
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
2-PHENYLPHENOL AND ITS SODIUM SALT
IDENTITY
Chemical names
2-phenylphenol; sodium 2-phenylphenate
Synonyms
for the free phenol; o-hydroxydiphenyl
Structural formulae
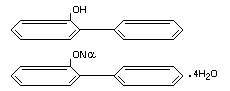 Other relevant chemical properties
The free phenol is a white, crystalline, free-flowing powder,
molecular weight 170.2. Mild phenolic odour. Less than 0.1g is soluble
in 1 ml of water. Freely soluble in ethanol, soluble in fats and oils.
It is dissolved or dispersed in wax formulations.
The sodium salt is a buff coloured solid. Molecular weight 264.3. Very
soluble in water and ethanol; practically insoluble in oils. Used as
water solution.
Purity
Specifications for purity of both compounds have been published by the
European Economic Community (E.E.C., 1967):
E231 2-phenylphenol
melting point 56 - 58°C
content not less than 99%
biphenylether not more than 0.3%
p-phenylphenol not more than 0.1%
alpha-naphthol not more than 0.01%
sulphate after ashing not more than 0.05%
E232 sodium orthophenylphenate
melting point of 2-phenylphenol precipitated by acidification of
sodium salt, not recrystallized and dried over sulphuric acid 56
- 58°C
ph 2% water solution must be between pH 11.1 and 11.8
content not less than 95% C12H ONa.4H2.0
biphenylether not more than 0.3%
p-phenylphenol not more than 0.1%
x-naphthol not more than 0.01%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
The biological data available on this compound have been evaluated by
the Joint FAO/WHO Expert Committee on Food Additives, and a monograph
entitled "Sodium o-phenylphenol" is included in the Sixth Report of
this Committee (FAO/WHO, 1962). A summary of the data then considered
as well as some limited additional information which has recently
become available is included below.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Tissue examination of rats which had received 2-phenylphenol orally
for two years revealed little tendency for storage of the compound.
The kidney was the only organ containing detectable amounts of the
compound; average values of 220 mg/kg tissue were found in the kidneys
of rats fed 20,000 ppm in the diet, and approximately 10 mg/kg tissue
in the kidneys of rats fed 2000 ppm (Hodge et al., 1952).
After administration to rabbits, 2-phenylphenol is highly conjugated
with glucuronic acid but it is not known whether it is converted to an
ethereal sulphate in this species. The glucuronide has been isolated
from rabbit urine and characterized by conversion to the
triacetylmethyl ester (Williams, 1959; Dodgson et al., 1948; Kamil et
al., 1951).
When administered orally to rats, 2-phenylphenol is partly converted
to 2,5-dihydroxybiphenyl which, like 2-phenylphenol, is excreted in
the urine as a glucuronide and ethereal sulphate. The total output of
2-phenylphenol within 48 hours amounted to about 70 percent as
conjugates of 2-phenylphenol and 2,5-dihydroxyphenol (Ernst, 1965).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Groups of 18 mice of each sex from two hybrid strains of mice were
given 2-phenylphenol for 18 months. The dose of 100 mg/kg was given to
the mice by gavage from the seventh day of age to the time of weaning
at four weeks of age and thereafter the chemical was added to the diet
in a corresponding amount of 280 ppm. There was no significant
increase in the incidence of tumours (Innes et al., 1969).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Rat oral 2700-3000 (approx.) MacIntosh, 1945
Hodge et al., 1952
Cat oral 500 (approx.) MacIntosh, 1945
Short-term studies
Dog
A preliminary study demonstrated that when daily doses of 1,000 mg/kg
body-weight of 2-phenylphenol were fed to two dogs, both died within a
month. Groups of two dogs each were then fed 2-phenylphenol for a
period of one year in daily amounts of 0, 20, 200, and 500 mg/kg body
weight. No effect related to the administration of 2-phenylphenol was
observed. Haematological values, urinary sugar and protein values,
organ weights and histopathological examination of the various tissues
did not differ from the normal range (Hodge et al., 1952).
Rat
Groups, each comprising 15 male rats, were fed 2-phenylphenol in daily
doses of 0, 2, 20, or 200 mg/kg body-weight for 32 days. There were no
toxic symptoms attributable to feeding 2-phenylphenol. The mean
growth-rate and the haemoglobin and white blood cell levels in all
groups were comparable to the controls (MacIntosh, 1945).
Five male and five female rats in each group were given by stomach
tube doses of 0, 50, 100, 200, and 500 mg/kg body-weight for five days
a week over a period of six months. The only abnormality observed was
a slight increase in average liver and kidney weights in the animals
fed the 500 mg/kg dose level (Hodge et al., 1952).
When diets containing 0, 1,000, 3,000, 10,000, or 20,000 ppm of
2-phenylphenol were fed for three months to groups of rats comprising
12 males and 12 females, slight retardation of growth was observed in
the 20,000 ppm group. There was no significant difference between the
mortality of control and test animals. There were doubtful increases
in weight of liver, kidney, and spleen of certain rats of the 10,000
and 20,000 ppm groups. No tissue changes were observed (Hodge et al.,
1952)
Long-term studies
Rat
Male and female rats (25 of each sex per group) were maintained for
two years on diets containing 0, 200, 2,000 and 20,000 ppm of
2-phenylphenol. The animals fed 200 and 2000 ppm showed no adverse
effects when compared with the controls, as judged by growth,
mortality, gross appearance, haematology, urinary sugar and protein
values, organ-weights, tissue content of 2-phenylphenol, and
histopathological examination of various tissues. The group fed 20,000
ppm of 2-phenylphenol differed from the controls by exhibiting slight
retardation of growth, histological kidney changes (marked tubular
dilation), and the presence of small amounts of 2-phenylphenol in the
kidney tissues (Hodge et al., 1952).
OBSERVATION IN MAN
A 5.0 percent solution of 2-phenylphenol in sesame oil and a 0.1
percent aqueous solution of the sodium salt tested on 200 subjects
caused neither primary skin irritation nor skin sensitization. The
sodium salt is slightly irritating in 0.5 percent aqueous solution and
decidedly irritating in 1.0 percent and 5.0 percent solutions (Hodge
et al., 1952).
COMMENT
The short and long-term studies in rats are sufficient to be taken as
a basis for the evaluation of 2-phenylphenol. The dog study was not
considered adequate. In addition, the studies on metabolism are
incomplete and there are no studies on reproduction. However, the
Meeting considered that the toxicological studies were adequate to
reaffirm the previous acceptable daily intake established by the Joint
FAO/WHO Expert Committee on Food Additives in its Sixth Report
(FAO/WHO, 1962).
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Dog: 500 mg/kg body-weight/day
Rat: 2000 ppm in the diet, equivalent to 100 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 1.0 mg/kg body-weight (based upon the previous adi. - FAO/WHO,
1962)
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
In Great Britain a dormant paint application to apple trees is
approved for control of canker.
Post-harvest treatments
2-phenylphenol has been used broadly for over 40 years in many
countries for its general antimicrobial properties. Due to its broad
spectrum bactericidal and fungistatic effect, and commercial
availability it is widely used. It is employed as a household
disinfectant, preservative in oil-water emulsions used in the textile
and metal industries, as well as a post-harvest treatment for fruits
and vegetables to prevent losses due to rots and moulds in storage and
transport of these foods. The sodium salt is used as a preservative
for protein-based and other adhesives, as well as for the post-harvest
use in fruits and vegetables.
Principal formulations in the U.S.A. are:
2-phenylphenol: 5% liquid; 98% wettable powder; 2% in wax.
Wax formulations are applied by commercial application equipment in
tandem with sorting and washing facilities. These are specifically
designed for the crop concerned as to technology of use, formulation
strength, time of immersion in the bath and monitoring of the process
to ensure maintenance of treatment criteria for effectiveness without
damage to the crop. Wax strength formulations vary, e.g. carrots 0.5%,
citrus 0.8%, cucumbers 2.5%, nectarines 0.2%, peaches 0.2%; peppers
2.5%, plums 2%, tomatoes 2.5%. A 5% liquid dip is used for crates,
field boxes, hampers, lugs and wood containers for fruits and
vegetables (U.S.D.A., 1967).
sodium o-phenyl phenate: 97% wettable powder, solutions ranging from
1.5% to 40%, wax emulsion from 0.7% to 2.0%.
The strength of solution used varies with the crop concerned and the
time of immersion to obtain protection and avoid phytotoxic reactions
in fruits or vegetables. The times of immersion will vary from a few
seconds to several minutes, but are precisely prescribed and
monitoring is required for each formulation and crop. Examples
includes apples 0.45% - 1.4% solution, bananas 2% applied to cut stem
area of crown of head area (no residue in fruit or skin), cantaloups
1.5%, carrots 0.1%, cherries 1.0% with wetting agent, citrus 0.5% wax
emulsion to 3% in water (plus caustic and hexamine), cucumbers 1%
- 2%, nectarines 1%, peaches 0.05 - 0.6%, pears 0.23% to 1.45%,
peppers 1%, pineapple 1.25% (1% in wax), plums 0.1%, sweet potatoes
0.37% - 0.5% solution or 0.7% wax emulsion, tomatoes 1% (U.S.D.A.,
1967).
These use patterns were available from the U.S.A. only.
RESIDUES RESULTING FROM SUPERVISED TRIALS
As mentioned above, the technology for use of these compounds has been
developed under commercial conditions requiring accurate control of
time of treatment which may be from as brief as 10 seconds (apples and
pears) to 15-18 minutes (peaches), at specified temperatures, pH etc.
The data pertaining to the conditions of these trials were collected
from several locations in the U.S.A. They are unpublished. They have
been collated and submitted with full details to FAO by the Dow
Chemical Company (Dow, 1969).
A review of several abstract journals revealed no published literature
bearing on this subject from other countries. It is known that both
compounds are used by several citrus exporting countries, but these
have not been identified, nor were use patterns, technology of use or
data on residues resulting from supervised trials supplied for
evaluation from other than the U.S.A.
The results of U.S.A. trials are summarized in Tables 1 and 2 (Dow,
1969).
TABLE I
Residues resulting from supervised trials with 2-phenylphenol
Percent 2-phenylphenol Range of residues as
Fruit or vegetable in wax* ppm o-phenylphenol
Not rinsed
Carrots 0.5 17.0
Cucumbers 0.5-2 4.2-5.6
Peppers 2 0.1-1.0
Plums 2 2.5-12.8
Sweet potatoes 1 4.0-5.7
Tomatoes 2-3 0.1-7.7
*Concentration varies according to crop and/or contact time
TABLE 2
Residues resulting from supervised trials with Sodium o-phenylphenate
Percent sodium o-phenylphenate Range of residues as
Fruit or vegetable in water* ppm o-phenylphenol
Rinsed Not rinsed
Apples 0.42-0.77 0.2-14.0
Cantaloups 1.5
edible portion 1.0-6.0
whole fruit 42.0-117.0
Carrots 0.1 2.8-11.0
Cherries 0.65-1.08 0.3-2
Citrus 2.0 5.5-9.3
Cucumbers 1.0 1.3-7.7
Nectarines 0.75-1.2 0.4-1.4
Peaches 0.1-0.12 9.0-15.0
Pears 0.4-0.99 1.0-25.0 1.0-22.0
Peppers 1.0 4.2-7.1
Pineapples 1.25 3.8-7.1
Plums 0.4 0.8-1.3
Sweet potatoes 0.5-1.0 0.5-11.0 1.7-3.4
Tomatoes 0.9 0.5-1.4
*Concentration varies according to crop and/or contact time
FATE OF RESIDUES
Carrots treated with 0.1% sodium o-phenylphenate, stored and steam or
abrasion peeled prior to canning in a commercial manner contained no
residues in the canned product. In cantaloups the residue is mostly in
the unedible portion (42.0-117 ppm in whole fruit, 2.8-11.0 ppm in
edible portion) (Dow, 1969). The value of OPP residues found in peel
of oranges varies slightly with variety. Shamoute oranges peel varied
between 5.9-23 ppm; Valencias between 8.8 and 21.3 ppm; grapefruit
peel 7.0-26.1 ppm; lemons 5.4-15.6 ppm in peel. The quantities of OPP
in pulp of citrus is very low. In many cases none could be detected,
but when peel contained the higher levels, OPP residues from trace to
0.4 ppm were found in pulp (Rajzman and Apfelbaum, 1968).
