
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
PARAQUAT
IDENTITY
Chemical name
1, 1'-Dimethyl-4,4'-bipyridylium ion
1, 1-Dimethyl-4,4'-bipyridirium
Synonyms
(Dichloride)-PP 148, Gramoxone, Preeglone, Weedol Di(methyl
sulphate)-PP 910, Aerial Gramoxone
Structural formula
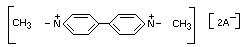 Other relevant chemical properties
Available as the di(methyl sulphate) or the dichloride which are white
crystalline solids; the di(methyl sulphate) is deliquescent. The salts
melt with decomposition in the region of 300°C. Both compounds are
stable in acid or neutral solutions and unstable in alkaline solution,
very soluble in water and decompose in ultraviolet light. They are
inactivated by inert clays and by anionic surfactants. Solutions of
paraquat become intensely purple on reduction, due to the formation of
a water-soluble, relatively stable free radical. The reduction is
autoxidizable, and solutions of the free radical absorb at 396 nm; the
unreduced form absorbs at 256 nm. Vigorous reduction gives tetrahydro
derivatives and ultimately the fully saturated base. The redox
potential (-446 mV) is independent of pH. Concentrated aqueous
solutions of paraquat corrode steel, tinplate, galvanized iron and
aluminium.
Purity
Technical, 90-95 percent
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption distribution and excretion
Paraquat is not readily absorbed from the gut (Daniel and Gage, 1966).
Following oral administration, peak concentrations of paraquat in the
blood are reached within one to six hours. Paraquat does not appear to
be selectively concentrated by any tissues in the body (Conning et
al., 1969).
Paraquat (14C-methyl labelled) administered to rats by an oral or
subcutaneous route was completely recovered in the excreta. Following
oral administration of 4-6 mg/kg body-weight of paraquat as the
dichloride salt, from 99-102 percent of the administered dose was
recovered in urine (6 percent) and faeces (93-95 per cent). Following
subcutaneous administration of 21-23 mg/kg body-weight (methyl
sulphate salt) from 85-112 per cent of the administered dose was
recovered in the urine and faeces, (in urine 73-96 percent and in
faeces 14-16 percent). Paraquat appeared to be poorly absorbed from
the gut. However, 30 percent of a dose of paraquat is present in rat
faeces as metabolic products. It has been suggested that paraquat may
be metabolized in vivo by microbial action within the gut. A small
proportion of these breakdown products may be absorbed from the gut
(Daniel and Gage, 1966).
Effect on enzymes and other biochemical parameters
Studies of the reaction of paraquat with liver cell preparation
suggest that cyclic reduction and reoxidation of the molecule may be a
primary mechanism in the effects noted. These effects include a slight
increase in oxygen uptake by mitochondria (possibly due to poor
penetration of the mitochondrial membrane), a stimulation of oxygen
uptake with NADH or ß-hydroxy-butyrate (but not succinate) as a
substrate in mitochondrial fragments inhibited with Amytal, and an
increase of NADPH oxidase in microsomes. Free radicals can be produced
from paraquat incubated anaerobically in the presence of NADPH and
microsomes derived from rat liver. Purified lipoamide dehydrogenase
from pig heart is able to reduce paraquat to the free radicals in the
presence of NADPH. Paraquat also increased the respiration of the
liver mitochondrial fragments. This action is attributed to the
activity of flavo-protein dehydrogenases. The property paraquat has of
undergoing cyclic reduction and oxidation suggests that it could
interfere in electron-transport processes, diverting electrons from
the system and reducing oxygen to water. The resting respiration of
mitochondria was almost unaffected by paraquat, probably because of
its inability to penetrate the mitochondrial membrane (Gage, 1968a).
Increased peroxidation occurs after paraquat treatment in plants and
has been shown to be associated with the peroxidation of microsomal
phospholipids in animals. An examination of lung lipids from rats
treated with paraquat revealed no diminution in the content of
unsaturated fatty acids. Preparations of rat liver either treated with
paraquat in vitro or taken from animals given paraquat in vivo,
showed no evidence of direct effect on fatty acid synthesis. Analysis
of lung lipids up to six days after poisoning with paraquat revealed
no significant changes in the composition of lung phospholipids. Large
doses of tocopheryl acetate, given to animals before but not after
exposure to paraquat, affords some protection against its toxic effect
(Conning et al., 1969).
In vitro binding studies have shown paraquat to bind to nucleic
acids and acidic mucopolysaccharides; the binding is lessened by
moderate salt concentrations. Paraquat in not bound by plasma protein
or tissue homogenates, but small amounts may be bound by macrophages.
In this instance, binding occurs in the cytoplasmic fraction (Conning
et al., 1969).
Manktelow (1967), in an attempt to explain the specific action of
paraquat on lung tissue, has proposed that it interferes with the
production of lung surfactant. Studies on the effects of expectorants
on pulmonary congestion in rats administered paraquat (ip, 10 and 20
mg/kg) confirmed this observation (Cambar and Aviado, 1970).
Paraquat was found to increase pulmonary resistance and moisture
content and to decrease pulmonary surfactant, pulmonary compliance and
respiratory minute volume. None of the expectorants examined had an
effect on all of the parameters investigated.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
Six groups of rats (ten males or ten females per group) were examined
for reproduction and teratogenic effects of paraquat at 0, 30 and 100
ppm in the diet fed to the parent (F0) generation only. Paraquat at
100 ppm was fed to F0 males only (mated to control females),F0
females only (mated to control males) and F0 males and F0 females
(mated to each other). The paraquat fed parental (F0) generation was
mated and produced three litters while exposed to paraquat. The F1
and F2 generations were not directly exposed to paraquat. The
long-term ingestion of paraquat did not influence growth or fertility
of the treated rats or of their offspring (Griffiths et al., 1966).
A single intraperitoneal injection to rats of paraquat (6.5 mg/kg
body-weight) on day 6 of gestation induced a high incidence of costal
cartilage malformation in the embryos. This defect was not noted when
injections were given on days 7-14 of gestation. A dose of 13 mg/kg on
days 6-14 of gestation did not give this defect, although in most
cases the dose was abortifacient (Khera and Whitta, 1968).
Special studies on acute toxicity of a metabolite
(N-methylisonicotinic acid)
The acute rat oral LD50 of an unneutralized solution of the
metabolite N-methylisonicotinic acid is between 2000 and 5000 mg/kg
body-weight (McElligott, 1966; Clark, 1965b). Neutralization of the
solution depressed the toxicity further (McElligott, 1966). The acute
rat ip LD50 of N-methylisonicotinic acid is approximately 500 mg/kg
body-weight (Clark, 1965b). Two of three male rats survived
intraperitoneal administration of 4000 mg/kg of neutralized
N-methylisonicotinic acid methyl sulphate (McElligott, 1966).
Special studies on subacute toxicity of metabolite
(N-methylisonicotinic acid)
Rat
One group of rats (seven males and seven females) was given
N-methylisonicotinic acid methyl sulphate by oral intubation for 21
days at a dose of 2 g/kg body-weight/day. Toxic effects were limited
to salivation, piloerection and occasional flaccidity. No effects on
blood chemistry and gross or microscopic pathology were observed
(McElligott, 1966).
Groups of rats (25 males and 25 females) were fed N-methylisonicotinic
acid methyl sulphate in the diet at concentrations of 0, 0.5, 2.0 and
4.0 percent for 90 days. Weight gains were reduced at the 4 percent
level in both males and females. Histopathological observations
include degenerative tubules in testes and degenerate cells in the
lumen of the tubules in the epididymus at the 4 percent level. No
abnormal effects were observed in mortality, body-weight, food
consumption, haematology and gross and microscopic pathology at the 2
percent level and below (Broadurst et al., 1966).
Rabbit
Three female rabbits treated dermally with N-methylisonicotinic acid
methyl sulphate powder at six alternate 24-hour periods displayed mild
desquamation but no systemic effects (McElligott, 1966).
Acute toxicity
LD50 levels of paraquat in different species are given in Table I.
After administration of acutely toxic doses to rats, all animals
displayed the same toxic signs; they appeared healthy for the first 24
hours, and then became subdued and lethargic; respiration became
progressively more difficult, and signs of anoxia were evident after
3-4 days; deaths occurred from 2-14 days after administration and
followed by inappetance and weight loss; lung congestion was evident
with varying degrees of consolidation. The pattern of mortality after
a single oral dose of paraquat indicates that there is a maximum death
rate in 2-5 days, with some deaths occurring at 10-12 days. Animals
dying in the second group had marked congestion of the lungs with an
oedematous fluid in many of the alveoli and excess macrophages in
others. Cellular proliferation around the bronchi and in the walls of
the alveoli was marked, and large tracts of the pulmonary tissue
contained a high proportion of mast cells, with consequent reduction
in the air-containing cavities.
TABLE I
Acute toxicity of paraquat in different species
LD50
(mg ion/kg
Animal Route Salt Form body-weight) Reference
Chicken oral chloride 262 Swan, 1959
Rat oral chloride 120-157 Swan, 1959
Clark, 1965a
Rat oral methylsulfate 100-110 Gaines, 1969
Rat oral methylsulfate 127-141 Swan, 1959
Clark, 1965a
Rat dermal methylsulfate 80-90 Gaines, 1969
Rat ip chloride 19 Clark, 1965a
Rat ip methylsulfate 16 Clark, 1965a
Rat sc methylsulfate 24 Swan, 1959
Rat (4 hr) chloride 6.4 mg/l Palazzolo et al.,
inhalation (LC50) 1964
Guinea Pig oral chloride 30 Swan, 1959
Rabbit oral methylsulfate 126 Swan, 1959
Rabbit dermal chloride 240 McElligott, 1965
Rabbit ip chloride 18.2 McElligott, 1965
Cat oral chloride 35 Swan, 1959
Sheep oral chloride 100 Walley, 1964
Cow oral chloride 50-75 Walley, 1964
In two tests, instillation of approximately 6-10 mg of paraquat
(dichloride or dimethyl sulphate salts) into the conjunctival sac of
rabbits resulted in temporary slight lachrymation and conjunctival
congestion one to three days after dozing. No permanent damage was
noted, although in some cases recovery was slow. (McElligott, 1965;
Clerk et al., 1966; Swan, 1959).
Short-term studies
Rabbit
Multiple percutaneous administration of paraquat to rabbits at doses
from 2.8 to 116 mg/kg body-weight daily for 20 days resulted in no
effects seen at 2.8 mg/kg/per day. At 7.3 mg/kg, all animals survived,
but there was lung congestion with consolidation of the alveoli. Two
of three animals dosed at 14.5 mg/kg died within 20 days. When the
site of application was covered by an occlusive dressing, amounts as
low as 2.8 mg/kg (1.6 mg cation kg) resulted in moist red skin with
sloughing of the skin (McElligott, 1965).
Multiple percutaneous non-occluded application of paraquat dichloride
to five male and five female rabbits at doses of 0, 0.6, 1.5 and 3.0
mg/kg body-weight/day for 20 days resulted in one male death at 1.5
mg/kg at 26 days and one male death at 3 mg/kg at 14 days. Signs of
inactivity, muscular weakness, lassitude, nasal discharge and
salivation were evident at the highest dose after 5-6 days. Local skin
reactions were evident at all doses, with recovery occurring only at
the 0.6 mg/kg level. No adverse affects were noted with regard to
body-weight or gross and microscopic examination of surviving animals.
Microscopic examinations of the dead animals revealed significant lung
damage (Palazzolo and Calandra, 1965).
Dog
Five groups of dogs (from two to four males and females per group)
were fed paraquat in their diet for 26 to 27 months at doses of 0, 10,
50, 125 and 250 ppm. Dietary feeding of paraquat dichloride at 250 ppm
over a two month period resulted in body-weight depression, depressed
food intake respiratory distress and death, with gross and
histopathologic changes in the heart, kidneys, brain and lung. At 125
ppm over a 27-month period, the following signs were evident: death;
depressed food intake body-weight; respiratory distress; and growths
and microscopic changes in the lungs. Changes in organ-weights and
decrease weights of spleen, brain and testes. Organ to body-weight
ratio increases were noted with liver, heart, brain, thyroid and
adrenal gland, while the spleen to body-weight ratio was decreased. No
effects were noted at 50 ppm (34 ppm of paraquat ion) (Cervenka et
al., 1964).
One group of dogs (three males and three females) were fed paraquat
dichloride 75 ppm in the diet for two years. Slight alterations were
seen in the lungs upon gross and microscopic examination which were
believed to be due to paraquat. No adverse effects were noted on
body-weight, food consumption, survival, behaviour, haematological
studies, blood chemistry, urinalysis, liver function tests,
organ-weights and ratios and growths and microscopic examination of
tissues other than lungs. The level of paraquat dichloride causing no
toxicological effect on the dog was 50 ppm (34 ppm paraquat cation)
(Baran and Calandra, 1965).
Grazing animals
Multiple daily oral administration to sheep at 20 mg/kg body-weight
for five days resulted in death of all animals within two weeks.
Multiple daily oral administration at 10 mg/kg for five days killed
one of six sheep in 26 days, while 5 mg/kg for fourteen days resulted
in listless animals; recovery was very slow (Walley, 1964).
Multiple daily oral administration to cattle of 20 mg/kg body-weight
for four days resulted in death within one week. Levels of 10 and 5
mg/kg body-weight orally for five and fourteen days, respectively,
resulted in no deaths, but animals were listless and unhealthy;
recovery was slow (Walley, 1964).
Five groups of two sheep each and three groups of one calf each were
exposed for four weeks to levels of 0, 1, 5, 10 or 20 ppm and 0.5 or
20 ppm of paraquat, respectively, in their drinking water. No adverse
toxicological effects wore noted after one month (Sarfaty, 1963).
Cattle suffered no toxic effects over a four-week period when grazed
on pasture immediately after it had been sprayed with paraquat. Horses
showed definite ill effects, including local lesions of the mouth and
increased mucus secretions (Calderbank et al., 1968).
Ewe lambs were grazed in pastures 30 days after treatment with 1-2
pounds of paraquat per acre with no effect on their growth or general
well being (Torell and Kay, 1964).
Subacute inhalation studies
One group of dogs (one male and one female), guinea pigs (five males
and five females) and rats (five males and five females) were exposed
to an aerosol of paraquat dichloride at a concentration of 0.1 mg/1,
six hours per day, five days per week, for three weeks. Growth
depression was evident among the guinea pigs and rats, and the female
dog lost weight. No effects wore noted with regard to untoward
behavioural reaction, haematology, blood chemistry and gross or
microscopic alterations of tissues (Palazzolo et al., 1965). Repeated
daily 6-hour exposures to rats of paraquat aerosols over a three-week
period produced signs of lung irritation, but no deaths, at 0.4 mg/l
(Gage, 1968b).
Long-term studies
Rat
Four groups of rats (30 males and 30 females, 60 of each sex were
controls) were fed diets containing paraquat dichloride at levels of
0, 50, 125 and 250 ppm for two years. No adverse effects were seen at
any level tested on growth, survival, behaviour, tumour incidence,
haematologic studies, urinalysis, organ weights, ratios of organ to
brain or organ to body-weight and gross pathologic examination.
Microscopic examination of tissues and organs at 0 and 250 ppm (170
ppm of paraquat cation) revealed no adverse effects (Kohn at al.,
1964).
OBSERVATIONS IN MAN
The hazard of paraquat to man has been associated with two general
areas: accidental ingestion and dermal contamination. Accidental oral
ingestion of paraquat in small quantities in man has generally
resulted in death (sometimes delayed). In almost all cases where death
occurred severe lung damage with proliferation of the alveolar walls
was evident. These lesions were occasionally accompanied by renal
failure as evidenced at autopsy by gross damage to the kidneys
(Bullivant, 1966; Fennelly et al., 1968; Goulding, 1968; Campbell,
1968; Duffy and O'Sullivan, 1968; Tilling, 1968; Matthew et al., 1968
and Cowie and Kahn, 1968). In two accidental poisoning cases, recovery
was complete (McKean, 1968; Lloyd, 1969).
One suicide case from subcutaneous injection of paraquat resulted in
delayed death in 17 to 18 days, with severe proliferation of the
epithelium of the lung. Renal macroscopic or microscopic pathological
changes were not evident (Almog and Tal, 1967; Herczeg and Reit,
1968).
Dermal contamination had been shown to result in damage and
discolouration of the fingernails. The damage included softening of
the nail at the base, with occasional loss of the nail. It appeared
that the damage was local because of the asymmetry of the lesion and
because the toenails were not affected (Samman and Johnston, 1969).
Following accidental instillation of paraquat into the eye, symptoms
of irritation and inflammation of the conjunctiva increase and large
areas of the conjunctiva and cornea may be shed. With treatment,
recovery is slow (Calderbank, 1968; Cant and Lewis, 1968).
COMMENT
Paraquat is not readily absorbed from the gastrointestinal tract.
After oral administration, it is excreted primarily in the faeces.
After subcutaneous administration, it is primarily excreted in urine.
The metabolic products formed by micro-organisms in the gut appear to
be more readily absorbed than paraquat. No information is available on
the toxicity of these metabolites formed by the gut flora. The soil
metabolite N-methylisonicotinic acid is of a low order of toxicity as
compared with the parent compound when tested in rats.
From the available experimental data on animals and clinical
experiences with man, it is evident that paraquat causes irreversible
proliferative changes in lung tissue. The level of 75 ppm when fed to
dogs over a two-year interval may be considered as a threshold dose on
the basis of this pulmonary effect. An adverse effect on a long-term
study in rats could not be demonstrated with levels up to 250 ppm.
