
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
DIPHENYLAMINE
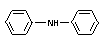 Explanation
Diphenylamine (DPA) was first evaluated by the 1969 JMPR (FAO/WHO
1970).1 No-effect levels in mouse, rat and dog were determined. An
ADI was allocated on the basis of the no-effect level observed in a
2-year study in the dog.
As new toxicological data had become available, diphenylamine was
reconsidered by the 1976 JMPR (FAO/WHO 1977), and an ADI was derived
from a no-effect level observed in a 6-month mouse study.
Further data have become available and are summarized in the
following monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Studies on Identification of Toxic Impurities in Commercial
Diphenylamine
Six samples of commercial diphenylamine (DPA) were subjected to
investigation for identification of minor components. Combined
chemical techniques (TLC, GLC/MS, IR,NMR) were used. Only one
impurity, o-cyclohexylaniline, was found in all the commercial DPA
samples at levels of 5-93 ppm. p-Cyclohexylaniline was detected in
only one sample. o-Biphenylamine was found in three samples
(3.2-32 ppm). p-Biphenylamine, a known carcinogen was found in four
samples (6.9-94 ppm). Another compound (band 1) was detected, but its
structure was not precisely determined. This compound was found to be
remarkably unstable to heat, light and oxygen, and rapidly polymerized
to give a deep purple, insoluble, resinous material. The components
E,F,G, isolated in the work of Crocker et al (1972), were comparable
to band 1 (unidentified compound), band 3 (o-cyclohexylaniline) and
band 5 (p-cyclohexylaniline), respectively, of this work (Safe
et al 1977).
1 See Annex 2 for WHO and FAO documentation.
Short-Term Studies
Pregnant Sprague-Dawley rats of known gestation were fed diets
containing 0, 1.5% or 2.5% diphenylamine of source I (DPA/I) aged
about 2 years from gestation day 14 to term, 2.5 DPA/I aged 3 months,
or 2.5% DPA/II aged 2 years. All viable newborn rats were removed from
mothers shortly after birth and two or three randomly selected
pairs of kidneys from each litter were prepared for histological
examination.
The histology of the kidneys from mothers was normal, and no
tubular dilatation or other abnormalities were observed. Cystic
changes in the proximal tubules were regularly induced by feeding 2.5%
2-year aged DPA/I. Lesions were uncommon and less severe after feeding
either 1.5% 2-year-aged DPA/I, 2.5% 3-month-aged DPA/I, or 2.5%
2-year-aged DPA/II.
In another portion of the study, three trace compounds
(designated E, F and G) were isolated by thin-layer chromatography
from 2-year-aged DPA/I, and also purified DPA/I was prepared. Rats
were given, by gastric tube in 70% ethanol, either 2-year-aged DPA/I
(20 mg/day), purified DPA/I (20 mg/day), contaminant E (20 µg/day),
contaminant F (20 µg/day), or contaminant G (20 µg/day).
Aged DPA/I and the contaminant E frequently induced moderate
cystic changes in the proximal tubules of the newborn rat.
Chromatographically pure DPA/I and the two other contaminants F and G
induced only mild cystic tubular changes (Crocker et al 1972).
COMMENTS
Diphenylamine (DPA) was first evaluated by the 1969 JMPR.
No-effect levels in mouse, rat and dog were determined. An ADI was
allocated on the basis of the no-effect level observed in a 2-year
study in the dog.
As new toxicological data had become available, diphenylamine was
reconsidered by the 1976 JMPR and an ADI was derived from a no-effect
level observed in a six-month mouse study.
The Meeting considered a study showing that an impurity (not
fully characterized) in commercial grade diphenylamine (DPA) is the
active nephrotoxic compound responsible for the in utero induction
of cystic tubular lesions in the kidneys of newborn rats of mothers
fed DPA in dietary concentrations of 2.5%. Another study described the
identification of some impurities in commercial samples (source not
specified) of DPA, showing that the level of primary amines is higher
than that specified (10 ppm) by the 1969 JMPR. The Meeting noted that,
in general, the purity of DPA used in toxicological studies has not
been reported, except for the long-term mouse study (FAO/WHO,1977) in
which 99.5% pure DPA was used. On the other hand, the specification of
purity (99.9%) reported by the 1969 JMPR appeared unrealistic. The
Meeting noted that no teratogenicity studies had been evaluated by
previous Meetings.
