
FAO/PL:1968/M/9/1
WHO/FOOD ADD./69.35
1968 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Geneva, 9-16 December,
1968.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Geneva, 1969
DICOFOL
IDENTITY
Chemical names
1,1 bis (4-chlorophenyl) 2,2,2-trichloroethanol;
2,2,2,-trichloro-1, 1-di (4-chlorophenyl) ethanol (IUPAC).
Synonyms
Kelthane(R)
Formula
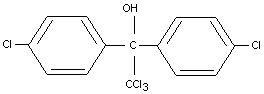 Other information on identity and properties
Analysis of technical dicofol is not known. Purity of technical
dicofol is 82-88 per cent. The United States Department of Agriculture
(USDA, 1968a) places the total acaricide market for 1964 in the United
States of America at about three million pounds active ingredients per
year, dicofol being about half of that amount. Resistance is still
today a cause for reduction of the use of dicofol, e.g. in Israel.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
No specific identification of the metabolites of dicofol in animals
has been made. However, on the basis of the known chemistry of the
compound, 4,4'-dichlorobenzophenone is considered to be a probable
metabolite. Thus, under alkaline conditions, dicofol is hydrolytically
cleaved to 4,4'-dichlorobenzophenone and chloroform (Rohm and Haas,
1968).
Upon ingestion of dicofol by mammals, storage of the compound occurs
in the adipose tissue. Rats were fed dicofol at a level of 32 ppm in
their diet for 12 weeks. After eight weeks the level of the compound
in the fat had reached equilibrium at concentrations of 25 ppm for the
males and 70 ppm for the females, which amounts corresponded to 75 per
cent and 200 per cent of the dietary levels. After 12 weeks, dicofol
was withdrawn from the diet and the level of stored material declined.
The rate of decline was greater for the male animals than for the
females. By 14 weeks after withdrawal the level of dicofol in the fat
was zero for the males but still remained at about 6 ppm for the
females. Feeding with higher or lower dose levels also showed that
dicofol was stored in the fat of the female rat to a greater degree
than in the male (Smith et al., 1959).
The effect of dicofol on plasma 17-hydroxy-corticosteroids in the dog
was determined on two dogs which were fed 300 ppm and 900 ppm over two
separate periods of one to two months' duration. The ability of the
adrenal cortex to elaborate 17-hydroxy-corticosteroids in response to
ACTH stimulation was slightly reduced at the 300 ppm level and
markedly reduced at the 900 ppm level. The results also showed that
following this treatment with dicofol, the ability of the adrenal
gland to return to the pre-treatment level of response to ACTH
proceeded slowly and, possibly, incompletely (Smith et al., 1959).
Acute toxicity (80 per cent technical product)
LD50 mg/kg
Animal Route body-weight Reference
Rat (M) Oral 809 Smith et al., 1959
Rat (F) Oral 684 Smith et al., 1959
Rabbit (M) Oral 1810 Smith et al., 1959
Dog (mixed) Oral >4000 Smith et al., 1959
Short-term studies
Rat
Dicofol was fed to groups, each containing 10 male and 10 female rats,
for 90 days at dietary concentrations of 0, 20, 100, 500, 1250 and
2500 ppm. Survival was adversely affected at 1250 ppm and above.
Growth was inhibited at 100 ppm and higher levels in the females but
only at 1250 ppm in the males. Increased liver to body weight ratios
occurred in the survivors in both sexes. Liver lesions were the most
consistent histopathological finding, but were only of scattered
incidence at dose levels below 1250 ppm (Smith et al., 1959).
Groups containing equal numbers of male and female rats were fed 0, 2,
5, 10, 15 and 20 ppm in their diets for 55 weeks. Growth, survival and
liver to body weight ratios were not affected at any dose level (Smith
et al., 1959).
Five groups, each of 10 male and 10 female rats were fed 0, 50, 200,
1000 and 3000 ppm in their diet for 13 weeks. Body weight and food
intake were reduced at the 200 ppm level and above, and at 3000 ppm
there was 100 per cent mortality. Haematological criteria were
comparable to the controls. Organ to body weight ratios were increased
in the case of the liver at the 200 ppm level and above and in the
case of the thyroid at the 1000 ppm level. Decreased uterus and
prostrate to body weight ratios were observed at 1000 ppm. Liver
glucose-6-phosphatase was depressed at 1000 ppm. while hexobarbital
oxidase activity showed a dose-related stimulation in all treated
groups. Histopathological examination revealed enlargement of
centrilobar cells and nuclei at 200 ppm and above. Electron microscopy
revealed smooth endoplasmic reticulum whorls to be present only at
1000 ppm and above (Verschuuren et al., 1968).
Dog
Groups each containing three dogs were given levels of dicofol of 100,
300 and 900 ppm for one year. Survival was affected only at 900 ppm.
Body weight gain was normal and haematological and histological
observations revealed no pathological effects (Smith et al., 1959).
Long-term studies
Rat
Dicofol was fed to 60 groups, each containing 10 male and 10 female
rats, at dietary levels of 0, 20, 100, 250, 500 and 1000 ppm for two
years. Growth depression occurred in male rats at 500 and 1000 ppm,
and in female rats progressively with increasing dietary concentration
at 250, 500 and 1000 ppm. A growth depression after three months,
recorded in female rats at 20 ppm (but not at 100 ppm) has not been
observed again in later studies. Absolute organ weights showed no
significant differences from the controls, with the exception of an
increase in the case of the livers and kidneys of the female rats fed
1000 ppm. Organ to body weight ratios were significantly increased for
the liver at 250 ppm and for the liver, kidney and heart at 500 ppm in
the females, but only for the liver at 500 ppm in the males.
Histopathological findings were confined to hydropic changes in the
liver which were regarded as reversible (Larson, 1957).
Special studies
(a) Reproduction
Mouse. Groups of varying numbers of mice were maintained throughout
five generations on dietary levels of 0, 7, 25, 100, 225 and 500 ppm
of dicofol. At the 500 ppm level the litter sizes, average weight of
the pups and also the values for fertility, viability and lactation
indices were lower than for the control group. However, all these
parameters were normal for the 225 ppm and lower levels (Brown,
1967a).
Rat. Four groups, each of 27 male and 27 female rats were fed
dietary levels of 0, 100, 500 and 1000 ppm of dicofol in a
two-generation reproduction study. There were no F1b pups surviving
at 21 days when the original parents were fed 500 or 1000 ppm. Litter
size from the 1000 ppm group was similar to the controls but over-all
mortality in the pups was greater. Considerable reduction in fertility
of the animals fed 500 and 1000 ppm was evident. No congenital defects
were observed in any of the F2a or F2b animals (Brown, 1964-65).
Groups of rats were maintained on diets containing 25 or 75 ppm of
dicofol through a three-generation study. The average number of pups
born per litter to parents receiving 75 ppm was slightly lower than
for the controls. There was no compound related effects relative to
body weight, fertility, gestation, viability or lactation indices at
either level, nor were there any congenital abnormalities evident in
either the viable or the still-born pups (Brown, 1967b).
(b) Toxicity study of a possible metabolite
The possible metabolite, 4,4'-dichlorobenzophenone was fed to groups
each containing 15 male and 15 female rats, at dietary levels of 0,
100 and 1250 ppm for three months. No effects were noted at either
level except somewhat reduced heart to body weight ratios found in the
males receiving 1250 ppm, but not in the females (Larson, 1965).
Comments
Short-term experiments on dogs and rats, reproduction studies on rats
and mice and long-term experiments on rats are reported. From the
results of the acute and short-term experiments a great species
difference in susceptibility is evident between dogs and rats.
No specific identification of possible metabolites has been made in
animals but dichlorbenzophenone is a probable metabolite. No human
studies have been reported.
Comparative metabolic studies in animals and man, including adrenal
function studies after oral administration are desirable.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 50 ppm, equivalent to 2.5 mg/kg per day.
Estimate of acceptable daily intake for man
0.0-0.025 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Kelthane is a miticide with systemic properties, used on a large
number of crops. It has no insecticidal activity. The following table
gives a review of application rates of dicofol and recommended
pre-harvest intervals.
Rate of application (kg/ha) Pre-harvest
interval
Crop Small plants Large plants (days)
Apples, pears, crabapples, 2.20 4.41-5.30 7
quince
Cherries 2.20 3.96 7
Peaches, apricots, 2.20 3.96 14
nectarines
Walnuts, filberts, pecans,
chestnuts, hickory nuts 1.76 4.85 14
and almonds, if hulls are
not fed to livestock
Almonds, if hulls are fed 1.76 3.09 Before nut
to livestock formation
Grapes, hops .66 1.60 7
Strawberries .44 2.96 2
Raspberries and other cane .66 1.60 2
berries
Beans .44 .73 7
Beans (dry) .44 1.00 45
Beans (California) .66 1.32 45
Cantaloupe, cucumbers,
pumpkins, squash, melons, .44 .73 2
water melons
Tomatoes, peppers .66 1.00 2
Alfalfa, clover, other .66 1.32
legumes for seed only
Corn (field) .88 1.76 Before
ears form
Plums, prunes, figs 1.76 2.50 7
(Adapted from Rohm and Haas, Kelthane 35 label)
Post-harvest treatments
No post-harvest uses of dicofol are known.
Other uses
Dicofol is used for control of mites on lawns, ornamentals and shade
trees, as well as against clover mites in buildings.
Residues resulting from supervised trials
Detailed residue data are available from United States trials with
dicofol on important crops and have been deposited with FAO. Rates of
application were similar to those given above. The typical data
presented below are representative:
Crop Number of Post-treatment Residue (ppm)
treatments interval (days) Range Average
Almonds (whole) 1 129-157 3.11-0.18 1.60
Apples 1 7 1.1
Beans (whole) 1 6 2.45
Carrots (roots) 1-2 5 0.00-0.25 0.10
Celery 1 6 1.32-4.45 2.88
Cherries 1 7 1.2 -1.8 1.5
Citrus (whole) 1-2 7 1.12-5.0 2.2
Cottonseed 1 15-76 0.00-0.1 0.0
Cucumbers 1 2 0.7 -2.3 1.6
Figs 1 7 1.86-5.32 3.92
Grapes 1 5 0.89-2.21 1.64
Hops (green) 1 7 3.0 -7.9 5.4
Lettuce 2 2 1.10-9.87 5.59
Mint (fresh hay) 1 7 35.8 -38.9 37.3
Peaches 1 14 4.9 -6.8 5.7
(continued)
Crop Number of Post-treatment Residue (ppm)
treatments interval (days) Range Average
Pears 2 7 1.1 -2.6 1.9
Pineapples 4 5 0.0 -0.05 <0.05
(pulp)
Plums, prunes 1 9 0.06-0.16 0.11
Raspberries 1 2 2.7
Spinach 1 4 2.88-3.90 3.39
Strawberries 1 3 0.13-0.90 0.37
Tea, raw leaves 1 7 18.00-31.00 25.0
1 7 0.00-18.5 5.1
Tomatoes 1 1 0.5 -0.57 0.53
Tropical fruits 5 4 0.0 0.0
Walnuts 1 7 0.57-0.92 0.86
Water melons 1 20 0.0
(heart)
Although dicofol is used for seed treatment no data on residues
are available.
Fate of residues
In soils
Dicofol is not used against pests in soil, but studies have been
conducted to determine residues in soil resulting from spray
application on crops. Dicofol residues in soil decrease rapidly at
first, then more slowly. After three monthly applications of 2.56
kg/ha, residues which had reached 1.13 ppm decreased to 0.25 ppm after
636 days, (Rohm and Haas, 1967).
