
BROMOPHOS JMPR 1972
IDENTITY
Chemical name
O,O-dimethyl-O-(2,5-dichloro-4-bromophenyl)
phosphorothioate.
Synonyms
Nexion(R), S 1942, SHG-1942, bromofos.
Structural formula
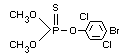 Other information on identity and properties
Molecular weight: 366.0
State: white crystals
Melting point: 53°C
Boiling point: 140-142°C at 10-2 torr.
Vapour pressure: 1.3 x 10-4 mm Hg at 20°C
Solubility: soluble in most organic solvents, e.g., toluene,
carbon tetrachloride, diethyl ether. Slightly
soluble in low molecular weight alcohols. Water
solubility - 40 ppm at 27°C.
Stability:
Stable in aqueous suspension. Hydrolyzes in distinct alkaline medium.
Purity of technical material:
O-4-bromo-2,5-dichlorophenyl-O,
O-dimethyl-phosphorothioate: approx. 95.0%;
O-4-bromo-2,3-dichlorophenyl-O,O-dimethyl-phosphorothioate:
approx. 3.0%;
O-6-bromo-2,5-dichlorophenyl-O,O-dimethyl-phosphorothioate:
approx. 1.0%;
O-dichlorophenyl-O,O-dimethyl-phosphorothioate:
approx. 1.0% (chlorine position not defined).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies on rats with 32P and 3H-labelled bromophos showed it is well
absorbed from the gastrointestinal tract. In the first 12 hours
following oral, intravenous or intraperitoneal administration, large
quantities of radioactivity were found in the stomach, intestine,
liver and kidneys, but after 24 hours the majority was found in urine
and faeces. Bromophos showed no tendency to accumulate in any organ.
The maximum blood level was found 7 hours after oral administration,
and the biological half life in rats was 14 hours. Approximately 96%
of the activity of a 10 mg/kg dose of 3H-bromophos was excreted in
urine and 1% in faeces during 24 hours following oral administration.
Excretion was complete in 96 hours, a total of 2% of activity
appearing in the faeces. In the same period, administration of
32P-bromophos resulted in the excretion of 63% of activity in urine
and 16% in faeces. During 8 hours following intraduodenal
administration of 5 mg/kg of 3H-bromophos, 25% of the activity was
excreted in bile in rats. Since only 1% of 32P-bromophos was excreted
in the same period, the biliary excretion was probably mainly
dichlorobromophenol or its metabolites. Biliary excreted 3H is
probably re-absorbed since only 2% of an oral dose was found in
faeces. The relatively high 32P content in faeces may result from
metabolism of bromophos in the intestine (Stiasni et al., 1967).
Biotransformation
Five urine metabolites were found after administration of
32P-bromophos whereas three were found after administration of
3H-bromophos. Analysis showed that the metabolites were phosphate,
dimethylthionophosphate, monodesmethyl-bromophos and
dichlorobromophenol. Two metabolites were unidentified. Bromophos
itself, its O-analogue bromoxon and monodesmethyl-bromoxon were not
found in urine (Stiasni et al., 1967).
Effect on enzymes and other biochemical parameters
Bromophos inhibits cholinesterase activity in rats and dogs, the
no-effect level when administered over a 100-day period being 0.63
mg/kg/day in rats and 1.5 mg/kg/day in dogs (Kinkel and Seume, 1963;
Kinkel et al., 1965; Kinkel and Dirks, 1966; Boehringer, 1968;
Leuschner et al., 1967). When a single oral dose of 800 or 1200
mg/kg was given to horses, plasma cholinesterase was inhibited even
after 16 days, although partial reactivation was found (Paton, 1965).
TOXICOLOGICAL STUDIES
Special studies on the metabolites
Several metabolites were found during investigations on rats and dogs
and on examination of metabolism of bromophos in plants. The most
important of these have been examined. A summary of acute oral
toxicities of bromophos metabolites is given in Table 1.
Short-term toxicity of metabolites
Groups of 10 male and 10 female rats were fed for 5 weeks on control
diet or diet providing 25, 100 or 400 mg/kg/day bromoxon, 250, 500 or
1000 mg/kg/day desmethyl-bromoxon or 250, 500 or 1000 mg/kg/day of
2,5-dichloro-4-bromophenol. At the highest dosage levels with each
metabolite the growth of animals was significantly inhibited. Plasma
and RBC cholinesterases were inhibited by bromoxon at 25 mg/kg
(maximum inhibition), and the plasma enzyme by desmethyl-bromoxon at
500 mg/kg. No enzyme inhibition occurred with dichloro-4-bromophenol.
The results of other investigations were normal; histopathological
studies were not carried out (Leuschner, 1968)
Groups of 10 male and 10 female rats were fed on diets providing 0,
125, 250 and 500 mg/kg/day of desmethyl-bromophos for 5 weeks. At 500
mg/kg, plasma cholinesterase was minimally inhibited. The weights of
liver and pituitary were above normal in male animals and histological
examination showed minimal centrilobular fatty infiltration of the
liver, the latter finding was considered to be reversible. Body weight
and food intake were reduced at the 500 mg/kg level. No abnormalities
were found at lower dosage levels (Leuschner et al., 1969).
Special studies on neurotoxicity
Six adult hens were administered 10 g/kg body-weight of bromophos
together with atropine and 2-PAM. Ataxia and incoordination were
present in all birds for several days, and three died. Histological
examination was said to reveal severe demyelination in all birds
(Byewater, 1966).
TABLE 1
Acute oral toxicities of bromophos metabolites
Compound Animal LD50 Reference
(mg/kg body-weight)
Bromoxon Mouse 2 100 Muacevic, 1965
Monodesmethyl-bromophos Rat 1 900-4 600 Muacevic, 1965
Mouse 1 800 1966
Desmethyl-bromophos Mouse 1 085 Muacevic, 1968
(triethylamine salt)
Desmethyl-bromoxon Mouse 2 150 Muacevic, 1965
O,O-dimethyl-O-(5,6-dichloro-4-bromophenyl)-thiophosphoric Mouse 2 850 Muacevic, 1965
acid ester
O,O-dimethyl-O-(4,6-dichloro-2-bromophenyl)-thiophosphoric Mouse about 2 000 Muacevic, 1965
acid ester
O,O-dimethyl-O-(2,4-dichloro-6-bromophenyl)-thiophosphoric Mouse >6 000 Muacevic, 1965
acid ester
O,O-dimethyl-thiophosphoric acid Mouse 4 700 Muacevic, 1965
sodium
2,5-dichloro-4-bromophenol Mouse 1 100-1 900 Muacevic, 1965
Rat 3 200-3 550 1966
Ten adult hens each received orally 1 g bromophos/kg/day until signs
of paralysis developed (between 12 and 56 days), at which time each
was examined for histological changes in the brain and spinal cord.
These were reported to consist of degeneration of ganglia cells and
demyelination (Kinkel and Hubner, 1966), but the suitability of the
material for accurate pathological interpretation was challenged
(Glees, 1966).
Two adult hens were each given 5.5 g/kg of bromophos in divided doses
over a period of 7 weeks. A 300 mg/kg dose produced transient
excitability and diarrhoea and the 4th of 5 and 4th of 8 consecutive
doses of 400 mg/kg day given at a 3-week interval caused an unsteady
gait and diarrhoea which persisted each time for 6-9 days after
administration. The hens completely recovered following this
treatment, indicating the lack of delayed neurotoxic effect (Barnes,
1966). Ten adult hens received two oral doses of 2 g/kg of bromophos
at 3-week intervals. A neuropathological examination carried out after
a 3-week observation period revealed no degenerative processes in the
CNS or peripheral nerves (Muacevic and Glees, 1967).
Groups of 10 adult hens were administered 0, 12.5 or 125 mg/kg of
bromophos/day in their food for 4 weeks. Clinically apparent
neurological signs were reversible when treatment ceased and a
neuropathological examination of 2 birds of each group showed no CNS
changes (Muacevic and Glees, 1968).
In order to investigate the occurrence of paralysis in dogs
administered bromophos for 2 years, and the finding on
neuropathological examination of localized ganglion cell degeneration
and other lesions in the CNS (Kinkel et al., 1965), an experiment
was carried out in which two groups of 3 male and 3 female dogs were
administered 0 and 87.5 mg/kg and 6 male and 6 female dogs 175 mg/kg
body-weight/day of bromophos orally in capsules for periods up to 270
days. One dog on 175 mg/kg had a single epileptiform fit and another
was killed after 62 doses because it was cachectic. An extensive
neuropathological examination of the central nervous system, spinal
ganglia and peripheral nerves failed to reveal any neuropathological
changes (Boehringer, 1968).
Special studies on pharmacology
Bromophos inhibits cholinesterase activity indirectly through its
metabolite bromoxon. No antidote effect was found with atropine
sulphate and aldoximes when rats and mice were administered high doses
of bromophos orally or intraperitoneally. The lack of effect was
probably due to the toxic effect of the high doses of solvent which
had to be used to administer the bromophos. However, cholinesterase
inhibition was diminished in mice by atropine and Toxogonin (Muacevic,
1965). Inhibition of brain cholinesterase in bromophos treated rats
could be prevented by intravenous injection of several reactivators
(Muacevic, 1968). Administration of atropine and 2-PAM stopped
development of signs of poisoning when 0.02-0.2 g/kg body-weight of
bromophos was administered to chickens. At 0.5 and 1.0 g/kg dosage
levels the development of signs of poisoning were delayed (Kinkel,
1964a).
Special studies on reproduction
Groups of 20 male and 20 female rats were fed on diets providing 0, 5,
20 and 80 mg bromophos/kg/day. These formed the parent generation of a
standard three generation study (F.D.A. advisory committee, 1970). The
80 mg/kg dosage level produced no clinical signs of poisoning but the
rate of body-weight gain was depressed in all generations,
particularly in males. The fertility and size and weight of litters
was unaffected, but the number of stillbirths in this group was high.
The survival rate of young was also reduced except in the first litter
and the parent generation. Animals consuming 5 or 20 mg/kg
bromophos/day were no different from control animals. No runts or
malformations were observed at any dosage or in any generation, and
the behaviour, appearance, food intake, results of haematological
investigations and adult survival were also unaffected. In this study
the cholinesterase activity of plasma and liver was significantly
depressed in animals receiving 5 mg/kg/day. The threshold dosage level
for RBC enzyme inhibition was between 5 and 30 mg/kg and for brain
enzyme between 5 and 20 mg/kg in males and 20 and 80 mg/kg in females
(Leuschner et al., 1967).
Special studies on teratogenicity
Five groups of 10 pregnant female rabbits were administered by gavage
25, 50, 100, 200 or 400 mg/kg body-weight of bromophos each day
between day 6 and 18 of pregnancy. Forty animals acted as controls.
Foetuses were removed and examined on day 30 of pregnancy. Twenty five
mg bromophos/kg/day orally had no untoward effect on foetuses or
parent animals. At higher dosage levels the parent animals became
debilitated and developed limb flaccidity and diarrhea, and the number
of fully-grown, live foetuses was reduced. In no group did the type or
number of malformations observed differ from that of the controls
(Leuschner and Leuschner, 1966a).
Acute toxicity
Studies on the acute toxicity of bromophos in several animal species
are summarized in Table 2.
TABLE 2 Acute toxicity of bromophos in animals
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 3 311 - 5 900 Muacevic, 1963; 1964;
1965; 1967;
Worth et al., 1967
Mouse i.p. 1 000 - 4 900 Oettel, 1963; Muacevic,
1964
Rat oral 3 750 - 8 000 Kinkel et al., 1966;
Oettel, 1963; Muacevic,
1967.
Rat i.p. 3 125 Kinkel and Sann, 1964.
Guinea pig oral >6 000 Muacevic, 1967
Rabbit oral 720 Muacevic, 1964
Fowl oral 9 700 Kinkel, 1964a
Dog oral >625 Worth et al., 1967
Potentiation of the acute toxicity of bromophos occurred with
bromophos-ethyl, diazinon, dichlorvos, dimethoate, malathion,
mevinphos, naleb, parathion and carbaryl. DDT, heptachlor and lindane
possessed strong antagonistic activity to the acute effects of
bromophos (Kinkel, 1964b; Muacevic 1964, 1965, 1966, 1967, 1968).
Short-term studies
Rat
Four groups of 6 male and 6 female rats received diets providing 0,
0.65, 1.25 and 2.5 mg bromophos per kg body-weight/day for 100 days.
The cholinesterase activity in RBC was not significantly affected at
any dosage level. Plasma enzyme was inhibited at the 1.25 mg/kg level,
but not at the lower level, and it returned to normal in two weeks
after the treatment was stopped. The behaviour, growth, food intake
and macroscopic appearance of organs were normal (Leuschner and
Leuschner, 1966b).
Four groups of 16 male rats received by gastric tube daily doses of 0,
188, 750 and 1 250 mg bromophos/kg body-weight/day for 100 days. The
rate of body-weight gain was reduced in all test groups while food
intake was lower only at the highest level. Animals in the 750 and
1 250 mg/kg groups became restless for an hour or so after treatment
but the degree of restlessness did not increase during the test.
Brain, liver and plasma cholinesterase were inhibited at all dosage
levels. Hydropic swelling of the hepatic cells was seen at all dosage
levels; this effect was dose related. In the 1 250 mg/kg group hyaline
droplets were seen in tubular cells and protein within the tubules of
the kidneys. Urine analysis and the bromosulphophthalein retention
test yielded negative results. Haematological findings and the
macroscopic appearance of internal organs were normal (Kinkel and
Seume, 1963).
Dog
Three groups of 2 male and 2 female beagle dogs received diets
providing 0.75, 1.5 and 3.0 mg bromophos/kg/day for 100 days. The
control group consisted of one male and one female. No or only slight
inhibition of the plasma and RBC cholinesterase activities (never
exceeding 36%) was observed at the three dosage levels; an inhibition
of 20% was suggested as no-effect level in this experiment.
Erythrocyte cholinesterase was more susceptible to inhibition than
plasma cholinesterase. In one dog exposed to the lowest dosage level,
35% inhibition of the RBC cholinesterase was observed, but this was
reduced to 20% when tested seven weeks later. Neither the brain nor
the liver cholinesterase was found to be inhibited when examined
(Kinkel and Dirks, 1966).
Six groups of 3 male and 3 female mongrel dogs aged 1-3 years and
weighing between 3.9 and 10.3 kg were administered 0, 11, 43.8, 87.5
and 175 mg bromophos/kg body-weight by gastric tube on every working
day for 2 years. Treatment at the 87.5 and 175 mg/kg levels made the
animals restless for some time after dosage. One animal on each of the
43.8 and 175 mg/kg levels died after suffering bites, and one on 87.5
and 3 on 175 mg/kg died after developing respiratory difficulties,
hoarseness, salivation and tremors followed by ataxia and then paresis
of the hind limbs. Two dogs of the 175 mg/kg group developed the same
signs but recovered, one after being taken off treatment and the other
while administration of bromophos continued. Histological examination
of the animals which died on the two highest dosage levels showed foci
of ganglion cell degeneration in the spinal cord; this involved only
small numbers of cells in the ganglia. In addition, spermatogenesis
was impaired with proliferation of Leydig's cells in 2 males of the
175 mg/kg group. Degeneration of the CNS in animals which did not die
during the test was insignificant or non-existent. Plasma, RBC, liver
and brain cholinesterase activities were depressed at all dosage
levels in this test. The food intake, growth, sexual cycle, urinary
ascorbic acid excretion and the results of haematological, liver
function and urine tests were similar in test and control groups. The
relative organ weights and results of macroscopic and microscopic
examination of organs were normal in the 11 and 43.8 mg/kg groups
(Kinkel et al., 1965).
In a nine month study (See special studies on
neurotoxicity) groups of mongrel dogs weighing 6-20 kg were
administered 0, 87.5 or 175 mg bromophos/kg body-weight
daily in capsules for 270 days. Body-weight and food intake were
reduced in accordance with the dose given. Although 2/6 bitches on
175 mg/kg/day were at no time on heat, histological examination of
the ovaries and uterus revealed no pathological findings.
Histological examination showed seminiferous tubule degeneration in
3 dogs receiving 175 mg/kg/day. Prostatic hypertrophy was also more
evident in test animals. The death of one dog of the high level group
was not regarded as being due to bromophos consumption. Erythrocyte
and plasma cholinesterase were inhibited in both test groups. The
results of haematological tests, serum analysis and urine analysis
were all normal (Boehringer, 1968).
Long-term studies
Rat
Groups of 6-month-old rats were administered 0, 87.5, 175 and 350 mg
bromophos/kg body-weight by gavage each working day for 2 years. The
groups consisted of 25 male and 25 female animals, but 3, 5, 5 and 17
animals were included in the test after the start in the 0, 87.5, 175
and 350 mg/kg dosage groups, respectively. After 8, 10, 12, 15, 18 and
21 months, two animals, one of each sex, were killed for
histopathological examination. The rate of body-weight gain was
depressed at the highest dosage level, and in this group proteinuria
was more evident than in controls; in addition, the number of
erythrocytes in the urinary sediment was increased but the kidneys
were histologically normal.
Cholinesterase was inhibited in the plasma, RBC, brain and liver by
all treatment levels. Survival was poor, with only 5, 4, 2 and 5 males
and 9, 6, 7 and 8 females of the 0, 87.5, 175 and 350 mg/kg groups,
respectively, surviving for 100 weeks. The behaviour of all test
groups was normal as were the results of haematological and liver
function tests. Urinary ascorbic acid excretion was unaltered by
bromophos administration. The weights of 8 organs and the results of
macroscopic and microscopic examinations revealed no lesion which
could be attributed to treatment. No tumours were reported to have
occurred in control or test groups (Kinkel et al., 1965).
OBSERVATIONS IN MAN
Workers exposed to bromophos during production and formulation for two
years showed no change in RBC cholinesterase levels as estimated at
monthly intervals (Boehringer, 1966). Eight subjects were engaged in
spraying bromophos for 6´ hours over a period of 2 days, and 5 sprayed
for 4 hours a day on 14 days during a 22-day period in trials at the
WHO Insecticide Testing Unit, Lagos. Protective clothing was worn. No
clinical symptoms occurred, but plasma cholinesterase levels were
slightly depressed, the lowest being 75% of the pre-exposure level.
Levels returned to normal after one month (WHO, 1967).
COMMENT
Bromophos is rapidly absorbed, metabolized and excreted, mainly in the
urine. The compound potentiates the activity of several
organo-phosphates and carbamates, but its acute effects are
antagonized by some chlorinated hydrocarbons. The data on
neurotoxicity are contradictory and hence unsatisfactory. However, the
studies said to give positive results were poor, and the Meeting felt
that the compound was unlikely to cause neuropathy.
A reproduction study in rats showed no effect at 20 mg/kg/day.
Increased stillbirths and decreased pup weight were evident at 80
mg/kg/day.
Short-term studies in rat showed no effects on plasma cholinesterase
at 0.63 mg/kg/day. Somewhat higher doses caused erythrocyte
cholinesterase depression. At much higher doses brain cholinesterase
depression occurred. Hydropic swelling of hepatic cells at 188
mg/kg/day and hyaline droplets in tubular cells and protein in kidney
tubules at 1 250 mg/kg/day were observed.
Short-term studies in dog indicated no effect on plasma cholinesterase
at 1.5 mg/kg/day. At 175 mg/kg/day impaired spermatogenesis was
observed.
A long-term study in rat did not reveal any tumours, but survival was
poor at termination of the study.
Although the studies were considered poor by present day standards,
the Meeting decided that data were adequate to permit establishment of
a temporary ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 0.63 mg/kg body-weight/day
Dog: 1.5 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.006 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bromophos is an insecticide with contact and stomach action. It is
effective against a broad spectrum of chewing and sucking insects and
possesses a very low mammalian toxicity. It can be used as an
emulsifiable concentrate, ULV concentrate, wettable powder, granular
or dust formulation, seed dressing, aerosol formulation and fog
solution. It is compatible with other insecticides and fungicides.
Bromophos is of moderate toxicity to bees and should not be sprayed on
flowering crops during the flight of bees.
Bromophos has been used in the majority of European countries as well
as Algeria, Canada, Kuwait, Mexico, New Zealand, Nicaragua, Pakistan,
South Africa, Syria and Venezuela.
Pre-harvest treatments
Bromophos is used on various crops, mainly fruit and vegetables, for
control of a large number of important sucking and chewing insect
pests, such as vegetable root maggots, aphids, sawflies, fruit flies,
codling moths, mangold fly and beetles.
According to the single crop and the main pest species occurring, the
recommended concentrations of spray wash are given between 0.02% and
0.1% a.i.; the application rates range from 0.4 kg to 1.5 kg a.i./ha.
Bromophos is suitable for the treatment of a number of crops, except
for certain susceptible varieties of grape, pear, melon, cucumber,
lettuce and cabbage, mainly as frequent short interval treatments. Use
in glasshouses is not recommended.
The withholding periods range from 1 day to 21 days, depending on
local conditions and crop.
Post-harvest treatments
Bromophos is registered and recommended for storage pest control in
Mexico, South Africa, Spain and the United Kingdom and is currently
being introduced into other European and overseas countries.
Application of between 6 ppm and 12 ppm is recommended for grain
protection against various species of weevil and beetle causing damage
to stored products.
Other uses
Bromophos is used for the indoor and outdoor control of pests of
hygienic importance. As it shows a good effect against larval as well
as against adult stages and as it has a very low mammalian toxicity,
bromophos has proved to be an effective and safe compound for public
health operations. Furthermore, this material is recommended in the
veterinary field against chicken mites and other ectoparasites on
domestic animals and is highly effective against blowflies on sheep.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials on a variety of
fruits, vegetables, field crops and stored wheat (Boehringer,
1965-1971). Summaries of much of this information, and additional data
as well, have been published recently (Eichler, 1971). Table 3
presents a summary of available data, together with relevant
information on rates of application, number of applications and
pre-harvest interval.
In one reported trial, stored wheat that had been treated with
bromophos at 3 rates was milled into flour and the flour baked into
bread (Boehringer, 1965-1971). Analysis at each step gave the results
shown in Table 4.
Thus, less than 10% of the original amount of pesticide applied to
wheat is carried through to the final product, bread.
Data are also available on residues resulting from supervised trials
in animals.
Four lambs were dipped in 0.5% bromophos weekly for 9 weeks. Omental
biopsies were performed up to 22 days after the final dipping and the
fat samples analyzed by microcoulometric gas chromatography. Residues
of bromophos ranged from 5-14 ppm on day 1 to 0.07-0.43 ppm on day 22.
Bromoxon and 2,5-dichloro-4-bromophenol were not determined (Clark
et al., 1966).