There is no information available on possible chemical alteration of
the nature of the residue. All residue analysis methods to date rely
on expressing the residue as 2-phenylphenol.
Evidence of residues in food in commerce or at consumption
Information on the residue content of food in commerce is rare. Only
one published paper has a bearing on this subject (Rajzman and
Apfelbaum, 1968). Citrus fruit treated in 22 packing houses in Israel
were collected, sampled and analysed. The residues in whole fruit
range from 1.8 to 8.3 ppm with 80% of the samples containing less than
5 ppm. The authors conclude that in view of the small number of
samples used, their finding of a maximum of 8.3 ppm should not be
considered as the maximum quantity which can occur in citrus treated
in the manner described.
There is no information available with regard to residues in food at
the time of consumption. It can be assumed there will be little loss
of residue in wax-treated fruits and vegetables. Since the treatment
is by post-harvest application, the amounts in wax-treated food
reaching the consumer can be considered as those reported as resulting
from supervised trials. Possibly slightly lower levels will remain in
food treated with water formulations.
METHODS OF RESIDUE ANALYSIS
Colorimetric procedures have most often been proposed for the
determination of residues of 2-phenylphenol. Tomkins and Isherwood
(1945) used coupling with diazotized sulphanilic acid in determining
residues in oranges and marmalade after a steam-distillation
extraction, with acid-alkali treatment clean-up stages. Gottleib and
Marsh (1946) suggested an aminophenazone procedure for the
determination of several phenolic fungicides. The results of a
collaborative study of this method as applied to the determination of
residues of 2-phenylphenol in apples, pears and citrus fruit have been
reported by Schiffman (1957); Hayward and Grierson (1960) used this
method on oranges. A similar procedure has been officially recommended
by the Communauté Economique Européene (van Elslande, 1967) for
residues in fruits and this should be suitable for regulatory purposes
at the present time. A modified steam-distillation extraction
apparatus has been described by Leinbach and Brekke (1961) who used an
indophenol colorimetric method for residues in purees of raspberry,
strawberry, prunes, loganberry and fig and in orange juice. The
coloration given by reacting 2-phenylphenol with titanium sulphate
(Caulfield and Robinson, 1953) is not sufficiently sensitive for
residue analysis. A spectrofluorometric procedure for the examination
of single fruits was proposed by the Cotta-Ramusino and Stacchini
(1966) which is sensitive to <0.1 mg of 2-phenylphenol for fruit,
Gunther et al. (1963) reported a procedure for the routine examination
of citrus fruits for biphenyl and 2-phenylphenol, determining the
latter colorimetrically after coupling with p-nitrofluoroborate, and
obtained recoveries ranging from 89 to 100 percent at the 0.5 to 10
ppm level.
Chromatographic methods available include a gas chromatographic
examination of concentrated orange juice (Thomas, 1960), applicable
over the range 1 to 10 ppm on 1 ml of sample and a thin-layer
chromatographic procedure for the detection of residues of biphenyl
and 2-phenylphenol in lemons (Chioffi, 1965). Mestres and Chave,
(1965) used extraction with cyclohexane in a Dean and Stark apparatus
before separating and determining biphenyl and 2-phenylphenol by gas
chromatography with flame-ionisation detection. These chromatographic
techniques should be developed and evaluated for application as
regulatory procedures.
NATIONAL TOLERANCES
Tolerance (ppm)
Country Crop as 2-phenylphenol
Canada Cherries, nectarines 5
Citrus fruits, cucumbers,
pepper (bell), pineapple,
tomatoes 10
Sweet potatoes 15
Carrots, peaches, plums 20
Apples, pears 25
Cantaloups 125
E.E.C. Citrus fruits 12
Netherlands Citrus fruits 10
United Kingdom Citrus fruit 70
Apples, pears, pineapples 10
Melons 125
NATIONAL TOLERANCES (continued)
Tolerance (ppm)
Country Crop as 2-phenylphenol
United States Cherries, nectarines 5
of America Citrus, citron, cucumbers, grapefruit, 10
kumquats, lemons, limes oranges,
peppers (bell), pineapple, tangelos,
tangerines, tomatoes
Sweet potatoes 15
Carrots, peaches, plums (fresh prunes) 20
Apples, pears 25
Cantaloups, of which not more than 125
10 ppm shall be in the edible portion.
APPRAISAL
The antimicrobial properties of 2-phenylphenol have been utilized in
many countries for over 40 years. Due to its broad spectrum fungicidal
and bactericidal properties and commercial availability it has been
used widely as a germicide. In addition to its current use as a
post-harvest pesticide it has been used in household disinfectants,
preservatives for water-oil emulsions in textile and metal industries.
The sodium salt is also used as a preservative in water base paints
and adhesives. The free phenol is used in the post-harvest treatment
of fruits and vegetables in wax formulations as a protectant in
shipment and storage. The sodium salt is dissolved in water and used
as a post-harvest rinse for fruit and vegetables. The tree phenol is
also used to treat harvesting containers, shipping and storage
facilities, fruit handling machinery and shipping containers.
The data on residues from supervised trials are based only on U.S.A.
technology of use and on the two products as manufactured by Dow
Chemical Company. These are proprietary compounds, manufactured by
several companies in many countries. No information was provided on
the use pattern or technology of use in other countries.
The residues from both compounds are expressed as 2-phenylphenol.
Highly specific methods of dipping and washing individual species of
fruits and vegetables in the protective baths have been developed,
with carefully prescribed times of immersion varying from as little as
10 seconds (apples and pears) to e.g. 6 minutes for citrus. A skilled
technology and monitoring of wash solutions is required to maintain
correct conditions for treatment. Consequently, amounts of residues
vary depending upon the type of formulation used, the technology of
treatment and the fruit or vegetable itself. Supervised trials in the
U.S.A. carried out in several types of commercial scale fruit and
vegetable handling facilities resulted in residues of (ppm) apples
0.2-14, cantaloups (whole fruit) 42-117, carrots 2.8-17 (removed by
processing), cherries 0.3-2, citrus 5.5-9.3, cucumbers 1.3-7.7,
nectarines 0.4-1.4, peaches 9-15, pears 1-22, peppers 4.2-7.1,
pineapples 3.8-7.1, plums 0.8-12.8, sweet potatoes 0.5-5.7, tomatoes
0.5-7.7. Variations of conditions of treatment and contact time could
presumably result in slightly higher residues.
Methods of analysis for residues are available and they should be
evaluated by collaborative studies. (This in not considered critical).
In general all methods are based on determining the 2-phenylphenol
content of an aqueous solution residue extract by colometric
measurement after coupling with 4-aminoantipyrine.
No data are available on residues on fruits and vegetables as
consumed, but since these are post-harvest uses it would be a fair
speculation to assume that the residues would be the average of the
ranges outlined above.
Since the technology of treatment is so critical, in approaching the
recommendation of tolerances, some leeway should be provided for error
in time of immersion, pH of bath and other factors in this technology.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Cherries, nectarines 3 )
)
Citrus, cucumbers, peppers (bell), )
pineapples and tomatoes 10 )
)
Sweet potatoes 15 )
)
Apples, plums, (fresh prunes) 15 ) ppm as 2-phenylphenol
)
Carrots, peaches 20 )
)
Pears 25 )
)
Cantaloups (whole fruit) 120 )
)
Cantaloups (edible portion) 10 )
FURTHER WORK OR INFORMATION
DESIRABLE
1. A reproduction study in experimental animals.
2. Metabolic studies in experimental animals and man.
3. Long-term studies using a larger number of animals.
4. Data on residue levels in raw agricultural commodities moving in
commerce.
5. Data on residues in food at the time of consumption.
6. Results of collaborative studies of chromatographic methods of
analysis.
REFERENCES
Benger, H. (1968) Fluorimetsche bestimmung von o-phenyl-phenol in
citrusfrüchten. Arch. Hyg. 152(3):225-30
Caulfield, P.H. and Robinson, R.J. (1953) Spectrophotometric
determination of o-phenylphenol with titanium sulphate. Analyt.
Chem. 25:982-3
Chioffi, V. (1965) Investigation of diphenyl and o-phenylphenol in
lemons by thin-layer chromatography. Boll. Lab. Chim. Provinciali
(Bologna), 16:366-76 Chem. Abs., 1966, 64:1254d.
Cotta-Ramusino, F. and Stacchini, A. (1966) Spectrofluorometric
determination of o-phenylphenol in citrus fruits. Fruits (Paris),
21:37-9; Chem. Abs., 64:14858h.
Dodgson, K.S., Garton, G.A., Stubbs, A.L. and Williams, R.T. (1948)
Studies on detoxication 15. On the glucuronides of stilboestrol,
hexoestrol and dienoestrol. Biochem. J., 42:357-65 (cited in
Williams 1959)
Dow. (1969) Unpub. three-volume submission of data submitted by Dow
Chemical Co., Midland, Mich., U.S.A. to FAO
E.E.C. (1967) Amtsblatt der Europaischen Gemeinschaften, No. G1203B,
11 July 1967, 10. Jahrgang Nr. 148.
Elslande van, R. (1967) Dosage des résidues d'orthophénylphénol et
d'orthophénylphénate de sodium dans les agrumes. J. Offic.
Communantés Européenes, 10: No.148, 7-9 (11.7.1967).
Ernst, W. (1965) Umwandlung und Ausscheidung von 2-hydroxydiphenyl
bei der Ratte. Arzneimittel. - Forsch., 15:632-6
FAO/WHO (1962) Evaluation of the toxicity of a number of
antimicrobials and antioxidants. Sixth Report of the Joint FAO/WHO
Expert Committee on Food Additives; FAO Nutrition Meetings Report
Series No.31; Wld Hlth Org. techn. Rep. Ser. No.228, pp. 78-81
Gottleib, S. and Marsh, P.B. (1946) Quantitative determination of
phenolic fungicides. Ind. Eng. Chem. Anal. Ed., 18:16-19
Gunther, F.A., Blinn, R.C. and Barkley, J.H. (1963) Procedure for
routine determination of biphenyl and o-phenylphenol on and in
citrus fruit. Analyst, 88:36-42
Hayward, F.W. and Grierson, W. (1960) Effects of treatment conditions
on o-phenylphenol residues in oranges. J. Agr. Fd. Chem. 8.308-10
Hodge, H.C., Maynard, E.A., Blanchet, H.J. Jr., Spencer, H.C. and
Rowe, V.K. (1952) Toxicological studies of orthophenylphenol
(Dowicide I) J. Pharmacol. exp. Ther., 104:202-10
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L.,
Fishbein, L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L.,
Gart, J.J., Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in mice.
A preliminary note. J. Nat. Cancer Inst. 42:1102-14
Kamil, I.A., Smith, J.N. and Williams, R.T. (1951) Studies in
detoxication. 41. A study of the optical rotations of the amides and
triacetyl methyl eaters of some biosynthetic substituted
phenylglucuronides. Biochem. J. 50:235-40 (cited in Williams,
1959)
Leinbach, L.R. and Brekke, J.E. (1961) A modified Gibbs method for the
determination of 1 ppm or less of o-phenylphenol in fruits. J.
Agr. Food Chem. 9:205-6
MacIntosh, F.C. (1945) The toxicity of diphenyl and o-phenyl-phenol.
Analyst, 70:334
Mestres, R. and Chave, C. (1965) Determination of biphenyl and
o-phenylphenol in citrus fruits. Trans. Soc. Pharm. Montpellier,
24:272-82. Chem. Abs., 63:15434g.
Rajzman, A. and Apfelbaum, A. (1968) Survey of o-phenylphenol
residues found in marketable citrus fruit. J. Sci. Fd. Agric. 19:740-4
Schiffman, C.D. (1957) Report on sodium orthophenylphenate residues on
apples, pears and citrus fruits. J. Assoc. Offic. Agric. Chem.,
40:238-42
Thomas, R. (1960) The detection and determination of diphenyl and
o-phenylphenol in concentrated orange juice by gas
chromatography. Analyst, 85:551-6
Tomkins, R.G. and Isherwood, F.A. (1945) The absorption of diphenyl
and o-phenylphenol by oranges from treated wraps. Analyst,
70:330-3
U.S.D.A. (1967) Summary of registered agricultural pesticide chemical
uses (2nd Edition), U.S. Department of Agriculture, Washington, D.C.
pp. 647, 727-8(a) (1967)
Williams, R.T. (1959) "Detoxication Mechanisms" London, Chapman and
Hall, p. 308
Other relevant chemical properties
The free phenol is a white, crystalline, free-flowing powder,
molecular weight 170.2. Mild phenolic odour. Less than 0.1g is soluble
in 1 ml of water. Freely soluble in ethanol, soluble in fats and oils.