In plants
No information is available on metabolism in plants.
In animals
Cows fed 2 ppm dicofol for 71 days averaged from 0.23-0.40 ppm
residues in their milk; when the same cows were fed 1.0 ppm, the level
was not detectable. Two body fat samples taken at the end of the study
contained 1.1 and 2.7 ppm (Zweig et al,, 1963).
A "Blue" cow fed 5 ppm dicofol for 17 days, excreted a maximum of
0.022 ppm dicofol per se and a maximum of 0.55 ppm dicofol plus
metabolites in the milk. Plateau values were obtained about six days
after start of dicofol feedings, and initial decay was also rapid.
From the fifth day after withdrawal of dicofol, the amounts of
material excreted were small, and the rate of decrease became low.
In a "White" cow fed 30 ppm dicofol for three days, 0.68 ppm dicofol
were found in kidney fat. Dicofol metabolites were found in kidney
fat, liver, udder, brain, kidney lungs, blood, heart, omentum, bone
marrow and muscles (Rohm and Haas, 1968).
The feeding of dicofol to steers produces no significant residues in
body fat at a level of 0.75 and 1.5 ppm. A feeding level of 3.0 ppm
produces a definite residue of 0.32 ppm (Peoples, 1967).
In tissues, milk and urine of a cow fed dicofol two groups of
metabolites were found, one more polar than dicofol and one less
polar. The metabolites of the first group were not identified. In the
second group, two substances were identified as 1,1 bis
(4-chlorophenyl) 2-chloro-ethylene and as 1,1 bis
(4-chlorophenyl)-2,2-dichloro-ethylene. Sixty per cent of total milk
residues is due to the latter substance, but it is suggested that it
may be present in technical dicofol rather than result from the
metabolic reduction of dicofol itself (Rohm and Haas, 1958). The
latter two compounds were also found as metabolites of DDT.
In storage and processing
Residues on fruits are reduced by washing. Wash-water of oranges
contained up to 0.05 ppm dicofol; 0.12-0.57 ppm remained in the peel.
Unwashed raspberries contained 0.7-2.9 ppm dicofol, residues which
were reduced to 0.7-1.4 after washing.
Peeling reduces residues to values below 1 ppm:
Fruit Residue (ppm)
Peel Pulp
Citrus fruit 0.29-12.0 0-0.27
Almonds 0-12.2 0-0.03
Cucumbers 0.09-0.4 0-0.17
Pineapples 5.5 -24.2 0-0.05
Tropical fruit 4.2 -9.7 0
(Rohm and Haas, private communication)
In canned or frozen fruits dicofol was found only in 1.3 per cent of
domestic samples, with an average ppm of 0.01. No dicofol was found in
samples imported into the United States of America (USDA, 1968b).
Brewed tea contained residues up to 0.0085 ppm. Under rigorous
treatment, such as preparing instant tea from tea leaves, or
distilling oil from mint leaves, dicofol may be broken down to
p,p'-dichlorobenzophenone and chloroform (Rohm and Haas, private
communication).
Evidence of residues in food in commerce or at consumption
Examination of market samples of food in the United States of America
showed that dicofol residues were found in 0.7 per cent of domestic
samples and 1.8 per cent of imported samples (Duggan and Dawson,
1967a). In fruit the following concentrations were found in the United
States of America from 1964 to 1967 (USDA, 1968b).
Domestic samples Imported samples
Fruit Incidence Average Incidence Average
per cent. (ppm) per cent. (ppm)
Small fruit
(strawberries,
cherries, plums, 5.1 0.03 2.1 0.02
grapes, cranberries,
etc.)
(continued)
Domestic samples Imported samples
Fruit Incidence Average Incidence Average
per cent. (ppm) per cent. (ppm)
Large fruit
(apples, 8.6 0.02 3.3 0.01
oranges, pears,
peaches, etc.)
Total diet studies in the United States of America from 1964 to 1967
showed the following results (Duggan and Weatherwax, 1967b; USDA,
1968b)
Year Positive per cent. Daily average
composites intake (mg)
1964-1965 0.5 0.003
1965-1966 3.7 0.002
1966-1967 5.6 0.012
In 41.9 per cent of fruit-composites (apples, oranges, pears,
peaches, etc.) dicofol was found in an average concentration of 0.031
ppm (USDA, 1968b). Dietary intake of dicofol in the United States of
America was 0.00004 mg/kg body weight/day in 1965; 0.00015 in 1966 and
0.00018 in 1967 with an average of 0.00013 for three years (USDA,
1968b).
In Canada 60 restaurant meals were examined. Dicofol was found in an
average concentration of 0.07 ppm in fruit and fruit salads, in
legumes, 0.08 ppm whereas only traces were found in milk (Swackhamer,
1965).
Methods of residue analysis
The methods are adequate for present purposes. Methods for
colorimetric determination of dicofol are based on the method of
Rosenthal, 1957. Dicofol is converted to chloroform with alkali.
Chloroform is separated from extraneous materials and produces, with a
water-pyridine-sodium-hydroxide mixture, a Fujiwara-type red dye,
which is read at 535mµ against a pyridine blank. This method may be
used for residue determination in plants, fruit and vegetables and has
been simplified for analysis of milk (Gordon, 1963). Residues in crops
and soil may also be determined, after chromatographical clean-up, by
GLC using an electron capture detector (Morgan, 1967). An ultra-violet
spectrophotometric method involves hydrolysing the dicofol to
4,4'-dichlorobenzophenone (Gunther and Blinn, 1957). A method omitting
the hydrolysis step and allowing the dicofol to be oxidized to
non-interfering compounds permits the measurement of
4,4'-dichlorobenzophenone present in mint-oil and mint-hay (Rohm and
Haas, private communication).
The sensitivity of the analytical procedures is 0.01 ppm.
National tolerances
Tolerance Pre-harvest
Country Crop (ppm) interval (days)
United States Berries 5
of America
German Federal Fruit, including
Republic grapes and hops 0.5 14
Vegetables 0.5
Beans 14
Cucumbers 4
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Dicofol is used exclusively to control phytophagous mites and although
closely related to DDT has virtually no insecticidal activity. Its
principal use is on apples, grapes and hops in Europe and North
America, and tea (spot treatments only) in the Far East, with some
minor use on citrus in these same areas. In North America, the rates
and frequencies of application are generally greater than in other
countries because of more serious mite problems. It is not used on
animals. Dicofol is a technical product containing 82-88 per cent of
the pure (active) compound. The impurities are known and their
relative concentration are constant.
The data available to the meeting were obtained solely in the United
States of America and did not include information about uses elsewhere
or figures for residues after such use, although figures were
available for some imports into the United States of America.
Very little is known about the metabolism of dicofol in plants and
animals. In animals, dicofol can be found as such together with
various metabolites. Precise figures for the amounts of these
compounds in experimental plants or animals, either from a single or
from repeated applications, have not been published.
1,1 bis (4-chlorophenyl) 2-chloro-ethylene and 1,1 bis
(4-chlorophenyl-2,-2-dichloro-ethylene) have been identified as
metabolites in animals. However, the latter may be present in the
technical product used. In mint oil and tea, 4,4-dichlorobenzophenone
has been identified.
The literature includes methods of residue analysis which measure
dicofol alone or together with some breakdown products. For the
determination of dicofol alone the sensitivity is 0.01 ppm. However,
no referee method of analysis has been evaluated.
Recommendations
Temporary tolerances
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Fruit, vegetables, hops, tea (from a
particular estate, for blending only) 5 ppm
Tea (blended) 1 ppm
Further work or information
Required before 30 June 1972:
1. Data from countries other than the United States of America on the
required rates and frequencies of application, pre-harvest
intervals, and the resultant residues.
2. Information on the nature of the terminal residues in plants,
animals, and their products.
Desirable:
1. Collaborative studies to establish a referee method.
2. Comparative metabolic studies in animals and man, including adrenal
function studies after oral administration.
REFERENCES
Brown, J. R. (1964-65) Toxicologic studies on the effects of kelthane
in the diet of albino rats on reproduction (first interim, second
interim and final reports). Departments of Physiological Hygiene,
University of Toronto. Unpublished reports
Brown, J. R. (1967a) Toxicological studies on
2,2-bis-chlorophenyl-2,2,2-trichloroethanol, kelthane. Brown
Biological Laboratories Ltd. Unpublished report
Brown, J. R. (1967b) Three-generation reproduction study on rats
receiving technical kelthane in their diet. Department of
Physiological Hygiene, University of Toronto. Unpublished report
Duggan, R. E. and Dawson, K. (1967a) Pesticides: A report on residues
in food. FDA Papers, 1: 2-5
Duggan, R. E. and Weatherwax, J. R. (1967b) Dietary intake of
pesticide chemicals. Science, 157: 1006-1010
Gordon, C. F., Haines, L. D. and Martin, J. J. (1963) Acaricide
residues: An improved method for kelthane residue analysis with
applications for determination of residues in milk. J. Agr. Food
Chem., 11: 84-86
Gunther, F. A. and Blinn, R. C. (1957) Ultraviolet spectrophotometric
microdetection of the acaricide 4,4'-dichloro-alpha-(trichlormethyl)
benzhydrol (SW 293). J.Agr. Food Chem., 5: 517-519
Larson, P. S. (1957) Two-year study on the effect of adding kelthane
to the diet of rats. Medical College of Virginia. Unpublished report
Larson, P. S. (1965) Toxicologic study on the effect of adding
dichlorobenzophenone to the diet of rats for a period of three months.
Department of Pharmacology. Medical College of Virginia. Unpublished
report
Morgan, N. L. (1967) The identification and relative retention times
of p,p'-kelthane and its breakdown product p,p'-dichlorobenzophenone
using GLC. Bull. environ. Contam. Toxicol., 2: 306-312
Peoples, S. A. (1967) Residues in the body fat of steers fed kelthane
in their ration. Dept. of Physiol. Sci. School of Veterinary Medicine,
University of California, Davis. Unpublished report
Rohm and Haas. (1958) Research Reports 13-27 and 11.141. Robin and
Haas Co.
Rohm and Haas. (1967) RAR Memorandum 521. Rohm and Hass Co.
Rohm and Haas Co. (1968) Kelthane. Unpublished report
Rosenthal, J., Frisone, G. J. and Gunther, F. A. (1957) Colorimetric
microdetermination of the acaricide
4,4'-dichloro-alpha-(trichloromethyl) benzhydrol (FW-293).J. Agr. Food
Chem., 5: 514-517
Smith, R. B. Jr, Larson, P. S., Finnegan, J. K., Haag, H. B.,
Hennigar, G. R, and Cobey, F. (1959) Toxicologic studies on 2,2
bis-(chlorophenyl)-2,2,2-trichloroethanol (kelthane). Toxicol. appl.
Pharmacol., 1: 119-134
Swackhamer, A. B. (1965) Report on pesticide residues in restaurant
meals in Canada. Food and Drug Directorate, Department of National
Health and Welfare, Ottawa, Canada. Pesticide Progress, 3: 108-114
USDA. (1968a) Agricultural Economic Report 131
USDA. (1968b) United States Department of Health, Education and
Welfare. The regulation of pesticides in the United States
Verschuuren, H. G., Kroes, R. and den Tonkelaar, E. M. (1968)
Toxiciteitsonderzoek met kelthaan bij ratten gedurende 90 dagen
(Toxicity experiment with kelthane in rats of 90 day duration).
