
WHO FOOD ADDITIVES SERIES: 51
First draft prepared by Mr Derek Renshaw
Food Standards Agency, London, England
Dr Carl Cerniglia
Division of Microbiology, National Center for Toxicological Research, Food and Drug Administration, Jefferson, Arkansas, USA
and
Professor Kunitoshi Mitsumori
Laboratory of Veterinary Pathology, School of Veterinary Medicine, Faculty of Agriculture, Tokyo University of Agriculture and Technology, Tokyo, Japan
The Committee considered neomycin at its forty-third, forty-seventh, fifty-second and fifty-eighth meetings (Annex 1, references 113, 125, 140 and 157). At its forty-third meeting, the Committee established a temporary ADI of 0–30 µg/kg bw on the basis of a NOEL of 6 mg/kg bw per day for ototoxicity in a 90-day study in guinea-pigs and a safety factor of 200. The ADI was made temporary in view of deficiencies in the genotoxicity data. Studies of mutagenicity were requested for evaluation in 1996.
At its forty-seventh meeting (Annex 1, reference 125), the Committee considered new data on genotoxicity for neomycin. On concluding that neomycin was not genotoxic, it established an ADI of 0–60 µg/kg bw on the basis of the NOEL of 6 mg/kg bw per day for ototoxicity in the 90-day study in guinea-pigs and a safety factor of 100.
Following a request by the Codex Committee on Residues of Veterinary Drugs in Foods at its Twelfth Session (Codex Alimentarius Commission, 2000), the Committee at its fifty-eighth meeting considered information on registration of injectable neomycin products and on their use with respect to good practice in the use of veterinary drugs. The Committee also considered data on the toxicity of neomycin in calves, but it concluded that the information was relevant only to the welfare of the target animals and therefore fell outside its mandate.
The Codex Committee on Residues of Veterinary Drugs in Foods at its Thirteenth Session (Codex Alimentarius Commission, 2001) requested the Committee to evaluate new data on the safety of neomycin. Two submissions were made, one addressing the microbiological aspects of the safety of neomycin to consumers and the other addressing the evidence for a link between the presence of a specific mutation to mitochondrial DNA in humans and increased susceptibility to amino-glycoside-induced ototoxicity.
The ADIs that could be derived from studies in bacteria in vitro, from studies of human flora-associated animals in vivo and from studies in humans were evaluated.
Neomycin is used to treat superficial infections in humans and is given orally to cattle, sheep, pigs, goats and poultry for bacterial gastrointestinal infections and by intramammary administration to treat mastitis. It is an aminoglycoside and is active against bacteria that grow aerobically. It is considered to be inactive against anaerobes, and its activity against facultative bacteria in vitro is lower in anaerobic environments than in air (Annex 1, reference 113).
Neomycin is produced by Streptomyces fradiae. Preparations are complexes consisting of neomycin A, neomycin B and neomycin C, generally containing more than 90% neomycin B, the remainder being mainly neomycin C. Neomycins B and C both contain three amino sugars attached by glycosidic linkage to the central hexose. Neomycin A, more appropriately referred to as neamine, is a hydrolysis product of either neomycin B or neomycin C and usually comprises less than 1% of the mixture. Neomycin A has a bicyclic ring system with four amino groups. Neomycin B has a total of six amino groups and consists of neamine and neobiosamine B, a disaccharide of D-ribose and neosamine B. Neomycin C is a stereoisomer and consists of neamine and neobiosamine C, a disaccharide from D-ribose and neosamine C (stereoisomer of neosamine B). When tested by antibiotic dilution techniques against aerobic and facultative bacteria, the activity of neomycin B is generally greater than that of neomycin C, which is greater than that of neamine.
The bacteriocidal effects of aminoglycosides are brought about by disruption of cellular transport mechanisms as a result of the formation of abnormal cell membrane channels by abnormal proteins (Prescott et al., 2000). Neomycin reacts with 30S ribosomal subunits of prokaryotic cells by electrostatic attraction. This blocks the formation of a complex of mRNA with formethionine and tRNA and induces misreading of the genetic code on the mRNA template (Brown, 1988). The result is a change in the conformation of the ribosomal binding protein, with concomitant errors in reading the code of the mRNA (Mingeot-Leclercq et al. 1999), and a non-functional protein is produced (Brown, 1988).
As it is a polycation, neomycin binds to negatively charged bacterial surface anions such as the lipopolysaccharide of gram-negative bacteria, teichoic acids of gram-positive bacteria and polar portions of phospholipid in both types of bacteria. Active transport is required for neomycin to traverse the membrane so that it can reach the ribosomal target site. Such transport mechanisms are lacking in a number of the anaerobes studied to date, and for this reason they are generally not sensitive to the aminoglycosides. Aminoglycosides are moved across the cytoplasmic membrane by the membrane potential after non-specific association with a transporter in the cytoplasmic membranes. Ribosomes and nucleic acids act as binding sinks for transported aminoglycosides and contribute significantly to total cell uptake. In the presence of sufficient concentrations of neomycin, transport may cause some release of cell components, including potassium, amino acids and nucleotides, from exposed bacterial cells by damaging the cell wall.
Aminoglycosides have additional effects on microorganisms, including inter-ference with the cellular electron transfer system, induction of RNA breakdown, disruption of polysomes into inactive monosomes, inhibition of translation, blocking of initiation of DNA replication, effects on DNA metabolism and damage to cell membranes (Brown, 1988; Prescott et al., 2000). At least some of these effects are likely to be due to the mistranslation of mRNA.
As noted above, the transport of aminoglycosides into facultative anaerobes is an energy-dependent system requiring electron transport (Bryan & Kwan, 1981). Under anaerobic conditions, the membrane potential of facultative bacteria is diminished, and transport of aminoglycosides is markedly impaired. Facultative bacteria that are sensitive to neomycin under aerobic conditions are much less susceptible under anaerobic conditions owing to the lack of an effective proton motive force for transport in these organisms.
Although aminoglycosides bind in vitro to both the cell surfaces and the ribosomes of Bacteroides and Clostridia, these and other obligate anaerobes are naturally insensitive to aminoglycosides. Their insensitivity is not related to the production of inactivating enzymes nor to mutations that reduce ribosomal affinity. In fact, protein synthesis by Bacteroides fragilis and Clostridium perfringens in vitro is inhibited by aminoglycosides, to which they are otherwise insensitive. Rather, the insensitivity of anaerobes appears to be due to insufficient transmembrane driving force or the amount of cell membrane transporter (Bryan et al., 1979; Bryan & Kwan, 1981; Rasmussen & Tally, 1993). Thus, the insensitivity results from an inability of the cells to accumulate sufficient intracellular concentrations of neomycin for it to be inhibitory.
There are three categories of resistance to aminoglycosides: that due to spontaneous mutation (rare, occurring with a probability of 10–8 to 10–10 in a cell population (Moellering 1983)); that due to altered cell permeability to the aminoglycoside; and that due to inheritance of plasmid-encoded resistance factors which specify inactivating enzymes. The latter is more commonly reported for clinically relevant species. Most of the acquired resistance to aminoglycosides in aerobic bacteria is due to the acquisition of inactivating enzymes which modify the drug bound to the transporter, preventing ribosomal binding. The plasmid resistance factors (R factors) against neomycin encode phosphotransferases, acetyltransferases and nucleotidyltransferases. These enzymes are in certain cases active against more than one aminoglycoside but do not necessarily cause complete inactivation. Thus, bacteria that inherit plasmids which encode these inactivating enzymes may become resistant or less sensitive to other aminoglycosides (Lechevalier, 1975; Davies, 1986; Moellering, 1983).
