
Concise International Chemical Assessment Document 16
AZODICARBONAMIDE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Concise International Chemical Assessment Document 16
AZODICARBONAMIDE
First draft prepared by
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom,
Dr S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire, United
Kingdom, and
Mrs E. Ball, Health and Safety Executive, Merseyside, United Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1999
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Azodicarbonamide.
(Concise international chemical assessment document ; 16)
1.Azo compounds 2.Occupational exposure 3.Risk assessment
I.International Programme on Chemical Safety II.Series
ISBN 92 4 153016 2 (NLM classification: QV 235)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1999
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
9.1. Case reports
9.2. Epidemiological studies
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for azodicarbonamide
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Health surveillance advice
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 -- SOURCE DOCUMENT
APPENDIX 2 -- CICAD PEER REVIEW
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994)
Assessing human health risks of chemicals: deriviation of
guidance values for health-based exposure limits. Geneva, World
Health Organization (Environmental Health Criteria 170).
Procedures
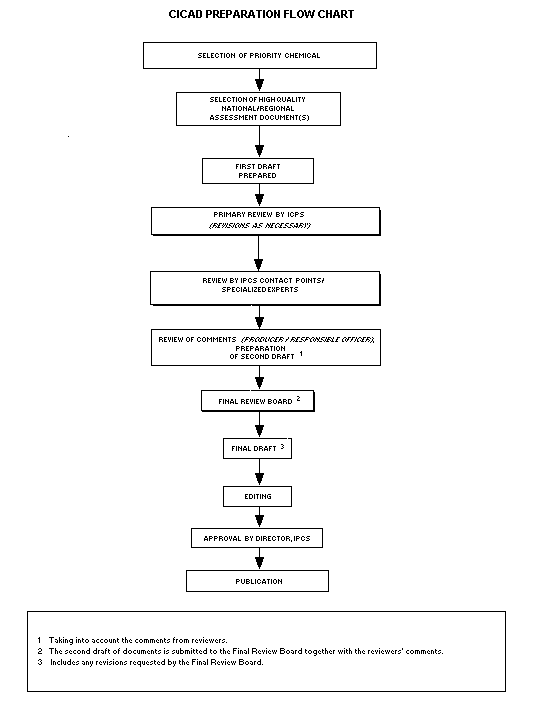
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on azodicarbonamide was based on a review of human
health (primarily occupational) concerns prepared by the United
Kingdom's Health and Safety Executive (Ball et al., 1996). Hence,
although this CICAD includes an assessment of the available
environmental data, the main focus is on risks to human health in the
working environment, including an emphasis on information from routes
that are relevant to occupational settings. Data identified up to June
1994 were covered in the review. A further literature search was
performed, up to July 1997, to identify any new information published
since this review was completed. The original source document did not
address environmental concerns; as literature searches have failed to
identify relevant studies in this area, an environmental risk
assessment has not been attempted. Information on the nature of the
peer review and availability of the source document is presented in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Tokyo, Japan, on 30
June - 2 July 1998. Participants at the Final Review Board meeting are
listed in Appendix 3. The International Chemical Safety Card (ICSC
0380) for azodicarbonamide, produced by the International Programme on
Chemical Safety (IPCS, 1993), has also been reproduced in this
document.
Toxicokinetic data on azodicarbonamide (CAS No. 123-77-3) are
limited, but the chemical appears to be well absorbed by the
inhalation and oral routes in rodents. Substantial quantities of the
substance remain unabsorbed from the gastrointestinal tract and are
passed out in the faeces. Azodicarbonamide is readily converted to
biurea, the only breakdown product identified, and it is likely that
systemic exposure is principally to this derivative rather than to the
parent compound. Elimination of absorbed azodicarbonamide/biurea is
rapid, occurring predominantly via the urine, and there is very little
systemic retention of biurea.
Azodicarbonamide is of low acute toxicity and does not cause
skin, eye, or respiratory tract irritation in experimental animals.
Results from a poorly conducted skin sensitization study were
negative, and there was no evidence of an asthmatic-type response in
guinea-pigs in one study. No adverse effects were observed in
experimental animals inhaling up to 200 mg/m3 for up to 13 weeks.
Repeated oral exposures resulted in the appearance of pyelonephritis
with casts and crystalline deposits in renal tubuli in several
species. However, the dose levels required to induce these effects
were high (>200 mg/kg body weight per day in studies of up to
1 year's duration). Although azodicarbonamide was found to be a
mutagen in bacterial systems, there is no evidence that this effect
would be expressed in vivo. The carcinogenicity and reproductive
toxicity of azodicarbonamide have not been examined in detail, but no
tumorigenic or antifertility effects were observed in early studies in
which animals were treated with the breakdown product biurea.
Developmental toxicity has not been studied.
Studies in humans have concentrated solely on the ability of
azodicarbonamide to induce asthma and skin sensitization. Evidence
that azodicarbonamide can induce asthma in humans has been found from
bronchial challenge studies with symptomatic individuals and from
health evaluations of employees at workplaces where azodicarbonamide
is manufactured or used. There are also indications that
azodicarbonamide may induce skin sensitization.
On the basis that azodicarbonamide is a human asthmagen and that
the concentrations required to induce asthma in a non-sensitive
individual or to provoke a response in a sensitive individual are
unknown, it is concluded that there is a risk to human health under
present occupational exposure conditions. The level of risk is
uncertain; hence, exposure levels should be reduced as much as
possible.
Data have been identified that indicate ethyl carbamate formation
in consumer products such as bread and beer following the addition of
azodicarbonamide. Exposure of the general public to azodicarbonamide
could not be evaluated because of the lack of available data.
Azodicarbonamide released to surface waters would partition to
the hydrosphere with no significant sorption to particulates. The
half-life for reaction with hydroxyl radicals in the atmosphere is
calculated to be 0.4 days. Azodicarbonamide was readily biodegradable
in two out of three tests with sewage sludge and was degraded in soil
by 20-70% over 14 days. No-observed-effect concentrations (NOECs) for
fish and the water flea have been reported at >50 and 5 mg/litre,
respectively. Lack of information on release to the environment
precludes a quantitative risk assessment.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Azodicarbonamide (CAS No. 123-77-3) is a synthetic chemical that
exists at ambient temperature as a yellow-orange crystalline solid. It
is poorly soluble in water at 20°C (<50 mg/litre), although it is
slightly soluble in hot water. It is insoluble in many organic
solvents, but it is soluble in N, N-dimethyl formamide and dimethyl
sulfoxide. It has a very low vapour pressure (2.53 × 10-11 kPa at
20°C). Additional physical/chemical properties are presented in the
International Chemical Safety Card reproduced in this document.
Common synonyms for azodicarbonamide are ADA, ADC,
azobiscarbonamide, azobiscarboxamide, azodicarbodiamide,
azodicarboxamide, azodiformamide, azobisformamide,
1,1'-azobisformamide, diazenedicarboxamide, and diazenedicarbonic acid
amide. Trade names are listed in the International Chemical Safety
Card appended to this document.
Azodicarbonamide's structural formula is shown below:
1. EXECUTIVE SUMMARY
This CICAD on azodicarbonamide was based on a review of human
health (primarily occupational) concerns prepared by the United
Kingdom's Health and Safety Executive (Ball et al., 1996). Hence,
although this CICAD includes an assessment of the available
environmental data, the main focus is on risks to human health in the
working environment, including an emphasis on information from routes
that are relevant to occupational settings. Data identified up to June
1994 were covered in the review. A further literature search was
performed, up to July 1997, to identify any new information published
since this review was completed. The original source document did not
address environmental concerns; as literature searches have failed to
identify relevant studies in this area, an environmental risk
assessment has not been attempted. Information on the nature of the
peer review and availability of the source document is presented in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Tokyo, Japan, on 30
June - 2 July 1998. Participants at the Final Review Board meeting are
listed in Appendix 3. The International Chemical Safety Card (ICSC
0380) for azodicarbonamide, produced by the International Programme on
Chemical Safety (IPCS, 1993), has also been reproduced in this
document.
Toxicokinetic data on azodicarbonamide (CAS No. 123-77-3) are
limited, but the chemical appears to be well absorbed by the
inhalation and oral routes in rodents. Substantial quantities of the
substance remain unabsorbed from the gastrointestinal tract and are
passed out in the faeces. Azodicarbonamide is readily converted to
biurea, the only breakdown product identified, and it is likely that
systemic exposure is principally to this derivative rather than to the
parent compound. Elimination of absorbed azodicarbonamide/biurea is
rapid, occurring predominantly via the urine, and there is very little
systemic retention of biurea.
Azodicarbonamide is of low acute toxicity and does not cause
skin, eye, or respiratory tract irritation in experimental animals.
Results from a poorly conducted skin sensitization study were
negative, and there was no evidence of an asthmatic-type response in
guinea-pigs in one study. No adverse effects were observed in
experimental animals inhaling up to 200 mg/m3 for up to 13 weeks.
Repeated oral exposures resulted in the appearance of pyelonephritis
with casts and crystalline deposits in renal tubuli in several
species. However, the dose levels required to induce these effects
were high (>200 mg/kg body weight per day in studies of up to
1 year's duration). Although azodicarbonamide was found to be a
mutagen in bacterial systems, there is no evidence that this effect
would be expressed in vivo. The carcinogenicity and reproductive
toxicity of azodicarbonamide have not been examined in detail, but no
tumorigenic or antifertility effects were observed in early studies in
which animals were treated with the breakdown product biurea.
Developmental toxicity has not been studied.
Studies in humans have concentrated solely on the ability of
azodicarbonamide to induce asthma and skin sensitization. Evidence
that azodicarbonamide can induce asthma in humans has been found from
bronchial challenge studies with symptomatic individuals and from
health evaluations of employees at workplaces where azodicarbonamide
is manufactured or used. There are also indications that
azodicarbonamide may induce skin sensitization.
On the basis that azodicarbonamide is a human asthmagen and that
the concentrations required to induce asthma in a non-sensitive
individual or to provoke a response in a sensitive individual are
unknown, it is concluded that there is a risk to human health under
present occupational exposure conditions. The level of risk is
uncertain; hence, exposure levels should be reduced as much as
possible.
Data have been identified that indicate ethyl carbamate formation
in consumer products such as bread and beer following the addition of
azodicarbonamide. Exposure of the general public to azodicarbonamide
could not be evaluated because of the lack of available data.
Azodicarbonamide released to surface waters would partition to
the hydrosphere with no significant sorption to particulates. The
half-life for reaction with hydroxyl radicals in the atmosphere is
calculated to be 0.4 days. Azodicarbonamide was readily biodegradable
in two out of three tests with sewage sludge and was degraded in soil
by 20-70% over 14 days. No-observed-effect concentrations (NOECs) for
fish and the water flea have been reported at >50 and 5 mg/litre,
respectively. Lack of information on release to the environment
precludes a quantitative risk assessment.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Azodicarbonamide (CAS No. 123-77-3) is a synthetic chemical that
exists at ambient temperature as a yellow-orange crystalline solid. It
is poorly soluble in water at 20°C (<50 mg/litre), although it is
slightly soluble in hot water. It is insoluble in many organic
solvents, but it is soluble in N, N-dimethyl formamide and dimethyl
sulfoxide. It has a very low vapour pressure (2.53 × 10-11 kPa at
20°C). Additional physical/chemical properties are presented in the
International Chemical Safety Card reproduced in this document.
Common synonyms for azodicarbonamide are ADA, ADC,
azobiscarbonamide, azobiscarboxamide, azodicarbodiamide,
azodicarboxamide, azodiformamide, azobisformamide,
1,1'-azobisformamide, diazenedicarboxamide, and diazenedicarbonic acid
amide. Trade names are listed in the International Chemical Safety
Card appended to this document.
Azodicarbonamide's structural formula is shown below:
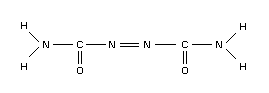 The conversion factors for azodicarbonamide at 20°C and 101.3 kPa
are as follows:
1 mg/m3 = 0.21 ppm
1 ppm = 4.8 mg/m3
3. ANALYTICAL METHODS
Two methods can be used to measure levels of azodicarbonamide in
workplace air. In the first, samples are collected on 37-mm Teflon
filters backed with polyethylene, which in turn is backed with a
cellulose pad (Ahrenholz & Neumeister, 1987). Sampling takes place at
2 litres/min for periods from 7 to 486 min. After sampling,
azodicarbonamide is recovered from the filter with dimethyl sulfoxide
and analysed by high-performance liquid chromatography. The limit of
quantification is given as 5 µg per sample. For a 15-min sample at 2
litres/min, this is equivalent to a quantification limit in air of
0.167 mg/m3; over 8 h, it is equivalent to 0.005 mg/m3.
In the second method, azodicarbonamide is collected on 37-mm
glass fibre filters at 15-20 litres/min (Vainiotalo & Pfaffli, 1988).
It is then eluted from the filter with dimethyl sulfoxide. Following
the addition of sodium hydroxide and glucose solutions, the
azodicarbonamide is reduced to hydrazine. This is reacted with
4-dimethylaminobenzaldehyde, and the resulting aldazine is measured
spectrophotometrically at 460 nm. The lower detection limit is given
as 0.001 mg/m3 for a 4-m3 air sample (equivalent to 4 µg per
sample). This is a considerably larger volume than would normally be
collected for assessing personal exposure samples. At a more typical
flow rate of 2 litres/min for 8 h, 960 litres of air would be sampled,
resulting in a detection limit of about 0.005 mg/m3. Sampling over 15
min at the same rate would give a detection limit of about 0.16
mg/m3.
Although there are published methods for measuring
azodicarbonamide and its metabolite biurea in rats (Bechtold et al.,
1989; see also Mewhinney et al., 1987), there are no reports
describing their measurement in human body fluids.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The principal end use of azodicarbonamide is as a blowing agent
in the rubber and plastics industries. It is used in the expansion of
a wide range of polymers, including polyvinyl chloride, polyolefins,
and natural and synthetic rubbers. The blowing action occurs when the
azodicarbonamide decomposes on heating (process temperatures
approx. 190-230°C) to yield gases (nitrogen, carbon monoxide, carbon
dioxide, and ammonia), solid residues, and sublimated substances.
Decomposition accelerators, in the form of metal salts and oxides, may
also be added to bring about decomposition at lower temperatures.
Azodicarbonamide has in the past been used in the United Kingdom
and Eire (but not other European Union member states) as a flour
improver in the bread-making industry, but this use is no longer
permitted. It is not known how common this practice is worldwide.
Azodicarbonamide is not used in other consumer products.
Azodicarbonamide is manufactured by the reaction of dihydrazine
sulfate and urea under high temperature and pressure. The product of
this reaction is then oxidized using sodium chlorate and centrifuged
to yield a slurry containing azodicarbonamide. The slurry is washed to
remove impurities and dried to obtain the azodicarbonamide powder.
This is then micronized to a fine powder (95% of particles <10 µm,
which is in the respirable range for humans) before packaging.
Very limited information is available on production volumes. The
Hazardous Substances Data Bank (HSDB, 1996) gives US production
figures of "greater than 4.54 tonnes." Until recently,
azodicarbonamide was produced in the United Kingdom; however, this
production has now stopped, and all azodicarbonamide used in the
United Kingdom is imported, predominantly through one large company.
Approximately 2500 tonnes are supplied to the United Kingdom market
each year. Both pure azodicarbonamide (approximately 2200 tonnes) and
pre-mixed formulations (300 tonnes) are supplied, the latter
containing between 10 and 95% azodicarbonamide, depending on the end
use application. "Masterbatch" products, in which the azodicarbonamide
is pelletized with polyolefins, and blended pastes (azodicarbonamide
and plasticizer) are also supplied to the rubber and plastics
industries. In addition to the main importer, there are some firms
that process azodicarbonamide into dust-suppressed powders, pastes,
and "Masterbatch" formulations before selling the processed
azodicarbonamide to the end users.
Recent studies have examined the contribution of azodicarbonamide
to the levels of ethyl carbamate in bread and beer (Canas et al.,
1997; Dennis et al., 1997a,b). It is not clear if unreacted
azodicarbonamide is present in these products; therefore, it is not
possible to assess the contribution that consumption of such products
might make to the overall body burden of azodicarbonamide.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
A calculated half-life of 0.4 days for reaction with hydroxyl
radicals in air has been reported.1
Azodicarbonamide added to five different soil types at 200 mg
nitrogen/kg soil (dry weight) was degraded (measured as recovery of
inorganic nitrogen) by between 21.1% and 66.1% over 14 days
(Frankenberger & Tabatabai, 1982).
Degradation of azodicarbonamide by sewage sludge organisms has
been investigated in three modified Sturm tests (Organisation for
Economic Co-operation and Development [OECD] Guideline 301B). The
compound was "readily biodegradable" in two out of the three tests and
was degraded by 21% over 30 days in the third test (Uniroyal,
1992).2
According to Mackay Level I fugacity modelling, azodicarbonamide
released to surface waters will partition to the hydrosphere with no
significant sorption to particulates.3
1 Bayer, unpublished calculated value (1988) based on the method of
Atkinson; value presented in IUCLID (European Union database),
version dated 7 February 1996.
2 Bayer, unpublished value (1991) presented in IUCLID (European
Union database), version dated 7 February 1996; no details
available.
3 Bayer remark in IUCLID (European Union database), version dated 7
February 1996.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
There are no data available on levels of azodicarbonamide in
ambient air, water, soils, or sediment.
6.2 Human exposure
The data available to the authors of this CICAD are restricted to
the occupational environment. It is estimated that several thousand
persons are exposed to azodicarbonamide in the workplace in the United
Kingdom. Of this total, it is estimated that only a few hundred
persons are exposed as part of their main work activity (i.e., those
involved in compounding, mixing, or raw material handling).
Data obtained by the United Kingdom's Health and Safety Executive
at a plant milling azodicarbonamide powder in micronizing mills (four
samples were collected in total) showed average personal exposures
during the day shift to be 11.8 and 9.8 mg/m3 and during the night
shift to be 2.3 and 2.8 mg/m3 because of the lower throughput at
night. Samples were collected over 4 h. The operators' tasks involved
bagging, weighing, and packaging the milled product.
In the published literature, Slovak (1981) reported time-weighted
average total dust levels in the range 2-5 mg/m3 for azodicarbonamide
manufacturing operations. However, no details were given concerning
occupational groups or tasks. A US National Institute for Occupational
Safety and Health study (Ahrenholz et al., 1985) examined personal
exposures of workers handling azodicarbonamide in a flooring factory.
The work involved the formulation of pastes or paints and required the
blending of azodicarbonamide powder with plasticizers, resins,
pigments, and other additives. Exposures to azodicarbonamide occurred
primarily while the workers were weighing and tipping the powder.
Short-term (sample duration <70 min) personal exposures were in the
range 0.15-12 mg/m3 (median 2.7 mg/m3). A second study (Ahrenholz &
Anderson, 1985) focused on the use of azodicarbonamide in the
injection moulding of plastics. The process involved blending
azodicarbonamide powder with resins. Two sets of full-shift
measurements were reported, with median 8-h time-weighted average
levels of 6.2 µg/m3 (range, not detectable to 280 µg/m3) and 25
µg/m3 (range, trace to 752 µg/m3), respectively.
No data are available relating to dermal exposure levels.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
No information is available on the toxicokinetics of
azodicarbonamide in humans.
Most of the toxicokinetic data available for azodicarbonamide
come from animal studies (Mewhinney et al., 1987). Absorption of
azodicarbonamide has been demonstrated following both a single
inhalation exposure of up to 6 h (34% of dose) and a single oral
administration (10-33% of dose) of radiolabelled azodicarbonamide to
rats. In contrast, approximately 90% of a single intratracheally
instilled dose was apparently absorbed. The difference in absorption
between inhaled and intratracheally instilled azodicarbonamide could
be related to the fact that much of the inhaled azodicarbonamide did
not reach the lower respiratory tract. Half an hour after a 6-h
nose-only exposure of rats to 25 mg/m3 of a dry aerosol (average
mass aerodynamic diameter 3.4 µm), 78% of the calculated total intake
was located in the gastrointestinal tract.
Following exposure by both inhalation and oral routes,
substantial quantities of the substance remain unabsorbed from the
gastrointestinal tract and are passed out in the faeces.
Azodicarbonamide is readily converted to biurea, the only breakdown
product identified, and it is likely that systemic exposure is
principally to this derivative rather than to the parent compound.
Elimination of absorbed azodicarbonamide/biurea is rapid, occurring
predominantly via the urine, and there is very little systemic
retention of biurea.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
It is noted that some of the available toxicological studies
were conducted using biurea. Azodicarbonamide readily undergoes
reduction in the presence of thiol groups to form the stable compound
biurea. Given that thiol groups are also present in many biological
molecules, there is the potential for this reaction to take place
wherever azodicarbonamide encounters thiol groups in biological
systems. This has been demonstrated in an experiment in which
radiolabelled azodicarbonamide was added to fresh rat blood (Mewhinney
et al., 1987). All radioactivity was in the form of biurea within 5
min when untreated blood was used. Radioactivity associated with
azodicarbonamide was detected only in blood to which 5 mg unlabelled
azodicarbonamide/ml blood was added. This level is very much greater
than the levels that humans are likely to encounter.
8.1 Single exposure
Azodicarbonamide is of low acute toxicity by all relevant routes
of exposure. The LC50 was greater than 6100 mg/m3 in rats and mice
exposed to a dry aerosol (median mass aerodynamic diameter
5.8 ± 2.25 µm [geometric standard deviation]) of azodicarbonamide for
4 h (IRDC, 1982a,b). No mortality was observed in rats given oral
doses of up to 5000 mg/kg body weight (Loeser, 1976). The dermal LD50
was >2000 mg/kg body weight in rabbits following application of this
substance under an occlusive dressing for 24 h (MB Research
Laboratories Inc., 1986). Few specific toxic effects were observed in
any single exposure study.
8.2 Irritation and sensitization
Although most studies were of uncertain quality and in many cases
would not comply with modern regulatory standards, results of several
skin and eye irritation studies indicate that azodicarbonamide should
not be regarded as a skin or eye irritant (Kimmerle, 1965; Conning,
1966; Mihail, 1977; MB Research Laboratories Inc., 1986). In a study
of respiratory irritation, in which guinea-pigs were exposed head only
to azodicarbonamide in plethysmography tubes, either no changes or
effects of doubtful significance were reported for various lung
function parameters, indicating that irritation was minimal at
concentrations up to 97 mg/m3 for 1 h (Shopp et al., 1987).
Owing to the poor quality of the only available skin
sensitization study (the concentration used for induction and
challenge -- a 1% solution in dimethyl formamide -- was very low; only
four guinea-pigs were used in the test group; and test sites were not
occluded), it was not possible to draw any conclusions regarding the
ability of azodicarbonamide to induce skin sensitization in animals
(Stevens, 1967). No evidence of pulmonary irritation or
asthmatic-type-reactions (no changes in specific airways conductance,
no evidence of histopathological effects on the upper or lower
respiratory tract, and no evidence of circulating antibodies) was
obtained in one study in which groups of 10 guinea-pigs were
repeatedly exposed by inhalation to unconjugated azodicarbonamide at
0, 51, or 200 mg/m3 for 6 h/day, 5 days/week, for 4 weeks (Gerlach et
al., 1989).
8.3 Short-term exposure
Azodicarbonamide is of relatively low toxicity to animals
repeatedly exposed by the inhalation or oral routes. In well-conducted
2-week inhalation studies, groups of 10 male and 10 female F344/N rats
or B6C3F1 mice were exposed to dry aerosols (median mass aerodynamic
diameter 2 µm) at 0, 2, 10, 50, 100, or 200 mg/m3 for 6 h/day, 5
days/week (Medinsky et al., 1990). Investigations included analyses of
methaemoglobin and blood cholinesterase and extensive macroscopic and
microscopic pathology; no changes of toxicological significance were
seen.
Groups of five male and five female mice received 0, 625, 1250,
5000, or 10 000 mg azodicarbonamide/kg body weight per day by oral
gavage in corn oil, 5 days/week for 2 weeks.1 Mortalities were
observed at 1250 mg/kg body weight per day and above (presumably
treatment related). Histopathologically, at 1250 mg/kg body weight per
day and above, pyelonephritis with casts was seen in renal tubuli, and
crystalline deposits were observed in renal tubuli and the urinary
bladder.
A similar study was conducted in rats; again, there was a
dose-related increase in mortality at 1250 mg/kg body weight per day
and above and a similar profile of renal lesions, although effects
were seen at 1250 mg/kg body weight per day and above in males and at
2500 mg/kg body weight per day and above in females.1
In one early and very briefly reported study, signs of toxicity,
the nature of which was not reported, were seen in male rats given 300
mg azodicarbonamide/kg body weight per day for 5 days but not in rats
given 200 mg/kg body weight per day (Kimmerle, 1965).
In another study designed to look for adverse effects in the
thyroid (but only investigating the uptake of iodine in the thyroid
gland and serum protein-bound iodine), no clear evidence of thyroid
toxicity was found in rats given low-iodine diets containing 1, 5, or
10% azodicarbonamide or 5 or 10% biurea for periods ranging between 1
and 4 weeks (Gafford et al., 1971).
There are no data relating to the effects of repeated dermal
exposures.
1 IRDC, unpublished data; cited in BG Chemie (1995).
8.4 Long-term exposure
8.4.1 Subchronic exposure
Groups of 10 male and 10 female F344/N rats or B6C3F1 mice were
exposed to dry aerosols (median mass aerodynamic diameter 2 µm) at 0,
50, 100, or 200 mg/m3 for 6 h/day, 5 days/week, for 13 weeks
(Medinsky et al., 1990). Investigations included analyses of various
enzyme activities in urine, haematology, blood cholinesterase, serum
triiodothyronine and thyroxine, vaginal cytology, sperm morphology,
levels of azodicarbonamide and biurea in lungs and kidneys (Mewhinney
et al., 1987), and extensive macroscopic and microscopic pathology; no
changes of toxicological significance were seen.
Groups of 10 male mice received 1, 78, 156, 312, 625, or 1250 mg
azodicarbonamide/kg body weight per day and groups of 10 female mice
received 0, 156, 312, 625, 1250, or 2500 mg azodicarbonamide/kg body
weight per day by oral gavage in corn oil, 5 days/week for 13 weeks.1
In contrast to the 2-week study (see section 8.3), there were no
mortalities and no histopathological abnormalities.
Groups of 10 male rats received 1, 100, 500, or 2500 mg
azodicarbonamide/kg body weight per day and groups of 10 female mice
received 0, 200, 1000, or 5000 mg azodicarbonamide/kg body weight per
day by oral gavage in corn oil, 5 days/week for 13 weeks.1 Mortality
was observed only at 2500 mg/kg body weight per day in males and at
5000 mg/kg body weight per day in females. Histopathologically,
pyelonephritis and crystalline deposits were observed in males at 2500
mg/kg body weight per day and in females at 5000 mg/kg body weight per
day only.
8.4.2 Chronic exposure and carcinogenicity
The effects of long-term exposure to azodicarbonamide have not
been well studied, and no conventional carcinogenicity studies are
available. The only data come from 1- and 2-year studies in which rats
and dogs received diets containing various amounts of biurea. In the
1-year study, rats and dogs ate diets containing 5 or 10% biurea (Oser
et al., 1965). One high-dose rat died, and body weight gain was
slightly depressed in high-dose males. No other signs of toxicity were
observed in rats at necropsy. Most dogs from both dose groups died.
