour pour un sujet de 50 kg) établie par le
JMPR.
Les dérivés du triphénylétain exercent des effets délétères sur
les organismes aquatiques à très faible concentration. Par exemple, on
observé l'apparition d'organes mâles chez des femelles de gastéropodes
japonais à la concentration d'environ 1 ng/litre (concentration sans
effet ou NOEC, non déterminée) et des effets toxiques ont été observés
chez les larves d'une sorte de vairon, Pimephales promelas, à la
concentration de 0,23 µg/litre (concentration la plus faible
produisant un effet ou LOEC). On estime que le triphénylétain perturbe
les fonctions endocriniennes; en effet, l'apparition d'organes sexuels
mâles chez les gastéropodes femelles est probablement due à un trouble
hormonal.
On n'a pas établi de NOEC relative au changement de sexe chez les
mollusques par suite d'une exposition au triphénylétain. On a constaté
expérimentalement, en procédant à des injections, que le
triphénylétain avait une activité du même ordre que celle du
tributylétain vis-à-vis du genre Thais. Chez les mollusques du
genre Nucella il est moins actif que le tributylétain, mais sa
bioaccumulation est supérieure. De ces expérimentations, on peut
conclure que la NOEC du triphénylétain doit être de quelques ng/litre
tout au plus. Cette estimation est corroborée par la fréquence de
l'appartion, en situation réelle, d'organes mâles chez des mollusques
femelles du genre Thais exposés aux concentrations ambiantes. Dans
l'environnement, les résidus de triphénylétain accompagnent ceux de
tributylétain, aussi ne peut-on évaluer leur contribution respective
au phénomène d'apparition d'organes mâles chez les gastéropodes
femelles du genre Thais. Dans ces conditions, on peut conclure que
l'utilisation de l'un ou l'autre de ces organostanniques dans les
peintures antisalissures conduit de tout manière à la décimation des
invertébrés marins.
RESUMEN DE ORIENTACION
Este CICAD sobre los compuestos del trifenilestaño se basa en el
examen preparado por el Comité Nacional para los Documentos
Internacionales Concisos sobre Evaluación de Sustancias Químicas
(Comité Nacional para los CICAD, 1997). En el presente examen se citan
numerosos estudios críticos de los efectos en la salud procedentes de
monografías sobre residuos de los plaguicidas preparadas por la
Organización de las Naciones Unidas para la Agricultura y la
Alimentación (FAO, 1991a,b) y la Organización Mundial de la Salud
(OMS, 1992). Estas monografías contienen los resúmenes de los
numerosos estudios que los fabricantes presentaron a la OMS para su
evaluación, además de resúmenes de los documentos publicados. En el
caso de los estudios presentados por los fabricantes, los documentos
originales son privados y los autores del examen preparado por el
Comité Nacional para los CICAD (1977), los autores del proyecto del
CICAD o la Junta de Evaluación Final del CICAD no pudieron disponer de
ellos. Por consiguiente, este CICAD se basa inevitablemente en las
evaluaciones realizadas en la reunión conjunta FAO/OMS sobre residuos
de plaguicidas (JMPR) para los estudios citados de los resúmenes de
datos privados.
Se obtuvo amplia información sobre los efectos en el medio
ambiente de un examen acerca de los efectos ecológicos de los
compuestos de estaño con tres grupos orgánicos, preparado por el
Comité Consultivo sobre Plaguicidas de la Dirección de Salud y
Seguridad del Reino Unido (HSE, 1992). Otros datos proceden de una
búsqueda en las bases de datos Medline y Toxline Plus hasta octubre de
1997. La información relativa al carácter de los procesos de examen y
la disponibilidad de los documentos originales figura en el apéndice
1. La información acerca del examen colegiado de este CICAD se
presenta en el apéndice 2. Su aprobación tuvo lugar como evaluación
internacional en una reunión de la Junta de Evaluación Final,
celebrada en Tokio, Japón, del 30 de junio al 2 de julio de 1998. La
lista de participantes en esta reunión aparece en el apéndice 3. La
Ficha internacional de seguridad química (ICSC 1283) para el hidróxido
de trifenilestaño (TPTH), preparada por el Programa Internacional de
Seguridad de las Sustancias Químicas (IPCS, 1996) también se reproduce
en el presente documento.