The Meeting decided to change the ADI to temporary status.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse : 10 ppm in the diet, equivalent to 1.5 mg/kg bw.
Rat : 100 ppm in the diet, equivalent to 5 mg/kg bw.
Dog : 100 ppm in the diet, equivalent to 2.5 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1984)
A teratogenicity study.
Desirable
1. Short-term studies with special attention to the formation of
Heinz bodies (from 1976 JMPR).
2. Mutagenicity tests.
3. Information on the nature and level of impurities in technical
grade diphenylamine.
REFERENCES
Crocker, J.F.S., Brown, D.M., Borch, R.F., Vernier, R.L. Renal cystic
1972 disease induced in newborn rats by diphenylamine
derivatives. Am. J. Pathol. 66(2):343-348.
Safe, S., Hutzinger, O., Crocker, J.F.S., Digout, S.C. Identification
1977 of toxic impurities in commercial diphenylamine.
Bull.Environ.Contam. Toxicol. 17:204-207.
Explanation
Diphenylamine (DPA) was first evaluated by the 1969 JMPR (FAO/WHO
1970).1 No-effect levels in mouse, rat and dog were determined. An
ADI was allocated on the basis of the no-effect level observed in a
2-year study in the dog.
As new toxicological data had become available, diphenylamine was
reconsidered by the 1976 JMPR (FAO/WHO 1977), and an ADI was derived
from a no-effect level observed in a 6-month mouse study.
Further data have become available and are summarized in the
following monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Studies on Identification of Toxic Impurities in Commercial
Diphenylamine
Six samples of commercial diphenylamine (DPA) were subjected to
investigation for identification of minor components. Combined
chemical techniques (TLC, GLC/MS, IR,NMR) were used. Only one
impurity, o-cyclohexylaniline, was found in all the commercial DPA
samples at levels of 5-93 ppm. p-Cyclohexylaniline was detected in
only one sample. o-Biphenylamine was found in three samples
(3.2-32 ppm). p-Biphenylamine, a known carcinogen was found in four
samples (6.9-94 ppm). Another compound (band 1) was detected, but its
structure was not precisely determined. This compound was found to be
remarkably unstable to heat, light and oxygen, and rapidly polymerized
to give a deep purple, insoluble, resinous material. The components
E,F,G, isolated in the work of Crocker et al (1972), were comparable
to band 1 (unidentified compound), band 3 (o-cyclohexylaniline) and
band 5 (p-cyclohexylaniline), respectively, of this work (Safe
et al 1977).
1 See Annex 2 for WHO and FAO documentation.
Short-Term Studies
Pregnant Sprague-Dawley rats of known gestation were fed diets
containing 0, 1.5% or 2.5% diphenylamine of source I (DPA/I) aged
about 2 years from gestation day 14 to term, 2.5 DPA/I aged 3 months,
or 2.5% DPA/II aged 2 years. All viable newborn rats were removed from
mothers shortly after birth and two or three randomly selected
pairs of kidneys from each litter were prepared for histological
examination.
The histology of the kidneys from mothers was normal, and no
tubular dilatation or other abnormalities were observed. Cystic
changes in the proximal tubules were regularly induced by feeding 2.5%
2-year aged DPA/I. Lesions were uncommon and less severe after feeding
either 1.5% 2-year-aged DPA/I, 2.5% 3-month-aged DPA/I, or 2.5%
2-year-aged DPA/II.
In another portion of the study, three trace compounds
(designated E, F and G) were isolated by thin-layer chromatography
from 2-year-aged DPA/I, and also purified DPA/I was prepared. Rats
were given, by gastric tube in 70% ethanol, either 2-year-aged DPA/I
(20 mg/day), purified DPA/I (20 mg/day), contaminant E (20 µg/day),
contaminant F (20 µg/day), or contaminant G (20 µg/day).
Aged DPA/I and the contaminant E frequently induced moderate
cystic changes in the proximal tubules of the newborn rat.
Chromatographically pure DPA/I and the two other contaminants F and G
induced only mild cystic tubular changes (Crocker et al 1972).
COMMENTS
Diphenylamine (DPA) was first evaluated by the 1969 JMPR.
