
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
FENITROTHION
IDENTITY
Chemical name
dimethyl 3-methyl-4-nitrophenyl phosphorothionate
Synonyms
0,0-dimethyl 0-(3-methyl-nitrophenyl) phosphorothioate, Sumithion(R),
Folition(R), Accothion(R), Danathion(R) (Denmark), Metathion(R)
(Czechoslovakia), methylnitrophos (countries in Eastern Europe)
Structural formula
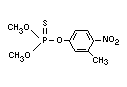 Other relevant chemical properties
Properties of fenitrothion - melting point 0.3°C, boiling point
109°C/0.1 mm Hg; d420 1.3084; yellowish-brown liquid with unpleasant
odour; soluble in alcohols, ethers, ketones, aromatic hydrocarbons;
insoluble in water; completely stable for 2 years, at 20-25°C; storage
temperature should not exceed 40°C; unstable in alkaline media.
Formulations - 95% concentrate, 50% emulsifiable concentrate, 40%
wettable powder, 2.5% and 5% dust. Deburol(R) (Ciba) contains 15%
fenitrothion and 62% mineral oil. One company indicates that their 95%
concentrate contains 95% fenitrothion (minimum) and 0.5%
3-methyl-4-nitrophenol (maximum).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Various comprehensive studies in mouse, rat, and guinea-pig have dealt
with the pharmacodynamic and biochemical aspects of fenitrothion and
its metabolites (Nishizawa et al., 1961; Miyamoto, 1964a, 1964b, 1969;
Miyamoto et al., 1963a; Vandanis and Crawford 1964; Hollingworth et
al., 1967 Hladká and Nosál, 1967 and Douch et al., 1968).
Fenitrothion is presumably rapidly absorbed from the mammalian
intestinal tract as evidenced by the appearance of radioactivity in
blood from guinea-pigs and rats administered phosphorus32 -labelled
fenitrothion orally. The presence of the oxygen analogue was
demonstrated in all tissues examined (brain, heart, lung, liver,
kidney, spleen and muscle) and it was detectable in blood one minute
after an intravenous injection of fenitrothion. This oxygen analogue
(II of Fig. 1) is the important metabolite with respect to toxicity.
It is formed in the microsomal fraction of the cell, the main organs
responsible for the transformation being the liver and kidney.
Fenitrooxon is further metabolized as indicated in Fig. 1. The major
excretion product found is 3-methyl-4-nitrophenol (VII) which can
further be oxidized to 3-carboxy-4-nitrophenol (VIII). Other
metabolites are the demethyl-derivatives (V and VI) which, with
increasing doses, are excreted in increasing amounts. A total of nine
metabolites has been isolated, the majority of which can also be
identified. In vitro studies showed that formation of the oxygen
analogue (II) was dependent on the availability of reduced nicotine
adenine dinucleotide phosphate (NADPH2) and oxygen. Liver slices
incubated with fenitrothion did not produce measurable amounts of
fenitrooxon, while liver homogenates and the supernatant fraction of
such homogenates showed appreciable activation of added fenitrothion.
No correlation between the toxicity and rate of formation of the
oxygen analogue could, however, be demonstrated (Miyamoto et al.,
1963a; Miyamoto, 1969).
No observations are available in these studies on the distribution
into fatty tissue: however, residue studies on milk, meat and fat from
cattle indicate amounts of approximately 0.001 ppm in these samples
(Miyamoto and Sato, 1969).
Fenitrothion and its metabolites are excreted mainly in the urine
(90-95 per cent). Up to 10 per cent was recovered in faeces. Within
three days nearly complete recovery of an orally administered dose (15
mg/kg) could be obtained. The metabolic, pattern of fenitrothion as it
appears from these studies is shown in Fig. 1. The ratios between the
amounts of metabolites was dependent upon the dose given, as is shown
in Table 1, which gives the percentage distribution of metabolites in
mouse urine after various doses of fenitrothion (Hollingworth et al.,
1967).
Effect on enzymes and other biochemical parameters
As with other organophosphorous compounds fenitrothion acts in the
animal organism as a cholinesterase inhibitor, probably after
conversion to the oxygen-analogue. Some evidence presented indicates
that the cholinesterase inhibiting effect in brain depends more on the
rate of penetration into the brain than on the rate of oxidation and
decomposition of fenitrothion (Miyamoto, 1969).
Other relevant chemical properties
Properties of fenitrothion - melting point 0.3°C, boiling point
109°C/0.1 mm Hg; d420 1.3084; yellowish-brown liquid with unpleasant
odour; soluble in alcohols, ethers, ketones, aromatic hydrocarbons;
insoluble in water; completely stable for 2 years, at 20-25°C; storage
temperature should not exceed 40°C; unstable in alkaline media.
Formulations - 95% concentrate, 50% emulsifiable concentrate, 40%
wettable powder, 2.5% and 5% dust. Deburol(R) (Ciba) contains 15%
fenitrothion and 62% mineral oil. One company indicates that their 95%
concentrate contains 95% fenitrothion (minimum) and 0.5%
3-methyl-4-nitrophenol (maximum).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Various comprehensive studies in mouse, rat, and guinea-pig have dealt
with the pharmacodynamic and biochemical aspects of fenitrothion and
its metabolites (Nishizawa et al., 1961; Miyamoto, 1964a, 1964b, 1969;
Miyamoto et al., 1963a; Vandanis and Crawford 1964; Hollingworth et
al., 1967 Hladká and Nosál, 1967 and Douch et al., 1968).
Fenitrothion is presumably rapidly absorbed from the mammalian
intestinal tract as evidenced by the appearance of radioactivity in
blood from guinea-pigs and rats administered phosphorus32 -labelled
fenitrothion orally. The presence of the oxygen analogue was
demonstrated in all tissues examined (brain, heart, lung, liver,
kidney, spleen and muscle) and it was detectable in blood one minute
after an intravenous injection of fenitrothion. This oxygen analogue
(II of Fig. 1) is the important metabolite with respect to toxicity.
It is formed in the microsomal fraction of the cell, the main organs
responsible for the transformation being the liver and kidney.
Fenitrooxon is further metabolized as indicated in Fig. 1. The major
excretion product found is 3-methyl-4-nitrophenol (VII) which can
further be oxidized to 3-carboxy-4-nitrophenol (VIII). Other
metabolites are the demethyl-derivatives (V and VI) which, with
increasing doses, are excreted in increasing amounts. A total of nine
metabolites has been isolated, the majority of which can also be
identified. In vitro studies showed that formation of the oxygen
analogue (II) was dependent on the availability of reduced nicotine
adenine dinucleotide phosphate (NADPH2) and oxygen. Liver slices
incubated with fenitrothion did not produce measurable amounts of
fenitrooxon, while liver homogenates and the supernatant fraction of
such homogenates showed appreciable activation of added fenitrothion.
No correlation between the toxicity and rate of formation of the
oxygen analogue could, however, be demonstrated (Miyamoto et al.,
1963a; Miyamoto, 1969).
No observations are available in these studies on the distribution
into fatty tissue: however, residue studies on milk, meat and fat from
cattle indicate amounts of approximately 0.001 ppm in these samples
(Miyamoto and Sato, 1969).
Fenitrothion and its metabolites are excreted mainly in the urine
(90-95 per cent). Up to 10 per cent was recovered in faeces. Within
three days nearly complete recovery of an orally administered dose (15
mg/kg) could be obtained. The metabolic, pattern of fenitrothion as it
appears from these studies is shown in Fig. 1. The ratios between the
amounts of metabolites was dependent upon the dose given, as is shown
in Table 1, which gives the percentage distribution of metabolites in
mouse urine after various doses of fenitrothion (Hollingworth et al.,
1967).
Effect on enzymes and other biochemical parameters
As with other organophosphorous compounds fenitrothion acts in the
animal organism as a cholinesterase inhibitor, probably after
conversion to the oxygen-analogue. Some evidence presented indicates
that the cholinesterase inhibiting effect in brain depends more on the
rate of penetration into the brain than on the rate of oxidation and
decomposition of fenitrothion (Miyamoto, 1969).
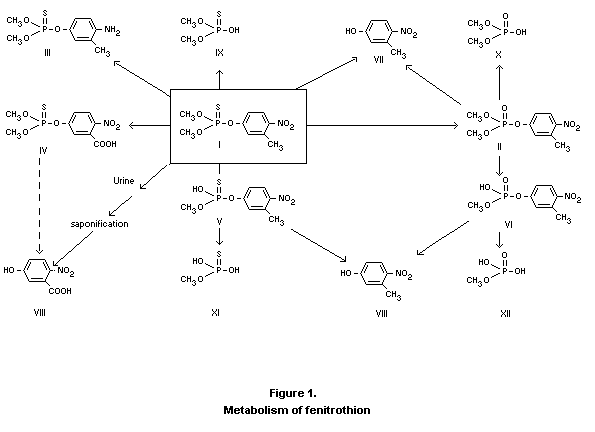 TABLE I
Percentage distribution of metabolites of fenitrothion in mouse urine after
administering various doses
Metabolite Percentage of radioactive metabolite excreted
in urine after giving P52 - labelled fenitrothion
at the indicated dose levels in mg/kg body-weight
3 17 200 850
Phosphoric acid 2.0 2.4 1.9 1.2
Methylphosphoric acid 1.5 2.5 2.4 1.1
Dimethylphosphoric acid 32.2 21.4 5.8 3.0
Dimethylphosphorothioic acid 12.8 20.3 8.7 6.6
Phosphate analogue (II) 2.7 1.6 3.3 2.5
O-demethylphosphate analogue (VI) 26.1 28.4 24.6 17.1
O-demethylphosphorothioate
analogue (V) 20.5 20.1 50.9 66.1
Unknown 2.2 2.5 2.4 2.4
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Hen
Three hens protected against acute anti-cholinesterase effects with
atropine and pralidoxime were given a single oral dose of 400 mg/kg
body-weight of fenitrothion. No symptoms of paralysis occurred during
an observation period of 28 days. Histologic examination of spinal
cord and sciatic nerves revealed no pathological lesions in two hens
and a few scattered degenerated fibres in the spinal cord of the third
(Carshalton, 1962).
Seven hens protected in the same way were given an oral dose of 250
mg/kg body-weight of fenitrothion and three other hens were given 500
mg/kg. Two of the 500 mg/kg group died within 1-2 days, while the
remaining eight hens did not show any sign of paralysis during an
observation period of six weeks (Kimmerle, 1962b).
Special studies on potentiation
Rat
Female rats were given intraperitoneal doses of a combination of
fenitrothion and the following organophosphorus compounds: parathion,
parathion-methyl, demeton, disulfoton, malathion, EPN,
azinphos-methyl, carbophenothion, mevinphos, dioxathion, schraden,
ethion, diazinon, Folex, coumaphos, and fenchlorphos as well as the
carbamate carbaryl. No sign of a potentiating effect was demonstrated
(DuBois and Kinoshita, 1963).
When male rats were given acute oral doses of mixtures of fenitrothion
and phosphamidon a marked potentiation of toxicity occurred as
evidenced by increased mortality. In female rats a potentiation of
toxicity occurred as evidenced by increased mortality. In female rats
a potentiation occurred only in mixtures containing relatively low
concentrations of fenitrothion, the potention effect diminishing as
the concentration of fenitrothion was increased. It was concluded that
potentiation is associated with a non-linear phosphorothioate
conversion (Braid and Nix, 1968).
Special studies on the metabolite fenitrooxon
The metabolite fenitrooxon is more toxic than the parent compound (cf.
Tables II and III).
TABLE II
Acute toxicity of fenitrooxon
Acute
Animal Route LD50 mg/kg References
body-weight
Mouse oral 90 Miyamoto, 1969
Mouse oral 120 Hollingworth et al., 1967
Rat oral 24 Miyamoto, 1969
Rat i.v. 3.3 Miyamoto, 1969
Guinea-pig oral 221 Miyamoto, 1969
Guinea-pig i.v. 32 Miyamoto, 1969
Acute toxicity
The symptoms of acute toxicity are the same as for other
organophosphorus compounds, doses close to the LD50 producing
symptoms which developed more rapidly after intravenous than after
oral administration. The compound is considerably less toxic to
mammals than its close structural analogue parathion-methyl (Miyamoto
et al., 1963b). Table II gives the LD50 in several species:
TABLE III
LD50 mg/kg
Animal Route body-weight References
Mouse (M) oral 1336 Carshalton, 1964
Mouse (F) oral 1416 Carshalton, 1964
Mouse (M) i.p. 115 DuBois and Puchala, 1960
Mouse (F) i.p. 110 DuBois and Puchala, 1960
Mouse i.v. 220 Miyamoto et al, 1963b
Rat (M) oral 740 Gaines, 1969
Rat (F) oral 570 Gaines, 1969
Rat (M) i.p. 135 DuBois and Puchala, 1960
Rat (M) i.p. 160 DuBois and Puchala, 1960
Rat i.v. 33 Miyamoto et al, 1963b
Guinea-pig (M) oral 500 DuBois and Puchala, 1960
Guinea-pig oral 1850 Miyamoto et al, 1963b
Guinea-pig (M) i.p. 110 DuBois and Puchala, 1960
Guinea-pig i.v. 112 Miyamoto et al, 1963b
Cat oral 142 Nishizawa et al, 1961
Short-term studies
Dog
In what was described as a preliminary test, groups of dogs, each
comprising one male and one female animal, were given daily oral doses
by capsule of 0, 2, 9 or 40 mg/kg body-weight of fenitrothion for
periods up to 98 days. Body-weights, blood biochemistry,
cholinesterase levels and haemograms were checked at intervals. At the
2 mg/kg level there was no effect with respect to any other of the
parameters mentioned. At 9 mg/kg a slight depression after 60 days and
at 40 mg/kg a moderate depression after 29 days occurred in whole
blood, plasma and red-cell cholinesterase. At 40 mg/kg there were also
marked toxic symptoms typical of cholinergic stimulation, and the dogs
in this group were sacrificed below the end of the 98-day period
(Cooper, 1966).
Rat
Groups of male rats (16 or 17 in number) were fed 0, 32, 63, 125, 250,
and 500 ppm of fenitrothion in the diet for 90 days. Mortality, food
intake, growth, general behaviour, urinalysis, average organ-weights
and histpathology were comparable to the controls in the groups fed
32, 63, 125, and 250 ppm. All the animals fed 500 ppm showed clinical
symptoms of anti-cholinesterase poisoning and there were minimal
symptoms in four animals in the 250 ppm group. In the 500 ppm group
the average organ-weights of the testes and brain were increased in
comparison with those of the control group. After interim sacrifice
every month of four rats from each group, measurement of the
cholinesterase activity of plasma, red cells, brain cortex, liver and
kidney showed a dose-dependent depression, the lowest being in the
brain. The cholinesterase activity in the 32 and 63 ppm groups
generally increased after 60 days of dosing to a level within the
normal limits, the beet recovery being in the plasma, kidney and
brain, less in the red cells and the liver (Misu et al., 1966).
Two groups, each of 20 male rats, were dosed by stomach tube on six
days a week for six months with 10 mg/kg and 11 mg/kg body-weight of
fenitrothion, respectively. During the first weeks the rats showed a
temporary deterioration of general condition and loss of weight.
Haematology and urinalysis during the experiment and gross and
microscopic pathology at its termination did not reveal any
abnormalities (Klimmer, 1961).
Male rats were given daily oral doses of 13 mg/kg body-weight of
fenitrothion for 28 days. Red-cell cholinesterase activity showed
severe depression, but there was recovery 30 days after withdrawal of
fenitrothion (Kimmerle, 1962a).
Male rats were fed 5, 10, and 20 ppm of fenitrothion in the diet for
an unspecified period. Brain and red cell cholinesterase activity was
normal in the 5 ppm group, whereas the 10 ppm group showed a slight
depression of red cell activity after five weeks with recovery two
weeks after withdrawal.
The 20 ppm group showed some depression of activity both in red cells
and in brain and the recovery in the brain remained incomplete two
weeks after withdrawal (Carshalton, 1964).
Other reported studies with fenitrothion in the rat lasting 90 days
indicate that the levels causing no appreciable effect on the
cholinesterase activity of plasma, red-cells and whole blood were 20
ppm in the diet and 10 ppm in drinking water. The only exception was a
moderate inhibition of the activity in plasma among the male animals
when given 10 ppm in drinking water. The above mentioned levels and
higher, namely 92.8 ppm in the diet and 46.2 ppm and 215 ppm in
drinking water, caused no effect on food or water intake, weight-gain,
average organ-weights, haemogram and blood biochemistry. However, 92.8
ppm of fenitrothion in the diet and 46.4 ppm in drinking water had a
moderate effect on whole blood and red-cell cholinesterase and a more
marked effect on plasma cholinesterase. The cholinesterase activity
recovered between 30-40 days after withdrawal of fenitrothion. The
levels of 430 ppm in the diet and 1000 ppm in drinking water caused a
depression of body-weight gains (Cooper, 1966).
Long-term studies
Rat
An interim report on what appears to be a two-year feeding study in
rats is available. Groups of nine or 10 male rats were fed 0, 25, 100
or 400 ppm of fenitrothion in their diet and were sacrificed after
feeding these levels for 63 weeks. As a positive control a group was
also fed 800 ppm of malathion. At the 400 ppm level of fenitrothion,
food intake and body-weight gain were increased and only a few animals
survived the 63 week period. At this level there was a 100 per cent
depression of red-cell cholinesterase. At the 100 ppm level a slight
(10-30 per cent) cholinesterase depression occurred in the brain and a
moderate depression (30-65 per cent) in the red blood cells and
plasma. At 25 ppm there was no effect on cholinesterase nor was there
any effect with regard to any other parameters evaluated (Ueda and
Nishimura, 1966).
OBSERVATIONS IN MAN
In a field spraying operation in Southern Nigeria including a village
using a five per cent spray of fenitrothion, examination of 18
villagers one week later did not reveal any clinical symptoms of
toxicity or plasma cholinesterase depression. The same was true of the
three spraymen examined on the first, second and sixth day after
spraying relative to a pre-spraying level (Vandekar, 1965).
In another field spraying trial in Northern Nigeria, 10,000 huts in
which about 16,500 people lived were sprayed. Field test
cholinesterase determinations on whole blood did not show any
appreciable difference in cholinesterase levels of 535 villagers
tasted before spraying and 299 villagers tested 5-30 days after
spraying. After one week of intensive spraying five out of 20 spraymen
developed a 50 per cent depression of cholinesterase which returned to
a stable level after a period of rest. One sprayman developed symptoms
of toxicity which lasted only a few hours and disappeared without
treatment (Wilford et al., 1965).
Fenitrothion was given to a total of 24 human subjects in single oral
doses of from 2.5 to 20 mg (0.042 to 0.33 mg/kg body-weight for a 60
kg man). The excretion of the metabolite, 3-methyl-4-nitrophenol, in
the urine was almost complete within 24 hours, the maximum excretion
occurring in the first 12 hours. The percentage of the dose excreted
during this time depended to a certain extent upon the size of the
dose administered, being about 70 per cent of theoretical after a
0.042 mg/kg dose and about 50 per cent after a 0.33 mg/kg dose. Both
plasma and cholinesterase activity were not depressed below normal
except possibly in one person given a 0.33 mg/kg dose, where some
depression of plasma cholinesterase was apparent after six and 24
hours (about 65 per cent of the pre-test level). When repeated doses
of 2.5 or 5 mg approximately 0.04 to 0.08 mg/kg) were given to five
individuals, four times at 24 hour intervals, most of the nitrocresol
metabolite appeared in the urine within the interval 0 to 12 hours
after administration. After receiving the third and fourth dose there
was a trend towards a rise in red cell cholinesterase activity but in
no cases was there any evidence of reduction below normal levels of
the activity of this enzyme in either plasma or red cells (Nosal and
Hladka, 1968).
COMMENT
Adequate information is available on the toxicity, biochemistry and
metabolism of fenitrothion, in three species of rodent. Information is
also available in man including field spraying studies and a
metabolism study. Only an interim report comprising a 63-week study in
rats in available and there are no 1-2 year studies in a non-rodent
mammalian species. The short-term studies in the rat, using
cholinesterase inhibition criteria, provide a no-effect level. No
reproduction or teratogenicity studies are available and in view of
the similarity in the structure of fenitrothion to parathion-methyl,
it is important that such studies be undertaken. For this reason and
because no adequate long-term studies are available it was decided to
give only a temporary acceptable daily intake to this compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 ppm in the diet, equivalent to 0.25 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenitrothion is a broad-spectrum insecticide having a much lower acute
mammalian toxicity than many similar organophosphorus insecticides.
Its action is by direct contact or an a stomach poison. It can be
applied as an emulsifiable concentrate, wettable powder, granular
formulation or dust.
It is toxic to bees and should not be sprayed on flowering crops.
Fenitrothion can be combined with all conventional insecticides and
fungicides except those having an alkaline reaction.
The insecticide has been used throughout Europe, East Pakistan, East
Africa, United Arab Republic, Japan, Republic of China, New Zealand,
Canada, and Brazil. It is not registered for use in the United States.
Pre-harvest treatments
Fenitrothion is used on a wide variety of crops including fruits,
field crops, vegetables, rice, cotton, cereals, cocoa, tea, and coffee
for control of stem borers, hoppers, leaf miners, leaf rollers,
whiteflies, fruit flies, mealybugs, mirids and bugs, thrips, aphids,
mites, lady beetles, caterpillars, and soft scale insects.
The recommended concentrations and rates of application vary for the
different crops and pest species to be controlled. Concentrations of
sprays range from 0.05 to 0.1%. active ingredient (a.i.) and rates of
application from 0.5 to 2.0 kg a.i./hectare. The chemical is generally
tolerated by most crops although high dosages may injure cotton, and
phytotoxicity on Brassica crops and orchard fruits has been
encountered.
Safety intervals prior to harvest range from 10 to 21 days and differ
depending on the country and the crop.
Post-harvest treatments
No post-harvest treatments are made in Europe. Admixture of 1 to 5 ppm
of fenitrothion has been recommended to protect grains such as rice,
wheat, and barley for months against various weevils and beetles. Bag
treatment is acceptable in Brazil for protecting stored grains and
post-harvest treatments are under development in various countries.
Other uses
Fenitrothion is used to control grasshoppers, locusts, and
caterpillars on pastures and spruce budworm in forests. It also
provides control of household and other pests, such as mosquitoes and
their larvae, houseflies, cockroaches, lice, bedbugs, and poultry
mites. Its action is rapid, and its residual activity is good.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Typical maximum residues after treatment of a variety of crops are
given in Table IV.
TABLE IV
Maximum residues after treatment of crops
Maximum
Dosage residues at
active Pre-harvest harvest, ppm
Crop ingredient interval, days respectively Reference
Rice 1000 g/ha 41 0.005 Sumitomo 1969
Rice 0.07% spray or Miyamoto, Sato
5% granules 1-2 months n.d. 1965
Apple 0.05% spray 21 0.05 Sumitomo 1969
Apple 0.2% spray 7 and 14 0.5 and 0.35 Bayer 1969
Apple, Golden 0.15% spray 10 and 15 1.0 and 0.75 Bayer 1969
cherry 0.2% spray 7 and 14 0.5 and 0.2 Bayer 1969
Grape* 0.05% spray 10 0.50 Sumitomo 1969
Tomato* 0.05% spray 7 0.18 Sumitomo 1969
Cocoa 0.1% spray 14 0.10 Miyamoto et al
1965b
Red cabbage 0.15%; 1000 7 Outside leaves
1/ha 0.65
Cabbage less
outside leaves
0.25 Bayer 1969
Sugar beets 0.15%; 1000 8 n.d. Bayer 1969
1/ha
TABLE IV (cont'd)
Maximum residues after treatment of crops
Maximum
Dosage residues at
active Pre-harvest harvest, ppm
Crop ingredient interval, days respectively Reference
Green tea 0.1% spray 14 0.27 Sumitomo 1969
Cauliflower 0.1%; 1000 7 n.d. Bayer 1969
1/ha
Lettuce 0.1%; 1000 7 and 14 1.05 and 0.3 Bayer 1969
1/ha
Lettuce 0.05%; 600 1, 7, and 14 0.65, 0.06, Möllhoff 1968
1/ha 0.01
Peas and pod 0.25%; 560 0 and 5 0.7 and <0.15 Bayer 1969
1/ha
*These green-house trials gave residues higher than those of field
trials.
FATE OF RESIDUES
General comments
Fenitrothion may be metabolized as shown in Figure 1. The major route
in animals appears to be through splitting of the P-O-aryl bond to
give the corresponding dimethyl esters of phosphorothioic and
phosphoric acids. Another means of degradation is demethylation of the
methoxy group to give desmethyl fenitrothion. Although formation of
the oxygen analogue, fenitrooxon, is minor compared to products formed
via hydrolysis, fenitrooxon must be taken into account because the
mammalian toxicity of oxons is generally much higher than that of
parent thiono pesticides. In plants the metabolism of fenitrothion
appears to be similar to that in animals.
In animals
Orally administered 32P-labelled fenitrothion was readily absorbed
from the digestive tract of guinea pigs or rats and the major portion
of the radioactivity excreted in the urine. Neither fenitrothion nor
fenitrooxon was detected and desmethyl fenitrothion, dimethyl
phosphorothioic and dimethyl phosphoric acids were identified in the
urine (Miyamoto et al., 1963a).
Following intravenous injection of radioactive 32P fenitrothion into
guinea pigs and rats, fenitrothion rapidly disappeared from the blood.
