
FAO Nutrition Meetings
Report Series No. 40A,B,C
WHO/Food Add./67.29
TOXICOLOGICAL EVALUATION OF SOME
ANTIMICROBIALS, ANTIOXIDANTS, EMULSIFIERS,
STABILIZERS, FLOUR-TREATMENT AGENTS, ACIDS AND BASES
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met at Rome,
13-20 December, 19651 Geneva, 11-18 October, 19662
1 Ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1966 No. 40;
Wld Hlth Org. techn. Rep. Ser., 1966, 339
2 Tenth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1967, in press;
Food and Agriculture Organization of the United Nations
World Health Organization
1967
AZODICARBONAMIDE
Synonym Azobisformamide
Chemical name Azodicarbonamide
Empirical formula C2H4O2N4
Structural formula
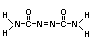 Molecular weight 116.10
Definition Azodicarbonamide, after drying, contains
not less than 98.6 per cent
C2H4O2N4.
Description Azodicarbonamide occurs as a yellow to
orange red, odourless, crystalline
powder.
Uses As a strengthening agent for flour in a
mixture usually containing 1 part by
weight of azodicarbonamide with 9 parts
by weight of a mixture of starch and
tricalcium phosphate.
Biological Data
Biochemical aspects
When azodicarbonamide reacts with flour, it behaves as a hydrogen
acceptor and it is rapidly and completely converted into biurea, which
is stable under baking conditions. Reaction between azodicarbonamide
and flour only occurs on wetting. Forty-five minutes after treatment
of a flour with 8.25 ppm of azodicarbonamide, less than 0.1 ppm of
azodicarbonamide could be detected in the dough. When 14C-labelled
azodicarbonamide was used for breadmaking, the activity remained in
the bread and there was no liberation of labelled carbon dioxide.
Biurea labelled with 14C was unaffected by pepsin or trypsin
in vitro. When administered orally to rats in doses of 33 mg,
labelled biurea was recovered quantitatively within 120 hours from
faeces and urine. Absorption from the intestinal tract was about 20
per cent. No radioactivity was detectable in the blood after 24 hours;
no tissues were found to accumulate biurea (Joiner et al., 1963; Oser
et al., 1965).
Special studies
Study of the amino-acid pattern of gluten obtained from flours
that were either untreated or treated with azodicarbonamide at 11 ppm
or 110 ppm revealed no significant changes (Oser et al., 1965).
No alteration in the amounts of thiamine, riboflavin, or niacin
present in natural or enriched flour or bread made from such flours,
was observed after treatment with 11 ppm of azodicarbonamide (Joiner
et al., 1963; Oser et al., 1965).
Acute toxicity
Mouse. Single doses of 0, 1, 2, 4 and 6 g/kg body-weight were
administered orally to groups of 5 mice. No adverse effects were
observed (Joiner, 1965).
Single doses of 0, 250 and 500 mg/kg body-weight were
administered intraperitoneally to groups of 5 mice. Moderate
depression and some diarrhoea, but no deaths, occurred. Single doses
of 0, 750, 1000 and 1250 mg/kg body-weight were administered to groups
of 5 mice intraperitoneally. Diarrhoea, cyanosis, dyspnoea and
depression were observed, with 1, 4, 4 deaths in the three groups at
48 hours respectively (Oser, 1965).
Rat. Single doses of O, 1, 3 and 6 g/kg body-weight were
administered to groups of 5 rats by intragastric intubation. No ill
effects were observed (Joiner, 1965).
Short-term studies
Rat. Doses of 0, l00 and 1000 mg/kg body-weight were
administered daily by intragastric intubation to groups of 10 rats for
a period of 8 weeks. No adverse effect was observed (Joiner, 1965).
Dog. A daily dose, of 60 mg/kg body-weight was administered in
the diet for 8 weeks to a dog, without demonstrable ill effect on
growth rate, haematology and general health (Joiner, 1965).
Rabbit. Intradermal and patch tests for skin sensitivity to
azodicarbonamide were carried out on groups of 10 rabbits.
Azodicarbonamide showed no activity as a primary skin irritant. No
other effect was observed (United States Testing, 1952).
Man. Despite large-scale industrial and bakery use of
azodicarbonamide no dermatological problems associated with such use,
have been reported.
