
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
TOXICOLOGICAL EVALUATION OF SOME
FOOD COLOURS, ENZYMES, FLAVOUR
ENHANCERS, THICKENING AGENTS, AND
CERTAIN FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES 6
The evaluations contained in this publication were prepared by the
Joint FAO/WHO Expert Committee on Food Additives which met in Rome,
4-13 June 19741
World Health Organization Geneva 1975
1 Eighteenth Report of the Joint FAO/WHO Expert Committee on
Food Additives, Wld Hlth Org. techn. Rep. Ser., 1974, No. 557.
FAO Nutrition Meetings Report Series, 1974, No. 54.
ALLURA RED AC
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Orally administered dye is rapidly absorbed as evidenced by
coloration of eyes and skin within two hours after intubation of 5 g
into dogs (Anonymous, 1965b). Five rats were fed 5.19% dye in the diet
and the excretion products determined in urine and faeces. About 29%
of the total dye consumed was recovered unchanged from the faeces,
less than 0.1% unchanged dye was found in the urine. Thus 71% appears
to have been metabolized but it was found impossible to identify any
metabolites chemically either in urine or faeces. It is suggested that
azo reduction by gut flora will form principally 2-methoxy-5 methyl-
aniline-4-sulfonic acid (cresidine-4-sulfonic acid)
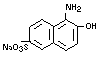
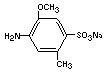 and 1-amino-2-naphthol-6-sulfonic acid
and 1-amino-2-naphthol-6-sulfonic acid
 Some of the cresidine sulfonic acid may also be further acetylated.
All these metabolites are likely to be excreted in the urine and very
little is likely to be excreted in the bile (Anonymous, 1970d).
TOXICOLOGICAL STUDIES
Special studies on reproduction and teratogenicity
Rat
Groups of 10 male and 20 female rats received 0%, 0.37%, 1.39% or
5.19% dye in their diet through two parental (F1A was the P2
generation) and two filial generations. Mating occurred after 27 weeks
on either control or test diet both for the P1 and P2 generation.
The fertility indices were low for the controls and test animals in
the F1A and F1B generation as well as in the low test level F2A and
all test levels of F2B generation. Growth was suppressed slightly for
the lowest test level F1B and high test level F1A + F1B pups as well
as for the high test level F2A and F2B pups. Other indices, litter
size, pup weight at 24 hours, were comparable in each group in each
generation. No consistent pathological changes were noted in the P1,
P2, F1A, F1B and F2B generations. No evidence was seen of
teratogenic or embryotoxic effects regarding implantation sites,
resorption sites, and live fetuses indices. No difference from
controls was noted with regard to appearance, anatomy and structure of
test fetuses (Anonymous, 1969).
Groups of 24, 19, 20, 21 and 16 pregnant rats received
respectively 0, 15, 30, 100 and 200 mg/kg of the dye by gavage daily
during pregnancy days 0-19. Dams were delivered by section on day 20
of pregnancy. Preliminary gross observations indicate no dye-induced
effects in terms of early or late deaths, resorptions per litter,
pre-implantation loss, number of fetuses per litter and average fetus
weight (Anonymous, 1974b).
Rabbit
In three groups of 14 rabbits, Allura Red was given in doses of
0, 200 and 700 mg/kg bw by gavage from day 6 to 18 of pregnancy. There
were no indications of a compound-related effect with regard to
appearance and behaviour, body weight or in gross necropsy findings
for the maternal does. No adverse effects on implantation and litter
data were noted nor any fetal abnormalities (Anonymous, 1974a).
Acute toxicity
LD50
Animal Route (mg/kg bw) Reference
Rat oral (gavage) > 10 000 Anonymous, 1965a
Rabbit dermal > 10 000 Anonymous, 1967
Dog oral (intubation) > 5 000 Anonymous, 1965b
Tests for dermal irritation on intact and abraded rabbit skin
showed no gross irritation at levels of 0.316, 1.0, 3.16 and 10 g/kg
bw but resulted in persistent skin staining (Anonymous, 1967). Daily
application at rates of 0.5 g/kg, five days a week for 15 applications
on abraded and 65 applications on intact skin, of 0.1% and 1%
solutions in water or as a hydrophilic ointment revealed no compound-
related effect on general appearance, body weight, clinical laboratory
studies, gross and microscopic pathology. No dermal irritation was
noted except that produced by the hydrophilic ointment base
(Anonymous, 1968).
