
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
FENITROTHION
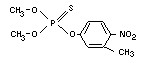 Explanation
The Joint Meeting evaluated fenitrothion in 1969, 1974, 1976,
1977 and 1979 (FAO/WHO 1970, 1975, 1977 and 1978, 1980). 1 An ADI was
partly supported by studies conducted by Industrial Bio-Test
Laboratories (IBT), for which no replacement studies, independently
obtained validations or additional data have been submitted to the
JMPR. Accordingly, the ADI for fenitrothion was re-evaluated.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
COMMENTS
The non-rodent mammalian toxicity studies (Burtner et al 1974;
Lindberg et al 1972; Mastalski 1971 a,b,c) the rabbit teratology
study (Ladd 1971), and the 21-day study using human volunteers
(Garofolo et al 1972) were all performed by IBT and were used as the
basis for the ADI. No independently obtained validations, replacement
studies or other additional data have been submitted to the JMPR
concerning these data. Considering the absence of adequate data on
non-rodent mammalian species, including humans, as well as the need
for an adequate teratology study, the present ADI is retained as a
temporary ADI, using a slightly increased safety factor, pending
receipt of data validations and/or additional data.
1 See Annex 2 for WHO and FAO documentation.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 5 ppm in the diet, equivalent to 0.25 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.001 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1984)
1. A 12-month toxicity study in the dog.
2. A teratology study in a suitable species.
3. Further observations in humans.
REFERENCES
Burtner, B.R., Kennedy, G.L. and Keplinger, M.L. 90-day subacute oral
1974 study. One-year chronic oral cholinesterase activity study,
and two-year chronic oral toxicity study with Sumithion
technical in beagle dogs. Report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Sumitomo Chemical Co. Ltd. (Unpublished)
Garofolo, M., Palazzolo, R.J. and Sanders, R.G. A study on the effects
1972 of sumithon on plasma and erythrocyte cholinesterase
activity in human subjects during subacute oral
administration. Report from Industrial Bio-Test
Laboratories, submitted to the World Health Organization by
Sumitomo Chemical Co. Ltd. (Unpublished)
Ladd, R. Teratogenic study with sumithion(R) technical in albino
1971 rabbits. Report from Industrial Bio-Test Laboratories
submitted to the World Health Organization by Sumitomo
Chemical Co. Ltd. (Unpublished)
Lindberg, D.C., Wright, P.L. and Keplinger, M.L. 90-day subacute oral
1972 toxicity study with sumithion technical in beagle dogs.
Report submitted to the World Health Organization by
Sumitomo Chemical Co. Ltd. (Unpublished)
Mastalski, K. Acute oral toxicity study with Sumioxon pure in beagle
1971a dogs. Report from Industrial Bio-Test Laboratories,
submitted to the World Health Organization by Sumitomo
Chemical Co. Ltd. (Unpublished)
1971b Acute oral toxicity study with p-nitro metacresol in beagle
dogs. Report from Industrial Bio-Test Laboratories submitted
to the World Health Organization by Sumitomo Chemical Co.
Ltd. (Unpublished)
1971c Acute oral toxicity study with sumithion technical grade in
beagle dogs. Report from Industrial Bio-Test Laboratories
submitted to the World Health Organization by Sumitomo
Chemical Co. Ltd. (Unpublished)
Explanation
The Joint Meeting evaluated fenitrothion in 1969, 1974, 1976,
1977 and 1979 (FAO/WHO 1970, 1975, 1977 and 1978, 1980). 1 An ADI was
partly supported by studies conducted by Industrial Bio-Test
Laboratories (IBT), for which no replacement studies, independently
obtained validations or additional data have been submitted to the
JMPR. Accordingly, the ADI for fenitrothion was re-evaluated.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
COMMENTS
The non-rodent mammalian toxicity studies (Burtner et al 1974;
Lindberg et al 1972; Mastalski 1971 a,b,c) the rabbit teratology
study (Ladd 1971), and the 21-day study using human volunteers
(Garofolo et al 1972) were all performed by IBT and were used as the
basis for the ADI. No independently obtained validations, replacement
studies or other additional data have been submitted to the JMPR
concerning these data. Considering the absence of adequate data on
non-rodent mammalian species, including humans, as well as the need
for an adequate teratology study, the present ADI is retained as a
temporary ADI, using a slightly increased safety factor, pending
receipt of data validations and/or additional data.
