
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
FERBAM
Chemical name
Ferric dimethyldithiocarbamate.
Synonym
Fermate
Empirical formula
C9H18N3S6Fe
Structural formula
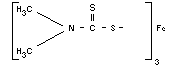 BIOLOGICAL DATA
Biochemical aspects
The compound does not appear to be stored in the tissues of rats
and dogs, but in the rat the feeding of ferbam increases the skeletal
stores of iron (Hodge et al., 1956).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 1 000 Zapp, 1950
Mouse Intraperitoneal 54 315 and 3 000* Hodge et al., 1952
Kligman & Rosenweig,
1948
Zapp, 1950
Rat Oral 5 700-17 000(ALD)** Hodge et al., 1952
Hodge et al., 1956
Zapp, 1950
Rat Intraperitoneal 2 700 Hodge et al., 1952
* Difference due to use of different vehicles.
** ALD = approximate lethal dose.
Short-term studies
Rat. Groups of 10 male and 10 female weanling rats were fed
diets containing 100, 500, 2500 and 500 ppm of ferbam for one month.
At 100 ppm no growth depression was found. At the 3 higher levels,
growth depression was found, while in the 5000 ppm group the mortality
rate was increased. No significant histopathological changes were seen
in any of the animals (Hodge et al., 1952).
Groups of 20 rats, 10 males and 10 females, were maintained for
one month on a diet containing 2500 ppm ferbam. Post-mortem
examination revealed no thyroid abnormalities (Hodge et al., 1952).
Dog. One dog was given ferbam and ziram together for one month,
each at a dosage of 5 mg/kg body-weight per day, without adverse
effect, except slight anaemia. Another remained healthy - again except
for slight anaemia - when given ferbam alone for one month at a dosage
of 25 mg/kg body-weight per day and also on 50 mg/kg body-weight per
day for one week, although an attempt to raise the dosage to 100 mg/kg
body-weight per day immediately provoked severe vomiting and malaise
(Hodge et al., 1952).
Groups, each of 2 adult dogs, were given daily doses of 0.5, 5
and 25 mg of ferbam per kg body-weight for one year. Convulsions
occurred at the highest dose level. Urine analysis, blood picture
organ weights and histology (including the thyroid) were normal (Hodge
et al., 1956).
Long-term studies
Rat. Weanling rats in groups of 25 males and 25 females were
fed for 2 years diets containing 25, 250 and 2500 ppm of ferbam.
Growth-rate was depressed and the life-span shortened only at the
highest concentration. In this group, moreover, neurological changes
appeared in the animals after 2 months; cystic brain lesions developed
and in some of the males the testes atrophied. The thyroid glands were
normal in all groups and there was no increase in tumour incidence
(Hodge et al., 1956).
Comments on experimental studies reported
For ferbam, as for most of the dithiocarbamates, short- and
long-term studies in animals have been reported, but for all of them
biochemical data are inadequate.
EVALUATION
The chemical nature of the residues of the dithiocarbamates in
or on the plant has not been ascertained. The compounds themselves
have effects on the thyroid, nervous system and blood in animals. In
the absence of information about their mode of action an acceptable
intake for man cannot be estimated.
Further work required
Determination and evaluation of toxicity of the residues
occurring in the plant. Extension of the long-term studies, including
reproduction studies which should concern at least 2 species. Special
attention should be given to neurological changes, goitrogenicity, and
occurrence of anaemia.
REFERENCES
Hodge. H. C., Maynard. E. A., Downs, W., Blanchet, H. J. & Jones,
C. K. (1952) J. Amer. pharm. Ass., sci. Ed., 41, 662
Hodge, H. C., Maynard, E. A., Downs, W. L., Coye, R. D. & Steadman,
L. T. (1956) J. Phamacol. exp. Ther., 118, 174
Kligman, A. M. & Rosenweig, W. (1948) J. invest. Derm., 10, 59
Zapp, J. R. (1950) United States food and drug residue tolerance
hearings, FDC, 57, 7757-7789
BIOLOGICAL DATA
Biochemical aspects
The compound does not appear to be stored in the tissues of rats
and dogs, but in the rat the feeding of ferbam increases the skeletal
stores of iron (Hodge et al., 1956).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 1 000 Zapp, 1950
Mouse Intraperitoneal 54 315 and 3 000* Hodge et al., 1952
Kligman & Rosenweig,
1948
Zapp, 1950
Rat Oral 5 700-17 000(ALD)** Hodge et al., 1952
Hodge et al., 1956
Zapp, 1950
Rat Intraperitoneal 2 700 Hodge et al., 1952
* Difference due to use of different vehicles.
** ALD = approximate lethal dose.
Short-term studies
Rat. Groups of 10 male and 10 female weanling rats were fed
diets containing 100, 500, 2500 and 500 ppm of ferbam for one month.
At 100 ppm no growth depression was found. At the 3 higher levels,
growth depression was found, while in the 5000 ppm group the mortality
rate was increased. No significant histopathological changes were seen
in any of the animals (Hodge et al., 1952).
