
WHO/Food Add./24.65
FAO Nutrition Meetings
Report Series No. 38A
SPECIFICATIONS FOR IDENTITY AND
PURITY AND TOXICOLOGICAL EVALUATION
OF SOME ANTIMICROBIALS AND
ANTIOXIDANTS
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met 8-17
December 1964a
a Eighth Report of the Joint FAO/WHO Expert Committee on Food
Additives, Wld Hlth Org. techn. Rep. Ser., 1965, 309; FAO
Nutrition Meetings Report Series 1965, 38.
DIPHENYL
CHEMICAL NAMES Biphenyl; phenylbenzene
EMPIRICAL FORMULA C12H10
STRUCTURAL FORMULA
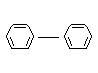 MOLECULAR WEIGHT 154.21
USE As a fungistatic agent in wrapping material to
inhibit growth of moulds on citrus fruits.
DESCRIPTION White or pale yellow to amber, crystalline
solid or leaflets having a characteristic
odour.
IDENTIFICATION TESTS
A. Solubility: Water: Insoluble
Ether: Soluble
Ethanol: Soluble
B. Melting point: About 69°
C. Boiling point: About 225°
PURITY TESTS
Arsenic: Not more than 3 mg/kg.
Lead: Not more than 10 mg/kg.
Solidification point: Not below 68.5°
Distillation range: It distils completely within a 2.5° range between
252.5° and 257.5°.
ASSAY
Required.
Biological Data
Biochemical aspects
Diphenyl is metabolized in rats1,2 and rabbits2 to various phenolic
compounds, mainly 4-hydroxydiphenyl which is excreted in the urine in
both free and conjugated form (ethereal sulfate and glucuronate).
This demonstration of a common mechanism in 2 species of animals
suggests that human metabolism would follow similar paths, but no
report of this has been found in the literature.
Diphenyl was found to be less toxic when given with a diet containing
a supplement of 1-cystine or dl-methionine, but diphenyl is not highly
conjugated with cystine.
Acute toxicity
Animal Route LD50 References
(mg/kg body-weight)
Rat oral 3500-5000 3,4
Rabbit oral 2400 3
Short-term studies
Rabbit. Five rabbits were each given doses of 1 g/kg body-weight by
stomach tube in a 25% dilution in olive oil 2 or 3 times a week until
they died. Deaths occurred within 5 to 20 weeks. The blood picture
showed no significant change, but the gain in body-weight was less
than in the controls, and retention of urea occurred near the terminal
stage of intoxication.3
Rat. Eleven young female rats were fed for 32 days on a diet low in
casein and containing 1% diphenyl. A marked growth retardation was
observed. Histological examination of 2 animals showed the beginning
of fatty changes in the liver and mild toxic changes in the kidney.1
In a small group of weanling rats fed with a diet containing 0.3%
diphenyl, weight increase was less than in control animals.5
Administration of diphenyl to young rats for 4 weeks in doses of 2, 20
and 200 mg/kg body-weight per day induced neither growth retardation
nor significant change in the blood picture.6
Oral administration of 0.3 mg/kg body-weight daily to 10 rats for 12
days did not induce growth retardation.7
Groups of young rats were fed for 3 months on rations containing 0%,
0.01% and 0.1% of diphenyl. No significant differences were observed
in the growth rates, food efficiency, organ weights, blood urea or
histology of tissues between treated and control rats. At the level
of 0.1% only a slight polyuria was noted.8
Monkey. Small groups of monkeys were fed for a year on rations
containing 0.01%, 0.1% and 1% diphenyl. The only significant change
was a slight increase in weight of the liver at the intake level of
1%.8
Dog. Nine dogs divided into 3 groups received diphenyl in corn oil
daily at the rate of 0, 2.5 and 25 mg/kg body-weight 5 days a week for
52 weeks. One dog on 2.5 mg/kg and 1 on 25 mg/kg lost about 1 kg in
weight each. The other animals gained in weight. Blood and urine
were unchanged. No gross or microscopic pathological changes were
found in any of the tissues examined.9
Long-term studies
Rat. A long-term feeding experiment with rats made use of dietary
levels of 0.01%, 0.1% and 1% diphenyl for a 2-year period.8
Unfortunately this study failed to supply the desired information for
two reasons: (a) a severe respiratory infection caused a high
mortality among the controls; (b) rats receiving 0.1% and 1% diphenyl
exhibited tubular dilatation of the kidneys, as did two of the control
rats.