National Institute of Public Health, Utrecht. Unpublished report
Zweig, G., Pye, E. L. and Peoples, S. A. (1963) Residues in butter fat
and body fat of dairy cows fed at two levels of kelthane (1.0 and 2.0
ppm). J. Agr. Food Chem., 11: 72-79
Other information on identity and properties
Analysis of technical dicofol is not known. Purity of technical
dicofol is 82-88 per cent. The United States Department of Agriculture
(USDA, 1968a) places the total acaricide market for 1964 in the United
States of America at about three million pounds active ingredients per
year, dicofol being about half of that amount. Resistance is still
today a cause for reduction of the use of dicofol, e.g. in Israel.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
No specific identification of the metabolites of dicofol in animals
has been made. However, on the basis of the known chemistry of the
compound, 4,4'-dichlorobenzophenone is considered to be a probable
metabolite. Thus, under alkaline conditions, dicofol is hydrolytically
cleaved to 4,4'-dichlorobenzophenone and chloroform (Rohm and Haas,
1968).
Upon ingestion of dicofol by mammals, storage of the compound occurs
in the adipose tissue. Rats were fed dicofol at a level of 32 ppm in
their diet for 12 weeks. After eight weeks the level of the compound
in the fat had reached equilibrium at concentrations of 25 ppm for the
males and 70 ppm for the females, which amounts corresponded to 75 per
cent and 200 per cent of the dietary levels. After 12 weeks, dicofol
was withdrawn from the diet and the level of stored material declined.
The rate of decline was greater for the male animals than for the
females. By 14 weeks after withdrawal the level of dicofol in the fat
was zero for the males but still remained at about 6 ppm for the
females. Feeding with higher or lower dose levels also showed that
dicofol was stored in the fat of the female rat to a greater degree
than in the male (Smith et al., 1959).
The effect of dicofol on plasma 17-hydroxy-corticosteroids in the dog
was determined on two dogs which were fed 300 ppm and 900 ppm over two
separate periods of one to two months' duration. The ability of the
adrenal cortex to elaborate 17-hydroxy-corticosteroids in response to
ACTH stimulation was slightly reduced at the 300 ppm level and
markedly reduced at the 900 ppm level. The results also showed that
following this treatment with dicofol, the ability of the adrenal
gland to return to the pre-treatment level of response to ACTH
proceeded slowly and, possibly, incompletely (Smith et al., 1959).
Acute toxicity (80 per cent technical product)
LD50 mg/kg
Animal Route body-weight Reference
Rat (M) Oral 809 Smith et al., 1959
Rat (F) Oral 684 Smith et al., 1959
Rabbit (M) Oral 1810 Smith et al., 1959
Dog (mixed) Oral >4000 Smith et al., 1959
Short-term studies
Rat
Dicofol was fed to groups, each containing 10 male and 10 female rats,
for 90 days at dietary concentrations of 0, 20, 100, 500, 1250 and
2500 ppm. Survival was adversely affected at 1250 ppm and above.
Growth was inhibited at 100 ppm and higher levels in the females but
only at 1250 ppm in the males. Increased liver to body weight ratios
occurred in the survivors in both sexes. Liver lesions were the most
consistent histopathological finding, but were only of scattered
incidence at dose levels below 1250 ppm (Smith et al., 1959).
Groups containing equal numbers of male and female rats were fed 0, 2,
5, 10, 15 and 20 ppm in their diets for 55 weeks. Growth, survival and
liver to body weight ratios were not affected at any dose level (Smith
et al., 1959).
Five groups, each of 10 male and 10 female rats were fed 0, 50, 200,
1000 and 3000 ppm in their diet for 13 weeks. Body weight and food
intake were reduced at the 200 ppm level and above, and at 3000 ppm
there was 100 per cent mortality. Haematological criteria were
comparable to the controls. Organ to body weight ratios were increased
in the case of the liver at the 200 ppm level and above and in the
case of the thyroid at the 1000 ppm level. Decreased uterus and
prostrate to body weight ratios were observed at 1000 ppm. Liver
glucose-6-phosphatase was depressed at 1000 ppm. while hexobarbital
oxidase activity showed a dose-related stimulation in all treated
groups. Histopathological examination revealed enlargement of
centrilobar cells and nuclei at 200 ppm and above. Electron microscopy
revealed smooth endoplasmic reticulum whorls to be present only at
1000 ppm and above (Verschuuren et al., 1968).
Dog
Groups each containing three dogs were given levels of dicofol of 100,
300 and 900 ppm for one year. Survival was affected only at 900 ppm.
Body weight gain was normal and haematological and histological
observations revealed no pathological effects (Smith et al., 1959).
Long-term studies
Rat
Dicofol was fed to 60 groups, each containing 10 male and 10 female
rats, at dietary levels of 0, 20, 100, 250, 500 and 1000 ppm for two
years. Growth depression occurred in male rats at 500 and 1000 ppm,
and in female rats progressively with increasing dietary concentration
at 250, 500 and 1000 ppm. A growth depression after three months,
recorded in female rats at 20 ppm (but not at 100 ppm) has not been
observed again in later studies. Absolute organ weights showed no
significant differences from the controls, with the exception of an
increase in the case of the livers and kidneys of the female rats fed
1000 ppm. Organ to body weight ratios were significantly increased for
the liver at 250 ppm and for the liver, kidney and heart at 500 ppm in
the females, but only for the liver at 500 ppm in the males.
Histopathological findings were confined to hydropic changes in the
liver which were regarded as reversible (Larson, 1957).
Special studies
(a) Reproduction
Mouse. Groups of varying numbers of mice were maintained throughout
five generations on dietary levels of 0, 7, 25, 100, 225 and 500 ppm
of dicofol. At the 500 ppm level the litter sizes, average weight of
the pups and also the values for fertility, viability and lactation
indices were lower than for the control group. However, all these
parameters were normal for the 225 ppm and lower levels (Brown,
1967a).
Rat. Four groups, each of 27 male and 27 female rats were fed
dietary levels of 0, 100, 500 and 1000 ppm of dicofol in a
two-generation reproduction study. There were no F1b pups surviving
at 21 days when the original parents were fed 500 or 1000 ppm. Litter
size from the 1000 ppm group was similar to the controls but over-all
mortality in the pups was greater. Considerable reduction in fertility
of the animals fed 500 and 1000 ppm was evident. No congenital defects
were observed in any of the F2a or F2b animals (Brown, 1964-65).
Groups of rats were maintained on diets containing 25 or 75 ppm of
dicofol through a three-generation study. The average number of pups
born per litter to parents receiving 75 ppm was slightly lower than
for the controls. There was no compound related effects relative to
body weight, fertility, gestation, viability or lactation indices at
either level, nor were there any congenital abnormalities evident in
either the viable or the still-born pups (Brown, 1967b).
(b) Toxicity study of a possible metabolite
The possible metabolite, 4,4'-dichlorobenzophenone was fed to groups
each containing 15 male and 15 female rats, at dietary levels of 0,
100 and 1250 ppm for three months. No effects were noted at either
level except somewhat reduced heart to body weight ratios found in the
males receiving 1250 ppm, but not in the females (Larson, 1965).
Comments
Short-term experiments on dogs and rats, reproduction studies on rats
and mice and long-term experiments on rats are reported. From the
results of the acute and short-term experiments a great species
difference in susceptibility is evident between dogs and rats.
No specific identification of possible metabolites has been made in
animals but dichlorbenzophenone is a probable metabolite. No human
studies have been reported.
Comparative metabolic studies in animals and man, including adrenal
function studies after oral administration are desirable.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 50 ppm, equivalent to 2.5 mg/kg per day.
Estimate of acceptable daily intake for man
0.0-0.025 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Kelthane is a miticide with systemic properties, used on a large
number of crops. It has no insecticidal activity. The following table
gives a review of application rates of dicofol and recommended
pre-harvest intervals.
Rate of application (kg/ha) Pre-harvest
interval
Crop Small plants Large plants (days)
Apples, pears, crabapples, 2.20 4.41-5.30 7
quince
Cherries 2.20 3.96 7
Peaches, apricots, 2.20 3.96 14
nectarines
Walnuts, filberts, pecans,
chestnuts, hickory nuts 1.76 4.85 14
and almonds, if hulls are
not fed to livestock
Almonds, if hulls are fed 1.76 3.09 Before nut
to livestock formation
Grapes, hops .66 1.60 7
Strawberries .44 2.96 2
Raspberries and other cane .66 1.60 2
berries
Beans .44 .73 7
Beans (dry) .44 1.00 45
Beans (California) .66 1.32 45
Cantaloupe, cucumbers,
pumpkins, squash, melons, .44 .73 2
water melons
Tomatoes, peppers .66 1.00 2
Alfalfa, clover, other .66 1.32
legumes for seed only
Corn (field) .88 1.76 Before
ears form
Plums, prunes, figs 1.76 2.50 7
(Adapted from Rohm and Haas, Kelthane 35 label)
Post-harvest treatments
No post-harvest uses of dicofol are known.
Other uses
Dicofol is used for control of mites on lawns, ornamentals and shade
trees, as well as against clover mites in buildings.
Residues resulting from supervised trials
Detailed residue data are available from United States trials with
dicofol on important crops and have been deposited with FAO. Rates of
application were similar to those given above. The typical data
presented below are representative:
Crop Number of Post-treatment Residue (ppm)
treatments interval (days) Range Average
Almonds (whole) 1 129-157 3.11-0.18 1.60
Apples 1 7 1.1
Beans (whole) 1 6 2.45
Carrots (roots) 1-2 5 0.00-0.25 0.10
Celery 1 6 1.32-4.45 2.88
Cherries 1 7 1.2 -1.8 1.5
Citrus (whole) 1-2 7 1.12-5.0 2.2
Cottonseed 1 15-76 0.00-0.1 0.0
Cucumbers 1 2 0.7 -2.3 1.6
Figs 1 7 1.86-5.32 3.92
Grapes 1 5 0.89-2.21 1.64
Hops (green) 1 7 3.0 -7.9 5.4
Lettuce 2 2 1.10-9.87 5.59
Mint (fresh hay) 1 7 35.8 -38.9 37.3
Peaches 1 14 4.9 -6.8 5.7
(continued)
Crop Number of Post-treatment Residue (ppm)
treatments interval (days) Range Average
Pears 2 7 1.1 -2.6 1.9
Pineapples 4 5 0.0 -0.05 <0.05
(pulp)
Plums, prunes 1 9 0.06-0.16 0.11
Raspberries 1 2 2.7
Spinach 1 4 2.88-3.90 3.39
Strawberries 1 3 0.13-0.90 0.37
Tea, raw leaves 1 7 18.00-31.00 25.0
1 7 0.00-18.5 5.1
Tomatoes 1 1 0.5 -0.57 0.53
Tropical fruits 5 4 0.0 0.0
Walnuts 1 7 0.57-0.92 0.86
Water melons 1 20 0.0
(heart)
Although dicofol is used for seed treatment no data on residues
are available.
Fate of residues
In soils
Dicofol is not used against pests in soil, but studies have been
conducted to determine residues in soil resulting from spray
application on crops. Dicofol residues in soil decrease rapidly at
first, then more slowly. After three monthly applications of 2.56
kg/ha, residues which had reached 1.13 ppm decreased to 0.25 ppm after
636 days, (Rohm and Haas, 1967).
