
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
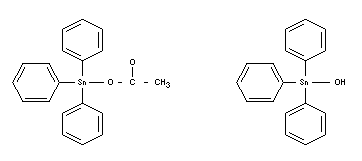
TRIPHENYLTIN COMPOUNDS
Chemical name
Triphenyltin acetate Triphenyltin hydroxide
Synonyms
Fentin
Empirical formulae
C20H18O2Sn C18H16OSn
Structural formulae
 BIOLOGICAL DATA
Biochemical aspects
After ingestion of triphenyltin by cows or sheep, the compound
is largely excreted with the faeces (Bügemann et al., 1964; Herok &
Götte, 1961). Three sheep given 10 mg daily of 113Sn-triphenyltin
acetate for 20 days were killed respectively 28, 52 and 218 days after
the beginning of the treatment. 113Sn was found in the milk (average
concentration 0.0017 ppm) during the treatment but 17 days after
withdrawal of the compound its concentration in the milk was near
detectable limits. Tin was present in the milk in at least two forms
other than triphenyltin acetate. During the treatment, the
concentration of 113Sn in the blood and in the wine were 2.9 µg/l and
7.5 µg/l respectively. In the sheep killed at 28 days, liver, kidney,
lungs, pancreas, gall-bladder and brain contained a greater amount of
113Sn than other organs. After 218 days the amount of 113Sn found in
the liver was still greater than in other organs (Herok & Götte,
1961).
Work on rats given 113Sn-triphenyltin showed that absorbed
triphenyltin was rapidly distributed through the tissues of the body
including the brain. Triphenyltin was eliminated relatively slowly and
it could be detected in the brain 38 days after a single dose (Heath,
1963).
In vitro studies have shown that triphenyltin inhibits
oxidative phosphorylation by isolated liver mitochondria and the
adenosine triphosphatase activity of brain microsomes (50% inhibition
by concentrations of 1 × 10-6M and 1 × 10-5M respectively) (W. N.
Aldridge, quoted by Stoner, 1965).
The oxygen consumption of cerebral slices of brains from rats in
a moribund condition due to triphenyltin was within normal limits (J.
E. Cremer, quoted by Stoner, 1965).
Studies on the effect of tin compounds on plants are in
progress in several laboratories. It has been shown that in the
presence of light and air several by-products of triphenyltin acetate
can be formed, such as diphenyltin and finally insoluble tin (Kroller,
1960). The tin content from the leaves can be transferred to the
ground. However, when 113Sn-triphenyltin acetate or hydroxide was
applied to potato foliage, no translocation of 113Sn from leaves
to tubers could be demonstrated above the limit of determination of
0.0001 ppm. Tin could be detected in the haulm, where the percentage
of the original compound decreased for 20 days (Anon., 1964). It has
also been shown that triphenyltin has no systemic action when applied
to celeriac and sugar-beet (Herok & Götte, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
Mouse Oral 81-93.3 Anon., 1961
Mouse Intraperitoneal 7.9 Stoner, 1965
Rat Oral 136* Klimmer, 1964
Rat Oral 491** Stoner, 1965
Rat Intraperitoneal 13.2 Klimmer, 1964
Rat Intraperitoneal 8.5 Stoner, 1965
Guinea-pig Oral 21 Klimmer, 1964
Guinea-pig Intraperitoneal 5.3 Klimmer, 1964
Guinea-pig Intraperitoneal 3.7 Stoner, 1965
(continued)
Animal Route LD50 mg/kg References
Rabbit Oral 30-50 Klimmer, 1964
Rabbit Intraperitoneal 10 Klimmer, 1964
* In tylose.
** In arachis oil.
In all species the main action of these organo-tin compounds is
thought to be on the central nervous system.
Cat. Intravenous administration of triphenyltin acetate at a
dose of 1 mg/kg produced an increase in blood-pressure and a short
interruption of respiration followed by stimulation of respiration and
clonic contractions of the limb muscles. Repeated administration of
1-2 mg/kg at 20-60 minute intervals led to arterial hypotension. A
decrease in the effect of noradrenaline on blood-pressure was also
found. Death took place after 4-14 mg/kg of triphenyltin acetate from
paralysis of the respiratory centres (Tauberger, 1963).
Short-term studies
Rat
(a) Triphenyltin acetate. Groups of 20-25 rats given
triphenyltin acetate by stomach-tube at doses equivalent to 5, 10, 25
and 50 ppm for 107-170 days. At 50 ppm 70% of the rats died in 7.49
days. Nervous symptoms, as well as blood, urinary or histopathological
changes were not observed at these lower dose levels (Klimmer, 1964).
In other experiments on rats no deaths occurred during a
10-week period on 200 ppm triphenyltin acetate. These rats were then
put on a diet containing 300 ppm and 5 out of 6 rats died after a
further 117-168 days (Stoner, 1965).
Groups of young rats, 10 of each sex, were given respectively
0, 5, 10, 25 and 50 ppm of triphenyltin acetate for 12 weeks. Decrease
of food intake and growth inhibition were recorded at 50 ppm as well
as growth inhibition in males at 25 ppm. At 10 ppm and above the
number of leucocytes in the blood was decreased and at 50 ppm the
haemoglobin was reduced. At the highest level there was a decrease of
the organ/heart weight ratio for the pituitary and pancreas in all
animals and uterus and ovary in the females. The same ratio for the
thyroid was decreased in all the females as well as in the males at
25 and 50 ppm. The water content of the brain in males and spinal cord
in females was significantly increased but only at 50 ppm. No
histological studies are yet available on this material (Verschuuren &
van Esch, 1964).
