
Pesticide residues in food - 2002 - Joint FAO/WHO Meeting on Pesticide Residues
First draft prepared by
E. Mendez
Office of Pesticide Programs, United States Environmental Protection Agency,
Washington DC, USA
Lindane (1alpha,2alpha,3beta,4alpha,5alpha,6beta-1,2,3,4,5,6-hexachlorocyclohexane) is a broad-spectrum organochlorine compound used against a wide range of soil-dwelling and plant-eating insects. It is commonly used on numerous crops, as a seed treatment, in warehouses and to control insect-borne diseases. Lindane is also used in the treatment of scabies and lice in humans. Lindane was last evaluated by the 1997 JMPR (Annex 1, reference 80), when a temporary ADI of 00.001 mg/kg bw was established on the basis of deaths and hepatic toxicity in a 2-year study of toxicity and carcinogenicity in rats. The ADI was made temporary because of concern about immunotoxic effects reported in mice given lindane (purity, 97%) at doses of 0.12 mg/kg bw per day and above. Lindane was re-evaluated at the present Meeting within the periodic review programme of the Codex Committee on Pesticide Residues.
Lindane is the gamma isomer of hexachlorocyclohexane. Five other isomers of hexachlorocyclo-hexane are commonly found in technical-grade lindane, but the gamma isomer is the predominant one, comprising at least 99% of the mixture.
Mice
Single doses of [14C]lindane were administered by gavage to female ICR mice that had been fasted for 12 h at a dose of 1 mg/kg bw (1 ΅Ci of labelled compound). About 54% of the administered dose was absorbed through the gastrointestinal tract within 15 min, and 71% had been absorbed within 1 h. Urinary excretion of the radiolabelled compound reached a maximum of 4.1% of the dose 60 min after administration (Ahdaya et al., 1981).
In a published study, five female C57 Bl/6 and six DBA/2 mice received lindane (purity, 99.9%) at a single oral dose of 20 mg/kg bw by gavage. Blood samples were collected 0, 5, 10, 15, 20, 30, 40, 50 and 60 min after administration to determine the concentration of lindane or its metabolites. A time-dependent increase in the blood concentration of lindane was seen in both strains within 40 min of treatment. After 40 min, the concentration in blood reached a plateau of 500 ng/ml (Liu & Morgan, 1986).
In a study designed to assess the accumulation of residues, groups of five female Swiss mice were given diets containing lindane (purity, 99.8%) at a concentration providing a dose of 1.5 mg/kg bw per day for 4 weeks, 1 mg/kg bw per day for 6 weeks or 3.1 mg/kg bw per day for 2, 4 or 6 weeks. Lindane was measured in whole brain, ovary, adrenal gland, liver, kidney, abdominal fat and femoral muscle. The accumulation of lindane residues in tissues displayed a time- and dose-related increase in all treated groups. For example, the accumulation was greater in animals receiving 3.1 mg/kg bw per day for 6 weeks than in animals given the same dose for shorter periods (2 or 4 weeks). Although the total intake of the compound remained constant at 43 mg, accumulation of lindane residues increased in a time-dependent manner (Table 1), suggesting that a steady state had not been reached. For all treatment groups, the highest lindane content was in fat, followed by brain, kidney, muscle, liver, adrenal and ovary (Lahiri et al., 1990).
Lindane (purity, 99.9%) was administered by gavage to six B6 and six D2 female mice at a dose of 20 mg/kg bw per day for 10 consecutive days. Blood samples were collected daily 1 h after administration or at the time of sacrifice after blood collection on day 10. Brain, liver, spleen and kidneys were collected for determination of lindane, and metabolites were measured in all tissues except brain. In order to determine the time course of lindane concentrations in blood, three additional B6 mice were given lindane at the same dose for 20 days.
Table 1. Lindane content (ppm) of various tissues of mice given diets containing lindane
|
Tissue |
Dose regimen |
||||
|
1.5 mg/kg bw |
1 mg/kg bw |
3.1 mg/kg bw |
3.1 mg/kg bw |
3.1 mg/kg bw |
|
|
Fat |
0.39 |
0.45 |
0.33 |
0.80 |
1.0 |
|
Brain |
0.30 |
0.34 |
0.23 |
0.56 |
0.63 |
|
Kidney |
0.19 |
0.26 |
0.15 |
0.37 |
0.48 |
|
Muscle |
0.16 |
0.20 |
0.10 |
0.24 |
0.32 |
|
Liver |
0.13 |
0.15 |
0.09 |
0.19 |
0.25 |
|
Adrenal |
0.02 |
0.05 |
0.01 |
0.15 |
0.19 |
|
Ovary |
0.009 |
0.02 |
0.008 |
0.10 |
0.12 |
From Lahiri et al. (1990)
The concentration of lindane in blood increased to a similar extent in the two strains during the first 4 days of the study. Subsequently, however, the concentration was higher in D2 mice than in B6 mice. All of the B6 mice but none of the D2 mice survived to the end of the study on day 10. One death was reported on day 6 of the study, and deaths occurring daily thereafter within 1 h after the last dose; the clinical signs of toxicity at that time included tremors, rapid respiration, spasms and convulsions. At the end of the study, the concentration of lindane in the blood of B6 mice was 41% lower than that in D2 mice. Furthermore, the concentration of lindane in the brains of D2 mice was statistically significantly higher (by 78%; p < 0.001) than in B6 mice, while the concentrations in liver, kidney and spleen were comparable in the two strains. In B6 mice dosed for 20 days, the concentration of lindane in blood continued to increase until the end of the study (Liu & Morgan, 1986).
Groups of 72 F1 female mice of the obese yellow, lean pseudoagouti and lean black phenotypes, 4 weeks of age, were given diets containing lindane (purity, > 99%) at a concentration of 0 or 160 ppm. Eighteen mice of each phenotype at each dose received a single oral dose of 18 mg/kg bw of [14C]lindane (55 ΅Ci; specific activity, 82 mCi/mmol; radiochemical purity, 97%) for 13, 26, 52 or 82 weeks, were then transferred to metabolism cages for collection of urine, faeces and expired air for 24 h and were killed 24 h later. Liver, kidney, blood and adipose tissue were collected at necropsy for determination of their radioactivity content.
Mice that had received lindane in their diet before administration of radiolabelled compound (for < 52 weeks; i.e. 56 weeks of age) excreted less radioactivity than concurrent controls, consistent with the reported increase in radioactivity in adipose tissue induced by lindane. Mice aged 86 weeks (treated for 82 weeks), regardless of phenotype, showed an excretion pattern similar to that of concurrent controls, representing 3047% of the administered dose. In general, obese yellow mice (control and treated) excreted substantially less radioactivity than their black and pseudoagouti counterparts. In tissues, the highest lindane content was that of adipose tissue at all times (Table 2). The main route of excretion was urine, which contained 3540% of the administered dose, while faeces contained 37% (Chadwick & Copeland, 1987).
Table 2. Content of radiolabel in tissues (per cent administered dose per organ [liver, kidney] or per dose [blood, adipose tissue])
|
Treatment week |
Liver |
Kidney |
Blood |
Adipose tissue |
||||||||
|
Black |
Pseudoagouti |
Yellow |
Black |
Pseudoagouti |
Yellow |
Black |
Pseudoagouti |
Yellow |
Black |
Pseudoagouti |
Yellow |
|
|
13 |
0.62 |
0.58 |
0.47 |
0.070 |
0.060 |
0.069 |
0.11 |
0.087 |
0.069 |
6.1 |
4.6 |
4.1 |
|
26 |
0.47 |
0.58 |
0.46 |
0.054 |
0.058 |
0.041 |
0.14 |
0.12 |
0.064 |
5.8 |
6.4 |
3.1 |
|
52 |
1.6 |
1.1 |
0.48 |
0.092 |
0.098 |
0.039 |
0.18 |
0.24 |
0.084 |
6.4 |
5.3 |
1.7 |
|
82 |
1.1 |
1.0 |
0.59 |
0.12 |
0.13 |
0.077 |
0.12 |
0.11 |
0.046 |
4.7 |
4.1 |
1.5 |
From Chadwick & Copeland (1987)
Rats
Six female Holtzman rats were given [14 C]lindane (purity, 99%) at a single oral dose of 1.7 mg and were killed 24 h later. Urine, faeces, liver, kidneys and adipose tissues were collected. By 24 h after administration, 13% of the administered dose was recovered in the excreta, urinary excretion accounting for 12% and faeces efor only 0.3%. Urine samples were further analysed to ascertain the metabolite profile. Most of the radioactivity recovered in urine was in the form of chlorophenols (both free and conjugated) and polar metabolites; the metabolites included 2,3,5-trichlorophenol, 2,4,6-trichlorophenol and 2,3,4,6-tetracholorophenol. Evaluation of the distribution of radioactivity indicated that adipose tissue accumulated lindane and/or its metabolites to a greater extent than kidney or liver (Chadwick et al., 1977).
The absorption, distribution, metabolism and excretion of [14C]lindane (radiochemical purity, 98%) were studied in groups of six female Sprague-Dawley rats given a single oral dose of 1.9 mg of lindane with or without pretreatment (in the diet) with 500 ppm Aroclor 1254, 360 ppm phenobarbital or beta-naphthoflavone 1 week before lindane administration. The excretion and distribution pattern of radioactivity were determined 24 h after treatment. Animals that had not been pretreated excreted a total of 22% of the administered dose in urine, faeces and expired air within 24 h. Urinary excretion accounted for 20% of the administered dose, while that in faeces and expired air represented 2% and 0.02%, respectively. Liver, kidneys and adipose tissue were collected from each animal. The highest concentration of radiolabel was found in adipose tissue, accounting for 10% of the administered dose, while the radioactivity detected in liver and kidneys amounted to 0.03% and 0.02%, respectively.
In animals pretreated with either Aroclor 1254 or phenobarbital, 83% and 78% of the administered dose was excreted, respectively, within 24 h, representing a fourfold increase over that in animals given lindane alone. In contrast, pretreatment with beta-naphthoflavone did not significantly alter the pattern of excretion of radioactivity when compared with the control group receiving no pretreatment (Chadwick et al., 1981).
Male Wistar rats received diets containing lindane (purity, 99.99%) at a concentration of 0 or 25 ppm for 6 months, and an additional group of rats received lindane in the diet for 6 months, followed by a 1-month recovery period. After the appropriate treatment period, four rats receiving 25 ppm lindane for 6 months and two rats each receiving 25 ppm lindane for 6 months with a 1-month recovery or basal diet for 6 months were given a single dose of 5 mg/kg bw per day of [3H]lindane (specific activity, 57 mCi/mmol per l) by gavage. The animals were killed 8 h after administration of radiolabelled compound, and samples were collected from the liver, kidney, renal cortex, fat, brain and testes. The highest concentration of radioactivity in the tissue samples was found in the renal cortex (31% of recovered radioactivity), followed by fat, liver, brain, testes and kidneys (Zhu et al., 1986).
In a study to compare the concentration of radioactivity in blood and urine after oral, intraperitoneal and intravenous administration of [14C]lindane in rats, intragastric administration resulted in rapid absorption, as evidenced by observation of peak concentrations in blood 510 h after treatment. Rapid absorption was coupled with rapid elimination from the blood compartment, where no radioactivity was detected 35 h after treatment. Approximately 80% of the administered radioactivity was excreted in the urine within the first 8 days (Sulik et al., 1988).
Six male Wistar rats received lindane (purity unspecified) at a dose of 8 mg/kg bw per day for 10 days, followed by [14C]lindane at a single oral dose of 90 ΅g/kg bw (2.5 ΅Ci). The rats were killed 24 or 72 h after administration of the radioactive dose. Urine, blood and faeces and 14 organs (e.g., brain, heart, lung, liver, spleen, kidney, muscle, fat) were collected at necropsy for determination of excretion and radiolabel deposition.
The highest concentration of radioactivity was detected in adipose tissue at 24 and 72 h (37% and 17% of the administered dose, respectively), followed by the kidneys (3.7%) and muscle (3.5%) at the 24-h evaluation period only. The remaining organs examined contained < 1% of the administered radioactivity. Urine contained 31% of the administered radiolabel at 24 h and 46% at 72 h after treatment. Most of the radioactivity recovered in excreta (92% in urine and 57% in faeces) was in the form of conjugated metabolites, while unmodified lindane comprised 36% of the administered dose (Seidler et al., 1971).
Lindane (purity, 99.9%) was administered to Chbb:THOM (SPF) rats by gavage at a dose of 15 mg/kg bw per day for 14, 28 or 56 days to investigate the effect of dosing duration on the distribution of lindane. Additionally, the distribution of lindane in blood, brain, liver, kidney and fat tissue was evaluated 5, 8 and 15 days after cessation of administration. The concentration in tissue samples increased during the first 2 weeks of treatment but declined thereafter. Although all the tissues had this pattern of accumulation and elimination, the liver and kidneys showed a more dramatic change in lindane deposition, as evidenced by a 10-fold decrease in radioactivity at the end of the study (day 71) when compared with the value at day 14. This suggests that lindane induces self-metabolism by induction of metabolic enzymes (Eichler et al., 1983).
In a study of the distribution of lindane, groups of five Wistar rats were given diets containing lindane (purity, > 99%) at a concentration of 100 or 800 ppm for 5, 10 or 15 days. Brain, liver, blood, kidney, heart, lung, spleen, muscle and epidydimal fat samples were collected at necropsy to determine the lindane content. In a concurrent set of experiments, the role of nutritional state on the distribution of lindane was evaluated. Animals received lindane for 2 weeks and were then assigned to three groups: one for immediate sacrifice, one given basal diet ad libitum for 1 week, and one given a restricted diet.
The experiments designed to yield information on the tissue distribution of lindane showed that, with the exception of fat tissue, the concentration of lindane did not increase with dose or duration. In the case of adipose tissue, a twofold increase in lindane content was reported in animals at 800 ppm as compared with those given 100 ppm. An increase was also noted with duration of treatment in the groups at 100 and 800 ppm, although the increase at the lower dose was slight.
Food restriction after administration of lindane did not result in a significant redistribution of lindane residues. As would be expected during a period of partial starvation, fat depots were mobilized (Srinivasan & Radhakrishnamurty, 1983).
In a study of the effects of a protein-deficient diet on the distribution of hexachlorocyclo-hexanes in rat tissues, groups of 120 male Druckrey rats received diets containing technical-grade material containing 6.5% lindane at a concentration providing a dose of 50 mg/kg bw per day, for 30 days and were then killed. The animals received a diet containing 5, 10 or 21% casein starting 28 days before initiation of dosing (a diet containing 21% casein is considered to be normal).
The pattern of distribution of lindane to various tissues was affected by the protein content of their diet. The concentrations in adipose tissue and adrenal glands were twofold higher in animals that received a protein-deficient diet (5% casein) than in those receiving a basal diet (21% casein). Animals receiving the basal diet had less overall distribution of lindane (relative to animals receiving the protein-deficient diet) to all tissues examined (kidney, thymus, adipose, adrenals, muscle, lungs, spleen, testes and blood), whereas the liver, heart and brain showed slight increases (Table 3) (Khanna et al., 1995).
Table 3. Effect of dietary protein content on distribution of lindane in tissues of rats
|
Tissue |
Concentration of lindane (΅g/g or ΅g/ml) |
||
|
5% casein |
10% casein |
21% casein |
|
|
Liver |
0.09 |
0.06 |
0.23 |
|
Kidney |
0.95 |
1.0 |
0.84 |
|
Thymus |
0.61 |
0.06 |
0.18 |
|
Adipose |
11 |
5.6 |
5.8 |
|
Adrenal |
3.2 |
0.86 |
0.96 |
|
Muscle |
0.50 |
0.22 |
0.26 |
|
Heart |
0.09 |
0.12 |
0.33 |
|
Lungs |
0.42 |
0.24 |
0.28 |
|
Spleen |
0.45 |
0.24 |
0.28 |
|
Testes |
0.05 |
0.03 |
0.01 |
|
Brain |
0.11 |
0.15 |
0.50 |
|
Blood |
0.28 |
0.21 |
0.23 |
From Khanna et al. (1995)
Six female Sprague-Dawley rats were each given 2 mg of lindane for 6 days and, on day 7, 2 mg of lindane containing [14C]lindane (1.6 ΅Ci). The rats were then placed in metabolism cages and their urine and feces collected for 24 h and analysed for radioactivity. Within the first 24 h, 58% of the administered dose was found in the excreta (Chadwick & Freal, 1972).
Groups of 36 female Sprague-Dawley rats received diets containing lindane (purity, 99.8%) at a concentration of 0, 130, 220 or 350 ppm. Six rats per dose were killed after 1, 2, 4, 8, 16 and 24 weeks of treatment, 24 h after receiving a single oral dose of lindane (2.1 mg after 1 and 2 weeks and 19 mg thereafter). A dose-dependent increase in the lindane content of fat and liver over that in concurrent controls was reported. However, the hepatic content declined with prolonged treatment. A plausible explanation for this effect is that lindane induces its own metabolism (Copeland & Chadwick 1979).
Lindane (20% emulsifiable concentrate) spiked with [14C]lindane was applied topically to groups of four male Crl:CD®(SD)BR rats at a dose of 0.1, 1 or 10 mg. Four rats at each dose were bled and killed 0.5, 1, 2, 4, 10 and 24 h after treatment. As the area was not rinsed, the animals were exposed continuously until sacrifice. Urine was collected at the time of sacrifice, and radioactivity was measured in urine, faeces, blood, application site (skin), skin wash, carcass, dose applicator and the cover of the application site.
No clinical signs of toxicity were observed during the study period. The absorption of [14C]lindane in the group given 0.1 mg ranged from 0.6% after 30 min to 28% 24 h after administration. The percentage of the dose absorbed appeared to decrease with increasing dose. The absorption in animals given 1 mg of lindane ranged from 1% at 30 min to 21% at the 24-h sacrifice, while animals given the highest dose of lindane (10 mg) had an absorption rate of 0.7% at 30 min and 5.1% at 24 h (Bosch, 1987).
Rabbits
Five male New Zealand white rabbits received [14C]lindane (purity, > 99%) in gelatin capsules twice a week for 26 weeks, resulting in doses of 3 mg/kg bw per day during the first 4 weeks of treatment, 6 mg/kg bw per day in weeks 515 and 12 mg/kg bw per day thereafter. Urine and faeces were collected daily for 32 weeks. After a 6-week recovery period (week 32 of the study), the animals were killed, and radioactivity was measured in various tissues. At the end of the dosing regimen (week 26), 54% of the administered radiolabel was recovered in the urine, while the faecal output amounted to 13% of the administered dose. During the recovery period, 3% and 1% of the administered radiolabel was recovered in urine and faeces, respectively. The highest concentration of lindane or its metabolites was found in fat tissue, followed by liver, kidney, muscle and brain (Karapally et al., 1973).
Lindane (20% emulsifiable concentrate containing 14C-lindane) was administered topically once to groups of four male Hra:(NZW)SPF rabbits at a dose of 0.5, 5 or 50 mg. Four rabbits at each dose were bled and killed 0.5, 1, 2, 4, 10 and 24 h after application. As the area had not been rinsed, they were exposed continuously until sacrifice. Urine was collected at the time of sacrifice, and radioactivity was measured in urine, faeces, blood, application site (skin), skin wash, carcass, dose applicator and the cover of the application site. The only signs of toxicity seen during the study were soft, mucoid faeces in 2/24 animals given 0.5 mg, 6/24 given 5 mg and 1/24 given 50 mg.
The absorption of [14C]lindane in animals given a topical application of 0.5 mg ranged from 6% 30 min after application to 57% after 24 h. The percentage of the dose absorbed appeared to decrease with increasing dose, as evidenced by absorption of 740% (30 min24 h after exposure) of the dose of 5 mg and absorption of 217% (30 min24 h after exposure) of the dose of 50 mg (Bosch, 1987).
Sheep
In a study designed to examine the potential transfer of lindane across the placenta, lindane (purity unspecified) was administered to groups of four ewes at a dose of 1 or 5 mg/kg bw per day for 10 days at mid-gestation. The ewes lambed 711 weeks after initiation of dosing. The concentrations of lindane in blood and fat were assayed at various times. Omental fat from ewes at the two doses contained 10 and 54 ppm lindane, respectively, during the first and second week after administration, while these values declined substantially after lambing, to 0.3 and 0.6 ppm. The concentrations of lindane in lambs (0.20.4 ppm) were slightly lower than those in ewes 4 weeks after parturition (Harrison & Mol, 1968).
Goats
Alpine goats were given lindane (purity, 99.8%) containing [14C]lindane in gelatine capsules at a dose of 1 (two goats) or 10 mg/kg bw per day (one goat) for 4 days. The animals were killed 24 h after the last administration, and radioactivity was measured in tissues, milk, urine, faeces, gut content and expired air. Within 4 days of treatment, 40% of the administered radiolabel had been excreted in the urine, while the faecal output accounted for 6% of the administered dose. The concentration of lindane in milk was 12% and reached a plateau after 3 days. It is noteworthy that 85% of the 14C-labelled residues found in milk were partitioned into fat. As seen in other species, the highest concentration of radioactivity in tissues was detected in adipose tissue (about 2% of the administered dose), followed by liver (Wilkes et al., 1987).
Pigs
Four Durok and Yorkshire boars were given diets containing lindane (purity unspecified) at concentrations providing a dose of 0, 1 or 2 mg/kg bw per day for 21 days. During the last week of the study, the animals were housed in metabolism cages for collection of urine and faeces. At necropsy, liver, kidney, back fat and longissimus dorsi samples were collected for analysis of their lindane content. The highest concentration was found in back fat, 0.19, 20 and 44 ppm being detected after treatment at 0, 1 and 2 mg/kg bw per day, respectively (Davey & Gerrits, 1969)
Groups of three pigs of each sex were given diets containing lindane (purity unspecified) at concentrations providing a dose of 0, 2 or 40 mg/kg bw per day. Blood and back fat were collected at 6-week intervals for determination of the lindane content. During most of the study, the concentrations in blood remained at < 0.01 ppm for animals at both doses. A time- and dose-dependent increase in lindane content was reported in the back fat samples. The highest concentration was detected in adipose tissue, followed by brain, kidney and muscle (Davey & Johnson, 1974).
