
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
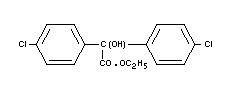
CHLOROBENZILATE
Chemical name
ethyl-2-hydroxy-2,2-di(p-chlorophenyl) acetate; ethyl
4,4'-dichlorobenzilate; ethyl 4,4'-dichlorodiphenylglycollate.
Empirical formula
C16H14O3Cl2
Structural formula
 BIOLOGICAL DATA
Biochemical aspects
Chlorobenzilate appears to undergo extensive hydrolysis in the
body to the acid form which is the main excretion product.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 729 Horn et al., 1955
(Technical product)
Mouse Oral 4 850 Gasser, 1952a;
Gasser, 1952b
Rat Oral 702 Horn et al., 1955
(Technical product)
Rat Oral 3 100 Gasser, 1952a;
Gasser, 1952b
Rat Oral 735 Horn et al., 1955
(25% xylene emulsion)
Short-term studies
Rat. Groups of rats, each of 20 males, were fed 40 and 800 ppm
of technical chlorobenzilate in the diet for 44 or 48 weeks (Geigy,
1953-55).
The rats receiving 40 ppm grew at about the same rate as the
controls, but those receiving 800 ppm were markedly retarded as to
growth. Several animals receiving 800 ppm exhibited red or swollen
eyelids and soft faeces, and one developed diarrhoea. During the test,
2 control rats and 5 rats receiving 800 ppm died, there were no deaths
among those receiving 40 ppm.
Gross autopsy of all survivors showed no characteristic changes
attributable to feeding of the compound. The weights of the liver,
kidneys, and testes of rats receiving 40 ppm, when expressed as
percentages of body-weight, were significantly greater than those of
the controls. The organ weights of rats receiving 800 ppm were not
evaluated statistically, though the absolute weights were less than at
40 ppm.
Microscopic examination of the main organs revealed no
significant histological changes attributable to the administration of
the compound, but non-specific histological changes were noted in the
pancreas and adrenals of some rats receiving 40 ppm and in the spleen,
pancreas and adrenals of the rats receiving 800 ppm (Geigy, 1953-55).
Dog. Groups of 2 dogs, one male and one female in each group,
were given 12.8 mg and 64.1 mg/kg body-weight per day for 35 weeks.
All dogs either maintained their body-weight or made slight
weight gains; they showed normal behaviour, had good appetite, and
showed no gross signs of toxicity throughout the entire study.
Biochemical and haematological results were within normal limits.
Gross and microscopic examination of the main organs revealed no
significant pathological changes at the end of the 35-week period
(Horn et al., 1955).
Long-term studies
Rat. Groups each of 40 rats (20 males and 20 females) were fed
500 ppm of technical chlorobenzilate for 2 years, and one group of 20
males was fed 50 ppm of the compound for 2 years (Horn et al., 1955).
Only the males receiving 500 ppm showed a significantly lower
weight gain as compared to the controls. Tissues from the main organs
were examined. The histopathological findings could not be attributed
to the feeding of chlorobenzilate for 2 years. The organ weights of
liver, kidneys and spleen were not significantly different from those
of the controls. In the males receiving 50 and 500 ppm there was an
increase in the frequency of atrophied testes (Horn et al., 1955).
Comments on experimental studies reported and evaluation
The maximum no-effect level in the rat appears to be about 50
ppm, but further animal studies are required to confirm this before an
evaluation can be made.
Further work required
Metabolic studies in animals. Additional long-term studies in
rats at lower dose-levels and long-term studies in other species.
REFERENCES
Gasser, R. (1952a) Compte Rendues, IIIe Congrès international de
phytopharmacie, Paris, p. 357
Gasser, R. (1952b) Experienta, 8, 65
Geigy Chemical Corp., Yonkers, New York, 1953-55, Unpublished data
Horn, J. J., Bruce, R. B. & Paynter, O. E. (1955) J. Agric. Food
Chem., 3, 752
BIOLOGICAL DATA
Biochemical aspects
Chlorobenzilate appears to undergo extensive hydrolysis in the
body to the acid form which is the main excretion product.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 729 Horn et al., 1955
(Technical product)
Mouse Oral 4 850 Gasser, 1952a;
Gasser, 1952b
Rat Oral 702 Horn et al., 1955
(Technical product)
Rat Oral 3 100 Gasser, 1952a;
Gasser, 1952b
Rat Oral 735 Horn et al., 1955
(25% xylene emulsion)
Short-term studies
Rat. Groups of rats, each of 20 males, were fed 40 and 800 ppm
of technical chlorobenzilate in the diet for 44 or 48 weeks (Geigy,
1953-55).
The rats receiving 40 ppm grew at about the same rate as the
controls, but those receiving 800 ppm were markedly retarded as to
growth. Several animals receiving 800 ppm exhibited red or swollen
eyelids and soft faeces, and one developed diarrhoea. During the test,
2 control rats and 5 rats receiving 800 ppm died, there were no deaths
among those receiving 40 ppm.
