
QUINTOZENE
First draft prepared by
D.J. Clegg
Carp, Ontario, Canada
and
A. Moretto
Istituto di Medicina del Lavoro,
Universita degli Studi di Padova, Padua, Italy
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Potentiation
Thyroid function
Studies of metabolites
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Quintozene (pentachloronitrobenzene) was evaluated
toxicologically by the JMPR in 1969, 1973, 1975, and 1977 (Annex I,
references 12, 20, 24, and 28). A temporary ADI of 0-0.001 mg/kg bw
was established in 1969. An ADI of 0-0.007 mg/kg bw was subsequently
allocated by the 1973 Meeting and confirmed by the 1977 Meeting.
Quintozene was re-evaluated by the present Meeting within the periodic
review programme of the CCPR. Data used in previous evaluations were
re-evaluated and new published and unpublished data included.
Quintozene produced in the past was frequently contaminated with high
levels (up to 11%) of hexachlorobenzene (HCB), a pesticide for which
the conditional ADI of 0-0.0006 mg/kg was withdrawn by the JMPR in
1978 (Annex I, reference 30). HCB is both tumorigenic and teratogenic,
and its presence in the older technical-grade quintozene was probably
responsible for the toxic effects that were observed. The present
evaluation of the data on the toxicity of quintozene was therefore
predicated mainly on new data from studies of quintozene containing
less than 0.1% HCB.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Of C57Bl/6 mice given quintozene at oral doses of 500 mg/kg bw
per day on days 7-11 of gestation, 81% had unilateral or bilateral
renal agenesis. When samples of blood, urine, liver, kidney, fat,
placentas, and fetuses were analysed for quintozene, pentachloro-
benzene, pentachloroaniline, pentachlorophenyl sulfide, and HCB, fat
contained the highest concentrations of all compounds. Fetuses
contained higher concentrations of metabolites, particularly methyl
pentachlorophenyl sulfide, than of quintozene; by 24 h after
treatment, quintozene was virtually undetectable, although metabolites
were still present. (Courtney, 1973).
Quintozene containing < 1.0% HCB and < 0.1% pentachlorophenol
was administered to pregnant C57Bl/6 mice as a single dose of
500 mg/kg bw per day on day 18 of gestation (two mice), as four
consecutive doses of 500 mg/kg bw per day on days 7-10 of gestation
(four mice) or as five consecutive doses of 500 mg/kg bw per day on
days 7-11 of gestation (five mice). The mice were killed 6 h after the
single administration and 24 h after the consecutive doses. Combined
fetal and placental tissue and amniotic fluid were analysed by
electron capture and gas-liquid chromatography. Quintozene,
pentachloroaniline, pentachlorophenyl, and HCB were detected at low
levels; the highest concentration was that of methyl pentachlorophenyl
sulfide, at 2.05 ppm. In the mice that received repeated doses, methyl
pentachlorophenyl sulfide was the major component of the residues
(0.84-1.19 ppm) but HCB was present at much higher concentrations
(5.15-8.10 ppm). Thus there was no evidence that quintozene or its
metabolites were concentrated or accumulated in the conceptus, but HCB
was accumulated.
In a further study, C57B1/6 mice received single doses of
500 mg/kg bw per day quintozene (similar grade to that administered to
pregnant mice) and were sacrificed and analysed after 2, 6, 24, or
48 h. The highest concentration of quintozene was seen at 2 h and
decreased with time. Administration of four or five doses did not
result in accumulation of quintozene or of pentachloroaniline or
pentachlorobenzene; peak levels of methyl pentachlorophenyl sulfide
occurred at 24 h. After repeated dosing, the concentrations were lower
than the 24-h peak level. HCB was accumulated, however, particularly
in ovaries; no compound accumulated in testes. The biliary contents of
pentachloroaniline and methyl pentachlorophenyl sulfide were highest
24 h after treatment but had decreased 48 h after single doses.
Repeated doses to pregnant mice resulted in increased levels of methyl
pentachlorophenyl sulfide in bile, but these were considerably lower
than the 24-h level seen after a single dose. The levels of methyl
pentachlorophenyl sulfide in fat peaked 24 h after a single dose. HCB
was accumulated in liver, kidney, bile, and particularly fat, in both
pregnant and non-pregnant mice after single or repeated doses
(Courtney et al., 1976).
Three male (341-363 g) and three female (224-228 g)
Osborne-Mendel rats that had been fasted for 16 h were given
14C-quintozene in cottonseed oil at 4.78-4.92 mg/kg bw (males) or
4.31-4.70 mg/kg bw (females) by oral intubation, providing doses equal
to 10.65-11.34 µCi per animal for males and 6.13-6.81 µCi for females.
Blood samples were obtained from the orbital sinus at 0, 0.5, 1, 2, 4,
8, 12, 24, 36, 48, 60, 72, 96, 120, and 144 h after treatment. Urine
and faeces were collected at 24-h intervals up to 144 h, volume and
weight being recorded for each sample; at term, livers, kidneys, and
gastrointestinal tracts were washed and excised. The carcasses were
frozen. The peak levels of radioabel in whole blood were maximal at
12 h, and the half-life was 21.8 h. Plasma and erythrocytes were
separated at 12, 60, and 144 h, when the ratios of radiolabel in
plasma to that in erythrocytes were 3, 2, and 0.7, respectively.
Urinary excretion at 24 h (expressed as percent of administered dose)
was 3.5-8.4% in males and 13.0-26.8% in females; by 72 h, males had
excreted 7.2-11.7% and females 21.6-36.7%. Low levels were still being
excreted in urine 120-144 h after treatment, males having excreted
7.8-12.3% and females 23.9-38.3% of the administered dose by 144 h.
Faecal excretion 24 h after treatment comprised 2.0-46.0% of the
administered dose in males and 21.3-51.2% in females; by 72 h,
53.1-84.6% had been excreted by males and 37.0-73.2% by females. Low
levels were still being excreted at 144 h, when males had excreted
56.6-90.8% of the administered dose and females 37.9-76.0%. At that
time, measurable amounts were found in liver (0.02-0.04% of the
administered dose; mean, 0.03%), kidney (0.01-0.06%; mean, 0.02%),
gastrointestinal tract washings (0.03-0.20%; mean, 0.08%), and
carcasses (0.12-0.33%; mean, 0.2%). Radiolabel was found in all
carcass samples. The overall percent recovery was 68.4-114%, with an
average of 85% (Adamovics & O'Grodnick, 1978).
Male and female Osborne-Mendel rats were given 5 mg/kg bw
14C-quintozene orally in cottonseed oil. Urine and faeces were
collected at 24-h intervals for 144 h, when livers, kidneys,
carcasses, and gastrointestinal tracts were washed and assayed. The
total 14C activity in urine was 10.5% for males and 33.4% for
females, and that in faeces was 72% for males and 53.7% for females,
indicating a sex difference. In all animals, 0.03% was found in liver,
0.02% in kidney, 0.2% in carcasses, and 0.08% in the gastrointestinal
wash (O'Grodnick et al., 1981).
Four groups of five laying, single-comb white Leghorn hens, 28
weeks of age, received 14C-quintozene at 0, 15.8, 39.4, or 78.9 mg
per hen (50 µCi per day; radiochemical purity, > 93%) in gelatine
capsules for six consecutive days. Body-weight gain, food intake, and
egg production were generally comparable in all treated groups. Hens
were sacrificed about 6 h after the final dose. Eggs and excreta were
collected daily, pooled by dose group and weighed. Radiolabel was
eliminated mainly in the excreta (87-94% of the administered dose)
over the course of the study, and the percent excretion was fairly
constant and comparable in all groups. The residue levels in egg yolk
increased with both dose and time: on day 3 (day 0 being the first day
of dosing), the residue levels were 0.456, 1.05, and 1.91 ppm at the
three doses, respectively; by day 5, the levels were 1.74, 3.52, and
5.75 ppm. The residues in the yolks of eggs removed from the oviduct
at sacrifice had even higher levels. The residues in egg white were
relatively consistent within doses at all intervals. The maximal
residues were 0.075, 0.241, and 0.291 ppm at the three doses,
respectively, representing < 0.01% of the administered dose. In
tissues, the maximal levels were observed in abdominal fat (2.64,
6.17, and 10.1 ppm with increasing dose), kidney (1.84, 5.05, and
7.29 ppm), skin with fat (1.68, 3.75, and 5.92 ppm), blood (0.82,
2.39, and 3.85 ppm), liver (0.87, 2.72, and 3.81 ppm), thigh muscle
(0.13, 0.36, and 0.71 ppm), and breast muscle (0.07, 0.17, and
0.298 ppm). Recovery in samples of excreta, egg yolk, liver, and fat
spiked with the high or low dose was > 96.3% (Daun, 1989).
Goats (strain unspecified) in their second lactation, producing
at least 0.75 kg of milk per day, were treated daily for five days
with 0 (dextrose), 20, or 50 mg/kg bw of admixed 14C-labelled and
unlabelled quintozene in gelatine capsules to give 1000 µCi per goat
per day. Goats were milked twice daily, and all of the urine and
faeces were collected. At sacrifice, about 6 h after the final dose,
blood, kidney, liver, muscle, urine, bile, and the gastrointestinal
tract and its contents were refrigerated (blood) or frozen.
Radiochemical purity (93%) and stability (unchanged over the course of
the study) were determined by thin-layer chromatography (TLC). Body
weights and food intake were not significantly altered. The mean
amounts of fortified samples recovered were 96.1-98.7% in faeces,
98-99.6% in urine, 100.5% in liver, 101.8% in milk, and 94.6% in
blood. The residues in milk of animals given 20 mg/kg bw per day were
3.21-3.88 ppm at the afternoon milking and 1.41-2.04 ppm at the
morning milking, representing 0.3-0.5% of the administered dose (mean,
0.35%). In animals at 50 mg/kg bw per day, afternoon milk contained
6.75-8.63 ppm and morning milk 5.23-8.36 ppm, representing a mean
recovery of 0.41% of the administered dose. Milk residues did not
increase with time. In faeces, 25.7 and 19.2% of the total
administered doses of 20 and 50 mg/kg bw per day was eliminated over
five days, the levels being higher in the morning samples. Urinary
excretion was 33.4 and 38.3% of the administered dose at these doses
throughout the study, again, with higher levels in morning than in
afternoon samples. At the end of treatment, about 1.3% of the
administered dose of 20 mg/kg bw per day and 1.09% of the higher dose
was found in blood, liver, kidneys, muscle, fat, bile, urine, and cage
rinses. The residue levels were 6.3 and 9.8 ppm in blood, 32.1 and
49.1 ppm in kidney, 25.9 and 45.2 ppm in liver, 17.4 and 27 ppm in
omental fat, 18.3 and 32.8 ppm in renal fat, 254 and 448 ppm in bile,
and 229 and 553 ppm in the urinary bladder (Daun, 1990).
Female goats (strain unspecified) received diets containing
2 mg/kg bw (two goats), 30 mg/kg bw (two goats), or 100 mg/kg bw (one
goat) of 14C-quintozene (radiopurity, 99.5%; purity of admixed
unlabelled quintozene, 98%), and one ewe (strain unspecified) received
30 mg/kg bw. Excreta were collected from one goat at 2 and that at
100 mg/kg bw and from the ewe after 72 h, from the two goats at
30 mg/kg bw at 96 h, and from one goat at 2 mg/kg bw at 144 h. After
the dose of 2 mg/kg bw per day, 78.8% had been recovered in urine,
14.0% in faeces, and 1.6% in milk by 144 h; after 72 h, 81.8% was
recovered from urine, 9.7% from faeces, 0.7% from milk, 1.2% from the
gastrointestinal tract and its contents, and 2.4% from the carcass.
After 30 mg/kg bw, 39.6% was recovered in urine, 42.9% in faeces, 0.2%
in milk, 0.8% in the gastrointestinal tract and contents, and 4.7% in
the carcass after 96 h. At 100 mg/kg bw, 37% was recovered in urine,
51.3% in faeces, 0.1% in milk, 1.7% in the gastrointestinal tract, and
3.1% in the carcass after 72 h. In the ewe, which was killed 72 h
after ingestion of 30 mg/kg bw, 64.6% of the administered dose was
found in urine, 25.4% in faeces, I% in the gastrointestinal tract and
contents, and 4% in the carcass. There is thus a tendency for faecal
excretion to increase with increasing dose in goats; however, the low
milk production by goats receiving 30 or 100 mg/kg bw confounds any
significance of excretion via the milk. Bile had the highest
concentrations, followed by fat, liver, and kidney, and the
concentrations appeared to be proportional to the dose (Aschbacher &
Feil, 1983).
(b) Biotransformation
Male rats (strain unspecified) fed quintozene (purity, 97.8%,
containing 1.8% HCB) at dietary levels of 50 or 500 ppm for seven
months, followed by two months on control diet, had only HCB in fat.
The levels of pentachloroaniline were 0.019 and 1.11 ppm and those of
methylpentachlorophenyl sulfide were 0.46 and 4.74 ppm at the two
dietary concentrations, respectively (Kuchar et al., 1969).
No quintozene was found in fat or faeces of male Charles River CD
rats that received 5, 50, or 500 ppm quintozene in the diet for 33
weeks and were then allowed to recover for 60 days, or in skeletal
muscle, liver, or kidney of males that received 500 ppm. No
metabolites were found in fat after the recovery period. The major
metabolite detected was methylpentachlorophenyl sulfide, which was
found in faeces of rats fed 500 ppm quintozene. Pentachloroaniline was
found in tissues of rats at 50 and 500 ppm and in faeces of those at
500 ppm, and pentachlorobenzene was present at low levels (up to
0.3 ppm at the 500-ppm dietary concentration) but was not seen in
faeces after recovery. HCB was present in all samples, with high
levels in muscle (29.7 ppm) and fat (up to 117 ppm) during treatment
at 500 ppm and recovery (22 ppm) (Borzelleca et al., 1971).
Male and female Osborne-Mendel rats received 5 mg/kg bw
14C-quintozene orally in cottonseed oil, and urine and faeces were
collected at 24-h intervals for 144 h. The samples were then extracted
with hexane and, in the case of urine, with methylene chloride and
analysed by gas chromatography and mass spectrometry. Pentachloro-
aniline was the major metabolite in urine, maximal levels being found
24-48 h after treatment. Low levels of pentachlorobenzene, quintozene,
and methylpentachlorophenyl sulfone were also detected in 0-48-h urine
samples. Pentachloroaniline was also the major metabolite in faeces,
maximal levels being found 24-48 h after treatment. Quintozene was
detected in 0-48-h samples, and methylpentachlorophenyl sulfone was
found at low levels in 24-48-h samples. As most of the radiolabel in
urine was not extracted in hexane, a further 10 female rats were given
14C-quintozene, and their urine was extracted with hexane and then
with methylene chloride. The latter extract contained 81% of the
radiolabel in urine. Three radioactive fractions were separated by
high-performance liquid chromatography (HPLC), which were identified
as N-acetyl- S-(pentachlorophenyl)cysteine (59%), pentachlorophenol
(5%), and, after hydrolysis, TLC, and autoradiography, pentachloro-
aniline (O'Grodnick et al., 1981).
Twenty-four-hour urine samples from the rats studied by Adamovics
and O'Grodnick (1978), described above, were combined over 0-120 h and
extracted with hexane. No pentachlorobenzene, HCB, or quintozene was
detected (limit of detection, 0.002 ppm) by gas chromatography, and no
HCB was detected by acid hydrolysis and hexane extraction (limit of
detection, 0.007 ppm). Pentachlorobenzene was detected by gas
chromatography at very low levels in acid-hydrolysed hexane-extracted
urine from females (0.04 ppm), and quintozene was detected in that of
males (0.052 ppm), only in 0-24-h samples. The major metabolite both
with and without acid hydrolysis and hexane extraction was
pentachloroaniline. A sex difference was noted, in that the levels in
male urine were 0.36, 0.21, 0.05, 0.04, and 0.01 ppm and those in
female urine 0.83, 1.03, 0.28, 0.05, and 0.02 ppm at 24, 48, 72, 96,
and 120 h, respectively, with no acidic hydrolysis of urine. A less
pronounced difference was seen when acid-hydrolysed urine was examined
(males: 0.44, 0.48, 0.07 < 0.007, and < 0.007 ppm and females: 0.71,
0.63, 0.05, 0.007, and < 0.007 ppm). Traces of methylpentachloro-
phenyl sulfide were seen in unhydrolysed urine from males at 24 and
48 h and from females at 48 h. In acid-hydrolysed hexane extracts,
traces were seen only in urine from females at 24 h. Faecal samples
contained neither pentachlorobenzene nor HCB. Measurable levels of
quintozene (maximum, 0.273 ppm) were seen at 24 and 48 h, penta-
chloroaniline at all intervals except 120-h urine from females, and
methylpentachlorophenyl sulfide in 48-h from animals of each sex
(maximum, 0.211 ppm). The levels of pentachloroaniline were increased
only in 24-h urine from females.
After recovery, the relative extractability of radiolabel into
hexane before and after acid hydrolysis of urine was determined. In
males, 8.0, 11.2, 16.3, 23.4, and 19.2% was extractable before
hydrolysis at 24, 48, 72, 96, and 120 h; the comparable figures in
females were 5.3, 14.8, 25.5, 25.5, and 26.3%. After acid hydrolysis,
the extractability of male urine was 34.5, 40.0, 25.0, 10.3, and 17.9%
and that of females was 13.9, 19.1, 10.2, 13.3, and 9.1%. The
percentages of radiolabel in aqueous extracts of urine after acid
hydrolysis were 57.5, 48.8, 58.7, 66.3, and 62.9% for males and 80.8,
66.1, 66.7, 61.3, and 64.6% for females at the five intervals,
respectively. Faecal extractability into hexane also differed between
the sexes, being lower in males during the first 48 h, comparable at
72 and 96 h, and lower in females at 120 h (Adamovics & O'Grodnick,
1978).
A further study was performed to identify metabolites that are
not extractable in hexane, i.e. the 50-60% of radiolabel still present
in the urine and the 70-80% still present in faeces. Although not
stated, it is assumed that the urine and faecal samples were obtained
from the same rats as in the previous study. Pooled urine with a pH
adjusted to 13-14 with sodium hydroxide was steam-distilled for 4 h in
a modified Bleidner apparatus with iso-octane as the organic phase.
The distilled aqueous phase was then extracted with ethyl acetate at
both alkaline and neutral pH. Urine samples were collected from male
rats 24-48 h after treatment; faecal samples were collected from
females 0-24 h after treatment and were treated similarly to urine
samples. Metabolites were identified by comparison with standards and
analysed by TLC, dry-column chromatography, and combined gas
chromatography-mass spectroscopy. In the urinary analyses, 22% of the
extractable 14C was found, along with pentachloroaniline, methyl-
pentachlorophenyl sulfide, and small amounts of methylpentachloro-
phenyl sulfone. (The latter was identified only tentatively.) The
aqueous layer, extracted with ethyl acetate after Bleidner
distillation, contained 27% of the extractable 14C, pentachloro-
phenyl, a small amount of a tetrachlorophenol, and a number of
unidentified compounds thought to be methylated phenols. The faecal
metabolites extracted by iso-octane were pentachloroaniline; the
aqueous extracts showed no migration of 14C from the origin
(O'Grodnick, 1978).
Three male and two female Osborne-Mendel rats that had been
fasted for 16 h were given 14C-quintozene at 5 mg/kg bw (10 µCi) in
cottonseed oil. Urine and faeces were collected for 0-24 and 24-48 h
after treatment and were extracted with hexane to remove previously
characterized organic-extractable metabolites (see above). Three polar
metabolites were identified in urine, only two of which are likely to
be real metabolites. The first, pentachlorophenyl- N-acetylcysteine,
was identified by its retention time in reverse-phase HPLC, its Rf
in three TLC systems, and comparison of the Rf of methylated
derivatives of pentachlorophenyl- N-acetylcysteine with that of known
standards. The sulfur analogue of octachlorodibenzo- para-dioxin
(octachlorothianthrene) was identified by mass spectroscopy, but this
is probably an artefact due to fragmentation of a quintozene
metabolite by mass spectrometric pyrolysis (at 350°C) to give a
pentachlorothiophenolate moiety that isomerizes to the octachloro-
thianthrene. This supposition is supported by the solubility of
octachlorodibenzo- para-dioxin in organic solvents and the
dissimilarity in retention times on HPLC and TLC for 14C activity
and thianthrene. (Mass spectroscopy of quintozene indicates a
pentachlorophenylthio moiety in all interrelated mass spectra.) A
third polar metabolite was not identified but may be an intermediate
in the formation of pentachlorophenyl- N-acetylcysteine. It is not a
glucuronide, on the basis of TLC in two solvent systems, failure to
yield a trimethylsilyl derivative on reaction with bis-trimethyl-
silyltrifluoroacetamide, and migration of 14C activity after
diazomethane treatment; it may be pentachlorophenyl cysteine. Faecal
samples extracted in hexane and then submitted to acid hydrolysis and
neutralization were also found to contain two metabolites after
Amberlite XAD-2 column chromatography and ethyl acetate extraction,
with subsequent dry-column chromatography. One was non-polar and had
migration characteristics similar to those of simple quintozene
metabolites such as pentachloroaniline; the other was polar and was
not identified (O'Grodnick, 1979).
In a pilot study with 10 female Osborne-Mendel rats given 5 mg
14C-quintozene (10 µCi per rat) by gavage, urine and faeces were
collected over 0-24, 24-48, and 48-72 h. Radiolabel was measured in
individual urine samples, and composite samples were extracted with
hexane and then methylene chloride after acidification to pH 1 with
hydrochloric acid or with methylene chloride only after acidification.
The methylene chloride fractions were subjected to TLC and HPLC. The
average recovery of radiolabel in urine after 72 h was 32.4 ± 11.7% of
the administered dose. In the extracts obtained with methylene
chloride alone, the major metabolite was N-acetyl- S-penta-
chlorocysteine (59%); pentachlorophenol was also found (5%), but 24%
of the radiolabel could not be identified by comparison with
standards. The extracted N-acetyl- S-pentachlorocysteine was
lyophilized and subjected to mass spectroscopy after refrigeration for
one month or immediately after HPLC. The refrigerated sample yielded a
cluster of Cl6 which corresponded to one of the major fragments of
octochlorothianthrene; this cluster was not seen in the other sample.
The first sample was then methylated with diazomethane, analysed by
mass spectroscopy, and found to be identical to methylated
N-acetyl- S-pentachlorophenyl cysteine. Mass spectroscopic analysis
of commercial pentachlorothiophenol, a metabolite of quintozene,
showed the presence of octachlorothianthrene. These data support the
hypothesis that the presence of octachlorothianthrene after mass
spectroscopy of quintozene metabolites is due to the procedure in
which N-acetyl- S-pentachlorophenol cysteine degrades to
pentachlorothiophenol, which then dimerizes to octochlorothianthrene.
The second major urinary metabolite appears to be a conjugate of
pentachloroaniline, since that compound is released by acid hydrolysis
(Adamovics, 1980).
Rabbits were given quintozene (purity unspecified) as an aqueous
suspension at doses of 1, 2, or 3 g by stomach tube. Anorexia was
seen. Urine was collected over 72 h after treatment, and each 24-h
sample was analysed. Pentachloroanilene comprised an average of 12-14%
of each of the administered doses, and N-acetyl-3-pentachloro-
phenyl-L-cysteine represented 5,14, and 4% of the three doses,
respectively; pentachlorphenyl was not detected. Faecal analysis
indicated absorption of 54, 38, and 41% of the doses (Betts et al.,
1955).
Tissues from three male beagle dogs fed quintozene (purity,
97.8%; containing 1.8% HCB, 0.4% 2,3,4,5-tetrachloronitrobenzene, and
< 0.1% pentachlorobenzene) for two years at dietary concentrations of
5 or 1080 ppm were collected, frozen, and analysed by gas chroma-
tography. Quintozene was not detected in muscle, kidney, fat, or liver
and was found in only small amounts (< 0.004 ppm) in the urine of
dogs at the high dose 24 h before sacrifice. In faeces taken at this
time, quintozene was found at 0.059 ppm in dogs at the low dose and at
14.1 ppm in those at the high dose. Pentachloroaniline was detected in
fat, liver, urine (> 1 ppm), and faeces (16.7 ppm in dogs at
1080 ppm). Methylpentachlorphenyl sulfide was found in urine
(< 0.001 ppm in dogs at both doses), muscle (0.227 ppm at the high
dose), liver (0.039 at the low and 0.322 ppm at the high dose), kidney
(1.08 ppm at the high dose), fat (0.03 at the low and 2.5 ppm at the
high dose), and faeces (0.134 at the low and 3.64 ppm at the high
dose). The sensitivity of the method was about 0.005 ppm (Kuchar
et al., 1969).
After two years' feeding of male dogs on diets containing 0, 5,
30, 180, or 1080 ppm quintozene, the compound was detected in faeces
at levels increasing from 0.2 to 16.7 ppm with dose; low levels were
also seen in urine (traces at 30 and 180 ppm and 0.004 ppm at the
highest dose). Methylpentachlorophenyl sulfide was the major
metabolite found in all tissues (kidney, brain, muscle, liver, spleen,
bile, fat, blood, faeces, and urine). Since low levels were noted in
some control tissues, the residues seen in brain, fat, and blood of
dogs at 5 ppm can be considered to be minimal or absent. Residues were
found in kidneys only in dogs at doses > 180 ppm. Spleen and bile,
which contained the highest levels of residues, were analysed only
from dogs at 1080 ppm. The levels in urine were comparable to those of
controls, but residues were present in faeces of dogs at all doses.
Pentachloroaniline was not detected in brain, skeletal muscle, or
spleen, but 0.018 ppm was seen in the kidneys of one dog at 180 ppm.
The levels in liver (0.039-0.057 ppm) were inconsistent, the lowest
being seen in dogs at the high dose. Bile had the highest
concentration, 1.92 ppm. The levels in fat (0.64 ppm) and blood
(0.008 ppm) were increased only in dogs at the highest dose. Faecal
levels increased with dose, reaching 16.7 ppm at the highest dietary
concentration; the low levels seen in urine also increased with dose,
from 0.002 at 5 ppm to 0.092 at 1080 ppm. Pentachlorobenzene was
detected in all tissues but not in urine, the levels increasing with
dose; the highest level, 5.12 ppm, was found in the fat of dogs fed
the highest dose. HCB was found in all treated animals, with the
highest level, 194 ppm, in the fat of dogs at the highest
concentration (Borzelleca et al., 1971).
Rhesus monkey were given 14C-labelled quintozene (free of HCB
and radiochemically pure) at single oral doses of 2 mg/kg bw (six
males and two females) or 91 mg/kg bw (one female) or daily doses of
2 ppm for 71 days (two monkeys of each sex). Sample extracts were
purified by column chromatography on silica gel and 400 ml each of 1:1
(v/v) benzene/chloroform, chloroform, acetonitrile, and methanol, and
further purified by TLC with hexane or (for tissues from animals at
the single dose of 2 mg/kg bw) 10% acetone in n-hexane. Zones from
the TLC plates were washed with methanol and analysed by gas-liquid
chromatography and mass spectroscopy. Quintozene and seven metabolites
were identified by comparison with standards, the metabolites being
pentachloroaniline, pentachlorobenzene, pentachlorophenyl, penta-
chlorothioanisole, pentachlorothiophenol, 2,3,4,5-tetrachloroaniline,
and methylated 2,3,5,6-tetrachlorophenol. Six further metabolites,
tetrachlorothioanisole, tetrachloroaminothioanisole, tetrachloro-
aminophenylmethyl sulfoxide, tetrachlorophenylmethyl sulfoxide,
bis-methylmercaptoaminotrichlorobenzene, and bis-methylmercapto-
tetrachloro-benzene, were characterized by their retention times and
mass spectra, but their identity was not verified by comparison with
synthesized compounds. In all treated animals, urinary and faecal
excretion of pentachloroaniline predominated, accounting for 36-55.4%
of the urinary extracts and 66-70.6% of the faecal extracts.
Pentachlorophenyl accounted for only 12.2-17.5% of the extract in
urine; pentachlorobenzene accounted for about 11% of urinary extracts
and 0.5-1.1% of faecal extracts; pentachlorothioanisole accounted for
9.7-10% of the extract in urine and 6.0-6.2% of the extract in faeces;
and bis-methylmercaptotetrachlorobenzene accounted for 9.2-9.8% of
urinary extracts and 7.1-9.3% of faecal extracts. The metabolites were
found mainly at levels of 0.01-1 ppm, whereas quintozene was found in
faeces at 12.9-16.3% (Kögel et al., 1979a).
In an extension of this study, an additional female monkey was
given 0.5 mg of 14C-quintozene in ethanol by stomach tube. Blood
samples were taken at 30-min intervals for 3 h, hourly from 3 to 7 h
after treatment, and then every 24 h until day 5. Urine and faeces
were collected daily. Two males in the previous study given the single
dose of 2 mg/kg bw were sacrificed at 24 and 48 h, respectively, and
autopsied, and urine and faeces were collected for 14 days from the
remaining animals. Urine and faeces were collected for 20 days from
the female given the single dose of 91 mg/kg bw in oil suspension, and
blood samples were taken on days 1, 2, 8, 10, 16, 18, 21, and 23 after
treatment. One monkey of each sex given 2 ppm for 71 days was killed
at the end of exposure and the remainder after nine days on normal
diet. The concentrations of quintozene and/or its metabolites in
tissues after the single dose of 2 mg/kg bw were highest in bile, with
280 ppm at 24 h and 14.4 ppm at 48 h; in the liver, 2.3 ppm was found
at 24 h and 0.5 ppm at 48 h; decreasing levels were seen at both times
in kidney, thymus, bone marrow, plasma, adrenal cortex, erythrocytes,
muscle, heart, and brain. Lymph nodes and fat both had 0.3 ppm at 24 h
but 0.4 and 0.6 ppm, respectively, at 48 h. The concentrations of
quintozene and its metabolite in tissues after 71 days of dietary
exposure to 2 ppm were lower in females than in males; the order of
concentrations in tissues remained similar, but the fat, thymus, bone
marrow, and adrenal cortex contents were slightly increased.
Accumulation was minimal and nonsignificant. A plateau level of
storage was attained after 3040 days (Kögel et al., 1979b).
In the experiment described above, Aschbacher and Feil (1983)
isolated the following radiolabelled compounds from urine after
pyrolysis, extraction, various forms of chromatography (e.g. TLC,
HPLC), and mass spectrometry: pentachloroaniline, tetrachloroaniline
(trace), tetrachloroaminophenol, pentachloroaniline sulfamate,
glucuronide conjugates, and, at higher doses, N-acetylcysteine
conjugates, pentachlorothiophenol, pentachlorothioanisole, and
bis-methylthiotetrachlorobenzene. The latter compound was not detected
in the ewe, but tetrachloro(methylthio)thiophenol was observed.
Pentachloroaniline and quintozene were found in faeces. Unextractable
residues were also present. The metabolism appeared to follow two
general pathways, one involving reduction of the nitro group to an
amine and formation of secondary metabolites (which appears to be the
principal route at low doses) and one involving replacement of the
nitro group with a sulfur-containing group (e.g. a thio, methylthio
glucuronide, or N-acetylcysteine), usually at higher doses.
A lactating goat (strain unspecified) was given 50 mg uniformly
ring-labelled (at 1040 dpm/µg) 14C-quintozene, for five consecutive
days. On day 2 of treatment, urine was passed through a reverse-phase
C-18 cartridge, eluted with methanol, and analysed by HPLC. Four
metabolites were identified by mass spectroscopy as pentachloroaniline
sulfamate (85%), N-hydroxylated pentachloroaniline (6%),
tetrachlorothioanisole (5%), and a pentachloroaniline conjugate,
probably either a cysteinyl or mercapturic acid conjugate (4%).
Kidneys, which contained 49.1 ppm 14C activity calculated as
quintozene, were extracted with chloroform, methanol, and water, and
the remaining pellet was treated with a protease. The chloroform
extract contained 46% of the radiolabel, the water contained 28%, and
the pellet released 1.9% (of 26%) of its radiolabel. Six renal
metabolites were identified in the chloroform fraction and two of them
also in the water fraction after HPLC and mass spectroscopy as
pentachloroanisole and pentachloroanisole glucuronide (total, < 80%),
pentachlorothiophenol, tetrachloro(methylthio)thiophenol, tetrachloro-
thioanisole, and tetrachloroaniline methyl sulfoxide. In liver
extracted by the same methods, 24% of total 14C was found in
chloroform and 20% in water; the 14C in the pellet was soluble in
water after treatment with protease. Six metabolites were identified
in the chloroform fraction and two of them also in the water fraction
after HPLC and mass spectroscopy as pentachloroaniline, pentachloro-
aniline glucuronide (major metabolites), pentachlorothiophenol dimer,
N-hydroxypentachloroaniline, pentachlorothiophenol, and
tetrachloro(methylthio)thiophenol. Milk and omental and renal fat
contained only pentochloroaniline (McManus, 1989). After similar
treatment, extraction, and identification techniques, goat muscle was
shown to contain 2.22 ppm calculated as quintozene, 46% pentachloro-
aniline, 11% pentachlorothiophenol, and 42% tetrachlorothioanisole and
tetrachlorophenyl methyl sulfoxide (McManus, 1990).
A Holstein cow was given the equivalent of 5 ppm quintozene
dissolved in ethanol in a grain supplement, the level administered
being based on a daily ration of 22.7 kg per cow, for three
consecutive days. Morning and evening milk samples were combined and
analysed one day before treatment, daily during treatment, and for
three days after treatment. Urine was collected over the test period.
Quintozene was determined by electron affinity gas chromatography,
with a sensitivity of about 0.01 ppm. No parent compound or
chlorinated metabolites were found in milk, but peaks identical to
pentachloroaniline and accounting for 45% of the administered dose
were detected in urine. No mercapturic acid metabolites were found in
acetone extracts of milk and urine evaporated and methylated with
boron trifluoride-methanol and then extracted with hexane
(St John et al., 1965).
Groups of three cows (strain unspecified) were given quintozene
in capsules at doses equivalent to 0, 0.1,1, or 10 ppm in the diet for
12-16 weeks. Fat biopsies were taken from one cow at each dose at
weeks 0, 1, 2, 4, 7, and 8; milk was collected twice a day and
analysed on days 0, 1, 7, 14, 21, 28, 35, 42, 49, and 56. At term,
subcutaneous and abdominal fat, muscle, liver, and kidney were
analysed. Quintozene was not detected consistently in fat biopsies or
tissues; it was found at concentrations of 0.001-0.006 ppm in cows at
all doses. Since the highest levels were seen at the lowest dietary
concentration and the levels were variable, contamination may have
accounted for all of the quintozene detected. It was concluded that
quintozene is not stored and is not excreted in the milk.
Pentachloroaniline was found consistently in the fat and milk (at
0.003-0.006 ppm) of cows given 10 ppm but inconsistently (in two of
six analyses) and at very low levels at lower doses (Borzelleca
et al., 1971).
Metabolites were identified in the samples taken from white
Leghorn hens in the study of Daun (1989) described above. Excreta were
centrifuged, and the supernatant was filtered and subjected to HPLC
and mass spectroscopy. Pentachlorothiophenol comprised 30% of the
radiolabel, S-(pentachlorophenyl)thiopyruvate accounted for 26.4%,
S-pentachlorphenyl thioacetate for 16.8%, pentachlorothiophenol
malonylcysteine for 19.1%, and two unidentified metabolites for 5.4
and 2.0%. Tissue samples were examined for metabolites after
combustion and extraction with methanol-water and chloroform. Solids
were treated with protease to release bound residues, which accounted
for 39% of the activity; all bound residues were released after
overnight digestion with 1N sodium hydroxide at 60°C, but similar
treatment with 2N hydrochloric acid freed only 16%.
The fat of hens at the high dose was extracted by blending
combusted samples with chloroform, evaporation, and redissolution in
hexane, and partitioning with acetonitrile. The last sample contained
91.3% of the radiolabel. The single HPLC peak was then examined by gas
chromatography and mass spectroscopy with chemical ionization using
methane. Five chlorine-containing compounds were detected and
identified as quintozene (48.3% of the radiolabel in the fat),
pentachloroaniline (16.1%), tetrachloroaniline methyl sulfoxide (31%),
tetrachlorothioanisole (0.6%), and an unknown metabolite apparently
with a three-chlorine cluster. Chloroform-extractable metabolites in
kidney were separated on HPLC and characterized by mass spectroscopy
as S-(pentachlorophenyl)cysteine (54%) and tetrachlorothioanisole
sulfone (46%). A methanol extract purified by C-18 solid-phase
extraction followed by HPLC and mass spectroscopy contained 40%
S-(pentachlorophenyl)-thioacetate and 60% N-hydroxy pentachloro-
aniline. Solid-phase extraction of chloroform and methanol-water
extracts on C-18, fractionation by HPLC, and characterization by mass
spectroscopy indicated the presence of S-pentachloropenylthioacetate
(7%) and pentachloro-thiophenyl in the chloroform extract and the
latter metabolite in the methanol-water extract (70%); the metabolites
in the two solvents comprised 71% of the residue. Pentachlorothio-
anisole sulfoxide (30%) was also seen in the methanol extract. The
metabolites in egg yolk were pentachlorobenzene (4%), pentachloro-
nitroaniline (70%), pentachlorothiophenol (18%), and pentochloro-
thioanisole (37.6%). Thigh muscle extracted with chloroform purified
on a silica cartridge contained 87% of the radiolabel, 88% as either
tetrachlorothioanisole sulfone or S-(pentachlorophenyl)thioacetate.
Two unidentified metabolites were present at 0.4 and 1.8%; another,
present at 8.3%, was tentatively identified as pentachlorothioanisole
on the basis of its retention time. The metabolites in skin fat were
the same as those seen in abdominal fat. The levels of metabolites in
breast muscle were insufficient to permit isolation and identi-
fication. Most of the metabolites thus appear to be tissue-specific;
however, pentachloroaniline was found in fat and egg yolk,
pentachlorothioanisole in thigh muscle and egg yolk, pentachloro-
thiophenol in liver, egg yolk, and excreta and S-(pentachloro-
phenyl)thioacetate in liver, kidney, excreta, and possibly thigh
muscle (Parkins, 1990).
In the same hens, bound residues were found in egg yolk (75.3% of
the total 14C activity), kidney (32.8%), liver (35.2%), breast
(51.3%), and thigh (25.9%). Hydrolysis with 1N sodium hydroxide at
600°C for 16 h was most effective in releasing bound residues, freeing
104% in liver, 79.9% in kidney, 75.4% in thigh muscle, 64.8% in breast
muscle, and 38.2% in yolk; in the kidney, however, pepsin and
bacterial protease released more bound 14C than basic hydrolysis,
presumably by cleavage of conjugated metabolites. Attempts to cleave
glyceride esters in yolk with lipase yielded only 5% hydrolysis. Use
of Raney nickel to release bound residues in kidney and yolk resulted
in 7 and 10%, respectively, indicating that bound residues in these
tissues are trapped within the biological matrix rather than
conjugated. When basic hydrolysates of kidney, liver, and egg yolk
were acidified, partitioned with chloroform, purified by florisil
solid-phase extraction, and examined by gas chromatography-mass
spectroscopy, the only metabolite found in kidney was pentachloro-
aniline; pentachloroaniline and pentachlorothioanisole were found in
liver, with trace amounts of pentachlorobenzene, HCB, quintozene, and
tetrachlorothiophenol. As most of these compounds do not have
functional moieties for conjugation, the hypothesis of biological
entrapment is strengthened. In egg yolk, the major bound residue was
pentachlorothioanisole, with trace amounts of pentachlorobenzene, HCB,
quintozene, and pentachloroanisole. The trace levels of pentachloro-
benzene and HCB in liver and egg yolk are not due to metabolism
of quintozene. The quintozene used in these studies contained 0.8%
HCB and 0.08% pentachlorobenzene and was stated to have been produced
before imposition of the 0.1% HCB limit (Parkins, 1991).
The proposed metabolic pathway of quintozene in animals is shown
in Figure 1.
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of quintozene are
shown in Table 1.
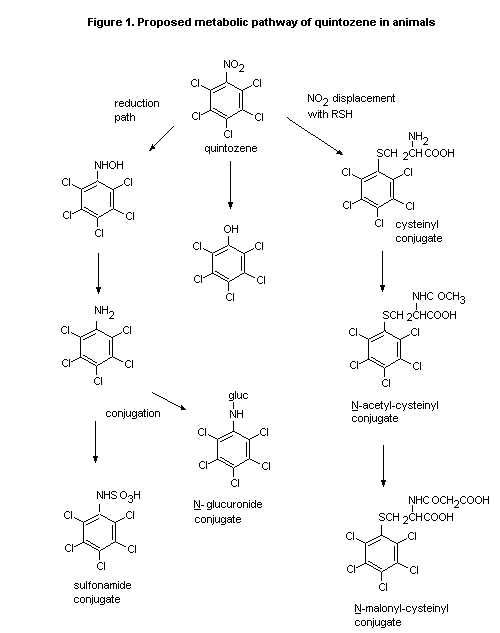 Table 1. Acute toxicity of quintozene
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Rat Male, female Oral > 5000a 99.4 Warshawsky (1994a)
Rat Oral > 30 000 NR Wit et al. (1960)
Rat Male, female Oral 1650 (male) 98.2 Finnegan et al. (1958)
1710 (female)
Rat NR Intraperitoneal 5000 NR Wit et al. (1960)
Rat Male, female Inhalation 1.7b 99.4 Hilaski (1994)
Rabbit Male Oral Erraticc 98.2 Finnegan et al. (1958)
Rabbit Male, female Dermal > 5000 99.4 Warshawsky (1994b)
Rabbit NR Dermal > 4 g 99.7 (1.8% Borzelleca et al. (1971)
HCB
Dog NR Oral > 2500 98.2 Finnegan et al. (1958)
75% wettable powder as 40% aqueous solution
Rat Male, female Oral 16 g (equal to NR Finnegan et al. (1958)
12 g/kg bw
quintozene)
NR, not reported
a Decreased defaecation at all doses; decreased activity at 1300, 1700, and 2000; soft stools at 2000
and 5000; and anogenital staining at 1700, 2000, and 5000; full recovery by day 6
b Mass median aerodynamic diameter, 3.6 µm; standard geometric deviation, 1.9; aerosol concentration,
1.3-2.2 mg/litre; air flow, 38 litres/min; decreased activity, increased salivation, and rapid
breathing
c Mortality rates: 0 at 350 mg/kg bw per day, 2 at 500 mg/kg bw per day, 1 at 650 mg/kg bw per day,
0 at 800 mg/kg bw per day, 3 at 1100 mg/kg bw per day, and 3 at 1400 mg/kg bw per day
(b) Short-term toxicity
Mice
Groups of 10 B6C3F1 mice of each sex, eight to nine weeks old,
were fed diets containing quintozene (purity, 99.6%; HCB content,
0.07%) at concentrations of 0,1250, 2500, 5000, 10 000, or 20 000 ppm
(males) and 0, 2500, 5000, 10,000, 20 000, or 40 000 ppm (females) for
13 weeks. Five animals per cage were checked twice daily, food
consumption was measured weekly by cage, and individual body weights
were recorded weekly. All mice were necropsied, livers were weighed,
and gross lesions, tissue masses, mandibular lymph nodes, mammary
glands, skin, salivary gland, sternebrae, thyroid, parathyroid, small
intestine, colon, liver, prostate, testis, ovary, uterus, lung and
bronchi, heart, oesophagus, stomach, brain, thymus, trachea, pancreas,
spleen, kidneys, adrenal, urinary bladder, pituitary, spinal cord (if
neurological signs occurred), and eyes (if grossly abnormal) were
examined histopathologically. All females at 40 000 ppm died during
the study. The final mean body weights in animals at 10 000 and
20 000 ppm were 7 and 8% lower than those of controls for males and 5
and 8% for females, respectively. Scattering of food was frequent, but
mice at the high dose may have consumed more food than controls.
Absolute liver weights were increased significantly in males at 1250,
2500, and 5000 ppm and in females at 2500, 5000, and 10 000 ppm.
Liver:body weight ratios were significantly increased in males at
doses > 2500 ppm and in females at > 5000 ppm. Clinical signs
were limited to small body size and emaciation of all males at
20 000 ppm and all females at 20 000 and 40 000 ppm. No
histopathological changes were seen in male mice, but females at
40 000 ppm had lymphoid depletion of the spleen, mesenteric lymph
nodes, or thymus. Effects in the lungs of all mice were consistent
with Sendai vital infection. There was no NOAEL since absolute liver
weights were increased at all doses (US National Toxicology Program,
1987).
Rats
An unspecified number of young rats (strain unspecified) were fed
diets containing 0 or 2000 ppm quintozene for 10 weeks. No gross
effects were noted, other than a decreased growth rate in males
(Wit et al., 1957).
Groups of five rats (strain unspecified) of each sex were fed
diets containing a 20% dust formulation of quintozene for 13 weeks to
give doses of 0, 0 (dust minus quintozene), 63.5, 635, 1250, 2500, or
5000 ppm. Animals were weighed weekly, and erythrocyte, haemoglobin,
and leukocyte determinations were performed at term. The liver,
kidney, and testis were weighed, and the heart, lung, liver, kidney,
spleen, stomach, small intestine, caecum, large intestine, thyroid,
adrenals, pancreas, and gonads were examined histologically. Rats at
5000 ppm lost weight during the first two weeks and were sacrificed in
poor condition at the start of week 3, although only one male had
died. Body weights were reduced to a statistically significant degree
in males and nonsignificantly in females at 2500 ppm. One male at 0
(powder control), one at 63.5 ppm, and one at 1250 ppm died.
Haematological parameters were not altered (no data reported). The
liver:body weight ratios were significantly increased in all treated
animals, except for females at 63.5 ppm. The kidney:body weight ratios
were increased in males at 1250 and 2500 ppm. 'Fine vacuolation' of
liver cytoplasm was seen in rats at 5000 ppm. There was no NOAEL
(Finnegan et al., 1958)
Groups of 10 male and 10 female rats (strain unspecified) were
fed diets containing quintozene at 0, 1000, 5000, or 10 000 ppm for 90
days. Growth was inhibited slightly at 5000 ppm and markedly at
10 000 ppm. The NOAEL was 1000 ppm, equivalent to 50 mg/kg bw per day
(Hoescht AG, 1964).
Four groups of 15 Charles River CD rats of each sex, seven weeks
of age, were fed diets containing quintozene (purity, 99.5%) at
concentrations of 0, 50, 3000, or 6000 ppm for 13 weeks. The average
intakes were 3.07, 187, and 381 mg/kg bw per day for males and 3.69,
223, and 455 mg/kg bw per day for females. Diets were analysed for
homogeneity (10 samples per dose), stability (on days 0 and 10), and
concentration (10 samples at weeks 1 and two samples at 2, 3, 4, 8,
and 12 weeks). The homogeneity of eight samples of diet containing
50 ppm was 95-108%, with two outlyers at 117 and 118%; the homogeneity
of 10 samples of the diet containing 3000 ppm was 92-101%, and that of
10 samples of diet at 6000 ppm was 93-98%. Duplicate analyses of
stability showed it to be within 10%. The concentrations of quintozene
in the diets were 98% (range, 91-103%) at 50 ppm, 94% (91-97%) at
3000 ppm, and 92% (85-95%) at 6000 ppm. Rats were observed twice a day
for toxic signs, morbidity, and mortality; food intake and body weight
were recorded weekly. Erythrocyte count, haemoglobin level,
haematocrit, total and differential leukocyte counts, platelet count,
reticulocyte count, mean corpuscular volume, mean corpuscular
haemoglobin, and mean corpuscular haemoglobin concentration; sodium,
potassium, chloride, calcium, inorganic phosphorus, alkaline
phosphatase, total bilirubin, aspartate aminotransferase, alanine
aminotransferase, creatine phosphokinase, urea nitrogen, creatinine,
total protein, albumin, globulin, cholesterol, and serum glucose; and
the colour, appearance, volume, specific gravity, microscopic
elements, pH, protein, glucose, ketones, bilirubin, occult blood,
nitrite, and urobilinogen in urine were reported for 10 rats of each
sex at each dose at week 13. Blood samples from the orbital sinus and
urine were collected during a fasting period. Ophthalmoscopic
examinations were performed on all rats before and on day 86 of the
study. The adrenals, heart, brain, kidney, liver, and gonads of all
rats were weighed post mortem. All animals were examined
macroscopically, and about 40 tissues were preserved. All tissues from
controls and from rats at the high dose and the livers, kidneys, and
lungs of animals at the low and middle doses were examined
microscopically. Lesions observed grossly were also examined.
There were no deaths during the study. Several minor signs (e.g.
hair loss and scabbing) occurred at low incidences but were not
dose-related. Malocclusion was seen in 1, 0, 6, and 4 males at the
four doses, respectively, but is unlikely to have been related to
treatment. Decreased body weight was noted in animals of each sex at
3000 and 6000 ppm and was statistically significant in males at
6000 ppm and in females at 3000 and 6000 ppm. Slightly decreased
terminal weight was seen in animals of each sex (by 8.1 and 6.1% in
males and females at the high dose, respectively) and was dose-
related. Body-weight gain was decreased by 16% in animals of each sex
at 6000 ppm and by 11 and 13% in males and females, respectively, at
3000 ppm. Food intake was reduced significantly in animals of each sex
at 6000 ppm during week 1 and sporadically in weeks 3, 4, and 8 in
males and week 5 in females. A consistent, nonsignificant reduction in
food intake was seen throughout the study in animals of each sex,
except in females at weeks 2 and 4; the food intake of males at
3000 ppm was significantly reduced only in week 1 but was consistently
less than that in controls except in week 10. Females had a
statistically significant reduction in food intake in weeks 5, 6, and
7 and a nonsignificant reduction at other times.
Ophthalmological examination showed no compound- or dose-related
effects, and no adverse effects were observed on haematological
parameters. Reduced alanine aminotransferase levels were seen in
animals of each sex at 3000 and 6000 ppm. In females, total protein
was increased at all doses, albumin was increased at 3000 ppm, and
globulin at 3000 and 6000 ppm; the cholesterol level was significantly
increased at 50 and 6000 ppm. In males, serum alkaline phosphatase
activity was increased at 50 ppm only; no changes were seen in serum
protein levels. The individual total protein levels in females were
greater than the range of levels in the controls in one rat per dose.
A similar pattern was seen for albumin and globulin, except that the
globulin level in two animals at 6000 ppm exceeded the maximal level
in controls. Although these mean values are statistically significant,
the effect was minimal and unlikely to be of toxicological
significance. The increase in cholesterol levels was seen at all doses
but was significant only at 50 and 6000 ppm. The changes were limited
to one sex, and the biological significance of this observation is
uncertain. The pH of the urine of males at 3000 and 6000 ppm was
lowered, but the values were within normal limits for rats. Other
parameters, e.g. a high ketone level in one male rat at 6000 ppm and
low levels of glucose (0.1 g/dl) in one male per dose, showed wide
individual variations and were not considered to be of toxicological
significance.
The absolute kidney weight was increased in males at 3000
(statistically significant) and 6000 (statistically nonsignificant)
ppm, and the kidney weights relative to those of the body and brain
were increased significantly in males at 3000 and 6000 ppm. The
absolute kidney weights were decreased in females at 50 ppm, and the
weights relative to body weight but not brain weight were
significantly decreased at 50 ppm and increased at 6000 ppm. The
apparent effect on the kidney:body weight ratio is. probably a
reflection of decreased body weight at 6000 ppm. The absolute liver
weights were nonsignificantly increased in animals of each sex at 3000
and 6000 ppm, the effect being dose-related in females. The liver:body
weight ratios were significantly increased in males at 3000 and
6000 ppm and in females at 6000 ppm, probably reflecting decreased
body weight. The liver:brain weight ratios were nonsignificantly
increased in animals of each sex at 3000 and 6000 ppm. Changes in the
weight of the brain relative to body weight in males at 3000 and
6000 ppm and of the heart relative to body weight in males at 6000 ppm
were also attributed to reduced body weights.
Males had increased incidences of chronic nephritis (1, 6, 5, and
4 out of 15 animals at 0, 50, 3000, and 6000 ppm, respectively), which
were not dose-related, and an increased incidence of mild haemorrhage
in mesenteric lymph nodes (4 at 6000 ppm; 0 in controls), but these
effects were unlikely to be related to treatment. Mandibular lymph
node haemorrhages occurred in 5/15 control and 0/15 male rats at
6000 ppm, and mediastinal lymph node haemorrhages were seen in 3/15
control and 1/15 males at 6000 ppm. Low incidences of haemorrhage seen
in medias final lymph nodes of seven females at 6000 ppm and three
controls and of mesenteric lymph nodes in six females at 6000 ppm and
no controls may indicate a slight effect, the significance of which is
not known. The only effects in liver were seen at 6000 ppm, comprising
hepatocellular hypertrophy in 7/15 males and 8/15 females and in none
of the controls. The NOAEL was 50 ppm, equal to 3.07 mg/kg bw per day,
on the basis of minimal changes in body-weight gain and liver weight
and changes in alanine aminotransferase activity at higher doses
(McGee, 1988).
Four groups of six acclimatized Charles River CD rats of each
sex, with initial body weights of 247-265 g for males and 229-244 g
for females, were treated on clipped dorsal skin, 6 h per day for 21
days with quintozene (purity, 98.72%) applied as a paste in deionized
water at 0, 30, 300, or 1000 mg/kg bw per day. The application site
was wrapped in gauze bandaging secured with non-irritating tape. After
each application the test site was washed with tepid water. Rats were
observed for mortality, moribundity, clinical signs, and behaviour
twice daily, and irritation was scored by the Draize method once
daily. Body weight was recorded before treatment and then weekly, and
food intake was measured weekly. Haematological and clinical chemical
tests were done at termination on blood obtained from the orbital
sinuses before fasting, and urine samples obtained during fasting were
analysed. The haematological parameters measured included erythrocyte
counts, haemoglobin, haematocrit, total and differential leukocyte
counts, mean corpuscular volume, mean corpuscular haemoglobin, mean
corpuscular haemoglobin concentration, and platelet counts. The
clinical chemical parameters measured were sodium, potassium,
chloride, calcium, inorganic phosphorus, alkaline phosphatase, total
bilirubin, aspartate and alanine aminotransferases, lactic
dehydrogenase, creatine phosphokinase, urea nitrogen, creatinine,
total protein, albumin, globulin, the albumin:globulin ratio,
cholesterol, and glucose; and the urinary parameters were colour,
appearance, volume, specific gravity, microscopic elements, pH,
protein, glucose, ketones, bilirubin, occult blood, nitrite,
urobilinogen, and leukocyte incidence. All rats were examined
macroscopically post mortem; kidneys, liver, and testes were
weighed, and 44 organs or tissues per rat were preserved, as were
gross lesions seen at macroscopic examination. Microscopic examination
was limited to liver, kidney and treated and untreated skin in animals
at 0 and 1000 mg/kg bw per day, and all gross lesions.
There were no deaths. Ventral hair loss was seen occasionally in
females, but the incidence was not dose-related. No behavioural
changes and no signs of dermal irritation were detected. No
compound-related changes in body weight were seen, although increased
body-weight gain was noted in males at 30 mg/kg bw per day
(statistically nonsignificant). Food intake was comparable in all
groups. There were no statistically significant changes in any
haematological parameters, and clinical chemistry parameters were
unaffected, except for a statistically significant, dose-related
decrease in alanine aminotransferase activity in males and in females
at 1000 mg/kg bw per day; a statistically significant decrease was
also seen at 1000 mg/kg bw per day, but there was no effect at lower
doses. Other changes (increased alkaline phosphatase activity and
decreased total bilirubin in females at 300 mg/kg bw per day) were not
dose-related. There were no significant changes in urinary parameters.
Organ weights also showed no statistically significant changes,
although testicular weights were reduced in two of five rats at
1000 mg/kg bw per day; these testes were not examined microscopically.
There were no compound- or dose-related microscopic effects on the
liver, kidney, or treated or untreated skin. The NOAEL was 300 mg/kg
bw per day (Goldenthal, 1992).
Dogs
Three groups of three mongrel dogs were fed dietary levels of 25,
200, or 1000 ppm quintozene for one year by mixing a commercial powder
containing 20% quintozene, 77% pyrax ABB (a pyrophyllite carrier), and
3% Armour sticker with the diet, which was mixed with water
immediately before feeding. Dogs were weighed weekly; haematological
parameters (unspecified but including total and differential leukocyte
counts) were measured at 0, 6, and 12 months; and the heart, lung,
liver, kidney, spleen, gastrointestinal tract, thyroid, adrenal,
pancreas, gonads, and bone marrow were examined histologically. One
dog at 200 ppm whelped at week 5 of the study, and two dogs at
1000 ppm whelped at weeks 7 and 8. Some pups were allowed to suckle
and were then sacrificed at four to six weeks of age and examined
histopathologically. There were no deaths. Body weight was variable
but apparently unaffected by quintozene. The only effects on
haematological parameters were increased eosinophil counts at 6 and 12
months in some animals (probably due to parasites) and low total
leukocyte counts at 12 months in dogs at 10 000 ppm. No lesions were
seen in the pups examined histopathologically. Hepatic-cell
enlargement with pale cytoplasmic staining was noted in all adult
dogs, but the severity was not dose-related. Periodic acid-Schiff and
Best carmine staining of the liver showed abundant glycogen around the
periphery of lobules. Staining for fat gave negative results. There
was no NOAEL, owing to the random whelping (Finnegan et al., 1958).
Four groups of three beagle dogs (Ridglan Farms, Inc.),
previously immunized and vaccinated against most common viruses and
infections, were fed diets containing 0, 40, 2000, or 4000 ppm
quintozene (purity, at least 96%) for four weeks. The diets were
prepared weekly, acetone being used as an aid to dispersion at 40 ppm
and also added to control diets. Homogeneity and stability were
measured in week 4, and the concentration of quintozene was measured
in the diets for weeks 2, 3, and 4. The homogeneity in 10 samples was
91-99% (mean, 95%) at 40 ppm, 92-103% (mean, 98%) at 2000 ppm, and
95-104% (mean, 98%) at 4000 ppm. The stability over 10 days (in
duplicate analyses) was 98-101%, and the dietary concentrations were
85-116% (mean, 100%) at 40 ppm, 91-108% (mean, 100%) at 2000 ppm, and
92-98% (mean, 94%) at 4000 ppm. Dogs were observed twice daily for
clinical signs, mortality, and moribundity. Body weights were measured
before treatment and then weekly; food intake was measured weekly.
Total and differential leukocyte counts, erythrocyte count,
haemoglobin, haematocrit, mean corpuscular volume, mean corpuscular
haemoglobin, mean corpuscular haemoglobin concentration, platelet
counts, and reticulocyte counts; and sodium, potassium, chloride,
calcium, inorganic phosphorus, alkaline phosphatase, total bilirubin,
aspartate and alanine aminotransferases, creatine phosphokinase, urea
nitrogen, creatinine, total protein, albumin, globulin, cholesterol,
and glucose were measured before and after four weeks of treatment.
Blood samples were taken from the jugular vein of dogs fasted
overnight, and urine collected during this period was analysed for
colour, appearance, volume, specific gravity, microscopic elements,
pH, protein, glucose, ketones, bilirubin, occult blood, nitrite, and
urobilinogen. The adrenals, brain, heart, kidney, liver, gonads,
pituitary, spleen, and thyroid-parathyroid were weighed and examined
macroscopically, and about 40 organs were preserved but were not
examined microscopically.
There were no deaths or dose-related clinical signs, and no
compound-related effects were observed on body weight. Food intake, on
the basis of grams per animal per day, was reduced in males at
4000 ppm throughout the study and in those at 40 and 2000 ppm during
week 1; it was also decreased in weeks 2 and 4 but was increased in
animals at 2000 ppm in weeks 2, 3, and 4. Food intake in females on
this basis was slightly reduced in all groups at all intervals, but
the decreases were not dose-related. Food intake on the basis of grams
per kilogram body weight was reduced in males at all doses in week 1
and in those at 40 and 3000 ppm at all periods; however, intake was
increased in animals at 2000 ppm in weeks 2, 3, and 4. In females,
intake on this basis was decreased in week 1 and at 4000 ppm in week
2; at other periods, intake was close to control values. Overall,
intake was reduced in all treated animals in week 1 and was
inconsistent thereafter. None of the changes in food intake is
statistically significant, and they are unlikely to be biologically
significant.
No significant haematological changes were observed in males. In
females, such changes were limited to decreased leukocyte counts in
animals at 40 and 4000 ppm and decreased mean corpuscular haemoglobin
in dogs at 2000 ppm. None of these changes is of toxicological
significance. Alanine aminotransferase activity was markedly decreased
in a dose-related manner in animals of each sex, and dose-related
increases in cholesterol levels were seen in females at all doses and
in males (131, 192, 199 and 167 mg/dl at 0, 40, 2000, and 4000 ppm).
No significant changes were observed in urinalysis. The absolute
weights of kidney and spleen were statistically significantly greater
than those of controls in male dogs at 2000 ppm and were nonsigni-
ficantly increased in dogs at 4000 ppm. Liver weights were also
nonsignificantly increased (not dose-related) at these doses. The
liver:body weight ratios of males at 2000 and 4000 ppm were
statistically significantly increased, as were those of the kidney at
2000 ppm; there was a considerable but nonsignificant increase at
4000 ppm. Spleen:body weight ratios were statistically nonsigni-
ficantly increased in males at 2000 and 4000 ppm. The thyroid-
parathyroid:body weight ratio was significantly increased in male dogs
at 2000 ppm and nonsignificantly in those at 4000 ppm. The ratios of
kidney, spleen, and thyroid-parathyroid to brain weights were
significantly increased in male dogs at 2000 ppm and nonsignificantly
at 4000 ppm; the liver:brain weight was significantly increased at
both 2000 and 4000 ppm. In females, the only statistically significant
change was in the liver:body weight ratio, which was increased at 2000
and 4000 ppm; nonsignificantly increased absolute liver weights were
also noted, and nonsignificant increases in brain and brain:body
weight ratios were seen at 2000 and 4000 ppm. The effects on organ
weights (particularly liver) and on clinical chemical parameters
indicate a probable NOAEL of 40 ppm, equivalent to 1 mg/kg bw per day;
however, in the absence of histopathological reports, the significance
of the findings cannot be fully assessed (Johnson, 1989).
Four groups of six beagle dogs of each sex (Ridglan Farms Inc.),
five to six months old and suitably immunized and vaccinated, were fed
diets containing 0, 15, 150, or 1500 ppm quintozene (purity, 99.4%,
with HCB at a maximum of 0.07% and a mean of 0.045%) for one year. The
homogeneity and stability of the diets were determined before the
study, and all diets were analysed for the percentage of the nominal
levels that were actually present. Diets were prepared weekly for four
weeks and monthly thereafter. Acetone was used as the dispersant in
the 15 and 150 ppm and control diets. The homogeneity in 10 samples
was 93-99% at 15 ppm, 85-107% at 150 ppm, and 89-110% at 1500 ppm,
with respective mean values of 95, 93, and 99%. Stability,
investigated over 10 days, was satisfactory. Duplicate analyses for
actual concentrations at each interval and dose showed mean values
over the study of 96, 94, and 96% and ranges of 87-104%, 89-110%, and
82-103% at 15, 150, and 1500 ppm, respectively. The actual intakes
were calculated from data on food intake and body weight to be 0.4,
4.3, and 40.1 mg/kg bw per day for males and 0.44, 4.22, and
41.48 mg/kg bw per day for females. Dogs were observed for mortality,
moribundity, and clinical signs twice daily. Body weights and food
intake were recorded weekly for 14 weeks and monthly thereafter.
