
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
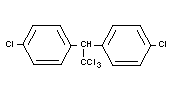
DDT
Chemical name
1,1,1-trichloro-2,2-di-(p-chlorophenyl) ethane;
trichloro-di-(4'-chlorophenyl) ethane.
Synonyms
Chlorophenothane; Zeidane, Gesarol, Neocid, Dicophane
Empirical formula
C14H9Cl5
Structural formula
 BIOLOGICAL DATA
Biochemical aspects
DDT is only slightly absorbed by the skin, but the degree of
absorption depends on the vehicle used. It may be absorbed after
inhalation.
After oral administration, most of the dose is found unchanged in
the faeces, but some absorption may occur, particularly in the
presence of lipids. The absorbed DDT is transformed into
2,2-di-(p-chlorophenyl)-1,1-dichloro-ethylene (DDE) (Pearce et al.,
1952; Mattson et al., 1953) and into 2,2-di-(p-chlorophenyl)-acetic
acid (DDA) (Spicer et al., 1947; Judah, 1949; Hayes et al., 1956).
Some unknown compounds are also found (Spicer et al., 1947; Hayes et
al., 1956, Rothe et al., 1957; Cueto et al., 1956). A certain amount
of DDT and DDE accumulates in the fat (Laug et al., 1950; Hayes et
al., 1958; Maier-Bode, 1960; Laug et al., 1951). DDA is excreted in
the urine and in a combined form in the bile. DDT is also found in the
milk (Spicer et al., 1947; Telford & Guthrie, 1945; Wilson et al.,
1946; Woodard et al., 1945; Smith et al., 1948; Biddulph et al., 1950;
Gannon & Decker, 1960). This solubility in fats, combined with the
relative chemical stability of the insecticide, explains its
cumulative nature. In the rat a concentration of 10 ppm in the diet
has been shown to interfere with the storage and metabolism of vitamin
A in the liver (Phillips, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 150-420* Negherbon, 1959
Rat Intravenous 40-60 Negherbon, 1959
Mouse Oral 150-400* Negherbon, 1959
Guinea-pig Oral 400 Negherbon, 1959
Rabbit Oral 250-400* Negherbon, 1959
Rabbit Intraperitoneal 2 100 Negherbon, 1959
Dog Intravenous Approximately Negherbon, 1959
50
Cat Oral 400-600* Negherbon, 1959
Monkey Oral >200 Negherbon, 1959
Horse Oral >300 Negherbon, 1959
Chicken Oral >1 300 Negherbon, 1959
* The LD50 dose of DDT varies within wide limits, depending on sex,
and the type of vehicle used.
The toxic effects, revealing involvement of the central nervous
system (such as hypersensitivity, excitability, generalized trembling,
convulsions, paralysis) appear within 5-10 minutes of intravenous
administration and after a latent period of some hours following oral
administration. Death occurs as a result of respiratory arrest in the
rat, rabbit, cat and monkey, and of ventricular fibrillation in the
dog. It is the consensus of the literature that the significant action
of DDT is on the nervous system; when ventricular fibrillation occurs,
it is precipitated by adrenaline released from the adrenal medulla by
stimulation of the sympathetic nervous system (Phillips & Gilman,
1946).
In rats given a single oral dose of DDT sufficient to kill about
half of them, the severity of symptoms corresponded with the
concentration of the unchanged compound in the brain (Dale et al.,
1963). Furthermore, approximately the same concentration of DDT is
found in the brain of rats killed by DDT no matter whether the dosage
is acute, subacute, or chronic (Hayes & Dale, 1964).
The fatal dose for man is difficult to establish but is generally
taken to be of the order of 500 mg/kg body-weight. A dose of the order
of 10 mg/kg body-weight can give rise in some subjects, but not in
all, to toxic symptoms (nausea, headache, sweating), and with doses of
16 mg/kg body-weight upwards, convulsions often occur (Anon., 1951).
Short-term studies
Rat. Rats, in groups of 8 males and 8 females, were gives diets
with 1, 5, 10 and 50 ppm of DDT for 23 weeks. At concentrations of 5
ppm and higher, histopathological changes in the liver were found
(Laug et al., 1950).
A series of experiments was carried out with small groups of
rats, 178 males and 104 females in all, which were given diets
containing DDT in concentrations from traces up to 5000 ppm for
periods ranging from one to 14 months. In some cases observation of
the animals continued after cessation of treatment. At levels above
400 ppm changes in growth rate were noted. Above 200 ppm liver
enlargement was seen. In the male animals with intakes of 5 ppm and
above, specific histological lesions in the liver were seen. These
lesions consisted of hypertrophy of the parenchymal cells, increased
lipid deposits, marginal localization of cytoplasmic granules and,
above all, the appearance of complex cytoplasmic inclusions of a lipid
nature, called "lipospheres". In the females these liver lesions were
only seen in diets containing 200 ppm or more. Necrotic lesions were
seen only at concentrations above 1000 ppm (Ortega et al., 1956). The
authors noted that lipospheres and other changes considered
characteristic of DDT were more prominent in male rats although
females store more of the compound and were more susceptible to
poisoning. For this and other reasons, they speculated that the
changes might be adaptive. They noted also that earlier work had
failed to demonstrate similar changes in other species (Ortega et al.,
1956). Later work in the same laboratory failed to demonstrate such
changes in monkeys treated with DDT for 7.5 years (Durham et al.,
1963).
In an electron-microscopical examination of the liver of rats fed
diets containing 5-1000 ppm of DDT for 2-18 months, the following
cytoplasmic abnormalities were found: slight proliferation of the
endoplasmic reticulum; peripheral distribution of the ribosomes and
intracytoplasmic inclusions consisting of aggregates of membranes very
rich in lipids (Ortega, 1962).
Daily oral doses of DDT 0.2 mg/kg for 10 days produced functional
changes in the conditional reflex pattern which appeared after 5-7
doses and persisted 5-7 days after the end of exposure (Andronova,
1956).
Behavioural studies have also been made on groups of rats fed
diets containing 100, 200, 400 and 600 ppm DDT. Problem solving and
speed of locomotion were unaffected by these doses of DDT. There was a
significant alteration in the patterns of locomotion in these rats and
their reactions to stress involving visual stimuli were reduced
(Khairy, 1959).
Dog. Dogs were given DDT orally. Those receiving 100 mg/kg
body-weight daily died within 7 weeks. Animals subjected to lower
doses survived and seemed normal even after 50 weeks (Draize et al.,
1944). When the experiment was continued for 3 years, the animals
receiving doses of 50 and 80 mg/kg body-weight developed jaundice and
haemorrhagic symptoms. Those receiving 10 mg/kg body-weight daily
showed no ill effects (Hayes et al., 1956; Lehman, 1952).
Dogs were given DDT by stomach-tube, in the form of a 10%
solution in peanut oil in doses ranging from 150 to 350 mg/kg
body-weight for about 90 days. Some of the animals died. The authors
noted neurological symptoms, showing involvement of the cerebellum as
revealed by histological lesions (Haymaker et al., 1946).
Three dogs were given intramuscular injections of DDT in the form
of a 10% solution in olive oil at the rate of 100 mg/kg body-weight
daily for 25 to 30 days. A control animal received 1 ml of olive oil
in the same way. The experimental animals showed a temporary loss of
weight. The kidneys were discoloured and on histological examination
showed tubular damage. At the same time, proliferation was observed in
the lymphoid tissues (lymph nodes, spleen, bone marrow) and the wall
of the small intestine, but the blood showed no leukaemic
characteristics (Gerebtzoff et al., 1950; Gerebtzoff & Philippot,
1952).
Monkey. Monkeys given DDT orally in a dose of 0.2 mg/kg
body-weight for 7-9 months developed symptoms of hepatitis. After a
year, the animals showed liver enlargement and hyperglycaemia
(Shillinger et al., 1955). These liver changes were not confirmed in
another experiment on monkeys lasting 7.5 years (Durham et al., 1963).
Long-term studies
Rat. Groups of 12 male rats were subjected for 2 years to diets
containing 100, 200, 400 and 800 ppm of DDT, in the form of a 10%
solution in corn oil. In another experiment groups each of 24 rats (12
males and 12 females) were given, during the same period, diets
containing 200, 400, 600 and 800 ppm. Also additional groups of 24
animals received 600 and 800 ppm incorporated in their feed in a dry
state. In the groups receiving 400 ppm and above, an increase in the
mortality rate was seen in relation to the dose. Apart from nervous
symptoms at doses of 400 ppm and above, typical liver lesions were
found at all concentrations. Hepatic cell tumours were seen in 4 out
of 75 animals and 11 other rats showed nodular adenomatoid hyperplasia
(Fitzhugh & Nelson, 1947).
In an experiment with rats fed for 2 years on a diet containing
10 ppm of DDT, histological liver lesions were also observed
(Fitzhugh, 1948). Groups of 80 young rats each (40 male and 40 female)
were fed 0.25, 12.5 and 25 ppm of DDT. The histological lesions of the
liver observed were always slight and, according to the authors,
non-specific, but nevertheless they were more frequent with DDT than
with aldrin or dieldrin, which were administered in the same
concentrations for comparative purposes (Treon & Cleveland, 1955).