These results indicate that the rat is an insensitive species and,
while the dog in affected by paraquat, its susceptibility may be lower
than man. Man must be considered more sensitive than other species
thus far examined. In man, the delayed occurrence of lesions in the
lung, renal failure and a local effect on corneal epithelium, nasal
mucosa, skin and fingernails suggest that paraquat my be considered a
pesticide whose handling be restricted to trained professional
personnel.
In addition, studies on prophylaxis and treatment of the toxic effects
of paraquat were considered to be urgently needed; reproduction
studies are limited to one species, the rat. For the above-mentioned
reasons, the Committee considered that it was possible to establish a
temporary acceptably daily intake for man, based upon the no-effect
level in the dog.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg body-weight/day
(corresponds to 9.1 mg paraquat ion/mg body-weight/day)
Dog: 50 ppm in the diet, equivalent to 1.25 mg/kg body-weight/day
(corresponds to 0.91 mg paraquat ion/kg body-weight/day)
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE IN MAN
0-0.001 mg/kg body-weight as paraquat dichloride (0-0.0007 mg/kg
body-weight expressed as paraquat ion)
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Herbicide and desiccant; rapidly absorbed by green plants but
inactivated on contact with soil. Widely used for pre-crop and
post-crop-emergence weed control, plantation weed control, aquatic
weed control, pasture renovation, pre-harvest desiccation of hops,
cotton defoliation and potato haulm and sugar cane desiccation.
FATE OF RESIDUES
In animals
The fate of 14C-paraquat administered orally to cattle was
investigated by Stevens and Watley (1966). They found that the bulk of
radioactivity was excreted in the faeces and amounts <5% were
present, largely as breakdown products, in the urine.
A group of three cows, orally treated with a single dose of paraquat
at 8 mg/kg, excreted between 0.003 and 0.008 percent of the dose in
the milk and 0.24 percent of the dose in the urine within the
seven-day testing interval (Stevens et al.,1966; Stevens et al.,
1964). The amount of ingested material in the milk was the same
irrespective of position of a radioactive label indicating that
paraquat itself or a metabolite(s) containing methyl groups and intact
ring structures was present.
Two calves grazed for three or seven days on pasture containing
residues of 300-400 ppm paraquat were found to have significant
residues of paraquat only in the gut and stomach tissues. The kidney
contained the highest tissue residues of 0.15 ppm, with traces found
in lungs, heart and liver (Litchfield, 1969).
In plants
From observations made with 14C-labelled material, paraquat is
transported to a slightly greater extent than diquat from the leaves
of potato plants to the tubers (Slade and Bell, 1966). Coates et al.
(1966) found appreciable movement in wheat, even in the roots. Slade
(1966) studied the degradation of labelled paraquat on tomato, broad
beans and maize plants; the degradation was found to be non-enzymic,
but could be attributed to sunlight. Using potato plants, experiments
showed that even if metabolism had occurred in the plant, no
degradation products were transported to the tubers.
In soil
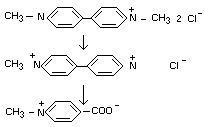
Paraquat has been shown to be degraded by soil microorganisms
(Funderburk and Bozarth, 1967) to demethylated paraquat
(1-methyl-4,4'-dipyridinium ion) and another compound characterized as
the 1-methyl-4-carboxy-pyridinium ion (N-methylisonicotinic acid).
The following degradation pathway by bacteria - demethylation of
parent molecule and ring cleavage of one of the heterocyclic rings to
eventually forms the carboxylated N-methyl-pyridinium ion.
Other relevant chemical properties
Available as the di(methyl sulphate) or the dichloride which are white
crystalline solids; the di(methyl sulphate) is deliquescent. The salts
melt with decomposition in the region of 300°C. Both compounds are
stable in acid or neutral solutions and unstable in alkaline solution,
very soluble in water and decompose in ultraviolet light. They are
inactivated by inert clays and by anionic surfactants. Solutions of
paraquat become intensely purple on reduction, due to the formation of
a water-soluble, relatively stable free radical. The reduction is
autoxidizable, and solutions of the free radical absorb at 396 nm; the
unreduced form absorbs at 256 nm. Vigorous reduction gives tetrahydro
derivatives and ultimately the fully saturated base. The redox
potential (-446 mV) is independent of pH. Concentrated aqueous
solutions of paraquat corrode steel, tinplate, galvanized iron and
aluminium.
Purity
Technical, 90-95 percent
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption distribution and excretion
Paraquat is not readily absorbed from the gut (Daniel and Gage, 1966).
Following oral administration, peak concentrations of paraquat in the
blood are reached within one to six hours. Paraquat does not appear to
be selectively concentrated by any tissues in the body (Conning et
al., 1969).
Paraquat (14C-methyl labelled) administered to rats by an oral or
subcutaneous route was completely recovered in the excreta. Following
oral administration of 4-6 mg/kg body-weight of paraquat as the
dichloride salt, from 99-102 percent of the administered dose was
recovered in urine (6 percent) and faeces (93-95 per cent). Following
subcutaneous administration of 21-23 mg/kg body-weight (methyl
sulphate salt) from 85-112 per cent of the administered dose was
recovered in the urine and faeces, (in urine 73-96 percent and in
faeces 14-16 percent). Paraquat appeared to be poorly absorbed from
the gut. However, 30 percent of a dose of paraquat is present in rat
faeces as metabolic products. It has been suggested that paraquat may
be metabolized in vivo by microbial action within the gut. A small
proportion of these breakdown products may be absorbed from the gut
(Daniel and Gage, 1966).
Effect on enzymes and other biochemical parameters
Studies of the reaction of paraquat with liver cell preparation
suggest that cyclic reduction and reoxidation of the molecule may be a
primary mechanism in the effects noted. These effects include a slight
increase in oxygen uptake by mitochondria (possibly due to poor
penetration of the mitochondrial membrane), a stimulation of oxygen
uptake with NADH or ß-hydroxy-butyrate (but not succinate) as a
substrate in mitochondrial fragments inhibited with Amytal, and an
increase of NADPH oxidase in microsomes. Free radicals can be produced
from paraquat incubated anaerobically in the presence of NADPH and
microsomes derived from rat liver. Purified lipoamide dehydrogenase
from pig heart is able to reduce paraquat to the free radicals in the
presence of NADPH. Paraquat also increased the respiration of the
liver mitochondrial fragments. This action is attributed to the
activity of flavo-protein dehydrogenases. The property paraquat has of
undergoing cyclic reduction and oxidation suggests that it could
interfere in electron-transport processes, diverting electrons from
the system and reducing oxygen to water. The resting respiration of
mitochondria was almost unaffected by paraquat, probably because of
its inability to penetrate the mitochondrial membrane (Gage, 1968a).
Increased peroxidation occurs after paraquat treatment in plants and
has been shown to be associated with the peroxidation of microsomal
phospholipids in animals. An examination of lung lipids from rats
treated with paraquat revealed no diminution in the content of
unsaturated fatty acids. Preparations of rat liver either treated with
paraquat in vitro or taken from animals given paraquat in vivo,
showed no evidence of direct effect on fatty acid synthesis. Analysis
of lung lipids up to six days after poisoning with paraquat revealed
no significant changes in the composition of lung phospholipids. Large
doses of tocopheryl acetate, given to animals before but not after
exposure to paraquat, affords some protection against its toxic effect
(Conning et al., 1969).
In vitro binding studies have shown paraquat to bind to nucleic
acids and acidic mucopolysaccharides; the binding is lessened by
moderate salt concentrations. Paraquat in not bound by plasma protein
or tissue homogenates, but small amounts may be bound by macrophages.
In this instance, binding occurs in the cytoplasmic fraction (Conning
et al., 1969).
Manktelow (1967), in an attempt to explain the specific action of
paraquat on lung tissue, has proposed that it interferes with the
production of lung surfactant. Studies on the effects of expectorants
on pulmonary congestion in rats administered paraquat (ip, 10 and 20
mg/kg) confirmed this observation (Cambar and Aviado, 1970).
Paraquat was found to increase pulmonary resistance and moisture
content and to decrease pulmonary surfactant, pulmonary compliance and
respiratory minute volume. None of the expectorants examined had an
effect on all of the parameters investigated.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
Six groups of rats (ten males or ten females per group) were examined
for reproduction and teratogenic effects of paraquat at 0, 30 and 100
ppm in the diet fed to the parent (F0) generation only. Paraquat at
100 ppm was fed to F0 males only (mated to control females),F0
females only (mated to control males) and F0 males and F0 females
(mated to each other). The paraquat fed parental (F0) generation was
mated and produced three litters while exposed to paraquat. The F1
and F2 generations were not directly exposed to paraquat. The
long-term ingestion of paraquat did not influence growth or fertility
of the treated rats or of their offspring (Griffiths et al., 1966).
A single intraperitoneal injection to rats of paraquat (6.5 mg/kg
body-weight) on day 6 of gestation induced a high incidence of costal
cartilage malformation in the embryos. This defect was not noted when
injections were given on days 7-14 of gestation. A dose of 13 mg/kg on
days 6-14 of gestation did not give this defect, although in most
cases the dose was abortifacient (Khera and Whitta, 1968).
Special studies on acute toxicity of a metabolite
(N-methylisonicotinic acid)
The acute rat oral LD50 of an unneutralized solution of the
metabolite N-methylisonicotinic acid is between 2000 and 5000 mg/kg
body-weight (McElligott, 1966; Clark, 1965b). Neutralization of the
solution depressed the toxicity further (McElligott, 1966). The acute
rat ip LD50 of N-methylisonicotinic acid is approximately 500 mg/kg
body-weight (Clark, 1965b). Two of three male rats survived
intraperitoneal administration of 4000 mg/kg of neutralized
N-methylisonicotinic acid methyl sulphate (McElligott, 1966).
Special studies on subacute toxicity of metabolite
(N-methylisonicotinic acid)
Rat
One group of rats (seven males and seven females) was given
N-methylisonicotinic acid methyl sulphate by oral intubation for 21
days at a dose of 2 g/kg body-weight/day. Toxic effects were limited
to salivation, piloerection and occasional flaccidity. No effects on
blood chemistry and gross or microscopic pathology were observed
(McElligott, 1966).
Groups of rats (25 males and 25 females) were fed N-methylisonicotinic
acid methyl sulphate in the diet at concentrations of 0, 0.5, 2.0 and
4.0 percent for 90 days. Weight gains were reduced at the 4 percent
level in both males and females. Histopathological observations
include degenerative tubules in testes and degenerate cells in the
lumen of the tubules in the epididymus at the 4 percent level. No
abnormal effects were observed in mortality, body-weight, food
consumption, haematology and gross and microscopic pathology at the 2
percent level and below (Broadurst et al., 1966).
Rabbit
Three female rabbits treated dermally with N-methylisonicotinic acid
methyl sulphate powder at six alternate 24-hour periods displayed mild
desquamation but no systemic effects (McElligott, 1966).
Acute toxicity
LD50 levels of paraquat in different species are given in Table I.
After administration of acutely toxic doses to rats, all animals
displayed the same toxic signs; they appeared healthy for the first 24
hours, and then became subdued and lethargic; respiration became
progressively more difficult, and signs of anoxia were evident after
3-4 days; deaths occurred from 2-14 days after administration and
followed by inappetance and weight loss; lung congestion was evident
with varying degrees of consolidation. The pattern of mortality after
a single oral dose of paraquat indicates that there is a maximum death
rate in 2-5 days, with some deaths occurring at 10-12 days. Animals
dying in the second group had marked congestion of the lungs with an
oedematous fluid in many of the alveoli and excess macrophages in
others. Cellular proliferation around the bronchi and in the walls of
the alveoli was marked, and large tracts of the pulmonary tissue
contained a high proportion of mast cells, with consequent reduction
in the air-containing cavities.
TABLE I
Acute toxicity of paraquat in different species
LD50
(mg ion/kg
Animal Route Salt Form body-weight) Reference
Chicken oral chloride 262 Swan, 1959
Rat oral chloride 120-157 Swan, 1959
Clark, 1965a
Rat oral methylsulfate 100-110 Gaines, 1969
Rat oral methylsulfate 127-141 Swan, 1959
Clark, 1965a
Rat dermal methylsulfate 80-90 Gaines, 1969
Rat ip chloride 19 Clark, 1965a
Rat ip methylsulfate 16 Clark, 1965a
Rat sc methylsulfate 24 Swan, 1959
Rat (4 hr) chloride 6.4 mg/l Palazzolo et al.,
inhalation (LC50) 1964
Guinea Pig oral chloride 30 Swan, 1959
Rabbit oral methylsulfate 126 Swan, 1959
Rabbit dermal chloride 240 McElligott, 1965
Rabbit ip chloride 18.2 McElligott, 1965
Cat oral chloride 35 Swan, 1959
Sheep oral chloride 100 Walley, 1964
Cow oral chloride 50-75 Walley, 1964
In two tests, instillation of approximately 6-10 mg of paraquat
(dichloride or dimethyl sulphate salts) into the conjunctival sac of
rabbits resulted in temporary slight lachrymation and conjunctival
congestion one to three days after dozing. No permanent damage was
noted, although in some cases recovery was slow. (McElligott, 1965;
Clerk et al., 1966; Swan, 1959).
Short-term studies
Rabbit
Multiple percutaneous administration of paraquat to rabbits at doses
from 2.8 to 116 mg/kg body-weight daily for 20 days resulted in no
effects seen at 2.8 mg/kg/per day. At 7.3 mg/kg, all animals survived,
but there was lung congestion with consolidation of the alveoli. Two
of three animals dosed at 14.5 mg/kg died within 20 days. When the
site of application was covered by an occlusive dressing, amounts as
low as 2.8 mg/kg (1.6 mg cation kg) resulted in moist red skin with
sloughing of the skin (McElligott, 1965).
Multiple percutaneous non-occluded application of paraquat dichloride
to five male and five female rabbits at doses of 0, 0.6, 1.5 and 3.0
mg/kg body-weight/day for 20 days resulted in one male death at 1.5
mg/kg at 26 days and one male death at 3 mg/kg at 14 days. Signs of
inactivity, muscular weakness, lassitude, nasal discharge and
salivation were evident at the highest dose after 5-6 days. Local skin
reactions were evident at all doses, with recovery occurring only at
the 0.6 mg/kg level. No adverse affects were noted with regard to
body-weight or gross and microscopic examination of surviving animals.
Microscopic examinations of the dead animals revealed significant lung
damage (Palazzolo and Calandra, 1965).
Dog
Five groups of dogs (from two to four males and females per group)
were fed paraquat in their diet for 26 to 27 months at doses of 0, 10,
50, 125 and 250 ppm. Dietary feeding of paraquat dichloride at 250 ppm
over a two month period resulted in body-weight depression, depressed
food intake respiratory distress and death, with gross and
histopathologic changes in the heart, kidneys, brain and lung. At 125
ppm over a 27-month period, the following signs were evident: death;
depressed food intake body-weight; respiratory distress; and growths
and microscopic changes in the lungs. Changes in organ-weights and
decrease weights of spleen, brain and testes. Organ to body-weight
ratio increases were noted with liver, heart, brain, thyroid and
adrenal gland, while the spleen to body-weight ratio was decreased. No
effects were noted at 50 ppm (34 ppm of paraquat ion) (Cervenka et
al., 1964).
One group of dogs (three males and three females) were fed paraquat
dichloride 75 ppm in the diet for two years. Slight alterations were
seen in the lungs upon gross and microscopic examination which were
believed to be due to paraquat. No adverse effects were noted on
body-weight, food consumption, survival, behaviour, haematological
studies, blood chemistry, urinalysis, liver function tests,
organ-weights and ratios and growths and microscopic examination of
tissues other than lungs. The level of paraquat dichloride causing no
toxicological effect on the dog was 50 ppm (34 ppm paraquat cation)
(Baran and Calandra, 1965).
Grazing animals
Multiple daily oral administration to sheep at 20 mg/kg body-weight
for five days resulted in death of all animals within two weeks.
Multiple daily oral administration at 10 mg/kg for five days killed
one of six sheep in 26 days, while 5 mg/kg for fourteen days resulted
in listless animals; recovery was very slow (Walley, 1964).
Multiple daily oral administration to cattle of 20 mg/kg body-weight
for four days resulted in death within one week. Levels of 10 and 5
mg/kg body-weight orally for five and fourteen days, respectively,
resulted in no deaths, but animals were listless and unhealthy;
recovery was slow (Walley, 1964).
Five groups of two sheep each and three groups of one calf each were
exposed for four weeks to levels of 0, 1, 5, 10 or 20 ppm and 0.5 or
20 ppm of paraquat, respectively, in their drinking water. No adverse
toxicological effects wore noted after one month (Sarfaty, 1963).
Cattle suffered no toxic effects over a four-week period when grazed
on pasture immediately after it had been sprayed with paraquat. Horses
showed definite ill effects, including local lesions of the mouth and
increased mucus secretions (Calderbank et al., 1968).
Ewe lambs were grazed in pastures 30 days after treatment with 1-2
pounds of paraquat per acre with no effect on their growth or general
well being (Torell and Kay, 1964).
Subacute inhalation studies
One group of dogs (one male and one female), guinea pigs (five males
and five females) and rats (five males and five females) were exposed
to an aerosol of paraquat dichloride at a concentration of 0.1 mg/1,
six hours per day, five days per week, for three weeks. Growth
depression was evident among the guinea pigs and rats, and the female
dog lost weight. No effects wore noted with regard to untoward
behavioural reaction, haematology, blood chemistry and gross or
microscopic alterations of tissues (Palazzolo et al., 1965). Repeated
daily 6-hour exposures to rats of paraquat aerosols over a three-week
period produced signs of lung irritation, but no deaths, at 0.4 mg/l
(Gage, 1968b).