No-effect levels in mouse, rat and dog were determined. An ADI was
allocated on the basis of the no-effect level observed in a 2-year
study in the dog.
As new toxicological data had become available, diphenylamine was
reconsidered by the 1976 JMPR and an ADI was derived from a no-effect
level observed in a six-month mouse study.
The Meeting considered a study showing that an impurity (not
fully characterized) in commercial grade diphenylamine (DPA) is the
active nephrotoxic compound responsible for the in utero induction
of cystic tubular lesions in the kidneys of newborn rats of mothers
fed DPA in dietary concentrations of 2.5%. Another study described the
identification of some impurities in commercial samples (source not
specified) of DPA, showing that the level of primary amines is higher
than that specified (10 ppm) by the 1969 JMPR. The Meeting noted that,
in general, the purity of DPA used in toxicological studies has not
been reported, except for the long-term mouse study (FAO/WHO,1977) in
which 99.5% pure DPA was used. On the other hand, the specification of
purity (99.9%) reported by the 1969 JMPR appeared unrealistic. The
Meeting noted that no teratogenicity studies had been evaluated by
previous Meetings.
The Meeting decided to change the ADI to temporary status.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse : 10 ppm in the diet, equivalent to 1.5 mg/kg bw.
Rat : 100 ppm in the diet, equivalent to 5 mg/kg bw.
Dog : 100 ppm in the diet, equivalent to 2.5 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1984)
A teratogenicity study.
Desirable
1. Short-term studies with special attention to the formation of
Heinz bodies (from 1976 JMPR).
2. Mutagenicity tests.
3. Information on the nature and level of impurities in technical
grade diphenylamine.
REFERENCES
Crocker, J.F.S., Brown, D.M., Borch, R.F., Vernier, R.L. Renal cystic
1972 disease induced in newborn rats by diphenylamine
derivatives. Am. J. Pathol. 66(2):343-348.
Safe, S., Hutzinger, O., Crocker, J.F.S., Digout, S.C. Identification
1977 of toxic impurities in commercial diphenylamine.
Bull.Environ.Contam. Toxicol. 17:204-207.
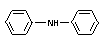 Explanation
Diphenylamine (DPA) was first evaluated by the 1969 JMPR (FAO/WHO
1970).1 No-effect levels in mouse, rat and dog were determined. An
ADI was allocated on the basis of the no-effect level observed in a
2-year study in the dog.
As new toxicological data had become available, diphenylamine was
reconsidered by the 1976 JMPR (FAO/WHO 1977), and an ADI was derived
from a no-effect level observed in a 6-month mouse study.
Further data have become available and are summarized in the
following monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Studies on Identification of Toxic Impurities in Commercial
Diphenylamine
Six samples of commercial diphenylamine (DPA) were subjected to
investigation for identification of minor components. Combined
chemical techniques (TLC, GLC/MS, IR,NMR) were used. Only one
impurity, o-cyclohexylaniline, was found in all the commercial DPA
samples at levels of 5-93 ppm. p-Cyclohexylaniline was detected in
only one sample. o-Biphenylamine was found in three samples
(3.2-32 ppm). p-Biphenylamine, a known carcinogen was found in four
samples (6.9-94 ppm). Another compound (band 1) was detected, but its
structure was not precisely determined. This compound was found to be
remarkably unstable to heat, light and oxygen, and rapidly polymerized
to give a deep purple, insoluble, resinous material. The components
E,F,G, isolated in the work of Crocker et al (1972), were comparable
to band 1 (unidentified compound), band 3 (o-cyclohexylaniline) and
band 5 (p-cyclohexylaniline), respectively, of this work (Safe
et al 1977).
1 See Annex 2 for WHO and FAO documentation.
Short-Term Studies
Pregnant Sprague-Dawley rats of known gestation were fed diets
containing 0, 1.5% or 2.5% diphenylamine of source I (DPA/I) aged
about 2 years from gestation day 14 to term, 2.5 DPA/I aged 3 months,
or 2.5% DPA/II aged 2 years. All viable newborn rats were removed from
mothers shortly after birth and two or three randomly selected
pairs of kidneys from each litter were prepared for histological
examination.
