
ANTHOCYANINS
Explanation
These compounds have not previously been reviewed by the Joint
FAO/WHO Expert Committee on Food Additives.
Introduction
Anthocyanins represent a large group of water-soluble plant
pigments of the 2-phenylbenzophyrylium (flavylium) structure
(Kuhnau, 1976). The class, "Anthocyanins", consists of some 200 or
more compounds (Parkinson & Brown, 1981) chemically combined to a
sugar moiety (glucose < rhamnose < galactose < xylose
< arabinose) of which the most common are:
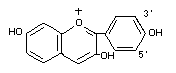 Anthocyanin structure
Carbon ring B substitution
Compound 3' 5'
pelargonidin -H -H
cyanidin -OH -H
delphinidin -OH -OH
peonidin -OCH3 -H
petunidin -OCH3 -OH
malvidin -OCH3 -OCH3
The blue to red colour imparted by the anthocyanins depends
largely upon the pH of the medium (Francis, 1977). The anthocyanins
normally exist as glycosides; the aglycone component alone is
extremely unstable.
The anthocyanin pigments present in grape-skin extract consist
of diglucosides, monoglucosides, acylated monoglucosides, and
acylated diglucosides of peonidin, malvidin, cyanidin, petunidin
and delphinidin. The amount of each compound varies depending upon
the variety of grape and climatic conditions.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Anthocyanins are poorly absorbed from the gastrointestinal
tract. Anthocyanins (notably delphinidin) extracted from concord
grapes were administered to rats by either gavage (100 mg) or by
percutaneous injection (50 mg) and the urine tested for unchanged
anthocyanins by an HCl-acid red test (Horwitt, 1933). Anthocyanin
was detected in the urine of rats administered anthocyanin by the
percutaneous route but not by gavage. In studies in dogs (Horwitt,
1933) administered anthocyanin (500 mg) by gastric fistula, no
urinary coloration was demonstrated. However, in the rabbit, 1-2%
of an oral dose of anthocyanin (500 mg) was present in the urine as
the unchanged pigment. It should be noted that the HCl-acid red
test used in this study would only detect unchanged anthocyanins
(Scheline, 1978). If the anthocyanins were transformed into
colourless pseudobases or pale anhydrolases prior to absorption and
excretion, they would not be detected (Kuhnau, 1976).
The absence of pigmented urine in normal individuals ingesting
anthocyanin-containing foods in humans coupled with the apparent
lack of metabolism of anthocyanins has been interpreted as showing
that gastrointestinal absorption of these compounds does not occur
(Clark & Mackay, 1950). Clinical studies have reported
anthocyaninuria in patients with a beet allergy, following the
ingestion of large amounts of beets (Zindler & Colovos, 1950).
However, this has been identified as betaninuria, and is related to
the excretion of betanin, rather than anthocyanins (Forrai et al,
1968).
Tissue disposition of anthocyanosides derived from Vaccinium
myrtillus (approximately 25% anthocyanins) was examined in
Charles River rats following intraperitoneal (i.p.) or intravenous
(i.v.) injection. Following acute administration by either route,
anthocyanins were found to distribute rapidly into the tissues.
Accumulation was primarily in the kidney, skin, liver, heart and
lung (Lietti & Forni, 1976). There was also some indication of
lymph node uptake of the anthocyanins. Elimination of the compound
occurred primarily via the kidney (25-29%/24 hours) and bile
(15-18%/24 hours). Because of the high urinary excretion rate in
these studies, the anthocyanins are considered to be eliminated by
both glomerular filtration and renal tubular excretion (Lietti &
Forni, 1976).
Metabolism
Studies in rats have shown that some anthocyanins (notably
pelargonidin, delphinidin, malvidin) were subject to degradation by
intestinal bacteria (Griffiths & Smith, 1972a, b). p-hydroxyphenyl-
lactic acid was detected in the urine of rats following the
oral administration of pelargonidin (a 3',3-diglycoside of
pelargonidin). Decoloration of "anthocyanin" by rat caecal cell
extracts has been reported (Haveland-Smith, 1981). Anthocyanin
extracts incubated with human faecal suspensions for 2-3 days
remained unchanged (as measured by a reduction in suspension
colour).
The presence of 2 unidentified metabolites in the urine of
rats after gavage with 100 mg of delphinidin has also been reported
(Scheline, 1978). Rats gavaged with malvidin (a 3',5'-diglycoside
of malvidin) had 3 unidentified metabolites present in the urine.
These studies suggest that some of the metabolites of anthocyanins
(aglycones) can be absorbed. Metabolism of anthocyanins may occur
to a limited degree by ring fission and/or glycoside hydrolysis of
the anthocyanins (Parkinson & Brown, 1981). Cyanidin, the most
widespread anthocyanin, has not been shown to be attacked by
intestinal bacteria (Scheline, 1968; Griffiths & Smith, 1972a).
Effects on enzymes and other biochemical parameters
Both pelargonidin and delphinidin have been shown to inhibit
aldoreductase in the lens of rats (Varma & Kinoshita, 1976). In
other studies, anthocyanin-3-monoglycosides (namely petunidin-,
delphinidin- and malvidin-) extracted from grapes were found to
increase the activity of alpha glucan phosphorylase and glutamic
acid dicarboxylase but inhibit glycerol dehydrogenase, malate
dehydrogenase and hexokinase (Carpenter et al., 1967).
Other studies have shown that anthocyanins are capable of
chelating ions such as copper (Somaatmadja et al., 1964) and iodide
(Moudgal et al., 1958). The iodide ion was observed in vitro to
form a stable complex with the anthocyanins (Moudgal et al., 1958).
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Cyanidin chloride was not mutagenic when examined in the Ames
assay using Salmonella typhimurium strain TA-98 with and without
metabolic activation (arochlor 1254 induced rat liver S-9 fraction)
(MacGregor & Jurd, 1978). Structure-activity testing of a large
group of flavonols for mutagenic response in this assay system
indicated that compounds of flavylium class were inactive.
Cyanidin and delphinidin were inactive in the Ames assay
system using 5 different strains of Salmonella typhimurium
(TA-1535, TA-100, TA-1537, TA-1538 and TA-98) with and without
activation (Brown & Dietrich, 1979).
Anthocyanin was tested in both the Ames test using
Salmonella typhimurium TA-1538 for mutagenicity and in another
in vitro test employing E. coli Wf2 for induction of DNA
damage. In both assay procedures with or without metabolic
activation (using either rat caecal extracts or rat liver
microsomes) anthocyanins were not found to induce any response
(Haveland-Smith, 1981). Negative findings were also reported for
the anthocyanins in a gene conversion assay using S. cerevisiae
D4 (Haveland-Smith, 1981).
Special studies on pharmacology
In rabbits administered anthocyanin glycosides 6 g/kg (oral)
or 500 mg/kg (i.p.) acutely, no adverse effect was noted on blood
pressure. However, 100-200 mg/kg i.v. was shown to elicit a
transcent hypotension accompanied by a decrease in respiratory
amplitude. At 25 mg/kg i.v., diuretic effects were also reported.
Anthocyanin also caused a vasodilation in the isolated rabbit heart
(Pourrat et al., 1967).
In mice, anthocyanins given in oral doses of 500 mg/kg
produced a sedative effect on the animals (Pourrat et al., 1967).
Improvements in visual acuity and darkness adaptation have
been reported in humans for a short period of time, after receiving
oral doses of up to 700 mg of the anthocyanins (Pourrat et al.,
1967).
Special studies on reproduction
A 2-generation reproduction study was performed in rats
(Sprague-Dawley) ingesting a grape-skin extract preparation that
was prepared by spray drying the liquid form of the extract after
addition of a carrier material (malto-dextrose). The preparation
contained approximately 3% anthocyanins. The test group received
dietary levels of 7.5% or 15% of the grape-skin extract throughout
the study. There were two concurrent control groups, one receiving
the basal diet, the other receiving a diet containing 9% of the
malto-dextrin used as a carrier to the grape-skin extract
preparation. The F2a generation (10/litter culled at 4 days) were
maintained for 21 days post-partum, then autopsied. No differences
in reproduction performance or indices including pup viability were
apparent between control and dosed groups. At the high-dose level,
both the F1a and F2a rats exhibited lower body weights than the
concurrent controls. Body weights of the F2 pups in the 7.5% group
were marginally depressed. However, it should be noted that the
decrease in body weights was accompanied by a concomitant decrease
in food intake. At week 6 and at termination of the studies,
haematological and blood serum chemistry and urinalyses were
carried out in the F1a group. There were no compound-related
effects. At week 18 of the study, rats in the F1a group were
sacrificed and absolute and relative organ weights determined, and
a complete histological study was carried out in the principal
organs and tissues. Decrease in organ weights of the liver, adrenal
and thyroid occurred in the 15% group. There were no compound-
related histological effects (Cox & Babish, 1978a).
