
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
DINOCAP
IDENTITY
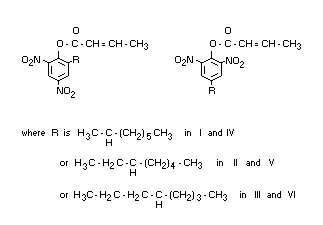
Chemical name
Dinocap is a mixture of dinocap-4 and dinocap-6 (British Standard 1831
: 1969).
Dinocap-4 is a mixture of the following isomers:
I 2,6-dinitro-4-(1-methylheptyl) phenyl crotonate
II 2,6-dinitro-4-(1-ethylhexyl) phenyl crotonate
III 2,6-dinitro-4-(1-propylpentyl) phenyl crotonate
Dinocap-6 is a mixture of the following isomers:
IV 2,4-dinitro-6-(1-methylheptyl) phenyl crotonate
V 2,4-dinitro-6-(1-ethylhexyl) phenyl crotonate
VI 2,4-dinitro-6-(1-propylpentyl) phenyl crotonate
The ratio of dinocap-4 to dinocap-6 in technical dinocap is likely to
be of the order of 1 : 2.
G.L.C. analyses of the mixture of methyl ethers prepared from the
unesterified precursor of dinocap gave the following ratio of isomers
present (Clifford et al., 1965):
I 12 per cent
II 15 per cent
III 11 per cent
IV 12 per cent
V 26 per cent
VI 24 per cent
Synonyms
Karathane(R), Crotothane(R)
Structural formula
 Other relevant chemical properties
Technical dinocap is a dark red, viscous liquid with very slight
solubility in water but good solubility in the usual organic solvents.
Information on vapour pressure is not available, but persistence on
surfaces is shorter than that of binapacryl.
Incomplete esterification in the manufacturing process leads to the
presence of the free phenols corresponding to the six esters mentioned
above; these are reported (Rohm and Haas, 1969) to form 5 to 6 per
cent of the technical product. A smaller proportion, less than 1 per
cent, consists of mono-nitrophenols. These three classes together form
about 80 per cent of the technical product. Of the remainder, about 4
per cent consists of a mixture of octenes, formed by dehydration of
the starting material, capryl alcohol, and another 2 per cent is
crotonic acid. There are also four unknowns that produce peaks on the
G.L.C. chart, totalling 2 to 3 per cent, and 10 to 12 per cent of the
technical product in 'not volatile'.
Formulations include wettable powders usually claimed to contain 25
per cent active ingredients; Karathane(R) W.P. is now claimed to
contain 19.5 per cent active ingredients; no further information is
available concerning Crotothane(R) W.P. Another commercial formulation
is a liquid concentrate; Karathane LC was formerly claimed to contain
48 per cent active ingredients, but is now claimed to contain 39 per
cent ; Liquid Crotothane is sold an containing 50 per cent active
ingredients. There are also dusts containing less than 10 per cent
a.i.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
No residues of dinocap were detected in the subcutaneous and
intraperitoneal fat obtained from an unspecified number of rats fed
500 ppm of dinocap in their diet for one month. The method of analysis
was sensitive to 1 ppm (Larson et al., 1959).
Effect on enzymes and other biochemical parameters
Dinocap was compared with 2,4-dinitrophenol in oxygen consumption
studies in rats. Six rats (four males and two females) were given a
single oral dose of 600 mg/kg body-weight of dinocap. Measurements
made at various intervals showed that a steady increase in oxygen
consumption occurred in the females, which reached a maximum of 63 per
cent of the zero-time level after 24 hours. Measurements made on two
of these animals after 48 hours showed that the level had fallen to 29
per cent. No increase in oxygen consumption occurred during 24 hours
in the males fed dinocap. A comparable study with six other rats (two
males and four females) given a single oral dose of 40 mg/kg
body-weight of 2,4-dinitrophenol resulted in a maximum increase in
oxygen consumption of 116 per cent for the females and 90 per cent for
the males after three hours, followed by a gradual decrease after that
time. Thus, dinocap produced an increase in oxygen consumption in
female rats only, this effect being of longer duration although lower
in magnitude than the effect which occurred in both sexes with
2,4-dinitrophenol (Larson et al., 1959).
The compound 4-isooctyl-2,6-dinitrophenol (a constituent of technical
dinocap) was found to be 7 to 25 times more potent than
2,4-dinitrophenol in stimulation of respiration of rat-liver
mitochondria. The author concluded that the pKa and the lipid
solubility of the compounds as well as the pH of the media were the
factors influencing this activity, and that there was no intrinsic
structure-activity relationship relative to the size of the octyl
group (Hemker, 1962).
TOXICOLOGICAL STUDIES
Special studies on cataract formation
Chicken
Chicks were fed dietary levels of dinocap or binapacryl which were
equivalent on a molar basis to levels of 500 or 1000 ppm of
2,4-dinitrophenol. No lens opacities resulted from 17 to 28 days
administration of either of these compounds at these levels. Feeding
2,4-dinitrophenol at 2000 ppm under the same conditions also did not
result in the formation of lens opacities. However, when the dietary
level of dinocap or binapacryl was increased to 2000 ppm molar
equivalent to dinitrophenol a distinct incidence of cataracts
occurred. When fed at 4000 ppm absolute dietary level no difference
could be observed between dinocap and binapacryl with regard to
incidence of cataract formation (Cervenka and Kay, 1963).
Duck
Dinocap was compared with 2,4-dinitrophenol in studies to determine if
cataracts were formed in ducks. Three series of studies were conducted
using the technical grade dinocap throughout.
In the first experiment groups comprising 10 ducklings, each of about
10 days of age, were fed dietary levels of 0, 50, 250, or 2500 ppm of
dinocap or 0, 25, 125, 250 or 1250 ppm of 2,4-dinitrophenol for 12
weeks. Survival was adversely affected at the 500 and 2500 ppm levels
of dinocap and the 1250 ppm level of 2,4-dinitrophenol. Growth was
depressed at 250 ppm and higher concentrations of dinocap and at all
concentrations of 2,4-dinitrophenol. The 1250 ppm level of
2,4-dinitrophenol produced cataracts within 24 hours, and all other
levels both of dinocap and 2,4-dinitrophenol produced cataracts within
seven weeks. No cataracts occurred in the controls. After the
cataracts had developed, withdrawal of the substances from the diet
for a five-week period did not result in regression of the cataracts
(Larson et al., 1959).
In the second experiment, groups each containing 10 ducklings,
received diets containing 0, 2, 5, 10 or 25 ppm of dinocap or 0, 25,
125, 250 or 1250 ppm of 2,4-dinitrophenol. Most of the ducks including
all of the controls developed cataracts by the end of seven weeks and
the test series was discarded. A conclusion was drawn that cataracts
had developed from some unknown cause (Larson et al., 1959).
The third experiment was conducted using the same conditions as have
been described for the second. Ophthalmologic examinations made after
5, 9 and 13 weeks on the test diet showed no cataracts in any treated
group (Larson et al., 1959).
In a more recent study, ducklings were fed 250 ppm of
dinitrooctylphenol (the isomer or mixtures thereof not specified) for
13 weeks and none developed cataracts. When 2,4-dinitrophenol was also
fed as a positive control to ducklings, cataracts developed in all
cases. The explanation of the erratic results from the earlier
cataract studies with dinocap has been suggested to be at least partly
related to the variation in composition of the technical product
relative to the presence of significant impurities. There may,
however, be other explanations (Swisher, 1969).
Acute toxicity
LD50
(technical grade*)
Animal Route mg/kg body-weight Reference
Rat (M) oral 980 Larson et al., 1959
Rat (M) iv. 23 Larson et al., 1959
Rat (F) oral 1190 Larson et al., 1959
Rabbit (M) oral 2000 Larson et al., 1959
(cont'd)
LD50
(technical grade*)
Animal Route mg/kg body-weight Reference
Dog oral 100 Larson et al., 1959
* Technical grade is here defined as:
2,4-dinitro-6-(2-octyl)-phenyl crotonate
2,4-dinitro-6-(3-octyl)-phenyl crotonate
2,4-dinitro-6-(4-octyl)-phenyl crotonate ....... 73 per cent
2,6-dinitro-4-(2-octyl)-phenyl crotonate
2,6-dinitro-4-(3-octyl)-phenyl crotonate
2,6-dinitro-4-(4-octyl)-phenyl crotonate
nitrocctylphenols (principally dinitro-) ....... 5 per cent
inert ingredients (octenes, crotonic
acid and related compounds) ................... 22 per cent
Short-term studies
Dog
Groups, each containing three dogs of unspecified sex and age, were
fed diets containing 10, 50, 100, 250 and 1000 ppm of technical
dinocap for one year. One dog in the 250 ppm group died within six
weeks, another was sacrificed after marked weight loss and the third
was transferred to a control diet. Two dogs in the 1000 ppm group died
within six weeks and the third was transferred to the control diet.
(No other control group was reported to have been used). Decreased
appetite and drastic weight loss preceded death. Moderate weight loss
was evident at the 100 ppm level but not at 50 ppm. Hepatic necrosis
occurred in the dogs fed 250 and 1000 ppm. Haematologic values were
normal at all dose-levels (Larson et al., 1959).
Rat
Groups containing 10 weanling rats of each sex were fed diets
containing 0, 10, 50, 250, 1000 and 2500 ppm of technical dinocap for
six months. Growth and survival were reduced at the 2500 ppm level and
growth was reduced at 1000 ppm. Enlarged spleens occurred in the males
receiving 2500 ppm. Haematological and microscopic examinations
revealed no changes attributable to treatment (Larson et al., 1959).
Long-term studies
Rat
Groups containing 10 weanling rats of each sex were fed diets
containing 0, 10, 50, 250, 500 and 1000 ppm of technical dinocap for
two years. There was decreased weight gain during the first year only
in the male rats fed 1000 ppm, but the effect on bodyweight during the
second year was not reported. No other effect of treatment, either
gross or histopathological was noted at any of the dose-levels studied
(Larson et al., 1959).
OBSERVATIONS IN MAN
Patch tests were performed on the forearms of 50 human subjects using
dinocap formulated either as an emulsion or as a wettable powder.
