
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
TOXICOLOGICAL EVALUATION OF CERTAIN
VETERINARY DRUG RESIDUES IN FOOD
WHO FOOD ADDITIVES SERIES 45
Prepared by the
Fifty-fourth meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 2000
DICYCLANIL
First draft prepared by
M.E.J. Pronk and G.J. Schefferlie
Centre for Substances and Risk Assessment,
National Institute of Public Health and the Environment,
Bilthoven, The Netherlands
Explanation
Biological data
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration studies
Developmental toxicity
Special studies: Pharmacological effects
Comments
Evaluation
References
1. EXPLANATION
Dicyclanil is a pyrimidine-derived regulator of insect growth
used for topical treatment of sheep to prevent larval infestation by
the blowfly (Lucilia cuprina). It is used as a pour-on formulation
containing 5% (w/v) of the drug. Data were provided on the use of
dicyclanil applied as a pour-on formulation to sheep at a maximum dose
of 0.1 g/kg bw. Dicyclanil has not previously been evaluated by the
Committee.
The chemical name of dicyclanil (CAS No. 112636-83-6) is
4,6-diamino-2-cyclopropylamino-pyrimidine-5-carbonitrile. The purity
of the technical-grade material used in the pivotal studies of
toxicity and pharmacology was 94.3%, unless otherwise stated.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Five groups of three male Han Wistar rats received a single dose
of 50 mg/kg bw technical-grade dicyclanil in polyethylene glycol
200:ethanol (5:3 v/v) by oral gavage. Blood samples were taken from
one group each at 2, 4, 8, 16, and 24 h after administration. The
maximum concentration of dicyclanil in plasma, approximately 20 µg/ml,
was found in the animals sampled at 8 h. The concentration in plasma
from animals sampled at 24 h ranged from 1.6 to 3.6 µg/ml. The study
was of unconventional design, without GLP or quality assurance
certification. The pharmacokinetics of dicyclanil could not be
calculated (Dubach-Powell, 1996).
[2-14C]Pyrimidyl-labelled dicyclanil (purity, 98%) in
polyethylene glycol 200: ethanol (5:3 v/v) was administered to
Tif:RAIf (SPF) rats by oral gavage for 7 consecutive days at a dose of
0.5 or 20 mg/kg bw per day. Urine and faeces were collected throughout
treatment, until the time of death. Three rats of each sex per dose
were killed 24 and 72 h after the last dose, and abdominal fat,
kidneys, liver, skeletal muscle, plasma, whole blood, gastrointestinal
tract with contents, and the residual carcass were taken for analysis.
All samples were analysed for radiolabel by liquid scintillation
counting. The study was certified for compliance with GLP and quality
assurance.
On the basis of the radiolabel in urine and tissues, absorption
from the gastrointestinal tract represented 80-85% of the administered
dose. Within 24 h after the last dose, 93-96% of the total dose had
been excreted, predominantly via the urine (79-83%) and to a lesser
extent via the faeces (6-12%). During the following 48 h, only an
additional 2-3% was excreted, indicating rapid elimination of the
absorbed material. The concentration of radiolabel in tissues 24 h
after the final dose of 0.5 mg/kg bw was < 4 µg/kg as dicyclanil
equivalents, except in liver (270 µg/kg), blood (170 µg/kg), kidneys
(37 µg/kg), and residual carcass (23 µg/kg). At 72 h, these
concentrations had declined to 40-80% of their values at 24 h, except
in blood, where the levels declined very slowly. The radiolabel in
blood was associated with erythrocytes. The concentrations of residues
in tissue were proportional to the dose. No differences were observed
between males and females (Hassler, 1994).
2.1.2 Biotransformation
In the study of Hassler (1994) described above, the metabolites
in urine, faeces, and tissues were characterized by thin-layer
chromatography and those in urine and faeces also by high-performance
liquid chromatography. Further investigations on the same samples
included solid-phase extraction, high-voltage electrophoresis, and
nuclear magnetic resonance, infrared, and mass spectroscopy. This
study was certified for compliance with GLP and quality assurance
(Thanei, 1996a).
The metabolic pattern in urine, faeces, and selected tissues
constituted up to 12 metabolite fractions and was essentially
independent of dose and sex. One major fraction, representing 48-54%
of the total dose, dominated the urinary metabolite pattern and was
identified as N-(4,6-diamino-5-cyano-pyrimidin-2-yl)propionamide.
The parent compound was also found in urine, where it accounted for
2-7% of the total dose. Other identified urinary metabolites were
2,4,6-triaminopyrimidin-5-carbonitrile (9-10%),
3-(4,6-diamino-5-cyanopyrimidin-2-ylamino)propionic acid (4-10%), and
2-(4,6-diamino-5-cyanopyrimidin-2-ylamino)-3-hydroxypropionic acid
(1-3%). All these metabolites were also identified in faeces, but at
markedly lower concentrations, each fraction representing < 3% of the
total dose. The parent compound accounted for approximately 1% of the
total dose in faeces. Besides polar metabolites,
2,4,6-triaminopyrimidin-5-carbonitrile was the major metabolite in
liver and kidneys, with smaller amounts of parent compound and
probably N-(4,6-diamino-5-cyanopyrimidin-2-yl)propionamide. A
similar but quantitatively different metabolic pattern was observed in
muscle and fat, which contained more nonpolar metabolites (most
pronounced in fat) (Hassler, 1994; Thanei, 1996a).
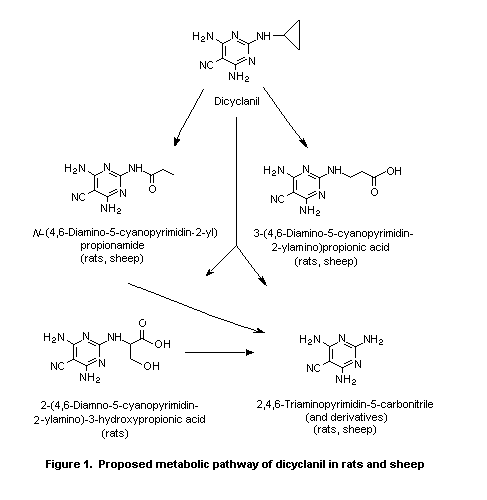
Biotransformation of dicyclanil in rats is limited to the
cyclopropyl ring, while the cyano group is metabolically stable.
Biotransformation in rats involves oxidative opening of the
cyclopropyl ring at various positions, followed by further oxidation
and cleavage of the cyclopropyl- N-bond (i.e. dealkylation). Most of
the metabolites are the result of more than one transformation. The
metabolic pathways of dicyclanil in sheep treated topically are
essentially the same as those in rats (Phillips, 1996; Thanei, 1996b;
Löffler, 1998). The proposed metabolic pathway of dicyclanil in rats
and sheep is given in Figure 1.
2.2 Toxicological studies
2.2.1 Acute toxicity
In studies with technical-grade dicyclanil in distilled water
containing 0.5% carboxymethylcellulose in 0.1% aqueous polysorbate 80
in male and female Tif:RAIf (SPF) rats, one study that followed OECD
test guideline 401 with GLP and quality assurance certification showed
an LD50 value after oral administration of 560 mg/kg bw in males and
of approximately 500 mg/kg bw in females. Common signs of toxicity
observed were piloerection, hunched posture, and dyspnoea. All animals
had reduced locomotor activity, with ataxia in some males. At
necropsy, involuted testes were found in two males that had received
200 mg/kg bw dicyclanil (Hartmann, 1992a). In a study that followed
OECD test guideline 402 with GLP and quality assurance certification,
the LD50 after dermal application was > 2000 mg/kg bw in both males
and females. The only signs of toxicity observed were piloerection and
hunched posture (Hartmann, 1992b). In a study that followed OECD test
guideline 403 with GLP and quality assurance certification, rats of
the same strain were exposed by nose only to a dicyclanil aerosol for
4 h (minimal mean aerodynamic diameter, 0.9-2.5 µm). The LC50 after
exposure by inhalation was 3400 mg/m3 for males and 3000 mg/m3 for
females. The toxic signs observed were piloerection, hunched posture,
dyspnoea, and reduced locomotor activity. Spotted lungs were found in
animals exposed to higher doses of dicyclanil, and abdominal
distention was found in males that survived the high dose (Hartmann,
1993). In all studies, the surviving animals recovered within 2-12
days.
 Three male New Zealand white rabbits (Chbb:NZW) received a
semi-occlusive topical application of 0.5 g of technical-grade
dicyclanil moistened with distilled water containing 0.5%
carboxymethylcellulose in 0.1% aqueous polysorbate 80 to their shaved
flanks. The study followed OECD test guideline 404, with GLP and
quality assurance certification. Only very slight erythema was
observed 1 ( n = 3) to 24 h ( n = 1) after removal of the patch
(Hagemann, 1992a).
In a study that followed OECD test guideline 405 with GLP and
quality assurance certification, three female New Zealand white
rabbits (Chbb:NZW) received an instillation of 0.1 ml (84 mg) of
technical-grade dicyclanil into the conjunctival sac of one eye. The
other eye served as control. The cornea appeared to be unaffected by
treatment; one animal showed an affected iris 1 h after instillation
but recovered within 24 h. Slight chemosis of the conjunctiva was
observed in two animals 1 h after instillation, but they recovered
within 24 h. Redness of the conjunctiva was seen in all animals,
although at different grades (score 1 or 2), and recovery to normal
occurred within 1-7 days (Hagemann, 1992b).
In 10 male and 10 female Pirbright white Tif:DHP guinea-pigs
submitted to an optimization test that followed OECD test guideline
406 with GLP and quality assurance certification, no significant skin
sensitization (1/20 positive) was observed after epidermal challenge
with 20% technical-grade dicyclanil in vaseline, this being a
subirritant dose. When the skin barrier was intentionally bypassed,
i.e. by intradermal challenge with a 0.1% solution of technical-grade
dicyclanil in 10% propylene glycol, 13 of 20 treated animals showed
positive reactions versus 3 of 20 animals given the vehicle
( p < 0.01) (Hagemann, 1993).
2.2.2 Short-term studies of toxicity
Rats
In a 28-day range-finding study, groups of five male and five
female Tif:RAIf (SPF) rats received technical-grade dicyclanil
(purity, > 98%) in the diet at a concentration of 0, 100, 500, or
2000 mg/kg of diet, equal to average achieved intakes of 0, 9.5, 50,
and 150 mg/kg bw per day for males and 0, 9.4, 49, and 160 mg/kg bw
per day for females. The study followed OECD test guideline 407
(adapted to the objectives of a range-finding study) without GLP or
quality assurance certification.
No treatment-related deaths occurred. Piloerection was observed
in animals of each sex at the highest dose during the second half of
the study. Dose-dependent reductions in food consumption, body-weight
gain, and final body weight were noted in all treated groups. These
were particularly marked in males at the high dose. Water consumption
was reduced in females at 500 and 2000 mg/kg of diet. Increased
incidences of anisocytosis and polychromasia of erythrocytes and a
slightly lower (not statistically significant) leukocyte count were
noted in males at the high dose. In both males and females at this
dose, higher plasma concentrations of urea and cholesterol and
increased activities of aspartate aminotransferase (twofold) and
alanine aminotransferase (five- to sevenfold) were noted.
Hypoglycaemia and lower plasma total bilirubin concentrations were
also observed in these animals, although the changes were not
statistically significant in females. Males at the high dose also had
slightly lower plasma globulin and calcium concentrations, while
females at this dose had an increased plasma phosphate concentration.
Consistent with the reduced body weights of all treated animals, the
absolute weights of a number of organs were decreased, whereas the
relative weights of a number of other organs were increased. The only
notable changes in organ weights which could be considered to be
treatment-related effects were reductions in the absolute and relative
weights of the prostate in males at the intermediate and high doses
and of the adrenals in females at the low and intermediate doses. No
macroscopic changes were found, apart from emaciation at the high
dose. Histopathologically, reduced spermatogenesis in the testes was
seen in 1/5 males at 500 mg/kg of diet and 5/5 males at 2000 mg/kg of
diet, accompanied by accumulation of cellular debris in the epididymal
duct in all those at the high dose. These animals also showed
immaturity of the prostate. In 1/5 females at 500 mg/kg of diet and in
4/5 females at 2000 mg/kg of diet, polyovular ovarian follicles, as
indicated by hypercellularity, were seen (Bachmann, 1991).
In a 3-month study of toxicity, groups of 10 male and 10 female
Tif:RAIf (SPF) rats received technical-grade dicyclanil in the diet at
a concentration of 0, 5, 25, 125, or 500 mg/kg of diet, equal to
average achieved intakes of 0, 0.31, 1.6, 8.0, and 33 mg/kg bw per day
for males and 0, 0.31, 1.7, 8.4, and 34 mg/kg bw per day for females.
Ten additional animals of each sex in the control and high-dose groups
were kept for a 4-week recovery period. The study followed OECD test
guideline 408, with GLP and quality assurance certification.
No treatment-related deaths or clinical signs were observed.
Slight reductions in body-weight gain and food consumption were
observed in animals of each sex at the high dose and in males at 125
mg/kg of diet. Owing to a compensatory increase in food intake during
the recovery period, the body weights of animals at the high dose were
comparable to those of the controls by the end of the recovery period.
Chemical analysis of blood revealed slightly lower plasma glucose
concentrations in males and females at 125 and 500 mg/kg of diet at
the end of the treatment period, but this was reversed within the
recovery period. Higher organ:body weight ratios were observed for
kidneys, brain, and testis in males at the high dose and for liver and
brain in females at this dose, these changes being reversible within
the 4-week recovery period. Although the relative epididymidal weights
were increased in males at doses > 25 mg/kg of diet, this was
considered not to be toxicologically significant because the absolute
epididymidal weights were not different from those of controls and
there were no abnormal histopathological findings. No
treatment-related ophthalmological or haematological changes were
observed, and no gross or microscopic alterations were observed. One
female at the high dose that was alllowed to recover had a mammary
tumour, which was considered to be of spontaneous origin. On the basis
of the reduction in body-weight gain, the NOEL was 25 mg/kg of diet,
equal to 1.6 mg/kg bw per day (Bachmann, 1993).
In a study that followed OECD test guideline 410 with GLP and
quality assurance certification, groups of five male and five female
Tif:RAIf (SPF) rats received dermal applications of technical-grade
dicyclanil at a dose of 0, 5, 30, 300, or 1000 mg/kg bw per day, 6
h/day on 5 days per week for 4 weeks. Dicyclanil was dissolved in
water containing 0.5% carboxymethylcellulose and 0.1% polysorbate 80
and was applied under an occlusive dressing to a clipped dorsal area
of the skin. At the end of each application period, the site was
washed with lukewarm water.
None of the animals died and no treatment-related clinical signs
were observed. Apart from a few incidental findings, no signs of local
skin irritation were seen. Male rats at 300 and 1000 mg/kg bw per day
showed dose-dependently decreased body weights and body-weight gain
and slightly lower food consumption. The plasma concentrations of
sodium and calcium were slightly reduced in these animals. Females at
the high dose showed increased absolute and relative liver weights. A
similar effect was observed in females at 300 mg/kg bw per day but did
not reach statistical significance. The absolute, but not the
relative, weight of the brain of females was increased at doses
> 30 mg/kg bw per day, without histopathological findings. No
treatment-related effects were noted on gross examination. Microscopic
examination showed hepatocyte hypertrophy in males at 1000 mg/kg bw
per day and in females at 300 and 1000 mg/kg bw per day. The NOEL was
30 mg/kg bw per day, on the basis of decreased body-weight gain and
changes in the liver (Marty, 1995).
Dogs
In a 28-day range-finding study, groups of two male and two
female beagles were given technical-grade dicyclanil (purity, > 98%)
in the diet at a concentration of 0, 200, 1000, or 2500 mg/kg of diet,
equal to average achieved intakes of 0, 5.6, 31, and 66 mg/kg bw per
day for males and 0, 6.2, 30, and 50 mg/kg bw per day for females. The
study followed OECD test guideline 409 (adapted to the objectives of a
range-finding study) without GLP (except for the pathology report) or
quality assurance certification.
No treatment-related deaths occurred. The clinical signs included
body tremors (in animals of each sex at 2500 mg/kg of diet), vomiting
(in animals of each sex at 2500 mg/kg of diet and in one male at 1000
mg/kg of diet), dyspnoea and slight apathy (in males at 2500 mg/kg of
diet). Animals at the high dose lost weight and had reduced food
consumption, and females at 1000 mg/kg of diet also showed a slight
reduction in food consumption. The reduction in food consumption did
not appear to be due to unpalatability. One male and one female at the
high dose showed a prolonged prothrombin time, and the other male at
the high dose had a slightly increased number of blood platelets.
Alanine aminotransferase activity was markedly increased and alkaline
phosphatase activity slightly increased in one male at the high dose
and in one female at the intermediate and one at the high dose;
aspartate aminotransferase activity was also increased in the female
at the high dose. Very slight decreases were observed in plasma
potassium concentrations in males at the intermediate and high doses,
in plasma glucose concentration in females at the high dose, and in
the plasma concentrations of urea, creatinine, and total bilirubin in
males and females at the high dose. Urinary analysis revealed an
increased incidence of proteinuria in males at the high dose and
females at the intermediate and high doses. The absolute and relative
weights of the testis and thymus were markedly decreased and those of
the heart slightly decreased at 2500 mg/kg of diet. In animals of each
sex at 1000 and 2500 mg/kg of diet, the absolute and relative weights
of the adrenal and kidney were increased, but without any
histopathological changes. Microscopic examination revealed hepatic
toxicity in one female at 1000 mg/kg of diet and in all animals at
2500 mg/kg of diet. The toxicity included focal or single-cell
necrosis, inflammatory-cell foci, and a brown granular pigment in
macrophages. One female at the intermediate dose also had mild
bile-duct hyperplasia, and one male at the high dose had periportal
hepatocyte enlargement. The testes of males at this dose showed
degeneration characterized by reduced numbers of tailed spermatids and
increased numbers of multinucleated or giant cells. Mild thymic
atrophy was noted in animals of each sex at the highest dose. The
heart of one male at this dose was mottled and showed inflammation,
fibrosis, and haemorrhage. Tubular basophilia or dilatation was
present in the kidneys of all animals at the high dose, two at the
intermediate dose, and one at the low dose, but not in controls. This
lesion is of minimal toxicological significance (Altmann, 1991).
Groups of four male and four female beagles received
technical-grade dicyclanil in the diet at a concentration of 0, 20,
100, 500, or 1500 mg/kg of diet for 3 months, equal to average
achieved intakes of 0, 0.61, 2.7, 14, and 42 mg/kg bw per day for
males and 0, 0.71, 3.5, 17, and 42 mg/kg bw per day for females. The
study followed OECD test guideline 409, with GLP and quality assurance
certification.
One male at the high dose was found dead at week 11 after general
deterioration in clinical condition accompanied by tonic-clonic
spasms. Post-mortem examination did not reveal the cause of death.
Animals at the high dose started to show clinical signs from week
9-11, including slight ataxia, unnaturally raised tails, and frequent
shaking. In addition, vomitus and traces of blood in the faeces were
observed. Ophthalmoscopic examinations revealed no treatment-related
changes. Animals at the high dose lost weight (males only) or had
reduced body-weight gain with reduced food intake. A very slight
reduction in food intake was also observed transiently in some animals
at 500 mg/kg of diet. Slightly reduced haemoglobin concentrations and
haematocrit values, associated with minor microcytosis and
hypochromasia of erythrocytes, were recorded in animals at the high
dose. Increased plasma cholesterol and phospholipid concentrations
were observed in animals at doses > 100 mg/kg of diet. Plasma
albumin concentrations were slightly decreased in males and females at
1500 mg/kg of diet and in females at 500 mg/kg of diet. Decreased
plasma calcium, potassium, urea, creatinine, and total bilirubin
concentrations were found in animals at the high dose. Urinary
analysis revealed no treatment-related effects. The mean absolute and
relative liver weights were increased in animals at the highest dose
and in females also at 20, 100 (not statistically significant), and
500 mg/kg of diet (not dose-related). The absolute and relative
adrenal weights were also slightly increased at the high dose. The
absolute and relative weights of the thymus (males), testis, and
spleen (both sexes) were decreased in animals at the high dose. The
kidney weights were increased in males (relative) and females
(absolute and relative) at 1500 mg/kg of diet. Macroscopic examination
showed no treatment-related effects. Microscopy of the liver showed
minimal to moderate focal or multifocal subcapsular inflammation with
fibrosis in 2/4 males and 3/4 females at the high dose. Enlarged
hepatocytes, diagnosed as cellular oedema, were observed in the
centrilobular and midzonal region of 3/4 females at 20, 100, and 500
mg/kg of diet and in 1/4 males and all females at 1500 mg/kg of diet.
No morphological signs of hepatocellular damage were apparent. Minimal
atrophy of the white pulp of the spleen was observed in 3/4 males at
500 and 1500 mg/kg of diet and in all females at 1500 mg/kg of diet.
Thymic atrophy was found in 3/4 males at 500 mg/kg of diet and in all
males at 1500 mg/kg of diet. Minimal atrophy of the lymphatic tissue
of the mesenteric lymph node was observed in 3/4 males at the high
dose, and minimal to marked atrophy of the glandular tissue of the
prostate was detected in 3/4 males at 100 mg/kg of diet, 1/4 at 500
mg/kg of diet, and 4/4 at 1500 mg/kg of diet. Examination of the
testes revealed minimal tubular atrophy in 3/4 males at the high dose,
associated with a marked reduction in spermatogenesis in all males of
this group. A dose-related increase in the frequency of inflammatory
changes associated with epithelial hyperplasia was found in the
urinary bladders of females at 100, 500, and 1500 mg/kg of diet
(Altmann, 1993). The Committee noted that hepatocyte oedema without
hepatocellular damage is not of toxicological significance. Hence, the
NOEL was 20 mg/kg of diet, equal to 0.61 mg/kg bw per day, on the
basis of increased plasma cholesterol concentrations and
histopathological findings in the prostate and urinary bladder.
In a 1-year study of toxicity, groups of four male and four
female beagles received technical-grade dicyclanil in the diet at a
concentration of 0, 5, 25, 150, or 750 mg/kg of diet, equal to average
achieved intakes of 0, 0.16, 0.71, 4.4, and 23 mg/kg bw per day for
males and 0, 0.15, 0.77, 5.1, and 23 mg/kg bw per day for females. Two
additional animals per sex in the control and high-dose groups were
maintained for a 4-week recovery period. The observations included
deaths, clinical signs, body weight, food consumption, ocular,
neurological, haematological, clinical chemical, and urinary
parameters, and macroscopic and microscopic end-points. The study
followed OECD test guideline 452, with GLP and quality assurance
certification.
One female at the high dose was found dead on day 13, with no
previous abnormal clinical signs. One male at the high dose was killed
on study day 32 after vomiting, showing marked apathy, lying in a
lateral position, and weight loss correlated to reduced food intake.
Vomiting was observed in females at the high dose, and the body-weight
gain (in two animals) and food consumption of females at the high dose
were slightly reduced. Ocular and neurological examinations revealed
no treatment-related effects, nor were there any changes in
haematological or urinary parameters. Throughout the treatment period,
the plasma cholesterol concentrations were increased in animals of
each sex at 750 mg/kg of diet (not statistically significant in
females) and in males at 150 mg/kg of diet; in males, the change was
not reversed after the 4-week recovery period. Males at the high dose
showed slightly lower plasma calcium concentrations. Both males and
females at the high dose had reduced plasma concentrations of
bilirubin and urea and reduced alkaline phosphatase activity. The
changes in blood chemistry were partially reversed during the recovery
period. The absolute and relative liver weights were increased in
animals at 750 mg/kg of diet, but only the change in absolute weight
in males was statistically significant. The absolute (statistically
significant) and relative weights of the heart were decreased in
females at the high dose. The weight changes were reversed by the end
of the 4-week recovery period. The macroscopic and microscopic
findings were confined to the two animals at the high dose that died
before the scheduled kill. The macroscopic findings consisted of a
scar in the liver and pale kidneys in the male and a mass and
haemorrhagic content in the abdominal cavity of the female.
Histopathologically, both animals showed marked diffuse liver necrosis
and kidney lesions (more severe in the male). The male also showed
testicular and prostatic atrophy, and the female had a thrombus in a
peritoneal blood vessel. The two animals suffered from acute, severe
liver failure and resulting cardiocirculatory disturbances, and the
male also showed stress due to weight loss. Their condition was very
different from that of the other treated dogs in this study, and
comparable acute, severe liver toxicity was not seen in the 28-day and
3-month studies in dogs treated with doses up to 2500 and 1500 mg/kg
of diet, respectively. The lesions seen in the two animals are
considered to be incidental findings. On the basis of increased plasma
cholesterol concentrations in males, the NOEL was 25 mg/kg of diet,
equal to 0.71 mg/kg bw per day (Altmann, 1995).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In an 18-month study of carcinogenicity, groups of 60 male and 60
female Tif:MAGf (SPF) mice were given diets containing technical-grade
dicyclanil at a concentration of 0, 10, 100, 500, or 1500 mg/kg of
diet, equal to average achieved intakes of 0, 1.1, 12, 59, and 210
mg/kg bw per day for males and 0, 1.1, 12, 65, and 200 mg/kg bw per
day for females. Ten animals per sex from each group were assigned for
evaluation of haematological parameters. The study followed OECD test
guideline 451 with GLP and quality assurance certification, but
histopathological examination of the animals at the high dose (i.e.
the group that was killed before study termination, see below) was
limited to the liver and lungs.
Clinical signs were observed only in males at the highest dose,
which injured themselves through vigorous scratching. A higher
mortality rate was noted among males at the high dose and to a lesser
extent in females at this dose. The self-inflicted injuries and poor
health of the animals at the high dose led to a decision to terminate
all surviving animals in this group during weeks 58-59. Doses < 500
mg/kg of diet did not affect survival. The body weights of males and
females at 1500 mg/kg of diet were reduced, with an approximately 50%
reduction in body-weight gain, and those of females at 500 mg/kg of
diet were reduced with a 30% reduction in body-weight gain. As food
intake was not affected, higher food consumption ratios were noted in
animals of each sex at 1500 mg/kg of diet and in females at 500 mg/kg
of diet. Haematological parameters were not affected by treatment. The
absolute and relative weights of the liver were increased in animals
of each sex at 500 mg/kg of diet (for males after adjustment for three
control outliers). In females at 500 mg/kg of diet, the relative
weights of the kidney, brain, and adrenals were increased, while the
absolute weights of these organs were not changed. The
treatment-related macroscopic findings included enlarged livers
(characterized histopathologically as hepatocellular hypertrophy) in
males at 500 mg/kg of diet and in both sexes at 1500 mg/kg of diet,
and masses and/or nodules of the liver in females at 500 and 1500
mg/kg of diet. Treatment-related microscopic findings were observed in
the liver, olfactory epithelium, adrenal gland, and bone marrow.
Kupffer cell pigmentation (mainly haemosiderin) and hepatocellular
necrosis were observed in males at > 100 mg/kg of diet. Increased
numbers of hepatocellular mitotic figures and multinucleated
hepatocytes were seen in the livers of males at the high dose, and the
frequency of foci of cellular change was increased in animals of each
sex at the highest dose. The incidence of heptocellular adenomas was
higher in females at 500 and 1500 mg/kg of diet (9/53 and 5/60,
respectively) than in controls (0/52). In addition, the incidence of
hepatocellular carcinomas was increased in females at the highest dose
(6/60 versus 0 in all other groups). Pigmentation of the olfactory
epithelium was observed at increasing incidence and severity in
animals of each sex at 100 and 500 mg/kg of diet, and males at these
doses showed an increased incidence of inflammatory cell infiltration
(cell type not specified) in the underlying Bowman's glands. At 500
mg/kg of diet, both males and females also showed increased incidences
of pigmentation of the adrenal glands, recorded as ceroid (i.e. a
partly oxidized form of lipofuscin) deposition, and of
hypercellularity of the bone marrow. Treatment with dicyclanil did not
affect the number of animals with malignant lymphomas. It was noted,
however, that females at 500 mg/kg of diet had more sites that
appeared to be infiltrated by malignant lymphoma cells than did
controls or animals at other doses (Bachmann, 1996a).
The Committee noted that the doses at which the liver adenomas
and carcinomas occurred exceeded the maximum tolerated dose for
females, and that there were signs of hepatocellular proliferation in
these animals which might have been involved in the hepatic
carcinogenesis observed. Pigmentation of the olfactory epithelium was
also observed in a long-term study in rats (Bachmann, 1996b; see
below) and was further investigated in a separate study (Weber, 1998;
see below). As the Committee considered the effects on the olfactory
epithelium to be of no biological significance (see below), the NOEL
in this study in mice was 10 mg/kg of diet, equal to 1.1 mg/kg bw per
day, on the basis of effects on the liver.
Rats
In a 2-year study of carcinogenicity and toxicity, groups of 80
Tif:RAIf (SPF) rats of each sex were given technical-grade dicyclanil
in the diet at a concentration of 0, 5, 25, 125, or 500 mg/kg of diet,
equal to average achieved intakes of 0, 0.19, 0.97, 4.8, and 22 mg/kg
bw per day for males and 0, 0.23, 1.2, 6.0, and 26 mg/kg bw per day
for females. Haematological, clinical chemical, and urinary analyses
were performed during weeks 13, 26, 52, 78, and 105 on 20, 10, and 10
animals of each sex per group, respectively. After 1 year, 10 animals
of each sex per group were killed. The study followed OECD test
guideline 453, with GLP and quality assurance certification.
Treatment with dicyclanil did not affect the incidence of
clinical signs or survival. Decreased food consumption was noted in
males and females at the highest dose. The body weights of males and
females at 500 mg/kg of diet were reduced (with an approximately 25%
reduction in body-weight gain), as were the body weights of animals at
125 mg/kg of diet (with a reduction in body-weight gain of < 10%).
Slight but statistically significant red blood cell dyscrasia and a
reduced number of monocytes were observed in males at the high dose
when compared with controls, but the changes were generally
inconsistent over time, not dose-related, and within the range of
historical controls and are therefore regarded as toxicologically
insignificant. Increased concentrations of inorganic phosphate were
observed in males and females at the high dose throughout the study,
although the increase was not statistically significant in females.
Males at 125 mg/kg of diet also had increased inorganic phosphate
concentrations, but these reached statistical significance only in
weeks 78 and 105. Lower concentrations of triglycerides were recorded
for males at 500 mg/kg of diet, although statistical significance was
achieved only in weeks 13 and 26. Urinary parameters were not
affected. As a result of the lower terminal body weights, almost all
of the relative organ weights were increased in males and females at
the highest dose, especially of the kidney, liver, and epididymides.
The absolute epididymal weights were also increased in males at the
high dose at 105 weeks. Males at 125 mg/kg of diet also had increased
relative liver weights, although these were within historical control
values. An increased incidence of liver cysts was observed in females
at the high dose, characterized primarily as unilocular or
multilocular biliary cysts on microscopic examination. In males at the
high dose, an increased incidence of masses and nodules in the
exocrine pancreas was noted, which were characterized
histopathologically as foci or areas of hyperplasia. Aside from the
histopathological findings in the pancreas and liver, the only other
treatment-related histopathological finding was an increased incidence
of pigmentation of the olfactory epithelium in males at > 25 mg/kg
of diet and in females at > 125 mg/kg of diet, the effects seen at
125 and 500 mg/kg of diet being more severe than at lower doses.
Dicyclanil did not affect the tumour incidence in this study
(Bachmann, 1996b).
Weber (1998) studied the histological nature and causes of the
pigmentation of the olfactory epithelium, using material from male
rats in the 2-year study (controls and rats given 500 mg/kg of diet)
and the 3-month study (controls and rats given 500 mg/kg of diet;
Bachmann, 1993). Material from male control rats of the same strain in
five other long-term studies was included to provide more reference
material.