Studies on Biurea
Short-term studies
Rat. Two test groups of 25 male and 25 female rats and a
control group of 10 male and 10 female rats were fed diets to which
was added 0, 5 or 10 per cent. biurea, for a period of one year. The
rate of weight gain of the male rats was slightly depressed during the
first 12 weeks in the test groups, but recovered later. The
appearance, behaviour, food intake and utilization, morbidity and
mortality in the test and control groups were not significantly
different. Haematological studies and blood biochemistry showed no
abnormalities. No abnormalities were seen at autopsy, relative organ
weights were within normal limits; histological examination of major
organs showed no difference between test and control groups (Oser et
al., 1965).
Dog. Four mongrel dogs (2 male and 2 female) were fed a diet
containing 5 per cent. (2 dogs) and 10 per cent. (2 dogs) of biurea,
respectively. Some difficulty was experienced in persuading the
animals to eat this diet. The 10 per cent. group thrived poorly and
died at 20 weeks; the 5 per cent. group survived and was sacrificed at
11 months. The only pathological change noted was the presence of
massive biurea calculi in the kidneys. No significant changes were
seen in haematological studies, nor in the gross or microscopical
study of the main organs (Oser et al., 1965).
Groups of 4 mongrel dogs were fed diets containing 77 per cent.
of bread to which was added 0, 750, 2370 or 7500 ppm of biurea, for 2
years. Weight gain and food intake showed no significant difference
between control and test groups. Detailed study of blood formation,
methaemoglobin levels and histopathological examination of major
organs showed no differences between control and test groups, except
for a greyish brown colour of the liver in two dogs, renal haemorrhage
in one dog receiving 750 ppm of biurea, and dilated renal pelves in
one dog receiving the highest dose (7500 ppm) of biurea (Oser et al.,
1965).
Long-term studies
Rat. Groups of 25 male and 25 female weanling rats were fed
diets containing 83 per cent. of bread to which was added 0, 750, 2370
or 7500 ppm of biurea, respectively, corresponding to biurea levels in
the diet equivalent to 0, 311, 983, or 3110 ppm. The first generation
was studied for a life span (two years); 10 males and 10 females were
selected at random from the F1 generation and kept on the diet and
observed for 28 weeks; the F2 generation was studied similarly and
the F3 generation for 14 weeks.
The appearance, behaviour, rate of weight gain and food intake in
each group of all generations showed no significant differences.
Reproduction and lactation were normal. Morbidity and mortality were
similar in all groups and showed no dose-related variations.
Haematological studies (including methaemoglobin levels) and gross and
microscopic examination of all the main organs revealed no significant
difference between test and control groups, except for one enlarged
kidney in a female rat receiving a high dose of biurea. A detailed
assessment of tumour incidence was made and nothing significant was
found (Oser et al., 1965).
Studies on flour overtreated with azodicarbonamide, and bread
baked from it
Short-term studies
Rat.Two groups of 10 rats were fed daily for 8 weeks by gastric
tube with untreated flour and with flour treated with azodicarbonamide
(120 ppm). No adverse effect was observed on the rate of weight gain,
blood formation, or the gross or microscopical appearance of the main
organs (Oser et al., 1965).
Dog. A group of 4 mongrel dogs was fed bread made from flour
treated with azodicarbonamide (100 ppm). The animals were observed for
two years. No abnormalities were found in weight gain, food intake,
appearance, behaviour, and morbidity. At autopsy no macroscopic or
microscopic change of any significance was found in the main organs
(Oser et al., 1965).
Long-term studies
Rat. Flour treated with azodicarbonamide (100 ppm) was made
into bread and this was fed to 25 male and 25 female weanling rats;
bread made from the untreated flour was fed to a control group of 25
male and 25 female rats. The animals were observed over the life-span
(2 years). Appearance, -behaviour, weight gain, food intake,
reproduction and lactation, morbidity and mortality rate were not
significantly different. Detailed studies on the blood and main
organs revealed no macroscopic-or microscopic abnormalities. Tumour
incidence was examined in detail and no alteration in the numbers or
patterns of tumours found was observed (Oser et al., 1965)
Comments
Azodicarbonamide has been extensively studied and the theoretical
point with regard to the possible effect of unconverted
azodicarbonamide was covered by experiments using overtreated flour or
bread made from it. The evidence strongly supports the view that
azodicarbonamide is rapidly and completely converted to biurea on
wetting and that this substance is stable in bread. Biurea itself is
metabolically inert, has low toxicity and does not present any
carcinogenic hazard. Azodicarbonamide has been adequately studied in
several species and is similarly free from carcinogenic hazard.