Necropsy revealed massive, multiple renal calculi, bladder calculi,
and chronic pyelonephritis. However, the dogs that were selected for
this study were of uncertain and variable origin; hence, no useful
results could be obtained from them. The main constituent (comprising
80-100%) of the calculi was identified as biurea.
In the 2-year study, rats and dogs ate diets containing either
bread baked with untreated flour but supplemented with 750, 2370, or
7500 mg biurea/kg or bread baked with flour containing 100 mg
1 IRDC, unpublished data; cited in BG Chemie (1995).
azodicarbonamide/kg (Oser et al., 1965). Controls received diets
containing bread baked with untreated flour. Given that
azodicarbonamide is readily converted to biurea (Joiner et al., 1963),
it is likely that the animals receiving the bread baked with
azodicarbonamide-treated flour were actually exposed to biurea. As
with the previous investigations, the dogs that were selected for this
study were of uncertain and variable origin; hence, no useful results
could be obtained from them. For rats, no treatment-related deaths
occurred, and no adverse effects were observed that were considered to
be treatment related. Assuming food consumption of 20 g/day and a mean
body weight of 350 g, the dietary inclusion levels correspond to
approximately 45, 140, and 450 mg biurea/kg body weight per day.
8.5 Genotoxicity and related end-points
Azodicarbonamide is mutagenic in vitro, inducing base-pair
mutations in bacteria with and without metabolic activation (Pharmakon
Research International, 1984a; Mortelmans et al., 1986; Hachiya,
1987).1 In contrast, several standard in vitro assays in mammalian
cell systems have yielded negative results; gene mutation assays in
Chinese hamster ovary cells, using the hypoxanthine guanine
phosphoribosyl transferase locus, and in mouse lymphoma cells, using
the thymidine kinase locus, have been conducted, along with an in
vitro liver unscheduled DNA synthesis assay and a sister chromatid
exchange assay in Chinese hamster ovary cells (Pharmakon Research
International, 1984b,c).1,2 A positive result was obtained in a
chromosomal aberration assay in Chinese hamster ovary cells, but the
result was not reproducible.2 Negative results were obtained in a
sex-linked recessive lethal assay in Drosophila (Yoon et al., 1985).
Two in vivo bone marrow micronucleus assays in mice conducted by the
intraperitoneal route (0 or 150 mg azodicarbonamide/kg body weight)
were available, both giving negative results (Pharmakon Research
International, 1984d; Hachiya, 1987).
1 T. Cameron, unpublished Ames and mouse lymphoma test results
(1990) from the short-term program sponsored by the Division of
Cancer Aetiology, National Cancer Institute (cited in Chemical
Carcinogenesis Research Information System [CCRIS] database,
US National Cancer Institute).
2 NTP, unpublished data on chromosome aberration and sister
chromatid exchange assays in Chinese hamster ovary cells for
azodicarbonamide, submitted to the National Toxicology Program,
National Institutes of Health, US Department of Health and Human
Services, Research Triangle Park, NC.
8.6 Reproductive and developmental toxicity
The only study that has been conducted1 is a three-generation
study in which rats were given diets containing up to 7500 mg
biurea/kg (equivalent to approximately 450 mg/kg body weight per day)
(Oser & Oser, 1963; Oser et al., 1965). For each generation, rats were
mated twice, and the first litter was sacrificed at weaning. From the
second litter, 10 males and 10 females were chosen at random to form
the parents for the next generation. The study finished with the
weaning of the F3 generation. For each generation, fertility index
(percentage of matings resulting in pregnancy), gestation index
(number of pregnancies resulting in live litters), viability index
(numbers of pups surviving 4 or more days), and lactation index
(numbers of pups alive at 4 days surviving to weaning) were
determined. No reproductive effects were observed.
The only other information available is that no organ weight or
histological changes were observed in the reproductive organs of rats
and mice repeatedly exposed for 13 weeks to up to 200 mg
azodicarbonamide/m3 by inhalation (see section 8.4.1, Medinsky et
al., 1990).
The developmental toxicity of azodicarbonamide has not been
studied.
8.7 Immunological and neurological effects
No studies are available that specifically investigate these
endpoints, and there is no relevant information from toxicity studies
in animals.
1 The authors of this CICAD have been informed that a reproductive
toxicity screening test is being conducted according to OECD test
method 421.
9. EFFECTS ON HUMANS
The effects of exposure to azodicarbonamide in humans have not
been fully evaluated. There are no data detailing the effects of
single exposures by any route. The most frequently reported effects of
repeated exposure to azodicarbonamide are respiratory symptoms as well
as, to a lesser extent, skin sensitization reactions. There are no
reports relating to the potential for azodicarbonamide to produce
other systemic adverse effects. The potential genotoxic, carcinogenic,
and reproductive effects of azodicarbonamide in exposed humans have
not been studied.
9.1 Case reports
A number of reports have been published of individual
azodicarbonamide workers alleging asthma induced by exposure to
azodicarbonamide. The strongest evidence comes from a study of two
individuals (one atopic and one non-atopic) who worked at the same
plastics factory for about 4 years (Malo et al., 1985; Pineau et al.,
1985). Both were intermittently exposed (1-2 weeks' duration, 3-4
times per year) to azodicarbonamide at work. A few months after their
first encounter with azodicarbonamide, both developed symptoms
described as "eye/nose irritation" at work, followed a few hours later
by nocturnal asthmatic symptoms. After a 1-month period free from
exposure, both subjects underwent lung provocation studies. Baseline
values for forced expiratory volume in 1 s (FEV1), forced vital
capacity (FVC), and the concentration of histamine required to produce
a 20% drop in FEV1 (PC20H) were obtained by spirometry. Both
subjects performed a control challenge using lactose and then a 50:50
mixture of lactose and azodicarbonamide for 15 s on the next day. On
both days, lung function was monitored to follow the time course of
any response. It was reported that the trial was not carried out
blind.
No effects on lung function were observed following challenge
with lactose alone. After the azodicarbonamide challenge, however, the
atopic individual developed a late respiratory response starting 3 h
after challenge and reaching a maximum 24% drop in FEV1 6 h after
challenge. A drop in PC20H was also reported, demonstrating increased
airway hyperreactivity, and this parameter did not return to the
baseline value until 6 weeks after challenge. The non-atopic
individual showed a dual response to azodicarbonamide. Peak reductions
in FEV1 of greater than 20% were recorded 30 min and 5-6 h after
exposure. No significant reduction in PC20H was reported for the
second individual. A control atopic subject with underlying asthma who
worked in the same industry but did not experience work-related
respiratory effects was also tested. His baseline PC20H was similar
to that of the atopic subject, but no change in lung function was
observed following a 15-min exposure to azodicarbonamide under similar
conditions (as this subject had less reactive airways, a much longer
exposure duration was utilized). Owing to the insolubility of
azodicarbonamide, skin prick tests were not performed.
Six other cases have been reported in the literature, but in each
case the evidence that azodicarbonamide was the cause of the
respiratory symptoms is less strong. In some cases, there had been
previous exposure in industries associated with potential exposure to
other asthmagenic substances; for others, the bronchial challenge test
was either poorly conducted or not conducted at all (Valentino &
Comai, 1985; Alt & Diller, 1988; Normand et al., 1989).
Three case reports on skin sensitization have been published. In
the most recently reported investigation, a male textile worker
exposed to azodicarbonamide in foam ear-plugs was patch tested to
discover the cause of a recurrent dermatitis of the ear (Nava et al.,
1983; Bonsall, 1984; Yates & Dixon, 1988). No response was elicited
with a number of standard (International Contact Dermatitis Research
Group standard series) allergens. However, the individual gave a
strong positive reaction to the ear-plugs at 48 and 96 h and also to
azodicarbonamide (a component of the ear-plugs) at a concentration of
1 and 5% in petrolatum but not at 0.1% in petrolatum. Ten control
subjects patch tested with 1 and 5% azodicarbonamide in petrolatum did
not respond, and the individual reported no further symptoms upon
discarding the ear-plugs.
9.2 Epidemiological studies
Workplace health surveys have also been carried out where
azodicarbonamide was either manufactured or used to investigate the
presence of respiratory symptoms in azodicarbonamide workers.
A prevalence study of occupational asthma was carried out among a
group of 151 workers at a factory manufacturing azodicarbonamide
(Slovak, 1981). Diagnosis of asthma was made on the basis of an
administered questionnaire and a detailed occupational history taken
by the author. The population was divided into three groups: those
classified as potentially sensitized, on the basis of questionnaire
results; those with daily exposure but without symptoms; and those
with no exposure to azodicarbonamide or any other known sensitizer. On
one day, pre- and post-shift spirometry was performed, and FEV1, FVC,
and the FEV1/FVC ratio were determined. Skin prick tests were also
attempted using both common allergens to determine atopic status and
azodicarbonamide at concentrations of 0.1, 1, and 5% in dimethyl
sulfoxide. Concurrent personal sampling measurements were made to
determine the levels of airborne azodicarbonamide to which individuals
were exposed.
Personal sampling indicated that, at the time of the
investigation, airborne concentrations of azodicarbonamide ranged
between 2 and 5 mg/m3, as 8-h time-weighted averages. From the
questionnaires and occupational histories, 28 individuals (18.5%) were
diagnosed as having asthma apparently related to azodicarbonamide
exposure. Twelve further cases of occupational asthma were identified
from company records of past employees. Skin prick tests with
azodicarbonamide could not be adequately performed owing to the
insolubility of the substance.
Of the 28 current workers classified as sensitized, over half
developed symptoms within 3 months of first exposure and 21/28 (75%)
within 1 year. Symptoms and signs included shortness of breath, chest
tightness, wheezing, cough, rhinitis, conjunctivitis, and rash.
Reactions were of an immediate type for 6/28 (21%) individuals, late
onset for 16/28 (57%), and dual onset for 6/28 workers. Of those
showing a dual response, all but one had initially shown a late onset
pattern. A total of 13/28 (46%) workers reported worsening of symptoms
with continuing exposure to azodicarbonamide and a shortening of the
time between returning to work and reappearance of symptoms. Eight out
of 13 workers exposed to azodicarbonamide for more than 3 months after
development of symptoms also developed sensitivity to previously
well-tolerated irritants (e.g., sulfur dioxide and tobacco smoke),
which persisted for over a month after removal from exposure to
azodicarbonamide. In five individuals, this airway hyperreactivity
persisted for over 3 years. There were no changes in FEV1 or FVC over
the work shift in any group. In view of the latency in development of
effects, late or dual onset of symptoms in 12/28 (43%) symptomatic
workers, increase in sensitivity with repeated exposure, and the
persisting lung hyperreactivity in workers with prolonged exposure
after developing symptoms, it seems likely that these individuals had
become sensitized to azodicarbonamide.
Ahrenholz & Anderson (1985) and Whitehead et al. (1987) conducted
detailed investigations of the workforce at a plastics factory
employing about 325 workers. Lung function tests and interviews to
gather information on occupational history, smoking habits, past
illnesses, and respiratory, nasal, eye, and skin irritation, including
the time course of any symptoms, were carried out with a large
percentage of the workforce. There were no clear differences in the
results of lung function studies between those exposed to
azodicarbonamide and non-exposed individuals. However, responses to
the questionnaire revealed a significant association between symptoms
of irritation, cough, wheezing, shortness of breath, and headache and
present or previous employment as an injection mould operator. There
was also a slight but not statistically significant increase in the
reporting of skin rash among those with current or previous work in
the injection moulding department. The prevalence of all the above
symptoms was reduced among those whose employment in this department
was limited to the period before azodicarbonamide was introduced or
after a change in the process significantly reduced the use of
azodicarbonamide at the plant.
Personal sampling showed that concentrations of airborne
azodicarbonamide ranged from below the limit of detection (0.001
mg/m3) to 0.32 mg/m3 (median 0.006 mg/m3; geometric mean 0.004
mg/m3) averaged over the full shift. The highest concentration of
azodicarbonamide recorded (for an injection mould operator) was 0.01
mg/m3. Toluene, styrene, phenols, and triphenyl phosphate were also
detected at concentrations at or below the odour threshold for each
substance.
Other personal sampling data for a group of 17 individuals
revealed levels of azodicarbonamide ranging from traces to 0.8 mg/m3
(median 0.03 mg/m3; geometric mean 0.02 mg/m3) averaged over the
full shift. The second highest value recorded was 0.4 mg/m3, and the
next highest, 0.06 mg/m3. A moderate although statistically
significant reduction in FEV1 (mean reduction of 64 ml) and FVC (mean
reduction of 77 ml) occurred following shifts in which workers were
exposed to azodicarbonamide. Coughing at work, wheeze, and chest
tightness were also reported, and symptoms were apparently worse
during the week than on Sunday.
A detailed investigation of the workforce at a plant making floor
coverings was conducted after nosebleeds, mucous membrane irritation,
and skin rashes were reported in workers handling azodicarbonamide
(Ahrenholz et al., 1985). Two surveys were carried out. The initial
survey revealed, in decreasing order of prevalence, symptoms of eye
irritation, nose irritation, cough, nocturnal cough, shortness of
breath, wheeze, and chest tightness. The more extensive follow-up
survey was conducted 6 weeks later. Pre- and post-shift auscultation,
lung function tests, and respiratory symptoms (recorded by
questionnaire) were recorded. Blood samples were also taken for
immunological investigations.
Responses to the questionnaire revealed 15/30 regularly exposed
workers experiencing occupationally related lower respiratory tract
symptoms (cough, wheeze, and shortness of breath) compared with 1/16
never-exposed workers. No significant differences in pre- and
post-shift FEV1 and FVC measurements were found. For those workers
apparently not exposed to azodicarbonamide or exposed indirectly
(working in the vicinity but not directly handling azodicarbonamide),
levels (8-h time-weighted average) ranged from <0.001 to 0.1 mg/m3.
However, during weighing and charging operations, peaks of between
0.15 and 12 mg/m3 (median 2.7 mg/m3) were measured for individuals
directly involved.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Azodicarbonamide has been tested under OECD Guideline protocols
in one species of fish and in the water flea ( Daphnia magna);
results are in an unpublished industry report, which has not been
peer-reviewed (see Table 1). A second study, not conducted to a
protocol or under Good Laboratory Practices, showed no effect of an
azodicarbonamide solution analysed at 8 mg/litre on the zebra fish
( Brachydanio rerio).1 There was no effect on oxygen consumption of
sewage sludge organisms exposed over 3 h to azodicarbonamide at
>10 000 mg/litre (this is substantially greater than the solubility
of the compound, and no information is available on how this
concentration was achieved).2 Overall, it is not possible to draw
firm conclusions from these studies.
1 IUCLID (European Union database), version dated 7 February 1996.
2 Bayer, unpublished value (1988) presented in IUCLID (European
Union database), version dated 7 February 1996; no details
available.
Table 1: Acute studies on toxicity to aquatic organisms.
Organism Protocol End-point Concentration Reference
(mg/litre)
Fathead minnow OECD 203 96-h NOEC >50 Uniroyal (1992)
(Pimephales promelas)
Water flea OECD 202 48-h NOEC 4.8 (measured) Uniroyal (1992)
(Daphnia magna) ~16 (nominal)
48-h EC50 11 (measured)
immobilization 29 (nominal)
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Some of the available toxicological studies have been conducted
using biurea rather than azodicarbonamide. However, azodicarbonamide
is readily converted to biurea in vivo. Hence, similar toxicological
properties would be expected.
Azodicarbonamide is of low acute toxicity by all routes, and,
although the animal studies are of uncertain quality, solid
azodicarbonamide would not be regarded as a skin or eye irritant. With
respect to respiratory tract irritation, no changes of toxicological
significance were seen in guinea-pigs exposed to azodicarbonamide
aerosol at concentrations up to 97 mg/m3 for 1 h.
No conclusions could be drawn regarding skin sensitization
potential from the available, poor-quality animal studies. Although
there are currently no validated animal studies investigating
asthmagenic potential, there was no evidence of pulmonary irritation
or asthmatic-type reactions in guinea-pigs exposed to up to 200 mg
azodicarbonamide aerosol/m3 for 6 h/day, 5 days/week, for 4 weeks.
Similarly, there were no changes of toxicological significance
seen among rats or mice exposed by inhalation to up to 200 mg
azodicarbonamide aerosol/m3 for 6 h/day, 5 days/week, for up to 13
weeks.
For repeated-dose studies using the oral route, data were
inconsistent. In 2-year studies in which rats received up to 450 mg
biurea/kg body weight per day, there were no adverse effects seen.
Unpublished information suggests that no adverse effects were seen in
male mice exposed to up to 1250 mg azodicarbonamide/kg body weight per
day and in female mice exposed to up to 2500 mg/kg body weight per day
for 13 weeks. However, shorter-term studies (2 weeks, also
unpublished) indicated histological lesions in the kidneys of rats and
mice of both sexes at 1250 mg/kg body weight per day or more. A
13-week study in rats indicated kidney lesions in males at 2500 mg/kg
body weight per day, with no adverse effects observed at the next
lowest exposure level, 500 mg/kg body weight per day. For female rats,
kidney lesions were observed at 5000 mg/kg body weight per day, and no
adverse effects were observed at 1000 mg/kg body weight per day. There
were no data in relation to repeated dermal exposure.
Azodicarbonamide has been identified as a mutagen in bacterial
systems, but it was not mutagenic in mammalian cell in vitro test
systems or in two mammalian assays in vivo using bone marrow. It is
therefore unlikely that the mutagenic properties displayed by
azodicarbonamide in bacterial systems will be expressed in vivo.
However, it is considered that a confirmatory in vivo study in a
second tissue is desirable.
There are no adequate data available relating to carcinogenic,
reproductive, or developmental effects; hence, it is not possible to
evaluate the risk to human health for these end-points.
Several bronchial challenge studies have been reported, but only
one provides reasonable evidence that the work-related asthmatic
symptoms were due specifically to azodicarbonamide. This report is
considered to show an asthmatic response and not an irritant response
to azodicarbonamide on challenge. Animal studies suggest that airborne
concentrations of up to 200 mg/m3 can be tolerated with little or no
pulmonary irritation, and it is unlikely that the levels used in the
bronchial challenge tests approached those used in animal studies. The
delay in response to azodicarbonamide challenge, the magnitude of
reduction in FEV1 accompanied by an increase in airway
hyperreactivity in one individual, and the fact that a control
individual with mildly hyperreactive airways did not respond to a much
more prolonged exposure under similar challenge conditions provide
further evidence for asthmagenicity. Further evidence of a link
between azodicarbonamide and respiratory problems is provided by the
results of workplace health evaluations. Although criticisms can be
levelled at individual studies, weight of evidence suggests that
azodicarbonamide can induce asthma in a significant proportion of
exposed people.
There are some case reports of individuals with skin reactions to
topically applied azodicarbonamide. For some of these, results are
questionable. However, in workplace health surveys, the incidence of
skin rash was found to be greater among workers regularly exposed to
azodicarbonamide. Although no firm conclusions could be drawn from the
poorly reported animal study, clear evidence of skin sensitization to
azodicarbonamide in one individual and supporting evidence of skin
problems from workplace health surveys lead to the conclusion that
azodicarbonamide should be considered as a human skin sensitizer.
In conclusion, the key toxic effect of azodicarbonamide in humans
is asthmagenicity. Evidence of this effect has been found from
bronchial challenge studies and workplace health evaluations. From the
information available, azodicarbonamide is considered to have a low
potential for irritancy; thus, it is considered that the respiratory
symptoms observed in these studies are most likely due to an
asthmatic-type response rather than respiratory tract irritancy. There
is no clear information on the levels that may have induced or
provoked the state of asthma.
There is also information to indicate that azodicarbonamide can
cause skin sensitization in humans.
11.1.2 Criteria for setting guidance values for azodicarbonamide
The main cause for concern relates to the risk of developing
occupational asthma. There is no information available relating to
dose-response relationships or levels associated with the induction of
a hypersensitive state or provocation of an asthmatic response. Hence,
it is not possible to reliably quantify the risk of developing
occupational asthma.
11.1.3 Sample risk characterization
Using data obtained from a factory in the United Kingdom and
published exposure data as an example (section 6.2), levels of
airborne azodicarbonamide measured over periods of <70 min to 4 h of
up to 12 mg/m3 have been observed. Short-term peak exposures could be
higher than this level.
In the United Kingdom occupational setting, it is recommended
that a maximum exposure limit (MEL) be assigned to substances for
which it has not been possible to identify a level of exposure that is
without adverse effects on health. This is a non-health-based
standard, and, in determining the most appropriate level for a MEL,
consideration is taken of the level of control that it is reasonably
practicable for industry to achieve. The MEL of 1 mg/m3
(8-h time-weighted average) was based on a level of control that was
deemed by tripartite agreement to be reasonably practicable under
workplace conditions within the United Kingdom. There is also a
continuing remit for industry to keep on reducing exposure levels as
advances in technology make this possible. For substances that are
asthmagens, it is also advisable to have a short-term exposure limit
(STEL) to restrict peak exposures, as they may have a role in the
induction and triggering of asthmatic phenomena. In the absence of any
specific data that might advise adequately on the numerical value of
the STEL, 3 mg/m3 (15-min reference period) has been established.
As azodicarbonamide is a skin sensitizer, where skin contact can
occur, there may be a risk of developing allergic dermatitis if
suitable personal protective equipment is not used.
There is evidence to suggest that azodicarbonamide has been added
to consumer products such as bread and beer. The limited toxicology
database and lack of exposure data make it difficult to adequately
assess the risk to humans potentially exposed; hence, there is a need
for further information.
11.2 Evaluation of environmental effects
Lack of information on release to the environment precludes a
quantitative risk assessment.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations by international bodies were not identified.
Information on international hazard classification and labelling is
included in the International Chemical Safety Card reproduced in this
document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0380) reproduced in this
document.
13.1 Human health hazards
Azodicarbonamide is of low acute toxicity, but repeated or
prolonged contact may cause asthma and skin sensitization.
13.2 Health surveillance advice
Physicians involved in worker health surveillance programmes
should be aware of the potential of azodicarbonamide as a human
asthmagen.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
AZODICARBONAMIDE ICSC:0380
October 1997
CAS# 123-77-3 Diazenedicarboxamide
RTECS # LQ1040000 1,1'-Azobisformamide
UN # 3242 C2H2N4O2/NH2CON=NCONH2
EC3 611-028-00-3
Molecular mass: 116.1
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/
EXPOSURE SYMPTOMS FIRE FIGHTING
FIRE Flammable. Gives off NO open flames, NO sparks, Foam, powder.
irritating or toxic fumes and NO smoking.
(or gases) in a fire.
EXPLOSION
EXPOSURE PREVENT DISPERSION OF
DUST! STRICT HYGIENE!
Inhalation Cough. Headache. Shortness of Local exhaust or breathing Fresh air, rest.
breath. Sore throat. Wheezing. protection. Refer for medical
Fatigue. Cramps. attention.
Skin Redness. Protective clothing. Remove contaminated clothes.
Rinse and then wash skin with
water and soap.
Eyes Redness. Pain. Safety goggles, or eye First rinse with plenty of
protection in combination water for several minutes
with breathing protection. (remove contact lenses if easily
possible), then take to a doctor.
Ingestion Do not eat, drink, or smoke Rinse mouth. Give
during work. plenty of water to drink.
Rest.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into sealable containers; EU Classification
if appropriate, moisten first to prevent dusting. Symbol: Xn
Carefully collect remainder, then remove to safe R: 42-44
place (extra personal protection; P2 filter S: (2-)22-24-37
respirator for harmful particles). UN Classification
UN Hazard Class: 4.1
UN Pack Group: II
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-41G19
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
ORANGE RED CRYSTALS OR YELLOW POWDER. The substance can be absorbed into the body by
inhalation of its aerosol.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on heating or on burning Evaporation at 20°C is negligible;
producing toxic fumes (nitrogen oxides). a harmful concentration of airborne particles
can, however, be reached quickly.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV not established. The substance irritates the eyes and the respiratory
tract. Inhalation of dust may cause asthmatic
reactions (see Notes).
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
Repeated or prolonged contact with skin may cause
dermatitis. Repeated or prolonged contact may cause
skin sensitazation. Repeated or prolonged inhalation
exposure may cause asthma.
PHYSICAL PROPERTIES
Melting point (decomposed): 225°C
Relative density (water = 1): 1.65
Solubility in water: none
ENVIRONMENTAL DATA
NOTES
The symptoms of asthma often do not become manifest until a few hours have passed and they are aggravated
by physical effort. Rest and medical observation are therefore essential. Anyone who has shown symptoms
of asthma due to this substance should avoid all further contact with this substance. Genitron AC,
Kempore 125, Porofor LK 1074 and Unifoam AZ are trade names.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the CEC or the IPCS is
responsible for the use which might be made of this information.
REFERENCES
Ahrenholz S, Anderson K (1985) Health hazard evaluation report of
Leon Plastics, Grand Rapids, MI. Cincinnati, OH, US Department of
Health and Human Services (Report No. HETA-83-156-1622; PB89-143200).
Ahrenholz S, Neumeister C (1987) Development of a sampling and
analytical method for azodicarbonamide. American Industrial Hygiene
Association journal, 48(5):442-446.
Ahrenholz S, Morawetz J, Liss G (1985) Health hazard evaluation
report of Armstrong World Plastics, Lancaster, PA. Cincinnati, OH,
US Department of Health and Human Services (Report No.
HETA-83-451-1547; PB86-105582).
Alt E, Diller W (1988) "Hyperreactivity cough" in occupational
medicine. Zentralblatt fuer Arbeitsmedizin, Arbeitsschutz
und Prophylaxe Ergonomie, 38 (Suppl. 2):22-26.
Ball E, Saleem A, Ogunbiyi A, Wiley K, Groves J (1996)
Azodicarbonamide; Criteria document for an occupational exposure
limit. Sudbury, Suffolk, Health and Safety Executive, HSE Books
(ISBN 0 7176 10926).
Bechtold W, Medinsky M, Cheng Y, Hobbs C (1989) Azodicarbonamide:
methods for the analysis in tissues of rats and inhalation
disposition. Xenobiotics, 19:1003-1012.
BG Chemie (1995) Azodicarbonamide. In: GDCh-Advisory Committee on
Existing Chemicals of Environmental Relevance, eds. Toxicological
evaluations, potential health hazards of existing chemicals. Vol.
8. Stuttgart, S. Hirzel (ISBN 0 387 58287 8).
Bonsall J (1984) Allergic contact dermatitis to azodicarbonamide.
Contact dermatitis, 10:42.
Canas B, Diachenko G, Nyman P (1997) Ethyl carbamate levels resulting
from azodicarbonamide use in bread. Food additives and contaminants,
14(1):89-94.
Conning D (1966) Azodicarbonamide (Genitron AC4). Toxicological
report. Unpublished report submitted to Imperial Chemical Industries
Limited, Macclesfield (Report No. TR/522).
Dennis M, Massey R, Ginn R, Parker I, Crews C, Zimmerli B, Zoller O,
Rhyn P, Osborne B (1997a) The effect of azodicarbonamide
concentrations on ethyl carbamate concentrations in bread and toast.
Food additives and contaminants, 14(1):95-100.
Dennis M, Massey R, Ginn R, Willetts P, Crews C, Parker I (1997b) The
contribution of azodicarbonamide to ethyl carbamate formation in bread
and beer. Food additives and contaminants, 14(1):101-108.