TABLE 3 Bromophos residues on several crops
Rate of No. of Pre-harvest
Crop application treatments interval Residue
(a.i.) (days) (ppm)
Cauliflower 0.06 g/m2 1 0 4.901
7 0.041
14 <0.021
Broccoli 0.05 g/plant 1 62 <0.10
Kidney beans 0.06 g/m2 1 0 0.62-1.50
7 0.26-0.56
Cucumbers 0.04 g/m2 1 0 0.15-0.45
7 0.03-0.05
Garden lettuce 0.015 g/m2 1 4 1.9-2.6
0.025 g/m2 1 4 0.34-0.60
7 0.09-0.26
Lambs lettuce 0.025 g/m2 1 7 0.94-1.17
Kohlrabi 0.04 g/m2 1 7 <0.02
0.06 g/plant 1 30 0.04
Leek 0.8 kg/ha 1 0 2.76-2.80
14 1.27-1.43
Carrots 0.25 g/m2 2 38 2.90-3.55
0.2 g/m2 2 45 0.89-1.03
80 0.58-0.59
Peas 0.04 g/m2 1 7 <0.03
Radish 0.25 g/m2 1 16 1.03-1.32
21 0.12-0.23
Celery 120 g/ha 4 75 <0.05 (stalks)
0.32-0.55 (leaves)
Spinach 0.04 g/m2 1 1 5.48-7.85
7 0.25-0.67
14 <0.03-0.16
TABLE 3 (Cont'd.)
Rate of No. of Pre-harvest
Crop application treatments interval Residue
(a.i.) (days) (ppm)
Red cabbage 0.05 g/m2 1 7 <0.08
White cabbage 0.05 g/m2 1 7 <0.06-0.10
Savoy cabbage 0.06 g/m2 1 1 1.18-2.70
7 0.57-1.00
14 0.03-0.42
Onions 0.1 g/m2 1 123 <0.02
Maize 2.0 kg/ha 1 75 <0.17
Sugar beets 0.06 g/m2 1 14 <0.15-0.22 (beet)
1.1-1.9 (leaves)
Wheat 114 g/ha 1 14 0.17
228 g/ha 2 45 <0.02
Rape 228 g/ha 1 49 0.09 (seed)
0.17 (oil)
Apples 2.0 g/tree 1 0 1.10-1.20
4 1.49-1.70
7 0.51-1.30
14 0.48-0.61
Pears 1.25 g/tree 1 8 0.59-0.77
Yellow plums 0.62 g/tree 1 7 0.512
Cherries 2.5 g/tree 1 1 1.103
7 0.363
Peaches 0.38 g/tree 1 7 0.03-0.13
14 <0.03-0.25
Blackcurrants 0.25 g/shrub 1 7 0.13-0.36
Redcurrants 0.25 g/shrub 1 7 0.64-0.74
0.8 kg/ha 1 0 0.56-0.61
7 0.11-0.13
TABLE 3 (Cont'd.)
Rate of No. of Pre-harvest
Crop application treatments interval Residue
(a.i.) (days) (ppm)
Blackberries 0.25 g/shrub 1 7 0.14-0.19
Strawberries 0.8 kg/ha 1 0 0.61-0.64
7 0.11-0.12
Gooseberries 0.8 kg/ha 1 0 0.20-0.25
7 0.06-0.07
0.25 g/shrub 1 7 0.40-0.43
Olives 4.5 g/tree 1 10 2.58-2.92
Olive oil 4.5 g/tree 1 10 3.00-3.15
Stored wheat 0.012 g/kg 1 0 8.0
122 5.6
365 5.3
1 average of 4 analyses; raw data not available.
2 average of 9 analyses; raw data not available.
3 average of 6 analyses; raw data not available.
TABLE 4 Bromophos residues in wheat and wheat products
Rate of Post-treatment Residues (ppm)
application interval (days) Wheat Flour Bread
6 ppm 270 1.32 1.20 0.38-0.41
12 ppm 270 3.00 2.65-2.90 0.78-0.87
25 ppm 880 4.57 3.98-4.25 1.14-1.16
Milk from ten cows was analyzed for residues after stall spraying at
0.5 g/m2 of wall surface. Five cows were present during the spraying
and five were removed. Milk from the latter cows did not contain
measurable amounts of bromophos (<0.02 ppm). Milk from the five cows
left in the stalls during treatment had residues of 0.032-0.042 ppm on
post-treatment day 1; 0.031-0.045 ppm on day 2; 0.026-0.034 ppm on day
3; and <0.020 on day 5 (Boehringer, 1965-1971).
FATE OF RESIDUES
General comments
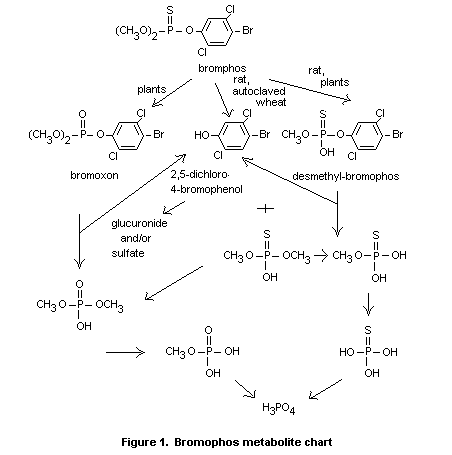
The metabolism or degradation of bromophos appears to be similar in
plants and animals, with the exception that hardly any bromoxon is
found in animals whereas low levels of bromoxon are likely to be
encountered in plant material. This is summarized in Figure 1.
Other information on identity and properties
Molecular weight: 366.0
State: white crystals
Melting point: 53°C
Boiling point: 140-142°C at 10-2 torr.
Vapour pressure: 1.3 x 10-4 mm Hg at 20°C
Solubility: soluble in most organic solvents, e.g., toluene,
carbon tetrachloride, diethyl ether. Slightly
soluble in low molecular weight alcohols. Water
solubility - 40 ppm at 27°C.
Stability:
Stable in aqueous suspension. Hydrolyzes in distinct alkaline medium.
Purity of technical material:
O-4-bromo-2,5-dichlorophenyl-O,
O-dimethyl-phosphorothioate: approx. 95.0%;
O-4-bromo-2,3-dichlorophenyl-O,O-dimethyl-phosphorothioate:
approx. 3.0%;
O-6-bromo-2,5-dichlorophenyl-O,O-dimethyl-phosphorothioate:
approx. 1.0%;
O-dichlorophenyl-O,O-dimethyl-phosphorothioate:
approx. 1.0% (chlorine position not defined).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies on rats with 32P and 3H-labelled bromophos showed it is well
absorbed from the gastrointestinal tract. In the first 12 hours
following oral, intravenous or intraperitoneal administration, large
quantities of radioactivity were found in the stomach, intestine,
liver and kidneys, but after 24 hours the majority was found in urine
and faeces. Bromophos showed no tendency to accumulate in any organ.
The maximum blood level was found 7 hours after oral administration,
and the biological half life in rats was 14 hours. Approximately 96%
of the activity of a 10 mg/kg dose of 3H-bromophos was excreted in
urine and 1% in faeces during 24 hours following oral administration.
Excretion was complete in 96 hours, a total of 2% of activity
appearing in the faeces. In the same period, administration of
32P-bromophos resulted in the excretion of 63% of activity in urine
and 16% in faeces. During 8 hours following intraduodenal
administration of 5 mg/kg of 3H-bromophos, 25% of the activity was
excreted in bile in rats. Since only 1% of 32P-bromophos was excreted
in the same period, the biliary excretion was probably mainly
dichlorobromophenol or its metabolites. Biliary excreted 3H is
probably re-absorbed since only 2% of an oral dose was found in
faeces. The relatively high 32P content in faeces may result from
metabolism of bromophos in the intestine (Stiasni et al., 1967).
Biotransformation
Five urine metabolites were found after administration of
32P-bromophos whereas three were found after administration of
3H-bromophos. Analysis showed that the metabolites were phosphate,
dimethylthionophosphate, monodesmethyl-bromophos and
dichlorobromophenol. Two metabolites were unidentified. Bromophos
itself, its O-analogue bromoxon and monodesmethyl-bromoxon were not
found in urine (Stiasni et al., 1967).
Effect on enzymes and other biochemical parameters
Bromophos inhibits cholinesterase activity in rats and dogs, the
no-effect level when administered over a 100-day period being 0.63
mg/kg/day in rats and 1.5 mg/kg/day in dogs (Kinkel and Seume, 1963;
Kinkel et al., 1965; Kinkel and Dirks, 1966; Boehringer, 1968;
Leuschner et al., 1967). When a single oral dose of 800 or 1200
mg/kg was given to horses, plasma cholinesterase was inhibited even
after 16 days, although partial reactivation was found (Paton, 1965).
TOXICOLOGICAL STUDIES
Special studies on the metabolites
Several metabolites were found during investigations on rats and dogs
and on examination of metabolism of bromophos in plants. The most
important of these have been examined. A summary of acute oral
toxicities of bromophos metabolites is given in Table 1.
Short-term toxicity of metabolites
Groups of 10 male and 10 female rats were fed for 5 weeks on control
diet or diet providing 25, 100 or 400 mg/kg/day bromoxon, 250, 500 or
1000 mg/kg/day desmethyl-bromoxon or 250, 500 or 1000 mg/kg/day of
2,5-dichloro-4-bromophenol. At the highest dosage levels with each
metabolite the growth of animals was significantly inhibited. Plasma
and RBC cholinesterases were inhibited by bromoxon at 25 mg/kg
(maximum inhibition), and the plasma enzyme by desmethyl-bromoxon at
500 mg/kg. No enzyme inhibition occurred with dichloro-4-bromophenol.
The results of other investigations were normal; histopathological
studies were not carried out (Leuschner, 1968)
Groups of 10 male and 10 female rats were fed on diets providing 0,
125, 250 and 500 mg/kg/day of desmethyl-bromophos for 5 weeks. At 500
mg/kg, plasma cholinesterase was minimally inhibited. The weights of
liver and pituitary were above normal in male animals and histological
examination showed minimal centrilobular fatty infiltration of the
liver, the latter finding was considered to be reversible. Body weight
and food intake were reduced at the 500 mg/kg level. No abnormalities
were found at lower dosage levels (Leuschner et al., 1969).
Special studies on neurotoxicity
Six adult hens were administered 10 g/kg body-weight of bromophos
together with atropine and 2-PAM. Ataxia and incoordination were
present in all birds for several days, and three died. Histological
examination was said to reveal severe demyelination in all birds
(Byewater, 1966).
TABLE 1
Acute oral toxicities of bromophos metabolites
Compound Animal LD50 Reference
(mg/kg body-weight)
Bromoxon Mouse 2 100 Muacevic, 1965
Monodesmethyl-bromophos Rat 1 900-4 600 Muacevic, 1965
Mouse 1 800 1966
Desmethyl-bromophos Mouse 1 085 Muacevic, 1968
(triethylamine salt)
Desmethyl-bromoxon Mouse 2 150 Muacevic, 1965
O,O-dimethyl-O-(5,6-dichloro-4-bromophenyl)-thiophosphoric Mouse 2 850 Muacevic, 1965
acid ester
O,O-dimethyl-O-(4,6-dichloro-2-bromophenyl)-thiophosphoric Mouse about 2 000 Muacevic, 1965
acid ester
O,O-dimethyl-O-(2,4-dichloro-6-bromophenyl)-thiophosphoric Mouse >6 000 Muacevic, 1965
acid ester
O,O-dimethyl-thiophosphoric acid Mouse 4 700 Muacevic, 1965
sodium
2,5-dichloro-4-bromophenol Mouse 1 100-1 900 Muacevic, 1965
Rat 3 200-3 550 1966
Ten adult hens each received orally 1 g bromophos/kg/day until signs
of paralysis developed (between 12 and 56 days), at which time each
was examined for histological changes in the brain and spinal cord.
These were reported to consist of degeneration of ganglia cells and
demyelination (Kinkel and Hubner, 1966), but the suitability of the
material for accurate pathological interpretation was challenged
(Glees, 1966).
Two adult hens were each given 5.5 g/kg of bromophos in divided doses
over a period of 7 weeks. A 300 mg/kg dose produced transient
excitability and diarrhoea and the 4th of 5 and 4th of 8 consecutive
doses of 400 mg/kg day given at a 3-week interval caused an unsteady
gait and diarrhoea which persisted each time for 6-9 days after
administration. The hens completely recovered following this
treatment, indicating the lack of delayed neurotoxic effect (Barnes,
1966). Ten adult hens received two oral doses of 2 g/kg of bromophos
at 3-week intervals. A neuropathological examination carried out after
a 3-week observation period revealed no degenerative processes in the
CNS or peripheral nerves (Muacevic and Glees, 1967).
Groups of 10 adult hens were administered 0, 12.5 or 125 mg/kg of
bromophos/day in their food for 4 weeks. Clinically apparent
neurological signs were reversible when treatment ceased and a
neuropathological examination of 2 birds of each group showed no CNS
changes (Muacevic and Glees, 1968).
In order to investigate the occurrence of paralysis in dogs
administered bromophos for 2 years, and the finding on
neuropathological examination of localized ganglion cell degeneration
and other lesions in the CNS (Kinkel et al., 1965), an experiment
was carried out in which two groups of 3 male and 3 female dogs were
administered 0 and 87.5 mg/kg and 6 male and 6 female dogs 175 mg/kg
body-weight/day of bromophos orally in capsules for periods up to 270
days. One dog on 175 mg/kg had a single epileptiform fit and another
was killed after 62 doses because it was cachectic. An extensive
neuropathological examination of the central nervous system, spinal
ganglia and peripheral nerves failed to reveal any neuropathological
changes (Boehringer, 1968).
Special studies on pharmacology
Bromophos inhibits cholinesterase activity indirectly through its
metabolite bromoxon. No antidote effect was found with atropine
sulphate and aldoximes when rats and mice were administered high doses
of bromophos orally or intraperitoneally. The lack of effect was
probably due to the toxic effect of the high doses of solvent which
had to be used to administer the bromophos. However, cholinesterase
inhibition was diminished in mice by atropine and Toxogonin (Muacevic,
1965). Inhibition of brain cholinesterase in bromophos treated rats
could be prevented by intravenous injection of several reactivators
(Muacevic, 1968). Administration of atropine and 2-PAM stopped
development of signs of poisoning when 0.02-0.2 g/kg body-weight of
bromophos was administered to chickens. At 0.5 and 1.0 g/kg dosage
levels the development of signs of poisoning were delayed (Kinkel,
1964a).
Special studies on reproduction
Groups of 20 male and 20 female rats were fed on diets providing 0, 5,
20 and 80 mg bromophos/kg/day. These formed the parent generation of a
standard three generation study (F.D.A. advisory committee, 1970). The
80 mg/kg dosage level produced no clinical signs of poisoning but the
rate of body-weight gain was depressed in all generations,
particularly in males. The fertility and size and weight of litters
was unaffected, but the number of stillbirths in this group was high.
The survival rate of young was also reduced except in the first litter
and the parent generation. Animals consuming 5 or 20 mg/kg
bromophos/day were no different from control animals. No runts or
malformations were observed at any dosage or in any generation, and
the behaviour, appearance, food intake, results of haematological
investigations and adult survival were also unaffected. In this study
the cholinesterase activity of plasma and liver was significantly
depressed in animals receiving 5 mg/kg/day. The threshold dosage level
for RBC enzyme inhibition was between 5 and 30 mg/kg and for brain
enzyme between 5 and 20 mg/kg in males and 20 and 80 mg/kg in females
(Leuschner et al., 1967).
Special studies on teratogenicity
Five groups of 10 pregnant female rabbits were administered by gavage
25, 50, 100, 200 or 400 mg/kg body-weight of bromophos each day
between day 6 and 18 of pregnancy. Forty animals acted as controls.
Foetuses were removed and examined on day 30 of pregnancy. Twenty five
mg bromophos/kg/day orally had no untoward effect on foetuses or
parent animals. At higher dosage levels the parent animals became
debilitated and developed limb flaccidity and diarrhea, and the number
of fully-grown, live foetuses was reduced. In no group did the type or
number of malformations observed differ from that of the controls
(Leuschner and Leuschner, 1966a).
Acute toxicity
Studies on the acute toxicity of bromophos in several animal species
are summarized in Table 2.
TABLE 2 Acute toxicity of bromophos in animals
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 3 311 - 5 900 Muacevic, 1963; 1964;
1965; 1967;
Worth et al., 1967
Mouse i.p. 1 000 - 4 900 Oettel, 1963; Muacevic,
1964
Rat oral 3 750 - 8 000 Kinkel et al., 1966;
Oettel, 1963; Muacevic,
1967.
Rat i.p. 3 125 Kinkel and Sann, 1964.
Guinea pig oral >6 000 Muacevic, 1967
Rabbit oral 720 Muacevic, 1964
Fowl oral 9 700 Kinkel, 1964a
Dog oral >625 Worth et al., 1967
Potentiation of the acute toxicity of bromophos occurred with
bromophos-ethyl, diazinon, dichlorvos, dimethoate, malathion,
mevinphos, naleb, parathion and carbaryl. DDT, heptachlor and lindane
possessed strong antagonistic activity to the acute effects of
bromophos (Kinkel, 1964b; Muacevic 1964, 1965, 1966, 1967, 1968).
Short-term studies
Rat
Four groups of 6 male and 6 female rats received diets providing 0,
0.65, 1.25 and 2.5 mg bromophos per kg body-weight/day for 100 days.
The cholinesterase activity in RBC was not significantly affected at
any dosage level. Plasma enzyme was inhibited at the 1.25 mg/kg level,
but not at the lower level, and it returned to normal in two weeks
after the treatment was stopped. The behaviour, growth, food intake
and macroscopic appearance of organs were normal (Leuschner and
Leuschner, 1966b).
Four groups of 16 male rats received by gastric tube daily doses of 0,
188, 750 and 1 250 mg bromophos/kg body-weight/day for 100 days. The
rate of body-weight gain was reduced in all test groups while food
intake was lower only at the highest level. Animals in the 750 and
1 250 mg/kg groups became restless for an hour or so after treatment
but the degree of restlessness did not increase during the test.
Brain, liver and plasma cholinesterase were inhibited at all dosage
levels. Hydropic swelling of the hepatic cells was seen at all dosage
levels; this effect was dose related. In the 1 250 mg/kg group hyaline
droplets were seen in tubular cells and protein within the tubules of
the kidneys. Urine analysis and the bromosulphophthalein retention
test yielded negative results. Haematological findings and the
macroscopic appearance of internal organs were normal (Kinkel and
Seume, 1963).
Dog
Three groups of 2 male and 2 female beagle dogs received diets
providing 0.75, 1.5 and 3.0 mg bromophos/kg/day for 100 days. The
control group consisted of one male and one female. No or only slight
inhibition of the plasma and RBC cholinesterase activities (never
exceeding 36%) was observed at the three dosage levels; an inhibition
of 20% was suggested as no-effect level in this experiment.
Erythrocyte cholinesterase was more susceptible to inhibition than
plasma cholinesterase. In one dog exposed to the lowest dosage level,
35% inhibition of the RBC cholinesterase was observed, but this was
reduced to 20% when tested seven weeks later. Neither the brain nor
the liver cholinesterase was found to be inhibited when examined
(Kinkel and Dirks, 1966).
Six groups of 3 male and 3 female mongrel dogs aged 1-3 years and
weighing between 3.9 and 10.3 kg were administered 0, 11, 43.8, 87.5
and 175 mg bromophos/kg body-weight by gastric tube on every working
day for 2 years. Treatment at the 87.5 and 175 mg/kg levels made the
animals restless for some time after dosage. One animal on each of the
43.8 and 175 mg/kg levels died after suffering bites, and one on 87.5
and 3 on 175 mg/kg died after developing respiratory difficulties,
hoarseness, salivation and tremors followed by ataxia and then paresis
of the hind limbs. Two dogs of the 175 mg/kg group developed the same
signs but recovered, one after being taken off treatment and the other
while administration of bromophos continued. Histological examination
of the animals which died on the two highest dosage levels showed foci
of ganglion cell degeneration in the spinal cord; this involved only
small numbers of cells in the ganglia. In addition, spermatogenesis
was impaired with proliferation of Leydig's cells in 2 males of the
175 mg/kg group. Degeneration of the CNS in animals which did not die
during the test was insignificant or non-existent. Plasma, RBC, liver
and brain cholinesterase activities were depressed at all dosage
levels in this test. The food intake, growth, sexual cycle, urinary
ascorbic acid excretion and the results of haematological, liver
function and urine tests were similar in test and control groups. The
relative organ weights and results of macroscopic and microscopic
examination of organs were normal in the 11 and 43.8 mg/kg groups
(Kinkel et al., 1965).
In a nine month study (See special studies on
neurotoxicity) groups of mongrel dogs weighing 6-20 kg were
administered 0, 87.5 or 175 mg bromophos/kg body-weight
daily in capsules for 270 days. Body-weight and food intake were
reduced in accordance with the dose given. Although 2/6 bitches on
175 mg/kg/day were at no time on heat, histological examination of
the ovaries and uterus revealed no pathological findings.
Histological examination showed seminiferous tubule degeneration in
3 dogs receiving 175 mg/kg/day. Prostatic hypertrophy was also more
evident in test animals. The death of one dog of the high level group
was not regarded as being due to bromophos consumption. Erythrocyte
and plasma cholinesterase were inhibited in both test groups. The
results of haematological tests, serum analysis and urine analysis
were all normal (Boehringer, 1968).
Long-term studies
Rat
Groups of 6-month-old rats were administered 0, 87.5, 175 and 350 mg
bromophos/kg body-weight by gavage each working day for 2 years. The
groups consisted of 25 male and 25 female animals, but 3, 5, 5 and 17
animals were included in the test after the start in the 0, 87.5, 175
and 350 mg/kg dosage groups, respectively. After 8, 10, 12, 15, 18 and
21 months, two animals, one of each sex, were killed for
histopathological examination. The rate of body-weight gain was
depressed at the highest dosage level, and in this group proteinuria
was more evident than in controls; in addition, the number of
erythrocytes in the urinary sediment was increased but the kidneys
were histologically normal.
Cholinesterase was inhibited in the plasma, RBC, brain and liver by
all treatment levels. Survival was poor, with only 5, 4, 2 and 5 males
and 9, 6, 7 and 8 females of the 0, 87.5, 175 and 350 mg/kg groups,
respectively, surviving for 100 weeks. The behaviour of all test
groups was normal as were the results of haematological and liver
function tests. Urinary ascorbic acid excretion was unaltered by
bromophos administration. The weights of 8 organs and the results of
macroscopic and microscopic examinations revealed no lesion which
could be attributed to treatment. No tumours were reported to have
occurred in control or test groups (Kinkel et al., 1965).
OBSERVATIONS IN MAN
Workers exposed to bromophos during production and formulation for two
years showed no change in RBC cholinesterase levels as estimated at
monthly intervals (Boehringer, 1966). Eight subjects were engaged in
spraying bromophos for 6´ hours over a period of 2 days, and 5 sprayed
for 4 hours a day on 14 days during a 22-day period in trials at the
WHO Insecticide Testing Unit, Lagos. Protective clothing was worn. No
clinical symptoms occurred, but plasma cholinesterase levels were
slightly depressed, the lowest being 75% of the pre-exposure level.
Levels returned to normal after one month (WHO, 1967).