Exposure was for 48 hours. Moderate irritation resulted from the
emulsion in 11 subjects and from the powder in three. Similar results
occurred when the opposite forearms were patched 12 days later, 25
subjects reacting to the emulsion and nine to the powder. Intensified
reactions resulted during succeeding days in three subjects (Larson et
al., 1959).
COMMENT
On the basis of animal data a no-effect level for rats has been
established. Since the chemical structure of 2,4-dinitrophenol is
similar to dinocap and because 2,4-dinitrophenol produces cataracts in
man, the possibility of cataract formation in man from dinocap is of
concern. For this reason dinocap should be used with great caution.
Inconclusive experiments in ducks gave a possible indication of
cataract formation. Additional studies with other species of animals
are needed to establish the exact dosage of dinocap which does not
produce cataracts. In addition, the data on the toxicology of dinocap
were obtained between 1954 and 1958, and are incomplete especially in
relation to metabolism. Skin sensitization is reported in human
subjects but no other data on man are available. The technical product
appears to be of variable composition.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Dinocap in a non-systematic fungicide; only its activity against
powdery mildews is of commercial importance. It also restricts the
development of populations of several tetranychid phytophagous mites,
and in view of the widespread resistance of such species to specific
acaricides, this role is very valuable.
Powdery mildews are important on apple, apricot and peach, and less so
on pear and cherry among deciduous fruits; dinocap has become the
leading fungicide for control on apple, apricot and pear, and second
only to sulphur on peach (Kirby, 1969). In the U.K., over 15,000
hectares of apples were sprayed with dinocap in 1967; a smaller area
of pears was also sprayed. For 10-14 day interval spraying, the usual
rate of use on apple in the U.K. is 2.24 kg of the wettable powder per
ha; according to the recent claim for Karathane WP, this is 0.44 kg
a.i, per ha. According to the U.S.A. manufacturers, U.S.A. regulations
allow up to 1.75 kg a.i. per ha to be applied on each occasion. In the
U.K., at least one week must elapse between the final application and
harvest on all edible crops; in the U.S.A. this period applies to
cucurbits, but 21 days must elapse on all fruits, except apricots and
peaches which need 45 days and almonds where use is allowed up to the
'jacket stage'.
Post-harvest use
Use of dinocap post harvest does not arise on perennial crops.
Other uses
The widespread occurrence of powdery mildews on almost all
ornamentals, annual and perrenial, leads to widespread use of dinocap
by nurserymen as well as public and private gardeners throughout the
summer and early autumn. No use on animals is known.
RESIDUES RESULTING FROM SUPERVISED TRIALS
On apples, trials on two cultivars in two States of the U.S.A. showed
that, in the absence of heavy rain, the half-life of dinocap was 1.9
days over a range of initial deposits from at least 50 to 200 ppm.
Heavy rainfall was found to reduce deposits very considerably (Rohm
and Haas, 1958, unpublished).
Trials conducted by or for Rohm and Haas Company provide the following
results for deposits and residues on various crops:
Pre-harvest Deposit or residue
No. of Rate interval Range Average
Crop applications g/a.i./ha days ppm ppm
Apple 3 380 28 Nil Nil
5 570 0 0.26-0.57 0.41
21 <0.05
1 380 0 0.29-0.40 0.33
(cont'd)
Pre-harvest Deposit or residue
No. of Rate interval Range Average
Crop applications g/a.i./ha days ppm ppm
14 Nil-0.07 0.04
21 Nil-0.01 0.01
( 2 190 )
( 27 No detectable residue
(12 130 )
6 850 0 0.20-0.43 0.33
7 0.06-0.12 0.09
14 No detectable residue
Pear 4 510 5 0.04-0.20 0.11
21 No detectable residue
Apricot 2 380 0 0.35-0.70 0.55
21 No detectable residue
Apricot 2 0 Nil-0.15 0.08
(dried)
14 No detectable residue
Peach 1 255 0 0.17-0.85 0.54
21 No detectable residue
3 255 18 No detectable residue
Blackberry 3 760 44 0.05-0.07 0.06
Boysenberry 1 380 0 0.36-0.51 0.41
7 No detectable residue
Raspberry 3 95 10 No detectable residue
Grape 1 95 0 Nil-1.15 0.54
7 0.17-0.95 0.33
(cont'd)
Pre-harvest Deposit or residue
No. of Rate interval Range Average
Crop applications g/a.i./ha days ppm ppm
14 Nil-0.37 0.13
21 Nil-0.50 0.12
28 No detectable residue
( 1 180 ) 0 0.02-0.10 0.04
( )
( 2 86 ) 20 Nil-0.03 0.01
Grape (juice) ( 1 180 )
( ) 29 Nil-0.01 0.01
( 2 86 )
Strawberry 3 380 0 0.81-1.43 1.02
10 0.08-0.15 0.11
21 No detectable residue
Cantaloup 18 130 3 (Pulp)Nil-0.08 0.027
Honeydew melon 18 130 3 (Pulp)No detectable residue
Cucumber ( 2 95 )
( ) 0 No detectable residue
( 1 130 )
Pumpkin 5 130 4 hours (Pulp)0.20-0.34 0.24
(whole)Nil-0.60 0.24
Squash 4 95 0 Nil-0.15 0.08
3 No detectable residue
Muskmelon ( 9 130 ) (Pulp)0.00-0.10 0.02
( ) 0 (Rind)0.00-0.10 0.04
( 5 195 )
FATE OF RESIDUES
The short half-life on foliage, approximately two days, already
mentioned means that dinocap readily disappears from leaves. A longer
half-life, about four days, on strawberries was found by Kilgore and
Cheng (1963) who made a single application 21 days before harvest.
Nothing has been found in the literature concerning breakdown
products.
METHODS OF RESIDUE ANALYSIS
Several colorimetric methods sensitive to about 0.1 ppm have been
proposed for the determination of residues of dinocap in apples,
strawberries, grapes, tomatoes and animal tissue (Rosenthal et al.,
1957; Skerrett and Baker, 1962; Kilgore and Cheng, 1963). However,
with the fuller recognition of the relative importance of the various
components of commercial formulations of dinocap (Clifford et al.,
1965; Kirby and Elvidge, 1965; Kirby and Hunter, 1965) a gas
chromatographic procedure to likely to prove more generally useful.
Clifford and Watkins (1968) have described suitable gas
chromatographic conditions for the examination of a range of
dinitroalkyl phenols. Boggs (1966) and Yip and Howard (1968) preferred
to convert the phenols to methyl others before gas chromatography with
electron capture detection. No complete extraction, clean-up and gas
chromatographic determination procedure can be recommended for
residues of the active ingredients of dinocap at the present time but
the development and establishment of such a procedure is recommended.
APPRAISAL
Dinocap is defined B.S. 1831:1969 as "a mixture of dinocap-4 and
dinocap-6"; it is a mixture of six isomeric dinitro-s-octylphenyl
crotonates, not merely one of them as previously defined (B.S.
1831:1965), The commercial "active material" also contains a small
percentage of unesterified dinitro-s-octylphenols, together with
mononitrophenols and some 20% of "inactive" ingredients.
Commercial preparations, both wettable powders and liquid
formulations, are used throughout the world for the control of powdery
mildews of apple, pear, peach, apricot, grape, soft fruits, cucurbits,
rose and other ornamentals. It is also a non-ovicidal acaricide and
its use for control of powdery mildews also provides control of
certain mites, notably the fruit tree red spider mite (European red
mite) as long as spraying is continued. Curative (not eradicant)
action against powdery mildews is nearly as good as protective action,
but there is no evidence of systemic activity even within the leaf.
Solubility in water is low, but persistence on plant surfaces
(half-life) is very short. Deposits from recommended spray rates
rarely reach 1 ppm on the day of application, and residues of the
order of 0.05 ppm are to be expected within 7 days.
Several colorimetric procedures have been used for dinocap residues,
but in view of the complex nature of the product, gas chromatographic
procedures are likely to give much more valuable information.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
The data were insufficient to enable recommendations to be made.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Additional studies with more animals to establish the exact dosage
of dinocap which does not produce cataracts.
2. Extension of the chronic toxicity experiment in dogs or other
non-rodent mammalian species.
3. Metabolic studies including determination of phenols in the blood.
4. Information on the compounds included in dinocap and assurance of a
standardized technical product.
5. Investigation aimed at clarifying physiological lesions with regard
to cellular respiratory control.
6. Reproduction studies in animals.
7. Information is required on the nature of terminal residues,
including the identity of the substances giving peaks by the GLC
method of examination.
8. Residue data are required from countries other than the U.S.A.
DESIRABLE
The development and establishment of a GLC method for the
determination of residues of the active ingredients.
REFERENCES
Boggs, H. (1966) Gas chromatography of dinitro herbicides. J. Assoc.
Offic. Anal. Chem. 49:772-3
Cervenka, H. and Kay, J.H. (1963) Cataractogenic studies. Unpublished
report from Industrial Bio-test Laboratories Inc., to Niagara Chemical
Division, FMC Corporation. Submitted by Farbwerke Hoechst AG.
Clifford, D.R., Watkins, D.A.M. and Woodcock, D. (1965) Composition
of commercial dinocap preparations. Chemy Ind., pp.1654-5
Clifford, D.R. and Watkins, D.A.M. (1968) The gas chromatography of
dinitroalkyl phenols. J. Gas Chromatog., 6:191-2
Hemker, H.C. (1962) Lipid solubility as a factor influencing the
activity of uncoupling phenols. Biochim. Biophys. Acta, 63:46-54
Kilgore, W.W. and Cheng, K.W. (1963) Extraction and determination of
Karathane residues in fruits. J. agric. Fd Chem., 11:477-79
Kirby, A.H.M. and Elvidge, J.A. (1965) Composition of commercial
dinocap preparations. Chemy Ind., 2103
Kirby, A.H.M. and Hunter, L.D. (1965) Identification of
dinitro-octylphenols in certain commercial fungicides. Nature,
208:189-90
Kirby, A.H.M. (1969) Fungicides for deciduous top fruit: a survey in
1968. Wd. Rev. Pest Central. 8:45-58
Larson, P.S., Finnegan, J.K., Smith, R.B. Jr., Haag. H.B., Hennigar,
G.R. and Patterson, W.M. (1959) Acute and chronic toxicity studies on
2,4-dinitro-6-(l-methylheptyl) phenyl crotonate (Karathane).