Long-term studies in mice are in progress (Frazer, 1966).
Evaluation
Acceptable level of treatment
Flour: 0-45 ppm
REFERENCES
Frazer, A. C. (1966) University of Birmingham work in progress
Joiner, R. R., Vidal, F. D. & Markes, H. C. (1963) Cereal Chemistry,
40, 539
Joiner, R. R., Unpublished report submitted by Wallace & Tiernan, 1965
Oser, B. L., Oser, M. & Morgareidge, K. (1965) Toxicol. appl.
Pharmacol., 7, 445
United States Testing Company (1952) Unpublished report
Molecular weight 116.10
Definition Azodicarbonamide, after drying, contains
not less than 98.6 per cent
C2H4O2N4.
Description Azodicarbonamide occurs as a yellow to
orange red, odourless, crystalline
powder.
Uses As a strengthening agent for flour in a
mixture usually containing 1 part by
weight of azodicarbonamide with 9 parts
by weight of a mixture of starch and
tricalcium phosphate.
Biological Data
Biochemical aspects
When azodicarbonamide reacts with flour, it behaves as a hydrogen
acceptor and it is rapidly and completely converted into biurea, which
is stable under baking conditions. Reaction between azodicarbonamide
and flour only occurs on wetting. Forty-five minutes after treatment
of a flour with 8.25 ppm of azodicarbonamide, less than 0.1 ppm of
azodicarbonamide could be detected in the dough. When 14C-labelled
azodicarbonamide was used for breadmaking, the activity remained in
the bread and there was no liberation of labelled carbon dioxide.
Biurea labelled with 14C was unaffected by pepsin or trypsin
in vitro. When administered orally to rats in doses of 33 mg,
labelled biurea was recovered quantitatively within 120 hours from
faeces and urine. Absorption from the intestinal tract was about 20
per cent. No radioactivity was detectable in the blood after 24 hours;
no tissues were found to accumulate biurea (Joiner et al., 1963; Oser
et al., 1965).
Special studies
Study of the amino-acid pattern of gluten obtained from flours
that were either untreated or treated with azodicarbonamide at 11 ppm
or 110 ppm revealed no significant changes (Oser et al., 1965).
No alteration in the amounts of thiamine, riboflavin, or niacin
present in natural or enriched flour or bread made from such flours,
was observed after treatment with 11 ppm of azodicarbonamide (Joiner
et al., 1963; Oser et al., 1965).
Acute toxicity
Mouse. Single doses of 0, 1, 2, 4 and 6 g/kg body-weight were
administered orally to groups of 5 mice. No adverse effects were
observed (Joiner, 1965).
Single doses of 0, 250 and 500 mg/kg body-weight were
administered intraperitoneally to groups of 5 mice. Moderate
depression and some diarrhoea, but no deaths, occurred. Single doses
of 0, 750, 1000 and 1250 mg/kg body-weight were administered to groups
of 5 mice intraperitoneally. Diarrhoea, cyanosis, dyspnoea and
depression were observed, with 1, 4, 4 deaths in the three groups at
48 hours respectively (Oser, 1965).
Rat. Single doses of O, 1, 3 and 6 g/kg body-weight were
administered to groups of 5 rats by intragastric intubation. No ill
effects were observed (Joiner, 1965).
Short-term studies
Rat. Doses of 0, l00 and 1000 mg/kg body-weight were
administered daily by intragastric intubation to groups of 10 rats for
a period of 8 weeks. No adverse effect was observed (Joiner, 1965).
Dog. A daily dose, of 60 mg/kg body-weight was administered in
the diet for 8 weeks to a dog, without demonstrable ill effect on
growth rate, haematology and general health (Joiner, 1965).
Rabbit. Intradermal and patch tests for skin sensitivity to
azodicarbonamide were carried out on groups of 10 rabbits.
Azodicarbonamide showed no activity as a primary skin irritant. No
other effect was observed (United States Testing, 1952).
Man. Despite large-scale industrial and bakery use of
azodicarbonamide no dermatological problems associated with such use,
have been reported.
Studies on Biurea
Short-term studies
Rat. Two test groups of 25 male and 25 female rats and a
control group of 10 male and 10 female rats were fed diets to which
was added 0, 5 or 10 per cent. biurea, for a period of one year. The
rate of weight gain of the male rats was slightly depressed during the
first 12 weeks in the test groups, but recovered later. The
appearance, behaviour, food intake and utilization, morbidity and
mortality in the test and control groups were not significantly
different. Haematological studies and blood biochemistry showed no
abnormalities. No abnormalities were seen at autopsy, relative organ
weights were within normal limits; histological examination of major
organs showed no difference between test and control groups (Oser et
al., 1965).