COMMENT
Bromophos is rapidly absorbed, metabolized and excreted, mainly in the
urine. The compound potentiates the activity of several
organo-phosphates and carbamates, but its acute effects are
antagonized by some chlorinated hydrocarbons. The data on
neurotoxicity are contradictory and hence unsatisfactory. However, the
studies said to give positive results were poor, and the Meeting felt
that the compound was unlikely to cause neuropathy.
A reproduction study in rats showed no effect at 20 mg/kg/day.
Increased stillbirths and decreased pup weight were evident at 80
mg/kg/day.
Short-term studies in rat showed no effects on plasma cholinesterase
at 0.63 mg/kg/day. Somewhat higher doses caused erythrocyte
cholinesterase depression. At much higher doses brain cholinesterase
depression occurred. Hydropic swelling of hepatic cells at 188
mg/kg/day and hyaline droplets in tubular cells and protein in kidney
tubules at 1 250 mg/kg/day were observed.
Short-term studies in dog indicated no effect on plasma cholinesterase
at 1.5 mg/kg/day. At 175 mg/kg/day impaired spermatogenesis was
observed.
A long-term study in rat did not reveal any tumours, but survival was
poor at termination of the study.
Although the studies were considered poor by present day standards,
the Meeting decided that data were adequate to permit establishment of
a temporary ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 0.63 mg/kg body-weight/day
Dog: 1.5 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.006 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bromophos is an insecticide with contact and stomach action. It is
effective against a broad spectrum of chewing and sucking insects and
possesses a very low mammalian toxicity. It can be used as an
emulsifiable concentrate, ULV concentrate, wettable powder, granular
or dust formulation, seed dressing, aerosol formulation and fog
solution. It is compatible with other insecticides and fungicides.
Bromophos is of moderate toxicity to bees and should not be sprayed on
flowering crops during the flight of bees.
Bromophos has been used in the majority of European countries as well
as Algeria, Canada, Kuwait, Mexico, New Zealand, Nicaragua, Pakistan,
South Africa, Syria and Venezuela.
Pre-harvest treatments
Bromophos is used on various crops, mainly fruit and vegetables, for
control of a large number of important sucking and chewing insect
pests, such as vegetable root maggots, aphids, sawflies, fruit flies,
codling moths, mangold fly and beetles.
According to the single crop and the main pest species occurring, the
recommended concentrations of spray wash are given between 0.02% and
0.1% a.i.; the application rates range from 0.4 kg to 1.5 kg a.i./ha.
Bromophos is suitable for the treatment of a number of crops, except
for certain susceptible varieties of grape, pear, melon, cucumber,
lettuce and cabbage, mainly as frequent short interval treatments. Use
in glasshouses is not recommended.
The withholding periods range from 1 day to 21 days, depending on
local conditions and crop.
Post-harvest treatments
Bromophos is registered and recommended for storage pest control in
Mexico, South Africa, Spain and the United Kingdom and is currently
being introduced into other European and overseas countries.
Application of between 6 ppm and 12 ppm is recommended for grain
protection against various species of weevil and beetle causing damage
to stored products.
Other uses
Bromophos is used for the indoor and outdoor control of pests of
hygienic importance. As it shows a good effect against larval as well
as against adult stages and as it has a very low mammalian toxicity,
bromophos has proved to be an effective and safe compound for public
health operations. Furthermore, this material is recommended in the
veterinary field against chicken mites and other ectoparasites on
domestic animals and is highly effective against blowflies on sheep.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials on a variety of
fruits, vegetables, field crops and stored wheat (Boehringer,
1965-1971). Summaries of much of this information, and additional data
as well, have been published recently (Eichler, 1971). Table 3
presents a summary of available data, together with relevant
information on rates of application, number of applications and
pre-harvest interval.
In one reported trial, stored wheat that had been treated with
bromophos at 3 rates was milled into flour and the flour baked into
bread (Boehringer, 1965-1971). Analysis at each step gave the results
shown in Table 4.
Thus, less than 10% of the original amount of pesticide applied to
wheat is carried through to the final product, bread.
Data are also available on residues resulting from supervised trials
in animals.
Four lambs were dipped in 0.5% bromophos weekly for 9 weeks. Omental
biopsies were performed up to 22 days after the final dipping and the
fat samples analyzed by microcoulometric gas chromatography. Residues
of bromophos ranged from 5-14 ppm on day 1 to 0.07-0.43 ppm on day 22.
Bromoxon and 2,5-dichloro-4-bromophenol were not determined (Clark
et al., 1966).
TABLE 3 Bromophos residues on several crops
Rate of No. of Pre-harvest
Crop application treatments interval Residue
(a.i.) (days) (ppm)
Cauliflower 0.06 g/m2 1 0 4.901
7 0.041
14 <0.021
Broccoli 0.05 g/plant 1 62 <0.10
Kidney beans 0.06 g/m2 1 0 0.62-1.50
7 0.26-0.56
Cucumbers 0.04 g/m2 1 0 0.15-0.45
7 0.03-0.05
Garden lettuce 0.015 g/m2 1 4 1.9-2.6
0.025 g/m2 1 4 0.34-0.60
7 0.09-0.26
Lambs lettuce 0.025 g/m2 1 7 0.94-1.17
Kohlrabi 0.04 g/m2 1 7 <0.02
0.06 g/plant 1 30 0.04
Leek 0.8 kg/ha 1 0 2.76-2.80
14 1.27-1.43
Carrots 0.25 g/m2 2 38 2.90-3.55
0.2 g/m2 2 45 0.89-1.03
80 0.58-0.59
Peas 0.04 g/m2 1 7 <0.03
Radish 0.25 g/m2 1 16 1.03-1.32
21 0.12-0.23
Celery 120 g/ha 4 75 <0.05 (stalks)
0.32-0.55 (leaves)
Spinach 0.04 g/m2 1 1 5.48-7.85
7 0.25-0.67
14 <0.03-0.16
TABLE 3 (Cont'd.)
Rate of No. of Pre-harvest
Crop application treatments interval Residue
(a.i.) (days) (ppm)
Red cabbage 0.05 g/m2 1 7 <0.08
White cabbage 0.05 g/m2 1 7 <0.06-0.10
Savoy cabbage 0.06 g/m2 1 1 1.18-2.70
7 0.57-1.00
14 0.03-0.42
Onions 0.1 g/m2 1 123 <0.02
Maize 2.0 kg/ha 1 75 <0.17
Sugar beets 0.06 g/m2 1 14 <0.15-0.22 (beet)
1.1-1.9 (leaves)
Wheat 114 g/ha 1 14 0.17
228 g/ha 2 45 <0.02
Rape 228 g/ha 1 49 0.09 (seed)
0.17 (oil)
Apples 2.0 g/tree 1 0 1.10-1.20
4 1.49-1.70
7 0.51-1.30
14 0.48-0.61
Pears 1.25 g/tree 1 8 0.59-0.77
Yellow plums 0.62 g/tree 1 7 0.512
Cherries 2.5 g/tree 1 1 1.103
7 0.363
Peaches 0.38 g/tree 1 7 0.03-0.13
14 <0.03-0.25
Blackcurrants 0.25 g/shrub 1 7 0.13-0.36
Redcurrants 0.25 g/shrub 1 7 0.64-0.74
0.8 kg/ha 1 0 0.56-0.61
7 0.11-0.13
TABLE 3 (Cont'd.)
Rate of No. of Pre-harvest
Crop application treatments interval Residue
(a.i.) (days) (ppm)
Blackberries 0.25 g/shrub 1 7 0.14-0.19
Strawberries 0.8 kg/ha 1 0 0.61-0.64
7 0.11-0.12
Gooseberries 0.8 kg/ha 1 0 0.20-0.25
7 0.06-0.07
0.25 g/shrub 1 7 0.40-0.43
Olives 4.5 g/tree 1 10 2.58-2.92
Olive oil 4.5 g/tree 1 10 3.00-3.15
Stored wheat 0.012 g/kg 1 0 8.0
122 5.6
365 5.3
1 average of 4 analyses; raw data not available.
2 average of 9 analyses; raw data not available.
3 average of 6 analyses; raw data not available.
TABLE 4 Bromophos residues in wheat and wheat products
Rate of Post-treatment Residues (ppm)
application interval (days) Wheat Flour Bread
6 ppm 270 1.32 1.20 0.38-0.41
12 ppm 270 3.00 2.65-2.90 0.78-0.87
25 ppm 880 4.57 3.98-4.25 1.14-1.16
Milk from ten cows was analyzed for residues after stall spraying at
0.5 g/m2 of wall surface. Five cows were present during the spraying
and five were removed. Milk from the latter cows did not contain
measurable amounts of bromophos (<0.02 ppm). Milk from the five cows
left in the stalls during treatment had residues of 0.032-0.042 ppm on
post-treatment day 1; 0.031-0.045 ppm on day 2; 0.026-0.034 ppm on day
3; and <0.020 on day 5 (Boehringer, 1965-1971).
FATE OF RESIDUES
General comments
The metabolism or degradation of bromophos appears to be similar in
plants and animals, with the exception that hardly any bromoxon is
found in animals whereas low levels of bromoxon are likely to be
encountered in plant material. This is summarized in Figure 1.
 In animals
The absorption, distribution and excretion of 32P and 3H-labelled
bromophos administered to rats by various routes are discussed in the
preceding section on BIOCHEMICAL ASPECTS.
The decomposition of 32P-labelled bromophos following cutaneous
application to lactating cows (20 mg/kg in alcohol or paraffin oil)
was studied by Dedek and Schwarz (1969). No bromoxon was detected in
blood or milk by thin-layer chromatography. Concentrations of 0.4 to
0.7 ppm of desmethyl-bromophos and traces of bromophos were present.
The two methyl groups of bromophos appear to be simultaneously split
off by mild alkaline hydrolysis and by the action of a
glutathione-dependent liver enzyme (Stenersen, 1969a, 1969b).
In plants
In studies on tomato plants using 32O and 3H-labelled bromophos, it
was found that it does not act systemically but penetrates from the
surface into the interior of the leaf and also from a nutrient
solution into the root. In addition to unchanged bromophos,
dichlorobromophenol was found as a main metabolite (13% of the total
dose applied after 7 days) and small amounts of bromoxon,
monodesmethyl-bromophos, dimethyl thionophosphate and inorganic
phosphate were detected (Stiasni et al., 1969).
The metabolism of bromophos-32P was studied in wheat-, carrot-, and
onion-seedlings and by soil microorganisms (Stenerson, 1969). The main
finding was that bisdesmethyl-bromophos had been produced in several
of the experiments, but no desmethyl-bromophos was detectable.
However, the identification of bisdesmethyl-bromophos was not certain.
In soil
Bromophos E.C. was applied one time at 0.5 g a.i./m2 to high moorland
soil (acid, high organic), Ingelheim sand and clay soil. Zero to 20
cm-deep samples were taken periodically for 26 weeks and analysed for
bromophos and dichloro-bromophenol residues, with the results shown in
table 5.
TABLE 5 Bromophos residues in soil
Residues (ppm)1
Type of Post-treatment Dichloro-bromophenol
soil time (weeks) Bromophos
High moorland 0 13.102 0.30
soil 1 9.00 0.26
3 8.18 1.14
6 6.95 0.57
9 2.15 1.72
13 0.75 0.53
26 0.58 0.93
Sandy soil 0 1.433 0.65
1 0.64 0.53
3 0.20 0.20
6 0.13 0.10
9 <0.02 0.10
13 <0.02 0.10
26 <0.02 0.10
Clay soil 0 1.544 0.59
1 0.81 0.29
3 0.20 0.21
6 0.09 <0.10
9 <0.02 <0.10
13 <0.02 <0.10
26 <0.02 <0.10
1 Values calculated as dry substance from the measured moisture
content.
2 Y = 1.006-0.056 log X; r = 0.9188
3 Y = 0.047-0.187 log X; r = 0.9777
4 Y = 0.095-0.201 log X; r = 0.9890
Degradation of bromophos is clearly much more rapid in sandy and clay
soils. When the sandy soil was treated once at the same rate with
bromophos granules, the residues (corrected for moisture in sample)
fell from 5.98 ppm at week 0 to <0.02 at week 26. Although initial
residues were higher than for E.C., no measurable residue remained
after 26 weeks (Boehringer, 1965-1971). The generally rapid
degradation in soil was further indicated by trials in Germany with 3%
coarse dust and 5% granular applied one time at 2, 4, or 8 kg/ha which
gave residues of 0.03-0.16 ppm after 92 days (Boehringer, 1965-1971)
and by elaborate tests in the United States using a range of
application rates, numbers of applications, sampling intervals and
soils in diverse geographic and climatic areas. There was no
appreciable build-up of residues or leaching down through soil to any
extent observed for either bromophos or dichlorobromophenol (Tepe,
1968).
In storage and processing
Bromophos was applied to stored wheat grains at 10 ppm and the
decomposition studied over a 10-week period. Although only traces of
dimethylphosphorothionate were found, indicating that
desmethyl-bromophos is the main phosphatase degradation product, large
amounts of monomethyl-phosphorothionate were detected. Bromoxon was
found only during the first 20 days in amounts up to 10 percent of the
original bromophos level. Degradation of bromophos proceeded rapidly
for about 7 days, then stopped for about 3 weeks due to the
accumulation of desmethyl-bromophos, which inhibits phosphatase
hydrolysis. After the desmethyl-bromophos had been degraded, bromophos
degradation resumed. The level of free dichlorobromophenol did not
increase significantly until degradation of desmethyl-bromophos was
well underway, suggesting the formation of the phenol from the
desmethyl or oxon compounds. When bromophos was applied to autoclaved
grains, only dimethyl-phosphorothionate and dichloro-bromophenol were
produced (Rowlands, 1966a).
Oxidation of bromophos to bromoxon occurred in the seed coats and
germs of wheat grains. Hydrolytic activity was found in the germ and
endosperm (Rowlands, 1966b).
METHODS OF RESIDUE ANALYSIS
Many general and specific chemical, biochemical and biological methods
of analysis have been developed for residues of bromophos. These have
recently been reviewed and summarized by Eichler (1971) and will not
be elaborated here. An excellent method has been developed by Leber
and Deckers (1968). It utilizes gas chromatography with a phosphorus
detector and can estimate quantities down to the range of 0.001 to
0.01 ppm with a 100-g sample. The method also includes procedures for
determining bromoxon either by colorimetry or by gas chromatography
with a sensitivity of 0.03 to 0.05 ppm. Thin-layer chromatographic
procedures are also given for identifying residues.
Since bromophos is among the pesticides listed as detectable by the
multi-residue gas chromatographic procedure of Abbott et al. (1970),
that method is the most suitable for regulatory use. The method of
Leber and Deckers (1968) would be suitable for confirmation of the
identity of residues.
NATIONAL TOLERANCES
Some examples of tolerances in various countries were reported to the
Joint Meeting and are listed in Table 6.
TABLE 6 Examples of national tolerances reported to the Meeting
Country Commodity Tolerance
(ppm)
Canada Apple 1.5
Germany, Federal Berries, pome fruit, leaf
Republic vegetables, cabbage 1.5
Stone fruit, legumes, 0.6
root vegetables
Field corn 0.2
Hungary 2
Netherlands 0.5
Carrots 1.5
Germany, Democratic Fruit, vegetables (except
Republic onions and potatoes) 11
Animal fat, vegetable oil,
potatoes, onions, meat, fish,
eggs, milk, grain, baby foods 0
South Africa Stored maize 8
1 not more than 0.1 ppm oxon.
APPRAISAL
Bromophos is a non-systemic halogen-containing organophosphorus
insecticide used on a wide variety of crops and animals to control
biting and sucking insects. It is also used to protect stored
products, as a seed protection agent for grain crops and as a vector
control agent in public health.
Supervised experiments with foliar, row or surface treatments on
fruits and vegetables have shown bromophos to have a generally low
persistence. However, the comparative rate of residue decline is
highly dependent on many factors, such as botanical species,
morphological structure, weather formulation, and method and time of
treatment. The lipophilic nature of the compound causes it to
penetrate the cuticular wax of certain crops (for example - apples and
pears) which delays release and degradation. This property makes it
necessary for tolerance recommendations to be made on an individual
commodity basis rather than on broad crop categories.
On the basis of a single trial in which four lambs were dipped in 0.5%
bromophos weekly for 9 weeks, it would appear that the compound did
not accumulate excessively in the fat and was eliminated fairly
rapidly after treatment ceased, dropping from about 10 ppm on day 1 to
about 2 ppm on day 8 and to about 0.2 ppm after 22 days. Milk cows
exposed to bromophos during stall treatment had low levels in the milk
(0.045 ppm maximum) which fell below the limit of detection (0.20 ppm)
after five days.
The metabolite of bromophos most likely to be found in plants and soil
is 2,5-dichloro-4-bromophenol. Small amounts of bromoxon and
monodesmethyl-bromophos were also found in tomato plants according to
one report, whereas different studies on wheat, carrot and onion
seedlings indicated the production of bisdesmethylbromophos but not
desmethyl-bromophos. The identity of the bisdesmethyl-bromophos was
not certain. In animals, bromophos is excreted rapidly via the urine
and the major metabolites found are dichloro-bromophenol and
monodesmethyl-bromophos. Extremely low levels of bromoxon may also
occur in blood.
Available multi-residue gas chromatographic procedures are suitable
for application for regulatory purpose and are recommended.
Although bromophos is recommended for use against ectoparasites of
animals and poultry, there was no data available on residues likely to
occur in meat (except sheep) or eggs and no recommendations for
tolerances could be made for these commodities.
RECOMMENDATIONS
TEMPORARY TOLERANCES
The following temporary tolerances are based on residues likely to be
found at harvest following currently recommended use patterns. For the
majority of fruits and vegetables, the recommended pre-harvest
interval is 7 days. In the case of whole milk, the practical residue
limit is at or about the limit of determination. The temporary
tolerances are expressed on bromophos.
ppm
Olives, olive oil 5
Apples, lamb's lettuce, leeks,
radishes 2
Carrots, celery, French beans, pears,
plums, red currants, savoy cabbage,
spinach 1
Blackberries, blackcurrants,
cherries, lettuce, gooseberries,
peaches, strawberries, sugarbeets
(roots), fat of meat of sheep 0.5
Wheat, rapeseed, rapeseed oil 0.2
Broccoli, cauliflower, cucumbers,
red cabbage, cabbage, kohlrabi,
onions, peas 0.1
Milk (whole) 0.02*
* at or about the limit of determination
Remarks
The temporary tolerance for wheat is based on residues likely to be
found at harvest.
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1977)
1. A study in dogs using animals of similar weight, age and origin
in control and test groups, with particular attention to renal
function and testicular pathology. The dosage levels should be
set to demonstrate the no-effect level.
2. An adequate study to assess the carcinogenic potential of
bromophos.
REQUIRED (before tolerances can be recommended)
1. Residue data from supervised trials for meat of domestic animals
other than sheep, paultry, eggs, any fruit or vegetables not
listed.
2. Further information on good agricultural practices for use on
stored grain and the effects of moisture and temperature on
residues in stored grain.
DESIRABLE
A study to determine dose levels causing no carboxylesterase
(aliesterase) activity depression.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales, 1966-1977.
III. Organophosphorous pesticides residues in the total diet. Pestic.
Sci., 1: 10-13.
Barnes, J.M. (1966) Mammalian toxicity report. WHO insecticide
evaluation and testing programme, stage I. (unpublished)
Boehringer, C.H. Sohn. (1965-1971) Residue investigation reports.
Boehringer, C.H. Sohn. (1966) Produktion und Formulierung von
Bromophos bei Laboratories Fher Spanien. Report dated 25/3/66.
(unpublished)
Boehringer, C.H. Sohn. (1968) Chronic oral toxicity tests on dogs
using the compound bromophos. (unpublished)
Byewater, J. (1966) Report by Biological Laboratories to Agricola
Chemicals Ltd. (unpublished)
Clark, D.E., Younger, R.L. and Ayala, C.H. (1966) Toxicosis and
residues in bromophos-dipped sheep. J. Agr. Fd. Chem., 14(6): 608-609.
Dedek, W. and Schwarz, H. (1969) Zum verhalten des mindertoxischen
Insektizids 32P-bromophos nach cutaner Applikation am Rind. Z.
Naturf., 24B: 744-747.
Eichler D. (1971) Bromophos and bromophos-ethyl residues. Residue
Reviews, 41: 65-112.
El-Sebae, A.H. and El-Sayed, A.M.K. (1969) Persistence of malathion
and bromophos on bean and clover crops. Z. für angew. Entomol., 63:
378.
Engst, von R., Knoll, R. and Ackermann, H. (1969) Zur
Rückstandsproblematik von Pflanzenschutz und
Schödlingsbekämpfungsmitteln (PSM) in der Kleinstkinderernährung.
Dtsch. Gesundheitsw., 24: 1744.
Food and Drug Administration. (1970) Advisory committee on protocols
for safety evaluations: Panel on reproduction report on reproduction
studies in the safety evaluation of food additives and pesticide
residues. Toxicology and Applied Pharmacology, 16: 264-296.
Glees, P. (1966) Neuropathological report to C.H. Boehringer Sohn.
(unpublished)
Kinkel, H.J. (1964a) Ermittlung der akuten peroralen Toxizität und
Untersuchungen über die Neurotoxizität des Präparates Bromophos (CELA
S 1942 = O,O-dimethyl-O-2,5-dichlor-bromophenylthionophosphat)
an Hühnern. Report Battelle Institut. (unpublished)
Kinkel, H.J. (1964b) Prüfung des Potenzierungseffektes: Bromophos-EPN.
Report Battelle Institut. (unpublished)
Kinkel, H.J. and Dirks, E. (1966) Investigations on the cholinesterase
activity in dogs after oral application of bromophos. Report Battelle
Institut. (unpublished)
Kinkel, H.J. and Hübner, K.H. (1966) Histopathologische Untersuchungen
an Hirn und Rückenmark bei Hühnern nach oraler Applikation von
Bromophos. Report Battelle Institut. (unpublished)
Kinkel, H.J., Hübner, K.H., Königsman, G. and Dirks, E. (1965) Chronic
oral toxicity assay of bromophos
(O,O-dimethyl-O-(2,5-dichloro-4-bromophenyl)-thionophosphate) on
dogs and rats. Report Battelle Institut. (unpublished)
Kinkel, H.J., Muacevic, G., Sehring, R. and Bodenstein, G. (1966) Zur
Toxikologie von Bromophos. Archiv. für Toxikologie, 22: 36-57.
Kinkel, H.J. and Sann, E. (1964) Ermittlung der akuten
intraperitonealen Toxizität und Untersuchungen über die Hemmürkung des
Präparates Bromophos (CELA S 1942) auf die Erythrozyten-Cholinesterase
bei der Ratte. Report Battelle Institut. (unpublished)
Kinkel, H.J. and Seume, F.W. (1963) Investigations on the toxicity of
O,O-dimethyl-O-2,5-dichloro-4-bromo-phenyl-thionophosphate (S
1942). Report Battelle Institut. (unpublished)
Leber, G. (1967) Ruckstandsbestimmung von bromophos in fettarmen
pflanzlichen material. Internal reports RU 2,22/02/10-8/22/67 and
12/20/67 C.H. Boehringer Sohn.