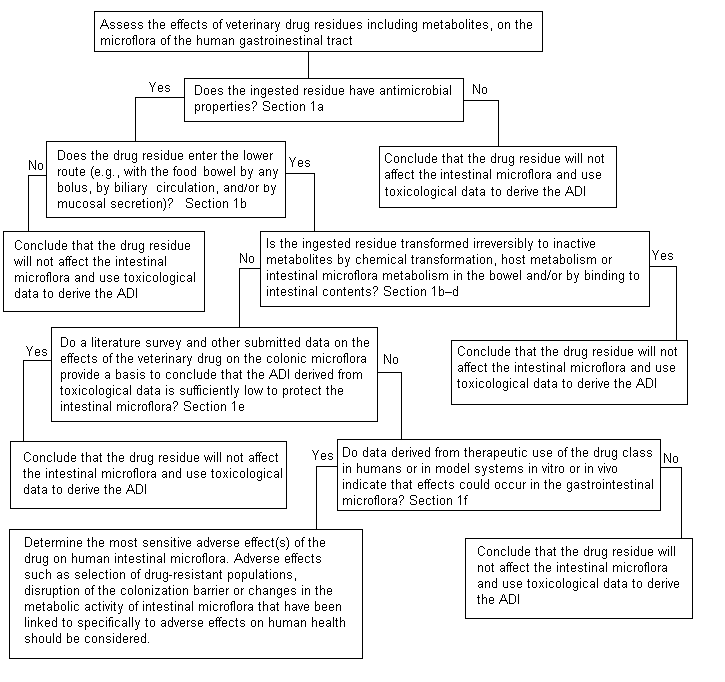
At its fifty-second meeting (Annex 1, reference 141), the Committee developed a decision tree to address the potential adverse impacts of antimicrobial residues on human intestinal microflora (Figure 1). At its present meeting, the Committee used this decision tree to answer the following questions in its assessment of neomycin.

Figure 1. Decision tree for determining adverse microbiological
effects of residues of antimicrobial drug in food-producing animals
1. ‘Does the ingested residue have antimicrobial properties?’
Yes, but the spectrum of activity against the major groups of intestinal bacteria is limited, as shown in the table of minimum inhibitory concentrations (MICs) for relevant intestinal bacteria tested under standard conditions specified by the National Committee of Clinical Laboratory Standards (USA; 1993) (see Table 1). Gastro-intestinal anaerobes are insensitive to neomycin. In a survey of gastrointestinal bacteria tested at standard and high inoculum densities, the lowest MIC50 values were recorded for the most sensitive relevant genera, Eubacterium (8 µg/ml) and Lactobacillus (64 µg/ml), respectively. The potency of neomycin against Escherichia coli in vitro decreased as the inoculum density increased or the oxygen availability decreased. The MIC50 for E. coli at a high inoculum density was 64 µg/ml under aerobic test conditions and 128 µg/ml under anaerobic conditions; the latter value was higher than those of other intestinal organisms. The MIC50 of 64 µg/ml was used by the Committee in its calculations, as Lactobacillus was the most sensitive relevant strain at high inoculum density.
Table 1. Minimum inhibitory concentrations (MICs) of neomycin against major bacterial groups tested under the conditions of the National Committee of Clinical Laboratory Standards of the USAa
|
Bacterial species and genus |
Strains |
MIC50 (µg/ml)b |
|||
|
Lowc |
Highd |
||||
|
Supplemented blood medium |
Wilkins–Chalgren-glucose medium |
Supplemented blood medium |
Wilkins–Chalgren-glucose medium |
||
|
Bacteroides |
15e,f |
> 128 |
> 128 |
> 128 |
> 128 |
|
Bifidobacterium |
12e |
16 |
128 |
||
|
Clostridium |
11e,5f |
> 128 |
128 |
> 128 |
> 128 |
|
Enterococcus |
10e,2f |
> 128 |
128 |
> 128 |
> 128 |
|
Escherichia coli (aerobic)g |
13e |
16 |
64 |
||
|
Escherichia coli (anaerobic)g |
13e |
> 128 |
> 128 |
||
|
Eubacterium |
9e |
8 |
> 128 |
||
|
Fusobacterium |
5e,3f |
16 |
32 |
> 128 |
128 |
|
Lactobacillus |
15e,2f |
>128 |
32 |
> 128 |
64 |
|
Peptostreptoccoccus/Peptococcus |
16e,14f |
>128 |
32 |
> 128 |
128 |
|
a |
Corrected version of the original tables that appeared in reference 141 in Annex 1 |
|
b |
MIC50 values are for all strains included in the assay, tested by the standard guideline of the National Committee of Clinical Laboratory Standards for determining MICs of anaerobes by the agar dilution technique. |
|
c |
The cell concentration of low-density inocula was 1 × 108 cells/ml. The amount of inoculum per spot on agar medium was approximately 2 × 105 cells, assuming 0.002 ml of culture per spot. |
|
d |
The cell concentration of high-density inocula was 1 × 1010 cells/ml. The amount of inoculum per spot on agar medium was approximately 2 × 107 cells, assuming 0.002 ml of culture per spot. |
|
e |
Number of tested strains of which the MIC values for Wilkins–Chalgren-glucose medium were used to calculate summary values. |
|
f |
Number of tested strains of which the MIC values for supplemented blood medium were used to calculate summary values. |
|
g |
The same set of 13 strains was tested aerobically and anaerobically with the same inocula and lots of media. The inocula used to determine the aerobic MIC were incubated at 37 °C aerobically. |
2. ‘Does the drug residue enter the lower bowel by any route?’
Yes. Most of an oral dose is excreted in faeces, without metabolism. Neomycin is poorly absorbed from the intestinal tract of humans and animals and from cows’ udders. The amount of orally administered neomycin recovered in urine was less than 10%. In healthy humans given a single oral dose of neomycin sulfate at > 1000 mg per person, the proportion of neomycin absorbed from the gastrointestinal tract was < 10% on the basis of blood and urine analysis (Poth et al., 1950; Kunin et al., 1960). Very young calves were found to absorb slightly more (Aschbacher & Feil, 1994). Neither neomycin B nor neomycin C was preferentially absorbed in dogs given either molecule orally (Freyburger & Johnson, 1956).
It is generally accepted that most of the neomycin that is ingested is excreted. As neomycin is a polycation, much of the ingested material is apparently adsorbed to faecal contents. Therefore, a conservative microbiological safety assessment would assume that 100% neomycin is excreted chemically unaltered in faeces.
3. ‘Is the ingested residue transformed irreversibly to inactive metabolites by chemical transformation, metabolism mediated by the host or intestinal microflora in the bowel or binding to intestinal contents?’
Neomycin undergoes negligible biotransformation after parenteral administration and binds avidly to intestinal and faecal contents. It is not clear what percentage of an oral dose is inactivated or whether the inactivation is dose-dependent. Although studies have been conducted of binding and inactivation of neomycin in faecal suspensions in vitro, the solids and soluble components of faecal specimens are complex and heterogeneous, varying substantially among individuals. Thus, these studies cannot be considered exhaustive for defining the kinetics or the physico-chemical mechanisms of binding or inactivation of neomycin. Nevertheless, they provide useful information for assessing the effects and safety of residues of neomycin in milk and tissues.
In an early study of binding of aminoglycosides to dog faeces, neomycin was incubated in a dilute suspension of faeces, and the amount of neomycin bound to faecal solids was calculated as the difference between the amount added to the suspension and the amount measured (by microbiological assay) in the supernatant after the mixture had been incubated and centrifuged. Initially, 75% of the neomycin was calculated to have been pelleted with faecal solids in the incubation mixture. After two washings of the pelleted solids and acid extraction (method unspecified), 47% of the added neomycin was calculated to be retained with the faecal solids. The same experiment was conducted with other aminoglycosides, including gentamycin, kanamycin and paromomycin, with similar results (Wagman et al., 1974).
