
THIODICARB
EXPLANATION
Thiodicarb is a non-systemic carbamate insecticide with a
relatively narrow spectrum of activity closely related to its first
metabolite, methomyl. It is specific against Lepidopterous pests,
controlling larvae at different stages as well as eggs in many
instances.
Thiodicarb was reviewed for the first time by the Joint Meeting
in 1985.
IDENTITY
CHEMICAL NAME
3,7,9,13-tetramethyl-5,11-dioxa-2,8,14-trithia-4,7,9,12-
tetraazapentadeca-3,12-diene-6,10-dione
IUPAC - Dimethyl N,N'-thiobis-[O-(methylcarbamoyl)
thiolacetohydroxamate]
CAS - Ethanimidothioic acid, N,N'-[thiobis[(methylimino)-
carbonyloxy]]bis, -dimethyl ester (CAS Registry No. 59669-26-0)
SYNONYMS
Dimethyl N,N'[thiobis[(methylimino)carbonyloxy]]bis
[ethanimidothioate]
LARVIN(R); NIVRAL(R); SEMEVIN(R)
EMPIRICAL FORMULA
C10H18N4O4S3
STRUCTURAL FORMULA
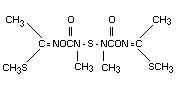 OTHER INFORMATION ON IDENTITY AND PROPERTIES
MOLECULAR WEIGHT: 354.5
PHYSICAL STATE
AND COLOUR: crystalline powder, white to light tan
ODOUR: slightly sulfurous
DENSITY (g/ml): 1.4424 at 20°C
VAPOUR PRESSURE: 4.3 × 10 -5 at 20°C
(mm Hg) 1.1 × 10 -4 at 35°C
2.1 × 10 -4 at 40°C
3.2 × 10 -4 at 45°C
4.2 × 10 -4 at 50°C
5.2 × 10 -4 at 55°C
MELTING POINT: 173-174°C
FLAMMABILITY: generates a flammable vapor space (methanol)
REACTIVITY: stable in light and ambient conditions. At
elevated temperatures (e.g. above 100°C), the
product decomposes into the following principal
degradation products; carbon dioxide, acetonitrile
and dimethyl disulfide. The decomposition process
is catalyzed by Lewis-acid type heavy metal salts.
Can be hydrolyzed under acid- or base-catalyzed
conditions. The principal degradation product from
the hydrolysis of thiodicarb is methomyl.
OXIDIZING OR
REDUCING ACTION: Neither the product nor any of the accompanying
impurities have the tendency to act as an
oxidizing or reducing agent.
pH: 1 g/100 ml H2O produces a pH of 6.65 at 21.5°C
SOLUBILITY
(wt. %): Xylene 0.1 at 50°C
0.4 at 68°C
1.0 at 92°C
2.5 at 106°C
5.0 at 119°C
Pyridine 1.2 at 25°C
6.4 at 45°C
13.4 at 60°C
Dichloromethane 10.2 at 0°C
11.4 at 5°C
16.1 at 30°C
Acetone 0.8 at 25°C
Acetonitrile 2.0 at 25°C
Ethyl acetate 0.2 at 25°C
Ethyl ether 0.1 at 25°C
Methanol 0.3 at 25°C
Tetrachloroethylene 0.1 at 25°C
Water (distilled) 35 ppm at 25°C
OCTANOL/WATER
PARTITION
COEFFICIENT: 1.65 (Log P by reverse phase TLC technique)
STORAGE
STABILITY: Minimum storage life is typically in excess of 2
years.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, excretion, and biotransformation
Thiodicarb, which essentially consists of two methomyl moieties
joined through their amino nitrogen by sulfur, is rapidly degraded to
S-methyl-N-[(methylcarbamoyl)oxy]thioacetamide (methomyl) in the rat
stomach. Over 30% of an oral dose of 16 mg thiodicarb per kg. b.w. was
converted to methomyl in the gut within 15 minutes. Only 40% of the
oral dose was found in the rat gut at 15 minutes, indicating that the
compound had been rapidly absorbed. Approximately 26% of the
radioactivity in the administered dose was found in other body
fractions, with the remainder of the dose (34%) apparently lost as
volatiles (Andrawes et al., 1977).
In rats given radiolabelled thiodicarb as a single oral dose of
40 mg/kg b.w., 80% of the administered dose was eliminated within 48
hours. After 4 days, 48% of the dose had been eliminated in the
respiratory gases, 32% in the urine, and 4.5% in the faeces; 11% of
the dose remained in the carcass, due to incorporation of
radiolabelled carbon dioxide and acetic acid metabolites into natural
products (Andrawes et al., 1977; Andrawes & Bailey 1979a; Feung
et al., 1980).
Thiodicarb is degraded in the stomach not only to methomyl but
also to some other unstable intermediates, including methomyl
methylol, methomyl oxime, methomyl sulfoxide, and methomyl sulfoxide
oxime, which are subsequently converted to acetonitrile and carbon
dioxide and primarily eliminated by respiration and in the urine.
Acetonitrile is the only metabolite retained to some extent in body
tissues and fluids; a very small fraction of the acetonitrile is
further degraded to carbon dioxide, acetic acid, and to acetamide,
which is suspected of being a mouse and rat carcinogen (Weisburger
et al., 1969; Fleischman et al., 1980). The ultimate metabolic
fate of methomyl in animals depends on its isomeric configuration. In
the rat the stable and predominant form is the syn isomer, which is
metabolized primarily to CO2, while partial conversion from the syn
isomer to the anti isomer leads primarily to acetonitrile, most of
which is respired unchanged (Huhtanen & Dorough 1976).
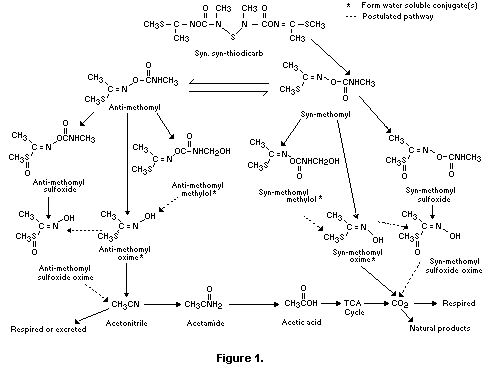
A proposed metabolic pathway for thiodicarb in animals is shown
in Figure 1.
In the cow, metabolites of a single oral dose of thiodicarb are
excreted in different proportions when compared to the rat, although
there are no substantial differences in the metabolic pathways
involved. During the initial 3-day period following administration of
radiolabelled thiodicarb (16 mg/kg) or methomyl (4 mg/kg) to rats,
31-32% of the administered dose was found in the urine. Only 5% of a
dose of thiodicarb (7.02 mg/kg) administered to a cow was excreted in
the urine during the same period. In rats, approximately 40-50% of the
dose was recovered as CO2 and/or acetonitrile from respired gases
during a 72 hour period, while similarly the cow eliminated 66% of the
applied dose in respired gases. The CO2:acetonitrile ratio in
respired air was 4- to 16-fold higher in the cow than in the rat. The
higher CO2:acetonitrile ratios in cows may represent a species-
specific tendency toward reduced formation of the anti methomyl
stereoisomer (Andrawes et al., 1977; Andrawes & Bailey, 1979b;
Khasawinah & College, 1978).
OTHER INFORMATION ON IDENTITY AND PROPERTIES
MOLECULAR WEIGHT: 354.5
PHYSICAL STATE
AND COLOUR: crystalline powder, white to light tan
ODOUR: slightly sulfurous
DENSITY (g/ml): 1.4424 at 20°C
VAPOUR PRESSURE: 4.3 × 10 -5 at 20°C
(mm Hg) 1.1 × 10 -4 at 35°C
2.1 × 10 -4 at 40°C
3.2 × 10 -4 at 45°C
4.2 × 10 -4 at 50°C
5.2 × 10 -4 at 55°C
MELTING POINT: 173-174°C
FLAMMABILITY: generates a flammable vapor space (methanol)
REACTIVITY: stable in light and ambient conditions. At
elevated temperatures (e.g. above 100°C), the
product decomposes into the following principal
degradation products; carbon dioxide, acetonitrile
and dimethyl disulfide. The decomposition process
is catalyzed by Lewis-acid type heavy metal salts.
Can be hydrolyzed under acid- or base-catalyzed
conditions. The principal degradation product from
the hydrolysis of thiodicarb is methomyl.
OXIDIZING OR
REDUCING ACTION: Neither the product nor any of the accompanying
impurities have the tendency to act as an
oxidizing or reducing agent.
pH: 1 g/100 ml H2O produces a pH of 6.65 at 21.5°C
SOLUBILITY
(wt. %): Xylene 0.1 at 50°C
0.4 at 68°C
1.0 at 92°C
2.5 at 106°C
5.0 at 119°C
Pyridine 1.2 at 25°C
6.4 at 45°C
13.4 at 60°C
Dichloromethane 10.2 at 0°C
11.4 at 5°C
16.1 at 30°C
Acetone 0.8 at 25°C
Acetonitrile 2.0 at 25°C
Ethyl acetate 0.2 at 25°C
Ethyl ether 0.1 at 25°C
Methanol 0.3 at 25°C
Tetrachloroethylene 0.1 at 25°C
Water (distilled) 35 ppm at 25°C
OCTANOL/WATER
PARTITION
COEFFICIENT: 1.65 (Log P by reverse phase TLC technique)
STORAGE
STABILITY: Minimum storage life is typically in excess of 2
years.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, excretion, and biotransformation
Thiodicarb, which essentially consists of two methomyl moieties
joined through their amino nitrogen by sulfur, is rapidly degraded to
S-methyl-N-[(methylcarbamoyl)oxy]thioacetamide (methomyl) in the rat
stomach. Over 30% of an oral dose of 16 mg thiodicarb per kg. b.w. was
converted to methomyl in the gut within 15 minutes. Only 40% of the
oral dose was found in the rat gut at 15 minutes, indicating that the
compound had been rapidly absorbed. Approximately 26% of the
radioactivity in the administered dose was found in other body
fractions, with the remainder of the dose (34%) apparently lost as
volatiles (Andrawes et al., 1977).
In rats given radiolabelled thiodicarb as a single oral dose of
40 mg/kg b.w., 80% of the administered dose was eliminated within 48
hours. After 4 days, 48% of the dose had been eliminated in the
respiratory gases, 32% in the urine, and 4.5% in the faeces; 11% of
the dose remained in the carcass, due to incorporation of
radiolabelled carbon dioxide and acetic acid metabolites into natural
products (Andrawes et al., 1977; Andrawes & Bailey 1979a; Feung
et al., 1980).
Thiodicarb is degraded in the stomach not only to methomyl but
also to some other unstable intermediates, including methomyl
methylol, methomyl oxime, methomyl sulfoxide, and methomyl sulfoxide
oxime, which are subsequently converted to acetonitrile and carbon
dioxide and primarily eliminated by respiration and in the urine.
Acetonitrile is the only metabolite retained to some extent in body
tissues and fluids; a very small fraction of the acetonitrile is
further degraded to carbon dioxide, acetic acid, and to acetamide,
which is suspected of being a mouse and rat carcinogen (Weisburger
et al., 1969; Fleischman et al., 1980). The ultimate metabolic
fate of methomyl in animals depends on its isomeric configuration. In
the rat the stable and predominant form is the syn isomer, which is
metabolized primarily to CO2, while partial conversion from the syn
isomer to the anti isomer leads primarily to acetonitrile, most of
which is respired unchanged (Huhtanen & Dorough 1976).
A proposed metabolic pathway for thiodicarb in animals is shown
in Figure 1.
In the cow, metabolites of a single oral dose of thiodicarb are
excreted in different proportions when compared to the rat, although
there are no substantial differences in the metabolic pathways
involved. During the initial 3-day period following administration of
radiolabelled thiodicarb (16 mg/kg) or methomyl (4 mg/kg) to rats,
31-32% of the administered dose was found in the urine. Only 5% of a
dose of thiodicarb (7.02 mg/kg) administered to a cow was excreted in
the urine during the same period. In rats, approximately 40-50% of the
dose was recovered as CO2 and/or acetonitrile from respired gases
during a 72 hour period, while similarly the cow eliminated 66% of the
applied dose in respired gases. The CO2:acetonitrile ratio in
respired air was 4- to 16-fold higher in the cow than in the rat. The
higher CO2:acetonitrile ratios in cows may represent a species-
specific tendency toward reduced formation of the anti methomyl
stereoisomer (Andrawes et al., 1977; Andrawes & Bailey, 1979b;
Khasawinah & College, 1978).
 Andrawes (1983) reported that oral administration of radiolabeled
acetonitrile to rats resulted in elimination of 65% of the applied
dose in expired air, with less than 1% expired as 14CO2. In a
similar study however, Huhtanen & Dorough (1976) found that in rats
9.1% of a dose of acetonitrile was eliminated as CO2 in respiratory
gases.
Two lactating Holstein cows/group were given approximately 0.004,
0.4, 1.4, or 4 mg 14C-thiodicarb per kg b.w. daily for 21 days. Milk
and urine were collected twice daily, and blood and faeces were
collected at 3- to 4-day intervals. One cow at each dosage level was
sacrificed 12 hours after the last treatment, and tissues (liver,
kidney, lung, spleen, heart, brain, ovary, udder, tongue, foreleg
muscle, hindleg muscle, neck muscle and omental fat) were collected
for analysis. The remaining 4 cows (1 at each dosage level) were kept
for an additional 7 days after termination of treatment. During this
post-treatment phase, milk and urine were collected twice daily as
before, and blood and faeces were collected daily. The same tissues
were collected after 7 days post-treatment. The radioactivity found in
all samples (i.e., tissue, milk, blood, urine and faeces) was dose
dependent. The concentration of acetonitrile in milk approached peak
levels within 7 days in all dose groups. The acetonitrile levels
appeared to remain nearly constant through the rest of the 21-day
treatment period, then declined sharply during the 7-day post-
treatment phase. Following 21 days of treatment, tissue levels of 14C
residues were highest in liver and lowest in adipose tissues. Seven
days after termination of dosing, radioactivity levels were lower in
all tissues except liver. The authors concluded that the predominant
end-products of thiodicarb catabolism were acetonitrile, acetamide,
and carbon dioxide. Acetamide, which is believed to be produced by
hydrolysis of acetonitrile, was found in trace amounts (< 0.01 ppm)
in milk at the highest feeding level. Approximately 24% of the
aqueous-extractable urinary-radioactivity was identified as acetamide
(Feung et al., 1980).
In a cow given a single dose (7.02 mg/kg b.w.)14C-acetyl
thiodicarb, 66, 11.4, 5.0, and 4.6% of the administered radioactivity
was eliminated, during 72 hours post-treatment, in respired air,
faeces, urine and milk, respectively, while 10.1% was retained in the
tissues (Khasawinah & College, 1978).
In a study using mature White Leghorn laying chickens, no
carbamate residues were found in tissues or eggs after 21 days of oral
administration of thiodicarb at dosages of 15.4, 28.6 and 102 ppm in
the diet. Acetonitrile, detected in the eggs and tissues (except fat)
at the 102-ppm level, corresponded to 0.2 ppm (egg yolk and whites),
0.5 ppm (liver), and 0.2-0.3 ppm (muscle). The level in eggs was
directly proportional to the dosage level administered. Acetamide was
not detected in egg yolks, but was found in egg whites (0.07 ppm), in
liver (0.06 ppm) and in muscle (0.01 ppm), when administered at 102
ppm thiodicarb (Andrawes & Bailey, 1980).
Based on the available data that 5.72% of the administered
syn-methomyl is converted to acetonitrile, a maximum of 5.72% of the
administered 14C-thiodicarb could therefore be available for
conversion to acetamide, since thiodicarb is metabolized predominantly
to syn-methomyl. On a weight-percent basis, the maximum acetamide
that could occur from ingestion of thiodicarb residues is 1.9% of the
administered dose (53:1 thiodicarb:acetamide on a weight-to-weight
basis) (Andrawes & Bailey, 1979a & 1979b; Huhtanen & Dorough, 1976).
Effects on enzymes and other biochemical parameters
The most significant biochemical effect of thiodicarb and its
carbamate metabolites is the ability to reversibly inhibit
acetylcholinesterase (ChE), a capability in common with other methyl
carbamate esters. The methylcarbamate-acetylcholinesterase complex
dissociates rapidly, and the acetylcholinesterase enzyme is released
unaltered. The transient nature of acetylcholinesterase inhibition by
thiodicarb makes it difficult to assay accurately, since inhibition is
reversed by sample dilution and by adding substrate (butyrylcholine or
acetylcholine) during assay procedures. Therefore, the assay procedure
must be very rapid and use less than optimal amounts of substrate. The
automated colorimetric method of Humiston & Wright (1967) is suitable
for such determination.
Groups of Fischer 344 rats (10 males and 10 females per group)
were administered thiodicarb in the diet at dosage levels of 0, 1, 3,
10, or 30 mg/kg b.w./day for 28 days. Rats were observed for mortality
and clinical symptoms of toxicity. Food consumption was determined
weekly and plasma- and erythrocyte-ChE measurements were analyzed on
days 0, 15, and 29. Brain ChE was determined on day 29.
Body weight and food consumption were significantly reduced in
females at > 10 mg/kg, b.w. Males were not similarly affected.
There was no mortality. Plasma and erythrocyte ChE were significantly
depressed in high-dose males and females. Plasma ChE recovered to
normal values by day 29, while red blood cell (RBC) ChE remained
significantly depressed in both sexes at > 30 mg/kg b.w./day. Brain
ChE was not depressed at any level (Mirro & DePass, 1982).
Toxicological studies
Special study on reproduction
Rat
Groups of Fischer 344 rats (20 female and 10 male rats/group)
were administered thiodicarb (purity 99+%) in the diet, at dosage
levels of 0, 0, 0.5, 1.0, 3, or 10 mg/kg b.w., in a three-generation
reproduction study. Each generation produced only one litter per
generation. The routine indices of fertility, gestation,
survivability, viability, and lactation were determined for all
generations, as well as selected gross and histopathological
evaluations for F2a and F3a generations. Rats were mated on a 1-male-
to-2-females ratio when approximately 100 days of age. Dams and pups
were observed daily for appearance and behaviour. Litter size was
reduced to 10 on day 4 postpartum. Brother-sister matings were
avoided.
There were no compound-related effects on any of the reproductive
parameters measured, except for an initial transitory body-weight
depression in F0 males. Gross and microscopic analyses were
reportedly unremarkable. Thiodicarb was without adverse effects on
reproduction at doses up to and including 10 mg/kg b.w. (Woodside
et al., 1979c).
Special studies on teratogenicity
Mouse
In a pilot study using pregnant Charles-River CD-1 mice,
thiodicarb (technical grade) administered orally from days 6 through
16 of gestation, at dosage levels of 0, 20, 60, 100, 150, 200, 300, or
400 mg/kg b.w., produced excessive maternal toxicity (body-weight
depression and mortality) at doses greater than 200 mg/kg b.w.
(Dangler & Rodwell, 1979).
Groups of Charles-River CD-1 mice (25 mated females/group) were
administered thiodicarb (technical grade) via oral gavage at dosage
levels of 0, 50, 100, or 200 mg/kg b.w. from days 6 through 16 of
gestation. Mating was accomplished on a 1-female-to-1-male schedule at
94 days of age. Day of copulation was also considered day 0 of
gestation. Animals were observed routinely for appearance, behaviour
and body-weight changes. Pups were delivered by Caesarean-section on
day 17 of gestation. All foetuses were weighed, sexed, examined for
external malformations, and subsequently subjected to visceral or
skeletal examination.
Six females in the high-dose group died during the study. Body
weights were unaffected by treatment. There was no evidence of
maternal or foetal toxicity at doses > 100 mg/kg b.w. and no
teratogenic effects at doses > 200 mg/kg b.w. (Janes et al.,
1980).
Rat
In a pilot teratology study using pregnant Charles-River CD COBS
rats, thiodicarb (technical grade), administered orally at dosage
levels of 0, 20, 40, 80, 120, or 160 mg/kg b.w. on days 6-19 of
gestation, produced mortality at doses > 40 mg/kg b.w. There were
no reported adverse effects on reproduction or foetal development
(Ziemke & Rodwell, 1979).
Groups of Charles-River CD COBS rats (25 mated females/group)
were administered thiodicarb (technical grade) via oral gavage at
dosage levels of 0, 10, 20, or 30 mg/kg b.w. on gestation days 6-19.
Females were mated 1-to-1 with males at 13 weeks of age. Day of
copulation was also considered day 0 of gestation. Animals were
observed routinely for appearance, behaviour, and body-weight changes.
Pups were delivered by Caesarean-section on gestation-day 20. All
foetuses were weighed, sexed, measured, and examined for external
malformations. One-third of the foetuses were fixed for visceral
examination and the remaining two-thirds of the foetuses were
subjected to skeletal examination. Early and late resorptions, number
of implantations and corpora lutea, and the number and location of
viable and non-viable foetuses, were recorded.
Clinical observations, apparent at 20 and 30 mg/kg b.w. within 4
hours after treatment, included inactivity, tremors, and clear oral
discharge. There was no mortality. Maternal body weights determined
on days 0, 6, 9, 12, 16, and 20 were significantly decreased at
> 20 mg/kg b.w, and mean foetal body-weights were significantly
reduced at > 10 mg/kg b.w. No teratogenic effects were produced,
although delayed maturation of foetuses (reduced ossification),
associated with maternal toxicity, was observed. There was no evidence
of visceral or skeletal anomalies attributable to thiodicarb at doses
up to and including 30 mg/kg b.w. (Tasker et al., 1979).
Groups of Fischer 344 rats (10 mated females/group) were
administered thiodicarb (purity 99+%) in the diet via 2 separate
dosing regimens: from day 0 to day 20 of gestation or from day 6 to
day 15 of gestation. Dose levels in each dosing schedule included 0,
0.5, 1.0, 3, or 100 mg/kg b.w. A positive control group received
625 mg/kg b.w. of aspirin via gavage on gestation day 10. Females were
mated with males on a 1-to-1 schedule in order to achieve 10 pregnant
females per dose. All pups were delivered by Caesarean-section on
gestation day 20 and examined for visceral and skeletal abnormalities.
Maternal body-weight gains determined on days 0 and 12 of
gestation were reduced in all treatment groups. However, these changes
were extremely variable at doses of 0.5-3.0 mg/kg b.w. (only two
measurements were included) and they were not considered to be
biologically significant except at the highest dose administered where
the body weights were uniformly depressed. Foetal body weight and
length were also significantly reduced in the high-dose group that was
administered thiodicarb throughout gestation (days 0-20). The aspirin-
exposed foetuses were similarly affected. Thiodicarb did not adversely
affect the number of implantations, resorptions, live foetuses per
litter, or pre-implantation losses. Resorption frequency was increased
in high- dose females given thiodicarb from days 6 through 15 of
gestation. The increase was based on the percentage of affected
litters as well as the resorptions per litter. Aspirin demonstrated
the same response. The incidences of visceral anaomalies were not
increased in either the aspririn or thiodicarb groups when compared to
controls. Skeletal variations were similar among all groups except for
an increased incidence of bilobed thoracic vertebral centra and poorly
ossified sternebrae (on a percentage of live-foetuses-per-litter
basis) in the 100 mg/kg b.w. group administered thiodicarb throughout
gestation. This effect was suggestive of delayed maturity in foetuses
derived from dams which demonstrated significant maternal toxicity
(i.e. decreased weight gain and decreased foetal size). This effect is
also known to occur spontaneously (at similar frequencies) in other
rat strains. The aspirin group exhibited several skeletal variations
(e.g. split/missing vertebral centra, extra vertebrae, extra ribs and
fused/wavy ribs), demonstrating the sensitivity of the study.
Thiodicarb was not teratogenic at any dose administered but it was
maternally toxic at 100 mg/kg b.w. (Woodside et al., 1979b).
Special studies on carcinogenicity (See also "Long-term studies")
Mouse
Groups of Charles-River COBS CD-1 mice (80/sex/group) were
administered thiodicarb (analytical grade, 99+% purity) in the diet
for 24 months at dosage levels of 0, 0, 1.0, 3, or 10 mg/kg b.w./day.
Animals were 42 days old at the start of the study. The mice were
examined daily for mortality and adverse physical/behavioural
condition. Routine evaluation of food consumption and body weights
were performed. At the conclusion of the study, or at death, organ
weights and gross and microscopic examination of tissues and organs of
all animals were performed. All were anesthetized with methyoxyflurane
and killed by cervical dislocation.
Mortality in high-dose mice was increased over controls in males
during the last 2 months of the study and in females during months 17
and 18.
Body weights and food consumption were not affected by treatment.
There were no significant differences among treatment and control
groups for the incidence of neoplasms or the identification of non-
neoplastic lesions occurring in dead animals, or interim- and final-
sacrifice animals. However, the overall incidence among control and
treatment groups of hepatocellular neoplasms was much greater than
reported in the literature for this strain of mouse (Table 1).
Table 1. Hepatocellular neoplasms in mice treated with thiodicarb
and in 2 control groups
Dose (mg/kg b.w.)
0 0 1 3 10
Males
Adenoma 18/80 19/76 22/78 24/78 24/80
Carcinoma 37/80 25/76 29/78 32/78 26/80
Females
Adenoma 2/80 5/77 2/78 2/76 1/75
Carcinoma 1/80 3/77 3/78 1/76 3/75
Furthermore, there was no decrease in time-to-tumour for the
observation of hepatocellular neoplasms (Table 2).
Table 2. Time-to-tumour (hepatocellular neoplasms) for male mice
treated with thiodicarb and for 2 control groups
Dose (mg/kg b.w.)
0 0 1 3 10
Animals which
died or were 6/53 10/49 12/51 22/48 13/54
moribund
Animals at 18-
month interim 16/20 15/20 16/20 15/20 13/20
sacrifice
Animals surviving
24 months 4/7 6/8 6/7 4/10 4/6
(terminal kill)
The other most frequently occurring neoplasm was alveologenic
tumour of the lungs. As with hepatocellular neoplasms, there was no
compound-related response within the incidence of this tumour type
(Table 3).
Table 3. Alveologenic tumours in mice treated with thiodicarb and
in 2 control groups
Dose (mg/kg b.w.)
0 0 1 3 10
Males
Alveologenic
tumours 23/80 23/76 31/78 15/78 22/80
Carcinoma 1/80 0/76 0/78 1/78 0/80
Females
Alveologenic
tumours 19/80 13/78 19/78 17/77 13/75
Carcinoma 2/80 0/78 0/78 0/77 0/75
The principle non-neoplastic lesions observed in all control and
treated groups included: lymphocytic infiltration or hyperplasia of
numerous tissues, generalized amyloidosis, hyaline degeneration of
sternal cartilage, haemosiderosis of various organs, subacute
sialoadenitis, glomerulosclerosis, and renal cystic tubular dilation.
These effects were not compound-related. Thiodicarb was not
carcinogenic to CD-1 mice at dietary doses up to and including
10 mg/kg b.w. (Woodside et al., 1980a).
Special studies on mutagenicity
Thiodicarb was evaluated in a series of mutagenicity assays
including the Ames, dominant-lethal, micronucleus, reverse-mutation,
mitotic-crossing-over, and DNA-damage test methods. It was negative
for mutagenic potential in all tests except for a positive response in
the mitotic gene conversion assay using Sacharomyces cerevisiae.
(See Table 4 for details.)
Table 4. Results of mutagenicity assays on thiodicarb
Test Test Concentration Purity Results Reference
System Object of thiodicarb
Ames test S. typhimurium 1, 10, 100, Unknown Negative Jagannath &
* TA98 500, and Brusick, 1978
TA100 1000 µg/plate
TA1535
TA1537
TA1538
Micronucleus Mouse, bone 5 and 10 Technical Negative Naismith &
test marrow mg/kg grade (1) Matthews,
1979a
Dominant Rat 0, 0.5, 1, Analytical Negative Woodside
lethal 3 & grade (2) et al., 1979c
10 mg/kg (99+%)
Reverse S. cerevisiae 2.5, 6.2, Technical Negative Naismith &
mutation 25, 62, & grade (3) Matthews,
250 µg/ml 1979b
Mitotic S. cerevisiae 2.5, 6.2, Technical Negative Naismith &
crossing 25, 62, & grade (3) Matthews,
over 250 µg/ml 1979e
Mitotic S. cerevisiae 2.5, 6.2, Technical (4) Naismith &
gene 25, 62, & grade Matthews,
conversion 250 µg/ml 1979c
Primary E. coli 0.001, 0.01, Technical Negative Naismith &
DNA W3110 0.1, 1, and grade (5) Matthews,
damage* p3478 10 mg/ml 1979d
Chromosomal Chinese hamster 10, 15, 20, Technical Negative Ivett &
aberrations ovary (CHO) 30 µg/ml 91.48% Brusick,
cells* w/o activ. 1985
10, 20, 30,
40 µg/ml
w/activation
Unscheduled Rat primary 0.5, 1.0, 2.5, Technical Negative Cifone &
DNA synthesis hepatocytes 5.0, 10.0, 91.48% Brusick,
25.0, 50.0, 1985a
100.0, 250.0
µg/ml
Table 4. (Con't)
Test Test Concentration Purity Results Reference
System Object of thiodicarb
Mouse lymphoma L5178Y (TK+/-) 5, 8, 10, Technical Positive Cifone &
forward mutation & 12 µg/ml 91.48% (6) Brusick,
assay* 1985b
* With and without metabolic activation
(1) The positive control (TEM) gave the expected response at 0.5 ml/kg.
(2) The positive control (TEM) gave the expected response at 0.25 mg/kg.
(3) The positive control (NQO) gave the expected response at 10-6 M.
(4) Increase in the heteroallelic trp 5-12/trp 5-27 diploid strain D7 at
25 µg/ml.
(5) The positive controls (ethylmethane sulfonate and diethylnitrosamine)
gave the expected response.
(6) The test material induced repeatable increases in the mutant frequency,
with and without metabolic activation.
Special studies on neurotoxicity
Although the Meeting considers the testing of methyl carbamates
for potential neurotoxicity to be inappropriate (Annex 1, FAO/WHO,
1985b, para. 2.8), the manufacturer conducted 2 separate studies, both
of which were negative.
Chicken
In a preliminary acute oral toxicity study using 10- to 14-month-
old White Leghorn hens, the LD50 for thiodicarb was calculated to be
582 mg/kg b.w. When atropine sulfate (15 mg/kg b.w.) was administered
15 minutes prior to dosing only 2/5 hens died after 5 days at a dose
of 830 mg/kg b.w. (Schwartz & Stevens, 1978).
Hens protected with 15 mg/kg b.w. atropine sulfate were
administered single doses of 660 mg/kg b.w. thiodicarb via oral
intubation. Vehicle (corn oil) and positive (TOCP) control groups were
also employed. A dose of 750 mg/kg b.w. TOCP in corn oil was used.
There were 10 hens in each control group and 40 in the thiodicarb
group. Hens were observed daily for pharmacologic and toxicologic
effects, including neurologic evaluation of leg weakness, gait, and
walking ability. On day 21 all positive controls and 10 test-group
birds were necropsied, perfused with 10% neutral buffered formalin,
and examined histologically. On day 22 eight new positive controls
were added to the study and the existing 30 treated birds were
re-dosed a second time with 660 mg/kg b.w. thiodicarb.