Minimal to moderate pigmentation was found in controls from the
long-term studies with dicyclanil and other compounds, but not in
controls from the 3-month study. Treatment with 500 mg/kg of diet
resulted in increased pigmentation, which was minimal after 3 months
and moderate to marked after 12 and 24 months, the degree of
pigmentation at the later times being similar. Staining indicated that
the pigment consisted mainly of oxidized lipofuscins and was located
in the olfactory epithelium and the underlying lamina propria. The
pigment appeared to be located in secondary lysosomes. Further
investigations by high-resolution microscopy indicated that the
supporting cells and secretory cells of Bowman glands were affected by
the pigmentation. Neuronal olfactory perikarya, nerve bundles of the
olfactory nerve in the olfactory mucosa, and the olfactory bulbs (in
the brain) were free of pigment accumulation. Apart from the
pigmentation, no other treatment-related morphological changes in the
olfactory mucosa were found. According to the author, the clinical
signs in the 2-year study gave no indication of a disturbed olfactory
sense due to dicyclanil treatment. In addition, the presence of
mucopolysaccharides in the Bowman glands indicated normal functioning
of the olfactory mucosa in the dicyclanil-treated rats. The author
concluded that the increased pigmentation of the olfactory epithelium
in male rats after treatment with dicyclanil results from accumulation
of lipofuscin in the cytoplasm of supporting cells and secretory cells
of the Bowman glands and is an enhancement of a natural age-related
process. In the absence of other morphological changes in the
olfactory mucosa, the author considered the pigmentation not to be
detrimental to the structure or function of the olfactory mucosa and,
therefore, is not to be regarded as adverse (Weber, 1998).
Recognizing that the effects on the olfactory epithelium were an
enhancement of a natural age-related process, the Committee noted that
dicyclanil had no effect on survival, behaviour, or general
well-being. As there were no other morphological changes in the
olfactory mucosa, the Committee concluded that the effect was of no
biological significance. Therefore, the NOEL in the 2-year study in
rats was 125 mg/kg of diet, equal to 22 mg/kg bw per day, on the basis
of changes in body weight and histopathological changes in the liver
and pancreas.
2.2.4 Genotoxicity
The results of studies for genotoxicity with technical-grade
dicyclanil are summarized in Table 1. The studies were of conventional
design, with GLP and quality assurance certification, except for the
study of Ogorek & Arni (1987).
2.2.5 Reproductive toxicity
(a) Multigeneration study
Rats
In a two-generation study of reproductive toxicity with two
litters per generation, groups of 30 male and 30 female Tif:RAIf (SPF)
rats received diets containing technical-grade dicyclanil at a
concentration of 0, 5, 30, 200, or 500 mg/kg of diet from 10 weeks
before the first mating until necropsy at the end of the lactation
period. Dams were allowed to litter and suckle their pups naturally.
When possible, the F1a litters were culled to four pups of each sex
per litter at day 4 post partum. After weaning, a number of F1a
pups were selected to be the F1 parents, and the remaining F1a
pups were necropsied. The F0 parents were then mated a second time,
and the resulting litters (F1b) were again culled to four pups of
each sex per litter. After weaning, both the F1b pups and the F0
parents were necropsied. The same procedure was followed for the F1
parents in order to produce F2a and F2b generations. The
observations included clinical signs, deaths, body weight, food
consumption, reproductive parameters, pup survival and development,
macroscopic appearance at necropsy, and, for parents in the control
and high-dose groups, histological appearance of the reproductive and
target organs. The study followed OECD test guideline 416, with GLP
and quality assurance certification.
Effects in F0 parents: The F0 parents showed no
treatment-related deaths or clinical signs and no effects on male or
female mating or fertility indices, on maternal gestation or
parturition indices, or on the duration of gestation. In the premating
period, males and females showed decreased body-weight gain and food
intake at 500 mg/kg of diet and, marginally, at 200 mg/kg of diet.
During both gestation periods, the overall body-weight gain of females
in all treated groups was similar to that of controls, while in both
lactation periods the overall body-weight gain of females was
increased at 500 mg/kg of diet and, marginally, at 200 mg/kg of diet.
Gross examination and histology revealed no treatment-related effects.
Secondary to the reduced body weights, the relative weights of most
organs were increased in males and females at 500 mg/kg of diet. At
this dose, the absolute weights of the heart and liver of males were
decreased.
Table 1. Results of assays for genotoxicity with dicyclanil
End-point Test object Concentration Result Reference
In vitro
Reverse S. typhimurium 20-5000 µg/plate Negativea Ogorek & Arni
mutation TA1537, TA98, (1987)
TA100
Reverse S. typhimurium 313-5000 µg/plate Negativeb Hertner (1992)
mutation TA1535, TA1537,
TA98, TA100,
E. coli WP2 uvrA
Gene mutation V79 Chinese 12.4-400 µg/ml Negative Geleick (1992)
hamster lung cells, - S9c
hprt locus 24.7-667 µg/ml
+ S9d,e
Chromosomal Chinese hamster 20.8-83.4 µg/ml Negative Hertner (1993a)
aberration ovary cells -S9c
166.75-667 µg/ml
+S9d,e
Unscheduled Primary rat 6.2-670 µg/mle Negative Hertner (1993c)
DNA synthesis hepatocytes
In vivo
Micronucleus Mouse bone 47-188 mg/kg bw; Negativef Hertner (1993b)
formation marrow once by oral gavage
a With and without rat liver S9 fraction; results of cytotoxicity test not given,
precipitation not reported
b With and without rat liver S9 fraction; precipitation at 5000 µg/plate
c Cytotoxic at the highest concentration
d Slightly cytotoxic at the highest concentration
e The highest concentration represents the limit of solubility in dimethyl sulfoxide.
f At all doses and all times, the ratio of polychromatic to normochromatic erythrocytes
did not deviate from that in controls; clinical signs of toxicity (including reduced
locomotor activity, unkempt fur, ataxia, piloerection, and diarrhoea) were observed
at the highest dose tested; doses > 312.5 mg/kg bw resulted in death.
Effects in F1 pups: At all doses and both matings, no
treatment-related effects were seen in the F1 offspring in terms of
sex ratio, clinical signs, litter size, the development of physical
landmarks (surface righting and eye opening), or macroscopic findings
at necropsy. A slight increase in the pup mortality rate was observed
on days 0-4 post partum at 500 mg/kg of diet (F1a) and at 30 and
200 mg/kg of diet (F1b). This was considered not to be
treatment-related because it could be attributed to two (F1a) or
three (F1b) litters and no dose-response relationship was found. At
500 mg/kg of diet, the weights of F1a pups were lower than those of
controls at birth and on days 14 and 21 post partum, owing to
reduced weight gain from day 4 post partum onwards. The weights of
F1b pups at the high dose were similar to those of controls from
birth through to day 14 post partum. Thereafter, the weight gain
decreased, resulting in lower pup weights on day 21 post partum.
Effects in F1 parents: In the F1 parents, no
treatment-related deaths or clinical signs and no effects on male or
female mating or fertility indices, on maternal gestation or
parturition indices, or on the duration of gestation were observed.
The body weights of the F1 parents at the high dose were lower than
those of controls throughout the study. Although the body weights of
males at 200 mg/kg of diet were reduced during the final part of the
study, the overall body-weight gain of these animals was not different
from that of controls. During both gestation periods, the overall
body-weight gain of all treated females was slightly lower than that
of controls, while in both lactation periods the overall body-weight
gain of females was increased at 500 mg/kg of diet and, marginally, at
200 mg/kg of diet. The absolute weights of most organs of males at the
high dose were decreased, with the exception of those of the testis
and brain. In females at the high dose, only the absolute weights of
the heart, liver, and kidney were decreased. At 200 mg/kg of diet, the
absolute weight of the liver was decreased in animals of each sex. In
contrast, secondary to the reduced body weights of these two groups,
the relative weights of most organs were increased, attaining
statistical significance for most organs at 500 mg/kg of diet and for
brain (both sexes), kidneys (males only), and testis at 200 mg/kg of
diet. Gross examination and histology revealed no treatment-related
effects.
Effects in F2 pups: At all doses and both matings, no
treatment-related effects were noted in the F2 offspring in terms of
sex ratio, mortality rate, clinical signs, litter size, the
development of physical landmarks (surface righting and eye opening),
or macroscopic findings at necropsy. The only treatment-related effect
observed was reduced weight gain in F2a and F2b pups at the
highest dose from day 4 post partum onwards, resulting in
significantly lower pup weights on days 14 and 21 post partum.
The NOEL for parental toxicity was 30 mg/kg of diet, equal to 2
mg/kg bw per day, on the basis of changes in body weight. The NOEL for
reproductive toxicity was 500 mg/kg of diet, equal to 24 mg/kg bw per
day, the highest dose tested. The NOEL for pup toxicity was 200 mg/kg
of diet, equal to 21 mg/kg bw per day, on the basis of reduced
body-weight gain (Khalil, 1995).
(b) Developmental toxicity
Rats
In a range-finding study, groups of eight pregnant Tif:RAIf (SPF)
rats received technical-grade dicyclanil (purity, > 98%) in 3%
aqueous corn starch by oral gavage at a dose of 0, 75, or 150 mg/kg bw
per day on days 6-15 of gestation. On day 21 of gestation, the dams
were killed and necropsied, and the fetuses were weighed, sexed, and
examined for external abnormalities.
Two dams at the high dose were killed in moribund condition. The
clinical signs in these animals and in a third animal at the high dose
included piloerection, salivation, dyspnoea, and hypotonia. Maternal
body-weight gain and gravid uterine weights were dose-dependently
decreased in all treated animals, but food consumption was
dose-dependently decreased only during the first week of treatment.
Post-implantation loss was dose-dependently increased by treatment,
owing to early resorptions. Three dams at the high dose resorbed their
entire litters. The number of viable fetuses and fetal body weights
were dose-dependently reduced. Three fetuses at the high dose showed
generalized oedema (FitzGerald, 1990a).
In the main study, groups of 24 pregnant Tif:RAIf (SPF) rats
received technical-grade dicyclanil in an aqueous solution of 0.5%
sodium carboxy-methylcellulose by oral gavage at a dose of 0, 1, 5,
25, or 75 mg/kg bw per day on days 6-15 of gestation. On gestation day
21, the dams were killed and necropsied, and the fetuses were weighed,
sexed, and examined for external, visceral, and skeletal
abnormalities. The study was of conventional design, with GLP and
quality assurance certification.
There were no deaths and no treatment-related clinical signs of
toxicity. Maternal body weight, body-weight gain, and food consumption
as well as net body-weight change and carcass weight at necropsy were
reduced at the highest dose. A marginal decrease in these parameters
was also observed at 25 mg/kg bw per day. One animal at 75 mg/kg bw
per day had total, early resorptions and showed haemorrhagic fluid in
the uterus at necropsy. In animals at 75 mg/kg bw per day, the gravid
uterine weight was reduced; the number of early postimplantation
losses was slightly increased and the number of fetuses per litter was
slightly lower, but without statistical significance. Effects on the
fetuses were observed only at the highest dose. These included reduced
fetal weight, a slight increase in the frequency of renal pelvic
dilatation, and a number of mainly sternebral defects and variations
due to poor or absent ossification. There was no evidence of
teratogenicity. The NOEL for maternal toxicity was 5 mg/kg bw per day,
on the basis of a reduction in body-weight gain. The NOEL for
developmental toxicity was 25 mg/kg bw per day, on the basis of
reduced fetal weight, increased renal pelvic dilatation, and increased
skeletal anomalies and variations consistent with a slight delay in
skeletal maturation (FitzGerald, 1993a).
Rabbits
In a range-finding study, groups of eight pregnant Russian
rabbits (strain unspecified) received technical-grade dicyclanil
(purity, > 98%) in 3% aqueous corn starch by oral gavage at a daily
dose of 0, 20, or 40 mg/kg bw on days 7-19 of gestation. On day 29 of
gestation, the dams were killed and necropsied, and the fetuses were
weighed, sexed, and examined for external abnormalities.
No treatment-related deaths or clinical signs were observed.
During treatment, the body-weight gain and food consumption of dams at
the high dose were considerably reduced. The gravid uterine weights
were not affected by treatment. The rate of pre-implantation loss was
slightly reduced and that of postimplantation loss slightly increased
at 40 mg/kg bw per day, which resulted in a comparable number of
fetuses to controls. The body weights of female fetuses were slightly
reduced at this dose. There were no remarkable external fetal changes
(FitzGerald, 1990b).
In the main study, groups of 19 pregnant Russian Chbb:HM rabbits
received technical-grade dicyclanil in an aqueous solution of 0.5%
sodium carboxy-methylcellulose by oral gavage at a daily dose of 0, 1,
3, 10, or 30 mg/kg bw on days 7-19 of gestation. On gestation day 29,
the dams were killed and necropsied, and the fetuses were weighed,
sexed, and examined for external, visceral, and skeletal
abnormalities. The study was of conventional design, with GLP and
quality assurance certification.
No deaths or treatment-related clinical signs of toxicity were
observed. Maternal body weight, body-weight gain, and food consumption
and the carcass weight at necropsy were reduced at 30 mg/kg bw per
day. Maternal body-weight gain was also reduced at 10 mg/kg bw per
day. Necropsy of the dams revealed no treatment-related effects, and
reproductive function was not affected. The body weights of fetuses at
the highest dose were reduced, and the fetuses showed skeletal
variations indicative of a slight delay in ossification. There was no
evidence of teratogenicity. The NOEL for maternal toxicity was 3 mg/kg
bw per day on the basis of reduced body-weight gain. The NOEL for
developmental toxicity was 10 mg/kg bw per day on the basis of reduced
fetal weight and increased skeletal variations consistent with delayed
ossification (FitzGerald, 1993b).
2.2.6 Special studies: Pharmacological effects
The effects of dicyclanil on the central nervous system
(centrally controlled behaviour, body temperature, locomotor activity,
hypnotic potentiation, motor coordination), the peripheral nervous
system, the autonomic nervous system and smooth muscles, the
cardiovascular and respiratory systems, and the gastrointestinal tract
were investigated in mice, rats, and guinea-pigs in vivo and
in vitro. The effect of dicyclanil on sperm morphology and motility
was also examined. All of the studies followed the Japanese Guidelines
for the Registration of Pharmaceuticals (General Pharmacology), with
GLP and quality assurance certification.
In vivo, technical-grade dicyclanil in polyethylene glycol
200:ethanol (5:3 v/v) was administered orally by gavage at a single
dose of 0, 1, 10, 50, or 100 mg/kg bw to male NMRI mice or male Han
Wistar rats. Doses < 100 mg/kg bw had no effect on the body
temperature of rats (Pfister & Gisin, 1996a), on the
hexobarbitone-induced sleeping time in mice (Pfister & Gisin, 1996b),
or on gastrointestinal motility in mice (Pfister & Gisin, 1996c).
In the modified Irwin test for general behavioural changes,
treatment of mice with dicyclanil at 100 mg/kg bw slightly inhibited
both exploratory activity and the startle response. The changes were
most evident 6 h after treatment, with complete recovery of the
startle response by 8 h and of exploratory activity by 24 h after
treatment. Mice treated with 1 or 10 mg/kg bw showed normal behaviour
(Pfister & Gisin, 1996d). After treatment with the vehicle, reduced
locomotor activity (static, mobile, and rearing activity and mobile
and active times) was seen in control mice over 24 h, being most
pronounced during the first 8 h. These parameters were less markedly
reduced after treatment with dicyclanil at 1 (not statistically
significant) or 10 mg/kg bw. Opposite effects were induced by
dicyclanil at 100 mg/kg bw, when the reduction in static activity was
more pronounced than in controls while changes in the other parameters
were comparable. All of the effects were fully reversed by 24 h after
dosing (Pfister & Hussherr, 1996a). Motor coordination in mice, as
assessed by recording the time the animals could retain their balance
on a rotating rod, was inhibited at 100 mg/kg bw but not at 1 or 10
mg/kg bw. The effect was significant 4 h after dosing but had
completely disappeared by 24 h (Pfister & Hussherr, 1996b).
Treatment of rats with 100 mg/kg bw (the only dose tested)
resulted in slight increases throughout the observation period (6-8 h
after dosing) in heart rate (statistically significant) and tidal and
minute lung volume (statistically significant only when compared with
values for vehicle-treated animals, which generally had lower values
than untreated animals). The blood pressure, electrocardiogram, and
respiratory rate remained unchanged (Pfister & Nordmann, 1996).
Treatment of rats with 50 mg/kg bw (the only dose tested) had no
effect on sperm motility, concentration, or morphology. Minor,
statistically nonsignificant increases in abnormal sperm morphology 6
weeks after dosing (sperm with head only and with abnormally shaped
hooks) were completely reversed by 12 weeks after dosing (Pfister &
Gisin, 1996e.
In vitro, a concentration of 0, 0.1, 0.3, 1, or 3 mmol/L of
technical-grade dicyclanil in dimethyl sulfoxide had no significant
effect on directly induced contractions of skeletal muscle or on
contractions induced indirectly by phrenic nerve stimulation in the
isolated phrenic nerve-diaphragm preparation from rats. The authors
concluded that dicylanil has no effect on the skeletal neuromuscular
junction (Pfister & Hussherr, 1996c). When dicyclanil was tested on
ileum isolated from guinea-pigs, dose-dependent antagonistic activity
against the smooth muscle contractions induced by histamine and
acetylcholine and against the nonspecific smooth muscle contractions
induced by barium chloride was observed at concentrations > 0.3, 1,
and > 0.3 mmol/L, respectively. All of the effects were fully and
rapidly reversible. Dicyclanil was considered to be only a weak
antagonist (Pfister & Gisin, 1996f).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term and long-term
toxicity, carcinogenicity, genotoxicity, reproductive toxicity, and
pharmacology of dicyclanil. All of the pivotal studies were carried
out according to appropriate standards for study protocol and conduct.
After repeated oral administration of radiolabelled dicyclanil to
rats, the radiolabel was rapidly and almost completely absorbed and
was rapidly distributed to the major organs and tissues. Elimination
was rapid (> 93% of the total dose within 24 h) and was virtually
complete within 3 days. The major route of elimination was the urine
(79-83% of the total dose within 24 h), while the faecal route was of
minor importance (6-12% of the total dose within 24 h).
Biotransformation in the rat involves oxidative opening of the
cyclopropyl ring at various positions, followed by further oxidation
and cleavage of the cyclopropyl-N bond (i.e. dealkylation). The
metabolic pathways of dicyclanil in sheep treated topically are
essentially the same as those in rats.
After oral administration of dicyclanil to rats, the LD50
values were 560 mg/kg bw in males and approximately 500 mg/kg bw in
females. Dicyclanil is moderately hazardous when given as a single
oral dose.
In a three-month study of toxicity, rats received dicyclanil in
the diet at a concentration of 0, 5, 25, 125, or 500 mg/kg of diet.
Male rats at 125 and 500 mg/kg of diet showed decreased food
consumption and body-weight gain and slightly decreased plasma glucose
concentrations. The weights of the kidneys, brain, and testis relative
to that of the body were increased in males at 500 mg/kg of diet, and
females at this dose showed decreased food consumption, body-weight
gain, and plasma glucose concentrations and increased relative weights
of the liver and brain; plasma glucose concentrations were also
reduced in females at 125 mg/kg of diet. The NOEL was 25 mg/kg of
diet, equal to 1.6 mg/kg bw per day, on the basis of the reduction in
body-weight gain.
In a 3-month study of toxicity, dogs received dicyclanil in the
diet at a concentration of 0, 20, 100, 500, or 1500 mg/kg of diet.
Clinical signs consisting mainly of neurotoxicity, decreased food
consumption and body-weight gain, and changes in clinical chemistry
and erythrocyte parameters were observed mainly at the highest dose.
Plasma cholesterol and phospholipid concentrations were increased in
animals at concentrations of 100 mg/kg of diet and above. The weights
of the spleen (males and females), thymus (males only), and testis
were decreased at 1500 mg/kg of diet. Atrophy of the spleen in males
and females and atrophy of the thymus, mesenteric lymph node, testis,
and prostate in males were observed at concentrations of 100 mg/kg of
diet and above. The weights of the liver, adrenals, and kidneys were
increased in animals at the highest dose; in females, the liver
weights were also increased at lower doses. Inflammatory changes were
seen in the urinary bladder of females at concentrations of 100 mg/kg
of diet and above and in the livers of males and females at 1500 mg/kg
of diet. Hepatocyte oedema was observed in females at all doses, but
hepatocellular damage was not seen at any dose in either sex. The
Committee noted that hepatocyte oedema without hepatocellular damage
is not of toxicological significance. Hence, the NOEL was 20 mg/kg of
diet, equal to 0.61 mg/kg bw per day, on the basis of increased plasma
cholesterol concentrations and histopathological findings in the
prostate and urinary bladder.
In a 1-year study, dogs received dicyclanil in the diet at a
concentration of 0, 5, 25, 150, or 750 mg/kg of diet. Males showed
slightly decreased plasma calcium concentrations and increased
absolute and relative liver weights at 750 mg/kg of diet. Plasma
cholesterol concentrations were increased in males at 150 and 750
mg/kg of diet, and this change was not reversed after a 4-week
recovery period. Females receiving the highest dose vomited and had
slightly reduced food consumption and body-weight gain, slightly
increased plasma cholesterol concentrations, increased absolute and
relative liver weights, and decreased absolute and relative heart
weights. Macroscopic and microscopic findings consisting of liver
necrosis, tubular lesions in the kidneys, testicular and prostatic
atrophy, and vascular thrombus, were found in one male and one female
at the highest dose that died before their scheduled sacrifice. These
animals suffered from acute, severe liver failure and resulting
cardiovascular disturbances and stress due to weight loss. Comparable
acute, severe liver toxicity was not observed in the 3-month study of
toxicity in dogs given feed containing dicyclanil at concentrations up
to 1500 mg/kg of diet. The findings in the two animals were therefore
considered to be incidental. The NOEL was 25 mg/kg of diet, equal to
0.71 mg/kg bw per day, on the basis of increased plasma cholesterol
concentrations in male dogs. The Committee noted that this NOEL is
supported by the NOEL in the 3-month study of toxicity in dogs. It
also noted that the histopathological findings observed in the 3-month
study were not seen in the 1-year study among animals that lived until
the time of scheduled sacrifice.
In a study of carcinogenicity, mice received diets containing
dicyclanil at a concentration of 0, 10, 100, 500, or 1500 mg/kg of
diet for 18 months. The animals at the highest dose were killed during
weeks 58-59 because of self-inflicted injuries and poor health. On the
basis of significant reductions in body-weight gain, the
concentrations of 500 and 1500 mg/kg of diet in females and 1500 mg/kg
of diet in males were considered to exceed the maximum tolerated dose.
The liver was the main target organ in both male and female mice. The
effects included Kupffer cell pigmentation (with haemosiderin) and
hepatocellular necrosis in males at doses of 100 mg/kg of diet and
higher, increased incidences of hepatocellular adenomas in females at
500 and 1500 mg/kg of diet, and hepatocellular carcinomas in females
at 1500 mg/kg of diet. The Committee noted that these liver tumours
were observed only at doses that exceeded the maximum tolerated dose
and that there were signs of hepatocellular proliferation in these
animals which might have been involved in the hepatic carcinogenesis
observed. Pigmentation of the olfactory epithelium (with oxidized
lipofuscins) was observed in animals of each sex at 100 and 500 mg/kg
of diet; in the males, this effect was accompanied by an increased
incidence of inflammatory cell infiltration in the underlying Bowman
glands. Males and females at 500 mg/kg of diet also showed
pigmentation of the adrenal glands (with partly oxidized lipofuscins)
and hypercellularity of the bone marrow. As the Committee considered
the effects on the olfactory epithelium to be of no biological
significance, the NOEL was 10 mg/kg of diet, equal to 1.1 mg/kg bw per
day, on the basis of the effects on the liver.
In a 2-year study of carcinogenicity and toxicity, rats received
diets containing dicyclanil at a concentration of 0, 5, 25, 125, or
500 mg/kg of diet. In animals at the highest dose, food consumption
and body-weight gain were decreased, and the relative weights of
almost all organs were increased as a result. Treatment-related
histopathological alterations were observed in the exocrine pancreas
(hyperplasia) in males at the highest dose, in the liver (biliary
cysts) in females at the highest dose, and in the olfactory epithelium
(pigmentation resulting from accumulation of oxidized lipofuscins) in
males at doses of 25 mg/kg of diet and above and in females at doses
of 125 mg/kg of diet and above. Although the latter represents
enhancement of a naturally occurring age-related process, treatment
had no effect on survival, behaviour, or general well-being, and there
were no other morphological changes in the olfactory mucosa.
Therefore, the Committee concluded that the effect on the olfactory
epithelium was of no biological significance. Dicyclanil did not
affect the incidence of tumours. The NOEL was 125 mg/kg of diet, equal
to 22 mg/kg bw per day, on the basis of changes in body weight and
histopathological changes in the liver and pancreas.
Dicyclanil has been tested in vitro for its ability to induce
reverse mutation in Salmonella typhimurium and Escherichia coli,
gene mutation in Chinese hamster lung cells, chromosomal aberrations
in Chinese hamster ovary cells, and unscheduled DNA synthesis in
primary rat hepatocytes. It has been tested in vivo for its ability
to induce micronuclei in bone-marrow cells of mice treated orally. The
results of all of these tests were negative. On the basis of these
data, the Committee concluded that dicyclanil is not genotoxic.
Dicyclanil increased the incidence of liver tumours in female
mice. However, as these tumours occurred in only one tissue of animals
of one sex and one species at doses that were above the maximum
tolerated dose, and dicyclanil is not genotoxic, the Committee
concluded that dicyclanil does not represent a carcinogenic risk for
humans.
In a two-generation study of reproductive toxicity, with two
litters per generation, rats were given dicyclanil in the diet at a
concentration of 0, 5, 30, 200, or 500 mg/kg of diet. Treatment
reduced the body-weight gain of the parental animals at the highest
dose and, marginally, at 200 mg/kg of diet. Secondary to this effect,
dicyclanil increased the relative weights of most organs in animals at
the highest dose and of the brain (males and females), kidneys (males
only), and testis at 200 mg/kg of diet. Reproductive parameters were
not affected. The only effect of dicyclanil on pups was to reduce
their weight gain from day 4 post partum onwards. The NOEL for
parental toxicity was 30 mg/kg of diet, equal to 2 mg/kg bw per day,
on the basis of changes in body weight. The NOEL for reproductive
toxicity was 500 mg/kg of diet, equal to 24 mg/kg bw per day, the
highest dose tested. The NOEL for toxicity to the pups was 200 mg/kg
of diet, equal to 21 mg/kg bw per day, on the basis of reduced
body-weight gain.
In a study of developmental toxicity in rats given dicyclanil at
a dose of 0, 1, 5, 25, or 75 mg/kg bw per day orally on days 6-15 of
gestation, the highest dose was toxic to the dams, as seen by
reductions in body-weight gain, food consumption, and the weight of
the gravid uterus. Marginal reductions in body-weight gain and food
consumption were also observed at 25 mg/kg bw per day. The effects on
the fetuses, observed only at the highest dose, were reduced fetal
weight, a slightly increased incidence of renal pelvic dilatation, and
a number of mainly sternebral defects and variations due to poor or
absent ossification. There was no evidence of teratogenicity. The NOEL
for maternal toxicity was 5 mg/kg bw per day on the basis of the
reduction in body-weight gain. The NOEL for developmental toxicity was
25 mg/kg bw per day on the basis of reduced fetal weight, increased
renal pelvic dilatation, increased skeletal anomalies, and variations
consistent with a slight delay in skeletal maturation.
In a study of developmental toxicity in rabbits given dicyclanil
at a dose of 0, 1, 3, 10, or 30 mg/kg bw per day orally on days 7-19
of gestation, dams at 30 mg/kg bw per day had reduced food consumption
and those at 10 and 30 mg/kg bw per day showed reduced body-weight
gain. The fetuses of dams at the highest dose had lower body weights
than controls and an increased incidence of skeletal variations
indicative of a slight delay in ossification. There was no evidence of
teratogenicity. The NOEL for maternal toxicity was 3 mg/kg bw per day
on the basis of reduced body-weight gain. The NOEL for developmental
toxicity was 10 mg/kg bw per day on the basis of reduced fetal weight
and skeletal variations consistent with delayed ossification.
In pharmacological tests in vitro, dicyclanil at doses up to
3 mmol/L had no effect on the skeletal neuromuscular junction. At
concentrations of 0.3 mmol/L and higher, it had slightly antagonistic
effects on smooth muscle contractions induced by agonists. In mice and
rats given a single oral dose of 0, 1, 10, 50, or 100 mg/kg bw, the
highest dose affected general behaviour, locomotor activity, motor
coordination, heart rate, and tidal and minute lung volume. Locomotor
activity was also affected at 10 mg/kg bw and, very slightly, at
1 mg/kg bw. The treatment had no effect on body temperature, hypnotic
potentiation, gastrointestinal motility, blood pressure, heart beat,
or respiratory rate.
4. EVALUATION
The Committee established an ADI of 0-7 mg/kg bw, on the basis of
the NOEL of 0.71 mg/kg bw per day for increased plasma cholesterol
concentrations in the 1-year study of toxicity in dogs and a safety
factor of 100. As is its usual practice, the Committee rounded the
value of the ADI to one significant figure.
5. REFERENCES
Altmann, B. (1991) CGA 183893 tech. 28-Day range finding toxicity
study in beagle dogs. Unpublished report (test no. 901202) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Altmann, B. (1993) CGA 183893 tech. 3-Month subchronic dietary
toxicity study in beagle dogs. Unpublished report (test no.
922813) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Altmann, B. (1995) CGA 183893 tech. 12-Month chronic dietary toxicity
study in beagle dogs. Unpublished report (test no. 922814) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Bachmann, M. (1991) CGA 183893 tech. 28-Days range finding study in
rats (administration in food). Unpublished report (test no.
901201) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Bachmann, M. (1993) CGA 183893 tech. 3-Month oral toxicity study in
rats (administration in food). Unpublished report (test no.
922810) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Bachmann, M. (1996a) CGA 183893 tech. 18-Month oncogenicity study in
mice. Unpublished report (test no. 922815) from Ciba-Geigy Ltd,
Stein, Switzerland. Submitted to WHO by Novartis Animal Health
Inc., Basel, Switzerland.
Bachmann, M. (1996b) CGA 183893 tech. 24-Month carcinogenicity and
chronic toxicity study in rats. Unpublished report (test no.
922816) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Dubach-Powell, J.R. (1996) Pharmacokinetic study of CGA 183893 tech.
in the rat. Unpublished letter report (RCC project no.
615295/Ciba project no. 952803) from Research & Consulting
Company, Itingen, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
FitzGerald, R.E. (1990a) Preliminary oral toxicity study with CGA
183893 technical in pregnant rats (Dose range-finding study).
Unpublished report (test no. 901211) from Ciba-Geigy Ltd, Stein,
Switzerland. Submitted to WHO by Novartis Animal Health Inc.,
Basel, Switzerland.
FitzGerald, R.E. (1990b) Preliminary toxicity study in pregnant
rabbits with CGA 183893 technical (oral administration) (Dose
rangefinding study). Unpublished report (test no. 901212) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
FitzGerald, R.E. (1993a) CGA 183893 technical: Rat oral
teratogenicity. Unpublished report (study no. 922821) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
FitzGerald, R.E. (1993b) CGA 183893 technical: Rabbit oral
teratogenicity. Unpublished report (study no. 922822) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Geleick, D. (1992) CGA 183893 tech. Gene mutation test with Chinese
hamster cells V79 (OECD conform) in vitro. Unpublished report
(test no. 922827) from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Hagemann, C. (1992a) CGA 183893 tech. Acute dermal
irritation/corrosion study in the rabbit. Unpublished report
(test no. 922803) from Ciba-Geigy Ltd, Stein, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Hagemann, C. (1992b) CGA 183893 tech. Acute eye irritation/corrosion
study in the rabbit. Unpublished report (test no. 922802).
CibaGeigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Hagemann, C. (1993) CGA 183893 tech. Skin sensitization test in the
guinea pig optimisation test. Unpubished report (test no. 922805)
from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by
Novartis Animal Health Inc., Basel, Switzerland.