Special studies on teratogenicity
The anthocyanin glycosides (an extract from currants,
blueberries and elderberries) were reported not to be teratogenic
in rats, mice or rabbits when given at dose levels of 1.5, 3 or
9 g/kg over 3 successive generations (Pourrat et al., 1967).
Acute toxicity
LD50 Reference
Animal Route (mg/kg bw)
Mice i.p. 4 110 Pourrat et al., 1967
i.v. 840 Pourrat et al., 1967
Oral 25 000 Pourrat et al., 1967
Rats i.p. 2 850 Pourrat et al., 1967
i.v. 240 Pourrat et al., 1967
Oral 20 000 Pourrat et al., 1967
Test animals were administered the anthocyanins (cyanidin,
petunidin and delphinidin mixture extracted from currants,
blueberries and elderberries) in doses from 0 to 25 000 mg/kg bw
for mice and from 0 to 20 000 mg/kg for rats. Following i.v. or
i.p. administration, toxic doses of anthocyanins produced sedation,
convulsions and finally death.
Short-term studies
Weanling male and female Wistar rats (20/group) were fed a
diet containing anthocyanin extract at levels equivalent to
3000 mg/day or 6000 mg/day for a period of 90 days. A group of
concurrent controls were also used in the study. The doses of
anthocyanin administered were estimated to be 5 and 10 times,
respectively, the level that a human would ingest. No differences
were observed between the test animals and controls in survival,
growth or histopathology of the principal tissues at the
termination of the study (Pourrat et al., 1967).
In another study, guinea-pigs received 3000 mg/kg of
anthocyanin in the diet for 15 days. No adverse effects were
reported (Pourrat et al., 1967).
Male and female beagle dogs (4/sex/dose) received either 0,
7.5% or 15% of grape-skin extract (approximately 2.39% anthocyanin
by weight) in the diet for 90 days. No differences were noted
between control and treated animals in body weights, growth,
survival, clinical chemistries (haematology, biochemistry or
urinalysis), organ weights or pathological lesions (gross or
microscopic) (Cox & Babish, 1978a).
OBSERVATIONS IN MAN
Man is naturally exposed to anthocyanins through the ingestion
of fruits and vegetables. Levels of exposure under normal dietary
conditions have not been established.
Information on the metabolism and toxicity of the anthocyanins
is limited. Its interpretation is complicated because the
anthocyanins represent a large group of chemically-related
substances and the effect observed with one defined anthocyanin may
not be applicable to another. The available information suggests
that anthocyanins are poorly absorbed from the gastrointestinal
tract. Metabolism is limited and may be due to the activity of the
intestinal bacterial flora. The metabolites of anthocyanins have
not been identified. However, the insensitivity of the assay
techniques used for measuring unmetabolized anthocyanins may result
in a significant underestimate of the degree of absorption and
metabolism of the anthocyanins (Kuhnau, 1976).
Comments
Toxicological studies are limited, and have been carried out
with mixtures extracted from a variety of fruits. The available
data indicate that such extracts are of a very low order of
toxicity. Diets containing 7.5% or 15% of a grape-skin extract
preparation (approximately 3% anthocyanin) had no effect on the
reproductive performance of rats in a 2-generation reproductive
study. The lower body weights of offspring were related to a
concomitant decrease in food intake. At the highest level tested,
there was a decreased organ weight of the liver, adrenal and
thyroid. There were no compound-related histological effects. No
compound-related effects were observed in a short-term study in
which dogs were fed diets containing 7.5% or 15% of the grape-skin
extract preparation.
EVALUATION
Level causing no toxicological effect (Grape-skin extract
preparation)
Rat (young): 7.5% of the diet equivalent to 7500 mg/kg bw.
Estimate of acceptable daily intake for man
0-2.5 mg/kg bw.*
* Anthocyanins (present in the grape-skin preparation at level
of approximately 3%).
REFERENCES
Brown, J. P. & Dietrich, P. S. (1979) Mutagenicity of plant
flavonols in the Salmonella/mammalian microsome test,
Mutation Research, 66, 223-240
Carpenter, J. A., Wang, Y.-P. & Powers, J. J. (1967) Effects of
anthocyanin pigments on certain enzymes, Proc. Soc. Exptl.
Biol. Med., 124, 702-706
Clark, W. G. & Mackay, E. W. (1950) The absorption and excretion of
rutin and related flavanoid substances, J. Amer. Med.
Assoc., 143, 1411-1415
Cox, G. E. & Babish, J. C. (1978a) Evaluation of the safety of
dietary administration of special grape color powder (type
BW-AT) on reproduction, lactation and maturation when fed to
Sprague-Dawley rats. Unpublished report No. 5417 by Food and
Drug Research Laboratories, Inc., submitted to the World
Health Organization by FDA
Cox, G. E. & Babish, J. C. (1978b) A 90-day feeding study of
special grape color powder (type BW-AT) to Beagle dogs.
Unpublished report No. 5417 by Food and Drug Research
Laboratories, Inc., submitted to the World Health Organization
by FDA
Forrai, G. Vágújfalvi, D. & Bölcskey, P. (1968) Betaninuria in
childhood, Acta Paediatrica Academiae Scientiarum
Hungaricae, 9, 43-51
Francies, F. J. (1977) Anthocyanins. In: Furia, E., ed.,
Current aspects of foods colorants, Cleveland, Ohio, CRC
Press, pp. 19-28
Griffiths, L. A. & Smith, G. E. (1972a) Metabolism of myricetin and
related compounds in the rat. Metabolite formation in vivo
and by the intestinal microflora in vitro, Biochem. J.,
183, 141-151
Griffiths, L. A. & Smith, G. E. (1972b) Metabolism of apigenin and
related compounds in the rat, Biochem. J., 128, 901-911
Haveland-Smith, R. B. (1981) Evaluation of the genotoxicity of some
natural food colors using bacterial assays, Mutation
Research, 91, 285-290
Horwitt, K. M. (1933) Observations on behavior of the anthocyanin
pigment from concord grapes in the animal body, Proc. Soc.
Exptl. Biol. Med., 30, 949-951
Kuhnau, J. (1976) The flavanoids. A class of semi-essential food
components: their role in human nutrition, World Rev. Nutr.
Diet., 24, 117-191
Lietti, A. & Forni, G. (1976) Studies on Vaccinium myrtillus
anthocyanosides. II. Aspects of anthocyanin pharmacokinetics
in the rat, Arzneim-Forsch., 26
MacGregor, J. T. & Jurd, L. (1978) Mutagenicity of plant
flavanoids: Structural requirements for mutagenic activity in
Salmonella typhimurium, Mutation Research, 54, 297-309
Moudgal, N. R., Raghupathy, E. & Sarma, P. S. (1958) Studies on
goitrogenic agents in foods. III. Goitrogenic action of some
glycosides isolated from edible nuts, J. Nutr., 66,
291-303
Parkinson, T. M. & Brown, J. P. (1981) Metabolic fate of food
colorants, Ann. Rev. Nutr., 1, 175-205
Pourrat, H., Bastide, P., Dorier, P. & Tronche, P. (1967)
Préparation et activité thérapeutique de quelques glycosides
d'anthocyanes, Chim. Thérap., 2, 33-38
Scheline, R. R. (1968) The metabolism of drugs and other organic
compounds by the intestinal microflora, Acta Pharmacol. et
Toxicol., 26, 332-342
Scheline, R. R. (1978) Mammalian metabolism of plant xenobiotics,
New York, Academic Press
Singleton, V. L. & Esau, P. (1969) Phenolic substances in grapes
and wine and their significance. In: Chichester, C. O.,
Mrak, E. M. & Stewart, G. F., eds, Advances in food
research, New York, Academic Press, Suppl. 1, pp. 31-38
Somaatmadja, D., Powers, J. J. & Hamdy, M. K. (1964) Anthocyanins.