Los compuestos de trifenilestaño son derivados trifenílicos del
estaño tetravalente. Son sólidos incoloros con presiones de vapor
bajas. Son lipófilos y su solubilidad en agua es escasa.
Los compuestos de trifenilestaño y tributilestaño se han
utilizado ampliamente desde los años sesenta como alguicidas y
molusquicidas en los productos antiincrustantes. Se ha restringido el
empleo de los compuestos de estaño con tres grupos orgánicos en las
pinturas antiincrustantes debido a sus efectos devastadores en la
industria de las ostras y a los más generales en el ecosistema
acuático. El trifenilestaño se utiliza como fungicida no sistémico de
acción fundamentalmente protectora.
El trifenilestaño se adsorbe fuertemente al sedimento y al suelo
y la desorción es escasa. Su semivida en agua se ha estimado en varios
días en junio y en 2-3 semanas en noviembre. Si bien los compuestos de
trifenilestaño se pueden degradar mediante defenilación escalonada y
excretarse en forma conjugada, se ha observado bioacumulación en los
peces y los caracoles, con factores de bioconcentración que oscilan
entre varios cientos y 32 500 (en el saco intestinal de Lymnaea
stagnalis).
Las concentraciones de los compuestos de trifenilestaño en el
medio ambiente varían en función de la manera, el momento y el lugar
de utilización de esos compuestos. Se han detectado concentraciones
que oscilan entre 0 ng/litro y casi 200 ng/litro en zonas de bahías o
puertos deportivos debido a la lixiviación a partir de las
embarcaciones tratadas con pinturas antiincrustantes que contienen
trifenilestaño. Las concentraciones de compuestos de trifenilestaño en
el medio ambiente se han reducido en los últimos años como
consecuencia del endurecimiento de las restricciones sobre su uso en
las pinturas antiincrustantes.
Administrados a ratas por vía oral, los compuestos de
trifenilestaño no se absorben con facilidad y se excretan
fundamentalmente en las heces y parcialmente en la orina. Se
metabolizan a difenilestaño, monofenilestaño y residuos ligados no
extraíbles. Los compuestos de trifenilestaño absorbidos se acumulan
sobre todo en el riñón y el hígado y en menor cantidad en otros
órganos. Tras su aplicación cutánea pueden penetrar a través de la
piel, de forma dependiente del tiempo y la concentración.
El trifenilestaño tiene diversos efectos en la salud de las
distintas especies animales, en particular en el sistema inmunitario,
efectos en la reproducción/desarrollo, con niveles próximos a los de
toxicidad materna (las concentraciones más bajas con efectos adversos
observados o LOAEL son en general del orden de mg/kg o inferiores),
hiperplasia/adenomas en los órganos endocrinos, apoptosis en las
células del timo, liberación de calcio en las células del retículo
sarcoplásmico e irritación ocular. Se están investigando los
mecanismos que provocan esos efectos; este perfil de toxicidad se
puede explicar por un mecanismo común.
Los compuestos de trifenilestaño tienen una toxicidad
moderadamente aguda en las ratas. No son carcinogénicos, pero algunos
datos ponen de manifiesto una acción coclastogénica.
Los efectos reproductivos y en el desarrollo son un aumento en el
número de implantaciones y fetos vivos (con 1,0 mg de acetato de
trifenilestaño (TPTA)/kg de peso corporal al día en un estudio de
administración con sonda realizado en conejos), reducción del tamaño
de la camada/peso de las crías y del peso relativo del timo o el bazo
en las crías destetadas (con 1,5 mg de TPTH/kg de peso corporal al día
en los alimentos en un estudio de reproducción en dos generaciones
realizado en ratas; la concentración sin efectos adversos observados o
NOAEL es de 0,4 mg/kg de peso corporal al día) y aborto y reducción
del peso fetal (con 0,9 mg de TPTH/kg de peso corporal al día en un
estudio de administración con sonda realizado en conejos).
La NOAEL más baja detectada en las pruebas de toxicidad fue de
0,1 mg de TPTH/kg de peso corporal al día para la toxicidad materna en
un estudio de administración con sonda realizado en conejos, basado en
la disminución del consumo de alimentos y del aumento del peso
corporal con 0,3 mg/kg de peso corporal al día. Se obtuvo el mismo
valor en un estudio inicial de dos años realizado con ratas, en el
cual se observó que con las dosis más altas se producía una ligera
disminución de la concentración de leucocitos. En un estudio de 52
semanas realizado con perros se estimó una NOAEL de 0,21 mg de TPTH/kg
de peso corporal al día, tomando como base una disminución del peso
relativo del hígado en las hembras con las dosis más altas.