Long-term studies
Rat
Four groups of rats (30 males and 30 females, 60 of each sex were
controls) were fed diets containing paraquat dichloride at levels of
0, 50, 125 and 250 ppm for two years. No adverse effects were seen at
any level tested on growth, survival, behaviour, tumour incidence,
haematologic studies, urinalysis, organ weights, ratios of organ to
brain or organ to body-weight and gross pathologic examination.
Microscopic examination of tissues and organs at 0 and 250 ppm (170
ppm of paraquat cation) revealed no adverse effects (Kohn at al.,
1964).
OBSERVATIONS IN MAN
The hazard of paraquat to man has been associated with two general
areas: accidental ingestion and dermal contamination. Accidental oral
ingestion of paraquat in small quantities in man has generally
resulted in death (sometimes delayed). In almost all cases where death
occurred severe lung damage with proliferation of the alveolar walls
was evident. These lesions were occasionally accompanied by renal
failure as evidenced at autopsy by gross damage to the kidneys
(Bullivant, 1966; Fennelly et al., 1968; Goulding, 1968; Campbell,
1968; Duffy and O'Sullivan, 1968; Tilling, 1968; Matthew et al., 1968
and Cowie and Kahn, 1968). In two accidental poisoning cases, recovery
was complete (McKean, 1968; Lloyd, 1969).
One suicide case from subcutaneous injection of paraquat resulted in
delayed death in 17 to 18 days, with severe proliferation of the
epithelium of the lung. Renal macroscopic or microscopic pathological
changes were not evident (Almog and Tal, 1967; Herczeg and Reit,
1968).
Dermal contamination had been shown to result in damage and
discolouration of the fingernails. The damage included softening of
the nail at the base, with occasional loss of the nail. It appeared
that the damage was local because of the asymmetry of the lesion and
because the toenails were not affected (Samman and Johnston, 1969).
Following accidental instillation of paraquat into the eye, symptoms
of irritation and inflammation of the conjunctiva increase and large
areas of the conjunctiva and cornea may be shed. With treatment,
recovery is slow (Calderbank, 1968; Cant and Lewis, 1968).
COMMENT
Paraquat is not readily absorbed from the gastrointestinal tract.
After oral administration, it is excreted primarily in the faeces.
After subcutaneous administration, it is primarily excreted in urine.
The metabolic products formed by micro-organisms in the gut appear to
be more readily absorbed than paraquat. No information is available on
the toxicity of these metabolites formed by the gut flora. The soil
metabolite N-methylisonicotinic acid is of a low order of toxicity as
compared with the parent compound when tested in rats.
From the available experimental data on animals and clinical
experiences with man, it is evident that paraquat causes irreversible
proliferative changes in lung tissue. The level of 75 ppm when fed to
dogs over a two-year interval may be considered as a threshold dose on
the basis of this pulmonary effect. An adverse effect on a long-term
study in rats could not be demonstrated with levels up to 250 ppm.
These results indicate that the rat is an insensitive species and,
while the dog in affected by paraquat, its susceptibility may be lower
than man. Man must be considered more sensitive than other species
thus far examined. In man, the delayed occurrence of lesions in the
lung, renal failure and a local effect on corneal epithelium, nasal
mucosa, skin and fingernails suggest that paraquat my be considered a
pesticide whose handling be restricted to trained professional
personnel.
In addition, studies on prophylaxis and treatment of the toxic effects
of paraquat were considered to be urgently needed; reproduction
studies are limited to one species, the rat. For the above-mentioned
reasons, the Committee considered that it was possible to establish a
temporary acceptably daily intake for man, based upon the no-effect
level in the dog.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg body-weight/day
(corresponds to 9.1 mg paraquat ion/mg body-weight/day)
Dog: 50 ppm in the diet, equivalent to 1.25 mg/kg body-weight/day
(corresponds to 0.91 mg paraquat ion/kg body-weight/day)
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE IN MAN
0-0.001 mg/kg body-weight as paraquat dichloride (0-0.0007 mg/kg
body-weight expressed as paraquat ion)
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Herbicide and desiccant; rapidly absorbed by green plants but
inactivated on contact with soil. Widely used for pre-crop and
post-crop-emergence weed control, plantation weed control, aquatic
weed control, pasture renovation, pre-harvest desiccation of hops,
cotton defoliation and potato haulm and sugar cane desiccation.
FATE OF RESIDUES
In animals
The fate of 14C-paraquat administered orally to cattle was
investigated by Stevens and Watley (1966). They found that the bulk of
radioactivity was excreted in the faeces and amounts <5% were
present, largely as breakdown products, in the urine.
A group of three cows, orally treated with a single dose of paraquat
at 8 mg/kg, excreted between 0.003 and 0.008 percent of the dose in
the milk and 0.24 percent of the dose in the urine within the
seven-day testing interval (Stevens et al.,1966; Stevens et al.,
1964). The amount of ingested material in the milk was the same
irrespective of position of a radioactive label indicating that
paraquat itself or a metabolite(s) containing methyl groups and intact
ring structures was present.
Two calves grazed for three or seven days on pasture containing
residues of 300-400 ppm paraquat were found to have significant
residues of paraquat only in the gut and stomach tissues. The kidney
contained the highest tissue residues of 0.15 ppm, with traces found
in lungs, heart and liver (Litchfield, 1969).
In plants
From observations made with 14C-labelled material, paraquat is
transported to a slightly greater extent than diquat from the leaves
of potato plants to the tubers (Slade and Bell, 1966). Coates et al.
(1966) found appreciable movement in wheat, even in the roots. Slade
(1966) studied the degradation of labelled paraquat on tomato, broad
beans and maize plants; the degradation was found to be non-enzymic,
but could be attributed to sunlight. Using potato plants, experiments
showed that even if metabolism had occurred in the plant, no
degradation products were transported to the tubers.
In soil
Paraquat has been shown to be degraded by soil microorganisms
(Funderburk and Bozarth, 1967) to demethylated paraquat
(1-methyl-4,4'-dipyridinium ion) and another compound characterized as
the 1-methyl-4-carboxy-pyridinium ion (N-methylisonicotinic acid).
The following degradation pathway by bacteria - demethylation of
parent molecule and ring cleavage of one of the heterocyclic rings to
eventually forms the carboxylated N-methyl-pyridinium ion.
 In sunlight
On the plant surface (and in solution), paraquat is rapidly broken
down photochemically. The two end products from ultraviolet
irradiation of solutions, both of which have very low toxicities in
mammals, are identified as N-methyl betaines of iso-nicotinic acid and
methylamine.
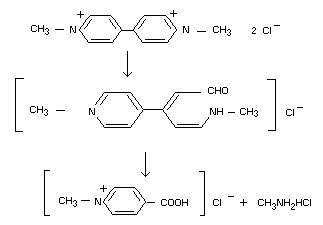
Slade (1965, 1966) investigated the degradation of paraquat by both
sunlight and the ultraviolet light from a mercury vapour lamp. Two
degradation products were identified, 1-methyl-4-carboxypyridinium ion
and methylamine hydrochloride; the following degradation pathway was
proposed:
In sunlight
On the plant surface (and in solution), paraquat is rapidly broken
down photochemically. The two end products from ultraviolet
irradiation of solutions, both of which have very low toxicities in
mammals, are identified as N-methyl betaines of iso-nicotinic acid and
methylamine.
Slade (1965, 1966) investigated the degradation of paraquat by both
sunlight and the ultraviolet light from a mercury vapour lamp. Two
degradation products were identified, 1-methyl-4-carboxypyridinium ion
and methylamine hydrochloride; the following degradation pathway was
proposed:
 In water
Paraquat applied to water for aquatic weed control purposes quickly
disappears due to uptake by weeds and absorption by soil, silt and
particulate suspended matter (Calderbank, 1968). No information is
available on the ultimate fate of the chemical in this environment.
The rate of disappearance in very variable, depending on the movement
of the water and the presence of mud or suspended matter, but
treatments within the range of 1-4 mg/litre in the water have resulted
in only 0.1 mg/litre or less of paraquat being detectable in from 6 to
14 days after application. Decomposition of the killed weed is rapid,
any remaining residue of paraquat thus liberated being subsequently
absorbed on the bottom mud. Such residues in the largely organic muds
may be more readily available to bacterial degradation than when
absorbed to clay minerals in soils.
Evidence of residues in food in commerce or at consumption
Only when the crop in sprayed directly are significant residues of
paraquat likely to be found. A summary of residues found in cotton
after use for desiccation purposes is given in Table II. (Calderbank,
1968)
TABLE II
Paraquat residues in, ten days after desiccation at 0.5 lb
paraquat/acre (U.S.A. results)
Fraction analysed Paraquat found, ppm
Cotton as picked, including
trash and bolls 2.0
Ginned seed 0.18
Acid-delinted seed 0.05
Mechanically reginned seed 0.08
Lint cotton 3.0
Hulls 0.13
Trash 3.7
Crude oil Non-detected
Meal 0.02
Data obtained following use of paraquat as a desiccant on several food
crops has also been published (Calderbank, 1968); a summary of these
results is given in Table III.
METHODS OF RESIDUE ANALYSIS
An ion-exchange method for determining paraquat residues has been
developed by Calderbank and Yuen (1965). The method depends on the
measurement of light absorption at 396 nm of reduced solutions of
paraquat after concentration and purification by cation-exchange
chromatography and has been used for a wide variety of food crops,
water, etc.; limit of sensitivity is 0.01 ppm. Radaelli and Bosetto
(1968) used it for the determination of residues in clays and mineral
soils. Paraquat and its photochemical decomposition product,
4-carboxyl-1-methyl pyridinium chloride, can be determined
polarographically (Slade and Jackson, 1971).
TABLE III
Summary of paraquat residues in food crops, desiccation uses
Rate of Average Residues
Application Paraquat
Crop (lb/acre) (ppm)
Barley 0.5 -1.0 3-10
Wheat 0.5 -1.0 1-2.5
Maize 0.5 -1.2 ND2/0.2
Rice (with husk) 0.15 -0.54 0.7-22
Rice (dehusked or polished) 0.15 -0.54 ND-0.2
Peas, beans, sunflower seed 0.35 -1.2 ND-2.0
Sorghum seed 0.25 -1.0 0.1-0.4
Cotton (as picked) 0.5 -1.0 2-3
Onions 0.5 -2.0 0.05 -0.5
Potatoes 0.5 -1.5 0.02 -0.13
Sugar cane juice 0.5 -2.0 ND
Seed oils (sesame,
sunflower, rape, cotton) up to 1.2 ND
1/ 3-21 days after application
2/ ND = none detected
The lesser duckweed (Lemna minor L) provides a simple sensitive
bioassay technique for determining paraquat residues in water
(Funderburk and Lawrence, 1963). Plant extracts containing paraquat
have been chromatographed on thin layers of silica gel by Slade (1966)
using 5 M ammonium chloride solution for development. Faust and Hunter
(1965) determined paraquat in natural surface water at 256 nm
following chemical clean-up by ion exchange.
NATIONAL TOLERANCES
Country Crop Tolerance
(ppm)
U.S.A. Potatoes 0.5
Apples, pears, apricots, avocados,
bananas, cherries, citrus fruits,
figs, grapes, papayas, peaches, 0.05
nectarines, plums, prunes (fresh)
maize, lettuce, melons, peppers,
tomatoes 0.05
maize and sorghum grain 0.05
maize, sorghum and soybean forage 0.05
Almond hulls 0.5
Almonds, filberts, macadamia 0.05
nuts and walnuts
Coffee beans, olives, soybeans 0.05
Cottonseed 0.5
Sugarbeet (roots and tops) 0.5
Information has also been received regarding -
Cotton (as picked) 2 mg/kg
APPRAISAL
Paraquat is very widely used for weed control in many crops, as an
aquatic herbicide and as a desiccant on cotton, potato haulm and sugar
cane. Paraquat in a stable compound in plants. Ultraviolet light,
sunlight and soil micro-organisms degraded paraquat to
N-methyl-isonicotinic acid and methylamine hydrochloride. Following
ingestion by cows, traces of paraquat or its metabolites are secreted
into the milk. Residues are very unlikely to accrue from soil or
pre-emergence applications but can occur following use for desiccation
purposes. The suggested tolerances are based on each desiccation
usage. The colorimetric procedure of Calderbank and Yuen (1965) should
be suitable for regulatory purposes.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to June 1974)
Cottonseed 0.2 ppm
Potatoes 0.1 ppm
Cottonseed meal 0.05 ppm
Cottonseed oil (edible) 0.05 ppm
Sugar cane juice 0.05 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Detailed comparative toxicity and metabolism studies in order to
elucidate the reason for the comparatively high sensitivity of man
to this compound.
2. Additional reproduction studies on at least one species.
3. Examination in several species of the toxic effects of metabolites
formed by the action of the gut flora.
DESIRABLE
Long-term oral studies on additional species.
REFERENCES
Almog, C.H. and Tal, E. (1967) Death from paraquat after subcutaneous
injection. Brit. Med. J., 3: 721
Baran, J. and Calandra, J.C. (1965) Two-year chronic oral toxicity of
paraquat-beagle dogs. Unpublished report from Industrial Bio-Test
(Aug. 1964) to ICI through Chevron Chemical Co. to FDA
Broadhurst, T.O., Griffiths, D. and McElligott, T.F. (1966) Ninety-day
oral toxicity of N-methyl-isonicotinic acid methosulfate - albino
rats. Unpublished report IHR/194 (April 1966) ICI Ltd. through Chevron
Chemical Co. to FDA
Bullivant, C.M. (1966) Accidental poisoning by paraquat: report of two
cases in man. Brit. Med. J., 1: 1272-1273
Calderbank, A. and Yuen, S.H. (1965) An Ion-exchange method for
determining paraquat residues in food crops. Analyst, 90: 99-106
Calderbank, A., McKenna, R.M., Stevens, M.A. and Walley, J.K. (1968)
Grazing trials on paraquat-treated pasture. J. Sci. Fd. Agric. 19:
246-250
Calderbank, A. (1968) The bipyridylium herbicides. Effects on man,
Advances in pest control research, Metcalf R.L. Ed. vol 8, p. 224
Interscience Publishers N.Y.
Cambar, P.J. and Aviado, D.M. (1970) Bronchopulmonary effects of
paraquat and expectorants. Arch. environm. Hlth, 20: 488-494
Campbell, S. (1968) Paraquat poisoning. Clinical Toxicol.,1: 245-249
Cant, J.S. and Lewis, D.R.H. (1968) Ocular damage due to paraquat and
diquat. Brit. Med. J., 2: 224
Cervenka, H., Kay, J.H. and Calandra, J.C. (1964) Chronic oral
toxicity of paraquat - beagle dogs. Unpublished report from Industrial
Bio-Test (Aug. 1964) to ICI through Chevron Chemical Co. to FDA
Clark, D.G. (1965a) The acute toxicity of paraquat. Unpublished report
IHR/170 (Jan. 1965) from ICI through Chevron Chemical Co. to FDA
Clark, D.G. (1965b) Unpublished report TR/438 (25 Jan. 1965) from ICI
Ltd. through Chevron Chemical Co. to FDA
Clark, D.G., McElligott, T.F. and Hurst, E.W. (1966) The toxicity of
paraquat. Brit. J. Indust. Med., 23: 26-32
Coates, G.E., Funderburk, H.H., Lawrence, J.M. and Davis, D.E. (1966)
Factors affecting persistence and inactivation of diquat and paraquat.
Weed Res.,6:58-66
Conning, D.M., Fletcher, K. and Swan, A.A.B. (1969) Paraquat and
related bipyridyls. Brit. Med. Bull.,25:245-249
Cowie, J. and Kahn, J. (1968) Acute renal failure in case of paraquat
poisoning. Brit. Med. J., 1: 749-750
Daniel, J.W. and Gage, J.C. (1966) Absorption and excretion of diquat
and paraquat in rats. Brit. J. Industr. Med.,23: 133-136
Duffy, B.S. and O'Sullivan, D.J. (1968) Paraquat poisoning. J. Irish
Med. Assoc.,61: 97-98
Faust, S.D. and Hunter, N.E. (1965) Chemical methods for the
determination of aquatic herbicides. J. Am. Water Works Assoc., 57:
1028-1037
Fennelley, J.J., Gallagher, J.T. and Carroll, R.J. (1968) Paraquat
poisoning in a pregnant woman. Brit. Med. J.,3: 722-723
Funderburk, H.H. and Lawrence, J.M. (1963) A sensitive method for
determination of low concentrations of diquat and paraquat. Nature,
199: 1011-1012
Funderburk, H.H. and Bozarth, G.A. (1967) Review of the metabolism and
decomposition of diquat and paraquat. J. Agric. Fd. Chem.,15:
563-567
Gage, J.C. (1968a) The action of paraquat and diquat on the
respiration of liver cell fractions. Biochem. J.,109: 757-761
Gage, J.C. (1968b) Toxicity of paraquat and diquat aerosols generated
by a size selective cyclone. Effect of particle size distribution.
Brit. J. Industr. Med.,25: 304-314
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol.,14: 515-534
Goulding, R. (1968) Paraquat poisoning. The Practitioner,200:739-740
Griffiths, D., Ponsford, D.C. and Hurst, E.W. (1966) A study of
reproduction in rats treated with paraquat in the diet. Unpublished
report IHR/185 (Jan. 1966) from ICI Ltd.