Fenitrothion and fenitrooxon were found in the tissues, and their
amounts decreased rapidly. The desmethyl compound and the dimethyl
esters mentioned in the foregoing paragraph were found mostly in the
liver and kidneys (Miyamoto, 1964a).
Excretion of metabolic products is rapid and chiefly in the form of
3-methyl-4-nitrophenol (the nitrocresol hydrolysis product) (Hladka
and Nosal, 1967; Nosal and Hladka, 1968); the cresol methyl may be
oxidized to COOH (Douch et al., 1968). The desmethyl compounds are
also excreted (Hollingworth et al., 1967; Miyamoto et al., 1963b).
Between 60 and 90% of the insecticide is excreted within two days,
chiefly in the aforementioned forms. Only up to 10% is excreted in the
feces. Detoxication via the desmethyl compounds is dose-dependent.
After oral administration of up to 40 grams of fenitrothion per
lactating cow, residues in the milk were as high as 0.4 ppm after 6
hours and below the limit of detection after one day (Hais and Franz,
1965). Detoxication in bovine rumens is rapid owing to reduction of
fenitrothion to the amino compound (Miyamoto et al., 1967).
Cows fed 3 mg/kg body weight of fenitrothion for seven consecutive
days produced milk having up to 0.002 ppm residue of fenitrothion on
the second day, and no residue one day after administration was
stopped. Less than 0.003 ppm aminofenitrothion and about 0.1 ppm
p-nitrocresol were detected during treatment, and no fenitrooxon was
found (Miyamoto et al., 1967).
Thirty calves (1-1.5 yr. av. wt. 243 kg) confined on a pasture sprayed
with 375 g/ha of fenitrothion (11.8 ppm initial residue on grass) were
periodically sacrificed and breast muscle and omental fat analysed. On
the first day residues in the meat and fat were about 0.01 ppm. No
residue of fenitrothion was found in the meat from the third day on
and only 0.004-0.007 ppm was found in the fat on the third day; these
amounts decreased almost to control levels by the seventh day
(Republic of Argentina, 1968; Miyamoto and Sato, 1969).
Lactating dairy cattle were fed 50 ppm of fenitrothion in the feed
(dry basis) for 29 days. No residue of fenitrothion, fenitrooxon, or
the cresol appeared in the milk. A maximum of 0.006 ppm of
aminofenitrothion was found (Bowman, 1969).
In plants
About 50% of 32P-labelled fenitrothion sprayed on rice plants
penetrated into the tissues in 24 hours; at the end of this period
only 10% was left, indicating rapid decomposition. Some fenitrooxon
formed but it disappeared from the tissues more rapidly than
fenitrothion. Rice grains harvested 46 days after treatment contained
0.0007 ppm fenitrothion and less than 1 ppm of p-nitrocresol and
dimethyl phosphorothioic acid (Miyamoto and Sato, 1965).
Fenitrothion does not appear to have much systemic action. After
treatment of rice plants with fenitrothion, more residue is found in
the bran than in the polished grains (Miyamato and Sato, 1965). Very
little active ingredient passed from peel to fruit in stored bananas
(Miyamoto et al., 1965a).
The half-life of fenitrothion in green plants ranges between the
values established for parathion and parathion-methyl, i.e. between
one and two days; the half-life of the oxon is estimated to be only a
few hours (Möllhoff, 1968).
Although the oxon may form in plants it occurs only during the first
few days after treatment and in proportions (ca. 1% smaller than those
in animals (Miyamoto et al., 1963; Miyamoto and Sato 1965; Möllhoff,
1968). Desmethyl compounds occur only in minor amounts in plants.
Phytotoxicity of fenitrothion on cabbage was ascribed to high
penetration of the insecticide and accumulation of the cresol
hydrolysis product (Tomizawa and Kobayashi, 1964).
In soil
The soil bacteria Bacillus subtilis, converted more than half of
radiolabelled 32P fenitrothion to the amino analogue under aerobic
conditions at 37°C in 24 hours. The desmethyl derivatives of
fenitrothion and aminofenitrothion as well as dimethyl phosphorothioic
acid formed. None of the oxon or oxons of the degradation products
were found (Miyamoto et al., 1966).
Fenitrothion is gradually inactivated by other bacterial species
including gram-positive and gram-negative rods, but not by fungi and
yeasts (Yasuno et al., 1965).
Fenitrothion is readily absorbed onto various kinds of soil and
decomposed by alkalinity or microorganisms (Muramoto, 1967).
Persistence in soil does not appear to be great.
In storage and processing
Fenitrothion shows promise for control of grain insects in storage.
Applied at 2 and 1 ppm to barley, it decreased to 0.4 ppm after 15
weeks' storage (Green and Tyler, 1966). On barley in silos the
half-life of fenitrothion was about 100 days (Green and Tyler, 1966)
and on stored bananas about 15 days (Miyamoto et al., 1965a). Applied
to wheat 11% moisture) at rates of 1, 2 and 4 ppm, 0.2, 0.4., and 1.1
ppm of fenitrothion remained, respectively, after 9 months of storage
at 25°C and 60% relative humidity (Kane and Green, 1968).
In extensive trials as a wheat protectant, fenitrothion levels
steadied below 2 ppm after about 2 months regardless of application
rate between 2.5 and 10 ppm. In one trial 6 ppm applied to grain
decreased to 2.4, 1.7, and 1.1 ppm, respectively, after 1´, 4, and 6
months. Flour made from the 2.4-ppm sample contained 0.3 ppm
fenitrothion, while only traces were found in the flour and bread from
the later samples (Cooper Technical Bureau, 1968).
Evidence of residues in food in commerce or at consumption
No report of residues of fenitrothion in food in commerce or at
consumption has been found. Reference is made under "In storage and
processing" to the finding of only "traces" of fenitrothion in flour
and bread prepared from grain containing 1.7 and 1.1 ppm of the
insecticide.
METHODS OF RESIDUE ANALYSIS
Metabolic studies on plants and mammals indicate that residues of
fenitrooxon and aminofenitrooxon, when they do form, occur in small
amount and are degraded or excreted more rapidly than the parent
compound. Once pilot experiments establish that no more than traces of
these compounds form on a given crop, there does not seem to be any
need to analyse for these compounds on that crop in commerce. (For
purposes of this discussion, a trace shall be considered less than 10%
of the tolerance level of the parent compound).
Analyses for the p-nitrocresol have been made and measurable amounts
of the compound are found following good agricultural practice. A
determination of the cresol is sometimes reported. However, if a pilot
experiment on a given crop shows no excessive amount of the cresol to
be present at harvest, there does not appear to be any need for its
analysis on that crop in commerce. Other metabolites are generally
rather polar and do not tend to be stored.
In essence then, the analyst will be concerned with residues of
fenitrothion itself unless pilot experiments indicate that other
metabolites should be taken into account. This same view is expressed
by Frehse and Möllhoff in a IUPAC report by Egan (1969).
Fenitrothion in so similar to parathion and methyl parathion that
analytical methods used for them may be used for fenitrothion.
However, many of the earlier methods lack specificity. Numerous
procedures for a wide variety of harvest products are based on
thin-layer chromatography, spectroscopy and gas chromatography.
Information relating to these analyses follow. No reference to
inter-laboratory collaborative studies on analytical procedures was
found.
Extraction and partitioning
It is not sufficient simply to wash the analytical samples since small
amounts of the active ingredient penetrate into the plant. It is
therefore important that complete extraction of the insecticide and
metabolites (e.g. by exhaustive Soxhlet extraction) be compared
against the extraction method used. Harvest products with a high
content of water have been macerated with acetone (Horler, 1966;
Möllhoff, 1967, 1968), acetonitrile (Coffin and Savary, 1964; Thier
and Bergner, 1966), or ethanol (Fischer, 1968). Very good recoveries
were obtained by Soxhlet extraction with chloroform-methanol (9:1 v/v)
(Bowman and Beroza, 1969). For milk, a combination of polar and
nonpolar extractants is required, e.g. acetone-methylene chloride
(Bowman and Beroza, 1969), ethanol-hexane (Franz and Kovac, 1965).
Extraction with methanol-acetonitrile also proved suitable for milk
samples (Miyamoto et al., 1967). For oil-containing harvest products
with a low water content, use is made of benzene (Dawson et al., 1964;
Horler, 1966), petroleum ether (Kovac and Sohler, 1965), hexane
(Horler, 1966), or chloroform (Yuen, 1966). On account of the
favourable partition coefficients (Bowman and Beroza, 1965; Kovac,
1963), fats and waxes are best removed by partitioning between hexane
and acetonitrile (Franz and Kovac, 1965; Miyamoto et al., 1967;
Möllhoff, 1967; Bowman and Beroza, 1969).
Qualitative analysis
Although no reports of fenitrothion being separated from similar
residues by column chromatography have been noted, separation is
possible with thin-layer or gas chromatography. Interfering active
ingredients are parathion, parathion-methyl, malathion, and fenthion
and the resolution is just sufficient for distinguishing fenitrothion
from these active ingredients (see e.g. Möllhoff, 1968). By comparing
gas chromatograms recorded with a phosphorus detector and an electron
capture detector, fenitrothion can often be clearly differentiated
from malathion and fenthion owing to differences in sensitivity.
Colorimetry
Colorimetric determinations are carried out by the method of Averell
and Norris (1948) or by determination of the cresol after
saponification of the active ingredient with alkali. The limits of
determination are about 0.05 ppm.
Thin-layer chromatography
Thin-layer chromatography has been used for clean-up of the extracts
with final determination by colorimetry after deleting the appropriate
spot from the plate, or for direct determination on the plate after
spraying. Quantitative evaluation is possible in the first instance;
in the second, the evaluation is semiquantitative and more suitable
for confirming identity. About 0.1 ppm of fenitrothion can be
determined in most harvested products. Determination of lesser amounts
is possible with procedures utilizing cholinesterase inhibition
(Ackermann, 1966; Mendoza et al., 1968; Schutzmann and Barthel, 1969;
Winterlin et al., 1968).
Column chromatography
For extract cleanup or separation of metabolites, columns are
suitable, especially those packed with deactivated silica gel (Bowman
and Beroza, 1969), deactivated Florisil (Möllhoff, 1967, 1968), or
deactivated acid aluminum oxide (Horler, 1966). Columns packed with
active Florisil (Beckman and Garber, 1969), magnesium oxide (Coffin
and Savary, 1964), polyethylene-impregnated aluminum oxide (Coffin and
Savary, 1964), and Sephadex LH-20 (Horler, 1968; Ruzicka, et al.,
1968) have also been used.
Gas chromatography
The most reliable and highly sensitive methods for fenitrothion
utilize gas chromatography with either the flame-photometric (Bowman
and Beroza, 1969) or the thermionic detector (Miyamoto et al., 1967,
Sato et al., 1968). These detectors respond to the phosphorus in the
molecule with very high specificity. No cleanup is usually required
with the flame-photometric detector when analysing for either
fenitrothion or its oxygen analogue unless a fatty food is being
analysed. In this case a simple hexane-acetonitrile partition is used.
A cleanup was used with the thermionic detector but it may often be
omitted. Limit of determination is usually 0.01-0.001 ppm.
In the analysis for fenitrooxon a separation from fenitrothion on
silica gel is used. The compound is then determined by gas
chromatography (Bowman and Beroza, 1969) or enzymatically (Miyamoto et
al., 1967). The cresol is determined by electron-capture gas
chromatography (Bowman and Beroza, 1969) or colorimetrically (Miyamoto
et al., 1967) after column separation. Analysis of fenitrothion,
fenitrooxon, and the cresol by electron-capture gas chromatography is
possible (Bowman and Beroza, 1969; Dawson et al., 1964; Horler, 1966;
Möllhoff, 1967) but less specific; sensitivity, about 0.1 ppm, can be
improved with a suitable cleanup.
Determination of metabolites
Gas chromatographic methods have been described for the simultaneous
determination of fenitrothion, fenitrooxon (Bowman and Beroza, 1969;
Möllhoff, 1968), 3-methyl-4-nitro phenol (Bowman and Beroza, 1969;
Miyamoto et al., 1967) and the amino compound of fenitrothion
(Miyamoto et al., 1967). As noted, for market controls determination
of fenitrothion is generally sufficient (Möllhoff, 1968).
NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Tolerance Withholding
Country Crop (ppm) period (days)
Austria 2.0 proposed
Belgium Top fruits and vegetables 0.5 14
China, Rice - 21
Republic of Vegetables - 10
Denmark Beets, apples, peas, clover - 15
Tolerance Withholding
Country Crop (ppm) period (days)
East Africa Mango, cereals - 7
Germany, Field crops, vegetables, fruits, and - 10
Eastern special crops
Crops for Infant food and remedies - 21
Germany Vegetables, root and field crops, 0.4 10
(Fed. Rep.) legumes, fruits, grapes
Finland - 21
France Vegetables, fruits - 15
Italy - 15
Netherlands Cereals, fruits 0.5 21
New Zealand - 14 (7 for
pasture)
Poland Top fruits (except strawberries and - 14
raspberries)
Portugal Vegetables, citrus, fruits, cocoa, coffee - 21
Pasture grass - 10
Spain Vegetables, citrus, fruits - 15-21
Sweden Peas, rape, wheat, barley - 10
Switzerland Top fruits 0.2 in
combination
with
Amidithion
APPRAISAL
Fenitrothion is a broad spectrum insecticide with a much lower acute
mammalian toxicity than many similar insecticides. It is not covered
by patents and is produced by at least six companies. Use of
fenitrothion is almost worldwide (excluding the U.S.A.) for such crops
as rice, fruits, vegetables, cotton, cereals, soybeans, coffee and
tea. Application rates are 0.5-2.0 kg a.i./ha. Waiting intervals are
generally 10-21 days. It is used against spruce budworm (in forests),
insects attacking man, and in some countries to protect stored
products. Its use on pasture land (375 g/ha) results in almost no
residues of fenitrothion in milk or meat (<0.01 ppm). The insecticide
is available as a 95% concentrate, 50% emulsifiable concentrate, 40%
wettable powder, 2.5 and 5% dusts. Composition of technical materials
is not known and is apt to vary depending on manufacturer. Residue
data are available mostly from Europe and Japan.
Although several metabolites of fenitrothion are known to form
(fenitrooxon, desmethyl fenitrothion, aminofenitrothion, and
3-methyl-4-nitrophenol), they do not accumulate in significant amounts
or they appear to be comparatively non-toxic. Unless experimental
trials indicate otherwise, fenitrothion appears to be the only toxic
residue to be determined in products in commerce.
Many methods are available for residue analysis of fenitrothion. The
best of these utilize gas chromatography with the flame photometric or
thermionic detector. Some of these methods may also be used for
analysis of metabolites. Sensitivity is usually better than 0.01 ppm.
Evaluation of these procedures for regulatory purposes is suggested.
Few data on residues in food in commerce or in diets have been
reported.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCE OR
PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to June 1973)
Apples, cherries, grapes, lettuce 0.5 ppm ) Preharvest interval to
) be such that these
Tomatoes 0.2 ppm ) tolerances are not
) exceeded)
Red cabbage, green tea 0.3 ppm
Cocoa 0.1 ppm
The 3-methyl-4-nitrophenol is not included in the recommendations at
this time but the Joint Meeting should review this aspect in due
course.
PRACTICAL RESIDUE LIMITS
Milk (whole) 0.002
Meat or fat 0.03
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Reproduction and teratogenicity studies in animals preferably in
non-human primates.
2. Adequate long-term studies in rodent and non-rodent mammalian
species.
3. Data on residue levels in raw agricultural commodities moving in
commerce and in total diet studies.
4. Data on disappearance of residues during storage, processing and
cooking.
5. Data on rate of residue decline in rice and pre-harvest interval.
6. Before use as a grain protectant, data on persistence of residues
in storage of the grains concerned and definitive data on residues
in bread are needed.
7. Data on occurrence of 4-nitro-3-methylphenol residues and their
significance toxicologically.
8. Information on ingredients in technical products produced by
several manufacturers.
DESIRABLE
1. Observations in man.
2. Evaluation of gas chromatographic methods for regulatory purposes.
REFERENCES
Ackermann, H. (1966) Enzymatischer Nachweis phosphororganischer
Insektizide nach dünnschicht-chromatographischer Trennung. Nahrung,
10:273-4
Averell, P.R. and Norris, M.V. (1948) Estimation of small amounts of
O,O-diethyl-O,p-nitro-phenyl thiophosphate. Anal. Chem. 20:753-6
Bayer (1969) (Farbenfabriken Bayer AG.). Fenitrothion. Unpub. Rept.
Beckman, H. and Garber, D. (1969) Recovery of 65 organophosphorus
pesticides from Florisil with a new solvent elution system. J. Ass.
Offic. Anal. Chem. 52:286-93
Bowman, M.C. and Beroza, M. (1965) Extraction p-values of
pesticides and related compounds in six binary solvent systems.
J. Ass. Offic. Agr. Chem. 48:943-52
Bowman, M.C. and Beroza, M. (1969) Determination of Accothion, its
oxygen analog, and its cresol in corn, grass and milk by gas
chromatography. J. Agr. Food Chem. 17:271-6
Bowman, M.C. (1969) Private communication
Braid, P.E. and Nix, M. (1968) Potentiation of toxicity of Sumithion
by phosphamidon in the rat. Canad. J. Physiol. Pharmacol. 46:145-9
Carshalton (1962) WHO insecticide evaluation and testing programme
- stage I. Mammalian toxicity report OMS 43=S5660, Test for neurotoxic
effects in hens. Unpub. Rept. from the Toxicology Research Unit,
Charshalton. Submitted by Farbenfabriken Bayer AG.
Carshalton. (1964) OMS 43. Summary. OMS 43. Mammalian toxicity.
Unpub. Rept. of the Toxicology Research Unit, Carshalton. Submitted
by Farbenfabriken Bayer AG.
Coffin, D.E. and Savary, G. (1964) Procedure for extraction and
cleanup of plant material prior to determination of organophosphate
residues. J. Ass. Offic. Agr. Chem. 47:875-81
Cooper. (1966) Toxicity of Sumithion. Unpub. Rept. of the Cooper
Technical Bureau. Submitted by Sumitomo Chemical Co., Ltd. Osaka
Cooper. (1968) Technical Bureau. Sumithion as a grain protectant.
Technical report by William Cooper and Nephews, Australia. Cited by
Sumitomo, 1969
Dawson, J.A., Donegan, L. and Thain, E.M. (1964) The determination of
parathion and related insecticides by gas-liquid chromatography
with special reference to fenitrothion residues in cocoa.
Analyst, 89:495-6
Douch, P.G.C., Hook, C.E.R. and Smith, J.N. (1968) Metabolism of
Folithion (dimethyl-4-nitro-3-methylphenyl phosphorothionate).
Australasian J. Pharm., 49: No.584. Suppl. Nr.66, 2.S.
DuBois, K.P. and Puchala, E. (1960) The acute toxicity and
anticholinesterase action of Bayer 41831. Unpub. Rept. from the
Department of Pharmacology, University or Chicago. Submitted by
Farbenfabriken Bayer AG.
DuBois, K.P. and Kinoshita, F. (1963) The acute toxicity of Bayer
41831 in combination with other anticholinesterase insecticides.
Unpub. Rept. from the Department of Pharmacology, University of
Chicago. Submitted by Farbenfabriken Bayer AG.
Fischer, R. (1968) Nachweis und quantitative Bestimmung von
Phosphor-Insektiziden in biologischem Material. III. Arch.
Toxicol., 23:129-35
Franz, J. and Kovac, J. (1965) Bestimmung toxischer Rückstände von
O,O-Dimethyl-O-(3-methyl-4-nitrophenyl)-thiophosphat in Milch. Z.
Anal. Chem. 210:354-8
Frehse, H. and Möllhoff, E. (1969) Organophosphorus insecticide
residue analysis. Evaluation. (A) Metabolic products. In report of
IUPAC Commission on the development, improvement, and standardization
of methods of pesticide residue analysis. Egan, H. J. Ass. Offic.
Anal. Chem. 52:306-9
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14:515-34
Green, A.A. and Tyler, P.S. (1966) A field comparison of malathion,
dichlorvos and fenitrothion for the control of Oryzaephilus
surinamensis (L) infesting stored barley. J. Stored Prod. Res.
1:273-85
Hais, K. and Franz, J. (1965) On the problem of Metathione residues
in milk after disinfestation. Cesk. Hyg. 10.205-8
Hladká, A. and Nosál, M. (1967) The determination of the exposition
to Metathion fenitrothion) on the basis of excreting its metabolite
p-nitro-m-cresol through urine in rats. Internat. Arch.
Gewerbepath. Gewerbehyg. 23:209-14
Hollingworth, R.M., Fukuto, T.R. and Metcalf, R.L. (1967) Selectivity
of Sumithion Compared with Methyl Parathion. Influence of structure
on anticholinesterase activity. Metabolism in the white mouse. J.
Agric. Food Chem., 15:235-49
Horler, D.F. (1966) Determination of fenitrothion on stored barley. J. Stored
Prod. Res. 1:287-90
Horler, D.F. (1968) Gel filtration on Sephadex LH-20. A general
clean-up method for pesticides extracted from grain. J. Sci. Food Agr.
19:229-31
Kane, J. and Green, A.A. (1968) The protection of bagged grain from
insect infestation using fenitrothion. J. Stored Prod. Res. 4 59.
Kimmerle, G. (1962a) Toxikologische Untersuchungen mit dem Wirkstoff
S 5660. Unpub. Rept. from Toxilkolisches und Gewerbehygienisches
Laboratorium. Submitted by Farbenfabriken Bayer AG.
Kimmerle, G. (1962b) S 5660. Unpub. Rept. on the neurotoxic effect
on hens from Toxikologisches und Gewerbehygienisches Laboratorium.
Submitted by Farbenfabriken Bayer AG.
Klimmer, O.R. (1961) Opinion on the toxicity of the compound "S 5660"
of Farbenfabriken Bayer AG., Leverkusen. Unpub. Rept. from
Pharmakologisches Institut der Rheinischen
Friedrich-Wilhelms-Universität, Bonn. Submitted by Farbenfabriken
Bayer AG.
Kovac, J. (1963) Bestimmung von
O,O-Dimethyl-O-(3-methyl-4-nitrophenyl)-thiophosphat in technischen
Produkten nach vorhergehender Abtrennung der Begleitstoffe mittels
Dünnschichtchromatographie. J. Chromatogr. 11:412-3
Kovac, J. and Sohler, E. (1965) Bestimmung von
O,O-Dimethyl-O-(3-methyl-4-nitrophenyl)-thiophosphat-Rückständen
in Obst und Gemüse nach vorangegangener Abtrennung der mitextrahierten
Farbstoffe durch Dünnschichtchromatographie. Z. Anal. Chem. 208.201-4
Mendoza, C.E., Wales, P.J., McLeod, H.A. and McKinley, W.P. (1968)
Thin-layer chromatographic-enzyme inhibition procedure to screen for
organophosphorus pesticides in plant extracts without elaborate
clean-up. Analyst 93:173-7
Misu, Y., Segawa, T., Kuruma, I., Kojima, M. and Takagi, H. (1966)
Subacute toxicity of O,O-dimethyl-O-(3-methyl-4-nitrophenyl)
phosphorothioate (Sumithion) in the rat. Toxicol. appl.
Pharmacol., 9:17-26
Miyamoto, J., Sato, Y., Kadota, T., Fujinami, A. and Endo, M. (1963a)
Studies on the mode of action of organophosphorus compounds. Part I.
Metabolic Fate of p32 labeled Sumithion and Methylparathion
in guinea-pig and white rat. Agric. Biol. Chem. (Tokyo), 27:381-9
Miyamoto, J., Sato, Y., Kadota, T. and Fujinami, A. (1963b) Studies
on the mode of action of organophosphorus compounds. Part II.
Inhibition of mammalian cholinesterase in vivo following the
administration of Sumithion and Methylparathion. Agric.
Biol. Chem. (Tokyo), 27:669-76
Miyamoto, J. (1964a) Studies on the mode of action of organophosphorus
compounds. Part III. Activation and degradation of Sumithion
and Methylparathion in vivo. Agric. Biol. Chem. (Tokyo),
28:411-21
Miyamoto, J. (1964b) Studies on the mode of action of organophosphorus
compounds. Part IV. Penetration of Sumithion, Methylparathion and
their oxygen analogs into guinea pig brain and inhibition of
cholinesterase in vivo. Agric. Biol. Chem. Tokyo), 28:422-30
Miyamoto, J. and Sato, Y. (1965) Determination of insecticide residue
in animal and plant tissues. II. Metabolic fate of Sumithion in rice
plant applied at the preheading stage and its residue in harvested
grains. Botyu-Kagaku, 30:45-9
Miyamoto, J., Kawaguchi, Y. and Sato, Y. (1965a) Determination of
insecticide residue in animal and plant tissues. I. Determination of
Sumithion residues in bananas grown in Formosa. Botyu-Kagaku, 30:9-12
Miyamoto, J., Sato, Y. and Fujikawa, K. (1965b) Determination of
insecticide residue in animal and plant tissues. III. Determination
of residual amount of Sumithion in cocoa beans grown in
Nigeria. Botyu-Kagaku 30:49-51
Miyamoto, J., Kitagawa, K. and Sato, Y. (1966) Metabolism of
organophosphorus insecticides by Bacillus subtilis, with
special emphasis on Sumithion. Jap. J. Exp. Med. 36:211-25
Miyamoto, J., Sato, Y. and Suzuki, S. (1967) Determination of
insecticide residue in animal and plant tissues. IV. Determination
of residual amount of Sumithion and some of its metabolites in fresh
milk. Botyu-Kagaku. 32:95-100
Miyamoto, J. (1969) Mechanism of low toxicity of Sumithion toward
mammals. Residue Reviews, 25:251-64
Miyamoto, J. and Sato, Y. (1969) Determination of insecticide residue
in animal and plant tissues. VI. Determination of Sumithion residue
in cattle tissues. Botyu Kagaku 34:3-6
Möllhoff, E. (1967) Gas chromatographic determination of residues of
E605 products and Agritox in plants and soil samples.