Short-term studies
Rat
Groups of 10 male and 10 female rats were fed diets containing 0,
0.37, 0.72, 1.39, 2.69 and 5.19% dye for six weeks. No evidence of
compound-related effects was noted as regards body weight, food
consumption, survival, organ weights, gross and microscopic pathology.
Haematology and urinalysis were normal and no evidence of Heinz body
formation was noted (Anonymous, 1966a).
Dog
Four groups of one male and one female beagle dog received 0,
125, 250 and 500 mg/kg daily in capsule form. No adverse effects were
noted on body weight, food consumption, survival, organ weights, gross
and histopathology, haematology and clinical chemistry. At the highest
level there were slight ill-defined hepatic parenchymal changes in
both dogs (Anonymous, 1966b).
Groups of four male and four female beagle dogs received 0, 0.37,
1.39 and 5.19% in their diet for 104 weeks. All animals remained
normal regarding appearance, behaviour, haematology and clinical
chemistry findings, gross and histopathology. Both faeces and urine
were coloured at all test levels. At 52 weeks the adrenal cortical
cells of the high level groups showed some vacuolation and brown
pigment deposition in the Kupffer cells of females at the two lower
test levels. These changes had disappeared by 104 weeks and special
histological examination of the eyes revealed no adverse changes
(Anonymous, 1970b).
Long-term studies
Mouse
A control group of 50 male and 50 female mice, a positive control
group of 25 male and 25 female mice and a test group of 50 male and 50
female mice were treated with 0.1 ml of either distilled water, 5%
test solution or 10 µg 3,4-benzpyrene in acetone twice weekly for 20
months. The results in the positive control group showed the mouse
strain used to be sensitive to benzpyrene carcinogenesis. Survival,
gross and histopathology of major organs were comparable in the
negative controls and test animals. Histology of the skin revealed
comparable incidence and degrees of severity of acanthosis,
hyperheratosis and dermatitis for the negative control and test groups
(Anonymous, 1970c).
Rat
Groups of 30 male and 30 female rats received in their diet 0,
0.37, 1.39 and 5.19% dye for 92 weeks. Moderate growth depression
occurred at the 5.19% level in both sexes. No other compound-related
effects were noted as regards appearance, behaviour, survival, organ
weights, clinical laboratory studies or gross and histopathology. No
evidence of Heinz body formation was noted apart from a slight
tendency to anaemia (Anonymous, 1970a).
Comments:
There is only very limited information available on the
metabolism of this dye and further metabolic investigations using
radio-labelled materials are planned. The available long-term study in
rats showed no adverse effects except moderate growth depression at
the highest level tested but was considered to be inadequate because
of the poor survival and small number of animals examined terminally.
The studies on reproduction and embryotoxicity including
teratogenicity revealed no significant adverse effects in the two
species investigated. A two-year study in a non-rodent species showed
no compound-related toxic effects. An adequate life span study in the
rat and the results of the planned studies on the metabolism of this
colour are needed.
EVALUATION
Not possible on the data provided.
REFERENCES
Anonymous (1965a) Unpublished Report No. 165-114 dated 7 December 1965
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1965b) Unpublished Report dated 23 November 1965 from
Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1966a) Unpublished Report No. 165-112 dated 18 November
1966 from Hazelton Laboratories Inc., submitted by Allied
Chemical Corporation
Anonymous (1966b) Unpublished Report No. 165-116 from Hazelton
Laboratories Inc., submitted by Allied Chemical Corporation
Anonymous (1967) Unpublished Report No. 165-119 dated 16 October 1967
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1968) Unpublished Report No. 165-120 dated 20 December 1968
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1969) Unpublished Report No. 165-125 dated 29 May 1965 from
Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970a) Unpublished Report No. 165-121 dated 2 March 1970
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970b) Unpublished Report No. 165-122 dated 2 March 1970
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970c) Unpublished Report No. 165-123 dated 2 March 1970
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970d) Unpublished Report No. 21855 dated 11 February 1970
from Buffalo Research Laboratory, submitted by Allied Chemical
Corporation
Anonymous (1974a) Unpublished Report No. 165-142 dated 15 June 1974
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1974b) Unpublished Report submitted by the United States
Food and Drug Administration
Some of the cresidine sulfonic acid may also be further acetylated.