Frankenberger W, Tabatabai M (1982) Transformations of amide nitrogen
in soils. Soil Science Society of America journal, 46:280-284.
Gafford F, Sharry P, Pittman J Jr (1971) Effect of azodicarbonamide
(1,1-azobisformamide) on thyroid function. Journal of clinical
endocrinology and metabolism, 32:659-662.
Gerlach R, Medinsky M, Hobbs C, Bice D, Bechtold W, Cheng Y, Gillett
N, Birnbaum L, Mauderly J (1989) Effect of four week repeated
inhalation exposure to unconjugated azodicarbonamide on specific and
non-specific airway sensitivity of the guinea pig. Journal of
applied toxicology, 9(3):145-153.
Hachiya N (1987) Evaluation of chemical genotoxicity by a series of
short term tests. Akita Igaku, 14:269-292.
HSDB (1996) Hazardous substances data bank. Bethesda, MD, US
National Library of Medicine. CHEM-BANK, SilverPlatter compact disk.
IPCS (1993) International Chemical Safety Card
-- Diazenedicarboxamide. Geneva, World Health Organization,
International Programme on Chemical Safety (ICSC 0380).
IRDC (1982a) Acute inhalation toxicity test with azodicarbonamide in
rats. Unpublished report submitted by the International Research and
Development Corporation to the National Toxicology Program, National
Institutes of Health, US Department of Health and Human Services,
Research Triangle Park, NC (Report No. 5703-115).
IRDC (1982b) Acute inhalation toxicity test with azodicarbonamide in
mice. Unpublished report submitted by the International Research and
Development Corporation to the National Toxicology Program, National
Institutes of Health, US Department of Health and Human Services,
Research Triangle Park, NC (Report No. 5703-111).
Joiner R, Vidal F, Marks H (1963) A new powdered agent for flour
maturing. Cereal chemistry, 40:539-553.
Kimmerle G (1965) Toxicological studies on Porofor ADC, Urazol and
plastic foam extracts. Unpublished report, Institute of Toxicology,
Bayer AG, Wuppertal-Elberfeld.
Loeser E (1976) Acute oral toxicity: Porofor ADC/K. Unpublished
report, Institute of Toxicology, Bayer AG, Wuppertal-Elberfeld.
Malo J, Pineau L, Cartier A (1985) Occupational asthma due to
azobisformamide. Clinical allergy, 15:261-264.
MB Research Laboratories Inc. (1986) Acute dermal toxicity in
rabbits. MB 86-83 93 B. Celogen AZ 120. Unpublished report submitted
to Uniroyal Chemical Company Inc., Middlebury, CN.
Medinsky M, Bechtold W, Birnbaurn L, Bond J, Burt D, Cheng Y, Gillett
N, Gulati D, Hobbs C, Pickrell J (1990) Effect of inhaled
azodicarbonamide on F344/N rats and B6C3F1 mice with 2-week and
13-week inhalation exposures. Fundamental and applied toxicology,
15:308-319.
Mewhinney J, Ayres P, Bechtold W, Dutcher J, Cheng Y, Bond J, Medinsky
M, Henderson R, Birnbaum L (1987) The fate of inhaled azodicarbonamide
in rats. Fundamental and applied toxicology, 8:372-381.
Mihail T (1977) Studies of skin and mucous membrane tolerance:
Porofor ADC/K. Unpublished report, Institute of Toxicology, Bayer
AG, Wuppertal-Elberfeld.
Mortelmans K, Haworth S, Lawlor T, Speck W, Trainer B, Zeiger E (1986)
Salmonella mutagenicity tests: 11. Results from the testing of 270
chemicals. Environmental mutagenesis, 8 (Suppl. 7):1-119.
Nava C, Beretta F, Elena A, Ghizzi A, Pattarin R, Villa L (1983)
Allergic dermatitis due to improvers and other flour additives.
Medicina del Lavoro, 74(5):376-379.
Normand J, Grange F, Hernandez C, Ganay A, Davezies P, Bergeret A,
Prost G (1989) Occupational asthma after exposure to azodicarbonamide:
report of four cases. British journal of industrial medicine,
46:60-62.
Oser B, Oser M (1963) Nutritional studies on rats on diets containing
high levels of partial ester emulsifiers. 11. Reproduction and
lactation. Journal of nutrition, 60:489-505.
Oser B, Oser M, Morgareidge K, Stemberg S (1965) Studies of the safety
of azodicarbonamide as a flour maturing agent. Toxicology and
applied pharmacology, 7:445-472.
Pharmakon Research International (1984a) Ames
Salmonella/microsome plate test (EPA/OECD). PH 301-UN-004-84.
Celogen AZ-130. Unpublished report submitted to Uniroyal Chemical
Company Inc., Middlebury, CN.
Pharmakon Research International (1984b) CHO/HGPRT mammalian cell
forward gene mutation assay. PH 314-UN-003-84. Celogen AZ.
Unpublished report submitted to Uniroyal Chemical Company Inc.,
Middlebury, CN.
Pharmakon Research International (1984c) Rat hepatocyte primary
culture/DNA repair test. PH 311-UN-003-84. Celogen AZ-130.
Unpublished report submitted to Uniroyal Chemical Company Inc.,
Middlebury, CN.
Pharmakon Research International (1984d) Micronucleus test (MNT)
OECD. PH-309A-UN-003-84. Celogen AZ-130. Unpublished report
submitted to Uniroyal Chemical Company Inc., Middlebury, CN.
Pineau L, Cartier A, Malo J-L (1985) Occupational asthma due to
azobisformamide. Journal of allergy and clinical immunology, 75:170.
Shopp G, Cheng Y, Gillet N, Bechtold W, Medinsky M, Hobbs C, Birnbaum
L, Mauderly J (1987) Acute inhalation exposure of azodicarbonamide in
the guinea pig. American Industrial Hygiene Association journal,
48(2):127-132.
Slovak A (1981) Occupational asthma caused by a plastics blowing
agent, azodicarbonamide. Thorax, 36(12):906-909.
Stevens M (1967) Use of the albino guinea pig to detect the skin
sensitising ability of chemicals. British journal of industrial
medicine, 24:189-202.
Uniroyal (1992) Unpublished report from the Uniroyal Chemical Company
Inc., Middlebury, CN.
Vainiotalo S, Pfaffli P (1988) Measurement of azodicarbonamide in
workroom air in the plastics processing industry. Annals of
occupational hygiene, 43:203-208.
Valentino M, Comai M (1985) Occupational asthma from azodicarbonamide:
Case report. Giornale Italiano di Medicina del Lavoro,
7(2-3):97-99.
Whitehead L, Robins T, Fine L, Hansen D (1987) Respiratory symptoms
associated with the use of azodicarbonamide foaming agent in a
plastics injection moulding facility. American journal of industrial
medicine, 11:83-92.
Yates V, Dixon J (1988) Contact dermatitis from azodicarbonamide in
earplugs. Contact dermatitis, 19(2):155-156.
Yoon J, Mason J, Valencia R, Woodruff R, Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. IV. Results of 45 coded compounds
tested for the National Toxicology Program. Environmental
mutagenesis, 7:349-367.
APPENDIX 1 -- SOURCE DOCUMENT
Ball et al. (1996): Azodicarbonamide; Criteria document for an
occupational exposure limit
The authors' first draft was initially reviewed internally by a
group of approximately 10 United Kingdom Health and Safety Executive
experts (mainly toxicologists, but also scientists involved in other
relevant disciplines, such as epidemiology and occupational hygiene).
The toxicology section of the amended draft was then reviewed by
toxicologists from the United Kingdom Department of Health.
Subsequently, the entire criteria document was reviewed by a
tripartite advisory committee to the United Kingdom Health and Safety
Commission, the Working Group for the Assessment of Toxic Chemicals
(WATCH). This committee is composed of experts in toxicology and
occupational health and hygiene from industry, trade unions, and
academia.
The members of the WATCH committee at the time of the peer review
were Mr S.R. Bailey, Independent Consultant; Professor J. Bridges,
University of Surrey; Dr H. Cross, Trade Unions Congress; Dr A
Fletcher, Trade Unions Congress; Dr I.G. Guest, Chemical Industries
Association; Dr A. Hay, Trade Unions Congress; Dr J. Leeser, Chemical
Industries Association; Dr L. Levy, Institute of Occupational Hygiene,
Birmingham; Mr A. Moses, Chemical Industries Association; Dr R. Owen,
Trade Unions Congress; and Dr M. Sharratt, University of Surrey.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on azodicarbonamide was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
József Fodor National Center of Public Health, Budapest, Hungary
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Institute of Public Health, Prague, Czech Republic
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands
United States Department of Health and Human Services (National
Institute for Occupational Safety and Health, Cincinnati, USA;
National Institute of Environmental Health Sciences, Research
Triangle Park, USA)
United States Environmental Protection Agency (Drinking Water
Program, Denver, USA)
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Tokyo, Japan, 30 June - 2 July 1998
Members
Dr R. Benson, Drinking Water Program, United States Environmental
Protection Agency, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Mr R. Cary, Health Directorate, Health and Safety Executive,
Merseyside, United Kingdom
Dr C. DeRosa, Agency for Toxic Substances and Disease Registry, Center
for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire, United
Kingdom
Dr H. Gibb, National Center for Environmental Assessment, United
States Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada ( Chairperson)
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan ( Vice-Chairperson)
Professor S.A. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia ( Rapporteur)
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute of
Occupational Medicine, Beijing, People's Republic of China
Observers
Professor F.M.C. Carpanini,1 Secretary-General, ECETOC (European
Centre for Ecotoxicology and Toxicology of Chemicals), Brussels,
Belgium
1 Invited but unable to attend.
Dr M. Ema, Division of Biological Evaluation, National Institute of
Health Sciences, Osakai, Japan
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Mr T. Jacob,1 Dupont, Washington, DC, USA
Dr H. Koeter, Organisation for Economic Co-operation and Development,
Paris, France
Mr H. Kondo, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Ms J. Matsui, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Mr R. Montaigne,1 European Chemical Industry Council (CEFIC),
Brussels, Belgium
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr H. Nishimura, Environmental Health Science Laboratory, National
Institute of Health Sciences, Osaka, Japan
Ms C. Ohtake, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr T. Suzuki, Division of Food, National Institute of Health Sciences,
Tokyo, Japan
Dr K. Takeda, Mitsubishikasei Institute of Toxicological and
Environmental Sciences, Yokohama, Japan
Dr K. Tasaka, Department of Chemistry, International Christian
University, Tokyo, Japan
Dr H. Yamada, Environment Conservation Division, National Research
Institute of Fisheries Science, Kanagawa, Japan
Dr M. Yamamoto, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
1 Invited but unable to attend.
Dr M. Yasuno, School of Environmental Science, The University of Shiga
Prefecture, Hikone, Japan
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif à l'azodicarbonamide a été préparé à partir
d'une étude du Health and Safety Executive du Royaume-Uni sur les
risques pour la santé humaine (risques professionnels pour
l'essentiel) (Ball et al., 1996). De ce fait, bien qu'il comporte un
volet sur l'évaluation des données écologiques disponibles, il est
principalement centré sur les risques pour la santé humaine sur les
lieux de travail et tout particulièrement sur les voies d'exposition
professionnelle à prendre en considération. L'analyse des données sur
lesquelles repose l'étude a été arrêtée à juin 1994. Un dépouillement
de la littérature a été ensuite effectué jusqu'à juillet 1997, à la
recherche de données qui auraient pu être publiées depuis la fin de
l'étude. Le document original ne prend pas en considérations les
problèmes d'ordre écologique et comme le dépouillement de la
littérature n'a pas permis de trouver trace de travaux qui leur soit
consacrés, on n'a pas cherché à procéder à une évaluation des risques
pour l'environnement. On trouvera à l'appendice 1 des indications sur
les sources documentaires utilisées et sur leur mode de dépouillement.
Les renseignements concernant l'examen du CICAD par des pairs font
l'objet de l'appendice 2. Ce CICAD a été approuvé en tant
qu'évaluation internationale lors d'une réunion du Comité d'évaluation
finale qui s'est tenue à Tokyo (Japon) du 30 juin au 2 juillet 1998.
La liste des participants à cette réunion figure à l'appendice 3. La
fiche d'information internationale sur la sécurité chimique (ICSC No
0380) établie par le Programme international sur la Sécurité chimique
(IPCS, 1993) est également reproduite dans ce document.
Les données toxicocinétiques sur l'azodicarbonamide (No CAS
123-77-3) sont limitées, mais ce composé se révèle être bien absorbé
chez les rongeurs après inhalation ou ingestion. Une fraction notable
de l'azocarbonamide traverse cependant les voies digestives sans être
résorbé et se retrouve dans les matières fécales. L'azodicarbonamide
est facilement transformé en biurée ou dicarbamylhydrazine, le seul
métabolite qui ait été identifié et il est probable que l'exposition
par la voie générale concerne ce dernier dérivé plutôt que la molécule
initiale. Après absorption, l'élimination de l'azocarbonamide ou de la
biurée est rapide et s'effectue principalement par la voie urinaire,
la biurée étant très peu retenue dans l'organisme.
L'azocarbonamide présente une faible toxicité aiguë et il ne
provoque pas d'irritation cutanée, oculaire ou respiratoire chez les
animaux d'expérience. Des résultats négatifs ont été obtenu lors d'une
étude de la sensibilisation cutanée, d'ailleurs mal conduite, et une
autre étude, effectuée sur des cobayes, n'a pas révélé de réaction de
nature asthmatiforme. Aucun effet indésirable n'a été observé chez des
animaux de laboratoire à qui on en avait fait inhaler pendant des
durées allant jusqu'à 13 semaines à des doses pouvant atteindre
200 mg/m3. Une exposition répétée par la voie orale a entraîné des
lésions évocatrices de pyélonéphrite avec présence intratubulaire de
cylindres et de dépôts cristallins chez plusieurs espèces. Cependant,
il a fallu une dose élevée pour provoquer ces effets (>200 mg/kg de
poids corporel par jour sur une durée pouvant aller jusqu'à 1 an).
L'azodicarbonamide s'est révélé mutagène sur des systèmes bactériens,
mais il n'est pas certain que cet effet se produise in vivo. On n'a
pas examiné en détail la cancérogénicité de l'azodicarbonamide, ni sa
toxicité génésique, mais des études anciennes au cours desquelles des
animaux avaient reçu son métabolite, la biurée, n'ont pas mis en
évidence d'effets tumorigènes ou stérilisants. On n'a pas étudié son
action toxique éventuelle sur le développement.
Les études relatives à la santé humaine portent uniquement sur
l'aptitude de l'azodicarbonamide à provoquer de l'asthme ou une
sensibilisation cutanée. Des épreuves d'exposition bronchique
effectuées sur des sujets symptomatiques de même que l'examen médical
de personnes employées à la fabrication d'azodicarbonamide ou sur des
lieux ou on en utilisait, ont révélé que le composé pouvait provoquer
de l'asthme chez l'Homme. Certains indices permettent également de
penser qu'il peut provoquer une sensibilisation cutanée.
En se basant sur le fait que l'azodicarbonamide peut provoquer un
asthme chez l'Homme et que l'on ignore à partir de quelle
concentration cet asthme risque d'apparaître chez un sujet non
sensible ou une réaction se manifester chez un sujet sensible, on est
arrivé à la conclusion que dans les conditions actuelles d'exposition
professionnelles, il y avait un risque pour la santé humaine. Compte
tenu de l'incertitude sur la concentration à partir de laquelle il y a
effectivement risque, il convient de réduire l'exposition le plus
possible.
On possède des données indiquant qu'il se forme du carbamate
d'éthyle dans certains produits de consommation comme le pain ou la
bière après addition d'azodicarbonamide. Faute de données, il n'a pas
été possible d'évaluer le degré d'exposition de la population générale
à l'azodicarbonamide.
En cas de décharge dans les eaux superficielles, l'azocarbonamide
se répartirait dans l'hydrosphère sans sorption importante aux
matières particulaires. Dans le cas d'une réaction sur les radicaux
hydroxyles de l'atmosphère, la demi-vie calculée est de 0,4 jour. Dans
deux essais sur trois effectués avec des boues d'égout, on a constaté
que l'azodicarbonamide se révélait facilement biodégradable et on
observé une décomposition à 20-70 % dans le sol en 14 jours. La
concentration sans effet observable pour les poissons et la puce d'eau
a été trouvée respectivement égale à >50 et 5 mg/litre. En
l'absence de renseignements sur la décharge d'azodicarbonamide dans
l'environnement, on ne peut procéder à une évaluation quantitative du
risque.
RESUMEN DE ORIENTACION
Este CICAD sobre la azodicarbonamida se basa en un examen de los
problemas relativos a la salud humana (fundamentalmente ocupacionales)
preparado por la Dirección de Salud y Seguridad del Reino Unido (Ball
et al., 1996). Por consiguiente, aunque el presente CICAD incluye una
evaluación de los datos ecológicos disponibles, se concentra sobre
todo en el riesgo para la salud humana en las condiciones del trabajo,
con particular atención a la información acerca de las rutas que son
de interés para el entorno ocupacional. En este examen se han
incorporado los datos identificados hasta junio de 1994. Se realizó
una ulterior búsqueda bibliográfica hasta julio de 1997 para localizar
la información nueva que se hubiera publicado desde la terminación del
examen. En el documento original no se abordaban los problemas
relativos al medio ambiente; dado que en la búsqueda bibliográfica no
se han encontrado estudios de interés de este sector, no se ha
intentado realizar una evaluación del riesgo para el medio ambiente.
La información acerca del carácter del examen colegiado del documento
original y su disponibilidad figura en el apéndice 1. La información
sobre el examen colegiado de este CICAD aparece en el apéndice 2. Este
CICAD se aprobó como evaluación internacional en una reunión de la
Junta de Evaluación Final celebrada en Tokio, Japón, del 30 de junio
al 2 de julio de 1998. La lista de participantes en esta reunión
figura en el apéndice 3. La Ficha internacional de seguridad química
(ICSC 0380) para la azodicarbonamida, preparada por el Programa
Internacional de Seguridad de las Sustancias Químicas (IPCS, 1993),
también se reproduce en el presente documento.
Los datos toxicocinéticos sobre la azodicarbonamida
(CAS No 123-77-3) son limitados, pero parece que se absorbe bien en
roedores por inhalación y por vía oral. Quedan sin absorber cantidades
importantes de la sustancia en el sistema gastrointestinal, que se
eliminan en las heces. La azodicarbonamida se convierte fácilmente en
biurea, único producto de la biodegradación identificado, y es
probable que haya exposición sistémica fundamentalmente a este
derivado y no al compuesto original. La eliminación de la
azodicarbonamida/biurea absorbida es rápida, sobre todo a través de la
orina, y hay una retención sistémica de biurea muy escasa.
La toxicidad aguda de la azodicarbonamida es baja y en los
animales de experimentación no produce irritación cutánea, ocular o
del aparato respiratorio. Los resultados de un estudio de
sensibilización cutáneo poco fidedigno fueron negativos y en otro
estudio no se obtuvieron pruebas de una respuesta de tipo asmático en
cobayas. No se detectaron efectos adversos en animales de
experimentación que inhalaron hasta 200 mg/m3 durante 13 semanas. La
exposición oral repetida provocó pielonefritis con cilindros y
depósitos cristalinos en los túbulos renales en varias especies. Sin
embargo, las dosis necesarias para inducir estos efectos fueron altas
(>200 mg/kg de peso corporal al día en estudios de hasta un año de
duración). Si bien se observó que la azodicarbonamida era mutagénica
en sistemas bacterianos, no hay pruebas de que este efecto aparezca
in vivo. No se han examinado con detalle la carcinogenicidad y la
toxicidad reproductiva de la azodicarbonamida, pero en estudios
iniciales en los cuales se trataron animales con el producto de
degradación, la biurea, no se detectaron efectos tumorígenos o
anticonceptivos. No se ha estudiado la toxicidad en el desarrollo.
Los estudios en el ser humano se han concentrado exclusivamente
en la capacidad de la azodicarbonamida para inducir asma y
sensibilización cutánea. En estudios de estímulo bronquial de personas
sintomáticas y en evaluaciones de la salud de los empleados en lugares
donde se fabrica o utiliza azodicarbonamida se ha comprobado que este
producto puede inducir asma en el ser humano. Existen asimismo
indicios de que la azodicarbonamida puede inducir sensibilización
cutánea.
A partir de la base de que la azodicarbonamida es un asmógeno
humano y de que no se conocen las concentraciones que se requieren
para inducir el asma en una persona no sensible o provocar una
respuesta en una persona sensible, se llega a la conclusión de que
existe un riesgo para la salud humana en las condiciones actuales de
exposición ocupacional. El nivel del riesgo es incierto; por
consiguiente, se deben reducir al máximo los niveles de exposición.
Se conocen datos que indican que se forma etilcarbamato en
productos de consumo como el pan y la cerveza después de la adición de
azodicarbonamida. La exposición del público general no se pudo evaluar
debido a la falta de datos disponibles.
La azodicarbonamida liberada en las aguas superficiales se
distribuiría en la hidrosfera con una sorción no significativa en
partículas. La semivida para la reacción en la atmósfera con los
radicales hidroxilo se calcula que es de 0,4 días. La biodegradación
de la azodicarbonamida fue fácil en dos de las tres pruebas realizadas
con lodos cloacales y la degradación en el suelo fue del 20%-70% en un
período de 14 días. No se han notificado concentraciones sin efectos
observados (NOEC) para peces y pulgas de agua a >50 y 5 mg/litro,
respectivamente. La falta de información sobre la liberación en el
medio ambiente impide una evaluación cuantitativa del riesgo.
The conversion factors for azodicarbonamide at 20°C and 101.3 kPa
are as follows:
1 mg/m3 = 0.21 ppm
1 ppm = 4.8 mg/m3
3. ANALYTICAL METHODS
Two methods can be used to measure levels of azodicarbonamide in
workplace air. In the first, samples are collected on 37-mm Teflon
filters backed with polyethylene, which in turn is backed with a
cellulose pad (Ahrenholz & Neumeister, 1987). Sampling takes place at
2 litres/min for periods from 7 to 486 min. After sampling,
azodicarbonamide is recovered from the filter with dimethyl sulfoxide
and analysed by high-performance liquid chromatography. The limit of
quantification is given as 5 µg per sample. For a 15-min sample at 2
litres/min, this is equivalent to a quantification limit in air of
0.167 mg/m3; over 8 h, it is equivalent to 0.005 mg/m3.
In the second method, azodicarbonamide is collected on 37-mm
glass fibre filters at 15-20 litres/min (Vainiotalo & Pfaffli, 1988).
It is then eluted from the filter with dimethyl sulfoxide. Following
the addition of sodium hydroxide and glucose solutions, the
azodicarbonamide is reduced to hydrazine. This is reacted with
4-dimethylaminobenzaldehyde, and the resulting aldazine is measured
spectrophotometrically at 460 nm. The lower detection limit is given
as 0.001 mg/m3 for a 4-m3 air sample (equivalent to 4 µg per
sample). This is a considerably larger volume than would normally be
collected for assessing personal exposure samples. At a more typical
flow rate of 2 litres/min for 8 h, 960 litres of air would be sampled,
resulting in a detection limit of about 0.005 mg/m3. Sampling over 15
min at the same rate would give a detection limit of about 0.16
mg/m3.
Although there are published methods for measuring
azodicarbonamide and its metabolite biurea in rats (Bechtold et al.,
1989; see also Mewhinney et al., 1987), there are no reports
describing their measurement in human body fluids.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The principal end use of azodicarbonamide is as a blowing agent
in the rubber and plastics industries. It is used in the expansion of
a wide range of polymers, including polyvinyl chloride, polyolefins,
and natural and synthetic rubbers. The blowing action occurs when the
azodicarbonamide decomposes on heating (process temperatures
approx. 190-230°C) to yield gases (nitrogen, carbon monoxide, carbon
dioxide, and ammonia), solid residues, and sublimated substances.
Decomposition accelerators, in the form of metal salts and oxides, may
also be added to bring about decomposition at lower temperatures.
Azodicarbonamide has in the past been used in the United Kingdom
and Eire (but not other European Union member states) as a flour
improver in the bread-making industry, but this use is no longer
permitted. It is not known how common this practice is worldwide.
Azodicarbonamide is not used in other consumer products.
Azodicarbonamide is manufactured by the reaction of dihydrazine
sulfate and urea under high temperature and pressure. The product of
this reaction is then oxidized using sodium chlorate and centrifuged
to yield a slurry containing azodicarbonamide. The slurry is washed to
remove impurities and dried to obtain the azodicarbonamide powder.
This is then micronized to a fine powder (95% of particles <10 µm,
which is in the respirable range for humans) before packaging.
Very limited information is available on production volumes. The
Hazardous Substances Data Bank (HSDB, 1996) gives US production
figures of "greater than 4.54 tonnes." Until recently,
azodicarbonamide was produced in the United Kingdom; however, this
production has now stopped, and all azodicarbonamide used in the
United Kingdom is imported, predominantly through one large company.
Approximately 2500 tonnes are supplied to the United Kingdom market
each year. Both pure azodicarbonamide (approximately 2200 tonnes) and
pre-mixed formulations (300 tonnes) are supplied, the latter
containing between 10 and 95% azodicarbonamide, depending on the end
use application. "Masterbatch" products, in which the azodicarbonamide
is pelletized with polyolefins, and blended pastes (azodicarbonamide
and plasticizer) are also supplied to the rubber and plastics
industries. In addition to the main importer, there are some firms
that process azodicarbonamide into dust-suppressed powders, pastes,
and "Masterbatch" formulations before selling the processed
azodicarbonamide to the end users.
Recent studies have examined the contribution of azodicarbonamide
to the levels of ethyl carbamate in bread and beer (Canas et al.,
1997; Dennis et al., 1997a,b). It is not clear if unreacted
azodicarbonamide is present in these products; therefore, it is not
possible to assess the contribution that consumption of such products
might make to the overall body burden of azodicarbonamide.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
A calculated half-life of 0.4 days for reaction with hydroxyl
radicals in air has been reported.1
Azodicarbonamide added to five different soil types at 200 mg
nitrogen/kg soil (dry weight) was degraded (measured as recovery of
inorganic nitrogen) by between 21.1% and 66.1% over 14 days
(Frankenberger & Tabatabai, 1982).
Degradation of azodicarbonamide by sewage sludge organisms has
been investigated in three modified Sturm tests (Organisation for
Economic Co-operation and Development [OECD] Guideline 301B). The
compound was "readily biodegradable" in two out of the three tests and
was degraded by 21% over 30 days in the third test (Uniroyal,
1992).2
According to Mackay Level I fugacity modelling, azodicarbonamide
released to surface waters will partition to the hydrosphere with no
significant sorption to particulates.3
1 Bayer, unpublished calculated value (1988) based on the method of
Atkinson; value presented in IUCLID (European Union database),
version dated 7 February 1996.
2 Bayer, unpublished value (1991) presented in IUCLID (European
Union database), version dated 7 February 1996; no details
available.
3 Bayer remark in IUCLID (European Union database), version dated 7
February 1996.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
There are no data available on levels of azodicarbonamide in
ambient air, water, soils, or sediment.
