
FAO/PL:1968/M/9/1
WHO/FOOD ADD./69.35
1968 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Geneva, 9-16 December,
1968.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Geneva, 1969
AZINPHOS-METHYL
This pesticide was evaluated for acceptable daily intake by the Joint
Meeting of the FAO Committee on Pesticides in Agriculture and the WHO
Expert Committee on Pesticide Residues (FAO/WHO, 1965).
Since that time information has become available on the identity of
the technical material and its residues in food and their evaluation.
Therefore the previously published monograph has been greatly expanded
and is reproduced in its entirety below.
IDENTITY
Chemical names
S-(3,4-dihydro-4-oxo-benzo[d]-[1,2,3]-triazin-3-ylmethyl)
dimethyl phosphorothiolthionate
S-(3,4-dihydro-4-oxo-benzo[d]-[1,2,3]-triazin-3-ylmethyl)
OO-dimethyl phosphorodithioate (IUPAC).
Synonyms
GuthionR, GusathionR, Bayer 17147.
Structural formula
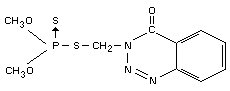 Other information on identity and properties
The pure material is a white, crystalline solid, M.P. 73-74°C; soluble
in water at 25°C (1:30 000) and soluble in most organic solvents. The
technical product is a brown waxy solid (M.P. 65-68°C). It decomposes
at elevated temperatures with the evolution of gas, and is rapidly
hydrolyzed by cold alkali to form anthranilic acid and is subject to
hydrolysis by acids. Suitable oxidizing agents convert it to the
oxygen analogue.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Azinphos-methyl is activated to gutoxon, a highly potent
cholinesterase inhibitor which has a molar I50 in rat brain of 2.99 ×
10-8 (Schrader, 1963). In vitro, the rate of degradation of gutoxon
by liver microsomal enzymes is not altered by species, or sex
variation in mammals (Johnsen and Dahm, 1966). Also the rate of in
vitro metabolism by the liver is similar in mammals, birds, and fish
(Murphy, 1966).
In vitro studies showed that azinphos-methyl is a poor brain
cholinesterase inhibitor in mammalian, avian, or piscine species but
gutoxon is a potent inhibitor; avian brain cholinesterase inhibition
being less than that in mammals, and fish brain cholinesterase being
the most susceptible to inhibition. Species differences in sensitivity
to gutoxon inhibition of brain cholinesterase are probably
sufficiently large to modify the influence of variation in metabolic
rates (Murphy et al., 1968).
Acute toxicity
LD50 (mg/kg
Animal Route body-weight) Reference
Mouse Oral 8* Sato, 1959
Mouse i.p. 8-10** Murphy, 1966
Rat Oral 11-25 DuBois et al., 1955
Gaines, 1960
Rat i.p. 5-11.6 DuBois et al., 1955
Guinea-pig Oral 80 DuBois et al., 1955
Guinea-pig i.p. 40 DuBois et al., 1955
* Given as azinphos-methyl emulsion.
** Given as a corn oil solution.
Short-term studies
Rat
Groups of 10 male rats were fed 0, 5, 10, 20, 50 or 100 ppm
azinphos-methyl in the diet for nine weeks. At 50, and 100 ppm slight
decrease in food intake, decrease in body-weight gain, and muscular
spasms and trembling were observed. Whole blood cholinesterase
activity was markedly decreased at 20, 50 and 100 ppm. At 5 and 10 ppm
the maximum depression which occurred, was 20 per cent below the
control values. (Huntingdon Research Centre, 1966.)
The addition of azinphos-methyl to the diet of groups of 10 young male
and 10 young female rats each, at levels of 2, 5 and 20 ppm did not
markedly alter the growth-rate over 60 days. The body-weight of the
male rats fed 20 ppm was about four per cent less than that of the
controls. In the rats fed 2 and 5 ppm for 120 days, cholinesterase
activity was unaffected, but at 20 ppm there was inhibition in the
brain (10 per cent) and in serum and erythrocytes (about 30
per cent). After 120 days no appreciable changes in the gross and
microscopic appearance of brain, heart, liver, spleen, adrenals,
stomach, intestines, skeletal muscle and bone-marrow were found. There
was no evidence of demyelination in the nervous system (Doull et al.,
1956).
When weanling male rats were fed diets containing 50 and 100 ppm of
azinphos-methyl for 16 weeks, approximately half of each group died.
All animals receiving azinphos-methyl in the diet showed marked
effects of cholinergic stimulation, including diarrhoea, salivation,
lacrimation, muscular tremors and fasciculations. These symptoms were
most marked during the first month on the diets. The rats fed 50 ppm
of azinphos-methyl weighed about 10 per cent less, and the rats fed
100 ppm, 18 per cent less than rats fed a normal diet. The
cholinesterase activity of the serum, brain, erythrocytes and
submaxillary glands of rats fed 50 and 100 ppm was markedly inhibited.
The inhibition was most marked in the erythrocytes and brain and the
animals did not fully recover from these effects during a three-week
period on a normal diet. Both gross and microscopic examination failed
to indicate any evidence of testicular atrophy due to the presence of
the high levels of azinphos-methyl in the diet (Doull et al., 1957a).
Dog
Groups of two dogs, one male and one female, given 5, 10, 20 and 50
ppm of azinphos-methyl in their diet did not show any loss of weight
nor any symptoms of azinphos-methyl poisoning during a 12-week period.
At levels of 5, 10 and 20 ppm there was no significant decrease, but
at 50 ppm there was a 25 per cent decrease in serum cholinesterase
activity at the end of the 12-week period. The erythrocyte
cholinesterase of the one male and one female dog began to be
inhibited at the 10 ppm level (Doull et al., 1967b).
Six groups of one male and one female dogs were fed 0, 20, 50, 100,
200 or 400 ppm of azinphos-methyl in a dry diet, for 19 weeks. Whole
blood cholinesterase depression was slight at 20 and 50 ppm, becoming
apparent after four weeks on the diet; however, reactivation occurred
by the sixth week at 20 ppm, and by the ninth week at 50 ppm. At the
100 ppm level and above, cholinesterase activity was reduced by more
than 50 per cent; reactivation was not apparent at 400 ppm, was
doubtful at 200 ppm, and was slight at 100 ppm after 17 weeks. (Loser
and Lorke, 1967.)
Four groups of four male and four female dogs along with a control
group, were fed azinphos-methyl in a dry diet for two years. At the
lowest level the dogs were fed 5 ppm throughout the two-year period.
At the second level they were fed 20 ppm for 36 weeks, followed by 50
ppm for 15 months. At the third level the dogs received 50 ppm
throughout the two-year period. At the top dose level they received 50
ppm for 36 weeks, followed by 100 ppm for 21 weeks, 150 ppm for 27
weeks and finally 300 ppm for 21 weeks. Mortality throughout was
comparable to the controls. At 300 ppm, tremors, muscular weakness and
abnormal quietness were noted, especially among the male animals.
Weight loss occurred to a slight degree at this dose level. Food
intake was slightly reduced in females at 150 and 300 ppm.
Cholinesterase depression was not apparent during the first three
months of the study. In the period three to nine months, red blood
cell cholinesterase was slightly depressed at 20 ppm, and more
markedly depressed at 50 ppm. In the second year of the study, plasma
cholinesterase depression was apparent in dogs receiving 50 ppm and
above. Red blood cell cholinesterase continued to be depressed at
intermediate and high dose levels, and minimal depression was also
seen at 5 ppm. Other parameters, including haematology, clinical
chemistry, urinalysis, organ to body-weight ratios, and gross and
histopathology were all comparable to controls. (Huntingdon Research
Centre, 1966b.)
Long-term studies
Rat
Groups of 40 male and 40 female rats were fed 2.5, 5 and 20 ppm for
two years and a top group was fed 50 ppm for 47 weeks and then 100
ppm. Two control groups were also maintained. At 100 ppm, toxic
symptoms in the form of salivation, diuresis, exophthalmos, loss of
balance and co-ordination, muscular fasciculation, and minor tremors
were observed in five females. Mortality, food intake, body-weight
gain, food utilization, urinalysis, and haematology were comparable to
controls. Depression of cholinesterase levels of red blood cells and
plasma occurred at 20 ppm and above, and plasma cholinesterase
depression was apparent up to 39 weeks at 5 ppm in the males. In the
females, consistent depression of plasma and red blood cell
cholinesterase was apparent only at the top dose. At 20 ppm plasma
cholinesterase depression occurred up to and including 39 weeks. At 5
ppm depression occurred at 10, 39 and 78 weeks. Red blood cell
cholinesterase was depressed up to 65 weeks at 20 ppm, but only up to
10 weeks at 5 ppm. Brain cholinesterase depression was significantly
depressed at the top dose level only. Organ to body-weight ratios
showed random variations, a possible increase with the liver occurring
at the top dose level. There was no indication that tumour incidence
was increased and it was concluded that azinphos-methyl was devoid of
carcinogenic activity in the rat. Gross and histopathology showed no
compound related effects. (Huntingdon Research Centre, 1966,)
Special studies
Reproduction
Mouse. Groups of six male and 24 female mice were fed 0, 5, 10, 25
and 50 ppm in the diet over three generations. Initial exposure to the
diet was for 30 days prior to the first mating of the Fo generation.
Because of the high mortality (15 out of 24) among the females at 50
ppm prior to mating, this dose level was eliminated after the first
mating. Fertility and litter size were not affected in the 50 ppm
group, but survival to weaning was significantly decreased. Up to and
including 25 ppm no adverse effects were apparent as judged by
fertility, gestation or lactation, litter size, or survival of
offspring to 30 days. Gross and microscopic examination of F3b
weanlings showed no compound related changes. (Root et al., 1965.)
Rabbit. Three groups of 10 pregnant female rabbits were fed 0, 5 or
25 ppm in the diet, from the eighth to the sixteenth day of pregnancy.
Five rabbits in each group were sacrificed on the twenty-ninth day of
pregnancy, the remainder being permitted to litter. No compound
related effects occurred with respect to litter size, stillbirths, sex
ratios, average foetal weights, incidence of immature foetuses, or
survival to 30 days. (Doull et al., 1966.)
Comments
Since the last evaluation by the FAO/WHO Joint Meeting (Rome, 15-22
March 1965), results of reproduction studies in mice, short-term
studies in dogs and long-term studies in rats have been provided. The
studies performed appear satisfactory. Further work desirable includes
studies on cholinesterase inhibition of plasma and erythrocytes in man
and metabolic studies in man.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effects
Rat: 2.5 ppm in the diet, equivalent to 0.125 mg/kg.
Dog: About 5 ppm in the dry diet, equivalent to 0.125 mg/kg.
Estimate of acceptable daily intake of azinphos-methyl for man
0-0.0025 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Azinphos-methyl is used for the pre-harvest control of a wide spectrum
of insects and mites attacking fruit, vegetable and forage crops.
Residues resulting from supervised trials
The following typical data are extracted from Chemagro internal
reports of trials made at various field stations.
Rate of application No. of Pre-harvest Residue
Crop (kg/ha) treatments interval (days) (ppm)
Alfalfa 0.50 1 5 0.4
Apples 0.28-0.35 7 7 0.75
Apricots 0.28-0.35 1 15 4.6
21 3.1
Broccoli 0.56 5 15 0.38
Brussels 0.56 1 7 0.6
sprouts 14 n.d.
Cabbage 0.56 3 15 n.d.
Cherries 0.28-0.35 1 7 0.18-0.74
14 n.d.-0.23
Clover 0.50 1 5 0.4
Grapes 0.28-0.35 1 15 0.6
22 0.4
0.28-0.35 2 16 5.1
23 3.7
Grapefruit 1.12 1 15 0.3-0.9
Peaches 0.28-0.35 1 19 1.0
31 0.4
Pears 0.28-0.35 1 8 0.3
Peas 0.56 3 7 n.d.
Plums 0.28-0.56 1 14 n.d.-0.25
Strawberries 1.12 1 7 0.9-1.5
14 0.7
Tomatoes 1.56 1 7 0.09
Plums 0.28-0.56 1 14 n.d.-0.25
Strawberries 1.12 1 7 0.9-1.5
14 0.7
Tomatoes 1.56 1 7 0.09
n.d. = not detectable.
Evidence of residues in food moving in commerce or at consumption
No data available.
Fate of residues
In plants. Azinphos-methyl has not been shown to exhibit systemic
action. No phosphorothiolate oxidation product could be found in or on
sprayed cotton leaves (Tietz et al., 1957, 1960). Two unidentified
phosphorus-containing metabolites, more lipophilic than the applied
chemical, appeared and gave positive tests for anthranilic acid
(Meagher et al., 1960). Further metabolites were recovered containing
the radioactive phosphorus in phospholipids and as hydrolysis
products.
With lettuce, most of the chemical remained on the surface and no
oxygen analogue was isolated (Magill et al., 1966). After 14 days, 95
per cent of the extracted residue was azinphos-methyl with four
components in the remaining five per cent Two were benzazimide and
methyl benzazimide sulfide while the remaining two were not
identified.
