
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 54
LINDANE
(Gamma-HCH)
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1991
This is a companion volume to Environmental Health Criteria 124:
Lindane
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data
Lindane (gamma-HCH) : health and safety guide.
(Health and safety guide ; no. 54)
1. Benzene hexachloride - standards I. Series
ISBN 92 4 151054 4 (NLM Classification: WA 240)
ISSN 0259-7268
(c) World Health Organization 1991
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Uses
2. SUMMARY AND EVALUATION
2.1. Environmental transport, distribution, and transformation
2.2. Environmental levels and human exposure
2.3. Kinetics and metabolism
2.4. Effects on organisms in the environment
2.5. Effects on experimental animals and in vitro test systems
2.6. Effects on human beings
3. CONCLUSIONS AND RECOMMENDATIONS
3.1. Conclusions
3.1.1. General population exposure
3.1.2. Subpopulations at special risk
3.1.3. Occupational exposure
3.1.4. Environmental effects
3.2. Recommendations
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and protection,
first aid
4.1.1. Advice to physicians
4.1.1.1 Symptoms of poisoning
4.1.1.2 Medical advice
4.1.2. Health surveillance advice
4.2. Safety in use
4.3. Explosion and fire hazards
4.3.1. Explosion hazards
4.3.2. Fire hazards
4.4. Storage
4.4.1. Leaking containers in store
4.5. Transport
4.6. Spillage and disposal
4.6.1. Spillage
4.6.2. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1. Previous evaluations by international bodies
7.2. Exposure limit values
7.3. Specific restrictions
7.4. Labelling, packaging, and transport
7.5. Waste disposal
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) documents produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is a Summary of Chemical Safety
Information which should be readily available, and should be clearly
explained, to all who could come into contact with the chemical. The
section on regulatory information has been extracted from the legal
file of the International Register of Potentially Toxic Chemicals
(IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Manager
International Programme on Chemical Safety
Division of Environmental Health
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Common name: lindane
Chemical structure:
gamma-
isomer
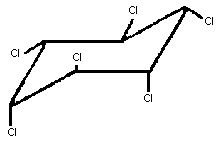 Chemical formula: C6H6Cl6
Relative molecular mass: 290.85
CAS chemical name: 1 alpha, 2 alpha, 3ß, 4 alpha, 5 alpha,
6ß-hexachlorocyclohexane
CAS registry number: 58-89-9
RTECS registry number: GV4900000
Definitions:
Name Definition Remarks
Lindane Product containing ISO-AFNOR (name for a product
not less than 99% not yet recognized by BSI)
gamma-HCH
Lindane = gamma-HCH Common name used for gamma-HCH
in the USSR only
gamma-HCH gamma isomer of ISO-AFNOR common name
1,2,3,4,5,6-hexa-
chlorocyclohexane
gamma-BHC gamma isomer of ISO BSI common name in English-
1,2,3,4,5,6-benzene speaking countries (recognized by
hexachloride ISO as synonym of gamma-HCH)
According to IUPAC rules the designation "benzene hexachloride" is
incorrect. Nevertheless, it is still widely used, especially in the
form of its abbreviation BHC. Therefore, gamma-BHC is another common
name that is approved by the ISO. The compound is called gamma-HCH by
the World Health Organization (WHO), but gamma-BHC by the Food and
Agriculture Organization of the United Nations (FAO). The synonym
hexachlorocyclohexane (gamma-isomer) is used by the Environmental
Protection Agency (EPA) and the American Conference of Governmental
Hygienists (ACGIH) in the USA.
Technical product
Common trade name: Large numbers of products containing lindane
are on the market throughout the world, under
hundreds of trade names; no attempt has been
made to list them.
Purity: In the past, the percentage of impurities in
technical lindane varied according to the
source. The isomers, alpha- and ß-HCH, were
the major impurities that occurred (see
Health and Safety Guide No. 53).
Nowadays, many countries, international
organizations, and manufacturers have set
strict purity requirements. The FAO
specification requires that technical lindane
should contain not less than 99.0% gamma-HCH.
For more details, see section 7.
1.2 Physical and Chemical Properties
Lindane is a white, crystalline solid, with a weak, or no, odour (the
characteristic smell of technical HCH is attributed to the impurities,
particularly heptachlorocyclohexane).
Some physical and chemical properties are given in the Summary of
Chemical Safety Information (section 6).
Lindane is stable to light, air, heat, carbon dioxide, and strong
acids. Dehydrochlorination of the compound may occur in the presence
of alkali, or on prolonged exposure to heat, forming trichlorobenzenes
and hydrochloric acid. Lindane is incompatible with strong bases and
powdered metals, such as iron, zinc, and aluminium. It is also
incompatible with oxidizing agents and can undergo oxidation, when in
contact with ozone.
1.3 Analytical Methods
Lindane can be determined separately from the other isomers after
extraction by liquid/liquid partition, column chromatography, and
detection by gas chromatography with electron-capture detection. The
high sensitivity of the analytical methods leads to the identification
of residue levels in the nanogram/kg or litre range.
1.4 Uses
Lindane has been used, since the early 1950s, as a broad-spectrum
insecticide for both agricultural and non-agricultural purposes. It
has been used in seed treatment, soil treatment, foliar applications,
the treatment of forests, timber, stored materials or products, and
against ectoparasites on animals and in public health.
In 1984, the global production amounted to 5000 metric tonnes.
In Japan, all use of HCH was prohibited in 1971. Several other
countries, e.g., the USA, have more or less severely restricted the
use of lindane, and specified the purity of the material to be used
(see section 7).
Lindane is offered to end-users in numerous formulations. The most
important of these are: wettable powders (up to 90% a.i.),
emulsifiable concentrates (not more than 20% a.i.), flowable
suspensions (in water), solutions in organic solvents (up to 50%
a.i.), dusts and powders (0.5-2% a.i.), granules and coarse dusts
(3-4% a.i.), ready-for-use baits, aerosols, and special formulations
for use in human and veterinary medicine.
Various lindane fumigation preparations for indoor use have been sold,
including fumigation strips, tablets, and smoke generators. They
contained practically pure lindane to which a small quantity of
binding material was added.
Lindane is often used in mixed formulations with other insecticides or
fungicides.
2. SUMMARY AND EVALUATION
2.1 Environmental Transport, Distribution, and Transformation
Lindane is strongly adsorbed on soils with a high organic matter
content. However, it can move downwards through the soil profile as a
result of rainfall or artificial irrigation, and there are strong
indications that volatilization is an important route of dissipation
under tropical, high-temperature conditions.
Rapid degradation (dechlorination) of lindane occurs on exposure to
ultraviolet radiation (UVR), forming pentachlorocyclohexenes
(gamma-PCCH) and tetrachlorocyclohexenes.
The half-life for the environmental degradation of lindane, under
humid or submerged conditions, and field conditions varied from a few
days up to 3 years, depending on various factors, such as soil type,
climate, and depth of application, among others. In European
agricultural soils, the half-life ranged between 40 and 70 days.
Biodegradation is much faster in non-sterilized soil than in
sterilized soil. Anaerobic conditions are most favourable for the
microbial metabolization of lindane. In water, degradation is mostly
by microorganisms in the sediments. The same degradation products are
formed.
The uptake and translocation of lindane and gamma-PCCH in plants is
limited, especially in soils with a high content of organic matter.
Residues are mainly found in the roots, only a small portion, if any,
being translocated into the stems, leaves, or fruits.
Rapid bioconcentration takes place in microorganisms, invertebrates,
fish, birds, and man, but biotransformation and elimination also occur
quite rapidly, when exposure is discontinued. In aquatic organisms,
uptake from water is more important than uptake from food. The
bioconcentration factors in aquatic organisms under laboratory
conditions ranged from approximately 10 up to 6000. Under field
conditions, the bioconcentration factors ranged from 10 up to 2600.
2.2 Environmental Levels and Human Exposure
Lindane is found in the air above the oceans, in concentrations of
0.039-0.68 ng/m3. In some countries, lindane was present in air in
concentrations of up to 11 ng/m3.
Lindane concentrations in surface water, estimated in a number of
countries in Europe, were mainly below 0.1 µg/litre. The
concentration of lindane in the Rhine and its tributaries in the
period 1969-74 varied between 0.01 and 0.4 µg/litre. Since 1974,
levels have been below 0.1µg/litre. In seawater, levels of between
0.001 and 0.02 µg/litre have been found.
Various studies have shown that concentrations of lindane in soil are
generally low (in the range of 0.001-0.01 mg/kg), except in waste
disposal areas.
Fish and shellfish contain gamma-HCH at concentrations ranging from
undetectable up to 2.5 mg/kg (on a fat basis), depending on such
factors as whether the organisms are living in fresh- or seawater and
whether they have a low or high fat content.
Total HCH concentrations were determined in ringed seals in the
Canadian Arctic over the period 1972-84. The mean concentrations in
the seals, which were initially approximately 130 µg/kg, increased,
during the period, to over 300 µg/kg blubber (wet weight). Levels of
about 330 and 440 µg/kg (wet weight) were found in the adipose tissue
of polar bears, in 1982 and 1984, respectively.
The levels of lindane in the livers of predator birds varied between
0.01 and 0.1 mg/kg. Eggs of sparrowhawks, collected in 1972-73 in the
Federal Republic of Germany, showed levels ranging from 0.6 up to
11.1 mg/kg (on a fat basis).
In drinking-water, lindane concentrations are generally below
0.001 µg/litre. Higher levels have been detected in only in a few
cases.
In industrialized countries, more than 90% of the human intake of
lindane originates from food. During the past 25 years, the lindane
concentrations have been determined in several food items, in a great
number of countries. Concentrations in cereals, fruits, vegetables,
pulses, and vegetable oils, were mainly in the range of undetectable
up to 0.5 mg/kg product. In milk, fat, meat, and eggs, the
concentrations ranged from undetectable up to 1.0 mg/kg product (on a
fat basis). In a few instances, higher concentrations were found. In
fish, the concentrations were generally far below 0.05 mg/kg product
(on a fat basis).
Total diet and/or market basket studies were carried out in a number
of countries to estimate the daily human intake of lindane. Intake
around 1970 was up to 0.05 µg/kg body weight per day; since then a
gradual decrease has taken place, with an intake of 0.003 µg/kg body
weight per day or less in 1980. In the mid-seventies, in the USA, the
daily intake of gamma-HCH by infants and toddlers decreased from 0.005
to 0.001 µg/kg body weight per day and from 0.01 to 0.005 µg/kg body
weight per day, respectively.
Determination of blood levels of lindane in the general population
have been carried out in different countries. In the Netherlands,
they were of the order of <0.1-0.2 µg/litre. However, much higher
levels were found in a number of other countries using technical HCH.
The mean concentrations in human adipose tissue, in various countries,
ranged from <0.1 to 0.2 mg/kg (on a fat basis).
The average concentrations of lindane found in human milk have
generally been rather low, ranging from <0.001 up to 0.1 mg/kg (on a
fat basis). A clear decrease has been seen over the years.
Lindane is distributed all over the world and can be detected in the
air, water, soil/sediment, aquatic and terrestrial organisms, and in
food. The concentrations in these different compartments are usually
low and are gradually decreasing. Thus, though human exposure occurs
via the daily food intake and lindane has been found in human blood,
adipose tissue, and breast milk, the figures are gradually decreasing.
