
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
QUINTOZENE
IDENTITY
Chemical name
pentachloronitrobenzene
Synonyms
PCNB, Brassicol(R), Terrachlor(R), Tritistan(R), Folosan(R),
Botrilex(R).
Structural formula
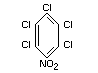 Other relevant chemical properties
Colourless crystalline needles practically insoluble in water, soluble
in benzene and chloroform. Technical quintozene is usually 97-99
percent pure. The main impurity, is hexachlorobenzene (1.5%) together
with lesser amounts of pentachlorobenzene and tetrachloronitrobenzene.
Vp 11.3 × 10-5mm Hg at 25°C. Quintozene shows high stability in the
soil. Quintozene is converted to pentachloroaniline (PCA) in moist
soil, the metabolite having somewhat lower fungicidal activity. In
animals the metabolites are pentachloroaniline and mercapturic acid
(Betts et al., 1955).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Fat samples from groups of ten rats (five males and five females) fed
diets containing 63.5, 635, 1250, or 2500 ppm of technical quintozene
for three months were examined for storage of quintozene. The apparent
storage ranged from an average of 43 ppm in the fat for the 63.5 ppm
diet to 1234 ppm for the 2500 ppm diet, there being a relatively
linear relationship between the levels of fat storage to the
concentration of quintozene in the diet. However, the neutron
activation method of analysis which was used would have shown the
presence of other chlorinated compounds as well an quintozene
(Finnegan et al., 1958).
Tissues from an unspecified number of dogs fed 5 or 1,080 ppm of
technical quintozene in their diet for 2 years were analysed by gas
chromatography for residues of quintozene and its metabolites. No
quintozene was found in fat, muscle, kidney, or liver tissues but two
metabolic products identified as pentachloroaniline and
methyl-pentachlorophenyl sulfide were found in these tissues.
Pentachloroaniline was found only in the fat and liver and in amounts
of less than 1 ppm for both dose-levels. Methyl-pentachlorophenyl
sulfide was found in the fat and liver of rats fed both dose levels
and was present as well in kidney and muscle in the 1080 ppm group,
the largest amount being 2.5 ppm in the fat of animals fed that level.
In another study on fat from an unspecified number of rats fed 50 or
500 ppm of technical quintozene in their diet for seven months, there
was less than 1 ppm of either of the metabolites in the fat of the
rats fed 50 ppm of quintozene, and approximately 1 ppm of
pentachloroaniline and 5 ppm of methyl-pentachlorophenyl sulfide for
the 500 ppm group (Kuchar et al., 1969).
Pentachloroaniline and another metabolite, a mercapturic acid, have
been isolated in urine from rabbits treated with quintozene. With a 2
g. dose an average of 62 percent of quintozene was unabsorbed and
excreted in the faeces. The average percentages excreted in urine as
pentachloroaniline and N-acetyl-S-pentachlorophenyl-L-cycteine were 12
and 14 percent respectively (Betts et al., 1955).
TOXICOLOGICAL STUDIES
Special studies on reproduction
A three-generation reproduction study was conducted with rats
receiving technical quintozene in their diet in concentrations of 0,
5, 50 and 500 ppm. Groups of 20 females were used and 2 litters were
produced in each generation. No significant differences were found
between the control- and quintozene-treated rats with respect to
fertility, gestation, viability or lactation indices, or in
weaning-weights. Histopathologic examination of the tissues of 10 pups
of each sex from the F2b generation showed no effect from the
treatment (Borzelloca and Larson, 1968b).
Special studies on carcinogenicity
Groups of 18 mice of each sex from two hybrid strains of mice were
given quintozene (the specifications not given) from 7 days of age for
18 months. The dose of 464 mg/kg was given to the mice by stomach tube
from the seventh day of age to the time weaning at four weeks of age
and thereafter the chemical was added to the diet in a corresponding
amount of 1,206 ppm. This level was a maximum tolerated dose for the
mice. There was a significantly elevated incidence of tumours, mostly
hepatomas, in both strains of mice (Innes. et al., 1969).
Acute toxicity
LD50
Animal Route mg/kg body-weight References
Rat (M) oral 1710* Finnegan, et al., 1958
(oil solution)
Rat (F) oral 1650* Finnegan, et al., 1958
(oil solution)
Rat oral >30,000 Wit, et al., 1957
(aqueous suspension)
Rat i.p. 5,000 Wit et al., 1957
(aqueous suspension)
* technical grade; defined as: pentachloronitrobenzene ... 98.2 percent
hexachlorobenzene ... 1.4 percent
traces of tetrachloro-
nitrobenzene and pentachlorobenzene
Short-term studies
Dog
Groups, each of three mongrel dogs, of unspecified sex were placed on
diets containing 25, 200 or 1,000 ppm of technical quintozene for one
year. No adverse effect was noted on body-weight or survival. No
haematologic changes were seen. Histopathologic changes were confined
to liver-cell enlargement with pale-staining cytoplasm at all
dose-levels, but there was no increasing severity of the lesions with
increasing exposure to quintozene (Finnegan et al., 1958).
Groups of four dogs of each sex were placed on diets containing 0, 5,
30, 180, or 1,080 ppm of technical quintozene for two years. No
adverse effect was noted on body-weight or survival. Increased
liver-weights, elevated serum-alkaline phosphatase and a moderate
degree of cholestatic hepatosis with secondary bile nephrosis occurred
in the dogs on the 1,080 ppm level. A minimum degree of cholestatic
hepatosis with secondary bile necrosis occurred at 180 ppm. No effect
was noted in the dogs fed 5 and 30 ppm of quintozene (Borzelloca and
Larson, 1968a).
Groups of six dogs, each comprising three male and three female
animals, were fed diets containing 0, 500, 1,000 or 5,000 ppm of
quintozene (the specification not given) for two years. Liver changes
occurred in all groups with the degree of damage related to the dose.
The 5,000 ppm level produced severe liver damage including fibrosis,
narrowing of hepatic cell cords, increased size of the periportal
areas and thick leucocyte infiltration. At the 1,000 and 500 ppm
dose-levels the changes were similar to those at the 5,000 ppm level
but to a lesser degree. The highest dose-level produced atrophy of
bone-marrow and reduced haematopoiesis (Hoechst, 1968).
Rat
Groups of 35 rats of each sex were fed diets containing 0, 63.5, 635,
1,250, 2,500 or 5,000 ppm of technical quintozene for three months.
Growth and survival were adversely affected in both sexes at the
dose-level of 5,000 ppm and in males growth was suppressed at 2,500
ppm. Liver to body-weight ratios were elevated at all dietary levels
except in the females fed 63.5 ppm. No haematologic changes were seen,
and histopathologic changes were limited to fine vacuolization of
liver-cell cytoplasm at 5000 ppm (Finnegan et al., 1958).
An unspecified number of young rats were fed diets containing 0 or
2,000 ppm of quintozene for 10 weeks. No gross effects, other than a
decreased growth rate in the males, were noted (Wit, et al., 1957).
Groups of 20 rats each comprising 10 male and 10 female animals were
fed diets containing 0, 1,000, 5,000 or 10,000 ppm of quintozene (the
specification not given) for 90 days. The animals grew slightly less
than controls at the 5,000 ppm dose-level and markedly less at the
10,000 ppm level (Hoechst, 1964).
Long-term studies
Rat
Groups of 10 rats of each sex were fed dicta containing 0, 25, 100,
300, 1,000 or 2,500 ppm of technical quintozene for two years. Growth
suppression occurred in the females at dose-levels of 100 ppm and
above; however, in the males growth depression occurred only at 2,500
ppm level. Haematologic and histopathologic observations in the test
animals were similar to the control group (Finnegan, et al., 1958).
OBSERVATIONS IN MAN
Quintozene (75 percent wettable powder) did not cause primary
irritation when applied to the skin of 50 human subjects; in 13 of
them sensitization was produced (Finnegan et al., 1958).
COMMENT
The acute and short-term studies and the reproduction studies in rats
are considered adequate. A preliminary report of studies in mice
indicates a potential for carcinogenicity in animals given a high dose
and further work is needed in other species. In the two-year study in
dogs severe morphologic changes were observed in the liver and
bone-marrow in the high dose-level groups. Furthermore, the apparent
erratic effect on growth an indicated in the studies with rats is not
explained. Additional studies should be done to delineate the exact
cause of these effects. Insufficient information in available on the
biological fate of the compound. For these reasons only a temporary
acceptable daily intake is established based on the two-year study in
the rats.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 25 ppm in the diet, equivalent to 1.25 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Quintozene is a fungicide mainly used for soil treatment or for
treatment of seeds and transplants but some crop applications are
recommended. The following table gives a review of application rates
of quintozene and recommended pre-harvest intervals.
TABLE I
Pre-harvest
Crop Rate Limitations interval days
Bananas 1.63% paste Apply to stems only - not to fruit
Beans 5.0 kg/ha Apply to foliage 21
0.5 g/kg seed Seed treatment 70
50g/100m of row Spray base of plants 21
40g/100m of row Soil treatment only 70
Cruciferous
vegetables 60 kg/ha Pre-planting 70
470g/100m row Row application prior to transplanting 70
Corn 0.5g/kg seed Seed treatment 70
Garlic 150g/100m row Soil treatment at planting 90
Lettuce (head) 150g/100m row When plants 5-7.5 cm tall 30
30g/100m row 2 treatments at 10 day intervals 20
Onions 40 kg/ha Pre-planting
TABLE I (cont'd)
Pre-harvest
Crop Rate Limitations interval days
Peanuts 250g/100m row Pre-planting 90
200g/100m row At pegging time 60
Peas 1g/kg Seed treatment
Peppers 70g/100m row At planting time 70
Potatoes 22 kg/ha Prior to planting 70
140g/100m row At planting time 70
Safflower,
Sorghum,
Soybeans,
Sugarbeet 1.5 g/kg Seed treatment only 70
Tomatoes 160g/100m row Prior to transplanting 70
Wheat 0.5g/kg seed Seed treatment 150
Post-harvest treatments
No post-harvest treatments with quintozene are known.
Other uses
Quintozene is used for control of fungi in alfalfa, clover, cotton,
ornamentals, bulbs, lawns, mushrooms and coffee crops.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Detailed residue data are available from United States trials with
quintozene on important crops and have been deposited with FAO. Rates
of application are similar to those given below. The typical data
presented below are representative:
TABLE II
Post-
Number treatment Residues (ppm)
Rate of interval
Crop kg/ha treatments days Range Average
Beans 1 1 60 0.003-0.004 0.003
10 1 60 0.005-0.006 0.005
Beans 1 1 60 <0.01 <0.01
Beans 1.5 1 60 <0.01 <0.01
Beans (small
white dry) 1 1 150 <0.01 <0.01
Beans (string) 0.5 1 70 <0.01 <0.01
Beans (navy) 8 4 120 0.003-0.152 0.07
Beans (Lima) 1.5 1 90 <0.01
Broccoli 20 1 0.003-0.018 0.012
40 1 140 0.002-0.021 0.013
Cabbage 20 1 140 0.000-0.014 0.007
40 1 140 0.000-0.020 0.007
Cottonseed 0.3 1 70 0.000-0.017 <0.017
Cottonseed 5.0 1 150 <0.012 <0.012
Cottonseed 2.5 1 150 <0.012 <0.012
5.0 1 180 0.004-0.032 0.014
Lettuce heads 100 1 180 0.00 -0.01 0.00
Lettuce outer
leaves 100 1 180 0.02 -0.11 0.06
Lettuce heads 2 8 0.00 0.00
2 16 0.00 0.00
Lettuce 18G 1 60 0.03 -0.05 0.03
(Greenhouse) 18WP 1 60 0.20 -0.30 0.26
18WP 1 70 0.09 -0.139 0.104
18G 1 70 0.048-0.093 0.058
TABLE II
Post-
Number treatment Residues (ppm)
Rate of interval
Crop kg/ha treatments days Range Average
Mushrooms 1.5 1 1 9.57 -9.68 9.6
1.5 1 3 2.75 -2.97 2.8
1.5 1 7 1.30 -1.36 1.34
Peanuts,kernels 10 1 130 0.063-0.154 0.104
kernels 100 1 130 0.208-0.212 0.210
Peanut shells 100 1 130 4.60 -5.21 4.96
Peppers 50 1 75 0.0 -0.008 0.002
50 2 35 0.0 -0.009 0.003
Potatoes 10 1 130 0.01 -0.066 0.027
10 1 130 0.066-0.125 0.088
10 1 130 0.00 -0.002 0.001
20 1 130 0.001-0.005 0.002
Tomatoes 3 2 50 0.00 -0.08 0.02
10 1 70 0.00 -0.01 0.01
50 1 100 0.00 -0.02 0.01
FATE OF RESIDUES
In animals
No data was available to show the residues in animal tissues or animal
products from the feeding of forage grown in soil treated with
quintozene. In view of the stability of the compound, its solubility
in lipids and its resistance to metabolism it seems highly likely that
significant residues of quintozene or pentachlor - derivatives do
occur in animal fats, milk and eggs.
Quintozene was reported in trace amounts in dairy produce and in oils,
fats and shortening in the U.S. total diet studies.
A paper by Kuchar et al. (1969) not available for consideration at the
time the original data was reviewed reports analytical studies of
metabolism of quintozene in beagle dogs, rats and plants.
Tissues from beagle dogs fed food containing 1,080 ppm quintozene in
their rations for 2 years were examined by GLC methods.
Pentachloroaniline (PCA) was identified in blood. Fat, liver, urine
and faeces yielded pentachloronitrobenzene (quintozene);
pentachlorobenzene (PCB); hexachlorobenzene (HCB) and
pentachloroaniline (PCA) and methyl pentachlorophenyl sulphide.
Quintozene was not detected in muscle, kidney, fat or liver of dogs
receiving 1,080 ppm in their diet over 2 years (234 gms quintozene in
all). HCB was the most prominent residue yielding 194 ppm in fatty
tissue. PCB occurred in a significant amounts only in fat (5.15 ppm)
and PCA only in faeces. A small amount of the quintozene fed is
excreted in the faeces (14 ppm).
No metabolites, only HCB, were found in tissues of rats fed quintozene
for 7 months at 500 ppm.
In plants
There is evidence of a slight systemic uptake by plants revealed by
analysis by spectrophotometric methods following application of
quintozene to soil. The following table gives typical examples from
the extensive data available (Olin Mathieson, 1969).