Pflanzenschutz-Nachrichten Bayer 20:557-74
Möllhoff, E. (1968) Beitrag zur Frage der Rückstände und ihrer
Bestimmung in Pflanzen nach Anwendung von Präparaten der E 605 - und
Agritox - Reihe. Pflanzenschutz-Nachrichten Bayer 21:331-58
Muramoto, N. (1967) Unpub. observations, cited by Sumitomo, 1969
Nishizawa, Y., Fujii, K., Kadota, T., Miyamoto, J. and Sakamoto, H.
(1961) Studies on the organophosphorus insecticides. Part VII.
Chemical and biological properties of new low toxic organophosphorus
insecticide. O,o-Dimethyl-O-(3-methyl-4-nitrophenyl) phosphorothioate.
Agric. Biol. Chem. (Japan), 25:605-10
Nosäl, M. and Hladkä, A. (1968) Determination of the exposure to
fenitrothion [O,O-dimethyl-O-(3-methyl-4-nitrophenyl) thiophosphate]
on the basis of the excretion of p-nitro-m-cresol by the urine
of the persons tested. Internat. Arch. Gewerbepath. Gewerbehyg.
25:28-38
Republic of Argentina (1968) - Unpub. Rept. prepared for Codex
Committee on Pesticide Residues
Ruzicka, J.H., Thomson, J., Wheals, B.B. and Wood, N.F. (1968) The
application of gel chromatography to the separation of
pesticides. I. Organophosphorus pesticides. J. Chromatogr.
34:14-20
Sato, Y., Miyamoto, J., and Suzuki, S. (1968) Determination of
insecticide residue in animal and plant tissues. V. A device to
increase the sensitivity of the gas chromatography detector to
organophosphorus insecticides. Bochu Kagaku 33:8-12
Schutzmann, R.L. and Barthel, W.F. (1969) Indoxyl acetate spray
reagent for fluorogenic detection of cholinesterase inhibitors in
environmental samples. J. Ass. Offic. Anal. Chem. 52:151-6
Sumitomo Chemical Company. (1969) Sumithion, Its toxicity, metabolism
and residues. Unpub. Rept.
Thier, H.P. and Bergner, K.G. (1966) Eine Schnellmethode zum Nachweis
wichtiger Schädlings-bekämpfungsmittel in Ost und Gemüse. Deutsche
Lebensmittel-Rundschau 62:399-402
Tomizawa, C. and Kobayashi, A. (1964) Relation between the behaviour
of parathion and Sumithion in cabbage leaves and their
phytotoxicity. Noyaku Seisan Gijutsu 11:30-32; Chem. Abstr. 62,
1387d (1965)
Ueda, K. and Nishimura, M. (1966) Interim report. Chronic toxicity of
Sumithion: two-year feeding study in rats. Unpub. Rept. from the
Department of Hygiene and Toxicology, Tokyo Dental College.
Submitted by Sumitomo Chemical Co., Ltd., Osaka.
Vandanis, A. and Crawford, L.G. (1964) Comparative metabolism of O,
O-dimethyl-O-p-nitrophenyl phosphorothioate (Methylparathion and
O, O-dimethyl-O-(3-methyl-1-nitrophenyl) phosphorothioate
(Sumithion) J. Econ. Entomol. 57:136-9
Vandekar, M. (1965) Observations on the toxicity of carbaryl,
Folithion and 3-isopropropyl-phenyl N-methylcarbamate in a
village-scale trial in Southern Nigeria. Bull. Wld. Hlth Org.
33.107-15
Wilford, K., Lietaert, P.E.A. and Foll, C.V. (1965) Toxicological
observations during large scale field trial of OMS-43 in Northern
Nigeria (preliminary report). WHO working paper 65/TOX/1 prepared
for an informal meeting on toxicology of insecticides. Geneva,
18-24 February 1965
Winterlin, W., Walker, G. and Frank, H. (1968) Detection of
cholinesterase-inhibiting pesticides following separation on
thin-layer chromatograms. J. Agr. Food Chem. 16:808-12
Yasuno, M., Hirakoshi, S. Sasa, M. and Uchida, M. (1965) Inactivation
of some organophosphorus insecticides by bacteria in polluted water.
Jap. J. Exp. Med. 35:545
Yuen, S.H. (1966) Absorptiometric determination of fenitrothion
residues in cocoa beans. Analyst 91:811-3
TABLE I
Percentage distribution of metabolites of fenitrothion in mouse urine after
administering various doses
Metabolite Percentage of radioactive metabolite excreted
in urine after giving P52 - labelled fenitrothion
at the indicated dose levels in mg/kg body-weight
3 17 200 850
Phosphoric acid 2.0 2.4 1.9 1.2
Methylphosphoric acid 1.5 2.5 2.4 1.1
Dimethylphosphoric acid 32.2 21.4 5.8 3.0
Dimethylphosphorothioic acid 12.8 20.3 8.7 6.6
Phosphate analogue (II) 2.7 1.6 3.3 2.5
O-demethylphosphate analogue (VI) 26.1 28.4 24.6 17.1
O-demethylphosphorothioate
analogue (V) 20.5 20.1 50.9 66.1
Unknown 2.2 2.5 2.4 2.4
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Hen
Three hens protected against acute anti-cholinesterase effects with
atropine and pralidoxime were given a single oral dose of 400 mg/kg
body-weight of fenitrothion. No symptoms of paralysis occurred during
an observation period of 28 days. Histologic examination of spinal
cord and sciatic nerves revealed no pathological lesions in two hens
and a few scattered degenerated fibres in the spinal cord of the third
(Carshalton, 1962).
Seven hens protected in the same way were given an oral dose of 250
mg/kg body-weight of fenitrothion and three other hens were given 500
mg/kg. Two of the 500 mg/kg group died within 1-2 days, while the
remaining eight hens did not show any sign of paralysis during an
observation period of six weeks (Kimmerle, 1962b).
Special studies on potentiation
Rat
Female rats were given intraperitoneal doses of a combination of
fenitrothion and the following organophosphorus compounds: parathion,
parathion-methyl, demeton, disulfoton, malathion, EPN,
azinphos-methyl, carbophenothion, mevinphos, dioxathion, schraden,
ethion, diazinon, Folex, coumaphos, and fenchlorphos as well as the
carbamate carbaryl. No sign of a potentiating effect was demonstrated
(DuBois and Kinoshita, 1963).
When male rats were given acute oral doses of mixtures of fenitrothion
and phosphamidon a marked potentiation of toxicity occurred as
evidenced by increased mortality. In female rats a potentiation of
toxicity occurred as evidenced by increased mortality. In female rats
a potentiation occurred only in mixtures containing relatively low
concentrations of fenitrothion, the potention effect diminishing as
the concentration of fenitrothion was increased. It was concluded that
potentiation is associated with a non-linear phosphorothioate
conversion (Braid and Nix, 1968).
Special studies on the metabolite fenitrooxon
The metabolite fenitrooxon is more toxic than the parent compound (cf.
Tables II and III).
TABLE II
Acute toxicity of fenitrooxon
Acute
Animal Route LD50 mg/kg References
body-weight
Mouse oral 90 Miyamoto, 1969
Mouse oral 120 Hollingworth et al., 1967
Rat oral 24 Miyamoto, 1969
Rat i.v. 3.3 Miyamoto, 1969
Guinea-pig oral 221 Miyamoto, 1969
Guinea-pig i.v. 32 Miyamoto, 1969
Acute toxicity
The symptoms of acute toxicity are the same as for other
organophosphorus compounds, doses close to the LD50 producing
symptoms which developed more rapidly after intravenous than after
oral administration. The compound is considerably less toxic to
mammals than its close structural analogue parathion-methyl (Miyamoto
et al., 1963b). Table II gives the LD50 in several species:
TABLE III
LD50 mg/kg
Animal Route body-weight References
Mouse (M) oral 1336 Carshalton, 1964
Mouse (F) oral 1416 Carshalton, 1964
Mouse (M) i.p. 115 DuBois and Puchala, 1960
Mouse (F) i.p. 110 DuBois and Puchala, 1960
Mouse i.v. 220 Miyamoto et al, 1963b
Rat (M) oral 740 Gaines, 1969
Rat (F) oral 570 Gaines, 1969
Rat (M) i.p. 135 DuBois and Puchala, 1960
Rat (M) i.p. 160 DuBois and Puchala, 1960
Rat i.v. 33 Miyamoto et al, 1963b
Guinea-pig (M) oral 500 DuBois and Puchala, 1960
Guinea-pig oral 1850 Miyamoto et al, 1963b
Guinea-pig (M) i.p. 110 DuBois and Puchala, 1960
Guinea-pig i.v. 112 Miyamoto et al, 1963b
Cat oral 142 Nishizawa et al, 1961
Short-term studies
Dog
In what was described as a preliminary test, groups of dogs, each
comprising one male and one female animal, were given daily oral doses
by capsule of 0, 2, 9 or 40 mg/kg body-weight of fenitrothion for
periods up to 98 days. Body-weights, blood biochemistry,
cholinesterase levels and haemograms were checked at intervals. At the
2 mg/kg level there was no effect with respect to any other of the
parameters mentioned. At 9 mg/kg a slight depression after 60 days and
at 40 mg/kg a moderate depression after 29 days occurred in whole
blood, plasma and red-cell cholinesterase. At 40 mg/kg there were also
marked toxic symptoms typical of cholinergic stimulation, and the dogs
in this group were sacrificed below the end of the 98-day period
(Cooper, 1966).
Rat
Groups of male rats (16 or 17 in number) were fed 0, 32, 63, 125, 250,
and 500 ppm of fenitrothion in the diet for 90 days. Mortality, food
intake, growth, general behaviour, urinalysis, average organ-weights
and histpathology were comparable to the controls in the groups fed
32, 63, 125, and 250 ppm. All the animals fed 500 ppm showed clinical
symptoms of anti-cholinesterase poisoning and there were minimal
symptoms in four animals in the 250 ppm group. In the 500 ppm group
the average organ-weights of the testes and brain were increased in
comparison with those of the control group. After interim sacrifice
every month of four rats from each group, measurement of the
cholinesterase activity of plasma, red cells, brain cortex, liver and
kidney showed a dose-dependent depression, the lowest being in the
brain. The cholinesterase activity in the 32 and 63 ppm groups
generally increased after 60 days of dosing to a level within the
normal limits, the beet recovery being in the plasma, kidney and
brain, less in the red cells and the liver (Misu et al., 1966).
Two groups, each of 20 male rats, were dosed by stomach tube on six
days a week for six months with 10 mg/kg and 11 mg/kg body-weight of
fenitrothion, respectively. During the first weeks the rats showed a
temporary deterioration of general condition and loss of weight.
Haematology and urinalysis during the experiment and gross and
microscopic pathology at its termination did not reveal any
abnormalities (Klimmer, 1961).
Male rats were given daily oral doses of 13 mg/kg body-weight of
fenitrothion for 28 days. Red-cell cholinesterase activity showed
severe depression, but there was recovery 30 days after withdrawal of
fenitrothion (Kimmerle, 1962a).
Male rats were fed 5, 10, and 20 ppm of fenitrothion in the diet for
an unspecified period. Brain and red cell cholinesterase activity was
normal in the 5 ppm group, whereas the 10 ppm group showed a slight
depression of red cell activity after five weeks with recovery two
weeks after withdrawal.
The 20 ppm group showed some depression of activity both in red cells
and in brain and the recovery in the brain remained incomplete two
weeks after withdrawal (Carshalton, 1964).
Other reported studies with fenitrothion in the rat lasting 90 days
indicate that the levels causing no appreciable effect on the
cholinesterase activity of plasma, red-cells and whole blood were 20
ppm in the diet and 10 ppm in drinking water. The only exception was a
moderate inhibition of the activity in plasma among the male animals
when given 10 ppm in drinking water. The above mentioned levels and
higher, namely 92.8 ppm in the diet and 46.2 ppm and 215 ppm in
drinking water, caused no effect on food or water intake, weight-gain,
average organ-weights, haemogram and blood biochemistry. However, 92.8
ppm of fenitrothion in the diet and 46.4 ppm in drinking water had a
moderate effect on whole blood and red-cell cholinesterase and a more
marked effect on plasma cholinesterase. The cholinesterase activity
recovered between 30-40 days after withdrawal of fenitrothion. The
levels of 430 ppm in the diet and 1000 ppm in drinking water caused a
depression of body-weight gains (Cooper, 1966).
Long-term studies
Rat
An interim report on what appears to be a two-year feeding study in
rats is available. Groups of nine or 10 male rats were fed 0, 25, 100
or 400 ppm of fenitrothion in their diet and were sacrificed after
feeding these levels for 63 weeks. As a positive control a group was
also fed 800 ppm of malathion. At the 400 ppm level of fenitrothion,
food intake and body-weight gain were increased and only a few animals
survived the 63 week period. At this level there was a 100 per cent
depression of red-cell cholinesterase. At the 100 ppm level a slight
(10-30 per cent) cholinesterase depression occurred in the brain and a
moderate depression (30-65 per cent) in the red blood cells and
plasma. At 25 ppm there was no effect on cholinesterase nor was there
any effect with regard to any other parameters evaluated (Ueda and
Nishimura, 1966).
OBSERVATIONS IN MAN
In a field spraying operation in Southern Nigeria including a village
using a five per cent spray of fenitrothion, examination of 18
villagers one week later did not reveal any clinical symptoms of
toxicity or plasma cholinesterase depression. The same was true of the
three spraymen examined on the first, second and sixth day after
spraying relative to a pre-spraying level (Vandekar, 1965).
In another field spraying trial in Northern Nigeria, 10,000 huts in
which about 16,500 people lived were sprayed. Field test
cholinesterase determinations on whole blood did not show any
appreciable difference in cholinesterase levels of 535 villagers
tasted before spraying and 299 villagers tested 5-30 days after
spraying. After one week of intensive spraying five out of 20 spraymen
developed a 50 per cent depression of cholinesterase which returned to
a stable level after a period of rest. One sprayman developed symptoms
of toxicity which lasted only a few hours and disappeared without
treatment (Wilford et al., 1965).
Fenitrothion was given to a total of 24 human subjects in single oral
doses of from 2.5 to 20 mg (0.042 to 0.33 mg/kg body-weight for a 60
kg man). The excretion of the metabolite, 3-methyl-4-nitrophenol, in
the urine was almost complete within 24 hours, the maximum excretion
occurring in the first 12 hours. The percentage of the dose excreted
during this time depended to a certain extent upon the size of the
dose administered, being about 70 per cent of theoretical after a
0.042 mg/kg dose and about 50 per cent after a 0.33 mg/kg dose. Both
plasma and cholinesterase activity were not depressed below normal
except possibly in one person given a 0.33 mg/kg dose, where some
depression of plasma cholinesterase was apparent after six and 24
hours (about 65 per cent of the pre-test level). When repeated doses
of 2.5 or 5 mg approximately 0.04 to 0.08 mg/kg) were given to five
individuals, four times at 24 hour intervals, most of the nitrocresol
metabolite appeared in the urine within the interval 0 to 12 hours
after administration. After receiving the third and fourth dose there
was a trend towards a rise in red cell cholinesterase activity but in
no cases was there any evidence of reduction below normal levels of
the activity of this enzyme in either plasma or red cells (Nosal and
Hladka, 1968).
COMMENT
Adequate information is available on the toxicity, biochemistry and
metabolism of fenitrothion, in three species of rodent. Information is
also available in man including field spraying studies and a
metabolism study. Only an interim report comprising a 63-week study in
rats in available and there are no 1-2 year studies in a non-rodent
mammalian species. The short-term studies in the rat, using
cholinesterase inhibition criteria, provide a no-effect level. No
reproduction or teratogenicity studies are available and in view of
the similarity in the structure of fenitrothion to parathion-methyl,
it is important that such studies be undertaken. For this reason and
because no adequate long-term studies are available it was decided to
give only a temporary acceptable daily intake to this compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 ppm in the diet, equivalent to 0.25 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenitrothion is a broad-spectrum insecticide having a much lower acute
mammalian toxicity than many similar organophosphorus insecticides.
Its action is by direct contact or an a stomach poison. It can be
applied as an emulsifiable concentrate, wettable powder, granular
formulation or dust.
It is toxic to bees and should not be sprayed on flowering crops.
Fenitrothion can be combined with all conventional insecticides and
fungicides except those having an alkaline reaction.
The insecticide has been used throughout Europe, East Pakistan, East
Africa, United Arab Republic, Japan, Republic of China, New Zealand,
Canada, and Brazil. It is not registered for use in the United States.
Pre-harvest treatments
Fenitrothion is used on a wide variety of crops including fruits,
field crops, vegetables, rice, cotton, cereals, cocoa, tea, and coffee
for control of stem borers, hoppers, leaf miners, leaf rollers,
whiteflies, fruit flies, mealybugs, mirids and bugs, thrips, aphids,
mites, lady beetles, caterpillars, and soft scale insects.
The recommended concentrations and rates of application vary for the
different crops and pest species to be controlled. Concentrations of
sprays range from 0.05 to 0.1%. active ingredient (a.i.) and rates of
application from 0.5 to 2.0 kg a.i./hectare. The chemical is generally
tolerated by most crops although high dosages may injure cotton, and
phytotoxicity on Brassica crops and orchard fruits has been
encountered.
Safety intervals prior to harvest range from 10 to 21 days and differ
depending on the country and the crop.
Post-harvest treatments
No post-harvest treatments are made in Europe. Admixture of 1 to 5 ppm
of fenitrothion has been recommended to protect grains such as rice,
wheat, and barley for months against various weevils and beetles. Bag
treatment is acceptable in Brazil for protecting stored grains and
post-harvest treatments are under development in various countries.
Other uses
Fenitrothion is used to control grasshoppers, locusts, and
caterpillars on pastures and spruce budworm in forests. It also
provides control of household and other pests, such as mosquitoes and
their larvae, houseflies, cockroaches, lice, bedbugs, and poultry
mites. Its action is rapid, and its residual activity is good.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Typical maximum residues after treatment of a variety of crops are
given in Table IV.
TABLE IV
Maximum residues after treatment of crops
Maximum
Dosage residues at
active Pre-harvest harvest, ppm
Crop ingredient interval, days respectively Reference
Rice 1000 g/ha 41 0.005 Sumitomo 1969
Rice 0.07% spray or Miyamoto, Sato
5% granules 1-2 months n.d. 1965
Apple 0.05% spray 21 0.05 Sumitomo 1969
Apple 0.2% spray 7 and 14 0.5 and 0.35 Bayer 1969
Apple, Golden 0.15% spray 10 and 15 1.0 and 0.75 Bayer 1969
cherry 0.2% spray 7 and 14 0.5 and 0.2 Bayer 1969
Grape* 0.05% spray 10 0.50 Sumitomo 1969
Tomato* 0.05% spray 7 0.18 Sumitomo 1969
Cocoa 0.1% spray 14 0.10 Miyamoto et al
1965b
Red cabbage 0.15%; 1000 7 Outside leaves
1/ha 0.65
Cabbage less
outside leaves
0.25 Bayer 1969
Sugar beets 0.15%; 1000 8 n.d. Bayer 1969
1/ha
TABLE IV (cont'd)
Maximum residues after treatment of crops
Maximum
Dosage residues at
active Pre-harvest harvest, ppm
Crop ingredient interval, days respectively Reference
Green tea 0.1% spray 14 0.27 Sumitomo 1969
Cauliflower 0.1%; 1000 7 n.d. Bayer 1969
1/ha
Lettuce 0.1%; 1000 7 and 14 1.05 and 0.3 Bayer 1969
1/ha
Lettuce 0.05%; 600 1, 7, and 14 0.65, 0.06, Möllhoff 1968
1/ha 0.01
Peas and pod 0.25%; 560 0 and 5 0.7 and <0.15 Bayer 1969
1/ha
*These green-house trials gave residues higher than those of field
trials.
FATE OF RESIDUES
General comments
Fenitrothion may be metabolized as shown in Figure 1. The major route
in animals appears to be through splitting of the P-O-aryl bond to
give the corresponding dimethyl esters of phosphorothioic and
phosphoric acids. Another means of degradation is demethylation of the
methoxy group to give desmethyl fenitrothion. Although formation of
the oxygen analogue, fenitrooxon, is minor compared to products formed
via hydrolysis, fenitrooxon must be taken into account because the
mammalian toxicity of oxons is generally much higher than that of
parent thiono pesticides. In plants the metabolism of fenitrothion
appears to be similar to that in animals.
In animals
Orally administered 32P-labelled fenitrothion was readily absorbed
from the digestive tract of guinea pigs or rats and the major portion
of the radioactivity excreted in the urine. Neither fenitrothion nor
fenitrooxon was detected and desmethyl fenitrothion, dimethyl
phosphorothioic and dimethyl phosphoric acids were identified in the
urine (Miyamoto et al., 1963a).
Following intravenous injection of radioactive 32P fenitrothion into
guinea pigs and rats, fenitrothion rapidly disappeared from the blood.
Fenitrothion and fenitrooxon were found in the tissues, and their
amounts decreased rapidly. The desmethyl compound and the dimethyl
esters mentioned in the foregoing paragraph were found mostly in the
liver and kidneys (Miyamoto, 1964a).
Excretion of metabolic products is rapid and chiefly in the form of
3-methyl-4-nitrophenol (the nitrocresol hydrolysis product) (Hladka
and Nosal, 1967; Nosal and Hladka, 1968); the cresol methyl may be
oxidized to COOH (Douch et al., 1968). The desmethyl compounds are
also excreted (Hollingworth et al., 1967; Miyamoto et al., 1963b).
Between 60 and 90% of the insecticide is excreted within two days,
chiefly in the aforementioned forms. Only up to 10% is excreted in the
feces. Detoxication via the desmethyl compounds is dose-dependent.
After oral administration of up to 40 grams of fenitrothion per
lactating cow, residues in the milk were as high as 0.4 ppm after 6
hours and below the limit of detection after one day (Hais and Franz,
1965). Detoxication in bovine rumens is rapid owing to reduction of
fenitrothion to the amino compound (Miyamoto et al., 1967).
Cows fed 3 mg/kg body weight of fenitrothion for seven consecutive
days produced milk having up to 0.002 ppm residue of fenitrothion on
the second day, and no residue one day after administration was
stopped. Less than 0.003 ppm aminofenitrothion and about 0.1 ppm
p-nitrocresol were detected during treatment, and no fenitrooxon was
found (Miyamoto et al., 1967).
Thirty calves (1-1.5 yr. av. wt. 243 kg) confined on a pasture sprayed
with 375 g/ha of fenitrothion (11.8 ppm initial residue on grass) were
periodically sacrificed and breast muscle and omental fat analysed. On
the first day residues in the meat and fat were about 0.01 ppm. No
residue of fenitrothion was found in the meat from the third day on
and only 0.004-0.007 ppm was found in the fat on the third day; these
amounts decreased almost to control levels by the seventh day
(Republic of Argentina, 1968; Miyamoto and Sato, 1969).
Lactating dairy cattle were fed 50 ppm of fenitrothion in the feed
(dry basis) for 29 days. No residue of fenitrothion, fenitrooxon, or
the cresol appeared in the milk. A maximum of 0.006 ppm of
aminofenitrothion was found (Bowman, 1969).
In plants
About 50% of 32P-labelled fenitrothion sprayed on rice plants
penetrated into the tissues in 24 hours; at the end of this period
only 10% was left, indicating rapid decomposition. Some fenitrooxon
formed but it disappeared from the tissues more rapidly than
fenitrothion. Rice grains harvested 46 days after treatment contained
0.0007 ppm fenitrothion and less than 1 ppm of p-nitrocresol and
dimethyl phosphorothioic acid (Miyamoto and Sato, 1965).
Fenitrothion does not appear to have much systemic action. After
treatment of rice plants with fenitrothion, more residue is found in
the bran than in the polished grains (Miyamato and Sato, 1965). Very
little active ingredient passed from peel to fruit in stored bananas
(Miyamoto et al., 1965a).
The half-life of fenitrothion in green plants ranges between the
values established for parathion and parathion-methyl, i.e. between
one and two days; the half-life of the oxon is estimated to be only a
few hours (Möllhoff, 1968).
Although the oxon may form in plants it occurs only during the first
few days after treatment and in proportions (ca. 1% smaller than those
in animals (Miyamoto et al., 1963; Miyamoto and Sato 1965; Möllhoff,
1968). Desmethyl compounds occur only in minor amounts in plants.
Phytotoxicity of fenitrothion on cabbage was ascribed to high
penetration of the insecticide and accumulation of the cresol
hydrolysis product (Tomizawa and Kobayashi, 1964).
In soil
The soil bacteria Bacillus subtilis, converted more than half of
radiolabelled 32P fenitrothion to the amino analogue under aerobic
conditions at 37°C in 24 hours. The desmethyl derivatives of
fenitrothion and aminofenitrothion as well as dimethyl phosphorothioic
acid formed. None of the oxon or oxons of the degradation products
were found (Miyamoto et al., 1966).
Fenitrothion is gradually inactivated by other bacterial species
including gram-positive and gram-negative rods, but not by fungi and
yeasts (Yasuno et al., 1965).