The vehicle controls provided no evidence of toxicity, while the
positive control group displayed clinical and microscopic symptoms of
delayed neuro-toxicity (increased number of swollen axons per cervical
spinal cord cross-section). Although some signs of neurological
impairment were initially displayed in the thiodicarb group, these
were not evident after 4 days in study and were not confirmed upon
histologic examination (Becci et al., 1979).
Due to the marginal response in thiodicarb-treated birds, the
entire study was repeated using 30 White Leghorn hens (8-12 months
old) dosed orally with 660 mg/kg b.w. thiodicarb (analytical grade).
There were 10 hens in each of the negative (corn oil) and positive
(TOTP) control groups. Thirty minutes prior to dosing, each group
received 25 mg/kg b.w. atropine sulfate via gavage. A dose of
1200 mg/kg b.w. TOTP was employed in the positive control group.
A total of 13/30 thiodicarb hens died on test, with no mortality
in either control group. Thiodicarb and the negative control group
provided no evidence of delayed neurotoxicity. In the TOTP group, 9/10
birds demonstrated lesions in the sciatic nerve and spinal cord,
consisting of axonal degeneration, necrosis, and demyelination (Myer
et al., 1980).
Special studies on skin and eye irritation
Rabbit
Thiodicarb is not irritating to the skin. When applied to the
shaved backs of rabbits, thiodicarb produced only slight erythema in a
few cases (Mallory et al., 1982i; Wentz & Wolfe, 1979).
Thiodicarb was applied to the shaved, intact skin of rabbits, 5
times a week for 3 consecutive weeks, at 4 dosage levels up to
4 g/kg/day. Mortality, behaviour, food consumption, body weight, and
gross and microscopic histopathology were unaffected by treatment.
Localized erythema and edema occurred in all treated groups.
Thiodicarb treatment was reported to produce a dose-related macrocytic
anaemia (Conroy et al., 1979b; Gallo & Stevens, 1980).
In a second dermal toxicity study in rabbits, using
2000 mg/kg/day as the highest dosage level, all criteria were
unaffected by treatment. Groups of 10 male and 10 female New Zealand
White rabbits were treated dermally with thiodicarb 5 days per week
for 3 consecutive weeks at dosage levels of 0, 250, 500, 1000, or
2000 mg/kg (2 control groups were utilized in this study). Thiodicarb
was administered to both intact and abraded dermal surfaces. There
were no effects in the study attributable to thiodicarb. The
occurrence of macrocytic anaemia, observed in the first study, was not
confirmed (Schardein, 1982).
Thiodicarb applied to the conjunctival sacs of rabbits did not
damage the cornea, but resulted in temporary eye irritation (Cameron &
Wolfe, 1979; Mallory et al., 1982a). In monkeys, thiodicarb caused
no eye irritation or damage (Weatherholtz, 1982). Methomyl was mildly
irritating to the skin of guinea-pigs and was a mild irritant to the
eye (Kaplan & Sherman, 1977). Methomyl oxime was not irritating to the
skin and was moderately irritating to the eye (Myers et al., 1984).
Special studies on dermal sensitization
The potential for thiodicarb to produce skin sensitization was
tested in guinea-pigs and human subjects. Guinea-pigs, given multiple
intradermal injections of thiodicarb in a modified Landsteiner
procedure, showed minimal sensitization reactions to a challenge
injection two weeks later (Conroy et al., 1979a & c). Repeated
application (via a modified Buehler method) of thiodicarb to the
shaved backs of guinea-pigs did not cause allergic sensitization
(Field, 1979b). In a similar test with human subjects, thiodicarb
produced no sensitization by repeated contact (Kamphake et al.,
1980). Methomyl was not a sensitizer when administered to guinea-pigs
(Kaplan & Sherman, 1977).
Acute toxicity
The acute toxicity of thiodicarb and its metabolites in several
animal species is summarized in Table 5.
Short-term studies
Mouse
Groups of Charles River CD-1 mice (5 males and 5 females per
group) were administered thiodicarb in the diet for 7 days at dietary
levels of 0, 15, 45, or 90 mg/kg b.w./day. Growth and mortality were
unaffected. In male mice, at the two highest dosage levels, an
increased absolute kidney weight was observed (Homan et al., 1977).
Groups of ICR-JCL (SPF) mice (10 per sex per group) were fed
thiodicarb (92.5% purity) in the diet for 4 weeks at dosage levels of
0, 30, 100, or 300 ppm (equal to 0, 4.2, 13.7, and 40.8 mg/kg b.w./day
for males and 0, 3.8, 12.3, and 36.3 mg/kg b.w./day for females).
There was no mortality during the study. Body weight, food consumption
and organ weights (brain, heart, liver, spleen, kidney, testes, and
ovaries) were unaffected by treatment (Yoshida et al. 1983a).
Table 5. Results of acute toxicity assays of thiodicarb and its metabolitea
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Thiodicarb Mouse both Oral corn oil 226 Myers et al., 1977b
(148-346)
Rat M Oral corn oil 74.8-129 Hallory et al.,1982b;1982e;1982g
(52.9-186) Myers et al.,1982b;1982c;1982d;1982e
F Oral corn oil 50-136 Mallory et al., 1982b;1982e;1982g
(34.9-192.1) Myers et al.,1982b;1982c;1982d;1982e
M Oral 0.25% methyl 46.5-83 Mallory et al., 1982c;1982f;1982h
cellulose or (33.4-108) Myers et al.,1982b;1982c;1982c;1982d
water
F Oral 0.25% methyl 39.1-55 Mallory et al., 1982e;1982f;1982h
cellulose or (29.4-65.5) Myers et al., 1982b;1982c;1982d
water
both Oral corn oil 74.6-215 Mallory et al., 1982b;1982e;1982g
(59.6-268.8) Myers et al., 1982b;1982c;1982d;1982e
both Oral 0.25% methyl 49.4-97 Mallory et al., 1982c;1982f;1982h
cellulose or (39.4-138.2) Myers et al., 1982b;1982c;1982d
water
Guinea- M Oral corn oil 160 Myers et al., 1977c
pig (94.3-271)
Rabbit both Oral capsule > 400 Myers et al., 1979a
both Oral corn oil 556 Myers et al., 1979a
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Chicken F Oral corn oil 582-660 Myer et al., 1980
Schwartz & Stevens, 1978
Monkey both Oral capsule 467.2 Weatherholtz et al., 1983
(329.6-662.1)
Rat M Dermal 2540 Field 1979a
4 hr. (1120-5750) Koehler & Dorman 1979
intact Myers et al., 1975;1979a
skin
Rabbit both Dermal > 6310 Lemen et al., 1979;
24 hr. Mallory et al., 1982d;
abraded Myers et al., 1978b;1979a
& intact
skin
Rat both I.P. 23 (15.9-33.1) Matthews 1979b
Mouse both I.P. 34 (21.9-52.7) Matthews 1979a
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Rat Inhalation > 2.0 Myers et al., 1975
148 min.
aerosol in
DMSO
Inhalation > 0.2 Myers et al., 1979a
360 min.
aerosol in
DMSO
both Inhalation 0.1155-0.220 Dickey et al., 1979
240 min. (0.1094- Myers et al., 1977a
dust 0.320)
Inhalation > 2.4 Myers et al., 1978a
60 min.
dust
Aqueous 1.04 Myers et al., 1982a
aerosol
Methomyl Rat M Oral corn oil 14.1-47.6 Kaplan & Sherman 1977;
peanut oil (8.0-72.7) Myers et al., 1976a;1981
PEG 400
F Oral corn oil 12.3-48.5 Kaplan & Sherman 1977;
peanut oil (7.6-70.2) Myers et al., 1979b;I981
Weil & Carpenter 1978
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Guinea- M Oral acetone 15*** Kaplan & Sherman 1977
pig peanut oil
Japanese Oral water;CMC 34 Kaplan & Sherman 1977
Quail
Dog M capsule 30*** Kaplan & Sherman 1977
Monkey both aqueous 40*** Kaplan & Sherman 1977
(Rhesus)
Chicken F Oral acetone/ 28 Kaplan & Sherman 1977
water
Hydroxymethyl Rat M Oral corn oil 200 Myers et al., 1978c
methomyl (123-326)
Oral corn oil 238 Myers et al., 1979b
(156-363)
Methomyl Rat M Oral corn oil 453 Myers et al., 1978a
sulfoxide (296-692)
Oral corn oil 476 Myers et al., 1979b
(311-727)
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Methomyl Rat M Oral corn oil 794 Myers et al., 1976b
oxide (486-1300)
Oral corn oil 1000 Myers et al., 1979b
(636-1572)
both Oral water 350 Myers et al., 1984
(311-394)
Acetonitrile Rat M Oral water 246 Pozzani et al., 1955
(160-378)
F Oral water 234 Pozzani et al., 1955
(203-270)
Oral water 38/0 Smyth 1947
Methomyl Rat M Dermal water > 200 X Kaplan & Sherman 1977
intact & 5 days
abraded
skin 6 h
Rabbit M Dermal water > 5000 Kaplan & Sherman 1977
intact
skin 24 h
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
M Dermal water 800-951 Myers et al., 1981
intact
& abraded
skin 24 h
F Dermal water 566-1130 Myers et al., 1981
intact
& abraded
skin 24 h
Methomyl Rat M Dermal corn oil > 1000 Myers et al., 1976b
oxime intact
skin 4 h
Dermal water > 2000 Meyers et al., 1984
intact
skin 24 h
Acetonitrile Rat i.p. 0.95 ml/kg Rogers 1959
(0.58-1.54)
Rat i.v. 1.68 ml/kg Rogers 1959
(0.08-3.21)
Rabbit Dermal 1.26 ml/kg Carpenter et al., 1958
Methomyl Rat M Inhaltion water 76 mg/l Kaplan & Sherman 1977
of aqueous
aerosol
4 h
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Methomyl Rat Inhalation water > 0.82 Myers et al., 1976b
oxime of mg/1
aqueous
aerosol
4 h
* 95% confidence limits (the widest range from each set of studies) are given in parentheses.
** Because particles of thiodicarb are not of respirable size, the material was ground to a dust
for these tests.
*** Minimum lethal dose.
Groups of ICR-JCL (SPF) mice, (28 per sex per group) were fed
thiodicarb (92.5% purity) in the diet for 13 weeks at dosage levels of
0, 30, 150, or 600 ppm (equal to 0, 3.8, 18.9, and 76.5 mg/kg b.w. for
males and 0, 4.3, 21.5, and 85.0 mg/kg b.w. for females). Mortality,
food consumption, body weight, water consumption, urinalysis,
haematology, and clinical chemistry were determined. Cholinesterase
levels were determined in plasma, red blood cells (RBC) and brain at
study termination, as were organ weights, gross pathology, and
histopathology. Brain ChE was stated to be depressed in 150-
and 600-ppm females and in 600-ppm males. Also reported were
significantly-increased relative liver weights in high-dose females.
There were no other compound-related effects reported. However,
individual animal data, gross pathology, or histopathology were not
submitted. Therefore, a no-observed-effect level cannot be assessed
from these data as presented to the 1985 Joint Meeting (Yoshida
et al., 1983b).
Rat
Groups of Harlan-Wistar rats (5 per sex per group) were fed
thiodicarb in the diet for 7 days at dosages of 0, 5, 25, or 100 mg/kg
b.w./day. Limited effects were noted on body weight and food
consumption, which were decreased in high-dose males. Relative kidney
weights were also reportedly increased in all treated males (Woodside
et al., 1975).
Groups of Wistar rats (5 per sex per group) were administered
thiodicarb in the diet at dosages of 0, 0, 18.5, 48.6, or 128 mg/kg
b.w./day for 7 days. Observations were made on mortality, food
consumption, body weight, and gross liver and kidney changes. Body
weights were reduced in all treated groups compared to controls.
Relative liver weights and absolute kidney weights were reduced in
high-dose rats (Woodside et al., 1978).
In a preliminary 13-week study 4 groups of Fischer 344 rats
(5 per sex per group) were administered thiodicarb in the diet at
dosage levels of 0, 5, 15, or 45 mg/kg b.w./day. One additional group
per sex received 90 mg/kg b.w./day for 80 days. Observations included:
mortality, food and water consumption, organ weights, and ChE activity
(RBC, plasma and brain). Blood samples for ChE measurements were
obtained via cardiac puncture. At necropsy, the brain was removed,
weighed, and submitted for ChE assay. Relative liver, adrenal, and
spleen weights were increased in all treated males, and relative
spleen weights were increased in mid- and high-dose females. Body
weights were reduced throughout the study in all treated males. ChE
activity was unaffected at any dose level, except for increased RBC
ChE activity in high-dose males and females (Woodside et al.,
1979a).
Groups of Fischer 344 rats (10 males and 10 females per group)
were administered thiodicarb (99% purity) in the diet at dosage levels
of 0, 0, 1.0, 3.0, 10 or 30 mg/kg b.w./day for 13 weeks. Observations
included mortality, clinical observations, food and water consumption,
body weight, organ weights, haematology, clinical chemistry,
urinalysis, ChE activity (plasma, RBC and brain-at-germination), and
gross and microscopic analysis. At both 10 and 30 mg/kg b.w./day, body
weight and water consumption decreased significantly among females,
with slight increases in males at the same dose levels. At 30 mg/kg
b.w., relative spleen weights were increased significantly in males,
while relative kidney weights were increased in high-dose females.
Urine volume was increased in males and decreased in females at the
high-dose level. Specific gravity of urine was also decreased in high-
dose females. Other clinical parameters were unaffected by treatment
except for decreased mean haemoglobin concentrations in high-dose
males. ChE activities were comparable among all groups except for a
significantly-increased RBC ChE in high-dose males. Gross and
microscopic analyses were unremarkable and typical of changes
associated with 4-month-old Fischer 344 rats. A NOEL was demonstrated
at 3 mg/kg b.w. (Homan et al., 1978b).
Dog
Groups of 9-month-old Beagle dogs (4 per sex per group) were fed
thiodicarb in the diet at targeted dosages of 0, 15, 45, or 90 mg/kg
b.w./day for 13 weeks. However, due to mortality at the highest dose,
the level was reduced to 76 and 86 mg/kg b.w. for females and males,
respectively. Animals were examined routinely for physical/behavioural
changes, food and water consumption, body-weight changes, haematologic
and blood chemistry parameters, and urinalysis. Organ weights, gross
necropsy and histologic effects were examined at terminal sacrifice.
ChE activities (plasma and RBC) were determined pre- treatment and on
days 28, 63, 79, and 93 of the study. Brain ChE was determined at
sacrifice.
Two females died in the high-dose group, and dose-related
cholinergic symptoms were observed in both sexes in the mid- and high-
dose groups. Symptoms included anorexia, vomiting, and loose stools.
However, ChE activity was unaffected throughout the study. Food
consumption decreased in high-dose females. Water consumption
increased in both sexes in the high-dose groups. Body weight was
decreased in females at the mid- and high-dose levels but there was no
effect on males. Urine volume was increased in mid- and high-dose
males. Decreases in RBC count, packed cell volume, and haemoglobin
were noted in both sexes in the mid- and high-dose groups, while
reticulocyte counts were increased in both sexes of the high-dose
groups only. These changes, together with bone-marrow hyperplasia,
decreased mean-corpuscular haemoglobin concentrations, and congested
spleen in high-dose groups, are suggestive of compensatory
haematopoiesis and anaemia. Relative liver weights and serum GPT
levels were increased in both sexes at the high dose. However, males
at the high dose demonstrated cloudy swelling, vacuolation and
degenerative liver effects. All treated groups produced evidence of
focal and diffuse inflammation of the liver. A clear NOEL was not
demonstrated in this study (Homan, et al., 1978a).
Groups of Beagle dogs, 6-7 months old (6 per sex per dose) were
administered thiodicarb in the diet at dosage levels of 0, 5, 15, or
45 mg/kg b.w./ day for 26 weeks. Animals were observed routinely for
survival, clinical appearance, food consumption, body-weight change,
ChE activity, haematologic and clinical chemistry parameters, and
urinalysis. Organ-weight changes and gross and microscopic pathologic
examinations were performed at terminal sacrifice. Cholinesterase
activity was twice determined pretreatment and once each on fasted and
non-fasted samples during weeks 8, 17, and 26. Brain ChE was
determined at sacrifice.
Thiodicarb did not affect body weight, food consumption,
urinalysis, organ weights, or gross or microscopic pathological
examination of tissues. Among the male dogs, there was a dose-related
increase in the combined incidence of soft stools, mucoid stools, and
diarrhoea. These were not evident in females. Treatment-related
changes in haematology included significantly-decreased haematocrit,
haemoglobin, and RBC levels in high-dose males and females. Dogs
receiving the highest dose had significantly-elevated levels of serum
GPT, plasma ChE, and RBC ChE as well as significantly-decreased levels
of calcium, total protein and globulin, with correspondingly-increased
albumin-to-globulin ratio. There were no treatment-related effects
reported at 15 mg/kg b.w./day (Wolfe 1981).
Long-term study
Rat
Six groups of Fischer 344 rats (120 rats per sex per dose) were
administered thiodicarb (analytical grade, 99+% purity) in the diet at
dosage levels of 0, 0, 0.5, 1, 3, or 10 mg/kg b.w. for 24 months.
Animals were 50 days of age when dosing began, and housed 3 per cage
for males and 5 per cage for females. Rats were examined daily for
mortality and adverse physical or behavioural changes. Food and water
consumption and body-weight changes were determined routinely. Interim
sacrifices were performed at 6, 12, and 19 months, utilizing 10 to 20
rats per sex. Haematology, clinical chemistry, and urinalysis
determinations were made prior to each scheduled sacrifice. Organ
weights and gross and microscopic evaluations were also performed at
each sacrifice and on dead or moribund animals (excluding organ
weights). Limited ophthalmological examinations were also performed.
Mortality was increased in high-dose males, but not females, from
the 15th to the 21st months. At termination of the study, mortality in
control males exceeded that in the high-dose males. Food consumption
was unaffected in treated rats, although sporadic decreases were
occasionally observed. Body weights were depressed in high-dose males
from the 15th to 20th months and in high-dose females from the 3rd to
the 21st months. An outbreak of sialodacry-oadenitis virus was
discovered at about the 18th month, which had contributed to a
generalized debilitated condition and to weight loss in all animals
for approximately 2-3 weeks. Haematological parameters were
unremarkable except for reduced erythrocyte counts, hematocrits and
haemaglobin levels in males fed > 1 mg/kg thiodicarb at the 12th
month, but not at 19 or 24 months. ChE activity (plasma, RBC, and
brain), determined after 48 hours on the control diet, was unaffected.
However, the time delay between compound administration and ChE
measurement is considered excessive for a carbamate and therefore this
result is not meaningful. The only change in ChE activity, consistent
with other thiodicarb-feeding studies, was a significant increase in
RBC ChE at 24 months in high-dose males. Clinical chemistry and
urinalysis determinations were not affected by treatment except for
transient reduction in urine volume at 12 months in females given
> 3 mg/kg. Although sporadic differences were observed at various
sacrifice intervals, there were no dose-related effects on absolute or
relative organ-weights.
There were no significant pathologic changes attributable to
thiodicarb at 6, 12, or 19 months, and no carcinogenic response at any
dose throughout the study. High-dose males presented evidence at 24
months of an increased incidence of pituitary cysts, while high-dose
females demonstrated haemosiderosis of the mediastinal lymph nodes.
Hepatocellular hyperplasia and interstitial prostatitis were increased
in treated males, although not significantly at the high dose; their
incidence was not dose-related, and there was not a significant
increase in liver neoplasms. High-dose males had an increased, but not
significant, incidence of epithelial hyperplasia and epithelial
thymomas. Mononuclear cell leukemia and interstitial cell adenomas
(in males) occurred spontaneously in all groups, in general agreement
with this strain of rat.
A NOEL of 3 mg/kg b.w. was established with no evidence of
oncogenic potential at doses up to and including 10 mg/kg b.w.
(Woodside et al., 1980b).
COMMENTS
Thiodicarb is rapidly absorbed, metabolized, and excreted, and
has not been demonstrated to accumulate in animal tissues. It is
degraded to methomyl, which is converted to the "oxime" (methyl
hydroxythioacetimidate) and ultimately to acetonitrile, carbon
dioxide, acetic acid, and acetamide. The ultimate metabolic fate in
animals depends on the isomeric configuration of methmyl (SYN or
ANTI).
The acute oral toxicity (LD50) in rats is 66-120 mg/kg b.w.,
depending on the vehicle used. In monkeys an oral LD50 is 467 mg/kg
b.w.
Thiodicarb causes reversible cholinesterase inhibition. Long-term
feeding and oncogenicity studies in rats and mice have revealed no
oncogenic potential in either species, and a NOEL of 3 mg/kg b.w. for
non-oncogenic effects was determined. No adverse reproduction or
teratogenic effects in rats or mice have been demonstrated.
Thiodicarb was not mutagenic in a wide variety of assays.
However, it was positive in the mitotic gene conversion assay using
Saccharomyces cerevisiae.
In two short-term feeding studies in dogs, thiodicarb
demonstrated adverse liver effects in 90 days at doses > 15 mg/kg
b.w., while in a 6-month study at doses up to 45 mg/kg b.w. no adverse
effects were observed at 15 mg/kg b.w. Considering the conflicting
information concerning liver effects in dogs, the Meeting required the
submission of the results from an ongoing 1-year dog study by 1987.
Therefore, a temporary ADI was allocated.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 60 ppm in the diet, equivalent to 3 mg/kg b.w.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.01 mg/kg b.w.
FURTHER WORK OR INFORMATION REQUIRED (by 1987)
l. Submission of ongoing 1-year dog feeding study known to be in
progress.
2. Information on possible effect on maternal body-weight gain
demonstrated in a rat teratology study.
DESIRED
1. Observations in man, including monitoring of acetamide in the
urine.
REFERENCES
Andrawes, N.R., College, P.R. & Bailey, R.H. Short-term fate of orally
(1977) administered UC 51762 in the rat. File No. 23509.
Unpublished report from Union Carbide Agricultural Products
Company, Inc. Submitted to WHO by the Union Carbide
Agricultural Products Co., Inc.
Andrawes, N.R. & Bailey, R.H. Metabolism of acetyl-l-l4C-LARVIN(R) in
(1979a) the rat. File No. 26925. Unpublished report from Union
Carbide Agricultural Products Company. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Andrawes, N.R. & Bailey, R.H. Metabolism of acetyl-1-l4C-methomyl in
(1979b) the rat. File No. 26945. Unpublished report from Union
Carbide Agricultural Products Company. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Andrawes, N.R. & Bailey, R.H. Fate of acetyl-1-14C-thiodicarb in laying
(1980) chickens under continuous feeding conditions. File No.
26414. Unpublished report from Union Carbide Agricultural
Products Company. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Andrawes, N.R. Acetonitrile metabolism in the rat. File No. 32291.
(1983) Unpublished report from Union Carbide Agricultural Products
Company, Inc. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Becci, P.J., Schwartz, C.S., & Parent, R.A. Evaluation of UC 51762 No.
(1979) 40-488 as a potential delayed neurotoxic agent following
oral administration to hens protected by atropine sulfate.
Report No. 6065. Unpublished report from Food and Drug
Research Laboratories. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Cameron, J.T. & Wolfe, G.W. Acute eye irritation study in rabbits.
(1979) Project No. 400-616. Unpublished report from Hazleton
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Carpenter, C.P., Pozzani, U.C., Weil, U.C., Palm, P.E. & Nair, J.H.
(1958) The toxicity of acetonitrile. Part II. Special report No.
21-100. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company Inc.
Cifone, M.A. & Brusick, D.J. Evaluation of thiodicarb in the rat
(1985a) primary hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. 20991 from Litton Bionetics, Inc.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Cifone, M.A. & Brusick, D.J. Mutagenicity of thiodicarb in a mouse
(1985b) lymphoma mutation assay. Unpublished report No. 20989 from
Litton Bionetics, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Conroy, W.J., DePass, L.R., Homan, E.R., Weil, C.S., Geary, D.L. &
(1979a) Frank, F.R. Dermal sensitization potential in guinea pig.
Special report No. 42-61. Unpublished report from the Mellon
Institute. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Conroy, W.J., DePass, L.R., Homan, E.R., Weil, C.S., Geary, D.L., &
(1979b) Frank, F.R. UC 51762, 16-dose rabbit dermal study. Special
report No. 42-52. Unpublished report from Mellon Insititute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Conroy, W.J., DePass, L.R., Homan, E.R., Weil, C.S., & Webb, G.A.
(1979c) Dermal sensitization potential in the guinea pig. Special
report No. 42-26. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Dangler, T.L. & Rodwell, D.E. Pilot teratology study in mice. Report
(1979) No. 369-030. Unpublished report from International Research
and Development Corporation. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Dickey, C.L., Nachreiner, D.J., DePass, L.R., Gad, S.C., Weil, C.S.,
(1979) Geary, D.L. & Frank, F.R. Acute and 9-day dust inhalation
study on rats with UC 51762 technical. Special Report No.
42-63. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Feung, C.S., College, P.R. & Chancey, E.L. Studies on the disposition
(1980) of 14C-thiodicarb in lactating cows. File No. 27350.
Unpublished report from Union Carbide Agricultural Products
Company, Inc. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Field, W.E. Acute dermal toxicity in rats. Project No. CDC-UC-012-79.
(1979a) Unpublished report from CDC Research, Inc. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Field, W.E. Skin sensitization in guinea pigs. Project No. CDC-UC-003-
(1979b) 79. Unpublished report from CDC Research, Inc. Submitted to
WHO by Union Carbide Agricultural Products Company, Inc.
Fleischman, R.W., Baker, J.R., Hagopian, M., Wade, G.G., Hayden, D.W.,
(1980) Smith, E.R., Weisburger, J.H. & Weisburger, E.K.
Carcinogenesis bioassay of acetamide, hexanamide, adipamide,
urea and phi-tolylurea in mice and rats. J. of Environ.
Pathol. and Toxicol., 3, 149-170.
Gallo, M.A. & Stevens, K.R. Evaluation of LARVIN(R) (UC 51762) in 21
(1980) day dermal toxicity study in rabbits. Project No. 02413-090.
Unpublished report from Foster D. Snell. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Homan, E.R., Weil, C.S., & Cox, E.F. Seven-day feeding study of UC
(1977) 51762 in mice. Special report No. 40-7. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Homan, E.R., Fowler, E.H., Reid, J.B., & Cox, E.F. Thirteen-week oral
(1978a) toxicity study of UC 51762 in dogs. Special report No.
41-98. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Homan, E.R., Maronpot, R.R., Reid, J.B., & Cox, E.F. Thirteen-week
(1978b) oral toxicity study of UC 51762 in rats. Special report No.
41-63. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Huhtanen, K. & Dorough, H.W. Isomerization and Beckman rearrangement
(1976) reactions in the metabolism of methomyl in rats. Pesticide
Biochem. Physiol. 6, 571-583.
Humiston, C.J. & Wright, C.J. An automated method for the
(1967) determination of cholinesterase activity. Tox. Appl.
Pharmacol. 10, 476-480.
Ivett, J.L. & Brusick, D.J. Clastogenic evaluation of 91.48% a.i.
(1985) thiodicarb in an in vivo cytogenetic assay measuring
chromosomal aberration frequencies in Chinese hamster ovary
(CHO) cells. Unpublished report No. 20990 from Litton
Bionetics, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Jagannath, D.R. & Brusick, D. Mutagenic evaluation of CHF 41-43 (UC
(1978) 51762) by the Ames Salmonella/microsome plate test.
Project No. 20838. Unpublished report from Litton Bionetics,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Janes, J.M., Rodwell, D.E., & Jessup, D.C. Teratology study in mice.
(1980) No. 369-032. Unpublished report from International Research
and Development Corporation. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Kamphake, J., Ragsdale, W.E., Weber, E.M., & Carabello, F.B. Repeated
(1980) insult patch test - human volunteers. Report No. 80-0589-
70,73. Unpublished report from Hilltop Research, Inc.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Kaplan, A.M. & Sherman, H. Toxicity Studies with Methyl N-
(1977) (methylamino)-carbamoyloxy ethamidothioate. Toxicol. Appl.
Pharmacol., 40, 1-17.
Khasawinah, A.M. & College, P.R. Fate of single oral dose of 14C-
(1978) acetyl UC 51762 in a lactating cow - metabolism into natural
products. File No. 25257. Unpublished report from Union
Carbide Agricultural Products Company, Inc. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Koehler, S. & Dorman, N. Clinical safety evaluation of LARVIN(R) UC
(1979) 51762 powder and LARVIN(R) 500 flowable. Study No. 2177.
Unpublished report from Food and Drug Research Laboratories.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Lemen, J.K., Wolfe, G.W., & Voelker, R.W. Acute dermal administration
(1979) in rabbits. Project No. 400-614. Unpublished report from
Hazleton Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) technical;
(1982a) primary eye irritation test in rabbits. Report No. PH 421-
UC-004-82. Unpublished report from Pharmakon Research
Laboratories, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - analytical
(1982b) standard; acute oral toxicity study in rats (14 Day). Report
No. PH 402-UC-010-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - analytical
(1982c) standard; acute oral toxicity study in rats (14 Day). Report
No. PH 402-UC-011-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical;
(1982d) acute dermal toxicity test in rabbits. Report No. PH 422-UC-
005-82. Unpublished report from Pharmakon Research
Laboratories, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982e) lab preparation; acute oral toxicity test in rats. Report
No. PH 402-UC-008-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982f) lab preparation; acute oral toxicity test in rats. Report
No. PH 402-UC-009-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982g) pilot plant - batch 34; acute oral toxicity test in rats.
Report No. PH 402-UC-006-82. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982h) pilot plant - batch 34; acute oral toxicity test in rats.
Report No. PH 402-UC-007-82. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. Primary dermal
(1982i) irritation study in rabbits. Report No. PH 420-UC-002-82.
Unpublished report from Pharmakon Research Laboratories,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Matthews, R.J. Acute intraperitoneal LD50 of UC 51762 in the mouse.
(1979a) Report No. PH 405-UC-001-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Matthews, R.J. Acute intraperitoneal LD50 of UC 51762 in the rat.
(1979b) Report No. PH 404-UC-001-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mirro, E.J. & DePass, R.L. Inclusion of UC 51762 in the diet of rats
(1982) for 28 days. Report No. 45-19. Unpublished report from Union
Carbide Bushy Run Research Center. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myer, J., Dean, W.P., & Jessup, D.C. Acute delayed neurotoxicity study
(1980) in hens. Report No. 369-047. Unpublished report from
International Research and Development Corporation.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Weil, C.S., & Carpenter, C.P. Acute toxicity studies of
(1975) UC 51762 in rats and rabbits. Special report No. 38-55.
Unpublished report from Mellon Institute. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Myers, R.C., Weil, C.S., & Cox, E.F. Acute oral LD50 study UC 45650
(1976a) (S-methyl O-(methylcarbamoyl)acetothiohydroximate) in rats.