Los compuestos de tributilestaño afectan al sistema inmunitario.
Se ha observado una disminución de la concentración de
inmunoglobulinas (incluso con la dosis más baja, es decir, 0,3 mg de
TPTH/kg de peso corporal al día, en un estudio de alimentación de dos
años realizado con ratas), linfopenia (con 0,3 mg de TPTH/kg de peso
corporal al día en otro estudio de alimentación de dos años realizado
con ratas o de 0,338 mg/m3 en un estudio de inhalación de 13 semanas
con ratas), atrofia del timo (con 1,5 mg de cloruro de trifenilestaño
(TPTCl)/kg de peso corporal al día en un estudio de alimentación de
dos semanas realizado con ratas destetadas) y atrofia del bazo (con 5
mg de TPTH/kg de peso corporal al día en un estudio de alimentación de
28 días realizado con ratones). En general, las hembras son más
susceptibles que los machos.
La JMPR tuvo en cuenta varios efectos finales al establecer la
ingesta diaria admisible (IDA) de trifenilestaño para la exposición
oral (FAO, 1991b; OMS, 1992). En primer lugar, se aplicó un factor de
incertidumbre de 200 a la concentración sin efectos observados (NOEL)
de 0,1 mg/kg de peso corporal al día (basada en el resultado de la
disminución de la concentración de leucocitos con las dosis más altas
obtenido en un estudio de dos años realizado con ratas) para llegar a
una IDA de 0-0,5 µg/kg de peso corporal. En segundo lugar, se aplicó
un factor de incertidumbre de 500 a una LOAEL de 0,3 mg/kg de peso
corporal al día en un estudio de dos años realizado en ratas en el que
se observó un aumento de la mortalidad y una reducción de la
concentración de inmunoglobulina sérica. Otras LOAEL que se tuvieron
en cuenta, junto con los efectos citados más arriba, son 0,4 mg/kg de
peso corporal al día en un estudio de la reproducción en dos
generaciones realizado con ratas (con los niveles más altos se observó
una disminución dependiente de la dosis de los pesos relativos del
bazo y el timo en los machos y las hembras destetados de la F1 y la
F2), 0,3 mg/kg de peso corporal al día en un estudio de corta
duración realizado en ratas (con las dosis más altas se detectó una
reducción de la concentración de leucocitos y de la IgG y un aumento
del peso relativo de los testículos), 0,21 mg/kg de peso corporal al
día en un estudio de corta duración realizado en perros (con las dosis
más altas se observó un aumento del peso relativo del hígado y una
disminución de la razón albúmina sérica/globulina) y 0,1 mg/kg de peso
corporal al día en un estudio de teratología en conejos (con las dosis
más altas se detectó toxicidad materna).
No se dispone de datos relativos a la exposición ocupacional a
los compuestos de trifenilestaño. En un pequeño número de informes de
casos de intoxicación se describen efectos neurotóxicos, que parecían
persistir. La exposición del público general a estos compuestos se
produce fundamentalmente por la ingestión de alimentos marinos
contaminados, en los cuales se han encontrado a veces concentraciones
de hasta 1 µg/g (en el músculo de algunas especies de peces). En 1997
se estimó en el Japón una ingesta de trifenilestaño a partir de
alimentos contaminados de alrededor del 11% de la IDA (es decir, 2,75
µg/día para una persona de 50 kg) establecida por la JMPR.
Los compuestos de trifenilestaño tienen efectos nocivos en los
organismos acuáticos a concentraciones muy bajas. Por ejemplo, se
observó imposexo en especies del género Thais (gasterópodos del
Japón) con concentraciones de alrededor de 1 ng/litro (no se determinó
la concentración sin efectos observados o NOEC) y se observó toxicidad
crónica en las larvas de Pimephales promelas con 0,23 µg/litro
(concentración más baja con efectos observados o LOEC). Se considera
que el trifenilestaño es un perturbador del sistema endocrino a causa
del imposexo, fenómeno en el cual se forman órganos sexuales
masculinos en los gasterópodos hembra, probablemente debido a un
trastorno hormonal.