VI. Chelation studies on anthocyanins and other related
compounds, J. Food Sci., 29, 655-660
Varma, S. D. & Kinoshita, J. H. (1976) Inhibition of lens aldose
reductase by flavanoids - their possible role in the
prevention of diabetic cataracts, Biochem. Pharmacol., 25,
2505-2513
Zindler, G. A. & Colovos, G. C. (1950) Anthocyaninuria and beet
allergy, Ann. Allergy, 8, 603-617
Anthocyanin structure
Carbon ring B substitution
Compound 3' 5'
pelargonidin -H -H
cyanidin -OH -H
delphinidin -OH -OH
peonidin -OCH3 -H
petunidin -OCH3 -OH
malvidin -OCH3 -OCH3
The blue to red colour imparted by the anthocyanins depends
largely upon the pH of the medium (Francis, 1977). The anthocyanins
normally exist as glycosides; the aglycone component alone is
extremely unstable.
The anthocyanin pigments present in grape-skin extract consist
of diglucosides, monoglucosides, acylated monoglucosides, and
acylated diglucosides of peonidin, malvidin, cyanidin, petunidin
and delphinidin. The amount of each compound varies depending upon
the variety of grape and climatic conditions.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Anthocyanins are poorly absorbed from the gastrointestinal
tract. Anthocyanins (notably delphinidin) extracted from concord
grapes were administered to rats by either gavage (100 mg) or by
percutaneous injection (50 mg) and the urine tested for unchanged
anthocyanins by an HCl-acid red test (Horwitt, 1933). Anthocyanin
was detected in the urine of rats administered anthocyanin by the
percutaneous route but not by gavage. In studies in dogs (Horwitt,
1933) administered anthocyanin (500 mg) by gastric fistula, no
urinary coloration was demonstrated. However, in the rabbit, 1-2%
of an oral dose of anthocyanin (500 mg) was present in the urine as
the unchanged pigment. It should be noted that the HCl-acid red
test used in this study would only detect unchanged anthocyanins
(Scheline, 1978). If the anthocyanins were transformed into
colourless pseudobases or pale anhydrolases prior to absorption and
excretion, they would not be detected (Kuhnau, 1976).
The absence of pigmented urine in normal individuals ingesting
anthocyanin-containing foods in humans coupled with the apparent
lack of metabolism of anthocyanins has been interpreted as showing
that gastrointestinal absorption of these compounds does not occur
(Clark & Mackay, 1950). Clinical studies have reported
anthocyaninuria in patients with a beet allergy, following the
ingestion of large amounts of beets (Zindler & Colovos, 1950).
However, this has been identified as betaninuria, and is related to
the excretion of betanin, rather than anthocyanins (Forrai et al,
1968).
Tissue disposition of anthocyanosides derived from Vaccinium
myrtillus (approximately 25% anthocyanins) was examined in
Charles River rats following intraperitoneal (i.p.) or intravenous
(i.v.) injection. Following acute administration by either route,
anthocyanins were found to distribute rapidly into the tissues.
Accumulation was primarily in the kidney, skin, liver, heart and
lung (Lietti & Forni, 1976). There was also some indication of
lymph node uptake of the anthocyanins. Elimination of the compound
occurred primarily via the kidney (25-29%/24 hours) and bile
(15-18%/24 hours). Because of the high urinary excretion rate in
these studies, the anthocyanins are considered to be eliminated by
both glomerular filtration and renal tubular excretion (Lietti &
Forni, 1976).
Metabolism
Studies in rats have shown that some anthocyanins (notably
pelargonidin, delphinidin, malvidin) were subject to degradation by
intestinal bacteria (Griffiths & Smith, 1972a, b). p-hydroxyphenyl-
lactic acid was detected in the urine of rats following the
oral administration of pelargonidin (a 3',3-diglycoside of
pelargonidin). Decoloration of "anthocyanin" by rat caecal cell
extracts has been reported (Haveland-Smith, 1981). Anthocyanin
extracts incubated with human faecal suspensions for 2-3 days
remained unchanged (as measured by a reduction in suspension
colour).
The presence of 2 unidentified metabolites in the urine of
rats after gavage with 100 mg of delphinidin has also been reported
(Scheline, 1978). Rats gavaged with malvidin (a 3',5'-diglycoside
of malvidin) had 3 unidentified metabolites present in the urine.
These studies suggest that some of the metabolites of anthocyanins
(aglycones) can be absorbed. Metabolism of anthocyanins may occur
to a limited degree by ring fission and/or glycoside hydrolysis of
the anthocyanins (Parkinson & Brown, 1981). Cyanidin, the most
widespread anthocyanin, has not been shown to be attacked by
intestinal bacteria (Scheline, 1968; Griffiths & Smith, 1972a).
Effects on enzymes and other biochemical parameters
Both pelargonidin and delphinidin have been shown to inhibit
aldoreductase in the lens of rats (Varma & Kinoshita, 1976). In
other studies, anthocyanin-3-monoglycosides (namely petunidin-,
delphinidin- and malvidin-) extracted from grapes were found to
increase the activity of alpha glucan phosphorylase and glutamic
acid dicarboxylase but inhibit glycerol dehydrogenase, malate
dehydrogenase and hexokinase (Carpenter et al., 1967).
Other studies have shown that anthocyanins are capable of
chelating ions such as copper (Somaatmadja et al., 1964) and iodide
(Moudgal et al., 1958). The iodide ion was observed in vitro to
form a stable complex with the anthocyanins (Moudgal et al., 1958).
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Cyanidin chloride was not mutagenic when examined in the Ames
assay using Salmonella typhimurium strain TA-98 with and without
metabolic activation (arochlor 1254 induced rat liver S-9 fraction)
(MacGregor & Jurd, 1978). Structure-activity testing of a large
group of flavonols for mutagenic response in this assay system
indicated that compounds of flavylium class were inactive.
Cyanidin and delphinidin were inactive in the Ames assay
system using 5 different strains of Salmonella typhimurium
(TA-1535, TA-100, TA-1537, TA-1538 and TA-98) with and without
activation (Brown & Dietrich, 1979).
Anthocyanin was tested in both the Ames test using
Salmonella typhimurium TA-1538 for mutagenicity and in another
in vitro test employing E. coli Wf2 for induction of DNA
damage. In both assay procedures with or without metabolic
activation (using either rat caecal extracts or rat liver
microsomes) anthocyanins were not found to induce any response
(Haveland-Smith, 1981). Negative findings were also reported for
the anthocyanins in a gene conversion assay using S. cerevisiae
D4 (Haveland-Smith, 1981).
Special studies on pharmacology
In rabbits administered anthocyanin glycosides 6 g/kg (oral)
or 500 mg/kg (i.p.) acutely, no adverse effect was noted on blood
pressure. However, 100-200 mg/kg i.v. was shown to elicit a
transcent hypotension accompanied by a decrease in respiratory
amplitude. At 25 mg/kg i.v., diuretic effects were also reported.
Anthocyanin also caused a vasodilation in the isolated rabbit heart
(Pourrat et al., 1967).
In mice, anthocyanins given in oral doses of 500 mg/kg
produced a sedative effect on the animals (Pourrat et al., 1967).
Improvements in visual acuity and darkness adaptation have
been reported in humans for a short period of time, after receiving
oral doses of up to 700 mg of the anthocyanins (Pourrat et al.,
1967).
Special studies on reproduction
A 2-generation reproduction study was performed in rats
(Sprague-Dawley) ingesting a grape-skin extract preparation that
was prepared by spray drying the liquid form of the extract after
addition of a carrier material (malto-dextrose). The preparation
contained approximately 3% anthocyanins. The test group received
dietary levels of 7.5% or 15% of the grape-skin extract throughout
the study. There were two concurrent control groups, one receiving
the basal diet, the other receiving a diet containing 9% of the
malto-dextrin used as a carrier to the grape-skin extract
preparation. The F2a generation (10/litter culled at 4 days) were
maintained for 21 days post-partum, then autopsied. No differences
in reproduction performance or indices including pup viability were
apparent between control and dosed groups. At the high-dose level,
both the F1a and F2a rats exhibited lower body weights than the
concurrent controls. Body weights of the F2 pups in the 7.5% group
were marginally depressed. However, it should be noted that the
decrease in body weights was accompanied by a concomitant decrease
in food intake. At week 6 and at termination of the studies,
haematological and blood serum chemistry and urinalyses were
carried out in the F1a group. There were no compound-related
effects. At week 18 of the study, rats in the F1a group were
sacrificed and absolute and relative organ weights determined, and
a complete histological study was carried out in the principal
organs and tissues. Decrease in organ weights of the liver, adrenal
and thyroid occurred in the 15% group. There were no compound-
related histological effects (Cox & Babish, 1978a).