Ophthalmology was performed before the test and in week 52.
Haematological and clinical chemical examinations were performed on
blood from the jugular vein of dogs fasted overnight at 6 and 12
months. Urinalysis was performed at 6 and 12 months on urine collected
during the fasting period. The same haematological, clinical chemical,
and urinary parameters were investigated as in the 28-day study
(Johnson, 1989). At termination (death before the end of the study or
sacrifice), the adrenals, brain, heart, kidneys, liver, gonads,
pituitary, spleen, and thyroid-parathyroids were examined grossly and
weighed. Adrenals, aorta, bone (rib), bone marrow (rib), brain (fore-,
mid-, and hind-), eye (with optic nerve), gall-bladder, oesophagus,
stomach, duodenum, jejunum, ileum, caecum, colon, rectum, ovaries,
testis with epididymides, heart, kidneys, liver, lung (with bronchi),
lymph nodes (tracheobronchial, mesenteric, and regional), mammary
gland (females only), pancreas, pituitary, prostate, salivary gland
(with mandibular lymph node), sciatic nerve, skeletal muscle (thigh),
skin, spinal cord (cervical, thoracic, and lumbar), spleen, thymic
region, trachea, urinary bladder, and uterus from all dogs were
preserved, processed, and examined microscopically.
In one male dog at 15 ppm that was sacrificed in extremis at
week 28, the clinical and pathological signs indicated a diagnosis of
generalized systemic blastomycosis. The death was not related to
treatment, and the clinical signs reported (including diarrhoea,
emesis, alopecia, lacrimation, and ocular discharge) did not indicate
dose- or compound-related incidence. One male at 150 ppm had
convulsions in weeks 39 and 48 and tremors in weeks 39-40, associated
with limb rigidity in weeks 39-40 and excessive salivation. No
abnormal neural effects were seen histopathologically at term. The
effect was isolated and is unlikely to have been compound-related.
Body weight and weight gain were unaffected. Food intake was variable,
but no clear trends were apparent. There were no compound-related
effects on haematological parameters, the only statistically
significant difference from control values being decreased haemoglobin
levels at 12 months in males at 15 ppm. Statistically significant
changes in clinical chemical parameters comprised increased alkaline
phosphatase activity at 1500 ppm in males at 6 and 12 months and in
females at 6 months; a considerable but not statistically significant
increase occurred in females at 12 months. Significantly decreased
alanine aminotransferase activity was seen in animals of each sex at
150 and 1500 ppm at 6 and 12 months; significantly decreased
creatinine was noted in males at these doses at 6 and 12 months and in
females at 1500 ppm at 6 months. Cholesterol levels were significantly
increased in males at 1500 ppm at 6 and 12 months and nonsignificantly
increased in females at 1500 ppm at 6 and 12 months. Globulin levels
were also significantly increased in males at 150 and 1500 ppm at 6
months, and blood urea nitrogen was decreased in females at 1500 ppm
at 12 months. No effects were seen on urinalysis.
Statistically significant increases in the absolute and relative
(to body and brain weight) weights of liver were seen in females at
1500 ppm and in relative weights in males at 1500 ppm; the absolute
liver weight in males was also increased but not significantly.
Nonsignificant increases in absolute and relative liver weights were
seen in animals of each sex at 150 ppm. A statistically significant
increase in the kidney:body weight ratio was seen in females at
1500 ppm. The maximal absolute adrenal weights were slightly increased
in males at 1500 ppm, and females showed a minimal, dose-related
increase in adrenal weights. Microscopic examination showed slight
hepatocellular hypertrophy in all dogs at 1500 ppm. No dose-related
effects were seen on alveolar macrophages, inflammation, or the
incidence of pneumonia. Adrenal changes in males were comparable in
all groups. In females at 1500 ppm, the incidence of lymphocytic
infiltration and vacuolar changes was increased, but these effects are
considered not to be of toxicological significance. The incidence of
pituitary cysts was increased in animals of each sex at 1500 ppm, but
such cysts were relatively common in control beagle dogs. The NOAEL
was probably 150 ppm, equal to 4.3 mg/kg bw per day in males and
4.22 mg/kg bw per day in females. Changes in clinical chemical
parameters seen at this dose (e.g. decreased alanine aminotransferase
activity and decreased creatinine) did not appear to be associated
with microscopic changes in tissues or organs (Goldenthal, 1990)
Four groups of three beagle dogs of each sex were fed diets
containing quintozene (purity, 98.8%) at concentrations of 0, 500,
1000, or 5000 ppm for two years. Erythrocyte counts, total and
differential leukocyte counts, haemoglobin, haematocrit, and Heinz
bodies; and the specific gravity, pH, appearance, colour, microscopic
sediment, albumin, glucose, and bilirubin in urine were reported at
26, 52, and 78 weeks and at terminal sacrifice. The heart, lung,
liver, kidney, spleen, pancreas, gonads, prostate, adrenal, thyroid,
brain, pituitary, eye with optic nerve, stomach, jejunum, colon, and
bone marrow were examined histologically. Of the animals at the
highest dose, three males died on days 214, 288, and 357 and two
females on days 317 and 472. Food intake was slightly reduced (mean,
4.6%) in animals at 1000 ppm and markedly (by about 30%) in those at
5000 ppm. All dogs at 5000 ppm lost weight, and their body-weight
gains (means, 2.75 kg at 500 and 2.62 kg at 1000 ppm) were lower than
that of controls (4.85 kg). Signs of toxicity at 5000 ppm included
lacrimation, conjunctival discharge, and milky corneal opacity; one
dog had corneal ulceration. One dog at 1000 ppm had conjunctivitis and
a second had corneal opacity for a short period. No effects were seen
at 500 ppm. Anaemia was induced in dogs at the high dose, with
characteristic reductions in haemoglobin, haematocrit, and erythrocyte
counts. Atrophy of bone marrow and reduced haematopoiesis were
observed at 5000 ppm. Severe histopathological changes in the liver
were seen at 5000 ppm and included fibrous narrowing of hepatic cell
cords, enlarged periportal areas, and increased leukocytic
infiltration. Similar but less intense effects were seen at 500 and
1000 ppm. There was no NOAEL (Scholz & Brunk, 1968).
Five groups of four beagle dogs of each sex, 4.5 months old, were
fed diets containing quintozene (purity, 98.2%; containing 1.4% HCB
and traces of tetrachloronitrobenzene and pentachlorobenzene) at
concentrations of 0, 5, 30, 180, or 1080 ppm for two years. Diets were
prepared weekly by dilution of the high dose, which was prepared by
blending corn-oil solutions of quintozene with the diet; the dietary
fat content was increased from 9 to 11%. Before feeding, an equal
weight of water was added to the food. Body weight and food intake
were determined weekly. The haematocrit, haemoglobin, and total and
differential leukocyte counts, and urinary reducing substances,
protein, specific gravity, and sediment were determined at 3, 6, 12,
18, and 24 months. Blood urea nitrogen, serum aspartate
aminotransferase, serum alkaline phosphatase, and cholinesterase,
prothrombin time, and bromsulphthalein retention time were determined
in controls and dogs at the high dose at 0, 3, 6, 12, 18 and 24
months. The frequency of oestrus was recorded. One dog of each sex at
each dose was sacrificed at 12 months for histopathological
examination. Organ and organ:body weight ratios were reported for
heart, spleen, liver, kidneys, and testes; the brain, lung, heart,
aorta, liver, spleen, kidney, stomach, ileum, jejunum, large
intestine, urinary bladder, bone marrow (sternal and long bone),
pituitary, thyroid, pancreas, adrenal, gonad, lymph node, and eye were
examined histologically.
No deaths were observed. The changes in body weight were not
significant, but weight gain tended to be reduced in females at 180
and 1080 ppm during weeks 6 and 13, and sporadically in all treated
males, which also had sporadic decreases in food intake. Neither body
weight nor food intake was consistently affected by treatment.
Slightly reduced haematocrit values were noted in males at 30 and
180 ppm at 78 weeks (statistically significant), and reduced
haemoglobin values were noted in males at 30 ppm; a nonsignificant
decrease in haematocrit and haemoglobin values was again seen in males
at 30 and 180 ppm at 104 weeks. The absence of any effect at 1080 ppm
indicates that these effects were probably random. No consistent
effects were seen on serum cholinesterase, the maximal inhibition
being 20% in females at 1080 ppm in week 13 and 23.6% in females at 30
ppm at week 52; all other depressions were < 15.4% and were random.
Mean serum aspartate aminotransferase activity was increased in
females at 1080 ppm at 13 and 78 weeks but decreased at 104 weeks
(statistically significant only at 13 weeks); it was also decreased in
males at 1080 ppm at 26 weeks and increased at 78 weeks. Serum
alkaline phosphatase activity was increased in males and females at 52
weeks and thereafter, but the increase achieved statistical
significance only at 52 weeks when data on animals of each sex were
combined. External lymph nodes and oestrus cycle were not affected by
treatment. Liver weight and the liver:body weight ratios of males and
females at 180 and 1080 ppm were nonsignificantly increased at one
year; at two years, a nonsignificant increase in absolute liver weight
was seen in males at 1080 ppm and a significant increase in females.
The increased liver:body weight ratio was significant only when data
for the two sexes were combined. The absolute and relative liver
weights were nonsignificantly reduced in females at 5, 30, and 180 ppm
but significantly reduced in males at 30 and 180 ppm. Testicular
weights were nonsignificantly reduced in dogs at 1080 ppm, and the
weight ratio was statistically significantly reduced. Other changes
(such as increased kidney weight ratio at 5 ppm and increased heart
ratio at 180 ppm in males) were random. Dogs at 180 ppm showed minimal
suppression of bile flow in liver cord cells; the effect was greater
in animals at 1080 ppm, resulting in moderate bile pigment
accumulation in cord cells. The effect was usually reversible. The
NOAEL was 30 ppm, equivalent to 0.75 mg/kg bw per day (Larson &
Borzelleca, 1968; Borzelleca et al., 1971).
Monkeys
Two rhesus monkeys of each sex were fed consecutive daily doses
of quintozene (purity, > 99.9%) on sucrose pellets, corresponding to
2 ppm of their daily diet, for 70 days. There were no controls. One
monkey of each sex was sacrificed on day 71. Haemoglobin, packed cell
volume, erythrocyte and total leukocyte counts, methaemoglobin, and
Heinz bodies, and sodium, potassium, bilirubin, creatinine, blood urea
nitrogen, total protein, serum aspartate and alanine aminotrans-
ferases, lactate dehydrogenase, cholesterol, luteinizing and
follicular-stimulating hormone levels, progesterone, and cortisol were
determined in blood samples taken before the test, on days 19 and 48,
and, in the surviving monkeys, on day 76. There were no changes in
haemoglobin values, packed cell volume, or erythrocyte or leukocyte
cell counts. The level of methaemoglobin was slightly elevated on day
1 but not thereafter. No change was seen in the incidence of Heinz
bodies. No changes were seen in clinical chemistry, although the data
on bilirubin and lactate dehydrogenase were highly variable; no time
effect relationship was apparent. At sacrifice, no histopathological
effects were seen in the liver, stomach, large or small intestine,
spleen, kidney, heart, lung, thymus, cerebrum, cerebellum, pons,
medulla, spinal cord, or bone marrow of the animals sacrificed on day
71. The NOAEL was > 2 ppm, equivalent to > 0.1 mg/kg bw per day
(Kögel et al., 1979b)
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 18 B6C3F1 and B6AKF1 mice of each sex were given
quintozene (purity and contaminants unspecified) at a dose of
464 mg/kg bw by stomach tube from seven days of age to the time of
weaning at four weeks of age and thereafter at a dose of 1206 ppm for
18 months. The latter was the maximal tolerated dose. There was a
significantly elevated incidence of tumours, mostly hepatomas, in both
strains (Innes et al., 1969).
Groups of 100 male and 100 female Swiss random strain mice were
fed diets containing quintozene (containing 2.7% HCB) at levels of 0,
100, 400, or 1200 ppm for 80 weeks. Body-weight gain was decreased in
animals of each sex at 1200 ppm. Liver:body weight ratios were
increased in males and females at 400 and 1200 ppm, and the
kidney:body weight ratio was increased in females at 1200 ppm. General
appearance, behaviour, and survival were not affected by treatment,
and the haematological indices were considered to be within normal
limits. A non-dose-related increase in nodular hyperplasia of the
liver was seen in all treated males. The hyperplastic areas had
essentially normal cell architecture and were regarded as
non-neoplastic. An increased incidence of subcutaneous fibrosarcomas
was observed in females at 1200 ppm. None of the other neoplasms
appeared to be related to treatment and were common in mice of this
strain. There was no NOAEL (van der Heijden & Til, 1974).
Groups of 50 male and 50 female mice (probably B6C3F1) were fed
diets containing two increasing doses of quintozene (purity, > 98%;
with 0.15% pentachlorobenzene, 0.25% 2,3,4,5-tetrachloronitrobenzene,
and 1% HCB), initially at 1075 and 2150 ppm for males and 2320 and
4640 ppm for females, increased to 3000 and 6000 ppm for males and
4500 and 9000 ppm for females during the last 315 days of the 546-day
(78-week) exposure period. A control group consisting of 20 male and
20 female mice was given only the vehicle, 2% corn oil. The total
duration of the study was 92 weeks, but some mice were killed and
autopsied earlier. Malignant tumours were seen at frequencies of 50%
in 25% of control males and 20% in 10% of control females, at 33% in
29% of males and 18% in 6% of females at the low dose, and at 29% in
20% of males and 57% in 19% of females at the high dose. Only the last
frequency was statistically significantly higher than expected; many
malignant histiocytic lymphomas were found in numerous organs of the
same animal. No significant differences were found between groups in
the numbers of animals with tumours, and no particular type of tumour
could be ascribed to the treatment (Wedig et al., 1976).
Three groups of 50 B6C3F1 mice of each sex, seven to eight weeks
old, were fed diets containing quintozene (purity, 99.6%, with 0.07%
HCB) at doses of 0, 2500, or 5000 ppm for 103 weeks, followed by a
one-week withdrawal period before terminal sacrifice. Mice were
observed twice daily, and five per cage were weighed weekly for 12
weeks and monthly thereafter. Food intake per cage was measured every
four weeks. The homogeneity of the diets was within 10%; the stability
of quintozene in the diet for two weeks was 100% at 25°C; and the mean
actual dietary concentrations, measured 14 times during the study,
were 2460 ppm (98.4%) with a range of 94-104.4% at 2500 ppm and
4980 ppm (99.6%) with a range of 94-104% at 5000 ppm. All animals
(including those that died before the end of the study) were
necropsied, unless autolysis or cannibalization had occurred. Tissue
masses, abnormal regional lymph nodes, mammary gland, salivary gland,
bone marrow, costochondral junction, thymus, larynx, trachea, lungs
and bronchi, heart, thyroid, parathyroid, oesophagus, stomach,
duodenum, jejunum, ileum, colon, mesenteric lymph nodes, liver,
gall-bladder, pancreas, spleen, kidney, adrenal, urinary bladder,
seminal vesicles, prostate, uterus, gonads, brain, pituitary, and
caecum from controls, female mice at the low dose, males and females
at the high dose, and all males at the low dose that died before term
were examined histologically. Gross lesions, livers, and nasal
cavities from all males at the low dose were also examined.
The terminal body weights of male mice were 96% of control values
at 2500 and 90% at 5000 ppm. The mean body weights of females at
5000 ppm were consistently 10% below control values by week 24 and
thereafter, but reached this level at 2500 ppm only after 92 weeks; at
term, the mean weights of females were 18 and 21% below control
values. No clinical signs were reported. Food intake may have exceeded
that of controls, but this was difficult to determine because of food
scattering. The survival of males at term, 31-34 mice, was comparable
in all groups. At term, 30 female controls, 18 at the low dose, and 14
at the high dose were still alive; however, five mice at the low dose
were accidentally drowned and two mice at 2500 ppm and one at 5000 ppm
died during the recovery period.
In male mice at 0, 2500, and 5000 ppm, the numbers with malignant
tumours were 19, 25, and 17 (25, 30, and 21 tumours) and those with
benign tumours were 19, 15, and 17 (23, 16, and 19 tumours). There was
no dose-effect relationship for the incidence of tumours in any
specific organ, the incidences generally being comparable between
groups. The incidence of non-neoplastic lesions in comparison with the
control incidence was not of toxicological significance. In female
mice at 0, 2500, and 5000 ppm, the numbers with malignant tumours were
15, 11, and 9 (15, 11, and 9 tumours) and those with benign tumours
were 16, 17, and 14 (18, 19, and 19 tumours). Tumours were not
concentrated in specific tissues and there was no dose-effect
relationship. Increased incidences of ovarian abscesses were seen,
with 24, 44, and 58% at 0, 2500, and 5000 ppm. Five of six abscesses
that were cultured contained Klebsiella. Hyperplasia of the
mediastinal lymph node was observed at incidences of 2, 9, and 20% and
liver haematopoiesis at 18, 42, and 46%; splenic haematopoiesis was
observed at incidences of 28, 48, and 54%. These effects may reflect
increased susceptibility to infection rather than a toxicological
effect.
Quintozene thus decreased body weight at 5000 ppm, equal to
953 mg/kg bw per day in males and 1358 mg/kg bw per day in females.
There was no increase in neoplasia, and the non-neoplastic
pathological effects in female mice were probably due to infection.
The NOAEL in male mice was 2500 ppm. In female mice at 2500 ppm, the
reduced body weight reached 10% only at 92 weeks and continued to
decrease, to 18%, until 103 weeks; as this effect is unlikely to be
compound-related, 2500 ppm was also the probable NOAEL for female
mice. Overall, the NOAEL was 2500 ppm, equal to 387 mg/kg bw per day
in male mice (US National Toxicology Program, 1987).
A group of 10 mice (strain not specified) of each sex received
topical applications on shaved skin of 0.2 ml of a 0.3% solution of
quintozene (purity and contaminants unspecified) in acetone twice
weekly for 12 weeks. The control group received acetone alone. After
application of quintozene, croton oil was applied to the site
(presumably twice weekly) for 20 weeks, and mice were sacrificed 20
weeks later. Papillomas were seen in 2/10, 6/10, 8/9, and 7/8 treated
males, in 6/10, 7/9, 7/7, and 7/4(?) treated females, in 1/10, 6/9,
5/8, and 1/7 control males, and in 0/10, 3/10, 4/8, and 4/7 control
females 10, 20, 30, and 40 weeks after the initial croton oil
application, respectively. The time to 50% tumour incidence was 13
weeks in treated males, 10 weeks in treated females, 16 weeks in male
controls, and unknown for control females. The total numbers of
tumours were 29 in treated males, 33 in treated females, 6 in control
males, and 7 in control females. Quintozene at the doses used in the
study thus did not induce tumours in the absence of a promoting agent
(Searle, 1966).
Rats
Groups of 10 rats of each sex (strain unspecified) were fed diets
containing 0, 25, 100, 300, 1000, or 2500 ppm quintozene (purity and
contaminants unspecified) prepared by blending a powder comprising 20%
quintozene, 77% pyrax ABB (a pyrophyllite carrier), and 3% Armour
'sticker' with Purina dog chow. Rats were weighed weekly, and
haematological parameters (unspecified) were determined at 11 and 24
months. At death and terminal sacrifice, heart, lung, liver, kidney,
spleen, stomach, small intestine, caecum, large intestine, thyroid,
adrenal, bladder, pancreas, and bone marrow were examined
histologically. At one year, survival in males was 7, 10, 8, 7, 9, and
10 animals, and that in females was 9, 6, 8,10, 10, and 8 animals at
0, 25, 100, 300, 1000, and 2500 ppm, respectively; by two years,
survival was 1, 4, 4, 3, 3, and 4 in males and 3, 2, 5, 5, 5, and 2 in
females. Body-weight changes (only means given) were sporadic;
statistically significant increases were seen in males at 25 ppm
(weeks 4-52) and 100 ppm (week 4) and in females at 100 ppm (weeks
2-78), 300 ppm (week 8 and weeks 52-78), 1000 ppm (week 26), and
2500 ppm (weeks 2-78); decreases were seen in males at 2500 ppm (weeks
2 and 8). The small numbers of animals make definite conclusions
difficult, but the dose of 2500 ppm appeared to decrease body weight
consistently in animals of each sex, as did the dose of 1000 ppm in
females. The decreases at all doses in females may be real, but males
were affected only at 2500 ppm. The haematological parameters were
stated to be within normal limits, and histopathological lesions were
stated to be limited to 'occasional lung abscesses and occurrences of
fatty changes in the liver. Lesions are not correlatable with dietary
concentration of quintozene but are of the type often seen in old
rats.' Individual data were not available. The NOAEL in this limited
study was 300 ppm, equivalent to 15 mg/kg bw per day (Finnegan
et al., 1958).
Groups of 50 male and 50 female Wistar rats were fed dietary
levels of 0, 100, 400 and 1200 ppm quintozene (containing 2.7% HCB)
for two years. The liver:- and kidney:body weight ratios were
increased in rats at 400 and 1200 ppm. Dose-related increases in the
incidences of single-cell necrosis and fatty metamorphosis of
hepatocytes were seen in animals of each sex at 400 and 1200 ppm, and
enlarged centrilobular hepatocytes were seen at all doses. There was
no increase in tumour incidence, and no adverse effects were observed
on general appearance, behaviour, body-weight gain, or food
consumption. Haematological, blood chemical, and urinary values were
considered to be within normal limits. There was no NOAEL in this
limited study (Sinkeldam et al., 1974).
Groups of 50 male and 50 female rats (strain unspecified) were
fed diets containing two decreasing doses of quintozene (purity, >
98%; with 0.15% pentachlorobenzene, 0.25% 2, 3, 4, 5-tetrachloro-
nitrobenzene, and 1% HCB): for the first 98 days, males received 7500
or 15 000 ppm and females, 11 000 or 22 000 ppm; during the last 448
days, males received 5000 or 10 000 ppm and females, 7250 or
14 500 ppm. A control group consisting of 20 male and 20 female rats
was given only the vehicle, 2% corn oil. The total exposure time was
546 days (78 weeks), and the duration of the study was 85-117 weeks.
Malignant tumours were seen at frequencies of 16% in 10% of control
males and 10% in 10% of control females, at 13% in 8.5% of males and
8% in 6% of females at the low dose, and at 23% in 12.5% of males and
9% in 6.5% of females at the high dose. No significant difference
between the groups was found in the total number of tumours or the
number of animals with tumours, and no particular type of tumour could
be ascribed to the treatment (Wedig et al., 1976).
Four groups of 50 Charles River CD rats of each sex, six to seven
weeks old, were fed diets containing quintozene (purity, 99.4%; <
0.07% HCB) at doses of 0, 20, 3000, or 6000 ppm for 24 months. An
additional 10 rats of each sex at each dose were treated similarly and
sacrificed at 12 months. As the homogeneity of the diet at the 20-ppm
dose was unsatisfactory in week 1 (range, 14-40 ppm), blending time
was increased for the week-2 diets, which improved the range to
16-22 ppm. From week 28, acetone was used as an aid to dispersion,
resulting in a variation of 16.3-18.9 ppm. The homogeneity was
acceptable at higher doses. The stability over 10 days was within 8%.
Duplicate analyses about every four weeks indicated that the mean
percentages of the nominal concentrations were 95 8.4 at 20 ppm, 100 ±
6.5 at 3000 ppm, and 101 ± 6.9 at 6000 ppm. Diets were prepared
weekly. Rats were observed twice daily for mortality, moribundity, and
overt signs of toxicity. Body weight and food intake were recorded for
individual rats weekly for 16 weeks and monthly thereafter.
Ophthalmological examinations were conducted before treatment and at
12 and 24 months. Five rats of each sex were screened microbio-
logically before the test. Erythrocyte count, total and differential
leukocyte counts, haemoglobin, haematocrit, platelet counts, mean
corpuscular volume, mean corpuscular haemoglobin, and mean corpuscular
haemoglobin concentration; and sodium, potassium, chloride, calcium,
inorganic phosphorus, alkaline phosphatase, bilirubin, aspartate and
alanine aminotransferases, creatine phosphokinase, urea nitrogen,
creatinine, total protein, albumin, globulin, cholesterol and glucose
were determined in orbital sinus blood from 10 rats of each sex at
each dose, which had been fasted overnight, at 6, 12, 18, and 24
months. The colour, appearance, volume, specific gravity, microscopic
elements, pH, protein, glucose, bilirubin, ketones, occult blood,
nitrite, and urobilinogen in urine were determined in samples
collected during the fasting period at 6, 12, 18, and 24 months. All
animals were necropsied, and adrenals, aorta, femur, bone marrow
(femur), brain (fore-, mid-, and hind-), eye (including optic nerve),
oesophagus, stomach, duodenum, jejunum, ileum, caecum, colon, rectum,
ovary, testis (with epididymides), heart, kidney, liver, lung, lymph
nodes, mammary gland (female only), pancreas, pituitary, prostate and
seminal vesicles, salivary gland (with lymph nodes), sciatic nerve,
skeletal muscle (thigh), skin, spinal cord (cervical, thoracic, and
lumbar), spleen, thymic region, thyroid, parathyroid, trachea, urinary
bladder, uterus, gross lesions, and tissue masses were collected. The
adrenals, brain, heart, kidney, liver, gonads, and thyroid-parathyroid
were weighed at sacrifice at 12 and 24 months. All tissues of control
rats, those at the high dose, and those that died or were sacrificed
were examined histologically at term. Tissue masses, gross lesions,
regional lymph nodes, and, in females only, lung were examined
histologically at interim sacrifice of animals at 20 and 3000 ppm; the
liver, kidney, lung, thyroids, parathyroids, tissue masses, and
regional lymph nodes of animals at these doses were examined at
terminal sacrifice.
Mortality was 52, 54, 42, and 16% in males and 42, 50, 46, and
48% in females at 0, 20, 3000, and 6000 ppm, respectively, excluding
rats sacrificed at 12 months. One of 60 rats at each dose had died by
12 months, the only clinical sign being a slightly increased incidence
of body surface staining in females at 6000 ppm and an increased
incidence of malocclusion in males at this dose from week 1 to term,
which was obviously not compound-related. Body weight was
significantly reduced in animals of each sex at 6000 ppm throughout
the study, the decrease being > 10% by week 2 in males and by week 24
in females. Slightly lower weights were noted in females at 3000 ppm
after 24 weeks (statistically significant in 14/21 weighings), but the
weight decreases in males were generally comparable to those of
controls, except for a slight (generally statistically nonsignificant)
reduction after 84 weeks. Food intake on the basis of grams per
kilogram body weight tended to be increased, especially in males, and
to a lesser degree females, at 6000 ppm. The average intakes of the
diets at 20, 3000, and 6000 ppm were 0.9, 141, and 303 mg/kg bw per
day in males and 1.14, 179, and 370 mg/kg bw per day in females. No
compound-related ophthalmological findings were seen.
Statistically significant haematological findings tended to be
sporadic and do not appear to be compound-induced. In males at
6000 ppm, haemoglobin and haematocrit were reduced at six months only,
and mean corpuscular volume was reduced at 6 and 12 months; platelet
counts were increased at 12 months in males at 20 ppm. In females,
erythrocyte counts were elevated at 18 months at 20 and 6000 ppm,
haemoglobin was depressed at 6 months at 3000 and 6000 ppm but was
increased at 18 months at 20 ppm, haematocrit was increased at 18
months at 20 ppm, mean corpuscular volume was decreased at 18 months
at 3000 ppm and at 6, 12, and 18 months at 6000 ppm, mean corpuscular
haemoglobin was decreased at 18 months at 3000 ppm and at 6 and 18
months at 6000 ppm, and mean corpuscular haemoglobin concentration was
depressed at 6 months at 6000 ppm. Lymphocyte counts were elevated at
12 months at 20 ppm.
The clinical chemical findings in males included increased
potassium levels at 24 months and decreased glucose at 6 months at
6000 ppm, which are probably random effects. Alanine aminotransferase
activity was reduced at 6 months at 20 ppm, at 6, 18, and 24 months at
3000 ppm, and at all intervals at 6000 ppm; however, it was increased
at 12 months at 3000 ppm. The decrease is considered to be compound-
related, and the amount is dose-related. In females at 6000 ppm,
slight decreases in chloride were seen at 6 and 12 months and slightly
increased calcium at 18 months. Alkaline phosphatase activity was
increased at 6 and 12 months and aspartate aminotransferase activity
was decreased at all intervals at 6000 ppm; alanine aminotransferase
activity was decreased in a dose-related manner at all intervals at
3000 and 6000 ppm. Cholesterol was increased at all intervals and
glucose was decreased at 6 months at 6000 ppm. Total protein was
increased at 18 months at 3000 and 6000 ppm. Thus, in animals of each
sex, compound-related effects on alanine aminotransferase activity
were seen at 3000 and 6000 ppm, and in females aspartate aminotrans-
ferase activity was decreased and cholesterol was increased at
6000 ppm. The effects on alkaline phosphatase activity may also be
compound-related. There were no apparent changes at any dose or
interval in urinary parameters, except possibly in volume; but water
contamination was likely to have occurred.
Macroscopic examination of tissues at 12 months showed an
increased incidence of tan-white foci in lungs of female rats at
6000 ppm (one control; 11 at 6000 ppm), and the incidence of
tan-white-yellow lung foci was increased in a dose-related manner in
animals of each sex at 3000 and 6000 ppm that died or were sacrificed
at 12-24 months. The incidence of accentuated lobulation of the liver
was increased in animals of each sex at 6000 ppm. Thyroid enlargement
was also noted, with incidences of 0, 0, 2, and 3 in males and 1, 0,
2, and 1 in females at 0, 20, 3000, and 6000 ppm. This finding is
questionable.
The absolute and relative (to brain and body weight) weights of
the kidney, liver, and testis were statistically significantly
increased in males at 3000 and 6000 ppm at the 12-month interim
sacrifice. The organ:body weight ratios were affected by decreases in
the body weight of males at 6000 ppm, but significant increases were
reported for heart, kidney, liver, thyroid-parathyroid, and testis at
this dose. At 3000 ppm, increased organ:body weight ratios were seen
for kidney and liver. The organ:brain weight ratios were increased for
kidney at 3000 and 6000 ppm and for testis at 6000 ppm. Significantly
increased organ:body weight ratios were seen for brain, kidney, liver,
and thyroid-parathyroid in females at 6000 ppm at the 12-month
sacrifice. In males at terminal sacrifice, the absolute weights of
liver were increased at 6000 ppm, of the thyroid-parathyroid at 3000
and 6000 ppm, and of the kidney at 3000 ppm. Increased organ:body
weight ratios were seen in males for brain at 6000 ppm, kidney at
3000 ppm, liver at 3000 and 6000 ppm, and thyroid-parathyroid at 3000
and 6000 ppm, and the organ:brain weight ratios were increased in
males for kidney at 3000 ppm, liver at 3000 and 6000 ppm, and
thyroid-parathyroid at 3000 and 6000 ppm. Females at 6000 ppm with
body weights nonsignificantly lower than those of controls had a
decreased absolute heart weight and an increased absolute thyroid-
parathyroid weight. Increased organ:body weight ratios were seen for
liver at 6000 ppm and thyroid-parathyroid at 3000 and 6000 ppm; the
heart:brain weight was decreased and the thyroid-parathyroid:brain
weight was increased at 6000 ppm. When nonsignificant changes are
included, the major effects were increases in liver and thyroid-
parathyroid weights in animals at 3000 and 6000 ppm at both interim
and final sacrifices.
There were no microscopic observations that would account for the
changes in kidney and testicular weights. No compound-related
microscopic pathological changes were seen up to 12 months. At 12-24
months, the incidence of hypertrophy in males was 0, 0, 13/48, and
30/49, that of hyperplasia 0, 0, 1, and 1, and that of hepatocellular
adenomas 1/49, 0/49, 1/48, and 4/49 rats at 0, 20, 3000, and 6000 ppm.
Increased incidences of alveolar macrophages, interstitial pneumonia,
and perivascular lymphoid infiltration were seen in the lungs of males
at 6000 ppm. Thyroid hypertrophy was seen in 3/49, 2/49, 20/48, and
33/49 rats, follicular adenomas in 0/49, 0/49, 6/48, and 5/49 rats,
follicular carcinomas in 0/49, 1/49, 0/48, and 2/49 rats, and
follicular hyperplasia in 2/49, 2/49, 7/48, and 8/49 rats at 0, 20,
3000, and 6000 ppm, respectively. The incidence of colloidal cysts was
also increased in males at 6000 ppm. In females at 12-24 months, liver
hypertrophy occurred in 0/50, 0/49, 19/50, and 29/47 rats, hyperplasia
in 1/50, 0/49, 5/50, and 3/47 rats, and hepatocellular adenomas in
1/50, 0/49, 1/50, and 1/47 rats at 0, 20, 3000, and 6000 ppm,
respectively. Increased incidences of alveolar macrophages and
interstitial pneumonia were seen at 3000 and 6000 ppm; the incidence
of perivascular lymphoid infiltration was not increased. Thyroid
hypertrophy was seen in 0/50, 0/49, 18/50, and 23/46 female rats,
follicular adenomas in 1/50, 0/49, 2/50, and 4/46, follicular
carcinomas in 1/50, 0/49, 0/50, and 1/46, and follicular hyperplasia
in 0/50, 0/49, 6/50, and 8/46 at 0, 20, 3000, and 6000 ppm,
respectively. In data on historical controls from 11 two-year studies,
the mean percentage incidences of follicular adenomas were 2.88% for
males and 0.3% for females, and those of follicular carcinomas were
1.73% for males and 0.60% for females. In the study described here,
the incidences of follicular adenoma were 0, 0, 12.5, and 10.2% for
males and 2, 0,12, and 8.7% for females at 0, 20, 3000, and 6000 ppm,
respectively, and those of follicular carcinoma were 0, 2, 0, and 4.1
in males, and 2, 0, 0, and 2.2 in females. The incidence of follicular
adenomas in animals of each sex at 3000 and 6000 ppm therefore exceeds
the historical mean, as does that of follicular carcinomas in males at
20 and 6000 ppm and in females in the control and 6000-ppm groups. The
incidence of follicular carcinomas in males at 20 ppm, control
females, and in females at 6000 ppm was one per group, which is within
the historical control range, as is the incidence of follicular
carcinomas in males at 6000 ppm: 2/49 as compared to 3/32 in one
historical control. Quintozene therefore induced thyroid adenomas and
may have induced thyroid carcinomas. The changes observed in the lung
are consistent with infections; the increased incidence of such
changes at high doses may indicate either reduced immune competence or
a stress-related increase in susceptibility to infection. The
hepatocellular hypertrophy (mainly centrilobular) in which
eosinophilic cytoplasm was noted is indistinguishable from the
hepatocellular hypertrophy arising from increased drug metabolizing
enzyme activity. The NOAEL was 20 ppm, equivalent to 1 mg/kg bw per
day (Goldenthal, 1991).
(d) Reproductive toxicity
Four groups of 25 weanling (28-day-old) Charles River CD rats of
each sex were fed diets containing quintozene (purity, 98.2%, with
1.4% HCB and trace levels of tetrachloronitrobenzene and
pentachlorobenzene) at concentrations of 0, 5, 50, or 500 ppm. The
diet was prepared weekly, using 10 ml of corn oil for 500 ppm
quintozene, and was appropriately diluted with additional feed. After
11 weeks on diet, groups of 20 F0 females were paired with a male at
the same dose, rotated weekly for three weeks if necessary. Matings,
numbers of pregnancies, litters born, pups 1, 4, and 21 days
post partum, and litter weight at day 21 were recorded. Litters were
culled to 10 pups on day 4. Indices of fertility (pregnancies per
mating × 100), gestation (total litters per number of pregnancies ×
100), viability (live pups at day 4 per live pups born × 100) and
lactation (weaned pups per number of remaining pups after culling on
day 4 × 100) were calculated. A second litter (F1b) was initiated 10
days after the birth of the final F1a litter, and these animals were
used as parental rats for the F2 and F3 generations. Autopsies
were performed on F0 adults after weaning of F1b pups, F1a pups
at weaning, F1b adults after weaning of F2b pups, and F2a pups
at weaning, this pattern being maintained throughout the study. Ten
F3b offspring of each sex were maintained on the diets until two
months old, when they were sacrificed, and the heart, lung, liver,
kidney, urinary bladder, spleen, stomach, small and large intestine,
caecum, lymph nodes, bone marrow, skeletal muscle, skin, brain,
pituitary, thymus, thyroid, adrenals, pancreas, and gonads were
examined histopathologically. The only effects were on the body weight
of adult females: No significant changes were observed between
controls and F0 or F1b females at 500 ppm, but the body weights of
F2b females were significantly decreased at 500 ( P < 0.05) and
50 ppm ( P < 0.01). The effect was not dose-related. The NOAEL was
probably 500 ppm, equivalent to 2.5 mg/kg bw per day (Larson &
Borzelleca, 1968; Borzelleca et al., 1971).
Four groups of 26 Charles River COBS CD rats of each sex, aged 55
days, were fed diets containing quintozene (purity, > 99%; 0.08%
HCB) at doses of 0, 20, 3000, or 6000 ppm for two litters per
generation for two generations. Parents for the second generation were
selected from the F1b litters, avoiding sibling matings. Diets were
prepared weekly in a twin-shell blender with an intensifier bar to mix
500 g with additional diet to achieve the required levels. As the
homogeneity of the diet at the low dose was poor in week 1, the mixing
time was increased to 20 min after an initial 2-min mixing with 250 g
untreated diet, the mixer bar being used for the first 10 min of the
final blending. This process improved the homogeneity, but the dietary
levels were reduced to 84% of the nominal concentration. The diets
containing the low dose were therefore again modified, by dissolving
the quintozene in acetone before preparing the premix and blending (in
a Hobart mixer) it for 5 min before mixture with additional diet. The
homogeneity and percentage concentration were then acceptable. Those
at the middle and high doses were acceptable from week 1. The control
diet was also mixed with acetone from week 4. Analysis of the diets at
least once every four weeks throughout the study showed good
concordance with nominal values. F0 parents were fed treated diets
for 81 days before pairing, and F1 parents were given the test diet
for 90 days between weaning and pairing. Rats were observed twice
daily for mortality and signs of toxicity; body weights were recorded
weekly until sacrifice, except during gestation when animals were
weighed on days 0, 7, 14, and 20. Food intake was measured weekly
except during mating and, for females, during gestation and lactation,
when data were obtained for days 0-7, 7-14, 14-20, and 0-20 of
gestation and 0-4, 4-7, 7-14, and 14-21 of lactation. Rats were paired
1:1, and copulation was determined by either vaginal lavage or the
presence of a copulatory plug. The day of detection of copulation was
designated day 0 of gestation, for a maximum of 21 days. The time
between end of lactation and re-pairing was 10 days. Males were
changed between F1a and F1b and between F2a and F2b pairings.
The duration of the gestation period and difficulties in parturition
were recorded. Litter size, the numbers of still- and live births, and
gross abnormalities were noted. On lactation day 4, litters were
culled to eight pups (four of each sex when possible). Pups and dams
were observed twice daily for abnormal behaviour (including nesting
and nursing) and deaths. Pups were sexed and weighed individually on
days 0, 4, 7, 14, and 21 of lactation.
At term, F0 males were sacrificed and necropsied, and their
tissues were preserved. All males that failed to sire a litter were
examined for the presence of sperm in the epididymides. F0 females
were sacrificed at end of lactation of F1b pups (or, if non-gravid,
25 days after separation from males). Uteri from apparently non-gravid
females were examined with 10% ammonium sulfide. F1a offspring were
discarded after weaning. F1b offspring were necropsied, only gross
pathological lesions and malformed pups being preserved. A similar
programme was followed for the F1 generation. In both generations,
the coagulating gland, cervix, ovaries, pituitary, prostate, seminal
vesicles, testes with epididymides, uterus, vagina, and gross lesions
were examined microscopically.
Three adults died: one F0 male at 3000 ppm in week 28, one
23-day-old F1 male at 6000 ppm, and one control F1 female at about
20 weeks of age. These deaths did not appear to be compound-related.
Brown abdominal staining and brown or yellow anogenital staining
(usually for only 1-3 weeks) were seen in F0 females at the middle
and high doses. A slightly increased incidence of hair loss also
occurred in this group. F1 males and females at 6000 ppm had an
increased incidence of small size, which generally disappeared by 15
(males) to 17 (females) weeks of age. The body weights of F0 males
at 3000 and 6000 ppm and of females at 6000 ppm were lower than those
of controls (statistically significant), and a slight (usually
nonsignificant) decrease occurred in females at 3000 ppm. F1 males
and females at 6000 ppm and females at 3000 ppm had significantly
reduced body weights in weeks 4-17. During gestation, significant
reductions in mean body weights were seen in females at 6000 ppm in
the F0/F1a (at 14-21 and 0-21 days), F0/F1b (on days 0 and 7),
F1/F2a (at all intervals), and F1/F2b (at 14-20 days)
generations. The mean body weights at 6000 ppm were also significantly
reduced during lactation in the F0/F1a (at 0-4, 7-14, 14-21, and
0-21 days), the F0/F1b (0-21 days only), the F1/F2a (7-14 days
only), and the F1/F2b (14-21 and 0-21 days) generations. In
animals at 3000 ppm, decreased body weights were seen in the
F1/F2b matings on days 4-7, 14-21, and 0-21. Food intake was
reduced before mating of adults at 6000 ppm and during the lactation
period in F0 and F1 females.
No compound-related effects on male copulation, male fertility
index, female fertility index, gestation index, copulatory interval,
or gestation length were seen in either generation, although greater
fertility than controls was seen in F2a (animals of each sex) and
F2b (females) animals. Males that failed to mate had motile and
morphologically normal sperm in the epididymides, apart from one male
in the F1/F2b group at the low dose which had non-motile,
morphologically abnormal sperm. The mean numbers of stillbirths and
live births, the sex ratios, and viability indices were comparable to
those of controls, except in F1/F2a offspring at the middle dose
in which the sex ratio was skewed in favour of males. In animals at
the high dose, the total number of live births and the body weights of
live offspring were significantly reduced; the body weights of
offspring were also significantly reduced in the F0/F1a (days 14
and 21), F0/F1b (females only, day 21), and F0/F2b (days 14
and 21) generations at 3000 ppm.
During lactation, an increased incidence of 'pale' pups was seen
at 3000 and 6000 ppm in the F0/F1a generation on day 14 and in
F1/F2a pups at 6000 ppm on days 7, 14, and 21. Missing tails were
seen in F1/F2a pups at 6000 ppm on days 14 and 21 and in
F1/F2b pups at low incidence on all days. Concentric rings on the
tail were seen at high incidence in F1/F2a pups at 6000 ppm on
days 7, 14, and 21.
The incidences of malformations in offspring at birth were
reported only in summary form. In the F1a litters, two dead
offspring at 3000 ppm had malformations consisting of mandibular
micrognathia in one, cleft palate in both, and an intraventricular
defect in one; no malformations were seen in dead F1b offspring. One
case of anophthalmia was noted among 86 F1b pups at 20 ppm at day
21. Malformations seen in two dead F2a pups in two litters at
6000 ppm were a bulbous pulmonary trunk, an intraventricular septal
defect, and aortic arch stenosis; no malformations were seen in dead
F2b offspring. One F2b control pup out of 118 had microphthalmia
and mandibular micrognathia, one at 6000 ppm had anophthalmia, and one
at this dose had situs inversus; these pups were from different
litters. The incidence of variants was virtually nil.
Terminal macroscopic examination indicated no treatment-related
effects in F0 parents. Tan-yellow foci in the lung were seen in some
F1 males and females at 6000 ppm and in two females at 3000 ppm. The
testicular weights of animals that failed to sire offspring were
slightly reduced in two F1 controls and one treated F1 male at
3000 ppm. Microscopic examination of F0 adults showed no
compound-related effects. In F1 animals, the tan-yellow foci in the
lungs were aggregates of alveolar macrophages, perivascular-
peribronchial lymphoid cell infiltration, and/or interstitial
pneumonia. These changes are typical of pulmonary viral infections.
Thus, quintozene is unlikely to have been toxic, although stress due
to its administration or effects on the immune system may have
resulted in increased susceptibility to infection in animals at
6000 ppm and possibly in females at 3000 ppm. The NOAEL was 20 ppm,
equivalent to 1 mg/kg bw per day, on the basis of changes in body
weight in pups and adults at 3000 ppm. No adverse effects were seen on
reproductive parameters (Schardein et al., 1991).
(e) Developmental toxicity
Mice
A group of 23 C57Bl/6 mice was given quintozene (containing 11%
HCB) at 500 mg/kg bw per day in corn oil on days 7-11 of gestation; 19
controls were available. Further groups received either pentachloro-
aniline at 100 or 200 mg/kg bw per day or tetrachloronitrobenzene at
200 mg/kg bw per day on days 7-18 of pregnancy. In mice treated with
quintozene, fetal mortality was slightly increased, and the incidence
of malformations was significantly increased. Renal agenesis was the
most frequent effect, and the incidences of anophthalmia (19.2%
greater than in controls), microphthalmia (9.8%), and cleft palate
were increased. Pentachloroaniline and tetrachloronitrobenzene did not
cause malformations or affect maternal body weight, the liver:body
weight ratio, fetal weight, or fetal mortality (Courtney et al.,
1976).
Groups of CD-1 mice were given quintozene (containing 11% HCB) at
doses of 250 or 500 mg/kg bw per day (five or 10 litters), penta-
chloroaniline at 250 mg/kg bw per day (nine litters), tetrachloro-
nitrobenzene at 200 mg/kg bw per day (six litters in one study and
nine in the other), quintozene fabricated to contain 11% HCB (nine
litters) at 500 mg/kg bw per day, HCB at 100 mg/kg bw per day (10
litters), or quintozene containing < 20 ppm HCB at 500 mg/kg bw per
day (10 litters) on days 7-16 of gestation. Maternal liver:body weight
ratios were increased at both doses (dose-related) of contaminated
quintozene and with the fabricated sample of quintozene; a
nonsignificant increase was seen with HCB. The incidence of
malformations per litter was significantly increased at 500 mg/kg bw
per day of contaminated quintozene (28.6% in comparison with 6.2, 2.0,
or 7.2% in corn oil:acetone [9:1] control groups at the same volume),
and to a lesser degree with HCB (13.6%) and with quintozene with <
20 ppm HCB. The major malformation observed with contaminated and
fabricated quintozene and HCB was cleft palate; this malformation was
also seen with quintozene containing < 20 ppm HCB, but the major
contributor to the incidence was clubbed foot. The last preparation
differed from the other quintozene samples in that it provided a
solution rather than a suspension; this may have increased absorption.
This group also showed signs of toxicity, expressed as abortion in
three of 13 mice on day 17 of gestation. A similar high incidence of
clubbed foot has not been seen in other studies of quintozene, and the
author suggested that it may have been due to increased uterine muscle
tone, with consequent pressure effects on the developing fetus
(Courtney et al., 1976).
A group of 30 CD1 timed-pregnant mice were given quintozene
(containing 5% HCB) orally at 750 mg/kg bw per day on gestation days
8-12; 40 timed-pregnant mice were used as controls. Day 20 of
gestation was considered to be postnatal day 1. Death occurred in 17%
of animals, and 73% were pregnant. Litter size was nonsignificantly
reduced on day 1, but pup weights were comparable to those of
controls, and by postnatal day 3, the mortality and weights of pups
were comparable to those of controls. There was no NOAEL (Kavlock
et al., 1987).
Rats
Groups of five to seven pregnant CD rats received quintozene
containing 11% HCB or < 1.0% HCB at 500 mg/kg bw per day or
pentachlorophenol or tetrachloronitrobenzene at 75 mg/kg bw per day on
days 7-18 of gestation. No effects were seen on maternal weight gain
or the liver:body weight ratio. Pentachlorophenol decreased fetal
weights. The incidence of malformations was minimal, and these
consisted of enlarged cerebral ventricles, umbilical hernias, and
slightly enlarged renal pelvis (Courtney et al., 1976).
Pregnant Charles River albino rats received quintozene in corn
oil by intubation at doses of 100-1563 ppm on days 6-15 of gestation
and were sacrificed on day 20. Corpora lutea, position and numbers of
dead and resorbed pups, fetal weights, and sex ratio were recorded.
One-half of the fetuses were stained with alizarin Red-S for skeletal
examination, and the remainder were either fixed in Bouin's fluid for
examination of soft tissues or preserved in formalin. No difference
was seen between control and treated groups for any parameter,
including renal pelvis dilatation, hydronephrosis, or hydroureter,
which occurred in all groups. No NOAEL could be determined because of
the unusual expression of doses (Jordan & Borzelleca, 1973).
Four groups of 25 mated Charles River COBS female rats were given
quintozene (purity, 97.3%; containing 0.025% HCB) at doses of 0, 30,
600, or 1200 mg/kg bw per day as 10 ml/kg bw of 0.2% carboxymethyl-
cellulose suspensions by gavage on days 6-15 of gestation. The
suspensions were prepared daily; analysis on days 7 and 14 of the
study indicated actual levels of 88-110% of the nominal concentration
and stability over 24 h. The day of mating was designated day 0 of
gestation. Rats were observed twice daily for mortality and changes in
appearance and behaviour. Maternal rats were weighed on gestation days
0, 6, 9, 12, 16, and 20, and food intake was measured daily and
calculated for days 6-9, 9-12, 12-16, 16-20, 6-15, and 6-20 of
gestation. Rats were killed on day 20 of gestation, and the uterus and
ovaries were examined in situ and gravid uteri weighed. The numbers
and locations of viable and non-viable fetuses and of early and late
resorptions and the numbers of implantations and corpora lutea were
recorded. Maternal tissues were examined macroscopically, and grossly
abnormal tissues were preserved. The uteri of apparently non-pregnant
rats were examined with 10% ammonium sulfide. Fetuses were weighted,
sexed, and examined for external malformations; half were preserved in
Bouin's solution for examination of soft tissues by Wilson's
technique, and the remainder were eviscerated and processed for
Alizarin R-S staining and subsequent skeletal examination (Dawson
technique).
There were no deaths or adverse clinical or behavioural effects.
A nonsignificant reduction in the body weights of animals at
1200 mg/kg bw per day was seen but which was only 1-2% after
correction for initial weight differences. Food intake was
significantly reduced at 6-9 days in animals at 1200 mg/kg bw per day,
but the reduction was only 10.8% when measured as grams per animal per
day or 8.7% when measured as grams per kilogram body weight per day.
The effect was probably due to unpalatability. At later intervals and
during days 6-15 and 6-20, food intake was comparable to that of
controls. The incidences of pregnancy were 24/25 controls, 23/25 at
30 mg/kg bw per day, 25/25 at 600 mg/kg bw per day, and 23/25 at
1200 mg/kg bw per day. There were no total resorptions, and all
pregnancies resulted in viable offspring; the viable litter sizes were
comparable at all doses. The numbers of early resorptions were
slightly increased in all test groups, but the incidence was within
the normal historical range. The incidences of late resorptions and
corpora lutea were comparable in all groups. The group mean individual
fetal weights were unaffected, and the sex ratio was normal. The
malformations seen in controls were anophthalmia in one fetus,
malpositioned ovary and kidney in another, and a vertebral anomaly
with an associated rib anomaly in a third. At 30 mg/kg bw per day, one
fetus had an encephalocoele and a malpositioned ovary, one had
anasarca, a bent scapula, and bent limb bones, and a third had a
folded retina. At 600 mg/kg bw per day, one pup had microphthalmia and
folded retinas; at 1200 mg/kg bw per day, two pups from separate
litters had malpositioned kidneys and ovaries. Thus, no pattern in the
incidence of malformations and no dose-response relationship was seen
in the occurrence of malformations. The incidence of variants was
within normal limits. The NOAEL for maternal, embryo- and fetotoxicity
and for embryofetal development was 1200 mg/kg bw per day, the highest
dose tested (Keller, 1988a).
Rabbits
Four groups of 16 artificially inseminated New Zealand white
rabbits, three months old at receipt and acclimatized for 111 days,
were given quintozene (purity, 97.3%) at doses of 0, 12.5, 125, or
250 mg/kg bw per day in 0.2% carboxymethylcellulose on days 7-19 of
gestation. Because of maternal toxicity, additional rabbits, five
months old at reception and acclimatized for 48 days, were added about
75 days later and intubated with 0 (16 rabbits), 6.25 (16 rabbits), or
125 (12 rabbits) mg/kg bw per day. Semen for artificial insemination
was provided by six males proven to be fertile and was used to
inseminate an equal number of females in each concurrent group, except
in the second study when two males were used to inseminate fewer
females at 125 mg/kg bw per day than the remaining four males. The day
of insemination was designated day 0 of gestation. Caesarian sections
were performed on day 29 of gestation. The stability and homogeneity
of the quintozene formulation were satisfactory, and the actual
concentrations were 95-110% of the nominal ones.
Survival, appearance, and behaviour were observed twice daily and
were reported daily for days 7-29 of gestation. Rabbits that died
before the end of the study were necropsied, and those that showed
signs of abortion or premature delivery were sacrificed. The fetuses
of these rabbits were examined externally and preserved; those of
rabbits that died within 24 h of the end of the study were evaluated.
Body weights were recorded on days 0, 7, 13, 20, 24, and 29 of
gestation, and food intake was measured daily and calculated for days
7-13, 13-20, 20-29, 7-20, and 0-29 of gestation. At termination, the
uterus and ovaries were examined, and gravid uterine weight, the
number and location of viable and non-viable fetuses, the incidence of
early and late resorptions and total implantations, and the number of
corpora lutea were recorded. Maternal thoracic and abdominal cavities
were examined macroscopically, and grossly abnormal tissues and organs
were preserved. Non-pregnant uteri were examined with 10% ammonium
sulfide solution. Fetuses were weighed, tagged, and examined
externally, then dissected, internally sexed, and examined for soft
tissue abnormalities, including mid-coronal brain slices. The
carcasses were processed, stained with Alizarin Red-S (Dawson
technique), and examined for skeletal abnormalities.
One rabbit at 12.5 mg/kg bw per day died on day 28 of gestation;
two died at 125 mg/kg bw per day, both aborting before death on day 28
of gestation; and five died at 250 mg/kg bw per day on days 19, 21,
26, 26, and 27 of gestation, the death on day 27 being preceded by
abortion. In the secondary study, no deaths occurred at any dose. The
known causes of death in the initial study were mucoid enteritis in
those at 125 mg/kg bw per day and pneumonia in three of the five that
died at 250 mg/kg bw per day; the other causes of death were not
determined. The incidences of abortion were: two controls on days 24
and 27, two at 12.5 mg/kg bw per day on days 26 and 27, three at
125 mg/kg bw per day on days 23, 28, and 28, and four at 250 mg/kg bw
per day on days 23, 24, 25, and 26. When animals that aborted before
death are included, the incidences were 2, 2, 5, and 5 at 0, 12.5,
125, and 250 mg/kg bw per day, respectively. In the secondary study,
one rabbit at 125 mg/kg bw per day aborted on day 26. Premature births
occurred in one doe in each control group and in one at 125 mg/kg bw
per day. Signs of toxicity included mucoid stools in 6/16 rabbits at
250 mg/kg bw per day and 1/16 rabbits at 125 mg/kg bw per day, an
increased incidence of absent stools in 4/16 controls, 8/16 at 125,
and 11/16 at 250 mg/kg bw per day, and an increased incidence of
anogenital staining in 3, 8, 9, and 10 animals at 0, 12.5, 125, and
250 mg/kg bw per day in the initial group and 2, 3, and 9 at 0, 6.25,
and 125 mg/kg bw per day in the secondary group.
Body weights were markedly reduced after treatment at 250 mg/kg
bw per day, the loss peaking on day 24 of gestation, with very slight
recovery by day 29. The body weight at term, corrected for gravid
uterine weight, was also markedly reduced. Body-weight gain was
consistently reduced throughout gestation. Animals at 125 mg/kg bw per
day had a greater weight loss on days 13-20 of gestation in both the
initial and, to a lesser extent, the secondary study; however, the
weight reduction in the second group was greater on days 24-29. Both
groups at this dose had lowered corrected body weights at term than
controls, the effect being more apparent in the second group. Food
intake was significantly reduced at most intervals, when expressed
either as grams per animal or grams per kilogram body weight per day.
Stillbirths occurred in 1/81, 1/85, 1/49, and 3/28 rabbits at 0, 12.5,
125, and 250 mg/kg bw per day and in 0/82, 0/101, and 0/68 at 0, 6.25
and 125 mg/kg bw per day. The incidences of total resorptions in
relation to total implantations were 5.8, 11.5, 9.1, and 22.2% at 0,
12.5, 125, and 250 mg/kg bw per day and 24.8, 10.6, and 2.7% at 0,
6.25, and 125 mg/kg bw per day. Mean fetal weight was reduced markedly
at 250 mg/kg bw per day. The sex ratio was normal in all groups. The
mean litter size, including both viable and non-viable pups, was 7.4,
6.5, 6.8, and 4.7 at 0, 12.5, 125, and 250 mg/kg bw per day and 5.8,
7.7, and 6.8 at 0, 6.25, and 125 mg/kg bw per day. Malformations were
seen in 4, 4, 9, 2, 3, 2, and 0 pups in 3, 3, 6, 2, 3, 2, and 0
litters at 0, 0 (second study), 6.25 (second study), 12.5, 125, 125
(second study), and 250 mg/kg bw per day. The high incidence of
malformations at 6.25 mg/kg bw per day was accounted for by three pups
with multiple malformations that were not seen at higher doses. As
artificial insemination occurred over three days, each male normally
providing semen for two females, the multiple malformations may be due
to genetic defects mediated through the male. The incidence of
variants was comparable between groups. (Keller, 1988b).
The probable NOAELs are difficult to determine, since the results
at 125 mg/kg bw per day were not consistent between the studies. The
deaths due to mucoid enteritis were probably not compound-related
since the five deaths at 250 mg/kg bw per day were not associated with
pneumonia or mucoid enteritis. Although the incidence of abortions was
increased at 125 and 250 mg/kg bw per day, two of the five abortions
at the lower dose occurred immediately before death due to mucoid
enteritis and may therefore not be compound-related. The only
consistent effect was a decrease in body-weight gain at 125 and
250 mg/kg bw per day. The NOAEL for maternal toxicity was thus
12.5 mg/kg bw per day. That for embryo- and fetotoxicity was 125 mg/kg
bw per day, on the basis of reduced pup weight, increased resorption
rates, and a slight increase in the incidence of stillbirths at
250 mg/kg bw per day. The NOAEL for malformations was 250 mg/kg bw per
day, although only 28 fetuses from six litters were available for
examination at this dose.
(f) Genotoxicity
The results of studies of the genotoxicity of quintozene are
reported in Table 2.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Six male New Zealand white rabbits, three months old, were
exposed to quintozene (purity, 99.42%) moistened with water at a dose
of 500 mg per rabbit on dipped, intact dorsal skin under a 2.5-cm2
gauze patch secured with tape. The dressings were removed after 4 h,
the sites were wiped with dry towels, and the animals were observed
for 72 h. No dermal irritation was observed after 30-60 min or 24, 48,
or 72 h (Warshawsky, 1994c).
Six male New Zealand white rabbits, about three months old,
received 0.0996 g of quintozene (purity, 99.42%) in the cupped
conjunctival sac of the right eye, and the eyelids were held together
for 1 s after administration. The eyes were not washed and were scored
for irritation (on the Draize scale) at 1, 24, 48, and 72 h. At 72 h,
they were examined with sodium fluorescein. After 1 h, all rabbits had
a clear discharge, and two also showed blanching. Conjunctival
redness, chemosis, and discharge were seen in all animals, the redness
persisting in four rabbits up to 24 h. All eyes were normal by 48 h.
The average group scores were 7/110 at 1 h and 1.7/110 at 24 h,
indicating that the compound is minimally irritating to the rabbit eye
(Warshawsky, 1994d).
Table 2. Results of tests for the genotoxicity of quintozene
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 0-6667 µg/plate 99.6 Negativea US National Toxicology
TA100, TA1535, TA1537 Program (1987)
Reverse mutation S. typhimurium, five strains NR NR Negative Simmon et al. (1976)
Mitotic recombination Saccharomyces cerevisiae NR NR Negative Simmon et al. (1976)
DNA repair E. coli NR NR Negative Simmon et al. (1976)
Gene mutation Mouse lymphoma 1.25-10 µg/ml 99.6 Negativea US National Toxicology
L5178Y tk+/tk- cells Program (1987)
Sister chromatid Chinese hamster ovary cells 0.75-7.5 µg/ml 99.6 Negativeb US National Toxicology
exchange Program (1987)
Sister chromatid Chinese hamster ovary cells 7.5-75 µg/ml 99.6 Negativec US National Toxicology
exchange Program (1987)
Chromosomal Chinese hamster ovary cells 2.4-75 µg/ml 99.6 Positivea,d US National Toxicology
aberration Program (1987)
In vivo
Dominant lethal Mice Three doses NR Negativee Jorgenson et al. (1976)
mutation
NR, not reported
a With and without metabolic activation; similar results in two experiments with two or three replications each
b Without metabolic activation
c With metabolic activation
d 98% of cells with simple chromosomal and chromatid breaks; 2% with complex rearrangements; response not dose-related
e Males treated for seven weeks; then, one male mated with two females for eight weeks
Four Hartley guinea-pigs of each sex were exposed to quintozene
(purity, 98%) diluted in acetone on four sites at concentrations of
75, 50, 25, 10, 5.0, 2.5, 1.0, and 0.5% and scored for irritation at
24 and 48 h. On the basis of this pilot study, induction doses of 75
and 50%, which caused mild irritation, and challenge doses of 5, 2.5,
and 0.5%, which caused virtually no irritation, were selected for the
main study. In the main study, groups of 10 guinea-pigs of each sex
received applications on clipped, intact skin under occluded 25-mm
chambers containing 0.3 ml for 6 h; after 22-26 h, the animals were
depilated, and irritation was scored within 2 h as slight patchy
erythema, slight but confluent or moderate patchy erythema (grade 1),
moderate erythema (grade 2), or severe erythema (grade 3). Animals
were re-scored 22-26 h after the initial scoring. Induction involved
three 6-h exposures on the same site over two weeks, the initial
exposure being to 75% and the other two exposures to 50% quintozene in
acetone. No dermal reactions were seen after the first dose; slight
patchy erythema was elicited in one male after the second dose and in
two males and two females after the third dose. One male was
sacrificed after the initial dose due to non-compound-related
ill-health. Vehicle controls showed no dermal reactions.