Groups of 15 weanling rats of each sex were fed 0.001%, 0.005%, 0.01%,
0.05%, O.1%, O.5% and 1% diphenyl in the diet. Growth was inhibited
particularly at the 0.5% and 1% levels, apparently because of
decreased food consumption daring the first 100 days of the test.
During 750 days there was a decrease in longevity in rats on the 0.5%
and 1% diets. At the other levels there was no significant evidence
of toxicity. With the diet containing 1% diphenyl, haemoglobin values
of male end female rats were lower in comparison with controls when
measured at 300 and 400 days respectively. The histopathological
changes observed in the kidneys of rats on the 0.5% and 1% diets were
irregular scarring, lymphocytic infiltration, tubular atrophy and
patchy tubular dilatation to the point of cyst formation in
association with hydronephrosis and sometimes albuminuria and
haemoglobinuria. These phenomena did not occur at a concentration of
O.1% diphenyl or less.10
In another long-term study, the onset end reversibility of kidney
damage in rats on diets containing 0.1%, 0.25% and 0.5% diphenyl were
investigated, and the findings quoted under reference 10 were
confirmed.11
In one experiment on 13 rats fed daily with a diet containing 0.025%
to 0.05% diphenyl, after 2 months 1 animal had squamous cell carcinoma
and 2 had papillomas of the forestomach.4
Comment on experimental studies reported
Most of the studies reported have been carried out in dogs and rats.
The value of negative results of short-term studies in groups of 3
dogs at levels of 2.5 and 25 mg/kg body-weight is limited.9
Long-term studies in rats provide a basis for evaluation.10,11
Metabolic studies on 2 species reveal a common mechanism of
detoxication, but no data are available on metabolism in man.
Evaluation
Level causing no significant toxicological effect in the rat
From consideration of the long-term studies in rats, the level causing
no significant toxicological effect when ingested over a period
approximating to the life span nay be assessed at 0.1%.
0.05% (= 500 ppm) in the diet is equivalent to 25 mg/kg body-weight
per day.
Estimate of acceptable daily intakes for man
mg/kg body-weight
Unconditional acceptance 0-0.05
Conditional acceptance 0.05-0.25
Comment
Diphenyl presents certain peculiar problems since it is primarily used
as a treatment for wrappers for fruit. Nevertheless, diphenyl
penetrates into the skin of the fruit and may consequently be included
in food or drink prepared from it. Fruit drinks are commonly consumed
in considerable quantities by children and by sick people. For these
reasons caution should be exercised and the pattern of use should be
closely studied.
Further Work Considered Desirable
1. Long-term studies in a species other than the rat, with careful
evaluation of tumour incidence.
2. Biochemical studies in man.
References
1. West, H. D. (1940) Proc. Soc. exp. Biol. (N.Y.), 43, 373
2. West, H. D., Lawson, J. R., Miller, I. H. & Mathura, R. (1955)
Fed. Proc., 140, 303
3. Deichmann, W. B., Kitzmiller, K. V., Dierker, M. & Witherup, S.
(1947) J. industr. Hyg., 29, 1
4. Pecehiai, L. & Saffiotei, U. (1957) Med. d. lavoro, 48, 247
5. West, H. D. & Jefferson, N. C. (1942) J. Nutr., 23, 425
6. MacIntosh, F. C. (1945) Analyst 70, 334
7. Rogliani, E. & Procaccini, S. (1956) Biochim. appl., 3, 193
8. Newell, G. W. (1953) Toxicological study of diphenyl in citrus
wraps (Report 1953 of the Standford Research Institute)