Hartmann, H.R. (1992a) CGA 183893 tech. Acute oral toxicity in the
rat. Unpublished report (test no. 922801) from Ciba-Geigy Ltd,
Stein, Switzerland. Submitted to WHO by Novartis Animal Health
Inc., Basel, Switzerland.
Hartmann, H.R. (1992b) CGA 183893 tech. Acute dermal toxicity in the
rat. Unpublished report (test no. 922804) from Ciba-Geigy Ltd,
Stein, Switzerland. Submitted to WHO by Novartis Animal Health
Inc., Basel, Switzerland.
Hartmann, H.R. (1993) CGA 183893 tech. Acute inhalation toxicity in
the rat. Unpublished report (test no. 922806) from Ciba-Geigy
Ltd, Stein, Switzerland. Submitted to WHO by Novartis Animal
Health Inc., Basel, Switzerland.
Hassler, S. (1994) Absorption, distribution and elimination of
[2-14C]pyrimidyl CGA 183893 in the rat after multiple oral
administration. Unpublished report (report no. 7/94; project no.
012AM01) from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to
WHO by Novartis Animal Health Inc., Basel, Switzerland.
Hertner, T. (1992) CGA 183893 tech. Salmonella and
Escherichia/liver-microsome test. Unpublished report (test no.
922825) from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Hertner, T. (1993a) CGA 183893 tech. Cytogenetic test on Chinese
hamster cells in vitro (EC conform). Unpublished report (test no.
922826) from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Hertner, T. (1993b) CGA 183893 tech. Autoradiographic DNA repair test
on rat hepatocytes (OECD conform) in vitro. Unpublished report
(test no. 922824) from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Hertner, T. (1993c) CGA 183893 tech. Micronucleus test, mouse (OECD
conform). Unpublished report (test no. 922823) from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by Novartis Animal
Health Inc., Basel, Switzerland.
Khalil, S. (1995) CGA 183893 technical: Rat dietary two-generation
reproduction study. Unpublished report (study no. 922818) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Löffler, A. (1998) The nature of residues in tissues of sheep after
single topical pour-on administration of [pyrimidine-2-14C]CGA
183893. Unpublished report (study no. 012AM04) from Novartis Crop
Protection AG, Basel, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Marty, J.H. (1995) CGA 183893 tech. 28-Day repeated dose dermal
toxicity study in the rat. Unpublished report (test no. 922807)
from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by
Novartis Animal Health Inc., Basel, Switzerland.
Ogorek, B. & Arni, P. (1987) Salmonella mutagenicity test with three
strains with CGA 183893 (Test for mutagenic properties in
bacteria). Unpublished report (experiment no. 871051) from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Pfister, T. & Gisin, M.(1996a) General pharmacology of CGA 183893
tech. Effect on the body temperature in the rat. Unpublished
report (project no. RCC 615317/CG 952803) from Research &
Consulting Company, Ltd, Itingen, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Pfister, T. & Gisin, M. (1996b) General pharmacology of CGA 183893
tech. Effect on the sleeping time induced by hexobarbitone in the
mouse. Unpublished report (project no. RCC 615330/CG 952803) from
Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Gisin, M. (1996c) General pharmacology of CGA 183893
tech. Effect on the intestinal motility (charcoal propulsion) in
the mouse. Unpublished report (project no. RCC 615385/CG 952803)
from Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Gisin, M. (1996d) General pharmacology of CGA 183893
tech. Modified Irwin screen test in the mouse. Unpublished report
(project no. RCC 615306/CG 952803) from Research & Consulting
Company, Ltd, Itingen, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Pfister, T. & Gisin, M. (1996e) General pharmacology of CGA 183893
tech. Effect on sperm motility, concentration and morphology in
the rat. Unpublished report (project no. RCC 615407/CG 952803)
from Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Gisin, M. (1996f) General pharmacology of CGA 183893
tech. Effect on the isolated ileum of the guinea pig. Unpublished
report (project no. RCC 615363/CG 952803) from Research &
Consulting Company, Ltd, Itingen, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Pfister, T. & Hussherr, D. (1996a) General pharmacology of CGA 183893
tech. Effect on the locomotor activity in the mouse. Unpublished
report (project no. RCC 615328/CG 952803) from Research &
Consulting Company, Ltd, Itingen, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Pfister, T. & Hussherr, D. (1996b) General pharmacology of CGA 183893
tech. Effect on motor co-ordination (rotarod) in the mouse.
Unpublished report (project no. RCC 615341/CG 952803) from
Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Hussherr, D. (1996c) General pharmacology of CGA 183893
tech. Effect on the isolated phrenic nerve-diaphragm preparation
of the rat. Unpublished report (project no. RCC 615352/CG 952803)
from Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Nordmann, A. (1996) General pharmacology of CGA 183893
tech. Effect on the cardiovascular and respiratory systems in the
rat. Unpublished report (project no. RCC 615374/CG 952803) from
Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Phillips, M. (1996) [Pyrimidine-2-14C]-CGA 183893. Nature of
metabolites in the excreta and tissues of sheep following single
pour-on administration. Unpublished report (No. 14072; project
no. 158665) from Inveresk Research, Tranent, Scotland. Submitted
to WHO by Novartis Animal Health Inc., Basel, Switzerland.
Thanei, P. (1996a) The metabolism of [2-14C]pyrimidyl CGA 183893 in
the rat. Unpublished report (No.7/96; project no. 012AM02) from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Thanei, P. (1996b) The nature of metabolites in tissues and excreta of
sheep after single topical administration of [2-14C]pyrimidyl
CGA 183893. Unpublished report (No. 9/96; project no. 012AM03)
from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Novartis Animal Health Inc., Basel, Switzerland.
Weber, E. (1998) CGA 183893 tech. Pigmentation of the olfactory mucosa
observed after dietary administration to rats. Unpublished report
(report no. CB 98/05) from Novartis Crop Protection AG, Basel,
Switzerland. Submitted to WHO by Novartis Animal Health Inc.,
Basel, Switzerland.
Three male New Zealand white rabbits (Chbb:NZW) received a
semi-occlusive topical application of 0.5 g of technical-grade
dicyclanil moistened with distilled water containing 0.5%
carboxymethylcellulose in 0.1% aqueous polysorbate 80 to their shaved
flanks. The study followed OECD test guideline 404, with GLP and
quality assurance certification. Only very slight erythema was
observed 1 ( n = 3) to 24 h ( n = 1) after removal of the patch
(Hagemann, 1992a).
In a study that followed OECD test guideline 405 with GLP and
quality assurance certification, three female New Zealand white
rabbits (Chbb:NZW) received an instillation of 0.1 ml (84 mg) of
technical-grade dicyclanil into the conjunctival sac of one eye. The
other eye served as control. The cornea appeared to be unaffected by
treatment; one animal showed an affected iris 1 h after instillation
but recovered within 24 h. Slight chemosis of the conjunctiva was
observed in two animals 1 h after instillation, but they recovered
within 24 h. Redness of the conjunctiva was seen in all animals,
although at different grades (score 1 or 2), and recovery to normal
occurred within 1-7 days (Hagemann, 1992b).
In 10 male and 10 female Pirbright white Tif:DHP guinea-pigs
submitted to an optimization test that followed OECD test guideline
406 with GLP and quality assurance certification, no significant skin
sensitization (1/20 positive) was observed after epidermal challenge
with 20% technical-grade dicyclanil in vaseline, this being a
subirritant dose. When the skin barrier was intentionally bypassed,
i.e. by intradermal challenge with a 0.1% solution of technical-grade
dicyclanil in 10% propylene glycol, 13 of 20 treated animals showed
positive reactions versus 3 of 20 animals given the vehicle
( p < 0.01) (Hagemann, 1993).
2.2.2 Short-term studies of toxicity
Rats
In a 28-day range-finding study, groups of five male and five
female Tif:RAIf (SPF) rats received technical-grade dicyclanil
(purity, > 98%) in the diet at a concentration of 0, 100, 500, or
2000 mg/kg of diet, equal to average achieved intakes of 0, 9.5, 50,
and 150 mg/kg bw per day for males and 0, 9.4, 49, and 160 mg/kg bw
per day for females. The study followed OECD test guideline 407
(adapted to the objectives of a range-finding study) without GLP or
quality assurance certification.
No treatment-related deaths occurred. Piloerection was observed
in animals of each sex at the highest dose during the second half of
the study. Dose-dependent reductions in food consumption, body-weight
gain, and final body weight were noted in all treated groups. These
were particularly marked in males at the high dose. Water consumption
was reduced in females at 500 and 2000 mg/kg of diet. Increased
incidences of anisocytosis and polychromasia of erythrocytes and a
slightly lower (not statistically significant) leukocyte count were
noted in males at the high dose. In both males and females at this
dose, higher plasma concentrations of urea and cholesterol and
increased activities of aspartate aminotransferase (twofold) and
alanine aminotransferase (five- to sevenfold) were noted.
Hypoglycaemia and lower plasma total bilirubin concentrations were
also observed in these animals, although the changes were not
statistically significant in females. Males at the high dose also had
slightly lower plasma globulin and calcium concentrations, while
females at this dose had an increased plasma phosphate concentration.
Consistent with the reduced body weights of all treated animals, the
absolute weights of a number of organs were decreased, whereas the
relative weights of a number of other organs were increased. The only
notable changes in organ weights which could be considered to be
treatment-related effects were reductions in the absolute and relative
weights of the prostate in males at the intermediate and high doses
and of the adrenals in females at the low and intermediate doses. No
macroscopic changes were found, apart from emaciation at the high
dose. Histopathologically, reduced spermatogenesis in the testes was
seen in 1/5 males at 500 mg/kg of diet and 5/5 males at 2000 mg/kg of
diet, accompanied by accumulation of cellular debris in the epididymal
duct in all those at the high dose. These animals also showed
immaturity of the prostate. In 1/5 females at 500 mg/kg of diet and in
4/5 females at 2000 mg/kg of diet, polyovular ovarian follicles, as
indicated by hypercellularity, were seen (Bachmann, 1991).
In a 3-month study of toxicity, groups of 10 male and 10 female
Tif:RAIf (SPF) rats received technical-grade dicyclanil in the diet at
a concentration of 0, 5, 25, 125, or 500 mg/kg of diet, equal to
average achieved intakes of 0, 0.31, 1.6, 8.0, and 33 mg/kg bw per day
for males and 0, 0.31, 1.7, 8.4, and 34 mg/kg bw per day for females.
Ten additional animals of each sex in the control and high-dose groups
were kept for a 4-week recovery period. The study followed OECD test
guideline 408, with GLP and quality assurance certification.
No treatment-related deaths or clinical signs were observed.
Slight reductions in body-weight gain and food consumption were
observed in animals of each sex at the high dose and in males at 125
mg/kg of diet. Owing to a compensatory increase in food intake during
the recovery period, the body weights of animals at the high dose were
comparable to those of the controls by the end of the recovery period.
Chemical analysis of blood revealed slightly lower plasma glucose
concentrations in males and females at 125 and 500 mg/kg of diet at
the end of the treatment period, but this was reversed within the
recovery period. Higher organ:body weight ratios were observed for
kidneys, brain, and testis in males at the high dose and for liver and
brain in females at this dose, these changes being reversible within
the 4-week recovery period. Although the relative epididymidal weights
were increased in males at doses > 25 mg/kg of diet, this was
considered not to be toxicologically significant because the absolute
epididymidal weights were not different from those of controls and
there were no abnormal histopathological findings. No
treatment-related ophthalmological or haematological changes were
observed, and no gross or microscopic alterations were observed. One
female at the high dose that was alllowed to recover had a mammary
tumour, which was considered to be of spontaneous origin. On the basis
of the reduction in body-weight gain, the NOEL was 25 mg/kg of diet,
equal to 1.6 mg/kg bw per day (Bachmann, 1993).
In a study that followed OECD test guideline 410 with GLP and
quality assurance certification, groups of five male and five female
Tif:RAIf (SPF) rats received dermal applications of technical-grade
dicyclanil at a dose of 0, 5, 30, 300, or 1000 mg/kg bw per day, 6
h/day on 5 days per week for 4 weeks. Dicyclanil was dissolved in
water containing 0.5% carboxymethylcellulose and 0.1% polysorbate 80
and was applied under an occlusive dressing to a clipped dorsal area
of the skin. At the end of each application period, the site was
washed with lukewarm water.
None of the animals died and no treatment-related clinical signs
were observed. Apart from a few incidental findings, no signs of local
skin irritation were seen. Male rats at 300 and 1000 mg/kg bw per day
showed dose-dependently decreased body weights and body-weight gain
and slightly lower food consumption. The plasma concentrations of
sodium and calcium were slightly reduced in these animals. Females at
the high dose showed increased absolute and relative liver weights. A
similar effect was observed in females at 300 mg/kg bw per day but did
not reach statistical significance. The absolute, but not the
relative, weight of the brain of females was increased at doses
> 30 mg/kg bw per day, without histopathological findings. No
treatment-related effects were noted on gross examination. Microscopic
examination showed hepatocyte hypertrophy in males at 1000 mg/kg bw
per day and in females at 300 and 1000 mg/kg bw per day. The NOEL was
30 mg/kg bw per day, on the basis of decreased body-weight gain and
changes in the liver (Marty, 1995).
Dogs
In a 28-day range-finding study, groups of two male and two
female beagles were given technical-grade dicyclanil (purity, > 98%)
in the diet at a concentration of 0, 200, 1000, or 2500 mg/kg of diet,
equal to average achieved intakes of 0, 5.6, 31, and 66 mg/kg bw per
day for males and 0, 6.2, 30, and 50 mg/kg bw per day for females. The
study followed OECD test guideline 409 (adapted to the objectives of a
range-finding study) without GLP (except for the pathology report) or
quality assurance certification.
No treatment-related deaths occurred. The clinical signs included
body tremors (in animals of each sex at 2500 mg/kg of diet), vomiting
(in animals of each sex at 2500 mg/kg of diet and in one male at 1000
mg/kg of diet), dyspnoea and slight apathy (in males at 2500 mg/kg of
diet). Animals at the high dose lost weight and had reduced food
consumption, and females at 1000 mg/kg of diet also showed a slight
reduction in food consumption. The reduction in food consumption did
not appear to be due to unpalatability. One male and one female at the
high dose showed a prolonged prothrombin time, and the other male at
the high dose had a slightly increased number of blood platelets.
Alanine aminotransferase activity was markedly increased and alkaline
phosphatase activity slightly increased in one male at the high dose
and in one female at the intermediate and one at the high dose;
aspartate aminotransferase activity was also increased in the female
at the high dose. Very slight decreases were observed in plasma
potassium concentrations in males at the intermediate and high doses,
in plasma glucose concentration in females at the high dose, and in
the plasma concentrations of urea, creatinine, and total bilirubin in
males and females at the high dose. Urinary analysis revealed an
increased incidence of proteinuria in males at the high dose and
females at the intermediate and high doses. The absolute and relative
weights of the testis and thymus were markedly decreased and those of
the heart slightly decreased at 2500 mg/kg of diet. In animals of each
sex at 1000 and 2500 mg/kg of diet, the absolute and relative weights
of the adrenal and kidney were increased, but without any
histopathological changes. Microscopic examination revealed hepatic
toxicity in one female at 1000 mg/kg of diet and in all animals at
2500 mg/kg of diet. The toxicity included focal or single-cell
necrosis, inflammatory-cell foci, and a brown granular pigment in
macrophages. One female at the intermediate dose also had mild
bile-duct hyperplasia, and one male at the high dose had periportal
hepatocyte enlargement. The testes of males at this dose showed
degeneration characterized by reduced numbers of tailed spermatids and
increased numbers of multinucleated or giant cells. Mild thymic
atrophy was noted in animals of each sex at the highest dose. The
heart of one male at this dose was mottled and showed inflammation,
fibrosis, and haemorrhage. Tubular basophilia or dilatation was
present in the kidneys of all animals at the high dose, two at the
intermediate dose, and one at the low dose, but not in controls. This
lesion is of minimal toxicological significance (Altmann, 1991).
Groups of four male and four female beagles received
technical-grade dicyclanil in the diet at a concentration of 0, 20,
100, 500, or 1500 mg/kg of diet for 3 months, equal to average
achieved intakes of 0, 0.61, 2.7, 14, and 42 mg/kg bw per day for
males and 0, 0.71, 3.5, 17, and 42 mg/kg bw per day for females. The
study followed OECD test guideline 409, with GLP and quality assurance
certification.
One male at the high dose was found dead at week 11 after general
deterioration in clinical condition accompanied by tonic-clonic
spasms. Post-mortem examination did not reveal the cause of death.
Animals at the high dose started to show clinical signs from week
9-11, including slight ataxia, unnaturally raised tails, and frequent
shaking. In addition, vomitus and traces of blood in the faeces were
observed. Ophthalmoscopic examinations revealed no treatment-related
changes. Animals at the high dose lost weight (males only) or had
reduced body-weight gain with reduced food intake. A very slight
reduction in food intake was also observed transiently in some animals
at 500 mg/kg of diet. Slightly reduced haemoglobin concentrations and
haematocrit values, associated with minor microcytosis and
hypochromasia of erythrocytes, were recorded in animals at the high
dose. Increased plasma cholesterol and phospholipid concentrations
were observed in animals at doses > 100 mg/kg of diet. Plasma
albumin concentrations were slightly decreased in males and females at
1500 mg/kg of diet and in females at 500 mg/kg of diet. Decreased
plasma calcium, potassium, urea, creatinine, and total bilirubin
concentrations were found in animals at the high dose. Urinary
analysis revealed no treatment-related effects. The mean absolute and
relative liver weights were increased in animals at the highest dose
and in females also at 20, 100 (not statistically significant), and
500 mg/kg of diet (not dose-related). The absolute and relative
adrenal weights were also slightly increased at the high dose. The
absolute and relative weights of the thymus (males), testis, and
spleen (both sexes) were decreased in animals at the high dose. The
kidney weights were increased in males (relative) and females
(absolute and relative) at 1500 mg/kg of diet. Macroscopic examination
showed no treatment-related effects. Microscopy of the liver showed
minimal to moderate focal or multifocal subcapsular inflammation with
fibrosis in 2/4 males and 3/4 females at the high dose. Enlarged
hepatocytes, diagnosed as cellular oedema, were observed in the
centrilobular and midzonal region of 3/4 females at 20, 100, and 500
mg/kg of diet and in 1/4 males and all females at 1500 mg/kg of diet.
No morphological signs of hepatocellular damage were apparent. Minimal
atrophy of the white pulp of the spleen was observed in 3/4 males at
500 and 1500 mg/kg of diet and in all females at 1500 mg/kg of diet.
Thymic atrophy was found in 3/4 males at 500 mg/kg of diet and in all
males at 1500 mg/kg of diet. Minimal atrophy of the lymphatic tissue
of the mesenteric lymph node was observed in 3/4 males at the high
dose, and minimal to marked atrophy of the glandular tissue of the
prostate was detected in 3/4 males at 100 mg/kg of diet, 1/4 at 500
mg/kg of diet, and 4/4 at 1500 mg/kg of diet. Examination of the
testes revealed minimal tubular atrophy in 3/4 males at the high dose,
associated with a marked reduction in spermatogenesis in all males of
this group. A dose-related increase in the frequency of inflammatory
changes associated with epithelial hyperplasia was found in the
urinary bladders of females at 100, 500, and 1500 mg/kg of diet
(Altmann, 1993). The Committee noted that hepatocyte oedema without
hepatocellular damage is not of toxicological significance. Hence, the
NOEL was 20 mg/kg of diet, equal to 0.61 mg/kg bw per day, on the
basis of increased plasma cholesterol concentrations and
histopathological findings in the prostate and urinary bladder.
In a 1-year study of toxicity, groups of four male and four
female beagles received technical-grade dicyclanil in the diet at a
concentration of 0, 5, 25, 150, or 750 mg/kg of diet, equal to average
achieved intakes of 0, 0.16, 0.71, 4.4, and 23 mg/kg bw per day for
males and 0, 0.15, 0.77, 5.1, and 23 mg/kg bw per day for females. Two
additional animals per sex in the control and high-dose groups were
maintained for a 4-week recovery period. The observations included
deaths, clinical signs, body weight, food consumption, ocular,
neurological, haematological, clinical chemical, and urinary
parameters, and macroscopic and microscopic end-points. The study
followed OECD test guideline 452, with GLP and quality assurance
certification.
One female at the high dose was found dead on day 13, with no
previous abnormal clinical signs. One male at the high dose was killed
on study day 32 after vomiting, showing marked apathy, lying in a
lateral position, and weight loss correlated to reduced food intake.
Vomiting was observed in females at the high dose, and the body-weight
gain (in two animals) and food consumption of females at the high dose
were slightly reduced. Ocular and neurological examinations revealed
no treatment-related effects, nor were there any changes in
haematological or urinary parameters. Throughout the treatment period,
the plasma cholesterol concentrations were increased in animals of
each sex at 750 mg/kg of diet (not statistically significant in
females) and in males at 150 mg/kg of diet; in males, the change was
not reversed after the 4-week recovery period. Males at the high dose
showed slightly lower plasma calcium concentrations. Both males and
females at the high dose had reduced plasma concentrations of
bilirubin and urea and reduced alkaline phosphatase activity. The
changes in blood chemistry were partially reversed during the recovery
period. The absolute and relative liver weights were increased in
animals at 750 mg/kg of diet, but only the change in absolute weight
in males was statistically significant. The absolute (statistically
significant) and relative weights of the heart were decreased in
females at the high dose. The weight changes were reversed by the end
of the 4-week recovery period. The macroscopic and microscopic
findings were confined to the two animals at the high dose that died
before the scheduled kill. The macroscopic findings consisted of a
scar in the liver and pale kidneys in the male and a mass and
haemorrhagic content in the abdominal cavity of the female.
Histopathologically, both animals showed marked diffuse liver necrosis
and kidney lesions (more severe in the male). The male also showed
testicular and prostatic atrophy, and the female had a thrombus in a
peritoneal blood vessel. The two animals suffered from acute, severe
liver failure and resulting cardiocirculatory disturbances, and the
male also showed stress due to weight loss. Their condition was very
different from that of the other treated dogs in this study, and
comparable acute, severe liver toxicity was not seen in the 28-day and
3-month studies in dogs treated with doses up to 2500 and 1500 mg/kg
of diet, respectively. The lesions seen in the two animals are
considered to be incidental findings. On the basis of increased plasma
cholesterol concentrations in males, the NOEL was 25 mg/kg of diet,
equal to 0.71 mg/kg bw per day (Altmann, 1995).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In an 18-month study of carcinogenicity, groups of 60 male and 60
female Tif:MAGf (SPF) mice were given diets containing technical-grade
dicyclanil at a concentration of 0, 10, 100, 500, or 1500 mg/kg of
diet, equal to average achieved intakes of 0, 1.1, 12, 59, and 210
mg/kg bw per day for males and 0, 1.1, 12, 65, and 200 mg/kg bw per
day for females. Ten animals per sex from each group were assigned for
evaluation of haematological parameters. The study followed OECD test
guideline 451 with GLP and quality assurance certification, but
histopathological examination of the animals at the high dose (i.e.
the group that was killed before study termination, see below) was
limited to the liver and lungs.
Clinical signs were observed only in males at the highest dose,
which injured themselves through vigorous scratching. A higher
mortality rate was noted among males at the high dose and to a lesser
extent in females at this dose. The self-inflicted injuries and poor
health of the animals at the high dose led to a decision to terminate
all surviving animals in this group during weeks 58-59. Doses < 500
mg/kg of diet did not affect survival. The body weights of males and
females at 1500 mg/kg of diet were reduced, with an approximately 50%
reduction in body-weight gain, and those of females at 500 mg/kg of
diet were reduced with a 30% reduction in body-weight gain. As food
intake was not affected, higher food consumption ratios were noted in
animals of each sex at 1500 mg/kg of diet and in females at 500 mg/kg
of diet. Haematological parameters were not affected by treatment. The
absolute and relative weights of the liver were increased in animals
of each sex at 500 mg/kg of diet (for males after adjustment for three
control outliers). In females at 500 mg/kg of diet, the relative
weights of the kidney, brain, and adrenals were increased, while the
absolute weights of these organs were not changed. The
treatment-related macroscopic findings included enlarged livers
(characterized histopathologically as hepatocellular hypertrophy) in
males at 500 mg/kg of diet and in both sexes at 1500 mg/kg of diet,
and masses and/or nodules of the liver in females at 500 and 1500
mg/kg of diet. Treatment-related microscopic findings were observed in
the liver, olfactory epithelium, adrenal gland, and bone marrow.
Kupffer cell pigmentation (mainly haemosiderin) and hepatocellular
necrosis were observed in males at > 100 mg/kg of diet. Increased
numbers of hepatocellular mitotic figures and multinucleated
hepatocytes were seen in the livers of males at the high dose, and the
frequency of foci of cellular change was increased in animals of each
sex at the highest dose. The incidence of heptocellular adenomas was
higher in females at 500 and 1500 mg/kg of diet (9/53 and 5/60,
respectively) than in controls (0/52). In addition, the incidence of
hepatocellular carcinomas was increased in females at the highest dose
(6/60 versus 0 in all other groups). Pigmentation of the olfactory
epithelium was observed at increasing incidence and severity in
animals of each sex at 100 and 500 mg/kg of diet, and males at these
doses showed an increased incidence of inflammatory cell infiltration
(cell type not specified) in the underlying Bowman's glands. At 500
mg/kg of diet, both males and females also showed increased incidences
of pigmentation of the adrenal glands, recorded as ceroid (i.e. a
partly oxidized form of lipofuscin) deposition, and of
hypercellularity of the bone marrow. Treatment with dicyclanil did not
affect the number of animals with malignant lymphomas. It was noted,
however, that females at 500 mg/kg of diet had more sites that
appeared to be infiltrated by malignant lymphoma cells than did
controls or animals at other doses (Bachmann, 1996a).
The Committee noted that the doses at which the liver adenomas
and carcinomas occurred exceeded the maximum tolerated dose for
females, and that there were signs of hepatocellular proliferation in
these animals which might have been involved in the hepatic
carcinogenesis observed. Pigmentation of the olfactory epithelium was
also observed in a long-term study in rats (Bachmann, 1996b; see
below) and was further investigated in a separate study (Weber, 1998;
see below). As the Committee considered the effects on the olfactory
epithelium to be of no biological significance (see below), the NOEL
in this study in mice was 10 mg/kg of diet, equal to 1.1 mg/kg bw per
day, on the basis of effects on the liver.
Rats
In a 2-year study of carcinogenicity and toxicity, groups of 80
Tif:RAIf (SPF) rats of each sex were given technical-grade dicyclanil
in the diet at a concentration of 0, 5, 25, 125, or 500 mg/kg of diet,
equal to average achieved intakes of 0, 0.19, 0.97, 4.8, and 22 mg/kg
bw per day for males and 0, 0.23, 1.2, 6.0, and 26 mg/kg bw per day
for females. Haematological, clinical chemical, and urinary analyses
were performed during weeks 13, 26, 52, 78, and 105 on 20, 10, and 10
animals of each sex per group, respectively. After 1 year, 10 animals
of each sex per group were killed. The study followed OECD test
guideline 453, with GLP and quality assurance certification.
Treatment with dicyclanil did not affect the incidence of
clinical signs or survival. Decreased food consumption was noted in
males and females at the highest dose. The body weights of males and
females at 500 mg/kg of diet were reduced (with an approximately 25%
reduction in body-weight gain), as were the body weights of animals at
125 mg/kg of diet (with a reduction in body-weight gain of < 10%).
Slight but statistically significant red blood cell dyscrasia and a
reduced number of monocytes were observed in males at the high dose
when compared with controls, but the changes were generally
inconsistent over time, not dose-related, and within the range of
historical controls and are therefore regarded as toxicologically
insignificant. Increased concentrations of inorganic phosphate were
observed in males and females at the high dose throughout the study,
although the increase was not statistically significant in females.
Males at 125 mg/kg of diet also had increased inorganic phosphate
concentrations, but these reached statistical significance only in
weeks 78 and 105. Lower concentrations of triglycerides were recorded
for males at 500 mg/kg of diet, although statistical significance was
achieved only in weeks 13 and 26. Urinary parameters were not
affected. As a result of the lower terminal body weights, almost all
of the relative organ weights were increased in males and females at
the highest dose, especially of the kidney, liver, and epididymides.
The absolute epididymal weights were also increased in males at the
high dose at 105 weeks. Males at 125 mg/kg of diet also had increased
relative liver weights, although these were within historical control
values. An increased incidence of liver cysts was observed in females
at the high dose, characterized primarily as unilocular or
multilocular biliary cysts on microscopic examination. In males at the
high dose, an increased incidence of masses and nodules in the
exocrine pancreas was noted, which were characterized
histopathologically as foci or areas of hyperplasia. Aside from the
histopathological findings in the pancreas and liver, the only other
treatment-related histopathological finding was an increased incidence
of pigmentation of the olfactory epithelium in males at > 25 mg/kg
of diet and in females at > 125 mg/kg of diet, the effects seen at
125 and 500 mg/kg of diet being more severe than at lower doses.
Dicyclanil did not affect the tumour incidence in this study
(Bachmann, 1996b).
Weber (1998) studied the histological nature and causes of the
pigmentation of the olfactory epithelium, using material from male
rats in the 2-year study (controls and rats given 500 mg/kg of diet)
and the 3-month study (controls and rats given 500 mg/kg of diet;
Bachmann, 1993). Material from male control rats of the same strain in
five other long-term studies was included to provide more reference
material.
Minimal to moderate pigmentation was found in controls from the
long-term studies with dicyclanil and other compounds, but not in
controls from the 3-month study. Treatment with 500 mg/kg of diet
resulted in increased pigmentation, which was minimal after 3 months
and moderate to marked after 12 and 24 months, the degree of
pigmentation at the later times being similar. Staining indicated that
the pigment consisted mainly of oxidized lipofuscins and was located
in the olfactory epithelium and the underlying lamina propria. The
pigment appeared to be located in secondary lysosomes. Further
investigations by high-resolution microscopy indicated that the
supporting cells and secretory cells of Bowman glands were affected by
the pigmentation. Neuronal olfactory perikarya, nerve bundles of the
olfactory nerve in the olfactory mucosa, and the olfactory bulbs (in
the brain) were free of pigment accumulation. Apart from the
pigmentation, no other treatment-related morphological changes in the
olfactory mucosa were found. According to the author, the clinical
signs in the 2-year study gave no indication of a disturbed olfactory
sense due to dicyclanil treatment. In addition, the presence of
mucopolysaccharides in the Bowman glands indicated normal functioning
of the olfactory mucosa in the dicyclanil-treated rats. The author
concluded that the increased pigmentation of the olfactory epithelium
in male rats after treatment with dicyclanil results from accumulation
of lipofuscin in the cytoplasm of supporting cells and secretory cells
of the Bowman glands and is an enhancement of a natural age-related
process. In the absence of other morphological changes in the
olfactory mucosa, the author considered the pigmentation not to be
detrimental to the structure or function of the olfactory mucosa and,
therefore, is not to be regarded as adverse (Weber, 1998).
Recognizing that the effects on the olfactory epithelium were an
enhancement of a natural age-related process, the Committee noted that
dicyclanil had no effect on survival, behaviour, or general
well-being. As there were no other morphological changes in the
olfactory mucosa, the Committee concluded that the effect was of no
biological significance. Therefore, the NOEL in the 2-year study in
rats was 125 mg/kg of diet, equal to 22 mg/kg bw per day, on the basis
of changes in body weight and histopathological changes in the liver
and pancreas.
2.2.4 Genotoxicity
The results of studies for genotoxicity with technical-grade
dicyclanil are summarized in Table 1. The studies were of conventional
design, with GLP and quality assurance certification, except for the
study of Ogorek & Arni (1987).