In plants
No information is available on metabolism in plants.
In animals
Cows fed 2 ppm dicofol for 71 days averaged from 0.23-0.40 ppm
residues in their milk; when the same cows were fed 1.0 ppm, the level
was not detectable. Two body fat samples taken at the end of the study
contained 1.1 and 2.7 ppm (Zweig et al,, 1963).
A "Blue" cow fed 5 ppm dicofol for 17 days, excreted a maximum of
0.022 ppm dicofol per se and a maximum of 0.55 ppm dicofol plus
metabolites in the milk. Plateau values were obtained about six days
after start of dicofol feedings, and initial decay was also rapid.
From the fifth day after withdrawal of dicofol, the amounts of
material excreted were small, and the rate of decrease became low.
In a "White" cow fed 30 ppm dicofol for three days, 0.68 ppm dicofol
were found in kidney fat. Dicofol metabolites were found in kidney
fat, liver, udder, brain, kidney lungs, blood, heart, omentum, bone
marrow and muscles (Rohm and Haas, 1968).
The feeding of dicofol to steers produces no significant residues in
body fat at a level of 0.75 and 1.5 ppm. A feeding level of 3.0 ppm
produces a definite residue of 0.32 ppm (Peoples, 1967).
In tissues, milk and urine of a cow fed dicofol two groups of
metabolites were found, one more polar than dicofol and one less
polar. The metabolites of the first group were not identified. In the
second group, two substances were identified as 1,1 bis
(4-chlorophenyl) 2-chloro-ethylene and as 1,1 bis
(4-chlorophenyl)-2,2-dichloro-ethylene. Sixty per cent of total milk
residues is due to the latter substance, but it is suggested that it
may be present in technical dicofol rather than result from the
metabolic reduction of dicofol itself (Rohm and Haas, 1958). The
latter two compounds were also found as metabolites of DDT.
In storage and processing
Residues on fruits are reduced by washing. Wash-water of oranges
contained up to 0.05 ppm dicofol; 0.12-0.57 ppm remained in the peel.
Unwashed raspberries contained 0.7-2.9 ppm dicofol, residues which
were reduced to 0.7-1.4 after washing.
Peeling reduces residues to values below 1 ppm:
Fruit Residue (ppm)
Peel Pulp
Citrus fruit 0.29-12.0 0-0.27
Almonds 0-12.2 0-0.03
Cucumbers 0.09-0.4 0-0.17
Pineapples 5.5 -24.2 0-0.05
Tropical fruit 4.2 -9.7 0
(Rohm and Haas, private communication)
In canned or frozen fruits dicofol was found only in 1.3 per cent of
domestic samples, with an average ppm of 0.01. No dicofol was found in
samples imported into the United States of America (USDA, 1968b).
Brewed tea contained residues up to 0.0085 ppm. Under rigorous
treatment, such as preparing instant tea from tea leaves, or
distilling oil from mint leaves, dicofol may be broken down to
p,p'-dichlorobenzophenone and chloroform (Rohm and Haas, private
communication).
Evidence of residues in food in commerce or at consumption
Examination of market samples of food in the United States of America
showed that dicofol residues were found in 0.7 per cent of domestic
samples and 1.8 per cent of imported samples (Duggan and Dawson,
1967a). In fruit the following concentrations were found in the United
States of America from 1964 to 1967 (USDA, 1968b).
Domestic samples Imported samples
Fruit Incidence Average Incidence Average
per cent. (ppm) per cent. (ppm)
Small fruit
(strawberries,
cherries, plums, 5.1 0.03 2.1 0.02
grapes, cranberries,
etc.)
(continued)
Domestic samples Imported samples
Fruit Incidence Average Incidence Average
per cent. (ppm) per cent. (ppm)
Large fruit
(apples, 8.6 0.02 3.3 0.01
oranges, pears,
peaches, etc.)
Total diet studies in the United States of America from 1964 to 1967
showed the following results (Duggan and Weatherwax, 1967b; USDA,
1968b)
Year Positive per cent. Daily average
composites intake (mg)
1964-1965 0.5 0.003
1965-1966 3.7 0.002
1966-1967 5.6 0.012
In 41.9 per cent of fruit-composites (apples, oranges, pears,
peaches, etc.) dicofol was found in an average concentration of 0.031
ppm (USDA, 1968b). Dietary intake of dicofol in the United States of
America was 0.00004 mg/kg body weight/day in 1965; 0.00015 in 1966 and
0.00018 in 1967 with an average of 0.00013 for three years (USDA,
1968b).
In Canada 60 restaurant meals were examined. Dicofol was found in an
average concentration of 0.07 ppm in fruit and fruit salads, in
legumes, 0.08 ppm whereas only traces were found in milk (Swackhamer,
1965).
Methods of residue analysis
The methods are adequate for present purposes. Methods for
colorimetric determination of dicofol are based on the method of
Rosenthal, 1957. Dicofol is converted to chloroform with alkali.
Chloroform is separated from extraneous materials and produces, with a
water-pyridine-sodium-hydroxide mixture, a Fujiwara-type red dye,
which is read at 535mµ against a pyridine blank. This method may be
used for residue determination in plants, fruit and vegetables and has
been simplified for analysis of milk (Gordon, 1963). Residues in crops
and soil may also be determined, after chromatographical clean-up, by
GLC using an electron capture detector (Morgan, 1967). An ultra-violet
spectrophotometric method involves hydrolysing the dicofol to
4,4'-dichlorobenzophenone (Gunther and Blinn, 1957). A method omitting
the hydrolysis step and allowing the dicofol to be oxidized to
non-interfering compounds permits the measurement of
4,4'-dichlorobenzophenone present in mint-oil and mint-hay (Rohm and
Haas, private communication).
The sensitivity of the analytical procedures is 0.01 ppm.
National tolerances
Tolerance Pre-harvest
Country Crop (ppm) interval (days)
United States Berries 5
of America
German Federal Fruit, including
Republic grapes and hops 0.5 14
Vegetables 0.5
Beans 14
Cucumbers 4
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Dicofol is used exclusively to control phytophagous mites and although
closely related to DDT has virtually no insecticidal activity. Its
principal use is on apples, grapes and hops in Europe and North
America, and tea (spot treatments only) in the Far East, with some
minor use on citrus in these same areas. In North America, the rates
and frequencies of application are generally greater than in other
countries because of more serious mite problems. It is not used on
animals. Dicofol is a technical product containing 82-88 per cent of
the pure (active) compound. The impurities are known and their
relative concentration are constant.
The data available to the meeting were obtained solely in the United
States of America and did not include information about uses elsewhere
or figures for residues after such use, although figures were
available for some imports into the United States of America.
Very little is known about the metabolism of dicofol in plants and
animals. In animals, dicofol can be found as such together with
various metabolites. Precise figures for the amounts of these
compounds in experimental plants or animals, either from a single or
from repeated applications, have not been published.
1,1 bis (4-chlorophenyl) 2-chloro-ethylene and 1,1 bis
(4-chlorophenyl-2,-2-dichloro-ethylene) have been identified as
metabolites in animals. However, the latter may be present in the
technical product used. In mint oil and tea, 4,4-dichlorobenzophenone
has been identified.
The literature includes methods of residue analysis which measure
dicofol alone or together with some breakdown products. For the
determination of dicofol alone the sensitivity is 0.01 ppm. However,
no referee method of analysis has been evaluated.
Recommendations
Temporary tolerances
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Fruit, vegetables, hops, tea (from a
particular estate, for blending only) 5 ppm
Tea (blended) 1 ppm
Further work or information
Required before 30 June 1972:
1. Data from countries other than the United States of America on the
required rates and frequencies of application, pre-harvest
intervals, and the resultant residues.
2. Information on the nature of the terminal residues in plants,
animals, and their products.
Desirable:
1. Collaborative studies to establish a referee method.
2. Comparative metabolic studies in animals and man, including adrenal
function studies after oral administration.
REFERENCES
Brown, J. R. (1964-65) Toxicologic studies on the effects of kelthane
in the diet of albino rats on reproduction (first interim, second
interim and final reports). Departments of Physiological Hygiene,
University of Toronto. Unpublished reports
Brown, J. R. (1967a) Toxicological studies on
2,2-bis-chlorophenyl-2,2,2-trichloroethanol, kelthane. Brown
Biological Laboratories Ltd. Unpublished report
Brown, J. R. (1967b) Three-generation reproduction study on rats
receiving technical kelthane in their diet. Department of
Physiological Hygiene, University of Toronto. Unpublished report
Duggan, R. E. and Dawson, K. (1967a) Pesticides: A report on residues
in food. FDA Papers, 1: 2-5
Duggan, R. E. and Weatherwax, J. R. (1967b) Dietary intake of
pesticide chemicals. Science, 157: 1006-1010
Gordon, C. F., Haines, L. D. and Martin, J. J. (1963) Acaricide
residues: An improved method for kelthane residue analysis with
applications for determination of residues in milk. J. Agr. Food
Chem., 11: 84-86
Gunther, F. A. and Blinn, R. C. (1957) Ultraviolet spectrophotometric
microdetection of the acaricide 4,4'-dichloro-alpha-(trichlormethyl)
benzhydrol (SW 293). J.Agr. Food Chem., 5: 517-519
Larson, P. S. (1957) Two-year study on the effect of adding kelthane
to the diet of rats. Medical College of Virginia. Unpublished report
Larson, P. S. (1965) Toxicologic study on the effect of adding
dichlorobenzophenone to the diet of rats for a period of three months.
Department of Pharmacology. Medical College of Virginia. Unpublished
report
Morgan, N. L. (1967) The identification and relative retention times
of p,p'-kelthane and its breakdown product p,p'-dichlorobenzophenone
using GLC. Bull. environ. Contam. Toxicol., 2: 306-312
Peoples, S. A. (1967) Residues in the body fat of steers fed kelthane
in their ration. Dept. of Physiol. Sci. School of Veterinary Medicine,
University of California, Davis. Unpublished report
Rohm and Haas. (1958) Research Reports 13-27 and 11.141. Robin and
Haas Co.
Rohm and Haas. (1967) RAR Memorandum 521. Rohm and Hass Co.
Rohm and Haas Co. (1968) Kelthane. Unpublished report
Rosenthal, J., Frisone, G. J. and Gunther, F. A. (1957) Colorimetric
microdetermination of the acaricide
4,4'-dichloro-alpha-(trichloromethyl) benzhydrol (FW-293).J. Agr. Food
Chem., 5: 514-517
Smith, R. B. Jr, Larson, P. S., Finnegan, J. K., Haag, H. B.,
Hennigar, G. R, and Cobey, F. (1959) Toxicologic studies on 2,2
bis-(chlorophenyl)-2,2,2-trichloroethanol (kelthane). Toxicol. appl.
Pharmacol., 1: 119-134
Swackhamer, A. B. (1965) Report on pesticide residues in restaurant
meals in Canada. Food and Drug Directorate, Department of National
Health and Welfare, Ottawa, Canada. Pesticide Progress, 3: 108-114
USDA. (1968a) Agricultural Economic Report 131
USDA. (1968b) United States Department of Health, Education and
Welfare. The regulation of pesticides in the United States
Verschuuren, H. G., Kroes, R. and den Tonkelaar, E. M. (1968)
Toxiciteitsonderzoek met kelthaan bij ratten gedurende 90 dagen
(Toxicity experiment with kelthane in rats of 90 day duration).
