
DINOCAP JMPR 1998
First draft prepared by
S.F.P. Warren
Pesticides Safety Directorate, Ministry of Agriculture, Fisheries and
Food,
Mallard House, Kings Pool, York, United Kingdom
Explanation
Evaluation for acceptable daily intake
Biological data
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies: Ocular toxicity
Comments
Toxicological evaluation
References
Explanation
Dinocap was evaluated by the JMPR in 1969, 1974, and 1989 (Annex
1, references 12, 22, 1nd 56). An ADI of 0-0.001 mg/kg bw was
allocated in 1989 on the basis of a NOAEL of 0.5 mg/kg bw per day in a
study of developmental toxicity in rabbits and a safety factor of 500.
At the present Meeting recent data on material of greater purity than
that tested previously were evaluated. Dinocap consists of 2,4- and
2,6-dinitro-octylphenyl crotonates in which the octyl moiety is either
1-methylheptyl, 1-ethylhexyl, or 1-propylpentyl. A number of the
studies that were reviewed were performed with the methylheptyl isomer
used as a model for dinocap.
Evaluation for Acceptable Daily Intake
1. Biological data
(a) Absorption, distribution, and excretion
In a comparison of the absorption, distribution, and excretion of
the hethylheptyl isomer of dinocap (DNHPC) in mice after oral or
dermal administration, 14C-phenyl-labelled material (specific
activity, 18.6 mCi/g; radioactive purity, 95%) was administered at 25
mg/kg bw to female CD-1 mice as a single oral or single dermal dose or
as repeated dermal doses for 4 h/day up to 10 days. For oral
administration, corn oil was used as the vehicle; for dermal
administration, Karathane formulation blank solvents were used. Blood
samples were collected from subgroups of three mice at appropriate
times after dosing. The dermal application site was not occluded, but
a collar was applied to prevent grooming. For repeated dermal
administration, the application site was changed on successive days. A
further study was conducted to measure total systemic absorption over
4 h after a single dermal dose of 10 or 25 mg/kg bw in groups of four
mice. There are no guidelines for the objectives of this study, but
the method was appropriate to the purpose.
The results are shown in Table 1. The peak blood concentrations
were about four times lower and the time to peak concentrations
slightly delayed after dermal dosing in comparison with oral
administration, and the area under the curve was about seven times
smaller than after oral administration. The peak blood levels after
repeated dermal administration were no higher than that after a single
dose, suggesting that clearance of dinocap was sufficiently rapid that
no accumulation occurred under these conditions, as reported
previously. No material balance was attempted in this part of the
study. In the study of dermal penetration, 97-99% of the radiolabel
was recovered; recovery from the faeces and urine was 16% at the low
dose and 25% at the high dose, with, in each case, slightly more in
urine than in faeces. Less than 1% remained at the skin application
site. The half-life for elimination of radiolabel from plasma was
about 6 h after either oral or dermal administration. These results
are consistent with relatively rapid absorption of 25% of the
administered dose of dinocap through the skin of mice within 4 h
(Evans & Hazelton, 1995)
Table 1. Absorption of the methylheptyl isomer of
dinocap in mice after oral and dermal administration
Treatment Dose Tmax Cmax AUC T1/2
(mg/kg bw) (h) (ppm) (ppm-h) (h)
Single oral 25 2-6 18-21 345 6
Single dermal 25 6-8 4 52 6.5
Repeated dermal 25 NA 2-4 NA NA
When compared with the pharmacokinetic data described for rabbits
in the 1989 JMPR monograph (Annex 1, reference 58), the peak plasma
concentrations were approximately similar after a 25-mg/kg oral dose
(15 ppm in rabbits and 20 ppm in mice), and the Tmax was slightly
achieved slightly later in mice (6 h) than in rabbits (3 h).
Penetration of 14C-labelled DNHPC through samples of human and
mouse skin was compared in vitro. Cryopreserved human skin from the
thigh, back, and lateral torso of three donors, respectively, was
stripped of fat and sliced at 350 µm; skin was also derived from eight
female CD-1 mice, shaved, and stripped of fat. Discs of skin were
inserted into flow-through diffusion cells, and the barrier integrity
was verified with tritiated water. 14C-DNHPC was made up in Karathane
formulation blank and applied to the skin surface at a rate reported
to be 548 µg/cm2. The receptor chamber contained Hanks' balanced salt
solution and 4% albumin at a flow-through rate of 1.5 ml/min. The skin
samples were exposed to the Karathane test material for 24 h then
swabbed with a mild detergent. The human skin samples were separated
into layers which were analysed individually; mouse skin was analysed
intact. As radiolabel recovery was not determined, the results are
expressed only in terms of the proportion of radiolabel actually
recovered.
Of the radiolabel recovered, only 0.2% was recovered from
receptor fluid after penetration through mouse skin and only 0.3%
after penetration through human skin; 68% of radiolabel was recovered
from within mouse skin, but only 10% from within human skin. Slightly
less than 90% of the material recovered from human skin was in washes
of the skin surface. Steady-state flux rates through mouse skin were
approximately twice those through human skin. A total of 68% of the
recovered dose penetrated the mouse stratum corneum, and 10.5%
penetrated the human stratum corneum. The finding that only 0.2% DNHPC
penetrated into receptor fluid from mouse skin diifers from that in
mice in vivo, described above, which showed 25% penetration of a 25
mg/kg dose of 14C-DNHPC through mouse skin within 4 h and only 1%
remaining in skin. No comparison of skin concentrations was presented,
but, assuming a dermal application area of 3 cm2 for a
30-g mouse in vivo, the surface concentration is approximately 250
µg/cm2. As this value is comparable with the 548 µg/cm2 found
in vitro, dermal surface concentrations are unlikely to account for
the difference. A comparison of the penetration of DNHPC though human
and mouse skin was not given; however, human skin is generally less
penetrable than that of mice (Ruegg, 1996).
In a study reported only in summary, 55% of an undefined dose of
radiolabelled dinocap was eliminated in the urine of four adult rhesus
monkeys after intramuscular injection; 52% was excreted within 24 h of
injection (Maibach, 1985).
In a further study, dermal absorption of 14C-DNHPC was evaluated
by treating groups of four female rhesus monkeys with a dose of 1.6 mg
(approximately 0.2 mg/kg bw) as a 1:3 v/v solution in acetone on 40 or
0.64 cm2 of abdominal skin. When the skin of one of two groups that
received the dose over 40 cm2 was washed only with water, the total
recovery of radiolabel was poor (approximately 30%). The recoveries
were slightly better (50%) in the group in which the skin was washed
with aqueous ethanol, and only the results for this group are
considered further for this concentration. Occlusion of the
application sites was not reported, but the animals were restrained in
metabolic chairs for the 6-h exposure. An additional group received a
single intravenous dose of 0.1 mg/kg bw DNHPC in dimethyl sulfoxide.
After 6 h, the application site was washed, and the animals were
transferred to metabolism cages. Samples of blood, faeces, and urine
were collected at frequent intervals over a four-day elimination
period. Dermal absorption was calculated by correcting measured
urinary or faecal radiolabel elimination for the proportions of the
total administered dose eliminated by these routes after intravenous
injection, a method which fails to address dermal depot formation. The
design of the study is not closely compliant with any guideline.
The plasma levels after dermal administration were low. Total
recovery of the application of 0.2 mg/kg to 0.64 cm2, equivalent to
2500 µg/cm2, was approximately 80%, and absorption was calculated to
be about 5% on the basis of both faecal and urinary elimination.
Recovery of the more dilute application (0.2 mg/kg bw to 40 cm2,
equivalent to 40 µg/cm2) was approximately 50%, and absorption was
calculated as 10% from urinary elimination and 20% from faecal
excretion (Wester & Maibach, 1985).
(b) Biotransformation
In a study conducted to characterize urinary metabolites, a
mixture of 13C- and 14C-2,4-DNHPC was administered by gavage in corn
oil to three Sprague-Dawley rats at 100 mg/kg bw and to 15 CD-1 mice
at 25 mg/kg bw. Pooled urine samples collected during the first 24 h
after administration, which contained 90% of the radiolabel eliminated
by the urinary route, were analysed for metabolites by
high-performance liquid chromatography and mass spectroscopy.
Approximately 30% of the radiolabel administered to rats and 58%
of that administered to mice was recovered in urine, and 94% of the
radiolabel in rat urine and 82% of that in mouse urine could be
allocated to identified metabolites. Structures were assigned to 12
metabolites in rats and 13 in mice. In both species, the pattern of
metabolites was consistent with a metabolic pathway involving
extensive initial hydrolysis of the croton ester, resulting in loss of
the crotonate group (which was not found in any urinary metabolite),
leaving dinitrooctylphenol. Subsequent metabolites appeared to be
formed by ß- or alpha-oxidation of the methylheptyl group. Small
proportions of radiolabel were eliminated as conjugates: 4.5% in rats
as acetyl conjugates and 7.7% in mice as unidentified conjugates but
including sulfates. Seven metabolites, accounting for 12% of the total
recovered dose, were identified as occurring in mice but not in rats;
6.5% of the administered dose was attributable to unidentified
conjugates, and the remainder were chain-shortened alcohols and
aldehydes attributed to intermediates of alpha-oxidation with a common
end-product, as found in rats. The metabolites were allocated to
alpha-oxidation products on the basis that they showed shortening of
the heptyl moiety, but they were not obviously consistent with ß-
oxidation. Overall, 85% of metabolites found in rats were also found
in mice, and 70% of those found in mice were also found in rats
(Potter, 1996).
The proposed metabolic pathways of dinocap in rats and mice are
shown in Figures 1 and 2.
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of various
formulations of dinocap are shown in Table 2. Purified technical-grade
dinocap appeared to be marginally less acutely toxic than the less
highly purified material described in the 1989 report (Annex 1,
reference 58). The purified material was moderately irritating to the
skin (Romanello et al., 1987d) and eye (Romanello et al., 1987d) and
was sensitizing to the skin when tested by the Buehler method
(Anderson & Baldwin, 1990a); the previous conclusions of the JMPR
remain unaltered by these data. Products containing dinocap were also
shown to be irritating to the skin and eye of rabbits (Bernacki &
Hamilton, 1992c; Krajewski et al., 1987b,c; Morrison & Hamilton, 1992;
Romanello et al., 1987e,f,g) and to have skin sensitizing potential
(Anderson & Baldwin, 1990b,c).
(b) Short-term studies of toxicity
Mice
In a dose range-finding study reported only in brief, purified
technical-grade dinocap (purity, 95.7%) was administered in the diet
of CD-1 mice (age at start of study unspecified) at concentrations of
25, 75, 225, 500, 1000, 2000, or 4000 ppm for 28 days. The full extent
of histological examination was not reported. Concentrations of 500
ppm and higher caused deaths, with total loss of males at 2000 or 4000
ppm and of females at 1000 ppm and higher. The results are shown in
Table 3. The liver appeared to be the principal target organ, showing
hepatocellular necrosis that appeared to be of only mild to moderate
severity when given at doses that caused deaths. The amount of detail
provided was insufficient to identify a NOAEL (Bernacki & Baldwin,
1987).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 60 male and 60 female CD-1 mice were fed diets
containing 0 (control), 15, 100, or 200 ppm dinocap (purity,
93.2-96.2% at intervals during the study) for 78 weeks. The mice were
seven to eight weeks old at the start of treatment and thus two weeks
older than required by the guideline; much of the most rapid phase of
growth was thus missed. The study design included ophthalmological
examination. After the deaths of 14 females at the highest dose during
week 1, that dose was reduced to 150 ppm from the start of week 2 for
females and week 3 for males. With the exception of the deaths in week
1, there was no treatment-related effect on survival, which was about
80% in controls and 72% at the highest dose (group with highest
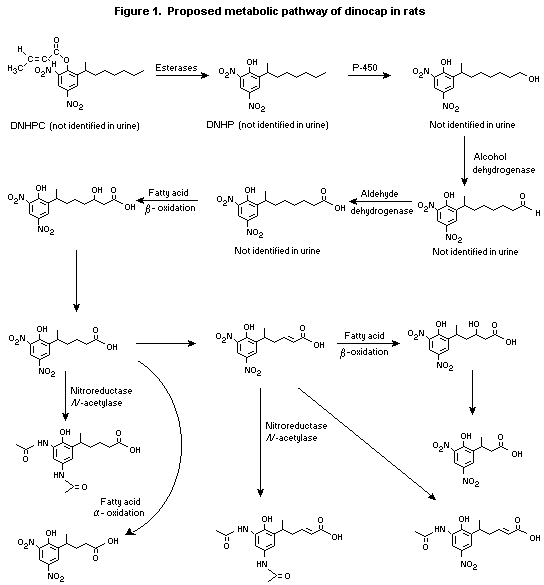
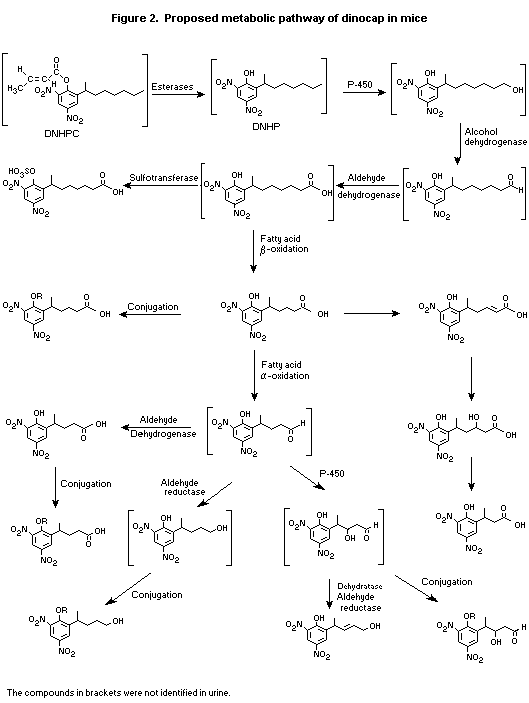 Table 2. Acute toxicity of formulations of dinocap
Species Strain Sex Route Purity LD50 or LC50 Reference
(%) (mg/kg bw
or mg/L)
Technical-grade dinocap
Mouse CD-1 M Oral 95 292 Morrison et al. (1987)
Rat Crl:CD M Oral 95 3100 Morrison et al. (1987)
Rat Crl:CD M Oral 95 > 500 Romanello et al. (1987a)
F < 5000
Rat Crl:CD M Inhalation 95 > 3 Ferguson (1997)
F 3
Rabbit New Zealand white M/F Dermal 95 > 5000 Romanello et al. (1987b)
Karathane LC fungicide-miticide
Mouse CD-1 M Oral 40.4 517 Onishi (1989)
F 413
Mouse CD-1 M/F Dermal 35.6 1020 Procopio & Parno (1995)
Rat Crl:CD M/F Oral 37.6 > 2000 Bernacki & Hamilton (1992a)
Rat Crl:CD M Oral 39 > 500 Lampe et al. (1987a)
F < 5000
Rat Crl:CD M/F Dermal 37.6 > 2000 Bernacki & Hamilton (1992b)
Rabbit New Zealand white M/F Dermal 39 > 5000 Lampe et al. (1987b)
Rat Crl:CD M/F Inhalation 50 0.9 Blair & Cavender (1979)
Karathane FN-57 fungicide-miticide
Mouse CD-1 M/F Oral 20 1401 Morrison & Hamilton (1991)
Rat Crl:CD M Oral 20 > 500 Romanello et al. (1987c)
F < 5000
Rat Crl:CD M/F Inhalation 20 > 4.9 Wanner & Hagan (1991)
Rabbit New Zealand white M/F Dermal 20 > 5000 Krajewski et al. (1987a)
M, male; F, female
Table 3. Results of a dose range-finding study in mice
Outcome Dose (ppm in diet)
0 25 75 225 500 1000 2000 4000
Mortality, males 0/10 0/10 0/10 0/10 0/10 5/10 10/10 10/10
Mortality, females 0/10 0/10 0/10 0/10 7/10 10/10 10/10 10/10
Final body weight, males (g) 34 33 33 32 29 25
Final body weight, females (g) 27 28 29 26 20
Liver weight, males (g) 2.0 2.0 2.1 1.9 1.8 1.7*
Liver weight, females (g) 1.5 1.6 1.7* 1.5 1.2*
Liver weight, males (% bw) 5.8 5.9 6.1 6.0 6.4* 6.8*
Liver weight, females (% bw) 5.5 5.7 6.0* 5.9* 5.9
Hepatocellular necrosis, males NR NR NR NR NR 3/10 9/10 6/10
Hepatocellular necrosis, females NR NR NR NR 5/10 6/10 7/10 3/10
NR, not reported
From Bernacki & Hamilton (1987)
* Statistically significant at 0.05 > p > 0.01
mortality) at 78 weeks. There were no overt treatment-related symptoms
of toxicity. Body-weight gain and food consumption were reduced in
males at 150 ppm and in females at 100 ppm (20% less than that of
controls) and 150 ppm. The efficiency of food use appeared to be
impaired only in males at the highest dose and not in females, thus
suggesting that the effect in females was at least partly a
consequence of palatability. Mean testis weight was reduced in males
at 150 ppm, and histopathological examination revealed a background
incidence of unilateral and bilateral testicular atrophy in all
groups, including controls, with incidences of 11/60, 4/60, 11/59, and
25/60 at 0, 15, 100, and 150 ppm, respectively. A moderate incidence
of individual cell necrosis was reported in mice in all groups,
possibly associated with mouse hepatitis virus infection indicated by
serological markers at 18 months, but the incidence of necrosis seen
in the 28-day study described previously was not reproduced. There was
no evidence of carcinogenicity in any tissue. The NOAEL was 15 ppm,
equivalent to 2.7 mg/kg bw per day, on the basis of reduced
body-weight gain with a corresponding reduction in food consumption in
females at 100 ppm (Moore, 1991).
(d) Genotoxicity
Studies of the genotoxicity of dinocap of lower purity than that
used in the studies reported in the previous monograph are summarized
in Table 4. The results are consistent with the previous conclusions
of the JMPR.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 26 Crl:CD rats of each sex received dinocap (purity,
96%) in the diet at concentrations of 40, 200, or 1000 ppm for two
generations. At weaning of the F1 animals, the highest dose was
reduced to 400 ppm because of a high rate of mortality in these pups.
The litters were culled to eight pups at day 4 post partum. In
addition to the normal guideline requirements, selected F1 males at
0, 40, or 200 ppm were used in a study of gonadal function (sperm
motility, sperm count, weight of cauda epididymis). Body-weight gain
and food consumption were retarded at 1000 ppm during the F0
pre-mating period and at 400 ppm during the F1 pre-mating period.
There was increased pup mortality at weaning among litters of the
group at 1000 ppm, until this dose was reduced to 400 ppm; the cause
of death could not be determined but the presence of yellow or red
discolouration of the contents of the gastrointestinal tract and
bladder suggested the presence of test material or its metabolites.
There were no specific effects on reproductive function or the ability
to rear young, and there was no effect on gonadal function. Increased
liver weights, with no evidence of a histological correlate, were
found among rats receiving 400 or 1000 ppm. The NOAEL was 200 ppm,
equal to 13 mg/kg bw per day (Morseth, 1990).
Table 4. Results of studies of the genotoxicity of dinocap
End-point Test object Dose Purity Results Reference
(%)
In vitro
Gene mutation Chinese hamster 3-10 µg/mla 83.9 Negative Foxall (1985)
ovary cells, hprt locus 15-25 µg/mlb Negative
Metaphase analysis Chinese hamster 1-10 µg/mla NR Negative Ivett & Myhr (1986)
ovary cells 5-20 µg/mlb Negative
In vivo
Metaphase analysis CD-1 mouse 12.6-126 mg/kg bw 83.9 Negative Sames et al. (1986)
bone marrow
a Without an exogenous metabolic system
b With an exogenous metabolic system
(ii) Developmental toxicity
Mice
Groups of 24 CD-1 mice presumed to be pregnant were given dinocap
(purity, 94.4%) in aqueous 1% tragacanth gum at doses of 0 (control),
4, 10, or 25 mg/kg bw per day by gavage on days 6-15 of gestation. On
day 18 of gestation, about half of the mice in each group were killed
and the fetuses were examined; the remaining dams were allowed to
deliver and rear their litters, which were culled on day 4 post
partum to two pups per litter. On day 43, these mice were evaluated
for swimming performance.
The results of this study are shown in Table 5. There were no
maternal deaths during the study and no overt signs of toxicity. The
body-weight gain of the pregnant mice was minimally impaired at 25
mg/kg bw per day on days 12-16 of gestation, although the weight
difference may have been a consequence of the reduced litter size.