For the primary challenge, chambers containing 5, 2.5, or 0.5%
quintozene were applied to each induced animal on previously untreated
sites. Reactions were observed at all three doses, the 5%
concentration inducing slight patchy erythema in eight males, grade 1
erythema in one male, and slight patchy erythema in nine females at
24 h; slight patchy erythema in seven males, grade 1 erythema in one
male, slight patchy erythema in nine females, and a grade 1 reaction
in one female were seen at 48 h. Comparable results were seen with 2.5
and 0.5%. The mean group scores were 0.5, 0.5, and 0.5 at 24 h and
0.5, 0.5, and 0.4 at 48 h with 5, 2.5, and 0.5%, respectively. Vehicle
controls given the same challenge doses under the same conditions had
slight patchy erythema at all concentrations, with mean group scores
of 0.3, 0.4, and 0.3 at 24 h and 0.3, 0.3, and 0.4 at 48 h.
Nine induced animals and five naive animals of each sex were
re-challenged at least six days after the initial challenge with 5 or
0.5% on previously unexposed sites. The mean group scores for induced
animals were 0.4 and 0.2 at 5% and 0.3 and 0.2 at 0.5% at 24 and 48 h,
respectively. Grade 1 reactions were seen in two guinea-pigs at 24 h
and one at 48 h with the 5% concentration and one at 24 and 48 h with
0.5% quintozene. The naive animals challenged with the 5% concen-
tration had mean irritation scores of 0.4 at 24 h (one animal with a
grade-1 score) and 0.2 at 48 h, and those exposed to 0.5% had scores
of 0.5 at 24 h and 0.2 at 48 h. The incidence and persistence of grade
1 scores in the challenged animals indicate that quintozene can induce
sensitization (Kreuzmann, 1988).
(ii) Potentiation
Combinations of quintozene at half or whole LD50 doses of
1740 mg/kg bw with terrozole (5-ethyoxy-3-trichloromethyl-
1,1,2,4-thiazole) at the LD50 dose of 1080 mg/kg bw or at LD2.3,
LD6.7, LD16, LD31, or one-half the LD50 were given to groups
of 10 male CD Charles River rats at 100 mg/kg bw quintozene and
appropriate amounts of terrazole. Mortality recorded over 14 days
showed an additive effect (Borzelleca et al., 1971).
(iii) Thyroid function
Groups of 75 male Charles River CD rats were fed diets containing
0, 20, or 6000 ppm quintozene (purity, 99.09%) for up to 90 days, and
groups of 15 rats per dose were sacrificed after 7, 14, 30, or 90
days; the remaining 15 rats were maintained for a further 90 days on
normal diet before sacrifice. The average intakes were 1 and 333 mg/kg
bw per day. Diets were prepared weekly (with acetone as the vehicle at
the 0 and 20 ppm levels) and analysed in duplicate during weeks 1-4,
8, and 12. The mean dietary concentrations were 81% of the nominal
(range, 72-98%) at 20 ppm and 91% (range, 86-99%) at 6000 ppm.
Mortality, moribundity, and clinical parameters were checked twice
daily and body weight and food intake weekly; blood samples were
collected from the abdominal aorta of non-fasted rats at sacrifice,
when complete macroscopic examinations were performed and the liver,
pituitary, and thyroid-parathyroid were weighed. These organs were
examined microscopically, as was the liver of all rats at terminal
sacrifice; the thymuses of a few animals were also examined.
Triiodothyronine, thyroxine, and thyroid-stimulating hormone were
measured in all rats at each dose and interval; reverse
triiodothyronine was measured in all rats at the end of treatment.
There were no deaths. The clinical signs, which occurred at low
incidence and were not dose-related, included malocclusion in seven
controls, six at 20 ppm, and five at 6000 ppm, and occasional hair
loss and redness of the eyes. Body weight and weight gain were reduced
in animals at 6000 ppm during treatment, but the weight gain exceeded
that of controls during the recovery period and by the time of
sacrifice exceeded that of controls. The main decrease in body-weight
gain occurred in week 1, when food intake was also significantly
decreased. At 6000 ppm, animals had reduced triiodothyronine and
thyroxine and increased thyroid-stimulating hormone values during most
of the treatment period, the effects being statistically significant
at most intervals. Reverse triiodothyronine was reduced at 90 days.
The triiodothyronine, thyroxine, and thyroid-stimulating hormone
values returned to normal during the recovery period. At 20 ppm, no
significant changes were seen, except for an increased thyroxine value
at seven days, which was considered to be a random effect. The
absolute weight of the pituitary was reduced at seven days in animals
at 6000 ppm; however, no other changes in the absolute or relative
weights of the pituitary occurred during the study, and no abnormal
microscopic changes were observed. The absolute weight of the liver
was nonsignificantly increased at 30 and 90 days in animals at this
dose but returned to normal after the recovery period; relative (to
body) weights were significantly increased at 14, 30, and 90 days, but
this effect may have been due to the decreased body weight seen at all
intervals except after the recovery period. The hepatocellular
hypertrophy seen after treatment for 90 days indicates that the
increased absolute liver weight at 30 and 90 days at 6000 ppm was
compound-related. The thyroid-parathyroid weights were not
significantly affected, except that the absolute weight was
significantly decreased at 30 days in animals at 6000 ppm and very
slightly decreased in those at 20 ppm. At 90 days, statistically
nonsignificant decreases were again seen at 20 and 6000 ppm (33 g in
controls versus 30 g at both 20 and 6000 ppm). After 90 days'
recovery, the thyroid-parathyroid weights were comparable to or
slightly greater than control values. Follicular epithelial
hypertrophy of the thyroid was observed at 6000 ppm, with trace (14
days), mild (30 days), or moderate (90 days) severity. At 20 ppm, mild
follicular epithelial hypertrophy was noted at 90 days. The decreased
thyroid weight may therefore reflect the colloid breakdown that occurs
in hypertrophic thyroid follicular cells, but this was not confirmed
microscopically. As the effects on the liver and thyroid were
reversible and only minimal effects were noted at 20 ppm, the NOAEL
was 20 ppm, equal to 1 mg/kg bw per day (Goldenthal, 1993).
(iv) Studies of metabolites
Four groups of 10 weanling female Sprague-Dawley rats were fed
diets containing pentachloronitrosobenzene, which is believed to be an
intermediate in the metabolic reduction of quintozene to pentachloro-
aniline, at doses of 0, 10, 50, or 100 ppm for 18 weeks. Rats were
weighed twice weekly, and food intake was monitored daily. Serum
alkaline phosphatase, aspartate and alanine aminotransferases, urinary
and liver porphyrins, liver glutathione, hepatic microsomal protein,
cytochrome P450, haemoglobin ferrihaemoglobin, packed cell volume, and
erythrocyte and total and differential leukocyte counts were
determined in all rats at the end of treatment. Organ:body weight
ratios were reported for liver, heart, spleen, and kidneys; the liver,
kidney, gastrointestinal tract, and urinary bladder were examined
histologically.
Body weights tended to be increased from week 2 onwards in all
treated groups, but statistical significance was not achieved and no
dose-effect relationship was seen. Food intake and food efficiency
were increased in all treated groups. The organ:body weight ratios
were comparable in all animals. A dose-effect relationship was
seen for ferrihaemoglobin levels, with values of 0, 0.5, 0.9, and
1.1 g/100 ml for controls and the three dose groups, respectively.
Serum alanine aminotransferase activity showed a dose-related,
statistically significant increase at all doses. Urinary porphyrin
patterns were similar over the last 24 h of the study. The hepatic
microsomal liver protein and hepatic and urinary porphyrin composition
was unchanged, but cytochrome P450 enzyme activity was significantly
increased at 100 ppm. At this dose, the concentrations of glutathione
and oxidized glutathione in liver were also increased. The liver was
normal histologically, but multilayered bladder epithelium of variable
thickness was seen, characterized by the presence of large superficial
cells. There was no sign of cellular proliferation (Renner et al.,
1981).
3. Observations in humans
Fifty human subjects received one 6.4-mm moistened cotton square
dipped into a 75% wettable powder formulation of quintozene (purity
unspecified) on the palmar surface of the forearm. The squares were
then covered with foil, which was taped into place. Exposure was for
48 h. No irritation was observed when the dressings were removed. When
the test was repeated two weeks later, four individuals showed
positive reactions, comprising erythema, itching, and (in three)
oedema and small vesicle formation. Of the individuals who showed no
reaction at the time the patch was removed, nine developed a delayed
reaction 8 h to several days after testing (Finnegan et al., 1958).
Workers who were still employed and had been exposed during the
production of quintozene for 2.5 years (one person), seven years (one
person), 8.5 years (one person), or 11 years (10 people) years had
undergone twice-yearly health checks, including monitoring of height,
weight, vision, blood pressure, X-ray (once per year only),
haematological parameters (erythrocyte count, haemoglobin,
haematocrit, total leukocyte count), an evaluation of subjective and
objective symptoms, life style, family history, and individual past
history, throughout the employment period. After 1989, hearing tests,
blood chemistry (serum aspartate and alanine aminotranferases,
gamma-glutamyl transpeptidase, alkaline phosphatase, partial
thromboplastin time, total cholesterol, and blood glucose),
(urinalysis (urea nitrogen, uric acid, glucose, protein, and occult
blood), and electrocardiography were added. No compound-related
effects were observed. A further group of nine individuals, with
exposure for three months (one person), one year (one person), two
years (one person), three years (one person), four years (one person),
seven years (one person), or 10 years (three people), who were engaged
in the manufacture of quintozene dust formulations were subjected to
similar examinations and showed no compound-related abnormalities. No
estimate of exposure or information on protective measures used (e.g.
clothing, gloves, and masks) was provided (Yamazaki, 1994).
Comments
Quintozene is absorbed relatively slowly after oral
administration to rats and mice, peak blood levels being observed
about 74 h after dosing. 14C from labelled quintozene is eliminated
mainly in the faeces of animals of each sex, although females excrete
greater amounts in the urine than males. In goats, low doses (30 mg/kg
bw) were excreted mainly in the urine, but faecal elimination
predominated at higher doses (100 mg/kg bw). Bile levels in ruminants
were high, and enterohepatic circulation is probable. Excretion was
more rapid and complete in chickens than in mammals. There was no
evidence of tissue accumulation in any species.
The biotransformation of quintozene was virtually complete. Two
general metabolic pathways were apparent, one involving reduction of
the nitro group to an amino group and subsequent formation of
secondary metabolites, and the second involving replacement of the
nitro group with a sulfur-containing group (e.g. a thio- or
methylthio-glucuronide or N-acetylcysteine group). Metabolic studies
in several species have been reported.
The acute oral LD50 of quintozene was > 1.5 g/kg bw in both
rats and dogs. The dermal LD50 was > 5 g/kg bw in rabbits, and the
4-h LC50 in rats was > 1.7 mg/litre air. Quintozene was mildly
irritating to the rabbit eye and not irritating to rabbit or human
skin, but it was a mild dermal sensitizing agent in guinea-pigs and
humans. WHO has classified quintozene as unlikely to present an acute
hazard in normal use.
In a single 13-week study in mice at dietary concentrations of 0,
1250 (males only), 2500, 5000, 10 000, 20 000, or 40 000 (females
only) ppm, 5-8% decreases in terminal body weight were seen at doses
> 10 000 ppm, and increased absolute liver weights were seen in
animals of each sex at all doses. All mice at 40 000 ppm died.
Four short-term studies in rats were available; in only one of
them was quintozene containing < 0.1% hexachlorobenzene used. Dietary
concentrations of 0, 50, 3000, or 6000 ppm administered for 13 weeks
resulted in an NOAEL of 50 ppm (equal to 3.1 mg/kg bw per day) on the
basis of a slight (6-8%) reduction in terminal body weight, increased
liver weight and decreased alanine aminotransferase activity at higher
doses. Five short-term studies in dogs (lasting four weeks to two
years) were available, in only one of which quintozene met the purity
specification. In this one-year study, at dietary concentrations of 0,
15, 150, or 1500 ppm, increased liver weight and hepatocellular
hypertrophy were seen at 1500 ppm, which were accompanied by increased
serum alkaline phosphatase and cholesterol levels and decreased
alanine aminotransferase activity and creatinine levels. The NOAEL was
150 ppm, equal to 4.2 mg/kg bw per day. A 70-day study in monkeys at a
dietary concentration of 2 ppm revealed no adverse effects. In a
21-day study in rabbits, with application to the skin of doses of 0,
30, 300, or 1000 mg/kg bw of quintozene for 6 h per day, significantly
decreased alanine aminotransferase activity was seen at 1000 mg/kg bw
per day. No irritation or other adverse effect was observed.
Four long-term (72-103-week) studies by oral administration in
mice were available, in only one of which did the compound meet the
contamination criterion. This 103-week study at dietary concentrations
of 0, 2500, or 5000 ppm showed decreased terminal body weights in
males (4% at 2500 ppm and 10% at 5000 ppm) and females (18 and 21%).
Since the body-weight losses in the females exceeded 10% at 2500 ppm
only after week 92 of the study, the NOAEL was 2500 ppm (equal to
360 mg/kg bw per day), although the interpretation was complicated by
Klebsiella infection. No evidence of carcinogenicity was seen. In a
study in which technical-grade quintozene (purity unspecified) was
applied dermally twice weekly (0.2 ml of 0.3% quintozene in acetone)
to mice for 12 weeks, followed by twice-weekly dermal applications of
croton oil for 20 weeks, no tumour induction was seen in the absence
of the promoting agent.
Of four long-term (two-year) studies in rats, only one met the
contaminant specification. In this study, at doses of 0, 20, 3000, or
6000 ppm, thyroid follicular adenomas were seen at 3000 and 6000 ppm
and possibly carcinomas at 6000 ppm (especially in males). Hepato-
cellular hypertrophy (mainly centrilobular), indistinguishable from
that arising from increases in drug-metabolizing enzyme activity, was
also seen at 3000 and 6000 ppm. The absolute and relative (to body and
brain) weights of the thyroid and parathyroid were increased at the
two highest doses in most instances. A special 90-day study on thyroid
hormones showed hepatocellular hypertrophy, which was only minimal,
after 90 days' exposure to 20 ppm. Complete reversibility was seen
after 90 days' withdrawal. Thyroid and parathyroid weights were
decreased only after 30 days' exposure. At 6000 ppm, triiodothyronine
and thyroxine levels were decreased and the levels of thyroid-
stimulating hormone were increased significantly at most intervals
during dosing; the level of reverse triiodothyronine was reduced at 90
days. All changes were reversible within 90 days after withdrawal. The
changes in thyroid hormone levels are consistent with increased
microsomal enzyme activity, resulting in increased excretion of
triiodothyronine and thyroxine and subsequent disruption of the
hypothalamic-pituitary-thyroid axis, causing increased circulating
levels of thyroid-stimulating hormone. Thyroid-stimulating hormone
initiates increased production of triiodothyronine and thyroxine, with
follicular hyperplasia in the thyroid, leading to adenoma and, more
rarely, carcinoma. Quintozene was thus carcinogenic. The induction of
thyroid tumours appears to be secondary to the disruption of thyroid
function, as indicated by the increased levels of thyroid-stimulating
hormone. The NOAEL for this effect was 20 ppm, equivalent to 1 mg/kg
bw per day.
One of two multigeneration studies in rats met the criterion for
the level of contamination. In this study, with dietary concentrations
of 0, 20, 3000, or 6000 ppm and two litters per generation for two
generations, dietary concentrations of 3000 and 6000 ppm reduced pup
and adult body weights. The NOAEL was 20 ppm, equivalent to 1 mg/kg bw
per day.
In studies of developmental toxicity, 13 mice (in part of a study
of the incidences of cleft palate and renal agenesis, subsequently
shown to be induced by hexachlorobenzene contamination) received doses
of 500 mg/kg bw per day by gavage on days 7-16 of gestation. Abortions
occurred, and a low incidence of cleft palate (2%) and a high
incidence (23%) of clubfoot were noted. Marked maternal toxicity was
seen. The administered material formed a solution, and not a
suspension as in all other studies. This study was not considered in
establishing the ADI. Of three studies of developmental toxicity in
rats, only one, with doses of 0, 30, 600, and 1200 mg/kg bw per day,
was acceptable. The NOAEL for maternal and developmental toxicity was
> 1200 mg/kg bw per day. There was no evidence of induction of
malformations. In rabbits given doses of 0, 12, 120, or 250 mg/kg bw
per day or 0, 6.2, or 120 mg/kg bw per day, maternal toxicity was seen
at 120 mg/kg bw per day and embryo- and fetotoxicity at 250 mg/kg bw
per day, including reduced pup weight, increased resorptions, and an
increased incidence of stillbirths; no increase in malformations was
seen at any dose.
In a number of studies (e.g. long-term studies in mice and rats
and the two-generation study of reproductive toxicity in rats), the
incidences of infection appeared to be slightly increased at high
doses. Although the reports of histopathological examinations do not
indicate effects on the immune system, it would be useful if studies
could be performed to investigate the potential for quintozene to
affect the immune system.
Quintozene has been tested in a range of studies for genotoxicity
in vitro. An acceptable assay is required to corroborate the
increase in chromosomal aberration frequency observed in cultured
cells.
An ADI of 0-0.01 mg/kg bw was allocated on the basis of the NOAEL
of 1 mg/kg bw per day in the two-year study, the study of thyroid
toxicity, and the two-generation study in rats, using a 100-fold
safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 2500 ppm, equal to 390 mg/kg bw per day (103-week study of
carcinogenicity)
Rat: 20 ppm, equivalent to 1 mg/kg bw per day (24-month study of
carcinogenicity, multigeneration study, and study of thyroid
toxicity) 1200 mg/kg bw per day (study of developmental
toxicity)
Rabbit: 12 mg/kg bw per day (maternal toxicity in study of
developmental toxicity) 120 mg/kg bw per day (embryo- and
fetotoxicity in a study of developmental toxicity)
Dog: 150 ppm, equivalent to 4.2 mg/kg bw per day (one-year study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw, for quintozene containing less than 0.1%
hexachlorobenzene
Studies that would provide information useful for continued evaluation
of the compound
1. Assays of genotoxicity in vivo to assess possible
chromosomal aberrations
2. Studies to assess the potential of quintozene to interfere
with the immune system
3. Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to quintozene
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Skin irritation, rabbit Not irritating
Eye irritation, rabbit Minimally irritating
Skin sensitization, guinea-pig Mild sensitizer
(modified Buehler)
Dermal irritation, human No irritation with 75% formulation
Skin sensitization, human Sensitization in 13/50 individuals
Inhalation, 4-h, toxicity, rat LC50 > 1.7 mg/litre air
Oral, toxicity, rat and dog > 1.5 g/kg bw
Medium-term (1-26 weeks) Dermal irritation, 21-day, rat No irritation; body weight, food intake, haematology,
clinical chemistry (except decreased alanine
aminotransferase), urinalysis, organ weights and liver
and kidney histology unaffected at doses
< 1000 mg/kg bw per day
Repeated oral, toxicity, 13-week, rat NOAEL = 3.1 mg/kg bw per day; minimal changes in
body and liver weight; reduced alanine
aminotransferase activity
Dietary, developmental toxicity, rabbit NOAEL = 12.5 mg/kg bw per day for maternal toxicity,
125 mg/kg bw per day for embryo- and fetotoxicity,
> 250 mg/kg bw per day for teratogenicity
Long-term (> one year) Dietary, toxicity, carcinogenicity, rat NOAEL = 1 mg/kg bw per day; follicular adenomas
of the thyroid and hepatocellular adenomas
References
Adamovics, J.A. (1980) The metabolic fate of pentachloronitrobenzen:
Pilot study. Unpublished report from Bio/dynamics Inc., Division
of Environmental and Analytical Chemistry, Olin Corporation
Report No. 79056. Submitted to WHO by Uniroyal Chemical Company,
Inc.
Adamovics, J.A. & O'Grodnick, J.S. (1978) Absorption and elimination
characteristics of 14C-labelled pentachloronitrobenzene in
rats-pilot study. Unpublished report from Bio/dynamics Inc.,
Division of Environmental and Analytical Chemistry, Olin
Corporation Report No. 77037. Submitted to WHO by Uniroyal
Chemical Company, Inc.
Aschbacher, P.W. & Feil, B.J. (1983) Metabolism of pentachloro-
nitrobenzene by goats and sheep. J. Agric. Food Chem.,
31, 1150-1158.
Betts, J.J., James, S.P. & Thorpe, W.V. (1955) The metabolism of
pentachloronitrobenzene and 2:3:4:6-tetrachloronitrobenzene and
the formation of mercapturic acids in the rabbit. Biochem. J.,
61, 611-617.
Borzelleca, J.F., Larson, P.S., Crawford, E.M., Hennigar, G.R., Jr,
Kuchar, E.J. & Klein, H.H. (1971) Toxicological and metabolic
studies on pentachloronitrobenzene. Toxicol. Appl. Pharmacol.,
18, 522-534.
Courtney, K.D. (1973) The effect of pentachloronitrobenzene on fetal
kidneys (Abstract 41). Toxicol. Appl. Pharmacol., 25, 455.
Courtney, K.D., Copeland, M.F. & Robbins, A. (1976) The effects of
pentachloronitrobenzene, HCB and related compounds on fetal
development. Toxicol. Appl. Pharmacol., 35, 239-256.
Daun, R.J. (1989) Pentachloronitrobenzene: Nature of the residue in
poultry-laying hens. Unpublished report from Hazelton
Laboratories America Inc., Project HLA 611-119. Submitted to WHO
by Uniroyal Chemical Company, Inc.
Daun, R.J. (1990) Pentachloronitrobenzene: Nature of the residue in
livestock-lactating goats. Unpublished report from Hazelton
Laboratories America Inc., Project HLA 611-118. Submitted to WHO
by Uniroyal Chemical Company, Inc.
Finnegan, J.K., Larson, P.S., Smith, R.B., Jr, Haag, H.B. & Hennigar,
G.R. (1958) Acute and chronic toxicity studies on pentachloro-
nitrobenzene. Arch. Int. Pharmacodyn., 115, 38-52.
Goldenthal, E.I. (1990) One year chronic dietary study in dogs.
Unpublished report No. 399-087 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Goldenthal, E.I. (1991). Two year dietary toxicity and oncogenicity
study in rats. Unpublished report No. 399-072 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Company, Inc.
Goldenthal, E.I. (1992). 21 day dermal toxicity study in rats.
Unpublished report No. 399-125 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Goldenthal, E.I. (1993). 90 day toxicity study in rats. Unpublished
report No. 399-122 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Company, Inc.
van der Heijden, C.A. & Til, H.P. (1974) Pentachloro-nitrobenzene
carcinogenicity study in mice. Unpublished report R4365 from
Central Institute for Nutrition and Food Research, Bilthoven,
Netherlands.
Hoescht AG (1964) Toxikologische Prüfung von Pentachlor-nitrobenzol.
3. Auf chronische Toxizität (90-Tage-Test) an Ratten. Unpublished
report prepared and submitted by Farbwerke Hoescht, AG.
Hilaski, R.J. (1994) EPA (FIFRA) Acute inhalation toxicity evaluation
on terraclor technical in rats. Unpublished report No. 399-146
from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Innes, J.R.M., Ulland, B.M., Valeric, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L., Gart,
J.J., Klein, M., Mitchell, I. & Peters, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in mice. A
preliminary note. J. Natl Cancer Inst., 42, 1101-1114.
Johnson, D.E. (1989) Twenty-eight day pilot study in dogs. Unpublished
report No. 399-093 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Company, Inc.
Jordan, R.L. & Borzelleca, J.F. (1973) Teratogenic studies with
pentachloronitrobenzene in rats (Abstract 40). Toxicol. Appl.
Pharmacol., 25, 454.
Jorgenson, T.A., Rushbrook, C.J. & Newell, G.W. (1976). In vivo
mutagenesis investigations of ten commercial pesticides
(Abstract 41). Toxicol. Appl. Pharmacol., 37, 109.
Kavlock, R.J., Short, R.D., Jr & Chernoff, N. (1987) Further
evaluation of an in vivo teratology screen. Teratog. Carcinog.
Mutag., 7, 7-16.
Keller, K.A. (1988a) Developmental toxicity study in rats. Unpublished
report No. 399-068 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Company, Inc.
Keller, K.A. (1988b) Developmental toxicity study in New Zealand white
rabbits. Unpublished report No. 399-070 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Company, Inc.
Kögel, W., Müller, F., Coulston, S. & Korte, F. (1979a)
Biotransformation of pentachloronitrobenzene-14C in rhesus
monkeys after single and chronic oral administration.
Chemosphere, 8, 97-105.
Kögel, W., Müller, F., Coulston, S. & Korte, F. (1979b) Fate and
effects of pentachloronitrobenzene in rhesus monkeys. J. Agric.
Food Chem., 27, 1181-1185.
Kreuzmann, J.J. (1988) Delayed contact hypersensitivity in guinea
pigs. Unpublished report No. 88-0052-21 from Hill Top Biolabs,
Inc. Submitted to WHO by Uniroyal Chemical Company, Inc.
Kuchar, E.J., Geenty, F.O., Griffith, W.P. & Thomas, R.J. (1969)
Analytical studies of metabolism of terraclor in beagle dogs,
rats and plants. J. Agric. Food Chem., 17, 1237-1242.
Larson, P.S. & Borzelleca, J.F. (1968) Toxicologic study on the
effects of adding terraclor to the diet of beagle dogs for a
period of two years. Unpublished report from the Department of
Pharmacology, Medical College of Virginia, Richmond, Virginia,
USA. Submitted to WHO by Uniroyal Chemical Company, Inc.
McGee, D.H. (1988) Thirteen week dietary toxicity study in rats with
PCNB (pentachloronitrobenzene). Unpublished report No. 399-071
from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Uniroyal Chemical
Company, Inc.
McManus, J.P. (1989) Metabolism of pentachloronitrobenzene in the
goat-metabolite identification. Unpublished report from Uniroyal
Chemical Company, Inc., Crop Protection Department, Chemistry
Section, R & D, Uniroyal Project 8761. Submitted to WHO Uniroyal
Chemical Company, Inc.
McManus, J.P. (1990) Metabolism of pentachloronitrobenzene in the
goat-identification of metabolites in muscle. Unpublished report
from Uniroyal Chemical Company, Inc., Crop Protection Department,
Chemistry Section, R & D, Uniroyal Project 8761a. Submitted to
WHO Uniroyal Chemical Company, Inc.
O'Grodnick, J.S. (1978) Characterization and identification of
14C-PCNB metabolites in rat urine and faeces. Unpublished
report from Bio/dynamics Inc., Division of Environmental and
Analytical Chemistry, Olin Corporation Report No. 77037-2.
Submitted to WHO by Uniroyal Chemical Company, Inc.
O'Grodnick, J.S. (1979) Identification of the polar metabolites of
14C-PCNB after oral administration to rats. Unpublished report
from Bio/dynamics Inc., Division of Environmental and Analytical
Chemistry, Olin Corporation Report No. 78054. Submitted to WHO by
Uniroyal Chemical Company, Inc.
O'Grodnick, J.S., Adamovics, J.A., Blake, S.H. & Wedig, J. (1981) The
metabolic fate of 14C-labelled pentachloronitrobenzene in
Osborne-Mendel rats. Chemosphere, 10, 67-72.
Parkins, M.D. (1990). Pentachloronitrobenzene: Nature of the residue
in poultry-laying hens. Unpublished report from Uniroyal Chemical
Company, Inc., Crop Protection Department, Chemistry Section, R.
& D., Project No. 8762. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Parkins, M.D. (1991) Pentachloronitrobenzene: Nature of the residue in
poultry-laying hens. Unpublished report from Uniroyal Chemical
Company, Inc., Crop Protection Department, Chemistry Section, R &
D, Project No. 8762. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Renner, G., Herforth, B., Gokel, J.M., Goerz, G. & Luderschmidt, C.
(1981) Subchronic toxicity studies on pentachloronitrosobenzene
(PCNO) in female rats. Ecotoxicol. Environ. Saf., 5, 281-290.
Schardein, J.L., York, R.G. & Laughlin, M.A. (1991) Two generation
reproduction study in rats. Unpublished report No. 399-086 from
International Research and Development Corporation, Mattawan,
Michigan, USA. Submitted to WHO by Uniroyal Chemical Company,
Inc.
Scholz, & Brunk, (1968) Kronischer orale toxizitätsprüfung von
pentachlornitrobenzol, 2-Jahres-Versuch an Hunden. Unpublished
report from Rewerke Hoescht AG. Submitted to WHO by Uniroyal
Chemical Company, Inc.
Searle, C.E. (1966) Tumor initiatory activity of some
chloromononitrobenzenes and other compounds. Cancer Res.,
26, 12-17.
Simmon, V.F., Poole, D.C. & Newell, G.W. (1976) In vitro mutagenic
studies of twenty pesticides (Abstract 42). Toxicol. Appl.
Pharmacol., 37, 109.
Sinkeldam, E.J., van der Heijden, C.A., de Groot, A.P. & Til, H.P.
(1974) Carcinogenicity study with pentrachloronitrobenzene in
rat. Unpublished report R442 from Central Institute for Nutrition
and Food Research, Bilthoven, Netherlands.
St John, L.E., Jr, Ammering, J.W., Wagner, D.G., Warner, R.G. & Lisk,
D.J. (1965) Fate of 4,6-dinitro-2-isobutylphenol, 2-chloro-4,6-
bis-(ethylamino)-S-triazine, and pentachloronitrobenzene in the
dairy cow. J. Dairy Sci., 48, 502-503.
US National Toxicology Program (1987) Toxicology and carcinogenesis
studies of pentachloronitrobenzene in B6C3F1 mice (feed studies)
(National Toxicology Program Technical Report Series No. 325),
Washington DC, US Department of Health and Human Services.
Warshawsky, L.D. (1994a) Acute oral toxicity study in rats.
Unpublished report No. 399-155 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Warshawsky, L.D. (1994b) Acute dermal toxicity study in rabbits.
Unpublished report No. 399-152 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Warshawsky, L.D. (1994c) Primary dermal irritation test in rabbits,
following a 4 hr exposure period. Unpublished report No. 399-153
from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Warshawsky, L.D. (1994d). Primary eye irritation study in rabbits.
Unpublished report No. 399-154 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Wedig, J.H., Sperling, F. & Miller, R. (1976) Unpublished report of an
analysis of data submitted by the National Cancer Institute from
Olin Corporation.
Wit, S.L., van Esch, G.J. & van Genderen, H. (1960) Toxicity of some
chloronitrobenzene compounds (trichlorodinitrobenzene,
trichloronitrobenzene, tetrachloronitrobenzene and
pentachloronitrobenzene) to laboratory rats and residues found in
crops treated with these fungicides. In: Proceedings of the
Fourth International Congress on Crop Protection, Hamburg,
September 1957, Vol. 2, Braunschweig, pp. 1665-1668.
Yamazaki, Y. (1994) Health report. Unpublished report from Ohmuta
Factory, Mitsui Toatsu Chemicals Inc. Submitted to WHO by
Uniroyal Chemical Company, Inc.
Table 1. Acute toxicity of quintozene
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Rat Male, female Oral > 5000a 99.4 Warshawsky (1994a)
Rat Oral > 30 000 NR Wit et al. (1960)
Rat Male, female Oral 1650 (male) 98.2 Finnegan et al. (1958)
1710 (female)
Rat NR Intraperitoneal 5000 NR Wit et al. (1960)
Rat Male, female Inhalation 1.7b 99.4 Hilaski (1994)
Rabbit Male Oral Erraticc 98.2 Finnegan et al. (1958)
Rabbit Male, female Dermal > 5000 99.4 Warshawsky (1994b)
Rabbit NR Dermal > 4 g 99.7 (1.8% Borzelleca et al. (1971)
HCB
Dog NR Oral > 2500 98.2 Finnegan et al. (1958)
75% wettable powder as 40% aqueous solution
Rat Male, female Oral 16 g (equal to NR Finnegan et al. (1958)
12 g/kg bw
quintozene)
NR, not reported
a Decreased defaecation at all doses; decreased activity at 1300, 1700, and 2000; soft stools at 2000
and 5000; and anogenital staining at 1700, 2000, and 5000; full recovery by day 6
b Mass median aerodynamic diameter, 3.6 µm; standard geometric deviation, 1.9; aerosol concentration,
1.3-2.2 mg/litre; air flow, 38 litres/min; decreased activity, increased salivation, and rapid
breathing
c Mortality rates: 0 at 350 mg/kg bw per day, 2 at 500 mg/kg bw per day, 1 at 650 mg/kg bw per day,
0 at 800 mg/kg bw per day, 3 at 1100 mg/kg bw per day, and 3 at 1400 mg/kg bw per day
(b) Short-term toxicity
Mice
Groups of 10 B6C3F1 mice of each sex, eight to nine weeks old,
were fed diets containing quintozene (purity, 99.6%; HCB content,
0.07%) at concentrations of 0,1250, 2500, 5000, 10 000, or 20 000 ppm
(males) and 0, 2500, 5000, 10,000, 20 000, or 40 000 ppm (females) for
13 weeks. Five animals per cage were checked twice daily, food
consumption was measured weekly by cage, and individual body weights
were recorded weekly. All mice were necropsied, livers were weighed,
and gross lesions, tissue masses, mandibular lymph nodes, mammary
glands, skin, salivary gland, sternebrae, thyroid, parathyroid, small
intestine, colon, liver, prostate, testis, ovary, uterus, lung and
bronchi, heart, oesophagus, stomach, brain, thymus, trachea, pancreas,
spleen, kidneys, adrenal, urinary bladder, pituitary, spinal cord (if
neurological signs occurred), and eyes (if grossly abnormal) were
examined histopathologically. All females at 40 000 ppm died during
the study. The final mean body weights in animals at 10 000 and
20 000 ppm were 7 and 8% lower than those of controls for males and 5
and 8% for females, respectively. Scattering of food was frequent, but
mice at the high dose may have consumed more food than controls.
Absolute liver weights were increased significantly in males at 1250,
2500, and 5000 ppm and in females at 2500, 5000, and 10 000 ppm.
Liver:body weight ratios were significantly increased in males at
doses > 2500 ppm and in females at > 5000 ppm. Clinical signs
were limited to small body size and emaciation of all males at
20 000 ppm and all females at 20 000 and 40 000 ppm. No
histopathological changes were seen in male mice, but females at
40 000 ppm had lymphoid depletion of the spleen, mesenteric lymph
nodes, or thymus. Effects in the lungs of all mice were consistent
with Sendai vital infection. There was no NOAEL since absolute liver
weights were increased at all doses (US National Toxicology Program,
1987).
Rats
An unspecified number of young rats (strain unspecified) were fed
diets containing 0 or 2000 ppm quintozene for 10 weeks. No gross
effects were noted, other than a decreased growth rate in males
(Wit et al., 1957).
Groups of five rats (strain unspecified) of each sex were fed
diets containing a 20% dust formulation of quintozene for 13 weeks to
give doses of 0, 0 (dust minus quintozene), 63.5, 635, 1250, 2500, or
5000 ppm. Animals were weighed weekly, and erythrocyte, haemoglobin,
and leukocyte determinations were performed at term. The liver,
kidney, and testis were weighed, and the heart, lung, liver, kidney,
spleen, stomach, small intestine, caecum, large intestine, thyroid,
adrenals, pancreas, and gonads were examined histologically. Rats at
5000 ppm lost weight during the first two weeks and were sacrificed in
poor condition at the start of week 3, although only one male had
died. Body weights were reduced to a statistically significant degree
in males and nonsignificantly in females at 2500 ppm. One male at 0
(powder control), one at 63.5 ppm, and one at 1250 ppm died.
Haematological parameters were not altered (no data reported). The
liver:body weight ratios were significantly increased in all treated
animals, except for females at 63.5 ppm. The kidney:body weight ratios
were increased in males at 1250 and 2500 ppm. 'Fine vacuolation' of
liver cytoplasm was seen in rats at 5000 ppm. There was no NOAEL
(Finnegan et al., 1958)
Groups of 10 male and 10 female rats (strain unspecified) were
fed diets containing quintozene at 0, 1000, 5000, or 10 000 ppm for 90
days. Growth was inhibited slightly at 5000 ppm and markedly at
10 000 ppm. The NOAEL was 1000 ppm, equivalent to 50 mg/kg bw per day
(Hoescht AG, 1964).
Four groups of 15 Charles River CD rats of each sex, seven weeks
of age, were fed diets containing quintozene (purity, 99.5%) at
concentrations of 0, 50, 3000, or 6000 ppm for 13 weeks. The average
intakes were 3.07, 187, and 381 mg/kg bw per day for males and 3.69,
223, and 455 mg/kg bw per day for females. Diets were analysed for
homogeneity (10 samples per dose), stability (on days 0 and 10), and
concentration (10 samples at weeks 1 and two samples at 2, 3, 4, 8,
and 12 weeks). The homogeneity of eight samples of diet containing
50 ppm was 95-108%, with two outlyers at 117 and 118%; the homogeneity
of 10 samples of the diet containing 3000 ppm was 92-101%, and that of
10 samples of diet at 6000 ppm was 93-98%. Duplicate analyses of
stability showed it to be within 10%. The concentrations of quintozene
in the diets were 98% (range, 91-103%) at 50 ppm, 94% (91-97%) at
3000 ppm, and 92% (85-95%) at 6000 ppm. Rats were observed twice a day
for toxic signs, morbidity, and mortality; food intake and body weight
were recorded weekly. Erythrocyte count, haemoglobin level,
haematocrit, total and differential leukocyte counts, platelet count,
reticulocyte count, mean corpuscular volume, mean corpuscular
haemoglobin, and mean corpuscular haemoglobin concentration; sodium,
potassium, chloride, calcium, inorganic phosphorus, alkaline
phosphatase, total bilirubin, aspartate aminotransferase, alanine
aminotransferase, creatine phosphokinase, urea nitrogen, creatinine,
total protein, albumin, globulin, cholesterol, and serum glucose; and
the colour, appearance, volume, specific gravity, microscopic
elements, pH, protein, glucose, ketones, bilirubin, occult blood,
nitrite, and urobilinogen in urine were reported for 10 rats of each
sex at each dose at week 13. Blood samples from the orbital sinus and
urine were collected during a fasting period. Ophthalmoscopic
examinations were performed on all rats before and on day 86 of the
study. The adrenals, heart, brain, kidney, liver, and gonads of all
rats were weighed post mortem. All animals were examined
macroscopically, and about 40 tissues were preserved. All tissues from
controls and from rats at the high dose and the livers, kidneys, and
lungs of animals at the low and middle doses were examined
microscopically. Lesions observed grossly were also examined.
There were no deaths during the study. Several minor signs (e.g.
hair loss and scabbing) occurred at low incidences but were not
dose-related. Malocclusion was seen in 1, 0, 6, and 4 males at the
four doses, respectively, but is unlikely to have been related to
treatment. Decreased body weight was noted in animals of each sex at
3000 and 6000 ppm and was statistically significant in males at
6000 ppm and in females at 3000 and 6000 ppm. Slightly decreased
terminal weight was seen in animals of each sex (by 8.1 and 6.1% in
males and females at the high dose, respectively) and was dose-
related. Body-weight gain was decreased by 16% in animals of each sex
at 6000 ppm and by 11 and 13% in males and females, respectively, at
3000 ppm. Food intake was reduced significantly in animals of each sex
at 6000 ppm during week 1 and sporadically in weeks 3, 4, and 8 in
males and week 5 in females. A consistent, nonsignificant reduction in
food intake was seen throughout the study in animals of each sex,
except in females at weeks 2 and 4; the food intake of males at
3000 ppm was significantly reduced only in week 1 but was consistently
less than that in controls except in week 10. Females had a
statistically significant reduction in food intake in weeks 5, 6, and
7 and a nonsignificant reduction at other times.
Ophthalmological examination showed no compound- or dose-related
effects, and no adverse effects were observed on haematological
parameters. Reduced alanine aminotransferase levels were seen in
animals of each sex at 3000 and 6000 ppm. In females, total protein
was increased at all doses, albumin was increased at 3000 ppm, and
globulin at 3000 and 6000 ppm; the cholesterol level was significantly
increased at 50 and 6000 ppm. In males, serum alkaline phosphatase
activity was increased at 50 ppm only; no changes were seen in serum
protein levels. The individual total protein levels in females were
greater than the range of levels in the controls in one rat per dose.
A similar pattern was seen for albumin and globulin, except that the
globulin level in two animals at 6000 ppm exceeded the maximal level
in controls. Although these mean values are statistically significant,
the effect was minimal and unlikely to be of toxicological
significance. The increase in cholesterol levels was seen at all doses
but was significant only at 50 and 6000 ppm. The changes were limited
to one sex, and the biological significance of this observation is
uncertain. The pH of the urine of males at 3000 and 6000 ppm was
lowered, but the values were within normal limits for rats. Other
parameters, e.g. a high ketone level in one male rat at 6000 ppm and
low levels of glucose (0.1 g/dl) in one male per dose, showed wide
individual variations and were not considered to be of toxicological
significance.
The absolute kidney weight was increased in males at 3000
(statistically significant) and 6000 (statistically nonsignificant)
ppm, and the kidney weights relative to those of the body and brain
were increased significantly in males at 3000 and 6000 ppm. The
absolute kidney weights were decreased in females at 50 ppm, and the
weights relative to body weight but not brain weight were
significantly decreased at 50 ppm and increased at 6000 ppm. The
apparent effect on the kidney:body weight ratio is. probably a
reflection of decreased body weight at 6000 ppm. The absolute liver
weights were nonsignificantly increased in animals of each sex at 3000
and 6000 ppm, the effect being dose-related in females. The liver:body
weight ratios were significantly increased in males at 3000 and
6000 ppm and in females at 6000 ppm, probably reflecting decreased
body weight. The liver:brain weight ratios were nonsignificantly
increased in animals of each sex at 3000 and 6000 ppm. Changes in the
weight of the brain relative to body weight in males at 3000 and
6000 ppm and of the heart relative to body weight in males at 6000 ppm
were also attributed to reduced body weights.
Males had increased incidences of chronic nephritis (1, 6, 5, and
4 out of 15 animals at 0, 50, 3000, and 6000 ppm, respectively), which
were not dose-related, and an increased incidence of mild haemorrhage
in mesenteric lymph nodes (4 at 6000 ppm; 0 in controls), but these
effects were unlikely to be related to treatment. Mandibular lymph
node haemorrhages occurred in 5/15 control and 0/15 male rats at
6000 ppm, and mediastinal lymph node haemorrhages were seen in 3/15
control and 1/15 males at 6000 ppm. Low incidences of haemorrhage seen
in medias final lymph nodes of seven females at 6000 ppm and three
controls and of mesenteric lymph nodes in six females at 6000 ppm and
no controls may indicate a slight effect, the significance of which is
not known. The only effects in liver were seen at 6000 ppm, comprising
hepatocellular hypertrophy in 7/15 males and 8/15 females and in none
of the controls. The NOAEL was 50 ppm, equal to 3.07 mg/kg bw per day,
on the basis of minimal changes in body-weight gain and liver weight
and changes in alanine aminotransferase activity at higher doses
(McGee, 1988).
Four groups of six acclimatized Charles River CD rats of each
sex, with initial body weights of 247-265 g for males and 229-244 g
for females, were treated on clipped dorsal skin, 6 h per day for 21
days with quintozene (purity, 98.72%) applied as a paste in deionized
water at 0, 30, 300, or 1000 mg/kg bw per day. The application site
was wrapped in gauze bandaging secured with non-irritating tape. After
each application the test site was washed with tepid water. Rats were
observed for mortality, moribundity, clinical signs, and behaviour
twice daily, and irritation was scored by the Draize method once
daily. Body weight was recorded before treatment and then weekly, and
food intake was measured weekly. Haematological and clinical chemical
tests were done at termination on blood obtained from the orbital
sinuses before fasting, and urine samples obtained during fasting were
analysed. The haematological parameters measured included erythrocyte
counts, haemoglobin, haematocrit, total and differential leukocyte
counts, mean corpuscular volume, mean corpuscular haemoglobin, mean
corpuscular haemoglobin concentration, and platelet counts. The
clinical chemical parameters measured were sodium, potassium,
chloride, calcium, inorganic phosphorus, alkaline phosphatase, total
bilirubin, aspartate and alanine aminotransferases, lactic
dehydrogenase, creatine phosphokinase, urea nitrogen, creatinine,
total protein, albumin, globulin, the albumin:globulin ratio,
cholesterol, and glucose; and the urinary parameters were colour,
appearance, volume, specific gravity, microscopic elements, pH,
protein, glucose, ketones, bilirubin, occult blood, nitrite,
urobilinogen, and leukocyte incidence. All rats were examined
macroscopically post mortem; kidneys, liver, and testes were
weighed, and 44 organs or tissues per rat were preserved, as were
gross lesions seen at macroscopic examination. Microscopic examination
was limited to liver, kidney and treated and untreated skin in animals
at 0 and 1000 mg/kg bw per day, and all gross lesions.
There were no deaths. Ventral hair loss was seen occasionally in
females, but the incidence was not dose-related. No behavioural
changes and no signs of dermal irritation were detected. No
compound-related changes in body weight were seen, although increased
body-weight gain was noted in males at 30 mg/kg bw per day
(statistically nonsignificant). Food intake was comparable in all
groups. There were no statistically significant changes in any
haematological parameters, and clinical chemistry parameters were
unaffected, except for a statistically significant, dose-related
decrease in alanine aminotransferase activity in males and in females
at 1000 mg/kg bw per day; a statistically significant decrease was
also seen at 1000 mg/kg bw per day, but there was no effect at lower
doses. Other changes (increased alkaline phosphatase activity and
decreased total bilirubin in females at 300 mg/kg bw per day) were not
dose-related. There were no significant changes in urinary parameters.
Organ weights also showed no statistically significant changes,
although testicular weights were reduced in two of five rats at
1000 mg/kg bw per day; these testes were not examined microscopically.
There were no compound- or dose-related microscopic effects on the
liver, kidney, or treated or untreated skin. The NOAEL was 300 mg/kg
bw per day (Goldenthal, 1992).
Dogs
Three groups of three mongrel dogs were fed dietary levels of 25,
200, or 1000 ppm quintozene for one year by mixing a commercial powder
containing 20% quintozene, 77% pyrax ABB (a pyrophyllite carrier), and
3% Armour sticker with the diet, which was mixed with water
immediately before feeding. Dogs were weighed weekly; haematological
parameters (unspecified but including total and differential leukocyte
counts) were measured at 0, 6, and 12 months; and the heart, lung,
liver, kidney, spleen, gastrointestinal tract, thyroid, adrenal,
pancreas, gonads, and bone marrow were examined histologically. One
dog at 200 ppm whelped at week 5 of the study, and two dogs at
1000 ppm whelped at weeks 7 and 8. Some pups were allowed to suckle
and were then sacrificed at four to six weeks of age and examined
histopathologically. There were no deaths. Body weight was variable
but apparently unaffected by quintozene. The only effects on
haematological parameters were increased eosinophil counts at 6 and 12
months in some animals (probably due to parasites) and low total
leukocyte counts at 12 months in dogs at 10 000 ppm. No lesions were
seen in the pups examined histopathologically. Hepatic-cell
enlargement with pale cytoplasmic staining was noted in all adult
dogs, but the severity was not dose-related. Periodic acid-Schiff and
Best carmine staining of the liver showed abundant glycogen around the
periphery of lobules. Staining for fat gave negative results. There
was no NOAEL, owing to the random whelping (Finnegan et al., 1958).
Four groups of three beagle dogs (Ridglan Farms, Inc.),
previously immunized and vaccinated against most common viruses and
infections, were fed diets containing 0, 40, 2000, or 4000 ppm
quintozene (purity, at least 96%) for four weeks. The diets were
prepared weekly, acetone being used as an aid to dispersion at 40 ppm
and also added to control diets. Homogeneity and stability were
measured in week 4, and the concentration of quintozene was measured
in the diets for weeks 2, 3, and 4. The homogeneity in 10 samples was
91-99% (mean, 95%) at 40 ppm, 92-103% (mean, 98%) at 2000 ppm, and
95-104% (mean, 98%) at 4000 ppm. The stability over 10 days (in
duplicate analyses) was 98-101%, and the dietary concentrations were
85-116% (mean, 100%) at 40 ppm, 91-108% (mean, 100%) at 2000 ppm, and
92-98% (mean, 94%) at 4000 ppm. Dogs were observed twice daily for
clinical signs, mortality, and moribundity. Body weights were measured
before treatment and then weekly; food intake was measured weekly.
Total and differential leukocyte counts, erythrocyte count,
haemoglobin, haematocrit, mean corpuscular volume, mean corpuscular
haemoglobin, mean corpuscular haemoglobin concentration, platelet
counts, and reticulocyte counts; and sodium, potassium, chloride,
calcium, inorganic phosphorus, alkaline phosphatase, total bilirubin,
aspartate and alanine aminotransferases, creatine phosphokinase, urea
nitrogen, creatinine, total protein, albumin, globulin, cholesterol,
and glucose were measured before and after four weeks of treatment.
Blood samples were taken from the jugular vein of dogs fasted
overnight, and urine collected during this period was analysed for
colour, appearance, volume, specific gravity, microscopic elements,
pH, protein, glucose, ketones, bilirubin, occult blood, nitrite, and
urobilinogen. The adrenals, brain, heart, kidney, liver, gonads,
pituitary, spleen, and thyroid-parathyroid were weighed and examined
macroscopically, and about 40 organs were preserved but were not
examined microscopically.
There were no deaths or dose-related clinical signs, and no
compound-related effects were observed on body weight. Food intake, on
the basis of grams per animal per day, was reduced in males at
4000 ppm throughout the study and in those at 40 and 2000 ppm during
week 1; it was also decreased in weeks 2 and 4 but was increased in
animals at 2000 ppm in weeks 2, 3, and 4. Food intake in females on
this basis was slightly reduced in all groups at all intervals, but
the decreases were not dose-related. Food intake on the basis of grams
per kilogram body weight was reduced in males at all doses in week 1
and in those at 40 and 3000 ppm at all periods; however, intake was
increased in animals at 2000 ppm in weeks 2, 3, and 4. In females,
intake on this basis was decreased in week 1 and at 4000 ppm in week
2; at other periods, intake was close to control values. Overall,
intake was reduced in all treated animals in week 1 and was
inconsistent thereafter. None of the changes in food intake is
statistically significant, and they are unlikely to be biologically
significant.
No significant haematological changes were observed in males. In
females, such changes were limited to decreased leukocyte counts in
animals at 40 and 4000 ppm and decreased mean corpuscular haemoglobin
in dogs at 2000 ppm. None of these changes is of toxicological
significance. Alanine aminotransferase activity was markedly decreased
in a dose-related manner in animals of each sex, and dose-related
increases in cholesterol levels were seen in females at all doses and
in males (131, 192, 199 and 167 mg/dl at 0, 40, 2000, and 4000 ppm).
No significant changes were observed in urinalysis. The absolute
weights of kidney and spleen were statistically significantly greater
than those of controls in male dogs at 2000 ppm and were nonsigni-
ficantly increased in dogs at 4000 ppm. Liver weights were also
nonsignificantly increased (not dose-related) at these doses. The
liver:body weight ratios of males at 2000 and 4000 ppm were
statistically significantly increased, as were those of the kidney at
2000 ppm; there was a considerable but nonsignificant increase at
4000 ppm. Spleen:body weight ratios were statistically nonsigni-
ficantly increased in males at 2000 and 4000 ppm. The thyroid-
parathyroid:body weight ratio was significantly increased in male dogs
at 2000 ppm and nonsignificantly in those at 4000 ppm. The ratios of
kidney, spleen, and thyroid-parathyroid to brain weights were
significantly increased in male dogs at 2000 ppm and nonsignificantly
at 4000 ppm; the liver:brain weight was significantly increased at
both 2000 and 4000 ppm. In females, the only statistically significant
change was in the liver:body weight ratio, which was increased at 2000
and 4000 ppm; nonsignificantly increased absolute liver weights were
also noted, and nonsignificant increases in brain and brain:body
weight ratios were seen at 2000 and 4000 ppm. The effects on organ
weights (particularly liver) and on clinical chemical parameters
indicate a probable NOAEL of 40 ppm, equivalent to 1 mg/kg bw per day;
however, in the absence of histopathological reports, the significance
of the findings cannot be fully assessed (Johnson, 1989).
Four groups of six beagle dogs of each sex (Ridglan Farms Inc.),
five to six months old and suitably immunized and vaccinated, were fed
diets containing 0, 15, 150, or 1500 ppm quintozene (purity, 99.4%,
with HCB at a maximum of 0.07% and a mean of 0.045%) for one year. The
homogeneity and stability of the diets were determined before the
study, and all diets were analysed for the percentage of the nominal
levels that were actually present. Diets were prepared weekly for four
weeks and monthly thereafter. Acetone was used as the dispersant in
the 15 and 150 ppm and control diets. The homogeneity in 10 samples
was 93-99% at 15 ppm, 85-107% at 150 ppm, and 89-110% at 1500 ppm,
with respective mean values of 95, 93, and 99%. Stability,
investigated over 10 days, was satisfactory. Duplicate analyses for
actual concentrations at each interval and dose showed mean values
over the study of 96, 94, and 96% and ranges of 87-104%, 89-110%, and
82-103% at 15, 150, and 1500 ppm, respectively. The actual intakes
were calculated from data on food intake and body weight to be 0.4,
4.3, and 40.1 mg/kg bw per day for males and 0.44, 4.22, and
41.48 mg/kg bw per day for females. Dogs were observed for mortality,
moribundity, and clinical signs twice daily. Body weights and food
intake were recorded weekly for 14 weeks and monthly thereafter.
Ophthalmology was performed before the test and in week 52.
Haematological and clinical chemical examinations were performed on
blood from the jugular vein of dogs fasted overnight at 6 and 12
months. Urinalysis was performed at 6 and 12 months on urine collected
during the fasting period. The same haematological, clinical chemical,
and urinary parameters were investigated as in the 28-day study
(Johnson, 1989). At termination (death before the end of the study or
sacrifice), the adrenals, brain, heart, kidneys, liver, gonads,
pituitary, spleen, and thyroid-parathyroids were examined grossly and
weighed. Adrenals, aorta, bone (rib), bone marrow (rib), brain (fore-,
mid-, and hind-), eye (with optic nerve), gall-bladder, oesophagus,
stomach, duodenum, jejunum, ileum, caecum, colon, rectum, ovaries,
testis with epididymides, heart, kidneys, liver, lung (with bronchi),
lymph nodes (tracheobronchial, mesenteric, and regional), mammary
gland (females only), pancreas, pituitary, prostate, salivary gland
(with mandibular lymph node), sciatic nerve, skeletal muscle (thigh),
skin, spinal cord (cervical, thoracic, and lumbar), spleen, thymic
region, trachea, urinary bladder, and uterus from all dogs were
preserved, processed, and examined microscopically.
In one male dog at 15 ppm that was sacrificed in extremis at
week 28, the clinical and pathological signs indicated a diagnosis of
generalized systemic blastomycosis. The death was not related to
treatment, and the clinical signs reported (including diarrhoea,
emesis, alopecia, lacrimation, and ocular discharge) did not indicate
dose- or compound-related incidence. One male at 150 ppm had
convulsions in weeks 39 and 48 and tremors in weeks 39-40, associated
with limb rigidity in weeks 39-40 and excessive salivation. No
abnormal neural effects were seen histopathologically at term. The
effect was isolated and is unlikely to have been compound-related.
Body weight and weight gain were unaffected. Food intake was variable,
but no clear trends were apparent. There were no compound-related
effects on haematological parameters, the only statistically
significant difference from control values being decreased haemoglobin
levels at 12 months in males at 15 ppm. Statistically significant
changes in clinical chemical parameters comprised increased alkaline
phosphatase activity at 1500 ppm in males at 6 and 12 months and in
females at 6 months; a considerable but not statistically significant
increase occurred in females at 12 months. Significantly decreased
alanine aminotransferase activity was seen in animals of each sex at
150 and 1500 ppm at 6 and 12 months; significantly decreased
creatinine was noted in males at these doses at 6 and 12 months and in
females at 1500 ppm at 6 months. Cholesterol levels were significantly
increased in males at 1500 ppm at 6 and 12 months and nonsignificantly
increased in females at 1500 ppm at 6 and 12 months. Globulin levels
were also significantly increased in males at 150 and 1500 ppm at 6
months, and blood urea nitrogen was decreased in females at 1500 ppm
at 12 months. No effects were seen on urinalysis.
Statistically significant increases in the absolute and relative
(to body and brain weight) weights of liver were seen in females at
1500 ppm and in relative weights in males at 1500 ppm; the absolute
liver weight in males was also increased but not significantly.
Nonsignificant increases in absolute and relative liver weights were
seen in animals of each sex at 150 ppm. A statistically significant
increase in the kidney:body weight ratio was seen in females at
1500 ppm. The maximal absolute adrenal weights were slightly increased
in males at 1500 ppm, and females showed a minimal, dose-related
increase in adrenal weights. Microscopic examination showed slight
hepatocellular hypertrophy in all dogs at 1500 ppm. No dose-related
effects were seen on alveolar macrophages, inflammation, or the
incidence of pneumonia. Adrenal changes in males were comparable in
all groups. In females at 1500 ppm, the incidence of lymphocytic
infiltration and vacuolar changes was increased, but these effects are
considered not to be of toxicological significance. The incidence of
pituitary cysts was increased in animals of each sex at 1500 ppm, but
such cysts were relatively common in control beagle dogs. The NOAEL
was probably 150 ppm, equal to 4.3 mg/kg bw per day in males and
4.22 mg/kg bw per day in females. Changes in clinical chemical
parameters seen at this dose (e.g. decreased alanine aminotransferase
activity and decreased creatinine) did not appear to be associated
with microscopic changes in tissues or organs (Goldenthal, 1990)
Four groups of three beagle dogs of each sex were fed diets
containing quintozene (purity, 98.8%) at concentrations of 0, 500,
1000, or 5000 ppm for two years. Erythrocyte counts, total and
differential leukocyte counts, haemoglobin, haematocrit, and Heinz
bodies; and the specific gravity, pH, appearance, colour, microscopic
sediment, albumin, glucose, and bilirubin in urine were reported at
26, 52, and 78 weeks and at terminal sacrifice. The heart, lung,
liver, kidney, spleen, pancreas, gonads, prostate, adrenal, thyroid,
brain, pituitary, eye with optic nerve, stomach, jejunum, colon, and
bone marrow were examined histologically. Of the animals at the
highest dose, three males died on days 214, 288, and 357 and two
females on days 317 and 472. Food intake was slightly reduced (mean,
4.6%) in animals at 1000 ppm and markedly (by about 30%) in those at
5000 ppm. All dogs at 5000 ppm lost weight, and their body-weight
gains (means, 2.75 kg at 500 and 2.62 kg at 1000 ppm) were lower than
that of controls (4.85 kg). Signs of toxicity at 5000 ppm included
lacrimation, conjunctival discharge, and milky corneal opacity; one
dog had corneal ulceration. One dog at 1000 ppm had conjunctivitis and
a second had corneal opacity for a short period. No effects were seen
at 500 ppm. Anaemia was induced in dogs at the high dose, with
characteristic reductions in haemoglobin, haematocrit, and erythrocyte
counts. Atrophy of bone marrow and reduced haematopoiesis were
observed at 5000 ppm. Severe histopathological changes in the liver
were seen at 5000 ppm and included fibrous narrowing of hepatic cell
cords, enlarged periportal areas, and increased leukocytic
infiltration. Similar but less intense effects were seen at 500 and
1000 ppm. There was no NOAEL (Scholz & Brunk, 1968).
Five groups of four beagle dogs of each sex, 4.5 months old, were
fed diets containing quintozene (purity, 98.2%; containing 1.4% HCB
and traces of tetrachloronitrobenzene and pentachlorobenzene) at
concentrations of 0, 5, 30, 180, or 1080 ppm for two years. Diets were
prepared weekly by dilution of the high dose, which was prepared by
blending corn-oil solutions of quintozene with the diet; the dietary
fat content was increased from 9 to 11%. Before feeding, an equal
weight of water was added to the food. Body weight and food intake
were determined weekly. The haematocrit, haemoglobin, and total and
differential leukocyte counts, and urinary reducing substances,
protein, specific gravity, and sediment were determined at 3, 6, 12,
18, and 24 months. Blood urea nitrogen, serum aspartate
aminotransferase, serum alkaline phosphatase, and cholinesterase,
prothrombin time, and bromsulphthalein retention time were determined
in controls and dogs at the high dose at 0, 3, 6, 12, 18 and 24
months. The frequency of oestrus was recorded. One dog of each sex at
each dose was sacrificed at 12 months for histopathological
examination. Organ and organ:body weight ratios were reported for
heart, spleen, liver, kidneys, and testes; the brain, lung, heart,
aorta, liver, spleen, kidney, stomach, ileum, jejunum, large
intestine, urinary bladder, bone marrow (sternal and long bone),
pituitary, thyroid, pancreas, adrenal, gonad, lymph node, and eye were
examined histologically.
No deaths were observed. The changes in body weight were not
significant, but weight gain tended to be reduced in females at 180
and 1080 ppm during weeks 6 and 13, and sporadically in all treated
males, which also had sporadic decreases in food intake. Neither body
weight nor food intake was consistently affected by treatment.
Slightly reduced haematocrit values were noted in males at 30 and
180 ppm at 78 weeks (statistically significant), and reduced
haemoglobin values were noted in males at 30 ppm; a nonsignificant
decrease in haematocrit and haemoglobin values was again seen in males
at 30 and 180 ppm at 104 weeks. The absence of any effect at 1080 ppm
indicates that these effects were probably random. No consistent
effects were seen on serum cholinesterase, the maximal inhibition
being 20% in females at 1080 ppm in week 13 and 23.6% in females at 30
ppm at week 52; all other depressions were < 15.4% and were random.
Mean serum aspartate aminotransferase activity was increased in
females at 1080 ppm at 13 and 78 weeks but decreased at 104 weeks
(statistically significant only at 13 weeks); it was also decreased in
males at 1080 ppm at 26 weeks and increased at 78 weeks. Serum
alkaline phosphatase activity was increased in males and females at 52
weeks and thereafter, but the increase achieved statistical
significance only at 52 weeks when data on animals of each sex were
combined. External lymph nodes and oestrus cycle were not affected by
treatment. Liver weight and the liver:body weight ratios of males and
females at 180 and 1080 ppm were nonsignificantly increased at one
year; at two years, a nonsignificant increase in absolute liver weight
was seen in males at 1080 ppm and a significant increase in females.
The increased liver:body weight ratio was significant only when data
for the two sexes were combined. The absolute and relative liver
weights were nonsignificantly reduced in females at 5, 30, and 180 ppm
but significantly reduced in males at 30 and 180 ppm. Testicular
weights were nonsignificantly reduced in dogs at 1080 ppm, and the
weight ratio was statistically significantly reduced. Other changes
(such as increased kidney weight ratio at 5 ppm and increased heart
ratio at 180 ppm in males) were random. Dogs at 180 ppm showed minimal
suppression of bile flow in liver cord cells; the effect was greater
in animals at 1080 ppm, resulting in moderate bile pigment
accumulation in cord cells. The effect was usually reversible. The
NOAEL was 30 ppm, equivalent to 0.75 mg/kg bw per day (Larson &
Borzelleca, 1968; Borzelleca et al., 1971).