No se ha establecido la NOEC del trifenilestaño para el imposexo
de los moluscos. El trifenilestaño tiene experimentalmente, mediante
inyección, un efecto semejante al del tributilestaño en el género
Thais. El primero es menos potente que el segundo en Nucella; sin
embargo, su bioacumulación es mayor. A partir de esta información se
puede estimar que la NOEC para el trifenilestaño será de varios
ng/litro o más baja. La prevalencia de imposexo en Thais observada
sobre el terreno en presencia de determinadas concentraciones en el
medio ambiente apoya esta estimación. Habida cuenta de que los
residuos de trifenilestaño y tributilestaño aparecen juntos en el
medio ambiente, no se puede evaluar su contribución relativa al
imposexo de las especies de Thais. Conforme a esta información, el
uso de trifenilestaño o de tributilestaño en la pintura
antiincrustante produciría una disminución de la población de los
invertebrados marinos.
See Also:
Toxicological Abbreviations
Triphenyltin compounds (FAO Meeting Report PL/1965/10/1)
 Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on triphenyltin compounds was based on a review
prepared by the National Committee for Concise International Chemical
Assessment Documents of Japan (CICAD National Committee, 1997). Many
critical studies on health effects in this review were cited from
monographs on pesticide residues prepared by the Food and Agriculture
Organisation of the United Nations (FAO, 1991a,b) and the World Health
Organization (WHO, 1992). These monographs report summaries of the
many studies submitted to WHO by manufacturers for evaluation, in
addition to summaries of published papers. In the case of studies
submitted by manufacturers, original papers are proprietary and were
not available to authors of the review prepared by the CICAD National
Committee (1997), to authors of the CICAD draft, or to the CICAD Final
Review Board. Therefore, this CICAD inevitably relies on the
evaluations made by the Joint FAO/WHO Meeting on Pesticide Residues
(JMPR) for those studies cited from summaries of proprietary data.
Extensive information on environmental effects was obtained from
a review of the environmental effects of triorganotin compounds,
prepared by the Advisory Committee on Pesticides of the Health and
Safety Executive of the United Kingdom (HSE, 1992). Additional data
were obtained through a search of Medline and Toxline Plus databases
up to October 1997. Information on the nature of the review processes
and the availability of the source documents is presented in Appendix
1. Information on the peer review of this CICAD is presented in
Appendix 2. This CICAD was approved as an international assessment at
a meeting of the Final Review Board, held in Tokyo, Japan, on 30 June
- 2 July 1998. Participants at the Final Review Board meeting are
listed in Appendix 3. The International Chemical Safety Card (ICSC
1283) for triphenyltin hydroxide (TPTH), produced by the International
Programme on Chemical Safety (IPCS, 1996), has also been reproduced in
this document.
Triphenyltin compounds are triphenyl derivatives of tetravalent
tin. They are colourless solids with low vapour pressures. They are
lipophilic and have low solubility in water.
Triphenyltin and tributyltin compounds have been used extensively
as algicides and molluscicides in antifouling products since the
1960s. Use of triorganotins in antifouling paints has been restricted
in many countries because of their catastrophic effects on the oyster
industry and more general effects on the aquatic ecosystem.
Triphenyltin is used as a non-systemic fungicide with mainly
protective action.
Triphenyltin is strongly adsorbed to sediment and soil, and
little desorption occurs. Its half-life in water has been estimated to
be several days in June and 2-3 weeks in November. Although
triphenyltin compounds can be degraded by stepwise dephenylation and
excreted in conjugated forms, they bioaccumulate in fish and snails,
with bioconcentration factors (BCFs) ranging from several hundred to
32 500 (in the intestinal sac of Lymnaea stagnalis).
Environmental concentrations of triphenyltin compounds vary
depending upon how, when, and where the compounds were used.
Concentrations ranging from 0 ng/litre to nearly 200 ng/litre have
been detected in bay areas or marinas as a result of leaching from
ships treated with triphenyltin-based antifouling paints.
Environmental concentrations of triphenyltin compounds have decreased
in recent years as a result of tightening restrictions on their use in
antifouling paints.
Triphenyltin compounds given orally to rats are not readily
absorbed and are excreted primarily in faeces and partly in urine.