Cattle
Four milking cows received diets containing lindane (purity unspecified) in gelatine capsules at doses ranging from 0.07 to 6.2 mg/kg bw per day for 70180 days. The lindane concentration in the milk was measured daily. A dose-dependent increase was reported, the concentration ranging from 0.07 to 10 ppm (Ely et al., 1952).
Mice
Five C57Bl/6 and six DBA/2 female mice received lindane (purity, 99.9%) at a dose of 20 mg/kg bw per day once or for 10 consecutive days, and the time course of the blood concentration of the metabolite 2,4,6-trichlorophenol was assayed over 1 h. On day 10 of the study, the animals were killed, and brain, liver, spleen, and kidney samples were collected for determination of metabolites. In animals given the single oral administration of lindane, the blood concentration of 2,4,6-trichlorophenol increased in both strains up to 100 ng/ml within 1 h. Two principal metabolites were identified in the blood after repeated oral administration of lindane: 2,4,6-trichlorophenol and 2,3,4,6-tetrachlorophenol, the former predominating (Liu & Morgan, 1986).
Rats
The synthesis of chlorinated phenols resulting from metabolism of lindane was examined in eight male Wistar rats that received a single oral dose of lindane (purity unspecified) at 0 or 68 mg/kg bw. Urine samples were collected daily for 4 days after treatment and analysed for lindane metabolites. In addition, the conjugation rates of metabolites were investigated. During the collection period, four major metabolites were identified consistently: 2,3-dichlorophenol, 2,4,6-trichlorophenol, 2,4,5-trichlorophenol and 2,3,5,6-tetrachlorophenol. The degree of conjugation was extensive, particularly during the first 24 h after administration of the test article. The conjugation rate ranged from 72% for 2,4,6-trichlorophenol to 42% for 2,4,5-trichlorophenol and declined in a time-dependent fashion. Under the conditions of this study, 2,3,5,6-tetrachloro-phenol was the predominant metabolite (12 ΅mol/l), followed by 2,4,6-trichlorophenol (0.51.5 ΅mol/l), 2,3-dichlorophenol (0.251 ΅mol/l) and 2,4,5-trichlorophenol (0.10.5 ΅mol/l) after 24 days. The phenolic metabolites were excreted primarily in the urine within 2448 h of administration (Baliková et al., 1989).
The profile of lindane metabolites in the brain was investigated in male Wistar rats given lindane (purity unspecified) at a single dose of 0, 30 or 60 mg/kg bw. Animals receiving the lower dose were killed 5 h after treatment, while those given the higher dose were killed 24 h after treatment. Brain samples were collected, and homogenates were analysed for the presence and identity of metabolites. The predominant metabolite was 2,4,6-trichlorophenol. After administration of 60 mg/kg bw, six major metabolites (1,2,3,5-tetrachlorobenzene, 1,2,4,5-tetrachlorobenzene, 1,2,3,4-tetrachlorobenzene, 2,3,4,5,6-pentachlorocyclohexene, pentachlorobenzene, 3,6/4,5-hexachlorocyclohexene) and lindane were detected. The metabolites in the cerebella of animals at the lower dose were investigated. In this structure, metabolism occurred primarily through dehydrochlorination, resulting in 3,6/4,5- and 3,5/4,6-pentachlorocyclohexene. Hexachlorobenzene was detected in all samples at concentrations of 0.51 ppb (Artigas et al., 1988).
Six female Holtzman rats received 1.7 mg of lindane (purity unspecified) containing 1.5 ΅Ci [14C]lindane. Urine samples were collected 24 h later and analysed for metabolite content, and the animals were killed. Liver samples were homogenized and analysed for microsomal enzyme activity in vitro. Of the radioactivity excreted in the urine 24 h after treatment, only 4.4% was identified as neutral metabolites, while 26%, 27% and 45% of the radioactivity was associated with free, conjugated and polar metabolites, respectively. The predominant phenols excreted in the urine were 2,4,6-trichlorophenol and 2,3,4,6-tetrachlorophenol, in a ratio of 2.7 (Chadwick et al., 1977).
Groups of male Wistar rats received lindane (purity unspecified) or its metabolites gamma-2,3,4,5,6 pentachlorocyclohexene, pentachlorobenzene and pentachlorophenol at a dose of 8 mg/kg bw per day orally for 19 days At necropsy, blood, liver, kidneys, adrenals, heart, spleen, brain, muscle and intestinal adipose tissue were collected and analysed. Excreta were pooled weekly and analysed with and without glucuronidase treatment.
The metabolites found in urine were pentachlorophenol, 2,3,4,6-tetrachlorophenol, 2,3,5,6-tetrachlorophenol and 2,4,6-trichlorophenol, as well as lindane. Only lindane was identified in faeces. The pattern of metabolites in blood paralleled that in urine, while the metabolites in liver consisted of gamma-pentachlorocyclohexene, pentachlorophenol, 2,3,4,6-tetrachlorophenol and 2,3,5,6-tetrachlorophenol. The predominant metabolite varied among the organs examined. While gamma-pentachlorocyclohexene was the predominant metabolite in kidneys, that in spleen was pentachlorophenol, those in the heart were 2,4,6-trichlorophenol, 2,3,4,6-tetrachlorophenol and gamma-pentachlorocyclohexene, and gamma-pentachlorocyclohexene was the only metabolite identified in brain. Glucuronidase treatment showed that trichlorophenols, 2,3,4,6-, 2,3,5,6- and 2,3,4,5-tetrachlorophenol and pentachlorophenol were conjugated, although not extensively (Engst et al., 1976).
Lindane (purity unspecified) was administered to six female Fischer 344 rats at a dose of 2 mg for 13 days, and on day 14 [14C]lindane (2 ΅Ci) was added to the dose. Urine and faeces were collected daily and assayed for metabolite content. Urine contained increasing concentrations of glucuronic acid conjugates from day 4 of treatment; sulfate conjugation appeared to be slightly inhibited during the first 5 days of treatment but increased to the level in concurrent controls thereafter (Chadwick et al., 1971).
Six female Sprague-Dawley rats received 0 or 2 mg of lindane (purity unspecified) orally for 6 days and 2 mg of lindane containing 1.6 ΅Ci [14C]lindane on day 7. Urine samples were collected at 24-h intervals throughout the study and analysed for metabolite content. In general, the pretreated animals excreted more radioactivity than concurrent controls and excreted substantially more trichlorophenols (65%, with 30% in controls). On day 8, the tetrachlorophenols excreted by controls consisted of approximately 25% 2,3,4,6-tetrachlorophenol, while pretreated animals excreted 45% (Chadwick & Freal, 1972).
Three novel lindane metabolites were identified in female Sprague-Dawley rats given diets containing lindane (purity unspecified) at a concentration of 400 ppm for 1 month. Urine samples were collected daily and analysed for lindane metabolites after acidification and extraction. Analysis of the urinary metabolite profile resulted in the identification of 2,3,4,5,6-pentacychlorocyclohexene-(2)-ol-1, 2,3,4,6-tetrachlorocyclohexenol and 2,4,5,6-tetrachlorocyclo-hexenol. These metabolites were excreted primarily as sulfate and glucuronide conjugates (Chadwick et al., 1978).
Rats received lindane (purity unspecified) at an oral dose of 40 mg/kg bw per day on days 1, 3 and 5, and urine and faeces were collected daily until study termination on day 7 and analysed for metabolites. Hexachlorobenzene was detected exclusively in the faeces. Formation of hexachlorobenzene is the result of sequential dehydrogenation of lindane (Gopalaswamy & Aiyar, 1986).
Humans
Human metabolism of lindane was investigated during biomonitoring of lindane production and forestry workers, by analysing the urine of workers potentially exposed to technical-grade material. The analysis revealed the presence of alpha-, beta-, gamma- and delta-hexachlorocyclohexane, traces of hexachlorobenzene and pentachlorobenzene, gamma- and delta-pentachlorocyclohexene, pentachlorophenol, 2,3,4,5-, 2,3,4,6- and 2,3,5,6-tetrachlorophenol, several trichlorophenols as well as glucuronides of the aforementioned metabolites. In addition, pentachlorocyclohexanes, tetrachlorophenol, hexachlorobenzene and pentachlorobenzene were identified in blood samples (Engst et al., 1978).
During biomonitoring, the urine of workers involved in the production of gamma-hexachlorocyclo-hexane (purity, 99.8%) from technical-grade material was examined. The major metabolites identified were 2,4,6-, 2,3,5- and 2,4,5-trichlorophenol, which were excreted in urine in equal proportions (Angerer et al., 1983).
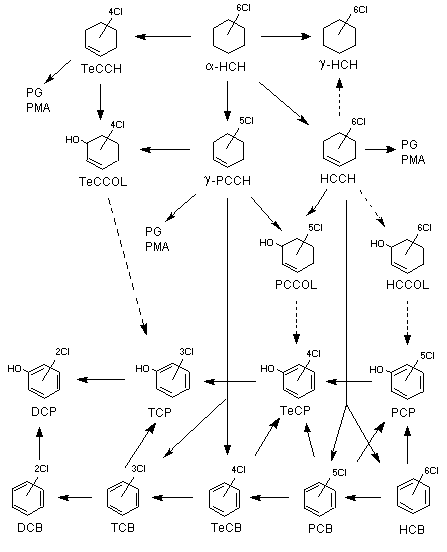
Figure 1 shows the proposed metabolism of lindane.

Figure 1. Major steps in the biotransformation of lindane
DCB, dichlorobenzene; DCP, dichlorophenol; HCB, hexachlorobenzene; HCCH, hexachlorocyclohexene;
HCCOL, hexachlorocyclohexenol; alpha-HCH, alpha-1,2,3,4,5,6-hexachlorocyclohexane; gamma-HCH,
gamma-1,2,3,4,5,6-hexachlorocyclohexane; PCB, pentachlorobenzene; gamma-PCCH, gamma-2,3,4,5,6-pentachlorocyclohexene;
PCCOL, 2,3,4,5,6-pentachlorocyclohexene-(2)-ol-(1); PCP, pentachlorophenol; TCB, trichlorobenzene; TCP, tricholorophenol;
TeCCH, 3,4,5,6-tetrachlorocyclohexene; TeCCOL, tetrachlorocyclohexenol; TeCB, tetrachlorobenzene; TeCP, tetrachlorophenol;
PG, glucuronide conjugate; PMA, sulfate conjugate
The effects of lindane on drug metabolizing enzymes were investigated in rodents. In an attempt to ascertain if various strains and species had different enzyme activities, CF1 and B6C3F1 mice and Mendel rats received diets containing lindane (purity unspecified) at concentrations of 50300 ppm for 3 days or 3 months. The animals were killed after dosing and the livers collected for assay of the specific activities of enzymes implicated in lindane metabolism.
In all three rodent strains, the basal glutathione-S-transferase activity was higher in males than in females. This difference in enzyme activity did not appear to correlate with susceptibility to lindane. In comparison with the other strains investigated, CF1 mice had the highest glutathione-S-transferase activity. Furthermore, female CF1 mice showed five- to sixfold induction of this enzyme after treatment with lindane at the highest dose (300 ppm). Treatment of animals with high doses of lindane induced more UDP-glucuronosyl transferase activity in rat liver microsomes than in mouse liver microsomes. Enhanced activity of this enzyme could result in an increase in the conjugation of phenol metabolites derived from lindane. In contrast, CF1 mice had more monooxygenase activity than rats, regardless of treatment. CF1 mice given lindane, however, had less epoxide hydrolase activity than rats. Coupled with the high monooxygenase activity, the reduction in epoxide hydroxylase activity could result in accumulation of the reactive epoxides produced during lindane metabolism (Oesch et al., 1982).
The potential cytotoxic and cell-transforming effects of lindane on BALB/c 3T3 cells were studied in vitro in the presence and absence of an exogenous metabolic activation system obtained from rats treated with phenobarbital. To determine the cytotoxic concentration, the cells were exposed to 10, 50, 100 and 200 ΅g/ml of lindane (purity, 99%), while doses of 10, 50 and 100 ΅g/ml were used to test for transformation.
In the absence of metabolic activation, lindane was not cytotoxic at any dose. However, in the presence of the metabolizing system, a dose-dependent increase in cytotoxicity was observed. Moreover, lindane showed statistically significant, dose-dependent cell transformation capacity at all doses tested, regardless of metabolic activation. This result suggests that lindane may act as a promoter in carcinogenesis (Perocco et al., 1995).
In a study designed to compare the formation of chlorophenol metabolites from hexachlorocyclohexane, male Swiss mice and female Wistar rats received a single intraperitoneal injection of 500 mg/kg bw Aroclor 1254 and were killed 5 days later. Microsome fractions were prepared from the liver to determine the extent of formation of chlorophenol metabolites after addition of lindane to the microsome fractions. In both rodent species, 2,6-dichlorophenol, 2,3,5-, 2,3,6- and 2,4,6-trichlorophenol, 2,3,4,5- and 2,3,5,6-tetrachlorophenol and pentachlorophenol were produced. While the rat microsome preparation produced more 2,4,6-trichlorophenol than that of the mice, the two species produced comparable amounts of 2,6-dichlorophenol (the predominant metabolite) (Munir et al., 1984).
The potential role of a hepatic microsomal mixed-function oxidase system in lindane dehydrogenation was evaluated in vitro. Female Sherman and Sprague-Dawley rats were pretreated with 2 mg of DDT (to stimulate lindane metabolism) for 2 weeks. They were then killed, and the livers were used to prepare microsomal fractions. Microsomal fractions from untreated rats were used as concurrent controls. Lindane was incubated with the microsomal fractions and NADPH-generating systems in the presence and absence of inhibitors. Molecular oxygen and reduced pyridine nucleotide coenzyme were required to attain maximum lindane dehydrogenation. Inhibition by SKF 525-A and carbon monoxide implicated the cytochrome P450 system in the dehydrogenation process. Conversely, the failure of cyanide to inhibit dehydrogenation suggested that the cytochrome b desaturase system is not involved. It was shown that DDT could enhance dehydrogenase activity (Chadwick et al., 1975).
The saturable, dose-dependent nature of lindane metabolism was studied by incubation of lindane with rat liver microsomes in vitro. The dehydrogenation of lindane to hexachlorocyclohexene and further hydroxylation to 2,3,4,6-tetrachlorocyclohexenol showed non-linear increases with dose and a significant non-linear decrease with time, indicating that the metabolism of lindane is both saturable and dose-dependent (Copeland, 1985).
In a study designed to examine the metabolism of lindane by liver microsomes, male Wistar rats were given phenobarbital, 3-methylcholanthrene, cobalt chloride, SKF 525-A or piperonyl butoxide. Rats given saline solution were used as controls. The rats were killed after treatment, and their livers were used to prepare a microsomal fraction which was subsequently incubated with lindane in the presence of NADPH.
The metabolism proceeded by dehydrogenation, dehydrochlorination and dechlorination. Inhibition by SKF 525-A, piperonyl butoxide, N2 or the absence of NADPH indicated that cytochrome P450 was involved in this reaction. This assertion was further supported by the fact that cyanide had no effect on dehydrogenation. Furthermore, the ability of phenobarbital to induce dehydrogenation suggests that the cytochrome b system is not involved in this reaction. Conversely, dehydrochlorination was inhibited by N2, carbon monoxide, piperonyl butoxide, potassium cyanide and the absence of NADPH, but not by SKF 525-A. In fact, SKF 525-A and cobalt chloride induced this reaction, while phenobarbital had no effect, suggesting that a specific species of cytochrome P450 and the cytochrome b5 system or another enzyme system are responsible for the dehydrochlorination of lindane (Yamamoto et al., 1983).
The metabolism of lindane by human liver microsomes was studied by incubating them with lindane and an NADPH generating system. The microsomes metabolized lindane to four major metabolites: gamma-hexachlorocyclohexene, gamma-pentachlorocyclohexene, beta-pentachlorocyclohexene and 2,4,6-trichlorophenol. The two major secondary metabolites identified were 2,3,4,6-tetrachlorophenol and pentachlorobenzene (Fitzloff et al., 1982).
In a study conducted to examine the anaerobic metabolism of lindane, rat liver microsomes were prepared and incubated with lindane in the presence of NADPH and nitrogen. Under the conditions of this study, lindane was dechlorinated to 3,5,6/5-tetrachlorocyclohexene, which may be an intermediate between lindane and 4-chloromercapturic acid, thereby indicating that dechlorination has a pivotal role in lindane metabolism in vivo (Kurihara et al., 1979a).
The formation of mercapturic acid in rats exposed to lindane was evaluated in vitro by administering lindane (purity unspecified) intraperitoneally at a dose of 17 or 34 ΅mol. A crude soluble enzyme fraction was obtained from the livers of the animals and incubated with glutathione. Under the conditions of this study, glutathione conjugation occurred directly on polychlorocyclohexenes. While most polychlorocyclohexenes are metabolized similarly in vivo and in vitro, hexachlorocyclohexene might be dechlorinated and dehydrochlorinated before glutathione conjugation in the cytosol (Kurihara et al., 1979b).
Studies of the acute toxicity of lindane are summarized in Table 4. Mice given lindane orally showed clinical signs indicative of effects on the central nervous system, including but not limited to hypoactivity, dyspnoea, ataxia and convulsions. Rats given lindane showed similar signs of toxicity within 30 min of treatment.
Table 4. Acute toxicity of technical-grade lindane
|
Species |
Strain |
Sex |
Route |
LD50 |
LC50 |
Reference |
|
Mouse |
B6C3F1 |
Male |
Oral |
56 |
Wolfe & Ralph (1980) |
|
|
Female |
77 |
|||||
|
CF1 |
Male |
Oral |
160 |
Wolfe & Dauvin (1980) |
||
|
Female |
110 |
|||||
|
Chbi:NMRI (SPF) |
Male |
Oral |
120 |
Paul et al. (1980) |
||
|
Female |
110 |
|||||
|
NMRI-EMD (SPF) |
Male & female |
Oral |
250 |
Frohberg et al. (1972) |
||
|
NMRI |
Male & female |
Intraperitoneal |
97 |
Frohberg et al. (1972) |
||
|
NMRI |
Male & female |
Intramuscular |
150 |
Frohberg et al. (1972) |
||
|
Rat |
Wistar |
Male |
Oral |
140 |
Frohberg et al. (1972) |
|
|
Female |
190 |
|||||
|
Wistar |
Male & female |
Dermal |
1000 |
Ullman et al. (1986a) |
||
|
Wistar |
Male & female |
Inhalation |
0.002 |
Ullman & Mohler (1986) |
||
|
Wistar |
Male & female |
Intraperitoneal |
69 |
Frohberg et al. (1972) |
||
|
Rabbit |
New Zealand white |
Male & female |
Dermal |
Not irritating |
Ullman et al. (1986b) |
|
|
New Zealand white |
Male & female |
Eye |
Not irritating |
Ullman et al. (1986c) |
||
|
Guinea-pig |
Dunkin-Hartley |
Male & female |
Dermal |
Not sensitizing |
Ullman et al. (1986d) |
Lindane of > 99 % purity was used in all studies.
Rats to which lindane was applied dermally at the LD50 showed signs of toxicity including dyspnoea, hunched posture and hypoactivity. Hypoactivity, hunched posture and emaciation were observed in rats exposed to lindane by inhalation. In mice and rats treated intraperitoneally with lindane, hypoactivity, staggering, tremors and dyspnoea were observed. The signs of toxicity shown by mice after intramuscular administration of lindane included hypoactivity and staggering.
Mice
In a 14-week study of toxicity, groups 45 CD1 mice of each sex were exposed (whole-body) to lindane (purity, 99.6%) by inhalation at a concentration of 0, 0.3, 1 or 10/5 mg/m3 for 6 h on 5 days/week. Owing to a high mortality rate, the high concentration was reduced from 10 to 5 mg/m3 at the beginning of the second week. The particle size achieved for the dust was 3.2 ΅m, with a geometric standard deviation of 1.7. Groups of 15 mice of each sex at each dose were killed during week 7 of the study, at the end of exposure in week 14 and at the end of the recovery period in week 20.
A substantial number of deaths occurred among animals at 10 mg/m3 during the first week of the study (12/45 females and 2/45 males), and three males and three females died after the concentration had been lowered to 5 mg/m3. No clinical signs of toxicity, changes in ophthalmic parameters or consistent changes in body weight were reported during the study. Females showed dose-dependent increases in glucose concentration, which were first detected during week 7 and attained statistical significance (p < 0.001) in those at 5 mg/m3 by week 20 of the study (177% of control). At this time, females at concentrations > 1 mg/m3 also showed a statistically significant increase in blood urea nitrogen (133% and 144% of control at 1 and 5 mg/m3, respectively). An increase in urinary potassium concentration was reported in animals at the highest concentration.
Bone-marrow myelograms from males exposed to 5 mg/m3 showed significant increases in sternum megarubricytes, total erythrocyte series, total eosinophils and myeloid:erythrocytic ratios as well as decreases in progranulocytes during week 7. Females at this concentration for 7 weeks showed decreased eosinophilic myelocytes in femoral smears and decreased eosinophilic metamyelocytes in sternum smears. During week 14, several parameters were affected in both males and females, including decreases in lymphocyte counts, increased total lymphocyte cell counts in femoral smears and increased rubiblast and polychromatophilic cell counts in both sternum and femoral smears. Gross necropsy of animals that were killed on schedule showed no remarkable findings. Males that died intercurrently had an increased incidence of kidney lesions (cysts, colour changes, hydronephrosis and increased size), distension of the urinary bladder and distension of the urethra in those at the highest concentration.