The histology of the kidneys from mothers was normal, and no
tubular dilatation or other abnormalities were observed. Cystic
changes in the proximal tubules were regularly induced by feeding 2.5%
2-year aged DPA/I. Lesions were uncommon and less severe after feeding
either 1.5% 2-year-aged DPA/I, 2.5% 3-month-aged DPA/I, or 2.5%
2-year-aged DPA/II.
In another portion of the study, three trace compounds
(designated E, F and G) were isolated by thin-layer chromatography
from 2-year-aged DPA/I, and also purified DPA/I was prepared. Rats
were given, by gastric tube in 70% ethanol, either 2-year-aged DPA/I
(20 mg/day), purified DPA/I (20 mg/day), contaminant E (20 µg/day),
contaminant F (20 µg/day), or contaminant G (20 µg/day).
Aged DPA/I and the contaminant E frequently induced moderate
cystic changes in the proximal tubules of the newborn rat.
Chromatographically pure DPA/I and the two other contaminants F and G
induced only mild cystic tubular changes (Crocker et al 1972).
COMMENTS
Diphenylamine (DPA) was first evaluated by the 1969 JMPR.
No-effect levels in mouse, rat and dog were determined. An ADI was
allocated on the basis of the no-effect level observed in a 2-year
study in the dog.
As new toxicological data had become available, diphenylamine was
reconsidered by the 1976 JMPR and an ADI was derived from a no-effect
level observed in a six-month mouse study.
The Meeting considered a study showing that an impurity (not
fully characterized) in commercial grade diphenylamine (DPA) is the
active nephrotoxic compound responsible for the in utero induction
of cystic tubular lesions in the kidneys of newborn rats of mothers
fed DPA in dietary concentrations of 2.5%. Another study described the
identification of some impurities in commercial samples (source not
specified) of DPA, showing that the level of primary amines is higher
than that specified (10 ppm) by the 1969 JMPR. The Meeting noted that,
in general, the purity of DPA used in toxicological studies has not
been reported, except for the long-term mouse study (FAO/WHO,1977) in
which 99.5% pure DPA was used. On the other hand, the specification of
purity (99.9%) reported by the 1969 JMPR appeared unrealistic. The
Meeting noted that no teratogenicity studies had been evaluated by
previous Meetings.
The Meeting decided to change the ADI to temporary status.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse : 10 ppm in the diet, equivalent to 1.5 mg/kg bw.
Rat : 100 ppm in the diet, equivalent to 5 mg/kg bw.
Dog : 100 ppm in the diet, equivalent to 2.5 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1984)
A teratogenicity study.
Desirable
1. Short-term studies with special attention to the formation of
Heinz bodies (from 1976 JMPR).
2. Mutagenicity tests.
3. Information on the nature and level of impurities in technical
grade diphenylamine.
REFERENCES
Crocker, J.F.S., Brown, D.M., Borch, R.F., Vernier, R.L. Renal cystic
1972 disease induced in newborn rats by diphenylamine
derivatives. Am. J. Pathol. 66(2):343-348.
Safe, S., Hutzinger, O., Crocker, J.F.S., Digout, S.C. Identification
1977 of toxic impurities in commercial diphenylamine.
Bull.Environ.Contam. Toxicol. 17:204-207.
Explanation
Diphenylamine (DPA) was first evaluated by the 1969 JMPR (FAO/WHO
1970).1 No-effect levels in mouse, rat and dog were determined. An
ADI was allocated on the basis of the no-effect level observed in a
2-year study in the dog.
As new toxicological data had become available, diphenylamine was
reconsidered by the 1976 JMPR (FAO/WHO 1977), and an ADI was derived
from a no-effect level observed in a 6-month mouse study.
Further data have become available and are summarized in the
following monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Studies on Identification of Toxic Impurities in Commercial
Diphenylamine
Six samples of commercial diphenylamine (DPA) were subjected to
investigation for identification of minor components. Combined
chemical techniques (TLC, GLC/MS, IR,NMR) were used. Only one
impurity, o-cyclohexylaniline, was found in all the commercial DPA
samples at levels of 5-93 ppm. p-Cyclohexylaniline was detected in
only one sample. o-Biphenylamine was found in three samples
(3.2-32 ppm). p-Biphenylamine, a known carcinogen was found in four
samples (6.9-94 ppm). Another compound (band 1) was detected, but its
structure was not precisely determined. This compound was found to be
remarkably unstable to heat, light and oxygen, and rapidly polymerized
to give a deep purple, insoluble, resinous material. The components
E,F,G, isolated in the work of Crocker et al (1972), were comparable
to band 1 (unidentified compound), band 3 (o-cyclohexylaniline) and
band 5 (p-cyclohexylaniline), respectively, of this work (Safe
et al 1977).