Experiments with 25 young rats did not show any harmful effects
after daily application by stomach-tube of a dose of 10 µg of DDT
equivalent to a dietary intake of 0.1-0.15 ppm for 17 months (Klimmer,
1955).
Monkey. Twenty-four Rhesus monkeys, 12 males and 12 females,
were divided into groups and fed over periods as long as 7.5 years or
more on diets containing respectively 5, 50, 200 and 5000 ppm of DDT.
All the animals subjected to the concentration of 5000 ppm showed
convulsions, accompanied by loss of appetite and a fall in weight. At
a concentration of 200 ppm or below (the latter dose corresponding to
daily doses of 2.2-5.54 mg/kg body-weight), no harmful effects were
observed and, in particular, no histological lesions in the liver or
disturbances in the functioning of that organ, as shown by the
bromsulfthalein test (Durham et al., 1963).
Observations on man. An experimental study was carried out of
the effects on man of prolonged ingestion of small doses of DDT (in
the form of oily solutions in capsules or emulsions in milk). The
authors used for this study, 51 volunteers; 17 received a normal diet,
17 received 3.5 mg/kg body-weight and 17 received 35 mg DDT daily. The
last dose is approximately 0.5 mg/kg body-weight. Administration was
continued for as long as 18 months. The authors noted that the
accumulation of the insecticide in the fatty tissues and the urinary
excretion of its metabolite, DDA, were proportional to the dose of DDT
ingested. A state of equilibrium was reached after about a year and
the concentration of DDT accumulated in the fatty tissues reached an
average of 234 ppm (101-367 ppm) in subjects who had ingested 35 mg of
DDT per day. Throughout the whole experiment, no subject complained of
malaise, nor did any ill-effects appear that could be attributed to
the ingestion of DDT (Hayes et al., 1956). The essential results were
confirmed in a separate investigation in which dosage with DDT lasted
for 21 months and the volunteers were observed for an additional 27
months. Fourteen men received 35 mg/man/day; 6 received 3.5
mg/man/day, and 4 men served as controls. The study also revealed the
relationship between storage of DDT And the urinary excretion of DDA
and demonstrated that the loss of DDT from storage in man following
cessation of dosage is slow - only about two-thirds in 27 months,
(Hayes et al., in preparation).
Observations were made on 40 workers engaged, over a period of
years, in the manufacturing of specialities based on DDT, under
conditions where suitable precautions had not been taken to protect
their skin. According to the DDA concentration found in the urine,
these subjects had absorbed daily as much DDT as volunteers who had
ingested 35 mg of the insecticide per day in the experiment mentioned
above. Thorough medical and biological tests failed to reveal any
toxic symptoms, even in workers exposed to the toxic product for 6.5
years (Ortelee, 1958).
More recent studies of the same and other workers, some with
longer exposures, confirmed the essential findings.
It has been shown by Durham et al. (1965) that the storage of DDT
in man is directly proportional to intake over a wide range of dosage
(0.04 to 35.0 mg/man/day) so that from the level in the fat the daily
intake can be estimate. From this relationship and from recent
measurements of DDT stored in human fat (Dale et al., 1965) it is
clear that the highest average intake of DDT of any human population
yet observed is about 0.68 mg/man/day. However, in the only country
where samples have been taken repeatedly, the level of storage of DDT
by the general population did not increase from 1950 through 1963, the
last year for which information is now available (Quinby et al.,
1965).
Comments on experimental studies reported
DDT has been studied extensively in several species of animals.
The rat is particularly susceptible. Concentrations as low as 5 and 10
ppm in the diet have caused changes in some male rats but these are
inconstant, reversible and they are always most prominent in the least
susceptible sex.
Of much greater importance is the enormous amount of work which
has been done on the effects of this compound in man. Doses equivalent
to 0.5 mg/kg body-weight daily have been given experimentally to a
group of significant size for as long as 21 months without effect
apart from storage in the fat.
EVALUATION
This has been based on the studies on man.
Estimate of acceptable daily intake for man
0-0.01 mg/kg/body-weight.
REFERENCES
Andronova, G. P. (1956) Dissertation, F. F. Erisman's State Research
Institute for Hygiene, Moscow
Anon. (1951) Report of the Committee on Pesticides of the Council on
Pharmacy and Chemistry, J. Amer. med. Ass., 145., 725
Biddulph, C., Bateman, G. Q., Bryson, M. J., Harris, J. R., Greenwood,
D. A., Binns, W., Miner, M. L., Harris, L. E. & Madsen, L. L. (1950)
Advances in Chemistry, 1, 237
Cueto, C., Barnes, A. G. & Mattson, A. M. (1956) J. Agr. Food Chem.,
4 (2), 943
Dale, W. E., Copeland, N. F. & Hayes, W. J., jr (1965) Bull. Wld.
Hlth Org. (In press)
Dale, W. E., Gaines, T. B. & Hayes, W. J., jr (1963) Science, 142,
1474
Draize, J. H., Woodard, G., Fitzhugh, O. G., Nelson, A. A., Smith, R.
B. & Calvery, H. O. (1944) J. Amer. chem. Soc., 22, 1503
Durham, W. F., Armstrong, J. F. & Quinby, G. E. (1965) J.
Environment. Hlth (In press)
Durham, W. F., Ortega, F. & Hayes, W. J., jr (1963) Arch. Int.
Pharmacodyn., 141, 111
Fitzhugh, O. G. (1948) Ind. Eng. Chem., 40, 704
Fitzhugh, O. G. & Nelson, A. A. (1947) J. Pharmacol. exp. Ther.,
89, 18
Gannon, N. & Decker. G. C. (1960) J. Econ. Ent., 53, 411
Gerebtzoff, M. A. & Philippot, E. (1952) Experientia (Basel, 8
(10), 395
Gerebtzoff, M. A., Philippot, E. & Dallemagne, M. J. (1950) C.R.
Soc. Biol. (Paris), 144, 1135
Hayes, W. J., jr & Dale, W. E. (1964) Toxicol. Appl. Pharmacol., 6
349
Hayes, W. J., jr, Dale, E. & Pirkle, C. I. (In preparation)
Hayes, W. J., jr, Durham, W. F. & Cueto, C., jr (1956) J. Amer. med.
Ass., 162 (9), 890
Hayes, W. J., jr, Walker, K. C., Elliott, J. W. & Durham, W. F. (1958)
Arch. industr. Hlth, 18, 398
Haymaker, W., Ginzler, A. M. & Ferguson, R. L. (1946) Amer. J. M.
Soc., 212, 423
Judah, J. D. (1949) Brit. J. Pharmacol., 4, 120
Khairy, M. (1959) Quart. J. exp. Psychol., 11, 84
Klimer, O. R. (1955) Arch. exp. Path. Pharmak., 227, 183
Laug, E. P., Kunze, F. M. & Prickett, C. S. (1951) Arch. industr.
Hyg., 3, 245
Laug, E. P., Nelson, A. A., Fitzhugh, O. G. & Kunze, F. M. (1950)
J. Pharmacol. exp. Ther., 98, 268
Lehman, A. J. (1952) Quart. Bull. Assoc. Food & Drug Officials,
U.S., 16, 3, 47, 126
Maier-Bode, H. (1960) Med. exp. (Basel), 1, 153
Mattson, A. M., Spillane, J. T., Baker, C. & Pearce, G. W. (1953)
Anal. Chem., 25, 1067
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia &
London, Saunders, vol. III
Ortega, P. (1962) Fed. Proc., 21, 306
Ortega, P., Hayes, W. J., jr, Durham, W. F. & Mattson, A. (1956)
Publ. Hlth Monogr. 43, 27 (33 references)
Ortelee, M. F. (1958) Arch. industr. Hlth, 18, 433
Pearce, G. W., Mattson, A. M. & Hayes, W. J., jr, (1952) Science,
116, 254
Phillips, W. E. G. (1963) Canad. J. Biochem., 41, 1793
Philips, F. & Gilman, A. (1946) J. Pharmacol. exp. Ther., 86, 213
Quinby, G. E., Hayes, W. J., jr, Armstrong, J. F. & Durham, W. F.
(1965) J. Amer. med. Ass., 191, 175
Rothe, C. F., Mattson. A. M., Nuelsein, R. M. & Hayes, W. J., jr
(1957) Arch. industr. Hlth, 16 (1), 82
Shillinger, U. I., Naumova, L. P. & Pererman, S. W. (1955)
Vep. Pitan., 14, 41
Smith, R. F., Hoskins, W. M. & Hullmer, O. H. (1948) J. econ. Ent.,
41, 759
Spicer, S. S., Sweeney, T. R., von Oettingen, W. F., Lillie, R. D. &
Neal, P. A. (1947) Vet. Med., 42 (8), 289
Telford, H. S. & Guthrie, J. E. (1945) Science, 102, 647
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Wilson. H. F. et al. (1946) J. econ. Ent., 39, 802
Woodard, G., Ofner, R. R. & Montgomery, C. M. (1945) Science, 102,
177
BIOLOGICAL DATA
Biochemical aspects
DDT is only slightly absorbed by the skin, but the degree of
absorption depends on the vehicle used. It may be absorbed after
inhalation.