TABLE III
Residues
Seeds or
Applied Soil Roots Leaves fruit
Crop kg/acre ppm ppm ppm ppm
Beans 1 1.2 0.59 0.034 0.003
1.5 0.68 1.12 0.017 0.013
1.5 0.59 2.27 N.D. 0.004
Snap beans 1.0 9.2 9.0 0.004 -
1.5 3.4 7.0 0.09 -
String beans 0.75 - 0.94 0.288 -
1.0 - 3.62 0.221 -
1.5 - 2.37 0.461 -
Field beans 1.5 7.77 19.33 0.12 -
Lettuce 4.0 - - 0.0 -
(field) 15.0 - - 0.02 -
50.0 - - 0.00 -
100.0 - - 0.00 -
100 - - 0.06 outer -
leaves
TABLE III
Residues
Seeds or
Applied Soil Roots Leaves fruit
Crop kg/acre ppm ppm ppm ppm
Lettuce 18 dust - - 0.05 -
(greenhouse)
0.4 Spray - - 0.03 -
18 Granule - - 0.02 -
Cabbage 2.0 - - 0.007 -
2.0 - - 0.008 -
Tomatoes 6.0 - - - 0.02
50.0 - - - 0.01
Potatoes 10.0 - - - 0.03
10.0 - - - 0.008
Alfalfa 10 - - 0.02 -
20 - - 0.05 -
Peanut 10 - - 0.235 -
1.0 - 0.44 0.003 -
Gorbach and Wagner (1967) showed, by using highly sensitive GLC
techniques capable of detecting both quintozene and PCA that potatoes
growing in soil treated with quintozene at rates from 25 to 800 kg/ha
showed significant residues of quintozene in the skin (up to 3 ppm at
800 kg/ha) but only insignificant traces of PCA (up to 0.4 ppm) in the
skin. The flesh of these potatoes contained no detectable residues of
quintozene (less than 0.01 ppm) and less than 0.1 ppm of PCA and
unidentified metabolites.
Gorbach (1969) reports that the most recent studies reveal no evidence
of translocation from soil into green parts of leafy plants. A
carefully controlled experiment, where all possible contamination by
splash or vapour was eliminated, revealed no uptake by parsley growing
in quintozene treated soil.
Kuchar et al. (1969) reports that the metabolic pathway in plants
appears to be the same mechanism as in animals. Studies using cotton
seed planted in soil containing 300 ppm quintozene yielded the
following residues in the young cotton plants two weeks later: (1)
pentachloranitrobenzene (quintozene) 155 ppm; (2) pentachlorobenzene
4 ppm; (3) methyl pentachlorophenyl sulphide 3 ppm; (4)
hexachlorobenzene 5 ppm; (5) pentachloroaniline 1.1 ppm; and (6) 2, 3,
4, 5 - tetrachloronitrobenzene 0.018 ppm. Residues (2), (4) and (6)
may have originated partly from impurities in the technical grade
quintozene (97.8% PCNB; 1.8% HCB; 0.1% PCB and 0.4% TCNB).
These authors were able to show that the two unidentified materials
reported by Gorbach and Wagner (1967) were in fact hexachlorobenzene
(HCB) and the metabolite methyl pentachlorphenyl sulphide.
In soils
Quintozene appears to persist for long periods in the soil as disease
control may be as long an 12 months (Hertzfield, 1967). Ko and Farley
(1969) show that in moist soil quintozene is gradually converted to
pentachloroaniline (PCA) and the conversion was greatly enhanced by
submergence of the soil in water. PCA was stable in both moist and
submerged soil and was inhibitory to micro-organisms but to a lesser
extent than quintozene. The long term action of quintozene is partly
due to its conversion to PCA.
Quintozene remains unchanged in sterilized, moist soil but disappears
from submerged sterilized soil with a half life of three weeks. No PCA
was detected in sterilized submerged soil. Studies by Ko and Farley
(1969) showed that soil micro-organisms are responsible for the
conversion of quintozene to PCA.
Evidence of residues in food in commerce or at consumption
The only data available on quintozene residues in food moving in
commerce was gathered in the U.S.A. The U.S.D.A./H.E.W. 1968 reports
that of 9,789 samples of leaf and stem vegetables produced in the
U.S.A. and examined for a wide range of pesticides, 89 samples (0.89%)
contained quintozene at levels ranging from trace quantities to
greater than 2.0 ppm. The following shows the range:
ppm %
Trace - 0.03 0.42
0.04 - 0.5 0.19
0.51 - 1.0 0.1
1.01 - 2.0 0.04
Above - 2.0 0.14
0.89%
Quintozene was reported to occur in trace amounts in the fat of dairy
produce and at a level of 0.021 ppm in a composite sample of oils,
fats and shortening in the total diet studies conducted in the U.S.A.
in 1963-68 (Duggan 1968, Corneliussen 1969). The total intake in the
diet was calculated to be no more than trace amounts (less than 0.001
mg).
METHODS OF RESIDUE ANALYSIS
A review of analytical methods is given in the book by Zweig (1964).
Klein and Gajan (1961) have carefully compared a colorimetric, a
polarographic and a gas chromatographic - colometric method for
residue analysis on lettuce, cabbage and beans. The colorimetric
method of Ackermann et al. (1958) is reported to be the most accurate
in the range below 5 ppm with a recovery of 94%. The latter method was
improved by Ackermann et al. (1963). This method is however the
slowest of the three methods and does not differentiate quintozene
from tetrachlorobenzene. The polarographic method (Bache and Lisk,
1960; Klein and Gajan, 1961 and Gorbach, 1961) is the most rapid
because of less stringent cleanup requirements. Gorbach (1961) used a
sublimation step in the cleanup before the polarographic determination
and thus eliminated many of the interfering substances.
With the polarographic method, recoveries averaged 81%. with a
standard deviation of 12%. The gas chromatographic-colometric method
(Klein and Gajan, 1961) yielded average recoveries of 90% with average
deviation of 15%.
For proper identification, extracts should be checked for quintozene
by paper chromatography (Mitchell, 1957, 1958) or by thin layer
chromatography (Gorbach, 1967). In the latter paper, methods are given
to separate and identify the metabolite pentachloroaniline.
All three methods can be recommended and can be selected according to
equipment available.
(a) The colorimetric method (Ackermann et al., 1963). The quintozene
residue in fat-free extract is hydrolized to nitrite with alcoholic
potassium hydroxide, the nitrite is used to diazotize procaine
hydrochloride and the diazonium salt coupled with l-naphthylamine to
give a magenta solution having absorption maximum at 525 mu.
(b) Polarographic method (Bache and Lisk, 1960; Klein and Gajan,
1961; Gorbach, 1961). The crop material is extracted with hexane. The
extract is filtered and dried and a part of the co-extractives are
removed by freezing and adsorption on Attaclay.
Chromatography of the concentrated extract using Florisil removes the
remainder of the interfering substances. The solvent is then
evaporated and the residue dissolved in isopropyl alcohol. After
adding sodium acetate and acetic acid for supporting electrolyte and
deoxygenating the solution, the polarogram in recorded from 0.00 to
1.15 volts against saturated calomel electrode. The half-wave
potential for quintozene in -0.47 volts.
The later papers recommend a number of modifications to the
polarographic procedure.
(c) Gas Chromatographic - Microcolometric Method (Klein and Gajan,
1961; Gorbach, 1961). The extraction is carried out as described for
the polarographic method. The concentrated extract is evaporated to
dryness and the residue dissolved in hexane is injected into the
instrument.
The multi-detection procedure for determining chlorinated residues in
non-fatty foods based on JAOAC, 49 222 (1966) paragraph 24.213 which
in official for a number of pesticides is satisfactory for the
determination of quintozene residues.
A method using electron capture gas chromatography (Methratta T.P. et
al., 1967) suitable for determining quintozene residues in plants,
seeds and soil has a sensitivity of 0.01 ppm and is relatively free of
interference from plant extracts. The method is probably suitable for
development towards greater sensitivity.
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
Germany (Fed.Rep.) Cabbage, lettuce 1.0
onions, cucumber 1.0
horse radish 1.0
leaf vegetables 3.0
bananas (peeled) 0.1
Netherlands Fruit and vegetables 5.0
United States of America Bananas, beans, broccoli, Originally on "no
brussels sprouts, cabbage, residue basis". At
cauliflower, cotton, present under review.
garlic, lettuce, peanuts,
peas, peppers, potatoes,
tomatoes, wheat.
APPRAISAL
Quintozene or pentachloronitrobenzene (PCNB) is a versatile fungicide
used chiefly against soil fungi in agriculture and horticulture. It
was first developed in 1930 as a seed dressing for wheat. It is used
in many countries as a soil fungicide against Sclerotinia,
Rhizoctonia, Botrytis, Sclerotium, Fusrium and similar fungi of
vegetable and forage crops. Application by means of wettable powder,
emulsion concentrate or dust ranges from 10 to 55 kg/ha or 15 to 600g
per 100m of row. Seed dressings applied in the form of dust range from
30g to 300 g per 100 kg of seed. Treatments other than those applied
to the soil are usually aimed at the base of the plant but some
foliage applications are made, particularly to lettuce, beans and
mushrooms. Pre-harvest intervals are four to eight weeks.
The data available to the meeting were obtained solely in the United
States of America and did not include information about residues
following use elsewhere. Some information is available about residues
in foods in commerce.
Quintozene has high stability in soil and under neutral conditions
remains stable for exceptionally long periods.
The literature includes a number of methods of residue analysis based
on electron-capture gas chromatography and spectrophotometric methods.
The sensitivity of the methods is reported to be 0.01 ppm but the
spectrophotometric method does not determine the metabolite
pentachloroaniline (PCA). Quintozene and PCA may be determined by
multi residue methods for chlorinated compounds but no regulatory
methods have been evaluated, and there is reason to believe that the
recovery from many food commodities may be low unless special
provision is made for extraction and cleanup. Further work on the
development of an acceptable regulatory method is required.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to 1973)
Residues of the metabolite pentachloroaniline to be included.
Bananas (pulp) 0.01 ppm
(whole) 1.0
Beans 0.01
Beans (navy) 0.2
Broccoli 0.02
Cabbage 0.02
Cottonseed 0.03
Lettuce 0.3
(cont'd)
Mushrooms 10.0
Peanuts (kernels) 0.3
(whole) 5.0
Peppers 0.01
Potatoes 0.2
Tomatoes 0.1
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Carcinogenicity studies in two species of animals.
2. Studies to explain the cause of growth depression in rats and the
effect on liver and bone-marrow in dogs.
3. Further studies on the metabolism and on the metabolites,
particularly pentachloroaniline.
4. Data from countries other than the United States of America on the
required rates and frequencies of application, pre-harvest
intervals and the resulting residues.
5. Information on residues in edible animal tissues and in animal
products resulting from the feeding of plant products (including
forage) treated with quintozene in accordance with normal
agricultural practice.
6. Information on the frequency and level of quintozene residues in
food commodities in commerce.
7. Information on the level of metabolites, particularly
pentachloroaniline in plants and animals.
DESIRABLE
1. Development of analytical methods for greater sensitivity and
evaluation for regulatory purposes.
2. Information on the residue levels in root crops, especially
carrots, grown in soil treated previously with quintozene in crop
rotation.
REFERENCES
Ackermann, H.J. et al. (1958) Spectrophotometric determination of
pentachloronitrobenzene on food and forage Crops. J. Agric. and
Food Chem. 6:747-50 (Oct.)
Ackermann, H.J. et al. (1963) Modifications to the spectrophotometric
analysis of PCNB in Soil and Crops. J. Agric. and Food Chem.
11(4) 297 (July/Aug. 1963)
Bache, C.A. and Lisk, D.I. (1960) Journal of Agriculture and Food
Chemistry 8, 459
Betts, J.J., James, Sybil P. and Thorpe, W.V. (1955) The metabolism of
pentachloronitrobenzene and tetrachloronitrobenzene and the
formation of mercapturic acid in the rabbit Biochem. J. 61 (4)
611-7
Borzelloca, J.F. and Larson, P.S. (1968a) Toxicologic study on the
effect of adding Terrachlor to the diet of beagle dogs for a period of
two years. Unpub. Rept. from the Medical College of Virginia submitted
by Olin Mathieson Chemical Corporation
Borzelloca, J.F. and Larson, P.S. (1968b) Three generation
reproduction study on rats receiving Terrachlor in their diet. Unpub.
Rept. from the Medical College of Virginia submitted by Olin Mathieson
Chemical Corporation
Corneliussen, P.E. (1969) Residues in food and feed - pesticide
residues in total diet samples (IV). Pesticides Monitoring Journal 2
(4) 140-52
Duggan, R.E. (1968) Residues in food and feed 1963-67. Pesticides
Monitoring Journal 2 (1) 2-46
Gorbach, S. (1961) Acta Chemica (Academiae Scientarum Hungaricae) 28
(1-3), 199-206
Gorbach, S. and Wagner, U. (1967) Pentachloronitrobenzene residues in
potatoes. J. Agric. and Food Chem. 15 (4) 654
Gorbach, S. (1969) Personal Communication to FAO
Hertzfield, E.G. (1967) Terrachlor - a new Soil Fungicide Agricultural
Chemicals 12 30-3
Hoechst. (1964) Toxikologische Prüfung von Pentachlornitrobenzol. 3.
Auf chronische Toxizität (90-Tage-Test) an Ratten. Unpub. Rept.
prepared and submitted by Farbwerke Hoechst, AG.
Hoechst. (1968) Chronische orale Toxizatätsprüfung von
Pentachlornitrobenzol. 2-Jahres-Versuch an Hunden. Unpub. Rept.
prepared and submitted by Farbwerke Hoechst AG.
Finnegan, J.K., Larson, P.S., Smith, R.B. Jr., Haag, H.B. and
Hennigar, G.R. (1958) Acute and chronic toxicity studies on
pentachloronitrobenzene. Arch. int. Pharmacodyn. 114:38-52
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L., Gart,
J.J., Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in
mice. A preliminary note. J. nat. Cancer Inst. 42:1101-14
Klein, A.K. and Gajan, R.J. (1961) Methods for the determination of
PCNB Residues. JAOAC 44:712
Ko, W.H. and Farley, J.D. (1969) Conversion of
pentachloronitrobenzene to pentachloroaniline in soil and the effect
of these compounds on soil Micro-organisms. Phytopathology 59 (1)
64-7
Kuchar, E.J., Geenty, Frances, O, Griffith, William P. and Thomas,
Raymond J. (1969) Analytical studies of metabolism of Terraclor in
beagle dogs, rats and plants, J. Agric. and Food Chem. 17 (6)
1237-40
Methratta, T.P., Montagna, R.W. and Griffith, W.P. (1967)
Determination of PCNB in crops and soil by electron-capture gas
chromatography. J. Agric. and Food Chem. 15 (4) 648 (July/Aug. 1967)
Mitchell, L.C. (1957) J. Assoc. Offic. Agric. Chemists 40, 294
Mitchell, L.C. (1958) J. Assoc. Offic. Agric, Chemists 41, 781
Olin Mathieson Chemical Corporation. (1969) Research reports on
quintozene
United States of America. (1969) Department of Agriculture Summary of
Registered Agricultural Pesticide Chemical Uses
United States of America. (1968) Department of Health, Education and
Welfare FDA Pesticide Analytical Manual Volume 1 Section 212.1
Wit, S.L., van Esch, G.J. and van Genderen, H. (1957) Toxicity of
some chloronitrobenzen compounds (trichlorodinitrobenzene,
trichloronitrobenzene, tetrachloronitrobenzene and
pentachloronitrobenzene) to laboratory rats and residues
found in crops treated with these fungicides. Proceedings of
the Fourth International Congress of Crop Protection,
Hamburg, September 1957. Vol. 2 (Braunschweig 1960): pp.