In animals. P32 and C14-labelled material administered orally to
dairy cows did not result in azinphos-methyl or the P=0 derivative in
the blood or milk (Everett et al., 1966). Excluded possible
metabolites were anthranilic acid, benzazimide, hydroxymethyl
benzazimide, mercaptomethyl benzazimide, N-methyl benzazimide, bis
(N-methyl benzazimide) sulfide and the corresponding disulfide.
However four unidentified components containing the benzazimide
moiety, appeared, one of which accounted for 90 per cent of the total
residue. They contribute a maximum of 0.017 ppm in the milk when
feeding the animal at the rate of 0.2 mg/kg, equivalent to 2.8 ppm in
the feed. At least two of the metabolites may be oxidation products of
an intermediate metabolite, bis (N-methylbenzazimide) sulfide. Thus
the residue in milk from feeding forage containing 2.8 ppm
azinphos-methyl will be 0.008 ppm expressed as mercaptomethyl
benzazimide while the residue in tissues will not exceed 0.1 ppm.
The fluorometric method of Adams and Anderson (1966) is suitable for
determining these metabolites.
In storage and processing. Residues of azinphos-methyl were stable
at frozen storage levels (-18 to -23°C) in samples of fruit, fodder
and vegetables (Chemagro, 1962). Washing oranges reduced the residues
from 1.0 ppm to 0.7 ppm (whole fruit), from 2.7 to 1.9 ppm in the
peel. While the juice and pulp were free of residues, the orange oil
contained 30 ppm (Anderson et al., 1963). Simple washing reduces
residues on the peel from 71 to 96 per cent, the greater amount with
the fresher residue (Gunther et al., 1963). Field-sprayed snap beans
bearing an initial residue of 1.09 ppm had a residue of 0.14 ppm after
washing, blanching and freezing (Carlin et al., 1966). After canning
the residue was reduced to 0.02 ppm. Soy-bean oil fortified with
azinphos-methyl and deodorized according to commercial procedures had
the residue reduced by 36 per cent (Thornton, 1967b). Sugar-cane
containing 0.1 to 0.6 ppm showed no residues in the molasses or sugar
when processed (FAO/WHO, 1965).
Methods of residue analysis
Colorimetric, gas chromatographic, fluorometric, infra-red and
polarographic methods have been developed. One commonly used procedure
is based on weak alkaline hydrolysis of the residue to liberate
anthranilic acid which is then diazotized and coupled with
N-(1-naphthyl)-ethylenediamine dihydrochloride and the resultant
purple colour measured at 555 mµ (Meagher et al., 1960; MacDougall,
1964; Cohen et al., 1966). Modifications have been made to shorten the
procedure (Cox, 1964). Miles (1964) has done this by direct coupling
of the benzotriazinyl-containing residue in an acetic-hydrochloric
acid mixture and measuring the blue-violet colour at 556 mµ. Smart
(1967) has modified the method by introducing an improved extraction
procedure.
Another colorimetric method involves the alkali cleavage and
measurement of the dimethyl phosphorodithioate as the copper complex
absorbance at 420 mµ. This measures the parent compound only and is
similar to the infra-red measurement using the P=S stretching
vibration at 654 cm-1 (15.25 microns) (Cohen et al., 1966),
The sensitivity of the anthranilic acid colorimetric method can be
increased to about 0.05 ppm by using 10 cm cells. The precision for
recovery of azinphos-methyl from a number of crops indicates that in
the range of 0.2 to 0.5 ppm the average deviation from the mean is
approximately six per cent.
A spectrophotofluorometric method is based on the measurement of the
anthranilic acid released on alkaline hydrolysis by determining
fluorometrically at an activating wavelength of 340 mµ and a
fluorescence of 400 mµ (Adams and Anderson, 1966). It has a
sensitivity of about 0.005 ppm for milk, 0.02 ppm for most animal
tissues and 0.03 ppm for fat.
More recently a gas chromatographic procedure has been developed for
the determination of residues in soy-beans using the potassium
chloride thermionic emission flame detector for phosphorus (Thornton,
1967a). This method is sensitive to 0.005 ppm and distinguishes
between azinphos-methyl and its P=O analogue.
A method based on the measurement of the formaldehyde released on acid
hydrolysis of the residue has been used (Giang and Schechter, 1958) as
well as a polarographic analysis (Bates, 1962).
National tolerances
Tolerance Pre-harvest
Country Crop (ppm) interval (days)
Australia 21
Austria 21
Benelux fruit, grapes 0.5
Brazil fruit, vegetables 2.0
cotton seed 0.5
Canada grapes 0.5
fruits, vegetables 1-2.0
Denmark 21
France 15
Germany fruit, grapes,
(Fed. Rep.) vegetables 0.4 14
Italy 0.4 20
New Zealand berry fruit,
leafy vegetables 21
pip and stone
fruit, root
crops, tomatoes 14
Norway 21
Portugal 21
South Africa citrus fruit 2.0 21
pome fruit 14
Switzerland fruit, grapes 1.0 21
United Kingdom 21
United States grapes 5.0
of America fruit, vegetables 2.0
cereals, soy-beans,
nuts, peas,
cucumbers, melons
peppers and
potatoes "zero"
RECOMMENDATION FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Since the publication of the monographs resulting from the 1965 Joint
Meeting, work has confirmed that the residue on plants is mainly
present as the original compound, azinphos-methyl. In the five per
cent of residue which is not accounted for as azinphos-methyl in
lettuce 14 days after treatment, out of four metabolites present two
were identified as benzazimide and methyl benzazimide.
Investigations with dairy cows indicate that no residues of
azinphos-methyl or the oxygen analogue will occur in milk from animals
consuming fodder likely to contain residues. However, four
non-phosphorus-containing metabolites, but still containing the
benzazimide moiety, may be present, although they have not been
precisely identified.
Although colorimetric and gas-liquid chromatographic methods have been
developed, none of these have been evaluated for regulatory or referee
purposes.
Recommendations
The following temporary tolerances (to be in effect until 1972) are
to apply to raw agricultural products moving in commerce unless
otherwise indicated. In the case of fruits and vegetables the
tolerances should be applied as soon as practicable after harvest and
in any event prior to actual retail to the public. In the case of
commodities entering international trade, the tolerances should be
applied by the importing country at the point of entry or as soon as
practicable thereafter. Furthermore, the tolerances should not apply
to the ethyl derivative nor to the oxygen analogue, the latter usually
being insignificant.
Temporary tolerances
Apricots and grapes 4 ppm
Other fruits 1 ppm
Vegetables 0.5 ppm
Further work or information
Required before 30 June 1972
1. Information on the nature of terminal residues in plants, animals
and their products.
2. Further data on residue levels in raw agricultural products moving
in commerce.
3. Data on disappearance of residues during storage and household
cooking of vegetables.
4. Data on the possible carry-over of residues into wine as a result
of the treatment of grapes.
5. Comparative evaluation of gas-liquid chromatographic and
spectrophotometric methods for the determination of azinphos-methyl
and its oxygen analogue for regulatory purposes.
Desirable
1. Studies on cholinesterase inhibition of plasm and erythrocytes in
man.
2. Metabolic studies in man.
3. Identification and toxicology of metabolites, especially those
having the benzazimide moiety, in milk.
4. Collaborative studies to establish a referee method.
REFERENCES
Adams, J. M. and Anderson, C. A. (1966) Spectrophotofluorometric
method for Guthion residues in milk and animal tissues. J. Agric. Food
Chem., 14: 53-55
Anderson, C. A., MacDougall, D., Kesterson, J. W., Hendrickson, R, and
Brooks, R. F. (1963) The effect of processing on Guthion residues in
oranges and orange products. J. Agric. Food Chem., 11: 422-424
Bates, J. A. R. (1962) Polarographic determination of azinphos-methyl
residues in certain crops. Analyst, 87: 786-790
Carlin, A. F., Hibbs, E. T. and Dahm, P. A. (1966) Insecticide
residues and sensory evaluation of canned and frozen snap beans field
sprayed with Guthion and DDT. Food Technol., 20: 80-83
Chemagro. (1962) Effect of frozen storage on Guthion residues in
various crops. Report No. 8682
Cohen, C. J., Betker, W. R., Wasleski, D. M. and Cavaghol, J. C.
(1966) Analysis of Guthion insecticide, J. Agric. Food Chem., 14:
315-318
Cox, W. S. (1964) Rapid determination of Guthion residues on crops.
J.A.O.A.C., 47: 280-282
Doull, J., Anido, P. and DuBois, K. P. (1957a) Effect of high dietary
levels of guthion on rats. University of Chicago. Unpublished report
Doull, J., Anido, P. and DuBois, K. P. (1957b) Determination of the
safe dietary level of guthion for dogs. University of Chicago.
Unpublished report
Doull, J., DiGiacomo, R. and Meskauskas, J. (1966) Short term breeding
studies with guthion in rabbits. University of Chicago. Unpublished
report
Doull, J., Rehfuss, P. A. and DuBois, K. P. (1956) The effects of
diets containing guthion (Bayer 17147) on rats. University of
Chicago. Unpublished report
DuBois, K. P., Thursh, D. R. and Murphy, S. D. (1955) The acute
mammalian toxicity and mechanism of action of Bayer 17147. University
of Chicago. Unpublished report
DuBois, K. P., Thursh, D. R. and Murphy, S. D. (1957) Studies on the
toxicity and pharmacologic actions of the dimethoxy ester of
benzotriazine dithiophosphoric acid (DBD guthion). J. Pharmacol.exp.
Ther., 119: 208-218
Everett, L. J., Anderson, C. A. and MacDougall, D. (1966) Nature and
extent of Guthion residues in milk and tissues resulting from treated
forage. J. Agric. Food Chem. 14: 47-53
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Mtg. Rept. PL/1965/10/1; WHO/Food Add./27.65
Gaines, T. B. (1960) The acute toxicity of pesticides to rats,
Toxicol. appl. Pharmacol., 2: 88-99
Giang, P. A. and Schechter, M. S. (1958) Colorimetric method for
estimation of Guthion residues in cottonseeds and cottonseed oil. J.
Agric. Food Chem., 6: 845-848
Gunther, F. A., Carman, G. E., Blinn, R. C. and Barkley, J. H. (1963)
Persistence of residues of Guthion on and in mature lemons and oranges
and in laboratory processed citrus "pulp" cattle feed. J. Agric. Food
Chem., 11: 424-427
Huntingdon Research Centre. (1966a) Toxicity of gusathion during
repeated administration to rats for two years. Unpublished report
Huntingdon Research Centre. (1966b) Gusathion (Bayer 17147), Chronic
oral toxicity studies in dogs. Unpublished report
Johnsen, R. E. and Dahm, P. A. (1966) Activation and degradation
efficiencies of liver microsomes from eight vertebrate species, using
organophosphates as substrates, J. Econ. Entomol., 59: 1437-1442
Löser, E. and Lorke, D. (1967) Die Aktivität der Cholinesterase bei
Hunden nach Verabreichung von Gusathion mit dem Futter. Farbenfabriken
Bayer. Unpublished report
MacDougall, D. (1964) Guthion. In: G. Zweig, Analytical Methods for
Pesticides, Plant Growth Regulators and Food Additives, vol. II,
Academic Press, New York-London
Magill, L. J. and Everett, L. J. (1966) Guthion-C14 study on lettuce.
Chemagro Report No. 18636
Meagher, W. R., Adams, J. M., Anderson, C. A. and MacDougall, D.
(1960) Colorimetric determination of Guthion residues in crops.
J. Agric. Food Chem., 8: 282-286
Miles, J. R. W. (1964) A new colorimetric method for determination of
residues of Guthion and Ethyl Guthion and their oxygen analogs.
J.A.O.A.C., 47: 882-885
Murphy, S. D. (1966) Liver metabolism and toxicity of thiophosphate
insecticides in mammalian, avian and piscine species. Proc. Soc. exp.
Biol. (N.Y.), 123: 392-398
Murphy, S. D., Lauwerys, R. R. and Cheever, K. L. (1968) Comparative
anticholinesterase action to organophosphorus insecticides in
vertebrates. Toxicol. appl. Pharmacol., 12: 22-35
Root, M., Vesselinovitch, D., Meskauskas, J, and Doull, J. (1965)
Effect of guthion in the diet on the reproduction and lactation of
mice. University of Chicago. Unpublished report
Sato, J. (1959) Studies on organic phosphorus. Gusathion and phosdrin.
1. The toxicity of gusathion and phosdrin. Kumamoto med. J., 12:
313-317 (Chem. Abstr., 54: 21473 (1960))
Schrader, G. (1963) Gusathion in Die Entwicklung neuer insektizider
Phosphosaure-Ester. Verlag Chemie, Weinheim., pp. 176-186
Smart, N. A. (1967) A modification of Miles' method for determining
azinphos-methyl residues in crops. Analyst, 92: 779-781
Thornton, J. S. (1967a) Determination of Guthion M-E in soybeans by
thermionic emission flame gas chromatography. Chemagro Report No.
20182
Thornton, J. S. (1967b) Effect of the oil deodorization process on
Guthion residues in soy bean oil (simulated). Chemagro Report No.