Special report No. 39-40. Unpublished report from Mellon
Institute. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Myers, R.C., Weil, C.S., & Cox, E.F. Acute toxicity study of S-methyl
(1976b) acetothiohydroximate UC 52702 in rats and rabbits. Special
report No. 39-25. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Carpenter, C.P., & Cox, E.F. Acute inhalation LD50 study
(1977a) of UC 51762 in rats. Special report No. 40-61. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., Carpenter, C.P., & Cox, E.F. Acute LD50 study of UC 51762
(1977b) in mice. Special report No. 40-6. Unpublished report from
Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., Carpenter, C.P., & Cox, E.F. Acute oral LD50 study in
(1977c) guinea pigs. Special report No. 40-142. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., Homan, E.R., Carpenter, C.P. & Cox, E.F. Miscellaneous
(1978a) toxicity studies. Special report No. 41-25. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., Homan, E.R., & Cox, E.F. Acute dermal toxicity of UC
(1978b) 51762 in rabbits. Special report No. 41-93. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., Homan, E.R., & Webb, G.A. Acute oral LD50 study of
(1978c) 1-methylthioacetaldehyde-O-(hydroxymethyl carbamoyl) oxide
(UC 58614) in rats. Special report No. 41-133. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., Homan, E.R., Weil, C.S., & Frank, F.R. UC
(1979a) 51762 technical; range-finding toxicity studies. Special
report No. 42-19. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Homan, E.R., Weil, C.S., & Frank, F.R. Miscellaneous
(1979b) toxicity studies. Special report No. 42-27. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. Methomyl technical; acute
(1981) toxicity and irritancy study. Report No. 44-75a. Unpublished
report from Union Carbide Bushy Run Research Center.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. Acute toxicity and irritancy
(1982a) studies. Report No. 45-24. Unpublished report from Union
Carbide Bushy Run Research Center. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. LARVIN(R) - analytical
(1982b) standard; acute oral toxicity study in rats. Report No.
45-152. Unpublished report from Union Carbide Bushy Run
Research Center. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. LARVIN(R) - technical lab
(1982c) preparation; acute oral toxicity study in rats. Report No.
45-151. Unpublished report from Union Carbide Bushy Run
Research Center. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. LARVIN(R) - technical pilot
(1982d) plant - batch 34; acute oral toxicity study in rats. Report
No. 45-151. Unpublished report from Union Carbide Bushy Run
Research Center. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., Weil, C.S., & Frank, F.R. LARVIN(R) - technical; acute
(1982e) oral toxicity study in rats. Report No. 45-40. Unpublished
report from Union Carbide Bushy Run Research Center.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Slesinski, R.S. & Frank, F.R. Methomyl oxime: acute
(1984) toxicity and irritancy study. Project report 47-185.
Unpublished report from Union Carbide Bushy Run Research
Center. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; micronucleus test.
(1979a) Report No. PH 309-UC-001-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; mitotic crossing
(1979b) over in Saccharomyces cerevisiae. Report No. PH302-UC-001-
79. Unpublished report from Pharmakon Research Laboratories,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; mitotic gene
(1979c) conversion in Saccharomyces cerevisiae. Report No. PH304-
UC-001-79. Unpublished report from Pharmakon Research
Laboratories, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; primary DNA
(1979d) damage. Report No. PH 305-AM-002-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; reverse mutation
(1978e) in Saccharomyces cerevisiae. Report No. PH 303-UC-001-79.
Unpublished report from Pharmakon Research Laboratories,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Pozzani, U.C., Nair, J.R., & Carpenter, C.P. The toxicity of
(1955) acetonitrile. Special report No. 18-34. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Rogers, L.D. Intraportal injection of acetonitrile. Report No. 22-2.
(1959) Unpublished report from Mellon Institute. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Schardein, J. 21-Day dermal toxicity study in rabbits. Report No.
(1982) 369-053. Unpublished report from International Research and
Development Corporation. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Schwartz, C.S. & Stevens, K.R. Acute oral LD50 in hens. Report No.
(1978) 6064. Unpublished report from Food and Drug Research
Laboratories. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Smyth, H.F. Range-finding toxicity test of acetonitrile. Special
(1947) report No. 10-23. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Tasker, E.J., Rodwell, D.E., & Jessup, D.C. Teratology study in rats.
(1979) Report No. 369-029. Unpublished report from International
Research and Development Corporation. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Weatherholtz, W.M. LARVIN(R) - technical; fourteen-day eye irritation
(1982) study in monkeys. Project No. 400-648. Unpublished report
from Hazleton Research Laboratories. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Weatherholtz, W.M., Rendon, F., & Gluck, S.E. Dose rangefinding study
(1983) in monkeys. Project No. 400-694. Unpublished report from
Hazleton Research Laboratories. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Weil, C.S. & Carpenter, C.P. Miscellaneous toxicity studies. Special
(1978) report No. 34-88. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Weisburger, J.H., Yamamoto, R.S., Glass, R.M., & Frankel, H.H.
(1969) Prevention by arginine glutamate of the carcinogenicity of
acetamide in rats. Toxicol. Appl. Pharmacol., 14,
163-175.
Wentz, K.L. & Wolfe, G.W. Primary skin irritation study in rabbits.
(1979) Project No. 400- 617. Unpublished report from Hazleton
Research Laboratories. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Wolfe, G.W. Subchronic toxicity study in dogs. Project No. 400-626.
(1981) Unpublished report from Hazleton Research Laboratories.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Woodside, M.D., Weil, C.S., & Cox, E.F. Seven-day feeding study of UC
(1975) 51762 in rats. Special report No. 38-136. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Reid, J.B., & Cox, E.F. Seven-day
(1978) feeding study of UC 51762 in rats. Special report No.
41-100. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1979a) UC 51762; dimethyl N,N'-(thiobis((methylimino)carbonoyloxy))
bis-(ethanimidothioate) inclusion in the diet of rats for
thirteen weeks. Special report No. 42-125. Unpublished
report from Mellon Institiute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1979b) UC 51762; rat teratology studies. Special report No. 42-48.
Unpublished report from Mellon Institute. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1979c) UC 51762 technical; inclusion in the diet of rats for three
generations and dominant lethal mutagenesis studies. Special
report No. 42-165. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1980a) UC 51762; chronic oncogenicity feeding study in mice.
Special report No. 43-10. Unpublished report from Mellon
Institute. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1980b) UC 51762; chronic toxicity and oncogenicity feeding study in
Fischer 344 rats. Special report No. 43-18. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Yoshida, A., Maita, K., Saito, T., & Miyaoka, T. LARVIN(R): 4-weeks
(1983a) range-finding study in mice. Unpublished report from The
Institute of Environmental Toxicology. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Yoshida, A., Takahashi, K., Kosaka, T., & Miyaoka, T. Thiodicarb: 3
(1983b) month oral sub-chronic toxicity study in mice (study I).
Unpublished report from The Institute of Environmental
Toxicology. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Ziemke, K.A. & Rodwell, D.E. Pilot teratology study in rats. Report
(1979) No. 369-028. Unpublished report from International Research
and Development Corporation. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Andrawes (1983) reported that oral administration of radiolabeled
acetonitrile to rats resulted in elimination of 65% of the applied
dose in expired air, with less than 1% expired as 14CO2. In a
similar study however, Huhtanen & Dorough (1976) found that in rats
9.1% of a dose of acetonitrile was eliminated as CO2 in respiratory
gases.
Two lactating Holstein cows/group were given approximately 0.004,
0.4, 1.4, or 4 mg 14C-thiodicarb per kg b.w. daily for 21 days. Milk
and urine were collected twice daily, and blood and faeces were
collected at 3- to 4-day intervals. One cow at each dosage level was
sacrificed 12 hours after the last treatment, and tissues (liver,
kidney, lung, spleen, heart, brain, ovary, udder, tongue, foreleg
muscle, hindleg muscle, neck muscle and omental fat) were collected
for analysis. The remaining 4 cows (1 at each dosage level) were kept
for an additional 7 days after termination of treatment. During this
post-treatment phase, milk and urine were collected twice daily as
before, and blood and faeces were collected daily. The same tissues
were collected after 7 days post-treatment. The radioactivity found in
all samples (i.e., tissue, milk, blood, urine and faeces) was dose
dependent. The concentration of acetonitrile in milk approached peak
levels within 7 days in all dose groups. The acetonitrile levels
appeared to remain nearly constant through the rest of the 21-day
treatment period, then declined sharply during the 7-day post-
treatment phase. Following 21 days of treatment, tissue levels of 14C
residues were highest in liver and lowest in adipose tissues. Seven
days after termination of dosing, radioactivity levels were lower in
all tissues except liver. The authors concluded that the predominant
end-products of thiodicarb catabolism were acetonitrile, acetamide,
and carbon dioxide. Acetamide, which is believed to be produced by
hydrolysis of acetonitrile, was found in trace amounts (< 0.01 ppm)
in milk at the highest feeding level. Approximately 24% of the
aqueous-extractable urinary-radioactivity was identified as acetamide
(Feung et al., 1980).
In a cow given a single dose (7.02 mg/kg b.w.)14C-acetyl
thiodicarb, 66, 11.4, 5.0, and 4.6% of the administered radioactivity
was eliminated, during 72 hours post-treatment, in respired air,
faeces, urine and milk, respectively, while 10.1% was retained in the
tissues (Khasawinah & College, 1978).
In a study using mature White Leghorn laying chickens, no
carbamate residues were found in tissues or eggs after 21 days of oral
administration of thiodicarb at dosages of 15.4, 28.6 and 102 ppm in
the diet. Acetonitrile, detected in the eggs and tissues (except fat)
at the 102-ppm level, corresponded to 0.2 ppm (egg yolk and whites),
0.5 ppm (liver), and 0.2-0.3 ppm (muscle). The level in eggs was
directly proportional to the dosage level administered. Acetamide was
not detected in egg yolks, but was found in egg whites (0.07 ppm), in
liver (0.06 ppm) and in muscle (0.01 ppm), when administered at 102
ppm thiodicarb (Andrawes & Bailey, 1980).
Based on the available data that 5.72% of the administered
syn-methomyl is converted to acetonitrile, a maximum of 5.72% of the
administered 14C-thiodicarb could therefore be available for
conversion to acetamide, since thiodicarb is metabolized predominantly
to syn-methomyl. On a weight-percent basis, the maximum acetamide
that could occur from ingestion of thiodicarb residues is 1.9% of the
administered dose (53:1 thiodicarb:acetamide on a weight-to-weight
basis) (Andrawes & Bailey, 1979a & 1979b; Huhtanen & Dorough, 1976).
Effects on enzymes and other biochemical parameters
The most significant biochemical effect of thiodicarb and its
carbamate metabolites is the ability to reversibly inhibit
acetylcholinesterase (ChE), a capability in common with other methyl
carbamate esters. The methylcarbamate-acetylcholinesterase complex
dissociates rapidly, and the acetylcholinesterase enzyme is released
unaltered. The transient nature of acetylcholinesterase inhibition by
thiodicarb makes it difficult to assay accurately, since inhibition is
reversed by sample dilution and by adding substrate (butyrylcholine or
acetylcholine) during assay procedures. Therefore, the assay procedure
must be very rapid and use less than optimal amounts of substrate. The
automated colorimetric method of Humiston & Wright (1967) is suitable
for such determination.
Groups of Fischer 344 rats (10 males and 10 females per group)
were administered thiodicarb in the diet at dosage levels of 0, 1, 3,
10, or 30 mg/kg b.w./day for 28 days. Rats were observed for mortality
and clinical symptoms of toxicity. Food consumption was determined
weekly and plasma- and erythrocyte-ChE measurements were analyzed on
days 0, 15, and 29. Brain ChE was determined on day 29.
Body weight and food consumption were significantly reduced in
females at > 10 mg/kg, b.w. Males were not similarly affected.
There was no mortality. Plasma and erythrocyte ChE were significantly
depressed in high-dose males and females. Plasma ChE recovered to
normal values by day 29, while red blood cell (RBC) ChE remained
significantly depressed in both sexes at > 30 mg/kg b.w./day. Brain
ChE was not depressed at any level (Mirro & DePass, 1982).
Toxicological studies
Special study on reproduction
Rat
Groups of Fischer 344 rats (20 female and 10 male rats/group)
were administered thiodicarb (purity 99+%) in the diet, at dosage
levels of 0, 0, 0.5, 1.0, 3, or 10 mg/kg b.w., in a three-generation
reproduction study. Each generation produced only one litter per
generation. The routine indices of fertility, gestation,
survivability, viability, and lactation were determined for all
generations, as well as selected gross and histopathological
evaluations for F2a and F3a generations. Rats were mated on a 1-male-
to-2-females ratio when approximately 100 days of age. Dams and pups
were observed daily for appearance and behaviour. Litter size was
reduced to 10 on day 4 postpartum. Brother-sister matings were
avoided.
There were no compound-related effects on any of the reproductive
parameters measured, except for an initial transitory body-weight
depression in F0 males. Gross and microscopic analyses were
reportedly unremarkable. Thiodicarb was without adverse effects on
reproduction at doses up to and including 10 mg/kg b.w. (Woodside
et al., 1979c).
Special studies on teratogenicity
Mouse
In a pilot study using pregnant Charles-River CD-1 mice,
thiodicarb (technical grade) administered orally from days 6 through
16 of gestation, at dosage levels of 0, 20, 60, 100, 150, 200, 300, or
400 mg/kg b.w., produced excessive maternal toxicity (body-weight
depression and mortality) at doses greater than 200 mg/kg b.w.
(Dangler & Rodwell, 1979).
Groups of Charles-River CD-1 mice (25 mated females/group) were
administered thiodicarb (technical grade) via oral gavage at dosage
levels of 0, 50, 100, or 200 mg/kg b.w. from days 6 through 16 of
gestation. Mating was accomplished on a 1-female-to-1-male schedule at
94 days of age. Day of copulation was also considered day 0 of
gestation. Animals were observed routinely for appearance, behaviour
and body-weight changes. Pups were delivered by Caesarean-section on
day 17 of gestation. All foetuses were weighed, sexed, examined for
external malformations, and subsequently subjected to visceral or
skeletal examination.
Six females in the high-dose group died during the study. Body
weights were unaffected by treatment. There was no evidence of
maternal or foetal toxicity at doses > 100 mg/kg b.w. and no
teratogenic effects at doses > 200 mg/kg b.w. (Janes et al.,
1980).
Rat
In a pilot teratology study using pregnant Charles-River CD COBS
rats, thiodicarb (technical grade), administered orally at dosage
levels of 0, 20, 40, 80, 120, or 160 mg/kg b.w. on days 6-19 of
gestation, produced mortality at doses > 40 mg/kg b.w. There were
no reported adverse effects on reproduction or foetal development
(Ziemke & Rodwell, 1979).
Groups of Charles-River CD COBS rats (25 mated females/group)
were administered thiodicarb (technical grade) via oral gavage at
dosage levels of 0, 10, 20, or 30 mg/kg b.w. on gestation days 6-19.
Females were mated 1-to-1 with males at 13 weeks of age. Day of
copulation was also considered day 0 of gestation. Animals were
observed routinely for appearance, behaviour, and body-weight changes.
Pups were delivered by Caesarean-section on gestation-day 20. All
foetuses were weighed, sexed, measured, and examined for external
malformations. One-third of the foetuses were fixed for visceral
examination and the remaining two-thirds of the foetuses were
subjected to skeletal examination. Early and late resorptions, number
of implantations and corpora lutea, and the number and location of
viable and non-viable foetuses, were recorded.
Clinical observations, apparent at 20 and 30 mg/kg b.w. within 4
hours after treatment, included inactivity, tremors, and clear oral
discharge. There was no mortality. Maternal body weights determined
on days 0, 6, 9, 12, 16, and 20 were significantly decreased at
> 20 mg/kg b.w, and mean foetal body-weights were significantly
reduced at > 10 mg/kg b.w. No teratogenic effects were produced,
although delayed maturation of foetuses (reduced ossification),
associated with maternal toxicity, was observed. There was no evidence
of visceral or skeletal anomalies attributable to thiodicarb at doses
up to and including 30 mg/kg b.w. (Tasker et al., 1979).
Groups of Fischer 344 rats (10 mated females/group) were
administered thiodicarb (purity 99+%) in the diet via 2 separate
dosing regimens: from day 0 to day 20 of gestation or from day 6 to
day 15 of gestation. Dose levels in each dosing schedule included 0,
0.5, 1.0, 3, or 100 mg/kg b.w. A positive control group received
625 mg/kg b.w. of aspirin via gavage on gestation day 10. Females were
mated with males on a 1-to-1 schedule in order to achieve 10 pregnant
females per dose. All pups were delivered by Caesarean-section on
gestation day 20 and examined for visceral and skeletal abnormalities.
Maternal body-weight gains determined on days 0 and 12 of
gestation were reduced in all treatment groups. However, these changes
were extremely variable at doses of 0.5-3.0 mg/kg b.w. (only two
measurements were included) and they were not considered to be
biologically significant except at the highest dose administered where
the body weights were uniformly depressed. Foetal body weight and
length were also significantly reduced in the high-dose group that was
administered thiodicarb throughout gestation (days 0-20). The aspirin-
exposed foetuses were similarly affected. Thiodicarb did not adversely
affect the number of implantations, resorptions, live foetuses per
litter, or pre-implantation losses. Resorption frequency was increased
in high- dose females given thiodicarb from days 6 through 15 of
gestation. The increase was based on the percentage of affected
litters as well as the resorptions per litter. Aspirin demonstrated
the same response. The incidences of visceral anaomalies were not
increased in either the aspririn or thiodicarb groups when compared to
controls. Skeletal variations were similar among all groups except for
an increased incidence of bilobed thoracic vertebral centra and poorly
ossified sternebrae (on a percentage of live-foetuses-per-litter
basis) in the 100 mg/kg b.w. group administered thiodicarb throughout
gestation. This effect was suggestive of delayed maturity in foetuses
derived from dams which demonstrated significant maternal toxicity
(i.e. decreased weight gain and decreased foetal size). This effect is
also known to occur spontaneously (at similar frequencies) in other
rat strains. The aspirin group exhibited several skeletal variations
(e.g. split/missing vertebral centra, extra vertebrae, extra ribs and
fused/wavy ribs), demonstrating the sensitivity of the study.
Thiodicarb was not teratogenic at any dose administered but it was
maternally toxic at 100 mg/kg b.w. (Woodside et al., 1979b).
Special studies on carcinogenicity (See also "Long-term studies")
Mouse
Groups of Charles-River COBS CD-1 mice (80/sex/group) were
administered thiodicarb (analytical grade, 99+% purity) in the diet
for 24 months at dosage levels of 0, 0, 1.0, 3, or 10 mg/kg b.w./day.
Animals were 42 days old at the start of the study. The mice were
examined daily for mortality and adverse physical/behavioural
condition. Routine evaluation of food consumption and body weights
were performed. At the conclusion of the study, or at death, organ
weights and gross and microscopic examination of tissues and organs of
all animals were performed. All were anesthetized with methyoxyflurane
and killed by cervical dislocation.
Mortality in high-dose mice was increased over controls in males
during the last 2 months of the study and in females during months 17
and 18.
Body weights and food consumption were not affected by treatment.
There were no significant differences among treatment and control
groups for the incidence of neoplasms or the identification of non-
neoplastic lesions occurring in dead animals, or interim- and final-
sacrifice animals. However, the overall incidence among control and
treatment groups of hepatocellular neoplasms was much greater than
reported in the literature for this strain of mouse (Table 1).
Table 1. Hepatocellular neoplasms in mice treated with thiodicarb
and in 2 control groups
Dose (mg/kg b.w.)
0 0 1 3 10
Males
Adenoma 18/80 19/76 22/78 24/78 24/80
Carcinoma 37/80 25/76 29/78 32/78 26/80
Females
Adenoma 2/80 5/77 2/78 2/76 1/75
Carcinoma 1/80 3/77 3/78 1/76 3/75
Furthermore, there was no decrease in time-to-tumour for the
observation of hepatocellular neoplasms (Table 2).
Table 2. Time-to-tumour (hepatocellular neoplasms) for male mice
treated with thiodicarb and for 2 control groups
Dose (mg/kg b.w.)
0 0 1 3 10
Animals which
died or were 6/53 10/49 12/51 22/48 13/54
moribund
Animals at 18-
month interim 16/20 15/20 16/20 15/20 13/20
sacrifice
Animals surviving
24 months 4/7 6/8 6/7 4/10 4/6
(terminal kill)
The other most frequently occurring neoplasm was alveologenic
tumour of the lungs. As with hepatocellular neoplasms, there was no
compound-related response within the incidence of this tumour type
(Table 3).
Table 3. Alveologenic tumours in mice treated with thiodicarb and
in 2 control groups
Dose (mg/kg b.w.)
0 0 1 3 10
Males
Alveologenic
tumours 23/80 23/76 31/78 15/78 22/80
Carcinoma 1/80 0/76 0/78 1/78 0/80
Females
Alveologenic
tumours 19/80 13/78 19/78 17/77 13/75
Carcinoma 2/80 0/78 0/78 0/77 0/75
The principle non-neoplastic lesions observed in all control and
treated groups included: lymphocytic infiltration or hyperplasia of
numerous tissues, generalized amyloidosis, hyaline degeneration of
sternal cartilage, haemosiderosis of various organs, subacute
sialoadenitis, glomerulosclerosis, and renal cystic tubular dilation.
These effects were not compound-related. Thiodicarb was not
carcinogenic to CD-1 mice at dietary doses up to and including
10 mg/kg b.w. (Woodside et al., 1980a).
Special studies on mutagenicity
Thiodicarb was evaluated in a series of mutagenicity assays
including the Ames, dominant-lethal, micronucleus, reverse-mutation,
mitotic-crossing-over, and DNA-damage test methods. It was negative
for mutagenic potential in all tests except for a positive response in
the mitotic gene conversion assay using Sacharomyces cerevisiae.
(See Table 4 for details.)
Table 4. Results of mutagenicity assays on thiodicarb
Test Test Concentration Purity Results Reference
System Object of thiodicarb
Ames test S. typhimurium 1, 10, 100, Unknown Negative Jagannath &
* TA98 500, and Brusick, 1978
TA100 1000 µg/plate
TA1535
TA1537
TA1538
Micronucleus Mouse, bone 5 and 10 Technical Negative Naismith &
test marrow mg/kg grade (1) Matthews,
1979a
Dominant Rat 0, 0.5, 1, Analytical Negative Woodside
lethal 3 & grade (2) et al., 1979c
10 mg/kg (99+%)
Reverse S. cerevisiae 2.5, 6.2, Technical Negative Naismith &
mutation 25, 62, & grade (3) Matthews,
250 µg/ml 1979b
Mitotic S. cerevisiae 2.5, 6.2, Technical Negative Naismith &
crossing 25, 62, & grade (3) Matthews,
over 250 µg/ml 1979e
Mitotic S. cerevisiae 2.5, 6.2, Technical (4) Naismith &
gene 25, 62, & grade Matthews,
conversion 250 µg/ml 1979c
Primary E. coli 0.001, 0.01, Technical Negative Naismith &
DNA W3110 0.1, 1, and grade (5) Matthews,
damage* p3478 10 mg/ml 1979d
Chromosomal Chinese hamster 10, 15, 20, Technical Negative Ivett &
aberrations ovary (CHO) 30 µg/ml 91.48% Brusick,
cells* w/o activ. 1985
10, 20, 30,
40 µg/ml
w/activation
Unscheduled Rat primary 0.5, 1.0, 2.5, Technical Negative Cifone &
DNA synthesis hepatocytes 5.0, 10.0, 91.48% Brusick,
25.0, 50.0, 1985a
100.0, 250.0
µg/ml
Table 4. (Con't)
Test Test Concentration Purity Results Reference
System Object of thiodicarb
Mouse lymphoma L5178Y (TK+/-) 5, 8, 10, Technical Positive Cifone &
forward mutation & 12 µg/ml 91.48% (6) Brusick,
assay* 1985b
* With and without metabolic activation
(1) The positive control (TEM) gave the expected response at 0.5 ml/kg.
(2) The positive control (TEM) gave the expected response at 0.25 mg/kg.
(3) The positive control (NQO) gave the expected response at 10-6 M.
(4) Increase in the heteroallelic trp 5-12/trp 5-27 diploid strain D7 at
25 µg/ml.
(5) The positive controls (ethylmethane sulfonate and diethylnitrosamine)
gave the expected response.
(6) The test material induced repeatable increases in the mutant frequency,
with and without metabolic activation.
Special studies on neurotoxicity
Although the Meeting considers the testing of methyl carbamates
for potential neurotoxicity to be inappropriate (Annex 1, FAO/WHO,
1985b, para. 2.8), the manufacturer conducted 2 separate studies, both
of which were negative.
Chicken
In a preliminary acute oral toxicity study using 10- to 14-month-
old White Leghorn hens, the LD50 for thiodicarb was calculated to be
582 mg/kg b.w. When atropine sulfate (15 mg/kg b.w.) was administered
15 minutes prior to dosing only 2/5 hens died after 5 days at a dose
of 830 mg/kg b.w. (Schwartz & Stevens, 1978).
Hens protected with 15 mg/kg b.w. atropine sulfate were
administered single doses of 660 mg/kg b.w. thiodicarb via oral
intubation. Vehicle (corn oil) and positive (TOCP) control groups were
also employed. A dose of 750 mg/kg b.w. TOCP in corn oil was used.
There were 10 hens in each control group and 40 in the thiodicarb
group. Hens were observed daily for pharmacologic and toxicologic
effects, including neurologic evaluation of leg weakness, gait, and
walking ability. On day 21 all positive controls and 10 test-group
birds were necropsied, perfused with 10% neutral buffered formalin,
and examined histologically. On day 22 eight new positive controls
were added to the study and the existing 30 treated birds were
re-dosed a second time with 660 mg/kg b.w. thiodicarb.
The vehicle controls provided no evidence of toxicity, while the
positive control group displayed clinical and microscopic symptoms of
delayed neuro-toxicity (increased number of swollen axons per cervical
spinal cord cross-section). Although some signs of neurological
impairment were initially displayed in the thiodicarb group, these
were not evident after 4 days in study and were not confirmed upon
histologic examination (Becci et al., 1979).
Due to the marginal response in thiodicarb-treated birds, the
entire study was repeated using 30 White Leghorn hens (8-12 months
old) dosed orally with 660 mg/kg b.w. thiodicarb (analytical grade).
There were 10 hens in each of the negative (corn oil) and positive
(TOTP) control groups. Thirty minutes prior to dosing, each group
received 25 mg/kg b.w. atropine sulfate via gavage. A dose of
1200 mg/kg b.w. TOTP was employed in the positive control group.
A total of 13/30 thiodicarb hens died on test, with no mortality
in either control group. Thiodicarb and the negative control group
provided no evidence of delayed neurotoxicity. In the TOTP group, 9/10
birds demonstrated lesions in the sciatic nerve and spinal cord,
consisting of axonal degeneration, necrosis, and demyelination (Myer
et al., 1980).
Special studies on skin and eye irritation
Rabbit
Thiodicarb is not irritating to the skin. When applied to the
shaved backs of rabbits, thiodicarb produced only slight erythema in a
few cases (Mallory et al., 1982i; Wentz & Wolfe, 1979).
Thiodicarb was applied to the shaved, intact skin of rabbits, 5
times a week for 3 consecutive weeks, at 4 dosage levels up to
4 g/kg/day. Mortality, behaviour, food consumption, body weight, and
gross and microscopic histopathology were unaffected by treatment.
Localized erythema and edema occurred in all treated groups.
Thiodicarb treatment was reported to produce a dose-related macrocytic
anaemia (Conroy et al., 1979b; Gallo & Stevens, 1980).
In a second dermal toxicity study in rabbits, using
2000 mg/kg/day as the highest dosage level, all criteria were
unaffected by treatment. Groups of 10 male and 10 female New Zealand
White rabbits were treated dermally with thiodicarb 5 days per week
for 3 consecutive weeks at dosage levels of 0, 250, 500, 1000, or
2000 mg/kg (2 control groups were utilized in this study). Thiodicarb
was administered to both intact and abraded dermal surfaces. There
were no effects in the study attributable to thiodicarb. The
occurrence of macrocytic anaemia, observed in the first study, was not
confirmed (Schardein, 1982).
Thiodicarb applied to the conjunctival sacs of rabbits did not
damage the cornea, but resulted in temporary eye irritation (Cameron &
Wolfe, 1979; Mallory et al., 1982a). In monkeys, thiodicarb caused
no eye irritation or damage (Weatherholtz, 1982). Methomyl was mildly
irritating to the skin of guinea-pigs and was a mild irritant to the
eye (Kaplan & Sherman, 1977). Methomyl oxime was not irritating to the
skin and was moderately irritating to the eye (Myers et al., 1984).
Special studies on dermal sensitization
The potential for thiodicarb to produce skin sensitization was
tested in guinea-pigs and human subjects. Guinea-pigs, given multiple
intradermal injections of thiodicarb in a modified Landsteiner
procedure, showed minimal sensitization reactions to a challenge
injection two weeks later (Conroy et al., 1979a & c). Repeated
application (via a modified Buehler method) of thiodicarb to the
shaved backs of guinea-pigs did not cause allergic sensitization
(Field, 1979b). In a similar test with human subjects, thiodicarb
produced no sensitization by repeated contact (Kamphake et al.,
1980). Methomyl was not a sensitizer when administered to guinea-pigs
(Kaplan & Sherman, 1977).
Acute toxicity
The acute toxicity of thiodicarb and its metabolites in several
animal species is summarized in Table 5.
Short-term studies
Mouse
Groups of Charles River CD-1 mice (5 males and 5 females per
group) were administered thiodicarb in the diet for 7 days at dietary
levels of 0, 15, 45, or 90 mg/kg b.w./day. Growth and mortality were
unaffected. In male mice, at the two highest dosage levels, an
increased absolute kidney weight was observed (Homan et al., 1977).
Groups of ICR-JCL (SPF) mice (10 per sex per group) were fed
thiodicarb (92.5% purity) in the diet for 4 weeks at dosage levels of
0, 30, 100, or 300 ppm (equal to 0, 4.2, 13.7, and 40.8 mg/kg b.w./day
for males and 0, 3.8, 12.3, and 36.3 mg/kg b.w./day for females).
There was no mortality during the study. Body weight, food consumption
and organ weights (brain, heart, liver, spleen, kidney, testes, and
ovaries) were unaffected by treatment (Yoshida et al. 1983a).
