
FENSULFOTHION JMPR 1972
IDENTITY
Chemical name
O,O-diethyl-O-[4-(methylsulfinyl)] phosphorothioate.
Synonyms
O,O-diethyl-O-[4-(methylsulfinyl)] monothiophosphate.
Dasanit(R), Terracur(R), Bay 25144.
Structural formula
 Other information on identity and properties
Physical state: yellow-brown liquid
Molecular weight: 308.35
Boiling point: 138 - 141°C at 0.01 mm Hg
Volatility: <0.01 mg/cu meter (20°C)
Specific gravity: D 20 = 1.202
4
Refractive index: n 25 = 1.54
D
Solubility: in water, at 20°C, 1200 ppm soluble in most
organic solvents except aliphatics
Stability: stable under normal conditions of storage and
use
Hydrolysis rate: half-life at 81°C and pH 2.5 - 6, 120 hours
Formulations used: granular 3, 5, 10 and 15%; liquid (EC) 720
g/l
Purity of fensulfothion 94-96; O,O-diethyl-
technical O[4(methylthio)-phenyl] phosphorothioate
material: 1-3; O,O-diethyl-O-4(methylsulfonyl)-
phenyl] phosphorothioate 0.2 - 0.6;
4-(methylsulfinyl) phenol 0.8 - 1.0; water
0.3 - 0.8 (all figures in % w)
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Following oral administration to rats fensulfothion is rapidly
absorbed, distributed and excreted. Following oral doses of 0.7 to 1.5
mg/kg, tissue residues reached maximum values within 8 hours and
residues were rapidly excreted within 24 hours, primarily in the
urine. Female rats appear to excrete the acutely administered dose
somewhat slower than males. These differences may account for the
greater susceptibility of females when administered acutely toxic
doses (Everett, 1968).
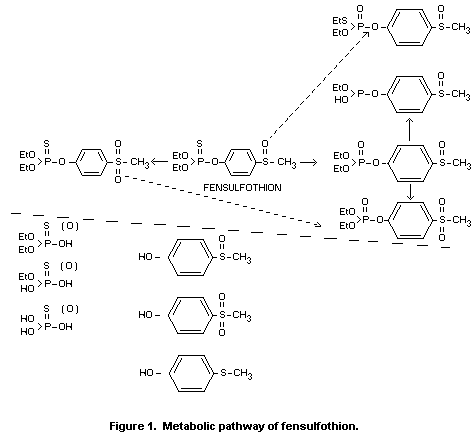
Biotransformation
The metabolic route is largely through oxidative and/or hydrolytic
pathways. In female rats, the formation of the oxygen analogue and its
sulfone followed by P-O-ethyl dealkylation is a significant pathway.
In male rats hydrolytic and/or oxidative cleavage of the
phosphorothionate of the sulfoxide or the sulfone is the more
prevalent route of detoxication. Although the presence of the
metabolite 4-methylthiophenol was detected in certain biological
systems, this reductive pathway is believed to be minor.
Fensulfothion, applied to plant stems or to roots in a water
dispersion, was absorbed slowly into the plant and converted to the
phosphate analogue and to the sulfone. After 9 days the sulfone
phosphate analogue was detected (Katague and Anderson, 1967; Benjamini
et al., 1959a). In air, slow oxidation to the sulfone and
isomerization of the phosphorothiolate was shown to occur (Benjamini
et al., 1959b). This conversion has not been demonstrated in mammals
with fensulfothion. A summary of the significant features of the
metabolic pathway of fensulfothion is shown in Figure 1.
Effect on enzymes and other biochemical parameters
Fensulfothion, like other organophosphorothionate esters, is a weak
cholinesterase inhibitor which, after being converted to the
corresponding phosphate ester of fensulfothion is from 500 to 2 000
times more active in inhibiting cholinesterase.
Following intraperitoneal administration of fensulfothion (0.9 mg/kg)
to rats, inhibition of cholinesterase was maximal within one hour.
Reversal of inhibition became evident within 6 hours and was
progressive for 5 days, at which time activity returned to near normal
values (Dubois & Kinoshita, 1964).
As with several other compounds of a similar structural nature,
cholinesterase activity in females is more sensitive to in vivo
anti-cholinesterase activity. This is possibly a result of a
difference in enzyme sensitivity or a sex difference in the rate of
metabolism. No other biochemical parameters appear to be affected by
fensulfothion.
TOXICOLOGICAL STUDIES
Special studies on metabolites
Acute toxicity of fensulfothion metabolites and related products in
rats is shown in Table 1.
Special studies on mutagenicity
A dominant lethal test using groups of male mice (12 mice/group) was
conducted by treating the animals with fensulfothion by
intraperitoneal injection with 0, 0.5 and 1.0 mg/kg. For 6 consecutive
weeks treated males were placed in a cage with 3 untreated females per
week. The females were sacrificed at mid-pregnancy and examined for
reproduction defects; i.e., implantation sites, resorption sites and
live embryos. There were no significant differences between the test
and controls on any parameter examined. Fensulfothion does not affect
the mutation rate as evidenced by the mouse dominant lethal test
(Industrial Biotest Laboratory, 1971b).
Other information on identity and properties
Physical state: yellow-brown liquid
Molecular weight: 308.35
Boiling point: 138 - 141°C at 0.01 mm Hg
Volatility: <0.01 mg/cu meter (20°C)
Specific gravity: D 20 = 1.202
4
Refractive index: n 25 = 1.54
D
Solubility: in water, at 20°C, 1200 ppm soluble in most
organic solvents except aliphatics
Stability: stable under normal conditions of storage and
use
Hydrolysis rate: half-life at 81°C and pH 2.5 - 6, 120 hours
Formulations used: granular 3, 5, 10 and 15%; liquid (EC) 720
g/l
Purity of fensulfothion 94-96; O,O-diethyl-
technical O[4(methylthio)-phenyl] phosphorothioate
material: 1-3; O,O-diethyl-O-4(methylsulfonyl)-
phenyl] phosphorothioate 0.2 - 0.6;
4-(methylsulfinyl) phenol 0.8 - 1.0; water
0.3 - 0.8 (all figures in % w)
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Following oral administration to rats fensulfothion is rapidly
absorbed, distributed and excreted. Following oral doses of 0.7 to 1.5
mg/kg, tissue residues reached maximum values within 8 hours and
residues were rapidly excreted within 24 hours, primarily in the
urine. Female rats appear to excrete the acutely administered dose
somewhat slower than males. These differences may account for the
greater susceptibility of females when administered acutely toxic
doses (Everett, 1968).
Biotransformation
The metabolic route is largely through oxidative and/or hydrolytic
pathways. In female rats, the formation of the oxygen analogue and its
sulfone followed by P-O-ethyl dealkylation is a significant pathway.
In male rats hydrolytic and/or oxidative cleavage of the
phosphorothionate of the sulfoxide or the sulfone is the more
prevalent route of detoxication. Although the presence of the
metabolite 4-methylthiophenol was detected in certain biological
systems, this reductive pathway is believed to be minor.
Fensulfothion, applied to plant stems or to roots in a water
dispersion, was absorbed slowly into the plant and converted to the
phosphate analogue and to the sulfone. After 9 days the sulfone
phosphate analogue was detected (Katague and Anderson, 1967; Benjamini
et al., 1959a). In air, slow oxidation to the sulfone and
isomerization of the phosphorothiolate was shown to occur (Benjamini
et al., 1959b). This conversion has not been demonstrated in mammals
with fensulfothion. A summary of the significant features of the
metabolic pathway of fensulfothion is shown in Figure 1.
Effect on enzymes and other biochemical parameters
Fensulfothion, like other organophosphorothionate esters, is a weak
cholinesterase inhibitor which, after being converted to the
corresponding phosphate ester of fensulfothion is from 500 to 2 000
times more active in inhibiting cholinesterase.
Following intraperitoneal administration of fensulfothion (0.9 mg/kg)
to rats, inhibition of cholinesterase was maximal within one hour.
Reversal of inhibition became evident within 6 hours and was
progressive for 5 days, at which time activity returned to near normal
values (Dubois & Kinoshita, 1964).
As with several other compounds of a similar structural nature,
cholinesterase activity in females is more sensitive to in vivo
anti-cholinesterase activity. This is possibly a result of a
difference in enzyme sensitivity or a sex difference in the rate of
metabolism. No other biochemical parameters appear to be affected by
fensulfothion.
TOXICOLOGICAL STUDIES
Special studies on metabolites
Acute toxicity of fensulfothion metabolites and related products in
rats is shown in Table 1.
Special studies on mutagenicity
A dominant lethal test using groups of male mice (12 mice/group) was
conducted by treating the animals with fensulfothion by
intraperitoneal injection with 0, 0.5 and 1.0 mg/kg. For 6 consecutive
weeks treated males were placed in a cage with 3 untreated females per
week. The females were sacrificed at mid-pregnancy and examined for
reproduction defects; i.e., implantation sites, resorption sites and
live embryos. There were no significant differences between the test
and controls on any parameter examined. Fensulfothion does not affect
the mutation rate as evidenced by the mouse dominant lethal test
(Industrial Biotest Laboratory, 1971b).
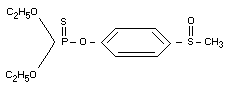 TABLE 1 Acute toxicity of fensulfothion metabolites and related
products in rats
LD50
(mg/kg) I501
ip
Name Formula M F (M)
Sulfide-P(S) R-S-CH3 2.52 3×10-4
5.5 1.53 5.4×10-4
O
Sulfoxide P(S) "
(fensulfothion) R-S-CH3 1.5 3×10-4
4.5 1.2 2.5×10-5
O
Sulfone P(S) "
R-S-CH3 1.6 5.1×10-7*
"
O
3.7 1.4 8.6×10 -5
Sulfide P(O) R1-S-CH3 2.0 1.6×10 -7
1.8 1.5 4.9×10 -7
O
"
Sulfoxide P(O) R1-S-CH3 1.2 1.6×10 -7
1.8 1.4 5.3×10 -8
O
"
Sulfone P(O) R1-S-CH3 0.9 4.8×10 -9
"
O
TABLE 1 Acute toxicity of fensulfothion metabolites and related
products in rats
LD50
(mg/kg) I501
ip
Name Formula M F (M)
Sulfide-P(S) R-S-CH3 2.52 3×10-4
5.5 1.53 5.4×10-4
O
Sulfoxide P(S) "
(fensulfothion) R-S-CH3 1.5 3×10-4
4.5 1.2 2.5×10-5
O
Sulfone P(S) "
R-S-CH3 1.6 5.1×10-7*
"
O
3.7 1.4 8.6×10 -5
Sulfide P(O) R1-S-CH3 2.0 1.6×10 -7
1.8 1.5 4.9×10 -7
O
"
Sulfoxide P(O) R1-S-CH3 1.2 1.6×10 -7
1.8 1.4 5.3×10 -8
O
"
Sulfone P(O) R1-S-CH3 0.9 4.8×10 -9
"
O
 1 Rat brain cholinesterase, molar concentration inducing
50% inhibitions.
2 Dubois & Kinoshita, 1964
3 Dubois & Jackson, 1967
* This extremely low value is probably due to contamination with the
P(O) analogue.
Special studies on neurotoxicity
Chickens were administered fensulfothion orally or by intraperitoneal
injection at dosage levels ranging from 0.005 to 0.05 gm/kg (the birds
were administered atropine and 2-PAM prior to fensulfothion). The hens
that survived the acute signs of poisoning did not show weakness or
ataxia. At the dosage levels tested fensulfothion does not induce a
delayed neurotoxic response, as seen with TOCP (Kimmerle, 1965b).
Chickens were fed fensulfothion in the diet at levels of 0, 1, 5, 20
and 100 ppm for 30 days. One day after treatment ended and again 30
days later hens were sacrificed and nerve tissue examined
histologically. Histological examination showed no evidence of
demyelination. The 100 ppm level caused death of half of the animals
and the survivors showed clinical signs of poisoning. Cholinesterase
inhibition in blood was depressed after 30 days of feeding but was
recovered after 4 weeks. There were no clinical or pathological signs
of delayed neurological disruption, as evidenced with TOCP (Kimmerle,
1965a; Grundmann, 1965).
Special studies on potentiation
Fensulfothion was administered intraperitoneally by simultaneous
administration of ´ LD50 doses to female rats in combination with 17
other anticholinesterase pesticides. There was no evidence of greater
than additive acute effects. This potentiation study was carried out
with parathion, malathion, EPN, phosdrin, ethion, fenchlorphos,
methylparathion, azinphos-methyl, chlorobenzilate, dithianon,
carbaryl, coumaphos, disulfoton, demeton, trithion, schradan, diazinon
(Dubois and Kinoshita, 1963).
Special studies on reproduction
Groups of mice (24 females and 6 males/group fed 0, 1, 5 and 20 ppm
and 40 females and 10 males/group for the first breeding and 24
females and 6 males for the second breeding fed 0, 2 and 4 ppm) were
fed fensulfothion and subjected to a standard 3-generation, 2-litter/
generation, reproduction study. The level of 20 ppm was lethal and was
discarded. At 5 ppm female mice of the Fo generation had an increased
mortality prior to mating. The survivors showed no effects of 5 ppm in
the diet on reproduction, gestation or lactation indices. A slight
reduction in lactation index was seen at 5 ppm in the F3b pups
surviving to weaning. Gross and microscopic examination of tissues of
the F3b groups showed no changes attributable to the inclusion of
fensulfothion in the diet. The effects of fensulfothion in the diet at
20 and 5 ppm, although these levels are toxic to mice, do not reflect
a hazard to reproduction (Doull et al., 1967).
Special studies on teratogenicity
Pregnant rabbits (9-11 rabbits/group) were administered fensulfothion
orally at dosage levels of 0.05 and 0.1 mg/kg per day on days to 16 of
gestation. On day 29 all animals were sacrificed and the young removed
by caesarean section. Fensulfothion did not affect pregnancy or fetal
mortality as indicated by the number of resorption sites, abortions or
dead fetuses. There was no evidence of abnormal fetal development from
gross observation or from examination of skeletal structure at 0.05
mg/kg/day. At 0.01 mg/kg/day there was a slight nonsignificant
increase in minor skeletal abnormalities. Fensulfothion is not
teratogenic to the rabbit (Industrial Biotest Laboratory, 1971a).
Acute toxicity
Acute toxicity of fensulfothion has been studied in several animal
species, a summary of results is given in Table 2.
TABLE 2 Acute toxicity of fensulfothion in animals
Species Sex Route LD50 Reference
(mg/kg)
Rat M oral 3.96-10.5 Dubois & Kinoshita, 1964
Kimerle, 1965c
Gaines, 1969
Solly & Harrison, 1971a
ip 5.5 Dabois & Kinoshita, 1964
Spencer, 1968
F oral 1-8-2.3 Dubois & Kinoshita, 1964
Kimmerle, 1965c
Gaines, 1969
Solly & Harrison, 1971a
F ip 0.9-1.5 Dubois & Kinoshita, 1964
Spencer, 1968
M dermal 14-30 Dubois & Kinoshita, 1964
Kimmerle, 1965c
Gaines, 1969
Rat F dermal 3.5-13 Dubois & Kinoshita, 1964
Kimmerle, 1965c
Gaines, 1969
TABLE 2 (Cont'd.)
Species Sex Route LD50 Reference
(mg/kg)
M inhalation 0.113 mg/l Kimmerle, 1966
(1 h)
0.030 mg/l
(4 h)
Mouse M ip 10.5 Dubois & Kinoshita, 1964
F ip 7.0 Ibid.
Guinea M oral 9.0 Ibid.
Pig
M ip 5.4 Ibid.
Sheep F oral 3.4 Solly Harrison, 1971a
Chick M oral 0.99 Sherman & Ross, 1961
Chicken p oral & ip 2.5-5.0 Kimmerle, 1965b
Signs of poisoning following acute intoxication are typical of other
organophosphate esters capable of inducing cholinergic signs of
poisoning. Signs of poisoning occur rapidly and animals return to
normal within 24 hours (Dubois & Kinoshita, 1964).
The administration of atropine (100 mg/kg) 10 minutes before
intraperitoneal administration of fensulfothion increased the LD50
from 1.5 to 2.0 mg/kg. Intraperitonal injection of PAM (100 mg/kg)
immediately after fensulfothion raised the LD50 to 6 mg/kg. Treatment
with both agents resulted in an LD50 of 7 mg/kg (Dubois & Kinoshita,
1964). Following oral administration of fensulfothion, atropine and
combinations of atropine and PAM or atropine and BH-6 were shown to
increase the LD50 values (Kimmerle, 1966). Atropine in combination
with reactivators of cholinesterase are effective antidotal agents for
the acute toxic effects of fensulfothion.