2.2.5 Reproductive toxicity
(a) Multigeneration study
Rats
In a two-generation study of reproductive toxicity with two
litters per generation, groups of 30 male and 30 female Tif:RAIf (SPF)
rats received diets containing technical-grade dicyclanil at a
concentration of 0, 5, 30, 200, or 500 mg/kg of diet from 10 weeks
before the first mating until necropsy at the end of the lactation
period. Dams were allowed to litter and suckle their pups naturally.
When possible, the F1a litters were culled to four pups of each sex
per litter at day 4 post partum. After weaning, a number of F1a
pups were selected to be the F1 parents, and the remaining F1a
pups were necropsied. The F0 parents were then mated a second time,
and the resulting litters (F1b) were again culled to four pups of
each sex per litter. After weaning, both the F1b pups and the F0
parents were necropsied. The same procedure was followed for the F1
parents in order to produce F2a and F2b generations. The
observations included clinical signs, deaths, body weight, food
consumption, reproductive parameters, pup survival and development,
macroscopic appearance at necropsy, and, for parents in the control
and high-dose groups, histological appearance of the reproductive and
target organs. The study followed OECD test guideline 416, with GLP
and quality assurance certification.
Effects in F0 parents: The F0 parents showed no
treatment-related deaths or clinical signs and no effects on male or
female mating or fertility indices, on maternal gestation or
parturition indices, or on the duration of gestation. In the premating
period, males and females showed decreased body-weight gain and food
intake at 500 mg/kg of diet and, marginally, at 200 mg/kg of diet.
During both gestation periods, the overall body-weight gain of females
in all treated groups was similar to that of controls, while in both
lactation periods the overall body-weight gain of females was
increased at 500 mg/kg of diet and, marginally, at 200 mg/kg of diet.
Gross examination and histology revealed no treatment-related effects.
Secondary to the reduced body weights, the relative weights of most
organs were increased in males and females at 500 mg/kg of diet. At
this dose, the absolute weights of the heart and liver of males were
decreased.
Table 1. Results of assays for genotoxicity with dicyclanil
End-point Test object Concentration Result Reference
In vitro
Reverse S. typhimurium 20-5000 µg/plate Negativea Ogorek & Arni
mutation TA1537, TA98, (1987)
TA100
Reverse S. typhimurium 313-5000 µg/plate Negativeb Hertner (1992)
mutation TA1535, TA1537,
TA98, TA100,
E. coli WP2 uvrA
Gene mutation V79 Chinese 12.4-400 µg/ml Negative Geleick (1992)
hamster lung cells, - S9c
hprt locus 24.7-667 µg/ml
+ S9d,e
Chromosomal Chinese hamster 20.8-83.4 µg/ml Negative Hertner (1993a)
aberration ovary cells -S9c
166.75-667 µg/ml
+S9d,e
Unscheduled Primary rat 6.2-670 µg/mle Negative Hertner (1993c)
DNA synthesis hepatocytes
In vivo
Micronucleus Mouse bone 47-188 mg/kg bw; Negativef Hertner (1993b)
formation marrow once by oral gavage
a With and without rat liver S9 fraction; results of cytotoxicity test not given,
precipitation not reported
b With and without rat liver S9 fraction; precipitation at 5000 µg/plate
c Cytotoxic at the highest concentration
d Slightly cytotoxic at the highest concentration
e The highest concentration represents the limit of solubility in dimethyl sulfoxide.
f At all doses and all times, the ratio of polychromatic to normochromatic erythrocytes
did not deviate from that in controls; clinical signs of toxicity (including reduced
locomotor activity, unkempt fur, ataxia, piloerection, and diarrhoea) were observed
at the highest dose tested; doses > 312.5 mg/kg bw resulted in death.
Effects in F1 pups: At all doses and both matings, no
treatment-related effects were seen in the F1 offspring in terms of
sex ratio, clinical signs, litter size, the development of physical
landmarks (surface righting and eye opening), or macroscopic findings
at necropsy. A slight increase in the pup mortality rate was observed
on days 0-4 post partum at 500 mg/kg of diet (F1a) and at 30 and
200 mg/kg of diet (F1b). This was considered not to be
treatment-related because it could be attributed to two (F1a) or
three (F1b) litters and no dose-response relationship was found. At
500 mg/kg of diet, the weights of F1a pups were lower than those of
controls at birth and on days 14 and 21 post partum, owing to
reduced weight gain from day 4 post partum onwards. The weights of
F1b pups at the high dose were similar to those of controls from
birth through to day 14 post partum. Thereafter, the weight gain
decreased, resulting in lower pup weights on day 21 post partum.
Effects in F1 parents: In the F1 parents, no
treatment-related deaths or clinical signs and no effects on male or
female mating or fertility indices, on maternal gestation or
parturition indices, or on the duration of gestation were observed.
The body weights of the F1 parents at the high dose were lower than
those of controls throughout the study. Although the body weights of
males at 200 mg/kg of diet were reduced during the final part of the
study, the overall body-weight gain of these animals was not different
from that of controls. During both gestation periods, the overall
body-weight gain of all treated females was slightly lower than that
of controls, while in both lactation periods the overall body-weight
gain of females was increased at 500 mg/kg of diet and, marginally, at
200 mg/kg of diet. The absolute weights of most organs of males at the
high dose were decreased, with the exception of those of the testis
and brain. In females at the high dose, only the absolute weights of
the heart, liver, and kidney were decreased. At 200 mg/kg of diet, the
absolute weight of the liver was decreased in animals of each sex. In
contrast, secondary to the reduced body weights of these two groups,
the relative weights of most organs were increased, attaining
statistical significance for most organs at 500 mg/kg of diet and for
brain (both sexes), kidneys (males only), and testis at 200 mg/kg of
diet. Gross examination and histology revealed no treatment-related
effects.
Effects in F2 pups: At all doses and both matings, no
treatment-related effects were noted in the F2 offspring in terms of
sex ratio, mortality rate, clinical signs, litter size, the
development of physical landmarks (surface righting and eye opening),
or macroscopic findings at necropsy. The only treatment-related effect
observed was reduced weight gain in F2a and F2b pups at the
highest dose from day 4 post partum onwards, resulting in
significantly lower pup weights on days 14 and 21 post partum.
The NOEL for parental toxicity was 30 mg/kg of diet, equal to 2
mg/kg bw per day, on the basis of changes in body weight. The NOEL for
reproductive toxicity was 500 mg/kg of diet, equal to 24 mg/kg bw per
day, the highest dose tested. The NOEL for pup toxicity was 200 mg/kg
of diet, equal to 21 mg/kg bw per day, on the basis of reduced
body-weight gain (Khalil, 1995).
(b) Developmental toxicity
Rats
In a range-finding study, groups of eight pregnant Tif:RAIf (SPF)
rats received technical-grade dicyclanil (purity, > 98%) in 3%
aqueous corn starch by oral gavage at a dose of 0, 75, or 150 mg/kg bw
per day on days 6-15 of gestation. On day 21 of gestation, the dams
were killed and necropsied, and the fetuses were weighed, sexed, and
examined for external abnormalities.
Two dams at the high dose were killed in moribund condition. The
clinical signs in these animals and in a third animal at the high dose
included piloerection, salivation, dyspnoea, and hypotonia. Maternal
body-weight gain and gravid uterine weights were dose-dependently
decreased in all treated animals, but food consumption was
dose-dependently decreased only during the first week of treatment.
Post-implantation loss was dose-dependently increased by treatment,
owing to early resorptions. Three dams at the high dose resorbed their
entire litters. The number of viable fetuses and fetal body weights
were dose-dependently reduced. Three fetuses at the high dose showed
generalized oedema (FitzGerald, 1990a).
In the main study, groups of 24 pregnant Tif:RAIf (SPF) rats
received technical-grade dicyclanil in an aqueous solution of 0.5%
sodium carboxy-methylcellulose by oral gavage at a dose of 0, 1, 5,
25, or 75 mg/kg bw per day on days 6-15 of gestation. On gestation day
21, the dams were killed and necropsied, and the fetuses were weighed,
sexed, and examined for external, visceral, and skeletal
abnormalities. The study was of conventional design, with GLP and
quality assurance certification.
There were no deaths and no treatment-related clinical signs of
toxicity. Maternal body weight, body-weight gain, and food consumption
as well as net body-weight change and carcass weight at necropsy were
reduced at the highest dose. A marginal decrease in these parameters
was also observed at 25 mg/kg bw per day. One animal at 75 mg/kg bw
per day had total, early resorptions and showed haemorrhagic fluid in
the uterus at necropsy. In animals at 75 mg/kg bw per day, the gravid
uterine weight was reduced; the number of early postimplantation
losses was slightly increased and the number of fetuses per litter was
slightly lower, but without statistical significance. Effects on the
fetuses were observed only at the highest dose. These included reduced
fetal weight, a slight increase in the frequency of renal pelvic
dilatation, and a number of mainly sternebral defects and variations
due to poor or absent ossification. There was no evidence of
teratogenicity. The NOEL for maternal toxicity was 5 mg/kg bw per day,
on the basis of a reduction in body-weight gain. The NOEL for
developmental toxicity was 25 mg/kg bw per day, on the basis of
reduced fetal weight, increased renal pelvic dilatation, and increased
skeletal anomalies and variations consistent with a slight delay in
skeletal maturation (FitzGerald, 1993a).
Rabbits
In a range-finding study, groups of eight pregnant Russian
rabbits (strain unspecified) received technical-grade dicyclanil
(purity, > 98%) in 3% aqueous corn starch by oral gavage at a daily
dose of 0, 20, or 40 mg/kg bw on days 7-19 of gestation. On day 29 of
gestation, the dams were killed and necropsied, and the fetuses were
weighed, sexed, and examined for external abnormalities.
No treatment-related deaths or clinical signs were observed.
During treatment, the body-weight gain and food consumption of dams at
the high dose were considerably reduced. The gravid uterine weights
were not affected by treatment. The rate of pre-implantation loss was
slightly reduced and that of postimplantation loss slightly increased
at 40 mg/kg bw per day, which resulted in a comparable number of
fetuses to controls. The body weights of female fetuses were slightly
reduced at this dose. There were no remarkable external fetal changes
(FitzGerald, 1990b).
In the main study, groups of 19 pregnant Russian Chbb:HM rabbits
received technical-grade dicyclanil in an aqueous solution of 0.5%
sodium carboxy-methylcellulose by oral gavage at a daily dose of 0, 1,
3, 10, or 30 mg/kg bw on days 7-19 of gestation. On gestation day 29,
the dams were killed and necropsied, and the fetuses were weighed,
sexed, and examined for external, visceral, and skeletal
abnormalities. The study was of conventional design, with GLP and
quality assurance certification.
No deaths or treatment-related clinical signs of toxicity were
observed. Maternal body weight, body-weight gain, and food consumption
and the carcass weight at necropsy were reduced at 30 mg/kg bw per
day. Maternal body-weight gain was also reduced at 10 mg/kg bw per
day. Necropsy of the dams revealed no treatment-related effects, and
reproductive function was not affected. The body weights of fetuses at
the highest dose were reduced, and the fetuses showed skeletal
variations indicative of a slight delay in ossification. There was no
evidence of teratogenicity. The NOEL for maternal toxicity was 3 mg/kg
bw per day on the basis of reduced body-weight gain. The NOEL for
developmental toxicity was 10 mg/kg bw per day on the basis of reduced
fetal weight and increased skeletal variations consistent with delayed
ossification (FitzGerald, 1993b).
2.2.6 Special studies: Pharmacological effects
The effects of dicyclanil on the central nervous system
(centrally controlled behaviour, body temperature, locomotor activity,
hypnotic potentiation, motor coordination), the peripheral nervous
system, the autonomic nervous system and smooth muscles, the
cardiovascular and respiratory systems, and the gastrointestinal tract
were investigated in mice, rats, and guinea-pigs in vivo and
in vitro. The effect of dicyclanil on sperm morphology and motility
was also examined. All of the studies followed the Japanese Guidelines
for the Registration of Pharmaceuticals (General Pharmacology), with
GLP and quality assurance certification.
In vivo, technical-grade dicyclanil in polyethylene glycol
200:ethanol (5:3 v/v) was administered orally by gavage at a single
dose of 0, 1, 10, 50, or 100 mg/kg bw to male NMRI mice or male Han
Wistar rats. Doses < 100 mg/kg bw had no effect on the body
temperature of rats (Pfister & Gisin, 1996a), on the
hexobarbitone-induced sleeping time in mice (Pfister & Gisin, 1996b),
or on gastrointestinal motility in mice (Pfister & Gisin, 1996c).
In the modified Irwin test for general behavioural changes,
treatment of mice with dicyclanil at 100 mg/kg bw slightly inhibited
both exploratory activity and the startle response. The changes were
most evident 6 h after treatment, with complete recovery of the
startle response by 8 h and of exploratory activity by 24 h after
treatment. Mice treated with 1 or 10 mg/kg bw showed normal behaviour
(Pfister & Gisin, 1996d). After treatment with the vehicle, reduced
locomotor activity (static, mobile, and rearing activity and mobile
and active times) was seen in control mice over 24 h, being most
pronounced during the first 8 h. These parameters were less markedly
reduced after treatment with dicyclanil at 1 (not statistically
significant) or 10 mg/kg bw. Opposite effects were induced by
dicyclanil at 100 mg/kg bw, when the reduction in static activity was
more pronounced than in controls while changes in the other parameters
were comparable. All of the effects were fully reversed by 24 h after
dosing (Pfister & Hussherr, 1996a). Motor coordination in mice, as
assessed by recording the time the animals could retain their balance
on a rotating rod, was inhibited at 100 mg/kg bw but not at 1 or 10
mg/kg bw. The effect was significant 4 h after dosing but had
completely disappeared by 24 h (Pfister & Hussherr, 1996b).
Treatment of rats with 100 mg/kg bw (the only dose tested)
resulted in slight increases throughout the observation period (6-8 h
after dosing) in heart rate (statistically significant) and tidal and
minute lung volume (statistically significant only when compared with
values for vehicle-treated animals, which generally had lower values
than untreated animals). The blood pressure, electrocardiogram, and
respiratory rate remained unchanged (Pfister & Nordmann, 1996).
Treatment of rats with 50 mg/kg bw (the only dose tested) had no
effect on sperm motility, concentration, or morphology. Minor,
statistically nonsignificant increases in abnormal sperm morphology 6
weeks after dosing (sperm with head only and with abnormally shaped
hooks) were completely reversed by 12 weeks after dosing (Pfister &
Gisin, 1996e.
In vitro, a concentration of 0, 0.1, 0.3, 1, or 3 mmol/L of
technical-grade dicyclanil in dimethyl sulfoxide had no significant
effect on directly induced contractions of skeletal muscle or on
contractions induced indirectly by phrenic nerve stimulation in the
isolated phrenic nerve-diaphragm preparation from rats. The authors
concluded that dicylanil has no effect on the skeletal neuromuscular
junction (Pfister & Hussherr, 1996c). When dicyclanil was tested on
ileum isolated from guinea-pigs, dose-dependent antagonistic activity
against the smooth muscle contractions induced by histamine and
acetylcholine and against the nonspecific smooth muscle contractions
induced by barium chloride was observed at concentrations > 0.3, 1,
and > 0.3 mmol/L, respectively. All of the effects were fully and
rapidly reversible. Dicyclanil was considered to be only a weak
antagonist (Pfister & Gisin, 1996f).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term and long-term
toxicity, carcinogenicity, genotoxicity, reproductive toxicity, and
pharmacology of dicyclanil. All of the pivotal studies were carried
out according to appropriate standards for study protocol and conduct.
After repeated oral administration of radiolabelled dicyclanil to
rats, the radiolabel was rapidly and almost completely absorbed and
was rapidly distributed to the major organs and tissues. Elimination
was rapid (> 93% of the total dose within 24 h) and was virtually
complete within 3 days. The major route of elimination was the urine
(79-83% of the total dose within 24 h), while the faecal route was of
minor importance (6-12% of the total dose within 24 h).
Biotransformation in the rat involves oxidative opening of the
cyclopropyl ring at various positions, followed by further oxidation
and cleavage of the cyclopropyl-N bond (i.e. dealkylation). The
metabolic pathways of dicyclanil in sheep treated topically are
essentially the same as those in rats.
After oral administration of dicyclanil to rats, the LD50
values were 560 mg/kg bw in males and approximately 500 mg/kg bw in
females. Dicyclanil is moderately hazardous when given as a single
oral dose.
In a three-month study of toxicity, rats received dicyclanil in
the diet at a concentration of 0, 5, 25, 125, or 500 mg/kg of diet.
Male rats at 125 and 500 mg/kg of diet showed decreased food
consumption and body-weight gain and slightly decreased plasma glucose
concentrations. The weights of the kidneys, brain, and testis relative
to that of the body were increased in males at 500 mg/kg of diet, and
females at this dose showed decreased food consumption, body-weight
gain, and plasma glucose concentrations and increased relative weights
of the liver and brain; plasma glucose concentrations were also
reduced in females at 125 mg/kg of diet. The NOEL was 25 mg/kg of
diet, equal to 1.6 mg/kg bw per day, on the basis of the reduction in
body-weight gain.
In a 3-month study of toxicity, dogs received dicyclanil in the
diet at a concentration of 0, 20, 100, 500, or 1500 mg/kg of diet.
Clinical signs consisting mainly of neurotoxicity, decreased food
consumption and body-weight gain, and changes in clinical chemistry
and erythrocyte parameters were observed mainly at the highest dose.
Plasma cholesterol and phospholipid concentrations were increased in
animals at concentrations of 100 mg/kg of diet and above. The weights
of the spleen (males and females), thymus (males only), and testis
were decreased at 1500 mg/kg of diet. Atrophy of the spleen in males
and females and atrophy of the thymus, mesenteric lymph node, testis,
and prostate in males were observed at concentrations of 100 mg/kg of
diet and above. The weights of the liver, adrenals, and kidneys were
increased in animals at the highest dose; in females, the liver
weights were also increased at lower doses. Inflammatory changes were
seen in the urinary bladder of females at concentrations of 100 mg/kg
of diet and above and in the livers of males and females at 1500 mg/kg
of diet. Hepatocyte oedema was observed in females at all doses, but
hepatocellular damage was not seen at any dose in either sex. The
Committee noted that hepatocyte oedema without hepatocellular damage
is not of toxicological significance. Hence, the NOEL was 20 mg/kg of
diet, equal to 0.61 mg/kg bw per day, on the basis of increased plasma
cholesterol concentrations and histopathological findings in the
prostate and urinary bladder.
In a 1-year study, dogs received dicyclanil in the diet at a
concentration of 0, 5, 25, 150, or 750 mg/kg of diet. Males showed
slightly decreased plasma calcium concentrations and increased
absolute and relative liver weights at 750 mg/kg of diet. Plasma
cholesterol concentrations were increased in males at 150 and 750
mg/kg of diet, and this change was not reversed after a 4-week
recovery period. Females receiving the highest dose vomited and had
slightly reduced food consumption and body-weight gain, slightly
increased plasma cholesterol concentrations, increased absolute and
relative liver weights, and decreased absolute and relative heart
weights. Macroscopic and microscopic findings consisting of liver
necrosis, tubular lesions in the kidneys, testicular and prostatic
atrophy, and vascular thrombus, were found in one male and one female
at the highest dose that died before their scheduled sacrifice. These
animals suffered from acute, severe liver failure and resulting
cardiovascular disturbances and stress due to weight loss. Comparable
acute, severe liver toxicity was not observed in the 3-month study of
toxicity in dogs given feed containing dicyclanil at concentrations up
to 1500 mg/kg of diet. The findings in the two animals were therefore
considered to be incidental. The NOEL was 25 mg/kg of diet, equal to
0.71 mg/kg bw per day, on the basis of increased plasma cholesterol
concentrations in male dogs. The Committee noted that this NOEL is
supported by the NOEL in the 3-month study of toxicity in dogs. It
also noted that the histopathological findings observed in the 3-month
study were not seen in the 1-year study among animals that lived until
the time of scheduled sacrifice.
In a study of carcinogenicity, mice received diets containing
dicyclanil at a concentration of 0, 10, 100, 500, or 1500 mg/kg of
diet for 18 months. The animals at the highest dose were killed during
weeks 58-59 because of self-inflicted injuries and poor health. On the
basis of significant reductions in body-weight gain, the
concentrations of 500 and 1500 mg/kg of diet in females and 1500 mg/kg
of diet in males were considered to exceed the maximum tolerated dose.
The liver was the main target organ in both male and female mice. The
effects included Kupffer cell pigmentation (with haemosiderin) and
hepatocellular necrosis in males at doses of 100 mg/kg of diet and
higher, increased incidences of hepatocellular adenomas in females at
500 and 1500 mg/kg of diet, and hepatocellular carcinomas in females
at 1500 mg/kg of diet. The Committee noted that these liver tumours
were observed only at doses that exceeded the maximum tolerated dose
and that there were signs of hepatocellular proliferation in these
animals which might have been involved in the hepatic carcinogenesis
observed. Pigmentation of the olfactory epithelium (with oxidized
lipofuscins) was observed in animals of each sex at 100 and 500 mg/kg
of diet; in the males, this effect was accompanied by an increased
incidence of inflammatory cell infiltration in the underlying Bowman
glands. Males and females at 500 mg/kg of diet also showed
pigmentation of the adrenal glands (with partly oxidized lipofuscins)
and hypercellularity of the bone marrow. As the Committee considered
the effects on the olfactory epithelium to be of no biological
significance, the NOEL was 10 mg/kg of diet, equal to 1.1 mg/kg bw per
day, on the basis of the effects on the liver.
In a 2-year study of carcinogenicity and toxicity, rats received
diets containing dicyclanil at a concentration of 0, 5, 25, 125, or
500 mg/kg of diet. In animals at the highest dose, food consumption
and body-weight gain were decreased, and the relative weights of
almost all organs were increased as a result. Treatment-related
histopathological alterations were observed in the exocrine pancreas
(hyperplasia) in males at the highest dose, in the liver (biliary
cysts) in females at the highest dose, and in the olfactory epithelium
(pigmentation resulting from accumulation of oxidized lipofuscins) in
males at doses of 25 mg/kg of diet and above and in females at doses
of 125 mg/kg of diet and above. Although the latter represents
enhancement of a naturally occurring age-related process, treatment
had no effect on survival, behaviour, or general well-being, and there
were no other morphological changes in the olfactory mucosa.
Therefore, the Committee concluded that the effect on the olfactory
epithelium was of no biological significance. Dicyclanil did not
affect the incidence of tumours. The NOEL was 125 mg/kg of diet, equal
to 22 mg/kg bw per day, on the basis of changes in body weight and
histopathological changes in the liver and pancreas.
Dicyclanil has been tested in vitro for its ability to induce
reverse mutation in Salmonella typhimurium and Escherichia coli,
gene mutation in Chinese hamster lung cells, chromosomal aberrations
in Chinese hamster ovary cells, and unscheduled DNA synthesis in
primary rat hepatocytes. It has been tested in vivo for its ability
to induce micronuclei in bone-marrow cells of mice treated orally. The
results of all of these tests were negative. On the basis of these
data, the Committee concluded that dicyclanil is not genotoxic.
Dicyclanil increased the incidence of liver tumours in female
mice. However, as these tumours occurred in only one tissue of animals
of one sex and one species at doses that were above the maximum
tolerated dose, and dicyclanil is not genotoxic, the Committee
concluded that dicyclanil does not represent a carcinogenic risk for
humans.
In a two-generation study of reproductive toxicity, with two
litters per generation, rats were given dicyclanil in the diet at a
concentration of 0, 5, 30, 200, or 500 mg/kg of diet. Treatment
reduced the body-weight gain of the parental animals at the highest
dose and, marginally, at 200 mg/kg of diet. Secondary to this effect,
dicyclanil increased the relative weights of most organs in animals at
the highest dose and of the brain (males and females), kidneys (males
only), and testis at 200 mg/kg of diet. Reproductive parameters were
not affected. The only effect of dicyclanil on pups was to reduce
their weight gain from day 4 post partum onwards. The NOEL for
parental toxicity was 30 mg/kg of diet, equal to 2 mg/kg bw per day,
on the basis of changes in body weight. The NOEL for reproductive
toxicity was 500 mg/kg of diet, equal to 24 mg/kg bw per day, the
highest dose tested. The NOEL for toxicity to the pups was 200 mg/kg
of diet, equal to 21 mg/kg bw per day, on the basis of reduced
body-weight gain.
In a study of developmental toxicity in rats given dicyclanil at
a dose of 0, 1, 5, 25, or 75 mg/kg bw per day orally on days 6-15 of
gestation, the highest dose was toxic to the dams, as seen by
reductions in body-weight gain, food consumption, and the weight of
the gravid uterus. Marginal reductions in body-weight gain and food
consumption were also observed at 25 mg/kg bw per day. The effects on
the fetuses, observed only at the highest dose, were reduced fetal
weight, a slightly increased incidence of renal pelvic dilatation, and
a number of mainly sternebral defects and variations due to poor or
absent ossification. There was no evidence of teratogenicity. The NOEL
for maternal toxicity was 5 mg/kg bw per day on the basis of the
reduction in body-weight gain. The NOEL for developmental toxicity was
25 mg/kg bw per day on the basis of reduced fetal weight, increased
renal pelvic dilatation, increased skeletal anomalies, and variations
consistent with a slight delay in skeletal maturation.
In a study of developmental toxicity in rabbits given dicyclanil
at a dose of 0, 1, 3, 10, or 30 mg/kg bw per day orally on days 7-19
of gestation, dams at 30 mg/kg bw per day had reduced food consumption
and those at 10 and 30 mg/kg bw per day showed reduced body-weight
gain. The fetuses of dams at the highest dose had lower body weights
than controls and an increased incidence of skeletal variations
indicative of a slight delay in ossification. There was no evidence of
teratogenicity. The NOEL for maternal toxicity was 3 mg/kg bw per day
on the basis of reduced body-weight gain. The NOEL for developmental
toxicity was 10 mg/kg bw per day on the basis of reduced fetal weight
and skeletal variations consistent with delayed ossification.
In pharmacological tests in vitro, dicyclanil at doses up to
3 mmol/L had no effect on the skeletal neuromuscular junction. At
concentrations of 0.3 mmol/L and higher, it had slightly antagonistic
effects on smooth muscle contractions induced by agonists. In mice and
rats given a single oral dose of 0, 1, 10, 50, or 100 mg/kg bw, the
highest dose affected general behaviour, locomotor activity, motor
coordination, heart rate, and tidal and minute lung volume. Locomotor
activity was also affected at 10 mg/kg bw and, very slightly, at
1 mg/kg bw. The treatment had no effect on body temperature, hypnotic
potentiation, gastrointestinal motility, blood pressure, heart beat,
or respiratory rate.
4. EVALUATION
The Committee established an ADI of 0-7 mg/kg bw, on the basis of
the NOEL of 0.71 mg/kg bw per day for increased plasma cholesterol
concentrations in the 1-year study of toxicity in dogs and a safety
factor of 100. As is its usual practice, the Committee rounded the
value of the ADI to one significant figure.
5. REFERENCES
Altmann, B. (1991) CGA 183893 tech. 28-Day range finding toxicity
study in beagle dogs. Unpublished report (test no. 901202) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Altmann, B. (1993) CGA 183893 tech. 3-Month subchronic dietary
toxicity study in beagle dogs. Unpublished report (test no.
922813) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Altmann, B. (1995) CGA 183893 tech. 12-Month chronic dietary toxicity
study in beagle dogs. Unpublished report (test no. 922814) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Bachmann, M. (1991) CGA 183893 tech. 28-Days range finding study in
rats (administration in food). Unpublished report (test no.
901201) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Bachmann, M. (1993) CGA 183893 tech. 3-Month oral toxicity study in
rats (administration in food). Unpublished report (test no.
922810) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Bachmann, M. (1996a) CGA 183893 tech. 18-Month oncogenicity study in
mice. Unpublished report (test no. 922815) from Ciba-Geigy Ltd,
Stein, Switzerland. Submitted to WHO by Novartis Animal Health
Inc., Basel, Switzerland.
Bachmann, M. (1996b) CGA 183893 tech. 24-Month carcinogenicity and
chronic toxicity study in rats. Unpublished report (test no.
922816) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Dubach-Powell, J.R. (1996) Pharmacokinetic study of CGA 183893 tech.
in the rat. Unpublished letter report (RCC project no.
615295/Ciba project no. 952803) from Research & Consulting
Company, Itingen, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
FitzGerald, R.E. (1990a) Preliminary oral toxicity study with CGA
183893 technical in pregnant rats (Dose range-finding study).
Unpublished report (test no. 901211) from Ciba-Geigy Ltd, Stein,
Switzerland. Submitted to WHO by Novartis Animal Health Inc.,
Basel, Switzerland.
FitzGerald, R.E. (1990b) Preliminary toxicity study in pregnant
rabbits with CGA 183893 technical (oral administration) (Dose
rangefinding study). Unpublished report (test no. 901212) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
FitzGerald, R.E. (1993a) CGA 183893 technical: Rat oral
teratogenicity. Unpublished report (study no. 922821) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
FitzGerald, R.E. (1993b) CGA 183893 technical: Rabbit oral
teratogenicity. Unpublished report (study no. 922822) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Geleick, D. (1992) CGA 183893 tech. Gene mutation test with Chinese
hamster cells V79 (OECD conform) in vitro. Unpublished report
(test no. 922827) from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Hagemann, C. (1992a) CGA 183893 tech. Acute dermal
irritation/corrosion study in the rabbit. Unpublished report
(test no. 922803) from Ciba-Geigy Ltd, Stein, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Hagemann, C. (1992b) CGA 183893 tech. Acute eye irritation/corrosion
study in the rabbit. Unpublished report (test no. 922802).
CibaGeigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Hagemann, C. (1993) CGA 183893 tech. Skin sensitization test in the
guinea pig optimisation test. Unpubished report (test no. 922805)
from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by
Novartis Animal Health Inc., Basel, Switzerland.
Hartmann, H.R. (1992a) CGA 183893 tech. Acute oral toxicity in the
rat. Unpublished report (test no. 922801) from Ciba-Geigy Ltd,
Stein, Switzerland. Submitted to WHO by Novartis Animal Health
Inc., Basel, Switzerland.
Hartmann, H.R. (1992b) CGA 183893 tech. Acute dermal toxicity in the
rat. Unpublished report (test no. 922804) from Ciba-Geigy Ltd,
Stein, Switzerland. Submitted to WHO by Novartis Animal Health
Inc., Basel, Switzerland.
Hartmann, H.R. (1993) CGA 183893 tech. Acute inhalation toxicity in
the rat. Unpublished report (test no. 922806) from Ciba-Geigy
Ltd, Stein, Switzerland. Submitted to WHO by Novartis Animal
Health Inc., Basel, Switzerland.
Hassler, S. (1994) Absorption, distribution and elimination of
[2-14C]pyrimidyl CGA 183893 in the rat after multiple oral
administration. Unpublished report (report no. 7/94; project no.
012AM01) from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to
WHO by Novartis Animal Health Inc., Basel, Switzerland.
Hertner, T. (1992) CGA 183893 tech. Salmonella and
Escherichia/liver-microsome test. Unpublished report (test no.
922825) from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Hertner, T. (1993a) CGA 183893 tech. Cytogenetic test on Chinese
hamster cells in vitro (EC conform). Unpublished report (test no.
922826) from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Hertner, T. (1993b) CGA 183893 tech. Autoradiographic DNA repair test
on rat hepatocytes (OECD conform) in vitro. Unpublished report
(test no. 922824) from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Hertner, T. (1993c) CGA 183893 tech. Micronucleus test, mouse (OECD
conform). Unpublished report (test no. 922823) from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by Novartis Animal
Health Inc., Basel, Switzerland.
Khalil, S. (1995) CGA 183893 technical: Rat dietary two-generation
reproduction study. Unpublished report (study no. 922818) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Löffler, A. (1998) The nature of residues in tissues of sheep after
single topical pour-on administration of [pyrimidine-2-14C]CGA
183893. Unpublished report (study no. 012AM04) from Novartis Crop
Protection AG, Basel, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Marty, J.H. (1995) CGA 183893 tech. 28-Day repeated dose dermal
toxicity study in the rat. Unpublished report (test no. 922807)
from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by
Novartis Animal Health Inc., Basel, Switzerland.
