
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
FORMOTHION
IDENTITY
Chemical name
S-(N-formyl-N-methylcarbamoylmethyl) dimethyl phosphorothiolothionate
Synonyms
S-(N-formyl-N-methylcarbamoylmethyl) O,O-dimethyl phosphorodithioate,
Anthio(R)
Structural formula
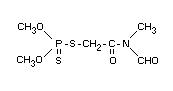 Other relevant chemical properties
Yellow oil with onion-like odour or white crystalline solid; d420
1.361; soluble in alcohols, ether, chloroform, ketones, aromatic
solvents; insoluble in paraffin solvents and water; stable in apolar
solvents, unstable at alkaline pH. Formothion is available as a 25
percent and 40 percent emulsifiable concentrate (ingredients other
than formothion undefined).
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Single oral doses of 10 mg/kg body-weight of formothion, labelled with
carbon14 in the carbamoyl group, were administered by stomach tube to
male rats. Autoradiographs indicated that the compound was readily
absorbed from the stomach. Within 30 minutes after giving the dose, a
high level of radioactivity was found in the liver and kidney,
although the major activity was still in the stomach. Six hours after
administration the activity in the stomach area was low; the main
activity then being in the kidneys, with less activity in the liver,
intestines, pancreas and thymus. After 24 hours from the time of
administration of the dose, distinct radioactivity was found only in
the thymus. The urine was the major source of excretion; it contained
98-99 percent of the radioactivity compared to the faeces which had
only 1-2 percent. A total of 51 per cent of the administered
radioactivity had been excreted in the urine after four hours and 96
percent after 24 hours. The presence of considerable radioactivity in
the bile indicated that there was also excretion of the compound and
its metabolites in the bile. However, because so little radioactivity
was encountered in the faeces nearly all of these compounds must be
re-absorbed in the intestine (Klotsche, 1969a).
Biotransformation
Formothion appears to be completely metabolized in male rats. The only
metabolite found, which is said to be of known structure is termed
"formothion acid", and this compound is eliminated in the urine more
rapidly than other polar metabolites of unknown structure.
("Formothion acid" to presumably O,O-dimethyl-S-carboxymethyl
phosphorodithioate or a phosphate analogue of that compound). All
these metabolites including "formothion acid" contain nearly all the
administered radioactivity, which indicates that they still contain
the carboxyl-carbon of the labelled formothion (Klotsche 1969a).
Although it had been stated that formothion "is converted to
dimethoate in the animal" (rat) (Carshalton, 1965), no dimethoate has
been demonstrated in the metabolic studies performed with formothion
in rats (Klotsche 1969a). However dimethoate has been identified in
plants treated with formothion (Klotsche 1969b).
Effect on enzymes and other biochemical parameters
Experiment in vitro with rat serum cholinesterase have
demonstrated that formothion has anti-cholinesterase activity
(Klotsche 1961). For human serum anti-cholinesterase the I50 was
found to be 1.91 × 10-5 (Zehnder, 1961).
Special studies on neurotoxicity
Chicken
Two groups, each containing three hens were given 100 mg/kg
body-weight of formothion by intramuscular injection; two more groups
were given 150 mg/kg. In one of each of the groups the hens were
protected against the lethal effects of formothion with pralidoxime
and atropine. All the unprotected and one of the protected hens in the
150 mg/kg group died from acute poisoning while the other hens
survived and did not show any clinical symptoms of neurotoxicity
during an observation period of 22 and 29 days after administration of
the 100 and 150 mg/kg doses, respectively. A positive control group
given an unspecified dose of triorthocresyl phosphate (TOCP) displayed
distinct signs of paralysis (Sandoz, 1964a; Klotsche 1966).
Special studies on potentiation
Rat
Single oral doses of formothion were administered jointly to rats with
the following organophosphorus compounds: diazinon, dimethoate,
malathion, parathion, phosphamidon and thiometon. There were no
effects other than additive except for the formothion - dimethoate
combination where there was some indication of a potentiating effect
(Klotsche 1969a),
Special studies on teratogenicity
Rabbit
Groups of 10 rabbits were given daily doses by stomach tube of 6 and
30 mg/kg bodyweight of formothion, from the sixth to the eighteenth
day of pregnancy. Several groups served as controls. Pregnancy rate,
number of implantations, live and dead foetuses, embryonic and foetal
deaths and resorptions were comparable in the test and control groups.
The same was true of the foetal and placental weights and the number
of malformations of organs and the skeletal systems in the foetuses
(Klotsche 1969a).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse (M) oral 190 Klotsche 1966
Mouse (F) oral 195 Klotsche 1966
Rat (M) oral 370-400 Klotsche 1966
Rat (F) oral 500-540 Klotsche 1966
Rat (F) oral 424 Carshalton, 1965
Rat (M) i.v. 36 Klotsche 1966
Guinea-pig oral 560 Sandoz, 1968
Rabbit (M) oral 570 Klotsche 1969a
Rabbit (M) i.v. 20 Klotsche 1969a
Cat (F) oral 213 Klotsche 1966
Chicken i.v. 20 Klotsche 1966
The symptoms of poisoning were the same as for other
anti-cholinesterase organophosphorus compounds. In most of the rat
experiments the onset of symptoms was delayed until 70-90 minutes
after the oral administration (Carshalton, 1965; Sandoz, 1968).
Short-term studies
Chicken
Three groups of young cocks, each group comprising five birds, were
fed for three weeks on grain impregnated with approximately 0, 65 and
330 mg/kg of formothion. In the group that received grain having 330
mg/kg, weight loss occurred in the first week, probably due to food
aversion, but the original weight was restored by the end of the test
period. Haematological examination revealed no abnormalities
indicative of toxic effects in any group. Serum cholinesterase was
normal in the group receiving the wheat containing 65 mg/kg but in
those on the 330 mg/kg level there was a reduction of 30 percent after
10 days and 40 percent after 21 days compared to the controls (Sandoz,
1963; Klotsche, 1966).
Dog
Groups of three dogs each comprising two males and one female were
given doses of 0, 2, 8 mg/kg body-weight of formothion orally in
gelatine capsules for six months. Another group was given 16 mg/kg for
three months and then 32 mg/kg for the remaining three months, while a
further group was given 100 mg/kg for a maximum period of nine weeks.
There were no deaths in the animals given doses of 32 mg/kg or less,
but two of the three dogs in the 100 mg/kg group died. These two dogs,
together with the three controls, one animal in the 2 mg/kg group and
the three animals in the 32 mg/kg group were submitted to gross and
histopathological examination at autopsy. In the 100 mg/kg group there
was a rapid drop in serum cholinesterase, typical symptoms of
anti-cholinesterase poisoning and weight loss. Decrease in eosinophile
leucocyte counts and great increase in relative adrenal weights were
found in the two dogs which died but the surviving dog made rapid
recovery of weight and serum cholinesterase upon withdrawal of
formothion. Food intake, haematology, urinalysis and liver function
tests showed no abnormalities attributable to formothion in the dogs
given 32 mg/kg or lower. A slight loss of weight was observed at 32
mg/kg. Weekly determination of serum cholinesterase activity gave
fluctuating values both in the controls and in the animals given up to
32 mg/kg (Klotsche, 1966).
Pheasant
Three groups of pheasants, each containing two male and two females,
received wheat grain treated with 0, 0.15 or 0.75 percent of
formothion for three weeks. The decrease in food intake in the group
on the high level was thought to be largely responsible for a
considerable loss of weight. The weights in the other groups remained
constant, or increased slightly. No toxic symptoms were noted and
haematology showed no pathological changes in any of the animals. The
activity of serum cholinesterase in the birds on the high strength
compared with that of the controls, was reduced by about 45 percent.
The transaminase values were normal, indicating that there was no
liver damage (Sandoz, 1964b; Klotsche, 1966).
Rat
Two groups, initially comprising 15 male rats per group, were given,
by stomach tube, doses of 35 mg/kg and 90 mg/kg body-weight of
formothion. The doses were administered six-times weekly for one month
and then five times weekly for two months. Only one of the 15 animals
survived 90 mg/kg while 13 survived 35 mg/kg. Serum cholinesterase
after three months was 25 percent and 46 percent of normal in the 90
and 35 mg/kg groups, respectively. No changes due to formothion were
found upon gross and histopathological examination of the dead or in
the surviving animals after sacrifice (Klotzsche, 1966; Klotsche,
1969a).
Groups of rats, initially comprising 25 male and 25 female animals
were given by stomach tube 0, 3, 5, 9 and 16 mg/kg body-weight of
formothion daily for six months. Levels of 3 and 5 mg/kg were
tolerated without any clinical symptoms, while the rats given 9 mg/kg
showed slight, and those given 16 mg/kg appreciable symptoms which
disappeared after four weeks of receiving the compound, indicating
that a tolerance to the compound developed. A decrease in weight-gain
for male rats was found in the 9 and 16 mg/kg groups. Haematological
and urine examination revealed no dose-dependent changes. One rat of
each sex from each group was sacrificed monthly. Some decrease in
serum cholinesterase activity was found in the test groups. In the 3
mg/kg group, though, the activity found from the fourth month and
onward was comparable to the controls. After six months the
cholinesterase activity in the 5 mg/kg group was approximately 50
percent and in the 16 mg/kg group 30 percent of that of the control
group. The average weights of liver and spleen (10 animals) were not
different in the test and control groups. Gross and histological
examinations (two animals from each group) revealed no changes which
could be attributed to formothion (Klotsche, 1966; Klotsche, 1969a).
Groups of varying numbers of rats were given formothion percutaneously
in daily doses of 0, 70 and 140 mg/kg body-weight for three weeks.
After this time, the surviving animals were sacrificed and these
animals and those which died during the treatment were subjected to
autopsy. Gross pathology showed no differences in organ-weights. The
animals which received 140 mg/kg displayed slight histological changes
in the liver (fine-droplet fatty infiltration, nuclear pyknosis,
single cell degeneration) and in the adrenal glands (increased
plasmavacuolization in the zone fasciculata) (Klotsche and Rüttiman,
1965).
Long-term studies
No information available.
COMMENT
Formothion seems to be rapidly and completely metabolized in the rat,
the only animal from which information on metabolism is available. The
analogy of this compound with dimethoate, a transformation product in
plants, was considered but there is no information that such a
transformation occurs in animals, the only metabolite characterized
being "formothion acid". Based upon a six-month study in the dog,
there is a no-effect level of 16 mg/kg body-weight per day. However,
the rat appears to be a more sensitive animal; an effect of 3 mg/kg
body-weight produced a depression of serum cholinesterase and a
no-effect level was not found. There is some indication of a possible
potentiation of formothion and dimethoate. No information is available
on long-term studies in animals or observations in man. The
experimental studies available are considered insufficient for
establishing an acceptable daily intake for man.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Formothion is a phosphorus-containing systemic insecticide with
activity as a contact and stomach poison. It has no ovicidal action.
It is used on a wide variety of crops (cotton, rice, cereals,
sugarcane, oil seeds, sugar-beets, citrus, grapes, fruits, vegetables,
potatoes, tomatoes, coffee, tea, and tobacco) against a wide range of
sucking pests and a number of biting and chewing insects (aphids,
fruit flies, olive flies, Mangold flies, white flies, thrips, Jassids,
mites, scale insects, and mealybugs). Waiting periods between last
application and harvest are generally one or two weeks, depending on
country and crop, but may be as much as six weeks. Formothion is
registered in more than 50 countries in Europe, South America, Africa,
Asia, and Australia.
Pre-harvest treatments
Formothion is usually applied as a 0.1-0.2% spray.
Post-harvest treatments
None are known.