Neomycin was incubated at various concnetrations with similarly dilute suspensions of faecal specimens obtained from nine healthy volunteers. As in the previous study, ‘binding’ of neomycin to faeces was calculated from the amount of added activity that remained in the supernatant solution after removal of faecal solids by centrifugation of the incubation mixture. The percentage of concentrations of neomycin up to 500 µg/ml that was bound was calculated to be 83–98%. The amount bound depended on the amount of neomycin added per gram of diluted faeces. The authors reported that no destruction or inactivation of neomycin occurred during incubation in the supernatant solutions, but data were not provided (Hazenberg et al., 1984). The same test system was used to compare the binding of various aminoglycosides and antibiotics used in selective decontamination of the bowel. Neomycin and polymyxin B showed substantially higher percentages of binding per unit weight of antibiotic than did tobramycin, gentamycin and cephradine (Hazenberg et al., 1983a).
Neomycin at various concentrations was incubated with suspensions of individual faecal specimens collected from eight healthy volunteers. The suspensions contained higher concentrations of faecal solids than in the studies described above, and two incubation times (0 and 24 h) were studied in duplicate. As in the other studies, the microbiological activity remaining in the supernatants was used to calculate the ‘per cent inactivation’ relative to the amount of added neomycin. The microbiological activity in the supernatants of pelleted faecal solids depended on the concentration of added neomycin, and the percentage of added neomycin that was ‘inactivated’ decreased with increasing concentrations of neomycin. Nearly 100% binding was seen in incubation mixtures in which the ratio of neomycin to faecal wet weight was < 5 mg to 1 g. This ratio was consistent with the observations of Hazenberg et al. (1984) and Wagman et al. (1974). The authors concluded that the ‘inactivation’ occurred during the time it took to mix the suspension and harvest the supernatant solution (roughly 50 min), because no difference in the per cent inactivation was seen at 0 and 24 h (Veringa & van der Waaij, 1984).
In all four studies summarized above, a microbiological assay was used to assess the concentration of neomycin. The recovery of neomycin from the test system was not reported. The only amounts of neomycin measured were the concentrations remaining in supernatant solutions after centrifugation of faecal solids. Thus, estimates of ‘per cent binding’ or ‘per cent inactivation’ reflect microbiological activity expressed as a percentage of the total neomycin activity added to the test system. The net decrease in the detectable microbiological activity of soluble neomycin could have been due to a number of competing reactions, including: inactivation by ionic binding to insoluble components of the slurry (including bacterial cells); inactivation by enzymatic modification (phosphorylation, acetylation); artefactual physical trapping of neomycin by faecal solids during centrifugation; active transport and uptake of neomycin by sensitive bacterial cells; and inactivation by binding to soluble components of the slurry. Whatever the cause, the detectable microbiological activity was reduced substantially within a short time in these dilute faecal incubations. This decrease in soluble microbiological activity, by whatever mechanism, was saturable. Thus, the faecal slurries had a finite capacity to inactivate neomycin. Overall, the results indicate that neomycin is partially or fully inactivated at the levels found as residues. The data are, however, difficult to verify, as discussed above, and the next question in the decision tree should be addressed.
4. ‘Do data on the effects of the drug on the colonic microflora provide a basis to conclude that the ADI derived from toxicological data is sufficiently low to protect the intestinal microflora?’
Yes. Orally administered neomycin is used as adjunct therapy (at a dose of 4–8 g/day) for hepatic coma and for ‘preparation’ or ‘selective decontamination’ of facultative anaerobes in the bowel for surgery (9–21 g with purgatives over 3 days). A reversible intestinal malabsorption syndrome with loss of digestive enzymes and flattening of intestinal villi has been described after oral dosing with neomycin at > 3 g/day for more than several days (Jacobson et al., 1960; Faloon et al., 1962). Malabsorption of fats, protein, cholesterol, carotenes, mono- and disaccharides, vitamin B12, sodium, calcium and iron have been reported, due in part to direct inhibition of absorption after interaction of neomycin with the nutrient. Neomycin is thought to disrupt micelle formation of bile acids and neutral sterols, thus leading to their increased excretion in faeces (Sirtori et al., 1991). In vitro, addition of neomycin to human bile and bile salt solutions resulted in precipitates of bile salts and bilirubin, indicating that there may be a relationship between binding in vitro and the malabsorption of fat observed when neomycin is given orally (Faloon et al., 1962). Prepared solutions of sodium glycocholate and sodium taurocholate are precipitated by the addition of neomycin, but the precipitation is reversed as the pH is raised, with maximum precipitation at pH 6.0. The extent to which this occurs after ingestion of the concentrations at which residues occur has not been determined. The doses at which malabsorption is observed are more than 1000 times higher than the toxicological ADI (0–60 µg/kg bw), and the calculated microbiological ADI is based on microbiological data.
In a study of the effects of antibiotics on human gut flora, changes in facultative bacteria, yeast and Clostridia were monitored in patients in hospital for non-gastrointestinal diseases. The bacterial populations in faecal specimens collected before, during and after treatment with neomycin were enumerated. Four patients received neomycin orally at 4 g/day for 7 days, and 10 patients received 2 g/day for 6 days. The populations of all the bacterial groups that were monitored decreased during treatment. The group most severely affected was enterococci, followed by E. coli non-lactose fermenters, lactobacilli, Clostridia and Proteus species. The numbers of yeasts and Klebsiella-like organisms increased, as did those of antibiotic-resistant populations (Daikos et al., 1968). In a study in which facultative bacteria and yeasts in faecal specimens from patients given neomycin orally were cultured, 2 g/day of neomycin reduced the numbers of facultative bacteria (Poth et al., 1950, 1951). In patients treated with neomycin orally at a dose of 3 or 5–6 g/day, although the total bacterial counts remained high (1010–1011 cells/g faeces), the number of enterococci fell by two to three orders of magnitude, and the decreases in the numbers of coliforms and Clostridia were even greater. The populations of bifidobacteria and Bacteroides were more constant (Finegold et al., 1965, 1983).
Neomycin was administered to healthy volunteers at a dose of 1 g three times daily for 5 days, and fresh stool samples were collected before, during and after treatment and monitored for facultative bacteria and strict anaerobes. The mean E. coli counts were not affected by treatment, but two of the eight volunteers showed substantial differences in counts. It was concluded that this regimen had little effect on the counts of aerobic bacteria in the colon (Arabi et al., 1979).
In all the studies summarized above, oral doses > 2000 mg were given per adult. The responses varied, but the effects on facultative bacteria reflected a general trend. The concentration of neomycin residues remaining after veterinary useafter, 3.6 mg, is > 500 times lower than the doses used in these studies. As the numbers of anaerobic bacteria are not detectably affected at therapeutic doses, there is little reason to expect that the populations in the lower bowel would be affected by daily ingestion of 3.6 mg.
The absence of any change in the population of anaerobic bacteria in humans given neomycin at doses > 2000 µg is consistent with recent findings in HFA mice given neomycin, in which no changes in the populations of subpopulations of E. coli, ‘neomycin-resistant E. coli’, enterococci, ‘neomycin-resistant enterococci’ or lactobacilli were found at the highest concentration of neomycin added to drinking-water (20 µg/ml) for 6 weeks (Kotarski, 2002). Assuming that a 40-g mouse ingests 5 ml of neomycin-containing water per day, the amount of neomycin ingested would be (20 µg/ml × 5 ml/day per 40-g mouse), or 150 mg/60 kg bw. Thus, the toxicological ADI of 0–3.6 mg/60-kg person (equal to 0–60 µg/kg bw) is sufficient to protect against changes in bacterial composition, including emergence of resistance.
The toxicological ADI of 0–60 µg/kg bw is further justified by the finding of Hazenberg et al. (1983b) that no changes in bacterial subgroups were induced in HFA mice given neomycin at 1000 µg/ml in water for 35 days, but changes were induced with 2000 µg/ml. The NOEL for changes in bacterial subpopulations in this study was thus 7500 mg/60-kg person. Although the study was not designed to determine a microbiological ADI, it nevertheless provides information suggesting that the NOEL for changes in a bacterial population is much higher than the current toxicological ADI. The available data (Table 2) thus support the toxicological ADI of 0–60 µg/kg bw (equal to 0–3.6 mg/60-kg person), which would protect the human gastrointestinal flora. The current toxicological ADI for neomycin should therefore not be changed, consistent with the positive answer to question 4 and recommen-dation 1(e) of the guidance for the decision tree (Annex 1, reference 147).