6.2 Human exposure
The data available to the authors of this CICAD are restricted to
the occupational environment. It is estimated that several thousand
persons are exposed to azodicarbonamide in the workplace in the United
Kingdom. Of this total, it is estimated that only a few hundred
persons are exposed as part of their main work activity (i.e., those
involved in compounding, mixing, or raw material handling).
Data obtained by the United Kingdom's Health and Safety Executive
at a plant milling azodicarbonamide powder in micronizing mills (four
samples were collected in total) showed average personal exposures
during the day shift to be 11.8 and 9.8 mg/m3 and during the night
shift to be 2.3 and 2.8 mg/m3 because of the lower throughput at
night. Samples were collected over 4 h. The operators' tasks involved
bagging, weighing, and packaging the milled product.
In the published literature, Slovak (1981) reported time-weighted
average total dust levels in the range 2-5 mg/m3 for azodicarbonamide
manufacturing operations. However, no details were given concerning
occupational groups or tasks. A US National Institute for Occupational
Safety and Health study (Ahrenholz et al., 1985) examined personal
exposures of workers handling azodicarbonamide in a flooring factory.
The work involved the formulation of pastes or paints and required the
blending of azodicarbonamide powder with plasticizers, resins,
pigments, and other additives. Exposures to azodicarbonamide occurred
primarily while the workers were weighing and tipping the powder.
Short-term (sample duration <70 min) personal exposures were in the
range 0.15-12 mg/m3 (median 2.7 mg/m3). A second study (Ahrenholz &
Anderson, 1985) focused on the use of azodicarbonamide in the
injection moulding of plastics. The process involved blending
azodicarbonamide powder with resins. Two sets of full-shift
measurements were reported, with median 8-h time-weighted average
levels of 6.2 µg/m3 (range, not detectable to 280 µg/m3) and 25
µg/m3 (range, trace to 752 µg/m3), respectively.
No data are available relating to dermal exposure levels.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
No information is available on the toxicokinetics of
azodicarbonamide in humans.
Most of the toxicokinetic data available for azodicarbonamide
come from animal studies (Mewhinney et al., 1987). Absorption of
azodicarbonamide has been demonstrated following both a single
inhalation exposure of up to 6 h (34% of dose) and a single oral
administration (10-33% of dose) of radiolabelled azodicarbonamide to
rats. In contrast, approximately 90% of a single intratracheally
instilled dose was apparently absorbed. The difference in absorption
between inhaled and intratracheally instilled azodicarbonamide could
be related to the fact that much of the inhaled azodicarbonamide did
not reach the lower respiratory tract. Half an hour after a 6-h
nose-only exposure of rats to 25 mg/m3 of a dry aerosol (average
mass aerodynamic diameter 3.4 µm), 78% of the calculated total intake
was located in the gastrointestinal tract.
Following exposure by both inhalation and oral routes,
substantial quantities of the substance remain unabsorbed from the
gastrointestinal tract and are passed out in the faeces.
Azodicarbonamide is readily converted to biurea, the only breakdown
product identified, and it is likely that systemic exposure is
principally to this derivative rather than to the parent compound.
Elimination of absorbed azodicarbonamide/biurea is rapid, occurring
predominantly via the urine, and there is very little systemic
retention of biurea.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
It is noted that some of the available toxicological studies
were conducted using biurea. Azodicarbonamide readily undergoes
reduction in the presence of thiol groups to form the stable compound
biurea. Given that thiol groups are also present in many biological
molecules, there is the potential for this reaction to take place
wherever azodicarbonamide encounters thiol groups in biological
systems. This has been demonstrated in an experiment in which
radiolabelled azodicarbonamide was added to fresh rat blood (Mewhinney
et al., 1987). All radioactivity was in the form of biurea within 5
min when untreated blood was used. Radioactivity associated with
azodicarbonamide was detected only in blood to which 5 mg unlabelled
azodicarbonamide/ml blood was added. This level is very much greater
than the levels that humans are likely to encounter.
8.1 Single exposure
Azodicarbonamide is of low acute toxicity by all relevant routes
of exposure. The LC50 was greater than 6100 mg/m3 in rats and mice
exposed to a dry aerosol (median mass aerodynamic diameter
5.8 ± 2.25 µm [geometric standard deviation]) of azodicarbonamide for
4 h (IRDC, 1982a,b). No mortality was observed in rats given oral
doses of up to 5000 mg/kg body weight (Loeser, 1976). The dermal LD50
was >2000 mg/kg body weight in rabbits following application of this
substance under an occlusive dressing for 24 h (MB Research
Laboratories Inc., 1986). Few specific toxic effects were observed in
any single exposure study.
8.2 Irritation and sensitization
Although most studies were of uncertain quality and in many cases
would not comply with modern regulatory standards, results of several
skin and eye irritation studies indicate that azodicarbonamide should
not be regarded as a skin or eye irritant (Kimmerle, 1965; Conning,
1966; Mihail, 1977; MB Research Laboratories Inc., 1986). In a study
of respiratory irritation, in which guinea-pigs were exposed head only
to azodicarbonamide in plethysmography tubes, either no changes or
effects of doubtful significance were reported for various lung
function parameters, indicating that irritation was minimal at
concentrations up to 97 mg/m3 for 1 h (Shopp et al., 1987).
Owing to the poor quality of the only available skin
sensitization study (the concentration used for induction and
challenge -- a 1% solution in dimethyl formamide -- was very low; only
four guinea-pigs were used in the test group; and test sites were not
occluded), it was not possible to draw any conclusions regarding the
ability of azodicarbonamide to induce skin sensitization in animals
(Stevens, 1967). No evidence of pulmonary irritation or
asthmatic-type-reactions (no changes in specific airways conductance,
no evidence of histopathological effects on the upper or lower
respiratory tract, and no evidence of circulating antibodies) was
obtained in one study in which groups of 10 guinea-pigs were
repeatedly exposed by inhalation to unconjugated azodicarbonamide at
0, 51, or 200 mg/m3 for 6 h/day, 5 days/week, for 4 weeks (Gerlach et
al., 1989).
8.3 Short-term exposure
Azodicarbonamide is of relatively low toxicity to animals
repeatedly exposed by the inhalation or oral routes. In well-conducted
2-week inhalation studies, groups of 10 male and 10 female F344/N rats
or B6C3F1 mice were exposed to dry aerosols (median mass aerodynamic
diameter 2 µm) at 0, 2, 10, 50, 100, or 200 mg/m3 for 6 h/day, 5
days/week (Medinsky et al., 1990). Investigations included analyses of
methaemoglobin and blood cholinesterase and extensive macroscopic and
microscopic pathology; no changes of toxicological significance were
seen.
Groups of five male and five female mice received 0, 625, 1250,
5000, or 10 000 mg azodicarbonamide/kg body weight per day by oral
gavage in corn oil, 5 days/week for 2 weeks.1 Mortalities were
observed at 1250 mg/kg body weight per day and above (presumably
treatment related). Histopathologically, at 1250 mg/kg body weight per
day and above, pyelonephritis with casts was seen in renal tubuli, and
crystalline deposits were observed in renal tubuli and the urinary
bladder.
A similar study was conducted in rats; again, there was a
dose-related increase in mortality at 1250 mg/kg body weight per day
and above and a similar profile of renal lesions, although effects
were seen at 1250 mg/kg body weight per day and above in males and at
2500 mg/kg body weight per day and above in females.1
In one early and very briefly reported study, signs of toxicity,
the nature of which was not reported, were seen in male rats given 300
mg azodicarbonamide/kg body weight per day for 5 days but not in rats
given 200 mg/kg body weight per day (Kimmerle, 1965).
In another study designed to look for adverse effects in the
thyroid (but only investigating the uptake of iodine in the thyroid
gland and serum protein-bound iodine), no clear evidence of thyroid
toxicity was found in rats given low-iodine diets containing 1, 5, or
10% azodicarbonamide or 5 or 10% biurea for periods ranging between 1
and 4 weeks (Gafford et al., 1971).
There are no data relating to the effects of repeated dermal
exposures.
1 IRDC, unpublished data; cited in BG Chemie (1995).
8.4 Long-term exposure
8.4.1 Subchronic exposure
Groups of 10 male and 10 female F344/N rats or B6C3F1 mice were
exposed to dry aerosols (median mass aerodynamic diameter 2 µm) at 0,
50, 100, or 200 mg/m3 for 6 h/day, 5 days/week, for 13 weeks
(Medinsky et al., 1990). Investigations included analyses of various
enzyme activities in urine, haematology, blood cholinesterase, serum
triiodothyronine and thyroxine, vaginal cytology, sperm morphology,
levels of azodicarbonamide and biurea in lungs and kidneys (Mewhinney
et al., 1987), and extensive macroscopic and microscopic pathology; no
changes of toxicological significance were seen.
Groups of 10 male mice received 1, 78, 156, 312, 625, or 1250 mg
azodicarbonamide/kg body weight per day and groups of 10 female mice
received 0, 156, 312, 625, 1250, or 2500 mg azodicarbonamide/kg body
weight per day by oral gavage in corn oil, 5 days/week for 13 weeks.1
In contrast to the 2-week study (see section 8.3), there were no
mortalities and no histopathological abnormalities.
Groups of 10 male rats received 1, 100, 500, or 2500 mg
azodicarbonamide/kg body weight per day and groups of 10 female mice
received 0, 200, 1000, or 5000 mg azodicarbonamide/kg body weight per
day by oral gavage in corn oil, 5 days/week for 13 weeks.1 Mortality
was observed only at 2500 mg/kg body weight per day in males and at
5000 mg/kg body weight per day in females. Histopathologically,
pyelonephritis and crystalline deposits were observed in males at 2500
mg/kg body weight per day and in females at 5000 mg/kg body weight per
day only.
8.4.2 Chronic exposure and carcinogenicity
The effects of long-term exposure to azodicarbonamide have not
been well studied, and no conventional carcinogenicity studies are
available. The only data come from 1- and 2-year studies in which rats
and dogs received diets containing various amounts of biurea. In the
1-year study, rats and dogs ate diets containing 5 or 10% biurea (Oser
et al., 1965). One high-dose rat died, and body weight gain was
slightly depressed in high-dose males. No other signs of toxicity were
observed in rats at necropsy. Most dogs from both dose groups died.
Necropsy revealed massive, multiple renal calculi, bladder calculi,
and chronic pyelonephritis. However, the dogs that were selected for
this study were of uncertain and variable origin; hence, no useful
results could be obtained from them. The main constituent (comprising
80-100%) of the calculi was identified as biurea.
In the 2-year study, rats and dogs ate diets containing either
bread baked with untreated flour but supplemented with 750, 2370, or
7500 mg biurea/kg or bread baked with flour containing 100 mg
1 IRDC, unpublished data; cited in BG Chemie (1995).
azodicarbonamide/kg (Oser et al., 1965). Controls received diets
containing bread baked with untreated flour. Given that
azodicarbonamide is readily converted to biurea (Joiner et al., 1963),
it is likely that the animals receiving the bread baked with
azodicarbonamide-treated flour were actually exposed to biurea. As
with the previous investigations, the dogs that were selected for this
study were of uncertain and variable origin; hence, no useful results
could be obtained from them. For rats, no treatment-related deaths
occurred, and no adverse effects were observed that were considered to
be treatment related. Assuming food consumption of 20 g/day and a mean
body weight of 350 g, the dietary inclusion levels correspond to
approximately 45, 140, and 450 mg biurea/kg body weight per day.
8.5 Genotoxicity and related end-points
Azodicarbonamide is mutagenic in vitro, inducing base-pair
mutations in bacteria with and without metabolic activation (Pharmakon
Research International, 1984a; Mortelmans et al., 1986; Hachiya,
1987).1 In contrast, several standard in vitro assays in mammalian
cell systems have yielded negative results; gene mutation assays in
Chinese hamster ovary cells, using the hypoxanthine guanine
phosphoribosyl transferase locus, and in mouse lymphoma cells, using
the thymidine kinase locus, have been conducted, along with an in
vitro liver unscheduled DNA synthesis assay and a sister chromatid
exchange assay in Chinese hamster ovary cells (Pharmakon Research
International, 1984b,c).1,2 A positive result was obtained in a
chromosomal aberration assay in Chinese hamster ovary cells, but the
result was not reproducible.2 Negative results were obtained in a
sex-linked recessive lethal assay in Drosophila (Yoon et al., 1985).
Two in vivo bone marrow micronucleus assays in mice conducted by the
intraperitoneal route (0 or 150 mg azodicarbonamide/kg body weight)
were available, both giving negative results (Pharmakon Research
International, 1984d; Hachiya, 1987).
1 T. Cameron, unpublished Ames and mouse lymphoma test results
(1990) from the short-term program sponsored by the Division of
Cancer Aetiology, National Cancer Institute (cited in Chemical
Carcinogenesis Research Information System [CCRIS] database,
US National Cancer Institute).
2 NTP, unpublished data on chromosome aberration and sister
chromatid exchange assays in Chinese hamster ovary cells for
azodicarbonamide, submitted to the National Toxicology Program,
National Institutes of Health, US Department of Health and Human
Services, Research Triangle Park, NC.
8.6 Reproductive and developmental toxicity
The only study that has been conducted1 is a three-generation
study in which rats were given diets containing up to 7500 mg
biurea/kg (equivalent to approximately 450 mg/kg body weight per day)
(Oser & Oser, 1963; Oser et al., 1965). For each generation, rats were
mated twice, and the first litter was sacrificed at weaning. From the
second litter, 10 males and 10 females were chosen at random to form
the parents for the next generation. The study finished with the
weaning of the F3 generation. For each generation, fertility index
(percentage of matings resulting in pregnancy), gestation index
(number of pregnancies resulting in live litters), viability index
(numbers of pups surviving 4 or more days), and lactation index
(numbers of pups alive at 4 days surviving to weaning) were
determined. No reproductive effects were observed.
The only other information available is that no organ weight or
histological changes were observed in the reproductive organs of rats
and mice repeatedly exposed for 13 weeks to up to 200 mg
azodicarbonamide/m3 by inhalation (see section 8.4.1, Medinsky et
al., 1990).
The developmental toxicity of azodicarbonamide has not been
studied.
8.7 Immunological and neurological effects
No studies are available that specifically investigate these
endpoints, and there is no relevant information from toxicity studies
in animals.
1 The authors of this CICAD have been informed that a reproductive
toxicity screening test is being conducted according to OECD test
method 421.
9. EFFECTS ON HUMANS
The effects of exposure to azodicarbonamide in humans have not
been fully evaluated. There are no data detailing the effects of
single exposures by any route. The most frequently reported effects of
repeated exposure to azodicarbonamide are respiratory symptoms as well
as, to a lesser extent, skin sensitization reactions. There are no
reports relating to the potential for azodicarbonamide to produce
other systemic adverse effects. The potential genotoxic, carcinogenic,
and reproductive effects of azodicarbonamide in exposed humans have
not been studied.
9.1 Case reports
A number of reports have been published of individual
azodicarbonamide workers alleging asthma induced by exposure to
azodicarbonamide. The strongest evidence comes from a study of two
individuals (one atopic and one non-atopic) who worked at the same
plastics factory for about 4 years (Malo et al., 1985; Pineau et al.,
1985). Both were intermittently exposed (1-2 weeks' duration, 3-4
times per year) to azodicarbonamide at work. A few months after their
first encounter with azodicarbonamide, both developed symptoms
described as "eye/nose irritation" at work, followed a few hours later
by nocturnal asthmatic symptoms. After a 1-month period free from
exposure, both subjects underwent lung provocation studies. Baseline
values for forced expiratory volume in 1 s (FEV1), forced vital
capacity (FVC), and the concentration of histamine required to produce
a 20% drop in FEV1 (PC20H) were obtained by spirometry. Both
subjects performed a control challenge using lactose and then a 50:50
mixture of lactose and azodicarbonamide for 15 s on the next day. On
both days, lung function was monitored to follow the time course of
any response. It was reported that the trial was not carried out
blind.
No effects on lung function were observed following challenge
with lactose alone. After the azodicarbonamide challenge, however, the
atopic individual developed a late respiratory response starting 3 h
after challenge and reaching a maximum 24% drop in FEV1 6 h after
challenge. A drop in PC20H was also reported, demonstrating increased
airway hyperreactivity, and this parameter did not return to the
baseline value until 6 weeks after challenge. The non-atopic
individual showed a dual response to azodicarbonamide. Peak reductions
in FEV1 of greater than 20% were recorded 30 min and 5-6 h after
exposure. No significant reduction in PC20H was reported for the
second individual. A control atopic subject with underlying asthma who
worked in the same industry but did not experience work-related
respiratory effects was also tested. His baseline PC20H was similar
to that of the atopic subject, but no change in lung function was
observed following a 15-min exposure to azodicarbonamide under similar
conditions (as this subject had less reactive airways, a much longer
exposure duration was utilized). Owing to the insolubility of
azodicarbonamide, skin prick tests were not performed.
Six other cases have been reported in the literature, but in each
case the evidence that azodicarbonamide was the cause of the
respiratory symptoms is less strong. In some cases, there had been
previous exposure in industries associated with potential exposure to
other asthmagenic substances; for others, the bronchial challenge test
was either poorly conducted or not conducted at all (Valentino &
Comai, 1985; Alt & Diller, 1988; Normand et al., 1989).
Three case reports on skin sensitization have been published. In
the most recently reported investigation, a male textile worker
exposed to azodicarbonamide in foam ear-plugs was patch tested to
discover the cause of a recurrent dermatitis of the ear (Nava et al.,
1983; Bonsall, 1984; Yates & Dixon, 1988). No response was elicited
with a number of standard (International Contact Dermatitis Research
Group standard series) allergens. However, the individual gave a
strong positive reaction to the ear-plugs at 48 and 96 h and also to
azodicarbonamide (a component of the ear-plugs) at a concentration of
1 and 5% in petrolatum but not at 0.1% in petrolatum. Ten control
subjects patch tested with 1 and 5% azodicarbonamide in petrolatum did
not respond, and the individual reported no further symptoms upon
discarding the ear-plugs.
9.2 Epidemiological studies
Workplace health surveys have also been carried out where
azodicarbonamide was either manufactured or used to investigate the
presence of respiratory symptoms in azodicarbonamide workers.
A prevalence study of occupational asthma was carried out among a
group of 151 workers at a factory manufacturing azodicarbonamide
(Slovak, 1981). Diagnosis of asthma was made on the basis of an
administered questionnaire and a detailed occupational history taken
by the author. The population was divided into three groups: those
classified as potentially sensitized, on the basis of questionnaire
results; those with daily exposure but without symptoms; and those
with no exposure to azodicarbonamide or any other known sensitizer. On
one day, pre- and post-shift spirometry was performed, and FEV1, FVC,
and the FEV1/FVC ratio were determined. Skin prick tests were also
attempted using both common allergens to determine atopic status and
azodicarbonamide at concentrations of 0.1, 1, and 5% in dimethyl
sulfoxide. Concurrent personal sampling measurements were made to
determine the levels of airborne azodicarbonamide to which individuals
were exposed.
Personal sampling indicated that, at the time of the
investigation, airborne concentrations of azodicarbonamide ranged
between 2 and 5 mg/m3, as 8-h time-weighted averages. From the
questionnaires and occupational histories, 28 individuals (18.5%) were
diagnosed as having asthma apparently related to azodicarbonamide
exposure. Twelve further cases of occupational asthma were identified
from company records of past employees. Skin prick tests with
azodicarbonamide could not be adequately performed owing to the
insolubility of the substance.
Of the 28 current workers classified as sensitized, over half
developed symptoms within 3 months of first exposure and 21/28 (75%)
within 1 year. Symptoms and signs included shortness of breath, chest
tightness, wheezing, cough, rhinitis, conjunctivitis, and rash.
Reactions were of an immediate type for 6/28 (21%) individuals, late
onset for 16/28 (57%), and dual onset for 6/28 workers. Of those
showing a dual response, all but one had initially shown a late onset
pattern. A total of 13/28 (46%) workers reported worsening of symptoms
with continuing exposure to azodicarbonamide and a shortening of the
time between returning to work and reappearance of symptoms. Eight out
of 13 workers exposed to azodicarbonamide for more than 3 months after
development of symptoms also developed sensitivity to previously
well-tolerated irritants (e.g., sulfur dioxide and tobacco smoke),
which persisted for over a month after removal from exposure to
azodicarbonamide. In five individuals, this airway hyperreactivity
persisted for over 3 years. There were no changes in FEV1 or FVC over
the work shift in any group. In view of the latency in development of
effects, late or dual onset of symptoms in 12/28 (43%) symptomatic
workers, increase in sensitivity with repeated exposure, and the
persisting lung hyperreactivity in workers with prolonged exposure
after developing symptoms, it seems likely that these individuals had
become sensitized to azodicarbonamide.
Ahrenholz & Anderson (1985) and Whitehead et al. (1987) conducted
detailed investigations of the workforce at a plastics factory
employing about 325 workers. Lung function tests and interviews to
gather information on occupational history, smoking habits, past
illnesses, and respiratory, nasal, eye, and skin irritation, including
the time course of any symptoms, were carried out with a large
percentage of the workforce. There were no clear differences in the
results of lung function studies between those exposed to
azodicarbonamide and non-exposed individuals. However, responses to
the questionnaire revealed a significant association between symptoms
of irritation, cough, wheezing, shortness of breath, and headache and
present or previous employment as an injection mould operator. There
was also a slight but not statistically significant increase in the
reporting of skin rash among those with current or previous work in
the injection moulding department. The prevalence of all the above
symptoms was reduced among those whose employment in this department
was limited to the period before azodicarbonamide was introduced or
after a change in the process significantly reduced the use of
azodicarbonamide at the plant.
Personal sampling showed that concentrations of airborne
azodicarbonamide ranged from below the limit of detection (0.001
mg/m3) to 0.32 mg/m3 (median 0.006 mg/m3; geometric mean 0.004
mg/m3) averaged over the full shift. The highest concentration of
azodicarbonamide recorded (for an injection mould operator) was 0.01
mg/m3. Toluene, styrene, phenols, and triphenyl phosphate were also
detected at concentrations at or below the odour threshold for each
substance.
Other personal sampling data for a group of 17 individuals
revealed levels of azodicarbonamide ranging from traces to 0.8 mg/m3
(median 0.03 mg/m3; geometric mean 0.02 mg/m3) averaged over the
full shift. The second highest value recorded was 0.4 mg/m3, and the
next highest, 0.06 mg/m3. A moderate although statistically
significant reduction in FEV1 (mean reduction of 64 ml) and FVC (mean
reduction of 77 ml) occurred following shifts in which workers were
exposed to azodicarbonamide. Coughing at work, wheeze, and chest
tightness were also reported, and symptoms were apparently worse
during the week than on Sunday.
A detailed investigation of the workforce at a plant making floor
coverings was conducted after nosebleeds, mucous membrane irritation,
and skin rashes were reported in workers handling azodicarbonamide
(Ahrenholz et al., 1985). Two surveys were carried out. The initial
survey revealed, in decreasing order of prevalence, symptoms of eye
irritation, nose irritation, cough, nocturnal cough, shortness of
breath, wheeze, and chest tightness. The more extensive follow-up
survey was conducted 6 weeks later. Pre- and post-shift auscultation,
lung function tests, and respiratory symptoms (recorded by
questionnaire) were recorded. Blood samples were also taken for
immunological investigations.
Responses to the questionnaire revealed 15/30 regularly exposed
workers experiencing occupationally related lower respiratory tract
symptoms (cough, wheeze, and shortness of breath) compared with 1/16
never-exposed workers. No significant differences in pre- and
post-shift FEV1 and FVC measurements were found. For those workers
apparently not exposed to azodicarbonamide or exposed indirectly
(working in the vicinity but not directly handling azodicarbonamide),
levels (8-h time-weighted average) ranged from <0.001 to 0.1 mg/m3.
However, during weighing and charging operations, peaks of between
0.15 and 12 mg/m3 (median 2.7 mg/m3) were measured for individuals
directly involved.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Azodicarbonamide has been tested under OECD Guideline protocols
in one species of fish and in the water flea ( Daphnia magna);
results are in an unpublished industry report, which has not been
peer-reviewed (see Table 1). A second study, not conducted to a
protocol or under Good Laboratory Practices, showed no effect of an
azodicarbonamide solution analysed at 8 mg/litre on the zebra fish
( Brachydanio rerio).1 There was no effect on oxygen consumption of
sewage sludge organisms exposed over 3 h to azodicarbonamide at
>10 000 mg/litre (this is substantially greater than the solubility
of the compound, and no information is available on how this
concentration was achieved).2 Overall, it is not possible to draw
firm conclusions from these studies.
1 IUCLID (European Union database), version dated 7 February 1996.
2 Bayer, unpublished value (1988) presented in IUCLID (European
Union database), version dated 7 February 1996; no details
available.
Table 1: Acute studies on toxicity to aquatic organisms.
Organism Protocol End-point Concentration Reference
(mg/litre)
Fathead minnow OECD 203 96-h NOEC >50 Uniroyal (1992)
(Pimephales promelas)
Water flea OECD 202 48-h NOEC 4.8 (measured) Uniroyal (1992)
(Daphnia magna) ~16 (nominal)
48-h EC50 11 (measured)
immobilization 29 (nominal)
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Some of the available toxicological studies have been conducted
using biurea rather than azodicarbonamide. However, azodicarbonamide
is readily converted to biurea in vivo. Hence, similar toxicological
properties would be expected.
Azodicarbonamide is of low acute toxicity by all routes, and,
although the animal studies are of uncertain quality, solid
azodicarbonamide would not be regarded as a skin or eye irritant. With
respect to respiratory tract irritation, no changes of toxicological
significance were seen in guinea-pigs exposed to azodicarbonamide
aerosol at concentrations up to 97 mg/m3 for 1 h.
No conclusions could be drawn regarding skin sensitization
potential from the available, poor-quality animal studies. Although
there are currently no validated animal studies investigating
asthmagenic potential, there was no evidence of pulmonary irritation
or asthmatic-type reactions in guinea-pigs exposed to up to 200 mg
azodicarbonamide aerosol/m3 for 6 h/day, 5 days/week, for 4 weeks.
Similarly, there were no changes of toxicological significance
seen among rats or mice exposed by inhalation to up to 200 mg
azodicarbonamide aerosol/m3 for 6 h/day, 5 days/week, for up to 13
weeks.
For repeated-dose studies using the oral route, data were
inconsistent. In 2-year studies in which rats received up to 450 mg
biurea/kg body weight per day, there were no adverse effects seen.
Unpublished information suggests that no adverse effects were seen in
male mice exposed to up to 1250 mg azodicarbonamide/kg body weight per
day and in female mice exposed to up to 2500 mg/kg body weight per day
for 13 weeks. However, shorter-term studies (2 weeks, also
unpublished) indicated histological lesions in the kidneys of rats and
mice of both sexes at 1250 mg/kg body weight per day or more. A
13-week study in rats indicated kidney lesions in males at 2500 mg/kg
body weight per day, with no adverse effects observed at the next
lowest exposure level, 500 mg/kg body weight per day. For female rats,
kidney lesions were observed at 5000 mg/kg body weight per day, and no
adverse effects were observed at 1000 mg/kg body weight per day. There
were no data in relation to repeated dermal exposure.
Azodicarbonamide has been identified as a mutagen in bacterial
systems, but it was not mutagenic in mammalian cell in vitro test
systems or in two mammalian assays in vivo using bone marrow. It is
therefore unlikely that the mutagenic properties displayed by
azodicarbonamide in bacterial systems will be expressed in vivo.