2.3 Kinetics and Metabolism
In rats, lindane was rapidly absorbed from the gastrointestinal tract
and distributed to all organs and tissues within a few hours. The
highest concentrations were found in the adipose tissue and skin. The
fat/blood ratio was of the order of 150-200, the liver/blood ratio,
5.3-9.6, and the brain/blood ratio, 4-6.5. The same fat/blood ratio
was found in inhalation studies on rats. These ratios show a sex
difference, the ratios being higher in females.
The uptake of lindane through the skin after dermal application was
slow and very low, which may explain the low toxicity of lindane
following dermal exposure.
Lindane is metabolized by four enzymatic reactions, mainly in the
liver, i.e., dehydrogenation to gamma-hexachlorocyclohexene,
dehydrochlorination to gamma-pentachlorocyclohexene, dechlorination to
gamma-tetrachlorohexene, and hydroxylation to hexachlorocyclohexanol.
The end-products of the biotransformation are di-, tri-, tetra-,
penta-, and hexachloro- compounds. These metabolites are mainly
excreted via the urine in the free form or conjugated with glucuronic
acid, sulfuric acid, or N-acetylcystein. Elimination is rather rapid,
with half-life times in the rat of approximately 3-4 days.
Bacteria and fungi metabolize lindane into tetra- and
pentachlorocyclohexene.
The rate of metabolic transformation in plants is low and the main
degradation pathway proceeds via pentachlorocyclohexene to tri- and
tetrachlorophenol, and conjugates with ß-glucose or other unknown
compounds.
There is no evidence that isomerization of lindane to alpha-HCH takes
place.
2.4 Effects on Organisms in the Environment
The toxicity of lindane for bacteria, algae, and protozoa is low: the
no-observed-effect level was generally 1 mg/litre. Effects on fungi
are variable, with no-observed-effect levels varying among species
from 1 to 30 mg/litre. Lindane is moderately toxic for invertebrates
and fish. The LC50 and EC50 values for these organisms were of
the order of 20-90 µg/litre. In short-term and long-term studies on
3 species of fish, the no-observed-effect level was 9 µg/litre.
Reproduction studies on 3 species of fish showed no-observed-effect
levels ranging from 2.1 to 23.4 µg/litre.
The LC50 values for both freshwater and marine crustacea varied
between 1 and 1100 µg/litre. A reproduction study on Daphnia magna
showed a dose-dependent depression of reproduction. The
no-observed-effect level was in the range of 11-19 µg/litre.
Reproduction of molluscs was not adversely affected at 1 mg/litre.
The LD50 for the honey bee was 0.56 µg/bee.
Acute oral LD50 values for a number of bird species were between 100
and 1000 mg/kg body weight. In short-term studies on birds, dose
levels of between 4 and 10 mg/kg diet did not produce any effects,
even on egg-shell quality. Egg production was decreased in laying
ducks treated with dose levels of up to 20 mg lindane/kg body weight.
All bats exposed to surface wood scrapings containing initial lindane
levels of 10-866 mg/m2, resulting from application at the recommended
rate, died within 17 days. Lindane at 20 mg/kg diet (the highest dose
tested) was a no-observed-effect level for mortality and reproductive
success in small field mammals.
No data were available on effects on populations and ecosystems.
2.5 Effects on Experimental Animals and In Vitro Test Systems
The acute oral toxicity of lindane is moderate, the LD50s for mice
and rats ranging from 60 to 250 mg/kg body weight, depending on the
vehicle used. The dermal LD50 for the rat is approximately
900 mg/kg body weight. Signs of poisoning are those of central
nervous system stimulation.
Lindane does not irritate or sensitize the skin, however, it is a
slight eye irritant.
In a 90-day study on the rat, a no-observed-effect level of 10 mg/kg
diet (equivalent to 0.5 mg/kg body weight) was established. At 50 and
250 mg/kg diet, an increase was seen in the weight of the liver,
kidneys, and thyroid. At 250 mg/kg diet, an increase in liver enzyme
activity also occurred. In another 90-day study on the rat, 4 mg
lindane/kg diet (equivalent to 0.2 mg/kg body weight) was a
no-observed-effect level, renal and hepatic toxicity being observed at
dose levels of 20 mg/kg diet, or more. Lindane increases the enzyme
activity in the liver, accelerating not only its own breakdown, but
also that of other compounds. In a 30-day feeding study on the rat,
no neurological effects were observed with 240 mg lindane/kg diet,
(equivalent to 12 mg/kg body weight). However, neurological effects
were seen when the same dose was administered by gavage.
A short-term toxicity study on mice was inadequate, and a
no-observed-effect level could not be established.
In a study on dogs, a dose-level of 15 mg lindane/kg diet (equivalent
to 0.6 mg/kg body weight), for 63 weeks, did not induce any effects.
A large number of parameters were examined in a 2-year study on dogs.
However, no substance-related abnormalities were apparent with dietary
levels of 50 mg/kg (equivalent to 2 mg/kg body weight), or less. In
the group administered 100 mg/kg diet, an increase in alkaline
phosphatase activity was observed, and, at 200 mg/kg diet,
abnormalities in the EEG tracings, indicative of non-specific neuronal
irritation, were seen.
Two old, long-term rat studies have been reported in which levels of
10-1600 mg/kg diet were tested. In one study, the no-observed-effect
level was 50 mg/kg diet (equivalent to 2.5 mg/kg body weight). At
100 mg/kg diet, an increase in liver weight, hepatocellular
hypertrophy, fatty degeneration, and necrosis were seen. In the
second study, a lindane concentration of 25 mg/kg diet (equivalent to
1.25 mg/kg body weight) did not induce any effects. With 50 mg/kg
diet, hepatocellular hypertrophy and fatty degeneration were seen.
Rats were exposed through inhalation to lindane at 0.02-4.54 mg/m3,
for 6 h/day, over 3 months. At the highest dose level, increases in
hepatic cytochrome P-450 values were observed. The no-observed-effect
level in this 3-month study was 4.54 mg/m3.
Lindane was investigated in tests covering all aspects of reproduction
(3-generation rat study) as well as embryotoxicity and teratogenicity,
using oral and/or parenteral applications (oral., sc, ip; mouse, rat,
dog, pig). It was found that lindane did not exhibit teratogenic
properties, after oral and parenteral application (extra ribs were
regarded as variations). Fetotoxic and/or maternal toxic effects were
observed after administration (by gavage) of lindane at 10 mg/kg body
weight; therefore, 5 mg/kg body weight was the no-observed-effect
level.
No effects on reproduction and maturation were seen in the
3-generation rat study with lindane levels of up to 100 mg/kg diet.
However, morphological changes in the liver, indicating enzyme
induction, occurred in the offspring of the third generation, with
50 mg/kg diet. The no-observed-effect level in this test was 25 mg/kg
diet (equivalent to 1.25 mg/kg body weight).
The mutagenicity of lindane has been adequately studied. It has been
extensively investigated for its ability to produce gene mutations in
bacteria and mammalian cells, and also in the sex-linked recessive
lethal assay in Drosophila melanogaster. Negative results were
obtained consistently. The ability of lindane to produce chromosome
damage and SCEs has also been investigated in mammalian cells, both
in vitro and in vivo. Again, negative results were obtained. The
results of assays for DNA damage in bacteria were negative. The
results of in vivo studies on rats and mice, to investigate covalent
binding of orally administered lindane to DNA in the liver, were also
negative. The very few positive results obtained were due to invalid
study design and/or unknown lindane qualities. Overall, lindane
appears not to have mutagenic potential.
Studies to define the carcinogenic potential of lindane have been
carried out on the mouse and rat at dose levels of up to 600 mg/kg
diet, and up to 1600 mg/kg diet, respectively. Hyperplastic nodules
and/or hepatocellular adenomas were found in studies on mice at levels
of 160 mg/kg diet, or more. In some studies, the dose levels exceeded
the maximum tolerated dose. Two studies on mice and one on rats, with
dose levels of up to 160 mg/kg diet, and 640 mg/kg diet, respectively,
did not show any increase in the incidence of tumours.
The results of studies on initiation-promotion, mode of action, and
mutagenicity indicate that the tumorigenic response observed with
gamma-HCH in mice results from a non-genetic mechanism.
2.6 Effects on Human Beings
Several cases of fatal poisoning and of non-fatal illness, caused by
lindane, have been reported. These were either accidental,
intentional (suicide), or occurred through gross neglect of safety
precautions or improper use of medical products containing lindane.
Symptomatology included: nausea, restlessness, headache, vomiting,
tremor, ataxia, tonic-clonic convulsions, and/or changes in the EEG
pattern.
These effects were reversible following cessation of exposure and/or
symptomatic treatment.
In spite of the extensive use of lindane over 40 years, only very few
cases of occupational poisoning have been reported. Even in workers
exposed for long periods in both the manufacture and application of
lindane, only an increase in the activity of drug-metabolizing enzymes
of the liver has been occasionally found.
There is no evidence for a relationship between lindane exposure and
the occurrence of blood dyscrasias, as has been suggested in some
publications.
It can be concluded from a few acute and short-term studies on human
beings, that a dose level of approximately 1.0 mg/kg body weight does
not induce poisoning, but that a dose level of 15-17 mg/kg body weight
will result in severe toxic symptoms.
Approximately 10% of a dermally applied dose is absorbed through the
human skin, but more is absorbed when the skin is damaged.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions (lindane >99% gamma-HCH)
3.1.1 General population exposure
Lindane is circulating in the environment and is present in
food-chains, and there is continual human exposure. However, the
daily intake and total exposure of the general population is gradually
decreasing and is clearly below the advised acceptable daily intake
(ADI), and, thus, of no health concern.
3.1.2 Subpopulations at special risk
The exposure of breast-fed babies to lindane in breast milk is
generally below the ADI and, thus, not a health concern. Though lower
levels of exposure would be preferred, this is not a limiting factor
for the use of natural breast feeding.
Prescriptions should be strictly followed in the therapeutic use of
lindane against scabies and for the control of body lice.
3.1.3 Occupational exposure
As long as the recommended precautions to minimize worker exposure are
observed, lindane can be handled safely.
3.1.4 Environmental effects
Under recommended conditions of application as wood treatment, lindane
is toxic to bats roosting in close contact with treated wood.
Apart from spills in the aquatic environment, there is no evidence to
suggest that the presence of lindane poses a significant hazard for
organisms in the environment.
3.2 Recommendations (lindane >99% gamma-HCH)
(a) In order to minimize environmental pollution by other
HCH-isomers, lindane (>99% gamma-HCH) must be used instead of
technical HCH.
(b) In order to avoid environmental pollution, by-products and
effluents from the manufacturing of lindane should be disposed of in
an appropriate way.
(c) In disposing of lindane, care should be taken to avoid
contamination of natural waters and soil.
(d) As with other pesticides, proper instructions on application
procedures and safety precautions should be given to those handling
lindane.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Lindane is an organochlorine insecticide. It is moderately toxic and
can be moderately hazardous for human beings, if incorrectly or
carelessly handled. It is rather persistent in the environment. It
is therefore essential that the correct precautions should be observed
in the handling and use of the compound.
For details see the Summary of Chemical Safety Information (section
6).