Males at the highest concentration showed histological evidence of fibronecrotic thymus regions and mediastinitis in week 7, ceroid degeneration of the adrenal gland cortex (3/10 and 1/10 control) and nodular cortical-cell hyperplasia in week 14. At the lowest concentration, 3/9 males had fibronecrotic thymus regions and mediastinitis. No treatment-related effects were seen in females killed in week 7, but females exposed to the highest concentration and killed in week 14 had a statistically significant increase in the incidence of spindle-cell hyperplasia in the adrenal glands (71%, with 10% in controls).
The absolute weight of the testes and those relative to body weight and brain weight were increased in animals at the highest concentration that were killed at interim sacrifice. Females at concentrations > 0.3 mg/m3 that were killed in weeks 7 and 20 showed dose-dependent increases in thymus weights. The NOAEL was 0.3 mg/m3, equivalent to 0.003 mg/l, on the basis of increased thymus weight, mediastinitis and fibronecrotic thymus regions (Klonne & Kintigh, 1988).
Rats
In a 28-day range-finding study, groups of 15 Wistar rats of each sex received diets containing lindane (purity, 99.5%) at a concentration of 0, 1, 10, 100 or 400 ppm and were observed twice daily for clinical signs of toxicity or death. Individual body weights, food consumption and food use efficiency were assessed at the beginning of the study and weekly thereafter. Haematological and clinical chemical parameters were evaluated only at the end of the study.
No deaths were reported during the study. An increased incidence of convulsions was the only clinical sign of toxicity observed and was found only in females at the highest concentration. A statistically significant decrease in body-weight gain was seen in females (by 56%) and males (by 25%) at this concentration, and a slight decrease in body-weight gain (8.5%) were seen in females at 100 ppm. Males at concentrations > 100 ppm had a higher urinary output with lower specific gravity and pH than controls, but the effects were inconsistent. Statistically significant (p < 0.05 or p < 0.001) decreases in haemoglobin concentration, erythrocyte count and packed cell volume were reported for males and females at 400 ppm, and males at concentrations > 100 ppm had increased (p < 0.05) platelet counts from the third week of the study. The clinical chemical parameters affected by lindane at 400 ppm included marginal increases in phosphorus, calcium, cholesterol and urea concentrations and a decreased albumin:globulin ratio in both males and females.
The concentration of lindane in serum, liver, kidney and brain, measured at the time of sacrifice, showed a dose-dependent increase in all tissues. Females at dietary concentrations > 100 ppm had higher concentrations in the brain than males; however, males had higher renal concentrations of lindane than females at all dietary concentrations. Pale kidneys and increased absolute and relative weights of the kidney were reported in males at concentrations > 100 ppm. At the highest concentration, males and females had increased absolute and relative weights of the liver and females had an increase in the relative spleen weight. Males given lindane showed a dose-dependent increase in the severity of hyaline droplet accumulation in the renal proximal tubule, which led to interstitial chronic nephritis and necrosis coupled with tubule regeneration at concentrations > 100 ppm. Changes in the histological appearance of the livers of animals that died both during the study and at scheduled sacrifice consisted of an increased incidence of periacinar hepatocytic hypertrophy in males at 100 and 400 ppm (2/10 and 10/16, respectively, with 0/10 controls) and in 12/12 females at 400 ppm (0/10 in controls). The NOAEL was 10 ppm, equal to 0.98 mg/kg bw per day, on the basis of a statistically significantly increase in the incidence of periacinar hepatocytic hypertrophy in males and increased platelet counts at 100 ppm, equal to 9.6 mg/kg bw per day (Amyes, 1990).
In a 6-week study, groups of five Crl:CD(SD)Br rats of each sex received diets containing lindane (purity, 99.6%) at a concentration of 0, 80, 200, 400 or 800 ppm. Animals were observed daily for signs of toxicity and twice a day for deaths or moribundity. Body weights, food consumption and food use efficiency were assessed at the beginning of the study and weekly thereafter. At the end of the study, the animals were killed and necropsied grossly. The adrenals, kidneys, liver and testes were weighed, and the kidneys, liver and gross pathological lesions were examined histologically.
Two females at 800 ppm died during the study, but the cause of death could not be determined as the only sign of toxicity was rough fur. Dose-dependent decreases in body-weight gain were reported for males, reaching a maximum (decrease of 15% in comparison with concurrent controls) in those at 800 ppm. The decreases in body-weight gain were paralleled by decreases in food consumption (915% lower than control) throughout the study. The absolute and relative weights of the kidneys were increased in a dose-dependent manner in males; females also showed an increased relative kidney weight, although the increase in males was more dramatic (36% increase in males, 5% in females at 800 ppm). The absolute and relative weights of the liver increased in a dose-dependent manner in males and females. The increases in relative liver weight reached 40% and 57% and attained statistical significance in males and females at 800 ppm, respectively. Gross necropsy revealed no concentration-related abnormalities. Hyaline droplet nephropathy was observed at all concentrations only in males. Liver hypertrophy and leukocyte foci were reported almost all treated animals. Males at concentrations > 200 ppm showed an increased incidence of mottled kidneys, and animals of each sex showed an increased incidence of mottled livers. The NOAEL was 80 ppm, equivalent to 8 mg/kg bw per day, on the basis of an increased incidence of hepatotoxicity and mottled kidneys at 200 ppm, equivalent to 20 mg/kg bw per day (Jones, 1988).
Groups of 20 Wistar rats of each sex received diets containing lindane (purity, 99.85%) at a concentration of 0, 0.2, 0.8, 4, 20 and 100 ppm for 90 days. Five rats of each sex per dose were maintained on basal diet for 6 weeks after the end of the dosing period for a recovery phase. Animals were observed twice a day for signs of toxicity and deaths, with comprehensive examinations (including palpation) and measurements of body weight and food consumption weekly. Ophthalmic and auditory evaluations were conducted before treatment, in week 12 and at the end of the recovery period. Haematological, clinical chemical and urinary analyses were carried out before initiation of the study, in weeks 6 and 13 and at the end of the recovery period. Brain cholinesterase activity was assessed in five rats of each sex per dose at the end of treatment. Cytochrome P450, N-demethylase, and carboxylesterase activities were measured in 10 rats of each sex per dose at the end of treatment and in five rats of each sex per dose after the recovery phase. All animals that died intercurrently or were killed on schedule were examined grossly and histologically. All gross lesions and 33 organs or tissues were examined microscopically, and the adrenal glands, brain, ovaries, testes, heart, kidneys, liver and thyroid glands were weighed.
One death was reported in the group at 4 ppm, which was considered to be incidental to treatment. No clinical signs of toxicity or changes in body weight, food consumption, food use efficiency or ophthalmic or auditory parameters were reported. No consistent changes in haematological or urine end-points were recorded. A statistically significant, dose-dependent, 1226% increase in urea concentration was reported in males at concentrations > 4 ppm in weeks 6 and 13, but this effect was not observed after the 6-week recovery period. Brain cholinesterase activity was not affected by treatment. Animals at the highest concentration showed increased activity of cytochrome P450, while N-demethylase activity was unaffected. The cytochrome P450 activity had returned to control values by the end of the recovery period.
A pattern of diffuse renal discolouration was observed in all males at concentrations > 20 ppm, severity increasing in a dose-dependent manner. This finding was correlated with hyaline droplet accumulation, tubule degeneration and distension, and an increased incidence of interstitial nephritis. The tubule-cell degeneration had resolved by the end of the recovery period. The absolute weight of the liver was slightly but statistically significantly increased in males at 100 ppm (by 13%; p < 0.05), while females showed a 10% increase (p < 0.05) at concentrations > 20 ppm. A statistically significant (p < 0.05 or 0.01) increase was found in the relative (to body weight) weights of the liver in males at 20 and 100 ppm (10 and 14%, respectively) and females at 100 ppm (10%; p < 0.01). These increases were consistent with the observations made during histopathological examination, in which a dose-related (but reversible) increase in the incidence and severity of hepatocellular hypertrophy was found in both sexes at 4, 20 and 100 ppm (2/30, 14/30 and 21/30 animals, respectively). The NOAEL was 100 ppm, equal to 7.6 mg/kg bw per day, the highest dose tested (Suter, 1983).
Lindane (purity, 99.9%) was administered by inhalation to groups of 12 male and 12 female Wistar rats at a nominal concentration of 0, 0.02, 0.1, 0.5 or 5 mg/m3 for 6 h/day for 90 days. Additional groups of 12 rats of each sex at 0 and 5 mg/m3 were treated for 90 days and allowed to recover for 6 weeks before they were killed. The analytical atmospheric concentrations were 0, 0.02, 0.12, 0.6 and 4.5 mg/m3, respectively. The arithmetic mean particle size of the aerosol was 1.1 ± 0.39 ΅m, and the geometric mean was 1.0 ± 1.4 ΅m. The animals were observed for deaths, signs of toxicity and food and water consumption once a day on 5 days/week. Haematology, clinical chemistry, urine analysis and measurements of cholinesterase inhibition and enzyme induction were conducted before, during and after exposure. The concentration of lindane was measured in the liver, brain, serum and peritoneal fat of five rats of each sex per dose at the end of the treatment and recovery periods. Gross and histological (31 organs or tissues) examinations were conducted on all animals at the end of treatment and recovery.
Lindane was detected in brain, liver, fat and serum of all treated rats. The chemical accumulated in fat, reaching concentrations of 130 mg/g and 58 mg/g in females and males at the highest dose, respectively. After the recovery period, traces of lindane were still detectable in these tissues. All rats survived to scheduled sacrifice. Slight diarrhoea and piloerection were observed in all males and females exposed to the highest concentration, beginning during week 3 of exposure and persisting until day 41 of the study. No exposure-related effects were seen on body-weight gain, food or water consumption or urine parameters. Although haematological parameters did not appear to be affected by treatment, data for individual animals were not provided, and the statistics could not be verified. The clinical chemical results, especially for Na+, K+ and Ca++, were highly variable. The cytochrome P450 activity was 340% and 170% of the control values in males and females at 5 mg/m3, respectively, after 90 days but similar to control levels after the recovery period. In animals at lower doses, the cytochrome P450 activity was comparable to that of concurrent controls. Bone-marrow myelograms from animals exposed to 5 mg/m3 showed significantly (p < 0.05) increased reticulocyte (+110%), stem cell (+31%) and myeloblast (+33%) counts in males, increased reticulocyte count (+55%) in females and decreased lymphocyte count (45%) in females. No doseresponse relationship could be established for these changes, however, as bone marrow from the other groups was not assayed.
Males exposed to 5 mg/m3 had significantly (p < 0.05 or 0.01) increased absolute (+7.8% to +12%) and relative (+19%) kidney weights as compared with controls, and the absolute and relative kidney weights of males exposed to 0.5 mg/m3 were increased by 89.8% and 6.98.2%, respectively, which were considered to be biologically significant even though they are not statistically significant. After the recovery phase, the kidney weights of exposed males were similar to those of controls. In females exposed to 5 mg/m3, the absolute and relative kidney weights were increased (p < 0.05) by 9.29.9% and 7.98.2%, respectively, as compared with controls. A statistically significant increase in relative testis weight was also reported at all doses, but the toxicological relevance of this observation is uncertain, as the absolute testis weight was statistically significantly increased (p < 0.01) only at the low concentration and no compound-related changes were seen in the histopathological evaluation. The absolute liver weights of males at the highest dose were not affected, but the relative liver weights were slightly (6.9%) higher than those of controls. In females at the highest dose, the absolute and relative liver weights were 12% and 11% higher, respectively, than those of controls. No differences in absolute and relative liver weights were seen between the exposed and control groups after the recovery period.
Kidney lesions were observed in 17% of control males, none of those at 0.02 mg/m3, 25% of those at 0.1 mg/m3, 83% of those at 0.50 mg/m3 and 82% of those at 5 mg/m3. The lesions included cloudy swelling of the tubule epithelium, dilated renal tubules containing protein and proliferated tubules. After the recovery phase, only cloudy swelling of the tubule epithelium was observed in two control animals and one at the highest concentration The NOAEL was 0.5 mg/m3, equal to 0.12 mg/kg bw per day, on the basis of diarrhoea, piloerection and changes in the bone-marrow myelogram at 5 mg/m3 (Hertel et al., 1983).
Rabbits
Groups of 40 male and 40 female New Zealand white rabbits received dermal applications of lindane (purity, 99.5%) in 5% aqueous carboxymethyl cellulose at a dose of 0, 10, 60 or 400 mg/kg bw per day under occlusion for 6 h/day, 5 days/week for 13 weeks. Owing to excessive toxicity, the highest dose was reduced to 350 mg/kg bw per day in week 9 and to 320 mg/kg bw per day from week 11 until the end of the study. Within each group, 10 animals of each sex per group were killed at week 6, 20 were killed at 13 weeks and 10 were dosed for 13 weeks and allowed a 6-week recovery.
Tremors and convulsions were observed in animals at the highest dose, from day 16 in males and day 19 in females. One female at 60 mg/kg bw per day showed these clinical signs on day 50 only. Clinical signs of toxicity were not observed in animals at the lowest dose. Reactions at the site of application were not reported. Of animals at the highest dose, 17 males and eight females died before scheduled sacrifice. Deaths were first observed after week 5. All animals at lower doses survived to scheduled sacrifice.
The body weights and body-weight gains of animals at the two lower doses were similar to those of controls throughout the study. Males and females at the highest dose began to lose weight after the first week of the study, so that their absolute body weights were 37% and 310%, respectively, lower than those of controls during the 13 weeks of treatment. During recovery, the body weights of the males remained 38% lower, while those of females were only 13% lower than those of controls. The data on body weights were not analysed statistically. The body-weight loss by rabbits at the highest dose correlated with generally reduced food consumption during treatment.
No treatment-related effects were observed on ophthalmic, urine or leukocyte parameters. Alkaline phosphatase activity was significantly increased in females at the highest dose at interim sacrifice (+34%; p < 0.05), and in males (+44%; p < 0.01) and females (+53%; p < 0.01) at terminal sacrifice. Females at this dose also had significantly increased gamma-glutamyl transferase activity (+38%; p < 0.01) at terminal sacrifice. Males at the highest dose showed significant (p < 0.05 or 0.01) reductions in haemoglobin concentration (7%), erythrocyte count (8.6%) and packed cell volume (5.7%) at terminal sacrifice, but these erythrocyte parameters were comparable to those of controls after recovery and were not affected in females.
At terminal sacrifice, males and females at the highest dose had slightly increased absolute kidney weights and significantly (p < 0.01) increased relative kidney weights as compared with controls. The absolute and relative kidney (left and right) weights were increased by 104106% and 112114%, respectively, in males and 105106% and 115116%, respectively, in females. Females at the highest dose also had significantly (p < 0.01) increased absolute (+27%) and relative (+3045%) liver weights at both sacrifices, which remained slightly (+1317%) elevated after recovery. The relative liver weights were significantly (+37%; p < 0.01) increased in males at the highest dose at terminal sacrifice. The absolute adrenal weights (left and right) were significantly (p < 0.05 or 0.01) increased at terminal sacrifice in males at the intermediate dose (+2023%) and highest dose (+4046%) and in females at the highest dose (+3334%). The relative adrenal weights were increased (p < 0.05 or 0.01) by 1922% in males at the intermediate dose and by 4657% in males and females at the highest dose. After the recovery period, the organ weights of the treated groups were similar to those of controls.
No treatment-related gross or histopathological lesions were observed in the kidneys, adrenals or skin. The incidence and severity of centrilobular hypertrophy of the liver was increased in males and females at the two higher doses at all sacrifices. At both the interim and terminal sacrifices, centrilobular hypertrophy was observed in 20% of males and 2530% of females at the intermediate dose and 80100% of males and 7390% of females at the highest dose. After recovery, this lesion was seen in 30% of males and 40% of females at the intermediate dose and in 50% of males and 29% of females at the highest dose. The NOAEL was 10 mg/kg bw per day on the basis of lesions in the liver and increased adrenal weights in males at 60 mg/kg bw per day (Brown, 1988).
Dogs
In a 90-day study, groups of four beagle dogs of each sex received diets containing lindane (purity, > 99%) at a concentration of 0, 25, 50 or 100 ppm. The animals were observed daily for signs of toxicity and deaths. The haematological parameters evaluated at the end of the study included erythrocyte count, haemoglobin concentration, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, total leukocyte count, reticulocyte count, platelet count, thromboplastin time and partial thromboplastin time. The clinical chemistry included measurements of glucose, urea, creatinine, total cholesterol, total bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, lactate dehydrogenase, total protein and electrolytes. Urine was also analysed.
No changes were found in the parameters evaluated. The NOAEL was 100 ppm, equivalent to 1 mg/kg bw per day, the highest dose tested (Noel et al., 1969).
Groups of four beagle dogs of each sex received diets containing lindane (purity, > 99%) at a concentration of 0, 25, 50 or 100 ppm for 104 weeks. The animals were observed daily for signs of toxicity and deaths; food consumption was measured twice a day and body weights were recorded weekly. Ophthalmic evaluations were performed before dosing and at 1, 3, 6, 12 and 24 months. Haematology, clinical chemistry and urine analysis were conducted twice before initiation of dosing and at 1, 3, 6, 12 and 24 months. At the end of the study, all animals were examined grossly and histologically (34 tissues or organs), and 15 organs were weighed. The concentrations of lindane were determined in fat, liver and brain samples from all animals.
One animal at the highest concentration died 393 days after the second of two brief convulsive episodes occurring 200 days apart. Each episode was followed by rapid recovery. Convulsions in 1/4 female controls and 2/4 males at 25 ppm were the only clinical sign of toxicity seen during the study. These observations are considered incidental, as similar effects were not seen at higher doses. Body weights, food consumption, urine parameters and electroencephalograms were unchanged. The only haematological change was a dose-dependent increase (> 17%) in platelet count in animals at concentrations > 50 ppm during week 4. In terms of clinical chemistry, a statistically significant increase in alkaline phosphatase activity (by 3653%) was found in animals at 100 ppm from week 25, which persisted until the end of the study. Alkaline phosphatase activity increased in a time-dependent fashion.
At gross necropsy, an increased incidence (3/8 animals) of enlarged liver was reported in dogs at 100 ppm, and the animals in this group had darker, more friable livers (6/8) than controls (0/8). Increased absolute and relative spleen weights (150160% and 140160% of control, respectively) were found in all treated groups. Histopathological changes were observed in the pituitary and adrenal glands but not in the spleen. In the pituitary, the changes consisted of an increased incidence of cysts in the pars distalis (2/8, 4/8, 5/8 and 3/8 animals at 0, 25, 50 and 100 ppm, respectively). The changes in the adrenals consisted of increased incidences of cytoplasmic vacuolation (4/8 and 3/8 animals at 50 and 100 ppm, respectively, with 1/8 in the control group). The NOAEL was 25 ppm, equal to 0.83 mg/kg bw per day, on the basis of an increased incidence of cytoplasmic vacuolation in the adrenal glands at 50 ppm, equal to 1.6 mg/kg bw per day (Rivett et al., 1971).
Mice
Groups of 2040 male dd mice received diets containing various isomers (purity, > 99%) alone or in combination at a concentration of 0, 100, 250 or 500 ppm for 24 weeks. The animals were weighed weekly and necropsied grossly at the end of the study, when the livers were weighed and examined by histopathology and electron microscopy.
Body weights were unaffected by lindane at any concentration, but the absolute and relative liver weights were increased by 16% and 33%, respectively, in animals at 500 ppm. Microscopic evaluation of these livers revealed hypertrophy but no tumours. The NOAEL for lindane was 500 ppm, equivalent to 25 mg/kg bw per day, the highest dose tested (Ito et al., 1973a).
In a study of the role of polychlorinated biphenyls in promoting liver tumorigenesis induced by benzene hexachloride and the effect of exposure to various benzene hexachloride isomers on liver tumorigenesis, groups of 2030 dd male mice were given diets containing alpha-, beta or gamma-benzene hexachloride (purity, > 99%) at a concentration of 0, 50, 100 or 250 ppm in the presence or absence of 250 ppm Kanechlor-500 (pentachlorobenzene-5) for 24 weeks. The animals were weighed weekly. At the end of the study, they were killed and necropsied grossly; the livers were weighed and examined microscopically. Animals that died intercurrently were discarded without further evaluation.
Exposure to pentachlorobenzene-5 in conjunction with all three isomers of benzene hexachloride at any concentration resulted in increases in absolute and relative liver weights of 87370% and 83-370%, respectively. In the absence of pentachlorobenzene-5, however, only alpha-benzene hexachloride at a concentration of 250 ppm elicited a 120% and 110% increase in absolute and relative liver weights, respectively. Histopathological evaluation revealed 2781% increases in the incidences of nodular hyperplasia and hepatocellular carcinoma after administration of alpha-benzene hexachloride at 250 ppm in the presence or absence of pentachlorobenzene-5. beta-Benzene hexachloride increased the incidence of liver tumours only at concentrations > 100 ppm and only in the presence of pentachlorobenzene-5. gamma-Benzene hexachloride (lindane) did not cause liver tumours at any concentration. The NOAEL for lindane was 250 ppm, equivalent to 12 mg/kg bw per day, the highest dose tested (Ito et al., 1973b).
Groups of 50 Chbb:NMRI (SPF) mice of each sex received diets containing lindane (purity, 99.5%) at a concentration of 12, 25 or 50 ppm for 80 weeks. The control group consisted of 100 mice of each sex. Food consumption and the body weights of 15 mice of each sex per dose were measured weekly for the first 26 weeks and then every 2 weeks. All animals were subjected to a full gross examination, and the brain, adrenals, gonads and urinary bladder and any gross lesions were examined histologically.
Mortality rates, food consumption and body weights were not affected by treatment. Animals at 50 ppm that died intercurrently had a higher incidence of enlarged spleens (5/19, with 1/28 in the control group), and those killed at the end of the study had a 10% increase in the incidence of pulmonary lesions, including mottled and pale lungs (5/41) over that in controls (2/86). There were two incidences of polymorphonuclear sarcoma and one of spindle-cell sarcoma among animals at the highest concentration, with none in the control group. The NOAEL was 25 ppm, equal to 3.9 mg/kg bw per day, on the basis of enlarged spleens and lung lesions at 50 ppm, equal to 7.8 mg/kg bw per day (Köllmer, 1975).