It is dissolved or dispersed in wax formulations.
The sodium salt is a buff coloured solid. Molecular weight 264.3. Very
soluble in water and ethanol; practically insoluble in oils. Used as
water solution.
Purity
Specifications for purity of both compounds have been published by the
European Economic Community (E.E.C., 1967):
E231 2-phenylphenol
melting point 56 - 58°C
content not less than 99%
biphenylether not more than 0.3%
p-phenylphenol not more than 0.1%
alpha-naphthol not more than 0.01%
sulphate after ashing not more than 0.05%
E232 sodium orthophenylphenate
melting point of 2-phenylphenol precipitated by acidification of
sodium salt, not recrystallized and dried over sulphuric acid 56
- 58°C
ph 2% water solution must be between pH 11.1 and 11.8
content not less than 95% C12H ONa.4H2.0
biphenylether not more than 0.3%
p-phenylphenol not more than 0.1%
x-naphthol not more than 0.01%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
The biological data available on this compound have been evaluated by
the Joint FAO/WHO Expert Committee on Food Additives, and a monograph
entitled "Sodium o-phenylphenol" is included in the Sixth Report of
this Committee (FAO/WHO, 1962). A summary of the data then considered
as well as some limited additional information which has recently
become available is included below.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Tissue examination of rats which had received 2-phenylphenol orally
for two years revealed little tendency for storage of the compound.
The kidney was the only organ containing detectable amounts of the
compound; average values of 220 mg/kg tissue were found in the kidneys
of rats fed 20,000 ppm in the diet, and approximately 10 mg/kg tissue
in the kidneys of rats fed 2000 ppm (Hodge et al., 1952).
After administration to rabbits, 2-phenylphenol is highly conjugated
with glucuronic acid but it is not known whether it is converted to an
ethereal sulphate in this species. The glucuronide has been isolated
from rabbit urine and characterized by conversion to the
triacetylmethyl ester (Williams, 1959; Dodgson et al., 1948; Kamil et
al., 1951).
When administered orally to rats, 2-phenylphenol is partly converted
to 2,5-dihydroxybiphenyl which, like 2-phenylphenol, is excreted in
the urine as a glucuronide and ethereal sulphate. The total output of
2-phenylphenol within 48 hours amounted to about 70 percent as
conjugates of 2-phenylphenol and 2,5-dihydroxyphenol (Ernst, 1965).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Groups of 18 mice of each sex from two hybrid strains of mice were
given 2-phenylphenol for 18 months. The dose of 100 mg/kg was given to
the mice by gavage from the seventh day of age to the time of weaning
at four weeks of age and thereafter the chemical was added to the diet
in a corresponding amount of 280 ppm. There was no significant
increase in the incidence of tumours (Innes et al., 1969).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Rat oral 2700-3000 (approx.) MacIntosh, 1945
Hodge et al., 1952
Cat oral 500 (approx.) MacIntosh, 1945
Short-term studies
Dog
A preliminary study demonstrated that when daily doses of 1,000 mg/kg
body-weight of 2-phenylphenol were fed to two dogs, both died within a
month. Groups of two dogs each were then fed 2-phenylphenol for a
period of one year in daily amounts of 0, 20, 200, and 500 mg/kg body
weight. No effect related to the administration of 2-phenylphenol was
observed. Haematological values, urinary sugar and protein values,
organ weights and histopathological examination of the various tissues
did not differ from the normal range (Hodge et al., 1952).
Rat
Groups, each comprising 15 male rats, were fed 2-phenylphenol in daily
doses of 0, 2, 20, or 200 mg/kg body-weight for 32 days. There were no
toxic symptoms attributable to feeding 2-phenylphenol. The mean
growth-rate and the haemoglobin and white blood cell levels in all
groups were comparable to the controls (MacIntosh, 1945).
Five male and five female rats in each group were given by stomach
tube doses of 0, 50, 100, 200, and 500 mg/kg body-weight for five days
a week over a period of six months. The only abnormality observed was
a slight increase in average liver and kidney weights in the animals
fed the 500 mg/kg dose level (Hodge et al., 1952).
When diets containing 0, 1,000, 3,000, 10,000, or 20,000 ppm of
2-phenylphenol were fed for three months to groups of rats comprising
12 males and 12 females, slight retardation of growth was observed in
the 20,000 ppm group. There was no significant difference between the
mortality of control and test animals. There were doubtful increases
in weight of liver, kidney, and spleen of certain rats of the 10,000
and 20,000 ppm groups. No tissue changes were observed (Hodge et al.,
1952)
Long-term studies
Rat
Male and female rats (25 of each sex per group) were maintained for
two years on diets containing 0, 200, 2,000 and 20,000 ppm of
2-phenylphenol. The animals fed 200 and 2000 ppm showed no adverse
effects when compared with the controls, as judged by growth,
mortality, gross appearance, haematology, urinary sugar and protein
values, organ-weights, tissue content of 2-phenylphenol, and
histopathological examination of various tissues. The group fed 20,000
ppm of 2-phenylphenol differed from the controls by exhibiting slight
retardation of growth, histological kidney changes (marked tubular
dilation), and the presence of small amounts of 2-phenylphenol in the
kidney tissues (Hodge et al., 1952).
OBSERVATION IN MAN
A 5.0 percent solution of 2-phenylphenol in sesame oil and a 0.1
percent aqueous solution of the sodium salt tested on 200 subjects
caused neither primary skin irritation nor skin sensitization. The
sodium salt is slightly irritating in 0.5 percent aqueous solution and
decidedly irritating in 1.0 percent and 5.0 percent solutions (Hodge
et al., 1952).
COMMENT
The short and long-term studies in rats are sufficient to be taken as
a basis for the evaluation of 2-phenylphenol. The dog study was not
considered adequate. In addition, the studies on metabolism are
incomplete and there are no studies on reproduction. However, the
Meeting considered that the toxicological studies were adequate to
reaffirm the previous acceptable daily intake established by the Joint
FAO/WHO Expert Committee on Food Additives in its Sixth Report
(FAO/WHO, 1962).
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Dog: 500 mg/kg body-weight/day
Rat: 2000 ppm in the diet, equivalent to 100 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 1.0 mg/kg body-weight (based upon the previous adi. - FAO/WHO,
1962)
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
In Great Britain a dormant paint application to apple trees is
approved for control of canker.
Post-harvest treatments
2-phenylphenol has been used broadly for over 40 years in many
countries for its general antimicrobial properties. Due to its broad
spectrum bactericidal and fungistatic effect, and commercial
availability it is widely used. It is employed as a household
disinfectant, preservative in oil-water emulsions used in the textile
and metal industries, as well as a post-harvest treatment for fruits
and vegetables to prevent losses due to rots and moulds in storage and
transport of these foods. The sodium salt is used as a preservative
for protein-based and other adhesives, as well as for the post-harvest
use in fruits and vegetables.
Principal formulations in the U.S.A. are:
2-phenylphenol: 5% liquid; 98% wettable powder; 2% in wax.
Wax formulations are applied by commercial application equipment in
tandem with sorting and washing facilities. These are specifically
designed for the crop concerned as to technology of use, formulation
strength, time of immersion in the bath and monitoring of the process
to ensure maintenance of treatment criteria for effectiveness without
damage to the crop. Wax strength formulations vary, e.g. carrots 0.5%,
citrus 0.8%, cucumbers 2.5%, nectarines 0.2%, peaches 0.2%; peppers
2.5%, plums 2%, tomatoes 2.5%. A 5% liquid dip is used for crates,
field boxes, hampers, lugs and wood containers for fruits and
vegetables (U.S.D.A., 1967).
sodium o-phenyl phenate: 97% wettable powder, solutions ranging from
1.5% to 40%, wax emulsion from 0.7% to 2.0%.
The strength of solution used varies with the crop concerned and the
time of immersion to obtain protection and avoid phytotoxic reactions
in fruits or vegetables. The times of immersion will vary from a few
seconds to several minutes, but are precisely prescribed and
monitoring is required for each formulation and crop. Examples
includes apples 0.45% - 1.4% solution, bananas 2% applied to cut stem
area of crown of head area (no residue in fruit or skin), cantaloups
1.5%, carrots 0.1%, cherries 1.0% with wetting agent, citrus 0.5% wax
emulsion to 3% in water (plus caustic and hexamine), cucumbers 1%
- 2%, nectarines 1%, peaches 0.05 - 0.6%, pears 0.23% to 1.45%,
peppers 1%, pineapple 1.25% (1% in wax), plums 0.1%, sweet potatoes
0.37% - 0.5% solution or 0.7% wax emulsion, tomatoes 1% (U.S.D.A.,
1967).
These use patterns were available from the U.S.A. only.
RESIDUES RESULTING FROM SUPERVISED TRIALS
As mentioned above, the technology for use of these compounds has been
developed under commercial conditions requiring accurate control of
time of treatment which may be from as brief as 10 seconds (apples and
pears) to 15-18 minutes (peaches), at specified temperatures, pH etc.
The data pertaining to the conditions of these trials were collected
from several locations in the U.S.A. They are unpublished. They have
been collated and submitted with full details to FAO by the Dow
Chemical Company (Dow, 1969).
A review of several abstract journals revealed no published literature
bearing on this subject from other countries. It is known that both
compounds are used by several citrus exporting countries, but these
have not been identified, nor were use patterns, technology of use or
data on residues resulting from supervised trials supplied for
evaluation from other than the U.S.A.
The results of U.S.A. trials are summarized in Tables 1 and 2 (Dow,
1969).
TABLE I
Residues resulting from supervised trials with 2-phenylphenol
Percent 2-phenylphenol Range of residues as
Fruit or vegetable in wax* ppm o-phenylphenol
Not rinsed
Carrots 0.5 17.0
Cucumbers 0.5-2 4.2-5.6
Peppers 2 0.1-1.0
Plums 2 2.5-12.8
Sweet potatoes 1 4.0-5.7
Tomatoes 2-3 0.1-7.7
*Concentration varies according to crop and/or contact time
TABLE 2
Residues resulting from supervised trials with Sodium o-phenylphenate
Percent sodium o-phenylphenate Range of residues as
Fruit or vegetable in water* ppm o-phenylphenol
Rinsed Not rinsed
Apples 0.42-0.77 0.2-14.0
Cantaloups 1.5
edible portion 1.0-6.0
whole fruit 42.0-117.0
Carrots 0.1 2.8-11.0
Cherries 0.65-1.08 0.3-2
Citrus 2.0 5.5-9.3
Cucumbers 1.0 1.3-7.7
Nectarines 0.75-1.2 0.4-1.4
Peaches 0.1-0.12 9.0-15.0
Pears 0.4-0.99 1.0-25.0 1.0-22.0
Peppers 1.0 4.2-7.1
Pineapples 1.25 3.8-7.1
Plums 0.4 0.8-1.3
Sweet potatoes 0.5-1.0 0.5-11.0 1.7-3.4
Tomatoes 0.9 0.5-1.4
*Concentration varies according to crop and/or contact time
FATE OF RESIDUES
Carrots treated with 0.1% sodium o-phenylphenate, stored and steam or
abrasion peeled prior to canning in a commercial manner contained no
residues in the canned product. In cantaloups the residue is mostly in
the unedible portion (42.0-117 ppm in whole fruit, 2.8-11.0 ppm in
edible portion) (Dow, 1969). The value of OPP residues found in peel
of oranges varies slightly with variety. Shamoute oranges peel varied
between 5.9-23 ppm; Valencias between 8.8 and 21.3 ppm; grapefruit
peel 7.0-26.1 ppm; lemons 5.4-15.6 ppm in peel. The quantities of OPP
in pulp of citrus is very low. In many cases none could be detected,
but when peel contained the higher levels, OPP residues from trace to
0.4 ppm were found in pulp (Rajzman and Apfelbaum, 1968).
