First draft prepared by
I. Dewhurst
Pesticides Safety Directorate, Ministry of Agriculture, Fisheries and Food,
Mallard House, Kings Pool, York, United Kingdom
Thiodicarb is a carbamate insecticide that acts by inhibiting acetylcholinesterase activity. It was evaluated by JMPR in 1985 and 1986 (Annex 1, references 44 and 47). An ADI of 00.03 mg/kg bw was established in 1986. It was considered by the 2000 JMPR within the periodic review programme of the Codex Committee on Pesticide Residues.
The thiodicarb used for biochemical and toxicity testing was usually supplied as an off-white powder with a purity of 9199.9%. The sponsor commented that there have been no significant changes in the composition of technical-grade thiodicarb over time. The compound is also known as Larvin technical and UC51762.
(a) Absorption, distribution, and excretion
A number of studies on the toxicokinetics of thiodicarb that were reviewed by the 1985 JMPR (Annex 1, reference 46) were not resubmitted. The findings of the studies were consistent with those of the more recent work evaluated at the present Meeting. For completeness, and to provide a comparison with the newer studies, sections of text from the 1985 JMPR monograph are reproduced here.
Thiodicarb, which consists essentially of two methomyl moieties joined through their amino nitrogen by sulfur, is rapidly degraded to S-methyl-N-[(methylcarbamoyl)oxy]thioacetamide (methomyl) in the rat stomach. Over 30% of an oral dose of 16 mg/kg bw was converted to methomyl in the gut within 15 min. Only 40% of the dose was found in the gut at 15 min, indicating that the compound had been rapidly absorbed. Approximately 26% of the radiolabel in the administered dose was found in other body fractions, the remainder of the dose (34%) apparently having been lost as volatile compounds.
In rats given radiolabelled thiodicarb as a single oral dose of 40 mg/kg bw, 80% of the dose was eliminated within 48 h. After 4 days, 48% of the dose had been eliminated in the respiratory gases, 32% in the urine, and 4.5% in the faeces; 11% of the dose remained in the carcass because of incorporation of radiolabelled carbon dioxide and acetic acid metabolites into natural products.
Thiodicarb is degraded in the stomach not only to methomyl but also to some other unstable intermediates, including methomyl methylol, methomyl oxime, methomyl sulfoxide, and methomyl sulfoxide oxime, which are subsequently converted to acetonitrile and carbon dioxide and eliminated primarily by respiration and in the urine. Acetonitrile is the only metabolite retained to some extent in body tissues and fluids; a small fraction of the acetonitrile is further degraded to carbon dioxide, acetic acid, and acetamide, which is suspected to be carcinogenic in mice and rats. The ultimate metabolic fate of methomyl in animals depends on its isomeric configuration. In rats, the stable and predominant form is the syn isomer, which is metabolized primarily to carbon dioxide, while partial conversion from the syn isomer to the anti isomer leads primarily to acetonitrile, most of which is respired unchanged.
On the basis of the finding that 5.7% of administered syn-methomyl is converted to acetonitrile, a maximum of 5.7% of the administered [14C]thiodicarb might therefore be available for conversion to acetamide, since thiodicarb is metabolized predominantly to syn-methomyl. On a weightpercent basis, the maximum amount of acetamide that could be produced after ingestion of thiodicarb residues is 1.9% of the administered dose (53:l thiodicarb:acetamide, weight:weight).
Rats
An extensive study of the toxicokinetics of thiodicarb in rats was performed. The study was divided into three phases: determination of the Tmax and Cmax in blood, plasma, and erythrocytes; a mass balance investigation of excretory pathways; and an investigation of tissue concentrations and metabolites.
In phase 1, groups of five male and five female Sprague-Dawley rats received a single dose of [acetimide-14C]thiodicarb (23 mCi/mmol per L) by gavage in Emulphor EL620 (8 ml/kg bw) at 2.4 or 18 mg/kg bw. The purity of the radiolabelled sample varied with analytical procedure from 88% (by high-performance liquid chromatography [HPLC]) to 93% (by thin-layer chromatography [TLC]), the remainder being primarily methomyl. Samples of urine and faeces were taken 6, 12, 24, and 48 h after dosing, and blood samples were taken from the tail vein 0.25, 0.5, 1, 2, 4, 8, 12, 24, 48, 72, and 96 h after dosing. At terminal sacrifice (96 h), samples of 12 tissues and the carcass were taken. Trapping of volatile compounds was not performed. Radiolabel was measured by liquid scintillation counting (LSC) after appropriate processing of samples. Animals receiving 18 mg/kg bw showed a range of clinical signs, including tremors, during the first 3 h. The results (Table 1) showed that thiodicarb at 2.4 mg/kg bw was absorbed very rapidly, but the plasma Tmax was slightly longer at 18 mg/kg bw, indicating saturation of absorption. However, as the ratio of plasma to erythrocyte Cmax was similar, there appeared to be no significant alteration of key kinetics with increasing dose. The Tmax for erythrocytes was later than that for plasma, which, with the high concentration of radiolabel present in erythrocytes at 96 h, indicates that the compounds responsible for the radiolabel in plasma might be metabolized further to the molecules associated with the erythrocytes. Females had a higher erythrocyte Cmax than males and delayed urinary excretion, indicating potential sex differences in metabolism. (The high concentration of radiolabel in eythrocytes may be associated with the haematological effects seen in the studies of toxicity, although no work has been performed to investigate the mechanism of this effect.)
Table 1. Toxicokinetics of [acetimide-14C]thiodicarb in groups of five rats of each sex (means)
|
Sex |
Dose |
Toxicokinetic parameter |
|||
|
T max (h) |
C max (΅g equivalent/g) |
||||
|
Plasma |
Erythrocytes |
Plasma |
Erythrocytes |
||
|
Male |
2.4 |
1 |
24 |
0.8 |
4.1 |
|
|
18 |
4 |
24 |
4.5 |
29 |
|
Female |
2.4 |
1 |
24 |
0.8 |
6.4 |
|
|
18 |
4 |
24 |
4.9 |
41 |
Table 1
(continued)|
Sex |
Dose |
Toxicokinetic parameter |
||||
|
Concentration (΅g equivalent/g) |
Urine (% of dose) |
|||||
|
Plasma |
Erythrocytes |
06 h |
612 h |
048 h |
||
|
Male |
2.4 |
0.1 |
3.4 |
5.0 |
2.3 |
10 |
|
|
18 |
1.4 |
24 |
13 |
7.9 |
28 |
|
Female |
2.4 |
0.1 |
5.3 |
1.6 |
5.2 |
10 |
|
|
18 |
1.4 |
36 |
7.4 |
3.4 |
22 |
From Hiles (1987)
In phase 2, similar groups of rats received a single dose of the same preparation of [acetimide-14C]thiodicarb by gavage at 2 or 16 mg/kg bw. Samples of urine and faeces were taken 6, 12, 24, 48, 72, 96, 120, 144, and 168 h after dosing, and blood samples were taken at terminal sacrifice (168 h) with samples of 12 tissues and the carcass. Trapped volatile compounds were sampled at the same times as urine by use of a 2:1 mixture of methyl-cellosolve:ethanolamine to collect carbon dioxide and of butylcellosolve at 10 °C to trap acetonitrile. Clinical signs, including tremors, were seen in animals given 16 mg/kg bw. The total recovery of radiolabel was about 90%, which is considered acceptable for a compound that gives off a large amount of volatile components. The results (Table 2) showed that thiodicarb was absorbed rapidly, 1850% of the administered dose being excreted within 12 h, mainly as exhaled volatile compounds. The elimination of carbon dioxide was considerably more rapid than that of acetonitrile. The ratio of exhaled carbon dioxide:acetonitrile varied with sex and dose, but the pattern was not consistent and no reliable conclusions can be drawn from this observation. The amount excreted in the urine of males was independent of dose, but in females urinary excretion was more rapid and extensive at 16 mg/kg bw than at 2 mg/kg bw. The radiolabel in tissues at 168 h represented 8% of the administered dose, and the proportion was independent of dose and sex. The highest concentrations of radiolabel were associated with erythrocytes; the major organs had lower concentrations than whole blood (Table 2).
Table 2. Excretion patterns and tissue concentrations in groups of five rats given thiodicarb by gavage (means)
|
Sex |
Dose |
Per cent of dose in |
|||||||
|
Carbon dioxide |
Acetonitrile |
Urine |
Faeces |
||||||
|
06 h |
0168 h |
012 h |
0168 h |
01 h |
1224 h |
0168 h |
0168 h |
||
|
Male |
2 |
18 |
24 |
5.7 |
14 |
27 |
3.7 |
34 |
5.3 |
|
|
16 |
9.7 |
15 |
8.5 |
27 |
24 |
2.2 |
34 |
4.4 |
|
Female |
2 |
8.0 |
13 |
14 |
38 |
7.8 |
1.1 |
21 |
9.2 |
|
|
16 |
13 |
23 |
6.4 |
20 |
20 |
5.4 |
31 |
4.3 |
Table 2 (continued)
|
Sex |
Dose |
Concentration (΅g equivalent per gram) |
Total recovery |
|||||||
|
Plasma |
Erythrocytes |
Whole blood |
Carcass |
Liver |
Spleen |
Kidney |
Brain |
|||
|
Male |
2 |
0.06 |
3.0 |
1.7 |
0.1 |
0.3 |
0.4 |
0.4 |
0.1 |
86 |
|
|
16 |
0.6 |
13 |
7.2 |
1.1 |
1.4 |
1.4 |
1.4 |
0.6 |
90 |
|
Female |
2 |
0.15 |
1.5 |
0.8 |
0.1 |
0.2 |
0.2 |
0.3 |
0.05 |
89 |
|
|
16 |
0.8 |
24 |
13 |
1.1 |
1.8 |
2.6 |
1.8 |
0.6 |
89 |
From Hiles (1987)
In phase 3, groups of three rats of each sex received a single dose of [acetimide-14C]thiodicarb at 2.4 or 18 mg/kg bw. The animals were killed at the times of the plasma Tmax/2, Tmax (on the basis of the results of phase 1), 24, or 48 h. Samples of urine and faeces were taken immediately before sacrifice or 6, 12, 24, and 48 h after dosing from appropriate groups. Blood samples were taken at terminal sacrifice, with samples of 12 tissues and the carcass. Clinical signs, including tremors, were seen in animals receiving 18 mg/kg bw, and salivation was seen in two animals receiving 2.4 mg/kg bw. The results (Table 3) were generally consistent with the findings in phases 1 and 2, with the highest concentrations of radiolabel in erythrocytes and higher concentrations of residues in females than in males. The value for plasma Tmax at 2.4 mg/kg bw was 1 h, as in phase 1, but at 18 mg/kg bw the Tmax was 24 h in males and 2 h in females, compared with 4 h in phase 1. The concentrations in major organs were similar to those in whole blood and plasma, the kidney, liver, and spleen having the highest concentrations, which would be expected for a compound that is extensively metabolized, rapidly excreted in urine, and a fraction of which is associated with erythrocytes. Thiodicarb and its metabolites penetrated to only a limited extent into the brain (Hiles, 1987).
Table 3. Tissue concentrations in samples from groups of three rats given thiodicarb by gavage (means)
|
Sex |
Dose |
Time |
Concentration (΅g equivalents/g) |
|||||||
|
Plasma |
Erythrocytes |
Whole blood |
Carcass |
Liver |
Spleen |
Kidnay |
Brain |
|||
|
Male |
2.4 |
Tmax |
1.1 |
2.4 |
1.6 |
0.4 |
1.8 |
0.9 |
2.4 |
0.3 |
|
|
|
24 h |
0.4 |
4.5 |
2.3 |
0.3 |
1.0 |
0.5 |
1.1 |
0.2 |
|
|
18 |
Tmax |
5.3 |
15 |
9.4 |
2.7 |
7.7 |
2.8 |
7.4 |
1.8 |
|
|
|
24 h |
7.2 |
27 |
17 |
2.6 |
8.3 |
4.6 |
6.1 |
1.8 |
|
Female |
2.4 |
Tmax |
1.8 |
3.6 |
2.6 |
0.5 |
1.9 |
0.8 |
2.9 |
0.4 |
|
|
|
24 h |
0.6 |
6.5 |
3.5 |
0.3 |
1.3 |
0.9 |
1.2 |
0.2 |
|
|
18 |
Tmax |
7.3 |
24 |
16 |
2.9 |
8.8 |
3.7 |
11 |
1.8 |
|
|
|
24 h |
6.4 |
46 |
25 |
3.3 |
8.8 |
6.1 |
6.2 |
1.7 |
From Hiles (1987); Tmax at 2.4 mg/kg bw was taken as 1 h and that at 18 mg/kg bw as 4 h.
Cynomolgus monkeys
The purpose of this study was to verify the metabolic pathway observed in rats and to quantify the metabolites (especially acetamide) in a species more closely resembling humans. The absorption, distribution, metabolism, and excretion of [acetimide-14C]thiodicarb (radiochemical purity, > 97%; specific activity, 17.7 mCi/mmol per L) and its metabolites were studied in four male cynomolgus monkeys (Macaca fascicularis) aged 12.5 years. The animals were placed into individual metabolic chambers immediately after a single oral dose at a nominal rate of 5 mg/kg bw administered by gavage in 1% carboxymethyl cellulose at 4 ml/kg bw in combination with unlabelled thiodicarb (purity, 99.95%). Urine was collected 6 and 24 h after dosing and then every 24 h up to 168 h. Faeces, cage debris, and cage washings were collected daily up to 7 days (168 h). Expired gases were trapped in two methanol traps, two potassium hydroxide traps, and a cold (70 °C) trap sequentially, and analysed 6, 24, and 48 h after dosing. Seven days after dosing, the monkeys were killed, and lungs, brain, gastrointestinal tract, liver, and samples of fat, muscle, and skin were collected. The radiolabelled compounds were analysed and quantified by a combination of TLC, HPLC, and LSC, with comparison to authentic standards. The presence of conjugates was investigated by incubation with glucuronidase and sulfatase preparations. The efficiency of extraction and capture was determined for each procedure and found to be > 97%.
Approximately 59% of the radiolabel was excreted during the first 24 h. Over the 7-day period, a mean of 31% of the administered dose was excreted in urine (including cage wash). Absorption appeared to be essentially complete, only 4.6% of the applied dose being eliminated in the faeces. During the first 48 h, a mean of 35% of the dose was eliminated as volatile components. At sacrifice 168 h after dosing, < 5% of the dose was found in the tissues (Table 4). Of the radiolabel retained in the tissues, most was in fat (1.3%; 0.5 ΅g equivalents/g), muscle (1.1%), and skin (1.1%; 0.5 ΅g equivalents/g). Liver (1.2 ΅g equivalents/g) had the highest concentration of radiolabel at 7 days, with 0.2 ΅g equivalents/g in whole blood and 0.15 ΅g equivalents/g in brain. The total radiolabel recovered in the four animals represented 7481% of the administered dose. The apparently low recovery might be due in part to the fact that volatile compounds were collected for only 48 h (Hawkins et al., 1993).
Table 4 Excretion of radiolabel by cynomolgus monkeys after a single oral dose of [acetimide-14C]thiodicarb (5 mg/kg bw)
|
|
Per cent of administered dose in |
|||||||
|
|
Acetonitrile |
Carbon dioxide |
||||||
|
|
06 h |
624 h |
2448 h |
Total |
06 h |
624 h |
2448 h |
Total |
|
|
2.0 |
3.8 |
2.9 |
8.8 |
13.6 |
11.5 |
2.2 |
27.3 |
|
|
1.9 |
3.0 |
1.7 |
6.6 |
23.0 |
5.9 |
1.1 |
30.1 |
|
|
1.7 |
3.4 |
1.5 |
6.6 |
16 |
6.9 |
2.5 |
25.2 |
|
|
2.1 |
5.9 |
4.2 |
12.3 |
17.2 |
10.3 |
1.2 |
28.7 |
|
Mean ± SD |
1.9 ± 0.2 |
4.0 ± 1.6 |
2.6 ± 1.6 |
8.6 ± 2.7 |
17 ± 4.0 |
8.6 ± 2.7 |
1.8 ± 0.7 |
28 ± 2.1 |
Table 4 (continued)
|
|
Per cent of administered dose in |
||||||
|
|
Expired air |
Urine |
Cage wash |
Urine + cage wash |
|||
|
|
06 h |
624 h |
2448 h |
Total |
|||
|
|
36.1 |
14.9 |
5.1 |
1.5 |
22.9 |
4.1 |
27.0 |
|
|
36.7 |
28.7 |
3.7 |
1.2 |
34.8 |
0.98 |
35.7 |
|
|
31.7 |
22.0 |
4.7 |
1.6 |
29.3 |
3.1 |
32.3 |
|
|
41.0 |
19.0 |
6.2 |
2.2 |
28.4 |
1.9 |
30.4 |
|
Mean ± SD |
36 ± 3.8 |
21 ± 5.8 |
4.9 ± 1.0 |
1.6 ± 0.4 |
29 ± 4.9 |
2.5 ± 1.4 |
31 ± 3.6 |
Table 4 (continued)
|
|
Per cent of administered dose in |
|||||
|
|
Faeces |
Cage debris |
Tissues |
Total (%) |
||
|
|
024 h |
2448 h |
Total |
|||
|
|
0.97 |
2.9 |
4.8 |
0.62 |
5.1 |
73.5 |
|
|
0.16 |
3.7 |
4.5 |
0.08 |
4.1 |
81.0 |
|
|
0.29 |
4.4 |
5.4 |
0.28 |
4.7 |
74.4 |
|
|
2.5 |
0.98 |
3.8 |
0.15 |
4.7 |
80.1 |
|
Mean ± SD |
0.98 ± 1.1 |
3.0 ± 1.5 |
4.6 ± 0.6 |
0.28 ± 0.2 |
4.7 ± 0.40 |
77 ± 3.8 |
From Hawkins et al. (1993)
Goats
The metabolic fate of [acetimide-14C]thiodicarb (specific activity, 18 mCi/mmol; radiochemical purity, 98.4%) was studied in two lactating goats after 7 days of dosing at 56 mg/kg bw per day by capsule twice daily after milking. A third animal served as a control. Volatile compounds were collected for about 10 h on day 6 of the dosing regime. Faeces, urine, and milk were collected twice daily during treatment and for 7 days after completion of dosing. Blood, gut contents, and tissue samples were taken for analysis within 18 h of the last dose. Volatile compounds, milk, urine, faeces, tissues, blood, and gut contents were analysed for total residues.
One goat became ill during the study after an injury to the oral cavity considered to be due to the balling gun used to deliver the capsules. The results (Table 5) showed that the primary route of excretion was as volatile compounds (carbon dioxide and acetonitrile). About 50% of the administered dose was recovered from both goats,; the deficit was probably due to incomplete capture of volatile compounds in the indirect respiration chamber used. The concentration of total radiolabel was fairly constant from 3 days after dosing in faeces, urine, and milk. The peak concentrations in milk were 15 and 20 mg/kg. Radiolabel was extensively distributed to tissues, and the highest mean concentrations were found in liver (25 mg/kg), kidney (13 mg/kg), and blood (11 mg/kg) (Hanlon & Norris, 1993).
Table 5. Total recovery of radiolabel from goats given [acetimide-14C]thiodicarb for 7 days
|
Animal |
Per cent of total radiolabel recovered |
|||||
|
Tissuesa |
Milk |
Urine |
Faeces |
Volatile compounds |
Total |
|
|
1 |
8.6 |
6.4 |
8.9 |
7.7 |
21 |
52 |
|
2 |
14 |
3.1 |
5.8 |
3.5 |
23 |
50 |
|
Mean |
11 |
4.7 |
7.3 |
5.6 |
22 |
51 |
From Hanlon & Norris (1993)
a
Including blood and gut contentsCows
Groups of two lactating Holstein cows were given diets containing [acetimide-14C]thiodicarb (radiochemical purity, 98.5%; specific activity, 29 mCi/mg) at a concentration of 0.1, 10, 30, or 100 ppm, equivalent to 0.004, 0.4, 1.4, and 4 mg/kg bw per day, for 21 days. Milk and urine were collected twice daily, and blood and faeces were collected at 34-day intervals. Volatile compounds were not collected. One cow at each dose was killed 12 h after the last treatment, and liver, kidney, lung, spleen, heart, brain, ovary, udder, tongue, foreleg muscle, hindleg muscle, neck muscle, and omental fat were collected for analysis. The remaining four cows (one at each dose) were kept for an additional 7 days after termination of treatment, and milk and urine were collected twice, blood and faeces were collected daily, and tissues were collected at termination. The concentration of radiolabel, determined by LSC, increased with increasing dose in all samples of tissue, milk, blood, urine, and faeces. The concentration in milk increased with duration of dosing, reaching a plateau after about 2 weeks at 0.1 and 10 ppm and after 1 week at 30 and 100 ppm. After 21 days of treatment, the concentrations of radiolabel in tissues were highest in liver and kidney (Table 6). Seven days after the last dose, the concentrations were lower in all tissues except liver and muscle. After treatment, the concentrations of radiolabel in milk declined, with an initial half-time of 34 days (Feung et al., 1980).
Table 6. Concentrations of radiolabel (103 dpm/g) in tissues of lactating cows given [acetimide-14C]thiodicarb for 21 days
|
Tissue |
Time after treatment (days) |
Dose (ppm) |
|||
|
0.1 |
10 |
30 |
100 |
||
|
Liver |
21 |
1100 |
1300 |
3900 |
|
|
21 + 7 |
|
360 |
1500 |
4700 |
|
|
Kidney |
21 |
|
340 |
770 |
1200 |
|
21 + 7 |
|
93 |
390 |
1000 |
|
|
Adipose |
21 |
|
13 |
34 |
320 |
|
21 + 7 |
|
|
10 |
|
|
|
Foreleg muscle |
21 |
|
60 |
140 |
510 |
|
21 + 7 |
|
21 |
160 |
260 |
|
|
Milk |
21 |
2 |
170 |
590 |
1600 |
|
21 + 7 |
0.7 |
20 |
82 |
260 |
|
From Feung et al. (1980)
A lactating Holstein cow received a single dose of [acetimide-14C]thiodicarb in a capsule at a dose of 7 mg/kg bw. During 72 h after treatment, 66% of the administered radiolabel was eliminated in respired air, 11% in faeces, 5.0% in urine, and 4.6% in milk; 10% was retained in the tissues, the highest concentration being found in liver (9 ΅g equivalents/g). Blood samples taken up to 24 h showed higher concentrations of radiolabel in plasma than in erythrocytes, but the trend was reversed subsequently. The peak concentration of radiolabel in milk was 7.3 ppm 18 h after dosing. Expired radiolabel was found mainly in carbon dioxide and acetonitrile in a ratio of 8:1 (Khasawinah & College, 1978).
Hens
The metabolic fate of [acetimide-14C]thiodicarb (21.4 mCi/mmol per L) in groups of three laying hens was studied after 21 days of dosing by capsule with a concentration of 15, 29, or 100 ppm, equivalent to 1, 1.5, and 6 mg/kg bw per day. Faeces and eggs were analysed during treatment and for 7 days after the end of dosing. A range of tissues were taken from one hen in each group 6 h, 3 days, and 7 days after the last dose. Samples of tissues, faeces, eggs, and blood were analysed for total radiolabel by LSC after appropriate extraction and processing. In hens given the highest dose, the concentration of total radiolabel reached a plateau within 1 day in faeces (14 ΅g equivalents/g), 2 days in egg white (2 ΅g equivalents/g), and 10 days in egg yolk (14 ΅g equivalents/g) (Table 7). Significant concentrations were present in eggshell, indicating incorporation as carbonate. The detectable concentrations of radiolabel in eggs declined during the withdrawal period with a half-time of < 1 day. The concentration of total radiolabel in eggs and tissues was proportional to the dose administered. Six hours after the last dose, the highest concentrations of radiolabelled residues in tissues were in erythrocytes, liver, kidney, skin, and fat. The concentrations in the various muscles were similar. The detectable concentrations of radiolabel declined during the withdrawal period with a half-time of 34 days (Table 8; Andrawes & Bailey, 1980).