Short-term studies
Rat
Groups of female rats (5/group) were administered fensulfothion by
intraperitoneal injection daily for 60 days at dosage levels of 0,
0.25, 0.50 and 0.75 mg/kg. Mortality occurred at the highest dose with
5 of 5 rats dead within one week. At 0.5 mg/kg/day, 4 of the 5 rats
survived the treatment. All rats showed significantly reduced growth
rates and reduced brain, serum and submaxillary gland cholinesterase
activity (Dubois & Kinoshita, 1964).
Groups of female rats (25 rats/group) were fed fensulfothion for 8
weeks at dosage levels of 0, 0.5, 1 and 2 ppm. RBC cholinesterase
inhibition was observed at 2 ppm while plasma and brain levels were
unaffected. No cholinesterase depression was observed at lower dose
levels. Mortality, growth, hematology and clinical chemistry
parameters were normal (Root et al., 1969).
Dog
Groups of dogs (2 males and 2 females/group) were fed fensulfothion in
the diet at levels of 0, 1, 2, 5 and 10 ppm for 12 weeks. Other groups
fed at levels of 5 and 10 ppm displayed cholinergic signs of poisoning
and were removed from the study. Inhibition of cholinesterase was
observed in serum and RBC at 2 ppm and above. At 5 ppm and 10 ppm
signs of cholinergic poisoning and weight loss were observed (Root
et al., 1964).
Groups of dogs (2 males and 2 females/group) were fed fensulfothion in
the diet at levels of 0, 1, 2 and 5 ppm for 2 years. Severe weight
loss and reduced food consumption accompanied by signs of cholinergic
poisoning were evident in the early weeks of the study at 5 ppm. After
the second month, food consumption increased and lost body weight was
regained. Slight cholinergic signs at 2 ppm were also observed at the
beginning of the study. Reduction of serum and RBC cholinesterase was
evident at 5 ppm throughout the study. Slight reduction was observed
at 2 ppm with no effects noted at 1 ppm. No mortality was incurred as
a result of feeding fensulfothion, and hematology and gross and
histopathologic examination of tissues and organs were normal (Doull
et al., 1966a).
Long-term studies
Rat
Groups of rats (24 male and 27 female/group) were fed fensulfothion in
the diet for 17 months at dietary levels of 0, 1, 5 and 20 ppm.
Mortality of males was increased at 5 and 20 ppm. Growth of males and
females was slightly impaired at 20 ppm. Cholinesterase depression was
observed to be dose dependent in serum (19%), RBC (21%), submaxillary
gland (18%) and brain (24%) in females at 1 ppm and above and in males
at 5 ppm and above. No effects were observed on gross and histological
examination of organs and tissues (Doull et al., 1966b).
COMMENT
Fensulfothion is acutely very toxic to mammals. The compound is
metabolized similarly in animals and plants to substances of greater
toxicity by oxidation of both the enolic leaving group and the
phosphorothionate moiety. No effects on reproduction in rats,
neurotoxicity in hens, mutagenicity or teratogenicity at low levels in
rodents, or potentiation with other organophosphate compounds have
been observed.
A no-effect level in long-term studies in rats has not been
established. In a 17-month study cholinesterase depression at 1 ppm in
plasma, RBC and brain was observed. By plotting effect versus dose, a
theoretical no-effect level of 0.5 ppm (0.025 mg/kg/day) may be
estimated. Somatic effects were not noted up to levels of about five
times this dose. In a 2-year study in dogs, no adverse effects at 1
ppm were observed.
No data are reported on the effects of fensulfothion in man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 1.0 ppm in the diet, equivalent to 0.025 mg/kg/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0003 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fensulfothion is a systemic organo-phosphorous nematicide and
insecticide, which is used against soil nematodes (free living, root
knot and cyst forming nematodes) and a broad spectrum of soil borne
insects in field crops, vegetables and fruit. It is also used against
nematodes in turf grasses, in flowers and in ornamentals. In most
cultures the material is applied before planting or sowing or at
planting; in some cultures fensulfothion is applied in the soil in
established cultures.
Fensulfothion is used a.o. in Canada, Germany, Japan, New Zealand and
the Philippines.
Pre-harvest treatments
Table 3 summarizes the recommendations in accordance with good
agricultural practice, including rates and methods of application
(applications to soil at or near sowing or planting time; broadcast
application or band treatment).
TABLE 3 Officially recommended and registered uses of fensulfothion
Country and Crop Pest Dosage
(a.i.) Application time Restrictions
or pre-harvest
interval
Canada1
Brassicas 15 g/100 m row at planting or
(broccoli, max. 2.5 kg/ha shortly after
Brussels sprouts, planting
cabbage, cauliflower,
rutabagas, turnips)
Maize corn root worm 6-12 g/100 m row at planting Do not feed or ensile
(=0.5-1 kg/ha) treated forage
Potatoes Colorado beetle 5.0 kg/ha pre-plant broadcast
wireworms 100 days interval
tuber flea beetle
larvae
Ctenicera spp.
Cuba, Ecuador
Banana 3-4.5 g/tree established tree
Germany (W)2
Sugarbeet beetfly 1.25-2.5 kg/ha at sowing
(Pegomya hyoscyami)
centipedes
Philippines
Rice (paddy)
in flooded fields 1 kg/ha 15 days after broadcast
planting
TABLE 3 (Cont'd.)
Country and Crop Pest Dosage
(a.i.) Application time Restrictions
or pre-harvest
interval
2 kg/ha (2x) 45 and 75 days broadcast, granular
after planting application to paddy
water
U.S.A.3
Field corn corn root worm 0.5.1 kg/ha at planting
40 inch row spacing at planting
Popcorn
Sweet corn 12 g/100 m row at planting
Onions onion maggot 1 kg/ha in furrow at Do not apply to green
(dry bulb) planting bunch onion.
Peanuts nematodes 2-4 kg/ha band, at planting Do not feed vines and
and/or at peggig hay to livestock.
southern corn
rootworm 2-4 g/100 m row Do not apply more than
7 kg/ha in one year
Pineapple nematodes 50 kg/ha broadcast
nematodes 50 kg/ha (drench) pre-plant
Potatoes 2-5.0 kg/ha pre-plant broadcast of granules
Sugarbeet 1-2 kg/ha at sowing
15 g/100 m row at sowing
TABLE 3 (Cont'd.)
Country and Crop Pest Dosage
(a.i.) Application time Restrictions
or pre-harvest
interval
Sugarcane nematodes 2-5 kg/ha at planting band (40-45 cm)
Tobacco wireworms, 2-10 kg/ha pre-plant broadcast
nematodes
Tomatoes nematodes 3.2-6.4 kg/ha pre-plant band
10-20 kg/ha broadcast
1 Canada Dept. of Agriculture Plant products Division, Production & Marketing Branch
Use Claim for pesticides registered under the Pest Control Products Act May, 1971 & Jan. 1972, No. 834-128.
2 Pflanzenschutamittel-Verzeichnis, 23 Auflage, April 1972, Biologischen Bundesanstalt für Land - und
Forstwirtschaft Braunschweig.
3 USDA summary of Registered Agricultural Pesticide Chemical uses III D 22 5-31-69; 8-14-70, 3-24-72.
Post-harvest treatments
Fensulfothion is not recommended for post-harvest treatments.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in
different countries on food crops grown under various conditions. In
most cases dosage rates were applied in accordance with label
instructions. However, in some experiments higher dosages were also
included. Data from these trials are summarized in table 4.
TABLE 4 Residues in crops treated with fensulfothion
Crop Rate of application No. of Pre-harvest Net Residue
(a.i.) applications interval (ppm)
(days)
bananas 3.0 - 4.5 g/tree 1 1 - 90 <0.01 - 0.02
corn 1.2 - 3.2 kg/ha 2 21 - 67 <0.05
0.18 - 0.27 g/row )
plus 1.1 - 1.7 kg/ha ) 2 + 1 29 - 31 <0.01
cotton seed 0.55 - 0.82 g/m row 1 157 - 206 <0.01
onions 0.05 - 0.23 g/m row 1 80 - 191 <0.05
pineapple 60 - 200 kg/ha (drench) 1 561 - 973 <0.01
potatoes 7 - 8 kg/ha 1 102 - 150 <0.05
rutabagas 1.1 - 2.7 kg/ha 2 - 6 19 - 84 <0.01 - 0.05
soybeans 0.18 - 0.27 g/m row 1 71 - 119 <0.01
sugar beets 2 - 4 kg/ha 1 177 - 182 <0.01 - 0.10
0.12 - 2 g/m 1-3 58 - 196 <0.03
sugar cane 6 - 8 kg/ha (40 cm band) 1 228 - 343 <0.01 - 0.02
sweet potatoes 6.7 - 11.8 kg/ha 1 101 - 189 <0.01 - 0.05
tobacco 2 - 16 kg/ha 1 53 - 159 0.1 - 10.5
tomatoes 20 - 30 kg/ha 1 34 - 121 <0.05
The residues in these trials were predominantly determined by GLC
utilizing thermionic detection. Included in the analysis are total
toxic (cholinesterinase inhibiting) metabolites. Samples were taken at
harvest and immediately frozen until analysis. Bananas were stored
several days at room temperature before freezing.
In a ryegrass/white clover mixture the residue decreased from 90 ppm
(dry weight) immediately after application to 20 ppm after 13 days and
12, 4 and 1 ppm after 27, 55 and 90 days, respectively (Brewerton,
1971). Similar figures are given by Solly (1968) from grassland in New
Zealand.
FATE OF RESIDUES
General comments
The breakdown of fensulfothion in plants and animals seem identical
since the same metabolites have been identified in both.
In animals and animal products
In milk from cows grazing in pasture containing mainly residues of
fensulfothion sulfone, the oxygen analogue sulfone was detected. An
oxidative pathway in the ruminant appears most likely. Milk from dairy
cattle grazing for 10 days on pasture with an initial residue of 41
ppm fensulfothion contained 0.02 ppm oxygen analogue sulfone on the
third day, which decreased to 0.01 ppm on the 10th day (Solly
et al., 1971c).
Omental fat of sheep grazing for six days in pasture containing 43 ppm
initial residue of fensulfothion, showed a residue of 0.003 ppm,
although the erythrocyte cholinesterase depression was approximately
80% (Solly et al., 1971b).
In plants
The systemic action of fensulfothion was first demonstrated after
topical application stems of cotton plants (Benjamini et al.,
1959b). Katague and Anderson (1967) studied the fate of fensulfothion
after stem injection in cotton with 32P fensulfothion. They
identified the following metabolites by TLC with authentic standards:
O,O diethyl-O-[4-(methyl sulfinyl) phenyl)] phosphate (= oxygen
analogue) and O,O diethyl-[-4-(methylsulfonyl) phenyl]
phosphorothionate (sulfone).
After root uptake traces of O,O-diethyl-O-[-4 methyl sulfonyl
phenyl] phosphate (oxygen analogue sulfone) were found. The S-ethyl
analogue was not detected in either study.
After stem injection of 32P fensulfothion in maize, beans and cotton
the S-ethyl analogue could not be detected, but the previously
mentioned three metabolites were found (Everett and Gronberg, 1967).
Thornton (1967) analysed maize fodder from field samples by GLC for
fensulfothion and its metabolites. The interval for harvest was 40-121
days. The percent distribution of fensulfothion and metabolites was:
fensulfothion: 0-38% (average 16%), sulfone: 0-34% (average 12%),
oxygen analogue: 14-68% (average 45%), oxygen analogue sulfone: 8-49%
(average 27%).
Thornton (1968) showed the presence of 4-methylsulfonyl phenol in
maize fodder, but none in the maize kernels and cobs. Solly and
Harrison (1971a) found the sulfone as a major metabolite in pasture
grass, with only traces of the oxygen analogue and the oxygen analogue
sulfone. The same main metabolites were found in cured tobacco (Olson,
1972).
In soil
In soil the breakdown of fensulfothion and metabolites is rather
rapid. Duffy (1968) found experimentally that one half of the
fensulfothion in soil was degraded in 14 days. In other tests, in
which fensulfothion was incorporated in the top six inches of soil,
residues were determined over a period of one year. The time required
to decrease the residue to one half of the initial concentration was
generally less than 30 days; in another test it was slightly longer
than 180 days (Chemagro, 1968).
In storage and processing (cooking)
Thornton (1971) treated cotton seed oil fortified with 5 ppm
fensulfothion and the oxygen analogue with a laboratory steam
stripping similar to the process used commercially. The oil was heated
to 230 - 240°C and steam was passed through it for 3 hours. Analysis
of the processed oil showed that 48% of fensulfothion and 75% of the
oxygen analogue was swept away or degraded. The steam was scrubbed
with chloroform; 5 - 10% of the original 5 ppm were trapped and
identified as unchanged fensulfothion or oxygen analogue.
Katague (1968) simulated the refining of raw sugar beets to
concentrated syrup after fortifying the beets with 32P fensulfothion
and unlabelled metabolites. In the concentrated syrup the loss of
residue was 96 - 100% for fensulfothion, sulfone, oxygen analogue and
oxygen analogue sulfone. It was determined that the maximum total
concentration expected in the wet pulp would be no more than 5.5% of
the concentration in the raw beet.
In fresh harvested rutabagas stored 20 - 25 days at 4°C the total
residue of fensulfothion and metabolites decreased 50 - 92%. Cooking
reduced the residues by 48 - 68%. From these figures it may be
concluded that rutabagas, treated at recommended rates and stored for
a few weeks and then cooked, would contain no detectable residues.
Studies have been carried out to determine the extent of carry over of
residues in the tobacco smoke. Olson (1965) found 1.6% of
fensulfothion added to the tobacco in the smoke. After fortification
of cigarette tobacco with fensulfothion and subsequent analysis of the
solvent used for scrubbing the smoke, 7.9% recovery of unchanged
fensulfothion was shown in the smoke (Katague, 1966).
Olson (1968) fortified separate samples with fensulfothion and
metabolites. Recoveries in the smoke were 3.5, 3.0, 2.4 and 1.4%
respectively of fensulfothion, sulfone, oxygen analogue and oxygen
analogue sulfone.
During frozen storage of onions, potatoes and turnips, residues were
stable for 770 days, in cowpea vines for at least 98 days (Chemagro,
1967, 1965).
Evidence of residues in food in commerce or at consumption
No data available.
METHODS OF RESIDUE ANALYSIS
Gas chromatographic methods for analysis of fensulfothion and
metabolites are the methods of choice for residue analysis of various
crops, animal tissue and milk. GLC methods utilizing a KCl thermionic
detector proved to be particularly suitable for regulatory purposes.
Methods have been developed and adapted for analysis of maize,
peanuts, vegetables, forage crops and oil crops (Katague and Olson,
1969; Olson, 1970). The course of the analysis may be summarized as
extraction of the crop with acetone followed by partitioning with
chloroform.
The dry residue from the extract is oxidized with m-chloroperbenzoic
acid, which converts fensulfothion, sulfone and oxygen analogue to
oxygen analogue sulfone. This is further purified by solvent
partitioning and injected into the gas chromatograph. Residues of
fensulfothion and its three metabolites are measured as a single peak
as oxygen analogue sulfone. The method is specific for fensulfothion
and its metabolites. Twenty-three insecticides and metabolites
containing phosphorus were shown not to interfere (Olson, 1971).
Limit of detection of the method is 0.05 ppm or lower for
fensultothion and metabolites. Recoveries in various crop samples, in
cattle and in milk, ranged respectively from 71 - 86%, 102 - 109% and
96 - 116%.
GLC methods have been developed for the determination of individual
metabolites (Williams et al., 1971; Bowman and Hill, 1971). These
methods are less suitable for regulatory purposes than the method
described above.
Multidetection GLC methods for determining residues of
organo-phosphorous compounds in various crop samples were investigated
by the U.S. Food and Drug Administration. Fensulfothion and its three
metabolites were included in the study.
Watts et al. (1969) described a new charcoal liquid chromatography
procedure for improved clean-up of 60 organo-phosphorous compounds
including fensulfothion. Bowman and Beroza (1971) determined retention
times for 146 organo-phosphorous or sulphur compounds, including
fensulfothion, using a column packed with Dexsil 300
(polycarborane-siloxane) on HCl washed chromosorb W. The column can
be purged at temperatures up to 400°C.
Gas chromatic methods for determination of fensulfothion and
metabolites in soil have been developed (Olson, 1968; Katague, 1966).
NATIONAL TOLERANCES
Examples of national tolerances of fensulfothion residues are reported
in Table 5.
APPRAISAL
Fensulfothion is a systemic organophosphorous nematicide and
insecticide which is used on a considerable scale in various countries
on a relatively wide range of crops. Main uses are as soil treatment,
either broadcast or band treatment, or as a drench, against soil borne
nematodes (free living, root knot and cyst forming nematodes) and a
considerable range of soil borne insects.
Technical fensulfothion contains 94-96% of the pure compound. The
impurities in the technical material are known. The main components
are: O,O-diethyl-O-[4-(methylthio)-phenyl]-phosphorothioate
(1-3%) and 4-(methylsulfinyl)-phenol (0.8-1.0%).