Arch. int. Pharmacodyn., 119:31-42
Rohm and Haas. (1969) Karathane: chemical description, registered uses
(labels), residue studies. Rohm and Haas Company, Philadelphia,
Penn., U.S.A.
Rosenthal, I., Gordon, C.F., Stanley, E.L. and Perlman, M.H.
(1957) Microdetermination of the fungicide
dinitrocapryl-phenylcrotonate in food crops and animal tissues.
J. Agr. Fd Chem., 5:914-18
Skerrett, E.J. and Baker, E.A. (1962) The determination of spray
residues of 'Karathane' 87:228-9
Swisher, E.M. (1969) Report of a study on cataract formation in ducks
conducted in 1966 at the Medical College of Virginia. Unpublished
information submitted by Rohm and Haas Co.
Yip, G. and Howard, S.F. (1968) Extraction and clean-up procedure
for the gas chromatographic determination of four dinitrophenolic
pesticides. J. Assoc. Offic. Anal, Chem., 51:24-28
Other relevant chemical properties
Technical dinocap is a dark red, viscous liquid with very slight
solubility in water but good solubility in the usual organic solvents.
Information on vapour pressure is not available, but persistence on
surfaces is shorter than that of binapacryl.
Incomplete esterification in the manufacturing process leads to the
presence of the free phenols corresponding to the six esters mentioned
above; these are reported (Rohm and Haas, 1969) to form 5 to 6 per
cent of the technical product. A smaller proportion, less than 1 per
cent, consists of mono-nitrophenols. These three classes together form
about 80 per cent of the technical product. Of the remainder, about 4
per cent consists of a mixture of octenes, formed by dehydration of
the starting material, capryl alcohol, and another 2 per cent is
crotonic acid. There are also four unknowns that produce peaks on the
G.L.C. chart, totalling 2 to 3 per cent, and 10 to 12 per cent of the
technical product in 'not volatile'.
Formulations include wettable powders usually claimed to contain 25
per cent active ingredients; Karathane(R) W.P. is now claimed to
contain 19.5 per cent active ingredients; no further information is
available concerning Crotothane(R) W.P. Another commercial formulation
is a liquid concentrate; Karathane LC was formerly claimed to contain
48 per cent active ingredients, but is now claimed to contain 39 per
cent ; Liquid Crotothane is sold an containing 50 per cent active
ingredients. There are also dusts containing less than 10 per cent
a.i.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
No residues of dinocap were detected in the subcutaneous and
intraperitoneal fat obtained from an unspecified number of rats fed
500 ppm of dinocap in their diet for one month. The method of analysis
was sensitive to 1 ppm (Larson et al., 1959).
Effect on enzymes and other biochemical parameters
Dinocap was compared with 2,4-dinitrophenol in oxygen consumption
studies in rats. Six rats (four males and two females) were given a
single oral dose of 600 mg/kg body-weight of dinocap. Measurements
made at various intervals showed that a steady increase in oxygen
consumption occurred in the females, which reached a maximum of 63 per
cent of the zero-time level after 24 hours. Measurements made on two
of these animals after 48 hours showed that the level had fallen to 29
per cent. No increase in oxygen consumption occurred during 24 hours
in the males fed dinocap. A comparable study with six other rats (two
males and four females) given a single oral dose of 40 mg/kg
body-weight of 2,4-dinitrophenol resulted in a maximum increase in
oxygen consumption of 116 per cent for the females and 90 per cent for
the males after three hours, followed by a gradual decrease after that
time. Thus, dinocap produced an increase in oxygen consumption in
female rats only, this effect being of longer duration although lower
in magnitude than the effect which occurred in both sexes with
2,4-dinitrophenol (Larson et al., 1959).
The compound 4-isooctyl-2,6-dinitrophenol (a constituent of technical
dinocap) was found to be 7 to 25 times more potent than
2,4-dinitrophenol in stimulation of respiration of rat-liver
mitochondria. The author concluded that the pKa and the lipid
solubility of the compounds as well as the pH of the media were the
factors influencing this activity, and that there was no intrinsic
structure-activity relationship relative to the size of the octyl
group (Hemker, 1962).
TOXICOLOGICAL STUDIES
Special studies on cataract formation
Chicken
Chicks were fed dietary levels of dinocap or binapacryl which were
equivalent on a molar basis to levels of 500 or 1000 ppm of
2,4-dinitrophenol. No lens opacities resulted from 17 to 28 days
administration of either of these compounds at these levels. Feeding
2,4-dinitrophenol at 2000 ppm under the same conditions also did not
result in the formation of lens opacities. However, when the dietary
level of dinocap or binapacryl was increased to 2000 ppm molar
equivalent to dinitrophenol a distinct incidence of cataracts
occurred. When fed at 4000 ppm absolute dietary level no difference
could be observed between dinocap and binapacryl with regard to
incidence of cataract formation (Cervenka and Kay, 1963).
Duck
Dinocap was compared with 2,4-dinitrophenol in studies to determine if
cataracts were formed in ducks. Three series of studies were conducted
using the technical grade dinocap throughout.
In the first experiment groups comprising 10 ducklings, each of about
10 days of age, were fed dietary levels of 0, 50, 250, or 2500 ppm of
dinocap or 0, 25, 125, 250 or 1250 ppm of 2,4-dinitrophenol for 12
weeks. Survival was adversely affected at the 500 and 2500 ppm levels
of dinocap and the 1250 ppm level of 2,4-dinitrophenol. Growth was
depressed at 250 ppm and higher concentrations of dinocap and at all
concentrations of 2,4-dinitrophenol. The 1250 ppm level of
2,4-dinitrophenol produced cataracts within 24 hours, and all other
levels both of dinocap and 2,4-dinitrophenol produced cataracts within
seven weeks. No cataracts occurred in the controls. After the
cataracts had developed, withdrawal of the substances from the diet
for a five-week period did not result in regression of the cataracts
(Larson et al., 1959).
In the second experiment, groups each containing 10 ducklings,
received diets containing 0, 2, 5, 10 or 25 ppm of dinocap or 0, 25,
125, 250 or 1250 ppm of 2,4-dinitrophenol. Most of the ducks including
all of the controls developed cataracts by the end of seven weeks and
the test series was discarded. A conclusion was drawn that cataracts
had developed from some unknown cause (Larson et al., 1959).
The third experiment was conducted using the same conditions as have
been described for the second. Ophthalmologic examinations made after
5, 9 and 13 weeks on the test diet showed no cataracts in any treated
group (Larson et al., 1959).
In a more recent study, ducklings were fed 250 ppm of
dinitrooctylphenol (the isomer or mixtures thereof not specified) for
13 weeks and none developed cataracts. When 2,4-dinitrophenol was also
fed as a positive control to ducklings, cataracts developed in all
cases. The explanation of the erratic results from the earlier
cataract studies with dinocap has been suggested to be at least partly
related to the variation in composition of the technical product
relative to the presence of significant impurities. There may,
however, be other explanations (Swisher, 1969).
Acute toxicity
LD50
(technical grade*)
Animal Route mg/kg body-weight Reference
Rat (M) oral 980 Larson et al., 1959
Rat (M) iv. 23 Larson et al., 1959
Rat (F) oral 1190 Larson et al., 1959
Rabbit (M) oral 2000 Larson et al., 1959
(cont'd)
LD50
(technical grade*)
Animal Route mg/kg body-weight Reference
Dog oral 100 Larson et al., 1959
* Technical grade is here defined as:
2,4-dinitro-6-(2-octyl)-phenyl crotonate
2,4-dinitro-6-(3-octyl)-phenyl crotonate
2,4-dinitro-6-(4-octyl)-phenyl crotonate ....... 73 per cent
2,6-dinitro-4-(2-octyl)-phenyl crotonate
2,6-dinitro-4-(3-octyl)-phenyl crotonate
2,6-dinitro-4-(4-octyl)-phenyl crotonate
nitrocctylphenols (principally dinitro-) ....... 5 per cent
inert ingredients (octenes, crotonic
acid and related compounds) ................... 22 per cent
Short-term studies
Dog
Groups, each containing three dogs of unspecified sex and age, were
fed diets containing 10, 50, 100, 250 and 1000 ppm of technical
dinocap for one year. One dog in the 250 ppm group died within six
weeks, another was sacrificed after marked weight loss and the third
was transferred to a control diet. Two dogs in the 1000 ppm group died
within six weeks and the third was transferred to the control diet.
(No other control group was reported to have been used). Decreased
appetite and drastic weight loss preceded death. Moderate weight loss
was evident at the 100 ppm level but not at 50 ppm. Hepatic necrosis
occurred in the dogs fed 250 and 1000 ppm. Haematologic values were
normal at all dose-levels (Larson et al., 1959).
Rat
Groups containing 10 weanling rats of each sex were fed diets
containing 0, 10, 50, 250, 1000 and 2500 ppm of technical dinocap for
six months. Growth and survival were reduced at the 2500 ppm level and
growth was reduced at 1000 ppm. Enlarged spleens occurred in the males
receiving 2500 ppm. Haematological and microscopic examinations
revealed no changes attributable to treatment (Larson et al., 1959).
Long-term studies
Rat
Groups containing 10 weanling rats of each sex were fed diets
containing 0, 10, 50, 250, 500 and 1000 ppm of technical dinocap for
two years. There was decreased weight gain during the first year only
in the male rats fed 1000 ppm, but the effect on bodyweight during the
second year was not reported. No other effect of treatment, either
gross or histopathological was noted at any of the dose-levels studied
(Larson et al., 1959).
OBSERVATIONS IN MAN
Patch tests were performed on the forearms of 50 human subjects using
dinocap formulated either as an emulsion or as a wettable powder.
Exposure was for 48 hours. Moderate irritation resulted from the
emulsion in 11 subjects and from the powder in three. Similar results
occurred when the opposite forearms were patched 12 days later, 25
subjects reacting to the emulsion and nine to the powder. Intensified
reactions resulted during succeeding days in three subjects (Larson et
al., 1959).