20834
Tietz, H., Metcalf, R. L. and Fukuto, T. R. (1957) Action of the
insecticide "Gusathion" on cotton plants, and the problem of residues
in cottonseed. Höfchen-Briefe, 10: 279-289 (English edition)
Tietz, H., Fukuto, T. R. and Metcalf, R. L. (1960) Untersuchungen mit
32P-markierten Verbindungen uber das Verhalten des Insektizids
GusathionR bei Baumwollpflanzen und das damit verbundene
Ruckstandsproblem bei Baumwollsamen. Verh. IV. Intern.
Pflanzenschutz-Kongr. Hamburg 1957, Bd. 2, biol. bundesanstalt f.
Land-und Forstwirtschaft, Braunschweig, S. 1645-1646
Other information on identity and properties
The pure material is a white, crystalline solid, M.P. 73-74°C; soluble
in water at 25°C (1:30 000) and soluble in most organic solvents. The
technical product is a brown waxy solid (M.P. 65-68°C). It decomposes
at elevated temperatures with the evolution of gas, and is rapidly
hydrolyzed by cold alkali to form anthranilic acid and is subject to
hydrolysis by acids. Suitable oxidizing agents convert it to the
oxygen analogue.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Azinphos-methyl is activated to gutoxon, a highly potent
cholinesterase inhibitor which has a molar I50 in rat brain of 2.99 ×
10-8 (Schrader, 1963). In vitro, the rate of degradation of gutoxon
by liver microsomal enzymes is not altered by species, or sex
variation in mammals (Johnsen and Dahm, 1966). Also the rate of in
vitro metabolism by the liver is similar in mammals, birds, and fish
(Murphy, 1966).
In vitro studies showed that azinphos-methyl is a poor brain
cholinesterase inhibitor in mammalian, avian, or piscine species but
gutoxon is a potent inhibitor; avian brain cholinesterase inhibition
being less than that in mammals, and fish brain cholinesterase being
the most susceptible to inhibition. Species differences in sensitivity
to gutoxon inhibition of brain cholinesterase are probably
sufficiently large to modify the influence of variation in metabolic
rates (Murphy et al., 1968).
Acute toxicity
LD50 (mg/kg
Animal Route body-weight) Reference
Mouse Oral 8* Sato, 1959
Mouse i.p. 8-10** Murphy, 1966
Rat Oral 11-25 DuBois et al., 1955
Gaines, 1960
Rat i.p. 5-11.6 DuBois et al., 1955
Guinea-pig Oral 80 DuBois et al., 1955
Guinea-pig i.p. 40 DuBois et al., 1955
* Given as azinphos-methyl emulsion.
** Given as a corn oil solution.
Short-term studies
Rat
Groups of 10 male rats were fed 0, 5, 10, 20, 50 or 100 ppm
azinphos-methyl in the diet for nine weeks. At 50, and 100 ppm slight
decrease in food intake, decrease in body-weight gain, and muscular
spasms and trembling were observed. Whole blood cholinesterase
activity was markedly decreased at 20, 50 and 100 ppm. At 5 and 10 ppm
the maximum depression which occurred, was 20 per cent below the
control values. (Huntingdon Research Centre, 1966.)
The addition of azinphos-methyl to the diet of groups of 10 young male
and 10 young female rats each, at levels of 2, 5 and 20 ppm did not
markedly alter the growth-rate over 60 days. The body-weight of the
male rats fed 20 ppm was about four per cent less than that of the
controls. In the rats fed 2 and 5 ppm for 120 days, cholinesterase
activity was unaffected, but at 20 ppm there was inhibition in the
brain (10 per cent) and in serum and erythrocytes (about 30
per cent). After 120 days no appreciable changes in the gross and
microscopic appearance of brain, heart, liver, spleen, adrenals,
stomach, intestines, skeletal muscle and bone-marrow were found. There
was no evidence of demyelination in the nervous system (Doull et al.,
1956).
When weanling male rats were fed diets containing 50 and 100 ppm of
azinphos-methyl for 16 weeks, approximately half of each group died.
All animals receiving azinphos-methyl in the diet showed marked
effects of cholinergic stimulation, including diarrhoea, salivation,
lacrimation, muscular tremors and fasciculations. These symptoms were
most marked during the first month on the diets. The rats fed 50 ppm
of azinphos-methyl weighed about 10 per cent less, and the rats fed
100 ppm, 18 per cent less than rats fed a normal diet. The
cholinesterase activity of the serum, brain, erythrocytes and
submaxillary glands of rats fed 50 and 100 ppm was markedly inhibited.
The inhibition was most marked in the erythrocytes and brain and the
animals did not fully recover from these effects during a three-week
period on a normal diet. Both gross and microscopic examination failed
to indicate any evidence of testicular atrophy due to the presence of
the high levels of azinphos-methyl in the diet (Doull et al., 1957a).
Dog
Groups of two dogs, one male and one female, given 5, 10, 20 and 50
ppm of azinphos-methyl in their diet did not show any loss of weight
nor any symptoms of azinphos-methyl poisoning during a 12-week period.
At levels of 5, 10 and 20 ppm there was no significant decrease, but
at 50 ppm there was a 25 per cent decrease in serum cholinesterase
activity at the end of the 12-week period. The erythrocyte
cholinesterase of the one male and one female dog began to be
inhibited at the 10 ppm level (Doull et al., 1967b).
Six groups of one male and one female dogs were fed 0, 20, 50, 100,
200 or 400 ppm of azinphos-methyl in a dry diet, for 19 weeks. Whole
blood cholinesterase depression was slight at 20 and 50 ppm, becoming
apparent after four weeks on the diet; however, reactivation occurred
by the sixth week at 20 ppm, and by the ninth week at 50 ppm. At the
100 ppm level and above, cholinesterase activity was reduced by more
than 50 per cent; reactivation was not apparent at 400 ppm, was
doubtful at 200 ppm, and was slight at 100 ppm after 17 weeks. (Loser
and Lorke, 1967.)
Four groups of four male and four female dogs along with a control
group, were fed azinphos-methyl in a dry diet for two years. At the
lowest level the dogs were fed 5 ppm throughout the two-year period.
At the second level they were fed 20 ppm for 36 weeks, followed by 50
ppm for 15 months. At the third level the dogs received 50 ppm
throughout the two-year period. At the top dose level they received 50
ppm for 36 weeks, followed by 100 ppm for 21 weeks, 150 ppm for 27
weeks and finally 300 ppm for 21 weeks. Mortality throughout was
comparable to the controls. At 300 ppm, tremors, muscular weakness and
abnormal quietness were noted, especially among the male animals.
Weight loss occurred to a slight degree at this dose level. Food
intake was slightly reduced in females at 150 and 300 ppm.
Cholinesterase depression was not apparent during the first three
months of the study. In the period three to nine months, red blood
cell cholinesterase was slightly depressed at 20 ppm, and more
markedly depressed at 50 ppm. In the second year of the study, plasma
cholinesterase depression was apparent in dogs receiving 50 ppm and
above. Red blood cell cholinesterase continued to be depressed at
intermediate and high dose levels, and minimal depression was also
seen at 5 ppm. Other parameters, including haematology, clinical
chemistry, urinalysis, organ to body-weight ratios, and gross and
histopathology were all comparable to controls. (Huntingdon Research
Centre, 1966b.)
Long-term studies
Rat
Groups of 40 male and 40 female rats were fed 2.5, 5 and 20 ppm for
two years and a top group was fed 50 ppm for 47 weeks and then 100
ppm. Two control groups were also maintained. At 100 ppm, toxic
symptoms in the form of salivation, diuresis, exophthalmos, loss of
balance and co-ordination, muscular fasciculation, and minor tremors
were observed in five females. Mortality, food intake, body-weight
gain, food utilization, urinalysis, and haematology were comparable to
controls. Depression of cholinesterase levels of red blood cells and
plasma occurred at 20 ppm and above, and plasma cholinesterase
depression was apparent up to 39 weeks at 5 ppm in the males. In the
females, consistent depression of plasma and red blood cell
cholinesterase was apparent only at the top dose. At 20 ppm plasma
cholinesterase depression occurred up to and including 39 weeks. At 5
ppm depression occurred at 10, 39 and 78 weeks. Red blood cell
cholinesterase was depressed up to 65 weeks at 20 ppm, but only up to
10 weeks at 5 ppm. Brain cholinesterase depression was significantly
depressed at the top dose level only. Organ to body-weight ratios
showed random variations, a possible increase with the liver occurring
at the top dose level. There was no indication that tumour incidence
was increased and it was concluded that azinphos-methyl was devoid of
carcinogenic activity in the rat. Gross and histopathology showed no
compound related effects. (Huntingdon Research Centre, 1966,)
Special studies
Reproduction
Mouse. Groups of six male and 24 female mice were fed 0, 5, 10, 25
and 50 ppm in the diet over three generations. Initial exposure to the
diet was for 30 days prior to the first mating of the Fo generation.
Because of the high mortality (15 out of 24) among the females at 50
ppm prior to mating, this dose level was eliminated after the first
mating. Fertility and litter size were not affected in the 50 ppm
group, but survival to weaning was significantly decreased. Up to and
including 25 ppm no adverse effects were apparent as judged by
fertility, gestation or lactation, litter size, or survival of
offspring to 30 days. Gross and microscopic examination of F3b
weanlings showed no compound related changes. (Root et al., 1965.)
Rabbit. Three groups of 10 pregnant female rabbits were fed 0, 5 or
25 ppm in the diet, from the eighth to the sixteenth day of pregnancy.
Five rabbits in each group were sacrificed on the twenty-ninth day of
pregnancy, the remainder being permitted to litter. No compound
related effects occurred with respect to litter size, stillbirths, sex
ratios, average foetal weights, incidence of immature foetuses, or
survival to 30 days. (Doull et al., 1966.)
Comments
Since the last evaluation by the FAO/WHO Joint Meeting (Rome, 15-22
March 1965), results of reproduction studies in mice, short-term
studies in dogs and long-term studies in rats have been provided. The
studies performed appear satisfactory. Further work desirable includes
studies on cholinesterase inhibition of plasma and erythrocytes in man
and metabolic studies in man.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effects
Rat: 2.5 ppm in the diet, equivalent to 0.125 mg/kg.
Dog: About 5 ppm in the dry diet, equivalent to 0.125 mg/kg.
Estimate of acceptable daily intake of azinphos-methyl for man
0-0.0025 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Azinphos-methyl is used for the pre-harvest control of a wide spectrum
of insects and mites attacking fruit, vegetable and forage crops.
Residues resulting from supervised trials
The following typical data are extracted from Chemagro internal
reports of trials made at various field stations.
Rate of application No. of Pre-harvest Residue
Crop (kg/ha) treatments interval (days) (ppm)
Alfalfa 0.50 1 5 0.4
Apples 0.28-0.35 7 7 0.75
Apricots 0.28-0.35 1 15 4.6
21 3.1
Broccoli 0.56 5 15 0.38
Brussels 0.56 1 7 0.6
sprouts 14 n.d.
Cabbage 0.56 3 15 n.d.
Cherries 0.28-0.35 1 7 0.18-0.74
14 n.d.-0.23
Clover 0.50 1 5 0.4
Grapes 0.28-0.35 1 15 0.6
22 0.4
0.28-0.35 2 16 5.1
23 3.7
Grapefruit 1.12 1 15 0.3-0.9
Peaches 0.28-0.35 1 19 1.0
31 0.4
Pears 0.28-0.35 1 8 0.3
Peas 0.56 3 7 n.d.
Plums 0.28-0.56 1 14 n.d.-0.25
Strawberries 1.12 1 7 0.9-1.5
14 0.7
Tomatoes 1.56 1 7 0.09
Plums 0.28-0.56 1 14 n.d.-0.25
Strawberries 1.12 1 7 0.9-1.5
14 0.7
Tomatoes 1.56 1 7 0.09
n.d. = not detectable.
Evidence of residues in food moving in commerce or at consumption
No data available.
Fate of residues
In plants. Azinphos-methyl has not been shown to exhibit systemic
action. No phosphorothiolate oxidation product could be found in or on
sprayed cotton leaves (Tietz et al., 1957, 1960). Two unidentified
phosphorus-containing metabolites, more lipophilic than the applied
chemical, appeared and gave positive tests for anthranilic acid
(Meagher et al., 1960). Further metabolites were recovered containing
the radioactive phosphorus in phospholipids and as hydrolysis
products.
With lettuce, most of the chemical remained on the surface and no
oxygen analogue was isolated (Magill et al., 1966). After 14 days, 95
per cent of the extracted residue was azinphos-methyl with four
components in the remaining five per cent Two were benzazimide and
methyl benzazimide sulfide while the remaining two were not
identified.
In animals. P32 and C14-labelled material administered orally to
dairy cows did not result in azinphos-methyl or the P=0 derivative in
the blood or milk (Everett et al., 1966). Excluded possible
metabolites were anthranilic acid, benzazimide, hydroxymethyl
benzazimide, mercaptomethyl benzazimide, N-methyl benzazimide, bis
(N-methyl benzazimide) sulfide and the corresponding disulfide.