Fensulfothion is marketed in different formulations, i.e. granular 3,
5, 10 and 15% and emulsifiable liquid (720 g/l).
The concentration/rates of application vary depending on pest, crop
and method of application; "normal" application rates are 1-5 kg a.i.
per ha.
TABLE 5 Examples of national tolerances reported to the Meeting1
Country Commodity Tolerance
(ppm)
U.S.A. peanut hulls 5
maize forage and fodder of field corn,
pop corn and sweet corn 1
maize grain, including field corn and
pop corn (kernels); fresh maize
(including sweet corn), kernels plus
cobs, with husks removed; onions,
(dry); potatoes; rutabagas (roots) and
tomatoes 0.1
peanuts (shelled); pineapple, pineapple
forage, sugar beets (roots and tops) 0.05
bananas (whole); sugarcane; meat, fat and
meat by-products of cattle, goats and sheep 0.02
Canada Brassicas (broccoli, Brussels sprouts,
cabbage, cauliflower); rutabagas;
turnips, potatoes; maize no residue
Germany, No official tolerances established.
Federal For the following crops tolerances
Republic are being considered:
sugarbeet.
New Zealand all food crops 0.1
1 Fensulfothion and cholinesterase inhibiting metabolites in or on the
commodities.
The residue data available were obtained from different countries and
regions with different climatic and soil conditions. The residue data
presented for fensulfothion included the three metabolites, sulfone,
oxygen analogue and oxygen analogue sulfone, and are with a few
exceptions representative for those likely to occur in conditions of
good agricultural practice.
Information is available on the fate of fensulfothion residues in
soil, in plants and to a lesser extent in animal products after
feeding animals on treated pasture or with treated crops.
Approved uses on peanuts and pineapple give rise to residues in peanut
kernels and in pineapple not exceeding 0.05 ppm (the proposed
tolerances). Hulls of peanuts and pineapple forage from such treated
crops will not contain residues in excess of 5 ppm and 0.05 ppm,
respectively. Likewise, the residue in sugarcane will not exceed 0.02
ppm. A soil application of fensulfothion in maize (including pop corn
and sweet corn), giving rise to residues in the kernels not exceeding
the proposed tolerance of 0.1 ppm, will result in residues in the
forage of 1 ppm or lower. Feeding of the materials mentioned in this
paragraph as a part of the ration for cattle or sheep will not give
rise to residues in meat or milk above the limit of determination
(i.e. 0.01 ppm).
Residues which may occur in food either from plant or animal origin,
after observing the recommended directions of use and the recommended
harvest intervals, consist largely of the oxygen analogue and the
oxygen analogue sulfone and to a smaller extent the parent chemical
and the sulfone. The breakdown products mentioned above could be
identified in radio-labelled studies and confirmed with other relevant
methods of analysis, e.g. TLC.
The breakdown of fensulfothion in plants and animals follows an
identical pattern; the same metabolites have been identified in both.
Information is available on the rate of decrease of the residue of
fensulfothion and metabolites in some crops and commodities during
storage and processing, including household cooking. In addition,
information on the extent of carryover of residues in tobacco smoke
from residues initially applied on tobacco was presented.
Little information is available on fensulfothion residues in food in
commerce.
Gas chromatographic procedures are available for specific
determination of fensulfothion and its main metabolites, or all
compounds combined and measure as a single peak as the oxygen analogue
sulfone. These methods are suitable for regulatory purposes as
required. Recommendations are given for the most appropriate
extraction and clean up procedures in food products of plant and
animal origin. The limit of determination is 0.05 ppm for all crops
and animal tissue and 0.01 ppm for milk.
RECOMMENDATIONS
TOLERANCES
The following tolerances are recommended for fensulfothion, including
the metabolites (oxygen analogue, oxygen analogue sulfone and
sulfone).
ppm
Maize grain, including kernels of field
corn and pop corn, onions, potatoes,
rutabagas (roots), tomatoes 0.1
Peanuts (shelled), pineapple, sugarbeet 0.05
Bananas (whole fruit) 0.02
Fat of meat and edible offal of cattle,
goats and sheep 0.02
FURTHER WORK OR INFORMATION
DESIRABLE
1. Teratogenicity studies at higher dosage levels.
2. Studies on human exposure.
REFERENCES
Benjamini, E., Metcalf, R.L. and Fukuto, T.R. (1959a) The chemistry
and mode of action of the insecticide O,O-diethyl
O-p-methylsulfinyl-phenyl phosphorothionate and its analogues. J.
Econ. Ent., 52: 94-98.
Benjamini, E., Metcalf, R.L. and Fukuto, T.R. (1959b) Contact and
systemic insecticidal properties of O,O-diethyl O,O-diethyl
O-p-methylsulfinyl-phenyl phosphorothionate and its analogues. J.
Econ. Ent., 52: 99-102.
Bowman, M.C. and Beroza, M. (1971) Use of Dexsil 300 on a specially
washed Chromosorb W for multicomponent residue determinations of
phosphorus- and sulfur-containing pesticides by flame photometric GLC.
Ass. off. analyt. Chem., 54: 1086-1092.
Bowman, M.C. and Hill, K.R. (1971) Determination of Dasanit and three
of its metabolites in corn, grass and milk. J. Agr. Fd. Chem., 19:
342-345.
Brewerton, H.V., Gibbs, M.M. and Perrott, D.C.F. (1971) Fensulfothion
and BAY 37289 residues on pasture. N.Z.J. Agric. Res., 11: 303-312.
Chemagro Division of Baychem. Corp. (1965, 1967, 1968) Reports 21252,
23119-23122, 23124, 23125, 23136. (unpublished)
Doull, J., DiGiacomo, R., Root, M. and Meskauskas, J. (1966a) Chronic
oral toxicity of BAYER 25141 to dogs. Report by the University of
Chicago submitted by Farbenfabriken Bayer, AG. (unpublished)
Doull, J., DiGiacomo, R., Root, M. and Meskauskas, J. (1966b) Chronic
oral toxicity of Bayer 25141 to rats. Report by the University of
Chicago submitted by Farbenfabriken Bayer, AG. (unpublished)
Doull, J., Root, M. and DiGiacomo, R. (1967) Effect of BAYER 25141 in
the diet on the reproduction and lactation of mice. Report by the
University of Chicago submitted by Farbenfabriken Bayer, AG.
(unpublished)
Dubois, K.P. and Jackson, P. (1967) Comparison of the acute toxicity
and anticholinesterase action of BAYER 25141 and some possible
metabolites. Report by the University of Chicago submitted by
Farbenfabriken Bayer, AG. (unpublished)
Dubois, K.P. and Kinoshita, F. (1963) The acute toxicity of BAYER
25141 in combination with other anticholinesterase insecticides.
Report by the University of Chicago submitted by Farbenfabriken Bayer,
AG. (unpublished)
Dubois, K.P. and Kinoshita, F. (1964) Acute toxicity and
anticholinesterase action of O,O-diethyl-O-p-(methylsulfinyl)
phenyl phosphorothioate (DMSP) and some related compounds. Tox. Appl.
Pharmacol., 6: 78-85.
Duffy, J.R. (1968) The metabolism of zinophos and BAY 25141 in soils.
Report on extra-mural research project 167. (unpublished)
Everett, L.J. and Gronberg, R.R. (1967) Chemagro Division of Baychem.
Corp., Kansas City, Mo. Metabolism of P32-labelled Dasanit in beans,
corn and cotton. Chemagro Report no. 21513. (unpublished)
Everett, L.J. (1968) Rat metabolism of P32 labelled Dasanit. Report
of the Chemagro Corporation submitted by Farbenfabriken Bayer, AG.
(unpublished)
Gaines, T.B. (1969) Acute toxicity of pesticides. Tox. Appl.
Pharmacol., 14: 515-534.
Grundmann, E. (1965) Histologische Untersuckung/S767. Report by
Institut für Experimentelle pathologie, submitted by Farbenfabriken
Bayer, AG. (unpublished)
Homeyer, B. (1970) Zun gegenwärtigen Stand der Bekämpfung von
Bodeninsekten. Pflanzenschutz-Nachrichten-Bayer, 23/1970: 233-239.
Homeyer, B. (1971) Terracur(R), ein breit wirkendes Boden insektizid
und Nematizid. Pflanzenschutz-Nachrichten Bayer, 24/1971: 371-410.
Industrial Biotest Laboratory. (1971) Teratogenic study with Dasanit
technical in albino rabbits. Report by the Industrial Biotest
Laboratory submitted by Farbenfabriken Bayer, AG. (unpublished)
Industrial Biotest Laboratory. (1971b) Mutagenic study with Dasanit in
albino mice. Report by the Industrial Biotest Laboratory submitted by
Farbenfabriken Bayer, AG. (unpublished)
Jung, H.F. and Iwaya, K. (1971) Terracur P(R), ein Insektizid und
Nematizid mit besonderer Eignung für den Reisanbau.
Pflanzenschutz-Nachrichten Bayer, 24/1971: 489-504.
Katague, D.B. (1966) Chemagro Division of Baychem. Corp. Reports nos.
17885, 17886, 18320, 18714. (unpublished)
Katague, D.B. and Anderson, C.A. (1967) Metabolism of P32-labelled
Dasanit in cotton plants. Bull. Env. Cont. Tox., 2: 228-235.
Katague, D.B. (1968) Chemagro Division of Baychem. Corp. Effect of
processing on Dasanit residues in sugar beets. Report no. 23015.
(unpublished)
Katague, D.B. and Olson, T.J. (1969) Chemagro Division of Baychem.
Corp. Determination of residues of Dasanit (BAY 25141) in corn by
thermionic emission gas chromatography. Report No. 20076.
(unpublished)
Kimmerle, G. (1965a) Neurotoxische Unkersuchungen Mit S-767-Wirkstoff.
Report by Institut für Toxikologie, Farbenfabriken Bayer, AG.
(unpublished)
Kimmerle, G. (1965b) Akute neurotoxizitatsuntersuchungen an huhnern.
Report by Institut für Toxikologie, Farbenfabriken Bayer, AG.
(unpublished)
Kimmerle, G. (1965c) S-767 (Production No. 3553) and Product 5121
(Production No. 3562). Report by Institut für Toxikologie,
Farbenfabriken Bayer, AG. (unpublished)
Kimmerle, G. (1966) S-767 Batch 1/64 Lo-Nr 214/antidotwirkung. Report
by Institut für Toxikologie, Farbenfabriken Bayer, AG. (unpublished)
Leuck, D.B. and Bowman, M.C. (1972) Persistence of residues of Dasanit
and its metabolites in coastal bermudagrass, forage corn and corn
silage. J. Econ. Ent., 65: 257-260.
Olson, T.J., (1965, 1968, 1969, 1970, 1971, 1972) Chemagro Division of
Baychem. Corp. Reports nos. 15188, 34051, 25464, 34164, 23055, 23037,
30278.
Proude, C.K. Ergebnisse sechsjähriger Arbeit mit Terracur P(R) in
Neuseeland. Pflanzenschutz-Nachrichten Bayer, 24/1971: 505-526.
Read, D.C. (1971) Bioactivity of Dasanit in a mineral soil, in
rutabagas in the field and in storage and effect of cooking on
toxicants in the roots. J. Econ. Ent., 64: 597-601.
Root, M., Taitel, C. and Doull, J. (1964) Subacute oral toxicity of
BAYER 25141 in male and female dogs. Report by The University of
Chicago, submitted by Farbenfabriken Bayer, AG. (unpublished)
Root, M., Kinoshita, F. and Flynn, M. (1969) Subacute oral toxicity of
Dasanit to female rats. Report by the University of Chicago, submitted
by Farbenfabriken Bayer, AG. (unpublished)
Thornton, J.S. and Olson, T.J. (1970) Chemagro Division of Baychem.
Corp. Determination of Dasanit residues in animal tissue by thermionic
emission flame gas chromatograhpy. Report no. 20750. (unpublished)
Thornton, J.S., (1971) Chemagro Division of Baychem Corp. Effect of
oil deodorization process on residues of Dasanit and Dasanit oxygen
analogue in cottonseed oil (simulated). Report no. 30299.
(unpublished)
Tucker, R.K. and Crabtree, D.G. (1970) Handbook of toxicity of
pesticides to wildlife. USDI. Resource Publication no. 84.
Watts, R.R., Storherr, R.W., Pardue, J.R. and Osgood, T. (1969)
Charcoal column clean-up method for many organo-phosphorus pesticide
residues in crop extracts. J. Ass. off. analyt. Chem., 52: 522-526.
Williams, I.H., Kore, R. and Finlayson, D.G. (1971) Determination of
residues of Dasanit and three metabolites by gas chromatography with
flame photometric detection. J. Agr. Fd. Chem., 19: 456-458.
1 Rat brain cholinesterase, molar concentration inducing
50% inhibitions.
2 Dubois & Kinoshita, 1964
3 Dubois & Jackson, 1967
* This extremely low value is probably due to contamination with the
P(O) analogue.
Special studies on neurotoxicity
Chickens were administered fensulfothion orally or by intraperitoneal
injection at dosage levels ranging from 0.005 to 0.05 gm/kg (the birds
were administered atropine and 2-PAM prior to fensulfothion). The hens
that survived the acute signs of poisoning did not show weakness or
ataxia. At the dosage levels tested fensulfothion does not induce a
delayed neurotoxic response, as seen with TOCP (Kimmerle, 1965b).
Chickens were fed fensulfothion in the diet at levels of 0, 1, 5, 20
and 100 ppm for 30 days. One day after treatment ended and again 30
days later hens were sacrificed and nerve tissue examined
histologically. Histological examination showed no evidence of
demyelination. The 100 ppm level caused death of half of the animals
and the survivors showed clinical signs of poisoning. Cholinesterase
inhibition in blood was depressed after 30 days of feeding but was
recovered after 4 weeks. There were no clinical or pathological signs
of delayed neurological disruption, as evidenced with TOCP (Kimmerle,
1965a; Grundmann, 1965).
Special studies on potentiation
Fensulfothion was administered intraperitoneally by simultaneous
administration of ´ LD50 doses to female rats in combination with 17
other anticholinesterase pesticides. There was no evidence of greater
than additive acute effects. This potentiation study was carried out
with parathion, malathion, EPN, phosdrin, ethion, fenchlorphos,
methylparathion, azinphos-methyl, chlorobenzilate, dithianon,
carbaryl, coumaphos, disulfoton, demeton, trithion, schradan, diazinon
(Dubois and Kinoshita, 1963).
Special studies on reproduction
Groups of mice (24 females and 6 males/group fed 0, 1, 5 and 20 ppm
and 40 females and 10 males/group for the first breeding and 24
females and 6 males for the second breeding fed 0, 2 and 4 ppm) were
fed fensulfothion and subjected to a standard 3-generation, 2-litter/
generation, reproduction study. The level of 20 ppm was lethal and was
discarded. At 5 ppm female mice of the Fo generation had an increased
mortality prior to mating. The survivors showed no effects of 5 ppm in
the diet on reproduction, gestation or lactation indices. A slight
reduction in lactation index was seen at 5 ppm in the F3b pups
surviving to weaning. Gross and microscopic examination of tissues of
the F3b groups showed no changes attributable to the inclusion of
fensulfothion in the diet. The effects of fensulfothion in the diet at
20 and 5 ppm, although these levels are toxic to mice, do not reflect
a hazard to reproduction (Doull et al., 1967).
Special studies on teratogenicity
Pregnant rabbits (9-11 rabbits/group) were administered fensulfothion
orally at dosage levels of 0.05 and 0.1 mg/kg per day on days to 16 of
gestation. On day 29 all animals were sacrificed and the young removed
by caesarean section. Fensulfothion did not affect pregnancy or fetal
mortality as indicated by the number of resorption sites, abortions or
dead fetuses. There was no evidence of abnormal fetal development from
gross observation or from examination of skeletal structure at 0.05
mg/kg/day. At 0.01 mg/kg/day there was a slight nonsignificant
increase in minor skeletal abnormalities. Fensulfothion is not
teratogenic to the rabbit (Industrial Biotest Laboratory, 1971a).
Acute toxicity
Acute toxicity of fensulfothion has been studied in several animal
species, a summary of results is given in Table 2.
TABLE 2 Acute toxicity of fensulfothion in animals
Species Sex Route LD50 Reference
(mg/kg)
Rat M oral 3.96-10.5 Dubois & Kinoshita, 1964
Kimerle, 1965c
Gaines, 1969
Solly & Harrison, 1971a
ip 5.5 Dabois & Kinoshita, 1964
Spencer, 1968
F oral 1-8-2.3 Dubois & Kinoshita, 1964
Kimmerle, 1965c
Gaines, 1969
Solly & Harrison, 1971a
F ip 0.9-1.5 Dubois & Kinoshita, 1964
Spencer, 1968
M dermal 14-30 Dubois & Kinoshita, 1964
Kimmerle, 1965c
Gaines, 1969
Rat F dermal 3.5-13 Dubois & Kinoshita, 1964
Kimmerle, 1965c
Gaines, 1969
TABLE 2 (Cont'd.)