Ogorek, B. & Arni, P. (1987) Salmonella mutagenicity test with three
strains with CGA 183893 (Test for mutagenic properties in
bacteria). Unpublished report (experiment no. 871051) from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Pfister, T. & Gisin, M.(1996a) General pharmacology of CGA 183893
tech. Effect on the body temperature in the rat. Unpublished
report (project no. RCC 615317/CG 952803) from Research &
Consulting Company, Ltd, Itingen, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Pfister, T. & Gisin, M. (1996b) General pharmacology of CGA 183893
tech. Effect on the sleeping time induced by hexobarbitone in the
mouse. Unpublished report (project no. RCC 615330/CG 952803) from
Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Gisin, M. (1996c) General pharmacology of CGA 183893
tech. Effect on the intestinal motility (charcoal propulsion) in
the mouse. Unpublished report (project no. RCC 615385/CG 952803)
from Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Gisin, M. (1996d) General pharmacology of CGA 183893
tech. Modified Irwin screen test in the mouse. Unpublished report
(project no. RCC 615306/CG 952803) from Research & Consulting
Company, Ltd, Itingen, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Pfister, T. & Gisin, M. (1996e) General pharmacology of CGA 183893
tech. Effect on sperm motility, concentration and morphology in
the rat. Unpublished report (project no. RCC 615407/CG 952803)
from Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Gisin, M. (1996f) General pharmacology of CGA 183893
tech. Effect on the isolated ileum of the guinea pig. Unpublished
report (project no. RCC 615363/CG 952803) from Research &
Consulting Company, Ltd, Itingen, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Pfister, T. & Hussherr, D. (1996a) General pharmacology of CGA 183893
tech. Effect on the locomotor activity in the mouse. Unpublished
report (project no. RCC 615328/CG 952803) from Research &
Consulting Company, Ltd, Itingen, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Pfister, T. & Hussherr, D. (1996b) General pharmacology of CGA 183893
tech. Effect on motor co-ordination (rotarod) in the mouse.
Unpublished report (project no. RCC 615341/CG 952803) from
Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Hussherr, D. (1996c) General pharmacology of CGA 183893
tech. Effect on the isolated phrenic nerve-diaphragm preparation
of the rat. Unpublished report (project no. RCC 615352/CG 952803)
from Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Nordmann, A. (1996) General pharmacology of CGA 183893
tech. Effect on the cardiovascular and respiratory systems in the
rat. Unpublished report (project no. RCC 615374/CG 952803) from
Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Phillips, M. (1996) [Pyrimidine-2-14C]-CGA 183893. Nature of
metabolites in the excreta and tissues of sheep following single
pour-on administration. Unpublished report (No. 14072; project
no. 158665) from Inveresk Research, Tranent, Scotland. Submitted
to WHO by Novartis Animal Health Inc., Basel, Switzerland.
Thanei, P. (1996a) The metabolism of [2-14C]pyrimidyl CGA 183893 in
the rat. Unpublished report (No.7/96; project no. 012AM02) from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Thanei, P. (1996b) The nature of metabolites in tissues and excreta of
sheep after single topical administration of [2-14C]pyrimidyl
CGA 183893. Unpublished report (No. 9/96; project no. 012AM03)
from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Novartis Animal Health Inc., Basel, Switzerland.
Weber, E. (1998) CGA 183893 tech. Pigmentation of the olfactory mucosa
observed after dietary administration to rats. Unpublished report
(report no. CB 98/05) from Novartis Crop Protection AG, Basel,
Switzerland. Submitted to WHO by Novartis Animal Health Inc.,
Basel, Switzerland.
 Three male New Zealand white rabbits (Chbb:NZW) received a
semi-occlusive topical application of 0.5 g of technical-grade
dicyclanil moistened with distilled water containing 0.5%
carboxymethylcellulose in 0.1% aqueous polysorbate 80 to their shaved
flanks. The study followed OECD test guideline 404, with GLP and
quality assurance certification. Only very slight erythema was
observed 1 ( n = 3) to 24 h ( n = 1) after removal of the patch
(Hagemann, 1992a).
In a study that followed OECD test guideline 405 with GLP and
quality assurance certification, three female New Zealand white
rabbits (Chbb:NZW) received an instillation of 0.1 ml (84 mg) of
technical-grade dicyclanil into the conjunctival sac of one eye. The
other eye served as control. The cornea appeared to be unaffected by
treatment; one animal showed an affected iris 1 h after instillation
but recovered within 24 h. Slight chemosis of the conjunctiva was
observed in two animals 1 h after instillation, but they recovered
within 24 h. Redness of the conjunctiva was seen in all animals,
although at different grades (score 1 or 2), and recovery to normal
occurred within 1-7 days (Hagemann, 1992b).
In 10 male and 10 female Pirbright white Tif:DHP guinea-pigs
submitted to an optimization test that followed OECD test guideline
406 with GLP and quality assurance certification, no significant skin
sensitization (1/20 positive) was observed after epidermal challenge
with 20% technical-grade dicyclanil in vaseline, this being a
subirritant dose. When the skin barrier was intentionally bypassed,
i.e. by intradermal challenge with a 0.1% solution of technical-grade
dicyclanil in 10% propylene glycol, 13 of 20 treated animals showed
positive reactions versus 3 of 20 animals given the vehicle
( p < 0.01) (Hagemann, 1993).
2.2.2 Short-term studies of toxicity
Rats
In a 28-day range-finding study, groups of five male and five
female Tif:RAIf (SPF) rats received technical-grade dicyclanil
(purity, > 98%) in the diet at a concentration of 0, 100, 500, or
2000 mg/kg of diet, equal to average achieved intakes of 0, 9.5, 50,
and 150 mg/kg bw per day for males and 0, 9.4, 49, and 160 mg/kg bw
per day for females. The study followed OECD test guideline 407
(adapted to the objectives of a range-finding study) without GLP or
quality assurance certification.
No treatment-related deaths occurred. Piloerection was observed
in animals of each sex at the highest dose during the second half of
the study. Dose-dependent reductions in food consumption, body-weight
gain, and final body weight were noted in all treated groups. These
were particularly marked in males at the high dose. Water consumption
was reduced in females at 500 and 2000 mg/kg of diet. Increased
incidences of anisocytosis and polychromasia of erythrocytes and a
slightly lower (not statistically significant) leukocyte count were
noted in males at the high dose. In both males and females at this
dose, higher plasma concentrations of urea and cholesterol and
increased activities of aspartate aminotransferase (twofold) and
alanine aminotransferase (five- to sevenfold) were noted.
Hypoglycaemia and lower plasma total bilirubin concentrations were
also observed in these animals, although the changes were not
statistically significant in females. Males at the high dose also had
slightly lower plasma globulin and calcium concentrations, while
females at this dose had an increased plasma phosphate concentration.
Consistent with the reduced body weights of all treated animals, the
absolute weights of a number of organs were decreased, whereas the
relative weights of a number of other organs were increased. The only
notable changes in organ weights which could be considered to be
treatment-related effects were reductions in the absolute and relative
weights of the prostate in males at the intermediate and high doses
and of the adrenals in females at the low and intermediate doses. No
macroscopic changes were found, apart from emaciation at the high
dose. Histopathologically, reduced spermatogenesis in the testes was
seen in 1/5 males at 500 mg/kg of diet and 5/5 males at 2000 mg/kg of
diet, accompanied by accumulation of cellular debris in the epididymal
duct in all those at the high dose. These animals also showed
immaturity of the prostate. In 1/5 females at 500 mg/kg of diet and in
4/5 females at 2000 mg/kg of diet, polyovular ovarian follicles, as
indicated by hypercellularity, were seen (Bachmann, 1991).
In a 3-month study of toxicity, groups of 10 male and 10 female
Tif:RAIf (SPF) rats received technical-grade dicyclanil in the diet at
a concentration of 0, 5, 25, 125, or 500 mg/kg of diet, equal to
average achieved intakes of 0, 0.31, 1.6, 8.0, and 33 mg/kg bw per day
for males and 0, 0.31, 1.7, 8.4, and 34 mg/kg bw per day for females.
Ten additional animals of each sex in the control and high-dose groups
were kept for a 4-week recovery period. The study followed OECD test
guideline 408, with GLP and quality assurance certification.
No treatment-related deaths or clinical signs were observed.
Slight reductions in body-weight gain and food consumption were
observed in animals of each sex at the high dose and in males at 125
mg/kg of diet. Owing to a compensatory increase in food intake during
the recovery period, the body weights of animals at the high dose were
comparable to those of the controls by the end of the recovery period.
Chemical analysis of blood revealed slightly lower plasma glucose
concentrations in males and females at 125 and 500 mg/kg of diet at
the end of the treatment period, but this was reversed within the
recovery period. Higher organ:body weight ratios were observed for
kidneys, brain, and testis in males at the high dose and for liver and
brain in females at this dose, these changes being reversible within
the 4-week recovery period. Although the relative epididymidal weights
were increased in males at doses > 25 mg/kg of diet, this was
considered not to be toxicologically significant because the absolute
epididymidal weights were not different from those of controls and
there were no abnormal histopathological findings. No
treatment-related ophthalmological or haematological changes were
observed, and no gross or microscopic alterations were observed. One
female at the high dose that was alllowed to recover had a mammary
tumour, which was considered to be of spontaneous origin. On the basis
of the reduction in body-weight gain, the NOEL was 25 mg/kg of diet,
equal to 1.6 mg/kg bw per day (Bachmann, 1993).
In a study that followed OECD test guideline 410 with GLP and
quality assurance certification, groups of five male and five female
Tif:RAIf (SPF) rats received dermal applications of technical-grade
dicyclanil at a dose of 0, 5, 30, 300, or 1000 mg/kg bw per day, 6
h/day on 5 days per week for 4 weeks. Dicyclanil was dissolved in
water containing 0.5% carboxymethylcellulose and 0.1% polysorbate 80
and was applied under an occlusive dressing to a clipped dorsal area
of the skin. At the end of each application period, the site was
washed with lukewarm water.
None of the animals died and no treatment-related clinical signs
were observed. Apart from a few incidental findings, no signs of local
skin irritation were seen. Male rats at 300 and 1000 mg/kg bw per day
showed dose-dependently decreased body weights and body-weight gain
and slightly lower food consumption. The plasma concentrations of
sodium and calcium were slightly reduced in these animals. Females at
the high dose showed increased absolute and relative liver weights. A
similar effect was observed in females at 300 mg/kg bw per day but did
not reach statistical significance. The absolute, but not the
relative, weight of the brain of females was increased at doses
> 30 mg/kg bw per day, without histopathological findings. No
treatment-related effects were noted on gross examination. Microscopic
examination showed hepatocyte hypertrophy in males at 1000 mg/kg bw
per day and in females at 300 and 1000 mg/kg bw per day. The NOEL was
30 mg/kg bw per day, on the basis of decreased body-weight gain and
changes in the liver (Marty, 1995).
Dogs
In a 28-day range-finding study, groups of two male and two
female beagles were given technical-grade dicyclanil (purity, > 98%)
in the diet at a concentration of 0, 200, 1000, or 2500 mg/kg of diet,
equal to average achieved intakes of 0, 5.6, 31, and 66 mg/kg bw per
day for males and 0, 6.2, 30, and 50 mg/kg bw per day for females. The
study followed OECD test guideline 409 (adapted to the objectives of a
range-finding study) without GLP (except for the pathology report) or
quality assurance certification.
No treatment-related deaths occurred. The clinical signs included
body tremors (in animals of each sex at 2500 mg/kg of diet), vomiting
(in animals of each sex at 2500 mg/kg of diet and in one male at 1000
mg/kg of diet), dyspnoea and slight apathy (in males at 2500 mg/kg of
diet). Animals at the high dose lost weight and had reduced food
consumption, and females at 1000 mg/kg of diet also showed a slight
reduction in food consumption. The reduction in food consumption did
not appear to be due to unpalatability. One male and one female at the
high dose showed a prolonged prothrombin time, and the other male at
the high dose had a slightly increased number of blood platelets.
Alanine aminotransferase activity was markedly increased and alkaline
phosphatase activity slightly increased in one male at the high dose
and in one female at the intermediate and one at the high dose;
aspartate aminotransferase activity was also increased in the female
at the high dose. Very slight decreases were observed in plasma
potassium concentrations in males at the intermediate and high doses,
in plasma glucose concentration in females at the high dose, and in
the plasma concentrations of urea, creatinine, and total bilirubin in
males and females at the high dose. Urinary analysis revealed an
increased incidence of proteinuria in males at the high dose and
females at the intermediate and high doses. The absolute and relative
weights of the testis and thymus were markedly decreased and those of
the heart slightly decreased at 2500 mg/kg of diet. In animals of each
sex at 1000 and 2500 mg/kg of diet, the absolute and relative weights
of the adrenal and kidney were increased, but without any
histopathological changes. Microscopic examination revealed hepatic
toxicity in one female at 1000 mg/kg of diet and in all animals at
2500 mg/kg of diet. The toxicity included focal or single-cell
necrosis, inflammatory-cell foci, and a brown granular pigment in
macrophages. One female at the intermediate dose also had mild
bile-duct hyperplasia, and one male at the high dose had periportal
hepatocyte enlargement. The testes of males at this dose showed
degeneration characterized by reduced numbers of tailed spermatids and
increased numbers of multinucleated or giant cells. Mild thymic
atrophy was noted in animals of each sex at the highest dose. The
heart of one male at this dose was mottled and showed inflammation,
fibrosis, and haemorrhage. Tubular basophilia or dilatation was
present in the kidneys of all animals at the high dose, two at the
intermediate dose, and one at the low dose, but not in controls. This
lesion is of minimal toxicological significance (Altmann, 1991).
Groups of four male and four female beagles received
technical-grade dicyclanil in the diet at a concentration of 0, 20,
100, 500, or 1500 mg/kg of diet for 3 months, equal to average
achieved intakes of 0, 0.61, 2.7, 14, and 42 mg/kg bw per day for
males and 0, 0.71, 3.5, 17, and 42 mg/kg bw per day for females. The
study followed OECD test guideline 409, with GLP and quality assurance
certification.
One male at the high dose was found dead at week 11 after general
deterioration in clinical condition accompanied by tonic-clonic
spasms. Post-mortem examination did not reveal the cause of death.
Animals at the high dose started to show clinical signs from week
9-11, including slight ataxia, unnaturally raised tails, and frequent
shaking. In addition, vomitus and traces of blood in the faeces were
observed. Ophthalmoscopic examinations revealed no treatment-related
changes. Animals at the high dose lost weight (males only) or had
reduced body-weight gain with reduced food intake. A very slight
reduction in food intake was also observed transiently in some animals
at 500 mg/kg of diet. Slightly reduced haemoglobin concentrations and
haematocrit values, associated with minor microcytosis and
hypochromasia of erythrocytes, were recorded in animals at the high
dose. Increased plasma cholesterol and phospholipid concentrations
were observed in animals at doses > 100 mg/kg of diet. Plasma
albumin concentrations were slightly decreased in males and females at
1500 mg/kg of diet and in females at 500 mg/kg of diet. Decreased
plasma calcium, potassium, urea, creatinine, and total bilirubin
concentrations were found in animals at the high dose. Urinary
analysis revealed no treatment-related effects. The mean absolute and
relative liver weights were increased in animals at the highest dose
and in females also at 20, 100 (not statistically significant), and
500 mg/kg of diet (not dose-related). The absolute and relative
adrenal weights were also slightly increased at the high dose. The
absolute and relative weights of the thymus (males), testis, and
spleen (both sexes) were decreased in animals at the high dose. The
kidney weights were increased in males (relative) and females
(absolute and relative) at 1500 mg/kg of diet. Macroscopic examination
showed no treatment-related effects. Microscopy of the liver showed
minimal to moderate focal or multifocal subcapsular inflammation with
fibrosis in 2/4 males and 3/4 females at the high dose. Enlarged
hepatocytes, diagnosed as cellular oedema, were observed in the
centrilobular and midzonal region of 3/4 females at 20, 100, and 500
mg/kg of diet and in 1/4 males and all females at 1500 mg/kg of diet.
No morphological signs of hepatocellular damage were apparent. Minimal
atrophy of the white pulp of the spleen was observed in 3/4 males at
500 and 1500 mg/kg of diet and in all females at 1500 mg/kg of diet.
Thymic atrophy was found in 3/4 males at 500 mg/kg of diet and in all
males at 1500 mg/kg of diet. Minimal atrophy of the lymphatic tissue
of the mesenteric lymph node was observed in 3/4 males at the high
dose, and minimal to marked atrophy of the glandular tissue of the
prostate was detected in 3/4 males at 100 mg/kg of diet, 1/4 at 500
mg/kg of diet, and 4/4 at 1500 mg/kg of diet. Examination of the
testes revealed minimal tubular atrophy in 3/4 males at the high dose,
associated with a marked reduction in spermatogenesis in all males of
this group. A dose-related increase in the frequency of inflammatory
changes associated with epithelial hyperplasia was found in the
urinary bladders of females at 100, 500, and 1500 mg/kg of diet
(Altmann, 1993). The Committee noted that hepatocyte oedema without
hepatocellular damage is not of toxicological significance. Hence, the
NOEL was 20 mg/kg of diet, equal to 0.61 mg/kg bw per day, on the
basis of increased plasma cholesterol concentrations and
histopathological findings in the prostate and urinary bladder.
In a 1-year study of toxicity, groups of four male and four
female beagles received technical-grade dicyclanil in the diet at a
concentration of 0, 5, 25, 150, or 750 mg/kg of diet, equal to average
achieved intakes of 0, 0.16, 0.71, 4.4, and 23 mg/kg bw per day for
males and 0, 0.15, 0.77, 5.1, and 23 mg/kg bw per day for females. Two
additional animals per sex in the control and high-dose groups were
maintained for a 4-week recovery period. The observations included
deaths, clinical signs, body weight, food consumption, ocular,
neurological, haematological, clinical chemical, and urinary
parameters, and macroscopic and microscopic end-points. The study
followed OECD test guideline 452, with GLP and quality assurance
certification.
One female at the high dose was found dead on day 13, with no
previous abnormal clinical signs. One male at the high dose was killed
on study day 32 after vomiting, showing marked apathy, lying in a
lateral position, and weight loss correlated to reduced food intake.
Vomiting was observed in females at the high dose, and the body-weight
gain (in two animals) and food consumption of females at the high dose
were slightly reduced. Ocular and neurological examinations revealed
no treatment-related effects, nor were there any changes in
haematological or urinary parameters. Throughout the treatment period,
the plasma cholesterol concentrations were increased in animals of
each sex at 750 mg/kg of diet (not statistically significant in
females) and in males at 150 mg/kg of diet; in males, the change was
not reversed after the 4-week recovery period. Males at the high dose
showed slightly lower plasma calcium concentrations. Both males and
females at the high dose had reduced plasma concentrations of
bilirubin and urea and reduced alkaline phosphatase activity. The
changes in blood chemistry were partially reversed during the recovery
period. The absolute and relative liver weights were increased in
animals at 750 mg/kg of diet, but only the change in absolute weight
in males was statistically significant. The absolute (statistically
significant) and relative weights of the heart were decreased in
females at the high dose. The weight changes were reversed by the end
of the 4-week recovery period. The macroscopic and microscopic
findings were confined to the two animals at the high dose that died
before the scheduled kill. The macroscopic findings consisted of a
scar in the liver and pale kidneys in the male and a mass and
haemorrhagic content in the abdominal cavity of the female.
Histopathologically, both animals showed marked diffuse liver necrosis
and kidney lesions (more severe in the male). The male also showed
testicular and prostatic atrophy, and the female had a thrombus in a
peritoneal blood vessel. The two animals suffered from acute, severe
liver failure and resulting cardiocirculatory disturbances, and the
male also showed stress due to weight loss. Their condition was very
different from that of the other treated dogs in this study, and
comparable acute, severe liver toxicity was not seen in the 28-day and
3-month studies in dogs treated with doses up to 2500 and 1500 mg/kg
of diet, respectively. The lesions seen in the two animals are
considered to be incidental findings. On the basis of increased plasma
cholesterol concentrations in males, the NOEL was 25 mg/kg of diet,
equal to 0.71 mg/kg bw per day (Altmann, 1995).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In an 18-month study of carcinogenicity, groups of 60 male and 60
female Tif:MAGf (SPF) mice were given diets containing technical-grade
dicyclanil at a concentration of 0, 10, 100, 500, or 1500 mg/kg of
diet, equal to average achieved intakes of 0, 1.1, 12, 59, and 210
mg/kg bw per day for males and 0, 1.1, 12, 65, and 200 mg/kg bw per
day for females. Ten animals per sex from each group were assigned for
evaluation of haematological parameters. The study followed OECD test
guideline 451 with GLP and quality assurance certification, but
histopathological examination of the animals at the high dose (i.e.
the group that was killed before study termination, see below) was
limited to the liver and lungs.
Clinical signs were observed only in males at the highest dose,
which injured themselves through vigorous scratching. A higher
mortality rate was noted among males at the high dose and to a lesser
extent in females at this dose. The self-inflicted injuries and poor
health of the animals at the high dose led to a decision to terminate
all surviving animals in this group during weeks 58-59. Doses < 500
mg/kg of diet did not affect survival. The body weights of males and
females at 1500 mg/kg of diet were reduced, with an approximately 50%
reduction in body-weight gain, and those of females at 500 mg/kg of
diet were reduced with a 30% reduction in body-weight gain. As food
intake was not affected, higher food consumption ratios were noted in
animals of each sex at 1500 mg/kg of diet and in females at 500 mg/kg
of diet. Haematological parameters were not affected by treatment. The
absolute and relative weights of the liver were increased in animals
of each sex at 500 mg/kg of diet (for males after adjustment for three
control outliers). In females at 500 mg/kg of diet, the relative
weights of the kidney, brain, and adrenals were increased, while the
absolute weights of these organs were not changed. The
treatment-related macroscopic findings included enlarged livers
(characterized histopathologically as hepatocellular hypertrophy) in
males at 500 mg/kg of diet and in both sexes at 1500 mg/kg of diet,
and masses and/or nodules of the liver in females at 500 and 1500
mg/kg of diet. Treatment-related microscopic findings were observed in
the liver, olfactory epithelium, adrenal gland, and bone marrow.
Kupffer cell pigmentation (mainly haemosiderin) and hepatocellular
necrosis were observed in males at > 100 mg/kg of diet. Increased
numbers of hepatocellular mitotic figures and multinucleated
hepatocytes were seen in the livers of males at the high dose, and the
frequency of foci of cellular change was increased in animals of each
sex at the highest dose. The incidence of heptocellular adenomas was
higher in females at 500 and 1500 mg/kg of diet (9/53 and 5/60,
respectively) than in controls (0/52). In addition, the incidence of
hepatocellular carcinomas was increased in females at the highest dose
(6/60 versus 0 in all other groups). Pigmentation of the olfactory
epithelium was observed at increasing incidence and severity in
animals of each sex at 100 and 500 mg/kg of diet, and males at these
doses showed an increased incidence of inflammatory cell infiltration
(cell type not specified) in the underlying Bowman's glands. At 500
mg/kg of diet, both males and females also showed increased incidences
of pigmentation of the adrenal glands, recorded as ceroid (i.e. a
partly oxidized form of lipofuscin) deposition, and of
hypercellularity of the bone marrow. Treatment with dicyclanil did not
affect the number of animals with malignant lymphomas. It was noted,
however, that females at 500 mg/kg of diet had more sites that
appeared to be infiltrated by malignant lymphoma cells than did
controls or animals at other doses (Bachmann, 1996a).
The Committee noted that the doses at which the liver adenomas
and carcinomas occurred exceeded the maximum tolerated dose for
females, and that there were signs of hepatocellular proliferation in
these animals which might have been involved in the hepatic
carcinogenesis observed. Pigmentation of the olfactory epithelium was
also observed in a long-term study in rats (Bachmann, 1996b; see
below) and was further investigated in a separate study (Weber, 1998;
see below). As the Committee considered the effects on the olfactory
epithelium to be of no biological significance (see below), the NOEL
in this study in mice was 10 mg/kg of diet, equal to 1.1 mg/kg bw per
day, on the basis of effects on the liver.
Rats
In a 2-year study of carcinogenicity and toxicity, groups of 80
Tif:RAIf (SPF) rats of each sex were given technical-grade dicyclanil
in the diet at a concentration of 0, 5, 25, 125, or 500 mg/kg of diet,
equal to average achieved intakes of 0, 0.19, 0.97, 4.8, and 22 mg/kg
bw per day for males and 0, 0.23, 1.2, 6.0, and 26 mg/kg bw per day
for females. Haematological, clinical chemical, and urinary analyses
were performed during weeks 13, 26, 52, 78, and 105 on 20, 10, and 10
animals of each sex per group, respectively. After 1 year, 10 animals
of each sex per group were killed. The study followed OECD test
guideline 453, with GLP and quality assurance certification.
Treatment with dicyclanil did not affect the incidence of
clinical signs or survival. Decreased food consumption was noted in
males and females at the highest dose. The body weights of males and
females at 500 mg/kg of diet were reduced (with an approximately 25%
reduction in body-weight gain), as were the body weights of animals at
125 mg/kg of diet (with a reduction in body-weight gain of < 10%).
Slight but statistically significant red blood cell dyscrasia and a
reduced number of monocytes were observed in males at the high dose
when compared with controls, but the changes were generally
inconsistent over time, not dose-related, and within the range of
historical controls and are therefore regarded as toxicologically
insignificant. Increased concentrations of inorganic phosphate were
observed in males and females at the high dose throughout the study,
although the increase was not statistically significant in females.
Males at 125 mg/kg of diet also had increased inorganic phosphate
concentrations, but these reached statistical significance only in
weeks 78 and 105. Lower concentrations of triglycerides were recorded
for males at 500 mg/kg of diet, although statistical significance was
achieved only in weeks 13 and 26. Urinary parameters were not
affected. As a result of the lower terminal body weights, almost all
of the relative organ weights were increased in males and females at
the highest dose, especially of the kidney, liver, and epididymides.
The absolute epididymal weights were also increased in males at the
high dose at 105 weeks. Males at 125 mg/kg of diet also had increased
relative liver weights, although these were within historical control
values. An increased incidence of liver cysts was observed in females
at the high dose, characterized primarily as unilocular or
multilocular biliary cysts on microscopic examination. In males at the
high dose, an increased incidence of masses and nodules in the
exocrine pancreas was noted, which were characterized
histopathologically as foci or areas of hyperplasia. Aside from the
histopathological findings in the pancreas and liver, the only other
treatment-related histopathological finding was an increased incidence
of pigmentation of the olfactory epithelium in males at > 25 mg/kg
of diet and in females at > 125 mg/kg of diet, the effects seen at
125 and 500 mg/kg of diet being more severe than at lower doses.
Dicyclanil did not affect the tumour incidence in this study
(Bachmann, 1996b).
Weber (1998) studied the histological nature and causes of the
pigmentation of the olfactory epithelium, using material from male
rats in the 2-year study (controls and rats given 500 mg/kg of diet)
and the 3-month study (controls and rats given 500 mg/kg of diet;
Bachmann, 1993). Material from male control rats of the same strain in
five other long-term studies was included to provide more reference
material.
Minimal to moderate pigmentation was found in controls from the
long-term studies with dicyclanil and other compounds, but not in
controls from the 3-month study. Treatment with 500 mg/kg of diet
resulted in increased pigmentation, which was minimal after 3 months
and moderate to marked after 12 and 24 months, the degree of
pigmentation at the later times being similar. Staining indicated that
the pigment consisted mainly of oxidized lipofuscins and was located
in the olfactory epithelium and the underlying lamina propria. The
pigment appeared to be located in secondary lysosomes. Further
investigations by high-resolution microscopy indicated that the
supporting cells and secretory cells of Bowman glands were affected by
the pigmentation. Neuronal olfactory perikarya, nerve bundles of the
olfactory nerve in the olfactory mucosa, and the olfactory bulbs (in
the brain) were free of pigment accumulation. Apart from the
pigmentation, no other treatment-related morphological changes in the
olfactory mucosa were found. According to the author, the clinical
signs in the 2-year study gave no indication of a disturbed olfactory
sense due to dicyclanil treatment. In addition, the presence of
mucopolysaccharides in the Bowman glands indicated normal functioning
of the olfactory mucosa in the dicyclanil-treated rats. The author
concluded that the increased pigmentation of the olfactory epithelium
in male rats after treatment with dicyclanil results from accumulation
of lipofuscin in the cytoplasm of supporting cells and secretory cells
of the Bowman glands and is an enhancement of a natural age-related
process. In the absence of other morphological changes in the
olfactory mucosa, the author considered the pigmentation not to be
detrimental to the structure or function of the olfactory mucosa and,
therefore, is not to be regarded as adverse (Weber, 1998).
Recognizing that the effects on the olfactory epithelium were an
enhancement of a natural age-related process, the Committee noted that
dicyclanil had no effect on survival, behaviour, or general
well-being. As there were no other morphological changes in the
olfactory mucosa, the Committee concluded that the effect was of no
biological significance. Therefore, the NOEL in the 2-year study in
rats was 125 mg/kg of diet, equal to 22 mg/kg bw per day, on the basis
of changes in body weight and histopathological changes in the liver
and pancreas.
2.2.4 Genotoxicity
The results of studies for genotoxicity with technical-grade
dicyclanil are summarized in Table 1. The studies were of conventional
design, with GLP and quality assurance certification, except for the
study of Ogorek & Arni (1987).
2.2.5 Reproductive toxicity
(a) Multigeneration study
Rats
In a two-generation study of reproductive toxicity with two
litters per generation, groups of 30 male and 30 female Tif:RAIf (SPF)
rats received diets containing technical-grade dicyclanil at a
concentration of 0, 5, 30, 200, or 500 mg/kg of diet from 10 weeks
before the first mating until necropsy at the end of the lactation
period. Dams were allowed to litter and suckle their pups naturally.
When possible, the F1a litters were culled to four pups of each sex
per litter at day 4 post partum. After weaning, a number of F1a
pups were selected to be the F1 parents, and the remaining F1a
pups were necropsied. The F0 parents were then mated a second time,
and the resulting litters (F1b) were again culled to four pups of
each sex per litter. After weaning, both the F1b pups and the F0
parents were necropsied. The same procedure was followed for the F1
parents in order to produce F2a and F2b generations. The
observations included clinical signs, deaths, body weight, food
consumption, reproductive parameters, pup survival and development,
macroscopic appearance at necropsy, and, for parents in the control
and high-dose groups, histological appearance of the reproductive and
target organs. The study followed OECD test guideline 416, with GLP
and quality assurance certification.
Effects in F0 parents: The F0 parents showed no
treatment-related deaths or clinical signs and no effects on male or
female mating or fertility indices, on maternal gestation or
parturition indices, or on the duration of gestation. In the premating
period, males and females showed decreased body-weight gain and food
intake at 500 mg/kg of diet and, marginally, at 200 mg/kg of diet.
During both gestation periods, the overall body-weight gain of females
in all treated groups was similar to that of controls, while in both
lactation periods the overall body-weight gain of females was
increased at 500 mg/kg of diet and, marginally, at 200 mg/kg of diet.
Gross examination and histology revealed no treatment-related effects.
Secondary to the reduced body weights, the relative weights of most
organs were increased in males and females at 500 mg/kg of diet. At
this dose, the absolute weights of the heart and liver of males were
decreased.