(b) Triphenyltin hydroxide. Groups of 10 or 20 rats of both
sexes were given 0, 5, 20, 50 and 100 ppm of triphenyltin hydroxide
for 28 days. Food intake and body growth were depressed at 20 ppm and
above. Death-rates were 9/10 at 100 ppm; 9/20 at 50 ppm and 1/20 at
both 20 and 5 ppm (van Esch & Arnoldussen, 1962).
Groups of 10 rats of each sex were given respectively 0, 5, 10
or 25 ppm of triphenyltin hydroxide in the diet for 12 weeks. The food
intake was comparable to the controls. The females showed growth
inhibition after six weeks but they recovered in spite of continuing
treatment. Growth in males was comparable to the controls. In the
females blood leucocytes were decreased. No significant changes in
water content were found in nervous tissues. At 25 ppm decrease of the
thyroid weight was noticed (Verschuuren et al., 1962). In a similar
experiment in which 10 rats of each sex were given 50 ppm of the same
compound, the following features were noticed: decreased food intake,
growth inhibition in both sexes, decrease in weight of the thyroid,
pituitary, uterus, ovary, prostate and pancreas as well as decrease of
haemoglobin and leucocytes. The water content increased in the spinal
cord but not in the brain. No histopathological details of these
studies are yet available (Verschuuren & van Esch, 1964).
Guinea-pig
(a) Triphenyltin acetate. In one experiment, 50 ppm led to the
death of all of nine animals in 17-31 days. In other experiments only
a group of 5 guinea-pigs given 1 ppm for 392 days did not show an
increased mortality rate. Even at 1 ppm food intake was reduced. When
death occurred it was preceded by loss of weight and generalized
weakness (Stoner, 1965). Neither in these experiments nor in others
(acute and short-term) in which guinea-pigs and rats have been treated
with triphenyltin has a significant increase in the water content of
the central nervous system been found nor the characteristic
histological lesion in the white matter produced by triethyltin (Magee
et al., 1957) been seen (Stoner, 1965).
In other experiments, groups of guinea-pigs, 10 of each sex,
were given respectively 5, 10, 20 and 50 ppm of triphenyltin acetate
for 12 weeks. In all groups, but for the males given 5 ppm, growth
inhibition was obvious. At the end of the treatment no animals at 50
ppm were alive; in the other groups the survival rates were: 20 ppm,
8/10 males and 8/10 females; 10 ppm, 9/10 males and 10/10 females; 5
ppm, 9/10 males and 10/10 females. In all the groups the Hb content of
the blood as well as the total number of leucocytes and erythrocytes
was decreased in comparison to untreated controls. Also basophilic
"stippling" in erythrocytes was noted and only in the experimental
group. At 20 and 50 ppm a significant increase of brain water was
noticed (Verschuuren & van Esch, 1964).
(b) Triphenyltin hydroxide. Groups of 3 males and 3 females
were given 0, 1, 2.5, 5 and 20 ppm of triphenyltin hydroxide for three
weeks. No changes in the growth rate nor the water content nor
histology of the nervous system were observed (Verschuuren & van Esch,
1964).
Groups of 10 males and 10 females were given 2.5, 5, 10 or 20 ppm
for 12 weeks. Growth inhibition was observed at the highest
dose-level. Hb and total leucocytes were decreased in all groups. The
organ/body-weight ratio of spleen, thymus, uterus and testes were
decreased and those of kidney and brain were increased, but no
increase in the water content of the nervous system was observed. In
another experiment in which 10 animals of each sex were given 50 ppm
of triphenyltin hydroxide, all the animals died within six weeks and
showed a significant increase of brain weight and water content of the
brain and spinal cord. No histological details are yet available on
these studies (Verschuuren & van Esch, 1964).
Long-term studies
No long-term experiments are so far reported.
Comments on experimental studies reported
Triphenyltin appears to be a non-systemic fungicide for celeriac,
sugar-beet and potatoes. Although some toxicological data are now
available they are insufficient to establish a no-effect level in a
sensitive species of animal. There are many unresolved problems
concerning this compound notably concerning its action on the nervous
system. The meeting was informed that work on these problems was in
progress. Until detailed reports of these studies are to hand no
statement can be made about the acceptability of these compounds to
man.
REFERENCES
Anon. (1961) Unpublished data from Sankyo Co., Ltd.
Anon. (1964) Unpublished data from Philips-Duphar
Brügemann, J., Berth, K. & Nieser, K. H. (1964) Zbl. Vet. Med.,
11, 4
van Esch, G. J. & Arnoldussen, A. M. (1962) Unpublished report of the
National Institute of Public Health, Utrecht, Tox 39/62
Heath, D. F. (1963) Radiation and radioisotopes applied to insects
of agricultural importance, IAEA, Vienna, p. 185
Herok, J. & Götte, H. (1961) Symp radioisotopes in animal biology,
Mexico City, p. 177
Herok, J. & Götte, H. (1963) Internal J. Appl. Radiation and
Isotopes, 14, 461
Klimmer, O. R. (1964) Zbl. Vet. Med., 11, 29
Kroller, E. (1960) Dtsch Lebensmitt Rdsch., 7, 190
Magee, P. N., Stoner, H. B. & Barnes, J. M. (1957) J. Path. Bact.,
73, 107.