1 See Annex 2 for WHO and FAO documentation.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 5 ppm in the diet, equivalent to 0.25 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.001 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1984)
1. A 12-month toxicity study in the dog.
2. A teratology study in a suitable species.
3. Further observations in humans.
REFERENCES
Burtner, B.R., Kennedy, G.L. and Keplinger, M.L. 90-day subacute oral
1974 study. One-year chronic oral cholinesterase activity study,
and two-year chronic oral toxicity study with Sumithion
technical in beagle dogs. Report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Sumitomo Chemical Co. Ltd. (Unpublished)
Garofolo, M., Palazzolo, R.J. and Sanders, R.G. A study on the effects
1972 of sumithon on plasma and erythrocyte cholinesterase
activity in human subjects during subacute oral
administration. Report from Industrial Bio-Test
Laboratories, submitted to the World Health Organization by
Sumitomo Chemical Co. Ltd. (Unpublished)
Ladd, R. Teratogenic study with sumithion(R) technical in albino
1971 rabbits. Report from Industrial Bio-Test Laboratories
submitted to the World Health Organization by Sumitomo
Chemical Co. Ltd. (Unpublished)
Lindberg, D.C., Wright, P.L. and Keplinger, M.L. 90-day subacute oral
1972 toxicity study with sumithion technical in beagle dogs.
Report submitted to the World Health Organization by
Sumitomo Chemical Co. Ltd. (Unpublished)
Mastalski, K. Acute oral toxicity study with Sumioxon pure in beagle
1971a dogs. Report from Industrial Bio-Test Laboratories,
submitted to the World Health Organization by Sumitomo
Chemical Co. Ltd. (Unpublished)
1971b Acute oral toxicity study with p-nitro metacresol in beagle
dogs. Report from Industrial Bio-Test Laboratories submitted
to the World Health Organization by Sumitomo Chemical Co.
Ltd. (Unpublished)
1971c Acute oral toxicity study with sumithion technical grade in
beagle dogs. Report from Industrial Bio-Test Laboratories
submitted to the World Health Organization by Sumitomo
Chemical Co. Ltd. (Unpublished)
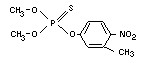 Explanation
The Joint Meeting evaluated fenitrothion in 1969, 1974, 1976,
1977 and 1979 (FAO/WHO 1970, 1975, 1977 and 1978, 1980). 1 An ADI was
partly supported by studies conducted by Industrial Bio-Test
Laboratories (IBT), for which no replacement studies, independently
obtained validations or additional data have been submitted to the
JMPR. Accordingly, the ADI for fenitrothion was re-evaluated.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
COMMENTS
The non-rodent mammalian toxicity studies (Burtner et al 1974;
Lindberg et al 1972; Mastalski 1971 a,b,c) the rabbit teratology
study (Ladd 1971), and the 21-day study using human volunteers
(Garofolo et al 1972) were all performed by IBT and were used as the
basis for the ADI. No independently obtained validations, replacement
studies or other additional data have been submitted to the JMPR
concerning these data. Considering the absence of adequate data on
non-rodent mammalian species, including humans, as well as the need
for an adequate teratology study, the present ADI is retained as a
temporary ADI, using a slightly increased safety factor, pending
receipt of data validations and/or additional data.
1 See Annex 2 for WHO and FAO documentation.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 5 ppm in the diet, equivalent to 0.25 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.001 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1984)
1. A 12-month toxicity study in the dog.
2. A teratology study in a suitable species.
3. Further observations in humans.
REFERENCES
Burtner, B.R., Kennedy, G.L. and Keplinger, M.L. 90-day subacute oral
1974 study. One-year chronic oral cholinesterase activity study,
and two-year chronic oral toxicity study with Sumithion
technical in beagle dogs. Report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Sumitomo Chemical Co. Ltd. (Unpublished)
Garofolo, M., Palazzolo, R.J. and Sanders, R.G. A study on the effects
1972 of sumithon on plasma and erythrocyte cholinesterase
activity in human subjects during subacute oral
administration. Report from Industrial Bio-Test
Laboratories, submitted to the World Health Organization by
Sumitomo Chemical Co. Ltd. (Unpublished)
Ladd, R. Teratogenic study with sumithion(R) technical in albino
1971 rabbits. Report from Industrial Bio-Test Laboratories
submitted to the World Health Organization by Sumitomo
Chemical Co. Ltd. (Unpublished)
Lindberg, D.C., Wright, P.L. and Keplinger, M.L. 90-day subacute oral
1972 toxicity study with sumithion technical in beagle dogs.