Gross examination showed no treatment-related findings. In animals
killed on day 18 of gestation (the normal end of a study of
teratogenicity), the average number of resorptions was increased in
dams at 25 mg/kg bw per day, with an associated decrease in litter
size. The incidence of resorptions was slightly increased in dams at
at 10 mg/kg bw per day, but the increase was within the range of
historical controls (0-1.6) All treated animals that delivered
naturally had a statistically significant increase in the duration of
gestation, although this was not considered to be toxicologically
significant.
The mean fetal weight was reduced, and there was an increased
incidence of fetuses with cleft palate and open eyelids at 10 and 25
mg/kg bw per day. There was an increased incidence of stillborn pups
and decreased pup body weight on days 7-21 post partum at 25 mg/kg
bw per day, and pup survival to day 4 post partum was reduced. The
incidence of litters with pups with head tilt and the incidence of
pups with cleft palate were increased at 25 mg/kg bw per day, although
all nine pups with cleft palate were from the same litter. Among the
pups that were allowed to survive until day 43 post partum, there
was an increased incidence of mice with head tilt, and ungroomed
coats, corneal opacity, and ptosis were seen in one or two males at
the highest dose. Body-weight gain and average body weights were
decreased in mice at this dose, and there was an increased incidence
of mice with altered swimming postures or ability. Of the 11 pups with
altered swimming posture, 10 had head tilt. A further three pups with
head tilt did not have altered swimming posture. The NOAEL for
developmental toxicity was 4 mg/kg bw per day on the basis of the
increased incidence of open eyelids and cleft palate in pups at 10
mg/kg bw per day. The NOAEL for maternal toxicity was 10 mg/kg bw per
day on the basis of minimally retarded weight gain during days 12-16
of gestation in dams at 25 mg/kg bw per day. Dinocap was teratogenic,
causing malformations at doses that had no effect on the dam (Lochry,
1989).
Table 5. Results of a study of developmental toxicity in mice
Parameter Dose (mg/kg bw per day)
0 4 10 25
Maternal weight gain (g), days 6-16 14.8 16.0 16.5 13.8
days 12-16 8.2 8.7 8.8 6.4
days 0-18 26.3 27.0 27.3 23.3
Caesarian-derived pups
No. of litters evaluated 12 12 12 9
Resorptions per litter 1.0 0.5 1.2* 1.8*
Litter size 11.2 11.2 10.8 8.7
Mean fetal weight (g) 1.4 1.35 1.30* 1.10**
Open eyelids, litters (total no. fetuses) 0 0 1 (1) 2 (3**)
Cleft palate, litters (total no. fetuses) 0 0 3 (4) 7 ** (65**)
Naturally-delivered pups
No. of litters evaluated 11 12 12 10
Gestation duration (days) 19.4 19.8* 19.8* 19.9**
Litter size, day 1 post partum 11.4 11.3 11.8 11.8
Head tilt (no. of litters) 0 0 0 3**
Cleft palate/pups dying days 0-21 post partum 0/3 0/1 0/0 9/16
Abnormal swimming ability, day 43, litters (no. fetuses) 0/11 0/11 0/12 5/9** (11/39)**
From Lochry (1989)
* Statistically significant at 0.05 > p > 0.01; ** statistically significant at 0.01 > p
A study was conducted to establish suitable doses for a study of
teratogenicity after dermal application to CD-1 mice. Fewer animals
were used that recommended in guideliness. Dinocap was applied as the
formulation Karathane LC XF, considered to be appropriate for
estimating the risk due to occupational exposure, but the doses used
were corrected for content and expressed as dinocap. Appropriate
dilutions were obtained with the formulation blank. In phase 1 of the
study, both untreated and vehicle control groups were used; in phase
2, only a vehicle control group was used. The dose volume of 290 µl/kg
bw was applied to one to five areas of shaven dorsal skin for 4 h each
day on days 6-15 of presumed gestation; the site was changed each day,
recommencing at the first site on the sixth day. Occlusion of the
application site was not mentioned, but a collar was placed to prevent
ingestion; no mention is made of how the mice were restrained during
application. After completion of the 4-h exposure each day, the test
material was washed off gently with soap and water.
Initially (phase 1), groups of eight presumed-pregnant mice were
given doses of 0 (control), 50, 80, or 100 mg/kg bw per day. Because
of excessive toxicity, similar groups of eight mice were given doses
of 0, 1, 4, 10, or 25 mg/kg bw per day by a similar protocol. The mice
were sacrificed on day 18 of presumed gestation; half of the fetuses
were used for skeletal examination, and the remaining half for
specific examination of otoliths and of the remaining skeleton. For
mice weighing 20-40 g, the dose volumes would have been 6-12 µl: the
practical difficulty of administering such small volumes may have
resulted in variations in accuracy. One, three, and four deaths
occurred during treatment at 50, 80, and 100 mg/kg bw per day,
respectively. Death was usually briefly (up to 24 h) preceded by signs
of toxicity, including dermal erythema, ataxia, red or tan vaginal
discharge, weight loss, and cold to touch. These doses all reduced
weight gain, with weight loss at the highest dose. The numbers of live
young were reduced at 50 and 80 mg/kg bw per day, and the litters of
the three surviving dams at 100 mg/kg bw per day contained only
resorbed conceptuses. Gross malformations were seen in 88% of fetuses
at 50 mg/kg bw per day and 100% of those at 80 mg/kg bw per day.
The principal treatment-related effects seen in phase 2 are shown
in Table 6. Little maternal toxicity occurred, and body-weight gain
was apparently unaffected by treatment. One mouse at the highest dose
was found dead on day 15 of gestation having been found stuporous with
an impaired righting reflex after dosing the day before. Death was
attributed to trauma, although no clear injury was detected at
necropsy. There was a slight incidence of skin irritation at the two
highest doses. The litter sizes, numbers of live fetuses per litter,
and fetal body weights were unaffected by treatment. There was no
change in the degree of skeletal ossification, but three fetuses of
one litter at the highest dose had cleft palate, and two fetuses of
another litter had open eyelids. Also at the highest dose, otolith
development was clearly impaired. These findings are consistent with
those of previous studies of the teratogenicity of dinocap in mice
after oral administration. In view of these results, no further study
by the dermal route was conducted. The study involved too few animals
to determine a NOAEL for teratogenicity in mice treated dermally,
particularly with respect to cleft palate; however, the quality of the
data on mean otolith scores in 49 fetuses may provide some degree of
confidence that the NOAEL for these effects is 10 mg/kg bw per day
(Foss, 1995).
Table 6. Results of a study for developmental toxicity in mice
Effect Dose (mg/kg bw per day)
0 1 4 10 25
No. of pregnant dams 8 8 7 8 6
Maternal weight gain on days 6-18 (g) 21.4 26.4 20.8 26.4 25.0
No. of fetuses 77 96 67 93 73
Cleft palate (no. of litters) 0 0 0 0 1
Open eyelids (no. of litters) 0 0 0 0 1
Mean otolith scorea 9.2 9.6 9.0 9.0 2.2
No. of fetuses examined 37 49 34 49 38
From Foss (1995)
a Three otoliths were scored for completeness on a scale of 1-4, to give a maximum
potential score of 12.
2,4-DNHPC and 2,6-DNHPC were not teratogenic in mice. Samples of
each isomer (purity, 95%) were tested seperately and in combination,
and the results were compared with those in mice receiving
technical-grade dinocap (purity, 84%), each group at a dose of 25
mg/kg bw per day in corn oil. Fewer pregnant animals were used than
recommended in the guidelines. Although litters born to
dinocap-treated mice showed a pattern of developmental defects typical
of those seen in the studies described above, the two isomers were
inactive at the same dose, both alone and in combination (Rogers et
al., 1987). In a separate study by the same group, otolith formation
was compared in the pups of mice and hamsters treated with dinocap
during gestation. Otolith formation was impaired in mice. Dinocap
affected otolith formation in hamsters, but only at a dose associated
with severe maternal and fetal toxicity (Rogers et al., 1989).
Rats
Groups of 25 Sprague-Dawley rats presumed to be pregnant received
dinocap (purity, 96%) as a suspension in aqueous methylcellulose at
doses of 0 (control), 10, 50, or 150 mg/kg bw per day by gavage on
days 6-15 of gestation. There were no deaths, and the only overt sign
of toxicity was an increased incidence of soft faeces in rats at 150
mg/kg bw per day. Maternal body-weight gain was impaired at 150 mg/kg
bw per day during the first two days of treatment, and food
consumption was slightly reduced in this group during treatment. Gross
examination revealed no treatment-related changes in pregnant or non-
pregnant females. There were no treatment-related changes in pup
weight and no increase in the incidence of malformations. There was an
apparent increase in the incidence of extra ribs at 150 mg/kg bw per
day, but seven of the 10 affected fetuses were from two litters. The
NOAEL for maternal and fetal toxicity was 50 mg/kg bw per day. There
was no evidence of teratogenicity at the highest dose of 150 mg/kg bw
per day (Solomon & Lutz, 1989).
Rabbits
Groups of 20 artificially inseminated New Zealand white rabbits
received dinocap (purity, 95.4%) in aqueous gum tragacanth by gavage
at doses of 0, 3, 12, 48, or 84 mg/kg bw per day on days 7-19 of
presumed pregnancy. Pups were delivered by caesarian section on day 29
and examined for developmental abnormalities in accordance with normal
guideline requirements. Two does at the high dose and one at the
intermediate dose died between days 24 and 29 of gestation, therefore
at least five days after dosing, but the deaths were considered to be
related to treatment. These animals all showed weight loss and
anorexia before death. The doe at the intermediate dose and one at the
high dose aborted; both does at the high dose that died had non-viable
litters (late resorptions). An additional five deaths were considered
unrelated to treatment and were primarily a consequence of intubation
errors.
The main results are shown in Table 7; statistical analyses were
not reported. There was an increased incidence of premature delivery
and abortion at 48 and 84 mg/kg bw per day; the premature deliveries
at the highest dose were primarily late resorptions. Clinical signs
(lack of faeces or dried faeces) and impaired weight gain and food
consumption were seen among does receiving these doses, litter sizes
and pup weights were reduced, and there was an increased incidence of
pups with skeletal malformations (vertebral assymetry and fused or
forked ribs). There was also a slight increase in delayed ossification
at a few sites in does at these doses. The NOAEL for maternal toxicity
was 3 mg/kg bw per day on the basis of weight-gain retardation during
treatment. The NOAEL for developmental toxicity was 12 mg/kg bw per
day on the basis of increased resorptions and reduced litter sizes at
48 mg/kg bw per day (Hoberman & Christian, 1987).
This conclusion is different from that of the 1989 JMPR (Annex 1,
reference 58) but is based on different data. In the latter review, an
increased incidence of hydrocephaly and neural tube defects was found
at doses > 3 mg/kg bw per day in the studies of Costlow & Kane
(1984 a,b). In the earlier studies, however, a less pure form of
dinocap (84%) was used, and the neural tube defects found by Costlow
and Kane (1984a) at a dose of 3 mg/kg bw per day were not found in the
second study (Costlow & Kane, 1984b) at 48 mg/kg bw per day nor in
studies by dermal administration in which the increased numbers of
Table 7. Results of a study of developmental toxicity in rabbits
Result Dose (mg/kg bw per day)
0 3 12 48 84
Adult animals
No. pregnant 17 17 15 15 14
No. with abortions 0 0 0 3 1
No. with premature delivery 1 0 0 1 3
No. with absence of faeces (on any day) 0 0 0 0 4
No. with dried faeces (on any day) 3 0 2 7 17
Weight change, days 7-20 (g) 150 200 90 -330 -550
Food consumption, days 7-20 (g/day) 160 170 152 65 51
No. with gastric ulceration 0 0 1 3 4
Litters
No. evaluated 12 17 14 10 9
Mean no. of live fetuses 7.2 7.5 8.1 6.3 5.9
Dead or resorbed fetuses per litter (%) 11.8 4.9 7.1 20.3 27.0
Mean fetal weight (g) 40.9 42.7 40.0 37.6 35.8
Vertebral assymetry: no. of litters (pups) 1 (1) 1 (1) 1 (2) 5 (7) 3 (8)
No. of litters (pups) with rib malformation 1 (1) 0 2 (3) 2 (2) 2 (6)
No. of litters (pups) with skeletal malformations 4 (4) 3 (3) 4 (6) 5 (7) 6 (12)
From Hoberman (1987)
resorptions and delayed ossifications were similar to these found by
Hoberman & Christian (1987).
(f) Special studies: Ocular toxicity
A NOAEL of 15 ppm was observed for ocular toxicity in a two-year
study in dogs (Weatherholz et al., 1979) in the 1989 JMPR (Annex 1,
reference 38). At 60 ppm, four of eight dogs had slight to moderate
discolouration of the tapetum lucidum, although no retinal atrophy and
no changes in the vascularity of the retina were noted. The remaining
four dogs at this dose showed moderate to marked discolouration of the
tapetum, reduced vascularity of the retina, and retinal atrophy (three
dogs). At 120/240 ppm, the four surviving dogs had marked
discolouration of the tapetum, reduced vascularity of the retina, and
retinal atrophy. Retinal atrophy was therefore present only in dogs
with severe tapetal changes, and no dog showed retinal effects without
effects in the tapetum.
A review of the literature for compounds that are toxic to the
tapetum lucidum of dogs was submitted (Solomon, 1991). Of six
compounds known to affect the tapetum, three (zinc pyridinethione, SCH
19927, and rosaramicin) had no effect in atapetal dogs, and no ocular
effects were reported in other atapetal animals. The other three
compounds (dinocap, hydroxy pyridinethione, and diphenyl
thiocarbazine) affected the tapetum in dogs (with no information on
atapetal dogs) but did not cause ocular effects in atapetal species.
The author concluded that the retinal atrophy seen with dinocap was
secondary to damage to the tapetum lucidum. Since humans do not have a
tapetum, it was concluded that humans would not be susceptible to
retinal damage as a consequence of this effect. The review is brief,
and the adequacy of the methods used, the sensitivity, and the
comparability of the findings were not considered. The conclusions
cannot therefore be regarded as definitive, although the data support
the hypothesis. The NOAEL in the study of Weatherholz et al. (1979)
was therefore identified for other effects. The 1989 JMPR report
(Annex 1, reference 58) indicates that significant effects, including
deaths, occurred at doses of 180-240 ppm, giving a NOAEL of 60 ppm,
equivalent to 1.5 mg/kg bw per day.
Comments
Dinocap is well absorbed after oral exposure. A proportion
(5-25%) is absorbed after dermal exposure, varying with species and
concentration. No conclusions were drawn about the degree of dermal
absorption in humans from the results of a study in which mouse and
human skin were compared; however, human skin is generally regarded as
being less permeable to toxicants than that of mice.
The urinary metabolites of the methylheptyl isomer in rats and
mice have been extensively characterized; characterization of the
faecal metabolites was reported by the 1989 JMPR, which concluded that
the pattern of metabolites in faeces seen by thin-layer chromatography
was similar to that observed in squash and cucumbers.
The new data confirmed the generally low degree of acute toxicity
of dinocap in rats; mice, however, appear to be more sensitive than
rats to both acute and developmental effects. Dinocap is a skin
irritant and sensitizer. The available studies did not address the
uncoupling of oxidative phosphorylation, identified by the 1989 JMPR
as a potentially significant mode of action.
WHO has classified dinocap as 'slightly hazardous' (WHO, 1996).
In a study of carcinogenicity in mice at doses of 0, 15, 100, or
200 ppm, no evidence of carcinogenicity was found. The NOAEL was 15
ppm, equal to 2.7 mg/kg bw per day. The lack of carcinogenicity in
mice is consistent with the absence of carcinogenicity in rats
reported by the 1989 JMPR.
The results of tests for genotoxicity (on the less pure form of
dinocap) were negative.
A multigeneration study of reproductive toxicity at dietary
concentrations of 0, 40, 200, or 1000 ppm in rats showed no specific
effect on any reproductive parameters; the NOAEL was 200 ppm, equal to
13 mg/kg bw per day.
In a study of developmental toxicity in mice dosed by gavage at
0, 4, 10, or 25 mg/kg bw per day, impaired otolith formation was seen
at 25 mg/kg bw per day. A dose-related increase in the incidence of
open eyelids and cleft palate extended down to 10 mg/kg bw per day in
the absence of maternal toxicity. The NOAEL was 4 mg/kg bw per day.
Dermal application of 50, 80, or 100 mg/kg bw per day to mice proved
excessively toxic for an evaluation of developmental toxicity. A
further dermal study in mice at 0, 1, 4, 10, or 25 mg/kg bw per day
showed malformations,including impaired otolith formation, at 25 mg/kg
bw per day in the absence of maternal toxicity. The NOAEL for
developmental toxicity after dermal application to mice was 10 mg/kg
bw per day. The results of the recent studies of developmental
toxicity confirmed the teratogenic potential of purified dinocap in
mice, even when applied dermally. Impaired otolith development,
characteristic of the teratogenicity of dinocap in mice, was also seen
in hamsters at doses that are maternally toxic. Less specific
malformations were seen in rabbits at maternally toxic doses. The
present Meeting concluded that the NOAEL in the studies in rabbits
described by the 1989 JMPR was 3 mg/kg bw per day rather than
0.5 mg/kg bw per day, since the findings on which the putative effect
level was established do not appear to be repeatable or clearly
dose-related. The methylheptyl isomer has been shown not to be
teratogenic to mice. The reason for the species difference in the
teratogenicity of dinocap in rats and mice therefore cannot be deduced
from the data on the metabolism of the methylheptyl isomer.
The two-year study in dogs that was evaluated at the 1989 Joint
Meeting was also reassessed on the basis that the critical effect
(retinal atrophy) was secondary to effects on the tapetum lucidum.
Since the tapetum lucidum is not present in humans, or in rats or mice
in which no retinal effect was seen, the Meeting concluded that it
would be inappropriate to base the evaluation on this effect. The
NOAEL was 60 ppm, equivalent to 1.5 mg/kg bw per day.
Teratogenic effects in mice were considered to be the
toxicological end-point of greatest concern. Since dinocap was
teratogenic in mice after either oral or dermal administration and
since malformations were seen in at least three species, the Meeting
considered a high safety factor to be appropriate. An ADI of 0-0.008
mg/kg bw was established on the basis of the NOAEL of
4 mg/kg bw per day in the developmental toxicity study in mice and a
safety factor of 500.
Establishment of an acute reference dose (RfD) was considered to
be appropriate since teratogenicity may occur after a single exposure.
An acute RfD was established on the basis of the NOAEL of 4 mg/kg bw
per day for teratogenicity in mice and a safety factor of 500, to give
an acute RfD of 0.008 mg/kg bw, which is appropriate for women of
child-bearing age.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 15 ppm, equal to 2.7 mg/kg bw per day (toxicity in a
study of carcinogenicity)
4 mg/kg bw per day (developmental toxicity)
10 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rat: 200 ppm, equal to 6.4 mg/kg bw per day (toxicity in a
study of carcinogenicity)
50 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Rabbit: 3 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 60 ppm, equivalent to 1.5 mg/kg bw per day (study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.008 mg/kg bw
Estimate of acute reference dose
0.008 mg/kg bw
Information that would be useful for continued evaluation of the
compound
Further observations in humans
List of end-points for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption 60-69% absorbed, max. concentration at 2-6 h
Dermal absorption 5-25%
Distribution Widely distributed
Potential for accumulation Limited, < 0.3% in tissue after 7 days
Rate and extent of excretion Biphasic; half-life is 3 h for 1st phase, 44 h for 2nd
phase, oral administration, rabbit
Metabolism in animals Extensive; > 96% metabolized
Toxicologically significant compounds Metabolites assumed to be of similar toxicity to parent
(animals, plants and environment)
Acute toxicity
Rat: LD50 oral 3100 mg/kg bw
Rat: LD50 dermal > 5000 mg/kg bw
Rat: LC50 inhalation 3 mg/L
Skin irritation Irritating
Eye irritation Irritating
Skin sensitization Sensitizing
Short-term toxicity
Target/critical effect General toxicity
Lowest relevant oral NOAEL Dog: 1.5 mg/kg bw per day
Lowest relevant dermal NOAEL Mouse: 10 mg/kg bw per day (teratogenicity)
Lowest relevant inhalation NOAEL No data
Genotoxicity Not genotoxic in an adequate battery of tests
Long-term toxicity and carcinogenicity
Target/critical effect: Impaired weight gain
Lowest relevant NOAEL Mouse: 2.7 mg/kg bw per day (carcinogenicity)
Carcinogenicity Not carcinogenic
Reproductive toxicity
Reproduction target/critical effect No effect on fertility or ability to rear young
Lowest relevant reproductive NOAEL Rat: 13 mg/kg bw per day (multigeneration study)
Developmental target/critical effect Malformations
Lowest relevant developmental NOAEL Mouse: 4 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No data, but no concern raised in other studies
Other toxicological studies Inhibits oxidative phosphorylation; methylheptyl
isomer not teratogenic
Medical data No significant dinocap-related effects reported
Summary Value Study Safety factor
ADI 0-0.008 mg/kg bw Mouse, developmental toxicity 500
Acute reference dose 0.008 mg/kg bw Mouse, developmental toxicity 500
References
Anderson, D.M. & Baldwin, R.C. (1990a) Karathane LC fungicide/miticide
delayed contact hypersensitivity study in guinea pigs. Unpublished
report No. 89R-187 from Rohm & Haas Company, Toxicology Department,
Spring House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas
Company, Spring House, Pennsylvania, USA.