However four unidentified components containing the benzazimide
moiety, appeared, one of which accounted for 90 per cent of the total
residue. They contribute a maximum of 0.017 ppm in the milk when
feeding the animal at the rate of 0.2 mg/kg, equivalent to 2.8 ppm in
the feed. At least two of the metabolites may be oxidation products of
an intermediate metabolite, bis (N-methylbenzazimide) sulfide. Thus
the residue in milk from feeding forage containing 2.8 ppm
azinphos-methyl will be 0.008 ppm expressed as mercaptomethyl
benzazimide while the residue in tissues will not exceed 0.1 ppm.
The fluorometric method of Adams and Anderson (1966) is suitable for
determining these metabolites.
In storage and processing. Residues of azinphos-methyl were stable
at frozen storage levels (-18 to -23°C) in samples of fruit, fodder
and vegetables (Chemagro, 1962). Washing oranges reduced the residues
from 1.0 ppm to 0.7 ppm (whole fruit), from 2.7 to 1.9 ppm in the
peel. While the juice and pulp were free of residues, the orange oil
contained 30 ppm (Anderson et al., 1963). Simple washing reduces
residues on the peel from 71 to 96 per cent, the greater amount with
the fresher residue (Gunther et al., 1963). Field-sprayed snap beans
bearing an initial residue of 1.09 ppm had a residue of 0.14 ppm after
washing, blanching and freezing (Carlin et al., 1966). After canning
the residue was reduced to 0.02 ppm. Soy-bean oil fortified with
azinphos-methyl and deodorized according to commercial procedures had
the residue reduced by 36 per cent (Thornton, 1967b). Sugar-cane
containing 0.1 to 0.6 ppm showed no residues in the molasses or sugar
when processed (FAO/WHO, 1965).
Methods of residue analysis
Colorimetric, gas chromatographic, fluorometric, infra-red and
polarographic methods have been developed. One commonly used procedure
is based on weak alkaline hydrolysis of the residue to liberate
anthranilic acid which is then diazotized and coupled with
N-(1-naphthyl)-ethylenediamine dihydrochloride and the resultant
purple colour measured at 555 mµ (Meagher et al., 1960; MacDougall,
1964; Cohen et al., 1966). Modifications have been made to shorten the
procedure (Cox, 1964). Miles (1964) has done this by direct coupling
of the benzotriazinyl-containing residue in an acetic-hydrochloric
acid mixture and measuring the blue-violet colour at 556 mµ. Smart
(1967) has modified the method by introducing an improved extraction
procedure.
Another colorimetric method involves the alkali cleavage and
measurement of the dimethyl phosphorodithioate as the copper complex
absorbance at 420 mµ. This measures the parent compound only and is
similar to the infra-red measurement using the P=S stretching
vibration at 654 cm-1 (15.25 microns) (Cohen et al., 1966),
The sensitivity of the anthranilic acid colorimetric method can be
increased to about 0.05 ppm by using 10 cm cells. The precision for
recovery of azinphos-methyl from a number of crops indicates that in
the range of 0.2 to 0.5 ppm the average deviation from the mean is
approximately six per cent.
A spectrophotofluorometric method is based on the measurement of the
anthranilic acid released on alkaline hydrolysis by determining
fluorometrically at an activating wavelength of 340 mµ and a
fluorescence of 400 mµ (Adams and Anderson, 1966). It has a
sensitivity of about 0.005 ppm for milk, 0.02 ppm for most animal
tissues and 0.03 ppm for fat.
More recently a gas chromatographic procedure has been developed for
the determination of residues in soy-beans using the potassium
chloride thermionic emission flame detector for phosphorus (Thornton,
1967a). This method is sensitive to 0.005 ppm and distinguishes
between azinphos-methyl and its P=O analogue.
A method based on the measurement of the formaldehyde released on acid
hydrolysis of the residue has been used (Giang and Schechter, 1958) as
well as a polarographic analysis (Bates, 1962).
National tolerances
Tolerance Pre-harvest
Country Crop (ppm) interval (days)
Australia 21
Austria 21
Benelux fruit, grapes 0.5
Brazil fruit, vegetables 2.0
cotton seed 0.5
Canada grapes 0.5
fruits, vegetables 1-2.0
Denmark 21
France 15
Germany fruit, grapes,
(Fed. Rep.) vegetables 0.4 14
Italy 0.4 20
New Zealand berry fruit,
leafy vegetables 21
pip and stone
fruit, root
crops, tomatoes 14
Norway 21
Portugal 21
South Africa citrus fruit 2.0 21
pome fruit 14
Switzerland fruit, grapes 1.0 21
United Kingdom 21
United States grapes 5.0
of America fruit, vegetables 2.0
cereals, soy-beans,
nuts, peas,
cucumbers, melons
peppers and
potatoes "zero"
RECOMMENDATION FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Since the publication of the monographs resulting from the 1965 Joint
Meeting, work has confirmed that the residue on plants is mainly
present as the original compound, azinphos-methyl. In the five per
cent of residue which is not accounted for as azinphos-methyl in
lettuce 14 days after treatment, out of four metabolites present two
were identified as benzazimide and methyl benzazimide.
Investigations with dairy cows indicate that no residues of
azinphos-methyl or the oxygen analogue will occur in milk from animals
consuming fodder likely to contain residues. However, four
non-phosphorus-containing metabolites, but still containing the
benzazimide moiety, may be present, although they have not been
precisely identified.
Although colorimetric and gas-liquid chromatographic methods have been
developed, none of these have been evaluated for regulatory or referee
purposes.
Recommendations
The following temporary tolerances (to be in effect until 1972) are
to apply to raw agricultural products moving in commerce unless
otherwise indicated. In the case of fruits and vegetables the
tolerances should be applied as soon as practicable after harvest and
in any event prior to actual retail to the public. In the case of
commodities entering international trade, the tolerances should be
applied by the importing country at the point of entry or as soon as
practicable thereafter. Furthermore, the tolerances should not apply
to the ethyl derivative nor to the oxygen analogue, the latter usually
being insignificant.
Temporary tolerances
Apricots and grapes 4 ppm
Other fruits 1 ppm
Vegetables 0.5 ppm
Further work or information
Required before 30 June 1972
1. Information on the nature of terminal residues in plants, animals
and their products.
2. Further data on residue levels in raw agricultural products moving
in commerce.
3. Data on disappearance of residues during storage and household
cooking of vegetables.
4. Data on the possible carry-over of residues into wine as a result
of the treatment of grapes.
5. Comparative evaluation of gas-liquid chromatographic and
spectrophotometric methods for the determination of azinphos-methyl
and its oxygen analogue for regulatory purposes.
Desirable
1. Studies on cholinesterase inhibition of plasm and erythrocytes in
man.
2. Metabolic studies in man.
3. Identification and toxicology of metabolites, especially those
having the benzazimide moiety, in milk.
4. Collaborative studies to establish a referee method.
REFERENCES
Adams, J. M. and Anderson, C. A. (1966) Spectrophotofluorometric
method for Guthion residues in milk and animal tissues. J. Agric. Food
Chem., 14: 53-55
Anderson, C. A., MacDougall, D., Kesterson, J. W., Hendrickson, R, and
Brooks, R. F. (1963) The effect of processing on Guthion residues in
oranges and orange products. J. Agric. Food Chem., 11: 422-424
Bates, J. A. R. (1962) Polarographic determination of azinphos-methyl
residues in certain crops. Analyst, 87: 786-790
Carlin, A. F., Hibbs, E. T. and Dahm, P. A. (1966) Insecticide
residues and sensory evaluation of canned and frozen snap beans field
sprayed with Guthion and DDT. Food Technol., 20: 80-83
Chemagro. (1962) Effect of frozen storage on Guthion residues in
various crops. Report No. 8682
Cohen, C. J., Betker, W. R., Wasleski, D. M. and Cavaghol, J. C.
(1966) Analysis of Guthion insecticide, J. Agric. Food Chem., 14:
315-318
Cox, W. S. (1964) Rapid determination of Guthion residues on crops.
J.A.O.A.C., 47: 280-282
Doull, J., Anido, P. and DuBois, K. P. (1957a) Effect of high dietary
levels of guthion on rats. University of Chicago. Unpublished report
Doull, J., Anido, P. and DuBois, K. P. (1957b) Determination of the
safe dietary level of guthion for dogs. University of Chicago.
Unpublished report
Doull, J., DiGiacomo, R. and Meskauskas, J. (1966) Short term breeding
studies with guthion in rabbits. University of Chicago. Unpublished
report
Doull, J., Rehfuss, P. A. and DuBois, K. P. (1956) The effects of
diets containing guthion (Bayer 17147) on rats. University of
Chicago. Unpublished report
DuBois, K. P., Thursh, D. R. and Murphy, S. D. (1955) The acute
mammalian toxicity and mechanism of action of Bayer 17147. University
of Chicago. Unpublished report
DuBois, K. P., Thursh, D. R. and Murphy, S. D. (1957) Studies on the
toxicity and pharmacologic actions of the dimethoxy ester of
benzotriazine dithiophosphoric acid (DBD guthion). J. Pharmacol.exp.
Ther., 119: 208-218
Everett, L. J., Anderson, C. A. and MacDougall, D. (1966) Nature and
extent of Guthion residues in milk and tissues resulting from treated
forage. J. Agric. Food Chem. 14: 47-53
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Mtg. Rept. PL/1965/10/1; WHO/Food Add./27.65
Gaines, T. B. (1960) The acute toxicity of pesticides to rats,
Toxicol. appl. Pharmacol., 2: 88-99
Giang, P. A. and Schechter, M. S. (1958) Colorimetric method for
estimation of Guthion residues in cottonseeds and cottonseed oil. J.
Agric. Food Chem., 6: 845-848
Gunther, F. A., Carman, G. E., Blinn, R. C. and Barkley, J. H. (1963)
Persistence of residues of Guthion on and in mature lemons and oranges
and in laboratory processed citrus "pulp" cattle feed. J. Agric. Food
Chem., 11: 424-427
Huntingdon Research Centre. (1966a) Toxicity of gusathion during
repeated administration to rats for two years. Unpublished report
Huntingdon Research Centre. (1966b) Gusathion (Bayer 17147), Chronic
oral toxicity studies in dogs. Unpublished report
Johnsen, R. E. and Dahm, P. A. (1966) Activation and degradation
efficiencies of liver microsomes from eight vertebrate species, using
organophosphates as substrates, J. Econ. Entomol., 59: 1437-1442
Löser, E. and Lorke, D. (1967) Die Aktivität der Cholinesterase bei
Hunden nach Verabreichung von Gusathion mit dem Futter. Farbenfabriken
Bayer. Unpublished report
MacDougall, D. (1964) Guthion. In: G. Zweig, Analytical Methods for
Pesticides, Plant Growth Regulators and Food Additives, vol. II,
Academic Press, New York-London
Magill, L. J. and Everett, L. J. (1966) Guthion-C14 study on lettuce.
Chemagro Report No. 18636
Meagher, W. R., Adams, J. M., Anderson, C. A. and MacDougall, D.
(1960) Colorimetric determination of Guthion residues in crops.
J. Agric. Food Chem., 8: 282-286
Miles, J. R. W. (1964) A new colorimetric method for determination of
residues of Guthion and Ethyl Guthion and their oxygen analogs.
J.A.O.A.C., 47: 882-885
Murphy, S. D. (1966) Liver metabolism and toxicity of thiophosphate
insecticides in mammalian, avian and piscine species. Proc. Soc. exp.
Biol. (N.Y.), 123: 392-398
Murphy, S. D., Lauwerys, R. R. and Cheever, K. L. (1968) Comparative
anticholinesterase action to organophosphorus insecticides in
vertebrates. Toxicol. appl. Pharmacol., 12: 22-35
Root, M., Vesselinovitch, D., Meskauskas, J, and Doull, J. (1965)
Effect of guthion in the diet on the reproduction and lactation of
mice. University of Chicago. Unpublished report
Sato, J. (1959) Studies on organic phosphorus. Gusathion and phosdrin.
1. The toxicity of gusathion and phosdrin. Kumamoto med. J., 12:
313-317 (Chem. Abstr., 54: 21473 (1960))
Schrader, G. (1963) Gusathion in Die Entwicklung neuer insektizider
Phosphosaure-Ester. Verlag Chemie, Weinheim., pp. 176-186
Smart, N. A. (1967) A modification of Miles' method for determining
azinphos-methyl residues in crops. Analyst, 92: 779-781
Thornton, J. S. (1967a) Determination of Guthion M-E in soybeans by
thermionic emission flame gas chromatography. Chemagro Report No.
20182
Thornton, J. S. (1967b) Effect of the oil deodorization process on
Guthion residues in soy bean oil (simulated). Chemagro Report No.