Monkeys
Two rhesus monkeys of each sex were fed consecutive daily doses
of quintozene (purity, > 99.9%) on sucrose pellets, corresponding to
2 ppm of their daily diet, for 70 days. There were no controls. One
monkey of each sex was sacrificed on day 71. Haemoglobin, packed cell
volume, erythrocyte and total leukocyte counts, methaemoglobin, and
Heinz bodies, and sodium, potassium, bilirubin, creatinine, blood urea
nitrogen, total protein, serum aspartate and alanine aminotrans-
ferases, lactate dehydrogenase, cholesterol, luteinizing and
follicular-stimulating hormone levels, progesterone, and cortisol were
determined in blood samples taken before the test, on days 19 and 48,
and, in the surviving monkeys, on day 76. There were no changes in
haemoglobin values, packed cell volume, or erythrocyte or leukocyte
cell counts. The level of methaemoglobin was slightly elevated on day
1 but not thereafter. No change was seen in the incidence of Heinz
bodies. No changes were seen in clinical chemistry, although the data
on bilirubin and lactate dehydrogenase were highly variable; no time
effect relationship was apparent. At sacrifice, no histopathological
effects were seen in the liver, stomach, large or small intestine,
spleen, kidney, heart, lung, thymus, cerebrum, cerebellum, pons,
medulla, spinal cord, or bone marrow of the animals sacrificed on day
71. The NOAEL was > 2 ppm, equivalent to > 0.1 mg/kg bw per day
(Kögel et al., 1979b)
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 18 B6C3F1 and B6AKF1 mice of each sex were given
quintozene (purity and contaminants unspecified) at a dose of
464 mg/kg bw by stomach tube from seven days of age to the time of
weaning at four weeks of age and thereafter at a dose of 1206 ppm for
18 months. The latter was the maximal tolerated dose. There was a
significantly elevated incidence of tumours, mostly hepatomas, in both
strains (Innes et al., 1969).
Groups of 100 male and 100 female Swiss random strain mice were
fed diets containing quintozene (containing 2.7% HCB) at levels of 0,
100, 400, or 1200 ppm for 80 weeks. Body-weight gain was decreased in
animals of each sex at 1200 ppm. Liver:body weight ratios were
increased in males and females at 400 and 1200 ppm, and the
kidney:body weight ratio was increased in females at 1200 ppm. General
appearance, behaviour, and survival were not affected by treatment,
and the haematological indices were considered to be within normal
limits. A non-dose-related increase in nodular hyperplasia of the
liver was seen in all treated males. The hyperplastic areas had
essentially normal cell architecture and were regarded as
non-neoplastic. An increased incidence of subcutaneous fibrosarcomas
was observed in females at 1200 ppm. None of the other neoplasms
appeared to be related to treatment and were common in mice of this
strain. There was no NOAEL (van der Heijden & Til, 1974).
Groups of 50 male and 50 female mice (probably B6C3F1) were fed
diets containing two increasing doses of quintozene (purity, > 98%;
with 0.15% pentachlorobenzene, 0.25% 2,3,4,5-tetrachloronitrobenzene,
and 1% HCB), initially at 1075 and 2150 ppm for males and 2320 and
4640 ppm for females, increased to 3000 and 6000 ppm for males and
4500 and 9000 ppm for females during the last 315 days of the 546-day
(78-week) exposure period. A control group consisting of 20 male and
20 female mice was given only the vehicle, 2% corn oil. The total
duration of the study was 92 weeks, but some mice were killed and
autopsied earlier. Malignant tumours were seen at frequencies of 50%
in 25% of control males and 20% in 10% of control females, at 33% in
29% of males and 18% in 6% of females at the low dose, and at 29% in
20% of males and 57% in 19% of females at the high dose. Only the last
frequency was statistically significantly higher than expected; many
malignant histiocytic lymphomas were found in numerous organs of the
same animal. No significant differences were found between groups in
the numbers of animals with tumours, and no particular type of tumour
could be ascribed to the treatment (Wedig et al., 1976).
Three groups of 50 B6C3F1 mice of each sex, seven to eight weeks
old, were fed diets containing quintozene (purity, 99.6%, with 0.07%
HCB) at doses of 0, 2500, or 5000 ppm for 103 weeks, followed by a
one-week withdrawal period before terminal sacrifice. Mice were
observed twice daily, and five per cage were weighed weekly for 12
weeks and monthly thereafter. Food intake per cage was measured every
four weeks. The homogeneity of the diets was within 10%; the stability
of quintozene in the diet for two weeks was 100% at 25°C; and the mean
actual dietary concentrations, measured 14 times during the study,
were 2460 ppm (98.4%) with a range of 94-104.4% at 2500 ppm and
4980 ppm (99.6%) with a range of 94-104% at 5000 ppm. All animals
(including those that died before the end of the study) were
necropsied, unless autolysis or cannibalization had occurred. Tissue
masses, abnormal regional lymph nodes, mammary gland, salivary gland,
bone marrow, costochondral junction, thymus, larynx, trachea, lungs
and bronchi, heart, thyroid, parathyroid, oesophagus, stomach,
duodenum, jejunum, ileum, colon, mesenteric lymph nodes, liver,
gall-bladder, pancreas, spleen, kidney, adrenal, urinary bladder,
seminal vesicles, prostate, uterus, gonads, brain, pituitary, and
caecum from controls, female mice at the low dose, males and females
at the high dose, and all males at the low dose that died before term
were examined histologically. Gross lesions, livers, and nasal
cavities from all males at the low dose were also examined.
The terminal body weights of male mice were 96% of control values
at 2500 and 90% at 5000 ppm. The mean body weights of females at
5000 ppm were consistently 10% below control values by week 24 and
thereafter, but reached this level at 2500 ppm only after 92 weeks; at
term, the mean weights of females were 18 and 21% below control
values. No clinical signs were reported. Food intake may have exceeded
that of controls, but this was difficult to determine because of food
scattering. The survival of males at term, 31-34 mice, was comparable
in all groups. At term, 30 female controls, 18 at the low dose, and 14
at the high dose were still alive; however, five mice at the low dose
were accidentally drowned and two mice at 2500 ppm and one at 5000 ppm
died during the recovery period.
In male mice at 0, 2500, and 5000 ppm, the numbers with malignant
tumours were 19, 25, and 17 (25, 30, and 21 tumours) and those with
benign tumours were 19, 15, and 17 (23, 16, and 19 tumours). There was
no dose-effect relationship for the incidence of tumours in any
specific organ, the incidences generally being comparable between
groups. The incidence of non-neoplastic lesions in comparison with the
control incidence was not of toxicological significance. In female
mice at 0, 2500, and 5000 ppm, the numbers with malignant tumours were
15, 11, and 9 (15, 11, and 9 tumours) and those with benign tumours
were 16, 17, and 14 (18, 19, and 19 tumours). Tumours were not
concentrated in specific tissues and there was no dose-effect
relationship. Increased incidences of ovarian abscesses were seen,
with 24, 44, and 58% at 0, 2500, and 5000 ppm. Five of six abscesses
that were cultured contained Klebsiella. Hyperplasia of the
mediastinal lymph node was observed at incidences of 2, 9, and 20% and
liver haematopoiesis at 18, 42, and 46%; splenic haematopoiesis was
observed at incidences of 28, 48, and 54%. These effects may reflect
increased susceptibility to infection rather than a toxicological
effect.
Quintozene thus decreased body weight at 5000 ppm, equal to
953 mg/kg bw per day in males and 1358 mg/kg bw per day in females.
There was no increase in neoplasia, and the non-neoplastic
pathological effects in female mice were probably due to infection.
The NOAEL in male mice was 2500 ppm. In female mice at 2500 ppm, the
reduced body weight reached 10% only at 92 weeks and continued to
decrease, to 18%, until 103 weeks; as this effect is unlikely to be
compound-related, 2500 ppm was also the probable NOAEL for female
mice. Overall, the NOAEL was 2500 ppm, equal to 387 mg/kg bw per day
in male mice (US National Toxicology Program, 1987).
A group of 10 mice (strain not specified) of each sex received
topical applications on shaved skin of 0.2 ml of a 0.3% solution of
quintozene (purity and contaminants unspecified) in acetone twice
weekly for 12 weeks. The control group received acetone alone. After
application of quintozene, croton oil was applied to the site
(presumably twice weekly) for 20 weeks, and mice were sacrificed 20
weeks later. Papillomas were seen in 2/10, 6/10, 8/9, and 7/8 treated
males, in 6/10, 7/9, 7/7, and 7/4(?) treated females, in 1/10, 6/9,
5/8, and 1/7 control males, and in 0/10, 3/10, 4/8, and 4/7 control
females 10, 20, 30, and 40 weeks after the initial croton oil
application, respectively. The time to 50% tumour incidence was 13
weeks in treated males, 10 weeks in treated females, 16 weeks in male
controls, and unknown for control females. The total numbers of
tumours were 29 in treated males, 33 in treated females, 6 in control
males, and 7 in control females. Quintozene at the doses used in the
study thus did not induce tumours in the absence of a promoting agent
(Searle, 1966).
Rats
Groups of 10 rats of each sex (strain unspecified) were fed diets
containing 0, 25, 100, 300, 1000, or 2500 ppm quintozene (purity and
contaminants unspecified) prepared by blending a powder comprising 20%
quintozene, 77% pyrax ABB (a pyrophyllite carrier), and 3% Armour
'sticker' with Purina dog chow. Rats were weighed weekly, and
haematological parameters (unspecified) were determined at 11 and 24
months. At death and terminal sacrifice, heart, lung, liver, kidney,
spleen, stomach, small intestine, caecum, large intestine, thyroid,
adrenal, bladder, pancreas, and bone marrow were examined
histologically. At one year, survival in males was 7, 10, 8, 7, 9, and
10 animals, and that in females was 9, 6, 8,10, 10, and 8 animals at
0, 25, 100, 300, 1000, and 2500 ppm, respectively; by two years,
survival was 1, 4, 4, 3, 3, and 4 in males and 3, 2, 5, 5, 5, and 2 in
females. Body-weight changes (only means given) were sporadic;
statistically significant increases were seen in males at 25 ppm
(weeks 4-52) and 100 ppm (week 4) and in females at 100 ppm (weeks
2-78), 300 ppm (week 8 and weeks 52-78), 1000 ppm (week 26), and
2500 ppm (weeks 2-78); decreases were seen in males at 2500 ppm (weeks
2 and 8). The small numbers of animals make definite conclusions
difficult, but the dose of 2500 ppm appeared to decrease body weight
consistently in animals of each sex, as did the dose of 1000 ppm in
females. The decreases at all doses in females may be real, but males
were affected only at 2500 ppm. The haematological parameters were
stated to be within normal limits, and histopathological lesions were
stated to be limited to 'occasional lung abscesses and occurrences of
fatty changes in the liver. Lesions are not correlatable with dietary
concentration of quintozene but are of the type often seen in old
rats.' Individual data were not available. The NOAEL in this limited
study was 300 ppm, equivalent to 15 mg/kg bw per day (Finnegan
et al., 1958).
Groups of 50 male and 50 female Wistar rats were fed dietary
levels of 0, 100, 400 and 1200 ppm quintozene (containing 2.7% HCB)
for two years. The liver:- and kidney:body weight ratios were
increased in rats at 400 and 1200 ppm. Dose-related increases in the
incidences of single-cell necrosis and fatty metamorphosis of
hepatocytes were seen in animals of each sex at 400 and 1200 ppm, and
enlarged centrilobular hepatocytes were seen at all doses. There was
no increase in tumour incidence, and no adverse effects were observed
on general appearance, behaviour, body-weight gain, or food
consumption. Haematological, blood chemical, and urinary values were
considered to be within normal limits. There was no NOAEL in this
limited study (Sinkeldam et al., 1974).
Groups of 50 male and 50 female rats (strain unspecified) were
fed diets containing two decreasing doses of quintozene (purity, >
98%; with 0.15% pentachlorobenzene, 0.25% 2, 3, 4, 5-tetrachloro-
nitrobenzene, and 1% HCB): for the first 98 days, males received 7500
or 15 000 ppm and females, 11 000 or 22 000 ppm; during the last 448
days, males received 5000 or 10 000 ppm and females, 7250 or
14 500 ppm. A control group consisting of 20 male and 20 female rats
was given only the vehicle, 2% corn oil. The total exposure time was
546 days (78 weeks), and the duration of the study was 85-117 weeks.
Malignant tumours were seen at frequencies of 16% in 10% of control
males and 10% in 10% of control females, at 13% in 8.5% of males and
8% in 6% of females at the low dose, and at 23% in 12.5% of males and
9% in 6.5% of females at the high dose. No significant difference
between the groups was found in the total number of tumours or the
number of animals with tumours, and no particular type of tumour could
be ascribed to the treatment (Wedig et al., 1976).
Four groups of 50 Charles River CD rats of each sex, six to seven
weeks old, were fed diets containing quintozene (purity, 99.4%; <
0.07% HCB) at doses of 0, 20, 3000, or 6000 ppm for 24 months. An
additional 10 rats of each sex at each dose were treated similarly and
sacrificed at 12 months. As the homogeneity of the diet at the 20-ppm
dose was unsatisfactory in week 1 (range, 14-40 ppm), blending time
was increased for the week-2 diets, which improved the range to
16-22 ppm. From week 28, acetone was used as an aid to dispersion,
resulting in a variation of 16.3-18.9 ppm. The homogeneity was
acceptable at higher doses. The stability over 10 days was within 8%.
Duplicate analyses about every four weeks indicated that the mean
percentages of the nominal concentrations were 95 8.4 at 20 ppm, 100 ±
6.5 at 3000 ppm, and 101 ± 6.9 at 6000 ppm. Diets were prepared
weekly. Rats were observed twice daily for mortality, moribundity, and
overt signs of toxicity. Body weight and food intake were recorded for
individual rats weekly for 16 weeks and monthly thereafter.
Ophthalmological examinations were conducted before treatment and at
12 and 24 months. Five rats of each sex were screened microbio-
logically before the test. Erythrocyte count, total and differential
leukocyte counts, haemoglobin, haematocrit, platelet counts, mean
corpuscular volume, mean corpuscular haemoglobin, and mean corpuscular
haemoglobin concentration; and sodium, potassium, chloride, calcium,
inorganic phosphorus, alkaline phosphatase, bilirubin, aspartate and
alanine aminotransferases, creatine phosphokinase, urea nitrogen,
creatinine, total protein, albumin, globulin, cholesterol and glucose
were determined in orbital sinus blood from 10 rats of each sex at
each dose, which had been fasted overnight, at 6, 12, 18, and 24
months. The colour, appearance, volume, specific gravity, microscopic
elements, pH, protein, glucose, bilirubin, ketones, occult blood,
nitrite, and urobilinogen in urine were determined in samples
collected during the fasting period at 6, 12, 18, and 24 months. All
animals were necropsied, and adrenals, aorta, femur, bone marrow
(femur), brain (fore-, mid-, and hind-), eye (including optic nerve),
oesophagus, stomach, duodenum, jejunum, ileum, caecum, colon, rectum,
ovary, testis (with epididymides), heart, kidney, liver, lung, lymph
nodes, mammary gland (female only), pancreas, pituitary, prostate and
seminal vesicles, salivary gland (with lymph nodes), sciatic nerve,
skeletal muscle (thigh), skin, spinal cord (cervical, thoracic, and
lumbar), spleen, thymic region, thyroid, parathyroid, trachea, urinary
bladder, uterus, gross lesions, and tissue masses were collected. The
adrenals, brain, heart, kidney, liver, gonads, and thyroid-parathyroid
were weighed at sacrifice at 12 and 24 months. All tissues of control
rats, those at the high dose, and those that died or were sacrificed
were examined histologically at term. Tissue masses, gross lesions,
regional lymph nodes, and, in females only, lung were examined
histologically at interim sacrifice of animals at 20 and 3000 ppm; the
liver, kidney, lung, thyroids, parathyroids, tissue masses, and
regional lymph nodes of animals at these doses were examined at
terminal sacrifice.
Mortality was 52, 54, 42, and 16% in males and 42, 50, 46, and
48% in females at 0, 20, 3000, and 6000 ppm, respectively, excluding
rats sacrificed at 12 months. One of 60 rats at each dose had died by
12 months, the only clinical sign being a slightly increased incidence
of body surface staining in females at 6000 ppm and an increased
incidence of malocclusion in males at this dose from week 1 to term,
which was obviously not compound-related. Body weight was
significantly reduced in animals of each sex at 6000 ppm throughout
the study, the decrease being > 10% by week 2 in males and by week 24
in females. Slightly lower weights were noted in females at 3000 ppm
after 24 weeks (statistically significant in 14/21 weighings), but the
weight decreases in males were generally comparable to those of
controls, except for a slight (generally statistically nonsignificant)
reduction after 84 weeks. Food intake on the basis of grams per
kilogram body weight tended to be increased, especially in males, and
to a lesser degree females, at 6000 ppm. The average intakes of the
diets at 20, 3000, and 6000 ppm were 0.9, 141, and 303 mg/kg bw per
day in males and 1.14, 179, and 370 mg/kg bw per day in females. No
compound-related ophthalmological findings were seen.
Statistically significant haematological findings tended to be
sporadic and do not appear to be compound-induced. In males at
6000 ppm, haemoglobin and haematocrit were reduced at six months only,
and mean corpuscular volume was reduced at 6 and 12 months; platelet
counts were increased at 12 months in males at 20 ppm. In females,
erythrocyte counts were elevated at 18 months at 20 and 6000 ppm,
haemoglobin was depressed at 6 months at 3000 and 6000 ppm but was
increased at 18 months at 20 ppm, haematocrit was increased at 18
months at 20 ppm, mean corpuscular volume was decreased at 18 months
at 3000 ppm and at 6, 12, and 18 months at 6000 ppm, mean corpuscular
haemoglobin was decreased at 18 months at 3000 ppm and at 6 and 18
months at 6000 ppm, and mean corpuscular haemoglobin concentration was
depressed at 6 months at 6000 ppm. Lymphocyte counts were elevated at
12 months at 20 ppm.
The clinical chemical findings in males included increased
potassium levels at 24 months and decreased glucose at 6 months at
6000 ppm, which are probably random effects. Alanine aminotransferase
activity was reduced at 6 months at 20 ppm, at 6, 18, and 24 months at
3000 ppm, and at all intervals at 6000 ppm; however, it was increased
at 12 months at 3000 ppm. The decrease is considered to be compound-
related, and the amount is dose-related. In females at 6000 ppm,
slight decreases in chloride were seen at 6 and 12 months and slightly
increased calcium at 18 months. Alkaline phosphatase activity was
increased at 6 and 12 months and aspartate aminotransferase activity
was decreased at all intervals at 6000 ppm; alanine aminotransferase
activity was decreased in a dose-related manner at all intervals at
3000 and 6000 ppm. Cholesterol was increased at all intervals and
glucose was decreased at 6 months at 6000 ppm. Total protein was
increased at 18 months at 3000 and 6000 ppm. Thus, in animals of each
sex, compound-related effects on alanine aminotransferase activity
were seen at 3000 and 6000 ppm, and in females aspartate aminotrans-
ferase activity was decreased and cholesterol was increased at
6000 ppm. The effects on alkaline phosphatase activity may also be
compound-related. There were no apparent changes at any dose or
interval in urinary parameters, except possibly in volume; but water
contamination was likely to have occurred.
Macroscopic examination of tissues at 12 months showed an
increased incidence of tan-white foci in lungs of female rats at
6000 ppm (one control; 11 at 6000 ppm), and the incidence of
tan-white-yellow lung foci was increased in a dose-related manner in
animals of each sex at 3000 and 6000 ppm that died or were sacrificed
at 12-24 months. The incidence of accentuated lobulation of the liver
was increased in animals of each sex at 6000 ppm. Thyroid enlargement
was also noted, with incidences of 0, 0, 2, and 3 in males and 1, 0,
2, and 1 in females at 0, 20, 3000, and 6000 ppm. This finding is
questionable.
The absolute and relative (to brain and body weight) weights of
the kidney, liver, and testis were statistically significantly
increased in males at 3000 and 6000 ppm at the 12-month interim
sacrifice. The organ:body weight ratios were affected by decreases in
the body weight of males at 6000 ppm, but significant increases were
reported for heart, kidney, liver, thyroid-parathyroid, and testis at
this dose. At 3000 ppm, increased organ:body weight ratios were seen
for kidney and liver. The organ:brain weight ratios were increased for
kidney at 3000 and 6000 ppm and for testis at 6000 ppm. Significantly
increased organ:body weight ratios were seen for brain, kidney, liver,
and thyroid-parathyroid in females at 6000 ppm at the 12-month
sacrifice. In males at terminal sacrifice, the absolute weights of
liver were increased at 6000 ppm, of the thyroid-parathyroid at 3000
and 6000 ppm, and of the kidney at 3000 ppm. Increased organ:body
weight ratios were seen in males for brain at 6000 ppm, kidney at
3000 ppm, liver at 3000 and 6000 ppm, and thyroid-parathyroid at 3000
and 6000 ppm, and the organ:brain weight ratios were increased in
males for kidney at 3000 ppm, liver at 3000 and 6000 ppm, and
thyroid-parathyroid at 3000 and 6000 ppm. Females at 6000 ppm with
body weights nonsignificantly lower than those of controls had a
decreased absolute heart weight and an increased absolute thyroid-
parathyroid weight. Increased organ:body weight ratios were seen for
liver at 6000 ppm and thyroid-parathyroid at 3000 and 6000 ppm; the
heart:brain weight was decreased and the thyroid-parathyroid:brain
weight was increased at 6000 ppm. When nonsignificant changes are
included, the major effects were increases in liver and thyroid-
parathyroid weights in animals at 3000 and 6000 ppm at both interim
and final sacrifices.
There were no microscopic observations that would account for the
changes in kidney and testicular weights. No compound-related
microscopic pathological changes were seen up to 12 months. At 12-24
months, the incidence of hypertrophy in males was 0, 0, 13/48, and
30/49, that of hyperplasia 0, 0, 1, and 1, and that of hepatocellular
adenomas 1/49, 0/49, 1/48, and 4/49 rats at 0, 20, 3000, and 6000 ppm.
Increased incidences of alveolar macrophages, interstitial pneumonia,
and perivascular lymphoid infiltration were seen in the lungs of males
at 6000 ppm. Thyroid hypertrophy was seen in 3/49, 2/49, 20/48, and
33/49 rats, follicular adenomas in 0/49, 0/49, 6/48, and 5/49 rats,
follicular carcinomas in 0/49, 1/49, 0/48, and 2/49 rats, and
follicular hyperplasia in 2/49, 2/49, 7/48, and 8/49 rats at 0, 20,
3000, and 6000 ppm, respectively. The incidence of colloidal cysts was
also increased in males at 6000 ppm. In females at 12-24 months, liver
hypertrophy occurred in 0/50, 0/49, 19/50, and 29/47 rats, hyperplasia
in 1/50, 0/49, 5/50, and 3/47 rats, and hepatocellular adenomas in
1/50, 0/49, 1/50, and 1/47 rats at 0, 20, 3000, and 6000 ppm,
respectively. Increased incidences of alveolar macrophages and
interstitial pneumonia were seen at 3000 and 6000 ppm; the incidence
of perivascular lymphoid infiltration was not increased. Thyroid
hypertrophy was seen in 0/50, 0/49, 18/50, and 23/46 female rats,
follicular adenomas in 1/50, 0/49, 2/50, and 4/46, follicular
carcinomas in 1/50, 0/49, 0/50, and 1/46, and follicular hyperplasia
in 0/50, 0/49, 6/50, and 8/46 at 0, 20, 3000, and 6000 ppm,
respectively. In data on historical controls from 11 two-year studies,
the mean percentage incidences of follicular adenomas were 2.88% for
males and 0.3% for females, and those of follicular carcinomas were
1.73% for males and 0.60% for females. In the study described here,
the incidences of follicular adenoma were 0, 0, 12.5, and 10.2% for
males and 2, 0,12, and 8.7% for females at 0, 20, 3000, and 6000 ppm,
respectively, and those of follicular carcinoma were 0, 2, 0, and 4.1
in males, and 2, 0, 0, and 2.2 in females. The incidence of follicular
adenomas in animals of each sex at 3000 and 6000 ppm therefore exceeds
the historical mean, as does that of follicular carcinomas in males at
20 and 6000 ppm and in females in the control and 6000-ppm groups. The
incidence of follicular carcinomas in males at 20 ppm, control
females, and in females at 6000 ppm was one per group, which is within
the historical control range, as is the incidence of follicular
carcinomas in males at 6000 ppm: 2/49 as compared to 3/32 in one
historical control. Quintozene therefore induced thyroid adenomas and
may have induced thyroid carcinomas. The changes observed in the lung
are consistent with infections; the increased incidence of such
changes at high doses may indicate either reduced immune competence or
a stress-related increase in susceptibility to infection. The
hepatocellular hypertrophy (mainly centrilobular) in which
eosinophilic cytoplasm was noted is indistinguishable from the
hepatocellular hypertrophy arising from increased drug metabolizing
enzyme activity. The NOAEL was 20 ppm, equivalent to 1 mg/kg bw per
day (Goldenthal, 1991).
(d) Reproductive toxicity
Four groups of 25 weanling (28-day-old) Charles River CD rats of
each sex were fed diets containing quintozene (purity, 98.2%, with
1.4% HCB and trace levels of tetrachloronitrobenzene and
pentachlorobenzene) at concentrations of 0, 5, 50, or 500 ppm. The
diet was prepared weekly, using 10 ml of corn oil for 500 ppm
quintozene, and was appropriately diluted with additional feed. After
11 weeks on diet, groups of 20 F0 females were paired with a male at
the same dose, rotated weekly for three weeks if necessary. Matings,
numbers of pregnancies, litters born, pups 1, 4, and 21 days
post partum, and litter weight at day 21 were recorded. Litters were
culled to 10 pups on day 4. Indices of fertility (pregnancies per
mating × 100), gestation (total litters per number of pregnancies ×
100), viability (live pups at day 4 per live pups born × 100) and
lactation (weaned pups per number of remaining pups after culling on
day 4 × 100) were calculated. A second litter (F1b) was initiated 10
days after the birth of the final F1a litter, and these animals were
used as parental rats for the F2 and F3 generations. Autopsies
were performed on F0 adults after weaning of F1b pups, F1a pups
at weaning, F1b adults after weaning of F2b pups, and F2a pups
at weaning, this pattern being maintained throughout the study. Ten
F3b offspring of each sex were maintained on the diets until two
months old, when they were sacrificed, and the heart, lung, liver,
kidney, urinary bladder, spleen, stomach, small and large intestine,
caecum, lymph nodes, bone marrow, skeletal muscle, skin, brain,
pituitary, thymus, thyroid, adrenals, pancreas, and gonads were
examined histopathologically. The only effects were on the body weight
of adult females: No significant changes were observed between
controls and F0 or F1b females at 500 ppm, but the body weights of
F2b females were significantly decreased at 500 ( P < 0.05) and
50 ppm ( P < 0.01). The effect was not dose-related. The NOAEL was
probably 500 ppm, equivalent to 2.5 mg/kg bw per day (Larson &
Borzelleca, 1968; Borzelleca et al., 1971).
Four groups of 26 Charles River COBS CD rats of each sex, aged 55
days, were fed diets containing quintozene (purity, > 99%; 0.08%
HCB) at doses of 0, 20, 3000, or 6000 ppm for two litters per
generation for two generations. Parents for the second generation were
selected from the F1b litters, avoiding sibling matings. Diets were
prepared weekly in a twin-shell blender with an intensifier bar to mix
500 g with additional diet to achieve the required levels. As the
homogeneity of the diet at the low dose was poor in week 1, the mixing
time was increased to 20 min after an initial 2-min mixing with 250 g
untreated diet, the mixer bar being used for the first 10 min of the
final blending. This process improved the homogeneity, but the dietary
levels were reduced to 84% of the nominal concentration. The diets
containing the low dose were therefore again modified, by dissolving
the quintozene in acetone before preparing the premix and blending (in
a Hobart mixer) it for 5 min before mixture with additional diet. The
homogeneity and percentage concentration were then acceptable. Those
at the middle and high doses were acceptable from week 1. The control
diet was also mixed with acetone from week 4. Analysis of the diets at
least once every four weeks throughout the study showed good
concordance with nominal values. F0 parents were fed treated diets
for 81 days before pairing, and F1 parents were given the test diet
for 90 days between weaning and pairing. Rats were observed twice
daily for mortality and signs of toxicity; body weights were recorded
weekly until sacrifice, except during gestation when animals were
weighed on days 0, 7, 14, and 20. Food intake was measured weekly
except during mating and, for females, during gestation and lactation,
when data were obtained for days 0-7, 7-14, 14-20, and 0-20 of
gestation and 0-4, 4-7, 7-14, and 14-21 of lactation. Rats were paired
1:1, and copulation was determined by either vaginal lavage or the
presence of a copulatory plug. The day of detection of copulation was
designated day 0 of gestation, for a maximum of 21 days. The time
between end of lactation and re-pairing was 10 days. Males were
changed between F1a and F1b and between F2a and F2b pairings.
The duration of the gestation period and difficulties in parturition
were recorded. Litter size, the numbers of still- and live births, and
gross abnormalities were noted. On lactation day 4, litters were
culled to eight pups (four of each sex when possible). Pups and dams
were observed twice daily for abnormal behaviour (including nesting
and nursing) and deaths. Pups were sexed and weighed individually on
days 0, 4, 7, 14, and 21 of lactation.
At term, F0 males were sacrificed and necropsied, and their
tissues were preserved. All males that failed to sire a litter were
examined for the presence of sperm in the epididymides. F0 females
were sacrificed at end of lactation of F1b pups (or, if non-gravid,
25 days after separation from males). Uteri from apparently non-gravid
females were examined with 10% ammonium sulfide. F1a offspring were
discarded after weaning. F1b offspring were necropsied, only gross
pathological lesions and malformed pups being preserved. A similar
programme was followed for the F1 generation. In both generations,
the coagulating gland, cervix, ovaries, pituitary, prostate, seminal
vesicles, testes with epididymides, uterus, vagina, and gross lesions
were examined microscopically.
Three adults died: one F0 male at 3000 ppm in week 28, one
23-day-old F1 male at 6000 ppm, and one control F1 female at about
20 weeks of age. These deaths did not appear to be compound-related.
Brown abdominal staining and brown or yellow anogenital staining
(usually for only 1-3 weeks) were seen in F0 females at the middle
and high doses. A slightly increased incidence of hair loss also
occurred in this group. F1 males and females at 6000 ppm had an
increased incidence of small size, which generally disappeared by 15
(males) to 17 (females) weeks of age. The body weights of F0 males
at 3000 and 6000 ppm and of females at 6000 ppm were lower than those
of controls (statistically significant), and a slight (usually
nonsignificant) decrease occurred in females at 3000 ppm. F1 males
and females at 6000 ppm and females at 3000 ppm had significantly
reduced body weights in weeks 4-17. During gestation, significant
reductions in mean body weights were seen in females at 6000 ppm in
the F0/F1a (at 14-21 and 0-21 days), F0/F1b (on days 0 and 7),
F1/F2a (at all intervals), and F1/F2b (at 14-20 days)
generations. The mean body weights at 6000 ppm were also significantly
reduced during lactation in the F0/F1a (at 0-4, 7-14, 14-21, and
0-21 days), the F0/F1b (0-21 days only), the F1/F2a (7-14 days
only), and the F1/F2b (14-21 and 0-21 days) generations. In
animals at 3000 ppm, decreased body weights were seen in the
F1/F2b matings on days 4-7, 14-21, and 0-21. Food intake was
reduced before mating of adults at 6000 ppm and during the lactation
period in F0 and F1 females.
No compound-related effects on male copulation, male fertility
index, female fertility index, gestation index, copulatory interval,
or gestation length were seen in either generation, although greater
fertility than controls was seen in F2a (animals of each sex) and
F2b (females) animals. Males that failed to mate had motile and
morphologically normal sperm in the epididymides, apart from one male
in the F1/F2b group at the low dose which had non-motile,
morphologically abnormal sperm. The mean numbers of stillbirths and
live births, the sex ratios, and viability indices were comparable to
those of controls, except in F1/F2a offspring at the middle dose
in which the sex ratio was skewed in favour of males. In animals at
the high dose, the total number of live births and the body weights of
live offspring were significantly reduced; the body weights of
offspring were also significantly reduced in the F0/F1a (days 14
and 21), F0/F1b (females only, day 21), and F0/F2b (days 14
and 21) generations at 3000 ppm.
During lactation, an increased incidence of 'pale' pups was seen
at 3000 and 6000 ppm in the F0/F1a generation on day 14 and in
F1/F2a pups at 6000 ppm on days 7, 14, and 21. Missing tails were
seen in F1/F2a pups at 6000 ppm on days 14 and 21 and in
F1/F2b pups at low incidence on all days. Concentric rings on the
tail were seen at high incidence in F1/F2a pups at 6000 ppm on
days 7, 14, and 21.
The incidences of malformations in offspring at birth were
reported only in summary form. In the F1a litters, two dead
offspring at 3000 ppm had malformations consisting of mandibular
micrognathia in one, cleft palate in both, and an intraventricular
defect in one; no malformations were seen in dead F1b offspring. One
case of anophthalmia was noted among 86 F1b pups at 20 ppm at day
21. Malformations seen in two dead F2a pups in two litters at
6000 ppm were a bulbous pulmonary trunk, an intraventricular septal
defect, and aortic arch stenosis; no malformations were seen in dead
F2b offspring. One F2b control pup out of 118 had microphthalmia
and mandibular micrognathia, one at 6000 ppm had anophthalmia, and one
at this dose had situs inversus; these pups were from different
litters. The incidence of variants was virtually nil.
Terminal macroscopic examination indicated no treatment-related
effects in F0 parents. Tan-yellow foci in the lung were seen in some
F1 males and females at 6000 ppm and in two females at 3000 ppm. The
testicular weights of animals that failed to sire offspring were
slightly reduced in two F1 controls and one treated F1 male at
3000 ppm. Microscopic examination of F0 adults showed no
compound-related effects. In F1 animals, the tan-yellow foci in the
lungs were aggregates of alveolar macrophages, perivascular-
peribronchial lymphoid cell infiltration, and/or interstitial
pneumonia. These changes are typical of pulmonary viral infections.
Thus, quintozene is unlikely to have been toxic, although stress due
to its administration or effects on the immune system may have
resulted in increased susceptibility to infection in animals at
6000 ppm and possibly in females at 3000 ppm. The NOAEL was 20 ppm,
equivalent to 1 mg/kg bw per day, on the basis of changes in body
weight in pups and adults at 3000 ppm. No adverse effects were seen on
reproductive parameters (Schardein et al., 1991).
(e) Developmental toxicity
Mice
A group of 23 C57Bl/6 mice was given quintozene (containing 11%
HCB) at 500 mg/kg bw per day in corn oil on days 7-11 of gestation; 19
controls were available. Further groups received either pentachloro-
aniline at 100 or 200 mg/kg bw per day or tetrachloronitrobenzene at
200 mg/kg bw per day on days 7-18 of pregnancy. In mice treated with
quintozene, fetal mortality was slightly increased, and the incidence
of malformations was significantly increased. Renal agenesis was the
most frequent effect, and the incidences of anophthalmia (19.2%
greater than in controls), microphthalmia (9.8%), and cleft palate
were increased. Pentachloroaniline and tetrachloronitrobenzene did not
cause malformations or affect maternal body weight, the liver:body
weight ratio, fetal weight, or fetal mortality (Courtney et al.,
1976).
Groups of CD-1 mice were given quintozene (containing 11% HCB) at
doses of 250 or 500 mg/kg bw per day (five or 10 litters), penta-
chloroaniline at 250 mg/kg bw per day (nine litters), tetrachloro-
nitrobenzene at 200 mg/kg bw per day (six litters in one study and
nine in the other), quintozene fabricated to contain 11% HCB (nine
litters) at 500 mg/kg bw per day, HCB at 100 mg/kg bw per day (10
litters), or quintozene containing < 20 ppm HCB at 500 mg/kg bw per
day (10 litters) on days 7-16 of gestation. Maternal liver:body weight
ratios were increased at both doses (dose-related) of contaminated
quintozene and with the fabricated sample of quintozene; a
nonsignificant increase was seen with HCB. The incidence of
malformations per litter was significantly increased at 500 mg/kg bw
per day of contaminated quintozene (28.6% in comparison with 6.2, 2.0,
or 7.2% in corn oil:acetone [9:1] control groups at the same volume),
and to a lesser degree with HCB (13.6%) and with quintozene with <
20 ppm HCB. The major malformation observed with contaminated and
fabricated quintozene and HCB was cleft palate; this malformation was
also seen with quintozene containing < 20 ppm HCB, but the major
contributor to the incidence was clubbed foot. The last preparation
differed from the other quintozene samples in that it provided a
solution rather than a suspension; this may have increased absorption.
This group also showed signs of toxicity, expressed as abortion in
three of 13 mice on day 17 of gestation. A similar high incidence of
clubbed foot has not been seen in other studies of quintozene, and the
author suggested that it may have been due to increased uterine muscle
tone, with consequent pressure effects on the developing fetus
(Courtney et al., 1976).
A group of 30 CD1 timed-pregnant mice were given quintozene
(containing 5% HCB) orally at 750 mg/kg bw per day on gestation days
8-12; 40 timed-pregnant mice were used as controls. Day 20 of
gestation was considered to be postnatal day 1. Death occurred in 17%
of animals, and 73% were pregnant. Litter size was nonsignificantly
reduced on day 1, but pup weights were comparable to those of
controls, and by postnatal day 3, the mortality and weights of pups
were comparable to those of controls. There was no NOAEL (Kavlock
et al., 1987).
Rats
Groups of five to seven pregnant CD rats received quintozene
containing 11% HCB or < 1.0% HCB at 500 mg/kg bw per day or
pentachlorophenol or tetrachloronitrobenzene at 75 mg/kg bw per day on
days 7-18 of gestation. No effects were seen on maternal weight gain
or the liver:body weight ratio. Pentachlorophenol decreased fetal
weights. The incidence of malformations was minimal, and these
consisted of enlarged cerebral ventricles, umbilical hernias, and
slightly enlarged renal pelvis (Courtney et al., 1976).
Pregnant Charles River albino rats received quintozene in corn
oil by intubation at doses of 100-1563 ppm on days 6-15 of gestation
and were sacrificed on day 20. Corpora lutea, position and numbers of
dead and resorbed pups, fetal weights, and sex ratio were recorded.
One-half of the fetuses were stained with alizarin Red-S for skeletal
examination, and the remainder were either fixed in Bouin's fluid for
examination of soft tissues or preserved in formalin. No difference
was seen between control and treated groups for any parameter,
including renal pelvis dilatation, hydronephrosis, or hydroureter,
which occurred in all groups. No NOAEL could be determined because of
the unusual expression of doses (Jordan & Borzelleca, 1973).
Four groups of 25 mated Charles River COBS female rats were given
quintozene (purity, 97.3%; containing 0.025% HCB) at doses of 0, 30,
600, or 1200 mg/kg bw per day as 10 ml/kg bw of 0.2% carboxymethyl-
cellulose suspensions by gavage on days 6-15 of gestation. The
suspensions were prepared daily; analysis on days 7 and 14 of the
study indicated actual levels of 88-110% of the nominal concentration
and stability over 24 h. The day of mating was designated day 0 of
gestation. Rats were observed twice daily for mortality and changes in
appearance and behaviour. Maternal rats were weighed on gestation days
0, 6, 9, 12, 16, and 20, and food intake was measured daily and
calculated for days 6-9, 9-12, 12-16, 16-20, 6-15, and 6-20 of
gestation. Rats were killed on day 20 of gestation, and the uterus and
ovaries were examined in situ and gravid uteri weighed. The numbers
and locations of viable and non-viable fetuses and of early and late
resorptions and the numbers of implantations and corpora lutea were
recorded. Maternal tissues were examined macroscopically, and grossly
abnormal tissues were preserved. The uteri of apparently non-pregnant
rats were examined with 10% ammonium sulfide. Fetuses were weighted,
sexed, and examined for external malformations; half were preserved in
Bouin's solution for examination of soft tissues by Wilson's
technique, and the remainder were eviscerated and processed for
Alizarin R-S staining and subsequent skeletal examination (Dawson
technique).
There were no deaths or adverse clinical or behavioural effects.
A nonsignificant reduction in the body weights of animals at
1200 mg/kg bw per day was seen but which was only 1-2% after
correction for initial weight differences. Food intake was
significantly reduced at 6-9 days in animals at 1200 mg/kg bw per day,
but the reduction was only 10.8% when measured as grams per animal per
day or 8.7% when measured as grams per kilogram body weight per day.
The effect was probably due to unpalatability. At later intervals and
during days 6-15 and 6-20, food intake was comparable to that of
controls. The incidences of pregnancy were 24/25 controls, 23/25 at
30 mg/kg bw per day, 25/25 at 600 mg/kg bw per day, and 23/25 at
1200 mg/kg bw per day. There were no total resorptions, and all
pregnancies resulted in viable offspring; the viable litter sizes were
comparable at all doses. The numbers of early resorptions were
slightly increased in all test groups, but the incidence was within
the normal historical range. The incidences of late resorptions and
corpora lutea were comparable in all groups. The group mean individual
fetal weights were unaffected, and the sex ratio was normal. The
malformations seen in controls were anophthalmia in one fetus,
malpositioned ovary and kidney in another, and a vertebral anomaly
with an associated rib anomaly in a third. At 30 mg/kg bw per day, one
fetus had an encephalocoele and a malpositioned ovary, one had
anasarca, a bent scapula, and bent limb bones, and a third had a
folded retina. At 600 mg/kg bw per day, one pup had microphthalmia and
folded retinas; at 1200 mg/kg bw per day, two pups from separate
litters had malpositioned kidneys and ovaries. Thus, no pattern in the
incidence of malformations and no dose-response relationship was seen
in the occurrence of malformations. The incidence of variants was
within normal limits. The NOAEL for maternal, embryo- and fetotoxicity
and for embryofetal development was 1200 mg/kg bw per day, the highest
dose tested (Keller, 1988a).
Rabbits
Four groups of 16 artificially inseminated New Zealand white
rabbits, three months old at receipt and acclimatized for 111 days,
were given quintozene (purity, 97.3%) at doses of 0, 12.5, 125, or
250 mg/kg bw per day in 0.2% carboxymethylcellulose on days 7-19 of
gestation. Because of maternal toxicity, additional rabbits, five
months old at reception and acclimatized for 48 days, were added about
75 days later and intubated with 0 (16 rabbits), 6.25 (16 rabbits), or
125 (12 rabbits) mg/kg bw per day. Semen for artificial insemination
was provided by six males proven to be fertile and was used to
inseminate an equal number of females in each concurrent group, except
in the second study when two males were used to inseminate fewer
females at 125 mg/kg bw per day than the remaining four males. The day
of insemination was designated day 0 of gestation. Caesarian sections
were performed on day 29 of gestation. The stability and homogeneity
of the quintozene formulation were satisfactory, and the actual
concentrations were 95-110% of the nominal ones.
Survival, appearance, and behaviour were observed twice daily and
were reported daily for days 7-29 of gestation. Rabbits that died
before the end of the study were necropsied, and those that showed
signs of abortion or premature delivery were sacrificed. The fetuses
of these rabbits were examined externally and preserved; those of
rabbits that died within 24 h of the end of the study were evaluated.
Body weights were recorded on days 0, 7, 13, 20, 24, and 29 of
gestation, and food intake was measured daily and calculated for days
7-13, 13-20, 20-29, 7-20, and 0-29 of gestation. At termination, the
uterus and ovaries were examined, and gravid uterine weight, the
number and location of viable and non-viable fetuses, the incidence of
early and late resorptions and total implantations, and the number of
corpora lutea were recorded. Maternal thoracic and abdominal cavities
were examined macroscopically, and grossly abnormal tissues and organs
were preserved. Non-pregnant uteri were examined with 10% ammonium
sulfide solution. Fetuses were weighed, tagged, and examined
externally, then dissected, internally sexed, and examined for soft
tissue abnormalities, including mid-coronal brain slices. The
carcasses were processed, stained with Alizarin Red-S (Dawson
technique), and examined for skeletal abnormalities.
One rabbit at 12.5 mg/kg bw per day died on day 28 of gestation;
two died at 125 mg/kg bw per day, both aborting before death on day 28
of gestation; and five died at 250 mg/kg bw per day on days 19, 21,
26, 26, and 27 of gestation, the death on day 27 being preceded by
abortion. In the secondary study, no deaths occurred at any dose. The
known causes of death in the initial study were mucoid enteritis in
those at 125 mg/kg bw per day and pneumonia in three of the five that
died at 250 mg/kg bw per day; the other causes of death were not
determined. The incidences of abortion were: two controls on days 24
and 27, two at 12.5 mg/kg bw per day on days 26 and 27, three at
125 mg/kg bw per day on days 23, 28, and 28, and four at 250 mg/kg bw
per day on days 23, 24, 25, and 26. When animals that aborted before
death are included, the incidences were 2, 2, 5, and 5 at 0, 12.5,
125, and 250 mg/kg bw per day, respectively. In the secondary study,
one rabbit at 125 mg/kg bw per day aborted on day 26. Premature births
occurred in one doe in each control group and in one at 125 mg/kg bw
per day. Signs of toxicity included mucoid stools in 6/16 rabbits at
250 mg/kg bw per day and 1/16 rabbits at 125 mg/kg bw per day, an
increased incidence of absent stools in 4/16 controls, 8/16 at 125,
and 11/16 at 250 mg/kg bw per day, and an increased incidence of
anogenital staining in 3, 8, 9, and 10 animals at 0, 12.5, 125, and
250 mg/kg bw per day in the initial group and 2, 3, and 9 at 0, 6.25,
and 125 mg/kg bw per day in the secondary group.
Body weights were markedly reduced after treatment at 250 mg/kg
bw per day, the loss peaking on day 24 of gestation, with very slight
recovery by day 29. The body weight at term, corrected for gravid
uterine weight, was also markedly reduced. Body-weight gain was
consistently reduced throughout gestation. Animals at 125 mg/kg bw per
day had a greater weight loss on days 13-20 of gestation in both the
initial and, to a lesser extent, the secondary study; however, the
weight reduction in the second group was greater on days 24-29. Both
groups at this dose had lowered corrected body weights at term than
controls, the effect being more apparent in the second group. Food
intake was significantly reduced at most intervals, when expressed
either as grams per animal or grams per kilogram body weight per day.
Stillbirths occurred in 1/81, 1/85, 1/49, and 3/28 rabbits at 0, 12.5,
125, and 250 mg/kg bw per day and in 0/82, 0/101, and 0/68 at 0, 6.25
and 125 mg/kg bw per day. The incidences of total resorptions in
relation to total implantations were 5.8, 11.5, 9.1, and 22.2% at 0,
12.5, 125, and 250 mg/kg bw per day and 24.8, 10.6, and 2.7% at 0,
6.25, and 125 mg/kg bw per day. Mean fetal weight was reduced markedly
at 250 mg/kg bw per day. The sex ratio was normal in all groups. The
mean litter size, including both viable and non-viable pups, was 7.4,
6.5, 6.8, and 4.7 at 0, 12.5, 125, and 250 mg/kg bw per day and 5.8,
7.7, and 6.8 at 0, 6.25, and 125 mg/kg bw per day. Malformations were
seen in 4, 4, 9, 2, 3, 2, and 0 pups in 3, 3, 6, 2, 3, 2, and 0
litters at 0, 0 (second study), 6.25 (second study), 12.5, 125, 125
(second study), and 250 mg/kg bw per day. The high incidence of
malformations at 6.25 mg/kg bw per day was accounted for by three pups
with multiple malformations that were not seen at higher doses. As
artificial insemination occurred over three days, each male normally
providing semen for two females, the multiple malformations may be due
to genetic defects mediated through the male. The incidence of
variants was comparable between groups. (Keller, 1988b).
The probable NOAELs are difficult to determine, since the results
at 125 mg/kg bw per day were not consistent between the studies. The
deaths due to mucoid enteritis were probably not compound-related
since the five deaths at 250 mg/kg bw per day were not associated with
pneumonia or mucoid enteritis. Although the incidence of abortions was
increased at 125 and 250 mg/kg bw per day, two of the five abortions
at the lower dose occurred immediately before death due to mucoid
enteritis and may therefore not be compound-related. The only
consistent effect was a decrease in body-weight gain at 125 and
250 mg/kg bw per day. The NOAEL for maternal toxicity was thus
12.5 mg/kg bw per day. That for embryo- and fetotoxicity was 125 mg/kg
bw per day, on the basis of reduced pup weight, increased resorption
rates, and a slight increase in the incidence of stillbirths at
250 mg/kg bw per day. The NOAEL for malformations was 250 mg/kg bw per
day, although only 28 fetuses from six litters were available for
examination at this dose.
(f) Genotoxicity
The results of studies of the genotoxicity of quintozene are
reported in Table 2.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Six male New Zealand white rabbits, three months old, were
exposed to quintozene (purity, 99.42%) moistened with water at a dose
of 500 mg per rabbit on dipped, intact dorsal skin under a 2.5-cm2
gauze patch secured with tape. The dressings were removed after 4 h,
the sites were wiped with dry towels, and the animals were observed
for 72 h. No dermal irritation was observed after 30-60 min or 24, 48,
or 72 h (Warshawsky, 1994c).
Six male New Zealand white rabbits, about three months old,
received 0.0996 g of quintozene (purity, 99.42%) in the cupped
conjunctival sac of the right eye, and the eyelids were held together
for 1 s after administration. The eyes were not washed and were scored
for irritation (on the Draize scale) at 1, 24, 48, and 72 h. At 72 h,
they were examined with sodium fluorescein. After 1 h, all rabbits had
a clear discharge, and two also showed blanching. Conjunctival
redness, chemosis, and discharge were seen in all animals, the redness
persisting in four rabbits up to 24 h. All eyes were normal by 48 h.
The average group scores were 7/110 at 1 h and 1.7/110 at 24 h,
indicating that the compound is minimally irritating to the rabbit eye
(Warshawsky, 1994d).
Table 2. Results of tests for the genotoxicity of quintozene
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 0-6667 µg/plate 99.6 Negativea US National Toxicology
TA100, TA1535, TA1537 Program (1987)
Reverse mutation S. typhimurium, five strains NR NR Negative Simmon et al. (1976)
Mitotic recombination Saccharomyces cerevisiae NR NR Negative Simmon et al. (1976)
DNA repair E. coli NR NR Negative Simmon et al. (1976)
Gene mutation Mouse lymphoma 1.25-10 µg/ml 99.6 Negativea US National Toxicology
L5178Y tk+/tk- cells Program (1987)
Sister chromatid Chinese hamster ovary cells 0.75-7.5 µg/ml 99.6 Negativeb US National Toxicology
exchange Program (1987)
Sister chromatid Chinese hamster ovary cells 7.5-75 µg/ml 99.6 Negativec US National Toxicology
exchange Program (1987)
Chromosomal Chinese hamster ovary cells 2.4-75 µg/ml 99.6 Positivea,d US National Toxicology
aberration Program (1987)
In vivo
Dominant lethal Mice Three doses NR Negativee Jorgenson et al. (1976)
mutation
NR, not reported
a With and without metabolic activation; similar results in two experiments with two or three replications each
b Without metabolic activation
c With metabolic activation
d 98% of cells with simple chromosomal and chromatid breaks; 2% with complex rearrangements; response not dose-related
e Males treated for seven weeks; then, one male mated with two females for eight weeks
Four Hartley guinea-pigs of each sex were exposed to quintozene
(purity, 98%) diluted in acetone on four sites at concentrations of
75, 50, 25, 10, 5.0, 2.5, 1.0, and 0.5% and scored for irritation at
24 and 48 h. On the basis of this pilot study, induction doses of 75
and 50%, which caused mild irritation, and challenge doses of 5, 2.5,
and 0.5%, which caused virtually no irritation, were selected for the
main study. In the main study, groups of 10 guinea-pigs of each sex
received applications on clipped, intact skin under occluded 25-mm
chambers containing 0.3 ml for 6 h; after 22-26 h, the animals were
depilated, and irritation was scored within 2 h as slight patchy
erythema, slight but confluent or moderate patchy erythema (grade 1),
moderate erythema (grade 2), or severe erythema (grade 3). Animals
were re-scored 22-26 h after the initial scoring. Induction involved
three 6-h exposures on the same site over two weeks, the initial
exposure being to 75% and the other two exposures to 50% quintozene in
acetone. No dermal reactions were seen after the first dose; slight
patchy erythema was elicited in one male after the second dose and in
two males and two females after the third dose. One male was
sacrificed after the initial dose due to non-compound-related
ill-health. Vehicle controls showed no dermal reactions.
For the primary challenge, chambers containing 5, 2.5, or 0.5%
quintozene were applied to each induced animal on previously untreated
sites. Reactions were observed at all three doses, the 5%
concentration inducing slight patchy erythema in eight males, grade 1
erythema in one male, and slight patchy erythema in nine females at
24 h; slight patchy erythema in seven males, grade 1 erythema in one
male, slight patchy erythema in nine females, and a grade 1 reaction
in one female were seen at 48 h. Comparable results were seen with 2.5
and 0.5%. The mean group scores were 0.5, 0.5, and 0.5 at 24 h and
0.5, 0.5, and 0.4 at 48 h with 5, 2.5, and 0.5%, respectively. Vehicle
controls given the same challenge doses under the same conditions had
slight patchy erythema at all concentrations, with mean group scores
of 0.3, 0.4, and 0.3 at 24 h and 0.3, 0.3, and 0.4 at 48 h.
Nine induced animals and five naive animals of each sex were
re-challenged at least six days after the initial challenge with 5 or
0.5% on previously unexposed sites. The mean group scores for induced
animals were 0.4 and 0.2 at 5% and 0.3 and 0.2 at 0.5% at 24 and 48 h,
respectively. Grade 1 reactions were seen in two guinea-pigs at 24 h
and one at 48 h with the 5% concentration and one at 24 and 48 h with
0.5% quintozene. The naive animals challenged with the 5% concen-
tration had mean irritation scores of 0.4 at 24 h (one animal with a
grade-1 score) and 0.2 at 48 h, and those exposed to 0.5% had scores
of 0.5 at 24 h and 0.2 at 48 h. The incidence and persistence of grade
1 scores in the challenged animals indicate that quintozene can induce
sensitization (Kreuzmann, 1988).
(ii) Potentiation
Combinations of quintozene at half or whole LD50 doses of
1740 mg/kg bw with terrozole (5-ethyoxy-3-trichloromethyl-
1,1,2,4-thiazole) at the LD50 dose of 1080 mg/kg bw or at LD2.3,
LD6.7, LD16, LD31, or one-half the LD50 were given to groups
of 10 male CD Charles River rats at 100 mg/kg bw quintozene and
appropriate amounts of terrazole. Mortality recorded over 14 days
showed an additive effect (Borzelleca et al., 1971).
(iii) Thyroid function
Groups of 75 male Charles River CD rats were fed diets containing
0, 20, or 6000 ppm quintozene (purity, 99.09%) for up to 90 days, and
groups of 15 rats per dose were sacrificed after 7, 14, 30, or 90
days; the remaining 15 rats were maintained for a further 90 days on
normal diet before sacrifice. The average intakes were 1 and 333 mg/kg
bw per day. Diets were prepared weekly (with acetone as the vehicle at
the 0 and 20 ppm levels) and analysed in duplicate during weeks 1-4,
8, and 12. The mean dietary concentrations were 81% of the nominal
(range, 72-98%) at 20 ppm and 91% (range, 86-99%) at 6000 ppm.
Mortality, moribundity, and clinical parameters were checked twice
daily and body weight and food intake weekly; blood samples were
collected from the abdominal aorta of non-fasted rats at sacrifice,
when complete macroscopic examinations were performed and the liver,
pituitary, and thyroid-parathyroid were weighed. These organs were
examined microscopically, as was the liver of all rats at terminal
sacrifice; the thymuses of a few animals were also examined.
Triiodothyronine, thyroxine, and thyroid-stimulating hormone were
measured in all rats at each dose and interval; reverse
triiodothyronine was measured in all rats at the end of treatment.
There were no deaths. The clinical signs, which occurred at low
incidence and were not dose-related, included malocclusion in seven
controls, six at 20 ppm, and five at 6000 ppm, and occasional hair
loss and redness of the eyes. Body weight and weight gain were reduced
in animals at 6000 ppm during treatment, but the weight gain exceeded
that of controls during the recovery period and by the time of
sacrifice exceeded that of controls. The main decrease in body-weight
gain occurred in week 1, when food intake was also significantly
decreased. At 6000 ppm, animals had reduced triiodothyronine and
thyroxine and increased thyroid-stimulating hormone values during most
of the treatment period, the effects being statistically significant
at most intervals. Reverse triiodothyronine was reduced at 90 days.
The triiodothyronine, thyroxine, and thyroid-stimulating hormone
values returned to normal during the recovery period. At 20 ppm, no
significant changes were seen, except for an increased thyroxine value
at seven days, which was considered to be a random effect. The
absolute weight of the pituitary was reduced at seven days in animals
at 6000 ppm; however, no other changes in the absolute or relative
weights of the pituitary occurred during the study, and no abnormal
microscopic changes were observed. The absolute weight of the liver
was nonsignificantly increased at 30 and 90 days in animals at this
dose but returned to normal after the recovery period; relative (to
body) weights were significantly increased at 14, 30, and 90 days, but
this effect may have been due to the decreased body weight seen at all
intervals except after the recovery period. The hepatocellular
hypertrophy seen after treatment for 90 days indicates that the
increased absolute liver weight at 30 and 90 days at 6000 ppm was
compound-related. The thyroid-parathyroid weights were not
significantly affected, except that the absolute weight was
significantly decreased at 30 days in animals at 6000 ppm and very
slightly decreased in those at 20 ppm. At 90 days, statistically
nonsignificant decreases were again seen at 20 and 6000 ppm (33 g in
controls versus 30 g at both 20 and 6000 ppm). After 90 days'
recovery, the thyroid-parathyroid weights were comparable to or
slightly greater than control values. Follicular epithelial
hypertrophy of the thyroid was observed at 6000 ppm, with trace (14
days), mild (30 days), or moderate (90 days) severity. At 20 ppm, mild
follicular epithelial hypertrophy was noted at 90 days. The decreased
thyroid weight may therefore reflect the colloid breakdown that occurs
in hypertrophic thyroid follicular cells, but this was not confirmed
microscopically. As the effects on the liver and thyroid were
reversible and only minimal effects were noted at 20 ppm, the NOAEL
was 20 ppm, equal to 1 mg/kg bw per day (Goldenthal, 1993).
(iv) Studies of metabolites
Four groups of 10 weanling female Sprague-Dawley rats were fed
diets containing pentachloronitrosobenzene, which is believed to be an
intermediate in the metabolic reduction of quintozene to pentachloro-
aniline, at doses of 0, 10, 50, or 100 ppm for 18 weeks. Rats were
weighed twice weekly, and food intake was monitored daily. Serum
alkaline phosphatase, aspartate and alanine aminotransferases, urinary
and liver porphyrins, liver glutathione, hepatic microsomal protein,
cytochrome P450, haemoglobin ferrihaemoglobin, packed cell volume, and
erythrocyte and total and differential leukocyte counts were
determined in all rats at the end of treatment. Organ:body weight
ratios were reported for liver, heart, spleen, and kidneys; the liver,
kidney, gastrointestinal tract, and urinary bladder were examined
histologically.
Body weights tended to be increased from week 2 onwards in all
treated groups, but statistical significance was not achieved and no
dose-effect relationship was seen. Food intake and food efficiency
were increased in all treated groups. The organ:body weight ratios
were comparable in all animals. A dose-effect relationship was
seen for ferrihaemoglobin levels, with values of 0, 0.5, 0.9, and
1.1 g/100 ml for controls and the three dose groups, respectively.
Serum alanine aminotransferase activity showed a dose-related,
statistically significant increase at all doses. Urinary porphyrin
patterns were similar over the last 24 h of the study. The hepatic
microsomal liver protein and hepatic and urinary porphyrin composition
was unchanged, but cytochrome P450 enzyme activity was significantly
increased at 100 ppm. At this dose, the concentrations of glutathione
and oxidized glutathione in liver were also increased. The liver was
normal histologically, but multilayered bladder epithelium of variable
thickness was seen, characterized by the presence of large superficial
cells. There was no sign of cellular proliferation (Renner et al.,
1981).
3. Observations in humans
Fifty human subjects received one 6.4-mm moistened cotton square
dipped into a 75% wettable powder formulation of quintozene (purity
unspecified) on the palmar surface of the forearm. The squares were
then covered with foil, which was taped into place. Exposure was for
48 h. No irritation was observed when the dressings were removed. When
the test was repeated two weeks later, four individuals showed
positive reactions, comprising erythema, itching, and (in three)
oedema and small vesicle formation. Of the individuals who showed no
reaction at the time the patch was removed, nine developed a delayed
reaction 8 h to several days after testing (Finnegan et al., 1958).
Workers who were still employed and had been exposed during the
production of quintozene for 2.5 years (one person), seven years (one
person), 8.5 years (one person), or 11 years (10 people) years had
undergone twice-yearly health checks, including monitoring of height,
weight, vision, blood pressure, X-ray (once per year only),
haematological parameters (erythrocyte count, haemoglobin,
haematocrit, total leukocyte count), an evaluation of subjective and
objective symptoms, life style, family history, and individual past
history, throughout the employment period. After 1989, hearing tests,
blood chemistry (serum aspartate and alanine aminotranferases,
gamma-glutamyl transpeptidase, alkaline phosphatase, partial
thromboplastin time, total cholesterol, and blood glucose),
(urinalysis (urea nitrogen, uric acid, glucose, protein, and occult
blood), and electrocardiography were added. No compound-related
effects were observed. A further group of nine individuals, with
exposure for three months (one person), one year (one person), two
years (one person), three years (one person), four years (one person),
seven years (one person), or 10 years (three people), who were engaged
in the manufacture of quintozene dust formulations were subjected to
similar examinations and showed no compound-related abnormalities. No
estimate of exposure or information on protective measures used (e.g.
clothing, gloves, and masks) was provided (Yamazaki, 1994).
Comments
Quintozene is absorbed relatively slowly after oral
administration to rats and mice, peak blood levels being observed
about 74 h after dosing. 14C from labelled quintozene is eliminated
mainly in the faeces of animals of each sex, although females excrete
greater amounts in the urine than males. In goats, low doses (30 mg/kg
bw) were excreted mainly in the urine, but faecal elimination
predominated at higher doses (100 mg/kg bw). Bile levels in ruminants
were high, and enterohepatic circulation is probable. Excretion was
more rapid and complete in chickens than in mammals. There was no
evidence of tissue accumulation in any species.
The biotransformation of quintozene was virtually complete. Two
general metabolic pathways were apparent, one involving reduction of
the nitro group to an amino group and subsequent formation of
secondary metabolites, and the second involving replacement of the
nitro group with a sulfur-containing group (e.g. a thio- or
methylthio-glucuronide or N-acetylcysteine group). Metabolic studies
in several species have been reported.
The acute oral LD50 of quintozene was > 1.5 g/kg bw in both
rats and dogs. The dermal LD50 was > 5 g/kg bw in rabbits, and the
4-h LC50 in rats was > 1.7 mg/litre air. Quintozene was mildly
irritating to the rabbit eye and not irritating to rabbit or human
skin, but it was a mild dermal sensitizing agent in guinea-pigs and
humans. WHO has classified quintozene as unlikely to present an acute
hazard in normal use.