Other uses
Formothion is applied as a spray on ornamental plants and tobacco.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues of formothion, after normal application (0.1-0.2% spray),
generally decrease to less than 1 ppm after 10 days. Persistence of
residues depends on type of crop and environmental factors (rainfall,
temperature). Residues are usually formothion and dimethoate
(O,O-dimethyl S-(N-methylcarbamoylmethyl) phosphorodithioate), the
latter being formed by loss of the N-formyl group. (Dimethoate was
evaluated by FAO/WHO in 1967). The oxygen analogue of dimethoate,
dimethoxon, may also form.
In trials on fruits and vegetables, residues often diminish to 0.1 ppm
or less in 14 days. In some instances (sugar-beets and potatoes) no
residues were found after treatment. In typical trials following
normal practice, the maximum residues found are listed in the
following table:
Typical maximum residues found following normal application
Maximum residues
when sampled,
Spray Day respectively, ppma/
Crop application sampled Formothion Dimethoate
Sugar-beets 0.2% 0,7,10 nd,nd,nd nd,nd,nd
Cherriesb/ 0.2% 0,14,21 0.4,nd,nd 1.6,0.3,0.1
Tomatoes 0.15% 0,29 0.2,nd 0.4,0.06
Grapes 0.15% 0,56 0.8,nd 0.8,0.05
Plums 0.15% 0,16 0.6,<0.05 0.15,<0.05
Coffee leavesc/ 0.5%(2X) 1/4,14,28 61,nd,nd 53,7,0.6
Strawberries 0.2% 0,14,21 0.2,nd,nd 0.4,0.3,0.1
Potatoes 0.2% 3,7 nd,nd nd,nd
Apples 0.2% 1,7 0.25,nd 0.25,0.1
Black currants 0.2% 0,7 0.6,nd 1.2,1.6d/
Snap beans 0.2% 0.5,7 tr,nd 0.75,0.35
Wheat 700 cc/ha 7,14,21 nd,nd,nd 0.12,0.23,0.11
Sugarcane plant ca. 0.3 kg/ha 30 nd nd
(4X)
Typical maximum residues found following normal application (continued)
Maximum residues
when sampled,
Spray Day respectively, ppma/
Crop application sampled Formothion Dimethoate
Olives 0.12% (2X) 10,20 nd,nd nd,nd
Olive oil 0.12% 10,20 nd,nd nd,nd
Oranges
peel 0.12% 15,29 0,17,0.31 1.30,1.70
pulp 0.12% 15,29 nd,nd tr,tr
whole fruit 0.12% 15,29 0.12,nd 0.55,0.18
Grapefruit
peel 0.12% 15,29,96 0.35,0.14,tr 2.10,1.20,0.22
pulp 0.12% 15,29,96 nd,nd,nd 0.14,tr,nd
whole fruit 0.12% 15,29,96 tr,0.08,tr 0.49,0.27,tr
leaves 0.12% 96 nd 0.63
Cole crops 0.2% 60-105 nd nd
a/ tr - trace; nd - none detected. Fingings of more than 0.1 ppm dimethoxon are
given in footnotes.
b/ Up to 0.35 ppm dimethoxon found in other trials. Oxon generally less than
0.1 ppm at 21 days.
c/ Deliberate overdose to study degradation. Test conducted in greenhouse.
d/ 0.2 ppm dimethoxon
FATE OF RESIDUES
According to Sandoz (Anon., 1969) the active residues after treatment
of plants with formothion are formothion, dimethoate, and dimethoxon.
The data of Table I indicate that formothion has usually degraded to
dimethoate almost completely after seven days with the exception of
residues on the peel of oranges and grapefruits, on which 0.17 and
0.35 ppm residues of formothion were found after 15 days.
A recent study on the metabolism of 32P-labelled dimethoate is of
interest because dimethoate is the principal persistent residue of
formothion. It was applied to bean plants four ways, and the only
significant residue besides dimethoate was the oxygen analogue (Lucier
and Menzer, 1968).
Information on the fate of residues in mammals is scanty. However
14C-labelled formothion administered orally to the rat was completely
metabolized, and elimination was essentially complete in 24 hours; the
only metabolite of well known structure is said to be formothion acid
(Anon., 1969); presumably this is the thiocarboxy derivative
CH3O S
\ "
P-S-CH2COOH
/
CH3O
which is also a metabolite of dimethoate in mammals (FAO/WHO, 1968).
Evidence of residues in food in commerce or at consumption
No definite data available. However, the statement is made that
residues are rapidly degraded by high temperature, especially by
cooking.
METHODS OF RESIDUE ANALYSIS
A residue method for formothion should determine the parent
insecticide and the two metabolites, dimethoate and dimethoxon.
The method utilized to obtain almost all the data of this report
requires a thorough cleanup of the plant extract (several column
chromatographies and binary solvent partitions) followed by paper
chromatography with formamide as the immobile phase (Faderl, 1962).
Recoveries are 70-80% for formothion, 85-95% for dimethoate and 70-80%
for dimethoxon. Sensitivities are 0.04-0.06 ppm. Determinations are
semi-quantitative (± 25%) below 3 ppm. A thin-layer chromatographic
procedure is also described but it does not appear to have any
significant advantage over the paper procedure (Anon., 1969). The
paper chromatographic method, although acceptable, is not the method
of choice today.
Since formothion is usually converted to dimethoate within a week and
the waiting interval after treatment is at least one week, only
dimethoate (and possibly dimethoxon) is likely to remain at harvest.
Methods for the analysis of dimethoate (and dimethoxon) already cited
(FAO/WHO, 1968) should therefore be useful for formothion and perhaps
directly applicable. An international interlaboratory study on methods
for dimethoate residues in crops has recommended a colorimetric
procedure devised by Frehse (Joint Dimethoate Residues Panel, 1968).
Use of a gas chromatographic method with flame-photometric or
thermionic detection should give improved accuracy, sensitivity,
specificity, and reliability in analyzing for residues of formothion
and its metabolites. Such a method will be more rapid and less subject
to error because little or no cleanup is needed. As part of method
development an exhaustive extraction (e.g. Soxhlet) should be used to
check on completeness of extraction (Bowman et al., 1968). Gas
chromatographic methods for qualitative detection of both formothion
and dimethoate have appeared (Askew et al., 1969; Ruzicka et al.,
1967; Ebing, 1968). A gas chromatographic procedure for multiresidue
detection and analysis of organophosphorus pesticides is likely to
prove suitable, and such a procedure should be established.
NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Tolerance
(ppm) Withholding
Country (Various fruits and vegetables) period (weeks)
Belgium - 2
Denmark - 1
E.E.C. 0.6 (proposed) -
France - 1
Germany (Fed. Rep.) 0.6* 2
Great Britain - 1
Netherlands 0.5 2
Sweden - 3 (proposed)
Switzerland 0.3 6**
* 0.5 ppm dimethoate + 0.1 ppm formothion
** For cherry flies, 3 weeks
APPRAISAL
Formothion is a phosphorus-containing systemic insecticide used on a
wide variety of crops in many countries to control many sucking pests
and some biting and chewing insects. It is usually applied as a
0.1-0.2 percent spray prepared from a 25 or 40 percent emulsifiable
concentrate (ingredients other than formothion undefined). Residues
are generally 0.3-0.5 ppm or less after one or two weeks and at
harvest usually consist of dimethoate (to which it degrades) and
occasionally traces of dimethoxon.
Information on mammalian metabolism is scanty. However, if the residue
at harvest is dimethoate and its oxon, the information on dimethoate
already on record (FAC/WHO 1968) should suffice.
Formothion and dimethoate should be considered together since their
significant residues appear to be identical at harvest; that is, the
effect of applying dimethoate and formothion will be additive.
No data are available on residues in meat and milk, in total diets, in
foods in commerce, or in cooked or processed foods.
A sensitive and reliable analytical method in needed for regulatory
purposes. A gas chromatographic analysis with a flame photometric or
thermionic detector in suggested. Specificity is high and sensitivity
is usually 0.01 ppm or better. Methods of this kind have been
published and should be evaluated.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TEMPORARY TOLERANCES
Pre-harvest Tolerance
Crop interval, days ppm
Strawberries 14 0.3
Blackcurrants 7 2.0
Insufficient data were available on which to base recommendations for
grapes, wheat and citrus fruit.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for the parent
compound can be established)
1. Short-term studies in a non-rodent mammalian species with
cholinesterase determination.
2. Long-term studies in rats.
3. Information on ingredients in technical products produced by
several manufacturers.
4. Data on animal metabolism and residues in meat and milk of animals
consuming agricultural products treated in accordance with good
agricultural practice.
5. Data on disappearance of residues during storage, processing and
cooking.
6. Data on residue levels in raw agricultural commodities moving in
commerce and in total diet studies.
DESIRABLE
1. Metabolic studies in non-rodent mammalian species.
2. Observations in man.
3. Evaluation of a gas chromatographic method for residue analysis and
for regulatory purposes.
REFERENCES
Anon. (1969) Formothion. An organophosphorus systemic insecticide.
Submitted by the Swiss delegation to the Codex Committee on Pesticide
Residues
Askew, J., Ruzicka, J.H. and Wheals, B.B. (1969) A general method for
the determination of organophosphorus pesticide residues in river
waters and effluents by gas, thin-layer and gel chromatography.
Analyst 94, 275-83
Bowman, M.C., Leuck, D.B. and Beroza, M. (1968) Procedures for
extracting residues of phosphorus insecticides and metabolites
from field-treated crops. J. Agr. Food Chem. 16, 796-802
Carshalton. (1965) WHO insecticide evaluation and testing programme.
Stage I. Toxicity report. OMS 968. Unpub. Rept. from the Toxicology
Research Unit, Carshalton
Ebing, W. (1968) Gaschromatischer Rückstandnachweis von 47
phosphorhaltigen Insektizid-Wirketoffen nach einer Einheitverfahren.
Pflanzenschutz-Berichte 38, 1-22
Faderl, N. (1962) Methode zur Bestimmung von Mikromengen organischer
Phosphorinsektizide. Mitt. Geb. Lebensmittelunters. Hyg. 53,
154-75
FAO/WHO. (1968) 1967 evaluations of some pesticide residues in food.
FAO/PL:1967/M/11/1; WHO/Food Add. 68.30.
Joint Dimethoate Residues Panel. (1968) The determination of dimethoate
residues in fruits and vegetables; report. Analyst 93, 756-66
Klotsche, C. (1961) Formothion, ein neuer systemicher
phosphorsaureester geringerer Giftigkeit. Mitt. Lebensmitt. Hyg.,
52; 340-9
Klotsche, C. and Rüttiman, G. (1965) Subacute dermal toxicity of
Anthio (containing 24% of formothion as active ingredient). Unpub.
Rept. produced and submitted by Sandoz, Ltd., Basle
Klotsche, C. (1966) Toxikologische Untersuchungen mit dem
systemischen phosphorsaureester Formothion. Int. Arch. Gewerbepath.
Gewerbehyg. 22.246-61
Klotsche, C. (1969a) Formothion. An organophosphorus insecticide.
Unpub. Rept. on animal toxicology submitted by Sandoz, Ltd., Basle
Klotsche, C. (1969b) Formothion. An organophosphorus insecticide.
Unpub. Rept. on residues in plants submitted by Sandoz, Ltd., Basle
Lucier, G.W. and Menzer, R.E. (1968) Metabolism of dimethoate in
bean plants in relation to its mode of application. J. Agr. Food
Chem. 16, 936-45
Ruzicka, J., Thomson, J. and Wheals, B.B. (1967) The
gas-chromatographic examination of organophosphorus pesticides and
their oxidation products. J. Chromatogr. 30, 92-9
Sandoz. (1963) Toxicity of Anthio to birds. Unpub. Rept. produced
and submitted by Sandoz, Ltd., Basle
Sandoz. (1964a) Neurotoxicity of Anthio. Unpub. Rept. produced and
submitted by Sandoz, Ltd., Basle
Sandoz. (1964b) Toxicity of Anthio to birds (pheasants) Unpub.