Special studies on teratogenicity
The anthocyanin glycosides (an extract from currants,
blueberries and elderberries) were reported not to be teratogenic
in rats, mice or rabbits when given at dose levels of 1.5, 3 or
9 g/kg over 3 successive generations (Pourrat et al., 1967).
Acute toxicity
LD50 Reference
Animal Route (mg/kg bw)
Mice i.p. 4 110 Pourrat et al., 1967
i.v. 840 Pourrat et al., 1967
Oral 25 000 Pourrat et al., 1967
Rats i.p. 2 850 Pourrat et al., 1967
i.v. 240 Pourrat et al., 1967
Oral 20 000 Pourrat et al., 1967
Test animals were administered the anthocyanins (cyanidin,
petunidin and delphinidin mixture extracted from currants,
blueberries and elderberries) in doses from 0 to 25 000 mg/kg bw
for mice and from 0 to 20 000 mg/kg for rats. Following i.v. or
i.p. administration, toxic doses of anthocyanins produced sedation,
convulsions and finally death.
Short-term studies
Weanling male and female Wistar rats (20/group) were fed a
diet containing anthocyanin extract at levels equivalent to
3000 mg/day or 6000 mg/day for a period of 90 days. A group of
concurrent controls were also used in the study. The doses of
anthocyanin administered were estimated to be 5 and 10 times,
respectively, the level that a human would ingest. No differences
were observed between the test animals and controls in survival,
growth or histopathology of the principal tissues at the
termination of the study (Pourrat et al., 1967).
In another study, guinea-pigs received 3000 mg/kg of
anthocyanin in the diet for 15 days. No adverse effects were
reported (Pourrat et al., 1967).
Male and female beagle dogs (4/sex/dose) received either 0,
7.5% or 15% of grape-skin extract (approximately 2.39% anthocyanin
by weight) in the diet for 90 days. No differences were noted
between control and treated animals in body weights, growth,
survival, clinical chemistries (haematology, biochemistry or
urinalysis), organ weights or pathological lesions (gross or
microscopic) (Cox & Babish, 1978a).
OBSERVATIONS IN MAN
Man is naturally exposed to anthocyanins through the ingestion
of fruits and vegetables. Levels of exposure under normal dietary
conditions have not been established.
Information on the metabolism and toxicity of the anthocyanins
is limited. Its interpretation is complicated because the
anthocyanins represent a large group of chemically-related
substances and the effect observed with one defined anthocyanin may
not be applicable to another. The available information suggests
that anthocyanins are poorly absorbed from the gastrointestinal
tract. Metabolism is limited and may be due to the activity of the
intestinal bacterial flora. The metabolites of anthocyanins have
not been identified. However, the insensitivity of the assay
techniques used for measuring unmetabolized anthocyanins may result
in a significant underestimate of the degree of absorption and
metabolism of the anthocyanins (Kuhnau, 1976).
Comments
Toxicological studies are limited, and have been carried out
with mixtures extracted from a variety of fruits. The available
data indicate that such extracts are of a very low order of
toxicity. Diets containing 7.5% or 15% of a grape-skin extract
preparation (approximately 3% anthocyanin) had no effect on the
reproductive performance of rats in a 2-generation reproductive
study. The lower body weights of offspring were related to a
concomitant decrease in food intake. At the highest level tested,
there was a decreased organ weight of the liver, adrenal and
thyroid. There were no compound-related histological effects. No
compound-related effects were observed in a short-term study in
which dogs were fed diets containing 7.5% or 15% of the grape-skin
extract preparation.
EVALUATION
Level causing no toxicological effect (Grape-skin extract
preparation)
Rat (young): 7.5% of the diet equivalent to 7500 mg/kg bw.
Estimate of acceptable daily intake for man
0-2.5 mg/kg bw.*
* Anthocyanins (present in the grape-skin preparation at level
of approximately 3%).
REFERENCES
Brown, J. P. & Dietrich, P. S. (1979) Mutagenicity of plant
flavonols in the Salmonella/mammalian microsome test,
Mutation Research, 66, 223-240
Carpenter, J. A., Wang, Y.-P. & Powers, J. J. (1967) Effects of
anthocyanin pigments on certain enzymes, Proc. Soc. Exptl.
Biol. Med., 124, 702-706
Clark, W. G. & Mackay, E. W. (1950) The absorption and excretion of
rutin and related flavanoid substances, J. Amer. Med.
Assoc., 143, 1411-1415
Cox, G. E. & Babish, J. C. (1978a) Evaluation of the safety of
dietary administration of special grape color powder (type
BW-AT) on reproduction, lactation and maturation when fed to
Sprague-Dawley rats. Unpublished report No. 5417 by Food and
Drug Research Laboratories, Inc., submitted to the World
Health Organization by FDA
Cox, G. E. & Babish, J. C. (1978b) A 90-day feeding study of
special grape color powder (type BW-AT) to Beagle dogs.
Unpublished report No. 5417 by Food and Drug Research
Laboratories, Inc., submitted to the World Health Organization
by FDA
Forrai, G. Vágújfalvi, D. & Bölcskey, P. (1968) Betaninuria in
childhood, Acta Paediatrica Academiae Scientiarum
Hungaricae, 9, 43-51
Francies, F. J. (1977) Anthocyanins. In: Furia, E., ed.,
Current aspects of foods colorants, Cleveland, Ohio, CRC
Press, pp. 19-28
Griffiths, L. A. & Smith, G. E. (1972a) Metabolism of myricetin and
related compounds in the rat. Metabolite formation in vivo
and by the intestinal microflora in vitro, Biochem. J.,
183, 141-151
Griffiths, L. A. & Smith, G. E. (1972b) Metabolism of apigenin and
related compounds in the rat, Biochem. J., 128, 901-911
Haveland-Smith, R. B. (1981) Evaluation of the genotoxicity of some
natural food colors using bacterial assays, Mutation
Research, 91, 285-290
Horwitt, K. M. (1933) Observations on behavior of the anthocyanin
pigment from concord grapes in the animal body, Proc. Soc.
Exptl. Biol. Med., 30, 949-951
Kuhnau, J. (1976) The flavanoids. A class of semi-essential food
components: their role in human nutrition, World Rev. Nutr.
Diet., 24, 117-191
Lietti, A. & Forni, G. (1976) Studies on Vaccinium myrtillus
anthocyanosides. II. Aspects of anthocyanin pharmacokinetics
in the rat, Arzneim-Forsch., 26
MacGregor, J. T. & Jurd, L. (1978) Mutagenicity of plant
flavanoids: Structural requirements for mutagenic activity in
Salmonella typhimurium, Mutation Research, 54, 297-309
Moudgal, N. R., Raghupathy, E. & Sarma, P. S. (1958) Studies on
goitrogenic agents in foods. III. Goitrogenic action of some
glycosides isolated from edible nuts, J. Nutr., 66,
291-303
Parkinson, T. M. & Brown, J. P. (1981) Metabolic fate of food
colorants, Ann. Rev. Nutr., 1, 175-205
Pourrat, H., Bastide, P., Dorier, P. & Tronche, P. (1967)
Préparation et activité thérapeutique de quelques glycosides
d'anthocyanes, Chim. Thérap., 2, 33-38
Scheline, R. R. (1968) The metabolism of drugs and other organic
compounds by the intestinal microflora, Acta Pharmacol. et
Toxicol., 26, 332-342
Scheline, R. R. (1978) Mammalian metabolism of plant xenobiotics,
New York, Academic Press
Singleton, V. L. & Esau, P. (1969) Phenolic substances in grapes
and wine and their significance. In: Chichester, C. O.,
Mrak, E. M. & Stewart, G. F., eds, Advances in food
research, New York, Academic Press, Suppl. 1, pp. 31-38
Somaatmadja, D., Powers, J. J. & Hamdy, M. K. (1964) Anthocyanins.