Fenitrothion is readily absorbed onto various kinds of soil and
decomposed by alkalinity or microorganisms (Muramoto, 1967).
Persistence in soil does not appear to be great.
In storage and processing
Fenitrothion shows promise for control of grain insects in storage.
Applied at 2 and 1 ppm to barley, it decreased to 0.4 ppm after 15
weeks' storage (Green and Tyler, 1966). On barley in silos the
half-life of fenitrothion was about 100 days (Green and Tyler, 1966)
and on stored bananas about 15 days (Miyamoto et al., 1965a). Applied
to wheat 11% moisture) at rates of 1, 2 and 4 ppm, 0.2, 0.4., and 1.1
ppm of fenitrothion remained, respectively, after 9 months of storage
at 25°C and 60% relative humidity (Kane and Green, 1968).
In extensive trials as a wheat protectant, fenitrothion levels
steadied below 2 ppm after about 2 months regardless of application
rate between 2.5 and 10 ppm. In one trial 6 ppm applied to grain
decreased to 2.4, 1.7, and 1.1 ppm, respectively, after 1´, 4, and 6
months. Flour made from the 2.4-ppm sample contained 0.3 ppm
fenitrothion, while only traces were found in the flour and bread from
the later samples (Cooper Technical Bureau, 1968).
Evidence of residues in food in commerce or at consumption
No report of residues of fenitrothion in food in commerce or at
consumption has been found. Reference is made under "In storage and
processing" to the finding of only "traces" of fenitrothion in flour
and bread prepared from grain containing 1.7 and 1.1 ppm of the
insecticide.
METHODS OF RESIDUE ANALYSIS
Metabolic studies on plants and mammals indicate that residues of
fenitrooxon and aminofenitrooxon, when they do form, occur in small
amount and are degraded or excreted more rapidly than the parent
compound. Once pilot experiments establish that no more than traces of
these compounds form on a given crop, there does not seem to be any
need to analyse for these compounds on that crop in commerce. (For
purposes of this discussion, a trace shall be considered less than 10%
of the tolerance level of the parent compound).
Analyses for the p-nitrocresol have been made and measurable amounts
of the compound are found following good agricultural practice. A
determination of the cresol is sometimes reported. However, if a pilot
experiment on a given crop shows no excessive amount of the cresol to
be present at harvest, there does not appear to be any need for its
analysis on that crop in commerce. Other metabolites are generally
rather polar and do not tend to be stored.
In essence then, the analyst will be concerned with residues of
fenitrothion itself unless pilot experiments indicate that other
metabolites should be taken into account. This same view is expressed
by Frehse and Möllhoff in a IUPAC report by Egan (1969).
Fenitrothion in so similar to parathion and methyl parathion that
analytical methods used for them may be used for fenitrothion.
However, many of the earlier methods lack specificity. Numerous
procedures for a wide variety of harvest products are based on
thin-layer chromatography, spectroscopy and gas chromatography.
Information relating to these analyses follow. No reference to
inter-laboratory collaborative studies on analytical procedures was
found.
Extraction and partitioning
It is not sufficient simply to wash the analytical samples since small
amounts of the active ingredient penetrate into the plant. It is
therefore important that complete extraction of the insecticide and
metabolites (e.g. by exhaustive Soxhlet extraction) be compared
against the extraction method used. Harvest products with a high
content of water have been macerated with acetone (Horler, 1966;
Möllhoff, 1967, 1968), acetonitrile (Coffin and Savary, 1964; Thier
and Bergner, 1966), or ethanol (Fischer, 1968). Very good recoveries
were obtained by Soxhlet extraction with chloroform-methanol (9:1 v/v)
(Bowman and Beroza, 1969). For milk, a combination of polar and
nonpolar extractants is required, e.g. acetone-methylene chloride
(Bowman and Beroza, 1969), ethanol-hexane (Franz and Kovac, 1965).
Extraction with methanol-acetonitrile also proved suitable for milk
samples (Miyamoto et al., 1967). For oil-containing harvest products
with a low water content, use is made of benzene (Dawson et al., 1964;
Horler, 1966), petroleum ether (Kovac and Sohler, 1965), hexane
(Horler, 1966), or chloroform (Yuen, 1966). On account of the
favourable partition coefficients (Bowman and Beroza, 1965; Kovac,
1963), fats and waxes are best removed by partitioning between hexane
and acetonitrile (Franz and Kovac, 1965; Miyamoto et al., 1967;
Möllhoff, 1967; Bowman and Beroza, 1969).
Qualitative analysis
Although no reports of fenitrothion being separated from similar
residues by column chromatography have been noted, separation is
possible with thin-layer or gas chromatography. Interfering active
ingredients are parathion, parathion-methyl, malathion, and fenthion
and the resolution is just sufficient for distinguishing fenitrothion
from these active ingredients (see e.g. Möllhoff, 1968). By comparing
gas chromatograms recorded with a phosphorus detector and an electron
capture detector, fenitrothion can often be clearly differentiated
from malathion and fenthion owing to differences in sensitivity.
Colorimetry
Colorimetric determinations are carried out by the method of Averell
and Norris (1948) or by determination of the cresol after
saponification of the active ingredient with alkali. The limits of
determination are about 0.05 ppm.
Thin-layer chromatography
Thin-layer chromatography has been used for clean-up of the extracts
with final determination by colorimetry after deleting the appropriate
spot from the plate, or for direct determination on the plate after
spraying. Quantitative evaluation is possible in the first instance;
in the second, the evaluation is semiquantitative and more suitable
for confirming identity. About 0.1 ppm of fenitrothion can be
determined in most harvested products. Determination of lesser amounts
is possible with procedures utilizing cholinesterase inhibition
(Ackermann, 1966; Mendoza et al., 1968; Schutzmann and Barthel, 1969;
Winterlin et al., 1968).
Column chromatography
For extract cleanup or separation of metabolites, columns are
suitable, especially those packed with deactivated silica gel (Bowman
and Beroza, 1969), deactivated Florisil (Möllhoff, 1967, 1968), or
deactivated acid aluminum oxide (Horler, 1966). Columns packed with
active Florisil (Beckman and Garber, 1969), magnesium oxide (Coffin
and Savary, 1964), polyethylene-impregnated aluminum oxide (Coffin and
Savary, 1964), and Sephadex LH-20 (Horler, 1968; Ruzicka, et al.,
1968) have also been used.
Gas chromatography
The most reliable and highly sensitive methods for fenitrothion
utilize gas chromatography with either the flame-photometric (Bowman
and Beroza, 1969) or the thermionic detector (Miyamoto et al., 1967,
Sato et al., 1968). These detectors respond to the phosphorus in the
molecule with very high specificity. No cleanup is usually required
with the flame-photometric detector when analysing for either
fenitrothion or its oxygen analogue unless a fatty food is being
analysed. In this case a simple hexane-acetonitrile partition is used.
A cleanup was used with the thermionic detector but it may often be
omitted. Limit of determination is usually 0.01-0.001 ppm.
In the analysis for fenitrooxon a separation from fenitrothion on
silica gel is used. The compound is then determined by gas
chromatography (Bowman and Beroza, 1969) or enzymatically (Miyamoto et
al., 1967). The cresol is determined by electron-capture gas
chromatography (Bowman and Beroza, 1969) or colorimetrically (Miyamoto
et al., 1967) after column separation. Analysis of fenitrothion,
fenitrooxon, and the cresol by electron-capture gas chromatography is
possible (Bowman and Beroza, 1969; Dawson et al., 1964; Horler, 1966;
Möllhoff, 1967) but less specific; sensitivity, about 0.1 ppm, can be
improved with a suitable cleanup.
Determination of metabolites
Gas chromatographic methods have been described for the simultaneous
determination of fenitrothion, fenitrooxon (Bowman and Beroza, 1969;
Möllhoff, 1968), 3-methyl-4-nitro phenol (Bowman and Beroza, 1969;
Miyamoto et al., 1967) and the amino compound of fenitrothion
(Miyamoto et al., 1967). As noted, for market controls determination
of fenitrothion is generally sufficient (Möllhoff, 1968).
NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Tolerance Withholding
Country Crop (ppm) period (days)
Austria 2.0 proposed
Belgium Top fruits and vegetables 0.5 14
China, Rice - 21
Republic of Vegetables - 10
Denmark Beets, apples, peas, clover - 15
Tolerance Withholding
Country Crop (ppm) period (days)
East Africa Mango, cereals - 7
Germany, Field crops, vegetables, fruits, and - 10
Eastern special crops
Crops for Infant food and remedies - 21
Germany Vegetables, root and field crops, 0.4 10
(Fed. Rep.) legumes, fruits, grapes
Finland - 21
France Vegetables, fruits - 15
Italy - 15
Netherlands Cereals, fruits 0.5 21
New Zealand - 14 (7 for
pasture)
Poland Top fruits (except strawberries and - 14
raspberries)
Portugal Vegetables, citrus, fruits, cocoa, coffee - 21
Pasture grass - 10
Spain Vegetables, citrus, fruits - 15-21
Sweden Peas, rape, wheat, barley - 10
Switzerland Top fruits 0.2 in
combination
with
Amidithion
APPRAISAL
Fenitrothion is a broad spectrum insecticide with a much lower acute
mammalian toxicity than many similar insecticides. It is not covered
by patents and is produced by at least six companies. Use of
fenitrothion is almost worldwide (excluding the U.S.A.) for such crops
as rice, fruits, vegetables, cotton, cereals, soybeans, coffee and
tea. Application rates are 0.5-2.0 kg a.i./ha. Waiting intervals are
generally 10-21 days. It is used against spruce budworm (in forests),
insects attacking man, and in some countries to protect stored
products. Its use on pasture land (375 g/ha) results in almost no
residues of fenitrothion in milk or meat (<0.01 ppm). The insecticide
is available as a 95% concentrate, 50% emulsifiable concentrate, 40%
wettable powder, 2.5 and 5% dusts. Composition of technical materials
is not known and is apt to vary depending on manufacturer. Residue
data are available mostly from Europe and Japan.
Although several metabolites of fenitrothion are known to form
(fenitrooxon, desmethyl fenitrothion, aminofenitrothion, and
3-methyl-4-nitrophenol), they do not accumulate in significant amounts
or they appear to be comparatively non-toxic. Unless experimental
trials indicate otherwise, fenitrothion appears to be the only toxic
residue to be determined in products in commerce.
Many methods are available for residue analysis of fenitrothion. The
best of these utilize gas chromatography with the flame photometric or
thermionic detector. Some of these methods may also be used for
analysis of metabolites. Sensitivity is usually better than 0.01 ppm.
Evaluation of these procedures for regulatory purposes is suggested.
Few data on residues in food in commerce or in diets have been
reported.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCE OR
PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to June 1973)
Apples, cherries, grapes, lettuce 0.5 ppm ) Preharvest interval to
) be such that these
Tomatoes 0.2 ppm ) tolerances are not
) exceeded)
Red cabbage, green tea 0.3 ppm
Cocoa 0.1 ppm
The 3-methyl-4-nitrophenol is not included in the recommendations at
this time but the Joint Meeting should review this aspect in due
course.
PRACTICAL RESIDUE LIMITS
Milk (whole) 0.002
Meat or fat 0.03
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Reproduction and teratogenicity studies in animals preferably in
non-human primates.
2. Adequate long-term studies in rodent and non-rodent mammalian
species.
3. Data on residue levels in raw agricultural commodities moving in
commerce and in total diet studies.
4. Data on disappearance of residues during storage, processing and
cooking.
5. Data on rate of residue decline in rice and pre-harvest interval.
6. Before use as a grain protectant, data on persistence of residues
in storage of the grains concerned and definitive data on residues
in bread are needed.
7. Data on occurrence of 4-nitro-3-methylphenol residues and their
significance toxicologically.
8. Information on ingredients in technical products produced by
several manufacturers.
DESIRABLE
1. Observations in man.
2. Evaluation of gas chromatographic methods for regulatory purposes.
REFERENCES
Ackermann, H. (1966) Enzymatischer Nachweis phosphororganischer
Insektizide nach dünnschicht-chromatographischer Trennung. Nahrung,
10:273-4
Averell, P.R. and Norris, M.V. (1948) Estimation of small amounts of
O,O-diethyl-O,p-nitro-phenyl thiophosphate. Anal. Chem. 20:753-6
Bayer (1969) (Farbenfabriken Bayer AG.). Fenitrothion. Unpub. Rept.
Beckman, H. and Garber, D. (1969) Recovery of 65 organophosphorus
pesticides from Florisil with a new solvent elution system. J. Ass.
Offic. Anal. Chem. 52:286-93
Bowman, M.C. and Beroza, M. (1965) Extraction p-values of
pesticides and related compounds in six binary solvent systems.
J. Ass. Offic. Agr. Chem. 48:943-52
Bowman, M.C. and Beroza, M. (1969) Determination of Accothion, its
oxygen analog, and its cresol in corn, grass and milk by gas
chromatography. J. Agr. Food Chem. 17:271-6
Bowman, M.C. (1969) Private communication
Braid, P.E. and Nix, M. (1968) Potentiation of toxicity of Sumithion
by phosphamidon in the rat. Canad. J. Physiol. Pharmacol. 46:145-9
Carshalton (1962) WHO insecticide evaluation and testing programme
- stage I. Mammalian toxicity report OMS 43=S5660, Test for neurotoxic
effects in hens. Unpub. Rept. from the Toxicology Research Unit,
Charshalton. Submitted by Farbenfabriken Bayer AG.
Carshalton. (1964) OMS 43. Summary. OMS 43. Mammalian toxicity.
Unpub. Rept. of the Toxicology Research Unit, Carshalton. Submitted
by Farbenfabriken Bayer AG.
Coffin, D.E. and Savary, G. (1964) Procedure for extraction and
cleanup of plant material prior to determination of organophosphate
residues. J. Ass. Offic. Agr. Chem. 47:875-81
Cooper. (1966) Toxicity of Sumithion. Unpub. Rept. of the Cooper
Technical Bureau. Submitted by Sumitomo Chemical Co., Ltd. Osaka
Cooper. (1968) Technical Bureau. Sumithion as a grain protectant.
Technical report by William Cooper and Nephews, Australia. Cited by
Sumitomo, 1969
Dawson, J.A., Donegan, L. and Thain, E.M. (1964) The determination of
parathion and related insecticides by gas-liquid chromatography
with special reference to fenitrothion residues in cocoa.
Analyst, 89:495-6
Douch, P.G.C., Hook, C.E.R. and Smith, J.N. (1968) Metabolism of
Folithion (dimethyl-4-nitro-3-methylphenyl phosphorothionate).
Australasian J. Pharm., 49: No.584. Suppl. Nr.66, 2.S.
DuBois, K.P. and Puchala, E. (1960) The acute toxicity and
anticholinesterase action of Bayer 41831. Unpub. Rept. from the
Department of Pharmacology, University or Chicago. Submitted by
Farbenfabriken Bayer AG.
DuBois, K.P. and Kinoshita, F. (1963) The acute toxicity of Bayer
41831 in combination with other anticholinesterase insecticides.
Unpub. Rept. from the Department of Pharmacology, University of
Chicago. Submitted by Farbenfabriken Bayer AG.
Fischer, R. (1968) Nachweis und quantitative Bestimmung von
Phosphor-Insektiziden in biologischem Material. III. Arch.
Toxicol., 23:129-35
Franz, J. and Kovac, J. (1965) Bestimmung toxischer Rückstände von
O,O-Dimethyl-O-(3-methyl-4-nitrophenyl)-thiophosphat in Milch. Z.
Anal. Chem. 210:354-8
Frehse, H. and Möllhoff, E. (1969) Organophosphorus insecticide
residue analysis. Evaluation. (A) Metabolic products. In report of
IUPAC Commission on the development, improvement, and standardization
of methods of pesticide residue analysis. Egan, H. J. Ass. Offic.
Anal. Chem. 52:306-9
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14:515-34
Green, A.A. and Tyler, P.S. (1966) A field comparison of malathion,
dichlorvos and fenitrothion for the control of Oryzaephilus
surinamensis (L) infesting stored barley. J. Stored Prod. Res.
1:273-85
Hais, K. and Franz, J. (1965) On the problem of Metathione residues
in milk after disinfestation. Cesk. Hyg. 10.205-8
Hladká, A. and Nosál, M. (1967) The determination of the exposition
to Metathion fenitrothion) on the basis of excreting its metabolite
p-nitro-m-cresol through urine in rats. Internat. Arch.
Gewerbepath. Gewerbehyg. 23:209-14
Hollingworth, R.M., Fukuto, T.R. and Metcalf, R.L. (1967) Selectivity
of Sumithion Compared with Methyl Parathion. Influence of structure
on anticholinesterase activity. Metabolism in the white mouse. J.
Agric. Food Chem., 15:235-49
Horler, D.F. (1966) Determination of fenitrothion on stored barley. J. Stored
Prod. Res. 1:287-90
Horler, D.F. (1968) Gel filtration on Sephadex LH-20. A general
clean-up method for pesticides extracted from grain. J. Sci. Food Agr.
19:229-31
Kane, J. and Green, A.A. (1968) The protection of bagged grain from
insect infestation using fenitrothion. J. Stored Prod. Res. 4 59.
Kimmerle, G. (1962a) Toxikologische Untersuchungen mit dem Wirkstoff
S 5660. Unpub. Rept. from Toxilkolisches und Gewerbehygienisches
Laboratorium. Submitted by Farbenfabriken Bayer AG.
Kimmerle, G. (1962b) S 5660. Unpub. Rept. on the neurotoxic effect
on hens from Toxikologisches und Gewerbehygienisches Laboratorium.
Submitted by Farbenfabriken Bayer AG.
Klimmer, O.R. (1961) Opinion on the toxicity of the compound "S 5660"
of Farbenfabriken Bayer AG., Leverkusen. Unpub. Rept. from
Pharmakologisches Institut der Rheinischen
Friedrich-Wilhelms-Universität, Bonn. Submitted by Farbenfabriken
Bayer AG.
Kovac, J. (1963) Bestimmung von
O,O-Dimethyl-O-(3-methyl-4-nitrophenyl)-thiophosphat in technischen
Produkten nach vorhergehender Abtrennung der Begleitstoffe mittels
Dünnschichtchromatographie. J. Chromatogr. 11:412-3
Kovac, J. and Sohler, E. (1965) Bestimmung von
O,O-Dimethyl-O-(3-methyl-4-nitrophenyl)-thiophosphat-Rückständen
in Obst und Gemüse nach vorangegangener Abtrennung der mitextrahierten
Farbstoffe durch Dünnschichtchromatographie. Z. Anal. Chem. 208.201-4
Mendoza, C.E., Wales, P.J., McLeod, H.A. and McKinley, W.P. (1968)
Thin-layer chromatographic-enzyme inhibition procedure to screen for
organophosphorus pesticides in plant extracts without elaborate
clean-up. Analyst 93:173-7
Misu, Y., Segawa, T., Kuruma, I., Kojima, M. and Takagi, H. (1966)
Subacute toxicity of O,O-dimethyl-O-(3-methyl-4-nitrophenyl)
phosphorothioate (Sumithion) in the rat. Toxicol. appl.
Pharmacol., 9:17-26
Miyamoto, J., Sato, Y., Kadota, T., Fujinami, A. and Endo, M. (1963a)
Studies on the mode of action of organophosphorus compounds. Part I.
Metabolic Fate of p32 labeled Sumithion and Methylparathion
in guinea-pig and white rat. Agric. Biol. Chem. (Tokyo), 27:381-9
Miyamoto, J., Sato, Y., Kadota, T. and Fujinami, A. (1963b) Studies
on the mode of action of organophosphorus compounds. Part II.
Inhibition of mammalian cholinesterase in vivo following the
administration of Sumithion and Methylparathion. Agric.
Biol. Chem. (Tokyo), 27:669-76
Miyamoto, J. (1964a) Studies on the mode of action of organophosphorus
compounds. Part III. Activation and degradation of Sumithion
and Methylparathion in vivo. Agric. Biol. Chem. (Tokyo),
28:411-21
Miyamoto, J. (1964b) Studies on the mode of action of organophosphorus
compounds. Part IV. Penetration of Sumithion, Methylparathion and
their oxygen analogs into guinea pig brain and inhibition of
cholinesterase in vivo. Agric. Biol. Chem. Tokyo), 28:422-30
Miyamoto, J. and Sato, Y. (1965) Determination of insecticide residue
in animal and plant tissues. II. Metabolic fate of Sumithion in rice
plant applied at the preheading stage and its residue in harvested
grains. Botyu-Kagaku, 30:45-9
Miyamoto, J., Kawaguchi, Y. and Sato, Y. (1965a) Determination of
insecticide residue in animal and plant tissues. I. Determination of
Sumithion residues in bananas grown in Formosa. Botyu-Kagaku, 30:9-12
Miyamoto, J., Sato, Y. and Fujikawa, K. (1965b) Determination of
insecticide residue in animal and plant tissues. III. Determination
of residual amount of Sumithion in cocoa beans grown in
Nigeria. Botyu-Kagaku 30:49-51
Miyamoto, J., Kitagawa, K. and Sato, Y. (1966) Metabolism of
organophosphorus insecticides by Bacillus subtilis, with
special emphasis on Sumithion. Jap. J. Exp. Med. 36:211-25
Miyamoto, J., Sato, Y. and Suzuki, S. (1967) Determination of
insecticide residue in animal and plant tissues. IV. Determination
of residual amount of Sumithion and some of its metabolites in fresh
milk. Botyu-Kagaku. 32:95-100
Miyamoto, J. (1969) Mechanism of low toxicity of Sumithion toward
mammals. Residue Reviews, 25:251-64
Miyamoto, J. and Sato, Y. (1969) Determination of insecticide residue
in animal and plant tissues. VI. Determination of Sumithion residue
in cattle tissues. Botyu Kagaku 34:3-6
Möllhoff, E. (1967) Gas chromatographic determination of residues of
E605 products and Agritox in plants and soil samples.
Pflanzenschutz-Nachrichten Bayer 20:557-74
Möllhoff, E. (1968) Beitrag zur Frage der Rückstände und ihrer
Bestimmung in Pflanzen nach Anwendung von Präparaten der E 605 - und
Agritox - Reihe. Pflanzenschutz-Nachrichten Bayer 21:331-58
Muramoto, N. (1967) Unpub. observations, cited by Sumitomo, 1969
Nishizawa, Y., Fujii, K., Kadota, T., Miyamoto, J. and Sakamoto, H.
(1961) Studies on the organophosphorus insecticides. Part VII.
Chemical and biological properties of new low toxic organophosphorus
insecticide. O,o-Dimethyl-O-(3-methyl-4-nitrophenyl) phosphorothioate.
Agric. Biol. Chem. (Japan), 25:605-10
Nosäl, M. and Hladkä, A. (1968) Determination of the exposure to
fenitrothion [O,O-dimethyl-O-(3-methyl-4-nitrophenyl) thiophosphate]
on the basis of the excretion of p-nitro-m-cresol by the urine
of the persons tested. Internat. Arch. Gewerbepath. Gewerbehyg.
25:28-38
Republic of Argentina (1968) - Unpub. Rept. prepared for Codex
Committee on Pesticide Residues
Ruzicka, J.H., Thomson, J., Wheals, B.B. and Wood, N.F. (1968) The
application of gel chromatography to the separation of
pesticides. I. Organophosphorus pesticides. J. Chromatogr.
34:14-20
Sato, Y., Miyamoto, J., and Suzuki, S. (1968) Determination of
insecticide residue in animal and plant tissues. V. A device to
increase the sensitivity of the gas chromatography detector to
organophosphorus insecticides. Bochu Kagaku 33:8-12
Schutzmann, R.L. and Barthel, W.F. (1969) Indoxyl acetate spray
reagent for fluorogenic detection of cholinesterase inhibitors in
environmental samples. J. Ass. Offic. Anal. Chem. 52:151-6
Sumitomo Chemical Company. (1969) Sumithion, Its toxicity, metabolism
and residues. Unpub. Rept.
Thier, H.P. and Bergner, K.G. (1966) Eine Schnellmethode zum Nachweis
wichtiger Schädlings-bekämpfungsmittel in Ost und Gemüse. Deutsche
Lebensmittel-Rundschau 62:399-402
Tomizawa, C. and Kobayashi, A. (1964) Relation between the behaviour
of parathion and Sumithion in cabbage leaves and their
phytotoxicity. Noyaku Seisan Gijutsu 11:30-32; Chem. Abstr. 62,
1387d (1965)
Ueda, K. and Nishimura, M. (1966) Interim report. Chronic toxicity of
Sumithion: two-year feeding study in rats. Unpub. Rept. from the
Department of Hygiene and Toxicology, Tokyo Dental College.
Submitted by Sumitomo Chemical Co., Ltd., Osaka.
Vandanis, A. and Crawford, L.G. (1964) Comparative metabolism of O,
O-dimethyl-O-p-nitrophenyl phosphorothioate (Methylparathion and
O, O-dimethyl-O-(3-methyl-1-nitrophenyl) phosphorothioate
(Sumithion) J. Econ. Entomol. 57:136-9
Vandekar, M. (1965) Observations on the toxicity of carbaryl,
Folithion and 3-isopropropyl-phenyl N-methylcarbamate in a
village-scale trial in Southern Nigeria. Bull. Wld. Hlth Org.