In a single 13-week study in mice at dietary concentrations of 0,
1250 (males only), 2500, 5000, 10 000, 20 000, or 40 000 (females
only) ppm, 5-8% decreases in terminal body weight were seen at doses
> 10 000 ppm, and increased absolute liver weights were seen in
animals of each sex at all doses. All mice at 40 000 ppm died.
Four short-term studies in rats were available; in only one of
them was quintozene containing < 0.1% hexachlorobenzene used. Dietary
concentrations of 0, 50, 3000, or 6000 ppm administered for 13 weeks
resulted in an NOAEL of 50 ppm (equal to 3.1 mg/kg bw per day) on the
basis of a slight (6-8%) reduction in terminal body weight, increased
liver weight and decreased alanine aminotransferase activity at higher
doses. Five short-term studies in dogs (lasting four weeks to two
years) were available, in only one of which quintozene met the purity
specification. In this one-year study, at dietary concentrations of 0,
15, 150, or 1500 ppm, increased liver weight and hepatocellular
hypertrophy were seen at 1500 ppm, which were accompanied by increased
serum alkaline phosphatase and cholesterol levels and decreased
alanine aminotransferase activity and creatinine levels. The NOAEL was
150 ppm, equal to 4.2 mg/kg bw per day. A 70-day study in monkeys at a
dietary concentration of 2 ppm revealed no adverse effects. In a
21-day study in rabbits, with application to the skin of doses of 0,
30, 300, or 1000 mg/kg bw of quintozene for 6 h per day, significantly
decreased alanine aminotransferase activity was seen at 1000 mg/kg bw
per day. No irritation or other adverse effect was observed.
Four long-term (72-103-week) studies by oral administration in
mice were available, in only one of which did the compound meet the
contamination criterion. This 103-week study at dietary concentrations
of 0, 2500, or 5000 ppm showed decreased terminal body weights in
males (4% at 2500 ppm and 10% at 5000 ppm) and females (18 and 21%).
Since the body-weight losses in the females exceeded 10% at 2500 ppm
only after week 92 of the study, the NOAEL was 2500 ppm (equal to
360 mg/kg bw per day), although the interpretation was complicated by
Klebsiella infection. No evidence of carcinogenicity was seen. In a
study in which technical-grade quintozene (purity unspecified) was
applied dermally twice weekly (0.2 ml of 0.3% quintozene in acetone)
to mice for 12 weeks, followed by twice-weekly dermal applications of
croton oil for 20 weeks, no tumour induction was seen in the absence
of the promoting agent.
Of four long-term (two-year) studies in rats, only one met the
contaminant specification. In this study, at doses of 0, 20, 3000, or
6000 ppm, thyroid follicular adenomas were seen at 3000 and 6000 ppm
and possibly carcinomas at 6000 ppm (especially in males). Hepato-
cellular hypertrophy (mainly centrilobular), indistinguishable from
that arising from increases in drug-metabolizing enzyme activity, was
also seen at 3000 and 6000 ppm. The absolute and relative (to body and
brain) weights of the thyroid and parathyroid were increased at the
two highest doses in most instances. A special 90-day study on thyroid
hormones showed hepatocellular hypertrophy, which was only minimal,
after 90 days' exposure to 20 ppm. Complete reversibility was seen
after 90 days' withdrawal. Thyroid and parathyroid weights were
decreased only after 30 days' exposure. At 6000 ppm, triiodothyronine
and thyroxine levels were decreased and the levels of thyroid-
stimulating hormone were increased significantly at most intervals
during dosing; the level of reverse triiodothyronine was reduced at 90
days. All changes were reversible within 90 days after withdrawal. The
changes in thyroid hormone levels are consistent with increased
microsomal enzyme activity, resulting in increased excretion of
triiodothyronine and thyroxine and subsequent disruption of the
hypothalamic-pituitary-thyroid axis, causing increased circulating
levels of thyroid-stimulating hormone. Thyroid-stimulating hormone
initiates increased production of triiodothyronine and thyroxine, with
follicular hyperplasia in the thyroid, leading to adenoma and, more
rarely, carcinoma. Quintozene was thus carcinogenic. The induction of
thyroid tumours appears to be secondary to the disruption of thyroid
function, as indicated by the increased levels of thyroid-stimulating
hormone. The NOAEL for this effect was 20 ppm, equivalent to 1 mg/kg
bw per day.
One of two multigeneration studies in rats met the criterion for
the level of contamination. In this study, with dietary concentrations
of 0, 20, 3000, or 6000 ppm and two litters per generation for two
generations, dietary concentrations of 3000 and 6000 ppm reduced pup
and adult body weights. The NOAEL was 20 ppm, equivalent to 1 mg/kg bw
per day.
In studies of developmental toxicity, 13 mice (in part of a study
of the incidences of cleft palate and renal agenesis, subsequently
shown to be induced by hexachlorobenzene contamination) received doses
of 500 mg/kg bw per day by gavage on days 7-16 of gestation. Abortions
occurred, and a low incidence of cleft palate (2%) and a high
incidence (23%) of clubfoot were noted. Marked maternal toxicity was
seen. The administered material formed a solution, and not a
suspension as in all other studies. This study was not considered in
establishing the ADI. Of three studies of developmental toxicity in
rats, only one, with doses of 0, 30, 600, and 1200 mg/kg bw per day,
was acceptable. The NOAEL for maternal and developmental toxicity was
> 1200 mg/kg bw per day. There was no evidence of induction of
malformations. In rabbits given doses of 0, 12, 120, or 250 mg/kg bw
per day or 0, 6.2, or 120 mg/kg bw per day, maternal toxicity was seen
at 120 mg/kg bw per day and embryo- and fetotoxicity at 250 mg/kg bw
per day, including reduced pup weight, increased resorptions, and an
increased incidence of stillbirths; no increase in malformations was
seen at any dose.
In a number of studies (e.g. long-term studies in mice and rats
and the two-generation study of reproductive toxicity in rats), the
incidences of infection appeared to be slightly increased at high
doses. Although the reports of histopathological examinations do not
indicate effects on the immune system, it would be useful if studies
could be performed to investigate the potential for quintozene to
affect the immune system.
Quintozene has been tested in a range of studies for genotoxicity
in vitro. An acceptable assay is required to corroborate the
increase in chromosomal aberration frequency observed in cultured
cells.
An ADI of 0-0.01 mg/kg bw was allocated on the basis of the NOAEL
of 1 mg/kg bw per day in the two-year study, the study of thyroid
toxicity, and the two-generation study in rats, using a 100-fold
safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 2500 ppm, equal to 390 mg/kg bw per day (103-week study of
carcinogenicity)
Rat: 20 ppm, equivalent to 1 mg/kg bw per day (24-month study of
carcinogenicity, multigeneration study, and study of thyroid
toxicity) 1200 mg/kg bw per day (study of developmental
toxicity)
Rabbit: 12 mg/kg bw per day (maternal toxicity in study of
developmental toxicity) 120 mg/kg bw per day (embryo- and
fetotoxicity in a study of developmental toxicity)
Dog: 150 ppm, equivalent to 4.2 mg/kg bw per day (one-year study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw, for quintozene containing less than 0.1%
hexachlorobenzene
Studies that would provide information useful for continued evaluation
of the compound
1. Assays of genotoxicity in vivo to assess possible
chromosomal aberrations
2. Studies to assess the potential of quintozene to interfere
with the immune system
3. Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to quintozene
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Skin irritation, rabbit Not irritating
Eye irritation, rabbit Minimally irritating
Skin sensitization, guinea-pig Mild sensitizer
(modified Buehler)
Dermal irritation, human No irritation with 75% formulation
Skin sensitization, human Sensitization in 13/50 individuals
Inhalation, 4-h, toxicity, rat LC50 > 1.7 mg/litre air
Oral, toxicity, rat and dog > 1.5 g/kg bw
Medium-term (1-26 weeks) Dermal irritation, 21-day, rat No irritation; body weight, food intake, haematology,
clinical chemistry (except decreased alanine
aminotransferase), urinalysis, organ weights and liver
and kidney histology unaffected at doses
< 1000 mg/kg bw per day
Repeated oral, toxicity, 13-week, rat NOAEL = 3.1 mg/kg bw per day; minimal changes in
body and liver weight; reduced alanine
aminotransferase activity
Dietary, developmental toxicity, rabbit NOAEL = 12.5 mg/kg bw per day for maternal toxicity,
125 mg/kg bw per day for embryo- and fetotoxicity,
> 250 mg/kg bw per day for teratogenicity
Long-term (> one year) Dietary, toxicity, carcinogenicity, rat NOAEL = 1 mg/kg bw per day; follicular adenomas
of the thyroid and hepatocellular adenomas
References
Adamovics, J.A. (1980) The metabolic fate of pentachloronitrobenzen:
Pilot study. Unpublished report from Bio/dynamics Inc., Division
of Environmental and Analytical Chemistry, Olin Corporation
Report No. 79056. Submitted to WHO by Uniroyal Chemical Company,
Inc.
Adamovics, J.A. & O'Grodnick, J.S. (1978) Absorption and elimination
characteristics of 14C-labelled pentachloronitrobenzene in
rats-pilot study. Unpublished report from Bio/dynamics Inc.,
Division of Environmental and Analytical Chemistry, Olin
Corporation Report No. 77037. Submitted to WHO by Uniroyal
Chemical Company, Inc.
Aschbacher, P.W. & Feil, B.J. (1983) Metabolism of pentachloro-
nitrobenzene by goats and sheep. J. Agric. Food Chem.,
31, 1150-1158.
Betts, J.J., James, S.P. & Thorpe, W.V. (1955) The metabolism of
pentachloronitrobenzene and 2:3:4:6-tetrachloronitrobenzene and
the formation of mercapturic acids in the rabbit. Biochem. J.,
61, 611-617.
Borzelleca, J.F., Larson, P.S., Crawford, E.M., Hennigar, G.R., Jr,
Kuchar, E.J. & Klein, H.H. (1971) Toxicological and metabolic
studies on pentachloronitrobenzene. Toxicol. Appl. Pharmacol.,
18, 522-534.
Courtney, K.D. (1973) The effect of pentachloronitrobenzene on fetal
kidneys (Abstract 41). Toxicol. Appl. Pharmacol., 25, 455.
Courtney, K.D., Copeland, M.F. & Robbins, A. (1976) The effects of
pentachloronitrobenzene, HCB and related compounds on fetal
development. Toxicol. Appl. Pharmacol., 35, 239-256.
Daun, R.J. (1989) Pentachloronitrobenzene: Nature of the residue in
poultry-laying hens. Unpublished report from Hazelton
Laboratories America Inc., Project HLA 611-119. Submitted to WHO
by Uniroyal Chemical Company, Inc.
Daun, R.J. (1990) Pentachloronitrobenzene: Nature of the residue in
livestock-lactating goats. Unpublished report from Hazelton
Laboratories America Inc., Project HLA 611-118. Submitted to WHO
by Uniroyal Chemical Company, Inc.
Finnegan, J.K., Larson, P.S., Smith, R.B., Jr, Haag, H.B. & Hennigar,
G.R. (1958) Acute and chronic toxicity studies on pentachloro-
nitrobenzene. Arch. Int. Pharmacodyn., 115, 38-52.
Goldenthal, E.I. (1990) One year chronic dietary study in dogs.
Unpublished report No. 399-087 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Goldenthal, E.I. (1991). Two year dietary toxicity and oncogenicity
study in rats. Unpublished report No. 399-072 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Company, Inc.
Goldenthal, E.I. (1992). 21 day dermal toxicity study in rats.
Unpublished report No. 399-125 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Goldenthal, E.I. (1993). 90 day toxicity study in rats. Unpublished
report No. 399-122 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Company, Inc.
van der Heijden, C.A. & Til, H.P. (1974) Pentachloro-nitrobenzene
carcinogenicity study in mice. Unpublished report R4365 from
Central Institute for Nutrition and Food Research, Bilthoven,
Netherlands.
Hoescht AG (1964) Toxikologische Prüfung von Pentachlor-nitrobenzol.
3. Auf chronische Toxizität (90-Tage-Test) an Ratten. Unpublished
report prepared and submitted by Farbwerke Hoescht, AG.
Hilaski, R.J. (1994) EPA (FIFRA) Acute inhalation toxicity evaluation
on terraclor technical in rats. Unpublished report No. 399-146
from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Innes, J.R.M., Ulland, B.M., Valeric, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L., Gart,
J.J., Klein, M., Mitchell, I. & Peters, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in mice. A
preliminary note. J. Natl Cancer Inst., 42, 1101-1114.
Johnson, D.E. (1989) Twenty-eight day pilot study in dogs. Unpublished
report No. 399-093 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Company, Inc.
Jordan, R.L. & Borzelleca, J.F. (1973) Teratogenic studies with
pentachloronitrobenzene in rats (Abstract 40). Toxicol. Appl.
Pharmacol., 25, 454.
Jorgenson, T.A., Rushbrook, C.J. & Newell, G.W. (1976). In vivo
mutagenesis investigations of ten commercial pesticides
(Abstract 41). Toxicol. Appl. Pharmacol., 37, 109.
Kavlock, R.J., Short, R.D., Jr & Chernoff, N. (1987) Further
evaluation of an in vivo teratology screen. Teratog. Carcinog.
Mutag., 7, 7-16.
Keller, K.A. (1988a) Developmental toxicity study in rats. Unpublished
report No. 399-068 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Company, Inc.
Keller, K.A. (1988b) Developmental toxicity study in New Zealand white
rabbits. Unpublished report No. 399-070 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Company, Inc.
Kögel, W., Müller, F., Coulston, S. & Korte, F. (1979a)
Biotransformation of pentachloronitrobenzene-14C in rhesus
monkeys after single and chronic oral administration.
Chemosphere, 8, 97-105.
Kögel, W., Müller, F., Coulston, S. & Korte, F. (1979b) Fate and
effects of pentachloronitrobenzene in rhesus monkeys. J. Agric.
Food Chem., 27, 1181-1185.
Kreuzmann, J.J. (1988) Delayed contact hypersensitivity in guinea
pigs. Unpublished report No. 88-0052-21 from Hill Top Biolabs,
Inc. Submitted to WHO by Uniroyal Chemical Company, Inc.
Kuchar, E.J., Geenty, F.O., Griffith, W.P. & Thomas, R.J. (1969)
Analytical studies of metabolism of terraclor in beagle dogs,
rats and plants. J. Agric. Food Chem., 17, 1237-1242.
Larson, P.S. & Borzelleca, J.F. (1968) Toxicologic study on the
effects of adding terraclor to the diet of beagle dogs for a
period of two years. Unpublished report from the Department of
Pharmacology, Medical College of Virginia, Richmond, Virginia,
USA. Submitted to WHO by Uniroyal Chemical Company, Inc.
McGee, D.H. (1988) Thirteen week dietary toxicity study in rats with
PCNB (pentachloronitrobenzene). Unpublished report No. 399-071
from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Uniroyal Chemical
Company, Inc.
McManus, J.P. (1989) Metabolism of pentachloronitrobenzene in the
goat-metabolite identification. Unpublished report from Uniroyal
Chemical Company, Inc., Crop Protection Department, Chemistry
Section, R & D, Uniroyal Project 8761. Submitted to WHO Uniroyal
Chemical Company, Inc.
McManus, J.P. (1990) Metabolism of pentachloronitrobenzene in the
goat-identification of metabolites in muscle. Unpublished report
from Uniroyal Chemical Company, Inc., Crop Protection Department,
Chemistry Section, R & D, Uniroyal Project 8761a. Submitted to
WHO Uniroyal Chemical Company, Inc.
O'Grodnick, J.S. (1978) Characterization and identification of
14C-PCNB metabolites in rat urine and faeces. Unpublished
report from Bio/dynamics Inc., Division of Environmental and
Analytical Chemistry, Olin Corporation Report No. 77037-2.
Submitted to WHO by Uniroyal Chemical Company, Inc.
O'Grodnick, J.S. (1979) Identification of the polar metabolites of
14C-PCNB after oral administration to rats. Unpublished report
from Bio/dynamics Inc., Division of Environmental and Analytical
Chemistry, Olin Corporation Report No. 78054. Submitted to WHO by
Uniroyal Chemical Company, Inc.
O'Grodnick, J.S., Adamovics, J.A., Blake, S.H. & Wedig, J. (1981) The
metabolic fate of 14C-labelled pentachloronitrobenzene in
Osborne-Mendel rats. Chemosphere, 10, 67-72.
Parkins, M.D. (1990). Pentachloronitrobenzene: Nature of the residue
in poultry-laying hens. Unpublished report from Uniroyal Chemical
Company, Inc., Crop Protection Department, Chemistry Section, R.
& D., Project No. 8762. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Parkins, M.D. (1991) Pentachloronitrobenzene: Nature of the residue in
poultry-laying hens. Unpublished report from Uniroyal Chemical
Company, Inc., Crop Protection Department, Chemistry Section, R &
D, Project No. 8762. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Renner, G., Herforth, B., Gokel, J.M., Goerz, G. & Luderschmidt, C.
(1981) Subchronic toxicity studies on pentachloronitrosobenzene
(PCNO) in female rats. Ecotoxicol. Environ. Saf., 5, 281-290.
Schardein, J.L., York, R.G. & Laughlin, M.A. (1991) Two generation
reproduction study in rats. Unpublished report No. 399-086 from
International Research and Development Corporation, Mattawan,
Michigan, USA. Submitted to WHO by Uniroyal Chemical Company,
Inc.
Scholz, & Brunk, (1968) Kronischer orale toxizitätsprüfung von
pentachlornitrobenzol, 2-Jahres-Versuch an Hunden. Unpublished
report from Rewerke Hoescht AG. Submitted to WHO by Uniroyal
Chemical Company, Inc.
Searle, C.E. (1966) Tumor initiatory activity of some
chloromononitrobenzenes and other compounds. Cancer Res.,
26, 12-17.
Simmon, V.F., Poole, D.C. & Newell, G.W. (1976) In vitro mutagenic
studies of twenty pesticides (Abstract 42). Toxicol. Appl.
Pharmacol., 37, 109.
Sinkeldam, E.J., van der Heijden, C.A., de Groot, A.P. & Til, H.P.
(1974) Carcinogenicity study with pentrachloronitrobenzene in
rat. Unpublished report R442 from Central Institute for Nutrition
and Food Research, Bilthoven, Netherlands.
St John, L.E., Jr, Ammering, J.W., Wagner, D.G., Warner, R.G. & Lisk,
D.J. (1965) Fate of 4,6-dinitro-2-isobutylphenol, 2-chloro-4,6-
bis-(ethylamino)-S-triazine, and pentachloronitrobenzene in the
dairy cow. J. Dairy Sci., 48, 502-503.
US National Toxicology Program (1987) Toxicology and carcinogenesis
studies of pentachloronitrobenzene in B6C3F1 mice (feed studies)
(National Toxicology Program Technical Report Series No. 325),
Washington DC, US Department of Health and Human Services.
Warshawsky, L.D. (1994a) Acute oral toxicity study in rats.
Unpublished report No. 399-155 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Warshawsky, L.D. (1994b) Acute dermal toxicity study in rabbits.
Unpublished report No. 399-152 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Warshawsky, L.D. (1994c) Primary dermal irritation test in rabbits,
following a 4 hr exposure period. Unpublished report No. 399-153
from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Warshawsky, L.D. (1994d). Primary eye irritation study in rabbits.
Unpublished report No. 399-154 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Wedig, J.H., Sperling, F. & Miller, R. (1976) Unpublished report of an
analysis of data submitted by the National Cancer Institute from
Olin Corporation.
Wit, S.L., van Esch, G.J. & van Genderen, H. (1960) Toxicity of some
chloronitrobenzene compounds (trichlorodinitrobenzene,
trichloronitrobenzene, tetrachloronitrobenzene and
pentachloronitrobenzene) to laboratory rats and residues found in
crops treated with these fungicides. In: Proceedings of the
Fourth International Congress on Crop Protection, Hamburg,
September 1957, Vol. 2, Braunschweig, pp. 1665-1668.
Yamazaki, Y. (1994) Health report. Unpublished report from Ohmuta
Factory, Mitsui Toatsu Chemicals Inc. Submitted to WHO by
Uniroyal Chemical Company, Inc.
 Table 1. Acute toxicity of quintozene
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Rat Male, female Oral > 5000a 99.4 Warshawsky (1994a)
Rat Oral > 30 000 NR Wit et al. (1960)
Rat Male, female Oral 1650 (male) 98.2 Finnegan et al. (1958)
1710 (female)
Rat NR Intraperitoneal 5000 NR Wit et al. (1960)
Rat Male, female Inhalation 1.7b 99.4 Hilaski (1994)
Rabbit Male Oral Erraticc 98.2 Finnegan et al. (1958)
Rabbit Male, female Dermal > 5000 99.4 Warshawsky (1994b)
Rabbit NR Dermal > 4 g 99.7 (1.8% Borzelleca et al. (1971)
HCB
Dog NR Oral > 2500 98.2 Finnegan et al. (1958)
75% wettable powder as 40% aqueous solution
Rat Male, female Oral 16 g (equal to NR Finnegan et al. (1958)
12 g/kg bw
quintozene)
NR, not reported
a Decreased defaecation at all doses; decreased activity at 1300, 1700, and 2000; soft stools at 2000
and 5000; and anogenital staining at 1700, 2000, and 5000; full recovery by day 6
b Mass median aerodynamic diameter, 3.6 µm; standard geometric deviation, 1.9; aerosol concentration,
1.3-2.2 mg/litre; air flow, 38 litres/min; decreased activity, increased salivation, and rapid
breathing
c Mortality rates: 0 at 350 mg/kg bw per day, 2 at 500 mg/kg bw per day, 1 at 650 mg/kg bw per day,
0 at 800 mg/kg bw per day, 3 at 1100 mg/kg bw per day, and 3 at 1400 mg/kg bw per day
(b) Short-term toxicity
Mice
Groups of 10 B6C3F1 mice of each sex, eight to nine weeks old,
were fed diets containing quintozene (purity, 99.6%; HCB content,
0.07%) at concentrations of 0,1250, 2500, 5000, 10 000, or 20 000 ppm
(males) and 0, 2500, 5000, 10,000, 20 000, or 40 000 ppm (females) for
13 weeks. Five animals per cage were checked twice daily, food
consumption was measured weekly by cage, and individual body weights
were recorded weekly. All mice were necropsied, livers were weighed,
and gross lesions, tissue masses, mandibular lymph nodes, mammary
glands, skin, salivary gland, sternebrae, thyroid, parathyroid, small
intestine, colon, liver, prostate, testis, ovary, uterus, lung and
bronchi, heart, oesophagus, stomach, brain, thymus, trachea, pancreas,
spleen, kidneys, adrenal, urinary bladder, pituitary, spinal cord (if
neurological signs occurred), and eyes (if grossly abnormal) were
examined histopathologically. All females at 40 000 ppm died during
the study. The final mean body weights in animals at 10 000 and
20 000 ppm were 7 and 8% lower than those of controls for males and 5
and 8% for females, respectively. Scattering of food was frequent, but
mice at the high dose may have consumed more food than controls.
Absolute liver weights were increased significantly in males at 1250,
2500, and 5000 ppm and in females at 2500, 5000, and 10 000 ppm.
Liver:body weight ratios were significantly increased in males at
doses > 2500 ppm and in females at > 5000 ppm. Clinical signs
were limited to small body size and emaciation of all males at
20 000 ppm and all females at 20 000 and 40 000 ppm. No
histopathological changes were seen in male mice, but females at
40 000 ppm had lymphoid depletion of the spleen, mesenteric lymph
nodes, or thymus. Effects in the lungs of all mice were consistent
with Sendai vital infection. There was no NOAEL since absolute liver
weights were increased at all doses (US National Toxicology Program,
1987).
Rats
An unspecified number of young rats (strain unspecified) were fed
diets containing 0 or 2000 ppm quintozene for 10 weeks. No gross
effects were noted, other than a decreased growth rate in males
(Wit et al., 1957).
Groups of five rats (strain unspecified) of each sex were fed
diets containing a 20% dust formulation of quintozene for 13 weeks to
give doses of 0, 0 (dust minus quintozene), 63.5, 635, 1250, 2500, or
5000 ppm. Animals were weighed weekly, and erythrocyte, haemoglobin,
and leukocyte determinations were performed at term. The liver,
kidney, and testis were weighed, and the heart, lung, liver, kidney,
spleen, stomach, small intestine, caecum, large intestine, thyroid,
adrenals, pancreas, and gonads were examined histologically. Rats at
5000 ppm lost weight during the first two weeks and were sacrificed in
poor condition at the start of week 3, although only one male had
died. Body weights were reduced to a statistically significant degree
in males and nonsignificantly in females at 2500 ppm. One male at 0
(powder control), one at 63.5 ppm, and one at 1250 ppm died.
Haematological parameters were not altered (no data reported). The
liver:body weight ratios were significantly increased in all treated
animals, except for females at 63.5 ppm. The kidney:body weight ratios
were increased in males at 1250 and 2500 ppm. 'Fine vacuolation' of
liver cytoplasm was seen in rats at 5000 ppm. There was no NOAEL
(Finnegan et al., 1958)
Groups of 10 male and 10 female rats (strain unspecified) were
fed diets containing quintozene at 0, 1000, 5000, or 10 000 ppm for 90
days. Growth was inhibited slightly at 5000 ppm and markedly at
10 000 ppm. The NOAEL was 1000 ppm, equivalent to 50 mg/kg bw per day
(Hoescht AG, 1964).
Four groups of 15 Charles River CD rats of each sex, seven weeks
of age, were fed diets containing quintozene (purity, 99.5%) at
concentrations of 0, 50, 3000, or 6000 ppm for 13 weeks. The average
intakes were 3.07, 187, and 381 mg/kg bw per day for males and 3.69,
223, and 455 mg/kg bw per day for females. Diets were analysed for
homogeneity (10 samples per dose), stability (on days 0 and 10), and
concentration (10 samples at weeks 1 and two samples at 2, 3, 4, 8,
and 12 weeks). The homogeneity of eight samples of diet containing
50 ppm was 95-108%, with two outlyers at 117 and 118%; the homogeneity
of 10 samples of the diet containing 3000 ppm was 92-101%, and that of
10 samples of diet at 6000 ppm was 93-98%. Duplicate analyses of
stability showed it to be within 10%. The concentrations of quintozene
in the diets were 98% (range, 91-103%) at 50 ppm, 94% (91-97%) at
3000 ppm, and 92% (85-95%) at 6000 ppm. Rats were observed twice a day
for toxic signs, morbidity, and mortality; food intake and body weight
were recorded weekly. Erythrocyte count, haemoglobin level,
haematocrit, total and differential leukocyte counts, platelet count,
reticulocyte count, mean corpuscular volume, mean corpuscular
haemoglobin, and mean corpuscular haemoglobin concentration; sodium,
potassium, chloride, calcium, inorganic phosphorus, alkaline
phosphatase, total bilirubin, aspartate aminotransferase, alanine
aminotransferase, creatine phosphokinase, urea nitrogen, creatinine,
total protein, albumin, globulin, cholesterol, and serum glucose; and
the colour, appearance, volume, specific gravity, microscopic
elements, pH, protein, glucose, ketones, bilirubin, occult blood,
nitrite, and urobilinogen in urine were reported for 10 rats of each
sex at each dose at week 13. Blood samples from the orbital sinus and
urine were collected during a fasting period. Ophthalmoscopic
examinations were performed on all rats before and on day 86 of the
study. The adrenals, heart, brain, kidney, liver, and gonads of all
rats were weighed post mortem. All animals were examined
macroscopically, and about 40 tissues were preserved. All tissues from
controls and from rats at the high dose and the livers, kidneys, and
lungs of animals at the low and middle doses were examined
microscopically. Lesions observed grossly were also examined.
There were no deaths during the study. Several minor signs (e.g.
hair loss and scabbing) occurred at low incidences but were not
dose-related. Malocclusion was seen in 1, 0, 6, and 4 males at the
four doses, respectively, but is unlikely to have been related to
treatment. Decreased body weight was noted in animals of each sex at
3000 and 6000 ppm and was statistically significant in males at
6000 ppm and in females at 3000 and 6000 ppm. Slightly decreased
terminal weight was seen in animals of each sex (by 8.1 and 6.1% in
males and females at the high dose, respectively) and was dose-
related. Body-weight gain was decreased by 16% in animals of each sex
at 6000 ppm and by 11 and 13% in males and females, respectively, at
3000 ppm. Food intake was reduced significantly in animals of each sex
at 6000 ppm during week 1 and sporadically in weeks 3, 4, and 8 in
males and week 5 in females. A consistent, nonsignificant reduction in
food intake was seen throughout the study in animals of each sex,
except in females at weeks 2 and 4; the food intake of males at
3000 ppm was significantly reduced only in week 1 but was consistently
less than that in controls except in week 10. Females had a
statistically significant reduction in food intake in weeks 5, 6, and
7 and a nonsignificant reduction at other times.
Ophthalmological examination showed no compound- or dose-related
effects, and no adverse effects were observed on haematological
parameters. Reduced alanine aminotransferase levels were seen in
animals of each sex at 3000 and 6000 ppm. In females, total protein
was increased at all doses, albumin was increased at 3000 ppm, and
globulin at 3000 and 6000 ppm; the cholesterol level was significantly
increased at 50 and 6000 ppm. In males, serum alkaline phosphatase
activity was increased at 50 ppm only; no changes were seen in serum
protein levels. The individual total protein levels in females were
greater than the range of levels in the controls in one rat per dose.
A similar pattern was seen for albumin and globulin, except that the
globulin level in two animals at 6000 ppm exceeded the maximal level
in controls. Although these mean values are statistically significant,
the effect was minimal and unlikely to be of toxicological
significance. The increase in cholesterol levels was seen at all doses
but was significant only at 50 and 6000 ppm. The changes were limited
to one sex, and the biological significance of this observation is
uncertain. The pH of the urine of males at 3000 and 6000 ppm was
lowered, but the values were within normal limits for rats. Other
parameters, e.g. a high ketone level in one male rat at 6000 ppm and
low levels of glucose (0.1 g/dl) in one male per dose, showed wide
individual variations and were not considered to be of toxicological
significance.
The absolute kidney weight was increased in males at 3000
(statistically significant) and 6000 (statistically nonsignificant)
ppm, and the kidney weights relative to those of the body and brain
were increased significantly in males at 3000 and 6000 ppm. The
absolute kidney weights were decreased in females at 50 ppm, and the
weights relative to body weight but not brain weight were
significantly decreased at 50 ppm and increased at 6000 ppm. The
apparent effect on the kidney:body weight ratio is. probably a
reflection of decreased body weight at 6000 ppm. The absolute liver
weights were nonsignificantly increased in animals of each sex at 3000
and 6000 ppm, the effect being dose-related in females. The liver:body
weight ratios were significantly increased in males at 3000 and
6000 ppm and in females at 6000 ppm, probably reflecting decreased
body weight. The liver:brain weight ratios were nonsignificantly
increased in animals of each sex at 3000 and 6000 ppm. Changes in the
weight of the brain relative to body weight in males at 3000 and
6000 ppm and of the heart relative to body weight in males at 6000 ppm
were also attributed to reduced body weights.
Males had increased incidences of chronic nephritis (1, 6, 5, and
4 out of 15 animals at 0, 50, 3000, and 6000 ppm, respectively), which
were not dose-related, and an increased incidence of mild haemorrhage
in mesenteric lymph nodes (4 at 6000 ppm; 0 in controls), but these
effects were unlikely to be related to treatment. Mandibular lymph
node haemorrhages occurred in 5/15 control and 0/15 male rats at
6000 ppm, and mediastinal lymph node haemorrhages were seen in 3/15
control and 1/15 males at 6000 ppm. Low incidences of haemorrhage seen
in medias final lymph nodes of seven females at 6000 ppm and three
controls and of mesenteric lymph nodes in six females at 6000 ppm and
no controls may indicate a slight effect, the significance of which is
not known. The only effects in liver were seen at 6000 ppm, comprising
hepatocellular hypertrophy in 7/15 males and 8/15 females and in none
of the controls. The NOAEL was 50 ppm, equal to 3.07 mg/kg bw per day,
on the basis of minimal changes in body-weight gain and liver weight
and changes in alanine aminotransferase activity at higher doses
(McGee, 1988).
Four groups of six acclimatized Charles River CD rats of each
sex, with initial body weights of 247-265 g for males and 229-244 g
for females, were treated on clipped dorsal skin, 6 h per day for 21
days with quintozene (purity, 98.72%) applied as a paste in deionized
water at 0, 30, 300, or 1000 mg/kg bw per day. The application site
was wrapped in gauze bandaging secured with non-irritating tape. After
each application the test site was washed with tepid water. Rats were
observed for mortality, moribundity, clinical signs, and behaviour
twice daily, and irritation was scored by the Draize method once
daily. Body weight was recorded before treatment and then weekly, and
food intake was measured weekly. Haematological and clinical chemical
tests were done at termination on blood obtained from the orbital
sinuses before fasting, and urine samples obtained during fasting were
analysed. The haematological parameters measured included erythrocyte
counts, haemoglobin, haematocrit, total and differential leukocyte
counts, mean corpuscular volume, mean corpuscular haemoglobin, mean
corpuscular haemoglobin concentration, and platelet counts. The
clinical chemical parameters measured were sodium, potassium,
chloride, calcium, inorganic phosphorus, alkaline phosphatase, total
bilirubin, aspartate and alanine aminotransferases, lactic
dehydrogenase, creatine phosphokinase, urea nitrogen, creatinine,
total protein, albumin, globulin, the albumin:globulin ratio,
cholesterol, and glucose; and the urinary parameters were colour,
appearance, volume, specific gravity, microscopic elements, pH,
protein, glucose, ketones, bilirubin, occult blood, nitrite,
urobilinogen, and leukocyte incidence. All rats were examined
macroscopically post mortem; kidneys, liver, and testes were
weighed, and 44 organs or tissues per rat were preserved, as were
gross lesions seen at macroscopic examination. Microscopic examination
was limited to liver, kidney and treated and untreated skin in animals
at 0 and 1000 mg/kg bw per day, and all gross lesions.
There were no deaths. Ventral hair loss was seen occasionally in
females, but the incidence was not dose-related. No behavioural
changes and no signs of dermal irritation were detected. No
compound-related changes in body weight were seen, although increased
body-weight gain was noted in males at 30 mg/kg bw per day
(statistically nonsignificant). Food intake was comparable in all
groups. There were no statistically significant changes in any
haematological parameters, and clinical chemistry parameters were
unaffected, except for a statistically significant, dose-related
decrease in alanine aminotransferase activity in males and in females
at 1000 mg/kg bw per day; a statistically significant decrease was
also seen at 1000 mg/kg bw per day, but there was no effect at lower
doses. Other changes (increased alkaline phosphatase activity and
decreased total bilirubin in females at 300 mg/kg bw per day) were not
dose-related. There were no significant changes in urinary parameters.
Organ weights also showed no statistically significant changes,
although testicular weights were reduced in two of five rats at
1000 mg/kg bw per day; these testes were not examined microscopically.
There were no compound- or dose-related microscopic effects on the
liver, kidney, or treated or untreated skin. The NOAEL was 300 mg/kg
bw per day (Goldenthal, 1992).
Dogs
Three groups of three mongrel dogs were fed dietary levels of 25,
200, or 1000 ppm quintozene for one year by mixing a commercial powder
containing 20% quintozene, 77% pyrax ABB (a pyrophyllite carrier), and
3% Armour sticker with the diet, which was mixed with water
immediately before feeding. Dogs were weighed weekly; haematological
parameters (unspecified but including total and differential leukocyte
counts) were measured at 0, 6, and 12 months; and the heart, lung,
liver, kidney, spleen, gastrointestinal tract, thyroid, adrenal,
pancreas, gonads, and bone marrow were examined histologically. One
dog at 200 ppm whelped at week 5 of the study, and two dogs at
1000 ppm whelped at weeks 7 and 8. Some pups were allowed to suckle
and were then sacrificed at four to six weeks of age and examined
histopathologically. There were no deaths. Body weight was variable
but apparently unaffected by quintozene. The only effects on
haematological parameters were increased eosinophil counts at 6 and 12
months in some animals (probably due to parasites) and low total
leukocyte counts at 12 months in dogs at 10 000 ppm. No lesions were
seen in the pups examined histopathologically. Hepatic-cell
enlargement with pale cytoplasmic staining was noted in all adult
dogs, but the severity was not dose-related. Periodic acid-Schiff and
Best carmine staining of the liver showed abundant glycogen around the
periphery of lobules. Staining for fat gave negative results. There
was no NOAEL, owing to the random whelping (Finnegan et al., 1958).
Four groups of three beagle dogs (Ridglan Farms, Inc.),
previously immunized and vaccinated against most common viruses and
infections, were fed diets containing 0, 40, 2000, or 4000 ppm
quintozene (purity, at least 96%) for four weeks. The diets were
prepared weekly, acetone being used as an aid to dispersion at 40 ppm
and also added to control diets. Homogeneity and stability were
measured in week 4, and the concentration of quintozene was measured
in the diets for weeks 2, 3, and 4. The homogeneity in 10 samples was
91-99% (mean, 95%) at 40 ppm, 92-103% (mean, 98%) at 2000 ppm, and
95-104% (mean, 98%) at 4000 ppm. The stability over 10 days (in
duplicate analyses) was 98-101%, and the dietary concentrations were
85-116% (mean, 100%) at 40 ppm, 91-108% (mean, 100%) at 2000 ppm, and
92-98% (mean, 94%) at 4000 ppm. Dogs were observed twice daily for
clinical signs, mortality, and moribundity. Body weights were measured
before treatment and then weekly; food intake was measured weekly.
Total and differential leukocyte counts, erythrocyte count,
haemoglobin, haematocrit, mean corpuscular volume, mean corpuscular
haemoglobin, mean corpuscular haemoglobin concentration, platelet
counts, and reticulocyte counts; and sodium, potassium, chloride,
calcium, inorganic phosphorus, alkaline phosphatase, total bilirubin,
aspartate and alanine aminotransferases, creatine phosphokinase, urea
nitrogen, creatinine, total protein, albumin, globulin, cholesterol,
and glucose were measured before and after four weeks of treatment.
Blood samples were taken from the jugular vein of dogs fasted
overnight, and urine collected during this period was analysed for
colour, appearance, volume, specific gravity, microscopic elements,
pH, protein, glucose, ketones, bilirubin, occult blood, nitrite, and
urobilinogen. The adrenals, brain, heart, kidney, liver, gonads,
pituitary, spleen, and thyroid-parathyroid were weighed and examined
macroscopically, and about 40 organs were preserved but were not
examined microscopically.
There were no deaths or dose-related clinical signs, and no
compound-related effects were observed on body weight. Food intake, on
the basis of grams per animal per day, was reduced in males at
4000 ppm throughout the study and in those at 40 and 2000 ppm during
week 1; it was also decreased in weeks 2 and 4 but was increased in
animals at 2000 ppm in weeks 2, 3, and 4. Food intake in females on
this basis was slightly reduced in all groups at all intervals, but
the decreases were not dose-related. Food intake on the basis of grams
per kilogram body weight was reduced in males at all doses in week 1
and in those at 40 and 3000 ppm at all periods; however, intake was
increased in animals at 2000 ppm in weeks 2, 3, and 4. In females,
intake on this basis was decreased in week 1 and at 4000 ppm in week
2; at other periods, intake was close to control values. Overall,
intake was reduced in all treated animals in week 1 and was
inconsistent thereafter. None of the changes in food intake is
statistically significant, and they are unlikely to be biologically
significant.
No significant haematological changes were observed in males. In
females, such changes were limited to decreased leukocyte counts in
animals at 40 and 4000 ppm and decreased mean corpuscular haemoglobin
in dogs at 2000 ppm. None of these changes is of toxicological
significance. Alanine aminotransferase activity was markedly decreased
in a dose-related manner in animals of each sex, and dose-related
increases in cholesterol levels were seen in females at all doses and
in males (131, 192, 199 and 167 mg/dl at 0, 40, 2000, and 4000 ppm).
No significant changes were observed in urinalysis. The absolute
weights of kidney and spleen were statistically significantly greater
than those of controls in male dogs at 2000 ppm and were nonsigni-
ficantly increased in dogs at 4000 ppm. Liver weights were also
nonsignificantly increased (not dose-related) at these doses. The
liver:body weight ratios of males at 2000 and 4000 ppm were
statistically significantly increased, as were those of the kidney at
2000 ppm; there was a considerable but nonsignificant increase at
4000 ppm. Spleen:body weight ratios were statistically nonsigni-
ficantly increased in males at 2000 and 4000 ppm. The thyroid-
parathyroid:body weight ratio was significantly increased in male dogs
at 2000 ppm and nonsignificantly in those at 4000 ppm. The ratios of
kidney, spleen, and thyroid-parathyroid to brain weights were
significantly increased in male dogs at 2000 ppm and nonsignificantly
at 4000 ppm; the liver:brain weight was significantly increased at
both 2000 and 4000 ppm. In females, the only statistically significant
change was in the liver:body weight ratio, which was increased at 2000
and 4000 ppm; nonsignificantly increased absolute liver weights were
also noted, and nonsignificant increases in brain and brain:body
weight ratios were seen at 2000 and 4000 ppm. The effects on organ
weights (particularly liver) and on clinical chemical parameters
indicate a probable NOAEL of 40 ppm, equivalent to 1 mg/kg bw per day;
however, in the absence of histopathological reports, the significance
of the findings cannot be fully assessed (Johnson, 1989).
Four groups of six beagle dogs of each sex (Ridglan Farms Inc.),
five to six months old and suitably immunized and vaccinated, were fed
diets containing 0, 15, 150, or 1500 ppm quintozene (purity, 99.4%,
with HCB at a maximum of 0.07% and a mean of 0.045%) for one year. The
homogeneity and stability of the diets were determined before the
study, and all diets were analysed for the percentage of the nominal
levels that were actually present. Diets were prepared weekly for four
weeks and monthly thereafter. Acetone was used as the dispersant in
the 15 and 150 ppm and control diets. The homogeneity in 10 samples
was 93-99% at 15 ppm, 85-107% at 150 ppm, and 89-110% at 1500 ppm,
with respective mean values of 95, 93, and 99%. Stability,
investigated over 10 days, was satisfactory. Duplicate analyses for
actual concentrations at each interval and dose showed mean values
over the study of 96, 94, and 96% and ranges of 87-104%, 89-110%, and
82-103% at 15, 150, and 1500 ppm, respectively. The actual intakes
were calculated from data on food intake and body weight to be 0.4,
4.3, and 40.1 mg/kg bw per day for males and 0.44, 4.22, and
41.48 mg/kg bw per day for females. Dogs were observed for mortality,
moribundity, and clinical signs twice daily. Body weights and food
intake were recorded weekly for 14 weeks and monthly thereafter.
Ophthalmology was performed before the test and in week 52.
Haematological and clinical chemical examinations were performed on
blood from the jugular vein of dogs fasted overnight at 6 and 12
months. Urinalysis was performed at 6 and 12 months on urine collected
during the fasting period. The same haematological, clinical chemical,
and urinary parameters were investigated as in the 28-day study
(Johnson, 1989). At termination (death before the end of the study or
sacrifice), the adrenals, brain, heart, kidneys, liver, gonads,
pituitary, spleen, and thyroid-parathyroids were examined grossly and
weighed. Adrenals, aorta, bone (rib), bone marrow (rib), brain (fore-,
mid-, and hind-), eye (with optic nerve), gall-bladder, oesophagus,
stomach, duodenum, jejunum, ileum, caecum, colon, rectum, ovaries,
testis with epididymides, heart, kidneys, liver, lung (with bronchi),
lymph nodes (tracheobronchial, mesenteric, and regional), mammary
gland (females only), pancreas, pituitary, prostate, salivary gland
(with mandibular lymph node), sciatic nerve, skeletal muscle (thigh),
skin, spinal cord (cervical, thoracic, and lumbar), spleen, thymic
region, trachea, urinary bladder, and uterus from all dogs were
preserved, processed, and examined microscopically.
In one male dog at 15 ppm that was sacrificed in extremis at
week 28, the clinical and pathological signs indicated a diagnosis of
generalized systemic blastomycosis. The death was not related to
treatment, and the clinical signs reported (including diarrhoea,
emesis, alopecia, lacrimation, and ocular discharge) did not indicate
dose- or compound-related incidence. One male at 150 ppm had
convulsions in weeks 39 and 48 and tremors in weeks 39-40, associated
with limb rigidity in weeks 39-40 and excessive salivation. No
abnormal neural effects were seen histopathologically at term. The
effect was isolated and is unlikely to have been compound-related.
Body weight and weight gain were unaffected. Food intake was variable,
but no clear trends were apparent. There were no compound-related
effects on haematological parameters, the only statistically
significant difference from control values being decreased haemoglobin
levels at 12 months in males at 15 ppm. Statistically significant
changes in clinical chemical parameters comprised increased alkaline
phosphatase activity at 1500 ppm in males at 6 and 12 months and in
females at 6 months; a considerable but not statistically significant
increase occurred in females at 12 months. Significantly decreased
alanine aminotransferase activity was seen in animals of each sex at
150 and 1500 ppm at 6 and 12 months; significantly decreased
creatinine was noted in males at these doses at 6 and 12 months and in
females at 1500 ppm at 6 months. Cholesterol levels were significantly
increased in males at 1500 ppm at 6 and 12 months and nonsignificantly
increased in females at 1500 ppm at 6 and 12 months. Globulin levels
were also significantly increased in males at 150 and 1500 ppm at 6
months, and blood urea nitrogen was decreased in females at 1500 ppm
at 12 months. No effects were seen on urinalysis.
Statistically significant increases in the absolute and relative
(to body and brain weight) weights of liver were seen in females at
1500 ppm and in relative weights in males at 1500 ppm; the absolute
liver weight in males was also increased but not significantly.
Nonsignificant increases in absolute and relative liver weights were
seen in animals of each sex at 150 ppm. A statistically significant
increase in the kidney:body weight ratio was seen in females at
1500 ppm. The maximal absolute adrenal weights were slightly increased
in males at 1500 ppm, and females showed a minimal, dose-related
increase in adrenal weights. Microscopic examination showed slight
hepatocellular hypertrophy in all dogs at 1500 ppm. No dose-related
effects were seen on alveolar macrophages, inflammation, or the
incidence of pneumonia. Adrenal changes in males were comparable in
all groups. In females at 1500 ppm, the incidence of lymphocytic
infiltration and vacuolar changes was increased, but these effects are
considered not to be of toxicological significance. The incidence of
pituitary cysts was increased in animals of each sex at 1500 ppm, but
such cysts were relatively common in control beagle dogs. The NOAEL
was probably 150 ppm, equal to 4.3 mg/kg bw per day in males and
4.22 mg/kg bw per day in females. Changes in clinical chemical
parameters seen at this dose (e.g. decreased alanine aminotransferase
activity and decreased creatinine) did not appear to be associated
with microscopic changes in tissues or organs (Goldenthal, 1990)
Four groups of three beagle dogs of each sex were fed diets
containing quintozene (purity, 98.8%) at concentrations of 0, 500,
1000, or 5000 ppm for two years. Erythrocyte counts, total and
differential leukocyte counts, haemoglobin, haematocrit, and Heinz
bodies; and the specific gravity, pH, appearance, colour, microscopic
sediment, albumin, glucose, and bilirubin in urine were reported at
26, 52, and 78 weeks and at terminal sacrifice. The heart, lung,
liver, kidney, spleen, pancreas, gonads, prostate, adrenal, thyroid,
brain, pituitary, eye with optic nerve, stomach, jejunum, colon, and
bone marrow were examined histologically. Of the animals at the
highest dose, three males died on days 214, 288, and 357 and two
females on days 317 and 472. Food intake was slightly reduced (mean,
4.6%) in animals at 1000 ppm and markedly (by about 30%) in those at
5000 ppm. All dogs at 5000 ppm lost weight, and their body-weight
gains (means, 2.75 kg at 500 and 2.62 kg at 1000 ppm) were lower than
that of controls (4.85 kg). Signs of toxicity at 5000 ppm included
lacrimation, conjunctival discharge, and milky corneal opacity; one
dog had corneal ulceration. One dog at 1000 ppm had conjunctivitis and
a second had corneal opacity for a short period. No effects were seen
at 500 ppm. Anaemia was induced in dogs at the high dose, with
characteristic reductions in haemoglobin, haematocrit, and erythrocyte
counts. Atrophy of bone marrow and reduced haematopoiesis were
observed at 5000 ppm. Severe histopathological changes in the liver
were seen at 5000 ppm and included fibrous narrowing of hepatic cell
cords, enlarged periportal areas, and increased leukocytic
infiltration. Similar but less intense effects were seen at 500 and
1000 ppm. There was no NOAEL (Scholz & Brunk, 1968).
Five groups of four beagle dogs of each sex, 4.5 months old, were
fed diets containing quintozene (purity, 98.2%; containing 1.4% HCB
and traces of tetrachloronitrobenzene and pentachlorobenzene) at
concentrations of 0, 5, 30, 180, or 1080 ppm for two years. Diets were
prepared weekly by dilution of the high dose, which was prepared by
blending corn-oil solutions of quintozene with the diet; the dietary
fat content was increased from 9 to 11%. Before feeding, an equal
weight of water was added to the food. Body weight and food intake
were determined weekly. The haematocrit, haemoglobin, and total and
differential leukocyte counts, and urinary reducing substances,
protein, specific gravity, and sediment were determined at 3, 6, 12,
18, and 24 months. Blood urea nitrogen, serum aspartate
aminotransferase, serum alkaline phosphatase, and cholinesterase,
prothrombin time, and bromsulphthalein retention time were determined
in controls and dogs at the high dose at 0, 3, 6, 12, 18 and 24
months. The frequency of oestrus was recorded. One dog of each sex at
each dose was sacrificed at 12 months for histopathological
examination. Organ and organ:body weight ratios were reported for
heart, spleen, liver, kidneys, and testes; the brain, lung, heart,
aorta, liver, spleen, kidney, stomach, ileum, jejunum, large
intestine, urinary bladder, bone marrow (sternal and long bone),
pituitary, thyroid, pancreas, adrenal, gonad, lymph node, and eye were
examined histologically.
No deaths were observed. The changes in body weight were not
significant, but weight gain tended to be reduced in females at 180
and 1080 ppm during weeks 6 and 13, and sporadically in all treated
males, which also had sporadic decreases in food intake. Neither body
weight nor food intake was consistently affected by treatment.
Slightly reduced haematocrit values were noted in males at 30 and
180 ppm at 78 weeks (statistically significant), and reduced
haemoglobin values were noted in males at 30 ppm; a nonsignificant
decrease in haematocrit and haemoglobin values was again seen in males
at 30 and 180 ppm at 104 weeks. The absence of any effect at 1080 ppm
indicates that these effects were probably random. No consistent
effects were seen on serum cholinesterase, the maximal inhibition
being 20% in females at 1080 ppm in week 13 and 23.6% in females at 30
ppm at week 52; all other depressions were < 15.4% and were random.
Mean serum aspartate aminotransferase activity was increased in
females at 1080 ppm at 13 and 78 weeks but decreased at 104 weeks
(statistically significant only at 13 weeks); it was also decreased in
males at 1080 ppm at 26 weeks and increased at 78 weeks. Serum
alkaline phosphatase activity was increased in males and females at 52
weeks and thereafter, but the increase achieved statistical
significance only at 52 weeks when data on animals of each sex were
combined. External lymph nodes and oestrus cycle were not affected by
treatment. Liver weight and the liver:body weight ratios of males and
females at 180 and 1080 ppm were nonsignificantly increased at one
year; at two years, a nonsignificant increase in absolute liver weight
was seen in males at 1080 ppm and a significant increase in females.
The increased liver:body weight ratio was significant only when data
for the two sexes were combined. The absolute and relative liver
weights were nonsignificantly reduced in females at 5, 30, and 180 ppm
but significantly reduced in males at 30 and 180 ppm. Testicular
weights were nonsignificantly reduced in dogs at 1080 ppm, and the
weight ratio was statistically significantly reduced. Other changes
(such as increased kidney weight ratio at 5 ppm and increased heart
ratio at 180 ppm in males) were random. Dogs at 180 ppm showed minimal
suppression of bile flow in liver cord cells; the effect was greater
in animals at 1080 ppm, resulting in moderate bile pigment
accumulation in cord cells. The effect was usually reversible. The
NOAEL was 30 ppm, equivalent to 0.75 mg/kg bw per day (Larson &
Borzelleca, 1968; Borzelleca et al., 1971).
Monkeys
Two rhesus monkeys of each sex were fed consecutive daily doses
of quintozene (purity, > 99.9%) on sucrose pellets, corresponding to
2 ppm of their daily diet, for 70 days. There were no controls. One
monkey of each sex was sacrificed on day 71. Haemoglobin, packed cell
volume, erythrocyte and total leukocyte counts, methaemoglobin, and
Heinz bodies, and sodium, potassium, bilirubin, creatinine, blood urea
nitrogen, total protein, serum aspartate and alanine aminotrans-
ferases, lactate dehydrogenase, cholesterol, luteinizing and
follicular-stimulating hormone levels, progesterone, and cortisol were
determined in blood samples taken before the test, on days 19 and 48,
and, in the surviving monkeys, on day 76. There were no changes in
haemoglobin values, packed cell volume, or erythrocyte or leukocyte
cell counts. The level of methaemoglobin was slightly elevated on day
1 but not thereafter. No change was seen in the incidence of Heinz
bodies. No changes were seen in clinical chemistry, although the data
on bilirubin and lactate dehydrogenase were highly variable; no time
effect relationship was apparent. At sacrifice, no histopathological
effects were seen in the liver, stomach, large or small intestine,
spleen, kidney, heart, lung, thymus, cerebrum, cerebellum, pons,
medulla, spinal cord, or bone marrow of the animals sacrificed on day
71. The NOAEL was > 2 ppm, equivalent to > 0.1 mg/kg bw per day
(Kögel et al., 1979b)
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 18 B6C3F1 and B6AKF1 mice of each sex were given
quintozene (purity and contaminants unspecified) at a dose of
464 mg/kg bw by stomach tube from seven days of age to the time of
weaning at four weeks of age and thereafter at a dose of 1206 ppm for
18 months. The latter was the maximal tolerated dose. There was a
significantly elevated incidence of tumours, mostly hepatomas, in both
strains (Innes et al., 1969).
Groups of 100 male and 100 female Swiss random strain mice were
fed diets containing quintozene (containing 2.7% HCB) at levels of 0,
100, 400, or 1200 ppm for 80 weeks. Body-weight gain was decreased in
animals of each sex at 1200 ppm. Liver:body weight ratios were
increased in males and females at 400 and 1200 ppm, and the
kidney:body weight ratio was increased in females at 1200 ppm. General
appearance, behaviour, and survival were not affected by treatment,
and the haematological indices were considered to be within normal
limits. A non-dose-related increase in nodular hyperplasia of the
liver was seen in all treated males. The hyperplastic areas had
essentially normal cell architecture and were regarded as
non-neoplastic. An increased incidence of subcutaneous fibrosarcomas
was observed in females at 1200 ppm. None of the other neoplasms
appeared to be related to treatment and were common in mice of this
strain. There was no NOAEL (van der Heijden & Til, 1974).
Groups of 50 male and 50 female mice (probably B6C3F1) were fed
diets containing two increasing doses of quintozene (purity, > 98%;
with 0.15% pentachlorobenzene, 0.25% 2,3,4,5-tetrachloronitrobenzene,
and 1% HCB), initially at 1075 and 2150 ppm for males and 2320 and
4640 ppm for females, increased to 3000 and 6000 ppm for males and
4500 and 9000 ppm for females during the last 315 days of the 546-day
(78-week) exposure period. A control group consisting of 20 male and
20 female mice was given only the vehicle, 2% corn oil. The total
duration of the study was 92 weeks, but some mice were killed and
autopsied earlier. Malignant tumours were seen at frequencies of 50%
in 25% of control males and 20% in 10% of control females, at 33% in
29% of males and 18% in 6% of females at the low dose, and at 29% in
20% of males and 57% in 19% of females at the high dose. Only the last
frequency was statistically significantly higher than expected; many
malignant histiocytic lymphomas were found in numerous organs of the
same animal. No significant differences were found between groups in
the numbers of animals with tumours, and no particular type of tumour
could be ascribed to the treatment (Wedig et al., 1976).
Three groups of 50 B6C3F1 mice of each sex, seven to eight weeks
old, were fed diets containing quintozene (purity, 99.6%, with 0.07%
HCB) at doses of 0, 2500, or 5000 ppm for 103 weeks, followed by a
one-week withdrawal period before terminal sacrifice. Mice were
observed twice daily, and five per cage were weighed weekly for 12
weeks and monthly thereafter. Food intake per cage was measured every
four weeks. The homogeneity of the diets was within 10%; the stability
of quintozene in the diet for two weeks was 100% at 25°C; and the mean
actual dietary concentrations, measured 14 times during the study,
were 2460 ppm (98.4%) with a range of 94-104.4% at 2500 ppm and
4980 ppm (99.6%) with a range of 94-104% at 5000 ppm. All animals
(including those that died before the end of the study) were
necropsied, unless autolysis or cannibalization had occurred. Tissue
masses, abnormal regional lymph nodes, mammary gland, salivary gland,
bone marrow, costochondral junction, thymus, larynx, trachea, lungs
and bronchi, heart, thyroid, parathyroid, oesophagus, stomach,
duodenum, jejunum, ileum, colon, mesenteric lymph nodes, liver,
gall-bladder, pancreas, spleen, kidney, adrenal, urinary bladder,
seminal vesicles, prostate, uterus, gonads, brain, pituitary, and
caecum from controls, female mice at the low dose, males and females
at the high dose, and all males at the low dose that died before term
were examined histologically. Gross lesions, livers, and nasal
cavities from all males at the low dose were also examined.
The terminal body weights of male mice were 96% of control values
at 2500 and 90% at 5000 ppm. The mean body weights of females at
5000 ppm were consistently 10% below control values by week 24 and
thereafter, but reached this level at 2500 ppm only after 92 weeks; at
term, the mean weights of females were 18 and 21% below control
values. No clinical signs were reported. Food intake may have exceeded
that of controls, but this was difficult to determine because of food
scattering. The survival of males at term, 31-34 mice, was comparable
in all groups. At term, 30 female controls, 18 at the low dose, and 14
at the high dose were still alive; however, five mice at the low dose
were accidentally drowned and two mice at 2500 ppm and one at 5000 ppm
died during the recovery period.
In male mice at 0, 2500, and 5000 ppm, the numbers with malignant
tumours were 19, 25, and 17 (25, 30, and 21 tumours) and those with
benign tumours were 19, 15, and 17 (23, 16, and 19 tumours). There was
no dose-effect relationship for the incidence of tumours in any
specific organ, the incidences generally being comparable between
groups. The incidence of non-neoplastic lesions in comparison with the
control incidence was not of toxicological significance. In female
mice at 0, 2500, and 5000 ppm, the numbers with malignant tumours were
15, 11, and 9 (15, 11, and 9 tumours) and those with benign tumours
were 16, 17, and 14 (18, 19, and 19 tumours). Tumours were not
concentrated in specific tissues and there was no dose-effect
relationship. Increased incidences of ovarian abscesses were seen,
with 24, 44, and 58% at 0, 2500, and 5000 ppm. Five of six abscesses
that were cultured contained Klebsiella. Hyperplasia of the
mediastinal lymph node was observed at incidences of 2, 9, and 20% and
liver haematopoiesis at 18, 42, and 46%; splenic haematopoiesis was
observed at incidences of 28, 48, and 54%. These effects may reflect
increased susceptibility to infection rather than a toxicological
effect.
Quintozene thus decreased body weight at 5000 ppm, equal to
953 mg/kg bw per day in males and 1358 mg/kg bw per day in females.
There was no increase in neoplasia, and the non-neoplastic
pathological effects in female mice were probably due to infection.
The NOAEL in male mice was 2500 ppm. In female mice at 2500 ppm, the
reduced body weight reached 10% only at 92 weeks and continued to
decrease, to 18%, until 103 weeks; as this effect is unlikely to be
compound-related, 2500 ppm was also the probable NOAEL for female
mice. Overall, the NOAEL was 2500 ppm, equal to 387 mg/kg bw per day
in male mice (US National Toxicology Program, 1987).
A group of 10 mice (strain not specified) of each sex received
topical applications on shaved skin of 0.2 ml of a 0.3% solution of
quintozene (purity and contaminants unspecified) in acetone twice
weekly for 12 weeks. The control group received acetone alone. After
application of quintozene, croton oil was applied to the site
(presumably twice weekly) for 20 weeks, and mice were sacrificed 20
weeks later. Papillomas were seen in 2/10, 6/10, 8/9, and 7/8 treated
males, in 6/10, 7/9, 7/7, and 7/4(?) treated females, in 1/10, 6/9,
5/8, and 1/7 control males, and in 0/10, 3/10, 4/8, and 4/7 control
females 10, 20, 30, and 40 weeks after the initial croton oil
application, respectively. The time to 50% tumour incidence was 13
weeks in treated males, 10 weeks in treated females, 16 weeks in male
controls, and unknown for control females. The total numbers of
tumours were 29 in treated males, 33 in treated females, 6 in control
males, and 7 in control females. Quintozene at the doses used in the
study thus did not induce tumours in the absence of a promoting agent
(Searle, 1966).
Rats
Groups of 10 rats of each sex (strain unspecified) were fed diets
containing 0, 25, 100, 300, 1000, or 2500 ppm quintozene (purity and
contaminants unspecified) prepared by blending a powder comprising 20%
quintozene, 77% pyrax ABB (a pyrophyllite carrier), and 3% Armour
'sticker' with Purina dog chow. Rats were weighed weekly, and
haematological parameters (unspecified) were determined at 11 and 24
months. At death and terminal sacrifice, heart, lung, liver, kidney,
spleen, stomach, small intestine, caecum, large intestine, thyroid,
adrenal, bladder, pancreas, and bone marrow were examined
histologically. At one year, survival in males was 7, 10, 8, 7, 9, and
10 animals, and that in females was 9, 6, 8,10, 10, and 8 animals at
0, 25, 100, 300, 1000, and 2500 ppm, respectively; by two years,
survival was 1, 4, 4, 3, 3, and 4 in males and 3, 2, 5, 5, 5, and 2 in
females. Body-weight changes (only means given) were sporadic;
statistically significant increases were seen in males at 25 ppm
(weeks 4-52) and 100 ppm (week 4) and in females at 100 ppm (weeks
2-78), 300 ppm (week 8 and weeks 52-78), 1000 ppm (week 26), and
2500 ppm (weeks 2-78); decreases were seen in males at 2500 ppm (weeks
2 and 8). The small numbers of animals make definite conclusions
difficult, but the dose of 2500 ppm appeared to decrease body weight
consistently in animals of each sex, as did the dose of 1000 ppm in
females. The decreases at all doses in females may be real, but males
were affected only at 2500 ppm. The haematological parameters were
stated to be within normal limits, and histopathological lesions were
stated to be limited to 'occasional lung abscesses and occurrences of
fatty changes in the liver. Lesions are not correlatable with dietary
concentration of quintozene but are of the type often seen in old
rats.' Individual data were not available. The NOAEL in this limited
study was 300 ppm, equivalent to 15 mg/kg bw per day (Finnegan
et al., 1958).