4.1.1 Advice to physicians
4.1.1.1 Symptoms of poisoning
Lindane is readily absorbed and toxic after ingestion, by skin contact
(especially liquid formulations), and by inhalation of dust from
powder concentrates. It acts as a stimulant of the central nervous
system.
Following accidental ingestion or over-exposure, symptoms may include
headache, dizziness, nausea, vomiting, weakness in the legs,
stimulation of the central nervous system with clonic jerks and
convulsions, sometimes leading to death.
Respiratory depression may lead to respiratory acidosis, and, if
necessary, blood gases should be checked. The use of an ECG monitor
is recommended, if the symptoms are severe.
4.1.1.2 Medical advice
Medical treatment is largely symptomatic and supportive, and directed
against convulsions and hypoxia. If swallowed, the stomach should be
emptied, as soon as possible, by inducing vomiting and/or, when
possible, by careful gastric lavage. When the product is mixed with
an oil or solvent, special care must be taken to avoid aspiration into
the lungs, and subsequent aspiration pneumonitis. The best solution
is careful gastric lavage, using a cuffed endotracheal tube. Other
means of gastric decontamination, such as induced vomiting, should
only be used when a serious life-threatening intoxication is suspected
and no specialized medical care is available. This should be followed
by intragastric administration of up to 50 g (3-4 tablespoons) of
activated charcoal and 30 g of magnesium or sodium sulfate in a 30%
aqueous solution. Oily purgatives are contraindicated. No fats,
oils, or milk should be given.
If convulsions occur, anticonvulsants should be given, e.g., diazepam,
10 mg slowly, intravenously (children 1-5 mg), repeated as necessary;
or thiopental sodium, or hexobarbital sodium, slowly, intravenously in
a dose of 10 mg/kg, with a maximum total dose of up to 750 mg for an
adult, or paraldehyde (5 ml) by intramuscular injection. The
short-acting anti-convulsants should always be followed by
phenobarbital, given orally at 3 mg/kg (up to 200 mg for an adult), or
phenobarbital sodium, given intramuscularly at 3 mg/kg (also up to
200 mg for an adult). When close monitoring of the respiratory status
is possible, the dose may be increased, if needed, to suppress
convulsions.
Morphine and its derivatives, atropine, adrenaline, and noradrenaline
should never be given.
An unobstructed airway must be maintained. Respiratory inadequacy,
which may be accentuated by barbiturate anticonvulsants, should be
corrected, and oxygen and/or artificial ventilation may be needed.
4.1.2 Health surveillance advice
Pre-employment, and annual general medical examinations are advised
for regularly exposed workers.
4.2 Safety in Use
Manufacture and formulation
All efforts should be made to control exposure by the enclosure of
dusty operations, the use of exhaust ventilation, and good
housekeeping. Use full protective clothing.
Handling liquid formulations:
Wear protective neoprene or PVC gloves, cotton overalls, a rubber
apron, rubber boots, and a face-shield.
Handling powder formulations:
Avoid raising a dust cloud. Wear protective gloves and an appropriate
dust mask or respirator. Follow the advice relating to personal
hygiene.
Ground spray application:
Wear hat or cap, cotton overalls or a long-sleeved cotton shirt, long
trousers, and boots or shoes. When there is a risk of accidental
contamination by the spray, an impermeable hood and jacket should also
be worn. At all times, avoid exposure to, and inhalation of, the
spray mist. Do not spray into the wind.
Read and observe the instructions applying to the equipment being
used. Pay proper regard to wind speed and direction. Always spray
downwind. Do not spray when there are other people immediately
downwind.
Applications for termite control in buildings:
Reduce exposure of the applicator by keeping windows open and by the
use of portable exhausts in basements. Wear full protective
equipment. Never handle concentrate material in any part of a house
or building. Store away from clothing, bedding, dishes, food, and
animal feed, before application. Observe re-entry period, where
applicable.
After application:
Ensure that equipment is thoroughly cleaned and stored away ready for
use the next time. Carry out any essential maintenance.
Partly-used containers must be reclosed and returned to storage.
Empty containers should be disposed of as advised in section 4.6.2.
Change out of working clothes and take a bath or shower. Launder
clothing before re-use, keeping separate from household laundry.
4.3 Explosion and Fire Hazards
4.3.1 Explosion hazards
The explosion hazard will depend on the solvent used in the
formulation or on the characteristics of the dust.
4.3.2 Fire hazards
Liquid products containing organic solvents may be flammable.
Extinguish fires with alcohol-resistant foam, carbon dioxide, or
powder. With sufficient burning or external heat, lindane will
decompose, emitting toxic fumes, e.g., phosgene, hydrogen chloride,
and carbon monoxide. Fire-fighters should be equipped with
self-contained breathing apparatus, eye protection, and full
protective clothing.
The use of water spray should be confined to the cooling of unaffected
stock, thus avoiding the accumulation of polluted run-off from the
site.
4.4 Storage
Products should be stored in locked buildings, preferably dedicated to
insecticides. Keep products out of reach of children and unauthorized
personnel. Do not store near foodstuffs or animal feed.
4.4.1 Leaking containers in store
Take precautions and use appropriate personal protection. Empty any
product remaining in damaged or leaking containers into a clean empty
drum, which should then be tightly closed and suitably labelled.
Sweep up spillage with sawdust, sand, or earth (moisten for powders),
and dispose of safely.
When empty, leaky drums should be rinsed three times with at least
1 litre of water per 20-litre drum. Swirl round to rinse the walls,
empty, and add the rinsings to the sawdust or earth. Puncture or
crush the container to prevent re-use.
4.5 Transport
Comply with any local requirements regarding movement of hazardous
goods or wastes. Do not transport in the same compartment as
foodstuffs or animal feed. Make sure that containers are in good
condition and labels undamaged, before despatch.
4.6 Spillage and Disposal
4.6.1 Spillage
Before dealing with any spillage, precautions should be taken, as
required, and appropriate personal protection should be used. Sweep
up solid products and absorb remaining spilled product with moist
sawdust, sand, or earth; transfer in suitable container to safe place
for disposal. Prevent liquid from spreading or contaminating other
cargo and vegetation, and avoid pollution of surface waters and ground
water by using the most suitable available material, e.g., earth or
sand. Since lindane is toxic for fish, care should be taken to avoid
run-off into surface waters and drains.
4.6.2 Disposal
Surplus product, contaminated absorbents, and containers should be
disposed of in an appropriate way. Lindane is not readily decomposed
chemically or biologically and is relatively persistent. Waste
material should be burned only in a proper incinerator designed for
organochlorine waste disposal, with effluent gas scrubbing. If this
is not possible, bury in an approved dump or landfill, where there is
no risk of contamination of surface or ground water, as long as local
legislation is not contravened. Puncture and/or crush containers to
prevent re-use.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Lindane may pose a toxic hazard for aquatic and terrestrial species.
It may enter the food chain and give rise to bioaccumulation and
biomagnification; it is also rather persistent in the environment. In
the event of a major environmental contamination incident, appropriate
monitoring should be carried out.
Industrial discharges from manufacturing, formulation, and technical
applications should not be allowed to pollute the environment and
should be treated properly.
Any spillage or unused product should be prevented from spreading to
vegetation or waterways and should be treated and disposed of
properly.
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, lindane. It should be displayed at, or
near, entrances to areas where there is potential exposure to
lindane, and on processing equipment and containers. The summary
should be translated into the appropriate language(s). All persons
potentially exposed to the chemical should also have the instructions
in the summary clearly explained.
Space is available for insertion of the National Occupational
Exposure Limit, the address and telephone number of the National
Poison Control Centre, and for local trade names.
LINDANE
Chemical formula: C6H6Cl6 CAS registry number: 58-89-9
CAS chemical name: 1 alpha, 2 alpha, 3ß, 4 alpha, 5 alpha, 6ß-hexachlorocyclohexane RTECS registry number: GV4900000
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Melting point (°C) 112.8 White crystalline solid; weak chemical odour;
Density (20°C) (g/ml) 1.85 stable to light, air, heat, carbon dioxide,
Vapour pressure (mmHg) (20°C) 3.26 x 10-5 and strong acids; dechlorination may occur in
Relative molecular mass 290.85 the presence of alkali, or on prolonged exposure to
n-Octanol/water partition heat; corrosive to aluminium; used as a
coefficient (log Pow) 3.2-3.7 broad-spectrum insecticide for agricultural and
Solubility in water non-agricultural applications
(mg/litre) (20°C) 10 (slightly soluble)
Solubility in:
- ethanol 6.7%
- mineral oils slightly
- acetone, aromatic, and
chlorinated solvents soluble
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
SKIN: Overexposure may cause Avoid skin contact, wear Remove contaminated clothing immediately
poisoning protective clothing, PVC or and launder before re-use; wash skin with
neoprene gloves, rubber boots water and soap
EYES: Irritation, redness Wear face-shield or goggles Flush with clean water for 15 minutes; if
irritation persists, seek medical attention
INHALATION: Dust may Wear appropriate dust mask or
irritate respirator; use appropriate
ventilation in buildings
INGESTION: Unlikely Do not eat, drink, or smoke during
occupational hazard work
Accidental or intentional Obtain medical attention immediately; if
ingestion may cause lethal gastric lavage is not possible, in a rural
poisoning situation, induce vomiting; keep at rest,
lying face downwards
ENVIRONMENT: Toxic for Do not spill on animal feed or in
aquatic and terrestrial life; water ways
bioaccumulates
SPILLAGE STORAGE FIRE AND EXPLOSION
Take appropriate personal Products should be stored in Technical material is not flammable;
precautions; prevent liquid locked buildings, preferably liquid formulations may burn; emulsifiable
from spreading or contaminating dedicated to insecticides concentrates are miscible with water;
other cargo, vegetation, or extinguish fires with alcohol-resistant
waterways, with a barrier of the foam, carbon dioxide, or powder; with
most suitable available material, Keep products out of reach of sufficient burning or external heat, the
e.g., earth or sand; absorb spilled children and unauthorized product will decompose, emitting toxic
liquid with sawdust, sand, or personnel; do not store near fumes; the smoke and fumes could be
earth; sweep up and place it in a foodstuffs or animal feed injurious through inhalation, or absorption
closeable container for later safe through the skin; therefore, protective
disposal clothing and self-contained breathing
apparatus will be required; confine the use
of water spray to the cooling of unaffected stock;
polluted water should not be allowed to
pollute the environment, but should be
disposed of properly
WASTE DISPOSAL NATIONAL INFORMATION
Lindane is not readily decomposed National Occupational Exposure UN No. 2761, 2762, 2995, 2996
chemically or biologically and is Limit:
rather persistent; waste material
should be burned in a proper
incinerator designed for
organochlorine waste disposal;
if this is not possible, bury in an National Poison Control Centre:
approved dump or landfill where there
is no risk of contamination of surface
or ground water; comply with any
local legislation regarding disposal
of toxic wastes Local Trade Names:
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file. A full reference to the original national document from which
the information was extracted can be obtained from IRPTC. When no
effective date appears in the IRPTC legal file, the year of the
reference from which the data are taken is indicated by (r).
The reader should be aware that regulatory decisions about chemicals,
taken in a certain country, can only be fully understood in the
framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application
7.1 Previous Evaluations by International Bodies
The International Agency for Research on Cancer (IARC) evaluated the
hexachlorocyclohexanes in 1987 and concluded that, for the technical
grade and the alpha-isomer, there is sufficient evidence for
carcinogenicity for animals; evidence is limited for the ß- and
gamma-isomers. There is inadequate evidence for their carcinogenicity
for human beings. Hexachlorocyclohexanes were classified in Group 2B.