They are metabolized to diphenyltin, monophenyltin, and
non-extractable bound residues. Absorbed triphenyltin compounds
accumulate in kidney and liver to the greatest extent, with smaller
amounts in other organs. Triphenyltin compounds applied dermally can
penetrate through the skin in a time- and dose-dependent manner.
Triphenyltin exerts a variety of health effects in various animal
species, including effects on the immune system,
reproductive/developmental effects at levels near those that are
maternally toxic (most lowest-observed-adverse-effect levels, or
LOAELs, are in the several mg/kg range or lower), hyperplasia/adenomas
in endocrine organs, apoptosis in thymus cells, calcium release in
sarcoplasmic reticulum cells, and eye irritation. The underlying
mechanisms of these effects are under investigation; a common
mechanism may explain this toxicity profile.
Triphenyltin compounds are moderately acutely toxic to rats. They
are not carcinogenic, but some data show that they are co-clastogenic.
Reproductive and developmental effects include a decrease in the
number of implantations and live fetuses (at 1.0 mg triphenyltin
acetate [TPTA]/kg body weight per day in a rabbit gavage study),
reduction in litter size/pup weight and in relative thymus or spleen
weight in the weanlings (at 1.5 mg TPTH/kg body weight per day in the
diet in a two-generation reproduction study in rats;
no-observed-adverse-effect level, or NOAEL, 0.4 mg/kg body weight per
day), and abortion and reduction in fetal weight (at 0.9 mg TPTH/kg
body weight per day in a rabbit gavage study).
The lowest NOAEL detected in the toxicity tests was 0.1 mg
TPTH/kg body weight per day for maternal toxicity in a rabbit gavage
study, based on decreased food consumption and body weight gain at 0.3
mg/kg body weight per day. The same value was obtained in an early
2-year rat study in which a slight decrease in white blood cells was
seen at higher doses. In a 52-week dog study, the NOAEL was estimated
to be 0.21 mg TPTH/kg body weight per day based on a decrease in
relative liver weight in females at higher doses.
Triphenyltin compounds affect the immune system. A decrease in
immunoglobulin (Ig) concentrations (even at the lowest dose level,
i.e., 0.3 mg TPTH/kg body weight per day in a 2-year feeding study in
rats), lymphopenia (at 0.3 mg TPTH/kg body weight per day in another
2-year feeding study in rats or at 0.338 mg TPTH/m3 in a 13-week
inhalation study in rats), thymus atrophy (at 1.5 mg triphenyltin
chloride [TPTCl]/kg body weight per day in a 2-week feeding study with
weanling rats), and splenic atrophy (at 5 mg TPTH/kg body weight per
day in a 28-day feeding study in mice) have been observed. Females are
generally more susceptible than males.
Several end-points were taken into consideration by JMPR in
establishing the acceptable daily intake (ADI) of triphenyltin for
oral exposure (FAO, 1991b; WHO, 1992). First, a 200-fold uncertainty
factor was applied to the no-observed-effect level (NOEL) of 0.1 mg/kg
body weight per day (based on a finding of reduced white blood cell
counts at higher doses in a 2-year study in rats) to arrive at an ADI
of 0-0.5 µg/kg body weight. Secondly, a 500-fold uncertainty factor
was applied to a LOAEL of 0.3 mg/kg body weight per day in a 2-year
study in rats in which increased mortality and reduced serum
immunoglobulin levels were noted. Other NOAELs that were taken into
consideration together with the above effect levels are 0.4 mg/kg body
weight per day in a two-generation reproduction study with rats (a
dose-related decrease in spleen and thymus weights in F1 and F2 male
and female weanlings was observed at higher levels), 0.3 mg/kg body
weight per day in a short-term study in rats (reduction in white blood
cells, decrease in IgG, and increase in relative testes weight were
seen at higher levels), 0.21 mg/kg body weight per day in a short-term
dog study (increase in relative liver weight and decrease in serum
albumin/globulin ratio were seen at higher levels), and 0.1 mg/kg body
weight per day in a teratology study in rabbits (maternal toxicity was
seen at higher levels).
There are no data concerning occupational exposure to
triphenyltin compounds. A few poisoning case reports describe
neurotoxic effects, which appeared to persist. Exposure of the general
public to triphenyltin compounds occurs mostly from ingestion of
contaminated seafood, which in some cases has been found to contain
triphenyltin levels as high as 1 µg/g (in muscle of some fish
species). Triphenyltin intake from contaminated foods in Japan in 1997
was estimated to be around 11% of the ADI (i.e., 2.75 µg/day for a
50-kg person) established by JMPR.