All these metabolites are likely to be excreted in the urine and very
little is likely to be excreted in the bile (Anonymous, 1970d).
TOXICOLOGICAL STUDIES
Special studies on reproduction and teratogenicity
Rat
Groups of 10 male and 20 female rats received 0%, 0.37%, 1.39% or
5.19% dye in their diet through two parental (F1A was the P2
generation) and two filial generations. Mating occurred after 27 weeks
on either control or test diet both for the P1 and P2 generation.
The fertility indices were low for the controls and test animals in
the F1A and F1B generation as well as in the low test level F2A and
all test levels of F2B generation. Growth was suppressed slightly for
the lowest test level F1B and high test level F1A + F1B pups as well
as for the high test level F2A and F2B pups. Other indices, litter
size, pup weight at 24 hours, were comparable in each group in each
generation. No consistent pathological changes were noted in the P1,
P2, F1A, F1B and F2B generations. No evidence was seen of
teratogenic or embryotoxic effects regarding implantation sites,
resorption sites, and live fetuses indices. No difference from
controls was noted with regard to appearance, anatomy and structure of
test fetuses (Anonymous, 1969).
Groups of 24, 19, 20, 21 and 16 pregnant rats received
respectively 0, 15, 30, 100 and 200 mg/kg of the dye by gavage daily
during pregnancy days 0-19. Dams were delivered by section on day 20
of pregnancy. Preliminary gross observations indicate no dye-induced
effects in terms of early or late deaths, resorptions per litter,
pre-implantation loss, number of fetuses per litter and average fetus
weight (Anonymous, 1974b).
Rabbit
In three groups of 14 rabbits, Allura Red was given in doses of
0, 200 and 700 mg/kg bw by gavage from day 6 to 18 of pregnancy. There
were no indications of a compound-related effect with regard to
appearance and behaviour, body weight or in gross necropsy findings
for the maternal does. No adverse effects on implantation and litter
data were noted nor any fetal abnormalities (Anonymous, 1974a).
Acute toxicity
LD50
Animal Route (mg/kg bw) Reference
Rat oral (gavage) > 10 000 Anonymous, 1965a
Rabbit dermal > 10 000 Anonymous, 1967
Dog oral (intubation) > 5 000 Anonymous, 1965b
Tests for dermal irritation on intact and abraded rabbit skin
showed no gross irritation at levels of 0.316, 1.0, 3.16 and 10 g/kg
bw but resulted in persistent skin staining (Anonymous, 1967). Daily
application at rates of 0.5 g/kg, five days a week for 15 applications
on abraded and 65 applications on intact skin, of 0.1% and 1%
solutions in water or as a hydrophilic ointment revealed no compound-
related effect on general appearance, body weight, clinical laboratory
studies, gross and microscopic pathology. No dermal irritation was
noted except that produced by the hydrophilic ointment base
(Anonymous, 1968).
Short-term studies
Rat
Groups of 10 male and 10 female rats were fed diets containing 0,
0.37, 0.72, 1.39, 2.69 and 5.19% dye for six weeks. No evidence of
compound-related effects was noted as regards body weight, food
consumption, survival, organ weights, gross and microscopic pathology.
Haematology and urinalysis were normal and no evidence of Heinz body
formation was noted (Anonymous, 1966a).
Dog
Four groups of one male and one female beagle dog received 0,
125, 250 and 500 mg/kg daily in capsule form. No adverse effects were
noted on body weight, food consumption, survival, organ weights, gross
and histopathology, haematology and clinical chemistry. At the highest
level there were slight ill-defined hepatic parenchymal changes in
both dogs (Anonymous, 1966b).
Groups of four male and four female beagle dogs received 0, 0.37,
1.39 and 5.19% in their diet for 104 weeks. All animals remained
normal regarding appearance, behaviour, haematology and clinical
chemistry findings, gross and histopathology. Both faeces and urine
were coloured at all test levels. At 52 weeks the adrenal cortical
cells of the high level groups showed some vacuolation and brown
pigment deposition in the Kupffer cells of females at the two lower
test levels. These changes had disappeared by 104 weeks and special
histological examination of the eyes revealed no adverse changes
(Anonymous, 1970b).