Table 5. Results of acute toxicity assays of thiodicarb and its metabolitea
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Thiodicarb Mouse both Oral corn oil 226 Myers et al., 1977b
(148-346)
Rat M Oral corn oil 74.8-129 Hallory et al.,1982b;1982e;1982g
(52.9-186) Myers et al.,1982b;1982c;1982d;1982e
F Oral corn oil 50-136 Mallory et al., 1982b;1982e;1982g
(34.9-192.1) Myers et al.,1982b;1982c;1982d;1982e
M Oral 0.25% methyl 46.5-83 Mallory et al., 1982c;1982f;1982h
cellulose or (33.4-108) Myers et al.,1982b;1982c;1982c;1982d
water
F Oral 0.25% methyl 39.1-55 Mallory et al., 1982e;1982f;1982h
cellulose or (29.4-65.5) Myers et al., 1982b;1982c;1982d
water
both Oral corn oil 74.6-215 Mallory et al., 1982b;1982e;1982g
(59.6-268.8) Myers et al., 1982b;1982c;1982d;1982e
both Oral 0.25% methyl 49.4-97 Mallory et al., 1982c;1982f;1982h
cellulose or (39.4-138.2) Myers et al., 1982b;1982c;1982d
water
Guinea- M Oral corn oil 160 Myers et al., 1977c
pig (94.3-271)
Rabbit both Oral capsule > 400 Myers et al., 1979a
both Oral corn oil 556 Myers et al., 1979a
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Chicken F Oral corn oil 582-660 Myer et al., 1980
Schwartz & Stevens, 1978
Monkey both Oral capsule 467.2 Weatherholtz et al., 1983
(329.6-662.1)
Rat M Dermal 2540 Field 1979a
4 hr. (1120-5750) Koehler & Dorman 1979
intact Myers et al., 1975;1979a
skin
Rabbit both Dermal > 6310 Lemen et al., 1979;
24 hr. Mallory et al., 1982d;
abraded Myers et al., 1978b;1979a
& intact
skin
Rat both I.P. 23 (15.9-33.1) Matthews 1979b
Mouse both I.P. 34 (21.9-52.7) Matthews 1979a
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Rat Inhalation > 2.0 Myers et al., 1975
148 min.
aerosol in
DMSO
Inhalation > 0.2 Myers et al., 1979a
360 min.
aerosol in
DMSO
both Inhalation 0.1155-0.220 Dickey et al., 1979
240 min. (0.1094- Myers et al., 1977a
dust 0.320)
Inhalation > 2.4 Myers et al., 1978a
60 min.
dust
Aqueous 1.04 Myers et al., 1982a
aerosol
Methomyl Rat M Oral corn oil 14.1-47.6 Kaplan & Sherman 1977;
peanut oil (8.0-72.7) Myers et al., 1976a;1981
PEG 400
F Oral corn oil 12.3-48.5 Kaplan & Sherman 1977;
peanut oil (7.6-70.2) Myers et al., 1979b;I981
Weil & Carpenter 1978
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Guinea- M Oral acetone 15*** Kaplan & Sherman 1977
pig peanut oil
Japanese Oral water;CMC 34 Kaplan & Sherman 1977
Quail
Dog M capsule 30*** Kaplan & Sherman 1977
Monkey both aqueous 40*** Kaplan & Sherman 1977
(Rhesus)
Chicken F Oral acetone/ 28 Kaplan & Sherman 1977
water
Hydroxymethyl Rat M Oral corn oil 200 Myers et al., 1978c
methomyl (123-326)
Oral corn oil 238 Myers et al., 1979b
(156-363)
Methomyl Rat M Oral corn oil 453 Myers et al., 1978a
sulfoxide (296-692)
Oral corn oil 476 Myers et al., 1979b
(311-727)
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Methomyl Rat M Oral corn oil 794 Myers et al., 1976b
oxide (486-1300)
Oral corn oil 1000 Myers et al., 1979b
(636-1572)
both Oral water 350 Myers et al., 1984
(311-394)
Acetonitrile Rat M Oral water 246 Pozzani et al., 1955
(160-378)
F Oral water 234 Pozzani et al., 1955
(203-270)
Oral water 38/0 Smyth 1947
Methomyl Rat M Dermal water > 200 X Kaplan & Sherman 1977
intact & 5 days
abraded
skin 6 h
Rabbit M Dermal water > 5000 Kaplan & Sherman 1977
intact
skin 24 h
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
M Dermal water 800-951 Myers et al., 1981
intact
& abraded
skin 24 h
F Dermal water 566-1130 Myers et al., 1981
intact
& abraded
skin 24 h
Methomyl Rat M Dermal corn oil > 1000 Myers et al., 1976b
oxime intact
skin 4 h
Dermal water > 2000 Meyers et al., 1984
intact
skin 24 h
Acetonitrile Rat i.p. 0.95 ml/kg Rogers 1959
(0.58-1.54)
Rat i.v. 1.68 ml/kg Rogers 1959
(0.08-3.21)
Rabbit Dermal 1.26 ml/kg Carpenter et al., 1958
Methomyl Rat M Inhaltion water 76 mg/l Kaplan & Sherman 1977
of aqueous
aerosol
4 h
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Methomyl Rat Inhalation water > 0.82 Myers et al., 1976b
oxime of mg/1
aqueous
aerosol
4 h
* 95% confidence limits (the widest range from each set of studies) are given in parentheses.
** Because particles of thiodicarb are not of respirable size, the material was ground to a dust
for these tests.
*** Minimum lethal dose.
Groups of ICR-JCL (SPF) mice, (28 per sex per group) were fed
thiodicarb (92.5% purity) in the diet for 13 weeks at dosage levels of
0, 30, 150, or 600 ppm (equal to 0, 3.8, 18.9, and 76.5 mg/kg b.w. for
males and 0, 4.3, 21.5, and 85.0 mg/kg b.w. for females). Mortality,
food consumption, body weight, water consumption, urinalysis,
haematology, and clinical chemistry were determined. Cholinesterase
levels were determined in plasma, red blood cells (RBC) and brain at
study termination, as were organ weights, gross pathology, and
histopathology. Brain ChE was stated to be depressed in 150-
and 600-ppm females and in 600-ppm males. Also reported were
significantly-increased relative liver weights in high-dose females.
There were no other compound-related effects reported. However,
individual animal data, gross pathology, or histopathology were not
submitted. Therefore, a no-observed-effect level cannot be assessed
from these data as presented to the 1985 Joint Meeting (Yoshida
et al., 1983b).
Rat
Groups of Harlan-Wistar rats (5 per sex per group) were fed
thiodicarb in the diet for 7 days at dosages of 0, 5, 25, or 100 mg/kg
b.w./day. Limited effects were noted on body weight and food
consumption, which were decreased in high-dose males. Relative kidney
weights were also reportedly increased in all treated males (Woodside
et al., 1975).
Groups of Wistar rats (5 per sex per group) were administered
thiodicarb in the diet at dosages of 0, 0, 18.5, 48.6, or 128 mg/kg
b.w./day for 7 days. Observations were made on mortality, food
consumption, body weight, and gross liver and kidney changes. Body
weights were reduced in all treated groups compared to controls.
Relative liver weights and absolute kidney weights were reduced in
high-dose rats (Woodside et al., 1978).
In a preliminary 13-week study 4 groups of Fischer 344 rats
(5 per sex per group) were administered thiodicarb in the diet at
dosage levels of 0, 5, 15, or 45 mg/kg b.w./day. One additional group
per sex received 90 mg/kg b.w./day for 80 days. Observations included:
mortality, food and water consumption, organ weights, and ChE activity
(RBC, plasma and brain). Blood samples for ChE measurements were
obtained via cardiac puncture. At necropsy, the brain was removed,
weighed, and submitted for ChE assay. Relative liver, adrenal, and
spleen weights were increased in all treated males, and relative
spleen weights were increased in mid- and high-dose females. Body
weights were reduced throughout the study in all treated males. ChE
activity was unaffected at any dose level, except for increased RBC
ChE activity in high-dose males and females (Woodside et al.,
1979a).
Groups of Fischer 344 rats (10 males and 10 females per group)
were administered thiodicarb (99% purity) in the diet at dosage levels
of 0, 0, 1.0, 3.0, 10 or 30 mg/kg b.w./day for 13 weeks. Observations
included mortality, clinical observations, food and water consumption,
body weight, organ weights, haematology, clinical chemistry,
urinalysis, ChE activity (plasma, RBC and brain-at-germination), and
gross and microscopic analysis. At both 10 and 30 mg/kg b.w./day, body
weight and water consumption decreased significantly among females,
with slight increases in males at the same dose levels. At 30 mg/kg
b.w., relative spleen weights were increased significantly in males,
while relative kidney weights were increased in high-dose females.
Urine volume was increased in males and decreased in females at the
high-dose level. Specific gravity of urine was also decreased in high-
dose females. Other clinical parameters were unaffected by treatment
except for decreased mean haemoglobin concentrations in high-dose
males. ChE activities were comparable among all groups except for a
significantly-increased RBC ChE in high-dose males. Gross and
microscopic analyses were unremarkable and typical of changes
associated with 4-month-old Fischer 344 rats. A NOEL was demonstrated
at 3 mg/kg b.w. (Homan et al., 1978b).
Dog
Groups of 9-month-old Beagle dogs (4 per sex per group) were fed
thiodicarb in the diet at targeted dosages of 0, 15, 45, or 90 mg/kg
b.w./day for 13 weeks. However, due to mortality at the highest dose,
the level was reduced to 76 and 86 mg/kg b.w. for females and males,
respectively. Animals were examined routinely for physical/behavioural
changes, food and water consumption, body-weight changes, haematologic
and blood chemistry parameters, and urinalysis. Organ weights, gross
necropsy and histologic effects were examined at terminal sacrifice.
ChE activities (plasma and RBC) were determined pre- treatment and on
days 28, 63, 79, and 93 of the study. Brain ChE was determined at
sacrifice.
Two females died in the high-dose group, and dose-related
cholinergic symptoms were observed in both sexes in the mid- and high-
dose groups. Symptoms included anorexia, vomiting, and loose stools.
However, ChE activity was unaffected throughout the study. Food
consumption decreased in high-dose females. Water consumption
increased in both sexes in the high-dose groups. Body weight was
decreased in females at the mid- and high-dose levels but there was no
effect on males. Urine volume was increased in mid- and high-dose
males. Decreases in RBC count, packed cell volume, and haemoglobin
were noted in both sexes in the mid- and high-dose groups, while
reticulocyte counts were increased in both sexes of the high-dose
groups only. These changes, together with bone-marrow hyperplasia,
decreased mean-corpuscular haemoglobin concentrations, and congested
spleen in high-dose groups, are suggestive of compensatory
haematopoiesis and anaemia. Relative liver weights and serum GPT
levels were increased in both sexes at the high dose. However, males
at the high dose demonstrated cloudy swelling, vacuolation and
degenerative liver effects. All treated groups produced evidence of
focal and diffuse inflammation of the liver. A clear NOEL was not
demonstrated in this study (Homan, et al., 1978a).
Groups of Beagle dogs, 6-7 months old (6 per sex per dose) were
administered thiodicarb in the diet at dosage levels of 0, 5, 15, or
45 mg/kg b.w./ day for 26 weeks. Animals were observed routinely for
survival, clinical appearance, food consumption, body-weight change,
ChE activity, haematologic and clinical chemistry parameters, and
urinalysis. Organ-weight changes and gross and microscopic pathologic
examinations were performed at terminal sacrifice. Cholinesterase
activity was twice determined pretreatment and once each on fasted and
non-fasted samples during weeks 8, 17, and 26. Brain ChE was
determined at sacrifice.
Thiodicarb did not affect body weight, food consumption,
urinalysis, organ weights, or gross or microscopic pathological
examination of tissues. Among the male dogs, there was a dose-related
increase in the combined incidence of soft stools, mucoid stools, and
diarrhoea. These were not evident in females. Treatment-related
changes in haematology included significantly-decreased haematocrit,
haemoglobin, and RBC levels in high-dose males and females. Dogs
receiving the highest dose had significantly-elevated levels of serum
GPT, plasma ChE, and RBC ChE as well as significantly-decreased levels
of calcium, total protein and globulin, with correspondingly-increased
albumin-to-globulin ratio. There were no treatment-related effects
reported at 15 mg/kg b.w./day (Wolfe 1981).
Long-term study
Rat
Six groups of Fischer 344 rats (120 rats per sex per dose) were
administered thiodicarb (analytical grade, 99+% purity) in the diet at
dosage levels of 0, 0, 0.5, 1, 3, or 10 mg/kg b.w. for 24 months.
Animals were 50 days of age when dosing began, and housed 3 per cage
for males and 5 per cage for females. Rats were examined daily for
mortality and adverse physical or behavioural changes. Food and water
consumption and body-weight changes were determined routinely. Interim
sacrifices were performed at 6, 12, and 19 months, utilizing 10 to 20
rats per sex. Haematology, clinical chemistry, and urinalysis
determinations were made prior to each scheduled sacrifice. Organ
weights and gross and microscopic evaluations were also performed at
each sacrifice and on dead or moribund animals (excluding organ
weights). Limited ophthalmological examinations were also performed.
Mortality was increased in high-dose males, but not females, from
the 15th to the 21st months. At termination of the study, mortality in
control males exceeded that in the high-dose males. Food consumption
was unaffected in treated rats, although sporadic decreases were
occasionally observed. Body weights were depressed in high-dose males
from the 15th to 20th months and in high-dose females from the 3rd to
the 21st months. An outbreak of sialodacry-oadenitis virus was
discovered at about the 18th month, which had contributed to a
generalized debilitated condition and to weight loss in all animals
for approximately 2-3 weeks. Haematological parameters were
unremarkable except for reduced erythrocyte counts, hematocrits and
haemaglobin levels in males fed > 1 mg/kg thiodicarb at the 12th
month, but not at 19 or 24 months. ChE activity (plasma, RBC, and
brain), determined after 48 hours on the control diet, was unaffected.
However, the time delay between compound administration and ChE
measurement is considered excessive for a carbamate and therefore this
result is not meaningful. The only change in ChE activity, consistent
with other thiodicarb-feeding studies, was a significant increase in
RBC ChE at 24 months in high-dose males. Clinical chemistry and
urinalysis determinations were not affected by treatment except for
transient reduction in urine volume at 12 months in females given
> 3 mg/kg. Although sporadic differences were observed at various
sacrifice intervals, there were no dose-related effects on absolute or
relative organ-weights.
There were no significant pathologic changes attributable to
thiodicarb at 6, 12, or 19 months, and no carcinogenic response at any
dose throughout the study. High-dose males presented evidence at 24
months of an increased incidence of pituitary cysts, while high-dose
females demonstrated haemosiderosis of the mediastinal lymph nodes.
Hepatocellular hyperplasia and interstitial prostatitis were increased
in treated males, although not significantly at the high dose; their
incidence was not dose-related, and there was not a significant
increase in liver neoplasms. High-dose males had an increased, but not
significant, incidence of epithelial hyperplasia and epithelial
thymomas. Mononuclear cell leukemia and interstitial cell adenomas
(in males) occurred spontaneously in all groups, in general agreement
with this strain of rat.
A NOEL of 3 mg/kg b.w. was established with no evidence of
oncogenic potential at doses up to and including 10 mg/kg b.w.
(Woodside et al., 1980b).
COMMENTS
Thiodicarb is rapidly absorbed, metabolized, and excreted, and
has not been demonstrated to accumulate in animal tissues. It is
degraded to methomyl, which is converted to the "oxime" (methyl
hydroxythioacetimidate) and ultimately to acetonitrile, carbon
dioxide, acetic acid, and acetamide. The ultimate metabolic fate in
animals depends on the isomeric configuration of methmyl (SYN or
ANTI).
The acute oral toxicity (LD50) in rats is 66-120 mg/kg b.w.,
depending on the vehicle used. In monkeys an oral LD50 is 467 mg/kg
b.w.
Thiodicarb causes reversible cholinesterase inhibition. Long-term
feeding and oncogenicity studies in rats and mice have revealed no
oncogenic potential in either species, and a NOEL of 3 mg/kg b.w. for
non-oncogenic effects was determined. No adverse reproduction or
teratogenic effects in rats or mice have been demonstrated.
Thiodicarb was not mutagenic in a wide variety of assays.
However, it was positive in the mitotic gene conversion assay using
Saccharomyces cerevisiae.
In two short-term feeding studies in dogs, thiodicarb
demonstrated adverse liver effects in 90 days at doses > 15 mg/kg
b.w., while in a 6-month study at doses up to 45 mg/kg b.w. no adverse
effects were observed at 15 mg/kg b.w. Considering the conflicting
information concerning liver effects in dogs, the Meeting required the
submission of the results from an ongoing 1-year dog study by 1987.
Therefore, a temporary ADI was allocated.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 60 ppm in the diet, equivalent to 3 mg/kg b.w.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.01 mg/kg b.w.
FURTHER WORK OR INFORMATION REQUIRED (by 1987)
l. Submission of ongoing 1-year dog feeding study known to be in
progress.
2. Information on possible effect on maternal body-weight gain
demonstrated in a rat teratology study.
DESIRED
1. Observations in man, including monitoring of acetamide in the
urine.
REFERENCES
Andrawes, N.R., College, P.R. & Bailey, R.H. Short-term fate of orally
(1977) administered UC 51762 in the rat. File No. 23509.
Unpublished report from Union Carbide Agricultural Products
Company, Inc. Submitted to WHO by the Union Carbide
Agricultural Products Co., Inc.
Andrawes, N.R. & Bailey, R.H. Metabolism of acetyl-l-l4C-LARVIN(R) in
(1979a) the rat. File No. 26925. Unpublished report from Union
Carbide Agricultural Products Company. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Andrawes, N.R. & Bailey, R.H. Metabolism of acetyl-1-l4C-methomyl in
(1979b) the rat. File No. 26945. Unpublished report from Union
Carbide Agricultural Products Company. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Andrawes, N.R. & Bailey, R.H. Fate of acetyl-1-14C-thiodicarb in laying
(1980) chickens under continuous feeding conditions. File No.
26414. Unpublished report from Union Carbide Agricultural
Products Company. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Andrawes, N.R. Acetonitrile metabolism in the rat. File No. 32291.
(1983) Unpublished report from Union Carbide Agricultural Products
Company, Inc. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Becci, P.J., Schwartz, C.S., & Parent, R.A. Evaluation of UC 51762 No.
(1979) 40-488 as a potential delayed neurotoxic agent following
oral administration to hens protected by atropine sulfate.
Report No. 6065. Unpublished report from Food and Drug
Research Laboratories. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Cameron, J.T. & Wolfe, G.W. Acute eye irritation study in rabbits.
(1979) Project No. 400-616. Unpublished report from Hazleton
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Carpenter, C.P., Pozzani, U.C., Weil, U.C., Palm, P.E. & Nair, J.H.
(1958) The toxicity of acetonitrile. Part II. Special report No.
21-100. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company Inc.
Cifone, M.A. & Brusick, D.J. Evaluation of thiodicarb in the rat
(1985a) primary hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. 20991 from Litton Bionetics, Inc.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Cifone, M.A. & Brusick, D.J. Mutagenicity of thiodicarb in a mouse
(1985b) lymphoma mutation assay. Unpublished report No. 20989 from
Litton Bionetics, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Conroy, W.J., DePass, L.R., Homan, E.R., Weil, C.S., Geary, D.L. &
(1979a) Frank, F.R. Dermal sensitization potential in guinea pig.
Special report No. 42-61. Unpublished report from the Mellon
Institute. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Conroy, W.J., DePass, L.R., Homan, E.R., Weil, C.S., Geary, D.L., &
(1979b) Frank, F.R. UC 51762, 16-dose rabbit dermal study. Special
report No. 42-52. Unpublished report from Mellon Insititute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Conroy, W.J., DePass, L.R., Homan, E.R., Weil, C.S., & Webb, G.A.
(1979c) Dermal sensitization potential in the guinea pig. Special
report No. 42-26. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Dangler, T.L. & Rodwell, D.E. Pilot teratology study in mice. Report
(1979) No. 369-030. Unpublished report from International Research
and Development Corporation. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Dickey, C.L., Nachreiner, D.J., DePass, L.R., Gad, S.C., Weil, C.S.,
(1979) Geary, D.L. & Frank, F.R. Acute and 9-day dust inhalation
study on rats with UC 51762 technical. Special Report No.
42-63. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Feung, C.S., College, P.R. & Chancey, E.L. Studies on the disposition
(1980) of 14C-thiodicarb in lactating cows. File No. 27350.
Unpublished report from Union Carbide Agricultural Products
Company, Inc. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Field, W.E. Acute dermal toxicity in rats. Project No. CDC-UC-012-79.
(1979a) Unpublished report from CDC Research, Inc. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Field, W.E. Skin sensitization in guinea pigs. Project No. CDC-UC-003-
(1979b) 79. Unpublished report from CDC Research, Inc. Submitted to
WHO by Union Carbide Agricultural Products Company, Inc.
Fleischman, R.W., Baker, J.R., Hagopian, M., Wade, G.G., Hayden, D.W.,
(1980) Smith, E.R., Weisburger, J.H. & Weisburger, E.K.
Carcinogenesis bioassay of acetamide, hexanamide, adipamide,
urea and phi-tolylurea in mice and rats. J. of Environ.
Pathol. and Toxicol., 3, 149-170.
Gallo, M.A. & Stevens, K.R. Evaluation of LARVIN(R) (UC 51762) in 21
(1980) day dermal toxicity study in rabbits. Project No. 02413-090.
Unpublished report from Foster D. Snell. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Homan, E.R., Weil, C.S., & Cox, E.F. Seven-day feeding study of UC
(1977) 51762 in mice. Special report No. 40-7. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Homan, E.R., Fowler, E.H., Reid, J.B., & Cox, E.F. Thirteen-week oral
(1978a) toxicity study of UC 51762 in dogs. Special report No.
41-98. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Homan, E.R., Maronpot, R.R., Reid, J.B., & Cox, E.F. Thirteen-week
(1978b) oral toxicity study of UC 51762 in rats. Special report No.
41-63. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Huhtanen, K. & Dorough, H.W. Isomerization and Beckman rearrangement
(1976) reactions in the metabolism of methomyl in rats. Pesticide
Biochem. Physiol. 6, 571-583.
Humiston, C.J. & Wright, C.J. An automated method for the
(1967) determination of cholinesterase activity. Tox. Appl.
Pharmacol. 10, 476-480.
Ivett, J.L. & Brusick, D.J. Clastogenic evaluation of 91.48% a.i.
(1985) thiodicarb in an in vivo cytogenetic assay measuring
chromosomal aberration frequencies in Chinese hamster ovary
(CHO) cells. Unpublished report No. 20990 from Litton
Bionetics, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Jagannath, D.R. & Brusick, D. Mutagenic evaluation of CHF 41-43 (UC
(1978) 51762) by the Ames Salmonella/microsome plate test.
Project No. 20838. Unpublished report from Litton Bionetics,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Janes, J.M., Rodwell, D.E., & Jessup, D.C. Teratology study in mice.
(1980) No. 369-032. Unpublished report from International Research
and Development Corporation. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Kamphake, J., Ragsdale, W.E., Weber, E.M., & Carabello, F.B. Repeated
(1980) insult patch test - human volunteers. Report No. 80-0589-
70,73. Unpublished report from Hilltop Research, Inc.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Kaplan, A.M. & Sherman, H. Toxicity Studies with Methyl N-
(1977) (methylamino)-carbamoyloxy ethamidothioate. Toxicol. Appl.
Pharmacol., 40, 1-17.
Khasawinah, A.M. & College, P.R. Fate of single oral dose of 14C-
(1978) acetyl UC 51762 in a lactating cow - metabolism into natural
products. File No. 25257. Unpublished report from Union
Carbide Agricultural Products Company, Inc. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Koehler, S. & Dorman, N. Clinical safety evaluation of LARVIN(R) UC
(1979) 51762 powder and LARVIN(R) 500 flowable. Study No. 2177.
Unpublished report from Food and Drug Research Laboratories.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Lemen, J.K., Wolfe, G.W., & Voelker, R.W. Acute dermal administration
(1979) in rabbits. Project No. 400-614. Unpublished report from
Hazleton Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) technical;
(1982a) primary eye irritation test in rabbits. Report No. PH 421-
UC-004-82. Unpublished report from Pharmakon Research
Laboratories, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - analytical
(1982b) standard; acute oral toxicity study in rats (14 Day). Report
No. PH 402-UC-010-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - analytical
(1982c) standard; acute oral toxicity study in rats (14 Day). Report
No. PH 402-UC-011-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical;
(1982d) acute dermal toxicity test in rabbits. Report No. PH 422-UC-
005-82. Unpublished report from Pharmakon Research
Laboratories, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982e) lab preparation; acute oral toxicity test in rats. Report
No. PH 402-UC-008-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982f) lab preparation; acute oral toxicity test in rats. Report
No. PH 402-UC-009-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982g) pilot plant - batch 34; acute oral toxicity test in rats.
Report No. PH 402-UC-006-82. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982h) pilot plant - batch 34; acute oral toxicity test in rats.
Report No. PH 402-UC-007-82. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. Primary dermal
(1982i) irritation study in rabbits. Report No. PH 420-UC-002-82.
Unpublished report from Pharmakon Research Laboratories,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Matthews, R.J. Acute intraperitoneal LD50 of UC 51762 in the mouse.
(1979a) Report No. PH 405-UC-001-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Matthews, R.J. Acute intraperitoneal LD50 of UC 51762 in the rat.
(1979b) Report No. PH 404-UC-001-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mirro, E.J. & DePass, R.L. Inclusion of UC 51762 in the diet of rats
(1982) for 28 days. Report No. 45-19. Unpublished report from Union
Carbide Bushy Run Research Center. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myer, J., Dean, W.P., & Jessup, D.C. Acute delayed neurotoxicity study
(1980) in hens. Report No. 369-047. Unpublished report from
International Research and Development Corporation.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Weil, C.S., & Carpenter, C.P. Acute toxicity studies of
(1975) UC 51762 in rats and rabbits. Special report No. 38-55.
Unpublished report from Mellon Institute. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Myers, R.C., Weil, C.S., & Cox, E.F. Acute oral LD50 study UC 45650
(1976a) (S-methyl O-(methylcarbamoyl)acetothiohydroximate) in rats.
Special report No. 39-40. Unpublished report from Mellon
Institute. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Myers, R.C., Weil, C.S., & Cox, E.F. Acute toxicity study of S-methyl
(1976b) acetothiohydroximate UC 52702 in rats and rabbits. Special
report No. 39-25. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Carpenter, C.P., & Cox, E.F. Acute inhalation LD50 study
(1977a) of UC 51762 in rats. Special report No. 40-61. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., Carpenter, C.P., & Cox, E.F. Acute LD50 study of UC 51762
(1977b) in mice. Special report No. 40-6. Unpublished report from
Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., Carpenter, C.P., & Cox, E.F. Acute oral LD50 study in
(1977c) guinea pigs. Special report No. 40-142. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., Homan, E.R., Carpenter, C.P. & Cox, E.F. Miscellaneous
(1978a) toxicity studies. Special report No. 41-25. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., Homan, E.R., & Cox, E.F. Acute dermal toxicity of UC
(1978b) 51762 in rabbits. Special report No. 41-93. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., Homan, E.R., & Webb, G.A. Acute oral LD50 study of
(1978c) 1-methylthioacetaldehyde-O-(hydroxymethyl carbamoyl) oxide
(UC 58614) in rats. Special report No. 41-133. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., Homan, E.R., Weil, C.S., & Frank, F.R. UC
(1979a) 51762 technical; range-finding toxicity studies. Special
report No. 42-19. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Homan, E.R., Weil, C.S., & Frank, F.R. Miscellaneous
(1979b) toxicity studies. Special report No. 42-27. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. Methomyl technical; acute
(1981) toxicity and irritancy study. Report No. 44-75a. Unpublished
report from Union Carbide Bushy Run Research Center.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. Acute toxicity and irritancy
(1982a) studies. Report No. 45-24. Unpublished report from Union
Carbide Bushy Run Research Center. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. LARVIN(R) - analytical
(1982b) standard; acute oral toxicity study in rats. Report No.
45-152. Unpublished report from Union Carbide Bushy Run
Research Center. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. LARVIN(R) - technical lab
(1982c) preparation; acute oral toxicity study in rats. Report No.
45-151. Unpublished report from Union Carbide Bushy Run
Research Center. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. LARVIN(R) - technical pilot
(1982d) plant - batch 34; acute oral toxicity study in rats. Report
No. 45-151. Unpublished report from Union Carbide Bushy Run
Research Center. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., Weil, C.S., & Frank, F.R. LARVIN(R) - technical; acute
(1982e) oral toxicity study in rats. Report No. 45-40. Unpublished
report from Union Carbide Bushy Run Research Center.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Slesinski, R.S. & Frank, F.R. Methomyl oxime: acute
(1984) toxicity and irritancy study. Project report 47-185.
Unpublished report from Union Carbide Bushy Run Research
Center. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; micronucleus test.
(1979a) Report No. PH 309-UC-001-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; mitotic crossing
(1979b) over in Saccharomyces cerevisiae. Report No. PH302-UC-001-
79. Unpublished report from Pharmakon Research Laboratories,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; mitotic gene
(1979c) conversion in Saccharomyces cerevisiae. Report No. PH304-
UC-001-79. Unpublished report from Pharmakon Research
Laboratories, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; primary DNA
(1979d) damage. Report No. PH 305-AM-002-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; reverse mutation
(1978e) in Saccharomyces cerevisiae. Report No. PH 303-UC-001-79.
Unpublished report from Pharmakon Research Laboratories,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Pozzani, U.C., Nair, J.R., & Carpenter, C.P. The toxicity of
(1955) acetonitrile. Special report No. 18-34. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Rogers, L.D. Intraportal injection of acetonitrile. Report No. 22-2.
(1959) Unpublished report from Mellon Institute. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Schardein, J. 21-Day dermal toxicity study in rabbits. Report No.
(1982) 369-053. Unpublished report from International Research and
Development Corporation. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Schwartz, C.S. & Stevens, K.R. Acute oral LD50 in hens. Report No.
(1978) 6064. Unpublished report from Food and Drug Research
Laboratories. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Smyth, H.F. Range-finding toxicity test of acetonitrile. Special
(1947) report No. 10-23. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Tasker, E.J., Rodwell, D.E., & Jessup, D.C. Teratology study in rats.
(1979) Report No. 369-029. Unpublished report from International
Research and Development Corporation. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Weatherholtz, W.M. LARVIN(R) - technical; fourteen-day eye irritation
(1982) study in monkeys. Project No. 400-648. Unpublished report
from Hazleton Research Laboratories. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Weatherholtz, W.M., Rendon, F., & Gluck, S.E. Dose rangefinding study
(1983) in monkeys. Project No. 400-694. Unpublished report from
Hazleton Research Laboratories. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Weil, C.S. & Carpenter, C.P. Miscellaneous toxicity studies. Special
(1978) report No. 34-88. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Weisburger, J.H., Yamamoto, R.S., Glass, R.M., & Frankel, H.H.
(1969) Prevention by arginine glutamate of the carcinogenicity of
acetamide in rats. Toxicol. Appl. Pharmacol., 14,
163-175.
Wentz, K.L. & Wolfe, G.W. Primary skin irritation study in rabbits.
(1979) Project No. 400- 617. Unpublished report from Hazleton
Research Laboratories. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Wolfe, G.W. Subchronic toxicity study in dogs. Project No. 400-626.
(1981) Unpublished report from Hazleton Research Laboratories.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Woodside, M.D., Weil, C.S., & Cox, E.F. Seven-day feeding study of UC
(1975) 51762 in rats. Special report No. 38-136. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Reid, J.B., & Cox, E.F. Seven-day
(1978) feeding study of UC 51762 in rats. Special report No.
41-100. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1979a) UC 51762; dimethyl N,N'-(thiobis((methylimino)carbonoyloxy))
bis-(ethanimidothioate) inclusion in the diet of rats for
thirteen weeks. Special report No. 42-125. Unpublished
report from Mellon Institiute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1979b) UC 51762; rat teratology studies. Special report No. 42-48.
Unpublished report from Mellon Institute. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1979c) UC 51762 technical; inclusion in the diet of rats for three
generations and dominant lethal mutagenesis studies. Special
report No. 42-165. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1980a) UC 51762; chronic oncogenicity feeding study in mice.
Special report No. 43-10. Unpublished report from Mellon
Institute. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1980b) UC 51762; chronic toxicity and oncogenicity feeding study in
Fischer 344 rats. Special report No. 43-18. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Yoshida, A., Maita, K., Saito, T., & Miyaoka, T. LARVIN(R): 4-weeks
(1983a) range-finding study in mice. Unpublished report from The
Institute of Environmental Toxicology. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Yoshida, A., Takahashi, K., Kosaka, T., & Miyaoka, T. Thiodicarb: 3
(1983b) month oral sub-chronic toxicity study in mice (study I).