Report submitted to the World Health Organization by
Sumitomo Chemical Co. Ltd. (Unpublished)
Mastalski, K. Acute oral toxicity study with Sumioxon pure in beagle
1971a dogs. Report from Industrial Bio-Test Laboratories,
submitted to the World Health Organization by Sumitomo
Chemical Co. Ltd. (Unpublished)
1971b Acute oral toxicity study with p-nitro metacresol in beagle
dogs. Report from Industrial Bio-Test Laboratories submitted
to the World Health Organization by Sumitomo Chemical Co.
Ltd. (Unpublished)
1971c Acute oral toxicity study with sumithion technical grade in
beagle dogs. Report from Industrial Bio-Test Laboratories
submitted to the World Health Organization by Sumitomo
Chemical Co. Ltd. (Unpublished)
Explanation
The Joint Meeting evaluated fenitrothion in 1969, 1974, 1976,
1977 and 1979 (FAO/WHO 1970, 1975, 1977 and 1978, 1980). 1 An ADI was
partly supported by studies conducted by Industrial Bio-Test
Laboratories (IBT), for which no replacement studies, independently
obtained validations or additional data have been submitted to the
JMPR. Accordingly, the ADI for fenitrothion was re-evaluated.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
COMMENTS
The non-rodent mammalian toxicity studies (Burtner et al 1974;
Lindberg et al 1972; Mastalski 1971 a,b,c) the rabbit teratology
study (Ladd 1971), and the 21-day study using human volunteers
(Garofolo et al 1972) were all performed by IBT and were used as the
basis for the ADI. No independently obtained validations, replacement
studies or other additional data have been submitted to the JMPR
concerning these data. Considering the absence of adequate data on
non-rodent mammalian species, including humans, as well as the need
for an adequate teratology study, the present ADI is retained as a
temporary ADI, using a slightly increased safety factor, pending
receipt of data validations and/or additional data.
1 See Annex 2 for WHO and FAO documentation.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 5 ppm in the diet, equivalent to 0.25 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.001 mg/kg bw.
FURTHER WORK OR INFORMATION
Required (by 1984)
1. A 12-month toxicity study in the dog.
2. A teratology study in a suitable species.
3. Further observations in humans.
REFERENCES
Burtner, B.R., Kennedy, G.L. and Keplinger, M.L. 90-day subacute oral
1974 study. One-year chronic oral cholinesterase activity study,
and two-year chronic oral toxicity study with Sumithion
technical in beagle dogs. Report from Industrial Bio-Test
Laboratories, Inc., submitted to the World Health
Organization by Sumitomo Chemical Co. Ltd. (Unpublished)
Garofolo, M., Palazzolo, R.J. and Sanders, R.G. A study on the effects
1972 of sumithon on plasma and erythrocyte cholinesterase
activity in human subjects during subacute oral
administration. Report from Industrial Bio-Test
Laboratories, submitted to the World Health Organization by
Sumitomo Chemical Co. Ltd. (Unpublished)
Ladd, R. Teratogenic study with sumithion(R) technical in albino
1971 rabbits. Report from Industrial Bio-Test Laboratories
submitted to the World Health Organization by Sumitomo
Chemical Co. Ltd. (Unpublished)
Lindberg, D.C., Wright, P.L. and Keplinger, M.L. 90-day subacute oral
1972 toxicity study with sumithion technical in beagle dogs.
Report submitted to the World Health Organization by
Sumitomo Chemical Co. Ltd. (Unpublished)
Mastalski, K. Acute oral toxicity study with Sumioxon pure in beagle
1971a dogs. Report from Industrial Bio-Test Laboratories,
submitted to the World Health Organization by Sumitomo
Chemical Co. Ltd. (Unpublished)
1971b Acute oral toxicity study with p-nitro metacresol in beagle
dogs. Report from Industrial Bio-Test Laboratories submitted
to the World Health Organization by Sumitomo Chemical Co.
Ltd. (Unpublished)
1971c Acute oral toxicity study with sumithion technical grade in
beagle dogs. Report from Industrial Bio-Test Laboratories
submitted to the World Health Organization by Sumitomo
Chemical Co. Ltd. (Unpublished)
