
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
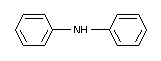
DIPHENYLAMINE
IDENTITY
Chemical name
Diphenylamine
Synonyms
DPA
Structural formula
 Purity
Minimum 99.9 per cent by cryoscopic method. Primary amines as aniline,
not more than 10 parts per million.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Diphenylamine is absorbed from the digestive tract of the rat, rabbit,
dog and man; however no information is available on the extent of
absorption (DeEdu, 1961; Alexander et al., 1965).
The metabolism of diphenylamine has been studied in these four species
and is essentially the same in all of them. A slight amount of
unchanged diphenylamine has been detected in the urine of rabbits and
of man (but not rats) after oral administration of the compound. The
major metabolites are 4-hydroxydiphenylamine and
4,4'-dihydroxydiphenylamine. Both of these compounds have been
identified as conjugates in the urine of human subjects which had
received a single oral dose of 100 mg of diphenylamine, but no free
hydroxylated derivatives are excreted in man. In the rat, rabbit and
dog, 4-hroxydiphenylamine is excreted partly unchanged and partly as
conjugates with glucuronic acid and sulphuric acid, and these
conjugates have been isolated from rabbit urine. Hydroxylation in the
ortho-position occurs only in the rabbit and then only to a slight
extent. N-hydroxydiphenylamine has not been detected from any species
and there is no evidence for its formation (Alexander et al., 1965;
Booth, 1963).
The N-glucuronide of 4-hydroxydiphenylamine has been reported to have
been detected in the urine or rats after oral administration of
diphenylamine. However, the method described for characterizing the
compound would not have distinguished it from the O-glucuronide
(DeEds, 1961).
Studies with carbon14-labelled diphenylamine indicate that the
compound in rapidly metabolized and excreted by the rat. After 48
hours, 75 per cent of an intraperitoneal dose appears in the urine. An
intravenous dose results in the excretion of 25 per cent of the
radio-activity in bile after six hours (Alexander et al., 1965).
Hydroxylated derivatives of diphenylamine have been detected in rat
and dog faeces and represent excretion via the bile, since acid
hydrolysed bile was found to contain 4-hydroxydiphenylamine. The
possibility that intestinal bacteria may be also hydroxylate
unabsorbed diphenylamine has, however, not been ruled out (DeEds.
1961).
No information is available on the relative amounts of urinary or
faecal metabolites derived from an oral dose of diphenylamine.
Special studies on carcinogenicity
Mouse
Two groups, each containing 40-50 male and 40-50 female mice weighing
30-35 g, were selected for this study. The animals received
subcutaneous injections of 0.5 ml of a 25 per cent solution of
diphenylamine in trioctanoin (approximately 4000 mg/kg body-weight)
once every two weeks on alternate sides of the body. After two months
it was found that the compound accumulated when given this frequently
and the injections were then given once every three weeks until the
animals had received injections for a total period of 80 weeks. A
control group of mice received injections of the solvent vehicle
alone. There was no significant difference in the incidence of
malignant or benign tumours between the test and the control groups,
nor was the injection of diphenylamine associated with the development
of tumours at the injection site. A related study involving oral
feeding of diphenylamine to mice also involved tissue examination for
tumours and this study is described under "Long-term studies" (Univ.
of Birmingham, 1966).
An 80 week oral feeding study in mice is currently in progress and the
study will include an evaluation for carcinogenicity. The results from
a preliminary progress report of this study are given under
"Short-term studies" (Golberg, 1969).
Rat
It is known that bacteria in the human stomach will reduce nitrates to
nitrites. Studies in 31 patients showed that these nitrites will react
with diphenylamine to form diphenylnitrosamine (Sander and Seif,
1969). However, it has previously been demonstrated with studies in
the rat that, unlike many nitrosamines, diphenylnitrosamine is not a
carcinogen (Druckrey et al., 1961).
Special studies on reproduction
Rat
A two-generation rat reproduction study was conducted with dietary
levels of 0, 1000, 2500 and 5000 ppm of diphenylamine. Treatment was
began at weaning. At 100 days of age, 12 females and three males were
selected from each of these dose level groups and grouped as three
females and one male per cage. Once a week for three weeks the males
were isolated among the three groups of females, after which time the
males were removed and the females placed in individual cages. When
all the litters were weaned, the females were given a 2-3 week rest
and then remated. In addition offspring from the first mating were
mated once, as described above, for a second generation study. Feeding
of diphenylamine did not influence the number of litters born or the
incidence of mortality of the offspring. It was uncertain if
diphenylamine was responsible for the reduced litter-size and weight
of the young at 21 days which was observed in the group fed 5000 ppm
of diphenylamine (Thomas et al., 1967a; Booth, 1963).
Acute toxicity
The oral LD50 of diphenylamine to male rats is approximately 3,200
mg/kg body-weight (American Cyanamid Co., 1956).
Short-term studies
Dog
Groups of four dogs (two males and two females) were fed diets
containing 0, 100, 1000 and 10,000 ppm of diphenylamine for a period
of two years. The dogs were about eight months of age at the
commencement of the study. Decreased body-weight gain occurred in the
1000 and 10,000 ppm groups although food consumption was normal. A
pronounced anaemia developed in the 10,000 ppm group and mild anaemia
in the 1000 ppm group. The bromsulphthalein (BSP) test of liver
function from day 618 to day 627 indicated a moderate degree of liver
damage at 10,000 ppm. The phenolsulphonaphthalein (PSP) test of kidney
function gave normal values, and the urine gave negative tests for
albumin and glucose. All organ-weight changes and microscopic lesions
were limited to the 10,000 ppm level. These manifestations consisted
of peripherolubular fatty change in the liver with a marked increase
in liver-weight and other-extractable lipids; a mild haemosiderosis of
the spleen, kidneys and bone marrow, and a slight increase in kidney
weight (Thomas et al., 1967b).
Mouse
A preliminary progress report is available on the feeding of dietary
levels of 0, 50, 100 and 250 ppm of diphenylamine to groups of mice.
Over a 2-3 month period there has been no difference in body-weight or
incidence of mortality between control and test groups. The feeding
will be continued for a total of 80 weeks and will include
haematological examinations and histological evaluation at autopsy
(Golberg, 1969).
Rat
Groups of 10 male rats were fed diets containing 0, 100, 1000, or
10,000 ppm of diphenylamine for 30 days. There were no deaths in any
group, and no signs of toxicity at 100 and 1000 ppm. The animals fed
10,000 ppm made only about one-half the weight gain of the controls
even though the food intakes of the two groups did not differ. Food
intake and weight gain at 1000 ppm were significantly higher than
those of the controls. Autopsy of animals at 100 and 1000 ppm
disclosed no gross pathologic lesions that could be attributed to
feeding of diphenylamine. At 10,000 ppm the animals had dark and
roughened spleens, and three had hyperaemic kidneys. Paleness of the
extremities in most of the rats fed the highest dose level was noted
but no chemical analysis was performed to determine if methaemoglobin
was present in the blood. No histological examination was made of the
tissues (American Cyanamid Company, 1956).
Groups of six female rats were fed diets containing 0, 250, 1000,
5000, 10,000, or 15,000 ppm of diphenylamine for 226 days. Inhibition
of growth occurred at dietary levels of 5000 ppm or more. At necropsy
no gross change was noted except for enlargement of the kidneys in
animals which had received 15,000 ppm. Microscopic examination of
tissues revealed the formation of foci of dilated renal tubules and
pigmentation of the liver and kidney suggestive of blood destruction
at levels of 5000 ppm and above (Thomas et al., 1957; Booth, 1963).
An unspecified number of rats, including weanling rats of both sexes
and adult male rats weighing 250 grams, were fed a diet containing 2.5
per cent of diphenylamine for periods up to 12 months. Morphologic
alterations of the renal tubules were found in all animals. These
alterations varied from tubular dilation to overt cyst formation.
Glomerular filtration rate and maximum urinary osmolality were
decreased and the degree of decrement corresponded to the degree of
involvement. The animals with polycystic kidneys had an increased
susceptibility to pyelonephritis (Kime et al., 1962).
Long-term studies
Mouse
Groups of mice (40-50 animals of each sex) were fed diets containing
0, 100, 300, 1000, and 5000 ppm of diphenylamine for 80 weeks. The
study began when the animals were 4-5 weeks of age. Increased
mortality occurred on diets containing 300 ppm or more of
diphenylamine. Abnormalities in the liver (chronic inflammatory change
and iron pigment deposition), kidneys (iron deposition) and spleen
(iron deposition, fibrosis, lymph follicle hypoplasia) occurred at
5000 ppm, and also in the spleen at 1000 ppm. Although the total
tumour incidence was significantly increased in the 100 ppm group,
this increase appeared incidental and not associated with
diphenylamine intake. The effect of subcutaneous injections of
diphenylamine to another group of mice in this study is described
under "Special studies on carcinogenicity". In this experiment except
for one isolated period between weeks 30 and 40 with respect to the
female test group, the incidence of mortality was not different from a
control group (Univ. of Birmingham, 1966).
Rat
Groups of 40 rats each comprising 20 males and 20 females were fed
diets containing 0, 10, 100, 1000, 5000, or 10,000 ppm of
diphenylamine, commencing at weaning and continuing for two years.
There was no evidence of toxicity among animals at the 100 ppm level
and below. There was a decreased rate of growth at 5000 and 10,000
ppm, the effect at the latter level being at least partly due to
reduced food intake. Doubtful effect on growth occurred at 1000 ppm in
the females. A moderate degree of anaemia occurred at 10,000 ppm,
which was reversible upon returning the animals to the control diet.
Total white cell count and differential white cell count remained
normal at all dose levels. Kidney damage in the form of dilated
tubules was produced at levels above 1000 ppm, and to a lesser degree
at 1000 ppm. The incidence and type of tumours found were unrelated to
treatment with diphenylamine (Thomas et al., 1967a).
COMMENT
Dihydroxylated products of metabolism have been identified in the
urine of laboratory animals and human subjects; N-hydroxylation was
not observed. Short-term studies with an insufficient number of rats
have been carried out; at higher concentrations of diphenylamine,
morphologic alterations of the renal tubules were found. There is
insufficient information on the possible formation of methaemoglobin
which might be expected with an aromatic amine. In the long-term
studies on mice, an increased tumour incidence appeared incidental,
but subcutaneous injections of diphenylamine in trioctanoin solution
demonstrated no significant difference between test and control
groups. In the long-term studies on rats, the incidence and type of
tumours were unrelated to treatment with diphenylamine. A study on the
carcinogenicity in mice after oral ingestion is in progress. The
short-term studies on dogs and the lone-term studies on rats form the
experimental basis for the established adi.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 100 ppm in the diet, equivalent to 2.5 mg/kg body-weight/day
Mouse: 100 ppm in the diet, equivalent to 15 mg/kg body-weight/day
Rat: 100 ppm in the diet, equivalent to 5 mg/kg body-weight/day
Estimate of acceptable daily intake for man
0.0-0.025 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
DPA is used to prevent a storage disorder of apples known as scald. It
is the only known use of DPA. The incidence and severity of the
disease varies, depending upon locality, seasonal conditions prior to
and at harvest. Varietal differences in susceptibility and severity of
the condition are frequent, as well as a requirement for greater
concentrations of CPA (and higher residues) for them to prevent
losses. The protective action of DPA is believed to be due to the
antioxidant effect on alpha-farnesene a sesquiterpene which occurs in
the natural coatings of apples (Huelin and Murray, 1966).