Groups of 50 male and 50 female Wistar rats were fed dietary
levels of 0, 100, 400 and 1200 ppm quintozene (containing 2.7% HCB)
for two years. The liver:- and kidney:body weight ratios were
increased in rats at 400 and 1200 ppm. Dose-related increases in the
incidences of single-cell necrosis and fatty metamorphosis of
hepatocytes were seen in animals of each sex at 400 and 1200 ppm, and
enlarged centrilobular hepatocytes were seen at all doses. There was
no increase in tumour incidence, and no adverse effects were observed
on general appearance, behaviour, body-weight gain, or food
consumption. Haematological, blood chemical, and urinary values were
considered to be within normal limits. There was no NOAEL in this
limited study (Sinkeldam et al., 1974).
Groups of 50 male and 50 female rats (strain unspecified) were
fed diets containing two decreasing doses of quintozene (purity, >
98%; with 0.15% pentachlorobenzene, 0.25% 2, 3, 4, 5-tetrachloro-
nitrobenzene, and 1% HCB): for the first 98 days, males received 7500
or 15 000 ppm and females, 11 000 or 22 000 ppm; during the last 448
days, males received 5000 or 10 000 ppm and females, 7250 or
14 500 ppm. A control group consisting of 20 male and 20 female rats
was given only the vehicle, 2% corn oil. The total exposure time was
546 days (78 weeks), and the duration of the study was 85-117 weeks.
Malignant tumours were seen at frequencies of 16% in 10% of control
males and 10% in 10% of control females, at 13% in 8.5% of males and
8% in 6% of females at the low dose, and at 23% in 12.5% of males and
9% in 6.5% of females at the high dose. No significant difference
between the groups was found in the total number of tumours or the
number of animals with tumours, and no particular type of tumour could
be ascribed to the treatment (Wedig et al., 1976).
Four groups of 50 Charles River CD rats of each sex, six to seven
weeks old, were fed diets containing quintozene (purity, 99.4%; <
0.07% HCB) at doses of 0, 20, 3000, or 6000 ppm for 24 months. An
additional 10 rats of each sex at each dose were treated similarly and
sacrificed at 12 months. As the homogeneity of the diet at the 20-ppm
dose was unsatisfactory in week 1 (range, 14-40 ppm), blending time
was increased for the week-2 diets, which improved the range to
16-22 ppm. From week 28, acetone was used as an aid to dispersion,
resulting in a variation of 16.3-18.9 ppm. The homogeneity was
acceptable at higher doses. The stability over 10 days was within 8%.
Duplicate analyses about every four weeks indicated that the mean
percentages of the nominal concentrations were 95 8.4 at 20 ppm, 100 ±
6.5 at 3000 ppm, and 101 ± 6.9 at 6000 ppm. Diets were prepared
weekly. Rats were observed twice daily for mortality, moribundity, and
overt signs of toxicity. Body weight and food intake were recorded for
individual rats weekly for 16 weeks and monthly thereafter.
Ophthalmological examinations were conducted before treatment and at
12 and 24 months. Five rats of each sex were screened microbio-
logically before the test. Erythrocyte count, total and differential
leukocyte counts, haemoglobin, haematocrit, platelet counts, mean
corpuscular volume, mean corpuscular haemoglobin, and mean corpuscular
haemoglobin concentration; and sodium, potassium, chloride, calcium,
inorganic phosphorus, alkaline phosphatase, bilirubin, aspartate and
alanine aminotransferases, creatine phosphokinase, urea nitrogen,
creatinine, total protein, albumin, globulin, cholesterol and glucose
were determined in orbital sinus blood from 10 rats of each sex at
each dose, which had been fasted overnight, at 6, 12, 18, and 24
months. The colour, appearance, volume, specific gravity, microscopic
elements, pH, protein, glucose, bilirubin, ketones, occult blood,
nitrite, and urobilinogen in urine were determined in samples
collected during the fasting period at 6, 12, 18, and 24 months. All
animals were necropsied, and adrenals, aorta, femur, bone marrow
(femur), brain (fore-, mid-, and hind-), eye (including optic nerve),
oesophagus, stomach, duodenum, jejunum, ileum, caecum, colon, rectum,
ovary, testis (with epididymides), heart, kidney, liver, lung, lymph
nodes, mammary gland (female only), pancreas, pituitary, prostate and
seminal vesicles, salivary gland (with lymph nodes), sciatic nerve,
skeletal muscle (thigh), skin, spinal cord (cervical, thoracic, and
lumbar), spleen, thymic region, thyroid, parathyroid, trachea, urinary
bladder, uterus, gross lesions, and tissue masses were collected. The
adrenals, brain, heart, kidney, liver, gonads, and thyroid-parathyroid
were weighed at sacrifice at 12 and 24 months. All tissues of control
rats, those at the high dose, and those that died or were sacrificed
were examined histologically at term. Tissue masses, gross lesions,
regional lymph nodes, and, in females only, lung were examined
histologically at interim sacrifice of animals at 20 and 3000 ppm; the
liver, kidney, lung, thyroids, parathyroids, tissue masses, and
regional lymph nodes of animals at these doses were examined at
terminal sacrifice.
Mortality was 52, 54, 42, and 16% in males and 42, 50, 46, and
48% in females at 0, 20, 3000, and 6000 ppm, respectively, excluding
rats sacrificed at 12 months. One of 60 rats at each dose had died by
12 months, the only clinical sign being a slightly increased incidence
of body surface staining in females at 6000 ppm and an increased
incidence of malocclusion in males at this dose from week 1 to term,
which was obviously not compound-related. Body weight was
significantly reduced in animals of each sex at 6000 ppm throughout
the study, the decrease being > 10% by week 2 in males and by week 24
in females. Slightly lower weights were noted in females at 3000 ppm
after 24 weeks (statistically significant in 14/21 weighings), but the
weight decreases in males were generally comparable to those of
controls, except for a slight (generally statistically nonsignificant)
reduction after 84 weeks. Food intake on the basis of grams per
kilogram body weight tended to be increased, especially in males, and
to a lesser degree females, at 6000 ppm. The average intakes of the
diets at 20, 3000, and 6000 ppm were 0.9, 141, and 303 mg/kg bw per
day in males and 1.14, 179, and 370 mg/kg bw per day in females. No
compound-related ophthalmological findings were seen.
Statistically significant haematological findings tended to be
sporadic and do not appear to be compound-induced. In males at
6000 ppm, haemoglobin and haematocrit were reduced at six months only,
and mean corpuscular volume was reduced at 6 and 12 months; platelet
counts were increased at 12 months in males at 20 ppm. In females,
erythrocyte counts were elevated at 18 months at 20 and 6000 ppm,
haemoglobin was depressed at 6 months at 3000 and 6000 ppm but was
increased at 18 months at 20 ppm, haematocrit was increased at 18
months at 20 ppm, mean corpuscular volume was decreased at 18 months
at 3000 ppm and at 6, 12, and 18 months at 6000 ppm, mean corpuscular
haemoglobin was decreased at 18 months at 3000 ppm and at 6 and 18
months at 6000 ppm, and mean corpuscular haemoglobin concentration was
depressed at 6 months at 6000 ppm. Lymphocyte counts were elevated at
12 months at 20 ppm.
The clinical chemical findings in males included increased
potassium levels at 24 months and decreased glucose at 6 months at
6000 ppm, which are probably random effects. Alanine aminotransferase
activity was reduced at 6 months at 20 ppm, at 6, 18, and 24 months at
3000 ppm, and at all intervals at 6000 ppm; however, it was increased
at 12 months at 3000 ppm. The decrease is considered to be compound-
related, and the amount is dose-related. In females at 6000 ppm,
slight decreases in chloride were seen at 6 and 12 months and slightly
increased calcium at 18 months. Alkaline phosphatase activity was
increased at 6 and 12 months and aspartate aminotransferase activity
was decreased at all intervals at 6000 ppm; alanine aminotransferase
activity was decreased in a dose-related manner at all intervals at
3000 and 6000 ppm. Cholesterol was increased at all intervals and
glucose was decreased at 6 months at 6000 ppm. Total protein was
increased at 18 months at 3000 and 6000 ppm. Thus, in animals of each
sex, compound-related effects on alanine aminotransferase activity
were seen at 3000 and 6000 ppm, and in females aspartate aminotrans-
ferase activity was decreased and cholesterol was increased at
6000 ppm. The effects on alkaline phosphatase activity may also be
compound-related. There were no apparent changes at any dose or
interval in urinary parameters, except possibly in volume; but water
contamination was likely to have occurred.
Macroscopic examination of tissues at 12 months showed an
increased incidence of tan-white foci in lungs of female rats at
6000 ppm (one control; 11 at 6000 ppm), and the incidence of
tan-white-yellow lung foci was increased in a dose-related manner in
animals of each sex at 3000 and 6000 ppm that died or were sacrificed
at 12-24 months. The incidence of accentuated lobulation of the liver
was increased in animals of each sex at 6000 ppm. Thyroid enlargement
was also noted, with incidences of 0, 0, 2, and 3 in males and 1, 0,
2, and 1 in females at 0, 20, 3000, and 6000 ppm. This finding is
questionable.
The absolute and relative (to brain and body weight) weights of
the kidney, liver, and testis were statistically significantly
increased in males at 3000 and 6000 ppm at the 12-month interim
sacrifice. The organ:body weight ratios were affected by decreases in
the body weight of males at 6000 ppm, but significant increases were
reported for heart, kidney, liver, thyroid-parathyroid, and testis at
this dose. At 3000 ppm, increased organ:body weight ratios were seen
for kidney and liver. The organ:brain weight ratios were increased for
kidney at 3000 and 6000 ppm and for testis at 6000 ppm. Significantly
increased organ:body weight ratios were seen for brain, kidney, liver,
and thyroid-parathyroid in females at 6000 ppm at the 12-month
sacrifice. In males at terminal sacrifice, the absolute weights of
liver were increased at 6000 ppm, of the thyroid-parathyroid at 3000
and 6000 ppm, and of the kidney at 3000 ppm. Increased organ:body
weight ratios were seen in males for brain at 6000 ppm, kidney at
3000 ppm, liver at 3000 and 6000 ppm, and thyroid-parathyroid at 3000
and 6000 ppm, and the organ:brain weight ratios were increased in
males for kidney at 3000 ppm, liver at 3000 and 6000 ppm, and
thyroid-parathyroid at 3000 and 6000 ppm. Females at 6000 ppm with
body weights nonsignificantly lower than those of controls had a
decreased absolute heart weight and an increased absolute thyroid-
parathyroid weight. Increased organ:body weight ratios were seen for
liver at 6000 ppm and thyroid-parathyroid at 3000 and 6000 ppm; the
heart:brain weight was decreased and the thyroid-parathyroid:brain
weight was increased at 6000 ppm. When nonsignificant changes are
included, the major effects were increases in liver and thyroid-
parathyroid weights in animals at 3000 and 6000 ppm at both interim
and final sacrifices.
There were no microscopic observations that would account for the
changes in kidney and testicular weights. No compound-related
microscopic pathological changes were seen up to 12 months. At 12-24
months, the incidence of hypertrophy in males was 0, 0, 13/48, and
30/49, that of hyperplasia 0, 0, 1, and 1, and that of hepatocellular
adenomas 1/49, 0/49, 1/48, and 4/49 rats at 0, 20, 3000, and 6000 ppm.
Increased incidences of alveolar macrophages, interstitial pneumonia,
and perivascular lymphoid infiltration were seen in the lungs of males
at 6000 ppm. Thyroid hypertrophy was seen in 3/49, 2/49, 20/48, and
33/49 rats, follicular adenomas in 0/49, 0/49, 6/48, and 5/49 rats,
follicular carcinomas in 0/49, 1/49, 0/48, and 2/49 rats, and
follicular hyperplasia in 2/49, 2/49, 7/48, and 8/49 rats at 0, 20,
3000, and 6000 ppm, respectively. The incidence of colloidal cysts was
also increased in males at 6000 ppm. In females at 12-24 months, liver
hypertrophy occurred in 0/50, 0/49, 19/50, and 29/47 rats, hyperplasia
in 1/50, 0/49, 5/50, and 3/47 rats, and hepatocellular adenomas in
1/50, 0/49, 1/50, and 1/47 rats at 0, 20, 3000, and 6000 ppm,
respectively. Increased incidences of alveolar macrophages and
interstitial pneumonia were seen at 3000 and 6000 ppm; the incidence
of perivascular lymphoid infiltration was not increased. Thyroid
hypertrophy was seen in 0/50, 0/49, 18/50, and 23/46 female rats,
follicular adenomas in 1/50, 0/49, 2/50, and 4/46, follicular
carcinomas in 1/50, 0/49, 0/50, and 1/46, and follicular hyperplasia
in 0/50, 0/49, 6/50, and 8/46 at 0, 20, 3000, and 6000 ppm,
respectively. In data on historical controls from 11 two-year studies,
the mean percentage incidences of follicular adenomas were 2.88% for
males and 0.3% for females, and those of follicular carcinomas were
1.73% for males and 0.60% for females. In the study described here,
the incidences of follicular adenoma were 0, 0, 12.5, and 10.2% for
males and 2, 0,12, and 8.7% for females at 0, 20, 3000, and 6000 ppm,
respectively, and those of follicular carcinoma were 0, 2, 0, and 4.1
in males, and 2, 0, 0, and 2.2 in females. The incidence of follicular
adenomas in animals of each sex at 3000 and 6000 ppm therefore exceeds
the historical mean, as does that of follicular carcinomas in males at
20 and 6000 ppm and in females in the control and 6000-ppm groups. The
incidence of follicular carcinomas in males at 20 ppm, control
females, and in females at 6000 ppm was one per group, which is within
the historical control range, as is the incidence of follicular
carcinomas in males at 6000 ppm: 2/49 as compared to 3/32 in one
historical control. Quintozene therefore induced thyroid adenomas and
may have induced thyroid carcinomas. The changes observed in the lung
are consistent with infections; the increased incidence of such
changes at high doses may indicate either reduced immune competence or
a stress-related increase in susceptibility to infection. The
hepatocellular hypertrophy (mainly centrilobular) in which
eosinophilic cytoplasm was noted is indistinguishable from the
hepatocellular hypertrophy arising from increased drug metabolizing
enzyme activity. The NOAEL was 20 ppm, equivalent to 1 mg/kg bw per
day (Goldenthal, 1991).
(d) Reproductive toxicity
Four groups of 25 weanling (28-day-old) Charles River CD rats of
each sex were fed diets containing quintozene (purity, 98.2%, with
1.4% HCB and trace levels of tetrachloronitrobenzene and
pentachlorobenzene) at concentrations of 0, 5, 50, or 500 ppm. The
diet was prepared weekly, using 10 ml of corn oil for 500 ppm
quintozene, and was appropriately diluted with additional feed. After
11 weeks on diet, groups of 20 F0 females were paired with a male at
the same dose, rotated weekly for three weeks if necessary. Matings,
numbers of pregnancies, litters born, pups 1, 4, and 21 days
post partum, and litter weight at day 21 were recorded. Litters were
culled to 10 pups on day 4. Indices of fertility (pregnancies per
mating × 100), gestation (total litters per number of pregnancies ×
100), viability (live pups at day 4 per live pups born × 100) and
lactation (weaned pups per number of remaining pups after culling on
day 4 × 100) were calculated. A second litter (F1b) was initiated 10
days after the birth of the final F1a litter, and these animals were
used as parental rats for the F2 and F3 generations. Autopsies
were performed on F0 adults after weaning of F1b pups, F1a pups
at weaning, F1b adults after weaning of F2b pups, and F2a pups
at weaning, this pattern being maintained throughout the study. Ten
F3b offspring of each sex were maintained on the diets until two
months old, when they were sacrificed, and the heart, lung, liver,
kidney, urinary bladder, spleen, stomach, small and large intestine,
caecum, lymph nodes, bone marrow, skeletal muscle, skin, brain,
pituitary, thymus, thyroid, adrenals, pancreas, and gonads were
examined histopathologically. The only effects were on the body weight
of adult females: No significant changes were observed between
controls and F0 or F1b females at 500 ppm, but the body weights of
F2b females were significantly decreased at 500 ( P < 0.05) and
50 ppm ( P < 0.01). The effect was not dose-related. The NOAEL was
probably 500 ppm, equivalent to 2.5 mg/kg bw per day (Larson &
Borzelleca, 1968; Borzelleca et al., 1971).
Four groups of 26 Charles River COBS CD rats of each sex, aged 55
days, were fed diets containing quintozene (purity, > 99%; 0.08%
HCB) at doses of 0, 20, 3000, or 6000 ppm for two litters per
generation for two generations. Parents for the second generation were
selected from the F1b litters, avoiding sibling matings. Diets were
prepared weekly in a twin-shell blender with an intensifier bar to mix
500 g with additional diet to achieve the required levels. As the
homogeneity of the diet at the low dose was poor in week 1, the mixing
time was increased to 20 min after an initial 2-min mixing with 250 g
untreated diet, the mixer bar being used for the first 10 min of the
final blending. This process improved the homogeneity, but the dietary
levels were reduced to 84% of the nominal concentration. The diets
containing the low dose were therefore again modified, by dissolving
the quintozene in acetone before preparing the premix and blending (in
a Hobart mixer) it for 5 min before mixture with additional diet. The
homogeneity and percentage concentration were then acceptable. Those
at the middle and high doses were acceptable from week 1. The control
diet was also mixed with acetone from week 4. Analysis of the diets at
least once every four weeks throughout the study showed good
concordance with nominal values. F0 parents were fed treated diets
for 81 days before pairing, and F1 parents were given the test diet
for 90 days between weaning and pairing. Rats were observed twice
daily for mortality and signs of toxicity; body weights were recorded
weekly until sacrifice, except during gestation when animals were
weighed on days 0, 7, 14, and 20. Food intake was measured weekly
except during mating and, for females, during gestation and lactation,
when data were obtained for days 0-7, 7-14, 14-20, and 0-20 of
gestation and 0-4, 4-7, 7-14, and 14-21 of lactation. Rats were paired
1:1, and copulation was determined by either vaginal lavage or the
presence of a copulatory plug. The day of detection of copulation was
designated day 0 of gestation, for a maximum of 21 days. The time
between end of lactation and re-pairing was 10 days. Males were
changed between F1a and F1b and between F2a and F2b pairings.
The duration of the gestation period and difficulties in parturition
were recorded. Litter size, the numbers of still- and live births, and
gross abnormalities were noted. On lactation day 4, litters were
culled to eight pups (four of each sex when possible). Pups and dams
were observed twice daily for abnormal behaviour (including nesting
and nursing) and deaths. Pups were sexed and weighed individually on
days 0, 4, 7, 14, and 21 of lactation.
At term, F0 males were sacrificed and necropsied, and their
tissues were preserved. All males that failed to sire a litter were
examined for the presence of sperm in the epididymides. F0 females
were sacrificed at end of lactation of F1b pups (or, if non-gravid,
25 days after separation from males). Uteri from apparently non-gravid
females were examined with 10% ammonium sulfide. F1a offspring were
discarded after weaning. F1b offspring were necropsied, only gross
pathological lesions and malformed pups being preserved. A similar
programme was followed for the F1 generation. In both generations,
the coagulating gland, cervix, ovaries, pituitary, prostate, seminal
vesicles, testes with epididymides, uterus, vagina, and gross lesions
were examined microscopically.
Three adults died: one F0 male at 3000 ppm in week 28, one
23-day-old F1 male at 6000 ppm, and one control F1 female at about
20 weeks of age. These deaths did not appear to be compound-related.
Brown abdominal staining and brown or yellow anogenital staining
(usually for only 1-3 weeks) were seen in F0 females at the middle
and high doses. A slightly increased incidence of hair loss also
occurred in this group. F1 males and females at 6000 ppm had an
increased incidence of small size, which generally disappeared by 15
(males) to 17 (females) weeks of age. The body weights of F0 males
at 3000 and 6000 ppm and of females at 6000 ppm were lower than those
of controls (statistically significant), and a slight (usually
nonsignificant) decrease occurred in females at 3000 ppm. F1 males
and females at 6000 ppm and females at 3000 ppm had significantly
reduced body weights in weeks 4-17. During gestation, significant
reductions in mean body weights were seen in females at 6000 ppm in
the F0/F1a (at 14-21 and 0-21 days), F0/F1b (on days 0 and 7),
F1/F2a (at all intervals), and F1/F2b (at 14-20 days)
generations. The mean body weights at 6000 ppm were also significantly
reduced during lactation in the F0/F1a (at 0-4, 7-14, 14-21, and
0-21 days), the F0/F1b (0-21 days only), the F1/F2a (7-14 days
only), and the F1/F2b (14-21 and 0-21 days) generations. In
animals at 3000 ppm, decreased body weights were seen in the
F1/F2b matings on days 4-7, 14-21, and 0-21. Food intake was
reduced before mating of adults at 6000 ppm and during the lactation
period in F0 and F1 females.
No compound-related effects on male copulation, male fertility
index, female fertility index, gestation index, copulatory interval,
or gestation length were seen in either generation, although greater
fertility than controls was seen in F2a (animals of each sex) and
F2b (females) animals. Males that failed to mate had motile and
morphologically normal sperm in the epididymides, apart from one male
in the F1/F2b group at the low dose which had non-motile,
morphologically abnormal sperm. The mean numbers of stillbirths and
live births, the sex ratios, and viability indices were comparable to
those of controls, except in F1/F2a offspring at the middle dose
in which the sex ratio was skewed in favour of males. In animals at
the high dose, the total number of live births and the body weights of
live offspring were significantly reduced; the body weights of
offspring were also significantly reduced in the F0/F1a (days 14
and 21), F0/F1b (females only, day 21), and F0/F2b (days 14
and 21) generations at 3000 ppm.
During lactation, an increased incidence of 'pale' pups was seen
at 3000 and 6000 ppm in the F0/F1a generation on day 14 and in
F1/F2a pups at 6000 ppm on days 7, 14, and 21. Missing tails were
seen in F1/F2a pups at 6000 ppm on days 14 and 21 and in
F1/F2b pups at low incidence on all days. Concentric rings on the
tail were seen at high incidence in F1/F2a pups at 6000 ppm on
days 7, 14, and 21.
The incidences of malformations in offspring at birth were
reported only in summary form. In the F1a litters, two dead
offspring at 3000 ppm had malformations consisting of mandibular
micrognathia in one, cleft palate in both, and an intraventricular
defect in one; no malformations were seen in dead F1b offspring. One
case of anophthalmia was noted among 86 F1b pups at 20 ppm at day
21. Malformations seen in two dead F2a pups in two litters at
6000 ppm were a bulbous pulmonary trunk, an intraventricular septal
defect, and aortic arch stenosis; no malformations were seen in dead
F2b offspring. One F2b control pup out of 118 had microphthalmia
and mandibular micrognathia, one at 6000 ppm had anophthalmia, and one
at this dose had situs inversus; these pups were from different
litters. The incidence of variants was virtually nil.
Terminal macroscopic examination indicated no treatment-related
effects in F0 parents. Tan-yellow foci in the lung were seen in some
F1 males and females at 6000 ppm and in two females at 3000 ppm. The
testicular weights of animals that failed to sire offspring were
slightly reduced in two F1 controls and one treated F1 male at
3000 ppm. Microscopic examination of F0 adults showed no
compound-related effects. In F1 animals, the tan-yellow foci in the
lungs were aggregates of alveolar macrophages, perivascular-
peribronchial lymphoid cell infiltration, and/or interstitial
pneumonia. These changes are typical of pulmonary viral infections.
Thus, quintozene is unlikely to have been toxic, although stress due
to its administration or effects on the immune system may have
resulted in increased susceptibility to infection in animals at
6000 ppm and possibly in females at 3000 ppm. The NOAEL was 20 ppm,
equivalent to 1 mg/kg bw per day, on the basis of changes in body
weight in pups and adults at 3000 ppm. No adverse effects were seen on
reproductive parameters (Schardein et al., 1991).
(e) Developmental toxicity
Mice
A group of 23 C57Bl/6 mice was given quintozene (containing 11%
HCB) at 500 mg/kg bw per day in corn oil on days 7-11 of gestation; 19
controls were available. Further groups received either pentachloro-
aniline at 100 or 200 mg/kg bw per day or tetrachloronitrobenzene at
200 mg/kg bw per day on days 7-18 of pregnancy. In mice treated with
quintozene, fetal mortality was slightly increased, and the incidence
of malformations was significantly increased. Renal agenesis was the
most frequent effect, and the incidences of anophthalmia (19.2%
greater than in controls), microphthalmia (9.8%), and cleft palate
were increased. Pentachloroaniline and tetrachloronitrobenzene did not
cause malformations or affect maternal body weight, the liver:body
weight ratio, fetal weight, or fetal mortality (Courtney et al.,
1976).
Groups of CD-1 mice were given quintozene (containing 11% HCB) at
doses of 250 or 500 mg/kg bw per day (five or 10 litters), penta-
chloroaniline at 250 mg/kg bw per day (nine litters), tetrachloro-
nitrobenzene at 200 mg/kg bw per day (six litters in one study and
nine in the other), quintozene fabricated to contain 11% HCB (nine
litters) at 500 mg/kg bw per day, HCB at 100 mg/kg bw per day (10
litters), or quintozene containing < 20 ppm HCB at 500 mg/kg bw per
day (10 litters) on days 7-16 of gestation. Maternal liver:body weight
ratios were increased at both doses (dose-related) of contaminated
quintozene and with the fabricated sample of quintozene; a
nonsignificant increase was seen with HCB. The incidence of
malformations per litter was significantly increased at 500 mg/kg bw
per day of contaminated quintozene (28.6% in comparison with 6.2, 2.0,
or 7.2% in corn oil:acetone [9:1] control groups at the same volume),
and to a lesser degree with HCB (13.6%) and with quintozene with <
20 ppm HCB. The major malformation observed with contaminated and
fabricated quintozene and HCB was cleft palate; this malformation was
also seen with quintozene containing < 20 ppm HCB, but the major
contributor to the incidence was clubbed foot. The last preparation
differed from the other quintozene samples in that it provided a
solution rather than a suspension; this may have increased absorption.
This group also showed signs of toxicity, expressed as abortion in
three of 13 mice on day 17 of gestation. A similar high incidence of
clubbed foot has not been seen in other studies of quintozene, and the
author suggested that it may have been due to increased uterine muscle
tone, with consequent pressure effects on the developing fetus
(Courtney et al., 1976).
A group of 30 CD1 timed-pregnant mice were given quintozene
(containing 5% HCB) orally at 750 mg/kg bw per day on gestation days
8-12; 40 timed-pregnant mice were used as controls. Day 20 of
gestation was considered to be postnatal day 1. Death occurred in 17%
of animals, and 73% were pregnant. Litter size was nonsignificantly
reduced on day 1, but pup weights were comparable to those of
controls, and by postnatal day 3, the mortality and weights of pups
were comparable to those of controls. There was no NOAEL (Kavlock
et al., 1987).
Rats
Groups of five to seven pregnant CD rats received quintozene
containing 11% HCB or < 1.0% HCB at 500 mg/kg bw per day or
pentachlorophenol or tetrachloronitrobenzene at 75 mg/kg bw per day on
days 7-18 of gestation. No effects were seen on maternal weight gain
or the liver:body weight ratio. Pentachlorophenol decreased fetal
weights. The incidence of malformations was minimal, and these
consisted of enlarged cerebral ventricles, umbilical hernias, and
slightly enlarged renal pelvis (Courtney et al., 1976).
Pregnant Charles River albino rats received quintozene in corn
oil by intubation at doses of 100-1563 ppm on days 6-15 of gestation
and were sacrificed on day 20. Corpora lutea, position and numbers of
dead and resorbed pups, fetal weights, and sex ratio were recorded.
One-half of the fetuses were stained with alizarin Red-S for skeletal
examination, and the remainder were either fixed in Bouin's fluid for
examination of soft tissues or preserved in formalin. No difference
was seen between control and treated groups for any parameter,
including renal pelvis dilatation, hydronephrosis, or hydroureter,
which occurred in all groups. No NOAEL could be determined because of
the unusual expression of doses (Jordan & Borzelleca, 1973).
Four groups of 25 mated Charles River COBS female rats were given
quintozene (purity, 97.3%; containing 0.025% HCB) at doses of 0, 30,
600, or 1200 mg/kg bw per day as 10 ml/kg bw of 0.2% carboxymethyl-
cellulose suspensions by gavage on days 6-15 of gestation. The
suspensions were prepared daily; analysis on days 7 and 14 of the
study indicated actual levels of 88-110% of the nominal concentration
and stability over 24 h. The day of mating was designated day 0 of
gestation. Rats were observed twice daily for mortality and changes in
appearance and behaviour. Maternal rats were weighed on gestation days
0, 6, 9, 12, 16, and 20, and food intake was measured daily and
calculated for days 6-9, 9-12, 12-16, 16-20, 6-15, and 6-20 of
gestation. Rats were killed on day 20 of gestation, and the uterus and
ovaries were examined in situ and gravid uteri weighed. The numbers
and locations of viable and non-viable fetuses and of early and late
resorptions and the numbers of implantations and corpora lutea were
recorded. Maternal tissues were examined macroscopically, and grossly
abnormal tissues were preserved. The uteri of apparently non-pregnant
rats were examined with 10% ammonium sulfide. Fetuses were weighted,
sexed, and examined for external malformations; half were preserved in
Bouin's solution for examination of soft tissues by Wilson's
technique, and the remainder were eviscerated and processed for
Alizarin R-S staining and subsequent skeletal examination (Dawson
technique).
There were no deaths or adverse clinical or behavioural effects.
A nonsignificant reduction in the body weights of animals at
1200 mg/kg bw per day was seen but which was only 1-2% after
correction for initial weight differences. Food intake was
significantly reduced at 6-9 days in animals at 1200 mg/kg bw per day,
but the reduction was only 10.8% when measured as grams per animal per
day or 8.7% when measured as grams per kilogram body weight per day.
The effect was probably due to unpalatability. At later intervals and
during days 6-15 and 6-20, food intake was comparable to that of
controls. The incidences of pregnancy were 24/25 controls, 23/25 at
30 mg/kg bw per day, 25/25 at 600 mg/kg bw per day, and 23/25 at
1200 mg/kg bw per day. There were no total resorptions, and all
pregnancies resulted in viable offspring; the viable litter sizes were
comparable at all doses. The numbers of early resorptions were
slightly increased in all test groups, but the incidence was within
the normal historical range. The incidences of late resorptions and
corpora lutea were comparable in all groups. The group mean individual
fetal weights were unaffected, and the sex ratio was normal. The
malformations seen in controls were anophthalmia in one fetus,
malpositioned ovary and kidney in another, and a vertebral anomaly
with an associated rib anomaly in a third. At 30 mg/kg bw per day, one
fetus had an encephalocoele and a malpositioned ovary, one had
anasarca, a bent scapula, and bent limb bones, and a third had a
folded retina. At 600 mg/kg bw per day, one pup had microphthalmia and
folded retinas; at 1200 mg/kg bw per day, two pups from separate
litters had malpositioned kidneys and ovaries. Thus, no pattern in the
incidence of malformations and no dose-response relationship was seen
in the occurrence of malformations. The incidence of variants was
within normal limits. The NOAEL for maternal, embryo- and fetotoxicity
and for embryofetal development was 1200 mg/kg bw per day, the highest
dose tested (Keller, 1988a).
Rabbits
Four groups of 16 artificially inseminated New Zealand white
rabbits, three months old at receipt and acclimatized for 111 days,
were given quintozene (purity, 97.3%) at doses of 0, 12.5, 125, or
250 mg/kg bw per day in 0.2% carboxymethylcellulose on days 7-19 of
gestation. Because of maternal toxicity, additional rabbits, five
months old at reception and acclimatized for 48 days, were added about
75 days later and intubated with 0 (16 rabbits), 6.25 (16 rabbits), or
125 (12 rabbits) mg/kg bw per day. Semen for artificial insemination
was provided by six males proven to be fertile and was used to
inseminate an equal number of females in each concurrent group, except
in the second study when two males were used to inseminate fewer
females at 125 mg/kg bw per day than the remaining four males. The day
of insemination was designated day 0 of gestation. Caesarian sections
were performed on day 29 of gestation. The stability and homogeneity
of the quintozene formulation were satisfactory, and the actual
concentrations were 95-110% of the nominal ones.
Survival, appearance, and behaviour were observed twice daily and
were reported daily for days 7-29 of gestation. Rabbits that died
before the end of the study were necropsied, and those that showed
signs of abortion or premature delivery were sacrificed. The fetuses
of these rabbits were examined externally and preserved; those of
rabbits that died within 24 h of the end of the study were evaluated.
Body weights were recorded on days 0, 7, 13, 20, 24, and 29 of
gestation, and food intake was measured daily and calculated for days
7-13, 13-20, 20-29, 7-20, and 0-29 of gestation. At termination, the
uterus and ovaries were examined, and gravid uterine weight, the
number and location of viable and non-viable fetuses, the incidence of
early and late resorptions and total implantations, and the number of
corpora lutea were recorded. Maternal thoracic and abdominal cavities
were examined macroscopically, and grossly abnormal tissues and organs
were preserved. Non-pregnant uteri were examined with 10% ammonium
sulfide solution. Fetuses were weighed, tagged, and examined
externally, then dissected, internally sexed, and examined for soft
tissue abnormalities, including mid-coronal brain slices. The
carcasses were processed, stained with Alizarin Red-S (Dawson
technique), and examined for skeletal abnormalities.
One rabbit at 12.5 mg/kg bw per day died on day 28 of gestation;
two died at 125 mg/kg bw per day, both aborting before death on day 28
of gestation; and five died at 250 mg/kg bw per day on days 19, 21,
26, 26, and 27 of gestation, the death on day 27 being preceded by
abortion. In the secondary study, no deaths occurred at any dose. The
known causes of death in the initial study were mucoid enteritis in
those at 125 mg/kg bw per day and pneumonia in three of the five that
died at 250 mg/kg bw per day; the other causes of death were not
determined. The incidences of abortion were: two controls on days 24
and 27, two at 12.5 mg/kg bw per day on days 26 and 27, three at
125 mg/kg bw per day on days 23, 28, and 28, and four at 250 mg/kg bw
per day on days 23, 24, 25, and 26. When animals that aborted before
death are included, the incidences were 2, 2, 5, and 5 at 0, 12.5,
125, and 250 mg/kg bw per day, respectively. In the secondary study,
one rabbit at 125 mg/kg bw per day aborted on day 26. Premature births
occurred in one doe in each control group and in one at 125 mg/kg bw
per day. Signs of toxicity included mucoid stools in 6/16 rabbits at
250 mg/kg bw per day and 1/16 rabbits at 125 mg/kg bw per day, an
increased incidence of absent stools in 4/16 controls, 8/16 at 125,
and 11/16 at 250 mg/kg bw per day, and an increased incidence of
anogenital staining in 3, 8, 9, and 10 animals at 0, 12.5, 125, and
250 mg/kg bw per day in the initial group and 2, 3, and 9 at 0, 6.25,
and 125 mg/kg bw per day in the secondary group.
Body weights were markedly reduced after treatment at 250 mg/kg
bw per day, the loss peaking on day 24 of gestation, with very slight
recovery by day 29. The body weight at term, corrected for gravid
uterine weight, was also markedly reduced. Body-weight gain was
consistently reduced throughout gestation. Animals at 125 mg/kg bw per
day had a greater weight loss on days 13-20 of gestation in both the
initial and, to a lesser extent, the secondary study; however, the
weight reduction in the second group was greater on days 24-29. Both
groups at this dose had lowered corrected body weights at term than
controls, the effect being more apparent in the second group. Food
intake was significantly reduced at most intervals, when expressed
either as grams per animal or grams per kilogram body weight per day.
Stillbirths occurred in 1/81, 1/85, 1/49, and 3/28 rabbits at 0, 12.5,
125, and 250 mg/kg bw per day and in 0/82, 0/101, and 0/68 at 0, 6.25
and 125 mg/kg bw per day. The incidences of total resorptions in
relation to total implantations were 5.8, 11.5, 9.1, and 22.2% at 0,
12.5, 125, and 250 mg/kg bw per day and 24.8, 10.6, and 2.7% at 0,
6.25, and 125 mg/kg bw per day. Mean fetal weight was reduced markedly
at 250 mg/kg bw per day. The sex ratio was normal in all groups. The
mean litter size, including both viable and non-viable pups, was 7.4,
6.5, 6.8, and 4.7 at 0, 12.5, 125, and 250 mg/kg bw per day and 5.8,
7.7, and 6.8 at 0, 6.25, and 125 mg/kg bw per day. Malformations were
seen in 4, 4, 9, 2, 3, 2, and 0 pups in 3, 3, 6, 2, 3, 2, and 0
litters at 0, 0 (second study), 6.25 (second study), 12.5, 125, 125
(second study), and 250 mg/kg bw per day. The high incidence of
malformations at 6.25 mg/kg bw per day was accounted for by three pups
with multiple malformations that were not seen at higher doses. As
artificial insemination occurred over three days, each male normally
providing semen for two females, the multiple malformations may be due
to genetic defects mediated through the male. The incidence of
variants was comparable between groups. (Keller, 1988b).
The probable NOAELs are difficult to determine, since the results
at 125 mg/kg bw per day were not consistent between the studies. The
deaths due to mucoid enteritis were probably not compound-related
since the five deaths at 250 mg/kg bw per day were not associated with
pneumonia or mucoid enteritis. Although the incidence of abortions was
increased at 125 and 250 mg/kg bw per day, two of the five abortions
at the lower dose occurred immediately before death due to mucoid
enteritis and may therefore not be compound-related. The only
consistent effect was a decrease in body-weight gain at 125 and
250 mg/kg bw per day. The NOAEL for maternal toxicity was thus
12.5 mg/kg bw per day. That for embryo- and fetotoxicity was 125 mg/kg
bw per day, on the basis of reduced pup weight, increased resorption
rates, and a slight increase in the incidence of stillbirths at
250 mg/kg bw per day. The NOAEL for malformations was 250 mg/kg bw per
day, although only 28 fetuses from six litters were available for
examination at this dose.
(f) Genotoxicity
The results of studies of the genotoxicity of quintozene are
reported in Table 2.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Six male New Zealand white rabbits, three months old, were
exposed to quintozene (purity, 99.42%) moistened with water at a dose
of 500 mg per rabbit on dipped, intact dorsal skin under a 2.5-cm2
gauze patch secured with tape. The dressings were removed after 4 h,
the sites were wiped with dry towels, and the animals were observed
for 72 h. No dermal irritation was observed after 30-60 min or 24, 48,
or 72 h (Warshawsky, 1994c).
Six male New Zealand white rabbits, about three months old,
received 0.0996 g of quintozene (purity, 99.42%) in the cupped
conjunctival sac of the right eye, and the eyelids were held together
for 1 s after administration. The eyes were not washed and were scored
for irritation (on the Draize scale) at 1, 24, 48, and 72 h. At 72 h,
they were examined with sodium fluorescein. After 1 h, all rabbits had
a clear discharge, and two also showed blanching. Conjunctival
redness, chemosis, and discharge were seen in all animals, the redness
persisting in four rabbits up to 24 h. All eyes were normal by 48 h.
The average group scores were 7/110 at 1 h and 1.7/110 at 24 h,
indicating that the compound is minimally irritating to the rabbit eye
(Warshawsky, 1994d).
Table 2. Results of tests for the genotoxicity of quintozene
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 0-6667 µg/plate 99.6 Negativea US National Toxicology
TA100, TA1535, TA1537 Program (1987)
Reverse mutation S. typhimurium, five strains NR NR Negative Simmon et al. (1976)
Mitotic recombination Saccharomyces cerevisiae NR NR Negative Simmon et al. (1976)
DNA repair E. coli NR NR Negative Simmon et al. (1976)
Gene mutation Mouse lymphoma 1.25-10 µg/ml 99.6 Negativea US National Toxicology
L5178Y tk+/tk- cells Program (1987)
Sister chromatid Chinese hamster ovary cells 0.75-7.5 µg/ml 99.6 Negativeb US National Toxicology
exchange Program (1987)
Sister chromatid Chinese hamster ovary cells 7.5-75 µg/ml 99.6 Negativec US National Toxicology
exchange Program (1987)
Chromosomal Chinese hamster ovary cells 2.4-75 µg/ml 99.6 Positivea,d US National Toxicology
aberration Program (1987)
In vivo
Dominant lethal Mice Three doses NR Negativee Jorgenson et al. (1976)
mutation
NR, not reported
a With and without metabolic activation; similar results in two experiments with two or three replications each
b Without metabolic activation
c With metabolic activation
d 98% of cells with simple chromosomal and chromatid breaks; 2% with complex rearrangements; response not dose-related
e Males treated for seven weeks; then, one male mated with two females for eight weeks
Four Hartley guinea-pigs of each sex were exposed to quintozene
(purity, 98%) diluted in acetone on four sites at concentrations of
75, 50, 25, 10, 5.0, 2.5, 1.0, and 0.5% and scored for irritation at
24 and 48 h. On the basis of this pilot study, induction doses of 75
and 50%, which caused mild irritation, and challenge doses of 5, 2.5,
and 0.5%, which caused virtually no irritation, were selected for the
main study. In the main study, groups of 10 guinea-pigs of each sex
received applications on clipped, intact skin under occluded 25-mm
chambers containing 0.3 ml for 6 h; after 22-26 h, the animals were
depilated, and irritation was scored within 2 h as slight patchy
erythema, slight but confluent or moderate patchy erythema (grade 1),
moderate erythema (grade 2), or severe erythema (grade 3). Animals
were re-scored 22-26 h after the initial scoring. Induction involved
three 6-h exposures on the same site over two weeks, the initial
exposure being to 75% and the other two exposures to 50% quintozene in
acetone. No dermal reactions were seen after the first dose; slight
patchy erythema was elicited in one male after the second dose and in
two males and two females after the third dose. One male was
sacrificed after the initial dose due to non-compound-related
ill-health. Vehicle controls showed no dermal reactions.
For the primary challenge, chambers containing 5, 2.5, or 0.5%
quintozene were applied to each induced animal on previously untreated
sites. Reactions were observed at all three doses, the 5%
concentration inducing slight patchy erythema in eight males, grade 1
erythema in one male, and slight patchy erythema in nine females at
24 h; slight patchy erythema in seven males, grade 1 erythema in one
male, slight patchy erythema in nine females, and a grade 1 reaction
in one female were seen at 48 h. Comparable results were seen with 2.5
and 0.5%. The mean group scores were 0.5, 0.5, and 0.5 at 24 h and
0.5, 0.5, and 0.4 at 48 h with 5, 2.5, and 0.5%, respectively. Vehicle
controls given the same challenge doses under the same conditions had
slight patchy erythema at all concentrations, with mean group scores
of 0.3, 0.4, and 0.3 at 24 h and 0.3, 0.3, and 0.4 at 48 h.
Nine induced animals and five naive animals of each sex were
re-challenged at least six days after the initial challenge with 5 or
0.5% on previously unexposed sites. The mean group scores for induced
animals were 0.4 and 0.2 at 5% and 0.3 and 0.2 at 0.5% at 24 and 48 h,
respectively. Grade 1 reactions were seen in two guinea-pigs at 24 h
and one at 48 h with the 5% concentration and one at 24 and 48 h with
0.5% quintozene. The naive animals challenged with the 5% concen-
tration had mean irritation scores of 0.4 at 24 h (one animal with a
grade-1 score) and 0.2 at 48 h, and those exposed to 0.5% had scores
of 0.5 at 24 h and 0.2 at 48 h. The incidence and persistence of grade
1 scores in the challenged animals indicate that quintozene can induce
sensitization (Kreuzmann, 1988).
(ii) Potentiation
Combinations of quintozene at half or whole LD50 doses of
1740 mg/kg bw with terrozole (5-ethyoxy-3-trichloromethyl-
1,1,2,4-thiazole) at the LD50 dose of 1080 mg/kg bw or at LD2.3,
LD6.7, LD16, LD31, or one-half the LD50 were given to groups
of 10 male CD Charles River rats at 100 mg/kg bw quintozene and
appropriate amounts of terrazole. Mortality recorded over 14 days
showed an additive effect (Borzelleca et al., 1971).
(iii) Thyroid function
Groups of 75 male Charles River CD rats were fed diets containing
0, 20, or 6000 ppm quintozene (purity, 99.09%) for up to 90 days, and
groups of 15 rats per dose were sacrificed after 7, 14, 30, or 90
days; the remaining 15 rats were maintained for a further 90 days on
normal diet before sacrifice. The average intakes were 1 and 333 mg/kg
bw per day. Diets were prepared weekly (with acetone as the vehicle at
the 0 and 20 ppm levels) and analysed in duplicate during weeks 1-4,
8, and 12. The mean dietary concentrations were 81% of the nominal
(range, 72-98%) at 20 ppm and 91% (range, 86-99%) at 6000 ppm.
Mortality, moribundity, and clinical parameters were checked twice
daily and body weight and food intake weekly; blood samples were
collected from the abdominal aorta of non-fasted rats at sacrifice,
when complete macroscopic examinations were performed and the liver,
pituitary, and thyroid-parathyroid were weighed. These organs were
examined microscopically, as was the liver of all rats at terminal
sacrifice; the thymuses of a few animals were also examined.
Triiodothyronine, thyroxine, and thyroid-stimulating hormone were
measured in all rats at each dose and interval; reverse
triiodothyronine was measured in all rats at the end of treatment.
There were no deaths. The clinical signs, which occurred at low
incidence and were not dose-related, included malocclusion in seven
controls, six at 20 ppm, and five at 6000 ppm, and occasional hair
loss and redness of the eyes. Body weight and weight gain were reduced
in animals at 6000 ppm during treatment, but the weight gain exceeded
that of controls during the recovery period and by the time of
sacrifice exceeded that of controls. The main decrease in body-weight
gain occurred in week 1, when food intake was also significantly
decreased. At 6000 ppm, animals had reduced triiodothyronine and
thyroxine and increased thyroid-stimulating hormone values during most
of the treatment period, the effects being statistically significant
at most intervals. Reverse triiodothyronine was reduced at 90 days.
The triiodothyronine, thyroxine, and thyroid-stimulating hormone
values returned to normal during the recovery period. At 20 ppm, no
significant changes were seen, except for an increased thyroxine value
at seven days, which was considered to be a random effect. The
absolute weight of the pituitary was reduced at seven days in animals
at 6000 ppm; however, no other changes in the absolute or relative
weights of the pituitary occurred during the study, and no abnormal
microscopic changes were observed. The absolute weight of the liver
was nonsignificantly increased at 30 and 90 days in animals at this
dose but returned to normal after the recovery period; relative (to
body) weights were significantly increased at 14, 30, and 90 days, but
this effect may have been due to the decreased body weight seen at all
intervals except after the recovery period. The hepatocellular
hypertrophy seen after treatment for 90 days indicates that the
increased absolute liver weight at 30 and 90 days at 6000 ppm was
compound-related. The thyroid-parathyroid weights were not
significantly affected, except that the absolute weight was
significantly decreased at 30 days in animals at 6000 ppm and very
slightly decreased in those at 20 ppm. At 90 days, statistically
nonsignificant decreases were again seen at 20 and 6000 ppm (33 g in
controls versus 30 g at both 20 and 6000 ppm). After 90 days'
recovery, the thyroid-parathyroid weights were comparable to or
slightly greater than control values. Follicular epithelial
hypertrophy of the thyroid was observed at 6000 ppm, with trace (14
days), mild (30 days), or moderate (90 days) severity. At 20 ppm, mild
follicular epithelial hypertrophy was noted at 90 days. The decreased
thyroid weight may therefore reflect the colloid breakdown that occurs
in hypertrophic thyroid follicular cells, but this was not confirmed
microscopically. As the effects on the liver and thyroid were
reversible and only minimal effects were noted at 20 ppm, the NOAEL
was 20 ppm, equal to 1 mg/kg bw per day (Goldenthal, 1993).
(iv) Studies of metabolites
Four groups of 10 weanling female Sprague-Dawley rats were fed
diets containing pentachloronitrosobenzene, which is believed to be an
intermediate in the metabolic reduction of quintozene to pentachloro-
aniline, at doses of 0, 10, 50, or 100 ppm for 18 weeks. Rats were
weighed twice weekly, and food intake was monitored daily. Serum
alkaline phosphatase, aspartate and alanine aminotransferases, urinary
and liver porphyrins, liver glutathione, hepatic microsomal protein,
cytochrome P450, haemoglobin ferrihaemoglobin, packed cell volume, and
erythrocyte and total and differential leukocyte counts were
determined in all rats at the end of treatment. Organ:body weight
ratios were reported for liver, heart, spleen, and kidneys; the liver,
kidney, gastrointestinal tract, and urinary bladder were examined
histologically.
Body weights tended to be increased from week 2 onwards in all
treated groups, but statistical significance was not achieved and no
dose-effect relationship was seen. Food intake and food efficiency
were increased in all treated groups. The organ:body weight ratios
were comparable in all animals. A dose-effect relationship was
seen for ferrihaemoglobin levels, with values of 0, 0.5, 0.9, and
1.1 g/100 ml for controls and the three dose groups, respectively.
Serum alanine aminotransferase activity showed a dose-related,
statistically significant increase at all doses. Urinary porphyrin
patterns were similar over the last 24 h of the study. The hepatic
microsomal liver protein and hepatic and urinary porphyrin composition
was unchanged, but cytochrome P450 enzyme activity was significantly
increased at 100 ppm. At this dose, the concentrations of glutathione
and oxidized glutathione in liver were also increased. The liver was
normal histologically, but multilayered bladder epithelium of variable
thickness was seen, characterized by the presence of large superficial
cells. There was no sign of cellular proliferation (Renner et al.,
1981).
3. Observations in humans
Fifty human subjects received one 6.4-mm moistened cotton square
dipped into a 75% wettable powder formulation of quintozene (purity
unspecified) on the palmar surface of the forearm. The squares were
then covered with foil, which was taped into place. Exposure was for
48 h. No irritation was observed when the dressings were removed. When
the test was repeated two weeks later, four individuals showed
positive reactions, comprising erythema, itching, and (in three)
oedema and small vesicle formation. Of the individuals who showed no
reaction at the time the patch was removed, nine developed a delayed
reaction 8 h to several days after testing (Finnegan et al., 1958).
Workers who were still employed and had been exposed during the
production of quintozene for 2.5 years (one person), seven years (one
person), 8.5 years (one person), or 11 years (10 people) years had
undergone twice-yearly health checks, including monitoring of height,
weight, vision, blood pressure, X-ray (once per year only),
haematological parameters (erythrocyte count, haemoglobin,
haematocrit, total leukocyte count), an evaluation of subjective and
objective symptoms, life style, family history, and individual past
history, throughout the employment period. After 1989, hearing tests,
blood chemistry (serum aspartate and alanine aminotranferases,
gamma-glutamyl transpeptidase, alkaline phosphatase, partial
thromboplastin time, total cholesterol, and blood glucose),
(urinalysis (urea nitrogen, uric acid, glucose, protein, and occult
blood), and electrocardiography were added. No compound-related
effects were observed. A further group of nine individuals, with
exposure for three months (one person), one year (one person), two
years (one person), three years (one person), four years (one person),
seven years (one person), or 10 years (three people), who were engaged
in the manufacture of quintozene dust formulations were subjected to
similar examinations and showed no compound-related abnormalities. No
estimate of exposure or information on protective measures used (e.g.
clothing, gloves, and masks) was provided (Yamazaki, 1994).
Comments
Quintozene is absorbed relatively slowly after oral
administration to rats and mice, peak blood levels being observed
about 74 h after dosing. 14C from labelled quintozene is eliminated
mainly in the faeces of animals of each sex, although females excrete
greater amounts in the urine than males. In goats, low doses (30 mg/kg
bw) were excreted mainly in the urine, but faecal elimination
predominated at higher doses (100 mg/kg bw). Bile levels in ruminants
were high, and enterohepatic circulation is probable. Excretion was
more rapid and complete in chickens than in mammals. There was no
evidence of tissue accumulation in any species.
The biotransformation of quintozene was virtually complete. Two
general metabolic pathways were apparent, one involving reduction of
the nitro group to an amino group and subsequent formation of
secondary metabolites, and the second involving replacement of the
nitro group with a sulfur-containing group (e.g. a thio- or
methylthio-glucuronide or N-acetylcysteine group). Metabolic studies
in several species have been reported.
The acute oral LD50 of quintozene was > 1.5 g/kg bw in both
rats and dogs. The dermal LD50 was > 5 g/kg bw in rabbits, and the
4-h LC50 in rats was > 1.7 mg/litre air. Quintozene was mildly
irritating to the rabbit eye and not irritating to rabbit or human
skin, but it was a mild dermal sensitizing agent in guinea-pigs and
humans. WHO has classified quintozene as unlikely to present an acute
hazard in normal use.
In a single 13-week study in mice at dietary concentrations of 0,
1250 (males only), 2500, 5000, 10 000, 20 000, or 40 000 (females
only) ppm, 5-8% decreases in terminal body weight were seen at doses
> 10 000 ppm, and increased absolute liver weights were seen in
animals of each sex at all doses. All mice at 40 000 ppm died.
Four short-term studies in rats were available; in only one of
them was quintozene containing < 0.1% hexachlorobenzene used. Dietary
concentrations of 0, 50, 3000, or 6000 ppm administered for 13 weeks
resulted in an NOAEL of 50 ppm (equal to 3.1 mg/kg bw per day) on the
basis of a slight (6-8%) reduction in terminal body weight, increased
liver weight and decreased alanine aminotransferase activity at higher
doses. Five short-term studies in dogs (lasting four weeks to two
years) were available, in only one of which quintozene met the purity
specification. In this one-year study, at dietary concentrations of 0,
15, 150, or 1500 ppm, increased liver weight and hepatocellular
hypertrophy were seen at 1500 ppm, which were accompanied by increased
serum alkaline phosphatase and cholesterol levels and decreased
alanine aminotransferase activity and creatinine levels. The NOAEL was
150 ppm, equal to 4.2 mg/kg bw per day. A 70-day study in monkeys at a
dietary concentration of 2 ppm revealed no adverse effects. In a
21-day study in rabbits, with application to the skin of doses of 0,
30, 300, or 1000 mg/kg bw of quintozene for 6 h per day, significantly
decreased alanine aminotransferase activity was seen at 1000 mg/kg bw
per day. No irritation or other adverse effect was observed.
Four long-term (72-103-week) studies by oral administration in
mice were available, in only one of which did the compound meet the
contamination criterion. This 103-week study at dietary concentrations
of 0, 2500, or 5000 ppm showed decreased terminal body weights in
males (4% at 2500 ppm and 10% at 5000 ppm) and females (18 and 21%).
Since the body-weight losses in the females exceeded 10% at 2500 ppm
only after week 92 of the study, the NOAEL was 2500 ppm (equal to
360 mg/kg bw per day), although the interpretation was complicated by
Klebsiella infection. No evidence of carcinogenicity was seen. In a
study in which technical-grade quintozene (purity unspecified) was
applied dermally twice weekly (0.2 ml of 0.3% quintozene in acetone)
to mice for 12 weeks, followed by twice-weekly dermal applications of
croton oil for 20 weeks, no tumour induction was seen in the absence
of the promoting agent.
Of four long-term (two-year) studies in rats, only one met the
contaminant specification. In this study, at doses of 0, 20, 3000, or
6000 ppm, thyroid follicular adenomas were seen at 3000 and 6000 ppm
and possibly carcinomas at 6000 ppm (especially in males). Hepato-
cellular hypertrophy (mainly centrilobular), indistinguishable from
that arising from increases in drug-metabolizing enzyme activity, was
also seen at 3000 and 6000 ppm. The absolute and relative (to body and
brain) weights of the thyroid and parathyroid were increased at the
two highest doses in most instances. A special 90-day study on thyroid
hormones showed hepatocellular hypertrophy, which was only minimal,
after 90 days' exposure to 20 ppm. Complete reversibility was seen
after 90 days' withdrawal. Thyroid and parathyroid weights were
decreased only after 30 days' exposure. At 6000 ppm, triiodothyronine
and thyroxine levels were decreased and the levels of thyroid-
stimulating hormone were increased significantly at most intervals
during dosing; the level of reverse triiodothyronine was reduced at 90
days. All changes were reversible within 90 days after withdrawal. The
changes in thyroid hormone levels are consistent with increased
microsomal enzyme activity, resulting in increased excretion of
triiodothyronine and thyroxine and subsequent disruption of the
hypothalamic-pituitary-thyroid axis, causing increased circulating
levels of thyroid-stimulating hormone. Thyroid-stimulating hormone
initiates increased production of triiodothyronine and thyroxine, with
follicular hyperplasia in the thyroid, leading to adenoma and, more
rarely, carcinoma. Quintozene was thus carcinogenic. The induction of
thyroid tumours appears to be secondary to the disruption of thyroid
function, as indicated by the increased levels of thyroid-stimulating
hormone. The NOAEL for this effect was 20 ppm, equivalent to 1 mg/kg
bw per day.
One of two multigeneration studies in rats met the criterion for
the level of contamination. In this study, with dietary concentrations
of 0, 20, 3000, or 6000 ppm and two litters per generation for two
generations, dietary concentrations of 3000 and 6000 ppm reduced pup
and adult body weights. The NOAEL was 20 ppm, equivalent to 1 mg/kg bw
per day.
In studies of developmental toxicity, 13 mice (in part of a study
of the incidences of cleft palate and renal agenesis, subsequently
shown to be induced by hexachlorobenzene contamination) received doses
of 500 mg/kg bw per day by gavage on days 7-16 of gestation. Abortions
occurred, and a low incidence of cleft palate (2%) and a high
incidence (23%) of clubfoot were noted. Marked maternal toxicity was
seen. The administered material formed a solution, and not a
suspension as in all other studies. This study was not considered in
establishing the ADI. Of three studies of developmental toxicity in
rats, only one, with doses of 0, 30, 600, and 1200 mg/kg bw per day,
was acceptable. The NOAEL for maternal and developmental toxicity was
> 1200 mg/kg bw per day. There was no evidence of induction of
malformations. In rabbits given doses of 0, 12, 120, or 250 mg/kg bw
per day or 0, 6.2, or 120 mg/kg bw per day, maternal toxicity was seen
at 120 mg/kg bw per day and embryo- and fetotoxicity at 250 mg/kg bw
per day, including reduced pup weight, increased resorptions, and an
increased incidence of stillbirths; no increase in malformations was
seen at any dose.
In a number of studies (e.g. long-term studies in mice and rats
and the two-generation study of reproductive toxicity in rats), the
incidences of infection appeared to be slightly increased at high
doses. Although the reports of histopathological examinations do not
indicate effects on the immune system, it would be useful if studies
could be performed to investigate the potential for quintozene to
affect the immune system.
Quintozene has been tested in a range of studies for genotoxicity
in vitro. An acceptable assay is required to corroborate the
increase in chromosomal aberration frequency observed in cultured
cells.
An ADI of 0-0.01 mg/kg bw was allocated on the basis of the NOAEL
of 1 mg/kg bw per day in the two-year study, the study of thyroid
toxicity, and the two-generation study in rats, using a 100-fold
safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 2500 ppm, equal to 390 mg/kg bw per day (103-week study of
carcinogenicity)
Rat: 20 ppm, equivalent to 1 mg/kg bw per day (24-month study of
carcinogenicity, multigeneration study, and study of thyroid
toxicity) 1200 mg/kg bw per day (study of developmental
toxicity)
Rabbit: 12 mg/kg bw per day (maternal toxicity in study of
developmental toxicity) 120 mg/kg bw per day (embryo- and
fetotoxicity in a study of developmental toxicity)
Dog: 150 ppm, equivalent to 4.2 mg/kg bw per day (one-year study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw, for quintozene containing less than 0.1%
hexachlorobenzene
Studies that would provide information useful for continued evaluation
of the compound
1. Assays of genotoxicity in vivo to assess possible
chromosomal aberrations
2. Studies to assess the potential of quintozene to interfere
with the immune system
3. Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to quintozene
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Skin irritation, rabbit Not irritating
Eye irritation, rabbit Minimally irritating
Skin sensitization, guinea-pig Mild sensitizer
(modified Buehler)
Dermal irritation, human No irritation with 75% formulation
Skin sensitization, human Sensitization in 13/50 individuals
Inhalation, 4-h, toxicity, rat LC50 > 1.7 mg/litre air
Oral, toxicity, rat and dog > 1.5 g/kg bw
Medium-term (1-26 weeks) Dermal irritation, 21-day, rat No irritation; body weight, food intake, haematology,
clinical chemistry (except decreased alanine
aminotransferase), urinalysis, organ weights and liver
and kidney histology unaffected at doses
< 1000 mg/kg bw per day
Repeated oral, toxicity, 13-week, rat NOAEL = 3.1 mg/kg bw per day; minimal changes in
body and liver weight; reduced alanine
aminotransferase activity
Dietary, developmental toxicity, rabbit NOAEL = 12.5 mg/kg bw per day for maternal toxicity,
125 mg/kg bw per day for embryo- and fetotoxicity,
> 250 mg/kg bw per day for teratogenicity
Long-term (> one year) Dietary, toxicity, carcinogenicity, rat NOAEL = 1 mg/kg bw per day; follicular adenomas
of the thyroid and hepatocellular adenomas
References
Adamovics, J.A. (1980) The metabolic fate of pentachloronitrobenzen:
Pilot study. Unpublished report from Bio/dynamics Inc., Division
of Environmental and Analytical Chemistry, Olin Corporation
Report No. 79056. Submitted to WHO by Uniroyal Chemical Company,
Inc.
Adamovics, J.A. & O'Grodnick, J.S. (1978) Absorption and elimination
characteristics of 14C-labelled pentachloronitrobenzene in
rats-pilot study. Unpublished report from Bio/dynamics Inc.,
Division of Environmental and Analytical Chemistry, Olin
Corporation Report No. 77037. Submitted to WHO by Uniroyal
Chemical Company, Inc.
Aschbacher, P.W. & Feil, B.J. (1983) Metabolism of pentachloro-
nitrobenzene by goats and sheep. J. Agric. Food Chem.,
31, 1150-1158.
Betts, J.J., James, S.P. & Thorpe, W.V. (1955) The metabolism of
pentachloronitrobenzene and 2:3:4:6-tetrachloronitrobenzene and
the formation of mercapturic acids in the rabbit. Biochem. J.,
61, 611-617.
Borzelleca, J.F., Larson, P.S., Crawford, E.M., Hennigar, G.R., Jr,
Kuchar, E.J. & Klein, H.H. (1971) Toxicological and metabolic
studies on pentachloronitrobenzene. Toxicol. Appl. Pharmacol.,
18, 522-534.
Courtney, K.D. (1973) The effect of pentachloronitrobenzene on fetal
kidneys (Abstract 41). Toxicol. Appl. Pharmacol., 25, 455.
Courtney, K.D., Copeland, M.F. & Robbins, A. (1976) The effects of
pentachloronitrobenzene, HCB and related compounds on fetal
development. Toxicol. Appl. Pharmacol., 35, 239-256.