Long-term studies
Mouse
A control group of 50 male and 50 female mice, a positive control
group of 25 male and 25 female mice and a test group of 50 male and 50
female mice were treated with 0.1 ml of either distilled water, 5%
test solution or 10 µg 3,4-benzpyrene in acetone twice weekly for 20
months. The results in the positive control group showed the mouse
strain used to be sensitive to benzpyrene carcinogenesis. Survival,
gross and histopathology of major organs were comparable in the
negative controls and test animals. Histology of the skin revealed
comparable incidence and degrees of severity of acanthosis,
hyperheratosis and dermatitis for the negative control and test groups
(Anonymous, 1970c).
Rat
Groups of 30 male and 30 female rats received in their diet 0,
0.37, 1.39 and 5.19% dye for 92 weeks. Moderate growth depression
occurred at the 5.19% level in both sexes. No other compound-related
effects were noted as regards appearance, behaviour, survival, organ
weights, clinical laboratory studies or gross and histopathology. No
evidence of Heinz body formation was noted apart from a slight
tendency to anaemia (Anonymous, 1970a).
Comments:
There is only very limited information available on the
metabolism of this dye and further metabolic investigations using
radio-labelled materials are planned. The available long-term study in
rats showed no adverse effects except moderate growth depression at
the highest level tested but was considered to be inadequate because
of the poor survival and small number of animals examined terminally.
The studies on reproduction and embryotoxicity including
teratogenicity revealed no significant adverse effects in the two
species investigated. A two-year study in a non-rodent species showed
no compound-related toxic effects. An adequate life span study in the
rat and the results of the planned studies on the metabolism of this
colour are needed.
EVALUATION
Not possible on the data provided.
REFERENCES
Anonymous (1965a) Unpublished Report No. 165-114 dated 7 December 1965
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1965b) Unpublished Report dated 23 November 1965 from
Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1966a) Unpublished Report No. 165-112 dated 18 November
1966 from Hazelton Laboratories Inc., submitted by Allied
Chemical Corporation
Anonymous (1966b) Unpublished Report No. 165-116 from Hazelton
Laboratories Inc., submitted by Allied Chemical Corporation
Anonymous (1967) Unpublished Report No. 165-119 dated 16 October 1967
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1968) Unpublished Report No. 165-120 dated 20 December 1968
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1969) Unpublished Report No. 165-125 dated 29 May 1965 from
Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970a) Unpublished Report No. 165-121 dated 2 March 1970
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970b) Unpublished Report No. 165-122 dated 2 March 1970
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970c) Unpublished Report No. 165-123 dated 2 March 1970
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970d) Unpublished Report No. 21855 dated 11 February 1970
from Buffalo Research Laboratory, submitted by Allied Chemical
Corporation
Anonymous (1974a) Unpublished Report No. 165-142 dated 15 June 1974
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1974b) Unpublished Report submitted by the United States
Food and Drug Administration
 and 1-amino-2-naphthol-6-sulfonic acid
and 1-amino-2-naphthol-6-sulfonic acid
 Some of the cresidine sulfonic acid may also be further acetylated.
All these metabolites are likely to be excreted in the urine and very
little is likely to be excreted in the bile (Anonymous, 1970d).
TOXICOLOGICAL STUDIES
Special studies on reproduction and teratogenicity
Rat
Groups of 10 male and 20 female rats received 0%, 0.37%, 1.39% or
5.19% dye in their diet through two parental (F1A was the P2
generation) and two filial generations. Mating occurred after 27 weeks
on either control or test diet both for the P1 and P2 generation.
The fertility indices were low for the controls and test animals in
the F1A and F1B generation as well as in the low test level F2A and
all test levels of F2B generation. Growth was suppressed slightly for
the lowest test level F1B and high test level F1A + F1B pups as well
as for the high test level F2A and F2B pups. Other indices, litter
size, pup weight at 24 hours, were comparable in each group in each
generation. No consistent pathological changes were noted in the P1,
P2, F1A, F1B and F2B generations. No evidence was seen of
teratogenic or embryotoxic effects regarding implantation sites,
resorption sites, and live fetuses indices. No difference from
controls was noted with regard to appearance, anatomy and structure of
test fetuses (Anonymous, 1969).