Anderson, D.M.& Baldwin, R.C. (1990b) Karathane FN-57
fungicide/miticide delayed contact hypersensitivity study in guinea
pigs. Unpublished study No. 89R-188 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Anderson, D.M. & Baldwin, R.C. (1990c) Karathane technical (HPK)
fungicide/miticide delayed contact hypersensitivity study in guinea
pigs. Unpublished report No. 89R-189 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Bernacki, H.J. & Baldwin, R.C. (1987) Karathane technical (dinocap):
Range finding four-week dietary toxicity study in mice. Unpublished
report No. 87R-175. from Rohm & Haas Co., Toxicology Department,
Spring House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co.,
Spring House, Pennsylvania, USA.
Bernacki, H.J. & Hamilton, J.D. (1992a) Karathane LC
fungicide/miticide: Skin irritation study in rabbits. Unpublished
report No. 91R-170 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Bernacki, H.J. & Hamilton, J.D. (1992b) Karathane LC
fungicide/miticide: Acute dermal toxicity study in male and female
rats. Unpublished report No. 91R-171 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Bernacki, H.J. & Hamilton, J.D. (1992c) Karathane LC
fungicide/miticide: Acute oral toxicity study in male and female rats.
Unpublished report No. 91R-173 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Blair, M. & Cavender, F.L. (1979) TD 77-310 acute inhalation study in
rats. Unpublished report No. 285-029 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Rohm & Haas Co., Spring House, Pennsylvania, USA.
Costlow, R.D. & Kane, W.W. (1984a) Teratology study with Karathane in
rabbits. Unpublished report No. 83R-22 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Costlow, R.D. & Kane, W.W. (1984b) Teratology study with Karathane in
rabbits. Unpublished report No. 83R-113 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA
Evans, K.W. & Hazelton, G.A. (1995) Blood pharmacokinetics and dermal
absorption of Karathane in female mice. Unpublished report No. 95R-105
from Rohm & Haas Co., Toxicology Department, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Ferguson, J.S. (1997) Karathane technical: Acute inhalation toxicity
study in rats. Unpublished report No. 96R-126 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Foss, J.A. (1995) Dermal dosage-range developmental toxicity study of
Karathane LC XF in mice. Unpublished report No. 018-023P from Argus
Research Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA Rohm & Haas
Co. report No. 95RC-024.
Foxall, S. (1985) Karathane technical: CHO/HGPRT gene mutation assay.
Unpublished report No. 85R-204 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Hoberman, A. & Christian, M. (1987) A developmental toxicity study of
Karathane fungicide/miticide (technical) administered via stomach tube
to New Zealand white rabbits. Unpublished report No. 018-012 from
Argus Research Laboratories, Inc., Horsham, Pennsylvania, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Rohm & Haas Co. report No. 86RC-71.
Ivett, J. & Myhr, B. (1986) Clastogenic evaluation of Karathane
technical in an in-vitro cytogenetic assay measuring chromosomal
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished study project No. 20990 from Litton Bionetics, Inc.,
Kensington, Massachusetts, USA. Rohm & Haas Co. report No. 85RC-62.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1987a) Karathane
FN-57 fungicide/miticide: Acute dermal toxicity study in male and
female rabbits. Unpublished report No. 87R-138 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1987b) Karathane
FN-57 fungicide/miticide: Eye irritation study in rabbits. Unpublished
report No. 87R-114 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1987c) Karathane
FN-57 fungicide/miticide: Skin irritation study in male rabbits.
Unpublished report No. 87R-139 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Lampe, K.R., Morrison, R.D. & Baldwin, R.C. (1987a) Karathane LC
fungicide-miticide: Acute oral toxicity study in male and female rats.
Unpublished report No. 87R-132 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Lampe, K.R., Morrison, R.D. & Baldwin, R.C. (1987b) Karathane LC
fungicide-miticide: Acute dermal toxicity study in male and female
rats. Unpublished report No. 87R-133 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Lochry, E. (1989) Dinocap (high purity technical): An oral (gavage)
developmental toxicity study in CD-1(ICR)BR mice including a postnatal
phase. Unpublished report No. 018-015 from Argus Research
Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO by
Rohm & Haas Co., Spring House, Pennsylvania, USA. Rohm & Haas Co.
report No. 88P-335.
Maibach, H. (1985) Elimination of 14C labeled Karathane in rhesus
monkeys following a single intramuscular injected dose. Unpublished
report No. 85RC-18 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Moore, M.R. (1991) Dinocap (technical purified): 78-Week dietary
oncogenicity study in mice. Unpublished report No. 417-445 from
Hazleton Laboratories America, Inc., Rockville, Massachusetts, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Rohm & Haas Co. report No. 88RC-090
Morrison, R.D. & Hamilton, J.D. (1991) Karathane FN-57
fungicide/miticide: Acute oral toxicity study in male and female mice.
Unpublished report No. 91R-001 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Morrison, R.D. & Hamilton, J.D. (1992) Karathane LC
fungicide/miticide: Eye irritation study in rabbits. Unpublished
report No. 91R-172 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Morrison, R.D., Krzywicki, K.M. & Hazelton, G.A. (1987) Karathane
technical purified: Acute oral toxicity study in male rats and mice.
Unpublished report No. 86R-149A,B from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Morseth, S. (1990) Dinocap (technical purified): Two-generation
reproduction study in rats. Unpublished report No. 417-442 from
Hazleton Laboratories America, Inc., Vienna, Virginia, USA. Submitted
to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA. Rohm &
Haas Co. report No. 88RC-092.
Onishi, M. (1989) Karathane LC (HPK) fungicide/fiticide: Acute oral
toxicity study in mice. Unpublished study from Shin Nippon Biomedical
Laboratories Ltd, Kagoshima, Japan. Rohm & Haas Co. report No.
90RC-1001. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Potter, D. (1996) Identification of
2,4-dinitro-6-(1-methylheptyl)phenyl crotonate metabolites in rat and
mouse urine. Unpublished report No. 95R-064 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Procopio, K.R. & Parno, J.R. (1995) Karathane LC: Acute dermal
toxicity study in male and female mice. Unpublished report No. 94R-244
from Rohm & Haas Co., Toxicology Department, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Rogers, J.M., Gray, E.L., Carver, B.D. & Kavlock, R.J. (1987)
Developmental toxicity of dinocap in the mouse is not due to two
isomers of the major active ingredient. Teratol. Carcinog. Mutag.,
7, 341-346.
Rogers, J.M., Burkhead, L.M. & Barbee B.D. (1989) Effects of dinocap
on otolith formation: Evaluation of mouse and hamster fetuses at term.
Teratology, 39, 515-523.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987a) Karathane
technical purified: Acute oral toxicity study in male and female rats.
Unpublished report No. 87R-124 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987b) Karathane
technical purified: Acute dermal toxicity study in male and female
rabbits. Unpublished report No. 87R-126 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987c) Karathane
FN-57 fungicide-miticide: Acute oral toxicity study in male and female
rats. Unpublished report No. 87R-137 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. 1987d) Karathane
technical purified: Skin irritation study in male rabbits. Unpublished
report No. 87R-125 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987e) Karathane LC
fungicide/miticide: Rabbit skin irritation study. Unpublished report
No. 87R-134 from Rohm & Haas Co., Toxicology Department, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987f) Karathane
technical purified: Eye irritation study in male rabbits. Unpublished
report No. 87R-111 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987g) Karathane LC
fungicide/miticide: Eye irritation study in rabbits. Unpublished
report No. 87R-135 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Ruegg, C.E. (1996) In vitro percutaneous absorption and cutaneous
distribution of Karathane LC XF across intact human and mouse skin.
Unpublished report No. M95-024 from In Vitro Technologies, Inc.,
Baltimore, Maryland, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA. Rohm & Haas Co. report No. 95RC-187..
Sames, J.L., Yu, R.Y. & McCarthy, K.L. (1986) Karathane: In-vivo
cytogenetic study in mice. Unpublished report No. 85R-235 from Rohm &
Haas Co., Toxicology Department, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Solomon, H.M. (1991) Karathane fungicide/miticide: Ocular toxicity in
dogs. Unpublished report No. 91M-663 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Solomon, H.M. & Lutz, M.F. (1989) Dinocap: Oral (gavage) developmental
toxicity study in rats. Unpublished report No. 88R-236 from Rohm &
Haas Co., Toxicology Department, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Wanner, F.J. & Hagan, J.V. (1991) Karathane FN-57: Acute inhalation
study in rats. Unpublished report No. 91R-002 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Weatherholz, W.D., Kundzins, W., Alsaker, R.A., Voelker, R.W. &
Peterson, K. (1979) 104-Week toxicity study in dogs: Karathane
technical. Unpublished report from Hazleton Laboratories America,
Inc., Vienna, Virginia, USA. Submitted to WHO by Rohm & Haas Co.,
Spring House, Pennsylvania, USA. Rohm & Haas Co. report 79RC-45.
Wester, R.W. & Maibach, H.I. (1985) Karathane: Percutaneous absorption
of 14C Karathane (14C-DNHPC) in rhesus monkey following single
topical application. Unpublished report No. 85RC-049 from Rohm & Haas
Co., Toxicology Department, Spring House, Pennsylvania, USA. Submitted
to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
Table 2. Acute toxicity of formulations of dinocap
Species Strain Sex Route Purity LD50 or LC50 Reference
(%) (mg/kg bw
or mg/L)
Technical-grade dinocap
Mouse CD-1 M Oral 95 292 Morrison et al. (1987)
Rat Crl:CD M Oral 95 3100 Morrison et al. (1987)
Rat Crl:CD M Oral 95 > 500 Romanello et al. (1987a)
F < 5000
Rat Crl:CD M Inhalation 95 > 3 Ferguson (1997)
F 3
Rabbit New Zealand white M/F Dermal 95 > 5000 Romanello et al. (1987b)
Karathane LC fungicide-miticide
Mouse CD-1 M Oral 40.4 517 Onishi (1989)
F 413
Mouse CD-1 M/F Dermal 35.6 1020 Procopio & Parno (1995)
Rat Crl:CD M/F Oral 37.6 > 2000 Bernacki & Hamilton (1992a)
Rat Crl:CD M Oral 39 > 500 Lampe et al. (1987a)
F < 5000
Rat Crl:CD M/F Dermal 37.6 > 2000 Bernacki & Hamilton (1992b)
Rabbit New Zealand white M/F Dermal 39 > 5000 Lampe et al. (1987b)
Rat Crl:CD M/F Inhalation 50 0.9 Blair & Cavender (1979)
Karathane FN-57 fungicide-miticide
Mouse CD-1 M/F Oral 20 1401 Morrison & Hamilton (1991)
Rat Crl:CD M Oral 20 > 500 Romanello et al. (1987c)
F < 5000
Rat Crl:CD M/F Inhalation 20 > 4.9 Wanner & Hagan (1991)
Rabbit New Zealand white M/F Dermal 20 > 5000 Krajewski et al. (1987a)
M, male; F, female
Table 3. Results of a dose range-finding study in mice
Outcome Dose (ppm in diet)
0 25 75 225 500 1000 2000 4000
Mortality, males 0/10 0/10 0/10 0/10 0/10 5/10 10/10 10/10
Mortality, females 0/10 0/10 0/10 0/10 7/10 10/10 10/10 10/10
Final body weight, males (g) 34 33 33 32 29 25
Final body weight, females (g) 27 28 29 26 20
Liver weight, males (g) 2.0 2.0 2.1 1.9 1.8 1.7*
Liver weight, females (g) 1.5 1.6 1.7* 1.5 1.2*
Liver weight, males (% bw) 5.8 5.9 6.1 6.0 6.4* 6.8*
Liver weight, females (% bw) 5.5 5.7 6.0* 5.9* 5.9
Hepatocellular necrosis, males NR NR NR NR NR 3/10 9/10 6/10
Hepatocellular necrosis, females NR NR NR NR 5/10 6/10 7/10 3/10
NR, not reported
From Bernacki & Hamilton (1987)
* Statistically significant at 0.05 > p > 0.01
mortality) at 78 weeks. There were no overt treatment-related symptoms
of toxicity. Body-weight gain and food consumption were reduced in
males at 150 ppm and in females at 100 ppm (20% less than that of
controls) and 150 ppm. The efficiency of food use appeared to be
impaired only in males at the highest dose and not in females, thus
suggesting that the effect in females was at least partly a
consequence of palatability. Mean testis weight was reduced in males
at 150 ppm, and histopathological examination revealed a background
incidence of unilateral and bilateral testicular atrophy in all
groups, including controls, with incidences of 11/60, 4/60, 11/59, and
25/60 at 0, 15, 100, and 150 ppm, respectively. A moderate incidence
of individual cell necrosis was reported in mice in all groups,
possibly associated with mouse hepatitis virus infection indicated by
serological markers at 18 months, but the incidence of necrosis seen
in the 28-day study described previously was not reproduced. There was
no evidence of carcinogenicity in any tissue. The NOAEL was 15 ppm,
equivalent to 2.7 mg/kg bw per day, on the basis of reduced
body-weight gain with a corresponding reduction in food consumption in
females at 100 ppm (Moore, 1991).
(d) Genotoxicity
Studies of the genotoxicity of dinocap of lower purity than that
used in the studies reported in the previous monograph are summarized
in Table 4. The results are consistent with the previous conclusions
of the JMPR.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 26 Crl:CD rats of each sex received dinocap (purity,
96%) in the diet at concentrations of 40, 200, or 1000 ppm for two
generations. At weaning of the F1 animals, the highest dose was
reduced to 400 ppm because of a high rate of mortality in these pups.
The litters were culled to eight pups at day 4 post partum. In
addition to the normal guideline requirements, selected F1 males at
0, 40, or 200 ppm were used in a study of gonadal function (sperm
motility, sperm count, weight of cauda epididymis). Body-weight gain
and food consumption were retarded at 1000 ppm during the F0
pre-mating period and at 400 ppm during the F1 pre-mating period.
There was increased pup mortality at weaning among litters of the
group at 1000 ppm, until this dose was reduced to 400 ppm; the cause
of death could not be determined but the presence of yellow or red
discolouration of the contents of the gastrointestinal tract and
bladder suggested the presence of test material or its metabolites.
There were no specific effects on reproductive function or the ability
to rear young, and there was no effect on gonadal function. Increased
liver weights, with no evidence of a histological correlate, were
found among rats receiving 400 or 1000 ppm. The NOAEL was 200 ppm,
equal to 13 mg/kg bw per day (Morseth, 1990).
Table 4. Results of studies of the genotoxicity of dinocap
End-point Test object Dose Purity Results Reference
(%)
In vitro
Gene mutation Chinese hamster 3-10 µg/mla 83.9 Negative Foxall (1985)
ovary cells, hprt locus 15-25 µg/mlb Negative
Metaphase analysis Chinese hamster 1-10 µg/mla NR Negative Ivett & Myhr (1986)
ovary cells 5-20 µg/mlb Negative
In vivo
Metaphase analysis CD-1 mouse 12.6-126 mg/kg bw 83.9 Negative Sames et al. (1986)
bone marrow
a Without an exogenous metabolic system
b With an exogenous metabolic system
(ii) Developmental toxicity
Mice
Groups of 24 CD-1 mice presumed to be pregnant were given dinocap
(purity, 94.4%) in aqueous 1% tragacanth gum at doses of 0 (control),
4, 10, or 25 mg/kg bw per day by gavage on days 6-15 of gestation. On
day 18 of gestation, about half of the mice in each group were killed
and the fetuses were examined; the remaining dams were allowed to
deliver and rear their litters, which were culled on day 4 post
partum to two pups per litter. On day 43, these mice were evaluated
for swimming performance.
The results of this study are shown in Table 5. There were no
maternal deaths during the study and no overt signs of toxicity. The
body-weight gain of the pregnant mice was minimally impaired at 25
mg/kg bw per day on days 12-16 of gestation, although the weight
difference may have been a consequence of the reduced litter size.
Gross examination showed no treatment-related findings. In animals
killed on day 18 of gestation (the normal end of a study of
teratogenicity), the average number of resorptions was increased in
dams at 25 mg/kg bw per day, with an associated decrease in litter
size. The incidence of resorptions was slightly increased in dams at
at 10 mg/kg bw per day, but the increase was within the range of
historical controls (0-1.6) All treated animals that delivered
naturally had a statistically significant increase in the duration of
gestation, although this was not considered to be toxicologically
significant.
The mean fetal weight was reduced, and there was an increased
incidence of fetuses with cleft palate and open eyelids at 10 and 25
mg/kg bw per day. There was an increased incidence of stillborn pups
and decreased pup body weight on days 7-21 post partum at 25 mg/kg
bw per day, and pup survival to day 4 post partum was reduced. The
incidence of litters with pups with head tilt and the incidence of
pups with cleft palate were increased at 25 mg/kg bw per day, although
all nine pups with cleft palate were from the same litter. Among the
pups that were allowed to survive until day 43 post partum, there
was an increased incidence of mice with head tilt, and ungroomed
coats, corneal opacity, and ptosis were seen in one or two males at
the highest dose. Body-weight gain and average body weights were
decreased in mice at this dose, and there was an increased incidence
of mice with altered swimming postures or ability. Of the 11 pups with
altered swimming posture, 10 had head tilt. A further three pups with
head tilt did not have altered swimming posture. The NOAEL for
developmental toxicity was 4 mg/kg bw per day on the basis of the
increased incidence of open eyelids and cleft palate in pups at 10
mg/kg bw per day. The NOAEL for maternal toxicity was 10 mg/kg bw per
day on the basis of minimally retarded weight gain during days 12-16
of gestation in dams at 25 mg/kg bw per day. Dinocap was teratogenic,
causing malformations at doses that had no effect on the dam (Lochry,
1989).
Table 5. Results of a study of developmental toxicity in mice
Parameter Dose (mg/kg bw per day)
0 4 10 25
Maternal weight gain (g), days 6-16 14.8 16.0 16.5 13.8
days 12-16 8.2 8.7 8.8 6.4
days 0-18 26.3 27.0 27.3 23.3
Caesarian-derived pups
No. of litters evaluated 12 12 12 9
Resorptions per litter 1.0 0.5 1.2* 1.8*
Litter size 11.2 11.2 10.8 8.7
Mean fetal weight (g) 1.4 1.35 1.30* 1.10**
Open eyelids, litters (total no. fetuses) 0 0 1 (1) 2 (3**)
Cleft palate, litters (total no. fetuses) 0 0 3 (4) 7 ** (65**)
Naturally-delivered pups
No. of litters evaluated 11 12 12 10
Gestation duration (days) 19.4 19.8* 19.8* 19.9**
Litter size, day 1 post partum 11.4 11.3 11.8 11.8
Head tilt (no. of litters) 0 0 0 3**
Cleft palate/pups dying days 0-21 post partum 0/3 0/1 0/0 9/16
Abnormal swimming ability, day 43, litters (no. fetuses) 0/11 0/11 0/12 5/9** (11/39)**
From Lochry (1989)
* Statistically significant at 0.05 > p > 0.01; ** statistically significant at 0.01 > p
A study was conducted to establish suitable doses for a study of
teratogenicity after dermal application to CD-1 mice. Fewer animals
were used that recommended in guideliness. Dinocap was applied as the
formulation Karathane LC XF, considered to be appropriate for
estimating the risk due to occupational exposure, but the doses used
were corrected for content and expressed as dinocap. Appropriate
dilutions were obtained with the formulation blank. In phase 1 of the
study, both untreated and vehicle control groups were used; in phase
2, only a vehicle control group was used. The dose volume of 290 µl/kg
bw was applied to one to five areas of shaven dorsal skin for 4 h each
day on days 6-15 of presumed gestation; the site was changed each day,
recommencing at the first site on the sixth day. Occlusion of the
application site was not mentioned, but a collar was placed to prevent
ingestion; no mention is made of how the mice were restrained during
application. After completion of the 4-h exposure each day, the test
material was washed off gently with soap and water.