After oral administration, most of the dose is found unchanged in
the faeces, but some absorption may occur, particularly in the
presence of lipids. The absorbed DDT is transformed into
2,2-di-(p-chlorophenyl)-1,1-dichloro-ethylene (DDE) (Pearce et al.,
1952; Mattson et al., 1953) and into 2,2-di-(p-chlorophenyl)-acetic
acid (DDA) (Spicer et al., 1947; Judah, 1949; Hayes et al., 1956).
Some unknown compounds are also found (Spicer et al., 1947; Hayes et
al., 1956, Rothe et al., 1957; Cueto et al., 1956). A certain amount
of DDT and DDE accumulates in the fat (Laug et al., 1950; Hayes et
al., 1958; Maier-Bode, 1960; Laug et al., 1951). DDA is excreted in
the urine and in a combined form in the bile. DDT is also found in the
milk (Spicer et al., 1947; Telford & Guthrie, 1945; Wilson et al.,
1946; Woodard et al., 1945; Smith et al., 1948; Biddulph et al., 1950;
Gannon & Decker, 1960). This solubility in fats, combined with the
relative chemical stability of the insecticide, explains its
cumulative nature. In the rat a concentration of 10 ppm in the diet
has been shown to interfere with the storage and metabolism of vitamin
A in the liver (Phillips, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 150-420* Negherbon, 1959
Rat Intravenous 40-60 Negherbon, 1959
Mouse Oral 150-400* Negherbon, 1959
Guinea-pig Oral 400 Negherbon, 1959
Rabbit Oral 250-400* Negherbon, 1959
Rabbit Intraperitoneal 2 100 Negherbon, 1959
Dog Intravenous Approximately Negherbon, 1959
50
Cat Oral 400-600* Negherbon, 1959
Monkey Oral >200 Negherbon, 1959
Horse Oral >300 Negherbon, 1959
Chicken Oral >1 300 Negherbon, 1959
* The LD50 dose of DDT varies within wide limits, depending on sex,
and the type of vehicle used.
The toxic effects, revealing involvement of the central nervous
system (such as hypersensitivity, excitability, generalized trembling,
convulsions, paralysis) appear within 5-10 minutes of intravenous
administration and after a latent period of some hours following oral
administration. Death occurs as a result of respiratory arrest in the
rat, rabbit, cat and monkey, and of ventricular fibrillation in the
dog. It is the consensus of the literature that the significant action
of DDT is on the nervous system; when ventricular fibrillation occurs,
it is precipitated by adrenaline released from the adrenal medulla by
stimulation of the sympathetic nervous system (Phillips & Gilman,
1946).
In rats given a single oral dose of DDT sufficient to kill about
half of them, the severity of symptoms corresponded with the
concentration of the unchanged compound in the brain (Dale et al.,
1963). Furthermore, approximately the same concentration of DDT is
found in the brain of rats killed by DDT no matter whether the dosage
is acute, subacute, or chronic (Hayes & Dale, 1964).
The fatal dose for man is difficult to establish but is generally
taken to be of the order of 500 mg/kg body-weight. A dose of the order
of 10 mg/kg body-weight can give rise in some subjects, but not in
all, to toxic symptoms (nausea, headache, sweating), and with doses of
16 mg/kg body-weight upwards, convulsions often occur (Anon., 1951).
Short-term studies
Rat. Rats, in groups of 8 males and 8 females, were gives diets
with 1, 5, 10 and 50 ppm of DDT for 23 weeks. At concentrations of 5
ppm and higher, histopathological changes in the liver were found
(Laug et al., 1950).
A series of experiments was carried out with small groups of
rats, 178 males and 104 females in all, which were given diets
containing DDT in concentrations from traces up to 5000 ppm for
periods ranging from one to 14 months. In some cases observation of
the animals continued after cessation of treatment. At levels above
400 ppm changes in growth rate were noted. Above 200 ppm liver
enlargement was seen. In the male animals with intakes of 5 ppm and
above, specific histological lesions in the liver were seen. These
lesions consisted of hypertrophy of the parenchymal cells, increased
lipid deposits, marginal localization of cytoplasmic granules and,
above all, the appearance of complex cytoplasmic inclusions of a lipid
nature, called "lipospheres". In the females these liver lesions were
only seen in diets containing 200 ppm or more. Necrotic lesions were
seen only at concentrations above 1000 ppm (Ortega et al., 1956). The
authors noted that lipospheres and other changes considered
characteristic of DDT were more prominent in male rats although
females store more of the compound and were more susceptible to
poisoning. For this and other reasons, they speculated that the
changes might be adaptive. They noted also that earlier work had
failed to demonstrate similar changes in other species (Ortega et al.,
1956). Later work in the same laboratory failed to demonstrate such
changes in monkeys treated with DDT for 7.5 years (Durham et al.,
1963).
In an electron-microscopical examination of the liver of rats fed
diets containing 5-1000 ppm of DDT for 2-18 months, the following
cytoplasmic abnormalities were found: slight proliferation of the
endoplasmic reticulum; peripheral distribution of the ribosomes and
intracytoplasmic inclusions consisting of aggregates of membranes very
rich in lipids (Ortega, 1962).
Daily oral doses of DDT 0.2 mg/kg for 10 days produced functional
changes in the conditional reflex pattern which appeared after 5-7
doses and persisted 5-7 days after the end of exposure (Andronova,
1956).
Behavioural studies have also been made on groups of rats fed
diets containing 100, 200, 400 and 600 ppm DDT. Problem solving and
speed of locomotion were unaffected by these doses of DDT. There was a
significant alteration in the patterns of locomotion in these rats and
their reactions to stress involving visual stimuli were reduced
(Khairy, 1959).
Dog. Dogs were given DDT orally. Those receiving 100 mg/kg
body-weight daily died within 7 weeks. Animals subjected to lower
doses survived and seemed normal even after 50 weeks (Draize et al.,
1944). When the experiment was continued for 3 years, the animals
receiving doses of 50 and 80 mg/kg body-weight developed jaundice and
haemorrhagic symptoms. Those receiving 10 mg/kg body-weight daily
showed no ill effects (Hayes et al., 1956; Lehman, 1952).
Dogs were given DDT by stomach-tube, in the form of a 10%
solution in peanut oil in doses ranging from 150 to 350 mg/kg
body-weight for about 90 days. Some of the animals died. The authors
noted neurological symptoms, showing involvement of the cerebellum as
revealed by histological lesions (Haymaker et al., 1946).
Three dogs were given intramuscular injections of DDT in the form
of a 10% solution in olive oil at the rate of 100 mg/kg body-weight
daily for 25 to 30 days. A control animal received 1 ml of olive oil
in the same way. The experimental animals showed a temporary loss of
weight. The kidneys were discoloured and on histological examination
showed tubular damage. At the same time, proliferation was observed in
the lymphoid tissues (lymph nodes, spleen, bone marrow) and the wall
of the small intestine, but the blood showed no leukaemic
characteristics (Gerebtzoff et al., 1950; Gerebtzoff & Philippot,
1952).
Monkey. Monkeys given DDT orally in a dose of 0.2 mg/kg
body-weight for 7-9 months developed symptoms of hepatitis. After a
year, the animals showed liver enlargement and hyperglycaemia
(Shillinger et al., 1955). These liver changes were not confirmed in
another experiment on monkeys lasting 7.5 years (Durham et al., 1963).
Long-term studies
Rat. Groups of 12 male rats were subjected for 2 years to diets
containing 100, 200, 400 and 800 ppm of DDT, in the form of a 10%
solution in corn oil. In another experiment groups each of 24 rats (12
males and 12 females) were given, during the same period, diets
containing 200, 400, 600 and 800 ppm. Also additional groups of 24
animals received 600 and 800 ppm incorporated in their feed in a dry
state. In the groups receiving 400 ppm and above, an increase in the
mortality rate was seen in relation to the dose. Apart from nervous
symptoms at doses of 400 ppm and above, typical liver lesions were
found at all concentrations. Hepatic cell tumours were seen in 4 out
of 75 animals and 11 other rats showed nodular adenomatoid hyperplasia
(Fitzhugh & Nelson, 1947).
In an experiment with rats fed for 2 years on a diet containing
10 ppm of DDT, histological liver lesions were also observed
(Fitzhugh, 1948). Groups of 80 young rats each (40 male and 40 female)
were fed 0.25, 12.5 and 25 ppm of DDT. The histological lesions of the
liver observed were always slight and, according to the authors,
non-specific, but nevertheless they were more frequent with DDT than
with aldrin or dieldrin, which were administered in the same
concentrations for comparative purposes (Treon & Cleveland, 1955).