Unpublished report from The Institute of Environmental
Toxicology. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Ziemke, K.A. & Rodwell, D.E. Pilot teratology study in rats. Report
(1979) No. 369-028. Unpublished report from International Research
and Development Corporation. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
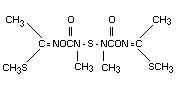 OTHER INFORMATION ON IDENTITY AND PROPERTIES
MOLECULAR WEIGHT: 354.5
PHYSICAL STATE
AND COLOUR: crystalline powder, white to light tan
ODOUR: slightly sulfurous
DENSITY (g/ml): 1.4424 at 20°C
VAPOUR PRESSURE: 4.3 × 10 -5 at 20°C
(mm Hg) 1.1 × 10 -4 at 35°C
2.1 × 10 -4 at 40°C
3.2 × 10 -4 at 45°C
4.2 × 10 -4 at 50°C
5.2 × 10 -4 at 55°C
MELTING POINT: 173-174°C
FLAMMABILITY: generates a flammable vapor space (methanol)
REACTIVITY: stable in light and ambient conditions. At
elevated temperatures (e.g. above 100°C), the
product decomposes into the following principal
degradation products; carbon dioxide, acetonitrile
and dimethyl disulfide. The decomposition process
is catalyzed by Lewis-acid type heavy metal salts.
Can be hydrolyzed under acid- or base-catalyzed
conditions. The principal degradation product from
the hydrolysis of thiodicarb is methomyl.
OXIDIZING OR
REDUCING ACTION: Neither the product nor any of the accompanying
impurities have the tendency to act as an
oxidizing or reducing agent.
pH: 1 g/100 ml H2O produces a pH of 6.65 at 21.5°C
SOLUBILITY
(wt. %): Xylene 0.1 at 50°C
0.4 at 68°C
1.0 at 92°C
2.5 at 106°C
5.0 at 119°C
Pyridine 1.2 at 25°C
6.4 at 45°C
13.4 at 60°C
Dichloromethane 10.2 at 0°C
11.4 at 5°C
16.1 at 30°C
Acetone 0.8 at 25°C
Acetonitrile 2.0 at 25°C
Ethyl acetate 0.2 at 25°C
Ethyl ether 0.1 at 25°C
Methanol 0.3 at 25°C
Tetrachloroethylene 0.1 at 25°C
Water (distilled) 35 ppm at 25°C
OCTANOL/WATER
PARTITION
COEFFICIENT: 1.65 (Log P by reverse phase TLC technique)
STORAGE
STABILITY: Minimum storage life is typically in excess of 2
years.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, excretion, and biotransformation
Thiodicarb, which essentially consists of two methomyl moieties
joined through their amino nitrogen by sulfur, is rapidly degraded to
S-methyl-N-[(methylcarbamoyl)oxy]thioacetamide (methomyl) in the rat
stomach. Over 30% of an oral dose of 16 mg thiodicarb per kg. b.w. was
converted to methomyl in the gut within 15 minutes. Only 40% of the
oral dose was found in the rat gut at 15 minutes, indicating that the
compound had been rapidly absorbed. Approximately 26% of the
radioactivity in the administered dose was found in other body
fractions, with the remainder of the dose (34%) apparently lost as
volatiles (Andrawes et al., 1977).
In rats given radiolabelled thiodicarb as a single oral dose of
40 mg/kg b.w., 80% of the administered dose was eliminated within 48
hours. After 4 days, 48% of the dose had been eliminated in the
respiratory gases, 32% in the urine, and 4.5% in the faeces; 11% of
the dose remained in the carcass, due to incorporation of
radiolabelled carbon dioxide and acetic acid metabolites into natural
products (Andrawes et al., 1977; Andrawes & Bailey 1979a; Feung
et al., 1980).
Thiodicarb is degraded in the stomach not only to methomyl but
also to some other unstable intermediates, including methomyl
methylol, methomyl oxime, methomyl sulfoxide, and methomyl sulfoxide
oxime, which are subsequently converted to acetonitrile and carbon
dioxide and primarily eliminated by respiration and in the urine.
Acetonitrile is the only metabolite retained to some extent in body
tissues and fluids; a very small fraction of the acetonitrile is
further degraded to carbon dioxide, acetic acid, and to acetamide,
which is suspected of being a mouse and rat carcinogen (Weisburger
et al., 1969; Fleischman et al., 1980). The ultimate metabolic
fate of methomyl in animals depends on its isomeric configuration. In
the rat the stable and predominant form is the syn isomer, which is
metabolized primarily to CO2, while partial conversion from the syn
isomer to the anti isomer leads primarily to acetonitrile, most of
which is respired unchanged (Huhtanen & Dorough 1976).
A proposed metabolic pathway for thiodicarb in animals is shown
in Figure 1.
In the cow, metabolites of a single oral dose of thiodicarb are
excreted in different proportions when compared to the rat, although
there are no substantial differences in the metabolic pathways
involved. During the initial 3-day period following administration of
radiolabelled thiodicarb (16 mg/kg) or methomyl (4 mg/kg) to rats,
31-32% of the administered dose was found in the urine. Only 5% of a
dose of thiodicarb (7.02 mg/kg) administered to a cow was excreted in
the urine during the same period. In rats, approximately 40-50% of the
dose was recovered as CO2 and/or acetonitrile from respired gases
during a 72 hour period, while similarly the cow eliminated 66% of the
applied dose in respired gases. The CO2:acetonitrile ratio in
respired air was 4- to 16-fold higher in the cow than in the rat. The
higher CO2:acetonitrile ratios in cows may represent a species-
specific tendency toward reduced formation of the anti methomyl
stereoisomer (Andrawes et al., 1977; Andrawes & Bailey, 1979b;
Khasawinah & College, 1978).
OTHER INFORMATION ON IDENTITY AND PROPERTIES
MOLECULAR WEIGHT: 354.5
PHYSICAL STATE
AND COLOUR: crystalline powder, white to light tan
ODOUR: slightly sulfurous
DENSITY (g/ml): 1.4424 at 20°C
VAPOUR PRESSURE: 4.3 × 10 -5 at 20°C
(mm Hg) 1.1 × 10 -4 at 35°C
2.1 × 10 -4 at 40°C
3.2 × 10 -4 at 45°C
4.2 × 10 -4 at 50°C
5.2 × 10 -4 at 55°C
MELTING POINT: 173-174°C
FLAMMABILITY: generates a flammable vapor space (methanol)
REACTIVITY: stable in light and ambient conditions. At
elevated temperatures (e.g. above 100°C), the
product decomposes into the following principal
degradation products; carbon dioxide, acetonitrile
and dimethyl disulfide. The decomposition process
is catalyzed by Lewis-acid type heavy metal salts.
Can be hydrolyzed under acid- or base-catalyzed
conditions. The principal degradation product from
the hydrolysis of thiodicarb is methomyl.
OXIDIZING OR
REDUCING ACTION: Neither the product nor any of the accompanying
impurities have the tendency to act as an
oxidizing or reducing agent.
pH: 1 g/100 ml H2O produces a pH of 6.65 at 21.5°C
SOLUBILITY
(wt. %): Xylene 0.1 at 50°C
0.4 at 68°C
1.0 at 92°C
2.5 at 106°C
5.0 at 119°C
Pyridine 1.2 at 25°C
6.4 at 45°C
13.4 at 60°C
Dichloromethane 10.2 at 0°C
11.4 at 5°C
16.1 at 30°C
Acetone 0.8 at 25°C
Acetonitrile 2.0 at 25°C
Ethyl acetate 0.2 at 25°C
Ethyl ether 0.1 at 25°C
Methanol 0.3 at 25°C
Tetrachloroethylene 0.1 at 25°C
Water (distilled) 35 ppm at 25°C
OCTANOL/WATER
PARTITION
COEFFICIENT: 1.65 (Log P by reverse phase TLC technique)
STORAGE
STABILITY: Minimum storage life is typically in excess of 2
years.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, excretion, and biotransformation
Thiodicarb, which essentially consists of two methomyl moieties
joined through their amino nitrogen by sulfur, is rapidly degraded to
S-methyl-N-[(methylcarbamoyl)oxy]thioacetamide (methomyl) in the rat
stomach. Over 30% of an oral dose of 16 mg thiodicarb per kg. b.w. was
converted to methomyl in the gut within 15 minutes. Only 40% of the
oral dose was found in the rat gut at 15 minutes, indicating that the
compound had been rapidly absorbed. Approximately 26% of the
radioactivity in the administered dose was found in other body
fractions, with the remainder of the dose (34%) apparently lost as
volatiles (Andrawes et al., 1977).
In rats given radiolabelled thiodicarb as a single oral dose of
40 mg/kg b.w., 80% of the administered dose was eliminated within 48
hours. After 4 days, 48% of the dose had been eliminated in the
respiratory gases, 32% in the urine, and 4.5% in the faeces; 11% of
the dose remained in the carcass, due to incorporation of
radiolabelled carbon dioxide and acetic acid metabolites into natural
products (Andrawes et al., 1977; Andrawes & Bailey 1979a; Feung
et al., 1980).
Thiodicarb is degraded in the stomach not only to methomyl but
also to some other unstable intermediates, including methomyl
methylol, methomyl oxime, methomyl sulfoxide, and methomyl sulfoxide
oxime, which are subsequently converted to acetonitrile and carbon
dioxide and primarily eliminated by respiration and in the urine.
Acetonitrile is the only metabolite retained to some extent in body
tissues and fluids; a very small fraction of the acetonitrile is
further degraded to carbon dioxide, acetic acid, and to acetamide,
which is suspected of being a mouse and rat carcinogen (Weisburger
et al., 1969; Fleischman et al., 1980). The ultimate metabolic
fate of methomyl in animals depends on its isomeric configuration. In
the rat the stable and predominant form is the syn isomer, which is
metabolized primarily to CO2, while partial conversion from the syn
isomer to the anti isomer leads primarily to acetonitrile, most of
which is respired unchanged (Huhtanen & Dorough 1976).
A proposed metabolic pathway for thiodicarb in animals is shown
in Figure 1.
In the cow, metabolites of a single oral dose of thiodicarb are
excreted in different proportions when compared to the rat, although
there are no substantial differences in the metabolic pathways
involved. During the initial 3-day period following administration of
radiolabelled thiodicarb (16 mg/kg) or methomyl (4 mg/kg) to rats,
31-32% of the administered dose was found in the urine. Only 5% of a
dose of thiodicarb (7.02 mg/kg) administered to a cow was excreted in
the urine during the same period. In rats, approximately 40-50% of the
dose was recovered as CO2 and/or acetonitrile from respired gases
during a 72 hour period, while similarly the cow eliminated 66% of the
applied dose in respired gases. The CO2:acetonitrile ratio in
respired air was 4- to 16-fold higher in the cow than in the rat. The
higher CO2:acetonitrile ratios in cows may represent a species-
specific tendency toward reduced formation of the anti methomyl
stereoisomer (Andrawes et al., 1977; Andrawes & Bailey, 1979b;
Khasawinah & College, 1978).
 Andrawes (1983) reported that oral administration of radiolabeled
acetonitrile to rats resulted in elimination of 65% of the applied
dose in expired air, with less than 1% expired as 14CO2. In a
similar study however, Huhtanen & Dorough (1976) found that in rats
9.1% of a dose of acetonitrile was eliminated as CO2 in respiratory
gases.
Two lactating Holstein cows/group were given approximately 0.004,
0.4, 1.4, or 4 mg 14C-thiodicarb per kg b.w. daily for 21 days. Milk
and urine were collected twice daily, and blood and faeces were
collected at 3- to 4-day intervals. One cow at each dosage level was
sacrificed 12 hours after the last treatment, and tissues (liver,
kidney, lung, spleen, heart, brain, ovary, udder, tongue, foreleg
muscle, hindleg muscle, neck muscle and omental fat) were collected
for analysis. The remaining 4 cows (1 at each dosage level) were kept
for an additional 7 days after termination of treatment. During this
post-treatment phase, milk and urine were collected twice daily as
before, and blood and faeces were collected daily. The same tissues
were collected after 7 days post-treatment. The radioactivity found in
all samples (i.e., tissue, milk, blood, urine and faeces) was dose
dependent. The concentration of acetonitrile in milk approached peak
levels within 7 days in all dose groups. The acetonitrile levels
appeared to remain nearly constant through the rest of the 21-day
treatment period, then declined sharply during the 7-day post-
treatment phase. Following 21 days of treatment, tissue levels of 14C
residues were highest in liver and lowest in adipose tissues. Seven
days after termination of dosing, radioactivity levels were lower in
all tissues except liver. The authors concluded that the predominant
end-products of thiodicarb catabolism were acetonitrile, acetamide,
and carbon dioxide. Acetamide, which is believed to be produced by
hydrolysis of acetonitrile, was found in trace amounts (< 0.01 ppm)
in milk at the highest feeding level. Approximately 24% of the
aqueous-extractable urinary-radioactivity was identified as acetamide
(Feung et al., 1980).
In a cow given a single dose (7.02 mg/kg b.w.)14C-acetyl
thiodicarb, 66, 11.4, 5.0, and 4.6% of the administered radioactivity
was eliminated, during 72 hours post-treatment, in respired air,
faeces, urine and milk, respectively, while 10.1% was retained in the
tissues (Khasawinah & College, 1978).
In a study using mature White Leghorn laying chickens, no
carbamate residues were found in tissues or eggs after 21 days of oral
administration of thiodicarb at dosages of 15.4, 28.6 and 102 ppm in
the diet. Acetonitrile, detected in the eggs and tissues (except fat)
at the 102-ppm level, corresponded to 0.2 ppm (egg yolk and whites),
0.5 ppm (liver), and 0.2-0.3 ppm (muscle). The level in eggs was
directly proportional to the dosage level administered. Acetamide was
not detected in egg yolks, but was found in egg whites (0.07 ppm), in
liver (0.06 ppm) and in muscle (0.01 ppm), when administered at 102
ppm thiodicarb (Andrawes & Bailey, 1980).
Based on the available data that 5.72% of the administered
syn-methomyl is converted to acetonitrile, a maximum of 5.72% of the
administered 14C-thiodicarb could therefore be available for
conversion to acetamide, since thiodicarb is metabolized predominantly
to syn-methomyl. On a weight-percent basis, the maximum acetamide
that could occur from ingestion of thiodicarb residues is 1.9% of the
administered dose (53:1 thiodicarb:acetamide on a weight-to-weight
basis) (Andrawes & Bailey, 1979a & 1979b; Huhtanen & Dorough, 1976).
Effects on enzymes and other biochemical parameters
The most significant biochemical effect of thiodicarb and its
carbamate metabolites is the ability to reversibly inhibit
acetylcholinesterase (ChE), a capability in common with other methyl
carbamate esters. The methylcarbamate-acetylcholinesterase complex
dissociates rapidly, and the acetylcholinesterase enzyme is released
unaltered. The transient nature of acetylcholinesterase inhibition by
thiodicarb makes it difficult to assay accurately, since inhibition is
reversed by sample dilution and by adding substrate (butyrylcholine or
acetylcholine) during assay procedures. Therefore, the assay procedure
must be very rapid and use less than optimal amounts of substrate. The
automated colorimetric method of Humiston & Wright (1967) is suitable
for such determination.
Groups of Fischer 344 rats (10 males and 10 females per group)
were administered thiodicarb in the diet at dosage levels of 0, 1, 3,
10, or 30 mg/kg b.w./day for 28 days. Rats were observed for mortality
and clinical symptoms of toxicity. Food consumption was determined
weekly and plasma- and erythrocyte-ChE measurements were analyzed on
days 0, 15, and 29. Brain ChE was determined on day 29.
Body weight and food consumption were significantly reduced in
females at > 10 mg/kg, b.w. Males were not similarly affected.
There was no mortality. Plasma and erythrocyte ChE were significantly
depressed in high-dose males and females. Plasma ChE recovered to
normal values by day 29, while red blood cell (RBC) ChE remained
significantly depressed in both sexes at > 30 mg/kg b.w./day. Brain
ChE was not depressed at any level (Mirro & DePass, 1982).
Toxicological studies
Special study on reproduction
Rat
Groups of Fischer 344 rats (20 female and 10 male rats/group)
were administered thiodicarb (purity 99+%) in the diet, at dosage
levels of 0, 0, 0.5, 1.0, 3, or 10 mg/kg b.w., in a three-generation
reproduction study. Each generation produced only one litter per
generation. The routine indices of fertility, gestation,
survivability, viability, and lactation were determined for all
generations, as well as selected gross and histopathological
evaluations for F2a and F3a generations. Rats were mated on a 1-male-
to-2-females ratio when approximately 100 days of age. Dams and pups
were observed daily for appearance and behaviour. Litter size was
reduced to 10 on day 4 postpartum. Brother-sister matings were
avoided.
There were no compound-related effects on any of the reproductive
parameters measured, except for an initial transitory body-weight
depression in F0 males. Gross and microscopic analyses were
reportedly unremarkable. Thiodicarb was without adverse effects on
reproduction at doses up to and including 10 mg/kg b.w. (Woodside
et al., 1979c).
Special studies on teratogenicity
Mouse
In a pilot study using pregnant Charles-River CD-1 mice,
thiodicarb (technical grade) administered orally from days 6 through
16 of gestation, at dosage levels of 0, 20, 60, 100, 150, 200, 300, or
400 mg/kg b.w., produced excessive maternal toxicity (body-weight
depression and mortality) at doses greater than 200 mg/kg b.w.
(Dangler & Rodwell, 1979).
Groups of Charles-River CD-1 mice (25 mated females/group) were
administered thiodicarb (technical grade) via oral gavage at dosage
levels of 0, 50, 100, or 200 mg/kg b.w. from days 6 through 16 of
gestation. Mating was accomplished on a 1-female-to-1-male schedule at
94 days of age. Day of copulation was also considered day 0 of
gestation. Animals were observed routinely for appearance, behaviour
and body-weight changes. Pups were delivered by Caesarean-section on
day 17 of gestation. All foetuses were weighed, sexed, examined for
external malformations, and subsequently subjected to visceral or
skeletal examination.
Six females in the high-dose group died during the study. Body
weights were unaffected by treatment. There was no evidence of
maternal or foetal toxicity at doses > 100 mg/kg b.w. and no
teratogenic effects at doses > 200 mg/kg b.w. (Janes et al.,
1980).
Rat
In a pilot teratology study using pregnant Charles-River CD COBS
rats, thiodicarb (technical grade), administered orally at dosage
levels of 0, 20, 40, 80, 120, or 160 mg/kg b.w. on days 6-19 of
gestation, produced mortality at doses > 40 mg/kg b.w. There were
no reported adverse effects on reproduction or foetal development
(Ziemke & Rodwell, 1979).
Groups of Charles-River CD COBS rats (25 mated females/group)
were administered thiodicarb (technical grade) via oral gavage at
dosage levels of 0, 10, 20, or 30 mg/kg b.w. on gestation days 6-19.
Females were mated 1-to-1 with males at 13 weeks of age. Day of
copulation was also considered day 0 of gestation. Animals were
observed routinely for appearance, behaviour, and body-weight changes.
Pups were delivered by Caesarean-section on gestation-day 20. All
foetuses were weighed, sexed, measured, and examined for external
malformations. One-third of the foetuses were fixed for visceral
examination and the remaining two-thirds of the foetuses were
subjected to skeletal examination. Early and late resorptions, number
of implantations and corpora lutea, and the number and location of
viable and non-viable foetuses, were recorded.
Clinical observations, apparent at 20 and 30 mg/kg b.w. within 4
hours after treatment, included inactivity, tremors, and clear oral
discharge. There was no mortality. Maternal body weights determined
on days 0, 6, 9, 12, 16, and 20 were significantly decreased at
> 20 mg/kg b.w, and mean foetal body-weights were significantly
reduced at > 10 mg/kg b.w. No teratogenic effects were produced,
although delayed maturation of foetuses (reduced ossification),
associated with maternal toxicity, was observed. There was no evidence
of visceral or skeletal anomalies attributable to thiodicarb at doses
up to and including 30 mg/kg b.w. (Tasker et al., 1979).
Groups of Fischer 344 rats (10 mated females/group) were
administered thiodicarb (purity 99+%) in the diet via 2 separate
dosing regimens: from day 0 to day 20 of gestation or from day 6 to
day 15 of gestation. Dose levels in each dosing schedule included 0,
0.5, 1.0, 3, or 100 mg/kg b.w. A positive control group received
625 mg/kg b.w. of aspirin via gavage on gestation day 10. Females were
mated with males on a 1-to-1 schedule in order to achieve 10 pregnant
females per dose. All pups were delivered by Caesarean-section on
gestation day 20 and examined for visceral and skeletal abnormalities.
Maternal body-weight gains determined on days 0 and 12 of
gestation were reduced in all treatment groups. However, these changes
were extremely variable at doses of 0.5-3.0 mg/kg b.w. (only two
measurements were included) and they were not considered to be
biologically significant except at the highest dose administered where
the body weights were uniformly depressed. Foetal body weight and
length were also significantly reduced in the high-dose group that was
administered thiodicarb throughout gestation (days 0-20). The aspirin-
exposed foetuses were similarly affected. Thiodicarb did not adversely
affect the number of implantations, resorptions, live foetuses per
litter, or pre-implantation losses. Resorption frequency was increased
in high- dose females given thiodicarb from days 6 through 15 of
gestation. The increase was based on the percentage of affected
litters as well as the resorptions per litter. Aspirin demonstrated
the same response. The incidences of visceral anaomalies were not
increased in either the aspririn or thiodicarb groups when compared to
controls. Skeletal variations were similar among all groups except for
an increased incidence of bilobed thoracic vertebral centra and poorly
ossified sternebrae (on a percentage of live-foetuses-per-litter
basis) in the 100 mg/kg b.w. group administered thiodicarb throughout
gestation. This effect was suggestive of delayed maturity in foetuses
derived from dams which demonstrated significant maternal toxicity
(i.e. decreased weight gain and decreased foetal size). This effect is
also known to occur spontaneously (at similar frequencies) in other
rat strains. The aspirin group exhibited several skeletal variations
(e.g. split/missing vertebral centra, extra vertebrae, extra ribs and
fused/wavy ribs), demonstrating the sensitivity of the study.
Thiodicarb was not teratogenic at any dose administered but it was
maternally toxic at 100 mg/kg b.w. (Woodside et al., 1979b).
Special studies on carcinogenicity (See also "Long-term studies")
Mouse
Groups of Charles-River COBS CD-1 mice (80/sex/group) were
administered thiodicarb (analytical grade, 99+% purity) in the diet
for 24 months at dosage levels of 0, 0, 1.0, 3, or 10 mg/kg b.w./day.
Animals were 42 days old at the start of the study. The mice were
examined daily for mortality and adverse physical/behavioural
condition. Routine evaluation of food consumption and body weights
were performed. At the conclusion of the study, or at death, organ
weights and gross and microscopic examination of tissues and organs of
all animals were performed. All were anesthetized with methyoxyflurane
and killed by cervical dislocation.
Mortality in high-dose mice was increased over controls in males
during the last 2 months of the study and in females during months 17
and 18.
Body weights and food consumption were not affected by treatment.
There were no significant differences among treatment and control
groups for the incidence of neoplasms or the identification of non-
neoplastic lesions occurring in dead animals, or interim- and final-
sacrifice animals. However, the overall incidence among control and
treatment groups of hepatocellular neoplasms was much greater than
reported in the literature for this strain of mouse (Table 1).
Table 1. Hepatocellular neoplasms in mice treated with thiodicarb
and in 2 control groups
Dose (mg/kg b.w.)
0 0 1 3 10
Males
Adenoma 18/80 19/76 22/78 24/78 24/80
Carcinoma 37/80 25/76 29/78 32/78 26/80
Females
Adenoma 2/80 5/77 2/78 2/76 1/75
Carcinoma 1/80 3/77 3/78 1/76 3/75
Furthermore, there was no decrease in time-to-tumour for the
observation of hepatocellular neoplasms (Table 2).
Table 2. Time-to-tumour (hepatocellular neoplasms) for male mice
treated with thiodicarb and for 2 control groups
Dose (mg/kg b.w.)
0 0 1 3 10
Animals which
died or were 6/53 10/49 12/51 22/48 13/54
moribund
Animals at 18-
month interim 16/20 15/20 16/20 15/20 13/20
sacrifice
Animals surviving
24 months 4/7 6/8 6/7 4/10 4/6
(terminal kill)
The other most frequently occurring neoplasm was alveologenic
tumour of the lungs. As with hepatocellular neoplasms, there was no
compound-related response within the incidence of this tumour type
(Table 3).
Table 3. Alveologenic tumours in mice treated with thiodicarb and
in 2 control groups
Dose (mg/kg b.w.)
0 0 1 3 10
Males
Alveologenic
tumours 23/80 23/76 31/78 15/78 22/80
Carcinoma 1/80 0/76 0/78 1/78 0/80
Females
Alveologenic
tumours 19/80 13/78 19/78 17/77 13/75
Carcinoma 2/80 0/78 0/78 0/77 0/75
The principle non-neoplastic lesions observed in all control and
treated groups included: lymphocytic infiltration or hyperplasia of
numerous tissues, generalized amyloidosis, hyaline degeneration of
sternal cartilage, haemosiderosis of various organs, subacute
sialoadenitis, glomerulosclerosis, and renal cystic tubular dilation.
These effects were not compound-related. Thiodicarb was not
carcinogenic to CD-1 mice at dietary doses up to and including
10 mg/kg b.w. (Woodside et al., 1980a).
Special studies on mutagenicity
Thiodicarb was evaluated in a series of mutagenicity assays
including the Ames, dominant-lethal, micronucleus, reverse-mutation,
mitotic-crossing-over, and DNA-damage test methods. It was negative
for mutagenic potential in all tests except for a positive response in
the mitotic gene conversion assay using Sacharomyces cerevisiae.
(See Table 4 for details.)
Table 4. Results of mutagenicity assays on thiodicarb
Test Test Concentration Purity Results Reference
System Object of thiodicarb
Ames test S. typhimurium 1, 10, 100, Unknown Negative Jagannath &
* TA98 500, and Brusick, 1978
TA100 1000 µg/plate
TA1535
TA1537
TA1538
Micronucleus Mouse, bone 5 and 10 Technical Negative Naismith &
test marrow mg/kg grade (1) Matthews,
1979a
Dominant Rat 0, 0.5, 1, Analytical Negative Woodside
lethal 3 & grade (2) et al., 1979c
10 mg/kg (99+%)
Reverse S. cerevisiae 2.5, 6.2, Technical Negative Naismith &
mutation 25, 62, & grade (3) Matthews,
250 µg/ml 1979b
Mitotic S. cerevisiae 2.5, 6.2, Technical Negative Naismith &
crossing 25, 62, & grade (3) Matthews,
over 250 µg/ml 1979e
Mitotic S. cerevisiae 2.5, 6.2, Technical (4) Naismith &
gene 25, 62, & grade Matthews,
conversion 250 µg/ml 1979c
Primary E. coli 0.001, 0.01, Technical Negative Naismith &
DNA W3110 0.1, 1, and grade (5) Matthews,
damage* p3478 10 mg/ml 1979d
Chromosomal Chinese hamster 10, 15, 20, Technical Negative Ivett &
aberrations ovary (CHO) 30 µg/ml 91.48% Brusick,
cells* w/o activ. 1985
10, 20, 30,
40 µg/ml
w/activation
Unscheduled Rat primary 0.5, 1.0, 2.5, Technical Negative Cifone &
DNA synthesis hepatocytes 5.0, 10.0, 91.48% Brusick,
25.0, 50.0, 1985a
100.0, 250.0
µg/ml
Table 4. (Con't)
Test Test Concentration Purity Results Reference
System Object of thiodicarb
Mouse lymphoma L5178Y (TK+/-) 5, 8, 10, Technical Positive Cifone &
forward mutation & 12 µg/ml 91.48% (6) Brusick,
assay* 1985b
* With and without metabolic activation
(1) The positive control (TEM) gave the expected response at 0.5 ml/kg.
(2) The positive control (TEM) gave the expected response at 0.25 mg/kg.
(3) The positive control (NQO) gave the expected response at 10-6 M.
(4) Increase in the heteroallelic trp 5-12/trp 5-27 diploid strain D7 at
25 µg/ml.
(5) The positive controls (ethylmethane sulfonate and diethylnitrosamine)
gave the expected response.
(6) The test material induced repeatable increases in the mutant frequency,
with and without metabolic activation.
Special studies on neurotoxicity
Although the Meeting considers the testing of methyl carbamates
for potential neurotoxicity to be inappropriate (Annex 1, FAO/WHO,
1985b, para. 2.8), the manufacturer conducted 2 separate studies, both
of which were negative.
Chicken
In a preliminary acute oral toxicity study using 10- to 14-month-
old White Leghorn hens, the LD50 for thiodicarb was calculated to be
582 mg/kg b.w. When atropine sulfate (15 mg/kg b.w.) was administered
15 minutes prior to dosing only 2/5 hens died after 5 days at a dose
of 830 mg/kg b.w. (Schwartz & Stevens, 1978).
Hens protected with 15 mg/kg b.w. atropine sulfate were
administered single doses of 660 mg/kg b.w. thiodicarb via oral
intubation. Vehicle (corn oil) and positive (TOCP) control groups were
also employed. A dose of 750 mg/kg b.w. TOCP in corn oil was used.
There were 10 hens in each control group and 40 in the thiodicarb
group. Hens were observed daily for pharmacologic and toxicologic
effects, including neurologic evaluation of leg weakness, gait, and
walking ability. On day 21 all positive controls and 10 test-group
birds were necropsied, perfused with 10% neutral buffered formalin,
and examined histologically. On day 22 eight new positive controls
were added to the study and the existing 30 treated birds were
re-dosed a second time with 660 mg/kg b.w. thiodicarb.
The vehicle controls provided no evidence of toxicity, while the
positive control group displayed clinical and microscopic symptoms of
delayed neuro-toxicity (increased number of swollen axons per cervical
spinal cord cross-section). Although some signs of neurological
impairment were initially displayed in the thiodicarb group, these
were not evident after 4 days in study and were not confirmed upon
histologic examination (Becci et al., 1979).
Due to the marginal response in thiodicarb-treated birds, the
entire study was repeated using 30 White Leghorn hens (8-12 months
old) dosed orally with 660 mg/kg b.w. thiodicarb (analytical grade).
There were 10 hens in each of the negative (corn oil) and positive
(TOTP) control groups. Thirty minutes prior to dosing, each group
received 25 mg/kg b.w. atropine sulfate via gavage. A dose of
1200 mg/kg b.w. TOTP was employed in the positive control group.
A total of 13/30 thiodicarb hens died on test, with no mortality
in either control group. Thiodicarb and the negative control group
provided no evidence of delayed neurotoxicity. In the TOTP group, 9/10
birds demonstrated lesions in the sciatic nerve and spinal cord,
consisting of axonal degeneration, necrosis, and demyelination (Myer
et al., 1980).
Special studies on skin and eye irritation
Rabbit
Thiodicarb is not irritating to the skin. When applied to the
shaved backs of rabbits, thiodicarb produced only slight erythema in a
few cases (Mallory et al., 1982i; Wentz & Wolfe, 1979).
Thiodicarb was applied to the shaved, intact skin of rabbits, 5
times a week for 3 consecutive weeks, at 4 dosage levels up to
4 g/kg/day. Mortality, behaviour, food consumption, body weight, and
gross and microscopic histopathology were unaffected by treatment.