Species Sex Route LD50 Reference
(mg/kg)
M inhalation 0.113 mg/l Kimmerle, 1966
(1 h)
0.030 mg/l
(4 h)
Mouse M ip 10.5 Dubois & Kinoshita, 1964
F ip 7.0 Ibid.
Guinea M oral 9.0 Ibid.
Pig
M ip 5.4 Ibid.
Sheep F oral 3.4 Solly Harrison, 1971a
Chick M oral 0.99 Sherman & Ross, 1961
Chicken p oral & ip 2.5-5.0 Kimmerle, 1965b
Signs of poisoning following acute intoxication are typical of other
organophosphate esters capable of inducing cholinergic signs of
poisoning. Signs of poisoning occur rapidly and animals return to
normal within 24 hours (Dubois & Kinoshita, 1964).
The administration of atropine (100 mg/kg) 10 minutes before
intraperitoneal administration of fensulfothion increased the LD50
from 1.5 to 2.0 mg/kg. Intraperitonal injection of PAM (100 mg/kg)
immediately after fensulfothion raised the LD50 to 6 mg/kg. Treatment
with both agents resulted in an LD50 of 7 mg/kg (Dubois & Kinoshita,
1964). Following oral administration of fensulfothion, atropine and
combinations of atropine and PAM or atropine and BH-6 were shown to
increase the LD50 values (Kimmerle, 1966). Atropine in combination
with reactivators of cholinesterase are effective antidotal agents for
the acute toxic effects of fensulfothion.
Short-term studies
Rat
Groups of female rats (5/group) were administered fensulfothion by
intraperitoneal injection daily for 60 days at dosage levels of 0,
0.25, 0.50 and 0.75 mg/kg. Mortality occurred at the highest dose with
5 of 5 rats dead within one week. At 0.5 mg/kg/day, 4 of the 5 rats
survived the treatment. All rats showed significantly reduced growth
rates and reduced brain, serum and submaxillary gland cholinesterase
activity (Dubois & Kinoshita, 1964).
Groups of female rats (25 rats/group) were fed fensulfothion for 8
weeks at dosage levels of 0, 0.5, 1 and 2 ppm. RBC cholinesterase
inhibition was observed at 2 ppm while plasma and brain levels were
unaffected. No cholinesterase depression was observed at lower dose
levels. Mortality, growth, hematology and clinical chemistry
parameters were normal (Root et al., 1969).
Dog
Groups of dogs (2 males and 2 females/group) were fed fensulfothion in
the diet at levels of 0, 1, 2, 5 and 10 ppm for 12 weeks. Other groups
fed at levels of 5 and 10 ppm displayed cholinergic signs of poisoning
and were removed from the study. Inhibition of cholinesterase was
observed in serum and RBC at 2 ppm and above. At 5 ppm and 10 ppm
signs of cholinergic poisoning and weight loss were observed (Root
et al., 1964).
Groups of dogs (2 males and 2 females/group) were fed fensulfothion in
the diet at levels of 0, 1, 2 and 5 ppm for 2 years. Severe weight
loss and reduced food consumption accompanied by signs of cholinergic
poisoning were evident in the early weeks of the study at 5 ppm. After
the second month, food consumption increased and lost body weight was
regained. Slight cholinergic signs at 2 ppm were also observed at the
beginning of the study. Reduction of serum and RBC cholinesterase was
evident at 5 ppm throughout the study. Slight reduction was observed
at 2 ppm with no effects noted at 1 ppm. No mortality was incurred as
a result of feeding fensulfothion, and hematology and gross and
histopathologic examination of tissues and organs were normal (Doull
et al., 1966a).
Long-term studies
Rat
Groups of rats (24 male and 27 female/group) were fed fensulfothion in
the diet for 17 months at dietary levels of 0, 1, 5 and 20 ppm.
Mortality of males was increased at 5 and 20 ppm. Growth of males and
females was slightly impaired at 20 ppm. Cholinesterase depression was
observed to be dose dependent in serum (19%), RBC (21%), submaxillary
gland (18%) and brain (24%) in females at 1 ppm and above and in males
at 5 ppm and above. No effects were observed on gross and histological
examination of organs and tissues (Doull et al., 1966b).
COMMENT
Fensulfothion is acutely very toxic to mammals. The compound is
metabolized similarly in animals and plants to substances of greater
toxicity by oxidation of both the enolic leaving group and the
phosphorothionate moiety. No effects on reproduction in rats,
neurotoxicity in hens, mutagenicity or teratogenicity at low levels in
rodents, or potentiation with other organophosphate compounds have
been observed.
A no-effect level in long-term studies in rats has not been
established. In a 17-month study cholinesterase depression at 1 ppm in
plasma, RBC and brain was observed. By plotting effect versus dose, a
theoretical no-effect level of 0.5 ppm (0.025 mg/kg/day) may be
estimated. Somatic effects were not noted up to levels of about five
times this dose. In a 2-year study in dogs, no adverse effects at 1
ppm were observed.
No data are reported on the effects of fensulfothion in man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 1.0 ppm in the diet, equivalent to 0.025 mg/kg/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0003 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fensulfothion is a systemic organo-phosphorous nematicide and
insecticide, which is used against soil nematodes (free living, root
knot and cyst forming nematodes) and a broad spectrum of soil borne
insects in field crops, vegetables and fruit. It is also used against
nematodes in turf grasses, in flowers and in ornamentals. In most
cultures the material is applied before planting or sowing or at
planting; in some cultures fensulfothion is applied in the soil in
established cultures.
Fensulfothion is used a.o. in Canada, Germany, Japan, New Zealand and
the Philippines.
Pre-harvest treatments
Table 3 summarizes the recommendations in accordance with good
agricultural practice, including rates and methods of application
(applications to soil at or near sowing or planting time; broadcast
application or band treatment).
TABLE 3 Officially recommended and registered uses of fensulfothion
Country and Crop Pest Dosage
(a.i.) Application time Restrictions
or pre-harvest
interval
Canada1
Brassicas 15 g/100 m row at planting or
(broccoli, max. 2.5 kg/ha shortly after
Brussels sprouts, planting
cabbage, cauliflower,
rutabagas, turnips)
Maize corn root worm 6-12 g/100 m row at planting Do not feed or ensile
(=0.5-1 kg/ha) treated forage
Potatoes Colorado beetle 5.0 kg/ha pre-plant broadcast
wireworms 100 days interval
tuber flea beetle
larvae
Ctenicera spp.
Cuba, Ecuador
Banana 3-4.5 g/tree established tree
Germany (W)2
Sugarbeet beetfly 1.25-2.5 kg/ha at sowing
(Pegomya hyoscyami)
centipedes
Philippines
Rice (paddy)
in flooded fields 1 kg/ha 15 days after broadcast
planting
TABLE 3 (Cont'd.)
Country and Crop Pest Dosage
(a.i.) Application time Restrictions
or pre-harvest
interval
2 kg/ha (2x) 45 and 75 days broadcast, granular
after planting application to paddy
water
U.S.A.3
Field corn corn root worm 0.5.1 kg/ha at planting
40 inch row spacing at planting
Popcorn
Sweet corn 12 g/100 m row at planting
Onions onion maggot 1 kg/ha in furrow at Do not apply to green
(dry bulb) planting bunch onion.
Peanuts nematodes 2-4 kg/ha band, at planting Do not feed vines and
and/or at peggig hay to livestock.
southern corn
rootworm 2-4 g/100 m row Do not apply more than
7 kg/ha in one year
Pineapple nematodes 50 kg/ha broadcast
nematodes 50 kg/ha (drench) pre-plant
Potatoes 2-5.0 kg/ha pre-plant broadcast of granules
Sugarbeet 1-2 kg/ha at sowing
15 g/100 m row at sowing
TABLE 3 (Cont'd.)
Country and Crop Pest Dosage
(a.i.) Application time Restrictions
or pre-harvest
interval
Sugarcane nematodes 2-5 kg/ha at planting band (40-45 cm)
Tobacco wireworms, 2-10 kg/ha pre-plant broadcast
nematodes
Tomatoes nematodes 3.2-6.4 kg/ha pre-plant band
10-20 kg/ha broadcast
1 Canada Dept. of Agriculture Plant products Division, Production & Marketing Branch
Use Claim for pesticides registered under the Pest Control Products Act May, 1971 & Jan. 1972, No. 834-128.
2 Pflanzenschutamittel-Verzeichnis, 23 Auflage, April 1972, Biologischen Bundesanstalt für Land - und
Forstwirtschaft Braunschweig.
3 USDA summary of Registered Agricultural Pesticide Chemical uses III D 22 5-31-69; 8-14-70, 3-24-72.
Post-harvest treatments
Fensulfothion is not recommended for post-harvest treatments.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in
different countries on food crops grown under various conditions. In
most cases dosage rates were applied in accordance with label
instructions. However, in some experiments higher dosages were also
included. Data from these trials are summarized in table 4.
TABLE 4 Residues in crops treated with fensulfothion
Crop Rate of application No. of Pre-harvest Net Residue
(a.i.) applications interval (ppm)
(days)
bananas 3.0 - 4.5 g/tree 1 1 - 90 <0.01 - 0.02
corn 1.2 - 3.2 kg/ha 2 21 - 67 <0.05
0.18 - 0.27 g/row )
plus 1.1 - 1.7 kg/ha ) 2 + 1 29 - 31 <0.01
cotton seed 0.55 - 0.82 g/m row 1 157 - 206 <0.01
onions 0.05 - 0.23 g/m row 1 80 - 191 <0.05
pineapple 60 - 200 kg/ha (drench) 1 561 - 973 <0.01
potatoes 7 - 8 kg/ha 1 102 - 150 <0.05
rutabagas 1.1 - 2.7 kg/ha 2 - 6 19 - 84 <0.01 - 0.05
soybeans 0.18 - 0.27 g/m row 1 71 - 119 <0.01
sugar beets 2 - 4 kg/ha 1 177 - 182 <0.01 - 0.10
0.12 - 2 g/m 1-3 58 - 196 <0.03
sugar cane 6 - 8 kg/ha (40 cm band) 1 228 - 343 <0.01 - 0.02
sweet potatoes 6.7 - 11.8 kg/ha 1 101 - 189 <0.01 - 0.05
tobacco 2 - 16 kg/ha 1 53 - 159 0.1 - 10.5
tomatoes 20 - 30 kg/ha 1 34 - 121 <0.05
The residues in these trials were predominantly determined by GLC
utilizing thermionic detection. Included in the analysis are total
toxic (cholinesterinase inhibiting) metabolites. Samples were taken at
harvest and immediately frozen until analysis. Bananas were stored
several days at room temperature before freezing.
In a ryegrass/white clover mixture the residue decreased from 90 ppm
(dry weight) immediately after application to 20 ppm after 13 days and
12, 4 and 1 ppm after 27, 55 and 90 days, respectively (Brewerton,
1971). Similar figures are given by Solly (1968) from grassland in New
Zealand.
FATE OF RESIDUES
General comments
The breakdown of fensulfothion in plants and animals seem identical
since the same metabolites have been identified in both.
In animals and animal products
In milk from cows grazing in pasture containing mainly residues of
fensulfothion sulfone, the oxygen analogue sulfone was detected. An
oxidative pathway in the ruminant appears most likely. Milk from dairy
cattle grazing for 10 days on pasture with an initial residue of 41
ppm fensulfothion contained 0.02 ppm oxygen analogue sulfone on the
third day, which decreased to 0.01 ppm on the 10th day (Solly
et al., 1971c).
Omental fat of sheep grazing for six days in pasture containing 43 ppm
initial residue of fensulfothion, showed a residue of 0.003 ppm,
although the erythrocyte cholinesterase depression was approximately
80% (Solly et al., 1971b).
In plants
The systemic action of fensulfothion was first demonstrated after
topical application stems of cotton plants (Benjamini et al.,
1959b). Katague and Anderson (1967) studied the fate of fensulfothion
after stem injection in cotton with 32P fensulfothion. They
identified the following metabolites by TLC with authentic standards:
O,O diethyl-O-[4-(methyl sulfinyl) phenyl)] phosphate (= oxygen
analogue) and O,O diethyl-[-4-(methylsulfonyl) phenyl]
phosphorothionate (sulfone).
After root uptake traces of O,O-diethyl-O-[-4 methyl sulfonyl
phenyl] phosphate (oxygen analogue sulfone) were found. The S-ethyl
analogue was not detected in either study.
After stem injection of 32P fensulfothion in maize, beans and cotton
the S-ethyl analogue could not be detected, but the previously
mentioned three metabolites were found (Everett and Gronberg, 1967).
Thornton (1967) analysed maize fodder from field samples by GLC for
fensulfothion and its metabolites. The interval for harvest was 40-121
days. The percent distribution of fensulfothion and metabolites was:
fensulfothion: 0-38% (average 16%), sulfone: 0-34% (average 12%),
oxygen analogue: 14-68% (average 45%), oxygen analogue sulfone: 8-49%
(average 27%).
Thornton (1968) showed the presence of 4-methylsulfonyl phenol in
maize fodder, but none in the maize kernels and cobs. Solly and
Harrison (1971a) found the sulfone as a major metabolite in pasture
grass, with only traces of the oxygen analogue and the oxygen analogue
sulfone. The same main metabolites were found in cured tobacco (Olson,
1972).
In soil
In soil the breakdown of fensulfothion and metabolites is rather
rapid. Duffy (1968) found experimentally that one half of the
fensulfothion in soil was degraded in 14 days. In other tests, in
which fensulfothion was incorporated in the top six inches of soil,
residues were determined over a period of one year. The time required
to decrease the residue to one half of the initial concentration was
generally less than 30 days; in another test it was slightly longer
than 180 days (Chemagro, 1968).
In storage and processing (cooking)
Thornton (1971) treated cotton seed oil fortified with 5 ppm
fensulfothion and the oxygen analogue with a laboratory steam
stripping similar to the process used commercially. The oil was heated
to 230 - 240°C and steam was passed through it for 3 hours. Analysis
of the processed oil showed that 48% of fensulfothion and 75% of the
oxygen analogue was swept away or degraded. The steam was scrubbed
with chloroform; 5 - 10% of the original 5 ppm were trapped and
identified as unchanged fensulfothion or oxygen analogue.
Katague (1968) simulated the refining of raw sugar beets to
concentrated syrup after fortifying the beets with 32P fensulfothion
and unlabelled metabolites. In the concentrated syrup the loss of
residue was 96 - 100% for fensulfothion, sulfone, oxygen analogue and
oxygen analogue sulfone. It was determined that the maximum total
concentration expected in the wet pulp would be no more than 5.5% of
the concentration in the raw beet.
In fresh harvested rutabagas stored 20 - 25 days at 4°C the total
residue of fensulfothion and metabolites decreased 50 - 92%. Cooking
reduced the residues by 48 - 68%. From these figures it may be
concluded that rutabagas, treated at recommended rates and stored for
a few weeks and then cooked, would contain no detectable residues.
Studies have been carried out to determine the extent of carry over of
residues in the tobacco smoke. Olson (1965) found 1.6% of
fensulfothion added to the tobacco in the smoke. After fortification
of cigarette tobacco with fensulfothion and subsequent analysis of the
solvent used for scrubbing the smoke, 7.9% recovery of unchanged
fensulfothion was shown in the smoke (Katague, 1966).
Olson (1968) fortified separate samples with fensulfothion and
metabolites. Recoveries in the smoke were 3.5, 3.0, 2.4 and 1.4%
respectively of fensulfothion, sulfone, oxygen analogue and oxygen
analogue sulfone.
During frozen storage of onions, potatoes and turnips, residues were
stable for 770 days, in cowpea vines for at least 98 days (Chemagro,
1967, 1965).
Evidence of residues in food in commerce or at consumption
No data available.
METHODS OF RESIDUE ANALYSIS
Gas chromatographic methods for analysis of fensulfothion and
metabolites are the methods of choice for residue analysis of various
crops, animal tissue and milk. GLC methods utilizing a KCl thermionic
detector proved to be particularly suitable for regulatory purposes.
Methods have been developed and adapted for analysis of maize,
peanuts, vegetables, forage crops and oil crops (Katague and Olson,
1969; Olson, 1970). The course of the analysis may be summarized as
extraction of the crop with acetone followed by partitioning with
chloroform.
The dry residue from the extract is oxidized with m-chloroperbenzoic
acid, which converts fensulfothion, sulfone and oxygen analogue to
oxygen analogue sulfone. This is further purified by solvent
partitioning and injected into the gas chromatograph. Residues of
fensulfothion and its three metabolites are measured as a single peak
as oxygen analogue sulfone. The method is specific for fensulfothion
and its metabolites. Twenty-three insecticides and metabolites
containing phosphorus were shown not to interfere (Olson, 1971).
Limit of detection of the method is 0.05 ppm or lower for
fensultothion and metabolites. Recoveries in various crop samples, in
cattle and in milk, ranged respectively from 71 - 86%, 102 - 109% and
96 - 116%.