Herczeg, E. and Reit, A. (1968) Lungenveranderunger bei todlich
verlaufener paraquatvergiftung. Zbl. allg. Path. Anat., 111: 325-328
Khera, L.S. and Whitta, L.L. (1968) Embryopathic effects of diquat and
paraquat in the rat. Ind. Med. Surg., 37: 553
Kohn, F.E., Kay, J.H. and Calandra, J.C. (1964) Two-year chronic oral
toxicity of paraquat-albino rats. Unpublished report from Industrial
Bio-Test Laboratories (July 1964) to ICI through Chevron Chemical Co.
to FDA
Litchfield, M.H. (1969) Grazing trial on paraquat-treated pasture.
Unpublished report IHR/257 (April 1969) ICI Ltd.
Lloyd, E.L. (1969) Recovery after taking Weedol. Brit. Med. J., 2:
189
Manktelow, B.W. (1967) The loss of pulmonary surfactant in paraquat
poisoning. Brit. J. Exptl. Path. 48: 366-369
Matthew, H., Logan, A., Woodrugg, M.F.A. and Heard, B, (1968) Paraquat
poisoning - lung transplantation. Brit. Med. J.,3: 759-763
McElligott, T.F. (1965) The dermal toxicity of paraquat. Unpublished
ICI report IHR/172 (Jan 1965; through Chevron Chemical Co. to FDA
McElligott, T.F. (1966) Toxicological report - N-methyl isonicotinic
acid methosulphate. Unpublished report TR/512 (April 1966) ICI Ltd.
through Chevron Chemical Co. to FDA
McKean, W.I. (1968) Recovery from paraquat poisoning. Brit. Med.
J.,3:292
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. (1964) Acute aerosol
inhalation toxicity of paraquat dichloride. Unpublished report from
Bio-Test Lab. Inc. to ICI (15 April 1964) through Chevron Chemical Co.
to FDA
Palazzolo, R.J. and Calandra, J.C. (1965) Sub-acute dermal toxicity of
paraquat dichloride. Unpublished report by Industrial Bio-Test Lab.
(Aug. 1966) through California Chemical Co. to FDA
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. (1965) Sub-acute
aerosol inhalation toxicity of paraquat dichloride. Unpublished report
from Indus. Bio-Test Lab. (Jan. 1965) to ICI Ltd. through Chevron
Chemical Co. to FDA
Radaelli, L. and Bosetto, M. (1968) Determination of paraquat in clays
and mineral soils. Ricerca Scient., 38: 855-857
Samman, P.D. and Johnston, E.N.M. (1969) Nail damage associated with
handling of paraquat and diquat. Brit. Med. J., 1: 818-819
Sarfaty, A.B. (1963) Observational trials on toxicity to sheep and
cattle. Unpublished report Chevron Chemical Co. to FDA
Slade, P. (1965) Photochemical degradation of paraquat. Nature, 207:
515-516
Slade, P. (1966) The fate of paraquat applied to plants. Weed Res.,
6: 158-167
Slade, P. and Bell, E.G. (1966) Movement of paraquat in plants. Weed
Res., 6: 267-274
Slade, P. and Jackson, L.S. (1971) Pestic. Sci., in preparation
Stevens, M.A., Walker, G.H. and Walley, J.K. (1964) The excretion of
14C paraquat by the cow. Unpublished report IHR/164 (Oct. 1964) by
ICI through Chevron Chemical Co. to FDA
Stevens, M.A. and Walley, J.K. (1966) Tissue and milk residues arising
from the ingestion of single doses of diquat and paraquat by cattle.
J. Sci. Food Agric., 17: 472-475
Swan, A.A.B. (1959) Unpublished report by ICI TR/241 (Dec. 1959)
submitted through Chevron Chemical Co. to FDA
Tilling, W. (1968) Paraquat - intoxikation. Dtsch. Medizin.
Wchschr.,50: 2439-2441
Torell, D. and Kay, B.L. (1964) Pasturing of sheep on paraquat treated
forage. Unpublished report - 383-42 Chevron Chemical Co. to FDA
Walley, J.K. (1964) Paraquat toxicity in sheep and cattle. Unpublished
report IHR/166 (April 1964) submitted from ICI through Chevron
Chemical Co. to FDA
In water
Paraquat applied to water for aquatic weed control purposes quickly
disappears due to uptake by weeds and absorption by soil, silt and
particulate suspended matter (Calderbank, 1968). No information is
available on the ultimate fate of the chemical in this environment.
The rate of disappearance in very variable, depending on the movement
of the water and the presence of mud or suspended matter, but
treatments within the range of 1-4 mg/litre in the water have resulted
in only 0.1 mg/litre or less of paraquat being detectable in from 6 to
14 days after application. Decomposition of the killed weed is rapid,
any remaining residue of paraquat thus liberated being subsequently
absorbed on the bottom mud. Such residues in the largely organic muds
may be more readily available to bacterial degradation than when
absorbed to clay minerals in soils.
Evidence of residues in food in commerce or at consumption
Only when the crop in sprayed directly are significant residues of
paraquat likely to be found. A summary of residues found in cotton
after use for desiccation purposes is given in Table II. (Calderbank,
1968)
TABLE II
Paraquat residues in, ten days after desiccation at 0.5 lb
paraquat/acre (U.S.A. results)
Fraction analysed Paraquat found, ppm
Cotton as picked, including
trash and bolls 2.0
Ginned seed 0.18
Acid-delinted seed 0.05
Mechanically reginned seed 0.08
Lint cotton 3.0
Hulls 0.13
Trash 3.7
Crude oil Non-detected
Meal 0.02
Data obtained following use of paraquat as a desiccant on several food
crops has also been published (Calderbank, 1968); a summary of these
results is given in Table III.
METHODS OF RESIDUE ANALYSIS
An ion-exchange method for determining paraquat residues has been
developed by Calderbank and Yuen (1965). The method depends on the
measurement of light absorption at 396 nm of reduced solutions of
paraquat after concentration and purification by cation-exchange
chromatography and has been used for a wide variety of food crops,
water, etc.; limit of sensitivity is 0.01 ppm. Radaelli and Bosetto
(1968) used it for the determination of residues in clays and mineral
soils. Paraquat and its photochemical decomposition product,
4-carboxyl-1-methyl pyridinium chloride, can be determined
polarographically (Slade and Jackson, 1971).
TABLE III
Summary of paraquat residues in food crops, desiccation uses
Rate of Average Residues
Application Paraquat
Crop (lb/acre) (ppm)
Barley 0.5 -1.0 3-10
Wheat 0.5 -1.0 1-2.5
Maize 0.5 -1.2 ND2/0.2
Rice (with husk) 0.15 -0.54 0.7-22
Rice (dehusked or polished) 0.15 -0.54 ND-0.2
Peas, beans, sunflower seed 0.35 -1.2 ND-2.0
Sorghum seed 0.25 -1.0 0.1-0.4
Cotton (as picked) 0.5 -1.0 2-3
Onions 0.5 -2.0 0.05 -0.5
Potatoes 0.5 -1.5 0.02 -0.13
Sugar cane juice 0.5 -2.0 ND
Seed oils (sesame,
sunflower, rape, cotton) up to 1.2 ND
1/ 3-21 days after application
2/ ND = none detected
The lesser duckweed (Lemna minor L) provides a simple sensitive
bioassay technique for determining paraquat residues in water
(Funderburk and Lawrence, 1963). Plant extracts containing paraquat
have been chromatographed on thin layers of silica gel by Slade (1966)
using 5 M ammonium chloride solution for development. Faust and Hunter
(1965) determined paraquat in natural surface water at 256 nm
following chemical clean-up by ion exchange.
NATIONAL TOLERANCES
Country Crop Tolerance
(ppm)
U.S.A. Potatoes 0.5
Apples, pears, apricots, avocados,
bananas, cherries, citrus fruits,
figs, grapes, papayas, peaches, 0.05
nectarines, plums, prunes (fresh)
maize, lettuce, melons, peppers,
tomatoes 0.05
maize and sorghum grain 0.05
maize, sorghum and soybean forage 0.05
Almond hulls 0.5
Almonds, filberts, macadamia 0.05
nuts and walnuts
Coffee beans, olives, soybeans 0.05
Cottonseed 0.5
Sugarbeet (roots and tops) 0.5
Information has also been received regarding -
Cotton (as picked) 2 mg/kg
APPRAISAL
Paraquat is very widely used for weed control in many crops, as an
aquatic herbicide and as a desiccant on cotton, potato haulm and sugar
cane. Paraquat in a stable compound in plants. Ultraviolet light,
sunlight and soil micro-organisms degraded paraquat to
N-methyl-isonicotinic acid and methylamine hydrochloride. Following
ingestion by cows, traces of paraquat or its metabolites are secreted
into the milk. Residues are very unlikely to accrue from soil or
pre-emergence applications but can occur following use for desiccation
purposes. The suggested tolerances are based on each desiccation
usage. The colorimetric procedure of Calderbank and Yuen (1965) should
be suitable for regulatory purposes.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to June 1974)
Cottonseed 0.2 ppm
Potatoes 0.1 ppm
Cottonseed meal 0.05 ppm
Cottonseed oil (edible) 0.05 ppm
Sugar cane juice 0.05 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Detailed comparative toxicity and metabolism studies in order to
elucidate the reason for the comparatively high sensitivity of man
to this compound.
2. Additional reproduction studies on at least one species.
3. Examination in several species of the toxic effects of metabolites
formed by the action of the gut flora.
DESIRABLE
Long-term oral studies on additional species.
REFERENCES
Almog, C.H. and Tal, E. (1967) Death from paraquat after subcutaneous
injection. Brit. Med. J., 3: 721
Baran, J. and Calandra, J.C. (1965) Two-year chronic oral toxicity of
paraquat-beagle dogs. Unpublished report from Industrial Bio-Test
(Aug. 1964) to ICI through Chevron Chemical Co. to FDA
Broadhurst, T.O., Griffiths, D. and McElligott, T.F. (1966) Ninety-day
oral toxicity of N-methyl-isonicotinic acid methosulfate - albino
rats. Unpublished report IHR/194 (April 1966) ICI Ltd. through Chevron
Chemical Co. to FDA
Bullivant, C.M. (1966) Accidental poisoning by paraquat: report of two
cases in man. Brit. Med. J., 1: 1272-1273
Calderbank, A. and Yuen, S.H. (1965) An Ion-exchange method for
determining paraquat residues in food crops. Analyst, 90: 99-106
Calderbank, A., McKenna, R.M., Stevens, M.A. and Walley, J.K. (1968)
Grazing trials on paraquat-treated pasture. J. Sci. Fd. Agric. 19:
246-250
Calderbank, A. (1968) The bipyridylium herbicides. Effects on man,
Advances in pest control research, Metcalf R.L. Ed. vol 8, p. 224
Interscience Publishers N.Y.
Cambar, P.J. and Aviado, D.M. (1970) Bronchopulmonary effects of
paraquat and expectorants. Arch. environm. Hlth, 20: 488-494
Campbell, S. (1968) Paraquat poisoning. Clinical Toxicol.,1: 245-249
Cant, J.S. and Lewis, D.R.H. (1968) Ocular damage due to paraquat and
diquat. Brit. Med. J., 2: 224
Cervenka, H., Kay, J.H. and Calandra, J.C. (1964) Chronic oral
toxicity of paraquat - beagle dogs. Unpublished report from Industrial
Bio-Test (Aug. 1964) to ICI through Chevron Chemical Co. to FDA
Clark, D.G. (1965a) The acute toxicity of paraquat. Unpublished report
IHR/170 (Jan. 1965) from ICI through Chevron Chemical Co. to FDA
Clark, D.G. (1965b) Unpublished report TR/438 (25 Jan. 1965) from ICI
Ltd. through Chevron Chemical Co. to FDA
Clark, D.G., McElligott, T.F. and Hurst, E.W. (1966) The toxicity of
paraquat. Brit. J. Indust. Med., 23: 26-32
Coates, G.E., Funderburk, H.H., Lawrence, J.M. and Davis, D.E. (1966)
Factors affecting persistence and inactivation of diquat and paraquat.
Weed Res.,6:58-66
Conning, D.M., Fletcher, K. and Swan, A.A.B. (1969) Paraquat and
related bipyridyls. Brit. Med. Bull.,25:245-249
Cowie, J. and Kahn, J. (1968) Acute renal failure in case of paraquat
poisoning. Brit. Med. J., 1: 749-750
Daniel, J.W. and Gage, J.C. (1966) Absorption and excretion of diquat
and paraquat in rats. Brit. J. Industr. Med.,23: 133-136
Duffy, B.S. and O'Sullivan, D.J. (1968) Paraquat poisoning. J. Irish
Med. Assoc.,61: 97-98
Faust, S.D. and Hunter, N.E. (1965) Chemical methods for the
determination of aquatic herbicides. J. Am. Water Works Assoc., 57:
1028-1037
Fennelley, J.J., Gallagher, J.T. and Carroll, R.J. (1968) Paraquat
poisoning in a pregnant woman. Brit. Med. J.,3: 722-723
Funderburk, H.H. and Lawrence, J.M. (1963) A sensitive method for
determination of low concentrations of diquat and paraquat. Nature,
199: 1011-1012
Funderburk, H.H. and Bozarth, G.A. (1967) Review of the metabolism and
decomposition of diquat and paraquat. J. Agric. Fd. Chem.,15:
563-567
Gage, J.C. (1968a) The action of paraquat and diquat on the
respiration of liver cell fractions. Biochem. J.,109: 757-761
Gage, J.C. (1968b) Toxicity of paraquat and diquat aerosols generated
by a size selective cyclone. Effect of particle size distribution.
Brit. J. Industr. Med.,25: 304-314
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol.,14: 515-534
Goulding, R. (1968) Paraquat poisoning. The Practitioner,200:739-740
Griffiths, D., Ponsford, D.C. and Hurst, E.W. (1966) A study of
reproduction in rats treated with paraquat in the diet. Unpublished
report IHR/185 (Jan. 1966) from ICI Ltd.
Herczeg, E. and Reit, A. (1968) Lungenveranderunger bei todlich
verlaufener paraquatvergiftung. Zbl. allg. Path. Anat., 111: 325-328
Khera, L.S. and Whitta, L.L. (1968) Embryopathic effects of diquat and
paraquat in the rat. Ind. Med. Surg., 37: 553
Kohn, F.E., Kay, J.H. and Calandra, J.C. (1964) Two-year chronic oral
toxicity of paraquat-albino rats. Unpublished report from Industrial
Bio-Test Laboratories (July 1964) to ICI through Chevron Chemical Co.
to FDA
Litchfield, M.H. (1969) Grazing trial on paraquat-treated pasture.
Unpublished report IHR/257 (April 1969) ICI Ltd.
Lloyd, E.L. (1969) Recovery after taking Weedol. Brit. Med. J., 2:
189
Manktelow, B.W. (1967) The loss of pulmonary surfactant in paraquat
poisoning. Brit. J. Exptl. Path. 48: 366-369
Matthew, H., Logan, A., Woodrugg, M.F.A. and Heard, B, (1968) Paraquat
poisoning - lung transplantation. Brit. Med. J.,3: 759-763
McElligott, T.F. (1965) The dermal toxicity of paraquat. Unpublished
ICI report IHR/172 (Jan 1965; through Chevron Chemical Co. to FDA
McElligott, T.F. (1966) Toxicological report - N-methyl isonicotinic
acid methosulphate. Unpublished report TR/512 (April 1966) ICI Ltd.
through Chevron Chemical Co. to FDA
McKean, W.I. (1968) Recovery from paraquat poisoning. Brit. Med.
J.,3:292
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. (1964) Acute aerosol
inhalation toxicity of paraquat dichloride. Unpublished report from
Bio-Test Lab. Inc. to ICI (15 April 1964) through Chevron Chemical Co.
to FDA
Palazzolo, R.J. and Calandra, J.C. (1965) Sub-acute dermal toxicity of
paraquat dichloride. Unpublished report by Industrial Bio-Test Lab.
(Aug. 1966) through California Chemical Co. to FDA
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. (1965) Sub-acute
aerosol inhalation toxicity of paraquat dichloride. Unpublished report
from Indus. Bio-Test Lab. (Jan. 1965) to ICI Ltd. through Chevron
Chemical Co. to FDA
Radaelli, L. and Bosetto, M. (1968) Determination of paraquat in clays
and mineral soils. Ricerca Scient., 38: 855-857
Samman, P.D. and Johnston, E.N.M. (1969) Nail damage associated with
handling of paraquat and diquat. Brit. Med. J., 1: 818-819
Sarfaty, A.B. (1963) Observational trials on toxicity to sheep and
cattle. Unpublished report Chevron Chemical Co. to FDA
Slade, P. (1965) Photochemical degradation of paraquat. Nature, 207:
515-516
Slade, P. (1966) The fate of paraquat applied to plants. Weed Res.,
6: 158-167
Slade, P. and Bell, E.G. (1966) Movement of paraquat in plants. Weed
Res., 6: 267-274
Slade, P. and Jackson, L.S. (1971) Pestic. Sci., in preparation
Stevens, M.A., Walker, G.H. and Walley, J.K. (1964) The excretion of
14C paraquat by the cow. Unpublished report IHR/164 (Oct. 1964) by
ICI through Chevron Chemical Co. to FDA
Stevens, M.A. and Walley, J.K. (1966) Tissue and milk residues arising
from the ingestion of single doses of diquat and paraquat by cattle.
J. Sci. Food Agric., 17: 472-475
Swan, A.A.B. (1959) Unpublished report by ICI TR/241 (Dec. 1959)
submitted through Chevron Chemical Co. to FDA
Tilling, W. (1968) Paraquat - intoxikation. Dtsch. Medizin.