COMMENT
On the basis of animal data a no-effect level for rats has been
established. Since the chemical structure of 2,4-dinitrophenol is
similar to dinocap and because 2,4-dinitrophenol produces cataracts in
man, the possibility of cataract formation in man from dinocap is of
concern. For this reason dinocap should be used with great caution.
Inconclusive experiments in ducks gave a possible indication of
cataract formation. Additional studies with other species of animals
are needed to establish the exact dosage of dinocap which does not
produce cataracts. In addition, the data on the toxicology of dinocap
were obtained between 1954 and 1958, and are incomplete especially in
relation to metabolism. Skin sensitization is reported in human
subjects but no other data on man are available. The technical product
appears to be of variable composition.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Dinocap in a non-systematic fungicide; only its activity against
powdery mildews is of commercial importance. It also restricts the
development of populations of several tetranychid phytophagous mites,
and in view of the widespread resistance of such species to specific
acaricides, this role is very valuable.
Powdery mildews are important on apple, apricot and peach, and less so
on pear and cherry among deciduous fruits; dinocap has become the
leading fungicide for control on apple, apricot and pear, and second
only to sulphur on peach (Kirby, 1969). In the U.K., over 15,000
hectares of apples were sprayed with dinocap in 1967; a smaller area
of pears was also sprayed. For 10-14 day interval spraying, the usual
rate of use on apple in the U.K. is 2.24 kg of the wettable powder per
ha; according to the recent claim for Karathane WP, this is 0.44 kg
a.i, per ha. According to the U.S.A. manufacturers, U.S.A. regulations
allow up to 1.75 kg a.i. per ha to be applied on each occasion. In the
U.K., at least one week must elapse between the final application and
harvest on all edible crops; in the U.S.A. this period applies to
cucurbits, but 21 days must elapse on all fruits, except apricots and
peaches which need 45 days and almonds where use is allowed up to the
'jacket stage'.
Post-harvest use
Use of dinocap post harvest does not arise on perennial crops.
Other uses
The widespread occurrence of powdery mildews on almost all
ornamentals, annual and perrenial, leads to widespread use of dinocap
by nurserymen as well as public and private gardeners throughout the
summer and early autumn. No use on animals is known.
RESIDUES RESULTING FROM SUPERVISED TRIALS
On apples, trials on two cultivars in two States of the U.S.A. showed
that, in the absence of heavy rain, the half-life of dinocap was 1.9
days over a range of initial deposits from at least 50 to 200 ppm.
Heavy rainfall was found to reduce deposits very considerably (Rohm
and Haas, 1958, unpublished).
Trials conducted by or for Rohm and Haas Company provide the following
results for deposits and residues on various crops:
Pre-harvest Deposit or residue
No. of Rate interval Range Average
Crop applications g/a.i./ha days ppm ppm
Apple 3 380 28 Nil Nil
5 570 0 0.26-0.57 0.41
21 <0.05
1 380 0 0.29-0.40 0.33
(cont'd)
Pre-harvest Deposit or residue
No. of Rate interval Range Average
Crop applications g/a.i./ha days ppm ppm
14 Nil-0.07 0.04
21 Nil-0.01 0.01
( 2 190 )
( 27 No detectable residue
(12 130 )
6 850 0 0.20-0.43 0.33
7 0.06-0.12 0.09
14 No detectable residue
Pear 4 510 5 0.04-0.20 0.11
21 No detectable residue
Apricot 2 380 0 0.35-0.70 0.55
21 No detectable residue
Apricot 2 0 Nil-0.15 0.08
(dried)
14 No detectable residue
Peach 1 255 0 0.17-0.85 0.54
21 No detectable residue
3 255 18 No detectable residue
Blackberry 3 760 44 0.05-0.07 0.06
Boysenberry 1 380 0 0.36-0.51 0.41
7 No detectable residue
Raspberry 3 95 10 No detectable residue
Grape 1 95 0 Nil-1.15 0.54
7 0.17-0.95 0.33
(cont'd)
Pre-harvest Deposit or residue
No. of Rate interval Range Average
Crop applications g/a.i./ha days ppm ppm
14 Nil-0.37 0.13
21 Nil-0.50 0.12
28 No detectable residue
( 1 180 ) 0 0.02-0.10 0.04
( )
( 2 86 ) 20 Nil-0.03 0.01
Grape (juice) ( 1 180 )
( ) 29 Nil-0.01 0.01
( 2 86 )
Strawberry 3 380 0 0.81-1.43 1.02
10 0.08-0.15 0.11
21 No detectable residue
Cantaloup 18 130 3 (Pulp)Nil-0.08 0.027
Honeydew melon 18 130 3 (Pulp)No detectable residue
Cucumber ( 2 95 )
( ) 0 No detectable residue
( 1 130 )
Pumpkin 5 130 4 hours (Pulp)0.20-0.34 0.24
(whole)Nil-0.60 0.24
Squash 4 95 0 Nil-0.15 0.08
3 No detectable residue
Muskmelon ( 9 130 ) (Pulp)0.00-0.10 0.02
( ) 0 (Rind)0.00-0.10 0.04
( 5 195 )
FATE OF RESIDUES
The short half-life on foliage, approximately two days, already
mentioned means that dinocap readily disappears from leaves. A longer
half-life, about four days, on strawberries was found by Kilgore and
Cheng (1963) who made a single application 21 days before harvest.
Nothing has been found in the literature concerning breakdown
products.
METHODS OF RESIDUE ANALYSIS
Several colorimetric methods sensitive to about 0.1 ppm have been
proposed for the determination of residues of dinocap in apples,
strawberries, grapes, tomatoes and animal tissue (Rosenthal et al.,
1957; Skerrett and Baker, 1962; Kilgore and Cheng, 1963). However,
with the fuller recognition of the relative importance of the various
components of commercial formulations of dinocap (Clifford et al.,
1965; Kirby and Elvidge, 1965; Kirby and Hunter, 1965) a gas
chromatographic procedure to likely to prove more generally useful.
Clifford and Watkins (1968) have described suitable gas
chromatographic conditions for the examination of a range of
dinitroalkyl phenols. Boggs (1966) and Yip and Howard (1968) preferred
to convert the phenols to methyl others before gas chromatography with
electron capture detection. No complete extraction, clean-up and gas
chromatographic determination procedure can be recommended for
residues of the active ingredients of dinocap at the present time but
the development and establishment of such a procedure is recommended.
APPRAISAL
Dinocap is defined B.S. 1831:1969 as "a mixture of dinocap-4 and
dinocap-6"; it is a mixture of six isomeric dinitro-s-octylphenyl
crotonates, not merely one of them as previously defined (B.S.
1831:1965), The commercial "active material" also contains a small
percentage of unesterified dinitro-s-octylphenols, together with
mononitrophenols and some 20% of "inactive" ingredients.
Commercial preparations, both wettable powders and liquid
formulations, are used throughout the world for the control of powdery
mildews of apple, pear, peach, apricot, grape, soft fruits, cucurbits,
rose and other ornamentals. It is also a non-ovicidal acaricide and
its use for control of powdery mildews also provides control of
certain mites, notably the fruit tree red spider mite (European red
mite) as long as spraying is continued. Curative (not eradicant)
action against powdery mildews is nearly as good as protective action,
but there is no evidence of systemic activity even within the leaf.
Solubility in water is low, but persistence on plant surfaces
(half-life) is very short. Deposits from recommended spray rates
rarely reach 1 ppm on the day of application, and residues of the
order of 0.05 ppm are to be expected within 7 days.
Several colorimetric procedures have been used for dinocap residues,
but in view of the complex nature of the product, gas chromatographic
procedures are likely to give much more valuable information.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
The data were insufficient to enable recommendations to be made.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Additional studies with more animals to establish the exact dosage
of dinocap which does not produce cataracts.
2. Extension of the chronic toxicity experiment in dogs or other
non-rodent mammalian species.
3. Metabolic studies including determination of phenols in the blood.
4. Information on the compounds included in dinocap and assurance of a
standardized technical product.
5. Investigation aimed at clarifying physiological lesions with regard
to cellular respiratory control.
6. Reproduction studies in animals.
7. Information is required on the nature of terminal residues,
including the identity of the substances giving peaks by the GLC
method of examination.
8. Residue data are required from countries other than the U.S.A.
DESIRABLE
The development and establishment of a GLC method for the
determination of residues of the active ingredients.
REFERENCES
Boggs, H. (1966) Gas chromatography of dinitro herbicides. J. Assoc.
Offic. Anal. Chem. 49:772-3
Cervenka, H. and Kay, J.H. (1963) Cataractogenic studies. Unpublished
report from Industrial Bio-test Laboratories Inc., to Niagara Chemical
Division, FMC Corporation. Submitted by Farbwerke Hoechst AG.
Clifford, D.R., Watkins, D.A.M. and Woodcock, D. (1965) Composition
of commercial dinocap preparations. Chemy Ind., pp.1654-5
Clifford, D.R. and Watkins, D.A.M. (1968) The gas chromatography of
dinitroalkyl phenols. J. Gas Chromatog., 6:191-2
Hemker, H.C. (1962) Lipid solubility as a factor influencing the
activity of uncoupling phenols. Biochim. Biophys. Acta, 63:46-54
Kilgore, W.W. and Cheng, K.W. (1963) Extraction and determination of
Karathane residues in fruits. J. agric. Fd Chem., 11:477-79
Kirby, A.H.M. and Elvidge, J.A. (1965) Composition of commercial
dinocap preparations. Chemy Ind., 2103
Kirby, A.H.M. and Hunter, L.D. (1965) Identification of
dinitro-octylphenols in certain commercial fungicides. Nature,
208:189-90
Kirby, A.H.M. (1969) Fungicides for deciduous top fruit: a survey in
1968. Wd. Rev. Pest Central. 8:45-58
Larson, P.S., Finnegan, J.K., Smith, R.B. Jr., Haag. H.B., Hennigar,
G.R. and Patterson, W.M. (1959) Acute and chronic toxicity studies on
2,4-dinitro-6-(l-methylheptyl) phenyl crotonate (Karathane).
Arch. int. Pharmacodyn., 119:31-42
Rohm and Haas. (1969) Karathane: chemical description, registered uses
(labels), residue studies. Rohm and Haas Company, Philadelphia,
Penn., U.S.A.