Rept. produced and submitted by Sandoz, Ltd., Basle
Sandoz. (1968) Formothion. Unpub. summary report produced and
submitted by Sandoz, Ltd., Basle
Zehnder, K. (1961) Personal communication cited by Klotsche, 1961
Other relevant chemical properties
Yellow oil with onion-like odour or white crystalline solid; d420
1.361; soluble in alcohols, ether, chloroform, ketones, aromatic
solvents; insoluble in paraffin solvents and water; stable in apolar
solvents, unstable at alkaline pH. Formothion is available as a 25
percent and 40 percent emulsifiable concentrate (ingredients other
than formothion undefined).
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Single oral doses of 10 mg/kg body-weight of formothion, labelled with
carbon14 in the carbamoyl group, were administered by stomach tube to
male rats. Autoradiographs indicated that the compound was readily
absorbed from the stomach. Within 30 minutes after giving the dose, a
high level of radioactivity was found in the liver and kidney,
although the major activity was still in the stomach. Six hours after
administration the activity in the stomach area was low; the main
activity then being in the kidneys, with less activity in the liver,
intestines, pancreas and thymus. After 24 hours from the time of
administration of the dose, distinct radioactivity was found only in
the thymus. The urine was the major source of excretion; it contained
98-99 percent of the radioactivity compared to the faeces which had
only 1-2 percent. A total of 51 per cent of the administered
radioactivity had been excreted in the urine after four hours and 96
percent after 24 hours. The presence of considerable radioactivity in
the bile indicated that there was also excretion of the compound and
its metabolites in the bile. However, because so little radioactivity
was encountered in the faeces nearly all of these compounds must be
re-absorbed in the intestine (Klotsche, 1969a).
Biotransformation
Formothion appears to be completely metabolized in male rats. The only
metabolite found, which is said to be of known structure is termed
"formothion acid", and this compound is eliminated in the urine more
rapidly than other polar metabolites of unknown structure.
("Formothion acid" to presumably O,O-dimethyl-S-carboxymethyl
phosphorodithioate or a phosphate analogue of that compound). All
these metabolites including "formothion acid" contain nearly all the
administered radioactivity, which indicates that they still contain
the carboxyl-carbon of the labelled formothion (Klotsche 1969a).
Although it had been stated that formothion "is converted to
dimethoate in the animal" (rat) (Carshalton, 1965), no dimethoate has
been demonstrated in the metabolic studies performed with formothion
in rats (Klotsche 1969a). However dimethoate has been identified in
plants treated with formothion (Klotsche 1969b).
Effect on enzymes and other biochemical parameters
Experiment in vitro with rat serum cholinesterase have
demonstrated that formothion has anti-cholinesterase activity
(Klotsche 1961). For human serum anti-cholinesterase the I50 was
found to be 1.91 × 10-5 (Zehnder, 1961).
Special studies on neurotoxicity
Chicken
Two groups, each containing three hens were given 100 mg/kg
body-weight of formothion by intramuscular injection; two more groups
were given 150 mg/kg. In one of each of the groups the hens were
protected against the lethal effects of formothion with pralidoxime
and atropine. All the unprotected and one of the protected hens in the
150 mg/kg group died from acute poisoning while the other hens
survived and did not show any clinical symptoms of neurotoxicity
during an observation period of 22 and 29 days after administration of
the 100 and 150 mg/kg doses, respectively. A positive control group
given an unspecified dose of triorthocresyl phosphate (TOCP) displayed
distinct signs of paralysis (Sandoz, 1964a; Klotsche 1966).
Special studies on potentiation
Rat
Single oral doses of formothion were administered jointly to rats with
the following organophosphorus compounds: diazinon, dimethoate,
malathion, parathion, phosphamidon and thiometon. There were no
effects other than additive except for the formothion - dimethoate
combination where there was some indication of a potentiating effect
(Klotsche 1969a),
Special studies on teratogenicity
Rabbit
Groups of 10 rabbits were given daily doses by stomach tube of 6 and
30 mg/kg bodyweight of formothion, from the sixth to the eighteenth
day of pregnancy. Several groups served as controls. Pregnancy rate,
number of implantations, live and dead foetuses, embryonic and foetal
deaths and resorptions were comparable in the test and control groups.
The same was true of the foetal and placental weights and the number
of malformations of organs and the skeletal systems in the foetuses
(Klotsche 1969a).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse (M) oral 190 Klotsche 1966
Mouse (F) oral 195 Klotsche 1966
Rat (M) oral 370-400 Klotsche 1966
Rat (F) oral 500-540 Klotsche 1966
Rat (F) oral 424 Carshalton, 1965
Rat (M) i.v. 36 Klotsche 1966
Guinea-pig oral 560 Sandoz, 1968
Rabbit (M) oral 570 Klotsche 1969a
Rabbit (M) i.v. 20 Klotsche 1969a
Cat (F) oral 213 Klotsche 1966
Chicken i.v. 20 Klotsche 1966
The symptoms of poisoning were the same as for other
anti-cholinesterase organophosphorus compounds. In most of the rat
experiments the onset of symptoms was delayed until 70-90 minutes
after the oral administration (Carshalton, 1965; Sandoz, 1968).
Short-term studies
Chicken
Three groups of young cocks, each group comprising five birds, were
fed for three weeks on grain impregnated with approximately 0, 65 and
330 mg/kg of formothion. In the group that received grain having 330
mg/kg, weight loss occurred in the first week, probably due to food
aversion, but the original weight was restored by the end of the test
period. Haematological examination revealed no abnormalities
indicative of toxic effects in any group. Serum cholinesterase was
normal in the group receiving the wheat containing 65 mg/kg but in
those on the 330 mg/kg level there was a reduction of 30 percent after
10 days and 40 percent after 21 days compared to the controls (Sandoz,
1963; Klotsche, 1966).
Dog
Groups of three dogs each comprising two males and one female were
given doses of 0, 2, 8 mg/kg body-weight of formothion orally in
gelatine capsules for six months. Another group was given 16 mg/kg for
three months and then 32 mg/kg for the remaining three months, while a
further group was given 100 mg/kg for a maximum period of nine weeks.
There were no deaths in the animals given doses of 32 mg/kg or less,
but two of the three dogs in the 100 mg/kg group died. These two dogs,
together with the three controls, one animal in the 2 mg/kg group and
the three animals in the 32 mg/kg group were submitted to gross and
histopathological examination at autopsy. In the 100 mg/kg group there
was a rapid drop in serum cholinesterase, typical symptoms of
anti-cholinesterase poisoning and weight loss. Decrease in eosinophile
leucocyte counts and great increase in relative adrenal weights were
found in the two dogs which died but the surviving dog made rapid
recovery of weight and serum cholinesterase upon withdrawal of
formothion. Food intake, haematology, urinalysis and liver function
tests showed no abnormalities attributable to formothion in the dogs
given 32 mg/kg or lower. A slight loss of weight was observed at 32
mg/kg. Weekly determination of serum cholinesterase activity gave
fluctuating values both in the controls and in the animals given up to
32 mg/kg (Klotsche, 1966).
Pheasant
Three groups of pheasants, each containing two male and two females,
received wheat grain treated with 0, 0.15 or 0.75 percent of
formothion for three weeks. The decrease in food intake in the group
on the high level was thought to be largely responsible for a
considerable loss of weight. The weights in the other groups remained
constant, or increased slightly. No toxic symptoms were noted and
haematology showed no pathological changes in any of the animals. The
activity of serum cholinesterase in the birds on the high strength
compared with that of the controls, was reduced by about 45 percent.
The transaminase values were normal, indicating that there was no
liver damage (Sandoz, 1964b; Klotsche, 1966).
Rat
Two groups, initially comprising 15 male rats per group, were given,
by stomach tube, doses of 35 mg/kg and 90 mg/kg body-weight of
formothion. The doses were administered six-times weekly for one month
and then five times weekly for two months. Only one of the 15 animals
survived 90 mg/kg while 13 survived 35 mg/kg. Serum cholinesterase
after three months was 25 percent and 46 percent of normal in the 90
and 35 mg/kg groups, respectively. No changes due to formothion were
found upon gross and histopathological examination of the dead or in
the surviving animals after sacrifice (Klotzsche, 1966; Klotsche,
1969a).
Groups of rats, initially comprising 25 male and 25 female animals
were given by stomach tube 0, 3, 5, 9 and 16 mg/kg body-weight of
formothion daily for six months. Levels of 3 and 5 mg/kg were
tolerated without any clinical symptoms, while the rats given 9 mg/kg
showed slight, and those given 16 mg/kg appreciable symptoms which
disappeared after four weeks of receiving the compound, indicating
that a tolerance to the compound developed. A decrease in weight-gain
for male rats was found in the 9 and 16 mg/kg groups. Haematological
and urine examination revealed no dose-dependent changes. One rat of
each sex from each group was sacrificed monthly. Some decrease in
serum cholinesterase activity was found in the test groups. In the 3
mg/kg group, though, the activity found from the fourth month and
onward was comparable to the controls. After six months the
cholinesterase activity in the 5 mg/kg group was approximately 50
percent and in the 16 mg/kg group 30 percent of that of the control
group. The average weights of liver and spleen (10 animals) were not
different in the test and control groups. Gross and histological
examinations (two animals from each group) revealed no changes which
could be attributed to formothion (Klotsche, 1966; Klotsche, 1969a).
Groups of varying numbers of rats were given formothion percutaneously
in daily doses of 0, 70 and 140 mg/kg body-weight for three weeks.
After this time, the surviving animals were sacrificed and these
animals and those which died during the treatment were subjected to
autopsy. Gross pathology showed no differences in organ-weights. The
animals which received 140 mg/kg displayed slight histological changes
in the liver (fine-droplet fatty infiltration, nuclear pyknosis,
single cell degeneration) and in the adrenal glands (increased
plasmavacuolization in the zone fasciculata) (Klotsche and Rüttiman,
1965).
Long-term studies
No information available.
COMMENT
Formothion seems to be rapidly and completely metabolized in the rat,
the only animal from which information on metabolism is available. The
analogy of this compound with dimethoate, a transformation product in
plants, was considered but there is no information that such a
transformation occurs in animals, the only metabolite characterized
being "formothion acid". Based upon a six-month study in the dog,
there is a no-effect level of 16 mg/kg body-weight per day. However,
the rat appears to be a more sensitive animal; an effect of 3 mg/kg
body-weight produced a depression of serum cholinesterase and a
no-effect level was not found. There is some indication of a possible
potentiation of formothion and dimethoate. No information is available
on long-term studies in animals or observations in man. The
experimental studies available are considered insufficient for
establishing an acceptable daily intake for man.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Formothion is a phosphorus-containing systemic insecticide with
activity as a contact and stomach poison. It has no ovicidal action.
It is used on a wide variety of crops (cotton, rice, cereals,
sugarcane, oil seeds, sugar-beets, citrus, grapes, fruits, vegetables,
potatoes, tomatoes, coffee, tea, and tobacco) against a wide range of
sucking pests and a number of biting and chewing insects (aphids,
fruit flies, olive flies, Mangold flies, white flies, thrips, Jassids,
mites, scale insects, and mealybugs). Waiting periods between last
application and harvest are generally one or two weeks, depending on
country and crop, but may be as much as six weeks. Formothion is
registered in more than 50 countries in Europe, South America, Africa,
Asia, and Australia.
Pre-harvest treatments
Formothion is usually applied as a 0.1-0.2% spray.
Post-harvest treatments
None are known.
Other uses
Formothion is applied as a spray on ornamental plants and tobacco.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues of formothion, after normal application (0.1-0.2% spray),
generally decrease to less than 1 ppm after 10 days. Persistence of
residues depends on type of crop and environmental factors (rainfall,
temperature). Residues are usually formothion and dimethoate
(O,O-dimethyl S-(N-methylcarbamoylmethyl) phosphorodithioate), the
latter being formed by loss of the N-formyl group. (Dimethoate was
evaluated by FAO/WHO in 1967). The oxygen analogue of dimethoate,
dimethoxon, may also form.