9. Hazleton. L. H., Kundzins, N., Howard, J. W. & Johnson, C. D.
(1956) Studies on diphenyl in the dog. In: XXth International
Physiological Congress, Brussels, July 29 - August 4, 1956. Abstracts
of communications, p. 412
10. Ambrose, A. M., Booth, A. N., DeEds, F. & Cox, A. J. (1960) Food
Res., 25, 328
11. Booth, A. N., Ambrose, A. M. & DeEds, F. (1956) Fed. Proc., 15,
403
MOLECULAR WEIGHT 154.21
USE As a fungistatic agent in wrapping material to
inhibit growth of moulds on citrus fruits.
DESCRIPTION White or pale yellow to amber, crystalline
solid or leaflets having a characteristic
odour.
IDENTIFICATION TESTS
A. Solubility: Water: Insoluble
Ether: Soluble
Ethanol: Soluble
B. Melting point: About 69°
C. Boiling point: About 225°
PURITY TESTS
Arsenic: Not more than 3 mg/kg.
Lead: Not more than 10 mg/kg.
Solidification point: Not below 68.5°
Distillation range: It distils completely within a 2.5° range between
252.5° and 257.5°.
ASSAY
Required.
Biological Data
Biochemical aspects
Diphenyl is metabolized in rats1,2 and rabbits2 to various phenolic
compounds, mainly 4-hydroxydiphenyl which is excreted in the urine in
both free and conjugated form (ethereal sulfate and glucuronate).
This demonstration of a common mechanism in 2 species of animals
suggests that human metabolism would follow similar paths, but no
report of this has been found in the literature.
Diphenyl was found to be less toxic when given with a diet containing
a supplement of 1-cystine or dl-methionine, but diphenyl is not highly
conjugated with cystine.
Acute toxicity
Animal Route LD50 References
(mg/kg body-weight)
Rat oral 3500-5000 3,4
Rabbit oral 2400 3
Short-term studies
Rabbit. Five rabbits were each given doses of 1 g/kg body-weight by
stomach tube in a 25% dilution in olive oil 2 or 3 times a week until
they died. Deaths occurred within 5 to 20 weeks. The blood picture
showed no significant change, but the gain in body-weight was less
than in the controls, and retention of urea occurred near the terminal
stage of intoxication.3
Rat. Eleven young female rats were fed for 32 days on a diet low in
casein and containing 1% diphenyl. A marked growth retardation was
observed. Histological examination of 2 animals showed the beginning
of fatty changes in the liver and mild toxic changes in the kidney.1
In a small group of weanling rats fed with a diet containing 0.3%
diphenyl, weight increase was less than in control animals.5
Administration of diphenyl to young rats for 4 weeks in doses of 2, 20
and 200 mg/kg body-weight per day induced neither growth retardation
nor significant change in the blood picture.6
Oral administration of 0.3 mg/kg body-weight daily to 10 rats for 12
days did not induce growth retardation.7
Groups of young rats were fed for 3 months on rations containing 0%,
0.01% and 0.1% of diphenyl. No significant differences were observed
in the growth rates, food efficiency, organ weights, blood urea or
histology of tissues between treated and control rats. At the level
of 0.1% only a slight polyuria was noted.8
Monkey. Small groups of monkeys were fed for a year on rations
containing 0.01%, 0.1% and 1% diphenyl. The only significant change
was a slight increase in weight of the liver at the intake level of
1%.8
Dog. Nine dogs divided into 3 groups received diphenyl in corn oil
daily at the rate of 0, 2.5 and 25 mg/kg body-weight 5 days a week for
52 weeks. One dog on 2.5 mg/kg and 1 on 25 mg/kg lost about 1 kg in
weight each. The other animals gained in weight. Blood and urine
were unchanged. No gross or microscopic pathological changes were
found in any of the tissues examined.9
Long-term studies
Rat. A long-term feeding experiment with rats made use of dietary
levels of 0.01%, 0.1% and 1% diphenyl for a 2-year period.8
Unfortunately this study failed to supply the desired information for
two reasons: (a) a severe respiratory infection caused a high
mortality among the controls; (b) rats receiving 0.1% and 1% diphenyl
exhibited tubular dilatation of the kidneys, as did two of the control
rats.