VI. Chelation studies on anthocyanins and other related
compounds, J. Food Sci., 29, 655-660
Varma, S. D. & Kinoshita, J. H. (1976) Inhibition of lens aldose
reductase by flavanoids - their possible role in the
prevention of diabetic cataracts, Biochem. Pharmacol., 25,
2505-2513
Zindler, G. A. & Colovos, G. C. (1950) Anthocyaninuria and beet
allergy, Ann. Allergy, 8, 603-617
 Anthocyanin structure
Carbon ring B substitution
Compound 3' 5'
pelargonidin -H -H
cyanidin -OH -H
delphinidin -OH -OH
peonidin -OCH3 -H
petunidin -OCH3 -OH
malvidin -OCH3 -OCH3
The blue to red colour imparted by the anthocyanins depends
largely upon the pH of the medium (Francis, 1977). The anthocyanins
normally exist as glycosides; the aglycone component alone is
extremely unstable.
The anthocyanin pigments present in grape-skin extract consist
of diglucosides, monoglucosides, acylated monoglucosides, and
acylated diglucosides of peonidin, malvidin, cyanidin, petunidin
and delphinidin. The amount of each compound varies depending upon
the variety of grape and climatic conditions.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Anthocyanins are poorly absorbed from the gastrointestinal
tract. Anthocyanins (notably delphinidin) extracted from concord
grapes were administered to rats by either gavage (100 mg) or by
percutaneous injection (50 mg) and the urine tested for unchanged
anthocyanins by an HCl-acid red test (Horwitt, 1933). Anthocyanin
was detected in the urine of rats administered anthocyanin by the
percutaneous route but not by gavage. In studies in dogs (Horwitt,
1933) administered anthocyanin (500 mg) by gastric fistula, no
urinary coloration was demonstrated. However, in the rabbit, 1-2%
of an oral dose of anthocyanin (500 mg) was present in the urine as
the unchanged pigment. It should be noted that the HCl-acid red
test used in this study would only detect unchanged anthocyanins
(Scheline, 1978). If the anthocyanins were transformed into
colourless pseudobases or pale anhydrolases prior to absorption and
excretion, they would not be detected (Kuhnau, 1976).
The absence of pigmented urine in normal individuals ingesting
anthocyanin-containing foods in humans coupled with the apparent
lack of metabolism of anthocyanins has been interpreted as showing
that gastrointestinal absorption of these compounds does not occur
(Clark & Mackay, 1950). Clinical studies have reported
anthocyaninuria in patients with a beet allergy, following the
ingestion of large amounts of beets (Zindler & Colovos, 1950).
However, this has been identified as betaninuria, and is related to
the excretion of betanin, rather than anthocyanins (Forrai et al,
1968).
Tissue disposition of anthocyanosides derived from Vaccinium
myrtillus (approximately 25% anthocyanins) was examined in
Charles River rats following intraperitoneal (i.p.) or intravenous
(i.v.) injection. Following acute administration by either route,
anthocyanins were found to distribute rapidly into the tissues.
Accumulation was primarily in the kidney, skin, liver, heart and
lung (Lietti & Forni, 1976). There was also some indication of
lymph node uptake of the anthocyanins. Elimination of the compound
occurred primarily via the kidney (25-29%/24 hours) and bile
(15-18%/24 hours). Because of the high urinary excretion rate in
these studies, the anthocyanins are considered to be eliminated by
both glomerular filtration and renal tubular excretion (Lietti &
Forni, 1976).
Metabolism
Studies in rats have shown that some anthocyanins (notably
pelargonidin, delphinidin, malvidin) were subject to degradation by
intestinal bacteria (Griffiths & Smith, 1972a, b). p-hydroxyphenyl-
lactic acid was detected in the urine of rats following the
oral administration of pelargonidin (a 3',3-diglycoside of
pelargonidin). Decoloration of "anthocyanin" by rat caecal cell
extracts has been reported (Haveland-Smith, 1981). Anthocyanin
extracts incubated with human faecal suspensions for 2-3 days
remained unchanged (as measured by a reduction in suspension
colour).
The presence of 2 unidentified metabolites in the urine of
rats after gavage with 100 mg of delphinidin has also been reported
(Scheline, 1978). Rats gavaged with malvidin (a 3',5'-diglycoside
of malvidin) had 3 unidentified metabolites present in the urine.
These studies suggest that some of the metabolites of anthocyanins
(aglycones) can be absorbed. Metabolism of anthocyanins may occur
to a limited degree by ring fission and/or glycoside hydrolysis of
the anthocyanins (Parkinson & Brown, 1981). Cyanidin, the most
widespread anthocyanin, has not been shown to be attacked by
intestinal bacteria (Scheline, 1968; Griffiths & Smith, 1972a).
Effects on enzymes and other biochemical parameters
Both pelargonidin and delphinidin have been shown to inhibit
aldoreductase in the lens of rats (Varma & Kinoshita, 1976). In
other studies, anthocyanin-3-monoglycosides (namely petunidin-,
delphinidin- and malvidin-) extracted from grapes were found to
increase the activity of alpha glucan phosphorylase and glutamic
acid dicarboxylase but inhibit glycerol dehydrogenase, malate
dehydrogenase and hexokinase (Carpenter et al., 1967).
Other studies have shown that anthocyanins are capable of
chelating ions such as copper (Somaatmadja et al., 1964) and iodide
(Moudgal et al., 1958). The iodide ion was observed in vitro to
form a stable complex with the anthocyanins (Moudgal et al., 1958).
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Cyanidin chloride was not mutagenic when examined in the Ames
assay using Salmonella typhimurium strain TA-98 with and without
metabolic activation (arochlor 1254 induced rat liver S-9 fraction)
(MacGregor & Jurd, 1978). Structure-activity testing of a large
group of flavonols for mutagenic response in this assay system
indicated that compounds of flavylium class were inactive.
Cyanidin and delphinidin were inactive in the Ames assay
system using 5 different strains of Salmonella typhimurium
(TA-1535, TA-100, TA-1537, TA-1538 and TA-98) with and without
activation (Brown & Dietrich, 1979).
Anthocyanin was tested in both the Ames test using
Salmonella typhimurium TA-1538 for mutagenicity and in another
in vitro test employing E. coli Wf2 for induction of DNA
damage. In both assay procedures with or without metabolic
activation (using either rat caecal extracts or rat liver
microsomes) anthocyanins were not found to induce any response
(Haveland-Smith, 1981). Negative findings were also reported for
the anthocyanins in a gene conversion assay using S. cerevisiae
D4 (Haveland-Smith, 1981).
Special studies on pharmacology
In rabbits administered anthocyanin glycosides 6 g/kg (oral)
or 500 mg/kg (i.p.) acutely, no adverse effect was noted on blood
pressure. However, 100-200 mg/kg i.v. was shown to elicit a
transcent hypotension accompanied by a decrease in respiratory
amplitude. At 25 mg/kg i.v., diuretic effects were also reported.
Anthocyanin also caused a vasodilation in the isolated rabbit heart
(Pourrat et al., 1967).
In mice, anthocyanins given in oral doses of 500 mg/kg
produced a sedative effect on the animals (Pourrat et al., 1967).
Improvements in visual acuity and darkness adaptation have
been reported in humans for a short period of time, after receiving
oral doses of up to 700 mg of the anthocyanins (Pourrat et al.,
1967).
Special studies on reproduction
A 2-generation reproduction study was performed in rats
(Sprague-Dawley) ingesting a grape-skin extract preparation that
was prepared by spray drying the liquid form of the extract after
addition of a carrier material (malto-dextrose). The preparation
contained approximately 3% anthocyanins. The test group received
dietary levels of 7.5% or 15% of the grape-skin extract throughout
the study. There were two concurrent control groups, one receiving
the basal diet, the other receiving a diet containing 9% of the
malto-dextrin used as a carrier to the grape-skin extract
preparation. The F2a generation (10/litter culled at 4 days) were
maintained for 21 days post-partum, then autopsied. No differences
in reproduction performance or indices including pup viability were
apparent between control and dosed groups. At the high-dose level,
both the F1a and F2a rats exhibited lower body weights than the
concurrent controls. Body weights of the F2 pups in the 7.5% group
were marginally depressed. However, it should be noted that the
decrease in body weights was accompanied by a concomitant decrease
in food intake. At week 6 and at termination of the studies,
haematological and blood serum chemistry and urinalyses were
carried out in the F1a group. There were no compound-related
effects. At week 18 of the study, rats in the F1a group were
sacrificed and absolute and relative organ weights determined, and
a complete histological study was carried out in the principal
organs and tissues. Decrease in organ weights of the liver, adrenal
and thyroid occurred in the 15% group. There were no compound-
related histological effects (Cox & Babish, 1978a).