Table 1. Results of assays for genotoxicity with dicyclanil
End-point Test object Concentration Result Reference
In vitro
Reverse S. typhimurium 20-5000 µg/plate Negativea Ogorek & Arni
mutation TA1537, TA98, (1987)
TA100
Reverse S. typhimurium 313-5000 µg/plate Negativeb Hertner (1992)
mutation TA1535, TA1537,
TA98, TA100,
E. coli WP2 uvrA
Gene mutation V79 Chinese 12.4-400 µg/ml Negative Geleick (1992)
hamster lung cells, - S9c
hprt locus 24.7-667 µg/ml
+ S9d,e
Chromosomal Chinese hamster 20.8-83.4 µg/ml Negative Hertner (1993a)
aberration ovary cells -S9c
166.75-667 µg/ml
+S9d,e
Unscheduled Primary rat 6.2-670 µg/mle Negative Hertner (1993c)
DNA synthesis hepatocytes
In vivo
Micronucleus Mouse bone 47-188 mg/kg bw; Negativef Hertner (1993b)
formation marrow once by oral gavage
a With and without rat liver S9 fraction; results of cytotoxicity test not given,
precipitation not reported
b With and without rat liver S9 fraction; precipitation at 5000 µg/plate
c Cytotoxic at the highest concentration
d Slightly cytotoxic at the highest concentration
e The highest concentration represents the limit of solubility in dimethyl sulfoxide.
f At all doses and all times, the ratio of polychromatic to normochromatic erythrocytes
did not deviate from that in controls; clinical signs of toxicity (including reduced
locomotor activity, unkempt fur, ataxia, piloerection, and diarrhoea) were observed
at the highest dose tested; doses > 312.5 mg/kg bw resulted in death.
Effects in F1 pups: At all doses and both matings, no
treatment-related effects were seen in the F1 offspring in terms of
sex ratio, clinical signs, litter size, the development of physical
landmarks (surface righting and eye opening), or macroscopic findings
at necropsy. A slight increase in the pup mortality rate was observed
on days 0-4 post partum at 500 mg/kg of diet (F1a) and at 30 and
200 mg/kg of diet (F1b). This was considered not to be
treatment-related because it could be attributed to two (F1a) or
three (F1b) litters and no dose-response relationship was found. At
500 mg/kg of diet, the weights of F1a pups were lower than those of
controls at birth and on days 14 and 21 post partum, owing to
reduced weight gain from day 4 post partum onwards. The weights of
F1b pups at the high dose were similar to those of controls from
birth through to day 14 post partum. Thereafter, the weight gain
decreased, resulting in lower pup weights on day 21 post partum.
Effects in F1 parents: In the F1 parents, no
treatment-related deaths or clinical signs and no effects on male or
female mating or fertility indices, on maternal gestation or
parturition indices, or on the duration of gestation were observed.
The body weights of the F1 parents at the high dose were lower than
those of controls throughout the study. Although the body weights of
males at 200 mg/kg of diet were reduced during the final part of the
study, the overall body-weight gain of these animals was not different
from that of controls. During both gestation periods, the overall
body-weight gain of all treated females was slightly lower than that
of controls, while in both lactation periods the overall body-weight
gain of females was increased at 500 mg/kg of diet and, marginally, at
200 mg/kg of diet. The absolute weights of most organs of males at the
high dose were decreased, with the exception of those of the testis
and brain. In females at the high dose, only the absolute weights of
the heart, liver, and kidney were decreased. At 200 mg/kg of diet, the
absolute weight of the liver was decreased in animals of each sex. In
contrast, secondary to the reduced body weights of these two groups,
the relative weights of most organs were increased, attaining
statistical significance for most organs at 500 mg/kg of diet and for
brain (both sexes), kidneys (males only), and testis at 200 mg/kg of
diet. Gross examination and histology revealed no treatment-related
effects.
Effects in F2 pups: At all doses and both matings, no
treatment-related effects were noted in the F2 offspring in terms of
sex ratio, mortality rate, clinical signs, litter size, the
development of physical landmarks (surface righting and eye opening),
or macroscopic findings at necropsy. The only treatment-related effect
observed was reduced weight gain in F2a and F2b pups at the
highest dose from day 4 post partum onwards, resulting in
significantly lower pup weights on days 14 and 21 post partum.
The NOEL for parental toxicity was 30 mg/kg of diet, equal to 2
mg/kg bw per day, on the basis of changes in body weight. The NOEL for
reproductive toxicity was 500 mg/kg of diet, equal to 24 mg/kg bw per
day, the highest dose tested. The NOEL for pup toxicity was 200 mg/kg
of diet, equal to 21 mg/kg bw per day, on the basis of reduced
body-weight gain (Khalil, 1995).
(b) Developmental toxicity
Rats
In a range-finding study, groups of eight pregnant Tif:RAIf (SPF)
rats received technical-grade dicyclanil (purity, > 98%) in 3%
aqueous corn starch by oral gavage at a dose of 0, 75, or 150 mg/kg bw
per day on days 6-15 of gestation. On day 21 of gestation, the dams
were killed and necropsied, and the fetuses were weighed, sexed, and
examined for external abnormalities.
Two dams at the high dose were killed in moribund condition. The
clinical signs in these animals and in a third animal at the high dose
included piloerection, salivation, dyspnoea, and hypotonia. Maternal
body-weight gain and gravid uterine weights were dose-dependently
decreased in all treated animals, but food consumption was
dose-dependently decreased only during the first week of treatment.
Post-implantation loss was dose-dependently increased by treatment,
owing to early resorptions. Three dams at the high dose resorbed their
entire litters. The number of viable fetuses and fetal body weights
were dose-dependently reduced. Three fetuses at the high dose showed
generalized oedema (FitzGerald, 1990a).
In the main study, groups of 24 pregnant Tif:RAIf (SPF) rats
received technical-grade dicyclanil in an aqueous solution of 0.5%
sodium carboxy-methylcellulose by oral gavage at a dose of 0, 1, 5,
25, or 75 mg/kg bw per day on days 6-15 of gestation. On gestation day
21, the dams were killed and necropsied, and the fetuses were weighed,
sexed, and examined for external, visceral, and skeletal
abnormalities. The study was of conventional design, with GLP and
quality assurance certification.
There were no deaths and no treatment-related clinical signs of
toxicity. Maternal body weight, body-weight gain, and food consumption
as well as net body-weight change and carcass weight at necropsy were
reduced at the highest dose. A marginal decrease in these parameters
was also observed at 25 mg/kg bw per day. One animal at 75 mg/kg bw
per day had total, early resorptions and showed haemorrhagic fluid in
the uterus at necropsy. In animals at 75 mg/kg bw per day, the gravid
uterine weight was reduced; the number of early postimplantation
losses was slightly increased and the number of fetuses per litter was
slightly lower, but without statistical significance. Effects on the
fetuses were observed only at the highest dose. These included reduced
fetal weight, a slight increase in the frequency of renal pelvic
dilatation, and a number of mainly sternebral defects and variations
due to poor or absent ossification. There was no evidence of
teratogenicity. The NOEL for maternal toxicity was 5 mg/kg bw per day,
on the basis of a reduction in body-weight gain. The NOEL for
developmental toxicity was 25 mg/kg bw per day, on the basis of
reduced fetal weight, increased renal pelvic dilatation, and increased
skeletal anomalies and variations consistent with a slight delay in
skeletal maturation (FitzGerald, 1993a).
Rabbits
In a range-finding study, groups of eight pregnant Russian
rabbits (strain unspecified) received technical-grade dicyclanil
(purity, > 98%) in 3% aqueous corn starch by oral gavage at a daily
dose of 0, 20, or 40 mg/kg bw on days 7-19 of gestation. On day 29 of
gestation, the dams were killed and necropsied, and the fetuses were
weighed, sexed, and examined for external abnormalities.
No treatment-related deaths or clinical signs were observed.
During treatment, the body-weight gain and food consumption of dams at
the high dose were considerably reduced. The gravid uterine weights
were not affected by treatment. The rate of pre-implantation loss was
slightly reduced and that of postimplantation loss slightly increased
at 40 mg/kg bw per day, which resulted in a comparable number of
fetuses to controls. The body weights of female fetuses were slightly
reduced at this dose. There were no remarkable external fetal changes
(FitzGerald, 1990b).
In the main study, groups of 19 pregnant Russian Chbb:HM rabbits
received technical-grade dicyclanil in an aqueous solution of 0.5%
sodium carboxy-methylcellulose by oral gavage at a daily dose of 0, 1,
3, 10, or 30 mg/kg bw on days 7-19 of gestation. On gestation day 29,
the dams were killed and necropsied, and the fetuses were weighed,
sexed, and examined for external, visceral, and skeletal
abnormalities. The study was of conventional design, with GLP and
quality assurance certification.
No deaths or treatment-related clinical signs of toxicity were
observed. Maternal body weight, body-weight gain, and food consumption
and the carcass weight at necropsy were reduced at 30 mg/kg bw per
day. Maternal body-weight gain was also reduced at 10 mg/kg bw per
day. Necropsy of the dams revealed no treatment-related effects, and
reproductive function was not affected. The body weights of fetuses at
the highest dose were reduced, and the fetuses showed skeletal
variations indicative of a slight delay in ossification. There was no
evidence of teratogenicity. The NOEL for maternal toxicity was 3 mg/kg
bw per day on the basis of reduced body-weight gain. The NOEL for
developmental toxicity was 10 mg/kg bw per day on the basis of reduced
fetal weight and increased skeletal variations consistent with delayed
ossification (FitzGerald, 1993b).
2.2.6 Special studies: Pharmacological effects
The effects of dicyclanil on the central nervous system
(centrally controlled behaviour, body temperature, locomotor activity,
hypnotic potentiation, motor coordination), the peripheral nervous
system, the autonomic nervous system and smooth muscles, the
cardiovascular and respiratory systems, and the gastrointestinal tract
were investigated in mice, rats, and guinea-pigs in vivo and
in vitro. The effect of dicyclanil on sperm morphology and motility
was also examined. All of the studies followed the Japanese Guidelines
for the Registration of Pharmaceuticals (General Pharmacology), with
GLP and quality assurance certification.
In vivo, technical-grade dicyclanil in polyethylene glycol
200:ethanol (5:3 v/v) was administered orally by gavage at a single
dose of 0, 1, 10, 50, or 100 mg/kg bw to male NMRI mice or male Han
Wistar rats. Doses < 100 mg/kg bw had no effect on the body
temperature of rats (Pfister & Gisin, 1996a), on the
hexobarbitone-induced sleeping time in mice (Pfister & Gisin, 1996b),
or on gastrointestinal motility in mice (Pfister & Gisin, 1996c).
In the modified Irwin test for general behavioural changes,
treatment of mice with dicyclanil at 100 mg/kg bw slightly inhibited
both exploratory activity and the startle response. The changes were
most evident 6 h after treatment, with complete recovery of the
startle response by 8 h and of exploratory activity by 24 h after
treatment. Mice treated with 1 or 10 mg/kg bw showed normal behaviour
(Pfister & Gisin, 1996d). After treatment with the vehicle, reduced
locomotor activity (static, mobile, and rearing activity and mobile
and active times) was seen in control mice over 24 h, being most
pronounced during the first 8 h. These parameters were less markedly
reduced after treatment with dicyclanil at 1 (not statistically
significant) or 10 mg/kg bw. Opposite effects were induced by
dicyclanil at 100 mg/kg bw, when the reduction in static activity was
more pronounced than in controls while changes in the other parameters
were comparable. All of the effects were fully reversed by 24 h after
dosing (Pfister & Hussherr, 1996a). Motor coordination in mice, as
assessed by recording the time the animals could retain their balance
on a rotating rod, was inhibited at 100 mg/kg bw but not at 1 or 10
mg/kg bw. The effect was significant 4 h after dosing but had
completely disappeared by 24 h (Pfister & Hussherr, 1996b).
Treatment of rats with 100 mg/kg bw (the only dose tested)
resulted in slight increases throughout the observation period (6-8 h
after dosing) in heart rate (statistically significant) and tidal and
minute lung volume (statistically significant only when compared with
values for vehicle-treated animals, which generally had lower values
than untreated animals). The blood pressure, electrocardiogram, and
respiratory rate remained unchanged (Pfister & Nordmann, 1996).
Treatment of rats with 50 mg/kg bw (the only dose tested) had no
effect on sperm motility, concentration, or morphology. Minor,
statistically nonsignificant increases in abnormal sperm morphology 6
weeks after dosing (sperm with head only and with abnormally shaped
hooks) were completely reversed by 12 weeks after dosing (Pfister &
Gisin, 1996e.
In vitro, a concentration of 0, 0.1, 0.3, 1, or 3 mmol/L of
technical-grade dicyclanil in dimethyl sulfoxide had no significant
effect on directly induced contractions of skeletal muscle or on
contractions induced indirectly by phrenic nerve stimulation in the
isolated phrenic nerve-diaphragm preparation from rats. The authors
concluded that dicylanil has no effect on the skeletal neuromuscular
junction (Pfister & Hussherr, 1996c). When dicyclanil was tested on
ileum isolated from guinea-pigs, dose-dependent antagonistic activity
against the smooth muscle contractions induced by histamine and
acetylcholine and against the nonspecific smooth muscle contractions
induced by barium chloride was observed at concentrations > 0.3, 1,
and > 0.3 mmol/L, respectively. All of the effects were fully and
rapidly reversible. Dicyclanil was considered to be only a weak
antagonist (Pfister & Gisin, 1996f).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term and long-term
toxicity, carcinogenicity, genotoxicity, reproductive toxicity, and
pharmacology of dicyclanil. All of the pivotal studies were carried
out according to appropriate standards for study protocol and conduct.
After repeated oral administration of radiolabelled dicyclanil to
rats, the radiolabel was rapidly and almost completely absorbed and
was rapidly distributed to the major organs and tissues. Elimination
was rapid (> 93% of the total dose within 24 h) and was virtually
complete within 3 days. The major route of elimination was the urine
(79-83% of the total dose within 24 h), while the faecal route was of
minor importance (6-12% of the total dose within 24 h).
Biotransformation in the rat involves oxidative opening of the
cyclopropyl ring at various positions, followed by further oxidation
and cleavage of the cyclopropyl-N bond (i.e. dealkylation). The
metabolic pathways of dicyclanil in sheep treated topically are
essentially the same as those in rats.
After oral administration of dicyclanil to rats, the LD50
values were 560 mg/kg bw in males and approximately 500 mg/kg bw in
females. Dicyclanil is moderately hazardous when given as a single
oral dose.
In a three-month study of toxicity, rats received dicyclanil in
the diet at a concentration of 0, 5, 25, 125, or 500 mg/kg of diet.
Male rats at 125 and 500 mg/kg of diet showed decreased food
consumption and body-weight gain and slightly decreased plasma glucose
concentrations. The weights of the kidneys, brain, and testis relative
to that of the body were increased in males at 500 mg/kg of diet, and
females at this dose showed decreased food consumption, body-weight
gain, and plasma glucose concentrations and increased relative weights
of the liver and brain; plasma glucose concentrations were also
reduced in females at 125 mg/kg of diet. The NOEL was 25 mg/kg of
diet, equal to 1.6 mg/kg bw per day, on the basis of the reduction in
body-weight gain.
In a 3-month study of toxicity, dogs received dicyclanil in the
diet at a concentration of 0, 20, 100, 500, or 1500 mg/kg of diet.
Clinical signs consisting mainly of neurotoxicity, decreased food
consumption and body-weight gain, and changes in clinical chemistry
and erythrocyte parameters were observed mainly at the highest dose.
Plasma cholesterol and phospholipid concentrations were increased in
animals at concentrations of 100 mg/kg of diet and above. The weights
of the spleen (males and females), thymus (males only), and testis
were decreased at 1500 mg/kg of diet. Atrophy of the spleen in males
and females and atrophy of the thymus, mesenteric lymph node, testis,
and prostate in males were observed at concentrations of 100 mg/kg of
diet and above. The weights of the liver, adrenals, and kidneys were
increased in animals at the highest dose; in females, the liver
weights were also increased at lower doses. Inflammatory changes were
seen in the urinary bladder of females at concentrations of 100 mg/kg
of diet and above and in the livers of males and females at 1500 mg/kg
of diet. Hepatocyte oedema was observed in females at all doses, but
hepatocellular damage was not seen at any dose in either sex. The
Committee noted that hepatocyte oedema without hepatocellular damage
is not of toxicological significance. Hence, the NOEL was 20 mg/kg of
diet, equal to 0.61 mg/kg bw per day, on the basis of increased plasma
cholesterol concentrations and histopathological findings in the
prostate and urinary bladder.
In a 1-year study, dogs received dicyclanil in the diet at a
concentration of 0, 5, 25, 150, or 750 mg/kg of diet. Males showed
slightly decreased plasma calcium concentrations and increased
absolute and relative liver weights at 750 mg/kg of diet. Plasma
cholesterol concentrations were increased in males at 150 and 750
mg/kg of diet, and this change was not reversed after a 4-week
recovery period. Females receiving the highest dose vomited and had
slightly reduced food consumption and body-weight gain, slightly
increased plasma cholesterol concentrations, increased absolute and
relative liver weights, and decreased absolute and relative heart
weights. Macroscopic and microscopic findings consisting of liver
necrosis, tubular lesions in the kidneys, testicular and prostatic
atrophy, and vascular thrombus, were found in one male and one female
at the highest dose that died before their scheduled sacrifice. These
animals suffered from acute, severe liver failure and resulting
cardiovascular disturbances and stress due to weight loss. Comparable
acute, severe liver toxicity was not observed in the 3-month study of
toxicity in dogs given feed containing dicyclanil at concentrations up
to 1500 mg/kg of diet. The findings in the two animals were therefore
considered to be incidental. The NOEL was 25 mg/kg of diet, equal to
0.71 mg/kg bw per day, on the basis of increased plasma cholesterol
concentrations in male dogs. The Committee noted that this NOEL is
supported by the NOEL in the 3-month study of toxicity in dogs. It
also noted that the histopathological findings observed in the 3-month
study were not seen in the 1-year study among animals that lived until
the time of scheduled sacrifice.
In a study of carcinogenicity, mice received diets containing
dicyclanil at a concentration of 0, 10, 100, 500, or 1500 mg/kg of
diet for 18 months. The animals at the highest dose were killed during
weeks 58-59 because of self-inflicted injuries and poor health. On the
basis of significant reductions in body-weight gain, the
concentrations of 500 and 1500 mg/kg of diet in females and 1500 mg/kg
of diet in males were considered to exceed the maximum tolerated dose.
The liver was the main target organ in both male and female mice. The
effects included Kupffer cell pigmentation (with haemosiderin) and
hepatocellular necrosis in males at doses of 100 mg/kg of diet and
higher, increased incidences of hepatocellular adenomas in females at
500 and 1500 mg/kg of diet, and hepatocellular carcinomas in females
at 1500 mg/kg of diet. The Committee noted that these liver tumours
were observed only at doses that exceeded the maximum tolerated dose
and that there were signs of hepatocellular proliferation in these
animals which might have been involved in the hepatic carcinogenesis
observed. Pigmentation of the olfactory epithelium (with oxidized
lipofuscins) was observed in animals of each sex at 100 and 500 mg/kg
of diet; in the males, this effect was accompanied by an increased
incidence of inflammatory cell infiltration in the underlying Bowman
glands. Males and females at 500 mg/kg of diet also showed
pigmentation of the adrenal glands (with partly oxidized lipofuscins)
and hypercellularity of the bone marrow. As the Committee considered
the effects on the olfactory epithelium to be of no biological
significance, the NOEL was 10 mg/kg of diet, equal to 1.1 mg/kg bw per
day, on the basis of the effects on the liver.
In a 2-year study of carcinogenicity and toxicity, rats received
diets containing dicyclanil at a concentration of 0, 5, 25, 125, or
500 mg/kg of diet. In animals at the highest dose, food consumption
and body-weight gain were decreased, and the relative weights of
almost all organs were increased as a result. Treatment-related
histopathological alterations were observed in the exocrine pancreas
(hyperplasia) in males at the highest dose, in the liver (biliary
cysts) in females at the highest dose, and in the olfactory epithelium
(pigmentation resulting from accumulation of oxidized lipofuscins) in
males at doses of 25 mg/kg of diet and above and in females at doses
of 125 mg/kg of diet and above. Although the latter represents
enhancement of a naturally occurring age-related process, treatment
had no effect on survival, behaviour, or general well-being, and there
were no other morphological changes in the olfactory mucosa.
Therefore, the Committee concluded that the effect on the olfactory
epithelium was of no biological significance. Dicyclanil did not
affect the incidence of tumours. The NOEL was 125 mg/kg of diet, equal
to 22 mg/kg bw per day, on the basis of changes in body weight and
histopathological changes in the liver and pancreas.
Dicyclanil has been tested in vitro for its ability to induce
reverse mutation in Salmonella typhimurium and Escherichia coli,
gene mutation in Chinese hamster lung cells, chromosomal aberrations
in Chinese hamster ovary cells, and unscheduled DNA synthesis in
primary rat hepatocytes. It has been tested in vivo for its ability
to induce micronuclei in bone-marrow cells of mice treated orally. The
results of all of these tests were negative. On the basis of these
data, the Committee concluded that dicyclanil is not genotoxic.
Dicyclanil increased the incidence of liver tumours in female
mice. However, as these tumours occurred in only one tissue of animals
of one sex and one species at doses that were above the maximum
tolerated dose, and dicyclanil is not genotoxic, the Committee
concluded that dicyclanil does not represent a carcinogenic risk for
humans.
In a two-generation study of reproductive toxicity, with two
litters per generation, rats were given dicyclanil in the diet at a
concentration of 0, 5, 30, 200, or 500 mg/kg of diet. Treatment
reduced the body-weight gain of the parental animals at the highest
dose and, marginally, at 200 mg/kg of diet. Secondary to this effect,
dicyclanil increased the relative weights of most organs in animals at
the highest dose and of the brain (males and females), kidneys (males
only), and testis at 200 mg/kg of diet. Reproductive parameters were
not affected. The only effect of dicyclanil on pups was to reduce
their weight gain from day 4 post partum onwards. The NOEL for
parental toxicity was 30 mg/kg of diet, equal to 2 mg/kg bw per day,
on the basis of changes in body weight. The NOEL for reproductive
toxicity was 500 mg/kg of diet, equal to 24 mg/kg bw per day, the
highest dose tested. The NOEL for toxicity to the pups was 200 mg/kg
of diet, equal to 21 mg/kg bw per day, on the basis of reduced
body-weight gain.
In a study of developmental toxicity in rats given dicyclanil at
a dose of 0, 1, 5, 25, or 75 mg/kg bw per day orally on days 6-15 of
gestation, the highest dose was toxic to the dams, as seen by
reductions in body-weight gain, food consumption, and the weight of
the gravid uterus. Marginal reductions in body-weight gain and food
consumption were also observed at 25 mg/kg bw per day. The effects on
the fetuses, observed only at the highest dose, were reduced fetal
weight, a slightly increased incidence of renal pelvic dilatation, and
a number of mainly sternebral defects and variations due to poor or
absent ossification. There was no evidence of teratogenicity. The NOEL
for maternal toxicity was 5 mg/kg bw per day on the basis of the
reduction in body-weight gain. The NOEL for developmental toxicity was
25 mg/kg bw per day on the basis of reduced fetal weight, increased
renal pelvic dilatation, increased skeletal anomalies, and variations
consistent with a slight delay in skeletal maturation.
In a study of developmental toxicity in rabbits given dicyclanil
at a dose of 0, 1, 3, 10, or 30 mg/kg bw per day orally on days 7-19
of gestation, dams at 30 mg/kg bw per day had reduced food consumption
and those at 10 and 30 mg/kg bw per day showed reduced body-weight
gain. The fetuses of dams at the highest dose had lower body weights
than controls and an increased incidence of skeletal variations
indicative of a slight delay in ossification. There was no evidence of
teratogenicity. The NOEL for maternal toxicity was 3 mg/kg bw per day
on the basis of reduced body-weight gain. The NOEL for developmental
toxicity was 10 mg/kg bw per day on the basis of reduced fetal weight
and skeletal variations consistent with delayed ossification.
In pharmacological tests in vitro, dicyclanil at doses up to
3 mmol/L had no effect on the skeletal neuromuscular junction. At
concentrations of 0.3 mmol/L and higher, it had slightly antagonistic
effects on smooth muscle contractions induced by agonists. In mice and
rats given a single oral dose of 0, 1, 10, 50, or 100 mg/kg bw, the
highest dose affected general behaviour, locomotor activity, motor
coordination, heart rate, and tidal and minute lung volume. Locomotor
activity was also affected at 10 mg/kg bw and, very slightly, at
1 mg/kg bw. The treatment had no effect on body temperature, hypnotic
potentiation, gastrointestinal motility, blood pressure, heart beat,
or respiratory rate.
4. EVALUATION
The Committee established an ADI of 0-7 mg/kg bw, on the basis of
the NOEL of 0.71 mg/kg bw per day for increased plasma cholesterol
concentrations in the 1-year study of toxicity in dogs and a safety
factor of 100. As is its usual practice, the Committee rounded the
value of the ADI to one significant figure.
5. REFERENCES
Altmann, B. (1991) CGA 183893 tech. 28-Day range finding toxicity
study in beagle dogs. Unpublished report (test no. 901202) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Altmann, B. (1993) CGA 183893 tech. 3-Month subchronic dietary
toxicity study in beagle dogs. Unpublished report (test no.
922813) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Altmann, B. (1995) CGA 183893 tech. 12-Month chronic dietary toxicity
study in beagle dogs. Unpublished report (test no. 922814) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Bachmann, M. (1991) CGA 183893 tech. 28-Days range finding study in
rats (administration in food). Unpublished report (test no.
901201) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Bachmann, M. (1993) CGA 183893 tech. 3-Month oral toxicity study in
rats (administration in food). Unpublished report (test no.
922810) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Bachmann, M. (1996a) CGA 183893 tech. 18-Month oncogenicity study in
mice. Unpublished report (test no. 922815) from Ciba-Geigy Ltd,
Stein, Switzerland. Submitted to WHO by Novartis Animal Health
Inc., Basel, Switzerland.
Bachmann, M. (1996b) CGA 183893 tech. 24-Month carcinogenicity and
chronic toxicity study in rats. Unpublished report (test no.
922816) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Dubach-Powell, J.R. (1996) Pharmacokinetic study of CGA 183893 tech.
in the rat. Unpublished letter report (RCC project no.
615295/Ciba project no. 952803) from Research & Consulting
Company, Itingen, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
FitzGerald, R.E. (1990a) Preliminary oral toxicity study with CGA
183893 technical in pregnant rats (Dose range-finding study).
Unpublished report (test no. 901211) from Ciba-Geigy Ltd, Stein,
Switzerland. Submitted to WHO by Novartis Animal Health Inc.,
Basel, Switzerland.
FitzGerald, R.E. (1990b) Preliminary toxicity study in pregnant
rabbits with CGA 183893 technical (oral administration) (Dose
rangefinding study). Unpublished report (test no. 901212) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
FitzGerald, R.E. (1993a) CGA 183893 technical: Rat oral
teratogenicity. Unpublished report (study no. 922821) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
FitzGerald, R.E. (1993b) CGA 183893 technical: Rabbit oral
teratogenicity. Unpublished report (study no. 922822) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Geleick, D. (1992) CGA 183893 tech. Gene mutation test with Chinese
hamster cells V79 (OECD conform) in vitro. Unpublished report
(test no. 922827) from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Hagemann, C. (1992a) CGA 183893 tech. Acute dermal
irritation/corrosion study in the rabbit. Unpublished report
(test no. 922803) from Ciba-Geigy Ltd, Stein, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Hagemann, C. (1992b) CGA 183893 tech. Acute eye irritation/corrosion
study in the rabbit. Unpublished report (test no. 922802).
CibaGeigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Hagemann, C. (1993) CGA 183893 tech. Skin sensitization test in the
guinea pig optimisation test. Unpubished report (test no. 922805)
from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by
Novartis Animal Health Inc., Basel, Switzerland.
Hartmann, H.R. (1992a) CGA 183893 tech. Acute oral toxicity in the
rat. Unpublished report (test no. 922801) from Ciba-Geigy Ltd,
Stein, Switzerland. Submitted to WHO by Novartis Animal Health
Inc., Basel, Switzerland.
Hartmann, H.R. (1992b) CGA 183893 tech. Acute dermal toxicity in the
rat. Unpublished report (test no. 922804) from Ciba-Geigy Ltd,
Stein, Switzerland. Submitted to WHO by Novartis Animal Health
Inc., Basel, Switzerland.
Hartmann, H.R. (1993) CGA 183893 tech. Acute inhalation toxicity in
the rat. Unpublished report (test no. 922806) from Ciba-Geigy
Ltd, Stein, Switzerland. Submitted to WHO by Novartis Animal
Health Inc., Basel, Switzerland.
Hassler, S. (1994) Absorption, distribution and elimination of
[2-14C]pyrimidyl CGA 183893 in the rat after multiple oral
administration. Unpublished report (report no. 7/94; project no.
012AM01) from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to
WHO by Novartis Animal Health Inc., Basel, Switzerland.
Hertner, T. (1992) CGA 183893 tech. Salmonella and
Escherichia/liver-microsome test. Unpublished report (test no.
922825) from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Hertner, T. (1993a) CGA 183893 tech. Cytogenetic test on Chinese
hamster cells in vitro (EC conform). Unpublished report (test no.
922826) from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Hertner, T. (1993b) CGA 183893 tech. Autoradiographic DNA repair test
on rat hepatocytes (OECD conform) in vitro. Unpublished report
(test no. 922824) from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Hertner, T. (1993c) CGA 183893 tech. Micronucleus test, mouse (OECD
conform). Unpublished report (test no. 922823) from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by Novartis Animal
Health Inc., Basel, Switzerland.
Khalil, S. (1995) CGA 183893 technical: Rat dietary two-generation
reproduction study. Unpublished report (study no. 922818) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Löffler, A. (1998) The nature of residues in tissues of sheep after
single topical pour-on administration of [pyrimidine-2-14C]CGA
183893. Unpublished report (study no. 012AM04) from Novartis Crop
Protection AG, Basel, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Marty, J.H. (1995) CGA 183893 tech. 28-Day repeated dose dermal
toxicity study in the rat. Unpublished report (test no. 922807)
from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by
Novartis Animal Health Inc., Basel, Switzerland.
Ogorek, B. & Arni, P. (1987) Salmonella mutagenicity test with three
strains with CGA 183893 (Test for mutagenic properties in
bacteria). Unpublished report (experiment no. 871051) from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Pfister, T. & Gisin, M.(1996a) General pharmacology of CGA 183893
tech. Effect on the body temperature in the rat. Unpublished
report (project no. RCC 615317/CG 952803) from Research &
Consulting Company, Ltd, Itingen, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Pfister, T. & Gisin, M. (1996b) General pharmacology of CGA 183893
tech. Effect on the sleeping time induced by hexobarbitone in the
mouse. Unpublished report (project no. RCC 615330/CG 952803) from
Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Gisin, M. (1996c) General pharmacology of CGA 183893
tech. Effect on the intestinal motility (charcoal propulsion) in
the mouse. Unpublished report (project no. RCC 615385/CG 952803)
from Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Gisin, M. (1996d) General pharmacology of CGA 183893
tech. Modified Irwin screen test in the mouse. Unpublished report
(project no. RCC 615306/CG 952803) from Research & Consulting
Company, Ltd, Itingen, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Pfister, T. & Gisin, M. (1996e) General pharmacology of CGA 183893
tech. Effect on sperm motility, concentration and morphology in
the rat. Unpublished report (project no. RCC 615407/CG 952803)
from Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Gisin, M. (1996f) General pharmacology of CGA 183893
tech. Effect on the isolated ileum of the guinea pig. Unpublished
report (project no. RCC 615363/CG 952803) from Research &
Consulting Company, Ltd, Itingen, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Pfister, T. & Hussherr, D. (1996a) General pharmacology of CGA 183893
tech. Effect on the locomotor activity in the mouse. Unpublished
report (project no. RCC 615328/CG 952803) from Research &
Consulting Company, Ltd, Itingen, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Pfister, T. & Hussherr, D. (1996b) General pharmacology of CGA 183893
tech. Effect on motor co-ordination (rotarod) in the mouse.