In trials on fruits and vegetables, residues often diminish to 0.1 ppm
or less in 14 days. In some instances (sugar-beets and potatoes) no
residues were found after treatment. In typical trials following
normal practice, the maximum residues found are listed in the
following table:
Typical maximum residues found following normal application
Maximum residues
when sampled,
Spray Day respectively, ppma/
Crop application sampled Formothion Dimethoate
Sugar-beets 0.2% 0,7,10 nd,nd,nd nd,nd,nd
Cherriesb/ 0.2% 0,14,21 0.4,nd,nd 1.6,0.3,0.1
Tomatoes 0.15% 0,29 0.2,nd 0.4,0.06
Grapes 0.15% 0,56 0.8,nd 0.8,0.05
Plums 0.15% 0,16 0.6,<0.05 0.15,<0.05
Coffee leavesc/ 0.5%(2X) 1/4,14,28 61,nd,nd 53,7,0.6
Strawberries 0.2% 0,14,21 0.2,nd,nd 0.4,0.3,0.1
Potatoes 0.2% 3,7 nd,nd nd,nd
Apples 0.2% 1,7 0.25,nd 0.25,0.1
Black currants 0.2% 0,7 0.6,nd 1.2,1.6d/
Snap beans 0.2% 0.5,7 tr,nd 0.75,0.35
Wheat 700 cc/ha 7,14,21 nd,nd,nd 0.12,0.23,0.11
Sugarcane plant ca. 0.3 kg/ha 30 nd nd
(4X)
Typical maximum residues found following normal application (continued)
Maximum residues
when sampled,
Spray Day respectively, ppma/
Crop application sampled Formothion Dimethoate
Olives 0.12% (2X) 10,20 nd,nd nd,nd
Olive oil 0.12% 10,20 nd,nd nd,nd
Oranges
peel 0.12% 15,29 0,17,0.31 1.30,1.70
pulp 0.12% 15,29 nd,nd tr,tr
whole fruit 0.12% 15,29 0.12,nd 0.55,0.18
Grapefruit
peel 0.12% 15,29,96 0.35,0.14,tr 2.10,1.20,0.22
pulp 0.12% 15,29,96 nd,nd,nd 0.14,tr,nd
whole fruit 0.12% 15,29,96 tr,0.08,tr 0.49,0.27,tr
leaves 0.12% 96 nd 0.63
Cole crops 0.2% 60-105 nd nd
a/ tr - trace; nd - none detected. Fingings of more than 0.1 ppm dimethoxon are
given in footnotes.
b/ Up to 0.35 ppm dimethoxon found in other trials. Oxon generally less than
0.1 ppm at 21 days.
c/ Deliberate overdose to study degradation. Test conducted in greenhouse.
d/ 0.2 ppm dimethoxon
FATE OF RESIDUES
According to Sandoz (Anon., 1969) the active residues after treatment
of plants with formothion are formothion, dimethoate, and dimethoxon.
The data of Table I indicate that formothion has usually degraded to
dimethoate almost completely after seven days with the exception of
residues on the peel of oranges and grapefruits, on which 0.17 and
0.35 ppm residues of formothion were found after 15 days.
A recent study on the metabolism of 32P-labelled dimethoate is of
interest because dimethoate is the principal persistent residue of
formothion. It was applied to bean plants four ways, and the only
significant residue besides dimethoate was the oxygen analogue (Lucier
and Menzer, 1968).
Information on the fate of residues in mammals is scanty. However
14C-labelled formothion administered orally to the rat was completely
metabolized, and elimination was essentially complete in 24 hours; the
only metabolite of well known structure is said to be formothion acid
(Anon., 1969); presumably this is the thiocarboxy derivative
CH3O S
\ "
P-S-CH2COOH
/
CH3O
which is also a metabolite of dimethoate in mammals (FAO/WHO, 1968).
Evidence of residues in food in commerce or at consumption
No definite data available. However, the statement is made that
residues are rapidly degraded by high temperature, especially by
cooking.
METHODS OF RESIDUE ANALYSIS
A residue method for formothion should determine the parent
insecticide and the two metabolites, dimethoate and dimethoxon.
The method utilized to obtain almost all the data of this report
requires a thorough cleanup of the plant extract (several column
chromatographies and binary solvent partitions) followed by paper
chromatography with formamide as the immobile phase (Faderl, 1962).
Recoveries are 70-80% for formothion, 85-95% for dimethoate and 70-80%
for dimethoxon. Sensitivities are 0.04-0.06 ppm. Determinations are
semi-quantitative (± 25%) below 3 ppm. A thin-layer chromatographic
procedure is also described but it does not appear to have any
significant advantage over the paper procedure (Anon., 1969). The
paper chromatographic method, although acceptable, is not the method
of choice today.
Since formothion is usually converted to dimethoate within a week and
the waiting interval after treatment is at least one week, only
dimethoate (and possibly dimethoxon) is likely to remain at harvest.
Methods for the analysis of dimethoate (and dimethoxon) already cited
(FAO/WHO, 1968) should therefore be useful for formothion and perhaps
directly applicable. An international interlaboratory study on methods
for dimethoate residues in crops has recommended a colorimetric
procedure devised by Frehse (Joint Dimethoate Residues Panel, 1968).
Use of a gas chromatographic method with flame-photometric or
thermionic detection should give improved accuracy, sensitivity,
specificity, and reliability in analyzing for residues of formothion
and its metabolites. Such a method will be more rapid and less subject
to error because little or no cleanup is needed. As part of method
development an exhaustive extraction (e.g. Soxhlet) should be used to
check on completeness of extraction (Bowman et al., 1968). Gas
chromatographic methods for qualitative detection of both formothion
and dimethoate have appeared (Askew et al., 1969; Ruzicka et al.,
1967; Ebing, 1968). A gas chromatographic procedure for multiresidue
detection and analysis of organophosphorus pesticides is likely to
prove suitable, and such a procedure should be established.
NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Tolerance
(ppm) Withholding
Country (Various fruits and vegetables) period (weeks)
Belgium - 2
Denmark - 1
E.E.C. 0.6 (proposed) -
France - 1
Germany (Fed. Rep.) 0.6* 2
Great Britain - 1
Netherlands 0.5 2
Sweden - 3 (proposed)
Switzerland 0.3 6**
* 0.5 ppm dimethoate + 0.1 ppm formothion
** For cherry flies, 3 weeks
APPRAISAL
Formothion is a phosphorus-containing systemic insecticide used on a
wide variety of crops in many countries to control many sucking pests
and some biting and chewing insects. It is usually applied as a
0.1-0.2 percent spray prepared from a 25 or 40 percent emulsifiable
concentrate (ingredients other than formothion undefined). Residues
are generally 0.3-0.5 ppm or less after one or two weeks and at
harvest usually consist of dimethoate (to which it degrades) and
occasionally traces of dimethoxon.
Information on mammalian metabolism is scanty. However, if the residue
at harvest is dimethoate and its oxon, the information on dimethoate
already on record (FAC/WHO 1968) should suffice.
Formothion and dimethoate should be considered together since their
significant residues appear to be identical at harvest; that is, the
effect of applying dimethoate and formothion will be additive.
No data are available on residues in meat and milk, in total diets, in
foods in commerce, or in cooked or processed foods.
A sensitive and reliable analytical method in needed for regulatory
purposes. A gas chromatographic analysis with a flame photometric or
thermionic detector in suggested. Specificity is high and sensitivity
is usually 0.01 ppm or better. Methods of this kind have been
published and should be evaluated.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TEMPORARY TOLERANCES
Pre-harvest Tolerance
Crop interval, days ppm
Strawberries 14 0.3
Blackcurrants 7 2.0
Insufficient data were available on which to base recommendations for
grapes, wheat and citrus fruit.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for the parent
compound can be established)
1. Short-term studies in a non-rodent mammalian species with
cholinesterase determination.
2. Long-term studies in rats.
3. Information on ingredients in technical products produced by
several manufacturers.
4. Data on animal metabolism and residues in meat and milk of animals
consuming agricultural products treated in accordance with good
agricultural practice.
5. Data on disappearance of residues during storage, processing and
cooking.
6. Data on residue levels in raw agricultural commodities moving in
commerce and in total diet studies.
DESIRABLE
1. Metabolic studies in non-rodent mammalian species.
2. Observations in man.
3. Evaluation of a gas chromatographic method for residue analysis and
for regulatory purposes.
REFERENCES
Anon. (1969) Formothion. An organophosphorus systemic insecticide.
Submitted by the Swiss delegation to the Codex Committee on Pesticide
Residues
Askew, J., Ruzicka, J.H. and Wheals, B.B. (1969) A general method for
the determination of organophosphorus pesticide residues in river
waters and effluents by gas, thin-layer and gel chromatography.
Analyst 94, 275-83
Bowman, M.C., Leuck, D.B. and Beroza, M. (1968) Procedures for
extracting residues of phosphorus insecticides and metabolites
from field-treated crops. J. Agr. Food Chem. 16, 796-802
Carshalton. (1965) WHO insecticide evaluation and testing programme.
Stage I. Toxicity report. OMS 968. Unpub. Rept. from the Toxicology
Research Unit, Carshalton
Ebing, W. (1968) Gaschromatischer Rückstandnachweis von 47
phosphorhaltigen Insektizid-Wirketoffen nach einer Einheitverfahren.
Pflanzenschutz-Berichte 38, 1-22
Faderl, N. (1962) Methode zur Bestimmung von Mikromengen organischer
Phosphorinsektizide. Mitt. Geb. Lebensmittelunters. Hyg. 53,
154-75
FAO/WHO. (1968) 1967 evaluations of some pesticide residues in food.
FAO/PL:1967/M/11/1; WHO/Food Add. 68.30.
Joint Dimethoate Residues Panel. (1968) The determination of dimethoate
residues in fruits and vegetables; report. Analyst 93, 756-66
Klotsche, C. (1961) Formothion, ein neuer systemicher
phosphorsaureester geringerer Giftigkeit. Mitt. Lebensmitt. Hyg.,
52; 340-9
Klotsche, C. and Rüttiman, G. (1965) Subacute dermal toxicity of
Anthio (containing 24% of formothion as active ingredient). Unpub.
Rept. produced and submitted by Sandoz, Ltd., Basle
Klotsche, C. (1966) Toxikologische Untersuchungen mit dem
systemischen phosphorsaureester Formothion. Int. Arch. Gewerbepath.
Gewerbehyg. 22.246-61
Klotsche, C. (1969a) Formothion. An organophosphorus insecticide.
Unpub. Rept. on animal toxicology submitted by Sandoz, Ltd., Basle
Klotsche, C. (1969b) Formothion. An organophosphorus insecticide.
Unpub. Rept. on residues in plants submitted by Sandoz, Ltd., Basle
Lucier, G.W. and Menzer, R.E. (1968) Metabolism of dimethoate in
bean plants in relation to its mode of application. J. Agr. Food
Chem. 16, 936-45
Ruzicka, J., Thomson, J. and Wheals, B.B. (1967) The
gas-chromatographic examination of organophosphorus pesticides and
their oxidation products. J. Chromatogr. 30, 92-9
Sandoz. (1963) Toxicity of Anthio to birds. Unpub. Rept. produced
and submitted by Sandoz, Ltd., Basle
Sandoz. (1964a) Neurotoxicity of Anthio. Unpub. Rept. produced and
submitted by Sandoz, Ltd., Basle
Sandoz. (1964b) Toxicity of Anthio to birds (pheasants) Unpub.