Table 7. Distribution of radiolabel in faeces and eggs of hens given[acetimide-14C]thiodicarb by capsule for 21 days and after a 7-day withdrawal period (΅g equivalent/g)
|
Day |
Conceration in capsule (ppm) |
|||||||||
|
15 |
29 |
102 |
||||||||
|
Faeces |
Yolk |
White |
Faeces |
Yolk |
White |
Faeces |
Yolk |
White |
Shell |
|
|
1 |
1.6 |
|
|
3.2 |
|
|
14 |
|
|
|
|
2 |
1.3 |
|
|
3.7 |
|
|
15 |
1.0 |
2.0 |
2.1 |
|
6 |
|
|
|
|
|
|
|
10 |
1.5 |
2.5 |
|
12 |
|
1.4 |
0.2 |
|
4.1 |
0.6 |
|
15 |
2.0 |
3.1 |
|
21 |
1.4 |
1.5 |
0.2 |
4.5 |
4.6 |
0.6 |
13 |
13 |
1.6 |
1.5 |
|
21 + 1 |
0.4 |
|
|
2.0 |
|
|
10 |
14 |
1.3 |
1.5 |
|
21 + 3 |
0.1 |
|
|
0.3 |
|
|
1.1 |
10 |
0.2 |
0.4 |
|
21 + 7 |
0.1 |
|
|
0.2 |
|
|
0.4 |
2.2 |
0.1 |
0.2 |
From Andrawes & Bailey (1980); , sample not analysed
Table 8. Distribution of radiolabel in tissues of hens fed capsules containing [acetimide-14C]thiodicarb for 21 days, at 6 h and 3 and 7 days after the last dose (΅g equivalent/g)
|
Tissue |
Concentration in capsule (ppm) |
||||||||
|
15 |
29 |
102 |
|||||||
|
6 h |
3 days |
7 days |
6 h |
3 days |
7 days |
6 h |
3 days |
7 days |
|
|
Breast muscle |
0.5 |
0.2 |
0.1 |
1.0 |
0.5 |
0.4 |
3.4 |
1.9 |
1.3 |
|
Abdominal fat |
1.3 |
0.8 |
0.6 |
2.9 |
1.9 |
1.6 |
6.6 |
7.5 |
6.1 |
|
Skin |
0.6 |
0.4 |
0.3 |
1.7 |
1.1 |
0.7 |
4.3 |
4.0 |
1.6 |
|
Kidney |
1.4 |
0.7 |
0.3 |
3.4 |
1.7 |
0.9 |
8.5 |
4.7 |
1.7 |
|
Liver |
1.5 |
0.4 |
0.3 |
3.5 |
1.1 |
0.6 |
11 |
4.6 |
1.6 |
|
Plasma |
0.4 |
0.1 |
0.1 |
1.2 |
0.2 |
0.1 |
3.9 |
0.9 |
0.3 |
|
Erythrocytes |
2.3 |
1.4 |
0.7 |
7.5 |
4.5 |
2.7 |
20 |
18 |
8.4 |
From Andrawes & Bailey (1980
Rats
Samples obtained from phase 3 of the study of Hiles (1987; see above) were subsequently processed by solvent extraction, incubation with bovine peptidase or beta-glucuronidase and sulfatase from Helix pomata, and acid hydrolysis. The samples were then analysed by TLC with gas chromatography or HPLC, and the Rf values were compared with standards of a range of postulated thiodicarb metabolites. Most of the urinary radiolabel (86102%) was extracted into an aqueous solvent, although trace amounts (< 7%) were extracted into dichloromethane, a pattern that was independent of dose and time of sampling. The main components of the extracts are shown in Table 9. Many were not characterized, as they did not co-chromatograph with standards and were volatile. The pattern of metabolites in organic extracts differed by sex. Incubation with the glucuronidase and sulfatase mix did not alter the pattern of metabolites, indicating that no conjugates were present in urine. The residues in liver and kidney were extracted mainly (5878%) into an aqueous fraction, with traces (< 4%) in chloroform; 1838% of the radiolabel was unextractable. Erythrocytes showed no residue in the chloroform layer and small amounts in the aqueous layer (6% for females and 14% for males); about 75% of the residue remained in the sample even after acid (6 M HCl) hydrolysis. None of the constituents of the liver, kidney, or erythrocyte residues was identified (Hiles, 1987).
Table 9. Distribution of radiolabel in urine samples from rats given thiodicarb by gavage
|
Fraction |
Compound |
Per cent of urinary activity (mean) |
|
|
Males |
Females |
||
|
Dichloromethane |
Thiodicarb |
0.02 |
0.04 |
|
|
Methomyl |
0.12 |
1.2 |
|
|
Methomyl oxime |
0.32 |
0.91 |
|
|
U1 |
0.16 |
2.2 |
|
|
Methomyl sulfoxide and methomyl oxime sulfoxide |
0.29 |
0.34 |
|
Aqueous |
U2 (solvent front) |
8.9 |
11 |
|
|
U3 |
4.2 |
6.4 |
|
|
U4 |
67 |
67 |
From Hiles (1987)
Cynomolgus monkeys
In samples from the study of Hawkins et al. (1993), extensive metabolic degradation of thiodicarb was observed. During the 48-h period over which volatile compounds were collected, a mean of 28% of the dose was eliminated as carbon dioxide and 8.6% as acetonitrile. More than 18 metabolites were found in the urine, none of which accounted for > 5% of the dose. The metabolites identified corresponded to acetonitrile (1.22.9% of dose), acetic acid (0.40.9% of dose), and acetamide (0.81.0% of dose). No thiodicarb or its major degradation product methomyl was identified. Chromatography with reference standards also confirmed the absence of syn and anti forms of methomyl oxime, methomyl sulfoxide, and methomyl oxime sulfoxide in the urine. After treatment with Escherichia coli beta-glucuronidase, the propotion of radiolabel associated with the major polar component (M1, consisting of six individual compounds) decreased from 12% to 4.2% of the dose. The concentrations associated with eight components increased, including those corresponding to acetic acid (0.52.1% of the dose) and acetonitrile (3.23.8%), indicating some conjugation. Similar changes were not seen after incubation with glucuronidase and sulfatase from H. pomata. Faecal metabolites were not characterized because they contained too little radiolabel.
The major polar metabolites (M1 and M2) were detected in extracts of whole blood at 0.090.16 ΅g equivalents/g and in liver at 0.51.1 ΅g equivalents/g. In liver, a component corresponding to acetic acid (0.120.1 ΅g equivalents/g) was confirmed by both reverse-phase and ion-exchange HPLC; it was not found in blood. None of the radiolabelled components in whole blood or liver corresponded to thiodicarb, the syn or anti forms of methomyl, methomyl oxime, methomyl oxime sulfoxide, acetonitrile, or acetamide (Hawkins et al., 1993).
Goats
Samples from the study of Hanlon & Norris (1993) were investigated by a range of techniques to determine the metabolic profile of [acetimide-14C]thiodicarb in two goats. Urine samples were analysed directly by HPLC and TLC; milk samples were extracted with acetonitrile and hexane; the stomach contents and faeces were extracted with methanol; and selected tissues were extracted with water and then methanol. The resulting solids were treated with protease to liberate additional residues, and fractions were treated subsequently with various enzymes and/or base, as appropriate. Various extracts were analysed by a combination of HPLC and TLC. The gut contents of the two animals contained acetonitrile (15 and 24%), acetamide (5.8 and 7.1%), thiodicarb (6.3 and 5.5%), and methomyl (3.2 and 7.5%). Faeces were found to contain thiodicarb and methomyl as the major radiolabelled residues. In urine, only acetonitrile and acetamide were identified; no thiodicarb or methomyl was found. Between 70 and 91% of the radiolabelled residues was extractable from liver, kidney, and muscle with water. No thiodicarb, methomyl, or methomyl metabolites were found in any of these tissues. Acetonitrile and acetamide were detected in liver, kidney, and muscle; one of the radioactive components was a water-soluble polar compound which was transformed into acetic acid after alkaline hydrolysis (Table 10).
Table 10. Residues in edible tissues of goats as % total 14C-residues (TRR) and ppm thiodicarb equivalents
|
Metabolite |
Liver |
Kidney |
Muscle |
|||
|
|
% TRR |
ppm |
% TRR |
ppm |
% TRR |
ppm |
|
Acetonitrile |
32 |
8.2 |
23 |
2.9 |
72 |
3.1 |
|
Acetamide |
5.9 |
1.5 |
10 |
1.3 |
14 |
0.62 |
|
Acetic acida |
57 |
14 |
43 |
5.4 |
11 |
0.49 |
|
Total |
95 |
24 |
76 |
9.6 |
97 |
4.2 |
From Hanlon & Norris (1993)
a
After alkaline hydrolysis of an unknown polar compoundMost of the recoverable radiolabel was extracted from fat and milk samples with acetonitrile and hexane. The main constituents in fat, although there was considerable inter-animal variation, were saponifiable fatty acids and saponifiable lipids, indicating that radiolabel had been incorporated into normal metabolism. Milk (Table 11) contained low concentrations of radiolabel (< 5 ppm of any individual component) incorporated into natural products (lactose, lipids, and fatty acids) and a significant proportion of acetonitrile (Hanlon & Norris, 1993).
Table 11. Residues in milk of goats as % total 14C-residues (TRR) and ppm thiodicarb equivalents
|
Metabolite |
Goat 1 |
Goat 2 |
||
|
% TRR |
ppm |
% TRR |
ppm |
|
|
Acetonitrile |
29 |
4.1 |
18 |
1.8 |
|
Acetamide |
0.0 |
0.0 |
4.0 |
0.4 |
|
Lactose |
11 |
1.6 |
23 |
2.3 |
|
Acetic acid |
0.0 |
0.0 |
4.0 |
0.41 |
|
Non-saponifiable fatty acids |
0.6 |
0.1 |
0.3 |
0.03 |
|
Saponifiable fatty acids |
18 |
2.6 |
8.2 |
0.8 |
|
Other saponifiable lipids |
14 |
2.6 |
9.7 |
1.0 |
|
Losses |
8.4 |
1.2 |
7.8 |
0.8 |
|
Solids (bound) |
1.0 |
0.1 |
2.5 |
0.2 |
|
Total |
82 |
12 |
77 |
7.9 |
From Hanlon & Norris (1993)
Cows
Samples from the study of Feung et al. (1980; see above) were analysed for metabolites by TLC, infra-red, nuclear magnetic resonance, and gas chromatography after a range of extraction procedures. No parent compound or related carbamates or oximes was found in tissues, milk, or urine. Much of the residue in tissues (4080%) was not readily extractable, and a significant proportion of the extractable residues could not be identified, although they had properties similar to those of lipids and proteins. The main compounds identified in tissues were acetamide and acetonitrile; the ratio varied with tissue, but was typically 1:1. The main compound identified in milk was acetonitrile, the concentration reaching a peak within 7 days in all groups and approaching 1 ppm at the highest dose. The concentration of acetonitrile remained nearly constant throughout the rest of the 21-day treatment period, then declined during the 7-day post-treatment phase. Acetamide was found in trace amounts (0.0l ppm) in the milk of cows at the highest feeding concentration. About 24% of the aqueous extractable urinary radiolabel was identified as acetamide, with a similar amount of acetonitrile (Feung et al., 1980). These results are generally consistent with those of Khasawinah & College (1978). Although the earlier workers reported significant concentrations of acetamide in the milk from a cow given a single dose equivalent to 327 ppm, the findings were not quantified.
Hens
Samples from the study of Andrawes & Bailey (1980; see above) were analysed for metabolites by TLC, gel permeation chromatography, and gasliquid chromatography after a range of extraction procedures. The results for eggs produced during the withdrawal period were not presented. There was no evidence that thiodicarb or related carbamate or oxime compounds were present. Much of the radioactivity was unextractable or unidentifiable, as it was associated with lipids (Table 12). The main residue identified was acetonitrile, with lower concentrations of acetamide (Andrawes & Bailey, 1980).
Table 12. Radiolabelled compounds in liver, breast muscle, and eggs of hens given [acetimide-14C]-thiodicarb at an equivalent of 102 ppm for 21 days (g equivalent/g)
|
Metabolite |
Livera |
Breast musclea |
Eggs (day 21) |
|||||
|
6 h |
3 days |
7 days |
6 h |
3 days |
7 days |
Yolk |
White |
|
|
Acetonitrile |
0.4 |
0.08 |
0.02 |
0.3 |
0.01 |
0.001 |
0.2 |
0.2 |
|
Acetamide |
0.05 |
NC |
NC |
0.01 |
NC |
NC |
NC |
0.03 |
|
Unknown lipids |
2.5 |
0.8 |
0.5 |
0.4 |
0.4 |
0.2 |
10.1 |
NA |
|
Unknown polar compounds |
0.8 |
0.2 |
0.04 |
0.06 |
0.05 |
0.03 |
NA |
NA |
|
Unextractable compounds |
4.7 |
2.0 |
0.2 |
1.3 |
1.4 |
1.0 |
1.6 |
0.4 |
From Andrawes & Bailey (1980); NC, not confirmed; NA, not analysed
a
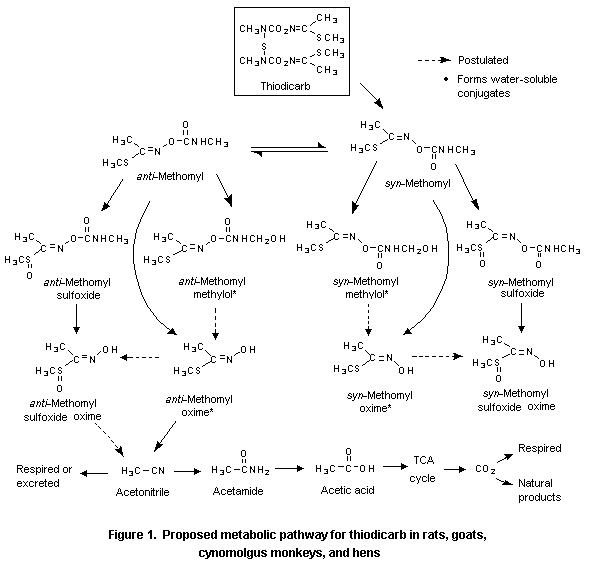
Time after last doseThe results of the very extensive work on goats are consistent with the findings in rats, cynomolgus monkeys, cows, and hens. Orally administered thiodicarb is initially hydrolysed to methomyl, which is then hydrolysed to acetonitrile, acetamide, and acetic acid. Further reactions result in incorporation of the acetyl carbon of thiodicarb into normal metabolism (Figure 1).

(c) Effects on enzymes and other biochemical parameters
Thiodicarb is a rapidly reversible inhibitor of acetylcholinesterase. In a study to determine the times to peak effect and recovery, groups of nine male and nine female Sprague-Dawley rats received a single dose of thiodicarb (purity, 95.1%) at 0, 5, 20, or 40 mg/kg bw in 0.5% methyl cellulose by gavage. Three animals of each sex per group were tested in a limited battery of observational tests for function (FOB) at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, and 24 h. Blood was drawn from the tail vein for determination of cholinesterase activity from three animals of each sex before dosing and 0.5, 1, 2, 4, 6, and 8 h after dosing. No deaths or clinical signs of toxicity were seen other than in the FOB. Reduced body weight was seen between days 1 and 2 in males and females receiving 20 or 40 mg/kg bw. There was considerable variation in the findings of the FOB between animals and over time. Reductions in locomotor activity, degree of arousal, ataxic gait, tremors, lachrymation, and salivation were seen at 0.5 h, but recovery was essentially complete by 3 h. The time to maximal effect varied with the end-point but was generally within 0.51.5 h. Plasma and to a lesser extent erythrocyte cholinesterase activity was inhibited at 5, 20, and 40 mg/kg bw. The peak inhibition occurred before 2 h, followed by recovery and a second phase of reduced activity; this was also seen to a certain extent in controls (Table 13). The study was not designed to determine a NOAEL. It shows that blood samples for determination of cholinesterase activity in studies with thiodicarb should be taken within 2 h of the last dose or feed (Beyrouty, 2000a).
Table 13. Cholinesterase activity in rats given thiodicarb by gavage (as % of pre-dosing values; mean of three animals)
|
Sex |
Dose |
Cholinesterase activity (% of pretreatment value) |
|||||||||||
|
Plasma |
Erythrocytes |
||||||||||||
|
0.5 h |
1 h |
2 h |
4 h |
6 h |
8 h |
0.5 h |
1 h |
2 h |
4 h |
6 h |
8 h |
||
|
Male |
0 |
89 |
94 |
90 |
97 |
89 |
83 |
79 |
108 |
91 |
84 |
88 |
96 |
|
|
5 |
54 |
54 |
61a |
85 |
86 |
84 |
81 |
53 |
99a |
74 |
91 |
65 |
|
|
20 |
48 |
42 |
52 |
93 |
61 |
159b |
72 |
88 |
86 |
73 |
60 |
62 |
|
|
40 |
19 |
25 |
27 |
69 |
38 |
60 |
60 |
72 |
98 |
93 |
67 |
51 |
|
Female |
0 |
96 |
130 |
98 |
116 |
92 |
108 |
117 |
126 |
113 |
111 |
112 |
130a |
|
|
5 |
49 |
76 |
63 |
91 |
87 |
91 |
87 |
101 |
99 |
129 |
91 |
123 |
|
|
20 |
34 |
46 |
37 |
115 |
56 |
95 |
67 |
66 |
86 |
64 |
76 |
67 |
|
|
40 |
29 |
41 |
30 |
65 |
77 |
54 |
67 |
48 |
93 |
67 |
106 |
78 |
From Beyrouty (2000a)
a
Only two animalsb
Only one animalThe acute toxicity of thiodicarb has been investigated in a large number of studies in which compounds of a range of purity and various vehicles were used. After oral administration, females were generally more sensitive than males, and the toxicity of thiodicarb given by this route was greater when an aqueous vehicle was used rather than corn oil. Thiodicarb was moderately toxic after oral administration and was classified as moderately hazardous by WHO. It was also moderately toxic after inhalation but showed little toxicity when applied dermally. The results are summarized in Table 14.
The deaths that occurred were usually within the first few hours after exposure. In a number of studies, the pattern of deaths was such that an LD50 could not be determined for one or both sexes by standard procedures. The clinical signs of toxicity were consistent with inhibition of acetylcholinesterase activity. A common finding at gross post-mortem examination was haemorrhaged or red lungs.
Table 14. Acute toxicity of thiodicarb
|
Material |
Species, strain |
Sex |
Route |
Vehicle |
LD50 or LC50 |
Purity |
Reference |
|
Thiodicarb |
Mouse, OF1 |
Male |
Gavage |
Distilled water |
73 (55-98) |
96 |
Dange (1995) |
|
Female |
79 (62-101) |
|
|||||
|
Larvin technical |
Rat, Wistar |
Male |
Gavage |
Corn oil |
84.1 (61-115) |
95 |
Myers et al. (1982a) |
|
Female |
50.0 (35-72) |
||||||
|
Larvin analytical standard |
Rat, Sprague-Dawley |
Male and female |
Gavage |
Corn oil |
156 (107226) |
99.8 |
Mallory et al. (1982a) |
|
Larvin analytical standard |
Rat, Sprague-Dawley |
Male and female |
Gavage |
Corn oil |
215 (172-269) |
94 |
Mallory et al. (1982b) |
|
Larvin technical pilot plant batch |
Rat, Sprague-Dawley |
Male and female |
Gavage |
Corn oil |
162 (123-214) |
94 |
Mallory et al. (1982c) |
|
Larvin technical |
Rat, Sprague-Dawley |
Male and female |
Gavage |
Methyl cellulose |
93 (73-119) |
92 |
Mallory et al. (1982d) |
|
Larvin analytical standard |
Rat, Sprague-Dawley |
Male and female |
Gavage |
Methyl cellulose |
97 (66-138) |
99.8 |
Mallory et al. (1982e) |
|
Larvin technical laboratory preparation |
Rat, Sprague-Dawley |
Male and female |
Gavage |
Methyl cellulose |
65 (52-81) |
94 |
Mallory et al. (1982f) |
|
Larvin technical pilot plant batch |
Rat, Sprague-Dawley |
Male |
Gavage |
Methyl cellulose |
83 (64-108) |
94 |
Mallory et al. (1982g) |
|
Female |
51 (34-75) |
||||||
|
Larvin analytical standard |
Rat, Wistar |
Male |
Gavage |
Corn oil |
129 (89-186) |
99.8 |
Myers et al. (1982b) |
|
Female |
59 (41-86) |
||||||
|
Larvin analytical standard |
Rat, Wistar |
Male |
Gavage |
Methyl cellulose |
69 (56-84) |
99.8 |
Myers et al. (1982b) |
|
Female |
39 (29-52) |
||||||
|
Larvin technical laboratory preparation |
Rat, Wistar |
Male |
Gavage |
Corn oil |
75 (53-106) |
94 |
Myers et al. (1982c) |
|
Female |
72 (49-105) |
||||||
|
Larvin technical laboratory preparation |
Rat, Wistar |
Male |
Gavage |
Methyl cellulose |
46.5 (33-65) |
94 |
Myers et al. (1982c) |
|
Female |
50.9 (46-56) |
||||||
|
Larvin technical pilot plant batch |
Rat, Wistar |
Male |
Gavage |
Corn oil |
96 (60-154) |
94 |
Myers et al. (1982d) |
|
Female |
57 (4083) |
||||||
|
Larvin technical pilot plant batch |
Rat, Wistar |
Male |
Gavage |
Methyl cellulose |
51 (48-65) |
94 |
Myers et al. (1982d) |
|
Female |
36 (26-60) |
||||||
|
Thiodicarb |
Rat Crl:CD® (SD) IGS BR albino |
Male |
Gavage |
Deionized water |
71 (43-119) |
95.1 |
Kern (1999) |
|
Female |
56 (48-66) |
||||||
|
UC51762 |
Rat |
Male |
Dermal |
Corn oil |
> 640 |
³ 99 |
Field (1979a) |
|
Female |
> 640 |
||||||
|
UC51762 |
Rat, albino |
Male |
Dermal |
Corn oil |
> 1600 |
NR |
Myers et al. (1975) |
|
UC51762 |
Rat, albino |
Male |
Dermal |
Corn oil |
2540 |
³ 99 |
Myers et al. (1979) |
|
Larvin technical |
Rabbit, New Zealand white |
Male |
Dermal |
Saline |
> 6310 |
91.7 |
Lemen et al. (1979) |
|
Female |
> 6310 |
||||||
|
Larvin technical |
Rabbit, New Zealand white |
Male |
Dermal |
Saline |
> 2000 |
92 |
Mallory et al. (1982h) |
|
Female |
> 2000 |
||||||
|
Thiodicarb |
Rabbit, Hra: New Zealand white |
Male |
Dermal |
Water |
> 2000 |
96.6 |
Glaza (1996a) |
|
Female |
> 2000 |
Thiodicarb has been tested for dermal irritancy in rabbits in three studies on both intact and abraded sites. Thiodicarb was not significantly irritating, the maximum response being slight erythema after 24 h of exposure (Wentz & Wolfe, 1979; Mallory et al., 1982i; Glaza, 1996b).
The ocular irritancy of thiodicarb was examined in three studies in rabbits. Thiodicarb produced transient, moderate irritation in rinsed and unrinsed eyes (Cameron & Wolfe, 1979; Mallory et al., 1982j; Glaza, 1996c). A study in cynomolgus monkeys (Macaca fascicularis) resulted in slight conjunctival redness, which had regressed by day 4 (Weatherholtz et al., 1982).
The dermal sensitizing potential of thiodicarb was investigated in three studies in guinea-pigs given topical or intra-dermal applications. Thiodicarb had some skin sensitizing potential (Table 15).