Alcohol suspensions have been used in some of the earlier work
reported here, but it is believed that only wettable powders, oil
emulsions and impregnated papers are in use now. Data are available
from Australia, New Zealand, U.S.A. and the U.K. DPA is also used in
Canada, and probably in many other apple producing countries.
Pre-harvest treatments
Mature apples on the trees are sprayed a few days before harvest if
the first symptoms of the disease are detected at this stage, or if
weather conditions suggest treatments should be applied. An aqueous
suspension is applied at concentrations of 500 to 2000 ppm.
Post-harvest treatments
Harvest fruit is (a) dipped in aqueous suspension (500 to 3000 ppm),
(b) sprayed in boxes, pallet loads, bins or conveyors (1000 to 2000
ppm), boxes immersed in suspensions or emulsions, or (c) individual
apples wrapped in impregnated paper (1 to 2 mg/wrap).
Most fruit treated is held in storage for from 80 to 200 days or more
before marketing.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues in whole fruit from tree sprays are usually comparatively
low, less than 1 ppm (Bruce et al., 1958; Harvey and Clark, 1959), but
can commonly be as high as 6 ppm on a particular variety (Gutenmann
and Lisk, 1963) at the rates outlined above. Initial residues from
post-harvest sprays and dips have been reported as high as 63 ppm, but
these decline rapidly in storage. Most post-harvest spray residues
after storage are in the range of 2 to 6 ppm (Harvey and Clark, 1959;
Bache et al., 1962), but have been reported as high as 7.7 ppm (Harvey
and Clark, 1959). Dip deposits from 2000 ppm suspension can have
initial residues as high as 12 ppm, usually most are above 8 ppm
(Bruce et al., 1958). In one variety, an initial residue of 62.6 ppm
declined after 120 days in storage to 10 ppm. 1000 ppm dip residues
are not likely to be higher than 4 ppm. Residues from impregnated
paper wraps are less than 4 ppm (Bruce et al., 1958). One-half the
total DPA content of apples was found in peel (Bruce et al., 1958),
but 90% can be found in the outer 2 to 4 mm of the fruit (Harvey and
Clark, 1959). Little migration of DPA occurs, either laterally or into
the apple flesh (Hall et al., 1961). This has recently been confirmed
using autoradiography and 14C-DPA solutions (Wilson 1969, unpublished
thesis).
FATE OF RESIDUES
The disappearance of DPA, from either physical or chemical standpoint
does not appear to be accounted for. It is assumed that most losses in
storage are due to volatilization, but this is not verified by
available literature.
Cooking studies have not been reported. The post-storage residues
noted above can be presumed to be those occurring on fruit at the time
of consumption.
Five years of data on commercial scale treatments and holding periods,
involving six varieties of apples are available from Cornell
University (Bache et al., 1962) resulting in average residues after
storage of up to 6 ppm required in order to obtain effective scald
control. Residues of between 8 to 9 ppm also are associated with the
most effective treatments. In 1965 Cornell University (Smock, R.M.
1969, unpublished) analysed commercial lots of treated apples
collected from eight locations in New York State. Treatments applied
by box flooding or immersion varied from 1000 to 2000 ppm DPA.
Variations in residues in individual apples depending upon location
within the box varied considerably, but no consistent pattern was
established. The highest residue reported from a sample at the top of
a box was 6.91 ppm (corrected).
METHODS OF RESIDUE ANALYSIS
The earliest development work on the use of DPA in New Zealand
utilized the method of Yatsu described by Harvey (1958). It involves
vanadium pentoxide-sulphuric acid oxidation of DPA to produce a blue
colour measured at 600 nanometers (nm). Sensitivity is approximately
0.5 ppm for apples. Bruce et al. (1958) developed a method of coupling
the amino with diazotized 2,4-dinitroaniline, measured at 530 nm. This
method is sensitive to at least 0.1 ppm in apples. This method is
likely to be acceptable for use by analysts in regulatory
laboratories. Recently, a procedure for gas chromatography electron
capture has been described (Guttenmann and Lisk, 1963). It involves
bromination of DPA to produce presumptive 2,2', 4,4',
6.6'-hexabromo-DPA and its subsequent GLC determination. Sensitivity
of about 0.02 ppm is claimed.
There is a need to monitor to DPA content of solutions during
commercial treatments in post-harvest applications in order to
determine the point in time for renewal of solutions. The GLC
procedure was not found to be reliable for residue values of 0-25 ppm
for whole apples. A colorimetric procedure was adapted whereby DPA
extracted from blended apple tissues with acetone was reacted with
varnadium pentoxide and quantitatively determined
spectrophotometrically at 605 mu. This procedure was modified for
monitoring the DPA content of commercial applicator systems (Wilson,
1969).
NATIONAL TOLERANCES
Country Commodity Tolerance (ppm)
Australia Apples 10
Canada Apples 10
United States of America Apples 10
Milk and meat zero
APPRAISAL
Diphenylamine (DPA) has a minimum purity of 99.9 per cent. Primary
amines such as aniline are stated as not more than 10 ppm of the
technical grade material.
DPA is used to prevent losses from a storage disease of apples known
as "scald", which varies in severity and incidence due to locality,
weather and in different varieties of apples. The protective action of
DPA is attributed to antioxidant effect on alpha-farnesene, a
sesquiterpene which occurs in the natural coating of apples.
Pre-harvest emulsion sprays applied to mature apples very close to
harvest and post-harvest treatments of bulk apples on sorting rollers,
in boxes, pallets, bins, etc. are applied as emulsions or wettable
powders. Impregnated paper wraps are also used.
The residue is believed to be DPA alone. No degradation products or
metabolites in fruit are identified, nor are they presumed to exist.
Losses are attributed to volatilization. The amount of residue
immediately after treatment varies with the technology of treatment,
but can very from 63 ppm to as low as 1 ppm. All treated apples are
held in storage from 80 to over 200 days. Residues decline in storage
to an average range of less than 1 to 8 ppm, with an occasional
residue in one variety as high as 10 ppm after 120 days, storage
reported. About half the residue is in the peel, and 90% in the outer
2 to 4 mm of fruit. Little migration occurs afterwards.
No results of cooking studies are available. Residues reaching the
consumer can be assumed to be in the order of those mentioned above.
Five years of data collected from commercial scale treatments and
storage holding periods suggest average residues will be 6 ppm, with
some at 8 to 9 ppm. The most recent commercial sampling from eight
locations in New York State resulted in the highest residue found as
6.9 ppm.
An analytical method based on coupling the amine with diazotized
2,4-dinitroaniline, measured at 530 nm is sensitive to 0.1 ppm in
apples. This method is likely to be acceptable for most regulatory
laboratories. A GLC electron capture method is also available. It
involves bromination of DPA to produce presumptive 2, 2', 4, 4', 6,
6'-hexabromo DPA.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TOLERANCES
Apples 10 ppm
FURTHER WORK OR INFORMATION
DESIRABLE
1. Experiments to determine if methaemoglobin is formed in animals.
2. Short-term studies using an adequate number of rats.
3. Additional metabolic studies in a non-rodent mammalian species.
4. The results of the carcinogenicity study in mice which is currently
in progress.
REFERENCES
Alexander, W.E., Ryan, A.J. and Wright, S.E. (1964) Metabolism of
diphenylamine in the rat and rabbit. Experientia 20:223-4
Alexander, W.E., Ryan, A.J. and Wright, S.E. (1965) Metabolism of
diphenylamine in rat, rabbit and man. Food Cosmet. Toxicol. 3:571-9
American Cyanamid Co. (1956) Diphenylamine : limited release toxicity
studies. Unpub. rept. submitted by C.B. Shaffer
Bache, C.A., Smock, R.M., Yatsu, L., Mooney. C., and Lisk, D.J. (1962)
Diphenylamine residues on apples in relation to scald control.
Proc. Amer. Soc. Hort. Sci. 81:57-60
Booth, A.N. (1963) Summary of toxicological data. Chronic toxicity
studies on diphenylamine. Food Cosmet. Toxicol 1:331-3
Bruce, R.B., Howard, J.W., and Zink, J.B. (1958) Determination of
diphenylamine residues on apples. J. Agr. Food Chem. 6:597-600
DeEds, F. (1961) Chronic toxicity studies on diphenylamine. Unpub.
rept. on data for use in a petition requesting tolerance for
diphenylamine used in prevention of "scald" of apples during
storage. Western Utilization R. and D. Div. U.S.D.A., Albany Calif.
Druckrey, H., Preussmann, R., Schmähl, D. and Miller, M. (1961)
Chemische Konstitution und Carcinogens Wirkung bei Nitrosaminen.
Naturwissenschaften 48:134-5
Golberg, L. (1969) Determination of the toxicity of diphenylamine in mice.
First progress report. Unpub. rept. Inst. Exp. Pathol. Toxicol.,
Albany, New York
Gutenmann, W.H., and Lisk, D.J. (1963) Rapid determination of
diphenylamine in apples by direct bromination and gas chromatography.
J. Agr. Food Chem. 11:468-70
Hall, E.G., Scott, K.J., and Coote, G.G. (1961) Control of superficial
scald on Granny Smith apples with diphenylamine. Australian J. Agr.
Res. 12:834-52
Harvey, H.E. (1958) Determination of diphenylamine residues on apples.
New Zealand J. Sci. 1:378-82
Harvey, H.E., and Clerk, P.J. (1959) Diphenylamine residues on apples.
New Zealand J. Sci. 2.266-72
Huelin, F.E., and Murray, K.E. (1966) alpha-Farnesene in the natural
coating of apples. Nature 210.1260-61
Kime, S.W. Jr., McNamara, J.J., Luse, S., Farmer, S., Silbert, C. and
Brieker, N.S. (1962) Experimental polyeystic renal disease in rats :
electron microscopy, function and susceptibility to pyelonephritis.
J. Lab. Clin. Med. 60:64-78
Sander, von J., and Seif, F. (1969) Bakterielle Reduktion von Nitrat
im Magen des Menschen als Ursache einer Nitrosamin-Bildung.
Arzneimittel-Forsch. 19:1091-98
Thomas, J.O., Cox, A.J. Jr. and DeEds, F. (1957) Kidney cysts produced
by diphenylamine. Stanford Med. Bull. 15:90-93
Thomas, J.O., Ribelin, W.E., Wilson, R.H., Keppler, D.C. and DeEds, F.
(1967a) Chronic toxicity of diphenylamine to albino rats. Toxicol.
appl. Pharmacol. 10:362-74
Thomas. J.O., Ribelin. W.E., Woodward, J.R. and DeEds, F. (1967b)
The chronic toxicity of diphenylamine for dogs. Toxicol. appl.
Pharmacol. 11:184-94
University of Birmingham. (1966) Studies on the long-term effect of
diphenylamine in mice. Unpub. rept. Toxicol. Unit, Dept. Med. Biochem.