33.107-15
Wilford, K., Lietaert, P.E.A. and Foll, C.V. (1965) Toxicological
observations during large scale field trial of OMS-43 in Northern
Nigeria (preliminary report). WHO working paper 65/TOX/1 prepared
for an informal meeting on toxicology of insecticides. Geneva,
18-24 February 1965
Winterlin, W., Walker, G. and Frank, H. (1968) Detection of
cholinesterase-inhibiting pesticides following separation on
thin-layer chromatograms. J. Agr. Food Chem. 16:808-12
Yasuno, M., Hirakoshi, S. Sasa, M. and Uchida, M. (1965) Inactivation
of some organophosphorus insecticides by bacteria in polluted water.
Jap. J. Exp. Med. 35:545
Yuen, S.H. (1966) Absorptiometric determination of fenitrothion
residues in cocoa beans. Analyst 91:811-3
 Other relevant chemical properties
Properties of fenitrothion - melting point 0.3°C, boiling point
109°C/0.1 mm Hg; d420 1.3084; yellowish-brown liquid with unpleasant
odour; soluble in alcohols, ethers, ketones, aromatic hydrocarbons;
insoluble in water; completely stable for 2 years, at 20-25°C; storage
temperature should not exceed 40°C; unstable in alkaline media.
Formulations - 95% concentrate, 50% emulsifiable concentrate, 40%
wettable powder, 2.5% and 5% dust. Deburol(R) (Ciba) contains 15%
fenitrothion and 62% mineral oil. One company indicates that their 95%
concentrate contains 95% fenitrothion (minimum) and 0.5%
3-methyl-4-nitrophenol (maximum).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Various comprehensive studies in mouse, rat, and guinea-pig have dealt
with the pharmacodynamic and biochemical aspects of fenitrothion and
its metabolites (Nishizawa et al., 1961; Miyamoto, 1964a, 1964b, 1969;
Miyamoto et al., 1963a; Vandanis and Crawford 1964; Hollingworth et
al., 1967 Hladká and Nosál, 1967 and Douch et al., 1968).
Fenitrothion is presumably rapidly absorbed from the mammalian
intestinal tract as evidenced by the appearance of radioactivity in
blood from guinea-pigs and rats administered phosphorus32 -labelled
fenitrothion orally. The presence of the oxygen analogue was
demonstrated in all tissues examined (brain, heart, lung, liver,
kidney, spleen and muscle) and it was detectable in blood one minute
after an intravenous injection of fenitrothion. This oxygen analogue
(II of Fig. 1) is the important metabolite with respect to toxicity.
It is formed in the microsomal fraction of the cell, the main organs
responsible for the transformation being the liver and kidney.
Fenitrooxon is further metabolized as indicated in Fig. 1. The major
excretion product found is 3-methyl-4-nitrophenol (VII) which can
further be oxidized to 3-carboxy-4-nitrophenol (VIII). Other
metabolites are the demethyl-derivatives (V and VI) which, with
increasing doses, are excreted in increasing amounts. A total of nine
metabolites has been isolated, the majority of which can also be
identified. In vitro studies showed that formation of the oxygen
analogue (II) was dependent on the availability of reduced nicotine
adenine dinucleotide phosphate (NADPH2) and oxygen. Liver slices
incubated with fenitrothion did not produce measurable amounts of
fenitrooxon, while liver homogenates and the supernatant fraction of
such homogenates showed appreciable activation of added fenitrothion.
No correlation between the toxicity and rate of formation of the
oxygen analogue could, however, be demonstrated (Miyamoto et al.,
1963a; Miyamoto, 1969).
No observations are available in these studies on the distribution
into fatty tissue: however, residue studies on milk, meat and fat from
cattle indicate amounts of approximately 0.001 ppm in these samples
(Miyamoto and Sato, 1969).
Fenitrothion and its metabolites are excreted mainly in the urine
(90-95 per cent). Up to 10 per cent was recovered in faeces. Within
three days nearly complete recovery of an orally administered dose (15
mg/kg) could be obtained. The metabolic, pattern of fenitrothion as it
appears from these studies is shown in Fig. 1. The ratios between the
amounts of metabolites was dependent upon the dose given, as is shown
in Table 1, which gives the percentage distribution of metabolites in
mouse urine after various doses of fenitrothion (Hollingworth et al.,
1967).
Effect on enzymes and other biochemical parameters
As with other organophosphorous compounds fenitrothion acts in the
animal organism as a cholinesterase inhibitor, probably after
conversion to the oxygen-analogue. Some evidence presented indicates
that the cholinesterase inhibiting effect in brain depends more on the
rate of penetration into the brain than on the rate of oxidation and
decomposition of fenitrothion (Miyamoto, 1969).
Other relevant chemical properties
Properties of fenitrothion - melting point 0.3°C, boiling point
109°C/0.1 mm Hg; d420 1.3084; yellowish-brown liquid with unpleasant
odour; soluble in alcohols, ethers, ketones, aromatic hydrocarbons;
insoluble in water; completely stable for 2 years, at 20-25°C; storage
temperature should not exceed 40°C; unstable in alkaline media.
Formulations - 95% concentrate, 50% emulsifiable concentrate, 40%
wettable powder, 2.5% and 5% dust. Deburol(R) (Ciba) contains 15%
fenitrothion and 62% mineral oil. One company indicates that their 95%
concentrate contains 95% fenitrothion (minimum) and 0.5%
3-methyl-4-nitrophenol (maximum).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Various comprehensive studies in mouse, rat, and guinea-pig have dealt
with the pharmacodynamic and biochemical aspects of fenitrothion and
its metabolites (Nishizawa et al., 1961; Miyamoto, 1964a, 1964b, 1969;
Miyamoto et al., 1963a; Vandanis and Crawford 1964; Hollingworth et
al., 1967 Hladká and Nosál, 1967 and Douch et al., 1968).
Fenitrothion is presumably rapidly absorbed from the mammalian
intestinal tract as evidenced by the appearance of radioactivity in
blood from guinea-pigs and rats administered phosphorus32 -labelled
fenitrothion orally. The presence of the oxygen analogue was
demonstrated in all tissues examined (brain, heart, lung, liver,
kidney, spleen and muscle) and it was detectable in blood one minute
after an intravenous injection of fenitrothion. This oxygen analogue
(II of Fig. 1) is the important metabolite with respect to toxicity.
It is formed in the microsomal fraction of the cell, the main organs
responsible for the transformation being the liver and kidney.
Fenitrooxon is further metabolized as indicated in Fig. 1. The major
excretion product found is 3-methyl-4-nitrophenol (VII) which can
further be oxidized to 3-carboxy-4-nitrophenol (VIII). Other
metabolites are the demethyl-derivatives (V and VI) which, with
increasing doses, are excreted in increasing amounts. A total of nine
metabolites has been isolated, the majority of which can also be
identified. In vitro studies showed that formation of the oxygen
analogue (II) was dependent on the availability of reduced nicotine
adenine dinucleotide phosphate (NADPH2) and oxygen. Liver slices
incubated with fenitrothion did not produce measurable amounts of
fenitrooxon, while liver homogenates and the supernatant fraction of
such homogenates showed appreciable activation of added fenitrothion.
No correlation between the toxicity and rate of formation of the
oxygen analogue could, however, be demonstrated (Miyamoto et al.,
1963a; Miyamoto, 1969).
No observations are available in these studies on the distribution
into fatty tissue: however, residue studies on milk, meat and fat from
cattle indicate amounts of approximately 0.001 ppm in these samples
(Miyamoto and Sato, 1969).
Fenitrothion and its metabolites are excreted mainly in the urine
(90-95 per cent). Up to 10 per cent was recovered in faeces. Within
three days nearly complete recovery of an orally administered dose (15
mg/kg) could be obtained. The metabolic, pattern of fenitrothion as it
appears from these studies is shown in Fig. 1. The ratios between the
amounts of metabolites was dependent upon the dose given, as is shown
in Table 1, which gives the percentage distribution of metabolites in
mouse urine after various doses of fenitrothion (Hollingworth et al.,
1967).
Effect on enzymes and other biochemical parameters
As with other organophosphorous compounds fenitrothion acts in the
animal organism as a cholinesterase inhibitor, probably after
conversion to the oxygen-analogue. Some evidence presented indicates
that the cholinesterase inhibiting effect in brain depends more on the
rate of penetration into the brain than on the rate of oxidation and
decomposition of fenitrothion (Miyamoto, 1969).
 TABLE I
Percentage distribution of metabolites of fenitrothion in mouse urine after
administering various doses
Metabolite Percentage of radioactive metabolite excreted
in urine after giving P52 - labelled fenitrothion
at the indicated dose levels in mg/kg body-weight
3 17 200 850
Phosphoric acid 2.0 2.4 1.9 1.2
Methylphosphoric acid 1.5 2.5 2.4 1.1
Dimethylphosphoric acid 32.2 21.4 5.8 3.0
Dimethylphosphorothioic acid 12.8 20.3 8.7 6.6
Phosphate analogue (II) 2.7 1.6 3.3 2.5
O-demethylphosphate analogue (VI) 26.1 28.4 24.6 17.1
O-demethylphosphorothioate
analogue (V) 20.5 20.1 50.9 66.1
Unknown 2.2 2.5 2.4 2.4
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Hen
Three hens protected against acute anti-cholinesterase effects with
atropine and pralidoxime were given a single oral dose of 400 mg/kg
body-weight of fenitrothion. No symptoms of paralysis occurred during
an observation period of 28 days. Histologic examination of spinal
cord and sciatic nerves revealed no pathological lesions in two hens
and a few scattered degenerated fibres in the spinal cord of the third
(Carshalton, 1962).
Seven hens protected in the same way were given an oral dose of 250
mg/kg body-weight of fenitrothion and three other hens were given 500
mg/kg. Two of the 500 mg/kg group died within 1-2 days, while the
remaining eight hens did not show any sign of paralysis during an
observation period of six weeks (Kimmerle, 1962b).
Special studies on potentiation
Rat
Female rats were given intraperitoneal doses of a combination of
fenitrothion and the following organophosphorus compounds: parathion,
parathion-methyl, demeton, disulfoton, malathion, EPN,
azinphos-methyl, carbophenothion, mevinphos, dioxathion, schraden,
ethion, diazinon, Folex, coumaphos, and fenchlorphos as well as the
carbamate carbaryl. No sign of a potentiating effect was demonstrated
(DuBois and Kinoshita, 1963).
When male rats were given acute oral doses of mixtures of fenitrothion
and phosphamidon a marked potentiation of toxicity occurred as
evidenced by increased mortality. In female rats a potentiation of
toxicity occurred as evidenced by increased mortality. In female rats
a potentiation occurred only in mixtures containing relatively low
concentrations of fenitrothion, the potention effect diminishing as
the concentration of fenitrothion was increased. It was concluded that
potentiation is associated with a non-linear phosphorothioate
conversion (Braid and Nix, 1968).
Special studies on the metabolite fenitrooxon
The metabolite fenitrooxon is more toxic than the parent compound (cf.
Tables II and III).
TABLE II
Acute toxicity of fenitrooxon
Acute
Animal Route LD50 mg/kg References
body-weight
Mouse oral 90 Miyamoto, 1969
Mouse oral 120 Hollingworth et al., 1967
Rat oral 24 Miyamoto, 1969
Rat i.v. 3.3 Miyamoto, 1969
Guinea-pig oral 221 Miyamoto, 1969
Guinea-pig i.v. 32 Miyamoto, 1969
Acute toxicity
The symptoms of acute toxicity are the same as for other
organophosphorus compounds, doses close to the LD50 producing
symptoms which developed more rapidly after intravenous than after
oral administration. The compound is considerably less toxic to
mammals than its close structural analogue parathion-methyl (Miyamoto
et al., 1963b). Table II gives the LD50 in several species:
TABLE III
LD50 mg/kg
Animal Route body-weight References
Mouse (M) oral 1336 Carshalton, 1964
Mouse (F) oral 1416 Carshalton, 1964
Mouse (M) i.p. 115 DuBois and Puchala, 1960
Mouse (F) i.p. 110 DuBois and Puchala, 1960
Mouse i.v. 220 Miyamoto et al, 1963b
Rat (M) oral 740 Gaines, 1969
Rat (F) oral 570 Gaines, 1969
Rat (M) i.p. 135 DuBois and Puchala, 1960
Rat (M) i.p. 160 DuBois and Puchala, 1960
Rat i.v. 33 Miyamoto et al, 1963b
Guinea-pig (M) oral 500 DuBois and Puchala, 1960
Guinea-pig oral 1850 Miyamoto et al, 1963b
Guinea-pig (M) i.p. 110 DuBois and Puchala, 1960
Guinea-pig i.v. 112 Miyamoto et al, 1963b
Cat oral 142 Nishizawa et al, 1961
Short-term studies
Dog
In what was described as a preliminary test, groups of dogs, each
comprising one male and one female animal, were given daily oral doses
by capsule of 0, 2, 9 or 40 mg/kg body-weight of fenitrothion for
periods up to 98 days. Body-weights, blood biochemistry,
cholinesterase levels and haemograms were checked at intervals. At the
2 mg/kg level there was no effect with respect to any other of the
parameters mentioned. At 9 mg/kg a slight depression after 60 days and
at 40 mg/kg a moderate depression after 29 days occurred in whole
blood, plasma and red-cell cholinesterase. At 40 mg/kg there were also
marked toxic symptoms typical of cholinergic stimulation, and the dogs
in this group were sacrificed below the end of the 98-day period
(Cooper, 1966).
Rat
Groups of male rats (16 or 17 in number) were fed 0, 32, 63, 125, 250,
and 500 ppm of fenitrothion in the diet for 90 days. Mortality, food
intake, growth, general behaviour, urinalysis, average organ-weights
and histpathology were comparable to the controls in the groups fed
32, 63, 125, and 250 ppm. All the animals fed 500 ppm showed clinical
symptoms of anti-cholinesterase poisoning and there were minimal
symptoms in four animals in the 250 ppm group. In the 500 ppm group
the average organ-weights of the testes and brain were increased in
comparison with those of the control group. After interim sacrifice
every month of four rats from each group, measurement of the
cholinesterase activity of plasma, red cells, brain cortex, liver and
kidney showed a dose-dependent depression, the lowest being in the
brain. The cholinesterase activity in the 32 and 63 ppm groups
generally increased after 60 days of dosing to a level within the
normal limits, the beet recovery being in the plasma, kidney and
brain, less in the red cells and the liver (Misu et al., 1966).
Two groups, each of 20 male rats, were dosed by stomach tube on six
days a week for six months with 10 mg/kg and 11 mg/kg body-weight of
fenitrothion, respectively. During the first weeks the rats showed a
temporary deterioration of general condition and loss of weight.
Haematology and urinalysis during the experiment and gross and
microscopic pathology at its termination did not reveal any
abnormalities (Klimmer, 1961).
Male rats were given daily oral doses of 13 mg/kg body-weight of
fenitrothion for 28 days. Red-cell cholinesterase activity showed
severe depression, but there was recovery 30 days after withdrawal of
fenitrothion (Kimmerle, 1962a).
Male rats were fed 5, 10, and 20 ppm of fenitrothion in the diet for
an unspecified period. Brain and red cell cholinesterase activity was
normal in the 5 ppm group, whereas the 10 ppm group showed a slight
depression of red cell activity after five weeks with recovery two
weeks after withdrawal.
The 20 ppm group showed some depression of activity both in red cells
and in brain and the recovery in the brain remained incomplete two
weeks after withdrawal (Carshalton, 1964).
Other reported studies with fenitrothion in the rat lasting 90 days
indicate that the levels causing no appreciable effect on the
cholinesterase activity of plasma, red-cells and whole blood were 20
ppm in the diet and 10 ppm in drinking water. The only exception was a
moderate inhibition of the activity in plasma among the male animals
when given 10 ppm in drinking water. The above mentioned levels and
higher, namely 92.8 ppm in the diet and 46.2 ppm and 215 ppm in
drinking water, caused no effect on food or water intake, weight-gain,
average organ-weights, haemogram and blood biochemistry. However, 92.8
ppm of fenitrothion in the diet and 46.4 ppm in drinking water had a
moderate effect on whole blood and red-cell cholinesterase and a more
marked effect on plasma cholinesterase. The cholinesterase activity
recovered between 30-40 days after withdrawal of fenitrothion. The
levels of 430 ppm in the diet and 1000 ppm in drinking water caused a
depression of body-weight gains (Cooper, 1966).
Long-term studies
Rat
An interim report on what appears to be a two-year feeding study in
rats is available. Groups of nine or 10 male rats were fed 0, 25, 100
or 400 ppm of fenitrothion in their diet and were sacrificed after
feeding these levels for 63 weeks. As a positive control a group was
also fed 800 ppm of malathion. At the 400 ppm level of fenitrothion,
food intake and body-weight gain were increased and only a few animals
survived the 63 week period. At this level there was a 100 per cent
depression of red-cell cholinesterase. At the 100 ppm level a slight
(10-30 per cent) cholinesterase depression occurred in the brain and a
moderate depression (30-65 per cent) in the red blood cells and
plasma. At 25 ppm there was no effect on cholinesterase nor was there
any effect with regard to any other parameters evaluated (Ueda and
Nishimura, 1966).
OBSERVATIONS IN MAN
In a field spraying operation in Southern Nigeria including a village
using a five per cent spray of fenitrothion, examination of 18
villagers one week later did not reveal any clinical symptoms of
toxicity or plasma cholinesterase depression. The same was true of the
three spraymen examined on the first, second and sixth day after
spraying relative to a pre-spraying level (Vandekar, 1965).
In another field spraying trial in Northern Nigeria, 10,000 huts in
which about 16,500 people lived were sprayed. Field test
cholinesterase determinations on whole blood did not show any
appreciable difference in cholinesterase levels of 535 villagers
tasted before spraying and 299 villagers tested 5-30 days after
spraying. After one week of intensive spraying five out of 20 spraymen
developed a 50 per cent depression of cholinesterase which returned to
a stable level after a period of rest. One sprayman developed symptoms
of toxicity which lasted only a few hours and disappeared without
treatment (Wilford et al., 1965).
Fenitrothion was given to a total of 24 human subjects in single oral
doses of from 2.5 to 20 mg (0.042 to 0.33 mg/kg body-weight for a 60
kg man). The excretion of the metabolite, 3-methyl-4-nitrophenol, in
the urine was almost complete within 24 hours, the maximum excretion
occurring in the first 12 hours. The percentage of the dose excreted
during this time depended to a certain extent upon the size of the
dose administered, being about 70 per cent of theoretical after a
0.042 mg/kg dose and about 50 per cent after a 0.33 mg/kg dose. Both
plasma and cholinesterase activity were not depressed below normal
except possibly in one person given a 0.33 mg/kg dose, where some
depression of plasma cholinesterase was apparent after six and 24
hours (about 65 per cent of the pre-test level). When repeated doses
of 2.5 or 5 mg approximately 0.04 to 0.08 mg/kg) were given to five
individuals, four times at 24 hour intervals, most of the nitrocresol
metabolite appeared in the urine within the interval 0 to 12 hours
after administration. After receiving the third and fourth dose there
was a trend towards a rise in red cell cholinesterase activity but in
no cases was there any evidence of reduction below normal levels of
the activity of this enzyme in either plasma or red cells (Nosal and
Hladka, 1968).
COMMENT
Adequate information is available on the toxicity, biochemistry and
metabolism of fenitrothion, in three species of rodent. Information is
also available in man including field spraying studies and a
metabolism study. Only an interim report comprising a 63-week study in
rats in available and there are no 1-2 year studies in a non-rodent
mammalian species. The short-term studies in the rat, using
cholinesterase inhibition criteria, provide a no-effect level. No
reproduction or teratogenicity studies are available and in view of
the similarity in the structure of fenitrothion to parathion-methyl,
it is important that such studies be undertaken. For this reason and
because no adequate long-term studies are available it was decided to
give only a temporary acceptable daily intake to this compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 ppm in the diet, equivalent to 0.25 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenitrothion is a broad-spectrum insecticide having a much lower acute
mammalian toxicity than many similar organophosphorus insecticides.
Its action is by direct contact or an a stomach poison. It can be
applied as an emulsifiable concentrate, wettable powder, granular
formulation or dust.
It is toxic to bees and should not be sprayed on flowering crops.
Fenitrothion can be combined with all conventional insecticides and
fungicides except those having an alkaline reaction.
The insecticide has been used throughout Europe, East Pakistan, East
Africa, United Arab Republic, Japan, Republic of China, New Zealand,
Canada, and Brazil. It is not registered for use in the United States.
Pre-harvest treatments
Fenitrothion is used on a wide variety of crops including fruits,
field crops, vegetables, rice, cotton, cereals, cocoa, tea, and coffee
for control of stem borers, hoppers, leaf miners, leaf rollers,
whiteflies, fruit flies, mealybugs, mirids and bugs, thrips, aphids,
mites, lady beetles, caterpillars, and soft scale insects.
The recommended concentrations and rates of application vary for the
different crops and pest species to be controlled. Concentrations of
sprays range from 0.05 to 0.1%. active ingredient (a.i.) and rates of
application from 0.5 to 2.0 kg a.i./hectare. The chemical is generally
tolerated by most crops although high dosages may injure cotton, and
phytotoxicity on Brassica crops and orchard fruits has been
encountered.
Safety intervals prior to harvest range from 10 to 21 days and differ
depending on the country and the crop.
Post-harvest treatments
No post-harvest treatments are made in Europe. Admixture of 1 to 5 ppm
of fenitrothion has been recommended to protect grains such as rice,
wheat, and barley for months against various weevils and beetles. Bag
treatment is acceptable in Brazil for protecting stored grains and
post-harvest treatments are under development in various countries.
Other uses
Fenitrothion is used to control grasshoppers, locusts, and
caterpillars on pastures and spruce budworm in forests. It also
provides control of household and other pests, such as mosquitoes and
their larvae, houseflies, cockroaches, lice, bedbugs, and poultry
mites. Its action is rapid, and its residual activity is good.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Typical maximum residues after treatment of a variety of crops are
given in Table IV.
TABLE IV
Maximum residues after treatment of crops
Maximum
Dosage residues at
active Pre-harvest harvest, ppm
Crop ingredient interval, days respectively Reference
Rice 1000 g/ha 41 0.005 Sumitomo 1969
Rice 0.07% spray or Miyamoto, Sato
5% granules 1-2 months n.d. 1965
Apple 0.05% spray 21 0.05 Sumitomo 1969
Apple 0.2% spray 7 and 14 0.5 and 0.35 Bayer 1969
Apple, Golden 0.15% spray 10 and 15 1.0 and 0.75 Bayer 1969
cherry 0.2% spray 7 and 14 0.5 and 0.2 Bayer 1969
Grape* 0.05% spray 10 0.50 Sumitomo 1969
Tomato* 0.05% spray 7 0.18 Sumitomo 1969
Cocoa 0.1% spray 14 0.10 Miyamoto et al
1965b
Red cabbage 0.15%; 1000 7 Outside leaves
1/ha 0.65
Cabbage less
outside leaves
0.25 Bayer 1969
Sugar beets 0.15%; 1000 8 n.d. Bayer 1969
1/ha
TABLE IV (cont'd)
Maximum residues after treatment of crops
Maximum
Dosage residues at
active Pre-harvest harvest, ppm
Crop ingredient interval, days respectively Reference
Green tea 0.1% spray 14 0.27 Sumitomo 1969
Cauliflower 0.1%; 1000 7 n.d. Bayer 1969
1/ha
Lettuce 0.1%; 1000 7 and 14 1.05 and 0.3 Bayer 1969
1/ha
Lettuce 0.05%; 600 1, 7, and 14 0.65, 0.06, Möllhoff 1968
1/ha 0.01
Peas and pod 0.25%; 560 0 and 5 0.7 and <0.15 Bayer 1969
1/ha
*These green-house trials gave residues higher than those of field
trials.
FATE OF RESIDUES
General comments
Fenitrothion may be metabolized as shown in Figure 1. The major route
in animals appears to be through splitting of the P-O-aryl bond to
give the corresponding dimethyl esters of phosphorothioic and
phosphoric acids. Another means of degradation is demethylation of the
methoxy group to give desmethyl fenitrothion. Although formation of
the oxygen analogue, fenitrooxon, is minor compared to products formed
via hydrolysis, fenitrooxon must be taken into account because the
mammalian toxicity of oxons is generally much higher than that of
parent thiono pesticides. In plants the metabolism of fenitrothion
appears to be similar to that in animals.
In animals
Orally administered 32P-labelled fenitrothion was readily absorbed
from the digestive tract of guinea pigs or rats and the major portion
of the radioactivity excreted in the urine. Neither fenitrothion nor
fenitrooxon was detected and desmethyl fenitrothion, dimethyl
phosphorothioic and dimethyl phosphoric acids were identified in the
urine (Miyamoto et al., 1963a).
Following intravenous injection of radioactive 32P fenitrothion into
guinea pigs and rats, fenitrothion rapidly disappeared from the blood.
Fenitrothion and fenitrooxon were found in the tissues, and their
amounts decreased rapidly. The desmethyl compound and the dimethyl
esters mentioned in the foregoing paragraph were found mostly in the
liver and kidneys (Miyamoto, 1964a).
Excretion of metabolic products is rapid and chiefly in the form of
3-methyl-4-nitrophenol (the nitrocresol hydrolysis product) (Hladka
and Nosal, 1967; Nosal and Hladka, 1968); the cresol methyl may be
oxidized to COOH (Douch et al., 1968). The desmethyl compounds are
also excreted (Hollingworth et al., 1967; Miyamoto et al., 1963b).
Between 60 and 90% of the insecticide is excreted within two days,
chiefly in the aforementioned forms. Only up to 10% is excreted in the
feces. Detoxication via the desmethyl compounds is dose-dependent.