Groups of 50 B6CF3 mice of each sex were given diets containing lindane at a concentration of 0, 80 or 160 ppm, equal to 0, 11 and 23 mg/kg bw per day, for 80 weeks and observed for an additional 1011 weeks. Food consumption was determined weekly for the first 16 weeks and every 4 weeks thereafter. Animals were observed for signs of toxicity and deaths twice daily. Detailed examinations, including palpation, were conducted once a week. A full gross examination and histopathological examination of gross lesions, adrenal glands, bone (with marrow), brain, bronchi, colon, duodenum, heart kidneys, liver, lungs, lymph nodes, mammary glands, ovaries, pancreas, parathyroid, pituitary, prostate, salivary glands, spleen, stomach, testes, thymus, thyroid, trachea and uterus were conducted for mice that were killed before or at the end of the study. All organs examined histopathologically were weighed.
Survival and body weights were unaffected by treatment. Alopecia, rough fur and distended abdomens were observed in treated animals. During the second year of the study, the females appeared to be more excitable and males showed more aggressive behaviour (fighting). High incidences of hepatocellular carcinomas were reported in all groups of males, including controls (20%, 39% and 20% of controls and at the two concentrations, respectively). In females, the incidences of this lesion were 0%, 4% and 7% for controls and treated animals, respectively (Table 5). Under the conditions of this study, lindane was not carcinogenic (Steinberg et al., 1977).
Table 5. Incidences of neoplastic lesions in the livers of B6C3F1 mice fed diets containing lindane
|
Neoplasm |
Dietary concentration (ppm) |
|||||
|
0 |
80 |
160 |
||||
|
Males |
Females |
Males |
Females |
Males |
Females |
|
|
Neoplastic nodule |
1/10 |
1/10 |
0/49 |
2/47 |
1/46 |
0/46 |
|
Hepatocellular carcinoma |
2/10 |
0/10 |
19/49 |
2/47 |
9/46 |
3/46 |
From Steinberg et al. (1977)
The carcinogenic potential of lindane in mice was further investigated in groups of 50 Chbi:NMRI (SPF) mice of each sex given diets containing lindane (purity unspecified) at a concentration of 12, 25 or 50 ppm for 80 weeks. The concurrent control group consisted of 100 mice of each sex. The animals were observed daily for signs of toxicity or tumours. Animals killed at the end of the study and those dying intercurrently were necropsied, and the brain, heart, lungs, liver, spleen, kidneys, suprarenal glands, gonads, bladder and any gross lesions identified at necropsy were examined histologically. In addition, 1015 liver samples from four mice of each sex per dose were examined by electron microscopy.
Clinical signs of toxicity, body weight, food consumption and mortality rates were unaffected by treatment. The tumour incidence in the treated groups (1323%) was comparable to that of concurrent controls (24%). The commonest neoplastic lesions were lymphocytic leukaemia or lymphosarcoma and primary lung tumours. These two lesions occurred at comparable frequencies in treated and control groups: 47% versus 8.5% for lymphocytic leukaemia or lymphosarcoma and 811% versus 10% for primary lung tumour. The incidences of liver-cell adenomas were also comparable in treated (2%) and control groups (2.5%). The only malignancy found in the liver was a malignant hæmangioendothelioma in an animal at 12 ppm (equal to 2 mg/kg bw per day). The histological findings in the liver were confirmed by electron microscopy, which showed no difference between the treated groups and the control. The NOAEL was 50 ppm, equal to 7.8 mg/kg bw per day, the highest dose tested (Weisse & Herbst, 1977).
Groups of 3696 female mice of the agouti, pseudoagouti and black strains were given diets containing lindane at a concentration of 0 or 160 ppm, equivalent to 0 and 23 mg/kg bw per day, for up to 24 months. These concentrations were selected on the basis of a preliminary study in which no deaths occurred after 1 month. Additional groups of 4896 agouti and black mice were fed treated or control diets for 6 months and then fed control diet for 6 or 18 months for evaluation of recovery.
Clinical signs of toxicity and information on survival were not reported. No apparent effects on body weight or food consumption were observed, but limited data were presented. At 6 and 12 months, the benzo[a]pyrene monooxygenase activity in the liver was increased by 1.61.8 times in the agouti, 2.72.8 times in the pseudoagouti and 2.1 times in the black strains in comparison with controls. The weights of the liver were increased by 1531% in the agouti, 1422% in the pseudoagouti and 1216% in the black strains at interval sacrifices up to 24 months. After the recovery period, the weights of the liver in treated mice were similar to those of controls.
No evidence was found for an increased incidence or decreased latency of liver tumours in the black strain at any time during the study or in the pseudoagouti strain at the 18-month sacrifice; however, at 18 months, 0/34 control and 12/36 (33%) treated agouti mice had developed hepatocellular adenomas, and one carcinoma was found in each of the treated and control groups. Treated agouti and pseudoagouti mice showed clear increases in the incidences of adenomas and slight increases in the incidences of carcinomas at 24 months (Table 6).
Table 6. Incidencesa of hepatocellular adenoma and carcinoma in (YS Χ VY) F1 hybrid mice given diets containing lindane
|
Treatment period (months) |
Control |
Treated |
||||||||||
|
Yellow |
Pseudoagouti |
Black |
Yellow |
Pseudoagouti |
Black |
|||||||
|
No. |
% |
No. |
% |
No. |
% |
No. |
% |
No. |
% |
No. |
% |
|
|
Adenomas |
||||||||||||
|
6 |
0/48 |
0/48 |
0/48 |
0/48 |
0/48 |
0/48 |
||||||
|
12 |
2/48 |
4 |
0/46 |
0/48 |
3/48 |
6 |
0/48 |
0/48 |
||||
|
18 |
0/34 |
2/35 |
6 |
4/35 |
11 |
12/36 |
33 |
0/33 |
2/36 |
6 |
||
|
24 |
8/93 |
9 |
5/95 |
5 |
6/96 |
6 |
33/94 |
35 |
11/95 |
12 |
3/96 |
3 |
|
Carcinomas |
||||||||||||
|
6 |
0/48 |
0/48 |
0/48 |
0/58 |
0/48 |
0/48 |
||||||
|
12 |
0/48 |
0/48 |
0/48 |
0/48 |
0/48 |
0/48 |
||||||
|
18 |
1/34 |
3 |
1/34 |
3 |
0/35 |
1/36 |
3 |
0/33 |
0/36 |
|||
|
24 |
12/93 |
13 |
2/95 |
2 |
3/96 |
3 |
16/94 |
17 |
5/95 |
5 |
1/96 |
1 |
|
Combined incidence of adenoma and carcinoma |
||||||||||||
|
20/93 |
22 |
7/95 |
7 |
9/96 |
9 |
49/94 |
52 |
16/95 |
17 |
4/96 |
4 |
|
From Wolff et al. (1987)
a Mice bearing tumours/mice examined
Increased incidences of Clara cell hyperplasia in the lung were seen at all sacrifice intervals for each strain, and the incidence of lung tumours was increased at later times in the agouti and pseudoagouti strains (Table 7). After recovery, the incidences of Clara cell hyperplasia (agouti and black) and lung tumours (agouti) remained slightly elevated as compared with the controls (Wolff et al., 1987).
Table 7. Incidencesa of Clara cell hyperplasia and lung tumours in (YS Χ VY) F1 hybrid mice given diets containing lindane
|
Treatment period (months) |
Control |
Treated |
||||||||||
|
Yellow |
Pseudoagouti |
Black |
Yellow |
Pseudoagouti |
Black |
|||||||
|
No. |
% |
No. |
% |
No. |
% |
No. |
% |
No. |
% |
No. |
% |
|
|
Hyperplasia |
||||||||||||
|
6 |
8/48 |
17 |
4/48 |
8 |
5/48 |
10 |
37/48 |
77 |
24/48 |
50 |
27/48 |
56 |
|
12 |
15/48 |
31 |
8/46 |
17 |
7/48 |
14 |
44/48 |
92 |
35/46 |
76 |
43/48 |
90 |
|
18 |
2/34 |
6 |
2/35 |
6 |
0/35 |
33/36 |
92 |
27/34 |
79 |
32/36 |
89 |
|
|
24 |
14/95 |
15 |
10/95 |
10 |
10/96 |
10 |
68/95 |
72 |
71/96 |
76 |
76/95 |
82 |
|
Lung tumours |
||||||||||||
|
6 |
2/48 |
4 |
1/48 |
2 |
1/48 |
2 |
1/48 |
2 |
0/48 |
0/48 |
||
|
12 |
0/48 |
1/46 |
2 |
1/48 |
2 |
1/48 |
2 |
0/46 |
0/46 |
|||
|
18 |
0/34 |
2/35 |
6 |
0/35 |
6/36 |
17 |
2/34 |
6 |
4/36 |
11 |
||
|
24 |
4/95 |
4 |
6/95 |
6 |
2/96 |
2 |
18/95 |
19 |
13/94 |
14 |
3/96 |
3 |
From Wolff et al. (1987)
a Mice bearing lesions/mice examined
Groups of 50 Crl:CD-1®(ICR)BR mice of each sex received diets containing lindane (purity, 99.78%) at a concentration of 0, 10, 40 or 160 ppm for 78 weeks. The animals were observed for clinical signs of toxicity and deaths twice daily, and comprehensive examinations (including palpation) were conducted weekly. Body weight and food consumption were measured weekly during the first 14 weeks and monthly thereafter. Haematological evaluations were conducted at the end of the study period. All animals were necropsied grossly, and 43 tissues and any gross lesions from animals in the control group and at the highest dose and those of animals dying intercurrently were examined histologically.
No significant treatment-related effects were seen on clinical signs, mortality rates, body weight, body-weight gain, food consumption or haematological parameters. The relative weight of the uterus and cervix of animals at 160 ppm that died intercurrently were decreased by 50% (p < 0.05), and these animals also had a decreased incidence of uterine cysts (30%) when compared with controls (79%). Given the lack of corroborative histopathological findings, these effects are considered not to be adverse or toxicologically relevant. At the end of the study, males at the highest concentration had a statistically significant increase in the incidences of centrilobular hepatocyte hypertrophy (34%; 5% in controls; p < 0.01) and eosinophilic foci of hepatocellular alteration (21%; 5% in controls; p < 0.05). Although no histopathological changes were seen in the livers of females, a statistically significant (p < 0.05) increase in the incidence of bronchiolaralveolar adenomas was reported at 160 ppm (22%; 6% in controls) in females only (Table 8). It should be noted, however, that these changes were not dose-dependent, and the tumour response was variable. Furthermore, the incidence of this finding in control animals was at the low end of the range for other controls in the same laboratory (6%). Nonetheless, the incidence in females at the highest dose (19%) exceeded the rate in other controls. When the slides were re-examined, two additional adenomas were identified in both the control and the high-concentration group. While the incidence of carcinomas was not increased when compared with concurrent controls, the combined incidence of adenomas and carcinomas at the highest concentration was increased (29%; 12% in controls; p < 0.05). The NOAEL was 40 ppm, equal to 5.2 mg/kg bw per day, on the basis of an increase incidence of alveolarbronchiolar adenomas in females at 160 ppm, equal to 21 mg/kg bw per day (Chase, 2000).
Table 8. Incidences of alveolarbronchiolar tumours in mice given diets containing lindane
|
Tumour |
Dietary concentration (ppm) |
|||||||
|
0 |
10 |
40 |
160 |
|||||
|
No. |
% |
No. |
% |
No. |
% |
No. |
% |
|
|
Males |
||||||||
|
Adenomas |
16/49 |
33 |
15/48 |
31 |
11/49 |
22 |
8/48 |
17 |
|
Carcinomas |
0/49 |
1/48 |
2 |
3/49 |
6 |
0/48 |
||
|
Combined adenomas and carcinomas |
16/49 |
33 |
16/48 |
33 |
14/49 |
29 |
8/48 |
17 |
|
Females |
||||||||
|
Adenomas |
5/48 |
10 |
7/46 |
15 |
7/47 |
15 |
13/48* |
27 |
|
Carcinomas |
1/48 |
2 |
2/46 |
4 |
2/47 |
4 |
1/48 |
2 |
|
Combined adenomas and carcinomas |
6/48 |
12 |
8/46 |
17 |
9/47 |
19 |
14/48* |
29 |
From Chase (2000)
* p < 0.05
Rats
Groups of 50 male and 50 female Wistar rats received diets containing lindane (purity, 99.75%) at a concentration of 0, 1, 10, 100 or 400 ppm for 1 year for the study of toxicity. An additional 15 rats of each sex per group were designated for interim sacrifice (followed by gross necropsy and histopathological examination) after 30 days and 26, 52 and 78 weeks (52 weeks of treatment followed by a 26-week recovery period). For the study of carcinogenicity, 55 rats of each sex per dose received the diets containing lindane at the same concentrations for 102 weeks, and five rats of each sex per dose were used to assess the concentrations of lindane in liver, kidney, brain and blood. The animals were necropsied and evaluated histologically at the end of the study. An additional 25 animals of each sex were examined to establish baseline parameters for haematology, clinical chemistry and urine analysis. Ten rats of each sex from this group were killed, and their kidneys, liver and lungs were examined histologically. During both phases of the study, blood and urine were collected in weeks 3, 12, 24, 51, 77 and 103 from 10 rats of each sex per dose.
During the phase of the study in which toxicity was studied, no treatment-related clinical signs of toxicity were reported. Alopecia, swollen limbs and aggressive behaviour were observed sporadically at similar rates in control and treated groups. Mortality rates were not affected. Although statistically significant decreases in body-weight gain were reported sporadically for animals of each sex at concentrations > 100 ppm, only females at the highest concentration had statistically significant decreases at week 52. These decreases in body-weight gain were found in conjunction with a marginal (7%) decrease in food consumption, which was not statistically or biologically significant. Food use efficiency was not affected by treatment. Haematological and clinical chemical parameters were not affected. Statistically significant increases in urine volume, with decreased pH and specific gravity, were reported in males at the highest concentration during the first 25 weeks of the study but were no longer present at week 52. A statistically significant increase in urea and creatinine concentrations was reported in males at dietary concentrations > 100 ppm during week 12 but was apparent only in animals at 400 ppm at week 24 and had resolved by the end of the dosing period (52 weeks), as was the case for the increase in urinary output. No consistent changes in organ weights (absolute or relative to body weight) were reported. Gross necropsy after the 30-day and 26-week interim sacrifices revealed an increased incidence of pale kidneys in males (30 days: 0/10, 5/10 and 6/10 in controls, 100 and 400 ppm, respectively; 26 weeks: 0/10 and 3/10 in controls and at 400 ppm). This finding was not present at necropsy at week 52 or week 78. Periacinar hepatocyte hypertrophy was observed in animals of each sex at concentrations > 100 ppm, and renal lesions suggestive of alpha2u-globulin-mediated nephrotoxicity were found in males at concentrations > 10 ppm. After a 26-week recovery period, the histopathological renal changes had resolved, and periacinar hepatocyte hypertrophy was observed only in females at 100 ppm with no doseresponse relationship.
In the phase of the study designed to investigate carcinogenicity, the clinical signs of toxicity consisted of convulsions in 11 females at the highest concentration. The survival rates at the end of the study were 36%, 36%, 31%, 20% and 16% for males and 49%, 38%, 44%, 35% and 18% for females at 0, 1, 10, 100 and 400 ppm, respectively. The survival rate of males at the highest concentration was similar to that of controls through week 93, but the survival rate of females at this concentration was significantly decreased, 50% survival being reached at week 89, compared with week 104 for the control group. Body-weight gain was significantly (p < 0.01) decreased for males at 100 and 400 ppm during the first few weeks of the study as compared with controls. Because the final body weights of males at 100 ppm were similar to those of controls, the initial reduction in weight gain was considered not biologically significant. The final body weights of males at 400 ppm were significantly (14%; p < 0.05) lower than those of controls. The body weights and body-weight gains of treated females were similar to those of controls throughout the study. Food consumption by the groups at the highest concentration was decreased by 15% in males and 19% in females during the first week of the study, but the total food consumption over the entire study was similar to control levels.
Platelet counts were significantly (p < 0.05 or 0.01) increased (by 20%) in males at 100 and 400 ppm at week 12 and in males and females at these concentrations at week 24 but not at later times. Males and females at 400 ppm showed significant (p < 0.05 or 0.01) decreases in erythrocyte parameters at week 104 as compared with the controls: the haemoglobin concentration was reduced by 16% and 18%, the erythrocyte counts by 14% and 21% and the packed cell volume by 16% and 18% in males and females, respectively. Significant (p < 0.05 or 0.01) changes in clinical chemical parameters were observed in males and females at the highest concentration during the first year of the study. Inorganic phosphorus was increased by 7.338% and calcium by 3.410% in males and females; cholesterol was increased by 45110% and urea by 2054% in females; and the albumin:globulin ratio was decreased by 8.318% in females. All parameters were similar to control values by week 104.
Males and females at the highest concentration had increased absolute and relative liver weights at all interim sacrifices, although statistical significance was not always reached. At the end of the study, the absolute and relative liver weights were significantly (p < 0.01) increased by 21% and 38%, respectively, in males and by 32% and 34%, respectively, in females. In animals at 100 ppm, the absolute liver weights were increased by 8.611% (not significant) and the relative liver weights by 1418% (p < 0.05 or 0.01) for animals of each sex at week 104. Significant (p < 0.05 or 0.01) increases in absolute and relative spleen weights were also seen at week 52 and in relative spleen weights at week 104, but the sex of the animals was not identified. The incidence of periacinar hepatocytic hypertrophy was significantly increased in animals at 100 and 400 ppm (Table 9), with 25/50 males and 19/50 females affected at 100 ppm and 40/50 males and 43/50 females at 400 ppm. No treatment-related histopathological lesions were observed in the spleen or bone marrow. Renal lesions indicative of alpha2u-globulin accumulation were observed in males at dietary concentrations > 10 ppm, but these were considered not to be relevant to human risk assessment.
Table 9. Incidence of periacinar hepatocytic hypertrophy in rats given diets containing lindane
|
Observation period |
Dietary concentration (ppm) |
||||
|
0 |
1 |
10 |
100 |
400 |
|
|
Males |
|||||
|
30 days |
0 |
0 |
0 |
2/10 |
10/10*** |
|
26 weeks |
0 |
0 |
0 |
4/10 |
10/10*** |
|
52 weeks |
0 |
3/10 |
3/10 |
9/10*** |
9/9*** |
|
78 weeks |
0 |
0 |
2/9 |
0 |
1/8 |
|
104 weeks |
1/18 |
0 |
1/16 |
6/9** |
8/9*** |
|
Unscheduled deathsa |
0/35 |
0/34 |
5/35 |
22/44*** |
33/44*** |
|
Females |
|||||
|
30 days |
0 |
0 |
0 |
0 |
9/10*** |
|
26 weeks |
0 |
0 |
0 |
3/10 |
9/9*** |
|
52 weeks |
0 |
0 |
0 |
5/9* |
8/8*** |
|
78 weeks |
0 |
0 |
1/8 |
5/9* |
2/9 |
|
104 weeks |
1/23 |
1/18 |
3/21 |
11/19*** |
8/9*** |
|
Unscheduled deathsa |
1/31 |
0/33 |
2/32 |
8/33* |
38/48*** |
From Amyes (1990)
* p < 0.05; ** p < 0.01; *** p < 0.001
a Calculated by the Meeting
Males at the highest concentration also showed an increased incidence of adrenal phaeochromocytomas (11/41; 6/32 in controls), the percentages of animals with benign and malignant tumours being 14%, 12%, 19%, 14% and 26% at 0, 1, 10, 100 and 400 ppm, respectively. The differences were statistically significant, depending on the test used. The NOAEL was 10 ppm, equal to 0.47 mg/kg bw per day, on the basis of toxic effects on the liver, deaths and increased spleen weights at 100 ppm, equal to 4.7 mg/kg bw per day (Amyes, 1990).
The results of studies for genotoxicity with lindane are summarized in Table 10.