Pharmacol., Birmingham, England
Wilson, L.G. (1969) Unpublished Ph.D. thesis. Ann Arbor, Mich. 17 Jan. Univ.
Microfilms 69-20-958
Purity
Minimum 99.9 per cent by cryoscopic method. Primary amines as aniline,
not more than 10 parts per million.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Diphenylamine is absorbed from the digestive tract of the rat, rabbit,
dog and man; however no information is available on the extent of
absorption (DeEdu, 1961; Alexander et al., 1965).
The metabolism of diphenylamine has been studied in these four species
and is essentially the same in all of them. A slight amount of
unchanged diphenylamine has been detected in the urine of rabbits and
of man (but not rats) after oral administration of the compound. The
major metabolites are 4-hydroxydiphenylamine and
4,4'-dihydroxydiphenylamine. Both of these compounds have been
identified as conjugates in the urine of human subjects which had
received a single oral dose of 100 mg of diphenylamine, but no free
hydroxylated derivatives are excreted in man. In the rat, rabbit and
dog, 4-hroxydiphenylamine is excreted partly unchanged and partly as
conjugates with glucuronic acid and sulphuric acid, and these
conjugates have been isolated from rabbit urine. Hydroxylation in the
ortho-position occurs only in the rabbit and then only to a slight
extent. N-hydroxydiphenylamine has not been detected from any species
and there is no evidence for its formation (Alexander et al., 1965;
Booth, 1963).
The N-glucuronide of 4-hydroxydiphenylamine has been reported to have
been detected in the urine or rats after oral administration of
diphenylamine. However, the method described for characterizing the
compound would not have distinguished it from the O-glucuronide
(DeEds, 1961).
Studies with carbon14-labelled diphenylamine indicate that the
compound in rapidly metabolized and excreted by the rat. After 48
hours, 75 per cent of an intraperitoneal dose appears in the urine. An
intravenous dose results in the excretion of 25 per cent of the
radio-activity in bile after six hours (Alexander et al., 1965).
Hydroxylated derivatives of diphenylamine have been detected in rat
and dog faeces and represent excretion via the bile, since acid
hydrolysed bile was found to contain 4-hydroxydiphenylamine. The
possibility that intestinal bacteria may be also hydroxylate
unabsorbed diphenylamine has, however, not been ruled out (DeEds.
1961).
No information is available on the relative amounts of urinary or
faecal metabolites derived from an oral dose of diphenylamine.
Special studies on carcinogenicity
Mouse
Two groups, each containing 40-50 male and 40-50 female mice weighing
30-35 g, were selected for this study. The animals received
subcutaneous injections of 0.5 ml of a 25 per cent solution of
diphenylamine in trioctanoin (approximately 4000 mg/kg body-weight)
once every two weeks on alternate sides of the body. After two months
it was found that the compound accumulated when given this frequently
and the injections were then given once every three weeks until the
animals had received injections for a total period of 80 weeks. A
control group of mice received injections of the solvent vehicle
alone. There was no significant difference in the incidence of
malignant or benign tumours between the test and the control groups,
nor was the injection of diphenylamine associated with the development
of tumours at the injection site. A related study involving oral
feeding of diphenylamine to mice also involved tissue examination for
tumours and this study is described under "Long-term studies" (Univ.
of Birmingham, 1966).
An 80 week oral feeding study in mice is currently in progress and the
study will include an evaluation for carcinogenicity. The results from
a preliminary progress report of this study are given under
"Short-term studies" (Golberg, 1969).
Rat
It is known that bacteria in the human stomach will reduce nitrates to
nitrites. Studies in 31 patients showed that these nitrites will react
with diphenylamine to form diphenylnitrosamine (Sander and Seif,
1969). However, it has previously been demonstrated with studies in
the rat that, unlike many nitrosamines, diphenylnitrosamine is not a
carcinogen (Druckrey et al., 1961).
Special studies on reproduction
Rat
A two-generation rat reproduction study was conducted with dietary
levels of 0, 1000, 2500 and 5000 ppm of diphenylamine. Treatment was
began at weaning. At 100 days of age, 12 females and three males were
selected from each of these dose level groups and grouped as three
females and one male per cage. Once a week for three weeks the males
were isolated among the three groups of females, after which time the
males were removed and the females placed in individual cages. When
all the litters were weaned, the females were given a 2-3 week rest
and then remated. In addition offspring from the first mating were
mated once, as described above, for a second generation study. Feeding
of diphenylamine did not influence the number of litters born or the
incidence of mortality of the offspring. It was uncertain if
diphenylamine was responsible for the reduced litter-size and weight
of the young at 21 days which was observed in the group fed 5000 ppm
of diphenylamine (Thomas et al., 1967a; Booth, 1963).
Acute toxicity
The oral LD50 of diphenylamine to male rats is approximately 3,200
mg/kg body-weight (American Cyanamid Co., 1956).
Short-term studies
Dog
Groups of four dogs (two males and two females) were fed diets
containing 0, 100, 1000 and 10,000 ppm of diphenylamine for a period
of two years. The dogs were about eight months of age at the
commencement of the study. Decreased body-weight gain occurred in the
1000 and 10,000 ppm groups although food consumption was normal. A
pronounced anaemia developed in the 10,000 ppm group and mild anaemia
in the 1000 ppm group. The bromsulphthalein (BSP) test of liver
function from day 618 to day 627 indicated a moderate degree of liver
damage at 10,000 ppm. The phenolsulphonaphthalein (PSP) test of kidney
function gave normal values, and the urine gave negative tests for
albumin and glucose. All organ-weight changes and microscopic lesions
were limited to the 10,000 ppm level. These manifestations consisted
of peripherolubular fatty change in the liver with a marked increase
in liver-weight and other-extractable lipids; a mild haemosiderosis of
the spleen, kidneys and bone marrow, and a slight increase in kidney
weight (Thomas et al., 1967b).
Mouse
A preliminary progress report is available on the feeding of dietary
levels of 0, 50, 100 and 250 ppm of diphenylamine to groups of mice.
Over a 2-3 month period there has been no difference in body-weight or
incidence of mortality between control and test groups. The feeding
will be continued for a total of 80 weeks and will include
haematological examinations and histological evaluation at autopsy
(Golberg, 1969).
Rat
Groups of 10 male rats were fed diets containing 0, 100, 1000, or
10,000 ppm of diphenylamine for 30 days. There were no deaths in any
group, and no signs of toxicity at 100 and 1000 ppm. The animals fed
10,000 ppm made only about one-half the weight gain of the controls
even though the food intakes of the two groups did not differ. Food
intake and weight gain at 1000 ppm were significantly higher than
those of the controls. Autopsy of animals at 100 and 1000 ppm
disclosed no gross pathologic lesions that could be attributed to
feeding of diphenylamine. At 10,000 ppm the animals had dark and
roughened spleens, and three had hyperaemic kidneys. Paleness of the
extremities in most of the rats fed the highest dose level was noted
but no chemical analysis was performed to determine if methaemoglobin
was present in the blood. No histological examination was made of the
tissues (American Cyanamid Company, 1956).
Groups of six female rats were fed diets containing 0, 250, 1000,
5000, 10,000, or 15,000 ppm of diphenylamine for 226 days. Inhibition
of growth occurred at dietary levels of 5000 ppm or more. At necropsy
no gross change was noted except for enlargement of the kidneys in
animals which had received 15,000 ppm. Microscopic examination of
tissues revealed the formation of foci of dilated renal tubules and
pigmentation of the liver and kidney suggestive of blood destruction
at levels of 5000 ppm and above (Thomas et al., 1957; Booth, 1963).
An unspecified number of rats, including weanling rats of both sexes
and adult male rats weighing 250 grams, were fed a diet containing 2.5
per cent of diphenylamine for periods up to 12 months. Morphologic
alterations of the renal tubules were found in all animals. These
alterations varied from tubular dilation to overt cyst formation.
Glomerular filtration rate and maximum urinary osmolality were
decreased and the degree of decrement corresponded to the degree of
involvement. The animals with polycystic kidneys had an increased
susceptibility to pyelonephritis (Kime et al., 1962).
Long-term studies
Mouse
Groups of mice (40-50 animals of each sex) were fed diets containing
0, 100, 300, 1000, and 5000 ppm of diphenylamine for 80 weeks. The
study began when the animals were 4-5 weeks of age. Increased
mortality occurred on diets containing 300 ppm or more of
diphenylamine. Abnormalities in the liver (chronic inflammatory change
and iron pigment deposition), kidneys (iron deposition) and spleen
(iron deposition, fibrosis, lymph follicle hypoplasia) occurred at
5000 ppm, and also in the spleen at 1000 ppm. Although the total
tumour incidence was significantly increased in the 100 ppm group,
this increase appeared incidental and not associated with
diphenylamine intake. The effect of subcutaneous injections of
diphenylamine to another group of mice in this study is described
under "Special studies on carcinogenicity". In this experiment except
for one isolated period between weeks 30 and 40 with respect to the
female test group, the incidence of mortality was not different from a
control group (Univ. of Birmingham, 1966).
Rat
Groups of 40 rats each comprising 20 males and 20 females were fed
diets containing 0, 10, 100, 1000, 5000, or 10,000 ppm of
diphenylamine, commencing at weaning and continuing for two years.
There was no evidence of toxicity among animals at the 100 ppm level
and below. There was a decreased rate of growth at 5000 and 10,000
ppm, the effect at the latter level being at least partly due to
reduced food intake. Doubtful effect on growth occurred at 1000 ppm in
the females. A moderate degree of anaemia occurred at 10,000 ppm,
which was reversible upon returning the animals to the control diet.
Total white cell count and differential white cell count remained
normal at all dose levels. Kidney damage in the form of dilated
tubules was produced at levels above 1000 ppm, and to a lesser degree
at 1000 ppm. The incidence and type of tumours found were unrelated to
treatment with diphenylamine (Thomas et al., 1967a).
COMMENT
Dihydroxylated products of metabolism have been identified in the
urine of laboratory animals and human subjects; N-hydroxylation was
not observed. Short-term studies with an insufficient number of rats
have been carried out; at higher concentrations of diphenylamine,
morphologic alterations of the renal tubules were found. There is
insufficient information on the possible formation of methaemoglobin
which might be expected with an aromatic amine. In the long-term
studies on mice, an increased tumour incidence appeared incidental,
but subcutaneous injections of diphenylamine in trioctanoin solution
demonstrated no significant difference between test and control
groups. In the long-term studies on rats, the incidence and type of
tumours were unrelated to treatment with diphenylamine. A study on the
carcinogenicity in mice after oral ingestion is in progress. The
short-term studies on dogs and the lone-term studies on rats form the
experimental basis for the established adi.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 100 ppm in the diet, equivalent to 2.5 mg/kg body-weight/day
Mouse: 100 ppm in the diet, equivalent to 15 mg/kg body-weight/day
Rat: 100 ppm in the diet, equivalent to 5 mg/kg body-weight/day
Estimate of acceptable daily intake for man
0.0-0.025 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
DPA is used to prevent a storage disorder of apples known as scald. It
is the only known use of DPA. The incidence and severity of the
disease varies, depending upon locality, seasonal conditions prior to
and at harvest. Varietal differences in susceptibility and severity of
the condition are frequent, as well as a requirement for greater
concentrations of CPA (and higher residues) for them to prevent
losses. The protective action of DPA is believed to be due to the
antioxidant effect on alpha-farnesene a sesquiterpene which occurs in
the natural coatings of apples (Huelin and Murray, 1966).