National Institute of Public Health, Utrecht. Unpublished report
Zweig, G., Pye, E. L. and Peoples, S. A. (1963) Residues in butter fat
and body fat of dairy cows fed at two levels of kelthane (1.0 and 2.0
ppm). J. Agr. Food Chem., 11: 72-79
 Other information on identity and properties
Analysis of technical dicofol is not known. Purity of technical
dicofol is 82-88 per cent. The United States Department of Agriculture
(USDA, 1968a) places the total acaricide market for 1964 in the United
States of America at about three million pounds active ingredients per
year, dicofol being about half of that amount. Resistance is still
today a cause for reduction of the use of dicofol, e.g. in Israel.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
No specific identification of the metabolites of dicofol in animals
has been made. However, on the basis of the known chemistry of the
compound, 4,4'-dichlorobenzophenone is considered to be a probable
metabolite. Thus, under alkaline conditions, dicofol is hydrolytically
cleaved to 4,4'-dichlorobenzophenone and chloroform (Rohm and Haas,
1968).
Upon ingestion of dicofol by mammals, storage of the compound occurs
in the adipose tissue. Rats were fed dicofol at a level of 32 ppm in
their diet for 12 weeks. After eight weeks the level of the compound
in the fat had reached equilibrium at concentrations of 25 ppm for the
males and 70 ppm for the females, which amounts corresponded to 75 per
cent and 200 per cent of the dietary levels. After 12 weeks, dicofol
was withdrawn from the diet and the level of stored material declined.
The rate of decline was greater for the male animals than for the
females. By 14 weeks after withdrawal the level of dicofol in the fat
was zero for the males but still remained at about 6 ppm for the
females. Feeding with higher or lower dose levels also showed that
dicofol was stored in the fat of the female rat to a greater degree
than in the male (Smith et al., 1959).
The effect of dicofol on plasma 17-hydroxy-corticosteroids in the dog
was determined on two dogs which were fed 300 ppm and 900 ppm over two
separate periods of one to two months' duration. The ability of the
adrenal cortex to elaborate 17-hydroxy-corticosteroids in response to
ACTH stimulation was slightly reduced at the 300 ppm level and
markedly reduced at the 900 ppm level. The results also showed that
following this treatment with dicofol, the ability of the adrenal
gland to return to the pre-treatment level of response to ACTH
proceeded slowly and, possibly, incompletely (Smith et al., 1959).
Acute toxicity (80 per cent technical product)
LD50 mg/kg
Animal Route body-weight Reference
Rat (M) Oral 809 Smith et al., 1959
Rat (F) Oral 684 Smith et al., 1959
Rabbit (M) Oral 1810 Smith et al., 1959
Dog (mixed) Oral >4000 Smith et al., 1959
Short-term studies
Rat
Dicofol was fed to groups, each containing 10 male and 10 female rats,
for 90 days at dietary concentrations of 0, 20, 100, 500, 1250 and
2500 ppm. Survival was adversely affected at 1250 ppm and above.
Growth was inhibited at 100 ppm and higher levels in the females but
only at 1250 ppm in the males. Increased liver to body weight ratios
occurred in the survivors in both sexes. Liver lesions were the most
consistent histopathological finding, but were only of scattered
incidence at dose levels below 1250 ppm (Smith et al., 1959).
Groups containing equal numbers of male and female rats were fed 0, 2,
5, 10, 15 and 20 ppm in their diets for 55 weeks. Growth, survival and
liver to body weight ratios were not affected at any dose level (Smith
et al., 1959).
Five groups, each of 10 male and 10 female rats were fed 0, 50, 200,
1000 and 3000 ppm in their diet for 13 weeks. Body weight and food
intake were reduced at the 200 ppm level and above, and at 3000 ppm
there was 100 per cent mortality. Haematological criteria were
comparable to the controls. Organ to body weight ratios were increased
in the case of the liver at the 200 ppm level and above and in the
case of the thyroid at the 1000 ppm level. Decreased uterus and
prostrate to body weight ratios were observed at 1000 ppm. Liver
glucose-6-phosphatase was depressed at 1000 ppm. while hexobarbital
oxidase activity showed a dose-related stimulation in all treated
groups. Histopathological examination revealed enlargement of
centrilobar cells and nuclei at 200 ppm and above. Electron microscopy
revealed smooth endoplasmic reticulum whorls to be present only at
1000 ppm and above (Verschuuren et al., 1968).
Dog
Groups each containing three dogs were given levels of dicofol of 100,
300 and 900 ppm for one year. Survival was affected only at 900 ppm.
Body weight gain was normal and haematological and histological
observations revealed no pathological effects (Smith et al., 1959).
Long-term studies
Rat
Dicofol was fed to 60 groups, each containing 10 male and 10 female
rats, at dietary levels of 0, 20, 100, 250, 500 and 1000 ppm for two
years. Growth depression occurred in male rats at 500 and 1000 ppm,
and in female rats progressively with increasing dietary concentration
at 250, 500 and 1000 ppm. A growth depression after three months,
recorded in female rats at 20 ppm (but not at 100 ppm) has not been
observed again in later studies. Absolute organ weights showed no
significant differences from the controls, with the exception of an
increase in the case of the livers and kidneys of the female rats fed
1000 ppm. Organ to body weight ratios were significantly increased for
the liver at 250 ppm and for the liver, kidney and heart at 500 ppm in
the females, but only for the liver at 500 ppm in the males.
Histopathological findings were confined to hydropic changes in the
liver which were regarded as reversible (Larson, 1957).
Special studies
(a) Reproduction
Mouse. Groups of varying numbers of mice were maintained throughout
five generations on dietary levels of 0, 7, 25, 100, 225 and 500 ppm
of dicofol. At the 500 ppm level the litter sizes, average weight of
the pups and also the values for fertility, viability and lactation
indices were lower than for the control group. However, all these
parameters were normal for the 225 ppm and lower levels (Brown,
1967a).
Rat. Four groups, each of 27 male and 27 female rats were fed
dietary levels of 0, 100, 500 and 1000 ppm of dicofol in a
two-generation reproduction study. There were no F1b pups surviving
at 21 days when the original parents were fed 500 or 1000 ppm. Litter
size from the 1000 ppm group was similar to the controls but over-all
mortality in the pups was greater. Considerable reduction in fertility
of the animals fed 500 and 1000 ppm was evident. No congenital defects
were observed in any of the F2a or F2b animals (Brown, 1964-65).
Groups of rats were maintained on diets containing 25 or 75 ppm of
dicofol through a three-generation study. The average number of pups
born per litter to parents receiving 75 ppm was slightly lower than
for the controls. There was no compound related effects relative to
body weight, fertility, gestation, viability or lactation indices at
either level, nor were there any congenital abnormalities evident in
either the viable or the still-born pups (Brown, 1967b).
(b) Toxicity study of a possible metabolite
The possible metabolite, 4,4'-dichlorobenzophenone was fed to groups
each containing 15 male and 15 female rats, at dietary levels of 0,
100 and 1250 ppm for three months. No effects were noted at either
level except somewhat reduced heart to body weight ratios found in the
males receiving 1250 ppm, but not in the females (Larson, 1965).
Comments
Short-term experiments on dogs and rats, reproduction studies on rats
and mice and long-term experiments on rats are reported. From the
results of the acute and short-term experiments a great species
difference in susceptibility is evident between dogs and rats.
No specific identification of possible metabolites has been made in
animals but dichlorbenzophenone is a probable metabolite. No human
studies have been reported.
Comparative metabolic studies in animals and man, including adrenal
function studies after oral administration are desirable.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 50 ppm, equivalent to 2.5 mg/kg per day.
Estimate of acceptable daily intake for man
0.0-0.025 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Kelthane is a miticide with systemic properties, used on a large
number of crops. It has no insecticidal activity. The following table
gives a review of application rates of dicofol and recommended
pre-harvest intervals.
Rate of application (kg/ha) Pre-harvest
interval
Crop Small plants Large plants (days)
Apples, pears, crabapples, 2.20 4.41-5.30 7
quince
Cherries 2.20 3.96 7
Peaches, apricots, 2.20 3.96 14
nectarines
Walnuts, filberts, pecans,
chestnuts, hickory nuts 1.76 4.85 14
and almonds, if hulls are
not fed to livestock
Almonds, if hulls are fed 1.76 3.09 Before nut
to livestock formation
Grapes, hops .66 1.60 7
Strawberries .44 2.96 2
Raspberries and other cane .66 1.60 2
berries
Beans .44 .73 7
Beans (dry) .44 1.00 45
Beans (California) .66 1.32 45
Cantaloupe, cucumbers,
pumpkins, squash, melons, .44 .73 2
water melons
Tomatoes, peppers .66 1.00 2
Alfalfa, clover, other .66 1.32
legumes for seed only
Corn (field) .88 1.76 Before
ears form
Plums, prunes, figs 1.76 2.50 7
(Adapted from Rohm and Haas, Kelthane 35 label)
Post-harvest treatments
No post-harvest uses of dicofol are known.
Other uses
Dicofol is used for control of mites on lawns, ornamentals and shade
trees, as well as against clover mites in buildings.
Residues resulting from supervised trials
Detailed residue data are available from United States trials with
dicofol on important crops and have been deposited with FAO. Rates of
application were similar to those given above. The typical data
presented below are representative:
Crop Number of Post-treatment Residue (ppm)
treatments interval (days) Range Average
Almonds (whole) 1 129-157 3.11-0.18 1.60
Apples 1 7 1.1
Beans (whole) 1 6 2.45
Carrots (roots) 1-2 5 0.00-0.25 0.10
Celery 1 6 1.32-4.45 2.88
Cherries 1 7 1.2 -1.8 1.5
Citrus (whole) 1-2 7 1.12-5.0 2.2
Cottonseed 1 15-76 0.00-0.1 0.0
Cucumbers 1 2 0.7 -2.3 1.6
Figs 1 7 1.86-5.32 3.92
Grapes 1 5 0.89-2.21 1.64
Hops (green) 1 7 3.0 -7.9 5.4
Lettuce 2 2 1.10-9.87 5.59
Mint (fresh hay) 1 7 35.8 -38.9 37.3
Peaches 1 14 4.9 -6.8 5.7
(continued)
Crop Number of Post-treatment Residue (ppm)
treatments interval (days) Range Average
Pears 2 7 1.1 -2.6 1.9
Pineapples 4 5 0.0 -0.05 <0.05
(pulp)
Plums, prunes 1 9 0.06-0.16 0.11
Raspberries 1 2 2.7
Spinach 1 4 2.88-3.90 3.39
Strawberries 1 3 0.13-0.90 0.37
Tea, raw leaves 1 7 18.00-31.00 25.0
1 7 0.00-18.5 5.1
Tomatoes 1 1 0.5 -0.57 0.53
Tropical fruits 5 4 0.0 0.0
Walnuts 1 7 0.57-0.92 0.86
Water melons 1 20 0.0
(heart)
Although dicofol is used for seed treatment no data on residues
are available.
Fate of residues
In soils
Dicofol is not used against pests in soil, but studies have been
conducted to determine residues in soil resulting from spray
application on crops. Dicofol residues in soil decrease rapidly at
first, then more slowly. After three monthly applications of 2.56
kg/ha, residues which had reached 1.13 ppm decreased to 0.25 ppm after
636 days, (Rohm and Haas, 1967).
In plants
No information is available on metabolism in plants.