Experiments with 25 young rats did not show any harmful effects
after daily application by stomach-tube of a dose of 10 µg of DDT
equivalent to a dietary intake of 0.1-0.15 ppm for 17 months (Klimmer,
1955).
Monkey. Twenty-four Rhesus monkeys, 12 males and 12 females,
were divided into groups and fed over periods as long as 7.5 years or
more on diets containing respectively 5, 50, 200 and 5000 ppm of DDT.
All the animals subjected to the concentration of 5000 ppm showed
convulsions, accompanied by loss of appetite and a fall in weight. At
a concentration of 200 ppm or below (the latter dose corresponding to
daily doses of 2.2-5.54 mg/kg body-weight), no harmful effects were
observed and, in particular, no histological lesions in the liver or
disturbances in the functioning of that organ, as shown by the
bromsulfthalein test (Durham et al., 1963).
Observations on man. An experimental study was carried out of
the effects on man of prolonged ingestion of small doses of DDT (in
the form of oily solutions in capsules or emulsions in milk). The
authors used for this study, 51 volunteers; 17 received a normal diet,
17 received 3.5 mg/kg body-weight and 17 received 35 mg DDT daily. The
last dose is approximately 0.5 mg/kg body-weight. Administration was
continued for as long as 18 months. The authors noted that the
accumulation of the insecticide in the fatty tissues and the urinary
excretion of its metabolite, DDA, were proportional to the dose of DDT
ingested. A state of equilibrium was reached after about a year and
the concentration of DDT accumulated in the fatty tissues reached an
average of 234 ppm (101-367 ppm) in subjects who had ingested 35 mg of
DDT per day. Throughout the whole experiment, no subject complained of
malaise, nor did any ill-effects appear that could be attributed to
the ingestion of DDT (Hayes et al., 1956). The essential results were
confirmed in a separate investigation in which dosage with DDT lasted
for 21 months and the volunteers were observed for an additional 27
months. Fourteen men received 35 mg/man/day; 6 received 3.5
mg/man/day, and 4 men served as controls. The study also revealed the
relationship between storage of DDT And the urinary excretion of DDA
and demonstrated that the loss of DDT from storage in man following
cessation of dosage is slow - only about two-thirds in 27 months,
(Hayes et al., in preparation).
Observations were made on 40 workers engaged, over a period of
years, in the manufacturing of specialities based on DDT, under
conditions where suitable precautions had not been taken to protect
their skin. According to the DDA concentration found in the urine,
these subjects had absorbed daily as much DDT as volunteers who had
ingested 35 mg of the insecticide per day in the experiment mentioned
above. Thorough medical and biological tests failed to reveal any
toxic symptoms, even in workers exposed to the toxic product for 6.5
years (Ortelee, 1958).
More recent studies of the same and other workers, some with
longer exposures, confirmed the essential findings.
It has been shown by Durham et al. (1965) that the storage of DDT
in man is directly proportional to intake over a wide range of dosage
(0.04 to 35.0 mg/man/day) so that from the level in the fat the daily
intake can be estimate. From this relationship and from recent
measurements of DDT stored in human fat (Dale et al., 1965) it is
clear that the highest average intake of DDT of any human population
yet observed is about 0.68 mg/man/day. However, in the only country
where samples have been taken repeatedly, the level of storage of DDT
by the general population did not increase from 1950 through 1963, the
last year for which information is now available (Quinby et al.,
1965).
Comments on experimental studies reported
DDT has been studied extensively in several species of animals.
The rat is particularly susceptible. Concentrations as low as 5 and 10
ppm in the diet have caused changes in some male rats but these are
inconstant, reversible and they are always most prominent in the least
susceptible sex.
Of much greater importance is the enormous amount of work which
has been done on the effects of this compound in man. Doses equivalent
to 0.5 mg/kg body-weight daily have been given experimentally to a
group of significant size for as long as 21 months without effect
apart from storage in the fat.
EVALUATION
This has been based on the studies on man.
Estimate of acceptable daily intake for man
0-0.01 mg/kg/body-weight.
REFERENCES
Andronova, G. P. (1956) Dissertation, F. F. Erisman's State Research
Institute for Hygiene, Moscow
Anon. (1951) Report of the Committee on Pesticides of the Council on
Pharmacy and Chemistry, J. Amer. med. Ass., 145., 725
Biddulph, C., Bateman, G. Q., Bryson, M. J., Harris, J. R., Greenwood,
D. A., Binns, W., Miner, M. L., Harris, L. E. & Madsen, L. L. (1950)
Advances in Chemistry, 1, 237
Cueto, C., Barnes, A. G. & Mattson, A. M. (1956) J. Agr. Food Chem.,
4 (2), 943
Dale, W. E., Copeland, N. F. & Hayes, W. J., jr (1965) Bull. Wld.
Hlth Org. (In press)
Dale, W. E., Gaines, T. B. & Hayes, W. J., jr (1963) Science, 142,
1474
Draize, J. H., Woodard, G., Fitzhugh, O. G., Nelson, A. A., Smith, R.
B. & Calvery, H. O. (1944) J. Amer. chem. Soc., 22, 1503
Durham, W. F., Armstrong, J. F. & Quinby, G. E. (1965) J.
Environment. Hlth (In press)
Durham, W. F., Ortega, F. & Hayes, W. J., jr (1963) Arch. Int.
Pharmacodyn., 141, 111
Fitzhugh, O. G. (1948) Ind. Eng. Chem., 40, 704
Fitzhugh, O. G. & Nelson, A. A. (1947) J. Pharmacol. exp. Ther.,
89, 18
Gannon, N. & Decker. G. C. (1960) J. Econ. Ent., 53, 411
Gerebtzoff, M. A. & Philippot, E. (1952) Experientia (Basel, 8
(10), 395
Gerebtzoff, M. A., Philippot, E. & Dallemagne, M. J. (1950) C.R.
Soc. Biol. (Paris), 144, 1135
Hayes, W. J., jr & Dale, W. E. (1964) Toxicol. Appl. Pharmacol., 6
349
Hayes, W. J., jr, Dale, E. & Pirkle, C. I. (In preparation)
Hayes, W. J., jr, Durham, W. F. & Cueto, C., jr (1956) J. Amer. med.
Ass., 162 (9), 890
Hayes, W. J., jr, Walker, K. C., Elliott, J. W. & Durham, W. F. (1958)
Arch. industr. Hlth, 18, 398
Haymaker, W., Ginzler, A. M. & Ferguson, R. L. (1946) Amer. J. M.
Soc., 212, 423
Judah, J. D. (1949) Brit. J. Pharmacol., 4, 120
Khairy, M. (1959) Quart. J. exp. Psychol., 11, 84
Klimer, O. R. (1955) Arch. exp. Path. Pharmak., 227, 183
Laug, E. P., Kunze, F. M. & Prickett, C. S. (1951) Arch. industr.
Hyg., 3, 245
Laug, E. P., Nelson, A. A., Fitzhugh, O. G. & Kunze, F. M. (1950)
J. Pharmacol. exp. Ther., 98, 268
Lehman, A. J. (1952) Quart. Bull. Assoc. Food & Drug Officials,
U.S., 16, 3, 47, 126
Maier-Bode, H. (1960) Med. exp. (Basel), 1, 153
Mattson, A. M., Spillane, J. T., Baker, C. & Pearce, G. W. (1953)
Anal. Chem., 25, 1067
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia &
London, Saunders, vol. III
Ortega, P. (1962) Fed. Proc., 21, 306
Ortega, P., Hayes, W. J., jr, Durham, W. F. & Mattson, A. (1956)
Publ. Hlth Monogr. 43, 27 (33 references)
Ortelee, M. F. (1958) Arch. industr. Hlth, 18, 433
Pearce, G. W., Mattson, A. M. & Hayes, W. J., jr, (1952) Science,
116, 254
Phillips, W. E. G. (1963) Canad. J. Biochem., 41, 1793
Philips, F. & Gilman, A. (1946) J. Pharmacol. exp. Ther., 86, 213
Quinby, G. E., Hayes, W. J., jr, Armstrong, J. F. & Durham, W. F.
(1965) J. Amer. med. Ass., 191, 175
Rothe, C. F., Mattson. A. M., Nuelsein, R. M. & Hayes, W. J., jr
(1957) Arch. industr. Hlth, 16 (1), 82
Shillinger, U. I., Naumova, L. P. & Pererman, S. W. (1955)
Vep. Pitan., 14, 41
Smith, R. F., Hoskins, W. M. & Hullmer, O. H. (1948) J. econ. Ent.,
41, 759
Spicer, S. S., Sweeney, T. R., von Oettingen, W. F., Lillie, R. D. &
Neal, P. A. (1947) Vet. Med., 42 (8), 289
Telford, H. S. & Guthrie, J. E. (1945) Science, 102, 647
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Wilson. H. F. et al. (1946) J. econ. Ent., 39, 802
Woodard, G., Ofner, R. R. & Montgomery, C. M. (1945) Science, 102,
177
 BIOLOGICAL DATA
Biochemical aspects
DDT is only slightly absorbed by the skin, but the degree of
absorption depends on the vehicle used. It may be absorbed after
inhalation.