Stoner, H. B. (1965) To be published
Tauberger, G. (1963) Med. Exp., 9, 393
Verschuuren, H. G. & van Esch, G. J. (1964) Unpublished report of the
National Institute or Public Health, Utrecht
Verschuuren, H. G. van Esch, G. J. & Arnoldussen, A. M. (1962)
Unpublished report of the National Institute of Public Health, 161/162
BIOLOGICAL DATA
Biochemical aspects
After ingestion of triphenyltin by cows or sheep, the compound
is largely excreted with the faeces (Bügemann et al., 1964; Herok &
Götte, 1961). Three sheep given 10 mg daily of 113Sn-triphenyltin
acetate for 20 days were killed respectively 28, 52 and 218 days after
the beginning of the treatment. 113Sn was found in the milk (average
concentration 0.0017 ppm) during the treatment but 17 days after
withdrawal of the compound its concentration in the milk was near
detectable limits. Tin was present in the milk in at least two forms
other than triphenyltin acetate. During the treatment, the
concentration of 113Sn in the blood and in the wine were 2.9 µg/l and
7.5 µg/l respectively. In the sheep killed at 28 days, liver, kidney,
lungs, pancreas, gall-bladder and brain contained a greater amount of
113Sn than other organs. After 218 days the amount of 113Sn found in
the liver was still greater than in other organs (Herok & Götte,
1961).
Work on rats given 113Sn-triphenyltin showed that absorbed
triphenyltin was rapidly distributed through the tissues of the body
including the brain. Triphenyltin was eliminated relatively slowly and
it could be detected in the brain 38 days after a single dose (Heath,
1963).
In vitro studies have shown that triphenyltin inhibits
oxidative phosphorylation by isolated liver mitochondria and the
adenosine triphosphatase activity of brain microsomes (50% inhibition
by concentrations of 1 × 10-6M and 1 × 10-5M respectively) (W. N.
Aldridge, quoted by Stoner, 1965).
The oxygen consumption of cerebral slices of brains from rats in
a moribund condition due to triphenyltin was within normal limits (J.
E. Cremer, quoted by Stoner, 1965).
Studies on the effect of tin compounds on plants are in
progress in several laboratories. It has been shown that in the
presence of light and air several by-products of triphenyltin acetate
can be formed, such as diphenyltin and finally insoluble tin (Kroller,
1960). The tin content from the leaves can be transferred to the
ground. However, when 113Sn-triphenyltin acetate or hydroxide was
applied to potato foliage, no translocation of 113Sn from leaves
to tubers could be demonstrated above the limit of determination of
0.0001 ppm. Tin could be detected in the haulm, where the percentage
of the original compound decreased for 20 days (Anon., 1964). It has
also been shown that triphenyltin has no systemic action when applied
to celeriac and sugar-beet (Herok & Götte, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
Mouse Oral 81-93.3 Anon., 1961
Mouse Intraperitoneal 7.9 Stoner, 1965
Rat Oral 136* Klimmer, 1964
Rat Oral 491** Stoner, 1965
Rat Intraperitoneal 13.2 Klimmer, 1964
Rat Intraperitoneal 8.5 Stoner, 1965
Guinea-pig Oral 21 Klimmer, 1964
Guinea-pig Intraperitoneal 5.3 Klimmer, 1964
Guinea-pig Intraperitoneal 3.7 Stoner, 1965
(continued)
Animal Route LD50 mg/kg References
Rabbit Oral 30-50 Klimmer, 1964
Rabbit Intraperitoneal 10 Klimmer, 1964
* In tylose.
** In arachis oil.
In all species the main action of these organo-tin compounds is
thought to be on the central nervous system.
Cat. Intravenous administration of triphenyltin acetate at a
dose of 1 mg/kg produced an increase in blood-pressure and a short
interruption of respiration followed by stimulation of respiration and
clonic contractions of the limb muscles. Repeated administration of
1-2 mg/kg at 20-60 minute intervals led to arterial hypotension. A
decrease in the effect of noradrenaline on blood-pressure was also
found. Death took place after 4-14 mg/kg of triphenyltin acetate from
paralysis of the respiratory centres (Tauberger, 1963).
Short-term studies
Rat
(a) Triphenyltin acetate. Groups of 20-25 rats given
triphenyltin acetate by stomach-tube at doses equivalent to 5, 10, 25
and 50 ppm for 107-170 days. At 50 ppm 70% of the rats died in 7.49
days. Nervous symptoms, as well as blood, urinary or histopathological
changes were not observed at these lower dose levels (Klimmer, 1964).
In other experiments on rats no deaths occurred during a
10-week period on 200 ppm triphenyltin acetate. These rats were then
put on a diet containing 300 ppm and 5 out of 6 rats died after a
further 117-168 days (Stoner, 1965).
Groups of young rats, 10 of each sex, were given respectively
0, 5, 10, 25 and 50 ppm of triphenyltin acetate for 12 weeks. Decrease
of food intake and growth inhibition were recorded at 50 ppm as well
as growth inhibition in males at 25 ppm. At 10 ppm and above the
number of leucocytes in the blood was decreased and at 50 ppm the
haemoglobin was reduced. At the highest level there was a decrease of
the organ/heart weight ratio for the pituitary and pancreas in all
animals and uterus and ovary in the females. The same ratio for the
thyroid was decreased in all the females as well as in the males at
25 and 50 ppm. The water content of the brain in males and spinal cord
in females was significantly increased but only at 50 ppm. No
histological studies are yet available on this material (Verschuuren &
van Esch, 1964).