Table 15. Results of studies of dermal sensitization in guinea-pigs exposed to thiodicarb
|
Method |
Animals |
Exposure |
Rest |
Challenge |
Result |
Reference |
|
Modified Landsteiner |
Male Hartley (14/group) |
Intradermal, 9 times in 3 weeks; 0.1 ml as 0.1% solution in saline |
14 days |
1 intradermal injection (0.05 ml) |
Weak response in 6/14 |
Conroy et al. (1979a) |
|
Buehler |
Male Hartley (10/group) |
Topical, 0.5 ml, 9 applications over 3 weeks (24 h each) 50% (vehicle not specified) |
17 days |
Twice within 24 h (1 day in between) |
Negative |
Field (1979b) |
|
Buehler |
Male and female Hartley (5/sex) |
Topical, 0.4 ml (75% in mineral oil), 3 applications within 3 weeks (6 h each) |
14 days |
0.4 ml (6 h), 7 days later: 0.4 ml |
Weak response in 3/10 at second challenge |
Rush (1991) |
A repeated-insult patch test for contact sensitivity was performed in 43 female and eight male volunteers aged 16 to > 60 years. Informed consent was obtained. Technical-grade thiodicarb (purity not specified) was diluted in distilled water to make an approximate 50% slurry. Induction involved nine exposures to 8 mg/24 h over 3 weeks. Reactions to the induction patches were scored 48 or 72 h after each application. After 2 weeks recovery, a challenge was applied on a naive site with 8 mg, and the scores were recorded 48 and 96 h later. No erythema or oedema was observed, indicating that technical-grade thiodicarb is not a sensitizing agent in humans when applied topically (Carabello et al., 1980).
(b) Short-term studies of toxicity
(i) Oral administration
Mice
Groups of 10 male and 10 female ICR-JCL (SPF) mice, 5 weeks of age, were fed diets containing thiodicarb (purity, 92.5%) at a concentration of 0, 30, 100, or 300 ppm, equal to 0, 4.3, 14, and 44 mg/kg bw per day for males and 0, 4.7, 16, and 46 mg/kg bw per day for females, for 4 weeks. Neither haematology nor clinical chemistry was performed. No deaths occurred during the study. Body weight, food consumption, clinical signs, and organ weights (brain, heart, liver, spleen, kidney, testes, and ovaries) were unaffected by treatment. The cholinesterase activity in plasma, erythrocytes, and brain was similar in all groups. Within the limits of the study protocol, the NOAEL was 300 ppm, equal to 44 mg/kg bw per day, the highest dose tested (Yoshida et al., 1983a).
Groups of 10 male and 10 female CD-1 mice received thiodicarb (purity, 96%) in the diet at a concentration of 0, 30, 1750, 3500, or 7000 ppm for 4 weeks. The animals were observed for deaths, clinical signs, body weight, and food consumption. Blood samples for analysis of plasma and erythrocyte cholinesterase activity were taken at week 4. Brain samples were removed at necropsy, rapidly frozen, and assayed for acetylcholinesterase activity. No haematological, clinical chemistry, or histological investigations were performed. The homogeneity and achieved concentrations were acceptable; the actual intakes are given in Table 16. Animals at the highest dose were often found to be thin and pale during the first half of the study, and the body-weight gain and food consumption of these animals were reduced. Increases in the absolute weights of the liver and spleen were seen at concentrations ³ 1750 ppm. Cholinesterase activities were similar in all groups, but the wide variation in control values for brain (2.5-fold) and erythrocytes (8-fold) hinders interpretation. Within the limits of the study protocol, the NOAEL was 30 ppm, equal to 6.2 mg/kg bw per day, on the basis of increased spleen weight at 1750 ppm, equal to 350 mg/kg bw per day (Atkinson et al., 1991).
Table 16. Findings in mice receiving thiodicarb in the diet for 4 weeks (means)
|
End-point |
Dose (ppm) |
|||||||||
|
Males |
Females |
|||||||||
|
0 |
30 |
1750 |
3500 |
7000 |
0 |
30 |
1750 |
3500 |
7000 |
|
|
Actual intake (mg/kg bw per day) |
0 |
6 |
350 |
730 |
1500 |
0 |
8 |
490 |
950 |
2000 |
|
Body weight (g) |
34 |
31 |
34 |
32 |
27* |
26 |
26 |
27 |
26 |
23* |
|
Food consumption (g/week) |
45 |
42 |
43 |
44 |
41 |
46 |
45 |
48 |
46 |
43 |
|
Erythrocyte cholinesterase activity (IU/ml) |
1.9 |
2.2 |
2.8 |
2.6 |
2.2 |
2.4 |
2.8 |
2.2 |
1.7 |
2.1 |
|
Brain cholinesterase activity (IU/mg) |
18 |
18 |
21 |
18 |
18 |
22 |
15 |
18 |
21 |
20 |
|
Spleen weight (g) |
0.12 |
0.11 |
0.15 |
0.16* |
0.18* |
0.16 |
0.18 |
0.21* |
0.25* |
0.22* |
|
Spleen weight corrected for bw (g) |
0.11 |
0.11 |
0.14* |
0.16* |
0.20* |
0.16 |
0.18 |
0.21* |
0.24* |
0.23* |
|
Liver weight (g) |
2.0 |
1.8 |
2.3* |
2.1 |
1.8 |
1.4 |
1.3 |
1.7* |
1.6* |
1.6* |
|
Liver weight corrected for bw (g) |
1.8 |
1.9 |
2.2* |
2.0 |
2.2 |
1.4 |
1.3 |
1.6 |
1.6 |
1.7* |
From Atkinson et al. (1991)
* p < 0.05
Groups of 28 male and 28 female ICR-JCL (SPF) mice were fed diets containing thiodicarb (purity, 92.5%) at a concentration of 0, 30, 150, or 600 ppm, equal to 0, 3.8, 19, and 77 mg/kg bw for males and 0, 4.3, 22,and 85 mg/kg bw for females, for 13 weeks. Deaths, food consumption, body weight, water consumption, and urinary, haematological, and clinical chemical end-points were determined. Cholinesterase activity was determined in plasma, erythrocytes, and brain at the end of the study, as were organ weights and gross and histopathological changes. Brain acetylcholinesterase activity was depressed in females at 150 and 600 ppm and in males at 600 ppm. Females at the highest dose also had significantly increased relative liver weights. No other compound-related effects were reported. Data on individual animals and details of gross and histopathological examinations were not submitted. Within the limits of the study design and reporting, the NOAEL was 30 ppm, equal to 4.3 mg/kg bw per day, on the basis of inhibition of brain acetylcholinesterase activity at 150 ppm (Yoshida et al., 1983b).
Rats
Groups of five male and five female Harlan-Wistar rats were fed diets containing thiodicarb (purity unknown) at a dose of 0, 5, 25, or 100 mg/kg bw per day for 7 days. Slight decreases in body weight and food consumption were reported in males at the highest dose The relative kidney weights were increased in all treated males (Woodside et al., 1975).
Groups of five male and five female Wistar rats were given diets containing thiodicarb (purity, > 99%) at unspecified concentrations, providing doses of 0, 0, 18, 49, and 130 mg/kg bw per day for males and 0, 21, 50, and 130 mg/kg bw per day for females, for 7 days. Observations were made for deaths, food consumption, body weight, and gross changes in the liver and kidney. Body-weight gain was reduced in all treated groups in a dose-related fashion, and body-weight loss was seen during some or all of the dosing period in all groups except males at the low dose. Food consumption was reduced by about 10% in groups at the low dose and by about 50% in animals at the highest dose. Cholinesterase activity and histopatho-logical changes were not examined. No NOAEL could be identified owing to body-weight loss in females at 18 mg/kg bw per day, the lowest dose tested (Woodside et al., 1978).
In a range-finding study, groups of five male and five female Sprague-Dawley rats received thiodicarb (purity, 96%) in the diet at a concentration of 0, 100, 300, 900, or 1800 ppm for 2 weeks. The investigations were limited to deaths, clinical signs, body weight, food consumption, organ weights, gross findings at necropsy, and determinations of cholinesterase activity. Analyses of the diet showed it to be acceptable, and the intakes were 0, 15, 46, 120, and 210 mg/kg bw per day for males and 0, 16, 50, 140, and 210 mg/kg bw per day for females. Body-weight gain was reduced by 1555% in a dose-related manner in animals at 900 and 1800 ppm. Food consumption paralleled body weight. Plasma cholinesterase activity was reduced by > 30% in males receiving concentrations ³ 300 ppm and in females at the highest concentration. Erythrocyte cholinesterase activity was similar in all groups. Brain acetylcholinesterase activity was reduced in females receiving 900 or 1800 ppm (Table 17); although this reduction was not statistically significant, a comparison of individual values indicates a clear effect in some animals. The weight of the spleen relative to body weight was increased markedly at the two higher concentrations and in males receiving 900 ppm. Within the limits of the investigations, the NOAEL was 300 ppm, equal to 46 mg/kg bw per day, on the basis of reduced body weight and inhibition of brain acetylcholinesterase activity at 900 ppm, equal to 120 mg/kg bw per day (Atkinson & Perry, 1991).
Table 17. Findings in rats given diets containing thiodicarb for 2 weeks (means)
|
Finding |
Dose (ppm) |
|||||||||
|
Males |
Females |
|||||||||
|
0 |
100 |
300 |
900 |
1800 |
0 |
100 |
300 |
900 |
1800 |
|
|
Brain cholinesterase activity (IU/mg) |
1.4 |
1.5 |
1.6 |
1.3 |
1.4 |
1.4 |
1.2 |
1.4 |
1.1 |
1.1 |
|
Range |
|
|
|
|
|
1.11.7 |
1.11.3 |
1.31.7 |
0.71.4 |
0.91.4 |
|
Spleen weight (g) |
0.8 |
0.9 |
0.7 |
0.9 |
0.9 |
0.6 |
0.5 |
0.6 |
0.6 |
1.1* |
|
Spleen weight corrected for bw (g) |
0.8 |
0.8 |
0.7 |
0.9 |
1.1 |
0.6 |
0.5 |
0.5 |
0.6 |
1.1* |
From Atkinson & Perry (1991)
* p < 0.05
Groups of 10 male and 10 female Fischer 344 rats received thiodicarb (purity unknown) at various (unspecified) concentrations in the diet for 4 weeks to provide an intake of 0, 1, 3, 10, or 30 mg/kg bw per day for 29 days. Blood samples were taken for determination of erythrocyte and plasma cholinesterase activity on days 8, 15, and 29; brain cholinesterase activity was determined in samples taken at necropsy. The animals were not fasted before sampling, and processing for cholinesterase activity was rapid. There were no deaths or clinical signs. Body-weight gain was reduced significantly from the start of dosing in females receiving 10 mg/kg bw per day and in animals of each sex at 30 mg/kg bw per day, by 15% in males and 50% in females. Food consumption was reduced by about 10% in females receiving 10 or 30 mg/kg bw per day. Brain acetylcholinesterase activity was not inhibited. Plasma and erythrocyte cholinesterase activities were decreased only at the two higher doses, the greatest degree of inhibition being seen at day 8, when the findings were statistically significant (p < 0.05). The erythrocyte cholinesterase activity was 70% of the control value on day 8, 80% on day 15, and 95% on day 29 in males, and 75% of control values on day 8, 80% on day 15, and 90% on day 29 in females. The NOAEL was 3 mg/kg bw per day on the basis of reduced body-weight gain in females at 10 mg/kg bw per day (DePass et al., 1982).
In a preliminary study, groups of five male and five female Fischer 344 rats were given diets containing thiodicarb (purity, 99.8%) at unspecified concentrations to provide an intake of 0, 5, 15, or 45 mg/kg bw per day for 13 weeks. An additional group received 90 mg/kg bw per day for 80 days. The animals were observed for deaths, food and water consumption, organ weights, and erythrocyte, plasma, and brain cholinesterase activity. No haematology or histology was performed. Blood samples were taken for determination of cholinesterase activity by cardiac puncture 24 h after the last feed. At necropsy 24 h after the last feed, the brain was removed, weighed, and assayed for acetylcholinesterase activity. The relative weights of the liver, adrenal gland, and spleen were increased in all treated males, and that of the spleen was increased in females at the two higher doses. Body weights were reduced in a dose-related manner throughout the study in all treated males, but those of females were unaffected by treatment. The only effect on cholinesterase activity was increased activity in erythrocytes in males and females at the high dose, although it is likely that significant reactivation occurred between the last feed and sampling. The reporting and investigations in this study were limited, and no NOAEL could be identified, as effects on liver and spleen weights and body-weight gain were seen in males at 5 mg/kg bw per day, the lowest dose tested (Woodside et al., 1979a).
Groups of 10 male and 10 female Fischer 344 rats were given diets containing thiodicarb (purity, 99%) at unspecified concentrations to provide an intake of 0, 0, 1, 3, 10, or 30 mg/kg bw per day for 13 weeks. The animals were observed for deaths, clinical signs, food and water consumption, body weight, organ weights, haematological, clinical chemical, and urinary end-points, and plasma, erythrocyte, and brain cholinesterase activity, and underwent gross and microscopic examination. Samples for determination of cholinesterase activity were taken after 24 h on control diet, which may have resulted in a significant degree of reactivation. Animals at 1 or 3 mg/kg bw per day were not examined for haematological or clinical chemical end-points. Histological examination was limited to controls and animals at the highest dose. At both 10 and 30 mg/kg bw per day, body weight and water consumption were significantly decreased among females and slightly increased in males. At 30 mg/kg bw per day, the relative weight of the spleen was significantly increased in males, and the relative weight of the kidney was increased in females. Urine volume was increased in males and decreased in females, and the specific gravity of urine was decreased in females at this dose. Other clinical parameters were unaffected by treatment. Slightly (7%) decreased mean haemoglobin concentrations were seen in males at the high dose. Cholinesterase activities were comparable in all groups, although the control values showed wide variation. No remarkable gross or microscopic findings were made. Within the limits of the protocol, the NOAEL was 3 mg/kg bw per day (Homan et al., 1978a).
Groups of 10 male and 10 female 4-week-old Sprague-Dawley rats received diets containing thiodicarb (purity, 96%) at a concentration of 60, 250, or 1200 ppm for 13 weeks. The animals were observed routinely for clinical signs, body weight, and food consumption. During week 13, samples were taken for haematology, clinical chemistry, determination of cholinesterase activity, and urine analysis. All animals underwent gross necropsy; 12 organs were weighed, and a wide range of tissues from controls and animals at the highest dose, the liver, kidney, and lungs from animals at the intermediate and low doses, and the spleens of females at the intermediate and low doses were examined histologically. The homogeneity and achieved dietary intakes were acceptable; the actual intakes are listed in Table 18. There were no deaths or clinical signs of toxicity. Body-weight gain, food consumption, urine volume and pH, and cholinesterase activities were all reduced in animals at the highest dose. Erythrocyte parameters were affected variously in females at the highest dose (Table 18). The serum potassium concentration was reduced in a dose-related manner in females, and this finding is considered to be of potential biological significance, as electrolyte concentrations are normally tightly controlled. The relative weight of the spleen was statistically significantly increased in rats at the highest dose and in males at the low dose. The frequency of pigment deposition in the spleen was increased in animals at the highest dose. The NOAEL was 60 ppm, equal to 6 mg/kg bw per day, on the basis of the dose-related, statistically significant decrease in serum potassium concentration at the next highest dose in females. The increased spleen weight in males at 60 ppm was considered not to be biologically adverse as the effect was not related to dose and was seen in only one sex (Atkinson & Robb, 1992).
Table 18. Findings in rats given diets containing thiodicarb for 13 weeks (means)
|
Finding |
Dose (ppm) |
|||||||
|
Males |
Females |
|||||||
|
0 |
60 |
250 |
1200 |
0 |
60 |
250 |
1200 |
|
|
Intake (mg/kg bw per day) |
0 |
4.5 |
24 |
151 |
0 |
6.5 |
21 |
140 |
|
Body-weight gain (g) |
380 |
410 |
3600 |
250* |
190 |
190 |
200 |
150* |
|
Erythrocyte count (1012/L) |
8.3 |
8.3 |
8.4 |
8.0 |
7.8 |
8.0 |
7.6 |
6.8* |
|
Erythrocyte volume fraction (%) |
42 |
41 |
42 |
41 |
40 |
42 |
39 |
37* |
|
Haemoglobin (g/dL) |
15 |
15 |
15 |
15 |
15 |
16 |
14 |
14* |
|
Mean corpuscular volume (fL) |
50 |
50 |
50 |
52 |
52 |
52 |
52 |
54* |
|
Erythrocyte cholinesterase activity (IU/ml) |
300 |
240 |
260 |
240 |
420 |
370 |
350 |
340 |
|
Brain cholinesterase activity (IU/mg) |
1.4 |
1.4 |
1.4 |
1.1* |
1.4 |
1.3 |
1.3 |
1.1* |
|
Serum potassium (mmol/L) |
4.1 |
4.2 |
4.0 |
4.0 |
4.1 |
3.9 |
3.6* |
3.5* |
|
Spleen weight (corrected for bw; g) |
0.84 |
0.99* |
0.90 |
1.1* |
0.63 |
0.64 |
0.67 |
0.86* |
|
Liver cytoplasmic rarefaction |
8/10 |
9/10 |
9/10 |
2/10* |
6/9 |
6/10 |
8/10 |
3/10 |
|
Spleen, brown pigment |
2/10 |
|
|
5/10 |
1/9 |
1/10 |
2/10 |
7/10* |
From Atkinson & Robb (1992)
* p < 0.05
Dogs
Groups of four male and four female 9-month-old beagles were fed diets containing thiodicarb (purity, 99.8%) at a concentration of 0, 400500, 13001800, or 25003500 ppm for 13 weeks to provide targeted doses of 0, 15, 45, and 90 mg/kg bw per day. Owing to a high mortality rate at the highest dose, it was reduced to 76 mg/kg bw per day for females and 86 mg/kg bw per day for males. The animals were examined routinely for physical and behavioural changes, food and water consumption, changes in body weight, and haematological, (limited) clinical chemical, and urinary parameters. Organ weights and gross and histological changes (excluding nerves) were determined at necropsy. Plasma and erythrocyte cholinesterase activity was determined twice before treatment and on days 28, 63, 79, and 93 of the study. Brain acetylcholinesterase activity was determined at sacrifice. The time between last exposure and sampling for cholinesterase activity was not reported.
The homogeneity, stability, and achieved intakes from the diets were not reported. Two females at the high dose died after 26 and 36 doses, and dose-related signs including anorexia, vomiting, and loose stools were observed in animals at the intermediate and high doses. Cholinesterase activity was not markedly affected at any time during the study; a 1015% reduction in brain acetylcholinesterase activity in all groups of treated males did not show a doseresponse relationship and was within the range of variation in controls. Food consumption was decreased by 20% in females at the intermediate dose and 35% in those at the high dose. Decreased body weight was seen in females at these two doses and in males at the highest dose. Water consumption was increased in animals at the intermediate and high doses, with a doubling in males. Urine volume was increased in males at the intermediate and high doses and in females at the high dose. Decreases in the erythrocytes count, packed cell volume, and haemoglobin were seen in animals at the intermediate and high doses, while the reticulocyte counts and mean corpuscular volume were increased in animals at the high dose. These changes, with bone-marrow hyperplasia and congested spleens seen at the high dose, suggest macrocytic anaemia and compensatory haematopoiesis. The relative weight of the liver was increased by 1520%, and serum alanine aminotransferase activity was elevated by fourfold in males and 10-fold in females at the high dose. Males at this dose had increased incidences of splenic congestion, hepatocellular vacuolation, and pigmented Kupffer cells. Two animals in each treated group had focal, diffuse inflammation of the liver, with no doseresponse relationship, while the incidence in controls was zero. In the absence of other findings and the absence of a doseresponse relationship, the hepatic lesions seen at the lowest dose were considered not to be adverse. The NOAEL was 15 mg/kg bw per day on the basis of clinical signs and alterations in erythrocyte parameters at higher doses (Homan et al., 1978b).
Groups of six male and six female beagles, 67 months old, were given diets containing thiodicarb (two batches; purity, 99.8% and > 95%) at variable concentrations (not specified) to provide targeted doses of 0, 5, 15, and 45 mg/kg bw per day, for 26 weeks. The animals were observed routinely for survival, clinical appearance, food consumption, ophthalmic end-points, body weight, haematological end-points (mean corpuscular volume not determined), and clinical chemical and urinary parameters. Organ weights and gross and microscopic lesions were determined at terminal sacrifice. Cholinesterase activity was determined twice before treatment and in samples from fasted and non-fasted animals during weeks 8, 17, and 26. Brain cholinesterase activity was determined at sacrifice.
The homogeneity and the achieved dietary levels were not reported. Thiodicarb did not significantly affect body weight, food consumption, urinary parameters, or the gross or microscopic appearance of tissues. Males, but not females, showed a dose-related increase in the combined incidence of soft stools, mucoid stools, and diarrhoea. The absolute and relative weights of the liver were increased in males at the highest dose, but no histopathological correlates were found. The treatment-related changes in haematological end-points included significantly decreased erythrocyte volume fraction, haemoglobin, and erythrocyte count in males at the high dose and in females at the two higher doses (Table 19). Dogs receiving the highest dose had elevated serum alanine aminotransferase and plasma and erythrocyte cholinesterase activity at week 26 and decreased concentrations of total protein and globulin, with a correspondingly increased albumin:globulin ratio. There were no notable differences in cholinesterase activity in samples from fasted and non-fasted animals The NOAEL was 5 mg/kg bw per day on the basis of altered erythrocyte parameters at 15 mg/kg bw per day and above (Wolfe, 1981).
Table 19. Findings in dogs given thiodicarb in the diet for 26 weeks (means)
|
Finding |
Dose (mg/kg bw per day) |
|||||||
|
Males |
Females |
|||||||
|
0 |
5 |
15 |
45 |
0 |
5 |
15 |
45 |
|
|
Before treatment |
||||||||
|
Erythrocyte cholinesterase activity (IU/ml) |
8.8 |
7.3 |
7.3 |
8.1 |
7.5 |
8.7 |
9.5 |
9.8 |
|
Erythrocyte count (1012/ L) |
6.3 |
6.1 |
6.1 |
6.3 |
6.1 |
6.1 |
5.8 |
6.0 |
|
Haematocrit (%) |
45 |
46 |
44 |
46 |
46 |
48 |
45 |
45 |
|
Week 8 |
||||||||
|
Erythrocyte cholinesterase avtivity (IU/ml) |
9.7 |
9.8 |
11 |
9.1 |
9.6 |
12 |
9.6 |
11 |
|
Erythrocyte count (1012/ L) |
6.8 |
6.7 |
6.8 |
5.8 |
7.1 |
7.3 |
6.3* |
6.1* |
|
Haematocrit (%) |
44 |
45 |
44 |
42 |
48 |
49 |
44 |
44 |
|
Week 13 |
||||||||
|
Erythrocyte count (1012/ L) |
6.9 |
7.0 |
7.1 |
6.0 |
7.2 |
7.2 |
6.4* |
6.4 |
|
Erythrocyte volume fraction (%) |
45 |
47 |
46 |
42 |
49 |
49 |
44 |
46 |
|
Haemoglobin (g/dL) |
15 |
16 |
16 |
14 |
16 |
17 |
15 |
16 |
|
Serum alanine aminotransferase activity (IU/L) |
17 |
20 |
19 |
87 |
16 |
13 |
16 |
45 |
|
Week 26 |
||||||||
|
Erythrocyte cholinesterase activity (IU/ml) |
9.6 |
8.0 |
9.3 |
11 |
7.8 |
9.7 |
11 |
12* |
|
Erythrocyte count (1012/ L) |
7.4 |
7.2 |
7.3 |
6.1* |
7.3 |
7.7 |
6.9 |
6.5 |
|
Haematocrit (%) |
50 |
50 |
50 |
44* |
51 |
53 |
48 |
47 |
|
Haemoglobin (g/dL) |
17 |
17 |
17 |
15* |
17 |
18 |
16 |
16 |
|
Serum alanine aminotransferase activity (IU/L) |
|
|
20 |
89 |
19 |
17 |
18 |
46 |
|
Brain cholinesterase activity (IU/g) |
7.8 |
8.0 |
8.1 |
7.0 |
8.1 |
8.3 |
9.0 |
8.6 |
|
Total protein (mg/ml) |
6.2 |
6.2 |
6.1 |
5.6* |
6.1 |
6.1 |
6.2 |
6.1 |
|
Albumin:globulin ratio |
1.1 |
1.0 |
1.0 |
1.2 |
1.0 |
1.1 |
1.0 |
1.3 |
|
Liver weight (g) |
230 |
260 |
260 |
270 |
210 |
190 |
200 |
220 |
|
Liver weight (% bw) |
2.2 |
2.3 |
2.4 |
2.5 |
2.4 |
2.3 |
2.5 |
2.7 |
From Wolfe (1981); , archive page illegible, typical control value about 22
Groups of six male and six female beagle dogs aged 2933 weeks were given diets containing thiodicarb (purity, 91.6%) at a concentration of 0, 160, 490, or 1500 ppm, equal to 4.4, 13, and 38 mg/kg bw per day for males and 4.5, 14, and 40 mg/kg bw per day for females, for 1 year. The observations included deaths, body weight, appearance and behaviour, and food and compound consumption. Clinical chemical (before treatment and at weeks 13, 26, and 52) and ophthalmic end-points, gross findings at necropsy, organ weights, and detailed histological appearance were determined. Blood samples for measurement of erythrocyte and plasma cholinesterase activity were taken from non-fasted animals on three occasions before treatment and at weeks 5, 13, 26, and 52. Brain acetylcholinesterase activity was assayed in samples obtained at termination.