Leber, G. (1968) Ruckstandsbestimmung von bromophos in tierischem
material. Internal Report RU 2,22/14/80-3/28/68. C.H. Boehringer Sohn.
Leber, G. and Deckers, W. (1968) Determination of residues of
bromophos and bromophos-ethyl. Proc. Brit. Insecticide Fungicide
Conf., Brighton, England, 4: 570.
Leuschner, F. (1968) Uber die subakute Toxizität von 3
Bromophos-metaboliten bei peroraler Verabreichung an Wister-Ratten.
Report C.H. Boehringer Sohn. (unpublished)
Leuschner, F. and Leuschner, A. (1966a) Untersuchungen über die
Wirkung von Bromophos auf den Foetus und das trächtige weibliche
Kaninchen bei peroraler Applikation. Report C.H. Boehringer Sohn.
(unpublished)
Leuschner, F. and Leuschner, A. (1966b) Untersuchüngen über die
Einwirkung von Bromophos auf die Acetyl-cholinesterase bei
Wistar-Ratten. Report C.H. Boehringer Sohn. (unpublished)
Leuschner, F., Leuschner, A. and Pöppe, E. (1967) Chronischer
Reproduktionsversuch über 3 Generationen an Wistar-Ratten bei
fortdauernder Verabreichung von Bromophos. Report C.H. Boehringer
Sohn. (unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W. and Otto, H. (1969)
Über die subakute Toxizität von Desmethylbromophos bei peroraler
Verabreichung an Sprague-Dawley Ratten. Report C.H. Boehringer Sohn.
(unpublished)
Muacevic, G. (1963) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1964) Reports C.H. Boehringer Sohn. (unpublished
Muacevic, G. (1965) Reaktivierungsversuche bei Bromophos. Report C.H.
Boehringer Sohn. (unpublished)
Muacevic, G. (1966) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1967) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1968) Die Reaktivierbarkeit der Cholinesterase durch
verschiedene Aldoxime nach Applikation von Bromophos im Rattengehirn
(in vivo-Versuche). Report C.H. Boehringer Sohn. (unpublished)
Muacevic, G. and Glees, P. (1967) Report on bromophos (1942) fowl
trial. C.H. Boehringer Sohn. (unpublished)
Muacevic, G. and Glees, P. (1968) Bericht über die Prüfung von
Bromophos an Hühnern. Report C.H. Boehringer Sohn. (unpublished)
Oettel, H. (1963) Report to C.H. Boehringer Sohn. Gewerbehyg.
Pharmakol. Institut der BASF. (unpublished)
Paton, I.M. (1965) Necropsy parasite recovery data controlled test.
Report Jensen-Salsbery Laboratories. (unpublished)
Rowlands, D.G. (1966a) The activation and detoxification of three
organic phosphorothionate insecticides applied to stored wheat grains.
J. stored Prod. Res., 2: 105-116.
Rowlands, D.G. (1966b) The metabolism of bromophos in stored wheat
grains. J. stored Prod. Res., 2: 1-12.
Rygg, T. and Somme, L. (1967) Rester av fosformidler i gulrot. Norsk
Landbruk, 13: 14.
Stenersen, J. (1969a) Degradation of 32P-bromophos by micro-organisms
and seedlings. Bull. Environ. Contam. Toxicol., 4: 104-112.
Stenersen, J. (1969b) Demethylation of the insecticide bromophos by a
glutathione-dependent liver enzyme and by alkaline buffers. J. Econ.
Entomol., 62(5): 1043-1045.
Stiasni, M., Rehbinder, D. and Deckers, W. (1967) Absorption,
distribution, and metabolism of
O-(4-bromo-2,5-dichlorophenyl)-O,O-dimethylphosphorothioate
(bromophos) in the rat. J. Agr. Fd. Chem., 15: 474-478.
Stiasni, M., Deckers, W., Schmidt, R. and Simon, H. (1969)
Translocation, penetration and metabolism of O-(4-bromo-2,5-
dichlorophenyl)-O,O-dimethylphosphorothioate (bromophos) in tomato
plants. J. Agr. Fd. Chem., 17(5): 1017-1020.
Tepe, J.B. (1968) Data from soil persistence and degradation study.
Eli Lilly and Company, communication to CELA G.m.b.H.
World Health Organization. (1967) Safe use of pesticides in public
health. Sixteenth report of the WHO expert committee on insecticides.
World Health Organization Technical Report Series No. 356.
Worth, H.M., Kehr, C.C. and Gibson, W.R. (1967) Effect of a single
dose of bromophos. Report Eli Lilly and Co. (unpublished)
In animals
The absorption, distribution and excretion of 32P and 3H-labelled
bromophos administered to rats by various routes are discussed in the
preceding section on BIOCHEMICAL ASPECTS.
The decomposition of 32P-labelled bromophos following cutaneous
application to lactating cows (20 mg/kg in alcohol or paraffin oil)
was studied by Dedek and Schwarz (1969). No bromoxon was detected in
blood or milk by thin-layer chromatography. Concentrations of 0.4 to
0.7 ppm of desmethyl-bromophos and traces of bromophos were present.
The two methyl groups of bromophos appear to be simultaneously split
off by mild alkaline hydrolysis and by the action of a
glutathione-dependent liver enzyme (Stenersen, 1969a, 1969b).
In plants
In studies on tomato plants using 32O and 3H-labelled bromophos, it
was found that it does not act systemically but penetrates from the
surface into the interior of the leaf and also from a nutrient
solution into the root. In addition to unchanged bromophos,
dichlorobromophenol was found as a main metabolite (13% of the total
dose applied after 7 days) and small amounts of bromoxon,
monodesmethyl-bromophos, dimethyl thionophosphate and inorganic
phosphate were detected (Stiasni et al., 1969).
The metabolism of bromophos-32P was studied in wheat-, carrot-, and
onion-seedlings and by soil microorganisms (Stenerson, 1969). The main
finding was that bisdesmethyl-bromophos had been produced in several
of the experiments, but no desmethyl-bromophos was detectable.
However, the identification of bisdesmethyl-bromophos was not certain.
In soil
Bromophos E.C. was applied one time at 0.5 g a.i./m2 to high moorland
soil (acid, high organic), Ingelheim sand and clay soil. Zero to 20
cm-deep samples were taken periodically for 26 weeks and analysed for
bromophos and dichloro-bromophenol residues, with the results shown in
table 5.
TABLE 5 Bromophos residues in soil
Residues (ppm)1
Type of Post-treatment Dichloro-bromophenol
soil time (weeks) Bromophos
High moorland 0 13.102 0.30
soil 1 9.00 0.26
3 8.18 1.14
6 6.95 0.57
9 2.15 1.72
13 0.75 0.53
26 0.58 0.93
Sandy soil 0 1.433 0.65
1 0.64 0.53
3 0.20 0.20
6 0.13 0.10
9 <0.02 0.10
13 <0.02 0.10
26 <0.02 0.10
Clay soil 0 1.544 0.59
1 0.81 0.29
3 0.20 0.21
6 0.09 <0.10
9 <0.02 <0.10
13 <0.02 <0.10
26 <0.02 <0.10
1 Values calculated as dry substance from the measured moisture
content.
2 Y = 1.006-0.056 log X; r = 0.9188
3 Y = 0.047-0.187 log X; r = 0.9777
4 Y = 0.095-0.201 log X; r = 0.9890
Degradation of bromophos is clearly much more rapid in sandy and clay
soils. When the sandy soil was treated once at the same rate with
bromophos granules, the residues (corrected for moisture in sample)
fell from 5.98 ppm at week 0 to <0.02 at week 26. Although initial
residues were higher than for E.C., no measurable residue remained
after 26 weeks (Boehringer, 1965-1971). The generally rapid
degradation in soil was further indicated by trials in Germany with 3%
coarse dust and 5% granular applied one time at 2, 4, or 8 kg/ha which
gave residues of 0.03-0.16 ppm after 92 days (Boehringer, 1965-1971)
and by elaborate tests in the United States using a range of
application rates, numbers of applications, sampling intervals and
soils in diverse geographic and climatic areas. There was no
appreciable build-up of residues or leaching down through soil to any
extent observed for either bromophos or dichlorobromophenol (Tepe,
1968).
In storage and processing
Bromophos was applied to stored wheat grains at 10 ppm and the
decomposition studied over a 10-week period. Although only traces of
dimethylphosphorothionate were found, indicating that
desmethyl-bromophos is the main phosphatase degradation product, large
amounts of monomethyl-phosphorothionate were detected. Bromoxon was
found only during the first 20 days in amounts up to 10 percent of the
original bromophos level. Degradation of bromophos proceeded rapidly
for about 7 days, then stopped for about 3 weeks due to the
accumulation of desmethyl-bromophos, which inhibits phosphatase
hydrolysis. After the desmethyl-bromophos had been degraded, bromophos
degradation resumed. The level of free dichlorobromophenol did not
increase significantly until degradation of desmethyl-bromophos was
well underway, suggesting the formation of the phenol from the
desmethyl or oxon compounds. When bromophos was applied to autoclaved
grains, only dimethyl-phosphorothionate and dichloro-bromophenol were
produced (Rowlands, 1966a).
Oxidation of bromophos to bromoxon occurred in the seed coats and
germs of wheat grains. Hydrolytic activity was found in the germ and
endosperm (Rowlands, 1966b).
METHODS OF RESIDUE ANALYSIS
Many general and specific chemical, biochemical and biological methods
of analysis have been developed for residues of bromophos. These have
recently been reviewed and summarized by Eichler (1971) and will not
be elaborated here. An excellent method has been developed by Leber
and Deckers (1968). It utilizes gas chromatography with a phosphorus
detector and can estimate quantities down to the range of 0.001 to
0.01 ppm with a 100-g sample. The method also includes procedures for
determining bromoxon either by colorimetry or by gas chromatography
with a sensitivity of 0.03 to 0.05 ppm. Thin-layer chromatographic
procedures are also given for identifying residues.
Since bromophos is among the pesticides listed as detectable by the
multi-residue gas chromatographic procedure of Abbott et al. (1970),
that method is the most suitable for regulatory use. The method of
Leber and Deckers (1968) would be suitable for confirmation of the
identity of residues.
NATIONAL TOLERANCES
Some examples of tolerances in various countries were reported to the
Joint Meeting and are listed in Table 6.
TABLE 6 Examples of national tolerances reported to the Meeting
Country Commodity Tolerance
(ppm)
Canada Apple 1.5
Germany, Federal Berries, pome fruit, leaf
Republic vegetables, cabbage 1.5
Stone fruit, legumes, 0.6
root vegetables
Field corn 0.2
Hungary 2
Netherlands 0.5
Carrots 1.5
Germany, Democratic Fruit, vegetables (except
Republic onions and potatoes) 11
Animal fat, vegetable oil,
potatoes, onions, meat, fish,
eggs, milk, grain, baby foods 0
South Africa Stored maize 8
1 not more than 0.1 ppm oxon.
APPRAISAL
Bromophos is a non-systemic halogen-containing organophosphorus
insecticide used on a wide variety of crops and animals to control
biting and sucking insects. It is also used to protect stored
products, as a seed protection agent for grain crops and as a vector
control agent in public health.
Supervised experiments with foliar, row or surface treatments on
fruits and vegetables have shown bromophos to have a generally low
persistence. However, the comparative rate of residue decline is
highly dependent on many factors, such as botanical species,
morphological structure, weather formulation, and method and time of
treatment. The lipophilic nature of the compound causes it to
penetrate the cuticular wax of certain crops (for example - apples and
pears) which delays release and degradation. This property makes it
necessary for tolerance recommendations to be made on an individual
commodity basis rather than on broad crop categories.
On the basis of a single trial in which four lambs were dipped in 0.5%
bromophos weekly for 9 weeks, it would appear that the compound did
not accumulate excessively in the fat and was eliminated fairly
rapidly after treatment ceased, dropping from about 10 ppm on day 1 to
about 2 ppm on day 8 and to about 0.2 ppm after 22 days. Milk cows
exposed to bromophos during stall treatment had low levels in the milk
(0.045 ppm maximum) which fell below the limit of detection (0.20 ppm)
after five days.
The metabolite of bromophos most likely to be found in plants and soil
is 2,5-dichloro-4-bromophenol. Small amounts of bromoxon and
monodesmethyl-bromophos were also found in tomato plants according to
one report, whereas different studies on wheat, carrot and onion
seedlings indicated the production of bisdesmethylbromophos but not
desmethyl-bromophos. The identity of the bisdesmethyl-bromophos was
not certain. In animals, bromophos is excreted rapidly via the urine
and the major metabolites found are dichloro-bromophenol and
monodesmethyl-bromophos. Extremely low levels of bromoxon may also
occur in blood.
Available multi-residue gas chromatographic procedures are suitable
for application for regulatory purpose and are recommended.
Although bromophos is recommended for use against ectoparasites of
animals and poultry, there was no data available on residues likely to
occur in meat (except sheep) or eggs and no recommendations for
tolerances could be made for these commodities.
RECOMMENDATIONS
TEMPORARY TOLERANCES
The following temporary tolerances are based on residues likely to be
found at harvest following currently recommended use patterns. For the
majority of fruits and vegetables, the recommended pre-harvest
interval is 7 days. In the case of whole milk, the practical residue
limit is at or about the limit of determination. The temporary
tolerances are expressed on bromophos.
ppm
Olives, olive oil 5
Apples, lamb's lettuce, leeks,
radishes 2
Carrots, celery, French beans, pears,
plums, red currants, savoy cabbage,
spinach 1
Blackberries, blackcurrants,
cherries, lettuce, gooseberries,
peaches, strawberries, sugarbeets
(roots), fat of meat of sheep 0.5
Wheat, rapeseed, rapeseed oil 0.2
Broccoli, cauliflower, cucumbers,
red cabbage, cabbage, kohlrabi,
onions, peas 0.1
Milk (whole) 0.02*
* at or about the limit of determination
Remarks
The temporary tolerance for wheat is based on residues likely to be
found at harvest.
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1977)
1. A study in dogs using animals of similar weight, age and origin
in control and test groups, with particular attention to renal
function and testicular pathology. The dosage levels should be
set to demonstrate the no-effect level.
2. An adequate study to assess the carcinogenic potential of
bromophos.
REQUIRED (before tolerances can be recommended)
1. Residue data from supervised trials for meat of domestic animals
other than sheep, paultry, eggs, any fruit or vegetables not
listed.
2. Further information on good agricultural practices for use on
stored grain and the effects of moisture and temperature on
residues in stored grain.
DESIRABLE
A study to determine dose levels causing no carboxylesterase
(aliesterase) activity depression.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales, 1966-1977.
III. Organophosphorous pesticides residues in the total diet. Pestic.
Sci., 1: 10-13.
Barnes, J.M. (1966) Mammalian toxicity report. WHO insecticide
evaluation and testing programme, stage I. (unpublished)
Boehringer, C.H. Sohn. (1965-1971) Residue investigation reports.
Boehringer, C.H. Sohn. (1966) Produktion und Formulierung von
Bromophos bei Laboratories Fher Spanien. Report dated 25/3/66.
(unpublished)
Boehringer, C.H. Sohn. (1968) Chronic oral toxicity tests on dogs
using the compound bromophos. (unpublished)
Byewater, J. (1966) Report by Biological Laboratories to Agricola
Chemicals Ltd. (unpublished)
Clark, D.E., Younger, R.L. and Ayala, C.H. (1966) Toxicosis and
residues in bromophos-dipped sheep. J. Agr. Fd. Chem., 14(6): 608-609.
Dedek, W. and Schwarz, H. (1969) Zum verhalten des mindertoxischen
Insektizids 32P-bromophos nach cutaner Applikation am Rind. Z.
Naturf., 24B: 744-747.
Eichler D. (1971) Bromophos and bromophos-ethyl residues. Residue
Reviews, 41: 65-112.
El-Sebae, A.H. and El-Sayed, A.M.K. (1969) Persistence of malathion
and bromophos on bean and clover crops. Z. für angew. Entomol., 63:
378.
Engst, von R., Knoll, R. and Ackermann, H. (1969) Zur
Rückstandsproblematik von Pflanzenschutz und
Schödlingsbekämpfungsmitteln (PSM) in der Kleinstkinderernährung.
Dtsch. Gesundheitsw., 24: 1744.
Food and Drug Administration. (1970) Advisory committee on protocols
for safety evaluations: Panel on reproduction report on reproduction
studies in the safety evaluation of food additives and pesticide
residues. Toxicology and Applied Pharmacology, 16: 264-296.
Glees, P. (1966) Neuropathological report to C.H. Boehringer Sohn.
(unpublished)
Kinkel, H.J. (1964a) Ermittlung der akuten peroralen Toxizität und
Untersuchungen über die Neurotoxizität des Präparates Bromophos (CELA
S 1942 = O,O-dimethyl-O-2,5-dichlor-bromophenylthionophosphat)
an Hühnern. Report Battelle Institut. (unpublished)
Kinkel, H.J. (1964b) Prüfung des Potenzierungseffektes: Bromophos-EPN.
Report Battelle Institut. (unpublished)
Kinkel, H.J. and Dirks, E. (1966) Investigations on the cholinesterase
activity in dogs after oral application of bromophos. Report Battelle
Institut. (unpublished)
Kinkel, H.J. and Hübner, K.H. (1966) Histopathologische Untersuchungen
an Hirn und Rückenmark bei Hühnern nach oraler Applikation von
Bromophos. Report Battelle Institut. (unpublished)
Kinkel, H.J., Hübner, K.H., Königsman, G. and Dirks, E. (1965) Chronic
oral toxicity assay of bromophos
(O,O-dimethyl-O-(2,5-dichloro-4-bromophenyl)-thionophosphate) on
dogs and rats. Report Battelle Institut. (unpublished)
Kinkel, H.J., Muacevic, G., Sehring, R. and Bodenstein, G. (1966) Zur
Toxikologie von Bromophos. Archiv. für Toxikologie, 22: 36-57.
Kinkel, H.J. and Sann, E. (1964) Ermittlung der akuten
intraperitonealen Toxizität und Untersuchungen über die Hemmürkung des
Präparates Bromophos (CELA S 1942) auf die Erythrozyten-Cholinesterase
bei der Ratte. Report Battelle Institut. (unpublished)
Kinkel, H.J. and Seume, F.W. (1963) Investigations on the toxicity of
O,O-dimethyl-O-2,5-dichloro-4-bromo-phenyl-thionophosphate (S
1942). Report Battelle Institut. (unpublished)
Leber, G. (1967) Ruckstandsbestimmung von bromophos in fettarmen
pflanzlichen material. Internal reports RU 2,22/02/10-8/22/67 and
12/20/67 C.H. Boehringer Sohn.
Leber, G. (1968) Ruckstandsbestimmung von bromophos in tierischem
material. Internal Report RU 2,22/14/80-3/28/68. C.H. Boehringer Sohn.
Leber, G. and Deckers, W. (1968) Determination of residues of
bromophos and bromophos-ethyl. Proc. Brit. Insecticide Fungicide
Conf., Brighton, England, 4: 570.
Leuschner, F. (1968) Uber die subakute Toxizität von 3
Bromophos-metaboliten bei peroraler Verabreichung an Wister-Ratten.
Report C.H. Boehringer Sohn. (unpublished)
Leuschner, F. and Leuschner, A. (1966a) Untersuchungen über die
Wirkung von Bromophos auf den Foetus und das trächtige weibliche
Kaninchen bei peroraler Applikation. Report C.H. Boehringer Sohn.
(unpublished)
Leuschner, F. and Leuschner, A. (1966b) Untersuchüngen über die
Einwirkung von Bromophos auf die Acetyl-cholinesterase bei
Wistar-Ratten. Report C.H. Boehringer Sohn. (unpublished)
Leuschner, F., Leuschner, A. and Pöppe, E. (1967) Chronischer
Reproduktionsversuch über 3 Generationen an Wistar-Ratten bei
fortdauernder Verabreichung von Bromophos. Report C.H. Boehringer
Sohn. (unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W. and Otto, H. (1969)
Über die subakute Toxizität von Desmethylbromophos bei peroraler
Verabreichung an Sprague-Dawley Ratten. Report C.H. Boehringer Sohn.
(unpublished)
Muacevic, G. (1963) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1964) Reports C.H. Boehringer Sohn. (unpublished
Muacevic, G. (1965) Reaktivierungsversuche bei Bromophos. Report C.H.
Boehringer Sohn. (unpublished)
Muacevic, G. (1966) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1967) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1968) Die Reaktivierbarkeit der Cholinesterase durch
verschiedene Aldoxime nach Applikation von Bromophos im Rattengehirn
(in vivo-Versuche). Report C.H. Boehringer Sohn. (unpublished)
Muacevic, G. and Glees, P. (1967) Report on bromophos (1942) fowl
trial. C.H. Boehringer Sohn. (unpublished)
Muacevic, G. and Glees, P. (1968) Bericht über die Prüfung von
Bromophos an Hühnern. Report C.H. Boehringer Sohn. (unpublished)
Oettel, H. (1963) Report to C.H. Boehringer Sohn. Gewerbehyg.
Pharmakol. Institut der BASF. (unpublished)
Paton, I.M. (1965) Necropsy parasite recovery data controlled test.
Report Jensen-Salsbery Laboratories. (unpublished)
Rowlands, D.G. (1966a) The activation and detoxification of three
organic phosphorothionate insecticides applied to stored wheat grains.
J. stored Prod. Res., 2: 105-116.
Rowlands, D.G. (1966b) The metabolism of bromophos in stored wheat
grains. J. stored Prod. Res., 2: 1-12.
Rygg, T. and Somme, L. (1967) Rester av fosformidler i gulrot. Norsk
Landbruk, 13: 14.
Stenersen, J. (1969a) Degradation of 32P-bromophos by micro-organisms
and seedlings. Bull. Environ. Contam. Toxicol., 4: 104-112.
Stenersen, J. (1969b) Demethylation of the insecticide bromophos by a
glutathione-dependent liver enzyme and by alkaline buffers. J. Econ.
Entomol., 62(5): 1043-1045.
Stiasni, M., Rehbinder, D. and Deckers, W. (1967) Absorption,
distribution, and metabolism of
O-(4-bromo-2,5-dichlorophenyl)-O,O-dimethylphosphorothioate
(bromophos) in the rat. J. Agr. Fd. Chem., 15: 474-478.
Stiasni, M., Deckers, W., Schmidt, R. and Simon, H. (1969)
Translocation, penetration and metabolism of O-(4-bromo-2,5-
dichlorophenyl)-O,O-dimethylphosphorothioate (bromophos) in tomato
plants. J. Agr. Fd. Chem., 17(5): 1017-1020.
Tepe, J.B. (1968) Data from soil persistence and degradation study.
Eli Lilly and Company, communication to CELA G.m.b.H.