Initially (phase 1), groups of eight presumed-pregnant mice were
given doses of 0 (control), 50, 80, or 100 mg/kg bw per day. Because
of excessive toxicity, similar groups of eight mice were given doses
of 0, 1, 4, 10, or 25 mg/kg bw per day by a similar protocol. The mice
were sacrificed on day 18 of presumed gestation; half of the fetuses
were used for skeletal examination, and the remaining half for
specific examination of otoliths and of the remaining skeleton. For
mice weighing 20-40 g, the dose volumes would have been 6-12 µl: the
practical difficulty of administering such small volumes may have
resulted in variations in accuracy. One, three, and four deaths
occurred during treatment at 50, 80, and 100 mg/kg bw per day,
respectively. Death was usually briefly (up to 24 h) preceded by signs
of toxicity, including dermal erythema, ataxia, red or tan vaginal
discharge, weight loss, and cold to touch. These doses all reduced
weight gain, with weight loss at the highest dose. The numbers of live
young were reduced at 50 and 80 mg/kg bw per day, and the litters of
the three surviving dams at 100 mg/kg bw per day contained only
resorbed conceptuses. Gross malformations were seen in 88% of fetuses
at 50 mg/kg bw per day and 100% of those at 80 mg/kg bw per day.
The principal treatment-related effects seen in phase 2 are shown
in Table 6. Little maternal toxicity occurred, and body-weight gain
was apparently unaffected by treatment. One mouse at the highest dose
was found dead on day 15 of gestation having been found stuporous with
an impaired righting reflex after dosing the day before. Death was
attributed to trauma, although no clear injury was detected at
necropsy. There was a slight incidence of skin irritation at the two
highest doses. The litter sizes, numbers of live fetuses per litter,
and fetal body weights were unaffected by treatment. There was no
change in the degree of skeletal ossification, but three fetuses of
one litter at the highest dose had cleft palate, and two fetuses of
another litter had open eyelids. Also at the highest dose, otolith
development was clearly impaired. These findings are consistent with
those of previous studies of the teratogenicity of dinocap in mice
after oral administration. In view of these results, no further study
by the dermal route was conducted. The study involved too few animals
to determine a NOAEL for teratogenicity in mice treated dermally,
particularly with respect to cleft palate; however, the quality of the
data on mean otolith scores in 49 fetuses may provide some degree of
confidence that the NOAEL for these effects is 10 mg/kg bw per day
(Foss, 1995).
Table 6. Results of a study for developmental toxicity in mice
Effect Dose (mg/kg bw per day)
0 1 4 10 25
No. of pregnant dams 8 8 7 8 6
Maternal weight gain on days 6-18 (g) 21.4 26.4 20.8 26.4 25.0
No. of fetuses 77 96 67 93 73
Cleft palate (no. of litters) 0 0 0 0 1
Open eyelids (no. of litters) 0 0 0 0 1
Mean otolith scorea 9.2 9.6 9.0 9.0 2.2
No. of fetuses examined 37 49 34 49 38
From Foss (1995)
a Three otoliths were scored for completeness on a scale of 1-4, to give a maximum
potential score of 12.
2,4-DNHPC and 2,6-DNHPC were not teratogenic in mice. Samples of
each isomer (purity, 95%) were tested seperately and in combination,
and the results were compared with those in mice receiving
technical-grade dinocap (purity, 84%), each group at a dose of 25
mg/kg bw per day in corn oil. Fewer pregnant animals were used than
recommended in the guidelines. Although litters born to
dinocap-treated mice showed a pattern of developmental defects typical
of those seen in the studies described above, the two isomers were
inactive at the same dose, both alone and in combination (Rogers et
al., 1987). In a separate study by the same group, otolith formation
was compared in the pups of mice and hamsters treated with dinocap
during gestation. Otolith formation was impaired in mice. Dinocap
affected otolith formation in hamsters, but only at a dose associated
with severe maternal and fetal toxicity (Rogers et al., 1989).
Rats
Groups of 25 Sprague-Dawley rats presumed to be pregnant received
dinocap (purity, 96%) as a suspension in aqueous methylcellulose at
doses of 0 (control), 10, 50, or 150 mg/kg bw per day by gavage on
days 6-15 of gestation. There were no deaths, and the only overt sign
of toxicity was an increased incidence of soft faeces in rats at 150
mg/kg bw per day. Maternal body-weight gain was impaired at 150 mg/kg
bw per day during the first two days of treatment, and food
consumption was slightly reduced in this group during treatment. Gross
examination revealed no treatment-related changes in pregnant or non-
pregnant females. There were no treatment-related changes in pup
weight and no increase in the incidence of malformations. There was an
apparent increase in the incidence of extra ribs at 150 mg/kg bw per
day, but seven of the 10 affected fetuses were from two litters. The
NOAEL for maternal and fetal toxicity was 50 mg/kg bw per day. There
was no evidence of teratogenicity at the highest dose of 150 mg/kg bw
per day (Solomon & Lutz, 1989).
Rabbits
Groups of 20 artificially inseminated New Zealand white rabbits
received dinocap (purity, 95.4%) in aqueous gum tragacanth by gavage
at doses of 0, 3, 12, 48, or 84 mg/kg bw per day on days 7-19 of
presumed pregnancy. Pups were delivered by caesarian section on day 29
and examined for developmental abnormalities in accordance with normal
guideline requirements. Two does at the high dose and one at the
intermediate dose died between days 24 and 29 of gestation, therefore
at least five days after dosing, but the deaths were considered to be
related to treatment. These animals all showed weight loss and
anorexia before death. The doe at the intermediate dose and one at the
high dose aborted; both does at the high dose that died had non-viable
litters (late resorptions). An additional five deaths were considered
unrelated to treatment and were primarily a consequence of intubation
errors.
The main results are shown in Table 7; statistical analyses were
not reported. There was an increased incidence of premature delivery
and abortion at 48 and 84 mg/kg bw per day; the premature deliveries
at the highest dose were primarily late resorptions. Clinical signs
(lack of faeces or dried faeces) and impaired weight gain and food
consumption were seen among does receiving these doses, litter sizes
and pup weights were reduced, and there was an increased incidence of
pups with skeletal malformations (vertebral assymetry and fused or
forked ribs). There was also a slight increase in delayed ossification
at a few sites in does at these doses. The NOAEL for maternal toxicity
was 3 mg/kg bw per day on the basis of weight-gain retardation during
treatment. The NOAEL for developmental toxicity was 12 mg/kg bw per
day on the basis of increased resorptions and reduced litter sizes at
48 mg/kg bw per day (Hoberman & Christian, 1987).
This conclusion is different from that of the 1989 JMPR (Annex 1,
reference 58) but is based on different data. In the latter review, an
increased incidence of hydrocephaly and neural tube defects was found
at doses > 3 mg/kg bw per day in the studies of Costlow & Kane
(1984 a,b). In the earlier studies, however, a less pure form of
dinocap (84%) was used, and the neural tube defects found by Costlow
and Kane (1984a) at a dose of 3 mg/kg bw per day were not found in the
second study (Costlow & Kane, 1984b) at 48 mg/kg bw per day nor in
studies by dermal administration in which the increased numbers of
Table 7. Results of a study of developmental toxicity in rabbits
Result Dose (mg/kg bw per day)
0 3 12 48 84
Adult animals
No. pregnant 17 17 15 15 14
No. with abortions 0 0 0 3 1
No. with premature delivery 1 0 0 1 3
No. with absence of faeces (on any day) 0 0 0 0 4
No. with dried faeces (on any day) 3 0 2 7 17
Weight change, days 7-20 (g) 150 200 90 -330 -550
Food consumption, days 7-20 (g/day) 160 170 152 65 51
No. with gastric ulceration 0 0 1 3 4
Litters
No. evaluated 12 17 14 10 9
Mean no. of live fetuses 7.2 7.5 8.1 6.3 5.9
Dead or resorbed fetuses per litter (%) 11.8 4.9 7.1 20.3 27.0
Mean fetal weight (g) 40.9 42.7 40.0 37.6 35.8
Vertebral assymetry: no. of litters (pups) 1 (1) 1 (1) 1 (2) 5 (7) 3 (8)
No. of litters (pups) with rib malformation 1 (1) 0 2 (3) 2 (2) 2 (6)
No. of litters (pups) with skeletal malformations 4 (4) 3 (3) 4 (6) 5 (7) 6 (12)
From Hoberman (1987)
resorptions and delayed ossifications were similar to these found by
Hoberman & Christian (1987).
(f) Special studies: Ocular toxicity
A NOAEL of 15 ppm was observed for ocular toxicity in a two-year
study in dogs (Weatherholz et al., 1979) in the 1989 JMPR (Annex 1,
reference 38). At 60 ppm, four of eight dogs had slight to moderate
discolouration of the tapetum lucidum, although no retinal atrophy and
no changes in the vascularity of the retina were noted. The remaining
four dogs at this dose showed moderate to marked discolouration of the
tapetum, reduced vascularity of the retina, and retinal atrophy (three
dogs). At 120/240 ppm, the four surviving dogs had marked
discolouration of the tapetum, reduced vascularity of the retina, and
retinal atrophy. Retinal atrophy was therefore present only in dogs
with severe tapetal changes, and no dog showed retinal effects without
effects in the tapetum.
A review of the literature for compounds that are toxic to the
tapetum lucidum of dogs was submitted (Solomon, 1991). Of six
compounds known to affect the tapetum, three (zinc pyridinethione, SCH
19927, and rosaramicin) had no effect in atapetal dogs, and no ocular
effects were reported in other atapetal animals. The other three
compounds (dinocap, hydroxy pyridinethione, and diphenyl
thiocarbazine) affected the tapetum in dogs (with no information on
atapetal dogs) but did not cause ocular effects in atapetal species.
The author concluded that the retinal atrophy seen with dinocap was
secondary to damage to the tapetum lucidum. Since humans do not have a
tapetum, it was concluded that humans would not be susceptible to
retinal damage as a consequence of this effect. The review is brief,
and the adequacy of the methods used, the sensitivity, and the
comparability of the findings were not considered. The conclusions
cannot therefore be regarded as definitive, although the data support
the hypothesis. The NOAEL in the study of Weatherholz et al. (1979)
was therefore identified for other effects. The 1989 JMPR report
(Annex 1, reference 58) indicates that significant effects, including
deaths, occurred at doses of 180-240 ppm, giving a NOAEL of 60 ppm,
equivalent to 1.5 mg/kg bw per day.
Comments
Dinocap is well absorbed after oral exposure. A proportion
(5-25%) is absorbed after dermal exposure, varying with species and
concentration. No conclusions were drawn about the degree of dermal
absorption in humans from the results of a study in which mouse and
human skin were compared; however, human skin is generally regarded as
being less permeable to toxicants than that of mice.
The urinary metabolites of the methylheptyl isomer in rats and
mice have been extensively characterized; characterization of the
faecal metabolites was reported by the 1989 JMPR, which concluded that
the pattern of metabolites in faeces seen by thin-layer chromatography
was similar to that observed in squash and cucumbers.
The new data confirmed the generally low degree of acute toxicity
of dinocap in rats; mice, however, appear to be more sensitive than
rats to both acute and developmental effects. Dinocap is a skin
irritant and sensitizer. The available studies did not address the
uncoupling of oxidative phosphorylation, identified by the 1989 JMPR
as a potentially significant mode of action.
WHO has classified dinocap as 'slightly hazardous' (WHO, 1996).
In a study of carcinogenicity in mice at doses of 0, 15, 100, or
200 ppm, no evidence of carcinogenicity was found. The NOAEL was 15
ppm, equal to 2.7 mg/kg bw per day. The lack of carcinogenicity in
mice is consistent with the absence of carcinogenicity in rats
reported by the 1989 JMPR.
The results of tests for genotoxicity (on the less pure form of
dinocap) were negative.
A multigeneration study of reproductive toxicity at dietary
concentrations of 0, 40, 200, or 1000 ppm in rats showed no specific
effect on any reproductive parameters; the NOAEL was 200 ppm, equal to
13 mg/kg bw per day.
In a study of developmental toxicity in mice dosed by gavage at
0, 4, 10, or 25 mg/kg bw per day, impaired otolith formation was seen
at 25 mg/kg bw per day. A dose-related increase in the incidence of
open eyelids and cleft palate extended down to 10 mg/kg bw per day in
the absence of maternal toxicity. The NOAEL was 4 mg/kg bw per day.
Dermal application of 50, 80, or 100 mg/kg bw per day to mice proved
excessively toxic for an evaluation of developmental toxicity. A
further dermal study in mice at 0, 1, 4, 10, or 25 mg/kg bw per day
showed malformations,including impaired otolith formation, at 25 mg/kg
bw per day in the absence of maternal toxicity. The NOAEL for
developmental toxicity after dermal application to mice was 10 mg/kg
bw per day. The results of the recent studies of developmental
toxicity confirmed the teratogenic potential of purified dinocap in
mice, even when applied dermally. Impaired otolith development,
characteristic of the teratogenicity of dinocap in mice, was also seen
in hamsters at doses that are maternally toxic. Less specific
malformations were seen in rabbits at maternally toxic doses. The
present Meeting concluded that the NOAEL in the studies in rabbits
described by the 1989 JMPR was 3 mg/kg bw per day rather than
0.5 mg/kg bw per day, since the findings on which the putative effect
level was established do not appear to be repeatable or clearly
dose-related. The methylheptyl isomer has been shown not to be
teratogenic to mice. The reason for the species difference in the
teratogenicity of dinocap in rats and mice therefore cannot be deduced
from the data on the metabolism of the methylheptyl isomer.
The two-year study in dogs that was evaluated at the 1989 Joint
Meeting was also reassessed on the basis that the critical effect
(retinal atrophy) was secondary to effects on the tapetum lucidum.
Since the tapetum lucidum is not present in humans, or in rats or mice
in which no retinal effect was seen, the Meeting concluded that it
would be inappropriate to base the evaluation on this effect. The
NOAEL was 60 ppm, equivalent to 1.5 mg/kg bw per day.
Teratogenic effects in mice were considered to be the
toxicological end-point of greatest concern. Since dinocap was
teratogenic in mice after either oral or dermal administration and
since malformations were seen in at least three species, the Meeting
considered a high safety factor to be appropriate. An ADI of 0-0.008
mg/kg bw was established on the basis of the NOAEL of
4 mg/kg bw per day in the developmental toxicity study in mice and a
safety factor of 500.
Establishment of an acute reference dose (RfD) was considered to
be appropriate since teratogenicity may occur after a single exposure.
An acute RfD was established on the basis of the NOAEL of 4 mg/kg bw
per day for teratogenicity in mice and a safety factor of 500, to give
an acute RfD of 0.008 mg/kg bw, which is appropriate for women of
child-bearing age.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 15 ppm, equal to 2.7 mg/kg bw per day (toxicity in a
study of carcinogenicity)
4 mg/kg bw per day (developmental toxicity)
10 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rat: 200 ppm, equal to 6.4 mg/kg bw per day (toxicity in a
study of carcinogenicity)
50 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Rabbit: 3 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 60 ppm, equivalent to 1.5 mg/kg bw per day (study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.008 mg/kg bw
Estimate of acute reference dose
0.008 mg/kg bw
Information that would be useful for continued evaluation of the
compound
Further observations in humans
List of end-points for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption 60-69% absorbed, max. concentration at 2-6 h
Dermal absorption 5-25%
Distribution Widely distributed
Potential for accumulation Limited, < 0.3% in tissue after 7 days
Rate and extent of excretion Biphasic; half-life is 3 h for 1st phase, 44 h for 2nd
phase, oral administration, rabbit
Metabolism in animals Extensive; > 96% metabolized
Toxicologically significant compounds Metabolites assumed to be of similar toxicity to parent
(animals, plants and environment)
Acute toxicity
Rat: LD50 oral 3100 mg/kg bw
Rat: LD50 dermal > 5000 mg/kg bw
Rat: LC50 inhalation 3 mg/L
Skin irritation Irritating
Eye irritation Irritating
Skin sensitization Sensitizing
Short-term toxicity
Target/critical effect General toxicity
Lowest relevant oral NOAEL Dog: 1.5 mg/kg bw per day
Lowest relevant dermal NOAEL Mouse: 10 mg/kg bw per day (teratogenicity)
Lowest relevant inhalation NOAEL No data
Genotoxicity Not genotoxic in an adequate battery of tests
Long-term toxicity and carcinogenicity
Target/critical effect: Impaired weight gain
Lowest relevant NOAEL Mouse: 2.7 mg/kg bw per day (carcinogenicity)
Carcinogenicity Not carcinogenic
Reproductive toxicity
Reproduction target/critical effect No effect on fertility or ability to rear young
Lowest relevant reproductive NOAEL Rat: 13 mg/kg bw per day (multigeneration study)
Developmental target/critical effect Malformations
Lowest relevant developmental NOAEL Mouse: 4 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No data, but no concern raised in other studies
Other toxicological studies Inhibits oxidative phosphorylation; methylheptyl
isomer not teratogenic
Medical data No significant dinocap-related effects reported
Summary Value Study Safety factor
ADI 0-0.008 mg/kg bw Mouse, developmental toxicity 500
Acute reference dose 0.008 mg/kg bw Mouse, developmental toxicity 500
References
Anderson, D.M. & Baldwin, R.C. (1990a) Karathane LC fungicide/miticide
delayed contact hypersensitivity study in guinea pigs. Unpublished
report No. 89R-187 from Rohm & Haas Company, Toxicology Department,
Spring House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas
Company, Spring House, Pennsylvania, USA.
Anderson, D.M.& Baldwin, R.C. (1990b) Karathane FN-57
fungicide/miticide delayed contact hypersensitivity study in guinea
pigs. Unpublished study No. 89R-188 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Anderson, D.M. & Baldwin, R.C. (1990c) Karathane technical (HPK)
fungicide/miticide delayed contact hypersensitivity study in guinea
pigs. Unpublished report No. 89R-189 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Bernacki, H.J. & Baldwin, R.C. (1987) Karathane technical (dinocap):
Range finding four-week dietary toxicity study in mice. Unpublished
report No. 87R-175. from Rohm & Haas Co., Toxicology Department,
Spring House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co.,
Spring House, Pennsylvania, USA.
Bernacki, H.J. & Hamilton, J.D. (1992a) Karathane LC
fungicide/miticide: Skin irritation study in rabbits. Unpublished
report No. 91R-170 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Bernacki, H.J. & Hamilton, J.D. (1992b) Karathane LC
fungicide/miticide: Acute dermal toxicity study in male and female
rats. Unpublished report No. 91R-171 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Bernacki, H.J. & Hamilton, J.D. (1992c) Karathane LC
fungicide/miticide: Acute oral toxicity study in male and female rats.
Unpublished report No. 91R-173 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Blair, M. & Cavender, F.L. (1979) TD 77-310 acute inhalation study in
rats. Unpublished report No. 285-029 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Rohm & Haas Co., Spring House, Pennsylvania, USA.
Costlow, R.D. & Kane, W.W. (1984a) Teratology study with Karathane in
rabbits. Unpublished report No. 83R-22 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Costlow, R.D. & Kane, W.W. (1984b) Teratology study with Karathane in
rabbits. Unpublished report No. 83R-113 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA
Evans, K.W. & Hazelton, G.A. (1995) Blood pharmacokinetics and dermal
absorption of Karathane in female mice. Unpublished report No. 95R-105
from Rohm & Haas Co., Toxicology Department, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Ferguson, J.S. (1997) Karathane technical: Acute inhalation toxicity
study in rats. Unpublished report No. 96R-126 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Foss, J.A. (1995) Dermal dosage-range developmental toxicity study of
Karathane LC XF in mice. Unpublished report No. 018-023P from Argus
Research Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA Rohm & Haas
Co. report No. 95RC-024.
Foxall, S. (1985) Karathane technical: CHO/HGPRT gene mutation assay.
Unpublished report No. 85R-204 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Hoberman, A. & Christian, M. (1987) A developmental toxicity study of
Karathane fungicide/miticide (technical) administered via stomach tube
to New Zealand white rabbits. Unpublished report No. 018-012 from
Argus Research Laboratories, Inc., Horsham, Pennsylvania, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Rohm & Haas Co. report No. 86RC-71.