There is no information available on possible chemical alteration of
the nature of the residue. All residue analysis methods to date rely
on expressing the residue as 2-phenylphenol.
Evidence of residues in food in commerce or at consumption
Information on the residue content of food in commerce is rare. Only
one published paper has a bearing on this subject (Rajzman and
Apfelbaum, 1968). Citrus fruit treated in 22 packing houses in Israel
were collected, sampled and analysed. The residues in whole fruit
range from 1.8 to 8.3 ppm with 80% of the samples containing less than
5 ppm. The authors conclude that in view of the small number of
samples used, their finding of a maximum of 8.3 ppm should not be
considered as the maximum quantity which can occur in citrus treated
in the manner described.
There is no information available with regard to residues in food at
the time of consumption. It can be assumed there will be little loss
of residue in wax-treated fruits and vegetables. Since the treatment
is by post-harvest application, the amounts in wax-treated food
reaching the consumer can be considered as those reported as resulting
from supervised trials. Possibly slightly lower levels will remain in
food treated with water formulations.
METHODS OF RESIDUE ANALYSIS
Colorimetric procedures have most often been proposed for the
determination of residues of 2-phenylphenol. Tomkins and Isherwood
(1945) used coupling with diazotized sulphanilic acid in determining
residues in oranges and marmalade after a steam-distillation
extraction, with acid-alkali treatment clean-up stages. Gottleib and
Marsh (1946) suggested an aminophenazone procedure for the
determination of several phenolic fungicides. The results of a
collaborative study of this method as applied to the determination of
residues of 2-phenylphenol in apples, pears and citrus fruit have been
reported by Schiffman (1957); Hayward and Grierson (1960) used this
method on oranges. A similar procedure has been officially recommended
by the Communauté Economique Européene (van Elslande, 1967) for
residues in fruits and this should be suitable for regulatory purposes
at the present time. A modified steam-distillation extraction
apparatus has been described by Leinbach and Brekke (1961) who used an
indophenol colorimetric method for residues in purees of raspberry,
strawberry, prunes, loganberry and fig and in orange juice. The
coloration given by reacting 2-phenylphenol with titanium sulphate
(Caulfield and Robinson, 1953) is not sufficiently sensitive for
residue analysis. A spectrofluorometric procedure for the examination
of single fruits was proposed by the Cotta-Ramusino and Stacchini
(1966) which is sensitive to <0.1 mg of 2-phenylphenol for fruit,
Gunther et al. (1963) reported a procedure for the routine examination
of citrus fruits for biphenyl and 2-phenylphenol, determining the
latter colorimetrically after coupling with p-nitrofluoroborate, and
obtained recoveries ranging from 89 to 100 percent at the 0.5 to 10
ppm level.
Chromatographic methods available include a gas chromatographic
examination of concentrated orange juice (Thomas, 1960), applicable
over the range 1 to 10 ppm on 1 ml of sample and a thin-layer
chromatographic procedure for the detection of residues of biphenyl
and 2-phenylphenol in lemons (Chioffi, 1965). Mestres and Chave,
(1965) used extraction with cyclohexane in a Dean and Stark apparatus
before separating and determining biphenyl and 2-phenylphenol by gas
chromatography with flame-ionisation detection. These chromatographic
techniques should be developed and evaluated for application as
regulatory procedures.
NATIONAL TOLERANCES
Tolerance (ppm)
Country Crop as 2-phenylphenol
Canada Cherries, nectarines 5
Citrus fruits, cucumbers,
pepper (bell), pineapple,
tomatoes 10
Sweet potatoes 15
Carrots, peaches, plums 20
Apples, pears 25
Cantaloups 125
E.E.C. Citrus fruits 12
Netherlands Citrus fruits 10
United Kingdom Citrus fruit 70
Apples, pears, pineapples 10
Melons 125
NATIONAL TOLERANCES (continued)
Tolerance (ppm)
Country Crop as 2-phenylphenol
United States Cherries, nectarines 5
of America Citrus, citron, cucumbers, grapefruit, 10
kumquats, lemons, limes oranges,
peppers (bell), pineapple, tangelos,
tangerines, tomatoes
Sweet potatoes 15
Carrots, peaches, plums (fresh prunes) 20
Apples, pears 25
Cantaloups, of which not more than 125
10 ppm shall be in the edible portion.
APPRAISAL
The antimicrobial properties of 2-phenylphenol have been utilized in
many countries for over 40 years. Due to its broad spectrum fungicidal
and bactericidal properties and commercial availability it has been
used widely as a germicide. In addition to its current use as a
post-harvest pesticide it has been used in household disinfectants,
preservatives for water-oil emulsions in textile and metal industries.
The sodium salt is also used as a preservative in water base paints
and adhesives. The free phenol is used in the post-harvest treatment
of fruits and vegetables in wax formulations as a protectant in
shipment and storage. The sodium salt is dissolved in water and used
as a post-harvest rinse for fruit and vegetables. The tree phenol is
also used to treat harvesting containers, shipping and storage
facilities, fruit handling machinery and shipping containers.
The data on residues from supervised trials are based only on U.S.A.
technology of use and on the two products as manufactured by Dow
Chemical Company. These are proprietary compounds, manufactured by
several companies in many countries. No information was provided on
the use pattern or technology of use in other countries.
The residues from both compounds are expressed as 2-phenylphenol.
Highly specific methods of dipping and washing individual species of
fruits and vegetables in the protective baths have been developed,
with carefully prescribed times of immersion varying from as little as
10 seconds (apples and pears) to e.g. 6 minutes for citrus. A skilled
technology and monitoring of wash solutions is required to maintain
correct conditions for treatment. Consequently, amounts of residues
vary depending upon the type of formulation used, the technology of
treatment and the fruit or vegetable itself. Supervised trials in the
U.S.A. carried out in several types of commercial scale fruit and
vegetable handling facilities resulted in residues of (ppm) apples
0.2-14, cantaloups (whole fruit) 42-117, carrots 2.8-17 (removed by
processing), cherries 0.3-2, citrus 5.5-9.3, cucumbers 1.3-7.7,
nectarines 0.4-1.4, peaches 9-15, pears 1-22, peppers 4.2-7.1,
pineapples 3.8-7.1, plums 0.8-12.8, sweet potatoes 0.5-5.7, tomatoes
0.5-7.7. Variations of conditions of treatment and contact time could
presumably result in slightly higher residues.
Methods of analysis for residues are available and they should be
evaluated by collaborative studies. (This in not considered critical).
In general all methods are based on determining the 2-phenylphenol
content of an aqueous solution residue extract by colometric
measurement after coupling with 4-aminoantipyrine.
No data are available on residues on fruits and vegetables as
consumed, but since these are post-harvest uses it would be a fair
speculation to assume that the residues would be the average of the
ranges outlined above.
Since the technology of treatment is so critical, in approaching the
recommendation of tolerances, some leeway should be provided for error
in time of immersion, pH of bath and other factors in this technology.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Cherries, nectarines 3 )
)
Citrus, cucumbers, peppers (bell), )
pineapples and tomatoes 10 )
)
Sweet potatoes 15 )
)
Apples, plums, (fresh prunes) 15 ) ppm as 2-phenylphenol
)
Carrots, peaches 20 )
)
Pears 25 )
)
Cantaloups (whole fruit) 120 )
)
Cantaloups (edible portion) 10 )
FURTHER WORK OR INFORMATION
DESIRABLE
1. A reproduction study in experimental animals.
2. Metabolic studies in experimental animals and man.
3. Long-term studies using a larger number of animals.
4. Data on residue levels in raw agricultural commodities moving in
commerce.
5. Data on residues in food at the time of consumption.
6. Results of collaborative studies of chromatographic methods of
analysis.
REFERENCES
Benger, H. (1968) Fluorimetsche bestimmung von o-phenyl-phenol in
citrusfrüchten. Arch. Hyg. 152(3):225-30
Caulfield, P.H. and Robinson, R.J. (1953) Spectrophotometric
determination of o-phenylphenol with titanium sulphate. Analyt.
Chem. 25:982-3
Chioffi, V. (1965) Investigation of diphenyl and o-phenylphenol in
lemons by thin-layer chromatography. Boll. Lab. Chim. Provinciali
(Bologna), 16:366-76 Chem. Abs., 1966, 64:1254d.
Cotta-Ramusino, F. and Stacchini, A. (1966) Spectrofluorometric
determination of o-phenylphenol in citrus fruits. Fruits (Paris),
21:37-9; Chem. Abs., 64:14858h.
Dodgson, K.S., Garton, G.A., Stubbs, A.L. and Williams, R.T. (1948)
Studies on detoxication 15. On the glucuronides of stilboestrol,
hexoestrol and dienoestrol. Biochem. J., 42:357-65 (cited in
Williams 1959)
Dow. (1969) Unpub. three-volume submission of data submitted by Dow
Chemical Co., Midland, Mich., U.S.A. to FAO
E.E.C. (1967) Amtsblatt der Europaischen Gemeinschaften, No. G1203B,
11 July 1967, 10. Jahrgang Nr. 148.
Elslande van, R. (1967) Dosage des résidues d'orthophénylphénol et
d'orthophénylphénate de sodium dans les agrumes. J. Offic.
Communantés Européenes, 10: No.148, 7-9 (11.7.1967).
Ernst, W. (1965) Umwandlung und Ausscheidung von 2-hydroxydiphenyl
bei der Ratte. Arzneimittel. - Forsch., 15:632-6
FAO/WHO (1962) Evaluation of the toxicity of a number of
antimicrobials and antioxidants. Sixth Report of the Joint FAO/WHO
Expert Committee on Food Additives; FAO Nutrition Meetings Report
Series No.31; Wld Hlth Org. techn. Rep. Ser. No.228, pp. 78-81
Gottleib, S. and Marsh, P.B. (1946) Quantitative determination of
phenolic fungicides. Ind. Eng. Chem. Anal. Ed., 18:16-19
Gunther, F.A., Blinn, R.C. and Barkley, J.H. (1963) Procedure for
routine determination of biphenyl and o-phenylphenol on and in
citrus fruit. Analyst, 88:36-42
Hayward, F.W. and Grierson, W. (1960) Effects of treatment conditions
on o-phenylphenol residues in oranges. J. Agr. Fd. Chem. 8.308-10
Hodge, H.C., Maynard, E.A., Blanchet, H.J. Jr., Spencer, H.C. and
Rowe, V.K. (1952) Toxicological studies of orthophenylphenol
(Dowicide I) J. Pharmacol. exp. Ther., 104:202-10
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L.,
Fishbein, L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L.,
Gart, J.J., Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in mice.
A preliminary note. J. Nat. Cancer Inst. 42:1102-14
Kamil, I.A., Smith, J.N. and Williams, R.T. (1951) Studies in
detoxication. 41. A study of the optical rotations of the amides and
triacetyl methyl eaters of some biosynthetic substituted
phenylglucuronides. Biochem. J. 50:235-40 (cited in Williams,
1959)
Leinbach, L.R. and Brekke, J.E. (1961) A modified Gibbs method for the
determination of 1 ppm or less of o-phenylphenol in fruits. J.
Agr. Food Chem. 9:205-6
MacIntosh, F.C. (1945) The toxicity of diphenyl and o-phenyl-phenol.
Analyst, 70:334
Mestres, R. and Chave, C. (1965) Determination of biphenyl and
o-phenylphenol in citrus fruits. Trans. Soc. Pharm. Montpellier,
24:272-82. Chem. Abs., 63:15434g.
Rajzman, A. and Apfelbaum, A. (1968) Survey of o-phenylphenol
residues found in marketable citrus fruit. J. Sci. Fd. Agric. 19:740-4
Schiffman, C.D. (1957) Report on sodium orthophenylphenate residues on
apples, pears and citrus fruits. J. Assoc. Offic. Agric. Chem.,
40:238-42
Thomas, R. (1960) The detection and determination of diphenyl and
o-phenylphenol in concentrated orange juice by gas
chromatography. Analyst, 85:551-6
Tomkins, R.G. and Isherwood, F.A. (1945) The absorption of diphenyl
and o-phenylphenol by oranges from treated wraps. Analyst,
70:330-3
U.S.D.A. (1967) Summary of registered agricultural pesticide chemical
uses (2nd Edition), U.S. Department of Agriculture, Washington, D.C.
pp. 647, 727-8(a) (1967)
Williams, R.T. (1959) "Detoxication Mechanisms" London, Chapman and
Hall, p. 308
 Other relevant chemical properties
The free phenol is a white, crystalline, free-flowing powder,
molecular weight 170.2. Mild phenolic odour. Less than 0.1g is soluble
in 1 ml of water. Freely soluble in ethanol, soluble in fats and oils.