Table 2. ADIs that could be derived from various studies on neomycin
|
Type of study |
No-effect observations |
ADI (mg/60-kg equivalent bw per day)a |
Reference |
|
MIC test for > 100 strains of intestinal bacteria (high inoculum density) |
MIC50 of most sensitive relevant genus, Lacto-bacillus, under conditions of high inoculum density = 64 µg/ml. Inactivation in colon due to binding not taken into account, as minimum bactericidal concentrations of sensitive aerobic facultative bacteria increased 30– 640-fold when faeces were added to test broth. |
ADI = (64 µg/ml × 200 g) / (1.0 × 1.0 × 60-kg person) |
Van Saene et al. (1985); |
|
HFA mice dosed orally in drinking-water |
No changes in E. coli, gram-negative anaerobes, total number of resistant anaerobes at 125 mg/kg bw per day |
ADI = 125 mg/kg equivalent bw × 60 kg 7500 |
Hazenburg et al. (1981) |
|
Humans |
No changes in numbers of Bacteroides, Clostridium, neomycin-resistant E. coli at oral dose of 3 g/day for 5 days |
3000 |
Finegold et al. (1965) |
|
Adapted from Cerniglia & Kotarski (1999) |
|
|
a |
ADIs are deriuved only from comparisons of MICs with data derived in vivo, to indicate the magnitude of the differences. Additional, relevant, unpublished information that may be available from registration files should be taken into account in making final decisions and establishing an appropriate ADI. |
High systemic doses of aminoglycosides can be ototoxic and nephrotoxic in humans and other mammals. In some countries, neomycin is not allowed for parenteral use as a veterinary drug because of concern about toxicity to target animals. In those countries that permit this use, it is generally restricted for treatment of serious gram-negative infections that are resistant to less toxic medications.
Deafness occurred in one of two calves treated with neomycin at an intramuscular dose of 2.2 mg/kg bw twice daily for 13 days and in one of two calves given 4.5 mg/kg bw intramuscularly twice daily for 12 days. Nephrotoxicity was seen in all four neomycin-treated calves. No adverse effects were seen in two control calves given benzylpenicillin at an intramuscular dose of 3600 U/kg bw twice daily for 7 days (Crowell et al., 1981).
Neomycin is considered to be the most toxic of the authorized aminoglycoside drugs (Riviere et al., 1991). Assessment of the damage to cultures of hair cells from the outer cochlea of neonate mice showed neomycin to be the most potent of several aminoglycoside and aminocyclitol drugs investigated. The order of potency was neomycin > gentamycin > dihydrostreptomycin > amikacin > neamine > spectinomycin (Kotecha & Richardson, 1994).
Aminoglycoside-induced ototoxicity may affect one or both ears (Lerner et al., 1998). Ototoxicity is more likely to occur after parenteral use than after oral administration. A review of studies of ototoxicity in humans (Govaerts et al., 1990) showed wide variation in estimates of the incidence of ototoxicity in patients treated with aminoglycosides, ranging from 0% to 63% for cochlear toxicity and 0% to 72% for vestibular toxicity. In a prospective study of 32 patients given long-term treatment with neomycin (neomycin sulfate at 500 mg per person every 6 h for 3 months), 6.7% of the patients developed cochlear toxicity, as determined by high-frequency audiometry (Rappaport et al., 1986). The risk of ototoxicity after orally administered neomycin is increased for patients with impaired renal function or gastrointestinal inflammation (Kavanagh & McCabe, 1983). Loss of hearing in the high frequency region was experienced by 9 of 17 children (53%) aged 2–7 years with gastroenteritis who had been given neomycin orally at doses of 50–100 mg/kg bw per day for 6–9 days (Zelenka et al., 1966).
High concentrations of aminoglycosides in plasma can result in transfer to the perilymph and endolymph of the inner ear. As diffusion back into the bloodstream is slow, the drug can accumulate in the perilymph and endolymph. The accumulation is dose-dependent but saturable, and the aminoglycoside can stay in the perilymph for prolonged periods. The half-life of aminoglycosides in perilymph is 10–15 times longer than that in serum (Lortholary et al., 1995; Chambers & Sande, 1996)
Aminoglycosides can damage the vestibular and cochlear sensory hair cells of the vestibular epithelium and the organ of Corti, which can result in impairment of auricular function, including loss of balance and irreversible deafness. The sensory cells do not regenerate, and retrograde degeneration of the auditory nerve (eighth cranial nerve) follows. The nerve cells become affected only when the hair cells are missing. Aminoglycoside-induced cochlear toxicity is one of the commonest causes of acquired deafness in humans but is less common in neonates and children than in adults (Matz, 1993; Chambers & Sande, 1996; Lerner et al., 1998).
Although all aminoglycosides can affect both cochlear and vestibular function, some preferential toxicity is evident. In the case of neomycin, it is principally auditory function that is affected. Tinnitus is often the first symptom of effects on the cochlea, and there may be auditory impairment if exposure to the drug is not discontinued within a few days. Perception of high-frequency sounds is lost first; if exposure to the drug continues, perception of lower frequencies is lost progressively (Langman, 1993; Chambers & Sande, 1996)
Although auditory (cochlear) toxicity is more common, neomycin can also cause vestibular toxicity. The symptoms of severe vestibular toxicity include nausea, vomiting, vertigo, nystagmus and difficulty with gait (Lerner et al., 1998).
The Committee assessed data on the ototoxicity of neomycin at its forty-third meeting (Annex 1, reference 114). Several studies of the effects of neomycin on guinea-pigs (Riskaer et al., 1956; Brummett et al., 1985) and on cats (Hawkins, 1952; Hawkins et al., 1953) were considered. In addition human case studies were taken into account (Waisbren & Spink, 1950; Lindsay et al., 1960; Halpern & Heller, 1961; King, 1962; Fields, 1964; Greenberg & Momary, 1965). The Committee could not identify a NOEL from the studies in cats but identified a NOEL of 6 mg/kg bw per day for ototoxicity in guinea-pigs given neomycin orally. The Committee at its forty-seventh meeting established an ADI of 0–60 µg/kg bw by applying a safety factor of 100 to the NOEL of 6 mg/kg bw per day from the study on guinea-pigs (Annex 1, reference 126).
Two groups of patients with aminoglycoside-induced ototoxicity have been identified: one in which the effect was the result of prolonged or high-dose exposure to the drug and a second that in which signs appeared after minimal exposure, suggesting an idiosyncratic susceptibility (Bacino et al., 1995). Clusters of susceptible individuals exist in certain families, suggesting that genetic factors might play a role. Families with raised susceptibility to the drug were reported in the literature as early as 1957 (Horiguchi & Moriyama, 1957). After a maternal inheritance pattern of aminoglycoside-associated deafness was identified in several Japanese (Higashi, 1989) and Chinese (Hu et al., 1991) families and in a large Arab–Israeli family (Jaber et al., 1992), the hypothesis of mitochondrial inheritance of the susceptibility was raised. The observations that every known mitochondrial genetic disease has sensorineural hearing loss or deafness as a common phenotype and that aminoglycoside-poisoned hair cells show mitochondrial dysmorphology are consistent with this hypothesis (Hutchin et al., 1993).