Localized erythema and edema occurred in all treated groups.
Thiodicarb treatment was reported to produce a dose-related macrocytic
anaemia (Conroy et al., 1979b; Gallo & Stevens, 1980).
In a second dermal toxicity study in rabbits, using
2000 mg/kg/day as the highest dosage level, all criteria were
unaffected by treatment. Groups of 10 male and 10 female New Zealand
White rabbits were treated dermally with thiodicarb 5 days per week
for 3 consecutive weeks at dosage levels of 0, 250, 500, 1000, or
2000 mg/kg (2 control groups were utilized in this study). Thiodicarb
was administered to both intact and abraded dermal surfaces. There
were no effects in the study attributable to thiodicarb. The
occurrence of macrocytic anaemia, observed in the first study, was not
confirmed (Schardein, 1982).
Thiodicarb applied to the conjunctival sacs of rabbits did not
damage the cornea, but resulted in temporary eye irritation (Cameron &
Wolfe, 1979; Mallory et al., 1982a). In monkeys, thiodicarb caused
no eye irritation or damage (Weatherholtz, 1982). Methomyl was mildly
irritating to the skin of guinea-pigs and was a mild irritant to the
eye (Kaplan & Sherman, 1977). Methomyl oxime was not irritating to the
skin and was moderately irritating to the eye (Myers et al., 1984).
Special studies on dermal sensitization
The potential for thiodicarb to produce skin sensitization was
tested in guinea-pigs and human subjects. Guinea-pigs, given multiple
intradermal injections of thiodicarb in a modified Landsteiner
procedure, showed minimal sensitization reactions to a challenge
injection two weeks later (Conroy et al., 1979a & c). Repeated
application (via a modified Buehler method) of thiodicarb to the
shaved backs of guinea-pigs did not cause allergic sensitization
(Field, 1979b). In a similar test with human subjects, thiodicarb
produced no sensitization by repeated contact (Kamphake et al.,
1980). Methomyl was not a sensitizer when administered to guinea-pigs
(Kaplan & Sherman, 1977).
Acute toxicity
The acute toxicity of thiodicarb and its metabolites in several
animal species is summarized in Table 5.
Short-term studies
Mouse
Groups of Charles River CD-1 mice (5 males and 5 females per
group) were administered thiodicarb in the diet for 7 days at dietary
levels of 0, 15, 45, or 90 mg/kg b.w./day. Growth and mortality were
unaffected. In male mice, at the two highest dosage levels, an
increased absolute kidney weight was observed (Homan et al., 1977).
Groups of ICR-JCL (SPF) mice (10 per sex per group) were fed
thiodicarb (92.5% purity) in the diet for 4 weeks at dosage levels of
0, 30, 100, or 300 ppm (equal to 0, 4.2, 13.7, and 40.8 mg/kg b.w./day
for males and 0, 3.8, 12.3, and 36.3 mg/kg b.w./day for females).
There was no mortality during the study. Body weight, food consumption
and organ weights (brain, heart, liver, spleen, kidney, testes, and
ovaries) were unaffected by treatment (Yoshida et al. 1983a).
Table 5. Results of acute toxicity assays of thiodicarb and its metabolitea
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Thiodicarb Mouse both Oral corn oil 226 Myers et al., 1977b
(148-346)
Rat M Oral corn oil 74.8-129 Hallory et al.,1982b;1982e;1982g
(52.9-186) Myers et al.,1982b;1982c;1982d;1982e
F Oral corn oil 50-136 Mallory et al., 1982b;1982e;1982g
(34.9-192.1) Myers et al.,1982b;1982c;1982d;1982e
M Oral 0.25% methyl 46.5-83 Mallory et al., 1982c;1982f;1982h
cellulose or (33.4-108) Myers et al.,1982b;1982c;1982c;1982d
water
F Oral 0.25% methyl 39.1-55 Mallory et al., 1982e;1982f;1982h
cellulose or (29.4-65.5) Myers et al., 1982b;1982c;1982d
water
both Oral corn oil 74.6-215 Mallory et al., 1982b;1982e;1982g
(59.6-268.8) Myers et al., 1982b;1982c;1982d;1982e
both Oral 0.25% methyl 49.4-97 Mallory et al., 1982c;1982f;1982h
cellulose or (39.4-138.2) Myers et al., 1982b;1982c;1982d
water
Guinea- M Oral corn oil 160 Myers et al., 1977c
pig (94.3-271)
Rabbit both Oral capsule > 400 Myers et al., 1979a
both Oral corn oil 556 Myers et al., 1979a
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Chicken F Oral corn oil 582-660 Myer et al., 1980
Schwartz & Stevens, 1978
Monkey both Oral capsule 467.2 Weatherholtz et al., 1983
(329.6-662.1)
Rat M Dermal 2540 Field 1979a
4 hr. (1120-5750) Koehler & Dorman 1979
intact Myers et al., 1975;1979a
skin
Rabbit both Dermal > 6310 Lemen et al., 1979;
24 hr. Mallory et al., 1982d;
abraded Myers et al., 1978b;1979a
& intact
skin
Rat both I.P. 23 (15.9-33.1) Matthews 1979b
Mouse both I.P. 34 (21.9-52.7) Matthews 1979a
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Rat Inhalation > 2.0 Myers et al., 1975
148 min.
aerosol in
DMSO
Inhalation > 0.2 Myers et al., 1979a
360 min.
aerosol in
DMSO
both Inhalation 0.1155-0.220 Dickey et al., 1979
240 min. (0.1094- Myers et al., 1977a
dust 0.320)
Inhalation > 2.4 Myers et al., 1978a
60 min.
dust
Aqueous 1.04 Myers et al., 1982a
aerosol
Methomyl Rat M Oral corn oil 14.1-47.6 Kaplan & Sherman 1977;
peanut oil (8.0-72.7) Myers et al., 1976a;1981
PEG 400
F Oral corn oil 12.3-48.5 Kaplan & Sherman 1977;
peanut oil (7.6-70.2) Myers et al., 1979b;I981
Weil & Carpenter 1978
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Guinea- M Oral acetone 15*** Kaplan & Sherman 1977
pig peanut oil
Japanese Oral water;CMC 34 Kaplan & Sherman 1977
Quail
Dog M capsule 30*** Kaplan & Sherman 1977
Monkey both aqueous 40*** Kaplan & Sherman 1977
(Rhesus)
Chicken F Oral acetone/ 28 Kaplan & Sherman 1977
water
Hydroxymethyl Rat M Oral corn oil 200 Myers et al., 1978c
methomyl (123-326)
Oral corn oil 238 Myers et al., 1979b
(156-363)
Methomyl Rat M Oral corn oil 453 Myers et al., 1978a
sulfoxide (296-692)
Oral corn oil 476 Myers et al., 1979b
(311-727)
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Methomyl Rat M Oral corn oil 794 Myers et al., 1976b
oxide (486-1300)
Oral corn oil 1000 Myers et al., 1979b
(636-1572)
both Oral water 350 Myers et al., 1984
(311-394)
Acetonitrile Rat M Oral water 246 Pozzani et al., 1955
(160-378)
F Oral water 234 Pozzani et al., 1955
(203-270)
Oral water 38/0 Smyth 1947
Methomyl Rat M Dermal water > 200 X Kaplan & Sherman 1977
intact & 5 days
abraded
skin 6 h
Rabbit M Dermal water > 5000 Kaplan & Sherman 1977
intact
skin 24 h
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
M Dermal water 800-951 Myers et al., 1981
intact
& abraded
skin 24 h
F Dermal water 566-1130 Myers et al., 1981
intact
& abraded
skin 24 h
Methomyl Rat M Dermal corn oil > 1000 Myers et al., 1976b
oxime intact
skin 4 h
Dermal water > 2000 Meyers et al., 1984
intact
skin 24 h
Acetonitrile Rat i.p. 0.95 ml/kg Rogers 1959
(0.58-1.54)
Rat i.v. 1.68 ml/kg Rogers 1959
(0.08-3.21)
Rabbit Dermal 1.26 ml/kg Carpenter et al., 1958
Methomyl Rat M Inhaltion water 76 mg/l Kaplan & Sherman 1977
of aqueous
aerosol
4 h
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Methomyl Rat Inhalation water > 0.82 Myers et al., 1976b
oxime of mg/1
aqueous
aerosol
4 h
* 95% confidence limits (the widest range from each set of studies) are given in parentheses.
** Because particles of thiodicarb are not of respirable size, the material was ground to a dust
for these tests.
*** Minimum lethal dose.
Groups of ICR-JCL (SPF) mice, (28 per sex per group) were fed
thiodicarb (92.5% purity) in the diet for 13 weeks at dosage levels of
0, 30, 150, or 600 ppm (equal to 0, 3.8, 18.9, and 76.5 mg/kg b.w. for
males and 0, 4.3, 21.5, and 85.0 mg/kg b.w. for females). Mortality,
food consumption, body weight, water consumption, urinalysis,
haematology, and clinical chemistry were determined. Cholinesterase
levels were determined in plasma, red blood cells (RBC) and brain at
study termination, as were organ weights, gross pathology, and
histopathology. Brain ChE was stated to be depressed in 150-
and 600-ppm females and in 600-ppm males. Also reported were
significantly-increased relative liver weights in high-dose females.
There were no other compound-related effects reported. However,
individual animal data, gross pathology, or histopathology were not
submitted. Therefore, a no-observed-effect level cannot be assessed
from these data as presented to the 1985 Joint Meeting (Yoshida
et al., 1983b).
Rat
Groups of Harlan-Wistar rats (5 per sex per group) were fed
thiodicarb in the diet for 7 days at dosages of 0, 5, 25, or 100 mg/kg
b.w./day. Limited effects were noted on body weight and food
consumption, which were decreased in high-dose males. Relative kidney
weights were also reportedly increased in all treated males (Woodside
et al., 1975).
Groups of Wistar rats (5 per sex per group) were administered
thiodicarb in the diet at dosages of 0, 0, 18.5, 48.6, or 128 mg/kg
b.w./day for 7 days. Observations were made on mortality, food
consumption, body weight, and gross liver and kidney changes. Body
weights were reduced in all treated groups compared to controls.
Relative liver weights and absolute kidney weights were reduced in
high-dose rats (Woodside et al., 1978).
In a preliminary 13-week study 4 groups of Fischer 344 rats
(5 per sex per group) were administered thiodicarb in the diet at
dosage levels of 0, 5, 15, or 45 mg/kg b.w./day. One additional group
per sex received 90 mg/kg b.w./day for 80 days. Observations included:
mortality, food and water consumption, organ weights, and ChE activity
(RBC, plasma and brain). Blood samples for ChE measurements were
obtained via cardiac puncture. At necropsy, the brain was removed,
weighed, and submitted for ChE assay. Relative liver, adrenal, and
spleen weights were increased in all treated males, and relative
spleen weights were increased in mid- and high-dose females. Body
weights were reduced throughout the study in all treated males. ChE
activity was unaffected at any dose level, except for increased RBC
ChE activity in high-dose males and females (Woodside et al.,
1979a).
Groups of Fischer 344 rats (10 males and 10 females per group)
were administered thiodicarb (99% purity) in the diet at dosage levels
of 0, 0, 1.0, 3.0, 10 or 30 mg/kg b.w./day for 13 weeks. Observations
included mortality, clinical observations, food and water consumption,
body weight, organ weights, haematology, clinical chemistry,
urinalysis, ChE activity (plasma, RBC and brain-at-germination), and
gross and microscopic analysis. At both 10 and 30 mg/kg b.w./day, body
weight and water consumption decreased significantly among females,
with slight increases in males at the same dose levels. At 30 mg/kg
b.w., relative spleen weights were increased significantly in males,
while relative kidney weights were increased in high-dose females.
Urine volume was increased in males and decreased in females at the
high-dose level. Specific gravity of urine was also decreased in high-
dose females. Other clinical parameters were unaffected by treatment
except for decreased mean haemoglobin concentrations in high-dose
males. ChE activities were comparable among all groups except for a
significantly-increased RBC ChE in high-dose males. Gross and
microscopic analyses were unremarkable and typical of changes
associated with 4-month-old Fischer 344 rats. A NOEL was demonstrated
at 3 mg/kg b.w. (Homan et al., 1978b).
Dog
Groups of 9-month-old Beagle dogs (4 per sex per group) were fed
thiodicarb in the diet at targeted dosages of 0, 15, 45, or 90 mg/kg
b.w./day for 13 weeks. However, due to mortality at the highest dose,
the level was reduced to 76 and 86 mg/kg b.w. for females and males,
respectively. Animals were examined routinely for physical/behavioural
changes, food and water consumption, body-weight changes, haematologic
and blood chemistry parameters, and urinalysis. Organ weights, gross
necropsy and histologic effects were examined at terminal sacrifice.
ChE activities (plasma and RBC) were determined pre- treatment and on
days 28, 63, 79, and 93 of the study. Brain ChE was determined at
sacrifice.
Two females died in the high-dose group, and dose-related
cholinergic symptoms were observed in both sexes in the mid- and high-
dose groups. Symptoms included anorexia, vomiting, and loose stools.
However, ChE activity was unaffected throughout the study. Food
consumption decreased in high-dose females. Water consumption
increased in both sexes in the high-dose groups. Body weight was
decreased in females at the mid- and high-dose levels but there was no
effect on males. Urine volume was increased in mid- and high-dose
males. Decreases in RBC count, packed cell volume, and haemoglobin
were noted in both sexes in the mid- and high-dose groups, while
reticulocyte counts were increased in both sexes of the high-dose
groups only. These changes, together with bone-marrow hyperplasia,
decreased mean-corpuscular haemoglobin concentrations, and congested
spleen in high-dose groups, are suggestive of compensatory
haematopoiesis and anaemia. Relative liver weights and serum GPT
levels were increased in both sexes at the high dose. However, males
at the high dose demonstrated cloudy swelling, vacuolation and
degenerative liver effects. All treated groups produced evidence of
focal and diffuse inflammation of the liver. A clear NOEL was not
demonstrated in this study (Homan, et al., 1978a).
Groups of Beagle dogs, 6-7 months old (6 per sex per dose) were
administered thiodicarb in the diet at dosage levels of 0, 5, 15, or
45 mg/kg b.w./ day for 26 weeks. Animals were observed routinely for
survival, clinical appearance, food consumption, body-weight change,
ChE activity, haematologic and clinical chemistry parameters, and
urinalysis. Organ-weight changes and gross and microscopic pathologic
examinations were performed at terminal sacrifice. Cholinesterase
activity was twice determined pretreatment and once each on fasted and
non-fasted samples during weeks 8, 17, and 26. Brain ChE was
determined at sacrifice.
Thiodicarb did not affect body weight, food consumption,
urinalysis, organ weights, or gross or microscopic pathological
examination of tissues. Among the male dogs, there was a dose-related
increase in the combined incidence of soft stools, mucoid stools, and
diarrhoea. These were not evident in females. Treatment-related
changes in haematology included significantly-decreased haematocrit,
haemoglobin, and RBC levels in high-dose males and females. Dogs
receiving the highest dose had significantly-elevated levels of serum
GPT, plasma ChE, and RBC ChE as well as significantly-decreased levels
of calcium, total protein and globulin, with correspondingly-increased
albumin-to-globulin ratio. There were no treatment-related effects
reported at 15 mg/kg b.w./day (Wolfe 1981).
Long-term study
Rat
Six groups of Fischer 344 rats (120 rats per sex per dose) were
administered thiodicarb (analytical grade, 99+% purity) in the diet at
dosage levels of 0, 0, 0.5, 1, 3, or 10 mg/kg b.w. for 24 months.
Animals were 50 days of age when dosing began, and housed 3 per cage
for males and 5 per cage for females. Rats were examined daily for
mortality and adverse physical or behavioural changes. Food and water
consumption and body-weight changes were determined routinely. Interim
sacrifices were performed at 6, 12, and 19 months, utilizing 10 to 20
rats per sex. Haematology, clinical chemistry, and urinalysis
determinations were made prior to each scheduled sacrifice. Organ
weights and gross and microscopic evaluations were also performed at
each sacrifice and on dead or moribund animals (excluding organ
weights). Limited ophthalmological examinations were also performed.
Mortality was increased in high-dose males, but not females, from
the 15th to the 21st months. At termination of the study, mortality in
control males exceeded that in the high-dose males. Food consumption
was unaffected in treated rats, although sporadic decreases were
occasionally observed. Body weights were depressed in high-dose males
from the 15th to 20th months and in high-dose females from the 3rd to
the 21st months. An outbreak of sialodacry-oadenitis virus was
discovered at about the 18th month, which had contributed to a
generalized debilitated condition and to weight loss in all animals
for approximately 2-3 weeks. Haematological parameters were
unremarkable except for reduced erythrocyte counts, hematocrits and
haemaglobin levels in males fed > 1 mg/kg thiodicarb at the 12th
month, but not at 19 or 24 months. ChE activity (plasma, RBC, and
brain), determined after 48 hours on the control diet, was unaffected.
However, the time delay between compound administration and ChE
measurement is considered excessive for a carbamate and therefore this
result is not meaningful. The only change in ChE activity, consistent
with other thiodicarb-feeding studies, was a significant increase in
RBC ChE at 24 months in high-dose males. Clinical chemistry and
urinalysis determinations were not affected by treatment except for
transient reduction in urine volume at 12 months in females given
> 3 mg/kg. Although sporadic differences were observed at various
sacrifice intervals, there were no dose-related effects on absolute or
relative organ-weights.
There were no significant pathologic changes attributable to
thiodicarb at 6, 12, or 19 months, and no carcinogenic response at any
dose throughout the study. High-dose males presented evidence at 24
months of an increased incidence of pituitary cysts, while high-dose
females demonstrated haemosiderosis of the mediastinal lymph nodes.
Hepatocellular hyperplasia and interstitial prostatitis were increased
in treated males, although not significantly at the high dose; their
incidence was not dose-related, and there was not a significant
increase in liver neoplasms. High-dose males had an increased, but not
significant, incidence of epithelial hyperplasia and epithelial
thymomas. Mononuclear cell leukemia and interstitial cell adenomas
(in males) occurred spontaneously in all groups, in general agreement
with this strain of rat.
A NOEL of 3 mg/kg b.w. was established with no evidence of
oncogenic potential at doses up to and including 10 mg/kg b.w.
(Woodside et al., 1980b).
COMMENTS
Thiodicarb is rapidly absorbed, metabolized, and excreted, and
has not been demonstrated to accumulate in animal tissues. It is
degraded to methomyl, which is converted to the "oxime" (methyl
hydroxythioacetimidate) and ultimately to acetonitrile, carbon
dioxide, acetic acid, and acetamide. The ultimate metabolic fate in
animals depends on the isomeric configuration of methmyl (SYN or
ANTI).
The acute oral toxicity (LD50) in rats is 66-120 mg/kg b.w.,
depending on the vehicle used. In monkeys an oral LD50 is 467 mg/kg
b.w.
Thiodicarb causes reversible cholinesterase inhibition. Long-term
feeding and oncogenicity studies in rats and mice have revealed no
oncogenic potential in either species, and a NOEL of 3 mg/kg b.w. for
non-oncogenic effects was determined. No adverse reproduction or
teratogenic effects in rats or mice have been demonstrated.
Thiodicarb was not mutagenic in a wide variety of assays.
However, it was positive in the mitotic gene conversion assay using
Saccharomyces cerevisiae.
In two short-term feeding studies in dogs, thiodicarb
demonstrated adverse liver effects in 90 days at doses > 15 mg/kg
b.w., while in a 6-month study at doses up to 45 mg/kg b.w. no adverse
effects were observed at 15 mg/kg b.w. Considering the conflicting
information concerning liver effects in dogs, the Meeting required the
submission of the results from an ongoing 1-year dog study by 1987.
Therefore, a temporary ADI was allocated.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 60 ppm in the diet, equivalent to 3 mg/kg b.w.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.01 mg/kg b.w.
FURTHER WORK OR INFORMATION REQUIRED (by 1987)
l. Submission of ongoing 1-year dog feeding study known to be in
progress.
2. Information on possible effect on maternal body-weight gain
demonstrated in a rat teratology study.
DESIRED
1. Observations in man, including monitoring of acetamide in the
urine.
REFERENCES
Andrawes, N.R., College, P.R. & Bailey, R.H. Short-term fate of orally
(1977) administered UC 51762 in the rat. File No. 23509.
Unpublished report from Union Carbide Agricultural Products
Company, Inc. Submitted to WHO by the Union Carbide
Agricultural Products Co., Inc.
Andrawes, N.R. & Bailey, R.H. Metabolism of acetyl-l-l4C-LARVIN(R) in
(1979a) the rat. File No. 26925. Unpublished report from Union
Carbide Agricultural Products Company. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Andrawes, N.R. & Bailey, R.H. Metabolism of acetyl-1-l4C-methomyl in
(1979b) the rat. File No. 26945. Unpublished report from Union
Carbide Agricultural Products Company. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Andrawes, N.R. & Bailey, R.H. Fate of acetyl-1-14C-thiodicarb in laying
(1980) chickens under continuous feeding conditions. File No.
26414. Unpublished report from Union Carbide Agricultural
Products Company. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Andrawes, N.R. Acetonitrile metabolism in the rat. File No. 32291.
(1983) Unpublished report from Union Carbide Agricultural Products
Company, Inc. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Becci, P.J., Schwartz, C.S., & Parent, R.A. Evaluation of UC 51762 No.
(1979) 40-488 as a potential delayed neurotoxic agent following
oral administration to hens protected by atropine sulfate.
Report No. 6065. Unpublished report from Food and Drug
Research Laboratories. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Cameron, J.T. & Wolfe, G.W. Acute eye irritation study in rabbits.
(1979) Project No. 400-616. Unpublished report from Hazleton
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Carpenter, C.P., Pozzani, U.C., Weil, U.C., Palm, P.E. & Nair, J.H.
(1958) The toxicity of acetonitrile. Part II. Special report No.
21-100. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company Inc.
Cifone, M.A. & Brusick, D.J. Evaluation of thiodicarb in the rat
(1985a) primary hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. 20991 from Litton Bionetics, Inc.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Cifone, M.A. & Brusick, D.J. Mutagenicity of thiodicarb in a mouse
(1985b) lymphoma mutation assay. Unpublished report No. 20989 from
Litton Bionetics, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Conroy, W.J., DePass, L.R., Homan, E.R., Weil, C.S., Geary, D.L. &
(1979a) Frank, F.R. Dermal sensitization potential in guinea pig.
Special report No. 42-61. Unpublished report from the Mellon
Institute. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Conroy, W.J., DePass, L.R., Homan, E.R., Weil, C.S., Geary, D.L., &
(1979b) Frank, F.R. UC 51762, 16-dose rabbit dermal study. Special
report No. 42-52. Unpublished report from Mellon Insititute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Conroy, W.J., DePass, L.R., Homan, E.R., Weil, C.S., & Webb, G.A.
(1979c) Dermal sensitization potential in the guinea pig. Special
report No. 42-26. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Dangler, T.L. & Rodwell, D.E. Pilot teratology study in mice. Report
(1979) No. 369-030. Unpublished report from International Research
and Development Corporation. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Dickey, C.L., Nachreiner, D.J., DePass, L.R., Gad, S.C., Weil, C.S.,
(1979) Geary, D.L. & Frank, F.R. Acute and 9-day dust inhalation
study on rats with UC 51762 technical. Special Report No.
42-63. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Feung, C.S., College, P.R. & Chancey, E.L. Studies on the disposition
(1980) of 14C-thiodicarb in lactating cows. File No. 27350.
Unpublished report from Union Carbide Agricultural Products
Company, Inc. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Field, W.E. Acute dermal toxicity in rats. Project No. CDC-UC-012-79.
(1979a) Unpublished report from CDC Research, Inc. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Field, W.E. Skin sensitization in guinea pigs. Project No. CDC-UC-003-
(1979b) 79. Unpublished report from CDC Research, Inc. Submitted to
WHO by Union Carbide Agricultural Products Company, Inc.
Fleischman, R.W., Baker, J.R., Hagopian, M., Wade, G.G., Hayden, D.W.,
(1980) Smith, E.R., Weisburger, J.H. & Weisburger, E.K.
Carcinogenesis bioassay of acetamide, hexanamide, adipamide,
urea and phi-tolylurea in mice and rats. J. of Environ.
Pathol. and Toxicol., 3, 149-170.
Gallo, M.A. & Stevens, K.R. Evaluation of LARVIN(R) (UC 51762) in 21
(1980) day dermal toxicity study in rabbits. Project No. 02413-090.
Unpublished report from Foster D. Snell. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Homan, E.R., Weil, C.S., & Cox, E.F. Seven-day feeding study of UC
(1977) 51762 in mice. Special report No. 40-7. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Homan, E.R., Fowler, E.H., Reid, J.B., & Cox, E.F. Thirteen-week oral
(1978a) toxicity study of UC 51762 in dogs. Special report No.
41-98. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Homan, E.R., Maronpot, R.R., Reid, J.B., & Cox, E.F. Thirteen-week
(1978b) oral toxicity study of UC 51762 in rats. Special report No.
41-63. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Huhtanen, K. & Dorough, H.W. Isomerization and Beckman rearrangement
(1976) reactions in the metabolism of methomyl in rats. Pesticide
Biochem. Physiol. 6, 571-583.
Humiston, C.J. & Wright, C.J. An automated method for the
(1967) determination of cholinesterase activity. Tox. Appl.
Pharmacol. 10, 476-480.
Ivett, J.L. & Brusick, D.J. Clastogenic evaluation of 91.48% a.i.
(1985) thiodicarb in an in vivo cytogenetic assay measuring
chromosomal aberration frequencies in Chinese hamster ovary
(CHO) cells. Unpublished report No. 20990 from Litton
Bionetics, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Jagannath, D.R. & Brusick, D. Mutagenic evaluation of CHF 41-43 (UC
(1978) 51762) by the Ames Salmonella/microsome plate test.
Project No. 20838. Unpublished report from Litton Bionetics,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Janes, J.M., Rodwell, D.E., & Jessup, D.C. Teratology study in mice.
(1980) No. 369-032. Unpublished report from International Research
and Development Corporation. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Kamphake, J., Ragsdale, W.E., Weber, E.M., & Carabello, F.B. Repeated
(1980) insult patch test - human volunteers. Report No. 80-0589-
70,73. Unpublished report from Hilltop Research, Inc.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Kaplan, A.M. & Sherman, H. Toxicity Studies with Methyl N-
(1977) (methylamino)-carbamoyloxy ethamidothioate. Toxicol. Appl.
Pharmacol., 40, 1-17.
Khasawinah, A.M. & College, P.R. Fate of single oral dose of 14C-
(1978) acetyl UC 51762 in a lactating cow - metabolism into natural
products. File No. 25257. Unpublished report from Union
Carbide Agricultural Products Company, Inc. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Koehler, S. & Dorman, N. Clinical safety evaluation of LARVIN(R) UC
(1979) 51762 powder and LARVIN(R) 500 flowable. Study No. 2177.
Unpublished report from Food and Drug Research Laboratories.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Lemen, J.K., Wolfe, G.W., & Voelker, R.W. Acute dermal administration
(1979) in rabbits. Project No. 400-614. Unpublished report from
Hazleton Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) technical;
(1982a) primary eye irritation test in rabbits. Report No. PH 421-
UC-004-82. Unpublished report from Pharmakon Research
Laboratories, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - analytical
(1982b) standard; acute oral toxicity study in rats (14 Day). Report
No. PH 402-UC-010-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - analytical
(1982c) standard; acute oral toxicity study in rats (14 Day). Report
No. PH 402-UC-011-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical;
(1982d) acute dermal toxicity test in rabbits. Report No. PH 422-UC-
005-82. Unpublished report from Pharmakon Research
Laboratories, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982e) lab preparation; acute oral toxicity test in rats. Report
No. PH 402-UC-008-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982f) lab preparation; acute oral toxicity test in rats. Report
No. PH 402-UC-009-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982g) pilot plant - batch 34; acute oral toxicity test in rats.
Report No. PH 402-UC-006-82. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982h) pilot plant - batch 34; acute oral toxicity test in rats.
Report No. PH 402-UC-007-82. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. Primary dermal
(1982i) irritation study in rabbits. Report No. PH 420-UC-002-82.
Unpublished report from Pharmakon Research Laboratories,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Matthews, R.J. Acute intraperitoneal LD50 of UC 51762 in the mouse.
(1979a) Report No. PH 405-UC-001-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Matthews, R.J. Acute intraperitoneal LD50 of UC 51762 in the rat.
(1979b) Report No. PH 404-UC-001-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mirro, E.J. & DePass, R.L. Inclusion of UC 51762 in the diet of rats
(1982) for 28 days. Report No. 45-19. Unpublished report from Union
Carbide Bushy Run Research Center. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myer, J., Dean, W.P., & Jessup, D.C. Acute delayed neurotoxicity study
(1980) in hens. Report No. 369-047. Unpublished report from
International Research and Development Corporation.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Weil, C.S., & Carpenter, C.P. Acute toxicity studies of
(1975) UC 51762 in rats and rabbits. Special report No. 38-55.
Unpublished report from Mellon Institute. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Myers, R.C., Weil, C.S., & Cox, E.F. Acute oral LD50 study UC 45650
(1976a) (S-methyl O-(methylcarbamoyl)acetothiohydroximate) in rats.
Special report No. 39-40. Unpublished report from Mellon
Institute. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Myers, R.C., Weil, C.S., & Cox, E.F. Acute toxicity study of S-methyl
(1976b) acetothiohydroximate UC 52702 in rats and rabbits. Special
report No. 39-25. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Carpenter, C.P., & Cox, E.F. Acute inhalation LD50 study
(1977a) of UC 51762 in rats. Special report No. 40-61. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., Carpenter, C.P., & Cox, E.F. Acute LD50 study of UC 51762
(1977b) in mice. Special report No. 40-6. Unpublished report from
Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., Carpenter, C.P., & Cox, E.F. Acute oral LD50 study in
(1977c) guinea pigs. Special report No. 40-142. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., Homan, E.R., Carpenter, C.P. & Cox, E.F. Miscellaneous
(1978a) toxicity studies. Special report No. 41-25. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., Homan, E.R., & Cox, E.F. Acute dermal toxicity of UC
(1978b) 51762 in rabbits. Special report No. 41-93. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., Homan, E.R., & Webb, G.A. Acute oral LD50 study of
(1978c) 1-methylthioacetaldehyde-O-(hydroxymethyl carbamoyl) oxide
(UC 58614) in rats. Special report No. 41-133. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., Homan, E.R., Weil, C.S., & Frank, F.R. UC
(1979a) 51762 technical; range-finding toxicity studies. Special
report No. 42-19. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Homan, E.R., Weil, C.S., & Frank, F.R. Miscellaneous
(1979b) toxicity studies. Special report No. 42-27. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. Methomyl technical; acute
(1981) toxicity and irritancy study. Report No. 44-75a. Unpublished
report from Union Carbide Bushy Run Research Center.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. Acute toxicity and irritancy
(1982a) studies. Report No. 45-24. Unpublished report from Union
Carbide Bushy Run Research Center. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. LARVIN(R) - analytical
(1982b) standard; acute oral toxicity study in rats. Report No.
45-152. Unpublished report from Union Carbide Bushy Run
Research Center. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. LARVIN(R) - technical lab
(1982c) preparation; acute oral toxicity study in rats. Report No.