After oral administration, most of the dose is found unchanged in
the faeces, but some absorption may occur, particularly in the
presence of lipids. The absorbed DDT is transformed into
2,2-di-(p-chlorophenyl)-1,1-dichloro-ethylene (DDE) (Pearce et al.,
1952; Mattson et al., 1953) and into 2,2-di-(p-chlorophenyl)-acetic
acid (DDA) (Spicer et al., 1947; Judah, 1949; Hayes et al., 1956).
Some unknown compounds are also found (Spicer et al., 1947; Hayes et
al., 1956, Rothe et al., 1957; Cueto et al., 1956). A certain amount
of DDT and DDE accumulates in the fat (Laug et al., 1950; Hayes et
al., 1958; Maier-Bode, 1960; Laug et al., 1951). DDA is excreted in
the urine and in a combined form in the bile. DDT is also found in the
milk (Spicer et al., 1947; Telford & Guthrie, 1945; Wilson et al.,
1946; Woodard et al., 1945; Smith et al., 1948; Biddulph et al., 1950;
Gannon & Decker, 1960). This solubility in fats, combined with the
relative chemical stability of the insecticide, explains its
cumulative nature. In the rat a concentration of 10 ppm in the diet
has been shown to interfere with the storage and metabolism of vitamin
A in the liver (Phillips, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 150-420* Negherbon, 1959
Rat Intravenous 40-60 Negherbon, 1959
Mouse Oral 150-400* Negherbon, 1959
Guinea-pig Oral 400 Negherbon, 1959
Rabbit Oral 250-400* Negherbon, 1959
Rabbit Intraperitoneal 2 100 Negherbon, 1959
Dog Intravenous Approximately Negherbon, 1959
50
Cat Oral 400-600* Negherbon, 1959
Monkey Oral >200 Negherbon, 1959
Horse Oral >300 Negherbon, 1959
Chicken Oral >1 300 Negherbon, 1959
* The LD50 dose of DDT varies within wide limits, depending on sex,
and the type of vehicle used.
The toxic effects, revealing involvement of the central nervous
system (such as hypersensitivity, excitability, generalized trembling,
convulsions, paralysis) appear within 5-10 minutes of intravenous
administration and after a latent period of some hours following oral
administration. Death occurs as a result of respiratory arrest in the
rat, rabbit, cat and monkey, and of ventricular fibrillation in the
dog. It is the consensus of the literature that the significant action
of DDT is on the nervous system; when ventricular fibrillation occurs,
it is precipitated by adrenaline released from the adrenal medulla by
stimulation of the sympathetic nervous system (Phillips & Gilman,
1946).
In rats given a single oral dose of DDT sufficient to kill about
half of them, the severity of symptoms corresponded with the
concentration of the unchanged compound in the brain (Dale et al.,
1963). Furthermore, approximately the same concentration of DDT is
found in the brain of rats killed by DDT no matter whether the dosage
is acute, subacute, or chronic (Hayes & Dale, 1964).
The fatal dose for man is difficult to establish but is generally
taken to be of the order of 500 mg/kg body-weight. A dose of the order
of 10 mg/kg body-weight can give rise in some subjects, but not in
all, to toxic symptoms (nausea, headache, sweating), and with doses of
16 mg/kg body-weight upwards, convulsions often occur (Anon., 1951).
Short-term studies
Rat. Rats, in groups of 8 males and 8 females, were gives diets
with 1, 5, 10 and 50 ppm of DDT for 23 weeks. At concentrations of 5
ppm and higher, histopathological changes in the liver were found
(Laug et al., 1950).
A series of experiments was carried out with small groups of
rats, 178 males and 104 females in all, which were given diets
containing DDT in concentrations from traces up to 5000 ppm for
periods ranging from one to 14 months. In some cases observation of
the animals continued after cessation of treatment. At levels above
400 ppm changes in growth rate were noted. Above 200 ppm liver
enlargement was seen. In the male animals with intakes of 5 ppm and
above, specific histological lesions in the liver were seen. These
lesions consisted of hypertrophy of the parenchymal cells, increased
lipid deposits, marginal localization of cytoplasmic granules and,
above all, the appearance of complex cytoplasmic inclusions of a lipid
nature, called "lipospheres". In the females these liver lesions were
only seen in diets containing 200 ppm or more. Necrotic lesions were
seen only at concentrations above 1000 ppm (Ortega et al., 1956). The
authors noted that lipospheres and other changes considered
characteristic of DDT were more prominent in male rats although
females store more of the compound and were more susceptible to
poisoning. For this and other reasons, they speculated that the
changes might be adaptive. They noted also that earlier work had
failed to demonstrate similar changes in other species (Ortega et al.,
1956). Later work in the same laboratory failed to demonstrate such
changes in monkeys treated with DDT for 7.5 years (Durham et al.,
1963).
In an electron-microscopical examination of the liver of rats fed
diets containing 5-1000 ppm of DDT for 2-18 months, the following
cytoplasmic abnormalities were found: slight proliferation of the
endoplasmic reticulum; peripheral distribution of the ribosomes and
intracytoplasmic inclusions consisting of aggregates of membranes very
rich in lipids (Ortega, 1962).
Daily oral doses of DDT 0.2 mg/kg for 10 days produced functional
changes in the conditional reflex pattern which appeared after 5-7
doses and persisted 5-7 days after the end of exposure (Andronova,
1956).
Behavioural studies have also been made on groups of rats fed
diets containing 100, 200, 400 and 600 ppm DDT. Problem solving and
speed of locomotion were unaffected by these doses of DDT. There was a
significant alteration in the patterns of locomotion in these rats and
their reactions to stress involving visual stimuli were reduced
(Khairy, 1959).
Dog. Dogs were given DDT orally. Those receiving 100 mg/kg
body-weight daily died within 7 weeks. Animals subjected to lower
doses survived and seemed normal even after 50 weeks (Draize et al.,
1944). When the experiment was continued for 3 years, the animals
receiving doses of 50 and 80 mg/kg body-weight developed jaundice and
haemorrhagic symptoms. Those receiving 10 mg/kg body-weight daily
showed no ill effects (Hayes et al., 1956; Lehman, 1952).
Dogs were given DDT by stomach-tube, in the form of a 10%
solution in peanut oil in doses ranging from 150 to 350 mg/kg
body-weight for about 90 days. Some of the animals died. The authors
noted neurological symptoms, showing involvement of the cerebellum as
revealed by histological lesions (Haymaker et al., 1946).
Three dogs were given intramuscular injections of DDT in the form
of a 10% solution in olive oil at the rate of 100 mg/kg body-weight
daily for 25 to 30 days. A control animal received 1 ml of olive oil
in the same way. The experimental animals showed a temporary loss of
weight. The kidneys were discoloured and on histological examination
showed tubular damage. At the same time, proliferation was observed in
the lymphoid tissues (lymph nodes, spleen, bone marrow) and the wall
of the small intestine, but the blood showed no leukaemic
characteristics (Gerebtzoff et al., 1950; Gerebtzoff & Philippot,
1952).
Monkey. Monkeys given DDT orally in a dose of 0.2 mg/kg
body-weight for 7-9 months developed symptoms of hepatitis. After a
year, the animals showed liver enlargement and hyperglycaemia
(Shillinger et al., 1955). These liver changes were not confirmed in
another experiment on monkeys lasting 7.5 years (Durham et al., 1963).
Long-term studies
Rat. Groups of 12 male rats were subjected for 2 years to diets
containing 100, 200, 400 and 800 ppm of DDT, in the form of a 10%
solution in corn oil. In another experiment groups each of 24 rats (12
males and 12 females) were given, during the same period, diets
containing 200, 400, 600 and 800 ppm. Also additional groups of 24
animals received 600 and 800 ppm incorporated in their feed in a dry
state. In the groups receiving 400 ppm and above, an increase in the
mortality rate was seen in relation to the dose. Apart from nervous
symptoms at doses of 400 ppm and above, typical liver lesions were
found at all concentrations. Hepatic cell tumours were seen in 4 out
of 75 animals and 11 other rats showed nodular adenomatoid hyperplasia
(Fitzhugh & Nelson, 1947).
In an experiment with rats fed for 2 years on a diet containing
10 ppm of DDT, histological liver lesions were also observed
(Fitzhugh, 1948). Groups of 80 young rats each (40 male and 40 female)
were fed 0.25, 12.5 and 25 ppm of DDT. The histological lesions of the
liver observed were always slight and, according to the authors,
non-specific, but nevertheless they were more frequent with DDT than
with aldrin or dieldrin, which were administered in the same
concentrations for comparative purposes (Treon & Cleveland, 1955).