Alcohol suspensions have been used in some of the earlier work
reported here, but it is believed that only wettable powders, oil
emulsions and impregnated papers are in use now. Data are available
from Australia, New Zealand, U.S.A. and the U.K. DPA is also used in
Canada, and probably in many other apple producing countries.
Pre-harvest treatments
Mature apples on the trees are sprayed a few days before harvest if
the first symptoms of the disease are detected at this stage, or if
weather conditions suggest treatments should be applied. An aqueous
suspension is applied at concentrations of 500 to 2000 ppm.
Post-harvest treatments
Harvest fruit is (a) dipped in aqueous suspension (500 to 3000 ppm),
(b) sprayed in boxes, pallet loads, bins or conveyors (1000 to 2000
ppm), boxes immersed in suspensions or emulsions, or (c) individual
apples wrapped in impregnated paper (1 to 2 mg/wrap).
Most fruit treated is held in storage for from 80 to 200 days or more
before marketing.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues in whole fruit from tree sprays are usually comparatively
low, less than 1 ppm (Bruce et al., 1958; Harvey and Clark, 1959), but
can commonly be as high as 6 ppm on a particular variety (Gutenmann
and Lisk, 1963) at the rates outlined above. Initial residues from
post-harvest sprays and dips have been reported as high as 63 ppm, but
these decline rapidly in storage. Most post-harvest spray residues
after storage are in the range of 2 to 6 ppm (Harvey and Clark, 1959;
Bache et al., 1962), but have been reported as high as 7.7 ppm (Harvey
and Clark, 1959). Dip deposits from 2000 ppm suspension can have
initial residues as high as 12 ppm, usually most are above 8 ppm
(Bruce et al., 1958). In one variety, an initial residue of 62.6 ppm
declined after 120 days in storage to 10 ppm. 1000 ppm dip residues
are not likely to be higher than 4 ppm. Residues from impregnated
paper wraps are less than 4 ppm (Bruce et al., 1958). One-half the
total DPA content of apples was found in peel (Bruce et al., 1958),
but 90% can be found in the outer 2 to 4 mm of the fruit (Harvey and
Clark, 1959). Little migration of DPA occurs, either laterally or into
the apple flesh (Hall et al., 1961). This has recently been confirmed
using autoradiography and 14C-DPA solutions (Wilson 1969, unpublished
thesis).
FATE OF RESIDUES
The disappearance of DPA, from either physical or chemical standpoint
does not appear to be accounted for. It is assumed that most losses in
storage are due to volatilization, but this is not verified by
available literature.
Cooking studies have not been reported. The post-storage residues
noted above can be presumed to be those occurring on fruit at the time
of consumption.
Five years of data on commercial scale treatments and holding periods,
involving six varieties of apples are available from Cornell
University (Bache et al., 1962) resulting in average residues after
storage of up to 6 ppm required in order to obtain effective scald
control. Residues of between 8 to 9 ppm also are associated with the
most effective treatments. In 1965 Cornell University (Smock, R.M.
1969, unpublished) analysed commercial lots of treated apples
collected from eight locations in New York State. Treatments applied
by box flooding or immersion varied from 1000 to 2000 ppm DPA.
Variations in residues in individual apples depending upon location
within the box varied considerably, but no consistent pattern was
established. The highest residue reported from a sample at the top of
a box was 6.91 ppm (corrected).
METHODS OF RESIDUE ANALYSIS
The earliest development work on the use of DPA in New Zealand
utilized the method of Yatsu described by Harvey (1958). It involves
vanadium pentoxide-sulphuric acid oxidation of DPA to produce a blue
colour measured at 600 nanometers (nm). Sensitivity is approximately
0.5 ppm for apples. Bruce et al. (1958) developed a method of coupling
the amino with diazotized 2,4-dinitroaniline, measured at 530 nm. This
method is sensitive to at least 0.1 ppm in apples. This method is
likely to be acceptable for use by analysts in regulatory
laboratories. Recently, a procedure for gas chromatography electron
capture has been described (Guttenmann and Lisk, 1963). It involves
bromination of DPA to produce presumptive 2,2', 4,4',
6.6'-hexabromo-DPA and its subsequent GLC determination. Sensitivity
of about 0.02 ppm is claimed.
There is a need to monitor to DPA content of solutions during
commercial treatments in post-harvest applications in order to
determine the point in time for renewal of solutions. The GLC
procedure was not found to be reliable for residue values of 0-25 ppm
for whole apples. A colorimetric procedure was adapted whereby DPA
extracted from blended apple tissues with acetone was reacted with
varnadium pentoxide and quantitatively determined
spectrophotometrically at 605 mu. This procedure was modified for
monitoring the DPA content of commercial applicator systems (Wilson,
1969).
NATIONAL TOLERANCES
Country Commodity Tolerance (ppm)
Australia Apples 10
Canada Apples 10
United States of America Apples 10
Milk and meat zero
APPRAISAL
Diphenylamine (DPA) has a minimum purity of 99.9 per cent. Primary
amines such as aniline are stated as not more than 10 ppm of the
technical grade material.
DPA is used to prevent losses from a storage disease of apples known
as "scald", which varies in severity and incidence due to locality,
weather and in different varieties of apples. The protective action of
DPA is attributed to antioxidant effect on alpha-farnesene, a
sesquiterpene which occurs in the natural coating of apples.
Pre-harvest emulsion sprays applied to mature apples very close to
harvest and post-harvest treatments of bulk apples on sorting rollers,
in boxes, pallets, bins, etc. are applied as emulsions or wettable
powders. Impregnated paper wraps are also used.
The residue is believed to be DPA alone. No degradation products or
metabolites in fruit are identified, nor are they presumed to exist.
Losses are attributed to volatilization. The amount of residue
immediately after treatment varies with the technology of treatment,
but can very from 63 ppm to as low as 1 ppm. All treated apples are
held in storage from 80 to over 200 days. Residues decline in storage
to an average range of less than 1 to 8 ppm, with an occasional
residue in one variety as high as 10 ppm after 120 days, storage
reported. About half the residue is in the peel, and 90% in the outer
2 to 4 mm of fruit. Little migration occurs afterwards.
No results of cooking studies are available. Residues reaching the
consumer can be assumed to be in the order of those mentioned above.
Five years of data collected from commercial scale treatments and
storage holding periods suggest average residues will be 6 ppm, with
some at 8 to 9 ppm. The most recent commercial sampling from eight
locations in New York State resulted in the highest residue found as
6.9 ppm.
An analytical method based on coupling the amine with diazotized
2,4-dinitroaniline, measured at 530 nm is sensitive to 0.1 ppm in
apples. This method is likely to be acceptable for most regulatory
laboratories. A GLC electron capture method is also available. It
involves bromination of DPA to produce presumptive 2, 2', 4, 4', 6,
6'-hexabromo DPA.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TOLERANCES
Apples 10 ppm
FURTHER WORK OR INFORMATION
DESIRABLE
1. Experiments to determine if methaemoglobin is formed in animals.
2. Short-term studies using an adequate number of rats.
3. Additional metabolic studies in a non-rodent mammalian species.
4. The results of the carcinogenicity study in mice which is currently
in progress.
REFERENCES
Alexander, W.E., Ryan, A.J. and Wright, S.E. (1964) Metabolism of
diphenylamine in the rat and rabbit. Experientia 20:223-4
Alexander, W.E., Ryan, A.J. and Wright, S.E. (1965) Metabolism of
diphenylamine in rat, rabbit and man. Food Cosmet. Toxicol. 3:571-9
American Cyanamid Co. (1956) Diphenylamine : limited release toxicity
studies. Unpub. rept. submitted by C.B. Shaffer
Bache, C.A., Smock, R.M., Yatsu, L., Mooney. C., and Lisk, D.J. (1962)
Diphenylamine residues on apples in relation to scald control.
Proc. Amer. Soc. Hort. Sci. 81:57-60
Booth, A.N. (1963) Summary of toxicological data. Chronic toxicity
studies on diphenylamine. Food Cosmet. Toxicol 1:331-3
Bruce, R.B., Howard, J.W., and Zink, J.B. (1958) Determination of
diphenylamine residues on apples. J. Agr. Food Chem. 6:597-600
DeEds, F. (1961) Chronic toxicity studies on diphenylamine. Unpub.
rept. on data for use in a petition requesting tolerance for
diphenylamine used in prevention of "scald" of apples during
storage. Western Utilization R. and D. Div. U.S.D.A., Albany Calif.
Druckrey, H., Preussmann, R., Schmähl, D. and Miller, M. (1961)
Chemische Konstitution und Carcinogens Wirkung bei Nitrosaminen.
Naturwissenschaften 48:134-5
Golberg, L. (1969) Determination of the toxicity of diphenylamine in mice.
First progress report. Unpub. rept. Inst. Exp. Pathol. Toxicol.,
Albany, New York
Gutenmann, W.H., and Lisk, D.J. (1963) Rapid determination of
diphenylamine in apples by direct bromination and gas chromatography.
J. Agr. Food Chem. 11:468-70
Hall, E.G., Scott, K.J., and Coote, G.G. (1961) Control of superficial
scald on Granny Smith apples with diphenylamine. Australian J. Agr.
Res. 12:834-52
Harvey, H.E. (1958) Determination of diphenylamine residues on apples.
New Zealand J. Sci. 1:378-82
Harvey, H.E., and Clerk, P.J. (1959) Diphenylamine residues on apples.
New Zealand J. Sci. 2.266-72
Huelin, F.E., and Murray, K.E. (1966) alpha-Farnesene in the natural
coating of apples. Nature 210.1260-61
Kime, S.W. Jr., McNamara, J.J., Luse, S., Farmer, S., Silbert, C. and
Brieker, N.S. (1962) Experimental polyeystic renal disease in rats :
electron microscopy, function and susceptibility to pyelonephritis.
J. Lab. Clin. Med. 60:64-78
Sander, von J., and Seif, F. (1969) Bakterielle Reduktion von Nitrat
im Magen des Menschen als Ursache einer Nitrosamin-Bildung.
Arzneimittel-Forsch. 19:1091-98
Thomas, J.O., Cox, A.J. Jr. and DeEds, F. (1957) Kidney cysts produced
by diphenylamine. Stanford Med. Bull. 15:90-93
Thomas, J.O., Ribelin, W.E., Wilson, R.H., Keppler, D.C. and DeEds, F.
(1967a) Chronic toxicity of diphenylamine to albino rats. Toxicol.
appl. Pharmacol. 10:362-74
Thomas. J.O., Ribelin. W.E., Woodward, J.R. and DeEds, F. (1967b)
The chronic toxicity of diphenylamine for dogs. Toxicol. appl.
Pharmacol. 11:184-94
University of Birmingham. (1966) Studies on the long-term effect of
diphenylamine in mice. Unpub. rept. Toxicol. Unit, Dept. Med. Biochem.
Pharmacol., Birmingham, England
Wilson, L.G. (1969) Unpublished Ph.D. thesis. Ann Arbor, Mich. 17 Jan. Univ.