Gross autopsy of all survivors showed no characteristic changes
attributable to feeding of the compound. The weights of the liver,
kidneys, and testes of rats receiving 40 ppm, when expressed as
percentages of body-weight, were significantly greater than those of
the controls. The organ weights of rats receiving 800 ppm were not
evaluated statistically, though the absolute weights were less than at
40 ppm.
Microscopic examination of the main organs revealed no
significant histological changes attributable to the administration of
the compound, but non-specific histological changes were noted in the
pancreas and adrenals of some rats receiving 40 ppm and in the spleen,
pancreas and adrenals of the rats receiving 800 ppm (Geigy, 1953-55).
Dog. Groups of 2 dogs, one male and one female in each group,
were given 12.8 mg and 64.1 mg/kg body-weight per day for 35 weeks.
All dogs either maintained their body-weight or made slight
weight gains; they showed normal behaviour, had good appetite, and
showed no gross signs of toxicity throughout the entire study.
Biochemical and haematological results were within normal limits.
Gross and microscopic examination of the main organs revealed no
significant pathological changes at the end of the 35-week period
(Horn et al., 1955).
Long-term studies
Rat. Groups each of 40 rats (20 males and 20 females) were fed
500 ppm of technical chlorobenzilate for 2 years, and one group of 20
males was fed 50 ppm of the compound for 2 years (Horn et al., 1955).
Only the males receiving 500 ppm showed a significantly lower
weight gain as compared to the controls. Tissues from the main organs
were examined. The histopathological findings could not be attributed
to the feeding of chlorobenzilate for 2 years. The organ weights of
liver, kidneys and spleen were not significantly different from those
of the controls. In the males receiving 50 and 500 ppm there was an
increase in the frequency of atrophied testes (Horn et al., 1955).
Comments on experimental studies reported and evaluation
The maximum no-effect level in the rat appears to be about 50
ppm, but further animal studies are required to confirm this before an
evaluation can be made.
Further work required
Metabolic studies in animals. Additional long-term studies in
rats at lower dose-levels and long-term studies in other species.
REFERENCES
Gasser, R. (1952a) Compte Rendues, IIIe Congrès international de
phytopharmacie, Paris, p. 357
Gasser, R. (1952b) Experienta, 8, 65
Geigy Chemical Corp., Yonkers, New York, 1953-55, Unpublished data
Horn, J. J., Bruce, R. B. & Paynter, O. E. (1955) J. Agric. Food
Chem., 3, 752
 BIOLOGICAL DATA
Biochemical aspects
Chlorobenzilate appears to undergo extensive hydrolysis in the
body to the acid form which is the main excretion product.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 729 Horn et al., 1955
(Technical product)
Mouse Oral 4 850 Gasser, 1952a;
Gasser, 1952b
Rat Oral 702 Horn et al., 1955
(Technical product)
Rat Oral 3 100 Gasser, 1952a;
Gasser, 1952b
Rat Oral 735 Horn et al., 1955
(25% xylene emulsion)
Short-term studies
Rat. Groups of rats, each of 20 males, were fed 40 and 800 ppm
of technical chlorobenzilate in the diet for 44 or 48 weeks (Geigy,
1953-55).
The rats receiving 40 ppm grew at about the same rate as the
controls, but those receiving 800 ppm were markedly retarded as to
growth. Several animals receiving 800 ppm exhibited red or swollen
eyelids and soft faeces, and one developed diarrhoea. During the test,
2 control rats and 5 rats receiving 800 ppm died, there were no deaths
among those receiving 40 ppm.
Gross autopsy of all survivors showed no characteristic changes
attributable to feeding of the compound. The weights of the liver,
kidneys, and testes of rats receiving 40 ppm, when expressed as
percentages of body-weight, were significantly greater than those of
the controls. The organ weights of rats receiving 800 ppm were not
evaluated statistically, though the absolute weights were less than at
40 ppm.
Microscopic examination of the main organs revealed no
significant histological changes attributable to the administration of
the compound, but non-specific histological changes were noted in the
pancreas and adrenals of some rats receiving 40 ppm and in the spleen,
pancreas and adrenals of the rats receiving 800 ppm (Geigy, 1953-55).
Dog. Groups of 2 dogs, one male and one female in each group,
were given 12.8 mg and 64.1 mg/kg body-weight per day for 35 weeks.
All dogs either maintained their body-weight or made slight
weight gains; they showed normal behaviour, had good appetite, and
showed no gross signs of toxicity throughout the entire study.
Biochemical and haematological results were within normal limits.
Gross and microscopic examination of the main organs revealed no
significant pathological changes at the end of the 35-week period
(Horn et al., 1955).