Ivett, J. & Myhr, B. (1986) Clastogenic evaluation of Karathane
technical in an in-vitro cytogenetic assay measuring chromosomal
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished study project No. 20990 from Litton Bionetics, Inc.,
Kensington, Massachusetts, USA. Rohm & Haas Co. report No. 85RC-62.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1987a) Karathane
FN-57 fungicide/miticide: Acute dermal toxicity study in male and
female rabbits. Unpublished report No. 87R-138 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1987b) Karathane
FN-57 fungicide/miticide: Eye irritation study in rabbits. Unpublished
report No. 87R-114 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1987c) Karathane
FN-57 fungicide/miticide: Skin irritation study in male rabbits.
Unpublished report No. 87R-139 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Lampe, K.R., Morrison, R.D. & Baldwin, R.C. (1987a) Karathane LC
fungicide-miticide: Acute oral toxicity study in male and female rats.
Unpublished report No. 87R-132 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Lampe, K.R., Morrison, R.D. & Baldwin, R.C. (1987b) Karathane LC
fungicide-miticide: Acute dermal toxicity study in male and female
rats. Unpublished report No. 87R-133 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Lochry, E. (1989) Dinocap (high purity technical): An oral (gavage)
developmental toxicity study in CD-1(ICR)BR mice including a postnatal
phase. Unpublished report No. 018-015 from Argus Research
Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO by
Rohm & Haas Co., Spring House, Pennsylvania, USA. Rohm & Haas Co.
report No. 88P-335.
Maibach, H. (1985) Elimination of 14C labeled Karathane in rhesus
monkeys following a single intramuscular injected dose. Unpublished
report No. 85RC-18 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Moore, M.R. (1991) Dinocap (technical purified): 78-Week dietary
oncogenicity study in mice. Unpublished report No. 417-445 from
Hazleton Laboratories America, Inc., Rockville, Massachusetts, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Rohm & Haas Co. report No. 88RC-090
Morrison, R.D. & Hamilton, J.D. (1991) Karathane FN-57
fungicide/miticide: Acute oral toxicity study in male and female mice.
Unpublished report No. 91R-001 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Morrison, R.D. & Hamilton, J.D. (1992) Karathane LC
fungicide/miticide: Eye irritation study in rabbits. Unpublished
report No. 91R-172 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Morrison, R.D., Krzywicki, K.M. & Hazelton, G.A. (1987) Karathane
technical purified: Acute oral toxicity study in male rats and mice.
Unpublished report No. 86R-149A,B from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Morseth, S. (1990) Dinocap (technical purified): Two-generation
reproduction study in rats. Unpublished report No. 417-442 from
Hazleton Laboratories America, Inc., Vienna, Virginia, USA. Submitted
to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA. Rohm &
Haas Co. report No. 88RC-092.
Onishi, M. (1989) Karathane LC (HPK) fungicide/fiticide: Acute oral
toxicity study in mice. Unpublished study from Shin Nippon Biomedical
Laboratories Ltd, Kagoshima, Japan. Rohm & Haas Co. report No.
90RC-1001. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Potter, D. (1996) Identification of
2,4-dinitro-6-(1-methylheptyl)phenyl crotonate metabolites in rat and
mouse urine. Unpublished report No. 95R-064 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Procopio, K.R. & Parno, J.R. (1995) Karathane LC: Acute dermal
toxicity study in male and female mice. Unpublished report No. 94R-244
from Rohm & Haas Co., Toxicology Department, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Rogers, J.M., Gray, E.L., Carver, B.D. & Kavlock, R.J. (1987)
Developmental toxicity of dinocap in the mouse is not due to two
isomers of the major active ingredient. Teratol. Carcinog. Mutag.,
7, 341-346.
Rogers, J.M., Burkhead, L.M. & Barbee B.D. (1989) Effects of dinocap
on otolith formation: Evaluation of mouse and hamster fetuses at term.
Teratology, 39, 515-523.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987a) Karathane
technical purified: Acute oral toxicity study in male and female rats.
Unpublished report No. 87R-124 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987b) Karathane
technical purified: Acute dermal toxicity study in male and female
rabbits. Unpublished report No. 87R-126 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987c) Karathane
FN-57 fungicide-miticide: Acute oral toxicity study in male and female
rats. Unpublished report No. 87R-137 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. 1987d) Karathane
technical purified: Skin irritation study in male rabbits. Unpublished
report No. 87R-125 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987e) Karathane LC
fungicide/miticide: Rabbit skin irritation study. Unpublished report
No. 87R-134 from Rohm & Haas Co., Toxicology Department, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987f) Karathane
technical purified: Eye irritation study in male rabbits. Unpublished
report No. 87R-111 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987g) Karathane LC
fungicide/miticide: Eye irritation study in rabbits. Unpublished
report No. 87R-135 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Ruegg, C.E. (1996) In vitro percutaneous absorption and cutaneous
distribution of Karathane LC XF across intact human and mouse skin.
Unpublished report No. M95-024 from In Vitro Technologies, Inc.,
Baltimore, Maryland, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA. Rohm & Haas Co. report No. 95RC-187..
Sames, J.L., Yu, R.Y. & McCarthy, K.L. (1986) Karathane: In-vivo
cytogenetic study in mice. Unpublished report No. 85R-235 from Rohm &
Haas Co., Toxicology Department, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Solomon, H.M. (1991) Karathane fungicide/miticide: Ocular toxicity in
dogs. Unpublished report No. 91M-663 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Solomon, H.M. & Lutz, M.F. (1989) Dinocap: Oral (gavage) developmental
toxicity study in rats. Unpublished report No. 88R-236 from Rohm &
Haas Co., Toxicology Department, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Wanner, F.J. & Hagan, J.V. (1991) Karathane FN-57: Acute inhalation
study in rats. Unpublished report No. 91R-002 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Weatherholz, W.D., Kundzins, W., Alsaker, R.A., Voelker, R.W. &
Peterson, K. (1979) 104-Week toxicity study in dogs: Karathane
technical. Unpublished report from Hazleton Laboratories America,
Inc., Vienna, Virginia, USA. Submitted to WHO by Rohm & Haas Co.,
Spring House, Pennsylvania, USA. Rohm & Haas Co. report 79RC-45.
Wester, R.W. & Maibach, H.I. (1985) Karathane: Percutaneous absorption
of 14C Karathane (14C-DNHPC) in rhesus monkey following single
topical application. Unpublished report No. 85RC-049 from Rohm & Haas
Co., Toxicology Department, Spring House, Pennsylvania, USA. Submitted
to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.

 Table 2. Acute toxicity of formulations of dinocap
Species Strain Sex Route Purity LD50 or LC50 Reference
(%) (mg/kg bw
or mg/L)
Technical-grade dinocap
Mouse CD-1 M Oral 95 292 Morrison et al. (1987)
Rat Crl:CD M Oral 95 3100 Morrison et al. (1987)
Rat Crl:CD M Oral 95 > 500 Romanello et al. (1987a)
F < 5000
Rat Crl:CD M Inhalation 95 > 3 Ferguson (1997)
F 3
Rabbit New Zealand white M/F Dermal 95 > 5000 Romanello et al. (1987b)
Karathane LC fungicide-miticide
Mouse CD-1 M Oral 40.4 517 Onishi (1989)
F 413
Mouse CD-1 M/F Dermal 35.6 1020 Procopio & Parno (1995)
Rat Crl:CD M/F Oral 37.6 > 2000 Bernacki & Hamilton (1992a)
Rat Crl:CD M Oral 39 > 500 Lampe et al. (1987a)
F < 5000
Rat Crl:CD M/F Dermal 37.6 > 2000 Bernacki & Hamilton (1992b)
Rabbit New Zealand white M/F Dermal 39 > 5000 Lampe et al. (1987b)
Rat Crl:CD M/F Inhalation 50 0.9 Blair & Cavender (1979)
Karathane FN-57 fungicide-miticide
Mouse CD-1 M/F Oral 20 1401 Morrison & Hamilton (1991)
Rat Crl:CD M Oral 20 > 500 Romanello et al. (1987c)
F < 5000
Rat Crl:CD M/F Inhalation 20 > 4.9 Wanner & Hagan (1991)
Rabbit New Zealand white M/F Dermal 20 > 5000 Krajewski et al. (1987a)
M, male; F, female
Table 3. Results of a dose range-finding study in mice
Outcome Dose (ppm in diet)
0 25 75 225 500 1000 2000 4000
Mortality, males 0/10 0/10 0/10 0/10 0/10 5/10 10/10 10/10
Mortality, females 0/10 0/10 0/10 0/10 7/10 10/10 10/10 10/10
Final body weight, males (g) 34 33 33 32 29 25
Final body weight, females (g) 27 28 29 26 20
Liver weight, males (g) 2.0 2.0 2.1 1.9 1.8 1.7*
Liver weight, females (g) 1.5 1.6 1.7* 1.5 1.2*
Liver weight, males (% bw) 5.8 5.9 6.1 6.0 6.4* 6.8*
Liver weight, females (% bw) 5.5 5.7 6.0* 5.9* 5.9
Hepatocellular necrosis, males NR NR NR NR NR 3/10 9/10 6/10
Hepatocellular necrosis, females NR NR NR NR 5/10 6/10 7/10 3/10
NR, not reported
From Bernacki & Hamilton (1987)
* Statistically significant at 0.05 > p > 0.01
mortality) at 78 weeks. There were no overt treatment-related symptoms
of toxicity. Body-weight gain and food consumption were reduced in
males at 150 ppm and in females at 100 ppm (20% less than that of
controls) and 150 ppm. The efficiency of food use appeared to be
impaired only in males at the highest dose and not in females, thus
suggesting that the effect in females was at least partly a
consequence of palatability. Mean testis weight was reduced in males
at 150 ppm, and histopathological examination revealed a background
incidence of unilateral and bilateral testicular atrophy in all
groups, including controls, with incidences of 11/60, 4/60, 11/59, and
25/60 at 0, 15, 100, and 150 ppm, respectively. A moderate incidence
of individual cell necrosis was reported in mice in all groups,
possibly associated with mouse hepatitis virus infection indicated by
serological markers at 18 months, but the incidence of necrosis seen
in the 28-day study described previously was not reproduced. There was
no evidence of carcinogenicity in any tissue. The NOAEL was 15 ppm,
equivalent to 2.7 mg/kg bw per day, on the basis of reduced
body-weight gain with a corresponding reduction in food consumption in
females at 100 ppm (Moore, 1991).
(d) Genotoxicity
Studies of the genotoxicity of dinocap of lower purity than that
used in the studies reported in the previous monograph are summarized
in Table 4. The results are consistent with the previous conclusions
of the JMPR.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 26 Crl:CD rats of each sex received dinocap (purity,
96%) in the diet at concentrations of 40, 200, or 1000 ppm for two
generations. At weaning of the F1 animals, the highest dose was
reduced to 400 ppm because of a high rate of mortality in these pups.
The litters were culled to eight pups at day 4 post partum. In
addition to the normal guideline requirements, selected F1 males at
0, 40, or 200 ppm were used in a study of gonadal function (sperm
motility, sperm count, weight of cauda epididymis). Body-weight gain
and food consumption were retarded at 1000 ppm during the F0
pre-mating period and at 400 ppm during the F1 pre-mating period.
There was increased pup mortality at weaning among litters of the
group at 1000 ppm, until this dose was reduced to 400 ppm; the cause
of death could not be determined but the presence of yellow or red
discolouration of the contents of the gastrointestinal tract and
bladder suggested the presence of test material or its metabolites.
There were no specific effects on reproductive function or the ability
to rear young, and there was no effect on gonadal function. Increased
liver weights, with no evidence of a histological correlate, were
found among rats receiving 400 or 1000 ppm. The NOAEL was 200 ppm,
equal to 13 mg/kg bw per day (Morseth, 1990).
Table 4. Results of studies of the genotoxicity of dinocap
End-point Test object Dose Purity Results Reference
(%)
In vitro
Gene mutation Chinese hamster 3-10 µg/mla 83.9 Negative Foxall (1985)
ovary cells, hprt locus 15-25 µg/mlb Negative
Metaphase analysis Chinese hamster 1-10 µg/mla NR Negative Ivett & Myhr (1986)
ovary cells 5-20 µg/mlb Negative
In vivo
Metaphase analysis CD-1 mouse 12.6-126 mg/kg bw 83.9 Negative Sames et al. (1986)
bone marrow
a Without an exogenous metabolic system
b With an exogenous metabolic system
(ii) Developmental toxicity
Mice
Groups of 24 CD-1 mice presumed to be pregnant were given dinocap
(purity, 94.4%) in aqueous 1% tragacanth gum at doses of 0 (control),
4, 10, or 25 mg/kg bw per day by gavage on days 6-15 of gestation. On
day 18 of gestation, about half of the mice in each group were killed
and the fetuses were examined; the remaining dams were allowed to
deliver and rear their litters, which were culled on day 4 post
partum to two pups per litter. On day 43, these mice were evaluated
for swimming performance.
The results of this study are shown in Table 5. There were no
maternal deaths during the study and no overt signs of toxicity. The
body-weight gain of the pregnant mice was minimally impaired at 25
mg/kg bw per day on days 12-16 of gestation, although the weight
difference may have been a consequence of the reduced litter size.
Gross examination showed no treatment-related findings. In animals
killed on day 18 of gestation (the normal end of a study of
teratogenicity), the average number of resorptions was increased in
dams at 25 mg/kg bw per day, with an associated decrease in litter
size. The incidence of resorptions was slightly increased in dams at
at 10 mg/kg bw per day, but the increase was within the range of
historical controls (0-1.6) All treated animals that delivered
naturally had a statistically significant increase in the duration of
gestation, although this was not considered to be toxicologically
significant.
The mean fetal weight was reduced, and there was an increased
incidence of fetuses with cleft palate and open eyelids at 10 and 25
mg/kg bw per day. There was an increased incidence of stillborn pups
and decreased pup body weight on days 7-21 post partum at 25 mg/kg
bw per day, and pup survival to day 4 post partum was reduced. The
incidence of litters with pups with head tilt and the incidence of
pups with cleft palate were increased at 25 mg/kg bw per day, although
all nine pups with cleft palate were from the same litter. Among the
pups that were allowed to survive until day 43 post partum, there
was an increased incidence of mice with head tilt, and ungroomed
coats, corneal opacity, and ptosis were seen in one or two males at
the highest dose. Body-weight gain and average body weights were
decreased in mice at this dose, and there was an increased incidence
of mice with altered swimming postures or ability. Of the 11 pups with
altered swimming posture, 10 had head tilt. A further three pups with
head tilt did not have altered swimming posture. The NOAEL for
developmental toxicity was 4 mg/kg bw per day on the basis of the
increased incidence of open eyelids and cleft palate in pups at 10
mg/kg bw per day. The NOAEL for maternal toxicity was 10 mg/kg bw per
day on the basis of minimally retarded weight gain during days 12-16
of gestation in dams at 25 mg/kg bw per day. Dinocap was teratogenic,
causing malformations at doses that had no effect on the dam (Lochry,
1989).
Table 5. Results of a study of developmental toxicity in mice
Parameter Dose (mg/kg bw per day)
0 4 10 25
Maternal weight gain (g), days 6-16 14.8 16.0 16.5 13.8
days 12-16 8.2 8.7 8.8 6.4
days 0-18 26.3 27.0 27.3 23.3
Caesarian-derived pups
No. of litters evaluated 12 12 12 9
Resorptions per litter 1.0 0.5 1.2* 1.8*
Litter size 11.2 11.2 10.8 8.7
Mean fetal weight (g) 1.4 1.35 1.30* 1.10**
Open eyelids, litters (total no. fetuses) 0 0 1 (1) 2 (3**)
Cleft palate, litters (total no. fetuses) 0 0 3 (4) 7 ** (65**)
Naturally-delivered pups
No. of litters evaluated 11 12 12 10
Gestation duration (days) 19.4 19.8* 19.8* 19.9**
Litter size, day 1 post partum 11.4 11.3 11.8 11.8
Head tilt (no. of litters) 0 0 0 3**
Cleft palate/pups dying days 0-21 post partum 0/3 0/1 0/0 9/16
Abnormal swimming ability, day 43, litters (no. fetuses) 0/11 0/11 0/12 5/9** (11/39)**
From Lochry (1989)
* Statistically significant at 0.05 > p > 0.01; ** statistically significant at 0.01 > p
A study was conducted to establish suitable doses for a study of
teratogenicity after dermal application to CD-1 mice. Fewer animals
were used that recommended in guideliness. Dinocap was applied as the
formulation Karathane LC XF, considered to be appropriate for
estimating the risk due to occupational exposure, but the doses used
were corrected for content and expressed as dinocap. Appropriate
dilutions were obtained with the formulation blank. In phase 1 of the
study, both untreated and vehicle control groups were used; in phase
2, only a vehicle control group was used. The dose volume of 290 µl/kg
bw was applied to one to five areas of shaven dorsal skin for 4 h each
day on days 6-15 of presumed gestation; the site was changed each day,
recommencing at the first site on the sixth day. Occlusion of the
application site was not mentioned, but a collar was placed to prevent
ingestion; no mention is made of how the mice were restrained during
application. After completion of the 4-h exposure each day, the test
material was washed off gently with soap and water.
Initially (phase 1), groups of eight presumed-pregnant mice were
given doses of 0 (control), 50, 80, or 100 mg/kg bw per day. Because
of excessive toxicity, similar groups of eight mice were given doses
of 0, 1, 4, 10, or 25 mg/kg bw per day by a similar protocol. The mice
were sacrificed on day 18 of presumed gestation; half of the fetuses
were used for skeletal examination, and the remaining half for
specific examination of otoliths and of the remaining skeleton. For
mice weighing 20-40 g, the dose volumes would have been 6-12 µl: the
practical difficulty of administering such small volumes may have
resulted in variations in accuracy. One, three, and four deaths
occurred during treatment at 50, 80, and 100 mg/kg bw per day,
respectively. Death was usually briefly (up to 24 h) preceded by signs
of toxicity, including dermal erythema, ataxia, red or tan vaginal
discharge, weight loss, and cold to touch. These doses all reduced
weight gain, with weight loss at the highest dose. The numbers of live
young were reduced at 50 and 80 mg/kg bw per day, and the litters of
the three surviving dams at 100 mg/kg bw per day contained only
resorbed conceptuses. Gross malformations were seen in 88% of fetuses
at 50 mg/kg bw per day and 100% of those at 80 mg/kg bw per day.
The principal treatment-related effects seen in phase 2 are shown
in Table 6. Little maternal toxicity occurred, and body-weight gain
was apparently unaffected by treatment. One mouse at the highest dose
was found dead on day 15 of gestation having been found stuporous with
an impaired righting reflex after dosing the day before. Death was
attributed to trauma, although no clear injury was detected at
necropsy. There was a slight incidence of skin irritation at the two
highest doses. The litter sizes, numbers of live fetuses per litter,
and fetal body weights were unaffected by treatment. There was no
change in the degree of skeletal ossification, but three fetuses of
one litter at the highest dose had cleft palate, and two fetuses of
another litter had open eyelids. Also at the highest dose, otolith
development was clearly impaired. These findings are consistent with
those of previous studies of the teratogenicity of dinocap in mice
after oral administration. In view of these results, no further study
by the dermal route was conducted. The study involved too few animals
to determine a NOAEL for teratogenicity in mice treated dermally,
particularly with respect to cleft palate; however, the quality of the
data on mean otolith scores in 49 fetuses may provide some degree of
confidence that the NOAEL for these effects is 10 mg/kg bw per day
(Foss, 1995).
Table 6. Results of a study for developmental toxicity in mice
Effect Dose (mg/kg bw per day)
0 1 4 10 25
No. of pregnant dams 8 8 7 8 6
Maternal weight gain on days 6-18 (g) 21.4 26.4 20.8 26.4 25.0
No. of fetuses 77 96 67 93 73
Cleft palate (no. of litters) 0 0 0 0 1
Open eyelids (no. of litters) 0 0 0 0 1
Mean otolith scorea 9.2 9.6 9.0 9.0 2.2
No. of fetuses examined 37 49 34 49 38
From Foss (1995)
a Three otoliths were scored for completeness on a scale of 1-4, to give a maximum
potential score of 12.