Microfilms 69-20-958
 Purity
Minimum 99.9 per cent by cryoscopic method. Primary amines as aniline,
not more than 10 parts per million.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Diphenylamine is absorbed from the digestive tract of the rat, rabbit,
dog and man; however no information is available on the extent of
absorption (DeEdu, 1961; Alexander et al., 1965).
The metabolism of diphenylamine has been studied in these four species
and is essentially the same in all of them. A slight amount of
unchanged diphenylamine has been detected in the urine of rabbits and
of man (but not rats) after oral administration of the compound. The
major metabolites are 4-hydroxydiphenylamine and
4,4'-dihydroxydiphenylamine. Both of these compounds have been
identified as conjugates in the urine of human subjects which had
received a single oral dose of 100 mg of diphenylamine, but no free
hydroxylated derivatives are excreted in man. In the rat, rabbit and
dog, 4-hroxydiphenylamine is excreted partly unchanged and partly as
conjugates with glucuronic acid and sulphuric acid, and these
conjugates have been isolated from rabbit urine. Hydroxylation in the
ortho-position occurs only in the rabbit and then only to a slight
extent. N-hydroxydiphenylamine has not been detected from any species
and there is no evidence for its formation (Alexander et al., 1965;
Booth, 1963).
The N-glucuronide of 4-hydroxydiphenylamine has been reported to have
been detected in the urine or rats after oral administration of
diphenylamine. However, the method described for characterizing the
compound would not have distinguished it from the O-glucuronide
(DeEds, 1961).
Studies with carbon14-labelled diphenylamine indicate that the
compound in rapidly metabolized and excreted by the rat. After 48
hours, 75 per cent of an intraperitoneal dose appears in the urine. An
intravenous dose results in the excretion of 25 per cent of the
radio-activity in bile after six hours (Alexander et al., 1965).
Hydroxylated derivatives of diphenylamine have been detected in rat
and dog faeces and represent excretion via the bile, since acid
hydrolysed bile was found to contain 4-hydroxydiphenylamine. The
possibility that intestinal bacteria may be also hydroxylate
unabsorbed diphenylamine has, however, not been ruled out (DeEds.
1961).
No information is available on the relative amounts of urinary or
faecal metabolites derived from an oral dose of diphenylamine.
Special studies on carcinogenicity
Mouse
Two groups, each containing 40-50 male and 40-50 female mice weighing
30-35 g, were selected for this study. The animals received
subcutaneous injections of 0.5 ml of a 25 per cent solution of
diphenylamine in trioctanoin (approximately 4000 mg/kg body-weight)
once every two weeks on alternate sides of the body. After two months
it was found that the compound accumulated when given this frequently
and the injections were then given once every three weeks until the
animals had received injections for a total period of 80 weeks. A
control group of mice received injections of the solvent vehicle
alone. There was no significant difference in the incidence of
malignant or benign tumours between the test and the control groups,
nor was the injection of diphenylamine associated with the development
of tumours at the injection site. A related study involving oral
feeding of diphenylamine to mice also involved tissue examination for
tumours and this study is described under "Long-term studies" (Univ.
of Birmingham, 1966).
An 80 week oral feeding study in mice is currently in progress and the
study will include an evaluation for carcinogenicity. The results from
a preliminary progress report of this study are given under
"Short-term studies" (Golberg, 1969).
Rat
It is known that bacteria in the human stomach will reduce nitrates to
nitrites. Studies in 31 patients showed that these nitrites will react
with diphenylamine to form diphenylnitrosamine (Sander and Seif,
1969). However, it has previously been demonstrated with studies in
the rat that, unlike many nitrosamines, diphenylnitrosamine is not a
carcinogen (Druckrey et al., 1961).
Special studies on reproduction
Rat
A two-generation rat reproduction study was conducted with dietary
levels of 0, 1000, 2500 and 5000 ppm of diphenylamine. Treatment was
began at weaning. At 100 days of age, 12 females and three males were
selected from each of these dose level groups and grouped as three
females and one male per cage. Once a week for three weeks the males
were isolated among the three groups of females, after which time the
males were removed and the females placed in individual cages. When
all the litters were weaned, the females were given a 2-3 week rest
and then remated. In addition offspring from the first mating were
mated once, as described above, for a second generation study. Feeding
of diphenylamine did not influence the number of litters born or the
incidence of mortality of the offspring. It was uncertain if
diphenylamine was responsible for the reduced litter-size and weight
of the young at 21 days which was observed in the group fed 5000 ppm
of diphenylamine (Thomas et al., 1967a; Booth, 1963).
Acute toxicity
The oral LD50 of diphenylamine to male rats is approximately 3,200
mg/kg body-weight (American Cyanamid Co., 1956).
Short-term studies
Dog
Groups of four dogs (two males and two females) were fed diets
containing 0, 100, 1000 and 10,000 ppm of diphenylamine for a period
of two years. The dogs were about eight months of age at the
commencement of the study. Decreased body-weight gain occurred in the
1000 and 10,000 ppm groups although food consumption was normal. A
pronounced anaemia developed in the 10,000 ppm group and mild anaemia
in the 1000 ppm group. The bromsulphthalein (BSP) test of liver
function from day 618 to day 627 indicated a moderate degree of liver
damage at 10,000 ppm. The phenolsulphonaphthalein (PSP) test of kidney
function gave normal values, and the urine gave negative tests for
albumin and glucose. All organ-weight changes and microscopic lesions
were limited to the 10,000 ppm level. These manifestations consisted
of peripherolubular fatty change in the liver with a marked increase
in liver-weight and other-extractable lipids; a mild haemosiderosis of
the spleen, kidneys and bone marrow, and a slight increase in kidney
weight (Thomas et al., 1967b).
Mouse
A preliminary progress report is available on the feeding of dietary
levels of 0, 50, 100 and 250 ppm of diphenylamine to groups of mice.
Over a 2-3 month period there has been no difference in body-weight or
incidence of mortality between control and test groups. The feeding
will be continued for a total of 80 weeks and will include
haematological examinations and histological evaluation at autopsy
(Golberg, 1969).
Rat
Groups of 10 male rats were fed diets containing 0, 100, 1000, or
10,000 ppm of diphenylamine for 30 days. There were no deaths in any
group, and no signs of toxicity at 100 and 1000 ppm. The animals fed
10,000 ppm made only about one-half the weight gain of the controls
even though the food intakes of the two groups did not differ. Food
intake and weight gain at 1000 ppm were significantly higher than
those of the controls. Autopsy of animals at 100 and 1000 ppm
disclosed no gross pathologic lesions that could be attributed to
feeding of diphenylamine. At 10,000 ppm the animals had dark and
roughened spleens, and three had hyperaemic kidneys. Paleness of the
extremities in most of the rats fed the highest dose level was noted
but no chemical analysis was performed to determine if methaemoglobin
was present in the blood. No histological examination was made of the
tissues (American Cyanamid Company, 1956).
Groups of six female rats were fed diets containing 0, 250, 1000,
5000, 10,000, or 15,000 ppm of diphenylamine for 226 days. Inhibition
of growth occurred at dietary levels of 5000 ppm or more. At necropsy
no gross change was noted except for enlargement of the kidneys in
animals which had received 15,000 ppm. Microscopic examination of
tissues revealed the formation of foci of dilated renal tubules and
pigmentation of the liver and kidney suggestive of blood destruction
at levels of 5000 ppm and above (Thomas et al., 1957; Booth, 1963).
An unspecified number of rats, including weanling rats of both sexes
and adult male rats weighing 250 grams, were fed a diet containing 2.5
per cent of diphenylamine for periods up to 12 months. Morphologic
alterations of the renal tubules were found in all animals. These
alterations varied from tubular dilation to overt cyst formation.
Glomerular filtration rate and maximum urinary osmolality were
decreased and the degree of decrement corresponded to the degree of
involvement. The animals with polycystic kidneys had an increased
susceptibility to pyelonephritis (Kime et al., 1962).
Long-term studies
Mouse
Groups of mice (40-50 animals of each sex) were fed diets containing
0, 100, 300, 1000, and 5000 ppm of diphenylamine for 80 weeks. The
study began when the animals were 4-5 weeks of age. Increased
mortality occurred on diets containing 300 ppm or more of
diphenylamine. Abnormalities in the liver (chronic inflammatory change
and iron pigment deposition), kidneys (iron deposition) and spleen
(iron deposition, fibrosis, lymph follicle hypoplasia) occurred at
5000 ppm, and also in the spleen at 1000 ppm. Although the total
tumour incidence was significantly increased in the 100 ppm group,
this increase appeared incidental and not associated with
diphenylamine intake. The effect of subcutaneous injections of
diphenylamine to another group of mice in this study is described
under "Special studies on carcinogenicity". In this experiment except
for one isolated period between weeks 30 and 40 with respect to the
female test group, the incidence of mortality was not different from a
control group (Univ. of Birmingham, 1966).
Rat
Groups of 40 rats each comprising 20 males and 20 females were fed
diets containing 0, 10, 100, 1000, 5000, or 10,000 ppm of
diphenylamine, commencing at weaning and continuing for two years.
There was no evidence of toxicity among animals at the 100 ppm level
and below. There was a decreased rate of growth at 5000 and 10,000
ppm, the effect at the latter level being at least partly due to
reduced food intake. Doubtful effect on growth occurred at 1000 ppm in
the females. A moderate degree of anaemia occurred at 10,000 ppm,
which was reversible upon returning the animals to the control diet.
Total white cell count and differential white cell count remained
normal at all dose levels. Kidney damage in the form of dilated
tubules was produced at levels above 1000 ppm, and to a lesser degree
at 1000 ppm. The incidence and type of tumours found were unrelated to
treatment with diphenylamine (Thomas et al., 1967a).
COMMENT
Dihydroxylated products of metabolism have been identified in the
urine of laboratory animals and human subjects; N-hydroxylation was
not observed. Short-term studies with an insufficient number of rats
have been carried out; at higher concentrations of diphenylamine,
morphologic alterations of the renal tubules were found. There is
insufficient information on the possible formation of methaemoglobin
which might be expected with an aromatic amine. In the long-term
studies on mice, an increased tumour incidence appeared incidental,
but subcutaneous injections of diphenylamine in trioctanoin solution
demonstrated no significant difference between test and control
groups. In the long-term studies on rats, the incidence and type of
tumours were unrelated to treatment with diphenylamine. A study on the
carcinogenicity in mice after oral ingestion is in progress. The
short-term studies on dogs and the lone-term studies on rats form the
experimental basis for the established adi.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 100 ppm in the diet, equivalent to 2.5 mg/kg body-weight/day
Mouse: 100 ppm in the diet, equivalent to 15 mg/kg body-weight/day
Rat: 100 ppm in the diet, equivalent to 5 mg/kg body-weight/day
Estimate of acceptable daily intake for man
0.0-0.025 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
DPA is used to prevent a storage disorder of apples known as scald. It
is the only known use of DPA. The incidence and severity of the
disease varies, depending upon locality, seasonal conditions prior to
and at harvest. Varietal differences in susceptibility and severity of
the condition are frequent, as well as a requirement for greater
concentrations of CPA (and higher residues) for them to prevent
losses. The protective action of DPA is believed to be due to the
antioxidant effect on alpha-farnesene a sesquiterpene which occurs in
the natural coatings of apples (Huelin and Murray, 1966).