The homogeneity and achieved incorporation were acceptable. No effects were seen on mortality rate, body-weight gain, urinary parameters, or water consumption. No consistent clinical signs were seen, but tremors occurred in one male and one female receiving the highest dose. Food consumption was reduced by < 10% in males at the beginning of the study and occasionally in females at the highest dose. Slight decreases in erythrocyte count, erythrocyte volume fraction, and haemoglobin were seen in animals at the high dose at all three observation times (Table 20). Plasma, erythrocyte, and brain cholinesterase activity was significantly inhibited (> 25%) in animals at the high dose, and animals at the intermediate dose had significant reductions in erythrocyte cholinesterase activity at week 13. Supplementary investigations showed that the time to peak inhibition of erythrocyte cholinesterase activity was 2 h, indicating that sampling at week 52 (3.5 h after feeding) was less than optimal. No changes were seen that would suggest a compound-related effect on any other measured clinical parameter. The weights of the liver and spleen in treated females were increased in a dose-related manner when compared with controls, and increases were also seen in the organ:body, and organ:brain weight ratios in females, with statistical significance only at the high dose. Such changes were not seen in males. No effects were observed on ophthalmic end-points or gross or histological appearance. As thiodicarb is a carbamate, the Meeting assumed that the inhibition of cholinesterase activity would not worsen with duration of dosing and that the late sampling at week 52 therefore did not compromise the study significantly. The NOAEL was 160 ppm, equal to 4.4 mg/kg bw per day, on the basis of inhibition of erythrocyte cholinesterase activity at 490 ppm (Hamada et al., 1986).
Table 20. Findings in dogs given thiodicarb in the diet for 52 weeks (means)
|
Finding |
Dose (ppm) |
|||||||
|
Males |
Females |
|||||||
|
0 |
160 |
490 |
1500 |
0 |
160 |
490 |
1500 |
|
|
Week 6 |
||||||||
|
Erythrocyte cholinesterase activity (΅mol/ml) |
10 |
9.6 |
9.9 |
11 |
9.8 |
10 |
9.8 |
9.7 |
|
Erythrocyte count (1012/L) |
6.9 |
6.3 |
6.5 |
6.5 |
7.2 |
7.4 |
7.3 |
7.4 |
|
Week 5 |
||||||||
|
Erythrocyte cholinesterase activity (΅mol/ml) |
7.6 |
7.7 |
6.7 |
5.1 |
8.7 |
8.1 |
7.0 |
5.1* |
|
Week 13 |
||||||||
|
Body weight (kg) |
9.9 |
9.9 |
9.7 |
10 |
8.8 |
8.9 |
9.3 |
8.8 |
|
Erythrocyte count (1012/L) |
7.0 |
6.3 |
6.4 |
5.7* |
6.3 |
6.6 |
6.6 |
5.6 |
|
Erythrocyte volume fraction (%) |
46 |
42 |
43 |
39 |
41 |
44 |
45 |
39 |
|
Haemoglobin (g/dL) |
16 |
14 |
15 |
13 |
14 |
15 |
16 |
13 |
|
Erythrocyte cholinesterase activity (΅mol/ml) |
8.9 |
7.7 |
6.5 |
4.9* |
9.3 |
8.9 |
6.7* |
5.3* |
|
Week 26 |
||||||||
|
Erythrocyte count (1012/L) |
6.9 |
6.6 |
6.4 |
5.8* |
6.5 |
6.1 |
6.3 |
5.7 |
|
Erythrocyte volume fraction (%) |
45 |
43 |
43 |
40 |
43 |
41 |
42 |
39 |
|
Haemoglobin (g/dL) |
15 |
15 |
15 |
13* |
15 |
14 |
15 |
14 |
|
Erythrocyte cholinesterase activity (΅mol/ml) |
8.2 |
7.8 |
7.7 |
6.5 |
9.3 |
8.0 |
7.6 |
6.3* |
|
Week 52 |
||||||||
|
Body weight (kg) |
9.8 |
10 |
10 |
10 |
9.4 |
9.4 |
10 |
9.4 |
|
Erythrocyte count (1012/L) |
6.9 |
6.7 |
6.4 |
6.1 |
7.0 |
6.5 |
6.4 |
5.7 |
|
Erythrocyte volume fraction (%) |
45 |
44 |
42 |
41 |
46 |
43 |
43 |
39 |
|
Haemoglobin (g/dL) |
16 |
15 |
15 |
14 |
16 |
15 |
15 |
13 |
|
Erythrocyte cholinesterase activity (΅mol/ml) |
9.0 |
8.7 |
8.2 |
7.4 |
9.2 |
9.7 |
9.1 |
9.4 |
|
Brain cholinesterase activity (΅mol/g) |
7.5 |
8.0 |
7.2 |
5.1* |
8.2 |
8.7 |
8.0 |
5.9* |
|
Spleen weight (g) |
22 |
23 |
22 |
24 |
21 |
24 |
26 |
31 |
|
Spleen weight (% bw) |
0.22 |
0.22 |
0.22 |
0.24 |
0.21 |
0.26 |
0.26 |
0.33* |
|
Liver weight (g) |
240 |
260 |
240 |
250 |
240 |
260 |
290 |
290 |
|
Liver weight (% bw) |
2.4 |
2.5 |
2.4 |
2.5 |
2.5 |
2.8 |
2.9 |
3.1* |
|
Spleen, pigment |
4/6 |
6/6 |
5/6 |
5/6 |
6/6 |
6/6 |
5/6 |
6/6 |
From Hamada et al. (1986)
(ii) Dermal application
Groups of five male New Zealand white rabbits were given dermal applications of thiodicarb (purity, 99%) as a paste in 0.9% saline at 1000 or 4000 mg/kg bw per day on 5 days/week for 3 weeks. The application site, which represented 10% of the body area, was abraded, covered, and kept occluded for 6 h before being washed. The investigations were limited to clinical signs, body weight, food consumption, gross and histological changes at necropsy (major organs only, excluding nerves), and haematological and cholinesterase activity determinations. Samples for measurement of cholinesterase activity were taken 24 h after the last application and were thus of minimal value. The death of a single rabbit at 4000 mg/kg bw per day was attributed to infection. The incidences of soft stools and diarrhoea were increased in both treated groups after approximately five applications. Body-weight gain and food consumption were significantly decreased in animals at 4000 mg/kg bw per day. At this dose, the erythrocyte count was decreased by about 20% and the haemoglobin concentration was reduced; the reticulocyte count and mean corpuscular volume were increased but were stated to be within the normal range. Erythrocyte and plasma cholinesterase activities were similar in all groups, but brain acetylcholinesterase activity was inhibited by about 25% in both treated groups, although there was considerable intra-group variation. No treatment-related changes in gross or histological appearance were found. No NOAEL could be identified because brain acetylcholinesterase activity was inhibited at both doses (Conroy et al., 1979b).
Groups of five male and five female New Zealand white rabbits received dermal applications of thiodicarb (purity, 97.4100%) as a paste in 0.9% saline at a dose of 0, 1000, 2000, or 4000 mg/kg bw per day on 5 days/week for 3 weeks. The application site was abraded in four groups and unabraded in the remaining four groups and was kept occluded for 6 h before being washed. Clinical signs, body weight, food consumption, gross and hostological findings at necropsy, and haematological and clinical chemical parameters were measured. Cholinesterase activity was not assayed, and the reporting of findings was limited. Eleven deaths occurred, spread evenly over the groups. Body-weight gain and food consumption were similar in all groups. The frequency of diarrhoea and abdominal distension was increased in all treated groups after week 2. Evidence of macrocytic anaemia was found in all treated groups, with decreased erythrocyte counts (1530%), erythrocyte volume fraction, and haemoglobin and increased mean corpuscular haemoglobin concentration and mean corpuscular volume. The cholesterol concentration doubled in animals at 4000 mg/kg bw per day, and there was a dose-related increase in potassium concentration (1227%) at all three doses. No treatment-related changes were found at gross or histological examination, although the weight of the liver was increased by 35% at the highest dose. No NOAEL could be identified because cholinesterase activity was not determined and evidence of macrocytic anaemia and increased potassium concentration at all doses (Gallo & Stevens, 1980).
Groups of five male and five female New Zealand white rabbits received dermal applications of thiodicarb (purity, > 99%) as a paste in 0.9% saline at a dose of 0, 250, 500, 1000, or 2000 mg/kg bw per day on 5 days/week for 3 weeks. The application site was abraded in four groups and unabraded in the remaining groups and was kept occluded for 6 h before being washed. Clinical signs, body weight, food consumption, gross and histological changes at necropsy, and haematological and clinical chemical changes were observed. Cholinesterase activity was not assayed. There were no deaths. Body-weight gain, clinical signs, haematological, clinical chemical, and urinary end-points, and food consumption were similar in all groups. There were no treatment-related gross or histological changes. The NOAEL was 2000 mg/kg bw per day, but the lack of determination of cholinesterase activity is a serious omission (Schardein, 1982).
(iii) Inhalation
Groups of 10 male and 10 female Sprague-Dawley rats were exposed nine times to fine dusts containing thiodicarb (purity unspecified) for 6 h/day, 5 days/week. The atmospheres were respirable (mass median aerodynamic diameter, < 2 ΅m) and the mean gravimetric concentrations were 0, 5, 18, and 54 mg/m3. The animals were observed for signs of toxicity, body weight, and food consumption. At sacrifice on the morning after the last exposure, they were examined grossly; lung, liver, kidney, and brain were removed for weighing, and samples of blood and brain were taken for determination of cholinesterase activity. At the highest level of exposure, signs typical of cholinergic effects were seen, comprising tremors, pin-point pupils, lachrymation, diarrhoea, and abnormal righting reflex and response to stimulus. A similar but less marked range of signs was observed at 18 mg/m3. At 5 mg/m3, slight tremors and pin-point pupils were seen consistently in animals of each sex. Significant inhibition of erythrocytes cholinesterase activity (20%) and of brain acetylcholinesterase activity (30%) was seen at 54 mg/m3 but not at lower concentrations. Given the presence of clinical signs and the time between the last exposure and sampling for cholinesterase activity, it is likely that considerable reactivation had occurred. No NOAEL could be identified, as clinical signs of toxicity were seen at 5 mg/m3, the lowest concentration tested (Dickey et al., 1979).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 80 CD-l mice of each sex aged 42 days were given diets containing thiodicarb (analytical grade; purity, > 99%) for up to 24 months at a concentration of 0 (two control groups), 412, 1234, or 42106 ppm to provide intakes of 0, 1, 3, and 10 mg/kg bw per day. The mice were observed for deaths, adverse physical and behavioural condition, food consumption, and body weight. Twenty mice of each sex per group were killed at week 80 and the surviving animals at the end of the study at 105 weeks. The observations comprised organ weights and gross and microscopic examination of tissues from all animals. Clinical chemistry, haematology, and determination of cholinesterase activity were not done.
The homogeneity, stability, achieved incorporation, and clinical signs were reported. The mortality rate varied considerably among groups; for animals at the high dose, it was increased over the control rate in females during the last 6 months of the study and in males during months 17 and 18. More than 50% of animals in all groups were still alive at 19 months. Body weights and food consumption were not affected by treatment. The treated and control groups did not differ significantly in the incidences of neoplasms or non-neoplastic lesions. The NOAEL was 3 mg/kg bw per day on the basis of an increased mortality rate in males and females at 10 mg/kg bw per day (Woodside et al., 1980a). The description was taken partially from the 1985 JMPR monograph, as the full pathology report was not available.
Groups of 65 CD-1 mice of each sex received diets containing thiodicarb (purity, 96%) at a concentration of 0, 1636, 236482, or 34176032 ppm to give intakes of 0, 5, 70, and 1000 mg/kg bw per day. Satellite groups consisting of 15 animals of each sex were killed at 52 weeks and examined grossly and in limited histological examinations. The main (carcinogenicity) study was planned to last 104 weeks but was terminated at 97 weeks owing to the poor survival in females at the highest dose. All animals were examined for clinical signs, body weight, and food and water consumption. Limited clinical chemical parameters and haematological end-points were determined for 10 animals from each group after 52 weeks. Differential blood cell counts were made after approximately 52, 77, and 96 weeks for all surviving animals in the main groups. Full histological evaluations were performed on all decedent and all control and high-dose animals in the carcinogenicity study; additionally, liver, kidney, lung, and spleen from surviving animals at the low and intermediate doses were examined. Organ weights were recorded for animals at interim sacrifice and for 10 of each sex per group at termination. Cholinesterase activity was not investigated, as no inhibition was found in a range-finding study at doses up to 7500 ppm.
The achieved doses and homogeneity were acceptable. The survival rate was reduced markedly in animals at the highest dose (Table 21), although there were no clinical signs associated with treatment. Body-weight gain was reduced at the highest dose, in males from the start of dosing and in females after about 10 months. Food and water consumption were similar in all groups. Increased leukocyte counts and altered erythrocyte parameters were seen in animals at the highest dose at week 52. The differential counts at weeks 77 and 96 were unremarkable, but the absolute numbers of cells were not given. Increases serum alanine aminotransferase activity and serum bilirubin concentration were seen in animals at the highest dose. Marked increases in the absolute and relative weights of the liver and spleen were seen in the groups at the highest dose at 52 and 97 weeks and to a lesser extent in females at the intermediate dose at 97 weeks; kidney weights were also increased in these females.
Table 21. Findings in mice given thiodicarb in the diet for 97 weeks (means)
|
Finding |
Dose (mg/kg bw per day) |
|||||||
|
Males |
Females |
|||||||
|
0 |
5 |
70 |
1000 |
0 |
5 |
70 |
1000 |
|
|
Survival at 97 weeks (%) |
66 |
60 |
63 |
51 |
60 |
72 |
65 |
37* |
|
Body weight at 52 weeks (g) |
44 |
45 |
44 |
37* |
36 |
36 |
37 |
35 |
|
Erythrocyte count (1012/L) |
9.2 |
9.1 |
8.9 |
5.8* |
8.9 |
8.9 |
8.9 |
7.0 |
|
Erythrocyte volume fraction (%) |
0.43 |
0.43 |
0.42 |
0.32* |
0.43 |
0.43 |
0.42 |
0.37* |
|
Haemoglobin concentration (g/dL) |
14 |
14 |
14 |
10* |
14 |
14 |
14 |
13* |
|
Mean corpuscular volume (fL) |
47 |
48 |
47 |
55 |
49 |
48 |
48 |
54 |
|
Leukocyte count |
5.5 |
5.5 |
5.6 |
7.7* |
4.0 |
4.2 |
3.8 |
5.0 |
|
Alanine aminotransferase activity (IU/L) |
55 |
50 |
68 |
79 |
41 |
49 |
53 |
95* |
|
Total bilirubin concentration (΅mol/L) |
1.8 |
1.7 |
2.0 |
5.3* |
2.1 |
1.9 |
2.4 |
3.3* |
|
Liver weight (week 52; g) |
2.3 |
2.2 |
2.4 |
2.9* |
1.8 |
1.8 |
1.9 |
2.7* |
|
Liver weight (week 97; g) |
2.3 |
2.2 |
2.1 |
3.8* |
1.7 |
1.8 |
2.1* |
4.1* |
|
Spleen weight (week 52; g) |
0.1 |
0.1 |
0.1 |
0.4* |
0.1 |
0.1 |
0.1 |
0.4* |
|
Spleen weight (week 97; g) |
0.1 |
0.1 |
0.1 |
0.4* |
0.1 |
0.1 |
0.2* |
0.4* |
|
Kidney weight (week 52; g) |
0.8 |
0.8 |
0.8 |
0.7* |
0.5 |
0.5 |
0.5 |
0.6* |
|
Kidney weight (week 97; g) |
0.8 |
0.8 |
0.8 |
0.7 |
0.5 |
0.5 |
0.5 |
0.7* |
From Atkinson et al. (1993) and Rieth (1995a)
* p < 0.05
Necropsy of animals killed after 52 or 97 weeks of dosing showed higher incidences of lesions in the liver and spleen in males and females at the highest dose (Table 22). Histological changes were seen in the spleen and liver at 52 weeks. At week 97, altered histological appearance was noted in a number of major organs in these animals and in the spleen, liver, and sternum of animals at the intermediate dose. Males and females at the highest dose had increased incidences of hepatocellular adenomas and carcinomas at week 97, and males also had an increased incidence of hepatocellular adenomas at week 52. The total tumour incidence was increased in both males and females at the highest dose. No statistically significant increase in tumour incidence was seen at 5 or 70 mg/kg bw per day, although the hepatocellular adenoma incidence was slightly increased in males receiving 70 mg/kg bw per day to a level (22%) which is marginally above the historical control range (018%).
Table 22. Incidences of gross and histological findings in mice given thiodicarb in the diet for 52 or 97 weeks (means)
|
Finding |
Dose (mg/kg bw per day) |
|||||||
|
Males |
Females |
|||||||
|
0 |
5 |
70 |
1000 |
0 |
5 |
70 |
1000 |
|
|
Week 52 |
||||||||
|
Hepatocellular hypertrophy |
0/13 |
0/10 |
0/12 |
11/11* |
0/12 |
0/11 |
0/13 |
11/11* |
|
Liver, single-cell necrosis |
0/13 |
0/10 |
1/12 |
10/11* |
0/12 |
0/11 |
0/13 |
7/11* |
|
Hepatocellular adenoma |
1/13 |
0/10 |
1/12 |
5/11* |
0/12 |
0/11 |
0/13 |
1/11 |
|
Spleen, haemosiderosis |
0/13 |
0/10 |
0/12 |
10/11* |
0/12 |
0/11 |
1/13 |
11/11* |
|
Spleen, extramedullary haematopoiesis |
0/13 |
2/10 |
1/12 |
11/11* |
0/12 |
0/11 |
0/13 |
11/11* |
|
Total to week 97 |
||||||||
|
Liver masses |
7/50 |
5/50 |
15/50* |
37/50* |
1/50 |
2/50 |
3/50 |
29/50* |
|
Lymph nodes, enlarged |
6/50 |
5/50 |
6/50 |
14/50* |
6/50 |
2/50 |
3/50 |
12/50 |
|
Seminal vesicles, enlarged horns |
21/50 |
18/50 |
26/50 |
2/50* |
|
|
|
|
|
Spleen, enlarged |
8/50 |
5/50 |
4/50 |
28/50* |
9/50 |
4/50 |
15/50 |
19/50* |
|
Uterine horn(s), enlarged |
|
|
|
|
21/50 |
24/50 |
18/50 |
9/50* |
|
Adrenal hyperplasia |
13/49 |
5/22 |
4/22 |
2/50* |
25/49 |
5/14 |
13/28 |
19/50 |
|
Cardiomyopathy |
20/50 |
10/23 |
12/22 |
33/50* |
9/50 |
4/15 |
13/28 |
15/50 |
|
Kidney, mineral deposits (cortical/pupillary) |
4/50 |
5/50 |
8/50 |
21/50* |
1/50 |
6/50 |
2/50 |
19/50* |
|
Nephropathy ( ³ 3) |
2/50 |
1/50 |
3/50 |
12/50* |
12/50 |
6/50 |
8/50 |
6/50* |
|
Kidney, papillary oedema |
0/50 |
0/50 |
1/50 |
29/50* |
1/50 |
4/50 |
0/50 |
12/50* |
|
Urothelial hyperplasia |
0/50 |
1/50 |
4/50 |
20/50* |
0/50 |
0/50 |
0/50 |
11/50* |
|
Hepatocyte pleiomorphism |
5/50 |
10/50 |
13/50* |
29/50* |
2/50 |
3/50 |
1/50 |
30/50* |
|
Bile-duct hyperplasia |
2/50 |
1/50 |
1/50 |
12/50* |
0/50 |
1/50 |
0/50 |
13/50* |
|
Liver, pigmented macrophages |
0/50 |
0/50 |
1/50 |
48/50* |
4/50 |
3/50 |
1/50 |
46/50* |
|
Spleen, haemosiderosis |
0/50 |
3/50 |
5/50* |
36/50* |
5/50 |
3/50 |
4/50 |
31/50* |
|
Spleen, extramedullary haematopoiesis |
14/50 |
14/50 |
21/50 |
41/50* |
15/50 |
16/50 |
31/50* |
38/50* |
|
Spleen, white pulp depletion |
5/50 |
1/50 |
3/50 |
20/50* |
4/50 |
0/50 |
4/50 |
12/50* |
|
Sternum, increased haematopoiesis |
10/50 |
4/23 |
2/22 |
4/50 |
1/50 |
2/15 |
8/15* |
7/15* |
|
Thymic atrophy |
3/46 |
5/20 |
1/16 |
12/40* |
5/46 |
1/14 |
2/24 |
5/49 |
|
Hepatocellular carcinoma |
2/50 |
0/50 |
3/50 |
14/50* |
0/50 |
0/50 |
1/50 |
8/50* |
|
Hepatocellular adenoma |
6/50 |
7/50 |
11/50 |
23/50* |
1/50 |
1/50 |
2/50 |
20/50* |
|
Animals with tumours |
25/50 |
|
|
43/50* |
32/50 |
|
|
42/50* |
|
Animals with malignant tumours |
8/50 |
|
|
24/50* |
20/50 |
|
|
27/50 |
From Atkinson et al. (1993); Rieth (1995a)
* p < 0.05
Limited examination
The liver tumours seen in this study were considered to be a result of a prolonged exposure to high concentrations of a xenobiotic. The only dose that resulted in a statistically significant increase in the incidence of tumours, 1000 mg/kg bw per day, is considered to have exceeded the maximum tolerated dose for the following reasons:
The NOAEL was 5 mg/kg bw per day on the basis of increases in splenic extramedullary haematopoiesis and haemosiderosis, liver weights, liver masses, and hepatocellular pleiomorphism in animals receiving 70 mg/kg bw per day (Atkinson et al., 1993; Rieth, 1995a).
Rats
Groups of 15 male and 15 female Sprague Dawley rats received diets containing thiodicarb (purity, 96%) at a concentration of 0, 60, 200, or 900 ppm, equal to of 0, 4, 14, and 70 mg/kg bw per day for males and 5, 19, and 97 mg/kg bw per day for females, for 52 weeks. Clinical chemistry, haematology, urine, and cholinesterase activity were measured in 10 animals per group at weeks 25 and 51. Animals were not fasted before sampling. Ophthalmoscopy was performed before treatment and at week 51. All animals underwent gross necropsy. Extensive histological examinations were performed on all control and high-dose animals, and liver, lung, kidneys, and spleen from animals at the intermediate and low doses were also examined. Homogeneity and the achieved levels of incorporation were acceptable.
There were no deaths, clinical signs, ophthalmic changes, or gross findings at necropsy related to treatment. Body-weight gain was reduced by up to 30% from the beginning of the study in animals at 900 ppm, despite a marginal increase in food consumption. Altered erythrocyte parameters and increased total serum bilirubin concentration were seen in animals at the highest dose at 25 and 51 weeks; the total serum bilirubin concentration was also increased in females at the intermediate dose at 51 weeks (Table 23). Erythrocyte cholinesterase activity was reduced in males at the highest dose; brain acetylcholinesterase activity was similar in all groups. Urine pH was reduced in animals at the highest dose at both 25 and 52 weeks; variations in specific gravity were generally linked to changes in volume and were considered not to indicate impaired renal function. The absolute and relative weights of the spleen were increased in females at the highest and intermediate doses. Histological examination showed the spleen to be the main target organ, with increased haemosiderosis and extramedullary haematopoiesis at all doses in females, although with no clear doseresponse relationship, and in males at the highest dose. The frequencies of thymic and adrenal lesions were also increased in females at the highest dose. The LOAEL was 60 ppm, equal to 5 mg/kg bw per day, because of the increased incidences of haemosiderosis and extramedullary haematopoiesis in females at all doses. These findings were consistent with the effects of thiodicarb on erythrocytes seen in a number of studies but not at 60 ppm in the parallel 2-year study (Atkinson et al., 1994a).