However, it is considered that a confirmatory in vivo study in a
second tissue is desirable.
There are no adequate data available relating to carcinogenic,
reproductive, or developmental effects; hence, it is not possible to
evaluate the risk to human health for these end-points.
Several bronchial challenge studies have been reported, but only
one provides reasonable evidence that the work-related asthmatic
symptoms were due specifically to azodicarbonamide. This report is
considered to show an asthmatic response and not an irritant response
to azodicarbonamide on challenge. Animal studies suggest that airborne
concentrations of up to 200 mg/m3 can be tolerated with little or no
pulmonary irritation, and it is unlikely that the levels used in the
bronchial challenge tests approached those used in animal studies. The
delay in response to azodicarbonamide challenge, the magnitude of
reduction in FEV1 accompanied by an increase in airway
hyperreactivity in one individual, and the fact that a control
individual with mildly hyperreactive airways did not respond to a much
more prolonged exposure under similar challenge conditions provide
further evidence for asthmagenicity. Further evidence of a link
between azodicarbonamide and respiratory problems is provided by the
results of workplace health evaluations. Although criticisms can be
levelled at individual studies, weight of evidence suggests that
azodicarbonamide can induce asthma in a significant proportion of
exposed people.
There are some case reports of individuals with skin reactions to
topically applied azodicarbonamide. For some of these, results are
questionable. However, in workplace health surveys, the incidence of
skin rash was found to be greater among workers regularly exposed to
azodicarbonamide. Although no firm conclusions could be drawn from the
poorly reported animal study, clear evidence of skin sensitization to
azodicarbonamide in one individual and supporting evidence of skin
problems from workplace health surveys lead to the conclusion that
azodicarbonamide should be considered as a human skin sensitizer.
In conclusion, the key toxic effect of azodicarbonamide in humans
is asthmagenicity. Evidence of this effect has been found from
bronchial challenge studies and workplace health evaluations. From the
information available, azodicarbonamide is considered to have a low
potential for irritancy; thus, it is considered that the respiratory
symptoms observed in these studies are most likely due to an
asthmatic-type response rather than respiratory tract irritancy. There
is no clear information on the levels that may have induced or
provoked the state of asthma.
There is also information to indicate that azodicarbonamide can
cause skin sensitization in humans.
11.1.2 Criteria for setting guidance values for azodicarbonamide
The main cause for concern relates to the risk of developing
occupational asthma. There is no information available relating to
dose-response relationships or levels associated with the induction of
a hypersensitive state or provocation of an asthmatic response. Hence,
it is not possible to reliably quantify the risk of developing
occupational asthma.
11.1.3 Sample risk characterization
Using data obtained from a factory in the United Kingdom and
published exposure data as an example (section 6.2), levels of
airborne azodicarbonamide measured over periods of <70 min to 4 h of
up to 12 mg/m3 have been observed. Short-term peak exposures could be
higher than this level.
In the United Kingdom occupational setting, it is recommended
that a maximum exposure limit (MEL) be assigned to substances for
which it has not been possible to identify a level of exposure that is
without adverse effects on health. This is a non-health-based
standard, and, in determining the most appropriate level for a MEL,
consideration is taken of the level of control that it is reasonably
practicable for industry to achieve. The MEL of 1 mg/m3
(8-h time-weighted average) was based on a level of control that was
deemed by tripartite agreement to be reasonably practicable under
workplace conditions within the United Kingdom. There is also a
continuing remit for industry to keep on reducing exposure levels as
advances in technology make this possible. For substances that are
asthmagens, it is also advisable to have a short-term exposure limit
(STEL) to restrict peak exposures, as they may have a role in the
induction and triggering of asthmatic phenomena. In the absence of any
specific data that might advise adequately on the numerical value of
the STEL, 3 mg/m3 (15-min reference period) has been established.
As azodicarbonamide is a skin sensitizer, where skin contact can
occur, there may be a risk of developing allergic dermatitis if
suitable personal protective equipment is not used.
There is evidence to suggest that azodicarbonamide has been added
to consumer products such as bread and beer. The limited toxicology
database and lack of exposure data make it difficult to adequately
assess the risk to humans potentially exposed; hence, there is a need
for further information.
11.2 Evaluation of environmental effects
Lack of information on release to the environment precludes a
quantitative risk assessment.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations by international bodies were not identified.
Information on international hazard classification and labelling is
included in the International Chemical Safety Card reproduced in this
document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0380) reproduced in this
document.
13.1 Human health hazards
Azodicarbonamide is of low acute toxicity, but repeated or
prolonged contact may cause asthma and skin sensitization.
13.2 Health surveillance advice
Physicians involved in worker health surveillance programmes
should be aware of the potential of azodicarbonamide as a human
asthmagen.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
AZODICARBONAMIDE ICSC:0380
October 1997
CAS# 123-77-3 Diazenedicarboxamide
RTECS # LQ1040000 1,1'-Azobisformamide
UN # 3242 C2H2N4O2/NH2CON=NCONH2
EC3 611-028-00-3
Molecular mass: 116.1
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/
EXPOSURE SYMPTOMS FIRE FIGHTING
FIRE Flammable. Gives off NO open flames, NO sparks, Foam, powder.
irritating or toxic fumes and NO smoking.
(or gases) in a fire.
EXPLOSION
EXPOSURE PREVENT DISPERSION OF
DUST! STRICT HYGIENE!
Inhalation Cough. Headache. Shortness of Local exhaust or breathing Fresh air, rest.
breath. Sore throat. Wheezing. protection. Refer for medical
Fatigue. Cramps. attention.
Skin Redness. Protective clothing. Remove contaminated clothes.
Rinse and then wash skin with
water and soap.
Eyes Redness. Pain. Safety goggles, or eye First rinse with plenty of
protection in combination water for several minutes
with breathing protection. (remove contact lenses if easily
possible), then take to a doctor.
Ingestion Do not eat, drink, or smoke Rinse mouth. Give
during work. plenty of water to drink.
Rest.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into sealable containers; EU Classification
if appropriate, moisten first to prevent dusting. Symbol: Xn
Carefully collect remainder, then remove to safe R: 42-44
place (extra personal protection; P2 filter S: (2-)22-24-37
respirator for harmful particles). UN Classification
UN Hazard Class: 4.1
UN Pack Group: II
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-41G19
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
ORANGE RED CRYSTALS OR YELLOW POWDER. The substance can be absorbed into the body by
inhalation of its aerosol.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on heating or on burning Evaporation at 20°C is negligible;
producing toxic fumes (nitrogen oxides). a harmful concentration of airborne particles
can, however, be reached quickly.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV not established. The substance irritates the eyes and the respiratory
tract. Inhalation of dust may cause asthmatic
reactions (see Notes).
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
Repeated or prolonged contact with skin may cause
dermatitis. Repeated or prolonged contact may cause
skin sensitazation. Repeated or prolonged inhalation
exposure may cause asthma.
PHYSICAL PROPERTIES
Melting point (decomposed): 225°C
Relative density (water = 1): 1.65
Solubility in water: none
ENVIRONMENTAL DATA
NOTES
The symptoms of asthma often do not become manifest until a few hours have passed and they are aggravated
by physical effort. Rest and medical observation are therefore essential. Anyone who has shown symptoms
of asthma due to this substance should avoid all further contact with this substance. Genitron AC,
Kempore 125, Porofor LK 1074 and Unifoam AZ are trade names.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the CEC or the IPCS is
responsible for the use which might be made of this information.
REFERENCES
Ahrenholz S, Anderson K (1985) Health hazard evaluation report of
Leon Plastics, Grand Rapids, MI. Cincinnati, OH, US Department of
Health and Human Services (Report No. HETA-83-156-1622; PB89-143200).
Ahrenholz S, Neumeister C (1987) Development of a sampling and
analytical method for azodicarbonamide. American Industrial Hygiene
Association journal, 48(5):442-446.
Ahrenholz S, Morawetz J, Liss G (1985) Health hazard evaluation
report of Armstrong World Plastics, Lancaster, PA. Cincinnati, OH,
US Department of Health and Human Services (Report No.
HETA-83-451-1547; PB86-105582).
Alt E, Diller W (1988) "Hyperreactivity cough" in occupational
medicine. Zentralblatt fuer Arbeitsmedizin, Arbeitsschutz
und Prophylaxe Ergonomie, 38 (Suppl. 2):22-26.
Ball E, Saleem A, Ogunbiyi A, Wiley K, Groves J (1996)
Azodicarbonamide; Criteria document for an occupational exposure
limit. Sudbury, Suffolk, Health and Safety Executive, HSE Books
(ISBN 0 7176 10926).
Bechtold W, Medinsky M, Cheng Y, Hobbs C (1989) Azodicarbonamide:
methods for the analysis in tissues of rats and inhalation
disposition. Xenobiotics, 19:1003-1012.
BG Chemie (1995) Azodicarbonamide. In: GDCh-Advisory Committee on
Existing Chemicals of Environmental Relevance, eds. Toxicological
evaluations, potential health hazards of existing chemicals. Vol.
8. Stuttgart, S. Hirzel (ISBN 0 387 58287 8).
Bonsall J (1984) Allergic contact dermatitis to azodicarbonamide.
Contact dermatitis, 10:42.
Canas B, Diachenko G, Nyman P (1997) Ethyl carbamate levels resulting
from azodicarbonamide use in bread. Food additives and contaminants,
14(1):89-94.
Conning D (1966) Azodicarbonamide (Genitron AC4). Toxicological
report. Unpublished report submitted to Imperial Chemical Industries
Limited, Macclesfield (Report No. TR/522).
Dennis M, Massey R, Ginn R, Parker I, Crews C, Zimmerli B, Zoller O,
Rhyn P, Osborne B (1997a) The effect of azodicarbonamide
concentrations on ethyl carbamate concentrations in bread and toast.
Food additives and contaminants, 14(1):95-100.
Dennis M, Massey R, Ginn R, Willetts P, Crews C, Parker I (1997b) The
contribution of azodicarbonamide to ethyl carbamate formation in bread
and beer. Food additives and contaminants, 14(1):101-108.
Frankenberger W, Tabatabai M (1982) Transformations of amide nitrogen
in soils. Soil Science Society of America journal, 46:280-284.
Gafford F, Sharry P, Pittman J Jr (1971) Effect of azodicarbonamide
(1,1-azobisformamide) on thyroid function. Journal of clinical
endocrinology and metabolism, 32:659-662.
Gerlach R, Medinsky M, Hobbs C, Bice D, Bechtold W, Cheng Y, Gillett
N, Birnbaum L, Mauderly J (1989) Effect of four week repeated
inhalation exposure to unconjugated azodicarbonamide on specific and
non-specific airway sensitivity of the guinea pig. Journal of
applied toxicology, 9(3):145-153.
Hachiya N (1987) Evaluation of chemical genotoxicity by a series of
short term tests. Akita Igaku, 14:269-292.
HSDB (1996) Hazardous substances data bank. Bethesda, MD, US
National Library of Medicine. CHEM-BANK, SilverPlatter compact disk.
IPCS (1993) International Chemical Safety Card
-- Diazenedicarboxamide. Geneva, World Health Organization,
International Programme on Chemical Safety (ICSC 0380).
IRDC (1982a) Acute inhalation toxicity test with azodicarbonamide in
rats. Unpublished report submitted by the International Research and
Development Corporation to the National Toxicology Program, National
Institutes of Health, US Department of Health and Human Services,
Research Triangle Park, NC (Report No. 5703-115).
IRDC (1982b) Acute inhalation toxicity test with azodicarbonamide in
mice. Unpublished report submitted by the International Research and
Development Corporation to the National Toxicology Program, National
Institutes of Health, US Department of Health and Human Services,
Research Triangle Park, NC (Report No. 5703-111).
Joiner R, Vidal F, Marks H (1963) A new powdered agent for flour
maturing. Cereal chemistry, 40:539-553.
Kimmerle G (1965) Toxicological studies on Porofor ADC, Urazol and
plastic foam extracts. Unpublished report, Institute of Toxicology,
Bayer AG, Wuppertal-Elberfeld.
Loeser E (1976) Acute oral toxicity: Porofor ADC/K. Unpublished
report, Institute of Toxicology, Bayer AG, Wuppertal-Elberfeld.
Malo J, Pineau L, Cartier A (1985) Occupational asthma due to
azobisformamide. Clinical allergy, 15:261-264.
MB Research Laboratories Inc. (1986) Acute dermal toxicity in
rabbits. MB 86-83 93 B. Celogen AZ 120. Unpublished report submitted
to Uniroyal Chemical Company Inc., Middlebury, CN.
Medinsky M, Bechtold W, Birnbaurn L, Bond J, Burt D, Cheng Y, Gillett
N, Gulati D, Hobbs C, Pickrell J (1990) Effect of inhaled
azodicarbonamide on F344/N rats and B6C3F1 mice with 2-week and
13-week inhalation exposures. Fundamental and applied toxicology,
15:308-319.
Mewhinney J, Ayres P, Bechtold W, Dutcher J, Cheng Y, Bond J, Medinsky
M, Henderson R, Birnbaum L (1987) The fate of inhaled azodicarbonamide
in rats. Fundamental and applied toxicology, 8:372-381.
Mihail T (1977) Studies of skin and mucous membrane tolerance:
Porofor ADC/K. Unpublished report, Institute of Toxicology, Bayer
AG, Wuppertal-Elberfeld.
Mortelmans K, Haworth S, Lawlor T, Speck W, Trainer B, Zeiger E (1986)
Salmonella mutagenicity tests: 11. Results from the testing of 270
chemicals. Environmental mutagenesis, 8 (Suppl. 7):1-119.
Nava C, Beretta F, Elena A, Ghizzi A, Pattarin R, Villa L (1983)
Allergic dermatitis due to improvers and other flour additives.
Medicina del Lavoro, 74(5):376-379.
Normand J, Grange F, Hernandez C, Ganay A, Davezies P, Bergeret A,
Prost G (1989) Occupational asthma after exposure to azodicarbonamide:
report of four cases. British journal of industrial medicine,
46:60-62.
Oser B, Oser M (1963) Nutritional studies on rats on diets containing
high levels of partial ester emulsifiers. 11. Reproduction and
lactation. Journal of nutrition, 60:489-505.
Oser B, Oser M, Morgareidge K, Stemberg S (1965) Studies of the safety
of azodicarbonamide as a flour maturing agent. Toxicology and
applied pharmacology, 7:445-472.
Pharmakon Research International (1984a) Ames
Salmonella/microsome plate test (EPA/OECD). PH 301-UN-004-84.
Celogen AZ-130. Unpublished report submitted to Uniroyal Chemical
Company Inc., Middlebury, CN.
Pharmakon Research International (1984b) CHO/HGPRT mammalian cell
forward gene mutation assay. PH 314-UN-003-84. Celogen AZ.
Unpublished report submitted to Uniroyal Chemical Company Inc.,
Middlebury, CN.
Pharmakon Research International (1984c) Rat hepatocyte primary
culture/DNA repair test. PH 311-UN-003-84. Celogen AZ-130.
Unpublished report submitted to Uniroyal Chemical Company Inc.,
Middlebury, CN.
Pharmakon Research International (1984d) Micronucleus test (MNT)
OECD. PH-309A-UN-003-84. Celogen AZ-130. Unpublished report
submitted to Uniroyal Chemical Company Inc., Middlebury, CN.
Pineau L, Cartier A, Malo J-L (1985) Occupational asthma due to
azobisformamide. Journal of allergy and clinical immunology, 75:170.
Shopp G, Cheng Y, Gillet N, Bechtold W, Medinsky M, Hobbs C, Birnbaum
L, Mauderly J (1987) Acute inhalation exposure of azodicarbonamide in
the guinea pig. American Industrial Hygiene Association journal,
48(2):127-132.
Slovak A (1981) Occupational asthma caused by a plastics blowing
agent, azodicarbonamide. Thorax, 36(12):906-909.
Stevens M (1967) Use of the albino guinea pig to detect the skin
sensitising ability of chemicals. British journal of industrial
medicine, 24:189-202.
Uniroyal (1992) Unpublished report from the Uniroyal Chemical Company
Inc., Middlebury, CN.
Vainiotalo S, Pfaffli P (1988) Measurement of azodicarbonamide in
workroom air in the plastics processing industry. Annals of
occupational hygiene, 43:203-208.
Valentino M, Comai M (1985) Occupational asthma from azodicarbonamide:
Case report. Giornale Italiano di Medicina del Lavoro,
7(2-3):97-99.
Whitehead L, Robins T, Fine L, Hansen D (1987) Respiratory symptoms
associated with the use of azodicarbonamide foaming agent in a
plastics injection moulding facility. American journal of industrial
medicine, 11:83-92.
Yates V, Dixon J (1988) Contact dermatitis from azodicarbonamide in
earplugs. Contact dermatitis, 19(2):155-156.
Yoon J, Mason J, Valencia R, Woodruff R, Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. IV. Results of 45 coded compounds
tested for the National Toxicology Program. Environmental
mutagenesis, 7:349-367.
APPENDIX 1 -- SOURCE DOCUMENT
Ball et al. (1996): Azodicarbonamide; Criteria document for an
occupational exposure limit
The authors' first draft was initially reviewed internally by a
group of approximately 10 United Kingdom Health and Safety Executive
experts (mainly toxicologists, but also scientists involved in other
relevant disciplines, such as epidemiology and occupational hygiene).
The toxicology section of the amended draft was then reviewed by
toxicologists from the United Kingdom Department of Health.
Subsequently, the entire criteria document was reviewed by a
tripartite advisory committee to the United Kingdom Health and Safety
Commission, the Working Group for the Assessment of Toxic Chemicals
(WATCH). This committee is composed of experts in toxicology and
occupational health and hygiene from industry, trade unions, and
academia.
The members of the WATCH committee at the time of the peer review
were Mr S.R. Bailey, Independent Consultant; Professor J. Bridges,
University of Surrey; Dr H. Cross, Trade Unions Congress; Dr A
Fletcher, Trade Unions Congress; Dr I.G. Guest, Chemical Industries
Association; Dr A. Hay, Trade Unions Congress; Dr J. Leeser, Chemical
Industries Association; Dr L. Levy, Institute of Occupational Hygiene,
Birmingham; Mr A. Moses, Chemical Industries Association; Dr R. Owen,
Trade Unions Congress; and Dr M. Sharratt, University of Surrey.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on azodicarbonamide was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
József Fodor National Center of Public Health, Budapest, Hungary
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Institute of Public Health, Prague, Czech Republic
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands
United States Department of Health and Human Services (National
Institute for Occupational Safety and Health, Cincinnati, USA;
National Institute of Environmental Health Sciences, Research
Triangle Park, USA)
United States Environmental Protection Agency (Drinking Water
Program, Denver, USA)
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Tokyo, Japan, 30 June - 2 July 1998
Members
Dr R. Benson, Drinking Water Program, United States Environmental
Protection Agency, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Mr R. Cary, Health Directorate, Health and Safety Executive,
Merseyside, United Kingdom
Dr C. DeRosa, Agency for Toxic Substances and Disease Registry, Center
for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire, United
Kingdom
Dr H. Gibb, National Center for Environmental Assessment, United
States Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada ( Chairperson)
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan ( Vice-Chairperson)
Professor S.A. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia ( Rapporteur)
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute of
Occupational Medicine, Beijing, People's Republic of China
Observers
Professor F.M.C. Carpanini,1 Secretary-General, ECETOC (European
Centre for Ecotoxicology and Toxicology of Chemicals), Brussels,
Belgium
1 Invited but unable to attend.
Dr M. Ema, Division of Biological Evaluation, National Institute of
Health Sciences, Osakai, Japan
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Mr T. Jacob,1 Dupont, Washington, DC, USA
Dr H. Koeter, Organisation for Economic Co-operation and Development,
Paris, France
Mr H. Kondo, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Ms J. Matsui, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Mr R. Montaigne,1 European Chemical Industry Council (CEFIC),
Brussels, Belgium
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr H. Nishimura, Environmental Health Science Laboratory, National
Institute of Health Sciences, Osaka, Japan
Ms C. Ohtake, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr T. Suzuki, Division of Food, National Institute of Health Sciences,
Tokyo, Japan
Dr K. Takeda, Mitsubishikasei Institute of Toxicological and
Environmental Sciences, Yokohama, Japan
Dr K. Tasaka, Department of Chemistry, International Christian
University, Tokyo, Japan
Dr H. Yamada, Environment Conservation Division, National Research
Institute of Fisheries Science, Kanagawa, Japan
Dr M. Yamamoto, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
1 Invited but unable to attend.
Dr M. Yasuno, School of Environmental Science, The University of Shiga
Prefecture, Hikone, Japan
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif à l'azodicarbonamide a été préparé à partir
d'une étude du Health and Safety Executive du Royaume-Uni sur les
risques pour la santé humaine (risques professionnels pour
l'essentiel) (Ball et al., 1996). De ce fait, bien qu'il comporte un
volet sur l'évaluation des données écologiques disponibles, il est
principalement centré sur les risques pour la santé humaine sur les
lieux de travail et tout particulièrement sur les voies d'exposition
professionnelle à prendre en considération. L'analyse des données sur
lesquelles repose l'étude a été arrêtée à juin 1994. Un dépouillement
de la littérature a été ensuite effectué jusqu'à juillet 1997, à la
recherche de données qui auraient pu être publiées depuis la fin de
l'étude. Le document original ne prend pas en considérations les
problèmes d'ordre écologique et comme le dépouillement de la
littérature n'a pas permis de trouver trace de travaux qui leur soit
consacrés, on n'a pas cherché à procéder à une évaluation des risques
pour l'environnement. On trouvera à l'appendice 1 des indications sur
les sources documentaires utilisées et sur leur mode de dépouillement.
Les renseignements concernant l'examen du CICAD par des pairs font
l'objet de l'appendice 2. Ce CICAD a été approuvé en tant
qu'évaluation internationale lors d'une réunion du Comité d'évaluation
finale qui s'est tenue à Tokyo (Japon) du 30 juin au 2 juillet 1998.
La liste des participants à cette réunion figure à l'appendice 3. La
fiche d'information internationale sur la sécurité chimique (ICSC No
0380) établie par le Programme international sur la Sécurité chimique
(IPCS, 1993) est également reproduite dans ce document.
Les données toxicocinétiques sur l'azodicarbonamide (No CAS
123-77-3) sont limitées, mais ce composé se révèle être bien absorbé
chez les rongeurs après inhalation ou ingestion. Une fraction notable
de l'azocarbonamide traverse cependant les voies digestives sans être
résorbé et se retrouve dans les matières fécales. L'azodicarbonamide
est facilement transformé en biurée ou dicarbamylhydrazine, le seul
métabolite qui ait été identifié et il est probable que l'exposition
par la voie générale concerne ce dernier dérivé plutôt que la molécule
initiale. Après absorption, l'élimination de l'azocarbonamide ou de la
biurée est rapide et s'effectue principalement par la voie urinaire,
la biurée étant très peu retenue dans l'organisme.
L'azocarbonamide présente une faible toxicité aiguë et il ne
provoque pas d'irritation cutanée, oculaire ou respiratoire chez les
animaux d'expérience. Des résultats négatifs ont été obtenu lors d'une
étude de la sensibilisation cutanée, d'ailleurs mal conduite, et une
autre étude, effectuée sur des cobayes, n'a pas révélé de réaction de
nature asthmatiforme. Aucun effet indésirable n'a été observé chez des
animaux de laboratoire à qui on en avait fait inhaler pendant des
durées allant jusqu'à 13 semaines à des doses pouvant atteindre
200 mg/m3. Une exposition répétée par la voie orale a entraîné des
lésions évocatrices de pyélonéphrite avec présence intratubulaire de
cylindres et de dépôts cristallins chez plusieurs espèces. Cependant,
il a fallu une dose élevée pour provoquer ces effets (>200 mg/kg de
poids corporel par jour sur une durée pouvant aller jusqu'à 1 an).
L'azodicarbonamide s'est révélé mutagène sur des systèmes bactériens,
mais il n'est pas certain que cet effet se produise in vivo. On n'a
pas examiné en détail la cancérogénicité de l'azodicarbonamide, ni sa
toxicité génésique, mais des études anciennes au cours desquelles des
animaux avaient reçu son métabolite, la biurée, n'ont pas mis en
évidence d'effets tumorigènes ou stérilisants. On n'a pas étudié son
action toxique éventuelle sur le développement.
Les études relatives à la santé humaine portent uniquement sur
l'aptitude de l'azodicarbonamide à provoquer de l'asthme ou une
sensibilisation cutanée. Des épreuves d'exposition bronchique
effectuées sur des sujets symptomatiques de même que l'examen médical
de personnes employées à la fabrication d'azodicarbonamide ou sur des
lieux ou on en utilisait, ont révélé que le composé pouvait provoquer
de l'asthme chez l'Homme. Certains indices permettent également de
penser qu'il peut provoquer une sensibilisation cutanée.
En se basant sur le fait que l'azodicarbonamide peut provoquer un
asthme chez l'Homme et que l'on ignore à partir de quelle
concentration cet asthme risque d'apparaître chez un sujet non
sensible ou une réaction se manifester chez un sujet sensible, on est
arrivé à la conclusion que dans les conditions actuelles d'exposition
professionnelles, il y avait un risque pour la santé humaine. Compte
tenu de l'incertitude sur la concentration à partir de laquelle il y a
effectivement risque, il convient de réduire l'exposition le plus
possible.
On possède des données indiquant qu'il se forme du carbamate
d'éthyle dans certains produits de consommation comme le pain ou la
bière après addition d'azodicarbonamide. Faute de données, il n'a pas
été possible d'évaluer le degré d'exposition de la population générale
à l'azodicarbonamide.
En cas de décharge dans les eaux superficielles, l'azocarbonamide
se répartirait dans l'hydrosphère sans sorption importante aux
matières particulaires. Dans le cas d'une réaction sur les radicaux
hydroxyles de l'atmosphère, la demi-vie calculée est de 0,4 jour. Dans
deux essais sur trois effectués avec des boues d'égout, on a constaté
que l'azodicarbonamide se révélait facilement biodégradable et on
observé une décomposition à 20-70 % dans le sol en 14 jours. La
concentration sans effet observable pour les poissons et la puce d'eau
a été trouvée respectivement égale à >50 et 5 mg/litre. En
l'absence de renseignements sur la décharge d'azodicarbonamide dans
l'environnement, on ne peut procéder à une évaluation quantitative du
risque.
RESUMEN DE ORIENTACION
Este CICAD sobre la azodicarbonamida se basa en un examen de los
problemas relativos a la salud humana (fundamentalmente ocupacionales)
preparado por la Dirección de Salud y Seguridad del Reino Unido (Ball
et al., 1996). Por consiguiente, aunque el presente CICAD incluye una
evaluación de los datos ecológicos disponibles, se concentra sobre
todo en el riesgo para la salud humana en las condiciones del trabajo,
con particular atención a la información acerca de las rutas que son
de interés para el entorno ocupacional. En este examen se han
incorporado los datos identificados hasta junio de 1994. Se realizó
una ulterior búsqueda bibliográfica hasta julio de 1997 para localizar
la información nueva que se hubiera publicado desde la terminación del
examen. En el documento original no se abordaban los problemas
relativos al medio ambiente; dado que en la búsqueda bibliográfica no
se han encontrado estudios de interés de este sector, no se ha
intentado realizar una evaluación del riesgo para el medio ambiente.