20834
Tietz, H., Metcalf, R. L. and Fukuto, T. R. (1957) Action of the
insecticide "Gusathion" on cotton plants, and the problem of residues
in cottonseed. Höfchen-Briefe, 10: 279-289 (English edition)
Tietz, H., Fukuto, T. R. and Metcalf, R. L. (1960) Untersuchungen mit
32P-markierten Verbindungen uber das Verhalten des Insektizids
GusathionR bei Baumwollpflanzen und das damit verbundene
Ruckstandsproblem bei Baumwollsamen. Verh. IV. Intern.
Pflanzenschutz-Kongr. Hamburg 1957, Bd. 2, biol. bundesanstalt f.
Land-und Forstwirtschaft, Braunschweig, S. 1645-1646
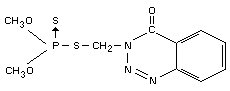 Other information on identity and properties
The pure material is a white, crystalline solid, M.P. 73-74°C; soluble
in water at 25°C (1:30 000) and soluble in most organic solvents. The
technical product is a brown waxy solid (M.P. 65-68°C). It decomposes
at elevated temperatures with the evolution of gas, and is rapidly
hydrolyzed by cold alkali to form anthranilic acid and is subject to
hydrolysis by acids. Suitable oxidizing agents convert it to the
oxygen analogue.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Azinphos-methyl is activated to gutoxon, a highly potent
cholinesterase inhibitor which has a molar I50 in rat brain of 2.99 ×
10-8 (Schrader, 1963). In vitro, the rate of degradation of gutoxon
by liver microsomal enzymes is not altered by species, or sex
variation in mammals (Johnsen and Dahm, 1966). Also the rate of in
vitro metabolism by the liver is similar in mammals, birds, and fish
(Murphy, 1966).
In vitro studies showed that azinphos-methyl is a poor brain
cholinesterase inhibitor in mammalian, avian, or piscine species but
gutoxon is a potent inhibitor; avian brain cholinesterase inhibition
being less than that in mammals, and fish brain cholinesterase being
the most susceptible to inhibition. Species differences in sensitivity
to gutoxon inhibition of brain cholinesterase are probably
sufficiently large to modify the influence of variation in metabolic
rates (Murphy et al., 1968).
Acute toxicity
LD50 (mg/kg
Animal Route body-weight) Reference
Mouse Oral 8* Sato, 1959
Mouse i.p. 8-10** Murphy, 1966
Rat Oral 11-25 DuBois et al., 1955
Gaines, 1960
Rat i.p. 5-11.6 DuBois et al., 1955
Guinea-pig Oral 80 DuBois et al., 1955
Guinea-pig i.p. 40 DuBois et al., 1955
* Given as azinphos-methyl emulsion.
** Given as a corn oil solution.
Short-term studies
Rat
Groups of 10 male rats were fed 0, 5, 10, 20, 50 or 100 ppm
azinphos-methyl in the diet for nine weeks. At 50, and 100 ppm slight
decrease in food intake, decrease in body-weight gain, and muscular
spasms and trembling were observed. Whole blood cholinesterase
activity was markedly decreased at 20, 50 and 100 ppm. At 5 and 10 ppm
the maximum depression which occurred, was 20 per cent below the
control values. (Huntingdon Research Centre, 1966.)
The addition of azinphos-methyl to the diet of groups of 10 young male
and 10 young female rats each, at levels of 2, 5 and 20 ppm did not
markedly alter the growth-rate over 60 days. The body-weight of the
male rats fed 20 ppm was about four per cent less than that of the
controls. In the rats fed 2 and 5 ppm for 120 days, cholinesterase
activity was unaffected, but at 20 ppm there was inhibition in the
brain (10 per cent) and in serum and erythrocytes (about 30
per cent). After 120 days no appreciable changes in the gross and
microscopic appearance of brain, heart, liver, spleen, adrenals,
stomach, intestines, skeletal muscle and bone-marrow were found. There
was no evidence of demyelination in the nervous system (Doull et al.,
1956).
When weanling male rats were fed diets containing 50 and 100 ppm of
azinphos-methyl for 16 weeks, approximately half of each group died.
All animals receiving azinphos-methyl in the diet showed marked
effects of cholinergic stimulation, including diarrhoea, salivation,
lacrimation, muscular tremors and fasciculations. These symptoms were
most marked during the first month on the diets. The rats fed 50 ppm
of azinphos-methyl weighed about 10 per cent less, and the rats fed
100 ppm, 18 per cent less than rats fed a normal diet. The
cholinesterase activity of the serum, brain, erythrocytes and
submaxillary glands of rats fed 50 and 100 ppm was markedly inhibited.
The inhibition was most marked in the erythrocytes and brain and the
animals did not fully recover from these effects during a three-week
period on a normal diet. Both gross and microscopic examination failed
to indicate any evidence of testicular atrophy due to the presence of
the high levels of azinphos-methyl in the diet (Doull et al., 1957a).
Dog
Groups of two dogs, one male and one female, given 5, 10, 20 and 50
ppm of azinphos-methyl in their diet did not show any loss of weight
nor any symptoms of azinphos-methyl poisoning during a 12-week period.
At levels of 5, 10 and 20 ppm there was no significant decrease, but
at 50 ppm there was a 25 per cent decrease in serum cholinesterase
activity at the end of the 12-week period. The erythrocyte
cholinesterase of the one male and one female dog began to be
inhibited at the 10 ppm level (Doull et al., 1967b).
Six groups of one male and one female dogs were fed 0, 20, 50, 100,
200 or 400 ppm of azinphos-methyl in a dry diet, for 19 weeks. Whole
blood cholinesterase depression was slight at 20 and 50 ppm, becoming
apparent after four weeks on the diet; however, reactivation occurred
by the sixth week at 20 ppm, and by the ninth week at 50 ppm. At the
100 ppm level and above, cholinesterase activity was reduced by more
than 50 per cent; reactivation was not apparent at 400 ppm, was
doubtful at 200 ppm, and was slight at 100 ppm after 17 weeks. (Loser
and Lorke, 1967.)
Four groups of four male and four female dogs along with a control
group, were fed azinphos-methyl in a dry diet for two years. At the
lowest level the dogs were fed 5 ppm throughout the two-year period.
At the second level they were fed 20 ppm for 36 weeks, followed by 50
ppm for 15 months. At the third level the dogs received 50 ppm
throughout the two-year period. At the top dose level they received 50
ppm for 36 weeks, followed by 100 ppm for 21 weeks, 150 ppm for 27
weeks and finally 300 ppm for 21 weeks. Mortality throughout was
comparable to the controls. At 300 ppm, tremors, muscular weakness and
abnormal quietness were noted, especially among the male animals.
Weight loss occurred to a slight degree at this dose level. Food
intake was slightly reduced in females at 150 and 300 ppm.
Cholinesterase depression was not apparent during the first three
months of the study. In the period three to nine months, red blood
cell cholinesterase was slightly depressed at 20 ppm, and more
markedly depressed at 50 ppm. In the second year of the study, plasma
cholinesterase depression was apparent in dogs receiving 50 ppm and
above. Red blood cell cholinesterase continued to be depressed at
intermediate and high dose levels, and minimal depression was also
seen at 5 ppm. Other parameters, including haematology, clinical
chemistry, urinalysis, organ to body-weight ratios, and gross and
histopathology were all comparable to controls. (Huntingdon Research
Centre, 1966b.)
Long-term studies
Rat
Groups of 40 male and 40 female rats were fed 2.5, 5 and 20 ppm for
two years and a top group was fed 50 ppm for 47 weeks and then 100
ppm. Two control groups were also maintained. At 100 ppm, toxic
symptoms in the form of salivation, diuresis, exophthalmos, loss of
balance and co-ordination, muscular fasciculation, and minor tremors
were observed in five females. Mortality, food intake, body-weight
gain, food utilization, urinalysis, and haematology were comparable to
controls. Depression of cholinesterase levels of red blood cells and
plasma occurred at 20 ppm and above, and plasma cholinesterase
depression was apparent up to 39 weeks at 5 ppm in the males. In the
females, consistent depression of plasma and red blood cell
cholinesterase was apparent only at the top dose. At 20 ppm plasma
cholinesterase depression occurred up to and including 39 weeks. At 5
ppm depression occurred at 10, 39 and 78 weeks. Red blood cell
cholinesterase was depressed up to 65 weeks at 20 ppm, but only up to
10 weeks at 5 ppm. Brain cholinesterase depression was significantly
depressed at the top dose level only. Organ to body-weight ratios
showed random variations, a possible increase with the liver occurring
at the top dose level. There was no indication that tumour incidence
was increased and it was concluded that azinphos-methyl was devoid of
carcinogenic activity in the rat. Gross and histopathology showed no
compound related effects. (Huntingdon Research Centre, 1966,)
Special studies
Reproduction
Mouse. Groups of six male and 24 female mice were fed 0, 5, 10, 25
and 50 ppm in the diet over three generations. Initial exposure to the
diet was for 30 days prior to the first mating of the Fo generation.
Because of the high mortality (15 out of 24) among the females at 50
ppm prior to mating, this dose level was eliminated after the first
mating. Fertility and litter size were not affected in the 50 ppm
group, but survival to weaning was significantly decreased. Up to and
including 25 ppm no adverse effects were apparent as judged by
fertility, gestation or lactation, litter size, or survival of
offspring to 30 days. Gross and microscopic examination of F3b
weanlings showed no compound related changes. (Root et al., 1965.)
Rabbit. Three groups of 10 pregnant female rabbits were fed 0, 5 or
25 ppm in the diet, from the eighth to the sixteenth day of pregnancy.
Five rabbits in each group were sacrificed on the twenty-ninth day of
pregnancy, the remainder being permitted to litter. No compound
related effects occurred with respect to litter size, stillbirths, sex
ratios, average foetal weights, incidence of immature foetuses, or
survival to 30 days. (Doull et al., 1966.)
Comments
Since the last evaluation by the FAO/WHO Joint Meeting (Rome, 15-22
March 1965), results of reproduction studies in mice, short-term
studies in dogs and long-term studies in rats have been provided. The
studies performed appear satisfactory. Further work desirable includes
studies on cholinesterase inhibition of plasma and erythrocytes in man
and metabolic studies in man.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effects
Rat: 2.5 ppm in the diet, equivalent to 0.125 mg/kg.
Dog: About 5 ppm in the dry diet, equivalent to 0.125 mg/kg.
Estimate of acceptable daily intake of azinphos-methyl for man
0-0.0025 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Azinphos-methyl is used for the pre-harvest control of a wide spectrum
of insects and mites attacking fruit, vegetable and forage crops.
Residues resulting from supervised trials
The following typical data are extracted from Chemagro internal
reports of trials made at various field stations.
Rate of application No. of Pre-harvest Residue
Crop (kg/ha) treatments interval (days) (ppm)
Alfalfa 0.50 1 5 0.4
Apples 0.28-0.35 7 7 0.75
Apricots 0.28-0.35 1 15 4.6
21 3.1
Broccoli 0.56 5 15 0.38
Brussels 0.56 1 7 0.6
sprouts 14 n.d.
Cabbage 0.56 3 15 n.d.
Cherries 0.28-0.35 1 7 0.18-0.74
14 n.d.-0.23
Clover 0.50 1 5 0.4
Grapes 0.28-0.35 1 15 0.6
22 0.4
0.28-0.35 2 16 5.1
23 3.7
Grapefruit 1.12 1 15 0.3-0.9
Peaches 0.28-0.35 1 19 1.0
31 0.4
Pears 0.28-0.35 1 8 0.3
Peas 0.56 3 7 n.d.
Plums 0.28-0.56 1 14 n.d.-0.25
Strawberries 1.12 1 7 0.9-1.5
14 0.7
Tomatoes 1.56 1 7 0.09
Plums 0.28-0.56 1 14 n.d.-0.25
Strawberries 1.12 1 7 0.9-1.5
14 0.7
Tomatoes 1.56 1 7 0.09
n.d. = not detectable.
Evidence of residues in food moving in commerce or at consumption
No data available.
Fate of residues
In plants. Azinphos-methyl has not been shown to exhibit systemic
action. No phosphorothiolate oxidation product could be found in or on
sprayed cotton leaves (Tietz et al., 1957, 1960). Two unidentified
phosphorus-containing metabolites, more lipophilic than the applied
chemical, appeared and gave positive tests for anthranilic acid
(Meagher et al., 1960). Further metabolites were recovered containing
the radioactive phosphorus in phospholipids and as hydrolysis
products.
With lettuce, most of the chemical remained on the surface and no
oxygen analogue was isolated (Magill et al., 1966). After 14 days, 95
per cent of the extracted residue was azinphos-methyl with four
components in the remaining five per cent Two were benzazimide and
methyl benzazimide sulfide while the remaining two were not
identified.
In animals. P32 and C14-labelled material administered orally to
dairy cows did not result in azinphos-methyl or the P=0 derivative in
the blood or milk (Everett et al., 1966). Excluded possible
metabolites were anthranilic acid, benzazimide, hydroxymethyl
benzazimide, mercaptomethyl benzazimide, N-methyl benzazimide, bis
(N-methyl benzazimide) sulfide and the corresponding disulfide.