Experiments with 25 young rats did not show any harmful effects
after daily application by stomach-tube of a dose of 10 µg of DDT
equivalent to a dietary intake of 0.1-0.15 ppm for 17 months (Klimmer,
1955).
Monkey. Twenty-four Rhesus monkeys, 12 males and 12 females,
were divided into groups and fed over periods as long as 7.5 years or
more on diets containing respectively 5, 50, 200 and 5000 ppm of DDT.
All the animals subjected to the concentration of 5000 ppm showed
convulsions, accompanied by loss of appetite and a fall in weight. At
a concentration of 200 ppm or below (the latter dose corresponding to
daily doses of 2.2-5.54 mg/kg body-weight), no harmful effects were
observed and, in particular, no histological lesions in the liver or
disturbances in the functioning of that organ, as shown by the
bromsulfthalein test (Durham et al., 1963).
Observations on man. An experimental study was carried out of
the effects on man of prolonged ingestion of small doses of DDT (in
the form of oily solutions in capsules or emulsions in milk). The
authors used for this study, 51 volunteers; 17 received a normal diet,
17 received 3.5 mg/kg body-weight and 17 received 35 mg DDT daily. The
last dose is approximately 0.5 mg/kg body-weight. Administration was
continued for as long as 18 months. The authors noted that the
accumulation of the insecticide in the fatty tissues and the urinary
excretion of its metabolite, DDA, were proportional to the dose of DDT
ingested. A state of equilibrium was reached after about a year and
the concentration of DDT accumulated in the fatty tissues reached an
average of 234 ppm (101-367 ppm) in subjects who had ingested 35 mg of
DDT per day. Throughout the whole experiment, no subject complained of
malaise, nor did any ill-effects appear that could be attributed to
the ingestion of DDT (Hayes et al., 1956). The essential results were
confirmed in a separate investigation in which dosage with DDT lasted
for 21 months and the volunteers were observed for an additional 27
months. Fourteen men received 35 mg/man/day; 6 received 3.5
mg/man/day, and 4 men served as controls. The study also revealed the
relationship between storage of DDT And the urinary excretion of DDA
and demonstrated that the loss of DDT from storage in man following
cessation of dosage is slow - only about two-thirds in 27 months,
(Hayes et al., in preparation).
Observations were made on 40 workers engaged, over a period of
years, in the manufacturing of specialities based on DDT, under
conditions where suitable precautions had not been taken to protect
their skin. According to the DDA concentration found in the urine,
these subjects had absorbed daily as much DDT as volunteers who had
ingested 35 mg of the insecticide per day in the experiment mentioned
above. Thorough medical and biological tests failed to reveal any
toxic symptoms, even in workers exposed to the toxic product for 6.5
years (Ortelee, 1958).
More recent studies of the same and other workers, some with
longer exposures, confirmed the essential findings.
It has been shown by Durham et al. (1965) that the storage of DDT
in man is directly proportional to intake over a wide range of dosage
(0.04 to 35.0 mg/man/day) so that from the level in the fat the daily
intake can be estimate. From this relationship and from recent
measurements of DDT stored in human fat (Dale et al., 1965) it is
clear that the highest average intake of DDT of any human population
yet observed is about 0.68 mg/man/day. However, in the only country
where samples have been taken repeatedly, the level of storage of DDT
by the general population did not increase from 1950 through 1963, the
last year for which information is now available (Quinby et al.,
1965).
Comments on experimental studies reported
DDT has been studied extensively in several species of animals.
The rat is particularly susceptible. Concentrations as low as 5 and 10
ppm in the diet have caused changes in some male rats but these are
inconstant, reversible and they are always most prominent in the least
susceptible sex.
Of much greater importance is the enormous amount of work which
has been done on the effects of this compound in man. Doses equivalent
to 0.5 mg/kg body-weight daily have been given experimentally to a
group of significant size for as long as 21 months without effect
apart from storage in the fat.
EVALUATION
This has been based on the studies on man.
Estimate of acceptable daily intake for man
0-0.01 mg/kg/body-weight.
REFERENCES
Andronova, G. P. (1956) Dissertation, F. F. Erisman's State Research
Institute for Hygiene, Moscow
Anon. (1951) Report of the Committee on Pesticides of the Council on
Pharmacy and Chemistry, J. Amer. med. Ass., 145., 725
Biddulph, C., Bateman, G. Q., Bryson, M. J., Harris, J. R., Greenwood,
D. A., Binns, W., Miner, M. L., Harris, L. E. & Madsen, L. L. (1950)
Advances in Chemistry, 1, 237
Cueto, C., Barnes, A. G. & Mattson, A. M. (1956) J. Agr. Food Chem.,
4 (2), 943
Dale, W. E., Copeland, N. F. & Hayes, W. J., jr (1965) Bull. Wld.
Hlth Org. (In press)
Dale, W. E., Gaines, T. B. & Hayes, W. J., jr (1963) Science, 142,
1474
Draize, J. H., Woodard, G., Fitzhugh, O. G., Nelson, A. A., Smith, R.
B. & Calvery, H. O. (1944) J. Amer. chem. Soc., 22, 1503
Durham, W. F., Armstrong, J. F. & Quinby, G. E. (1965) J.
Environment. Hlth (In press)
Durham, W. F., Ortega, F. & Hayes, W. J., jr (1963) Arch. Int.
Pharmacodyn., 141, 111
Fitzhugh, O. G. (1948) Ind. Eng. Chem., 40, 704
Fitzhugh, O. G. & Nelson, A. A. (1947) J. Pharmacol. exp. Ther.,
89, 18
Gannon, N. & Decker. G. C. (1960) J. Econ. Ent., 53, 411
Gerebtzoff, M. A. & Philippot, E. (1952) Experientia (Basel, 8
(10), 395
Gerebtzoff, M. A., Philippot, E. & Dallemagne, M. J. (1950) C.R.
Soc. Biol. (Paris), 144, 1135
Hayes, W. J., jr & Dale, W. E. (1964) Toxicol. Appl. Pharmacol., 6
349
Hayes, W. J., jr, Dale, E. & Pirkle, C. I. (In preparation)
Hayes, W. J., jr, Durham, W. F. & Cueto, C., jr (1956) J. Amer. med.
Ass., 162 (9), 890
Hayes, W. J., jr, Walker, K. C., Elliott, J. W. & Durham, W. F. (1958)
Arch. industr. Hlth, 18, 398
Haymaker, W., Ginzler, A. M. & Ferguson, R. L. (1946) Amer. J. M.
Soc., 212, 423
Judah, J. D. (1949) Brit. J. Pharmacol., 4, 120
Khairy, M. (1959) Quart. J. exp. Psychol., 11, 84
Klimer, O. R. (1955) Arch. exp. Path. Pharmak., 227, 183
Laug, E. P., Kunze, F. M. & Prickett, C. S. (1951) Arch. industr.
Hyg., 3, 245
Laug, E. P., Nelson, A. A., Fitzhugh, O. G. & Kunze, F. M. (1950)
J. Pharmacol. exp. Ther., 98, 268
Lehman, A. J. (1952) Quart. Bull. Assoc. Food & Drug Officials,
U.S., 16, 3, 47, 126
Maier-Bode, H. (1960) Med. exp. (Basel), 1, 153
Mattson, A. M., Spillane, J. T., Baker, C. & Pearce, G. W. (1953)
Anal. Chem., 25, 1067
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia &
London, Saunders, vol. III
Ortega, P. (1962) Fed. Proc., 21, 306
Ortega, P., Hayes, W. J., jr, Durham, W. F. & Mattson, A. (1956)
Publ. Hlth Monogr. 43, 27 (33 references)
Ortelee, M. F. (1958) Arch. industr. Hlth, 18, 433
Pearce, G. W., Mattson, A. M. & Hayes, W. J., jr, (1952) Science,
116, 254
Phillips, W. E. G. (1963) Canad. J. Biochem., 41, 1793
Philips, F. & Gilman, A. (1946) J. Pharmacol. exp. Ther., 86, 213
Quinby, G. E., Hayes, W. J., jr, Armstrong, J. F. & Durham, W. F.
(1965) J. Amer. med. Ass., 191, 175
Rothe, C. F., Mattson. A. M., Nuelsein, R. M. & Hayes, W. J., jr
(1957) Arch. industr. Hlth, 16 (1), 82
Shillinger, U. I., Naumova, L. P. & Pererman, S. W. (1955)
Vep. Pitan., 14, 41
Smith, R. F., Hoskins, W. M. & Hullmer, O. H. (1948) J. econ. Ent.,
41, 759
Spicer, S. S., Sweeney, T. R., von Oettingen, W. F., Lillie, R. D. &
Neal, P. A. (1947) Vet. Med., 42 (8), 289
Telford, H. S. & Guthrie, J. E. (1945) Science, 102, 647
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Wilson. H. F. et al. (1946) J. econ. Ent., 39, 802
Woodard, G., Ofner, R. R. & Montgomery, C. M. (1945) Science, 102,
177
BIOLOGICAL DATA
Biochemical aspects
DDT is only slightly absorbed by the skin, but the degree of
absorption depends on the vehicle used. It may be absorbed after
inhalation.