Groups of 15 weanling rats of each sex were fed 0.001%, 0.005%, 0.01%,
0.05%, O.1%, O.5% and 1% diphenyl in the diet. Growth was inhibited
particularly at the 0.5% and 1% levels, apparently because of
decreased food consumption daring the first 100 days of the test.
During 750 days there was a decrease in longevity in rats on the 0.5%
and 1% diets. At the other levels there was no significant evidence
of toxicity. With the diet containing 1% diphenyl, haemoglobin values
of male end female rats were lower in comparison with controls when
measured at 300 and 400 days respectively. The histopathological
changes observed in the kidneys of rats on the 0.5% and 1% diets were
irregular scarring, lymphocytic infiltration, tubular atrophy and
patchy tubular dilatation to the point of cyst formation in
association with hydronephrosis and sometimes albuminuria and
haemoglobinuria. These phenomena did not occur at a concentration of
O.1% diphenyl or less.10
In another long-term study, the onset end reversibility of kidney
damage in rats on diets containing 0.1%, 0.25% and 0.5% diphenyl were
investigated, and the findings quoted under reference 10 were
confirmed.11
In one experiment on 13 rats fed daily with a diet containing 0.025%
to 0.05% diphenyl, after 2 months 1 animal had squamous cell carcinoma
and 2 had papillomas of the forestomach.4
Comment on experimental studies reported
Most of the studies reported have been carried out in dogs and rats.
The value of negative results of short-term studies in groups of 3
dogs at levels of 2.5 and 25 mg/kg body-weight is limited.9
Long-term studies in rats provide a basis for evaluation.10,11
Metabolic studies on 2 species reveal a common mechanism of
detoxication, but no data are available on metabolism in man.
Evaluation
Level causing no significant toxicological effect in the rat
From consideration of the long-term studies in rats, the level causing
no significant toxicological effect when ingested over a period
approximating to the life span nay be assessed at 0.1%.
0.05% (= 500 ppm) in the diet is equivalent to 25 mg/kg body-weight
per day.
Estimate of acceptable daily intakes for man
mg/kg body-weight
Unconditional acceptance 0-0.05
Conditional acceptance 0.05-0.25
Comment
Diphenyl presents certain peculiar problems since it is primarily used
as a treatment for wrappers for fruit. Nevertheless, diphenyl
penetrates into the skin of the fruit and may consequently be included
in food or drink prepared from it. Fruit drinks are commonly consumed
in considerable quantities by children and by sick people. For these
reasons caution should be exercised and the pattern of use should be
closely studied.
Further Work Considered Desirable
1. Long-term studies in a species other than the rat, with careful
evaluation of tumour incidence.
2. Biochemical studies in man.
References
1. West, H. D. (1940) Proc. Soc. exp. Biol. (N.Y.), 43, 373
2. West, H. D., Lawson, J. R., Miller, I. H. & Mathura, R. (1955)
Fed. Proc., 140, 303
3. Deichmann, W. B., Kitzmiller, K. V., Dierker, M. & Witherup, S.
(1947) J. industr. Hyg., 29, 1
4. Pecehiai, L. & Saffiotei, U. (1957) Med. d. lavoro, 48, 247
5. West, H. D. & Jefferson, N. C. (1942) J. Nutr., 23, 425
6. MacIntosh, F. C. (1945) Analyst 70, 334
7. Rogliani, E. & Procaccini, S. (1956) Biochim. appl., 3, 193
8. Newell, G. W. (1953) Toxicological study of diphenyl in citrus
wraps (Report 1953 of the Standford Research Institute)
9. Hazleton. L. H., Kundzins, N., Howard, J. W. & Johnson, C. D.
(1956) Studies on diphenyl in the dog. In: XXth International
Physiological Congress, Brussels, July 29 - August 4, 1956. Abstracts
of communications, p. 412
10. Ambrose, A. M., Booth, A. N., DeEds, F. & Cox, A. J. (1960) Food
Res., 25, 328
11. Booth, A. N., Ambrose, A. M. & DeEds, F. (1956) Fed. Proc., 15,
403
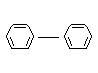 MOLECULAR WEIGHT 154.21
USE As a fungistatic agent in wrapping material to
inhibit growth of moulds on citrus fruits.