Triphenyltin compounds exert deleterious effects on aquatic
organisms at very low concentrations. For example, imposex of rock
shells (Japanese gastropods) was seen at around 1 ng/litre
(no-observed-effect concentration, or NOEC, not determined), and
chronic toxicity to fathead minnow ( Pimephales promelas) larvae was
observed at 0.23 µg/litre (lowest-observed-effect concentration, or
LOEC). Triphenyltin is considered to be an endocrine disruptor,
because imposex, a phenomenon in which female gastropods develop male
sex organs, is probably caused by hormonal disturbance.
No NOEC for triphenyltin has been established for imposex in
molluscs. Experimentally, by injection, triphenyltin has a potency
similar to that of tributyltin in the genus Thais. Triphenyltin is
less potent than tributyltin in Nucella; however, triphenyltin shows
greater bioaccumulation than tributyltin. From this, it can be
estimated that the NOEC for triphenyltin will be a few ng/litre or
lower. The observed prevalence of imposex in Thais in the field with
ambient concentrations supports this estimate. Because residues of
triphenyltin and tributyltin occur together in the environment, their
relative contribution to observed imposex cannot be assessed for
Thais species. Use of either triphenyltin or tributyltin in
antifouling paint would lead to population declines of marine
invertebrates on this basis.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Triphenyltin compounds are triphenyl derivatives of tetravalent
tin. They conform to the general formula (C6H5)3Sn-X, where X is an
anion or an anionic group, such as chloride, hydroxide, and acetate.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
1. EXECUTIVE SUMMARY
This CICAD on triphenyltin compounds was based on a review
prepared by the National Committee for Concise International Chemical
Assessment Documents of Japan (CICAD National Committee, 1997). Many
critical studies on health effects in this review were cited from
monographs on pesticide residues prepared by the Food and Agriculture
Organisation of the United Nations (FAO, 1991a,b) and the World Health
Organization (WHO, 1992). These monographs report summaries of the
many studies submitted to WHO by manufacturers for evaluation, in
addition to summaries of published papers. In the case of studies
submitted by manufacturers, original papers are proprietary and were
not available to authors of the review prepared by the CICAD National
Committee (1997), to authors of the CICAD draft, or to the CICAD Final
Review Board. Therefore, this CICAD inevitably relies on the
evaluations made by the Joint FAO/WHO Meeting on Pesticide Residues
(JMPR) for those studies cited from summaries of proprietary data.
Extensive information on environmental effects was obtained from
a review of the environmental effects of triorganotin compounds,
prepared by the Advisory Committee on Pesticides of the Health and
Safety Executive of the United Kingdom (HSE, 1992). Additional data
were obtained through a search of Medline and Toxline Plus databases
up to October 1997. Information on the nature of the review processes
and the availability of the source documents is presented in Appendix
1. Information on the peer review of this CICAD is presented in
Appendix 2. This CICAD was approved as an international assessment at
a meeting of the Final Review Board, held in Tokyo, Japan, on 30 June
- 2 July 1998. Participants at the Final Review Board meeting are
listed in Appendix 3. The International Chemical Safety Card (ICSC
1283) for triphenyltin hydroxide (TPTH), produced by the International
Programme on Chemical Safety (IPCS, 1996), has also been reproduced in
this document.
Triphenyltin compounds are triphenyl derivatives of tetravalent
tin. They are colourless solids with low vapour pressures. They are
lipophilic and have low solubility in water.
Triphenyltin and tributyltin compounds have been used extensively
as algicides and molluscicides in antifouling products since the
1960s. Use of triorganotins in antifouling paints has been restricted
in many countries because of their catastrophic effects on the oyster
industry and more general effects on the aquatic ecosystem.
Triphenyltin is used as a non-systemic fungicide with mainly
protective action.
Triphenyltin is strongly adsorbed to sediment and soil, and
little desorption occurs. Its half-life in water has been estimated to
be several days in June and 2-3 weeks in November. Although
triphenyltin compounds can be degraded by stepwise dephenylation and
excreted in conjugated forms, they bioaccumulate in fish and snails,
with bioconcentration factors (BCFs) ranging from several hundred to
32 500 (in the intestinal sac of Lymnaea stagnalis).