Rept. produced and submitted by Sandoz, Ltd., Basle
Sandoz. (1968) Formothion. Unpub. summary report produced and
submitted by Sandoz, Ltd., Basle
Zehnder, K. (1961) Personal communication cited by Klotsche, 1961
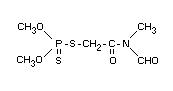 Other relevant chemical properties
Yellow oil with onion-like odour or white crystalline solid; d420
1.361; soluble in alcohols, ether, chloroform, ketones, aromatic
solvents; insoluble in paraffin solvents and water; stable in apolar
solvents, unstable at alkaline pH. Formothion is available as a 25
percent and 40 percent emulsifiable concentrate (ingredients other
than formothion undefined).
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Single oral doses of 10 mg/kg body-weight of formothion, labelled with
carbon14 in the carbamoyl group, were administered by stomach tube to
male rats. Autoradiographs indicated that the compound was readily
absorbed from the stomach. Within 30 minutes after giving the dose, a
high level of radioactivity was found in the liver and kidney,
although the major activity was still in the stomach. Six hours after
administration the activity in the stomach area was low; the main
activity then being in the kidneys, with less activity in the liver,
intestines, pancreas and thymus. After 24 hours from the time of
administration of the dose, distinct radioactivity was found only in
the thymus. The urine was the major source of excretion; it contained
98-99 percent of the radioactivity compared to the faeces which had
only 1-2 percent. A total of 51 per cent of the administered
radioactivity had been excreted in the urine after four hours and 96
percent after 24 hours. The presence of considerable radioactivity in
the bile indicated that there was also excretion of the compound and
its metabolites in the bile. However, because so little radioactivity
was encountered in the faeces nearly all of these compounds must be
re-absorbed in the intestine (Klotsche, 1969a).
Biotransformation
Formothion appears to be completely metabolized in male rats. The only
metabolite found, which is said to be of known structure is termed
"formothion acid", and this compound is eliminated in the urine more
rapidly than other polar metabolites of unknown structure.
("Formothion acid" to presumably O,O-dimethyl-S-carboxymethyl
phosphorodithioate or a phosphate analogue of that compound). All
these metabolites including "formothion acid" contain nearly all the
administered radioactivity, which indicates that they still contain
the carboxyl-carbon of the labelled formothion (Klotsche 1969a).
Although it had been stated that formothion "is converted to
dimethoate in the animal" (rat) (Carshalton, 1965), no dimethoate has
been demonstrated in the metabolic studies performed with formothion
in rats (Klotsche 1969a). However dimethoate has been identified in
plants treated with formothion (Klotsche 1969b).
Effect on enzymes and other biochemical parameters
Experiment in vitro with rat serum cholinesterase have
demonstrated that formothion has anti-cholinesterase activity
(Klotsche 1961). For human serum anti-cholinesterase the I50 was
found to be 1.91 × 10-5 (Zehnder, 1961).
Special studies on neurotoxicity
Chicken
Two groups, each containing three hens were given 100 mg/kg
body-weight of formothion by intramuscular injection; two more groups
were given 150 mg/kg. In one of each of the groups the hens were
protected against the lethal effects of formothion with pralidoxime
and atropine. All the unprotected and one of the protected hens in the
150 mg/kg group died from acute poisoning while the other hens
survived and did not show any clinical symptoms of neurotoxicity
during an observation period of 22 and 29 days after administration of
the 100 and 150 mg/kg doses, respectively. A positive control group
given an unspecified dose of triorthocresyl phosphate (TOCP) displayed
distinct signs of paralysis (Sandoz, 1964a; Klotsche 1966).
Special studies on potentiation
Rat
Single oral doses of formothion were administered jointly to rats with
the following organophosphorus compounds: diazinon, dimethoate,
malathion, parathion, phosphamidon and thiometon. There were no
effects other than additive except for the formothion - dimethoate
combination where there was some indication of a potentiating effect
(Klotsche 1969a),
Special studies on teratogenicity
Rabbit
Groups of 10 rabbits were given daily doses by stomach tube of 6 and
30 mg/kg bodyweight of formothion, from the sixth to the eighteenth
day of pregnancy. Several groups served as controls. Pregnancy rate,
number of implantations, live and dead foetuses, embryonic and foetal
deaths and resorptions were comparable in the test and control groups.
The same was true of the foetal and placental weights and the number
of malformations of organs and the skeletal systems in the foetuses
(Klotsche 1969a).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse (M) oral 190 Klotsche 1966
Mouse (F) oral 195 Klotsche 1966
Rat (M) oral 370-400 Klotsche 1966
Rat (F) oral 500-540 Klotsche 1966
Rat (F) oral 424 Carshalton, 1965
Rat (M) i.v. 36 Klotsche 1966
Guinea-pig oral 560 Sandoz, 1968
Rabbit (M) oral 570 Klotsche 1969a
Rabbit (M) i.v. 20 Klotsche 1969a
Cat (F) oral 213 Klotsche 1966
Chicken i.v. 20 Klotsche 1966
The symptoms of poisoning were the same as for other
anti-cholinesterase organophosphorus compounds. In most of the rat
experiments the onset of symptoms was delayed until 70-90 minutes
after the oral administration (Carshalton, 1965; Sandoz, 1968).
Short-term studies
Chicken
Three groups of young cocks, each group comprising five birds, were
fed for three weeks on grain impregnated with approximately 0, 65 and
330 mg/kg of formothion. In the group that received grain having 330
mg/kg, weight loss occurred in the first week, probably due to food
aversion, but the original weight was restored by the end of the test
period. Haematological examination revealed no abnormalities
indicative of toxic effects in any group. Serum cholinesterase was
normal in the group receiving the wheat containing 65 mg/kg but in
those on the 330 mg/kg level there was a reduction of 30 percent after
10 days and 40 percent after 21 days compared to the controls (Sandoz,
1963; Klotsche, 1966).
Dog
Groups of three dogs each comprising two males and one female were
given doses of 0, 2, 8 mg/kg body-weight of formothion orally in
gelatine capsules for six months. Another group was given 16 mg/kg for
three months and then 32 mg/kg for the remaining three months, while a
further group was given 100 mg/kg for a maximum period of nine weeks.
There were no deaths in the animals given doses of 32 mg/kg or less,
but two of the three dogs in the 100 mg/kg group died. These two dogs,
together with the three controls, one animal in the 2 mg/kg group and
the three animals in the 32 mg/kg group were submitted to gross and
histopathological examination at autopsy. In the 100 mg/kg group there
was a rapid drop in serum cholinesterase, typical symptoms of
anti-cholinesterase poisoning and weight loss. Decrease in eosinophile
leucocyte counts and great increase in relative adrenal weights were
found in the two dogs which died but the surviving dog made rapid
recovery of weight and serum cholinesterase upon withdrawal of
formothion. Food intake, haematology, urinalysis and liver function
tests showed no abnormalities attributable to formothion in the dogs
given 32 mg/kg or lower. A slight loss of weight was observed at 32
mg/kg. Weekly determination of serum cholinesterase activity gave
fluctuating values both in the controls and in the animals given up to
32 mg/kg (Klotsche, 1966).
Pheasant
Three groups of pheasants, each containing two male and two females,
received wheat grain treated with 0, 0.15 or 0.75 percent of
formothion for three weeks. The decrease in food intake in the group
on the high level was thought to be largely responsible for a
considerable loss of weight. The weights in the other groups remained
constant, or increased slightly. No toxic symptoms were noted and
haematology showed no pathological changes in any of the animals. The
activity of serum cholinesterase in the birds on the high strength
compared with that of the controls, was reduced by about 45 percent.
The transaminase values were normal, indicating that there was no
liver damage (Sandoz, 1964b; Klotsche, 1966).
Rat
Two groups, initially comprising 15 male rats per group, were given,
by stomach tube, doses of 35 mg/kg and 90 mg/kg body-weight of
formothion. The doses were administered six-times weekly for one month
and then five times weekly for two months. Only one of the 15 animals
survived 90 mg/kg while 13 survived 35 mg/kg. Serum cholinesterase
after three months was 25 percent and 46 percent of normal in the 90
and 35 mg/kg groups, respectively. No changes due to formothion were
found upon gross and histopathological examination of the dead or in
the surviving animals after sacrifice (Klotzsche, 1966; Klotsche,
1969a).
Groups of rats, initially comprising 25 male and 25 female animals
were given by stomach tube 0, 3, 5, 9 and 16 mg/kg body-weight of
formothion daily for six months. Levels of 3 and 5 mg/kg were
tolerated without any clinical symptoms, while the rats given 9 mg/kg
showed slight, and those given 16 mg/kg appreciable symptoms which
disappeared after four weeks of receiving the compound, indicating
that a tolerance to the compound developed. A decrease in weight-gain
for male rats was found in the 9 and 16 mg/kg groups. Haematological
and urine examination revealed no dose-dependent changes. One rat of
each sex from each group was sacrificed monthly. Some decrease in
serum cholinesterase activity was found in the test groups. In the 3
mg/kg group, though, the activity found from the fourth month and
onward was comparable to the controls. After six months the
cholinesterase activity in the 5 mg/kg group was approximately 50
percent and in the 16 mg/kg group 30 percent of that of the control
group. The average weights of liver and spleen (10 animals) were not
different in the test and control groups. Gross and histological
examinations (two animals from each group) revealed no changes which
could be attributed to formothion (Klotsche, 1966; Klotsche, 1969a).
Groups of varying numbers of rats were given formothion percutaneously
in daily doses of 0, 70 and 140 mg/kg body-weight for three weeks.
After this time, the surviving animals were sacrificed and these
animals and those which died during the treatment were subjected to
autopsy. Gross pathology showed no differences in organ-weights. The
animals which received 140 mg/kg displayed slight histological changes
in the liver (fine-droplet fatty infiltration, nuclear pyknosis,
single cell degeneration) and in the adrenal glands (increased
plasmavacuolization in the zone fasciculata) (Klotsche and Rüttiman,
1965).
Long-term studies
No information available.
COMMENT
Formothion seems to be rapidly and completely metabolized in the rat,
the only animal from which information on metabolism is available. The
analogy of this compound with dimethoate, a transformation product in
plants, was considered but there is no information that such a
transformation occurs in animals, the only metabolite characterized
being "formothion acid". Based upon a six-month study in the dog,
there is a no-effect level of 16 mg/kg body-weight per day. However,
the rat appears to be a more sensitive animal; an effect of 3 mg/kg
body-weight produced a depression of serum cholinesterase and a
no-effect level was not found. There is some indication of a possible
potentiation of formothion and dimethoate. No information is available
on long-term studies in animals or observations in man. The
experimental studies available are considered insufficient for
establishing an acceptable daily intake for man.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Formothion is a phosphorus-containing systemic insecticide with
activity as a contact and stomach poison. It has no ovicidal action.
It is used on a wide variety of crops (cotton, rice, cereals,
sugarcane, oil seeds, sugar-beets, citrus, grapes, fruits, vegetables,
potatoes, tomatoes, coffee, tea, and tobacco) against a wide range of
sucking pests and a number of biting and chewing insects (aphids,
fruit flies, olive flies, Mangold flies, white flies, thrips, Jassids,
mites, scale insects, and mealybugs). Waiting periods between last
application and harvest are generally one or two weeks, depending on
country and crop, but may be as much as six weeks. Formothion is
registered in more than 50 countries in Europe, South America, Africa,
Asia, and Australia.
Pre-harvest treatments
Formothion is usually applied as a 0.1-0.2% spray.
Post-harvest treatments
None are known.
Other uses
Formothion is applied as a spray on ornamental plants and tobacco.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues of formothion, after normal application (0.1-0.2% spray),
generally decrease to less than 1 ppm after 10 days. Persistence of
residues depends on type of crop and environmental factors (rainfall,
temperature). Residues are usually formothion and dimethoate
(O,O-dimethyl S-(N-methylcarbamoylmethyl) phosphorodithioate), the
latter being formed by loss of the N-formyl group. (Dimethoate was
evaluated by FAO/WHO in 1967). The oxygen analogue of dimethoate,
dimethoxon, may also form.