However four unidentified components containing the benzazimide
moiety, appeared, one of which accounted for 90 per cent of the total
residue. They contribute a maximum of 0.017 ppm in the milk when
feeding the animal at the rate of 0.2 mg/kg, equivalent to 2.8 ppm in
the feed. At least two of the metabolites may be oxidation products of
an intermediate metabolite, bis (N-methylbenzazimide) sulfide. Thus
the residue in milk from feeding forage containing 2.8 ppm
azinphos-methyl will be 0.008 ppm expressed as mercaptomethyl
benzazimide while the residue in tissues will not exceed 0.1 ppm.
The fluorometric method of Adams and Anderson (1966) is suitable for
determining these metabolites.
In storage and processing. Residues of azinphos-methyl were stable
at frozen storage levels (-18 to -23°C) in samples of fruit, fodder
and vegetables (Chemagro, 1962). Washing oranges reduced the residues
from 1.0 ppm to 0.7 ppm (whole fruit), from 2.7 to 1.9 ppm in the
peel. While the juice and pulp were free of residues, the orange oil
contained 30 ppm (Anderson et al., 1963). Simple washing reduces
residues on the peel from 71 to 96 per cent, the greater amount with
the fresher residue (Gunther et al., 1963). Field-sprayed snap beans
bearing an initial residue of 1.09 ppm had a residue of 0.14 ppm after
washing, blanching and freezing (Carlin et al., 1966). After canning
the residue was reduced to 0.02 ppm. Soy-bean oil fortified with
azinphos-methyl and deodorized according to commercial procedures had
the residue reduced by 36 per cent (Thornton, 1967b). Sugar-cane
containing 0.1 to 0.6 ppm showed no residues in the molasses or sugar
when processed (FAO/WHO, 1965).
Methods of residue analysis
Colorimetric, gas chromatographic, fluorometric, infra-red and
polarographic methods have been developed. One commonly used procedure
is based on weak alkaline hydrolysis of the residue to liberate
anthranilic acid which is then diazotized and coupled with
N-(1-naphthyl)-ethylenediamine dihydrochloride and the resultant
purple colour measured at 555 mµ (Meagher et al., 1960; MacDougall,
1964; Cohen et al., 1966). Modifications have been made to shorten the
procedure (Cox, 1964). Miles (1964) has done this by direct coupling
of the benzotriazinyl-containing residue in an acetic-hydrochloric
acid mixture and measuring the blue-violet colour at 556 mµ. Smart
(1967) has modified the method by introducing an improved extraction
procedure.
Another colorimetric method involves the alkali cleavage and
measurement of the dimethyl phosphorodithioate as the copper complex
absorbance at 420 mµ. This measures the parent compound only and is
similar to the infra-red measurement using the P=S stretching
vibration at 654 cm-1 (15.25 microns) (Cohen et al., 1966),
The sensitivity of the anthranilic acid colorimetric method can be
increased to about 0.05 ppm by using 10 cm cells. The precision for
recovery of azinphos-methyl from a number of crops indicates that in
the range of 0.2 to 0.5 ppm the average deviation from the mean is
approximately six per cent.
A spectrophotofluorometric method is based on the measurement of the
anthranilic acid released on alkaline hydrolysis by determining
fluorometrically at an activating wavelength of 340 mµ and a
fluorescence of 400 mµ (Adams and Anderson, 1966). It has a
sensitivity of about 0.005 ppm for milk, 0.02 ppm for most animal
tissues and 0.03 ppm for fat.
More recently a gas chromatographic procedure has been developed for
the determination of residues in soy-beans using the potassium
chloride thermionic emission flame detector for phosphorus (Thornton,
1967a). This method is sensitive to 0.005 ppm and distinguishes
between azinphos-methyl and its P=O analogue.
A method based on the measurement of the formaldehyde released on acid
hydrolysis of the residue has been used (Giang and Schechter, 1958) as
well as a polarographic analysis (Bates, 1962).
National tolerances
Tolerance Pre-harvest
Country Crop (ppm) interval (days)
Australia 21
Austria 21
Benelux fruit, grapes 0.5
Brazil fruit, vegetables 2.0
cotton seed 0.5
Canada grapes 0.5
fruits, vegetables 1-2.0
Denmark 21
France 15
Germany fruit, grapes,
(Fed. Rep.) vegetables 0.4 14
Italy 0.4 20
New Zealand berry fruit,
leafy vegetables 21
pip and stone
fruit, root
crops, tomatoes 14
Norway 21
Portugal 21
South Africa citrus fruit 2.0 21
pome fruit 14
Switzerland fruit, grapes 1.0 21
United Kingdom 21
United States grapes 5.0
of America fruit, vegetables 2.0
cereals, soy-beans,
nuts, peas,
cucumbers, melons
peppers and
potatoes "zero"
RECOMMENDATION FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Since the publication of the monographs resulting from the 1965 Joint
Meeting, work has confirmed that the residue on plants is mainly
present as the original compound, azinphos-methyl. In the five per
cent of residue which is not accounted for as azinphos-methyl in
lettuce 14 days after treatment, out of four metabolites present two
were identified as benzazimide and methyl benzazimide.
Investigations with dairy cows indicate that no residues of
azinphos-methyl or the oxygen analogue will occur in milk from animals
consuming fodder likely to contain residues. However, four
non-phosphorus-containing metabolites, but still containing the
benzazimide moiety, may be present, although they have not been
precisely identified.
Although colorimetric and gas-liquid chromatographic methods have been
developed, none of these have been evaluated for regulatory or referee
purposes.
Recommendations
The following temporary tolerances (to be in effect until 1972) are
to apply to raw agricultural products moving in commerce unless
otherwise indicated. In the case of fruits and vegetables the
tolerances should be applied as soon as practicable after harvest and
in any event prior to actual retail to the public. In the case of
commodities entering international trade, the tolerances should be
applied by the importing country at the point of entry or as soon as
practicable thereafter. Furthermore, the tolerances should not apply
to the ethyl derivative nor to the oxygen analogue, the latter usually
being insignificant.
Temporary tolerances
Apricots and grapes 4 ppm
Other fruits 1 ppm
Vegetables 0.5 ppm
Further work or information
Required before 30 June 1972
1. Information on the nature of terminal residues in plants, animals
and their products.
2. Further data on residue levels in raw agricultural products moving
in commerce.
3. Data on disappearance of residues during storage and household
cooking of vegetables.
4. Data on the possible carry-over of residues into wine as a result
of the treatment of grapes.
5. Comparative evaluation of gas-liquid chromatographic and
spectrophotometric methods for the determination of azinphos-methyl
and its oxygen analogue for regulatory purposes.
Desirable
1. Studies on cholinesterase inhibition of plasm and erythrocytes in
man.
2. Metabolic studies in man.
3. Identification and toxicology of metabolites, especially those
having the benzazimide moiety, in milk.
4. Collaborative studies to establish a referee method.
REFERENCES
Adams, J. M. and Anderson, C. A. (1966) Spectrophotofluorometric
method for Guthion residues in milk and animal tissues. J. Agric. Food
Chem., 14: 53-55
Anderson, C. A., MacDougall, D., Kesterson, J. W., Hendrickson, R, and
Brooks, R. F. (1963) The effect of processing on Guthion residues in
oranges and orange products. J. Agric. Food Chem., 11: 422-424
Bates, J. A. R. (1962) Polarographic determination of azinphos-methyl
residues in certain crops. Analyst, 87: 786-790
Carlin, A. F., Hibbs, E. T. and Dahm, P. A. (1966) Insecticide
residues and sensory evaluation of canned and frozen snap beans field
sprayed with Guthion and DDT. Food Technol., 20: 80-83
Chemagro. (1962) Effect of frozen storage on Guthion residues in
various crops. Report No. 8682
Cohen, C. J., Betker, W. R., Wasleski, D. M. and Cavaghol, J. C.
(1966) Analysis of Guthion insecticide, J. Agric. Food Chem., 14:
315-318
Cox, W. S. (1964) Rapid determination of Guthion residues on crops.
J.A.O.A.C., 47: 280-282
Doull, J., Anido, P. and DuBois, K. P. (1957a) Effect of high dietary
levels of guthion on rats. University of Chicago. Unpublished report
Doull, J., Anido, P. and DuBois, K. P. (1957b) Determination of the
safe dietary level of guthion for dogs. University of Chicago.
Unpublished report
Doull, J., DiGiacomo, R. and Meskauskas, J. (1966) Short term breeding
studies with guthion in rabbits. University of Chicago. Unpublished
report
Doull, J., Rehfuss, P. A. and DuBois, K. P. (1956) The effects of
diets containing guthion (Bayer 17147) on rats. University of
Chicago. Unpublished report
DuBois, K. P., Thursh, D. R. and Murphy, S. D. (1955) The acute
mammalian toxicity and mechanism of action of Bayer 17147. University
of Chicago. Unpublished report
DuBois, K. P., Thursh, D. R. and Murphy, S. D. (1957) Studies on the
toxicity and pharmacologic actions of the dimethoxy ester of
benzotriazine dithiophosphoric acid (DBD guthion). J. Pharmacol.exp.
Ther., 119: 208-218
Everett, L. J., Anderson, C. A. and MacDougall, D. (1966) Nature and
extent of Guthion residues in milk and tissues resulting from treated
forage. J. Agric. Food Chem. 14: 47-53
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Mtg. Rept. PL/1965/10/1; WHO/Food Add./27.65
Gaines, T. B. (1960) The acute toxicity of pesticides to rats,
Toxicol. appl. Pharmacol., 2: 88-99
Giang, P. A. and Schechter, M. S. (1958) Colorimetric method for
estimation of Guthion residues in cottonseeds and cottonseed oil. J.
Agric. Food Chem., 6: 845-848
Gunther, F. A., Carman, G. E., Blinn, R. C. and Barkley, J. H. (1963)
Persistence of residues of Guthion on and in mature lemons and oranges
and in laboratory processed citrus "pulp" cattle feed. J. Agric. Food
Chem., 11: 424-427
Huntingdon Research Centre. (1966a) Toxicity of gusathion during
repeated administration to rats for two years. Unpublished report
Huntingdon Research Centre. (1966b) Gusathion (Bayer 17147), Chronic
oral toxicity studies in dogs. Unpublished report
Johnsen, R. E. and Dahm, P. A. (1966) Activation and degradation
efficiencies of liver microsomes from eight vertebrate species, using
organophosphates as substrates, J. Econ. Entomol., 59: 1437-1442
Löser, E. and Lorke, D. (1967) Die Aktivität der Cholinesterase bei
Hunden nach Verabreichung von Gusathion mit dem Futter. Farbenfabriken
Bayer. Unpublished report
MacDougall, D. (1964) Guthion. In: G. Zweig, Analytical Methods for
Pesticides, Plant Growth Regulators and Food Additives, vol. II,
Academic Press, New York-London
Magill, L. J. and Everett, L. J. (1966) Guthion-C14 study on lettuce.
Chemagro Report No. 18636
Meagher, W. R., Adams, J. M., Anderson, C. A. and MacDougall, D.
(1960) Colorimetric determination of Guthion residues in crops.
J. Agric. Food Chem., 8: 282-286
Miles, J. R. W. (1964) A new colorimetric method for determination of
residues of Guthion and Ethyl Guthion and their oxygen analogs.
J.A.O.A.C., 47: 882-885
Murphy, S. D. (1966) Liver metabolism and toxicity of thiophosphate
insecticides in mammalian, avian and piscine species. Proc. Soc. exp.
Biol. (N.Y.), 123: 392-398
Murphy, S. D., Lauwerys, R. R. and Cheever, K. L. (1968) Comparative
anticholinesterase action to organophosphorus insecticides in
vertebrates. Toxicol. appl. Pharmacol., 12: 22-35
Root, M., Vesselinovitch, D., Meskauskas, J, and Doull, J. (1965)
Effect of guthion in the diet on the reproduction and lactation of
mice. University of Chicago. Unpublished report
Sato, J. (1959) Studies on organic phosphorus. Gusathion and phosdrin.
1. The toxicity of gusathion and phosdrin. Kumamoto med. J., 12:
313-317 (Chem. Abstr., 54: 21473 (1960))
Schrader, G. (1963) Gusathion in Die Entwicklung neuer insektizider
Phosphosaure-Ester. Verlag Chemie, Weinheim., pp. 176-186
Smart, N. A. (1967) A modification of Miles' method for determining
azinphos-methyl residues in crops. Analyst, 92: 779-781
Thornton, J. S. (1967a) Determination of Guthion M-E in soybeans by
thermionic emission flame gas chromatography. Chemagro Report No.
20182
Thornton, J. S. (1967b) Effect of the oil deodorization process on
Guthion residues in soy bean oil (simulated). Chemagro Report No.
20834
Tietz, H., Metcalf, R. L. and Fukuto, T. R. (1957) Action of the
insecticide "Gusathion" on cotton plants, and the problem of residues
in cottonseed. Höfchen-Briefe, 10: 279-289 (English edition)
Tietz, H., Fukuto, T. R. and Metcalf, R. L. (1960) Untersuchungen mit
32P-markierten Verbindungen uber das Verhalten des Insektizids
GusathionR bei Baumwollpflanzen und das damit verbundene
Ruckstandsproblem bei Baumwollsamen. Verh. IV. Intern.