Rosenthal, I., Gordon, C.F., Stanley, E.L. and Perlman, M.H.
(1957) Microdetermination of the fungicide
dinitrocapryl-phenylcrotonate in food crops and animal tissues.
J. Agr. Fd Chem., 5:914-18
Skerrett, E.J. and Baker, E.A. (1962) The determination of spray
residues of 'Karathane' 87:228-9
Swisher, E.M. (1969) Report of a study on cataract formation in ducks
conducted in 1966 at the Medical College of Virginia. Unpublished
information submitted by Rohm and Haas Co.
Yip, G. and Howard, S.F. (1968) Extraction and clean-up procedure
for the gas chromatographic determination of four dinitrophenolic
pesticides. J. Assoc. Offic. Anal, Chem., 51:24-28
 Other relevant chemical properties
Technical dinocap is a dark red, viscous liquid with very slight
solubility in water but good solubility in the usual organic solvents.
Information on vapour pressure is not available, but persistence on
surfaces is shorter than that of binapacryl.
Incomplete esterification in the manufacturing process leads to the
presence of the free phenols corresponding to the six esters mentioned
above; these are reported (Rohm and Haas, 1969) to form 5 to 6 per
cent of the technical product. A smaller proportion, less than 1 per
cent, consists of mono-nitrophenols. These three classes together form
about 80 per cent of the technical product. Of the remainder, about 4
per cent consists of a mixture of octenes, formed by dehydration of
the starting material, capryl alcohol, and another 2 per cent is
crotonic acid. There are also four unknowns that produce peaks on the
G.L.C. chart, totalling 2 to 3 per cent, and 10 to 12 per cent of the
technical product in 'not volatile'.
Formulations include wettable powders usually claimed to contain 25
per cent active ingredients; Karathane(R) W.P. is now claimed to
contain 19.5 per cent active ingredients; no further information is
available concerning Crotothane(R) W.P. Another commercial formulation
is a liquid concentrate; Karathane LC was formerly claimed to contain
48 per cent active ingredients, but is now claimed to contain 39 per
cent ; Liquid Crotothane is sold an containing 50 per cent active
ingredients. There are also dusts containing less than 10 per cent
a.i.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
No residues of dinocap were detected in the subcutaneous and
intraperitoneal fat obtained from an unspecified number of rats fed
500 ppm of dinocap in their diet for one month. The method of analysis
was sensitive to 1 ppm (Larson et al., 1959).
Effect on enzymes and other biochemical parameters
Dinocap was compared with 2,4-dinitrophenol in oxygen consumption
studies in rats. Six rats (four males and two females) were given a
single oral dose of 600 mg/kg body-weight of dinocap. Measurements
made at various intervals showed that a steady increase in oxygen
consumption occurred in the females, which reached a maximum of 63 per
cent of the zero-time level after 24 hours. Measurements made on two
of these animals after 48 hours showed that the level had fallen to 29
per cent. No increase in oxygen consumption occurred during 24 hours
in the males fed dinocap. A comparable study with six other rats (two
males and four females) given a single oral dose of 40 mg/kg
body-weight of 2,4-dinitrophenol resulted in a maximum increase in
oxygen consumption of 116 per cent for the females and 90 per cent for
the males after three hours, followed by a gradual decrease after that
time. Thus, dinocap produced an increase in oxygen consumption in
female rats only, this effect being of longer duration although lower
in magnitude than the effect which occurred in both sexes with
2,4-dinitrophenol (Larson et al., 1959).
The compound 4-isooctyl-2,6-dinitrophenol (a constituent of technical
dinocap) was found to be 7 to 25 times more potent than
2,4-dinitrophenol in stimulation of respiration of rat-liver
mitochondria. The author concluded that the pKa and the lipid
solubility of the compounds as well as the pH of the media were the
factors influencing this activity, and that there was no intrinsic
structure-activity relationship relative to the size of the octyl
group (Hemker, 1962).
TOXICOLOGICAL STUDIES
Special studies on cataract formation
Chicken
Chicks were fed dietary levels of dinocap or binapacryl which were
equivalent on a molar basis to levels of 500 or 1000 ppm of
2,4-dinitrophenol. No lens opacities resulted from 17 to 28 days
administration of either of these compounds at these levels. Feeding
2,4-dinitrophenol at 2000 ppm under the same conditions also did not
result in the formation of lens opacities. However, when the dietary
level of dinocap or binapacryl was increased to 2000 ppm molar
equivalent to dinitrophenol a distinct incidence of cataracts
occurred. When fed at 4000 ppm absolute dietary level no difference
could be observed between dinocap and binapacryl with regard to
incidence of cataract formation (Cervenka and Kay, 1963).
Duck
Dinocap was compared with 2,4-dinitrophenol in studies to determine if
cataracts were formed in ducks. Three series of studies were conducted
using the technical grade dinocap throughout.
In the first experiment groups comprising 10 ducklings, each of about
10 days of age, were fed dietary levels of 0, 50, 250, or 2500 ppm of
dinocap or 0, 25, 125, 250 or 1250 ppm of 2,4-dinitrophenol for 12
weeks. Survival was adversely affected at the 500 and 2500 ppm levels
of dinocap and the 1250 ppm level of 2,4-dinitrophenol. Growth was
depressed at 250 ppm and higher concentrations of dinocap and at all
concentrations of 2,4-dinitrophenol. The 1250 ppm level of
2,4-dinitrophenol produced cataracts within 24 hours, and all other
levels both of dinocap and 2,4-dinitrophenol produced cataracts within
seven weeks. No cataracts occurred in the controls. After the
cataracts had developed, withdrawal of the substances from the diet
for a five-week period did not result in regression of the cataracts
(Larson et al., 1959).
In the second experiment, groups each containing 10 ducklings,
received diets containing 0, 2, 5, 10 or 25 ppm of dinocap or 0, 25,
125, 250 or 1250 ppm of 2,4-dinitrophenol. Most of the ducks including
all of the controls developed cataracts by the end of seven weeks and
the test series was discarded. A conclusion was drawn that cataracts
had developed from some unknown cause (Larson et al., 1959).
The third experiment was conducted using the same conditions as have
been described for the second. Ophthalmologic examinations made after
5, 9 and 13 weeks on the test diet showed no cataracts in any treated
group (Larson et al., 1959).
In a more recent study, ducklings were fed 250 ppm of
dinitrooctylphenol (the isomer or mixtures thereof not specified) for
13 weeks and none developed cataracts. When 2,4-dinitrophenol was also
fed as a positive control to ducklings, cataracts developed in all
cases. The explanation of the erratic results from the earlier
cataract studies with dinocap has been suggested to be at least partly
related to the variation in composition of the technical product
relative to the presence of significant impurities. There may,
however, be other explanations (Swisher, 1969).
Acute toxicity
LD50
(technical grade*)
Animal Route mg/kg body-weight Reference
Rat (M) oral 980 Larson et al., 1959
Rat (M) iv. 23 Larson et al., 1959
Rat (F) oral 1190 Larson et al., 1959
Rabbit (M) oral 2000 Larson et al., 1959
(cont'd)
LD50
(technical grade*)
Animal Route mg/kg body-weight Reference
Dog oral 100 Larson et al., 1959
* Technical grade is here defined as:
2,4-dinitro-6-(2-octyl)-phenyl crotonate
2,4-dinitro-6-(3-octyl)-phenyl crotonate
2,4-dinitro-6-(4-octyl)-phenyl crotonate ....... 73 per cent
2,6-dinitro-4-(2-octyl)-phenyl crotonate
2,6-dinitro-4-(3-octyl)-phenyl crotonate
2,6-dinitro-4-(4-octyl)-phenyl crotonate
nitrocctylphenols (principally dinitro-) ....... 5 per cent
inert ingredients (octenes, crotonic
acid and related compounds) ................... 22 per cent
Short-term studies
Dog
Groups, each containing three dogs of unspecified sex and age, were
fed diets containing 10, 50, 100, 250 and 1000 ppm of technical
dinocap for one year. One dog in the 250 ppm group died within six
weeks, another was sacrificed after marked weight loss and the third
was transferred to a control diet. Two dogs in the 1000 ppm group died
within six weeks and the third was transferred to the control diet.
(No other control group was reported to have been used). Decreased
appetite and drastic weight loss preceded death. Moderate weight loss
was evident at the 100 ppm level but not at 50 ppm. Hepatic necrosis
occurred in the dogs fed 250 and 1000 ppm. Haematologic values were
normal at all dose-levels (Larson et al., 1959).
Rat
Groups containing 10 weanling rats of each sex were fed diets
containing 0, 10, 50, 250, 1000 and 2500 ppm of technical dinocap for
six months. Growth and survival were reduced at the 2500 ppm level and
growth was reduced at 1000 ppm. Enlarged spleens occurred in the males
receiving 2500 ppm. Haematological and microscopic examinations
revealed no changes attributable to treatment (Larson et al., 1959).
Long-term studies
Rat
Groups containing 10 weanling rats of each sex were fed diets
containing 0, 10, 50, 250, 500 and 1000 ppm of technical dinocap for
two years. There was decreased weight gain during the first year only
in the male rats fed 1000 ppm, but the effect on bodyweight during the
second year was not reported. No other effect of treatment, either
gross or histopathological was noted at any of the dose-levels studied
(Larson et al., 1959).
OBSERVATIONS IN MAN
Patch tests were performed on the forearms of 50 human subjects using
dinocap formulated either as an emulsion or as a wettable powder.
Exposure was for 48 hours. Moderate irritation resulted from the
emulsion in 11 subjects and from the powder in three. Similar results
occurred when the opposite forearms were patched 12 days later, 25
subjects reacting to the emulsion and nine to the powder. Intensified
reactions resulted during succeeding days in three subjects (Larson et
al., 1959).
COMMENT
On the basis of animal data a no-effect level for rats has been
established. Since the chemical structure of 2,4-dinitrophenol is
similar to dinocap and because 2,4-dinitrophenol produces cataracts in
man, the possibility of cataract formation in man from dinocap is of
concern. For this reason dinocap should be used with great caution.