It is dissolved or dispersed in wax formulations.
The sodium salt is a buff coloured solid. Molecular weight 264.3. Very
soluble in water and ethanol; practically insoluble in oils. Used as
water solution.
Purity
Specifications for purity of both compounds have been published by the
European Economic Community (E.E.C., 1967):
E231 2-phenylphenol
melting point 56 - 58°C
content not less than 99%
biphenylether not more than 0.3%
p-phenylphenol not more than 0.1%
alpha-naphthol not more than 0.01%
sulphate after ashing not more than 0.05%
E232 sodium orthophenylphenate
melting point of 2-phenylphenol precipitated by acidification of
sodium salt, not recrystallized and dried over sulphuric acid 56
- 58°C
ph 2% water solution must be between pH 11.1 and 11.8
content not less than 95% C12H ONa.4H2.0
biphenylether not more than 0.3%
p-phenylphenol not more than 0.1%
x-naphthol not more than 0.01%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
The biological data available on this compound have been evaluated by
the Joint FAO/WHO Expert Committee on Food Additives, and a monograph
entitled "Sodium o-phenylphenol" is included in the Sixth Report of
this Committee (FAO/WHO, 1962). A summary of the data then considered
as well as some limited additional information which has recently
become available is included below.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Tissue examination of rats which had received 2-phenylphenol orally
for two years revealed little tendency for storage of the compound.
The kidney was the only organ containing detectable amounts of the
compound; average values of 220 mg/kg tissue were found in the kidneys
of rats fed 20,000 ppm in the diet, and approximately 10 mg/kg tissue
in the kidneys of rats fed 2000 ppm (Hodge et al., 1952).
After administration to rabbits, 2-phenylphenol is highly conjugated
with glucuronic acid but it is not known whether it is converted to an
ethereal sulphate in this species. The glucuronide has been isolated
from rabbit urine and characterized by conversion to the
triacetylmethyl ester (Williams, 1959; Dodgson et al., 1948; Kamil et
al., 1951).
When administered orally to rats, 2-phenylphenol is partly converted
to 2,5-dihydroxybiphenyl which, like 2-phenylphenol, is excreted in
the urine as a glucuronide and ethereal sulphate. The total output of
2-phenylphenol within 48 hours amounted to about 70 percent as
conjugates of 2-phenylphenol and 2,5-dihydroxyphenol (Ernst, 1965).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Groups of 18 mice of each sex from two hybrid strains of mice were
given 2-phenylphenol for 18 months. The dose of 100 mg/kg was given to
the mice by gavage from the seventh day of age to the time of weaning
at four weeks of age and thereafter the chemical was added to the diet
in a corresponding amount of 280 ppm. There was no significant
increase in the incidence of tumours (Innes et al., 1969).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Rat oral 2700-3000 (approx.) MacIntosh, 1945
Hodge et al., 1952
Cat oral 500 (approx.) MacIntosh, 1945
Short-term studies
Dog
A preliminary study demonstrated that when daily doses of 1,000 mg/kg
body-weight of 2-phenylphenol were fed to two dogs, both died within a
month. Groups of two dogs each were then fed 2-phenylphenol for a
period of one year in daily amounts of 0, 20, 200, and 500 mg/kg body
weight. No effect related to the administration of 2-phenylphenol was
observed. Haematological values, urinary sugar and protein values,
organ weights and histopathological examination of the various tissues
did not differ from the normal range (Hodge et al., 1952).
Rat
Groups, each comprising 15 male rats, were fed 2-phenylphenol in daily
doses of 0, 2, 20, or 200 mg/kg body-weight for 32 days. There were no
toxic symptoms attributable to feeding 2-phenylphenol. The mean
growth-rate and the haemoglobin and white blood cell levels in all
groups were comparable to the controls (MacIntosh, 1945).
Five male and five female rats in each group were given by stomach
tube doses of 0, 50, 100, 200, and 500 mg/kg body-weight for five days
a week over a period of six months. The only abnormality observed was
a slight increase in average liver and kidney weights in the animals
fed the 500 mg/kg dose level (Hodge et al., 1952).
When diets containing 0, 1,000, 3,000, 10,000, or 20,000 ppm of
2-phenylphenol were fed for three months to groups of rats comprising
12 males and 12 females, slight retardation of growth was observed in
the 20,000 ppm group. There was no significant difference between the
mortality of control and test animals. There were doubtful increases
in weight of liver, kidney, and spleen of certain rats of the 10,000
and 20,000 ppm groups. No tissue changes were observed (Hodge et al.,
1952)
Long-term studies
Rat
Male and female rats (25 of each sex per group) were maintained for
two years on diets containing 0, 200, 2,000 and 20,000 ppm of
2-phenylphenol. The animals fed 200 and 2000 ppm showed no adverse
effects when compared with the controls, as judged by growth,
mortality, gross appearance, haematology, urinary sugar and protein
values, organ-weights, tissue content of 2-phenylphenol, and
histopathological examination of various tissues. The group fed 20,000
ppm of 2-phenylphenol differed from the controls by exhibiting slight
retardation of growth, histological kidney changes (marked tubular
dilation), and the presence of small amounts of 2-phenylphenol in the
kidney tissues (Hodge et al., 1952).
OBSERVATION IN MAN
A 5.0 percent solution of 2-phenylphenol in sesame oil and a 0.1
percent aqueous solution of the sodium salt tested on 200 subjects
caused neither primary skin irritation nor skin sensitization. The
sodium salt is slightly irritating in 0.5 percent aqueous solution and
decidedly irritating in 1.0 percent and 5.0 percent solutions (Hodge
et al., 1952).
COMMENT
The short and long-term studies in rats are sufficient to be taken as
a basis for the evaluation of 2-phenylphenol. The dog study was not
considered adequate. In addition, the studies on metabolism are
incomplete and there are no studies on reproduction. However, the
Meeting considered that the toxicological studies were adequate to
reaffirm the previous acceptable daily intake established by the Joint
FAO/WHO Expert Committee on Food Additives in its Sixth Report
(FAO/WHO, 1962).
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Dog: 500 mg/kg body-weight/day
Rat: 2000 ppm in the diet, equivalent to 100 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 1.0 mg/kg body-weight (based upon the previous adi. - FAO/WHO,
1962)
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
In Great Britain a dormant paint application to apple trees is
approved for control of canker.
Post-harvest treatments
2-phenylphenol has been used broadly for over 40 years in many
countries for its general antimicrobial properties. Due to its broad
spectrum bactericidal and fungistatic effect, and commercial
availability it is widely used. It is employed as a household
disinfectant, preservative in oil-water emulsions used in the textile
and metal industries, as well as a post-harvest treatment for fruits
and vegetables to prevent losses due to rots and moulds in storage and
transport of these foods. The sodium salt is used as a preservative
for protein-based and other adhesives, as well as for the post-harvest
use in fruits and vegetables.
Principal formulations in the U.S.A. are:
2-phenylphenol: 5% liquid; 98% wettable powder; 2% in wax.
Wax formulations are applied by commercial application equipment in
tandem with sorting and washing facilities. These are specifically
designed for the crop concerned as to technology of use, formulation
strength, time of immersion in the bath and monitoring of the process
to ensure maintenance of treatment criteria for effectiveness without
damage to the crop. Wax strength formulations vary, e.g. carrots 0.5%,
citrus 0.8%, cucumbers 2.5%, nectarines 0.2%, peaches 0.2%; peppers
2.5%, plums 2%, tomatoes 2.5%. A 5% liquid dip is used for crates,
field boxes, hampers, lugs and wood containers for fruits and
vegetables (U.S.D.A., 1967).
sodium o-phenyl phenate: 97% wettable powder, solutions ranging from
1.5% to 40%, wax emulsion from 0.7% to 2.0%.
The strength of solution used varies with the crop concerned and the
time of immersion to obtain protection and avoid phytotoxic reactions
in fruits or vegetables. The times of immersion will vary from a few
seconds to several minutes, but are precisely prescribed and
monitoring is required for each formulation and crop. Examples
includes apples 0.45% - 1.4% solution, bananas 2% applied to cut stem
area of crown of head area (no residue in fruit or skin), cantaloups
1.5%, carrots 0.1%, cherries 1.0% with wetting agent, citrus 0.5% wax
emulsion to 3% in water (plus caustic and hexamine), cucumbers 1%
- 2%, nectarines 1%, peaches 0.05 - 0.6%, pears 0.23% to 1.45%,
peppers 1%, pineapple 1.25% (1% in wax), plums 0.1%, sweet potatoes
0.37% - 0.5% solution or 0.7% wax emulsion, tomatoes 1% (U.S.D.A.,
1967).
These use patterns were available from the U.S.A. only.
RESIDUES RESULTING FROM SUPERVISED TRIALS
As mentioned above, the technology for use of these compounds has been
developed under commercial conditions requiring accurate control of
time of treatment which may be from as brief as 10 seconds (apples and
pears) to 15-18 minutes (peaches), at specified temperatures, pH etc.
The data pertaining to the conditions of these trials were collected
from several locations in the U.S.A. They are unpublished. They have
been collated and submitted with full details to FAO by the Dow
Chemical Company (Dow, 1969).
A review of several abstract journals revealed no published literature
bearing on this subject from other countries. It is known that both
compounds are used by several citrus exporting countries, but these
have not been identified, nor were use patterns, technology of use or
data on residues resulting from supervised trials supplied for
evaluation from other than the U.S.A.
The results of U.S.A. trials are summarized in Tables 1 and 2 (Dow,
1969).
TABLE I
Residues resulting from supervised trials with 2-phenylphenol
Percent 2-phenylphenol Range of residues as
Fruit or vegetable in wax* ppm o-phenylphenol
Not rinsed
Carrots 0.5 17.0
Cucumbers 0.5-2 4.2-5.6
Peppers 2 0.1-1.0
Plums 2 2.5-12.8
Sweet potatoes 1 4.0-5.7
Tomatoes 2-3 0.1-7.7
*Concentration varies according to crop and/or contact time
TABLE 2
Residues resulting from supervised trials with Sodium o-phenylphenate
Percent sodium o-phenylphenate Range of residues as
Fruit or vegetable in water* ppm o-phenylphenol
Rinsed Not rinsed
Apples 0.42-0.77 0.2-14.0
Cantaloups 1.5
edible portion 1.0-6.0
whole fruit 42.0-117.0
Carrots 0.1 2.8-11.0
Cherries 0.65-1.08 0.3-2
Citrus 2.0 5.5-9.3
Cucumbers 1.0 1.3-7.7
Nectarines 0.75-1.2 0.4-1.4
Peaches 0.1-0.12 9.0-15.0
Pears 0.4-0.99 1.0-25.0 1.0-22.0
Peppers 1.0 4.2-7.1
Pineapples 1.25 3.8-7.1
Plums 0.4 0.8-1.3
Sweet potatoes 0.5-1.0 0.5-11.0 1.7-3.4
Tomatoes 0.9 0.5-1.4
*Concentration varies according to crop and/or contact time
FATE OF RESIDUES
Carrots treated with 0.1% sodium o-phenylphenate, stored and steam or
abrasion peeled prior to canning in a commercial manner contained no
residues in the canned product. In cantaloups the residue is mostly in
the unedible portion (42.0-117 ppm in whole fruit, 2.8-11.0 ppm in
edible portion) (Dow, 1969). The value of OPP residues found in peel
of oranges varies slightly with variety. Shamoute oranges peel varied
between 5.9-23 ppm; Valencias between 8.8 and 21.3 ppm; grapefruit
peel 7.0-26.1 ppm; lemons 5.4-15.6 ppm in peel. The quantities of OPP
in pulp of citrus is very low. In many cases none could be detected,
but when peel contained the higher levels, OPP residues from trace to
0.4 ppm were found in pulp (Rajzman and Apfelbaum, 1968).
There is no information available on possible chemical alteration of
the nature of the residue. All residue analysis methods to date rely
on expressing the residue as 2-phenylphenol.