After oral administration, most of the dose is found unchanged in
the faeces, but some absorption may occur, particularly in the
presence of lipids. The absorbed DDT is transformed into
2,2-di-(p-chlorophenyl)-1,1-dichloro-ethylene (DDE) (Pearce et al.,
1952; Mattson et al., 1953) and into 2,2-di-(p-chlorophenyl)-acetic
acid (DDA) (Spicer et al., 1947; Judah, 1949; Hayes et al., 1956).
Some unknown compounds are also found (Spicer et al., 1947; Hayes et
al., 1956, Rothe et al., 1957; Cueto et al., 1956). A certain amount
of DDT and DDE accumulates in the fat (Laug et al., 1950; Hayes et
al., 1958; Maier-Bode, 1960; Laug et al., 1951). DDA is excreted in
the urine and in a combined form in the bile. DDT is also found in the
milk (Spicer et al., 1947; Telford & Guthrie, 1945; Wilson et al.,
1946; Woodard et al., 1945; Smith et al., 1948; Biddulph et al., 1950;
Gannon & Decker, 1960). This solubility in fats, combined with the
relative chemical stability of the insecticide, explains its
cumulative nature. In the rat a concentration of 10 ppm in the diet
has been shown to interfere with the storage and metabolism of vitamin
A in the liver (Phillips, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 150-420* Negherbon, 1959
Rat Intravenous 40-60 Negherbon, 1959
Mouse Oral 150-400* Negherbon, 1959
Guinea-pig Oral 400 Negherbon, 1959
Rabbit Oral 250-400* Negherbon, 1959
Rabbit Intraperitoneal 2 100 Negherbon, 1959
Dog Intravenous Approximately Negherbon, 1959
50
Cat Oral 400-600* Negherbon, 1959
Monkey Oral >200 Negherbon, 1959
Horse Oral >300 Negherbon, 1959
Chicken Oral >1 300 Negherbon, 1959
* The LD50 dose of DDT varies within wide limits, depending on sex,
and the type of vehicle used.
The toxic effects, revealing involvement of the central nervous
system (such as hypersensitivity, excitability, generalized trembling,
convulsions, paralysis) appear within 5-10 minutes of intravenous
administration and after a latent period of some hours following oral
administration. Death occurs as a result of respiratory arrest in the
rat, rabbit, cat and monkey, and of ventricular fibrillation in the
dog. It is the consensus of the literature that the significant action
of DDT is on the nervous system; when ventricular fibrillation occurs,
it is precipitated by adrenaline released from the adrenal medulla by
stimulation of the sympathetic nervous system (Phillips & Gilman,
1946).
In rats given a single oral dose of DDT sufficient to kill about
half of them, the severity of symptoms corresponded with the
concentration of the unchanged compound in the brain (Dale et al.,
1963). Furthermore, approximately the same concentration of DDT is
found in the brain of rats killed by DDT no matter whether the dosage
is acute, subacute, or chronic (Hayes & Dale, 1964).
The fatal dose for man is difficult to establish but is generally
taken to be of the order of 500 mg/kg body-weight. A dose of the order
of 10 mg/kg body-weight can give rise in some subjects, but not in
all, to toxic symptoms (nausea, headache, sweating), and with doses of
16 mg/kg body-weight upwards, convulsions often occur (Anon., 1951).
Short-term studies
Rat. Rats, in groups of 8 males and 8 females, were gives diets
with 1, 5, 10 and 50 ppm of DDT for 23 weeks. At concentrations of 5
ppm and higher, histopathological changes in the liver were found
(Laug et al., 1950).
A series of experiments was carried out with small groups of
rats, 178 males and 104 females in all, which were given diets
containing DDT in concentrations from traces up to 5000 ppm for
periods ranging from one to 14 months. In some cases observation of
the animals continued after cessation of treatment. At levels above
400 ppm changes in growth rate were noted. Above 200 ppm liver
enlargement was seen. In the male animals with intakes of 5 ppm and
above, specific histological lesions in the liver were seen. These
lesions consisted of hypertrophy of the parenchymal cells, increased
lipid deposits, marginal localization of cytoplasmic granules and,
above all, the appearance of complex cytoplasmic inclusions of a lipid
nature, called "lipospheres". In the females these liver lesions were
only seen in diets containing 200 ppm or more. Necrotic lesions were
seen only at concentrations above 1000 ppm (Ortega et al., 1956). The
authors noted that lipospheres and other changes considered
characteristic of DDT were more prominent in male rats although
females store more of the compound and were more susceptible to
poisoning. For this and other reasons, they speculated that the
changes might be adaptive. They noted also that earlier work had
failed to demonstrate similar changes in other species (Ortega et al.,
1956). Later work in the same laboratory failed to demonstrate such
changes in monkeys treated with DDT for 7.5 years (Durham et al.,
1963).
In an electron-microscopical examination of the liver of rats fed
diets containing 5-1000 ppm of DDT for 2-18 months, the following
cytoplasmic abnormalities were found: slight proliferation of the
endoplasmic reticulum; peripheral distribution of the ribosomes and
intracytoplasmic inclusions consisting of aggregates of membranes very
rich in lipids (Ortega, 1962).
Daily oral doses of DDT 0.2 mg/kg for 10 days produced functional
changes in the conditional reflex pattern which appeared after 5-7
doses and persisted 5-7 days after the end of exposure (Andronova,
1956).
Behavioural studies have also been made on groups of rats fed
diets containing 100, 200, 400 and 600 ppm DDT. Problem solving and
speed of locomotion were unaffected by these doses of DDT. There was a
significant alteration in the patterns of locomotion in these rats and
their reactions to stress involving visual stimuli were reduced
(Khairy, 1959).
Dog. Dogs were given DDT orally. Those receiving 100 mg/kg
body-weight daily died within 7 weeks. Animals subjected to lower
doses survived and seemed normal even after 50 weeks (Draize et al.,
1944). When the experiment was continued for 3 years, the animals
receiving doses of 50 and 80 mg/kg body-weight developed jaundice and
haemorrhagic symptoms. Those receiving 10 mg/kg body-weight daily
showed no ill effects (Hayes et al., 1956; Lehman, 1952).
Dogs were given DDT by stomach-tube, in the form of a 10%
solution in peanut oil in doses ranging from 150 to 350 mg/kg
body-weight for about 90 days. Some of the animals died. The authors
noted neurological symptoms, showing involvement of the cerebellum as
revealed by histological lesions (Haymaker et al., 1946).
Three dogs were given intramuscular injections of DDT in the form
of a 10% solution in olive oil at the rate of 100 mg/kg body-weight
daily for 25 to 30 days. A control animal received 1 ml of olive oil
in the same way. The experimental animals showed a temporary loss of
weight. The kidneys were discoloured and on histological examination
showed tubular damage. At the same time, proliferation was observed in
the lymphoid tissues (lymph nodes, spleen, bone marrow) and the wall
of the small intestine, but the blood showed no leukaemic
characteristics (Gerebtzoff et al., 1950; Gerebtzoff & Philippot,
1952).
Monkey. Monkeys given DDT orally in a dose of 0.2 mg/kg
body-weight for 7-9 months developed symptoms of hepatitis. After a
year, the animals showed liver enlargement and hyperglycaemia
(Shillinger et al., 1955). These liver changes were not confirmed in
another experiment on monkeys lasting 7.5 years (Durham et al., 1963).
Long-term studies
Rat. Groups of 12 male rats were subjected for 2 years to diets
containing 100, 200, 400 and 800 ppm of DDT, in the form of a 10%
solution in corn oil. In another experiment groups each of 24 rats (12
males and 12 females) were given, during the same period, diets
containing 200, 400, 600 and 800 ppm. Also additional groups of 24
animals received 600 and 800 ppm incorporated in their feed in a dry
state. In the groups receiving 400 ppm and above, an increase in the
mortality rate was seen in relation to the dose. Apart from nervous
symptoms at doses of 400 ppm and above, typical liver lesions were
found at all concentrations. Hepatic cell tumours were seen in 4 out
of 75 animals and 11 other rats showed nodular adenomatoid hyperplasia
(Fitzhugh & Nelson, 1947).
In an experiment with rats fed for 2 years on a diet containing
10 ppm of DDT, histological liver lesions were also observed
(Fitzhugh, 1948). Groups of 80 young rats each (40 male and 40 female)
were fed 0.25, 12.5 and 25 ppm of DDT. The histological lesions of the
liver observed were always slight and, according to the authors,
non-specific, but nevertheless they were more frequent with DDT than
with aldrin or dieldrin, which were administered in the same
concentrations for comparative purposes (Treon & Cleveland, 1955).
Experiments with 25 young rats did not show any harmful effects
after daily application by stomach-tube of a dose of 10 µg of DDT
equivalent to a dietary intake of 0.1-0.15 ppm for 17 months (Klimmer,
1955).