La información acerca del carácter del examen colegiado del documento
original y su disponibilidad figura en el apéndice 1. La información
sobre el examen colegiado de este CICAD aparece en el apéndice 2. Este
CICAD se aprobó como evaluación internacional en una reunión de la
Junta de Evaluación Final celebrada en Tokio, Japón, del 30 de junio
al 2 de julio de 1998. La lista de participantes en esta reunión
figura en el apéndice 3. La Ficha internacional de seguridad química
(ICSC 0380) para la azodicarbonamida, preparada por el Programa
Internacional de Seguridad de las Sustancias Químicas (IPCS, 1993),
también se reproduce en el presente documento.
Los datos toxicocinéticos sobre la azodicarbonamida
(CAS No 123-77-3) son limitados, pero parece que se absorbe bien en
roedores por inhalación y por vía oral. Quedan sin absorber cantidades
importantes de la sustancia en el sistema gastrointestinal, que se
eliminan en las heces. La azodicarbonamida se convierte fácilmente en
biurea, único producto de la biodegradación identificado, y es
probable que haya exposición sistémica fundamentalmente a este
derivado y no al compuesto original. La eliminación de la
azodicarbonamida/biurea absorbida es rápida, sobre todo a través de la
orina, y hay una retención sistémica de biurea muy escasa.
La toxicidad aguda de la azodicarbonamida es baja y en los
animales de experimentación no produce irritación cutánea, ocular o
del aparato respiratorio. Los resultados de un estudio de
sensibilización cutáneo poco fidedigno fueron negativos y en otro
estudio no se obtuvieron pruebas de una respuesta de tipo asmático en
cobayas. No se detectaron efectos adversos en animales de
experimentación que inhalaron hasta 200 mg/m3 durante 13 semanas. La
exposición oral repetida provocó pielonefritis con cilindros y
depósitos cristalinos en los túbulos renales en varias especies. Sin
embargo, las dosis necesarias para inducir estos efectos fueron altas
(>200 mg/kg de peso corporal al día en estudios de hasta un año de
duración). Si bien se observó que la azodicarbonamida era mutagénica
en sistemas bacterianos, no hay pruebas de que este efecto aparezca
in vivo. No se han examinado con detalle la carcinogenicidad y la
toxicidad reproductiva de la azodicarbonamida, pero en estudios
iniciales en los cuales se trataron animales con el producto de
degradación, la biurea, no se detectaron efectos tumorígenos o
anticonceptivos. No se ha estudiado la toxicidad en el desarrollo.
Los estudios en el ser humano se han concentrado exclusivamente
en la capacidad de la azodicarbonamida para inducir asma y
sensibilización cutánea. En estudios de estímulo bronquial de personas
sintomáticas y en evaluaciones de la salud de los empleados en lugares
donde se fabrica o utiliza azodicarbonamida se ha comprobado que este
producto puede inducir asma en el ser humano. Existen asimismo
indicios de que la azodicarbonamida puede inducir sensibilización
cutánea.
A partir de la base de que la azodicarbonamida es un asmógeno
humano y de que no se conocen las concentraciones que se requieren
para inducir el asma en una persona no sensible o provocar una
respuesta en una persona sensible, se llega a la conclusión de que
existe un riesgo para la salud humana en las condiciones actuales de
exposición ocupacional. El nivel del riesgo es incierto; por
consiguiente, se deben reducir al máximo los niveles de exposición.
Se conocen datos que indican que se forma etilcarbamato en
productos de consumo como el pan y la cerveza después de la adición de
azodicarbonamida. La exposición del público general no se pudo evaluar
debido a la falta de datos disponibles.
La azodicarbonamida liberada en las aguas superficiales se
distribuiría en la hidrosfera con una sorción no significativa en
partículas. La semivida para la reacción en la atmósfera con los
radicales hidroxilo se calcula que es de 0,4 días. La biodegradación
de la azodicarbonamida fue fácil en dos de las tres pruebas realizadas
con lodos cloacales y la degradación en el suelo fue del 20%-70% en un
período de 14 días. No se han notificado concentraciones sin efectos
observados (NOEC) para peces y pulgas de agua a >50 y 5 mg/litro,
respectivamente. La falta de información sobre la liberación en el
medio ambiente impide una evaluación cuantitativa del riesgo.
See Also:
Toxicological Abbreviations
Azodicarbonamide (ICSC)
Azodicarbonamide (FAO Nutrition Meetings Report Series 40abc)
AZODICARBONAMIDE (JECFA Evaluation)
 1. EXECUTIVE SUMMARY
This CICAD on azodicarbonamide was based on a review of human
health (primarily occupational) concerns prepared by the United
Kingdom's Health and Safety Executive (Ball et al., 1996). Hence,
although this CICAD includes an assessment of the available
environmental data, the main focus is on risks to human health in the
working environment, including an emphasis on information from routes
that are relevant to occupational settings. Data identified up to June
1994 were covered in the review. A further literature search was
performed, up to July 1997, to identify any new information published
since this review was completed. The original source document did not
address environmental concerns; as literature searches have failed to
identify relevant studies in this area, an environmental risk
assessment has not been attempted. Information on the nature of the
peer review and availability of the source document is presented in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Tokyo, Japan, on 30
June - 2 July 1998. Participants at the Final Review Board meeting are
listed in Appendix 3. The International Chemical Safety Card (ICSC
0380) for azodicarbonamide, produced by the International Programme on
Chemical Safety (IPCS, 1993), has also been reproduced in this
document.
Toxicokinetic data on azodicarbonamide (CAS No.
1. EXECUTIVE SUMMARY
This CICAD on azodicarbonamide was based on a review of human
health (primarily occupational) concerns prepared by the United
Kingdom's Health and Safety Executive (Ball et al., 1996). Hence,
although this CICAD includes an assessment of the available
environmental data, the main focus is on risks to human health in the
working environment, including an emphasis on information from routes
that are relevant to occupational settings. Data identified up to June
1994 were covered in the review. A further literature search was
performed, up to July 1997, to identify any new information published
since this review was completed. The original source document did not
address environmental concerns; as literature searches have failed to
identify relevant studies in this area, an environmental risk
assessment has not been attempted. Information on the nature of the
peer review and availability of the source document is presented in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Tokyo, Japan, on 30
June - 2 July 1998. Participants at the Final Review Board meeting are
listed in Appendix 3. The International Chemical Safety Card (ICSC
0380) for azodicarbonamide, produced by the International Programme on
Chemical Safety (IPCS, 1993), has also been reproduced in this
document.
Toxicokinetic data on azodicarbonamide (CAS No. 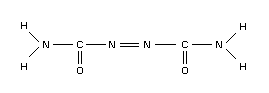 The conversion factors for azodicarbonamide at 20°C and 101.3 kPa
are as follows:
1 mg/m3 = 0.21 ppm
1 ppm = 4.8 mg/m3
3. ANALYTICAL METHODS
Two methods can be used to measure levels of azodicarbonamide in
workplace air. In the first, samples are collected on 37-mm Teflon
filters backed with polyethylene, which in turn is backed with a
cellulose pad (Ahrenholz & Neumeister, 1987). Sampling takes place at
2 litres/min for periods from 7 to 486 min. After sampling,
azodicarbonamide is recovered from the filter with dimethyl sulfoxide
and analysed by high-performance liquid chromatography. The limit of
quantification is given as 5 µg per sample. For a 15-min sample at 2
litres/min, this is equivalent to a quantification limit in air of
0.167 mg/m3; over 8 h, it is equivalent to 0.005 mg/m3.
In the second method, azodicarbonamide is collected on 37-mm
glass fibre filters at 15-20 litres/min (Vainiotalo & Pfaffli, 1988).
It is then eluted from the filter with dimethyl sulfoxide. Following
the addition of sodium hydroxide and glucose solutions, the
azodicarbonamide is reduced to hydrazine. This is reacted with
4-dimethylaminobenzaldehyde, and the resulting aldazine is measured
spectrophotometrically at 460 nm. The lower detection limit is given
as 0.001 mg/m3 for a 4-m3 air sample (equivalent to 4 µg per
sample). This is a considerably larger volume than would normally be
collected for assessing personal exposure samples. At a more typical
flow rate of 2 litres/min for 8 h, 960 litres of air would be sampled,
resulting in a detection limit of about 0.005 mg/m3. Sampling over 15
min at the same rate would give a detection limit of about 0.16
mg/m3.
Although there are published methods for measuring
azodicarbonamide and its metabolite biurea in rats (Bechtold et al.,
1989; see also Mewhinney et al., 1987), there are no reports
describing their measurement in human body fluids.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The principal end use of azodicarbonamide is as a blowing agent
in the rubber and plastics industries. It is used in the expansion of
a wide range of polymers, including polyvinyl chloride, polyolefins,
and natural and synthetic rubbers. The blowing action occurs when the
azodicarbonamide decomposes on heating (process temperatures
approx. 190-230°C) to yield gases (nitrogen, carbon monoxide, carbon
dioxide, and ammonia), solid residues, and sublimated substances.
Decomposition accelerators, in the form of metal salts and oxides, may
also be added to bring about decomposition at lower temperatures.
Azodicarbonamide has in the past been used in the United Kingdom
and Eire (but not other European Union member states) as a flour
improver in the bread-making industry, but this use is no longer
permitted. It is not known how common this practice is worldwide.
Azodicarbonamide is not used in other consumer products.
Azodicarbonamide is manufactured by the reaction of dihydrazine
sulfate and urea under high temperature and pressure. The product of
this reaction is then oxidized using sodium chlorate and centrifuged
to yield a slurry containing azodicarbonamide. The slurry is washed to
remove impurities and dried to obtain the azodicarbonamide powder.
This is then micronized to a fine powder (95% of particles <10 µm,
which is in the respirable range for humans) before packaging.
Very limited information is available on production volumes. The
Hazardous Substances Data Bank (HSDB, 1996) gives US production
figures of "greater than 4.54 tonnes." Until recently,
azodicarbonamide was produced in the United Kingdom; however, this
production has now stopped, and all azodicarbonamide used in the
United Kingdom is imported, predominantly through one large company.
Approximately 2500 tonnes are supplied to the United Kingdom market
each year. Both pure azodicarbonamide (approximately 2200 tonnes) and
pre-mixed formulations (300 tonnes) are supplied, the latter
containing between 10 and 95% azodicarbonamide, depending on the end
use application. "Masterbatch" products, in which the azodicarbonamide
is pelletized with polyolefins, and blended pastes (azodicarbonamide
and plasticizer) are also supplied to the rubber and plastics
industries. In addition to the main importer, there are some firms
that process azodicarbonamide into dust-suppressed powders, pastes,
and "Masterbatch" formulations before selling the processed
azodicarbonamide to the end users.
Recent studies have examined the contribution of azodicarbonamide
to the levels of ethyl carbamate in bread and beer (Canas et al.,
1997; Dennis et al., 1997a,b). It is not clear if unreacted
azodicarbonamide is present in these products; therefore, it is not
possible to assess the contribution that consumption of such products
might make to the overall body burden of azodicarbonamide.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
A calculated half-life of 0.4 days for reaction with hydroxyl
radicals in air has been reported.1
Azodicarbonamide added to five different soil types at 200 mg
nitrogen/kg soil (dry weight) was degraded (measured as recovery of
inorganic nitrogen) by between 21.1% and 66.1% over 14 days
(Frankenberger & Tabatabai, 1982).
Degradation of azodicarbonamide by sewage sludge organisms has
been investigated in three modified Sturm tests (Organisation for
Economic Co-operation and Development [OECD] Guideline 301B). The
compound was "readily biodegradable" in two out of the three tests and
was degraded by 21% over 30 days in the third test (Uniroyal,
1992).2
According to Mackay Level I fugacity modelling, azodicarbonamide
released to surface waters will partition to the hydrosphere with no
significant sorption to particulates.3
1 Bayer, unpublished calculated value (1988) based on the method of
Atkinson; value presented in IUCLID (European Union database),
version dated 7 February 1996.
2 Bayer, unpublished value (1991) presented in IUCLID (European
Union database), version dated 7 February 1996; no details
available.
3 Bayer remark in IUCLID (European Union database), version dated 7
February 1996.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
There are no data available on levels of azodicarbonamide in
ambient air, water, soils, or sediment.
6.2 Human exposure
The data available to the authors of this CICAD are restricted to
the occupational environment. It is estimated that several thousand
persons are exposed to azodicarbonamide in the workplace in the United
Kingdom. Of this total, it is estimated that only a few hundred
persons are exposed as part of their main work activity (i.e., those
involved in compounding, mixing, or raw material handling).
Data obtained by the United Kingdom's Health and Safety Executive
at a plant milling azodicarbonamide powder in micronizing mills (four
samples were collected in total) showed average personal exposures
during the day shift to be 11.8 and 9.8 mg/m3 and during the night
shift to be 2.3 and 2.8 mg/m3 because of the lower throughput at
night. Samples were collected over 4 h. The operators' tasks involved
bagging, weighing, and packaging the milled product.
In the published literature, Slovak (1981) reported time-weighted
average total dust levels in the range 2-5 mg/m3 for azodicarbonamide
manufacturing operations. However, no details were given concerning
occupational groups or tasks. A US National Institute for Occupational
Safety and Health study (Ahrenholz et al., 1985) examined personal
exposures of workers handling azodicarbonamide in a flooring factory.
The work involved the formulation of pastes or paints and required the
blending of azodicarbonamide powder with plasticizers, resins,
pigments, and other additives. Exposures to azodicarbonamide occurred
primarily while the workers were weighing and tipping the powder.
Short-term (sample duration <70 min) personal exposures were in the
range 0.15-12 mg/m3 (median 2.7 mg/m3). A second study (Ahrenholz &
Anderson, 1985) focused on the use of azodicarbonamide in the
injection moulding of plastics. The process involved blending
azodicarbonamide powder with resins. Two sets of full-shift
measurements were reported, with median 8-h time-weighted average
levels of 6.2 µg/m3 (range, not detectable to 280 µg/m3) and 25
µg/m3 (range, trace to 752 µg/m3), respectively.
No data are available relating to dermal exposure levels.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
No information is available on the toxicokinetics of
azodicarbonamide in humans.
Most of the toxicokinetic data available for azodicarbonamide
come from animal studies (Mewhinney et al., 1987). Absorption of
azodicarbonamide has been demonstrated following both a single
inhalation exposure of up to 6 h (34% of dose) and a single oral
administration (10-33% of dose) of radiolabelled azodicarbonamide to
rats. In contrast, approximately 90% of a single intratracheally
instilled dose was apparently absorbed. The difference in absorption
between inhaled and intratracheally instilled azodicarbonamide could
be related to the fact that much of the inhaled azodicarbonamide did
not reach the lower respiratory tract. Half an hour after a 6-h
nose-only exposure of rats to 25 mg/m3 of a dry aerosol (average
mass aerodynamic diameter 3.4 µm), 78% of the calculated total intake
was located in the gastrointestinal tract.
Following exposure by both inhalation and oral routes,
substantial quantities of the substance remain unabsorbed from the
gastrointestinal tract and are passed out in the faeces.
Azodicarbonamide is readily converted to biurea, the only breakdown
product identified, and it is likely that systemic exposure is
principally to this derivative rather than to the parent compound.
Elimination of absorbed azodicarbonamide/biurea is rapid, occurring
predominantly via the urine, and there is very little systemic
retention of biurea.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
It is noted that some of the available toxicological studies
were conducted using biurea. Azodicarbonamide readily undergoes
reduction in the presence of thiol groups to form the stable compound
biurea. Given that thiol groups are also present in many biological
molecules, there is the potential for this reaction to take place
wherever azodicarbonamide encounters thiol groups in biological
systems. This has been demonstrated in an experiment in which
radiolabelled azodicarbonamide was added to fresh rat blood (Mewhinney
et al., 1987). All radioactivity was in the form of biurea within 5
min when untreated blood was used. Radioactivity associated with
azodicarbonamide was detected only in blood to which 5 mg unlabelled
azodicarbonamide/ml blood was added. This level is very much greater
than the levels that humans are likely to encounter.
8.1 Single exposure
Azodicarbonamide is of low acute toxicity by all relevant routes
of exposure. The LC50 was greater than 6100 mg/m3 in rats and mice
exposed to a dry aerosol (median mass aerodynamic diameter
5.8 ± 2.25 µm [geometric standard deviation]) of azodicarbonamide for
4 h (IRDC, 1982a,b). No mortality was observed in rats given oral
doses of up to 5000 mg/kg body weight (Loeser, 1976). The dermal LD50
was >2000 mg/kg body weight in rabbits following application of this
substance under an occlusive dressing for 24 h (MB Research
Laboratories Inc., 1986). Few specific toxic effects were observed in
any single exposure study.
8.2 Irritation and sensitization
Although most studies were of uncertain quality and in many cases
would not comply with modern regulatory standards, results of several
skin and eye irritation studies indicate that azodicarbonamide should
not be regarded as a skin or eye irritant (Kimmerle, 1965; Conning,
1966; Mihail, 1977; MB Research Laboratories Inc., 1986). In a study
of respiratory irritation, in which guinea-pigs were exposed head only
to azodicarbonamide in plethysmography tubes, either no changes or
effects of doubtful significance were reported for various lung
function parameters, indicating that irritation was minimal at
concentrations up to 97 mg/m3 for 1 h (Shopp et al., 1987).
Owing to the poor quality of the only available skin
sensitization study (the concentration used for induction and
challenge -- a 1% solution in dimethyl formamide -- was very low; only
four guinea-pigs were used in the test group; and test sites were not
occluded), it was not possible to draw any conclusions regarding the
ability of azodicarbonamide to induce skin sensitization in animals
(Stevens, 1967). No evidence of pulmonary irritation or
asthmatic-type-reactions (no changes in specific airways conductance,
no evidence of histopathological effects on the upper or lower
respiratory tract, and no evidence of circulating antibodies) was
obtained in one study in which groups of 10 guinea-pigs were
repeatedly exposed by inhalation to unconjugated azodicarbonamide at
0, 51, or 200 mg/m3 for 6 h/day, 5 days/week, for 4 weeks (Gerlach et
al., 1989).
8.3 Short-term exposure
Azodicarbonamide is of relatively low toxicity to animals
repeatedly exposed by the inhalation or oral routes. In well-conducted
2-week inhalation studies, groups of 10 male and 10 female F344/N rats
or B6C3F1 mice were exposed to dry aerosols (median mass aerodynamic
diameter 2 µm) at 0, 2, 10, 50, 100, or 200 mg/m3 for 6 h/day, 5
days/week (Medinsky et al., 1990). Investigations included analyses of
methaemoglobin and blood cholinesterase and extensive macroscopic and
microscopic pathology; no changes of toxicological significance were
seen.
Groups of five male and five female mice received 0, 625, 1250,
5000, or 10 000 mg azodicarbonamide/kg body weight per day by oral
gavage in corn oil, 5 days/week for 2 weeks.1 Mortalities were
observed at 1250 mg/kg body weight per day and above (presumably
treatment related). Histopathologically, at 1250 mg/kg body weight per
day and above, pyelonephritis with casts was seen in renal tubuli, and
crystalline deposits were observed in renal tubuli and the urinary
bladder.
A similar study was conducted in rats; again, there was a
dose-related increase in mortality at 1250 mg/kg body weight per day
and above and a similar profile of renal lesions, although effects
were seen at 1250 mg/kg body weight per day and above in males and at
2500 mg/kg body weight per day and above in females.1
In one early and very briefly reported study, signs of toxicity,
the nature of which was not reported, were seen in male rats given 300
mg azodicarbonamide/kg body weight per day for 5 days but not in rats
given 200 mg/kg body weight per day (Kimmerle, 1965).
In another study designed to look for adverse effects in the
thyroid (but only investigating the uptake of iodine in the thyroid
gland and serum protein-bound iodine), no clear evidence of thyroid
toxicity was found in rats given low-iodine diets containing 1, 5, or
10% azodicarbonamide or 5 or 10% biurea for periods ranging between 1
and 4 weeks (Gafford et al., 1971).
There are no data relating to the effects of repeated dermal
exposures.
1 IRDC, unpublished data; cited in BG Chemie (1995).
8.4 Long-term exposure
8.4.1 Subchronic exposure
Groups of 10 male and 10 female F344/N rats or B6C3F1 mice were
exposed to dry aerosols (median mass aerodynamic diameter 2 µm) at 0,
50, 100, or 200 mg/m3 for 6 h/day, 5 days/week, for 13 weeks
(Medinsky et al., 1990). Investigations included analyses of various
enzyme activities in urine, haematology, blood cholinesterase, serum
triiodothyronine and thyroxine, vaginal cytology, sperm morphology,
levels of azodicarbonamide and biurea in lungs and kidneys (Mewhinney
et al., 1987), and extensive macroscopic and microscopic pathology; no
changes of toxicological significance were seen.
Groups of 10 male mice received 1, 78, 156, 312, 625, or 1250 mg
azodicarbonamide/kg body weight per day and groups of 10 female mice
received 0, 156, 312, 625, 1250, or 2500 mg azodicarbonamide/kg body
weight per day by oral gavage in corn oil, 5 days/week for 13 weeks.1
In contrast to the 2-week study (see section 8.3), there were no
mortalities and no histopathological abnormalities.
Groups of 10 male rats received 1, 100, 500, or 2500 mg
azodicarbonamide/kg body weight per day and groups of 10 female mice
received 0, 200, 1000, or 5000 mg azodicarbonamide/kg body weight per
day by oral gavage in corn oil, 5 days/week for 13 weeks.1 Mortality
was observed only at 2500 mg/kg body weight per day in males and at
5000 mg/kg body weight per day in females. Histopathologically,
pyelonephritis and crystalline deposits were observed in males at 2500
mg/kg body weight per day and in females at 5000 mg/kg body weight per
day only.
8.4.2 Chronic exposure and carcinogenicity
The effects of long-term exposure to azodicarbonamide have not
been well studied, and no conventional carcinogenicity studies are
available. The only data come from 1- and 2-year studies in which rats
and dogs received diets containing various amounts of biurea. In the
1-year study, rats and dogs ate diets containing 5 or 10% biurea (Oser
et al., 1965). One high-dose rat died, and body weight gain was
slightly depressed in high-dose males. No other signs of toxicity were
observed in rats at necropsy. Most dogs from both dose groups died.
Necropsy revealed massive, multiple renal calculi, bladder calculi,
and chronic pyelonephritis. However, the dogs that were selected for
this study were of uncertain and variable origin; hence, no useful
results could be obtained from them. The main constituent (comprising
80-100%) of the calculi was identified as biurea.
In the 2-year study, rats and dogs ate diets containing either
bread baked with untreated flour but supplemented with 750, 2370, or
7500 mg biurea/kg or bread baked with flour containing 100 mg
1 IRDC, unpublished data; cited in BG Chemie (1995).
azodicarbonamide/kg (Oser et al., 1965). Controls received diets
containing bread baked with untreated flour. Given that
azodicarbonamide is readily converted to biurea (Joiner et al., 1963),
it is likely that the animals receiving the bread baked with
azodicarbonamide-treated flour were actually exposed to biurea. As
with the previous investigations, the dogs that were selected for this
study were of uncertain and variable origin; hence, no useful results
could be obtained from them. For rats, no treatment-related deaths
occurred, and no adverse effects were observed that were considered to
be treatment related. Assuming food consumption of 20 g/day and a mean
body weight of 350 g, the dietary inclusion levels correspond to
approximately 45, 140, and 450 mg biurea/kg body weight per day.
8.5 Genotoxicity and related end-points
Azodicarbonamide is mutagenic in vitro, inducing base-pair
mutations in bacteria with and without metabolic activation (Pharmakon
Research International, 1984a; Mortelmans et al., 1986; Hachiya,
1987).1 In contrast, several standard in vitro assays in mammalian
cell systems have yielded negative results; gene mutation assays in
Chinese hamster ovary cells, using the hypoxanthine guanine
phosphoribosyl transferase locus, and in mouse lymphoma cells, using
the thymidine kinase locus, have been conducted, along with an in
vitro liver unscheduled DNA synthesis assay and a sister chromatid
exchange assay in Chinese hamster ovary cells (Pharmakon Research
International, 1984b,c).1,2 A positive result was obtained in a
chromosomal aberration assay in Chinese hamster ovary cells, but the
result was not reproducible.2 Negative results were obtained in a
sex-linked recessive lethal assay in Drosophila (Yoon et al., 1985).
Two in vivo bone marrow micronucleus assays in mice conducted by the
intraperitoneal route (0 or 150 mg azodicarbonamide/kg body weight)
were available, both giving negative results (Pharmakon Research
International, 1984d; Hachiya, 1987).
1 T. Cameron, unpublished Ames and mouse lymphoma test results
(1990) from the short-term program sponsored by the Division of
Cancer Aetiology, National Cancer Institute (cited in Chemical
Carcinogenesis Research Information System [CCRIS] database,
US National Cancer Institute).
2 NTP, unpublished data on chromosome aberration and sister
chromatid exchange assays in Chinese hamster ovary cells for
azodicarbonamide, submitted to the National Toxicology Program,
National Institutes of Health, US Department of Health and Human
Services, Research Triangle Park, NC.
8.6 Reproductive and developmental toxicity
The only study that has been conducted1 is a three-generation
study in which rats were given diets containing up to 7500 mg
biurea/kg (equivalent to approximately 450 mg/kg body weight per day)
(Oser & Oser, 1963; Oser et al., 1965). For each generation, rats were
mated twice, and the first litter was sacrificed at weaning. From the
second litter, 10 males and 10 females were chosen at random to form
the parents for the next generation. The study finished with the
weaning of the F3 generation. For each generation, fertility index
(percentage of matings resulting in pregnancy), gestation index
(number of pregnancies resulting in live litters), viability index
(numbers of pups surviving 4 or more days), and lactation index
(numbers of pups alive at 4 days surviving to weaning) were
determined. No reproductive effects were observed.
The only other information available is that no organ weight or
histological changes were observed in the reproductive organs of rats
and mice repeatedly exposed for 13 weeks to up to 200 mg
azodicarbonamide/m3 by inhalation (see section 8.4.1, Medinsky et
al., 1990).
The developmental toxicity of azodicarbonamide has not been
studied.
8.7 Immunological and neurological effects
No studies are available that specifically investigate these
endpoints, and there is no relevant information from toxicity studies
in animals.
1 The authors of this CICAD have been informed that a reproductive
toxicity screening test is being conducted according to OECD test
method 421.
9. EFFECTS ON HUMANS
The effects of exposure to azodicarbonamide in humans have not
been fully evaluated. There are no data detailing the effects of
single exposures by any route. The most frequently reported effects of
repeated exposure to azodicarbonamide are respiratory symptoms as well
as, to a lesser extent, skin sensitization reactions. There are no
reports relating to the potential for azodicarbonamide to produce
other systemic adverse effects. The potential genotoxic, carcinogenic,
and reproductive effects of azodicarbonamide in exposed humans have
not been studied.