World Health Organization. (1967) Safe use of pesticides in public
health. Sixteenth report of the WHO expert committee on insecticides.
World Health Organization Technical Report Series No. 356.
Worth, H.M., Kehr, C.C. and Gibson, W.R. (1967) Effect of a single
dose of bromophos. Report Eli Lilly and Co. (unpublished)
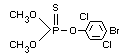 Other information on identity and properties
Molecular weight: 366.0
State: white crystals
Melting point: 53°C
Boiling point: 140-142°C at 10-2 torr.
Vapour pressure: 1.3 x 10-4 mm Hg at 20°C
Solubility: soluble in most organic solvents, e.g., toluene,
carbon tetrachloride, diethyl ether. Slightly
soluble in low molecular weight alcohols. Water
solubility - 40 ppm at 27°C.
Stability:
Stable in aqueous suspension. Hydrolyzes in distinct alkaline medium.
Purity of technical material:
O-4-bromo-2,5-dichlorophenyl-O,
O-dimethyl-phosphorothioate: approx. 95.0%;
O-4-bromo-2,3-dichlorophenyl-O,O-dimethyl-phosphorothioate:
approx. 3.0%;
O-6-bromo-2,5-dichlorophenyl-O,O-dimethyl-phosphorothioate:
approx. 1.0%;
O-dichlorophenyl-O,O-dimethyl-phosphorothioate:
approx. 1.0% (chlorine position not defined).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies on rats with 32P and 3H-labelled bromophos showed it is well
absorbed from the gastrointestinal tract. In the first 12 hours
following oral, intravenous or intraperitoneal administration, large
quantities of radioactivity were found in the stomach, intestine,
liver and kidneys, but after 24 hours the majority was found in urine
and faeces. Bromophos showed no tendency to accumulate in any organ.
The maximum blood level was found 7 hours after oral administration,
and the biological half life in rats was 14 hours. Approximately 96%
of the activity of a 10 mg/kg dose of 3H-bromophos was excreted in
urine and 1% in faeces during 24 hours following oral administration.
Excretion was complete in 96 hours, a total of 2% of activity
appearing in the faeces. In the same period, administration of
32P-bromophos resulted in the excretion of 63% of activity in urine
and 16% in faeces. During 8 hours following intraduodenal
administration of 5 mg/kg of 3H-bromophos, 25% of the activity was
excreted in bile in rats. Since only 1% of 32P-bromophos was excreted
in the same period, the biliary excretion was probably mainly
dichlorobromophenol or its metabolites. Biliary excreted 3H is
probably re-absorbed since only 2% of an oral dose was found in
faeces. The relatively high 32P content in faeces may result from
metabolism of bromophos in the intestine (Stiasni et al., 1967).
Biotransformation
Five urine metabolites were found after administration of
32P-bromophos whereas three were found after administration of
3H-bromophos. Analysis showed that the metabolites were phosphate,
dimethylthionophosphate, monodesmethyl-bromophos and
dichlorobromophenol. Two metabolites were unidentified. Bromophos
itself, its O-analogue bromoxon and monodesmethyl-bromoxon were not
found in urine (Stiasni et al., 1967).
Effect on enzymes and other biochemical parameters
Bromophos inhibits cholinesterase activity in rats and dogs, the
no-effect level when administered over a 100-day period being 0.63
mg/kg/day in rats and 1.5 mg/kg/day in dogs (Kinkel and Seume, 1963;
Kinkel et al., 1965; Kinkel and Dirks, 1966; Boehringer, 1968;
Leuschner et al., 1967). When a single oral dose of 800 or 1200
mg/kg was given to horses, plasma cholinesterase was inhibited even
after 16 days, although partial reactivation was found (Paton, 1965).
TOXICOLOGICAL STUDIES
Special studies on the metabolites
Several metabolites were found during investigations on rats and dogs
and on examination of metabolism of bromophos in plants. The most
important of these have been examined. A summary of acute oral
toxicities of bromophos metabolites is given in Table 1.
Short-term toxicity of metabolites
Groups of 10 male and 10 female rats were fed for 5 weeks on control
diet or diet providing 25, 100 or 400 mg/kg/day bromoxon, 250, 500 or
1000 mg/kg/day desmethyl-bromoxon or 250, 500 or 1000 mg/kg/day of
2,5-dichloro-4-bromophenol. At the highest dosage levels with each
metabolite the growth of animals was significantly inhibited. Plasma
and RBC cholinesterases were inhibited by bromoxon at 25 mg/kg
(maximum inhibition), and the plasma enzyme by desmethyl-bromoxon at
500 mg/kg. No enzyme inhibition occurred with dichloro-4-bromophenol.
The results of other investigations were normal; histopathological
studies were not carried out (Leuschner, 1968)
Groups of 10 male and 10 female rats were fed on diets providing 0,
125, 250 and 500 mg/kg/day of desmethyl-bromophos for 5 weeks. At 500
mg/kg, plasma cholinesterase was minimally inhibited. The weights of
liver and pituitary were above normal in male animals and histological
examination showed minimal centrilobular fatty infiltration of the
liver, the latter finding was considered to be reversible. Body weight
and food intake were reduced at the 500 mg/kg level. No abnormalities
were found at lower dosage levels (Leuschner et al., 1969).
Special studies on neurotoxicity
Six adult hens were administered 10 g/kg body-weight of bromophos
together with atropine and 2-PAM. Ataxia and incoordination were
present in all birds for several days, and three died. Histological
examination was said to reveal severe demyelination in all birds
(Byewater, 1966).
TABLE 1
Acute oral toxicities of bromophos metabolites
Compound Animal LD50 Reference
(mg/kg body-weight)
Bromoxon Mouse 2 100 Muacevic, 1965
Monodesmethyl-bromophos Rat 1 900-4 600 Muacevic, 1965
Mouse 1 800 1966
Desmethyl-bromophos Mouse 1 085 Muacevic, 1968
(triethylamine salt)
Desmethyl-bromoxon Mouse 2 150 Muacevic, 1965
O,O-dimethyl-O-(5,6-dichloro-4-bromophenyl)-thiophosphoric Mouse 2 850 Muacevic, 1965
acid ester
O,O-dimethyl-O-(4,6-dichloro-2-bromophenyl)-thiophosphoric Mouse about 2 000 Muacevic, 1965
acid ester
O,O-dimethyl-O-(2,4-dichloro-6-bromophenyl)-thiophosphoric Mouse >6 000 Muacevic, 1965
acid ester
O,O-dimethyl-thiophosphoric acid Mouse 4 700 Muacevic, 1965
sodium
2,5-dichloro-4-bromophenol Mouse 1 100-1 900 Muacevic, 1965
Rat 3 200-3 550 1966
Ten adult hens each received orally 1 g bromophos/kg/day until signs
of paralysis developed (between 12 and 56 days), at which time each
was examined for histological changes in the brain and spinal cord.
These were reported to consist of degeneration of ganglia cells and
demyelination (Kinkel and Hubner, 1966), but the suitability of the
material for accurate pathological interpretation was challenged
(Glees, 1966).
Two adult hens were each given 5.5 g/kg of bromophos in divided doses
over a period of 7 weeks. A 300 mg/kg dose produced transient
excitability and diarrhoea and the 4th of 5 and 4th of 8 consecutive
doses of 400 mg/kg day given at a 3-week interval caused an unsteady
gait and diarrhoea which persisted each time for 6-9 days after
administration. The hens completely recovered following this
treatment, indicating the lack of delayed neurotoxic effect (Barnes,
1966). Ten adult hens received two oral doses of 2 g/kg of bromophos
at 3-week intervals. A neuropathological examination carried out after
a 3-week observation period revealed no degenerative processes in the
CNS or peripheral nerves (Muacevic and Glees, 1967).
Groups of 10 adult hens were administered 0, 12.5 or 125 mg/kg of
bromophos/day in their food for 4 weeks. Clinically apparent
neurological signs were reversible when treatment ceased and a
neuropathological examination of 2 birds of each group showed no CNS
changes (Muacevic and Glees, 1968).
In order to investigate the occurrence of paralysis in dogs
administered bromophos for 2 years, and the finding on
neuropathological examination of localized ganglion cell degeneration
and other lesions in the CNS (Kinkel et al., 1965), an experiment
was carried out in which two groups of 3 male and 3 female dogs were
administered 0 and 87.5 mg/kg and 6 male and 6 female dogs 175 mg/kg
body-weight/day of bromophos orally in capsules for periods up to 270
days. One dog on 175 mg/kg had a single epileptiform fit and another
was killed after 62 doses because it was cachectic. An extensive
neuropathological examination of the central nervous system, spinal
ganglia and peripheral nerves failed to reveal any neuropathological
changes (Boehringer, 1968).
Special studies on pharmacology
Bromophos inhibits cholinesterase activity indirectly through its
metabolite bromoxon. No antidote effect was found with atropine
sulphate and aldoximes when rats and mice were administered high doses
of bromophos orally or intraperitoneally. The lack of effect was
probably due to the toxic effect of the high doses of solvent which
had to be used to administer the bromophos. However, cholinesterase
inhibition was diminished in mice by atropine and Toxogonin (Muacevic,
1965). Inhibition of brain cholinesterase in bromophos treated rats
could be prevented by intravenous injection of several reactivators
(Muacevic, 1968). Administration of atropine and 2-PAM stopped
development of signs of poisoning when 0.02-0.2 g/kg body-weight of
bromophos was administered to chickens. At 0.5 and 1.0 g/kg dosage
levels the development of signs of poisoning were delayed (Kinkel,
1964a).
Special studies on reproduction
Groups of 20 male and 20 female rats were fed on diets providing 0, 5,
20 and 80 mg bromophos/kg/day. These formed the parent generation of a
standard three generation study (F.D.A. advisory committee, 1970). The
80 mg/kg dosage level produced no clinical signs of poisoning but the
rate of body-weight gain was depressed in all generations,
particularly in males. The fertility and size and weight of litters
was unaffected, but the number of stillbirths in this group was high.
The survival rate of young was also reduced except in the first litter
and the parent generation. Animals consuming 5 or 20 mg/kg
bromophos/day were no different from control animals. No runts or
malformations were observed at any dosage or in any generation, and
the behaviour, appearance, food intake, results of haematological
investigations and adult survival were also unaffected. In this study
the cholinesterase activity of plasma and liver was significantly
depressed in animals receiving 5 mg/kg/day. The threshold dosage level
for RBC enzyme inhibition was between 5 and 30 mg/kg and for brain
enzyme between 5 and 20 mg/kg in males and 20 and 80 mg/kg in females
(Leuschner et al., 1967).
Special studies on teratogenicity
Five groups of 10 pregnant female rabbits were administered by gavage
25, 50, 100, 200 or 400 mg/kg body-weight of bromophos each day
between day 6 and 18 of pregnancy. Forty animals acted as controls.
Foetuses were removed and examined on day 30 of pregnancy. Twenty five
mg bromophos/kg/day orally had no untoward effect on foetuses or
parent animals. At higher dosage levels the parent animals became
debilitated and developed limb flaccidity and diarrhea, and the number
of fully-grown, live foetuses was reduced. In no group did the type or
number of malformations observed differ from that of the controls
(Leuschner and Leuschner, 1966a).
Acute toxicity
Studies on the acute toxicity of bromophos in several animal species
are summarized in Table 2.
TABLE 2 Acute toxicity of bromophos in animals
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 3 311 - 5 900 Muacevic, 1963; 1964;
1965; 1967;
Worth et al., 1967
Mouse i.p. 1 000 - 4 900 Oettel, 1963; Muacevic,
1964
Rat oral 3 750 - 8 000 Kinkel et al., 1966;
Oettel, 1963; Muacevic,
1967.
Rat i.p. 3 125 Kinkel and Sann, 1964.
Guinea pig oral >6 000 Muacevic, 1967
Rabbit oral 720 Muacevic, 1964
Fowl oral 9 700 Kinkel, 1964a
Dog oral >625 Worth et al., 1967
Potentiation of the acute toxicity of bromophos occurred with
bromophos-ethyl, diazinon, dichlorvos, dimethoate, malathion,
mevinphos, naleb, parathion and carbaryl. DDT, heptachlor and lindane
possessed strong antagonistic activity to the acute effects of
bromophos (Kinkel, 1964b; Muacevic 1964, 1965, 1966, 1967, 1968).
Short-term studies
Rat
Four groups of 6 male and 6 female rats received diets providing 0,
0.65, 1.25 and 2.5 mg bromophos per kg body-weight/day for 100 days.
The cholinesterase activity in RBC was not significantly affected at
any dosage level. Plasma enzyme was inhibited at the 1.25 mg/kg level,
but not at the lower level, and it returned to normal in two weeks
after the treatment was stopped. The behaviour, growth, food intake
and macroscopic appearance of organs were normal (Leuschner and
Leuschner, 1966b).
Four groups of 16 male rats received by gastric tube daily doses of 0,
188, 750 and 1 250 mg bromophos/kg body-weight/day for 100 days. The
rate of body-weight gain was reduced in all test groups while food
intake was lower only at the highest level. Animals in the 750 and
1 250 mg/kg groups became restless for an hour or so after treatment
but the degree of restlessness did not increase during the test.
Brain, liver and plasma cholinesterase were inhibited at all dosage
levels. Hydropic swelling of the hepatic cells was seen at all dosage
levels; this effect was dose related. In the 1 250 mg/kg group hyaline
droplets were seen in tubular cells and protein within the tubules of
the kidneys. Urine analysis and the bromosulphophthalein retention
test yielded negative results. Haematological findings and the
macroscopic appearance of internal organs were normal (Kinkel and
Seume, 1963).
Dog
Three groups of 2 male and 2 female beagle dogs received diets
providing 0.75, 1.5 and 3.0 mg bromophos/kg/day for 100 days. The
control group consisted of one male and one female. No or only slight
inhibition of the plasma and RBC cholinesterase activities (never
exceeding 36%) was observed at the three dosage levels; an inhibition
of 20% was suggested as no-effect level in this experiment.
Erythrocyte cholinesterase was more susceptible to inhibition than
plasma cholinesterase. In one dog exposed to the lowest dosage level,
35% inhibition of the RBC cholinesterase was observed, but this was
reduced to 20% when tested seven weeks later. Neither the brain nor
the liver cholinesterase was found to be inhibited when examined
(Kinkel and Dirks, 1966).
Six groups of 3 male and 3 female mongrel dogs aged 1-3 years and
weighing between 3.9 and 10.3 kg were administered 0, 11, 43.8, 87.5
and 175 mg bromophos/kg body-weight by gastric tube on every working
day for 2 years. Treatment at the 87.5 and 175 mg/kg levels made the
animals restless for some time after dosage. One animal on each of the
43.8 and 175 mg/kg levels died after suffering bites, and one on 87.5
and 3 on 175 mg/kg died after developing respiratory difficulties,
hoarseness, salivation and tremors followed by ataxia and then paresis
of the hind limbs. Two dogs of the 175 mg/kg group developed the same
signs but recovered, one after being taken off treatment and the other
while administration of bromophos continued. Histological examination
of the animals which died on the two highest dosage levels showed foci
of ganglion cell degeneration in the spinal cord; this involved only
small numbers of cells in the ganglia. In addition, spermatogenesis
was impaired with proliferation of Leydig's cells in 2 males of the
175 mg/kg group. Degeneration of the CNS in animals which did not die
during the test was insignificant or non-existent. Plasma, RBC, liver
and brain cholinesterase activities were depressed at all dosage
levels in this test. The food intake, growth, sexual cycle, urinary
ascorbic acid excretion and the results of haematological, liver
function and urine tests were similar in test and control groups. The
relative organ weights and results of macroscopic and microscopic
examination of organs were normal in the 11 and 43.8 mg/kg groups
(Kinkel et al., 1965).
In a nine month study (See special studies on
neurotoxicity) groups of mongrel dogs weighing 6-20 kg were
administered 0, 87.5 or 175 mg bromophos/kg body-weight
daily in capsules for 270 days. Body-weight and food intake were
reduced in accordance with the dose given. Although 2/6 bitches on
175 mg/kg/day were at no time on heat, histological examination of
the ovaries and uterus revealed no pathological findings.
Histological examination showed seminiferous tubule degeneration in
3 dogs receiving 175 mg/kg/day. Prostatic hypertrophy was also more
evident in test animals. The death of one dog of the high level group
was not regarded as being due to bromophos consumption. Erythrocyte
and plasma cholinesterase were inhibited in both test groups. The
results of haematological tests, serum analysis and urine analysis
were all normal (Boehringer, 1968).
Long-term studies
Rat
Groups of 6-month-old rats were administered 0, 87.5, 175 and 350 mg
bromophos/kg body-weight by gavage each working day for 2 years. The
groups consisted of 25 male and 25 female animals, but 3, 5, 5 and 17
animals were included in the test after the start in the 0, 87.5, 175
and 350 mg/kg dosage groups, respectively. After 8, 10, 12, 15, 18 and
21 months, two animals, one of each sex, were killed for
histopathological examination. The rate of body-weight gain was
depressed at the highest dosage level, and in this group proteinuria
was more evident than in controls; in addition, the number of
erythrocytes in the urinary sediment was increased but the kidneys
were histologically normal.
Cholinesterase was inhibited in the plasma, RBC, brain and liver by
all treatment levels. Survival was poor, with only 5, 4, 2 and 5 males
and 9, 6, 7 and 8 females of the 0, 87.5, 175 and 350 mg/kg groups,
respectively, surviving for 100 weeks. The behaviour of all test
groups was normal as were the results of haematological and liver
function tests. Urinary ascorbic acid excretion was unaltered by
bromophos administration. The weights of 8 organs and the results of
macroscopic and microscopic examinations revealed no lesion which
could be attributed to treatment. No tumours were reported to have
occurred in control or test groups (Kinkel et al., 1965).
OBSERVATIONS IN MAN
Workers exposed to bromophos during production and formulation for two
years showed no change in RBC cholinesterase levels as estimated at
monthly intervals (Boehringer, 1966). Eight subjects were engaged in
spraying bromophos for 6´ hours over a period of 2 days, and 5 sprayed
for 4 hours a day on 14 days during a 22-day period in trials at the
WHO Insecticide Testing Unit, Lagos. Protective clothing was worn. No
clinical symptoms occurred, but plasma cholinesterase levels were
slightly depressed, the lowest being 75% of the pre-exposure level.
Levels returned to normal after one month (WHO, 1967).
COMMENT
Bromophos is rapidly absorbed, metabolized and excreted, mainly in the
urine. The compound potentiates the activity of several
organo-phosphates and carbamates, but its acute effects are
antagonized by some chlorinated hydrocarbons. The data on
neurotoxicity are contradictory and hence unsatisfactory. However, the
studies said to give positive results were poor, and the Meeting felt
that the compound was unlikely to cause neuropathy.
A reproduction study in rats showed no effect at 20 mg/kg/day.
Increased stillbirths and decreased pup weight were evident at 80
mg/kg/day.
Short-term studies in rat showed no effects on plasma cholinesterase
at 0.63 mg/kg/day. Somewhat higher doses caused erythrocyte
cholinesterase depression. At much higher doses brain cholinesterase
depression occurred. Hydropic swelling of hepatic cells at 188
mg/kg/day and hyaline droplets in tubular cells and protein in kidney
tubules at 1 250 mg/kg/day were observed.
Short-term studies in dog indicated no effect on plasma cholinesterase
at 1.5 mg/kg/day. At 175 mg/kg/day impaired spermatogenesis was
observed.
A long-term study in rat did not reveal any tumours, but survival was
poor at termination of the study.
Although the studies were considered poor by present day standards,
the Meeting decided that data were adequate to permit establishment of
a temporary ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 0.63 mg/kg body-weight/day
Dog: 1.5 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.006 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bromophos is an insecticide with contact and stomach action. It is
effective against a broad spectrum of chewing and sucking insects and
possesses a very low mammalian toxicity. It can be used as an
emulsifiable concentrate, ULV concentrate, wettable powder, granular
or dust formulation, seed dressing, aerosol formulation and fog
solution. It is compatible with other insecticides and fungicides.
Bromophos is of moderate toxicity to bees and should not be sprayed on
flowering crops during the flight of bees.
Bromophos has been used in the majority of European countries as well
as Algeria, Canada, Kuwait, Mexico, New Zealand, Nicaragua, Pakistan,
South Africa, Syria and Venezuela.
Pre-harvest treatments
Bromophos is used on various crops, mainly fruit and vegetables, for
control of a large number of important sucking and chewing insect
pests, such as vegetable root maggots, aphids, sawflies, fruit flies,
codling moths, mangold fly and beetles.
According to the single crop and the main pest species occurring, the
recommended concentrations of spray wash are given between 0.02% and
0.1% a.i.; the application rates range from 0.4 kg to 1.5 kg a.i./ha.
Bromophos is suitable for the treatment of a number of crops, except
for certain susceptible varieties of grape, pear, melon, cucumber,
lettuce and cabbage, mainly as frequent short interval treatments. Use
in glasshouses is not recommended.
The withholding periods range from 1 day to 21 days, depending on
local conditions and crop.
Post-harvest treatments
Bromophos is registered and recommended for storage pest control in
Mexico, South Africa, Spain and the United Kingdom and is currently
being introduced into other European and overseas countries.
Application of between 6 ppm and 12 ppm is recommended for grain
protection against various species of weevil and beetle causing damage
to stored products.
Other uses
Bromophos is used for the indoor and outdoor control of pests of
hygienic importance. As it shows a good effect against larval as well
as against adult stages and as it has a very low mammalian toxicity,
bromophos has proved to be an effective and safe compound for public
health operations. Furthermore, this material is recommended in the
veterinary field against chicken mites and other ectoparasites on
domestic animals and is highly effective against blowflies on sheep.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials on a variety of
fruits, vegetables, field crops and stored wheat (Boehringer,
1965-1971). Summaries of much of this information, and additional data
as well, have been published recently (Eichler, 1971). Table 3
presents a summary of available data, together with relevant
information on rates of application, number of applications and
pre-harvest interval.
In one reported trial, stored wheat that had been treated with
bromophos at 3 rates was milled into flour and the flour baked into
bread (Boehringer, 1965-1971). Analysis at each step gave the results
shown in Table 4.
Thus, less than 10% of the original amount of pesticide applied to
wheat is carried through to the final product, bread.
Data are also available on residues resulting from supervised trials
in animals.
Four lambs were dipped in 0.5% bromophos weekly for 9 weeks. Omental
biopsies were performed up to 22 days after the final dipping and the
fat samples analyzed by microcoulometric gas chromatography. Residues
of bromophos ranged from 5-14 ppm on day 1 to 0.07-0.43 ppm on day 22.
Bromoxon and 2,5-dichloro-4-bromophenol were not determined (Clark
et al., 1966).