Monkey. Twenty-four Rhesus monkeys, 12 males and 12 females,
were divided into groups and fed over periods as long as 7.5 years or
more on diets containing respectively 5, 50, 200 and 5000 ppm of DDT.
All the animals subjected to the concentration of 5000 ppm showed
convulsions, accompanied by loss of appetite and a fall in weight. At
a concentration of 200 ppm or below (the latter dose corresponding to
daily doses of 2.2-5.54 mg/kg body-weight), no harmful effects were
observed and, in particular, no histological lesions in the liver or
disturbances in the functioning of that organ, as shown by the
bromsulfthalein test (Durham et al., 1963).
Observations on man. An experimental study was carried out of
the effects on man of prolonged ingestion of small doses of DDT (in
the form of oily solutions in capsules or emulsions in milk). The
authors used for this study, 51 volunteers; 17 received a normal diet,
17 received 3.5 mg/kg body-weight and 17 received 35 mg DDT daily. The
last dose is approximately 0.5 mg/kg body-weight. Administration was
continued for as long as 18 months. The authors noted that the
accumulation of the insecticide in the fatty tissues and the urinary
excretion of its metabolite, DDA, were proportional to the dose of DDT
ingested. A state of equilibrium was reached after about a year and
the concentration of DDT accumulated in the fatty tissues reached an
average of 234 ppm (101-367 ppm) in subjects who had ingested 35 mg of
DDT per day. Throughout the whole experiment, no subject complained of
malaise, nor did any ill-effects appear that could be attributed to
the ingestion of DDT (Hayes et al., 1956). The essential results were
confirmed in a separate investigation in which dosage with DDT lasted
for 21 months and the volunteers were observed for an additional 27
months. Fourteen men received 35 mg/man/day; 6 received 3.5
mg/man/day, and 4 men served as controls. The study also revealed the
relationship between storage of DDT And the urinary excretion of DDA
and demonstrated that the loss of DDT from storage in man following
cessation of dosage is slow - only about two-thirds in 27 months,
(Hayes et al., in preparation).
Observations were made on 40 workers engaged, over a period of
years, in the manufacturing of specialities based on DDT, under
conditions where suitable precautions had not been taken to protect
their skin. According to the DDA concentration found in the urine,
these subjects had absorbed daily as much DDT as volunteers who had
ingested 35 mg of the insecticide per day in the experiment mentioned
above. Thorough medical and biological tests failed to reveal any
toxic symptoms, even in workers exposed to the toxic product for 6.5
years (Ortelee, 1958).
More recent studies of the same and other workers, some with
longer exposures, confirmed the essential findings.
It has been shown by Durham et al. (1965) that the storage of DDT
in man is directly proportional to intake over a wide range of dosage
(0.04 to 35.0 mg/man/day) so that from the level in the fat the daily
intake can be estimate. From this relationship and from recent
measurements of DDT stored in human fat (Dale et al., 1965) it is
clear that the highest average intake of DDT of any human population
yet observed is about 0.68 mg/man/day. However, in the only country
where samples have been taken repeatedly, the level of storage of DDT
by the general population did not increase from 1950 through 1963, the
last year for which information is now available (Quinby et al.,
1965).
Comments on experimental studies reported
DDT has been studied extensively in several species of animals.
The rat is particularly susceptible. Concentrations as low as 5 and 10
ppm in the diet have caused changes in some male rats but these are
inconstant, reversible and they are always most prominent in the least
susceptible sex.
Of much greater importance is the enormous amount of work which
has been done on the effects of this compound in man. Doses equivalent
to 0.5 mg/kg body-weight daily have been given experimentally to a
group of significant size for as long as 21 months without effect
apart from storage in the fat.
EVALUATION
This has been based on the studies on man.
Estimate of acceptable daily intake for man
0-0.01 mg/kg/body-weight.
REFERENCES
Andronova, G. P. (1956) Dissertation, F. F. Erisman's State Research
Institute for Hygiene, Moscow
Anon. (1951) Report of the Committee on Pesticides of the Council on
Pharmacy and Chemistry, J. Amer. med. Ass., 145., 725
Biddulph, C., Bateman, G. Q., Bryson, M. J., Harris, J. R., Greenwood,
D. A., Binns, W., Miner, M. L., Harris, L. E. & Madsen, L. L. (1950)
Advances in Chemistry, 1, 237
Cueto, C., Barnes, A. G. & Mattson, A. M. (1956) J. Agr. Food Chem.,
4 (2), 943
Dale, W. E., Copeland, N. F. & Hayes, W. J., jr (1965) Bull. Wld.
Hlth Org. (In press)
Dale, W. E., Gaines, T. B. & Hayes, W. J., jr (1963) Science, 142,
1474
Draize, J. H., Woodard, G., Fitzhugh, O. G., Nelson, A. A., Smith, R.
B. & Calvery, H. O. (1944) J. Amer. chem. Soc., 22, 1503
Durham, W. F., Armstrong, J. F. & Quinby, G. E. (1965) J.
Environment. Hlth (In press)
Durham, W. F., Ortega, F. & Hayes, W. J., jr (1963) Arch. Int.
Pharmacodyn., 141, 111
Fitzhugh, O. G. (1948) Ind. Eng. Chem., 40, 704
Fitzhugh, O. G. & Nelson, A. A. (1947) J. Pharmacol. exp. Ther.,
89, 18
Gannon, N. & Decker. G. C. (1960) J. Econ. Ent., 53, 411
Gerebtzoff, M. A. & Philippot, E. (1952) Experientia (Basel, 8
(10), 395
Gerebtzoff, M. A., Philippot, E. & Dallemagne, M. J. (1950) C.R.
Soc. Biol. (Paris), 144, 1135
Hayes, W. J., jr & Dale, W. E. (1964) Toxicol. Appl. Pharmacol., 6
349
Hayes, W. J., jr, Dale, E. & Pirkle, C. I. (In preparation)
Hayes, W. J., jr, Durham, W. F. & Cueto, C., jr (1956) J. Amer. med.
Ass., 162 (9), 890
Hayes, W. J., jr, Walker, K. C., Elliott, J. W. & Durham, W. F. (1958)
Arch. industr. Hlth, 18, 398
Haymaker, W., Ginzler, A. M. & Ferguson, R. L. (1946) Amer. J. M.
Soc., 212, 423
Judah, J. D. (1949) Brit. J. Pharmacol., 4, 120
Khairy, M. (1959) Quart. J. exp. Psychol., 11, 84
Klimer, O. R. (1955) Arch. exp. Path. Pharmak., 227, 183
Laug, E. P., Kunze, F. M. & Prickett, C. S. (1951) Arch. industr.
Hyg., 3, 245
Laug, E. P., Nelson, A. A., Fitzhugh, O. G. & Kunze, F. M. (1950)
J. Pharmacol. exp. Ther., 98, 268
Lehman, A. J. (1952) Quart. Bull. Assoc. Food & Drug Officials,
U.S., 16, 3, 47, 126
Maier-Bode, H. (1960) Med. exp. (Basel), 1, 153
Mattson, A. M., Spillane, J. T., Baker, C. & Pearce, G. W. (1953)
Anal. Chem., 25, 1067
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia &
London, Saunders, vol. III
Ortega, P. (1962) Fed. Proc., 21, 306
Ortega, P., Hayes, W. J., jr, Durham, W. F. & Mattson, A. (1956)
Publ. Hlth Monogr. 43, 27 (33 references)
Ortelee, M. F. (1958) Arch. industr. Hlth, 18, 433
Pearce, G. W., Mattson, A. M. & Hayes, W. J., jr, (1952) Science,
116, 254
Phillips, W. E. G. (1963) Canad. J. Biochem., 41, 1793
Philips, F. & Gilman, A. (1946) J. Pharmacol. exp. Ther., 86, 213
Quinby, G. E., Hayes, W. J., jr, Armstrong, J. F. & Durham, W. F.
(1965) J. Amer. med. Ass., 191, 175
Rothe, C. F., Mattson. A. M., Nuelsein, R. M. & Hayes, W. J., jr
(1957) Arch. industr. Hlth, 16 (1), 82
Shillinger, U. I., Naumova, L. P. & Pererman, S. W. (1955)
Vep. Pitan., 14, 41
Smith, R. F., Hoskins, W. M. & Hullmer, O. H. (1948) J. econ. Ent.,
41, 759
Spicer, S. S., Sweeney, T. R., von Oettingen, W. F., Lillie, R. D. &
Neal, P. A. (1947) Vet. Med., 42 (8), 289
Telford, H. S. & Guthrie, J. E. (1945) Science, 102, 647
Treon, J. F. & Cleveland, F. P. (1955) J. Agr. Food Chem., 3, 402
Wilson. H. F. et al. (1946) J. econ. Ent., 39, 802
Woodard, G., Ofner, R. R. & Montgomery, C. M. (1945) Science, 102,
177