Unpublished report (project no. RCC 615341/CG 952803) from
Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Hussherr, D. (1996c) General pharmacology of CGA 183893
tech. Effect on the isolated phrenic nerve-diaphragm preparation
of the rat. Unpublished report (project no. RCC 615352/CG 952803)
from Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Nordmann, A. (1996) General pharmacology of CGA 183893
tech. Effect on the cardiovascular and respiratory systems in the
rat. Unpublished report (project no. RCC 615374/CG 952803) from
Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Phillips, M. (1996) [Pyrimidine-2-14C]-CGA 183893. Nature of
metabolites in the excreta and tissues of sheep following single
pour-on administration. Unpublished report (No. 14072; project
no. 158665) from Inveresk Research, Tranent, Scotland. Submitted
to WHO by Novartis Animal Health Inc., Basel, Switzerland.
Thanei, P. (1996a) The metabolism of [2-14C]pyrimidyl CGA 183893 in
the rat. Unpublished report (No.7/96; project no. 012AM02) from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Thanei, P. (1996b) The nature of metabolites in tissues and excreta of
sheep after single topical administration of [2-14C]pyrimidyl
CGA 183893. Unpublished report (No. 9/96; project no. 012AM03)
from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Novartis Animal Health Inc., Basel, Switzerland.
Weber, E. (1998) CGA 183893 tech. Pigmentation of the olfactory mucosa
observed after dietary administration to rats. Unpublished report
(report no. CB 98/05) from Novartis Crop Protection AG, Basel,
Switzerland. Submitted to WHO by Novartis Animal Health Inc.,
Basel, Switzerland.
Three male New Zealand white rabbits (Chbb:NZW) received a
semi-occlusive topical application of 0.5 g of technical-grade
dicyclanil moistened with distilled water containing 0.5%
carboxymethylcellulose in 0.1% aqueous polysorbate 80 to their shaved
flanks. The study followed OECD test guideline 404, with GLP and
quality assurance certification. Only very slight erythema was
observed 1 ( n = 3) to 24 h ( n = 1) after removal of the patch
(Hagemann, 1992a).
In a study that followed OECD test guideline 405 with GLP and
quality assurance certification, three female New Zealand white
rabbits (Chbb:NZW) received an instillation of 0.1 ml (84 mg) of
technical-grade dicyclanil into the conjunctival sac of one eye. The
other eye served as control. The cornea appeared to be unaffected by
treatment; one animal showed an affected iris 1 h after instillation
but recovered within 24 h. Slight chemosis of the conjunctiva was
observed in two animals 1 h after instillation, but they recovered
within 24 h. Redness of the conjunctiva was seen in all animals,
although at different grades (score 1 or 2), and recovery to normal
occurred within 1-7 days (Hagemann, 1992b).
In 10 male and 10 female Pirbright white Tif:DHP guinea-pigs
submitted to an optimization test that followed OECD test guideline
406 with GLP and quality assurance certification, no significant skin
sensitization (1/20 positive) was observed after epidermal challenge
with 20% technical-grade dicyclanil in vaseline, this being a
subirritant dose. When the skin barrier was intentionally bypassed,
i.e. by intradermal challenge with a 0.1% solution of technical-grade
dicyclanil in 10% propylene glycol, 13 of 20 treated animals showed
positive reactions versus 3 of 20 animals given the vehicle
( p < 0.01) (Hagemann, 1993).
2.2.2 Short-term studies of toxicity
Rats
In a 28-day range-finding study, groups of five male and five
female Tif:RAIf (SPF) rats received technical-grade dicyclanil
(purity, > 98%) in the diet at a concentration of 0, 100, 500, or
2000 mg/kg of diet, equal to average achieved intakes of 0, 9.5, 50,
and 150 mg/kg bw per day for males and 0, 9.4, 49, and 160 mg/kg bw
per day for females. The study followed OECD test guideline 407
(adapted to the objectives of a range-finding study) without GLP or
quality assurance certification.
No treatment-related deaths occurred. Piloerection was observed
in animals of each sex at the highest dose during the second half of
the study. Dose-dependent reductions in food consumption, body-weight
gain, and final body weight were noted in all treated groups. These
were particularly marked in males at the high dose. Water consumption
was reduced in females at 500 and 2000 mg/kg of diet. Increased
incidences of anisocytosis and polychromasia of erythrocytes and a
slightly lower (not statistically significant) leukocyte count were
noted in males at the high dose. In both males and females at this
dose, higher plasma concentrations of urea and cholesterol and
increased activities of aspartate aminotransferase (twofold) and
alanine aminotransferase (five- to sevenfold) were noted.
Hypoglycaemia and lower plasma total bilirubin concentrations were
also observed in these animals, although the changes were not
statistically significant in females. Males at the high dose also had
slightly lower plasma globulin and calcium concentrations, while
females at this dose had an increased plasma phosphate concentration.
Consistent with the reduced body weights of all treated animals, the
absolute weights of a number of organs were decreased, whereas the
relative weights of a number of other organs were increased. The only
notable changes in organ weights which could be considered to be
treatment-related effects were reductions in the absolute and relative
weights of the prostate in males at the intermediate and high doses
and of the adrenals in females at the low and intermediate doses. No
macroscopic changes were found, apart from emaciation at the high
dose. Histopathologically, reduced spermatogenesis in the testes was
seen in 1/5 males at 500 mg/kg of diet and 5/5 males at 2000 mg/kg of
diet, accompanied by accumulation of cellular debris in the epididymal
duct in all those at the high dose. These animals also showed
immaturity of the prostate. In 1/5 females at 500 mg/kg of diet and in
4/5 females at 2000 mg/kg of diet, polyovular ovarian follicles, as
indicated by hypercellularity, were seen (Bachmann, 1991).
In a 3-month study of toxicity, groups of 10 male and 10 female
Tif:RAIf (SPF) rats received technical-grade dicyclanil in the diet at
a concentration of 0, 5, 25, 125, or 500 mg/kg of diet, equal to
average achieved intakes of 0, 0.31, 1.6, 8.0, and 33 mg/kg bw per day
for males and 0, 0.31, 1.7, 8.4, and 34 mg/kg bw per day for females.
Ten additional animals of each sex in the control and high-dose groups
were kept for a 4-week recovery period. The study followed OECD test
guideline 408, with GLP and quality assurance certification.
No treatment-related deaths or clinical signs were observed.
Slight reductions in body-weight gain and food consumption were
observed in animals of each sex at the high dose and in males at 125
mg/kg of diet. Owing to a compensatory increase in food intake during
the recovery period, the body weights of animals at the high dose were
comparable to those of the controls by the end of the recovery period.
Chemical analysis of blood revealed slightly lower plasma glucose
concentrations in males and females at 125 and 500 mg/kg of diet at
the end of the treatment period, but this was reversed within the
recovery period. Higher organ:body weight ratios were observed for
kidneys, brain, and testis in males at the high dose and for liver and
brain in females at this dose, these changes being reversible within
the 4-week recovery period. Although the relative epididymidal weights
were increased in males at doses > 25 mg/kg of diet, this was
considered not to be toxicologically significant because the absolute
epididymidal weights were not different from those of controls and
there were no abnormal histopathological findings. No
treatment-related ophthalmological or haematological changes were
observed, and no gross or microscopic alterations were observed. One
female at the high dose that was alllowed to recover had a mammary
tumour, which was considered to be of spontaneous origin. On the basis
of the reduction in body-weight gain, the NOEL was 25 mg/kg of diet,
equal to 1.6 mg/kg bw per day (Bachmann, 1993).
In a study that followed OECD test guideline 410 with GLP and
quality assurance certification, groups of five male and five female
Tif:RAIf (SPF) rats received dermal applications of technical-grade
dicyclanil at a dose of 0, 5, 30, 300, or 1000 mg/kg bw per day, 6
h/day on 5 days per week for 4 weeks. Dicyclanil was dissolved in
water containing 0.5% carboxymethylcellulose and 0.1% polysorbate 80
and was applied under an occlusive dressing to a clipped dorsal area
of the skin. At the end of each application period, the site was
washed with lukewarm water.
None of the animals died and no treatment-related clinical signs
were observed. Apart from a few incidental findings, no signs of local
skin irritation were seen. Male rats at 300 and 1000 mg/kg bw per day
showed dose-dependently decreased body weights and body-weight gain
and slightly lower food consumption. The plasma concentrations of
sodium and calcium were slightly reduced in these animals. Females at
the high dose showed increased absolute and relative liver weights. A
similar effect was observed in females at 300 mg/kg bw per day but did
not reach statistical significance. The absolute, but not the
relative, weight of the brain of females was increased at doses
> 30 mg/kg bw per day, without histopathological findings. No
treatment-related effects were noted on gross examination. Microscopic
examination showed hepatocyte hypertrophy in males at 1000 mg/kg bw
per day and in females at 300 and 1000 mg/kg bw per day. The NOEL was
30 mg/kg bw per day, on the basis of decreased body-weight gain and
changes in the liver (Marty, 1995).
Dogs
In a 28-day range-finding study, groups of two male and two
female beagles were given technical-grade dicyclanil (purity, > 98%)
in the diet at a concentration of 0, 200, 1000, or 2500 mg/kg of diet,
equal to average achieved intakes of 0, 5.6, 31, and 66 mg/kg bw per
day for males and 0, 6.2, 30, and 50 mg/kg bw per day for females. The
study followed OECD test guideline 409 (adapted to the objectives of a
range-finding study) without GLP (except for the pathology report) or
quality assurance certification.
No treatment-related deaths occurred. The clinical signs included
body tremors (in animals of each sex at 2500 mg/kg of diet), vomiting
(in animals of each sex at 2500 mg/kg of diet and in one male at 1000
mg/kg of diet), dyspnoea and slight apathy (in males at 2500 mg/kg of
diet). Animals at the high dose lost weight and had reduced food
consumption, and females at 1000 mg/kg of diet also showed a slight
reduction in food consumption. The reduction in food consumption did
not appear to be due to unpalatability. One male and one female at the
high dose showed a prolonged prothrombin time, and the other male at
the high dose had a slightly increased number of blood platelets.
Alanine aminotransferase activity was markedly increased and alkaline
phosphatase activity slightly increased in one male at the high dose
and in one female at the intermediate and one at the high dose;
aspartate aminotransferase activity was also increased in the female
at the high dose. Very slight decreases were observed in plasma
potassium concentrations in males at the intermediate and high doses,
in plasma glucose concentration in females at the high dose, and in
the plasma concentrations of urea, creatinine, and total bilirubin in
males and females at the high dose. Urinary analysis revealed an
increased incidence of proteinuria in males at the high dose and
females at the intermediate and high doses. The absolute and relative
weights of the testis and thymus were markedly decreased and those of
the heart slightly decreased at 2500 mg/kg of diet. In animals of each
sex at 1000 and 2500 mg/kg of diet, the absolute and relative weights
of the adrenal and kidney were increased, but without any
histopathological changes. Microscopic examination revealed hepatic
toxicity in one female at 1000 mg/kg of diet and in all animals at
2500 mg/kg of diet. The toxicity included focal or single-cell
necrosis, inflammatory-cell foci, and a brown granular pigment in
macrophages. One female at the intermediate dose also had mild
bile-duct hyperplasia, and one male at the high dose had periportal
hepatocyte enlargement. The testes of males at this dose showed
degeneration characterized by reduced numbers of tailed spermatids and
increased numbers of multinucleated or giant cells. Mild thymic
atrophy was noted in animals of each sex at the highest dose. The
heart of one male at this dose was mottled and showed inflammation,
fibrosis, and haemorrhage. Tubular basophilia or dilatation was
present in the kidneys of all animals at the high dose, two at the
intermediate dose, and one at the low dose, but not in controls. This
lesion is of minimal toxicological significance (Altmann, 1991).
Groups of four male and four female beagles received
technical-grade dicyclanil in the diet at a concentration of 0, 20,
100, 500, or 1500 mg/kg of diet for 3 months, equal to average
achieved intakes of 0, 0.61, 2.7, 14, and 42 mg/kg bw per day for
males and 0, 0.71, 3.5, 17, and 42 mg/kg bw per day for females. The
study followed OECD test guideline 409, with GLP and quality assurance
certification.
One male at the high dose was found dead at week 11 after general
deterioration in clinical condition accompanied by tonic-clonic
spasms. Post-mortem examination did not reveal the cause of death.
Animals at the high dose started to show clinical signs from week
9-11, including slight ataxia, unnaturally raised tails, and frequent
shaking. In addition, vomitus and traces of blood in the faeces were
observed. Ophthalmoscopic examinations revealed no treatment-related
changes. Animals at the high dose lost weight (males only) or had
reduced body-weight gain with reduced food intake. A very slight
reduction in food intake was also observed transiently in some animals
at 500 mg/kg of diet. Slightly reduced haemoglobin concentrations and
haematocrit values, associated with minor microcytosis and
hypochromasia of erythrocytes, were recorded in animals at the high
dose. Increased plasma cholesterol and phospholipid concentrations
were observed in animals at doses > 100 mg/kg of diet. Plasma
albumin concentrations were slightly decreased in males and females at
1500 mg/kg of diet and in females at 500 mg/kg of diet. Decreased
plasma calcium, potassium, urea, creatinine, and total bilirubin
concentrations were found in animals at the high dose. Urinary
analysis revealed no treatment-related effects. The mean absolute and
relative liver weights were increased in animals at the highest dose
and in females also at 20, 100 (not statistically significant), and
500 mg/kg of diet (not dose-related). The absolute and relative
adrenal weights were also slightly increased at the high dose. The
absolute and relative weights of the thymus (males), testis, and
spleen (both sexes) were decreased in animals at the high dose. The
kidney weights were increased in males (relative) and females
(absolute and relative) at 1500 mg/kg of diet. Macroscopic examination
showed no treatment-related effects. Microscopy of the liver showed
minimal to moderate focal or multifocal subcapsular inflammation with
fibrosis in 2/4 males and 3/4 females at the high dose. Enlarged
hepatocytes, diagnosed as cellular oedema, were observed in the
centrilobular and midzonal region of 3/4 females at 20, 100, and 500
mg/kg of diet and in 1/4 males and all females at 1500 mg/kg of diet.
No morphological signs of hepatocellular damage were apparent. Minimal
atrophy of the white pulp of the spleen was observed in 3/4 males at
500 and 1500 mg/kg of diet and in all females at 1500 mg/kg of diet.
Thymic atrophy was found in 3/4 males at 500 mg/kg of diet and in all
males at 1500 mg/kg of diet. Minimal atrophy of the lymphatic tissue
of the mesenteric lymph node was observed in 3/4 males at the high
dose, and minimal to marked atrophy of the glandular tissue of the
prostate was detected in 3/4 males at 100 mg/kg of diet, 1/4 at 500
mg/kg of diet, and 4/4 at 1500 mg/kg of diet. Examination of the
testes revealed minimal tubular atrophy in 3/4 males at the high dose,
associated with a marked reduction in spermatogenesis in all males of
this group. A dose-related increase in the frequency of inflammatory
changes associated with epithelial hyperplasia was found in the
urinary bladders of females at 100, 500, and 1500 mg/kg of diet
(Altmann, 1993). The Committee noted that hepatocyte oedema without
hepatocellular damage is not of toxicological significance. Hence, the
NOEL was 20 mg/kg of diet, equal to 0.61 mg/kg bw per day, on the
basis of increased plasma cholesterol concentrations and
histopathological findings in the prostate and urinary bladder.
In a 1-year study of toxicity, groups of four male and four
female beagles received technical-grade dicyclanil in the diet at a
concentration of 0, 5, 25, 150, or 750 mg/kg of diet, equal to average
achieved intakes of 0, 0.16, 0.71, 4.4, and 23 mg/kg bw per day for
males and 0, 0.15, 0.77, 5.1, and 23 mg/kg bw per day for females. Two
additional animals per sex in the control and high-dose groups were
maintained for a 4-week recovery period. The observations included
deaths, clinical signs, body weight, food consumption, ocular,
neurological, haematological, clinical chemical, and urinary
parameters, and macroscopic and microscopic end-points. The study
followed OECD test guideline 452, with GLP and quality assurance
certification.
One female at the high dose was found dead on day 13, with no
previous abnormal clinical signs. One male at the high dose was killed
on study day 32 after vomiting, showing marked apathy, lying in a
lateral position, and weight loss correlated to reduced food intake.
Vomiting was observed in females at the high dose, and the body-weight
gain (in two animals) and food consumption of females at the high dose
were slightly reduced. Ocular and neurological examinations revealed
no treatment-related effects, nor were there any changes in
haematological or urinary parameters. Throughout the treatment period,
the plasma cholesterol concentrations were increased in animals of
each sex at 750 mg/kg of diet (not statistically significant in
females) and in males at 150 mg/kg of diet; in males, the change was
not reversed after the 4-week recovery period. Males at the high dose
showed slightly lower plasma calcium concentrations. Both males and
females at the high dose had reduced plasma concentrations of
bilirubin and urea and reduced alkaline phosphatase activity. The
changes in blood chemistry were partially reversed during the recovery
period. The absolute and relative liver weights were increased in
animals at 750 mg/kg of diet, but only the change in absolute weight
in males was statistically significant. The absolute (statistically
significant) and relative weights of the heart were decreased in
females at the high dose. The weight changes were reversed by the end
of the 4-week recovery period. The macroscopic and microscopic
findings were confined to the two animals at the high dose that died
before the scheduled kill. The macroscopic findings consisted of a
scar in the liver and pale kidneys in the male and a mass and
haemorrhagic content in the abdominal cavity of the female.
Histopathologically, both animals showed marked diffuse liver necrosis
and kidney lesions (more severe in the male). The male also showed
testicular and prostatic atrophy, and the female had a thrombus in a
peritoneal blood vessel. The two animals suffered from acute, severe
liver failure and resulting cardiocirculatory disturbances, and the
male also showed stress due to weight loss. Their condition was very
different from that of the other treated dogs in this study, and
comparable acute, severe liver toxicity was not seen in the 28-day and
3-month studies in dogs treated with doses up to 2500 and 1500 mg/kg
of diet, respectively. The lesions seen in the two animals are
considered to be incidental findings. On the basis of increased plasma
cholesterol concentrations in males, the NOEL was 25 mg/kg of diet,
equal to 0.71 mg/kg bw per day (Altmann, 1995).
2.2.3 Long-term studies of toxicity and carcinogenicity
Mice
In an 18-month study of carcinogenicity, groups of 60 male and 60
female Tif:MAGf (SPF) mice were given diets containing technical-grade
dicyclanil at a concentration of 0, 10, 100, 500, or 1500 mg/kg of
diet, equal to average achieved intakes of 0, 1.1, 12, 59, and 210
mg/kg bw per day for males and 0, 1.1, 12, 65, and 200 mg/kg bw per
day for females. Ten animals per sex from each group were assigned for
evaluation of haematological parameters. The study followed OECD test
guideline 451 with GLP and quality assurance certification, but
histopathological examination of the animals at the high dose (i.e.
the group that was killed before study termination, see below) was
limited to the liver and lungs.
Clinical signs were observed only in males at the highest dose,
which injured themselves through vigorous scratching. A higher
mortality rate was noted among males at the high dose and to a lesser
extent in females at this dose. The self-inflicted injuries and poor
health of the animals at the high dose led to a decision to terminate
all surviving animals in this group during weeks 58-59. Doses < 500
mg/kg of diet did not affect survival. The body weights of males and
females at 1500 mg/kg of diet were reduced, with an approximately 50%
reduction in body-weight gain, and those of females at 500 mg/kg of
diet were reduced with a 30% reduction in body-weight gain. As food
intake was not affected, higher food consumption ratios were noted in
animals of each sex at 1500 mg/kg of diet and in females at 500 mg/kg
of diet. Haematological parameters were not affected by treatment. The
absolute and relative weights of the liver were increased in animals
of each sex at 500 mg/kg of diet (for males after adjustment for three
control outliers). In females at 500 mg/kg of diet, the relative
weights of the kidney, brain, and adrenals were increased, while the
absolute weights of these organs were not changed. The
treatment-related macroscopic findings included enlarged livers
(characterized histopathologically as hepatocellular hypertrophy) in
males at 500 mg/kg of diet and in both sexes at 1500 mg/kg of diet,
and masses and/or nodules of the liver in females at 500 and 1500
mg/kg of diet. Treatment-related microscopic findings were observed in
the liver, olfactory epithelium, adrenal gland, and bone marrow.
Kupffer cell pigmentation (mainly haemosiderin) and hepatocellular
necrosis were observed in males at > 100 mg/kg of diet. Increased
numbers of hepatocellular mitotic figures and multinucleated
hepatocytes were seen in the livers of males at the high dose, and the
frequency of foci of cellular change was increased in animals of each
sex at the highest dose. The incidence of heptocellular adenomas was
higher in females at 500 and 1500 mg/kg of diet (9/53 and 5/60,
respectively) than in controls (0/52). In addition, the incidence of
hepatocellular carcinomas was increased in females at the highest dose
(6/60 versus 0 in all other groups). Pigmentation of the olfactory
epithelium was observed at increasing incidence and severity in
animals of each sex at 100 and 500 mg/kg of diet, and males at these
doses showed an increased incidence of inflammatory cell infiltration
(cell type not specified) in the underlying Bowman's glands. At 500
mg/kg of diet, both males and females also showed increased incidences
of pigmentation of the adrenal glands, recorded as ceroid (i.e. a
partly oxidized form of lipofuscin) deposition, and of
hypercellularity of the bone marrow. Treatment with dicyclanil did not
affect the number of animals with malignant lymphomas. It was noted,
however, that females at 500 mg/kg of diet had more sites that
appeared to be infiltrated by malignant lymphoma cells than did
controls or animals at other doses (Bachmann, 1996a).
The Committee noted that the doses at which the liver adenomas
and carcinomas occurred exceeded the maximum tolerated dose for
females, and that there were signs of hepatocellular proliferation in
these animals which might have been involved in the hepatic
carcinogenesis observed. Pigmentation of the olfactory epithelium was
also observed in a long-term study in rats (Bachmann, 1996b; see
below) and was further investigated in a separate study (Weber, 1998;
see below). As the Committee considered the effects on the olfactory
epithelium to be of no biological significance (see below), the NOEL
in this study in mice was 10 mg/kg of diet, equal to 1.1 mg/kg bw per
day, on the basis of effects on the liver.
Rats
In a 2-year study of carcinogenicity and toxicity, groups of 80
Tif:RAIf (SPF) rats of each sex were given technical-grade dicyclanil
in the diet at a concentration of 0, 5, 25, 125, or 500 mg/kg of diet,
equal to average achieved intakes of 0, 0.19, 0.97, 4.8, and 22 mg/kg
bw per day for males and 0, 0.23, 1.2, 6.0, and 26 mg/kg bw per day
for females. Haematological, clinical chemical, and urinary analyses
were performed during weeks 13, 26, 52, 78, and 105 on 20, 10, and 10
animals of each sex per group, respectively. After 1 year, 10 animals
of each sex per group were killed. The study followed OECD test
guideline 453, with GLP and quality assurance certification.
Treatment with dicyclanil did not affect the incidence of
clinical signs or survival. Decreased food consumption was noted in
males and females at the highest dose. The body weights of males and
females at 500 mg/kg of diet were reduced (with an approximately 25%
reduction in body-weight gain), as were the body weights of animals at
125 mg/kg of diet (with a reduction in body-weight gain of < 10%).
Slight but statistically significant red blood cell dyscrasia and a
reduced number of monocytes were observed in males at the high dose
when compared with controls, but the changes were generally
inconsistent over time, not dose-related, and within the range of
historical controls and are therefore regarded as toxicologically
insignificant. Increased concentrations of inorganic phosphate were
observed in males and females at the high dose throughout the study,
although the increase was not statistically significant in females.
Males at 125 mg/kg of diet also had increased inorganic phosphate
concentrations, but these reached statistical significance only in
weeks 78 and 105. Lower concentrations of triglycerides were recorded
for males at 500 mg/kg of diet, although statistical significance was
achieved only in weeks 13 and 26. Urinary parameters were not
affected. As a result of the lower terminal body weights, almost all
of the relative organ weights were increased in males and females at
the highest dose, especially of the kidney, liver, and epididymides.
The absolute epididymal weights were also increased in males at the
high dose at 105 weeks. Males at 125 mg/kg of diet also had increased
relative liver weights, although these were within historical control
values. An increased incidence of liver cysts was observed in females
at the high dose, characterized primarily as unilocular or
multilocular biliary cysts on microscopic examination. In males at the
high dose, an increased incidence of masses and nodules in the
exocrine pancreas was noted, which were characterized
histopathologically as foci or areas of hyperplasia. Aside from the
histopathological findings in the pancreas and liver, the only other
treatment-related histopathological finding was an increased incidence
of pigmentation of the olfactory epithelium in males at > 25 mg/kg
of diet and in females at > 125 mg/kg of diet, the effects seen at
125 and 500 mg/kg of diet being more severe than at lower doses.
Dicyclanil did not affect the tumour incidence in this study
(Bachmann, 1996b).
Weber (1998) studied the histological nature and causes of the
pigmentation of the olfactory epithelium, using material from male
rats in the 2-year study (controls and rats given 500 mg/kg of diet)
and the 3-month study (controls and rats given 500 mg/kg of diet;
Bachmann, 1993). Material from male control rats of the same strain in
five other long-term studies was included to provide more reference
material.
Minimal to moderate pigmentation was found in controls from the
long-term studies with dicyclanil and other compounds, but not in
controls from the 3-month study. Treatment with 500 mg/kg of diet
resulted in increased pigmentation, which was minimal after 3 months
and moderate to marked after 12 and 24 months, the degree of
pigmentation at the later times being similar. Staining indicated that
the pigment consisted mainly of oxidized lipofuscins and was located
in the olfactory epithelium and the underlying lamina propria. The
pigment appeared to be located in secondary lysosomes. Further
investigations by high-resolution microscopy indicated that the
supporting cells and secretory cells of Bowman glands were affected by
the pigmentation. Neuronal olfactory perikarya, nerve bundles of the
olfactory nerve in the olfactory mucosa, and the olfactory bulbs (in
the brain) were free of pigment accumulation. Apart from the
pigmentation, no other treatment-related morphological changes in the
olfactory mucosa were found. According to the author, the clinical
signs in the 2-year study gave no indication of a disturbed olfactory
sense due to dicyclanil treatment. In addition, the presence of
mucopolysaccharides in the Bowman glands indicated normal functioning
of the olfactory mucosa in the dicyclanil-treated rats. The author
concluded that the increased pigmentation of the olfactory epithelium
in male rats after treatment with dicyclanil results from accumulation
of lipofuscin in the cytoplasm of supporting cells and secretory cells
of the Bowman glands and is an enhancement of a natural age-related
process. In the absence of other morphological changes in the
olfactory mucosa, the author considered the pigmentation not to be
detrimental to the structure or function of the olfactory mucosa and,
therefore, is not to be regarded as adverse (Weber, 1998).
Recognizing that the effects on the olfactory epithelium were an
enhancement of a natural age-related process, the Committee noted that
dicyclanil had no effect on survival, behaviour, or general
well-being. As there were no other morphological changes in the
olfactory mucosa, the Committee concluded that the effect was of no
biological significance. Therefore, the NOEL in the 2-year study in
rats was 125 mg/kg of diet, equal to 22 mg/kg bw per day, on the basis
of changes in body weight and histopathological changes in the liver
and pancreas.
2.2.4 Genotoxicity
The results of studies for genotoxicity with technical-grade
dicyclanil are summarized in Table 1. The studies were of conventional
design, with GLP and quality assurance certification, except for the
study of Ogorek & Arni (1987).
2.2.5 Reproductive toxicity
(a) Multigeneration study
Rats
In a two-generation study of reproductive toxicity with two
litters per generation, groups of 30 male and 30 female Tif:RAIf (SPF)
rats received diets containing technical-grade dicyclanil at a
concentration of 0, 5, 30, 200, or 500 mg/kg of diet from 10 weeks
before the first mating until necropsy at the end of the lactation
period. Dams were allowed to litter and suckle their pups naturally.
When possible, the F1a litters were culled to four pups of each sex
per litter at day 4 post partum. After weaning, a number of F1a
pups were selected to be the F1 parents, and the remaining F1a
pups were necropsied. The F0 parents were then mated a second time,
and the resulting litters (F1b) were again culled to four pups of
each sex per litter. After weaning, both the F1b pups and the F0
parents were necropsied. The same procedure was followed for the F1
parents in order to produce F2a and F2b generations. The
observations included clinical signs, deaths, body weight, food
consumption, reproductive parameters, pup survival and development,
macroscopic appearance at necropsy, and, for parents in the control
and high-dose groups, histological appearance of the reproductive and
target organs. The study followed OECD test guideline 416, with GLP
and quality assurance certification.
Effects in F0 parents: The F0 parents showed no
treatment-related deaths or clinical signs and no effects on male or
female mating or fertility indices, on maternal gestation or
parturition indices, or on the duration of gestation. In the premating
period, males and females showed decreased body-weight gain and food
intake at 500 mg/kg of diet and, marginally, at 200 mg/kg of diet.
During both gestation periods, the overall body-weight gain of females
in all treated groups was similar to that of controls, while in both
lactation periods the overall body-weight gain of females was
increased at 500 mg/kg of diet and, marginally, at 200 mg/kg of diet.
Gross examination and histology revealed no treatment-related effects.
Secondary to the reduced body weights, the relative weights of most
organs were increased in males and females at 500 mg/kg of diet. At
this dose, the absolute weights of the heart and liver of males were
decreased.
Table 1. Results of assays for genotoxicity with dicyclanil
End-point Test object Concentration Result Reference
In vitro
Reverse S. typhimurium 20-5000 µg/plate Negativea Ogorek & Arni
mutation TA1537, TA98, (1987)
TA100
Reverse S. typhimurium 313-5000 µg/plate Negativeb Hertner (1992)
mutation TA1535, TA1537,
TA98, TA100,
E. coli WP2 uvrA
Gene mutation V79 Chinese 12.4-400 µg/ml Negative Geleick (1992)
hamster lung cells, - S9c
hprt locus 24.7-667 µg/ml
+ S9d,e
Chromosomal Chinese hamster 20.8-83.4 µg/ml Negative Hertner (1993a)
aberration ovary cells -S9c
166.75-667 µg/ml
+S9d,e
Unscheduled Primary rat 6.2-670 µg/mle Negative Hertner (1993c)
DNA synthesis hepatocytes
In vivo
Micronucleus Mouse bone 47-188 mg/kg bw; Negativef Hertner (1993b)
formation marrow once by oral gavage
a With and without rat liver S9 fraction; results of cytotoxicity test not given,
precipitation not reported
b With and without rat liver S9 fraction; precipitation at 5000 µg/plate
c Cytotoxic at the highest concentration
d Slightly cytotoxic at the highest concentration
e The highest concentration represents the limit of solubility in dimethyl sulfoxide.
f At all doses and all times, the ratio of polychromatic to normochromatic erythrocytes
did not deviate from that in controls; clinical signs of toxicity (including reduced
locomotor activity, unkempt fur, ataxia, piloerection, and diarrhoea) were observed
at the highest dose tested; doses > 312.5 mg/kg bw resulted in death.
Effects in F1 pups: At all doses and both matings, no
treatment-related effects were seen in the F1 offspring in terms of
sex ratio, clinical signs, litter size, the development of physical
landmarks (surface righting and eye opening), or macroscopic findings
at necropsy. A slight increase in the pup mortality rate was observed
on days 0-4 post partum at 500 mg/kg of diet (F1a) and at 30 and
200 mg/kg of diet (F1b). This was considered not to be
treatment-related because it could be attributed to two (F1a) or
three (F1b) litters and no dose-response relationship was found. At
500 mg/kg of diet, the weights of F1a pups were lower than those of
controls at birth and on days 14 and 21 post partum, owing to
reduced weight gain from day 4 post partum onwards. The weights of
F1b pups at the high dose were similar to those of controls from
birth through to day 14 post partum. Thereafter, the weight gain
decreased, resulting in lower pup weights on day 21 post partum.