1 See Annex 2 for WHO and FAO documentation.
Short-Term Studies
Pregnant Sprague-Dawley rats of known gestation were fed diets
containing 0, 1.5% or 2.5% diphenylamine of source I (DPA/I) aged
about 2 years from gestation day 14 to term, 2.5 DPA/I aged 3 months,
or 2.5% DPA/II aged 2 years. All viable newborn rats were removed from
mothers shortly after birth and two or three randomly selected
pairs of kidneys from each litter were prepared for histological
examination.
The histology of the kidneys from mothers was normal, and no
tubular dilatation or other abnormalities were observed. Cystic
changes in the proximal tubules were regularly induced by feeding 2.5%
2-year aged DPA/I. Lesions were uncommon and less severe after feeding
either 1.5% 2-year-aged DPA/I, 2.5% 3-month-aged DPA/I, or 2.5%
2-year-aged DPA/II.
In another portion of the study, three trace compounds
(designated E, F and G) were isolated by thin-layer chromatography
from 2-year-aged DPA/I, and also purified DPA/I was prepared. Rats
were given, by gastric tube in 70% ethanol, either 2-year-aged DPA/I
(20 mg/day), purified DPA/I (20 mg/day), contaminant E (20 µg/day),
contaminant F (20 µg/day), or contaminant G (20 µg/day).
Aged DPA/I and the contaminant E frequently induced moderate
cystic changes in the proximal tubules of the newborn rat.
Chromatographically pure DPA/I and the two other contaminants F and G
induced only mild cystic tubular changes (Crocker et al 1972).
COMMENTS
Diphenylamine (DPA) was first evaluated by the 1969 JMPR.
No-effect levels in mouse, rat and dog were determined. An ADI was
allocated on the basis of the no-effect level observed in a 2-year
study in the dog.
As new toxicological data had become available, diphenylamine was
reconsidered by the 1976 JMPR and an ADI was derived from a no-effect
level observed in a six-month mouse study.
The Meeting considered a study showing that an impurity (not
fully characterized) in commercial grade diphenylamine (DPA) is the
active nephrotoxic compound responsible for the in utero induction
of cystic tubular lesions in the kidneys of newborn rats of mothers
fed DPA in dietary concentrations of 2.5%. Another study described the
identification of some impurities in commercial samples (source not
specified) of DPA, showing that the level of primary amines is higher
than that specified (10 ppm) by the 1969 JMPR. The Meeting noted that,
in general, the purity of DPA used in toxicological studies has not
been reported, except for the long-term mouse study (FAO/WHO,1977) in
which 99.5% pure DPA was used. On the other hand, the specification of
purity (99.9%) reported by the 1969 JMPR appeared unrealistic. The
Meeting noted that no teratogenicity studies had been evaluated by
previous Meetings.
The Meeting decided to change the ADI to temporary status.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse : 10 ppm in the diet, equivalent to 1.5 mg/kg bw.
Rat : 100 ppm in the diet, equivalent to 5 mg/kg bw.
Dog : 100 ppm in the diet, equivalent to 2.5 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1984)
A teratogenicity study.
Desirable
1. Short-term studies with special attention to the formation of
Heinz bodies (from 1976 JMPR).
2. Mutagenicity tests.
3. Information on the nature and level of impurities in technical
grade diphenylamine.
REFERENCES
Crocker, J.F.S., Brown, D.M., Borch, R.F., Vernier, R.L. Renal cystic
1972 disease induced in newborn rats by diphenylamine
derivatives. Am. J. Pathol. 66(2):343-348.
Safe, S., Hutzinger, O., Crocker, J.F.S., Digout, S.C. Identification
1977 of toxic impurities in commercial diphenylamine.
Bull.Environ.Contam. Toxicol. 17:204-207.