1665-8
Zweig, Gunter. (1964) Analytical Methods for Pesticides and Plant
Growth Regulators and Food Additives Vol. III Academic Press. New
York
Other relevant chemical properties
Colourless crystalline needles practically insoluble in water, soluble
in benzene and chloroform. Technical quintozene is usually 97-99
percent pure. The main impurity, is hexachlorobenzene (1.5%) together
with lesser amounts of pentachlorobenzene and tetrachloronitrobenzene.
Vp 11.3 × 10-5mm Hg at 25°C. Quintozene shows high stability in the
soil. Quintozene is converted to pentachloroaniline (PCA) in moist
soil, the metabolite having somewhat lower fungicidal activity. In
animals the metabolites are pentachloroaniline and mercapturic acid
(Betts et al., 1955).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Fat samples from groups of ten rats (five males and five females) fed
diets containing 63.5, 635, 1250, or 2500 ppm of technical quintozene
for three months were examined for storage of quintozene. The apparent
storage ranged from an average of 43 ppm in the fat for the 63.5 ppm
diet to 1234 ppm for the 2500 ppm diet, there being a relatively
linear relationship between the levels of fat storage to the
concentration of quintozene in the diet. However, the neutron
activation method of analysis which was used would have shown the
presence of other chlorinated compounds as well an quintozene
(Finnegan et al., 1958).
Tissues from an unspecified number of dogs fed 5 or 1,080 ppm of
technical quintozene in their diet for 2 years were analysed by gas
chromatography for residues of quintozene and its metabolites. No
quintozene was found in fat, muscle, kidney, or liver tissues but two
metabolic products identified as pentachloroaniline and
methyl-pentachlorophenyl sulfide were found in these tissues.
Pentachloroaniline was found only in the fat and liver and in amounts
of less than 1 ppm for both dose-levels. Methyl-pentachlorophenyl
sulfide was found in the fat and liver of rats fed both dose levels
and was present as well in kidney and muscle in the 1080 ppm group,
the largest amount being 2.5 ppm in the fat of animals fed that level.
In another study on fat from an unspecified number of rats fed 50 or
500 ppm of technical quintozene in their diet for seven months, there
was less than 1 ppm of either of the metabolites in the fat of the
rats fed 50 ppm of quintozene, and approximately 1 ppm of
pentachloroaniline and 5 ppm of methyl-pentachlorophenyl sulfide for
the 500 ppm group (Kuchar et al., 1969).
Pentachloroaniline and another metabolite, a mercapturic acid, have
been isolated in urine from rabbits treated with quintozene. With a 2
g. dose an average of 62 percent of quintozene was unabsorbed and
excreted in the faeces. The average percentages excreted in urine as
pentachloroaniline and N-acetyl-S-pentachlorophenyl-L-cycteine were 12
and 14 percent respectively (Betts et al., 1955).
TOXICOLOGICAL STUDIES
Special studies on reproduction
A three-generation reproduction study was conducted with rats
receiving technical quintozene in their diet in concentrations of 0,
5, 50 and 500 ppm. Groups of 20 females were used and 2 litters were
produced in each generation. No significant differences were found
between the control- and quintozene-treated rats with respect to
fertility, gestation, viability or lactation indices, or in
weaning-weights. Histopathologic examination of the tissues of 10 pups
of each sex from the F2b generation showed no effect from the
treatment (Borzelloca and Larson, 1968b).
Special studies on carcinogenicity
Groups of 18 mice of each sex from two hybrid strains of mice were
given quintozene (the specifications not given) from 7 days of age for
18 months. The dose of 464 mg/kg was given to the mice by stomach tube
from the seventh day of age to the time weaning at four weeks of age
and thereafter the chemical was added to the diet in a corresponding
amount of 1,206 ppm. This level was a maximum tolerated dose for the
mice. There was a significantly elevated incidence of tumours, mostly
hepatomas, in both strains of mice (Innes. et al., 1969).
Acute toxicity
LD50
Animal Route mg/kg body-weight References
Rat (M) oral 1710* Finnegan, et al., 1958
(oil solution)
Rat (F) oral 1650* Finnegan, et al., 1958
(oil solution)
Rat oral >30,000 Wit, et al., 1957
(aqueous suspension)
Rat i.p. 5,000 Wit et al., 1957
(aqueous suspension)
* technical grade; defined as: pentachloronitrobenzene ... 98.2 percent
hexachlorobenzene ... 1.4 percent
traces of tetrachloro-
nitrobenzene and pentachlorobenzene
Short-term studies
Dog
Groups, each of three mongrel dogs, of unspecified sex were placed on
diets containing 25, 200 or 1,000 ppm of technical quintozene for one
year. No adverse effect was noted on body-weight or survival. No
haematologic changes were seen. Histopathologic changes were confined
to liver-cell enlargement with pale-staining cytoplasm at all
dose-levels, but there was no increasing severity of the lesions with
increasing exposure to quintozene (Finnegan et al., 1958).
Groups of four dogs of each sex were placed on diets containing 0, 5,
30, 180, or 1,080 ppm of technical quintozene for two years. No
adverse effect was noted on body-weight or survival. Increased
liver-weights, elevated serum-alkaline phosphatase and a moderate
degree of cholestatic hepatosis with secondary bile nephrosis occurred
in the dogs on the 1,080 ppm level. A minimum degree of cholestatic
hepatosis with secondary bile necrosis occurred at 180 ppm. No effect
was noted in the dogs fed 5 and 30 ppm of quintozene (Borzelloca and
Larson, 1968a).
Groups of six dogs, each comprising three male and three female
animals, were fed diets containing 0, 500, 1,000 or 5,000 ppm of
quintozene (the specification not given) for two years. Liver changes
occurred in all groups with the degree of damage related to the dose.
The 5,000 ppm level produced severe liver damage including fibrosis,
narrowing of hepatic cell cords, increased size of the periportal
areas and thick leucocyte infiltration. At the 1,000 and 500 ppm
dose-levels the changes were similar to those at the 5,000 ppm level
but to a lesser degree. The highest dose-level produced atrophy of
bone-marrow and reduced haematopoiesis (Hoechst, 1968).
Rat
Groups of 35 rats of each sex were fed diets containing 0, 63.5, 635,
1,250, 2,500 or 5,000 ppm of technical quintozene for three months.
Growth and survival were adversely affected in both sexes at the
dose-level of 5,000 ppm and in males growth was suppressed at 2,500
ppm. Liver to body-weight ratios were elevated at all dietary levels
except in the females fed 63.5 ppm. No haematologic changes were seen,
and histopathologic changes were limited to fine vacuolization of
liver-cell cytoplasm at 5000 ppm (Finnegan et al., 1958).
An unspecified number of young rats were fed diets containing 0 or
2,000 ppm of quintozene for 10 weeks. No gross effects, other than a
decreased growth rate in the males, were noted (Wit, et al., 1957).
Groups of 20 rats each comprising 10 male and 10 female animals were
fed diets containing 0, 1,000, 5,000 or 10,000 ppm of quintozene (the
specification not given) for 90 days. The animals grew slightly less
than controls at the 5,000 ppm dose-level and markedly less at the
10,000 ppm level (Hoechst, 1964).
Long-term studies
Rat
Groups of 10 rats of each sex were fed dicta containing 0, 25, 100,
300, 1,000 or 2,500 ppm of technical quintozene for two years. Growth
suppression occurred in the females at dose-levels of 100 ppm and
above; however, in the males growth depression occurred only at 2,500
ppm level. Haematologic and histopathologic observations in the test
animals were similar to the control group (Finnegan, et al., 1958).
OBSERVATIONS IN MAN
Quintozene (75 percent wettable powder) did not cause primary
irritation when applied to the skin of 50 human subjects; in 13 of
them sensitization was produced (Finnegan et al., 1958).
COMMENT
The acute and short-term studies and the reproduction studies in rats
are considered adequate. A preliminary report of studies in mice
indicates a potential for carcinogenicity in animals given a high dose
and further work is needed in other species. In the two-year study in
dogs severe morphologic changes were observed in the liver and
bone-marrow in the high dose-level groups. Furthermore, the apparent
erratic effect on growth an indicated in the studies with rats is not
explained. Additional studies should be done to delineate the exact
cause of these effects. Insufficient information in available on the
biological fate of the compound. For these reasons only a temporary
acceptable daily intake is established based on the two-year study in
the rats.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 25 ppm in the diet, equivalent to 1.25 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Quintozene is a fungicide mainly used for soil treatment or for
treatment of seeds and transplants but some crop applications are
recommended. The following table gives a review of application rates
of quintozene and recommended pre-harvest intervals.
TABLE I
Pre-harvest
Crop Rate Limitations interval days
Bananas 1.63% paste Apply to stems only - not to fruit
Beans 5.0 kg/ha Apply to foliage 21
0.5 g/kg seed Seed treatment 70
50g/100m of row Spray base of plants 21
40g/100m of row Soil treatment only 70
Cruciferous
vegetables 60 kg/ha Pre-planting 70
470g/100m row Row application prior to transplanting 70
Corn 0.5g/kg seed Seed treatment 70
Garlic 150g/100m row Soil treatment at planting 90
Lettuce (head) 150g/100m row When plants 5-7.5 cm tall 30
30g/100m row 2 treatments at 10 day intervals 20
Onions 40 kg/ha Pre-planting
TABLE I (cont'd)
Pre-harvest
Crop Rate Limitations interval days
Peanuts 250g/100m row Pre-planting 90
200g/100m row At pegging time 60
Peas 1g/kg Seed treatment
Peppers 70g/100m row At planting time 70
Potatoes 22 kg/ha Prior to planting 70
140g/100m row At planting time 70
Safflower,
Sorghum,
Soybeans,
Sugarbeet 1.5 g/kg Seed treatment only 70
Tomatoes 160g/100m row Prior to transplanting 70
Wheat 0.5g/kg seed Seed treatment 150
Post-harvest treatments
No post-harvest treatments with quintozene are known.
Other uses
Quintozene is used for control of fungi in alfalfa, clover, cotton,
ornamentals, bulbs, lawns, mushrooms and coffee crops.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Detailed residue data are available from United States trials with
quintozene on important crops and have been deposited with FAO. Rates
of application are similar to those given below. The typical data
presented below are representative:
TABLE II
Post-
Number treatment Residues (ppm)
Rate of interval
Crop kg/ha treatments days Range Average
Beans 1 1 60 0.003-0.004 0.003
10 1 60 0.005-0.006 0.005
Beans 1 1 60 <0.01 <0.01
Beans 1.5 1 60 <0.01 <0.01
Beans (small
white dry) 1 1 150 <0.01 <0.01
Beans (string) 0.5 1 70 <0.01 <0.01
Beans (navy) 8 4 120 0.003-0.152 0.07
Beans (Lima) 1.5 1 90 <0.01
Broccoli 20 1 0.003-0.018 0.012
40 1 140 0.002-0.021 0.013
Cabbage 20 1 140 0.000-0.014 0.007
40 1 140 0.000-0.020 0.007
Cottonseed 0.3 1 70 0.000-0.017 <0.017
Cottonseed 5.0 1 150 <0.012 <0.012
Cottonseed 2.5 1 150 <0.012 <0.012
5.0 1 180 0.004-0.032 0.014
Lettuce heads 100 1 180 0.00 -0.01 0.00
Lettuce outer
leaves 100 1 180 0.02 -0.11 0.06
Lettuce heads 2 8 0.00 0.00
2 16 0.00 0.00
Lettuce 18G 1 60 0.03 -0.05 0.03
(Greenhouse) 18WP 1 60 0.20 -0.30 0.26
18WP 1 70 0.09 -0.139 0.104
18G 1 70 0.048-0.093 0.058
TABLE II
Post-
Number treatment Residues (ppm)
Rate of interval
Crop kg/ha treatments days Range Average
Mushrooms 1.5 1 1 9.57 -9.68 9.6
1.5 1 3 2.75 -2.97 2.8
1.5 1 7 1.30 -1.36 1.34
Peanuts,kernels 10 1 130 0.063-0.154 0.104
kernels 100 1 130 0.208-0.212 0.210
Peanut shells 100 1 130 4.60 -5.21 4.96
Peppers 50 1 75 0.0 -0.008 0.002
50 2 35 0.0 -0.009 0.003
Potatoes 10 1 130 0.01 -0.066 0.027
10 1 130 0.066-0.125 0.088
10 1 130 0.00 -0.002 0.001
20 1 130 0.001-0.005 0.002
Tomatoes 3 2 50 0.00 -0.08 0.02
10 1 70 0.00 -0.01 0.01
50 1 100 0.00 -0.02 0.01
FATE OF RESIDUES
In animals
No data was available to show the residues in animal tissues or animal
products from the feeding of forage grown in soil treated with
quintozene. In view of the stability of the compound, its solubility
in lipids and its resistance to metabolism it seems highly likely that
significant residues of quintozene or pentachlor - derivatives do
occur in animal fats, milk and eggs.
Quintozene was reported in trace amounts in dairy produce and in oils,
fats and shortening in the U.S. total diet studies.
A paper by Kuchar et al. (1969) not available for consideration at the
time the original data was reviewed reports analytical studies of
metabolism of quintozene in beagle dogs, rats and plants.
Tissues from beagle dogs fed food containing 1,080 ppm quintozene in
their rations for 2 years were examined by GLC methods.
Pentachloroaniline (PCA) was identified in blood. Fat, liver, urine
and faeces yielded pentachloronitrobenzene (quintozene);
pentachlorobenzene (PCB); hexachlorobenzene (HCB) and
pentachloroaniline (PCA) and methyl pentachlorophenyl sulphide.
Quintozene was not detected in muscle, kidney, fat or liver of dogs
receiving 1,080 ppm in their diet over 2 years (234 gms quintozene in
all). HCB was the most prominent residue yielding 194 ppm in fatty
tissue. PCB occurred in a significant amounts only in fat (5.15 ppm)
and PCA only in faeces. A small amount of the quintozene fed is
excreted in the faeces (14 ppm).
No metabolites, only HCB, were found in tissues of rats fed quintozene
for 7 months at 500 ppm.
In plants
There is evidence of a slight systemic uptake by plants revealed by
analysis by spectrophotometric methods following application of
quintozene to soil. The following table gives typical examples from
the extensive data available (Olin Mathieson, 1969).