WHO (1990) classified technical lindane as "moderately hazardous" in
normal use (on the basis of an LD50 of 88 mg/kg).
WHO/FAO (1975) issued a data sheet on lindane (No. 12), dealing with
labelling, safe handling, transport, storage, disposal,
decontamination, training, and medical supervision of workers,
first-aid, and medical treatment.
Lindane was evaluated by the Joint FAO/WHO Meeting on Pesticide
Residues in 1966, 1967, 1968, 1969, 1973, 1974, 1975, 1977, 1979, and
1989. A maximum acceptable daily intake (ADI) of lindane in human
beings was established at 0-0.008 mg/kg body weight by the 1989 Joint
Meeting. This value was was based on a no-observed-effect level of:
- 10 mg/kg diet, equivalent to 0.75 mg/kg body weight per day in
the rat,
and
- 1.6 mg/kg body weight per day in the dog.
Maximum residue limits (MRLs) have been recommended by the FAO/ WHO
Codex Committee for more than 35 commodities, ranging from 0.05 mg/kg
on potatoes to 3 mg/kg on strawberries. A level of 0.5 mg/kg was
recommended for most fruit and vegetables.
7.2 Exposure Limit Values
Some exposure limit values for lindane are given in the Table on pp.
34-35.
7.3 Specific Restrictions
In Japan, all uses of HCH and lindane were prohibited in 1971. The
main reason was the environmental pollution with alpha-HCH and ß-HCH
that resulted from the previous extensive use of technical HCH.
Agricultural uses of technical HCH have been prohibited in most
countries, because of environmental pollution with alpha-HCH and
ß-HCH.
The European Community legislation prohibits the placing on the
market, and the use, of HCH containing less than 99% of the
gamma-isomer. The European Community legislation also prohibits the
placing on the market of cosmetics containing HCH.
In several other countries, the use of lindane has been more or less
severely restricted, e.g., Argentina, Brazil, Czechoslovakia, and the
USA.
In the Federal Republic of Germany, lindane may not be handled by
adolescents or by pregnant or nursing women.
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit descriptiona Value Effective
organization date
AIR Workplace Argentina Threshold limit value (TLV)
- Time-weighted average (TWA) 0.5 mg/m3 1979
- Short-term exposure level (STEL) 1.5 mg/m3
Germany, Maximum work-site concentration (MAK)
Federal - 8-h Time-weighted average (TWA) 0.5 mg/m3 1985
Republic of - Short-term exposure level (STEL) 5.0 mg/m3
(30-min) (1 x per shift)
United Kingdom Maximum exposure limit
- 8-h Time-weighted average (TWA) 0.5 mg/m3 1985
- Short-term exposure level (STEL) 1.5 mg/m3
<> (10-min Time-weighted average)
USA Permissible exposure limit (PEL)
- Time-weighted average (TWA) 0.5 mg/m3 1986
USSR Maximum allowable concentration (MAC)
- Ceiling value (CLV) 0.05 mg/m3 1983
FOOD Intake from FAO/WHO Acceptable daily intake (ADI) 0-0.008 mg/kg 1989
per kg of body weight
FOOD Plant FAO/WHO Maximum residue limit (MRL)
35 food commodities, ranging from.... 0.05 to 3 mg/kg 1979
Medium Specification Country/ Exposure limit description Value Effective
organization date
FEED European Maximum residue limit (MRL)
Community All feed, 0.2 mg/kg 1989
except fats 2 mg/kg
WATER Drinking- WHO Guideline level 0.3 µg/litre 1983
WATER Drinking- European Surface water for the preparation of
Community drinking-water - total pesticides:
-Quality A1 0.001 mg/litre 1980
-Quality A2 0.0025 mg/litre
-Quality A3 0.005 mg/litre
Mexico Maximum permissible concentration (MPC) 1973
- Receiving water treated for drinking 0.056 mg/litre
USA Maximum permissible concentration (MPC) 0.004 mg/litre 1975
WATER Ambient Mexico Maximum permissible concentration (MPC) 1973
- Coastal 0.2 µg/litre
- Estuaries 0.002 mg/litre
SOIL USSR Maximum acceptable level 0.1 mg/kg 1973
a TWA = time-weighted average over one working day (usually 8 h).
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies lindane in:
- Hazard Class 6.1: poisonous substance;
- Packing Group III: substance presenting a relatively low risk of
poisoning in transport, when the active ingredient ranges from 44
to 100% (solid) or 15 to 100% (liquid).
The label should be as follows:
Class III:
Chemical formula: C6H6Cl6
Relative molecular mass: 290.85
CAS chemical name: 1 alpha, 2 alpha, 3ß, 4 alpha, 5 alpha,
6ß-hexachlorocyclohexane
CAS registry number: 58-89-9
RTECS registry number: GV4900000
Definitions:
Name Definition Remarks
Lindane Product containing ISO-AFNOR (name for a product
not less than 99% not yet recognized by BSI)
gamma-HCH
Lindane = gamma-HCH Common name used for gamma-HCH
in the USSR only
gamma-HCH gamma isomer of ISO-AFNOR common name
1,2,3,4,5,6-hexa-
chlorocyclohexane
gamma-BHC gamma isomer of ISO BSI common name in English-
1,2,3,4,5,6-benzene speaking countries (recognized by
hexachloride ISO as synonym of gamma-HCH)
According to IUPAC rules the designation "benzene hexachloride" is
incorrect. Nevertheless, it is still widely used, especially in the
form of its abbreviation BHC. Therefore, gamma-BHC is another common
name that is approved by the ISO. The compound is called gamma-HCH by
the World Health Organization (WHO), but gamma-BHC by the Food and
Agriculture Organization of the United Nations (FAO). The synonym
hexachlorocyclohexane (gamma-isomer) is used by the Environmental
Protection Agency (EPA) and the American Conference of Governmental
Hygienists (ACGIH) in the USA.
Technical product
Common trade name: Large numbers of products containing lindane
are on the market throughout the world, under
hundreds of trade names; no attempt has been
made to list them.
Purity: In the past, the percentage of impurities in
technical lindane varied according to the
source. The isomers, alpha- and ß-HCH, were
the major impurities that occurred (see
Health and Safety Guide No. 53).
Nowadays, many countries, international
organizations, and manufacturers have set
strict purity requirements. The FAO
specification requires that technical lindane
should contain not less than 99.0% gamma-HCH.
For more details, see section 7.
1.2 Physical and Chemical Properties
Lindane is a white, crystalline solid, with a weak, or no, odour (the
characteristic smell of technical HCH is attributed to the impurities,
particularly heptachlorocyclohexane).
Some physical and chemical properties are given in the Summary of
Chemical Safety Information (section 6).
Lindane is stable to light, air, heat, carbon dioxide, and strong
acids. Dehydrochlorination of the compound may occur in the presence
of alkali, or on prolonged exposure to heat, forming trichlorobenzenes
and hydrochloric acid. Lindane is incompatible with strong bases and
powdered metals, such as iron, zinc, and aluminium. It is also
incompatible with oxidizing agents and can undergo oxidation, when in
contact with ozone.
1.3 Analytical Methods
Lindane can be determined separately from the other isomers after
extraction by liquid/liquid partition, column chromatography, and
detection by gas chromatography with electron-capture detection. The
high sensitivity of the analytical methods leads to the identification
of residue levels in the nanogram/kg or litre range.
1.4 Uses
Lindane has been used, since the early 1950s, as a broad-spectrum
insecticide for both agricultural and non-agricultural purposes. It
has been used in seed treatment, soil treatment, foliar applications,
the treatment of forests, timber, stored materials or products, and
against ectoparasites on animals and in public health.
In 1984, the global production amounted to 5000 metric tonnes.
In Japan, all use of HCH was prohibited in 1971. Several other
countries, e.g., the USA, have more or less severely restricted the
use of lindane, and specified the purity of the material to be used
(see section 7).
Lindane is offered to end-users in numerous formulations. The most
important of these are: wettable powders (up to 90% a.i.),
emulsifiable concentrates (not more than 20% a.i.), flowable
suspensions (in water), solutions in organic solvents (up to 50%
a.i.), dusts and powders (0.5-2% a.i.), granules and coarse dusts
(3-4% a.i.), ready-for-use baits, aerosols, and special formulations
for use in human and veterinary medicine.
Various lindane fumigation preparations for indoor use have been sold,
including fumigation strips, tablets, and smoke generators. They
contained practically pure lindane to which a small quantity of
binding material was added.
Lindane is often used in mixed formulations with other insecticides or
fungicides.
2. SUMMARY AND EVALUATION
2.1 Environmental Transport, Distribution, and Transformation
Lindane is strongly adsorbed on soils with a high organic matter
content. However, it can move downwards through the soil profile as a
result of rainfall or artificial irrigation, and there are strong
indications that volatilization is an important route of dissipation
under tropical, high-temperature conditions.
Rapid degradation (dechlorination) of lindane occurs on exposure to
ultraviolet radiation (UVR), forming pentachlorocyclohexenes
(gamma-PCCH) and tetrachlorocyclohexenes.
The half-life for the environmental degradation of lindane, under
humid or submerged conditions, and field conditions varied from a few
days up to 3 years, depending on various factors, such as soil type,
climate, and depth of application, among others. In European
agricultural soils, the half-life ranged between 40 and 70 days.
Biodegradation is much faster in non-sterilized soil than in
sterilized soil. Anaerobic conditions are most favourable for the
microbial metabolization of lindane. In water, degradation is mostly
by microorganisms in the sediments. The same degradation products are
formed.
The uptake and translocation of lindane and gamma-PCCH in plants is
limited, especially in soils with a high content of organic matter.
Residues are mainly found in the roots, only a small portion, if any,
being translocated into the stems, leaves, or fruits.
Rapid bioconcentration takes place in microorganisms, invertebrates,
fish, birds, and man, but biotransformation and elimination also occur
quite rapidly, when exposure is discontinued. In aquatic organisms,
uptake from water is more important than uptake from food. The
bioconcentration factors in aquatic organisms under laboratory
conditions ranged from approximately 10 up to 6000. Under field
conditions, the bioconcentration factors ranged from 10 up to 2600.
2.2 Environmental Levels and Human Exposure
Lindane is found in the air above the oceans, in concentrations of
0.039-0.68 ng/m3. In some countries, lindane was present in air in
concentrations of up to 11 ng/m3.
Lindane concentrations in surface water, estimated in a number of
countries in Europe, were mainly below 0.1 µg/litre. The
concentration of lindane in the Rhine and its tributaries in the
period 1969-74 varied between 0.01 and 0.4 µg/litre. Since 1974,
levels have been below 0.1µg/litre. In seawater, levels of between
0.001 and 0.02 µg/litre have been found.
Various studies have shown that concentrations of lindane in soil are
generally low (in the range of 0.001-0.01 mg/kg), except in waste
disposal areas.
Fish and shellfish contain gamma-HCH at concentrations ranging from
undetectable up to 2.5 mg/kg (on a fat basis), depending on such
factors as whether the organisms are living in fresh- or seawater and
whether they have a low or high fat content.