Daun, R.J. (1989) Pentachloronitrobenzene: Nature of the residue in
poultry-laying hens. Unpublished report from Hazelton
Laboratories America Inc., Project HLA 611-119. Submitted to WHO
by Uniroyal Chemical Company, Inc.
Daun, R.J. (1990) Pentachloronitrobenzene: Nature of the residue in
livestock-lactating goats. Unpublished report from Hazelton
Laboratories America Inc., Project HLA 611-118. Submitted to WHO
by Uniroyal Chemical Company, Inc.
Finnegan, J.K., Larson, P.S., Smith, R.B., Jr, Haag, H.B. & Hennigar,
G.R. (1958) Acute and chronic toxicity studies on pentachloro-
nitrobenzene. Arch. Int. Pharmacodyn., 115, 38-52.
Goldenthal, E.I. (1990) One year chronic dietary study in dogs.
Unpublished report No. 399-087 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Goldenthal, E.I. (1991). Two year dietary toxicity and oncogenicity
study in rats. Unpublished report No. 399-072 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Company, Inc.
Goldenthal, E.I. (1992). 21 day dermal toxicity study in rats.
Unpublished report No. 399-125 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Goldenthal, E.I. (1993). 90 day toxicity study in rats. Unpublished
report No. 399-122 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Company, Inc.
van der Heijden, C.A. & Til, H.P. (1974) Pentachloro-nitrobenzene
carcinogenicity study in mice. Unpublished report R4365 from
Central Institute for Nutrition and Food Research, Bilthoven,
Netherlands.
Hoescht AG (1964) Toxikologische Prüfung von Pentachlor-nitrobenzol.
3. Auf chronische Toxizität (90-Tage-Test) an Ratten. Unpublished
report prepared and submitted by Farbwerke Hoescht, AG.
Hilaski, R.J. (1994) EPA (FIFRA) Acute inhalation toxicity evaluation
on terraclor technical in rats. Unpublished report No. 399-146
from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Innes, J.R.M., Ulland, B.M., Valeric, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L., Gart,
J.J., Klein, M., Mitchell, I. & Peters, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in mice. A
preliminary note. J. Natl Cancer Inst., 42, 1101-1114.
Johnson, D.E. (1989) Twenty-eight day pilot study in dogs. Unpublished
report No. 399-093 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Company, Inc.
Jordan, R.L. & Borzelleca, J.F. (1973) Teratogenic studies with
pentachloronitrobenzene in rats (Abstract 40). Toxicol. Appl.
Pharmacol., 25, 454.
Jorgenson, T.A., Rushbrook, C.J. & Newell, G.W. (1976). In vivo
mutagenesis investigations of ten commercial pesticides
(Abstract 41). Toxicol. Appl. Pharmacol., 37, 109.
Kavlock, R.J., Short, R.D., Jr & Chernoff, N. (1987) Further
evaluation of an in vivo teratology screen. Teratog. Carcinog.
Mutag., 7, 7-16.
Keller, K.A. (1988a) Developmental toxicity study in rats. Unpublished
report No. 399-068 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Company, Inc.
Keller, K.A. (1988b) Developmental toxicity study in New Zealand white
rabbits. Unpublished report No. 399-070 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Company, Inc.
Kögel, W., Müller, F., Coulston, S. & Korte, F. (1979a)
Biotransformation of pentachloronitrobenzene-14C in rhesus
monkeys after single and chronic oral administration.
Chemosphere, 8, 97-105.
Kögel, W., Müller, F., Coulston, S. & Korte, F. (1979b) Fate and
effects of pentachloronitrobenzene in rhesus monkeys. J. Agric.
Food Chem., 27, 1181-1185.
Kreuzmann, J.J. (1988) Delayed contact hypersensitivity in guinea
pigs. Unpublished report No. 88-0052-21 from Hill Top Biolabs,
Inc. Submitted to WHO by Uniroyal Chemical Company, Inc.
Kuchar, E.J., Geenty, F.O., Griffith, W.P. & Thomas, R.J. (1969)
Analytical studies of metabolism of terraclor in beagle dogs,
rats and plants. J. Agric. Food Chem., 17, 1237-1242.
Larson, P.S. & Borzelleca, J.F. (1968) Toxicologic study on the
effects of adding terraclor to the diet of beagle dogs for a
period of two years. Unpublished report from the Department of
Pharmacology, Medical College of Virginia, Richmond, Virginia,
USA. Submitted to WHO by Uniroyal Chemical Company, Inc.
McGee, D.H. (1988) Thirteen week dietary toxicity study in rats with
PCNB (pentachloronitrobenzene). Unpublished report No. 399-071
from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Uniroyal Chemical
Company, Inc.
McManus, J.P. (1989) Metabolism of pentachloronitrobenzene in the
goat-metabolite identification. Unpublished report from Uniroyal
Chemical Company, Inc., Crop Protection Department, Chemistry
Section, R & D, Uniroyal Project 8761. Submitted to WHO Uniroyal
Chemical Company, Inc.
McManus, J.P. (1990) Metabolism of pentachloronitrobenzene in the
goat-identification of metabolites in muscle. Unpublished report
from Uniroyal Chemical Company, Inc., Crop Protection Department,
Chemistry Section, R & D, Uniroyal Project 8761a. Submitted to
WHO Uniroyal Chemical Company, Inc.
O'Grodnick, J.S. (1978) Characterization and identification of
14C-PCNB metabolites in rat urine and faeces. Unpublished
report from Bio/dynamics Inc., Division of Environmental and
Analytical Chemistry, Olin Corporation Report No. 77037-2.
Submitted to WHO by Uniroyal Chemical Company, Inc.
O'Grodnick, J.S. (1979) Identification of the polar metabolites of
14C-PCNB after oral administration to rats. Unpublished report
from Bio/dynamics Inc., Division of Environmental and Analytical
Chemistry, Olin Corporation Report No. 78054. Submitted to WHO by
Uniroyal Chemical Company, Inc.
O'Grodnick, J.S., Adamovics, J.A., Blake, S.H. & Wedig, J. (1981) The
metabolic fate of 14C-labelled pentachloronitrobenzene in
Osborne-Mendel rats. Chemosphere, 10, 67-72.
Parkins, M.D. (1990). Pentachloronitrobenzene: Nature of the residue
in poultry-laying hens. Unpublished report from Uniroyal Chemical
Company, Inc., Crop Protection Department, Chemistry Section, R.
& D., Project No. 8762. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Parkins, M.D. (1991) Pentachloronitrobenzene: Nature of the residue in
poultry-laying hens. Unpublished report from Uniroyal Chemical
Company, Inc., Crop Protection Department, Chemistry Section, R &
D, Project No. 8762. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Renner, G., Herforth, B., Gokel, J.M., Goerz, G. & Luderschmidt, C.
(1981) Subchronic toxicity studies on pentachloronitrosobenzene
(PCNO) in female rats. Ecotoxicol. Environ. Saf., 5, 281-290.
Schardein, J.L., York, R.G. & Laughlin, M.A. (1991) Two generation
reproduction study in rats. Unpublished report No. 399-086 from
International Research and Development Corporation, Mattawan,
Michigan, USA. Submitted to WHO by Uniroyal Chemical Company,
Inc.
Scholz, & Brunk, (1968) Kronischer orale toxizitätsprüfung von
pentachlornitrobenzol, 2-Jahres-Versuch an Hunden. Unpublished
report from Rewerke Hoescht AG. Submitted to WHO by Uniroyal
Chemical Company, Inc.
Searle, C.E. (1966) Tumor initiatory activity of some
chloromononitrobenzenes and other compounds. Cancer Res.,
26, 12-17.
Simmon, V.F., Poole, D.C. & Newell, G.W. (1976) In vitro mutagenic
studies of twenty pesticides (Abstract 42). Toxicol. Appl.
Pharmacol., 37, 109.
Sinkeldam, E.J., van der Heijden, C.A., de Groot, A.P. & Til, H.P.
(1974) Carcinogenicity study with pentrachloronitrobenzene in
rat. Unpublished report R442 from Central Institute for Nutrition
and Food Research, Bilthoven, Netherlands.
St John, L.E., Jr, Ammering, J.W., Wagner, D.G., Warner, R.G. & Lisk,
D.J. (1965) Fate of 4,6-dinitro-2-isobutylphenol, 2-chloro-4,6-
bis-(ethylamino)-S-triazine, and pentachloronitrobenzene in the
dairy cow. J. Dairy Sci., 48, 502-503.
US National Toxicology Program (1987) Toxicology and carcinogenesis
studies of pentachloronitrobenzene in B6C3F1 mice (feed studies)
(National Toxicology Program Technical Report Series No. 325),
Washington DC, US Department of Health and Human Services.
Warshawsky, L.D. (1994a) Acute oral toxicity study in rats.
Unpublished report No. 399-155 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Warshawsky, L.D. (1994b) Acute dermal toxicity study in rabbits.
Unpublished report No. 399-152 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Warshawsky, L.D. (1994c) Primary dermal irritation test in rabbits,
following a 4 hr exposure period. Unpublished report No. 399-153
from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Warshawsky, L.D. (1994d). Primary eye irritation study in rabbits.
Unpublished report No. 399-154 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Wedig, J.H., Sperling, F. & Miller, R. (1976) Unpublished report of an
analysis of data submitted by the National Cancer Institute from
Olin Corporation.
Wit, S.L., van Esch, G.J. & van Genderen, H. (1960) Toxicity of some
chloronitrobenzene compounds (trichlorodinitrobenzene,
trichloronitrobenzene, tetrachloronitrobenzene and
pentachloronitrobenzene) to laboratory rats and residues found in
crops treated with these fungicides. In: Proceedings of the
Fourth International Congress on Crop Protection, Hamburg,
September 1957, Vol. 2, Braunschweig, pp. 1665-1668.
Yamazaki, Y. (1994) Health report. Unpublished report from Ohmuta
Factory, Mitsui Toatsu Chemicals Inc. Submitted to WHO by
Uniroyal Chemical Company, Inc.
Table 1. Acute toxicity of quintozene
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Rat Male, female Oral > 5000a 99.4 Warshawsky (1994a)
Rat Oral > 30 000 NR Wit et al. (1960)
Rat Male, female Oral 1650 (male) 98.2 Finnegan et al. (1958)
1710 (female)
Rat NR Intraperitoneal 5000 NR Wit et al. (1960)
Rat Male, female Inhalation 1.7b 99.4 Hilaski (1994)
Rabbit Male Oral Erraticc 98.2 Finnegan et al. (1958)
Rabbit Male, female Dermal > 5000 99.4 Warshawsky (1994b)
Rabbit NR Dermal > 4 g 99.7 (1.8% Borzelleca et al. (1971)
HCB
Dog NR Oral > 2500 98.2 Finnegan et al. (1958)
75% wettable powder as 40% aqueous solution
Rat Male, female Oral 16 g (equal to NR Finnegan et al. (1958)
12 g/kg bw
quintozene)
NR, not reported
a Decreased defaecation at all doses; decreased activity at 1300, 1700, and 2000; soft stools at 2000
and 5000; and anogenital staining at 1700, 2000, and 5000; full recovery by day 6
b Mass median aerodynamic diameter, 3.6 µm; standard geometric deviation, 1.9; aerosol concentration,
1.3-2.2 mg/litre; air flow, 38 litres/min; decreased activity, increased salivation, and rapid
breathing
c Mortality rates: 0 at 350 mg/kg bw per day, 2 at 500 mg/kg bw per day, 1 at 650 mg/kg bw per day,
0 at 800 mg/kg bw per day, 3 at 1100 mg/kg bw per day, and 3 at 1400 mg/kg bw per day
(b) Short-term toxicity
Mice
Groups of 10 B6C3F1 mice of each sex, eight to nine weeks old,
were fed diets containing quintozene (purity, 99.6%; HCB content,
0.07%) at concentrations of 0,1250, 2500, 5000, 10 000, or 20 000 ppm
(males) and 0, 2500, 5000, 10,000, 20 000, or 40 000 ppm (females) for
13 weeks. Five animals per cage were checked twice daily, food
consumption was measured weekly by cage, and individual body weights
were recorded weekly. All mice were necropsied, livers were weighed,
and gross lesions, tissue masses, mandibular lymph nodes, mammary
glands, skin, salivary gland, sternebrae, thyroid, parathyroid, small
intestine, colon, liver, prostate, testis, ovary, uterus, lung and
bronchi, heart, oesophagus, stomach, brain, thymus, trachea, pancreas,
spleen, kidneys, adrenal, urinary bladder, pituitary, spinal cord (if
neurological signs occurred), and eyes (if grossly abnormal) were
examined histopathologically. All females at 40 000 ppm died during
the study. The final mean body weights in animals at 10 000 and
20 000 ppm were 7 and 8% lower than those of controls for males and 5
and 8% for females, respectively. Scattering of food was frequent, but
mice at the high dose may have consumed more food than controls.
Absolute liver weights were increased significantly in males at 1250,
2500, and 5000 ppm and in females at 2500, 5000, and 10 000 ppm.
Liver:body weight ratios were significantly increased in males at
doses > 2500 ppm and in females at > 5000 ppm. Clinical signs
were limited to small body size and emaciation of all males at
20 000 ppm and all females at 20 000 and 40 000 ppm. No
histopathological changes were seen in male mice, but females at
40 000 ppm had lymphoid depletion of the spleen, mesenteric lymph
nodes, or thymus. Effects in the lungs of all mice were consistent
with Sendai vital infection. There was no NOAEL since absolute liver
weights were increased at all doses (US National Toxicology Program,
1987).
Rats
An unspecified number of young rats (strain unspecified) were fed
diets containing 0 or 2000 ppm quintozene for 10 weeks. No gross
effects were noted, other than a decreased growth rate in males
(Wit et al., 1957).
Groups of five rats (strain unspecified) of each sex were fed
diets containing a 20% dust formulation of quintozene for 13 weeks to
give doses of 0, 0 (dust minus quintozene), 63.5, 635, 1250, 2500, or
5000 ppm. Animals were weighed weekly, and erythrocyte, haemoglobin,
and leukocyte determinations were performed at term. The liver,
kidney, and testis were weighed, and the heart, lung, liver, kidney,
spleen, stomach, small intestine, caecum, large intestine, thyroid,
adrenals, pancreas, and gonads were examined histologically. Rats at
5000 ppm lost weight during the first two weeks and were sacrificed in
poor condition at the start of week 3, although only one male had
died. Body weights were reduced to a statistically significant degree
in males and nonsignificantly in females at 2500 ppm. One male at 0
(powder control), one at 63.5 ppm, and one at 1250 ppm died.
Haematological parameters were not altered (no data reported). The
liver:body weight ratios were significantly increased in all treated
animals, except for females at 63.5 ppm. The kidney:body weight ratios
were increased in males at 1250 and 2500 ppm. 'Fine vacuolation' of
liver cytoplasm was seen in rats at 5000 ppm. There was no NOAEL
(Finnegan et al., 1958)
Groups of 10 male and 10 female rats (strain unspecified) were
fed diets containing quintozene at 0, 1000, 5000, or 10 000 ppm for 90
days. Growth was inhibited slightly at 5000 ppm and markedly at
10 000 ppm. The NOAEL was 1000 ppm, equivalent to 50 mg/kg bw per day
(Hoescht AG, 1964).
Four groups of 15 Charles River CD rats of each sex, seven weeks
of age, were fed diets containing quintozene (purity, 99.5%) at
concentrations of 0, 50, 3000, or 6000 ppm for 13 weeks. The average
intakes were 3.07, 187, and 381 mg/kg bw per day for males and 3.69,
223, and 455 mg/kg bw per day for females. Diets were analysed for
homogeneity (10 samples per dose), stability (on days 0 and 10), and
concentration (10 samples at weeks 1 and two samples at 2, 3, 4, 8,
and 12 weeks). The homogeneity of eight samples of diet containing
50 ppm was 95-108%, with two outlyers at 117 and 118%; the homogeneity
of 10 samples of the diet containing 3000 ppm was 92-101%, and that of
10 samples of diet at 6000 ppm was 93-98%. Duplicate analyses of
stability showed it to be within 10%. The concentrations of quintozene
in the diets were 98% (range, 91-103%) at 50 ppm, 94% (91-97%) at
3000 ppm, and 92% (85-95%) at 6000 ppm. Rats were observed twice a day
for toxic signs, morbidity, and mortality; food intake and body weight
were recorded weekly. Erythrocyte count, haemoglobin level,
haematocrit, total and differential leukocyte counts, platelet count,
reticulocyte count, mean corpuscular volume, mean corpuscular
haemoglobin, and mean corpuscular haemoglobin concentration; sodium,
potassium, chloride, calcium, inorganic phosphorus, alkaline
phosphatase, total bilirubin, aspartate aminotransferase, alanine
aminotransferase, creatine phosphokinase, urea nitrogen, creatinine,
total protein, albumin, globulin, cholesterol, and serum glucose; and
the colour, appearance, volume, specific gravity, microscopic
elements, pH, protein, glucose, ketones, bilirubin, occult blood,
nitrite, and urobilinogen in urine were reported for 10 rats of each
sex at each dose at week 13. Blood samples from the orbital sinus and
urine were collected during a fasting period. Ophthalmoscopic
examinations were performed on all rats before and on day 86 of the
study. The adrenals, heart, brain, kidney, liver, and gonads of all
rats were weighed post mortem. All animals were examined
macroscopically, and about 40 tissues were preserved. All tissues from
controls and from rats at the high dose and the livers, kidneys, and
lungs of animals at the low and middle doses were examined
microscopically. Lesions observed grossly were also examined.
There were no deaths during the study. Several minor signs (e.g.
hair loss and scabbing) occurred at low incidences but were not
dose-related. Malocclusion was seen in 1, 0, 6, and 4 males at the
four doses, respectively, but is unlikely to have been related to
treatment. Decreased body weight was noted in animals of each sex at
3000 and 6000 ppm and was statistically significant in males at
6000 ppm and in females at 3000 and 6000 ppm. Slightly decreased
terminal weight was seen in animals of each sex (by 8.1 and 6.1% in
males and females at the high dose, respectively) and was dose-
related. Body-weight gain was decreased by 16% in animals of each sex
at 6000 ppm and by 11 and 13% in males and females, respectively, at
3000 ppm. Food intake was reduced significantly in animals of each sex
at 6000 ppm during week 1 and sporadically in weeks 3, 4, and 8 in
males and week 5 in females. A consistent, nonsignificant reduction in
food intake was seen throughout the study in animals of each sex,
except in females at weeks 2 and 4; the food intake of males at
3000 ppm was significantly reduced only in week 1 but was consistently
less than that in controls except in week 10. Females had a
statistically significant reduction in food intake in weeks 5, 6, and
7 and a nonsignificant reduction at other times.
Ophthalmological examination showed no compound- or dose-related
effects, and no adverse effects were observed on haematological
parameters. Reduced alanine aminotransferase levels were seen in
animals of each sex at 3000 and 6000 ppm. In females, total protein
was increased at all doses, albumin was increased at 3000 ppm, and
globulin at 3000 and 6000 ppm; the cholesterol level was significantly
increased at 50 and 6000 ppm. In males, serum alkaline phosphatase
activity was increased at 50 ppm only; no changes were seen in serum
protein levels. The individual total protein levels in females were
greater than the range of levels in the controls in one rat per dose.
A similar pattern was seen for albumin and globulin, except that the
globulin level in two animals at 6000 ppm exceeded the maximal level
in controls. Although these mean values are statistically significant,
the effect was minimal and unlikely to be of toxicological
significance. The increase in cholesterol levels was seen at all doses
but was significant only at 50 and 6000 ppm. The changes were limited
to one sex, and the biological significance of this observation is
uncertain. The pH of the urine of males at 3000 and 6000 ppm was
lowered, but the values were within normal limits for rats. Other
parameters, e.g. a high ketone level in one male rat at 6000 ppm and
low levels of glucose (0.1 g/dl) in one male per dose, showed wide
individual variations and were not considered to be of toxicological
significance.
The absolute kidney weight was increased in males at 3000
(statistically significant) and 6000 (statistically nonsignificant)
ppm, and the kidney weights relative to those of the body and brain
were increased significantly in males at 3000 and 6000 ppm. The
absolute kidney weights were decreased in females at 50 ppm, and the
weights relative to body weight but not brain weight were
significantly decreased at 50 ppm and increased at 6000 ppm. The
apparent effect on the kidney:body weight ratio is. probably a
reflection of decreased body weight at 6000 ppm. The absolute liver
weights were nonsignificantly increased in animals of each sex at 3000
and 6000 ppm, the effect being dose-related in females. The liver:body
weight ratios were significantly increased in males at 3000 and
6000 ppm and in females at 6000 ppm, probably reflecting decreased
body weight. The liver:brain weight ratios were nonsignificantly
increased in animals of each sex at 3000 and 6000 ppm. Changes in the
weight of the brain relative to body weight in males at 3000 and
6000 ppm and of the heart relative to body weight in males at 6000 ppm
were also attributed to reduced body weights.
Males had increased incidences of chronic nephritis (1, 6, 5, and
4 out of 15 animals at 0, 50, 3000, and 6000 ppm, respectively), which
were not dose-related, and an increased incidence of mild haemorrhage
in mesenteric lymph nodes (4 at 6000 ppm; 0 in controls), but these
effects were unlikely to be related to treatment. Mandibular lymph
node haemorrhages occurred in 5/15 control and 0/15 male rats at
6000 ppm, and mediastinal lymph node haemorrhages were seen in 3/15
control and 1/15 males at 6000 ppm. Low incidences of haemorrhage seen
in medias final lymph nodes of seven females at 6000 ppm and three
controls and of mesenteric lymph nodes in six females at 6000 ppm and
no controls may indicate a slight effect, the significance of which is
not known. The only effects in liver were seen at 6000 ppm, comprising
hepatocellular hypertrophy in 7/15 males and 8/15 females and in none
of the controls. The NOAEL was 50 ppm, equal to 3.07 mg/kg bw per day,
on the basis of minimal changes in body-weight gain and liver weight
and changes in alanine aminotransferase activity at higher doses
(McGee, 1988).
Four groups of six acclimatized Charles River CD rats of each
sex, with initial body weights of 247-265 g for males and 229-244 g
for females, were treated on clipped dorsal skin, 6 h per day for 21
days with quintozene (purity, 98.72%) applied as a paste in deionized
water at 0, 30, 300, or 1000 mg/kg bw per day. The application site
was wrapped in gauze bandaging secured with non-irritating tape. After
each application the test site was washed with tepid water. Rats were
observed for mortality, moribundity, clinical signs, and behaviour
twice daily, and irritation was scored by the Draize method once
daily. Body weight was recorded before treatment and then weekly, and
food intake was measured weekly. Haematological and clinical chemical
tests were done at termination on blood obtained from the orbital
sinuses before fasting, and urine samples obtained during fasting were
analysed. The haematological parameters measured included erythrocyte
counts, haemoglobin, haematocrit, total and differential leukocyte
counts, mean corpuscular volume, mean corpuscular haemoglobin, mean
corpuscular haemoglobin concentration, and platelet counts. The
clinical chemical parameters measured were sodium, potassium,
chloride, calcium, inorganic phosphorus, alkaline phosphatase, total
bilirubin, aspartate and alanine aminotransferases, lactic
dehydrogenase, creatine phosphokinase, urea nitrogen, creatinine,
total protein, albumin, globulin, the albumin:globulin ratio,
cholesterol, and glucose; and the urinary parameters were colour,
appearance, volume, specific gravity, microscopic elements, pH,
protein, glucose, ketones, bilirubin, occult blood, nitrite,
urobilinogen, and leukocyte incidence. All rats were examined
macroscopically post mortem; kidneys, liver, and testes were
weighed, and 44 organs or tissues per rat were preserved, as were
gross lesions seen at macroscopic examination. Microscopic examination
was limited to liver, kidney and treated and untreated skin in animals
at 0 and 1000 mg/kg bw per day, and all gross lesions.
There were no deaths. Ventral hair loss was seen occasionally in
females, but the incidence was not dose-related. No behavioural
changes and no signs of dermal irritation were detected. No
compound-related changes in body weight were seen, although increased
body-weight gain was noted in males at 30 mg/kg bw per day
(statistically nonsignificant). Food intake was comparable in all
groups. There were no statistically significant changes in any
haematological parameters, and clinical chemistry parameters were
unaffected, except for a statistically significant, dose-related
decrease in alanine aminotransferase activity in males and in females
at 1000 mg/kg bw per day; a statistically significant decrease was
also seen at 1000 mg/kg bw per day, but there was no effect at lower
doses. Other changes (increased alkaline phosphatase activity and
decreased total bilirubin in females at 300 mg/kg bw per day) were not
dose-related. There were no significant changes in urinary parameters.
Organ weights also showed no statistically significant changes,
although testicular weights were reduced in two of five rats at
1000 mg/kg bw per day; these testes were not examined microscopically.
There were no compound- or dose-related microscopic effects on the
liver, kidney, or treated or untreated skin. The NOAEL was 300 mg/kg
bw per day (Goldenthal, 1992).
Dogs
Three groups of three mongrel dogs were fed dietary levels of 25,
200, or 1000 ppm quintozene for one year by mixing a commercial powder
containing 20% quintozene, 77% pyrax ABB (a pyrophyllite carrier), and
3% Armour sticker with the diet, which was mixed with water
immediately before feeding. Dogs were weighed weekly; haematological
parameters (unspecified but including total and differential leukocyte
counts) were measured at 0, 6, and 12 months; and the heart, lung,
liver, kidney, spleen, gastrointestinal tract, thyroid, adrenal,
pancreas, gonads, and bone marrow were examined histologically. One
dog at 200 ppm whelped at week 5 of the study, and two dogs at
1000 ppm whelped at weeks 7 and 8. Some pups were allowed to suckle
and were then sacrificed at four to six weeks of age and examined
histopathologically. There were no deaths. Body weight was variable
but apparently unaffected by quintozene. The only effects on
haematological parameters were increased eosinophil counts at 6 and 12
months in some animals (probably due to parasites) and low total
leukocyte counts at 12 months in dogs at 10 000 ppm. No lesions were
seen in the pups examined histopathologically. Hepatic-cell
enlargement with pale cytoplasmic staining was noted in all adult
dogs, but the severity was not dose-related. Periodic acid-Schiff and
Best carmine staining of the liver showed abundant glycogen around the
periphery of lobules. Staining for fat gave negative results. There
was no NOAEL, owing to the random whelping (Finnegan et al., 1958).
Four groups of three beagle dogs (Ridglan Farms, Inc.),
previously immunized and vaccinated against most common viruses and
infections, were fed diets containing 0, 40, 2000, or 4000 ppm
quintozene (purity, at least 96%) for four weeks. The diets were
prepared weekly, acetone being used as an aid to dispersion at 40 ppm
and also added to control diets. Homogeneity and stability were
measured in week 4, and the concentration of quintozene was measured
in the diets for weeks 2, 3, and 4. The homogeneity in 10 samples was
91-99% (mean, 95%) at 40 ppm, 92-103% (mean, 98%) at 2000 ppm, and
95-104% (mean, 98%) at 4000 ppm. The stability over 10 days (in
duplicate analyses) was 98-101%, and the dietary concentrations were
85-116% (mean, 100%) at 40 ppm, 91-108% (mean, 100%) at 2000 ppm, and
92-98% (mean, 94%) at 4000 ppm. Dogs were observed twice daily for
clinical signs, mortality, and moribundity. Body weights were measured
before treatment and then weekly; food intake was measured weekly.
Total and differential leukocyte counts, erythrocyte count,
haemoglobin, haematocrit, mean corpuscular volume, mean corpuscular
haemoglobin, mean corpuscular haemoglobin concentration, platelet
counts, and reticulocyte counts; and sodium, potassium, chloride,
calcium, inorganic phosphorus, alkaline phosphatase, total bilirubin,
aspartate and alanine aminotransferases, creatine phosphokinase, urea
nitrogen, creatinine, total protein, albumin, globulin, cholesterol,
and glucose were measured before and after four weeks of treatment.
Blood samples were taken from the jugular vein of dogs fasted
overnight, and urine collected during this period was analysed for
colour, appearance, volume, specific gravity, microscopic elements,
pH, protein, glucose, ketones, bilirubin, occult blood, nitrite, and
urobilinogen. The adrenals, brain, heart, kidney, liver, gonads,
pituitary, spleen, and thyroid-parathyroid were weighed and examined
macroscopically, and about 40 organs were preserved but were not
examined microscopically.
There were no deaths or dose-related clinical signs, and no
compound-related effects were observed on body weight. Food intake, on
the basis of grams per animal per day, was reduced in males at
4000 ppm throughout the study and in those at 40 and 2000 ppm during
week 1; it was also decreased in weeks 2 and 4 but was increased in
animals at 2000 ppm in weeks 2, 3, and 4. Food intake in females on
this basis was slightly reduced in all groups at all intervals, but
the decreases were not dose-related. Food intake on the basis of grams
per kilogram body weight was reduced in males at all doses in week 1
and in those at 40 and 3000 ppm at all periods; however, intake was
increased in animals at 2000 ppm in weeks 2, 3, and 4. In females,
intake on this basis was decreased in week 1 and at 4000 ppm in week
2; at other periods, intake was close to control values. Overall,
intake was reduced in all treated animals in week 1 and was
inconsistent thereafter. None of the changes in food intake is
statistically significant, and they are unlikely to be biologically
significant.
No significant haematological changes were observed in males. In
females, such changes were limited to decreased leukocyte counts in
animals at 40 and 4000 ppm and decreased mean corpuscular haemoglobin
in dogs at 2000 ppm. None of these changes is of toxicological
significance. Alanine aminotransferase activity was markedly decreased
in a dose-related manner in animals of each sex, and dose-related
increases in cholesterol levels were seen in females at all doses and
in males (131, 192, 199 and 167 mg/dl at 0, 40, 2000, and 4000 ppm).
No significant changes were observed in urinalysis. The absolute
weights of kidney and spleen were statistically significantly greater
than those of controls in male dogs at 2000 ppm and were nonsigni-
ficantly increased in dogs at 4000 ppm. Liver weights were also
nonsignificantly increased (not dose-related) at these doses. The
liver:body weight ratios of males at 2000 and 4000 ppm were
statistically significantly increased, as were those of the kidney at
2000 ppm; there was a considerable but nonsignificant increase at
4000 ppm. Spleen:body weight ratios were statistically nonsigni-
ficantly increased in males at 2000 and 4000 ppm. The thyroid-
parathyroid:body weight ratio was significantly increased in male dogs
at 2000 ppm and nonsignificantly in those at 4000 ppm. The ratios of
kidney, spleen, and thyroid-parathyroid to brain weights were
significantly increased in male dogs at 2000 ppm and nonsignificantly
at 4000 ppm; the liver:brain weight was significantly increased at
both 2000 and 4000 ppm. In females, the only statistically significant
change was in the liver:body weight ratio, which was increased at 2000
and 4000 ppm; nonsignificantly increased absolute liver weights were
also noted, and nonsignificant increases in brain and brain:body
weight ratios were seen at 2000 and 4000 ppm. The effects on organ
weights (particularly liver) and on clinical chemical parameters
indicate a probable NOAEL of 40 ppm, equivalent to 1 mg/kg bw per day;
however, in the absence of histopathological reports, the significance
of the findings cannot be fully assessed (Johnson, 1989).
Four groups of six beagle dogs of each sex (Ridglan Farms Inc.),
five to six months old and suitably immunized and vaccinated, were fed
diets containing 0, 15, 150, or 1500 ppm quintozene (purity, 99.4%,
with HCB at a maximum of 0.07% and a mean of 0.045%) for one year. The
homogeneity and stability of the diets were determined before the
study, and all diets were analysed for the percentage of the nominal
levels that were actually present. Diets were prepared weekly for four
weeks and monthly thereafter. Acetone was used as the dispersant in
the 15 and 150 ppm and control diets. The homogeneity in 10 samples
was 93-99% at 15 ppm, 85-107% at 150 ppm, and 89-110% at 1500 ppm,
with respective mean values of 95, 93, and 99%. Stability,
investigated over 10 days, was satisfactory. Duplicate analyses for
actual concentrations at each interval and dose showed mean values
over the study of 96, 94, and 96% and ranges of 87-104%, 89-110%, and
82-103% at 15, 150, and 1500 ppm, respectively. The actual intakes
were calculated from data on food intake and body weight to be 0.4,
4.3, and 40.1 mg/kg bw per day for males and 0.44, 4.22, and
41.48 mg/kg bw per day for females. Dogs were observed for mortality,
moribundity, and clinical signs twice daily. Body weights and food
intake were recorded weekly for 14 weeks and monthly thereafter.
Ophthalmology was performed before the test and in week 52.
Haematological and clinical chemical examinations were performed on
blood from the jugular vein of dogs fasted overnight at 6 and 12
months. Urinalysis was performed at 6 and 12 months on urine collected
during the fasting period. The same haematological, clinical chemical,
and urinary parameters were investigated as in the 28-day study
(Johnson, 1989). At termination (death before the end of the study or
sacrifice), the adrenals, brain, heart, kidneys, liver, gonads,
pituitary, spleen, and thyroid-parathyroids were examined grossly and
weighed. Adrenals, aorta, bone (rib), bone marrow (rib), brain (fore-,
mid-, and hind-), eye (with optic nerve), gall-bladder, oesophagus,
stomach, duodenum, jejunum, ileum, caecum, colon, rectum, ovaries,
testis with epididymides, heart, kidneys, liver, lung (with bronchi),
lymph nodes (tracheobronchial, mesenteric, and regional), mammary
gland (females only), pancreas, pituitary, prostate, salivary gland
(with mandibular lymph node), sciatic nerve, skeletal muscle (thigh),
skin, spinal cord (cervical, thoracic, and lumbar), spleen, thymic
region, trachea, urinary bladder, and uterus from all dogs were
preserved, processed, and examined microscopically.
In one male dog at 15 ppm that was sacrificed in extremis at
week 28, the clinical and pathological signs indicated a diagnosis of
generalized systemic blastomycosis. The death was not related to
treatment, and the clinical signs reported (including diarrhoea,
emesis, alopecia, lacrimation, and ocular discharge) did not indicate
dose- or compound-related incidence. One male at 150 ppm had
convulsions in weeks 39 and 48 and tremors in weeks 39-40, associated
with limb rigidity in weeks 39-40 and excessive salivation. No
abnormal neural effects were seen histopathologically at term. The
effect was isolated and is unlikely to have been compound-related.
Body weight and weight gain were unaffected. Food intake was variable,
but no clear trends were apparent. There were no compound-related
effects on haematological parameters, the only statistically
significant difference from control values being decreased haemoglobin
levels at 12 months in males at 15 ppm. Statistically significant
changes in clinical chemical parameters comprised increased alkaline
phosphatase activity at 1500 ppm in males at 6 and 12 months and in
females at 6 months; a considerable but not statistically significant
increase occurred in females at 12 months. Significantly decreased
alanine aminotransferase activity was seen in animals of each sex at
150 and 1500 ppm at 6 and 12 months; significantly decreased
creatinine was noted in males at these doses at 6 and 12 months and in
females at 1500 ppm at 6 months. Cholesterol levels were significantly
increased in males at 1500 ppm at 6 and 12 months and nonsignificantly
increased in females at 1500 ppm at 6 and 12 months. Globulin levels
were also significantly increased in males at 150 and 1500 ppm at 6
months, and blood urea nitrogen was decreased in females at 1500 ppm
at 12 months. No effects were seen on urinalysis.
Statistically significant increases in the absolute and relative
(to body and brain weight) weights of liver were seen in females at
1500 ppm and in relative weights in males at 1500 ppm; the absolute
liver weight in males was also increased but not significantly.
Nonsignificant increases in absolute and relative liver weights were
seen in animals of each sex at 150 ppm. A statistically significant
increase in the kidney:body weight ratio was seen in females at
1500 ppm. The maximal absolute adrenal weights were slightly increased
in males at 1500 ppm, and females showed a minimal, dose-related
increase in adrenal weights. Microscopic examination showed slight
hepatocellular hypertrophy in all dogs at 1500 ppm. No dose-related
effects were seen on alveolar macrophages, inflammation, or the
incidence of pneumonia. Adrenal changes in males were comparable in
all groups. In females at 1500 ppm, the incidence of lymphocytic
infiltration and vacuolar changes was increased, but these effects are
considered not to be of toxicological significance. The incidence of
pituitary cysts was increased in animals of each sex at 1500 ppm, but
such cysts were relatively common in control beagle dogs. The NOAEL
was probably 150 ppm, equal to 4.3 mg/kg bw per day in males and
4.22 mg/kg bw per day in females. Changes in clinical chemical
parameters seen at this dose (e.g. decreased alanine aminotransferase
activity and decreased creatinine) did not appear to be associated
with microscopic changes in tissues or organs (Goldenthal, 1990)
Four groups of three beagle dogs of each sex were fed diets
containing quintozene (purity, 98.8%) at concentrations of 0, 500,
1000, or 5000 ppm for two years. Erythrocyte counts, total and
differential leukocyte counts, haemoglobin, haematocrit, and Heinz
bodies; and the specific gravity, pH, appearance, colour, microscopic
sediment, albumin, glucose, and bilirubin in urine were reported at
26, 52, and 78 weeks and at terminal sacrifice. The heart, lung,
liver, kidney, spleen, pancreas, gonads, prostate, adrenal, thyroid,
brain, pituitary, eye with optic nerve, stomach, jejunum, colon, and
bone marrow were examined histologically. Of the animals at the
highest dose, three males died on days 214, 288, and 357 and two
females on days 317 and 472. Food intake was slightly reduced (mean,
4.6%) in animals at 1000 ppm and markedly (by about 30%) in those at
5000 ppm. All dogs at 5000 ppm lost weight, and their body-weight
gains (means, 2.75 kg at 500 and 2.62 kg at 1000 ppm) were lower than
that of controls (4.85 kg). Signs of toxicity at 5000 ppm included
lacrimation, conjunctival discharge, and milky corneal opacity; one
dog had corneal ulceration. One dog at 1000 ppm had conjunctivitis and
a second had corneal opacity for a short period. No effects were seen
at 500 ppm. Anaemia was induced in dogs at the high dose, with
characteristic reductions in haemoglobin, haematocrit, and erythrocyte
counts. Atrophy of bone marrow and reduced haematopoiesis were
observed at 5000 ppm. Severe histopathological changes in the liver
were seen at 5000 ppm and included fibrous narrowing of hepatic cell
cords, enlarged periportal areas, and increased leukocytic
infiltration. Similar but less intense effects were seen at 500 and
1000 ppm. There was no NOAEL (Scholz & Brunk, 1968).
Five groups of four beagle dogs of each sex, 4.5 months old, were
fed diets containing quintozene (purity, 98.2%; containing 1.4% HCB
and traces of tetrachloronitrobenzene and pentachlorobenzene) at
concentrations of 0, 5, 30, 180, or 1080 ppm for two years. Diets were
prepared weekly by dilution of the high dose, which was prepared by
blending corn-oil solutions of quintozene with the diet; the dietary
fat content was increased from 9 to 11%. Before feeding, an equal
weight of water was added to the food. Body weight and food intake
were determined weekly. The haematocrit, haemoglobin, and total and
differential leukocyte counts, and urinary reducing substances,
protein, specific gravity, and sediment were determined at 3, 6, 12,
18, and 24 months. Blood urea nitrogen, serum aspartate
aminotransferase, serum alkaline phosphatase, and cholinesterase,
prothrombin time, and bromsulphthalein retention time were determined
in controls and dogs at the high dose at 0, 3, 6, 12, 18 and 24
months. The frequency of oestrus was recorded. One dog of each sex at
each dose was sacrificed at 12 months for histopathological
examination. Organ and organ:body weight ratios were reported for
heart, spleen, liver, kidneys, and testes; the brain, lung, heart,
aorta, liver, spleen, kidney, stomach, ileum, jejunum, large
intestine, urinary bladder, bone marrow (sternal and long bone),
pituitary, thyroid, pancreas, adrenal, gonad, lymph node, and eye were
examined histologically.
No deaths were observed. The changes in body weight were not
significant, but weight gain tended to be reduced in females at 180
and 1080 ppm during weeks 6 and 13, and sporadically in all treated
males, which also had sporadic decreases in food intake. Neither body
weight nor food intake was consistently affected by treatment.
Slightly reduced haematocrit values were noted in males at 30 and
180 ppm at 78 weeks (statistically significant), and reduced
haemoglobin values were noted in males at 30 ppm; a nonsignificant
decrease in haematocrit and haemoglobin values was again seen in males
at 30 and 180 ppm at 104 weeks. The absence of any effect at 1080 ppm
indicates that these effects were probably random. No consistent
effects were seen on serum cholinesterase, the maximal inhibition
being 20% in females at 1080 ppm in week 13 and 23.6% in females at 30
ppm at week 52; all other depressions were < 15.4% and were random.
Mean serum aspartate aminotransferase activity was increased in
females at 1080 ppm at 13 and 78 weeks but decreased at 104 weeks
(statistically significant only at 13 weeks); it was also decreased in
males at 1080 ppm at 26 weeks and increased at 78 weeks. Serum
alkaline phosphatase activity was increased in males and females at 52
weeks and thereafter, but the increase achieved statistical
significance only at 52 weeks when data on animals of each sex were
combined. External lymph nodes and oestrus cycle were not affected by
treatment. Liver weight and the liver:body weight ratios of males and
females at 180 and 1080 ppm were nonsignificantly increased at one
year; at two years, a nonsignificant increase in absolute liver weight
was seen in males at 1080 ppm and a significant increase in females.
The increased liver:body weight ratio was significant only when data
for the two sexes were combined. The absolute and relative liver
weights were nonsignificantly reduced in females at 5, 30, and 180 ppm
but significantly reduced in males at 30 and 180 ppm. Testicular
weights were nonsignificantly reduced in dogs at 1080 ppm, and the
weight ratio was statistically significantly reduced. Other changes
(such as increased kidney weight ratio at 5 ppm and increased heart
ratio at 180 ppm in males) were random. Dogs at 180 ppm showed minimal
suppression of bile flow in liver cord cells; the effect was greater
in animals at 1080 ppm, resulting in moderate bile pigment
accumulation in cord cells. The effect was usually reversible. The
NOAEL was 30 ppm, equivalent to 0.75 mg/kg bw per day (Larson &
Borzelleca, 1968; Borzelleca et al., 1971).
Monkeys
Two rhesus monkeys of each sex were fed consecutive daily doses
of quintozene (purity, > 99.9%) on sucrose pellets, corresponding to
2 ppm of their daily diet, for 70 days. There were no controls. One
monkey of each sex was sacrificed on day 71. Haemoglobin, packed cell
volume, erythrocyte and total leukocyte counts, methaemoglobin, and
Heinz bodies, and sodium, potassium, bilirubin, creatinine, blood urea
nitrogen, total protein, serum aspartate and alanine aminotrans-
ferases, lactate dehydrogenase, cholesterol, luteinizing and
follicular-stimulating hormone levels, progesterone, and cortisol were
determined in blood samples taken before the test, on days 19 and 48,
and, in the surviving monkeys, on day 76. There were no changes in
haemoglobin values, packed cell volume, or erythrocyte or leukocyte
cell counts. The level of methaemoglobin was slightly elevated on day
1 but not thereafter. No change was seen in the incidence of Heinz
bodies. No changes were seen in clinical chemistry, although the data
on bilirubin and lactate dehydrogenase were highly variable; no time
effect relationship was apparent. At sacrifice, no histopathological
effects were seen in the liver, stomach, large or small intestine,
spleen, kidney, heart, lung, thymus, cerebrum, cerebellum, pons,
medulla, spinal cord, or bone marrow of the animals sacrificed on day
71. The NOAEL was > 2 ppm, equivalent to > 0.1 mg/kg bw per day
(Kögel et al., 1979b)
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 18 B6C3F1 and B6AKF1 mice of each sex were given
quintozene (purity and contaminants unspecified) at a dose of
464 mg/kg bw by stomach tube from seven days of age to the time of
weaning at four weeks of age and thereafter at a dose of 1206 ppm for
18 months. The latter was the maximal tolerated dose. There was a
significantly elevated incidence of tumours, mostly hepatomas, in both
strains (Innes et al., 1969).
Groups of 100 male and 100 female Swiss random strain mice were
fed diets containing quintozene (containing 2.7% HCB) at levels of 0,
100, 400, or 1200 ppm for 80 weeks. Body-weight gain was decreased in
animals of each sex at 1200 ppm. Liver:body weight ratios were
increased in males and females at 400 and 1200 ppm, and the
kidney:body weight ratio was increased in females at 1200 ppm. General
appearance, behaviour, and survival were not affected by treatment,
and the haematological indices were considered to be within normal
limits. A non-dose-related increase in nodular hyperplasia of the
liver was seen in all treated males. The hyperplastic areas had
essentially normal cell architecture and were regarded as
non-neoplastic. An increased incidence of subcutaneous fibrosarcomas
was observed in females at 1200 ppm. None of the other neoplasms
appeared to be related to treatment and were common in mice of this
strain. There was no NOAEL (van der Heijden & Til, 1974).
Groups of 50 male and 50 female mice (probably B6C3F1) were fed
diets containing two increasing doses of quintozene (purity, > 98%;
with 0.15% pentachlorobenzene, 0.25% 2,3,4,5-tetrachloronitrobenzene,
and 1% HCB), initially at 1075 and 2150 ppm for males and 2320 and
4640 ppm for females, increased to 3000 and 6000 ppm for males and
4500 and 9000 ppm for females during the last 315 days of the 546-day
(78-week) exposure period. A control group consisting of 20 male and
20 female mice was given only the vehicle, 2% corn oil. The total
duration of the study was 92 weeks, but some mice were killed and
autopsied earlier. Malignant tumours were seen at frequencies of 50%
in 25% of control males and 20% in 10% of control females, at 33% in
29% of males and 18% in 6% of females at the low dose, and at 29% in
20% of males and 57% in 19% of females at the high dose. Only the last
frequency was statistically significantly higher than expected; many
malignant histiocytic lymphomas were found in numerous organs of the
same animal. No significant differences were found between groups in
the numbers of animals with tumours, and no particular type of tumour
could be ascribed to the treatment (Wedig et al., 1976).
Three groups of 50 B6C3F1 mice of each sex, seven to eight weeks
old, were fed diets containing quintozene (purity, 99.6%, with 0.07%
HCB) at doses of 0, 2500, or 5000 ppm for 103 weeks, followed by a
one-week withdrawal period before terminal sacrifice. Mice were
observed twice daily, and five per cage were weighed weekly for 12
weeks and monthly thereafter. Food intake per cage was measured every
four weeks. The homogeneity of the diets was within 10%; the stability
of quintozene in the diet for two weeks was 100% at 25°C; and the mean
actual dietary concentrations, measured 14 times during the study,
were 2460 ppm (98.4%) with a range of 94-104.4% at 2500 ppm and
4980 ppm (99.6%) with a range of 94-104% at 5000 ppm. All animals
(including those that died before the end of the study) were
necropsied, unless autolysis or cannibalization had occurred. Tissue
masses, abnormal regional lymph nodes, mammary gland, salivary gland,
bone marrow, costochondral junction, thymus, larynx, trachea, lungs
and bronchi, heart, thyroid, parathyroid, oesophagus, stomach,
duodenum, jejunum, ileum, colon, mesenteric lymph nodes, liver,
gall-bladder, pancreas, spleen, kidney, adrenal, urinary bladder,
seminal vesicles, prostate, uterus, gonads, brain, pituitary, and
caecum from controls, female mice at the low dose, males and females
at the high dose, and all males at the low dose that died before term
were examined histologically. Gross lesions, livers, and nasal
cavities from all males at the low dose were also examined.
The terminal body weights of male mice were 96% of control values
at 2500 and 90% at 5000 ppm. The mean body weights of females at
5000 ppm were consistently 10% below control values by week 24 and
thereafter, but reached this level at 2500 ppm only after 92 weeks; at
term, the mean weights of females were 18 and 21% below control
values. No clinical signs were reported. Food intake may have exceeded
that of controls, but this was difficult to determine because of food
scattering. The survival of males at term, 31-34 mice, was comparable
in all groups. At term, 30 female controls, 18 at the low dose, and 14
at the high dose were still alive; however, five mice at the low dose
were accidentally drowned and two mice at 2500 ppm and one at 5000 ppm
died during the recovery period.
In male mice at 0, 2500, and 5000 ppm, the numbers with malignant
tumours were 19, 25, and 17 (25, 30, and 21 tumours) and those with
benign tumours were 19, 15, and 17 (23, 16, and 19 tumours). There was
no dose-effect relationship for the incidence of tumours in any
specific organ, the incidences generally being comparable between
groups. The incidence of non-neoplastic lesions in comparison with the
control incidence was not of toxicological significance. In female
mice at 0, 2500, and 5000 ppm, the numbers with malignant tumours were
15, 11, and 9 (15, 11, and 9 tumours) and those with benign tumours
were 16, 17, and 14 (18, 19, and 19 tumours). Tumours were not
concentrated in specific tissues and there was no dose-effect
relationship. Increased incidences of ovarian abscesses were seen,
with 24, 44, and 58% at 0, 2500, and 5000 ppm. Five of six abscesses
that were cultured contained Klebsiella. Hyperplasia of the
mediastinal lymph node was observed at incidences of 2, 9, and 20% and
liver haematopoiesis at 18, 42, and 46%; splenic haematopoiesis was
observed at incidences of 28, 48, and 54%. These effects may reflect
increased susceptibility to infection rather than a toxicological
effect.
Quintozene thus decreased body weight at 5000 ppm, equal to
953 mg/kg bw per day in males and 1358 mg/kg bw per day in females.
There was no increase in neoplasia, and the non-neoplastic
pathological effects in female mice were probably due to infection.
The NOAEL in male mice was 2500 ppm. In female mice at 2500 ppm, the
reduced body weight reached 10% only at 92 weeks and continued to
decrease, to 18%, until 103 weeks; as this effect is unlikely to be
compound-related, 2500 ppm was also the probable NOAEL for female
mice. Overall, the NOAEL was 2500 ppm, equal to 387 mg/kg bw per day
in male mice (US National Toxicology Program, 1987).
A group of 10 mice (strain not specified) of each sex received
topical applications on shaved skin of 0.2 ml of a 0.3% solution of
quintozene (purity and contaminants unspecified) in acetone twice
weekly for 12 weeks. The control group received acetone alone. After
application of quintozene, croton oil was applied to the site
(presumably twice weekly) for 20 weeks, and mice were sacrificed 20
weeks later. Papillomas were seen in 2/10, 6/10, 8/9, and 7/8 treated
males, in 6/10, 7/9, 7/7, and 7/4(?) treated females, in 1/10, 6/9,
5/8, and 1/7 control males, and in 0/10, 3/10, 4/8, and 4/7 control
females 10, 20, 30, and 40 weeks after the initial croton oil
application, respectively. The time to 50% tumour incidence was 13
weeks in treated males, 10 weeks in treated females, 16 weeks in male
controls, and unknown for control females. The total numbers of
tumours were 29 in treated males, 33 in treated females, 6 in control
males, and 7 in control females. Quintozene at the doses used in the
study thus did not induce tumours in the absence of a promoting agent
(Searle, 1966).
Rats
Groups of 10 rats of each sex (strain unspecified) were fed diets
containing 0, 25, 100, 300, 1000, or 2500 ppm quintozene (purity and
contaminants unspecified) prepared by blending a powder comprising 20%
quintozene, 77% pyrax ABB (a pyrophyllite carrier), and 3% Armour
'sticker' with Purina dog chow. Rats were weighed weekly, and
haematological parameters (unspecified) were determined at 11 and 24
months. At death and terminal sacrifice, heart, lung, liver, kidney,
spleen, stomach, small intestine, caecum, large intestine, thyroid,
adrenal, bladder, pancreas, and bone marrow were examined
histologically. At one year, survival in males was 7, 10, 8, 7, 9, and
10 animals, and that in females was 9, 6, 8,10, 10, and 8 animals at
0, 25, 100, 300, 1000, and 2500 ppm, respectively; by two years,
survival was 1, 4, 4, 3, 3, and 4 in males and 3, 2, 5, 5, 5, and 2 in
females. Body-weight changes (only means given) were sporadic;
statistically significant increases were seen in males at 25 ppm
(weeks 4-52) and 100 ppm (week 4) and in females at 100 ppm (weeks
2-78), 300 ppm (week 8 and weeks 52-78), 1000 ppm (week 26), and
2500 ppm (weeks 2-78); decreases were seen in males at 2500 ppm (weeks
2 and 8). The small numbers of animals make definite conclusions
difficult, but the dose of 2500 ppm appeared to decrease body weight
consistently in animals of each sex, as did the dose of 1000 ppm in
females. The decreases at all doses in females may be real, but males
were affected only at 2500 ppm. The haematological parameters were
stated to be within normal limits, and histopathological lesions were
stated to be limited to 'occasional lung abscesses and occurrences of
fatty changes in the liver. Lesions are not correlatable with dietary
concentration of quintozene but are of the type often seen in old
rats.' Individual data were not available. The NOAEL in this limited
study was 300 ppm, equivalent to 15 mg/kg bw per day (Finnegan
et al., 1958).
Groups of 50 male and 50 female Wistar rats were fed dietary
levels of 0, 100, 400 and 1200 ppm quintozene (containing 2.7% HCB)
for two years. The liver:- and kidney:body weight ratios were
increased in rats at 400 and 1200 ppm. Dose-related increases in the
incidences of single-cell necrosis and fatty metamorphosis of
hepatocytes were seen in animals of each sex at 400 and 1200 ppm, and
enlarged centrilobular hepatocytes were seen at all doses. There was
no increase in tumour incidence, and no adverse effects were observed
on general appearance, behaviour, body-weight gain, or food
consumption. Haematological, blood chemical, and urinary values were
considered to be within normal limits. There was no NOAEL in this
limited study (Sinkeldam et al., 1974).
Groups of 50 male and 50 female rats (strain unspecified) were
fed diets containing two decreasing doses of quintozene (purity, >
98%; with 0.15% pentachlorobenzene, 0.25% 2, 3, 4, 5-tetrachloro-
nitrobenzene, and 1% HCB): for the first 98 days, males received 7500
or 15 000 ppm and females, 11 000 or 22 000 ppm; during the last 448
days, males received 5000 or 10 000 ppm and females, 7250 or
14 500 ppm. A control group consisting of 20 male and 20 female rats
was given only the vehicle, 2% corn oil. The total exposure time was
546 days (78 weeks), and the duration of the study was 85-117 weeks.
Malignant tumours were seen at frequencies of 16% in 10% of control
males and 10% in 10% of control females, at 13% in 8.5% of males and
8% in 6% of females at the low dose, and at 23% in 12.5% of males and
9% in 6.5% of females at the high dose. No significant difference
between the groups was found in the total number of tumours or the
number of animals with tumours, and no particular type of tumour could
be ascribed to the treatment (Wedig et al., 1976).
Four groups of 50 Charles River CD rats of each sex, six to seven
weeks old, were fed diets containing quintozene (purity, 99.4%; <
0.07% HCB) at doses of 0, 20, 3000, or 6000 ppm for 24 months. An
additional 10 rats of each sex at each dose were treated similarly and
sacrificed at 12 months. As the homogeneity of the diet at the 20-ppm
dose was unsatisfactory in week 1 (range, 14-40 ppm), blending time
was increased for the week-2 diets, which improved the range to
16-22 ppm. From week 28, acetone was used as an aid to dispersion,
resulting in a variation of 16.3-18.9 ppm. The homogeneity was
acceptable at higher doses. The stability over 10 days was within 8%.
Duplicate analyses about every four weeks indicated that the mean
percentages of the nominal concentrations were 95 8.4 at 20 ppm, 100 ±
6.5 at 3000 ppm, and 101 ± 6.9 at 6000 ppm. Diets were prepared
weekly. Rats were observed twice daily for mortality, moribundity, and
overt signs of toxicity. Body weight and food intake were recorded for
individual rats weekly for 16 weeks and monthly thereafter.
Ophthalmological examinations were conducted before treatment and at
12 and 24 months. Five rats of each sex were screened microbio-
logically before the test. Erythrocyte count, total and differential
leukocyte counts, haemoglobin, haematocrit, platelet counts, mean
corpuscular volume, mean corpuscular haemoglobin, and mean corpuscular
haemoglobin concentration; and sodium, potassium, chloride, calcium,
inorganic phosphorus, alkaline phosphatase, bilirubin, aspartate and
alanine aminotransferases, creatine phosphokinase, urea nitrogen,
creatinine, total protein, albumin, globulin, cholesterol and glucose
were determined in orbital sinus blood from 10 rats of each sex at
each dose, which had been fasted overnight, at 6, 12, 18, and 24
months. The colour, appearance, volume, specific gravity, microscopic
elements, pH, protein, glucose, bilirubin, ketones, occult blood,
nitrite, and urobilinogen in urine were determined in samples
collected during the fasting period at 6, 12, 18, and 24 months. All
animals were necropsied, and adrenals, aorta, femur, bone marrow
(femur), brain (fore-, mid-, and hind-), eye (including optic nerve),
oesophagus, stomach, duodenum, jejunum, ileum, caecum, colon, rectum,
ovary, testis (with epididymides), heart, kidney, liver, lung, lymph
nodes, mammary gland (female only), pancreas, pituitary, prostate and
seminal vesicles, salivary gland (with lymph nodes), sciatic nerve,
skeletal muscle (thigh), skin, spinal cord (cervical, thoracic, and
lumbar), spleen, thymic region, thyroid, parathyroid, trachea, urinary
bladder, uterus, gross lesions, and tissue masses were collected. The
adrenals, brain, heart, kidney, liver, gonads, and thyroid-parathyroid
were weighed at sacrifice at 12 and 24 months. All tissues of control
rats, those at the high dose, and those that died or were sacrificed
were examined histologically at term. Tissue masses, gross lesions,
regional lymph nodes, and, in females only, lung were examined
histologically at interim sacrifice of animals at 20 and 3000 ppm; the
liver, kidney, lung, thyroids, parathyroids, tissue masses, and
regional lymph nodes of animals at these doses were examined at
terminal sacrifice.
Mortality was 52, 54, 42, and 16% in males and 42, 50, 46, and
48% in females at 0, 20, 3000, and 6000 ppm, respectively, excluding
rats sacrificed at 12 months. One of 60 rats at each dose had died by
12 months, the only clinical sign being a slightly increased incidence
of body surface staining in females at 6000 ppm and an increased
incidence of malocclusion in males at this dose from week 1 to term,
which was obviously not compound-related. Body weight was
significantly reduced in animals of each sex at 6000 ppm throughout
the study, the decrease being > 10% by week 2 in males and by week 24
in females. Slightly lower weights were noted in females at 3000 ppm
after 24 weeks (statistically significant in 14/21 weighings), but the
weight decreases in males were generally comparable to those of
controls, except for a slight (generally statistically nonsignificant)
reduction after 84 weeks. Food intake on the basis of grams per
kilogram body weight tended to be increased, especially in males, and
to a lesser degree females, at 6000 ppm. The average intakes of the
diets at 20, 3000, and 6000 ppm were 0.9, 141, and 303 mg/kg bw per
day in males and 1.14, 179, and 370 mg/kg bw per day in females. No
compound-related ophthalmological findings were seen.
Statistically significant haematological findings tended to be
sporadic and do not appear to be compound-induced. In males at
6000 ppm, haemoglobin and haematocrit were reduced at six months only,
and mean corpuscular volume was reduced at 6 and 12 months; platelet
counts were increased at 12 months in males at 20 ppm. In females,
erythrocyte counts were elevated at 18 months at 20 and 6000 ppm,
haemoglobin was depressed at 6 months at 3000 and 6000 ppm but was
increased at 18 months at 20 ppm, haematocrit was increased at 18
months at 20 ppm, mean corpuscular volume was decreased at 18 months
at 3000 ppm and at 6, 12, and 18 months at 6000 ppm, mean corpuscular
haemoglobin was decreased at 18 months at 3000 ppm and at 6 and 18
months at 6000 ppm, and mean corpuscular haemoglobin concentration was
depressed at 6 months at 6000 ppm. Lymphocyte counts were elevated at
12 months at 20 ppm.
The clinical chemical findings in males included increased
potassium levels at 24 months and decreased glucose at 6 months at
6000 ppm, which are probably random effects. Alanine aminotransferase
activity was reduced at 6 months at 20 ppm, at 6, 18, and 24 months at
3000 ppm, and at all intervals at 6000 ppm; however, it was increased
at 12 months at 3000 ppm. The decrease is considered to be compound-
related, and the amount is dose-related. In females at 6000 ppm,
slight decreases in chloride were seen at 6 and 12 months and slightly
increased calcium at 18 months. Alkaline phosphatase activity was
increased at 6 and 12 months and aspartate aminotransferase activity
was decreased at all intervals at 6000 ppm; alanine aminotransferase
activity was decreased in a dose-related manner at all intervals at
3000 and 6000 ppm. Cholesterol was increased at all intervals and
glucose was decreased at 6 months at 6000 ppm. Total protein was
increased at 18 months at 3000 and 6000 ppm. Thus, in animals of each
sex, compound-related effects on alanine aminotransferase activity
were seen at 3000 and 6000 ppm, and in females aspartate aminotrans-
ferase activity was decreased and cholesterol was increased at
6000 ppm. The effects on alkaline phosphatase activity may also be
compound-related. There were no apparent changes at any dose or
interval in urinary parameters, except possibly in volume; but water
contamination was likely to have occurred.
Macroscopic examination of tissues at 12 months showed an
increased incidence of tan-white foci in lungs of female rats at
6000 ppm (one control; 11 at 6000 ppm), and the incidence of
tan-white-yellow lung foci was increased in a dose-related manner in
animals of each sex at 3000 and 6000 ppm that died or were sacrificed
at 12-24 months. The incidence of accentuated lobulation of the liver
was increased in animals of each sex at 6000 ppm. Thyroid enlargement
was also noted, with incidences of 0, 0, 2, and 3 in males and 1, 0,
2, and 1 in females at 0, 20, 3000, and 6000 ppm. This finding is
questionable.
The absolute and relative (to brain and body weight) weights of
the kidney, liver, and testis were statistically significantly
increased in males at 3000 and 6000 ppm at the 12-month interim
sacrifice. The organ:body weight ratios were affected by decreases in
the body weight of males at 6000 ppm, but significant increases were
reported for heart, kidney, liver, thyroid-parathyroid, and testis at
this dose. At 3000 ppm, increased organ:body weight ratios were seen
for kidney and liver. The organ:brain weight ratios were increased for
kidney at 3000 and 6000 ppm and for testis at 6000 ppm. Significantly
increased organ:body weight ratios were seen for brain, kidney, liver,
and thyroid-parathyroid in females at 6000 ppm at the 12-month
sacrifice. In males at terminal sacrifice, the absolute weights of
liver were increased at 6000 ppm, of the thyroid-parathyroid at 3000
and 6000 ppm, and of the kidney at 3000 ppm. Increased organ:body
weight ratios were seen in males for brain at 6000 ppm, kidney at
3000 ppm, liver at 3000 and 6000 ppm, and thyroid-parathyroid at 3000
and 6000 ppm, and the organ:brain weight ratios were increased in
males for kidney at 3000 ppm, liver at 3000 and 6000 ppm, and
thyroid-parathyroid at 3000 and 6000 ppm. Females at 6000 ppm with
body weights nonsignificantly lower than those of controls had a
decreased absolute heart weight and an increased absolute thyroid-
parathyroid weight. Increased organ:body weight ratios were seen for
liver at 6000 ppm and thyroid-parathyroid at 3000 and 6000 ppm; the
heart:brain weight was decreased and the thyroid-parathyroid:brain
weight was increased at 6000 ppm. When nonsignificant changes are
included, the major effects were increases in liver and thyroid-
parathyroid weights in animals at 3000 and 6000 ppm at both interim
and final sacrifices.