DESCRIPTION White or pale yellow to amber, crystalline
solid or leaflets having a characteristic
odour.
IDENTIFICATION TESTS
A. Solubility: Water: Insoluble
Ether: Soluble
Ethanol: Soluble
B. Melting point: About 69°
C. Boiling point: About 225°
PURITY TESTS
Arsenic: Not more than 3 mg/kg.
Lead: Not more than 10 mg/kg.
Solidification point: Not below 68.5°
Distillation range: It distils completely within a 2.5° range between
252.5° and 257.5°.
ASSAY
Required.
Biological Data
Biochemical aspects
Diphenyl is metabolized in rats1,2 and rabbits2 to various phenolic
compounds, mainly 4-hydroxydiphenyl which is excreted in the urine in
both free and conjugated form (ethereal sulfate and glucuronate).
This demonstration of a common mechanism in 2 species of animals
suggests that human metabolism would follow similar paths, but no
report of this has been found in the literature.
Diphenyl was found to be less toxic when given with a diet containing
a supplement of 1-cystine or dl-methionine, but diphenyl is not highly
conjugated with cystine.
Acute toxicity
Animal Route LD50 References
(mg/kg body-weight)
Rat oral 3500-5000 3,4
Rabbit oral 2400 3
Short-term studies
Rabbit. Five rabbits were each given doses of 1 g/kg body-weight by
stomach tube in a 25% dilution in olive oil 2 or 3 times a week until
they died. Deaths occurred within 5 to 20 weeks. The blood picture
showed no significant change, but the gain in body-weight was less
than in the controls, and retention of urea occurred near the terminal
stage of intoxication.3
Rat. Eleven young female rats were fed for 32 days on a diet low in
casein and containing 1% diphenyl. A marked growth retardation was
observed. Histological examination of 2 animals showed the beginning
of fatty changes in the liver and mild toxic changes in the kidney.1
In a small group of weanling rats fed with a diet containing 0.3%
diphenyl, weight increase was less than in control animals.5
Administration of diphenyl to young rats for 4 weeks in doses of 2, 20
and 200 mg/kg body-weight per day induced neither growth retardation
nor significant change in the blood picture.6
Oral administration of 0.3 mg/kg body-weight daily to 10 rats for 12
days did not induce growth retardation.7
Groups of young rats were fed for 3 months on rations containing 0%,
0.01% and 0.1% of diphenyl. No significant differences were observed
in the growth rates, food efficiency, organ weights, blood urea or
histology of tissues between treated and control rats. At the level
of 0.1% only a slight polyuria was noted.8
Monkey. Small groups of monkeys were fed for a year on rations
containing 0.01%, 0.1% and 1% diphenyl. The only significant change
was a slight increase in weight of the liver at the intake level of
1%.8
Dog. Nine dogs divided into 3 groups received diphenyl in corn oil
daily at the rate of 0, 2.5 and 25 mg/kg body-weight 5 days a week for
52 weeks. One dog on 2.5 mg/kg and 1 on 25 mg/kg lost about 1 kg in
weight each. The other animals gained in weight. Blood and urine
were unchanged. No gross or microscopic pathological changes were
found in any of the tissues examined.9
Long-term studies
Rat. A long-term feeding experiment with rats made use of dietary
levels of 0.01%, 0.1% and 1% diphenyl for a 2-year period.8
Unfortunately this study failed to supply the desired information for
two reasons: (a) a severe respiratory infection caused a high
mortality among the controls; (b) rats receiving 0.1% and 1% diphenyl
exhibited tubular dilatation of the kidneys, as did two of the control
rats.