In trials on fruits and vegetables, residues often diminish to 0.1 ppm
or less in 14 days. In some instances (sugar-beets and potatoes) no
residues were found after treatment. In typical trials following
normal practice, the maximum residues found are listed in the
following table:
Typical maximum residues found following normal application
Maximum residues
when sampled,
Spray Day respectively, ppma/
Crop application sampled Formothion Dimethoate
Sugar-beets 0.2% 0,7,10 nd,nd,nd nd,nd,nd
Cherriesb/ 0.2% 0,14,21 0.4,nd,nd 1.6,0.3,0.1
Tomatoes 0.15% 0,29 0.2,nd 0.4,0.06
Grapes 0.15% 0,56 0.8,nd 0.8,0.05
Plums 0.15% 0,16 0.6,<0.05 0.15,<0.05
Coffee leavesc/ 0.5%(2X) 1/4,14,28 61,nd,nd 53,7,0.6
Strawberries 0.2% 0,14,21 0.2,nd,nd 0.4,0.3,0.1
Potatoes 0.2% 3,7 nd,nd nd,nd
Apples 0.2% 1,7 0.25,nd 0.25,0.1
Black currants 0.2% 0,7 0.6,nd 1.2,1.6d/
Snap beans 0.2% 0.5,7 tr,nd 0.75,0.35
Wheat 700 cc/ha 7,14,21 nd,nd,nd 0.12,0.23,0.11
Sugarcane plant ca. 0.3 kg/ha 30 nd nd
(4X)
Typical maximum residues found following normal application (continued)
Maximum residues
when sampled,
Spray Day respectively, ppma/
Crop application sampled Formothion Dimethoate
Olives 0.12% (2X) 10,20 nd,nd nd,nd
Olive oil 0.12% 10,20 nd,nd nd,nd
Oranges
peel 0.12% 15,29 0,17,0.31 1.30,1.70
pulp 0.12% 15,29 nd,nd tr,tr
whole fruit 0.12% 15,29 0.12,nd 0.55,0.18
Grapefruit
peel 0.12% 15,29,96 0.35,0.14,tr 2.10,1.20,0.22
pulp 0.12% 15,29,96 nd,nd,nd 0.14,tr,nd
whole fruit 0.12% 15,29,96 tr,0.08,tr 0.49,0.27,tr
leaves 0.12% 96 nd 0.63
Cole crops 0.2% 60-105 nd nd
a/ tr - trace; nd - none detected. Fingings of more than 0.1 ppm dimethoxon are
given in footnotes.
b/ Up to 0.35 ppm dimethoxon found in other trials. Oxon generally less than
0.1 ppm at 21 days.
c/ Deliberate overdose to study degradation. Test conducted in greenhouse.
d/ 0.2 ppm dimethoxon
FATE OF RESIDUES
According to Sandoz (Anon., 1969) the active residues after treatment
of plants with formothion are formothion, dimethoate, and dimethoxon.
The data of Table I indicate that formothion has usually degraded to
dimethoate almost completely after seven days with the exception of
residues on the peel of oranges and grapefruits, on which 0.17 and
0.35 ppm residues of formothion were found after 15 days.
A recent study on the metabolism of 32P-labelled dimethoate is of
interest because dimethoate is the principal persistent residue of
formothion. It was applied to bean plants four ways, and the only
significant residue besides dimethoate was the oxygen analogue (Lucier
and Menzer, 1968).
Information on the fate of residues in mammals is scanty. However
14C-labelled formothion administered orally to the rat was completely
metabolized, and elimination was essentially complete in 24 hours; the
only metabolite of well known structure is said to be formothion acid
(Anon., 1969); presumably this is the thiocarboxy derivative
CH3O S
\ "
P-S-CH2COOH
/
CH3O
which is also a metabolite of dimethoate in mammals (FAO/WHO, 1968).
Evidence of residues in food in commerce or at consumption
No definite data available. However, the statement is made that
residues are rapidly degraded by high temperature, especially by
cooking.
METHODS OF RESIDUE ANALYSIS
A residue method for formothion should determine the parent
insecticide and the two metabolites, dimethoate and dimethoxon.
The method utilized to obtain almost all the data of this report
requires a thorough cleanup of the plant extract (several column
chromatographies and binary solvent partitions) followed by paper
chromatography with formamide as the immobile phase (Faderl, 1962).
Recoveries are 70-80% for formothion, 85-95% for dimethoate and 70-80%
for dimethoxon. Sensitivities are 0.04-0.06 ppm. Determinations are
semi-quantitative (± 25%) below 3 ppm. A thin-layer chromatographic
procedure is also described but it does not appear to have any
significant advantage over the paper procedure (Anon., 1969). The
paper chromatographic method, although acceptable, is not the method
of choice today.
Since formothion is usually converted to dimethoate within a week and
the waiting interval after treatment is at least one week, only
dimethoate (and possibly dimethoxon) is likely to remain at harvest.
Methods for the analysis of dimethoate (and dimethoxon) already cited
(FAO/WHO, 1968) should therefore be useful for formothion and perhaps
directly applicable. An international interlaboratory study on methods
for dimethoate residues in crops has recommended a colorimetric
procedure devised by Frehse (Joint Dimethoate Residues Panel, 1968).
Use of a gas chromatographic method with flame-photometric or
thermionic detection should give improved accuracy, sensitivity,
specificity, and reliability in analyzing for residues of formothion
and its metabolites. Such a method will be more rapid and less subject
to error because little or no cleanup is needed. As part of method
development an exhaustive extraction (e.g. Soxhlet) should be used to
check on completeness of extraction (Bowman et al., 1968). Gas
chromatographic methods for qualitative detection of both formothion
and dimethoate have appeared (Askew et al., 1969; Ruzicka et al.,
1967; Ebing, 1968). A gas chromatographic procedure for multiresidue
detection and analysis of organophosphorus pesticides is likely to
prove suitable, and such a procedure should be established.
NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Tolerance
(ppm) Withholding
Country (Various fruits and vegetables) period (weeks)
Belgium - 2
Denmark - 1
E.E.C. 0.6 (proposed) -
France - 1
Germany (Fed. Rep.) 0.6* 2
Great Britain - 1
Netherlands 0.5 2
Sweden - 3 (proposed)
Switzerland 0.3 6**
* 0.5 ppm dimethoate + 0.1 ppm formothion
** For cherry flies, 3 weeks
APPRAISAL
Formothion is a phosphorus-containing systemic insecticide used on a
wide variety of crops in many countries to control many sucking pests
and some biting and chewing insects. It is usually applied as a
0.1-0.2 percent spray prepared from a 25 or 40 percent emulsifiable
concentrate (ingredients other than formothion undefined). Residues
are generally 0.3-0.5 ppm or less after one or two weeks and at
harvest usually consist of dimethoate (to which it degrades) and
occasionally traces of dimethoxon.
Information on mammalian metabolism is scanty. However, if the residue
at harvest is dimethoate and its oxon, the information on dimethoate
already on record (FAC/WHO 1968) should suffice.
Formothion and dimethoate should be considered together since their
significant residues appear to be identical at harvest; that is, the
effect of applying dimethoate and formothion will be additive.
No data are available on residues in meat and milk, in total diets, in
foods in commerce, or in cooked or processed foods.
A sensitive and reliable analytical method in needed for regulatory
purposes. A gas chromatographic analysis with a flame photometric or
thermionic detector in suggested. Specificity is high and sensitivity
is usually 0.01 ppm or better. Methods of this kind have been
published and should be evaluated.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TEMPORARY TOLERANCES
Pre-harvest Tolerance
Crop interval, days ppm
Strawberries 14 0.3
Blackcurrants 7 2.0
Insufficient data were available on which to base recommendations for
grapes, wheat and citrus fruit.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for the parent
compound can be established)
1. Short-term studies in a non-rodent mammalian species with
cholinesterase determination.
2. Long-term studies in rats.
3. Information on ingredients in technical products produced by
several manufacturers.
4. Data on animal metabolism and residues in meat and milk of animals
consuming agricultural products treated in accordance with good
agricultural practice.
5. Data on disappearance of residues during storage, processing and
cooking.
6. Data on residue levels in raw agricultural commodities moving in
commerce and in total diet studies.
DESIRABLE
1. Metabolic studies in non-rodent mammalian species.
2. Observations in man.
3. Evaluation of a gas chromatographic method for residue analysis and
for regulatory purposes.
REFERENCES
Anon. (1969) Formothion. An organophosphorus systemic insecticide.
Submitted by the Swiss delegation to the Codex Committee on Pesticide
Residues
Askew, J., Ruzicka, J.H. and Wheals, B.B. (1969) A general method for
the determination of organophosphorus pesticide residues in river
waters and effluents by gas, thin-layer and gel chromatography.
Analyst 94, 275-83
Bowman, M.C., Leuck, D.B. and Beroza, M. (1968) Procedures for
extracting residues of phosphorus insecticides and metabolites
from field-treated crops. J. Agr. Food Chem. 16, 796-802
Carshalton. (1965) WHO insecticide evaluation and testing programme.
Stage I. Toxicity report. OMS 968. Unpub. Rept. from the Toxicology
Research Unit, Carshalton
Ebing, W. (1968) Gaschromatischer Rückstandnachweis von 47
phosphorhaltigen Insektizid-Wirketoffen nach einer Einheitverfahren.
Pflanzenschutz-Berichte 38, 1-22
Faderl, N. (1962) Methode zur Bestimmung von Mikromengen organischer
Phosphorinsektizide. Mitt. Geb. Lebensmittelunters. Hyg. 53,
154-75
FAO/WHO. (1968) 1967 evaluations of some pesticide residues in food.
FAO/PL:1967/M/11/1; WHO/Food Add. 68.30.
Joint Dimethoate Residues Panel. (1968) The determination of dimethoate
residues in fruits and vegetables; report. Analyst 93, 756-66
Klotsche, C. (1961) Formothion, ein neuer systemicher
phosphorsaureester geringerer Giftigkeit. Mitt. Lebensmitt. Hyg.,
52; 340-9
Klotsche, C. and Rüttiman, G. (1965) Subacute dermal toxicity of
Anthio (containing 24% of formothion as active ingredient). Unpub.
Rept. produced and submitted by Sandoz, Ltd., Basle
Klotsche, C. (1966) Toxikologische Untersuchungen mit dem
systemischen phosphorsaureester Formothion. Int. Arch. Gewerbepath.
Gewerbehyg. 22.246-61
Klotsche, C. (1969a) Formothion. An organophosphorus insecticide.
Unpub. Rept. on animal toxicology submitted by Sandoz, Ltd., Basle
Klotsche, C. (1969b) Formothion. An organophosphorus insecticide.
Unpub. Rept. on residues in plants submitted by Sandoz, Ltd., Basle
Lucier, G.W. and Menzer, R.E. (1968) Metabolism of dimethoate in
bean plants in relation to its mode of application. J. Agr. Food
Chem. 16, 936-45
Ruzicka, J., Thomson, J. and Wheals, B.B. (1967) The
gas-chromatographic examination of organophosphorus pesticides and
their oxidation products. J. Chromatogr. 30, 92-9
Sandoz. (1963) Toxicity of Anthio to birds. Unpub. Rept. produced
and submitted by Sandoz, Ltd., Basle
Sandoz. (1964a) Neurotoxicity of Anthio. Unpub. Rept. produced and
submitted by Sandoz, Ltd., Basle
Sandoz. (1964b) Toxicity of Anthio to birds (pheasants) Unpub.