After oral administration of up to 40 grams of fenitrothion per
lactating cow, residues in the milk were as high as 0.4 ppm after 6
hours and below the limit of detection after one day (Hais and Franz,
1965). Detoxication in bovine rumens is rapid owing to reduction of
fenitrothion to the amino compound (Miyamoto et al., 1967).
Cows fed 3 mg/kg body weight of fenitrothion for seven consecutive
days produced milk having up to 0.002 ppm residue of fenitrothion on
the second day, and no residue one day after administration was
stopped. Less than 0.003 ppm aminofenitrothion and about 0.1 ppm
p-nitrocresol were detected during treatment, and no fenitrooxon was
found (Miyamoto et al., 1967).
Thirty calves (1-1.5 yr. av. wt. 243 kg) confined on a pasture sprayed
with 375 g/ha of fenitrothion (11.8 ppm initial residue on grass) were
periodically sacrificed and breast muscle and omental fat analysed. On
the first day residues in the meat and fat were about 0.01 ppm. No
residue of fenitrothion was found in the meat from the third day on
and only 0.004-0.007 ppm was found in the fat on the third day; these
amounts decreased almost to control levels by the seventh day
(Republic of Argentina, 1968; Miyamoto and Sato, 1969).
Lactating dairy cattle were fed 50 ppm of fenitrothion in the feed
(dry basis) for 29 days. No residue of fenitrothion, fenitrooxon, or
the cresol appeared in the milk. A maximum of 0.006 ppm of
aminofenitrothion was found (Bowman, 1969).
In plants
About 50% of 32P-labelled fenitrothion sprayed on rice plants
penetrated into the tissues in 24 hours; at the end of this period
only 10% was left, indicating rapid decomposition. Some fenitrooxon
formed but it disappeared from the tissues more rapidly than
fenitrothion. Rice grains harvested 46 days after treatment contained
0.0007 ppm fenitrothion and less than 1 ppm of p-nitrocresol and
dimethyl phosphorothioic acid (Miyamoto and Sato, 1965).
Fenitrothion does not appear to have much systemic action. After
treatment of rice plants with fenitrothion, more residue is found in
the bran than in the polished grains (Miyamato and Sato, 1965). Very
little active ingredient passed from peel to fruit in stored bananas
(Miyamoto et al., 1965a).
The half-life of fenitrothion in green plants ranges between the
values established for parathion and parathion-methyl, i.e. between
one and two days; the half-life of the oxon is estimated to be only a
few hours (Möllhoff, 1968).
Although the oxon may form in plants it occurs only during the first
few days after treatment and in proportions (ca. 1% smaller than those
in animals (Miyamoto et al., 1963; Miyamoto and Sato 1965; Möllhoff,
1968). Desmethyl compounds occur only in minor amounts in plants.
Phytotoxicity of fenitrothion on cabbage was ascribed to high
penetration of the insecticide and accumulation of the cresol
hydrolysis product (Tomizawa and Kobayashi, 1964).
In soil
The soil bacteria Bacillus subtilis, converted more than half of
radiolabelled 32P fenitrothion to the amino analogue under aerobic
conditions at 37°C in 24 hours. The desmethyl derivatives of
fenitrothion and aminofenitrothion as well as dimethyl phosphorothioic
acid formed. None of the oxon or oxons of the degradation products
were found (Miyamoto et al., 1966).
Fenitrothion is gradually inactivated by other bacterial species
including gram-positive and gram-negative rods, but not by fungi and
yeasts (Yasuno et al., 1965).
Fenitrothion is readily absorbed onto various kinds of soil and
decomposed by alkalinity or microorganisms (Muramoto, 1967).
Persistence in soil does not appear to be great.
In storage and processing
Fenitrothion shows promise for control of grain insects in storage.
Applied at 2 and 1 ppm to barley, it decreased to 0.4 ppm after 15
weeks' storage (Green and Tyler, 1966). On barley in silos the
half-life of fenitrothion was about 100 days (Green and Tyler, 1966)
and on stored bananas about 15 days (Miyamoto et al., 1965a). Applied
to wheat 11% moisture) at rates of 1, 2 and 4 ppm, 0.2, 0.4., and 1.1
ppm of fenitrothion remained, respectively, after 9 months of storage
at 25°C and 60% relative humidity (Kane and Green, 1968).
In extensive trials as a wheat protectant, fenitrothion levels
steadied below 2 ppm after about 2 months regardless of application
rate between 2.5 and 10 ppm. In one trial 6 ppm applied to grain
decreased to 2.4, 1.7, and 1.1 ppm, respectively, after 1´, 4, and 6
months. Flour made from the 2.4-ppm sample contained 0.3 ppm
fenitrothion, while only traces were found in the flour and bread from
the later samples (Cooper Technical Bureau, 1968).
Evidence of residues in food in commerce or at consumption
No report of residues of fenitrothion in food in commerce or at
consumption has been found. Reference is made under "In storage and
processing" to the finding of only "traces" of fenitrothion in flour
and bread prepared from grain containing 1.7 and 1.1 ppm of the
insecticide.
METHODS OF RESIDUE ANALYSIS
Metabolic studies on plants and mammals indicate that residues of
fenitrooxon and aminofenitrooxon, when they do form, occur in small
amount and are degraded or excreted more rapidly than the parent
compound. Once pilot experiments establish that no more than traces of
these compounds form on a given crop, there does not seem to be any
need to analyse for these compounds on that crop in commerce. (For
purposes of this discussion, a trace shall be considered less than 10%
of the tolerance level of the parent compound).
Analyses for the p-nitrocresol have been made and measurable amounts
of the compound are found following good agricultural practice. A
determination of the cresol is sometimes reported. However, if a pilot
experiment on a given crop shows no excessive amount of the cresol to
be present at harvest, there does not appear to be any need for its
analysis on that crop in commerce. Other metabolites are generally
rather polar and do not tend to be stored.
In essence then, the analyst will be concerned with residues of
fenitrothion itself unless pilot experiments indicate that other
metabolites should be taken into account. This same view is expressed
by Frehse and Möllhoff in a IUPAC report by Egan (1969).
Fenitrothion in so similar to parathion and methyl parathion that
analytical methods used for them may be used for fenitrothion.
However, many of the earlier methods lack specificity. Numerous
procedures for a wide variety of harvest products are based on
thin-layer chromatography, spectroscopy and gas chromatography.
Information relating to these analyses follow. No reference to
inter-laboratory collaborative studies on analytical procedures was
found.
Extraction and partitioning
It is not sufficient simply to wash the analytical samples since small
amounts of the active ingredient penetrate into the plant. It is
therefore important that complete extraction of the insecticide and
metabolites (e.g. by exhaustive Soxhlet extraction) be compared
against the extraction method used. Harvest products with a high
content of water have been macerated with acetone (Horler, 1966;
Möllhoff, 1967, 1968), acetonitrile (Coffin and Savary, 1964; Thier
and Bergner, 1966), or ethanol (Fischer, 1968). Very good recoveries
were obtained by Soxhlet extraction with chloroform-methanol (9:1 v/v)
(Bowman and Beroza, 1969). For milk, a combination of polar and
nonpolar extractants is required, e.g. acetone-methylene chloride
(Bowman and Beroza, 1969), ethanol-hexane (Franz and Kovac, 1965).
Extraction with methanol-acetonitrile also proved suitable for milk
samples (Miyamoto et al., 1967). For oil-containing harvest products
with a low water content, use is made of benzene (Dawson et al., 1964;
Horler, 1966), petroleum ether (Kovac and Sohler, 1965), hexane
(Horler, 1966), or chloroform (Yuen, 1966). On account of the
favourable partition coefficients (Bowman and Beroza, 1965; Kovac,
1963), fats and waxes are best removed by partitioning between hexane
and acetonitrile (Franz and Kovac, 1965; Miyamoto et al., 1967;
Möllhoff, 1967; Bowman and Beroza, 1969).
Qualitative analysis
Although no reports of fenitrothion being separated from similar
residues by column chromatography have been noted, separation is
possible with thin-layer or gas chromatography. Interfering active
ingredients are parathion, parathion-methyl, malathion, and fenthion
and the resolution is just sufficient for distinguishing fenitrothion
from these active ingredients (see e.g. Möllhoff, 1968). By comparing
gas chromatograms recorded with a phosphorus detector and an electron
capture detector, fenitrothion can often be clearly differentiated
from malathion and fenthion owing to differences in sensitivity.
Colorimetry
Colorimetric determinations are carried out by the method of Averell
and Norris (1948) or by determination of the cresol after
saponification of the active ingredient with alkali. The limits of
determination are about 0.05 ppm.
Thin-layer chromatography
Thin-layer chromatography has been used for clean-up of the extracts
with final determination by colorimetry after deleting the appropriate
spot from the plate, or for direct determination on the plate after
spraying. Quantitative evaluation is possible in the first instance;
in the second, the evaluation is semiquantitative and more suitable
for confirming identity. About 0.1 ppm of fenitrothion can be
determined in most harvested products. Determination of lesser amounts
is possible with procedures utilizing cholinesterase inhibition
(Ackermann, 1966; Mendoza et al., 1968; Schutzmann and Barthel, 1969;
Winterlin et al., 1968).
Column chromatography
For extract cleanup or separation of metabolites, columns are
suitable, especially those packed with deactivated silica gel (Bowman
and Beroza, 1969), deactivated Florisil (Möllhoff, 1967, 1968), or
deactivated acid aluminum oxide (Horler, 1966). Columns packed with
active Florisil (Beckman and Garber, 1969), magnesium oxide (Coffin
and Savary, 1964), polyethylene-impregnated aluminum oxide (Coffin and
Savary, 1964), and Sephadex LH-20 (Horler, 1968; Ruzicka, et al.,
1968) have also been used.
Gas chromatography
The most reliable and highly sensitive methods for fenitrothion
utilize gas chromatography with either the flame-photometric (Bowman
and Beroza, 1969) or the thermionic detector (Miyamoto et al., 1967,
Sato et al., 1968). These detectors respond to the phosphorus in the
molecule with very high specificity. No cleanup is usually required
with the flame-photometric detector when analysing for either
fenitrothion or its oxygen analogue unless a fatty food is being
analysed. In this case a simple hexane-acetonitrile partition is used.
A cleanup was used with the thermionic detector but it may often be
omitted. Limit of determination is usually 0.01-0.001 ppm.
In the analysis for fenitrooxon a separation from fenitrothion on
silica gel is used. The compound is then determined by gas
chromatography (Bowman and Beroza, 1969) or enzymatically (Miyamoto et
al., 1967). The cresol is determined by electron-capture gas
chromatography (Bowman and Beroza, 1969) or colorimetrically (Miyamoto
et al., 1967) after column separation. Analysis of fenitrothion,
fenitrooxon, and the cresol by electron-capture gas chromatography is
possible (Bowman and Beroza, 1969; Dawson et al., 1964; Horler, 1966;
Möllhoff, 1967) but less specific; sensitivity, about 0.1 ppm, can be
improved with a suitable cleanup.
Determination of metabolites
Gas chromatographic methods have been described for the simultaneous
determination of fenitrothion, fenitrooxon (Bowman and Beroza, 1969;
Möllhoff, 1968), 3-methyl-4-nitro phenol (Bowman and Beroza, 1969;
Miyamoto et al., 1967) and the amino compound of fenitrothion
(Miyamoto et al., 1967). As noted, for market controls determination
of fenitrothion is generally sufficient (Möllhoff, 1968).
NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Tolerance Withholding
Country Crop (ppm) period (days)
Austria 2.0 proposed
Belgium Top fruits and vegetables 0.5 14
China, Rice - 21
Republic of Vegetables - 10
Denmark Beets, apples, peas, clover - 15
Tolerance Withholding
Country Crop (ppm) period (days)
East Africa Mango, cereals - 7
Germany, Field crops, vegetables, fruits, and - 10
Eastern special crops
Crops for Infant food and remedies - 21
Germany Vegetables, root and field crops, 0.4 10
(Fed. Rep.) legumes, fruits, grapes
Finland - 21
France Vegetables, fruits - 15
Italy - 15
Netherlands Cereals, fruits 0.5 21
New Zealand - 14 (7 for
pasture)
Poland Top fruits (except strawberries and - 14
raspberries)
Portugal Vegetables, citrus, fruits, cocoa, coffee - 21
Pasture grass - 10
Spain Vegetables, citrus, fruits - 15-21
Sweden Peas, rape, wheat, barley - 10
Switzerland Top fruits 0.2 in
combination
with
Amidithion
APPRAISAL
Fenitrothion is a broad spectrum insecticide with a much lower acute
mammalian toxicity than many similar insecticides. It is not covered
by patents and is produced by at least six companies. Use of
fenitrothion is almost worldwide (excluding the U.S.A.) for such crops
as rice, fruits, vegetables, cotton, cereals, soybeans, coffee and
tea. Application rates are 0.5-2.0 kg a.i./ha. Waiting intervals are
generally 10-21 days. It is used against spruce budworm (in forests),
insects attacking man, and in some countries to protect stored
products. Its use on pasture land (375 g/ha) results in almost no
residues of fenitrothion in milk or meat (<0.01 ppm). The insecticide
is available as a 95% concentrate, 50% emulsifiable concentrate, 40%
wettable powder, 2.5 and 5% dusts. Composition of technical materials
is not known and is apt to vary depending on manufacturer. Residue
data are available mostly from Europe and Japan.
Although several metabolites of fenitrothion are known to form
(fenitrooxon, desmethyl fenitrothion, aminofenitrothion, and
3-methyl-4-nitrophenol), they do not accumulate in significant amounts
or they appear to be comparatively non-toxic. Unless experimental
trials indicate otherwise, fenitrothion appears to be the only toxic
residue to be determined in products in commerce.
Many methods are available for residue analysis of fenitrothion. The
best of these utilize gas chromatography with the flame photometric or
thermionic detector. Some of these methods may also be used for
analysis of metabolites. Sensitivity is usually better than 0.01 ppm.
Evaluation of these procedures for regulatory purposes is suggested.
Few data on residues in food in commerce or in diets have been
reported.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCE OR
PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to June 1973)
Apples, cherries, grapes, lettuce 0.5 ppm ) Preharvest interval to
) be such that these
Tomatoes 0.2 ppm ) tolerances are not
) exceeded)
Red cabbage, green tea 0.3 ppm
Cocoa 0.1 ppm
The 3-methyl-4-nitrophenol is not included in the recommendations at
this time but the Joint Meeting should review this aspect in due
course.
PRACTICAL RESIDUE LIMITS
Milk (whole) 0.002
Meat or fat 0.03
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Reproduction and teratogenicity studies in animals preferably in
non-human primates.
2. Adequate long-term studies in rodent and non-rodent mammalian
species.
3. Data on residue levels in raw agricultural commodities moving in
commerce and in total diet studies.
4. Data on disappearance of residues during storage, processing and
cooking.
5. Data on rate of residue decline in rice and pre-harvest interval.
6. Before use as a grain protectant, data on persistence of residues
in storage of the grains concerned and definitive data on residues
in bread are needed.
7. Data on occurrence of 4-nitro-3-methylphenol residues and their
significance toxicologically.
8. Information on ingredients in technical products produced by
several manufacturers.
DESIRABLE
1. Observations in man.
2. Evaluation of gas chromatographic methods for regulatory purposes.
REFERENCES
Ackermann, H. (1966) Enzymatischer Nachweis phosphororganischer
Insektizide nach dünnschicht-chromatographischer Trennung. Nahrung,
10:273-4
Averell, P.R. and Norris, M.V. (1948) Estimation of small amounts of
O,O-diethyl-O,p-nitro-phenyl thiophosphate. Anal. Chem. 20:753-6
Bayer (1969) (Farbenfabriken Bayer AG.). Fenitrothion. Unpub. Rept.
Beckman, H. and Garber, D. (1969) Recovery of 65 organophosphorus
pesticides from Florisil with a new solvent elution system. J. Ass.
Offic. Anal. Chem. 52:286-93
Bowman, M.C. and Beroza, M. (1965) Extraction p-values of
pesticides and related compounds in six binary solvent systems.
J. Ass. Offic. Agr. Chem. 48:943-52
Bowman, M.C. and Beroza, M. (1969) Determination of Accothion, its
oxygen analog, and its cresol in corn, grass and milk by gas
chromatography. J. Agr. Food Chem. 17:271-6
Bowman, M.C. (1969) Private communication
Braid, P.E. and Nix, M. (1968) Potentiation of toxicity of Sumithion
by phosphamidon in the rat. Canad. J. Physiol. Pharmacol. 46:145-9
Carshalton (1962) WHO insecticide evaluation and testing programme
- stage I. Mammalian toxicity report OMS 43=S5660, Test for neurotoxic
effects in hens. Unpub. Rept. from the Toxicology Research Unit,
Charshalton. Submitted by Farbenfabriken Bayer AG.
Carshalton. (1964) OMS 43. Summary. OMS 43. Mammalian toxicity.
Unpub. Rept. of the Toxicology Research Unit, Carshalton. Submitted
by Farbenfabriken Bayer AG.
Coffin, D.E. and Savary, G. (1964) Procedure for extraction and
cleanup of plant material prior to determination of organophosphate
residues. J. Ass. Offic. Agr. Chem. 47:875-81
Cooper. (1966) Toxicity of Sumithion. Unpub. Rept. of the Cooper
Technical Bureau. Submitted by Sumitomo Chemical Co., Ltd. Osaka
Cooper. (1968) Technical Bureau. Sumithion as a grain protectant.
Technical report by William Cooper and Nephews, Australia. Cited by
Sumitomo, 1969
Dawson, J.A., Donegan, L. and Thain, E.M. (1964) The determination of
parathion and related insecticides by gas-liquid chromatography
with special reference to fenitrothion residues in cocoa.
Analyst, 89:495-6
Douch, P.G.C., Hook, C.E.R. and Smith, J.N. (1968) Metabolism of
Folithion (dimethyl-4-nitro-3-methylphenyl phosphorothionate).
Australasian J. Pharm., 49: No.584. Suppl. Nr.66, 2.S.
DuBois, K.P. and Puchala, E. (1960) The acute toxicity and
anticholinesterase action of Bayer 41831. Unpub. Rept. from the
Department of Pharmacology, University or Chicago. Submitted by
Farbenfabriken Bayer AG.
DuBois, K.P. and Kinoshita, F. (1963) The acute toxicity of Bayer
41831 in combination with other anticholinesterase insecticides.
Unpub. Rept. from the Department of Pharmacology, University of
Chicago. Submitted by Farbenfabriken Bayer AG.
Fischer, R. (1968) Nachweis und quantitative Bestimmung von
Phosphor-Insektiziden in biologischem Material. III. Arch.
Toxicol., 23:129-35
Franz, J. and Kovac, J. (1965) Bestimmung toxischer Rückstände von
O,O-Dimethyl-O-(3-methyl-4-nitrophenyl)-thiophosphat in Milch. Z.
Anal. Chem. 210:354-8
Frehse, H. and Möllhoff, E. (1969) Organophosphorus insecticide
residue analysis. Evaluation. (A) Metabolic products. In report of
IUPAC Commission on the development, improvement, and standardization
of methods of pesticide residue analysis. Egan, H. J. Ass. Offic.
Anal. Chem. 52:306-9
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14:515-34
Green, A.A. and Tyler, P.S. (1966) A field comparison of malathion,
dichlorvos and fenitrothion for the control of Oryzaephilus
surinamensis (L) infesting stored barley. J. Stored Prod. Res.
1:273-85
Hais, K. and Franz, J. (1965) On the problem of Metathione residues
in milk after disinfestation. Cesk. Hyg. 10.205-8
Hladká, A. and Nosál, M. (1967) The determination of the exposition
to Metathion fenitrothion) on the basis of excreting its metabolite
p-nitro-m-cresol through urine in rats. Internat. Arch.
Gewerbepath. Gewerbehyg. 23:209-14
Hollingworth, R.M., Fukuto, T.R. and Metcalf, R.L. (1967) Selectivity
of Sumithion Compared with Methyl Parathion. Influence of structure
on anticholinesterase activity. Metabolism in the white mouse. J.
Agric. Food Chem., 15:235-49
Horler, D.F. (1966) Determination of fenitrothion on stored barley. J. Stored
Prod. Res. 1:287-90
Horler, D.F. (1968) Gel filtration on Sephadex LH-20. A general
clean-up method for pesticides extracted from grain. J. Sci. Food Agr.
19:229-31
Kane, J. and Green, A.A. (1968) The protection of bagged grain from
insect infestation using fenitrothion. J. Stored Prod. Res. 4 59.
Kimmerle, G. (1962a) Toxikologische Untersuchungen mit dem Wirkstoff
S 5660. Unpub. Rept. from Toxilkolisches und Gewerbehygienisches
Laboratorium. Submitted by Farbenfabriken Bayer AG.
Kimmerle, G. (1962b) S 5660. Unpub. Rept. on the neurotoxic effect
on hens from Toxikologisches und Gewerbehygienisches Laboratorium.
Submitted by Farbenfabriken Bayer AG.
Klimmer, O.R. (1961) Opinion on the toxicity of the compound "S 5660"
of Farbenfabriken Bayer AG., Leverkusen. Unpub. Rept. from
Pharmakologisches Institut der Rheinischen
Friedrich-Wilhelms-Universität, Bonn. Submitted by Farbenfabriken
Bayer AG.
Kovac, J. (1963) Bestimmung von
O,O-Dimethyl-O-(3-methyl-4-nitrophenyl)-thiophosphat in technischen
Produkten nach vorhergehender Abtrennung der Begleitstoffe mittels
Dünnschichtchromatographie. J. Chromatogr. 11:412-3
Kovac, J. and Sohler, E. (1965) Bestimmung von
O,O-Dimethyl-O-(3-methyl-4-nitrophenyl)-thiophosphat-Rückständen
in Obst und Gemüse nach vorangegangener Abtrennung der mitextrahierten
Farbstoffe durch Dünnschichtchromatographie. Z. Anal. Chem. 208.201-4
Mendoza, C.E., Wales, P.J., McLeod, H.A. and McKinley, W.P. (1968)
Thin-layer chromatographic-enzyme inhibition procedure to screen for
organophosphorus pesticides in plant extracts without elaborate
clean-up. Analyst 93:173-7
Misu, Y., Segawa, T., Kuruma, I., Kojima, M. and Takagi, H. (1966)
Subacute toxicity of O,O-dimethyl-O-(3-methyl-4-nitrophenyl)
phosphorothioate (Sumithion) in the rat. Toxicol. appl.
Pharmacol., 9:17-26
Miyamoto, J., Sato, Y., Kadota, T., Fujinami, A. and Endo, M. (1963a)
Studies on the mode of action of organophosphorus compounds. Part I.
Metabolic Fate of p32 labeled Sumithion and Methylparathion
in guinea-pig and white rat. Agric. Biol. Chem. (Tokyo), 27:381-9
Miyamoto, J., Sato, Y., Kadota, T. and Fujinami, A. (1963b) Studies
on the mode of action of organophosphorus compounds. Part II.
Inhibition of mammalian cholinesterase in vivo following the
administration of Sumithion and Methylparathion. Agric.
Biol. Chem. (Tokyo), 27:669-76
Miyamoto, J. (1964a) Studies on the mode of action of organophosphorus
compounds. Part III. Activation and degradation of Sumithion
and Methylparathion in vivo. Agric. Biol. Chem. (Tokyo),
28:411-21
Miyamoto, J. (1964b) Studies on the mode of action of organophosphorus
compounds. Part IV. Penetration of Sumithion, Methylparathion and
their oxygen analogs into guinea pig brain and inhibition of
cholinesterase in vivo. Agric. Biol. Chem. Tokyo), 28:422-30
Miyamoto, J. and Sato, Y. (1965) Determination of insecticide residue
in animal and plant tissues. II. Metabolic fate of Sumithion in rice
plant applied at the preheading stage and its residue in harvested
grains. Botyu-Kagaku, 30:45-9
Miyamoto, J., Kawaguchi, Y. and Sato, Y. (1965a) Determination of
insecticide residue in animal and plant tissues. I. Determination of
Sumithion residues in bananas grown in Formosa. Botyu-Kagaku, 30:9-12
Miyamoto, J., Sato, Y. and Fujikawa, K. (1965b) Determination of
insecticide residue in animal and plant tissues. III. Determination
of residual amount of Sumithion in cocoa beans grown in
Nigeria. Botyu-Kagaku 30:49-51
Miyamoto, J., Kitagawa, K. and Sato, Y. (1966) Metabolism of
organophosphorus insecticides by Bacillus subtilis, with
special emphasis on Sumithion. Jap. J. Exp. Med. 36:211-25
Miyamoto, J., Sato, Y. and Suzuki, S. (1967) Determination of
insecticide residue in animal and plant tissues. IV. Determination
of residual amount of Sumithion and some of its metabolites in fresh
milk. Botyu-Kagaku. 32:95-100
Miyamoto, J. (1969) Mechanism of low toxicity of Sumithion toward
mammals. Residue Reviews, 25:251-64
Miyamoto, J. and Sato, Y. (1969) Determination of insecticide residue
in animal and plant tissues. VI. Determination of Sumithion residue
in cattle tissues. Botyu Kagaku 34:3-6
Möllhoff, E. (1967) Gas chromatographic determination of residues of
E605 products and Agritox in plants and soil samples.