Dog. Four mongrel dogs (2 male and 2 female) were fed a diet
containing 5 per cent. (2 dogs) and 10 per cent. (2 dogs) of biurea,
respectively. Some difficulty was experienced in persuading the
animals to eat this diet. The 10 per cent. group thrived poorly and
died at 20 weeks; the 5 per cent. group survived and was sacrificed at
11 months. The only pathological change noted was the presence of
massive biurea calculi in the kidneys. No significant changes were
seen in haematological studies, nor in the gross or microscopical
study of the main organs (Oser et al., 1965).
Groups of 4 mongrel dogs were fed diets containing 77 per cent.
of bread to which was added 0, 750, 2370 or 7500 ppm of biurea, for 2
years. Weight gain and food intake showed no significant difference
between control and test groups. Detailed study of blood formation,
methaemoglobin levels and histopathological examination of major
organs showed no differences between control and test groups, except
for a greyish brown colour of the liver in two dogs, renal haemorrhage
in one dog receiving 750 ppm of biurea, and dilated renal pelves in
one dog receiving the highest dose (7500 ppm) of biurea (Oser et al.,
1965).
Long-term studies
Rat. Groups of 25 male and 25 female weanling rats were fed
diets containing 83 per cent. of bread to which was added 0, 750, 2370
or 7500 ppm of biurea, respectively, corresponding to biurea levels in
the diet equivalent to 0, 311, 983, or 3110 ppm. The first generation
was studied for a life span (two years); 10 males and 10 females were
selected at random from the F1 generation and kept on the diet and
observed for 28 weeks; the F2 generation was studied similarly and
the F3 generation for 14 weeks.
The appearance, behaviour, rate of weight gain and food intake in
each group of all generations showed no significant differences.
Reproduction and lactation were normal. Morbidity and mortality were
similar in all groups and showed no dose-related variations.
Haematological studies (including methaemoglobin levels) and gross and
microscopic examination of all the main organs revealed no significant
difference between test and control groups, except for one enlarged
kidney in a female rat receiving a high dose of biurea. A detailed
assessment of tumour incidence was made and nothing significant was
found (Oser et al., 1965).
Studies on flour overtreated with azodicarbonamide, and bread
baked from it
Short-term studies
Rat.Two groups of 10 rats were fed daily for 8 weeks by gastric
tube with untreated flour and with flour treated with azodicarbonamide
(120 ppm). No adverse effect was observed on the rate of weight gain,
blood formation, or the gross or microscopical appearance of the main
organs (Oser et al., 1965).
Dog. A group of 4 mongrel dogs was fed bread made from flour
treated with azodicarbonamide (100 ppm). The animals were observed for
two years. No abnormalities were found in weight gain, food intake,
appearance, behaviour, and morbidity. At autopsy no macroscopic or
microscopic change of any significance was found in the main organs
(Oser et al., 1965).
Long-term studies
Rat. Flour treated with azodicarbonamide (100 ppm) was made
into bread and this was fed to 25 male and 25 female weanling rats;
bread made from the untreated flour was fed to a control group of 25
male and 25 female rats. The animals were observed over the life-span
(2 years). Appearance, -behaviour, weight gain, food intake,
reproduction and lactation, morbidity and mortality rate were not
significantly different. Detailed studies on the blood and main
organs revealed no macroscopic-or microscopic abnormalities. Tumour
incidence was examined in detail and no alteration in the numbers or
patterns of tumours found was observed (Oser et al., 1965)
Comments
Azodicarbonamide has been extensively studied and the theoretical
point with regard to the possible effect of unconverted
azodicarbonamide was covered by experiments using overtreated flour or
bread made from it. The evidence strongly supports the view that
azodicarbonamide is rapidly and completely converted to biurea on
wetting and that this substance is stable in bread. Biurea itself is
metabolically inert, has low toxicity and does not present any
carcinogenic hazard. Azodicarbonamide has been adequately studied in
several species and is similarly free from carcinogenic hazard.
Long-term studies in mice are in progress (Frazer, 1966).