Alcohol suspensions have been used in some of the earlier work
reported here, but it is believed that only wettable powders, oil
emulsions and impregnated papers are in use now. Data are available
from Australia, New Zealand, U.S.A. and the U.K. DPA is also used in
Canada, and probably in many other apple producing countries.
Pre-harvest treatments
Mature apples on the trees are sprayed a few days before harvest if
the first symptoms of the disease are detected at this stage, or if
weather conditions suggest treatments should be applied. An aqueous
suspension is applied at concentrations of 500 to 2000 ppm.
Post-harvest treatments
Harvest fruit is (a) dipped in aqueous suspension (500 to 3000 ppm),
(b) sprayed in boxes, pallet loads, bins or conveyors (1000 to 2000
ppm), boxes immersed in suspensions or emulsions, or (c) individual
apples wrapped in impregnated paper (1 to 2 mg/wrap).
Most fruit treated is held in storage for from 80 to 200 days or more
before marketing.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues in whole fruit from tree sprays are usually comparatively
low, less than 1 ppm (Bruce et al., 1958; Harvey and Clark, 1959), but
can commonly be as high as 6 ppm on a particular variety (Gutenmann
and Lisk, 1963) at the rates outlined above. Initial residues from
post-harvest sprays and dips have been reported as high as 63 ppm, but
these decline rapidly in storage. Most post-harvest spray residues
after storage are in the range of 2 to 6 ppm (Harvey and Clark, 1959;
Bache et al., 1962), but have been reported as high as 7.7 ppm (Harvey
and Clark, 1959). Dip deposits from 2000 ppm suspension can have
initial residues as high as 12 ppm, usually most are above 8 ppm
(Bruce et al., 1958). In one variety, an initial residue of 62.6 ppm
declined after 120 days in storage to 10 ppm. 1000 ppm dip residues
are not likely to be higher than 4 ppm. Residues from impregnated
paper wraps are less than 4 ppm (Bruce et al., 1958). One-half the
total DPA content of apples was found in peel (Bruce et al., 1958),
but 90% can be found in the outer 2 to 4 mm of the fruit (Harvey and
Clark, 1959). Little migration of DPA occurs, either laterally or into
the apple flesh (Hall et al., 1961). This has recently been confirmed
using autoradiography and 14C-DPA solutions (Wilson 1969, unpublished
thesis).
FATE OF RESIDUES
The disappearance of DPA, from either physical or chemical standpoint
does not appear to be accounted for. It is assumed that most losses in
storage are due to volatilization, but this is not verified by
available literature.
Cooking studies have not been reported. The post-storage residues
noted above can be presumed to be those occurring on fruit at the time
of consumption.
Five years of data on commercial scale treatments and holding periods,
involving six varieties of apples are available from Cornell
University (Bache et al., 1962) resulting in average residues after
storage of up to 6 ppm required in order to obtain effective scald
control. Residues of between 8 to 9 ppm also are associated with the
most effective treatments. In 1965 Cornell University (Smock, R.M.
1969, unpublished) analysed commercial lots of treated apples
collected from eight locations in New York State. Treatments applied
by box flooding or immersion varied from 1000 to 2000 ppm DPA.
Variations in residues in individual apples depending upon location
within the box varied considerably, but no consistent pattern was
established. The highest residue reported from a sample at the top of
a box was 6.91 ppm (corrected).
METHODS OF RESIDUE ANALYSIS
The earliest development work on the use of DPA in New Zealand
utilized the method of Yatsu described by Harvey (1958). It involves
vanadium pentoxide-sulphuric acid oxidation of DPA to produce a blue
colour measured at 600 nanometers (nm). Sensitivity is approximately
0.5 ppm for apples. Bruce et al. (1958) developed a method of coupling
the amino with diazotized 2,4-dinitroaniline, measured at 530 nm. This
method is sensitive to at least 0.1 ppm in apples. This method is
likely to be acceptable for use by analysts in regulatory
laboratories. Recently, a procedure for gas chromatography electron
capture has been described (Guttenmann and Lisk, 1963). It involves
bromination of DPA to produce presumptive 2,2', 4,4',
6.6'-hexabromo-DPA and its subsequent GLC determination. Sensitivity
of about 0.02 ppm is claimed.
There is a need to monitor to DPA content of solutions during
commercial treatments in post-harvest applications in order to
determine the point in time for renewal of solutions. The GLC
procedure was not found to be reliable for residue values of 0-25 ppm
for whole apples. A colorimetric procedure was adapted whereby DPA
extracted from blended apple tissues with acetone was reacted with
varnadium pentoxide and quantitatively determined
spectrophotometrically at 605 mu. This procedure was modified for
monitoring the DPA content of commercial applicator systems (Wilson,
1969).
NATIONAL TOLERANCES
Country Commodity Tolerance (ppm)
Australia Apples 10
Canada Apples 10
United States of America Apples 10
Milk and meat zero
APPRAISAL
Diphenylamine (DPA) has a minimum purity of 99.9 per cent. Primary
amines such as aniline are stated as not more than 10 ppm of the
technical grade material.
DPA is used to prevent losses from a storage disease of apples known
as "scald", which varies in severity and incidence due to locality,
weather and in different varieties of apples. The protective action of
DPA is attributed to antioxidant effect on alpha-farnesene, a
sesquiterpene which occurs in the natural coating of apples.
Pre-harvest emulsion sprays applied to mature apples very close to
harvest and post-harvest treatments of bulk apples on sorting rollers,
in boxes, pallets, bins, etc. are applied as emulsions or wettable
powders. Impregnated paper wraps are also used.
The residue is believed to be DPA alone. No degradation products or
metabolites in fruit are identified, nor are they presumed to exist.
Losses are attributed to volatilization. The amount of residue
immediately after treatment varies with the technology of treatment,
but can very from 63 ppm to as low as 1 ppm. All treated apples are
held in storage from 80 to over 200 days. Residues decline in storage
to an average range of less than 1 to 8 ppm, with an occasional
residue in one variety as high as 10 ppm after 120 days, storage
reported. About half the residue is in the peel, and 90% in the outer
2 to 4 mm of fruit. Little migration occurs afterwards.
No results of cooking studies are available. Residues reaching the
consumer can be assumed to be in the order of those mentioned above.
Five years of data collected from commercial scale treatments and
storage holding periods suggest average residues will be 6 ppm, with
some at 8 to 9 ppm. The most recent commercial sampling from eight
locations in New York State resulted in the highest residue found as
6.9 ppm.
An analytical method based on coupling the amine with diazotized
2,4-dinitroaniline, measured at 530 nm is sensitive to 0.1 ppm in
apples. This method is likely to be acceptable for most regulatory
laboratories. A GLC electron capture method is also available. It
involves bromination of DPA to produce presumptive 2, 2', 4, 4', 6,
6'-hexabromo DPA.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TOLERANCES
Apples 10 ppm
FURTHER WORK OR INFORMATION
DESIRABLE
1. Experiments to determine if methaemoglobin is formed in animals.
2. Short-term studies using an adequate number of rats.
3. Additional metabolic studies in a non-rodent mammalian species.
4. The results of the carcinogenicity study in mice which is currently
in progress.
REFERENCES
Alexander, W.E., Ryan, A.J. and Wright, S.E. (1964) Metabolism of
diphenylamine in the rat and rabbit. Experientia 20:223-4
Alexander, W.E., Ryan, A.J. and Wright, S.E. (1965) Metabolism of
diphenylamine in rat, rabbit and man. Food Cosmet. Toxicol. 3:571-9
American Cyanamid Co. (1956) Diphenylamine : limited release toxicity
studies. Unpub. rept. submitted by C.B. Shaffer
Bache, C.A., Smock, R.M., Yatsu, L., Mooney. C., and Lisk, D.J. (1962)
Diphenylamine residues on apples in relation to scald control.
Proc. Amer. Soc. Hort. Sci. 81:57-60
Booth, A.N. (1963) Summary of toxicological data. Chronic toxicity
studies on diphenylamine. Food Cosmet. Toxicol 1:331-3
Bruce, R.B., Howard, J.W., and Zink, J.B. (1958) Determination of
diphenylamine residues on apples. J. Agr. Food Chem. 6:597-600
DeEds, F. (1961) Chronic toxicity studies on diphenylamine. Unpub.
rept. on data for use in a petition requesting tolerance for
diphenylamine used in prevention of "scald" of apples during
storage. Western Utilization R. and D. Div. U.S.D.A., Albany Calif.
Druckrey, H., Preussmann, R., Schmähl, D. and Miller, M. (1961)
Chemische Konstitution und Carcinogens Wirkung bei Nitrosaminen.
Naturwissenschaften 48:134-5
Golberg, L. (1969) Determination of the toxicity of diphenylamine in mice.
First progress report. Unpub. rept. Inst. Exp. Pathol. Toxicol.,
Albany, New York
Gutenmann, W.H., and Lisk, D.J. (1963) Rapid determination of
diphenylamine in apples by direct bromination and gas chromatography.
J. Agr. Food Chem. 11:468-70
Hall, E.G., Scott, K.J., and Coote, G.G. (1961) Control of superficial
scald on Granny Smith apples with diphenylamine. Australian J. Agr.
Res. 12:834-52
Harvey, H.E. (1958) Determination of diphenylamine residues on apples.
New Zealand J. Sci. 1:378-82
Harvey, H.E., and Clerk, P.J. (1959) Diphenylamine residues on apples.
New Zealand J. Sci. 2.266-72
Huelin, F.E., and Murray, K.E. (1966) alpha-Farnesene in the natural
coating of apples. Nature 210.1260-61
Kime, S.W. Jr., McNamara, J.J., Luse, S., Farmer, S., Silbert, C. and
Brieker, N.S. (1962) Experimental polyeystic renal disease in rats :
electron microscopy, function and susceptibility to pyelonephritis.
J. Lab. Clin. Med. 60:64-78
Sander, von J., and Seif, F. (1969) Bakterielle Reduktion von Nitrat
im Magen des Menschen als Ursache einer Nitrosamin-Bildung.
Arzneimittel-Forsch. 19:1091-98
Thomas, J.O., Cox, A.J. Jr. and DeEds, F. (1957) Kidney cysts produced
by diphenylamine. Stanford Med. Bull. 15:90-93
Thomas, J.O., Ribelin, W.E., Wilson, R.H., Keppler, D.C. and DeEds, F.
(1967a) Chronic toxicity of diphenylamine to albino rats. Toxicol.
appl. Pharmacol. 10:362-74
Thomas. J.O., Ribelin. W.E., Woodward, J.R. and DeEds, F. (1967b)
The chronic toxicity of diphenylamine for dogs. Toxicol. appl.
Pharmacol. 11:184-94
University of Birmingham. (1966) Studies on the long-term effect of
diphenylamine in mice. Unpub. rept. Toxicol. Unit, Dept. Med. Biochem.
Pharmacol., Birmingham, England
Wilson, L.G. (1969) Unpublished Ph.D. thesis. Ann Arbor, Mich. 17 Jan. Univ.