In animals
Cows fed 2 ppm dicofol for 71 days averaged from 0.23-0.40 ppm
residues in their milk; when the same cows were fed 1.0 ppm, the level
was not detectable. Two body fat samples taken at the end of the study
contained 1.1 and 2.7 ppm (Zweig et al,, 1963).
A "Blue" cow fed 5 ppm dicofol for 17 days, excreted a maximum of
0.022 ppm dicofol per se and a maximum of 0.55 ppm dicofol plus
metabolites in the milk. Plateau values were obtained about six days
after start of dicofol feedings, and initial decay was also rapid.
From the fifth day after withdrawal of dicofol, the amounts of
material excreted were small, and the rate of decrease became low.
In a "White" cow fed 30 ppm dicofol for three days, 0.68 ppm dicofol
were found in kidney fat. Dicofol metabolites were found in kidney
fat, liver, udder, brain, kidney lungs, blood, heart, omentum, bone
marrow and muscles (Rohm and Haas, 1968).
The feeding of dicofol to steers produces no significant residues in
body fat at a level of 0.75 and 1.5 ppm. A feeding level of 3.0 ppm
produces a definite residue of 0.32 ppm (Peoples, 1967).
In tissues, milk and urine of a cow fed dicofol two groups of
metabolites were found, one more polar than dicofol and one less
polar. The metabolites of the first group were not identified. In the
second group, two substances were identified as 1,1 bis
(4-chlorophenyl) 2-chloro-ethylene and as 1,1 bis
(4-chlorophenyl)-2,2-dichloro-ethylene. Sixty per cent of total milk
residues is due to the latter substance, but it is suggested that it
may be present in technical dicofol rather than result from the
metabolic reduction of dicofol itself (Rohm and Haas, 1958). The
latter two compounds were also found as metabolites of DDT.
In storage and processing
Residues on fruits are reduced by washing. Wash-water of oranges
contained up to 0.05 ppm dicofol; 0.12-0.57 ppm remained in the peel.
Unwashed raspberries contained 0.7-2.9 ppm dicofol, residues which
were reduced to 0.7-1.4 after washing.
Peeling reduces residues to values below 1 ppm:
Fruit Residue (ppm)
Peel Pulp
Citrus fruit 0.29-12.0 0-0.27
Almonds 0-12.2 0-0.03
Cucumbers 0.09-0.4 0-0.17
Pineapples 5.5 -24.2 0-0.05
Tropical fruit 4.2 -9.7 0
(Rohm and Haas, private communication)
In canned or frozen fruits dicofol was found only in 1.3 per cent of
domestic samples, with an average ppm of 0.01. No dicofol was found in
samples imported into the United States of America (USDA, 1968b).
Brewed tea contained residues up to 0.0085 ppm. Under rigorous
treatment, such as preparing instant tea from tea leaves, or
distilling oil from mint leaves, dicofol may be broken down to
p,p'-dichlorobenzophenone and chloroform (Rohm and Haas, private
communication).
Evidence of residues in food in commerce or at consumption
Examination of market samples of food in the United States of America
showed that dicofol residues were found in 0.7 per cent of domestic
samples and 1.8 per cent of imported samples (Duggan and Dawson,
1967a). In fruit the following concentrations were found in the United
States of America from 1964 to 1967 (USDA, 1968b).
Domestic samples Imported samples
Fruit Incidence Average Incidence Average
per cent. (ppm) per cent. (ppm)
Small fruit
(strawberries,
cherries, plums, 5.1 0.03 2.1 0.02
grapes, cranberries,
etc.)
(continued)
Domestic samples Imported samples
Fruit Incidence Average Incidence Average
per cent. (ppm) per cent. (ppm)
Large fruit
(apples, 8.6 0.02 3.3 0.01
oranges, pears,
peaches, etc.)
Total diet studies in the United States of America from 1964 to 1967
showed the following results (Duggan and Weatherwax, 1967b; USDA,
1968b)
Year Positive per cent. Daily average
composites intake (mg)
1964-1965 0.5 0.003
1965-1966 3.7 0.002
1966-1967 5.6 0.012
In 41.9 per cent of fruit-composites (apples, oranges, pears,
peaches, etc.) dicofol was found in an average concentration of 0.031
ppm (USDA, 1968b). Dietary intake of dicofol in the United States of
America was 0.00004 mg/kg body weight/day in 1965; 0.00015 in 1966 and
0.00018 in 1967 with an average of 0.00013 for three years (USDA,
1968b).
In Canada 60 restaurant meals were examined. Dicofol was found in an
average concentration of 0.07 ppm in fruit and fruit salads, in
legumes, 0.08 ppm whereas only traces were found in milk (Swackhamer,
1965).
Methods of residue analysis
The methods are adequate for present purposes. Methods for
colorimetric determination of dicofol are based on the method of
Rosenthal, 1957. Dicofol is converted to chloroform with alkali.
Chloroform is separated from extraneous materials and produces, with a
water-pyridine-sodium-hydroxide mixture, a Fujiwara-type red dye,
which is read at 535mµ against a pyridine blank. This method may be
used for residue determination in plants, fruit and vegetables and has
been simplified for analysis of milk (Gordon, 1963). Residues in crops
and soil may also be determined, after chromatographical clean-up, by
GLC using an electron capture detector (Morgan, 1967). An ultra-violet
spectrophotometric method involves hydrolysing the dicofol to
4,4'-dichlorobenzophenone (Gunther and Blinn, 1957). A method omitting
the hydrolysis step and allowing the dicofol to be oxidized to
non-interfering compounds permits the measurement of
4,4'-dichlorobenzophenone present in mint-oil and mint-hay (Rohm and
Haas, private communication).
The sensitivity of the analytical procedures is 0.01 ppm.
National tolerances
Tolerance Pre-harvest
Country Crop (ppm) interval (days)
United States Berries 5
of America
German Federal Fruit, including
Republic grapes and hops 0.5 14
Vegetables 0.5
Beans 14
Cucumbers 4
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Dicofol is used exclusively to control phytophagous mites and although
closely related to DDT has virtually no insecticidal activity. Its
principal use is on apples, grapes and hops in Europe and North
America, and tea (spot treatments only) in the Far East, with some
minor use on citrus in these same areas. In North America, the rates
and frequencies of application are generally greater than in other
countries because of more serious mite problems. It is not used on
animals. Dicofol is a technical product containing 82-88 per cent of
the pure (active) compound. The impurities are known and their
relative concentration are constant.
The data available to the meeting were obtained solely in the United
States of America and did not include information about uses elsewhere
or figures for residues after such use, although figures were
available for some imports into the United States of America.
Very little is known about the metabolism of dicofol in plants and
animals. In animals, dicofol can be found as such together with
various metabolites. Precise figures for the amounts of these
compounds in experimental plants or animals, either from a single or
from repeated applications, have not been published.
1,1 bis (4-chlorophenyl) 2-chloro-ethylene and 1,1 bis
(4-chlorophenyl-2,-2-dichloro-ethylene) have been identified as
metabolites in animals. However, the latter may be present in the
technical product used. In mint oil and tea, 4,4-dichlorobenzophenone
has been identified.
The literature includes methods of residue analysis which measure
dicofol alone or together with some breakdown products. For the
determination of dicofol alone the sensitivity is 0.01 ppm. However,
no referee method of analysis has been evaluated.
Recommendations
Temporary tolerances
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Fruit, vegetables, hops, tea (from a
particular estate, for blending only) 5 ppm
Tea (blended) 1 ppm
Further work or information
Required before 30 June 1972:
1. Data from countries other than the United States of America on the
required rates and frequencies of application, pre-harvest
intervals, and the resultant residues.
2. Information on the nature of the terminal residues in plants,
animals, and their products.
Desirable:
1. Collaborative studies to establish a referee method.
2. Comparative metabolic studies in animals and man, including adrenal
function studies after oral administration.
REFERENCES
Brown, J. R. (1964-65) Toxicologic studies on the effects of kelthane
in the diet of albino rats on reproduction (first interim, second
interim and final reports). Departments of Physiological Hygiene,
University of Toronto. Unpublished reports
Brown, J. R. (1967a) Toxicological studies on
2,2-bis-chlorophenyl-2,2,2-trichloroethanol, kelthane. Brown
Biological Laboratories Ltd. Unpublished report
Brown, J. R. (1967b) Three-generation reproduction study on rats
receiving technical kelthane in their diet. Department of
Physiological Hygiene, University of Toronto. Unpublished report
Duggan, R. E. and Dawson, K. (1967a) Pesticides: A report on residues
in food. FDA Papers, 1: 2-5
Duggan, R. E. and Weatherwax, J. R. (1967b) Dietary intake of
pesticide chemicals. Science, 157: 1006-1010
Gordon, C. F., Haines, L. D. and Martin, J. J. (1963) Acaricide
residues: An improved method for kelthane residue analysis with
applications for determination of residues in milk. J. Agr. Food
Chem., 11: 84-86
Gunther, F. A. and Blinn, R. C. (1957) Ultraviolet spectrophotometric
microdetection of the acaricide 4,4'-dichloro-alpha-(trichlormethyl)
benzhydrol (SW 293). J.Agr. Food Chem., 5: 517-519
Larson, P. S. (1957) Two-year study on the effect of adding kelthane
to the diet of rats. Medical College of Virginia. Unpublished report
Larson, P. S. (1965) Toxicologic study on the effect of adding
dichlorobenzophenone to the diet of rats for a period of three months.
Department of Pharmacology. Medical College of Virginia. Unpublished
report
Morgan, N. L. (1967) The identification and relative retention times
of p,p'-kelthane and its breakdown product p,p'-dichlorobenzophenone
using GLC. Bull. environ. Contam. Toxicol., 2: 306-312
Peoples, S. A. (1967) Residues in the body fat of steers fed kelthane
in their ration. Dept. of Physiol. Sci. School of Veterinary Medicine,
University of California, Davis. Unpublished report
Rohm and Haas. (1958) Research Reports 13-27 and 11.141. Robin and
Haas Co.
Rohm and Haas. (1967) RAR Memorandum 521. Rohm and Hass Co.
Rohm and Haas Co. (1968) Kelthane. Unpublished report
Rosenthal, J., Frisone, G. J. and Gunther, F. A. (1957) Colorimetric
microdetermination of the acaricide
4,4'-dichloro-alpha-(trichloromethyl) benzhydrol (FW-293).J. Agr. Food
Chem., 5: 514-517
Smith, R. B. Jr, Larson, P. S., Finnegan, J. K., Haag, H. B.,
Hennigar, G. R, and Cobey, F. (1959) Toxicologic studies on 2,2
bis-(chlorophenyl)-2,2,2-trichloroethanol (kelthane). Toxicol. appl.
Pharmacol., 1: 119-134
Swackhamer, A. B. (1965) Report on pesticide residues in restaurant
meals in Canada. Food and Drug Directorate, Department of National
Health and Welfare, Ottawa, Canada. Pesticide Progress, 3: 108-114
USDA. (1968a) Agricultural Economic Report 131
USDA. (1968b) United States Department of Health, Education and
Welfare. The regulation of pesticides in the United States
Verschuuren, H. G., Kroes, R. and den Tonkelaar, E. M. (1968)
Toxiciteitsonderzoek met kelthaan bij ratten gedurende 90 dagen
(Toxicity experiment with kelthane in rats of 90 day duration).