Table 10. Results of studies of genotoxicity with lindane
|
End-point |
Test object |
Concentration |
Purity (%) |
Results |
Reference |
|
In vitro |
|||||
|
Reverse mutationa |
S. typhimurium TA1535, TA1538, TA100, TA98 |
0.93210 ΅g/plate in DMSO |
>99 |
Negative |
Röhrborn (1977a) |
|
Reverse mutationa |
S. typhimurium TA1535, TA1538 |
0.315 mg/ml in DMSO |
> 99 |
Positive ± S9b |
Röhrborn (1975) |
|
Reverse mutationc |
S. typhimurium TA1535, TA1537, TA100, TA98; E. coli WP2 uvrA |
165000 ΅g/plate |
NR |
Negative |
Oesch (1980) |
|
Gene mutationd |
V79 Chinese hamster Hprt locus |
± S9, 0.5500 ΅g/ml |
99.8 |
Negative |
Glatt (1984) |
|
Gene mutatione |
V79 Chinese hamster Hprt locus |
+S9, 5500 ΅g/ml |
99.9 |
Negative |
Oesch & Glatt (1985) |
|
Chromosomal aberrationsf |
Chinese hamster ovary cells |
±S9, 25300 ΅g/ml |
99.7 |
Positiveg |
Murli (1990) |
|
DNA repairf |
Fischer 344 rat hepatocytes |
0.0515 ΅g/ml in DMSO |
99.7 |
Negative |
Cifone & McKeon (1990) |
|
Aneuploidy |
S. cerevisiae D61.M |
0.0030.17 mmol/l |
99.5 |
Negative |
Albertini et al. (1988) |
|
In vivo |
|||||
|
Chromosomal aberrationc |
Chinese hamster, bone marrow |
012 mg/kg bw per day for 5 days by gavage |
> 99 |
Positiveh |
Röhrborn (1976) |
|
Sister chromatid exchangei |
CF-1 mice, bone marrow |
Males: 0, 2, 10, 50 mg/kg bw per day |
99.8 |
Negative |
Guenard (1984a) |
|
Females: 1.6, 8, 40 mg/kg bw per day |
|||||
|
Sister chromatid exchangej |
CF-1 mice, bone marrow |
0, 1.3, 6.4, 32 mg/kg bw per day |
99.8 |
Positivek |
Guenard (1984b) |
|
Dominant lethal mutationl |
NMRI mice |
0, 12.5, 25, 50 mg/kg bw per day |
NR |
Negative |
Frohberg & Bauer |
|
Dominant lethal mutationm |
Rat (Chbb:THOM) |
0, 1.5, 7, 15 mg/kg bw per day, 8 weeks |
99.95 |
Negative |
Röhrborn (1977b) |
|
NR, not reported; S9, 9000 Χ g rat liver supernatant; DMSO, dimethylsulfoxide |
|
|
a |
Not compliant with GLP; no positive controls included |
|
b |
Dose-related mutagenic and toxic effects resulting in a decrease in the number of bacteria from 1.5 Χ 108 to 2.5 Χ 104; mutagenicity seen only in the presence of cytotoxicity or compound precipitate |
|
c |
Not compliant with GLP; positive controls included |
|
d |
Compliant with GLP; satisfies OECD guideline 476; positive controls included |
|
e |
Conducted under anaerobic conditions; compliant with GLP; satisfies OECD guideline 476; positive controls included; mutagenicity seen only in the presence of cytotoxicity or compound precipitate |
|
f |
Compliant with GLP; satisfies US EPA/FIFRA §84-2 (1989) guideline; positive controls included |
|
g |
Slight increase in percentage of cells with aberrations at 38 and 100 ΅g/ml S9; mutagenicity seen only in the presence of cytotoxicity or compound precipitate |
|
h |
1846% increase in aberrant metaphases including gaps at highest and lowest doses tested (p > 0.07); no increase in aberrant metaphases excluding gaps; mutagenicity seen only in the presence of cytotoxicity or compound precipitate |
|
i |
Compliant with GLP; conducted according to OECD short-term toxicology group, draft no. 14 US proposal. Lindane was administered orally to CF-1 mice, 2 h after subcutaneous implantation of 5-bromodeoxyuridine tablets. |
|
j |
Compliant with GLP; conducted according to OECD short-term toxicology group, draft no. 14 US proposal. Lindane was administered by intraperitoneal injection to CF-1 mice, 2 h after subcutaneous implantation of 5-bromodeoxyuridine tablets. |
|
k |
All treated females had significantly increased means of sister chromatid exchange per animal. |
|
l |
Not compliant with GLP. Lindane was administered by intraperitoneal injection to NMRI mice; 2/10 animals at the highest dose died intercurrently; examination of one of the decedents post mortem revealed a full urinary bladder containing sediment and obstruction of the urinary passages. |
|
m |
Not compliant with GLP. Lindane was administered by gavage to Chbb:THOM rats. |
Mice
Lindane (purity, 99.78%) was administered by gavage to groups of 12 female CD-1 mice at a dose of 0, 1 or 3 mg/kg bw per day 15 days before mating and was continued until postnatal day 21. Two mice at 3 mg/kg bw per day had to be killed in extremis. Although the authors stated that these sacrifices were necessitated due to accidental injury during intubation, the results of necropsy did not support this statement, and the Meeting considered these deaths to be due to the compound. No effects were seen on reproductive parameters including mating performance, fertility, length of gestation or parturition or gestation index. No effects were seen on litter size, pup viability, pup body weight or clinical signs of toxicity. The parental NOAEL was 1 mg/kg per day on the basis of deaths at 3 mg/kg per day. The NOAEL for offspring was 3 mg/kg per day, the highest dose tested (Reader, 1998)
Rats
In a range-finding study for a multigeneration study in rats, groups of six Charles River CD rats of each sex received diets containing lindane (purity, 99.67%) at a concentration of 0, 20, 100, 200 or 400 ppm, starting 15 days before mating and continuing until postnatal day 4.
Parental toxicity was manifested by the deaths of three females at 400 ppm. Alopecia was observed in all three decedents and in two of the three surviving females. Decreased body-weight gain was noted in females at 400 ppm when compared with concurrent controls. Although dose-related, the decreases in body-weight gain never exceeded 8%. Food consumption was decreased in males during the first week of dosing at 400 ppm and in females at concentrations > 200 ppm throughout the pre-mating period; however, food consumption was measured only during this period and may have continued during gestation and lactation. A decline in food use efficiency was also seen in these animals during this period. No compound-related changes in estrus, mating, fertility, conception rate, pre-coital interval or gestation length were observed. Necropsy of parental animals revealed increased absolute and relative liver weight for males at 400 ppm (9% and 8%, respectively) and females at 200 and 400 ppm (200: 23% and 15% increases in absolute and relative liver:body weights, respectively, 400 ppm: 20% and 51% absolute and liver:body weight, respectively).
No clinical signs of toxicity were seen in the offspring, although a 13% decrease in implantation sites and decreased numbers of live pups per litter (1232% decrease) on postnatal days 2 and 4 were reported at concentrations > 200 ppm. In addition, the number of live births was decreased by 16% at 400 ppm. The offspring of animals at concentrations > 100 ppm had a 1023% decrease in body weight between postnatal day 1 and 4. An increase in the female:male ratio was seen at 400 ppm. At necropsy, no milk was found in the stomachs of pups that died before postnatal day 4. The NOAEL for parental toxicity was 100 ppm, equal to 7.4 mg/kg bw per day, on the basis of decreased body-weight gain, food consumption and food use efficiency. The NOAEL for developmental toxicity was 20 ppm, equal to 1.6 mg/kg bw per day, on the basis of a 1023% decrease in body weight (Higgins, 1989).
In a multigeneration study, Charles River rats received diets containing lindane (purity, 99.5%) at a concentration of 0, 1, 20 or 150 ppm, starting at least 10 weeks before mating and continuing for two generations. The parental (F0) generation, comprising 30 rats of each sex per dose, received lindane in the diet for 10 weeks before mating until sacrifice about 3 weeks after weaning of the F1 litter. These animals were mated 1:1 (avoiding sibling matings) to produce the F1 generation, and 30 rats of each sex per dose from the F1 generation were selected to produce the F2 generation. These animals received lindane in the diet at the same concentration as the F0 generation, beginning on postnatal day 21, for at least 10 weeks before mating until sacrifice about 3 weeks after weaning of the F2 generation.
Animals in the F0 generation were observed daily for deaths, moribundity and signs of toxicity. Males were weighed weekly until sacrifice, and females were weighed weekly until mating was detected, on days 0, 6, 13 and 20 of gestation and on postnatal days 1, 4, 7, 14, 21 and 25. Food consumption was measured weekly only during the pre-mating period. All animals were necropsied grossly, and the kidneys, liver and reproductive organs were collected and weighed. The reproductive organs of animals in the control and high-concentration groups and the livers and kidneys of all treated animals were evaluated histologically.
For the F1 and F2 generations, the numbers of dead and live pups per litter, individual pup weights, sex and mortality, moribundity and signs of toxicity were recorded. On postnatal day 4, the litters were reduced to eight pups each, with four of each sex if possible. Pups were weighed on postnatal days 1, 4 (before culling), 7, 14, 21 and 25 and sexed on postnatal days 1, 4 (before and after culling), 14 and 25. Developmental landmarks such as pinna unfolding, hair growth, tooth eruption and eye opening were evaluated on a litter basis by recording day of onset and completion of the parameter. Pups culled on postnatal day 4, found dead or not selected as parental animals for the next generation were necropsied grossly.
The signs of toxicity in the F0 generation consisted of a significant decrease (11%) in body-weight gain in females on day 20 of gestation and postnatal day 1 at the highest dietary concentration. The effects seen at necropsy included significant increases in absolute and relative kidney:body weights of males at concentrations > 20 ppm and increased absolute and relative liver:body weights of females at 150 ppm. Gross examination revealed a 35% increase in the incidence of pale kidneys in animals at 150 ppm.
The effects of lindane on F1 offspring included significant decreases in body weights (< 11%) and increases in the absolute and relative kidney:body weights (12 and 19%, respectively) and relative liver:body weight (8%) in males at 150 ppm at terminal sacrifice. In addition, males at this concentration showed an increased incidence of hydronephrosis (7/30; 0/28 in controls). Males also had statistically significant increases in the incidence of periacinar hepatocellular hypertrophy (Table 11) and renal changes consistent with hyaline droplet formation at concentrations > 20 ppm at terminal sacrifice in adulthood.
Table 11. Incidence of periacinar hepatocytic hypertrophy in a multigeneration study of reproductive toxicity in rats given diets containing lindane
|
Generation |
Sex |
Dietary concentration (ppm) |
|||
|
0 |
1 |
20 |
150 |
||
|
F0 adults |
Male |
0/30 |
1/30 |
1/30 |
9/29** |
|
Female |
0/29 |
1/30 |
1/30 |
14/30** |
|
|
F1 adults |
Male |
0/28 |
2/30 |
6/30* |
6/30* |
|
Female |
0/30 |
0/29 |
1/29 |
11/28** |
|
From King (1991)
* p < 0.05; ** p < 0.001
The evidence of toxicity in the F2 generation consisted of a slight (8%) but statistically significant (p < 0.01) decrease in pup weight on postnatal day 1, statistically significantly (p < 0.001) decreased body-weight gain (8%) throughout lactation and statistically significant (p < 0.01) delays in the onset and completion of tooth eruption (10 and 12%, respectively) and hair growth (24%) at 150 ppm.
Reproductive parameters, such as length of gestation, post-implantation index and mating, fertility and liveborn indices were unaffected by treatment. However, at the highest concentration, 3/28 litters in the first generation and 2/27 litters in the second generation died or were killed for humane reasons. The NOAELs for parental and reproductive toxicity were 150 ppm, equal to 13 mg/kg bw per day, the highest concentration tested. The NOAEL for toxicity to offspring was 20 ppm, equal to 1.7 mg/kg bw per day, on the basis of developmental delays (tooth eruption and hair growth), decreased pup weight and decreased viability index (King, 1991).
Mice
In a study of developmental toxicity, groups of 25 NMRI-EMD (SPF) mice were given lindane (purity, > 99%) by gavage at a dose of 0, 12, 30 or 60 mg/kg bw per day in 0.5% carboxymethylcellulose. One group was treated on days 615 of gestation and the other on days 1113. All animals were killed on day 18 of gestation.
Animals given doses > 30 mg/kg bw per day on days 615 showed decreased activity, dyspnoea and occasional staggering 1530 min after administration. Animals receiving 60 mg/kg bw per day also had a statistically significant (p < 0.05), 25% decrease in body weight, an increased mortality rate (12/25; 0/25 in controls) and a statistically significant increase in the abortion rate (22/25; 0/25 in controls). The pregnancy rate of animals given 30 and 60 mg/kg bw per day dose groups declined by 12% and 14%, respectively.
Animals treated on days 1113 of gestation showed no clinical signs of maternal toxicity. Mortality rate, body weights and pregnancy rates were unaffected by treatment. However, animals at 12 and 30 mg/kg bw per day had higher abortion rates (14% and 6%, respectively) than concurrent controls.
The number of live fetuses per dam was decreased in animals treated on days 615 of gestation with 12 or 60 mg/kg bw per day (8.4 and 7.7, respectively, with 9.5 in controls). The weight of fetuses exposed in utero on these days was lower at 60 mg/kg bw per day than in controls. In fetuses exposed in utero on days 1113 of gestation, the only treatment-related effect was a decrease in the number of live fetuses per dam in the group receiving 12 mg/kg bw per day (8.4, with 9.5 in controls).
No compound-related effects were seen in terms of fetal length, sex ratio or the incidence or types of malformations at any dose with either treatment regimen. The NOAEL for maternal toxicity was 12 mg/kg bw per day on the basis of decreased activity, dyspnoea and staggering after dosing as well as decreased pregnancy rates at 30 mg/kg bw per day. No NOAEL could be identified for developmental toxicity as a decreased number of live fetuses per dam was seen at the lowest dose tested (Fröhberg & Bauer, 1972b).
Rats
In a study of developmental toxicity in rats, lindane (purity, > 99%) was administered at a dose of 0, 5, 10 or 20 mg/kg bw per day in 0.5% carboxymethylcellulose to groups of 20 CFY rats by gavage on days 615 of gestation. The dams were killed on day 20 of gestation and subjected to gross necropsy and histopathological examination; several major organs were weighed, and the ovaries and uterine contents were evaluated to assess the number of corpora lutea, number of viable young, resorption sites, litter weights and any fetal abnormalities.
Parental toxicity was seen in the form of the deaths of two dams at the highest dose on days 1214 of gestation and decreased food consumption at 10 and 20 mg/kg bw per day (by 18% and 31%, respectively). Body-weight gain was decreased by 31% and 54% at the two higher doses, respectively. Gross appearance, organ weights and pregnancy rates were unaffected by treatment.
The evidence of developmental toxicity was limited to a statistically significant increase in supernumerary ribs at the highest dose (41%, with 13% in controls on a litter basis; 54%, with 17% in controls on a fetus basis). Although not statistically significant, the increase on a litter basis at the intermediate dose (32%, with 13% in controls) is considered biologically relevant. The NOAEL for maternal toxicity was 5 mg/kg bw per day on the basis of decreased body-weight gain and food consumption at 10 mg/kg bw per day. The NOAEL for developmental toxicity was 5 mg/kg bw per day on the basis of an increased incidence of supernumerary ribs on both a fetal and litter basis at 10 mg/kg bw per day (Palmer & Lovell, 1971).
Rabbits
In a study of developmental toxicity, groups of 13 presumed pregnant New Zealand white rabbits were given lindane (purity not stated) by gavage in 0.5% carboxymethylcellulose at a dose of 0, 5, 10 or 20 mg/kg bw per day on days 618 of gestation. The dams were killed on day 29 and subjected to gross necropsy, and all fetuses were examined for visceral and skeletal malformations and variations. The results of external examination of the fetuses were not reported.
All does survived to scheduled sacrifice. All treated animals showed signs of toxicity, evidenced as tachypnoea and lethargy. The maternal body weight and food consumption were similar in treated and control groups. The results of gross necropsy were unremarkable. The weights of the organs were similar in treated and control groups. No treatment-related effects were observed in any group on the number of corpora lutea, number of implantation sites, number of live fetuses per dam, pre- and post-implantation losses, fetal body weights or fetal sex ratios. No treatment-related visceral or skeletal malformations or variations were observed in any of the fetuses, except for a slight increase (85%; controls, 63%) in the incidence of rabbits with 13 ribs at 20 mg/kg bw per day. No NOAEL could be identified for maternal toxicity as tachypnea and lethargy were seen at 5 mg/kg bw per day. The NOAEL for developmental toxicity was 10 mg/kg bw per day on the basis of the incidence of fetuses with 13 ribs (Palmer & Neuff, 1971).
Dogs
Beagle dogs received diets containing lindane (purity unspecified) at concentrations providing a dose of 0, 7.5 or 15 mg/kg bw per day from day 1 or 5 of gestation. No sign of maternal toxicity was reported. An increased incidence of stillbirths was reported in both treated groups (1831%; 2% in controls). The NOAEL for maternal toxicity was 15 mg/kg bw per day, and that for developmental toxicity was 7.5 mg/kg bw per day on the basis of the increased incidence of stillbirths (Earl et al., 1973).
Mice
In a study of the effects of lindane on mitochrondrial side-chain cleavage of cholesterol, mice received diets containing lindane at 0.02 mg for 4 weeks, 0.07 mg for 2 weeks, 0.03 mg for 6 weeks or 0.07 mg for 4 weeks. Treatment resulted in a decrease in cholesterol side-chain cleavage, which led to a decrease in the conversion of cholesterol to pregnolone and ultimately progesterone. A dose-dependent, 1634% increase in cholesterol concentration with a concomitant 2476% decrease in pregnolone and 840% decrease in progesterone were seen after lindane administration (Sircar & Lahiri, 1989).
Intraperitoneal injection of lindane at 15 mg/kg bw per day for 7 days resulted in a modest increase in estrone metabolism and inhibition of its uterotropic effect (Welch et al., 1971).
Lindane (purity, 99.8%) was administered to groups of six pregnant Swiss mice at a dose of 11 mg on days 14 of gestation. No signs of maternal toxicity were reported, but the ovarian weight of all lindane-treated animals was low, and no implantations were observed even when a vaginal plug and sperm had been detected in the vaginal smear. Another group of six mice received the same dose of lindane on days 14 of gestation in conjunction with 0.6 ΅g of estrogen on day 4 and 0.33 ΅g of progesterone on days 13 and 510. The animals were killed and necropsied on day 11. Mice that received hormonal treatment during exposure to lindane showed a normal implantation rate, while those given lindane alone showed complete lack of implantation (Sircar & Lahiri, 1989).
Rats
In a study of the possible antiestrogenic activity of lindane, 21-day-old female Fischer 344 rats received lindane (purity unspecified) at a dose of 0 (six rats), 5 (six rats), 10 (eight rats), 20 (12 rats) or 40 mg/kg bw per day (12 rats) by gavage for 14 weeks. All rats were evaluated for vaginal opening and for staging in the estrous cycle from vaginal smears. Beginning in week 11, naso-anal length was measured daily in order to estimate obesity (Lee index). All animals were killed by decapitation after week 15 on the day of vaginal proestrus between 13:00 and 14:00 h, and blood, liver, uterus, ovaries, brain and pituitary gland were collected and weighed.
Seven rats at 40 mg/kg bw per day dose died before the end of the study. Animals at 20 and 40 mg/kg bw per day showed statistically significant increases in body weight and body-weight gain from week 11. The increases for animals at 20 mg/kg bw per day were 1020% of control values for body weight and 280960% of control values for body-weight gain; the increase in body weight for animals at 40 mg/kg bw per day was 260280% of control values. All treated animals were obese from week 12 (Lee indices, 101106% of control values), and these findings correlated with increases in food consumption and use efficiency at doses > 20 mg/kg bw per day. Vaginal opening was delayed for females at doses > 10 mg/kg bw per day, and they showed a statistically significant decrease in the number of proestrus days in a 25-day interval, with five proestrus days in the control group and 2.53.5 days in treated groups. Food consumption was increased during the last day of vaginal diestrus and the day of vaginal proestrus. Liver weights were increased in a dose-dependent manner, while uterine, ovarian and pituitary weights were decreased The NOAEL was 5 mg/kg bw per day on the basis of delays in vaginal opening and disruption of estrous cyclicity (Chadwick et al., 1988).
Two studies were conducted to evaluate the effect of lindane on hormonal control of reproductive function in female rats. In the first experiment, 21-day-old female rats received lindane (purity unspecified) by gavage at a dose of 0 (six rats), 5 (six rats), 10 (eight rats), 20 (12 rats) or 40 mg/kg bw per day (12 rats) for a maximum of 109 days, until they were 130 days of age. Beginning on day 28, the animals were examined for vaginal opening, and then vaginal smears were obtained to determine the stage in the estrus cycle. Animals that showed two consecutive, regular ovarian cycles between 110 and 125 days of age were killed on the day of vaginal proestrus, and blood was collected and assayed for luteinizing hormone, follicle-stimulating hormone, prolactin, estradiol and progesterone. In addition, the pituitary was removed, weighed and sonicated to determine pituitary levels of luteinizing hormone, prolactin and follicle-stimulating hormone. Uterine and ovarian weights were also obtained.
While animals at 5 or 20 mg/kg bw per day showed no delay in vaginal opening, those at 10 and 40 mg/kg bw per day had statistically significant delays of 4 days and 7 days, respectively, in vaginal opening. These delays could not be attributed to decreases in body weight as the body weights of rats at doses > 20 mg/kg bw per day were higher than those of controls. Regular vaginal cycles were delayed at all doses, although they appear to normalize after 90 days in animals at 5 mg/kg bw per day and at 110 days of age at doses > 10 mg/kg bw per day. The absolute and relative uterine weights were decreased at doses > 20 mg/kg bw per day; a decrease was also seen at 5 mg/kg bw per day but not at 10 mg/kg bw per day. The absolute uterus weights decreased by 27% and 64% at 20 and 40 mg/kg bw per day, respectively, and the relative uterine weights decreased by 8%, 40% and 68% at 5, 20 and 40 mg/kg bw per day, respectively. The pituitary weights were decreased by 1428% at doses > 10 mg/kg bw per day. The circulating serum concentrations of luteinizing hormone and prolactin decreased in a statistically significant manner at doses > 20 mg/kg bw per day, but that of follicle-stimulating hormone was unaffected by treatment. While the serum estradiol concentrations at 5 and 20 mg/kg bw per day were comparable to those of the control group, a statistically significant (p < 0.05) increase was reported at 10 mg/kg bw per day and a decrease (p < 0.05) at 40 mg/kg per day. In the pituitary, however, the luteinizing hormone concentration decreased and that of follicle-stimulating hormone increased at doses > 20 mg/kg bw per day, while the concentration of prolactin decreased at 40 mg/kg bw per day.
In the second experiment, four groups of eight 21-day-old female rats received lindane (purity unspecified) in peanut oil at a dose of 30 mg/kg bw per day for 7 days, and another four groups of eight rats received peanut oil. On day 27, two groups given lindane and two given peanut oil received a subcutaneous injection of 10 ΅g of estradiol benzoate in corn oil. The remaining groups received subcutaneous injections of corn oil only (see Table 12 for group assignment). The rats were killed 6 or 30 h after injection of estradiol benzoate; blood was collected for hormone assays, as in the first experiment, and the uterus and ovaries were weighed.