Evaluation
Acceptable level of treatment
Flour: 0-45 ppm
REFERENCES
Frazer, A. C. (1966) University of Birmingham work in progress
Joiner, R. R., Vidal, F. D. & Markes, H. C. (1963) Cereal Chemistry,
40, 539
Joiner, R. R., Unpublished report submitted by Wallace & Tiernan, 1965
Oser, B. L., Oser, M. & Morgareidge, K. (1965) Toxicol. appl.
Pharmacol., 7, 445
United States Testing Company (1952) Unpublished report
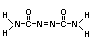 Molecular weight 116.10
Definition Azodicarbonamide, after drying, contains
not less than 98.6 per cent
C2H4O2N4.
Description Azodicarbonamide occurs as a yellow to
orange red, odourless, crystalline
powder.
Uses As a strengthening agent for flour in a
mixture usually containing 1 part by
weight of azodicarbonamide with 9 parts
by weight of a mixture of starch and
tricalcium phosphate.
Biological Data
Biochemical aspects
When azodicarbonamide reacts with flour, it behaves as a hydrogen
acceptor and it is rapidly and completely converted into biurea, which
is stable under baking conditions. Reaction between azodicarbonamide
and flour only occurs on wetting. Forty-five minutes after treatment
of a flour with 8.25 ppm of azodicarbonamide, less than 0.1 ppm of
azodicarbonamide could be detected in the dough. When 14C-labelled
azodicarbonamide was used for breadmaking, the activity remained in
the bread and there was no liberation of labelled carbon dioxide.
Biurea labelled with 14C was unaffected by pepsin or trypsin
in vitro. When administered orally to rats in doses of 33 mg,
labelled biurea was recovered quantitatively within 120 hours from
faeces and urine. Absorption from the intestinal tract was about 20
per cent. No radioactivity was detectable in the blood after 24 hours;
no tissues were found to accumulate biurea (Joiner et al., 1963; Oser
et al., 1965).
Special studies
Study of the amino-acid pattern of gluten obtained from flours
that were either untreated or treated with azodicarbonamide at 11 ppm
or 110 ppm revealed no significant changes (Oser et al., 1965).
No alteration in the amounts of thiamine, riboflavin, or niacin
present in natural or enriched flour or bread made from such flours,
was observed after treatment with 11 ppm of azodicarbonamide (Joiner
et al., 1963; Oser et al., 1965).
Acute toxicity
Mouse. Single doses of 0, 1, 2, 4 and 6 g/kg body-weight were
administered orally to groups of 5 mice. No adverse effects were
observed (Joiner, 1965).
Single doses of 0, 250 and 500 mg/kg body-weight were
administered intraperitoneally to groups of 5 mice. Moderate
depression and some diarrhoea, but no deaths, occurred. Single doses
of 0, 750, 1000 and 1250 mg/kg body-weight were administered to groups
of 5 mice intraperitoneally. Diarrhoea, cyanosis, dyspnoea and
depression were observed, with 1, 4, 4 deaths in the three groups at
48 hours respectively (Oser, 1965).
Rat. Single doses of O, 1, 3 and 6 g/kg body-weight were
administered to groups of 5 rats by intragastric intubation. No ill
effects were observed (Joiner, 1965).
Short-term studies
Rat. Doses of 0, l00 and 1000 mg/kg body-weight were
administered daily by intragastric intubation to groups of 10 rats for
a period of 8 weeks. No adverse effect was observed (Joiner, 1965).
Dog. A daily dose, of 60 mg/kg body-weight was administered in
the diet for 8 weeks to a dog, without demonstrable ill effect on
growth rate, haematology and general health (Joiner, 1965).
Rabbit. Intradermal and patch tests for skin sensitivity to
azodicarbonamide were carried out on groups of 10 rabbits.
Azodicarbonamide showed no activity as a primary skin irritant. No
other effect was observed (United States Testing, 1952).
Man. Despite large-scale industrial and bakery use of
azodicarbonamide no dermatological problems associated with such use,
have been reported.
Studies on Biurea
Short-term studies
Rat. Two test groups of 25 male and 25 female rats and a
control group of 10 male and 10 female rats were fed diets to which
was added 0, 5 or 10 per cent. biurea, for a period of one year. The
rate of weight gain of the male rats was slightly depressed during the
first 12 weeks in the test groups, but recovered later. The
appearance, behaviour, food intake and utilization, morbidity and
mortality in the test and control groups were not significantly
different. Haematological studies and blood biochemistry showed no
abnormalities. No abnormalities were seen at autopsy, relative organ
weights were within normal limits; histological examination of major
organs showed no difference between test and control groups (Oser et
al., 1965).