As the bacteriocidal activity of aminoglycosides was known to involve interaction with ribosomal RNA in bacteria and as mitochondrial ribosomes are structurally more similar to bacterial ribosomes than to mammalian cytosolic ribosomes, it was suspected that susceptibility to aminoglycoside-induced ototoxicity might be due to a mutation of mitochondrial DNA at a site corresponding to the production of ribosomal RNA. This mutation was sought in a large Arab–Israeli family in which 55 members had maternally inherited sensorineural deafness, which could be traced back through five generations to one common female ancestor (Prezant et al., 1993). The mutation was thought to be homoplasmic, as family members had either severe hearing loss or normal hearing, with no intermediate impairment. Formal segregation analysis predicted that the simultaneous inheritance of a mutation to mitochondrial DNA and an autosomal recessive mutation caused the disease phenotype. The entire mitochondrial DNA from the blood of two deaf patients from the Arab–Israeli family was sequenced, as was DNA corresponding to the mitochondrial 12S and 16S rRNA genes (between nucleotides 648–1601 and 1671–3229, respectively) from two Chinese patients who were members of other families with maternally inherited aminoglycoside-induced deafness. The results were compared with the sequences for 278 control individuals, consisting of 35 Arab–Israelis and whites, Asians and blacks in equal proportions. The investigation of the Arab–Israeli family also included sequencing of mitochondrial DNA from one non-deaf family member and a non-deaf, unrelated Arab, and all the sequences were compared with the published ‘Cambridge sequence’ of the 16 569-base pair human mitochondrial genome (Anderson et al., 1981). Several rare sequence variations (mutations) were detected in mitochondrial DNA from each of the four deaf individuals. Only one mutation was common to all three families: an adenine to guanine (A to G) homoplasmic point mutation at nucleotide 1555 (A1555G mutation), which is in a region of the 12S rRNA gene that codes for a highly conserved domain of the small rRNA. This mutation was not found in any of the 278 controls, whereas the other mutations were each found in a few control individuals. Aminoglycosides are known to bind to this region of the 12S rRNA gene in bacteria (Moazed & Noller, 1987; Gravel et al., 1987). Furthermore, the A1555G mutation was the only one of the identified mutations that affected a sequence of bases that was evolutionarily conserved. The sequence has been identified in mice, rats, cattle and humans. It codes for a domain of the small rRNA that is essentially the same in bacteria, plants, invertebrates and mammals.
The homoplasmic A1555G mutation was detected in mitochondrial DNA from the blood of seven of 41 (17%) unrelated individuals in the USA of diverse ethnic origins who had hearing loss that had developed after they had received amino-glycosides (Fischel-Ghodsian et al., 1997). The ethnic groups of the persons with the mutation included whites, Hispanics and Asians. Four of the seven individuals with the mutation had a family history of aminoglycoside-induced ototoxicity. In three of those with the mutation, the onset of deafness had occurred a number of years (17 years in one case) after exposure to aminoglycosides.
Mitochondrial DNA from blood and hair of members of two Japanese and three Chinese families (the subjects of previous reports by Higashi, 1989 and by Hu et al., 1991) with maternally inherited hypersensitivity to aminoglycosides showed the same A1555G mutation in all the deaf individuals investigated. The mutation was not found in 414 unaffected people, including 274 Asians, who were studied as controls. The mutation was also detected in four of 74 people in a Chinese hospital who had gone deaf after treatment with aminoglycosides (Hutchin et al., 1993).
Three Mongolian families with maternal inheritance of susceptibility to streptomycin-induced ototoxicity were identified at a school for the deaf. Blood samples from five deaf members of these three families (three from one family and one from each of the other families) were screened for A633G and A1555G mutations of the 12S r RNA gene and the A1736G mutation of the 16S rRNA gene. Control blood samples from 400 Mongolians with normal hearing was also screened. The A1555G mutation was detected in four of the five deaf subjects, including the three from the same family, but in none of the 400 control individuals. The other mutations investigated were present in four deaf subjects (including the three members of the same family and the person without the A1555G mutation), but were also present in the control population at an individual prevalence of approximately 5% (Pandya et al., 1997).
The A1555G mutation to the 12S rRNA gene in mitochondrial DNA was also found in 12 families in a village in Zaire where 53 of the 348 inhabitants were deaf. All of the families were said to have a common female ancestor who had lived about 150 years previously. Verbal tradition indicated that many members of the family had suddenly become profoundly deaf in 1954, and the high incidence of deafness had continued to the present. It seems unlikely that exposure to aminoglycosides played a part in this incident, as only one of the affected individuals had ever been treated with an aminoglycoside (Matthijs et al., 1996).
The A1555G mutation has thus been detected in the mitochondria of families with high rates of deafness in China (Hutchin et al., 1993; Prezant et al., 1993), Israel (Prezant, et al., 1993), Japan (Hutchin et al., 1993), Mongolia (Pandya et al., 1997), the USA (Fischel-Ghodsian et al., 1997) and Zaire (Matthijs et al., 1996). In some but not all cases the deafness was associated with prior exposure to aminoglycosides.
The A1555G mutation was detected in 10 of 319 unrelated Japanese out-patients with sensorineural hearing loss; 21 of the patients had received aminoglycosides by injection. Hearing loss was also found in 14 of 140 patients with cochlear implants; 22 of these patients had received aminoglycosides. In both series, the frequency of hearing loss was higher in the patients with a history of aminoglycoside use (33% in the out-patients and 59% in those with cochlear implants) (Usami et al., 2000).
A Japanese family with maternally inherited sensorineural hearing loss was reported, which had no history of aminoglycoside injection. There was no other known etiology for the deafness in this family. The A1555G mutation was detected in mitochondrial DNA from blood from all three family members with hearing loss who were investigated (Iwasaki et al., 2000).
The A1555G mutation was detected in mitochondrial DNA from leukocytes of four of 46 deaf–mute Japanese persons; the A3243G mutation was not detected in any of them. Two of the 46 deaf people investigated reported on questionnaires that they had received streptomycin injections, and both of these people had the A1555G mutation. The A1555G mutation was not detected in blood samples from 27 people with normal hearing or 110 patients with adult-onset sensorineural hearing loss. The mutation therefore appeared to be a contributing factor to prelingual deafness but not to late-onset sensorineural deafness (Oshima et al., 2001).
Seventy Spanish families with sensorineural deafness, comprising 36 congenital cases and 34 of late onset, were investigated for the presence of the A1555G mutation in mitochondrial DNA and compared with 200 control subjects. The mutation was found in the 19 of the 70 families with maternally inherited sensorineural deafness but in none of the controls. In 12 of the 19 affected families, all the members with the mutation who had received aminoglycosides became deaf, representing 30% of the deaf people in these families. None of the deaf people in the seven other affected families had been treated with aminoglycosides. Overall, 18% of the people with both the mutation and deafness had been treated with aminoglycosides. The median age at onset of deafness was significantly lower (p < 0.001) in people with the mutation who were treated with aminoglycosides (5.6 years) than in those with the mutation who did not receive aminoglycosides (41 years). The probability that a person with the mutation would develop deafness by the age of 30 years was estimated to be 96% if they were treated with an aminoglycoside and 40% if they were untreated (Estivill et al., 1997).
Mitochondrial DNA from two Italian sisters and three of their children who had developed profound high-frequency hearing loss after receiving aminoglycosides did not have the A1555G mutation. Nevertheless, sequencing of the 12S rRNA gene showed that they all had a thymidine deletion around nucleotide position 961 (Casano et al., 1999).
An investigation was conducted of the 12S rRNA gene in peripheral blood from 35 Chinese students in a school for the deaf, none of whom had the A1555G mutation. Comparison of the base sequences with the standard ‘Cambridge’ mitochondrial sequence and with results for 100 control subjects with normal hearing showed that only three of the patients had mutations to this gene (T1243C, T1520C and 961DeltaT+Cn) that were not also found in controls. The mutations were not found in areas of the gene known to be critical for aminoglycoside binding in the bacterial homologue; however, one of them (T1520C) was in the mitochondrial D-loop, which is also the site of the A1555G mutation found in other studies. The authors suggested that the effect of these mutations might be similar to that of the A1555G mutation: altering the three-dimensional structure of the gene to increase susceptibility to aminoglycoside-induced ototoxicity. One of the three unique mutations detected was an insertion at nucleotide 961 of multiple sequences coding for cytosine (961DeltaT+Cn). The authors claimed that such heteroplasmic mutations to mitochondrial DNA are usually restricted to only a few tissues, suggesting the possibility that some deaf people with no unique mutations to 12S rRNA in their blood cells might have significant mutations in sensory cells in their ears that contributed to their deafness (Bacino et al., 1995).