Evidence of residues in food in commerce or at consumption
Information on the residue content of food in commerce is rare. Only
one published paper has a bearing on this subject (Rajzman and
Apfelbaum, 1968). Citrus fruit treated in 22 packing houses in Israel
were collected, sampled and analysed. The residues in whole fruit
range from 1.8 to 8.3 ppm with 80% of the samples containing less than
5 ppm. The authors conclude that in view of the small number of
samples used, their finding of a maximum of 8.3 ppm should not be
considered as the maximum quantity which can occur in citrus treated
in the manner described.
There is no information available with regard to residues in food at
the time of consumption. It can be assumed there will be little loss
of residue in wax-treated fruits and vegetables. Since the treatment
is by post-harvest application, the amounts in wax-treated food
reaching the consumer can be considered as those reported as resulting
from supervised trials. Possibly slightly lower levels will remain in
food treated with water formulations.
METHODS OF RESIDUE ANALYSIS
Colorimetric procedures have most often been proposed for the
determination of residues of 2-phenylphenol. Tomkins and Isherwood
(1945) used coupling with diazotized sulphanilic acid in determining
residues in oranges and marmalade after a steam-distillation
extraction, with acid-alkali treatment clean-up stages. Gottleib and
Marsh (1946) suggested an aminophenazone procedure for the
determination of several phenolic fungicides. The results of a
collaborative study of this method as applied to the determination of
residues of 2-phenylphenol in apples, pears and citrus fruit have been
reported by Schiffman (1957); Hayward and Grierson (1960) used this
method on oranges. A similar procedure has been officially recommended
by the Communauté Economique Européene (van Elslande, 1967) for
residues in fruits and this should be suitable for regulatory purposes
at the present time. A modified steam-distillation extraction
apparatus has been described by Leinbach and Brekke (1961) who used an
indophenol colorimetric method for residues in purees of raspberry,
strawberry, prunes, loganberry and fig and in orange juice. The
coloration given by reacting 2-phenylphenol with titanium sulphate
(Caulfield and Robinson, 1953) is not sufficiently sensitive for
residue analysis. A spectrofluorometric procedure for the examination
of single fruits was proposed by the Cotta-Ramusino and Stacchini
(1966) which is sensitive to <0.1 mg of 2-phenylphenol for fruit,
Gunther et al. (1963) reported a procedure for the routine examination
of citrus fruits for biphenyl and 2-phenylphenol, determining the
latter colorimetrically after coupling with p-nitrofluoroborate, and
obtained recoveries ranging from 89 to 100 percent at the 0.5 to 10
ppm level.
Chromatographic methods available include a gas chromatographic
examination of concentrated orange juice (Thomas, 1960), applicable
over the range 1 to 10 ppm on 1 ml of sample and a thin-layer
chromatographic procedure for the detection of residues of biphenyl
and 2-phenylphenol in lemons (Chioffi, 1965). Mestres and Chave,
(1965) used extraction with cyclohexane in a Dean and Stark apparatus
before separating and determining biphenyl and 2-phenylphenol by gas
chromatography with flame-ionisation detection. These chromatographic
techniques should be developed and evaluated for application as
regulatory procedures.
NATIONAL TOLERANCES
Tolerance (ppm)
Country Crop as 2-phenylphenol
Canada Cherries, nectarines 5
Citrus fruits, cucumbers,
pepper (bell), pineapple,
tomatoes 10
Sweet potatoes 15
Carrots, peaches, plums 20
Apples, pears 25
Cantaloups 125
E.E.C. Citrus fruits 12
Netherlands Citrus fruits 10
United Kingdom Citrus fruit 70
Apples, pears, pineapples 10
Melons 125
NATIONAL TOLERANCES (continued)
Tolerance (ppm)
Country Crop as 2-phenylphenol
United States Cherries, nectarines 5
of America Citrus, citron, cucumbers, grapefruit, 10
kumquats, lemons, limes oranges,
peppers (bell), pineapple, tangelos,
tangerines, tomatoes
Sweet potatoes 15
Carrots, peaches, plums (fresh prunes) 20
Apples, pears 25
Cantaloups, of which not more than 125
10 ppm shall be in the edible portion.
APPRAISAL
The antimicrobial properties of 2-phenylphenol have been utilized in
many countries for over 40 years. Due to its broad spectrum fungicidal
and bactericidal properties and commercial availability it has been
used widely as a germicide. In addition to its current use as a
post-harvest pesticide it has been used in household disinfectants,
preservatives for water-oil emulsions in textile and metal industries.
The sodium salt is also used as a preservative in water base paints
and adhesives. The free phenol is used in the post-harvest treatment
of fruits and vegetables in wax formulations as a protectant in
shipment and storage. The sodium salt is dissolved in water and used
as a post-harvest rinse for fruit and vegetables. The tree phenol is
also used to treat harvesting containers, shipping and storage
facilities, fruit handling machinery and shipping containers.
The data on residues from supervised trials are based only on U.S.A.
technology of use and on the two products as manufactured by Dow
Chemical Company. These are proprietary compounds, manufactured by
several companies in many countries. No information was provided on
the use pattern or technology of use in other countries.
The residues from both compounds are expressed as 2-phenylphenol.
Highly specific methods of dipping and washing individual species of
fruits and vegetables in the protective baths have been developed,
with carefully prescribed times of immersion varying from as little as
10 seconds (apples and pears) to e.g. 6 minutes for citrus. A skilled
technology and monitoring of wash solutions is required to maintain
correct conditions for treatment. Consequently, amounts of residues
vary depending upon the type of formulation used, the technology of
treatment and the fruit or vegetable itself. Supervised trials in the
U.S.A. carried out in several types of commercial scale fruit and
vegetable handling facilities resulted in residues of (ppm) apples
0.2-14, cantaloups (whole fruit) 42-117, carrots 2.8-17 (removed by
processing), cherries 0.3-2, citrus 5.5-9.3, cucumbers 1.3-7.7,
nectarines 0.4-1.4, peaches 9-15, pears 1-22, peppers 4.2-7.1,
pineapples 3.8-7.1, plums 0.8-12.8, sweet potatoes 0.5-5.7, tomatoes
0.5-7.7. Variations of conditions of treatment and contact time could
presumably result in slightly higher residues.
Methods of analysis for residues are available and they should be
evaluated by collaborative studies. (This in not considered critical).
In general all methods are based on determining the 2-phenylphenol
content of an aqueous solution residue extract by colometric
measurement after coupling with 4-aminoantipyrine.
No data are available on residues on fruits and vegetables as
consumed, but since these are post-harvest uses it would be a fair
speculation to assume that the residues would be the average of the
ranges outlined above.
Since the technology of treatment is so critical, in approaching the
recommendation of tolerances, some leeway should be provided for error
in time of immersion, pH of bath and other factors in this technology.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Cherries, nectarines 3 )
)
Citrus, cucumbers, peppers (bell), )
pineapples and tomatoes 10 )
)
Sweet potatoes 15 )
)
Apples, plums, (fresh prunes) 15 ) ppm as 2-phenylphenol
)
Carrots, peaches 20 )
)
Pears 25 )
)
Cantaloups (whole fruit) 120 )
)
Cantaloups (edible portion) 10 )
FURTHER WORK OR INFORMATION
DESIRABLE
1. A reproduction study in experimental animals.
2. Metabolic studies in experimental animals and man.
3. Long-term studies using a larger number of animals.
4. Data on residue levels in raw agricultural commodities moving in
commerce.
5. Data on residues in food at the time of consumption.
6. Results of collaborative studies of chromatographic methods of
analysis.
REFERENCES
Benger, H. (1968) Fluorimetsche bestimmung von o-phenyl-phenol in
citrusfrüchten. Arch. Hyg. 152(3):225-30
Caulfield, P.H. and Robinson, R.J. (1953) Spectrophotometric
determination of o-phenylphenol with titanium sulphate. Analyt.
Chem. 25:982-3
Chioffi, V. (1965) Investigation of diphenyl and o-phenylphenol in
lemons by thin-layer chromatography. Boll. Lab. Chim. Provinciali
(Bologna), 16:366-76 Chem. Abs., 1966, 64:1254d.
Cotta-Ramusino, F. and Stacchini, A. (1966) Spectrofluorometric
determination of o-phenylphenol in citrus fruits. Fruits (Paris),
21:37-9; Chem. Abs., 64:14858h.
Dodgson, K.S., Garton, G.A., Stubbs, A.L. and Williams, R.T. (1948)
Studies on detoxication 15. On the glucuronides of stilboestrol,
hexoestrol and dienoestrol. Biochem. J., 42:357-65 (cited in
Williams 1959)
Dow. (1969) Unpub. three-volume submission of data submitted by Dow
Chemical Co., Midland, Mich., U.S.A. to FAO
E.E.C. (1967) Amtsblatt der Europaischen Gemeinschaften, No. G1203B,
11 July 1967, 10. Jahrgang Nr. 148.
Elslande van, R. (1967) Dosage des résidues d'orthophénylphénol et
d'orthophénylphénate de sodium dans les agrumes. J. Offic.
Communantés Européenes, 10: No.148, 7-9 (11.7.1967).
Ernst, W. (1965) Umwandlung und Ausscheidung von 2-hydroxydiphenyl
bei der Ratte. Arzneimittel. - Forsch., 15:632-6
FAO/WHO (1962) Evaluation of the toxicity of a number of
antimicrobials and antioxidants. Sixth Report of the Joint FAO/WHO
Expert Committee on Food Additives; FAO Nutrition Meetings Report
Series No.31; Wld Hlth Org. techn. Rep. Ser. No.228, pp. 78-81
Gottleib, S. and Marsh, P.B. (1946) Quantitative determination of
phenolic fungicides. Ind. Eng. Chem. Anal. Ed., 18:16-19
Gunther, F.A., Blinn, R.C. and Barkley, J.H. (1963) Procedure for
routine determination of biphenyl and o-phenylphenol on and in
citrus fruit. Analyst, 88:36-42
Hayward, F.W. and Grierson, W. (1960) Effects of treatment conditions
on o-phenylphenol residues in oranges. J. Agr. Fd. Chem. 8.308-10
Hodge, H.C., Maynard, E.A., Blanchet, H.J. Jr., Spencer, H.C. and
Rowe, V.K. (1952) Toxicological studies of orthophenylphenol
(Dowicide I) J. Pharmacol. exp. Ther., 104:202-10
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L.,
Fishbein, L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L.,
Gart, J.J., Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in mice.
A preliminary note. J. Nat. Cancer Inst. 42:1102-14
Kamil, I.A., Smith, J.N. and Williams, R.T. (1951) Studies in
detoxication. 41. A study of the optical rotations of the amides and
triacetyl methyl eaters of some biosynthetic substituted
phenylglucuronides. Biochem. J. 50:235-40 (cited in Williams,
1959)
Leinbach, L.R. and Brekke, J.E. (1961) A modified Gibbs method for the
determination of 1 ppm or less of o-phenylphenol in fruits. J.
Agr. Food Chem. 9:205-6
MacIntosh, F.C. (1945) The toxicity of diphenyl and o-phenyl-phenol.
Analyst, 70:334
Mestres, R. and Chave, C. (1965) Determination of biphenyl and
o-phenylphenol in citrus fruits. Trans. Soc. Pharm. Montpellier,
24:272-82. Chem. Abs., 63:15434g.
Rajzman, A. and Apfelbaum, A. (1968) Survey of o-phenylphenol
residues found in marketable citrus fruit. J. Sci. Fd. Agric. 19:740-4
Schiffman, C.D. (1957) Report on sodium orthophenylphenate residues on
apples, pears and citrus fruits. J. Assoc. Offic. Agric. Chem.,
40:238-42
Thomas, R. (1960) The detection and determination of diphenyl and
o-phenylphenol in concentrated orange juice by gas
chromatography. Analyst, 85:551-6
Tomkins, R.G. and Isherwood, F.A. (1945) The absorption of diphenyl
and o-phenylphenol by oranges from treated wraps. Analyst,
70:330-3
U.S.D.A. (1967) Summary of registered agricultural pesticide chemical
uses (2nd Edition), U.S. Department of Agriculture, Washington, D.C.
pp. 647, 727-8(a) (1967)
Williams, R.T. (1959) "Detoxication Mechanisms" London, Chapman and
Hall, p. 308
Other relevant chemical properties
The free phenol is a white, crystalline, free-flowing powder,
molecular weight 170.2. Mild phenolic odour. Less than 0.1g is soluble
in 1 ml of water. Freely soluble in ethanol, soluble in fats and oils.