Wchschr.,50: 2439-2441
Torell, D. and Kay, B.L. (1964) Pasturing of sheep on paraquat treated
forage. Unpublished report - 383-42 Chevron Chemical Co. to FDA
Walley, J.K. (1964) Paraquat toxicity in sheep and cattle. Unpublished
report IHR/166 (April 1964) submitted from ICI through Chevron
Chemical Co. to FDA
 Other relevant chemical properties
Available as the di(methyl sulphate) or the dichloride which are white
crystalline solids; the di(methyl sulphate) is deliquescent. The salts
melt with decomposition in the region of 300°C. Both compounds are
stable in acid or neutral solutions and unstable in alkaline solution,
very soluble in water and decompose in ultraviolet light. They are
inactivated by inert clays and by anionic surfactants. Solutions of
paraquat become intensely purple on reduction, due to the formation of
a water-soluble, relatively stable free radical. The reduction is
autoxidizable, and solutions of the free radical absorb at 396 nm; the
unreduced form absorbs at 256 nm. Vigorous reduction gives tetrahydro
derivatives and ultimately the fully saturated base. The redox
potential (-446 mV) is independent of pH. Concentrated aqueous
solutions of paraquat corrode steel, tinplate, galvanized iron and
aluminium.
Purity
Technical, 90-95 percent
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption distribution and excretion
Paraquat is not readily absorbed from the gut (Daniel and Gage, 1966).
Following oral administration, peak concentrations of paraquat in the
blood are reached within one to six hours. Paraquat does not appear to
be selectively concentrated by any tissues in the body (Conning et
al., 1969).
Paraquat (14C-methyl labelled) administered to rats by an oral or
subcutaneous route was completely recovered in the excreta. Following
oral administration of 4-6 mg/kg body-weight of paraquat as the
dichloride salt, from 99-102 percent of the administered dose was
recovered in urine (6 percent) and faeces (93-95 per cent). Following
subcutaneous administration of 21-23 mg/kg body-weight (methyl
sulphate salt) from 85-112 per cent of the administered dose was
recovered in the urine and faeces, (in urine 73-96 percent and in
faeces 14-16 percent). Paraquat appeared to be poorly absorbed from
the gut. However, 30 percent of a dose of paraquat is present in rat
faeces as metabolic products. It has been suggested that paraquat may
be metabolized in vivo by microbial action within the gut. A small
proportion of these breakdown products may be absorbed from the gut
(Daniel and Gage, 1966).
Effect on enzymes and other biochemical parameters
Studies of the reaction of paraquat with liver cell preparation
suggest that cyclic reduction and reoxidation of the molecule may be a
primary mechanism in the effects noted. These effects include a slight
increase in oxygen uptake by mitochondria (possibly due to poor
penetration of the mitochondrial membrane), a stimulation of oxygen
uptake with NADH or ß-hydroxy-butyrate (but not succinate) as a
substrate in mitochondrial fragments inhibited with Amytal, and an
increase of NADPH oxidase in microsomes. Free radicals can be produced
from paraquat incubated anaerobically in the presence of NADPH and
microsomes derived from rat liver. Purified lipoamide dehydrogenase
from pig heart is able to reduce paraquat to the free radicals in the
presence of NADPH. Paraquat also increased the respiration of the
liver mitochondrial fragments. This action is attributed to the
activity of flavo-protein dehydrogenases. The property paraquat has of
undergoing cyclic reduction and oxidation suggests that it could
interfere in electron-transport processes, diverting electrons from
the system and reducing oxygen to water. The resting respiration of
mitochondria was almost unaffected by paraquat, probably because of
its inability to penetrate the mitochondrial membrane (Gage, 1968a).
Increased peroxidation occurs after paraquat treatment in plants and
has been shown to be associated with the peroxidation of microsomal
phospholipids in animals. An examination of lung lipids from rats
treated with paraquat revealed no diminution in the content of
unsaturated fatty acids. Preparations of rat liver either treated with
paraquat in vitro or taken from animals given paraquat in vivo,
showed no evidence of direct effect on fatty acid synthesis. Analysis
of lung lipids up to six days after poisoning with paraquat revealed
no significant changes in the composition of lung phospholipids. Large
doses of tocopheryl acetate, given to animals before but not after
exposure to paraquat, affords some protection against its toxic effect
(Conning et al., 1969).
In vitro binding studies have shown paraquat to bind to nucleic
acids and acidic mucopolysaccharides; the binding is lessened by
moderate salt concentrations. Paraquat in not bound by plasma protein
or tissue homogenates, but small amounts may be bound by macrophages.
In this instance, binding occurs in the cytoplasmic fraction (Conning
et al., 1969).
Manktelow (1967), in an attempt to explain the specific action of
paraquat on lung tissue, has proposed that it interferes with the
production of lung surfactant. Studies on the effects of expectorants
on pulmonary congestion in rats administered paraquat (ip, 10 and 20
mg/kg) confirmed this observation (Cambar and Aviado, 1970).
Paraquat was found to increase pulmonary resistance and moisture
content and to decrease pulmonary surfactant, pulmonary compliance and
respiratory minute volume. None of the expectorants examined had an
effect on all of the parameters investigated.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
Six groups of rats (ten males or ten females per group) were examined
for reproduction and teratogenic effects of paraquat at 0, 30 and 100
ppm in the diet fed to the parent (F0) generation only. Paraquat at
100 ppm was fed to F0 males only (mated to control females),F0
females only (mated to control males) and F0 males and F0 females
(mated to each other). The paraquat fed parental (F0) generation was
mated and produced three litters while exposed to paraquat. The F1
and F2 generations were not directly exposed to paraquat. The
long-term ingestion of paraquat did not influence growth or fertility
of the treated rats or of their offspring (Griffiths et al., 1966).
A single intraperitoneal injection to rats of paraquat (6.5 mg/kg
body-weight) on day 6 of gestation induced a high incidence of costal
cartilage malformation in the embryos. This defect was not noted when
injections were given on days 7-14 of gestation. A dose of 13 mg/kg on
days 6-14 of gestation did not give this defect, although in most
cases the dose was abortifacient (Khera and Whitta, 1968).
Special studies on acute toxicity of a metabolite
(N-methylisonicotinic acid)
The acute rat oral LD50 of an unneutralized solution of the
metabolite N-methylisonicotinic acid is between 2000 and 5000 mg/kg
body-weight (McElligott, 1966; Clark, 1965b). Neutralization of the
solution depressed the toxicity further (McElligott, 1966). The acute
rat ip LD50 of N-methylisonicotinic acid is approximately 500 mg/kg
body-weight (Clark, 1965b). Two of three male rats survived
intraperitoneal administration of 4000 mg/kg of neutralized
N-methylisonicotinic acid methyl sulphate (McElligott, 1966).
Special studies on subacute toxicity of metabolite
(N-methylisonicotinic acid)
Rat
One group of rats (seven males and seven females) was given
N-methylisonicotinic acid methyl sulphate by oral intubation for 21
days at a dose of 2 g/kg body-weight/day. Toxic effects were limited
to salivation, piloerection and occasional flaccidity. No effects on
blood chemistry and gross or microscopic pathology were observed
(McElligott, 1966).
Groups of rats (25 males and 25 females) were fed N-methylisonicotinic
acid methyl sulphate in the diet at concentrations of 0, 0.5, 2.0 and
4.0 percent for 90 days. Weight gains were reduced at the 4 percent
level in both males and females. Histopathological observations
include degenerative tubules in testes and degenerate cells in the
lumen of the tubules in the epididymus at the 4 percent level. No
abnormal effects were observed in mortality, body-weight, food
consumption, haematology and gross and microscopic pathology at the 2
percent level and below (Broadurst et al., 1966).
Rabbit
Three female rabbits treated dermally with N-methylisonicotinic acid
methyl sulphate powder at six alternate 24-hour periods displayed mild
desquamation but no systemic effects (McElligott, 1966).
Acute toxicity
LD50 levels of paraquat in different species are given in Table I.
After administration of acutely toxic doses to rats, all animals
displayed the same toxic signs; they appeared healthy for the first 24
hours, and then became subdued and lethargic; respiration became
progressively more difficult, and signs of anoxia were evident after
3-4 days; deaths occurred from 2-14 days after administration and
followed by inappetance and weight loss; lung congestion was evident
with varying degrees of consolidation. The pattern of mortality after
a single oral dose of paraquat indicates that there is a maximum death
rate in 2-5 days, with some deaths occurring at 10-12 days. Animals
dying in the second group had marked congestion of the lungs with an
oedematous fluid in many of the alveoli and excess macrophages in
others. Cellular proliferation around the bronchi and in the walls of
the alveoli was marked, and large tracts of the pulmonary tissue
contained a high proportion of mast cells, with consequent reduction
in the air-containing cavities.
TABLE I
Acute toxicity of paraquat in different species
LD50
(mg ion/kg
Animal Route Salt Form body-weight) Reference
Chicken oral chloride 262 Swan, 1959
Rat oral chloride 120-157 Swan, 1959
Clark, 1965a
Rat oral methylsulfate 100-110 Gaines, 1969
Rat oral methylsulfate 127-141 Swan, 1959
Clark, 1965a
Rat dermal methylsulfate 80-90 Gaines, 1969
Rat ip chloride 19 Clark, 1965a
Rat ip methylsulfate 16 Clark, 1965a
Rat sc methylsulfate 24 Swan, 1959
Rat (4 hr) chloride 6.4 mg/l Palazzolo et al.,
inhalation (LC50) 1964
Guinea Pig oral chloride 30 Swan, 1959
Rabbit oral methylsulfate 126 Swan, 1959
Rabbit dermal chloride 240 McElligott, 1965
Rabbit ip chloride 18.2 McElligott, 1965
Cat oral chloride 35 Swan, 1959
Sheep oral chloride 100 Walley, 1964
Cow oral chloride 50-75 Walley, 1964
In two tests, instillation of approximately 6-10 mg of paraquat
(dichloride or dimethyl sulphate salts) into the conjunctival sac of
rabbits resulted in temporary slight lachrymation and conjunctival
congestion one to three days after dozing. No permanent damage was
noted, although in some cases recovery was slow. (McElligott, 1965;
Clerk et al., 1966; Swan, 1959).
Short-term studies
Rabbit
Multiple percutaneous administration of paraquat to rabbits at doses
from 2.8 to 116 mg/kg body-weight daily for 20 days resulted in no
effects seen at 2.8 mg/kg/per day. At 7.3 mg/kg, all animals survived,
but there was lung congestion with consolidation of the alveoli. Two
of three animals dosed at 14.5 mg/kg died within 20 days. When the
site of application was covered by an occlusive dressing, amounts as
low as 2.8 mg/kg (1.6 mg cation kg) resulted in moist red skin with
sloughing of the skin (McElligott, 1965).
Multiple percutaneous non-occluded application of paraquat dichloride
to five male and five female rabbits at doses of 0, 0.6, 1.5 and 3.0
mg/kg body-weight/day for 20 days resulted in one male death at 1.5
mg/kg at 26 days and one male death at 3 mg/kg at 14 days. Signs of
inactivity, muscular weakness, lassitude, nasal discharge and
salivation were evident at the highest dose after 5-6 days. Local skin
reactions were evident at all doses, with recovery occurring only at
the 0.6 mg/kg level. No adverse affects were noted with regard to
body-weight or gross and microscopic examination of surviving animals.
Microscopic examinations of the dead animals revealed significant lung
damage (Palazzolo and Calandra, 1965).
Dog
Five groups of dogs (from two to four males and females per group)
were fed paraquat in their diet for 26 to 27 months at doses of 0, 10,
50, 125 and 250 ppm. Dietary feeding of paraquat dichloride at 250 ppm
over a two month period resulted in body-weight depression, depressed
food intake respiratory distress and death, with gross and
histopathologic changes in the heart, kidneys, brain and lung. At 125
ppm over a 27-month period, the following signs were evident: death;
depressed food intake body-weight; respiratory distress; and growths
and microscopic changes in the lungs. Changes in organ-weights and
decrease weights of spleen, brain and testes. Organ to body-weight
ratio increases were noted with liver, heart, brain, thyroid and
adrenal gland, while the spleen to body-weight ratio was decreased. No
effects were noted at 50 ppm (34 ppm of paraquat ion) (Cervenka et
al., 1964).
One group of dogs (three males and three females) were fed paraquat
dichloride 75 ppm in the diet for two years. Slight alterations were
seen in the lungs upon gross and microscopic examination which were
believed to be due to paraquat. No adverse effects were noted on
body-weight, food consumption, survival, behaviour, haematological
studies, blood chemistry, urinalysis, liver function tests,
organ-weights and ratios and growths and microscopic examination of
tissues other than lungs. The level of paraquat dichloride causing no
toxicological effect on the dog was 50 ppm (34 ppm paraquat cation)
(Baran and Calandra, 1965).
Grazing animals
Multiple daily oral administration to sheep at 20 mg/kg body-weight
for five days resulted in death of all animals within two weeks.
Multiple daily oral administration at 10 mg/kg for five days killed
one of six sheep in 26 days, while 5 mg/kg for fourteen days resulted
in listless animals; recovery was very slow (Walley, 1964).
Multiple daily oral administration to cattle of 20 mg/kg body-weight
for four days resulted in death within one week. Levels of 10 and 5
mg/kg body-weight orally for five and fourteen days, respectively,
resulted in no deaths, but animals were listless and unhealthy;
recovery was slow (Walley, 1964).
Five groups of two sheep each and three groups of one calf each were
exposed for four weeks to levels of 0, 1, 5, 10 or 20 ppm and 0.5 or
20 ppm of paraquat, respectively, in their drinking water. No adverse
toxicological effects wore noted after one month (Sarfaty, 1963).
Cattle suffered no toxic effects over a four-week period when grazed
on pasture immediately after it had been sprayed with paraquat. Horses
showed definite ill effects, including local lesions of the mouth and
increased mucus secretions (Calderbank et al., 1968).
Ewe lambs were grazed in pastures 30 days after treatment with 1-2
pounds of paraquat per acre with no effect on their growth or general
well being (Torell and Kay, 1964).
Subacute inhalation studies
One group of dogs (one male and one female), guinea pigs (five males
and five females) and rats (five males and five females) were exposed
to an aerosol of paraquat dichloride at a concentration of 0.1 mg/1,
six hours per day, five days per week, for three weeks. Growth
depression was evident among the guinea pigs and rats, and the female
dog lost weight. No effects wore noted with regard to untoward
behavioural reaction, haematology, blood chemistry and gross or
microscopic alterations of tissues (Palazzolo et al., 1965). Repeated
daily 6-hour exposures to rats of paraquat aerosols over a three-week
period produced signs of lung irritation, but no deaths, at 0.4 mg/l
(Gage, 1968b).
Long-term studies
Rat
Four groups of rats (30 males and 30 females, 60 of each sex were
controls) were fed diets containing paraquat dichloride at levels of
0, 50, 125 and 250 ppm for two years. No adverse effects were seen at
any level tested on growth, survival, behaviour, tumour incidence,
haematologic studies, urinalysis, organ weights, ratios of organ to
brain or organ to body-weight and gross pathologic examination.
Microscopic examination of tissues and organs at 0 and 250 ppm (170
ppm of paraquat cation) revealed no adverse effects (Kohn at al.,
1964).
OBSERVATIONS IN MAN
The hazard of paraquat to man has been associated with two general
areas: accidental ingestion and dermal contamination. Accidental oral
ingestion of paraquat in small quantities in man has generally
resulted in death (sometimes delayed). In almost all cases where death
occurred severe lung damage with proliferation of the alveolar walls
was evident. These lesions were occasionally accompanied by renal
failure as evidenced at autopsy by gross damage to the kidneys
(Bullivant, 1966; Fennelly et al., 1968; Goulding, 1968; Campbell,
1968; Duffy and O'Sullivan, 1968; Tilling, 1968; Matthew et al., 1968
and Cowie and Kahn, 1968). In two accidental poisoning cases, recovery
was complete (McKean, 1968; Lloyd, 1969).
One suicide case from subcutaneous injection of paraquat resulted in
delayed death in 17 to 18 days, with severe proliferation of the
epithelium of the lung. Renal macroscopic or microscopic pathological
changes were not evident (Almog and Tal, 1967; Herczeg and Reit,
1968).
Dermal contamination had been shown to result in damage and
discolouration of the fingernails. The damage included softening of
the nail at the base, with occasional loss of the nail. It appeared
that the damage was local because of the asymmetry of the lesion and
because the toenails were not affected (Samman and Johnston, 1969).
Following accidental instillation of paraquat into the eye, symptoms
of irritation and inflammation of the conjunctiva increase and large
areas of the conjunctiva and cornea may be shed. With treatment,
recovery is slow (Calderbank, 1968; Cant and Lewis, 1968).
COMMENT
Paraquat is not readily absorbed from the gastrointestinal tract.
After oral administration, it is excreted primarily in the faeces.
After subcutaneous administration, it is primarily excreted in urine.
The metabolic products formed by micro-organisms in the gut appear to
be more readily absorbed than paraquat. No information is available on
the toxicity of these metabolites formed by the gut flora. The soil
metabolite N-methylisonicotinic acid is of a low order of toxicity as
compared with the parent compound when tested in rats.
From the available experimental data on animals and clinical
experiences with man, it is evident that paraquat causes irreversible
proliferative changes in lung tissue. The level of 75 ppm when fed to
dogs over a two-year interval may be considered as a threshold dose on
the basis of this pulmonary effect. An adverse effect on a long-term
study in rats could not be demonstrated with levels up to 250 ppm.