National Institute of Public Health, Utrecht. Unpublished report
Zweig, G., Pye, E. L. and Peoples, S. A. (1963) Residues in butter fat
and body fat of dairy cows fed at two levels of kelthane (1.0 and 2.0
ppm). J. Agr. Food Chem., 11: 72-79
Other information on identity and properties
Analysis of technical dicofol is not known. Purity of technical
dicofol is 82-88 per cent. The United States Department of Agriculture
(USDA, 1968a) places the total acaricide market for 1964 in the United
States of America at about three million pounds active ingredients per
year, dicofol being about half of that amount. Resistance is still
today a cause for reduction of the use of dicofol, e.g. in Israel.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
No specific identification of the metabolites of dicofol in animals
has been made. However, on the basis of the known chemistry of the
compound, 4,4'-dichlorobenzophenone is considered to be a probable
metabolite. Thus, under alkaline conditions, dicofol is hydrolytically
cleaved to 4,4'-dichlorobenzophenone and chloroform (Rohm and Haas,
1968).
Upon ingestion of dicofol by mammals, storage of the compound occurs
in the adipose tissue. Rats were fed dicofol at a level of 32 ppm in
their diet for 12 weeks. After eight weeks the level of the compound
in the fat had reached equilibrium at concentrations of 25 ppm for the
males and 70 ppm for the females, which amounts corresponded to 75 per
cent and 200 per cent of the dietary levels. After 12 weeks, dicofol
was withdrawn from the diet and the level of stored material declined.
The rate of decline was greater for the male animals than for the
females. By 14 weeks after withdrawal the level of dicofol in the fat
was zero for the males but still remained at about 6 ppm for the
females. Feeding with higher or lower dose levels also showed that
dicofol was stored in the fat of the female rat to a greater degree
than in the male (Smith et al., 1959).
The effect of dicofol on plasma 17-hydroxy-corticosteroids in the dog
was determined on two dogs which were fed 300 ppm and 900 ppm over two
separate periods of one to two months' duration. The ability of the
adrenal cortex to elaborate 17-hydroxy-corticosteroids in response to
ACTH stimulation was slightly reduced at the 300 ppm level and
markedly reduced at the 900 ppm level. The results also showed that
following this treatment with dicofol, the ability of the adrenal
gland to return to the pre-treatment level of response to ACTH
proceeded slowly and, possibly, incompletely (Smith et al., 1959).
Acute toxicity (80 per cent technical product)
LD50 mg/kg
Animal Route body-weight Reference
Rat (M) Oral 809 Smith et al., 1959
Rat (F) Oral 684 Smith et al., 1959
Rabbit (M) Oral 1810 Smith et al., 1959
Dog (mixed) Oral >4000 Smith et al., 1959
Short-term studies
Rat
Dicofol was fed to groups, each containing 10 male and 10 female rats,
for 90 days at dietary concentrations of 0, 20, 100, 500, 1250 and
2500 ppm. Survival was adversely affected at 1250 ppm and above.
Growth was inhibited at 100 ppm and higher levels in the females but
only at 1250 ppm in the males. Increased liver to body weight ratios
occurred in the survivors in both sexes. Liver lesions were the most
consistent histopathological finding, but were only of scattered
incidence at dose levels below 1250 ppm (Smith et al., 1959).
Groups containing equal numbers of male and female rats were fed 0, 2,
5, 10, 15 and 20 ppm in their diets for 55 weeks. Growth, survival and
liver to body weight ratios were not affected at any dose level (Smith
et al., 1959).
Five groups, each of 10 male and 10 female rats were fed 0, 50, 200,
1000 and 3000 ppm in their diet for 13 weeks. Body weight and food
intake were reduced at the 200 ppm level and above, and at 3000 ppm
there was 100 per cent mortality. Haematological criteria were
comparable to the controls. Organ to body weight ratios were increased
in the case of the liver at the 200 ppm level and above and in the
case of the thyroid at the 1000 ppm level. Decreased uterus and
prostrate to body weight ratios were observed at 1000 ppm. Liver
glucose-6-phosphatase was depressed at 1000 ppm. while hexobarbital
oxidase activity showed a dose-related stimulation in all treated
groups. Histopathological examination revealed enlargement of
centrilobar cells and nuclei at 200 ppm and above. Electron microscopy
revealed smooth endoplasmic reticulum whorls to be present only at
1000 ppm and above (Verschuuren et al., 1968).
Dog
Groups each containing three dogs were given levels of dicofol of 100,
300 and 900 ppm for one year. Survival was affected only at 900 ppm.
Body weight gain was normal and haematological and histological
observations revealed no pathological effects (Smith et al., 1959).
Long-term studies
Rat
Dicofol was fed to 60 groups, each containing 10 male and 10 female
rats, at dietary levels of 0, 20, 100, 250, 500 and 1000 ppm for two
years. Growth depression occurred in male rats at 500 and 1000 ppm,
and in female rats progressively with increasing dietary concentration
at 250, 500 and 1000 ppm. A growth depression after three months,
recorded in female rats at 20 ppm (but not at 100 ppm) has not been
observed again in later studies. Absolute organ weights showed no
significant differences from the controls, with the exception of an
increase in the case of the livers and kidneys of the female rats fed
1000 ppm. Organ to body weight ratios were significantly increased for
the liver at 250 ppm and for the liver, kidney and heart at 500 ppm in
the females, but only for the liver at 500 ppm in the males.
Histopathological findings were confined to hydropic changes in the
liver which were regarded as reversible (Larson, 1957).
Special studies
(a) Reproduction
Mouse. Groups of varying numbers of mice were maintained throughout
five generations on dietary levels of 0, 7, 25, 100, 225 and 500 ppm
of dicofol. At the 500 ppm level the litter sizes, average weight of
the pups and also the values for fertility, viability and lactation
indices were lower than for the control group. However, all these
parameters were normal for the 225 ppm and lower levels (Brown,
1967a).
Rat. Four groups, each of 27 male and 27 female rats were fed
dietary levels of 0, 100, 500 and 1000 ppm of dicofol in a
two-generation reproduction study. There were no F1b pups surviving
at 21 days when the original parents were fed 500 or 1000 ppm. Litter
size from the 1000 ppm group was similar to the controls but over-all
mortality in the pups was greater. Considerable reduction in fertility
of the animals fed 500 and 1000 ppm was evident. No congenital defects
were observed in any of the F2a or F2b animals (Brown, 1964-65).
Groups of rats were maintained on diets containing 25 or 75 ppm of
dicofol through a three-generation study. The average number of pups
born per litter to parents receiving 75 ppm was slightly lower than
for the controls. There was no compound related effects relative to
body weight, fertility, gestation, viability or lactation indices at
either level, nor were there any congenital abnormalities evident in
either the viable or the still-born pups (Brown, 1967b).
(b) Toxicity study of a possible metabolite
The possible metabolite, 4,4'-dichlorobenzophenone was fed to groups
each containing 15 male and 15 female rats, at dietary levels of 0,
100 and 1250 ppm for three months. No effects were noted at either
level except somewhat reduced heart to body weight ratios found in the
males receiving 1250 ppm, but not in the females (Larson, 1965).
Comments
Short-term experiments on dogs and rats, reproduction studies on rats
and mice and long-term experiments on rats are reported. From the
results of the acute and short-term experiments a great species
difference in susceptibility is evident between dogs and rats.
No specific identification of possible metabolites has been made in
animals but dichlorbenzophenone is a probable metabolite. No human
studies have been reported.
Comparative metabolic studies in animals and man, including adrenal
function studies after oral administration are desirable.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 50 ppm, equivalent to 2.5 mg/kg per day.
Estimate of acceptable daily intake for man
0.0-0.025 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Kelthane is a miticide with systemic properties, used on a large
number of crops. It has no insecticidal activity. The following table
gives a review of application rates of dicofol and recommended
pre-harvest intervals.
Rate of application (kg/ha) Pre-harvest
interval
Crop Small plants Large plants (days)
Apples, pears, crabapples, 2.20 4.41-5.30 7
quince
Cherries 2.20 3.96 7
Peaches, apricots, 2.20 3.96 14
nectarines
Walnuts, filberts, pecans,
chestnuts, hickory nuts 1.76 4.85 14
and almonds, if hulls are
not fed to livestock
Almonds, if hulls are fed 1.76 3.09 Before nut
to livestock formation
Grapes, hops .66 1.60 7
Strawberries .44 2.96 2
Raspberries and other cane .66 1.60 2
berries
Beans .44 .73 7
Beans (dry) .44 1.00 45
Beans (California) .66 1.32 45
Cantaloupe, cucumbers,
pumpkins, squash, melons, .44 .73 2
water melons
Tomatoes, peppers .66 1.00 2
Alfalfa, clover, other .66 1.32
legumes for seed only
Corn (field) .88 1.76 Before
ears form
Plums, prunes, figs 1.76 2.50 7
(Adapted from Rohm and Haas, Kelthane 35 label)
Post-harvest treatments
No post-harvest uses of dicofol are known.
Other uses
Dicofol is used for control of mites on lawns, ornamentals and shade
trees, as well as against clover mites in buildings.
Residues resulting from supervised trials
Detailed residue data are available from United States trials with
dicofol on important crops and have been deposited with FAO. Rates of
application were similar to those given above. The typical data
presented below are representative:
Crop Number of Post-treatment Residue (ppm)
treatments interval (days) Range Average
Almonds (whole) 1 129-157 3.11-0.18 1.60
Apples 1 7 1.1
Beans (whole) 1 6 2.45
Carrots (roots) 1-2 5 0.00-0.25 0.10
Celery 1 6 1.32-4.45 2.88
Cherries 1 7 1.2 -1.8 1.5
Citrus (whole) 1-2 7 1.12-5.0 2.2
Cottonseed 1 15-76 0.00-0.1 0.0
Cucumbers 1 2 0.7 -2.3 1.6
Figs 1 7 1.86-5.32 3.92
Grapes 1 5 0.89-2.21 1.64
Hops (green) 1 7 3.0 -7.9 5.4
Lettuce 2 2 1.10-9.87 5.59
Mint (fresh hay) 1 7 35.8 -38.9 37.3
Peaches 1 14 4.9 -6.8 5.7
(continued)
Crop Number of Post-treatment Residue (ppm)
treatments interval (days) Range Average
Pears 2 7 1.1 -2.6 1.9
Pineapples 4 5 0.0 -0.05 <0.05
(pulp)
Plums, prunes 1 9 0.06-0.16 0.11
Raspberries 1 2 2.7
Spinach 1 4 2.88-3.90 3.39
Strawberries 1 3 0.13-0.90 0.37
Tea, raw leaves 1 7 18.00-31.00 25.0
1 7 0.00-18.5 5.1
Tomatoes 1 1 0.5 -0.57 0.53
Tropical fruits 5 4 0.0 0.0
Walnuts 1 7 0.57-0.92 0.86
Water melons 1 20 0.0
(heart)
Although dicofol is used for seed treatment no data on residues
are available.
Fate of residues
In soils
Dicofol is not used against pests in soil, but studies have been
conducted to determine residues in soil resulting from spray
application on crops. Dicofol residues in soil decrease rapidly at
first, then more slowly. After three monthly applications of 2.56
kg/ha, residues which had reached 1.13 ppm decreased to 0.25 ppm after
636 days, (Rohm and Haas, 1967).
In plants
No information is available on metabolism in plants.
In animals
Cows fed 2 ppm dicofol for 71 days averaged from 0.23-0.40 ppm
residues in their milk; when the same cows were fed 1.0 ppm, the level
was not detectable. Two body fat samples taken at the end of the study
contained 1.1 and 2.7 ppm (Zweig et al,, 1963).