TABLE III
Residues
Seeds or
Applied Soil Roots Leaves fruit
Crop kg/acre ppm ppm ppm ppm
Beans 1 1.2 0.59 0.034 0.003
1.5 0.68 1.12 0.017 0.013
1.5 0.59 2.27 N.D. 0.004
Snap beans 1.0 9.2 9.0 0.004 -
1.5 3.4 7.0 0.09 -
String beans 0.75 - 0.94 0.288 -
1.0 - 3.62 0.221 -
1.5 - 2.37 0.461 -
Field beans 1.5 7.77 19.33 0.12 -
Lettuce 4.0 - - 0.0 -
(field) 15.0 - - 0.02 -
50.0 - - 0.00 -
100.0 - - 0.00 -
100 - - 0.06 outer -
leaves
TABLE III
Residues
Seeds or
Applied Soil Roots Leaves fruit
Crop kg/acre ppm ppm ppm ppm
Lettuce 18 dust - - 0.05 -
(greenhouse)
0.4 Spray - - 0.03 -
18 Granule - - 0.02 -
Cabbage 2.0 - - 0.007 -
2.0 - - 0.008 -
Tomatoes 6.0 - - - 0.02
50.0 - - - 0.01
Potatoes 10.0 - - - 0.03
10.0 - - - 0.008
Alfalfa 10 - - 0.02 -
20 - - 0.05 -
Peanut 10 - - 0.235 -
1.0 - 0.44 0.003 -
Gorbach and Wagner (1967) showed, by using highly sensitive GLC
techniques capable of detecting both quintozene and PCA that potatoes
growing in soil treated with quintozene at rates from 25 to 800 kg/ha
showed significant residues of quintozene in the skin (up to 3 ppm at
800 kg/ha) but only insignificant traces of PCA (up to 0.4 ppm) in the
skin. The flesh of these potatoes contained no detectable residues of
quintozene (less than 0.01 ppm) and less than 0.1 ppm of PCA and
unidentified metabolites.
Gorbach (1969) reports that the most recent studies reveal no evidence
of translocation from soil into green parts of leafy plants. A
carefully controlled experiment, where all possible contamination by
splash or vapour was eliminated, revealed no uptake by parsley growing
in quintozene treated soil.
Kuchar et al. (1969) reports that the metabolic pathway in plants
appears to be the same mechanism as in animals. Studies using cotton
seed planted in soil containing 300 ppm quintozene yielded the
following residues in the young cotton plants two weeks later: (1)
pentachloranitrobenzene (quintozene) 155 ppm; (2) pentachlorobenzene
4 ppm; (3) methyl pentachlorophenyl sulphide 3 ppm; (4)
hexachlorobenzene 5 ppm; (5) pentachloroaniline 1.1 ppm; and (6) 2, 3,
4, 5 - tetrachloronitrobenzene 0.018 ppm. Residues (2), (4) and (6)
may have originated partly from impurities in the technical grade
quintozene (97.8% PCNB; 1.8% HCB; 0.1% PCB and 0.4% TCNB).
These authors were able to show that the two unidentified materials
reported by Gorbach and Wagner (1967) were in fact hexachlorobenzene
(HCB) and the metabolite methyl pentachlorphenyl sulphide.
In soils
Quintozene appears to persist for long periods in the soil as disease
control may be as long an 12 months (Hertzfield, 1967). Ko and Farley
(1969) show that in moist soil quintozene is gradually converted to
pentachloroaniline (PCA) and the conversion was greatly enhanced by
submergence of the soil in water. PCA was stable in both moist and
submerged soil and was inhibitory to micro-organisms but to a lesser
extent than quintozene. The long term action of quintozene is partly
due to its conversion to PCA.
Quintozene remains unchanged in sterilized, moist soil but disappears
from submerged sterilized soil with a half life of three weeks. No PCA
was detected in sterilized submerged soil. Studies by Ko and Farley
(1969) showed that soil micro-organisms are responsible for the
conversion of quintozene to PCA.
Evidence of residues in food in commerce or at consumption
The only data available on quintozene residues in food moving in
commerce was gathered in the U.S.A. The U.S.D.A./H.E.W. 1968 reports
that of 9,789 samples of leaf and stem vegetables produced in the
U.S.A. and examined for a wide range of pesticides, 89 samples (0.89%)
contained quintozene at levels ranging from trace quantities to
greater than 2.0 ppm. The following shows the range:
ppm %
Trace - 0.03 0.42
0.04 - 0.5 0.19
0.51 - 1.0 0.1
1.01 - 2.0 0.04
Above - 2.0 0.14
0.89%
Quintozene was reported to occur in trace amounts in the fat of dairy
produce and at a level of 0.021 ppm in a composite sample of oils,
fats and shortening in the total diet studies conducted in the U.S.A.
in 1963-68 (Duggan 1968, Corneliussen 1969). The total intake in the
diet was calculated to be no more than trace amounts (less than 0.001
mg).
METHODS OF RESIDUE ANALYSIS
A review of analytical methods is given in the book by Zweig (1964).
Klein and Gajan (1961) have carefully compared a colorimetric, a
polarographic and a gas chromatographic - colometric method for
residue analysis on lettuce, cabbage and beans. The colorimetric
method of Ackermann et al. (1958) is reported to be the most accurate
in the range below 5 ppm with a recovery of 94%. The latter method was
improved by Ackermann et al. (1963). This method is however the
slowest of the three methods and does not differentiate quintozene
from tetrachlorobenzene. The polarographic method (Bache and Lisk,
1960; Klein and Gajan, 1961 and Gorbach, 1961) is the most rapid
because of less stringent cleanup requirements. Gorbach (1961) used a
sublimation step in the cleanup before the polarographic determination
and thus eliminated many of the interfering substances.
With the polarographic method, recoveries averaged 81%. with a
standard deviation of 12%. The gas chromatographic-colometric method
(Klein and Gajan, 1961) yielded average recoveries of 90% with average
deviation of 15%.
For proper identification, extracts should be checked for quintozene
by paper chromatography (Mitchell, 1957, 1958) or by thin layer
chromatography (Gorbach, 1967). In the latter paper, methods are given
to separate and identify the metabolite pentachloroaniline.
All three methods can be recommended and can be selected according to
equipment available.
(a) The colorimetric method (Ackermann et al., 1963). The quintozene
residue in fat-free extract is hydrolized to nitrite with alcoholic
potassium hydroxide, the nitrite is used to diazotize procaine
hydrochloride and the diazonium salt coupled with l-naphthylamine to
give a magenta solution having absorption maximum at 525 mu.
(b) Polarographic method (Bache and Lisk, 1960; Klein and Gajan,
1961; Gorbach, 1961). The crop material is extracted with hexane. The
extract is filtered and dried and a part of the co-extractives are
removed by freezing and adsorption on Attaclay.
Chromatography of the concentrated extract using Florisil removes the
remainder of the interfering substances. The solvent is then
evaporated and the residue dissolved in isopropyl alcohol. After
adding sodium acetate and acetic acid for supporting electrolyte and
deoxygenating the solution, the polarogram in recorded from 0.00 to
1.15 volts against saturated calomel electrode. The half-wave
potential for quintozene in -0.47 volts.
The later papers recommend a number of modifications to the
polarographic procedure.
(c) Gas Chromatographic - Microcolometric Method (Klein and Gajan,
1961; Gorbach, 1961). The extraction is carried out as described for
the polarographic method. The concentrated extract is evaporated to
dryness and the residue dissolved in hexane is injected into the
instrument.
The multi-detection procedure for determining chlorinated residues in
non-fatty foods based on JAOAC, 49 222 (1966) paragraph 24.213 which
in official for a number of pesticides is satisfactory for the
determination of quintozene residues.
A method using electron capture gas chromatography (Methratta T.P. et
al., 1967) suitable for determining quintozene residues in plants,
seeds and soil has a sensitivity of 0.01 ppm and is relatively free of
interference from plant extracts. The method is probably suitable for
development towards greater sensitivity.
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
Germany (Fed.Rep.) Cabbage, lettuce 1.0
onions, cucumber 1.0
horse radish 1.0
leaf vegetables 3.0
bananas (peeled) 0.1
Netherlands Fruit and vegetables 5.0
United States of America Bananas, beans, broccoli, Originally on "no
brussels sprouts, cabbage, residue basis". At
cauliflower, cotton, present under review.
garlic, lettuce, peanuts,
peas, peppers, potatoes,
tomatoes, wheat.
APPRAISAL
Quintozene or pentachloronitrobenzene (PCNB) is a versatile fungicide
used chiefly against soil fungi in agriculture and horticulture. It
was first developed in 1930 as a seed dressing for wheat. It is used
in many countries as a soil fungicide against Sclerotinia,
Rhizoctonia, Botrytis, Sclerotium, Fusrium and similar fungi of
vegetable and forage crops. Application by means of wettable powder,
emulsion concentrate or dust ranges from 10 to 55 kg/ha or 15 to 600g
per 100m of row. Seed dressings applied in the form of dust range from
30g to 300 g per 100 kg of seed. Treatments other than those applied
to the soil are usually aimed at the base of the plant but some
foliage applications are made, particularly to lettuce, beans and
mushrooms. Pre-harvest intervals are four to eight weeks.
The data available to the meeting were obtained solely in the United
States of America and did not include information about residues
following use elsewhere. Some information is available about residues
in foods in commerce.
Quintozene has high stability in soil and under neutral conditions
remains stable for exceptionally long periods.
The literature includes a number of methods of residue analysis based
on electron-capture gas chromatography and spectrophotometric methods.
The sensitivity of the methods is reported to be 0.01 ppm but the
spectrophotometric method does not determine the metabolite
pentachloroaniline (PCA). Quintozene and PCA may be determined by
multi residue methods for chlorinated compounds but no regulatory
methods have been evaluated, and there is reason to believe that the
recovery from many food commodities may be low unless special
provision is made for extraction and cleanup. Further work on the
development of an acceptable regulatory method is required.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to 1973)
Residues of the metabolite pentachloroaniline to be included.
Bananas (pulp) 0.01 ppm
(whole) 1.0
Beans 0.01
Beans (navy) 0.2
Broccoli 0.02
Cabbage 0.02
Cottonseed 0.03
Lettuce 0.3
(cont'd)
Mushrooms 10.0
Peanuts (kernels) 0.3
(whole) 5.0
Peppers 0.01
Potatoes 0.2
Tomatoes 0.1
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Carcinogenicity studies in two species of animals.
2. Studies to explain the cause of growth depression in rats and the
effect on liver and bone-marrow in dogs.
3. Further studies on the metabolism and on the metabolites,
particularly pentachloroaniline.
4. Data from countries other than the United States of America on the
required rates and frequencies of application, pre-harvest
intervals and the resulting residues.
5. Information on residues in edible animal tissues and in animal
products resulting from the feeding of plant products (including
forage) treated with quintozene in accordance with normal
agricultural practice.
6. Information on the frequency and level of quintozene residues in
food commodities in commerce.
7. Information on the level of metabolites, particularly
pentachloroaniline in plants and animals.
DESIRABLE
1. Development of analytical methods for greater sensitivity and
evaluation for regulatory purposes.
2. Information on the residue levels in root crops, especially
carrots, grown in soil treated previously with quintozene in crop
rotation.
REFERENCES
Ackermann, H.J. et al. (1958) Spectrophotometric determination of
pentachloronitrobenzene on food and forage Crops. J. Agric. and
Food Chem. 6:747-50 (Oct.)
Ackermann, H.J. et al. (1963) Modifications to the spectrophotometric
analysis of PCNB in Soil and Crops. J. Agric. and Food Chem.
11(4) 297 (July/Aug. 1963)
Bache, C.A. and Lisk, D.I. (1960) Journal of Agriculture and Food
Chemistry 8, 459
Betts, J.J., James, Sybil P. and Thorpe, W.V. (1955) The metabolism of
pentachloronitrobenzene and tetrachloronitrobenzene and the
formation of mercapturic acid in the rabbit Biochem. J. 61 (4)
611-7
Borzelloca, J.F. and Larson, P.S. (1968a) Toxicologic study on the
effect of adding Terrachlor to the diet of beagle dogs for a period of
two years. Unpub. Rept. from the Medical College of Virginia submitted
by Olin Mathieson Chemical Corporation
Borzelloca, J.F. and Larson, P.S. (1968b) Three generation
reproduction study on rats receiving Terrachlor in their diet. Unpub.
Rept. from the Medical College of Virginia submitted by Olin Mathieson
Chemical Corporation
Corneliussen, P.E. (1969) Residues in food and feed - pesticide
residues in total diet samples (IV). Pesticides Monitoring Journal 2
(4) 140-52
Duggan, R.E. (1968) Residues in food and feed 1963-67. Pesticides
Monitoring Journal 2 (1) 2-46
Gorbach, S. (1961) Acta Chemica (Academiae Scientarum Hungaricae) 28
(1-3), 199-206
Gorbach, S. and Wagner, U. (1967) Pentachloronitrobenzene residues in
potatoes. J. Agric. and Food Chem. 15 (4) 654
Gorbach, S. (1969) Personal Communication to FAO
Hertzfield, E.G. (1967) Terrachlor - a new Soil Fungicide Agricultural
Chemicals 12 30-3
Hoechst. (1964) Toxikologische Prüfung von Pentachlornitrobenzol. 3.
Auf chronische Toxizität (90-Tage-Test) an Ratten. Unpub. Rept.
prepared and submitted by Farbwerke Hoechst, AG.
Hoechst. (1968) Chronische orale Toxizatätsprüfung von
Pentachlornitrobenzol. 2-Jahres-Versuch an Hunden. Unpub. Rept.
prepared and submitted by Farbwerke Hoechst AG.
Finnegan, J.K., Larson, P.S., Smith, R.B. Jr., Haag, H.B. and
Hennigar, G.R. (1958) Acute and chronic toxicity studies on
pentachloronitrobenzene. Arch. int. Pharmacodyn. 114:38-52
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L., Gart,
J.J., Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in
mice. A preliminary note. J. nat. Cancer Inst. 42:1101-14
Klein, A.K. and Gajan, R.J. (1961) Methods for the determination of
PCNB Residues. JAOAC 44:712
Ko, W.H. and Farley, J.D. (1969) Conversion of
pentachloronitrobenzene to pentachloroaniline in soil and the effect
of these compounds on soil Micro-organisms. Phytopathology 59 (1)
64-7
Kuchar, E.J., Geenty, Frances, O, Griffith, William P. and Thomas,
Raymond J. (1969) Analytical studies of metabolism of Terraclor in
beagle dogs, rats and plants, J. Agric. and Food Chem. 17 (6)
1237-40
Methratta, T.P., Montagna, R.W. and Griffith, W.P. (1967)
Determination of PCNB in crops and soil by electron-capture gas
chromatography. J. Agric. and Food Chem. 15 (4) 648 (July/Aug. 1967)
Mitchell, L.C. (1957) J. Assoc. Offic. Agric. Chemists 40, 294
Mitchell, L.C. (1958) J. Assoc. Offic. Agric, Chemists 41, 781
Olin Mathieson Chemical Corporation. (1969) Research reports on
quintozene
United States of America. (1969) Department of Agriculture Summary of
Registered Agricultural Pesticide Chemical Uses
United States of America. (1968) Department of Health, Education and
Welfare FDA Pesticide Analytical Manual Volume 1 Section 212.1
Wit, S.L., van Esch, G.J. and van Genderen, H. (1957) Toxicity of
some chloronitrobenzen compounds (trichlorodinitrobenzene,
trichloronitrobenzene, tetrachloronitrobenzene and
pentachloronitrobenzene) to laboratory rats and residues
found in crops treated with these fungicides. Proceedings of
the Fourth International Congress of Crop Protection,
Hamburg, September 1957. Vol. 2 (Braunschweig 1960): pp.