Dog. Four mongrel dogs (2 male and 2 female) were fed a diet
containing 5 per cent. (2 dogs) and 10 per cent. (2 dogs) of biurea,
respectively. Some difficulty was experienced in persuading the
animals to eat this diet. The 10 per cent. group thrived poorly and
died at 20 weeks; the 5 per cent. group survived and was sacrificed at
11 months. The only pathological change noted was the presence of
massive biurea calculi in the kidneys. No significant changes were
seen in haematological studies, nor in the gross or microscopical
study of the main organs (Oser et al., 1965).
Groups of 4 mongrel dogs were fed diets containing 77 per cent.
of bread to which was added 0, 750, 2370 or 7500 ppm of biurea, for 2
years. Weight gain and food intake showed no significant difference
between control and test groups. Detailed study of blood formation,
methaemoglobin levels and histopathological examination of major
organs showed no differences between control and test groups, except
for a greyish brown colour of the liver in two dogs, renal haemorrhage
in one dog receiving 750 ppm of biurea, and dilated renal pelves in
one dog receiving the highest dose (7500 ppm) of biurea (Oser et al.,
1965).
Long-term studies
Rat. Groups of 25 male and 25 female weanling rats were fed
diets containing 83 per cent. of bread to which was added 0, 750, 2370
or 7500 ppm of biurea, respectively, corresponding to biurea levels in
the diet equivalent to 0, 311, 983, or 3110 ppm. The first generation
was studied for a life span (two years); 10 males and 10 females were
selected at random from the F1 generation and kept on the diet and
observed for 28 weeks; the F2 generation was studied similarly and
the F3 generation for 14 weeks.
The appearance, behaviour, rate of weight gain and food intake in
each group of all generations showed no significant differences.
Reproduction and lactation were normal. Morbidity and mortality were
similar in all groups and showed no dose-related variations.
Haematological studies (including methaemoglobin levels) and gross and
microscopic examination of all the main organs revealed no significant
difference between test and control groups, except for one enlarged
kidney in a female rat receiving a high dose of biurea. A detailed
assessment of tumour incidence was made and nothing significant was
found (Oser et al., 1965).
Studies on flour overtreated with azodicarbonamide, and bread
baked from it
Short-term studies
Rat.Two groups of 10 rats were fed daily for 8 weeks by gastric
tube with untreated flour and with flour treated with azodicarbonamide
(120 ppm). No adverse effect was observed on the rate of weight gain,
blood formation, or the gross or microscopical appearance of the main
organs (Oser et al., 1965).
Dog. A group of 4 mongrel dogs was fed bread made from flour
treated with azodicarbonamide (100 ppm). The animals were observed for
two years. No abnormalities were found in weight gain, food intake,
appearance, behaviour, and morbidity. At autopsy no macroscopic or
microscopic change of any significance was found in the main organs
(Oser et al., 1965).
Long-term studies
Rat. Flour treated with azodicarbonamide (100 ppm) was made
into bread and this was fed to 25 male and 25 female weanling rats;
bread made from the untreated flour was fed to a control group of 25
male and 25 female rats. The animals were observed over the life-span
(2 years). Appearance, -behaviour, weight gain, food intake,
reproduction and lactation, morbidity and mortality rate were not
significantly different. Detailed studies on the blood and main
organs revealed no macroscopic-or microscopic abnormalities. Tumour
incidence was examined in detail and no alteration in the numbers or
patterns of tumours found was observed (Oser et al., 1965)
Comments
Azodicarbonamide has been extensively studied and the theoretical
point with regard to the possible effect of unconverted
azodicarbonamide was covered by experiments using overtreated flour or
bread made from it. The evidence strongly supports the view that
azodicarbonamide is rapidly and completely converted to biurea on
wetting and that this substance is stable in bread. Biurea itself is
metabolically inert, has low toxicity and does not present any
carcinogenic hazard. Azodicarbonamide has been adequately studied in
several species and is similarly free from carcinogenic hazard.
Long-term studies in mice are in progress (Frazer, 1966).
Evaluation
Acceptable level of treatment
Flour: 0-45 ppm
REFERENCES
Frazer, A. C. (1966) University of Birmingham work in progress
Joiner, R. R., Vidal, F. D. & Markes, H. C. (1963) Cereal Chemistry,
40, 539
Joiner, R. R., Unpublished report submitted by Wallace & Tiernan, 1965
Oser, B. L., Oser, M. & Morgareidge, K. (1965) Toxicol. appl.