TABLE 3 Bromophos residues on several crops
Rate of No. of Pre-harvest
Crop application treatments interval Residue
(a.i.) (days) (ppm)
Cauliflower 0.06 g/m2 1 0 4.901
7 0.041
14 <0.021
Broccoli 0.05 g/plant 1 62 <0.10
Kidney beans 0.06 g/m2 1 0 0.62-1.50
7 0.26-0.56
Cucumbers 0.04 g/m2 1 0 0.15-0.45
7 0.03-0.05
Garden lettuce 0.015 g/m2 1 4 1.9-2.6
0.025 g/m2 1 4 0.34-0.60
7 0.09-0.26
Lambs lettuce 0.025 g/m2 1 7 0.94-1.17
Kohlrabi 0.04 g/m2 1 7 <0.02
0.06 g/plant 1 30 0.04
Leek 0.8 kg/ha 1 0 2.76-2.80
14 1.27-1.43
Carrots 0.25 g/m2 2 38 2.90-3.55
0.2 g/m2 2 45 0.89-1.03
80 0.58-0.59
Peas 0.04 g/m2 1 7 <0.03
Radish 0.25 g/m2 1 16 1.03-1.32
21 0.12-0.23
Celery 120 g/ha 4 75 <0.05 (stalks)
0.32-0.55 (leaves)
Spinach 0.04 g/m2 1 1 5.48-7.85
7 0.25-0.67
14 <0.03-0.16
TABLE 3 (Cont'd.)
Rate of No. of Pre-harvest
Crop application treatments interval Residue
(a.i.) (days) (ppm)
Red cabbage 0.05 g/m2 1 7 <0.08
White cabbage 0.05 g/m2 1 7 <0.06-0.10
Savoy cabbage 0.06 g/m2 1 1 1.18-2.70
7 0.57-1.00
14 0.03-0.42
Onions 0.1 g/m2 1 123 <0.02
Maize 2.0 kg/ha 1 75 <0.17
Sugar beets 0.06 g/m2 1 14 <0.15-0.22 (beet)
1.1-1.9 (leaves)
Wheat 114 g/ha 1 14 0.17
228 g/ha 2 45 <0.02
Rape 228 g/ha 1 49 0.09 (seed)
0.17 (oil)
Apples 2.0 g/tree 1 0 1.10-1.20
4 1.49-1.70
7 0.51-1.30
14 0.48-0.61
Pears 1.25 g/tree 1 8 0.59-0.77
Yellow plums 0.62 g/tree 1 7 0.512
Cherries 2.5 g/tree 1 1 1.103
7 0.363
Peaches 0.38 g/tree 1 7 0.03-0.13
14 <0.03-0.25
Blackcurrants 0.25 g/shrub 1 7 0.13-0.36
Redcurrants 0.25 g/shrub 1 7 0.64-0.74
0.8 kg/ha 1 0 0.56-0.61
7 0.11-0.13
TABLE 3 (Cont'd.)
Rate of No. of Pre-harvest
Crop application treatments interval Residue
(a.i.) (days) (ppm)
Blackberries 0.25 g/shrub 1 7 0.14-0.19
Strawberries 0.8 kg/ha 1 0 0.61-0.64
7 0.11-0.12
Gooseberries 0.8 kg/ha 1 0 0.20-0.25
7 0.06-0.07
0.25 g/shrub 1 7 0.40-0.43
Olives 4.5 g/tree 1 10 2.58-2.92
Olive oil 4.5 g/tree 1 10 3.00-3.15
Stored wheat 0.012 g/kg 1 0 8.0
122 5.6
365 5.3
1 average of 4 analyses; raw data not available.
2 average of 9 analyses; raw data not available.
3 average of 6 analyses; raw data not available.
TABLE 4 Bromophos residues in wheat and wheat products
Rate of Post-treatment Residues (ppm)
application interval (days) Wheat Flour Bread
6 ppm 270 1.32 1.20 0.38-0.41
12 ppm 270 3.00 2.65-2.90 0.78-0.87
25 ppm 880 4.57 3.98-4.25 1.14-1.16
Milk from ten cows was analyzed for residues after stall spraying at
0.5 g/m2 of wall surface. Five cows were present during the spraying
and five were removed. Milk from the latter cows did not contain
measurable amounts of bromophos (<0.02 ppm). Milk from the five cows
left in the stalls during treatment had residues of 0.032-0.042 ppm on
post-treatment day 1; 0.031-0.045 ppm on day 2; 0.026-0.034 ppm on day
3; and <0.020 on day 5 (Boehringer, 1965-1971).
FATE OF RESIDUES
General comments
The metabolism or degradation of bromophos appears to be similar in
plants and animals, with the exception that hardly any bromoxon is
found in animals whereas low levels of bromoxon are likely to be
encountered in plant material. This is summarized in Figure 1.
Other information on identity and properties
Molecular weight: 366.0
State: white crystals
Melting point: 53°C
Boiling point: 140-142°C at 10-2 torr.
Vapour pressure: 1.3 x 10-4 mm Hg at 20°C
Solubility: soluble in most organic solvents, e.g., toluene,
carbon tetrachloride, diethyl ether. Slightly
soluble in low molecular weight alcohols. Water
solubility - 40 ppm at 27°C.
Stability:
Stable in aqueous suspension. Hydrolyzes in distinct alkaline medium.
Purity of technical material:
O-4-bromo-2,5-dichlorophenyl-O,
O-dimethyl-phosphorothioate: approx. 95.0%;
O-4-bromo-2,3-dichlorophenyl-O,O-dimethyl-phosphorothioate:
approx. 3.0%;
O-6-bromo-2,5-dichlorophenyl-O,O-dimethyl-phosphorothioate:
approx. 1.0%;
O-dichlorophenyl-O,O-dimethyl-phosphorothioate:
approx. 1.0% (chlorine position not defined).
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Studies on rats with 32P and 3H-labelled bromophos showed it is well
absorbed from the gastrointestinal tract. In the first 12 hours
following oral, intravenous or intraperitoneal administration, large
quantities of radioactivity were found in the stomach, intestine,
liver and kidneys, but after 24 hours the majority was found in urine
and faeces. Bromophos showed no tendency to accumulate in any organ.
The maximum blood level was found 7 hours after oral administration,
and the biological half life in rats was 14 hours. Approximately 96%
of the activity of a 10 mg/kg dose of 3H-bromophos was excreted in
urine and 1% in faeces during 24 hours following oral administration.
Excretion was complete in 96 hours, a total of 2% of activity
appearing in the faeces. In the same period, administration of
32P-bromophos resulted in the excretion of 63% of activity in urine
and 16% in faeces. During 8 hours following intraduodenal
administration of 5 mg/kg of 3H-bromophos, 25% of the activity was
excreted in bile in rats. Since only 1% of 32P-bromophos was excreted
in the same period, the biliary excretion was probably mainly
dichlorobromophenol or its metabolites. Biliary excreted 3H is
probably re-absorbed since only 2% of an oral dose was found in
faeces. The relatively high 32P content in faeces may result from
metabolism of bromophos in the intestine (Stiasni et al., 1967).
Biotransformation
Five urine metabolites were found after administration of
32P-bromophos whereas three were found after administration of
3H-bromophos. Analysis showed that the metabolites were phosphate,
dimethylthionophosphate, monodesmethyl-bromophos and
dichlorobromophenol. Two metabolites were unidentified. Bromophos
itself, its O-analogue bromoxon and monodesmethyl-bromoxon were not
found in urine (Stiasni et al., 1967).
Effect on enzymes and other biochemical parameters
Bromophos inhibits cholinesterase activity in rats and dogs, the
no-effect level when administered over a 100-day period being 0.63
mg/kg/day in rats and 1.5 mg/kg/day in dogs (Kinkel and Seume, 1963;
Kinkel et al., 1965; Kinkel and Dirks, 1966; Boehringer, 1968;
Leuschner et al., 1967). When a single oral dose of 800 or 1200
mg/kg was given to horses, plasma cholinesterase was inhibited even
after 16 days, although partial reactivation was found (Paton, 1965).
TOXICOLOGICAL STUDIES
Special studies on the metabolites
Several metabolites were found during investigations on rats and dogs
and on examination of metabolism of bromophos in plants. The most
important of these have been examined. A summary of acute oral
toxicities of bromophos metabolites is given in Table 1.
Short-term toxicity of metabolites
Groups of 10 male and 10 female rats were fed for 5 weeks on control
diet or diet providing 25, 100 or 400 mg/kg/day bromoxon, 250, 500 or
1000 mg/kg/day desmethyl-bromoxon or 250, 500 or 1000 mg/kg/day of
2,5-dichloro-4-bromophenol. At the highest dosage levels with each
metabolite the growth of animals was significantly inhibited. Plasma
and RBC cholinesterases were inhibited by bromoxon at 25 mg/kg
(maximum inhibition), and the plasma enzyme by desmethyl-bromoxon at
500 mg/kg. No enzyme inhibition occurred with dichloro-4-bromophenol.
The results of other investigations were normal; histopathological
studies were not carried out (Leuschner, 1968)
Groups of 10 male and 10 female rats were fed on diets providing 0,
125, 250 and 500 mg/kg/day of desmethyl-bromophos for 5 weeks. At 500
mg/kg, plasma cholinesterase was minimally inhibited. The weights of
liver and pituitary were above normal in male animals and histological
examination showed minimal centrilobular fatty infiltration of the
liver, the latter finding was considered to be reversible. Body weight
and food intake were reduced at the 500 mg/kg level. No abnormalities
were found at lower dosage levels (Leuschner et al., 1969).
Special studies on neurotoxicity
Six adult hens were administered 10 g/kg body-weight of bromophos
together with atropine and 2-PAM. Ataxia and incoordination were
present in all birds for several days, and three died. Histological
examination was said to reveal severe demyelination in all birds
(Byewater, 1966).
TABLE 1
Acute oral toxicities of bromophos metabolites
Compound Animal LD50 Reference
(mg/kg body-weight)
Bromoxon Mouse 2 100 Muacevic, 1965
Monodesmethyl-bromophos Rat 1 900-4 600 Muacevic, 1965
Mouse 1 800 1966
Desmethyl-bromophos Mouse 1 085 Muacevic, 1968
(triethylamine salt)
Desmethyl-bromoxon Mouse 2 150 Muacevic, 1965
O,O-dimethyl-O-(5,6-dichloro-4-bromophenyl)-thiophosphoric Mouse 2 850 Muacevic, 1965
acid ester
O,O-dimethyl-O-(4,6-dichloro-2-bromophenyl)-thiophosphoric Mouse about 2 000 Muacevic, 1965
acid ester
O,O-dimethyl-O-(2,4-dichloro-6-bromophenyl)-thiophosphoric Mouse >6 000 Muacevic, 1965
acid ester
O,O-dimethyl-thiophosphoric acid Mouse 4 700 Muacevic, 1965
sodium
2,5-dichloro-4-bromophenol Mouse 1 100-1 900 Muacevic, 1965
Rat 3 200-3 550 1966
Ten adult hens each received orally 1 g bromophos/kg/day until signs
of paralysis developed (between 12 and 56 days), at which time each
was examined for histological changes in the brain and spinal cord.
These were reported to consist of degeneration of ganglia cells and
demyelination (Kinkel and Hubner, 1966), but the suitability of the
material for accurate pathological interpretation was challenged
(Glees, 1966).
Two adult hens were each given 5.5 g/kg of bromophos in divided doses
over a period of 7 weeks. A 300 mg/kg dose produced transient
excitability and diarrhoea and the 4th of 5 and 4th of 8 consecutive
doses of 400 mg/kg day given at a 3-week interval caused an unsteady
gait and diarrhoea which persisted each time for 6-9 days after
administration. The hens completely recovered following this
treatment, indicating the lack of delayed neurotoxic effect (Barnes,
1966). Ten adult hens received two oral doses of 2 g/kg of bromophos
at 3-week intervals. A neuropathological examination carried out after
a 3-week observation period revealed no degenerative processes in the
CNS or peripheral nerves (Muacevic and Glees, 1967).
Groups of 10 adult hens were administered 0, 12.5 or 125 mg/kg of
bromophos/day in their food for 4 weeks. Clinically apparent
neurological signs were reversible when treatment ceased and a
neuropathological examination of 2 birds of each group showed no CNS
changes (Muacevic and Glees, 1968).
In order to investigate the occurrence of paralysis in dogs
administered bromophos for 2 years, and the finding on
neuropathological examination of localized ganglion cell degeneration
and other lesions in the CNS (Kinkel et al., 1965), an experiment
was carried out in which two groups of 3 male and 3 female dogs were
administered 0 and 87.5 mg/kg and 6 male and 6 female dogs 175 mg/kg
body-weight/day of bromophos orally in capsules for periods up to 270
days. One dog on 175 mg/kg had a single epileptiform fit and another
was killed after 62 doses because it was cachectic. An extensive
neuropathological examination of the central nervous system, spinal
ganglia and peripheral nerves failed to reveal any neuropathological
changes (Boehringer, 1968).
Special studies on pharmacology
Bromophos inhibits cholinesterase activity indirectly through its
metabolite bromoxon. No antidote effect was found with atropine
sulphate and aldoximes when rats and mice were administered high doses
of bromophos orally or intraperitoneally. The lack of effect was
probably due to the toxic effect of the high doses of solvent which
had to be used to administer the bromophos. However, cholinesterase
inhibition was diminished in mice by atropine and Toxogonin (Muacevic,
1965). Inhibition of brain cholinesterase in bromophos treated rats
could be prevented by intravenous injection of several reactivators
(Muacevic, 1968). Administration of atropine and 2-PAM stopped
development of signs of poisoning when 0.02-0.2 g/kg body-weight of
bromophos was administered to chickens. At 0.5 and 1.0 g/kg dosage
levels the development of signs of poisoning were delayed (Kinkel,
1964a).
Special studies on reproduction
Groups of 20 male and 20 female rats were fed on diets providing 0, 5,
20 and 80 mg bromophos/kg/day. These formed the parent generation of a
standard three generation study (F.D.A. advisory committee, 1970). The
80 mg/kg dosage level produced no clinical signs of poisoning but the
rate of body-weight gain was depressed in all generations,
particularly in males. The fertility and size and weight of litters
was unaffected, but the number of stillbirths in this group was high.
The survival rate of young was also reduced except in the first litter
and the parent generation. Animals consuming 5 or 20 mg/kg
bromophos/day were no different from control animals. No runts or
malformations were observed at any dosage or in any generation, and
the behaviour, appearance, food intake, results of haematological
investigations and adult survival were also unaffected. In this study
the cholinesterase activity of plasma and liver was significantly
depressed in animals receiving 5 mg/kg/day. The threshold dosage level
for RBC enzyme inhibition was between 5 and 30 mg/kg and for brain
enzyme between 5 and 20 mg/kg in males and 20 and 80 mg/kg in females
(Leuschner et al., 1967).
Special studies on teratogenicity
Five groups of 10 pregnant female rabbits were administered by gavage
25, 50, 100, 200 or 400 mg/kg body-weight of bromophos each day
between day 6 and 18 of pregnancy. Forty animals acted as controls.
Foetuses were removed and examined on day 30 of pregnancy. Twenty five
mg bromophos/kg/day orally had no untoward effect on foetuses or
parent animals. At higher dosage levels the parent animals became
debilitated and developed limb flaccidity and diarrhea, and the number
of fully-grown, live foetuses was reduced. In no group did the type or
number of malformations observed differ from that of the controls
(Leuschner and Leuschner, 1966a).
Acute toxicity
Studies on the acute toxicity of bromophos in several animal species
are summarized in Table 2.
TABLE 2 Acute toxicity of bromophos in animals
Animal Route LD50 References
(mg/kg
body-weight)
Mouse oral 3 311 - 5 900 Muacevic, 1963; 1964;
1965; 1967;
Worth et al., 1967
Mouse i.p. 1 000 - 4 900 Oettel, 1963; Muacevic,
1964
Rat oral 3 750 - 8 000 Kinkel et al., 1966;
Oettel, 1963; Muacevic,
1967.
Rat i.p. 3 125 Kinkel and Sann, 1964.
Guinea pig oral >6 000 Muacevic, 1967
Rabbit oral 720 Muacevic, 1964
Fowl oral 9 700 Kinkel, 1964a
Dog oral >625 Worth et al., 1967
Potentiation of the acute toxicity of bromophos occurred with
bromophos-ethyl, diazinon, dichlorvos, dimethoate, malathion,
mevinphos, naleb, parathion and carbaryl. DDT, heptachlor and lindane
possessed strong antagonistic activity to the acute effects of
bromophos (Kinkel, 1964b; Muacevic 1964, 1965, 1966, 1967, 1968).
Short-term studies
Rat
Four groups of 6 male and 6 female rats received diets providing 0,
0.65, 1.25 and 2.5 mg bromophos per kg body-weight/day for 100 days.
The cholinesterase activity in RBC was not significantly affected at
any dosage level. Plasma enzyme was inhibited at the 1.25 mg/kg level,
but not at the lower level, and it returned to normal in two weeks
after the treatment was stopped. The behaviour, growth, food intake
and macroscopic appearance of organs were normal (Leuschner and
Leuschner, 1966b).
Four groups of 16 male rats received by gastric tube daily doses of 0,
188, 750 and 1 250 mg bromophos/kg body-weight/day for 100 days. The
rate of body-weight gain was reduced in all test groups while food
intake was lower only at the highest level. Animals in the 750 and
1 250 mg/kg groups became restless for an hour or so after treatment
but the degree of restlessness did not increase during the test.
Brain, liver and plasma cholinesterase were inhibited at all dosage
levels. Hydropic swelling of the hepatic cells was seen at all dosage
levels; this effect was dose related. In the 1 250 mg/kg group hyaline
droplets were seen in tubular cells and protein within the tubules of
the kidneys. Urine analysis and the bromosulphophthalein retention
test yielded negative results. Haematological findings and the
macroscopic appearance of internal organs were normal (Kinkel and
Seume, 1963).
Dog
Three groups of 2 male and 2 female beagle dogs received diets
providing 0.75, 1.5 and 3.0 mg bromophos/kg/day for 100 days. The
control group consisted of one male and one female. No or only slight
inhibition of the plasma and RBC cholinesterase activities (never
exceeding 36%) was observed at the three dosage levels; an inhibition
of 20% was suggested as no-effect level in this experiment.
Erythrocyte cholinesterase was more susceptible to inhibition than
plasma cholinesterase. In one dog exposed to the lowest dosage level,
35% inhibition of the RBC cholinesterase was observed, but this was
reduced to 20% when tested seven weeks later. Neither the brain nor
the liver cholinesterase was found to be inhibited when examined
(Kinkel and Dirks, 1966).
Six groups of 3 male and 3 female mongrel dogs aged 1-3 years and
weighing between 3.9 and 10.3 kg were administered 0, 11, 43.8, 87.5
and 175 mg bromophos/kg body-weight by gastric tube on every working
day for 2 years. Treatment at the 87.5 and 175 mg/kg levels made the
animals restless for some time after dosage. One animal on each of the
43.8 and 175 mg/kg levels died after suffering bites, and one on 87.5
and 3 on 175 mg/kg died after developing respiratory difficulties,
hoarseness, salivation and tremors followed by ataxia and then paresis
of the hind limbs. Two dogs of the 175 mg/kg group developed the same
signs but recovered, one after being taken off treatment and the other
while administration of bromophos continued. Histological examination
of the animals which died on the two highest dosage levels showed foci
of ganglion cell degeneration in the spinal cord; this involved only
small numbers of cells in the ganglia. In addition, spermatogenesis
was impaired with proliferation of Leydig's cells in 2 males of the
175 mg/kg group. Degeneration of the CNS in animals which did not die
during the test was insignificant or non-existent. Plasma, RBC, liver
and brain cholinesterase activities were depressed at all dosage
levels in this test. The food intake, growth, sexual cycle, urinary
ascorbic acid excretion and the results of haematological, liver
function and urine tests were similar in test and control groups. The
relative organ weights and results of macroscopic and microscopic
examination of organs were normal in the 11 and 43.8 mg/kg groups
(Kinkel et al., 1965).
In a nine month study (See special studies on
neurotoxicity) groups of mongrel dogs weighing 6-20 kg were
administered 0, 87.5 or 175 mg bromophos/kg body-weight
daily in capsules for 270 days. Body-weight and food intake were
reduced in accordance with the dose given. Although 2/6 bitches on
175 mg/kg/day were at no time on heat, histological examination of
the ovaries and uterus revealed no pathological findings.
Histological examination showed seminiferous tubule degeneration in
3 dogs receiving 175 mg/kg/day. Prostatic hypertrophy was also more
evident in test animals. The death of one dog of the high level group
was not regarded as being due to bromophos consumption. Erythrocyte
and plasma cholinesterase were inhibited in both test groups. The
results of haematological tests, serum analysis and urine analysis
were all normal (Boehringer, 1968).
Long-term studies
Rat
Groups of 6-month-old rats were administered 0, 87.5, 175 and 350 mg
bromophos/kg body-weight by gavage each working day for 2 years. The
groups consisted of 25 male and 25 female animals, but 3, 5, 5 and 17
animals were included in the test after the start in the 0, 87.5, 175
and 350 mg/kg dosage groups, respectively. After 8, 10, 12, 15, 18 and
21 months, two animals, one of each sex, were killed for
histopathological examination. The rate of body-weight gain was
depressed at the highest dosage level, and in this group proteinuria
was more evident than in controls; in addition, the number of
erythrocytes in the urinary sediment was increased but the kidneys
were histologically normal.
Cholinesterase was inhibited in the plasma, RBC, brain and liver by
all treatment levels. Survival was poor, with only 5, 4, 2 and 5 males
and 9, 6, 7 and 8 females of the 0, 87.5, 175 and 350 mg/kg groups,
respectively, surviving for 100 weeks. The behaviour of all test
groups was normal as were the results of haematological and liver
function tests. Urinary ascorbic acid excretion was unaltered by
bromophos administration. The weights of 8 organs and the results of
macroscopic and microscopic examinations revealed no lesion which
could be attributed to treatment. No tumours were reported to have
occurred in control or test groups (Kinkel et al., 1965).
OBSERVATIONS IN MAN
Workers exposed to bromophos during production and formulation for two
years showed no change in RBC cholinesterase levels as estimated at
monthly intervals (Boehringer, 1966). Eight subjects were engaged in
spraying bromophos for 6´ hours over a period of 2 days, and 5 sprayed
for 4 hours a day on 14 days during a 22-day period in trials at the
WHO Insecticide Testing Unit, Lagos. Protective clothing was worn. No
clinical symptoms occurred, but plasma cholinesterase levels were
slightly depressed, the lowest being 75% of the pre-exposure level.
Levels returned to normal after one month (WHO, 1967).
COMMENT
Bromophos is rapidly absorbed, metabolized and excreted, mainly in the
urine. The compound potentiates the activity of several
organo-phosphates and carbamates, but its acute effects are
antagonized by some chlorinated hydrocarbons. The data on
neurotoxicity are contradictory and hence unsatisfactory. However, the
studies said to give positive results were poor, and the Meeting felt
that the compound was unlikely to cause neuropathy.
A reproduction study in rats showed no effect at 20 mg/kg/day.
Increased stillbirths and decreased pup weight were evident at 80
mg/kg/day.