(b) Triphenyltin hydroxide. Groups of 10 or 20 rats of both
sexes were given 0, 5, 20, 50 and 100 ppm of triphenyltin hydroxide
for 28 days. Food intake and body growth were depressed at 20 ppm and
above. Death-rates were 9/10 at 100 ppm; 9/20 at 50 ppm and 1/20 at
both 20 and 5 ppm (van Esch & Arnoldussen, 1962).
Groups of 10 rats of each sex were given respectively 0, 5, 10
or 25 ppm of triphenyltin hydroxide in the diet for 12 weeks. The food
intake was comparable to the controls. The females showed growth
inhibition after six weeks but they recovered in spite of continuing
treatment. Growth in males was comparable to the controls. In the
females blood leucocytes were decreased. No significant changes in
water content were found in nervous tissues. At 25 ppm decrease of the
thyroid weight was noticed (Verschuuren et al., 1962). In a similar
experiment in which 10 rats of each sex were given 50 ppm of the same
compound, the following features were noticed: decreased food intake,
growth inhibition in both sexes, decrease in weight of the thyroid,
pituitary, uterus, ovary, prostate and pancreas as well as decrease of
haemoglobin and leucocytes. The water content increased in the spinal
cord but not in the brain. No histopathological details of these
studies are yet available (Verschuuren & van Esch, 1964).
Guinea-pig
(a) Triphenyltin acetate. In one experiment, 50 ppm led to the
death of all of nine animals in 17-31 days. In other experiments only
a group of 5 guinea-pigs given 1 ppm for 392 days did not show an
increased mortality rate. Even at 1 ppm food intake was reduced. When
death occurred it was preceded by loss of weight and generalized
weakness (Stoner, 1965). Neither in these experiments nor in others
(acute and short-term) in which guinea-pigs and rats have been treated
with triphenyltin has a significant increase in the water content of
the central nervous system been found nor the characteristic
histological lesion in the white matter produced by triethyltin (Magee
et al., 1957) been seen (Stoner, 1965).
In other experiments, groups of guinea-pigs, 10 of each sex,
were given respectively 5, 10, 20 and 50 ppm of triphenyltin acetate
for 12 weeks. In all groups, but for the males given 5 ppm, growth
inhibition was obvious. At the end of the treatment no animals at 50
ppm were alive; in the other groups the survival rates were: 20 ppm,
8/10 males and 8/10 females; 10 ppm, 9/10 males and 10/10 females; 5
ppm, 9/10 males and 10/10 females. In all the groups the Hb content of
the blood as well as the total number of leucocytes and erythrocytes
was decreased in comparison to untreated controls. Also basophilic
"stippling" in erythrocytes was noted and only in the experimental
group. At 20 and 50 ppm a significant increase of brain water was
noticed (Verschuuren & van Esch, 1964).
(b) Triphenyltin hydroxide. Groups of 3 males and 3 females
were given 0, 1, 2.5, 5 and 20 ppm of triphenyltin hydroxide for three
weeks. No changes in the growth rate nor the water content nor
histology of the nervous system were observed (Verschuuren & van Esch,
1964).
Groups of 10 males and 10 females were given 2.5, 5, 10 or 20 ppm
for 12 weeks. Growth inhibition was observed at the highest
dose-level. Hb and total leucocytes were decreased in all groups. The
organ/body-weight ratio of spleen, thymus, uterus and testes were
decreased and those of kidney and brain were increased, but no
increase in the water content of the nervous system was observed. In
another experiment in which 10 animals of each sex were given 50 ppm
of triphenyltin hydroxide, all the animals died within six weeks and
showed a significant increase of brain weight and water content of the
brain and spinal cord. No histological details are yet available on
these studies (Verschuuren & van Esch, 1964).
Long-term studies
No long-term experiments are so far reported.
Comments on experimental studies reported
Triphenyltin appears to be a non-systemic fungicide for celeriac,
sugar-beet and potatoes. Although some toxicological data are now
available they are insufficient to establish a no-effect level in a
sensitive species of animal. There are many unresolved problems
concerning this compound notably concerning its action on the nervous
system. The meeting was informed that work on these problems was in
progress. Until detailed reports of these studies are to hand no
statement can be made about the acceptability of these compounds to
man.
REFERENCES
Anon. (1961) Unpublished data from Sankyo Co., Ltd.
Anon. (1964) Unpublished data from Philips-Duphar
Brügemann, J., Berth, K. & Nieser, K. H. (1964) Zbl. Vet. Med.,
11, 4
van Esch, G. J. & Arnoldussen, A. M. (1962) Unpublished report of the
National Institute of Public Health, Utrecht, Tox 39/62
Heath, D. F. (1963) Radiation and radioisotopes applied to insects
of agricultural importance, IAEA, Vienna, p. 185
Herok, J. & Götte, H. (1961) Symp radioisotopes in animal biology,
Mexico City, p. 177
Herok, J. & Götte, H. (1963) Internal J. Appl. Radiation and
Isotopes, 14, 461
Klimmer, O. R. (1964) Zbl. Vet. Med., 11, 29
Kroller, E. (1960) Dtsch Lebensmitt Rdsch., 7, 190
Magee, P. N., Stoner, H. B. & Barnes, J. M. (1957) J. Path. Bact.,
73, 107.