Total HCH concentrations were determined in ringed seals in the
Canadian Arctic over the period 1972-84. The mean concentrations in
the seals, which were initially approximately 130 µg/kg, increased,
during the period, to over 300 µg/kg blubber (wet weight). Levels of
about 330 and 440 µg/kg (wet weight) were found in the adipose tissue
of polar bears, in 1982 and 1984, respectively.
The levels of lindane in the livers of predator birds varied between
0.01 and 0.1 mg/kg. Eggs of sparrowhawks, collected in 1972-73 in the
Federal Republic of Germany, showed levels ranging from 0.6 up to
11.1 mg/kg (on a fat basis).
In drinking-water, lindane concentrations are generally below
0.001 µg/litre. Higher levels have been detected in only in a few
cases.
In industrialized countries, more than 90% of the human intake of
lindane originates from food. During the past 25 years, the lindane
concentrations have been determined in several food items, in a great
number of countries. Concentrations in cereals, fruits, vegetables,
pulses, and vegetable oils, were mainly in the range of undetectable
up to 0.5 mg/kg product. In milk, fat, meat, and eggs, the
concentrations ranged from undetectable up to 1.0 mg/kg product (on a
fat basis). In a few instances, higher concentrations were found. In
fish, the concentrations were generally far below 0.05 mg/kg product
(on a fat basis).
Total diet and/or market basket studies were carried out in a number
of countries to estimate the daily human intake of lindane. Intake
around 1970 was up to 0.05 µg/kg body weight per day; since then a
gradual decrease has taken place, with an intake of 0.003 µg/kg body
weight per day or less in 1980. In the mid-seventies, in the USA, the
daily intake of gamma-HCH by infants and toddlers decreased from 0.005
to 0.001 µg/kg body weight per day and from 0.01 to 0.005 µg/kg body
weight per day, respectively.
Determination of blood levels of lindane in the general population
have been carried out in different countries. In the Netherlands,
they were of the order of <0.1-0.2 µg/litre. However, much higher
levels were found in a number of other countries using technical HCH.
The mean concentrations in human adipose tissue, in various countries,
ranged from <0.1 to 0.2 mg/kg (on a fat basis).
The average concentrations of lindane found in human milk have
generally been rather low, ranging from <0.001 up to 0.1 mg/kg (on a
fat basis). A clear decrease has been seen over the years.
Lindane is distributed all over the world and can be detected in the
air, water, soil/sediment, aquatic and terrestrial organisms, and in
food. The concentrations in these different compartments are usually
low and are gradually decreasing. Thus, though human exposure occurs
via the daily food intake and lindane has been found in human blood,
adipose tissue, and breast milk, the figures are gradually decreasing.
2.3 Kinetics and Metabolism
In rats, lindane was rapidly absorbed from the gastrointestinal tract
and distributed to all organs and tissues within a few hours. The
highest concentrations were found in the adipose tissue and skin. The
fat/blood ratio was of the order of 150-200, the liver/blood ratio,
5.3-9.6, and the brain/blood ratio, 4-6.5. The same fat/blood ratio
was found in inhalation studies on rats. These ratios show a sex
difference, the ratios being higher in females.
The uptake of lindane through the skin after dermal application was
slow and very low, which may explain the low toxicity of lindane
following dermal exposure.
Lindane is metabolized by four enzymatic reactions, mainly in the
liver, i.e., dehydrogenation to gamma-hexachlorocyclohexene,
dehydrochlorination to gamma-pentachlorocyclohexene, dechlorination to
gamma-tetrachlorohexene, and hydroxylation to hexachlorocyclohexanol.
The end-products of the biotransformation are di-, tri-, tetra-,
penta-, and hexachloro- compounds. These metabolites are mainly
excreted via the urine in the free form or conjugated with glucuronic
acid, sulfuric acid, or N-acetylcystein. Elimination is rather rapid,
with half-life times in the rat of approximately 3-4 days.
Bacteria and fungi metabolize lindane into tetra- and
pentachlorocyclohexene.
The rate of metabolic transformation in plants is low and the main
degradation pathway proceeds via pentachlorocyclohexene to tri- and
tetrachlorophenol, and conjugates with ß-glucose or other unknown
compounds.
There is no evidence that isomerization of lindane to alpha-HCH takes
place.
2.4 Effects on Organisms in the Environment
The toxicity of lindane for bacteria, algae, and protozoa is low: the
no-observed-effect level was generally 1 mg/litre. Effects on fungi
are variable, with no-observed-effect levels varying among species
from 1 to 30 mg/litre. Lindane is moderately toxic for invertebrates
and fish. The LC50 and EC50 values for these organisms were of
the order of 20-90 µg/litre. In short-term and long-term studies on
3 species of fish, the no-observed-effect level was 9 µg/litre.
Reproduction studies on 3 species of fish showed no-observed-effect
levels ranging from 2.1 to 23.4 µg/litre.
The LC50 values for both freshwater and marine crustacea varied
between 1 and 1100 µg/litre. A reproduction study on Daphnia magna
showed a dose-dependent depression of reproduction. The
no-observed-effect level was in the range of 11-19 µg/litre.
Reproduction of molluscs was not adversely affected at 1 mg/litre.
The LD50 for the honey bee was 0.56 µg/bee.
Acute oral LD50 values for a number of bird species were between 100
and 1000 mg/kg body weight. In short-term studies on birds, dose
levels of between 4 and 10 mg/kg diet did not produce any effects,
even on egg-shell quality. Egg production was decreased in laying
ducks treated with dose levels of up to 20 mg lindane/kg body weight.
All bats exposed to surface wood scrapings containing initial lindane
levels of 10-866 mg/m2, resulting from application at the recommended
rate, died within 17 days. Lindane at 20 mg/kg diet (the highest dose
tested) was a no-observed-effect level for mortality and reproductive
success in small field mammals.
No data were available on effects on populations and ecosystems.
2.5 Effects on Experimental Animals and In Vitro Test Systems
The acute oral toxicity of lindane is moderate, the LD50s for mice
and rats ranging from 60 to 250 mg/kg body weight, depending on the
vehicle used. The dermal LD50 for the rat is approximately
900 mg/kg body weight. Signs of poisoning are those of central
nervous system stimulation.
Lindane does not irritate or sensitize the skin, however, it is a
slight eye irritant.
In a 90-day study on the rat, a no-observed-effect level of 10 mg/kg
diet (equivalent to 0.5 mg/kg body weight) was established. At 50 and
250 mg/kg diet, an increase was seen in the weight of the liver,
kidneys, and thyroid. At 250 mg/kg diet, an increase in liver enzyme
activity also occurred. In another 90-day study on the rat, 4 mg
lindane/kg diet (equivalent to 0.2 mg/kg body weight) was a
no-observed-effect level, renal and hepatic toxicity being observed at
dose levels of 20 mg/kg diet, or more. Lindane increases the enzyme
activity in the liver, accelerating not only its own breakdown, but
also that of other compounds. In a 30-day feeding study on the rat,
no neurological effects were observed with 240 mg lindane/kg diet,
(equivalent to 12 mg/kg body weight). However, neurological effects
were seen when the same dose was administered by gavage.
A short-term toxicity study on mice was inadequate, and a
no-observed-effect level could not be established.
In a study on dogs, a dose-level of 15 mg lindane/kg diet (equivalent
to 0.6 mg/kg body weight), for 63 weeks, did not induce any effects.
A large number of parameters were examined in a 2-year study on dogs.
However, no substance-related abnormalities were apparent with dietary
levels of 50 mg/kg (equivalent to 2 mg/kg body weight), or less. In
the group administered 100 mg/kg diet, an increase in alkaline
phosphatase activity was observed, and, at 200 mg/kg diet,
abnormalities in the EEG tracings, indicative of non-specific neuronal
irritation, were seen.
Two old, long-term rat studies have been reported in which levels of
10-1600 mg/kg diet were tested. In one study, the no-observed-effect
level was 50 mg/kg diet (equivalent to 2.5 mg/kg body weight). At
100 mg/kg diet, an increase in liver weight, hepatocellular
hypertrophy, fatty degeneration, and necrosis were seen. In the
second study, a lindane concentration of 25 mg/kg diet (equivalent to
1.25 mg/kg body weight) did not induce any effects. With 50 mg/kg
diet, hepatocellular hypertrophy and fatty degeneration were seen.
Rats were exposed through inhalation to lindane at 0.02-4.54 mg/m3,
for 6 h/day, over 3 months. At the highest dose level, increases in
hepatic cytochrome P-450 values were observed. The no-observed-effect
level in this 3-month study was 4.54 mg/m3.
Lindane was investigated in tests covering all aspects of reproduction
(3-generation rat study) as well as embryotoxicity and teratogenicity,
using oral and/or parenteral applications (oral., sc, ip; mouse, rat,
dog, pig). It was found that lindane did not exhibit teratogenic
properties, after oral and parenteral application (extra ribs were
regarded as variations). Fetotoxic and/or maternal toxic effects were
observed after administration (by gavage) of lindane at 10 mg/kg body
weight; therefore, 5 mg/kg body weight was the no-observed-effect
level.
No effects on reproduction and maturation were seen in the
3-generation rat study with lindane levels of up to 100 mg/kg diet.
However, morphological changes in the liver, indicating enzyme
induction, occurred in the offspring of the third generation, with
50 mg/kg diet. The no-observed-effect level in this test was 25 mg/kg
diet (equivalent to 1.25 mg/kg body weight).
The mutagenicity of lindane has been adequately studied. It has been
extensively investigated for its ability to produce gene mutations in
bacteria and mammalian cells, and also in the sex-linked recessive
lethal assay in Drosophila melanogaster. Negative results were
obtained consistently. The ability of lindane to produce chromosome
damage and SCEs has also been investigated in mammalian cells, both
in vitro and in vivo. Again, negative results were obtained. The
results of assays for DNA damage in bacteria were negative. The
results of in vivo studies on rats and mice, to investigate covalent
binding of orally administered lindane to DNA in the liver, were also
negative. The very few positive results obtained were due to invalid
study design and/or unknown lindane qualities. Overall, lindane
appears not to have mutagenic potential.
Studies to define the carcinogenic potential of lindane have been
carried out on the mouse and rat at dose levels of up to 600 mg/kg
diet, and up to 1600 mg/kg diet, respectively. Hyperplastic nodules
and/or hepatocellular adenomas were found in studies on mice at levels
of 160 mg/kg diet, or more. In some studies, the dose levels exceeded
the maximum tolerated dose. Two studies on mice and one on rats, with
dose levels of up to 160 mg/kg diet, and 640 mg/kg diet, respectively,
did not show any increase in the incidence of tumours.
The results of studies on initiation-promotion, mode of action, and
mutagenicity indicate that the tumorigenic response observed with
gamma-HCH in mice results from a non-genetic mechanism.
2.6 Effects on Human Beings
Several cases of fatal poisoning and of non-fatal illness, caused by
lindane, have been reported. These were either accidental,
intentional (suicide), or occurred through gross neglect of safety
precautions or improper use of medical products containing lindane.
Symptomatology included: nausea, restlessness, headache, vomiting,
tremor, ataxia, tonic-clonic convulsions, and/or changes in the EEG
pattern.
These effects were reversible following cessation of exposure and/or
symptomatic treatment.