Inconclusive experiments in ducks gave a possible indication of
cataract formation. Additional studies with other species of animals
are needed to establish the exact dosage of dinocap which does not
produce cataracts. In addition, the data on the toxicology of dinocap
were obtained between 1954 and 1958, and are incomplete especially in
relation to metabolism. Skin sensitization is reported in human
subjects but no other data on man are available. The technical product
appears to be of variable composition.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Dinocap in a non-systematic fungicide; only its activity against
powdery mildews is of commercial importance. It also restricts the
development of populations of several tetranychid phytophagous mites,
and in view of the widespread resistance of such species to specific
acaricides, this role is very valuable.
Powdery mildews are important on apple, apricot and peach, and less so
on pear and cherry among deciduous fruits; dinocap has become the
leading fungicide for control on apple, apricot and pear, and second
only to sulphur on peach (Kirby, 1969). In the U.K., over 15,000
hectares of apples were sprayed with dinocap in 1967; a smaller area
of pears was also sprayed. For 10-14 day interval spraying, the usual
rate of use on apple in the U.K. is 2.24 kg of the wettable powder per
ha; according to the recent claim for Karathane WP, this is 0.44 kg
a.i, per ha. According to the U.S.A. manufacturers, U.S.A. regulations
allow up to 1.75 kg a.i. per ha to be applied on each occasion. In the
U.K., at least one week must elapse between the final application and
harvest on all edible crops; in the U.S.A. this period applies to
cucurbits, but 21 days must elapse on all fruits, except apricots and
peaches which need 45 days and almonds where use is allowed up to the
'jacket stage'.
Post-harvest use
Use of dinocap post harvest does not arise on perennial crops.
Other uses
The widespread occurrence of powdery mildews on almost all
ornamentals, annual and perrenial, leads to widespread use of dinocap
by nurserymen as well as public and private gardeners throughout the
summer and early autumn. No use on animals is known.
RESIDUES RESULTING FROM SUPERVISED TRIALS
On apples, trials on two cultivars in two States of the U.S.A. showed
that, in the absence of heavy rain, the half-life of dinocap was 1.9
days over a range of initial deposits from at least 50 to 200 ppm.
Heavy rainfall was found to reduce deposits very considerably (Rohm
and Haas, 1958, unpublished).
Trials conducted by or for Rohm and Haas Company provide the following
results for deposits and residues on various crops:
Pre-harvest Deposit or residue
No. of Rate interval Range Average
Crop applications g/a.i./ha days ppm ppm
Apple 3 380 28 Nil Nil
5 570 0 0.26-0.57 0.41
21 <0.05
1 380 0 0.29-0.40 0.33
(cont'd)
Pre-harvest Deposit or residue
No. of Rate interval Range Average
Crop applications g/a.i./ha days ppm ppm
14 Nil-0.07 0.04
21 Nil-0.01 0.01
( 2 190 )
( 27 No detectable residue
(12 130 )
6 850 0 0.20-0.43 0.33
7 0.06-0.12 0.09
14 No detectable residue
Pear 4 510 5 0.04-0.20 0.11
21 No detectable residue
Apricot 2 380 0 0.35-0.70 0.55
21 No detectable residue
Apricot 2 0 Nil-0.15 0.08
(dried)
14 No detectable residue
Peach 1 255 0 0.17-0.85 0.54
21 No detectable residue
3 255 18 No detectable residue
Blackberry 3 760 44 0.05-0.07 0.06
Boysenberry 1 380 0 0.36-0.51 0.41
7 No detectable residue
Raspberry 3 95 10 No detectable residue
Grape 1 95 0 Nil-1.15 0.54
7 0.17-0.95 0.33
(cont'd)
Pre-harvest Deposit or residue
No. of Rate interval Range Average
Crop applications g/a.i./ha days ppm ppm
14 Nil-0.37 0.13
21 Nil-0.50 0.12
28 No detectable residue
( 1 180 ) 0 0.02-0.10 0.04
( )
( 2 86 ) 20 Nil-0.03 0.01
Grape (juice) ( 1 180 )
( ) 29 Nil-0.01 0.01
( 2 86 )
Strawberry 3 380 0 0.81-1.43 1.02
10 0.08-0.15 0.11
21 No detectable residue
Cantaloup 18 130 3 (Pulp)Nil-0.08 0.027
Honeydew melon 18 130 3 (Pulp)No detectable residue
Cucumber ( 2 95 )
( ) 0 No detectable residue
( 1 130 )
Pumpkin 5 130 4 hours (Pulp)0.20-0.34 0.24
(whole)Nil-0.60 0.24
Squash 4 95 0 Nil-0.15 0.08
3 No detectable residue
Muskmelon ( 9 130 ) (Pulp)0.00-0.10 0.02
( ) 0 (Rind)0.00-0.10 0.04
( 5 195 )
FATE OF RESIDUES
The short half-life on foliage, approximately two days, already
mentioned means that dinocap readily disappears from leaves. A longer
half-life, about four days, on strawberries was found by Kilgore and
Cheng (1963) who made a single application 21 days before harvest.
Nothing has been found in the literature concerning breakdown
products.
METHODS OF RESIDUE ANALYSIS
Several colorimetric methods sensitive to about 0.1 ppm have been
proposed for the determination of residues of dinocap in apples,
strawberries, grapes, tomatoes and animal tissue (Rosenthal et al.,
1957; Skerrett and Baker, 1962; Kilgore and Cheng, 1963). However,
with the fuller recognition of the relative importance of the various
components of commercial formulations of dinocap (Clifford et al.,
1965; Kirby and Elvidge, 1965; Kirby and Hunter, 1965) a gas
chromatographic procedure to likely to prove more generally useful.
Clifford and Watkins (1968) have described suitable gas
chromatographic conditions for the examination of a range of
dinitroalkyl phenols. Boggs (1966) and Yip and Howard (1968) preferred
to convert the phenols to methyl others before gas chromatography with
electron capture detection. No complete extraction, clean-up and gas
chromatographic determination procedure can be recommended for
residues of the active ingredients of dinocap at the present time but
the development and establishment of such a procedure is recommended.
APPRAISAL
Dinocap is defined B.S. 1831:1969 as "a mixture of dinocap-4 and
dinocap-6"; it is a mixture of six isomeric dinitro-s-octylphenyl
crotonates, not merely one of them as previously defined (B.S.
1831:1965), The commercial "active material" also contains a small
percentage of unesterified dinitro-s-octylphenols, together with
mononitrophenols and some 20% of "inactive" ingredients.
Commercial preparations, both wettable powders and liquid
formulations, are used throughout the world for the control of powdery
mildews of apple, pear, peach, apricot, grape, soft fruits, cucurbits,
rose and other ornamentals. It is also a non-ovicidal acaricide and
its use for control of powdery mildews also provides control of
certain mites, notably the fruit tree red spider mite (European red
mite) as long as spraying is continued. Curative (not eradicant)
action against powdery mildews is nearly as good as protective action,
but there is no evidence of systemic activity even within the leaf.
Solubility in water is low, but persistence on plant surfaces
(half-life) is very short. Deposits from recommended spray rates
rarely reach 1 ppm on the day of application, and residues of the
order of 0.05 ppm are to be expected within 7 days.
Several colorimetric procedures have been used for dinocap residues,
but in view of the complex nature of the product, gas chromatographic
procedures are likely to give much more valuable information.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
The data were insufficient to enable recommendations to be made.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Additional studies with more animals to establish the exact dosage
of dinocap which does not produce cataracts.
2. Extension of the chronic toxicity experiment in dogs or other
non-rodent mammalian species.
3. Metabolic studies including determination of phenols in the blood.
4. Information on the compounds included in dinocap and assurance of a
standardized technical product.
5. Investigation aimed at clarifying physiological lesions with regard
to cellular respiratory control.
6. Reproduction studies in animals.
7. Information is required on the nature of terminal residues,
including the identity of the substances giving peaks by the GLC
method of examination.
8. Residue data are required from countries other than the U.S.A.
DESIRABLE
The development and establishment of a GLC method for the
determination of residues of the active ingredients.
REFERENCES
Boggs, H. (1966) Gas chromatography of dinitro herbicides. J. Assoc.
Offic. Anal. Chem. 49:772-3
Cervenka, H. and Kay, J.H. (1963) Cataractogenic studies. Unpublished
report from Industrial Bio-test Laboratories Inc., to Niagara Chemical
Division, FMC Corporation. Submitted by Farbwerke Hoechst AG.
Clifford, D.R., Watkins, D.A.M. and Woodcock, D. (1965) Composition
of commercial dinocap preparations. Chemy Ind., pp.1654-5
Clifford, D.R. and Watkins, D.A.M. (1968) The gas chromatography of
dinitroalkyl phenols. J. Gas Chromatog., 6:191-2
Hemker, H.C. (1962) Lipid solubility as a factor influencing the
activity of uncoupling phenols. Biochim. Biophys. Acta, 63:46-54
Kilgore, W.W. and Cheng, K.W. (1963) Extraction and determination of
Karathane residues in fruits. J. agric. Fd Chem., 11:477-79
Kirby, A.H.M. and Elvidge, J.A. (1965) Composition of commercial
dinocap preparations. Chemy Ind., 2103
Kirby, A.H.M. and Hunter, L.D. (1965) Identification of
dinitro-octylphenols in certain commercial fungicides. Nature,
208:189-90
Kirby, A.H.M. (1969) Fungicides for deciduous top fruit: a survey in
1968. Wd. Rev. Pest Central. 8:45-58
Larson, P.S., Finnegan, J.K., Smith, R.B. Jr., Haag. H.B., Hennigar,
G.R. and Patterson, W.M. (1959) Acute and chronic toxicity studies on
2,4-dinitro-6-(l-methylheptyl) phenyl crotonate (Karathane).
Arch. int. Pharmacodyn., 119:31-42
Rohm and Haas. (1969) Karathane: chemical description, registered uses
(labels), residue studies. Rohm and Haas Company, Philadelphia,
Penn., U.S.A.
Rosenthal, I., Gordon, C.F., Stanley, E.L. and Perlman, M.H.
(1957) Microdetermination of the fungicide
dinitrocapryl-phenylcrotonate in food crops and animal tissues.
J. Agr. Fd Chem., 5:914-18
Skerrett, E.J. and Baker, E.A. (1962) The determination of spray
residues of 'Karathane' 87:228-9
Swisher, E.M. (1969) Report of a study on cataract formation in ducks
conducted in 1966 at the Medical College of Virginia. Unpublished
information submitted by Rohm and Haas Co.