Microfilms 69-20-958
Purity
Minimum 99.9 per cent by cryoscopic method. Primary amines as aniline,
not more than 10 parts per million.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Diphenylamine is absorbed from the digestive tract of the rat, rabbit,
dog and man; however no information is available on the extent of
absorption (DeEdu, 1961; Alexander et al., 1965).
The metabolism of diphenylamine has been studied in these four species
and is essentially the same in all of them. A slight amount of
unchanged diphenylamine has been detected in the urine of rabbits and
of man (but not rats) after oral administration of the compound. The
major metabolites are 4-hydroxydiphenylamine and
4,4'-dihydroxydiphenylamine. Both of these compounds have been
identified as conjugates in the urine of human subjects which had
received a single oral dose of 100 mg of diphenylamine, but no free
hydroxylated derivatives are excreted in man. In the rat, rabbit and
dog, 4-hroxydiphenylamine is excreted partly unchanged and partly as
conjugates with glucuronic acid and sulphuric acid, and these
conjugates have been isolated from rabbit urine. Hydroxylation in the
ortho-position occurs only in the rabbit and then only to a slight
extent. N-hydroxydiphenylamine has not been detected from any species
and there is no evidence for its formation (Alexander et al., 1965;
Booth, 1963).
The N-glucuronide of 4-hydroxydiphenylamine has been reported to have
been detected in the urine or rats after oral administration of
diphenylamine. However, the method described for characterizing the
compound would not have distinguished it from the O-glucuronide
(DeEds, 1961).
Studies with carbon14-labelled diphenylamine indicate that the
compound in rapidly metabolized and excreted by the rat. After 48
hours, 75 per cent of an intraperitoneal dose appears in the urine. An
intravenous dose results in the excretion of 25 per cent of the
radio-activity in bile after six hours (Alexander et al., 1965).
Hydroxylated derivatives of diphenylamine have been detected in rat
and dog faeces and represent excretion via the bile, since acid
hydrolysed bile was found to contain 4-hydroxydiphenylamine. The
possibility that intestinal bacteria may be also hydroxylate
unabsorbed diphenylamine has, however, not been ruled out (DeEds.
1961).
No information is available on the relative amounts of urinary or
faecal metabolites derived from an oral dose of diphenylamine.
Special studies on carcinogenicity
Mouse
Two groups, each containing 40-50 male and 40-50 female mice weighing
30-35 g, were selected for this study. The animals received
subcutaneous injections of 0.5 ml of a 25 per cent solution of
diphenylamine in trioctanoin (approximately 4000 mg/kg body-weight)
once every two weeks on alternate sides of the body. After two months
it was found that the compound accumulated when given this frequently
and the injections were then given once every three weeks until the
animals had received injections for a total period of 80 weeks. A
control group of mice received injections of the solvent vehicle
alone. There was no significant difference in the incidence of
malignant or benign tumours between the test and the control groups,
nor was the injection of diphenylamine associated with the development
of tumours at the injection site. A related study involving oral
feeding of diphenylamine to mice also involved tissue examination for
tumours and this study is described under "Long-term studies" (Univ.
of Birmingham, 1966).
An 80 week oral feeding study in mice is currently in progress and the
study will include an evaluation for carcinogenicity. The results from
a preliminary progress report of this study are given under
"Short-term studies" (Golberg, 1969).
Rat
It is known that bacteria in the human stomach will reduce nitrates to
nitrites. Studies in 31 patients showed that these nitrites will react
with diphenylamine to form diphenylnitrosamine (Sander and Seif,
1969). However, it has previously been demonstrated with studies in
the rat that, unlike many nitrosamines, diphenylnitrosamine is not a
carcinogen (Druckrey et al., 1961).
Special studies on reproduction
Rat
A two-generation rat reproduction study was conducted with dietary
levels of 0, 1000, 2500 and 5000 ppm of diphenylamine. Treatment was
began at weaning. At 100 days of age, 12 females and three males were
selected from each of these dose level groups and grouped as three
females and one male per cage. Once a week for three weeks the males
were isolated among the three groups of females, after which time the
males were removed and the females placed in individual cages. When
all the litters were weaned, the females were given a 2-3 week rest
and then remated. In addition offspring from the first mating were
mated once, as described above, for a second generation study. Feeding
of diphenylamine did not influence the number of litters born or the
incidence of mortality of the offspring. It was uncertain if
diphenylamine was responsible for the reduced litter-size and weight
of the young at 21 days which was observed in the group fed 5000 ppm
of diphenylamine (Thomas et al., 1967a; Booth, 1963).
Acute toxicity
The oral LD50 of diphenylamine to male rats is approximately 3,200
mg/kg body-weight (American Cyanamid Co., 1956).
Short-term studies
Dog
Groups of four dogs (two males and two females) were fed diets
containing 0, 100, 1000 and 10,000 ppm of diphenylamine for a period
of two years. The dogs were about eight months of age at the
commencement of the study. Decreased body-weight gain occurred in the
1000 and 10,000 ppm groups although food consumption was normal. A
pronounced anaemia developed in the 10,000 ppm group and mild anaemia
in the 1000 ppm group. The bromsulphthalein (BSP) test of liver
function from day 618 to day 627 indicated a moderate degree of liver
damage at 10,000 ppm. The phenolsulphonaphthalein (PSP) test of kidney
function gave normal values, and the urine gave negative tests for
albumin and glucose. All organ-weight changes and microscopic lesions
were limited to the 10,000 ppm level. These manifestations consisted
of peripherolubular fatty change in the liver with a marked increase
in liver-weight and other-extractable lipids; a mild haemosiderosis of
the spleen, kidneys and bone marrow, and a slight increase in kidney
weight (Thomas et al., 1967b).
Mouse
A preliminary progress report is available on the feeding of dietary
levels of 0, 50, 100 and 250 ppm of diphenylamine to groups of mice.
Over a 2-3 month period there has been no difference in body-weight or
incidence of mortality between control and test groups. The feeding
will be continued for a total of 80 weeks and will include
haematological examinations and histological evaluation at autopsy
(Golberg, 1969).
Rat
Groups of 10 male rats were fed diets containing 0, 100, 1000, or
10,000 ppm of diphenylamine for 30 days. There were no deaths in any
group, and no signs of toxicity at 100 and 1000 ppm. The animals fed
10,000 ppm made only about one-half the weight gain of the controls
even though the food intakes of the two groups did not differ. Food
intake and weight gain at 1000 ppm were significantly higher than
those of the controls. Autopsy of animals at 100 and 1000 ppm
disclosed no gross pathologic lesions that could be attributed to
feeding of diphenylamine. At 10,000 ppm the animals had dark and
roughened spleens, and three had hyperaemic kidneys. Paleness of the
extremities in most of the rats fed the highest dose level was noted
but no chemical analysis was performed to determine if methaemoglobin
was present in the blood. No histological examination was made of the
tissues (American Cyanamid Company, 1956).
Groups of six female rats were fed diets containing 0, 250, 1000,
5000, 10,000, or 15,000 ppm of diphenylamine for 226 days. Inhibition
of growth occurred at dietary levels of 5000 ppm or more. At necropsy
no gross change was noted except for enlargement of the kidneys in
animals which had received 15,000 ppm. Microscopic examination of
tissues revealed the formation of foci of dilated renal tubules and
pigmentation of the liver and kidney suggestive of blood destruction
at levels of 5000 ppm and above (Thomas et al., 1957; Booth, 1963).
An unspecified number of rats, including weanling rats of both sexes
and adult male rats weighing 250 grams, were fed a diet containing 2.5
per cent of diphenylamine for periods up to 12 months. Morphologic
alterations of the renal tubules were found in all animals. These
alterations varied from tubular dilation to overt cyst formation.
Glomerular filtration rate and maximum urinary osmolality were
decreased and the degree of decrement corresponded to the degree of
involvement. The animals with polycystic kidneys had an increased
susceptibility to pyelonephritis (Kime et al., 1962).
Long-term studies
Mouse
Groups of mice (40-50 animals of each sex) were fed diets containing
0, 100, 300, 1000, and 5000 ppm of diphenylamine for 80 weeks. The
study began when the animals were 4-5 weeks of age. Increased
mortality occurred on diets containing 300 ppm or more of
diphenylamine. Abnormalities in the liver (chronic inflammatory change
and iron pigment deposition), kidneys (iron deposition) and spleen
(iron deposition, fibrosis, lymph follicle hypoplasia) occurred at
5000 ppm, and also in the spleen at 1000 ppm. Although the total
tumour incidence was significantly increased in the 100 ppm group,
this increase appeared incidental and not associated with
diphenylamine intake. The effect of subcutaneous injections of
diphenylamine to another group of mice in this study is described
under "Special studies on carcinogenicity". In this experiment except
for one isolated period between weeks 30 and 40 with respect to the
female test group, the incidence of mortality was not different from a
control group (Univ. of Birmingham, 1966).
Rat
Groups of 40 rats each comprising 20 males and 20 females were fed
diets containing 0, 10, 100, 1000, 5000, or 10,000 ppm of
diphenylamine, commencing at weaning and continuing for two years.
There was no evidence of toxicity among animals at the 100 ppm level
and below. There was a decreased rate of growth at 5000 and 10,000
ppm, the effect at the latter level being at least partly due to
reduced food intake. Doubtful effect on growth occurred at 1000 ppm in
the females. A moderate degree of anaemia occurred at 10,000 ppm,
which was reversible upon returning the animals to the control diet.
Total white cell count and differential white cell count remained
normal at all dose levels. Kidney damage in the form of dilated
tubules was produced at levels above 1000 ppm, and to a lesser degree
at 1000 ppm. The incidence and type of tumours found were unrelated to
treatment with diphenylamine (Thomas et al., 1967a).
COMMENT
Dihydroxylated products of metabolism have been identified in the
urine of laboratory animals and human subjects; N-hydroxylation was
not observed. Short-term studies with an insufficient number of rats
have been carried out; at higher concentrations of diphenylamine,
morphologic alterations of the renal tubules were found. There is
insufficient information on the possible formation of methaemoglobin
which might be expected with an aromatic amine. In the long-term
studies on mice, an increased tumour incidence appeared incidental,
but subcutaneous injections of diphenylamine in trioctanoin solution
demonstrated no significant difference between test and control
groups. In the long-term studies on rats, the incidence and type of
tumours were unrelated to treatment with diphenylamine. A study on the
carcinogenicity in mice after oral ingestion is in progress. The
short-term studies on dogs and the lone-term studies on rats form the
experimental basis for the established adi.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 100 ppm in the diet, equivalent to 2.5 mg/kg body-weight/day
Mouse: 100 ppm in the diet, equivalent to 15 mg/kg body-weight/day
Rat: 100 ppm in the diet, equivalent to 5 mg/kg body-weight/day
Estimate of acceptable daily intake for man
0.0-0.025 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
DPA is used to prevent a storage disorder of apples known as scald. It
is the only known use of DPA. The incidence and severity of the
disease varies, depending upon locality, seasonal conditions prior to
and at harvest. Varietal differences in susceptibility and severity of
the condition are frequent, as well as a requirement for greater
concentrations of CPA (and higher residues) for them to prevent
losses. The protective action of DPA is believed to be due to the
antioxidant effect on alpha-farnesene a sesquiterpene which occurs in
the natural coatings of apples (Huelin and Murray, 1966).