Pharmacol., 7, 445
United States Testing Company (1952) Unpublished report
Molecular weight 116.10
Definition Azodicarbonamide, after drying, contains
not less than 98.6 per cent
C2H4O2N4.
Description Azodicarbonamide occurs as a yellow to
orange red, odourless, crystalline
powder.
Uses As a strengthening agent for flour in a
mixture usually containing 1 part by
weight of azodicarbonamide with 9 parts
by weight of a mixture of starch and
tricalcium phosphate.
Biological Data
Biochemical aspects
When azodicarbonamide reacts with flour, it behaves as a hydrogen
acceptor and it is rapidly and completely converted into biurea, which
is stable under baking conditions. Reaction between azodicarbonamide
and flour only occurs on wetting. Forty-five minutes after treatment
of a flour with 8.25 ppm of azodicarbonamide, less than 0.1 ppm of
azodicarbonamide could be detected in the dough. When 14C-labelled
azodicarbonamide was used for breadmaking, the activity remained in
the bread and there was no liberation of labelled carbon dioxide.
Biurea labelled with 14C was unaffected by pepsin or trypsin
in vitro. When administered orally to rats in doses of 33 mg,
labelled biurea was recovered quantitatively within 120 hours from
faeces and urine. Absorption from the intestinal tract was about 20
per cent. No radioactivity was detectable in the blood after 24 hours;
no tissues were found to accumulate biurea (Joiner et al., 1963; Oser
et al., 1965).
Special studies
Study of the amino-acid pattern of gluten obtained from flours
that were either untreated or treated with azodicarbonamide at 11 ppm
or 110 ppm revealed no significant changes (Oser et al., 1965).
No alteration in the amounts of thiamine, riboflavin, or niacin
present in natural or enriched flour or bread made from such flours,
was observed after treatment with 11 ppm of azodicarbonamide (Joiner
et al., 1963; Oser et al., 1965).
Acute toxicity
Mouse. Single doses of 0, 1, 2, 4 and 6 g/kg body-weight were
administered orally to groups of 5 mice. No adverse effects were
observed (Joiner, 1965).
Single doses of 0, 250 and 500 mg/kg body-weight were
administered intraperitoneally to groups of 5 mice. Moderate
depression and some diarrhoea, but no deaths, occurred. Single doses
of 0, 750, 1000 and 1250 mg/kg body-weight were administered to groups
of 5 mice intraperitoneally. Diarrhoea, cyanosis, dyspnoea and
depression were observed, with 1, 4, 4 deaths in the three groups at
48 hours respectively (Oser, 1965).
Rat. Single doses of O, 1, 3 and 6 g/kg body-weight were
administered to groups of 5 rats by intragastric intubation. No ill
effects were observed (Joiner, 1965).
Short-term studies
Rat. Doses of 0, l00 and 1000 mg/kg body-weight were
administered daily by intragastric intubation to groups of 10 rats for
a period of 8 weeks. No adverse effect was observed (Joiner, 1965).
Dog. A daily dose, of 60 mg/kg body-weight was administered in
the diet for 8 weeks to a dog, without demonstrable ill effect on
growth rate, haematology and general health (Joiner, 1965).
Rabbit. Intradermal and patch tests for skin sensitivity to
azodicarbonamide were carried out on groups of 10 rabbits.
Azodicarbonamide showed no activity as a primary skin irritant. No
other effect was observed (United States Testing, 1952).
Man. Despite large-scale industrial and bakery use of
azodicarbonamide no dermatological problems associated with such use,
have been reported.
Studies on Biurea
Short-term studies
Rat. Two test groups of 25 male and 25 female rats and a
control group of 10 male and 10 female rats were fed diets to which
was added 0, 5 or 10 per cent. biurea, for a period of one year. The
rate of weight gain of the male rats was slightly depressed during the
first 12 weeks in the test groups, but recovered later. The
appearance, behaviour, food intake and utilization, morbidity and
mortality in the test and control groups were not significantly
different. Haematological studies and blood biochemistry showed no
abnormalities. No abnormalities were seen at autopsy, relative organ
weights were within normal limits; histological examination of major
organs showed no difference between test and control groups (Oser et
al., 1965).