1665-8
Zweig, Gunter. (1964) Analytical Methods for Pesticides and Plant
Growth Regulators and Food Additives Vol. III Academic Press. New
York
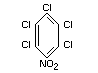 Other relevant chemical properties
Colourless crystalline needles practically insoluble in water, soluble
in benzene and chloroform. Technical quintozene is usually 97-99
percent pure. The main impurity, is hexachlorobenzene (1.5%) together
with lesser amounts of pentachlorobenzene and tetrachloronitrobenzene.
Vp 11.3 × 10-5mm Hg at 25°C. Quintozene shows high stability in the
soil. Quintozene is converted to pentachloroaniline (PCA) in moist
soil, the metabolite having somewhat lower fungicidal activity. In
animals the metabolites are pentachloroaniline and mercapturic acid
(Betts et al., 1955).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Fat samples from groups of ten rats (five males and five females) fed
diets containing 63.5, 635, 1250, or 2500 ppm of technical quintozene
for three months were examined for storage of quintozene. The apparent
storage ranged from an average of 43 ppm in the fat for the 63.5 ppm
diet to 1234 ppm for the 2500 ppm diet, there being a relatively
linear relationship between the levels of fat storage to the
concentration of quintozene in the diet. However, the neutron
activation method of analysis which was used would have shown the
presence of other chlorinated compounds as well an quintozene
(Finnegan et al., 1958).
Tissues from an unspecified number of dogs fed 5 or 1,080 ppm of
technical quintozene in their diet for 2 years were analysed by gas
chromatography for residues of quintozene and its metabolites. No
quintozene was found in fat, muscle, kidney, or liver tissues but two
metabolic products identified as pentachloroaniline and
methyl-pentachlorophenyl sulfide were found in these tissues.
Pentachloroaniline was found only in the fat and liver and in amounts
of less than 1 ppm for both dose-levels. Methyl-pentachlorophenyl
sulfide was found in the fat and liver of rats fed both dose levels
and was present as well in kidney and muscle in the 1080 ppm group,
the largest amount being 2.5 ppm in the fat of animals fed that level.
In another study on fat from an unspecified number of rats fed 50 or
500 ppm of technical quintozene in their diet for seven months, there
was less than 1 ppm of either of the metabolites in the fat of the
rats fed 50 ppm of quintozene, and approximately 1 ppm of
pentachloroaniline and 5 ppm of methyl-pentachlorophenyl sulfide for
the 500 ppm group (Kuchar et al., 1969).
Pentachloroaniline and another metabolite, a mercapturic acid, have
been isolated in urine from rabbits treated with quintozene. With a 2
g. dose an average of 62 percent of quintozene was unabsorbed and
excreted in the faeces. The average percentages excreted in urine as
pentachloroaniline and N-acetyl-S-pentachlorophenyl-L-cycteine were 12
and 14 percent respectively (Betts et al., 1955).
TOXICOLOGICAL STUDIES
Special studies on reproduction
A three-generation reproduction study was conducted with rats
receiving technical quintozene in their diet in concentrations of 0,
5, 50 and 500 ppm. Groups of 20 females were used and 2 litters were
produced in each generation. No significant differences were found
between the control- and quintozene-treated rats with respect to
fertility, gestation, viability or lactation indices, or in
weaning-weights. Histopathologic examination of the tissues of 10 pups
of each sex from the F2b generation showed no effect from the
treatment (Borzelloca and Larson, 1968b).
Special studies on carcinogenicity
Groups of 18 mice of each sex from two hybrid strains of mice were
given quintozene (the specifications not given) from 7 days of age for
18 months. The dose of 464 mg/kg was given to the mice by stomach tube
from the seventh day of age to the time weaning at four weeks of age
and thereafter the chemical was added to the diet in a corresponding
amount of 1,206 ppm. This level was a maximum tolerated dose for the
mice. There was a significantly elevated incidence of tumours, mostly
hepatomas, in both strains of mice (Innes. et al., 1969).
Acute toxicity
LD50
Animal Route mg/kg body-weight References
Rat (M) oral 1710* Finnegan, et al., 1958
(oil solution)
Rat (F) oral 1650* Finnegan, et al., 1958
(oil solution)
Rat oral >30,000 Wit, et al., 1957
(aqueous suspension)
Rat i.p. 5,000 Wit et al., 1957
(aqueous suspension)
* technical grade; defined as: pentachloronitrobenzene ... 98.2 percent
hexachlorobenzene ... 1.4 percent
traces of tetrachloro-
nitrobenzene and pentachlorobenzene
Short-term studies
Dog
Groups, each of three mongrel dogs, of unspecified sex were placed on
diets containing 25, 200 or 1,000 ppm of technical quintozene for one
year. No adverse effect was noted on body-weight or survival. No
haematologic changes were seen. Histopathologic changes were confined
to liver-cell enlargement with pale-staining cytoplasm at all
dose-levels, but there was no increasing severity of the lesions with
increasing exposure to quintozene (Finnegan et al., 1958).
Groups of four dogs of each sex were placed on diets containing 0, 5,
30, 180, or 1,080 ppm of technical quintozene for two years. No
adverse effect was noted on body-weight or survival. Increased
liver-weights, elevated serum-alkaline phosphatase and a moderate
degree of cholestatic hepatosis with secondary bile nephrosis occurred
in the dogs on the 1,080 ppm level. A minimum degree of cholestatic
hepatosis with secondary bile necrosis occurred at 180 ppm. No effect
was noted in the dogs fed 5 and 30 ppm of quintozene (Borzelloca and
Larson, 1968a).
Groups of six dogs, each comprising three male and three female
animals, were fed diets containing 0, 500, 1,000 or 5,000 ppm of
quintozene (the specification not given) for two years. Liver changes
occurred in all groups with the degree of damage related to the dose.
The 5,000 ppm level produced severe liver damage including fibrosis,
narrowing of hepatic cell cords, increased size of the periportal
areas and thick leucocyte infiltration. At the 1,000 and 500 ppm
dose-levels the changes were similar to those at the 5,000 ppm level
but to a lesser degree. The highest dose-level produced atrophy of
bone-marrow and reduced haematopoiesis (Hoechst, 1968).
Rat
Groups of 35 rats of each sex were fed diets containing 0, 63.5, 635,
1,250, 2,500 or 5,000 ppm of technical quintozene for three months.
Growth and survival were adversely affected in both sexes at the
dose-level of 5,000 ppm and in males growth was suppressed at 2,500
ppm. Liver to body-weight ratios were elevated at all dietary levels
except in the females fed 63.5 ppm. No haematologic changes were seen,
and histopathologic changes were limited to fine vacuolization of
liver-cell cytoplasm at 5000 ppm (Finnegan et al., 1958).
An unspecified number of young rats were fed diets containing 0 or
2,000 ppm of quintozene for 10 weeks. No gross effects, other than a
decreased growth rate in the males, were noted (Wit, et al., 1957).
Groups of 20 rats each comprising 10 male and 10 female animals were
fed diets containing 0, 1,000, 5,000 or 10,000 ppm of quintozene (the
specification not given) for 90 days. The animals grew slightly less
than controls at the 5,000 ppm dose-level and markedly less at the
10,000 ppm level (Hoechst, 1964).
Long-term studies
Rat
Groups of 10 rats of each sex were fed dicta containing 0, 25, 100,
300, 1,000 or 2,500 ppm of technical quintozene for two years. Growth
suppression occurred in the females at dose-levels of 100 ppm and
above; however, in the males growth depression occurred only at 2,500
ppm level. Haematologic and histopathologic observations in the test
animals were similar to the control group (Finnegan, et al., 1958).
OBSERVATIONS IN MAN
Quintozene (75 percent wettable powder) did not cause primary
irritation when applied to the skin of 50 human subjects; in 13 of
them sensitization was produced (Finnegan et al., 1958).
COMMENT
The acute and short-term studies and the reproduction studies in rats
are considered adequate. A preliminary report of studies in mice
indicates a potential for carcinogenicity in animals given a high dose
and further work is needed in other species. In the two-year study in
dogs severe morphologic changes were observed in the liver and
bone-marrow in the high dose-level groups. Furthermore, the apparent
erratic effect on growth an indicated in the studies with rats is not
explained. Additional studies should be done to delineate the exact
cause of these effects. Insufficient information in available on the
biological fate of the compound. For these reasons only a temporary
acceptable daily intake is established based on the two-year study in
the rats.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 25 ppm in the diet, equivalent to 1.25 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Quintozene is a fungicide mainly used for soil treatment or for
treatment of seeds and transplants but some crop applications are
recommended. The following table gives a review of application rates
of quintozene and recommended pre-harvest intervals.
TABLE I
Pre-harvest
Crop Rate Limitations interval days
Bananas 1.63% paste Apply to stems only - not to fruit
Beans 5.0 kg/ha Apply to foliage 21
0.5 g/kg seed Seed treatment 70
50g/100m of row Spray base of plants 21
40g/100m of row Soil treatment only 70
Cruciferous
vegetables 60 kg/ha Pre-planting 70
470g/100m row Row application prior to transplanting 70
Corn 0.5g/kg seed Seed treatment 70
Garlic 150g/100m row Soil treatment at planting 90
Lettuce (head) 150g/100m row When plants 5-7.5 cm tall 30
30g/100m row 2 treatments at 10 day intervals 20
Onions 40 kg/ha Pre-planting
TABLE I (cont'd)
Pre-harvest
Crop Rate Limitations interval days
Peanuts 250g/100m row Pre-planting 90
200g/100m row At pegging time 60
Peas 1g/kg Seed treatment
Peppers 70g/100m row At planting time 70
Potatoes 22 kg/ha Prior to planting 70
140g/100m row At planting time 70
Safflower,
Sorghum,
Soybeans,
Sugarbeet 1.5 g/kg Seed treatment only 70
Tomatoes 160g/100m row Prior to transplanting 70
Wheat 0.5g/kg seed Seed treatment 150
Post-harvest treatments
No post-harvest treatments with quintozene are known.
Other uses
Quintozene is used for control of fungi in alfalfa, clover, cotton,
ornamentals, bulbs, lawns, mushrooms and coffee crops.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Detailed residue data are available from United States trials with
quintozene on important crops and have been deposited with FAO. Rates
of application are similar to those given below. The typical data
presented below are representative:
TABLE II
Post-
Number treatment Residues (ppm)
Rate of interval
Crop kg/ha treatments days Range Average
Beans 1 1 60 0.003-0.004 0.003
10 1 60 0.005-0.006 0.005
Beans 1 1 60 <0.01 <0.01
Beans 1.5 1 60 <0.01 <0.01
Beans (small
white dry) 1 1 150 <0.01 <0.01
Beans (string) 0.5 1 70 <0.01 <0.01
Beans (navy) 8 4 120 0.003-0.152 0.07
Beans (Lima) 1.5 1 90 <0.01
Broccoli 20 1 0.003-0.018 0.012
40 1 140 0.002-0.021 0.013
Cabbage 20 1 140 0.000-0.014 0.007
40 1 140 0.000-0.020 0.007
Cottonseed 0.3 1 70 0.000-0.017 <0.017
Cottonseed 5.0 1 150 <0.012 <0.012
Cottonseed 2.5 1 150 <0.012 <0.012
5.0 1 180 0.004-0.032 0.014
Lettuce heads 100 1 180 0.00 -0.01 0.00
Lettuce outer
leaves 100 1 180 0.02 -0.11 0.06
Lettuce heads 2 8 0.00 0.00
2 16 0.00 0.00
Lettuce 18G 1 60 0.03 -0.05 0.03
(Greenhouse) 18WP 1 60 0.20 -0.30 0.26
18WP 1 70 0.09 -0.139 0.104
18G 1 70 0.048-0.093 0.058
TABLE II
Post-
Number treatment Residues (ppm)
Rate of interval
Crop kg/ha treatments days Range Average
Mushrooms 1.5 1 1 9.57 -9.68 9.6
1.5 1 3 2.75 -2.97 2.8
1.5 1 7 1.30 -1.36 1.34
Peanuts,kernels 10 1 130 0.063-0.154 0.104
kernels 100 1 130 0.208-0.212 0.210
Peanut shells 100 1 130 4.60 -5.21 4.96
Peppers 50 1 75 0.0 -0.008 0.002
50 2 35 0.0 -0.009 0.003
Potatoes 10 1 130 0.01 -0.066 0.027
10 1 130 0.066-0.125 0.088
10 1 130 0.00 -0.002 0.001
20 1 130 0.001-0.005 0.002
Tomatoes 3 2 50 0.00 -0.08 0.02
10 1 70 0.00 -0.01 0.01
50 1 100 0.00 -0.02 0.01
FATE OF RESIDUES
In animals
No data was available to show the residues in animal tissues or animal
products from the feeding of forage grown in soil treated with
quintozene. In view of the stability of the compound, its solubility
in lipids and its resistance to metabolism it seems highly likely that
significant residues of quintozene or pentachlor - derivatives do
occur in animal fats, milk and eggs.
Quintozene was reported in trace amounts in dairy produce and in oils,
fats and shortening in the U.S. total diet studies.
A paper by Kuchar et al. (1969) not available for consideration at the
time the original data was reviewed reports analytical studies of
metabolism of quintozene in beagle dogs, rats and plants.
Tissues from beagle dogs fed food containing 1,080 ppm quintozene in
their rations for 2 years were examined by GLC methods.
Pentachloroaniline (PCA) was identified in blood. Fat, liver, urine
and faeces yielded pentachloronitrobenzene (quintozene);
pentachlorobenzene (PCB); hexachlorobenzene (HCB) and
pentachloroaniline (PCA) and methyl pentachlorophenyl sulphide.
Quintozene was not detected in muscle, kidney, fat or liver of dogs
receiving 1,080 ppm in their diet over 2 years (234 gms quintozene in
all). HCB was the most prominent residue yielding 194 ppm in fatty
tissue. PCB occurred in a significant amounts only in fat (5.15 ppm)
and PCA only in faeces. A small amount of the quintozene fed is
excreted in the faeces (14 ppm).
No metabolites, only HCB, were found in tissues of rats fed quintozene
for 7 months at 500 ppm.
In plants
There is evidence of a slight systemic uptake by plants revealed by
analysis by spectrophotometric methods following application of
quintozene to soil. The following table gives typical examples from
the extensive data available (Olin Mathieson, 1969).