2,4-DNHPC and 2,6-DNHPC were not teratogenic in mice. Samples of
each isomer (purity, 95%) were tested seperately and in combination,
and the results were compared with those in mice receiving
technical-grade dinocap (purity, 84%), each group at a dose of 25
mg/kg bw per day in corn oil. Fewer pregnant animals were used than
recommended in the guidelines. Although litters born to
dinocap-treated mice showed a pattern of developmental defects typical
of those seen in the studies described above, the two isomers were
inactive at the same dose, both alone and in combination (Rogers et
al., 1987). In a separate study by the same group, otolith formation
was compared in the pups of mice and hamsters treated with dinocap
during gestation. Otolith formation was impaired in mice. Dinocap
affected otolith formation in hamsters, but only at a dose associated
with severe maternal and fetal toxicity (Rogers et al., 1989).
Rats
Groups of 25 Sprague-Dawley rats presumed to be pregnant received
dinocap (purity, 96%) as a suspension in aqueous methylcellulose at
doses of 0 (control), 10, 50, or 150 mg/kg bw per day by gavage on
days 6-15 of gestation. There were no deaths, and the only overt sign
of toxicity was an increased incidence of soft faeces in rats at 150
mg/kg bw per day. Maternal body-weight gain was impaired at 150 mg/kg
bw per day during the first two days of treatment, and food
consumption was slightly reduced in this group during treatment. Gross
examination revealed no treatment-related changes in pregnant or non-
pregnant females. There were no treatment-related changes in pup
weight and no increase in the incidence of malformations. There was an
apparent increase in the incidence of extra ribs at 150 mg/kg bw per
day, but seven of the 10 affected fetuses were from two litters. The
NOAEL for maternal and fetal toxicity was 50 mg/kg bw per day. There
was no evidence of teratogenicity at the highest dose of 150 mg/kg bw
per day (Solomon & Lutz, 1989).
Rabbits
Groups of 20 artificially inseminated New Zealand white rabbits
received dinocap (purity, 95.4%) in aqueous gum tragacanth by gavage
at doses of 0, 3, 12, 48, or 84 mg/kg bw per day on days 7-19 of
presumed pregnancy. Pups were delivered by caesarian section on day 29
and examined for developmental abnormalities in accordance with normal
guideline requirements. Two does at the high dose and one at the
intermediate dose died between days 24 and 29 of gestation, therefore
at least five days after dosing, but the deaths were considered to be
related to treatment. These animals all showed weight loss and
anorexia before death. The doe at the intermediate dose and one at the
high dose aborted; both does at the high dose that died had non-viable
litters (late resorptions). An additional five deaths were considered
unrelated to treatment and were primarily a consequence of intubation
errors.
The main results are shown in Table 7; statistical analyses were
not reported. There was an increased incidence of premature delivery
and abortion at 48 and 84 mg/kg bw per day; the premature deliveries
at the highest dose were primarily late resorptions. Clinical signs
(lack of faeces or dried faeces) and impaired weight gain and food
consumption were seen among does receiving these doses, litter sizes
and pup weights were reduced, and there was an increased incidence of
pups with skeletal malformations (vertebral assymetry and fused or
forked ribs). There was also a slight increase in delayed ossification
at a few sites in does at these doses. The NOAEL for maternal toxicity
was 3 mg/kg bw per day on the basis of weight-gain retardation during
treatment. The NOAEL for developmental toxicity was 12 mg/kg bw per
day on the basis of increased resorptions and reduced litter sizes at
48 mg/kg bw per day (Hoberman & Christian, 1987).
This conclusion is different from that of the 1989 JMPR (Annex 1,
reference 58) but is based on different data. In the latter review, an
increased incidence of hydrocephaly and neural tube defects was found
at doses > 3 mg/kg bw per day in the studies of Costlow & Kane
(1984 a,b). In the earlier studies, however, a less pure form of
dinocap (84%) was used, and the neural tube defects found by Costlow
and Kane (1984a) at a dose of 3 mg/kg bw per day were not found in the
second study (Costlow & Kane, 1984b) at 48 mg/kg bw per day nor in
studies by dermal administration in which the increased numbers of
Table 7. Results of a study of developmental toxicity in rabbits
Result Dose (mg/kg bw per day)
0 3 12 48 84
Adult animals
No. pregnant 17 17 15 15 14
No. with abortions 0 0 0 3 1
No. with premature delivery 1 0 0 1 3
No. with absence of faeces (on any day) 0 0 0 0 4
No. with dried faeces (on any day) 3 0 2 7 17
Weight change, days 7-20 (g) 150 200 90 -330 -550
Food consumption, days 7-20 (g/day) 160 170 152 65 51
No. with gastric ulceration 0 0 1 3 4
Litters
No. evaluated 12 17 14 10 9
Mean no. of live fetuses 7.2 7.5 8.1 6.3 5.9
Dead or resorbed fetuses per litter (%) 11.8 4.9 7.1 20.3 27.0
Mean fetal weight (g) 40.9 42.7 40.0 37.6 35.8
Vertebral assymetry: no. of litters (pups) 1 (1) 1 (1) 1 (2) 5 (7) 3 (8)
No. of litters (pups) with rib malformation 1 (1) 0 2 (3) 2 (2) 2 (6)
No. of litters (pups) with skeletal malformations 4 (4) 3 (3) 4 (6) 5 (7) 6 (12)
From Hoberman (1987)
resorptions and delayed ossifications were similar to these found by
Hoberman & Christian (1987).
(f) Special studies: Ocular toxicity
A NOAEL of 15 ppm was observed for ocular toxicity in a two-year
study in dogs (Weatherholz et al., 1979) in the 1989 JMPR (Annex 1,
reference 38). At 60 ppm, four of eight dogs had slight to moderate
discolouration of the tapetum lucidum, although no retinal atrophy and
no changes in the vascularity of the retina were noted. The remaining
four dogs at this dose showed moderate to marked discolouration of the
tapetum, reduced vascularity of the retina, and retinal atrophy (three
dogs). At 120/240 ppm, the four surviving dogs had marked
discolouration of the tapetum, reduced vascularity of the retina, and
retinal atrophy. Retinal atrophy was therefore present only in dogs
with severe tapetal changes, and no dog showed retinal effects without
effects in the tapetum.
A review of the literature for compounds that are toxic to the
tapetum lucidum of dogs was submitted (Solomon, 1991). Of six
compounds known to affect the tapetum, three (zinc pyridinethione, SCH
19927, and rosaramicin) had no effect in atapetal dogs, and no ocular
effects were reported in other atapetal animals. The other three
compounds (dinocap, hydroxy pyridinethione, and diphenyl
thiocarbazine) affected the tapetum in dogs (with no information on
atapetal dogs) but did not cause ocular effects in atapetal species.
The author concluded that the retinal atrophy seen with dinocap was
secondary to damage to the tapetum lucidum. Since humans do not have a
tapetum, it was concluded that humans would not be susceptible to
retinal damage as a consequence of this effect. The review is brief,
and the adequacy of the methods used, the sensitivity, and the
comparability of the findings were not considered. The conclusions
cannot therefore be regarded as definitive, although the data support
the hypothesis. The NOAEL in the study of Weatherholz et al. (1979)
was therefore identified for other effects. The 1989 JMPR report
(Annex 1, reference 58) indicates that significant effects, including
deaths, occurred at doses of 180-240 ppm, giving a NOAEL of 60 ppm,
equivalent to 1.5 mg/kg bw per day.
Comments
Dinocap is well absorbed after oral exposure. A proportion
(5-25%) is absorbed after dermal exposure, varying with species and
concentration. No conclusions were drawn about the degree of dermal
absorption in humans from the results of a study in which mouse and
human skin were compared; however, human skin is generally regarded as
being less permeable to toxicants than that of mice.
The urinary metabolites of the methylheptyl isomer in rats and
mice have been extensively characterized; characterization of the
faecal metabolites was reported by the 1989 JMPR, which concluded that
the pattern of metabolites in faeces seen by thin-layer chromatography
was similar to that observed in squash and cucumbers.
The new data confirmed the generally low degree of acute toxicity
of dinocap in rats; mice, however, appear to be more sensitive than
rats to both acute and developmental effects. Dinocap is a skin
irritant and sensitizer. The available studies did not address the
uncoupling of oxidative phosphorylation, identified by the 1989 JMPR
as a potentially significant mode of action.
WHO has classified dinocap as 'slightly hazardous' (WHO, 1996).
In a study of carcinogenicity in mice at doses of 0, 15, 100, or
200 ppm, no evidence of carcinogenicity was found. The NOAEL was 15
ppm, equal to 2.7 mg/kg bw per day. The lack of carcinogenicity in
mice is consistent with the absence of carcinogenicity in rats
reported by the 1989 JMPR.
The results of tests for genotoxicity (on the less pure form of
dinocap) were negative.
A multigeneration study of reproductive toxicity at dietary
concentrations of 0, 40, 200, or 1000 ppm in rats showed no specific
effect on any reproductive parameters; the NOAEL was 200 ppm, equal to
13 mg/kg bw per day.
In a study of developmental toxicity in mice dosed by gavage at
0, 4, 10, or 25 mg/kg bw per day, impaired otolith formation was seen
at 25 mg/kg bw per day. A dose-related increase in the incidence of
open eyelids and cleft palate extended down to 10 mg/kg bw per day in
the absence of maternal toxicity. The NOAEL was 4 mg/kg bw per day.
Dermal application of 50, 80, or 100 mg/kg bw per day to mice proved
excessively toxic for an evaluation of developmental toxicity. A
further dermal study in mice at 0, 1, 4, 10, or 25 mg/kg bw per day
showed malformations,including impaired otolith formation, at 25 mg/kg
bw per day in the absence of maternal toxicity. The NOAEL for
developmental toxicity after dermal application to mice was 10 mg/kg
bw per day. The results of the recent studies of developmental
toxicity confirmed the teratogenic potential of purified dinocap in
mice, even when applied dermally. Impaired otolith development,
characteristic of the teratogenicity of dinocap in mice, was also seen
in hamsters at doses that are maternally toxic. Less specific
malformations were seen in rabbits at maternally toxic doses. The
present Meeting concluded that the NOAEL in the studies in rabbits
described by the 1989 JMPR was 3 mg/kg bw per day rather than
0.5 mg/kg bw per day, since the findings on which the putative effect
level was established do not appear to be repeatable or clearly
dose-related. The methylheptyl isomer has been shown not to be
teratogenic to mice. The reason for the species difference in the
teratogenicity of dinocap in rats and mice therefore cannot be deduced
from the data on the metabolism of the methylheptyl isomer.
The two-year study in dogs that was evaluated at the 1989 Joint
Meeting was also reassessed on the basis that the critical effect
(retinal atrophy) was secondary to effects on the tapetum lucidum.
Since the tapetum lucidum is not present in humans, or in rats or mice
in which no retinal effect was seen, the Meeting concluded that it
would be inappropriate to base the evaluation on this effect. The
NOAEL was 60 ppm, equivalent to 1.5 mg/kg bw per day.
Teratogenic effects in mice were considered to be the
toxicological end-point of greatest concern. Since dinocap was
teratogenic in mice after either oral or dermal administration and
since malformations were seen in at least three species, the Meeting
considered a high safety factor to be appropriate. An ADI of 0-0.008
mg/kg bw was established on the basis of the NOAEL of
4 mg/kg bw per day in the developmental toxicity study in mice and a
safety factor of 500.
Establishment of an acute reference dose (RfD) was considered to
be appropriate since teratogenicity may occur after a single exposure.
An acute RfD was established on the basis of the NOAEL of 4 mg/kg bw
per day for teratogenicity in mice and a safety factor of 500, to give
an acute RfD of 0.008 mg/kg bw, which is appropriate for women of
child-bearing age.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 15 ppm, equal to 2.7 mg/kg bw per day (toxicity in a
study of carcinogenicity)
4 mg/kg bw per day (developmental toxicity)
10 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rat: 200 ppm, equal to 6.4 mg/kg bw per day (toxicity in a
study of carcinogenicity)
50 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Rabbit: 3 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 60 ppm, equivalent to 1.5 mg/kg bw per day (study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.008 mg/kg bw
Estimate of acute reference dose
0.008 mg/kg bw
Information that would be useful for continued evaluation of the
compound
Further observations in humans
List of end-points for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption 60-69% absorbed, max. concentration at 2-6 h
Dermal absorption 5-25%
Distribution Widely distributed
Potential for accumulation Limited, < 0.3% in tissue after 7 days
Rate and extent of excretion Biphasic; half-life is 3 h for 1st phase, 44 h for 2nd
phase, oral administration, rabbit
Metabolism in animals Extensive; > 96% metabolized
Toxicologically significant compounds Metabolites assumed to be of similar toxicity to parent
(animals, plants and environment)
Acute toxicity
Rat: LD50 oral 3100 mg/kg bw
Rat: LD50 dermal > 5000 mg/kg bw
Rat: LC50 inhalation 3 mg/L
Skin irritation Irritating
Eye irritation Irritating
Skin sensitization Sensitizing
Short-term toxicity
Target/critical effect General toxicity
Lowest relevant oral NOAEL Dog: 1.5 mg/kg bw per day
Lowest relevant dermal NOAEL Mouse: 10 mg/kg bw per day (teratogenicity)
Lowest relevant inhalation NOAEL No data
Genotoxicity Not genotoxic in an adequate battery of tests
Long-term toxicity and carcinogenicity
Target/critical effect: Impaired weight gain
Lowest relevant NOAEL Mouse: 2.7 mg/kg bw per day (carcinogenicity)
Carcinogenicity Not carcinogenic
Reproductive toxicity
Reproduction target/critical effect No effect on fertility or ability to rear young
Lowest relevant reproductive NOAEL Rat: 13 mg/kg bw per day (multigeneration study)
Developmental target/critical effect Malformations
Lowest relevant developmental NOAEL Mouse: 4 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No data, but no concern raised in other studies
Other toxicological studies Inhibits oxidative phosphorylation; methylheptyl
isomer not teratogenic
Medical data No significant dinocap-related effects reported
Summary Value Study Safety factor
ADI 0-0.008 mg/kg bw Mouse, developmental toxicity 500
Acute reference dose 0.008 mg/kg bw Mouse, developmental toxicity 500
References
Anderson, D.M. & Baldwin, R.C. (1990a) Karathane LC fungicide/miticide
delayed contact hypersensitivity study in guinea pigs. Unpublished
report No. 89R-187 from Rohm & Haas Company, Toxicology Department,
Spring House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas
Company, Spring House, Pennsylvania, USA.
Anderson, D.M.& Baldwin, R.C. (1990b) Karathane FN-57
fungicide/miticide delayed contact hypersensitivity study in guinea
pigs. Unpublished study No. 89R-188 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Anderson, D.M. & Baldwin, R.C. (1990c) Karathane technical (HPK)
fungicide/miticide delayed contact hypersensitivity study in guinea
pigs. Unpublished report No. 89R-189 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Bernacki, H.J. & Baldwin, R.C. (1987) Karathane technical (dinocap):
Range finding four-week dietary toxicity study in mice. Unpublished
report No. 87R-175. from Rohm & Haas Co., Toxicology Department,
Spring House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co.,
Spring House, Pennsylvania, USA.
Bernacki, H.J. & Hamilton, J.D. (1992a) Karathane LC
fungicide/miticide: Skin irritation study in rabbits. Unpublished
report No. 91R-170 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Bernacki, H.J. & Hamilton, J.D. (1992b) Karathane LC
fungicide/miticide: Acute dermal toxicity study in male and female
rats. Unpublished report No. 91R-171 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Bernacki, H.J. & Hamilton, J.D. (1992c) Karathane LC
fungicide/miticide: Acute oral toxicity study in male and female rats.
Unpublished report No. 91R-173 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Blair, M. & Cavender, F.L. (1979) TD 77-310 acute inhalation study in
rats. Unpublished report No. 285-029 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Rohm & Haas Co., Spring House, Pennsylvania, USA.
Costlow, R.D. & Kane, W.W. (1984a) Teratology study with Karathane in
rabbits. Unpublished report No. 83R-22 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Costlow, R.D. & Kane, W.W. (1984b) Teratology study with Karathane in
rabbits. Unpublished report No. 83R-113 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA
Evans, K.W. & Hazelton, G.A. (1995) Blood pharmacokinetics and dermal
absorption of Karathane in female mice. Unpublished report No. 95R-105
from Rohm & Haas Co., Toxicology Department, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Ferguson, J.S. (1997) Karathane technical: Acute inhalation toxicity
study in rats. Unpublished report No. 96R-126 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Foss, J.A. (1995) Dermal dosage-range developmental toxicity study of
Karathane LC XF in mice. Unpublished report No. 018-023P from Argus
Research Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA Rohm & Haas
Co. report No. 95RC-024.
Foxall, S. (1985) Karathane technical: CHO/HGPRT gene mutation assay.
Unpublished report No. 85R-204 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Hoberman, A. & Christian, M. (1987) A developmental toxicity study of
Karathane fungicide/miticide (technical) administered via stomach tube
to New Zealand white rabbits. Unpublished report No. 018-012 from
Argus Research Laboratories, Inc., Horsham, Pennsylvania, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Rohm & Haas Co. report No. 86RC-71.
Ivett, J. & Myhr, B. (1986) Clastogenic evaluation of Karathane
technical in an in-vitro cytogenetic assay measuring chromosomal
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished study project No. 20990 from Litton Bionetics, Inc.,
Kensington, Massachusetts, USA. Rohm & Haas Co. report No. 85RC-62.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1987a) Karathane
FN-57 fungicide/miticide: Acute dermal toxicity study in male and
female rabbits. Unpublished report No. 87R-138 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1987b) Karathane
FN-57 fungicide/miticide: Eye irritation study in rabbits. Unpublished
report No. 87R-114 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1987c) Karathane
FN-57 fungicide/miticide: Skin irritation study in male rabbits.
Unpublished report No. 87R-139 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Lampe, K.R., Morrison, R.D. & Baldwin, R.C. (1987a) Karathane LC
fungicide-miticide: Acute oral toxicity study in male and female rats.
Unpublished report No. 87R-132 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Lampe, K.R., Morrison, R.D. & Baldwin, R.C. (1987b) Karathane LC
fungicide-miticide: Acute dermal toxicity study in male and female
rats. Unpublished report No. 87R-133 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Lochry, E. (1989) Dinocap (high purity technical): An oral (gavage)
developmental toxicity study in CD-1(ICR)BR mice including a postnatal
phase. Unpublished report No. 018-015 from Argus Research
Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO by
Rohm & Haas Co., Spring House, Pennsylvania, USA. Rohm & Haas Co.
report No. 88P-335.
Maibach, H. (1985) Elimination of 14C labeled Karathane in rhesus
monkeys following a single intramuscular injected dose. Unpublished
report No. 85RC-18 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Moore, M.R. (1991) Dinocap (technical purified): 78-Week dietary
oncogenicity study in mice. Unpublished report No. 417-445 from
Hazleton Laboratories America, Inc., Rockville, Massachusetts, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Rohm & Haas Co. report No. 88RC-090
Morrison, R.D. & Hamilton, J.D. (1991) Karathane FN-57
fungicide/miticide: Acute oral toxicity study in male and female mice.
Unpublished report No. 91R-001 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Morrison, R.D. & Hamilton, J.D. (1992) Karathane LC
fungicide/miticide: Eye irritation study in rabbits. Unpublished
report No. 91R-172 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Morrison, R.D., Krzywicki, K.M. & Hazelton, G.A. (1987) Karathane
technical purified: Acute oral toxicity study in male rats and mice.
Unpublished report No. 86R-149A,B from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Morseth, S. (1990) Dinocap (technical purified): Two-generation
reproduction study in rats. Unpublished report No. 417-442 from
Hazleton Laboratories America, Inc., Vienna, Virginia, USA. Submitted
to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA. Rohm &
Haas Co. report No. 88RC-092.
Onishi, M. (1989) Karathane LC (HPK) fungicide/fiticide: Acute oral
toxicity study in mice. Unpublished study from Shin Nippon Biomedical
Laboratories Ltd, Kagoshima, Japan. Rohm & Haas Co. report No.
90RC-1001. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Potter, D. (1996) Identification of
2,4-dinitro-6-(1-methylheptyl)phenyl crotonate metabolites in rat and
mouse urine. Unpublished report No. 95R-064 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Procopio, K.R. & Parno, J.R. (1995) Karathane LC: Acute dermal
toxicity study in male and female mice. Unpublished report No. 94R-244
from Rohm & Haas Co., Toxicology Department, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Rogers, J.M., Gray, E.L., Carver, B.D. & Kavlock, R.J. (1987)
Developmental toxicity of dinocap in the mouse is not due to two
isomers of the major active ingredient. Teratol. Carcinog. Mutag.,
7, 341-346.