Special studies on teratogenicity
The anthocyanin glycosides (an extract from currants,
blueberries and elderberries) were reported not to be teratogenic
in rats, mice or rabbits when given at dose levels of 1.5, 3 or
9 g/kg over 3 successive generations (Pourrat et al., 1967).
Acute toxicity
LD50 Reference
Animal Route (mg/kg bw)
Mice i.p. 4 110 Pourrat et al., 1967
i.v. 840 Pourrat et al., 1967
Oral 25 000 Pourrat et al., 1967
Rats i.p. 2 850 Pourrat et al., 1967
i.v. 240 Pourrat et al., 1967
Oral 20 000 Pourrat et al., 1967
Test animals were administered the anthocyanins (cyanidin,
petunidin and delphinidin mixture extracted from currants,
blueberries and elderberries) in doses from 0 to 25 000 mg/kg bw
for mice and from 0 to 20 000 mg/kg for rats. Following i.v. or
i.p. administration, toxic doses of anthocyanins produced sedation,
convulsions and finally death.
Short-term studies
Weanling male and female Wistar rats (20/group) were fed a
diet containing anthocyanin extract at levels equivalent to
3000 mg/day or 6000 mg/day for a period of 90 days. A group of
concurrent controls were also used in the study. The doses of
anthocyanin administered were estimated to be 5 and 10 times,
respectively, the level that a human would ingest. No differences
were observed between the test animals and controls in survival,
growth or histopathology of the principal tissues at the
termination of the study (Pourrat et al., 1967).
In another study, guinea-pigs received 3000 mg/kg of
anthocyanin in the diet for 15 days. No adverse effects were
reported (Pourrat et al., 1967).
Male and female beagle dogs (4/sex/dose) received either 0,
7.5% or 15% of grape-skin extract (approximately 2.39% anthocyanin
by weight) in the diet for 90 days. No differences were noted
between control and treated animals in body weights, growth,
survival, clinical chemistries (haematology, biochemistry or
urinalysis), organ weights or pathological lesions (gross or
microscopic) (Cox & Babish, 1978a).
OBSERVATIONS IN MAN
Man is naturally exposed to anthocyanins through the ingestion
of fruits and vegetables. Levels of exposure under normal dietary
conditions have not been established.
Information on the metabolism and toxicity of the anthocyanins
is limited. Its interpretation is complicated because the
anthocyanins represent a large group of chemically-related
substances and the effect observed with one defined anthocyanin may
not be applicable to another. The available information suggests
that anthocyanins are poorly absorbed from the gastrointestinal
tract. Metabolism is limited and may be due to the activity of the
intestinal bacterial flora. The metabolites of anthocyanins have
not been identified. However, the insensitivity of the assay
techniques used for measuring unmetabolized anthocyanins may result
in a significant underestimate of the degree of absorption and
metabolism of the anthocyanins (Kuhnau, 1976).
Comments
Toxicological studies are limited, and have been carried out
with mixtures extracted from a variety of fruits. The available
data indicate that such extracts are of a very low order of
toxicity. Diets containing 7.5% or 15% of a grape-skin extract
preparation (approximately 3% anthocyanin) had no effect on the
reproductive performance of rats in a 2-generation reproductive
study. The lower body weights of offspring were related to a
concomitant decrease in food intake. At the highest level tested,
there was a decreased organ weight of the liver, adrenal and
thyroid. There were no compound-related histological effects. No
compound-related effects were observed in a short-term study in
which dogs were fed diets containing 7.5% or 15% of the grape-skin
extract preparation.
EVALUATION
Level causing no toxicological effect (Grape-skin extract
preparation)
Rat (young): 7.5% of the diet equivalent to 7500 mg/kg bw.
Estimate of acceptable daily intake for man
0-2.5 mg/kg bw.*
* Anthocyanins (present in the grape-skin preparation at level
of approximately 3%).
REFERENCES
Brown, J. P. & Dietrich, P. S. (1979) Mutagenicity of plant
flavonols in the Salmonella/mammalian microsome test,
Mutation Research, 66, 223-240
Carpenter, J. A., Wang, Y.-P. & Powers, J. J. (1967) Effects of
anthocyanin pigments on certain enzymes, Proc. Soc. Exptl.
Biol. Med., 124, 702-706
Clark, W. G. & Mackay, E. W. (1950) The absorption and excretion of
rutin and related flavanoid substances, J. Amer. Med.
Assoc., 143, 1411-1415
Cox, G. E. & Babish, J. C. (1978a) Evaluation of the safety of
dietary administration of special grape color powder (type
BW-AT) on reproduction, lactation and maturation when fed to
Sprague-Dawley rats. Unpublished report No. 5417 by Food and
Drug Research Laboratories, Inc., submitted to the World
Health Organization by FDA
Cox, G. E. & Babish, J. C. (1978b) A 90-day feeding study of
special grape color powder (type BW-AT) to Beagle dogs.
Unpublished report No. 5417 by Food and Drug Research
Laboratories, Inc., submitted to the World Health Organization
by FDA
Forrai, G. Vágújfalvi, D. & Bölcskey, P. (1968) Betaninuria in
childhood, Acta Paediatrica Academiae Scientiarum
Hungaricae, 9, 43-51
Francies, F. J. (1977) Anthocyanins. In: Furia, E., ed.,
Current aspects of foods colorants, Cleveland, Ohio, CRC
Press, pp. 19-28
Griffiths, L. A. & Smith, G. E. (1972a) Metabolism of myricetin and
related compounds in the rat. Metabolite formation in vivo
and by the intestinal microflora in vitro, Biochem. J.,
183, 141-151
Griffiths, L. A. & Smith, G. E. (1972b) Metabolism of apigenin and
related compounds in the rat, Biochem. J., 128, 901-911
Haveland-Smith, R. B. (1981) Evaluation of the genotoxicity of some
natural food colors using bacterial assays, Mutation
Research, 91, 285-290
Horwitt, K. M. (1933) Observations on behavior of the anthocyanin
pigment from concord grapes in the animal body, Proc. Soc.
Exptl. Biol. Med., 30, 949-951
Kuhnau, J. (1976) The flavanoids. A class of semi-essential food
components: their role in human nutrition, World Rev. Nutr.
Diet., 24, 117-191
Lietti, A. & Forni, G. (1976) Studies on Vaccinium myrtillus
anthocyanosides. II. Aspects of anthocyanin pharmacokinetics
in the rat, Arzneim-Forsch., 26
MacGregor, J. T. & Jurd, L. (1978) Mutagenicity of plant
flavanoids: Structural requirements for mutagenic activity in
Salmonella typhimurium, Mutation Research, 54, 297-309
Moudgal, N. R., Raghupathy, E. & Sarma, P. S. (1958) Studies on
goitrogenic agents in foods. III. Goitrogenic action of some
glycosides isolated from edible nuts, J. Nutr., 66,
291-303
Parkinson, T. M. & Brown, J. P. (1981) Metabolic fate of food
colorants, Ann. Rev. Nutr., 1, 175-205
Pourrat, H., Bastide, P., Dorier, P. & Tronche, P. (1967)
Préparation et activité thérapeutique de quelques glycosides
d'anthocyanes, Chim. Thérap., 2, 33-38
Scheline, R. R. (1968) The metabolism of drugs and other organic
compounds by the intestinal microflora, Acta Pharmacol. et
Toxicol., 26, 332-342
Scheline, R. R. (1978) Mammalian metabolism of plant xenobiotics,
New York, Academic Press
Singleton, V. L. & Esau, P. (1969) Phenolic substances in grapes
and wine and their significance. In: Chichester, C. O.,
Mrak, E. M. & Stewart, G. F., eds, Advances in food
research, New York, Academic Press, Suppl. 1, pp. 31-38
Somaatmadja, D., Powers, J. J. & Hamdy, M. K. (1964) Anthocyanins.