Groups of 24, 19, 20, 21 and 16 pregnant rats received
respectively 0, 15, 30, 100 and 200 mg/kg of the dye by gavage daily
during pregnancy days 0-19. Dams were delivered by section on day 20
of pregnancy. Preliminary gross observations indicate no dye-induced
effects in terms of early or late deaths, resorptions per litter,
pre-implantation loss, number of fetuses per litter and average fetus
weight (Anonymous, 1974b).
Rabbit
In three groups of 14 rabbits, Allura Red was given in doses of
0, 200 and 700 mg/kg bw by gavage from day 6 to 18 of pregnancy. There
were no indications of a compound-related effect with regard to
appearance and behaviour, body weight or in gross necropsy findings
for the maternal does. No adverse effects on implantation and litter
data were noted nor any fetal abnormalities (Anonymous, 1974a).
Acute toxicity
LD50
Animal Route (mg/kg bw) Reference
Rat oral (gavage) > 10 000 Anonymous, 1965a
Rabbit dermal > 10 000 Anonymous, 1967
Dog oral (intubation) > 5 000 Anonymous, 1965b
Tests for dermal irritation on intact and abraded rabbit skin
showed no gross irritation at levels of 0.316, 1.0, 3.16 and 10 g/kg
bw but resulted in persistent skin staining (Anonymous, 1967). Daily
application at rates of 0.5 g/kg, five days a week for 15 applications
on abraded and 65 applications on intact skin, of 0.1% and 1%
solutions in water or as a hydrophilic ointment revealed no compound-
related effect on general appearance, body weight, clinical laboratory
studies, gross and microscopic pathology. No dermal irritation was
noted except that produced by the hydrophilic ointment base
(Anonymous, 1968).
Short-term studies
Rat
Groups of 10 male and 10 female rats were fed diets containing 0,
0.37, 0.72, 1.39, 2.69 and 5.19% dye for six weeks. No evidence of
compound-related effects was noted as regards body weight, food
consumption, survival, organ weights, gross and microscopic pathology.
Haematology and urinalysis were normal and no evidence of Heinz body
formation was noted (Anonymous, 1966a).
Dog
Four groups of one male and one female beagle dog received 0,
125, 250 and 500 mg/kg daily in capsule form. No adverse effects were
noted on body weight, food consumption, survival, organ weights, gross
and histopathology, haematology and clinical chemistry. At the highest
level there were slight ill-defined hepatic parenchymal changes in
both dogs (Anonymous, 1966b).
Groups of four male and four female beagle dogs received 0, 0.37,
1.39 and 5.19% in their diet for 104 weeks. All animals remained
normal regarding appearance, behaviour, haematology and clinical
chemistry findings, gross and histopathology. Both faeces and urine
were coloured at all test levels. At 52 weeks the adrenal cortical
cells of the high level groups showed some vacuolation and brown
pigment deposition in the Kupffer cells of females at the two lower
test levels. These changes had disappeared by 104 weeks and special
histological examination of the eyes revealed no adverse changes
(Anonymous, 1970b).
Long-term studies
Mouse
A control group of 50 male and 50 female mice, a positive control
group of 25 male and 25 female mice and a test group of 50 male and 50
female mice were treated with 0.1 ml of either distilled water, 5%
test solution or 10 µg 3,4-benzpyrene in acetone twice weekly for 20
months. The results in the positive control group showed the mouse
strain used to be sensitive to benzpyrene carcinogenesis. Survival,
gross and histopathology of major organs were comparable in the
negative controls and test animals. Histology of the skin revealed
comparable incidence and degrees of severity of acanthosis,
hyperheratosis and dermatitis for the negative control and test groups
(Anonymous, 1970c).
Rat
Groups of 30 male and 30 female rats received in their diet 0,
0.37, 1.39 and 5.19% dye for 92 weeks. Moderate growth depression
occurred at the 5.19% level in both sexes. No other compound-related
effects were noted as regards appearance, behaviour, survival, organ
weights, clinical laboratory studies or gross and histopathology. No
evidence of Heinz body formation was noted apart from a slight
tendency to anaemia (Anonymous, 1970a).
Comments:
There is only very limited information available on the
metabolism of this dye and further metabolic investigations using
radio-labelled materials are planned. The available long-term study in
rats showed no adverse effects except moderate growth depression at
the highest level tested but was considered to be inadequate because
of the poor survival and small number of animals examined terminally.