Environmental concentrations of triphenyltin compounds vary
depending upon how, when, and where the compounds were used.
Concentrations ranging from 0 ng/litre to nearly 200 ng/litre have
been detected in bay areas or marinas as a result of leaching from
ships treated with triphenyltin-based antifouling paints.
Environmental concentrations of triphenyltin compounds have decreased
in recent years as a result of tightening restrictions on their use in
antifouling paints.
Triphenyltin compounds given orally to rats are not readily
absorbed and are excreted primarily in faeces and partly in urine.
They are metabolized to diphenyltin, monophenyltin, and
non-extractable bound residues. Absorbed triphenyltin compounds
accumulate in kidney and liver to the greatest extent, with smaller
amounts in other organs. Triphenyltin compounds applied dermally can
penetrate through the skin in a time- and dose-dependent manner.
Triphenyltin exerts a variety of health effects in various animal
species, including effects on the immune system,
reproductive/developmental effects at levels near those that are
maternally toxic (most lowest-observed-adverse-effect levels, or
LOAELs, are in the several mg/kg range or lower), hyperplasia/adenomas
in endocrine organs, apoptosis in thymus cells, calcium release in
sarcoplasmic reticulum cells, and eye irritation. The underlying
mechanisms of these effects are under investigation; a common
mechanism may explain this toxicity profile.
Triphenyltin compounds are moderately acutely toxic to rats. They
are not carcinogenic, but some data show that they are co-clastogenic.
Reproductive and developmental effects include a decrease in the
number of implantations and live fetuses (at 1.0 mg triphenyltin
acetate [TPTA]/kg body weight per day in a rabbit gavage study),
reduction in litter size/pup weight and in relative thymus or spleen
weight in the weanlings (at 1.5 mg TPTH/kg body weight per day in the
diet in a two-generation reproduction study in rats;
no-observed-adverse-effect level, or NOAEL, 0.4 mg/kg body weight per
day), and abortion and reduction in fetal weight (at 0.9 mg TPTH/kg
body weight per day in a rabbit gavage study).
The lowest NOAEL detected in the toxicity tests was 0.1 mg
TPTH/kg body weight per day for maternal toxicity in a rabbit gavage
study, based on decreased food consumption and body weight gain at 0.3
mg/kg body weight per day. The same value was obtained in an early
2-year rat study in which a slight decrease in white blood cells was
seen at higher doses. In a 52-week dog study, the NOAEL was estimated
to be 0.21 mg TPTH/kg body weight per day based on a decrease in
relative liver weight in females at higher doses.
Triphenyltin compounds affect the immune system. A decrease in
immunoglobulin (Ig) concentrations (even at the lowest dose level,
i.e., 0.3 mg TPTH/kg body weight per day in a 2-year feeding study in
rats), lymphopenia (at 0.3 mg TPTH/kg body weight per day in another
2-year feeding study in rats or at 0.338 mg TPTH/m3 in a 13-week
inhalation study in rats), thymus atrophy (at 1.5 mg triphenyltin
chloride [TPTCl]/kg body weight per day in a 2-week feeding study with
weanling rats), and splenic atrophy (at 5 mg TPTH/kg body weight per
day in a 28-day feeding study in mice) have been observed. Females are
generally more susceptible than males.
Several end-points were taken into consideration by JMPR in
establishing the acceptable daily intake (ADI) of triphenyltin for
oral exposure (FAO, 1991b; WHO, 1992). First, a 200-fold uncertainty
factor was applied to the no-observed-effect level (NOEL) of 0.1 mg/kg
body weight per day (based on a finding of reduced white blood cell
counts at higher doses in a 2-year study in rats) to arrive at an ADI
of 0-0.5 µg/kg body weight. Secondly, a 500-fold uncertainty factor
was applied to a LOAEL of 0.3 mg/kg body weight per day in a 2-year
study in rats in which increased mortality and reduced serum
immunoglobulin levels were noted. Other NOAELs that were taken into
consideration together with the above effect levels are 0.4 mg/kg body
weight per day in a two-generation reproduction study with rats (a
dose-related decrease in spleen and thymus weights in F1 and F2 male
and female weanlings was observed at higher levels), 0.3 mg/kg body
weight per day in a short-term study in rats (reduction in white blood
cells, decrease in IgG, and increase in relative testes weight were
seen at higher levels), 0.21 mg/kg body weight per day in a short-term
dog study (increase in relative liver weight and decrease in serum
albumin/globulin ratio were seen at higher levels), and 0.1 mg/kg body
weight per day in a teratology study in rabbits (maternal toxicity was
seen at higher levels).