These results indicate that the rat is an insensitive species and,
while the dog in affected by paraquat, its susceptibility may be lower
than man. Man must be considered more sensitive than other species
thus far examined. In man, the delayed occurrence of lesions in the
lung, renal failure and a local effect on corneal epithelium, nasal
mucosa, skin and fingernails suggest that paraquat my be considered a
pesticide whose handling be restricted to trained professional
personnel.
In addition, studies on prophylaxis and treatment of the toxic effects
of paraquat were considered to be urgently needed; reproduction
studies are limited to one species, the rat. For the above-mentioned
reasons, the Committee considered that it was possible to establish a
temporary acceptably daily intake for man, based upon the no-effect
level in the dog.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg body-weight/day
(corresponds to 9.1 mg paraquat ion/mg body-weight/day)
Dog: 50 ppm in the diet, equivalent to 1.25 mg/kg body-weight/day
(corresponds to 0.91 mg paraquat ion/kg body-weight/day)
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE IN MAN
0-0.001 mg/kg body-weight as paraquat dichloride (0-0.0007 mg/kg
body-weight expressed as paraquat ion)
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Herbicide and desiccant; rapidly absorbed by green plants but
inactivated on contact with soil. Widely used for pre-crop and
post-crop-emergence weed control, plantation weed control, aquatic
weed control, pasture renovation, pre-harvest desiccation of hops,
cotton defoliation and potato haulm and sugar cane desiccation.
FATE OF RESIDUES
In animals
The fate of 14C-paraquat administered orally to cattle was
investigated by Stevens and Watley (1966). They found that the bulk of
radioactivity was excreted in the faeces and amounts <5% were
present, largely as breakdown products, in the urine.
A group of three cows, orally treated with a single dose of paraquat
at 8 mg/kg, excreted between 0.003 and 0.008 percent of the dose in
the milk and 0.24 percent of the dose in the urine within the
seven-day testing interval (Stevens et al.,1966; Stevens et al.,
1964). The amount of ingested material in the milk was the same
irrespective of position of a radioactive label indicating that
paraquat itself or a metabolite(s) containing methyl groups and intact
ring structures was present.
Two calves grazed for three or seven days on pasture containing
residues of 300-400 ppm paraquat were found to have significant
residues of paraquat only in the gut and stomach tissues. The kidney
contained the highest tissue residues of 0.15 ppm, with traces found
in lungs, heart and liver (Litchfield, 1969).
In plants
From observations made with 14C-labelled material, paraquat is
transported to a slightly greater extent than diquat from the leaves
of potato plants to the tubers (Slade and Bell, 1966). Coates et al.
(1966) found appreciable movement in wheat, even in the roots. Slade
(1966) studied the degradation of labelled paraquat on tomato, broad
beans and maize plants; the degradation was found to be non-enzymic,
but could be attributed to sunlight. Using potato plants, experiments
showed that even if metabolism had occurred in the plant, no
degradation products were transported to the tubers.
In soil
Paraquat has been shown to be degraded by soil microorganisms
(Funderburk and Bozarth, 1967) to demethylated paraquat
(1-methyl-4,4'-dipyridinium ion) and another compound characterized as
the 1-methyl-4-carboxy-pyridinium ion (N-methylisonicotinic acid).
The following degradation pathway by bacteria - demethylation of
parent molecule and ring cleavage of one of the heterocyclic rings to
eventually forms the carboxylated N-methyl-pyridinium ion.
Other relevant chemical properties
Available as the di(methyl sulphate) or the dichloride which are white
crystalline solids; the di(methyl sulphate) is deliquescent. The salts
melt with decomposition in the region of 300°C. Both compounds are
stable in acid or neutral solutions and unstable in alkaline solution,
very soluble in water and decompose in ultraviolet light. They are
inactivated by inert clays and by anionic surfactants. Solutions of
paraquat become intensely purple on reduction, due to the formation of
a water-soluble, relatively stable free radical. The reduction is
autoxidizable, and solutions of the free radical absorb at 396 nm; the
unreduced form absorbs at 256 nm. Vigorous reduction gives tetrahydro
derivatives and ultimately the fully saturated base. The redox
potential (-446 mV) is independent of pH. Concentrated aqueous
solutions of paraquat corrode steel, tinplate, galvanized iron and
aluminium.
Purity
Technical, 90-95 percent
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption distribution and excretion
Paraquat is not readily absorbed from the gut (Daniel and Gage, 1966).
Following oral administration, peak concentrations of paraquat in the
blood are reached within one to six hours. Paraquat does not appear to
be selectively concentrated by any tissues in the body (Conning et
al., 1969).
Paraquat (14C-methyl labelled) administered to rats by an oral or
subcutaneous route was completely recovered in the excreta. Following
oral administration of 4-6 mg/kg body-weight of paraquat as the
dichloride salt, from 99-102 percent of the administered dose was
recovered in urine (6 percent) and faeces (93-95 per cent). Following
subcutaneous administration of 21-23 mg/kg body-weight (methyl
sulphate salt) from 85-112 per cent of the administered dose was
recovered in the urine and faeces, (in urine 73-96 percent and in
faeces 14-16 percent). Paraquat appeared to be poorly absorbed from
the gut. However, 30 percent of a dose of paraquat is present in rat
faeces as metabolic products. It has been suggested that paraquat may
be metabolized in vivo by microbial action within the gut. A small
proportion of these breakdown products may be absorbed from the gut
(Daniel and Gage, 1966).
Effect on enzymes and other biochemical parameters
Studies of the reaction of paraquat with liver cell preparation
suggest that cyclic reduction and reoxidation of the molecule may be a
primary mechanism in the effects noted. These effects include a slight
increase in oxygen uptake by mitochondria (possibly due to poor
penetration of the mitochondrial membrane), a stimulation of oxygen
uptake with NADH or ß-hydroxy-butyrate (but not succinate) as a
substrate in mitochondrial fragments inhibited with Amytal, and an
increase of NADPH oxidase in microsomes. Free radicals can be produced
from paraquat incubated anaerobically in the presence of NADPH and
microsomes derived from rat liver. Purified lipoamide dehydrogenase
from pig heart is able to reduce paraquat to the free radicals in the
presence of NADPH. Paraquat also increased the respiration of the
liver mitochondrial fragments. This action is attributed to the
activity of flavo-protein dehydrogenases. The property paraquat has of
undergoing cyclic reduction and oxidation suggests that it could
interfere in electron-transport processes, diverting electrons from
the system and reducing oxygen to water. The resting respiration of
mitochondria was almost unaffected by paraquat, probably because of
its inability to penetrate the mitochondrial membrane (Gage, 1968a).
Increased peroxidation occurs after paraquat treatment in plants and
has been shown to be associated with the peroxidation of microsomal
phospholipids in animals. An examination of lung lipids from rats
treated with paraquat revealed no diminution in the content of
unsaturated fatty acids. Preparations of rat liver either treated with
paraquat in vitro or taken from animals given paraquat in vivo,
showed no evidence of direct effect on fatty acid synthesis. Analysis
of lung lipids up to six days after poisoning with paraquat revealed
no significant changes in the composition of lung phospholipids. Large
doses of tocopheryl acetate, given to animals before but not after
exposure to paraquat, affords some protection against its toxic effect
(Conning et al., 1969).
In vitro binding studies have shown paraquat to bind to nucleic
acids and acidic mucopolysaccharides; the binding is lessened by
moderate salt concentrations. Paraquat in not bound by plasma protein
or tissue homogenates, but small amounts may be bound by macrophages.
In this instance, binding occurs in the cytoplasmic fraction (Conning
et al., 1969).
Manktelow (1967), in an attempt to explain the specific action of
paraquat on lung tissue, has proposed that it interferes with the
production of lung surfactant. Studies on the effects of expectorants
on pulmonary congestion in rats administered paraquat (ip, 10 and 20
mg/kg) confirmed this observation (Cambar and Aviado, 1970).
Paraquat was found to increase pulmonary resistance and moisture
content and to decrease pulmonary surfactant, pulmonary compliance and
respiratory minute volume. None of the expectorants examined had an
effect on all of the parameters investigated.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
Six groups of rats (ten males or ten females per group) were examined
for reproduction and teratogenic effects of paraquat at 0, 30 and 100
ppm in the diet fed to the parent (F0) generation only. Paraquat at
100 ppm was fed to F0 males only (mated to control females),F0
females only (mated to control males) and F0 males and F0 females
(mated to each other). The paraquat fed parental (F0) generation was
mated and produced three litters while exposed to paraquat. The F1
and F2 generations were not directly exposed to paraquat. The
long-term ingestion of paraquat did not influence growth or fertility
of the treated rats or of their offspring (Griffiths et al., 1966).
A single intraperitoneal injection to rats of paraquat (6.5 mg/kg
body-weight) on day 6 of gestation induced a high incidence of costal
cartilage malformation in the embryos. This defect was not noted when
injections were given on days 7-14 of gestation. A dose of 13 mg/kg on
days 6-14 of gestation did not give this defect, although in most
cases the dose was abortifacient (Khera and Whitta, 1968).
Special studies on acute toxicity of a metabolite
(N-methylisonicotinic acid)
The acute rat oral LD50 of an unneutralized solution of the
metabolite N-methylisonicotinic acid is between 2000 and 5000 mg/kg
body-weight (McElligott, 1966; Clark, 1965b). Neutralization of the
solution depressed the toxicity further (McElligott, 1966). The acute
rat ip LD50 of N-methylisonicotinic acid is approximately 500 mg/kg
body-weight (Clark, 1965b). Two of three male rats survived
intraperitoneal administration of 4000 mg/kg of neutralized
N-methylisonicotinic acid methyl sulphate (McElligott, 1966).
Special studies on subacute toxicity of metabolite
(N-methylisonicotinic acid)
Rat
One group of rats (seven males and seven females) was given
N-methylisonicotinic acid methyl sulphate by oral intubation for 21
days at a dose of 2 g/kg body-weight/day. Toxic effects were limited
to salivation, piloerection and occasional flaccidity. No effects on
blood chemistry and gross or microscopic pathology were observed
(McElligott, 1966).
Groups of rats (25 males and 25 females) were fed N-methylisonicotinic
acid methyl sulphate in the diet at concentrations of 0, 0.5, 2.0 and
4.0 percent for 90 days. Weight gains were reduced at the 4 percent
level in both males and females. Histopathological observations
include degenerative tubules in testes and degenerate cells in the
lumen of the tubules in the epididymus at the 4 percent level. No
abnormal effects were observed in mortality, body-weight, food
consumption, haematology and gross and microscopic pathology at the 2
percent level and below (Broadurst et al., 1966).
Rabbit
Three female rabbits treated dermally with N-methylisonicotinic acid
methyl sulphate powder at six alternate 24-hour periods displayed mild
desquamation but no systemic effects (McElligott, 1966).
Acute toxicity
LD50 levels of paraquat in different species are given in Table I.
After administration of acutely toxic doses to rats, all animals
displayed the same toxic signs; they appeared healthy for the first 24
hours, and then became subdued and lethargic; respiration became
progressively more difficult, and signs of anoxia were evident after
3-4 days; deaths occurred from 2-14 days after administration and
followed by inappetance and weight loss; lung congestion was evident
with varying degrees of consolidation. The pattern of mortality after
a single oral dose of paraquat indicates that there is a maximum death
rate in 2-5 days, with some deaths occurring at 10-12 days. Animals
dying in the second group had marked congestion of the lungs with an
oedematous fluid in many of the alveoli and excess macrophages in
others. Cellular proliferation around the bronchi and in the walls of
the alveoli was marked, and large tracts of the pulmonary tissue
contained a high proportion of mast cells, with consequent reduction
in the air-containing cavities.
TABLE I
Acute toxicity of paraquat in different species
LD50
(mg ion/kg
Animal Route Salt Form body-weight) Reference
Chicken oral chloride 262 Swan, 1959
Rat oral chloride 120-157 Swan, 1959
Clark, 1965a
Rat oral methylsulfate 100-110 Gaines, 1969
Rat oral methylsulfate 127-141 Swan, 1959
Clark, 1965a
Rat dermal methylsulfate 80-90 Gaines, 1969
Rat ip chloride 19 Clark, 1965a
Rat ip methylsulfate 16 Clark, 1965a
Rat sc methylsulfate 24 Swan, 1959
Rat (4 hr) chloride 6.4 mg/l Palazzolo et al.,
inhalation (LC50) 1964
Guinea Pig oral chloride 30 Swan, 1959
Rabbit oral methylsulfate 126 Swan, 1959
Rabbit dermal chloride 240 McElligott, 1965
Rabbit ip chloride 18.2 McElligott, 1965
Cat oral chloride 35 Swan, 1959
Sheep oral chloride 100 Walley, 1964
Cow oral chloride 50-75 Walley, 1964
In two tests, instillation of approximately 6-10 mg of paraquat
(dichloride or dimethyl sulphate salts) into the conjunctival sac of
rabbits resulted in temporary slight lachrymation and conjunctival
congestion one to three days after dozing. No permanent damage was
noted, although in some cases recovery was slow. (McElligott, 1965;
Clerk et al., 1966; Swan, 1959).
Short-term studies
Rabbit
Multiple percutaneous administration of paraquat to rabbits at doses
from 2.8 to 116 mg/kg body-weight daily for 20 days resulted in no
effects seen at 2.8 mg/kg/per day. At 7.3 mg/kg, all animals survived,
but there was lung congestion with consolidation of the alveoli. Two
of three animals dosed at 14.5 mg/kg died within 20 days. When the
site of application was covered by an occlusive dressing, amounts as
low as 2.8 mg/kg (1.6 mg cation kg) resulted in moist red skin with
sloughing of the skin (McElligott, 1965).
Multiple percutaneous non-occluded application of paraquat dichloride
to five male and five female rabbits at doses of 0, 0.6, 1.5 and 3.0
mg/kg body-weight/day for 20 days resulted in one male death at 1.5
mg/kg at 26 days and one male death at 3 mg/kg at 14 days. Signs of
inactivity, muscular weakness, lassitude, nasal discharge and
salivation were evident at the highest dose after 5-6 days. Local skin
reactions were evident at all doses, with recovery occurring only at
the 0.6 mg/kg level. No adverse affects were noted with regard to
body-weight or gross and microscopic examination of surviving animals.
Microscopic examinations of the dead animals revealed significant lung
damage (Palazzolo and Calandra, 1965).
Dog
Five groups of dogs (from two to four males and females per group)
were fed paraquat in their diet for 26 to 27 months at doses of 0, 10,
50, 125 and 250 ppm. Dietary feeding of paraquat dichloride at 250 ppm
over a two month period resulted in body-weight depression, depressed
food intake respiratory distress and death, with gross and
histopathologic changes in the heart, kidneys, brain and lung. At 125
ppm over a 27-month period, the following signs were evident: death;
depressed food intake body-weight; respiratory distress; and growths
and microscopic changes in the lungs. Changes in organ-weights and
decrease weights of spleen, brain and testes. Organ to body-weight
ratio increases were noted with liver, heart, brain, thyroid and
adrenal gland, while the spleen to body-weight ratio was decreased. No
effects were noted at 50 ppm (34 ppm of paraquat ion) (Cervenka et
al., 1964).
One group of dogs (three males and three females) were fed paraquat
dichloride 75 ppm in the diet for two years. Slight alterations were
seen in the lungs upon gross and microscopic examination which were
believed to be due to paraquat. No adverse effects were noted on
body-weight, food consumption, survival, behaviour, haematological
studies, blood chemistry, urinalysis, liver function tests,
organ-weights and ratios and growths and microscopic examination of
tissues other than lungs. The level of paraquat dichloride causing no
toxicological effect on the dog was 50 ppm (34 ppm paraquat cation)
(Baran and Calandra, 1965).
Grazing animals
Multiple daily oral administration to sheep at 20 mg/kg body-weight
for five days resulted in death of all animals within two weeks.
Multiple daily oral administration at 10 mg/kg for five days killed
one of six sheep in 26 days, while 5 mg/kg for fourteen days resulted
in listless animals; recovery was very slow (Walley, 1964).
Multiple daily oral administration to cattle of 20 mg/kg body-weight
for four days resulted in death within one week. Levels of 10 and 5
mg/kg body-weight orally for five and fourteen days, respectively,
resulted in no deaths, but animals were listless and unhealthy;
recovery was slow (Walley, 1964).
Five groups of two sheep each and three groups of one calf each were
exposed for four weeks to levels of 0, 1, 5, 10 or 20 ppm and 0.5 or
20 ppm of paraquat, respectively, in their drinking water. No adverse
toxicological effects wore noted after one month (Sarfaty, 1963).
Cattle suffered no toxic effects over a four-week period when grazed
on pasture immediately after it had been sprayed with paraquat. Horses
showed definite ill effects, including local lesions of the mouth and
increased mucus secretions (Calderbank et al., 1968).
Ewe lambs were grazed in pastures 30 days after treatment with 1-2
pounds of paraquat per acre with no effect on their growth or general
well being (Torell and Kay, 1964).
Subacute inhalation studies
One group of dogs (one male and one female), guinea pigs (five males
and five females) and rats (five males and five females) were exposed
to an aerosol of paraquat dichloride at a concentration of 0.1 mg/1,
six hours per day, five days per week, for three weeks. Growth
depression was evident among the guinea pigs and rats, and the female
dog lost weight. No effects wore noted with regard to untoward
behavioural reaction, haematology, blood chemistry and gross or
microscopic alterations of tissues (Palazzolo et al., 1965). Repeated
daily 6-hour exposures to rats of paraquat aerosols over a three-week
period produced signs of lung irritation, but no deaths, at 0.4 mg/l
(Gage, 1968b).