Table 12. Treatment given to groups of eight rats in a study to evaluate the effect of lindane on hormonal control of reproductive function
|
Gavage on postnatal day 21 |
Subcutaneous injection |
Time of sacrifice (h) |
|
Peanut oil |
Corn oil |
6 |
|
Peanut oil |
Corn oil |
30 |
|
Peanut oil |
Estradiol benzoate |
6 |
|
Peanut oil |
Estradiol benzoate |
30 |
|
Lindane |
Corn oil |
6 |
|
Lindane |
Corn oil |
30 |
|
Lindane |
Estradiol benzoate |
6 |
|
Lindane |
Estradiol benzoate |
30 |
From Cooper et al. (1989)
Both control and lindane-treated animals given estradiol benzoate showed increased uterine weights at sacrifice. In animals killed 6 h after hormone treatment, the uterine weights were similar in the two groups; however, 30 h after hormone treatment, the uterine weight of lindane-exposed animals was lower than that of controls (i.e. the gain in uterine weight was greater for control than lindane-treated animals). This effect cannot be attributed to differences in body weight as control and lindane-treated animals had similar body weights. At the 6-h evaluation, the pituitary weights of animals given lindane followed by corn oil were lower than those of animals given peanut oil followed by corn oil. This effect was not seen, however, at the 30-h examination. Conversely, estradiol benzoate treatment increased pituitary weights in both control and lindane-treated groups 30 h later. Lindane appeared to have an antagonistic effect to estradiol benzoate, as animals given peanut oil followed by estradiol benzoate had heavier pituitary glands than animals given lindane before estradiol benzoate (Table 13).
Table 13. Body weights, organ weights and serum estradiol concentrations in rats given lindane or peanut oil with and without estradiol benzoate
|
Treatment |
Body weight (g) |
Pituitary weight (mg) |
Uterine weight (mg) |
Serum estradiol concentration (pg/ml) |
|
Peanut oil, corn oil, 6-h sacrifice |
66 ± 2.0 |
3.9 ± 0.06 |
65 ± 4.5 |
ND |
|
Peanut oil, corn oil, 30-h sacrifice |
69 ± 2.0 |
4.2 ± 0.24 |
64 ± 4.1 |
ND |
|
Peanut oil, estradiol benzoate, 6-h sacrifice |
61 ± 1.4 |
3.9 ± 0.12 |
85 ± 3.0a |
190 ± 40 |
|
Peanut oil, estradiol benzoate, 30-h sacrifice |
72 ± 2.2 |
4.8 ± 0.17a |
140 ± 3.4a |
110 ± 40 |
|
Lindane, peanut oil, 6-h sacrifice |
61 ± 2.0 |
3.4 ± 0.15b |
53 ± 1.8 |
ND |
|
Lindane, peanut oil, 30-h sacrifice |
65 ± 2.5 |
3.6 ± 0.11 |
58 ± 3.4 |
ND |
|
Lindane, estradiol benzoate, 6-h sacrifice |
61 ± 2.6 |
3.6 ± 0.12 |
84 ± 7.4a |
250 ± 36 |
|
Lindane, estradiol benzoate, 30-h sacrifice |
63 ± 2.2 |
4.2 ± 0.11ab |
110 ± 2.9a,b |
50 ± 14 |
|
|
From Cooper et al. (1989); ND, not detected |
|
a |
Mean significantly different (p < 0.05) from that for animals not receiving estradiol benzoate and killed at the same time |
|
b |
Mean significantly different (p < 0.05) from that for animals given peanut oil and estradiol benzoate and killed at the same time |
The circulating serum estradiol concentrations were comparable in control and treated animals at the 6- and 30-h examinations. While the concentrations of serum luteinizing hormone and prolactin were not affected by lindane in the absence of estradiol benzoate (peanut oil- and corn oil-treated animals compared with lindane and corn oil-treated animals), estradiol benzoate increased the concentrations of these two hormones, regardless of lindane treatment. Nonetheless, the concentrations of luteinizing hormone and prolactin in the serum of animals that had not been given lindane were higher than those of animals given lindane before estradiol benzoate (Table 14). As for pituitary weights, this suggests that lindane antagonizes the effect of estradiol benzoate.
Table 14. Serum luteinizing hormone and prolactin concentrations in rats treated with peanut oil or lindane with and without estradiol benzoate
|
Luteinizing hormone |
Prolactin |
|
Peanut oil, corn oil, 30 h < peanut oil, estradiol benzoate, 30 h** |
Peanut oil, corn oil, 6 h < peanut oil, estradiol benzoate, 6 h** |
|
Lindane, corn oil, 30 h < lindane, estradiol benzoate, 30 h** |
Peanut oil, corn oil, 30 h < peanut oil, estradiol benzoate, 30 h** |
|
Lindane, estradiol benzoate, 30 h < peanut oil, estradiol benzoate, 30 h* |
Lindane, corn oil, 30 h < lindane, estradiol benzoate, 30 h** |
From Cooper et al. (1989)
* p < 0.05; ** p < 0.01
The pituitary concentrations of luteinizing hormone, prolactin and follicle-stimulating hormone decreased in estradiol benzoate-injected animals, regardless of prior exposure to lindane (Table 15). Nevertheless, these hormones were found in higher concentrations in the pituitaries of lindane-exposed animals than in controls, further confirming the antagonism of lindane with respect to estradiol benzoate (Cooper et al., 1989).
Table 15. Pituitary luteinizing hormone, follicle-stimulating hormone and prolactin concentrations in rats treated with peanut oil or lindane with and without estradiol benzoate
|
Luteinizing hormone |
Follicle-stimulating hormone |
Prolactin |
|
Lindane, estradiol benzoate, 30 h < peanut oil, corn oil, 30 h** |
Peanut oil, estradiol benzoate, 30 h < peanut oil, corn oil, 30 h** |
Peanut oil, estradiol benzoate, 6 h < peanut oil, corn oil, 6 h |
|
Lindane, estradiol benzoate, 30 h < peanut oil, corn oil, 30 h** |
Lindane, estradiol benzoate, 30 h < lindane, corn oil, 30 h** |
Peanut oil, estradiol benzoate, 30 h < peanut oil, corn oil, 30 h** |
|
Peanut oil, estradiol benzoate, 30 h < lindane, estradiol benzoate, 30 h** |
Peanut oil, estradiol benzoate, 30 h < lindane, estradiol benzoate, 30 h* |
Lindane, estradiol benzoate < lindane, corn oil, 6 h* |
|
Lindane, estradiol benzoate, 30 h < lindane, corn oil, 30 h* |
||
|
Lindane, corn oil, 30 h < peanut oil, corn oil, 30 h** |
||
|
Peanut oil, estradiol benzoate, 30 h < lindane, estradiol benzoate, 30 h* |
From Cooper et al. (1989)
*p < 0.05; ** p < 0.01
The effects seen in the study described above are in contrast to those in a previous study, in which groups of six ovariectomized albino rats were given peanut oil only, lindane (unspecified purity) in oil at 20 mg/kg bw per day, 1 ΅g of estradiol dipropionate intraperitoneally or 1 ΅g of estradiol dipropionate intraperitoneally plus lindane at 20 mg/kg bw per day orally for 30 days. The uterus, cervix and vagina of all animals were weighed, examined microscopically and analysed for glycogen content; and the erythrocyte count, total leukocyte count and haemoglobin concentrations were recorded.
Lindane alone did not affect the uterine, cervical or vaginal weights, but it did increase the glycogen content of these organs, by 27%, 61% and 23%, respectively. Animals given estradiol dipropionate showed substantial increases in uterine (320%), cervical (500%) and vaginal (170%) weights and increases in the glycogen content of 290%, 260% and 360%, respectively. Although simultaneous treatment with lindane and estradiol dipropionate resulted in increases of 260%, 450% and 170% in the weights of the uterus, cervix and vagina, respectively, and increases of 190%, 230% and 330% in the glycogen content of the three organs, respectively, the increases were not as large as with estradiol dipropionate alone. Histopathological evaluation of these ovariectomized animals showed infantile uterine, cervical and vaginal tissues. Treatment with lindane alone did not alter this observation, but treatment with estradiol alone or estradiol plus lindane led to the development (maturation) of reproductive tissue to a comparable extent. Limited haematological evaluation showed increased haemoglobin concentrations and erythrocyte counts in animals treated with estradiol, lindane or estradiol plus lindane, while the total leukocyte count was unaffected (Raizada et al., 1980).
In a study designed to examine the effect of lindane on sexual receptivity, groups of five to nine female Fischer CD 344 rats were given lindane (purity unspecified) by intraperitoneal injection at a dose of 0, 25, 33, 50 or 70 mg/kg bw in the morning of proestrus (between 10:00 and 11.00 h) or picrotoxin at 0, 1, 2 or 2.5 mg/kg bw. The sexual receptivity of females was tested by housing them with experienced studs on the evening of proestrus (between 19:00 and 21:00 h) for about 10 min or > 10 mounts. A successful mount was defined as a male accomplishing pelvic contact and a pelvic thrust. The lordosis:mount ratio was used to calculate female sexual receptivity. Females showing typical signs of mating behaviour (i.e. hopping and darting) were considered proceptive.
Statistically significant decreases in the lordosis:mount ratio coupled with decreased proceptive behaviour were reported at doses > 33 mg/kg bw. These decreases could not be attributed to decreases in overall activity, as the lindane-treated females kicked and avoided the males. They also showed normal levels of rearing and vocalization. Lordosis occurred within three mounts in controls and females at 25 mg/kg bw but only after seven mounts at 33 mg/kg bw, eight mounts at 50 mg/kg bw and six mounts at 75 mg/kg bw. Lordosis and proceptive behaviour were unaffected by picrotoxin, indicating that the changes in sexual receptivity were not dependent on gamma-aminobutyric acid. The NOAEL was 25 mg/kg bw on the basis of decreased sexual receptivity and proceptive behaviour (Uphouse, 1987).
In a study to examine the effect of lindane during diestrus on the reproductive cycle, groups of 610 adult, regularly cycling female Fischer CD 344 rats were given lindane (purity unspecified) at a dose of 0, 10, 25, 33 or 50 mg/kg bw by intraperitoneal injection on the afternoon of diestrus. Vaginal smears were obtained throughout 68 days after treatment, and the animals were observed for sexual receptivity on the day of predicted proestrus. Sexual receptivity was estimated by calculating the lordosis:mount ratio. Proceptivity was defined as displaying typical mating behaviour, such as hopping and darting. The ability of lindane to compete with estradiol for receptor binding was evaluated by adding [3H]estradiol with various concentrations of lindane to the cytosol fractions obtained from the hypothalamus, pre-optic area, pituitary and uterus.
[3H]Estradiol binding to its receptors in brain and uterus was unaffected by treatment with lindane. The length of the estrous cycle was increased by 68 days after treatment with lindane at doses > 25 mg/kg bw. On the predicted day of proestrus, a statistically significant decrease in the lordosis:mount ratio was reported for females at doses > 33 mg/kg bw. Evaluation by vaginal smear revealed that these animals did not have vaginal proestrus. However, the lordosis:mount ratio of treated animals in proestrus was comparable to that of control animals in proestrus (Uphouse & Williams, 1989).
Lindane (purity, 99.8%) was administered intraperitoneally to female CF rats twice a week for 4 weeks at a dose of 5 or 10 mg/kg bw per day. Exposure at both doses resulted in a lengthening of proestrus (five to seven times longer than control) and delays in ovulation. Vaginal cells exfoliated at proestrus had higher glycogen, succinate dehydrogenase and alkaline phosphatase contents. Cells exfoliated at estrus were characterized by increased acid phosphatase activity and increased mucopolysaccharide concentrations. Under the conditions of this study, lindane appeared to have estrogenic properties (Lahiri et al., 1985).
The effects of lindane on rat testes were studied in various experiments. Srinivasan et al. (1988) reported that administration of lindane at 800 ppm for 2 weeks resulted in tubular atrophy, oligospermia, increased numbers of necrotic spermatogenic cells and narrowed seminiferous tubules. Dickshith & Datta (1972) reported that intratesticular injection of 0.25 mg of lindane inhibited spermatogenesis and caused testicular hypertrophy in 7/12 rats and atrophy in the remaining five rats. The testicular hypertrophy was characterized by a doubling of testis size and weight, massive degeneration of seminiferous tubules and misshapen spermatozoa. The testicular atrophy was characterized by a 33% decrease in testis size, shrunken seminiferous tubules and oligospermia. In a study in which lindane was administered for 90 days at a dose of 18 mg/kg bw per day, massive damage to testicular tissue was reported, including tubular atrophy, spermatogenic cell necrosis and enlargement of interstitial cells (Dikshith et al., 1978).
Groups of eight CD-1 mice of each sex were given diets containing lindane (purity, 99.78%) at a concentration of 0, 10, 40 or 160 ppm for 39 weeks. A positive control group was given a single intraperitoneal dose of cyclophosphamide at 50 mg/kg bw 2 days before blood sampling. Blood was collected from the retro-orbital sinus of groups of four anaesthetized rats of each sex per dose on consecutive days, and the lymphocyte populations were identified with antibodies to CD3, CD4 and CD8 for T lymphocytes, CD19 for B lymphocytes and DX5 for natural killer (NK) cells.
Males consistently had higher lymphocyte counts than females (by 76%), accounted for primarily by higher counts of CD19+ B lymphocytes (125% of control). Cyclophosphamide treatment resulted in a 53% decrease in the absolute number of lymphocytes. Of the lymphocyte subpopulations, CD19+ B lymphocytes appeared to be the most significantly affected by cyclophosphamide, with a decrease of 64%, followed by NK cells, which were decreased by approximately 50%. Consequently, the percentage of B lymphocytes in the blood decreased while that of T lymphocytes increased; however, the absolute number of T lymphocytes did not increase and therefore the change in the proportion of B to T lymphocyte was due to susceptibility of B lymphocytes and not to proliferation of T lymphocytes. Females responded differently to cyclophosphamide: the absolute lymphocyte count increased by 74% as a result of a 92% increase in CD19+ B lymphocytes and a 102% increase in NK cells. A statistically significant (p < 0.05) 55% increase in NK cells was reported in females given lindane at 160 ppm. This was the only lymphocyte parameter altered by lindane. Under the conditions of this study, lindane did not affect the number or proportion of circulating lymphocytes (Wing, 2000).
Rats
The accumulation of alpha2u-globulin was evaluated in the kidneys of groups of 10 Fischer 344 rats of each sex given diets containing lindane (purity unspecified) at a concentration of 0, 1, 10, 100 or 400 ppm for 30 days. Slides of renal tissue from males at all dietary concentrations and from females only at 0 and 400 ppm were treated with anti-alpha2u-globulin antibodies in order to detect the presence of this protein. In addition to immunostaining, the slides from males at 0, 1 and 400 ppm and females at 0 and 400 ppm were stained with Masson Trichrome and haematoxylin and eosin to detect the presence of hyaline droplets.
alpha2u-Globulin was detected by immunostaining in males in all groups (including controls). The extent of immunostaining, and consequently of alpha2u-globulin, increased in a dose-dependent manner. No immunostaining was reported in females. Masson Trichrome staining revealed small hyaline droplets in males at 0 and 1 ppm and in females at 0 and 400 ppm. Large hyaline droplets were observed in males at 400 ppm. Haematoxylin and eosin staining showed evidence of hyaline droplet accumulation, multifocal cortical tubular necrosis, cortical tubular regeneration and granular casts at junctions of proximal tubules and the ascending loop of Henle in males at 400 ppm. Immunohistochemistry and histochemistry revealed changes in renal tissue from males consistent with alpha2u-globulin nephrotoxicity. The absence of changes in females suggests that the nephrotoxicity caused by lindane in males is related to accumulation of alpha2u-globulin in their kidneys (Dietrich & Swenberg, 1989).
Rats
In a study of acute neurotoxicity, groups of 10 Crl:CD®BR rats of each sex were given single doses of lindane (purity, 99.78%) by gavage at a concentration of 0, 6, 20 or 60 mg/kg bw. A battery of functional observational tests (FOB) and tests for motor activity were performed before and within 3 h (time of peak effect) of dosing (day 0) and on days 7 and 14 after dosing. Body weights were recorded before treatment, weekly during the study and on the FOB assessment days. Clinical signs were recorded at least once daily. At study termination, all animals were killed and fixed by whole-body perfusion, and designated tissues of the nervous system were processed for microscopic evaluation.
All animals survived to scheduled termination. One male at 60 mg/kg bw had convulsions within 2.8 h of treatment. Clinical signs were also observed in females at 60 mg/kg bw within 24 h of treatment and included staining of fur, stained urogenital region, hunched posture and piloerection; these effects persisted for 4 days. Significant treatment-related decreases in body-weight gain were observed for males at 60 mg/kg bw when compared with the control group during the first week of the study. Females at this dose also had slightly lower body-weight gains throughout the study. The food consumption of males and females at 60 mg/kg bw was significantly lower than that of controls during week 1 of the study. The food conversion ratios were similar for the treated and control groups.
At the first FOB assessment 3 h after treatment, males and females at 60 mg/kg bw had piloerection (1 male, 2 females), decreased rectal temperature (1 male, 1 female), increased hind-limb foot splay and hunched posture (4 males, 7 females). Males at this dose also had increased respiration (3 males, 1 female), and one showed tremor and twitching, while females had increased incidences of walking on tiptoe (10), licking behaviour (3), decreased foot splay (3) and absence of grooming (8). Females at 20 mg/kg bw also showed decreased grooming behaviour and increased fore-limb grip strength (3). Motor activity was significantly decreased in three males and three females at 60 mg/kg bw 3 h after treatment. The FOB assessment and motor activity in the group given 6 mg/kg bw were comparable to those of controls (Table 16).
Table 16. Mean motor activity (in s) during a 1-h session in male and female rats treated with lindane by gavage
|
Day |
Dose of lindane (mg/kg bw) |
|||||||
|
Males |
Females |
|||||||
|
0 |
6 |
20 |
60 |
0 |
6 |
20 |
60 |
|
|
Before treatment |
660 |
700 |
520 |
700 |
620 |
750 |
790 |
740 |
|
1 |
170 |
300 |
160 |
85 |
350 |
440 |
320 |
130** |
|
8 |
700 |
830 |
590 |
930 |
470 |
490 |
550 |
440 |
|
15 |
640 |
780 |
660 |
910* |
560 |
630 |
580 |
540 |
From Hughes (1999a)
*p < 0.05; **p < 0.01
No neuropathological lesions were observed on histological examination in the peripheral or central nervous system of treated animals. The NOAEL was 6 mg/kg bw per day on the basis of increased forelimb grip strength and decreased grooming behaviour at higher doses (Hughes, 1999a).
In a short-term study of neurotoxicity, groups of 10 Crl:CD®BR rats of each sex were were given diets containing lindane (purity, 99.78%) at a concentration of 0, 20, 100 or 500 ppm for 13 weeks. Owing to severe toxic reactions to treatment at 500 ppm, the dose was reduced to 400 ppm on day 11. These doses resulted in average daily intakes of 0, 1.4, 7.1 and 28 mg/kg bw per day for males and 0, 1.6, 7.9 and 30 mg/kg bw per day for females. FOB and motor activity tests were performed before and after 4, 8 and 13 weeks of treatment. Body weights were recorded before treatment, weekly during the study and on the FOB assessment days. Clinical signs were recorded at least once daily. At study termination, all animals were killed and fixed by whole-body perfusion, and designated tissues of the nervous system were processed for microscopic evaluation.
Three females at 500/400 ppm died before the scheduled termination, one on day 11 of the study, one during week 10 and one during week 13, and these deaths were attributed to treatment with lindane. The clinical signs before death included weight loss, swollen muzzle with scabbing, hunched posture, piloerection and staining of the anogenital region. Surviving females at this concentration showed hypersensitivity to touch, staining of the urogenital region and scabbing of the toes.
Significant (p < 0.05 or p < 0.01) treatment-related decreases in body weight were observed in males and females (14% and 23%, respectively) at 500/400 ppm, and decreased body-weight gains (70% for males and 180% for females, p < 0.01), food consumption (35% for males and 50% for females, p < 0.05 or p < 0.01, respectively) and food conversion ratios were observed for males and females at 500 ppm during the first week of the study when compared with the control group. Male rats tended to recover from these effects once the dose had been lowered, but the food consumption of females was slightly depressed throughout the remainder of the study. Females at 100 ppm had significantly lower body-weight gain (40%, p < 0.05) than the control group during the first week of the study, and this effect persisted, although not at a level of significance, throughout the study. These animals also had significantly decreased food consumption (16%, p < 0.01) during the first week of the study, and this trend continued. The weight of the liver was also increased in animals at 500/400 ppm, but no additional information was given.
During the FOB assessment, males and females at 500/400 ppm were perceived as difficult to handle and showed piloerection and hunched posture. Females at this concentration had missing claws (3), tended to urinate more often than controls, had increased grooming behaviour, rearing and motor activity, and one female had convulsions. Five to seven females in each treated group were seen to walk on tiptoe, and the difference in incidence from controls (1/10) was significant at the highest dietary concentration. Five females at 100 ppm also showed increased grooming behavior at week 4, and one animal in this group was extremely difficult to handle. The assessments of fore-limb and hind-limb grip strength and of hind-limb splay revealed no differences between any of the treated groups and the controls. Motor activity was also similar in treated and control groups.
No neuropathological end-points attributable to lindane were observed in the histological examinations of peripheral and central nervous system tissues from treated animals. The NOAEL was 100 ppm, equivalent to 7.1 mg/kg bw per day, on the basis of hypersensitivity to touch and hunched posture (Hughes, 1999b).
In a study of developmental neurotoxicity, groups of presumed pregnant Hsd Brl Han:Wist (Han Wistar) rats received diets containing lindane (purity, 99.78%) at a concentration of 0, 10, 50 or 120 ppm from day 6 of gestation through day 10 of lactation. These concentrations resulted in doses to F0 maternal animals of 0.80.9, 4.24.6 and 8.010 mg/kg per day, respectively, during gestation and 1.21.7, 5.68.3 and 1419 mg/kg, respectively, during lactation. Ten F1 offspring of each sex were evaluated in FOB and motor activity tests and for auditory startle response, learning and memory, developmental landmarks such as vaginal perforation and balano-preputial separation and brain weights and histopathological appearance on days 11 and 65, including morphometrics.