TABLE III
Residues
Seeds or
Applied Soil Roots Leaves fruit
Crop kg/acre ppm ppm ppm ppm
Beans 1 1.2 0.59 0.034 0.003
1.5 0.68 1.12 0.017 0.013
1.5 0.59 2.27 N.D. 0.004
Snap beans 1.0 9.2 9.0 0.004 -
1.5 3.4 7.0 0.09 -
String beans 0.75 - 0.94 0.288 -
1.0 - 3.62 0.221 -
1.5 - 2.37 0.461 -
Field beans 1.5 7.77 19.33 0.12 -
Lettuce 4.0 - - 0.0 -
(field) 15.0 - - 0.02 -
50.0 - - 0.00 -
100.0 - - 0.00 -
100 - - 0.06 outer -
leaves
TABLE III
Residues
Seeds or
Applied Soil Roots Leaves fruit
Crop kg/acre ppm ppm ppm ppm
Lettuce 18 dust - - 0.05 -
(greenhouse)
0.4 Spray - - 0.03 -
18 Granule - - 0.02 -
Cabbage 2.0 - - 0.007 -
2.0 - - 0.008 -
Tomatoes 6.0 - - - 0.02
50.0 - - - 0.01
Potatoes 10.0 - - - 0.03
10.0 - - - 0.008
Alfalfa 10 - - 0.02 -
20 - - 0.05 -
Peanut 10 - - 0.235 -
1.0 - 0.44 0.003 -
Gorbach and Wagner (1967) showed, by using highly sensitive GLC
techniques capable of detecting both quintozene and PCA that potatoes
growing in soil treated with quintozene at rates from 25 to 800 kg/ha
showed significant residues of quintozene in the skin (up to 3 ppm at
800 kg/ha) but only insignificant traces of PCA (up to 0.4 ppm) in the
skin. The flesh of these potatoes contained no detectable residues of
quintozene (less than 0.01 ppm) and less than 0.1 ppm of PCA and
unidentified metabolites.
Gorbach (1969) reports that the most recent studies reveal no evidence
of translocation from soil into green parts of leafy plants. A
carefully controlled experiment, where all possible contamination by
splash or vapour was eliminated, revealed no uptake by parsley growing
in quintozene treated soil.
Kuchar et al. (1969) reports that the metabolic pathway in plants
appears to be the same mechanism as in animals. Studies using cotton
seed planted in soil containing 300 ppm quintozene yielded the
following residues in the young cotton plants two weeks later: (1)
pentachloranitrobenzene (quintozene) 155 ppm; (2) pentachlorobenzene
4 ppm; (3) methyl pentachlorophenyl sulphide 3 ppm; (4)
hexachlorobenzene 5 ppm; (5) pentachloroaniline 1.1 ppm; and (6) 2, 3,
4, 5 - tetrachloronitrobenzene 0.018 ppm. Residues (2), (4) and (6)
may have originated partly from impurities in the technical grade
quintozene (97.8% PCNB; 1.8% HCB; 0.1% PCB and 0.4% TCNB).
These authors were able to show that the two unidentified materials
reported by Gorbach and Wagner (1967) were in fact hexachlorobenzene
(HCB) and the metabolite methyl pentachlorphenyl sulphide.
In soils
Quintozene appears to persist for long periods in the soil as disease
control may be as long an 12 months (Hertzfield, 1967). Ko and Farley
(1969) show that in moist soil quintozene is gradually converted to
pentachloroaniline (PCA) and the conversion was greatly enhanced by
submergence of the soil in water. PCA was stable in both moist and
submerged soil and was inhibitory to micro-organisms but to a lesser
extent than quintozene. The long term action of quintozene is partly
due to its conversion to PCA.
Quintozene remains unchanged in sterilized, moist soil but disappears
from submerged sterilized soil with a half life of three weeks. No PCA
was detected in sterilized submerged soil. Studies by Ko and Farley
(1969) showed that soil micro-organisms are responsible for the
conversion of quintozene to PCA.
Evidence of residues in food in commerce or at consumption
The only data available on quintozene residues in food moving in
commerce was gathered in the U.S.A. The U.S.D.A./H.E.W. 1968 reports
that of 9,789 samples of leaf and stem vegetables produced in the
U.S.A. and examined for a wide range of pesticides, 89 samples (0.89%)
contained quintozene at levels ranging from trace quantities to
greater than 2.0 ppm. The following shows the range:
ppm %
Trace - 0.03 0.42
0.04 - 0.5 0.19
0.51 - 1.0 0.1
1.01 - 2.0 0.04
Above - 2.0 0.14
0.89%
Quintozene was reported to occur in trace amounts in the fat of dairy
produce and at a level of 0.021 ppm in a composite sample of oils,
fats and shortening in the total diet studies conducted in the U.S.A.
in 1963-68 (Duggan 1968, Corneliussen 1969). The total intake in the
diet was calculated to be no more than trace amounts (less than 0.001
mg).
METHODS OF RESIDUE ANALYSIS
A review of analytical methods is given in the book by Zweig (1964).
Klein and Gajan (1961) have carefully compared a colorimetric, a
polarographic and a gas chromatographic - colometric method for
residue analysis on lettuce, cabbage and beans. The colorimetric
method of Ackermann et al. (1958) is reported to be the most accurate
in the range below 5 ppm with a recovery of 94%. The latter method was
improved by Ackermann et al. (1963). This method is however the
slowest of the three methods and does not differentiate quintozene
from tetrachlorobenzene. The polarographic method (Bache and Lisk,
1960; Klein and Gajan, 1961 and Gorbach, 1961) is the most rapid
because of less stringent cleanup requirements. Gorbach (1961) used a
sublimation step in the cleanup before the polarographic determination
and thus eliminated many of the interfering substances.
With the polarographic method, recoveries averaged 81%. with a
standard deviation of 12%. The gas chromatographic-colometric method
(Klein and Gajan, 1961) yielded average recoveries of 90% with average
deviation of 15%.
For proper identification, extracts should be checked for quintozene
by paper chromatography (Mitchell, 1957, 1958) or by thin layer
chromatography (Gorbach, 1967). In the latter paper, methods are given
to separate and identify the metabolite pentachloroaniline.
All three methods can be recommended and can be selected according to
equipment available.
(a) The colorimetric method (Ackermann et al., 1963). The quintozene
residue in fat-free extract is hydrolized to nitrite with alcoholic
potassium hydroxide, the nitrite is used to diazotize procaine
hydrochloride and the diazonium salt coupled with l-naphthylamine to
give a magenta solution having absorption maximum at 525 mu.
(b) Polarographic method (Bache and Lisk, 1960; Klein and Gajan,
1961; Gorbach, 1961). The crop material is extracted with hexane. The
extract is filtered and dried and a part of the co-extractives are
removed by freezing and adsorption on Attaclay.
Chromatography of the concentrated extract using Florisil removes the
remainder of the interfering substances. The solvent is then
evaporated and the residue dissolved in isopropyl alcohol. After
adding sodium acetate and acetic acid for supporting electrolyte and
deoxygenating the solution, the polarogram in recorded from 0.00 to
1.15 volts against saturated calomel electrode. The half-wave
potential for quintozene in -0.47 volts.
The later papers recommend a number of modifications to the
polarographic procedure.
(c) Gas Chromatographic - Microcolometric Method (Klein and Gajan,
1961; Gorbach, 1961). The extraction is carried out as described for
the polarographic method. The concentrated extract is evaporated to
dryness and the residue dissolved in hexane is injected into the
instrument.
The multi-detection procedure for determining chlorinated residues in
non-fatty foods based on JAOAC, 49 222 (1966) paragraph 24.213 which
in official for a number of pesticides is satisfactory for the
determination of quintozene residues.
A method using electron capture gas chromatography (Methratta T.P. et
al., 1967) suitable for determining quintozene residues in plants,
seeds and soil has a sensitivity of 0.01 ppm and is relatively free of
interference from plant extracts. The method is probably suitable for
development towards greater sensitivity.
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
Germany (Fed.Rep.) Cabbage, lettuce 1.0
onions, cucumber 1.0
horse radish 1.0
leaf vegetables 3.0
bananas (peeled) 0.1
Netherlands Fruit and vegetables 5.0
United States of America Bananas, beans, broccoli, Originally on "no
brussels sprouts, cabbage, residue basis". At
cauliflower, cotton, present under review.
garlic, lettuce, peanuts,
peas, peppers, potatoes,
tomatoes, wheat.
APPRAISAL
Quintozene or pentachloronitrobenzene (PCNB) is a versatile fungicide
used chiefly against soil fungi in agriculture and horticulture. It
was first developed in 1930 as a seed dressing for wheat. It is used
in many countries as a soil fungicide against Sclerotinia,
Rhizoctonia, Botrytis, Sclerotium, Fusrium and similar fungi of
vegetable and forage crops. Application by means of wettable powder,
emulsion concentrate or dust ranges from 10 to 55 kg/ha or 15 to 600g
per 100m of row. Seed dressings applied in the form of dust range from
30g to 300 g per 100 kg of seed. Treatments other than those applied
to the soil are usually aimed at the base of the plant but some
foliage applications are made, particularly to lettuce, beans and
mushrooms. Pre-harvest intervals are four to eight weeks.
The data available to the meeting were obtained solely in the United
States of America and did not include information about residues
following use elsewhere. Some information is available about residues
in foods in commerce.
Quintozene has high stability in soil and under neutral conditions
remains stable for exceptionally long periods.
The literature includes a number of methods of residue analysis based
on electron-capture gas chromatography and spectrophotometric methods.
The sensitivity of the methods is reported to be 0.01 ppm but the
spectrophotometric method does not determine the metabolite
pentachloroaniline (PCA). Quintozene and PCA may be determined by
multi residue methods for chlorinated compounds but no regulatory
methods have been evaluated, and there is reason to believe that the
recovery from many food commodities may be low unless special
provision is made for extraction and cleanup. Further work on the
development of an acceptable regulatory method is required.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to 1973)
Residues of the metabolite pentachloroaniline to be included.
Bananas (pulp) 0.01 ppm
(whole) 1.0
Beans 0.01
Beans (navy) 0.2
Broccoli 0.02
Cabbage 0.02
Cottonseed 0.03
Lettuce 0.3
(cont'd)
Mushrooms 10.0
Peanuts (kernels) 0.3
(whole) 5.0
Peppers 0.01
Potatoes 0.2
Tomatoes 0.1
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Carcinogenicity studies in two species of animals.
2. Studies to explain the cause of growth depression in rats and the
effect on liver and bone-marrow in dogs.
3. Further studies on the metabolism and on the metabolites,
particularly pentachloroaniline.
4. Data from countries other than the United States of America on the
required rates and frequencies of application, pre-harvest
intervals and the resulting residues.
5. Information on residues in edible animal tissues and in animal
products resulting from the feeding of plant products (including
forage) treated with quintozene in accordance with normal
agricultural practice.
6. Information on the frequency and level of quintozene residues in
food commodities in commerce.
7. Information on the level of metabolites, particularly
pentachloroaniline in plants and animals.
DESIRABLE
1. Development of analytical methods for greater sensitivity and
evaluation for regulatory purposes.
2. Information on the residue levels in root crops, especially
carrots, grown in soil treated previously with quintozene in crop
rotation.
REFERENCES
Ackermann, H.J. et al. (1958) Spectrophotometric determination of
pentachloronitrobenzene on food and forage Crops. J. Agric. and
Food Chem. 6:747-50 (Oct.)
Ackermann, H.J. et al. (1963) Modifications to the spectrophotometric
analysis of PCNB in Soil and Crops. J. Agric. and Food Chem.
11(4) 297 (July/Aug. 1963)
Bache, C.A. and Lisk, D.I. (1960) Journal of Agriculture and Food
Chemistry 8, 459
Betts, J.J., James, Sybil P. and Thorpe, W.V. (1955) The metabolism of
pentachloronitrobenzene and tetrachloronitrobenzene and the
formation of mercapturic acid in the rabbit Biochem. J. 61 (4)
611-7
Borzelloca, J.F. and Larson, P.S. (1968a) Toxicologic study on the
effect of adding Terrachlor to the diet of beagle dogs for a period of
two years. Unpub. Rept. from the Medical College of Virginia submitted
by Olin Mathieson Chemical Corporation
Borzelloca, J.F. and Larson, P.S. (1968b) Three generation
reproduction study on rats receiving Terrachlor in their diet. Unpub.
Rept. from the Medical College of Virginia submitted by Olin Mathieson
Chemical Corporation
Corneliussen, P.E. (1969) Residues in food and feed - pesticide
residues in total diet samples (IV). Pesticides Monitoring Journal 2
(4) 140-52
Duggan, R.E. (1968) Residues in food and feed 1963-67. Pesticides
Monitoring Journal 2 (1) 2-46
Gorbach, S. (1961) Acta Chemica (Academiae Scientarum Hungaricae) 28
(1-3), 199-206
Gorbach, S. and Wagner, U. (1967) Pentachloronitrobenzene residues in
potatoes. J. Agric. and Food Chem. 15 (4) 654
Gorbach, S. (1969) Personal Communication to FAO
Hertzfield, E.G. (1967) Terrachlor - a new Soil Fungicide Agricultural
Chemicals 12 30-3
Hoechst. (1964) Toxikologische Prüfung von Pentachlornitrobenzol. 3.
Auf chronische Toxizität (90-Tage-Test) an Ratten. Unpub. Rept.
prepared and submitted by Farbwerke Hoechst, AG.
Hoechst. (1968) Chronische orale Toxizatätsprüfung von
Pentachlornitrobenzol. 2-Jahres-Versuch an Hunden. Unpub. Rept.
prepared and submitted by Farbwerke Hoechst AG.
Finnegan, J.K., Larson, P.S., Smith, R.B. Jr., Haag, H.B. and
Hennigar, G.R. (1958) Acute and chronic toxicity studies on
pentachloronitrobenzene. Arch. int. Pharmacodyn. 114:38-52
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L., Gart,
J.J., Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in
mice. A preliminary note. J. nat. Cancer Inst. 42:1101-14
Klein, A.K. and Gajan, R.J. (1961) Methods for the determination of
PCNB Residues. JAOAC 44:712
Ko, W.H. and Farley, J.D. (1969) Conversion of
pentachloronitrobenzene to pentachloroaniline in soil and the effect
of these compounds on soil Micro-organisms. Phytopathology 59 (1)
64-7
Kuchar, E.J., Geenty, Frances, O, Griffith, William P. and Thomas,
Raymond J. (1969) Analytical studies of metabolism of Terraclor in
beagle dogs, rats and plants, J. Agric. and Food Chem. 17 (6)
1237-40
Methratta, T.P., Montagna, R.W. and Griffith, W.P. (1967)
Determination of PCNB in crops and soil by electron-capture gas
chromatography. J. Agric. and Food Chem. 15 (4) 648 (July/Aug. 1967)
Mitchell, L.C. (1957) J. Assoc. Offic. Agric. Chemists 40, 294
Mitchell, L.C. (1958) J. Assoc. Offic. Agric, Chemists 41, 781
Olin Mathieson Chemical Corporation. (1969) Research reports on
quintozene
United States of America. (1969) Department of Agriculture Summary of
Registered Agricultural Pesticide Chemical Uses
United States of America. (1968) Department of Health, Education and
Welfare FDA Pesticide Analytical Manual Volume 1 Section 212.1
Wit, S.L., van Esch, G.J. and van Genderen, H. (1957) Toxicity of
some chloronitrobenzen compounds (trichlorodinitrobenzene,
trichloronitrobenzene, tetrachloronitrobenzene and
pentachloronitrobenzene) to laboratory rats and residues
found in crops treated with these fungicides. Proceedings of
the Fourth International Congress of Crop Protection,
Hamburg, September 1957. Vol. 2 (Braunschweig 1960): pp.