In spite of the extensive use of lindane over 40 years, only very few
cases of occupational poisoning have been reported. Even in workers
exposed for long periods in both the manufacture and application of
lindane, only an increase in the activity of drug-metabolizing enzymes
of the liver has been occasionally found.
There is no evidence for a relationship between lindane exposure and
the occurrence of blood dyscrasias, as has been suggested in some
publications.
It can be concluded from a few acute and short-term studies on human
beings, that a dose level of approximately 1.0 mg/kg body weight does
not induce poisoning, but that a dose level of 15-17 mg/kg body weight
will result in severe toxic symptoms.
Approximately 10% of a dermally applied dose is absorbed through the
human skin, but more is absorbed when the skin is damaged.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions (lindane >99% gamma-HCH)
3.1.1 General population exposure
Lindane is circulating in the environment and is present in
food-chains, and there is continual human exposure. However, the
daily intake and total exposure of the general population is gradually
decreasing and is clearly below the advised acceptable daily intake
(ADI), and, thus, of no health concern.
3.1.2 Subpopulations at special risk
The exposure of breast-fed babies to lindane in breast milk is
generally below the ADI and, thus, not a health concern. Though lower
levels of exposure would be preferred, this is not a limiting factor
for the use of natural breast feeding.
Prescriptions should be strictly followed in the therapeutic use of
lindane against scabies and for the control of body lice.
3.1.3 Occupational exposure
As long as the recommended precautions to minimize worker exposure are
observed, lindane can be handled safely.
3.1.4 Environmental effects
Under recommended conditions of application as wood treatment, lindane
is toxic to bats roosting in close contact with treated wood.
Apart from spills in the aquatic environment, there is no evidence to
suggest that the presence of lindane poses a significant hazard for
organisms in the environment.
3.2 Recommendations (lindane >99% gamma-HCH)
(a) In order to minimize environmental pollution by other
HCH-isomers, lindane (>99% gamma-HCH) must be used instead of
technical HCH.
(b) In order to avoid environmental pollution, by-products and
effluents from the manufacturing of lindane should be disposed of in
an appropriate way.
(c) In disposing of lindane, care should be taken to avoid
contamination of natural waters and soil.
(d) As with other pesticides, proper instructions on application
procedures and safety precautions should be given to those handling
lindane.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Lindane is an organochlorine insecticide. It is moderately toxic and
can be moderately hazardous for human beings, if incorrectly or
carelessly handled. It is rather persistent in the environment. It
is therefore essential that the correct precautions should be observed
in the handling and use of the compound.
For details see the Summary of Chemical Safety Information (section
6).
4.1.1 Advice to physicians
4.1.1.1 Symptoms of poisoning
Lindane is readily absorbed and toxic after ingestion, by skin contact
(especially liquid formulations), and by inhalation of dust from
powder concentrates. It acts as a stimulant of the central nervous
system.
Following accidental ingestion or over-exposure, symptoms may include
headache, dizziness, nausea, vomiting, weakness in the legs,
stimulation of the central nervous system with clonic jerks and
convulsions, sometimes leading to death.
Respiratory depression may lead to respiratory acidosis, and, if
necessary, blood gases should be checked. The use of an ECG monitor
is recommended, if the symptoms are severe.
4.1.1.2 Medical advice
Medical treatment is largely symptomatic and supportive, and directed
against convulsions and hypoxia. If swallowed, the stomach should be
emptied, as soon as possible, by inducing vomiting and/or, when
possible, by careful gastric lavage. When the product is mixed with
an oil or solvent, special care must be taken to avoid aspiration into
the lungs, and subsequent aspiration pneumonitis. The best solution
is careful gastric lavage, using a cuffed endotracheal tube. Other
means of gastric decontamination, such as induced vomiting, should
only be used when a serious life-threatening intoxication is suspected
and no specialized medical care is available. This should be followed
by intragastric administration of up to 50 g (3-4 tablespoons) of
activated charcoal and 30 g of magnesium or sodium sulfate in a 30%
aqueous solution. Oily purgatives are contraindicated. No fats,
oils, or milk should be given.
If convulsions occur, anticonvulsants should be given, e.g., diazepam,
10 mg slowly, intravenously (children 1-5 mg), repeated as necessary;
or thiopental sodium, or hexobarbital sodium, slowly, intravenously in
a dose of 10 mg/kg, with a maximum total dose of up to 750 mg for an
adult, or paraldehyde (5 ml) by intramuscular injection. The
short-acting anti-convulsants should always be followed by
phenobarbital, given orally at 3 mg/kg (up to 200 mg for an adult), or
phenobarbital sodium, given intramuscularly at 3 mg/kg (also up to
200 mg for an adult). When close monitoring of the respiratory status
is possible, the dose may be increased, if needed, to suppress
convulsions.
Morphine and its derivatives, atropine, adrenaline, and noradrenaline
should never be given.
An unobstructed airway must be maintained. Respiratory inadequacy,
which may be accentuated by barbiturate anticonvulsants, should be
corrected, and oxygen and/or artificial ventilation may be needed.
4.1.2 Health surveillance advice
Pre-employment, and annual general medical examinations are advised
for regularly exposed workers.
4.2 Safety in Use
Manufacture and formulation
All efforts should be made to control exposure by the enclosure of
dusty operations, the use of exhaust ventilation, and good
housekeeping. Use full protective clothing.
Handling liquid formulations:
Wear protective neoprene or PVC gloves, cotton overalls, a rubber
apron, rubber boots, and a face-shield.
Handling powder formulations:
Avoid raising a dust cloud. Wear protective gloves and an appropriate
dust mask or respirator. Follow the advice relating to personal
hygiene.
Ground spray application:
Wear hat or cap, cotton overalls or a long-sleeved cotton shirt, long
trousers, and boots or shoes. When there is a risk of accidental
contamination by the spray, an impermeable hood and jacket should also
be worn. At all times, avoid exposure to, and inhalation of, the
spray mist. Do not spray into the wind.
Read and observe the instructions applying to the equipment being
used. Pay proper regard to wind speed and direction. Always spray
downwind. Do not spray when there are other people immediately
downwind.
Applications for termite control in buildings:
Reduce exposure of the applicator by keeping windows open and by the
use of portable exhausts in basements. Wear full protective
equipment. Never handle concentrate material in any part of a house
or building. Store away from clothing, bedding, dishes, food, and
animal feed, before application. Observe re-entry period, where
applicable.
After application:
Ensure that equipment is thoroughly cleaned and stored away ready for
use the next time. Carry out any essential maintenance.
Partly-used containers must be reclosed and returned to storage.
Empty containers should be disposed of as advised in section 4.6.2.
Change out of working clothes and take a bath or shower. Launder
clothing before re-use, keeping separate from household laundry.
4.3 Explosion and Fire Hazards
4.3.1 Explosion hazards
The explosion hazard will depend on the solvent used in the
formulation or on the characteristics of the dust.
4.3.2 Fire hazards
Liquid products containing organic solvents may be flammable.
Extinguish fires with alcohol-resistant foam, carbon dioxide, or
powder. With sufficient burning or external heat, lindane will
decompose, emitting toxic fumes, e.g., phosgene, hydrogen chloride,
and carbon monoxide. Fire-fighters should be equipped with
self-contained breathing apparatus, eye protection, and full
protective clothing.
The use of water spray should be confined to the cooling of unaffected
stock, thus avoiding the accumulation of polluted run-off from the
site.
4.4 Storage
Products should be stored in locked buildings, preferably dedicated to
insecticides. Keep products out of reach of children and unauthorized
personnel. Do not store near foodstuffs or animal feed.
4.4.1 Leaking containers in store
Take precautions and use appropriate personal protection. Empty any
product remaining in damaged or leaking containers into a clean empty
drum, which should then be tightly closed and suitably labelled.
Sweep up spillage with sawdust, sand, or earth (moisten for powders),
and dispose of safely.
When empty, leaky drums should be rinsed three times with at least
1 litre of water per 20-litre drum. Swirl round to rinse the walls,
empty, and add the rinsings to the sawdust or earth. Puncture or
crush the container to prevent re-use.
4.5 Transport
Comply with any local requirements regarding movement of hazardous
goods or wastes. Do not transport in the same compartment as
foodstuffs or animal feed. Make sure that containers are in good
condition and labels undamaged, before despatch.
4.6 Spillage and Disposal
4.6.1 Spillage
Before dealing with any spillage, precautions should be taken, as
required, and appropriate personal protection should be used. Sweep
up solid products and absorb remaining spilled product with moist
sawdust, sand, or earth; transfer in suitable container to safe place
for disposal. Prevent liquid from spreading or contaminating other
cargo and vegetation, and avoid pollution of surface waters and ground
water by using the most suitable available material, e.g., earth or
sand. Since lindane is toxic for fish, care should be taken to avoid
run-off into surface waters and drains.
4.6.2 Disposal
Surplus product, contaminated absorbents, and containers should be
disposed of in an appropriate way. Lindane is not readily decomposed
chemically or biologically and is relatively persistent. Waste
material should be burned only in a proper incinerator designed for
organochlorine waste disposal, with effluent gas scrubbing. If this
is not possible, bury in an approved dump or landfill, where there is
no risk of contamination of surface or ground water, as long as local
legislation is not contravened. Puncture and/or crush containers to
prevent re-use.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Lindane may pose a toxic hazard for aquatic and terrestrial species.
It may enter the food chain and give rise to bioaccumulation and
biomagnification; it is also rather persistent in the environment. In
the event of a major environmental contamination incident, appropriate
monitoring should be carried out.
Industrial discharges from manufacturing, formulation, and technical
applications should not be allowed to pollute the environment and
should be treated properly.
Any spillage or unused product should be prevented from spreading to
vegetation or waterways and should be treated and disposed of
properly.
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, lindane. It should be displayed at, or
near, entrances to areas where there is potential exposure to
lindane, and on processing equipment and containers. The summary
should be translated into the appropriate language(s). All persons
potentially exposed to the chemical should also have the instructions
in the summary clearly explained.
Space is available for insertion of the National Occupational
Exposure Limit, the address and telephone number of the National
Poison Control Centre, and for local trade names.