Table 23. Findings in rats given thiodicarb in the diet for 52 weeks (means)
|
Finding |
Dose (ppm) |
|||||||
|
Males |
Females |
|||||||
|
0 |
60 |
200 |
900 |
0 |
60 |
200 |
900 |
|
|
Week 25 |
||||||||
|
Body weight (g) |
650 |
670 |
620 |
540* |
350 |
340 |
350 |
310* |
|
Erythrocyte count (1012/L) |
8.9 |
9.1 |
9.0 |
8.5 |
8.2 |
8.0 |
8.1 |
7.5* |
|
Erythrocyte volume fraction (%) |
0.46 |
0.44 |
0.45 |
0.43* |
0.43 |
0.42 |
0.42 |
0.40* |
|
Haemoglobin concentration (g/dL) |
16 |
15 |
15 |
14 |
15 |
15 |
15 |
14* |
|
Mean corpuscular volume (fL) |
52 |
49* |
51 |
50 |
52 |
53 |
52 |
54 |
|
Leukocyte count (109/L) |
12 |
13 |
13 |
12 |
9.8 |
8.0 |
10 |
10 |
|
Total bilirubin concentration (΅mol/L) |
1.2 |
1.1 |
1.1 |
1.4 |
1.2 |
1.2 |
1.4 |
2.1* |
|
Erythrocyte cholinesterase activity (IU/L) |
1100 |
980 |
1200 |
870 |
1400 |
1400 |
1500 |
1300 |
|
Urine pH |
7.9 |
8.2 |
7.8 |
6.8* |
7.7 |
7.2 |
6.9* |
6.4* |
|
Week 51/52 |
||||||||
|
Body weight (g) |
810 |
840 |
780 |
620* |
450 |
440 |
430 |
360* |
|
Erythrocyte count (1012/L) |
8.6 |
8.8 |
8.6 |
8.0* |
7.6 |
6.8* |
7.4 |
6.8* |
|
Erythrocyte volume fraction (%) |
0.44 |
0.43 |
0.43 |
0.40* |
0.40 |
0.37* |
0.39 |
0.37* |
|
Haemoglobin concentration (g/dL) |
15 |
15 |
15 |
14* |
15 |
14 |
14 |
13* |
|
Mean corpuscular volume (fL) |
51 |
49* |
51 |
49* |
53 |
56 |
53 |
55 |
|
Leukocyte count (109/L) |
12 |
13 |
11 |
11 |
6.7 |
6.7 |
9.7* |
8.8* |
|
Total bilirubin concentration (΅mol/L) |
1.8 |
1.9 |
2.1 |
2.4 |
1.1 |
0.9 |
1.7* |
1.6* |
|
Erythrocyte cholinesterase activity (IU/L) |
930 |
990 |
960 |
590* |
1400 |
1400 |
1400 |
1200 |
|
Brain cholinesterase activity (IU/L) |
14 000 |
14 000 |
14 000 |
14 000 |
14 000 |
16 000 |
13 000 |
14 000 |
|
Urine pH |
8.0 |
8.2 |
7.2 |
7.0* |
8.2 |
7.7 |
6.8* |
6.6* |
|
Spleen weight (g) |
1.1 |
1.2 |
1.1 |
1.1 |
0.7 |
0.6 |
0.8 |
0.7 |
|
Spleen weight (corrected for bw; g) |
1.1 |
1.2 |
1.1 |
1.2 |
0.6 |
0.6 |
0.8 |
0.8* |
|
Adrenal, haemorrhagic degeneration |
0/15 |
|
|
0/15 |
5/15 |
|
|
9/15 |
|
Thymus, cystic duct |
0/15 |
|
|
1/15 |
2/15 |
|
|
8/15* |
|
Spleen, haemosiderosis |
4/15 |
1/15 |
2/15 |
2/15 |
4/15 |
10/15* |
9/15* |
11/15* |
|
Spleen, increased extramedullary haematopoiesis |
7/15 |
7/15 |
6/15 |
14/15* |
3/15 |
7/15 |
8/15 |
9/15* |
|
Testicular tubular atrophy |
0/15 |
|
|
2/15 |
|
|
|
|
From Atkinson et al. (1994a)
Six groups of 120 male and 120 female Fischer 344 rats, 50 days of age, were given diets containing thiodicarb (analytical grade; purity, ³ 99%) at variable levels (not specified) to provide an intake of 0, 0, 0.5, 1, 3, or 10 mg/kg bw per day, for about 24 months. The animals were housed three per cage for males and five per cage for females and were observed daily for deaths and adverse physical or behavioural changes. Food and water consumption and body-weight changes were determined routinely. Ten mice of each sex per group were killed at 6 and 12 months and 20 of each sex per group at 19 months. Haematological, ophthalmic, limited clinical chemical, and urinary end-points were determined before each scheduled sacrifice. Organ weights and gross and microscopic lesions were evaluated at each sacrifice and on dead or moribund animals (excluding organ weights); for the low and intermediate doses, only tissues from animals in the main groups and tissues with gross lesions were examined histologically. Plasma, erythrocyte, and brain cholinesterase activity was determined in samples taken from 10 rats of each sex per group at each sacrifice time; however, the relevance of the results is questionable, as the animals received control diet for 48 h before being killed.
The study was terminated after 25 months for males and 26 months for females. The homogeneity, stability, and achieved incorporation were not reported. No clinical signs were reported. The mortality rate of males at the highest dose was increased at some stages of the study, but at termination the rate in control males exceeded that in males at the high dose (Table 24). Food consumption was similar in all groups, although sporadic decreases were observed in treated rats. The body weights of males at the high dose were depressed from month 15 to 20 and in females at this dose from month 3 to 21. An outbreak of sialodacryoadenitis virus was identified at about 18 months, which contributed to a generally debilitated condition and to weight loss in all animals for 23 weeks. Reduced erythrocyte count, erythrocyte volume fraction, and haemoglobin concentration were found in males at 12 months but not at 6, 19, or 24 months. Cholinesterase activity in plasma, erythrocytes, and brain, determined after 48 h on control diet, was not inhibited by treatment. The only change in cholinesterase activity, consistent with the findings in other studies with thiodicarb given in the feed, was a significant increase in erythrocyte cholinesterase activity at 24 months in males at the high dose. Clinical chemical and urinary end-points were not affected by treatment except for a transient reduction in urine volume at 12 months in females given doses > 3 mg/kg bw per day. Although sporadic differences were observed at various intervals, there were no dose-related effects on absolute or relative organ weights.
Table 24. Findings in rats given thiodicarb in the diet for 104 weeks (means)
|
Finding |
Mean intake (mg/kg bw per day) |
|||||||
|
Males |
Females |
|||||||
|
0 |
1 |
3 |
10 |
0 |
1 |
3 |
10 |
|
|
Survival at 80 weeks (%) |
91 |
91 |
94 |
83* |
90 |
92 |
93 |
91 |
|
Survival at 104 weeks (%) |
77 |
77 |
79 |
78 |
72 |
70 |
80 |
76 |
|
Body-weight gain at 80 weeks (g) |
270 |
270 |
270 |
270 |
160 |
170 |
160 |
150* |
|
24 months (10/group) |
||||||||
|
Erythroc yte count (1012/L) |
7.9 |
8.1 |
7.6 |
8.6 |
7.1 |
7.1 |
7.7 |
7.3 |
|
Erythrocyte volume fraction (%) |
45 |
45 |
44 |
48 |
40 |
41 |
44 |
41 |
|
Haemoglobin concentration (g/dL) |
15 |
15 |
15 |
16 |
14 |
14 |
15 |
14 |
|
Mean corpuscular volume (fL) |
60 |
57 |
61 |
57 |
60 |
60 |
58 |
58 |
|
24 months |
||||||||||
|
Dose (mg/kg bw per day) |
0 |
0 |
1 |
3 |
10 |
0a |
0b |
1 |
3 |
10 |
|
Testicular interstitial-cell adenoma |
81 |
87 |
88 |
90 |
91 |
|
|
|
|
|
|
Sternal marrow myeloid hyperplasia |
3 |
1 |
3 |
5 |
7 |
0 |
0 |
0 |
0 |
0 |
|
Sternal marrow erythroid hyperplasia |
3 |
5 |
2 |
3 |
13 |
0 |
0 |
1 |
0 |
0 |
|
Thymus lymphoid hyperplasia |
1 |
2 |
1 |
1 |
6 |
4 |
2 |
0 |
0 |
0 |
|
Thymus epithelial hyperplasia |
40 |
37 |
38 |
30 |
50 |
0 |
1 |
1 |
1 |
0 |
|
Thymoma |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
|
Mediastinal lymph node, haemosiderosis |
40 |
38 |
44 |
48 |
46 |
53 |
56 |
29 |
32 |
62 |
|
Liver, pericholangitis |
52 |
44 |
28 |
28 |
62 |
30 |
38 |
21 |
16 |
39 |
|
Pituitary cysts |
9 |
8 |
7 |
7 |
19 |
58 |
37 |
29 |
33 |
33 |
From Woodside et al. (1980b); , not investigated
* p < 0.05
No significant pathological changes were attributable to treatment at 6, 12, or 19 months, and no carcinogenic response was found at any dose throughout the study. Males at the high dose showed evidence at 24 months of increased incidences of pituitary cysts, sternal marrow hyperplasia, thymic hyperplasia, and thymomas, and both males and females at this dose had a marginal increase in the frequency of haemosiderosis of the mediastinal lymph nodes (Table 24). These findings indicated a pattern of effects at the high dose, some of which were consistent with a response to reduced erythrocyte counts, but they did not achieve statistical significance in comparison with the incidences in the two control groups. An apparent increase in the number of treated male rats with hepatocellular hyperplasia and neoplastic nodules was not confirmed by a re-evaluation of the slides by a pathologist who was unaware of the control or treated status of the rats from which the tissues came and using clearly defined criteria. The incidence of liver tumours was not significantly increased. Testicular interstitial-cell adenomas occurred spontaneously in all groups at high incidence, but there was no evidence of a treatment-related effect.
If the absence of reliable determinations of cholinesterase activity is not considered and in view of the acute nature of the effects of thiodicarb on this end-point, the NOAEL was 3 mg/kg bw per day on the basis of reduced survival, histological findings, reduced erythrocyte counts during the first year, and reduced body weight at 10 mg/kg bw per day. There was no evidence of carcinogenic potential at 10 mg/kg bw per day, the highest dose tested, even though it was not shown convincingly that the maximum tolerated dose had been achieved (Woodside et al., 1980b). [The summary was adapted from the 1985 JMPR, as full data on pathological findings at interim times were not available.]
Groups of 50 male and 50 female Sprague-Dawley rats received diets containing thiodicarb (purity, 9496%) at a concentration of 0, 60, 200, or 900 ppm, equal to mean intakes of 0, 3, 12, and 60 mg/kg bw per day for males and 0, 4, 15, and 80 mg/kg bw per day for females, for 104 weeks. Clinical chemical, haematological, and urinary end-points and cholinesterase activity were determined for 10 animals per group at weeks 79 and 104. Differential blood counts were made during weeks 53, 78, and 103. The animals were not fasted before blood sampling. Ophthalmoscopy was performed on 20 rats of each sex per group before treatment and at weeks 51 and 103. All animals were necropsied and examined grossly, and extensive histological examinations were performed on all animals that died or were killed, all controls and high-dose animals and gross lesions, liver, lung, kidneys, testes, and spleen from all animals at the intermediate and low doses.
The homogeneity and achieved intakes were acceptable. No clinical signs or ophthalmic changes were found that were related to treatment. The survival rate of animals at the highest dose was increased (Table 25). Body-weight gain was reduced by up to 30% in animals of each sex at 900 ppm, and the food consumption of these animals was reduced at the beginning of the study. At 79 weeks, altered erythrocyte parameters were seen in animals at the highest dose and in males at the intermediate dose; the leukocyte counts were increased in females at the highest dose primarily becuase of a two- to threefold increase in neutrophil count. The platelet counts were increased significantly in animals at the highest dose at 79 weeks. Similar haematological findings were present at 104 weeks, but there was greater inter-animal variation. Clinical chemistry showed no consistent pattern. Plasma cholinesterase activity was reduced by > 20% in all groups of males at weeks 79 and 104 and in females at the highest dose at week 104. Erythrocyte cholinesterase activity was statistically significantly reduced in males at the highest dose (18%) and in females at the highest (35%) and intermediate (17%) doses at week 104. Brain acetylcholinesterase activity was similar in all groups, with a maximum inhibition of 11% in males at the highest dose (not significant). Urinary pH was reduced in animals at the highest dose at 104 weeks; variations in specific gravity were generally linked to changes in volume. The absolute weight of the spleen and that relative to body weight were increased in animals at the highest dose; the liver weight was increased in females at this dose. Histological examination confirmed that the spleen was the main target organ, with increased haemosiderosis in females at the highest dose and an increase in the frequency of extramedullary haematopoiesis in males at the highest and intermediate doses. The incidences of muscular atrophy, sciatic nerve degeneration, pituitary adenomas, and cardiomyopathy were decreased in males at the highest dose, while those of testicular tubular atrophy and interstitial-cell adenoma were statistically significantly (p = 0.04, Fisher exact test) increased. The incidence of testicular atrophy in all groups including controls (3254%) was greater than the range in controls in previous experiments (1223%), but no cause was identified. The incidence of interstitial-cell adenoma (24%) in animals at the highest dose was greater than the range of 010% in previous controls. Interstitial-cell tumours are an age-related finding in rats, and the high survival rate of males at the highest dose may have contributed to the high incidence in this study. In a statistical analysis corrected for survival, it was determined that the finding might have been due to chance (odds ratio, 2.9; 95% confidence interval, 0.99.4; Rieth, 1995b). Given the unusual incidence of testicular lesions in this study, the increased survival rate of males at the highest dose, and the clear NOAEL for the interstitial-cell tumours at 200 ppm (12 mg/kg bw per day), the finding in males at the highest dose is considered not to indicate significant carcinogenic potential. The incidences of liver tumours were not increased in treated animals, and the total number of tumours was not increased in the groups at the highest dose, despite the higher survival rates .
Table 25. Findings in rats given thiodicarb in the diet for 104 weeks (means)
|
Finding |
Dose (ppm) |
|||||||
|
Males |
Females |
|||||||
|
0 |
60 ppm |
200 ppm |
900 ppm |
0 |
60 ppm |
200 ppm |
900 ppm |
|
|
Mean intake (mg/kg bw per day) |
0 |
3.3 |
12 |
60 |
0 |
4.4 |
15 |
80 |
|
Survival at 104 weeks (%) |
42 |
42 |
34 |
54 |
38 |
48 |
28 |
64* |
|
Body weight at 80 weeks (g) |
830 |
880 |
820 |
640* |
500 |
540 |
510 |
420* |
|
Week 79 (10/group) |
||||||||
|
Erythrocyte count (1012/L) |
8.6 |
8.6 |
8.2 |
7.9 |
7.3 |
7.6 |
7.6 |
6.8* |
|
Erythrocyte volume fraction (%) |
0.45 |
0.44 |
0.42* |
0.40* |
0.40 |
0.41 |
0.42 |
0.38* |
|
Haemoglobin concentration (g/dL) |
15 |
15 |
14* |
13* |
14 |
14 |
14 |
13* |
|
Mean corpuscular volume (fL) |
52 |
50 |
52 |
51 |
56 |
54 |
55 |
56 |
|
Leukocyte count (109/L) |
11 |
10 |
14 |
13 |
6.2 |
6.4 |
6.8 |
8.0* |
|
Total bilirubin concentration (΅mol/L) |
1.5 |
2.3 |
1.4 |
5.9 |
1.7 |
1.3 |
1.2 |
1.3 |
|
Plasma cholinesterase activity (IU/L) |
1400 |
900* |
1000* |
550 |
2800 |
3300 |
2700 |
2600 |
|
Erythrocyte cholinesterase activity (IU/L) |
920 |
1000 |
910 |
830 |
680 |
840 |
680 |
830 |
|
Week 104 (10/group) |
||||||||
|
Liver weight (g) |
24 |
26 |
23 |
21 |
17 |
19 |
18 |
20 |
|
Liver weight (relative to bw; g) |
23 |
24 |
22 |
23 |
17 |
18 |
18 |
22* |
|
Spleen weight (g) |
1.4 |
1.5 |
1.6 |
1.6 |
0.9 |
1.0 |
1.0 |
1.3 |
|
Spleen weight (relative to bw; g) |
1.4 |
1.4 |
1.6 |
1.7 |
0.9 |
1.0 |
1.0 |
1.3 |
|
Plasma cholinesterase activity (IU/L) |
1200 |
950 |
840 |
320* |
2000 |
2400 |
1800 |
1000* |
|
Erythrocyte cholinesterase activity (IU/L) |
1600 |
1800 |
1500 |
1300* |
1700 |
1500 |
1400* |
1100* |
|
Brain cholinesterase activity (IU/L) |
14 000 |
16 000 |
16 000 |
12 000 |
12 000 |
17 000 |
15 000 |
16 000 |
|
Urine pH |
7.4 |
6.8 |
7.0 |
7.0 |
7.3 |
6.9 |
7.3 |
6.6 |
|
Week 104 |
||||||||
|
Lumbar lymph node, sinusoidal oedema |
2/25 |
4/14 |
1/10 |
2/21 |
0/30 |
0/15 |
0/19 |
7/26* |
|
Brain, ventricular dilatation |
2/49 |
5/28 |
4/34 |
2/50 |
10/50 |
11/26 |
14/36 |
3/50* |
|
Cardiomyopathy |
48/49 |
28/29 |
31/35 |
40/50* |
33/50 |
12/26 |
25/36 |
37/50 |
|
Galactocoele |
0/49 |
0/28 |
0/33 |
1/50 |
0/50 |
2/26 |
2/36 |
6/50* |
|
Hind foot inflammation |
9/11 |
9/14 |
11/12 |
27/34 |
8/12 |
2/8 |
3/4 |
9/13 |
|
Tail, inflammation / abscess |
3/11 |
3/12 |
4/12 |
12/34 |
4/12 |
6/8 |
1/4 |
5/13 |
|
Spleen, haemosiderosis |
0/49 |
0/50 |
1/50 |
2/50 |
2/50 |
2/50 |
3/50 |
14/50* |
|
Spleen, extramedullary haematopoiesis |
10/49 |
12/50 |
20/50* |
26/50* |
28/50 |
31/50 |
21/50 |
28/50 |
|
Testes, tubular atrophy |
19/49 |
16/50 |
22/50 |
27/50* |
|
|
|
|
|
Sciatic nerve degeneration |
24/50 |
7/29 |
17/34 |
4/50* |
10/50 |
2/24 |
5/36 |
7/50 |
|
Thigh muscle atrophy |
13/49 |
4/29 |
1/34 |
4/50* |
2/50 |
2/24 |
2/36 |
2/50 |
|
Thyroid |
|
|
|
|
|
|
|
|
|
No abnormality |
27/49 |
20/29 |
24/31 |
32/50 |
28/50 |
20/25 |
23/35 |
41/49* |
|
Diffuse C-cell hyperplasia |
1/49 |
3/29 |
2/31 |
0/50 |
8/50 |
1/25 |
5/35 |
1/49* |
|
Focal C-cell hyperplasia |
5/49 |
0/29 |
0/31 |
5/50 |
3/50 |
1/25 |
2/35 |
5/49 |
|
Hepatocellular adenoma |
2/49 |
0/50 |
0/50 |
2/50 |
0/50 |
1/50 |
0/50 |
0/50 |
|
Hepatocellular carcinoma |
2/49 |
1/50 |
3/50 |
1/50 |
0/50 |
0/50 |
0/50 |
0/50 |
|
Thyroid C-cell carcinoma |
0/49 |
0/29 |
0/31 |
2/50 |
2/50 |
1/25 |
2/35 |
0/49 |
|
Thyroid C-cell adenoma |
7/49 |
2/29 |
2/31 |
3/50 |
5/50 |
1/25 |
2/35 |
0/49 |
|
Testes, interstitial-cell adenoma |
5/49 |
3/50 |
3/50 |
12/50* |
|
|
|
|
|
Total malignant tumours |
17/50 |
|
|
10/50 |
13/50 |
|
|
11/50 |
|
Total benign tumours |
40/50 |
|
|
34/50 |
45/50 |
|
|
39/50 |
From Atkinson et al. (1994b); not investigated
* p < 0.05
A nonsignificant increase in the incidence of C-cell carcinoma of the thyroid was found in males at the highest dose, paralleled by a low incidence of C-cell adenomas (Table 25). Thyroid C-cell carcinomas occurred occasionally in other animals at the test facility (range, 02%) but at incidences below that seen in the current study in both males at the highest dose and control females. The combined incidence of benign and malignant thyroid C-cell tumours in males at the highest dose is lower than that in concurrent controls; the incidence of C-cell hyperplasia was not increased in males at the highest dose, and the overall incidence of thyroid lesions was lower than that in concurrent controls. In the context of the overall findings for the thyroid, the observation of two thyroid C-cell carcinomas in males at the highest dose is considered to be of no biological significance. The NOAEL was 60 ppm, equal to 3 mg/kg bw per day, on the basis of increased splenic extramedullary haematopoiesis at higher doses in males. The NOAEL for tumorigenicity was 200 ppm, equal to 12 mg/kg bw per day (Atkinson et al., 1994b).
An extensive range of genotoxicity assays has been performed both in vitro and in vivo with thiodicarb (Table 26). Some of the reports and protocols were less than optimal by current standards, and very pure (> 99%) batches were used. The Meeting considered that, given the extent of the database, these shortcomings did not significantly compromise the overall assessment of genotoxicity. Positive findings were reported at cytotoxic concentrations in an assay for gene mutation in mouse lymphoma L5178Y cells and over a range of concentrations in an assay for mitotic gene conversion in Saccharomyces cerevisiae. Negative results were obtained in three studies in vivo .
Table 26. Results of assays for the genotoxicity of thiodicarb
|
End-point |
Test system |
Concentration |
Purity |
Results |
Reference |
|
In vitro |
|||||
|
Reverse mutation |
S. typhimurium TA1535, TA1537, TA1538, TA98, TA100 |
1, 10, 100, 500, 1000 ΅g/plate |
99.8 |
Negativea |
Jagannath & Brusick (1978) |
|
Gene mutation |
Mouse lymphoma L5178Y Tk+/ cells |
1.2, 2.5, 3, 5, 6, 7, 8, 10 ΅g/ml with S9 |
91.5 |
Weakly positivea |
Cifone & Brusick (1985a) |
|
Primary DNA damage |
E. coli W3110 (polA+) and p-3478 (polA) |
10, 100, 1000, 10 000 ΅g/ml |
> 99 |
Negativea |
Naismith & Matthews (1979a) |
|
Reverse mutation |
S. cerevisiae, diploid strain D7, homozygous for ilvI-92 |
2.5, 6, 25, 63, 250 ΅g/ml |
> 99 |
Negativeb |
Naismith & Matthews (1979b) |
|
Mitotic crossing-over |
S. cerevisiae, diploid strain D7, heterozygous for ade 2-40/ade 2-119 |
2.5, 6, 25, 63, 250 ΅g/ml |
> 99 |
Negativeb |
Naismith & Matthews (1979c) |
|
Mitotic gene conversion |
S. cerevisiae, diploid strain D7 heteroallelic for trp 5-1 2/trp 5-27 |
2.5, 6, 25, 63, 250 ΅g/ml |
> 99 |
Positiveb |
Naismith & Matthews (1979d) |
|
Gene conversion |
S. cerevisiae, strain D4 |
1, 10, 100, 500, 1000 ΅g/plate |
99.8 |
Negativea,c |
Jagannath & Brusick (1978) |
|
Unscheduled DNA synthesis |
Rat primary hepatocytes |
0.5, 1, 2.5, 5, 10, 25, 50, 100 ΅g/ml |
91.5 |
Negative |
Cifone & Brusick (1985b) |
|
Chromosomal aberrations |
Chinese hamster ovary cells |
10, 20, 30, 40 ΅g/ml |
91.5 |
Negativea |
Ivett & Brusick (1985) |
|
In vivo |
|||||
|
Unscheduled DNA synthesis |
CD-1 mouse primary hepatocytes |
20, 50 mg/kg bw by gavage |
95.4 |
Negative |
Clare (1996) |
|
Dominant lethal mutation |
Fischer 344 rats |
0, 0.5, 1.0, 3.0, 10, 30 mg/kg bw per day for 3 weeks |
> 99 |
Negative |
Woodside et al. (1979b) |
|
Micronucleus formation |
CF-1 mouse bone marrow |
5, 10 mg/kg/day intra-peritoneally for 2 days |
> 99 |
Negative |
Naismith & Matthews (1979e) |
S9, exogenous metabolic activation system derived from Aroclor 1254-induced rat liver preparations
a
With and without S9b
Without S9c
Poor positive control responseRats
In a study involving three generations with one litter per generation, which included an investigation for dominant lethal mutations, groups of 10 male and 20 female Fischer 344 rats received diets containing analytical-grade thiodicarb (purity, ³ 99%) at variable concentrations, equal to 0, 0, 0.5, 1, 3, and 10 mg/kg bw per day, for 56 days before mating (1:2) in the F0 generation and then continuously during production of the F1, F2, and F3 litters. The animals were observed for clinical signs, food consumption, body weight, time to mating, fertility, litter size, and pup viability. The reproductive organs of five male and five female F2 parents and five F3 weanlings of each sex per group were examined histologically. In the study of dominant lethal mutation, groups of 15 F2 males (~155 days old) were mated 1:1 with control females for 3 consecutive weeks. The females were killed on day 12 of gestation and the reproductive system was examined for corpora lutea, viable embryos, resorptions, and deaths. Triethylene melamine was given as a positive control at a dose of 0.25 mg/kg bw intraperitoneally.