Groups of 20 rats, 10 males and 10 females, were maintained for
one month on a diet containing 2500 ppm ferbam. Post-mortem
examination revealed no thyroid abnormalities (Hodge et al., 1952).
Dog. One dog was given ferbam and ziram together for one month,
each at a dosage of 5 mg/kg body-weight per day, without adverse
effect, except slight anaemia. Another remained healthy - again except
for slight anaemia - when given ferbam alone for one month at a dosage
of 25 mg/kg body-weight per day and also on 50 mg/kg body-weight per
day for one week, although an attempt to raise the dosage to 100 mg/kg
body-weight per day immediately provoked severe vomiting and malaise
(Hodge et al., 1952).
Groups, each of 2 adult dogs, were given daily doses of 0.5, 5
and 25 mg of ferbam per kg body-weight for one year. Convulsions
occurred at the highest dose level. Urine analysis, blood picture
organ weights and histology (including the thyroid) were normal (Hodge
et al., 1956).
Long-term studies
Rat. Weanling rats in groups of 25 males and 25 females were
fed for 2 years diets containing 25, 250 and 2500 ppm of ferbam.
Growth-rate was depressed and the life-span shortened only at the
highest concentration. In this group, moreover, neurological changes
appeared in the animals after 2 months; cystic brain lesions developed
and in some of the males the testes atrophied. The thyroid glands were
normal in all groups and there was no increase in tumour incidence
(Hodge et al., 1956).
Comments on experimental studies reported
For ferbam, as for most of the dithiocarbamates, short- and
long-term studies in animals have been reported, but for all of them
biochemical data are inadequate.
EVALUATION
The chemical nature of the residues of the dithiocarbamates in
or on the plant has not been ascertained. The compounds themselves
have effects on the thyroid, nervous system and blood in animals. In
the absence of information about their mode of action an acceptable
intake for man cannot be estimated.
Further work required
Determination and evaluation of toxicity of the residues
occurring in the plant. Extension of the long-term studies, including
reproduction studies which should concern at least 2 species. Special
attention should be given to neurological changes, goitrogenicity, and
occurrence of anaemia.
REFERENCES
Hodge. H. C., Maynard. E. A., Downs, W., Blanchet, H. J. & Jones,
C. K. (1952) J. Amer. pharm. Ass., sci. Ed., 41, 662
Hodge, H. C., Maynard, E. A., Downs, W. L., Coye, R. D. & Steadman,
L. T. (1956) J. Phamacol. exp. Ther., 118, 174
Kligman, A. M. & Rosenweig, W. (1948) J. invest. Derm., 10, 59
Zapp, J. R. (1950) United States food and drug residue tolerance
hearings, FDC, 57, 7757-7789
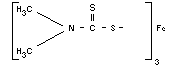 BIOLOGICAL DATA
Biochemical aspects
The compound does not appear to be stored in the tissues of rats
and dogs, but in the rat the feeding of ferbam increases the skeletal
stores of iron (Hodge et al., 1956).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 1 000 Zapp, 1950
Mouse Intraperitoneal 54 315 and 3 000* Hodge et al., 1952
Kligman & Rosenweig,
1948
Zapp, 1950
Rat Oral 5 700-17 000(ALD)** Hodge et al., 1952
Hodge et al., 1956
Zapp, 1950
Rat Intraperitoneal 2 700 Hodge et al., 1952
* Difference due to use of different vehicles.
** ALD = approximate lethal dose.
Short-term studies
Rat. Groups of 10 male and 10 female weanling rats were fed
diets containing 100, 500, 2500 and 500 ppm of ferbam for one month.
At 100 ppm no growth depression was found. At the 3 higher levels,
growth depression was found, while in the 5000 ppm group the mortality
rate was increased. No significant histopathological changes were seen
in any of the animals (Hodge et al., 1952).
Groups of 20 rats, 10 males and 10 females, were maintained for
one month on a diet containing 2500 ppm ferbam. Post-mortem
examination revealed no thyroid abnormalities (Hodge et al., 1952).
Dog. One dog was given ferbam and ziram together for one month,
each at a dosage of 5 mg/kg body-weight per day, without adverse
effect, except slight anaemia. Another remained healthy - again except
for slight anaemia - when given ferbam alone for one month at a dosage
of 25 mg/kg body-weight per day and also on 50 mg/kg body-weight per
day for one week, although an attempt to raise the dosage to 100 mg/kg
body-weight per day immediately provoked severe vomiting and malaise
(Hodge et al., 1952).
Groups, each of 2 adult dogs, were given daily doses of 0.5, 5
and 25 mg of ferbam per kg body-weight for one year. Convulsions
occurred at the highest dose level. Urine analysis, blood picture
organ weights and histology (including the thyroid) were normal (Hodge
et al., 1956).
Long-term studies
Rat. Weanling rats in groups of 25 males and 25 females were
fed for 2 years diets containing 25, 250 and 2500 ppm of ferbam.
Growth-rate was depressed and the life-span shortened only at the
highest concentration. In this group, moreover, neurological changes
appeared in the animals after 2 months; cystic brain lesions developed
and in some of the males the testes atrophied. The thyroid glands were
normal in all groups and there was no increase in tumour incidence
(Hodge et al., 1956).