1665-8
Zweig, Gunter. (1964) Analytical Methods for Pesticides and Plant
Growth Regulators and Food Additives Vol. III Academic Press. New
York
Other relevant chemical properties
Colourless crystalline needles practically insoluble in water, soluble
in benzene and chloroform. Technical quintozene is usually 97-99
percent pure. The main impurity, is hexachlorobenzene (1.5%) together
with lesser amounts of pentachlorobenzene and tetrachloronitrobenzene.
Vp 11.3 × 10-5mm Hg at 25°C. Quintozene shows high stability in the
soil. Quintozene is converted to pentachloroaniline (PCA) in moist
soil, the metabolite having somewhat lower fungicidal activity. In
animals the metabolites are pentachloroaniline and mercapturic acid
(Betts et al., 1955).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Fat samples from groups of ten rats (five males and five females) fed
diets containing 63.5, 635, 1250, or 2500 ppm of technical quintozene
for three months were examined for storage of quintozene. The apparent
storage ranged from an average of 43 ppm in the fat for the 63.5 ppm
diet to 1234 ppm for the 2500 ppm diet, there being a relatively
linear relationship between the levels of fat storage to the
concentration of quintozene in the diet. However, the neutron
activation method of analysis which was used would have shown the
presence of other chlorinated compounds as well an quintozene
(Finnegan et al., 1958).
Tissues from an unspecified number of dogs fed 5 or 1,080 ppm of
technical quintozene in their diet for 2 years were analysed by gas
chromatography for residues of quintozene and its metabolites. No
quintozene was found in fat, muscle, kidney, or liver tissues but two
metabolic products identified as pentachloroaniline and
methyl-pentachlorophenyl sulfide were found in these tissues.
Pentachloroaniline was found only in the fat and liver and in amounts
of less than 1 ppm for both dose-levels. Methyl-pentachlorophenyl
sulfide was found in the fat and liver of rats fed both dose levels
and was present as well in kidney and muscle in the 1080 ppm group,
the largest amount being 2.5 ppm in the fat of animals fed that level.
In another study on fat from an unspecified number of rats fed 50 or
500 ppm of technical quintozene in their diet for seven months, there
was less than 1 ppm of either of the metabolites in the fat of the
rats fed 50 ppm of quintozene, and approximately 1 ppm of
pentachloroaniline and 5 ppm of methyl-pentachlorophenyl sulfide for
the 500 ppm group (Kuchar et al., 1969).
Pentachloroaniline and another metabolite, a mercapturic acid, have
been isolated in urine from rabbits treated with quintozene. With a 2
g. dose an average of 62 percent of quintozene was unabsorbed and
excreted in the faeces. The average percentages excreted in urine as
pentachloroaniline and N-acetyl-S-pentachlorophenyl-L-cycteine were 12
and 14 percent respectively (Betts et al., 1955).
TOXICOLOGICAL STUDIES
Special studies on reproduction
A three-generation reproduction study was conducted with rats
receiving technical quintozene in their diet in concentrations of 0,
5, 50 and 500 ppm. Groups of 20 females were used and 2 litters were
produced in each generation. No significant differences were found
between the control- and quintozene-treated rats with respect to
fertility, gestation, viability or lactation indices, or in
weaning-weights. Histopathologic examination of the tissues of 10 pups
of each sex from the F2b generation showed no effect from the
treatment (Borzelloca and Larson, 1968b).
Special studies on carcinogenicity
Groups of 18 mice of each sex from two hybrid strains of mice were
given quintozene (the specifications not given) from 7 days of age for
18 months. The dose of 464 mg/kg was given to the mice by stomach tube
from the seventh day of age to the time weaning at four weeks of age
and thereafter the chemical was added to the diet in a corresponding
amount of 1,206 ppm. This level was a maximum tolerated dose for the
mice. There was a significantly elevated incidence of tumours, mostly
hepatomas, in both strains of mice (Innes. et al., 1969).
Acute toxicity
LD50
Animal Route mg/kg body-weight References
Rat (M) oral 1710* Finnegan, et al., 1958
(oil solution)
Rat (F) oral 1650* Finnegan, et al., 1958
(oil solution)
Rat oral >30,000 Wit, et al., 1957
(aqueous suspension)
Rat i.p. 5,000 Wit et al., 1957
(aqueous suspension)
* technical grade; defined as: pentachloronitrobenzene ... 98.2 percent
hexachlorobenzene ... 1.4 percent
traces of tetrachloro-
nitrobenzene and pentachlorobenzene
Short-term studies
Dog
Groups, each of three mongrel dogs, of unspecified sex were placed on
diets containing 25, 200 or 1,000 ppm of technical quintozene for one
year. No adverse effect was noted on body-weight or survival. No
haematologic changes were seen. Histopathologic changes were confined
to liver-cell enlargement with pale-staining cytoplasm at all
dose-levels, but there was no increasing severity of the lesions with
increasing exposure to quintozene (Finnegan et al., 1958).
Groups of four dogs of each sex were placed on diets containing 0, 5,
30, 180, or 1,080 ppm of technical quintozene for two years. No
adverse effect was noted on body-weight or survival. Increased
liver-weights, elevated serum-alkaline phosphatase and a moderate
degree of cholestatic hepatosis with secondary bile nephrosis occurred
in the dogs on the 1,080 ppm level. A minimum degree of cholestatic
hepatosis with secondary bile necrosis occurred at 180 ppm. No effect
was noted in the dogs fed 5 and 30 ppm of quintozene (Borzelloca and
Larson, 1968a).
Groups of six dogs, each comprising three male and three female
animals, were fed diets containing 0, 500, 1,000 or 5,000 ppm of
quintozene (the specification not given) for two years. Liver changes
occurred in all groups with the degree of damage related to the dose.
The 5,000 ppm level produced severe liver damage including fibrosis,
narrowing of hepatic cell cords, increased size of the periportal
areas and thick leucocyte infiltration. At the 1,000 and 500 ppm
dose-levels the changes were similar to those at the 5,000 ppm level
but to a lesser degree. The highest dose-level produced atrophy of
bone-marrow and reduced haematopoiesis (Hoechst, 1968).
Rat
Groups of 35 rats of each sex were fed diets containing 0, 63.5, 635,
1,250, 2,500 or 5,000 ppm of technical quintozene for three months.
Growth and survival were adversely affected in both sexes at the
dose-level of 5,000 ppm and in males growth was suppressed at 2,500
ppm. Liver to body-weight ratios were elevated at all dietary levels
except in the females fed 63.5 ppm. No haematologic changes were seen,
and histopathologic changes were limited to fine vacuolization of
liver-cell cytoplasm at 5000 ppm (Finnegan et al., 1958).
An unspecified number of young rats were fed diets containing 0 or
2,000 ppm of quintozene for 10 weeks. No gross effects, other than a
decreased growth rate in the males, were noted (Wit, et al., 1957).
Groups of 20 rats each comprising 10 male and 10 female animals were
fed diets containing 0, 1,000, 5,000 or 10,000 ppm of quintozene (the
specification not given) for 90 days. The animals grew slightly less
than controls at the 5,000 ppm dose-level and markedly less at the
10,000 ppm level (Hoechst, 1964).
Long-term studies
Rat
Groups of 10 rats of each sex were fed dicta containing 0, 25, 100,
300, 1,000 or 2,500 ppm of technical quintozene for two years. Growth
suppression occurred in the females at dose-levels of 100 ppm and
above; however, in the males growth depression occurred only at 2,500
ppm level. Haematologic and histopathologic observations in the test
animals were similar to the control group (Finnegan, et al., 1958).
OBSERVATIONS IN MAN
Quintozene (75 percent wettable powder) did not cause primary
irritation when applied to the skin of 50 human subjects; in 13 of
them sensitization was produced (Finnegan et al., 1958).
COMMENT
The acute and short-term studies and the reproduction studies in rats
are considered adequate. A preliminary report of studies in mice
indicates a potential for carcinogenicity in animals given a high dose
and further work is needed in other species. In the two-year study in
dogs severe morphologic changes were observed in the liver and
bone-marrow in the high dose-level groups. Furthermore, the apparent
erratic effect on growth an indicated in the studies with rats is not
explained. Additional studies should be done to delineate the exact
cause of these effects. Insufficient information in available on the
biological fate of the compound. For these reasons only a temporary
acceptable daily intake is established based on the two-year study in
the rats.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Rat: 25 ppm in the diet, equivalent to 1.25 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.001 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Quintozene is a fungicide mainly used for soil treatment or for
treatment of seeds and transplants but some crop applications are
recommended. The following table gives a review of application rates
of quintozene and recommended pre-harvest intervals.
TABLE I
Pre-harvest
Crop Rate Limitations interval days
Bananas 1.63% paste Apply to stems only - not to fruit
Beans 5.0 kg/ha Apply to foliage 21
0.5 g/kg seed Seed treatment 70
50g/100m of row Spray base of plants 21
40g/100m of row Soil treatment only 70
Cruciferous
vegetables 60 kg/ha Pre-planting 70
470g/100m row Row application prior to transplanting 70
Corn 0.5g/kg seed Seed treatment 70
Garlic 150g/100m row Soil treatment at planting 90
Lettuce (head) 150g/100m row When plants 5-7.5 cm tall 30
30g/100m row 2 treatments at 10 day intervals 20
Onions 40 kg/ha Pre-planting
TABLE I (cont'd)
Pre-harvest
Crop Rate Limitations interval days
Peanuts 250g/100m row Pre-planting 90
200g/100m row At pegging time 60
Peas 1g/kg Seed treatment
Peppers 70g/100m row At planting time 70
Potatoes 22 kg/ha Prior to planting 70
140g/100m row At planting time 70
Safflower,
Sorghum,
Soybeans,
Sugarbeet 1.5 g/kg Seed treatment only 70
Tomatoes 160g/100m row Prior to transplanting 70
Wheat 0.5g/kg seed Seed treatment 150
Post-harvest treatments
No post-harvest treatments with quintozene are known.
Other uses
Quintozene is used for control of fungi in alfalfa, clover, cotton,
ornamentals, bulbs, lawns, mushrooms and coffee crops.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Detailed residue data are available from United States trials with
quintozene on important crops and have been deposited with FAO. Rates
of application are similar to those given below. The typical data
presented below are representative:
TABLE II
Post-
Number treatment Residues (ppm)
Rate of interval
Crop kg/ha treatments days Range Average
Beans 1 1 60 0.003-0.004 0.003
10 1 60 0.005-0.006 0.005
Beans 1 1 60 <0.01 <0.01
Beans 1.5 1 60 <0.01 <0.01
Beans (small
white dry) 1 1 150 <0.01 <0.01
Beans (string) 0.5 1 70 <0.01 <0.01
Beans (navy) 8 4 120 0.003-0.152 0.07
Beans (Lima) 1.5 1 90 <0.01
Broccoli 20 1 0.003-0.018 0.012
40 1 140 0.002-0.021 0.013
Cabbage 20 1 140 0.000-0.014 0.007
40 1 140 0.000-0.020 0.007
Cottonseed 0.3 1 70 0.000-0.017 <0.017
Cottonseed 5.0 1 150 <0.012 <0.012
Cottonseed 2.5 1 150 <0.012 <0.012
5.0 1 180 0.004-0.032 0.014
Lettuce heads 100 1 180 0.00 -0.01 0.00
Lettuce outer
leaves 100 1 180 0.02 -0.11 0.06
Lettuce heads 2 8 0.00 0.00
2 16 0.00 0.00
Lettuce 18G 1 60 0.03 -0.05 0.03
(Greenhouse) 18WP 1 60 0.20 -0.30 0.26
18WP 1 70 0.09 -0.139 0.104
18G 1 70 0.048-0.093 0.058
TABLE II
Post-
Number treatment Residues (ppm)
Rate of interval
Crop kg/ha treatments days Range Average
Mushrooms 1.5 1 1 9.57 -9.68 9.6
1.5 1 3 2.75 -2.97 2.8
1.5 1 7 1.30 -1.36 1.34
Peanuts,kernels 10 1 130 0.063-0.154 0.104
kernels 100 1 130 0.208-0.212 0.210
Peanut shells 100 1 130 4.60 -5.21 4.96
Peppers 50 1 75 0.0 -0.008 0.002
50 2 35 0.0 -0.009 0.003
Potatoes 10 1 130 0.01 -0.066 0.027
10 1 130 0.066-0.125 0.088
10 1 130 0.00 -0.002 0.001
20 1 130 0.001-0.005 0.002
Tomatoes 3 2 50 0.00 -0.08 0.02
10 1 70 0.00 -0.01 0.01
50 1 100 0.00 -0.02 0.01
FATE OF RESIDUES
In animals
No data was available to show the residues in animal tissues or animal
products from the feeding of forage grown in soil treated with
quintozene. In view of the stability of the compound, its solubility
in lipids and its resistance to metabolism it seems highly likely that
significant residues of quintozene or pentachlor - derivatives do
occur in animal fats, milk and eggs.
Quintozene was reported in trace amounts in dairy produce and in oils,
fats and shortening in the U.S. total diet studies.
A paper by Kuchar et al. (1969) not available for consideration at the
time the original data was reviewed reports analytical studies of
metabolism of quintozene in beagle dogs, rats and plants.
Tissues from beagle dogs fed food containing 1,080 ppm quintozene in
their rations for 2 years were examined by GLC methods.
Pentachloroaniline (PCA) was identified in blood. Fat, liver, urine
and faeces yielded pentachloronitrobenzene (quintozene);
pentachlorobenzene (PCB); hexachlorobenzene (HCB) and
pentachloroaniline (PCA) and methyl pentachlorophenyl sulphide.
Quintozene was not detected in muscle, kidney, fat or liver of dogs
receiving 1,080 ppm in their diet over 2 years (234 gms quintozene in
all). HCB was the most prominent residue yielding 194 ppm in fatty
tissue. PCB occurred in a significant amounts only in fat (5.15 ppm)
and PCA only in faeces. A small amount of the quintozene fed is
excreted in the faeces (14 ppm).
No metabolites, only HCB, were found in tissues of rats fed quintozene
for 7 months at 500 ppm.
In plants
There is evidence of a slight systemic uptake by plants revealed by
analysis by spectrophotometric methods following application of
quintozene to soil. The following table gives typical examples from
the extensive data available (Olin Mathieson, 1969).