Rogers, J.M., Burkhead, L.M. & Barbee B.D. (1989) Effects of dinocap
on otolith formation: Evaluation of mouse and hamster fetuses at term.
Teratology, 39, 515-523.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987a) Karathane
technical purified: Acute oral toxicity study in male and female rats.
Unpublished report No. 87R-124 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987b) Karathane
technical purified: Acute dermal toxicity study in male and female
rabbits. Unpublished report No. 87R-126 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987c) Karathane
FN-57 fungicide-miticide: Acute oral toxicity study in male and female
rats. Unpublished report No. 87R-137 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. 1987d) Karathane
technical purified: Skin irritation study in male rabbits. Unpublished
report No. 87R-125 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987e) Karathane LC
fungicide/miticide: Rabbit skin irritation study. Unpublished report
No. 87R-134 from Rohm & Haas Co., Toxicology Department, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987f) Karathane
technical purified: Eye irritation study in male rabbits. Unpublished
report No. 87R-111 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987g) Karathane LC
fungicide/miticide: Eye irritation study in rabbits. Unpublished
report No. 87R-135 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Ruegg, C.E. (1996) In vitro percutaneous absorption and cutaneous
distribution of Karathane LC XF across intact human and mouse skin.
Unpublished report No. M95-024 from In Vitro Technologies, Inc.,
Baltimore, Maryland, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA. Rohm & Haas Co. report No. 95RC-187..
Sames, J.L., Yu, R.Y. & McCarthy, K.L. (1986) Karathane: In-vivo
cytogenetic study in mice. Unpublished report No. 85R-235 from Rohm &
Haas Co., Toxicology Department, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Solomon, H.M. (1991) Karathane fungicide/miticide: Ocular toxicity in
dogs. Unpublished report No. 91M-663 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Solomon, H.M. & Lutz, M.F. (1989) Dinocap: Oral (gavage) developmental
toxicity study in rats. Unpublished report No. 88R-236 from Rohm &
Haas Co., Toxicology Department, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Wanner, F.J. & Hagan, J.V. (1991) Karathane FN-57: Acute inhalation
study in rats. Unpublished report No. 91R-002 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Weatherholz, W.D., Kundzins, W., Alsaker, R.A., Voelker, R.W. &
Peterson, K. (1979) 104-Week toxicity study in dogs: Karathane
technical. Unpublished report from Hazleton Laboratories America,
Inc., Vienna, Virginia, USA. Submitted to WHO by Rohm & Haas Co.,
Spring House, Pennsylvania, USA. Rohm & Haas Co. report 79RC-45.
Wester, R.W. & Maibach, H.I. (1985) Karathane: Percutaneous absorption
of 14C Karathane (14C-DNHPC) in rhesus monkey following single
topical application. Unpublished report No. 85RC-049 from Rohm & Haas
Co., Toxicology Department, Spring House, Pennsylvania, USA. Submitted
to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
Table 2. Acute toxicity of formulations of dinocap
Species Strain Sex Route Purity LD50 or LC50 Reference
(%) (mg/kg bw
or mg/L)
Technical-grade dinocap
Mouse CD-1 M Oral 95 292 Morrison et al. (1987)
Rat Crl:CD M Oral 95 3100 Morrison et al. (1987)
Rat Crl:CD M Oral 95 > 500 Romanello et al. (1987a)
F < 5000
Rat Crl:CD M Inhalation 95 > 3 Ferguson (1997)
F 3
Rabbit New Zealand white M/F Dermal 95 > 5000 Romanello et al. (1987b)
Karathane LC fungicide-miticide
Mouse CD-1 M Oral 40.4 517 Onishi (1989)
F 413
Mouse CD-1 M/F Dermal 35.6 1020 Procopio & Parno (1995)
Rat Crl:CD M/F Oral 37.6 > 2000 Bernacki & Hamilton (1992a)
Rat Crl:CD M Oral 39 > 500 Lampe et al. (1987a)
F < 5000
Rat Crl:CD M/F Dermal 37.6 > 2000 Bernacki & Hamilton (1992b)
Rabbit New Zealand white M/F Dermal 39 > 5000 Lampe et al. (1987b)
Rat Crl:CD M/F Inhalation 50 0.9 Blair & Cavender (1979)
Karathane FN-57 fungicide-miticide
Mouse CD-1 M/F Oral 20 1401 Morrison & Hamilton (1991)
Rat Crl:CD M Oral 20 > 500 Romanello et al. (1987c)
F < 5000
Rat Crl:CD M/F Inhalation 20 > 4.9 Wanner & Hagan (1991)
Rabbit New Zealand white M/F Dermal 20 > 5000 Krajewski et al. (1987a)
M, male; F, female
Table 3. Results of a dose range-finding study in mice
Outcome Dose (ppm in diet)
0 25 75 225 500 1000 2000 4000
Mortality, males 0/10 0/10 0/10 0/10 0/10 5/10 10/10 10/10
Mortality, females 0/10 0/10 0/10 0/10 7/10 10/10 10/10 10/10
Final body weight, males (g) 34 33 33 32 29 25
Final body weight, females (g) 27 28 29 26 20
Liver weight, males (g) 2.0 2.0 2.1 1.9 1.8 1.7*
Liver weight, females (g) 1.5 1.6 1.7* 1.5 1.2*
Liver weight, males (% bw) 5.8 5.9 6.1 6.0 6.4* 6.8*
Liver weight, females (% bw) 5.5 5.7 6.0* 5.9* 5.9
Hepatocellular necrosis, males NR NR NR NR NR 3/10 9/10 6/10
Hepatocellular necrosis, females NR NR NR NR 5/10 6/10 7/10 3/10
NR, not reported
From Bernacki & Hamilton (1987)
* Statistically significant at 0.05 > p > 0.01
mortality) at 78 weeks. There were no overt treatment-related symptoms
of toxicity. Body-weight gain and food consumption were reduced in
males at 150 ppm and in females at 100 ppm (20% less than that of
controls) and 150 ppm. The efficiency of food use appeared to be
impaired only in males at the highest dose and not in females, thus
suggesting that the effect in females was at least partly a
consequence of palatability. Mean testis weight was reduced in males
at 150 ppm, and histopathological examination revealed a background
incidence of unilateral and bilateral testicular atrophy in all
groups, including controls, with incidences of 11/60, 4/60, 11/59, and
25/60 at 0, 15, 100, and 150 ppm, respectively. A moderate incidence
of individual cell necrosis was reported in mice in all groups,
possibly associated with mouse hepatitis virus infection indicated by
serological markers at 18 months, but the incidence of necrosis seen
in the 28-day study described previously was not reproduced. There was
no evidence of carcinogenicity in any tissue. The NOAEL was 15 ppm,
equivalent to 2.7 mg/kg bw per day, on the basis of reduced
body-weight gain with a corresponding reduction in food consumption in
females at 100 ppm (Moore, 1991).
(d) Genotoxicity
Studies of the genotoxicity of dinocap of lower purity than that
used in the studies reported in the previous monograph are summarized
in Table 4. The results are consistent with the previous conclusions
of the JMPR.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 26 Crl:CD rats of each sex received dinocap (purity,
96%) in the diet at concentrations of 40, 200, or 1000 ppm for two
generations. At weaning of the F1 animals, the highest dose was
reduced to 400 ppm because of a high rate of mortality in these pups.
The litters were culled to eight pups at day 4 post partum. In
addition to the normal guideline requirements, selected F1 males at
0, 40, or 200 ppm were used in a study of gonadal function (sperm
motility, sperm count, weight of cauda epididymis). Body-weight gain
and food consumption were retarded at 1000 ppm during the F0
pre-mating period and at 400 ppm during the F1 pre-mating period.
There was increased pup mortality at weaning among litters of the
group at 1000 ppm, until this dose was reduced to 400 ppm; the cause
of death could not be determined but the presence of yellow or red
discolouration of the contents of the gastrointestinal tract and
bladder suggested the presence of test material or its metabolites.
There were no specific effects on reproductive function or the ability
to rear young, and there was no effect on gonadal function. Increased
liver weights, with no evidence of a histological correlate, were
found among rats receiving 400 or 1000 ppm. The NOAEL was 200 ppm,
equal to 13 mg/kg bw per day (Morseth, 1990).
Table 4. Results of studies of the genotoxicity of dinocap
End-point Test object Dose Purity Results Reference
(%)
In vitro
Gene mutation Chinese hamster 3-10 µg/mla 83.9 Negative Foxall (1985)
ovary cells, hprt locus 15-25 µg/mlb Negative
Metaphase analysis Chinese hamster 1-10 µg/mla NR Negative Ivett & Myhr (1986)
ovary cells 5-20 µg/mlb Negative
In vivo
Metaphase analysis CD-1 mouse 12.6-126 mg/kg bw 83.9 Negative Sames et al. (1986)
bone marrow
a Without an exogenous metabolic system
b With an exogenous metabolic system
(ii) Developmental toxicity
Mice
Groups of 24 CD-1 mice presumed to be pregnant were given dinocap
(purity, 94.4%) in aqueous 1% tragacanth gum at doses of 0 (control),
4, 10, or 25 mg/kg bw per day by gavage on days 6-15 of gestation. On
day 18 of gestation, about half of the mice in each group were killed
and the fetuses were examined; the remaining dams were allowed to
deliver and rear their litters, which were culled on day 4 post
partum to two pups per litter. On day 43, these mice were evaluated
for swimming performance.
The results of this study are shown in Table 5. There were no
maternal deaths during the study and no overt signs of toxicity. The
body-weight gain of the pregnant mice was minimally impaired at 25
mg/kg bw per day on days 12-16 of gestation, although the weight
difference may have been a consequence of the reduced litter size.
Gross examination showed no treatment-related findings. In animals
killed on day 18 of gestation (the normal end of a study of
teratogenicity), the average number of resorptions was increased in
dams at 25 mg/kg bw per day, with an associated decrease in litter
size. The incidence of resorptions was slightly increased in dams at
at 10 mg/kg bw per day, but the increase was within the range of
historical controls (0-1.6) All treated animals that delivered
naturally had a statistically significant increase in the duration of
gestation, although this was not considered to be toxicologically
significant.
The mean fetal weight was reduced, and there was an increased
incidence of fetuses with cleft palate and open eyelids at 10 and 25
mg/kg bw per day. There was an increased incidence of stillborn pups
and decreased pup body weight on days 7-21 post partum at 25 mg/kg
bw per day, and pup survival to day 4 post partum was reduced. The
incidence of litters with pups with head tilt and the incidence of
pups with cleft palate were increased at 25 mg/kg bw per day, although
all nine pups with cleft palate were from the same litter. Among the
pups that were allowed to survive until day 43 post partum, there
was an increased incidence of mice with head tilt, and ungroomed
coats, corneal opacity, and ptosis were seen in one or two males at
the highest dose. Body-weight gain and average body weights were
decreased in mice at this dose, and there was an increased incidence
of mice with altered swimming postures or ability. Of the 11 pups with
altered swimming posture, 10 had head tilt. A further three pups with
head tilt did not have altered swimming posture. The NOAEL for
developmental toxicity was 4 mg/kg bw per day on the basis of the
increased incidence of open eyelids and cleft palate in pups at 10
mg/kg bw per day. The NOAEL for maternal toxicity was 10 mg/kg bw per
day on the basis of minimally retarded weight gain during days 12-16
of gestation in dams at 25 mg/kg bw per day. Dinocap was teratogenic,
causing malformations at doses that had no effect on the dam (Lochry,
1989).
Table 5. Results of a study of developmental toxicity in mice
Parameter Dose (mg/kg bw per day)
0 4 10 25
Maternal weight gain (g), days 6-16 14.8 16.0 16.5 13.8
days 12-16 8.2 8.7 8.8 6.4
days 0-18 26.3 27.0 27.3 23.3
Caesarian-derived pups
No. of litters evaluated 12 12 12 9
Resorptions per litter 1.0 0.5 1.2* 1.8*
Litter size 11.2 11.2 10.8 8.7
Mean fetal weight (g) 1.4 1.35 1.30* 1.10**
Open eyelids, litters (total no. fetuses) 0 0 1 (1) 2 (3**)
Cleft palate, litters (total no. fetuses) 0 0 3 (4) 7 ** (65**)
Naturally-delivered pups
No. of litters evaluated 11 12 12 10
Gestation duration (days) 19.4 19.8* 19.8* 19.9**
Litter size, day 1 post partum 11.4 11.3 11.8 11.8
Head tilt (no. of litters) 0 0 0 3**
Cleft palate/pups dying days 0-21 post partum 0/3 0/1 0/0 9/16
Abnormal swimming ability, day 43, litters (no. fetuses) 0/11 0/11 0/12 5/9** (11/39)**
From Lochry (1989)
* Statistically significant at 0.05 > p > 0.01; ** statistically significant at 0.01 > p
A study was conducted to establish suitable doses for a study of
teratogenicity after dermal application to CD-1 mice. Fewer animals
were used that recommended in guideliness. Dinocap was applied as the
formulation Karathane LC XF, considered to be appropriate for
estimating the risk due to occupational exposure, but the doses used
were corrected for content and expressed as dinocap. Appropriate
dilutions were obtained with the formulation blank. In phase 1 of the
study, both untreated and vehicle control groups were used; in phase
2, only a vehicle control group was used. The dose volume of 290 µl/kg
bw was applied to one to five areas of shaven dorsal skin for 4 h each
day on days 6-15 of presumed gestation; the site was changed each day,
recommencing at the first site on the sixth day. Occlusion of the
application site was not mentioned, but a collar was placed to prevent
ingestion; no mention is made of how the mice were restrained during
application. After completion of the 4-h exposure each day, the test
material was washed off gently with soap and water.
Initially (phase 1), groups of eight presumed-pregnant mice were
given doses of 0 (control), 50, 80, or 100 mg/kg bw per day. Because
of excessive toxicity, similar groups of eight mice were given doses
of 0, 1, 4, 10, or 25 mg/kg bw per day by a similar protocol. The mice
were sacrificed on day 18 of presumed gestation; half of the fetuses
were used for skeletal examination, and the remaining half for
specific examination of otoliths and of the remaining skeleton. For
mice weighing 20-40 g, the dose volumes would have been 6-12 µl: the
practical difficulty of administering such small volumes may have
resulted in variations in accuracy. One, three, and four deaths
occurred during treatment at 50, 80, and 100 mg/kg bw per day,
respectively. Death was usually briefly (up to 24 h) preceded by signs
of toxicity, including dermal erythema, ataxia, red or tan vaginal
discharge, weight loss, and cold to touch. These doses all reduced
weight gain, with weight loss at the highest dose. The numbers of live
young were reduced at 50 and 80 mg/kg bw per day, and the litters of
the three surviving dams at 100 mg/kg bw per day contained only
resorbed conceptuses. Gross malformations were seen in 88% of fetuses
at 50 mg/kg bw per day and 100% of those at 80 mg/kg bw per day.
The principal treatment-related effects seen in phase 2 are shown
in Table 6. Little maternal toxicity occurred, and body-weight gain
was apparently unaffected by treatment. One mouse at the highest dose
was found dead on day 15 of gestation having been found stuporous with
an impaired righting reflex after dosing the day before. Death was
attributed to trauma, although no clear injury was detected at
necropsy. There was a slight incidence of skin irritation at the two
highest doses. The litter sizes, numbers of live fetuses per litter,
and fetal body weights were unaffected by treatment. There was no
change in the degree of skeletal ossification, but three fetuses of
one litter at the highest dose had cleft palate, and two fetuses of
another litter had open eyelids. Also at the highest dose, otolith
development was clearly impaired. These findings are consistent with
those of previous studies of the teratogenicity of dinocap in mice
after oral administration. In view of these results, no further study
by the dermal route was conducted. The study involved too few animals
to determine a NOAEL for teratogenicity in mice treated dermally,
particularly with respect to cleft palate; however, the quality of the
data on mean otolith scores in 49 fetuses may provide some degree of
confidence that the NOAEL for these effects is 10 mg/kg bw per day
(Foss, 1995).
Table 6. Results of a study for developmental toxicity in mice
Effect Dose (mg/kg bw per day)
0 1 4 10 25
No. of pregnant dams 8 8 7 8 6
Maternal weight gain on days 6-18 (g) 21.4 26.4 20.8 26.4 25.0
No. of fetuses 77 96 67 93 73
Cleft palate (no. of litters) 0 0 0 0 1
Open eyelids (no. of litters) 0 0 0 0 1
Mean otolith scorea 9.2 9.6 9.0 9.0 2.2
No. of fetuses examined 37 49 34 49 38
From Foss (1995)
a Three otoliths were scored for completeness on a scale of 1-4, to give a maximum
potential score of 12.
2,4-DNHPC and 2,6-DNHPC were not teratogenic in mice. Samples of
each isomer (purity, 95%) were tested seperately and in combination,
and the results were compared with those in mice receiving
technical-grade dinocap (purity, 84%), each group at a dose of 25
mg/kg bw per day in corn oil. Fewer pregnant animals were used than
recommended in the guidelines. Although litters born to
dinocap-treated mice showed a pattern of developmental defects typical
of those seen in the studies described above, the two isomers were
inactive at the same dose, both alone and in combination (Rogers et
al., 1987). In a separate study by the same group, otolith formation
was compared in the pups of mice and hamsters treated with dinocap
during gestation. Otolith formation was impaired in mice. Dinocap
affected otolith formation in hamsters, but only at a dose associated
with severe maternal and fetal toxicity (Rogers et al., 1989).
Rats
Groups of 25 Sprague-Dawley rats presumed to be pregnant received
dinocap (purity, 96%) as a suspension in aqueous methylcellulose at
doses of 0 (control), 10, 50, or 150 mg/kg bw per day by gavage on
days 6-15 of gestation. There were no deaths, and the only overt sign
of toxicity was an increased incidence of soft faeces in rats at 150
mg/kg bw per day. Maternal body-weight gain was impaired at 150 mg/kg
bw per day during the first two days of treatment, and food
consumption was slightly reduced in this group during treatment. Gross
examination revealed no treatment-related changes in pregnant or non-
pregnant females. There were no treatment-related changes in pup
weight and no increase in the incidence of malformations. There was an
apparent increase in the incidence of extra ribs at 150 mg/kg bw per
day, but seven of the 10 affected fetuses were from two litters. The
NOAEL for maternal and fetal toxicity was 50 mg/kg bw per day. There
was no evidence of teratogenicity at the highest dose of 150 mg/kg bw
per day (Solomon & Lutz, 1989).
Rabbits
Groups of 20 artificially inseminated New Zealand white rabbits
received dinocap (purity, 95.4%) in aqueous gum tragacanth by gavage
at doses of 0, 3, 12, 48, or 84 mg/kg bw per day on days 7-19 of
presumed pregnancy. Pups were delivered by caesarian section on day 29
and examined for developmental abnormalities in accordance with normal
guideline requirements. Two does at the high dose and one at the
intermediate dose died between days 24 and 29 of gestation, therefore
at least five days after dosing, but the deaths were considered to be
related to treatment. These animals all showed weight loss and
anorexia before death. The doe at the intermediate dose and one at the
high dose aborted; both does at the high dose that died had non-viable
litters (late resorptions). An additional five deaths were considered
unrelated to treatment and were primarily a consequence of intubation
errors.
The main results are shown in Table 7; statistical analyses were
not reported. There was an increased incidence of premature delivery
and abortion at 48 and 84 mg/kg bw per day; the premature deliveries
at the highest dose were primarily late resorptions. Clinical signs
(lack of faeces or dried faeces) and impaired weight gain and food
consumption were seen among does receiving these doses, litter sizes
and pup weights were reduced, and there was an increased incidence of
pups with skeletal malformations (vertebral assymetry and fused or
forked ribs). There was also a slight increase in delayed ossification
at a few sites in does at these doses. The NOAEL for maternal toxicity
was 3 mg/kg bw per day on the basis of weight-gain retardation during
treatment. The NOAEL for developmental toxicity was 12 mg/kg bw per
day on the basis of increased resorptions and reduced litter sizes at
48 mg/kg bw per day (Hoberman & Christian, 1987).