Prezant et al. (1993) postulated that the mutation at position 1555 on mitochondrial DNA might change the three-dimensional structure of the small rRNA in such a way as to bring about greater binding of aminoglycosides and consequently increased toxicity. Hutchin et al. (1993) suggested that the mutation causes increased binding of aminoglycosides at the ribosome, resulting in inhibition or mis-translation of protein synthesis. As mitochondrial polypeptides comprise part of several enzyme complexes (oxidative phosphorylation complexes I, III, IV and V) involved in ATP production, reduced production of a crucial protein could result in reduced ATP production. Low ATP levels in the cochlea might lead to an imbalance of ion concentrations in the stria vascularis, endolymph or hair cells, which could cause accumulation of Ca2+ in the hair cells, leading to cell death.
In line with this proposed mode of action, it was observed that the rate of mitochondrial protein synthesis was decreased by about 30% in the presence of 2 mg/ml paramycin in lymphoblastoid cell lines from carriers of the A1555G mutation (from the Arab–Israeli family previously investigated), as compared with cell lines from control individuals (Guan et al., 2000). In contrast, comparison of the protein production by lymphoblastoid cell lines derived from deaf and non-deaf members of a family in Zaire who had the mutant (A1555G) mitochondrial DNA showed that exposure to 0.1 mg/ml of neomycin, gentamycin or streptomycin had no effect on the amount of protein translation in cells of either deaf or non-deaf individuals (Matthijs et al., 1996).
In preliminary studies, Southern blot analysis of DNA extracted from blood samples from the Arab–Israeli family previously described revealed no gross deletions, insertions or rearrangements of mitochondrial DNA (Jaber et al., 1992). Biochemical studies of the effects of aminoglycosides on oxidative phosphorylation complexes I, III, IV and V, which include all 13 mitochondrially encoded polypeptides, in lymphoblastoid cell lines derived from members of the family showed that mitochondrial protein synthesis was generally normal, but oxidative phosphorylation complex V showed more activity in cells from deaf family members than in those from their non-deaf siblings, and the activity of complex III was greater in cells from hearing and deaf family members than in control cells from unrelated Arabs (Prezant et al., 1992). Prezant et al. (1993) suggested that the development of deafness in this family might require the presence of both the mitochondrial A1555G mutation (causing increased activity of complex III) and homozygosity for an autosomal recessive mutation (causing increased activity of complex V), non-deaf family members being heterozygous for the recessive mutation. This hypothesis for the mechanism of deafness differs from that proposed by Prezant et al. (1992), as the increased activities of complexes III and V would be expected to result in increased ATP production rather than the hypothesized reduction. It is possible that the biochemical effects underlying deafness are expressed in the ear but not in lymphoblastoid cell lines.
The ADIs that could be derived from studies in bacteria in vitro, from studies of human flora-associated animals in vivo and from studies in humans were evaluated. The available relevant microbiological data indicated that the toxicological ADI of 0–60 µg/kg bw would protect the gastrointestinal flora of humans. The lowest ADI that could be set on the basis of the results of all the available microbiological studies would be 0–14 mg/kg bw (840 mg per person). This value is higher than the existing ADI of 0–60 µg/kg bw (3.6 mg per person), which was set on the basis of toxicological data. Intake of residues at levels that would expose consumers to up to 60 mg/kg bw (3.6 mg per person) would not be expected to have adverse effects on the gut microflora. Consequently, there is no need to change the current ADI on the basis of the microbiological data.
The Committee affirmed that recent papers indicate that there is a causal relationship between the presence of a point mutation in which the adenine at the 1555 position of the 12S rRNA gene on mitochondrial DNA is changed to a guanine (A1555G mutation) and the development of deafness. Hearing loss can be due to both genetic and environmental factors. Systemic exposure to a large dose of aminoglycosides can bring about deafness, and genetic factors may make some people more susceptible to the ototoxic effects of aminoglycosides than others. Although people with the A1555G mutation appeared to be more susceptible to aminoglycoside-induced ototoxicity, it was not clear whether any of them had received neomycin. Nevertheless, it was considered prudent to assume that the effects observed were relevant to all aminoglycosides, including neomycin.
Some families with the A1555G mutation appeared to be at increased risk of hearing loss even in the apparent absence of exposure to aminoglycosides. Thus, people with this mutation may be more susceptible to ototoxicity caused by a variety of environmental factors, one of which is exposure to aminoglycosides. The mutation is inherited from the mother and has been demonstrated in various ethnic groups, including Chinese, Japanese, Mongolian, Spanish and Arab–Israeli, and in individuals in the USA of diverse origin. Only a few families and individuals with the A1555G mutation have been identified worldwide.
The Committee recognized that people with the A1555G mutation might be susceptible to aminoglycoside-induced ototoxicity and might become deaf after receiving therapeutic doses of aminoglycosides. Since the dose and route of administration of aminoglycosides were not given in the reports of the studies in humans, a dose–response relationship could not be established for an increased risk of ototoxicity after administration of aminoglycosides to people with the A1555G mutation. No quantitative data were available for identifying a NOEL for the ototoxicity of neomycin or any other aminoglycoside in people with the A1555G mutation.
The Committee noted that the current ADI for neomycin of 0–60 µg/kg bw had been set by applying a safety factor of 100 to the NOEL of 6 mg/kg bw per day for ototoxicity in a 90-day study in guinea-pigs. This safety factor comprises a 10-fold factor to compensate for extrapolation of results from guinea-pigs to humans and another 10-fold factor to account for interindividual variation within the human population.
The Committee was aware that systemic exposure to large doses of amino-glycosides in excess of the recommended therapeutic doses could result in deafness in any person, irrespective of the presence of the mitochondrial DNA mutation. Nevertheless, deafness has been reported in people with the A1555G mutation given therapeutic doses of aminoglycosides. The recommended oral therapeutic dose of neomycin for adults is about 12 000 mg per person per day. The Committee noted that this dose is more than 3000 times greater than the current ADI for neomycin of 0–60 µg/kg bw (3.6 mg per person). This ADI is adequate to assure the health of all consumers, including those with the A1555G mutation.
The Committee concluded that there was no need to alter the ADI of neomycin to account for the possible susceptibility of the subpopulation with the A1555G mutation or to account for the microbiological properties of neomycin.
Anderson, S., Bankier, A.T., Barrell, B.G., de Bruijn, M.H.L., Coulson, A.R., Drouin, J., Eperon, I.C., Nierlich, D.P., Roe, B.A., Sanger, F., Schreier, P.H., Smith, A.J.H., Staden, R. & Young, I.G. (1981) Sequence and organisation of the mitochondrial genome. Nature, 290, 457–465.
Arabi, Y., Dimock, P., Burdon, D.W., Alexander-Williams, J. & Keighley, M.R.B. (1979) Influence of neomycin and metronidazole on colonic microflora of volunteers. J. Antimicrob. Chemother., 5, 531–537.
Aschbacher, P.W. & Feil, V.J. (1994) Neomycin metabolism in calves. J. Anim. Sci., 72, 683–689.
Bacino, C., Prezant, T.R., Bu, X., Fournier, P. & Fischel-Ghodsian, N. (1995) Susceptibility mutations in the mitochondrial small ribosomal RNA gene in aminoglycoside induced deafness. Pharmacogenetics, 5, 165–172.
Brown, S.A. (1988) Treatment of gram-negative infections. Vet. Clin. N. Am. Small Animal Pract., 18, 1141–1165.