No treatment-related effects were seen on clinical signs, body weight, reproductive performance, litter parameters, or fetal viability. The dominant lethal mutation rate was similar in treated and control groups. The results with the positive control were not entirely convincing, however, indicating that the study may have had low power. Cholinesterase activity was not measured. The NOAEL for general and reproductive toxicity was 10 mg/kg bw per day (Woodside et al., 1979b).
In a range-finding, one-generation study, 10 male and 10 female Crl:CDBR rats received diets containing technical-grade thiodicarb (purity, 94.5%) at a concentration of 0, 200, 600, 1800, or 3000 ppm. Dosing was begun 6 weeks before mating and was continued through gestation and weaning; 20 F1 pups of each sex per group were treated for 3 weeks after weaning. The treated diet was made available on the morning of the day of sacrifice. A wide range of parameters was studied, including cholinesterase activity in plasma, erythrocytes, and brain. The achieved doses, homogeneity, and stability were acceptable. Reduced body-weight gain was seen consistently in animals at doses ³ 600 ppm, and some F0 females at the highest dose were emaciated during the first week of treatment. Body-weight gain was reduced in rats at 200 ppm during some stages of the study. Pup survival to day 4 was reduced at 1800 ppm and to such an extent at 3000 ppm that only 12 females and 11 males received the treated diet after weaning. No effects were found on mating performance or pregnancy rate or at macroscopic examination. Brain cholinesterase activity was decreased by ~10% in F1 females receiving 3000 ppm, but this reduction was not statistically significant; no other notable effects were found on erythrocyte or brain cholinesterase activity. No NOAEL could be identified, but the reduced body-weight gain at 200 ppm was minimal and no specific effects were found on reproductive outcome (Henwood, 1991).
In a two-generation study with a single F1 litter and two F2 litters, groups of 28 Crl:CDBR rats of each sex received diets containing technical-grade thiodicarb (purity, 94.5%) at a concentration of 0, 100, 300, or 900 ppm, equal to achieved doses of 0, 5, 15, and 50 mg/kg bw per day for males and 0, 7, 22, and 72 mg/kg bw per day for females. Dosing was begun 10 weeks before mating and was continued until sacrifice. The animals were observed for clinical signs, deaths, food consumption, and reproductive performance and outcome. The litters were culled at day 4 to reduce the litter size to a maximum of four of each sex, and the pups were examined for viability, sex, weight, and external abnormalities. All animals that died during the study and 10 adults and weanlings of each sex per group killed as planned were examined macroscopically. The reproductive organs of 10 controls and high-dose adults of each sex per generation were examined microscopically. Samples of blood and brain were taken for cholinesterase assay from 10 adults of each sex, 10 day-21 pups of each sex per group, and one of each sex per litter per generation. The animals were not fasted before sampling, and the protocol involved techniques that would minimize the potential for reactivation.
Dietary analyses confirmed that the homogeneity, stability, and content of the diet were acceptable. No evidence was found of treatment-related effects on clinical signs, fertility, litter size at birth, sex ratio, or macroscopic or microscopic appearance. At 900 ppm, there were consistent effects on pup viability and survival, body-weight gain (20% reduction), food consumption (£ 20% reduction), and pups found dead with no milk in the stomach (Table 27). Similar but less widespread findings were seen at 300 ppm. At 100 ppm, pup weight was consistently reduced by < 10% (not statistically significant) but was compensated for in adulthood. The increased frequencies of pups with no milk in the stomach (F2b) and pups that died before the cull on day 4 (F1) did not show a doseresponse relationship and were not clearly related to treatment. Sporadic changes in erythrocyte and brain cholinesterase activity were seen in pups of dams at 900 ppm, although the changes were not consistent among generations and were not statistically significant. It is uncertain that this finding indicates that pups are more sensitive than adults, as pups received a higher dose on day 21 on a body-weight basis than adult animals given the same diet.
Table 27. Findings in rats given thiodicarb in the diet during mating, gestation and lactation
|
Finding |
Concentration (ppm) |
|||
|
0 |
100 |
300 |
900 |
|
|
F0F1 |
||||
|
Days to mating |
3.1 |
3.0 |
3.0 |
3.9 |
|
Pup weight at day 0 (g) |
6.1 |
5.9 |
5.4* |
5.1* |
|
Pup weight at day 21 (g) |
51 |
51 |
47* |
41* |
|
Pups with no milk in stomach |
7 |
4 |
12 |
26 |
|
Pup deaths days 04 |
4 |
17 |
9 |
47* |
|
Pup deaths days 521 |
0 |
1 |
1 |
3 |
|
Brain cholinesterase activity (%; M/F) |
|
|
|
|
|
Adults |
100 |
97/110 |
98/87 |
100/150 |
|
Pups |
100 |
120/120 |
120/91 |
140/100 |
|
Erythrocyte cholinesterase activity (%; M/F) |
|
|
|
|
|
Adults |
100 |
100/97 |
100/100 |
110/100 |
|
Pups |
100 |
94/110 |
91/110 |
94/96 |
|
F1F2a |
||||
|
Days to mating |
2.9 |
2.5 |
4.4 |
2.8 |
|
Pup weight at day 0 (g) |
6.2 |
6.0 |
5.4* |
5.0* |
|
Pup weight at day 21 (g) |
53 |
51 |
46* |
37* |
|
Pups with no milk in stomach |
8 |
8 |
21* |
59* |
|
Pup deaths day 04 |
6 |
8 |
67* |
120* |
|
Pup deaths day 521 |
0 |
1 |
0 |
6 |
|
Brain cholinesterase activity, pups (%; M/F) |
100 |
100/100 |
92/95 |
93/84 |
|
Erythrocyte cholinesterase activity, pups (%; M/F) |
100 |
100/91 |
110/93 |
91/83 |
|
F1F2b |
||||
|
Days to mating |
2.5 |
3.6 |
3.2 |
3.4 |
|
Pup weight at day 0 (g) |
6.2 |
5.7 |
5.1* |
5.1* |
|
Pup weight at day 21 (g) |
54 |
51 |
47* |
38* |
|
Pups with no milk in stomach |
7 |
27 |
9 |
31 |
|
Pup deaths day 04 |
6 |
11 |
24* |
91* |
|
Pup deaths day 521 |
1 |
4 |
1 |
11* |
|
Brain cholinesterase activity (%; M/F) |
|
|
|
|
|
Adults |
100 |
79/93 |
84/110 |
88/100 |
|
Pups |
100 |
100/91 |
93/91 |
75/86 |
|
Erythrocyte cholinesterase activity (%; M/F) |
|
|
|
|
|
Adults |
100 |
100/93 |
120/98 |
130/102 |
|
Pups |
100 |
97/97 |
95/100 |
77/79 |
From Henwood (1992); M, male; F, female; * p < 0.05
The NOAEL for reproductive toxicity was 100 ppm, equal to 5 mg/kg bw per day, on the basis of significantly reduced pup viability at 300 ppm. The overall NOAEL was also 100 ppm, on the basis of significantly reduced body weight in adults and pups at 300 ppm. The reductions in pup weight at 100 ppm were small and, although consistent, were considered not to be adverse (Henwood, 1992).
Mice
Groups of 25 mated female CD-1 mice received technical-grade thiodicarb (purity unspecified) at a dose of 0, 50, 100, or 200 mg/kg bw per day (based on day 6 weights) on days 616 of gestation by gavage in 0.5% aqueous methyl cellulose. The dams were observed for clinical signs, deaths, and body weight. At sacrifice on day 17, the uteri were examined for viable fetuses, implantations, and resorptions. The fetuses were sexed, weighed, and examined for external malformations. One-third of the fetuses were sectioned by Wilsons method for visceral investigations, and the remainder were stained in alizarin red and examined for skeletal effects. No information was provided to confirm the administered doses or on clinical signs or individual fetal variations. Six animals at the highest dose died on days 67, but the cause was not identified. No clinical signs were reported in animals at 50 or 100 mg/kg bw per day. There were no effects on maternal body weight, pregnancy outcome, fetal weights, or the incidence of malformations. The only finding indicative of an effect on the fetus was a small but dose-related increase in the incidence of accessory skull bones (up to three), but the highest incidence (2.3%) was within the range of previous controls at the test facility (02.6%), did not achieve statistical significance, and was considered not to be biologically significant. Within the limits of the study, the NOAEL for maternal toxicity was 100 mg/kg bw per day, on the basis of deaths at 200 mg/kg bw per day. The NOAEL for developmental toxicity was 200 mg/kg bw per day, the highest dose tested (Janes et al., 1980).
Rats
Groups of 1416 mated female Fischer 344 rats received diets containing technical-grade thiodicarb (purity, > 99%) at a dose of 0, 0.5, 1, 3, or 100 mg/kg bw per day during days 615 or 020 of gestation. Thiodicarb was incorporated into the diet at varying concentrations. A positive control group received aspirin at 625 mg/kg bw per day. The dams were observed for clinical signs, deaths, and body weight. At sacrifice on day 20, the uteri were examined for viable fetuses, implantations, and resorptions. The fetuses were sexed, weighed, and examined for external malformations; half the fetuses were examined for visceral anomalies and the other half for skeletal effects.
No information was provided to confirm the administered doses, and only limited details were given of the techniques used to examine fetuses and to determine clinical signs and individual fetal variations. There were no deaths. Maternal body-weight gain was reduced at 100 mg/kg bw per day by about 70% in both groups. A low pregnancy rate seen in some groups on days 615 was not reproduced on days 020, and is considered to be a chance finding. There were no effects on pregnancy outcome or the incidences of malformations or visceral variations. Fetal weights and crownrump lengths were reduced in animals at 100 mg/kg bw per day on days 020 but were unaltered when animals were treated only on days 615 of gestation. The incidences of some skeletal variations were increased, but the rates were within the range for previous controls and/or were not consistent with the two dosing schemes and are considered not to indicate specific fetotoxicity. The NOAEL for maternal toxicity was 3 mg/kg bw per day on the basis of reduced body-weight gain at 100 mg/kg bw per day. The NOAEL for developmental toxicity was 100 mg/kg bw per day, the highest dose tested (Woodside et al., 1979c).
Groups of 25 mated female CD rats received technical-grade thiodicarb (purity unspecified) at a dose of 0, 10, 20, or 30 mg/kg bw per day by gavage in 0.5% aqueous methyl cellulose on days 619 of gestation. The dams were observed for clinical signs, deaths, and body weight. At sacrifice on day 20, the uteri were examined for viable fetuses, implantations, and resorptions. The fetuses were sexed, weighed, and examined for external malformations; one-third of the fetuses were examined for visceral anomalies by Wilson sectioning, and the remainder were stained with alizarin red and examined for skeletal effects. Limited information was provided on clinical signs and individual fetuses. No deaths occurred, and there was no increase in the incidence of malformations. Clinical signs (tremors, discharge, matted coat) were reported in animals at 20 or 30 mg/kg bw per day, and dose-related reductions in maternal body-weight gain (by 1935%) and fetal weight (by 1130%) and reduced or delayed ossification of sternebrae, pubis, hyoid, skull, and vertebrae were seen all treated groups. No NOAEL could be identified for maternal toxicity, on the basis of reduced body-weight gain at all doses, or for fetotoxicity, on the basis of reduced weight and ossification at all doses. The NOAEL for teratogenicity was 30 mg/kg bw per day, the highest dose tested (Tasker et al., 1979).
Groups of 25 mated female Sprague-Dawley rats received technical-grade thiodicarb (purity, 94.7%) at a dose of 0, 1, 10, or 30 mg/kg bw per day by gavage in 0.5% aqueous methyl cellulose on days 615 of gestation. The doses were derived from a range-finding study in which maternal toxicity was observed at doses ³ 10 mg/kg bw per day, but no evidence of external malformations was found at 50 mg/kg bw per day. The dams were observed for clinical signs, deaths, food consumption, and body weight. At sacrifice on day 20, the uteri were examined for viable fetuses, implantations, and resorptions. The fetuses were sexed, weighed, and examined for external malformations; half of the fetuses were examined for visceral changes, and the remainder were stained with alizarin red and alcian blue to reveal skeletal effects. The dosing solutions were found to be acceptable. No deaths occurred. Clinical signs including tremors, salivation, and lethargy were reported in animals at 10 or 30 mg/kg bw per day from day 1 of dosing. Dose-related reductions in maternal body-weight gain (by 1329%) were seen at doses ³ 10 mg/kg bw per day during days 615. The food consumption of animals at the highest dose was reduced by about 10% during treatment. No effects were seen on fetal weight or litter size, and there was no treatment-related increase in the incidence of malformations or variations. The NOAEL for maternal toxicity was 1 mg/kg bw per day, on the basis of reduced body-weight gain and clinical signs at higher doses. The NOAEL for developmental toxicity was 30 mg/kg bw per day, the highest dose tested (Tyl et al., 1993).
The 10% effective dose (ED10) and the lower bound of the confidence interval for reduced maternal body weight and tremors were evaluated for the studies described above in a benchmark dose evaluation. Tremors were evaluated by a Weibull model and body weight by a quadratic polynomial. The ED10 was 6.4 or 8.1 mg/kg bw per day for tremors, with a lower bound of 3.8 mg/kg bw per day, and 3.67.9 mg/kg bw per day for effects on body weight, with a lower 95% confidence bound of 2.8. When these results were combined, the derived NOAEL for thiodicarb in developmental toxicity in rats was estimated to be 3 mg/kg bw per day (Starr, 1999).
Rabbits
Groups of 22 artificially inseminated New Zealand white rabbits received technical-grade thiodicarb (purity, 93%) at a dose of 0, 5, 20, or 40 mg/kg bw per day by gavage in 0.5% methyl cellulose on days 619 of gestation. The doses were derived from range-finding studies in which maternal toxicity occurred at doses ³ 20 mg/kg bw per day. The does were observed for clinical signs, deaths, food consumption, and body weight. At sacrifice on day 17, the uteri were examined for viable fetuses, implantations, and resorptions. All fetuses were sexed, weighed, and examined for external malformations, visceral malformations or variations, and skeletal effects. The dosing solutions were found to be satisfactory. Three animals at the highest dose and one at the intermediate dose died after aspirating the dosing solution. The clinical signs were similar in treated and control groups. Body-weight gain was reduced by 50% and food consumption by 12% in animals at 40 mg/kg bw per day between days 0 and 29 but were unaffected at lower doses. Fetal weights were slightly (~5%) reduced at the highest dose. Increased incidences of skeletal and soft-tissue malformations were seen at 20 mg/kg bw per day, but as the values were within the range for previous controls and were not seen at 40 mg/kg bw per day, they were considered not to be related to treatment. Three pups from two litters at the highest dose had 25 pre-sacral vertebrae; the incidence (2.9%) is greater than that in controls in previous experiments (01.9%), but as both dams had particularly low body-weight gains, this finding was considered to be secondary to maternal toxicity. The NOAEL for maternal toxicity was 20 mg/kg bw per day on the basis of reduced body-weight gain at 40 mg/kg bw per day. The NOAEL for developmental toxicity was 40 mg/kg bw per day, the highest dose tested (Rodwell, 1986).
(f) Special studies: Neurotoxicity
(i) Acute toxicity in rats
The acute neurotoxicity of thiodicarb was investigated in groups of 25 male and 20 female Sprague-Dawley rats given thiodicarb (purity, 95.1%) at a dose of 0, 5, 20, or 40 mg/kg bw by gavage in 0.5% methyl cellulose. The dosing solutions were confirmed as acceptable by chemical analyses. The groups were subdivided into 10 of each sex for a functional observational battery (FOB) on days 1 (3060 min. after dosing), 8, and 15, and groups 10 females and 15 males to determine cholinesterase activity. The FOB included observations in the home cage, during handling, in the open field, and of sensory responses, neuromuscular performance, locomotor activity, and body temperature. Brain cholinesterase activity was assayed in whole brain preparations from animals killed 1 or 24 h after dosing. Plasma and erythrocyte cholinesterase activity was determined in groups of five animals of each sex 1, 4, and 24 h after dosing and in groups of five males 2, 8, and 24 h after dosing. Erythrocyte activity was determined by subtracting the values for acetylthiocholine in plasma from those for haemolysed whole blood. Stained samples of an extensive range of tissues from the peripheral and central nervous systems from controls and high-dose groups were examined histologically after perfusion in situ on day 16.
No deaths occurred during the study. A range of clinical signs typical of anticholinesterase compounds was seen in animals receiving 20 or 40 mg/kg bw (Table 28). Performance in most aspects of the FOB on day 1 was significantly altered in the groups receiving 20 or 40 mg/kg bw. Body temperature and rearing activity were reduced in all treated groups, the degree of arousal and locomotor activity were reduced in all treated females, and the incidence of pin-point pupils was increased in all treated males. The results of the FOB at subsequent times were similar to those for controls and/or before treatment. Acetylcholinesterase activity in brain was reduced at all doses after 1 h but had recovered by 24 h. Erythrocyte cholinesterase activity was reduced in females at 1 and 4 h but was very variable in males. The weights of individual brain regions and the histological findings were similar in all groups. Degeneration of the sciatic nerve fibre was seen in 2/5 males at the highest dose and in 0/5 controls, but this finding was considered to be spontaneous as it was also seen in a control female and the frequency was not increased in animals given repeated doses of thiodicarb (see below). A single dose of thiodicarb thus produced transient but significant inhibition of brain acetylcholinesterase activity and the associated clinical signs at 5 mg/kg bw, the lowest dose tested. There was no evidence of irreversible neurological change at the highest dose tested, 40 mg/kg bw. No NOAEL could be identified (Beyrouty, 2000b).
Table 28. Results of observational tests for functional behaviour (FOB) and determinations of cholinesterase activity in rats given a single dose of thiodicarb by gavage
|
End-point |
Time |
Dose (mg/kg bw) |
|||||||
|
Males |
Females |
||||||||
|
0 |
5 |
20 |
40 |
0 |
5 |
20 |
40 |
||
|
Tremors (moderate) |
Day 1 |
0/10 |
0/10 |
9/10* |
9/10* |
0/10 |
0/10 |
4/10* |
10/10* |
|
Day 8 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
|
|
Lying on ventral surface |
Day 1 |
0/10 |
0/10 |
2/10 |
6/10* |
0/10 |
0/10 |
3/10* |
7/10* |
|
Day 8 |
4/10 |
5/10 |
3/10 |
4/10 |
2/10 |
3/10 |
0/10 |
1/10 |
|
|
Ataxic gait ³ moderate |
Day 1 |
0/10 |
0/10 |
4/10* |
8/10* |
0/10 |
0/10 |
4/10* |
7/10* |
|
Day 8 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
|
|
Locomotor activity slight |
Day 1 |
0/10 |
1/10 |
3/10* |
7/10* |
1/10 |
8/10* |
9/10* |
10/10* |
|
Day 8 |
4/10 |
6/10 |
5/10 |
6/10 |
0/10 |
1/10 |
0/10 |
0/10 |
|
|
Decreased arousal (moderate to severe) |
Day 1 |
2/10 |
3/10 |
9/10* |
9/10* |
1/10 |
4/10* |
9/10* |
10/10* |
|
Day 8 |
4/10 |
5/10 |
2/10 |
3/10 |
0/10 |
0/10 |
0/10 |
0/10 |
|
|
Pin-point pupils |
Day 1 |
0/10 |
2/10 |
3/10 |
8/10* |
0/10 |
0/10 |
3/10* |
6/10* |
|
Day 8 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
|
|
Salivation |
Day 1 |
0/10 |
0/10 |
8/10* |
8/10* |
0/10 |
0/10 |
3/10* |
6/10* |
|
Day 8 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
|
|
Decreased tail-pinch reaction |
Day 1 |
0/10 |
0/10 |
7/10* |
9/10* |
0/10 |
0/10 |
3/10 |
8/10* |
|
Day 8 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
|
|
Body temperature (°C; mean) |
Day 1 |
38 |
37* |
36* |
36* |
39 |
37* |
36* |
35* |
|
Day 8 |
38 |
38 |
38 |
38 |
38 |
38 |
38 |
38 |
|
|
Rearing in arena (mean) |
Day 1 |
6.2 |
3.1 |
1.3* |
0.6* |
11 |
4.7* |
2.0* |
0.5* |
|
Day 8 |
4.5 |
3.8 |
4.9 |
4.6 |
10 |
9.8 |
13 |
14 |
|
|
Front grip strength (g; mean) |
Before test |
480 |
480 |
490 |
460 |
410 |
410 |
410 |
430 |
|
Day 1 |
590 |
590 |
530 |
500 |
510 |
510 |
460 |
450 |
|
|
Day 8 |
560 |
580 |
550 |
540 |
470 |
520 |
490 |
490 |
|
|
Hind grip strength (g; mean) |
Before test |
360 |
380 |
360 |
330 |
330 |
300 |
330 |
330 |
|
Day 1 |
420 |
380 |
380 |
360 |
350 |
340 |
320 |
340 |
|
|
Day 8 |
420 |
440 |
440 |
420 |
360 |
390 |
400 |
390 |
|
|
Cholinesterase activity (mean) |
|||||||||
|
Plasma (IU/L) |
1 h |
200 |
120* |
81* |
52* |
720 |
390* |
270* |
150* |
|
4 h |
240 |
200 |
150* |
76* |
660 |
560 |
590 |
310 |
|
|
Erythrocytes (IU/L) |
1 h |
450 |
290* |
380 |
300* |
780 |
540* |
570* |
330* |
|
4 h |
480 |
380 |
280 |
500 |
860 |
660 |
650* |
460* |
|
|
24 h |
450 |
380 |
390 |
440 |
770 |
670 |
740 |
680 |
|
|
Brain (IU/g) |
1 h |
6.5 |
1.9* |
1.5* |
1.4* |
6.3 |
2.3* |
1.6* |
1.4* |
|
24 h |
6.4 |
6.4 |
6.4 |
6.2 |
6.5 |
6.4 |
6.5 |
5.4 |
|
From Beyrouty (2000b)
* p < 0.05
(ii) Short-term study of toxicity in rats
The neurotoxic potential of thiodicarb (purity, 95.1%) after short-term intake was studied in groups of 15 male and 15 female Sprague-Dawley rats given diets containing the compound at a concentration of 0, 100, 400, or 800 ppm, equal to mean intakes of 0, 6, 23, and 46 mg/kg bw per day for males and 0, 7, 27, and 53 mg/kg bw per day for females, for 13 weeks. The animals were observed for clinical signs, body weight, food consumption, and reactions in a FOB before dosing and during weeks 2, 7, and 13. The battery included observations in the home cage, during handling, in the open field, and of sensory responses, neuromuscular performance, locomotor activity, and body temperature. Samples for determination of plasma and erythrocyte cholinesterase activity were obtained from six animals of each sex per group before dosing, and in weeks 2, 7, and 13; and brain acetylcholinesterase activity was assayed in brain regions from the same animals at terminal sacrifice (time between last food consumption and sampling not stated). Stained samples of an extensive range of tissues of the peripheral and central nervous systems from five animals of each sex in the controls and high-dose groups were examined histologically after perfusion in situ.