9.1 Case reports
A number of reports have been published of individual
azodicarbonamide workers alleging asthma induced by exposure to
azodicarbonamide. The strongest evidence comes from a study of two
individuals (one atopic and one non-atopic) who worked at the same
plastics factory for about 4 years (Malo et al., 1985; Pineau et al.,
1985). Both were intermittently exposed (1-2 weeks' duration, 3-4
times per year) to azodicarbonamide at work. A few months after their
first encounter with azodicarbonamide, both developed symptoms
described as "eye/nose irritation" at work, followed a few hours later
by nocturnal asthmatic symptoms. After a 1-month period free from
exposure, both subjects underwent lung provocation studies. Baseline
values for forced expiratory volume in 1 s (FEV1), forced vital
capacity (FVC), and the concentration of histamine required to produce
a 20% drop in FEV1 (PC20H) were obtained by spirometry. Both
subjects performed a control challenge using lactose and then a 50:50
mixture of lactose and azodicarbonamide for 15 s on the next day. On
both days, lung function was monitored to follow the time course of
any response. It was reported that the trial was not carried out
blind.
No effects on lung function were observed following challenge
with lactose alone. After the azodicarbonamide challenge, however, the
atopic individual developed a late respiratory response starting 3 h
after challenge and reaching a maximum 24% drop in FEV1 6 h after
challenge. A drop in PC20H was also reported, demonstrating increased
airway hyperreactivity, and this parameter did not return to the
baseline value until 6 weeks after challenge. The non-atopic
individual showed a dual response to azodicarbonamide. Peak reductions
in FEV1 of greater than 20% were recorded 30 min and 5-6 h after
exposure. No significant reduction in PC20H was reported for the
second individual. A control atopic subject with underlying asthma who
worked in the same industry but did not experience work-related
respiratory effects was also tested. His baseline PC20H was similar
to that of the atopic subject, but no change in lung function was
observed following a 15-min exposure to azodicarbonamide under similar
conditions (as this subject had less reactive airways, a much longer
exposure duration was utilized). Owing to the insolubility of
azodicarbonamide, skin prick tests were not performed.
Six other cases have been reported in the literature, but in each
case the evidence that azodicarbonamide was the cause of the
respiratory symptoms is less strong. In some cases, there had been
previous exposure in industries associated with potential exposure to
other asthmagenic substances; for others, the bronchial challenge test
was either poorly conducted or not conducted at all (Valentino &
Comai, 1985; Alt & Diller, 1988; Normand et al., 1989).
Three case reports on skin sensitization have been published. In
the most recently reported investigation, a male textile worker
exposed to azodicarbonamide in foam ear-plugs was patch tested to
discover the cause of a recurrent dermatitis of the ear (Nava et al.,
1983; Bonsall, 1984; Yates & Dixon, 1988). No response was elicited
with a number of standard (International Contact Dermatitis Research
Group standard series) allergens. However, the individual gave a
strong positive reaction to the ear-plugs at 48 and 96 h and also to
azodicarbonamide (a component of the ear-plugs) at a concentration of
1 and 5% in petrolatum but not at 0.1% in petrolatum. Ten control
subjects patch tested with 1 and 5% azodicarbonamide in petrolatum did
not respond, and the individual reported no further symptoms upon
discarding the ear-plugs.
9.2 Epidemiological studies
Workplace health surveys have also been carried out where
azodicarbonamide was either manufactured or used to investigate the
presence of respiratory symptoms in azodicarbonamide workers.
A prevalence study of occupational asthma was carried out among a
group of 151 workers at a factory manufacturing azodicarbonamide
(Slovak, 1981). Diagnosis of asthma was made on the basis of an
administered questionnaire and a detailed occupational history taken
by the author. The population was divided into three groups: those
classified as potentially sensitized, on the basis of questionnaire
results; those with daily exposure but without symptoms; and those
with no exposure to azodicarbonamide or any other known sensitizer. On
one day, pre- and post-shift spirometry was performed, and FEV1, FVC,
and the FEV1/FVC ratio were determined. Skin prick tests were also
attempted using both common allergens to determine atopic status and
azodicarbonamide at concentrations of 0.1, 1, and 5% in dimethyl
sulfoxide. Concurrent personal sampling measurements were made to
determine the levels of airborne azodicarbonamide to which individuals
were exposed.
Personal sampling indicated that, at the time of the
investigation, airborne concentrations of azodicarbonamide ranged
between 2 and 5 mg/m3, as 8-h time-weighted averages. From the
questionnaires and occupational histories, 28 individuals (18.5%) were
diagnosed as having asthma apparently related to azodicarbonamide
exposure. Twelve further cases of occupational asthma were identified
from company records of past employees. Skin prick tests with
azodicarbonamide could not be adequately performed owing to the
insolubility of the substance.
Of the 28 current workers classified as sensitized, over half
developed symptoms within 3 months of first exposure and 21/28 (75%)
within 1 year. Symptoms and signs included shortness of breath, chest
tightness, wheezing, cough, rhinitis, conjunctivitis, and rash.
Reactions were of an immediate type for 6/28 (21%) individuals, late
onset for 16/28 (57%), and dual onset for 6/28 workers. Of those
showing a dual response, all but one had initially shown a late onset
pattern. A total of 13/28 (46%) workers reported worsening of symptoms
with continuing exposure to azodicarbonamide and a shortening of the
time between returning to work and reappearance of symptoms. Eight out
of 13 workers exposed to azodicarbonamide for more than 3 months after
development of symptoms also developed sensitivity to previously
well-tolerated irritants (e.g., sulfur dioxide and tobacco smoke),
which persisted for over a month after removal from exposure to
azodicarbonamide. In five individuals, this airway hyperreactivity
persisted for over 3 years. There were no changes in FEV1 or FVC over
the work shift in any group. In view of the latency in development of
effects, late or dual onset of symptoms in 12/28 (43%) symptomatic
workers, increase in sensitivity with repeated exposure, and the
persisting lung hyperreactivity in workers with prolonged exposure
after developing symptoms, it seems likely that these individuals had
become sensitized to azodicarbonamide.
Ahrenholz & Anderson (1985) and Whitehead et al. (1987) conducted
detailed investigations of the workforce at a plastics factory
employing about 325 workers. Lung function tests and interviews to
gather information on occupational history, smoking habits, past
illnesses, and respiratory, nasal, eye, and skin irritation, including
the time course of any symptoms, were carried out with a large
percentage of the workforce. There were no clear differences in the
results of lung function studies between those exposed to
azodicarbonamide and non-exposed individuals. However, responses to
the questionnaire revealed a significant association between symptoms
of irritation, cough, wheezing, shortness of breath, and headache and
present or previous employment as an injection mould operator. There
was also a slight but not statistically significant increase in the
reporting of skin rash among those with current or previous work in
the injection moulding department. The prevalence of all the above
symptoms was reduced among those whose employment in this department
was limited to the period before azodicarbonamide was introduced or
after a change in the process significantly reduced the use of
azodicarbonamide at the plant.
Personal sampling showed that concentrations of airborne
azodicarbonamide ranged from below the limit of detection (0.001
mg/m3) to 0.32 mg/m3 (median 0.006 mg/m3; geometric mean 0.004
mg/m3) averaged over the full shift. The highest concentration of
azodicarbonamide recorded (for an injection mould operator) was 0.01
mg/m3. Toluene, styrene, phenols, and triphenyl phosphate were also
detected at concentrations at or below the odour threshold for each
substance.
Other personal sampling data for a group of 17 individuals
revealed levels of azodicarbonamide ranging from traces to 0.8 mg/m3
(median 0.03 mg/m3; geometric mean 0.02 mg/m3) averaged over the
full shift. The second highest value recorded was 0.4 mg/m3, and the
next highest, 0.06 mg/m3. A moderate although statistically
significant reduction in FEV1 (mean reduction of 64 ml) and FVC (mean
reduction of 77 ml) occurred following shifts in which workers were
exposed to azodicarbonamide. Coughing at work, wheeze, and chest
tightness were also reported, and symptoms were apparently worse
during the week than on Sunday.
A detailed investigation of the workforce at a plant making floor
coverings was conducted after nosebleeds, mucous membrane irritation,
and skin rashes were reported in workers handling azodicarbonamide
(Ahrenholz et al., 1985). Two surveys were carried out. The initial
survey revealed, in decreasing order of prevalence, symptoms of eye
irritation, nose irritation, cough, nocturnal cough, shortness of
breath, wheeze, and chest tightness. The more extensive follow-up
survey was conducted 6 weeks later. Pre- and post-shift auscultation,
lung function tests, and respiratory symptoms (recorded by
questionnaire) were recorded. Blood samples were also taken for
immunological investigations.
Responses to the questionnaire revealed 15/30 regularly exposed
workers experiencing occupationally related lower respiratory tract
symptoms (cough, wheeze, and shortness of breath) compared with 1/16
never-exposed workers. No significant differences in pre- and
post-shift FEV1 and FVC measurements were found. For those workers
apparently not exposed to azodicarbonamide or exposed indirectly
(working in the vicinity but not directly handling azodicarbonamide),
levels (8-h time-weighted average) ranged from <0.001 to 0.1 mg/m3.
However, during weighing and charging operations, peaks of between
0.15 and 12 mg/m3 (median 2.7 mg/m3) were measured for individuals
directly involved.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Azodicarbonamide has been tested under OECD Guideline protocols
in one species of fish and in the water flea ( Daphnia magna);
results are in an unpublished industry report, which has not been
peer-reviewed (see Table 1). A second study, not conducted to a
protocol or under Good Laboratory Practices, showed no effect of an
azodicarbonamide solution analysed at 8 mg/litre on the zebra fish
( Brachydanio rerio).1 There was no effect on oxygen consumption of
sewage sludge organisms exposed over 3 h to azodicarbonamide at
>10 000 mg/litre (this is substantially greater than the solubility
of the compound, and no information is available on how this
concentration was achieved).2 Overall, it is not possible to draw
firm conclusions from these studies.
1 IUCLID (European Union database), version dated 7 February 1996.
2 Bayer, unpublished value (1988) presented in IUCLID (European
Union database), version dated 7 February 1996; no details
available.
Table 1: Acute studies on toxicity to aquatic organisms.
Organism Protocol End-point Concentration Reference
(mg/litre)
Fathead minnow OECD 203 96-h NOEC >50 Uniroyal (1992)
(Pimephales promelas)
Water flea OECD 202 48-h NOEC 4.8 (measured) Uniroyal (1992)
(Daphnia magna) ~16 (nominal)
48-h EC50 11 (measured)
immobilization 29 (nominal)
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Some of the available toxicological studies have been conducted
using biurea rather than azodicarbonamide. However, azodicarbonamide
is readily converted to biurea in vivo. Hence, similar toxicological
properties would be expected.
Azodicarbonamide is of low acute toxicity by all routes, and,
although the animal studies are of uncertain quality, solid
azodicarbonamide would not be regarded as a skin or eye irritant. With
respect to respiratory tract irritation, no changes of toxicological
significance were seen in guinea-pigs exposed to azodicarbonamide
aerosol at concentrations up to 97 mg/m3 for 1 h.
No conclusions could be drawn regarding skin sensitization
potential from the available, poor-quality animal studies. Although
there are currently no validated animal studies investigating
asthmagenic potential, there was no evidence of pulmonary irritation
or asthmatic-type reactions in guinea-pigs exposed to up to 200 mg
azodicarbonamide aerosol/m3 for 6 h/day, 5 days/week, for 4 weeks.
Similarly, there were no changes of toxicological significance
seen among rats or mice exposed by inhalation to up to 200 mg
azodicarbonamide aerosol/m3 for 6 h/day, 5 days/week, for up to 13
weeks.
For repeated-dose studies using the oral route, data were
inconsistent. In 2-year studies in which rats received up to 450 mg
biurea/kg body weight per day, there were no adverse effects seen.
Unpublished information suggests that no adverse effects were seen in
male mice exposed to up to 1250 mg azodicarbonamide/kg body weight per
day and in female mice exposed to up to 2500 mg/kg body weight per day
for 13 weeks. However, shorter-term studies (2 weeks, also
unpublished) indicated histological lesions in the kidneys of rats and
mice of both sexes at 1250 mg/kg body weight per day or more. A
13-week study in rats indicated kidney lesions in males at 2500 mg/kg
body weight per day, with no adverse effects observed at the next
lowest exposure level, 500 mg/kg body weight per day. For female rats,
kidney lesions were observed at 5000 mg/kg body weight per day, and no
adverse effects were observed at 1000 mg/kg body weight per day. There
were no data in relation to repeated dermal exposure.
Azodicarbonamide has been identified as a mutagen in bacterial
systems, but it was not mutagenic in mammalian cell in vitro test
systems or in two mammalian assays in vivo using bone marrow. It is
therefore unlikely that the mutagenic properties displayed by
azodicarbonamide in bacterial systems will be expressed in vivo.
However, it is considered that a confirmatory in vivo study in a
second tissue is desirable.
There are no adequate data available relating to carcinogenic,
reproductive, or developmental effects; hence, it is not possible to
evaluate the risk to human health for these end-points.
Several bronchial challenge studies have been reported, but only
one provides reasonable evidence that the work-related asthmatic
symptoms were due specifically to azodicarbonamide. This report is
considered to show an asthmatic response and not an irritant response
to azodicarbonamide on challenge. Animal studies suggest that airborne
concentrations of up to 200 mg/m3 can be tolerated with little or no
pulmonary irritation, and it is unlikely that the levels used in the
bronchial challenge tests approached those used in animal studies. The
delay in response to azodicarbonamide challenge, the magnitude of
reduction in FEV1 accompanied by an increase in airway
hyperreactivity in one individual, and the fact that a control
individual with mildly hyperreactive airways did not respond to a much
more prolonged exposure under similar challenge conditions provide
further evidence for asthmagenicity. Further evidence of a link
between azodicarbonamide and respiratory problems is provided by the
results of workplace health evaluations. Although criticisms can be
levelled at individual studies, weight of evidence suggests that
azodicarbonamide can induce asthma in a significant proportion of
exposed people.
There are some case reports of individuals with skin reactions to
topically applied azodicarbonamide. For some of these, results are
questionable. However, in workplace health surveys, the incidence of
skin rash was found to be greater among workers regularly exposed to
azodicarbonamide. Although no firm conclusions could be drawn from the
poorly reported animal study, clear evidence of skin sensitization to
azodicarbonamide in one individual and supporting evidence of skin
problems from workplace health surveys lead to the conclusion that
azodicarbonamide should be considered as a human skin sensitizer.
In conclusion, the key toxic effect of azodicarbonamide in humans
is asthmagenicity. Evidence of this effect has been found from
bronchial challenge studies and workplace health evaluations. From the
information available, azodicarbonamide is considered to have a low
potential for irritancy; thus, it is considered that the respiratory
symptoms observed in these studies are most likely due to an
asthmatic-type response rather than respiratory tract irritancy. There
is no clear information on the levels that may have induced or
provoked the state of asthma.
There is also information to indicate that azodicarbonamide can
cause skin sensitization in humans.
11.1.2 Criteria for setting guidance values for azodicarbonamide
The main cause for concern relates to the risk of developing
occupational asthma. There is no information available relating to
dose-response relationships or levels associated with the induction of
a hypersensitive state or provocation of an asthmatic response. Hence,
it is not possible to reliably quantify the risk of developing
occupational asthma.
11.1.3 Sample risk characterization
Using data obtained from a factory in the United Kingdom and
published exposure data as an example (section 6.2), levels of
airborne azodicarbonamide measured over periods of <70 min to 4 h of
up to 12 mg/m3 have been observed. Short-term peak exposures could be
higher than this level.
In the United Kingdom occupational setting, it is recommended
that a maximum exposure limit (MEL) be assigned to substances for
which it has not been possible to identify a level of exposure that is
without adverse effects on health. This is a non-health-based
standard, and, in determining the most appropriate level for a MEL,
consideration is taken of the level of control that it is reasonably
practicable for industry to achieve. The MEL of 1 mg/m3
(8-h time-weighted average) was based on a level of control that was
deemed by tripartite agreement to be reasonably practicable under
workplace conditions within the United Kingdom. There is also a
continuing remit for industry to keep on reducing exposure levels as
advances in technology make this possible. For substances that are
asthmagens, it is also advisable to have a short-term exposure limit
(STEL) to restrict peak exposures, as they may have a role in the
induction and triggering of asthmatic phenomena. In the absence of any
specific data that might advise adequately on the numerical value of
the STEL, 3 mg/m3 (15-min reference period) has been established.
As azodicarbonamide is a skin sensitizer, where skin contact can
occur, there may be a risk of developing allergic dermatitis if
suitable personal protective equipment is not used.
There is evidence to suggest that azodicarbonamide has been added
to consumer products such as bread and beer. The limited toxicology
database and lack of exposure data make it difficult to adequately
assess the risk to humans potentially exposed; hence, there is a need
for further information.
11.2 Evaluation of environmental effects
Lack of information on release to the environment precludes a
quantitative risk assessment.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations by international bodies were not identified.
Information on international hazard classification and labelling is
included in the International Chemical Safety Card reproduced in this
document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0380) reproduced in this
document.
13.1 Human health hazards
Azodicarbonamide is of low acute toxicity, but repeated or
prolonged contact may cause asthma and skin sensitization.
13.2 Health surveillance advice
Physicians involved in worker health surveillance programmes
should be aware of the potential of azodicarbonamide as a human
asthmagen.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
AZODICARBONAMIDE ICSC:0380
October 1997
CAS#
The conversion factors for azodicarbonamide at 20°C and 101.3 kPa
are as follows:
1 mg/m3 = 0.21 ppm
1 ppm = 4.8 mg/m3
3. ANALYTICAL METHODS
Two methods can be used to measure levels of azodicarbonamide in
workplace air. In the first, samples are collected on 37-mm Teflon
filters backed with polyethylene, which in turn is backed with a
cellulose pad (Ahrenholz & Neumeister, 1987). Sampling takes place at
2 litres/min for periods from 7 to 486 min. After sampling,
azodicarbonamide is recovered from the filter with dimethyl sulfoxide
and analysed by high-performance liquid chromatography. The limit of
quantification is given as 5 µg per sample. For a 15-min sample at 2
litres/min, this is equivalent to a quantification limit in air of
0.167 mg/m3; over 8 h, it is equivalent to 0.005 mg/m3.
In the second method, azodicarbonamide is collected on 37-mm
glass fibre filters at 15-20 litres/min (Vainiotalo & Pfaffli, 1988).
It is then eluted from the filter with dimethyl sulfoxide. Following
the addition of sodium hydroxide and glucose solutions, the
azodicarbonamide is reduced to hydrazine. This is reacted with
4-dimethylaminobenzaldehyde, and the resulting aldazine is measured
spectrophotometrically at 460 nm. The lower detection limit is given
as 0.001 mg/m3 for a 4-m3 air sample (equivalent to 4 µg per
sample). This is a considerably larger volume than would normally be
collected for assessing personal exposure samples. At a more typical
flow rate of 2 litres/min for 8 h, 960 litres of air would be sampled,
resulting in a detection limit of about 0.005 mg/m3. Sampling over 15
min at the same rate would give a detection limit of about 0.16
mg/m3.
Although there are published methods for measuring
azodicarbonamide and its metabolite biurea in rats (Bechtold et al.,
1989; see also Mewhinney et al., 1987), there are no reports
describing their measurement in human body fluids.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
The principal end use of azodicarbonamide is as a blowing agent
in the rubber and plastics industries. It is used in the expansion of
a wide range of polymers, including polyvinyl chloride, polyolefins,
and natural and synthetic rubbers. The blowing action occurs when the
azodicarbonamide decomposes on heating (process temperatures
approx. 190-230°C) to yield gases (nitrogen, carbon monoxide, carbon
dioxide, and ammonia), solid residues, and sublimated substances.
Decomposition accelerators, in the form of metal salts and oxides, may
also be added to bring about decomposition at lower temperatures.
Azodicarbonamide has in the past been used in the United Kingdom
and Eire (but not other European Union member states) as a flour
improver in the bread-making industry, but this use is no longer
permitted. It is not known how common this practice is worldwide.
Azodicarbonamide is not used in other consumer products.
Azodicarbonamide is manufactured by the reaction of dihydrazine
sulfate and urea under high temperature and pressure. The product of
this reaction is then oxidized using sodium chlorate and centrifuged
to yield a slurry containing azodicarbonamide. The slurry is washed to
remove impurities and dried to obtain the azodicarbonamide powder.
This is then micronized to a fine powder (95% of particles <10 µm,
which is in the respirable range for humans) before packaging.
Very limited information is available on production volumes. The
Hazardous Substances Data Bank (HSDB, 1996) gives US production
figures of "greater than 4.54 tonnes." Until recently,
azodicarbonamide was produced in the United Kingdom; however, this
production has now stopped, and all azodicarbonamide used in the
United Kingdom is imported, predominantly through one large company.
Approximately 2500 tonnes are supplied to the United Kingdom market
each year. Both pure azodicarbonamide (approximately 2200 tonnes) and
pre-mixed formulations (300 tonnes) are supplied, the latter
containing between 10 and 95% azodicarbonamide, depending on the end
use application. "Masterbatch" products, in which the azodicarbonamide
is pelletized with polyolefins, and blended pastes (azodicarbonamide
and plasticizer) are also supplied to the rubber and plastics
industries. In addition to the main importer, there are some firms
that process azodicarbonamide into dust-suppressed powders, pastes,
and "Masterbatch" formulations before selling the processed
azodicarbonamide to the end users.
Recent studies have examined the contribution of azodicarbonamide
to the levels of ethyl carbamate in bread and beer (Canas et al.,
1997; Dennis et al., 1997a,b). It is not clear if unreacted
azodicarbonamide is present in these products; therefore, it is not
possible to assess the contribution that consumption of such products
might make to the overall body burden of azodicarbonamide.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
A calculated half-life of 0.4 days for reaction with hydroxyl
radicals in air has been reported.1
Azodicarbonamide added to five different soil types at 200 mg
nitrogen/kg soil (dry weight) was degraded (measured as recovery of
inorganic nitrogen) by between 21.1% and 66.1% over 14 days
(Frankenberger & Tabatabai, 1982).
Degradation of azodicarbonamide by sewage sludge organisms has
been investigated in three modified Sturm tests (Organisation for
Economic Co-operation and Development [OECD] Guideline 301B). The
compound was "readily biodegradable" in two out of the three tests and
was degraded by 21% over 30 days in the third test (Uniroyal,
1992).2
According to Mackay Level I fugacity modelling, azodicarbonamide
released to surface waters will partition to the hydrosphere with no
significant sorption to particulates.3
1 Bayer, unpublished calculated value (1988) based on the method of
Atkinson; value presented in IUCLID (European Union database),
version dated 7 February 1996.
2 Bayer, unpublished value (1991) presented in IUCLID (European
Union database), version dated 7 February 1996; no details
available.
3 Bayer remark in IUCLID (European Union database), version dated 7
February 1996.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
There are no data available on levels of azodicarbonamide in
ambient air, water, soils, or sediment.
6.2 Human exposure
The data available to the authors of this CICAD are restricted to
the occupational environment. It is estimated that several thousand
persons are exposed to azodicarbonamide in the workplace in the United
Kingdom. Of this total, it is estimated that only a few hundred
persons are exposed as part of their main work activity (i.e., those
involved in compounding, mixing, or raw material handling).
Data obtained by the United Kingdom's Health and Safety Executive
at a plant milling azodicarbonamide powder in micronizing mills (four
samples were collected in total) showed average personal exposures
during the day shift to be 11.8 and 9.8 mg/m3 and during the night
shift to be 2.3 and 2.8 mg/m3 because of the lower throughput at
night. Samples were collected over 4 h. The operators' tasks involved
bagging, weighing, and packaging the milled product.
In the published literature, Slovak (1981) reported time-weighted
average total dust levels in the range 2-5 mg/m3 for azodicarbonamide
manufacturing operations. However, no details were given concerning
occupational groups or tasks. A US National Institute for Occupational
Safety and Health study (Ahrenholz et al., 1985) examined personal
exposures of workers handling azodicarbonamide in a flooring factory.
The work involved the formulation of pastes or paints and required the
blending of azodicarbonamide powder with plasticizers, resins,
pigments, and other additives. Exposures to azodicarbonamide occurred
primarily while the workers were weighing and tipping the powder.
Short-term (sample duration <70 min) personal exposures were in the
range 0.15-12 mg/m3 (median 2.7 mg/m3). A second study (Ahrenholz &
Anderson, 1985) focused on the use of azodicarbonamide in the
injection moulding of plastics. The process involved blending
azodicarbonamide powder with resins. Two sets of full-shift
measurements were reported, with median 8-h time-weighted average
levels of 6.2 µg/m3 (range, not detectable to 280 µg/m3) and 25
µg/m3 (range, trace to 752 µg/m3), respectively.
No data are available relating to dermal exposure levels.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
No information is available on the toxicokinetics of
azodicarbonamide in humans.
Most of the toxicokinetic data available for azodicarbonamide
come from animal studies (Mewhinney et al., 1987). Absorption of
azodicarbonamide has been demonstrated following both a single
inhalation exposure of up to 6 h (34% of dose) and a single oral
administration (10-33% of dose) of radiolabelled azodicarbonamide to
rats. In contrast, approximately 90% of a single intratracheally
instilled dose was apparently absorbed. The difference in absorption
between inhaled and intratracheally instilled azodicarbonamide could
be related to the fact that much of the inhaled azodicarbonamide did
not reach the lower respiratory tract. Half an hour after a 6-h
nose-only exposure of rats to 25 mg/m3 of a dry aerosol (average
mass aerodynamic diameter 3.4 µm), 78% of the calculated total intake
was located in the gastrointestinal tract.
Following exposure by both inhalation and oral routes,
substantial quantities of the substance remain unabsorbed from the
gastrointestinal tract and are passed out in the faeces.
Azodicarbonamide is readily converted to biurea, the only breakdown
product identified, and it is likely that systemic exposure is
principally to this derivative rather than to the parent compound.
Elimination of absorbed azodicarbonamide/biurea is rapid, occurring
predominantly via the urine, and there is very little systemic
retention of biurea.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
It is noted that some of the available toxicological studies
were conducted using biurea. Azodicarbonamide readily undergoes
reduction in the presence of thiol groups to form the stable compound
biurea. Given that thiol groups are also present in many biological
molecules, there is the potential for this reaction to take place
wherever azodicarbonamide encounters thiol groups in biological
systems. This has been demonstrated in an experiment in which
radiolabelled azodicarbonamide was added to fresh rat blood (Mewhinney
et al., 1987). All radioactivity was in the form of biurea within 5
min when untreated blood was used. Radioactivity associated with
azodicarbonamide was detected only in blood to which 5 mg unlabelled
azodicarbonamide/ml blood was added. This level is very much greater
than the levels that humans are likely to encounter.
8.1 Single exposure
Azodicarbonamide is of low acute toxicity by all relevant routes
of exposure. The LC50 was greater than 6100 mg/m3 in rats and mice
exposed to a dry aerosol (median mass aerodynamic diameter
5.8 ± 2.25 µm [geometric standard deviation]) of azodicarbonamide for
4 h (IRDC, 1982a,b). No mortality was observed in rats given oral
doses of up to 5000 mg/kg body weight (Loeser, 1976). The dermal LD50
was >2000 mg/kg body weight in rabbits following application of this
substance under an occlusive dressing for 24 h (MB Research
Laboratories Inc., 1986). Few specific toxic effects were observed in
any single exposure study.
8.2 Irritation and sensitization
Although most studies were of uncertain quality and in many cases
would not comply with modern regulatory standards, results of several
skin and eye irritation studies indicate that azodicarbonamide should
not be regarded as a skin or eye irritant (Kimmerle, 1965; Conning,
1966; Mihail, 1977; MB Research Laboratories Inc., 1986). In a study
of respiratory irritation, in which guinea-pigs were exposed head only
to azodicarbonamide in plethysmography tubes, either no changes or
effects of doubtful significance were reported for various lung
function parameters, indicating that irritation was minimal at
concentrations up to 97 mg/m3 for 1 h (Shopp et al., 1987).