Stoner, H. B. (1965) To be published
Tauberger, G. (1963) Med. Exp., 9, 393
Verschuuren, H. G. & van Esch, G. J. (1964) Unpublished report of the
National Institute or Public Health, Utrecht
Verschuuren, H. G. van Esch, G. J. & Arnoldussen, A. M. (1962)
Unpublished report of the National Institute of Public Health, 161/162
 BIOLOGICAL DATA
Biochemical aspects
After ingestion of triphenyltin by cows or sheep, the compound
is largely excreted with the faeces (Bügemann et al., 1964; Herok &
Götte, 1961). Three sheep given 10 mg daily of 113Sn-triphenyltin
acetate for 20 days were killed respectively 28, 52 and 218 days after
the beginning of the treatment. 113Sn was found in the milk (average
concentration 0.0017 ppm) during the treatment but 17 days after
withdrawal of the compound its concentration in the milk was near
detectable limits. Tin was present in the milk in at least two forms
other than triphenyltin acetate. During the treatment, the
concentration of 113Sn in the blood and in the wine were 2.9 µg/l and
7.5 µg/l respectively. In the sheep killed at 28 days, liver, kidney,
lungs, pancreas, gall-bladder and brain contained a greater amount of
113Sn than other organs. After 218 days the amount of 113Sn found in
the liver was still greater than in other organs (Herok & Götte,
1961).
Work on rats given 113Sn-triphenyltin showed that absorbed
triphenyltin was rapidly distributed through the tissues of the body
including the brain. Triphenyltin was eliminated relatively slowly and
it could be detected in the brain 38 days after a single dose (Heath,
1963).
In vitro studies have shown that triphenyltin inhibits
oxidative phosphorylation by isolated liver mitochondria and the
adenosine triphosphatase activity of brain microsomes (50% inhibition
by concentrations of 1 × 10-6M and 1 × 10-5M respectively) (W. N.
Aldridge, quoted by Stoner, 1965).
The oxygen consumption of cerebral slices of brains from rats in
a moribund condition due to triphenyltin was within normal limits (J.
E. Cremer, quoted by Stoner, 1965).
Studies on the effect of tin compounds on plants are in
progress in several laboratories. It has been shown that in the
presence of light and air several by-products of triphenyltin acetate
can be formed, such as diphenyltin and finally insoluble tin (Kroller,
1960). The tin content from the leaves can be transferred to the
ground. However, when 113Sn-triphenyltin acetate or hydroxide was
applied to potato foliage, no translocation of 113Sn from leaves
to tubers could be demonstrated above the limit of determination of
0.0001 ppm. Tin could be detected in the haulm, where the percentage
of the original compound decreased for 20 days (Anon., 1964). It has
also been shown that triphenyltin has no systemic action when applied
to celeriac and sugar-beet (Herok & Götte, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
Mouse Oral 81-93.3 Anon., 1961
Mouse Intraperitoneal 7.9 Stoner, 1965
Rat Oral 136* Klimmer, 1964
Rat Oral 491** Stoner, 1965
Rat Intraperitoneal 13.2 Klimmer, 1964
Rat Intraperitoneal 8.5 Stoner, 1965
Guinea-pig Oral 21 Klimmer, 1964
Guinea-pig Intraperitoneal 5.3 Klimmer, 1964
Guinea-pig Intraperitoneal 3.7 Stoner, 1965
(continued)
Animal Route LD50 mg/kg References
Rabbit Oral 30-50 Klimmer, 1964
Rabbit Intraperitoneal 10 Klimmer, 1964
* In tylose.
** In arachis oil.
In all species the main action of these organo-tin compounds is
thought to be on the central nervous system.
Cat. Intravenous administration of triphenyltin acetate at a
dose of 1 mg/kg produced an increase in blood-pressure and a short
interruption of respiration followed by stimulation of respiration and
clonic contractions of the limb muscles. Repeated administration of
1-2 mg/kg at 20-60 minute intervals led to arterial hypotension. A
decrease in the effect of noradrenaline on blood-pressure was also
found. Death took place after 4-14 mg/kg of triphenyltin acetate from
paralysis of the respiratory centres (Tauberger, 1963).
Short-term studies
Rat
(a) Triphenyltin acetate. Groups of 20-25 rats given
triphenyltin acetate by stomach-tube at doses equivalent to 5, 10, 25
and 50 ppm for 107-170 days. At 50 ppm 70% of the rats died in 7.49
days. Nervous symptoms, as well as blood, urinary or histopathological
changes were not observed at these lower dose levels (Klimmer, 1964).
In other experiments on rats no deaths occurred during a
10-week period on 200 ppm triphenyltin acetate. These rats were then
put on a diet containing 300 ppm and 5 out of 6 rats died after a
further 117-168 days (Stoner, 1965).
Groups of young rats, 10 of each sex, were given respectively
0, 5, 10, 25 and 50 ppm of triphenyltin acetate for 12 weeks. Decrease
of food intake and growth inhibition were recorded at 50 ppm as well
as growth inhibition in males at 25 ppm. At 10 ppm and above the
number of leucocytes in the blood was decreased and at 50 ppm the
haemoglobin was reduced. At the highest level there was a decrease of
the organ/heart weight ratio for the pituitary and pancreas in all
animals and uterus and ovary in the females. The same ratio for the
thyroid was decreased in all the females as well as in the males at
25 and 50 ppm. The water content of the brain in males and spinal cord
in females was significantly increased but only at 50 ppm. No
histological studies are yet available on this material (Verschuuren &
van Esch, 1964).