Short-term studies in rat showed no effects on plasma cholinesterase
at 0.63 mg/kg/day. Somewhat higher doses caused erythrocyte
cholinesterase depression. At much higher doses brain cholinesterase
depression occurred. Hydropic swelling of hepatic cells at 188
mg/kg/day and hyaline droplets in tubular cells and protein in kidney
tubules at 1 250 mg/kg/day were observed.
Short-term studies in dog indicated no effect on plasma cholinesterase
at 1.5 mg/kg/day. At 175 mg/kg/day impaired spermatogenesis was
observed.
A long-term study in rat did not reveal any tumours, but survival was
poor at termination of the study.
Although the studies were considered poor by present day standards,
the Meeting decided that data were adequate to permit establishment of
a temporary ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 0.63 mg/kg body-weight/day
Dog: 1.5 mg/kg body-weight/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.006 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bromophos is an insecticide with contact and stomach action. It is
effective against a broad spectrum of chewing and sucking insects and
possesses a very low mammalian toxicity. It can be used as an
emulsifiable concentrate, ULV concentrate, wettable powder, granular
or dust formulation, seed dressing, aerosol formulation and fog
solution. It is compatible with other insecticides and fungicides.
Bromophos is of moderate toxicity to bees and should not be sprayed on
flowering crops during the flight of bees.
Bromophos has been used in the majority of European countries as well
as Algeria, Canada, Kuwait, Mexico, New Zealand, Nicaragua, Pakistan,
South Africa, Syria and Venezuela.
Pre-harvest treatments
Bromophos is used on various crops, mainly fruit and vegetables, for
control of a large number of important sucking and chewing insect
pests, such as vegetable root maggots, aphids, sawflies, fruit flies,
codling moths, mangold fly and beetles.
According to the single crop and the main pest species occurring, the
recommended concentrations of spray wash are given between 0.02% and
0.1% a.i.; the application rates range from 0.4 kg to 1.5 kg a.i./ha.
Bromophos is suitable for the treatment of a number of crops, except
for certain susceptible varieties of grape, pear, melon, cucumber,
lettuce and cabbage, mainly as frequent short interval treatments. Use
in glasshouses is not recommended.
The withholding periods range from 1 day to 21 days, depending on
local conditions and crop.
Post-harvest treatments
Bromophos is registered and recommended for storage pest control in
Mexico, South Africa, Spain and the United Kingdom and is currently
being introduced into other European and overseas countries.
Application of between 6 ppm and 12 ppm is recommended for grain
protection against various species of weevil and beetle causing damage
to stored products.
Other uses
Bromophos is used for the indoor and outdoor control of pests of
hygienic importance. As it shows a good effect against larval as well
as against adult stages and as it has a very low mammalian toxicity,
bromophos has proved to be an effective and safe compound for public
health operations. Furthermore, this material is recommended in the
veterinary field against chicken mites and other ectoparasites on
domestic animals and is highly effective against blowflies on sheep.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials on a variety of
fruits, vegetables, field crops and stored wheat (Boehringer,
1965-1971). Summaries of much of this information, and additional data
as well, have been published recently (Eichler, 1971). Table 3
presents a summary of available data, together with relevant
information on rates of application, number of applications and
pre-harvest interval.
In one reported trial, stored wheat that had been treated with
bromophos at 3 rates was milled into flour and the flour baked into
bread (Boehringer, 1965-1971). Analysis at each step gave the results
shown in Table 4.
Thus, less than 10% of the original amount of pesticide applied to
wheat is carried through to the final product, bread.
Data are also available on residues resulting from supervised trials
in animals.
Four lambs were dipped in 0.5% bromophos weekly for 9 weeks. Omental
biopsies were performed up to 22 days after the final dipping and the
fat samples analyzed by microcoulometric gas chromatography. Residues
of bromophos ranged from 5-14 ppm on day 1 to 0.07-0.43 ppm on day 22.
Bromoxon and 2,5-dichloro-4-bromophenol were not determined (Clark
et al., 1966).
TABLE 3 Bromophos residues on several crops
Rate of No. of Pre-harvest
Crop application treatments interval Residue
(a.i.) (days) (ppm)
Cauliflower 0.06 g/m2 1 0 4.901
7 0.041
14 <0.021
Broccoli 0.05 g/plant 1 62 <0.10
Kidney beans 0.06 g/m2 1 0 0.62-1.50
7 0.26-0.56
Cucumbers 0.04 g/m2 1 0 0.15-0.45
7 0.03-0.05
Garden lettuce 0.015 g/m2 1 4 1.9-2.6
0.025 g/m2 1 4 0.34-0.60
7 0.09-0.26
Lambs lettuce 0.025 g/m2 1 7 0.94-1.17
Kohlrabi 0.04 g/m2 1 7 <0.02
0.06 g/plant 1 30 0.04
Leek 0.8 kg/ha 1 0 2.76-2.80
14 1.27-1.43
Carrots 0.25 g/m2 2 38 2.90-3.55
0.2 g/m2 2 45 0.89-1.03
80 0.58-0.59
Peas 0.04 g/m2 1 7 <0.03
Radish 0.25 g/m2 1 16 1.03-1.32
21 0.12-0.23
Celery 120 g/ha 4 75 <0.05 (stalks)
0.32-0.55 (leaves)
Spinach 0.04 g/m2 1 1 5.48-7.85
7 0.25-0.67
14 <0.03-0.16
TABLE 3 (Cont'd.)
Rate of No. of Pre-harvest
Crop application treatments interval Residue
(a.i.) (days) (ppm)
Red cabbage 0.05 g/m2 1 7 <0.08
White cabbage 0.05 g/m2 1 7 <0.06-0.10
Savoy cabbage 0.06 g/m2 1 1 1.18-2.70
7 0.57-1.00
14 0.03-0.42
Onions 0.1 g/m2 1 123 <0.02
Maize 2.0 kg/ha 1 75 <0.17
Sugar beets 0.06 g/m2 1 14 <0.15-0.22 (beet)
1.1-1.9 (leaves)
Wheat 114 g/ha 1 14 0.17
228 g/ha 2 45 <0.02
Rape 228 g/ha 1 49 0.09 (seed)
0.17 (oil)
Apples 2.0 g/tree 1 0 1.10-1.20
4 1.49-1.70
7 0.51-1.30
14 0.48-0.61
Pears 1.25 g/tree 1 8 0.59-0.77
Yellow plums 0.62 g/tree 1 7 0.512
Cherries 2.5 g/tree 1 1 1.103
7 0.363
Peaches 0.38 g/tree 1 7 0.03-0.13
14 <0.03-0.25
Blackcurrants 0.25 g/shrub 1 7 0.13-0.36
Redcurrants 0.25 g/shrub 1 7 0.64-0.74
0.8 kg/ha 1 0 0.56-0.61
7 0.11-0.13
TABLE 3 (Cont'd.)
Rate of No. of Pre-harvest
Crop application treatments interval Residue
(a.i.) (days) (ppm)
Blackberries 0.25 g/shrub 1 7 0.14-0.19
Strawberries 0.8 kg/ha 1 0 0.61-0.64
7 0.11-0.12
Gooseberries 0.8 kg/ha 1 0 0.20-0.25
7 0.06-0.07
0.25 g/shrub 1 7 0.40-0.43
Olives 4.5 g/tree 1 10 2.58-2.92
Olive oil 4.5 g/tree 1 10 3.00-3.15
Stored wheat 0.012 g/kg 1 0 8.0
122 5.6
365 5.3
1 average of 4 analyses; raw data not available.
2 average of 9 analyses; raw data not available.
3 average of 6 analyses; raw data not available.
TABLE 4 Bromophos residues in wheat and wheat products
Rate of Post-treatment Residues (ppm)
application interval (days) Wheat Flour Bread
6 ppm 270 1.32 1.20 0.38-0.41
12 ppm 270 3.00 2.65-2.90 0.78-0.87
25 ppm 880 4.57 3.98-4.25 1.14-1.16
Milk from ten cows was analyzed for residues after stall spraying at
0.5 g/m2 of wall surface. Five cows were present during the spraying
and five were removed. Milk from the latter cows did not contain
measurable amounts of bromophos (<0.02 ppm). Milk from the five cows
left in the stalls during treatment had residues of 0.032-0.042 ppm on
post-treatment day 1; 0.031-0.045 ppm on day 2; 0.026-0.034 ppm on day
3; and <0.020 on day 5 (Boehringer, 1965-1971).
FATE OF RESIDUES
General comments
The metabolism or degradation of bromophos appears to be similar in
plants and animals, with the exception that hardly any bromoxon is
found in animals whereas low levels of bromoxon are likely to be
encountered in plant material. This is summarized in Figure 1.
 In animals
The absorption, distribution and excretion of 32P and 3H-labelled
bromophos administered to rats by various routes are discussed in the
preceding section on BIOCHEMICAL ASPECTS.
The decomposition of 32P-labelled bromophos following cutaneous
application to lactating cows (20 mg/kg in alcohol or paraffin oil)
was studied by Dedek and Schwarz (1969). No bromoxon was detected in
blood or milk by thin-layer chromatography. Concentrations of 0.4 to
0.7 ppm of desmethyl-bromophos and traces of bromophos were present.
The two methyl groups of bromophos appear to be simultaneously split
off by mild alkaline hydrolysis and by the action of a
glutathione-dependent liver enzyme (Stenersen, 1969a, 1969b).
In plants
In studies on tomato plants using 32O and 3H-labelled bromophos, it
was found that it does not act systemically but penetrates from the
surface into the interior of the leaf and also from a nutrient
solution into the root. In addition to unchanged bromophos,
dichlorobromophenol was found as a main metabolite (13% of the total
dose applied after 7 days) and small amounts of bromoxon,
monodesmethyl-bromophos, dimethyl thionophosphate and inorganic
phosphate were detected (Stiasni et al., 1969).
The metabolism of bromophos-32P was studied in wheat-, carrot-, and
onion-seedlings and by soil microorganisms (Stenerson, 1969). The main
finding was that bisdesmethyl-bromophos had been produced in several
of the experiments, but no desmethyl-bromophos was detectable.
However, the identification of bisdesmethyl-bromophos was not certain.
In soil
Bromophos E.C. was applied one time at 0.5 g a.i./m2 to high moorland
soil (acid, high organic), Ingelheim sand and clay soil. Zero to 20
cm-deep samples were taken periodically for 26 weeks and analysed for
bromophos and dichloro-bromophenol residues, with the results shown in
table 5.
TABLE 5 Bromophos residues in soil
Residues (ppm)1
Type of Post-treatment Dichloro-bromophenol
soil time (weeks) Bromophos
High moorland 0 13.102 0.30
soil 1 9.00 0.26
3 8.18 1.14
6 6.95 0.57
9 2.15 1.72
13 0.75 0.53
26 0.58 0.93
Sandy soil 0 1.433 0.65
1 0.64 0.53
3 0.20 0.20
6 0.13 0.10
9 <0.02 0.10
13 <0.02 0.10
26 <0.02 0.10
Clay soil 0 1.544 0.59
1 0.81 0.29
3 0.20 0.21
6 0.09 <0.10
9 <0.02 <0.10
13 <0.02 <0.10
26 <0.02 <0.10
1 Values calculated as dry substance from the measured moisture
content.
2 Y = 1.006-0.056 log X; r = 0.9188
3 Y = 0.047-0.187 log X; r = 0.9777
4 Y = 0.095-0.201 log X; r = 0.9890
Degradation of bromophos is clearly much more rapid in sandy and clay
soils. When the sandy soil was treated once at the same rate with
bromophos granules, the residues (corrected for moisture in sample)
fell from 5.98 ppm at week 0 to <0.02 at week 26. Although initial
residues were higher than for E.C., no measurable residue remained
after 26 weeks (Boehringer, 1965-1971). The generally rapid
degradation in soil was further indicated by trials in Germany with 3%
coarse dust and 5% granular applied one time at 2, 4, or 8 kg/ha which
gave residues of 0.03-0.16 ppm after 92 days (Boehringer, 1965-1971)
and by elaborate tests in the United States using a range of
application rates, numbers of applications, sampling intervals and
soils in diverse geographic and climatic areas. There was no
appreciable build-up of residues or leaching down through soil to any
extent observed for either bromophos or dichlorobromophenol (Tepe,
1968).
In storage and processing
Bromophos was applied to stored wheat grains at 10 ppm and the
decomposition studied over a 10-week period. Although only traces of
dimethylphosphorothionate were found, indicating that
desmethyl-bromophos is the main phosphatase degradation product, large
amounts of monomethyl-phosphorothionate were detected. Bromoxon was
found only during the first 20 days in amounts up to 10 percent of the
original bromophos level. Degradation of bromophos proceeded rapidly
for about 7 days, then stopped for about 3 weeks due to the
accumulation of desmethyl-bromophos, which inhibits phosphatase
hydrolysis. After the desmethyl-bromophos had been degraded, bromophos
degradation resumed. The level of free dichlorobromophenol did not
increase significantly until degradation of desmethyl-bromophos was
well underway, suggesting the formation of the phenol from the
desmethyl or oxon compounds. When bromophos was applied to autoclaved
grains, only dimethyl-phosphorothionate and dichloro-bromophenol were
produced (Rowlands, 1966a).
Oxidation of bromophos to bromoxon occurred in the seed coats and
germs of wheat grains. Hydrolytic activity was found in the germ and
endosperm (Rowlands, 1966b).
METHODS OF RESIDUE ANALYSIS
Many general and specific chemical, biochemical and biological methods
of analysis have been developed for residues of bromophos. These have
recently been reviewed and summarized by Eichler (1971) and will not
be elaborated here. An excellent method has been developed by Leber
and Deckers (1968). It utilizes gas chromatography with a phosphorus
detector and can estimate quantities down to the range of 0.001 to
0.01 ppm with a 100-g sample. The method also includes procedures for
determining bromoxon either by colorimetry or by gas chromatography
with a sensitivity of 0.03 to 0.05 ppm. Thin-layer chromatographic
procedures are also given for identifying residues.
Since bromophos is among the pesticides listed as detectable by the
multi-residue gas chromatographic procedure of Abbott et al. (1970),
that method is the most suitable for regulatory use. The method of
Leber and Deckers (1968) would be suitable for confirmation of the
identity of residues.
NATIONAL TOLERANCES
Some examples of tolerances in various countries were reported to the
Joint Meeting and are listed in Table 6.
TABLE 6 Examples of national tolerances reported to the Meeting
Country Commodity Tolerance
(ppm)
Canada Apple 1.5
Germany, Federal Berries, pome fruit, leaf
Republic vegetables, cabbage 1.5
Stone fruit, legumes, 0.6
root vegetables
Field corn 0.2
Hungary 2
Netherlands 0.5
Carrots 1.5
Germany, Democratic Fruit, vegetables (except
Republic onions and potatoes) 11
Animal fat, vegetable oil,
potatoes, onions, meat, fish,
eggs, milk, grain, baby foods 0
South Africa Stored maize 8
1 not more than 0.1 ppm oxon.
APPRAISAL
Bromophos is a non-systemic halogen-containing organophosphorus
insecticide used on a wide variety of crops and animals to control
biting and sucking insects. It is also used to protect stored
products, as a seed protection agent for grain crops and as a vector
control agent in public health.
Supervised experiments with foliar, row or surface treatments on
fruits and vegetables have shown bromophos to have a generally low
persistence. However, the comparative rate of residue decline is
highly dependent on many factors, such as botanical species,
morphological structure, weather formulation, and method and time of
treatment. The lipophilic nature of the compound causes it to
penetrate the cuticular wax of certain crops (for example - apples and
pears) which delays release and degradation. This property makes it
necessary for tolerance recommendations to be made on an individual
commodity basis rather than on broad crop categories.
On the basis of a single trial in which four lambs were dipped in 0.5%
bromophos weekly for 9 weeks, it would appear that the compound did
not accumulate excessively in the fat and was eliminated fairly
rapidly after treatment ceased, dropping from about 10 ppm on day 1 to
about 2 ppm on day 8 and to about 0.2 ppm after 22 days. Milk cows
exposed to bromophos during stall treatment had low levels in the milk
(0.045 ppm maximum) which fell below the limit of detection (0.20 ppm)
after five days.
The metabolite of bromophos most likely to be found in plants and soil
is 2,5-dichloro-4-bromophenol. Small amounts of bromoxon and
monodesmethyl-bromophos were also found in tomato plants according to
one report, whereas different studies on wheat, carrot and onion
seedlings indicated the production of bisdesmethylbromophos but not
desmethyl-bromophos. The identity of the bisdesmethyl-bromophos was
not certain. In animals, bromophos is excreted rapidly via the urine
and the major metabolites found are dichloro-bromophenol and
monodesmethyl-bromophos. Extremely low levels of bromoxon may also
occur in blood.
Available multi-residue gas chromatographic procedures are suitable
for application for regulatory purpose and are recommended.
Although bromophos is recommended for use against ectoparasites of
animals and poultry, there was no data available on residues likely to
occur in meat (except sheep) or eggs and no recommendations for
tolerances could be made for these commodities.
RECOMMENDATIONS
TEMPORARY TOLERANCES
The following temporary tolerances are based on residues likely to be
found at harvest following currently recommended use patterns. For the
majority of fruits and vegetables, the recommended pre-harvest
interval is 7 days. In the case of whole milk, the practical residue
limit is at or about the limit of determination. The temporary
tolerances are expressed on bromophos.
ppm
Olives, olive oil 5
Apples, lamb's lettuce, leeks,
radishes 2
Carrots, celery, French beans, pears,
plums, red currants, savoy cabbage,
spinach 1
Blackberries, blackcurrants,
cherries, lettuce, gooseberries,
peaches, strawberries, sugarbeets
(roots), fat of meat of sheep 0.5
Wheat, rapeseed, rapeseed oil 0.2
Broccoli, cauliflower, cucumbers,
red cabbage, cabbage, kohlrabi,
onions, peas 0.1
Milk (whole) 0.02*
* at or about the limit of determination
Remarks
The temporary tolerance for wheat is based on residues likely to be
found at harvest.
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1977)
1. A study in dogs using animals of similar weight, age and origin
in control and test groups, with particular attention to renal
function and testicular pathology. The dosage levels should be
set to demonstrate the no-effect level.
2. An adequate study to assess the carcinogenic potential of
bromophos.
REQUIRED (before tolerances can be recommended)
1. Residue data from supervised trials for meat of domestic animals
other than sheep, paultry, eggs, any fruit or vegetables not
listed.
2. Further information on good agricultural practices for use on
stored grain and the effects of moisture and temperature on
residues in stored grain.
DESIRABLE
A study to determine dose levels causing no carboxylesterase
(aliesterase) activity depression.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales, 1966-1977.
III. Organophosphorous pesticides residues in the total diet. Pestic.
Sci., 1: 10-13.
Barnes, J.M. (1966) Mammalian toxicity report. WHO insecticide
evaluation and testing programme, stage I. (unpublished)
Boehringer, C.H. Sohn. (1965-1971) Residue investigation reports.
Boehringer, C.H. Sohn. (1966) Produktion und Formulierung von
Bromophos bei Laboratories Fher Spanien. Report dated 25/3/66.
(unpublished)
Boehringer, C.H. Sohn. (1968) Chronic oral toxicity tests on dogs
using the compound bromophos. (unpublished)
Byewater, J. (1966) Report by Biological Laboratories to Agricola
Chemicals Ltd. (unpublished)
Clark, D.E., Younger, R.L. and Ayala, C.H. (1966) Toxicosis and
residues in bromophos-dipped sheep. J. Agr. Fd. Chem., 14(6): 608-609.
Dedek, W. and Schwarz, H. (1969) Zum verhalten des mindertoxischen
Insektizids 32P-bromophos nach cutaner Applikation am Rind. Z.
Naturf., 24B: 744-747.
Eichler D. (1971) Bromophos and bromophos-ethyl residues. Residue
Reviews, 41: 65-112.
El-Sebae, A.H. and El-Sayed, A.M.K. (1969) Persistence of malathion
and bromophos on bean and clover crops. Z. für angew. Entomol., 63:
378.
Engst, von R., Knoll, R. and Ackermann, H. (1969) Zur
Rückstandsproblematik von Pflanzenschutz und
Schödlingsbekämpfungsmitteln (PSM) in der Kleinstkinderernährung.
Dtsch. Gesundheitsw., 24: 1744.
Food and Drug Administration. (1970) Advisory committee on protocols
for safety evaluations: Panel on reproduction report on reproduction
studies in the safety evaluation of food additives and pesticide
residues. Toxicology and Applied Pharmacology, 16: 264-296.
Glees, P. (1966) Neuropathological report to C.H. Boehringer Sohn.
(unpublished)
Kinkel, H.J. (1964a) Ermittlung der akuten peroralen Toxizität und
Untersuchungen über die Neurotoxizität des Präparates Bromophos (CELA
S 1942 = O,O-dimethyl-O-2,5-dichlor-bromophenylthionophosphat)
an Hühnern. Report Battelle Institut. (unpublished)
Kinkel, H.J. (1964b) Prüfung des Potenzierungseffektes: Bromophos-EPN.
Report Battelle Institut. (unpublished)
Kinkel, H.J. and Dirks, E. (1966) Investigations on the cholinesterase
activity in dogs after oral application of bromophos. Report Battelle
Institut. (unpublished)
Kinkel, H.J. and Hübner, K.H. (1966) Histopathologische Untersuchungen
an Hirn und Rückenmark bei Hühnern nach oraler Applikation von
Bromophos. Report Battelle Institut. (unpublished)
Kinkel, H.J., Hübner, K.H., Königsman, G. and Dirks, E. (1965) Chronic
oral toxicity assay of bromophos
(O,O-dimethyl-O-(2,5-dichloro-4-bromophenyl)-thionophosphate) on
dogs and rats. Report Battelle Institut. (unpublished)
Kinkel, H.J., Muacevic, G., Sehring, R. and Bodenstein, G. (1966) Zur
Toxikologie von Bromophos. Archiv. für Toxikologie, 22: 36-57.
Kinkel, H.J. and Sann, E. (1964) Ermittlung der akuten
intraperitonealen Toxizität und Untersuchungen über die Hemmürkung des
Präparates Bromophos (CELA S 1942) auf die Erythrozyten-Cholinesterase
bei der Ratte. Report Battelle Institut. (unpublished)
Kinkel, H.J. and Seume, F.W. (1963) Investigations on the toxicity of
O,O-dimethyl-O-2,5-dichloro-4-bromo-phenyl-thionophosphate (S
1942). Report Battelle Institut. (unpublished)
Leber, G. (1967) Ruckstandsbestimmung von bromophos in fettarmen
pflanzlichen material. Internal reports RU 2,22/02/10-8/22/67 and
12/20/67 C.H. Boehringer Sohn.
Leber, G. (1968) Ruckstandsbestimmung von bromophos in tierischem
material. Internal Report RU 2,22/14/80-3/28/68. C.H. Boehringer Sohn.
Leber, G. and Deckers, W. (1968) Determination of residues of
bromophos and bromophos-ethyl. Proc. Brit. Insecticide Fungicide
Conf., Brighton, England, 4: 570.