45-151. Unpublished report from Union Carbide Bushy Run
Research Center. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. LARVIN(R) - technical pilot
(1982d) plant - batch 34; acute oral toxicity study in rats. Report
No. 45-151. Unpublished report from Union Carbide Bushy Run
Research Center. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., Weil, C.S., & Frank, F.R. LARVIN(R) - technical; acute
(1982e) oral toxicity study in rats. Report No. 45-40. Unpublished
report from Union Carbide Bushy Run Research Center.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Slesinski, R.S. & Frank, F.R. Methomyl oxime: acute
(1984) toxicity and irritancy study. Project report 47-185.
Unpublished report from Union Carbide Bushy Run Research
Center. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; micronucleus test.
(1979a) Report No. PH 309-UC-001-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; mitotic crossing
(1979b) over in Saccharomyces cerevisiae. Report No. PH302-UC-001-
79. Unpublished report from Pharmakon Research Laboratories,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; mitotic gene
(1979c) conversion in Saccharomyces cerevisiae. Report No. PH304-
UC-001-79. Unpublished report from Pharmakon Research
Laboratories, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; primary DNA
(1979d) damage. Report No. PH 305-AM-002-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; reverse mutation
(1978e) in Saccharomyces cerevisiae. Report No. PH 303-UC-001-79.
Unpublished report from Pharmakon Research Laboratories,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Pozzani, U.C., Nair, J.R., & Carpenter, C.P. The toxicity of
(1955) acetonitrile. Special report No. 18-34. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Rogers, L.D. Intraportal injection of acetonitrile. Report No. 22-2.
(1959) Unpublished report from Mellon Institute. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Schardein, J. 21-Day dermal toxicity study in rabbits. Report No.
(1982) 369-053. Unpublished report from International Research and
Development Corporation. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Schwartz, C.S. & Stevens, K.R. Acute oral LD50 in hens. Report No.
(1978) 6064. Unpublished report from Food and Drug Research
Laboratories. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Smyth, H.F. Range-finding toxicity test of acetonitrile. Special
(1947) report No. 10-23. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Tasker, E.J., Rodwell, D.E., & Jessup, D.C. Teratology study in rats.
(1979) Report No. 369-029. Unpublished report from International
Research and Development Corporation. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Weatherholtz, W.M. LARVIN(R) - technical; fourteen-day eye irritation
(1982) study in monkeys. Project No. 400-648. Unpublished report
from Hazleton Research Laboratories. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Weatherholtz, W.M., Rendon, F., & Gluck, S.E. Dose rangefinding study
(1983) in monkeys. Project No. 400-694. Unpublished report from
Hazleton Research Laboratories. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Weil, C.S. & Carpenter, C.P. Miscellaneous toxicity studies. Special
(1978) report No. 34-88. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Weisburger, J.H., Yamamoto, R.S., Glass, R.M., & Frankel, H.H.
(1969) Prevention by arginine glutamate of the carcinogenicity of
acetamide in rats. Toxicol. Appl. Pharmacol., 14,
163-175.
Wentz, K.L. & Wolfe, G.W. Primary skin irritation study in rabbits.
(1979) Project No. 400- 617. Unpublished report from Hazleton
Research Laboratories. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Wolfe, G.W. Subchronic toxicity study in dogs. Project No. 400-626.
(1981) Unpublished report from Hazleton Research Laboratories.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Woodside, M.D., Weil, C.S., & Cox, E.F. Seven-day feeding study of UC
(1975) 51762 in rats. Special report No. 38-136. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Reid, J.B., & Cox, E.F. Seven-day
(1978) feeding study of UC 51762 in rats. Special report No.
41-100. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1979a) UC 51762; dimethyl N,N'-(thiobis((methylimino)carbonoyloxy))
bis-(ethanimidothioate) inclusion in the diet of rats for
thirteen weeks. Special report No. 42-125. Unpublished
report from Mellon Institiute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1979b) UC 51762; rat teratology studies. Special report No. 42-48.
Unpublished report from Mellon Institute. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1979c) UC 51762 technical; inclusion in the diet of rats for three
generations and dominant lethal mutagenesis studies. Special
report No. 42-165. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1980a) UC 51762; chronic oncogenicity feeding study in mice.
Special report No. 43-10. Unpublished report from Mellon
Institute. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1980b) UC 51762; chronic toxicity and oncogenicity feeding study in
Fischer 344 rats. Special report No. 43-18. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Yoshida, A., Maita, K., Saito, T., & Miyaoka, T. LARVIN(R): 4-weeks
(1983a) range-finding study in mice. Unpublished report from The
Institute of Environmental Toxicology. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Yoshida, A., Takahashi, K., Kosaka, T., & Miyaoka, T. Thiodicarb: 3
(1983b) month oral sub-chronic toxicity study in mice (study I).
Unpublished report from The Institute of Environmental
Toxicology. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Ziemke, K.A. & Rodwell, D.E. Pilot teratology study in rats. Report
(1979) No. 369-028. Unpublished report from International Research
and Development Corporation. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Andrawes (1983) reported that oral administration of radiolabeled
acetonitrile to rats resulted in elimination of 65% of the applied
dose in expired air, with less than 1% expired as 14CO2. In a
similar study however, Huhtanen & Dorough (1976) found that in rats
9.1% of a dose of acetonitrile was eliminated as CO2 in respiratory
gases.
Two lactating Holstein cows/group were given approximately 0.004,
0.4, 1.4, or 4 mg 14C-thiodicarb per kg b.w. daily for 21 days. Milk
and urine were collected twice daily, and blood and faeces were
collected at 3- to 4-day intervals. One cow at each dosage level was
sacrificed 12 hours after the last treatment, and tissues (liver,
kidney, lung, spleen, heart, brain, ovary, udder, tongue, foreleg
muscle, hindleg muscle, neck muscle and omental fat) were collected
for analysis. The remaining 4 cows (1 at each dosage level) were kept
for an additional 7 days after termination of treatment. During this
post-treatment phase, milk and urine were collected twice daily as
before, and blood and faeces were collected daily. The same tissues
were collected after 7 days post-treatment. The radioactivity found in
all samples (i.e., tissue, milk, blood, urine and faeces) was dose
dependent. The concentration of acetonitrile in milk approached peak
levels within 7 days in all dose groups. The acetonitrile levels
appeared to remain nearly constant through the rest of the 21-day
treatment period, then declined sharply during the 7-day post-
treatment phase. Following 21 days of treatment, tissue levels of 14C
residues were highest in liver and lowest in adipose tissues. Seven
days after termination of dosing, radioactivity levels were lower in
all tissues except liver. The authors concluded that the predominant
end-products of thiodicarb catabolism were acetonitrile, acetamide,
and carbon dioxide. Acetamide, which is believed to be produced by
hydrolysis of acetonitrile, was found in trace amounts (< 0.01 ppm)
in milk at the highest feeding level. Approximately 24% of the
aqueous-extractable urinary-radioactivity was identified as acetamide
(Feung et al., 1980).
In a cow given a single dose (7.02 mg/kg b.w.)14C-acetyl
thiodicarb, 66, 11.4, 5.0, and 4.6% of the administered radioactivity
was eliminated, during 72 hours post-treatment, in respired air,
faeces, urine and milk, respectively, while 10.1% was retained in the
tissues (Khasawinah & College, 1978).
In a study using mature White Leghorn laying chickens, no
carbamate residues were found in tissues or eggs after 21 days of oral
administration of thiodicarb at dosages of 15.4, 28.6 and 102 ppm in
the diet. Acetonitrile, detected in the eggs and tissues (except fat)
at the 102-ppm level, corresponded to 0.2 ppm (egg yolk and whites),
0.5 ppm (liver), and 0.2-0.3 ppm (muscle). The level in eggs was
directly proportional to the dosage level administered. Acetamide was
not detected in egg yolks, but was found in egg whites (0.07 ppm), in
liver (0.06 ppm) and in muscle (0.01 ppm), when administered at 102
ppm thiodicarb (Andrawes & Bailey, 1980).
Based on the available data that 5.72% of the administered
syn-methomyl is converted to acetonitrile, a maximum of 5.72% of the
administered 14C-thiodicarb could therefore be available for
conversion to acetamide, since thiodicarb is metabolized predominantly
to syn-methomyl. On a weight-percent basis, the maximum acetamide
that could occur from ingestion of thiodicarb residues is 1.9% of the
administered dose (53:1 thiodicarb:acetamide on a weight-to-weight
basis) (Andrawes & Bailey, 1979a & 1979b; Huhtanen & Dorough, 1976).
Effects on enzymes and other biochemical parameters
The most significant biochemical effect of thiodicarb and its
carbamate metabolites is the ability to reversibly inhibit
acetylcholinesterase (ChE), a capability in common with other methyl
carbamate esters. The methylcarbamate-acetylcholinesterase complex
dissociates rapidly, and the acetylcholinesterase enzyme is released
unaltered. The transient nature of acetylcholinesterase inhibition by
thiodicarb makes it difficult to assay accurately, since inhibition is
reversed by sample dilution and by adding substrate (butyrylcholine or
acetylcholine) during assay procedures. Therefore, the assay procedure
must be very rapid and use less than optimal amounts of substrate. The
automated colorimetric method of Humiston & Wright (1967) is suitable
for such determination.
Groups of Fischer 344 rats (10 males and 10 females per group)
were administered thiodicarb in the diet at dosage levels of 0, 1, 3,
10, or 30 mg/kg b.w./day for 28 days. Rats were observed for mortality
and clinical symptoms of toxicity. Food consumption was determined
weekly and plasma- and erythrocyte-ChE measurements were analyzed on
days 0, 15, and 29. Brain ChE was determined on day 29.
Body weight and food consumption were significantly reduced in
females at > 10 mg/kg, b.w. Males were not similarly affected.
There was no mortality. Plasma and erythrocyte ChE were significantly
depressed in high-dose males and females. Plasma ChE recovered to
normal values by day 29, while red blood cell (RBC) ChE remained
significantly depressed in both sexes at > 30 mg/kg b.w./day. Brain
ChE was not depressed at any level (Mirro & DePass, 1982).
Toxicological studies
Special study on reproduction
Rat
Groups of Fischer 344 rats (20 female and 10 male rats/group)
were administered thiodicarb (purity 99+%) in the diet, at dosage
levels of 0, 0, 0.5, 1.0, 3, or 10 mg/kg b.w., in a three-generation
reproduction study. Each generation produced only one litter per
generation. The routine indices of fertility, gestation,
survivability, viability, and lactation were determined for all
generations, as well as selected gross and histopathological
evaluations for F2a and F3a generations. Rats were mated on a 1-male-
to-2-females ratio when approximately 100 days of age. Dams and pups
were observed daily for appearance and behaviour. Litter size was
reduced to 10 on day 4 postpartum. Brother-sister matings were
avoided.
There were no compound-related effects on any of the reproductive
parameters measured, except for an initial transitory body-weight
depression in F0 males. Gross and microscopic analyses were
reportedly unremarkable. Thiodicarb was without adverse effects on
reproduction at doses up to and including 10 mg/kg b.w. (Woodside
et al., 1979c).
Special studies on teratogenicity
Mouse
In a pilot study using pregnant Charles-River CD-1 mice,
thiodicarb (technical grade) administered orally from days 6 through
16 of gestation, at dosage levels of 0, 20, 60, 100, 150, 200, 300, or
400 mg/kg b.w., produced excessive maternal toxicity (body-weight
depression and mortality) at doses greater than 200 mg/kg b.w.
(Dangler & Rodwell, 1979).
Groups of Charles-River CD-1 mice (25 mated females/group) were
administered thiodicarb (technical grade) via oral gavage at dosage
levels of 0, 50, 100, or 200 mg/kg b.w. from days 6 through 16 of
gestation. Mating was accomplished on a 1-female-to-1-male schedule at
94 days of age. Day of copulation was also considered day 0 of
gestation. Animals were observed routinely for appearance, behaviour
and body-weight changes. Pups were delivered by Caesarean-section on
day 17 of gestation. All foetuses were weighed, sexed, examined for
external malformations, and subsequently subjected to visceral or
skeletal examination.
Six females in the high-dose group died during the study. Body
weights were unaffected by treatment. There was no evidence of
maternal or foetal toxicity at doses > 100 mg/kg b.w. and no
teratogenic effects at doses > 200 mg/kg b.w. (Janes et al.,
1980).
Rat
In a pilot teratology study using pregnant Charles-River CD COBS
rats, thiodicarb (technical grade), administered orally at dosage
levels of 0, 20, 40, 80, 120, or 160 mg/kg b.w. on days 6-19 of
gestation, produced mortality at doses > 40 mg/kg b.w. There were
no reported adverse effects on reproduction or foetal development
(Ziemke & Rodwell, 1979).
Groups of Charles-River CD COBS rats (25 mated females/group)
were administered thiodicarb (technical grade) via oral gavage at
dosage levels of 0, 10, 20, or 30 mg/kg b.w. on gestation days 6-19.
Females were mated 1-to-1 with males at 13 weeks of age. Day of
copulation was also considered day 0 of gestation. Animals were
observed routinely for appearance, behaviour, and body-weight changes.
Pups were delivered by Caesarean-section on gestation-day 20. All
foetuses were weighed, sexed, measured, and examined for external
malformations. One-third of the foetuses were fixed for visceral
examination and the remaining two-thirds of the foetuses were
subjected to skeletal examination. Early and late resorptions, number
of implantations and corpora lutea, and the number and location of
viable and non-viable foetuses, were recorded.
Clinical observations, apparent at 20 and 30 mg/kg b.w. within 4
hours after treatment, included inactivity, tremors, and clear oral
discharge. There was no mortality. Maternal body weights determined
on days 0, 6, 9, 12, 16, and 20 were significantly decreased at
> 20 mg/kg b.w, and mean foetal body-weights were significantly
reduced at > 10 mg/kg b.w. No teratogenic effects were produced,
although delayed maturation of foetuses (reduced ossification),
associated with maternal toxicity, was observed. There was no evidence
of visceral or skeletal anomalies attributable to thiodicarb at doses
up to and including 30 mg/kg b.w. (Tasker et al., 1979).
Groups of Fischer 344 rats (10 mated females/group) were
administered thiodicarb (purity 99+%) in the diet via 2 separate
dosing regimens: from day 0 to day 20 of gestation or from day 6 to
day 15 of gestation. Dose levels in each dosing schedule included 0,
0.5, 1.0, 3, or 100 mg/kg b.w. A positive control group received
625 mg/kg b.w. of aspirin via gavage on gestation day 10. Females were
mated with males on a 1-to-1 schedule in order to achieve 10 pregnant
females per dose. All pups were delivered by Caesarean-section on
gestation day 20 and examined for visceral and skeletal abnormalities.
Maternal body-weight gains determined on days 0 and 12 of
gestation were reduced in all treatment groups. However, these changes
were extremely variable at doses of 0.5-3.0 mg/kg b.w. (only two
measurements were included) and they were not considered to be
biologically significant except at the highest dose administered where
the body weights were uniformly depressed. Foetal body weight and
length were also significantly reduced in the high-dose group that was
administered thiodicarb throughout gestation (days 0-20). The aspirin-
exposed foetuses were similarly affected. Thiodicarb did not adversely
affect the number of implantations, resorptions, live foetuses per
litter, or pre-implantation losses. Resorption frequency was increased
in high- dose females given thiodicarb from days 6 through 15 of
gestation. The increase was based on the percentage of affected
litters as well as the resorptions per litter. Aspirin demonstrated
the same response. The incidences of visceral anaomalies were not
increased in either the aspririn or thiodicarb groups when compared to
controls. Skeletal variations were similar among all groups except for
an increased incidence of bilobed thoracic vertebral centra and poorly
ossified sternebrae (on a percentage of live-foetuses-per-litter
basis) in the 100 mg/kg b.w. group administered thiodicarb throughout
gestation. This effect was suggestive of delayed maturity in foetuses
derived from dams which demonstrated significant maternal toxicity
(i.e. decreased weight gain and decreased foetal size). This effect is
also known to occur spontaneously (at similar frequencies) in other
rat strains. The aspirin group exhibited several skeletal variations
(e.g. split/missing vertebral centra, extra vertebrae, extra ribs and
fused/wavy ribs), demonstrating the sensitivity of the study.
Thiodicarb was not teratogenic at any dose administered but it was
maternally toxic at 100 mg/kg b.w. (Woodside et al., 1979b).
Special studies on carcinogenicity (See also "Long-term studies")
Mouse
Groups of Charles-River COBS CD-1 mice (80/sex/group) were
administered thiodicarb (analytical grade, 99+% purity) in the diet
for 24 months at dosage levels of 0, 0, 1.0, 3, or 10 mg/kg b.w./day.
Animals were 42 days old at the start of the study. The mice were
examined daily for mortality and adverse physical/behavioural
condition. Routine evaluation of food consumption and body weights
were performed. At the conclusion of the study, or at death, organ
weights and gross and microscopic examination of tissues and organs of
all animals were performed. All were anesthetized with methyoxyflurane
and killed by cervical dislocation.
Mortality in high-dose mice was increased over controls in males
during the last 2 months of the study and in females during months 17
and 18.
Body weights and food consumption were not affected by treatment.
There were no significant differences among treatment and control
groups for the incidence of neoplasms or the identification of non-
neoplastic lesions occurring in dead animals, or interim- and final-
sacrifice animals. However, the overall incidence among control and
treatment groups of hepatocellular neoplasms was much greater than
reported in the literature for this strain of mouse (Table 1).
Table 1. Hepatocellular neoplasms in mice treated with thiodicarb
and in 2 control groups
Dose (mg/kg b.w.)
0 0 1 3 10
Males
Adenoma 18/80 19/76 22/78 24/78 24/80
Carcinoma 37/80 25/76 29/78 32/78 26/80
Females
Adenoma 2/80 5/77 2/78 2/76 1/75
Carcinoma 1/80 3/77 3/78 1/76 3/75
Furthermore, there was no decrease in time-to-tumour for the
observation of hepatocellular neoplasms (Table 2).
Table 2. Time-to-tumour (hepatocellular neoplasms) for male mice
treated with thiodicarb and for 2 control groups
Dose (mg/kg b.w.)
0 0 1 3 10
Animals which
died or were 6/53 10/49 12/51 22/48 13/54
moribund
Animals at 18-
month interim 16/20 15/20 16/20 15/20 13/20
sacrifice
Animals surviving
24 months 4/7 6/8 6/7 4/10 4/6
(terminal kill)
The other most frequently occurring neoplasm was alveologenic
tumour of the lungs. As with hepatocellular neoplasms, there was no
compound-related response within the incidence of this tumour type
(Table 3).
Table 3. Alveologenic tumours in mice treated with thiodicarb and
in 2 control groups
Dose (mg/kg b.w.)
0 0 1 3 10
Males
Alveologenic
tumours 23/80 23/76 31/78 15/78 22/80
Carcinoma 1/80 0/76 0/78 1/78 0/80
Females
Alveologenic
tumours 19/80 13/78 19/78 17/77 13/75
Carcinoma 2/80 0/78 0/78 0/77 0/75
The principle non-neoplastic lesions observed in all control and
treated groups included: lymphocytic infiltration or hyperplasia of
numerous tissues, generalized amyloidosis, hyaline degeneration of
sternal cartilage, haemosiderosis of various organs, subacute
sialoadenitis, glomerulosclerosis, and renal cystic tubular dilation.
These effects were not compound-related. Thiodicarb was not
carcinogenic to CD-1 mice at dietary doses up to and including
10 mg/kg b.w. (Woodside et al., 1980a).
Special studies on mutagenicity
Thiodicarb was evaluated in a series of mutagenicity assays
including the Ames, dominant-lethal, micronucleus, reverse-mutation,
mitotic-crossing-over, and DNA-damage test methods. It was negative
for mutagenic potential in all tests except for a positive response in
the mitotic gene conversion assay using Sacharomyces cerevisiae.
(See Table 4 for details.)
Table 4. Results of mutagenicity assays on thiodicarb
Test Test Concentration Purity Results Reference
System Object of thiodicarb
Ames test S. typhimurium 1, 10, 100, Unknown Negative Jagannath &
* TA98 500, and Brusick, 1978
TA100 1000 µg/plate
TA1535
TA1537
TA1538
Micronucleus Mouse, bone 5 and 10 Technical Negative Naismith &
test marrow mg/kg grade (1) Matthews,
1979a
Dominant Rat 0, 0.5, 1, Analytical Negative Woodside
lethal 3 & grade (2) et al., 1979c
10 mg/kg (99+%)
Reverse S. cerevisiae 2.5, 6.2, Technical Negative Naismith &
mutation 25, 62, & grade (3) Matthews,
250 µg/ml 1979b
Mitotic S. cerevisiae 2.5, 6.2, Technical Negative Naismith &
crossing 25, 62, & grade (3) Matthews,
over 250 µg/ml 1979e
Mitotic S. cerevisiae 2.5, 6.2, Technical (4) Naismith &
gene 25, 62, & grade Matthews,
conversion 250 µg/ml 1979c
Primary E. coli 0.001, 0.01, Technical Negative Naismith &
DNA W3110 0.1, 1, and grade (5) Matthews,
damage* p3478 10 mg/ml 1979d
Chromosomal Chinese hamster 10, 15, 20, Technical Negative Ivett &
aberrations ovary (CHO) 30 µg/ml 91.48% Brusick,
cells* w/o activ. 1985
10, 20, 30,
40 µg/ml
w/activation
Unscheduled Rat primary 0.5, 1.0, 2.5, Technical Negative Cifone &
DNA synthesis hepatocytes 5.0, 10.0, 91.48% Brusick,
25.0, 50.0, 1985a
100.0, 250.0
µg/ml
Table 4. (Con't)
Test Test Concentration Purity Results Reference
System Object of thiodicarb
Mouse lymphoma L5178Y (TK+/-) 5, 8, 10, Technical Positive Cifone &
forward mutation & 12 µg/ml 91.48% (6) Brusick,
assay* 1985b
* With and without metabolic activation
(1) The positive control (TEM) gave the expected response at 0.5 ml/kg.
(2) The positive control (TEM) gave the expected response at 0.25 mg/kg.
(3) The positive control (NQO) gave the expected response at 10-6 M.
(4) Increase in the heteroallelic trp 5-12/trp 5-27 diploid strain D7 at
25 µg/ml.
(5) The positive controls (ethylmethane sulfonate and diethylnitrosamine)
gave the expected response.
(6) The test material induced repeatable increases in the mutant frequency,
with and without metabolic activation.
Special studies on neurotoxicity
Although the Meeting considers the testing of methyl carbamates
for potential neurotoxicity to be inappropriate (Annex 1, FAO/WHO,
1985b, para. 2.8), the manufacturer conducted 2 separate studies, both
of which were negative.
Chicken
In a preliminary acute oral toxicity study using 10- to 14-month-
old White Leghorn hens, the LD50 for thiodicarb was calculated to be
582 mg/kg b.w. When atropine sulfate (15 mg/kg b.w.) was administered
15 minutes prior to dosing only 2/5 hens died after 5 days at a dose
of 830 mg/kg b.w. (Schwartz & Stevens, 1978).
Hens protected with 15 mg/kg b.w. atropine sulfate were
administered single doses of 660 mg/kg b.w. thiodicarb via oral
intubation. Vehicle (corn oil) and positive (TOCP) control groups were
also employed. A dose of 750 mg/kg b.w. TOCP in corn oil was used.
There were 10 hens in each control group and 40 in the thiodicarb
group. Hens were observed daily for pharmacologic and toxicologic
effects, including neurologic evaluation of leg weakness, gait, and
walking ability. On day 21 all positive controls and 10 test-group
birds were necropsied, perfused with 10% neutral buffered formalin,
and examined histologically. On day 22 eight new positive controls
were added to the study and the existing 30 treated birds were
re-dosed a second time with 660 mg/kg b.w. thiodicarb.
The vehicle controls provided no evidence of toxicity, while the
positive control group displayed clinical and microscopic symptoms of
delayed neuro-toxicity (increased number of swollen axons per cervical
spinal cord cross-section). Although some signs of neurological
impairment were initially displayed in the thiodicarb group, these
were not evident after 4 days in study and were not confirmed upon
histologic examination (Becci et al., 1979).
Due to the marginal response in thiodicarb-treated birds, the
entire study was repeated using 30 White Leghorn hens (8-12 months
old) dosed orally with 660 mg/kg b.w. thiodicarb (analytical grade).
There were 10 hens in each of the negative (corn oil) and positive
(TOTP) control groups. Thirty minutes prior to dosing, each group
received 25 mg/kg b.w. atropine sulfate via gavage. A dose of
1200 mg/kg b.w. TOTP was employed in the positive control group.
A total of 13/30 thiodicarb hens died on test, with no mortality
in either control group. Thiodicarb and the negative control group
provided no evidence of delayed neurotoxicity. In the TOTP group, 9/10
birds demonstrated lesions in the sciatic nerve and spinal cord,
consisting of axonal degeneration, necrosis, and demyelination (Myer
et al., 1980).
Special studies on skin and eye irritation
Rabbit
Thiodicarb is not irritating to the skin. When applied to the
shaved backs of rabbits, thiodicarb produced only slight erythema in a
few cases (Mallory et al., 1982i; Wentz & Wolfe, 1979).
Thiodicarb was applied to the shaved, intact skin of rabbits, 5
times a week for 3 consecutive weeks, at 4 dosage levels up to
4 g/kg/day. Mortality, behaviour, food consumption, body weight, and
gross and microscopic histopathology were unaffected by treatment.
Localized erythema and edema occurred in all treated groups.
Thiodicarb treatment was reported to produce a dose-related macrocytic
anaemia (Conroy et al., 1979b; Gallo & Stevens, 1980).
In a second dermal toxicity study in rabbits, using
2000 mg/kg/day as the highest dosage level, all criteria were
unaffected by treatment. Groups of 10 male and 10 female New Zealand
White rabbits were treated dermally with thiodicarb 5 days per week
for 3 consecutive weeks at dosage levels of 0, 250, 500, 1000, or
2000 mg/kg (2 control groups were utilized in this study). Thiodicarb
was administered to both intact and abraded dermal surfaces. There
were no effects in the study attributable to thiodicarb. The
occurrence of macrocytic anaemia, observed in the first study, was not
confirmed (Schardein, 1982).
Thiodicarb applied to the conjunctival sacs of rabbits did not
damage the cornea, but resulted in temporary eye irritation (Cameron &
Wolfe, 1979; Mallory et al., 1982a). In monkeys, thiodicarb caused
no eye irritation or damage (Weatherholtz, 1982). Methomyl was mildly
irritating to the skin of guinea-pigs and was a mild irritant to the
eye (Kaplan & Sherman, 1977). Methomyl oxime was not irritating to the
skin and was moderately irritating to the eye (Myers et al., 1984).
Special studies on dermal sensitization
The potential for thiodicarb to produce skin sensitization was
tested in guinea-pigs and human subjects. Guinea-pigs, given multiple
intradermal injections of thiodicarb in a modified Landsteiner
procedure, showed minimal sensitization reactions to a challenge
injection two weeks later (Conroy et al., 1979a & c). Repeated
application (via a modified Buehler method) of thiodicarb to the
shaved backs of guinea-pigs did not cause allergic sensitization
(Field, 1979b). In a similar test with human subjects, thiodicarb
produced no sensitization by repeated contact (Kamphake et al.,
1980). Methomyl was not a sensitizer when administered to guinea-pigs
(Kaplan & Sherman, 1977).
Acute toxicity
The acute toxicity of thiodicarb and its metabolites in several
animal species is summarized in Table 5.
Short-term studies
Mouse
Groups of Charles River CD-1 mice (5 males and 5 females per
group) were administered thiodicarb in the diet for 7 days at dietary
levels of 0, 15, 45, or 90 mg/kg b.w./day. Growth and mortality were
unaffected. In male mice, at the two highest dosage levels, an
increased absolute kidney weight was observed (Homan et al., 1977).
Groups of ICR-JCL (SPF) mice (10 per sex per group) were fed
thiodicarb (92.5% purity) in the diet for 4 weeks at dosage levels of
0, 30, 100, or 300 ppm (equal to 0, 4.2, 13.7, and 40.8 mg/kg b.w./day
for males and 0, 3.8, 12.3, and 36.3 mg/kg b.w./day for females).
There was no mortality during the study. Body weight, food consumption
and organ weights (brain, heart, liver, spleen, kidney, testes, and
ovaries) were unaffected by treatment (Yoshida et al. 1983a).
Table 5. Results of acute toxicity assays of thiodicarb and its metabolitea
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Thiodicarb Mouse both Oral corn oil 226 Myers et al., 1977b
(148-346)
Rat M Oral corn oil 74.8-129 Hallory et al.,1982b;1982e;1982g
(52.9-186) Myers et al.,1982b;1982c;1982d;1982e
F Oral corn oil 50-136 Mallory et al., 1982b;1982e;1982g
(34.9-192.1) Myers et al.,1982b;1982c;1982d;1982e
M Oral 0.25% methyl 46.5-83 Mallory et al., 1982c;1982f;1982h
cellulose or (33.4-108) Myers et al.,1982b;1982c;1982c;1982d
water
F Oral 0.25% methyl 39.1-55 Mallory et al., 1982e;1982f;1982h
cellulose or (29.4-65.5) Myers et al., 1982b;1982c;1982d
water
both Oral corn oil 74.6-215 Mallory et al., 1982b;1982e;1982g
(59.6-268.8) Myers et al., 1982b;1982c;1982d;1982e
both Oral 0.25% methyl 49.4-97 Mallory et al., 1982c;1982f;1982h
cellulose or (39.4-138.2) Myers et al., 1982b;1982c;1982d
water
Guinea- M Oral corn oil 160 Myers et al., 1977c
pig (94.3-271)
Rabbit both Oral capsule > 400 Myers et al., 1979a
both Oral corn oil 556 Myers et al., 1979a
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Chicken F Oral corn oil 582-660 Myer et al., 1980
Schwartz & Stevens, 1978
Monkey both Oral capsule 467.2 Weatherholtz et al., 1983
(329.6-662.1)
Rat M Dermal 2540 Field 1979a
4 hr. (1120-5750) Koehler & Dorman 1979
intact Myers et al., 1975;1979a
skin
Rabbit both Dermal > 6310 Lemen et al., 1979;
24 hr. Mallory et al., 1982d;
abraded Myers et al., 1978b;1979a
& intact
skin
Rat both I.P. 23 (15.9-33.1) Matthews 1979b
Mouse both I.P. 34 (21.9-52.7) Matthews 1979a
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Rat Inhalation > 2.0 Myers et al., 1975
148 min.
aerosol in
DMSO
Inhalation > 0.2 Myers et al., 1979a
360 min.
aerosol in
DMSO
both Inhalation 0.1155-0.220 Dickey et al., 1979
240 min. (0.1094- Myers et al., 1977a
dust 0.320)
Inhalation > 2.4 Myers et al., 1978a
60 min.
dust
Aqueous 1.04 Myers et al., 1982a
aerosol
Methomyl Rat M Oral corn oil 14.1-47.6 Kaplan & Sherman 1977;
peanut oil (8.0-72.7) Myers et al., 1976a;1981
PEG 400
F Oral corn oil 12.3-48.5 Kaplan & Sherman 1977;
peanut oil (7.6-70.2) Myers et al., 1979b;I981
Weil & Carpenter 1978
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Guinea- M Oral acetone 15*** Kaplan & Sherman 1977
pig peanut oil
Japanese Oral water;CMC 34 Kaplan & Sherman 1977
Quail
Dog M capsule 30*** Kaplan & Sherman 1977
Monkey both aqueous 40*** Kaplan & Sherman 1977
(Rhesus)
Chicken F Oral acetone/ 28 Kaplan & Sherman 1977
water
Hydroxymethyl Rat M Oral corn oil 200 Myers et al., 1978c
methomyl (123-326)
Oral corn oil 238 Myers et al., 1979b
(156-363)
Methomyl Rat M Oral corn oil 453 Myers et al., 1978a
sulfoxide (296-692)
Oral corn oil 476 Myers et al., 1979b
(311-727)
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Methomyl Rat M Oral corn oil 794 Myers et al., 1976b
oxide (486-1300)
Oral corn oil 1000 Myers et al., 1979b
(636-1572)
both Oral water 350 Myers et al., 1984
(311-394)
Acetonitrile Rat M Oral water 246 Pozzani et al., 1955
(160-378)
F Oral water 234 Pozzani et al., 1955
(203-270)
Oral water 38/0 Smyth 1947
Methomyl Rat M Dermal water > 200 X Kaplan & Sherman 1977
intact & 5 days
abraded
skin 6 h
Rabbit M Dermal water > 5000 Kaplan & Sherman 1977
intact
skin 24 h
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
M Dermal water 800-951 Myers et al., 1981
intact
& abraded
skin 24 h
F Dermal water 566-1130 Myers et al., 1981
intact
& abraded
skin 24 h
Methomyl Rat M Dermal corn oil > 1000 Myers et al., 1976b
oxime intact
skin 4 h
Dermal water > 2000 Meyers et al., 1984
intact
skin 24 h
Acetonitrile Rat i.p. 0.95 ml/kg Rogers 1959
(0.58-1.54)
Rat i.v. 1.68 ml/kg Rogers 1959
(0.08-3.21)
Rabbit Dermal 1.26 ml/kg Carpenter et al., 1958
Methomyl Rat M Inhaltion water 76 mg/l Kaplan & Sherman 1977
of aqueous
aerosol
4 h
Table 5. (Con't)
Solvent LD50 LC50 Reference
Chemical Species Sex Route (vehicle) (mg/kg b.w.)* (mg/L)**
Methomyl Rat Inhalation water > 0.82 Myers et al., 1976b
oxime of mg/1
aqueous
aerosol
4 h
* 95% confidence limits (the widest range from each set of studies) are given in parentheses.