Pflanzenschutz-Nachrichten Bayer 20:557-74
Möllhoff, E. (1968) Beitrag zur Frage der Rückstände und ihrer
Bestimmung in Pflanzen nach Anwendung von Präparaten der E 605 - und
Agritox - Reihe. Pflanzenschutz-Nachrichten Bayer 21:331-58
Muramoto, N. (1967) Unpub. observations, cited by Sumitomo, 1969
Nishizawa, Y., Fujii, K., Kadota, T., Miyamoto, J. and Sakamoto, H.
(1961) Studies on the organophosphorus insecticides. Part VII.
Chemical and biological properties of new low toxic organophosphorus
insecticide. O,o-Dimethyl-O-(3-methyl-4-nitrophenyl) phosphorothioate.
Agric. Biol. Chem. (Japan), 25:605-10
Nosäl, M. and Hladkä, A. (1968) Determination of the exposure to
fenitrothion [O,O-dimethyl-O-(3-methyl-4-nitrophenyl) thiophosphate]
on the basis of the excretion of p-nitro-m-cresol by the urine
of the persons tested. Internat. Arch. Gewerbepath. Gewerbehyg.
25:28-38
Republic of Argentina (1968) - Unpub. Rept. prepared for Codex
Committee on Pesticide Residues
Ruzicka, J.H., Thomson, J., Wheals, B.B. and Wood, N.F. (1968) The
application of gel chromatography to the separation of
pesticides. I. Organophosphorus pesticides. J. Chromatogr.
34:14-20
Sato, Y., Miyamoto, J., and Suzuki, S. (1968) Determination of
insecticide residue in animal and plant tissues. V. A device to
increase the sensitivity of the gas chromatography detector to
organophosphorus insecticides. Bochu Kagaku 33:8-12
Schutzmann, R.L. and Barthel, W.F. (1969) Indoxyl acetate spray
reagent for fluorogenic detection of cholinesterase inhibitors in
environmental samples. J. Ass. Offic. Anal. Chem. 52:151-6
Sumitomo Chemical Company. (1969) Sumithion, Its toxicity, metabolism
and residues. Unpub. Rept.
Thier, H.P. and Bergner, K.G. (1966) Eine Schnellmethode zum Nachweis
wichtiger Schädlings-bekämpfungsmittel in Ost und Gemüse. Deutsche
Lebensmittel-Rundschau 62:399-402
Tomizawa, C. and Kobayashi, A. (1964) Relation between the behaviour
of parathion and Sumithion in cabbage leaves and their
phytotoxicity. Noyaku Seisan Gijutsu 11:30-32; Chem. Abstr. 62,
1387d (1965)
Ueda, K. and Nishimura, M. (1966) Interim report. Chronic toxicity of
Sumithion: two-year feeding study in rats. Unpub. Rept. from the
Department of Hygiene and Toxicology, Tokyo Dental College.
Submitted by Sumitomo Chemical Co., Ltd., Osaka.
Vandanis, A. and Crawford, L.G. (1964) Comparative metabolism of O,
O-dimethyl-O-p-nitrophenyl phosphorothioate (Methylparathion and
O, O-dimethyl-O-(3-methyl-1-nitrophenyl) phosphorothioate
(Sumithion) J. Econ. Entomol. 57:136-9
Vandekar, M. (1965) Observations on the toxicity of carbaryl,
Folithion and 3-isopropropyl-phenyl N-methylcarbamate in a
village-scale trial in Southern Nigeria. Bull. Wld. Hlth Org.
33.107-15
Wilford, K., Lietaert, P.E.A. and Foll, C.V. (1965) Toxicological
observations during large scale field trial of OMS-43 in Northern
Nigeria (preliminary report). WHO working paper 65/TOX/1 prepared
for an informal meeting on toxicology of insecticides. Geneva,
18-24 February 1965
Winterlin, W., Walker, G. and Frank, H. (1968) Detection of
cholinesterase-inhibiting pesticides following separation on
thin-layer chromatograms. J. Agr. Food Chem. 16:808-12
Yasuno, M., Hirakoshi, S. Sasa, M. and Uchida, M. (1965) Inactivation
of some organophosphorus insecticides by bacteria in polluted water.
Jap. J. Exp. Med. 35:545
Yuen, S.H. (1966) Absorptiometric determination of fenitrothion
residues in cocoa beans. Analyst 91:811-3
TABLE I
Percentage distribution of metabolites of fenitrothion in mouse urine after
administering various doses
Metabolite Percentage of radioactive metabolite excreted
in urine after giving P52 - labelled fenitrothion
at the indicated dose levels in mg/kg body-weight
3 17 200 850
Phosphoric acid 2.0 2.4 1.9 1.2
Methylphosphoric acid 1.5 2.5 2.4 1.1
Dimethylphosphoric acid 32.2 21.4 5.8 3.0
Dimethylphosphorothioic acid 12.8 20.3 8.7 6.6
Phosphate analogue (II) 2.7 1.6 3.3 2.5
O-demethylphosphate analogue (VI) 26.1 28.4 24.6 17.1
O-demethylphosphorothioate
analogue (V) 20.5 20.1 50.9 66.1
Unknown 2.2 2.5 2.4 2.4
TOXICOLOGICAL STUDIES
Special studies on neurotoxicity
Hen
Three hens protected against acute anti-cholinesterase effects with
atropine and pralidoxime were given a single oral dose of 400 mg/kg
body-weight of fenitrothion. No symptoms of paralysis occurred during
an observation period of 28 days. Histologic examination of spinal
cord and sciatic nerves revealed no pathological lesions in two hens
and a few scattered degenerated fibres in the spinal cord of the third
(Carshalton, 1962).
Seven hens protected in the same way were given an oral dose of 250
mg/kg body-weight of fenitrothion and three other hens were given 500
mg/kg. Two of the 500 mg/kg group died within 1-2 days, while the
remaining eight hens did not show any sign of paralysis during an
observation period of six weeks (Kimmerle, 1962b).
Special studies on potentiation
Rat
Female rats were given intraperitoneal doses of a combination of
fenitrothion and the following organophosphorus compounds: parathion,
parathion-methyl, demeton, disulfoton, malathion, EPN,
azinphos-methyl, carbophenothion, mevinphos, dioxathion, schraden,
ethion, diazinon, Folex, coumaphos, and fenchlorphos as well as the
carbamate carbaryl. No sign of a potentiating effect was demonstrated
(DuBois and Kinoshita, 1963).
When male rats were given acute oral doses of mixtures of fenitrothion
and phosphamidon a marked potentiation of toxicity occurred as
evidenced by increased mortality. In female rats a potentiation of
toxicity occurred as evidenced by increased mortality. In female rats
a potentiation occurred only in mixtures containing relatively low
concentrations of fenitrothion, the potention effect diminishing as
the concentration of fenitrothion was increased. It was concluded that
potentiation is associated with a non-linear phosphorothioate
conversion (Braid and Nix, 1968).
Special studies on the metabolite fenitrooxon
The metabolite fenitrooxon is more toxic than the parent compound (cf.
Tables II and III).
TABLE II
Acute toxicity of fenitrooxon
Acute
Animal Route LD50 mg/kg References
body-weight
Mouse oral 90 Miyamoto, 1969
Mouse oral 120 Hollingworth et al., 1967
Rat oral 24 Miyamoto, 1969
Rat i.v. 3.3 Miyamoto, 1969
Guinea-pig oral 221 Miyamoto, 1969
Guinea-pig i.v. 32 Miyamoto, 1969
Acute toxicity
The symptoms of acute toxicity are the same as for other
organophosphorus compounds, doses close to the LD50 producing
symptoms which developed more rapidly after intravenous than after
oral administration. The compound is considerably less toxic to
mammals than its close structural analogue parathion-methyl (Miyamoto
et al., 1963b). Table II gives the LD50 in several species:
TABLE III
LD50 mg/kg
Animal Route body-weight References
Mouse (M) oral 1336 Carshalton, 1964
Mouse (F) oral 1416 Carshalton, 1964
Mouse (M) i.p. 115 DuBois and Puchala, 1960
Mouse (F) i.p. 110 DuBois and Puchala, 1960
Mouse i.v. 220 Miyamoto et al, 1963b
Rat (M) oral 740 Gaines, 1969
Rat (F) oral 570 Gaines, 1969
Rat (M) i.p. 135 DuBois and Puchala, 1960
Rat (M) i.p. 160 DuBois and Puchala, 1960
Rat i.v. 33 Miyamoto et al, 1963b
Guinea-pig (M) oral 500 DuBois and Puchala, 1960
Guinea-pig oral 1850 Miyamoto et al, 1963b
Guinea-pig (M) i.p. 110 DuBois and Puchala, 1960
Guinea-pig i.v. 112 Miyamoto et al, 1963b
Cat oral 142 Nishizawa et al, 1961
Short-term studies
Dog
In what was described as a preliminary test, groups of dogs, each
comprising one male and one female animal, were given daily oral doses
by capsule of 0, 2, 9 or 40 mg/kg body-weight of fenitrothion for
periods up to 98 days. Body-weights, blood biochemistry,
cholinesterase levels and haemograms were checked at intervals. At the
2 mg/kg level there was no effect with respect to any other of the
parameters mentioned. At 9 mg/kg a slight depression after 60 days and
at 40 mg/kg a moderate depression after 29 days occurred in whole
blood, plasma and red-cell cholinesterase. At 40 mg/kg there were also
marked toxic symptoms typical of cholinergic stimulation, and the dogs
in this group were sacrificed below the end of the 98-day period
(Cooper, 1966).
Rat
Groups of male rats (16 or 17 in number) were fed 0, 32, 63, 125, 250,
and 500 ppm of fenitrothion in the diet for 90 days. Mortality, food
intake, growth, general behaviour, urinalysis, average organ-weights
and histpathology were comparable to the controls in the groups fed
32, 63, 125, and 250 ppm. All the animals fed 500 ppm showed clinical
symptoms of anti-cholinesterase poisoning and there were minimal
symptoms in four animals in the 250 ppm group. In the 500 ppm group
the average organ-weights of the testes and brain were increased in
comparison with those of the control group. After interim sacrifice
every month of four rats from each group, measurement of the
cholinesterase activity of plasma, red cells, brain cortex, liver and
kidney showed a dose-dependent depression, the lowest being in the
brain. The cholinesterase activity in the 32 and 63 ppm groups
generally increased after 60 days of dosing to a level within the
normal limits, the beet recovery being in the plasma, kidney and
brain, less in the red cells and the liver (Misu et al., 1966).
Two groups, each of 20 male rats, were dosed by stomach tube on six
days a week for six months with 10 mg/kg and 11 mg/kg body-weight of
fenitrothion, respectively. During the first weeks the rats showed a
temporary deterioration of general condition and loss of weight.
Haematology and urinalysis during the experiment and gross and
microscopic pathology at its termination did not reveal any
abnormalities (Klimmer, 1961).
Male rats were given daily oral doses of 13 mg/kg body-weight of
fenitrothion for 28 days. Red-cell cholinesterase activity showed
severe depression, but there was recovery 30 days after withdrawal of
fenitrothion (Kimmerle, 1962a).
Male rats were fed 5, 10, and 20 ppm of fenitrothion in the diet for
an unspecified period. Brain and red cell cholinesterase activity was
normal in the 5 ppm group, whereas the 10 ppm group showed a slight
depression of red cell activity after five weeks with recovery two
weeks after withdrawal.
The 20 ppm group showed some depression of activity both in red cells
and in brain and the recovery in the brain remained incomplete two
weeks after withdrawal (Carshalton, 1964).
Other reported studies with fenitrothion in the rat lasting 90 days
indicate that the levels causing no appreciable effect on the
cholinesterase activity of plasma, red-cells and whole blood were 20
ppm in the diet and 10 ppm in drinking water. The only exception was a
moderate inhibition of the activity in plasma among the male animals
when given 10 ppm in drinking water. The above mentioned levels and
higher, namely 92.8 ppm in the diet and 46.2 ppm and 215 ppm in
drinking water, caused no effect on food or water intake, weight-gain,
average organ-weights, haemogram and blood biochemistry. However, 92.8
ppm of fenitrothion in the diet and 46.4 ppm in drinking water had a
moderate effect on whole blood and red-cell cholinesterase and a more
marked effect on plasma cholinesterase. The cholinesterase activity
recovered between 30-40 days after withdrawal of fenitrothion. The
levels of 430 ppm in the diet and 1000 ppm in drinking water caused a
depression of body-weight gains (Cooper, 1966).
Long-term studies
Rat
An interim report on what appears to be a two-year feeding study in
rats is available. Groups of nine or 10 male rats were fed 0, 25, 100
or 400 ppm of fenitrothion in their diet and were sacrificed after
feeding these levels for 63 weeks. As a positive control a group was
also fed 800 ppm of malathion. At the 400 ppm level of fenitrothion,
food intake and body-weight gain were increased and only a few animals
survived the 63 week period. At this level there was a 100 per cent
depression of red-cell cholinesterase. At the 100 ppm level a slight
(10-30 per cent) cholinesterase depression occurred in the brain and a
moderate depression (30-65 per cent) in the red blood cells and
plasma. At 25 ppm there was no effect on cholinesterase nor was there
any effect with regard to any other parameters evaluated (Ueda and
Nishimura, 1966).
OBSERVATIONS IN MAN
In a field spraying operation in Southern Nigeria including a village
using a five per cent spray of fenitrothion, examination of 18
villagers one week later did not reveal any clinical symptoms of
toxicity or plasma cholinesterase depression. The same was true of the
three spraymen examined on the first, second and sixth day after
spraying relative to a pre-spraying level (Vandekar, 1965).
In another field spraying trial in Northern Nigeria, 10,000 huts in
which about 16,500 people lived were sprayed. Field test
cholinesterase determinations on whole blood did not show any
appreciable difference in cholinesterase levels of 535 villagers
tasted before spraying and 299 villagers tested 5-30 days after
spraying. After one week of intensive spraying five out of 20 spraymen
developed a 50 per cent depression of cholinesterase which returned to
a stable level after a period of rest. One sprayman developed symptoms
of toxicity which lasted only a few hours and disappeared without
treatment (Wilford et al., 1965).
Fenitrothion was given to a total of 24 human subjects in single oral
doses of from 2.5 to 20 mg (0.042 to 0.33 mg/kg body-weight for a 60
kg man). The excretion of the metabolite, 3-methyl-4-nitrophenol, in
the urine was almost complete within 24 hours, the maximum excretion
occurring in the first 12 hours. The percentage of the dose excreted
during this time depended to a certain extent upon the size of the
dose administered, being about 70 per cent of theoretical after a
0.042 mg/kg dose and about 50 per cent after a 0.33 mg/kg dose. Both
plasma and cholinesterase activity were not depressed below normal
except possibly in one person given a 0.33 mg/kg dose, where some
depression of plasma cholinesterase was apparent after six and 24
hours (about 65 per cent of the pre-test level). When repeated doses
of 2.5 or 5 mg approximately 0.04 to 0.08 mg/kg) were given to five
individuals, four times at 24 hour intervals, most of the nitrocresol
metabolite appeared in the urine within the interval 0 to 12 hours
after administration. After receiving the third and fourth dose there
was a trend towards a rise in red cell cholinesterase activity but in
no cases was there any evidence of reduction below normal levels of
the activity of this enzyme in either plasma or red cells (Nosal and
Hladka, 1968).
COMMENT
Adequate information is available on the toxicity, biochemistry and
metabolism of fenitrothion, in three species of rodent. Information is
also available in man including field spraying studies and a
metabolism study. Only an interim report comprising a 63-week study in
rats in available and there are no 1-2 year studies in a non-rodent
mammalian species. The short-term studies in the rat, using
cholinesterase inhibition criteria, provide a no-effect level. No
reproduction or teratogenicity studies are available and in view of
the similarity in the structure of fenitrothion to parathion-methyl,
it is important that such studies be undertaken. For this reason and
because no adequate long-term studies are available it was decided to
give only a temporary acceptable daily intake to this compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 ppm in the diet, equivalent to 0.25 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenitrothion is a broad-spectrum insecticide having a much lower acute
mammalian toxicity than many similar organophosphorus insecticides.
Its action is by direct contact or an a stomach poison. It can be
applied as an emulsifiable concentrate, wettable powder, granular
formulation or dust.
It is toxic to bees and should not be sprayed on flowering crops.
Fenitrothion can be combined with all conventional insecticides and
fungicides except those having an alkaline reaction.
The insecticide has been used throughout Europe, East Pakistan, East
Africa, United Arab Republic, Japan, Republic of China, New Zealand,
Canada, and Brazil. It is not registered for use in the United States.
Pre-harvest treatments
Fenitrothion is used on a wide variety of crops including fruits,
field crops, vegetables, rice, cotton, cereals, cocoa, tea, and coffee
for control of stem borers, hoppers, leaf miners, leaf rollers,
whiteflies, fruit flies, mealybugs, mirids and bugs, thrips, aphids,
mites, lady beetles, caterpillars, and soft scale insects.
The recommended concentrations and rates of application vary for the
different crops and pest species to be controlled. Concentrations of
sprays range from 0.05 to 0.1%. active ingredient (a.i.) and rates of
application from 0.5 to 2.0 kg a.i./hectare. The chemical is generally
tolerated by most crops although high dosages may injure cotton, and
phytotoxicity on Brassica crops and orchard fruits has been
encountered.
Safety intervals prior to harvest range from 10 to 21 days and differ
depending on the country and the crop.
Post-harvest treatments
No post-harvest treatments are made in Europe. Admixture of 1 to 5 ppm
of fenitrothion has been recommended to protect grains such as rice,
wheat, and barley for months against various weevils and beetles. Bag
treatment is acceptable in Brazil for protecting stored grains and
post-harvest treatments are under development in various countries.
Other uses
Fenitrothion is used to control grasshoppers, locusts, and
caterpillars on pastures and spruce budworm in forests. It also
provides control of household and other pests, such as mosquitoes and
their larvae, houseflies, cockroaches, lice, bedbugs, and poultry
mites. Its action is rapid, and its residual activity is good.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Typical maximum residues after treatment of a variety of crops are
given in Table IV.
TABLE IV
Maximum residues after treatment of crops
Maximum
Dosage residues at
active Pre-harvest harvest, ppm
Crop ingredient interval, days respectively Reference
Rice 1000 g/ha 41 0.005 Sumitomo 1969
Rice 0.07% spray or Miyamoto, Sato
5% granules 1-2 months n.d. 1965
Apple 0.05% spray 21 0.05 Sumitomo 1969
Apple 0.2% spray 7 and 14 0.5 and 0.35 Bayer 1969
Apple, Golden 0.15% spray 10 and 15 1.0 and 0.75 Bayer 1969
cherry 0.2% spray 7 and 14 0.5 and 0.2 Bayer 1969
Grape* 0.05% spray 10 0.50 Sumitomo 1969
Tomato* 0.05% spray 7 0.18 Sumitomo 1969
Cocoa 0.1% spray 14 0.10 Miyamoto et al
1965b
Red cabbage 0.15%; 1000 7 Outside leaves
1/ha 0.65
Cabbage less
outside leaves
0.25 Bayer 1969
Sugar beets 0.15%; 1000 8 n.d. Bayer 1969
1/ha
TABLE IV (cont'd)
Maximum residues after treatment of crops
Maximum
Dosage residues at
active Pre-harvest harvest, ppm
Crop ingredient interval, days respectively Reference
Green tea 0.1% spray 14 0.27 Sumitomo 1969
Cauliflower 0.1%; 1000 7 n.d. Bayer 1969
1/ha
Lettuce 0.1%; 1000 7 and 14 1.05 and 0.3 Bayer 1969
1/ha
Lettuce 0.05%; 600 1, 7, and 14 0.65, 0.06, Möllhoff 1968
1/ha 0.01
Peas and pod 0.25%; 560 0 and 5 0.7 and <0.15 Bayer 1969
1/ha
*These green-house trials gave residues higher than those of field
trials.
FATE OF RESIDUES
General comments
Fenitrothion may be metabolized as shown in Figure 1. The major route
in animals appears to be through splitting of the P-O-aryl bond to
give the corresponding dimethyl esters of phosphorothioic and
phosphoric acids. Another means of degradation is demethylation of the
methoxy group to give desmethyl fenitrothion. Although formation of
the oxygen analogue, fenitrooxon, is minor compared to products formed
via hydrolysis, fenitrooxon must be taken into account because the
mammalian toxicity of oxons is generally much higher than that of
parent thiono pesticides. In plants the metabolism of fenitrothion
appears to be similar to that in animals.
In animals
Orally administered 32P-labelled fenitrothion was readily absorbed
from the digestive tract of guinea pigs or rats and the major portion
of the radioactivity excreted in the urine. Neither fenitrothion nor
fenitrooxon was detected and desmethyl fenitrothion, dimethyl
phosphorothioic and dimethyl phosphoric acids were identified in the
urine (Miyamoto et al., 1963a).
Following intravenous injection of radioactive 32P fenitrothion into
guinea pigs and rats, fenitrothion rapidly disappeared from the blood.
Fenitrothion and fenitrooxon were found in the tissues, and their
amounts decreased rapidly. The desmethyl compound and the dimethyl
esters mentioned in the foregoing paragraph were found mostly in the
liver and kidneys (Miyamoto, 1964a).
Excretion of metabolic products is rapid and chiefly in the form of
3-methyl-4-nitrophenol (the nitrocresol hydrolysis product) (Hladka
and Nosal, 1967; Nosal and Hladka, 1968); the cresol methyl may be
oxidized to COOH (Douch et al., 1968). The desmethyl compounds are
also excreted (Hollingworth et al., 1967; Miyamoto et al., 1963b).
Between 60 and 90% of the insecticide is excreted within two days,
chiefly in the aforementioned forms. Only up to 10% is excreted in the
feces. Detoxication via the desmethyl compounds is dose-dependent.
After oral administration of up to 40 grams of fenitrothion per
lactating cow, residues in the milk were as high as 0.4 ppm after 6
hours and below the limit of detection after one day (Hais and Franz,
1965). Detoxication in bovine rumens is rapid owing to reduction of
fenitrothion to the amino compound (Miyamoto et al., 1967).
Cows fed 3 mg/kg body weight of fenitrothion for seven consecutive
days produced milk having up to 0.002 ppm residue of fenitrothion on
the second day, and no residue one day after administration was
stopped. Less than 0.003 ppm aminofenitrothion and about 0.1 ppm
p-nitrocresol were detected during treatment, and no fenitrooxon was
found (Miyamoto et al., 1967).
Thirty calves (1-1.5 yr. av. wt. 243 kg) confined on a pasture sprayed
with 375 g/ha of fenitrothion (11.8 ppm initial residue on grass) were
periodically sacrificed and breast muscle and omental fat analysed. On
the first day residues in the meat and fat were about 0.01 ppm. No
residue of fenitrothion was found in the meat from the third day on
and only 0.004-0.007 ppm was found in the fat on the third day; these
amounts decreased almost to control levels by the seventh day
(Republic of Argentina, 1968; Miyamoto and Sato, 1969).
Lactating dairy cattle were fed 50 ppm of fenitrothion in the feed
(dry basis) for 29 days. No residue of fenitrothion, fenitrooxon, or
the cresol appeared in the milk. A maximum of 0.006 ppm of
aminofenitrothion was found (Bowman, 1969).
In plants
About 50% of 32P-labelled fenitrothion sprayed on rice plants
penetrated into the tissues in 24 hours; at the end of this period
only 10% was left, indicating rapid decomposition. Some fenitrooxon
formed but it disappeared from the tissues more rapidly than
fenitrothion. Rice grains harvested 46 days after treatment contained
0.0007 ppm fenitrothion and less than 1 ppm of p-nitrocresol and
dimethyl phosphorothioic acid (Miyamoto and Sato, 1965).
Fenitrothion does not appear to have much systemic action. After
treatment of rice plants with fenitrothion, more residue is found in
the bran than in the polished grains (Miyamato and Sato, 1965). Very
little active ingredient passed from peel to fruit in stored bananas
(Miyamoto et al., 1965a).
The half-life of fenitrothion in green plants ranges between the
values established for parathion and parathion-methyl, i.e. between
one and two days; the half-life of the oxon is estimated to be only a
few hours (Möllhoff, 1968).
Although the oxon may form in plants it occurs only during the first
few days after treatment and in proportions (ca. 1% smaller than those
in animals (Miyamoto et al., 1963; Miyamoto and Sato 1965; Möllhoff,
1968). Desmethyl compounds occur only in minor amounts in plants.
Phytotoxicity of fenitrothion on cabbage was ascribed to high
penetration of the insecticide and accumulation of the cresol
hydrolysis product (Tomizawa and Kobayashi, 1964).
In soil
The soil bacteria Bacillus subtilis, converted more than half of
radiolabelled 32P fenitrothion to the amino analogue under aerobic
conditions at 37°C in 24 hours. The desmethyl derivatives of
fenitrothion and aminofenitrothion as well as dimethyl phosphorothioic
acid formed. None of the oxon or oxons of the degradation products
were found (Miyamoto et al., 1966).
Fenitrothion is gradually inactivated by other bacterial species
including gram-positive and gram-negative rods, but not by fungi and
yeasts (Yasuno et al., 1965).
Fenitrothion is readily absorbed onto various kinds of soil and
decomposed by alkalinity or microorganisms (Muramoto, 1967).
Persistence in soil does not appear to be great.
In storage and processing
Fenitrothion shows promise for control of grain insects in storage.
Applied at 2 and 1 ppm to barley, it decreased to 0.4 ppm after 15
weeks' storage (Green and Tyler, 1966). On barley in silos the
half-life of fenitrothion was about 100 days (Green and Tyler, 1966)
and on stored bananas about 15 days (Miyamoto et al., 1965a). Applied
to wheat 11% moisture) at rates of 1, 2 and 4 ppm, 0.2, 0.4., and 1.1
ppm of fenitrothion remained, respectively, after 9 months of storage
at 25°C and 60% relative humidity (Kane and Green, 1968).