There are no data concerning occupational exposure to
triphenyltin compounds. A few poisoning case reports describe
neurotoxic effects, which appeared to persist. Exposure of the general
public to triphenyltin compounds occurs mostly from ingestion of
contaminated seafood, which in some cases has been found to contain
triphenyltin levels as high as 1 µg/g (in muscle of some fish
species). Triphenyltin intake from contaminated foods in Japan in 1997
was estimated to be around 11% of the ADI (i.e., 2.75 µg/day for a
50-kg person) established by JMPR.
Triphenyltin compounds exert deleterious effects on aquatic
organisms at very low concentrations. For example, imposex of rock
shells (Japanese gastropods) was seen at around 1 ng/litre
(no-observed-effect concentration, or NOEC, not determined), and
chronic toxicity to fathead minnow ( Pimephales promelas) larvae was
observed at 0.23 µg/litre (lowest-observed-effect concentration, or
LOEC). Triphenyltin is considered to be an endocrine disruptor,
because imposex, a phenomenon in which female gastropods develop male
sex organs, is probably caused by hormonal disturbance.
No NOEC for triphenyltin has been established for imposex in
molluscs. Experimentally, by injection, triphenyltin has a potency
similar to that of tributyltin in the genus Thais. Triphenyltin is
less potent than tributyltin in Nucella; however, triphenyltin shows
greater bioaccumulation than tributyltin. From this, it can be
estimated that the NOEC for triphenyltin will be a few ng/litre or
lower. The observed prevalence of imposex in Thais in the field with
ambient concentrations supports this estimate. Because residues of
triphenyltin and tributyltin occur together in the environment, their
relative contribution to observed imposex cannot be assessed for
Thais species. Use of either triphenyltin or tributyltin in
antifouling paint would lead to population declines of marine
invertebrates on this basis.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Triphenyltin compounds are triphenyl derivatives of tetravalent
tin. They conform to the general formula (C6H5)3Sn-X, where X is an
anion or an anionic group, such as chloride, hydroxide, and acetate.
 Physical and chemical properties of triphenyltin compounds vary
depending upon the anion linked to tin. At ambient temperatures in the
pH range of 3-8, TPTA and TPTCl are hydrolysed to TPTH within 1 min;
as a consequence, the results of most studies with TPTA or TPTCl can
be applied to TPTH. Triphenyltin compounds are colourless solids with
low vapour pressures (<2 mPa at 50°C). The compounds are lipophilic
and have low water solubility (typically a few mg/litre at neutral
pH).
The identity and physical/chemical properties of TPTH, TPTA, and
TPTCl are given in Table 1. Additional properties of TPTH are
presented in the International Chemical Safety Card (ICSC 1283)
reproduced in this document.
Table 1: Identity and physical/chemical properties of some triphenyltin compounds.a
Triphenyltin hydroxide Triphenyltin acetate Triphenyltin chloride
Synonyms Fentin hydroxide; TPTH Fentin acetate; TPTA Fentin chloride; TPTCl
Chemical Abstracts
Physical and chemical properties of triphenyltin compounds vary
depending upon the anion linked to tin. At ambient temperatures in the
pH range of 3-8, TPTA and TPTCl are hydrolysed to TPTH within 1 min;
as a consequence, the results of most studies with TPTA or TPTCl can
be applied to TPTH. Triphenyltin compounds are colourless solids with
low vapour pressures (<2 mPa at 50°C). The compounds are lipophilic
and have low water solubility (typically a few mg/litre at neutral
pH).
The identity and physical/chemical properties of TPTH, TPTA, and
TPTCl are given in Table 1. Additional properties of TPTH are
presented in the International Chemical Safety Card (ICSC 1283)
reproduced in this document.
Table 1: Identity and physical/chemical properties of some triphenyltin compounds.a
Triphenyltin hydroxide Triphenyltin acetate Triphenyltin chloride
Synonyms Fentin hydroxide; TPTH Fentin acetate; TPTA Fentin chloride; TPTCl
Chemical Abstracts 