Long-term studies
Rat
Four groups of rats (30 males and 30 females, 60 of each sex were
controls) were fed diets containing paraquat dichloride at levels of
0, 50, 125 and 250 ppm for two years. No adverse effects were seen at
any level tested on growth, survival, behaviour, tumour incidence,
haematologic studies, urinalysis, organ weights, ratios of organ to
brain or organ to body-weight and gross pathologic examination.
Microscopic examination of tissues and organs at 0 and 250 ppm (170
ppm of paraquat cation) revealed no adverse effects (Kohn at al.,
1964).
OBSERVATIONS IN MAN
The hazard of paraquat to man has been associated with two general
areas: accidental ingestion and dermal contamination. Accidental oral
ingestion of paraquat in small quantities in man has generally
resulted in death (sometimes delayed). In almost all cases where death
occurred severe lung damage with proliferation of the alveolar walls
was evident. These lesions were occasionally accompanied by renal
failure as evidenced at autopsy by gross damage to the kidneys
(Bullivant, 1966; Fennelly et al., 1968; Goulding, 1968; Campbell,
1968; Duffy and O'Sullivan, 1968; Tilling, 1968; Matthew et al., 1968
and Cowie and Kahn, 1968). In two accidental poisoning cases, recovery
was complete (McKean, 1968; Lloyd, 1969).
One suicide case from subcutaneous injection of paraquat resulted in
delayed death in 17 to 18 days, with severe proliferation of the
epithelium of the lung. Renal macroscopic or microscopic pathological
changes were not evident (Almog and Tal, 1967; Herczeg and Reit,
1968).
Dermal contamination had been shown to result in damage and
discolouration of the fingernails. The damage included softening of
the nail at the base, with occasional loss of the nail. It appeared
that the damage was local because of the asymmetry of the lesion and
because the toenails were not affected (Samman and Johnston, 1969).
Following accidental instillation of paraquat into the eye, symptoms
of irritation and inflammation of the conjunctiva increase and large
areas of the conjunctiva and cornea may be shed. With treatment,
recovery is slow (Calderbank, 1968; Cant and Lewis, 1968).
COMMENT
Paraquat is not readily absorbed from the gastrointestinal tract.
After oral administration, it is excreted primarily in the faeces.
After subcutaneous administration, it is primarily excreted in urine.
The metabolic products formed by micro-organisms in the gut appear to
be more readily absorbed than paraquat. No information is available on
the toxicity of these metabolites formed by the gut flora. The soil
metabolite N-methylisonicotinic acid is of a low order of toxicity as
compared with the parent compound when tested in rats.
From the available experimental data on animals and clinical
experiences with man, it is evident that paraquat causes irreversible
proliferative changes in lung tissue. The level of 75 ppm when fed to
dogs over a two-year interval may be considered as a threshold dose on
the basis of this pulmonary effect. An adverse effect on a long-term
study in rats could not be demonstrated with levels up to 250 ppm.
These results indicate that the rat is an insensitive species and,
while the dog in affected by paraquat, its susceptibility may be lower
than man. Man must be considered more sensitive than other species
thus far examined. In man, the delayed occurrence of lesions in the
lung, renal failure and a local effect on corneal epithelium, nasal
mucosa, skin and fingernails suggest that paraquat my be considered a
pesticide whose handling be restricted to trained professional
personnel.
In addition, studies on prophylaxis and treatment of the toxic effects
of paraquat were considered to be urgently needed; reproduction
studies are limited to one species, the rat. For the above-mentioned
reasons, the Committee considered that it was possible to establish a
temporary acceptably daily intake for man, based upon the no-effect
level in the dog.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg body-weight/day
(corresponds to 9.1 mg paraquat ion/mg body-weight/day)
Dog: 50 ppm in the diet, equivalent to 1.25 mg/kg body-weight/day
(corresponds to 0.91 mg paraquat ion/kg body-weight/day)
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE IN MAN
0-0.001 mg/kg body-weight as paraquat dichloride (0-0.0007 mg/kg
body-weight expressed as paraquat ion)
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Herbicide and desiccant; rapidly absorbed by green plants but
inactivated on contact with soil. Widely used for pre-crop and
post-crop-emergence weed control, plantation weed control, aquatic
weed control, pasture renovation, pre-harvest desiccation of hops,
cotton defoliation and potato haulm and sugar cane desiccation.
FATE OF RESIDUES
In animals
The fate of 14C-paraquat administered orally to cattle was
investigated by Stevens and Watley (1966). They found that the bulk of
radioactivity was excreted in the faeces and amounts <5% were
present, largely as breakdown products, in the urine.
A group of three cows, orally treated with a single dose of paraquat
at 8 mg/kg, excreted between 0.003 and 0.008 percent of the dose in
the milk and 0.24 percent of the dose in the urine within the
seven-day testing interval (Stevens et al.,1966; Stevens et al.,
1964). The amount of ingested material in the milk was the same
irrespective of position of a radioactive label indicating that
paraquat itself or a metabolite(s) containing methyl groups and intact
ring structures was present.
Two calves grazed for three or seven days on pasture containing
residues of 300-400 ppm paraquat were found to have significant
residues of paraquat only in the gut and stomach tissues. The kidney
contained the highest tissue residues of 0.15 ppm, with traces found
in lungs, heart and liver (Litchfield, 1969).
In plants
From observations made with 14C-labelled material, paraquat is
transported to a slightly greater extent than diquat from the leaves
of potato plants to the tubers (Slade and Bell, 1966). Coates et al.
(1966) found appreciable movement in wheat, even in the roots. Slade
(1966) studied the degradation of labelled paraquat on tomato, broad
beans and maize plants; the degradation was found to be non-enzymic,
but could be attributed to sunlight. Using potato plants, experiments
showed that even if metabolism had occurred in the plant, no
degradation products were transported to the tubers.
In soil
Paraquat has been shown to be degraded by soil microorganisms
(Funderburk and Bozarth, 1967) to demethylated paraquat
(1-methyl-4,4'-dipyridinium ion) and another compound characterized as
the 1-methyl-4-carboxy-pyridinium ion (N-methylisonicotinic acid).
The following degradation pathway by bacteria - demethylation of
parent molecule and ring cleavage of one of the heterocyclic rings to
eventually forms the carboxylated N-methyl-pyridinium ion.
 In sunlight
On the plant surface (and in solution), paraquat is rapidly broken
down photochemically. The two end products from ultraviolet
irradiation of solutions, both of which have very low toxicities in
mammals, are identified as N-methyl betaines of iso-nicotinic acid and
methylamine.
Slade (1965, 1966) investigated the degradation of paraquat by both
sunlight and the ultraviolet light from a mercury vapour lamp. Two
degradation products were identified, 1-methyl-4-carboxypyridinium ion
and methylamine hydrochloride; the following degradation pathway was
proposed:
In sunlight
On the plant surface (and in solution), paraquat is rapidly broken
down photochemically. The two end products from ultraviolet
irradiation of solutions, both of which have very low toxicities in
mammals, are identified as N-methyl betaines of iso-nicotinic acid and
methylamine.
Slade (1965, 1966) investigated the degradation of paraquat by both
sunlight and the ultraviolet light from a mercury vapour lamp. Two
degradation products were identified, 1-methyl-4-carboxypyridinium ion
and methylamine hydrochloride; the following degradation pathway was
proposed:
 In water
Paraquat applied to water for aquatic weed control purposes quickly
disappears due to uptake by weeds and absorption by soil, silt and
particulate suspended matter (Calderbank, 1968). No information is
available on the ultimate fate of the chemical in this environment.
The rate of disappearance in very variable, depending on the movement
of the water and the presence of mud or suspended matter, but
treatments within the range of 1-4 mg/litre in the water have resulted
in only 0.1 mg/litre or less of paraquat being detectable in from 6 to
14 days after application. Decomposition of the killed weed is rapid,
any remaining residue of paraquat thus liberated being subsequently
absorbed on the bottom mud. Such residues in the largely organic muds
may be more readily available to bacterial degradation than when
absorbed to clay minerals in soils.
Evidence of residues in food in commerce or at consumption
Only when the crop in sprayed directly are significant residues of
paraquat likely to be found. A summary of residues found in cotton
after use for desiccation purposes is given in Table II. (Calderbank,
1968)
TABLE II
Paraquat residues in, ten days after desiccation at 0.5 lb
paraquat/acre (U.S.A. results)
Fraction analysed Paraquat found, ppm
Cotton as picked, including
trash and bolls 2.0
Ginned seed 0.18
Acid-delinted seed 0.05
Mechanically reginned seed 0.08
Lint cotton 3.0
Hulls 0.13
Trash 3.7
Crude oil Non-detected
Meal 0.02
Data obtained following use of paraquat as a desiccant on several food
crops has also been published (Calderbank, 1968); a summary of these
results is given in Table III.
METHODS OF RESIDUE ANALYSIS
An ion-exchange method for determining paraquat residues has been
developed by Calderbank and Yuen (1965). The method depends on the
measurement of light absorption at 396 nm of reduced solutions of
paraquat after concentration and purification by cation-exchange
chromatography and has been used for a wide variety of food crops,
water, etc.; limit of sensitivity is 0.01 ppm. Radaelli and Bosetto
(1968) used it for the determination of residues in clays and mineral
soils. Paraquat and its photochemical decomposition product,
4-carboxyl-1-methyl pyridinium chloride, can be determined
polarographically (Slade and Jackson, 1971).
TABLE III
Summary of paraquat residues in food crops, desiccation uses
Rate of Average Residues
Application Paraquat
Crop (lb/acre) (ppm)
Barley 0.5 -1.0 3-10
Wheat 0.5 -1.0 1-2.5
Maize 0.5 -1.2 ND2/0.2
Rice (with husk) 0.15 -0.54 0.7-22
Rice (dehusked or polished) 0.15 -0.54 ND-0.2
Peas, beans, sunflower seed 0.35 -1.2 ND-2.0
Sorghum seed 0.25 -1.0 0.1-0.4
Cotton (as picked) 0.5 -1.0 2-3
Onions 0.5 -2.0 0.05 -0.5
Potatoes 0.5 -1.5 0.02 -0.13
Sugar cane juice 0.5 -2.0 ND
Seed oils (sesame,
sunflower, rape, cotton) up to 1.2 ND
1/ 3-21 days after application
2/ ND = none detected
The lesser duckweed (Lemna minor L) provides a simple sensitive
bioassay technique for determining paraquat residues in water
(Funderburk and Lawrence, 1963). Plant extracts containing paraquat
have been chromatographed on thin layers of silica gel by Slade (1966)
using 5 M ammonium chloride solution for development. Faust and Hunter
(1965) determined paraquat in natural surface water at 256 nm
following chemical clean-up by ion exchange.
NATIONAL TOLERANCES
Country Crop Tolerance
(ppm)
U.S.A. Potatoes 0.5
Apples, pears, apricots, avocados,
bananas, cherries, citrus fruits,
figs, grapes, papayas, peaches, 0.05
nectarines, plums, prunes (fresh)
maize, lettuce, melons, peppers,
tomatoes 0.05
maize and sorghum grain 0.05
maize, sorghum and soybean forage 0.05
Almond hulls 0.5
Almonds, filberts, macadamia 0.05
nuts and walnuts
Coffee beans, olives, soybeans 0.05
Cottonseed 0.5
Sugarbeet (roots and tops) 0.5
Information has also been received regarding -
Cotton (as picked) 2 mg/kg
APPRAISAL
Paraquat is very widely used for weed control in many crops, as an
aquatic herbicide and as a desiccant on cotton, potato haulm and sugar
cane. Paraquat in a stable compound in plants. Ultraviolet light,
sunlight and soil micro-organisms degraded paraquat to
N-methyl-isonicotinic acid and methylamine hydrochloride. Following
ingestion by cows, traces of paraquat or its metabolites are secreted
into the milk. Residues are very unlikely to accrue from soil or
pre-emergence applications but can occur following use for desiccation
purposes. The suggested tolerances are based on each desiccation
usage. The colorimetric procedure of Calderbank and Yuen (1965) should
be suitable for regulatory purposes.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to June 1974)
Cottonseed 0.2 ppm
Potatoes 0.1 ppm
Cottonseed meal 0.05 ppm
Cottonseed oil (edible) 0.05 ppm
Sugar cane juice 0.05 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Detailed comparative toxicity and metabolism studies in order to
elucidate the reason for the comparatively high sensitivity of man
to this compound.
2. Additional reproduction studies on at least one species.
3. Examination in several species of the toxic effects of metabolites
formed by the action of the gut flora.
DESIRABLE
Long-term oral studies on additional species.
REFERENCES
Almog, C.H. and Tal, E. (1967) Death from paraquat after subcutaneous
injection. Brit. Med. J., 3: 721
Baran, J. and Calandra, J.C. (1965) Two-year chronic oral toxicity of
paraquat-beagle dogs. Unpublished report from Industrial Bio-Test
(Aug. 1964) to ICI through Chevron Chemical Co. to FDA
Broadhurst, T.O., Griffiths, D. and McElligott, T.F. (1966) Ninety-day
oral toxicity of N-methyl-isonicotinic acid methosulfate - albino
rats. Unpublished report IHR/194 (April 1966) ICI Ltd. through Chevron
Chemical Co. to FDA
Bullivant, C.M. (1966) Accidental poisoning by paraquat: report of two
cases in man. Brit. Med. J., 1: 1272-1273
Calderbank, A. and Yuen, S.H. (1965) An Ion-exchange method for
determining paraquat residues in food crops. Analyst, 90: 99-106
Calderbank, A., McKenna, R.M., Stevens, M.A. and Walley, J.K. (1968)
Grazing trials on paraquat-treated pasture. J. Sci. Fd. Agric. 19:
246-250
Calderbank, A. (1968) The bipyridylium herbicides. Effects on man,
Advances in pest control research, Metcalf R.L. Ed. vol 8, p. 224
Interscience Publishers N.Y.
Cambar, P.J. and Aviado, D.M. (1970) Bronchopulmonary effects of
paraquat and expectorants. Arch. environm. Hlth, 20: 488-494
Campbell, S. (1968) Paraquat poisoning. Clinical Toxicol.,1: 245-249
Cant, J.S. and Lewis, D.R.H. (1968) Ocular damage due to paraquat and
diquat. Brit. Med. J., 2: 224
Cervenka, H., Kay, J.H. and Calandra, J.C. (1964) Chronic oral
toxicity of paraquat - beagle dogs. Unpublished report from Industrial
Bio-Test (Aug. 1964) to ICI through Chevron Chemical Co. to FDA
Clark, D.G. (1965a) The acute toxicity of paraquat. Unpublished report
IHR/170 (Jan. 1965) from ICI through Chevron Chemical Co. to FDA
Clark, D.G. (1965b) Unpublished report TR/438 (25 Jan. 1965) from ICI
Ltd. through Chevron Chemical Co. to FDA
Clark, D.G., McElligott, T.F. and Hurst, E.W. (1966) The toxicity of
paraquat. Brit. J. Indust. Med., 23: 26-32
Coates, G.E., Funderburk, H.H., Lawrence, J.M. and Davis, D.E. (1966)
Factors affecting persistence and inactivation of diquat and paraquat.
Weed Res.,6:58-66
Conning, D.M., Fletcher, K. and Swan, A.A.B. (1969) Paraquat and
related bipyridyls. Brit. Med. Bull.,25:245-249
Cowie, J. and Kahn, J. (1968) Acute renal failure in case of paraquat
poisoning. Brit. Med. J., 1: 749-750
Daniel, J.W. and Gage, J.C. (1966) Absorption and excretion of diquat
and paraquat in rats. Brit. J. Industr. Med.,23: 133-136
Duffy, B.S. and O'Sullivan, D.J. (1968) Paraquat poisoning. J. Irish
Med. Assoc.,61: 97-98
Faust, S.D. and Hunter, N.E. (1965) Chemical methods for the
determination of aquatic herbicides. J. Am. Water Works Assoc., 57:
1028-1037
Fennelley, J.J., Gallagher, J.T. and Carroll, R.J. (1968) Paraquat
poisoning in a pregnant woman. Brit. Med. J.,3: 722-723
Funderburk, H.H. and Lawrence, J.M. (1963) A sensitive method for
determination of low concentrations of diquat and paraquat. Nature,
199: 1011-1012
Funderburk, H.H. and Bozarth, G.A. (1967) Review of the metabolism and
decomposition of diquat and paraquat. J. Agric. Fd. Chem.,15:
563-567
Gage, J.C. (1968a) The action of paraquat and diquat on the
respiration of liver cell fractions. Biochem. J.,109: 757-761
Gage, J.C. (1968b) Toxicity of paraquat and diquat aerosols generated
by a size selective cyclone. Effect of particle size distribution.
Brit. J. Industr. Med.,25: 304-314
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol.,14: 515-534
Goulding, R. (1968) Paraquat poisoning. The Practitioner,200:739-740
Griffiths, D., Ponsford, D.C. and Hurst, E.W. (1966) A study of
reproduction in rats treated with paraquat in the diet. Unpublished
report IHR/185 (Jan. 1966) from ICI Ltd.
Herczeg, E. and Reit, A. (1968) Lungenveranderunger bei todlich
verlaufener paraquatvergiftung. Zbl. allg. Path. Anat., 111: 325-328
Khera, L.S. and Whitta, L.L. (1968) Embryopathic effects of diquat and
paraquat in the rat. Ind. Med. Surg., 37: 553
Kohn, F.E., Kay, J.H. and Calandra, J.C. (1964) Two-year chronic oral
toxicity of paraquat-albino rats. Unpublished report from Industrial
Bio-Test Laboratories (July 1964) to ICI through Chevron Chemical Co.
to FDA
Litchfield, M.H. (1969) Grazing trial on paraquat-treated pasture.