Effects in F1 parents: In the F1 parents, no
treatment-related deaths or clinical signs and no effects on male or
female mating or fertility indices, on maternal gestation or
parturition indices, or on the duration of gestation were observed.
The body weights of the F1 parents at the high dose were lower than
those of controls throughout the study. Although the body weights of
males at 200 mg/kg of diet were reduced during the final part of the
study, the overall body-weight gain of these animals was not different
from that of controls. During both gestation periods, the overall
body-weight gain of all treated females was slightly lower than that
of controls, while in both lactation periods the overall body-weight
gain of females was increased at 500 mg/kg of diet and, marginally, at
200 mg/kg of diet. The absolute weights of most organs of males at the
high dose were decreased, with the exception of those of the testis
and brain. In females at the high dose, only the absolute weights of
the heart, liver, and kidney were decreased. At 200 mg/kg of diet, the
absolute weight of the liver was decreased in animals of each sex. In
contrast, secondary to the reduced body weights of these two groups,
the relative weights of most organs were increased, attaining
statistical significance for most organs at 500 mg/kg of diet and for
brain (both sexes), kidneys (males only), and testis at 200 mg/kg of
diet. Gross examination and histology revealed no treatment-related
effects.
Effects in F2 pups: At all doses and both matings, no
treatment-related effects were noted in the F2 offspring in terms of
sex ratio, mortality rate, clinical signs, litter size, the
development of physical landmarks (surface righting and eye opening),
or macroscopic findings at necropsy. The only treatment-related effect
observed was reduced weight gain in F2a and F2b pups at the
highest dose from day 4 post partum onwards, resulting in
significantly lower pup weights on days 14 and 21 post partum.
The NOEL for parental toxicity was 30 mg/kg of diet, equal to 2
mg/kg bw per day, on the basis of changes in body weight. The NOEL for
reproductive toxicity was 500 mg/kg of diet, equal to 24 mg/kg bw per
day, the highest dose tested. The NOEL for pup toxicity was 200 mg/kg
of diet, equal to 21 mg/kg bw per day, on the basis of reduced
body-weight gain (Khalil, 1995).
(b) Developmental toxicity
Rats
In a range-finding study, groups of eight pregnant Tif:RAIf (SPF)
rats received technical-grade dicyclanil (purity, > 98%) in 3%
aqueous corn starch by oral gavage at a dose of 0, 75, or 150 mg/kg bw
per day on days 6-15 of gestation. On day 21 of gestation, the dams
were killed and necropsied, and the fetuses were weighed, sexed, and
examined for external abnormalities.
Two dams at the high dose were killed in moribund condition. The
clinical signs in these animals and in a third animal at the high dose
included piloerection, salivation, dyspnoea, and hypotonia. Maternal
body-weight gain and gravid uterine weights were dose-dependently
decreased in all treated animals, but food consumption was
dose-dependently decreased only during the first week of treatment.
Post-implantation loss was dose-dependently increased by treatment,
owing to early resorptions. Three dams at the high dose resorbed their
entire litters. The number of viable fetuses and fetal body weights
were dose-dependently reduced. Three fetuses at the high dose showed
generalized oedema (FitzGerald, 1990a).
In the main study, groups of 24 pregnant Tif:RAIf (SPF) rats
received technical-grade dicyclanil in an aqueous solution of 0.5%
sodium carboxy-methylcellulose by oral gavage at a dose of 0, 1, 5,
25, or 75 mg/kg bw per day on days 6-15 of gestation. On gestation day
21, the dams were killed and necropsied, and the fetuses were weighed,
sexed, and examined for external, visceral, and skeletal
abnormalities. The study was of conventional design, with GLP and
quality assurance certification.
There were no deaths and no treatment-related clinical signs of
toxicity. Maternal body weight, body-weight gain, and food consumption
as well as net body-weight change and carcass weight at necropsy were
reduced at the highest dose. A marginal decrease in these parameters
was also observed at 25 mg/kg bw per day. One animal at 75 mg/kg bw
per day had total, early resorptions and showed haemorrhagic fluid in
the uterus at necropsy. In animals at 75 mg/kg bw per day, the gravid
uterine weight was reduced; the number of early postimplantation
losses was slightly increased and the number of fetuses per litter was
slightly lower, but without statistical significance. Effects on the
fetuses were observed only at the highest dose. These included reduced
fetal weight, a slight increase in the frequency of renal pelvic
dilatation, and a number of mainly sternebral defects and variations
due to poor or absent ossification. There was no evidence of
teratogenicity. The NOEL for maternal toxicity was 5 mg/kg bw per day,
on the basis of a reduction in body-weight gain. The NOEL for
developmental toxicity was 25 mg/kg bw per day, on the basis of
reduced fetal weight, increased renal pelvic dilatation, and increased
skeletal anomalies and variations consistent with a slight delay in
skeletal maturation (FitzGerald, 1993a).
Rabbits
In a range-finding study, groups of eight pregnant Russian
rabbits (strain unspecified) received technical-grade dicyclanil
(purity, > 98%) in 3% aqueous corn starch by oral gavage at a daily
dose of 0, 20, or 40 mg/kg bw on days 7-19 of gestation. On day 29 of
gestation, the dams were killed and necropsied, and the fetuses were
weighed, sexed, and examined for external abnormalities.
No treatment-related deaths or clinical signs were observed.
During treatment, the body-weight gain and food consumption of dams at
the high dose were considerably reduced. The gravid uterine weights
were not affected by treatment. The rate of pre-implantation loss was
slightly reduced and that of postimplantation loss slightly increased
at 40 mg/kg bw per day, which resulted in a comparable number of
fetuses to controls. The body weights of female fetuses were slightly
reduced at this dose. There were no remarkable external fetal changes
(FitzGerald, 1990b).
In the main study, groups of 19 pregnant Russian Chbb:HM rabbits
received technical-grade dicyclanil in an aqueous solution of 0.5%
sodium carboxy-methylcellulose by oral gavage at a daily dose of 0, 1,
3, 10, or 30 mg/kg bw on days 7-19 of gestation. On gestation day 29,
the dams were killed and necropsied, and the fetuses were weighed,
sexed, and examined for external, visceral, and skeletal
abnormalities. The study was of conventional design, with GLP and
quality assurance certification.
No deaths or treatment-related clinical signs of toxicity were
observed. Maternal body weight, body-weight gain, and food consumption
and the carcass weight at necropsy were reduced at 30 mg/kg bw per
day. Maternal body-weight gain was also reduced at 10 mg/kg bw per
day. Necropsy of the dams revealed no treatment-related effects, and
reproductive function was not affected. The body weights of fetuses at
the highest dose were reduced, and the fetuses showed skeletal
variations indicative of a slight delay in ossification. There was no
evidence of teratogenicity. The NOEL for maternal toxicity was 3 mg/kg
bw per day on the basis of reduced body-weight gain. The NOEL for
developmental toxicity was 10 mg/kg bw per day on the basis of reduced
fetal weight and increased skeletal variations consistent with delayed
ossification (FitzGerald, 1993b).
2.2.6 Special studies: Pharmacological effects
The effects of dicyclanil on the central nervous system
(centrally controlled behaviour, body temperature, locomotor activity,
hypnotic potentiation, motor coordination), the peripheral nervous
system, the autonomic nervous system and smooth muscles, the
cardiovascular and respiratory systems, and the gastrointestinal tract
were investigated in mice, rats, and guinea-pigs in vivo and
in vitro. The effect of dicyclanil on sperm morphology and motility
was also examined. All of the studies followed the Japanese Guidelines
for the Registration of Pharmaceuticals (General Pharmacology), with
GLP and quality assurance certification.
In vivo, technical-grade dicyclanil in polyethylene glycol
200:ethanol (5:3 v/v) was administered orally by gavage at a single
dose of 0, 1, 10, 50, or 100 mg/kg bw to male NMRI mice or male Han
Wistar rats. Doses < 100 mg/kg bw had no effect on the body
temperature of rats (Pfister & Gisin, 1996a), on the
hexobarbitone-induced sleeping time in mice (Pfister & Gisin, 1996b),
or on gastrointestinal motility in mice (Pfister & Gisin, 1996c).
In the modified Irwin test for general behavioural changes,
treatment of mice with dicyclanil at 100 mg/kg bw slightly inhibited
both exploratory activity and the startle response. The changes were
most evident 6 h after treatment, with complete recovery of the
startle response by 8 h and of exploratory activity by 24 h after
treatment. Mice treated with 1 or 10 mg/kg bw showed normal behaviour
(Pfister & Gisin, 1996d). After treatment with the vehicle, reduced
locomotor activity (static, mobile, and rearing activity and mobile
and active times) was seen in control mice over 24 h, being most
pronounced during the first 8 h. These parameters were less markedly
reduced after treatment with dicyclanil at 1 (not statistically
significant) or 10 mg/kg bw. Opposite effects were induced by
dicyclanil at 100 mg/kg bw, when the reduction in static activity was
more pronounced than in controls while changes in the other parameters
were comparable. All of the effects were fully reversed by 24 h after
dosing (Pfister & Hussherr, 1996a). Motor coordination in mice, as
assessed by recording the time the animals could retain their balance
on a rotating rod, was inhibited at 100 mg/kg bw but not at 1 or 10
mg/kg bw. The effect was significant 4 h after dosing but had
completely disappeared by 24 h (Pfister & Hussherr, 1996b).
Treatment of rats with 100 mg/kg bw (the only dose tested)
resulted in slight increases throughout the observation period (6-8 h
after dosing) in heart rate (statistically significant) and tidal and
minute lung volume (statistically significant only when compared with
values for vehicle-treated animals, which generally had lower values
than untreated animals). The blood pressure, electrocardiogram, and
respiratory rate remained unchanged (Pfister & Nordmann, 1996).
Treatment of rats with 50 mg/kg bw (the only dose tested) had no
effect on sperm motility, concentration, or morphology. Minor,
statistically nonsignificant increases in abnormal sperm morphology 6
weeks after dosing (sperm with head only and with abnormally shaped
hooks) were completely reversed by 12 weeks after dosing (Pfister &
Gisin, 1996e.
In vitro, a concentration of 0, 0.1, 0.3, 1, or 3 mmol/L of
technical-grade dicyclanil in dimethyl sulfoxide had no significant
effect on directly induced contractions of skeletal muscle or on
contractions induced indirectly by phrenic nerve stimulation in the
isolated phrenic nerve-diaphragm preparation from rats. The authors
concluded that dicylanil has no effect on the skeletal neuromuscular
junction (Pfister & Hussherr, 1996c). When dicyclanil was tested on
ileum isolated from guinea-pigs, dose-dependent antagonistic activity
against the smooth muscle contractions induced by histamine and
acetylcholine and against the nonspecific smooth muscle contractions
induced by barium chloride was observed at concentrations > 0.3, 1,
and > 0.3 mmol/L, respectively. All of the effects were fully and
rapidly reversible. Dicyclanil was considered to be only a weak
antagonist (Pfister & Gisin, 1996f).
3. COMMENTS
The Committee considered the results of studies on the
pharmacokinetics, metabolism, acute, short-term and long-term
toxicity, carcinogenicity, genotoxicity, reproductive toxicity, and
pharmacology of dicyclanil. All of the pivotal studies were carried
out according to appropriate standards for study protocol and conduct.
After repeated oral administration of radiolabelled dicyclanil to
rats, the radiolabel was rapidly and almost completely absorbed and
was rapidly distributed to the major organs and tissues. Elimination
was rapid (> 93% of the total dose within 24 h) and was virtually
complete within 3 days. The major route of elimination was the urine
(79-83% of the total dose within 24 h), while the faecal route was of
minor importance (6-12% of the total dose within 24 h).
Biotransformation in the rat involves oxidative opening of the
cyclopropyl ring at various positions, followed by further oxidation
and cleavage of the cyclopropyl-N bond (i.e. dealkylation). The
metabolic pathways of dicyclanil in sheep treated topically are
essentially the same as those in rats.
After oral administration of dicyclanil to rats, the LD50
values were 560 mg/kg bw in males and approximately 500 mg/kg bw in
females. Dicyclanil is moderately hazardous when given as a single
oral dose.
In a three-month study of toxicity, rats received dicyclanil in
the diet at a concentration of 0, 5, 25, 125, or 500 mg/kg of diet.
Male rats at 125 and 500 mg/kg of diet showed decreased food
consumption and body-weight gain and slightly decreased plasma glucose
concentrations. The weights of the kidneys, brain, and testis relative
to that of the body were increased in males at 500 mg/kg of diet, and
females at this dose showed decreased food consumption, body-weight
gain, and plasma glucose concentrations and increased relative weights
of the liver and brain; plasma glucose concentrations were also
reduced in females at 125 mg/kg of diet. The NOEL was 25 mg/kg of
diet, equal to 1.6 mg/kg bw per day, on the basis of the reduction in
body-weight gain.
In a 3-month study of toxicity, dogs received dicyclanil in the
diet at a concentration of 0, 20, 100, 500, or 1500 mg/kg of diet.
Clinical signs consisting mainly of neurotoxicity, decreased food
consumption and body-weight gain, and changes in clinical chemistry
and erythrocyte parameters were observed mainly at the highest dose.
Plasma cholesterol and phospholipid concentrations were increased in
animals at concentrations of 100 mg/kg of diet and above. The weights
of the spleen (males and females), thymus (males only), and testis
were decreased at 1500 mg/kg of diet. Atrophy of the spleen in males
and females and atrophy of the thymus, mesenteric lymph node, testis,
and prostate in males were observed at concentrations of 100 mg/kg of
diet and above. The weights of the liver, adrenals, and kidneys were
increased in animals at the highest dose; in females, the liver
weights were also increased at lower doses. Inflammatory changes were
seen in the urinary bladder of females at concentrations of 100 mg/kg
of diet and above and in the livers of males and females at 1500 mg/kg
of diet. Hepatocyte oedema was observed in females at all doses, but
hepatocellular damage was not seen at any dose in either sex. The
Committee noted that hepatocyte oedema without hepatocellular damage
is not of toxicological significance. Hence, the NOEL was 20 mg/kg of
diet, equal to 0.61 mg/kg bw per day, on the basis of increased plasma
cholesterol concentrations and histopathological findings in the
prostate and urinary bladder.
In a 1-year study, dogs received dicyclanil in the diet at a
concentration of 0, 5, 25, 150, or 750 mg/kg of diet. Males showed
slightly decreased plasma calcium concentrations and increased
absolute and relative liver weights at 750 mg/kg of diet. Plasma
cholesterol concentrations were increased in males at 150 and 750
mg/kg of diet, and this change was not reversed after a 4-week
recovery period. Females receiving the highest dose vomited and had
slightly reduced food consumption and body-weight gain, slightly
increased plasma cholesterol concentrations, increased absolute and
relative liver weights, and decreased absolute and relative heart
weights. Macroscopic and microscopic findings consisting of liver
necrosis, tubular lesions in the kidneys, testicular and prostatic
atrophy, and vascular thrombus, were found in one male and one female
at the highest dose that died before their scheduled sacrifice. These
animals suffered from acute, severe liver failure and resulting
cardiovascular disturbances and stress due to weight loss. Comparable
acute, severe liver toxicity was not observed in the 3-month study of
toxicity in dogs given feed containing dicyclanil at concentrations up
to 1500 mg/kg of diet. The findings in the two animals were therefore
considered to be incidental. The NOEL was 25 mg/kg of diet, equal to
0.71 mg/kg bw per day, on the basis of increased plasma cholesterol
concentrations in male dogs. The Committee noted that this NOEL is
supported by the NOEL in the 3-month study of toxicity in dogs. It
also noted that the histopathological findings observed in the 3-month
study were not seen in the 1-year study among animals that lived until
the time of scheduled sacrifice.
In a study of carcinogenicity, mice received diets containing
dicyclanil at a concentration of 0, 10, 100, 500, or 1500 mg/kg of
diet for 18 months. The animals at the highest dose were killed during
weeks 58-59 because of self-inflicted injuries and poor health. On the
basis of significant reductions in body-weight gain, the
concentrations of 500 and 1500 mg/kg of diet in females and 1500 mg/kg
of diet in males were considered to exceed the maximum tolerated dose.
The liver was the main target organ in both male and female mice. The
effects included Kupffer cell pigmentation (with haemosiderin) and
hepatocellular necrosis in males at doses of 100 mg/kg of diet and
higher, increased incidences of hepatocellular adenomas in females at
500 and 1500 mg/kg of diet, and hepatocellular carcinomas in females
at 1500 mg/kg of diet. The Committee noted that these liver tumours
were observed only at doses that exceeded the maximum tolerated dose
and that there were signs of hepatocellular proliferation in these
animals which might have been involved in the hepatic carcinogenesis
observed. Pigmentation of the olfactory epithelium (with oxidized
lipofuscins) was observed in animals of each sex at 100 and 500 mg/kg
of diet; in the males, this effect was accompanied by an increased
incidence of inflammatory cell infiltration in the underlying Bowman
glands. Males and females at 500 mg/kg of diet also showed
pigmentation of the adrenal glands (with partly oxidized lipofuscins)
and hypercellularity of the bone marrow. As the Committee considered
the effects on the olfactory epithelium to be of no biological
significance, the NOEL was 10 mg/kg of diet, equal to 1.1 mg/kg bw per
day, on the basis of the effects on the liver.
In a 2-year study of carcinogenicity and toxicity, rats received
diets containing dicyclanil at a concentration of 0, 5, 25, 125, or
500 mg/kg of diet. In animals at the highest dose, food consumption
and body-weight gain were decreased, and the relative weights of
almost all organs were increased as a result. Treatment-related
histopathological alterations were observed in the exocrine pancreas
(hyperplasia) in males at the highest dose, in the liver (biliary
cysts) in females at the highest dose, and in the olfactory epithelium
(pigmentation resulting from accumulation of oxidized lipofuscins) in
males at doses of 25 mg/kg of diet and above and in females at doses
of 125 mg/kg of diet and above. Although the latter represents
enhancement of a naturally occurring age-related process, treatment
had no effect on survival, behaviour, or general well-being, and there
were no other morphological changes in the olfactory mucosa.
Therefore, the Committee concluded that the effect on the olfactory
epithelium was of no biological significance. Dicyclanil did not
affect the incidence of tumours. The NOEL was 125 mg/kg of diet, equal
to 22 mg/kg bw per day, on the basis of changes in body weight and
histopathological changes in the liver and pancreas.
Dicyclanil has been tested in vitro for its ability to induce
reverse mutation in Salmonella typhimurium and Escherichia coli,
gene mutation in Chinese hamster lung cells, chromosomal aberrations
in Chinese hamster ovary cells, and unscheduled DNA synthesis in
primary rat hepatocytes. It has been tested in vivo for its ability
to induce micronuclei in bone-marrow cells of mice treated orally. The
results of all of these tests were negative. On the basis of these
data, the Committee concluded that dicyclanil is not genotoxic.
Dicyclanil increased the incidence of liver tumours in female
mice. However, as these tumours occurred in only one tissue of animals
of one sex and one species at doses that were above the maximum
tolerated dose, and dicyclanil is not genotoxic, the Committee
concluded that dicyclanil does not represent a carcinogenic risk for
humans.
In a two-generation study of reproductive toxicity, with two
litters per generation, rats were given dicyclanil in the diet at a
concentration of 0, 5, 30, 200, or 500 mg/kg of diet. Treatment
reduced the body-weight gain of the parental animals at the highest
dose and, marginally, at 200 mg/kg of diet. Secondary to this effect,
dicyclanil increased the relative weights of most organs in animals at
the highest dose and of the brain (males and females), kidneys (males
only), and testis at 200 mg/kg of diet. Reproductive parameters were
not affected. The only effect of dicyclanil on pups was to reduce
their weight gain from day 4 post partum onwards. The NOEL for
parental toxicity was 30 mg/kg of diet, equal to 2 mg/kg bw per day,
on the basis of changes in body weight. The NOEL for reproductive
toxicity was 500 mg/kg of diet, equal to 24 mg/kg bw per day, the
highest dose tested. The NOEL for toxicity to the pups was 200 mg/kg
of diet, equal to 21 mg/kg bw per day, on the basis of reduced
body-weight gain.
In a study of developmental toxicity in rats given dicyclanil at
a dose of 0, 1, 5, 25, or 75 mg/kg bw per day orally on days 6-15 of
gestation, the highest dose was toxic to the dams, as seen by
reductions in body-weight gain, food consumption, and the weight of
the gravid uterus. Marginal reductions in body-weight gain and food
consumption were also observed at 25 mg/kg bw per day. The effects on
the fetuses, observed only at the highest dose, were reduced fetal
weight, a slightly increased incidence of renal pelvic dilatation, and
a number of mainly sternebral defects and variations due to poor or
absent ossification. There was no evidence of teratogenicity. The NOEL
for maternal toxicity was 5 mg/kg bw per day on the basis of the
reduction in body-weight gain. The NOEL for developmental toxicity was
25 mg/kg bw per day on the basis of reduced fetal weight, increased
renal pelvic dilatation, increased skeletal anomalies, and variations
consistent with a slight delay in skeletal maturation.
In a study of developmental toxicity in rabbits given dicyclanil
at a dose of 0, 1, 3, 10, or 30 mg/kg bw per day orally on days 7-19
of gestation, dams at 30 mg/kg bw per day had reduced food consumption
and those at 10 and 30 mg/kg bw per day showed reduced body-weight
gain. The fetuses of dams at the highest dose had lower body weights
than controls and an increased incidence of skeletal variations
indicative of a slight delay in ossification. There was no evidence of
teratogenicity. The NOEL for maternal toxicity was 3 mg/kg bw per day
on the basis of reduced body-weight gain. The NOEL for developmental
toxicity was 10 mg/kg bw per day on the basis of reduced fetal weight
and skeletal variations consistent with delayed ossification.
In pharmacological tests in vitro, dicyclanil at doses up to
3 mmol/L had no effect on the skeletal neuromuscular junction. At
concentrations of 0.3 mmol/L and higher, it had slightly antagonistic
effects on smooth muscle contractions induced by agonists. In mice and
rats given a single oral dose of 0, 1, 10, 50, or 100 mg/kg bw, the
highest dose affected general behaviour, locomotor activity, motor
coordination, heart rate, and tidal and minute lung volume. Locomotor
activity was also affected at 10 mg/kg bw and, very slightly, at
1 mg/kg bw. The treatment had no effect on body temperature, hypnotic
potentiation, gastrointestinal motility, blood pressure, heart beat,
or respiratory rate.
4. EVALUATION
The Committee established an ADI of 0-7 mg/kg bw, on the basis of
the NOEL of 0.71 mg/kg bw per day for increased plasma cholesterol
concentrations in the 1-year study of toxicity in dogs and a safety
factor of 100. As is its usual practice, the Committee rounded the
value of the ADI to one significant figure.
5. REFERENCES
Altmann, B. (1991) CGA 183893 tech. 28-Day range finding toxicity
study in beagle dogs. Unpublished report (test no. 901202) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Altmann, B. (1993) CGA 183893 tech. 3-Month subchronic dietary
toxicity study in beagle dogs. Unpublished report (test no.
922813) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Altmann, B. (1995) CGA 183893 tech. 12-Month chronic dietary toxicity
study in beagle dogs. Unpublished report (test no. 922814) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Bachmann, M. (1991) CGA 183893 tech. 28-Days range finding study in
rats (administration in food). Unpublished report (test no.
901201) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Bachmann, M. (1993) CGA 183893 tech. 3-Month oral toxicity study in
rats (administration in food). Unpublished report (test no.
922810) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Bachmann, M. (1996a) CGA 183893 tech. 18-Month oncogenicity study in
mice. Unpublished report (test no. 922815) from Ciba-Geigy Ltd,
Stein, Switzerland. Submitted to WHO by Novartis Animal Health
Inc., Basel, Switzerland.
Bachmann, M. (1996b) CGA 183893 tech. 24-Month carcinogenicity and
chronic toxicity study in rats. Unpublished report (test no.
922816) from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Dubach-Powell, J.R. (1996) Pharmacokinetic study of CGA 183893 tech.
in the rat. Unpublished letter report (RCC project no.
615295/Ciba project no. 952803) from Research & Consulting
Company, Itingen, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
FitzGerald, R.E. (1990a) Preliminary oral toxicity study with CGA
183893 technical in pregnant rats (Dose range-finding study).
Unpublished report (test no. 901211) from Ciba-Geigy Ltd, Stein,
Switzerland. Submitted to WHO by Novartis Animal Health Inc.,
Basel, Switzerland.
FitzGerald, R.E. (1990b) Preliminary toxicity study in pregnant
rabbits with CGA 183893 technical (oral administration) (Dose
rangefinding study). Unpublished report (test no. 901212) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
FitzGerald, R.E. (1993a) CGA 183893 technical: Rat oral
teratogenicity. Unpublished report (study no. 922821) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
FitzGerald, R.E. (1993b) CGA 183893 technical: Rabbit oral
teratogenicity. Unpublished report (study no. 922822) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Geleick, D. (1992) CGA 183893 tech. Gene mutation test with Chinese
hamster cells V79 (OECD conform) in vitro. Unpublished report
(test no. 922827) from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Hagemann, C. (1992a) CGA 183893 tech. Acute dermal
irritation/corrosion study in the rabbit. Unpublished report
(test no. 922803) from Ciba-Geigy Ltd, Stein, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Hagemann, C. (1992b) CGA 183893 tech. Acute eye irritation/corrosion
study in the rabbit. Unpublished report (test no. 922802).
CibaGeigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Hagemann, C. (1993) CGA 183893 tech. Skin sensitization test in the
guinea pig optimisation test. Unpubished report (test no. 922805)
from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by
Novartis Animal Health Inc., Basel, Switzerland.
Hartmann, H.R. (1992a) CGA 183893 tech. Acute oral toxicity in the
rat. Unpublished report (test no. 922801) from Ciba-Geigy Ltd,
Stein, Switzerland. Submitted to WHO by Novartis Animal Health
Inc., Basel, Switzerland.
Hartmann, H.R. (1992b) CGA 183893 tech. Acute dermal toxicity in the
rat. Unpublished report (test no. 922804) from Ciba-Geigy Ltd,
Stein, Switzerland. Submitted to WHO by Novartis Animal Health
Inc., Basel, Switzerland.
Hartmann, H.R. (1993) CGA 183893 tech. Acute inhalation toxicity in
the rat. Unpublished report (test no. 922806) from Ciba-Geigy
Ltd, Stein, Switzerland. Submitted to WHO by Novartis Animal
Health Inc., Basel, Switzerland.
Hassler, S. (1994) Absorption, distribution and elimination of
[2-14C]pyrimidyl CGA 183893 in the rat after multiple oral
administration. Unpublished report (report no. 7/94; project no.
012AM01) from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to
WHO by Novartis Animal Health Inc., Basel, Switzerland.
Hertner, T. (1992) CGA 183893 tech. Salmonella and
Escherichia/liver-microsome test. Unpublished report (test no.
922825) from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Hertner, T. (1993a) CGA 183893 tech. Cytogenetic test on Chinese
hamster cells in vitro (EC conform). Unpublished report (test no.
922826) from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Hertner, T. (1993b) CGA 183893 tech. Autoradiographic DNA repair test
on rat hepatocytes (OECD conform) in vitro. Unpublished report
(test no. 922824) from Ciba-Geigy Ltd, Basel, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Hertner, T. (1993c) CGA 183893 tech. Micronucleus test, mouse (OECD
conform). Unpublished report (test no. 922823) from Ciba-Geigy
Ltd, Basel, Switzerland. Submitted to WHO by Novartis Animal
Health Inc., Basel, Switzerland.
Khalil, S. (1995) CGA 183893 technical: Rat dietary two-generation
reproduction study. Unpublished report (study no. 922818) from
Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Löffler, A. (1998) The nature of residues in tissues of sheep after
single topical pour-on administration of [pyrimidine-2-14C]CGA
183893. Unpublished report (study no. 012AM04) from Novartis Crop
Protection AG, Basel, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Marty, J.H. (1995) CGA 183893 tech. 28-Day repeated dose dermal
toxicity study in the rat. Unpublished report (test no. 922807)
from Ciba-Geigy Ltd, Stein, Switzerland. Submitted to WHO by
Novartis Animal Health Inc., Basel, Switzerland.
Ogorek, B. & Arni, P. (1987) Salmonella mutagenicity test with three
strains with CGA 183893 (Test for mutagenic properties in
bacteria). Unpublished report (experiment no. 871051) from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Pfister, T. & Gisin, M.(1996a) General pharmacology of CGA 183893
tech. Effect on the body temperature in the rat. Unpublished
report (project no. RCC 615317/CG 952803) from Research &
Consulting Company, Ltd, Itingen, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Pfister, T. & Gisin, M. (1996b) General pharmacology of CGA 183893
tech. Effect on the sleeping time induced by hexobarbitone in the
mouse. Unpublished report (project no. RCC 615330/CG 952803) from
Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Gisin, M. (1996c) General pharmacology of CGA 183893
tech. Effect on the intestinal motility (charcoal propulsion) in
the mouse. Unpublished report (project no. RCC 615385/CG 952803)
from Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Gisin, M. (1996d) General pharmacology of CGA 183893
tech. Modified Irwin screen test in the mouse. Unpublished report
(project no. RCC 615306/CG 952803) from Research & Consulting
Company, Ltd, Itingen, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Pfister, T. & Gisin, M. (1996e) General pharmacology of CGA 183893
tech. Effect on sperm motility, concentration and morphology in
the rat. Unpublished report (project no. RCC 615407/CG 952803)
from Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Gisin, M. (1996f) General pharmacology of CGA 183893
tech. Effect on the isolated ileum of the guinea pig. Unpublished
report (project no. RCC 615363/CG 952803) from Research &
Consulting Company, Ltd, Itingen, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Pfister, T. & Hussherr, D. (1996a) General pharmacology of CGA 183893
tech. Effect on the locomotor activity in the mouse. Unpublished
report (project no. RCC 615328/CG 952803) from Research &
Consulting Company, Ltd, Itingen, Switzerland. Submitted to WHO
by Novartis Animal Health Inc., Basel, Switzerland.
Pfister, T. & Hussherr, D. (1996b) General pharmacology of CGA 183893
tech. Effect on motor co-ordination (rotarod) in the mouse.
Unpublished report (project no. RCC 615341/CG 952803) from
Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Hussherr, D. (1996c) General pharmacology of CGA 183893
tech. Effect on the isolated phrenic nerve-diaphragm preparation
of the rat. Unpublished report (project no. RCC 615352/CG 952803)
from Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Pfister, T. & Nordmann, A. (1996) General pharmacology of CGA 183893
tech. Effect on the cardiovascular and respiratory systems in the
rat. Unpublished report (project no. RCC 615374/CG 952803) from
Research & Consulting Company, Ltd, Itingen, Switzerland.
Submitted to WHO by Novartis Animal Health Inc., Basel,
Switzerland.
Phillips, M. (1996) [Pyrimidine-2-14C]-CGA 183893. Nature of
metabolites in the excreta and tissues of sheep following single
pour-on administration. Unpublished report (No. 14072; project
no. 158665) from Inveresk Research, Tranent, Scotland. Submitted
to WHO by Novartis Animal Health Inc., Basel, Switzerland.
Thanei, P. (1996a) The metabolism of [2-14C]pyrimidyl CGA 183893 in
the rat. Unpublished report (No.7/96; project no. 012AM02) from
Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by Novartis
Animal Health Inc., Basel, Switzerland.
Thanei, P. (1996b) The nature of metabolites in tissues and excreta of
sheep after single topical administration of [2-14C]pyrimidyl
CGA 183893. Unpublished report (No. 9/96; project no. 012AM03)
from Ciba-Geigy Ltd, Basel, Switzerland. Submitted to WHO by
Novartis Animal Health Inc., Basel, Switzerland.
Weber, E. (1998) CGA 183893 tech. Pigmentation of the olfactory mucosa
observed after dietary administration to rats. Unpublished report
(report no. CB 98/05) from Novartis Crop Protection AG, Basel,
Switzerland. Submitted to WHO by Novartis Animal Health Inc.,
Basel, Switzerland.