Groups of 15 weanling rats of each sex were fed 0.001%, 0.005%, 0.01%,
0.05%, O.1%, O.5% and 1% diphenyl in the diet. Growth was inhibited
particularly at the 0.5% and 1% levels, apparently because of
decreased food consumption daring the first 100 days of the test.
During 750 days there was a decrease in longevity in rats on the 0.5%
and 1% diets. At the other levels there was no significant evidence
of toxicity. With the diet containing 1% diphenyl, haemoglobin values
of male end female rats were lower in comparison with controls when
measured at 300 and 400 days respectively. The histopathological
changes observed in the kidneys of rats on the 0.5% and 1% diets were
irregular scarring, lymphocytic infiltration, tubular atrophy and
patchy tubular dilatation to the point of cyst formation in
association with hydronephrosis and sometimes albuminuria and
haemoglobinuria. These phenomena did not occur at a concentration of
O.1% diphenyl or less.10
In another long-term study, the onset end reversibility of kidney
damage in rats on diets containing 0.1%, 0.25% and 0.5% diphenyl were
investigated, and the findings quoted under reference 10 were
confirmed.11
In one experiment on 13 rats fed daily with a diet containing 0.025%
to 0.05% diphenyl, after 2 months 1 animal had squamous cell carcinoma
and 2 had papillomas of the forestomach.4
Comment on experimental studies reported
Most of the studies reported have been carried out in dogs and rats.
The value of negative results of short-term studies in groups of 3
dogs at levels of 2.5 and 25 mg/kg body-weight is limited.9
Long-term studies in rats provide a basis for evaluation.10,11
Metabolic studies on 2 species reveal a common mechanism of
detoxication, but no data are available on metabolism in man.
Evaluation
Level causing no significant toxicological effect in the rat
From consideration of the long-term studies in rats, the level causing
no significant toxicological effect when ingested over a period
approximating to the life span nay be assessed at 0.1%.
0.05% (= 500 ppm) in the diet is equivalent to 25 mg/kg body-weight
per day.
Estimate of acceptable daily intakes for man
mg/kg body-weight
Unconditional acceptance 0-0.05
Conditional acceptance 0.05-0.25
Comment
Diphenyl presents certain peculiar problems since it is primarily used
as a treatment for wrappers for fruit. Nevertheless, diphenyl
penetrates into the skin of the fruit and may consequently be included
in food or drink prepared from it. Fruit drinks are commonly consumed
in considerable quantities by children and by sick people. For these
reasons caution should be exercised and the pattern of use should be
closely studied.
Further Work Considered Desirable
1. Long-term studies in a species other than the rat, with careful
evaluation of tumour incidence.
2. Biochemical studies in man.
References
1. West, H. D. (1940) Proc. Soc. exp. Biol. (N.Y.), 43, 373
2. West, H. D., Lawson, J. R., Miller, I. H. & Mathura, R. (1955)
Fed. Proc., 140, 303
3. Deichmann, W. B., Kitzmiller, K. V., Dierker, M. & Witherup, S.
(1947) J. industr. Hyg., 29, 1
4. Pecehiai, L. & Saffiotei, U. (1957) Med. d. lavoro, 48, 247
5. West, H. D. & Jefferson, N. C. (1942) J. Nutr., 23, 425
6. MacIntosh, F. C. (1945) Analyst 70, 334
7. Rogliani, E. & Procaccini, S. (1956) Biochim. appl., 3, 193
8. Newell, G. W. (1953) Toxicological study of diphenyl in citrus
wraps (Report 1953 of the Standford Research Institute)
9. Hazleton. L. H., Kundzins, N., Howard, J. W. & Johnson, C. D.
(1956) Studies on diphenyl in the dog. In: XXth International
Physiological Congress, Brussels, July 29 - August 4, 1956. Abstracts
of communications, p. 412
10. Ambrose, A. M., Booth, A. N., DeEds, F. & Cox, A. J. (1960) Food
Res., 25, 328
11. Booth, A. N., Ambrose, A. M. & DeEds, F. (1956) Fed. Proc., 15,
403
MOLECULAR WEIGHT 154.21
USE As a fungistatic agent in wrapping material to
inhibit growth of moulds on citrus fruits.