(b) Triphenyltin hydroxide. Groups of 10 or 20 rats of both
sexes were given 0, 5, 20, 50 and 100 ppm of triphenyltin hydroxide
for 28 days. Food intake and body growth were depressed at 20 ppm and
above. Death-rates were 9/10 at 100 ppm; 9/20 at 50 ppm and 1/20 at
both 20 and 5 ppm (van Esch & Arnoldussen, 1962).
Groups of 10 rats of each sex were given respectively 0, 5, 10
or 25 ppm of triphenyltin hydroxide in the diet for 12 weeks. The food
intake was comparable to the controls. The females showed growth
inhibition after six weeks but they recovered in spite of continuing
treatment. Growth in males was comparable to the controls. In the
females blood leucocytes were decreased. No significant changes in
water content were found in nervous tissues. At 25 ppm decrease of the
thyroid weight was noticed (Verschuuren et al., 1962). In a similar
experiment in which 10 rats of each sex were given 50 ppm of the same
compound, the following features were noticed: decreased food intake,
growth inhibition in both sexes, decrease in weight of the thyroid,
pituitary, uterus, ovary, prostate and pancreas as well as decrease of
haemoglobin and leucocytes. The water content increased in the spinal
cord but not in the brain. No histopathological details of these
studies are yet available (Verschuuren & van Esch, 1964).
Guinea-pig
(a) Triphenyltin acetate. In one experiment, 50 ppm led to the
death of all of nine animals in 17-31 days. In other experiments only
a group of 5 guinea-pigs given 1 ppm for 392 days did not show an
increased mortality rate. Even at 1 ppm food intake was reduced. When
death occurred it was preceded by loss of weight and generalized
weakness (Stoner, 1965). Neither in these experiments nor in others
(acute and short-term) in which guinea-pigs and rats have been treated
with triphenyltin has a significant increase in the water content of
the central nervous system been found nor the characteristic
histological lesion in the white matter produced by triethyltin (Magee
et al., 1957) been seen (Stoner, 1965).
In other experiments, groups of guinea-pigs, 10 of each sex,
were given respectively 5, 10, 20 and 50 ppm of triphenyltin acetate
for 12 weeks. In all groups, but for the males given 5 ppm, growth
inhibition was obvious. At the end of the treatment no animals at 50
ppm were alive; in the other groups the survival rates were: 20 ppm,
8/10 males and 8/10 females; 10 ppm, 9/10 males and 10/10 females; 5
ppm, 9/10 males and 10/10 females. In all the groups the Hb content of
the blood as well as the total number of leucocytes and erythrocytes
was decreased in comparison to untreated controls. Also basophilic
"stippling" in erythrocytes was noted and only in the experimental
group. At 20 and 50 ppm a significant increase of brain water was
noticed (Verschuuren & van Esch, 1964).
(b) Triphenyltin hydroxide. Groups of 3 males and 3 females
were given 0, 1, 2.5, 5 and 20 ppm of triphenyltin hydroxide for three
weeks. No changes in the growth rate nor the water content nor
histology of the nervous system were observed (Verschuuren & van Esch,
1964).
Groups of 10 males and 10 females were given 2.5, 5, 10 or 20 ppm
for 12 weeks. Growth inhibition was observed at the highest
dose-level. Hb and total leucocytes were decreased in all groups. The
organ/body-weight ratio of spleen, thymus, uterus and testes were
decreased and those of kidney and brain were increased, but no
increase in the water content of the nervous system was observed. In
another experiment in which 10 animals of each sex were given 50 ppm
of triphenyltin hydroxide, all the animals died within six weeks and
showed a significant increase of brain weight and water content of the
brain and spinal cord. No histological details are yet available on
these studies (Verschuuren & van Esch, 1964).
Long-term studies
No long-term experiments are so far reported.
Comments on experimental studies reported
Triphenyltin appears to be a non-systemic fungicide for celeriac,
sugar-beet and potatoes. Although some toxicological data are now
available they are insufficient to establish a no-effect level in a
sensitive species of animal. There are many unresolved problems
concerning this compound notably concerning its action on the nervous
system. The meeting was informed that work on these problems was in
progress. Until detailed reports of these studies are to hand no
statement can be made about the acceptability of these compounds to
man.
REFERENCES
Anon. (1961) Unpublished data from Sankyo Co., Ltd.
Anon. (1964) Unpublished data from Philips-Duphar
Brügemann, J., Berth, K. & Nieser, K. H. (1964) Zbl. Vet. Med.,
11, 4
van Esch, G. J. & Arnoldussen, A. M. (1962) Unpublished report of the
National Institute of Public Health, Utrecht, Tox 39/62
Heath, D. F. (1963) Radiation and radioisotopes applied to insects
of agricultural importance, IAEA, Vienna, p. 185
Herok, J. & Götte, H. (1961) Symp radioisotopes in animal biology,
Mexico City, p. 177
Herok, J. & Götte, H. (1963) Internal J. Appl. Radiation and
Isotopes, 14, 461
Klimmer, O. R. (1964) Zbl. Vet. Med., 11, 29
Kroller, E. (1960) Dtsch Lebensmitt Rdsch., 7, 190
Magee, P. N., Stoner, H. B. & Barnes, J. M. (1957) J. Path. Bact.,
73, 107.