A "Blue" cow fed 5 ppm dicofol for 17 days, excreted a maximum of
0.022 ppm dicofol per se and a maximum of 0.55 ppm dicofol plus
metabolites in the milk. Plateau values were obtained about six days
after start of dicofol feedings, and initial decay was also rapid.
From the fifth day after withdrawal of dicofol, the amounts of
material excreted were small, and the rate of decrease became low.
In a "White" cow fed 30 ppm dicofol for three days, 0.68 ppm dicofol
were found in kidney fat. Dicofol metabolites were found in kidney
fat, liver, udder, brain, kidney lungs, blood, heart, omentum, bone
marrow and muscles (Rohm and Haas, 1968).
The feeding of dicofol to steers produces no significant residues in
body fat at a level of 0.75 and 1.5 ppm. A feeding level of 3.0 ppm
produces a definite residue of 0.32 ppm (Peoples, 1967).
In tissues, milk and urine of a cow fed dicofol two groups of
metabolites were found, one more polar than dicofol and one less
polar. The metabolites of the first group were not identified. In the
second group, two substances were identified as 1,1 bis
(4-chlorophenyl) 2-chloro-ethylene and as 1,1 bis
(4-chlorophenyl)-2,2-dichloro-ethylene. Sixty per cent of total milk
residues is due to the latter substance, but it is suggested that it
may be present in technical dicofol rather than result from the
metabolic reduction of dicofol itself (Rohm and Haas, 1958). The
latter two compounds were also found as metabolites of DDT.
In storage and processing
Residues on fruits are reduced by washing. Wash-water of oranges
contained up to 0.05 ppm dicofol; 0.12-0.57 ppm remained in the peel.
Unwashed raspberries contained 0.7-2.9 ppm dicofol, residues which
were reduced to 0.7-1.4 after washing.
Peeling reduces residues to values below 1 ppm:
Fruit Residue (ppm)
Peel Pulp
Citrus fruit 0.29-12.0 0-0.27
Almonds 0-12.2 0-0.03
Cucumbers 0.09-0.4 0-0.17
Pineapples 5.5 -24.2 0-0.05
Tropical fruit 4.2 -9.7 0
(Rohm and Haas, private communication)
In canned or frozen fruits dicofol was found only in 1.3 per cent of
domestic samples, with an average ppm of 0.01. No dicofol was found in
samples imported into the United States of America (USDA, 1968b).
Brewed tea contained residues up to 0.0085 ppm. Under rigorous
treatment, such as preparing instant tea from tea leaves, or
distilling oil from mint leaves, dicofol may be broken down to
p,p'-dichlorobenzophenone and chloroform (Rohm and Haas, private
communication).
Evidence of residues in food in commerce or at consumption
Examination of market samples of food in the United States of America
showed that dicofol residues were found in 0.7 per cent of domestic
samples and 1.8 per cent of imported samples (Duggan and Dawson,
1967a). In fruit the following concentrations were found in the United
States of America from 1964 to 1967 (USDA, 1968b).
Domestic samples Imported samples
Fruit Incidence Average Incidence Average
per cent. (ppm) per cent. (ppm)
Small fruit
(strawberries,
cherries, plums, 5.1 0.03 2.1 0.02
grapes, cranberries,
etc.)
(continued)
Domestic samples Imported samples
Fruit Incidence Average Incidence Average
per cent. (ppm) per cent. (ppm)
Large fruit
(apples, 8.6 0.02 3.3 0.01
oranges, pears,
peaches, etc.)
Total diet studies in the United States of America from 1964 to 1967
showed the following results (Duggan and Weatherwax, 1967b; USDA,
1968b)
Year Positive per cent. Daily average
composites intake (mg)
1964-1965 0.5 0.003
1965-1966 3.7 0.002
1966-1967 5.6 0.012
In 41.9 per cent of fruit-composites (apples, oranges, pears,
peaches, etc.) dicofol was found in an average concentration of 0.031
ppm (USDA, 1968b). Dietary intake of dicofol in the United States of
America was 0.00004 mg/kg body weight/day in 1965; 0.00015 in 1966 and
0.00018 in 1967 with an average of 0.00013 for three years (USDA,
1968b).
In Canada 60 restaurant meals were examined. Dicofol was found in an
average concentration of 0.07 ppm in fruit and fruit salads, in
legumes, 0.08 ppm whereas only traces were found in milk (Swackhamer,
1965).
Methods of residue analysis
The methods are adequate for present purposes. Methods for
colorimetric determination of dicofol are based on the method of
Rosenthal, 1957. Dicofol is converted to chloroform with alkali.
Chloroform is separated from extraneous materials and produces, with a
water-pyridine-sodium-hydroxide mixture, a Fujiwara-type red dye,
which is read at 535mµ against a pyridine blank. This method may be
used for residue determination in plants, fruit and vegetables and has
been simplified for analysis of milk (Gordon, 1963). Residues in crops
and soil may also be determined, after chromatographical clean-up, by
GLC using an electron capture detector (Morgan, 1967). An ultra-violet
spectrophotometric method involves hydrolysing the dicofol to
4,4'-dichlorobenzophenone (Gunther and Blinn, 1957). A method omitting
the hydrolysis step and allowing the dicofol to be oxidized to
non-interfering compounds permits the measurement of
4,4'-dichlorobenzophenone present in mint-oil and mint-hay (Rohm and
Haas, private communication).
The sensitivity of the analytical procedures is 0.01 ppm.
National tolerances
Tolerance Pre-harvest
Country Crop (ppm) interval (days)
United States Berries 5
of America
German Federal Fruit, including
Republic grapes and hops 0.5 14
Vegetables 0.5
Beans 14
Cucumbers 4
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Dicofol is used exclusively to control phytophagous mites and although
closely related to DDT has virtually no insecticidal activity. Its
principal use is on apples, grapes and hops in Europe and North
America, and tea (spot treatments only) in the Far East, with some
minor use on citrus in these same areas. In North America, the rates
and frequencies of application are generally greater than in other
countries because of more serious mite problems. It is not used on
animals. Dicofol is a technical product containing 82-88 per cent of
the pure (active) compound. The impurities are known and their
relative concentration are constant.
The data available to the meeting were obtained solely in the United
States of America and did not include information about uses elsewhere
or figures for residues after such use, although figures were
available for some imports into the United States of America.
Very little is known about the metabolism of dicofol in plants and
animals. In animals, dicofol can be found as such together with
various metabolites. Precise figures for the amounts of these
compounds in experimental plants or animals, either from a single or
from repeated applications, have not been published.
1,1 bis (4-chlorophenyl) 2-chloro-ethylene and 1,1 bis
(4-chlorophenyl-2,-2-dichloro-ethylene) have been identified as
metabolites in animals. However, the latter may be present in the
technical product used. In mint oil and tea, 4,4-dichlorobenzophenone
has been identified.
The literature includes methods of residue analysis which measure
dicofol alone or together with some breakdown products. For the
determination of dicofol alone the sensitivity is 0.01 ppm. However,
no referee method of analysis has been evaluated.
Recommendations
Temporary tolerances
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruit and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
Fruit, vegetables, hops, tea (from a
particular estate, for blending only) 5 ppm
Tea (blended) 1 ppm
Further work or information
Required before 30 June 1972:
1. Data from countries other than the United States of America on the
required rates and frequencies of application, pre-harvest
intervals, and the resultant residues.
2. Information on the nature of the terminal residues in plants,
animals, and their products.
Desirable:
1. Collaborative studies to establish a referee method.
2. Comparative metabolic studies in animals and man, including adrenal
function studies after oral administration.
REFERENCES
Brown, J. R. (1964-65) Toxicologic studies on the effects of kelthane
in the diet of albino rats on reproduction (first interim, second
interim and final reports). Departments of Physiological Hygiene,
University of Toronto. Unpublished reports
Brown, J. R. (1967a) Toxicological studies on
2,2-bis-chlorophenyl-2,2,2-trichloroethanol, kelthane. Brown
Biological Laboratories Ltd. Unpublished report
Brown, J. R. (1967b) Three-generation reproduction study on rats
receiving technical kelthane in their diet. Department of
Physiological Hygiene, University of Toronto. Unpublished report
Duggan, R. E. and Dawson, K. (1967a) Pesticides: A report on residues
in food. FDA Papers, 1: 2-5
Duggan, R. E. and Weatherwax, J. R. (1967b) Dietary intake of
pesticide chemicals. Science, 157: 1006-1010
Gordon, C. F., Haines, L. D. and Martin, J. J. (1963) Acaricide
residues: An improved method for kelthane residue analysis with
applications for determination of residues in milk. J. Agr. Food
Chem., 11: 84-86
Gunther, F. A. and Blinn, R. C. (1957) Ultraviolet spectrophotometric
microdetection of the acaricide 4,4'-dichloro-alpha-(trichlormethyl)
benzhydrol (SW 293). J.Agr. Food Chem., 5: 517-519
Larson, P. S. (1957) Two-year study on the effect of adding kelthane
to the diet of rats. Medical College of Virginia. Unpublished report
Larson, P. S. (1965) Toxicologic study on the effect of adding
dichlorobenzophenone to the diet of rats for a period of three months.
Department of Pharmacology. Medical College of Virginia. Unpublished
report
Morgan, N. L. (1967) The identification and relative retention times
of p,p'-kelthane and its breakdown product p,p'-dichlorobenzophenone
using GLC. Bull. environ. Contam. Toxicol., 2: 306-312
Peoples, S. A. (1967) Residues in the body fat of steers fed kelthane
in their ration. Dept. of Physiol. Sci. School of Veterinary Medicine,
University of California, Davis. Unpublished report
Rohm and Haas. (1958) Research Reports 13-27 and 11.141. Robin and
Haas Co.
Rohm and Haas. (1967) RAR Memorandum 521. Rohm and Hass Co.
Rohm and Haas Co. (1968) Kelthane. Unpublished report
Rosenthal, J., Frisone, G. J. and Gunther, F. A. (1957) Colorimetric
microdetermination of the acaricide
4,4'-dichloro-alpha-(trichloromethyl) benzhydrol (FW-293).J. Agr. Food
Chem., 5: 514-517
Smith, R. B. Jr, Larson, P. S., Finnegan, J. K., Haag, H. B.,
Hennigar, G. R, and Cobey, F. (1959) Toxicologic studies on 2,2
bis-(chlorophenyl)-2,2,2-trichloroethanol (kelthane). Toxicol. appl.
Pharmacol., 1: 119-134
Swackhamer, A. B. (1965) Report on pesticide residues in restaurant
meals in Canada. Food and Drug Directorate, Department of National
Health and Welfare, Ottawa, Canada. Pesticide Progress, 3: 108-114
USDA. (1968a) Agricultural Economic Report 131
USDA. (1968b) United States Department of Health, Education and
Welfare. The regulation of pesticides in the United States
Verschuuren, H. G., Kroes, R. and den Tonkelaar, E. M. (1968)
Toxiciteitsonderzoek met kelthaan bij ratten gedurende 90 dagen
(Toxicity experiment with kelthane in rats of 90 day duration).
National Institute of Public Health, Utrecht. Unpublished report
Zweig, G., Pye, E. L. and Peoples, S. A. (1963) Residues in butter fat
and body fat of dairy cows fed at two levels of kelthane (1.0 and 2.0
ppm). J. Agr. Food Chem., 11: 72-79