GLC methods have been developed for the determination of individual
metabolites (Williams et al., 1971; Bowman and Hill, 1971). These
methods are less suitable for regulatory purposes than the method
described above.
Multidetection GLC methods for determining residues of
organo-phosphorous compounds in various crop samples were investigated
by the U.S. Food and Drug Administration. Fensulfothion and its three
metabolites were included in the study.
Watts et al. (1969) described a new charcoal liquid chromatography
procedure for improved clean-up of 60 organo-phosphorous compounds
including fensulfothion. Bowman and Beroza (1971) determined retention
times for 146 organo-phosphorous or sulphur compounds, including
fensulfothion, using a column packed with Dexsil 300
(polycarborane-siloxane) on HCl washed chromosorb W. The column can
be purged at temperatures up to 400°C.
Gas chromatic methods for determination of fensulfothion and
metabolites in soil have been developed (Olson, 1968; Katague, 1966).
NATIONAL TOLERANCES
Examples of national tolerances of fensulfothion residues are reported
in Table 5.
APPRAISAL
Fensulfothion is a systemic organophosphorous nematicide and
insecticide which is used on a considerable scale in various countries
on a relatively wide range of crops. Main uses are as soil treatment,
either broadcast or band treatment, or as a drench, against soil borne
nematodes (free living, root knot and cyst forming nematodes) and a
considerable range of soil borne insects.
Technical fensulfothion contains 94-96% of the pure compound. The
impurities in the technical material are known. The main components
are: O,O-diethyl-O-[4-(methylthio)-phenyl]-phosphorothioate
(1-3%) and 4-(methylsulfinyl)-phenol (0.8-1.0%).
Fensulfothion is marketed in different formulations, i.e. granular 3,
5, 10 and 15% and emulsifiable liquid (720 g/l).
The concentration/rates of application vary depending on pest, crop
and method of application; "normal" application rates are 1-5 kg a.i.
per ha.
TABLE 5 Examples of national tolerances reported to the Meeting1
Country Commodity Tolerance
(ppm)
U.S.A. peanut hulls 5
maize forage and fodder of field corn,
pop corn and sweet corn 1
maize grain, including field corn and
pop corn (kernels); fresh maize
(including sweet corn), kernels plus
cobs, with husks removed; onions,
(dry); potatoes; rutabagas (roots) and
tomatoes 0.1
peanuts (shelled); pineapple, pineapple
forage, sugar beets (roots and tops) 0.05
bananas (whole); sugarcane; meat, fat and
meat by-products of cattle, goats and sheep 0.02
Canada Brassicas (broccoli, Brussels sprouts,
cabbage, cauliflower); rutabagas;
turnips, potatoes; maize no residue
Germany, No official tolerances established.
Federal For the following crops tolerances
Republic are being considered:
sugarbeet.
New Zealand all food crops 0.1
1 Fensulfothion and cholinesterase inhibiting metabolites in or on the
commodities.
The residue data available were obtained from different countries and
regions with different climatic and soil conditions. The residue data
presented for fensulfothion included the three metabolites, sulfone,
oxygen analogue and oxygen analogue sulfone, and are with a few
exceptions representative for those likely to occur in conditions of
good agricultural practice.
Information is available on the fate of fensulfothion residues in
soil, in plants and to a lesser extent in animal products after
feeding animals on treated pasture or with treated crops.
Approved uses on peanuts and pineapple give rise to residues in peanut
kernels and in pineapple not exceeding 0.05 ppm (the proposed
tolerances). Hulls of peanuts and pineapple forage from such treated
crops will not contain residues in excess of 5 ppm and 0.05 ppm,
respectively. Likewise, the residue in sugarcane will not exceed 0.02
ppm. A soil application of fensulfothion in maize (including pop corn
and sweet corn), giving rise to residues in the kernels not exceeding
the proposed tolerance of 0.1 ppm, will result in residues in the
forage of 1 ppm or lower. Feeding of the materials mentioned in this
paragraph as a part of the ration for cattle or sheep will not give
rise to residues in meat or milk above the limit of determination
(i.e. 0.01 ppm).
Residues which may occur in food either from plant or animal origin,
after observing the recommended directions of use and the recommended
harvest intervals, consist largely of the oxygen analogue and the
oxygen analogue sulfone and to a smaller extent the parent chemical
and the sulfone. The breakdown products mentioned above could be
identified in radio-labelled studies and confirmed with other relevant
methods of analysis, e.g. TLC.
The breakdown of fensulfothion in plants and animals follows an
identical pattern; the same metabolites have been identified in both.
Information is available on the rate of decrease of the residue of
fensulfothion and metabolites in some crops and commodities during
storage and processing, including household cooking. In addition,
information on the extent of carryover of residues in tobacco smoke
from residues initially applied on tobacco was presented.
Little information is available on fensulfothion residues in food in
commerce.
Gas chromatographic procedures are available for specific
determination of fensulfothion and its main metabolites, or all
compounds combined and measure as a single peak as the oxygen analogue
sulfone. These methods are suitable for regulatory purposes as
required. Recommendations are given for the most appropriate
extraction and clean up procedures in food products of plant and
animal origin. The limit of determination is 0.05 ppm for all crops
and animal tissue and 0.01 ppm for milk.
RECOMMENDATIONS
TOLERANCES
The following tolerances are recommended for fensulfothion, including
the metabolites (oxygen analogue, oxygen analogue sulfone and
sulfone).
ppm
Maize grain, including kernels of field
corn and pop corn, onions, potatoes,
rutabagas (roots), tomatoes 0.1
Peanuts (shelled), pineapple, sugarbeet 0.05
Bananas (whole fruit) 0.02
Fat of meat and edible offal of cattle,
goats and sheep 0.02
FURTHER WORK OR INFORMATION
DESIRABLE
1. Teratogenicity studies at higher dosage levels.
2. Studies on human exposure.
REFERENCES
Benjamini, E., Metcalf, R.L. and Fukuto, T.R. (1959a) The chemistry
and mode of action of the insecticide O,O-diethyl
O-p-methylsulfinyl-phenyl phosphorothionate and its analogues. J.
Econ. Ent., 52: 94-98.
Benjamini, E., Metcalf, R.L. and Fukuto, T.R. (1959b) Contact and
systemic insecticidal properties of O,O-diethyl O,O-diethyl
O-p-methylsulfinyl-phenyl phosphorothionate and its analogues. J.
Econ. Ent., 52: 99-102.
Bowman, M.C. and Beroza, M. (1971) Use of Dexsil 300 on a specially
washed Chromosorb W for multicomponent residue determinations of
phosphorus- and sulfur-containing pesticides by flame photometric GLC.
Ass. off. analyt. Chem., 54: 1086-1092.
Bowman, M.C. and Hill, K.R. (1971) Determination of Dasanit and three
of its metabolites in corn, grass and milk. J. Agr. Fd. Chem., 19:
342-345.
Brewerton, H.V., Gibbs, M.M. and Perrott, D.C.F. (1971) Fensulfothion
and BAY 37289 residues on pasture. N.Z.J. Agric. Res., 11: 303-312.
Chemagro Division of Baychem. Corp. (1965, 1967, 1968) Reports 21252,
23119-23122, 23124, 23125, 23136. (unpublished)
Doull, J., DiGiacomo, R., Root, M. and Meskauskas, J. (1966a) Chronic
oral toxicity of BAYER 25141 to dogs. Report by the University of
Chicago submitted by Farbenfabriken Bayer, AG. (unpublished)
Doull, J., DiGiacomo, R., Root, M. and Meskauskas, J. (1966b) Chronic
oral toxicity of Bayer 25141 to rats. Report by the University of
Chicago submitted by Farbenfabriken Bayer, AG. (unpublished)
Doull, J., Root, M. and DiGiacomo, R. (1967) Effect of BAYER 25141 in
the diet on the reproduction and lactation of mice. Report by the
University of Chicago submitted by Farbenfabriken Bayer, AG.
(unpublished)
Dubois, K.P. and Jackson, P. (1967) Comparison of the acute toxicity
and anticholinesterase action of BAYER 25141 and some possible
metabolites. Report by the University of Chicago submitted by
Farbenfabriken Bayer, AG. (unpublished)
Dubois, K.P. and Kinoshita, F. (1963) The acute toxicity of BAYER
25141 in combination with other anticholinesterase insecticides.
Report by the University of Chicago submitted by Farbenfabriken Bayer,
AG. (unpublished)
Dubois, K.P. and Kinoshita, F. (1964) Acute toxicity and
anticholinesterase action of O,O-diethyl-O-p-(methylsulfinyl)
phenyl phosphorothioate (DMSP) and some related compounds. Tox. Appl.
Pharmacol., 6: 78-85.
Duffy, J.R. (1968) The metabolism of zinophos and BAY 25141 in soils.
Report on extra-mural research project 167. (unpublished)
Everett, L.J. and Gronberg, R.R. (1967) Chemagro Division of Baychem.
Corp., Kansas City, Mo. Metabolism of P32-labelled Dasanit in beans,
corn and cotton. Chemagro Report no. 21513. (unpublished)
Everett, L.J. (1968) Rat metabolism of P32 labelled Dasanit. Report
of the Chemagro Corporation submitted by Farbenfabriken Bayer, AG.
(unpublished)
Gaines, T.B. (1969) Acute toxicity of pesticides. Tox. Appl.
Pharmacol., 14: 515-534.
Grundmann, E. (1965) Histologische Untersuckung/S767. Report by
Institut für Experimentelle pathologie, submitted by Farbenfabriken
Bayer, AG. (unpublished)
Homeyer, B. (1970) Zun gegenwärtigen Stand der Bekämpfung von
Bodeninsekten. Pflanzenschutz-Nachrichten-Bayer, 23/1970: 233-239.
Homeyer, B. (1971) Terracur(R), ein breit wirkendes Boden insektizid
und Nematizid. Pflanzenschutz-Nachrichten Bayer, 24/1971: 371-410.
Industrial Biotest Laboratory. (1971) Teratogenic study with Dasanit
technical in albino rabbits. Report by the Industrial Biotest
Laboratory submitted by Farbenfabriken Bayer, AG. (unpublished)
Industrial Biotest Laboratory. (1971b) Mutagenic study with Dasanit in
albino mice. Report by the Industrial Biotest Laboratory submitted by
Farbenfabriken Bayer, AG. (unpublished)
Jung, H.F. and Iwaya, K. (1971) Terracur P(R), ein Insektizid und
Nematizid mit besonderer Eignung für den Reisanbau.
Pflanzenschutz-Nachrichten Bayer, 24/1971: 489-504.
Katague, D.B. (1966) Chemagro Division of Baychem. Corp. Reports nos.
17885, 17886, 18320, 18714. (unpublished)
Katague, D.B. and Anderson, C.A. (1967) Metabolism of P32-labelled
Dasanit in cotton plants. Bull. Env. Cont. Tox., 2: 228-235.
Katague, D.B. (1968) Chemagro Division of Baychem. Corp. Effect of
processing on Dasanit residues in sugar beets. Report no. 23015.
(unpublished)
Katague, D.B. and Olson, T.J. (1969) Chemagro Division of Baychem.
Corp. Determination of residues of Dasanit (BAY 25141) in corn by
thermionic emission gas chromatography. Report No. 20076.
(unpublished)
Kimmerle, G. (1965a) Neurotoxische Unkersuchungen Mit S-767-Wirkstoff.
Report by Institut für Toxikologie, Farbenfabriken Bayer, AG.
(unpublished)
Kimmerle, G. (1965b) Akute neurotoxizitatsuntersuchungen an huhnern.
Report by Institut für Toxikologie, Farbenfabriken Bayer, AG.
(unpublished)
Kimmerle, G. (1965c) S-767 (Production No. 3553) and Product 5121
(Production No. 3562). Report by Institut für Toxikologie,
Farbenfabriken Bayer, AG. (unpublished)
Kimmerle, G. (1966) S-767 Batch 1/64 Lo-Nr 214/antidotwirkung. Report
by Institut für Toxikologie, Farbenfabriken Bayer, AG. (unpublished)
Leuck, D.B. and Bowman, M.C. (1972) Persistence of residues of Dasanit
and its metabolites in coastal bermudagrass, forage corn and corn
silage. J. Econ. Ent., 65: 257-260.
Olson, T.J., (1965, 1968, 1969, 1970, 1971, 1972) Chemagro Division of
Baychem. Corp. Reports nos. 15188, 34051, 25464, 34164, 23055, 23037,
30278.
Proude, C.K. Ergebnisse sechsjähriger Arbeit mit Terracur P(R) in
Neuseeland. Pflanzenschutz-Nachrichten Bayer, 24/1971: 505-526.
Read, D.C. (1971) Bioactivity of Dasanit in a mineral soil, in
rutabagas in the field and in storage and effect of cooking on
toxicants in the roots. J. Econ. Ent., 64: 597-601.
Root, M., Taitel, C. and Doull, J. (1964) Subacute oral toxicity of
BAYER 25141 in male and female dogs. Report by The University of
Chicago, submitted by Farbenfabriken Bayer, AG. (unpublished)
Root, M., Kinoshita, F. and Flynn, M. (1969) Subacute oral toxicity of
Dasanit to female rats. Report by the University of Chicago, submitted
by Farbenfabriken Bayer, AG. (unpublished)
Thornton, J.S. and Olson, T.J. (1970) Chemagro Division of Baychem.
Corp. Determination of Dasanit residues in animal tissue by thermionic
emission flame gas chromatograhpy. Report no. 20750. (unpublished)
Thornton, J.S., (1971) Chemagro Division of Baychem Corp. Effect of
oil deodorization process on residues of Dasanit and Dasanit oxygen
analogue in cottonseed oil (simulated). Report no. 30299.
(unpublished)
Tucker, R.K. and Crabtree, D.G. (1970) Handbook of toxicity of
pesticides to wildlife. USDI. Resource Publication no. 84.
Watts, R.R., Storherr, R.W., Pardue, J.R. and Osgood, T. (1969)
Charcoal column clean-up method for many organo-phosphorus pesticide
residues in crop extracts. J. Ass. off. analyt. Chem., 52: 522-526.
Williams, I.H., Kore, R. and Finlayson, D.G. (1971) Determination of
residues of Dasanit and three metabolites by gas chromatography with
flame photometric detection. J. Agr. Fd. Chem., 19: 456-458.
 Other information on identity and properties
Physical state: yellow-brown liquid
Molecular weight: 308.35
Boiling point: 138 - 141°C at 0.01 mm Hg
Volatility: <0.01 mg/cu meter (20°C)
Specific gravity: D 20 = 1.202
4
Refractive index: n 25 = 1.54
D
Solubility: in water, at 20°C, 1200 ppm soluble in most
organic solvents except aliphatics
Stability: stable under normal conditions of storage and
use
Hydrolysis rate: half-life at 81°C and pH 2.5 - 6, 120 hours
Formulations used: granular 3, 5, 10 and 15%; liquid (EC) 720
g/l
Purity of fensulfothion 94-96; O,O-diethyl-
technical O[4(methylthio)-phenyl] phosphorothioate
material: 1-3; O,O-diethyl-O-4(methylsulfonyl)-
phenyl] phosphorothioate 0.2 - 0.6;
4-(methylsulfinyl) phenol 0.8 - 1.0; water
0.3 - 0.8 (all figures in % w)
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Following oral administration to rats fensulfothion is rapidly
absorbed, distributed and excreted. Following oral doses of 0.7 to 1.5
mg/kg, tissue residues reached maximum values within 8 hours and
residues were rapidly excreted within 24 hours, primarily in the
urine. Female rats appear to excrete the acutely administered dose
somewhat slower than males. These differences may account for the
greater susceptibility of females when administered acutely toxic
doses (Everett, 1968).
Biotransformation
The metabolic route is largely through oxidative and/or hydrolytic
pathways. In female rats, the formation of the oxygen analogue and its
sulfone followed by P-O-ethyl dealkylation is a significant pathway.
In male rats hydrolytic and/or oxidative cleavage of the
phosphorothionate of the sulfoxide or the sulfone is the more
prevalent route of detoxication. Although the presence of the
metabolite 4-methylthiophenol was detected in certain biological
systems, this reductive pathway is believed to be minor.
Fensulfothion, applied to plant stems or to roots in a water
dispersion, was absorbed slowly into the plant and converted to the
phosphate analogue and to the sulfone. After 9 days the sulfone
phosphate analogue was detected (Katague and Anderson, 1967; Benjamini
et al., 1959a). In air, slow oxidation to the sulfone and
isomerization of the phosphorothiolate was shown to occur (Benjamini
et al., 1959b). This conversion has not been demonstrated in mammals
with fensulfothion. A summary of the significant features of the
metabolic pathway of fensulfothion is shown in Figure 1.