The studies on reproduction and embryotoxicity including
teratogenicity revealed no significant adverse effects in the two
species investigated. A two-year study in a non-rodent species showed
no compound-related toxic effects. An adequate life span study in the
rat and the results of the planned studies on the metabolism of this
colour are needed.
EVALUATION
Not possible on the data provided.
REFERENCES
Anonymous (1965a) Unpublished Report No. 165-114 dated 7 December 1965
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1965b) Unpublished Report dated 23 November 1965 from
Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1966a) Unpublished Report No. 165-112 dated 18 November
1966 from Hazelton Laboratories Inc., submitted by Allied
Chemical Corporation
Anonymous (1966b) Unpublished Report No. 165-116 from Hazelton
Laboratories Inc., submitted by Allied Chemical Corporation
Anonymous (1967) Unpublished Report No. 165-119 dated 16 October 1967
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1968) Unpublished Report No. 165-120 dated 20 December 1968
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1969) Unpublished Report No. 165-125 dated 29 May 1965 from
Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970a) Unpublished Report No. 165-121 dated 2 March 1970
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970b) Unpublished Report No. 165-122 dated 2 March 1970
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970c) Unpublished Report No. 165-123 dated 2 March 1970
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970d) Unpublished Report No. 21855 dated 11 February 1970
from Buffalo Research Laboratory, submitted by Allied Chemical
Corporation
Anonymous (1974a) Unpublished Report No. 165-142 dated 15 June 1974
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1974b) Unpublished Report submitted by the United States
Food and Drug Administration
Some of the cresidine sulfonic acid may also be further acetylated.
All these metabolites are likely to be excreted in the urine and very
little is likely to be excreted in the bile (Anonymous, 1970d).
TOXICOLOGICAL STUDIES
Special studies on reproduction and teratogenicity
Rat
Groups of 10 male and 20 female rats received 0%, 0.37%, 1.39% or
5.19% dye in their diet through two parental (F1A was the P2
generation) and two filial generations. Mating occurred after 27 weeks
on either control or test diet both for the P1 and P2 generation.
The fertility indices were low for the controls and test animals in
the F1A and F1B generation as well as in the low test level F2A and
all test levels of F2B generation. Growth was suppressed slightly for
the lowest test level F1B and high test level F1A + F1B pups as well
as for the high test level F2A and F2B pups. Other indices, litter
size, pup weight at 24 hours, were comparable in each group in each
generation. No consistent pathological changes were noted in the P1,
P2, F1A, F1B and F2B generations. No evidence was seen of
teratogenic or embryotoxic effects regarding implantation sites,
resorption sites, and live fetuses indices. No difference from
controls was noted with regard to appearance, anatomy and structure of
test fetuses (Anonymous, 1969).
Groups of 24, 19, 20, 21 and 16 pregnant rats received
respectively 0, 15, 30, 100 and 200 mg/kg of the dye by gavage daily
during pregnancy days 0-19. Dams were delivered by section on day 20
of pregnancy. Preliminary gross observations indicate no dye-induced
effects in terms of early or late deaths, resorptions per litter,
pre-implantation loss, number of fetuses per litter and average fetus
weight (Anonymous, 1974b).
Rabbit
In three groups of 14 rabbits, Allura Red was given in doses of
0, 200 and 700 mg/kg bw by gavage from day 6 to 18 of pregnancy. There
were no indications of a compound-related effect with regard to
appearance and behaviour, body weight or in gross necropsy findings
for the maternal does. No adverse effects on implantation and litter
data were noted nor any fetal abnormalities (Anonymous, 1974a).
Acute toxicity
LD50
Animal Route (mg/kg bw) Reference
Rat oral (gavage) > 10 000 Anonymous, 1965a
Rabbit dermal > 10 000 Anonymous, 1967
Dog oral (intubation) > 5 000 Anonymous, 1965b
Tests for dermal irritation on intact and abraded rabbit skin
showed no gross irritation at levels of 0.316, 1.0, 3.16 and 10 g/kg
bw but resulted in persistent skin staining (Anonymous, 1967). Daily
application at rates of 0.5 g/kg, five days a week for 15 applications
on abraded and 65 applications on intact skin, of 0.1% and 1%
solutions in water or as a hydrophilic ointment revealed no compound-
related effect on general appearance, body weight, clinical laboratory
studies, gross and microscopic pathology. No dermal irritation was
noted except that produced by the hydrophilic ointment base
(Anonymous, 1968).