Rept. produced and submitted by Sandoz, Ltd., Basle
Sandoz. (1968) Formothion. Unpub. summary report produced and
submitted by Sandoz, Ltd., Basle
Zehnder, K. (1961) Personal communication cited by Klotsche, 1961
Other relevant chemical properties
Yellow oil with onion-like odour or white crystalline solid; d420
1.361; soluble in alcohols, ether, chloroform, ketones, aromatic
solvents; insoluble in paraffin solvents and water; stable in apolar
solvents, unstable at alkaline pH. Formothion is available as a 25
percent and 40 percent emulsifiable concentrate (ingredients other
than formothion undefined).
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Single oral doses of 10 mg/kg body-weight of formothion, labelled with
carbon14 in the carbamoyl group, were administered by stomach tube to
male rats. Autoradiographs indicated that the compound was readily
absorbed from the stomach. Within 30 minutes after giving the dose, a
high level of radioactivity was found in the liver and kidney,
although the major activity was still in the stomach. Six hours after
administration the activity in the stomach area was low; the main
activity then being in the kidneys, with less activity in the liver,
intestines, pancreas and thymus. After 24 hours from the time of
administration of the dose, distinct radioactivity was found only in
the thymus. The urine was the major source of excretion; it contained
98-99 percent of the radioactivity compared to the faeces which had
only 1-2 percent. A total of 51 per cent of the administered
radioactivity had been excreted in the urine after four hours and 96
percent after 24 hours. The presence of considerable radioactivity in
the bile indicated that there was also excretion of the compound and
its metabolites in the bile. However, because so little radioactivity
was encountered in the faeces nearly all of these compounds must be
re-absorbed in the intestine (Klotsche, 1969a).
Biotransformation
Formothion appears to be completely metabolized in male rats. The only
metabolite found, which is said to be of known structure is termed
"formothion acid", and this compound is eliminated in the urine more
rapidly than other polar metabolites of unknown structure.
("Formothion acid" to presumably O,O-dimethyl-S-carboxymethyl
phosphorodithioate or a phosphate analogue of that compound). All
these metabolites including "formothion acid" contain nearly all the
administered radioactivity, which indicates that they still contain
the carboxyl-carbon of the labelled formothion (Klotsche 1969a).
Although it had been stated that formothion "is converted to
dimethoate in the animal" (rat) (Carshalton, 1965), no dimethoate has
been demonstrated in the metabolic studies performed with formothion
in rats (Klotsche 1969a). However dimethoate has been identified in
plants treated with formothion (Klotsche 1969b).
Effect on enzymes and other biochemical parameters
Experiment in vitro with rat serum cholinesterase have
demonstrated that formothion has anti-cholinesterase activity
(Klotsche 1961). For human serum anti-cholinesterase the I50 was
found to be 1.91 × 10-5 (Zehnder, 1961).
Special studies on neurotoxicity
Chicken
Two groups, each containing three hens were given 100 mg/kg
body-weight of formothion by intramuscular injection; two more groups
were given 150 mg/kg. In one of each of the groups the hens were
protected against the lethal effects of formothion with pralidoxime
and atropine. All the unprotected and one of the protected hens in the
150 mg/kg group died from acute poisoning while the other hens
survived and did not show any clinical symptoms of neurotoxicity
during an observation period of 22 and 29 days after administration of
the 100 and 150 mg/kg doses, respectively. A positive control group
given an unspecified dose of triorthocresyl phosphate (TOCP) displayed
distinct signs of paralysis (Sandoz, 1964a; Klotsche 1966).
Special studies on potentiation
Rat
Single oral doses of formothion were administered jointly to rats with
the following organophosphorus compounds: diazinon, dimethoate,
malathion, parathion, phosphamidon and thiometon. There were no
effects other than additive except for the formothion - dimethoate
combination where there was some indication of a potentiating effect
(Klotsche 1969a),
Special studies on teratogenicity
Rabbit
Groups of 10 rabbits were given daily doses by stomach tube of 6 and
30 mg/kg bodyweight of formothion, from the sixth to the eighteenth
day of pregnancy. Several groups served as controls. Pregnancy rate,
number of implantations, live and dead foetuses, embryonic and foetal
deaths and resorptions were comparable in the test and control groups.
The same was true of the foetal and placental weights and the number
of malformations of organs and the skeletal systems in the foetuses
(Klotsche 1969a).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse (M) oral 190 Klotsche 1966
Mouse (F) oral 195 Klotsche 1966
Rat (M) oral 370-400 Klotsche 1966
Rat (F) oral 500-540 Klotsche 1966
Rat (F) oral 424 Carshalton, 1965
Rat (M) i.v. 36 Klotsche 1966
Guinea-pig oral 560 Sandoz, 1968
Rabbit (M) oral 570 Klotsche 1969a
Rabbit (M) i.v. 20 Klotsche 1969a
Cat (F) oral 213 Klotsche 1966
Chicken i.v. 20 Klotsche 1966
The symptoms of poisoning were the same as for other
anti-cholinesterase organophosphorus compounds. In most of the rat
experiments the onset of symptoms was delayed until 70-90 minutes
after the oral administration (Carshalton, 1965; Sandoz, 1968).
Short-term studies
Chicken
Three groups of young cocks, each group comprising five birds, were
fed for three weeks on grain impregnated with approximately 0, 65 and
330 mg/kg of formothion. In the group that received grain having 330
mg/kg, weight loss occurred in the first week, probably due to food
aversion, but the original weight was restored by the end of the test
period. Haematological examination revealed no abnormalities
indicative of toxic effects in any group. Serum cholinesterase was
normal in the group receiving the wheat containing 65 mg/kg but in
those on the 330 mg/kg level there was a reduction of 30 percent after
10 days and 40 percent after 21 days compared to the controls (Sandoz,
1963; Klotsche, 1966).
Dog
Groups of three dogs each comprising two males and one female were
given doses of 0, 2, 8 mg/kg body-weight of formothion orally in
gelatine capsules for six months. Another group was given 16 mg/kg for
three months and then 32 mg/kg for the remaining three months, while a
further group was given 100 mg/kg for a maximum period of nine weeks.
There were no deaths in the animals given doses of 32 mg/kg or less,
but two of the three dogs in the 100 mg/kg group died. These two dogs,
together with the three controls, one animal in the 2 mg/kg group and
the three animals in the 32 mg/kg group were submitted to gross and
histopathological examination at autopsy. In the 100 mg/kg group there
was a rapid drop in serum cholinesterase, typical symptoms of
anti-cholinesterase poisoning and weight loss. Decrease in eosinophile
leucocyte counts and great increase in relative adrenal weights were
found in the two dogs which died but the surviving dog made rapid
recovery of weight and serum cholinesterase upon withdrawal of
formothion. Food intake, haematology, urinalysis and liver function
tests showed no abnormalities attributable to formothion in the dogs
given 32 mg/kg or lower. A slight loss of weight was observed at 32
mg/kg. Weekly determination of serum cholinesterase activity gave
fluctuating values both in the controls and in the animals given up to
32 mg/kg (Klotsche, 1966).
Pheasant
Three groups of pheasants, each containing two male and two females,
received wheat grain treated with 0, 0.15 or 0.75 percent of
formothion for three weeks. The decrease in food intake in the group
on the high level was thought to be largely responsible for a
considerable loss of weight. The weights in the other groups remained
constant, or increased slightly. No toxic symptoms were noted and
haematology showed no pathological changes in any of the animals. The
activity of serum cholinesterase in the birds on the high strength
compared with that of the controls, was reduced by about 45 percent.
The transaminase values were normal, indicating that there was no
liver damage (Sandoz, 1964b; Klotsche, 1966).
Rat
Two groups, initially comprising 15 male rats per group, were given,
by stomach tube, doses of 35 mg/kg and 90 mg/kg body-weight of
formothion. The doses were administered six-times weekly for one month
and then five times weekly for two months. Only one of the 15 animals
survived 90 mg/kg while 13 survived 35 mg/kg. Serum cholinesterase
after three months was 25 percent and 46 percent of normal in the 90
and 35 mg/kg groups, respectively. No changes due to formothion were
found upon gross and histopathological examination of the dead or in
the surviving animals after sacrifice (Klotzsche, 1966; Klotsche,
1969a).
Groups of rats, initially comprising 25 male and 25 female animals
were given by stomach tube 0, 3, 5, 9 and 16 mg/kg body-weight of
formothion daily for six months. Levels of 3 and 5 mg/kg were
tolerated without any clinical symptoms, while the rats given 9 mg/kg
showed slight, and those given 16 mg/kg appreciable symptoms which
disappeared after four weeks of receiving the compound, indicating
that a tolerance to the compound developed. A decrease in weight-gain
for male rats was found in the 9 and 16 mg/kg groups. Haematological
and urine examination revealed no dose-dependent changes. One rat of
each sex from each group was sacrificed monthly. Some decrease in
serum cholinesterase activity was found in the test groups. In the 3
mg/kg group, though, the activity found from the fourth month and
onward was comparable to the controls. After six months the
cholinesterase activity in the 5 mg/kg group was approximately 50
percent and in the 16 mg/kg group 30 percent of that of the control
group. The average weights of liver and spleen (10 animals) were not
different in the test and control groups. Gross and histological
examinations (two animals from each group) revealed no changes which
could be attributed to formothion (Klotsche, 1966; Klotsche, 1969a).
Groups of varying numbers of rats were given formothion percutaneously
in daily doses of 0, 70 and 140 mg/kg body-weight for three weeks.
After this time, the surviving animals were sacrificed and these
animals and those which died during the treatment were subjected to
autopsy. Gross pathology showed no differences in organ-weights. The
animals which received 140 mg/kg displayed slight histological changes
in the liver (fine-droplet fatty infiltration, nuclear pyknosis,
single cell degeneration) and in the adrenal glands (increased
plasmavacuolization in the zone fasciculata) (Klotsche and Rüttiman,
1965).
Long-term studies
No information available.
COMMENT
Formothion seems to be rapidly and completely metabolized in the rat,
the only animal from which information on metabolism is available. The
analogy of this compound with dimethoate, a transformation product in
plants, was considered but there is no information that such a
transformation occurs in animals, the only metabolite characterized
being "formothion acid". Based upon a six-month study in the dog,
there is a no-effect level of 16 mg/kg body-weight per day. However,
the rat appears to be a more sensitive animal; an effect of 3 mg/kg
body-weight produced a depression of serum cholinesterase and a
no-effect level was not found. There is some indication of a possible
potentiation of formothion and dimethoate. No information is available
on long-term studies in animals or observations in man. The
experimental studies available are considered insufficient for
establishing an acceptable daily intake for man.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Formothion is a phosphorus-containing systemic insecticide with
activity as a contact and stomach poison. It has no ovicidal action.
It is used on a wide variety of crops (cotton, rice, cereals,
sugarcane, oil seeds, sugar-beets, citrus, grapes, fruits, vegetables,
potatoes, tomatoes, coffee, tea, and tobacco) against a wide range of
sucking pests and a number of biting and chewing insects (aphids,
fruit flies, olive flies, Mangold flies, white flies, thrips, Jassids,
mites, scale insects, and mealybugs). Waiting periods between last
application and harvest are generally one or two weeks, depending on
country and crop, but may be as much as six weeks. Formothion is
registered in more than 50 countries in Europe, South America, Africa,
Asia, and Australia.
Pre-harvest treatments
Formothion is usually applied as a 0.1-0.2% spray.
Post-harvest treatments
None are known.
Other uses
Formothion is applied as a spray on ornamental plants and tobacco.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues of formothion, after normal application (0.1-0.2% spray),
generally decrease to less than 1 ppm after 10 days. Persistence of
residues depends on type of crop and environmental factors (rainfall,
temperature). Residues are usually formothion and dimethoate
(O,O-dimethyl S-(N-methylcarbamoylmethyl) phosphorodithioate), the
latter being formed by loss of the N-formyl group. (Dimethoate was
evaluated by FAO/WHO in 1967). The oxygen analogue of dimethoate,
dimethoxon, may also form.