The homogeneity, stability, and achieved concentrations in the diet were confirmed as acceptable by chemical analyses. No deaths occurred during the study. Clinical signs and the results of the FOB were similar in all groups. Body-weight gain and food consumption were reduced from the start of the study in animals at 400 and 800 ppm (Table 29), but the pattern of results indicates that poor palatability may have been the main cause. Slight reductions in body temperature were seen in males at the highest dose at weeks 7 and 13. Plasma cholinesterase activity was unaffected by treatment. Erythrocyte cholinesterase activity was reduced by 1525% in rats at 800 ppm in week 2, but the reductions were not statistically significant. However, as blood samples were taken after the FOB some degree of reactivation may have occurred. Brain acetylcholinesterase activity was reduced in animals at 400 and 800 ppm, and, although the means were not statistically significantly different, the activity in animals was clearly > 20% below the range for controls. Slight reductions in brain acetylcholinesterase activity seen in rats at 100 ppm did not achieve statistical significance, and there was considerable overlap with control values. Despite the uncertainties with regard to potential reactivation between the last exposure and sampling, the reductions in brain cholinesterase activity at 400 and 800 ppm are considered to be treatment-related and adverse. Treatment had no effect on brain weight or morphological appearance, and the incidences of nerve fibre degeneration were similar in controls and animals at the highest dose. The NOAEL was 100 ppm, equal to 6 mg/kg bw per day, on the basis of inhibition of brain acetylcholinesterase activity at 400 ppm and above. No neuropathological changes were observed (Beyrouty, 2000c).
Table 29. Results of observational tests for functional behaviour (FOB) and determinations of cholinesterase activity in rats given diets containing thiodicarb for 13 weeks
|
End-point |
Time |
Concentration in diet (ppm) |
|||||||
|
Males |
Females |
||||||||
|
0 |
100 |
400 |
800 |
0 |
100 |
400 |
800 |
||
|
Body weight (g) |
Day 1 |
260 |
260 |
260 |
260 |
180 |
180 |
180 |
180 |
|
Day 15 |
340 |
340 |
330 |
320* |
220 |
220 |
210 |
210 |
|
|
Day 92 |
560 |
560 |
540 |
480* |
300 |
310 |
290 |
280 |
|
|
Food consumption (g/animal) |
Days 18 |
170 |
170 |
150* |
130* |
120 |
120 |
110* |
91* |
|
Days 1522 |
180 |
180 |
160* |
160* |
120 |
120 |
120 |
120 |
|
|
Days 8592 |
170 |
180 |
170 |
160* |
120 |
120 |
120 |
|
|
|
Body temperature (°C; mean) |
Week 2 |
38 |
38 |
38 |
38 |
39 |
38 |
39 |
38 |
|
Week 7 |
38 |
38 |
38 |
38 |
39 |
39 |
39 |
39 |
|
|
Week 13 |
38 |
38 |
38 |
38 |
39 |
39 |
39 |
39 |
|
|
Sciatic nerve fibre degeneration |
|
1/5 |
|
|
0/5 |
0/5 |
|
|
1/5 |
|
Lumbar cord nerve fibre degeneration |
|
2/5 |
|
|
2/5 |
2/5 |
|
|
1/5 |
|
Thoracic cord nerve fibre degeneration |
|
1/5 |
|
|
2/5 |
1/5 |
|
|
1/5 |
|
Cholinesterase activity (mean) |
|||||||||
|
Plasma (IU/L) |
Week 2 |
210 |
260 |
190 |
200 |
710 |
740 |
720 |
590 |
|
Week 13 |
180 |
240 |
180 |
200 |
1400 |
1350 |
1500 |
1300 |
|
|
Erythrocytes (IU/L) |
Week 2 |
660 |
580 |
560 |
480 |
930 |
940 |
920 |
790 |
|
Week 13 |
630 |
710 |
570 |
570 |
1300 |
1100 |
1200 |
1300 |
|
|
Brain (IU/g) |
Week 13 (mean) |
6.0 |
5.5 |
4.9 |
4.6 |
5.7 |
5.9 |
5.5 |
5.1 |
|
|
Range |
5.16.6 |
4.75.9 |
3.25.8 |
2.75.9 |
4.96.3 |
5.16.3 |
3.46.6 |
2.16.3 |
From Beyrouty (2000c)
* p < 0.05
The marked differences in the findings of the study of repeated dietary intake and acute exposure by gavage may be important for estimates of human risk from the diet (Bouvier, 2000).
(iii) Delayed neurotoxicity in hens
The 1985 JMPR monograph reported a preliminary study with 1014-month-old white Leghorn hens, in which the LD50 for thiodicarb was 580 mg/kg bw. When atropine sulfate was administered at 15 mg/kg bw 15 min before dosing, only two of five hens died after 5 days at a dose of 830 mg/kg bw (Schwartz & Stevens, 1978).
Forty hens protected with atropine sulfate at 15 mg/kg bw were given a single dose of thiodicarb at 660 mg/kg bw in corn oil by oral intubation. Groups of 10 controls received the vehicle or 750 mg/kg bw of tri-ortho-cresol phosphate in corn oil. The hens were observed daily for pharmacological and toxicological effects, including a neurological evaluation of leg weakness, gait, and walking ability. On day 21, all the positive controls and 10 treated birds were necropsied, perfused with 10% neutral buffered formalin, and examined histologically. On day 22, eight new positive controls were added to the study and the existing 30 treated birds were dosed a second time with thiodicarb. The vehicle controls showed no toxic effects, while the positive controls had clinical and microscopic symptoms of delayed neurotoxicity, seen as an increased number of swollen axons per cervical spinal cord cross-section. Although some signs of neurological impairment were seen initially in the group exposed to thiodicarb, these were no longer present after 4 days and were not confirmed by histological examination (Becci et al., 1979).
Owing to the marginal response in the thiodicarb-treated birds, the entire study was repeated with 30 white Leghorn hens aged 812 months, which received an oral dose of analytical-grade thiodicarb at 660 mg/kg bw in corn oil. Two control groups received either the vehicle or tri-ortho-tolyl phosphate at 1200 mg/kg bw. Thirty minutes before dosing, each bird received atropine sulfate at a dose of 25 mg/kg bw by gavage. Thirteen of 30 hens given thiodicarb died, but no deaths occurred in either control group. The groups given thiodicarb or the vehicle showed no evidence of delayed neurotoxicity, whereas nine given tri-ortho-tolyl phosphate had axonal degeneration, necrosis, and demyelination in the sciatic nerve and spinal cord (Myer et al., 1980).
(iv) Studies of antidotes
Groups of five male and five female Sprague-Dawley rats were given thiodicarb (purity, 93%) by gavage in 0.25% methylcellulose at doses between 15 and 225 mg/kg bw, and similar animals received thiodicarb followed immediately by intramuscular injections of atropine and/or 2-pyridine aldoxime hydrochloride, both at 10 mg. Atropine effectively protected against both clinical signs and death. 2-Pyridine aldoxime hydrochloride was ineffective alone and offered no clear benefit over atropine when the two were given in combination (Myers & Christopher, 1986).
(a) Exposure during field use
No published study on the adverse effects of use of thiodicarb was retrieved in a literature search.
(b) Medical surveillance of manufacturing plant personnel
Routine medical monitoring of persons working in thiodicarb manufacture and formulation revealed no adverse effects attributable to thiodicarb during 20 years of production (Bouvier & Kwiatkowski, 1999).
[acetimide-14C]Thiodicarb was rapidly absorbed from the gastrointestinal tract of rats, monkeys (Macaca fascicularis), goats, and cattle and was metabolized extensively to acetonitrile, CO2, and polar components of low relative molecular mass. The initial step in metabolism involves hydrolysis to methomyl, which also inhibits acetylcholinesterase activity. The concentration of radiolabel after administration of [acetimide-14C]thiodicarb at 2 mg/kg bw to rats reached a peak in plasma after 1 h, and it was excreted primarily as volatile components in exhaled air (40%) and urine (30%). The volatile compounds were identified as acetonitrile and CO2. Urine contained predominantly (90%) components that could be extracted in aqueous solvents, although most of the radiolabel in rat urinary was not identified. The residues in the carcass 7 days after dosing represented 79% of the administered dose. Rat erythrocytes contained a large amount of unextractable radiolabel 7 days after dosing, and a similar result was found in hens. Similar patterns of metabolism were seen in rats, monkeys, goats, chickens, and cattle. Investigations in hens, goats, and cattle showed that the radiolabel was incorporated into biomolecules such as lipids, sugars, and egg shell.
In goats given capsules providing doses of 56 mg/kg bw per day (dietary equivalents of 200300 ppm) for 7 days, the peak concentration of radioabel in milk was 1520 ppm, with no individual component representing > 5 ppm. In cows dosed once at 7 mg/kg bw (equivalent to 330 ppm), the peak concentration in milk was 7 ppm. In edible tissues, the highest concentrations of radiolabel were found in liver (25 ppm in goats; 9 ppm in cows). A similar distribution of radiolabel was seen in cows given thiodicarb in the diet at concentrations up to 100 ppm for 21 days. In hens given [acetimide-14C]thiodicarb at concentrations up to 100 ppm of diet for 21 days, the residues in edible tissues (up to 11 ppm in liver) and eggs (peak of 15 ppm in yolk) were present mainly as lipids or unextractable components, with < 1 ppm as acetonitrile or acetamide. Residues of thiodicarb, methomyl, or related carbamates or oximes were not detected in edible products of animals given [acetimide-14C]thiodicarb, and the main residues identified were acetonitrile, acetamide, and acetic acid.
The LD50 values for orally administered thiodicarb were 50100 mg/kg bw. Some studies showed that females were more susceptible than males. Thiodicarb was more toxic when administered orally in an aqueous vehicle than in corn oil. The toxicity of thiodicarb after inhalation varied considerably (LC50 values, 0.1> 2.0 mg/l), depending on the study design used. Overall, it appeared to be moderately toxic when inhaled. It showed little toxicity when applied dermally, with LD50 values typically > 2000 mg/kg bw. It did not significantly irritate the skin or eyes. Weak responses were seen in studies of skin sensitization in guinea-pigs, but an extensive study in humans given patch tests showed no evidence of sensitization. The Meeting concluded that thiodicarb is unlikely to sensitize human skin. WHO (1999) classified thiodicarb as moderately hazardous.
In studies in mammals treated by gavage, cholinergic toxicity was the primary effect. In rats, the time to peak inhibition of cholinesterase activity and effects in a battery of observational tests for function was less than 2 h, some effects first being seen at 0.5 h, with recovery within 24 h. A similar time-scale of effects was reported for inhibition of erythrocyte cholinesterase activity in dogs. These findings are consistent with the toxicokinetics of thiodicarb and of cholinesterase inhibition by carbamates in general, which show rapid reactivation. The Meeting considered that, in a number of studies with thiodicarb, the delay between the last exposure and sampling for erythrocyte or brain acetylcholinesterase activity (up to 48 h) was unacceptably long. Even in studies with repeated dietary doses in which attempts were made to minimize reactivation, thiodicarb often produced no consistent pattern of cholinesterase inhibition. Thiodicarb given in the diet to rodents did not produce cholinergic toxic effects, even at doses that caused significant cholinergic effects when administered by gavage. In studies with repeated doses and sequential measurements of erythrocyte cholinesterase activity over several months, there was no evidence of cumulative inhibition. Some studies showed an adaptive increase in cholinesterase activity.
The most extensive investigations of the cholinergic effects of thiodicarb are studies of neurotoxicity in rats given single or repeated doses. After a single dose by gavage, treatment-related effects were seen at 1 and 4 h but not at 24 h or subsequently. At a dose of 20 or 40 mg/kg bw, a range of effects was found in the a battery of observational tests for function and locomotor activity, with marked depression of cholinesterase activity (> 75%) in plasma, erythrocytes, and brain. At 5 mg/kg bw, the lowest dose tested, some signs of cholinergic effects were noted (pin-point pupils and reduced body temperature), with a significant depression (> 60%) of brain acetylcholinesterase activity. In a study in which rats received diets containing 0, 100, 400, or 800 ppm (equal to 6, 23, and 46 mg/kg bw per day) for 13 weeks, the only statistically significant findings were reductions in body-weight gain and food consumption at 400 and 800 ppm. The NOAEL was 100 ppm (equal to 6 mg/kg bw per day). No effects were observed on function or locomotor activity at any dose or time. As samples for determination of cholinesterase activity were not taken directly after feeding, some reactivation may have occurred. The lack of a clear decrease in brain acetylcholinesterase activity in animals receiving daily doses for 13 weeks that were nine times higher than the LOAEL of single dosing by gavage is probably due to the fact that spreading dietary intake over time led to a lower peak systemic concentration. No information was available on whether the bioavailability of thiodicarb residues in treated crops is reflected better by dietary or gavage treatment. In dogs treated in the diet, in which erythrocyte cholinesterase activity was determined about 2 h after dosing, significant inhibition was detected at ³ 490 ppm (equal to ³ 13 mg/kg bw per day), with a NOAEL of 160 ppm (equal to 4.5 mg/kg bw per day). The LOAELs for overt cholinergic effects (such as tremors) were 10 mg/kg bw per day in a study of developmental toxicity in rats treated by gavage, 20 mg/kg bw in a study of neurotoxicity in rats treated with a single dose by gavage, and 38 mg/kg bw per day in study of toxicity in dogs treated in the diet, with NOAELs of 1, 5, and 13 mg/kg bw per day, respectively.
Dermal exposure of rats to thiodicarb at a dose of 1000 mg/kg bw per day for 15 exposures over 3 weeks resulted in reduced (> 20%) brain acetylcholinesterase activity and alterations in haematological parameters that were qualitatively consistent with those observed in animals exposed orally.
The principal non-cholinergic effects of thiodicarb were reduced body-weight gain and food consumption and altered erythrocyte parameters with associated splenic lesions. These changes were seen in all species tested (mice, rats, and dogs), with no clear indication that any species was especially sensitive. The reduced food consumption and body-weight gain were generally, but not always, related, and were more prevalent at the beginning of a study, possibly indicating a local effect or unpalatability rather than systemic toxicity. Reductions in erythrocyte count, erythrocyte volume fraction, and haemoglobin concentration, sometimes associated with increased mean cell volume and reticulocyte number, were seen after 90 days of exposure at 45 mg/kg bw per day in dogs or 150 mg/kg bw per day in rats. The LOAEL in rats exposed for 79 weeks was 12 mg/kg bw per day. Increased relative spleen weight was seen in rats given thiodicarb for 2 weeks at doses ³ 120 mg/kg bw per day, which progressed with the duration of dosing, such that after 2 years exposure at 12 mg/kg bw per day there was an increased incidence of splenic extramedullary haematopoiesis. The effects on the spleen (haemosiderosis, increased weight, and extramedullary haematopoiesis) were consistent with macrocytic anaemia and the resulting homeostatic response. The overall NOAEL for erythrocytic and splenic effects was 3 mg/kg bw per day. Evidence of liver hypertrophy was seen in mice given thiodicarb at doses ³ 1800 ppm (equal to 350 mg/kg bw per day) for 4 weeks and in dogs receiving 90 mg/kg bw per day for 13 weeks or 45 mg/kg bw per day for 26 weeks; there was no consistent evidence of hepatotoxicity in rats. Occasional findings, such as fluctuations in potassium content (decreased in rats, increased in dogs) and alterations in urinary pH and volume, were not reproducible, were not associated with histopathological findings, and were considered to be of no significance for human risk assessment.
The carcinogenic potential of thiodicarb was investigated in two studies in mice and two studies in rats. In the first study in mice, the incidences of tumours were not increased at the highest dose tested (10 mg/kg bw per day), a dose which increased the mortality rate during some segments of the study. In the second study, mice received diets that provided doses up to 1000 mg/kg bw per day, which is more than 10 times the LD50 value after a single dose by gavage. Administration of the highest dose was associated with statistically significant increases in the incidences of hepatocellular carcinoma and adenoma. The incidence of hepatocellular adenoma in male mice receiving a dietary concentration equal to 70 mg/kg bw per day was increased (22%), and although not statistically significant it was marginally greater than the higher value of the range in historical controls (018%). This dose also increased the incidences of liver masses and hepatocellular pleiomorphism in males. Female mice given a dietary concentration equal to 70 mg/kg bw per day did not show increased incidences of neoplastic or non-neoplastic liver lesions. The NOAEL was 5 mg/kg bw per day. The Meeting concluded that the liver tumours were not relevant to human risk assessment, as the dose of 1000 mg/kg bw per day exceeded the maximum tolerated dose and the tumours occurred in an organ that showed significant non-neoplastic effects.
The first study of carcinogenicity in rats involved the Fischer 344 strain and a high dose equal to 10 mg/kg bw per day, and it was not clearly demonstrated that thiodicarb had been tested at the maximum tolerated dose. The only indication of tumorigenesis was a low incidence of thymomas (2%) in males receiving 10 mg/kg bw per day. The rate was greater than the value for concurrent controls, and the lesion was consistent with hyperplasia of lymphoid and epithelial cells of the thymus. However, the incidence of thymoma was not increased in females in this study nor in Sprague-Dawley rats of either sex. The NOAEL in the study with Fischer 344 rats was 3 mg/kg bw per day.
In the second study of carcinogenicity, Sprague-Dawley rats received diets containing thiodicarb at concentrations up to 900 ppm (equal to 60 mg/kg bw per day). The overall incidence of benign and malignant tumours was lower in animals at the highest dose than in controls even though the survival rate was higher. The incidences of tumours in the liver and thymus were not increased. The incidence of thyroid C-cell carcinomas in males at the highest dose (2/50) was marginally greater than the upper bound of the range in historical controls (02%), but it was not statistically significant, was associated with a reduced incidence of C-cell adenomas, and was not reproduced in females. Males at the highest dose also had an increased incidence of interstitial-cell adenoma of the testis (12/50), which was greater than that in historical controls (010%), and was associated with an increased incidence of testicular atrophy. Interstitial-cell tumours are an age-related finding in rats, and the long survival of these animals may have contributed to the finding. A statistical analysis corrected for survival showed that the finding could have been due to chance (odds ratio, 2.9; 95% confidence interval, 0.99.4). The NOAEL for tumours was 200 ppm (equal to 12 mg/kg bw per day), and the overall NOAEL was 60 ppm (equal to 3 mg/kg bw per day). The Meeting concluded that there was no consistent evidence that thiodicarb has significant carcinogenic potential in rats.
An extensive range of studies has been performed for genotoxicity with thiodicarb, both in vitro and in vivo. Positive findings were reported at cytotoxic concentrations in an assay for gene mutation in mouse lymphoma L5178Y cells and over a range of concentrations in an assay for mitotic gene conversion in Saccharomyces cerevisiae. Negative results were seen in seven further studies in vitro and in three conducted in vivo. The Meeting concluded that thiodicarb is unlikely to be genotoxic in vivo.
In view of the lack of genotoxicity in vivo and the finding of significant increases in the incidence of tumours only in mice and only at concentrations that were clearly toxic, the Meeting concluded that thiodicarb is not likely to pose a carcinogenic risk to humans.
In studies of reproductive toxicity in rats, thiodicarb did not adversely affect mating performance or litter size at birth when given at doses up to 3000 ppm (equivalent to 180 mg/kg bw per day). In a three-generation study of reproductive toxicity, there were no effects on pup survival or development at the highest dose, equivalent to 10 mg/kg bw per day. In a single-generation range-finding study and a two-generation study, pup weights and survival to day 4 were consistently reduced at doses of 15 mg/kg bw per day and above. An increased frequency of pups found dead with no milk in the stomach was seen at doses ³ 15 mg/kg bw per day, the cause of which was not determined. At 15 mg/kg bw per day, there was evidence of maternal toxicity, with a 1015% deficit in body weight. Measurements of cholinesterase activities in 21-day old F2b pups of dams exposed at 900 ppm (equal to 72 mg/kg bw per day) showed a statistically nonsignificant degree of inhibition, which was not seen in the parents or in other generations. The overall NOAEL in the three studies of reproductive toxicity was 10 mg/kg bw per day.
Thiodicarb was tested for developmental toxicity in mice, rats, and rabbits at doses up to 200, 100, and 40 mg/kg bw per day, respectively, all of which caused death or marked toxicity in dams. The LOAEL for maternal toxicity in the most sensitive species, rats, was 10 mg/kg bw per day, with a NOAEL of 1 mg/kg bw per day. Delayed ossification and reduced fetal weight were seen in one study at maternally toxic doses of ³ 10 mg/kg bw per day, but not in mice or rabbits or in two other studies in rats. The Meeting concluded that thiodicarb is not teratogenic.
The Meeting concluded that the existing database on thiodicarb is adequate to characterize the potential hazard to fetuses, infants, and children. Although thiodicarb is known to be neurotoxic in adults, the Meeting did not recommend that a study of developmental neurotoxicity be conducted since there was no clear evidence that offspring are more sensitive after pre- or postnatal exposure than adults in the same experiment.
As is to be expected of a carbamate, thiodicarb did not induce delayed neuropathy in hens administered a single dose of 660 mg/kg bw.
No studies have been performed in which thiodicarb was given orally to volunteers. Routine medical monitoring of persons working in thiodicarb manufacture and formulation revealed no adverse effects attributable to exposure to thiodicarb during 20 years of production.
The Meeting maintained the previously established ADI of 00.03 mg/kg bw, as it considered that it was still appropriate for use as a basis for assessing the risks associated with long-term intake. This conclusion was based on the NOAEL of 3 mg/kg bw per day for effects on erythrocytes and splenic haemosiderosis or extramedullary haematopoiesis in long-term studies of toxicity and carcinogenicity in rats and a safety factor of 100.
The Meeting concluded that the toxicological profile of thiodicarb requires establishment of an acute RfD and that the most appropriate end-points are cholinergic signs and inhibition of acetylcholinesterase activity. The Meeting established an acute RfD of 0.04 mg/kg bw for thiodicarb by applying a safety factor of 25 to the NOAEL of 1 mg/kg bw per day for clinical signs in the study of developmental toxicity in rats treated by gavage. A 25-fold safety factor was used because the data on thiodicarb indicate that the cholinergic effects are associated with the peak systemic concentration, and there is evidence that effects related to peak concentrations vary less between species and within populations than those related to a product of concentration and time (for additional details, see Annex 5). The acute RfD is supported by the NOAEL of 4.5 mg/kg bw per day for erythrocyte acetylcholinesterase activity in dogs 2 h after dosing and provides a margin of 125 on the LOAEL of 5 mg/kg bw per day for inhibition of acetylcholinesterase activity and related findings in the study of neurotoxicity in rats treated with a single dose by gavage.