Stoner, H. B. (1965) To be published
Tauberger, G. (1963) Med. Exp., 9, 393
Verschuuren, H. G. & van Esch, G. J. (1964) Unpublished report of the
National Institute or Public Health, Utrecht
Verschuuren, H. G. van Esch, G. J. & Arnoldussen, A. M. (1962)
Unpublished report of the National Institute of Public Health, 161/162
BIOLOGICAL DATA
Biochemical aspects
After ingestion of triphenyltin by cows or sheep, the compound
is largely excreted with the faeces (Bügemann et al., 1964; Herok &
Götte, 1961). Three sheep given 10 mg daily of 113Sn-triphenyltin
acetate for 20 days were killed respectively 28, 52 and 218 days after
the beginning of the treatment. 113Sn was found in the milk (average
concentration 0.0017 ppm) during the treatment but 17 days after
withdrawal of the compound its concentration in the milk was near
detectable limits. Tin was present in the milk in at least two forms
other than triphenyltin acetate. During the treatment, the
concentration of 113Sn in the blood and in the wine were 2.9 µg/l and
7.5 µg/l respectively. In the sheep killed at 28 days, liver, kidney,
lungs, pancreas, gall-bladder and brain contained a greater amount of
113Sn than other organs. After 218 days the amount of 113Sn found in
the liver was still greater than in other organs (Herok & Götte,
1961).
Work on rats given 113Sn-triphenyltin showed that absorbed
triphenyltin was rapidly distributed through the tissues of the body
including the brain. Triphenyltin was eliminated relatively slowly and
it could be detected in the brain 38 days after a single dose (Heath,
1963).
In vitro studies have shown that triphenyltin inhibits
oxidative phosphorylation by isolated liver mitochondria and the
adenosine triphosphatase activity of brain microsomes (50% inhibition
by concentrations of 1 × 10-6M and 1 × 10-5M respectively) (W. N.
Aldridge, quoted by Stoner, 1965).
The oxygen consumption of cerebral slices of brains from rats in
a moribund condition due to triphenyltin was within normal limits (J.
E. Cremer, quoted by Stoner, 1965).
Studies on the effect of tin compounds on plants are in
progress in several laboratories. It has been shown that in the
presence of light and air several by-products of triphenyltin acetate
can be formed, such as diphenyltin and finally insoluble tin (Kroller,
1960). The tin content from the leaves can be transferred to the
ground. However, when 113Sn-triphenyltin acetate or hydroxide was
applied to potato foliage, no translocation of 113Sn from leaves
to tubers could be demonstrated above the limit of determination of
0.0001 ppm. Tin could be detected in the haulm, where the percentage
of the original compound decreased for 20 days (Anon., 1964). It has
also been shown that triphenyltin has no systemic action when applied
to celeriac and sugar-beet (Herok & Götte, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
Mouse Oral 81-93.3 Anon., 1961
Mouse Intraperitoneal 7.9 Stoner, 1965
Rat Oral 136* Klimmer, 1964
Rat Oral 491** Stoner, 1965
Rat Intraperitoneal 13.2 Klimmer, 1964
Rat Intraperitoneal 8.5 Stoner, 1965
Guinea-pig Oral 21 Klimmer, 1964
Guinea-pig Intraperitoneal 5.3 Klimmer, 1964
Guinea-pig Intraperitoneal 3.7 Stoner, 1965
(continued)
Animal Route LD50 mg/kg References
Rabbit Oral 30-50 Klimmer, 1964
Rabbit Intraperitoneal 10 Klimmer, 1964
* In tylose.
** In arachis oil.
In all species the main action of these organo-tin compounds is
thought to be on the central nervous system.
Cat. Intravenous administration of triphenyltin acetate at a
dose of 1 mg/kg produced an increase in blood-pressure and a short
interruption of respiration followed by stimulation of respiration and
clonic contractions of the limb muscles. Repeated administration of
1-2 mg/kg at 20-60 minute intervals led to arterial hypotension. A
decrease in the effect of noradrenaline on blood-pressure was also
found. Death took place after 4-14 mg/kg of triphenyltin acetate from
paralysis of the respiratory centres (Tauberger, 1963).
Short-term studies
Rat
(a) Triphenyltin acetate. Groups of 20-25 rats given
triphenyltin acetate by stomach-tube at doses equivalent to 5, 10, 25
and 50 ppm for 107-170 days. At 50 ppm 70% of the rats died in 7.49
days. Nervous symptoms, as well as blood, urinary or histopathological
changes were not observed at these lower dose levels (Klimmer, 1964).
In other experiments on rats no deaths occurred during a
10-week period on 200 ppm triphenyltin acetate. These rats were then
put on a diet containing 300 ppm and 5 out of 6 rats died after a
further 117-168 days (Stoner, 1965).
Groups of young rats, 10 of each sex, were given respectively
0, 5, 10, 25 and 50 ppm of triphenyltin acetate for 12 weeks. Decrease
of food intake and growth inhibition were recorded at 50 ppm as well
as growth inhibition in males at 25 ppm. At 10 ppm and above the
number of leucocytes in the blood was decreased and at 50 ppm the
haemoglobin was reduced. At the highest level there was a decrease of
the organ/heart weight ratio for the pituitary and pancreas in all
animals and uterus and ovary in the females. The same ratio for the
thyroid was decreased in all the females as well as in the males at
25 and 50 ppm. The water content of the brain in males and spinal cord
in females was significantly increased but only at 50 ppm. No
histological studies are yet available on this material (Verschuuren &
van Esch, 1964).