LINDANE
Chemical formula: C6H6Cl6 CAS registry number: 58-89-9
CAS chemical name: 1 alpha, 2 alpha, 3ß, 4 alpha, 5 alpha, 6ß-hexachlorocyclohexane RTECS registry number: GV4900000
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Melting point (°C) 112.8 White crystalline solid; weak chemical odour;
Density (20°C) (g/ml) 1.85 stable to light, air, heat, carbon dioxide,
Vapour pressure (mmHg) (20°C) 3.26 x 10-5 and strong acids; dechlorination may occur in
Relative molecular mass 290.85 the presence of alkali, or on prolonged exposure to
n-Octanol/water partition heat; corrosive to aluminium; used as a
coefficient (log Pow) 3.2-3.7 broad-spectrum insecticide for agricultural and
Solubility in water non-agricultural applications
(mg/litre) (20°C) 10 (slightly soluble)
Solubility in:
- ethanol 6.7%
- mineral oils slightly
- acetone, aromatic, and
chlorinated solvents soluble
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
SKIN: Overexposure may cause Avoid skin contact, wear Remove contaminated clothing immediately
poisoning protective clothing, PVC or and launder before re-use; wash skin with
neoprene gloves, rubber boots water and soap
EYES: Irritation, redness Wear face-shield or goggles Flush with clean water for 15 minutes; if
irritation persists, seek medical attention
INHALATION: Dust may Wear appropriate dust mask or
irritate respirator; use appropriate
ventilation in buildings
INGESTION: Unlikely Do not eat, drink, or smoke during
occupational hazard work
Accidental or intentional Obtain medical attention immediately; if
ingestion may cause lethal gastric lavage is not possible, in a rural
poisoning situation, induce vomiting; keep at rest,
lying face downwards
ENVIRONMENT: Toxic for Do not spill on animal feed or in
aquatic and terrestrial life; water ways
bioaccumulates
SPILLAGE STORAGE FIRE AND EXPLOSION
Take appropriate personal Products should be stored in Technical material is not flammable;
precautions; prevent liquid locked buildings, preferably liquid formulations may burn; emulsifiable
from spreading or contaminating dedicated to insecticides concentrates are miscible with water;
other cargo, vegetation, or extinguish fires with alcohol-resistant
waterways, with a barrier of the foam, carbon dioxide, or powder; with
most suitable available material, Keep products out of reach of sufficient burning or external heat, the
e.g., earth or sand; absorb spilled children and unauthorized product will decompose, emitting toxic
liquid with sawdust, sand, or personnel; do not store near fumes; the smoke and fumes could be
earth; sweep up and place it in a foodstuffs or animal feed injurious through inhalation, or absorption
closeable container for later safe through the skin; therefore, protective
disposal clothing and self-contained breathing
apparatus will be required; confine the use
of water spray to the cooling of unaffected stock;
polluted water should not be allowed to
pollute the environment, but should be
disposed of properly
WASTE DISPOSAL NATIONAL INFORMATION
Lindane is not readily decomposed National Occupational Exposure UN No. 2761, 2762, 2995, 2996
chemically or biologically and is Limit:
rather persistent; waste material
should be burned in a proper
incinerator designed for
organochlorine waste disposal;
if this is not possible, bury in an National Poison Control Centre:
approved dump or landfill where there
is no risk of contamination of surface
or ground water; comply with any
local legislation regarding disposal
of toxic wastes Local Trade Names:
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file. A full reference to the original national document from which
the information was extracted can be obtained from IRPTC. When no
effective date appears in the IRPTC legal file, the year of the
reference from which the data are taken is indicated by (r).
The reader should be aware that regulatory decisions about chemicals,
taken in a certain country, can only be fully understood in the
framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application
7.1 Previous Evaluations by International Bodies
The International Agency for Research on Cancer (IARC) evaluated the
hexachlorocyclohexanes in 1987 and concluded that, for the technical
grade and the alpha-isomer, there is sufficient evidence for
carcinogenicity for animals; evidence is limited for the ß- and
gamma-isomers. There is inadequate evidence for their carcinogenicity
for human beings. Hexachlorocyclohexanes were classified in Group 2B.
WHO (1990) classified technical lindane as "moderately hazardous" in
normal use (on the basis of an LD50 of 88 mg/kg).
WHO/FAO (1975) issued a data sheet on lindane (No. 12), dealing with
labelling, safe handling, transport, storage, disposal,
decontamination, training, and medical supervision of workers,
first-aid, and medical treatment.
Lindane was evaluated by the Joint FAO/WHO Meeting on Pesticide
Residues in 1966, 1967, 1968, 1969, 1973, 1974, 1975, 1977, 1979, and
1989. A maximum acceptable daily intake (ADI) of lindane in human
beings was established at 0-0.008 mg/kg body weight by the 1989 Joint
Meeting. This value was was based on a no-observed-effect level of:
- 10 mg/kg diet, equivalent to 0.75 mg/kg body weight per day in
the rat,
and
- 1.6 mg/kg body weight per day in the dog.
Maximum residue limits (MRLs) have been recommended by the FAO/ WHO
Codex Committee for more than 35 commodities, ranging from 0.05 mg/kg
on potatoes to 3 mg/kg on strawberries. A level of 0.5 mg/kg was
recommended for most fruit and vegetables.
7.2 Exposure Limit Values
Some exposure limit values for lindane are given in the Table on pp.
34-35.
7.3 Specific Restrictions
In Japan, all uses of HCH and lindane were prohibited in 1971. The
main reason was the environmental pollution with alpha-HCH and ß-HCH
that resulted from the previous extensive use of technical HCH.
Agricultural uses of technical HCH have been prohibited in most
countries, because of environmental pollution with alpha-HCH and
ß-HCH.
The European Community legislation prohibits the placing on the
market, and the use, of HCH containing less than 99% of the
gamma-isomer. The European Community legislation also prohibits the
placing on the market of cosmetics containing HCH.
In several other countries, the use of lindane has been more or less
severely restricted, e.g., Argentina, Brazil, Czechoslovakia, and the
USA.
In the Federal Republic of Germany, lindane may not be handled by
adolescents or by pregnant or nursing women.
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit descriptiona Value Effective
organization date
AIR Workplace Argentina Threshold limit value (TLV)
- Time-weighted average (TWA) 0.5 mg/m3 1979
- Short-term exposure level (STEL) 1.5 mg/m3
Germany, Maximum work-site concentration (MAK)
Federal - 8-h Time-weighted average (TWA) 0.5 mg/m3 1985
Republic of - Short-term exposure level (STEL) 5.0 mg/m3
(30-min) (1 x per shift)
United Kingdom Maximum exposure limit
- 8-h Time-weighted average (TWA) 0.5 mg/m3 1985
- Short-term exposure level (STEL) 1.5 mg/m3
<> (10-min Time-weighted average)
USA Permissible exposure limit (PEL)
- Time-weighted average (TWA) 0.5 mg/m3 1986
USSR Maximum allowable concentration (MAC)
- Ceiling value (CLV) 0.05 mg/m3 1983
FOOD Intake from FAO/WHO Acceptable daily intake (ADI) 0-0.008 mg/kg 1989
per kg of body weight
FOOD Plant FAO/WHO Maximum residue limit (MRL)
35 food commodities, ranging from.... 0.05 to 3 mg/kg 1979
Medium Specification Country/ Exposure limit description Value Effective
organization date
FEED European Maximum residue limit (MRL)
Community All feed, 0.2 mg/kg 1989
except fats 2 mg/kg
WATER Drinking- WHO Guideline level 0.3 µg/litre 1983
WATER Drinking- European Surface water for the preparation of
Community drinking-water - total pesticides:
-Quality A1 0.001 mg/litre 1980
-Quality A2 0.0025 mg/litre
-Quality A3 0.005 mg/litre
Mexico Maximum permissible concentration (MPC) 1973
- Receiving water treated for drinking 0.056 mg/litre
USA Maximum permissible concentration (MPC) 0.004 mg/litre 1975
WATER Ambient Mexico Maximum permissible concentration (MPC) 1973
- Coastal 0.2 µg/litre
- Estuaries 0.002 mg/litre
SOIL USSR Maximum acceptable level 0.1 mg/kg 1973
a TWA = time-weighted average over one working day (usually 8 h).
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies lindane in:
- Hazard Class 6.1: poisonous substance;
- Packing Group III: substance presenting a relatively low risk of
poisoning in transport, when the active ingredient ranges from 44
to 100% (solid) or 15 to 100% (liquid).
The label should be as follows:
Class III:
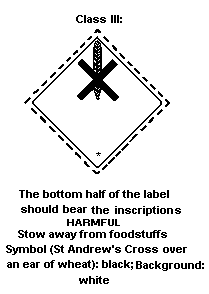 The FAO specifications for plant protection products for lindane are:
"... shall consist, essentially, of gamma-BHC as white or nearly white
granules, flakes, or powder, free from extraneous impurities or added
modifying agents, and with not more that a faint odour". FAO further
requires that it should contain not less than 99.0% gamma-HCH, and
that the melting point should be at least 112°C, not being depressed
when mixed with an equal amount of pure gamma-HCH. The acidity
maximum is 0.15% (calculated as sulfuric acid) and the loss on vacuum
drying maximum, 0.1%.
FAO also gives specifications for lindane dusts, dispersible powders,
solutions, and emulsifiable concentrates.
According to the WHO publication "Specifications for pesticides used
in public health", lindane should consist of at least 995 g
gamma-HCH/kg and should be in the form of white or near-white
granules, flakes, or powder, free from extraneous impurities or added
modifying agents. Analytical specifications are given, as well as
analytical methods.
Lindane should be packed in suitable, clean containers of specified
quality. All packages should bear, durably and legibly marked on the
container, the following:
- Manufacturer's name;
- Lindane specification;
- Batch number and date of test;
- Net weight of contents;
- Date of manufacture.
and the following minimum cautionary notice:
Keep well away from foodstuffs and animal feed and their
containers.
Similar requirements are given for water dispersible powders,
emulsifiable concentrates, and dustable powders containing gamma-HCH.
The European Economic Community (EEC) legislation requires the
labelling of lindane as a dangerous substance using the symbol:
The FAO specifications for plant protection products for lindane are:
"... shall consist, essentially, of gamma-BHC as white or nearly white
granules, flakes, or powder, free from extraneous impurities or added
modifying agents, and with not more that a faint odour". FAO further
requires that it should contain not less than 99.0% gamma-HCH, and
that the melting point should be at least 112°C, not being depressed
when mixed with an equal amount of pure gamma-HCH. The acidity
maximum is 0.15% (calculated as sulfuric acid) and the loss on vacuum
drying maximum, 0.1%.
FAO also gives specifications for lindane dusts, dispersible powders,
solutions, and emulsifiable concentrates.
According to the WHO publication "Specifications for pesticides used
in public health", lindane should consist of at least 995 g
gamma-HCH/kg and should be in the form of white or near-white
granules, flakes, or powder, free from extraneous impurities or added
modifying agents. Analytical specifications are given, as well as
analytical methods.
Lindane should be packed in suitable, clean containers of specified
quality. All packages should bear, durably and legibly marked on the
container, the following:
- Manufacturer's name;
- Lindane specification;
- Batch number and date of test;
- Net weight of contents;
- Date of manufacture.
and the following minimum cautionary notice:
Keep well away from foodstuffs and animal feed and their
containers.
Similar requirements are given for water dispersible powders,
emulsifiable concentrates, and dustable powders containing gamma-HCH.
The European Economic Community (EEC) legislation requires the
labelling of lindane as a dangerous substance using the symbol:
 The label must read:
Toxic by inhalation, in contact with skin and if swallowed;
irritating to eyes and skin; keep out of reach of children; keep
away from food, drink and animal feeding stuffs; if you feel
unwell, seek medical advice (show the label where possible).
The EEC legislation on the labelling of pesticide preparations
classifies lindane in Class 1c, for the purpose of determining the
label for preparations containing lindane.
7.5 Waste Disposal
In the USA, lindane is classified as a toxic pollutant and acute
hazardous waste, subject to handling, transport, treatment, storage,
and disposal regulations and permit and notification requirements. An
owner or operator of a hazardous waste incinerator must achieve 99.99%
destruction and removal efficiency for this substance. A ground-water
monitoring system must be installed and levels must be periodically
reported.
Aquatic environment
The EEC legislation has established limit values for the discharge of
HCH, during normal production, into the aquatic environment. The
limit values for emission standards (as of 1 October 1988) are:
g/1000 kg of product mg/litre water
HCH production plant 2 2
Lindane extraction plant 4 2
Production + extraction plant 5 2
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed. Rome, Codex Committee on Pesticide
Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage, and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man. Lyon, International Agency
for Research on Cancer.