Small differences from controls in absolute maternal body weights (78%) were observed at 120 ppm during gestation and early lactation (through day 11). The body-weight gains of dams at this concentration on days 620 of gestation were 6479% (p < 0.01) of the control values. The body-weight gain during lactation was similar in treated and control groups. During gestation, the food consumption of rats at 120 ppm was significantly (p < 0.01) lower (7492% of control) than that of the control group on days 1013, 1417 and 1819 of gestation. The food consumption of animals at the two lower concentrations during gestation and of all treated groups during lactation was similar to that of controls.
The absolute body weights of treated male and female pups at the two lower dietary concentrations were 1218% and 1620% less than those of controls, respectively, on days 411 of lactation, with recovery to < 10% by day 21. The body-weight gains on days 14 and 111 of lactation were 76% and 84%, respectively, of the control levels for males at 50 ppm, 79% and 79%, respectively, for females at 50 ppm, 60% and 73%, respectively for males at 120 ppm and 63% and 75%, respectively, for females at 120 ppm (p < 0.05 or 0.01). The body-weight gains of all treated groups were similar to those of controls during days 1121 of lactation. The body-weight gains after weaning were similar in treated and control groups, except for females at the two lower dietary concentrations. The differences in body weight for dams at 120 ppm were 10% less at the beginning of lactation and recovered to 6% less by the end of the study.
The dams at 120 ppm had more stillborn pups, as indicated by a live birth index of 77% compared with 99% for the control group. In addition, nine of their litters either died or were killed when moribund on days 14 of lactation. This resulted in a viability index for the group at 120 ppm of 71% compared with 89% for the controls. The mortality rate before day 4 of pups at the two higher concentrations, in litters surviving to weaning, was greater than in controls: three pups in 2/20 control litters; 18 pups in 8/22 litters at 50 ppm; 14 pups in 4/15 litters at 120 ppm. The survival of pups at 10 ppm was not affected at any time. No dose- or treatment-related differences were observed in the duration of gestation, number of pups per litter on day 1 or per cent of male offspring.
At necropsy, no treatment-related gross abnormalities were observed in the dams or offspring. The absolute and relative weights of the liver and kidney of offspring were not affected by treatment.
Dams at 120 ppm showed increased reactivity to handling during weeks 2 and 3 of treatment, and pups showed slower surface righting on day 4. There were no effects on measures of physical or sexual development. Increased motor activity was seen in offspring of each sex at 50 and 120 ppm during lactation. Some decrease in habituation of motor activity was also seen in females on day 22. While no effect was found on auditory startle reflex amplitude, a clear reduction in auditory startle response habituation was seen in offspring at 120 ppm on days 28 and 60. Slight decreases in absolute, but not relative, brain weights were found in female pups at 50 and 120 ppm on postnatal day 11 (910%), but the decrease was only 35% by day 65. Brain lengths and widths were similar in treated and control pups. Morphometric measurements did not show any significant differences in the sizes of the neocortex, hippocampus, corpus callosum or cerebellum on day 11 or 65. No histopathological lesions were found in the nervous system. The NOAEL for maternal toxicity was 50 ppm, equal to 4.2 mg/kg bw per day, on the basis of decreased body-weight gain, decreased food consumption and increased reactivity to handling. The NOAEL for toxicity to offspring was 10 ppm, equal to 0.8 mg/kg bw per day, on the basis of reduced pup survival, decreased body weight and body-weight gain during lactation, increased motor activity and decreased motor activity habituation (Myers, 1999).
The results of medical surveillance of workers employed in lindane production have generally not revealed any significant toxic effects. In one report, however, 16/37 workers exposed to lindane in a Hungarian fertilizer plant showed minor changes in neurological state (not described) and in electroencephalograms. Although the sponsor stated that the latter changes fell within the range of 1020% seen in the general Hungarian population, 41% of the workers had changes clearly in excess of that range (Czegledi & Avar, 1970).
Reports of acute toxicity during poisoning incidents with lindane abound in the literature. In general, the incidents were accidental, deliberate (suicide attempts) or due to gross misuse of the product. Poisoning incidents due to medical use of lindane have generally been associated with significant abuse. The commonest symptoms of lindane intoxication after oral ingestion are seizures, convulsions, vomiting and dizziness. Such acute symptoms usually resolved within 24 h when proper medical attention was given. In some instances, however, fatalities have been reported after exposure to lindane orally or by inhalation.
In two epidemiological studies (Saxena et al., 1980; Wassermann et al., 1982), high concentrations of lindane were found in women who went into premature labour. In one study, Saxena et al. (1980) reported that the concentrations in women going into premature labour were approximately three times higher than in the general population.
A casecontrol study was conducted in which the blood concentration of lindane was compared in infertile men and in the general male Israeli population. The concentrations of lindane were considerably higher in sterile men than in the general male population (Pines et al., 1987).
The potential link between exposure to chlorinated compounds and breast cancer was investigated in 7712 women participating in the Copenhagen City Heart Study, 240 of whom developed breast cancer during the subsequent two decades. While a correlation was drawn between breast cancer and exposure to beta-hexachlorocyclohexane and dieldrin, one could not be established for DDT metabolites, lindane or chlordane (Hoyer et al., 1998).
After oral administration, [14C]lindane was rapidly absorbed from the gastrointestinal tract of mice and rats and was extensively distributed throughout the body. In mice, radiolabel was detected in fat, brain, kidney, muscle, liver, adrenals and ovary tissue after administration in the diet. Adipose tissue had the highest concentration of lindane. A similar distribution pattern was observed in rats. The major route of excretion was urine, with a small proportion of an oral dose eliminated in the faeces. The half-life of lindane in rats was estimated to be 35 days, approximately 80% of the administered dose being excreted within 8 days.
Lindane undergoes extensive metabolism in mammals, proceeding through a pathway involving stepwise dehydrogenation, dechlorination and dehydrochlorination, which may be followed by conjugation with sulfate or glucuronide.
Lindane induces a number of metabolizing enzymes, including the cytochrome P450 system, glutathione-S-transferase and UDP-glucuronosyl transferase. In contrast, it inhibits, for example, epoxide hydrolysis at concentrations of 100 ppm and more.
Lindane was moderately acutely toxic when given orally, with LD50 values of 56 to 250 mg/kg bw in mice and 140190 mg/kg bw in rats. The LD50 and LC50 values after dermal and inhalation administration to rats were 1000 mg/kg bw and 0.002 mg/l, respectively. Lindane did not irritate the skin or eye in rabbits and did not sensitize the skin of guinea-pigs. WHO has classified lindane as moderately hazardous (WHO, 2000).
Lindane was toxic to the kidney and liver after administration orally, dermally or by inhalation in short-term and long-term studies of toxicity and studies of reproductive toxicity in rats. The renal toxicity of lindane was specific to male rats and was considered not to be relevant to human risk assessment since it is a consequence of accumulation of alpha2u-globulin, a protein that is not found in humans. Hepatocellular hypertrophy was observed in a number of studies in mice, rats and rabbits and was reversed only partially after recovery periods of up to 6 weeks. In a 2-year study of toxicity and carcinogenicity in rats, the NOAEL was 10 ppm in the diet (equal to 0.47 mg/kg bw per day) on the basis of increased liver weight, hepatocellular hypertrophy, increased spleen weight and deaths at 100 ppm (equal to 4.7 mg/kg bw per day).
Body weights and decrements in body-weight gain were reported in rats and rabbits, but not in mice. Decreased body-weight gain occurred at concentrations of 100 ppm (equal to 4.7 mg/kg bw per day) and higher.
In rats given lindane at a concentration of 400 ppm in the diet (equal to 35 mg/kg bw per day), marginal increases in blood phosphorus and calcium and a 45110% increase in cholesterol concentration, a 2054% increase in urea concentration and a statistically significant increase in platelet count were seen. In general, the haematological changes seen were marginal.
Acute administration by oral, dermal, intraperitoneal or intramuscular routes or by inhalation elicited effects characteristic of toxicity to the central nervous system, namely hypoactivity, dyspnoea, ataxia, convulsions and tremours. In addition, neurotoxic effects were observed after short- or long-term administration, including sensitivity to touch, aggressive behaviour, languor, piloerection, hunched posture, increased motor activity and paralysis of the hind quarters (rabbits only). In a study of acute neurotoxicity in rats, the NOAEL was 6 mg/kg bw on the basis of increased fore-limb grip strength and decreased grooming behaviour. In a 90-day study of neurotoxicity, the NOAEL was 100 ppm (equal to 7.1 mg/kg bw per day) on the basis of hypersensitivity to touch and hunched posture. In a study of developmental neurotoxicity, the NOAEL for maternal toxicity was 50 ppm (equal to 4.2 mg/kg bw per day) on the basis of decreased body weight, decreased food consumption and increased reactivity to handling, while the NOAEL for developmental toxicity was 10 ppm (equal to 0.8 mg/kg bw per day) on the basis of reduced pup survival, decreased body weight and body-weight gain during lactation, increased motor activity and decreased motor reflex.
Lindane did not induce a carcinogenic response in rats or dogs, but increased incidences of adenomas and carcinomas of the liver were observed in agouti and pseudoagouti mice at a dose of 23 mg/kg bw per day in a study of the role of genetic background in the latency and incidence of tumorigenesis. No tumours were observed in black mice in this study nor in any other strain of mice. In another study, a slightly increased incidence of lung adenomas was observed in female mice at the highest dose (21 mg/kg bw per day); however, there was a limited doseresponse relationship and this tumour is common in the strain of mice used, the incidence (27%) only slightly exceeding that in other control groups (19%).
Lindane was not genotoxic in vivo or in vitro. Genotoxicity was found only at cytotoxic concentrations or in the presence of lindane precipitate. The Meeting concluded that lindane is not genotoxic.
In the absence of genotoxicity and on the basis of the weight of the evidence from the studies of carcinogenicity, the Meeting concluded that lindane is not likely to pose a carcinogenic risk to humans. Further, in an epidemiological study designed to assess the potential association between breast cancer and exposure to chlorinated pesticides, no correlation with lindane was found.
In a multigeneration study of reproductive toxicity in rats, the NOAEL for parental toxicity was 150 ppm (equal to 13 mg/kg bw per day), the highest dose tested. The NOAEL for reproductive toxicity was 20 ppm (equal to 1.7 mg/kg bw per day), on the basis of a decreased litter viability index and delays in tooth eruption and hair growth.
Oral administration of lindane to pregnant rats resulted in a NOAEL for maternal toxicity of 5 mg/kg bw per day on the basis of decreased body-weight gain and food consumption. In this study, the NOAEL for developmental toxicity was 5 mg/kg bw per day on the basis of an increased incidence of supernumerary ribs. In a study of developmental toxicity in rabbits, a NOAEL for maternal toxicity was not identified; the LOAEL for maternal toxicity was 5 mg/kg bw per day, on the basis of tachypnoea and lethargy after several days of administration. The NOAEL for developmental toxicity was 10 mg/kg bw per day, on the basis of an increased incidence of fetuses with 13 ribs.
The Meeting reviewed several published studies of the effect of lindane on the endocrine system. Although lindane had anti-estrogenic properties in several studies, effects were reported only at doses of 5 mg/kg bw per day or more.
In view of the report of immunotoxicity in mice, a 39-week study was conducted in which mice were given lindane (purity, 99%) to examine its effects on the total number of leukocytes and on the relative proportion of lymphocyte populations. In females, administration at a dietary concentration of 160 ppm (equal to 24 mg/kg bw per day) resulted in a 55% increase in the natural killer cell population. In the absence of effects on other lymphocyte parameters, the Meeting concluded that lindane is not immunotoxic.
The Meeting concluded that the existing database is adequate to characterize the potential hazard of lindane to fetuses, infants, and children.
The Meeting established an ADI of 00.005 mg/kg bw on the basis of the NOAEL of 10 ppm, equal to 0.47 mg/kg bw per day, in the long-term study of toxicity and carcinogenicity in rats, in which an increased incidence of periacinar hepatocellular hypertrophy, increased liver and spleen weights and increased mortality occurred at higher doses, and a safety factor of 100.
The Meeting established an acute RfD of 0.06 mg/kg bw on the basis of the NOAEL of 6 mg/kg bw in the study of acute neurotoxicity in rats in which clinical signs of toxicity (increased fore-limb grip strength and decreased grooming behaviour) were observed at higher doses, and a safety factor of 100.
The LOAEL of 5 mg/kg bw per day in the study of developmental toxicity in rabbits was not used for establishing the acute RfD because the observed effects (tachypnoea and lethargy) occurred only after several exposures. Similarly, the NOAEL of 10 ppm, equal to 0.8 mg/kg bw per day, in the study of developmental neurotoxicity in rats was not used since the effects (decreased pup survival on postnatal day 4, decreased body-weight gain during lactation and changes in motor activity) could not be attributed to a single exposure.
|
Levels relevant to risk assessment |
||||
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
Long-term studies of toxicity and carcinogenicitya |
Toxicity |
25 ppm, equal to 3.9 mg/kg bw per day |
50 ppm, equal to 7.8 mg/kg bw per day |
|
Carcinogenicity |
50 ppm, equal to 7.8 mg/kg bw per dayb |
|
||
|
Rat |
28-day study of toxicitya |
Toxicity |
10 ppm, equal to 0.98 mg/kg bw per day |
100 ppm, equal to 9.6 mg/kg bw per day |
|
Long-term study of toxicity and carcinogenicitya |
Toxicity |
10 ppm, equal to 0.47 mg/kg bw per day |
100 ppm, equal to 4.7 mg/kg bw per day |
|
|
Carcinogenicity |
400 ppm, equal to 20 mg/kg bw per dayb |
|
||
|
Multigeneration study of reproductive toxicitya |
Parental toxicity |
150 ppm, equal to 13 mg/kg bw per dayb |
|
|
|
Reproductive toxicity |
20 ppm, equal to 1.7 mg/kg bw per day |
150 ppm, equal to 13 mg/kg bw per day |
||
|
Acute neurotoxicityc |
Neurotoxicity |
6 mg/kg bw |
20 mg/kg bw |
|
|
Developmental neurotoxicitya |
Maternal toxicity |
50 ppm, equal to 4.2 mg/kg bw per day |
120 ppm, equal to 8 mg/kg bw per day |
|
|
Developmental toxicity |
10 ppm, equal to 0.8 mg/kg bw per day |
50 ppm, equal to 4.2 mg/kg bw per day |
||
|
Rabbit |
Study of developmental toxicityc |
Maternal toxicity |
|
5 mg/kg bw per dayd |
|
Developmental toxicity |
10 mg/kg bw per day |
20 mg/kg bw per day |
||
|
Dog |
2-year study of toxicitya |
Toxicity |
25 ppm, equal to 0.83 mg/kg bw per day |
50 ppm, equal to 1.6 mg/kg bw per day |
a Dietary administration
b Highest dose tested
c Gavage
d Lowest dose tested
Estimate of acceptable daily intake for humans
00.005 mg/kg bw
Estimate of acute reference dose
0.06 mg/kg bw
Studies that would provide information useful for continued evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
|
Absorption, distribution, excretion and metabolism in mammals |
|
|
Rate and extent of oral absorption |
Rapid and extensive |
|
Distribution |
Extensive; highest concentration in adipose tissue |
|
Potential for accumulation |
Substantial potential for accumulation |
|
Rate and extent of excretion |
Slow (half-life of 35 days) |
|
Metabolism in animals |
Extensive; primarily excreted as sulfate and glucuronide conjugates |
|
Toxicologically significant compounds |
Lindane |
|
Acute toxicity |
|
|
Mouse, LD50, oral |
56250 mg/kg bw (varied with strain) |
|
Rat, LD50, oral |
140 mg/kg bw |
|
Rat, LD50, dermal |
1000 mg/kg bw |
|
Rat, LC50, inhalation |
0.002 mg/l, 4-h exposure (nose-only) |
|
Rabbit, dermal irritation |
Not irritating |
|
Rabbit, eye irritation |
Not irritating |
|
Guinea-pig, skin sensitization |
Not sensitizing (Magnuson & Klingman test) |
|
Short-term studies of toxicity |
|
|
Target/critical effect |
Periacinar hypertrophy, increased platelet count and decreased body-weight gain |
|
Lowest relevant oral NOAEL |
100 ppm, equal to 9.6 mg/kg bw per day |
|
Genotoxicity |
Not genotoxic |
|
Long-term studies of toxicity and carcinogenicity |
|
|
Target/critical effect |
Deaths, increased liver weight associated with hepatocellular hypertrophy, increased spleen weight and increased platelet count in rats |
|
Lowest relevant NOAEL |
10 ppm, equal to 0.47 mg/kg bw per day (rats) |
|
Carcinogenicity |
Unlikely to pose a carcinogenic risk to humans |
|
Reproductive toxicity |
|
|
Target/critical effect in reproductive toxicity |
Decreased litter viability index, decreased pup weight, delays in tooth eruption and hair growth |
|
Lowest relevant NOAEL for reproductive toxicity |
20 ppm, equal 1.7 mg/kg bw per day |
|
Parental target/critical effect |
None |
|
Lowest relevant parental NOAEL |
150 ppm, equal to 13 mg/kg bw per day (highest dose tested; rats). |
|
Target/critical effect in developmental toxicity |
Supernumerary ribs |
|
Lowest relevant NOAEL for developmental toxicity |
10 mg/kg bw per day (rabbits) |
|
Neurotoxicity |
|
|
Acute neurotoxicity |
NOAEL: 6 mg/kg bw; behavioural effects (rats) |
|
90-day neurotoxicity |
NOAEL: 100 ppm, equal to 7.1 mg/kg bw per day (hypersensitivity to touch and hunched posture; no neuropathology; rats) |
|
Developmental neurotoxicity |
Offspring NOAEL: 10 ppm, equal to 0.8 mg/kg bw per day (decreased pup survival, decreased body weight and body-weight gain during lactation, increased motor activity, decreased motor reflex; rats) |
|
Other studies |
|
|
Immunotoxicity |
No concern |
|
Medical data |
An epidemiological study indicated no correlation between exposure to lindane and breast cancer. |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
00.005 mg/kg bw |
2-year study of toxicity and carcinogenicity (rats) |
100 |
|
Acute RfD |
0.06 mg/kg bw |
Rats, acute neurotoxicity |
100 |
Ahdaya, S.M., Monroe, R.J. & Guthrie, F.E. (1981) Absorption and distribution of intubated insecticides in fasted mice. Pestic. Biochem. Physiol., 16, 3846.
Albertini, S., Friederich, U. & Würgler, F.E. (1988) Induction of mitotic chromosome loss in the diploid yeast Saccharomyces cerevisiae D61.M by genotoxic carcinogens and tumor promoters. Environ. Mol. Mutag., 11, 497508.
Amyes, S.J. (1990) Lindane: Combined oncogenicity and toxicity study by dietary administration to Wistar rats for 104 weeks. Unpublished report No. 90/CIL002/0839 from Life Science Research Ltd, Suffolk, England. Submitted to WHO by CIEL, Brussels, Belgium.
Angerer, J., Maass, R. & Heinrich, R. (1983) Occupational exposure to hexachlorocyclohexane. VI. Metabolism of gamma-hexachlorocyclohexane in man. Int. Arch. Occup. Environ. Health, 52, 5967.
Artigas, F., Martínez, E. & Gelp, R.E. (1988) Organochlorine pesticide by negative ion chemical ionization. Brain metabolites of lindane. Biomed. Environ. Mass Spectrom., 16, 279284.
Baliková, M., Kohlicek, J. & Rybka, K. (1989) Chlorinated phenols as metabolites of lindane. Evaluation of the degree of conjugation in rat urine. J. Anal. Toxicol., 13, 2730.
Bosch, A.L. (1987) Dermal absorption of 14C-lindane in male rats. Unpublished report No. 6188-103 from Hazleton Laboratories America, Inc., Madison, Wisconsin, USA. Submitted to WHO by CIEL, Brussels, Belgium.
Brown, D. (1988) Lindane: 13 week dermal toxicity study (with interim kill and recovery period) in the rabbit. Unpublished report No. 6164-580/6 from Hazelton UK, North Yokshire, England. Submitted to WHO by CIEL, Brussels, Belgium
Chadwick, R.W. & Copeland, M.F. (1987) Saturation of lindane metabolism in chronically treated (YS x VY) F1 hybrid mice. J. Toxicol. Environ. Health, 20, 411434.
Chadwick, R.W. & Freal, J.J. (1972) Comparative acceleration of lindane metabolism to chlorophenols by pretreatment of rats with lindane or with DTT and lindane. Food Cosmet. Toxicol., 10, 789795.
Chadwick, R.W., Cranmer, M.F. & Peoples, A.J. (1971) Comparative stimulation of gammaHCH metabolism by pretreatment of rats with gammaHCH, DDT, and DDT + gammaHCH. Toxicol. Appl. Pharmacol., 18, 685695.
Chadwick, R.W., Chuang, L.T. & Williams, K. (1975) Dehydrogenation: A previously unreported pathway of lindane metabolism in mammals. Pestic. Biochem. Physiol., 5, 575586.
Chadwick, R.W., Chadwick, C.J., Freal, J.J. & Bryden, C.C. (1977) Comparative enzyme induction and lindane metabolism in rats pre-treated with various organochlorine pesticides. Xenobiotica, 7, 235246.
Chadwick, R.W., Freal, J.J., Sovocool, J.W., Bryden, C.C. & Copeland, M.F. (1978) The identification of three previously unreported lindane metabolites from mammals. Chemosphere, 8, 633640.
Chadwick, R.W., Copeland, M.F., Mole, M.L., Nesnow, S. & Cooke, N. (1981) Comparative effect of pre-treatment with phenobarbital, Aroclor 1254, and beta-naphthoflavone on the metabolism of lindane. Pestic. Biochem. Physiol., 15, 120136.
Chadwick, R.W., Cooper, R.L., Chang, J., Rehnberg, G.L. & McElroy, W.K. ( 1988) Possible antiestrogenic activity of lindane in female rats. J. Biochem. Toxicol., 3, 147158.