Levels relevant to risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
2-year studies of toxicity and carcinogenicitya,b |
Toxicity |
5 mg/kg bw per day |
10mg/kg bw per day |
|
|
|
Carcinogenicity |
70 mg/kg bw per day |
1000 mg/kg bw per dayc |
|
|
Developmental toxicityd |
Maternal toxicity |
100 mg/kg bw per day |
200 mg/kg bw per day |
|
|
Embryo- and fetotoxicity |
200 mg/kg bw per day |
|
|
|
Rat |
2 year studies of toxicity and carcinogenicitya |
Toxicity |
60 ppm, equal to 3 mg/kg bw per day |
60 ppm, equal to 5 mg /kg bw per day (52 weeks) |
|
|
|
Carcinogenicity |
900 ppm, equal to 60 mg/kg bw per daye |
|
|
|
Multi-generation study of reproductive toxicitya,b |
Maternal and pup toxicity |
10 mg/kg bw per day |
300 ppm, equivalent to 15 mg/kg bw per day |
|
|
Developmental toxicityd |
Maternal toxicity |
1 mg/kg bw per day |
10 mg/kg bw per day |
|
|
|
Embryo- and fetotoxicity |
100 mg/kg bw per daye |
|
|
|
Acute neurotoxicityd |
|
|
5 mg/kg bw f |
|
Rabbit |
Developmental toxicityd |
Maternal toxicity |
20 mg/kg bw per day |
40 mg/kg bw per day |
|
|
|
Embryo- and fetotoxicity |
40 mg/kg bw per daye |
|
|
Dog |
1-year study of toxicitya |
Toxicity4.5 mg/kg bw per day |
160 ppm, equivalent to 13 mg/kg bw per day |
490 ppm, equivalent to |
a
Dietary administrationb
Two or more studies combinedc
Greater than the maximum tolerated dosed
Gavagee
Highest dose testedf
Lowest dose testedEstimate of acceptable daily intake for humans
00.03 mg/kg bw
Estimate of acute reference dose
0.04 mg/kg bw
Studies that would provide information valuable for continued evaluation of the compound
Further observations in humans
Summary of critical end-points
|
Absorption, distribution, excretion and metabolism in mammals |
|
|
Rate and extent of oral absorption |
Rapid (60% within 15 min) and extensive (> 70%) |
|
Distribution |
Extensive; highest concentration in erythrocytes |
|
Potential for accumulation |
Low, with the exception of erythrocytes |
|
Rate and extent of excretion |
Rapid (mainly in 012-h samples) and extensive; significant proportion as exhaled volatile compounds |
|
Metabolism in animals |
Very extensive, primary excretion as acetonitrile and CO2; retained radiolabel may be associated with simple carbon compounds incorporated into biomolecules |
|
Toxicologically significant compounds |
Thiodicarb and methomyl |
|
Acute toxicity |
|
|
Rats, LD50, oral |
50100 mg/kg bw (depending on vehicle) |
|
Rats, LD50, intraperitoneal |
No data |
|
Mice, LD50, oral |
75 mg/kg bw |
|
Skin sensitization (test method used) |
Negative or weak response in guinea-pigs (Buehler); negative in human patch test |
|
Short-term toxicity |
|
|
Target/critical effect |
Cholinesterase inhibition, effects on erythrocytes (mild macrocytic anaemia) and associated splenic findings |
|
Lowest relevant oral NOAEL |
4.5 mg/kg bw per day (52 weeks, dogs) |
|
Genotoxicity |
Negative in vivo |
|
Long-term toxicity and carcinogenicity |
|
|
Target/critical effect |
Macrocytic anaemia, splenic effects (haemosiderin deposition, extramedullary haematopoiesis); liver hyperplasia in mice |
|
Lowest relevant NOAEL |
3 mg/kg bw per day (rats) |
|
Carcinogenicity |
Liver tumours in mice at toxic doses; clear NOAELs identified. Unlikely to pose a risk to humans |
|
Reproductive toxicity |
|
|
Reproduction target/critical effect |
Reduced pup viability and weight |
|
Lowest relevant reproductive NOAEL |
10 mg/kg bw per day (rats) |
|
Developmental target/critical effect |
Not teratogenic; no specific embryo- or fetotoxicity |
|
Lowest relevant developmental NOAEL |
> 40 mg/kg bw per day (rabbits) |
|
Neurotoxicity |
|
|
Acute |
< 5 mg/kg bw; cholinesterase inhibition; no neuropathy |
|
90 days |
6 mg/kg bw per day; no neuropathy |
|
Delayed neuropathy |
None |
|
Medical data |
No adverse effects in production workers |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
00.03 mg/kg bw |
Rat, repeated doses |
100 |
|
Acute RfD |
0.04 mg/kg bw |
Rat, developmental and maternal toxicity |
25 |
References are identified with the codes GLP or QA to indicate whether they contained statements of compliance with the principles of Good Laboratory Practice or were subjected to internal Quality Assurance checks, respectively.
Andrawes, N.R. & Bailey, R.H. (1980) Fate of acetyl-1-14C thiodicarb in laying chickens under continuous feeding conditions. Unpublished report no. UCC 814C50, file no.27414 from Union Carbide Corporation. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Atkinson, C. & Perry, C. (1991) Thiodicarb, 2-week dietary range-finding study in rats. Unpublished report no. IRI 7652 Project No. 450420 from Inveresk Research International. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Atkinson, C. & Robb, D.T. (1992) Thiodicarb, 13-week dietary toxicity study in rats. Unpublished report no. IRI 7719 Project No. 450436 from Inveresk Research International. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Atkinson, C., Perry, C. & Hudson, P. (1991) Thiodicarb, 4-week dietary dose range-finding study in mice. Unpublished report no. IRI 7430 Project No. 451048 from Inveresk Research International. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Atkinson, C., Perry, C., Hudson, P., Snodgrass, E. & Ishwariah, V. (1993) Thiodicarb, 97-week dietary carcinogenicity study in mice with 52 week interim kill. Unpublished report no. IRI 7749 Project No. 439056 from Inveresk Research International. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Atkinson, C., Hudson, P. & Ishwariah, V. (1994a) Thiodicarb, 104-week dietary carcinogenicity study in rats with 52 week interim kill. Results after 52 weeks. Unpublished report no. IRI 7881 Project No. 450441 from Inveresk Research International. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Atkinson, C., Hudson, P., Willerton, J. & Ishwariah, V. (1994b) Thiodicarb, 104-week dietary carcinogenicity study in rats. Unpublished report no. IRI 11026 Project No. 450441 from Inveresk Research International. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Becci, P.J., Schwartz, C.S. & Parent, R.A. (1979) Evaluation of UC51762 No. 40-488 as a potential delayed neurotoxic agent following oral administration to hens protected by atropine sulfate. Unpublished report No. 6065 from Food and Drug Research Laboratories. Submitted to 1985 JMPR by Union Carbide Agricultural Products Co., Inc.
Beyrouty, P. (2000a) A time to peak behavioral and neurochemical effects study of a single oral administration of thiodicarb technical in rats. Unpublished report no. Project 97505 from ClinTrials BioResearch Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Beyrouty, P. (2000b) An acute study of the potential effects of orally administered thiodicarb technical on behavior, neurochemistry and neuromorphology in rats. Unpublished report no. Project 97508 from ClinTrials BioResearch Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Beyrouty, P. (2000c) A 13-week dietary study of the potential effects of orally administered thiodicarb technical on behavior, neurochemistry and neuromorphology in rats. Unpublished report no. Project 97509 from ClinTrials BioResearch Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Bouvier, G. (2000) Thiodicarb: Toxicology study endpoint selection for the acute reference dose. Position paper Submitted to WHO by Aventis Crop Science, France, 31 August 2000.
Bouvier, G. & Kwiatkowski, P. (1999) ThiodicarbToxicological evaluation. Unpublished document submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Cameron, J.T. & Wolfe, G.W. (1979) Acute eye irritation study in rabbits: Larvin insecticide technical (UC 51762). Unpublished report no. HLA Project No. 400-616 from Hazleton Laboratories America. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Carabello, F.B., Kampjake, J., Ragsdale, W.E. & Weber, E.M. (1980) Repeated insult patch test for Union Carbide Corporation. Unpublished report no. 80-0589-70,73 from Hill Top Research Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Cifone, M.A. & Brusick, D.J. (1985a) Mutagenicity of thiodicarb in a mouse lymphoma mutation assay. Unpublished report no. LBI Project No. 20989 from Litton Bionetics, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Cifone, M.A. & Brusick, D.J.(1985b) Mutagenicity of thiodicarb in the rat primary hepatocyte unscheduled DNA synthesis assay. Unpublished report no. LBI Project No. 20991 from Litton Bionetics, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Clare, C. (1996) Thiodicarb: Measurement of unscheduled DNA synthesis in the mouse liver using an in vivo/in vitro procedure. Unpublished report no. CH 198/97-1052 from Corning Hazleton. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Coate, W.B., Keenan, D.L., Dawkins, K. & Hardy, R.J. (1979) Acute inhalation toxicity study in rats, Larvin (UC 51762 technical dust). Unpublished report no. HLA Project No. 400-615 from Hazleton Laboratories America. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Conroy, W.J., Depass, L.R., Homan, E.R., Weil, C.S., Geary, D.L. & Frank, F.R. (1979a) UC 51762 16-dose rabbit dermal study. Unpublished report no. CHF 42-52 from Chemical Hygiene Fellowship, Carnegie Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Conroy, W.J., Depass, L.R., Homan, E.R., Weil, C.S., Geary, D.L. & Frank, F.R. (1979b) UC 51762 technical purified grade dermal sensitization potential in the guinea pig. Unpublished report no. CHF 42-61 from Chemical Hygiene Fellowship, Carnegie Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Dange, M. (1995) RPA 051762 oral LD50 in the mouse. Unpublished report no. SA 95154, 95187,95221 from Rhone-Poulenc Agrochimie. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
DePass, L.R., Mirro, E.J. & Frank, F.R. (1982.)UC 51762 inclusion in the diet of rats for 28 days. Unpublished report no. BRRC Report No. 45-19 from Bushy Run Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Dickey, C.L., Nachreiner, D.J., DePass, L.R., Gad, S.C., Weil, C.S., Geary, D.L. & Frank, F.R. (1979) UC 51762 technical: Acute and 9-day dust inhalation study on rats. Unpublished report no. CHF No. 42-63 from Chemical Hygiene Fellowship, Carnegie Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Feung, C.S., College, P.R. & Chancey, E.L. (1980) Studies on the disposition of 14C thiodicarb in lactating cows. Unpublished report no. UCC 814C50, file no.27350 from Union Carbide Corporation. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Field, W.E. (1979a) Acute dermal toxicity in rats, sample number UC-51762. Unpublished report no. CDC-UC-012-79 from CDC Research, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Field, W.E. (1979b) UC-51762 skin sensitization in guinea pigs. Unpublished report no. CDC-UC-003-79 from CDC Research, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Gallo, M.A. & Stevens, K.R. (1980) Evaluation of Larvin (UC 51762): 21-day dermal toxicity study in rabbits. Unpublished report no. Snell Project No. 02413-090 from Booz, Allen & Hamilton. Inc., Foster D. Snell Division. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Glaza, S.M. (1996a) Acute dermal toxicity study of thiodicarb technical in rabbits. Unpublished report no. CHW 50702036 from Corning Hazelton Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Glaza, S.M. (1996b) Primary dermal irritation study of thiodicarb technical in rabbits. Unpublished report no. CHW 50702037 from Corning Hazelton Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Glaza, S.M. (1996c) Primary eye irritation study of thiodicarb technical in rabbits. Unpublished report no. CHW 50702038 from Corning Hazelton Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Hamada, N.N., Hagan, W.H., Lewis, S.A., Alsaker, R.D., Vargas, K.J., Burns, J.M., Thakur, A.K. & Marshall, P.M. (1986) One year feeding study in dogs: thiodicarb technical. Unpublished report no. HLA Project No. 2100-126 from Hazelton Laboratories America. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Hanlon, C.M. & Norris, K.J. (1993) Metabolism of 14C-thiodicarb in lactating goats. Unpublished report no. Project 1245EC-91-170 from Analytical Development Corporation, Colorado. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Hawkins, D.R., Mayo, B.C., Pollard, A.D. & Haynes, L.M. (1993) 14C-Thiodicarb. The metabolism in monkeys. Unpublished report no. HRC/RNP/ 398/92/1553 from Huntingdon Research Center, Ltd. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Henwood, S.M. (1991) Range finding reproduction study with thiodicarb technical in rats. Unpublished report no. HLA6224-164 from Hazleton Wisconsin, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Henwood, S.M. (1992) Two generation reproduction study with thiodicarb technical in rats. Unpublished report no. 6224-166 from Hazleton Wisconsin, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Hiles, R.A. (1987) The metabolism of thiodicarb (acetyl-1-14C) in albino rats. Unpublished report no. HLA 6224-100 from Hazleton Laboratories America, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Homan, E.R., Maronpot, R.R., Reid, J.B. & Cox, E.F. (1978a) UC 51762 bis(1-methylthioacet-aldehyde-O-(N-methylcarbamoyl)oximo)sulfide: inclusion in the diet of rats for thirteen weeks. Unpublished report no. CHF 41-63 from Chemical Hygiene Fellowship, Carnegie-Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Homan, E.R., Fowler, E.H., Reid, J.B. & Cox, E.F. (1978b) UC 51762 bis(1-methylthioacet-aldehyde-O-(N-methylcarbamoyl)oximo)sulfide: inclusion in the diet of dogs for thirteen weeks. Unpublished report no. CHF 41-98 from Chemical Hygiene Fellowship, Carnegie-Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Ivett, J.L. & Brusick, D.J. (1985) Clastogenic evaluation of 91.48% ai thiodicarb in an in vitro cytogenetic assay measuring chromosomal aberration frequencies in Chinese hamster ovary (CHO) cells. Unpublished report no. LBI Project No 20990 from Litton Bionetics, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Jagannath, D.R. & Brusick, D.J. (1978) Mutagenic evaluation of CHF 41-43 (UC 51762) by the Ames Salmonella/microsome plate test. Unpublished report no. LBI Project No 20838 from Litton Bionetics, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Janes, J.M., Rodwell, D.E. & Jessup, D.C. (1980) UC 51762 teratology study in mice. Unpublished report no. IRDC 369-031from International Research and Development Corporation. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Kern, T.G. (1999) Acute oral toxicity study of thiodicarb in albino rats. Unpublished report no. WIL-21152 from WIL Research Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Khasawinah, A.M. & College, P.R. (1978) Fate of a single oral dose of 14C-acetyl UC 51762 in a lactating cowmetabolism into natural products. Unpublished report no. UCC 814C21, file no.25257 from Union Carbide Corporation. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Lemen, J.K., Wolfe, G.W. & Voelker, R.W. (1979) Acute dermal administration in rabbits: Larvin insecticide technical (UC 51762). Unpublished report No. HRL Project No. 400-614 from Hazleton Research Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Mallory, V.T., Naismith, R.W. & Matthews, R.J. (1982a) Acute oral toxicity study in rats (14 days): Larvin analytical standard. Unpublished report no. PRL Report No. PH 402-UC-010-82 from Pharmakon Research Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Mallory, V.T., Naismith, R.W. & Matthews, R.J. (1982b) Acute oral toxicity study in rats (14 days): Larvin analytical standard. Unpublished report no. PH 402-UC-008-82 from Pharmakon Research Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Mallory, V.T., Naismith, R.W. & Matthews, R.J. (1982c) Acute oral toxicity study in rats (14 days): Larvin technical pilot plant batch 34. Unpublished report no. PH 402-UC-006-82 from Pharmakon Research Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Mallory, V.T., Naismith, R.W. & Matthews, R.J. (1982d) Acute oral toxicity study in rats (14 days): Larvin technical. Unpublished report no. PH 402-UC-005-82 from Pharmakon Research Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Mallory, V.T., Naismith, R.W. & Matthews, R.J. (1982e) Acute oral toxicity study in rats (14 days): Larvin analytical standard. Unpublished report no. PH 402-UC-011-82 from Pharmakon Research Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Mallory, V.T., Naismith, R.W. & Matthews, R.J. (1982f) Acute oral toxicity study in rats (14 days): Larvin technical lab preparation. Unpublished report no. PH 402-UC-009-82 from Pharmakon Research Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Mallory, V.T., Naismith, R.W. & Matthews, R.J. (1982g) Acute oral toxicity study in rats (14 days): Larvin technical pilot plant batch 34. Unpublished report no. PH 402-UC-007-82 from Pharmakon Research Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Mallory, V.T., Naismith, R.W. & Matthews, R.J. (1982h) Acute dermal toxicity test in rabbits: Larvin technical. Unpublished report no. PH 422-UC-005-82 from Pharmakon Research Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Mallory, V.T., Naismith, R.W. & Matthews, R.J. (1982i) Primary dermal irritation study in rabbits: Larvin technical. Unpublished report no. PH 402-UC-002-82 from Pharmakon Research Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Mallory, V.T., Naismith, R.W. & Matthews, R.J. (1982j) Primary eye irritation test in rabbits: Larvin technical. Unpublished report no. PH 402-UC-004-82 from Pharmakon Research Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Meyer, J., Dean, W.P. & Jessup, D.C. (1980) Acute delayed neurotoxicity study in hens. Unpublished report No. 369-047 from Mellon Institute. Submitted to 1985 JMPR by Union Carbide Agricultural Products Co., Inc.
Meyers, R.C. & Christopher, S.M. (1986) Thiodicarb determination of antidotal effectiveness of 2-PAM and atropine in the rat. Unpublished report no. BRRC 48-180 from Bushy Run Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Myers, R.C., Weil, C.S. & Carpenter C.P. (1975) UC 51762: Bis(1-Methylthioacetaldehyde-O(N-methylcarbamoyl)oximino)sulfide rangefinding studies. Unpublished report no. CHF 38-55 from Chemical Hygiene Fellowship, Carnegie Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Myers, R.C., Carpenter, C.P. & Cox, E.F. (1977) Miscellaneous toxicity studies. Unpublished report no. 40-61 from Chemical Hygiene Fellowship, Carnegie Mellon Institute of Research . Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Myers, R.C., DePass, L.R., Homan, E.R., Weil, C.S. & Frank, F.R. (1979) UC 51762 Technical: rangefinding studies. Unpublished report no. CHF 42-19 from Chemical Hygiene Fellowship, Carnegie Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Myers, R.C., Weil, C.S. & Frank, F.R. (1982a) Larvin technical acute peroral toxicity study. Unpublished report no. BRRC 45-40 from Bushy Run Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Myers, R.C., Weil, C.S. & Frank, F.R. (1982b) Acute peroral toxicity study: Larvin analytical standard. Unpublished report no. BRRC 45-152 from Bushy Run Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Myers, R.C., Weil, C.S. & Frank, F.R. (1982c) Acute peroral toxicity study: Larvin technical-lab preparation Unpublished report no. BRRC 45-151 from Bushy Run Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Myers, R.C., Weil, C.S. & Frank, F.R. (1982d) Acute peroral toxicity study: Larvin technical-pilot plant batch 34. Unpublished report no. BRRC 45-141 from Bushy Run Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Nachreiner, D.J. & Dodd, D.E. (1988) Larvin technical DOT acute dust aerosol inhalation toxicity test in rats. Unpublished report no. Project ID No. 51-538 from Bushy Run Research Center. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Naismith, R.W. & Matthews, R.J. (1979a) UC 51762 technical; primary DNA damage Escherichia coli plate test. Unpublished report no. PH 305-AM-002-79 from Pharmakon Laboratories. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Naismith, R.W. & Matthews, R.J. (1979b) UC 51762 technical: Reverse mutation in Saccharomyces cerevisiae. Unpublished report no. PH 303-UC-001-79 from Pharmakon Laboratories. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Naismith, R.W. & Matthews, R.J. (1979c) UC 51762 technical: Mitotic crossing over in Saccharomyces cerevisiae. Unpublished report no. PH 302-UC-001-79 from Pharmakon Laboratories. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Naismith, R.W. & Matthews, R.J. (1979d) UC 51762 technical: Mitotic gene conversion in Saccharomyces cerevisiae. Unpublished report no. PH 304-UC-001-79 from Pharmakon Laboratories. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Naismith, R.W. & Matthews, R.J. (1979e) UC 51762 technical: Micronucleus test. Unpublished report no. PH 309-UC-001-79 from Pharmakon Laboratories. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Rieth, J.P. (1995a) 97 week dietary carcinogenicity study in mice with 52 week interim kill; supplemental subchronic data and background tumor incidences. Unpublished report. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Rieth, J.P. (1995b) 104-week dietary carcinogenicity study in rats; additional statistical analysis and background tumor incidence. Unpublished report. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Rodwell, D.E. (1986) A teratology study in rabbits with thiodicarb. Unpublished report no. WIL-95002 from WIL Research Laboratories. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Rush, R.E. (1991) Dermal sensitization study in guinea pigs with thiodicarb technical. Unpublished report no. SLS 3147.112 from Springborn Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Schardein, J. (1982) Larvin technical grade 21-day dermal toxicity study in rabbits. Unpublished report no. 369-053 from International Research and Development Corporation. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Schwartz, C.S. & Stevens, K.R. (1978) Acute oral LD50 in hens. Unpublished report No. 6064 from Food and Drug Research Laboratories. Submitted to 1985 JMPR by Union Carbide Agricultural Products Co., Inc.
Starr, T.B. (1999) Establishing a point of departure with benchmark dose methodology for use in reference dose calculations for thiodicarb. Unpublished report. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Tasker, E.J., Rodwell, D.E. & Jessup, D.C. (1979) UC 51762 teratology study in rats. Unpublished report no. 369-029 from International Research and Development Corporation. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Tyl, R.W., Marr, M.C. & Myers, C.B. (1993) Developmental toxicity evaluation of thiodicarb administered by gavage to CD (Sprague-Dawley) rats. Unpublished report no. RTI-65C-5345 from Research Triangle Institute. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Weatherholtz, W.M. , Rendon, F., Strausburg, J.C. & Colpean, B.R. (1982) Fourteen-day eye irritation study in monkeys. Larvin technical. Unpublished report no. 400-648 from Hazleton Laboratories, Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Wentz, K.L. & Wolfe, G.W. (1979) Primary skin irritation study in rabbits: Larvin insecticide technical (UC51762). Unpublished report no. 400-617 from Hazleton Laboratories Inc. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
WHO (1996) Environmental Health Criteria 178: Methomyl, Geneva.
WHO (1999) Recommended classification of pesticides by hazard and guidelines to classification 19981999 (WHO/PCS/98.21/Rev.1), Geneva, International Programme on Chemical Safety (www.who.int/pcs/pcs-act.htm).
Wolfe, G.W. (1981) Subchronic toxicity study in dog: Larvin thiodicarb. Unpublished report no. HRL 400-626 from Hazleton Research Laboratories. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (GLP).
Woodside, M.D., Weil, C.S. & Cox, E.F. (1975) UC 51762 dimethyl N,N-[thiobis[(methylimino)carbonoyl-oxy]]bis[ethanimidothioate] inclusion in the diet of rats for 7 days. Unpublished report no. 38-136 from Chemical Hygiene Fellowship, Carnegie-Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Woodside, M.D., DePass, L.R., Ried, J.B. & Cox, E.F. (1978) UC 51762 dimethyl N,N-[thiobis[(methylimino)-carbonoyloxy]]bis[ethanimidothioate] inclusion in the diet of rats for 7 days. Unpublished report no. 41-100 from Chemical Hygiene Fellowship, Carnegie-Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L. & Frank, F.R. (1979a) UC 51762 dimethyl N,N-[thiobis[(methylimino)carbonoyloxy]]bis[ethanimidothioate] inclusion in the diet of rats for thirteen weeks. Unpublished report no. 42-125 from Chemical Hygiene Fellowship, Carnegie-Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L. & Frank, F.R. (1979b) UC 51762 technical; inclusion in the diet of rats for three generations and dominant lethal mutagenesis studies. Unpublished report no. CHF 42-65 from Chemical Hygiene Fellowship, Carnegie-Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L. & Frank, F.R. (1979c) UC 51762 rat teratology studies. Unpublished report no. CHF 42-48 from Chemical Hygiene Fellowship, Carnegie-Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L. & Frank, F.R. (1980a) UC 51762; dimethyl N,N-(thiobis((methylimino) carbonoyloxy)) bis (ethanimidothioate): chronic oncogenicity feeding study in mice. Unpublished report no. CHF 43-10 from Chemical Hygiene Fellowship, Carnegie-Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L. & Frank, F.R. (1980b) UC 51762; dimethyl N,N-(thiobis((methylimino)carbonoyloxy))bis(ethanimidothioate): chronic toxicity and oncogenicity feeding study in Fisher 344 rats. Unpublished report no. CHF 43-18 from Chemical Hygiene Fellowship, Carnegie-Mellon Institute of Research. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
Yoshida, A., Maita, K., Saito, T. & Miyaoka, T. (1983a) Larvin: 4 week range finding study in mice. Unpublished report from Institute of Environmental Toxicology, Japan. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA.
Yoshida, A., Takahashi K., Kosaka, T., Miyaoka, T., Maita, K. & Shirasu, Y. (1983b) Thiodicarb: 3 month oral subchronic toxicity study in mice (study I). Unpublished report from Institute of Environmental Toxicology, Japan. Submitted to WHO by Rhone-Poulenc, Inc., Research Triangle Park, North Carolina, USA (QA).
See Also:
Toxicological Abbreviations
Thiodicarb (Pesticide residues in food: 1985 evaluations Part II Toxicology)
Thiodicarb (Pesticide residues in food: 1986 evaluations Part II Toxicology)