Short-term studies
Rat
Groups of 10 male and 10 female rats were fed diets containing 0,
0.37, 0.72, 1.39, 2.69 and 5.19% dye for six weeks. No evidence of
compound-related effects was noted as regards body weight, food
consumption, survival, organ weights, gross and microscopic pathology.
Haematology and urinalysis were normal and no evidence of Heinz body
formation was noted (Anonymous, 1966a).
Dog
Four groups of one male and one female beagle dog received 0,
125, 250 and 500 mg/kg daily in capsule form. No adverse effects were
noted on body weight, food consumption, survival, organ weights, gross
and histopathology, haematology and clinical chemistry. At the highest
level there were slight ill-defined hepatic parenchymal changes in
both dogs (Anonymous, 1966b).
Groups of four male and four female beagle dogs received 0, 0.37,
1.39 and 5.19% in their diet for 104 weeks. All animals remained
normal regarding appearance, behaviour, haematology and clinical
chemistry findings, gross and histopathology. Both faeces and urine
were coloured at all test levels. At 52 weeks the adrenal cortical
cells of the high level groups showed some vacuolation and brown
pigment deposition in the Kupffer cells of females at the two lower
test levels. These changes had disappeared by 104 weeks and special
histological examination of the eyes revealed no adverse changes
(Anonymous, 1970b).
Long-term studies
Mouse
A control group of 50 male and 50 female mice, a positive control
group of 25 male and 25 female mice and a test group of 50 male and 50
female mice were treated with 0.1 ml of either distilled water, 5%
test solution or 10 µg 3,4-benzpyrene in acetone twice weekly for 20
months. The results in the positive control group showed the mouse
strain used to be sensitive to benzpyrene carcinogenesis. Survival,
gross and histopathology of major organs were comparable in the
negative controls and test animals. Histology of the skin revealed
comparable incidence and degrees of severity of acanthosis,
hyperheratosis and dermatitis for the negative control and test groups
(Anonymous, 1970c).
Rat
Groups of 30 male and 30 female rats received in their diet 0,
0.37, 1.39 and 5.19% dye for 92 weeks. Moderate growth depression
occurred at the 5.19% level in both sexes. No other compound-related
effects were noted as regards appearance, behaviour, survival, organ
weights, clinical laboratory studies or gross and histopathology. No
evidence of Heinz body formation was noted apart from a slight
tendency to anaemia (Anonymous, 1970a).
Comments:
There is only very limited information available on the
metabolism of this dye and further metabolic investigations using
radio-labelled materials are planned. The available long-term study in
rats showed no adverse effects except moderate growth depression at
the highest level tested but was considered to be inadequate because
of the poor survival and small number of animals examined terminally.
The studies on reproduction and embryotoxicity including
teratogenicity revealed no significant adverse effects in the two
species investigated. A two-year study in a non-rodent species showed
no compound-related toxic effects. An adequate life span study in the
rat and the results of the planned studies on the metabolism of this
colour are needed.
EVALUATION
Not possible on the data provided.
REFERENCES
Anonymous (1965a) Unpublished Report No. 165-114 dated 7 December 1965
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1965b) Unpublished Report dated 23 November 1965 from
Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1966a) Unpublished Report No. 165-112 dated 18 November
1966 from Hazelton Laboratories Inc., submitted by Allied
Chemical Corporation
Anonymous (1966b) Unpublished Report No. 165-116 from Hazelton
Laboratories Inc., submitted by Allied Chemical Corporation
Anonymous (1967) Unpublished Report No. 165-119 dated 16 October 1967
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1968) Unpublished Report No. 165-120 dated 20 December 1968
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1969) Unpublished Report No. 165-125 dated 29 May 1965 from
Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970a) Unpublished Report No. 165-121 dated 2 March 1970
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970b) Unpublished Report No. 165-122 dated 2 March 1970
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970c) Unpublished Report No. 165-123 dated 2 March 1970
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1970d) Unpublished Report No. 21855 dated 11 February 1970
from Buffalo Research Laboratory, submitted by Allied Chemical
Corporation
Anonymous (1974a) Unpublished Report No. 165-142 dated 15 June 1974
from Hazelton Laboratories Inc., submitted by Allied Chemical
Corporation
Anonymous (1974b) Unpublished Report submitted by the United States
Food and Drug Administration