Chase, K. (2000) Lindane, carcinogenicity study by dietary administration to CD-1 mice for 78 weeks. Unpublished report No. 00 3512 from Huntingdon Life Sciences Ltd, Huntingdon, England.
Cifone, M.A. & McKeon, M. (1990) Mutagenicity test on lindane (technical) in the in vitro rat primary hepatocyte unscheduled DNA synthesis assay. Unpublished report No. 12024-0-447 from Hazleton Laboratories America, Inc., Kensington, Maryland, USA. Submitted to WHO by CIEL, Brussels, Belgium
Cooper, R.L., Chadwick, R.W., Rehnberg, G.L., Goldman, J.M., Booth, K.C., Hein, J.F. & McElroy, W.M. (1989) Effects of lindane on hormonal control of reproductive function in the female rat. Toxicol. Appl. Pharmacol., 99, 384394.
Copeland, M.F. (1985) Some factors affecting metabolism of lindane in the rat. Dissert. Abstracts Int., 45, 3170.
Copeland, M.F. & Chadwick, R.W. (1979) Bioisomerization of lindane in rats. J. Environ. Pathol. Toxicol., 2, 737749.
Czeglédi, J.G. & Avar, P. (1970) Occupational exposure to lindane: Clinical and laboratory findings. Br. J. Ind. Med., 27, 283286.
Davey, R.J. & Gerrits, R.J. (1969) Lindane residues in tissues and excreta of swine.
Davey, R.J. & Johnson, L.A. (1974) Tissue residues, blood chemistry and physiological response of lindane-treated swine. J. Anim. Sci., 38, 318324.
Dietrich, D.R. & Swenberg, J.A. (1989) Immunohistochemical localization of alpha2u-globulin in kidneys of rats treated with lindane. Unpublished report. Submitted to WHO by CIEL, Brussels, Belgium.
Dikshith, T.S.S. & Datta, K.K. (1972) Effect of intratesticular injection of lindane and endrin on the testes of rats. Acta Pharmacol. Toxicol., 31, 110.
Dikshith, T.S.S., Tandon, S.K., Datta, K.K., Gupta, P.K. & Behari, J.R. (1978) Comparative response of male rats to parathion and lindane: Histopathological and biochemical studies. Environ. Res., 17, 19.
Earl, F.L., Miller, E. & Van Loon, E.J. (1973) Reproductive, teratogenic, and neonatal effects of some pesticides and related compounds in beagle dogs and miniature swine. In: Deichmann, W.B., ed., Pesticides and Environment: A Continuing Controversy, New York: Intercontinental Medical Book Corp.
Eichler, D., Heupt, W. & Paul, W. (1983) Comparative study on the distribution of alpha and gamma-hexachlorocyclohexane in the rat with particular reference to the problem of isomerization. Xenobiotica, 13, 639647.
Ely, R.E., Moore, L.A., Mann, H.D. & Carter, R.H. (1952) The effect of various dosage levels of crystalline lindane on the concentration of lindane in cows milk. J. Dairy Sci., 35, 733737.
Engst, R., Macholz, R.M., Kujawa, M., Lewerenz, H. & Plass, R. (1976) The metabolism of lindane and its metabolites gamma-2,3,4,5,6-pentachlorocyclohexene, pentachlorobenzene, and pentachlorophenol in rats and the pathways of lindane metabolism. J. Environ. Sci. Health, B11, 95117.
Engst, R., Macholz, R.M. & Kujawa, M. (1978) Proof of metabolites of hexachlorocyclohexane in the human urine. (in German).
Fitzloff, J.F., Portig, J. & Stein, K. (1982) Lindane metabolism by human and rat liver microsomes. Xenobiotica, 12, 197202.
Frohberg, H. & Bauer, A. (1972a) Testing for mutagenic effects/dominant lethal test in male mice. Unpublished report No. 111AA-457-001 from E. Merck, Darmstadt, Germany. Submitted to WHO by CIEL, Brussels, Belgium.
Frohberg, H. & Bauer, A. (1972b) Lindane testing for teratogenic effects in mice following oral administration. Unpublished report No. 4/107/72 from E. Merck, Darmstadt, Germany. Submitted to WHO by CIEL, Brussels, Belgium.
Frohberg, H., von Eberstein, M., Engemann, I. & Weisse, G. (1972) Lindane testing for acute toxicity in rats following oral administration and intraperitoneal injection. Unpublished report No. 4/172/72 from E. Merck Co. Inc., Darmstadt, germany. Submitted to WHO by CIEL, Brussels, belgium.
Glatt, H.R. (1984) Mammalian cell (V79) mutagenicity tests on lindane. Unpublished report No. SP 540-VT21 from University of Mainz, Mainz, Germany. Submitted to WHO by CIEL, Brussels, Belgium.
Gopalaswamy, U.V. & Aiyar, A.S. (1986) Biotransformation and toxicity of lindane and its metabolite hexachlorobenzene in mammals. In: (IARC Scientific Publications No. 77), Lyon: IARCPress, pp. 267-276
Guenard, J. (1984a) In vitro sister chromatid exchange assay in CF1-mouse bone marrow cells with lindane (oral route). Unpublished report No. 025705 from Research & Consulting Company AG, Itingen, Switzerland. Submitted to WHO by CIEL, Brussels, Belgium.
Guenard, J. (1984b) In vitro sister chromatid exchange assay in CF1-mouse bone marrow cells with lindane (intraperitoneal injection). Unpublished report No. 025716 from Research & Consulting Company AG, Itingen, Switzerland. Submitted to WHO by CIEL, Brussels, Belgium.
Harrison, D.L. & Mol, J.C.M. (1968) Transfer of DDT and lindane from ewe to lamb. NZ Weed Pest. Contr. Conf., 21, 233239.
Hertel, R., Kürdel, W., Hochrainer, D. & Mohr, U. (1983) Lindane90 day inhalation study. Unpublished report No. 104264 from Frauenhofer Institut für Toxikologie und Aerosolforschung, Schmallenberg, Germany. Submitted to WHO by CIEL, Brussels, Belgium.
Higgins, C. (1989) Lindane: Preliminary study to assess effects on reproductive performance in rats. Unpublished report No. 90/CIL003/0515 from Life Science Research Ltd, Suffolk, England. Submitted to WHO by CIEL, Brussels, Belgium.
Hoyer, A., Grandjean, P., Jorgensen, T., Brock, J. & Hartvig, H. (1998) Organochlorine exposure and risk of breast cancer. Lancet, 352,18161820.
Hughes, E.W. (1999a) Neurotoxicity study by a single oral gavage administration to CD rats followed by a 14-day observation period. Unpublished report No. CIL/011 from Huntingdon Life Sciences Ltd, Huntingdon, England.
Hughes, E.W. (1999) 13-week neurotoxicity study in rats by dietary administration. Unpublished report No. CIL/012from Huntingdon Life Sciences Ltd, Huntingdon, England.
Ito, N., Nagasaki, H., Arai, M., Sugihara, S. & Makiura, S. (1973a) Histologic and ultrastructural studies on the hepatocarcinogenicity of benzene hexachloride in mice. J. Natl Cancer Inst., 51, 817826.
Ito, N., Nagasaki, H., Arai, M., Makiura, S., Sugihara, S. & Hirao, K. (1973b) Histopathologic studies on liver tumorigenesis induced in mice by technical polychlorinated biphenyls and its promoting effect on liver tumors induced by benzene hexachloride. J. Natl Cancer Inst., 51, 16371641.
Jones, R.P. (1988) Lindane: 42 day oral (dietary administration) dose range finding study in the rat. Unpublished report No.5203-460/9(R) from Hazleton Laboratories Europe Ltd, Harrogate, England. Submitted to WHO by CIEL, Brussels, Belgium.
Karapally, J.C., Saha, J.G. & Lee, W.Y. (1973) Metabolism of lindane-14C in the rabbit: Ether-soluble urinary metabolites. J. Agric. Food Chem., 21, 811818
Khanna, R.N., Gupta, R., Gupta, G.S.D. & Anand, M. (1995) Toxicokinetics and tissue distribution of HCH in rats fed on protein deficient diet. Toxicol. Environ. Chem., 48, 135143.
King, V.C. (1991) Lindane: Reproductive performance study in rats treated continuously through two successive generations. Unpublished report No. 91/CIL004/0948 from Life Science Research Ltd, Suffolk, England. Submitted to WHO by CIEL, Brussels, Belgium.
Klonne, D.R. & Kintigh, W.J. (1988) Lindane technical fourteen-week dust aerosol inhalation study on mice. Unpublished report No. 51-524 from Bushy Run Research Center, Export, Pennsylvania, USA. Submitted to WHO by CIEL, Brussels, Belgium.
Köllmer, H. (1975) Testing of the substance lindane for cancerogenic effects in mice using oral administration. Unpublished report from C.H. Boehringer Sohn, Ingelheim am Rhein, Germany. Submitted to WHO by CIEL, Brussels, Belgium.
Kurihara, N., Tanaka, K. & Nakajima, M. (1979a) Anaerobic metabolism of lindane and related compounds by liver microsomes. Adv. Pestic. Sci., 3, 557561.
Kurihara, N., Tanaka, K. & Nakajima, M. (1979b) Mercapturic acid formation from lindane in rats. Pestic. Biochem. Physiol., 10, 137150.
Lahiri, P., Chakravarty, J., Mondal, A. & Sircar, S. (1985) Effect of lindane on cytology and cytochemistry of exfoliated vaginal cells. Exp. Clin. Endocrinol., 85, 303308.
Lahiri, P., Chakravarty, J. & Sircar, S. (1990) Residue accumulation in mice chronically fed lindane (gamma-HCH). Proc. Indian Natl Sci. Acad., B56, 277280.
Liu, P.T. & Morgan, D.P. (1986) Comparative toxicity and biotransformation of lindane in C57BL/6 and DBA/2 mice. Life Sci., 39, 12371244.
Munir, K.M., Nair, J. & Bhide, S.V. (1984) Comparative formation of chlorophenol metabolites from hexachlorocyclohexane in mouse and rat in vivo and in vitro. Carcinogenesis, 5, 15191521.
Murli, H. (1990) Mutagenicity test on lindane (technical) in an in vitro cytogenetic assay measuring chromosomal aberration frequencies in Chinese hamster ovary (CHO) cells with multiple harvests under conditions of metabolic activation. Unpublished report No. 12024-0-437C from Hazleton Laboratories America Inc., Kensington, Maryland, USA. Submitted to WHO by CIEL, Brussels, Belgium.
Myers, D.P. (1999) Lindane: Developmental neurotoxicity study in the Han Wistar rat by dietary administration. Unpublished report No. CIL/022 from Huntingdon Life Sciences Ltd, Suffolk, England.
Noel, P.R.B., Osborne, B.E., Coote, S. & Street, A.E. (1969) Lindane toxicity studies in beagle dogs (initial studies and dietary intake for three months). Unpublished report No. 3059/69/485 from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by CIEL, Brussels, Belgium.
Oesch, F. (1980) Bacterial mutagenicity tests of lindane with mouse liver preparations as metabolizing systems. Unpublished report. University of Mainz, Mainz, Germany. Submitted to WHO by CIEL, Brussels, Belgium
Oesch, F. & Glatt, H.R. (1985) Mammalian cell (V79) mutagenicity test on lindane using anaerobic exposure conditions. Unpublished report No. SP 540-VT21-b from University of Mainz, Mainz, Germany. Submitted to WHO by CIEL, Brussels, Belgium.
Oesch, F., Friedberg, T., Herbst, M., Paul, W., Wilhelm, N. & Bentley, P. (1982) Effects of lindane treatment on drug metabolizing enzymes and liver weight of CF-1 mice in which it evoked hepatomas and in non-susceptible rodents. Chem. Biol. Interactions, 40, 114.
Palmer, A.K. & Lovell, M.R. (1971) Effect of lindane on pregnancy of the rat. Unpublished report No. 4307/71/463 from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by CIEL, Brussels, Belgium.
Palmer, A.K. & Neuff, A.M. (1971) Effect of lindane on pregnancy of the New Zealand white rabbit. Unpublished report No. 4308/71/464 from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by CIEL, Brussels, Belgium.
Paul, W., Knappen, F. & Stötzer, H. (1980) Testing for acute toxicity after oral administration in oily solution to Chbi:NMRI (SPF) mice. Unpublished report from C.H. Boehringer Sohn, Ingelheim am Rhein, Germany. Submitted to WHO by CIEL, Brussels, Belgium.
Perocco, P., Colacci, A., Del Ciello, C. & Grilli, S. (1995) Cytotoxic and cell transforming effects of the insecticide, lindane (gamma-hexachlorocyclohexane) on BALB/c 3T3 cells. Res. Commun. Mol. Pathol. Pharmacol., 89, 329339.
Pines, A., Cucos, S., Pnina, E.-H. & Ron, M. (1987) Some organochlorine insecticide and polychlorinated biphenyl blood residues in infertile males in the general Israeli population of the middle 1980s. Arch. Environ. Contam. Toxicol., 16, 587597.
Raizada, R.B., Saxena, I., Datta, K.K. & Dickshith, T.S.S. (1980) Weak estrogenic activity of lindane in rats. J. Toxicol. Environ. Health, 6, 483492.
Reader, S.C.J. (1998) Lindane preliminary one-generation reproductive study in Swiss mice by oral gavage administration. Unpublished report No. 98-3975 from Huntingdon Life Sciences Ltd, Suffolk, England. Submitted to WHO by CIEL, Brussels, Belgium.
Rivett, K.F., Sortwell, R.J., Spicer, E.J.F., Cheshire, P.J., Street, A.E. & Burrows, I.E. (1971) Lindane toxicity studies in beagle dogs (initial studies in dietary intake for 104 weeks). Unpublished report No. 4187/71/345 from Huntingdon Research Centre, Huntingdon, England. Submitted to WHO by CIEL, Brussels, Belgium.
Röhrborn, G. (1975) Scientific statement on mutagenicity of lindan. Unpublished report from University of Düsseldorf, Düsseldorf, Germany. Submitted to WHO by CIEL, Brussels, Belgium.
Röhrborn, G. (1976) Cytogenetic analysis of bone marrow of Chinese hamsters (Cricetulus griseus) after sub-acute treatment with lindan. Unpublished report from University of Düsseldorf, Düsseldorf, Germany. Submitted to WHO by CIEL, Brussels, Belgium.
Röhrborn, G. (1977a) Mutagenicity of lindane in the Salmonella/microsome test: Additional test with subbacteriostatic doses. Unpublished report from University of Düsseldorf, Düsseldorf, Germany. Submitted to WHO by CIEL, Brussels, Belgium.
Röhrborn, G. (1977b) Dominant lethal test after treatment of male rats with lindane. Unpublished report from University of Düsseldorf, Düsseldorf, Germany. Submitted to WHO by CIEL, Brussels, Belgium.
Saxena, M.C., Siddiqi, M.K.J., Bhargava, A.K., Seth, T.D., Krishnamurti, C.R. & Kutty, D. (1980) Role of chlorinated hydrocarbon pesticides in abortions and premature labour. Toxicology, 17, 323331.
Seidler, H., Macholz, R.M, Härtig, M., Kujawa, M. & Engst, R. (1971) Investigations on the metabolism of some insecticides and fungicides in the rat. 4 memos. Reduction and elimination of 14-C-lindan. Nahrung, 19, 473482 (in German).
Sircar, S. & Lahiri, P. (1989) Lindane (gamma-HCH) causes reproductive failure and fetotoxicity in mice. Toxicology, 59, 171177.
Srinivasan, K. & Radhakrishnamurty, R. (1983) Studies on the distribution of beta- and gamma-isomers of hexachlorocyclohexane in rat tissues. J. Environ. Sci. Health, B18, 401418.
Srinivasan, K., Ramesh, H.P. & Radhakrishnamurty, R. (1988) Changes induced by hexachlorocyclohexane isomers in rat liver and testis. Bull. Environ. Conta. Toxicol., 41, 531539.
Steinberg, M., Robens, J.F. & Williams, C.H. (1977) Bioassay of lindane for possible carcinogenicity. Unpublished report No. (NIH) 77-814 from National Cancer Institute, Bethesda, Maryland, USA. Submitted to WHO by CIEL, Brussels, Belgium.
Sulik, M., Derógowski, K., Kemona, A. & Sulik-Stefalska, E. (1988) Comparative investigations of the radioactivity on rats blood and urine after intravenous, intraperitoneal, and intragastric administration of gamma-benzene hexachloride-14C. Med. Prac., 4, 236240 (in Russian).
Suter, P. (1983) Three month toxicity study in rats with lindane. Unpublished report No. 005220 from Research Consulting Company AG, Itingen, Switzerland. Submitted to WHO by CIEL, Brussels, Belgium.
Ullmann, L. & Mohler, H. (1986) 4-hour acute aerosol inhalation toxicity study with lindane in rats. Unpublished report No. 061637 from Research & Consulting Company AG, Itingen, Switzerland. Submitted to WHO by CIEL, Brussels, Belgium.
Ullmann, L., Sacher, R. & Mohler, H. (1986a) Acute dermal toxicity study with lindane in rats. Unpublished report No. 061648 from Research & Consulting Company AG. Itingen, Switzerland. Submitted to WHO by CIEL, Brussels, Belgium.
Ullmann, L., Sacher, R. & Porricello, T. (1986b) Primary skin irritation study with lindane in rabbits (4-hour occlusive application). Unpublished report No. 061661 from Research & Consulting Company AG. Itingen, Switzerland. Submitted to WHO by CIEL, Brussels, Belgium.
Ullmann, L., Sacher, R. & Porricello, T. (1986c) Primary eye irritation with lindane in rabbits. Unpublished report No. 061672 from Research & Consulting Company AG. Itingen, Switzerland. Submitted to WHO by CIEL, Brussels, Belgium
Ullmann, L., Claire, R. & Bognar, G. (1986d) Test for delayed contact hypersensitivity in the albino guinea pig with lindane. Maximization test. Unpublished report No. 061650 from Research & Consulting Company AG. Itingen, Switzerland. Submitted to WHO by CIEL, Brussels, Belgium
Uphouse, L. (1987) Decreased rodent sexual receptivity after lindane. Toxicol. Lett., 39, 714.
Uphouse, L. & Williams, J. (1989) Diestrous treatment with lindane disrupts the female rat reproductive cycle. Toxicol. Lett., 48, 2128.
Wassermann, M., Ron, M., Bercovici, B., Wassermann, D., Cucos, S. & Pines, A. (1982) Premature delivery and organochlorine compounds: Polychlorinated biphenyls and some organochlorine insecticides. Environ. Res., 28, 106112.
Weisse, I. & Herbst, M. (1977) Carcinogenicity study of lindane in the mouse. Toxicology, 7, 233238.
Welch, R.M., Levin, W., Kuntzman, R., Jacobson, M. & Conney, A.H. (1971) Effect of halogenated hydrocarbons insecticides on the metabolism and uterotropic action of estrogens in rats and mice. Toxicol. Appl. Pharmacol., 19, 234246.
WHO (2000) The WHO recommended classification of pesticides by hazard and guidelines to classification 20002202 (WHO/PCS/01.5). Available from the International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland.
Wilkes et al. (1987) Metabolism study of 14C-lindane fed or topically applied to lactating goats. Unpublished report No. ADC 957 from Analytical Development Corp. Submitted to WHO by CIEL, Brussels, Belgium.
Wing, M.G. (2000) Investigation of the immunotoxic potential of lindane: Peripheral blood lymphocyte analysis on samples from mouse carcinogenicity study. Unpublished report No. CIL 024/994052 from Huntingdon Life Sciences Ltd, Huntingdon, England. Submitted to WHO by CIEL, Brussels, Belgium.
Wolfe, G.W. & Dauvin, E.M. (1980) Acute oral toxicity study in CF®-1 mice. Unpublished report No. 2146-100 from Hazleton Laboratories America, Inc., Vienna, Virginia, USA. Submitted to WHO by CIEL, Brussels, Belgium.
Wolfe, G.W. & Ralph, J.A. (1980) Acute oral toxicity study in B6C3F1 mice. Unpublished report No. 2146-101 from Hazleton Laboratories America, Inc., Vienna, Virginia, USA. Submitted to WHO by CIEL, Brussels, Belgium.
Wolff, G.L., Roberts, D.W., Morrissey, R.L., Greenman, D.L., Allen, R.R., Campbell, W.L., Bergman, H., Nesnow, S. & Firth, C.H. (1987) Tumorigenic responses to lindane in mice: Potentiation by a dominant mutation. Carcinogenesis, 8, 18891897.
Yamamoto, T., Egashirra, T., Yamanaka, Y., Yoshida, T. & Kuroiwa, Y. (1983) Initial metabolism of gamma-hexacholorocyclohexane (gamma-HCH) by rat liver microsomes. J. Pharm. Dyn., 6, 721728.
Zhu, J., Feng, Z. & Chen, J. (1986) Studies on the distribution and fate of [gamma-3H] hexachlorocyclohexane in rats. Pestic. Biochem. Physiol., 25, 414419.
See Also:
Toxicological Abbreviations
Lindane (EHC 124, 1991)
Lindane (HSG 54, 1991)
Lindane (ICSC)
Lindane (PIM 859)
Lindane (FAO Meeting Report PL/1965/10/1)
Lindane (FAO/PL:1967/M/11/1)
Lindane (FAO/PL:1968/M/9/1)
Lindane (FAO/PL:1969/M/17/1)
Lindane (WHO Pesticide Residues Series 3)
Lindane (WHO Pesticide Residues Series 4)
Lindane (WHO Pesticide Residues Series 5)
Lindane (Pesticide residues in food: 1977 evaluations)
Lindane (Pesticide residues in food: 1978 evaluations)
Lindane (Pesticide residues in food: 1979 evaluations)
Lindane (Pesticide residues in food: 1989 evaluations Part II Toxicology)
Lindane (Pesticide residues in food: 1997 evaluations Part II Toxicological & Environmental)