VI. Chelation studies on anthocyanins and other related
compounds, J. Food Sci., 29, 655-660
Varma, S. D. & Kinoshita, J. H. (1976) Inhibition of lens aldose
reductase by flavanoids - their possible role in the
prevention of diabetic cataracts, Biochem. Pharmacol., 25,
2505-2513
Zindler, G. A. & Colovos, G. C. (1950) Anthocyaninuria and beet
allergy, Ann. Allergy, 8, 603-617
Anthocyanin structure
Carbon ring B substitution
Compound 3' 5'
pelargonidin -H -H
cyanidin -OH -H
delphinidin -OH -OH
peonidin -OCH3 -H
petunidin -OCH3 -OH
malvidin -OCH3 -OCH3
The blue to red colour imparted by the anthocyanins depends
largely upon the pH of the medium (Francis, 1977). The anthocyanins
normally exist as glycosides; the aglycone component alone is
extremely unstable.
The anthocyanin pigments present in grape-skin extract consist
of diglucosides, monoglucosides, acylated monoglucosides, and
acylated diglucosides of peonidin, malvidin, cyanidin, petunidin
and delphinidin. The amount of each compound varies depending upon
the variety of grape and climatic conditions.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Anthocyanins are poorly absorbed from the gastrointestinal
tract. Anthocyanins (notably delphinidin) extracted from concord
grapes were administered to rats by either gavage (100 mg) or by
percutaneous injection (50 mg) and the urine tested for unchanged
anthocyanins by an HCl-acid red test (Horwitt, 1933). Anthocyanin
was detected in the urine of rats administered anthocyanin by the
percutaneous route but not by gavage. In studies in dogs (Horwitt,
1933) administered anthocyanin (500 mg) by gastric fistula, no
urinary coloration was demonstrated. However, in the rabbit, 1-2%
of an oral dose of anthocyanin (500 mg) was present in the urine as
the unchanged pigment. It should be noted that the HCl-acid red
test used in this study would only detect unchanged anthocyanins
(Scheline, 1978). If the anthocyanins were transformed into
colourless pseudobases or pale anhydrolases prior to absorption and
excretion, they would not be detected (Kuhnau, 1976).
The absence of pigmented urine in normal individuals ingesting
anthocyanin-containing foods in humans coupled with the apparent
lack of metabolism of anthocyanins has been interpreted as showing
that gastrointestinal absorption of these compounds does not occur
(Clark & Mackay, 1950). Clinical studies have reported
anthocyaninuria in patients with a beet allergy, following the
ingestion of large amounts of beets (Zindler & Colovos, 1950).
However, this has been identified as betaninuria, and is related to
the excretion of betanin, rather than anthocyanins (Forrai et al,
1968).
Tissue disposition of anthocyanosides derived from Vaccinium
myrtillus (approximately 25% anthocyanins) was examined in
Charles River rats following intraperitoneal (i.p.) or intravenous
(i.v.) injection. Following acute administration by either route,
anthocyanins were found to distribute rapidly into the tissues.
Accumulation was primarily in the kidney, skin, liver, heart and
lung (Lietti & Forni, 1976). There was also some indication of
lymph node uptake of the anthocyanins. Elimination of the compound
occurred primarily via the kidney (25-29%/24 hours) and bile
(15-18%/24 hours). Because of the high urinary excretion rate in
these studies, the anthocyanins are considered to be eliminated by
both glomerular filtration and renal tubular excretion (Lietti &
Forni, 1976).
Metabolism
Studies in rats have shown that some anthocyanins (notably
pelargonidin, delphinidin, malvidin) were subject to degradation by
intestinal bacteria (Griffiths & Smith, 1972a, b). p-hydroxyphenyl-
lactic acid was detected in the urine of rats following the
oral administration of pelargonidin (a 3',3-diglycoside of
pelargonidin). Decoloration of "anthocyanin" by rat caecal cell
extracts has been reported (Haveland-Smith, 1981). Anthocyanin
extracts incubated with human faecal suspensions for 2-3 days
remained unchanged (as measured by a reduction in suspension
colour).
The presence of 2 unidentified metabolites in the urine of
rats after gavage with 100 mg of delphinidin has also been reported
(Scheline, 1978). Rats gavaged with malvidin (a 3',5'-diglycoside
of malvidin) had 3 unidentified metabolites present in the urine.
These studies suggest that some of the metabolites of anthocyanins
(aglycones) can be absorbed. Metabolism of anthocyanins may occur
to a limited degree by ring fission and/or glycoside hydrolysis of
the anthocyanins (Parkinson & Brown, 1981). Cyanidin, the most
widespread anthocyanin, has not been shown to be attacked by
intestinal bacteria (Scheline, 1968; Griffiths & Smith, 1972a).
Effects on enzymes and other biochemical parameters
Both pelargonidin and delphinidin have been shown to inhibit
aldoreductase in the lens of rats (Varma & Kinoshita, 1976). In
other studies, anthocyanin-3-monoglycosides (namely petunidin-,
delphinidin- and malvidin-) extracted from grapes were found to
increase the activity of alpha glucan phosphorylase and glutamic
acid dicarboxylase but inhibit glycerol dehydrogenase, malate
dehydrogenase and hexokinase (Carpenter et al., 1967).
Other studies have shown that anthocyanins are capable of
chelating ions such as copper (Somaatmadja et al., 1964) and iodide
(Moudgal et al., 1958). The iodide ion was observed in vitro to
form a stable complex with the anthocyanins (Moudgal et al., 1958).
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
Cyanidin chloride was not mutagenic when examined in the Ames
assay using Salmonella typhimurium strain TA-98 with and without
metabolic activation (arochlor 1254 induced rat liver S-9 fraction)
(MacGregor & Jurd, 1978). Structure-activity testing of a large
group of flavonols for mutagenic response in this assay system
indicated that compounds of flavylium class were inactive.
Cyanidin and delphinidin were inactive in the Ames assay
system using 5 different strains of Salmonella typhimurium
(TA-1535, TA-100, TA-1537, TA-1538 and TA-98) with and without
activation (Brown & Dietrich, 1979).
Anthocyanin was tested in both the Ames test using
Salmonella typhimurium TA-1538 for mutagenicity and in another
in vitro test employing E. coli Wf2 for induction of DNA
damage. In both assay procedures with or without metabolic
activation (using either rat caecal extracts or rat liver
microsomes) anthocyanins were not found to induce any response
(Haveland-Smith, 1981). Negative findings were also reported for
the anthocyanins in a gene conversion assay using S. cerevisiae
D4 (Haveland-Smith, 1981).
Special studies on pharmacology
In rabbits administered anthocyanin glycosides 6 g/kg (oral)
or 500 mg/kg (i.p.) acutely, no adverse effect was noted on blood
pressure. However, 100-200 mg/kg i.v. was shown to elicit a
transcent hypotension accompanied by a decrease in respiratory
amplitude. At 25 mg/kg i.v., diuretic effects were also reported.
Anthocyanin also caused a vasodilation in the isolated rabbit heart
(Pourrat et al., 1967).
In mice, anthocyanins given in oral doses of 500 mg/kg
produced a sedative effect on the animals (Pourrat et al., 1967).
Improvements in visual acuity and darkness adaptation have
been reported in humans for a short period of time, after receiving
oral doses of up to 700 mg of the anthocyanins (Pourrat et al.,
1967).
Special studies on reproduction
A 2-generation reproduction study was performed in rats
(Sprague-Dawley) ingesting a grape-skin extract preparation that
was prepared by spray drying the liquid form of the extract after
addition of a carrier material (malto-dextrose). The preparation
contained approximately 3% anthocyanins. The test group received
dietary levels of 7.5% or 15% of the grape-skin extract throughout
the study. There were two concurrent control groups, one receiving
the basal diet, the other receiving a diet containing 9% of the
malto-dextrin used as a carrier to the grape-skin extract
preparation. The F2a generation (10/litter culled at 4 days) were
maintained for 21 days post-partum, then autopsied. No differences
in reproduction performance or indices including pup viability were
apparent between control and dosed groups. At the high-dose level,
both the F1a and F2a rats exhibited lower body weights than the
concurrent controls. Body weights of the F2 pups in the 7.5% group
were marginally depressed. However, it should be noted that the
decrease in body weights was accompanied by a concomitant decrease
in food intake. At week 6 and at termination of the studies,
haematological and blood serum chemistry and urinalyses were
carried out in the F1a group. There were no compound-related
effects. At week 18 of the study, rats in the F1a group were
sacrificed and absolute and relative organ weights determined, and
a complete histological study was carried out in the principal
organs and tissues. Decrease in organ weights of the liver, adrenal
and thyroid occurred in the 15% group. There were no compound-
related histological effects (Cox & Babish, 1978a).