(b) Triphenyltin hydroxide. Groups of 10 or 20 rats of both
sexes were given 0, 5, 20, 50 and 100 ppm of triphenyltin hydroxide
for 28 days. Food intake and body growth were depressed at 20 ppm and
above. Death-rates were 9/10 at 100 ppm; 9/20 at 50 ppm and 1/20 at
both 20 and 5 ppm (van Esch & Arnoldussen, 1962).
Groups of 10 rats of each sex were given respectively 0, 5, 10
or 25 ppm of triphenyltin hydroxide in the diet for 12 weeks. The food
intake was comparable to the controls. The females showed growth
inhibition after six weeks but they recovered in spite of continuing
treatment. Growth in males was comparable to the controls. In the
females blood leucocytes were decreased. No significant changes in
water content were found in nervous tissues. At 25 ppm decrease of the
thyroid weight was noticed (Verschuuren et al., 1962). In a similar
experiment in which 10 rats of each sex were given 50 ppm of the same
compound, the following features were noticed: decreased food intake,
growth inhibition in both sexes, decrease in weight of the thyroid,
pituitary, uterus, ovary, prostate and pancreas as well as decrease of
haemoglobin and leucocytes. The water content increased in the spinal
cord but not in the brain. No histopathological details of these
studies are yet available (Verschuuren & van Esch, 1964).
Guinea-pig
(a) Triphenyltin acetate. In one experiment, 50 ppm led to the
death of all of nine animals in 17-31 days. In other experiments only
a group of 5 guinea-pigs given 1 ppm for 392 days did not show an
increased mortality rate. Even at 1 ppm food intake was reduced. When
death occurred it was preceded by loss of weight and generalized
weakness (Stoner, 1965). Neither in these experiments nor in others
(acute and short-term) in which guinea-pigs and rats have been treated
with triphenyltin has a significant increase in the water content of
the central nervous system been found nor the characteristic
histological lesion in the white matter produced by triethyltin (Magee
et al., 1957) been seen (Stoner, 1965).
In other experiments, groups of guinea-pigs, 10 of each sex,
were given respectively 5, 10, 20 and 50 ppm of triphenyltin acetate
for 12 weeks. In all groups, but for the males given 5 ppm, growth
inhibition was obvious. At the end of the treatment no animals at 50
ppm were alive; in the other groups the survival rates were: 20 ppm,
8/10 males and 8/10 females; 10 ppm, 9/10 males and 10/10 females; 5
ppm, 9/10 males and 10/10 females. In all the groups the Hb content of
the blood as well as the total number of leucocytes and erythrocytes
was decreased in comparison to untreated controls. Also basophilic
"stippling" in erythrocytes was noted and only in the experimental
group. At 20 and 50 ppm a significant increase of brain water was
noticed (Verschuuren & van Esch, 1964).
(b) Triphenyltin hydroxide. Groups of 3 males and 3 females
were given 0, 1, 2.5, 5 and 20 ppm of triphenyltin hydroxide for three
weeks. No changes in the growth rate nor the water content nor
histology of the nervous system were observed (Verschuuren & van Esch,
1964).
Groups of 10 males and 10 females were given 2.5, 5, 10 or 20 ppm
for 12 weeks. Growth inhibition was observed at the highest
dose-level. Hb and total leucocytes were decreased in all groups. The
organ/body-weight ratio of spleen, thymus, uterus and testes were
decreased and those of kidney and brain were increased, but no
increase in the water content of the nervous system was observed. In
another experiment in which 10 animals of each sex were given 50 ppm
of triphenyltin hydroxide, all the animals died within six weeks and
showed a significant increase of brain weight and water content of the
brain and spinal cord. No histological details are yet available on
these studies (Verschuuren & van Esch, 1964).
Long-term studies
No long-term experiments are so far reported.
Comments on experimental studies reported
Triphenyltin appears to be a non-systemic fungicide for celeriac,
sugar-beet and potatoes. Although some toxicological data are now
available they are insufficient to establish a no-effect level in a
sensitive species of animal. There are many unresolved problems
concerning this compound notably concerning its action on the nervous
system. The meeting was informed that work on these problems was in
progress. Until detailed reports of these studies are to hand no
statement can be made about the acceptability of these compounds to
man.
REFERENCES
Anon. (1961) Unpublished data from Sankyo Co., Ltd.
Anon. (1964) Unpublished data from Philips-Duphar
Brügemann, J., Berth, K. & Nieser, K. H. (1964) Zbl. Vet. Med.,
11, 4
van Esch, G. J. & Arnoldussen, A. M. (1962) Unpublished report of the
National Institute of Public Health, Utrecht, Tox 39/62
Heath, D. F. (1963) Radiation and radioisotopes applied to insects
of agricultural importance, IAEA, Vienna, p. 185
Herok, J. & Götte, H. (1961) Symp radioisotopes in animal biology,
Mexico City, p. 177
Herok, J. & Götte, H. (1963) Internal J. Appl. Radiation and
Isotopes, 14, 461
Klimmer, O. R. (1964) Zbl. Vet. Med., 11, 29
Kroller, E. (1960) Dtsch Lebensmitt Rdsch., 7, 190
Magee, P. N., Stoner, H. B. & Barnes, J. M. (1957) J. Path. Bact.,
73, 107.
Stoner, H. B. (1965) To be published
Tauberger, G. (1963) Med. Exp., 9, 393
Verschuuren, H. G. & van Esch, G. J. (1964) Unpublished report of the
National Institute or Public Health, Utrecht
Verschuuren, H. G. van Esch, G. J. & Arnoldussen, A. M. (1962)
Unpublished report of the National Institute of Public Health, 161/162