Brummett, R.E., Hall, A.D. & Russell, K.B. (1985) Ninety day oral neomycin sulfate (U-4,567) ototoxicity study in the guinea-pig. Unpublished report No. 7263/85/068, study No. I-162, Upjohn Co., Pharmaceutical Research and Development, Kalamazoo, Michigan, USA.
Bryan, L.W. & Kwan, S. (1981) Mechanisms of aminoglycoside resistance of anaerobic bacteria and facultative bacteria grown anaerobically. J. Antimicrob. Chemother., 8 (Suppl. D), 1–8.
Bryan, L.E., Kowand, S.F. & van den Elzen, H.M. (1979) Mechanism of aminoglycoside antibiotic resistance in anaerobic bacteria: Clostridium perfringens and Bacteroides fragilis. Antimicrob. Agents Chemother., 15, 7–13.
Cerniglia, C.E. & Kotarski, S. (1999) Evaluation of veterinary drug residues in food for their potential to affect human intestinal microflora. Regul. Toxicol. Pharmacol., 29, 238–261.
Casano, R.A.M.S., Johnson, D.F., Bykhovskaya, Y., Torricelli, F., Bigozzi, M. & Fischel-Ghodsian, N. (1999) Inherited susceptibility to aminoglycoside ototoxicity: Genetic heterogeneity and chemical implications. Am. J. Otolaryngol., 20, 151–156.
Chambers, H.F. & Sande, M.A. (1996) Antimicrobial agents—The aminoglycosides. In: Sanford Goodman, L., Limbird, L.E., Milinoff, P.B., Gilman, A.G. & Hardman, J.G., eds, Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 9th Ed., New York: McGraw-Hill, pp. 1103–1121.
Codex Alimentarius Commission (2000) Report of the Twelfth Session of the Codex Committee on Residues of Veterinary Drugs in Foods, Washington DC, USA, 28–31 March 2000. Rome: Food and Agriculture Organization of the United Nations (unpublished document ALINORM 01/31).
Codex Alimentarius Commission (2001) Report of the Thirteenth Session of the Codex Committee on Residues of Veterinary Drugs in Foods, Charleston, SC, USA, 4–7 December 2001. Rome: Food and Agriculture Organization of the United Nations (unpublished document ALINORM 03/31).
Crowell, W.A., Divers, T.J., Byars, T.D., Marshall, A.E., Nusbaum, K.E. & Larsen. L. (1981) Neomycin toxicosis in calves. Am. J. Vet. Res., 42, 29–34.
Daikos, G.K., Kontomichalou, P., Bilalis, D. & Pimenidou, L. (1968) Intestinal flora ecology after oral use of antibiotics. Chemotherapy, 13, 146–160.
Davies, J.E. (1986) Aminoglycoside–aminocyclitol antibiotics and their modifying enyzmes. In: Lorian, V., ed., Antibiotics in Laboratory Medicine, Baltimore: Williams & Wilkins, pp. 790–809.
Estivill, X., Govea, N., Barcelo, A., Perello, E., Badenas, C., Romero, E., Moral, L., Scozzari, R., D’Urbano, L., Zeviani, M. & Torroni, A. (1997) Familial progressive sensorineural deafness is mainly due to the mtDNA A1555G mutation and is enhanced by treatment with aminoglycosides. Am. J. Hum. Genet., 62, 27–35.
Faloon, W.W., Paes, I.C., Woolfolk, D., Nankin, H., Wallace, K. & Haro, E.N. (1962) Effect of neomycin and kanamycin upon intestinal absorption. Ann. N.Y. Acad. Sci., 132, 879–887.
Finegold, S.M., Posnick, D.G., Miller, L.G. & Hewitt, W.L. (1965) The effect of various antibacterial compounds on the normal human fecal flora. Ernaehrungsforschung, 10, 316–341.
Finegold, S.M., Mathisen, G.E. & George, W.L. (1983) Changes in human intestinal flora related to the administration of antimicrobial agents. In: Hentges, D.J., ed., Human Intestinal Flora in Health and Disease, San Diego: Academic Press, pp. 355–446.
Fields, R.L. (1964) Neomycin ototoxicity. Report of a case due to rectal and colonic irrigations. Arch. Otolaryngol., 79: 67–70.
Fischel-Ghodsian, N., Prezant, T.R., Chaltraw, W.E., Wendt, K.A., Nelson, R.A., Arnos, K.S. & Falk, R.E. (1997) Mitochondrial gene mutation is a significant predisposing factor in aminoglycoside ototoxicity. Am. J. Otolaryngol., 18, 173–178.
Freyburger, W.A. & Johnson, L.E. (1956) Blood levels and urinary excretion of orally administered neomycins B and C in dogs. Antibiot. Chemother., 6, 586–588.
Govaerts, P.J., Claes, J., van de Hayning, P.H., Jorens, P.G., Marquet, J. & de Broe, M.E. (1990) Aminoglycoside-induced ototoxicity. Toxicol. Lett., 52, 227.
Gravel, M., Melancon, P. & Brakjer-Gingras, L. (1987) Cross-linking of streptomycin to the 16S-ribosomal RNA of Escherichia coli. Biochemistry, 26, 6227–6232.
Greenberg, L.H. & Momary, H., (1965) Audiotoxicity and nephrotoxicity due to orally administered neomycin. J. Am. Med. Assoc., 19, 827–828.
Guan, M.-X., Fischel-Ghodsian, N. & Attardi, G. (2000) A biochemical basis for the inherited susceptibility to aminoglycoside ototoxicity. Hum. Mol. Genet., 9, 1787–1793.
Halpern, E.B. & Heller, M.F. (1961) Ototoxicity of orally administered neomycin. Report of a case. Arch. Otolaryngol., 73, 675–677.
Hawkins, J.E. (1952) The ototoxicity of the neomycins. In: Transactions of the 11th Conference on the Chemotherapy of Tuberculosis, pp. 189–191.
Hawkins, J.E., Rahway, N.J. & Lurie, M.H. (1953) The ototoxicity of dihydrostreptomycin and neomycin in the cat. Ann. Otol. Rhinol. Laryngol., 62, 1128–1148.
Hazenberg, M.P., Baker, M. & Verschoor-Burggraaf, A. (1981) Effects of the human intestinal microflora on germ free mice. J. Appl. Bacteriol., 50, 95–106.
Hazenberg, M.P., van de Boom, M., Bakker, M. & van de Merwe, J.P. (1983a) Binding to faeces and influence on human anaerobes of antimicrobial agents used for selective decontamination. Antonie van Leeuwenhoek, 49, 111–117.
Hazenberg, M.P., van de Boom, M., Bakker, M. & van de Merwe, J.P. (1983b) Effect of antibiotics on the human intestinal flora in mice. Antonie van Leeuwenhoek, 49, 97–109.
Hazenberg, M.P., Pennock-Schroder, A.M., van den Boom, M. & van de Merwe, J.P. (1984) Binding to and antibacterial effect of ampicillin, neomycin and polymyxin B on human faeces. J. Hyg., 93, 27–34.
Higashi, K. (1989) Unique inheritance of streptomycin-induced deafness. Clin. Genet., 35, 433–436.
Horiguchi, S. & Moriyama, S. (1957),Familial occurrence of hearing disturbances due to the use of streptomycin. Otolaryngology (Tokyo), 29, 13–16.
Hu, D.N., Qui, W.Q., Wu, B.T., Fang, L.Z., Zhou, F., Gu, Y.P., Zhang, Q.H., Yan, J.H., Ding, Y.Q. & Wong, H. (1991) Genetic aspects of antibiotic induced deafness: Mitochondrial inheritance. J. Med. Genet., 28, 79–83.
Hutchin, T., Haworth, I., Higashi, K., Fischel-Ghodsian, N., Stoneking, M., Saha, N., Arnos, C. & Cortopassi, G., (1993) A molecular basis for human hypersensitivity to aminoglycoside antibiotics. Nucleic Acids Res., 21, 4174–4179.