Special studies on teratogenicity
The anthocyanin glycosides (an extract from currants,
blueberries and elderberries) were reported not to be teratogenic
in rats, mice or rabbits when given at dose levels of 1.5, 3 or
9 g/kg over 3 successive generations (Pourrat et al., 1967).
Acute toxicity
LD50 Reference
Animal Route (mg/kg bw)
Mice i.p. 4 110 Pourrat et al., 1967
i.v. 840 Pourrat et al., 1967
Oral 25 000 Pourrat et al., 1967
Rats i.p. 2 850 Pourrat et al., 1967
i.v. 240 Pourrat et al., 1967
Oral 20 000 Pourrat et al., 1967
Test animals were administered the anthocyanins (cyanidin,
petunidin and delphinidin mixture extracted from currants,
blueberries and elderberries) in doses from 0 to 25 000 mg/kg bw
for mice and from 0 to 20 000 mg/kg for rats. Following i.v. or
i.p. administration, toxic doses of anthocyanins produced sedation,
convulsions and finally death.
Short-term studies
Weanling male and female Wistar rats (20/group) were fed a
diet containing anthocyanin extract at levels equivalent to
3000 mg/day or 6000 mg/day for a period of 90 days. A group of
concurrent controls were also used in the study. The doses of
anthocyanin administered were estimated to be 5 and 10 times,
respectively, the level that a human would ingest. No differences
were observed between the test animals and controls in survival,
growth or histopathology of the principal tissues at the
termination of the study (Pourrat et al., 1967).
In another study, guinea-pigs received 3000 mg/kg of
anthocyanin in the diet for 15 days. No adverse effects were
reported (Pourrat et al., 1967).
Male and female beagle dogs (4/sex/dose) received either 0,
7.5% or 15% of grape-skin extract (approximately 2.39% anthocyanin
by weight) in the diet for 90 days. No differences were noted
between control and treated animals in body weights, growth,
survival, clinical chemistries (haematology, biochemistry or
urinalysis), organ weights or pathological lesions (gross or
microscopic) (Cox & Babish, 1978a).
OBSERVATIONS IN MAN
Man is naturally exposed to anthocyanins through the ingestion
of fruits and vegetables. Levels of exposure under normal dietary
conditions have not been established.
Information on the metabolism and toxicity of the anthocyanins
is limited. Its interpretation is complicated because the
anthocyanins represent a large group of chemically-related
substances and the effect observed with one defined anthocyanin may
not be applicable to another. The available information suggests
that anthocyanins are poorly absorbed from the gastrointestinal
tract. Metabolism is limited and may be due to the activity of the
intestinal bacterial flora. The metabolites of anthocyanins have
not been identified. However, the insensitivity of the assay
techniques used for measuring unmetabolized anthocyanins may result
in a significant underestimate of the degree of absorption and
metabolism of the anthocyanins (Kuhnau, 1976).
Comments
Toxicological studies are limited, and have been carried out
with mixtures extracted from a variety of fruits. The available
data indicate that such extracts are of a very low order of
toxicity. Diets containing 7.5% or 15% of a grape-skin extract
preparation (approximately 3% anthocyanin) had no effect on the
reproductive performance of rats in a 2-generation reproductive
study. The lower body weights of offspring were related to a
concomitant decrease in food intake. At the highest level tested,
there was a decreased organ weight of the liver, adrenal and
thyroid. There were no compound-related histological effects. No
compound-related effects were observed in a short-term study in
which dogs were fed diets containing 7.5% or 15% of the grape-skin
extract preparation.
EVALUATION
Level causing no toxicological effect (Grape-skin extract
preparation)
Rat (young): 7.5% of the diet equivalent to 7500 mg/kg bw.
Estimate of acceptable daily intake for man
0-2.5 mg/kg bw.*
* Anthocyanins (present in the grape-skin preparation at level
of approximately 3%).
REFERENCES
Brown, J. P. & Dietrich, P. S. (1979) Mutagenicity of plant
flavonols in the Salmonella/mammalian microsome test,
Mutation Research, 66, 223-240
Carpenter, J. A., Wang, Y.-P. & Powers, J. J. (1967) Effects of
anthocyanin pigments on certain enzymes, Proc. Soc. Exptl.
Biol. Med., 124, 702-706
Clark, W. G. & Mackay, E. W. (1950) The absorption and excretion of
rutin and related flavanoid substances, J. Amer. Med.
Assoc., 143, 1411-1415
Cox, G. E. & Babish, J. C. (1978a) Evaluation of the safety of
dietary administration of special grape color powder (type
BW-AT) on reproduction, lactation and maturation when fed to
Sprague-Dawley rats. Unpublished report No. 5417 by Food and
Drug Research Laboratories, Inc., submitted to the World
Health Organization by FDA
Cox, G. E. & Babish, J. C. (1978b) A 90-day feeding study of
special grape color powder (type BW-AT) to Beagle dogs.
Unpublished report No. 5417 by Food and Drug Research
Laboratories, Inc., submitted to the World Health Organization
by FDA
Forrai, G. Vágújfalvi, D. & Bölcskey, P. (1968) Betaninuria in
childhood, Acta Paediatrica Academiae Scientiarum
Hungaricae, 9, 43-51
Francies, F. J. (1977) Anthocyanins. In: Furia, E., ed.,
Current aspects of foods colorants, Cleveland, Ohio, CRC
Press, pp. 19-28
Griffiths, L. A. & Smith, G. E. (1972a) Metabolism of myricetin and
related compounds in the rat. Metabolite formation in vivo
and by the intestinal microflora in vitro, Biochem. J.,
183, 141-151
Griffiths, L. A. & Smith, G. E. (1972b) Metabolism of apigenin and
related compounds in the rat, Biochem. J., 128, 901-911
Haveland-Smith, R. B. (1981) Evaluation of the genotoxicity of some
natural food colors using bacterial assays, Mutation
Research, 91, 285-290
Horwitt, K. M. (1933) Observations on behavior of the anthocyanin
pigment from concord grapes in the animal body, Proc. Soc.
Exptl. Biol. Med., 30, 949-951
Kuhnau, J. (1976) The flavanoids. A class of semi-essential food
components: their role in human nutrition, World Rev. Nutr.
Diet., 24, 117-191
Lietti, A. & Forni, G. (1976) Studies on Vaccinium myrtillus
anthocyanosides. II. Aspects of anthocyanin pharmacokinetics
in the rat, Arzneim-Forsch., 26
MacGregor, J. T. & Jurd, L. (1978) Mutagenicity of plant
flavanoids: Structural requirements for mutagenic activity in
Salmonella typhimurium, Mutation Research, 54, 297-309
Moudgal, N. R., Raghupathy, E. & Sarma, P. S. (1958) Studies on
goitrogenic agents in foods. III. Goitrogenic action of some
glycosides isolated from edible nuts, J. Nutr., 66,
291-303
Parkinson, T. M. & Brown, J. P. (1981) Metabolic fate of food
colorants, Ann. Rev. Nutr., 1, 175-205
Pourrat, H., Bastide, P., Dorier, P. & Tronche, P. (1967)
Préparation et activité thérapeutique de quelques glycosides
d'anthocyanes, Chim. Thérap., 2, 33-38
Scheline, R. R. (1968) The metabolism of drugs and other organic
compounds by the intestinal microflora, Acta Pharmacol. et
Toxicol., 26, 332-342
Scheline, R. R. (1978) Mammalian metabolism of plant xenobiotics,
New York, Academic Press
Singleton, V. L. & Esau, P. (1969) Phenolic substances in grapes
and wine and their significance. In: Chichester, C. O.,
Mrak, E. M. & Stewart, G. F., eds, Advances in food
research, New York, Academic Press, Suppl. 1, pp. 31-38
Somaatmadja, D., Powers, J. J. & Hamdy, M. K. (1964) Anthocyanins.
VI. Chelation studies on anthocyanins and other related
compounds, J. Food Sci., 29, 655-660
Varma, S. D. & Kinoshita, J. H. (1976) Inhibition of lens aldose
reductase by flavanoids - their possible role in the
prevention of diabetic cataracts, Biochem. Pharmacol., 25,
2505-2513
Zindler, G. A. & Colovos, G. C. (1950) Anthocyaninuria and beet
allergy, Ann. Allergy, 8, 603-617