In trials on fruits and vegetables, residues often diminish to 0.1 ppm
or less in 14 days. In some instances (sugar-beets and potatoes) no
residues were found after treatment. In typical trials following
normal practice, the maximum residues found are listed in the
following table:
Typical maximum residues found following normal application
Maximum residues
when sampled,
Spray Day respectively, ppma/
Crop application sampled Formothion Dimethoate
Sugar-beets 0.2% 0,7,10 nd,nd,nd nd,nd,nd
Cherriesb/ 0.2% 0,14,21 0.4,nd,nd 1.6,0.3,0.1
Tomatoes 0.15% 0,29 0.2,nd 0.4,0.06
Grapes 0.15% 0,56 0.8,nd 0.8,0.05
Plums 0.15% 0,16 0.6,<0.05 0.15,<0.05
Coffee leavesc/ 0.5%(2X) 1/4,14,28 61,nd,nd 53,7,0.6
Strawberries 0.2% 0,14,21 0.2,nd,nd 0.4,0.3,0.1
Potatoes 0.2% 3,7 nd,nd nd,nd
Apples 0.2% 1,7 0.25,nd 0.25,0.1
Black currants 0.2% 0,7 0.6,nd 1.2,1.6d/
Snap beans 0.2% 0.5,7 tr,nd 0.75,0.35
Wheat 700 cc/ha 7,14,21 nd,nd,nd 0.12,0.23,0.11
Sugarcane plant ca. 0.3 kg/ha 30 nd nd
(4X)
Typical maximum residues found following normal application (continued)
Maximum residues
when sampled,
Spray Day respectively, ppma/
Crop application sampled Formothion Dimethoate
Olives 0.12% (2X) 10,20 nd,nd nd,nd
Olive oil 0.12% 10,20 nd,nd nd,nd
Oranges
peel 0.12% 15,29 0,17,0.31 1.30,1.70
pulp 0.12% 15,29 nd,nd tr,tr
whole fruit 0.12% 15,29 0.12,nd 0.55,0.18
Grapefruit
peel 0.12% 15,29,96 0.35,0.14,tr 2.10,1.20,0.22
pulp 0.12% 15,29,96 nd,nd,nd 0.14,tr,nd
whole fruit 0.12% 15,29,96 tr,0.08,tr 0.49,0.27,tr
leaves 0.12% 96 nd 0.63
Cole crops 0.2% 60-105 nd nd
a/ tr - trace; nd - none detected. Fingings of more than 0.1 ppm dimethoxon are
given in footnotes.
b/ Up to 0.35 ppm dimethoxon found in other trials. Oxon generally less than
0.1 ppm at 21 days.
c/ Deliberate overdose to study degradation. Test conducted in greenhouse.
d/ 0.2 ppm dimethoxon
FATE OF RESIDUES
According to Sandoz (Anon., 1969) the active residues after treatment
of plants with formothion are formothion, dimethoate, and dimethoxon.
The data of Table I indicate that formothion has usually degraded to
dimethoate almost completely after seven days with the exception of
residues on the peel of oranges and grapefruits, on which 0.17 and
0.35 ppm residues of formothion were found after 15 days.
A recent study on the metabolism of 32P-labelled dimethoate is of
interest because dimethoate is the principal persistent residue of
formothion. It was applied to bean plants four ways, and the only
significant residue besides dimethoate was the oxygen analogue (Lucier
and Menzer, 1968).
Information on the fate of residues in mammals is scanty. However
14C-labelled formothion administered orally to the rat was completely
metabolized, and elimination was essentially complete in 24 hours; the
only metabolite of well known structure is said to be formothion acid
(Anon., 1969); presumably this is the thiocarboxy derivative
CH3O S
\ "
P-S-CH2COOH
/
CH3O
which is also a metabolite of dimethoate in mammals (FAO/WHO, 1968).
Evidence of residues in food in commerce or at consumption
No definite data available. However, the statement is made that
residues are rapidly degraded by high temperature, especially by
cooking.
METHODS OF RESIDUE ANALYSIS
A residue method for formothion should determine the parent
insecticide and the two metabolites, dimethoate and dimethoxon.
The method utilized to obtain almost all the data of this report
requires a thorough cleanup of the plant extract (several column
chromatographies and binary solvent partitions) followed by paper
chromatography with formamide as the immobile phase (Faderl, 1962).
Recoveries are 70-80% for formothion, 85-95% for dimethoate and 70-80%
for dimethoxon. Sensitivities are 0.04-0.06 ppm. Determinations are
semi-quantitative (± 25%) below 3 ppm. A thin-layer chromatographic
procedure is also described but it does not appear to have any
significant advantage over the paper procedure (Anon., 1969). The
paper chromatographic method, although acceptable, is not the method
of choice today.
Since formothion is usually converted to dimethoate within a week and
the waiting interval after treatment is at least one week, only
dimethoate (and possibly dimethoxon) is likely to remain at harvest.
Methods for the analysis of dimethoate (and dimethoxon) already cited
(FAO/WHO, 1968) should therefore be useful for formothion and perhaps
directly applicable. An international interlaboratory study on methods
for dimethoate residues in crops has recommended a colorimetric
procedure devised by Frehse (Joint Dimethoate Residues Panel, 1968).
Use of a gas chromatographic method with flame-photometric or
thermionic detection should give improved accuracy, sensitivity,
specificity, and reliability in analyzing for residues of formothion
and its metabolites. Such a method will be more rapid and less subject
to error because little or no cleanup is needed. As part of method
development an exhaustive extraction (e.g. Soxhlet) should be used to
check on completeness of extraction (Bowman et al., 1968). Gas
chromatographic methods for qualitative detection of both formothion
and dimethoate have appeared (Askew et al., 1969; Ruzicka et al.,
1967; Ebing, 1968). A gas chromatographic procedure for multiresidue
detection and analysis of organophosphorus pesticides is likely to
prove suitable, and such a procedure should be established.
NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Tolerance
(ppm) Withholding
Country (Various fruits and vegetables) period (weeks)
Belgium - 2
Denmark - 1
E.E.C. 0.6 (proposed) -
France - 1
Germany (Fed. Rep.) 0.6* 2
Great Britain - 1
Netherlands 0.5 2
Sweden - 3 (proposed)
Switzerland 0.3 6**
* 0.5 ppm dimethoate + 0.1 ppm formothion
** For cherry flies, 3 weeks
APPRAISAL
Formothion is a phosphorus-containing systemic insecticide used on a
wide variety of crops in many countries to control many sucking pests
and some biting and chewing insects. It is usually applied as a
0.1-0.2 percent spray prepared from a 25 or 40 percent emulsifiable
concentrate (ingredients other than formothion undefined). Residues
are generally 0.3-0.5 ppm or less after one or two weeks and at
harvest usually consist of dimethoate (to which it degrades) and
occasionally traces of dimethoxon.
Information on mammalian metabolism is scanty. However, if the residue
at harvest is dimethoate and its oxon, the information on dimethoate
already on record (FAC/WHO 1968) should suffice.
Formothion and dimethoate should be considered together since their
significant residues appear to be identical at harvest; that is, the
effect of applying dimethoate and formothion will be additive.
No data are available on residues in meat and milk, in total diets, in
foods in commerce, or in cooked or processed foods.
A sensitive and reliable analytical method in needed for regulatory
purposes. A gas chromatographic analysis with a flame photometric or
thermionic detector in suggested. Specificity is high and sensitivity
is usually 0.01 ppm or better. Methods of this kind have been
published and should be evaluated.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TEMPORARY TOLERANCES
Pre-harvest Tolerance
Crop interval, days ppm
Strawberries 14 0.3
Blackcurrants 7 2.0
Insufficient data were available on which to base recommendations for
grapes, wheat and citrus fruit.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for the parent
compound can be established)
1. Short-term studies in a non-rodent mammalian species with
cholinesterase determination.
2. Long-term studies in rats.
3. Information on ingredients in technical products produced by
several manufacturers.
4. Data on animal metabolism and residues in meat and milk of animals
consuming agricultural products treated in accordance with good
agricultural practice.
5. Data on disappearance of residues during storage, processing and
cooking.
6. Data on residue levels in raw agricultural commodities moving in
commerce and in total diet studies.
DESIRABLE
1. Metabolic studies in non-rodent mammalian species.
2. Observations in man.
3. Evaluation of a gas chromatographic method for residue analysis and
for regulatory purposes.
REFERENCES
Anon. (1969) Formothion. An organophosphorus systemic insecticide.
Submitted by the Swiss delegation to the Codex Committee on Pesticide
Residues
Askew, J., Ruzicka, J.H. and Wheals, B.B. (1969) A general method for
the determination of organophosphorus pesticide residues in river
waters and effluents by gas, thin-layer and gel chromatography.
Analyst 94, 275-83
Bowman, M.C., Leuck, D.B. and Beroza, M. (1968) Procedures for
extracting residues of phosphorus insecticides and metabolites
from field-treated crops. J. Agr. Food Chem. 16, 796-802
Carshalton. (1965) WHO insecticide evaluation and testing programme.
Stage I. Toxicity report. OMS 968. Unpub. Rept. from the Toxicology
Research Unit, Carshalton
Ebing, W. (1968) Gaschromatischer Rückstandnachweis von 47
phosphorhaltigen Insektizid-Wirketoffen nach einer Einheitverfahren.
Pflanzenschutz-Berichte 38, 1-22
Faderl, N. (1962) Methode zur Bestimmung von Mikromengen organischer
Phosphorinsektizide. Mitt. Geb. Lebensmittelunters. Hyg. 53,
154-75
FAO/WHO. (1968) 1967 evaluations of some pesticide residues in food.
FAO/PL:1967/M/11/1; WHO/Food Add. 68.30.
Joint Dimethoate Residues Panel. (1968) The determination of dimethoate
residues in fruits and vegetables; report. Analyst 93, 756-66
Klotsche, C. (1961) Formothion, ein neuer systemicher
phosphorsaureester geringerer Giftigkeit. Mitt. Lebensmitt. Hyg.,
52; 340-9
Klotsche, C. and Rüttiman, G. (1965) Subacute dermal toxicity of
Anthio (containing 24% of formothion as active ingredient). Unpub.
Rept. produced and submitted by Sandoz, Ltd., Basle
Klotsche, C. (1966) Toxikologische Untersuchungen mit dem
systemischen phosphorsaureester Formothion. Int. Arch. Gewerbepath.
Gewerbehyg. 22.246-61
Klotsche, C. (1969a) Formothion. An organophosphorus insecticide.
Unpub. Rept. on animal toxicology submitted by Sandoz, Ltd., Basle
Klotsche, C. (1969b) Formothion. An organophosphorus insecticide.
Unpub. Rept. on residues in plants submitted by Sandoz, Ltd., Basle
Lucier, G.W. and Menzer, R.E. (1968) Metabolism of dimethoate in
bean plants in relation to its mode of application. J. Agr. Food
Chem. 16, 936-45
Ruzicka, J., Thomson, J. and Wheals, B.B. (1967) The
gas-chromatographic examination of organophosphorus pesticides and
their oxidation products. J. Chromatogr. 30, 92-9
Sandoz. (1963) Toxicity of Anthio to birds. Unpub. Rept. produced
and submitted by Sandoz, Ltd., Basle
Sandoz. (1964a) Neurotoxicity of Anthio. Unpub. Rept. produced and
submitted by Sandoz, Ltd., Basle
Sandoz. (1964b) Toxicity of Anthio to birds (pheasants) Unpub.
Rept. produced and submitted by Sandoz, Ltd., Basle
Sandoz. (1968) Formothion. Unpub. summary report produced and
submitted by Sandoz, Ltd., Basle
Zehnder, K. (1961) Personal communication cited by Klotsche, 1961