Comments on experimental studies reported
For ferbam, as for most of the dithiocarbamates, short- and
long-term studies in animals have been reported, but for all of them
biochemical data are inadequate.
EVALUATION
The chemical nature of the residues of the dithiocarbamates in
or on the plant has not been ascertained. The compounds themselves
have effects on the thyroid, nervous system and blood in animals. In
the absence of information about their mode of action an acceptable
intake for man cannot be estimated.
Further work required
Determination and evaluation of toxicity of the residues
occurring in the plant. Extension of the long-term studies, including
reproduction studies which should concern at least 2 species. Special
attention should be given to neurological changes, goitrogenicity, and
occurrence of anaemia.
REFERENCES
Hodge. H. C., Maynard. E. A., Downs, W., Blanchet, H. J. & Jones,
C. K. (1952) J. Amer. pharm. Ass., sci. Ed., 41, 662
Hodge, H. C., Maynard, E. A., Downs, W. L., Coye, R. D. & Steadman,
L. T. (1956) J. Phamacol. exp. Ther., 118, 174
Kligman, A. M. & Rosenweig, W. (1948) J. invest. Derm., 10, 59
Zapp, J. R. (1950) United States food and drug residue tolerance
hearings, FDC, 57, 7757-7789
BIOLOGICAL DATA
Biochemical aspects
The compound does not appear to be stored in the tissues of rats
and dogs, but in the rat the feeding of ferbam increases the skeletal
stores of iron (Hodge et al., 1956).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 1 000 Zapp, 1950
Mouse Intraperitoneal 54 315 and 3 000* Hodge et al., 1952
Kligman & Rosenweig,
1948
Zapp, 1950
Rat Oral 5 700-17 000(ALD)** Hodge et al., 1952
Hodge et al., 1956
Zapp, 1950
Rat Intraperitoneal 2 700 Hodge et al., 1952
* Difference due to use of different vehicles.
** ALD = approximate lethal dose.
Short-term studies
Rat. Groups of 10 male and 10 female weanling rats were fed
diets containing 100, 500, 2500 and 500 ppm of ferbam for one month.
At 100 ppm no growth depression was found. At the 3 higher levels,
growth depression was found, while in the 5000 ppm group the mortality
rate was increased. No significant histopathological changes were seen
in any of the animals (Hodge et al., 1952).
Groups of 20 rats, 10 males and 10 females, were maintained for
one month on a diet containing 2500 ppm ferbam. Post-mortem
examination revealed no thyroid abnormalities (Hodge et al., 1952).
Dog. One dog was given ferbam and ziram together for one month,
each at a dosage of 5 mg/kg body-weight per day, without adverse
effect, except slight anaemia. Another remained healthy - again except
for slight anaemia - when given ferbam alone for one month at a dosage
of 25 mg/kg body-weight per day and also on 50 mg/kg body-weight per
day for one week, although an attempt to raise the dosage to 100 mg/kg
body-weight per day immediately provoked severe vomiting and malaise
(Hodge et al., 1952).
Groups, each of 2 adult dogs, were given daily doses of 0.5, 5
and 25 mg of ferbam per kg body-weight for one year. Convulsions
occurred at the highest dose level. Urine analysis, blood picture
organ weights and histology (including the thyroid) were normal (Hodge
et al., 1956).
Long-term studies
Rat. Weanling rats in groups of 25 males and 25 females were
fed for 2 years diets containing 25, 250 and 2500 ppm of ferbam.
Growth-rate was depressed and the life-span shortened only at the
highest concentration. In this group, moreover, neurological changes
appeared in the animals after 2 months; cystic brain lesions developed
and in some of the males the testes atrophied. The thyroid glands were
normal in all groups and there was no increase in tumour incidence
(Hodge et al., 1956).
Comments on experimental studies reported
For ferbam, as for most of the dithiocarbamates, short- and
long-term studies in animals have been reported, but for all of them
biochemical data are inadequate.
EVALUATION
The chemical nature of the residues of the dithiocarbamates in
or on the plant has not been ascertained. The compounds themselves
have effects on the thyroid, nervous system and blood in animals. In
the absence of information about their mode of action an acceptable
intake for man cannot be estimated.
Further work required
Determination and evaluation of toxicity of the residues
occurring in the plant. Extension of the long-term studies, including
reproduction studies which should concern at least 2 species. Special
attention should be given to neurological changes, goitrogenicity, and
occurrence of anaemia.
REFERENCES
Hodge. H. C., Maynard. E. A., Downs, W., Blanchet, H. J. & Jones,
C. K. (1952) J. Amer. pharm. Ass., sci. Ed., 41, 662
Hodge, H. C., Maynard, E. A., Downs, W. L., Coye, R. D. & Steadman,
L. T. (1956) J. Phamacol. exp. Ther., 118, 174
Kligman, A. M. & Rosenweig, W. (1948) J. invest. Derm., 10, 59
Zapp, J. R. (1950) United States food and drug residue tolerance
hearings, FDC, 57, 7757-7789