Effect on enzymes and other biochemical parameters
Fensulfothion, like other organophosphorothionate esters, is a weak
cholinesterase inhibitor which, after being converted to the
corresponding phosphate ester of fensulfothion is from 500 to 2 000
times more active in inhibiting cholinesterase.
Following intraperitoneal administration of fensulfothion (0.9 mg/kg)
to rats, inhibition of cholinesterase was maximal within one hour.
Reversal of inhibition became evident within 6 hours and was
progressive for 5 days, at which time activity returned to near normal
values (Dubois & Kinoshita, 1964).
As with several other compounds of a similar structural nature,
cholinesterase activity in females is more sensitive to in vivo
anti-cholinesterase activity. This is possibly a result of a
difference in enzyme sensitivity or a sex difference in the rate of
metabolism. No other biochemical parameters appear to be affected by
fensulfothion.
TOXICOLOGICAL STUDIES
Special studies on metabolites
Acute toxicity of fensulfothion metabolites and related products in
rats is shown in Table 1.
Special studies on mutagenicity
A dominant lethal test using groups of male mice (12 mice/group) was
conducted by treating the animals with fensulfothion by
intraperitoneal injection with 0, 0.5 and 1.0 mg/kg. For 6 consecutive
weeks treated males were placed in a cage with 3 untreated females per
week. The females were sacrificed at mid-pregnancy and examined for
reproduction defects; i.e., implantation sites, resorption sites and
live embryos. There were no significant differences between the test
and controls on any parameter examined. Fensulfothion does not affect
the mutation rate as evidenced by the mouse dominant lethal test
(Industrial Biotest Laboratory, 1971b).
Other information on identity and properties
Physical state: yellow-brown liquid
Molecular weight: 308.35
Boiling point: 138 - 141°C at 0.01 mm Hg
Volatility: <0.01 mg/cu meter (20°C)
Specific gravity: D 20 = 1.202
4
Refractive index: n 25 = 1.54
D
Solubility: in water, at 20°C, 1200 ppm soluble in most
organic solvents except aliphatics
Stability: stable under normal conditions of storage and
use
Hydrolysis rate: half-life at 81°C and pH 2.5 - 6, 120 hours
Formulations used: granular 3, 5, 10 and 15%; liquid (EC) 720
g/l
Purity of fensulfothion 94-96; O,O-diethyl-
technical O[4(methylthio)-phenyl] phosphorothioate
material: 1-3; O,O-diethyl-O-4(methylsulfonyl)-
phenyl] phosphorothioate 0.2 - 0.6;
4-(methylsulfinyl) phenol 0.8 - 1.0; water
0.3 - 0.8 (all figures in % w)
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Following oral administration to rats fensulfothion is rapidly
absorbed, distributed and excreted. Following oral doses of 0.7 to 1.5
mg/kg, tissue residues reached maximum values within 8 hours and
residues were rapidly excreted within 24 hours, primarily in the
urine. Female rats appear to excrete the acutely administered dose
somewhat slower than males. These differences may account for the
greater susceptibility of females when administered acutely toxic
doses (Everett, 1968).
Biotransformation
The metabolic route is largely through oxidative and/or hydrolytic
pathways. In female rats, the formation of the oxygen analogue and its
sulfone followed by P-O-ethyl dealkylation is a significant pathway.
In male rats hydrolytic and/or oxidative cleavage of the
phosphorothionate of the sulfoxide or the sulfone is the more
prevalent route of detoxication. Although the presence of the
metabolite 4-methylthiophenol was detected in certain biological
systems, this reductive pathway is believed to be minor.
Fensulfothion, applied to plant stems or to roots in a water
dispersion, was absorbed slowly into the plant and converted to the
phosphate analogue and to the sulfone. After 9 days the sulfone
phosphate analogue was detected (Katague and Anderson, 1967; Benjamini
et al., 1959a). In air, slow oxidation to the sulfone and
isomerization of the phosphorothiolate was shown to occur (Benjamini
et al., 1959b). This conversion has not been demonstrated in mammals
with fensulfothion. A summary of the significant features of the
metabolic pathway of fensulfothion is shown in Figure 1.
Effect on enzymes and other biochemical parameters
Fensulfothion, like other organophosphorothionate esters, is a weak
cholinesterase inhibitor which, after being converted to the
corresponding phosphate ester of fensulfothion is from 500 to 2 000
times more active in inhibiting cholinesterase.
Following intraperitoneal administration of fensulfothion (0.9 mg/kg)
to rats, inhibition of cholinesterase was maximal within one hour.
Reversal of inhibition became evident within 6 hours and was
progressive for 5 days, at which time activity returned to near normal
values (Dubois & Kinoshita, 1964).
As with several other compounds of a similar structural nature,
cholinesterase activity in females is more sensitive to in vivo
anti-cholinesterase activity. This is possibly a result of a
difference in enzyme sensitivity or a sex difference in the rate of
metabolism. No other biochemical parameters appear to be affected by
fensulfothion.
TOXICOLOGICAL STUDIES
Special studies on metabolites
Acute toxicity of fensulfothion metabolites and related products in
rats is shown in Table 1.
Special studies on mutagenicity
A dominant lethal test using groups of male mice (12 mice/group) was
conducted by treating the animals with fensulfothion by
intraperitoneal injection with 0, 0.5 and 1.0 mg/kg. For 6 consecutive
weeks treated males were placed in a cage with 3 untreated females per
week. The females were sacrificed at mid-pregnancy and examined for
reproduction defects; i.e., implantation sites, resorption sites and
live embryos. There were no significant differences between the test
and controls on any parameter examined. Fensulfothion does not affect
the mutation rate as evidenced by the mouse dominant lethal test
(Industrial Biotest Laboratory, 1971b).
 TABLE 1 Acute toxicity of fensulfothion metabolites and related
products in rats
LD50
(mg/kg) I501
ip
Name Formula M F (M)
Sulfide-P(S) R-S-CH3 2.52 3×10-4
5.5 1.53 5.4×10-4
O
Sulfoxide P(S) "
(fensulfothion) R-S-CH3 1.5 3×10-4
4.5 1.2 2.5×10-5
O
Sulfone P(S) "
R-S-CH3 1.6 5.1×10-7*
"
O
3.7 1.4 8.6×10 -5
Sulfide P(O) R1-S-CH3 2.0 1.6×10 -7
1.8 1.5 4.9×10 -7
O
"
Sulfoxide P(O) R1-S-CH3 1.2 1.6×10 -7
1.8 1.4 5.3×10 -8
O
"
Sulfone P(O) R1-S-CH3 0.9 4.8×10 -9
"
O
TABLE 1 Acute toxicity of fensulfothion metabolites and related
products in rats
LD50
(mg/kg) I501
ip
Name Formula M F (M)
Sulfide-P(S) R-S-CH3 2.52 3×10-4
5.5 1.53 5.4×10-4
O
Sulfoxide P(S) "
(fensulfothion) R-S-CH3 1.5 3×10-4
4.5 1.2 2.5×10-5
O
Sulfone P(S) "
R-S-CH3 1.6 5.1×10-7*
"
O
3.7 1.4 8.6×10 -5
Sulfide P(O) R1-S-CH3 2.0 1.6×10 -7
1.8 1.5 4.9×10 -7
O
"
Sulfoxide P(O) R1-S-CH3 1.2 1.6×10 -7
1.8 1.4 5.3×10 -8
O
"
Sulfone P(O) R1-S-CH3 0.9 4.8×10 -9
"
O
 1 Rat brain cholinesterase, molar concentration inducing
50% inhibitions.
2 Dubois & Kinoshita, 1964
3 Dubois & Jackson, 1967
* This extremely low value is probably due to contamination with the
P(O) analogue.
Special studies on neurotoxicity
Chickens were administered fensulfothion orally or by intraperitoneal
injection at dosage levels ranging from 0.005 to 0.05 gm/kg (the birds
were administered atropine and 2-PAM prior to fensulfothion). The hens
that survived the acute signs of poisoning did not show weakness or
ataxia. At the dosage levels tested fensulfothion does not induce a
delayed neurotoxic response, as seen with TOCP (Kimmerle, 1965b).
Chickens were fed fensulfothion in the diet at levels of 0, 1, 5, 20
and 100 ppm for 30 days. One day after treatment ended and again 30
days later hens were sacrificed and nerve tissue examined
histologically. Histological examination showed no evidence of
demyelination. The 100 ppm level caused death of half of the animals
and the survivors showed clinical signs of poisoning. Cholinesterase
inhibition in blood was depressed after 30 days of feeding but was
recovered after 4 weeks. There were no clinical or pathological signs
of delayed neurological disruption, as evidenced with TOCP (Kimmerle,
1965a; Grundmann, 1965).
Special studies on potentiation
Fensulfothion was administered intraperitoneally by simultaneous
administration of ´ LD50 doses to female rats in combination with 17
other anticholinesterase pesticides. There was no evidence of greater
than additive acute effects. This potentiation study was carried out
with parathion, malathion, EPN, phosdrin, ethion, fenchlorphos,
methylparathion, azinphos-methyl, chlorobenzilate, dithianon,
carbaryl, coumaphos, disulfoton, demeton, trithion, schradan, diazinon
(Dubois and Kinoshita, 1963).
Special studies on reproduction
Groups of mice (24 females and 6 males/group fed 0, 1, 5 and 20 ppm
and 40 females and 10 males/group for the first breeding and 24
females and 6 males for the second breeding fed 0, 2 and 4 ppm) were
fed fensulfothion and subjected to a standard 3-generation, 2-litter/
generation, reproduction study. The level of 20 ppm was lethal and was
discarded. At 5 ppm female mice of the Fo generation had an increased
mortality prior to mating. The survivors showed no effects of 5 ppm in
the diet on reproduction, gestation or lactation indices. A slight
reduction in lactation index was seen at 5 ppm in the F3b pups
surviving to weaning. Gross and microscopic examination of tissues of
the F3b groups showed no changes attributable to the inclusion of
fensulfothion in the diet. The effects of fensulfothion in the diet at
20 and 5 ppm, although these levels are toxic to mice, do not reflect
a hazard to reproduction (Doull et al., 1967).
Special studies on teratogenicity
Pregnant rabbits (9-11 rabbits/group) were administered fensulfothion
orally at dosage levels of 0.05 and 0.1 mg/kg per day on days to 16 of
gestation. On day 29 all animals were sacrificed and the young removed
by caesarean section. Fensulfothion did not affect pregnancy or fetal
mortality as indicated by the number of resorption sites, abortions or
dead fetuses. There was no evidence of abnormal fetal development from
gross observation or from examination of skeletal structure at 0.05
mg/kg/day. At 0.01 mg/kg/day there was a slight nonsignificant
increase in minor skeletal abnormalities. Fensulfothion is not
teratogenic to the rabbit (Industrial Biotest Laboratory, 1971a).
Acute toxicity
Acute toxicity of fensulfothion has been studied in several animal
species, a summary of results is given in Table 2.
TABLE 2 Acute toxicity of fensulfothion in animals
Species Sex Route LD50 Reference
(mg/kg)
Rat M oral 3.96-10.5 Dubois & Kinoshita, 1964
Kimerle, 1965c
Gaines, 1969
Solly & Harrison, 1971a
ip 5.5 Dabois & Kinoshita, 1964
Spencer, 1968
F oral 1-8-2.3 Dubois & Kinoshita, 1964
Kimmerle, 1965c
Gaines, 1969
Solly & Harrison, 1971a
F ip 0.9-1.5 Dubois & Kinoshita, 1964
Spencer, 1968
M dermal 14-30 Dubois & Kinoshita, 1964
Kimmerle, 1965c
Gaines, 1969
Rat F dermal 3.5-13 Dubois & Kinoshita, 1964
Kimmerle, 1965c
Gaines, 1969
TABLE 2 (Cont'd.)
Species Sex Route LD50 Reference
(mg/kg)
M inhalation 0.113 mg/l Kimmerle, 1966
(1 h)
0.030 mg/l
(4 h)
Mouse M ip 10.5 Dubois & Kinoshita, 1964
F ip 7.0 Ibid.
Guinea M oral 9.0 Ibid.
Pig
M ip 5.4 Ibid.
Sheep F oral 3.4 Solly Harrison, 1971a
Chick M oral 0.99 Sherman & Ross, 1961
Chicken p oral & ip 2.5-5.0 Kimmerle, 1965b
Signs of poisoning following acute intoxication are typical of other
organophosphate esters capable of inducing cholinergic signs of
poisoning. Signs of poisoning occur rapidly and animals return to
normal within 24 hours (Dubois & Kinoshita, 1964).
The administration of atropine (100 mg/kg) 10 minutes before
intraperitoneal administration of fensulfothion increased the LD50
from 1.5 to 2.0 mg/kg. Intraperitonal injection of PAM (100 mg/kg)
immediately after fensulfothion raised the LD50 to 6 mg/kg. Treatment
with both agents resulted in an LD50 of 7 mg/kg (Dubois & Kinoshita,
1964). Following oral administration of fensulfothion, atropine and
combinations of atropine and PAM or atropine and BH-6 were shown to
increase the LD50 values (Kimmerle, 1966). Atropine in combination
with reactivators of cholinesterase are effective antidotal agents for
the acute toxic effects of fensulfothion.
Short-term studies
Rat
Groups of female rats (5/group) were administered fensulfothion by
intraperitoneal injection daily for 60 days at dosage levels of 0,
0.25, 0.50 and 0.75 mg/kg. Mortality occurred at the highest dose with
5 of 5 rats dead within one week. At 0.5 mg/kg/day, 4 of the 5 rats
survived the treatment. All rats showed significantly reduced growth
rates and reduced brain, serum and submaxillary gland cholinesterase
activity (Dubois & Kinoshita, 1964).
Groups of female rats (25 rats/group) were fed fensulfothion for 8
weeks at dosage levels of 0, 0.5, 1 and 2 ppm. RBC cholinesterase
inhibition was observed at 2 ppm while plasma and brain levels were
unaffected. No cholinesterase depression was observed at lower dose
levels. Mortality, growth, hematology and clinical chemistry
parameters were normal (Root et al., 1969).
Dog
Groups of dogs (2 males and 2 females/group) were fed fensulfothion in
the diet at levels of 0, 1, 2, 5 and 10 ppm for 12 weeks. Other groups
fed at levels of 5 and 10 ppm displayed cholinergic signs of poisoning
and were removed from the study. Inhibition of cholinesterase was
observed in serum and RBC at 2 ppm and above. At 5 ppm and 10 ppm
signs of cholinergic poisoning and weight loss were observed (Root
et al., 1964).
Groups of dogs (2 males and 2 females/group) were fed fensulfothion in
the diet at levels of 0, 1, 2 and 5 ppm for 2 years. Severe weight
loss and reduced food consumption accompanied by signs of cholinergic
poisoning were evident in the early weeks of the study at 5 ppm. After
the second month, food consumption increased and lost body weight was
regained. Slight cholinergic signs at 2 ppm were also observed at the
beginning of the study. Reduction of serum and RBC cholinesterase was
evident at 5 ppm throughout the study. Slight reduction was observed
at 2 ppm with no effects noted at 1 ppm. No mortality was incurred as
a result of feeding fensulfothion, and hematology and gross and
histopathologic examination of tissues and organs were normal (Doull
et al., 1966a).
Long-term studies
Rat
Groups of rats (24 male and 27 female/group) were fed fensulfothion in
the diet for 17 months at dietary levels of 0, 1, 5 and 20 ppm.
Mortality of males was increased at 5 and 20 ppm. Growth of males and
females was slightly impaired at 20 ppm. Cholinesterase depression was
observed to be dose dependent in serum (19%), RBC (21%), submaxillary
gland (18%) and brain (24%) in females at 1 ppm and above and in males
at 5 ppm and above. No effects were observed on gross and histological
examination of organs and tissues (Doull et al., 1966b).
COMMENT
Fensulfothion is acutely very toxic to mammals. The compound is
metabolized similarly in animals and plants to substances of greater
toxicity by oxidation of both the enolic leaving group and the
phosphorothionate moiety. No effects on reproduction in rats,
neurotoxicity in hens, mutagenicity or teratogenicity at low levels in
rodents, or potentiation with other organophosphate compounds have
been observed.
A no-effect level in long-term studies in rats has not been
established. In a 17-month study cholinesterase depression at 1 ppm in
plasma, RBC and brain was observed. By plotting effect versus dose, a
theoretical no-effect level of 0.5 ppm (0.025 mg/kg/day) may be
estimated. Somatic effects were not noted up to levels of about five
times this dose. In a 2-year study in dogs, no adverse effects at 1
ppm were observed.
No data are reported on the effects of fensulfothion in man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 1.0 ppm in the diet, equivalent to 0.025 mg/kg/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0003 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fensulfothion is a systemic organo-phosphorous nematicide and
insecticide, which is used against soil nematodes (free living, root
knot and cyst forming nematodes) and a broad spectrum of soil borne
insects in field crops, vegetables and fruit. It is also used against
nematodes in turf grasses, in flowers and in ornamentals. In most
cultures the material is applied before planting or sowing or at
planting; in some cultures fensulfothion is applied in the soil in
established cultures.