It is dissolved or dispersed in wax formulations.
The sodium salt is a buff coloured solid. Molecular weight 264.3. Very
soluble in water and ethanol; practically insoluble in oils. Used as
water solution.
Purity
Specifications for purity of both compounds have been published by the
European Economic Community (E.E.C., 1967):
E231 2-phenylphenol
melting point 56 - 58°C
content not less than 99%
biphenylether not more than 0.3%
p-phenylphenol not more than 0.1%
alpha-naphthol not more than 0.01%
sulphate after ashing not more than 0.05%
E232 sodium orthophenylphenate
melting point of 2-phenylphenol precipitated by acidification of
sodium salt, not recrystallized and dried over sulphuric acid 56
- 58°C
ph 2% water solution must be between pH 11.1 and 11.8
content not less than 95% C12H ONa.4H2.0
biphenylether not more than 0.3%
p-phenylphenol not more than 0.1%
x-naphthol not more than 0.01%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
The biological data available on this compound have been evaluated by
the Joint FAO/WHO Expert Committee on Food Additives, and a monograph
entitled "Sodium o-phenylphenol" is included in the Sixth Report of
this Committee (FAO/WHO, 1962). A summary of the data then considered
as well as some limited additional information which has recently
become available is included below.
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Tissue examination of rats which had received 2-phenylphenol orally
for two years revealed little tendency for storage of the compound.
The kidney was the only organ containing detectable amounts of the
compound; average values of 220 mg/kg tissue were found in the kidneys
of rats fed 20,000 ppm in the diet, and approximately 10 mg/kg tissue
in the kidneys of rats fed 2000 ppm (Hodge et al., 1952).
After administration to rabbits, 2-phenylphenol is highly conjugated
with glucuronic acid but it is not known whether it is converted to an
ethereal sulphate in this species. The glucuronide has been isolated
from rabbit urine and characterized by conversion to the
triacetylmethyl ester (Williams, 1959; Dodgson et al., 1948; Kamil et
al., 1951).
When administered orally to rats, 2-phenylphenol is partly converted
to 2,5-dihydroxybiphenyl which, like 2-phenylphenol, is excreted in
the urine as a glucuronide and ethereal sulphate. The total output of
2-phenylphenol within 48 hours amounted to about 70 percent as
conjugates of 2-phenylphenol and 2,5-dihydroxyphenol (Ernst, 1965).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Groups of 18 mice of each sex from two hybrid strains of mice were
given 2-phenylphenol for 18 months. The dose of 100 mg/kg was given to
the mice by gavage from the seventh day of age to the time of weaning
at four weeks of age and thereafter the chemical was added to the diet
in a corresponding amount of 280 ppm. There was no significant
increase in the incidence of tumours (Innes et al., 1969).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Rat oral 2700-3000 (approx.) MacIntosh, 1945
Hodge et al., 1952
Cat oral 500 (approx.) MacIntosh, 1945
Short-term studies
Dog
A preliminary study demonstrated that when daily doses of 1,000 mg/kg
body-weight of 2-phenylphenol were fed to two dogs, both died within a
month. Groups of two dogs each were then fed 2-phenylphenol for a
period of one year in daily amounts of 0, 20, 200, and 500 mg/kg body
weight. No effect related to the administration of 2-phenylphenol was
observed. Haematological values, urinary sugar and protein values,
organ weights and histopathological examination of the various tissues
did not differ from the normal range (Hodge et al., 1952).
Rat
Groups, each comprising 15 male rats, were fed 2-phenylphenol in daily
doses of 0, 2, 20, or 200 mg/kg body-weight for 32 days. There were no
toxic symptoms attributable to feeding 2-phenylphenol. The mean
growth-rate and the haemoglobin and white blood cell levels in all
groups were comparable to the controls (MacIntosh, 1945).
Five male and five female rats in each group were given by stomach
tube doses of 0, 50, 100, 200, and 500 mg/kg body-weight for five days
a week over a period of six months. The only abnormality observed was
a slight increase in average liver and kidney weights in the animals
fed the 500 mg/kg dose level (Hodge et al., 1952).
When diets containing 0, 1,000, 3,000, 10,000, or 20,000 ppm of
2-phenylphenol were fed for three months to groups of rats comprising
12 males and 12 females, slight retardation of growth was observed in
the 20,000 ppm group. There was no significant difference between the
mortality of control and test animals. There were doubtful increases
in weight of liver, kidney, and spleen of certain rats of the 10,000
and 20,000 ppm groups. No tissue changes were observed (Hodge et al.,
1952)
Long-term studies
Rat
Male and female rats (25 of each sex per group) were maintained for
two years on diets containing 0, 200, 2,000 and 20,000 ppm of
2-phenylphenol. The animals fed 200 and 2000 ppm showed no adverse
effects when compared with the controls, as judged by growth,
mortality, gross appearance, haematology, urinary sugar and protein
values, organ-weights, tissue content of 2-phenylphenol, and
histopathological examination of various tissues. The group fed 20,000
ppm of 2-phenylphenol differed from the controls by exhibiting slight
retardation of growth, histological kidney changes (marked tubular
dilation), and the presence of small amounts of 2-phenylphenol in the
kidney tissues (Hodge et al., 1952).
OBSERVATION IN MAN
A 5.0 percent solution of 2-phenylphenol in sesame oil and a 0.1
percent aqueous solution of the sodium salt tested on 200 subjects
caused neither primary skin irritation nor skin sensitization. The
sodium salt is slightly irritating in 0.5 percent aqueous solution and
decidedly irritating in 1.0 percent and 5.0 percent solutions (Hodge
et al., 1952).
COMMENT
The short and long-term studies in rats are sufficient to be taken as
a basis for the evaluation of 2-phenylphenol. The dog study was not
considered adequate. In addition, the studies on metabolism are
incomplete and there are no studies on reproduction. However, the
Meeting considered that the toxicological studies were adequate to
reaffirm the previous acceptable daily intake established by the Joint
FAO/WHO Expert Committee on Food Additives in its Sixth Report
(FAO/WHO, 1962).
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Dog: 500 mg/kg body-weight/day
Rat: 2000 ppm in the diet, equivalent to 100 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 1.0 mg/kg body-weight (based upon the previous adi. - FAO/WHO,
1962)
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
In Great Britain a dormant paint application to apple trees is
approved for control of canker.
Post-harvest treatments
2-phenylphenol has been used broadly for over 40 years in many
countries for its general antimicrobial properties. Due to its broad
spectrum bactericidal and fungistatic effect, and commercial
availability it is widely used. It is employed as a household
disinfectant, preservative in oil-water emulsions used in the textile
and metal industries, as well as a post-harvest treatment for fruits
and vegetables to prevent losses due to rots and moulds in storage and
transport of these foods. The sodium salt is used as a preservative
for protein-based and other adhesives, as well as for the post-harvest
use in fruits and vegetables.
Principal formulations in the U.S.A. are:
2-phenylphenol: 5% liquid; 98% wettable powder; 2% in wax.
Wax formulations are applied by commercial application equipment in
tandem with sorting and washing facilities. These are specifically
designed for the crop concerned as to technology of use, formulation
strength, time of immersion in the bath and monitoring of the process
to ensure maintenance of treatment criteria for effectiveness without
damage to the crop. Wax strength formulations vary, e.g. carrots 0.5%,
citrus 0.8%, cucumbers 2.5%, nectarines 0.2%, peaches 0.2%; peppers
2.5%, plums 2%, tomatoes 2.5%. A 5% liquid dip is used for crates,
field boxes, hampers, lugs and wood containers for fruits and
vegetables (U.S.D.A., 1967).
sodium o-phenyl phenate: 97% wettable powder, solutions ranging from
1.5% to 40%, wax emulsion from 0.7% to 2.0%.
The strength of solution used varies with the crop concerned and the
time of immersion to obtain protection and avoid phytotoxic reactions
in fruits or vegetables. The times of immersion will vary from a few
seconds to several minutes, but are precisely prescribed and
monitoring is required for each formulation and crop. Examples
includes apples 0.45% - 1.4% solution, bananas 2% applied to cut stem
area of crown of head area (no residue in fruit or skin), cantaloups
1.5%, carrots 0.1%, cherries 1.0% with wetting agent, citrus 0.5% wax
emulsion to 3% in water (plus caustic and hexamine), cucumbers 1%
- 2%, nectarines 1%, peaches 0.05 - 0.6%, pears 0.23% to 1.45%,
peppers 1%, pineapple 1.25% (1% in wax), plums 0.1%, sweet potatoes
0.37% - 0.5% solution or 0.7% wax emulsion, tomatoes 1% (U.S.D.A.,
1967).
These use patterns were available from the U.S.A. only.
RESIDUES RESULTING FROM SUPERVISED TRIALS
As mentioned above, the technology for use of these compounds has been
developed under commercial conditions requiring accurate control of
time of treatment which may be from as brief as 10 seconds (apples and
pears) to 15-18 minutes (peaches), at specified temperatures, pH etc.
The data pertaining to the conditions of these trials were collected
from several locations in the U.S.A. They are unpublished. They have
been collated and submitted with full details to FAO by the Dow
Chemical Company (Dow, 1969).
A review of several abstract journals revealed no published literature
bearing on this subject from other countries. It is known that both
compounds are used by several citrus exporting countries, but these
have not been identified, nor were use patterns, technology of use or
data on residues resulting from supervised trials supplied for
evaluation from other than the U.S.A.
The results of U.S.A. trials are summarized in Tables 1 and 2 (Dow,
1969).
TABLE I
Residues resulting from supervised trials with 2-phenylphenol
Percent 2-phenylphenol Range of residues as
Fruit or vegetable in wax* ppm o-phenylphenol
Not rinsed
Carrots 0.5 17.0
Cucumbers 0.5-2 4.2-5.6
Peppers 2 0.1-1.0
Plums 2 2.5-12.8
Sweet potatoes 1 4.0-5.7
Tomatoes 2-3 0.1-7.7
*Concentration varies according to crop and/or contact time
TABLE 2
Residues resulting from supervised trials with Sodium o-phenylphenate
Percent sodium o-phenylphenate Range of residues as
Fruit or vegetable in water* ppm o-phenylphenol
Rinsed Not rinsed
Apples 0.42-0.77 0.2-14.0
Cantaloups 1.5
edible portion 1.0-6.0
whole fruit 42.0-117.0
Carrots 0.1 2.8-11.0
Cherries 0.65-1.08 0.3-2
Citrus 2.0 5.5-9.3
Cucumbers 1.0 1.3-7.7
Nectarines 0.75-1.2 0.4-1.4
Peaches 0.1-0.12 9.0-15.0
Pears 0.4-0.99 1.0-25.0 1.0-22.0
Peppers 1.0 4.2-7.1
Pineapples 1.25 3.8-7.1
Plums 0.4 0.8-1.3
Sweet potatoes 0.5-1.0 0.5-11.0 1.7-3.4
Tomatoes 0.9 0.5-1.4
*Concentration varies according to crop and/or contact time
FATE OF RESIDUES
Carrots treated with 0.1% sodium o-phenylphenate, stored and steam or
abrasion peeled prior to canning in a commercial manner contained no
residues in the canned product. In cantaloups the residue is mostly in
the unedible portion (42.0-117 ppm in whole fruit, 2.8-11.0 ppm in
edible portion) (Dow, 1969). The value of OPP residues found in peel
of oranges varies slightly with variety. Shamoute oranges peel varied
between 5.9-23 ppm; Valencias between 8.8 and 21.3 ppm; grapefruit
peel 7.0-26.1 ppm; lemons 5.4-15.6 ppm in peel. The quantities of OPP
in pulp of citrus is very low. In many cases none could be detected,
but when peel contained the higher levels, OPP residues from trace to
0.4 ppm were found in pulp (Rajzman and Apfelbaum, 1968).
There is no information available on possible chemical alteration of
the nature of the residue. All residue analysis methods to date rely
on expressing the residue as 2-phenylphenol.