This conclusion is different from that of the 1989 JMPR (Annex 1,
reference 58) but is based on different data. In the latter review, an
increased incidence of hydrocephaly and neural tube defects was found
at doses > 3 mg/kg bw per day in the studies of Costlow & Kane
(1984 a,b). In the earlier studies, however, a less pure form of
dinocap (84%) was used, and the neural tube defects found by Costlow
and Kane (1984a) at a dose of 3 mg/kg bw per day were not found in the
second study (Costlow & Kane, 1984b) at 48 mg/kg bw per day nor in
studies by dermal administration in which the increased numbers of
Table 7. Results of a study of developmental toxicity in rabbits
Result Dose (mg/kg bw per day)
0 3 12 48 84
Adult animals
No. pregnant 17 17 15 15 14
No. with abortions 0 0 0 3 1
No. with premature delivery 1 0 0 1 3
No. with absence of faeces (on any day) 0 0 0 0 4
No. with dried faeces (on any day) 3 0 2 7 17
Weight change, days 7-20 (g) 150 200 90 -330 -550
Food consumption, days 7-20 (g/day) 160 170 152 65 51
No. with gastric ulceration 0 0 1 3 4
Litters
No. evaluated 12 17 14 10 9
Mean no. of live fetuses 7.2 7.5 8.1 6.3 5.9
Dead or resorbed fetuses per litter (%) 11.8 4.9 7.1 20.3 27.0
Mean fetal weight (g) 40.9 42.7 40.0 37.6 35.8
Vertebral assymetry: no. of litters (pups) 1 (1) 1 (1) 1 (2) 5 (7) 3 (8)
No. of litters (pups) with rib malformation 1 (1) 0 2 (3) 2 (2) 2 (6)
No. of litters (pups) with skeletal malformations 4 (4) 3 (3) 4 (6) 5 (7) 6 (12)
From Hoberman (1987)
resorptions and delayed ossifications were similar to these found by
Hoberman & Christian (1987).
(f) Special studies: Ocular toxicity
A NOAEL of 15 ppm was observed for ocular toxicity in a two-year
study in dogs (Weatherholz et al., 1979) in the 1989 JMPR (Annex 1,
reference 38). At 60 ppm, four of eight dogs had slight to moderate
discolouration of the tapetum lucidum, although no retinal atrophy and
no changes in the vascularity of the retina were noted. The remaining
four dogs at this dose showed moderate to marked discolouration of the
tapetum, reduced vascularity of the retina, and retinal atrophy (three
dogs). At 120/240 ppm, the four surviving dogs had marked
discolouration of the tapetum, reduced vascularity of the retina, and
retinal atrophy. Retinal atrophy was therefore present only in dogs
with severe tapetal changes, and no dog showed retinal effects without
effects in the tapetum.
A review of the literature for compounds that are toxic to the
tapetum lucidum of dogs was submitted (Solomon, 1991). Of six
compounds known to affect the tapetum, three (zinc pyridinethione, SCH
19927, and rosaramicin) had no effect in atapetal dogs, and no ocular
effects were reported in other atapetal animals. The other three
compounds (dinocap, hydroxy pyridinethione, and diphenyl
thiocarbazine) affected the tapetum in dogs (with no information on
atapetal dogs) but did not cause ocular effects in atapetal species.
The author concluded that the retinal atrophy seen with dinocap was
secondary to damage to the tapetum lucidum. Since humans do not have a
tapetum, it was concluded that humans would not be susceptible to
retinal damage as a consequence of this effect. The review is brief,
and the adequacy of the methods used, the sensitivity, and the
comparability of the findings were not considered. The conclusions
cannot therefore be regarded as definitive, although the data support
the hypothesis. The NOAEL in the study of Weatherholz et al. (1979)
was therefore identified for other effects. The 1989 JMPR report
(Annex 1, reference 58) indicates that significant effects, including
deaths, occurred at doses of 180-240 ppm, giving a NOAEL of 60 ppm,
equivalent to 1.5 mg/kg bw per day.
Comments
Dinocap is well absorbed after oral exposure. A proportion
(5-25%) is absorbed after dermal exposure, varying with species and
concentration. No conclusions were drawn about the degree of dermal
absorption in humans from the results of a study in which mouse and
human skin were compared; however, human skin is generally regarded as
being less permeable to toxicants than that of mice.
The urinary metabolites of the methylheptyl isomer in rats and
mice have been extensively characterized; characterization of the
faecal metabolites was reported by the 1989 JMPR, which concluded that
the pattern of metabolites in faeces seen by thin-layer chromatography
was similar to that observed in squash and cucumbers.
The new data confirmed the generally low degree of acute toxicity
of dinocap in rats; mice, however, appear to be more sensitive than
rats to both acute and developmental effects. Dinocap is a skin
irritant and sensitizer. The available studies did not address the
uncoupling of oxidative phosphorylation, identified by the 1989 JMPR
as a potentially significant mode of action.
WHO has classified dinocap as 'slightly hazardous' (WHO, 1996).
In a study of carcinogenicity in mice at doses of 0, 15, 100, or
200 ppm, no evidence of carcinogenicity was found. The NOAEL was 15
ppm, equal to 2.7 mg/kg bw per day. The lack of carcinogenicity in
mice is consistent with the absence of carcinogenicity in rats
reported by the 1989 JMPR.
The results of tests for genotoxicity (on the less pure form of
dinocap) were negative.
A multigeneration study of reproductive toxicity at dietary
concentrations of 0, 40, 200, or 1000 ppm in rats showed no specific
effect on any reproductive parameters; the NOAEL was 200 ppm, equal to
13 mg/kg bw per day.
In a study of developmental toxicity in mice dosed by gavage at
0, 4, 10, or 25 mg/kg bw per day, impaired otolith formation was seen
at 25 mg/kg bw per day. A dose-related increase in the incidence of
open eyelids and cleft palate extended down to 10 mg/kg bw per day in
the absence of maternal toxicity. The NOAEL was 4 mg/kg bw per day.
Dermal application of 50, 80, or 100 mg/kg bw per day to mice proved
excessively toxic for an evaluation of developmental toxicity. A
further dermal study in mice at 0, 1, 4, 10, or 25 mg/kg bw per day
showed malformations,including impaired otolith formation, at 25 mg/kg
bw per day in the absence of maternal toxicity. The NOAEL for
developmental toxicity after dermal application to mice was 10 mg/kg
bw per day. The results of the recent studies of developmental
toxicity confirmed the teratogenic potential of purified dinocap in
mice, even when applied dermally. Impaired otolith development,
characteristic of the teratogenicity of dinocap in mice, was also seen
in hamsters at doses that are maternally toxic. Less specific
malformations were seen in rabbits at maternally toxic doses. The
present Meeting concluded that the NOAEL in the studies in rabbits
described by the 1989 JMPR was 3 mg/kg bw per day rather than
0.5 mg/kg bw per day, since the findings on which the putative effect
level was established do not appear to be repeatable or clearly
dose-related. The methylheptyl isomer has been shown not to be
teratogenic to mice. The reason for the species difference in the
teratogenicity of dinocap in rats and mice therefore cannot be deduced
from the data on the metabolism of the methylheptyl isomer.
The two-year study in dogs that was evaluated at the 1989 Joint
Meeting was also reassessed on the basis that the critical effect
(retinal atrophy) was secondary to effects on the tapetum lucidum.
Since the tapetum lucidum is not present in humans, or in rats or mice
in which no retinal effect was seen, the Meeting concluded that it
would be inappropriate to base the evaluation on this effect. The
NOAEL was 60 ppm, equivalent to 1.5 mg/kg bw per day.
Teratogenic effects in mice were considered to be the
toxicological end-point of greatest concern. Since dinocap was
teratogenic in mice after either oral or dermal administration and
since malformations were seen in at least three species, the Meeting
considered a high safety factor to be appropriate. An ADI of 0-0.008
mg/kg bw was established on the basis of the NOAEL of
4 mg/kg bw per day in the developmental toxicity study in mice and a
safety factor of 500.
Establishment of an acute reference dose (RfD) was considered to
be appropriate since teratogenicity may occur after a single exposure.
An acute RfD was established on the basis of the NOAEL of 4 mg/kg bw
per day for teratogenicity in mice and a safety factor of 500, to give
an acute RfD of 0.008 mg/kg bw, which is appropriate for women of
child-bearing age.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 15 ppm, equal to 2.7 mg/kg bw per day (toxicity in a
study of carcinogenicity)
4 mg/kg bw per day (developmental toxicity)
10 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rat: 200 ppm, equal to 6.4 mg/kg bw per day (toxicity in a
study of carcinogenicity)
50 mg/kg bw per day (maternal and developmental
toxicity in a study of developmental toxicity)
Rabbit: 3 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 60 ppm, equivalent to 1.5 mg/kg bw per day (study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.008 mg/kg bw
Estimate of acute reference dose
0.008 mg/kg bw
Information that would be useful for continued evaluation of the
compound
Further observations in humans
List of end-points for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of oral absorption 60-69% absorbed, max. concentration at 2-6 h
Dermal absorption 5-25%
Distribution Widely distributed
Potential for accumulation Limited, < 0.3% in tissue after 7 days
Rate and extent of excretion Biphasic; half-life is 3 h for 1st phase, 44 h for 2nd
phase, oral administration, rabbit
Metabolism in animals Extensive; > 96% metabolized
Toxicologically significant compounds Metabolites assumed to be of similar toxicity to parent
(animals, plants and environment)
Acute toxicity
Rat: LD50 oral 3100 mg/kg bw
Rat: LD50 dermal > 5000 mg/kg bw
Rat: LC50 inhalation 3 mg/L
Skin irritation Irritating
Eye irritation Irritating
Skin sensitization Sensitizing
Short-term toxicity
Target/critical effect General toxicity
Lowest relevant oral NOAEL Dog: 1.5 mg/kg bw per day
Lowest relevant dermal NOAEL Mouse: 10 mg/kg bw per day (teratogenicity)
Lowest relevant inhalation NOAEL No data
Genotoxicity Not genotoxic in an adequate battery of tests
Long-term toxicity and carcinogenicity
Target/critical effect: Impaired weight gain
Lowest relevant NOAEL Mouse: 2.7 mg/kg bw per day (carcinogenicity)
Carcinogenicity Not carcinogenic
Reproductive toxicity
Reproduction target/critical effect No effect on fertility or ability to rear young
Lowest relevant reproductive NOAEL Rat: 13 mg/kg bw per day (multigeneration study)
Developmental target/critical effect Malformations
Lowest relevant developmental NOAEL Mouse: 4 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity No data, but no concern raised in other studies
Other toxicological studies Inhibits oxidative phosphorylation; methylheptyl
isomer not teratogenic
Medical data No significant dinocap-related effects reported
Summary Value Study Safety factor
ADI 0-0.008 mg/kg bw Mouse, developmental toxicity 500
Acute reference dose 0.008 mg/kg bw Mouse, developmental toxicity 500
References
Anderson, D.M. & Baldwin, R.C. (1990a) Karathane LC fungicide/miticide
delayed contact hypersensitivity study in guinea pigs. Unpublished
report No. 89R-187 from Rohm & Haas Company, Toxicology Department,
Spring House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas
Company, Spring House, Pennsylvania, USA.
Anderson, D.M.& Baldwin, R.C. (1990b) Karathane FN-57
fungicide/miticide delayed contact hypersensitivity study in guinea
pigs. Unpublished study No. 89R-188 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Anderson, D.M. & Baldwin, R.C. (1990c) Karathane technical (HPK)
fungicide/miticide delayed contact hypersensitivity study in guinea
pigs. Unpublished report No. 89R-189 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Bernacki, H.J. & Baldwin, R.C. (1987) Karathane technical (dinocap):
Range finding four-week dietary toxicity study in mice. Unpublished
report No. 87R-175. from Rohm & Haas Co., Toxicology Department,
Spring House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co.,
Spring House, Pennsylvania, USA.
Bernacki, H.J. & Hamilton, J.D. (1992a) Karathane LC
fungicide/miticide: Skin irritation study in rabbits. Unpublished
report No. 91R-170 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Bernacki, H.J. & Hamilton, J.D. (1992b) Karathane LC
fungicide/miticide: Acute dermal toxicity study in male and female
rats. Unpublished report No. 91R-171 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Bernacki, H.J. & Hamilton, J.D. (1992c) Karathane LC
fungicide/miticide: Acute oral toxicity study in male and female rats.
Unpublished report No. 91R-173 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Blair, M. & Cavender, F.L. (1979) TD 77-310 acute inhalation study in
rats. Unpublished report No. 285-029 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Rohm & Haas Co., Spring House, Pennsylvania, USA.
Costlow, R.D. & Kane, W.W. (1984a) Teratology study with Karathane in
rabbits. Unpublished report No. 83R-22 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Costlow, R.D. & Kane, W.W. (1984b) Teratology study with Karathane in
rabbits. Unpublished report No. 83R-113 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA
Evans, K.W. & Hazelton, G.A. (1995) Blood pharmacokinetics and dermal
absorption of Karathane in female mice. Unpublished report No. 95R-105
from Rohm & Haas Co., Toxicology Department, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Ferguson, J.S. (1997) Karathane technical: Acute inhalation toxicity
study in rats. Unpublished report No. 96R-126 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Foss, J.A. (1995) Dermal dosage-range developmental toxicity study of
Karathane LC XF in mice. Unpublished report No. 018-023P from Argus
Research Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA Rohm & Haas
Co. report No. 95RC-024.
Foxall, S. (1985) Karathane technical: CHO/HGPRT gene mutation assay.
Unpublished report No. 85R-204 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Hoberman, A. & Christian, M. (1987) A developmental toxicity study of
Karathane fungicide/miticide (technical) administered via stomach tube
to New Zealand white rabbits. Unpublished report No. 018-012 from
Argus Research Laboratories, Inc., Horsham, Pennsylvania, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Rohm & Haas Co. report No. 86RC-71.
Ivett, J. & Myhr, B. (1986) Clastogenic evaluation of Karathane
technical in an in-vitro cytogenetic assay measuring chromosomal
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished study project No. 20990 from Litton Bionetics, Inc.,
Kensington, Massachusetts, USA. Rohm & Haas Co. report No. 85RC-62.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1987a) Karathane
FN-57 fungicide/miticide: Acute dermal toxicity study in male and
female rabbits. Unpublished report No. 87R-138 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1987b) Karathane
FN-57 fungicide/miticide: Eye irritation study in rabbits. Unpublished
report No. 87R-114 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Krajewski, R.J., Morrison, R.D. & Baldwin, R.C. (1987c) Karathane
FN-57 fungicide/miticide: Skin irritation study in male rabbits.
Unpublished report No. 87R-139 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Lampe, K.R., Morrison, R.D. & Baldwin, R.C. (1987a) Karathane LC
fungicide-miticide: Acute oral toxicity study in male and female rats.
Unpublished report No. 87R-132 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Lampe, K.R., Morrison, R.D. & Baldwin, R.C. (1987b) Karathane LC
fungicide-miticide: Acute dermal toxicity study in male and female
rats. Unpublished report No. 87R-133 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA
Lochry, E. (1989) Dinocap (high purity technical): An oral (gavage)
developmental toxicity study in CD-1(ICR)BR mice including a postnatal
phase. Unpublished report No. 018-015 from Argus Research
Laboratories, Inc., Horsham, Pennsylvania, USA. Submitted to WHO by
Rohm & Haas Co., Spring House, Pennsylvania, USA. Rohm & Haas Co.
report No. 88P-335.
Maibach, H. (1985) Elimination of 14C labeled Karathane in rhesus
monkeys following a single intramuscular injected dose. Unpublished
report No. 85RC-18 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Moore, M.R. (1991) Dinocap (technical purified): 78-Week dietary
oncogenicity study in mice. Unpublished report No. 417-445 from
Hazleton Laboratories America, Inc., Rockville, Massachusetts, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Rohm & Haas Co. report No. 88RC-090
Morrison, R.D. & Hamilton, J.D. (1991) Karathane FN-57
fungicide/miticide: Acute oral toxicity study in male and female mice.
Unpublished report No. 91R-001 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Morrison, R.D. & Hamilton, J.D. (1992) Karathane LC
fungicide/miticide: Eye irritation study in rabbits. Unpublished
report No. 91R-172 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Morrison, R.D., Krzywicki, K.M. & Hazelton, G.A. (1987) Karathane
technical purified: Acute oral toxicity study in male rats and mice.
Unpublished report No. 86R-149A,B from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Morseth, S. (1990) Dinocap (technical purified): Two-generation
reproduction study in rats. Unpublished report No. 417-442 from
Hazleton Laboratories America, Inc., Vienna, Virginia, USA. Submitted
to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA. Rohm &
Haas Co. report No. 88RC-092.
Onishi, M. (1989) Karathane LC (HPK) fungicide/fiticide: Acute oral
toxicity study in mice. Unpublished study from Shin Nippon Biomedical
Laboratories Ltd, Kagoshima, Japan. Rohm & Haas Co. report No.
90RC-1001. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Potter, D. (1996) Identification of
2,4-dinitro-6-(1-methylheptyl)phenyl crotonate metabolites in rat and
mouse urine. Unpublished report No. 95R-064 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Procopio, K.R. & Parno, J.R. (1995) Karathane LC: Acute dermal
toxicity study in male and female mice. Unpublished report No. 94R-244
from Rohm & Haas Co., Toxicology Department, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Rogers, J.M., Gray, E.L., Carver, B.D. & Kavlock, R.J. (1987)
Developmental toxicity of dinocap in the mouse is not due to two
isomers of the major active ingredient. Teratol. Carcinog. Mutag.,
7, 341-346.
Rogers, J.M., Burkhead, L.M. & Barbee B.D. (1989) Effects of dinocap
on otolith formation: Evaluation of mouse and hamster fetuses at term.
Teratology, 39, 515-523.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987a) Karathane
technical purified: Acute oral toxicity study in male and female rats.
Unpublished report No. 87R-124 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987b) Karathane
technical purified: Acute dermal toxicity study in male and female
rabbits. Unpublished report No. 87R-126 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987c) Karathane
FN-57 fungicide-miticide: Acute oral toxicity study in male and female
rats. Unpublished report No. 87R-137 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. 1987d) Karathane
technical purified: Skin irritation study in male rabbits. Unpublished
report No. 87R-125 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987e) Karathane LC
fungicide/miticide: Rabbit skin irritation study. Unpublished report
No. 87R-134 from Rohm & Haas Co., Toxicology Department, Spring House,
Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring House,
Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987f) Karathane
technical purified: Eye irritation study in male rabbits. Unpublished
report No. 87R-111 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Romanello, A.S., Morrison, R.D. & Baldwin, R.C. (1987g) Karathane LC
fungicide/miticide: Eye irritation study in rabbits. Unpublished
report No. 87R-135 from Rohm & Haas Co., Toxicology Department, Spring
House, Pennsylvania, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA.
Ruegg, C.E. (1996) In vitro percutaneous absorption and cutaneous
distribution of Karathane LC XF across intact human and mouse skin.
Unpublished report No. M95-024 from In Vitro Technologies, Inc.,
Baltimore, Maryland, USA. Submitted to WHO by Rohm & Haas Co., Spring
House, Pennsylvania, USA. Rohm & Haas Co. report No. 95RC-187..
Sames, J.L., Yu, R.Y. & McCarthy, K.L. (1986) Karathane: In-vivo
cytogenetic study in mice. Unpublished report No. 85R-235 from Rohm &
Haas Co., Toxicology Department, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Solomon, H.M. (1991) Karathane fungicide/miticide: Ocular toxicity in
dogs. Unpublished report No. 91M-663 from Rohm & Haas Co., Toxicology
Department, Spring House, Pennsylvania, USA. Submitted to WHO by Rohm
& Haas Co., Spring House, Pennsylvania, USA.
Solomon, H.M. & Lutz, M.F. (1989) Dinocap: Oral (gavage) developmental
toxicity study in rats. Unpublished report No. 88R-236 from Rohm &
Haas Co., Toxicology Department, Spring House, Pennsylvania, USA.
Submitted to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Wanner, F.J. & Hagan, J.V. (1991) Karathane FN-57: Acute inhalation
study in rats. Unpublished report No. 91R-002 from Rohm & Haas Co.,
Toxicology Department, Spring House, Pennsylvania, USA. Submitted to
WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
Weatherholz, W.D., Kundzins, W., Alsaker, R.A., Voelker, R.W. &
Peterson, K. (1979) 104-Week toxicity study in dogs: Karathane
technical. Unpublished report from Hazleton Laboratories America,
Inc., Vienna, Virginia, USA. Submitted to WHO by Rohm & Haas Co.,
Spring House, Pennsylvania, USA. Rohm & Haas Co. report 79RC-45.
Wester, R.W. & Maibach, H.I. (1985) Karathane: Percutaneous absorption
of 14C Karathane (14C-DNHPC) in rhesus monkey following single
topical application. Unpublished report No. 85RC-049 from Rohm & Haas
Co., Toxicology Department, Spring House, Pennsylvania, USA. Submitted
to WHO by Rohm & Haas Co., Spring House, Pennsylvania, USA.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