Long-term studies
Rat. Groups each of 40 rats (20 males and 20 females) were fed
500 ppm of technical chlorobenzilate for 2 years, and one group of 20
males was fed 50 ppm of the compound for 2 years (Horn et al., 1955).
Only the males receiving 500 ppm showed a significantly lower
weight gain as compared to the controls. Tissues from the main organs
were examined. The histopathological findings could not be attributed
to the feeding of chlorobenzilate for 2 years. The organ weights of
liver, kidneys and spleen were not significantly different from those
of the controls. In the males receiving 50 and 500 ppm there was an
increase in the frequency of atrophied testes (Horn et al., 1955).
Comments on experimental studies reported and evaluation
The maximum no-effect level in the rat appears to be about 50
ppm, but further animal studies are required to confirm this before an
evaluation can be made.
Further work required
Metabolic studies in animals. Additional long-term studies in
rats at lower dose-levels and long-term studies in other species.
REFERENCES
Gasser, R. (1952a) Compte Rendues, IIIe Congrès international de
phytopharmacie, Paris, p. 357
Gasser, R. (1952b) Experienta, 8, 65
Geigy Chemical Corp., Yonkers, New York, 1953-55, Unpublished data
Horn, J. J., Bruce, R. B. & Paynter, O. E. (1955) J. Agric. Food
Chem., 3, 752
BIOLOGICAL DATA
Biochemical aspects
Chlorobenzilate appears to undergo extensive hydrolysis in the
body to the acid form which is the main excretion product.
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 729 Horn et al., 1955
(Technical product)
Mouse Oral 4 850 Gasser, 1952a;
Gasser, 1952b
Rat Oral 702 Horn et al., 1955
(Technical product)
Rat Oral 3 100 Gasser, 1952a;
Gasser, 1952b
Rat Oral 735 Horn et al., 1955
(25% xylene emulsion)
Short-term studies
Rat. Groups of rats, each of 20 males, were fed 40 and 800 ppm
of technical chlorobenzilate in the diet for 44 or 48 weeks (Geigy,
1953-55).
The rats receiving 40 ppm grew at about the same rate as the
controls, but those receiving 800 ppm were markedly retarded as to
growth. Several animals receiving 800 ppm exhibited red or swollen
eyelids and soft faeces, and one developed diarrhoea. During the test,
2 control rats and 5 rats receiving 800 ppm died, there were no deaths
among those receiving 40 ppm.
Gross autopsy of all survivors showed no characteristic changes
attributable to feeding of the compound. The weights of the liver,
kidneys, and testes of rats receiving 40 ppm, when expressed as
percentages of body-weight, were significantly greater than those of
the controls. The organ weights of rats receiving 800 ppm were not
evaluated statistically, though the absolute weights were less than at
40 ppm.
Microscopic examination of the main organs revealed no
significant histological changes attributable to the administration of
the compound, but non-specific histological changes were noted in the
pancreas and adrenals of some rats receiving 40 ppm and in the spleen,
pancreas and adrenals of the rats receiving 800 ppm (Geigy, 1953-55).
Dog. Groups of 2 dogs, one male and one female in each group,
were given 12.8 mg and 64.1 mg/kg body-weight per day for 35 weeks.
All dogs either maintained their body-weight or made slight
weight gains; they showed normal behaviour, had good appetite, and
showed no gross signs of toxicity throughout the entire study.
Biochemical and haematological results were within normal limits.
Gross and microscopic examination of the main organs revealed no
significant pathological changes at the end of the 35-week period
(Horn et al., 1955).
Long-term studies
Rat. Groups each of 40 rats (20 males and 20 females) were fed
500 ppm of technical chlorobenzilate for 2 years, and one group of 20
males was fed 50 ppm of the compound for 2 years (Horn et al., 1955).
Only the males receiving 500 ppm showed a significantly lower
weight gain as compared to the controls. Tissues from the main organs
were examined. The histopathological findings could not be attributed
to the feeding of chlorobenzilate for 2 years. The organ weights of
liver, kidneys and spleen were not significantly different from those
of the controls. In the males receiving 50 and 500 ppm there was an
increase in the frequency of atrophied testes (Horn et al., 1955).
Comments on experimental studies reported and evaluation
The maximum no-effect level in the rat appears to be about 50
ppm, but further animal studies are required to confirm this before an
evaluation can be made.
Further work required
Metabolic studies in animals. Additional long-term studies in
rats at lower dose-levels and long-term studies in other species.
REFERENCES
Gasser, R. (1952a) Compte Rendues, IIIe Congrès international de
phytopharmacie, Paris, p. 357
Gasser, R. (1952b) Experienta, 8, 65
Geigy Chemical Corp., Yonkers, New York, 1953-55, Unpublished data
Horn, J. J., Bruce, R. B. & Paynter, O. E. (1955) J. Agric. Food
Chem., 3, 752