Pflanzenschutz-Kongr. Hamburg 1957, Bd. 2, biol. bundesanstalt f.
Land-und Forstwirtschaft, Braunschweig, S. 1645-1646
Other information on identity and properties
The pure material is a white, crystalline solid, M.P. 73-74°C; soluble
in water at 25°C (1:30 000) and soluble in most organic solvents. The
technical product is a brown waxy solid (M.P. 65-68°C). It decomposes
at elevated temperatures with the evolution of gas, and is rapidly
hydrolyzed by cold alkali to form anthranilic acid and is subject to
hydrolysis by acids. Suitable oxidizing agents convert it to the
oxygen analogue.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Azinphos-methyl is activated to gutoxon, a highly potent
cholinesterase inhibitor which has a molar I50 in rat brain of 2.99 ×
10-8 (Schrader, 1963). In vitro, the rate of degradation of gutoxon
by liver microsomal enzymes is not altered by species, or sex
variation in mammals (Johnsen and Dahm, 1966). Also the rate of in
vitro metabolism by the liver is similar in mammals, birds, and fish
(Murphy, 1966).
In vitro studies showed that azinphos-methyl is a poor brain
cholinesterase inhibitor in mammalian, avian, or piscine species but
gutoxon is a potent inhibitor; avian brain cholinesterase inhibition
being less than that in mammals, and fish brain cholinesterase being
the most susceptible to inhibition. Species differences in sensitivity
to gutoxon inhibition of brain cholinesterase are probably
sufficiently large to modify the influence of variation in metabolic
rates (Murphy et al., 1968).
Acute toxicity
LD50 (mg/kg
Animal Route body-weight) Reference
Mouse Oral 8* Sato, 1959
Mouse i.p. 8-10** Murphy, 1966
Rat Oral 11-25 DuBois et al., 1955
Gaines, 1960
Rat i.p. 5-11.6 DuBois et al., 1955
Guinea-pig Oral 80 DuBois et al., 1955
Guinea-pig i.p. 40 DuBois et al., 1955
* Given as azinphos-methyl emulsion.
** Given as a corn oil solution.
Short-term studies
Rat
Groups of 10 male rats were fed 0, 5, 10, 20, 50 or 100 ppm
azinphos-methyl in the diet for nine weeks. At 50, and 100 ppm slight
decrease in food intake, decrease in body-weight gain, and muscular
spasms and trembling were observed. Whole blood cholinesterase
activity was markedly decreased at 20, 50 and 100 ppm. At 5 and 10 ppm
the maximum depression which occurred, was 20 per cent below the
control values. (Huntingdon Research Centre, 1966.)
The addition of azinphos-methyl to the diet of groups of 10 young male
and 10 young female rats each, at levels of 2, 5 and 20 ppm did not
markedly alter the growth-rate over 60 days. The body-weight of the
male rats fed 20 ppm was about four per cent less than that of the
controls. In the rats fed 2 and 5 ppm for 120 days, cholinesterase
activity was unaffected, but at 20 ppm there was inhibition in the
brain (10 per cent) and in serum and erythrocytes (about 30
per cent). After 120 days no appreciable changes in the gross and
microscopic appearance of brain, heart, liver, spleen, adrenals,
stomach, intestines, skeletal muscle and bone-marrow were found. There
was no evidence of demyelination in the nervous system (Doull et al.,
1956).
When weanling male rats were fed diets containing 50 and 100 ppm of
azinphos-methyl for 16 weeks, approximately half of each group died.
All animals receiving azinphos-methyl in the diet showed marked
effects of cholinergic stimulation, including diarrhoea, salivation,
lacrimation, muscular tremors and fasciculations. These symptoms were
most marked during the first month on the diets. The rats fed 50 ppm
of azinphos-methyl weighed about 10 per cent less, and the rats fed
100 ppm, 18 per cent less than rats fed a normal diet. The
cholinesterase activity of the serum, brain, erythrocytes and
submaxillary glands of rats fed 50 and 100 ppm was markedly inhibited.
The inhibition was most marked in the erythrocytes and brain and the
animals did not fully recover from these effects during a three-week
period on a normal diet. Both gross and microscopic examination failed
to indicate any evidence of testicular atrophy due to the presence of
the high levels of azinphos-methyl in the diet (Doull et al., 1957a).
Dog
Groups of two dogs, one male and one female, given 5, 10, 20 and 50
ppm of azinphos-methyl in their diet did not show any loss of weight
nor any symptoms of azinphos-methyl poisoning during a 12-week period.
At levels of 5, 10 and 20 ppm there was no significant decrease, but
at 50 ppm there was a 25 per cent decrease in serum cholinesterase
activity at the end of the 12-week period. The erythrocyte
cholinesterase of the one male and one female dog began to be
inhibited at the 10 ppm level (Doull et al., 1967b).
Six groups of one male and one female dogs were fed 0, 20, 50, 100,
200 or 400 ppm of azinphos-methyl in a dry diet, for 19 weeks. Whole
blood cholinesterase depression was slight at 20 and 50 ppm, becoming
apparent after four weeks on the diet; however, reactivation occurred
by the sixth week at 20 ppm, and by the ninth week at 50 ppm. At the
100 ppm level and above, cholinesterase activity was reduced by more
than 50 per cent; reactivation was not apparent at 400 ppm, was
doubtful at 200 ppm, and was slight at 100 ppm after 17 weeks. (Loser
and Lorke, 1967.)
Four groups of four male and four female dogs along with a control
group, were fed azinphos-methyl in a dry diet for two years. At the
lowest level the dogs were fed 5 ppm throughout the two-year period.
At the second level they were fed 20 ppm for 36 weeks, followed by 50
ppm for 15 months. At the third level the dogs received 50 ppm
throughout the two-year period. At the top dose level they received 50
ppm for 36 weeks, followed by 100 ppm for 21 weeks, 150 ppm for 27
weeks and finally 300 ppm for 21 weeks. Mortality throughout was
comparable to the controls. At 300 ppm, tremors, muscular weakness and
abnormal quietness were noted, especially among the male animals.
Weight loss occurred to a slight degree at this dose level. Food
intake was slightly reduced in females at 150 and 300 ppm.
Cholinesterase depression was not apparent during the first three
months of the study. In the period three to nine months, red blood
cell cholinesterase was slightly depressed at 20 ppm, and more
markedly depressed at 50 ppm. In the second year of the study, plasma
cholinesterase depression was apparent in dogs receiving 50 ppm and
above. Red blood cell cholinesterase continued to be depressed at
intermediate and high dose levels, and minimal depression was also
seen at 5 ppm. Other parameters, including haematology, clinical
chemistry, urinalysis, organ to body-weight ratios, and gross and
histopathology were all comparable to controls. (Huntingdon Research
Centre, 1966b.)
Long-term studies
Rat
Groups of 40 male and 40 female rats were fed 2.5, 5 and 20 ppm for
two years and a top group was fed 50 ppm for 47 weeks and then 100
ppm. Two control groups were also maintained. At 100 ppm, toxic
symptoms in the form of salivation, diuresis, exophthalmos, loss of
balance and co-ordination, muscular fasciculation, and minor tremors
were observed in five females. Mortality, food intake, body-weight
gain, food utilization, urinalysis, and haematology were comparable to
controls. Depression of cholinesterase levels of red blood cells and
plasma occurred at 20 ppm and above, and plasma cholinesterase
depression was apparent up to 39 weeks at 5 ppm in the males. In the
females, consistent depression of plasma and red blood cell
cholinesterase was apparent only at the top dose. At 20 ppm plasma
cholinesterase depression occurred up to and including 39 weeks. At 5
ppm depression occurred at 10, 39 and 78 weeks. Red blood cell
cholinesterase was depressed up to 65 weeks at 20 ppm, but only up to
10 weeks at 5 ppm. Brain cholinesterase depression was significantly
depressed at the top dose level only. Organ to body-weight ratios
showed random variations, a possible increase with the liver occurring
at the top dose level. There was no indication that tumour incidence
was increased and it was concluded that azinphos-methyl was devoid of
carcinogenic activity in the rat. Gross and histopathology showed no
compound related effects. (Huntingdon Research Centre, 1966,)
Special studies
Reproduction
Mouse. Groups of six male and 24 female mice were fed 0, 5, 10, 25
and 50 ppm in the diet over three generations. Initial exposure to the
diet was for 30 days prior to the first mating of the Fo generation.
Because of the high mortality (15 out of 24) among the females at 50
ppm prior to mating, this dose level was eliminated after the first
mating. Fertility and litter size were not affected in the 50 ppm
group, but survival to weaning was significantly decreased. Up to and
including 25 ppm no adverse effects were apparent as judged by
fertility, gestation or lactation, litter size, or survival of
offspring to 30 days. Gross and microscopic examination of F3b
weanlings showed no compound related changes. (Root et al., 1965.)
Rabbit. Three groups of 10 pregnant female rabbits were fed 0, 5 or
25 ppm in the diet, from the eighth to the sixteenth day of pregnancy.
Five rabbits in each group were sacrificed on the twenty-ninth day of
pregnancy, the remainder being permitted to litter. No compound
related effects occurred with respect to litter size, stillbirths, sex
ratios, average foetal weights, incidence of immature foetuses, or
survival to 30 days. (Doull et al., 1966.)
Comments
Since the last evaluation by the FAO/WHO Joint Meeting (Rome, 15-22
March 1965), results of reproduction studies in mice, short-term
studies in dogs and long-term studies in rats have been provided. The
studies performed appear satisfactory. Further work desirable includes
studies on cholinesterase inhibition of plasma and erythrocytes in man
and metabolic studies in man.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effects
Rat: 2.5 ppm in the diet, equivalent to 0.125 mg/kg.
Dog: About 5 ppm in the dry diet, equivalent to 0.125 mg/kg.
Estimate of acceptable daily intake of azinphos-methyl for man
0-0.0025 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Azinphos-methyl is used for the pre-harvest control of a wide spectrum
of insects and mites attacking fruit, vegetable and forage crops.
Residues resulting from supervised trials
The following typical data are extracted from Chemagro internal
reports of trials made at various field stations.
Rate of application No. of Pre-harvest Residue
Crop (kg/ha) treatments interval (days) (ppm)
Alfalfa 0.50 1 5 0.4
Apples 0.28-0.35 7 7 0.75
Apricots 0.28-0.35 1 15 4.6
21 3.1
Broccoli 0.56 5 15 0.38
Brussels 0.56 1 7 0.6
sprouts 14 n.d.
Cabbage 0.56 3 15 n.d.
Cherries 0.28-0.35 1 7 0.18-0.74
14 n.d.-0.23
Clover 0.50 1 5 0.4
Grapes 0.28-0.35 1 15 0.6
22 0.4
0.28-0.35 2 16 5.1
23 3.7
Grapefruit 1.12 1 15 0.3-0.9
Peaches 0.28-0.35 1 19 1.0
31 0.4
Pears 0.28-0.35 1 8 0.3
Peas 0.56 3 7 n.d.
Plums 0.28-0.56 1 14 n.d.-0.25
Strawberries 1.12 1 7 0.9-1.5
14 0.7
Tomatoes 1.56 1 7 0.09
Plums 0.28-0.56 1 14 n.d.-0.25
Strawberries 1.12 1 7 0.9-1.5
14 0.7
Tomatoes 1.56 1 7 0.09
n.d. = not detectable.
Evidence of residues in food moving in commerce or at consumption
No data available.
Fate of residues
In plants. Azinphos-methyl has not been shown to exhibit systemic
action. No phosphorothiolate oxidation product could be found in or on
sprayed cotton leaves (Tietz et al., 1957, 1960). Two unidentified
phosphorus-containing metabolites, more lipophilic than the applied
chemical, appeared and gave positive tests for anthranilic acid
(Meagher et al., 1960). Further metabolites were recovered containing
the radioactive phosphorus in phospholipids and as hydrolysis
products.
With lettuce, most of the chemical remained on the surface and no
oxygen analogue was isolated (Magill et al., 1966). After 14 days, 95
per cent of the extracted residue was azinphos-methyl with four
components in the remaining five per cent Two were benzazimide and
methyl benzazimide sulfide while the remaining two were not
identified.
In animals. P32 and C14-labelled material administered orally to
dairy cows did not result in azinphos-methyl or the P=0 derivative in
the blood or milk (Everett et al., 1966). Excluded possible
metabolites were anthranilic acid, benzazimide, hydroxymethyl
benzazimide, mercaptomethyl benzazimide, N-methyl benzazimide, bis
(N-methyl benzazimide) sulfide and the corresponding disulfide.
However four unidentified components containing the benzazimide
moiety, appeared, one of which accounted for 90 per cent of the total
residue. They contribute a maximum of 0.017 ppm in the milk when
feeding the animal at the rate of 0.2 mg/kg, equivalent to 2.8 ppm in
the feed. At least two of the metabolites may be oxidation products of
an intermediate metabolite, bis (N-methylbenzazimide) sulfide. Thus
the residue in milk from feeding forage containing 2.8 ppm
azinphos-methyl will be 0.008 ppm expressed as mercaptomethyl
benzazimide while the residue in tissues will not exceed 0.1 ppm.