DESCRIPTION White or pale yellow to amber, crystalline
solid or leaflets having a characteristic
odour.
IDENTIFICATION TESTS
A. Solubility: Water: Insoluble
Ether: Soluble
Ethanol: Soluble
B. Melting point: About 69°
C. Boiling point: About 225°
PURITY TESTS
Arsenic: Not more than 3 mg/kg.
Lead: Not more than 10 mg/kg.
Solidification point: Not below 68.5°
Distillation range: It distils completely within a 2.5° range between
252.5° and 257.5°.
ASSAY
Required.
Biological Data
Biochemical aspects
Diphenyl is metabolized in rats1,2 and rabbits2 to various phenolic
compounds, mainly 4-hydroxydiphenyl which is excreted in the urine in
both free and conjugated form (ethereal sulfate and glucuronate).
This demonstration of a common mechanism in 2 species of animals
suggests that human metabolism would follow similar paths, but no
report of this has been found in the literature.
Diphenyl was found to be less toxic when given with a diet containing
a supplement of 1-cystine or dl-methionine, but diphenyl is not highly
conjugated with cystine.
Acute toxicity
Animal Route LD50 References
(mg/kg body-weight)
Rat oral 3500-5000 3,4
Rabbit oral 2400 3
Short-term studies
Rabbit. Five rabbits were each given doses of 1 g/kg body-weight by
stomach tube in a 25% dilution in olive oil 2 or 3 times a week until
they died. Deaths occurred within 5 to 20 weeks. The blood picture
showed no significant change, but the gain in body-weight was less
than in the controls, and retention of urea occurred near the terminal
stage of intoxication.3
Rat. Eleven young female rats were fed for 32 days on a diet low in
casein and containing 1% diphenyl. A marked growth retardation was
observed. Histological examination of 2 animals showed the beginning
of fatty changes in the liver and mild toxic changes in the kidney.1
In a small group of weanling rats fed with a diet containing 0.3%
diphenyl, weight increase was less than in control animals.5
Administration of diphenyl to young rats for 4 weeks in doses of 2, 20
and 200 mg/kg body-weight per day induced neither growth retardation
nor significant change in the blood picture.6
Oral administration of 0.3 mg/kg body-weight daily to 10 rats for 12
days did not induce growth retardation.7
Groups of young rats were fed for 3 months on rations containing 0%,
0.01% and 0.1% of diphenyl. No significant differences were observed
in the growth rates, food efficiency, organ weights, blood urea or
histology of tissues between treated and control rats. At the level
of 0.1% only a slight polyuria was noted.8
Monkey. Small groups of monkeys were fed for a year on rations
containing 0.01%, 0.1% and 1% diphenyl. The only significant change
was a slight increase in weight of the liver at the intake level of
1%.8
Dog. Nine dogs divided into 3 groups received diphenyl in corn oil
daily at the rate of 0, 2.5 and 25 mg/kg body-weight 5 days a week for
52 weeks. One dog on 2.5 mg/kg and 1 on 25 mg/kg lost about 1 kg in
weight each. The other animals gained in weight. Blood and urine
were unchanged. No gross or microscopic pathological changes were
found in any of the tissues examined.9
Long-term studies
Rat. A long-term feeding experiment with rats made use of dietary
levels of 0.01%, 0.1% and 1% diphenyl for a 2-year period.8
Unfortunately this study failed to supply the desired information for
two reasons: (a) a severe respiratory infection caused a high
mortality among the controls; (b) rats receiving 0.1% and 1% diphenyl
exhibited tubular dilatation of the kidneys, as did two of the control
rats.