** Because particles of thiodicarb are not of respirable size, the material was ground to a dust
for these tests.
*** Minimum lethal dose.
Groups of ICR-JCL (SPF) mice, (28 per sex per group) were fed
thiodicarb (92.5% purity) in the diet for 13 weeks at dosage levels of
0, 30, 150, or 600 ppm (equal to 0, 3.8, 18.9, and 76.5 mg/kg b.w. for
males and 0, 4.3, 21.5, and 85.0 mg/kg b.w. for females). Mortality,
food consumption, body weight, water consumption, urinalysis,
haematology, and clinical chemistry were determined. Cholinesterase
levels were determined in plasma, red blood cells (RBC) and brain at
study termination, as were organ weights, gross pathology, and
histopathology. Brain ChE was stated to be depressed in 150-
and 600-ppm females and in 600-ppm males. Also reported were
significantly-increased relative liver weights in high-dose females.
There were no other compound-related effects reported. However,
individual animal data, gross pathology, or histopathology were not
submitted. Therefore, a no-observed-effect level cannot be assessed
from these data as presented to the 1985 Joint Meeting (Yoshida
et al., 1983b).
Rat
Groups of Harlan-Wistar rats (5 per sex per group) were fed
thiodicarb in the diet for 7 days at dosages of 0, 5, 25, or 100 mg/kg
b.w./day. Limited effects were noted on body weight and food
consumption, which were decreased in high-dose males. Relative kidney
weights were also reportedly increased in all treated males (Woodside
et al., 1975).
Groups of Wistar rats (5 per sex per group) were administered
thiodicarb in the diet at dosages of 0, 0, 18.5, 48.6, or 128 mg/kg
b.w./day for 7 days. Observations were made on mortality, food
consumption, body weight, and gross liver and kidney changes. Body
weights were reduced in all treated groups compared to controls.
Relative liver weights and absolute kidney weights were reduced in
high-dose rats (Woodside et al., 1978).
In a preliminary 13-week study 4 groups of Fischer 344 rats
(5 per sex per group) were administered thiodicarb in the diet at
dosage levels of 0, 5, 15, or 45 mg/kg b.w./day. One additional group
per sex received 90 mg/kg b.w./day for 80 days. Observations included:
mortality, food and water consumption, organ weights, and ChE activity
(RBC, plasma and brain). Blood samples for ChE measurements were
obtained via cardiac puncture. At necropsy, the brain was removed,
weighed, and submitted for ChE assay. Relative liver, adrenal, and
spleen weights were increased in all treated males, and relative
spleen weights were increased in mid- and high-dose females. Body
weights were reduced throughout the study in all treated males. ChE
activity was unaffected at any dose level, except for increased RBC
ChE activity in high-dose males and females (Woodside et al.,
1979a).
Groups of Fischer 344 rats (10 males and 10 females per group)
were administered thiodicarb (99% purity) in the diet at dosage levels
of 0, 0, 1.0, 3.0, 10 or 30 mg/kg b.w./day for 13 weeks. Observations
included mortality, clinical observations, food and water consumption,
body weight, organ weights, haematology, clinical chemistry,
urinalysis, ChE activity (plasma, RBC and brain-at-germination), and
gross and microscopic analysis. At both 10 and 30 mg/kg b.w./day, body
weight and water consumption decreased significantly among females,
with slight increases in males at the same dose levels. At 30 mg/kg
b.w., relative spleen weights were increased significantly in males,
while relative kidney weights were increased in high-dose females.
Urine volume was increased in males and decreased in females at the
high-dose level. Specific gravity of urine was also decreased in high-
dose females. Other clinical parameters were unaffected by treatment
except for decreased mean haemoglobin concentrations in high-dose
males. ChE activities were comparable among all groups except for a
significantly-increased RBC ChE in high-dose males. Gross and
microscopic analyses were unremarkable and typical of changes
associated with 4-month-old Fischer 344 rats. A NOEL was demonstrated
at 3 mg/kg b.w. (Homan et al., 1978b).
Dog
Groups of 9-month-old Beagle dogs (4 per sex per group) were fed
thiodicarb in the diet at targeted dosages of 0, 15, 45, or 90 mg/kg
b.w./day for 13 weeks. However, due to mortality at the highest dose,
the level was reduced to 76 and 86 mg/kg b.w. for females and males,
respectively. Animals were examined routinely for physical/behavioural
changes, food and water consumption, body-weight changes, haematologic
and blood chemistry parameters, and urinalysis. Organ weights, gross
necropsy and histologic effects were examined at terminal sacrifice.
ChE activities (plasma and RBC) were determined pre- treatment and on
days 28, 63, 79, and 93 of the study. Brain ChE was determined at
sacrifice.
Two females died in the high-dose group, and dose-related
cholinergic symptoms were observed in both sexes in the mid- and high-
dose groups. Symptoms included anorexia, vomiting, and loose stools.
However, ChE activity was unaffected throughout the study. Food
consumption decreased in high-dose females. Water consumption
increased in both sexes in the high-dose groups. Body weight was
decreased in females at the mid- and high-dose levels but there was no
effect on males. Urine volume was increased in mid- and high-dose
males. Decreases in RBC count, packed cell volume, and haemoglobin
were noted in both sexes in the mid- and high-dose groups, while
reticulocyte counts were increased in both sexes of the high-dose
groups only. These changes, together with bone-marrow hyperplasia,
decreased mean-corpuscular haemoglobin concentrations, and congested
spleen in high-dose groups, are suggestive of compensatory
haematopoiesis and anaemia. Relative liver weights and serum GPT
levels were increased in both sexes at the high dose. However, males
at the high dose demonstrated cloudy swelling, vacuolation and
degenerative liver effects. All treated groups produced evidence of
focal and diffuse inflammation of the liver. A clear NOEL was not
demonstrated in this study (Homan, et al., 1978a).
Groups of Beagle dogs, 6-7 months old (6 per sex per dose) were
administered thiodicarb in the diet at dosage levels of 0, 5, 15, or
45 mg/kg b.w./ day for 26 weeks. Animals were observed routinely for
survival, clinical appearance, food consumption, body-weight change,
ChE activity, haematologic and clinical chemistry parameters, and
urinalysis. Organ-weight changes and gross and microscopic pathologic
examinations were performed at terminal sacrifice. Cholinesterase
activity was twice determined pretreatment and once each on fasted and
non-fasted samples during weeks 8, 17, and 26. Brain ChE was
determined at sacrifice.
Thiodicarb did not affect body weight, food consumption,
urinalysis, organ weights, or gross or microscopic pathological
examination of tissues. Among the male dogs, there was a dose-related
increase in the combined incidence of soft stools, mucoid stools, and
diarrhoea. These were not evident in females. Treatment-related
changes in haematology included significantly-decreased haematocrit,
haemoglobin, and RBC levels in high-dose males and females. Dogs
receiving the highest dose had significantly-elevated levels of serum
GPT, plasma ChE, and RBC ChE as well as significantly-decreased levels
of calcium, total protein and globulin, with correspondingly-increased
albumin-to-globulin ratio. There were no treatment-related effects
reported at 15 mg/kg b.w./day (Wolfe 1981).
Long-term study
Rat
Six groups of Fischer 344 rats (120 rats per sex per dose) were
administered thiodicarb (analytical grade, 99+% purity) in the diet at
dosage levels of 0, 0, 0.5, 1, 3, or 10 mg/kg b.w. for 24 months.
Animals were 50 days of age when dosing began, and housed 3 per cage
for males and 5 per cage for females. Rats were examined daily for
mortality and adverse physical or behavioural changes. Food and water
consumption and body-weight changes were determined routinely. Interim
sacrifices were performed at 6, 12, and 19 months, utilizing 10 to 20
rats per sex. Haematology, clinical chemistry, and urinalysis
determinations were made prior to each scheduled sacrifice. Organ
weights and gross and microscopic evaluations were also performed at
each sacrifice and on dead or moribund animals (excluding organ
weights). Limited ophthalmological examinations were also performed.
Mortality was increased in high-dose males, but not females, from
the 15th to the 21st months. At termination of the study, mortality in
control males exceeded that in the high-dose males. Food consumption
was unaffected in treated rats, although sporadic decreases were
occasionally observed. Body weights were depressed in high-dose males
from the 15th to 20th months and in high-dose females from the 3rd to
the 21st months. An outbreak of sialodacry-oadenitis virus was
discovered at about the 18th month, which had contributed to a
generalized debilitated condition and to weight loss in all animals
for approximately 2-3 weeks. Haematological parameters were
unremarkable except for reduced erythrocyte counts, hematocrits and
haemaglobin levels in males fed > 1 mg/kg thiodicarb at the 12th
month, but not at 19 or 24 months. ChE activity (plasma, RBC, and
brain), determined after 48 hours on the control diet, was unaffected.
However, the time delay between compound administration and ChE
measurement is considered excessive for a carbamate and therefore this
result is not meaningful. The only change in ChE activity, consistent
with other thiodicarb-feeding studies, was a significant increase in
RBC ChE at 24 months in high-dose males. Clinical chemistry and
urinalysis determinations were not affected by treatment except for
transient reduction in urine volume at 12 months in females given
> 3 mg/kg. Although sporadic differences were observed at various
sacrifice intervals, there were no dose-related effects on absolute or
relative organ-weights.
There were no significant pathologic changes attributable to
thiodicarb at 6, 12, or 19 months, and no carcinogenic response at any
dose throughout the study. High-dose males presented evidence at 24
months of an increased incidence of pituitary cysts, while high-dose
females demonstrated haemosiderosis of the mediastinal lymph nodes.
Hepatocellular hyperplasia and interstitial prostatitis were increased
in treated males, although not significantly at the high dose; their
incidence was not dose-related, and there was not a significant
increase in liver neoplasms. High-dose males had an increased, but not
significant, incidence of epithelial hyperplasia and epithelial
thymomas. Mononuclear cell leukemia and interstitial cell adenomas
(in males) occurred spontaneously in all groups, in general agreement
with this strain of rat.
A NOEL of 3 mg/kg b.w. was established with no evidence of
oncogenic potential at doses up to and including 10 mg/kg b.w.
(Woodside et al., 1980b).
COMMENTS
Thiodicarb is rapidly absorbed, metabolized, and excreted, and
has not been demonstrated to accumulate in animal tissues. It is
degraded to methomyl, which is converted to the "oxime" (methyl
hydroxythioacetimidate) and ultimately to acetonitrile, carbon
dioxide, acetic acid, and acetamide. The ultimate metabolic fate in
animals depends on the isomeric configuration of methmyl (SYN or
ANTI).
The acute oral toxicity (LD50) in rats is 66-120 mg/kg b.w.,
depending on the vehicle used. In monkeys an oral LD50 is 467 mg/kg
b.w.
Thiodicarb causes reversible cholinesterase inhibition. Long-term
feeding and oncogenicity studies in rats and mice have revealed no
oncogenic potential in either species, and a NOEL of 3 mg/kg b.w. for
non-oncogenic effects was determined. No adverse reproduction or
teratogenic effects in rats or mice have been demonstrated.
Thiodicarb was not mutagenic in a wide variety of assays.
However, it was positive in the mitotic gene conversion assay using
Saccharomyces cerevisiae.
In two short-term feeding studies in dogs, thiodicarb
demonstrated adverse liver effects in 90 days at doses > 15 mg/kg
b.w., while in a 6-month study at doses up to 45 mg/kg b.w. no adverse
effects were observed at 15 mg/kg b.w. Considering the conflicting
information concerning liver effects in dogs, the Meeting required the
submission of the results from an ongoing 1-year dog study by 1987.
Therefore, a temporary ADI was allocated.
TOXICOLOGICAL EVALUATION
LEVEL CAUSING NO TOXICOLOGICAL EFFECT
Rat: 60 ppm in the diet, equivalent to 3 mg/kg b.w.
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.01 mg/kg b.w.
FURTHER WORK OR INFORMATION REQUIRED (by 1987)
l. Submission of ongoing 1-year dog feeding study known to be in
progress.
2. Information on possible effect on maternal body-weight gain
demonstrated in a rat teratology study.
DESIRED
1. Observations in man, including monitoring of acetamide in the
urine.
REFERENCES
Andrawes, N.R., College, P.R. & Bailey, R.H. Short-term fate of orally
(1977) administered UC 51762 in the rat. File No. 23509.
Unpublished report from Union Carbide Agricultural Products
Company, Inc. Submitted to WHO by the Union Carbide
Agricultural Products Co., Inc.
Andrawes, N.R. & Bailey, R.H. Metabolism of acetyl-l-l4C-LARVIN(R) in
(1979a) the rat. File No. 26925. Unpublished report from Union
Carbide Agricultural Products Company. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Andrawes, N.R. & Bailey, R.H. Metabolism of acetyl-1-l4C-methomyl in
(1979b) the rat. File No. 26945. Unpublished report from Union
Carbide Agricultural Products Company. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Andrawes, N.R. & Bailey, R.H. Fate of acetyl-1-14C-thiodicarb in laying
(1980) chickens under continuous feeding conditions. File No.
26414. Unpublished report from Union Carbide Agricultural
Products Company. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Andrawes, N.R. Acetonitrile metabolism in the rat. File No. 32291.
(1983) Unpublished report from Union Carbide Agricultural Products
Company, Inc. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Becci, P.J., Schwartz, C.S., & Parent, R.A. Evaluation of UC 51762 No.
(1979) 40-488 as a potential delayed neurotoxic agent following
oral administration to hens protected by atropine sulfate.
Report No. 6065. Unpublished report from Food and Drug
Research Laboratories. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Cameron, J.T. & Wolfe, G.W. Acute eye irritation study in rabbits.
(1979) Project No. 400-616. Unpublished report from Hazleton
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Carpenter, C.P., Pozzani, U.C., Weil, U.C., Palm, P.E. & Nair, J.H.
(1958) The toxicity of acetonitrile. Part II. Special report No.
21-100. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company Inc.
Cifone, M.A. & Brusick, D.J. Evaluation of thiodicarb in the rat
(1985a) primary hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. 20991 from Litton Bionetics, Inc.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Cifone, M.A. & Brusick, D.J. Mutagenicity of thiodicarb in a mouse
(1985b) lymphoma mutation assay. Unpublished report No. 20989 from
Litton Bionetics, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Conroy, W.J., DePass, L.R., Homan, E.R., Weil, C.S., Geary, D.L. &
(1979a) Frank, F.R. Dermal sensitization potential in guinea pig.
Special report No. 42-61. Unpublished report from the Mellon
Institute. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Conroy, W.J., DePass, L.R., Homan, E.R., Weil, C.S., Geary, D.L., &
(1979b) Frank, F.R. UC 51762, 16-dose rabbit dermal study. Special
report No. 42-52. Unpublished report from Mellon Insititute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Conroy, W.J., DePass, L.R., Homan, E.R., Weil, C.S., & Webb, G.A.
(1979c) Dermal sensitization potential in the guinea pig. Special
report No. 42-26. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Dangler, T.L. & Rodwell, D.E. Pilot teratology study in mice. Report
(1979) No. 369-030. Unpublished report from International Research
and Development Corporation. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Dickey, C.L., Nachreiner, D.J., DePass, L.R., Gad, S.C., Weil, C.S.,
(1979) Geary, D.L. & Frank, F.R. Acute and 9-day dust inhalation
study on rats with UC 51762 technical. Special Report No.
42-63. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Feung, C.S., College, P.R. & Chancey, E.L. Studies on the disposition
(1980) of 14C-thiodicarb in lactating cows. File No. 27350.
Unpublished report from Union Carbide Agricultural Products
Company, Inc. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Field, W.E. Acute dermal toxicity in rats. Project No. CDC-UC-012-79.
(1979a) Unpublished report from CDC Research, Inc. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Field, W.E. Skin sensitization in guinea pigs. Project No. CDC-UC-003-
(1979b) 79. Unpublished report from CDC Research, Inc. Submitted to
WHO by Union Carbide Agricultural Products Company, Inc.
Fleischman, R.W., Baker, J.R., Hagopian, M., Wade, G.G., Hayden, D.W.,
(1980) Smith, E.R., Weisburger, J.H. & Weisburger, E.K.
Carcinogenesis bioassay of acetamide, hexanamide, adipamide,
urea and phi-tolylurea in mice and rats. J. of Environ.
Pathol. and Toxicol., 3, 149-170.
Gallo, M.A. & Stevens, K.R. Evaluation of LARVIN(R) (UC 51762) in 21
(1980) day dermal toxicity study in rabbits. Project No. 02413-090.
Unpublished report from Foster D. Snell. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Homan, E.R., Weil, C.S., & Cox, E.F. Seven-day feeding study of UC
(1977) 51762 in mice. Special report No. 40-7. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Homan, E.R., Fowler, E.H., Reid, J.B., & Cox, E.F. Thirteen-week oral
(1978a) toxicity study of UC 51762 in dogs. Special report No.
41-98. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Homan, E.R., Maronpot, R.R., Reid, J.B., & Cox, E.F. Thirteen-week
(1978b) oral toxicity study of UC 51762 in rats. Special report No.
41-63. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Huhtanen, K. & Dorough, H.W. Isomerization and Beckman rearrangement
(1976) reactions in the metabolism of methomyl in rats. Pesticide
Biochem. Physiol. 6, 571-583.
Humiston, C.J. & Wright, C.J. An automated method for the
(1967) determination of cholinesterase activity. Tox. Appl.
Pharmacol. 10, 476-480.
Ivett, J.L. & Brusick, D.J. Clastogenic evaluation of 91.48% a.i.
(1985) thiodicarb in an in vivo cytogenetic assay measuring
chromosomal aberration frequencies in Chinese hamster ovary
(CHO) cells. Unpublished report No. 20990 from Litton
Bionetics, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Jagannath, D.R. & Brusick, D. Mutagenic evaluation of CHF 41-43 (UC
(1978) 51762) by the Ames Salmonella/microsome plate test.
Project No. 20838. Unpublished report from Litton Bionetics,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Janes, J.M., Rodwell, D.E., & Jessup, D.C. Teratology study in mice.
(1980) No. 369-032. Unpublished report from International Research
and Development Corporation. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Kamphake, J., Ragsdale, W.E., Weber, E.M., & Carabello, F.B. Repeated
(1980) insult patch test - human volunteers. Report No. 80-0589-
70,73. Unpublished report from Hilltop Research, Inc.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Kaplan, A.M. & Sherman, H. Toxicity Studies with Methyl N-
(1977) (methylamino)-carbamoyloxy ethamidothioate. Toxicol. Appl.
Pharmacol., 40, 1-17.
Khasawinah, A.M. & College, P.R. Fate of single oral dose of 14C-
(1978) acetyl UC 51762 in a lactating cow - metabolism into natural
products. File No. 25257. Unpublished report from Union
Carbide Agricultural Products Company, Inc. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Koehler, S. & Dorman, N. Clinical safety evaluation of LARVIN(R) UC
(1979) 51762 powder and LARVIN(R) 500 flowable. Study No. 2177.
Unpublished report from Food and Drug Research Laboratories.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Lemen, J.K., Wolfe, G.W., & Voelker, R.W. Acute dermal administration
(1979) in rabbits. Project No. 400-614. Unpublished report from
Hazleton Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) technical;
(1982a) primary eye irritation test in rabbits. Report No. PH 421-
UC-004-82. Unpublished report from Pharmakon Research
Laboratories, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - analytical
(1982b) standard; acute oral toxicity study in rats (14 Day). Report
No. PH 402-UC-010-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - analytical
(1982c) standard; acute oral toxicity study in rats (14 Day). Report
No. PH 402-UC-011-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical;
(1982d) acute dermal toxicity test in rabbits. Report No. PH 422-UC-
005-82. Unpublished report from Pharmakon Research
Laboratories, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982e) lab preparation; acute oral toxicity test in rats. Report
No. PH 402-UC-008-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982f) lab preparation; acute oral toxicity test in rats. Report
No. PH 402-UC-009-82. Unpublished report from Pharmakon
Research Laboratories, Inc. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982g) pilot plant - batch 34; acute oral toxicity test in rats.
Report No. PH 402-UC-006-82. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. LARVIN(R) - technical
(1982h) pilot plant - batch 34; acute oral toxicity test in rats.
Report No. PH 402-UC-007-82. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mallory, V.T., Naismith, R.W., & Matthews, R.J. Primary dermal
(1982i) irritation study in rabbits. Report No. PH 420-UC-002-82.
Unpublished report from Pharmakon Research Laboratories,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Matthews, R.J. Acute intraperitoneal LD50 of UC 51762 in the mouse.
(1979a) Report No. PH 405-UC-001-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Matthews, R.J. Acute intraperitoneal LD50 of UC 51762 in the rat.
(1979b) Report No. PH 404-UC-001-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Mirro, E.J. & DePass, R.L. Inclusion of UC 51762 in the diet of rats
(1982) for 28 days. Report No. 45-19. Unpublished report from Union
Carbide Bushy Run Research Center. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myer, J., Dean, W.P., & Jessup, D.C. Acute delayed neurotoxicity study
(1980) in hens. Report No. 369-047. Unpublished report from
International Research and Development Corporation.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Weil, C.S., & Carpenter, C.P. Acute toxicity studies of
(1975) UC 51762 in rats and rabbits. Special report No. 38-55.
Unpublished report from Mellon Institute. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Myers, R.C., Weil, C.S., & Cox, E.F. Acute oral LD50 study UC 45650
(1976a) (S-methyl O-(methylcarbamoyl)acetothiohydroximate) in rats.
Special report No. 39-40. Unpublished report from Mellon
Institute. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Myers, R.C., Weil, C.S., & Cox, E.F. Acute toxicity study of S-methyl
(1976b) acetothiohydroximate UC 52702 in rats and rabbits. Special
report No. 39-25. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Carpenter, C.P., & Cox, E.F. Acute inhalation LD50 study
(1977a) of UC 51762 in rats. Special report No. 40-61. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., Carpenter, C.P., & Cox, E.F. Acute LD50 study of UC 51762
(1977b) in mice. Special report No. 40-6. Unpublished report from
Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., Carpenter, C.P., & Cox, E.F. Acute oral LD50 study in
(1977c) guinea pigs. Special report No. 40-142. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., Homan, E.R., Carpenter, C.P. & Cox, E.F. Miscellaneous
(1978a) toxicity studies. Special report No. 41-25. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., Homan, E.R., & Cox, E.F. Acute dermal toxicity of UC
(1978b) 51762 in rabbits. Special report No. 41-93. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., Homan, E.R., & Webb, G.A. Acute oral LD50 study of
(1978c) 1-methylthioacetaldehyde-O-(hydroxymethyl carbamoyl) oxide
(UC 58614) in rats. Special report No. 41-133. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., Homan, E.R., Weil, C.S., & Frank, F.R. UC
(1979a) 51762 technical; range-finding toxicity studies. Special
report No. 42-19. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Homan, E.R., Weil, C.S., & Frank, F.R. Miscellaneous
(1979b) toxicity studies. Special report No. 42-27. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. Methomyl technical; acute
(1981) toxicity and irritancy study. Report No. 44-75a. Unpublished
report from Union Carbide Bushy Run Research Center.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. Acute toxicity and irritancy
(1982a) studies. Report No. 45-24. Unpublished report from Union
Carbide Bushy Run Research Center. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. LARVIN(R) - analytical
(1982b) standard; acute oral toxicity study in rats. Report No.
45-152. Unpublished report from Union Carbide Bushy Run
Research Center. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. LARVIN(R) - technical lab
(1982c) preparation; acute oral toxicity study in rats. Report No.
45-151. Unpublished report from Union Carbide Bushy Run
Research Center. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., DePass, L.R., & Frank, F.R. LARVIN(R) - technical pilot
(1982d) plant - batch 34; acute oral toxicity study in rats. Report
No. 45-151. Unpublished report from Union Carbide Bushy Run
Research Center. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Myers, R.C., Weil, C.S., & Frank, F.R. LARVIN(R) - technical; acute
(1982e) oral toxicity study in rats. Report No. 45-40. Unpublished
report from Union Carbide Bushy Run Research Center.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Myers, R.C., Slesinski, R.S. & Frank, F.R. Methomyl oxime: acute
(1984) toxicity and irritancy study. Project report 47-185.
Unpublished report from Union Carbide Bushy Run Research
Center. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; micronucleus test.
(1979a) Report No. PH 309-UC-001-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; mitotic crossing
(1979b) over in Saccharomyces cerevisiae. Report No. PH302-UC-001-
79. Unpublished report from Pharmakon Research Laboratories,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; mitotic gene
(1979c) conversion in Saccharomyces cerevisiae. Report No. PH304-
UC-001-79. Unpublished report from Pharmakon Research
Laboratories, Inc. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; primary DNA
(1979d) damage. Report No. PH 305-AM-002-79. Unpublished report from
Pharmakon Research Laboratories, Inc. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Naismith, R.W. & Matthews, R.J. UC 51762 technical; reverse mutation
(1978e) in Saccharomyces cerevisiae. Report No. PH 303-UC-001-79.
Unpublished report from Pharmakon Research Laboratories,
Inc. Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Pozzani, U.C., Nair, J.R., & Carpenter, C.P. The toxicity of
(1955) acetonitrile. Special report No. 18-34. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Rogers, L.D. Intraportal injection of acetonitrile. Report No. 22-2.
(1959) Unpublished report from Mellon Institute. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Schardein, J. 21-Day dermal toxicity study in rabbits. Report No.
(1982) 369-053. Unpublished report from International Research and
Development Corporation. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Schwartz, C.S. & Stevens, K.R. Acute oral LD50 in hens. Report No.
(1978) 6064. Unpublished report from Food and Drug Research
Laboratories. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Smyth, H.F. Range-finding toxicity test of acetonitrile. Special
(1947) report No. 10-23. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Tasker, E.J., Rodwell, D.E., & Jessup, D.C. Teratology study in rats.
(1979) Report No. 369-029. Unpublished report from International
Research and Development Corporation. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Weatherholtz, W.M. LARVIN(R) - technical; fourteen-day eye irritation
(1982) study in monkeys. Project No. 400-648. Unpublished report
from Hazleton Research Laboratories. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Weatherholtz, W.M., Rendon, F., & Gluck, S.E. Dose rangefinding study
(1983) in monkeys. Project No. 400-694. Unpublished report from
Hazleton Research Laboratories. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Weil, C.S. & Carpenter, C.P. Miscellaneous toxicity studies. Special
(1978) report No. 34-88. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Weisburger, J.H., Yamamoto, R.S., Glass, R.M., & Frankel, H.H.
(1969) Prevention by arginine glutamate of the carcinogenicity of
acetamide in rats. Toxicol. Appl. Pharmacol., 14,
163-175.
Wentz, K.L. & Wolfe, G.W. Primary skin irritation study in rabbits.
(1979) Project No. 400- 617. Unpublished report from Hazleton
Research Laboratories. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Wolfe, G.W. Subchronic toxicity study in dogs. Project No. 400-626.
(1981) Unpublished report from Hazleton Research Laboratories.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Woodside, M.D., Weil, C.S., & Cox, E.F. Seven-day feeding study of UC
(1975) 51762 in rats. Special report No. 38-136. Unpublished report
from Mellon Institute. Submitted to WHO by Union Carbide
Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Reid, J.B., & Cox, E.F. Seven-day
(1978) feeding study of UC 51762 in rats. Special report No.
41-100. Unpublished report from Mellon Institute. Submitted
to WHO by Union Carbide Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1979a) UC 51762; dimethyl N,N'-(thiobis((methylimino)carbonoyloxy))
bis-(ethanimidothioate) inclusion in the diet of rats for
thirteen weeks. Special report No. 42-125. Unpublished
report from Mellon Institiute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1979b) UC 51762; rat teratology studies. Special report No. 42-48.
Unpublished report from Mellon Institute. Submitted to WHO
by Union Carbide Agricultural Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1979c) UC 51762 technical; inclusion in the diet of rats for three
generations and dominant lethal mutagenesis studies. Special
report No. 42-165. Unpublished report from Mellon Institute.
Submitted to WHO by Union Carbide Agricultural Products
Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1980a) UC 51762; chronic oncogenicity feeding study in mice.
Special report No. 43-10. Unpublished report from Mellon
Institute. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Woodside, M.D., DePass, L.R., Weil, C.S., Geary, D.L., & Frank, F.R.
(1980b) UC 51762; chronic toxicity and oncogenicity feeding study in
Fischer 344 rats. Special report No. 43-18. Unpublished
report from Mellon Institute. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
Yoshida, A., Maita, K., Saito, T., & Miyaoka, T. LARVIN(R): 4-weeks
(1983a) range-finding study in mice. Unpublished report from The
Institute of Environmental Toxicology. Submitted to WHO by
Union Carbide Agricultural Products Company, Inc.
Yoshida, A., Takahashi, K., Kosaka, T., & Miyaoka, T. Thiodicarb: 3
(1983b) month oral sub-chronic toxicity study in mice (study I).
Unpublished report from The Institute of Environmental
Toxicology. Submitted to WHO by Union Carbide Agricultural
Products Company, Inc.
Ziemke, K.A. & Rodwell, D.E. Pilot teratology study in rats. Report
(1979) No. 369-028. Unpublished report from International Research
and Development Corporation. Submitted to WHO by Union
Carbide Agricultural Products Company, Inc.