In extensive trials as a wheat protectant, fenitrothion levels
steadied below 2 ppm after about 2 months regardless of application
rate between 2.5 and 10 ppm. In one trial 6 ppm applied to grain
decreased to 2.4, 1.7, and 1.1 ppm, respectively, after 1´, 4, and 6
months. Flour made from the 2.4-ppm sample contained 0.3 ppm
fenitrothion, while only traces were found in the flour and bread from
the later samples (Cooper Technical Bureau, 1968).
Evidence of residues in food in commerce or at consumption
No report of residues of fenitrothion in food in commerce or at
consumption has been found. Reference is made under "In storage and
processing" to the finding of only "traces" of fenitrothion in flour
and bread prepared from grain containing 1.7 and 1.1 ppm of the
insecticide.
METHODS OF RESIDUE ANALYSIS
Metabolic studies on plants and mammals indicate that residues of
fenitrooxon and aminofenitrooxon, when they do form, occur in small
amount and are degraded or excreted more rapidly than the parent
compound. Once pilot experiments establish that no more than traces of
these compounds form on a given crop, there does not seem to be any
need to analyse for these compounds on that crop in commerce. (For
purposes of this discussion, a trace shall be considered less than 10%
of the tolerance level of the parent compound).
Analyses for the p-nitrocresol have been made and measurable amounts
of the compound are found following good agricultural practice. A
determination of the cresol is sometimes reported. However, if a pilot
experiment on a given crop shows no excessive amount of the cresol to
be present at harvest, there does not appear to be any need for its
analysis on that crop in commerce. Other metabolites are generally
rather polar and do not tend to be stored.
In essence then, the analyst will be concerned with residues of
fenitrothion itself unless pilot experiments indicate that other
metabolites should be taken into account. This same view is expressed
by Frehse and Möllhoff in a IUPAC report by Egan (1969).
Fenitrothion in so similar to parathion and methyl parathion that
analytical methods used for them may be used for fenitrothion.
However, many of the earlier methods lack specificity. Numerous
procedures for a wide variety of harvest products are based on
thin-layer chromatography, spectroscopy and gas chromatography.
Information relating to these analyses follow. No reference to
inter-laboratory collaborative studies on analytical procedures was
found.
Extraction and partitioning
It is not sufficient simply to wash the analytical samples since small
amounts of the active ingredient penetrate into the plant. It is
therefore important that complete extraction of the insecticide and
metabolites (e.g. by exhaustive Soxhlet extraction) be compared
against the extraction method used. Harvest products with a high
content of water have been macerated with acetone (Horler, 1966;
Möllhoff, 1967, 1968), acetonitrile (Coffin and Savary, 1964; Thier
and Bergner, 1966), or ethanol (Fischer, 1968). Very good recoveries
were obtained by Soxhlet extraction with chloroform-methanol (9:1 v/v)
(Bowman and Beroza, 1969). For milk, a combination of polar and
nonpolar extractants is required, e.g. acetone-methylene chloride
(Bowman and Beroza, 1969), ethanol-hexane (Franz and Kovac, 1965).
Extraction with methanol-acetonitrile also proved suitable for milk
samples (Miyamoto et al., 1967). For oil-containing harvest products
with a low water content, use is made of benzene (Dawson et al., 1964;
Horler, 1966), petroleum ether (Kovac and Sohler, 1965), hexane
(Horler, 1966), or chloroform (Yuen, 1966). On account of the
favourable partition coefficients (Bowman and Beroza, 1965; Kovac,
1963), fats and waxes are best removed by partitioning between hexane
and acetonitrile (Franz and Kovac, 1965; Miyamoto et al., 1967;
Möllhoff, 1967; Bowman and Beroza, 1969).
Qualitative analysis
Although no reports of fenitrothion being separated from similar
residues by column chromatography have been noted, separation is
possible with thin-layer or gas chromatography. Interfering active
ingredients are parathion, parathion-methyl, malathion, and fenthion
and the resolution is just sufficient for distinguishing fenitrothion
from these active ingredients (see e.g. Möllhoff, 1968). By comparing
gas chromatograms recorded with a phosphorus detector and an electron
capture detector, fenitrothion can often be clearly differentiated
from malathion and fenthion owing to differences in sensitivity.
Colorimetry
Colorimetric determinations are carried out by the method of Averell
and Norris (1948) or by determination of the cresol after
saponification of the active ingredient with alkali. The limits of
determination are about 0.05 ppm.
Thin-layer chromatography
Thin-layer chromatography has been used for clean-up of the extracts
with final determination by colorimetry after deleting the appropriate
spot from the plate, or for direct determination on the plate after
spraying. Quantitative evaluation is possible in the first instance;
in the second, the evaluation is semiquantitative and more suitable
for confirming identity. About 0.1 ppm of fenitrothion can be
determined in most harvested products. Determination of lesser amounts
is possible with procedures utilizing cholinesterase inhibition
(Ackermann, 1966; Mendoza et al., 1968; Schutzmann and Barthel, 1969;
Winterlin et al., 1968).
Column chromatography
For extract cleanup or separation of metabolites, columns are
suitable, especially those packed with deactivated silica gel (Bowman
and Beroza, 1969), deactivated Florisil (Möllhoff, 1967, 1968), or
deactivated acid aluminum oxide (Horler, 1966). Columns packed with
active Florisil (Beckman and Garber, 1969), magnesium oxide (Coffin
and Savary, 1964), polyethylene-impregnated aluminum oxide (Coffin and
Savary, 1964), and Sephadex LH-20 (Horler, 1968; Ruzicka, et al.,
1968) have also been used.
Gas chromatography
The most reliable and highly sensitive methods for fenitrothion
utilize gas chromatography with either the flame-photometric (Bowman
and Beroza, 1969) or the thermionic detector (Miyamoto et al., 1967,
Sato et al., 1968). These detectors respond to the phosphorus in the
molecule with very high specificity. No cleanup is usually required
with the flame-photometric detector when analysing for either
fenitrothion or its oxygen analogue unless a fatty food is being
analysed. In this case a simple hexane-acetonitrile partition is used.
A cleanup was used with the thermionic detector but it may often be
omitted. Limit of determination is usually 0.01-0.001 ppm.
In the analysis for fenitrooxon a separation from fenitrothion on
silica gel is used. The compound is then determined by gas
chromatography (Bowman and Beroza, 1969) or enzymatically (Miyamoto et
al., 1967). The cresol is determined by electron-capture gas
chromatography (Bowman and Beroza, 1969) or colorimetrically (Miyamoto
et al., 1967) after column separation. Analysis of fenitrothion,
fenitrooxon, and the cresol by electron-capture gas chromatography is
possible (Bowman and Beroza, 1969; Dawson et al., 1964; Horler, 1966;
Möllhoff, 1967) but less specific; sensitivity, about 0.1 ppm, can be
improved with a suitable cleanup.
Determination of metabolites
Gas chromatographic methods have been described for the simultaneous
determination of fenitrothion, fenitrooxon (Bowman and Beroza, 1969;
Möllhoff, 1968), 3-methyl-4-nitro phenol (Bowman and Beroza, 1969;
Miyamoto et al., 1967) and the amino compound of fenitrothion
(Miyamoto et al., 1967). As noted, for market controls determination
of fenitrothion is generally sufficient (Möllhoff, 1968).
NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Tolerance Withholding
Country Crop (ppm) period (days)
Austria 2.0 proposed
Belgium Top fruits and vegetables 0.5 14
China, Rice - 21
Republic of Vegetables - 10
Denmark Beets, apples, peas, clover - 15
Tolerance Withholding
Country Crop (ppm) period (days)
East Africa Mango, cereals - 7
Germany, Field crops, vegetables, fruits, and - 10
Eastern special crops
Crops for Infant food and remedies - 21
Germany Vegetables, root and field crops, 0.4 10
(Fed. Rep.) legumes, fruits, grapes
Finland - 21
France Vegetables, fruits - 15
Italy - 15
Netherlands Cereals, fruits 0.5 21
New Zealand - 14 (7 for
pasture)
Poland Top fruits (except strawberries and - 14
raspberries)
Portugal Vegetables, citrus, fruits, cocoa, coffee - 21
Pasture grass - 10
Spain Vegetables, citrus, fruits - 15-21
Sweden Peas, rape, wheat, barley - 10
Switzerland Top fruits 0.2 in
combination
with
Amidithion
APPRAISAL
Fenitrothion is a broad spectrum insecticide with a much lower acute
mammalian toxicity than many similar insecticides. It is not covered
by patents and is produced by at least six companies. Use of
fenitrothion is almost worldwide (excluding the U.S.A.) for such crops
as rice, fruits, vegetables, cotton, cereals, soybeans, coffee and
tea. Application rates are 0.5-2.0 kg a.i./ha. Waiting intervals are
generally 10-21 days. It is used against spruce budworm (in forests),
insects attacking man, and in some countries to protect stored
products. Its use on pasture land (375 g/ha) results in almost no
residues of fenitrothion in milk or meat (<0.01 ppm). The insecticide
is available as a 95% concentrate, 50% emulsifiable concentrate, 40%
wettable powder, 2.5 and 5% dusts. Composition of technical materials
is not known and is apt to vary depending on manufacturer. Residue
data are available mostly from Europe and Japan.
Although several metabolites of fenitrothion are known to form
(fenitrooxon, desmethyl fenitrothion, aminofenitrothion, and
3-methyl-4-nitrophenol), they do not accumulate in significant amounts
or they appear to be comparatively non-toxic. Unless experimental
trials indicate otherwise, fenitrothion appears to be the only toxic
residue to be determined in products in commerce.
Many methods are available for residue analysis of fenitrothion. The
best of these utilize gas chromatography with the flame photometric or
thermionic detector. Some of these methods may also be used for
analysis of metabolites. Sensitivity is usually better than 0.01 ppm.
Evaluation of these procedures for regulatory purposes is suggested.
Few data on residues in food in commerce or in diets have been
reported.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCE OR
PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to June 1973)
Apples, cherries, grapes, lettuce 0.5 ppm ) Preharvest interval to
) be such that these
Tomatoes 0.2 ppm ) tolerances are not
) exceeded)
Red cabbage, green tea 0.3 ppm
Cocoa 0.1 ppm
The 3-methyl-4-nitrophenol is not included in the recommendations at
this time but the Joint Meeting should review this aspect in due
course.
PRACTICAL RESIDUE LIMITS
Milk (whole) 0.002
Meat or fat 0.03
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Reproduction and teratogenicity studies in animals preferably in
non-human primates.
2. Adequate long-term studies in rodent and non-rodent mammalian
species.
3. Data on residue levels in raw agricultural commodities moving in
commerce and in total diet studies.
4. Data on disappearance of residues during storage, processing and
cooking.
5. Data on rate of residue decline in rice and pre-harvest interval.
6. Before use as a grain protectant, data on persistence of residues
in storage of the grains concerned and definitive data on residues
in bread are needed.
7. Data on occurrence of 4-nitro-3-methylphenol residues and their
significance toxicologically.
8. Information on ingredients in technical products produced by
several manufacturers.
DESIRABLE
1. Observations in man.
2. Evaluation of gas chromatographic methods for regulatory purposes.
REFERENCES
Ackermann, H. (1966) Enzymatischer Nachweis phosphororganischer
Insektizide nach dünnschicht-chromatographischer Trennung. Nahrung,
10:273-4
Averell, P.R. and Norris, M.V. (1948) Estimation of small amounts of
O,O-diethyl-O,p-nitro-phenyl thiophosphate. Anal. Chem. 20:753-6
Bayer (1969) (Farbenfabriken Bayer AG.). Fenitrothion. Unpub. Rept.
Beckman, H. and Garber, D. (1969) Recovery of 65 organophosphorus
pesticides from Florisil with a new solvent elution system. J. Ass.
Offic. Anal. Chem. 52:286-93
Bowman, M.C. and Beroza, M. (1965) Extraction p-values of
pesticides and related compounds in six binary solvent systems.
J. Ass. Offic. Agr. Chem. 48:943-52
Bowman, M.C. and Beroza, M. (1969) Determination of Accothion, its
oxygen analog, and its cresol in corn, grass and milk by gas
chromatography. J. Agr. Food Chem. 17:271-6
Bowman, M.C. (1969) Private communication
Braid, P.E. and Nix, M. (1968) Potentiation of toxicity of Sumithion
by phosphamidon in the rat. Canad. J. Physiol. Pharmacol. 46:145-9
Carshalton (1962) WHO insecticide evaluation and testing programme
- stage I. Mammalian toxicity report OMS 43=S5660, Test for neurotoxic
effects in hens. Unpub. Rept. from the Toxicology Research Unit,
Charshalton. Submitted by Farbenfabriken Bayer AG.
Carshalton. (1964) OMS 43. Summary. OMS 43. Mammalian toxicity.
Unpub. Rept. of the Toxicology Research Unit, Carshalton. Submitted
by Farbenfabriken Bayer AG.
Coffin, D.E. and Savary, G. (1964) Procedure for extraction and
cleanup of plant material prior to determination of organophosphate
residues. J. Ass. Offic. Agr. Chem. 47:875-81
Cooper. (1966) Toxicity of Sumithion. Unpub. Rept. of the Cooper
Technical Bureau. Submitted by Sumitomo Chemical Co., Ltd. Osaka
Cooper. (1968) Technical Bureau. Sumithion as a grain protectant.
Technical report by William Cooper and Nephews, Australia. Cited by
Sumitomo, 1969
Dawson, J.A., Donegan, L. and Thain, E.M. (1964) The determination of
parathion and related insecticides by gas-liquid chromatography
with special reference to fenitrothion residues in cocoa.
Analyst, 89:495-6
Douch, P.G.C., Hook, C.E.R. and Smith, J.N. (1968) Metabolism of
Folithion (dimethyl-4-nitro-3-methylphenyl phosphorothionate).
Australasian J. Pharm., 49: No.584. Suppl. Nr.66, 2.S.
DuBois, K.P. and Puchala, E. (1960) The acute toxicity and
anticholinesterase action of Bayer 41831. Unpub. Rept. from the
Department of Pharmacology, University or Chicago. Submitted by
Farbenfabriken Bayer AG.
DuBois, K.P. and Kinoshita, F. (1963) The acute toxicity of Bayer
41831 in combination with other anticholinesterase insecticides.
Unpub. Rept. from the Department of Pharmacology, University of
Chicago. Submitted by Farbenfabriken Bayer AG.
Fischer, R. (1968) Nachweis und quantitative Bestimmung von
Phosphor-Insektiziden in biologischem Material. III. Arch.
Toxicol., 23:129-35
Franz, J. and Kovac, J. (1965) Bestimmung toxischer Rückstände von
O,O-Dimethyl-O-(3-methyl-4-nitrophenyl)-thiophosphat in Milch. Z.
Anal. Chem. 210:354-8
Frehse, H. and Möllhoff, E. (1969) Organophosphorus insecticide
residue analysis. Evaluation. (A) Metabolic products. In report of
IUPAC Commission on the development, improvement, and standardization
of methods of pesticide residue analysis. Egan, H. J. Ass. Offic.
Anal. Chem. 52:306-9
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14:515-34
Green, A.A. and Tyler, P.S. (1966) A field comparison of malathion,
dichlorvos and fenitrothion for the control of Oryzaephilus
surinamensis (L) infesting stored barley. J. Stored Prod. Res.
1:273-85
Hais, K. and Franz, J. (1965) On the problem of Metathione residues
in milk after disinfestation. Cesk. Hyg. 10.205-8
Hladká, A. and Nosál, M. (1967) The determination of the exposition
to Metathion fenitrothion) on the basis of excreting its metabolite
p-nitro-m-cresol through urine in rats. Internat. Arch.
Gewerbepath. Gewerbehyg. 23:209-14
Hollingworth, R.M., Fukuto, T.R. and Metcalf, R.L. (1967) Selectivity
of Sumithion Compared with Methyl Parathion. Influence of structure
on anticholinesterase activity. Metabolism in the white mouse. J.
Agric. Food Chem., 15:235-49
Horler, D.F. (1966) Determination of fenitrothion on stored barley. J. Stored
Prod. Res. 1:287-90
Horler, D.F. (1968) Gel filtration on Sephadex LH-20. A general
clean-up method for pesticides extracted from grain. J. Sci. Food Agr.
19:229-31
Kane, J. and Green, A.A. (1968) The protection of bagged grain from
insect infestation using fenitrothion. J. Stored Prod. Res. 4 59.
Kimmerle, G. (1962a) Toxikologische Untersuchungen mit dem Wirkstoff
S 5660. Unpub. Rept. from Toxilkolisches und Gewerbehygienisches
Laboratorium. Submitted by Farbenfabriken Bayer AG.
Kimmerle, G. (1962b) S 5660. Unpub. Rept. on the neurotoxic effect
on hens from Toxikologisches und Gewerbehygienisches Laboratorium.
Submitted by Farbenfabriken Bayer AG.
Klimmer, O.R. (1961) Opinion on the toxicity of the compound "S 5660"
of Farbenfabriken Bayer AG., Leverkusen. Unpub. Rept. from
Pharmakologisches Institut der Rheinischen
Friedrich-Wilhelms-Universität, Bonn. Submitted by Farbenfabriken
Bayer AG.
Kovac, J. (1963) Bestimmung von
O,O-Dimethyl-O-(3-methyl-4-nitrophenyl)-thiophosphat in technischen
Produkten nach vorhergehender Abtrennung der Begleitstoffe mittels
Dünnschichtchromatographie. J. Chromatogr. 11:412-3
Kovac, J. and Sohler, E. (1965) Bestimmung von
O,O-Dimethyl-O-(3-methyl-4-nitrophenyl)-thiophosphat-Rückständen
in Obst und Gemüse nach vorangegangener Abtrennung der mitextrahierten
Farbstoffe durch Dünnschichtchromatographie. Z. Anal. Chem. 208.201-4
Mendoza, C.E., Wales, P.J., McLeod, H.A. and McKinley, W.P. (1968)
Thin-layer chromatographic-enzyme inhibition procedure to screen for
organophosphorus pesticides in plant extracts without elaborate
clean-up. Analyst 93:173-7
Misu, Y., Segawa, T., Kuruma, I., Kojima, M. and Takagi, H. (1966)
Subacute toxicity of O,O-dimethyl-O-(3-methyl-4-nitrophenyl)
phosphorothioate (Sumithion) in the rat. Toxicol. appl.
Pharmacol., 9:17-26
Miyamoto, J., Sato, Y., Kadota, T., Fujinami, A. and Endo, M. (1963a)
Studies on the mode of action of organophosphorus compounds. Part I.
Metabolic Fate of p32 labeled Sumithion and Methylparathion
in guinea-pig and white rat. Agric. Biol. Chem. (Tokyo), 27:381-9
Miyamoto, J., Sato, Y., Kadota, T. and Fujinami, A. (1963b) Studies
on the mode of action of organophosphorus compounds. Part II.
Inhibition of mammalian cholinesterase in vivo following the
administration of Sumithion and Methylparathion. Agric.
Biol. Chem. (Tokyo), 27:669-76
Miyamoto, J. (1964a) Studies on the mode of action of organophosphorus
compounds. Part III. Activation and degradation of Sumithion
and Methylparathion in vivo. Agric. Biol. Chem. (Tokyo),
28:411-21
Miyamoto, J. (1964b) Studies on the mode of action of organophosphorus
compounds. Part IV. Penetration of Sumithion, Methylparathion and
their oxygen analogs into guinea pig brain and inhibition of
cholinesterase in vivo. Agric. Biol. Chem. Tokyo), 28:422-30
Miyamoto, J. and Sato, Y. (1965) Determination of insecticide residue
in animal and plant tissues. II. Metabolic fate of Sumithion in rice
plant applied at the preheading stage and its residue in harvested
grains. Botyu-Kagaku, 30:45-9
Miyamoto, J., Kawaguchi, Y. and Sato, Y. (1965a) Determination of
insecticide residue in animal and plant tissues. I. Determination of
Sumithion residues in bananas grown in Formosa. Botyu-Kagaku, 30:9-12
Miyamoto, J., Sato, Y. and Fujikawa, K. (1965b) Determination of
insecticide residue in animal and plant tissues. III. Determination
of residual amount of Sumithion in cocoa beans grown in
Nigeria. Botyu-Kagaku 30:49-51
Miyamoto, J., Kitagawa, K. and Sato, Y. (1966) Metabolism of
organophosphorus insecticides by Bacillus subtilis, with
special emphasis on Sumithion. Jap. J. Exp. Med. 36:211-25
Miyamoto, J., Sato, Y. and Suzuki, S. (1967) Determination of
insecticide residue in animal and plant tissues. IV. Determination
of residual amount of Sumithion and some of its metabolites in fresh
milk. Botyu-Kagaku. 32:95-100
Miyamoto, J. (1969) Mechanism of low toxicity of Sumithion toward
mammals. Residue Reviews, 25:251-64
Miyamoto, J. and Sato, Y. (1969) Determination of insecticide residue
in animal and plant tissues. VI. Determination of Sumithion residue
in cattle tissues. Botyu Kagaku 34:3-6
Möllhoff, E. (1967) Gas chromatographic determination of residues of
E605 products and Agritox in plants and soil samples.
Pflanzenschutz-Nachrichten Bayer 20:557-74
Möllhoff, E. (1968) Beitrag zur Frage der Rückstände und ihrer
Bestimmung in Pflanzen nach Anwendung von Präparaten der E 605 - und
Agritox - Reihe. Pflanzenschutz-Nachrichten Bayer 21:331-58
Muramoto, N. (1967) Unpub. observations, cited by Sumitomo, 1969
Nishizawa, Y., Fujii, K., Kadota, T., Miyamoto, J. and Sakamoto, H.
(1961) Studies on the organophosphorus insecticides. Part VII.
Chemical and biological properties of new low toxic organophosphorus
insecticide. O,o-Dimethyl-O-(3-methyl-4-nitrophenyl) phosphorothioate.
Agric. Biol. Chem. (Japan), 25:605-10
Nosäl, M. and Hladkä, A. (1968) Determination of the exposure to
fenitrothion [O,O-dimethyl-O-(3-methyl-4-nitrophenyl) thiophosphate]
on the basis of the excretion of p-nitro-m-cresol by the urine
of the persons tested. Internat. Arch. Gewerbepath. Gewerbehyg.
25:28-38
Republic of Argentina (1968) - Unpub. Rept. prepared for Codex
Committee on Pesticide Residues
Ruzicka, J.H., Thomson, J., Wheals, B.B. and Wood, N.F. (1968) The
application of gel chromatography to the separation of
pesticides. I. Organophosphorus pesticides. J. Chromatogr.
34:14-20
Sato, Y., Miyamoto, J., and Suzuki, S. (1968) Determination of
insecticide residue in animal and plant tissues. V. A device to
increase the sensitivity of the gas chromatography detector to
organophosphorus insecticides. Bochu Kagaku 33:8-12
Schutzmann, R.L. and Barthel, W.F. (1969) Indoxyl acetate spray
reagent for fluorogenic detection of cholinesterase inhibitors in
environmental samples. J. Ass. Offic. Anal. Chem. 52:151-6
Sumitomo Chemical Company. (1969) Sumithion, Its toxicity, metabolism
and residues. Unpub. Rept.
Thier, H.P. and Bergner, K.G. (1966) Eine Schnellmethode zum Nachweis
wichtiger Schädlings-bekämpfungsmittel in Ost und Gemüse. Deutsche
Lebensmittel-Rundschau 62:399-402
Tomizawa, C. and Kobayashi, A. (1964) Relation between the behaviour
of parathion and Sumithion in cabbage leaves and their
phytotoxicity. Noyaku Seisan Gijutsu 11:30-32; Chem. Abstr. 62,
1387d (1965)
Ueda, K. and Nishimura, M. (1966) Interim report. Chronic toxicity of
Sumithion: two-year feeding study in rats. Unpub. Rept. from the
Department of Hygiene and Toxicology, Tokyo Dental College.
Submitted by Sumitomo Chemical Co., Ltd., Osaka.
Vandanis, A. and Crawford, L.G. (1964) Comparative metabolism of O,
O-dimethyl-O-p-nitrophenyl phosphorothioate (Methylparathion and
O, O-dimethyl-O-(3-methyl-1-nitrophenyl) phosphorothioate
(Sumithion) J. Econ. Entomol. 57:136-9
Vandekar, M. (1965) Observations on the toxicity of carbaryl,
Folithion and 3-isopropropyl-phenyl N-methylcarbamate in a
village-scale trial in Southern Nigeria. Bull. Wld. Hlth Org.
33.107-15
Wilford, K., Lietaert, P.E.A. and Foll, C.V. (1965) Toxicological
observations during large scale field trial of OMS-43 in Northern
Nigeria (preliminary report). WHO working paper 65/TOX/1 prepared
for an informal meeting on toxicology of insecticides. Geneva,
18-24 February 1965
Winterlin, W., Walker, G. and Frank, H. (1968) Detection of
cholinesterase-inhibiting pesticides following separation on
thin-layer chromatograms. J. Agr. Food Chem. 16:808-12
Yasuno, M., Hirakoshi, S. Sasa, M. and Uchida, M. (1965) Inactivation
of some organophosphorus insecticides by bacteria in polluted water.
Jap. J. Exp. Med. 35:545
Yuen, S.H. (1966) Absorptiometric determination of fenitrothion
residues in cocoa beans. Analyst 91:811-3