Groups of 15 weanling rats of each sex were fed 0.001%, 0.005%, 0.01%,
0.05%, O.1%, O.5% and 1% diphenyl in the diet. Growth was inhibited
particularly at the 0.5% and 1% levels, apparently because of
decreased food consumption daring the first 100 days of the test.
During 750 days there was a decrease in longevity in rats on the 0.5%
and 1% diets. At the other levels there was no significant evidence
of toxicity. With the diet containing 1% diphenyl, haemoglobin values
of male end female rats were lower in comparison with controls when
measured at 300 and 400 days respectively. The histopathological
changes observed in the kidneys of rats on the 0.5% and 1% diets were
irregular scarring, lymphocytic infiltration, tubular atrophy and
patchy tubular dilatation to the point of cyst formation in
association with hydronephrosis and sometimes albuminuria and
haemoglobinuria. These phenomena did not occur at a concentration of
O.1% diphenyl or less.10
In another long-term study, the onset end reversibility of kidney
damage in rats on diets containing 0.1%, 0.25% and 0.5% diphenyl were
investigated, and the findings quoted under reference 10 were
confirmed.11
In one experiment on 13 rats fed daily with a diet containing 0.025%
to 0.05% diphenyl, after 2 months 1 animal had squamous cell carcinoma
and 2 had papillomas of the forestomach.4
Comment on experimental studies reported
Most of the studies reported have been carried out in dogs and rats.
The value of negative results of short-term studies in groups of 3
dogs at levels of 2.5 and 25 mg/kg body-weight is limited.9
Long-term studies in rats provide a basis for evaluation.10,11
Metabolic studies on 2 species reveal a common mechanism of
detoxication, but no data are available on metabolism in man.
Evaluation
Level causing no significant toxicological effect in the rat
From consideration of the long-term studies in rats, the level causing
no significant toxicological effect when ingested over a period
approximating to the life span nay be assessed at 0.1%.
0.05% (= 500 ppm) in the diet is equivalent to 25 mg/kg body-weight
per day.
Estimate of acceptable daily intakes for man
mg/kg body-weight
Unconditional acceptance 0-0.05
Conditional acceptance 0.05-0.25
Comment
Diphenyl presents certain peculiar problems since it is primarily used
as a treatment for wrappers for fruit. Nevertheless, diphenyl
penetrates into the skin of the fruit and may consequently be included
in food or drink prepared from it. Fruit drinks are commonly consumed
in considerable quantities by children and by sick people. For these
reasons caution should be exercised and the pattern of use should be
closely studied.
Further Work Considered Desirable
1. Long-term studies in a species other than the rat, with careful
evaluation of tumour incidence.
2. Biochemical studies in man.
References
1. West, H. D. (1940) Proc. Soc. exp. Biol. (N.Y.), 43, 373
2. West, H. D., Lawson, J. R., Miller, I. H. & Mathura, R. (1955)
Fed. Proc., 140, 303
3. Deichmann, W. B., Kitzmiller, K. V., Dierker, M. & Witherup, S.
(1947) J. industr. Hyg., 29, 1
4. Pecehiai, L. & Saffiotei, U. (1957) Med. d. lavoro, 48, 247
5. West, H. D. & Jefferson, N. C. (1942) J. Nutr., 23, 425
6. MacIntosh, F. C. (1945) Analyst 70, 334
7. Rogliani, E. & Procaccini, S. (1956) Biochim. appl., 3, 193
8. Newell, G. W. (1953) Toxicological study of diphenyl in citrus
wraps (Report 1953 of the Standford Research Institute)
9. Hazleton. L. H., Kundzins, N., Howard, J. W. & Johnson, C. D.
(1956) Studies on diphenyl in the dog. In: XXth International
Physiological Congress, Brussels, July 29 - August 4, 1956. Abstracts
of communications, p. 412
10. Ambrose, A. M., Booth, A. N., DeEds, F. & Cox, A. J. (1960) Food
Res., 25, 328
11. Booth, A. N., Ambrose, A. M. & DeEds, F. (1956) Fed. Proc., 15,
403