Alcohol suspensions have been used in some of the earlier work
reported here, but it is believed that only wettable powders, oil
emulsions and impregnated papers are in use now. Data are available
from Australia, New Zealand, U.S.A. and the U.K. DPA is also used in
Canada, and probably in many other apple producing countries.
Pre-harvest treatments
Mature apples on the trees are sprayed a few days before harvest if
the first symptoms of the disease are detected at this stage, or if
weather conditions suggest treatments should be applied. An aqueous
suspension is applied at concentrations of 500 to 2000 ppm.
Post-harvest treatments
Harvest fruit is (a) dipped in aqueous suspension (500 to 3000 ppm),
(b) sprayed in boxes, pallet loads, bins or conveyors (1000 to 2000
ppm), boxes immersed in suspensions or emulsions, or (c) individual
apples wrapped in impregnated paper (1 to 2 mg/wrap).
Most fruit treated is held in storage for from 80 to 200 days or more
before marketing.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues in whole fruit from tree sprays are usually comparatively
low, less than 1 ppm (Bruce et al., 1958; Harvey and Clark, 1959), but
can commonly be as high as 6 ppm on a particular variety (Gutenmann
and Lisk, 1963) at the rates outlined above. Initial residues from
post-harvest sprays and dips have been reported as high as 63 ppm, but
these decline rapidly in storage. Most post-harvest spray residues
after storage are in the range of 2 to 6 ppm (Harvey and Clark, 1959;
Bache et al., 1962), but have been reported as high as 7.7 ppm (Harvey
and Clark, 1959). Dip deposits from 2000 ppm suspension can have
initial residues as high as 12 ppm, usually most are above 8 ppm
(Bruce et al., 1958). In one variety, an initial residue of 62.6 ppm
declined after 120 days in storage to 10 ppm. 1000 ppm dip residues
are not likely to be higher than 4 ppm. Residues from impregnated
paper wraps are less than 4 ppm (Bruce et al., 1958). One-half the
total DPA content of apples was found in peel (Bruce et al., 1958),
but 90% can be found in the outer 2 to 4 mm of the fruit (Harvey and
Clark, 1959). Little migration of DPA occurs, either laterally or into
the apple flesh (Hall et al., 1961). This has recently been confirmed
using autoradiography and 14C-DPA solutions (Wilson 1969, unpublished
thesis).
FATE OF RESIDUES
The disappearance of DPA, from either physical or chemical standpoint
does not appear to be accounted for. It is assumed that most losses in
storage are due to volatilization, but this is not verified by
available literature.
Cooking studies have not been reported. The post-storage residues
noted above can be presumed to be those occurring on fruit at the time
of consumption.
Five years of data on commercial scale treatments and holding periods,
involving six varieties of apples are available from Cornell
University (Bache et al., 1962) resulting in average residues after
storage of up to 6 ppm required in order to obtain effective scald
control. Residues of between 8 to 9 ppm also are associated with the
most effective treatments. In 1965 Cornell University (Smock, R.M.
1969, unpublished) analysed commercial lots of treated apples
collected from eight locations in New York State. Treatments applied
by box flooding or immersion varied from 1000 to 2000 ppm DPA.
Variations in residues in individual apples depending upon location
within the box varied considerably, but no consistent pattern was
established. The highest residue reported from a sample at the top of
a box was 6.91 ppm (corrected).
METHODS OF RESIDUE ANALYSIS
The earliest development work on the use of DPA in New Zealand
utilized the method of Yatsu described by Harvey (1958). It involves
vanadium pentoxide-sulphuric acid oxidation of DPA to produce a blue
colour measured at 600 nanometers (nm). Sensitivity is approximately
0.5 ppm for apples. Bruce et al. (1958) developed a method of coupling
the amino with diazotized 2,4-dinitroaniline, measured at 530 nm. This
method is sensitive to at least 0.1 ppm in apples. This method is
likely to be acceptable for use by analysts in regulatory
laboratories. Recently, a procedure for gas chromatography electron
capture has been described (Guttenmann and Lisk, 1963). It involves
bromination of DPA to produce presumptive 2,2', 4,4',
6.6'-hexabromo-DPA and its subsequent GLC determination. Sensitivity
of about 0.02 ppm is claimed.
There is a need to monitor to DPA content of solutions during
commercial treatments in post-harvest applications in order to
determine the point in time for renewal of solutions. The GLC
procedure was not found to be reliable for residue values of 0-25 ppm
for whole apples. A colorimetric procedure was adapted whereby DPA
extracted from blended apple tissues with acetone was reacted with
varnadium pentoxide and quantitatively determined
spectrophotometrically at 605 mu. This procedure was modified for
monitoring the DPA content of commercial applicator systems (Wilson,
1969).
NATIONAL TOLERANCES
Country Commodity Tolerance (ppm)
Australia Apples 10
Canada Apples 10
United States of America Apples 10
Milk and meat zero
APPRAISAL
Diphenylamine (DPA) has a minimum purity of 99.9 per cent. Primary
amines such as aniline are stated as not more than 10 ppm of the
technical grade material.
DPA is used to prevent losses from a storage disease of apples known
as "scald", which varies in severity and incidence due to locality,
weather and in different varieties of apples. The protective action of
DPA is attributed to antioxidant effect on alpha-farnesene, a
sesquiterpene which occurs in the natural coating of apples.
Pre-harvest emulsion sprays applied to mature apples very close to
harvest and post-harvest treatments of bulk apples on sorting rollers,
in boxes, pallets, bins, etc. are applied as emulsions or wettable
powders. Impregnated paper wraps are also used.
The residue is believed to be DPA alone. No degradation products or
metabolites in fruit are identified, nor are they presumed to exist.
Losses are attributed to volatilization. The amount of residue
immediately after treatment varies with the technology of treatment,
but can very from 63 ppm to as low as 1 ppm. All treated apples are
held in storage from 80 to over 200 days. Residues decline in storage
to an average range of less than 1 to 8 ppm, with an occasional
residue in one variety as high as 10 ppm after 120 days, storage
reported. About half the residue is in the peel, and 90% in the outer
2 to 4 mm of fruit. Little migration occurs afterwards.
No results of cooking studies are available. Residues reaching the
consumer can be assumed to be in the order of those mentioned above.
Five years of data collected from commercial scale treatments and
storage holding periods suggest average residues will be 6 ppm, with
some at 8 to 9 ppm. The most recent commercial sampling from eight
locations in New York State resulted in the highest residue found as
6.9 ppm.
An analytical method based on coupling the amine with diazotized
2,4-dinitroaniline, measured at 530 nm is sensitive to 0.1 ppm in
apples. This method is likely to be acceptable for most regulatory
laboratories. A GLC electron capture method is also available. It
involves bromination of DPA to produce presumptive 2, 2', 4, 4', 6,
6'-hexabromo DPA.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TOLERANCES
Apples 10 ppm
FURTHER WORK OR INFORMATION
DESIRABLE
1. Experiments to determine if methaemoglobin is formed in animals.
2. Short-term studies using an adequate number of rats.
3. Additional metabolic studies in a non-rodent mammalian species.
4. The results of the carcinogenicity study in mice which is currently
in progress.
REFERENCES
Alexander, W.E., Ryan, A.J. and Wright, S.E. (1964) Metabolism of
diphenylamine in the rat and rabbit. Experientia 20:223-4
Alexander, W.E., Ryan, A.J. and Wright, S.E. (1965) Metabolism of
diphenylamine in rat, rabbit and man. Food Cosmet. Toxicol. 3:571-9
American Cyanamid Co. (1956) Diphenylamine : limited release toxicity
studies. Unpub. rept. submitted by C.B. Shaffer
Bache, C.A., Smock, R.M., Yatsu, L., Mooney. C., and Lisk, D.J. (1962)
Diphenylamine residues on apples in relation to scald control.
Proc. Amer. Soc. Hort. Sci. 81:57-60
Booth, A.N. (1963) Summary of toxicological data. Chronic toxicity
studies on diphenylamine. Food Cosmet. Toxicol 1:331-3
Bruce, R.B., Howard, J.W., and Zink, J.B. (1958) Determination of
diphenylamine residues on apples. J. Agr. Food Chem. 6:597-600
DeEds, F. (1961) Chronic toxicity studies on diphenylamine. Unpub.
rept. on data for use in a petition requesting tolerance for
diphenylamine used in prevention of "scald" of apples during
storage. Western Utilization R. and D. Div. U.S.D.A., Albany Calif.
Druckrey, H., Preussmann, R., Schmähl, D. and Miller, M. (1961)
Chemische Konstitution und Carcinogens Wirkung bei Nitrosaminen.
Naturwissenschaften 48:134-5
Golberg, L. (1969) Determination of the toxicity of diphenylamine in mice.
First progress report. Unpub. rept. Inst. Exp. Pathol. Toxicol.,
Albany, New York
Gutenmann, W.H., and Lisk, D.J. (1963) Rapid determination of
diphenylamine in apples by direct bromination and gas chromatography.
J. Agr. Food Chem. 11:468-70
Hall, E.G., Scott, K.J., and Coote, G.G. (1961) Control of superficial
scald on Granny Smith apples with diphenylamine. Australian J. Agr.
Res. 12:834-52
Harvey, H.E. (1958) Determination of diphenylamine residues on apples.
New Zealand J. Sci. 1:378-82
Harvey, H.E., and Clerk, P.J. (1959) Diphenylamine residues on apples.
New Zealand J. Sci. 2.266-72
Huelin, F.E., and Murray, K.E. (1966) alpha-Farnesene in the natural
coating of apples. Nature 210.1260-61
Kime, S.W. Jr., McNamara, J.J., Luse, S., Farmer, S., Silbert, C. and
Brieker, N.S. (1962) Experimental polyeystic renal disease in rats :
electron microscopy, function and susceptibility to pyelonephritis.
J. Lab. Clin. Med. 60:64-78
Sander, von J., and Seif, F. (1969) Bakterielle Reduktion von Nitrat
im Magen des Menschen als Ursache einer Nitrosamin-Bildung.
Arzneimittel-Forsch. 19:1091-98
Thomas, J.O., Cox, A.J. Jr. and DeEds, F. (1957) Kidney cysts produced
by diphenylamine. Stanford Med. Bull. 15:90-93
Thomas, J.O., Ribelin, W.E., Wilson, R.H., Keppler, D.C. and DeEds, F.
(1967a) Chronic toxicity of diphenylamine to albino rats. Toxicol.
appl. Pharmacol. 10:362-74
Thomas. J.O., Ribelin. W.E., Woodward, J.R. and DeEds, F. (1967b)
The chronic toxicity of diphenylamine for dogs. Toxicol. appl.
Pharmacol. 11:184-94
University of Birmingham. (1966) Studies on the long-term effect of
diphenylamine in mice. Unpub. rept. Toxicol. Unit, Dept. Med. Biochem.
Pharmacol., Birmingham, England
Wilson, L.G. (1969) Unpublished Ph.D. thesis. Ann Arbor, Mich. 17 Jan. Univ.
Microfilms 69-20-958