Iwasaki, S., Tamagawa, Y., Ocho, S. & Hoshino, T. (2000) Hereditary sensorineural hearing loss of unknown cause involving mitochondrial DNA 1555 mutation. Otorhinolaryngology, 62, 100–103.
Jaber, L., Shohat, M., Bu, X., Fischel-Ghodsian, N., Yang, H.-Y., Wang, S.-J. & Rotter, J.I. (1992) Sensoneural deafness inherited in a tissue-specific mitochondrial disorder. J. Med. Genet., 29, 86–90.
Jacobson, E.D., Chodos, R.B. & Faloon, W.W. (1960) An experimental malabsorption syndrome induced by neomycin. Am. J. Med., 28, 524–533.
Kavanagh, K.T. & McCabe, B.F. (1983) Ototoxicity of oral neomycin and vancomycin. Laryngoscope, 93, 649-653.
King, J.T. (1962) Severe deafness in an infant following oral administration of neomycin. J. Med. Assoc. Georgia, 51, 530–531.
Kotarski, S.F. (2002) Review of exploratory studies using neomycin to develop model test systems to examine the effects of antimicrobials on the human gut flora. Report No. ER-0802-7922-2002-001. Submitted to WHO by Pharmacia-Upjohn.
Kotecha, B. & Richardson, G.P. (1994) Ototoxicity in vitro: Effects of neomycin, gentamycin, dihydrostreptomycin, amikacin, spectinomycin, neamine, spermine and poly-L-lysine. Hearing Res., 73, 173–184.
Kunin, C.M., Chalmers, T.C. & Leevy, C.M. (1960) Absorption of orally administered neomycin and kanamycin. New Engl. J. Med., 262, 380–385.
Langman, A.W. (1993) Neomycin ototoxicity. Otolaryngology, 110, 441–444.
Lechevalier, H.A. (1975) The 25 years of neomycin. CRC Crit. Rev. Microbiol., 3, 359–397.
Lerner, S.A., Gaynes, R.P. & Nordstrom-Lerner, L. (1998) Aminoglycosides. In: Gorbach, S.L., Bartlett, J.G. & Blacklow, N.R., eds, Infectious Diseases, 2nd Ed., Philadelphia: W.B. Saunders Co., pp. 775–790.
Lindsay, J.R., Proctor, L.R. & Work, W.P. (1960) Histopathological inner ear changes in deafness due to neomycin in a human. Laryngoscope, 70, 382–392.
Lortholary, O., Tod, M., Cohen, Y. & Petitjean, O. (1995) Aminoglycosides. Med. Clin. N. Am., 79, 761–787.
Matthijs, G., Claes, S., Longo-Mbenza, B. & Cassiman, J.-J. (1996) Non-syndromic deafness associated with a mutation and a polymorphism in the mitochondrial 12S ribosomal RNA gene in a large Zairean pedigree. Eur. J. Hum. Genet., 4, 46–51.
Matz, G.J. (1993) Aminoglycoside cochleal ototoxicity. Ototoxicity, 26, 705–712.
Mingeot-Leclercq, M.-P., Glupczynski, Y. & Tulkens, P. (1999) Aminoglycosides: Activity and resistance. Antmicrob. Agents Chemother., 43, 727–737.
Moazed, D. & Noller, H.F. (1987) Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature, 327, 389–394.
Moellering, R.C., Jr (1983) In vitro antibacterial activity of the aminoglycoside antibiotics. Rev. Infect. Dis., 5 (Suppl. 2), S212–S232.
National Committee for Clinical Laboratory Standards (1993) Methods for Antimicrobial Susceptibility Testing, 3rd Ed., Villanova, Pennsylvania, p. 23.
Oshima, T., Kudo, T. & Ikeda, K. (2001) Point mutation of the mitochondrial genome in Japanese deaf-mutism. Otorhinolaryngology, 63, 329–332.
Pandya, A., Xia, X., Radnaabazar, J., Batsuuri, J., Dangaansuren, B., Fischel-Ghodsian, N. & Namce, W.E. (1997) Mutation in the mitochondrial 12S rRNA gene in two families from Mongolia with matrilineal aminoglycoside ototoxicity. J. Med. Genet., 34, 169–172.
Poth, E.J., Fromm, S.M., Wise, R.I. & Hsiang, C.M. (1950) Neomycin, a new intestinal antiseptic. Texas Rep. Biol. Med., 8, 353–360.
Poth, E.J., Martin, R.G., Fromm, S.M., Wise, R.I. & Hsiang, C.M. (1951) A critical analysis of neomycin as an intestinal antiseptic. Texas Rep. Biol. Med., 8, 631–644.
Prescott, J.F., Baggot, J.D. & Walker, R.D. (2000) Antimicrobial Therapy in Veterinary Medicine, 3rd Ed., Ames: Iowa State University Press, pp. 191–228.
Prezant, T.R., Shohat, M., Jaber, L., Pressman, S. & Fischel-Ghodsian, N. (1992) Biochemical characterisation of a pedigree with mitochondrially inherited deafness. Am. J. Med. Genet., 44, 465–472.
Prezant, T.R., Agapian, J.V., Bohlman, M.C., Bu, X., Oztas, S., Qui, W.-Q., Arnos, K., Cortopassi, G.A., Jaber, L., Rotter, J.I., Shohat, M. & Fischel-Ghodsian, N. (1993) Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced deafness and non-syndromic deafness. Nature Genet., 4, 289–293.
Rappaport, B.Z., Fausti, S.A., Schechter, M.A. & Frey, R.H. (1986) A prospective study of high-frequency auditory function in patients receiving oral neomycin. Scand. Audiol., 15, 67–71.
Rasmussen, B.A. & Tally, F.P. (1993) Antimicrobial resistance in Bacteroides. Clin. Lif. Dis., 16 (Suppl. 4), S390–S400.
Riskaer, N., Christensen, E., Petersen, E. & Weidman, H. (1956) The ototoxicity of neomycin. Experimental investigations. Acta Otolaryngol., 46, 137–152.
Riviere, J.E., Craigmill, A.L. & Sundloff, S.F. (1991) Handbook of Comparative Pharmacokinetics and Residues of Veterinary Antimicrobials, Boca Raton, Florida, CRC Press, Inc., pp. 263–275.
van Saene, J.J., van Saene, J.J., Stoutenbeek, C.P. & Lerk, C.F. (1985) Influence of feces on the activity of antimicrobial agents used for decontamination of the alimentary canal. Scand. J. Infect. Dis., 17, 295–300.
Sirtori, C.R., Manzoni, C. & Lovati, M.R. (1991) Mechanisms of lipid-lowering agents. Cardiology, 78, 226–235.
Usami, S.-I., Abe, S., Akita, J., Namba, A., Shinkawa, H., Ishii, M., Iwasaki, S., Hoshino, T., Ito, J., Doi, K., Kubo, T., Nakagawa, T., Komiyama, S., Tono, T. & Komune, S. (2000) Prevalence of mitochondrial gene mutations among hearing impaired patients. J. Med. Genet., 37, 38–40.
Veringa, E.M. & van der Waaij, D. (1984) Biological inactivation by faeces of antimicrobial drugs applicable in selective decontamination of the digestive tract. J. Antimicrob. Chemother., 14, 605–612.
Wagman, G.H., Bailey, J.V. & Weinstein, M.J. (1974) Binding of aminoglycosides to feces. Antimicrob. Agents Chemother., 6, 415–417.
Waisbren, B.A. & Spink, W.W. (1950) A clinical appraisal of neomycin. Ann. Intern. Med., 33, 1099–1119.
Zelenka, J., Tomes, D. & Jilkova, B (1966) [Possible ototoxic effects of neomycin administered per os in dyspepsia of the newborn. Experimental and audiometric study.] Pediatrie, 21, 573 (in French)
See Also:
Toxicological Abbreviations
Neomycin (WHO Food Additives Series 34)
Neomycin (WHO Food Additives Series 38)
NEOMYCIN (JECFA Evaluation)