Yip, G. and Howard, S.F. (1968) Extraction and clean-up procedure
for the gas chromatographic determination of four dinitrophenolic
pesticides. J. Assoc. Offic. Anal, Chem., 51:24-28
Other relevant chemical properties
Technical dinocap is a dark red, viscous liquid with very slight
solubility in water but good solubility in the usual organic solvents.
Information on vapour pressure is not available, but persistence on
surfaces is shorter than that of binapacryl.
Incomplete esterification in the manufacturing process leads to the
presence of the free phenols corresponding to the six esters mentioned
above; these are reported (Rohm and Haas, 1969) to form 5 to 6 per
cent of the technical product. A smaller proportion, less than 1 per
cent, consists of mono-nitrophenols. These three classes together form
about 80 per cent of the technical product. Of the remainder, about 4
per cent consists of a mixture of octenes, formed by dehydration of
the starting material, capryl alcohol, and another 2 per cent is
crotonic acid. There are also four unknowns that produce peaks on the
G.L.C. chart, totalling 2 to 3 per cent, and 10 to 12 per cent of the
technical product in 'not volatile'.
Formulations include wettable powders usually claimed to contain 25
per cent active ingredients; Karathane(R) W.P. is now claimed to
contain 19.5 per cent active ingredients; no further information is
available concerning Crotothane(R) W.P. Another commercial formulation
is a liquid concentrate; Karathane LC was formerly claimed to contain
48 per cent active ingredients, but is now claimed to contain 39 per
cent ; Liquid Crotothane is sold an containing 50 per cent active
ingredients. There are also dusts containing less than 10 per cent
a.i.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
No residues of dinocap were detected in the subcutaneous and
intraperitoneal fat obtained from an unspecified number of rats fed
500 ppm of dinocap in their diet for one month. The method of analysis
was sensitive to 1 ppm (Larson et al., 1959).
Effect on enzymes and other biochemical parameters
Dinocap was compared with 2,4-dinitrophenol in oxygen consumption
studies in rats. Six rats (four males and two females) were given a
single oral dose of 600 mg/kg body-weight of dinocap. Measurements
made at various intervals showed that a steady increase in oxygen
consumption occurred in the females, which reached a maximum of 63 per
cent of the zero-time level after 24 hours. Measurements made on two
of these animals after 48 hours showed that the level had fallen to 29
per cent. No increase in oxygen consumption occurred during 24 hours
in the males fed dinocap. A comparable study with six other rats (two
males and four females) given a single oral dose of 40 mg/kg
body-weight of 2,4-dinitrophenol resulted in a maximum increase in
oxygen consumption of 116 per cent for the females and 90 per cent for
the males after three hours, followed by a gradual decrease after that
time. Thus, dinocap produced an increase in oxygen consumption in
female rats only, this effect being of longer duration although lower
in magnitude than the effect which occurred in both sexes with
2,4-dinitrophenol (Larson et al., 1959).
The compound 4-isooctyl-2,6-dinitrophenol (a constituent of technical
dinocap) was found to be 7 to 25 times more potent than
2,4-dinitrophenol in stimulation of respiration of rat-liver
mitochondria. The author concluded that the pKa and the lipid
solubility of the compounds as well as the pH of the media were the
factors influencing this activity, and that there was no intrinsic
structure-activity relationship relative to the size of the octyl
group (Hemker, 1962).
TOXICOLOGICAL STUDIES
Special studies on cataract formation
Chicken
Chicks were fed dietary levels of dinocap or binapacryl which were
equivalent on a molar basis to levels of 500 or 1000 ppm of
2,4-dinitrophenol. No lens opacities resulted from 17 to 28 days
administration of either of these compounds at these levels. Feeding
2,4-dinitrophenol at 2000 ppm under the same conditions also did not
result in the formation of lens opacities. However, when the dietary
level of dinocap or binapacryl was increased to 2000 ppm molar
equivalent to dinitrophenol a distinct incidence of cataracts
occurred. When fed at 4000 ppm absolute dietary level no difference
could be observed between dinocap and binapacryl with regard to
incidence of cataract formation (Cervenka and Kay, 1963).
Duck
Dinocap was compared with 2,4-dinitrophenol in studies to determine if
cataracts were formed in ducks. Three series of studies were conducted
using the technical grade dinocap throughout.
In the first experiment groups comprising 10 ducklings, each of about
10 days of age, were fed dietary levels of 0, 50, 250, or 2500 ppm of
dinocap or 0, 25, 125, 250 or 1250 ppm of 2,4-dinitrophenol for 12
weeks. Survival was adversely affected at the 500 and 2500 ppm levels
of dinocap and the 1250 ppm level of 2,4-dinitrophenol. Growth was
depressed at 250 ppm and higher concentrations of dinocap and at all
concentrations of 2,4-dinitrophenol. The 1250 ppm level of
2,4-dinitrophenol produced cataracts within 24 hours, and all other
levels both of dinocap and 2,4-dinitrophenol produced cataracts within
seven weeks. No cataracts occurred in the controls. After the
cataracts had developed, withdrawal of the substances from the diet
for a five-week period did not result in regression of the cataracts
(Larson et al., 1959).
In the second experiment, groups each containing 10 ducklings,
received diets containing 0, 2, 5, 10 or 25 ppm of dinocap or 0, 25,
125, 250 or 1250 ppm of 2,4-dinitrophenol. Most of the ducks including
all of the controls developed cataracts by the end of seven weeks and
the test series was discarded. A conclusion was drawn that cataracts
had developed from some unknown cause (Larson et al., 1959).
The third experiment was conducted using the same conditions as have
been described for the second. Ophthalmologic examinations made after
5, 9 and 13 weeks on the test diet showed no cataracts in any treated
group (Larson et al., 1959).
In a more recent study, ducklings were fed 250 ppm of
dinitrooctylphenol (the isomer or mixtures thereof not specified) for
13 weeks and none developed cataracts. When 2,4-dinitrophenol was also
fed as a positive control to ducklings, cataracts developed in all
cases. The explanation of the erratic results from the earlier
cataract studies with dinocap has been suggested to be at least partly
related to the variation in composition of the technical product
relative to the presence of significant impurities. There may,
however, be other explanations (Swisher, 1969).
Acute toxicity
LD50
(technical grade*)
Animal Route mg/kg body-weight Reference
Rat (M) oral 980 Larson et al., 1959
Rat (M) iv. 23 Larson et al., 1959
Rat (F) oral 1190 Larson et al., 1959
Rabbit (M) oral 2000 Larson et al., 1959
(cont'd)
LD50
(technical grade*)
Animal Route mg/kg body-weight Reference
Dog oral 100 Larson et al., 1959
* Technical grade is here defined as:
2,4-dinitro-6-(2-octyl)-phenyl crotonate
2,4-dinitro-6-(3-octyl)-phenyl crotonate
2,4-dinitro-6-(4-octyl)-phenyl crotonate ....... 73 per cent
2,6-dinitro-4-(2-octyl)-phenyl crotonate
2,6-dinitro-4-(3-octyl)-phenyl crotonate
2,6-dinitro-4-(4-octyl)-phenyl crotonate
nitrocctylphenols (principally dinitro-) ....... 5 per cent
inert ingredients (octenes, crotonic
acid and related compounds) ................... 22 per cent
Short-term studies
Dog
Groups, each containing three dogs of unspecified sex and age, were
fed diets containing 10, 50, 100, 250 and 1000 ppm of technical
dinocap for one year. One dog in the 250 ppm group died within six
weeks, another was sacrificed after marked weight loss and the third
was transferred to a control diet. Two dogs in the 1000 ppm group died
within six weeks and the third was transferred to the control diet.
(No other control group was reported to have been used). Decreased
appetite and drastic weight loss preceded death. Moderate weight loss
was evident at the 100 ppm level but not at 50 ppm. Hepatic necrosis
occurred in the dogs fed 250 and 1000 ppm. Haematologic values were
normal at all dose-levels (Larson et al., 1959).
Rat
Groups containing 10 weanling rats of each sex were fed diets
containing 0, 10, 50, 250, 1000 and 2500 ppm of technical dinocap for
six months. Growth and survival were reduced at the 2500 ppm level and
growth was reduced at 1000 ppm. Enlarged spleens occurred in the males
receiving 2500 ppm. Haematological and microscopic examinations
revealed no changes attributable to treatment (Larson et al., 1959).
Long-term studies
Rat
Groups containing 10 weanling rats of each sex were fed diets
containing 0, 10, 50, 250, 500 and 1000 ppm of technical dinocap for
two years. There was decreased weight gain during the first year only
in the male rats fed 1000 ppm, but the effect on bodyweight during the
second year was not reported. No other effect of treatment, either
gross or histopathological was noted at any of the dose-levels studied
(Larson et al., 1959).
OBSERVATIONS IN MAN
Patch tests were performed on the forearms of 50 human subjects using
dinocap formulated either as an emulsion or as a wettable powder.
Exposure was for 48 hours. Moderate irritation resulted from the
emulsion in 11 subjects and from the powder in three. Similar results
occurred when the opposite forearms were patched 12 days later, 25
subjects reacting to the emulsion and nine to the powder. Intensified
reactions resulted during succeeding days in three subjects (Larson et
al., 1959).
COMMENT
On the basis of animal data a no-effect level for rats has been
established. Since the chemical structure of 2,4-dinitrophenol is
similar to dinocap and because 2,4-dinitrophenol produces cataracts in
man, the possibility of cataract formation in man from dinocap is of
concern. For this reason dinocap should be used with great caution.
Inconclusive experiments in ducks gave a possible indication of
cataract formation. Additional studies with other species of animals
are needed to establish the exact dosage of dinocap which does not
produce cataracts. In addition, the data on the toxicology of dinocap
were obtained between 1954 and 1958, and are incomplete especially in
relation to metabolism. Skin sensitization is reported in human
subjects but no other data on man are available. The technical product
appears to be of variable composition.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Dinocap in a non-systematic fungicide; only its activity against
powdery mildews is of commercial importance. It also restricts the
development of populations of several tetranychid phytophagous mites,
and in view of the widespread resistance of such species to specific
acaricides, this role is very valuable.