Fensulfothion is used a.o. in Canada, Germany, Japan, New Zealand and
the Philippines.
Pre-harvest treatments
Table 3 summarizes the recommendations in accordance with good
agricultural practice, including rates and methods of application
(applications to soil at or near sowing or planting time; broadcast
application or band treatment).
TABLE 3 Officially recommended and registered uses of fensulfothion
Country and Crop Pest Dosage
(a.i.) Application time Restrictions
or pre-harvest
interval
Canada1
Brassicas 15 g/100 m row at planting or
(broccoli, max. 2.5 kg/ha shortly after
Brussels sprouts, planting
cabbage, cauliflower,
rutabagas, turnips)
Maize corn root worm 6-12 g/100 m row at planting Do not feed or ensile
(=0.5-1 kg/ha) treated forage
Potatoes Colorado beetle 5.0 kg/ha pre-plant broadcast
wireworms 100 days interval
tuber flea beetle
larvae
Ctenicera spp.
Cuba, Ecuador
Banana 3-4.5 g/tree established tree
Germany (W)2
Sugarbeet beetfly 1.25-2.5 kg/ha at sowing
(Pegomya hyoscyami)
centipedes
Philippines
Rice (paddy)
in flooded fields 1 kg/ha 15 days after broadcast
planting
TABLE 3 (Cont'd.)
Country and Crop Pest Dosage
(a.i.) Application time Restrictions
or pre-harvest
interval
2 kg/ha (2x) 45 and 75 days broadcast, granular
after planting application to paddy
water
U.S.A.3
Field corn corn root worm 0.5.1 kg/ha at planting
40 inch row spacing at planting
Popcorn
Sweet corn 12 g/100 m row at planting
Onions onion maggot 1 kg/ha in furrow at Do not apply to green
(dry bulb) planting bunch onion.
Peanuts nematodes 2-4 kg/ha band, at planting Do not feed vines and
and/or at peggig hay to livestock.
southern corn
rootworm 2-4 g/100 m row Do not apply more than
7 kg/ha in one year
Pineapple nematodes 50 kg/ha broadcast
nematodes 50 kg/ha (drench) pre-plant
Potatoes 2-5.0 kg/ha pre-plant broadcast of granules
Sugarbeet 1-2 kg/ha at sowing
15 g/100 m row at sowing
TABLE 3 (Cont'd.)
Country and Crop Pest Dosage
(a.i.) Application time Restrictions
or pre-harvest
interval
Sugarcane nematodes 2-5 kg/ha at planting band (40-45 cm)
Tobacco wireworms, 2-10 kg/ha pre-plant broadcast
nematodes
Tomatoes nematodes 3.2-6.4 kg/ha pre-plant band
10-20 kg/ha broadcast
1 Canada Dept. of Agriculture Plant products Division, Production & Marketing Branch
Use Claim for pesticides registered under the Pest Control Products Act May, 1971 & Jan. 1972, No. 834-128.
2 Pflanzenschutamittel-Verzeichnis, 23 Auflage, April 1972, Biologischen Bundesanstalt für Land - und
Forstwirtschaft Braunschweig.
3 USDA summary of Registered Agricultural Pesticide Chemical uses III D 22 5-31-69; 8-14-70, 3-24-72.
Post-harvest treatments
Fensulfothion is not recommended for post-harvest treatments.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in
different countries on food crops grown under various conditions. In
most cases dosage rates were applied in accordance with label
instructions. However, in some experiments higher dosages were also
included. Data from these trials are summarized in table 4.
TABLE 4 Residues in crops treated with fensulfothion
Crop Rate of application No. of Pre-harvest Net Residue
(a.i.) applications interval (ppm)
(days)
bananas 3.0 - 4.5 g/tree 1 1 - 90 <0.01 - 0.02
corn 1.2 - 3.2 kg/ha 2 21 - 67 <0.05
0.18 - 0.27 g/row )
plus 1.1 - 1.7 kg/ha ) 2 + 1 29 - 31 <0.01
cotton seed 0.55 - 0.82 g/m row 1 157 - 206 <0.01
onions 0.05 - 0.23 g/m row 1 80 - 191 <0.05
pineapple 60 - 200 kg/ha (drench) 1 561 - 973 <0.01
potatoes 7 - 8 kg/ha 1 102 - 150 <0.05
rutabagas 1.1 - 2.7 kg/ha 2 - 6 19 - 84 <0.01 - 0.05
soybeans 0.18 - 0.27 g/m row 1 71 - 119 <0.01
sugar beets 2 - 4 kg/ha 1 177 - 182 <0.01 - 0.10
0.12 - 2 g/m 1-3 58 - 196 <0.03
sugar cane 6 - 8 kg/ha (40 cm band) 1 228 - 343 <0.01 - 0.02
sweet potatoes 6.7 - 11.8 kg/ha 1 101 - 189 <0.01 - 0.05
tobacco 2 - 16 kg/ha 1 53 - 159 0.1 - 10.5
tomatoes 20 - 30 kg/ha 1 34 - 121 <0.05
The residues in these trials were predominantly determined by GLC
utilizing thermionic detection. Included in the analysis are total
toxic (cholinesterinase inhibiting) metabolites. Samples were taken at
harvest and immediately frozen until analysis. Bananas were stored
several days at room temperature before freezing.
In a ryegrass/white clover mixture the residue decreased from 90 ppm
(dry weight) immediately after application to 20 ppm after 13 days and
12, 4 and 1 ppm after 27, 55 and 90 days, respectively (Brewerton,
1971). Similar figures are given by Solly (1968) from grassland in New
Zealand.
FATE OF RESIDUES
General comments
The breakdown of fensulfothion in plants and animals seem identical
since the same metabolites have been identified in both.
In animals and animal products
In milk from cows grazing in pasture containing mainly residues of
fensulfothion sulfone, the oxygen analogue sulfone was detected. An
oxidative pathway in the ruminant appears most likely. Milk from dairy
cattle grazing for 10 days on pasture with an initial residue of 41
ppm fensulfothion contained 0.02 ppm oxygen analogue sulfone on the
third day, which decreased to 0.01 ppm on the 10th day (Solly
et al., 1971c).
Omental fat of sheep grazing for six days in pasture containing 43 ppm
initial residue of fensulfothion, showed a residue of 0.003 ppm,
although the erythrocyte cholinesterase depression was approximately
80% (Solly et al., 1971b).
In plants
The systemic action of fensulfothion was first demonstrated after
topical application stems of cotton plants (Benjamini et al.,
1959b). Katague and Anderson (1967) studied the fate of fensulfothion
after stem injection in cotton with 32P fensulfothion. They
identified the following metabolites by TLC with authentic standards:
O,O diethyl-O-[4-(methyl sulfinyl) phenyl)] phosphate (= oxygen
analogue) and O,O diethyl-[-4-(methylsulfonyl) phenyl]
phosphorothionate (sulfone).
After root uptake traces of O,O-diethyl-O-[-4 methyl sulfonyl
phenyl] phosphate (oxygen analogue sulfone) were found. The S-ethyl
analogue was not detected in either study.
After stem injection of 32P fensulfothion in maize, beans and cotton
the S-ethyl analogue could not be detected, but the previously
mentioned three metabolites were found (Everett and Gronberg, 1967).
Thornton (1967) analysed maize fodder from field samples by GLC for
fensulfothion and its metabolites. The interval for harvest was 40-121
days. The percent distribution of fensulfothion and metabolites was:
fensulfothion: 0-38% (average 16%), sulfone: 0-34% (average 12%),
oxygen analogue: 14-68% (average 45%), oxygen analogue sulfone: 8-49%
(average 27%).
Thornton (1968) showed the presence of 4-methylsulfonyl phenol in
maize fodder, but none in the maize kernels and cobs. Solly and
Harrison (1971a) found the sulfone as a major metabolite in pasture
grass, with only traces of the oxygen analogue and the oxygen analogue
sulfone. The same main metabolites were found in cured tobacco (Olson,
1972).
In soil
In soil the breakdown of fensulfothion and metabolites is rather
rapid. Duffy (1968) found experimentally that one half of the
fensulfothion in soil was degraded in 14 days. In other tests, in
which fensulfothion was incorporated in the top six inches of soil,
residues were determined over a period of one year. The time required
to decrease the residue to one half of the initial concentration was
generally less than 30 days; in another test it was slightly longer
than 180 days (Chemagro, 1968).
In storage and processing (cooking)
Thornton (1971) treated cotton seed oil fortified with 5 ppm
fensulfothion and the oxygen analogue with a laboratory steam
stripping similar to the process used commercially. The oil was heated
to 230 - 240°C and steam was passed through it for 3 hours. Analysis
of the processed oil showed that 48% of fensulfothion and 75% of the
oxygen analogue was swept away or degraded. The steam was scrubbed
with chloroform; 5 - 10% of the original 5 ppm were trapped and
identified as unchanged fensulfothion or oxygen analogue.
Katague (1968) simulated the refining of raw sugar beets to
concentrated syrup after fortifying the beets with 32P fensulfothion
and unlabelled metabolites. In the concentrated syrup the loss of
residue was 96 - 100% for fensulfothion, sulfone, oxygen analogue and
oxygen analogue sulfone. It was determined that the maximum total
concentration expected in the wet pulp would be no more than 5.5% of
the concentration in the raw beet.
In fresh harvested rutabagas stored 20 - 25 days at 4°C the total
residue of fensulfothion and metabolites decreased 50 - 92%. Cooking
reduced the residues by 48 - 68%. From these figures it may be
concluded that rutabagas, treated at recommended rates and stored for
a few weeks and then cooked, would contain no detectable residues.
Studies have been carried out to determine the extent of carry over of
residues in the tobacco smoke. Olson (1965) found 1.6% of
fensulfothion added to the tobacco in the smoke. After fortification
of cigarette tobacco with fensulfothion and subsequent analysis of the
solvent used for scrubbing the smoke, 7.9% recovery of unchanged
fensulfothion was shown in the smoke (Katague, 1966).
Olson (1968) fortified separate samples with fensulfothion and
metabolites. Recoveries in the smoke were 3.5, 3.0, 2.4 and 1.4%
respectively of fensulfothion, sulfone, oxygen analogue and oxygen
analogue sulfone.
During frozen storage of onions, potatoes and turnips, residues were
stable for 770 days, in cowpea vines for at least 98 days (Chemagro,
1967, 1965).
Evidence of residues in food in commerce or at consumption
No data available.
METHODS OF RESIDUE ANALYSIS
Gas chromatographic methods for analysis of fensulfothion and
metabolites are the methods of choice for residue analysis of various
crops, animal tissue and milk. GLC methods utilizing a KCl thermionic
detector proved to be particularly suitable for regulatory purposes.
Methods have been developed and adapted for analysis of maize,
peanuts, vegetables, forage crops and oil crops (Katague and Olson,
1969; Olson, 1970). The course of the analysis may be summarized as
extraction of the crop with acetone followed by partitioning with
chloroform.
The dry residue from the extract is oxidized with m-chloroperbenzoic
acid, which converts fensulfothion, sulfone and oxygen analogue to
oxygen analogue sulfone. This is further purified by solvent
partitioning and injected into the gas chromatograph. Residues of
fensulfothion and its three metabolites are measured as a single peak
as oxygen analogue sulfone. The method is specific for fensulfothion
and its metabolites. Twenty-three insecticides and metabolites
containing phosphorus were shown not to interfere (Olson, 1971).
Limit of detection of the method is 0.05 ppm or lower for
fensultothion and metabolites. Recoveries in various crop samples, in
cattle and in milk, ranged respectively from 71 - 86%, 102 - 109% and
96 - 116%.
GLC methods have been developed for the determination of individual
metabolites (Williams et al., 1971; Bowman and Hill, 1971). These
methods are less suitable for regulatory purposes than the method
described above.
Multidetection GLC methods for determining residues of
organo-phosphorous compounds in various crop samples were investigated
by the U.S. Food and Drug Administration. Fensulfothion and its three
metabolites were included in the study.
Watts et al. (1969) described a new charcoal liquid chromatography
procedure for improved clean-up of 60 organo-phosphorous compounds
including fensulfothion. Bowman and Beroza (1971) determined retention
times for 146 organo-phosphorous or sulphur compounds, including
fensulfothion, using a column packed with Dexsil 300
(polycarborane-siloxane) on HCl washed chromosorb W. The column can
be purged at temperatures up to 400°C.
Gas chromatic methods for determination of fensulfothion and
metabolites in soil have been developed (Olson, 1968; Katague, 1966).
NATIONAL TOLERANCES
Examples of national tolerances of fensulfothion residues are reported
in Table 5.
APPRAISAL
Fensulfothion is a systemic organophosphorous nematicide and
insecticide which is used on a considerable scale in various countries
on a relatively wide range of crops. Main uses are as soil treatment,
either broadcast or band treatment, or as a drench, against soil borne
nematodes (free living, root knot and cyst forming nematodes) and a
considerable range of soil borne insects.
Technical fensulfothion contains 94-96% of the pure compound. The
impurities in the technical material are known. The main components
are: O,O-diethyl-O-[4-(methylthio)-phenyl]-phosphorothioate
(1-3%) and 4-(methylsulfinyl)-phenol (0.8-1.0%).
Fensulfothion is marketed in different formulations, i.e. granular 3,
5, 10 and 15% and emulsifiable liquid (720 g/l).
The concentration/rates of application vary depending on pest, crop
and method of application; "normal" application rates are 1-5 kg a.i.
per ha.
TABLE 5 Examples of national tolerances reported to the Meeting1
Country Commodity Tolerance
(ppm)
U.S.A. peanut hulls 5
maize forage and fodder of field corn,
pop corn and sweet corn 1
maize grain, including field corn and
pop corn (kernels); fresh maize
(including sweet corn), kernels plus
cobs, with husks removed; onions,
(dry); potatoes; rutabagas (roots) and
tomatoes 0.1
peanuts (shelled); pineapple, pineapple
forage, sugar beets (roots and tops) 0.05
bananas (whole); sugarcane; meat, fat and
meat by-products of cattle, goats and sheep 0.02
Canada Brassicas (broccoli, Brussels sprouts,
cabbage, cauliflower); rutabagas;
turnips, potatoes; maize no residue
Germany, No official tolerances established.
Federal For the following crops tolerances
Republic are being considered:
sugarbeet.
New Zealand all food crops 0.1
1 Fensulfothion and cholinesterase inhibiting metabolites in or on the
commodities.
The residue data available were obtained from different countries and
regions with different climatic and soil conditions. The residue data
presented for fensulfothion included the three metabolites, sulfone,
oxygen analogue and oxygen analogue sulfone, and are with a few
exceptions representative for those likely to occur in conditions of
good agricultural practice.
Information is available on the fate of fensulfothion residues in
soil, in plants and to a lesser extent in animal products after
feeding animals on treated pasture or with treated crops.
Approved uses on peanuts and pineapple give rise to residues in peanut
kernels and in pineapple not exceeding 0.05 ppm (the proposed
tolerances). Hulls of peanuts and pineapple forage from such treated
crops will not contain residues in excess of 5 ppm and 0.05 ppm,
respectively. Likewise, the residue in sugarcane will not exceed 0.02
ppm. A soil application of fensulfothion in maize (including pop corn
and sweet corn), giving rise to residues in the kernels not exceeding
the proposed tolerance of 0.1 ppm, will result in residues in the
forage of 1 ppm or lower. Feeding of the materials mentioned in this
paragraph as a part of the ration for cattle or sheep will not give
rise to residues in meat or milk above the limit of determination
(i.e. 0.01 ppm).
Residues which may occur in food either from plant or animal origin,
after observing the recommended directions of use and the recommended
harvest intervals, consist largely of the oxygen analogue and the
oxygen analogue sulfone and to a smaller extent the parent chemical
and the sulfone. The breakdown products mentioned above could be
identified in radio-labelled studies and confirmed with other relevant
methods of analysis, e.g. TLC.
The breakdown of fensulfothion in plants and animals follows an
identical pattern; the same metabolites have been identified in both.
Information is available on the rate of decrease of the residue of
fensulfothion and metabolites in some crops and commodities during
storage and processing, including household cooking. In addition,
information on the extent of carryover of residues in tobacco smoke
from residues initially applied on tobacco was presented.
Little information is available on fensulfothion residues in food in
commerce.
Gas chromatographic procedures are available for specific
determination of fensulfothion and its main metabolites, or all
compounds combined and measure as a single peak as the oxygen analogue
sulfone. These methods are suitable for regulatory purposes as
required. Recommendations are given for the most appropriate
extraction and clean up procedures in food products of plant and
animal origin. The limit of determination is 0.05 ppm for all crops
and animal tissue and 0.01 ppm for milk.