Dog. Four mongrel dogs (2 male and 2 female) were fed a diet
containing 5 per cent. (2 dogs) and 10 per cent. (2 dogs) of biurea,
respectively. Some difficulty was experienced in persuading the
animals to eat this diet. The 10 per cent. group thrived poorly and
died at 20 weeks; the 5 per cent. group survived and was sacrificed at
11 months. The only pathological change noted was the presence of
massive biurea calculi in the kidneys. No significant changes were
seen in haematological studies, nor in the gross or microscopical
study of the main organs (Oser et al., 1965).
Groups of 4 mongrel dogs were fed diets containing 77 per cent.
of bread to which was added 0, 750, 2370 or 7500 ppm of biurea, for 2
years. Weight gain and food intake showed no significant difference
between control and test groups. Detailed study of blood formation,
methaemoglobin levels and histopathological examination of major
organs showed no differences between control and test groups, except
for a greyish brown colour of the liver in two dogs, renal haemorrhage
in one dog receiving 750 ppm of biurea, and dilated renal pelves in
one dog receiving the highest dose (7500 ppm) of biurea (Oser et al.,
1965).
Long-term studies
Rat. Groups of 25 male and 25 female weanling rats were fed
diets containing 83 per cent. of bread to which was added 0, 750, 2370
or 7500 ppm of biurea, respectively, corresponding to biurea levels in
the diet equivalent to 0, 311, 983, or 3110 ppm. The first generation
was studied for a life span (two years); 10 males and 10 females were
selected at random from the F1 generation and kept on the diet and
observed for 28 weeks; the F2 generation was studied similarly and
the F3 generation for 14 weeks.
The appearance, behaviour, rate of weight gain and food intake in
each group of all generations showed no significant differences.
Reproduction and lactation were normal. Morbidity and mortality were
similar in all groups and showed no dose-related variations.
Haematological studies (including methaemoglobin levels) and gross and
microscopic examination of all the main organs revealed no significant
difference between test and control groups, except for one enlarged
kidney in a female rat receiving a high dose of biurea. A detailed
assessment of tumour incidence was made and nothing significant was
found (Oser et al., 1965).
Studies on flour overtreated with azodicarbonamide, and bread
baked from it
Short-term studies
Rat.Two groups of 10 rats were fed daily for 8 weeks by gastric
tube with untreated flour and with flour treated with azodicarbonamide
(120 ppm). No adverse effect was observed on the rate of weight gain,
blood formation, or the gross or microscopical appearance of the main
organs (Oser et al., 1965).
Dog. A group of 4 mongrel dogs was fed bread made from flour
treated with azodicarbonamide (100 ppm). The animals were observed for
two years. No abnormalities were found in weight gain, food intake,
appearance, behaviour, and morbidity. At autopsy no macroscopic or
microscopic change of any significance was found in the main organs
(Oser et al., 1965).
Long-term studies
Rat. Flour treated with azodicarbonamide (100 ppm) was made
into bread and this was fed to 25 male and 25 female weanling rats;
bread made from the untreated flour was fed to a control group of 25
male and 25 female rats. The animals were observed over the life-span
(2 years). Appearance, -behaviour, weight gain, food intake,
reproduction and lactation, morbidity and mortality rate were not
significantly different. Detailed studies on the blood and main
organs revealed no macroscopic-or microscopic abnormalities. Tumour
incidence was examined in detail and no alteration in the numbers or
patterns of tumours found was observed (Oser et al., 1965)
Comments
Azodicarbonamide has been extensively studied and the theoretical
point with regard to the possible effect of unconverted
azodicarbonamide was covered by experiments using overtreated flour or
bread made from it. The evidence strongly supports the view that
azodicarbonamide is rapidly and completely converted to biurea on
wetting and that this substance is stable in bread. Biurea itself is
metabolically inert, has low toxicity and does not present any
carcinogenic hazard. Azodicarbonamide has been adequately studied in
several species and is similarly free from carcinogenic hazard.
Long-term studies in mice are in progress (Frazer, 1966).
Evaluation
Acceptable level of treatment
Flour: 0-45 ppm
REFERENCES
Frazer, A. C. (1966) University of Birmingham work in progress
Joiner, R. R., Vidal, F. D. & Markes, H. C. (1963) Cereal Chemistry,
40, 539
Joiner, R. R., Unpublished report submitted by Wallace & Tiernan, 1965
Oser, B. L., Oser, M. & Morgareidge, K. (1965) Toxicol. appl.
Pharmacol., 7, 445
United States Testing Company (1952) Unpublished report