There were no microscopic observations that would account for the
changes in kidney and testicular weights. No compound-related
microscopic pathological changes were seen up to 12 months. At 12-24
months, the incidence of hypertrophy in males was 0, 0, 13/48, and
30/49, that of hyperplasia 0, 0, 1, and 1, and that of hepatocellular
adenomas 1/49, 0/49, 1/48, and 4/49 rats at 0, 20, 3000, and 6000 ppm.
Increased incidences of alveolar macrophages, interstitial pneumonia,
and perivascular lymphoid infiltration were seen in the lungs of males
at 6000 ppm. Thyroid hypertrophy was seen in 3/49, 2/49, 20/48, and
33/49 rats, follicular adenomas in 0/49, 0/49, 6/48, and 5/49 rats,
follicular carcinomas in 0/49, 1/49, 0/48, and 2/49 rats, and
follicular hyperplasia in 2/49, 2/49, 7/48, and 8/49 rats at 0, 20,
3000, and 6000 ppm, respectively. The incidence of colloidal cysts was
also increased in males at 6000 ppm. In females at 12-24 months, liver
hypertrophy occurred in 0/50, 0/49, 19/50, and 29/47 rats, hyperplasia
in 1/50, 0/49, 5/50, and 3/47 rats, and hepatocellular adenomas in
1/50, 0/49, 1/50, and 1/47 rats at 0, 20, 3000, and 6000 ppm,
respectively. Increased incidences of alveolar macrophages and
interstitial pneumonia were seen at 3000 and 6000 ppm; the incidence
of perivascular lymphoid infiltration was not increased. Thyroid
hypertrophy was seen in 0/50, 0/49, 18/50, and 23/46 female rats,
follicular adenomas in 1/50, 0/49, 2/50, and 4/46, follicular
carcinomas in 1/50, 0/49, 0/50, and 1/46, and follicular hyperplasia
in 0/50, 0/49, 6/50, and 8/46 at 0, 20, 3000, and 6000 ppm,
respectively. In data on historical controls from 11 two-year studies,
the mean percentage incidences of follicular adenomas were 2.88% for
males and 0.3% for females, and those of follicular carcinomas were
1.73% for males and 0.60% for females. In the study described here,
the incidences of follicular adenoma were 0, 0, 12.5, and 10.2% for
males and 2, 0,12, and 8.7% for females at 0, 20, 3000, and 6000 ppm,
respectively, and those of follicular carcinoma were 0, 2, 0, and 4.1
in males, and 2, 0, 0, and 2.2 in females. The incidence of follicular
adenomas in animals of each sex at 3000 and 6000 ppm therefore exceeds
the historical mean, as does that of follicular carcinomas in males at
20 and 6000 ppm and in females in the control and 6000-ppm groups. The
incidence of follicular carcinomas in males at 20 ppm, control
females, and in females at 6000 ppm was one per group, which is within
the historical control range, as is the incidence of follicular
carcinomas in males at 6000 ppm: 2/49 as compared to 3/32 in one
historical control. Quintozene therefore induced thyroid adenomas and
may have induced thyroid carcinomas. The changes observed in the lung
are consistent with infections; the increased incidence of such
changes at high doses may indicate either reduced immune competence or
a stress-related increase in susceptibility to infection. The
hepatocellular hypertrophy (mainly centrilobular) in which
eosinophilic cytoplasm was noted is indistinguishable from the
hepatocellular hypertrophy arising from increased drug metabolizing
enzyme activity. The NOAEL was 20 ppm, equivalent to 1 mg/kg bw per
day (Goldenthal, 1991).
(d) Reproductive toxicity
Four groups of 25 weanling (28-day-old) Charles River CD rats of
each sex were fed diets containing quintozene (purity, 98.2%, with
1.4% HCB and trace levels of tetrachloronitrobenzene and
pentachlorobenzene) at concentrations of 0, 5, 50, or 500 ppm. The
diet was prepared weekly, using 10 ml of corn oil for 500 ppm
quintozene, and was appropriately diluted with additional feed. After
11 weeks on diet, groups of 20 F0 females were paired with a male at
the same dose, rotated weekly for three weeks if necessary. Matings,
numbers of pregnancies, litters born, pups 1, 4, and 21 days
post partum, and litter weight at day 21 were recorded. Litters were
culled to 10 pups on day 4. Indices of fertility (pregnancies per
mating × 100), gestation (total litters per number of pregnancies ×
100), viability (live pups at day 4 per live pups born × 100) and
lactation (weaned pups per number of remaining pups after culling on
day 4 × 100) were calculated. A second litter (F1b) was initiated 10
days after the birth of the final F1a litter, and these animals were
used as parental rats for the F2 and F3 generations. Autopsies
were performed on F0 adults after weaning of F1b pups, F1a pups
at weaning, F1b adults after weaning of F2b pups, and F2a pups
at weaning, this pattern being maintained throughout the study. Ten
F3b offspring of each sex were maintained on the diets until two
months old, when they were sacrificed, and the heart, lung, liver,
kidney, urinary bladder, spleen, stomach, small and large intestine,
caecum, lymph nodes, bone marrow, skeletal muscle, skin, brain,
pituitary, thymus, thyroid, adrenals, pancreas, and gonads were
examined histopathologically. The only effects were on the body weight
of adult females: No significant changes were observed between
controls and F0 or F1b females at 500 ppm, but the body weights of
F2b females were significantly decreased at 500 ( P < 0.05) and
50 ppm ( P < 0.01). The effect was not dose-related. The NOAEL was
probably 500 ppm, equivalent to 2.5 mg/kg bw per day (Larson &
Borzelleca, 1968; Borzelleca et al., 1971).
Four groups of 26 Charles River COBS CD rats of each sex, aged 55
days, were fed diets containing quintozene (purity, > 99%; 0.08%
HCB) at doses of 0, 20, 3000, or 6000 ppm for two litters per
generation for two generations. Parents for the second generation were
selected from the F1b litters, avoiding sibling matings. Diets were
prepared weekly in a twin-shell blender with an intensifier bar to mix
500 g with additional diet to achieve the required levels. As the
homogeneity of the diet at the low dose was poor in week 1, the mixing
time was increased to 20 min after an initial 2-min mixing with 250 g
untreated diet, the mixer bar being used for the first 10 min of the
final blending. This process improved the homogeneity, but the dietary
levels were reduced to 84% of the nominal concentration. The diets
containing the low dose were therefore again modified, by dissolving
the quintozene in acetone before preparing the premix and blending (in
a Hobart mixer) it for 5 min before mixture with additional diet. The
homogeneity and percentage concentration were then acceptable. Those
at the middle and high doses were acceptable from week 1. The control
diet was also mixed with acetone from week 4. Analysis of the diets at
least once every four weeks throughout the study showed good
concordance with nominal values. F0 parents were fed treated diets
for 81 days before pairing, and F1 parents were given the test diet
for 90 days between weaning and pairing. Rats were observed twice
daily for mortality and signs of toxicity; body weights were recorded
weekly until sacrifice, except during gestation when animals were
weighed on days 0, 7, 14, and 20. Food intake was measured weekly
except during mating and, for females, during gestation and lactation,
when data were obtained for days 0-7, 7-14, 14-20, and 0-20 of
gestation and 0-4, 4-7, 7-14, and 14-21 of lactation. Rats were paired
1:1, and copulation was determined by either vaginal lavage or the
presence of a copulatory plug. The day of detection of copulation was
designated day 0 of gestation, for a maximum of 21 days. The time
between end of lactation and re-pairing was 10 days. Males were
changed between F1a and F1b and between F2a and F2b pairings.
The duration of the gestation period and difficulties in parturition
were recorded. Litter size, the numbers of still- and live births, and
gross abnormalities were noted. On lactation day 4, litters were
culled to eight pups (four of each sex when possible). Pups and dams
were observed twice daily for abnormal behaviour (including nesting
and nursing) and deaths. Pups were sexed and weighed individually on
days 0, 4, 7, 14, and 21 of lactation.
At term, F0 males were sacrificed and necropsied, and their
tissues were preserved. All males that failed to sire a litter were
examined for the presence of sperm in the epididymides. F0 females
were sacrificed at end of lactation of F1b pups (or, if non-gravid,
25 days after separation from males). Uteri from apparently non-gravid
females were examined with 10% ammonium sulfide. F1a offspring were
discarded after weaning. F1b offspring were necropsied, only gross
pathological lesions and malformed pups being preserved. A similar
programme was followed for the F1 generation. In both generations,
the coagulating gland, cervix, ovaries, pituitary, prostate, seminal
vesicles, testes with epididymides, uterus, vagina, and gross lesions
were examined microscopically.
Three adults died: one F0 male at 3000 ppm in week 28, one
23-day-old F1 male at 6000 ppm, and one control F1 female at about
20 weeks of age. These deaths did not appear to be compound-related.
Brown abdominal staining and brown or yellow anogenital staining
(usually for only 1-3 weeks) were seen in F0 females at the middle
and high doses. A slightly increased incidence of hair loss also
occurred in this group. F1 males and females at 6000 ppm had an
increased incidence of small size, which generally disappeared by 15
(males) to 17 (females) weeks of age. The body weights of F0 males
at 3000 and 6000 ppm and of females at 6000 ppm were lower than those
of controls (statistically significant), and a slight (usually
nonsignificant) decrease occurred in females at 3000 ppm. F1 males
and females at 6000 ppm and females at 3000 ppm had significantly
reduced body weights in weeks 4-17. During gestation, significant
reductions in mean body weights were seen in females at 6000 ppm in
the F0/F1a (at 14-21 and 0-21 days), F0/F1b (on days 0 and 7),
F1/F2a (at all intervals), and F1/F2b (at 14-20 days)
generations. The mean body weights at 6000 ppm were also significantly
reduced during lactation in the F0/F1a (at 0-4, 7-14, 14-21, and
0-21 days), the F0/F1b (0-21 days only), the F1/F2a (7-14 days
only), and the F1/F2b (14-21 and 0-21 days) generations. In
animals at 3000 ppm, decreased body weights were seen in the
F1/F2b matings on days 4-7, 14-21, and 0-21. Food intake was
reduced before mating of adults at 6000 ppm and during the lactation
period in F0 and F1 females.
No compound-related effects on male copulation, male fertility
index, female fertility index, gestation index, copulatory interval,
or gestation length were seen in either generation, although greater
fertility than controls was seen in F2a (animals of each sex) and
F2b (females) animals. Males that failed to mate had motile and
morphologically normal sperm in the epididymides, apart from one male
in the F1/F2b group at the low dose which had non-motile,
morphologically abnormal sperm. The mean numbers of stillbirths and
live births, the sex ratios, and viability indices were comparable to
those of controls, except in F1/F2a offspring at the middle dose
in which the sex ratio was skewed in favour of males. In animals at
the high dose, the total number of live births and the body weights of
live offspring were significantly reduced; the body weights of
offspring were also significantly reduced in the F0/F1a (days 14
and 21), F0/F1b (females only, day 21), and F0/F2b (days 14
and 21) generations at 3000 ppm.
During lactation, an increased incidence of 'pale' pups was seen
at 3000 and 6000 ppm in the F0/F1a generation on day 14 and in
F1/F2a pups at 6000 ppm on days 7, 14, and 21. Missing tails were
seen in F1/F2a pups at 6000 ppm on days 14 and 21 and in
F1/F2b pups at low incidence on all days. Concentric rings on the
tail were seen at high incidence in F1/F2a pups at 6000 ppm on
days 7, 14, and 21.
The incidences of malformations in offspring at birth were
reported only in summary form. In the F1a litters, two dead
offspring at 3000 ppm had malformations consisting of mandibular
micrognathia in one, cleft palate in both, and an intraventricular
defect in one; no malformations were seen in dead F1b offspring. One
case of anophthalmia was noted among 86 F1b pups at 20 ppm at day
21. Malformations seen in two dead F2a pups in two litters at
6000 ppm were a bulbous pulmonary trunk, an intraventricular septal
defect, and aortic arch stenosis; no malformations were seen in dead
F2b offspring. One F2b control pup out of 118 had microphthalmia
and mandibular micrognathia, one at 6000 ppm had anophthalmia, and one
at this dose had situs inversus; these pups were from different
litters. The incidence of variants was virtually nil.
Terminal macroscopic examination indicated no treatment-related
effects in F0 parents. Tan-yellow foci in the lung were seen in some
F1 males and females at 6000 ppm and in two females at 3000 ppm. The
testicular weights of animals that failed to sire offspring were
slightly reduced in two F1 controls and one treated F1 male at
3000 ppm. Microscopic examination of F0 adults showed no
compound-related effects. In F1 animals, the tan-yellow foci in the
lungs were aggregates of alveolar macrophages, perivascular-
peribronchial lymphoid cell infiltration, and/or interstitial
pneumonia. These changes are typical of pulmonary viral infections.
Thus, quintozene is unlikely to have been toxic, although stress due
to its administration or effects on the immune system may have
resulted in increased susceptibility to infection in animals at
6000 ppm and possibly in females at 3000 ppm. The NOAEL was 20 ppm,
equivalent to 1 mg/kg bw per day, on the basis of changes in body
weight in pups and adults at 3000 ppm. No adverse effects were seen on
reproductive parameters (Schardein et al., 1991).
(e) Developmental toxicity
Mice
A group of 23 C57Bl/6 mice was given quintozene (containing 11%
HCB) at 500 mg/kg bw per day in corn oil on days 7-11 of gestation; 19
controls were available. Further groups received either pentachloro-
aniline at 100 or 200 mg/kg bw per day or tetrachloronitrobenzene at
200 mg/kg bw per day on days 7-18 of pregnancy. In mice treated with
quintozene, fetal mortality was slightly increased, and the incidence
of malformations was significantly increased. Renal agenesis was the
most frequent effect, and the incidences of anophthalmia (19.2%
greater than in controls), microphthalmia (9.8%), and cleft palate
were increased. Pentachloroaniline and tetrachloronitrobenzene did not
cause malformations or affect maternal body weight, the liver:body
weight ratio, fetal weight, or fetal mortality (Courtney et al.,
1976).
Groups of CD-1 mice were given quintozene (containing 11% HCB) at
doses of 250 or 500 mg/kg bw per day (five or 10 litters), penta-
chloroaniline at 250 mg/kg bw per day (nine litters), tetrachloro-
nitrobenzene at 200 mg/kg bw per day (six litters in one study and
nine in the other), quintozene fabricated to contain 11% HCB (nine
litters) at 500 mg/kg bw per day, HCB at 100 mg/kg bw per day (10
litters), or quintozene containing < 20 ppm HCB at 500 mg/kg bw per
day (10 litters) on days 7-16 of gestation. Maternal liver:body weight
ratios were increased at both doses (dose-related) of contaminated
quintozene and with the fabricated sample of quintozene; a
nonsignificant increase was seen with HCB. The incidence of
malformations per litter was significantly increased at 500 mg/kg bw
per day of contaminated quintozene (28.6% in comparison with 6.2, 2.0,
or 7.2% in corn oil:acetone [9:1] control groups at the same volume),
and to a lesser degree with HCB (13.6%) and with quintozene with <
20 ppm HCB. The major malformation observed with contaminated and
fabricated quintozene and HCB was cleft palate; this malformation was
also seen with quintozene containing < 20 ppm HCB, but the major
contributor to the incidence was clubbed foot. The last preparation
differed from the other quintozene samples in that it provided a
solution rather than a suspension; this may have increased absorption.
This group also showed signs of toxicity, expressed as abortion in
three of 13 mice on day 17 of gestation. A similar high incidence of
clubbed foot has not been seen in other studies of quintozene, and the
author suggested that it may have been due to increased uterine muscle
tone, with consequent pressure effects on the developing fetus
(Courtney et al., 1976).
A group of 30 CD1 timed-pregnant mice were given quintozene
(containing 5% HCB) orally at 750 mg/kg bw per day on gestation days
8-12; 40 timed-pregnant mice were used as controls. Day 20 of
gestation was considered to be postnatal day 1. Death occurred in 17%
of animals, and 73% were pregnant. Litter size was nonsignificantly
reduced on day 1, but pup weights were comparable to those of
controls, and by postnatal day 3, the mortality and weights of pups
were comparable to those of controls. There was no NOAEL (Kavlock
et al., 1987).
Rats
Groups of five to seven pregnant CD rats received quintozene
containing 11% HCB or < 1.0% HCB at 500 mg/kg bw per day or
pentachlorophenol or tetrachloronitrobenzene at 75 mg/kg bw per day on
days 7-18 of gestation. No effects were seen on maternal weight gain
or the liver:body weight ratio. Pentachlorophenol decreased fetal
weights. The incidence of malformations was minimal, and these
consisted of enlarged cerebral ventricles, umbilical hernias, and
slightly enlarged renal pelvis (Courtney et al., 1976).
Pregnant Charles River albino rats received quintozene in corn
oil by intubation at doses of 100-1563 ppm on days 6-15 of gestation
and were sacrificed on day 20. Corpora lutea, position and numbers of
dead and resorbed pups, fetal weights, and sex ratio were recorded.
One-half of the fetuses were stained with alizarin Red-S for skeletal
examination, and the remainder were either fixed in Bouin's fluid for
examination of soft tissues or preserved in formalin. No difference
was seen between control and treated groups for any parameter,
including renal pelvis dilatation, hydronephrosis, or hydroureter,
which occurred in all groups. No NOAEL could be determined because of
the unusual expression of doses (Jordan & Borzelleca, 1973).
Four groups of 25 mated Charles River COBS female rats were given
quintozene (purity, 97.3%; containing 0.025% HCB) at doses of 0, 30,
600, or 1200 mg/kg bw per day as 10 ml/kg bw of 0.2% carboxymethyl-
cellulose suspensions by gavage on days 6-15 of gestation. The
suspensions were prepared daily; analysis on days 7 and 14 of the
study indicated actual levels of 88-110% of the nominal concentration
and stability over 24 h. The day of mating was designated day 0 of
gestation. Rats were observed twice daily for mortality and changes in
appearance and behaviour. Maternal rats were weighed on gestation days
0, 6, 9, 12, 16, and 20, and food intake was measured daily and
calculated for days 6-9, 9-12, 12-16, 16-20, 6-15, and 6-20 of
gestation. Rats were killed on day 20 of gestation, and the uterus and
ovaries were examined in situ and gravid uteri weighed. The numbers
and locations of viable and non-viable fetuses and of early and late
resorptions and the numbers of implantations and corpora lutea were
recorded. Maternal tissues were examined macroscopically, and grossly
abnormal tissues were preserved. The uteri of apparently non-pregnant
rats were examined with 10% ammonium sulfide. Fetuses were weighted,
sexed, and examined for external malformations; half were preserved in
Bouin's solution for examination of soft tissues by Wilson's
technique, and the remainder were eviscerated and processed for
Alizarin R-S staining and subsequent skeletal examination (Dawson
technique).
There were no deaths or adverse clinical or behavioural effects.
A nonsignificant reduction in the body weights of animals at
1200 mg/kg bw per day was seen but which was only 1-2% after
correction for initial weight differences. Food intake was
significantly reduced at 6-9 days in animals at 1200 mg/kg bw per day,
but the reduction was only 10.8% when measured as grams per animal per
day or 8.7% when measured as grams per kilogram body weight per day.
The effect was probably due to unpalatability. At later intervals and
during days 6-15 and 6-20, food intake was comparable to that of
controls. The incidences of pregnancy were 24/25 controls, 23/25 at
30 mg/kg bw per day, 25/25 at 600 mg/kg bw per day, and 23/25 at
1200 mg/kg bw per day. There were no total resorptions, and all
pregnancies resulted in viable offspring; the viable litter sizes were
comparable at all doses. The numbers of early resorptions were
slightly increased in all test groups, but the incidence was within
the normal historical range. The incidences of late resorptions and
corpora lutea were comparable in all groups. The group mean individual
fetal weights were unaffected, and the sex ratio was normal. The
malformations seen in controls were anophthalmia in one fetus,
malpositioned ovary and kidney in another, and a vertebral anomaly
with an associated rib anomaly in a third. At 30 mg/kg bw per day, one
fetus had an encephalocoele and a malpositioned ovary, one had
anasarca, a bent scapula, and bent limb bones, and a third had a
folded retina. At 600 mg/kg bw per day, one pup had microphthalmia and
folded retinas; at 1200 mg/kg bw per day, two pups from separate
litters had malpositioned kidneys and ovaries. Thus, no pattern in the
incidence of malformations and no dose-response relationship was seen
in the occurrence of malformations. The incidence of variants was
within normal limits. The NOAEL for maternal, embryo- and fetotoxicity
and for embryofetal development was 1200 mg/kg bw per day, the highest
dose tested (Keller, 1988a).
Rabbits
Four groups of 16 artificially inseminated New Zealand white
rabbits, three months old at receipt and acclimatized for 111 days,
were given quintozene (purity, 97.3%) at doses of 0, 12.5, 125, or
250 mg/kg bw per day in 0.2% carboxymethylcellulose on days 7-19 of
gestation. Because of maternal toxicity, additional rabbits, five
months old at reception and acclimatized for 48 days, were added about
75 days later and intubated with 0 (16 rabbits), 6.25 (16 rabbits), or
125 (12 rabbits) mg/kg bw per day. Semen for artificial insemination
was provided by six males proven to be fertile and was used to
inseminate an equal number of females in each concurrent group, except
in the second study when two males were used to inseminate fewer
females at 125 mg/kg bw per day than the remaining four males. The day
of insemination was designated day 0 of gestation. Caesarian sections
were performed on day 29 of gestation. The stability and homogeneity
of the quintozene formulation were satisfactory, and the actual
concentrations were 95-110% of the nominal ones.
Survival, appearance, and behaviour were observed twice daily and
were reported daily for days 7-29 of gestation. Rabbits that died
before the end of the study were necropsied, and those that showed
signs of abortion or premature delivery were sacrificed. The fetuses
of these rabbits were examined externally and preserved; those of
rabbits that died within 24 h of the end of the study were evaluated.
Body weights were recorded on days 0, 7, 13, 20, 24, and 29 of
gestation, and food intake was measured daily and calculated for days
7-13, 13-20, 20-29, 7-20, and 0-29 of gestation. At termination, the
uterus and ovaries were examined, and gravid uterine weight, the
number and location of viable and non-viable fetuses, the incidence of
early and late resorptions and total implantations, and the number of
corpora lutea were recorded. Maternal thoracic and abdominal cavities
were examined macroscopically, and grossly abnormal tissues and organs
were preserved. Non-pregnant uteri were examined with 10% ammonium
sulfide solution. Fetuses were weighed, tagged, and examined
externally, then dissected, internally sexed, and examined for soft
tissue abnormalities, including mid-coronal brain slices. The
carcasses were processed, stained with Alizarin Red-S (Dawson
technique), and examined for skeletal abnormalities.
One rabbit at 12.5 mg/kg bw per day died on day 28 of gestation;
two died at 125 mg/kg bw per day, both aborting before death on day 28
of gestation; and five died at 250 mg/kg bw per day on days 19, 21,
26, 26, and 27 of gestation, the death on day 27 being preceded by
abortion. In the secondary study, no deaths occurred at any dose. The
known causes of death in the initial study were mucoid enteritis in
those at 125 mg/kg bw per day and pneumonia in three of the five that
died at 250 mg/kg bw per day; the other causes of death were not
determined. The incidences of abortion were: two controls on days 24
and 27, two at 12.5 mg/kg bw per day on days 26 and 27, three at
125 mg/kg bw per day on days 23, 28, and 28, and four at 250 mg/kg bw
per day on days 23, 24, 25, and 26. When animals that aborted before
death are included, the incidences were 2, 2, 5, and 5 at 0, 12.5,
125, and 250 mg/kg bw per day, respectively. In the secondary study,
one rabbit at 125 mg/kg bw per day aborted on day 26. Premature births
occurred in one doe in each control group and in one at 125 mg/kg bw
per day. Signs of toxicity included mucoid stools in 6/16 rabbits at
250 mg/kg bw per day and 1/16 rabbits at 125 mg/kg bw per day, an
increased incidence of absent stools in 4/16 controls, 8/16 at 125,
and 11/16 at 250 mg/kg bw per day, and an increased incidence of
anogenital staining in 3, 8, 9, and 10 animals at 0, 12.5, 125, and
250 mg/kg bw per day in the initial group and 2, 3, and 9 at 0, 6.25,
and 125 mg/kg bw per day in the secondary group.
Body weights were markedly reduced after treatment at 250 mg/kg
bw per day, the loss peaking on day 24 of gestation, with very slight
recovery by day 29. The body weight at term, corrected for gravid
uterine weight, was also markedly reduced. Body-weight gain was
consistently reduced throughout gestation. Animals at 125 mg/kg bw per
day had a greater weight loss on days 13-20 of gestation in both the
initial and, to a lesser extent, the secondary study; however, the
weight reduction in the second group was greater on days 24-29. Both
groups at this dose had lowered corrected body weights at term than
controls, the effect being more apparent in the second group. Food
intake was significantly reduced at most intervals, when expressed
either as grams per animal or grams per kilogram body weight per day.
Stillbirths occurred in 1/81, 1/85, 1/49, and 3/28 rabbits at 0, 12.5,
125, and 250 mg/kg bw per day and in 0/82, 0/101, and 0/68 at 0, 6.25
and 125 mg/kg bw per day. The incidences of total resorptions in
relation to total implantations were 5.8, 11.5, 9.1, and 22.2% at 0,
12.5, 125, and 250 mg/kg bw per day and 24.8, 10.6, and 2.7% at 0,
6.25, and 125 mg/kg bw per day. Mean fetal weight was reduced markedly
at 250 mg/kg bw per day. The sex ratio was normal in all groups. The
mean litter size, including both viable and non-viable pups, was 7.4,
6.5, 6.8, and 4.7 at 0, 12.5, 125, and 250 mg/kg bw per day and 5.8,
7.7, and 6.8 at 0, 6.25, and 125 mg/kg bw per day. Malformations were
seen in 4, 4, 9, 2, 3, 2, and 0 pups in 3, 3, 6, 2, 3, 2, and 0
litters at 0, 0 (second study), 6.25 (second study), 12.5, 125, 125
(second study), and 250 mg/kg bw per day. The high incidence of
malformations at 6.25 mg/kg bw per day was accounted for by three pups
with multiple malformations that were not seen at higher doses. As
artificial insemination occurred over three days, each male normally
providing semen for two females, the multiple malformations may be due
to genetic defects mediated through the male. The incidence of
variants was comparable between groups. (Keller, 1988b).
The probable NOAELs are difficult to determine, since the results
at 125 mg/kg bw per day were not consistent between the studies. The
deaths due to mucoid enteritis were probably not compound-related
since the five deaths at 250 mg/kg bw per day were not associated with
pneumonia or mucoid enteritis. Although the incidence of abortions was
increased at 125 and 250 mg/kg bw per day, two of the five abortions
at the lower dose occurred immediately before death due to mucoid
enteritis and may therefore not be compound-related. The only
consistent effect was a decrease in body-weight gain at 125 and
250 mg/kg bw per day. The NOAEL for maternal toxicity was thus
12.5 mg/kg bw per day. That for embryo- and fetotoxicity was 125 mg/kg
bw per day, on the basis of reduced pup weight, increased resorption
rates, and a slight increase in the incidence of stillbirths at
250 mg/kg bw per day. The NOAEL for malformations was 250 mg/kg bw per
day, although only 28 fetuses from six litters were available for
examination at this dose.
(f) Genotoxicity
The results of studies of the genotoxicity of quintozene are
reported in Table 2.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Six male New Zealand white rabbits, three months old, were
exposed to quintozene (purity, 99.42%) moistened with water at a dose
of 500 mg per rabbit on dipped, intact dorsal skin under a 2.5-cm2
gauze patch secured with tape. The dressings were removed after 4 h,
the sites were wiped with dry towels, and the animals were observed
for 72 h. No dermal irritation was observed after 30-60 min or 24, 48,
or 72 h (Warshawsky, 1994c).
Six male New Zealand white rabbits, about three months old,
received 0.0996 g of quintozene (purity, 99.42%) in the cupped
conjunctival sac of the right eye, and the eyelids were held together
for 1 s after administration. The eyes were not washed and were scored
for irritation (on the Draize scale) at 1, 24, 48, and 72 h. At 72 h,
they were examined with sodium fluorescein. After 1 h, all rabbits had
a clear discharge, and two also showed blanching. Conjunctival
redness, chemosis, and discharge were seen in all animals, the redness
persisting in four rabbits up to 24 h. All eyes were normal by 48 h.
The average group scores were 7/110 at 1 h and 1.7/110 at 24 h,
indicating that the compound is minimally irritating to the rabbit eye
(Warshawsky, 1994d).
Table 2. Results of tests for the genotoxicity of quintozene
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 0-6667 µg/plate 99.6 Negativea US National Toxicology
TA100, TA1535, TA1537 Program (1987)
Reverse mutation S. typhimurium, five strains NR NR Negative Simmon et al. (1976)
Mitotic recombination Saccharomyces cerevisiae NR NR Negative Simmon et al. (1976)
DNA repair E. coli NR NR Negative Simmon et al. (1976)
Gene mutation Mouse lymphoma 1.25-10 µg/ml 99.6 Negativea US National Toxicology
L5178Y tk+/tk- cells Program (1987)
Sister chromatid Chinese hamster ovary cells 0.75-7.5 µg/ml 99.6 Negativeb US National Toxicology
exchange Program (1987)
Sister chromatid Chinese hamster ovary cells 7.5-75 µg/ml 99.6 Negativec US National Toxicology
exchange Program (1987)
Chromosomal Chinese hamster ovary cells 2.4-75 µg/ml 99.6 Positivea,d US National Toxicology
aberration Program (1987)
In vivo
Dominant lethal Mice Three doses NR Negativee Jorgenson et al. (1976)
mutation
NR, not reported
a With and without metabolic activation; similar results in two experiments with two or three replications each
b Without metabolic activation
c With metabolic activation
d 98% of cells with simple chromosomal and chromatid breaks; 2% with complex rearrangements; response not dose-related
e Males treated for seven weeks; then, one male mated with two females for eight weeks
Four Hartley guinea-pigs of each sex were exposed to quintozene
(purity, 98%) diluted in acetone on four sites at concentrations of
75, 50, 25, 10, 5.0, 2.5, 1.0, and 0.5% and scored for irritation at
24 and 48 h. On the basis of this pilot study, induction doses of 75
and 50%, which caused mild irritation, and challenge doses of 5, 2.5,
and 0.5%, which caused virtually no irritation, were selected for the
main study. In the main study, groups of 10 guinea-pigs of each sex
received applications on clipped, intact skin under occluded 25-mm
chambers containing 0.3 ml for 6 h; after 22-26 h, the animals were
depilated, and irritation was scored within 2 h as slight patchy
erythema, slight but confluent or moderate patchy erythema (grade 1),
moderate erythema (grade 2), or severe erythema (grade 3). Animals
were re-scored 22-26 h after the initial scoring. Induction involved
three 6-h exposures on the same site over two weeks, the initial
exposure being to 75% and the other two exposures to 50% quintozene in
acetone. No dermal reactions were seen after the first dose; slight
patchy erythema was elicited in one male after the second dose and in
two males and two females after the third dose. One male was
sacrificed after the initial dose due to non-compound-related
ill-health. Vehicle controls showed no dermal reactions.
For the primary challenge, chambers containing 5, 2.5, or 0.5%
quintozene were applied to each induced animal on previously untreated
sites. Reactions were observed at all three doses, the 5%
concentration inducing slight patchy erythema in eight males, grade 1
erythema in one male, and slight patchy erythema in nine females at
24 h; slight patchy erythema in seven males, grade 1 erythema in one
male, slight patchy erythema in nine females, and a grade 1 reaction
in one female were seen at 48 h. Comparable results were seen with 2.5
and 0.5%. The mean group scores were 0.5, 0.5, and 0.5 at 24 h and
0.5, 0.5, and 0.4 at 48 h with 5, 2.5, and 0.5%, respectively. Vehicle
controls given the same challenge doses under the same conditions had
slight patchy erythema at all concentrations, with mean group scores
of 0.3, 0.4, and 0.3 at 24 h and 0.3, 0.3, and 0.4 at 48 h.
Nine induced animals and five naive animals of each sex were
re-challenged at least six days after the initial challenge with 5 or
0.5% on previously unexposed sites. The mean group scores for induced
animals were 0.4 and 0.2 at 5% and 0.3 and 0.2 at 0.5% at 24 and 48 h,
respectively. Grade 1 reactions were seen in two guinea-pigs at 24 h
and one at 48 h with the 5% concentration and one at 24 and 48 h with
0.5% quintozene. The naive animals challenged with the 5% concen-
tration had mean irritation scores of 0.4 at 24 h (one animal with a
grade-1 score) and 0.2 at 48 h, and those exposed to 0.5% had scores
of 0.5 at 24 h and 0.2 at 48 h. The incidence and persistence of grade
1 scores in the challenged animals indicate that quintozene can induce
sensitization (Kreuzmann, 1988).
(ii) Potentiation
Combinations of quintozene at half or whole LD50 doses of
1740 mg/kg bw with terrozole (5-ethyoxy-3-trichloromethyl-
1,1,2,4-thiazole) at the LD50 dose of 1080 mg/kg bw or at LD2.3,
LD6.7, LD16, LD31, or one-half the LD50 were given to groups
of 10 male CD Charles River rats at 100 mg/kg bw quintozene and
appropriate amounts of terrazole. Mortality recorded over 14 days
showed an additive effect (Borzelleca et al., 1971).
(iii) Thyroid function
Groups of 75 male Charles River CD rats were fed diets containing
0, 20, or 6000 ppm quintozene (purity, 99.09%) for up to 90 days, and
groups of 15 rats per dose were sacrificed after 7, 14, 30, or 90
days; the remaining 15 rats were maintained for a further 90 days on
normal diet before sacrifice. The average intakes were 1 and 333 mg/kg
bw per day. Diets were prepared weekly (with acetone as the vehicle at
the 0 and 20 ppm levels) and analysed in duplicate during weeks 1-4,
8, and 12. The mean dietary concentrations were 81% of the nominal
(range, 72-98%) at 20 ppm and 91% (range, 86-99%) at 6000 ppm.
Mortality, moribundity, and clinical parameters were checked twice
daily and body weight and food intake weekly; blood samples were
collected from the abdominal aorta of non-fasted rats at sacrifice,
when complete macroscopic examinations were performed and the liver,
pituitary, and thyroid-parathyroid were weighed. These organs were
examined microscopically, as was the liver of all rats at terminal
sacrifice; the thymuses of a few animals were also examined.
Triiodothyronine, thyroxine, and thyroid-stimulating hormone were
measured in all rats at each dose and interval; reverse
triiodothyronine was measured in all rats at the end of treatment.
There were no deaths. The clinical signs, which occurred at low
incidence and were not dose-related, included malocclusion in seven
controls, six at 20 ppm, and five at 6000 ppm, and occasional hair
loss and redness of the eyes. Body weight and weight gain were reduced
in animals at 6000 ppm during treatment, but the weight gain exceeded
that of controls during the recovery period and by the time of
sacrifice exceeded that of controls. The main decrease in body-weight
gain occurred in week 1, when food intake was also significantly
decreased. At 6000 ppm, animals had reduced triiodothyronine and
thyroxine and increased thyroid-stimulating hormone values during most
of the treatment period, the effects being statistically significant
at most intervals. Reverse triiodothyronine was reduced at 90 days.
The triiodothyronine, thyroxine, and thyroid-stimulating hormone
values returned to normal during the recovery period. At 20 ppm, no
significant changes were seen, except for an increased thyroxine value
at seven days, which was considered to be a random effect. The
absolute weight of the pituitary was reduced at seven days in animals
at 6000 ppm; however, no other changes in the absolute or relative
weights of the pituitary occurred during the study, and no abnormal
microscopic changes were observed. The absolute weight of the liver
was nonsignificantly increased at 30 and 90 days in animals at this
dose but returned to normal after the recovery period; relative (to
body) weights were significantly increased at 14, 30, and 90 days, but
this effect may have been due to the decreased body weight seen at all
intervals except after the recovery period. The hepatocellular
hypertrophy seen after treatment for 90 days indicates that the
increased absolute liver weight at 30 and 90 days at 6000 ppm was
compound-related. The thyroid-parathyroid weights were not
significantly affected, except that the absolute weight was
significantly decreased at 30 days in animals at 6000 ppm and very
slightly decreased in those at 20 ppm. At 90 days, statistically
nonsignificant decreases were again seen at 20 and 6000 ppm (33 g in
controls versus 30 g at both 20 and 6000 ppm). After 90 days'
recovery, the thyroid-parathyroid weights were comparable to or
slightly greater than control values. Follicular epithelial
hypertrophy of the thyroid was observed at 6000 ppm, with trace (14
days), mild (30 days), or moderate (90 days) severity. At 20 ppm, mild
follicular epithelial hypertrophy was noted at 90 days. The decreased
thyroid weight may therefore reflect the colloid breakdown that occurs
in hypertrophic thyroid follicular cells, but this was not confirmed
microscopically. As the effects on the liver and thyroid were
reversible and only minimal effects were noted at 20 ppm, the NOAEL
was 20 ppm, equal to 1 mg/kg bw per day (Goldenthal, 1993).
(iv) Studies of metabolites
Four groups of 10 weanling female Sprague-Dawley rats were fed
diets containing pentachloronitrosobenzene, which is believed to be an
intermediate in the metabolic reduction of quintozene to pentachloro-
aniline, at doses of 0, 10, 50, or 100 ppm for 18 weeks. Rats were
weighed twice weekly, and food intake was monitored daily. Serum
alkaline phosphatase, aspartate and alanine aminotransferases, urinary
and liver porphyrins, liver glutathione, hepatic microsomal protein,
cytochrome P450, haemoglobin ferrihaemoglobin, packed cell volume, and
erythrocyte and total and differential leukocyte counts were
determined in all rats at the end of treatment. Organ:body weight
ratios were reported for liver, heart, spleen, and kidneys; the liver,
kidney, gastrointestinal tract, and urinary bladder were examined
histologically.
Body weights tended to be increased from week 2 onwards in all
treated groups, but statistical significance was not achieved and no
dose-effect relationship was seen. Food intake and food efficiency
were increased in all treated groups. The organ:body weight ratios
were comparable in all animals. A dose-effect relationship was
seen for ferrihaemoglobin levels, with values of 0, 0.5, 0.9, and
1.1 g/100 ml for controls and the three dose groups, respectively.
Serum alanine aminotransferase activity showed a dose-related,
statistically significant increase at all doses. Urinary porphyrin
patterns were similar over the last 24 h of the study. The hepatic
microsomal liver protein and hepatic and urinary porphyrin composition
was unchanged, but cytochrome P450 enzyme activity was significantly
increased at 100 ppm. At this dose, the concentrations of glutathione
and oxidized glutathione in liver were also increased. The liver was
normal histologically, but multilayered bladder epithelium of variable
thickness was seen, characterized by the presence of large superficial
cells. There was no sign of cellular proliferation (Renner et al.,
1981).
3. Observations in humans
Fifty human subjects received one 6.4-mm moistened cotton square
dipped into a 75% wettable powder formulation of quintozene (purity
unspecified) on the palmar surface of the forearm. The squares were
then covered with foil, which was taped into place. Exposure was for
48 h. No irritation was observed when the dressings were removed. When
the test was repeated two weeks later, four individuals showed
positive reactions, comprising erythema, itching, and (in three)
oedema and small vesicle formation. Of the individuals who showed no
reaction at the time the patch was removed, nine developed a delayed
reaction 8 h to several days after testing (Finnegan et al., 1958).
Workers who were still employed and had been exposed during the
production of quintozene for 2.5 years (one person), seven years (one
person), 8.5 years (one person), or 11 years (10 people) years had
undergone twice-yearly health checks, including monitoring of height,
weight, vision, blood pressure, X-ray (once per year only),
haematological parameters (erythrocyte count, haemoglobin,
haematocrit, total leukocyte count), an evaluation of subjective and
objective symptoms, life style, family history, and individual past
history, throughout the employment period. After 1989, hearing tests,
blood chemistry (serum aspartate and alanine aminotranferases,
gamma-glutamyl transpeptidase, alkaline phosphatase, partial
thromboplastin time, total cholesterol, and blood glucose),
(urinalysis (urea nitrogen, uric acid, glucose, protein, and occult
blood), and electrocardiography were added. No compound-related
effects were observed. A further group of nine individuals, with
exposure for three months (one person), one year (one person), two
years (one person), three years (one person), four years (one person),
seven years (one person), or 10 years (three people), who were engaged
in the manufacture of quintozene dust formulations were subjected to
similar examinations and showed no compound-related abnormalities. No
estimate of exposure or information on protective measures used (e.g.
clothing, gloves, and masks) was provided (Yamazaki, 1994).
Comments
Quintozene is absorbed relatively slowly after oral
administration to rats and mice, peak blood levels being observed
about 74 h after dosing. 14C from labelled quintozene is eliminated
mainly in the faeces of animals of each sex, although females excrete
greater amounts in the urine than males. In goats, low doses (30 mg/kg
bw) were excreted mainly in the urine, but faecal elimination
predominated at higher doses (100 mg/kg bw). Bile levels in ruminants
were high, and enterohepatic circulation is probable. Excretion was
more rapid and complete in chickens than in mammals. There was no
evidence of tissue accumulation in any species.
The biotransformation of quintozene was virtually complete. Two
general metabolic pathways were apparent, one involving reduction of
the nitro group to an amino group and subsequent formation of
secondary metabolites, and the second involving replacement of the
nitro group with a sulfur-containing group (e.g. a thio- or
methylthio-glucuronide or N-acetylcysteine group). Metabolic studies
in several species have been reported.
The acute oral LD50 of quintozene was > 1.5 g/kg bw in both
rats and dogs. The dermal LD50 was > 5 g/kg bw in rabbits, and the
4-h LC50 in rats was > 1.7 mg/litre air. Quintozene was mildly
irritating to the rabbit eye and not irritating to rabbit or human
skin, but it was a mild dermal sensitizing agent in guinea-pigs and
humans. WHO has classified quintozene as unlikely to present an acute
hazard in normal use.
In a single 13-week study in mice at dietary concentrations of 0,
1250 (males only), 2500, 5000, 10 000, 20 000, or 40 000 (females
only) ppm, 5-8% decreases in terminal body weight were seen at doses
> 10 000 ppm, and increased absolute liver weights were seen in
animals of each sex at all doses. All mice at 40 000 ppm died.
Four short-term studies in rats were available; in only one of
them was quintozene containing < 0.1% hexachlorobenzene used. Dietary
concentrations of 0, 50, 3000, or 6000 ppm administered for 13 weeks
resulted in an NOAEL of 50 ppm (equal to 3.1 mg/kg bw per day) on the
basis of a slight (6-8%) reduction in terminal body weight, increased
liver weight and decreased alanine aminotransferase activity at higher
doses. Five short-term studies in dogs (lasting four weeks to two
years) were available, in only one of which quintozene met the purity
specification. In this one-year study, at dietary concentrations of 0,
15, 150, or 1500 ppm, increased liver weight and hepatocellular
hypertrophy were seen at 1500 ppm, which were accompanied by increased
serum alkaline phosphatase and cholesterol levels and decreased
alanine aminotransferase activity and creatinine levels. The NOAEL was
150 ppm, equal to 4.2 mg/kg bw per day. A 70-day study in monkeys at a
dietary concentration of 2 ppm revealed no adverse effects. In a
21-day study in rabbits, with application to the skin of doses of 0,
30, 300, or 1000 mg/kg bw of quintozene for 6 h per day, significantly
decreased alanine aminotransferase activity was seen at 1000 mg/kg bw
per day. No irritation or other adverse effect was observed.
Four long-term (72-103-week) studies by oral administration in
mice were available, in only one of which did the compound meet the
contamination criterion. This 103-week study at dietary concentrations
of 0, 2500, or 5000 ppm showed decreased terminal body weights in
males (4% at 2500 ppm and 10% at 5000 ppm) and females (18 and 21%).
Since the body-weight losses in the females exceeded 10% at 2500 ppm
only after week 92 of the study, the NOAEL was 2500 ppm (equal to
360 mg/kg bw per day), although the interpretation was complicated by
Klebsiella infection. No evidence of carcinogenicity was seen. In a
study in which technical-grade quintozene (purity unspecified) was
applied dermally twice weekly (0.2 ml of 0.3% quintozene in acetone)
to mice for 12 weeks, followed by twice-weekly dermal applications of
croton oil for 20 weeks, no tumour induction was seen in the absence
of the promoting agent.
Of four long-term (two-year) studies in rats, only one met the
contaminant specification. In this study, at doses of 0, 20, 3000, or
6000 ppm, thyroid follicular adenomas were seen at 3000 and 6000 ppm
and possibly carcinomas at 6000 ppm (especially in males). Hepato-
cellular hypertrophy (mainly centrilobular), indistinguishable from
that arising from increases in drug-metabolizing enzyme activity, was
also seen at 3000 and 6000 ppm. The absolute and relative (to body and
brain) weights of the thyroid and parathyroid were increased at the
two highest doses in most instances. A special 90-day study on thyroid
hormones showed hepatocellular hypertrophy, which was only minimal,
after 90 days' exposure to 20 ppm. Complete reversibility was seen
after 90 days' withdrawal. Thyroid and parathyroid weights were
decreased only after 30 days' exposure. At 6000 ppm, triiodothyronine
and thyroxine levels were decreased and the levels of thyroid-
stimulating hormone were increased significantly at most intervals
during dosing; the level of reverse triiodothyronine was reduced at 90
days. All changes were reversible within 90 days after withdrawal. The
changes in thyroid hormone levels are consistent with increased
microsomal enzyme activity, resulting in increased excretion of
triiodothyronine and thyroxine and subsequent disruption of the
hypothalamic-pituitary-thyroid axis, causing increased circulating
levels of thyroid-stimulating hormone. Thyroid-stimulating hormone
initiates increased production of triiodothyronine and thyroxine, with
follicular hyperplasia in the thyroid, leading to adenoma and, more
rarely, carcinoma. Quintozene was thus carcinogenic. The induction of
thyroid tumours appears to be secondary to the disruption of thyroid
function, as indicated by the increased levels of thyroid-stimulating
hormone. The NOAEL for this effect was 20 ppm, equivalent to 1 mg/kg
bw per day.
One of two multigeneration studies in rats met the criterion for
the level of contamination. In this study, with dietary concentrations
of 0, 20, 3000, or 6000 ppm and two litters per generation for two
generations, dietary concentrations of 3000 and 6000 ppm reduced pup
and adult body weights. The NOAEL was 20 ppm, equivalent to 1 mg/kg bw
per day.
In studies of developmental toxicity, 13 mice (in part of a study
of the incidences of cleft palate and renal agenesis, subsequently
shown to be induced by hexachlorobenzene contamination) received doses
of 500 mg/kg bw per day by gavage on days 7-16 of gestation. Abortions
occurred, and a low incidence of cleft palate (2%) and a high
incidence (23%) of clubfoot were noted. Marked maternal toxicity was
seen. The administered material formed a solution, and not a
suspension as in all other studies. This study was not considered in
establishing the ADI. Of three studies of developmental toxicity in
rats, only one, with doses of 0, 30, 600, and 1200 mg/kg bw per day,
was acceptable. The NOAEL for maternal and developmental toxicity was
> 1200 mg/kg bw per day. There was no evidence of induction of
malformations. In rabbits given doses of 0, 12, 120, or 250 mg/kg bw
per day or 0, 6.2, or 120 mg/kg bw per day, maternal toxicity was seen
at 120 mg/kg bw per day and embryo- and fetotoxicity at 250 mg/kg bw
per day, including reduced pup weight, increased resorptions, and an
increased incidence of stillbirths; no increase in malformations was
seen at any dose.
In a number of studies (e.g. long-term studies in mice and rats
and the two-generation study of reproductive toxicity in rats), the
incidences of infection appeared to be slightly increased at high
doses. Although the reports of histopathological examinations do not
indicate effects on the immune system, it would be useful if studies
could be performed to investigate the potential for quintozene to
affect the immune system.
Quintozene has been tested in a range of studies for genotoxicity
in vitro. An acceptable assay is required to corroborate the
increase in chromosomal aberration frequency observed in cultured
cells.
An ADI of 0-0.01 mg/kg bw was allocated on the basis of the NOAEL
of 1 mg/kg bw per day in the two-year study, the study of thyroid
toxicity, and the two-generation study in rats, using a 100-fold
safety factor.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 2500 ppm, equal to 390 mg/kg bw per day (103-week study of
carcinogenicity)
Rat: 20 ppm, equivalent to 1 mg/kg bw per day (24-month study of
carcinogenicity, multigeneration study, and study of thyroid
toxicity) 1200 mg/kg bw per day (study of developmental
toxicity)
Rabbit: 12 mg/kg bw per day (maternal toxicity in study of
developmental toxicity) 120 mg/kg bw per day (embryo- and
fetotoxicity in a study of developmental toxicity)
Dog: 150 ppm, equivalent to 4.2 mg/kg bw per day (one-year study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw, for quintozene containing less than 0.1%
hexachlorobenzene
Studies that would provide information useful for continued evaluation
of the compound
1. Assays of genotoxicity in vivo to assess possible
chromosomal aberrations
2. Studies to assess the potential of quintozene to interfere
with the immune system
3. Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to quintozene
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Skin irritation, rabbit Not irritating
Eye irritation, rabbit Minimally irritating
Skin sensitization, guinea-pig Mild sensitizer
(modified Buehler)
Dermal irritation, human No irritation with 75% formulation
Skin sensitization, human Sensitization in 13/50 individuals
Inhalation, 4-h, toxicity, rat LC50 > 1.7 mg/litre air
Oral, toxicity, rat and dog > 1.5 g/kg bw
Medium-term (1-26 weeks) Dermal irritation, 21-day, rat No irritation; body weight, food intake, haematology,
clinical chemistry (except decreased alanine
aminotransferase), urinalysis, organ weights and liver
and kidney histology unaffected at doses
< 1000 mg/kg bw per day
Repeated oral, toxicity, 13-week, rat NOAEL = 3.1 mg/kg bw per day; minimal changes in
body and liver weight; reduced alanine
aminotransferase activity
Dietary, developmental toxicity, rabbit NOAEL = 12.5 mg/kg bw per day for maternal toxicity,
125 mg/kg bw per day for embryo- and fetotoxicity,
> 250 mg/kg bw per day for teratogenicity
Long-term (> one year) Dietary, toxicity, carcinogenicity, rat NOAEL = 1 mg/kg bw per day; follicular adenomas
of the thyroid and hepatocellular adenomas
References
Adamovics, J.A. (1980) The metabolic fate of pentachloronitrobenzen:
Pilot study. Unpublished report from Bio/dynamics Inc., Division
of Environmental and Analytical Chemistry, Olin Corporation
Report No. 79056. Submitted to WHO by Uniroyal Chemical Company,
Inc.
Adamovics, J.A. & O'Grodnick, J.S. (1978) Absorption and elimination
characteristics of 14C-labelled pentachloronitrobenzene in
rats-pilot study. Unpublished report from Bio/dynamics Inc.,
Division of Environmental and Analytical Chemistry, Olin
Corporation Report No. 77037. Submitted to WHO by Uniroyal
Chemical Company, Inc.
Aschbacher, P.W. & Feil, B.J. (1983) Metabolism of pentachloro-
nitrobenzene by goats and sheep. J. Agric. Food Chem.,
31, 1150-1158.
Betts, J.J., James, S.P. & Thorpe, W.V. (1955) The metabolism of
pentachloronitrobenzene and 2:3:4:6-tetrachloronitrobenzene and
the formation of mercapturic acids in the rabbit. Biochem. J.,
61, 611-617.
Borzelleca, J.F., Larson, P.S., Crawford, E.M., Hennigar, G.R., Jr,
Kuchar, E.J. & Klein, H.H. (1971) Toxicological and metabolic
studies on pentachloronitrobenzene. Toxicol. Appl. Pharmacol.,
18, 522-534.
Courtney, K.D. (1973) The effect of pentachloronitrobenzene on fetal
kidneys (Abstract 41). Toxicol. Appl. Pharmacol., 25, 455.
Courtney, K.D., Copeland, M.F. & Robbins, A. (1976) The effects of
pentachloronitrobenzene, HCB and related compounds on fetal
development. Toxicol. Appl. Pharmacol., 35, 239-256.
Daun, R.J. (1989) Pentachloronitrobenzene: Nature of the residue in
poultry-laying hens. Unpublished report from Hazelton
Laboratories America Inc., Project HLA 611-119. Submitted to WHO
by Uniroyal Chemical Company, Inc.
Daun, R.J. (1990) Pentachloronitrobenzene: Nature of the residue in
livestock-lactating goats. Unpublished report from Hazelton
Laboratories America Inc., Project HLA 611-118. Submitted to WHO
by Uniroyal Chemical Company, Inc.
Finnegan, J.K., Larson, P.S., Smith, R.B., Jr, Haag, H.B. & Hennigar,
G.R. (1958) Acute and chronic toxicity studies on pentachloro-
nitrobenzene. Arch. Int. Pharmacodyn., 115, 38-52.
Goldenthal, E.I. (1990) One year chronic dietary study in dogs.
Unpublished report No. 399-087 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Goldenthal, E.I. (1991). Two year dietary toxicity and oncogenicity
study in rats. Unpublished report No. 399-072 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Company, Inc.
Goldenthal, E.I. (1992). 21 day dermal toxicity study in rats.
Unpublished report No. 399-125 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Goldenthal, E.I. (1993). 90 day toxicity study in rats. Unpublished
report No. 399-122 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Company, Inc.
van der Heijden, C.A. & Til, H.P. (1974) Pentachloro-nitrobenzene
carcinogenicity study in mice. Unpublished report R4365 from
Central Institute for Nutrition and Food Research, Bilthoven,
Netherlands.
Hoescht AG (1964) Toxikologische Prüfung von Pentachlor-nitrobenzol.
3. Auf chronische Toxizität (90-Tage-Test) an Ratten. Unpublished
report prepared and submitted by Farbwerke Hoescht, AG.
Hilaski, R.J. (1994) EPA (FIFRA) Acute inhalation toxicity evaluation
on terraclor technical in rats. Unpublished report No. 399-146
from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Innes, J.R.M., Ulland, B.M., Valeric, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Pallota, A.J., Bates, R.R., Falk, H.L., Gart,
J.J., Klein, M., Mitchell, I. & Peters, J. (1969) Bioassay of
pesticides and industrial chemicals for tumorigenicity in mice. A
preliminary note. J. Natl Cancer Inst., 42, 1101-1114.
Johnson, D.E. (1989) Twenty-eight day pilot study in dogs. Unpublished
report No. 399-093 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Company, Inc.
Jordan, R.L. & Borzelleca, J.F. (1973) Teratogenic studies with
pentachloronitrobenzene in rats (Abstract 40). Toxicol. Appl.
Pharmacol., 25, 454.
Jorgenson, T.A., Rushbrook, C.J. & Newell, G.W. (1976). In vivo
mutagenesis investigations of ten commercial pesticides
(Abstract 41). Toxicol. Appl. Pharmacol., 37, 109.
Kavlock, R.J., Short, R.D., Jr & Chernoff, N. (1987) Further
evaluation of an in vivo teratology screen. Teratog. Carcinog.
Mutag., 7, 7-16.
Keller, K.A. (1988a) Developmental toxicity study in rats. Unpublished
report No. 399-068 from International Research and Development
Corporation, Mattawan, Michigan, USA. Submitted to WHO by
Uniroyal Chemical Company, Inc.
Keller, K.A. (1988b) Developmental toxicity study in New Zealand white
rabbits. Unpublished report No. 399-070 from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Uniroyal Chemical Company, Inc.
Kögel, W., Müller, F., Coulston, S. & Korte, F. (1979a)
Biotransformation of pentachloronitrobenzene-14C in rhesus
monkeys after single and chronic oral administration.
Chemosphere, 8, 97-105.
Kögel, W., Müller, F., Coulston, S. & Korte, F. (1979b) Fate and
effects of pentachloronitrobenzene in rhesus monkeys. J. Agric.
Food Chem., 27, 1181-1185.
Kreuzmann, J.J. (1988) Delayed contact hypersensitivity in guinea
pigs. Unpublished report No. 88-0052-21 from Hill Top Biolabs,
Inc. Submitted to WHO by Uniroyal Chemical Company, Inc.
Kuchar, E.J., Geenty, F.O., Griffith, W.P. & Thomas, R.J. (1969)
Analytical studies of metabolism of terraclor in beagle dogs,
rats and plants. J. Agric. Food Chem., 17, 1237-1242.
Larson, P.S. & Borzelleca, J.F. (1968) Toxicologic study on the
effects of adding terraclor to the diet of beagle dogs for a
period of two years. Unpublished report from the Department of
Pharmacology, Medical College of Virginia, Richmond, Virginia,
USA. Submitted to WHO by Uniroyal Chemical Company, Inc.
McGee, D.H. (1988) Thirteen week dietary toxicity study in rats with
PCNB (pentachloronitrobenzene). Unpublished report No. 399-071
from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Uniroyal Chemical
Company, Inc.
McManus, J.P. (1989) Metabolism of pentachloronitrobenzene in the
goat-metabolite identification. Unpublished report from Uniroyal
Chemical Company, Inc., Crop Protection Department, Chemistry
Section, R & D, Uniroyal Project 8761. Submitted to WHO Uniroyal
Chemical Company, Inc.
McManus, J.P. (1990) Metabolism of pentachloronitrobenzene in the
goat-identification of metabolites in muscle. Unpublished report
from Uniroyal Chemical Company, Inc., Crop Protection Department,
Chemistry Section, R & D, Uniroyal Project 8761a. Submitted to
WHO Uniroyal Chemical Company, Inc.
O'Grodnick, J.S. (1978) Characterization and identification of
14C-PCNB metabolites in rat urine and faeces. Unpublished
report from Bio/dynamics Inc., Division of Environmental and
Analytical Chemistry, Olin Corporation Report No. 77037-2.
Submitted to WHO by Uniroyal Chemical Company, Inc.
O'Grodnick, J.S. (1979) Identification of the polar metabolites of
14C-PCNB after oral administration to rats. Unpublished report
from Bio/dynamics Inc., Division of Environmental and Analytical
Chemistry, Olin Corporation Report No. 78054. Submitted to WHO by
Uniroyal Chemical Company, Inc.
O'Grodnick, J.S., Adamovics, J.A., Blake, S.H. & Wedig, J. (1981) The
metabolic fate of 14C-labelled pentachloronitrobenzene in
Osborne-Mendel rats. Chemosphere, 10, 67-72.
Parkins, M.D. (1990). Pentachloronitrobenzene: Nature of the residue
in poultry-laying hens. Unpublished report from Uniroyal Chemical
Company, Inc., Crop Protection Department, Chemistry Section, R.
& D., Project No. 8762. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Parkins, M.D. (1991) Pentachloronitrobenzene: Nature of the residue in
poultry-laying hens. Unpublished report from Uniroyal Chemical
Company, Inc., Crop Protection Department, Chemistry Section, R &
D, Project No. 8762. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Renner, G., Herforth, B., Gokel, J.M., Goerz, G. & Luderschmidt, C.
(1981) Subchronic toxicity studies on pentachloronitrosobenzene
(PCNO) in female rats. Ecotoxicol. Environ. Saf., 5, 281-290.
Schardein, J.L., York, R.G. & Laughlin, M.A. (1991) Two generation
reproduction study in rats. Unpublished report No. 399-086 from
International Research and Development Corporation, Mattawan,
Michigan, USA. Submitted to WHO by Uniroyal Chemical Company,
Inc.
Scholz, & Brunk, (1968) Kronischer orale toxizitätsprüfung von
pentachlornitrobenzol, 2-Jahres-Versuch an Hunden. Unpublished
report from Rewerke Hoescht AG. Submitted to WHO by Uniroyal
Chemical Company, Inc.
Searle, C.E. (1966) Tumor initiatory activity of some
chloromononitrobenzenes and other compounds. Cancer Res.,
26, 12-17.
Simmon, V.F., Poole, D.C. & Newell, G.W. (1976) In vitro mutagenic
studies of twenty pesticides (Abstract 42). Toxicol. Appl.
Pharmacol., 37, 109.
Sinkeldam, E.J., van der Heijden, C.A., de Groot, A.P. & Til, H.P.
(1974) Carcinogenicity study with pentrachloronitrobenzene in
rat. Unpublished report R442 from Central Institute for Nutrition
and Food Research, Bilthoven, Netherlands.
St John, L.E., Jr, Ammering, J.W., Wagner, D.G., Warner, R.G. & Lisk,
D.J. (1965) Fate of 4,6-dinitro-2-isobutylphenol, 2-chloro-4,6-
bis-(ethylamino)-S-triazine, and pentachloronitrobenzene in the
dairy cow. J. Dairy Sci., 48, 502-503.
US National Toxicology Program (1987) Toxicology and carcinogenesis
studies of pentachloronitrobenzene in B6C3F1 mice (feed studies)
(National Toxicology Program Technical Report Series No. 325),
Washington DC, US Department of Health and Human Services.
Warshawsky, L.D. (1994a) Acute oral toxicity study in rats.
Unpublished report No. 399-155 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Warshawsky, L.D. (1994b) Acute dermal toxicity study in rabbits.
Unpublished report No. 399-152 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Warshawsky, L.D. (1994c) Primary dermal irritation test in rabbits,
following a 4 hr exposure period. Unpublished report No. 399-153
from International Research and Development Corporation,
Mattawan, Michigan, USA. Submitted to WHO by Uniroyal Chemical
Company, Inc.
Warshawsky, L.D. (1994d). Primary eye irritation study in rabbits.
Unpublished report No. 399-154 from International Research and
Development Corporation, Mattawan, Michigan, USA. Submitted to
WHO by Uniroyal Chemical Company, Inc.
Wedig, J.H., Sperling, F. & Miller, R. (1976) Unpublished report of an
analysis of data submitted by the National Cancer Institute from
Olin Corporation.
Wit, S.L., van Esch, G.J. & van Genderen, H. (1960) Toxicity of some
chloronitrobenzene compounds (trichlorodinitrobenzene,
trichloronitrobenzene, tetrachloronitrobenzene and
pentachloronitrobenzene) to laboratory rats and residues found in
crops treated with these fungicides. In: Proceedings of the
Fourth International Congress on Crop Protection, Hamburg,
September 1957, Vol. 2, Braunschweig, pp. 1665-1668.
Yamazaki, Y. (1994) Health report. Unpublished report from Ohmuta
Factory, Mitsui Toatsu Chemicals Inc. Submitted to WHO by
Uniroyal Chemical Company, Inc.