Leuschner, F. (1968) Uber die subakute Toxizität von 3
Bromophos-metaboliten bei peroraler Verabreichung an Wister-Ratten.
Report C.H. Boehringer Sohn. (unpublished)
Leuschner, F. and Leuschner, A. (1966a) Untersuchungen über die
Wirkung von Bromophos auf den Foetus und das trächtige weibliche
Kaninchen bei peroraler Applikation. Report C.H. Boehringer Sohn.
(unpublished)
Leuschner, F. and Leuschner, A. (1966b) Untersuchüngen über die
Einwirkung von Bromophos auf die Acetyl-cholinesterase bei
Wistar-Ratten. Report C.H. Boehringer Sohn. (unpublished)
Leuschner, F., Leuschner, A. and Pöppe, E. (1967) Chronischer
Reproduktionsversuch über 3 Generationen an Wistar-Ratten bei
fortdauernder Verabreichung von Bromophos. Report C.H. Boehringer
Sohn. (unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W. and Otto, H. (1969)
Über die subakute Toxizität von Desmethylbromophos bei peroraler
Verabreichung an Sprague-Dawley Ratten. Report C.H. Boehringer Sohn.
(unpublished)
Muacevic, G. (1963) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1964) Reports C.H. Boehringer Sohn. (unpublished
Muacevic, G. (1965) Reaktivierungsversuche bei Bromophos. Report C.H.
Boehringer Sohn. (unpublished)
Muacevic, G. (1966) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1967) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1968) Die Reaktivierbarkeit der Cholinesterase durch
verschiedene Aldoxime nach Applikation von Bromophos im Rattengehirn
(in vivo-Versuche). Report C.H. Boehringer Sohn. (unpublished)
Muacevic, G. and Glees, P. (1967) Report on bromophos (1942) fowl
trial. C.H. Boehringer Sohn. (unpublished)
Muacevic, G. and Glees, P. (1968) Bericht über die Prüfung von
Bromophos an Hühnern. Report C.H. Boehringer Sohn. (unpublished)
Oettel, H. (1963) Report to C.H. Boehringer Sohn. Gewerbehyg.
Pharmakol. Institut der BASF. (unpublished)
Paton, I.M. (1965) Necropsy parasite recovery data controlled test.
Report Jensen-Salsbery Laboratories. (unpublished)
Rowlands, D.G. (1966a) The activation and detoxification of three
organic phosphorothionate insecticides applied to stored wheat grains.
J. stored Prod. Res., 2: 105-116.
Rowlands, D.G. (1966b) The metabolism of bromophos in stored wheat
grains. J. stored Prod. Res., 2: 1-12.
Rygg, T. and Somme, L. (1967) Rester av fosformidler i gulrot. Norsk
Landbruk, 13: 14.
Stenersen, J. (1969a) Degradation of 32P-bromophos by micro-organisms
and seedlings. Bull. Environ. Contam. Toxicol., 4: 104-112.
Stenersen, J. (1969b) Demethylation of the insecticide bromophos by a
glutathione-dependent liver enzyme and by alkaline buffers. J. Econ.
Entomol., 62(5): 1043-1045.
Stiasni, M., Rehbinder, D. and Deckers, W. (1967) Absorption,
distribution, and metabolism of
O-(4-bromo-2,5-dichlorophenyl)-O,O-dimethylphosphorothioate
(bromophos) in the rat. J. Agr. Fd. Chem., 15: 474-478.
Stiasni, M., Deckers, W., Schmidt, R. and Simon, H. (1969)
Translocation, penetration and metabolism of O-(4-bromo-2,5-
dichlorophenyl)-O,O-dimethylphosphorothioate (bromophos) in tomato
plants. J. Agr. Fd. Chem., 17(5): 1017-1020.
Tepe, J.B. (1968) Data from soil persistence and degradation study.
Eli Lilly and Company, communication to CELA G.m.b.H.
World Health Organization. (1967) Safe use of pesticides in public
health. Sixteenth report of the WHO expert committee on insecticides.
World Health Organization Technical Report Series No. 356.
Worth, H.M., Kehr, C.C. and Gibson, W.R. (1967) Effect of a single
dose of bromophos. Report Eli Lilly and Co. (unpublished)
In animals
The absorption, distribution and excretion of 32P and 3H-labelled
bromophos administered to rats by various routes are discussed in the
preceding section on BIOCHEMICAL ASPECTS.
The decomposition of 32P-labelled bromophos following cutaneous
application to lactating cows (20 mg/kg in alcohol or paraffin oil)
was studied by Dedek and Schwarz (1969). No bromoxon was detected in
blood or milk by thin-layer chromatography. Concentrations of 0.4 to
0.7 ppm of desmethyl-bromophos and traces of bromophos were present.
The two methyl groups of bromophos appear to be simultaneously split
off by mild alkaline hydrolysis and by the action of a
glutathione-dependent liver enzyme (Stenersen, 1969a, 1969b).
In plants
In studies on tomato plants using 32O and 3H-labelled bromophos, it
was found that it does not act systemically but penetrates from the
surface into the interior of the leaf and also from a nutrient
solution into the root. In addition to unchanged bromophos,
dichlorobromophenol was found as a main metabolite (13% of the total
dose applied after 7 days) and small amounts of bromoxon,
monodesmethyl-bromophos, dimethyl thionophosphate and inorganic
phosphate were detected (Stiasni et al., 1969).
The metabolism of bromophos-32P was studied in wheat-, carrot-, and
onion-seedlings and by soil microorganisms (Stenerson, 1969). The main
finding was that bisdesmethyl-bromophos had been produced in several
of the experiments, but no desmethyl-bromophos was detectable.
However, the identification of bisdesmethyl-bromophos was not certain.
In soil
Bromophos E.C. was applied one time at 0.5 g a.i./m2 to high moorland
soil (acid, high organic), Ingelheim sand and clay soil. Zero to 20
cm-deep samples were taken periodically for 26 weeks and analysed for
bromophos and dichloro-bromophenol residues, with the results shown in
table 5.
TABLE 5 Bromophos residues in soil
Residues (ppm)1
Type of Post-treatment Dichloro-bromophenol
soil time (weeks) Bromophos
High moorland 0 13.102 0.30
soil 1 9.00 0.26
3 8.18 1.14
6 6.95 0.57
9 2.15 1.72
13 0.75 0.53
26 0.58 0.93
Sandy soil 0 1.433 0.65
1 0.64 0.53
3 0.20 0.20
6 0.13 0.10
9 <0.02 0.10
13 <0.02 0.10
26 <0.02 0.10
Clay soil 0 1.544 0.59
1 0.81 0.29
3 0.20 0.21
6 0.09 <0.10
9 <0.02 <0.10
13 <0.02 <0.10
26 <0.02 <0.10
1 Values calculated as dry substance from the measured moisture
content.
2 Y = 1.006-0.056 log X; r = 0.9188
3 Y = 0.047-0.187 log X; r = 0.9777
4 Y = 0.095-0.201 log X; r = 0.9890
Degradation of bromophos is clearly much more rapid in sandy and clay
soils. When the sandy soil was treated once at the same rate with
bromophos granules, the residues (corrected for moisture in sample)
fell from 5.98 ppm at week 0 to <0.02 at week 26. Although initial
residues were higher than for E.C., no measurable residue remained
after 26 weeks (Boehringer, 1965-1971). The generally rapid
degradation in soil was further indicated by trials in Germany with 3%
coarse dust and 5% granular applied one time at 2, 4, or 8 kg/ha which
gave residues of 0.03-0.16 ppm after 92 days (Boehringer, 1965-1971)
and by elaborate tests in the United States using a range of
application rates, numbers of applications, sampling intervals and
soils in diverse geographic and climatic areas. There was no
appreciable build-up of residues or leaching down through soil to any
extent observed for either bromophos or dichlorobromophenol (Tepe,
1968).
In storage and processing
Bromophos was applied to stored wheat grains at 10 ppm and the
decomposition studied over a 10-week period. Although only traces of
dimethylphosphorothionate were found, indicating that
desmethyl-bromophos is the main phosphatase degradation product, large
amounts of monomethyl-phosphorothionate were detected. Bromoxon was
found only during the first 20 days in amounts up to 10 percent of the
original bromophos level. Degradation of bromophos proceeded rapidly
for about 7 days, then stopped for about 3 weeks due to the
accumulation of desmethyl-bromophos, which inhibits phosphatase
hydrolysis. After the desmethyl-bromophos had been degraded, bromophos
degradation resumed. The level of free dichlorobromophenol did not
increase significantly until degradation of desmethyl-bromophos was
well underway, suggesting the formation of the phenol from the
desmethyl or oxon compounds. When bromophos was applied to autoclaved
grains, only dimethyl-phosphorothionate and dichloro-bromophenol were
produced (Rowlands, 1966a).
Oxidation of bromophos to bromoxon occurred in the seed coats and
germs of wheat grains. Hydrolytic activity was found in the germ and
endosperm (Rowlands, 1966b).
METHODS OF RESIDUE ANALYSIS
Many general and specific chemical, biochemical and biological methods
of analysis have been developed for residues of bromophos. These have
recently been reviewed and summarized by Eichler (1971) and will not
be elaborated here. An excellent method has been developed by Leber
and Deckers (1968). It utilizes gas chromatography with a phosphorus
detector and can estimate quantities down to the range of 0.001 to
0.01 ppm with a 100-g sample. The method also includes procedures for
determining bromoxon either by colorimetry or by gas chromatography
with a sensitivity of 0.03 to 0.05 ppm. Thin-layer chromatographic
procedures are also given for identifying residues.
Since bromophos is among the pesticides listed as detectable by the
multi-residue gas chromatographic procedure of Abbott et al. (1970),
that method is the most suitable for regulatory use. The method of
Leber and Deckers (1968) would be suitable for confirmation of the
identity of residues.
NATIONAL TOLERANCES
Some examples of tolerances in various countries were reported to the
Joint Meeting and are listed in Table 6.
TABLE 6 Examples of national tolerances reported to the Meeting
Country Commodity Tolerance
(ppm)
Canada Apple 1.5
Germany, Federal Berries, pome fruit, leaf
Republic vegetables, cabbage 1.5
Stone fruit, legumes, 0.6
root vegetables
Field corn 0.2
Hungary 2
Netherlands 0.5
Carrots 1.5
Germany, Democratic Fruit, vegetables (except
Republic onions and potatoes) 11
Animal fat, vegetable oil,
potatoes, onions, meat, fish,
eggs, milk, grain, baby foods 0
South Africa Stored maize 8
1 not more than 0.1 ppm oxon.
APPRAISAL
Bromophos is a non-systemic halogen-containing organophosphorus
insecticide used on a wide variety of crops and animals to control
biting and sucking insects. It is also used to protect stored
products, as a seed protection agent for grain crops and as a vector
control agent in public health.
Supervised experiments with foliar, row or surface treatments on
fruits and vegetables have shown bromophos to have a generally low
persistence. However, the comparative rate of residue decline is
highly dependent on many factors, such as botanical species,
morphological structure, weather formulation, and method and time of
treatment. The lipophilic nature of the compound causes it to
penetrate the cuticular wax of certain crops (for example - apples and
pears) which delays release and degradation. This property makes it
necessary for tolerance recommendations to be made on an individual
commodity basis rather than on broad crop categories.
On the basis of a single trial in which four lambs were dipped in 0.5%
bromophos weekly for 9 weeks, it would appear that the compound did
not accumulate excessively in the fat and was eliminated fairly
rapidly after treatment ceased, dropping from about 10 ppm on day 1 to
about 2 ppm on day 8 and to about 0.2 ppm after 22 days. Milk cows
exposed to bromophos during stall treatment had low levels in the milk
(0.045 ppm maximum) which fell below the limit of detection (0.20 ppm)
after five days.
The metabolite of bromophos most likely to be found in plants and soil
is 2,5-dichloro-4-bromophenol. Small amounts of bromoxon and
monodesmethyl-bromophos were also found in tomato plants according to
one report, whereas different studies on wheat, carrot and onion
seedlings indicated the production of bisdesmethylbromophos but not
desmethyl-bromophos. The identity of the bisdesmethyl-bromophos was
not certain. In animals, bromophos is excreted rapidly via the urine
and the major metabolites found are dichloro-bromophenol and
monodesmethyl-bromophos. Extremely low levels of bromoxon may also
occur in blood.
Available multi-residue gas chromatographic procedures are suitable
for application for regulatory purpose and are recommended.
Although bromophos is recommended for use against ectoparasites of
animals and poultry, there was no data available on residues likely to
occur in meat (except sheep) or eggs and no recommendations for
tolerances could be made for these commodities.
RECOMMENDATIONS
TEMPORARY TOLERANCES
The following temporary tolerances are based on residues likely to be
found at harvest following currently recommended use patterns. For the
majority of fruits and vegetables, the recommended pre-harvest
interval is 7 days. In the case of whole milk, the practical residue
limit is at or about the limit of determination. The temporary
tolerances are expressed on bromophos.
ppm
Olives, olive oil 5
Apples, lamb's lettuce, leeks,
radishes 2
Carrots, celery, French beans, pears,
plums, red currants, savoy cabbage,
spinach 1
Blackberries, blackcurrants,
cherries, lettuce, gooseberries,
peaches, strawberries, sugarbeets
(roots), fat of meat of sheep 0.5
Wheat, rapeseed, rapeseed oil 0.2
Broccoli, cauliflower, cucumbers,
red cabbage, cabbage, kohlrabi,
onions, peas 0.1
Milk (whole) 0.02*
* at or about the limit of determination
Remarks
The temporary tolerance for wheat is based on residues likely to be
found at harvest.
FURTHER WORK OR INFORMATION
REQUIRED (by 30 June 1977)
1. A study in dogs using animals of similar weight, age and origin
in control and test groups, with particular attention to renal
function and testicular pathology. The dosage levels should be
set to demonstrate the no-effect level.
2. An adequate study to assess the carcinogenic potential of
bromophos.
REQUIRED (before tolerances can be recommended)
1. Residue data from supervised trials for meat of domestic animals
other than sheep, paultry, eggs, any fruit or vegetables not
listed.
2. Further information on good agricultural practices for use on
stored grain and the effects of moisture and temperature on
residues in stored grain.
DESIRABLE
A study to determine dose levels causing no carboxylesterase
(aliesterase) activity depression.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales, 1966-1977.
III. Organophosphorous pesticides residues in the total diet. Pestic.
Sci., 1: 10-13.
Barnes, J.M. (1966) Mammalian toxicity report. WHO insecticide
evaluation and testing programme, stage I. (unpublished)
Boehringer, C.H. Sohn. (1965-1971) Residue investigation reports.
Boehringer, C.H. Sohn. (1966) Produktion und Formulierung von
Bromophos bei Laboratories Fher Spanien. Report dated 25/3/66.
(unpublished)
Boehringer, C.H. Sohn. (1968) Chronic oral toxicity tests on dogs
using the compound bromophos. (unpublished)
Byewater, J. (1966) Report by Biological Laboratories to Agricola
Chemicals Ltd. (unpublished)
Clark, D.E., Younger, R.L. and Ayala, C.H. (1966) Toxicosis and
residues in bromophos-dipped sheep. J. Agr. Fd. Chem., 14(6): 608-609.
Dedek, W. and Schwarz, H. (1969) Zum verhalten des mindertoxischen
Insektizids 32P-bromophos nach cutaner Applikation am Rind. Z.
Naturf., 24B: 744-747.
Eichler D. (1971) Bromophos and bromophos-ethyl residues. Residue
Reviews, 41: 65-112.
El-Sebae, A.H. and El-Sayed, A.M.K. (1969) Persistence of malathion
and bromophos on bean and clover crops. Z. für angew. Entomol., 63:
378.
Engst, von R., Knoll, R. and Ackermann, H. (1969) Zur
Rückstandsproblematik von Pflanzenschutz und
Schödlingsbekämpfungsmitteln (PSM) in der Kleinstkinderernährung.
Dtsch. Gesundheitsw., 24: 1744.
Food and Drug Administration. (1970) Advisory committee on protocols
for safety evaluations: Panel on reproduction report on reproduction
studies in the safety evaluation of food additives and pesticide
residues. Toxicology and Applied Pharmacology, 16: 264-296.
Glees, P. (1966) Neuropathological report to C.H. Boehringer Sohn.
(unpublished)
Kinkel, H.J. (1964a) Ermittlung der akuten peroralen Toxizität und
Untersuchungen über die Neurotoxizität des Präparates Bromophos (CELA
S 1942 = O,O-dimethyl-O-2,5-dichlor-bromophenylthionophosphat)
an Hühnern. Report Battelle Institut. (unpublished)
Kinkel, H.J. (1964b) Prüfung des Potenzierungseffektes: Bromophos-EPN.
Report Battelle Institut. (unpublished)
Kinkel, H.J. and Dirks, E. (1966) Investigations on the cholinesterase
activity in dogs after oral application of bromophos. Report Battelle
Institut. (unpublished)
Kinkel, H.J. and Hübner, K.H. (1966) Histopathologische Untersuchungen
an Hirn und Rückenmark bei Hühnern nach oraler Applikation von
Bromophos. Report Battelle Institut. (unpublished)
Kinkel, H.J., Hübner, K.H., Königsman, G. and Dirks, E. (1965) Chronic
oral toxicity assay of bromophos
(O,O-dimethyl-O-(2,5-dichloro-4-bromophenyl)-thionophosphate) on
dogs and rats. Report Battelle Institut. (unpublished)
Kinkel, H.J., Muacevic, G., Sehring, R. and Bodenstein, G. (1966) Zur
Toxikologie von Bromophos. Archiv. für Toxikologie, 22: 36-57.
Kinkel, H.J. and Sann, E. (1964) Ermittlung der akuten
intraperitonealen Toxizität und Untersuchungen über die Hemmürkung des
Präparates Bromophos (CELA S 1942) auf die Erythrozyten-Cholinesterase
bei der Ratte. Report Battelle Institut. (unpublished)
Kinkel, H.J. and Seume, F.W. (1963) Investigations on the toxicity of
O,O-dimethyl-O-2,5-dichloro-4-bromo-phenyl-thionophosphate (S
1942). Report Battelle Institut. (unpublished)
Leber, G. (1967) Ruckstandsbestimmung von bromophos in fettarmen
pflanzlichen material. Internal reports RU 2,22/02/10-8/22/67 and
12/20/67 C.H. Boehringer Sohn.
Leber, G. (1968) Ruckstandsbestimmung von bromophos in tierischem
material. Internal Report RU 2,22/14/80-3/28/68. C.H. Boehringer Sohn.
Leber, G. and Deckers, W. (1968) Determination of residues of
bromophos and bromophos-ethyl. Proc. Brit. Insecticide Fungicide
Conf., Brighton, England, 4: 570.
Leuschner, F. (1968) Uber die subakute Toxizität von 3
Bromophos-metaboliten bei peroraler Verabreichung an Wister-Ratten.
Report C.H. Boehringer Sohn. (unpublished)
Leuschner, F. and Leuschner, A. (1966a) Untersuchungen über die
Wirkung von Bromophos auf den Foetus und das trächtige weibliche
Kaninchen bei peroraler Applikation. Report C.H. Boehringer Sohn.
(unpublished)
Leuschner, F. and Leuschner, A. (1966b) Untersuchüngen über die
Einwirkung von Bromophos auf die Acetyl-cholinesterase bei
Wistar-Ratten. Report C.H. Boehringer Sohn. (unpublished)
Leuschner, F., Leuschner, A. and Pöppe, E. (1967) Chronischer
Reproduktionsversuch über 3 Generationen an Wistar-Ratten bei
fortdauernder Verabreichung von Bromophos. Report C.H. Boehringer
Sohn. (unpublished)
Leuschner, F., Leuschner, A., Schwerdtfeger, W. and Otto, H. (1969)
Über die subakute Toxizität von Desmethylbromophos bei peroraler
Verabreichung an Sprague-Dawley Ratten. Report C.H. Boehringer Sohn.
(unpublished)
Muacevic, G. (1963) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1964) Reports C.H. Boehringer Sohn. (unpublished
Muacevic, G. (1965) Reaktivierungsversuche bei Bromophos. Report C.H.
Boehringer Sohn. (unpublished)
Muacevic, G. (1966) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1967) Reports C.H. Boehringer Sohn. (unpublished)
Muacevic, G. (1968) Die Reaktivierbarkeit der Cholinesterase durch
verschiedene Aldoxime nach Applikation von Bromophos im Rattengehirn
(in vivo-Versuche). Report C.H. Boehringer Sohn. (unpublished)
Muacevic, G. and Glees, P. (1967) Report on bromophos (1942) fowl
trial. C.H. Boehringer Sohn. (unpublished)
Muacevic, G. and Glees, P. (1968) Bericht über die Prüfung von
Bromophos an Hühnern. Report C.H. Boehringer Sohn. (unpublished)
Oettel, H. (1963) Report to C.H. Boehringer Sohn. Gewerbehyg.
Pharmakol. Institut der BASF. (unpublished)
Paton, I.M. (1965) Necropsy parasite recovery data controlled test.
Report Jensen-Salsbery Laboratories. (unpublished)
Rowlands, D.G. (1966a) The activation and detoxification of three
organic phosphorothionate insecticides applied to stored wheat grains.
J. stored Prod. Res., 2: 105-116.
Rowlands, D.G. (1966b) The metabolism of bromophos in stored wheat
grains. J. stored Prod. Res., 2: 1-12.
Rygg, T. and Somme, L. (1967) Rester av fosformidler i gulrot. Norsk
Landbruk, 13: 14.
Stenersen, J. (1969a) Degradation of 32P-bromophos by micro-organisms
and seedlings. Bull. Environ. Contam. Toxicol., 4: 104-112.
Stenersen, J. (1969b) Demethylation of the insecticide bromophos by a
glutathione-dependent liver enzyme and by alkaline buffers. J. Econ.
Entomol., 62(5): 1043-1045.
Stiasni, M., Rehbinder, D. and Deckers, W. (1967) Absorption,
distribution, and metabolism of
O-(4-bromo-2,5-dichlorophenyl)-O,O-dimethylphosphorothioate
(bromophos) in the rat. J. Agr. Fd. Chem., 15: 474-478.
Stiasni, M., Deckers, W., Schmidt, R. and Simon, H. (1969)
Translocation, penetration and metabolism of O-(4-bromo-2,5-
dichlorophenyl)-O,O-dimethylphosphorothioate (bromophos) in tomato
plants. J. Agr. Fd. Chem., 17(5): 1017-1020.
Tepe, J.B. (1968) Data from soil persistence and degradation study.
Eli Lilly and Company, communication to CELA G.m.b.H.
World Health Organization. (1967) Safe use of pesticides in public
health. Sixteenth report of the WHO expert committee on insecticides.
World Health Organization Technical Report Series No. 356.
Worth, H.M., Kehr, C.C. and Gibson, W.R. (1967) Effect of a single
dose of bromophos. Report Eli Lilly and Co. (unpublished)