IPCS/CEC (1990) International Chemical Safety Card No. 53: Lindane.
Luxembourg, Commission of the European Communities.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
for Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(unpublished document No. VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1989) Recommendations on the transport of dangerous
goods. 6th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (in preparation) EHC No. 124: Lindane. Geneva, World Health
Organization.
WHO (1990) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1990-91. Geneva, World
Health Organization (unpublished document WHO/PCS/90.1).
WHO (1990) Alpha- and beta-hexachlorocyclohexanes (Alpha- and
beta-HCHs) health and safety guide. Geneva, World Health
Organization. (Health and safety guide, No. 53)
WHO/FAO (1975) Data sheets on pesticides: No. 12: Lindane. Geneva,
World Health Organization (unpublished document).
WORTHING, C.R. & WALKER, S.B. (1987) The pesticide manual. 8th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
The label must read:
Toxic by inhalation, in contact with skin and if swallowed;
irritating to eyes and skin; keep out of reach of children; keep
away from food, drink and animal feeding stuffs; if you feel
unwell, seek medical advice (show the label where possible).
The EEC legislation on the labelling of pesticide preparations
classifies lindane in Class 1c, for the purpose of determining the
label for preparations containing lindane.
7.5 Waste Disposal
In the USA, lindane is classified as a toxic pollutant and acute
hazardous waste, subject to handling, transport, treatment, storage,
and disposal regulations and permit and notification requirements. An
owner or operator of a hazardous waste incinerator must achieve 99.99%
destruction and removal efficiency for this substance. A ground-water
monitoring system must be installed and levels must be periodically
reported.
Aquatic environment
The EEC legislation has established limit values for the discharge of
HCH, during normal production, into the aquatic environment. The
limit values for emission standards (as of 1 October 1988) are:
g/1000 kg of product mg/litre water
HCH production plant 2 2
Lindane extraction plant 4 2
Production + extraction plant 5 2
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed. Rome, Codex Committee on Pesticide
Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage, and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man. Lyon, International Agency
for Research on Cancer.
IPCS/CEC (1990) International Chemical Safety Card No. 53: Lindane.
Luxembourg, Commission of the European Communities.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
for Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(unpublished document No. VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1989) Recommendations on the transport of dangerous
goods. 6th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (in preparation) EHC No. 124: Lindane. Geneva, World Health
Organization.
WHO (1990) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1990-91. Geneva, World
Health Organization (unpublished document WHO/PCS/90.1).
WHO (1990) Alpha- and beta-hexachlorocyclohexanes (Alpha- and
beta-HCHs) health and safety guide. Geneva, World Health
Organization. (Health and safety guide, No. 53)
WHO/FAO (1975) Data sheets on pesticides: No. 12: Lindane. Geneva,
World Health Organization (unpublished document).
WORTHING, C.R. & WALKER, S.B. (1987) The pesticide manual. 8th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
See Also:
Toxicological Abbreviations
Lindane (EHC 124, 1991)
Lindane (ICSC)
Lindane (PIM 859)
Lindane (FAO Meeting Report PL/1965/10/1)
Lindane (FAO/PL:1967/M/11/1)
Lindane (JMPR Evaluations 2002 Part II Toxicological)
Lindane (FAO/PL:1968/M/9/1)
Lindane (FAO/PL:1969/M/17/1)
Lindane (WHO Pesticide Residues Series 3)
Lindane (WHO Pesticide Residues Series 4)
Lindane (WHO Pesticide Residues Series 5)
Lindane (Pesticide residues in food: 1977 evaluations)
Lindane (Pesticide residues in food: 1978 evaluations)
Lindane (Pesticide residues in food: 1979 evaluations)
Lindane (Pesticide residues in food: 1989 evaluations Part II Toxicology)
Lindane (Pesticide residues in food: 1997 evaluations Part II Toxicological & Environmental)
 Chemical formula: C6H6Cl6
Relative molecular mass: 290.85
CAS chemical name: 1 alpha, 2 alpha, 3ß, 4 alpha, 5 alpha,
6ß-hexachlorocyclohexane
CAS registry number:
Chemical formula: C6H6Cl6
Relative molecular mass: 290.85
CAS chemical name: 1 alpha, 2 alpha, 3ß, 4 alpha, 5 alpha,
6ß-hexachlorocyclohexane
CAS registry number:  The FAO specifications for plant protection products for lindane are:
"... shall consist, essentially, of gamma-BHC as white or nearly white
granules, flakes, or powder, free from extraneous impurities or added
modifying agents, and with not more that a faint odour". FAO further
requires that it should contain not less than 99.0% gamma-HCH, and
that the melting point should be at least 112°C, not being depressed
when mixed with an equal amount of pure gamma-HCH. The acidity
maximum is 0.15% (calculated as sulfuric acid) and the loss on vacuum
drying maximum, 0.1%.
FAO also gives specifications for lindane dusts, dispersible powders,
solutions, and emulsifiable concentrates.
According to the WHO publication "Specifications for pesticides used
in public health", lindane should consist of at least 995 g
gamma-HCH/kg and should be in the form of white or near-white
granules, flakes, or powder, free from extraneous impurities or added
modifying agents. Analytical specifications are given, as well as
analytical methods.
Lindane should be packed in suitable, clean containers of specified
quality. All packages should bear, durably and legibly marked on the
container, the following:
- Manufacturer's name;
- Lindane specification;
- Batch number and date of test;
- Net weight of contents;
- Date of manufacture.
and the following minimum cautionary notice:
Keep well away from foodstuffs and animal feed and their
containers.
Similar requirements are given for water dispersible powders,
emulsifiable concentrates, and dustable powders containing gamma-HCH.
The European Economic Community (EEC) legislation requires the
labelling of lindane as a dangerous substance using the symbol:
The FAO specifications for plant protection products for lindane are:
"... shall consist, essentially, of gamma-BHC as white or nearly white
granules, flakes, or powder, free from extraneous impurities or added
modifying agents, and with not more that a faint odour". FAO further
requires that it should contain not less than 99.0% gamma-HCH, and
that the melting point should be at least 112°C, not being depressed
when mixed with an equal amount of pure gamma-HCH. The acidity
maximum is 0.15% (calculated as sulfuric acid) and the loss on vacuum
drying maximum, 0.1%.
FAO also gives specifications for lindane dusts, dispersible powders,
solutions, and emulsifiable concentrates.
According to the WHO publication "Specifications for pesticides used
in public health", lindane should consist of at least 995 g
gamma-HCH/kg and should be in the form of white or near-white
granules, flakes, or powder, free from extraneous impurities or added
modifying agents. Analytical specifications are given, as well as
analytical methods.
Lindane should be packed in suitable, clean containers of specified
quality. All packages should bear, durably and legibly marked on the
container, the following:
- Manufacturer's name;
- Lindane specification;
- Batch number and date of test;
- Net weight of contents;
- Date of manufacture.
and the following minimum cautionary notice:
Keep well away from foodstuffs and animal feed and their
containers.
Similar requirements are given for water dispersible powders,
emulsifiable concentrates, and dustable powders containing gamma-HCH.
The European Economic Community (EEC) legislation requires the
labelling of lindane as a dangerous substance using the symbol:
 The label must read:
Toxic by inhalation, in contact with skin and if swallowed;
irritating to eyes and skin; keep out of reach of children; keep
away from food, drink and animal feeding stuffs; if you feel
unwell, seek medical advice (show the label where possible).
The EEC legislation on the labelling of pesticide preparations
classifies lindane in Class 1c, for the purpose of determining the
label for preparations containing lindane.
7.5 Waste Disposal
In the USA, lindane is classified as a toxic pollutant and acute
hazardous waste, subject to handling, transport, treatment, storage,
and disposal regulations and permit and notification requirements. An
owner or operator of a hazardous waste incinerator must achieve 99.99%
destruction and removal efficiency for this substance. A ground-water
monitoring system must be installed and levels must be periodically
reported.
Aquatic environment
The EEC legislation has established limit values for the discharge of
HCH, during normal production, into the aquatic environment. The
limit values for emission standards (as of 1 October 1988) are:
g/1000 kg of product mg/litre water
HCH production plant 2 2
Lindane extraction plant 4 2
Production + extraction plant 5 2
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed. Rome, Codex Committee on Pesticide
Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage, and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man. Lyon, International Agency
for Research on Cancer.
IPCS/CEC (1990) International Chemical Safety Card No. 53: Lindane.
Luxembourg, Commission of the European Communities.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
for Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(unpublished document No. VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1989) Recommendations on the transport of dangerous
goods. 6th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (in preparation) EHC No. 124: Lindane. Geneva, World Health
Organization.
WHO (1990) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1990-91. Geneva, World
Health Organization (unpublished document WHO/PCS/90.1).
WHO (1990) Alpha- and beta-hexachlorocyclohexanes (Alpha- and
beta-HCHs) health and safety guide. Geneva, World Health
Organization. (Health and safety guide, No. 53)
WHO/FAO (1975) Data sheets on pesticides: No. 12: Lindane. Geneva,
World Health Organization (unpublished document).
WORTHING, C.R. & WALKER, S.B. (1987) The pesticide manual. 8th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
The label must read:
Toxic by inhalation, in contact with skin and if swallowed;
irritating to eyes and skin; keep out of reach of children; keep
away from food, drink and animal feeding stuffs; if you feel
unwell, seek medical advice (show the label where possible).
The EEC legislation on the labelling of pesticide preparations
classifies lindane in Class 1c, for the purpose of determining the
label for preparations containing lindane.
7.5 Waste Disposal
In the USA, lindane is classified as a toxic pollutant and acute
hazardous waste, subject to handling, transport, treatment, storage,
and disposal regulations and permit and notification requirements. An
owner or operator of a hazardous waste incinerator must achieve 99.99%
destruction and removal efficiency for this substance. A ground-water
monitoring system must be installed and levels must be periodically
reported.
Aquatic environment
The EEC legislation has established limit values for the discharge of
HCH, during normal production, into the aquatic environment. The
limit values for emission standards (as of 1 October 1988) are:
g/1000 kg of product mg/litre water
HCH production plant 2 2
Lindane extraction plant 4 2
Production + extraction plant 5 2
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed. Rome, Codex Committee on Pesticide
Residues.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage, and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of
pesticides. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man. Lyon, International Agency
for Research on Cancer.
IPCS/CEC (1990) International Chemical Safety Card No. 53: Lindane.
Luxembourg, Commission of the European Communities.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
for Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization
(unpublished document No. VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1989) Recommendations on the transport of dangerous
goods. 6th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (in preparation) EHC No. 124: Lindane. Geneva, World Health
Organization.
WHO (1990) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1990-91. Geneva, World
Health Organization (unpublished document WHO/PCS/90.1).
WHO (1990) Alpha- and beta-hexachlorocyclohexanes (Alpha- and
beta-HCHs) health and safety guide. Geneva, World Health
Organization. (Health and safety guide, No. 53)
WHO/FAO (1975) Data sheets on pesticides: No. 12: Lindane. Geneva,
World Health Organization (unpublished document).
WORTHING, C.R. & WALKER, S.B. (1987) The pesticide manual. 8th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