Evidence of residues in food in commerce or at consumption
Information on the residue content of food in commerce is rare. Only
one published paper has a bearing on this subject (Rajzman and
Apfelbaum, 1968). Citrus fruit treated in 22 packing houses in Israel
were collected, sampled and analysed. The residues in whole fruit
range from 1.8 to 8.3 ppm with 80% of the samples containing less than
5 ppm. The authors conclude that in view of the small number of
samples used, their finding of a maximum of 8.3 ppm should not be
considered as the maximum quantity which can occur in citrus treated
in the manner described.
There is no information available with regard to residues in food at
the time of consumption. It can be assumed there will be little loss
of residue in wax-treated fruits and vegetables. Since the treatment
is by post-harvest application, the amounts in wax-treated food
reaching the consumer can be considered as those reported as resulting
from supervised trials. Possibly slightly lower levels will remain in
food treated with water formulations.
METHODS OF RESIDUE ANALYSIS
Colorimetric procedures have most often been proposed for the
determination of residues of 2-phenylphenol. Tomkins and Isherwood
(1945) used coupling with diazotized sulphanilic acid in determining
residues in oranges and marmalade after a steam-distillation
extraction, with acid-alkali treatment clean-up stages. Gottleib and
Marsh (1946) suggested an aminophenazone procedure for the
determination of several phenolic fungicides. The results of a
collaborative study of this method as applied to the determination of
residues of 2-phenylphenol in apples, pears and citrus fruit have been
reported by Schiffman (1957); Hayward and Grierson (1960) used this
method on oranges. A similar procedure has been officially recommended
by the Communauté Economique Européene (van Elslande, 1967) for
residues in fruits and this should be suitable for regulatory purposes
at the present time. A modified steam-distillation extraction
apparatus has been described by Leinbach and Brekke (1961) who used an
indophenol colorimetric method for residues in purees of raspberry,
strawberry, prunes, loganberry and fig and in orange juice. The
coloration given by reacting 2-phenylphenol with titanium sulphate
(Caulfield and Robinson, 1953) is not sufficiently sensitive for
residue analysis. A spectrofluorometric procedure for the examination
of single fruits was proposed by the Cotta-Ramusino and Stacchini
(1966) which is sensitive to <0.1 mg of 2-phenylphenol for fruit,
Gunther et al. (1963) reported a procedure for the routine examination
of citrus fruits for biphenyl and 2-phenylphenol, determining the
latter colorimetrically after coupling with p-nitrofluoroborate, and
obtained recoveries ranging from 89 to 100 percent at the 0.5 to 10
ppm level.
Chromatographic methods available include a gas chromatographic
examination of concentrated orange juice (Thomas, 1960), applicable
over the range 1 to 10 ppm on 1 ml of sample and a thin-layer
chromatographic procedure for the detection of residues of biphenyl
and 2-phenylphenol in lemons (Chioffi, 1965). Mestres and Chave,
(1965) used extraction with cyclohexane in a Dean and Stark apparatus
before separating and determining biphenyl and 2-phenylphenol by gas
chromatography with flame-ionisation detection. These chromatographic
techniques should be developed and evaluated for application as
regulatory procedures.
NATIONAL TOLERANCES
Tolerance (ppm)
Country Crop as 2-phenylphenol
Canada Cherries, nectarines 5
Citrus fruits, cucumbers,
pepper (bell), pineapple,
tomatoes 10
Sweet potatoes 15
Carrots, peaches, plums 20
Apples, pears 25
Cantaloups 125
E.E.C. Citrus fruits 12
Netherlands Citrus fruits 10
United Kingdom Citrus fruit 70
Apples, pears, pineapples 10
Melons 125
NATIONAL TOLERANCES (continued)
Tolerance (ppm)
Country Crop as 2-phenylphenol
United States Cherries, nectarines 5
of America Citrus, citron, cucumbers, grapefruit, 10
kumquats, lemons, limes oranges,
peppers (bell), pineapple, tangelos,
tangerines, tomatoes
Sweet potatoes 15
Carrots, peaches, plums (fresh prunes) 20
Apples, pears 25
Cantaloups, of which not more than 125
10 ppm shall be in the edible portion.
APPRAISAL
The antimicrobial properties of 2-phenylphenol have been utilized in
many countries for over 40 years. Due to its broad spectrum fungicidal
and bactericidal properties and commercial availability it has been
used widely as a germicide. In addition to its current use as a
post-harvest pesticide it has been used in household disinfectants,
preservatives for water-oil emulsions in textile and metal industries.
The sodium salt is also used as a preservative in water base paints
and adhesives. The free phenol is used in the post-harvest treatment
of fruits and vegetables in wax formulations as a protectant in
shipment and storage. The sodium salt is dissolved in water and used
as a post-harvest rinse for fruit and vegetables. The tree phenol is
also used to treat harvesting containers, shipping and storage
facilities, fruit handling machinery and shipping containers.
The data on residues from supervised trials are based only on U.S.A.
technology of use and on the two products as manufactured by Dow
Chemical Company. These are proprietary compounds, manufactured by
several companies in many countries. No information was provided on
the use pattern or technology of use in other countries.
The residues from both compounds are expressed as 2-phenylphenol.
Highly specific methods of dipping and washing individual species of
fruits and vegetables in the protective baths have been developed,
with carefully prescribed times of immersion varying from as little as
10 seconds (apples and pears) to e.g. 6 minutes for citrus. A skilled
technology and monitoring of wash solutions is required to maintain
correct conditions for treatment. Consequently, amounts of residues
vary depending upon the type of formulation used, the technology of
treatment and the fruit or vegetable itself. Supervised trials in the
U.S.A. carried out in several types of commercial scale fruit and
vegetable handling facilities resulted in residues of (ppm) apples
0.2-14, cantaloups (whole fruit) 42-117, carrots 2.8-17 (removed by
processing), cherries 0.3-2, citrus 5.5-9.3, cucumbers 1.3-7.7,
nectarines 0.4-1.4, peaches 9-15, pears 1-22, peppers 4.2-7.1,
pineapples 3.8-7.1, plums 0.8-12.8, sweet potatoes 0.5-5.7, tomatoes
0.5-7.7. Variations of conditions of treatment and contact time could
presumably result in slightly higher residues.
Methods of analysis for residues are available and they should be
evaluated by collaborative studies. (This in not considered critical).
In general all methods are based on determining the 2-phenylphenol
content of an aqueous solution residue extract by colometric
measurement after coupling with 4-aminoantipyrine.
No data are available on residues on fruits and vegetables as
consumed, but since these are post-harvest uses it would be a fair
speculation to assume that the residues would be the average of the
ranges outlined above.
Since the technology of treatment is so critical, in approaching the
recommendation of tolerances, some leeway should be provided for error
in time of immersion, pH of bath and other factors in this technology.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Cherries, nectarines 3 )
)
Citrus, cucumbers, peppers (bell), )
pineapples and tomatoes 10 )
)
Sweet potatoes 15 )
)
Apples, plums, (fresh prunes) 15 ) ppm as 2-phenylphenol
)
Carrots, peaches 20 )
)
Pears 25 )
)
Cantaloups (whole fruit) 120 )
)
Cantaloups (edible portion) 10 )
FURTHER WORK OR INFORMATION
DESIRABLE
1. A reproduction study in experimental animals.
2. Metabolic studies in experimental animals and man.
3. Long-term studies using a larger number of animals.
4. Data on residue levels in raw agricultural commodities moving in
commerce.
5. Data on residues in food at the time of consumption.
6. Results of collaborative studies of chromatographic methods of
analysis.
REFERENCES
Benger, H. (1968) Fluorimetsche bestimmung von o-phenyl-phenol in
citrusfrüchten. Arch. Hyg. 152(3):225-30
Caulfield, P.H. and Robinson, R.J. (1953) Spectrophotometric
determination of o-phenylphenol with titanium sulphate. Analyt.
Chem. 25:982-3
Chioffi, V. (1965) Investigation of diphenyl and o-phenylphenol in
lemons by thin-layer chromatography. Boll. Lab. Chim. Provinciali
(Bologna), 16:366-76 Chem. Abs., 1966, 64:1254d.
Cotta-Ramusino, F. and Stacchini, A. (1966) Spectrofluorometric
determination of o-phenylphenol in citrus fruits. Fruits (Paris),
21:37-9; Chem. Abs., 64:14858h.
Dodgson, K.S., Garton, G.A., Stubbs, A.L. and Williams, R.T. (1948)
Studies on detoxication 15. On the glucuronides of stilboestrol,
hexoestrol and dienoestrol. Biochem. J., 42:357-65 (cited in
Williams 1959)
Dow. (1969) Unpub. three-volume submission of data submitted by Dow
Chemical Co., Midland, Mich., U.S.A. to FAO
E.E.C. (1967) Amtsblatt der Europaischen Gemeinschaften, No. G1203B,
11 July 1967, 10. Jahrgang Nr. 148.
Elslande van, R. (1967) Dosage des résidues d'orthophénylphénol et
d'orthophénylphénate de sodium dans les agrumes. J. Offic.
Communantés Européenes, 10: No.148, 7-9 (11.7.1967).
Ernst, W. (1965) Umwandlung und Ausscheidung von 2-hydroxydiphenyl
bei der Ratte. Arzneimittel. - Forsch., 15:632-6
FAO/WHO (1962) Evaluation of the toxicity of a number of
antimicrobials and antioxidants. Sixth Report of the Joint FAO/WHO
Expert Committee on Food Additives; FAO Nutrition Meetings Report
Series No.31; Wld Hlth Org. techn. Rep. Ser. No.228, pp. 78-81
Gottleib, S. and Marsh, P.B. (1946) Quantitative determination of
phenolic fungicides. Ind. Eng. Chem. Anal. Ed., 18:16-19
Gunther, F.A., Blinn, R.C. and Barkley, J.H. (1963) Procedure for
routine determination of biphenyl and o-phenylphenol on and in
citrus fruit. Analyst, 88:36-42
Hayward, F.W. and Grierson, W. (1960) Effects of treatment conditions
on o-phenylphenol residues in oranges. J. Agr. Fd. Chem. 8.308-10
Hodge, H.C., Maynard, E.A., Blanchet, H.J. Jr., Spencer, H.C. and
Rowe, V.K. (1952) Toxicological studies of orthophenylphenol
(Dowicide I) J. Pharmacol. exp. Ther., 104:202-10
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L.,
Fishbein, L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L.,
Gart, J.J., Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in mice.
A preliminary note. J. Nat. Cancer Inst. 42:1102-14
Kamil, I.A., Smith, J.N. and Williams, R.T. (1951) Studies in
detoxication. 41. A study of the optical rotations of the amides and
triacetyl methyl eaters of some biosynthetic substituted
phenylglucuronides. Biochem. J. 50:235-40 (cited in Williams,
1959)
Leinbach, L.R. and Brekke, J.E. (1961) A modified Gibbs method for the
determination of 1 ppm or less of o-phenylphenol in fruits. J.
Agr. Food Chem. 9:205-6
MacIntosh, F.C. (1945) The toxicity of diphenyl and o-phenyl-phenol.
Analyst, 70:334
Mestres, R. and Chave, C. (1965) Determination of biphenyl and
o-phenylphenol in citrus fruits. Trans. Soc. Pharm. Montpellier,
24:272-82. Chem. Abs., 63:15434g.
Rajzman, A. and Apfelbaum, A. (1968) Survey of o-phenylphenol
residues found in marketable citrus fruit. J. Sci. Fd. Agric. 19:740-4
Schiffman, C.D. (1957) Report on sodium orthophenylphenate residues on
apples, pears and citrus fruits. J. Assoc. Offic. Agric. Chem.,
40:238-42
Thomas, R. (1960) The detection and determination of diphenyl and
o-phenylphenol in concentrated orange juice by gas
chromatography. Analyst, 85:551-6
Tomkins, R.G. and Isherwood, F.A. (1945) The absorption of diphenyl
and o-phenylphenol by oranges from treated wraps. Analyst,
70:330-3
U.S.D.A. (1967) Summary of registered agricultural pesticide chemical
uses (2nd Edition), U.S. Department of Agriculture, Washington, D.C.
pp. 647, 727-8(a) (1967)
Williams, R.T. (1959) "Detoxication Mechanisms" London, Chapman and
Hall, p. 308