Powdery mildews are important on apple, apricot and peach, and less so
on pear and cherry among deciduous fruits; dinocap has become the
leading fungicide for control on apple, apricot and pear, and second
only to sulphur on peach (Kirby, 1969). In the U.K., over 15,000
hectares of apples were sprayed with dinocap in 1967; a smaller area
of pears was also sprayed. For 10-14 day interval spraying, the usual
rate of use on apple in the U.K. is 2.24 kg of the wettable powder per
ha; according to the recent claim for Karathane WP, this is 0.44 kg
a.i, per ha. According to the U.S.A. manufacturers, U.S.A. regulations
allow up to 1.75 kg a.i. per ha to be applied on each occasion. In the
U.K., at least one week must elapse between the final application and
harvest on all edible crops; in the U.S.A. this period applies to
cucurbits, but 21 days must elapse on all fruits, except apricots and
peaches which need 45 days and almonds where use is allowed up to the
'jacket stage'.
Post-harvest use
Use of dinocap post harvest does not arise on perennial crops.
Other uses
The widespread occurrence of powdery mildews on almost all
ornamentals, annual and perrenial, leads to widespread use of dinocap
by nurserymen as well as public and private gardeners throughout the
summer and early autumn. No use on animals is known.
RESIDUES RESULTING FROM SUPERVISED TRIALS
On apples, trials on two cultivars in two States of the U.S.A. showed
that, in the absence of heavy rain, the half-life of dinocap was 1.9
days over a range of initial deposits from at least 50 to 200 ppm.
Heavy rainfall was found to reduce deposits very considerably (Rohm
and Haas, 1958, unpublished).
Trials conducted by or for Rohm and Haas Company provide the following
results for deposits and residues on various crops:
Pre-harvest Deposit or residue
No. of Rate interval Range Average
Crop applications g/a.i./ha days ppm ppm
Apple 3 380 28 Nil Nil
5 570 0 0.26-0.57 0.41
21 <0.05
1 380 0 0.29-0.40 0.33
(cont'd)
Pre-harvest Deposit or residue
No. of Rate interval Range Average
Crop applications g/a.i./ha days ppm ppm
14 Nil-0.07 0.04
21 Nil-0.01 0.01
( 2 190 )
( 27 No detectable residue
(12 130 )
6 850 0 0.20-0.43 0.33
7 0.06-0.12 0.09
14 No detectable residue
Pear 4 510 5 0.04-0.20 0.11
21 No detectable residue
Apricot 2 380 0 0.35-0.70 0.55
21 No detectable residue
Apricot 2 0 Nil-0.15 0.08
(dried)
14 No detectable residue
Peach 1 255 0 0.17-0.85 0.54
21 No detectable residue
3 255 18 No detectable residue
Blackberry 3 760 44 0.05-0.07 0.06
Boysenberry 1 380 0 0.36-0.51 0.41
7 No detectable residue
Raspberry 3 95 10 No detectable residue
Grape 1 95 0 Nil-1.15 0.54
7 0.17-0.95 0.33
(cont'd)
Pre-harvest Deposit or residue
No. of Rate interval Range Average
Crop applications g/a.i./ha days ppm ppm
14 Nil-0.37 0.13
21 Nil-0.50 0.12
28 No detectable residue
( 1 180 ) 0 0.02-0.10 0.04
( )
( 2 86 ) 20 Nil-0.03 0.01
Grape (juice) ( 1 180 )
( ) 29 Nil-0.01 0.01
( 2 86 )
Strawberry 3 380 0 0.81-1.43 1.02
10 0.08-0.15 0.11
21 No detectable residue
Cantaloup 18 130 3 (Pulp)Nil-0.08 0.027
Honeydew melon 18 130 3 (Pulp)No detectable residue
Cucumber ( 2 95 )
( ) 0 No detectable residue
( 1 130 )
Pumpkin 5 130 4 hours (Pulp)0.20-0.34 0.24
(whole)Nil-0.60 0.24
Squash 4 95 0 Nil-0.15 0.08
3 No detectable residue
Muskmelon ( 9 130 ) (Pulp)0.00-0.10 0.02
( ) 0 (Rind)0.00-0.10 0.04
( 5 195 )
FATE OF RESIDUES
The short half-life on foliage, approximately two days, already
mentioned means that dinocap readily disappears from leaves. A longer
half-life, about four days, on strawberries was found by Kilgore and
Cheng (1963) who made a single application 21 days before harvest.
Nothing has been found in the literature concerning breakdown
products.
METHODS OF RESIDUE ANALYSIS
Several colorimetric methods sensitive to about 0.1 ppm have been
proposed for the determination of residues of dinocap in apples,
strawberries, grapes, tomatoes and animal tissue (Rosenthal et al.,
1957; Skerrett and Baker, 1962; Kilgore and Cheng, 1963). However,
with the fuller recognition of the relative importance of the various
components of commercial formulations of dinocap (Clifford et al.,
1965; Kirby and Elvidge, 1965; Kirby and Hunter, 1965) a gas
chromatographic procedure to likely to prove more generally useful.
Clifford and Watkins (1968) have described suitable gas
chromatographic conditions for the examination of a range of
dinitroalkyl phenols. Boggs (1966) and Yip and Howard (1968) preferred
to convert the phenols to methyl others before gas chromatography with
electron capture detection. No complete extraction, clean-up and gas
chromatographic determination procedure can be recommended for
residues of the active ingredients of dinocap at the present time but
the development and establishment of such a procedure is recommended.
APPRAISAL
Dinocap is defined B.S. 1831:1969 as "a mixture of dinocap-4 and
dinocap-6"; it is a mixture of six isomeric dinitro-s-octylphenyl
crotonates, not merely one of them as previously defined (B.S.
1831:1965), The commercial "active material" also contains a small
percentage of unesterified dinitro-s-octylphenols, together with
mononitrophenols and some 20% of "inactive" ingredients.
Commercial preparations, both wettable powders and liquid
formulations, are used throughout the world for the control of powdery
mildews of apple, pear, peach, apricot, grape, soft fruits, cucurbits,
rose and other ornamentals. It is also a non-ovicidal acaricide and
its use for control of powdery mildews also provides control of
certain mites, notably the fruit tree red spider mite (European red
mite) as long as spraying is continued. Curative (not eradicant)
action against powdery mildews is nearly as good as protective action,
but there is no evidence of systemic activity even within the leaf.
Solubility in water is low, but persistence on plant surfaces
(half-life) is very short. Deposits from recommended spray rates
rarely reach 1 ppm on the day of application, and residues of the
order of 0.05 ppm are to be expected within 7 days.
Several colorimetric procedures have been used for dinocap residues,
but in view of the complex nature of the product, gas chromatographic
procedures are likely to give much more valuable information.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
The data were insufficient to enable recommendations to be made.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Additional studies with more animals to establish the exact dosage
of dinocap which does not produce cataracts.
2. Extension of the chronic toxicity experiment in dogs or other
non-rodent mammalian species.
3. Metabolic studies including determination of phenols in the blood.
4. Information on the compounds included in dinocap and assurance of a
standardized technical product.
5. Investigation aimed at clarifying physiological lesions with regard
to cellular respiratory control.
6. Reproduction studies in animals.
7. Information is required on the nature of terminal residues,
including the identity of the substances giving peaks by the GLC
method of examination.
8. Residue data are required from countries other than the U.S.A.
DESIRABLE
The development and establishment of a GLC method for the
determination of residues of the active ingredients.
REFERENCES
Boggs, H. (1966) Gas chromatography of dinitro herbicides. J. Assoc.
Offic. Anal. Chem. 49:772-3
Cervenka, H. and Kay, J.H. (1963) Cataractogenic studies. Unpublished
report from Industrial Bio-test Laboratories Inc., to Niagara Chemical
Division, FMC Corporation. Submitted by Farbwerke Hoechst AG.
Clifford, D.R., Watkins, D.A.M. and Woodcock, D. (1965) Composition
of commercial dinocap preparations. Chemy Ind., pp.1654-5
Clifford, D.R. and Watkins, D.A.M. (1968) The gas chromatography of
dinitroalkyl phenols. J. Gas Chromatog., 6:191-2
Hemker, H.C. (1962) Lipid solubility as a factor influencing the
activity of uncoupling phenols. Biochim. Biophys. Acta, 63:46-54
Kilgore, W.W. and Cheng, K.W. (1963) Extraction and determination of
Karathane residues in fruits. J. agric. Fd Chem., 11:477-79
Kirby, A.H.M. and Elvidge, J.A. (1965) Composition of commercial
dinocap preparations. Chemy Ind., 2103
Kirby, A.H.M. and Hunter, L.D. (1965) Identification of
dinitro-octylphenols in certain commercial fungicides. Nature,
208:189-90
Kirby, A.H.M. (1969) Fungicides for deciduous top fruit: a survey in
1968. Wd. Rev. Pest Central. 8:45-58
Larson, P.S., Finnegan, J.K., Smith, R.B. Jr., Haag. H.B., Hennigar,
G.R. and Patterson, W.M. (1959) Acute and chronic toxicity studies on
2,4-dinitro-6-(l-methylheptyl) phenyl crotonate (Karathane).
Arch. int. Pharmacodyn., 119:31-42
Rohm and Haas. (1969) Karathane: chemical description, registered uses
(labels), residue studies. Rohm and Haas Company, Philadelphia,
Penn., U.S.A.
Rosenthal, I., Gordon, C.F., Stanley, E.L. and Perlman, M.H.
(1957) Microdetermination of the fungicide
dinitrocapryl-phenylcrotonate in food crops and animal tissues.
J. Agr. Fd Chem., 5:914-18
Skerrett, E.J. and Baker, E.A. (1962) The determination of spray
residues of 'Karathane' 87:228-9
Swisher, E.M. (1969) Report of a study on cataract formation in ducks
conducted in 1966 at the Medical College of Virginia. Unpublished
information submitted by Rohm and Haas Co.
Yip, G. and Howard, S.F. (1968) Extraction and clean-up procedure
for the gas chromatographic determination of four dinitrophenolic
pesticides. J. Assoc. Offic. Anal, Chem., 51:24-28