The fluorometric method of Adams and Anderson (1966) is suitable for
determining these metabolites.
In storage and processing. Residues of azinphos-methyl were stable
at frozen storage levels (-18 to -23°C) in samples of fruit, fodder
and vegetables (Chemagro, 1962). Washing oranges reduced the residues
from 1.0 ppm to 0.7 ppm (whole fruit), from 2.7 to 1.9 ppm in the
peel. While the juice and pulp were free of residues, the orange oil
contained 30 ppm (Anderson et al., 1963). Simple washing reduces
residues on the peel from 71 to 96 per cent, the greater amount with
the fresher residue (Gunther et al., 1963). Field-sprayed snap beans
bearing an initial residue of 1.09 ppm had a residue of 0.14 ppm after
washing, blanching and freezing (Carlin et al., 1966). After canning
the residue was reduced to 0.02 ppm. Soy-bean oil fortified with
azinphos-methyl and deodorized according to commercial procedures had
the residue reduced by 36 per cent (Thornton, 1967b). Sugar-cane
containing 0.1 to 0.6 ppm showed no residues in the molasses or sugar
when processed (FAO/WHO, 1965).
Methods of residue analysis
Colorimetric, gas chromatographic, fluorometric, infra-red and
polarographic methods have been developed. One commonly used procedure
is based on weak alkaline hydrolysis of the residue to liberate
anthranilic acid which is then diazotized and coupled with
N-(1-naphthyl)-ethylenediamine dihydrochloride and the resultant
purple colour measured at 555 mµ (Meagher et al., 1960; MacDougall,
1964; Cohen et al., 1966). Modifications have been made to shorten the
procedure (Cox, 1964). Miles (1964) has done this by direct coupling
of the benzotriazinyl-containing residue in an acetic-hydrochloric
acid mixture and measuring the blue-violet colour at 556 mµ. Smart
(1967) has modified the method by introducing an improved extraction
procedure.
Another colorimetric method involves the alkali cleavage and
measurement of the dimethyl phosphorodithioate as the copper complex
absorbance at 420 mµ. This measures the parent compound only and is
similar to the infra-red measurement using the P=S stretching
vibration at 654 cm-1 (15.25 microns) (Cohen et al., 1966),
The sensitivity of the anthranilic acid colorimetric method can be
increased to about 0.05 ppm by using 10 cm cells. The precision for
recovery of azinphos-methyl from a number of crops indicates that in
the range of 0.2 to 0.5 ppm the average deviation from the mean is
approximately six per cent.
A spectrophotofluorometric method is based on the measurement of the
anthranilic acid released on alkaline hydrolysis by determining
fluorometrically at an activating wavelength of 340 mµ and a
fluorescence of 400 mµ (Adams and Anderson, 1966). It has a
sensitivity of about 0.005 ppm for milk, 0.02 ppm for most animal
tissues and 0.03 ppm for fat.
More recently a gas chromatographic procedure has been developed for
the determination of residues in soy-beans using the potassium
chloride thermionic emission flame detector for phosphorus (Thornton,
1967a). This method is sensitive to 0.005 ppm and distinguishes
between azinphos-methyl and its P=O analogue.
A method based on the measurement of the formaldehyde released on acid
hydrolysis of the residue has been used (Giang and Schechter, 1958) as
well as a polarographic analysis (Bates, 1962).
National tolerances
Tolerance Pre-harvest
Country Crop (ppm) interval (days)
Australia 21
Austria 21
Benelux fruit, grapes 0.5
Brazil fruit, vegetables 2.0
cotton seed 0.5
Canada grapes 0.5
fruits, vegetables 1-2.0
Denmark 21
France 15
Germany fruit, grapes,
(Fed. Rep.) vegetables 0.4 14
Italy 0.4 20
New Zealand berry fruit,
leafy vegetables 21
pip and stone
fruit, root
crops, tomatoes 14
Norway 21
Portugal 21
South Africa citrus fruit 2.0 21
pome fruit 14
Switzerland fruit, grapes 1.0 21
United Kingdom 21
United States grapes 5.0
of America fruit, vegetables 2.0
cereals, soy-beans,
nuts, peas,
cucumbers, melons
peppers and
potatoes "zero"
RECOMMENDATION FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Since the publication of the monographs resulting from the 1965 Joint
Meeting, work has confirmed that the residue on plants is mainly
present as the original compound, azinphos-methyl. In the five per
cent of residue which is not accounted for as azinphos-methyl in
lettuce 14 days after treatment, out of four metabolites present two
were identified as benzazimide and methyl benzazimide.
Investigations with dairy cows indicate that no residues of
azinphos-methyl or the oxygen analogue will occur in milk from animals
consuming fodder likely to contain residues. However, four
non-phosphorus-containing metabolites, but still containing the
benzazimide moiety, may be present, although they have not been
precisely identified.
Although colorimetric and gas-liquid chromatographic methods have been
developed, none of these have been evaluated for regulatory or referee
purposes.
Recommendations
The following temporary tolerances (to be in effect until 1972) are
to apply to raw agricultural products moving in commerce unless
otherwise indicated. In the case of fruits and vegetables the
tolerances should be applied as soon as practicable after harvest and
in any event prior to actual retail to the public. In the case of
commodities entering international trade, the tolerances should be
applied by the importing country at the point of entry or as soon as
practicable thereafter. Furthermore, the tolerances should not apply
to the ethyl derivative nor to the oxygen analogue, the latter usually
being insignificant.
Temporary tolerances
Apricots and grapes 4 ppm
Other fruits 1 ppm
Vegetables 0.5 ppm
Further work or information
Required before 30 June 1972
1. Information on the nature of terminal residues in plants, animals
and their products.
2. Further data on residue levels in raw agricultural products moving
in commerce.
3. Data on disappearance of residues during storage and household
cooking of vegetables.
4. Data on the possible carry-over of residues into wine as a result
of the treatment of grapes.
5. Comparative evaluation of gas-liquid chromatographic and
spectrophotometric methods for the determination of azinphos-methyl
and its oxygen analogue for regulatory purposes.
Desirable
1. Studies on cholinesterase inhibition of plasm and erythrocytes in
man.
2. Metabolic studies in man.
3. Identification and toxicology of metabolites, especially those
having the benzazimide moiety, in milk.
4. Collaborative studies to establish a referee method.
REFERENCES
Adams, J. M. and Anderson, C. A. (1966) Spectrophotofluorometric
method for Guthion residues in milk and animal tissues. J. Agric. Food
Chem., 14: 53-55
Anderson, C. A., MacDougall, D., Kesterson, J. W., Hendrickson, R, and
Brooks, R. F. (1963) The effect of processing on Guthion residues in
oranges and orange products. J. Agric. Food Chem., 11: 422-424
Bates, J. A. R. (1962) Polarographic determination of azinphos-methyl
residues in certain crops. Analyst, 87: 786-790
Carlin, A. F., Hibbs, E. T. and Dahm, P. A. (1966) Insecticide
residues and sensory evaluation of canned and frozen snap beans field
sprayed with Guthion and DDT. Food Technol., 20: 80-83
Chemagro. (1962) Effect of frozen storage on Guthion residues in
various crops. Report No. 8682
Cohen, C. J., Betker, W. R., Wasleski, D. M. and Cavaghol, J. C.
(1966) Analysis of Guthion insecticide, J. Agric. Food Chem., 14:
315-318
Cox, W. S. (1964) Rapid determination of Guthion residues on crops.
J.A.O.A.C., 47: 280-282
Doull, J., Anido, P. and DuBois, K. P. (1957a) Effect of high dietary
levels of guthion on rats. University of Chicago. Unpublished report
Doull, J., Anido, P. and DuBois, K. P. (1957b) Determination of the
safe dietary level of guthion for dogs. University of Chicago.
Unpublished report
Doull, J., DiGiacomo, R. and Meskauskas, J. (1966) Short term breeding
studies with guthion in rabbits. University of Chicago. Unpublished
report
Doull, J., Rehfuss, P. A. and DuBois, K. P. (1956) The effects of
diets containing guthion (Bayer 17147) on rats. University of
Chicago. Unpublished report
DuBois, K. P., Thursh, D. R. and Murphy, S. D. (1955) The acute
mammalian toxicity and mechanism of action of Bayer 17147. University
of Chicago. Unpublished report
DuBois, K. P., Thursh, D. R. and Murphy, S. D. (1957) Studies on the
toxicity and pharmacologic actions of the dimethoxy ester of
benzotriazine dithiophosphoric acid (DBD guthion). J. Pharmacol.exp.
Ther., 119: 208-218
Everett, L. J., Anderson, C. A. and MacDougall, D. (1966) Nature and
extent of Guthion residues in milk and tissues resulting from treated
forage. J. Agric. Food Chem. 14: 47-53
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Mtg. Rept. PL/1965/10/1; WHO/Food Add./27.65
Gaines, T. B. (1960) The acute toxicity of pesticides to rats,
Toxicol. appl. Pharmacol., 2: 88-99
Giang, P. A. and Schechter, M. S. (1958) Colorimetric method for
estimation of Guthion residues in cottonseeds and cottonseed oil. J.
Agric. Food Chem., 6: 845-848
Gunther, F. A., Carman, G. E., Blinn, R. C. and Barkley, J. H. (1963)
Persistence of residues of Guthion on and in mature lemons and oranges
and in laboratory processed citrus "pulp" cattle feed. J. Agric. Food
Chem., 11: 424-427
Huntingdon Research Centre. (1966a) Toxicity of gusathion during
repeated administration to rats for two years. Unpublished report
Huntingdon Research Centre. (1966b) Gusathion (Bayer 17147), Chronic
oral toxicity studies in dogs. Unpublished report
Johnsen, R. E. and Dahm, P. A. (1966) Activation and degradation
efficiencies of liver microsomes from eight vertebrate species, using
organophosphates as substrates, J. Econ. Entomol., 59: 1437-1442
Löser, E. and Lorke, D. (1967) Die Aktivität der Cholinesterase bei
Hunden nach Verabreichung von Gusathion mit dem Futter. Farbenfabriken
Bayer. Unpublished report
MacDougall, D. (1964) Guthion. In: G. Zweig, Analytical Methods for
Pesticides, Plant Growth Regulators and Food Additives, vol. II,
Academic Press, New York-London
Magill, L. J. and Everett, L. J. (1966) Guthion-C14 study on lettuce.
Chemagro Report No. 18636
Meagher, W. R., Adams, J. M., Anderson, C. A. and MacDougall, D.
(1960) Colorimetric determination of Guthion residues in crops.
J. Agric. Food Chem., 8: 282-286
Miles, J. R. W. (1964) A new colorimetric method for determination of
residues of Guthion and Ethyl Guthion and their oxygen analogs.
J.A.O.A.C., 47: 882-885
Murphy, S. D. (1966) Liver metabolism and toxicity of thiophosphate
insecticides in mammalian, avian and piscine species. Proc. Soc. exp.
Biol. (N.Y.), 123: 392-398
Murphy, S. D., Lauwerys, R. R. and Cheever, K. L. (1968) Comparative
anticholinesterase action to organophosphorus insecticides in
vertebrates. Toxicol. appl. Pharmacol., 12: 22-35
Root, M., Vesselinovitch, D., Meskauskas, J, and Doull, J. (1965)
Effect of guthion in the diet on the reproduction and lactation of
mice. University of Chicago. Unpublished report
Sato, J. (1959) Studies on organic phosphorus. Gusathion and phosdrin.
1. The toxicity of gusathion and phosdrin. Kumamoto med. J., 12:
313-317 (Chem. Abstr., 54: 21473 (1960))
Schrader, G. (1963) Gusathion in Die Entwicklung neuer insektizider
Phosphosaure-Ester. Verlag Chemie, Weinheim., pp. 176-186
Smart, N. A. (1967) A modification of Miles' method for determining
azinphos-methyl residues in crops. Analyst, 92: 779-781
Thornton, J. S. (1967a) Determination of Guthion M-E in soybeans by
thermionic emission flame gas chromatography. Chemagro Report No.
20182
Thornton, J. S. (1967b) Effect of the oil deodorization process on
Guthion residues in soy bean oil (simulated). Chemagro Report No.
20834
Tietz, H., Metcalf, R. L. and Fukuto, T. R. (1957) Action of the
insecticide "Gusathion" on cotton plants, and the problem of residues
in cottonseed. Höfchen-Briefe, 10: 279-289 (English edition)
Tietz, H., Fukuto, T. R. and Metcalf, R. L. (1960) Untersuchungen mit
32P-markierten Verbindungen uber das Verhalten des Insektizids
GusathionR bei Baumwollpflanzen und das damit verbundene
Ruckstandsproblem bei Baumwollsamen. Verh. IV. Intern.
Pflanzenschutz-Kongr. Hamburg 1957, Bd. 2, biol. bundesanstalt f.
Land-und Forstwirtschaft, Braunschweig, S. 1645-1646