Unpublished report IHR/257 (April 1969) ICI Ltd.
Lloyd, E.L. (1969) Recovery after taking Weedol. Brit. Med. J., 2:
189
Manktelow, B.W. (1967) The loss of pulmonary surfactant in paraquat
poisoning. Brit. J. Exptl. Path. 48: 366-369
Matthew, H., Logan, A., Woodrugg, M.F.A. and Heard, B, (1968) Paraquat
poisoning - lung transplantation. Brit. Med. J.,3: 759-763
McElligott, T.F. (1965) The dermal toxicity of paraquat. Unpublished
ICI report IHR/172 (Jan 1965; through Chevron Chemical Co. to FDA
McElligott, T.F. (1966) Toxicological report - N-methyl isonicotinic
acid methosulphate. Unpublished report TR/512 (April 1966) ICI Ltd.
through Chevron Chemical Co. to FDA
McKean, W.I. (1968) Recovery from paraquat poisoning. Brit. Med.
J.,3:292
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. (1964) Acute aerosol
inhalation toxicity of paraquat dichloride. Unpublished report from
Bio-Test Lab. Inc. to ICI (15 April 1964) through Chevron Chemical Co.
to FDA
Palazzolo, R.J. and Calandra, J.C. (1965) Sub-acute dermal toxicity of
paraquat dichloride. Unpublished report by Industrial Bio-Test Lab.
(Aug. 1966) through California Chemical Co. to FDA
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. (1965) Sub-acute
aerosol inhalation toxicity of paraquat dichloride. Unpublished report
from Indus. Bio-Test Lab. (Jan. 1965) to ICI Ltd. through Chevron
Chemical Co. to FDA
Radaelli, L. and Bosetto, M. (1968) Determination of paraquat in clays
and mineral soils. Ricerca Scient., 38: 855-857
Samman, P.D. and Johnston, E.N.M. (1969) Nail damage associated with
handling of paraquat and diquat. Brit. Med. J., 1: 818-819
Sarfaty, A.B. (1963) Observational trials on toxicity to sheep and
cattle. Unpublished report Chevron Chemical Co. to FDA
Slade, P. (1965) Photochemical degradation of paraquat. Nature, 207:
515-516
Slade, P. (1966) The fate of paraquat applied to plants. Weed Res.,
6: 158-167
Slade, P. and Bell, E.G. (1966) Movement of paraquat in plants. Weed
Res., 6: 267-274
Slade, P. and Jackson, L.S. (1971) Pestic. Sci., in preparation
Stevens, M.A., Walker, G.H. and Walley, J.K. (1964) The excretion of
14C paraquat by the cow. Unpublished report IHR/164 (Oct. 1964) by
ICI through Chevron Chemical Co. to FDA
Stevens, M.A. and Walley, J.K. (1966) Tissue and milk residues arising
from the ingestion of single doses of diquat and paraquat by cattle.
J. Sci. Food Agric., 17: 472-475
Swan, A.A.B. (1959) Unpublished report by ICI TR/241 (Dec. 1959)
submitted through Chevron Chemical Co. to FDA
Tilling, W. (1968) Paraquat - intoxikation. Dtsch. Medizin.
Wchschr.,50: 2439-2441
Torell, D. and Kay, B.L. (1964) Pasturing of sheep on paraquat treated
forage. Unpublished report - 383-42 Chevron Chemical Co. to FDA
Walley, J.K. (1964) Paraquat toxicity in sheep and cattle. Unpublished
report IHR/166 (April 1964) submitted from ICI through Chevron
Chemical Co. to FDA
In water
Paraquat applied to water for aquatic weed control purposes quickly
disappears due to uptake by weeds and absorption by soil, silt and
particulate suspended matter (Calderbank, 1968). No information is
available on the ultimate fate of the chemical in this environment.
The rate of disappearance in very variable, depending on the movement
of the water and the presence of mud or suspended matter, but
treatments within the range of 1-4 mg/litre in the water have resulted
in only 0.1 mg/litre or less of paraquat being detectable in from 6 to
14 days after application. Decomposition of the killed weed is rapid,
any remaining residue of paraquat thus liberated being subsequently
absorbed on the bottom mud. Such residues in the largely organic muds
may be more readily available to bacterial degradation than when
absorbed to clay minerals in soils.
Evidence of residues in food in commerce or at consumption
Only when the crop in sprayed directly are significant residues of
paraquat likely to be found. A summary of residues found in cotton
after use for desiccation purposes is given in Table II. (Calderbank,
1968)
TABLE II
Paraquat residues in, ten days after desiccation at 0.5 lb
paraquat/acre (U.S.A. results)
Fraction analysed Paraquat found, ppm
Cotton as picked, including
trash and bolls 2.0
Ginned seed 0.18
Acid-delinted seed 0.05
Mechanically reginned seed 0.08
Lint cotton 3.0
Hulls 0.13
Trash 3.7
Crude oil Non-detected
Meal 0.02
Data obtained following use of paraquat as a desiccant on several food
crops has also been published (Calderbank, 1968); a summary of these
results is given in Table III.
METHODS OF RESIDUE ANALYSIS
An ion-exchange method for determining paraquat residues has been
developed by Calderbank and Yuen (1965). The method depends on the
measurement of light absorption at 396 nm of reduced solutions of
paraquat after concentration and purification by cation-exchange
chromatography and has been used for a wide variety of food crops,
water, etc.; limit of sensitivity is 0.01 ppm. Radaelli and Bosetto
(1968) used it for the determination of residues in clays and mineral
soils. Paraquat and its photochemical decomposition product,
4-carboxyl-1-methyl pyridinium chloride, can be determined
polarographically (Slade and Jackson, 1971).
TABLE III
Summary of paraquat residues in food crops, desiccation uses
Rate of Average Residues
Application Paraquat
Crop (lb/acre) (ppm)
Barley 0.5 -1.0 3-10
Wheat 0.5 -1.0 1-2.5
Maize 0.5 -1.2 ND2/0.2
Rice (with husk) 0.15 -0.54 0.7-22
Rice (dehusked or polished) 0.15 -0.54 ND-0.2
Peas, beans, sunflower seed 0.35 -1.2 ND-2.0
Sorghum seed 0.25 -1.0 0.1-0.4
Cotton (as picked) 0.5 -1.0 2-3
Onions 0.5 -2.0 0.05 -0.5
Potatoes 0.5 -1.5 0.02 -0.13
Sugar cane juice 0.5 -2.0 ND
Seed oils (sesame,
sunflower, rape, cotton) up to 1.2 ND
1/ 3-21 days after application
2/ ND = none detected
The lesser duckweed (Lemna minor L) provides a simple sensitive
bioassay technique for determining paraquat residues in water
(Funderburk and Lawrence, 1963). Plant extracts containing paraquat
have been chromatographed on thin layers of silica gel by Slade (1966)
using 5 M ammonium chloride solution for development. Faust and Hunter
(1965) determined paraquat in natural surface water at 256 nm
following chemical clean-up by ion exchange.
NATIONAL TOLERANCES
Country Crop Tolerance
(ppm)
U.S.A. Potatoes 0.5
Apples, pears, apricots, avocados,
bananas, cherries, citrus fruits,
figs, grapes, papayas, peaches, 0.05
nectarines, plums, prunes (fresh)
maize, lettuce, melons, peppers,
tomatoes 0.05
maize and sorghum grain 0.05
maize, sorghum and soybean forage 0.05
Almond hulls 0.5
Almonds, filberts, macadamia 0.05
nuts and walnuts
Coffee beans, olives, soybeans 0.05
Cottonseed 0.5
Sugarbeet (roots and tops) 0.5
Information has also been received regarding -
Cotton (as picked) 2 mg/kg
APPRAISAL
Paraquat is very widely used for weed control in many crops, as an
aquatic herbicide and as a desiccant on cotton, potato haulm and sugar
cane. Paraquat in a stable compound in plants. Ultraviolet light,
sunlight and soil micro-organisms degraded paraquat to
N-methyl-isonicotinic acid and methylamine hydrochloride. Following
ingestion by cows, traces of paraquat or its metabolites are secreted
into the milk. Residues are very unlikely to accrue from soil or
pre-emergence applications but can occur following use for desiccation
purposes. The suggested tolerances are based on each desiccation
usage. The colorimetric procedure of Calderbank and Yuen (1965) should
be suitable for regulatory purposes.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
TEMPORARY TOLERANCES (effective to June 1974)
Cottonseed 0.2 ppm
Potatoes 0.1 ppm
Cottonseed meal 0.05 ppm
Cottonseed oil (edible) 0.05 ppm
Sugar cane juice 0.05 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Detailed comparative toxicity and metabolism studies in order to
elucidate the reason for the comparatively high sensitivity of man
to this compound.
2. Additional reproduction studies on at least one species.
3. Examination in several species of the toxic effects of metabolites
formed by the action of the gut flora.
DESIRABLE
Long-term oral studies on additional species.
REFERENCES
Almog, C.H. and Tal, E. (1967) Death from paraquat after subcutaneous
injection. Brit. Med. J., 3: 721
Baran, J. and Calandra, J.C. (1965) Two-year chronic oral toxicity of
paraquat-beagle dogs. Unpublished report from Industrial Bio-Test
(Aug. 1964) to ICI through Chevron Chemical Co. to FDA
Broadhurst, T.O., Griffiths, D. and McElligott, T.F. (1966) Ninety-day
oral toxicity of N-methyl-isonicotinic acid methosulfate - albino
rats. Unpublished report IHR/194 (April 1966) ICI Ltd. through Chevron
Chemical Co. to FDA
Bullivant, C.M. (1966) Accidental poisoning by paraquat: report of two
cases in man. Brit. Med. J., 1: 1272-1273
Calderbank, A. and Yuen, S.H. (1965) An Ion-exchange method for
determining paraquat residues in food crops. Analyst, 90: 99-106
Calderbank, A., McKenna, R.M., Stevens, M.A. and Walley, J.K. (1968)
Grazing trials on paraquat-treated pasture. J. Sci. Fd. Agric. 19:
246-250
Calderbank, A. (1968) The bipyridylium herbicides. Effects on man,
Advances in pest control research, Metcalf R.L. Ed. vol 8, p. 224
Interscience Publishers N.Y.
Cambar, P.J. and Aviado, D.M. (1970) Bronchopulmonary effects of
paraquat and expectorants. Arch. environm. Hlth, 20: 488-494
Campbell, S. (1968) Paraquat poisoning. Clinical Toxicol.,1: 245-249
Cant, J.S. and Lewis, D.R.H. (1968) Ocular damage due to paraquat and
diquat. Brit. Med. J., 2: 224
Cervenka, H., Kay, J.H. and Calandra, J.C. (1964) Chronic oral
toxicity of paraquat - beagle dogs. Unpublished report from Industrial
Bio-Test (Aug. 1964) to ICI through Chevron Chemical Co. to FDA
Clark, D.G. (1965a) The acute toxicity of paraquat. Unpublished report
IHR/170 (Jan. 1965) from ICI through Chevron Chemical Co. to FDA
Clark, D.G. (1965b) Unpublished report TR/438 (25 Jan. 1965) from ICI
Ltd. through Chevron Chemical Co. to FDA
Clark, D.G., McElligott, T.F. and Hurst, E.W. (1966) The toxicity of
paraquat. Brit. J. Indust. Med., 23: 26-32
Coates, G.E., Funderburk, H.H., Lawrence, J.M. and Davis, D.E. (1966)
Factors affecting persistence and inactivation of diquat and paraquat.
Weed Res.,6:58-66
Conning, D.M., Fletcher, K. and Swan, A.A.B. (1969) Paraquat and
related bipyridyls. Brit. Med. Bull.,25:245-249
Cowie, J. and Kahn, J. (1968) Acute renal failure in case of paraquat
poisoning. Brit. Med. J., 1: 749-750
Daniel, J.W. and Gage, J.C. (1966) Absorption and excretion of diquat
and paraquat in rats. Brit. J. Industr. Med.,23: 133-136
Duffy, B.S. and O'Sullivan, D.J. (1968) Paraquat poisoning. J. Irish
Med. Assoc.,61: 97-98
Faust, S.D. and Hunter, N.E. (1965) Chemical methods for the
determination of aquatic herbicides. J. Am. Water Works Assoc., 57:
1028-1037
Fennelley, J.J., Gallagher, J.T. and Carroll, R.J. (1968) Paraquat
poisoning in a pregnant woman. Brit. Med. J.,3: 722-723
Funderburk, H.H. and Lawrence, J.M. (1963) A sensitive method for
determination of low concentrations of diquat and paraquat. Nature,
199: 1011-1012
Funderburk, H.H. and Bozarth, G.A. (1967) Review of the metabolism and
decomposition of diquat and paraquat. J. Agric. Fd. Chem.,15:
563-567
Gage, J.C. (1968a) The action of paraquat and diquat on the
respiration of liver cell fractions. Biochem. J.,109: 757-761
Gage, J.C. (1968b) Toxicity of paraquat and diquat aerosols generated
by a size selective cyclone. Effect of particle size distribution.
Brit. J. Industr. Med.,25: 304-314
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol.,14: 515-534
Goulding, R. (1968) Paraquat poisoning. The Practitioner,200:739-740
Griffiths, D., Ponsford, D.C. and Hurst, E.W. (1966) A study of
reproduction in rats treated with paraquat in the diet. Unpublished
report IHR/185 (Jan. 1966) from ICI Ltd.
Herczeg, E. and Reit, A. (1968) Lungenveranderunger bei todlich
verlaufener paraquatvergiftung. Zbl. allg. Path. Anat., 111: 325-328
Khera, L.S. and Whitta, L.L. (1968) Embryopathic effects of diquat and
paraquat in the rat. Ind. Med. Surg., 37: 553
Kohn, F.E., Kay, J.H. and Calandra, J.C. (1964) Two-year chronic oral
toxicity of paraquat-albino rats. Unpublished report from Industrial
Bio-Test Laboratories (July 1964) to ICI through Chevron Chemical Co.
to FDA
Litchfield, M.H. (1969) Grazing trial on paraquat-treated pasture.
Unpublished report IHR/257 (April 1969) ICI Ltd.
Lloyd, E.L. (1969) Recovery after taking Weedol. Brit. Med. J., 2:
189
Manktelow, B.W. (1967) The loss of pulmonary surfactant in paraquat
poisoning. Brit. J. Exptl. Path. 48: 366-369
Matthew, H., Logan, A., Woodrugg, M.F.A. and Heard, B, (1968) Paraquat
poisoning - lung transplantation. Brit. Med. J.,3: 759-763
McElligott, T.F. (1965) The dermal toxicity of paraquat. Unpublished
ICI report IHR/172 (Jan 1965; through Chevron Chemical Co. to FDA
McElligott, T.F. (1966) Toxicological report - N-methyl isonicotinic
acid methosulphate. Unpublished report TR/512 (April 1966) ICI Ltd.
through Chevron Chemical Co. to FDA
McKean, W.I. (1968) Recovery from paraquat poisoning. Brit. Med.
J.,3:292
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. (1964) Acute aerosol
inhalation toxicity of paraquat dichloride. Unpublished report from
Bio-Test Lab. Inc. to ICI (15 April 1964) through Chevron Chemical Co.
to FDA
Palazzolo, R.J. and Calandra, J.C. (1965) Sub-acute dermal toxicity of
paraquat dichloride. Unpublished report by Industrial Bio-Test Lab.
(Aug. 1966) through California Chemical Co. to FDA
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. (1965) Sub-acute
aerosol inhalation toxicity of paraquat dichloride. Unpublished report
from Indus. Bio-Test Lab. (Jan. 1965) to ICI Ltd. through Chevron
Chemical Co. to FDA
Radaelli, L. and Bosetto, M. (1968) Determination of paraquat in clays
and mineral soils. Ricerca Scient., 38: 855-857
Samman, P.D. and Johnston, E.N.M. (1969) Nail damage associated with
handling of paraquat and diquat. Brit. Med. J., 1: 818-819
Sarfaty, A.B. (1963) Observational trials on toxicity to sheep and
cattle. Unpublished report Chevron Chemical Co. to FDA
Slade, P. (1965) Photochemical degradation of paraquat. Nature, 207:
515-516
Slade, P. (1966) The fate of paraquat applied to plants. Weed Res.,
6: 158-167
Slade, P. and Bell, E.G. (1966) Movement of paraquat in plants. Weed
Res., 6: 267-274
Slade, P. and Jackson, L.S. (1971) Pestic. Sci., in preparation
Stevens, M.A., Walker, G.H. and Walley, J.K. (1964) The excretion of
14C paraquat by the cow. Unpublished report IHR/164 (Oct. 1964) by
ICI through Chevron Chemical Co. to FDA
Stevens, M.A. and Walley, J.K. (1966) Tissue and milk residues arising
from the ingestion of single doses of diquat and paraquat by cattle.
J. Sci. Food Agric., 17: 472-475
Swan, A.A.B. (1959) Unpublished report by ICI TR/241 (Dec. 1959)
submitted through Chevron Chemical Co. to FDA
Tilling, W. (1968) Paraquat - intoxikation. Dtsch. Medizin.
Wchschr.,50: 2439-2441
Torell, D. and Kay, B.L. (1964) Pasturing of sheep on paraquat treated
forage. Unpublished report - 383-42 Chevron Chemical Co. to FDA
Walley, J.K. (1964) Paraquat toxicity in sheep and cattle. Unpublished
report IHR/166 (April 1964) submitted from ICI through Chevron
Chemical Co. to FDA