TABLE III
Residues
Seeds or
Applied Soil Roots Leaves fruit
Crop kg/acre ppm ppm ppm ppm
Beans 1 1.2 0.59 0.034 0.003
1.5 0.68 1.12 0.017 0.013
1.5 0.59 2.27 N.D. 0.004
Snap beans 1.0 9.2 9.0 0.004 -
1.5 3.4 7.0 0.09 -
String beans 0.75 - 0.94 0.288 -
1.0 - 3.62 0.221 -
1.5 - 2.37 0.461 -
Field beans 1.5 7.77 19.33 0.12 -
Lettuce 4.0 - - 0.0 -
(field) 15.0 - - 0.02 -
50.0 - - 0.00 -
100.0 - - 0.00 -
100 - - 0.06 outer -
leaves
TABLE III
Residues
Seeds or
Applied Soil Roots Leaves fruit
Crop kg/acre ppm ppm ppm ppm
Lettuce 18 dust - - 0.05 -
(greenhouse)
0.4 Spray - - 0.03 -
18 Granule - - 0.02 -
Cabbage 2.0 - - 0.007 -
2.0 - - 0.008 -
Tomatoes 6.0 - - - 0.02
50.0 - - - 0.01
Potatoes 10.0 - - - 0.03
10.0 - - - 0.008
Alfalfa 10 - - 0.02 -
20 - - 0.05 -
Peanut 10 - - 0.235 -
1.0 - 0.44 0.003 -
Gorbach and Wagner (1967) showed, by using highly sensitive GLC
techniques capable of detecting both quintozene and PCA that potatoes
growing in soil treated with quintozene at rates from 25 to 800 kg/ha
showed significant residues of quintozene in the skin (up to 3 ppm at
800 kg/ha) but only insignificant traces of PCA (up to 0.4 ppm) in the
skin. The flesh of these potatoes contained no detectable residues of
quintozene (less than 0.01 ppm) and less than 0.1 ppm of PCA and
unidentified metabolites.
Gorbach (1969) reports that the most recent studies reveal no evidence
of translocation from soil into green parts of leafy plants. A
carefully controlled experiment, where all possible contamination by
splash or vapour was eliminated, revealed no uptake by parsley growing
in quintozene treated soil.
Kuchar et al. (1969) reports that the metabolic pathway in plants
appears to be the same mechanism as in animals. Studies using cotton
seed planted in soil containing 300 ppm quintozene yielded the
following residues in the young cotton plants two weeks later: (1)
pentachloranitrobenzene (quintozene) 155 ppm; (2) pentachlorobenzene
4 ppm; (3) methyl pentachlorophenyl sulphide 3 ppm; (4)
hexachlorobenzene 5 ppm; (5) pentachloroaniline 1.1 ppm; and (6) 2, 3,
4, 5 - tetrachloronitrobenzene 0.018 ppm. Residues (2), (4) and (6)
may have originated partly from impurities in the technical grade
quintozene (97.8% PCNB; 1.8% HCB; 0.1% PCB and 0.4% TCNB).
These authors were able to show that the two unidentified materials
reported by Gorbach and Wagner (1967) were in fact hexachlorobenzene
(HCB) and the metabolite methyl pentachlorphenyl sulphide.
In soils
Quintozene appears to persist for long periods in the soil as disease
control may be as long an 12 months (Hertzfield, 1967). Ko and Farley
(1969) show that in moist soil quintozene is gradually converted to
pentachloroaniline (PCA) and the conversion was greatly enhanced by
submergence of the soil in water. PCA was stable in both moist and
submerged soil and was inhibitory to micro-organisms but to a lesser
extent than quintozene. The long term action of quintozene is partly
due to its conversion to PCA.
Quintozene remains unchanged in sterilized, moist soil but disappears
from submerged sterilized soil with a half life of three weeks. No PCA
was detected in sterilized submerged soil. Studies by Ko and Farley
(1969) showed that soil micro-organisms are responsible for the
conversion of quintozene to PCA.
Evidence of residues in food in commerce or at consumption
The only data available on quintozene residues in food moving in
commerce was gathered in the U.S.A. The U.S.D.A./H.E.W. 1968 reports
that of 9,789 samples of leaf and stem vegetables produced in the
U.S.A. and examined for a wide range of pesticides, 89 samples (0.89%)
contained quintozene at levels ranging from trace quantities to
greater than 2.0 ppm. The following shows the range:
ppm %
Trace - 0.03 0.42
0.04 - 0.5 0.19
0.51 - 1.0 0.1
1.01 - 2.0 0.04
Above - 2.0 0.14
0.89%
Quintozene was reported to occur in trace amounts in the fat of dairy
produce and at a level of 0.021 ppm in a composite sample of oils,
fats and shortening in the total diet studies conducted in the U.S.A.
in 1963-68 (Duggan 1968, Corneliussen 1969). The total intake in the
diet was calculated to be no more than trace amounts (less than 0.001
mg).
METHODS OF RESIDUE ANALYSIS
A review of analytical methods is given in the book by Zweig (1964).
Klein and Gajan (1961) have carefully compared a colorimetric, a
polarographic and a gas chromatographic - colometric method for
residue analysis on lettuce, cabbage and beans. The colorimetric
method of Ackermann et al. (1958) is reported to be the most accurate
in the range below 5 ppm with a recovery of 94%. The latter method was
improved by Ackermann et al. (1963). This method is however the
slowest of the three methods and does not differentiate quintozene
from tetrachlorobenzene. The polarographic method (Bache and Lisk,
1960; Klein and Gajan, 1961 and Gorbach, 1961) is the most rapid
because of less stringent cleanup requirements. Gorbach (1961) used a
sublimation step in the cleanup before the polarographic determination
and thus eliminated many of the interfering substances.
With the polarographic method, recoveries averaged 81%. with a
standard deviation of 12%. The gas chromatographic-colometric method
(Klein and Gajan, 1961) yielded average recoveries of 90% with average
deviation of 15%.
For proper identification, extracts should be checked for quintozene
by paper chromatography (Mitchell, 1957, 1958) or by thin layer
chromatography (Gorbach, 1967). In the latter paper, methods are given
to separate and identify the metabolite pentachloroaniline.
All three methods can be recommended and can be selected according to
equipment available.
(a) The colorimetric method (Ackermann et al., 1963). The quintozene
residue in fat-free extract is hydrolized to nitrite with alcoholic
potassium hydroxide, the nitrite is used to diazotize procaine
hydrochloride and the diazonium salt coupled with l-naphthylamine to
give a magenta solution having absorption maximum at 525 mu.
(b) Polarographic method (Bache and Lisk, 1960; Klein and Gajan,
1961; Gorbach, 1961). The crop material is extracted with hexane. The
extract is filtered and dried and a part of the co-extractives are
removed by freezing and adsorption on Attaclay.
Chromatography of the concentrated extract using Florisil removes the
remainder of the interfering substances. The solvent is then
evaporated and the residue dissolved in isopropyl alcohol. After
adding sodium acetate and acetic acid for supporting electrolyte and
deoxygenating the solution, the polarogram in recorded from 0.00 to
1.15 volts against saturated calomel electrode. The half-wave
potential for quintozene in -0.47 volts.
The later papers recommend a number of modifications to the
polarographic procedure.
(c) Gas Chromatographic - Microcolometric Method (Klein and Gajan,
1961; Gorbach, 1961). The extraction is carried out as described for
the polarographic method. The concentrated extract is evaporated to
dryness and the residue dissolved in hexane is injected into the
instrument.
The multi-detection procedure for determining chlorinated residues in
non-fatty foods based on JAOAC, 49 222 (1966) paragraph 24.213 which
in official for a number of pesticides is satisfactory for the
determination of quintozene residues.
A method using electron capture gas chromatography (Methratta T.P. et
al., 1967) suitable for determining quintozene residues in plants,
seeds and soil has a sensitivity of 0.01 ppm and is relatively free of
interference from plant extracts. The method is probably suitable for
development towards greater sensitivity.
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
Germany (Fed.Rep.) Cabbage, lettuce 1.0
onions, cucumber 1.0
horse radish 1.0
leaf vegetables 3.0
bananas (peeled) 0.1
Netherlands Fruit and vegetables 5.0
United States of America Bananas, beans, broccoli, Originally on "no
brussels sprouts, cabbage, residue basis". At
cauliflower, cotton, present under review.
garlic, lettuce, peanuts,
peas, peppers, potatoes,
tomatoes, wheat.
APPRAISAL
Quintozene or pentachloronitrobenzene (PCNB) is a versatile fungicide
used chiefly against soil fungi in agriculture and horticulture. It
was first developed in 1930 as a seed dressing for wheat. It is used
in many countries as a soil fungicide against Sclerotinia,
Rhizoctonia, Botrytis, Sclerotium, Fusrium and similar fungi of
vegetable and forage crops. Application by means of wettable powder,
emulsion concentrate or dust ranges from 10 to 55 kg/ha or 15 to 600g
per 100m of row. Seed dressings applied in the form of dust range from
30g to 300 g per 100 kg of seed. Treatments other than those applied
to the soil are usually aimed at the base of the plant but some
foliage applications are made, particularly to lettuce, beans and
mushrooms. Pre-harvest intervals are four to eight weeks.
The data available to the meeting were obtained solely in the United
States of America and did not include information about residues
following use elsewhere. Some information is available about residues
in foods in commerce.
Quintozene has high stability in soil and under neutral conditions
remains stable for exceptionally long periods.
The literature includes a number of methods of residue analysis based
on electron-capture gas chromatography and spectrophotometric methods.
The sensitivity of the methods is reported to be 0.01 ppm but the
spectrophotometric method does not determine the metabolite
pentachloroaniline (PCA). Quintozene and PCA may be determined by
multi residue methods for chlorinated compounds but no regulatory
methods have been evaluated, and there is reason to believe that the
recovery from many food commodities may be low unless special
provision is made for extraction and cleanup. Further work on the
development of an acceptable regulatory method is required.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to 1973)
Residues of the metabolite pentachloroaniline to be included.
Bananas (pulp) 0.01 ppm
(whole) 1.0
Beans 0.01
Beans (navy) 0.2
Broccoli 0.02
Cabbage 0.02
Cottonseed 0.03
Lettuce 0.3
(cont'd)
Mushrooms 10.0
Peanuts (kernels) 0.3
(whole) 5.0
Peppers 0.01
Potatoes 0.2
Tomatoes 0.1
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Carcinogenicity studies in two species of animals.
2. Studies to explain the cause of growth depression in rats and the
effect on liver and bone-marrow in dogs.
3. Further studies on the metabolism and on the metabolites,
particularly pentachloroaniline.
4. Data from countries other than the United States of America on the
required rates and frequencies of application, pre-harvest
intervals and the resulting residues.
5. Information on residues in edible animal tissues and in animal
products resulting from the feeding of plant products (including
forage) treated with quintozene in accordance with normal
agricultural practice.
6. Information on the frequency and level of quintozene residues in
food commodities in commerce.
7. Information on the level of metabolites, particularly
pentachloroaniline in plants and animals.
DESIRABLE
1. Development of analytical methods for greater sensitivity and
evaluation for regulatory purposes.
2. Information on the residue levels in root crops, especially
carrots, grown in soil treated previously with quintozene in crop
rotation.
REFERENCES
Ackermann, H.J. et al. (1958) Spectrophotometric determination of
pentachloronitrobenzene on food and forage Crops. J. Agric. and
Food Chem. 6:747-50 (Oct.)
Ackermann, H.J. et al. (1963) Modifications to the spectrophotometric
analysis of PCNB in Soil and Crops. J. Agric. and Food Chem.
11(4) 297 (July/Aug. 1963)
Bache, C.A. and Lisk, D.I. (1960) Journal of Agriculture and Food
Chemistry 8, 459
Betts, J.J., James, Sybil P. and Thorpe, W.V. (1955) The metabolism of
pentachloronitrobenzene and tetrachloronitrobenzene and the
formation of mercapturic acid in the rabbit Biochem. J. 61 (4)
611-7
Borzelloca, J.F. and Larson, P.S. (1968a) Toxicologic study on the
effect of adding Terrachlor to the diet of beagle dogs for a period of
two years. Unpub. Rept. from the Medical College of Virginia submitted
by Olin Mathieson Chemical Corporation
Borzelloca, J.F. and Larson, P.S. (1968b) Three generation
reproduction study on rats receiving Terrachlor in their diet. Unpub.
Rept. from the Medical College of Virginia submitted by Olin Mathieson
Chemical Corporation
Corneliussen, P.E. (1969) Residues in food and feed - pesticide
residues in total diet samples (IV). Pesticides Monitoring Journal 2
(4) 140-52
Duggan, R.E. (1968) Residues in food and feed 1963-67. Pesticides
Monitoring Journal 2 (1) 2-46
Gorbach, S. (1961) Acta Chemica (Academiae Scientarum Hungaricae) 28
(1-3), 199-206
Gorbach, S. and Wagner, U. (1967) Pentachloronitrobenzene residues in
potatoes. J. Agric. and Food Chem. 15 (4) 654
Gorbach, S. (1969) Personal Communication to FAO
Hertzfield, E.G. (1967) Terrachlor - a new Soil Fungicide Agricultural
Chemicals 12 30-3
Hoechst. (1964) Toxikologische Prüfung von Pentachlornitrobenzol. 3.
Auf chronische Toxizität (90-Tage-Test) an Ratten. Unpub. Rept.
prepared and submitted by Farbwerke Hoechst, AG.
Hoechst. (1968) Chronische orale Toxizatätsprüfung von
Pentachlornitrobenzol. 2-Jahres-Versuch an Hunden. Unpub. Rept.
prepared and submitted by Farbwerke Hoechst AG.
Finnegan, J.K., Larson, P.S., Smith, R.B. Jr., Haag, H.B. and
Hennigar, G.R. (1958) Acute and chronic toxicity studies on
pentachloronitrobenzene. Arch. int. Pharmacodyn. 114:38-52
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L., Gart,
J.J., Klein, M., Mitchell, I. and Peters, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in
mice. A preliminary note. J. nat. Cancer Inst. 42:1101-14
Klein, A.K. and Gajan, R.J. (1961) Methods for the determination of
PCNB Residues. JAOAC 44:712
Ko, W.H. and Farley, J.D. (1969) Conversion of
pentachloronitrobenzene to pentachloroaniline in soil and the effect
of these compounds on soil Micro-organisms. Phytopathology 59 (1)
64-7
Kuchar, E.J., Geenty, Frances, O, Griffith, William P. and Thomas,
Raymond J. (1969) Analytical studies of metabolism of Terraclor in
beagle dogs, rats and plants, J. Agric. and Food Chem. 17 (6)
1237-40
Methratta, T.P., Montagna, R.W. and Griffith, W.P. (1967)
Determination of PCNB in crops and soil by electron-capture gas
chromatography. J. Agric. and Food Chem. 15 (4) 648 (July/Aug. 1967)
Mitchell, L.C. (1957) J. Assoc. Offic. Agric. Chemists 40, 294
Mitchell, L.C. (1958) J. Assoc. Offic. Agric, Chemists 41, 781
Olin Mathieson Chemical Corporation. (1969) Research reports on
quintozene
United States of America. (1969) Department of Agriculture Summary of
Registered Agricultural Pesticide Chemical Uses
United States of America. (1968) Department of Health, Education and
Welfare FDA Pesticide Analytical Manual Volume 1 Section 212.1
Wit, S.L., van Esch, G.J. and van Genderen, H. (1957) Toxicity of
some chloronitrobenzen compounds (trichlorodinitrobenzene,
trichloronitrobenzene, tetrachloronitrobenzene and
pentachloronitrobenzene) to laboratory rats and residues
found in crops treated with these fungicides. Proceedings of
the Fourth International Congress of Crop Protection,
Hamburg, September 1957. Vol. 2 (Braunschweig 1960): pp.
1665-8
Zweig, Gunter. (1964) Analytical Methods for Pesticides and Plant
Growth Regulators and Food Additives Vol. III Academic Press. New
York