Owing to the poor quality of the only available skin
sensitization study (the concentration used for induction and
challenge -- a 1% solution in dimethyl formamide -- was very low; only
four guinea-pigs were used in the test group; and test sites were not
occluded), it was not possible to draw any conclusions regarding the
ability of azodicarbonamide to induce skin sensitization in animals
(Stevens, 1967). No evidence of pulmonary irritation or
asthmatic-type-reactions (no changes in specific airways conductance,
no evidence of histopathological effects on the upper or lower
respiratory tract, and no evidence of circulating antibodies) was
obtained in one study in which groups of 10 guinea-pigs were
repeatedly exposed by inhalation to unconjugated azodicarbonamide at
0, 51, or 200 mg/m3 for 6 h/day, 5 days/week, for 4 weeks (Gerlach et
al., 1989).
8.3 Short-term exposure
Azodicarbonamide is of relatively low toxicity to animals
repeatedly exposed by the inhalation or oral routes. In well-conducted
2-week inhalation studies, groups of 10 male and 10 female F344/N rats
or B6C3F1 mice were exposed to dry aerosols (median mass aerodynamic
diameter 2 µm) at 0, 2, 10, 50, 100, or 200 mg/m3 for 6 h/day, 5
days/week (Medinsky et al., 1990). Investigations included analyses of
methaemoglobin and blood cholinesterase and extensive macroscopic and
microscopic pathology; no changes of toxicological significance were
seen.
Groups of five male and five female mice received 0, 625, 1250,
5000, or 10 000 mg azodicarbonamide/kg body weight per day by oral
gavage in corn oil, 5 days/week for 2 weeks.1 Mortalities were
observed at 1250 mg/kg body weight per day and above (presumably
treatment related). Histopathologically, at 1250 mg/kg body weight per
day and above, pyelonephritis with casts was seen in renal tubuli, and
crystalline deposits were observed in renal tubuli and the urinary
bladder.
A similar study was conducted in rats; again, there was a
dose-related increase in mortality at 1250 mg/kg body weight per day
and above and a similar profile of renal lesions, although effects
were seen at 1250 mg/kg body weight per day and above in males and at
2500 mg/kg body weight per day and above in females.1
In one early and very briefly reported study, signs of toxicity,
the nature of which was not reported, were seen in male rats given 300
mg azodicarbonamide/kg body weight per day for 5 days but not in rats
given 200 mg/kg body weight per day (Kimmerle, 1965).
In another study designed to look for adverse effects in the
thyroid (but only investigating the uptake of iodine in the thyroid
gland and serum protein-bound iodine), no clear evidence of thyroid
toxicity was found in rats given low-iodine diets containing 1, 5, or
10% azodicarbonamide or 5 or 10% biurea for periods ranging between 1
and 4 weeks (Gafford et al., 1971).
There are no data relating to the effects of repeated dermal
exposures.
1 IRDC, unpublished data; cited in BG Chemie (1995).
8.4 Long-term exposure
8.4.1 Subchronic exposure
Groups of 10 male and 10 female F344/N rats or B6C3F1 mice were
exposed to dry aerosols (median mass aerodynamic diameter 2 µm) at 0,
50, 100, or 200 mg/m3 for 6 h/day, 5 days/week, for 13 weeks
(Medinsky et al., 1990). Investigations included analyses of various
enzyme activities in urine, haematology, blood cholinesterase, serum
triiodothyronine and thyroxine, vaginal cytology, sperm morphology,
levels of azodicarbonamide and biurea in lungs and kidneys (Mewhinney
et al., 1987), and extensive macroscopic and microscopic pathology; no
changes of toxicological significance were seen.
Groups of 10 male mice received 1, 78, 156, 312, 625, or 1250 mg
azodicarbonamide/kg body weight per day and groups of 10 female mice
received 0, 156, 312, 625, 1250, or 2500 mg azodicarbonamide/kg body
weight per day by oral gavage in corn oil, 5 days/week for 13 weeks.1
In contrast to the 2-week study (see section 8.3), there were no
mortalities and no histopathological abnormalities.
Groups of 10 male rats received 1, 100, 500, or 2500 mg
azodicarbonamide/kg body weight per day and groups of 10 female mice
received 0, 200, 1000, or 5000 mg azodicarbonamide/kg body weight per
day by oral gavage in corn oil, 5 days/week for 13 weeks.1 Mortality
was observed only at 2500 mg/kg body weight per day in males and at
5000 mg/kg body weight per day in females. Histopathologically,
pyelonephritis and crystalline deposits were observed in males at 2500
mg/kg body weight per day and in females at 5000 mg/kg body weight per
day only.
8.4.2 Chronic exposure and carcinogenicity
The effects of long-term exposure to azodicarbonamide have not
been well studied, and no conventional carcinogenicity studies are
available. The only data come from 1- and 2-year studies in which rats
and dogs received diets containing various amounts of biurea. In the
1-year study, rats and dogs ate diets containing 5 or 10% biurea (Oser
et al., 1965). One high-dose rat died, and body weight gain was
slightly depressed in high-dose males. No other signs of toxicity were
observed in rats at necropsy. Most dogs from both dose groups died.
Necropsy revealed massive, multiple renal calculi, bladder calculi,
and chronic pyelonephritis. However, the dogs that were selected for
this study were of uncertain and variable origin; hence, no useful
results could be obtained from them. The main constituent (comprising
80-100%) of the calculi was identified as biurea.
In the 2-year study, rats and dogs ate diets containing either
bread baked with untreated flour but supplemented with 750, 2370, or
7500 mg biurea/kg or bread baked with flour containing 100 mg
1 IRDC, unpublished data; cited in BG Chemie (1995).
azodicarbonamide/kg (Oser et al., 1965). Controls received diets
containing bread baked with untreated flour. Given that
azodicarbonamide is readily converted to biurea (Joiner et al., 1963),
it is likely that the animals receiving the bread baked with
azodicarbonamide-treated flour were actually exposed to biurea. As
with the previous investigations, the dogs that were selected for this
study were of uncertain and variable origin; hence, no useful results
could be obtained from them. For rats, no treatment-related deaths
occurred, and no adverse effects were observed that were considered to
be treatment related. Assuming food consumption of 20 g/day and a mean
body weight of 350 g, the dietary inclusion levels correspond to
approximately 45, 140, and 450 mg biurea/kg body weight per day.
8.5 Genotoxicity and related end-points
Azodicarbonamide is mutagenic in vitro, inducing base-pair
mutations in bacteria with and without metabolic activation (Pharmakon
Research International, 1984a; Mortelmans et al., 1986; Hachiya,
1987).1 In contrast, several standard in vitro assays in mammalian
cell systems have yielded negative results; gene mutation assays in
Chinese hamster ovary cells, using the hypoxanthine guanine
phosphoribosyl transferase locus, and in mouse lymphoma cells, using
the thymidine kinase locus, have been conducted, along with an in
vitro liver unscheduled DNA synthesis assay and a sister chromatid
exchange assay in Chinese hamster ovary cells (Pharmakon Research
International, 1984b,c).1,2 A positive result was obtained in a
chromosomal aberration assay in Chinese hamster ovary cells, but the
result was not reproducible.2 Negative results were obtained in a
sex-linked recessive lethal assay in Drosophila (Yoon et al., 1985).
Two in vivo bone marrow micronucleus assays in mice conducted by the
intraperitoneal route (0 or 150 mg azodicarbonamide/kg body weight)
were available, both giving negative results (Pharmakon Research
International, 1984d; Hachiya, 1987).
1 T. Cameron, unpublished Ames and mouse lymphoma test results
(1990) from the short-term program sponsored by the Division of
Cancer Aetiology, National Cancer Institute (cited in Chemical
Carcinogenesis Research Information System [CCRIS] database,
US National Cancer Institute).
2 NTP, unpublished data on chromosome aberration and sister
chromatid exchange assays in Chinese hamster ovary cells for
azodicarbonamide, submitted to the National Toxicology Program,
National Institutes of Health, US Department of Health and Human
Services, Research Triangle Park, NC.
8.6 Reproductive and developmental toxicity
The only study that has been conducted1 is a three-generation
study in which rats were given diets containing up to 7500 mg
biurea/kg (equivalent to approximately 450 mg/kg body weight per day)
(Oser & Oser, 1963; Oser et al., 1965). For each generation, rats were
mated twice, and the first litter was sacrificed at weaning. From the
second litter, 10 males and 10 females were chosen at random to form
the parents for the next generation. The study finished with the
weaning of the F3 generation. For each generation, fertility index
(percentage of matings resulting in pregnancy), gestation index
(number of pregnancies resulting in live litters), viability index
(numbers of pups surviving 4 or more days), and lactation index
(numbers of pups alive at 4 days surviving to weaning) were
determined. No reproductive effects were observed.
The only other information available is that no organ weight or
histological changes were observed in the reproductive organs of rats
and mice repeatedly exposed for 13 weeks to up to 200 mg
azodicarbonamide/m3 by inhalation (see section 8.4.1, Medinsky et
al., 1990).
The developmental toxicity of azodicarbonamide has not been
studied.
8.7 Immunological and neurological effects
No studies are available that specifically investigate these
endpoints, and there is no relevant information from toxicity studies
in animals.
1 The authors of this CICAD have been informed that a reproductive
toxicity screening test is being conducted according to OECD test
method 421.
9. EFFECTS ON HUMANS
The effects of exposure to azodicarbonamide in humans have not
been fully evaluated. There are no data detailing the effects of
single exposures by any route. The most frequently reported effects of
repeated exposure to azodicarbonamide are respiratory symptoms as well
as, to a lesser extent, skin sensitization reactions. There are no
reports relating to the potential for azodicarbonamide to produce
other systemic adverse effects. The potential genotoxic, carcinogenic,
and reproductive effects of azodicarbonamide in exposed humans have
not been studied.
9.1 Case reports
A number of reports have been published of individual
azodicarbonamide workers alleging asthma induced by exposure to
azodicarbonamide. The strongest evidence comes from a study of two
individuals (one atopic and one non-atopic) who worked at the same
plastics factory for about 4 years (Malo et al., 1985; Pineau et al.,
1985). Both were intermittently exposed (1-2 weeks' duration, 3-4
times per year) to azodicarbonamide at work. A few months after their
first encounter with azodicarbonamide, both developed symptoms
described as "eye/nose irritation" at work, followed a few hours later
by nocturnal asthmatic symptoms. After a 1-month period free from
exposure, both subjects underwent lung provocation studies. Baseline
values for forced expiratory volume in 1 s (FEV1), forced vital
capacity (FVC), and the concentration of histamine required to produce
a 20% drop in FEV1 (PC20H) were obtained by spirometry. Both
subjects performed a control challenge using lactose and then a 50:50
mixture of lactose and azodicarbonamide for 15 s on the next day. On
both days, lung function was monitored to follow the time course of
any response. It was reported that the trial was not carried out
blind.
No effects on lung function were observed following challenge
with lactose alone. After the azodicarbonamide challenge, however, the
atopic individual developed a late respiratory response starting 3 h
after challenge and reaching a maximum 24% drop in FEV1 6 h after
challenge. A drop in PC20H was also reported, demonstrating increased
airway hyperreactivity, and this parameter did not return to the
baseline value until 6 weeks after challenge. The non-atopic
individual showed a dual response to azodicarbonamide. Peak reductions
in FEV1 of greater than 20% were recorded 30 min and 5-6 h after
exposure. No significant reduction in PC20H was reported for the
second individual. A control atopic subject with underlying asthma who
worked in the same industry but did not experience work-related
respiratory effects was also tested. His baseline PC20H was similar
to that of the atopic subject, but no change in lung function was
observed following a 15-min exposure to azodicarbonamide under similar
conditions (as this subject had less reactive airways, a much longer
exposure duration was utilized). Owing to the insolubility of
azodicarbonamide, skin prick tests were not performed.
Six other cases have been reported in the literature, but in each
case the evidence that azodicarbonamide was the cause of the
respiratory symptoms is less strong. In some cases, there had been
previous exposure in industries associated with potential exposure to
other asthmagenic substances; for others, the bronchial challenge test
was either poorly conducted or not conducted at all (Valentino &
Comai, 1985; Alt & Diller, 1988; Normand et al., 1989).
Three case reports on skin sensitization have been published. In
the most recently reported investigation, a male textile worker
exposed to azodicarbonamide in foam ear-plugs was patch tested to
discover the cause of a recurrent dermatitis of the ear (Nava et al.,
1983; Bonsall, 1984; Yates & Dixon, 1988). No response was elicited
with a number of standard (International Contact Dermatitis Research
Group standard series) allergens. However, the individual gave a
strong positive reaction to the ear-plugs at 48 and 96 h and also to
azodicarbonamide (a component of the ear-plugs) at a concentration of
1 and 5% in petrolatum but not at 0.1% in petrolatum. Ten control
subjects patch tested with 1 and 5% azodicarbonamide in petrolatum did
not respond, and the individual reported no further symptoms upon
discarding the ear-plugs.
9.2 Epidemiological studies
Workplace health surveys have also been carried out where
azodicarbonamide was either manufactured or used to investigate the
presence of respiratory symptoms in azodicarbonamide workers.
A prevalence study of occupational asthma was carried out among a
group of 151 workers at a factory manufacturing azodicarbonamide
(Slovak, 1981). Diagnosis of asthma was made on the basis of an
administered questionnaire and a detailed occupational history taken
by the author. The population was divided into three groups: those
classified as potentially sensitized, on the basis of questionnaire
results; those with daily exposure but without symptoms; and those
with no exposure to azodicarbonamide or any other known sensitizer. On
one day, pre- and post-shift spirometry was performed, and FEV1, FVC,
and the FEV1/FVC ratio were determined. Skin prick tests were also
attempted using both common allergens to determine atopic status and
azodicarbonamide at concentrations of 0.1, 1, and 5% in dimethyl
sulfoxide. Concurrent personal sampling measurements were made to
determine the levels of airborne azodicarbonamide to which individuals
were exposed.
Personal sampling indicated that, at the time of the
investigation, airborne concentrations of azodicarbonamide ranged
between 2 and 5 mg/m3, as 8-h time-weighted averages. From the
questionnaires and occupational histories, 28 individuals (18.5%) were
diagnosed as having asthma apparently related to azodicarbonamide
exposure. Twelve further cases of occupational asthma were identified
from company records of past employees. Skin prick tests with
azodicarbonamide could not be adequately performed owing to the
insolubility of the substance.
Of the 28 current workers classified as sensitized, over half
developed symptoms within 3 months of first exposure and 21/28 (75%)
within 1 year. Symptoms and signs included shortness of breath, chest
tightness, wheezing, cough, rhinitis, conjunctivitis, and rash.
Reactions were of an immediate type for 6/28 (21%) individuals, late
onset for 16/28 (57%), and dual onset for 6/28 workers. Of those
showing a dual response, all but one had initially shown a late onset
pattern. A total of 13/28 (46%) workers reported worsening of symptoms
with continuing exposure to azodicarbonamide and a shortening of the
time between returning to work and reappearance of symptoms. Eight out
of 13 workers exposed to azodicarbonamide for more than 3 months after
development of symptoms also developed sensitivity to previously
well-tolerated irritants (e.g., sulfur dioxide and tobacco smoke),
which persisted for over a month after removal from exposure to
azodicarbonamide. In five individuals, this airway hyperreactivity
persisted for over 3 years. There were no changes in FEV1 or FVC over
the work shift in any group. In view of the latency in development of
effects, late or dual onset of symptoms in 12/28 (43%) symptomatic
workers, increase in sensitivity with repeated exposure, and the
persisting lung hyperreactivity in workers with prolonged exposure
after developing symptoms, it seems likely that these individuals had
become sensitized to azodicarbonamide.
Ahrenholz & Anderson (1985) and Whitehead et al. (1987) conducted
detailed investigations of the workforce at a plastics factory
employing about 325 workers. Lung function tests and interviews to
gather information on occupational history, smoking habits, past
illnesses, and respiratory, nasal, eye, and skin irritation, including
the time course of any symptoms, were carried out with a large
percentage of the workforce. There were no clear differences in the
results of lung function studies between those exposed to
azodicarbonamide and non-exposed individuals. However, responses to
the questionnaire revealed a significant association between symptoms
of irritation, cough, wheezing, shortness of breath, and headache and
present or previous employment as an injection mould operator. There
was also a slight but not statistically significant increase in the
reporting of skin rash among those with current or previous work in
the injection moulding department. The prevalence of all the above
symptoms was reduced among those whose employment in this department
was limited to the period before azodicarbonamide was introduced or
after a change in the process significantly reduced the use of
azodicarbonamide at the plant.
Personal sampling showed that concentrations of airborne
azodicarbonamide ranged from below the limit of detection (0.001
mg/m3) to 0.32 mg/m3 (median 0.006 mg/m3; geometric mean 0.004
mg/m3) averaged over the full shift. The highest concentration of
azodicarbonamide recorded (for an injection mould operator) was 0.01
mg/m3. Toluene, styrene, phenols, and triphenyl phosphate were also
detected at concentrations at or below the odour threshold for each
substance.
Other personal sampling data for a group of 17 individuals
revealed levels of azodicarbonamide ranging from traces to 0.8 mg/m3
(median 0.03 mg/m3; geometric mean 0.02 mg/m3) averaged over the
full shift. The second highest value recorded was 0.4 mg/m3, and the
next highest, 0.06 mg/m3. A moderate although statistically
significant reduction in FEV1 (mean reduction of 64 ml) and FVC (mean
reduction of 77 ml) occurred following shifts in which workers were
exposed to azodicarbonamide. Coughing at work, wheeze, and chest
tightness were also reported, and symptoms were apparently worse
during the week than on Sunday.
A detailed investigation of the workforce at a plant making floor
coverings was conducted after nosebleeds, mucous membrane irritation,
and skin rashes were reported in workers handling azodicarbonamide
(Ahrenholz et al., 1985). Two surveys were carried out. The initial
survey revealed, in decreasing order of prevalence, symptoms of eye
irritation, nose irritation, cough, nocturnal cough, shortness of
breath, wheeze, and chest tightness. The more extensive follow-up
survey was conducted 6 weeks later. Pre- and post-shift auscultation,
lung function tests, and respiratory symptoms (recorded by
questionnaire) were recorded. Blood samples were also taken for
immunological investigations.
Responses to the questionnaire revealed 15/30 regularly exposed
workers experiencing occupationally related lower respiratory tract
symptoms (cough, wheeze, and shortness of breath) compared with 1/16
never-exposed workers. No significant differences in pre- and
post-shift FEV1 and FVC measurements were found. For those workers
apparently not exposed to azodicarbonamide or exposed indirectly
(working in the vicinity but not directly handling azodicarbonamide),
levels (8-h time-weighted average) ranged from <0.001 to 0.1 mg/m3.
However, during weighing and charging operations, peaks of between
0.15 and 12 mg/m3 (median 2.7 mg/m3) were measured for individuals
directly involved.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Azodicarbonamide has been tested under OECD Guideline protocols
in one species of fish and in the water flea ( Daphnia magna);
results are in an unpublished industry report, which has not been
peer-reviewed (see Table 1). A second study, not conducted to a
protocol or under Good Laboratory Practices, showed no effect of an
azodicarbonamide solution analysed at 8 mg/litre on the zebra fish
( Brachydanio rerio).1 There was no effect on oxygen consumption of
sewage sludge organisms exposed over 3 h to azodicarbonamide at
>10 000 mg/litre (this is substantially greater than the solubility
of the compound, and no information is available on how this
concentration was achieved).2 Overall, it is not possible to draw
firm conclusions from these studies.
1 IUCLID (European Union database), version dated 7 February 1996.
2 Bayer, unpublished value (1988) presented in IUCLID (European
Union database), version dated 7 February 1996; no details
available.
Table 1: Acute studies on toxicity to aquatic organisms.
Organism Protocol End-point Concentration Reference
(mg/litre)
Fathead minnow OECD 203 96-h NOEC >50 Uniroyal (1992)
(Pimephales promelas)
Water flea OECD 202 48-h NOEC 4.8 (measured) Uniroyal (1992)
(Daphnia magna) ~16 (nominal)
48-h EC50 11 (measured)
immobilization 29 (nominal)
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Some of the available toxicological studies have been conducted
using biurea rather than azodicarbonamide. However, azodicarbonamide
is readily converted to biurea in vivo. Hence, similar toxicological
properties would be expected.
Azodicarbonamide is of low acute toxicity by all routes, and,
although the animal studies are of uncertain quality, solid
azodicarbonamide would not be regarded as a skin or eye irritant. With
respect to respiratory tract irritation, no changes of toxicological
significance were seen in guinea-pigs exposed to azodicarbonamide
aerosol at concentrations up to 97 mg/m3 for 1 h.
No conclusions could be drawn regarding skin sensitization
potential from the available, poor-quality animal studies. Although
there are currently no validated animal studies investigating
asthmagenic potential, there was no evidence of pulmonary irritation
or asthmatic-type reactions in guinea-pigs exposed to up to 200 mg
azodicarbonamide aerosol/m3 for 6 h/day, 5 days/week, for 4 weeks.
Similarly, there were no changes of toxicological significance
seen among rats or mice exposed by inhalation to up to 200 mg
azodicarbonamide aerosol/m3 for 6 h/day, 5 days/week, for up to 13
weeks.
For repeated-dose studies using the oral route, data were
inconsistent. In 2-year studies in which rats received up to 450 mg
biurea/kg body weight per day, there were no adverse effects seen.
Unpublished information suggests that no adverse effects were seen in
male mice exposed to up to 1250 mg azodicarbonamide/kg body weight per
day and in female mice exposed to up to 2500 mg/kg body weight per day
for 13 weeks. However, shorter-term studies (2 weeks, also
unpublished) indicated histological lesions in the kidneys of rats and
mice of both sexes at 1250 mg/kg body weight per day or more. A
13-week study in rats indicated kidney lesions in males at 2500 mg/kg
body weight per day, with no adverse effects observed at the next
lowest exposure level, 500 mg/kg body weight per day. For female rats,
kidney lesions were observed at 5000 mg/kg body weight per day, and no
adverse effects were observed at 1000 mg/kg body weight per day. There
were no data in relation to repeated dermal exposure.
Azodicarbonamide has been identified as a mutagen in bacterial
systems, but it was not mutagenic in mammalian cell in vitro test
systems or in two mammalian assays in vivo using bone marrow. It is
therefore unlikely that the mutagenic properties displayed by
azodicarbonamide in bacterial systems will be expressed in vivo.
However, it is considered that a confirmatory in vivo study in a
second tissue is desirable.
There are no adequate data available relating to carcinogenic,
reproductive, or developmental effects; hence, it is not possible to
evaluate the risk to human health for these end-points.
Several bronchial challenge studies have been reported, but only
one provides reasonable evidence that the work-related asthmatic
symptoms were due specifically to azodicarbonamide. This report is
considered to show an asthmatic response and not an irritant response
to azodicarbonamide on challenge. Animal studies suggest that airborne
concentrations of up to 200 mg/m3 can be tolerated with little or no
pulmonary irritation, and it is unlikely that the levels used in the
bronchial challenge tests approached those used in animal studies. The
delay in response to azodicarbonamide challenge, the magnitude of
reduction in FEV1 accompanied by an increase in airway
hyperreactivity in one individual, and the fact that a control
individual with mildly hyperreactive airways did not respond to a much
more prolonged exposure under similar challenge conditions provide
further evidence for asthmagenicity. Further evidence of a link
between azodicarbonamide and respiratory problems is provided by the
results of workplace health evaluations. Although criticisms can be
levelled at individual studies, weight of evidence suggests that
azodicarbonamide can induce asthma in a significant proportion of
exposed people.
There are some case reports of individuals with skin reactions to
topically applied azodicarbonamide. For some of these, results are
questionable. However, in workplace health surveys, the incidence of
skin rash was found to be greater among workers regularly exposed to
azodicarbonamide. Although no firm conclusions could be drawn from the
poorly reported animal study, clear evidence of skin sensitization to
azodicarbonamide in one individual and supporting evidence of skin
problems from workplace health surveys lead to the conclusion that
azodicarbonamide should be considered as a human skin sensitizer.
In conclusion, the key toxic effect of azodicarbonamide in humans
is asthmagenicity. Evidence of this effect has been found from
bronchial challenge studies and workplace health evaluations. From the
information available, azodicarbonamide is considered to have a low
potential for irritancy; thus, it is considered that the respiratory
symptoms observed in these studies are most likely due to an
asthmatic-type response rather than respiratory tract irritancy. There
is no clear information on the levels that may have induced or
provoked the state of asthma.
There is also information to indicate that azodicarbonamide can
cause skin sensitization in humans.
11.1.2 Criteria for setting guidance values for azodicarbonamide
The main cause for concern relates to the risk of developing
occupational asthma. There is no information available relating to
dose-response relationships or levels associated with the induction of
a hypersensitive state or provocation of an asthmatic response. Hence,
it is not possible to reliably quantify the risk of developing
occupational asthma.
11.1.3 Sample risk characterization
Using data obtained from a factory in the United Kingdom and
published exposure data as an example (section 6.2), levels of
airborne azodicarbonamide measured over periods of <70 min to 4 h of
up to 12 mg/m3 have been observed. Short-term peak exposures could be
higher than this level.
In the United Kingdom occupational setting, it is recommended
that a maximum exposure limit (MEL) be assigned to substances for
which it has not been possible to identify a level of exposure that is
without adverse effects on health. This is a non-health-based
standard, and, in determining the most appropriate level for a MEL,
consideration is taken of the level of control that it is reasonably
practicable for industry to achieve. The MEL of 1 mg/m3
(8-h time-weighted average) was based on a level of control that was
deemed by tripartite agreement to be reasonably practicable under
workplace conditions within the United Kingdom. There is also a
continuing remit for industry to keep on reducing exposure levels as
advances in technology make this possible. For substances that are
asthmagens, it is also advisable to have a short-term exposure limit
(STEL) to restrict peak exposures, as they may have a role in the
induction and triggering of asthmatic phenomena. In the absence of any
specific data that might advise adequately on the numerical value of
the STEL, 3 mg/m3 (15-min reference period) has been established.
As azodicarbonamide is a skin sensitizer, where skin contact can
occur, there may be a risk of developing allergic dermatitis if
suitable personal protective equipment is not used.
There is evidence to suggest that azodicarbonamide has been added
to consumer products such as bread and beer. The limited toxicology
database and lack of exposure data make it difficult to adequately
assess the risk to humans potentially exposed; hence, there is a need
for further information.
11.2 Evaluation of environmental effects
Lack of information on release to the environment precludes a
quantitative risk assessment.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations by international bodies were not identified.
Information on international hazard classification and labelling is
included in the International Chemical Safety Card reproduced in this
document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0380) reproduced in this
document.
13.1 Human health hazards
Azodicarbonamide is of low acute toxicity, but repeated or
prolonged contact may cause asthma and skin sensitization.
13.2 Health surveillance advice
Physicians involved in worker health surveillance programmes
should be aware of the potential of azodicarbonamide as a human
asthmagen.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
AZODICARBONAMIDE ICSC:0380
October 1997
CAS# 