RECOMMENDATIONS
TOLERANCES
The following tolerances are recommended for fensulfothion, including
the metabolites (oxygen analogue, oxygen analogue sulfone and
sulfone).
ppm
Maize grain, including kernels of field
corn and pop corn, onions, potatoes,
rutabagas (roots), tomatoes 0.1
Peanuts (shelled), pineapple, sugarbeet 0.05
Bananas (whole fruit) 0.02
Fat of meat and edible offal of cattle,
goats and sheep 0.02
FURTHER WORK OR INFORMATION
DESIRABLE
1. Teratogenicity studies at higher dosage levels.
2. Studies on human exposure.
REFERENCES
Benjamini, E., Metcalf, R.L. and Fukuto, T.R. (1959a) The chemistry
and mode of action of the insecticide O,O-diethyl
O-p-methylsulfinyl-phenyl phosphorothionate and its analogues. J.
Econ. Ent., 52: 94-98.
Benjamini, E., Metcalf, R.L. and Fukuto, T.R. (1959b) Contact and
systemic insecticidal properties of O,O-diethyl O,O-diethyl
O-p-methylsulfinyl-phenyl phosphorothionate and its analogues. J.
Econ. Ent., 52: 99-102.
Bowman, M.C. and Beroza, M. (1971) Use of Dexsil 300 on a specially
washed Chromosorb W for multicomponent residue determinations of
phosphorus- and sulfur-containing pesticides by flame photometric GLC.
Ass. off. analyt. Chem., 54: 1086-1092.
Bowman, M.C. and Hill, K.R. (1971) Determination of Dasanit and three
of its metabolites in corn, grass and milk. J. Agr. Fd. Chem., 19:
342-345.
Brewerton, H.V., Gibbs, M.M. and Perrott, D.C.F. (1971) Fensulfothion
and BAY 37289 residues on pasture. N.Z.J. Agric. Res., 11: 303-312.
Chemagro Division of Baychem. Corp. (1965, 1967, 1968) Reports 21252,
23119-23122, 23124, 23125, 23136. (unpublished)
Doull, J., DiGiacomo, R., Root, M. and Meskauskas, J. (1966a) Chronic
oral toxicity of BAYER 25141 to dogs. Report by the University of
Chicago submitted by Farbenfabriken Bayer, AG. (unpublished)
Doull, J., DiGiacomo, R., Root, M. and Meskauskas, J. (1966b) Chronic
oral toxicity of Bayer 25141 to rats. Report by the University of
Chicago submitted by Farbenfabriken Bayer, AG. (unpublished)
Doull, J., Root, M. and DiGiacomo, R. (1967) Effect of BAYER 25141 in
the diet on the reproduction and lactation of mice. Report by the
University of Chicago submitted by Farbenfabriken Bayer, AG.
(unpublished)
Dubois, K.P. and Jackson, P. (1967) Comparison of the acute toxicity
and anticholinesterase action of BAYER 25141 and some possible
metabolites. Report by the University of Chicago submitted by
Farbenfabriken Bayer, AG. (unpublished)
Dubois, K.P. and Kinoshita, F. (1963) The acute toxicity of BAYER
25141 in combination with other anticholinesterase insecticides.
Report by the University of Chicago submitted by Farbenfabriken Bayer,
AG. (unpublished)
Dubois, K.P. and Kinoshita, F. (1964) Acute toxicity and
anticholinesterase action of O,O-diethyl-O-p-(methylsulfinyl)
phenyl phosphorothioate (DMSP) and some related compounds. Tox. Appl.
Pharmacol., 6: 78-85.
Duffy, J.R. (1968) The metabolism of zinophos and BAY 25141 in soils.
Report on extra-mural research project 167. (unpublished)
Everett, L.J. and Gronberg, R.R. (1967) Chemagro Division of Baychem.
Corp., Kansas City, Mo. Metabolism of P32-labelled Dasanit in beans,
corn and cotton. Chemagro Report no. 21513. (unpublished)
Everett, L.J. (1968) Rat metabolism of P32 labelled Dasanit. Report
of the Chemagro Corporation submitted by Farbenfabriken Bayer, AG.
(unpublished)
Gaines, T.B. (1969) Acute toxicity of pesticides. Tox. Appl.
Pharmacol., 14: 515-534.
Grundmann, E. (1965) Histologische Untersuckung/S767. Report by
Institut für Experimentelle pathologie, submitted by Farbenfabriken
Bayer, AG. (unpublished)
Homeyer, B. (1970) Zun gegenwärtigen Stand der Bekämpfung von
Bodeninsekten. Pflanzenschutz-Nachrichten-Bayer, 23/1970: 233-239.
Homeyer, B. (1971) Terracur(R), ein breit wirkendes Boden insektizid
und Nematizid. Pflanzenschutz-Nachrichten Bayer, 24/1971: 371-410.
Industrial Biotest Laboratory. (1971) Teratogenic study with Dasanit
technical in albino rabbits. Report by the Industrial Biotest
Laboratory submitted by Farbenfabriken Bayer, AG. (unpublished)
Industrial Biotest Laboratory. (1971b) Mutagenic study with Dasanit in
albino mice. Report by the Industrial Biotest Laboratory submitted by
Farbenfabriken Bayer, AG. (unpublished)
Jung, H.F. and Iwaya, K. (1971) Terracur P(R), ein Insektizid und
Nematizid mit besonderer Eignung für den Reisanbau.
Pflanzenschutz-Nachrichten Bayer, 24/1971: 489-504.
Katague, D.B. (1966) Chemagro Division of Baychem. Corp. Reports nos.
17885, 17886, 18320, 18714. (unpublished)
Katague, D.B. and Anderson, C.A. (1967) Metabolism of P32-labelled
Dasanit in cotton plants. Bull. Env. Cont. Tox., 2: 228-235.
Katague, D.B. (1968) Chemagro Division of Baychem. Corp. Effect of
processing on Dasanit residues in sugar beets. Report no. 23015.
(unpublished)
Katague, D.B. and Olson, T.J. (1969) Chemagro Division of Baychem.
Corp. Determination of residues of Dasanit (BAY 25141) in corn by
thermionic emission gas chromatography. Report No. 20076.
(unpublished)
Kimmerle, G. (1965a) Neurotoxische Unkersuchungen Mit S-767-Wirkstoff.
Report by Institut für Toxikologie, Farbenfabriken Bayer, AG.
(unpublished)
Kimmerle, G. (1965b) Akute neurotoxizitatsuntersuchungen an huhnern.
Report by Institut für Toxikologie, Farbenfabriken Bayer, AG.
(unpublished)
Kimmerle, G. (1965c) S-767 (Production No. 3553) and Product 5121
(Production No. 3562). Report by Institut für Toxikologie,
Farbenfabriken Bayer, AG. (unpublished)
Kimmerle, G. (1966) S-767 Batch 1/64 Lo-Nr 214/antidotwirkung. Report
by Institut für Toxikologie, Farbenfabriken Bayer, AG. (unpublished)
Leuck, D.B. and Bowman, M.C. (1972) Persistence of residues of Dasanit
and its metabolites in coastal bermudagrass, forage corn and corn
silage. J. Econ. Ent., 65: 257-260.
Olson, T.J., (1965, 1968, 1969, 1970, 1971, 1972) Chemagro Division of
Baychem. Corp. Reports nos. 15188, 34051, 25464, 34164, 23055, 23037,
30278.
Proude, C.K. Ergebnisse sechsjähriger Arbeit mit Terracur P(R) in
Neuseeland. Pflanzenschutz-Nachrichten Bayer, 24/1971: 505-526.
Read, D.C. (1971) Bioactivity of Dasanit in a mineral soil, in
rutabagas in the field and in storage and effect of cooking on
toxicants in the roots. J. Econ. Ent., 64: 597-601.
Root, M., Taitel, C. and Doull, J. (1964) Subacute oral toxicity of
BAYER 25141 in male and female dogs. Report by The University of
Chicago, submitted by Farbenfabriken Bayer, AG. (unpublished)
Root, M., Kinoshita, F. and Flynn, M. (1969) Subacute oral toxicity of
Dasanit to female rats. Report by the University of Chicago, submitted
by Farbenfabriken Bayer, AG. (unpublished)
Thornton, J.S. and Olson, T.J. (1970) Chemagro Division of Baychem.
Corp. Determination of Dasanit residues in animal tissue by thermionic
emission flame gas chromatograhpy. Report no. 20750. (unpublished)
Thornton, J.S., (1971) Chemagro Division of Baychem Corp. Effect of
oil deodorization process on residues of Dasanit and Dasanit oxygen
analogue in cottonseed oil (simulated). Report no. 30299.
(unpublished)
Tucker, R.K. and Crabtree, D.G. (1970) Handbook of toxicity of
pesticides to wildlife. USDI. Resource Publication no. 84.
Watts, R.R., Storherr, R.W., Pardue, J.R. and Osgood, T. (1969)
Charcoal column clean-up method for many organo-phosphorus pesticide
residues in crop extracts. J. Ass. off. analyt. Chem., 52: 522-526.
Williams, I.H., Kore, R. and Finlayson, D.G. (1971) Determination of
residues of Dasanit and three metabolites by gas chromatography with
flame photometric detection. J. Agr. Fd. Chem., 19: 456-458.
1 Rat brain cholinesterase, molar concentration inducing
50% inhibitions.
2 Dubois & Kinoshita, 1964
3 Dubois & Jackson, 1967
* This extremely low value is probably due to contamination with the
P(O) analogue.
Special studies on neurotoxicity
Chickens were administered fensulfothion orally or by intraperitoneal
injection at dosage levels ranging from 0.005 to 0.05 gm/kg (the birds
were administered atropine and 2-PAM prior to fensulfothion). The hens
that survived the acute signs of poisoning did not show weakness or
ataxia. At the dosage levels tested fensulfothion does not induce a
delayed neurotoxic response, as seen with TOCP (Kimmerle, 1965b).
Chickens were fed fensulfothion in the diet at levels of 0, 1, 5, 20
and 100 ppm for 30 days. One day after treatment ended and again 30
days later hens were sacrificed and nerve tissue examined
histologically. Histological examination showed no evidence of
demyelination. The 100 ppm level caused death of half of the animals
and the survivors showed clinical signs of poisoning. Cholinesterase
inhibition in blood was depressed after 30 days of feeding but was
recovered after 4 weeks. There were no clinical or pathological signs
of delayed neurological disruption, as evidenced with TOCP (Kimmerle,
1965a; Grundmann, 1965).
Special studies on potentiation
Fensulfothion was administered intraperitoneally by simultaneous
administration of ´ LD50 doses to female rats in combination with 17
other anticholinesterase pesticides. There was no evidence of greater
than additive acute effects. This potentiation study was carried out
with parathion, malathion, EPN, phosdrin, ethion, fenchlorphos,
methylparathion, azinphos-methyl, chlorobenzilate, dithianon,
carbaryl, coumaphos, disulfoton, demeton, trithion, schradan, diazinon
(Dubois and Kinoshita, 1963).
Special studies on reproduction
Groups of mice (24 females and 6 males/group fed 0, 1, 5 and 20 ppm
and 40 females and 10 males/group for the first breeding and 24
females and 6 males for the second breeding fed 0, 2 and 4 ppm) were
fed fensulfothion and subjected to a standard 3-generation, 2-litter/
generation, reproduction study. The level of 20 ppm was lethal and was
discarded. At 5 ppm female mice of the Fo generation had an increased
mortality prior to mating. The survivors showed no effects of 5 ppm in
the diet on reproduction, gestation or lactation indices. A slight
reduction in lactation index was seen at 5 ppm in the F3b pups
surviving to weaning. Gross and microscopic examination of tissues of
the F3b groups showed no changes attributable to the inclusion of
fensulfothion in the diet. The effects of fensulfothion in the diet at
20 and 5 ppm, although these levels are toxic to mice, do not reflect
a hazard to reproduction (Doull et al., 1967).
Special studies on teratogenicity
Pregnant rabbits (9-11 rabbits/group) were administered fensulfothion
orally at dosage levels of 0.05 and 0.1 mg/kg per day on days to 16 of
gestation. On day 29 all animals were sacrificed and the young removed
by caesarean section. Fensulfothion did not affect pregnancy or fetal
mortality as indicated by the number of resorption sites, abortions or
dead fetuses. There was no evidence of abnormal fetal development from
gross observation or from examination of skeletal structure at 0.05
mg/kg/day. At 0.01 mg/kg/day there was a slight nonsignificant
increase in minor skeletal abnormalities. Fensulfothion is not
teratogenic to the rabbit (Industrial Biotest Laboratory, 1971a).
Acute toxicity
Acute toxicity of fensulfothion has been studied in several animal
species, a summary of results is given in Table 2.
TABLE 2 Acute toxicity of fensulfothion in animals
Species Sex Route LD50 Reference
(mg/kg)
Rat M oral 3.96-10.5 Dubois & Kinoshita, 1964
Kimerle, 1965c
Gaines, 1969
Solly & Harrison, 1971a
ip 5.5 Dabois & Kinoshita, 1964
Spencer, 1968
F oral 1-8-2.3 Dubois & Kinoshita, 1964
Kimmerle, 1965c
Gaines, 1969
Solly & Harrison, 1971a
F ip 0.9-1.5 Dubois & Kinoshita, 1964
Spencer, 1968
M dermal 14-30 Dubois & Kinoshita, 1964
Kimmerle, 1965c
Gaines, 1969
Rat F dermal 3.5-13 Dubois & Kinoshita, 1964
Kimmerle, 1965c
Gaines, 1969
TABLE 2 (Cont'd.)
Species Sex Route LD50 Reference
(mg/kg)
M inhalation 0.113 mg/l Kimmerle, 1966
(1 h)
0.030 mg/l
(4 h)
Mouse M ip 10.5 Dubois & Kinoshita, 1964
F ip 7.0 Ibid.
Guinea M oral 9.0 Ibid.
Pig
M ip 5.4 Ibid.
Sheep F oral 3.4 Solly Harrison, 1971a
Chick M oral 0.99 Sherman & Ross, 1961
Chicken p oral & ip 2.5-5.0 Kimmerle, 1965b
Signs of poisoning following acute intoxication are typical of other
organophosphate esters capable of inducing cholinergic signs of
poisoning. Signs of poisoning occur rapidly and animals return to
normal within 24 hours (Dubois & Kinoshita, 1964).
The administration of atropine (100 mg/kg) 10 minutes before
intraperitoneal administration of fensulfothion increased the LD50
from 1.5 to 2.0 mg/kg. Intraperitonal injection of PAM (100 mg/kg)
immediately after fensulfothion raised the LD50 to 6 mg/kg. Treatment
with both agents resulted in an LD50 of 7 mg/kg (Dubois & Kinoshita,
1964). Following oral administration of fensulfothion, atropine and
combinations of atropine and PAM or atropine and BH-6 were shown to
increase the LD50 values (Kimmerle, 1966). Atropine in combination
with reactivators of cholinesterase are effective antidotal agents for
the acute toxic effects of fensulfothion.
Short-term studies
Rat
Groups of female rats (5/group) were administered fensulfothion by
intraperitoneal injection daily for 60 days at dosage levels of 0,
0.25, 0.50 and 0.75 mg/kg. Mortality occurred at the highest dose with
5 of 5 rats dead within one week. At 0.5 mg/kg/day, 4 of the 5 rats
survived the treatment. All rats showed significantly reduced growth
rates and reduced brain, serum and submaxillary gland cholinesterase
activity (Dubois & Kinoshita, 1964).
Groups of female rats (25 rats/group) were fed fensulfothion for 8
weeks at dosage levels of 0, 0.5, 1 and 2 ppm. RBC cholinesterase
inhibition was observed at 2 ppm while plasma and brain levels were
unaffected. No cholinesterase depression was observed at lower dose
levels. Mortality, growth, hematology and clinical chemistry
parameters were normal (Root et al., 1969).
Dog
Groups of dogs (2 males and 2 females/group) were fed fensulfothion in
the diet at levels of 0, 1, 2, 5 and 10 ppm for 12 weeks. Other groups
fed at levels of 5 and 10 ppm displayed cholinergic signs of poisoning
and were removed from the study. Inhibition of cholinesterase was
observed in serum and RBC at 2 ppm and above. At 5 ppm and 10 ppm
signs of cholinergic poisoning and weight loss were observed (Root
et al., 1964).
Groups of dogs (2 males and 2 females/group) were fed fensulfothion in
the diet at levels of 0, 1, 2 and 5 ppm for 2 years. Severe weight
loss and reduced food consumption accompanied by signs of cholinergic
poisoning were evident in the early weeks of the study at 5 ppm. After
the second month, food consumption increased and lost body weight was
regained. Slight cholinergic signs at 2 ppm were also observed at the
beginning of the study. Reduction of serum and RBC cholinesterase was
evident at 5 ppm throughout the study. Slight reduction was observed
at 2 ppm with no effects noted at 1 ppm. No mortality was incurred as
a result of feeding fensulfothion, and hematology and gross and
histopathologic examination of tissues and organs were normal (Doull
et al., 1966a).
Long-term studies
Rat
Groups of rats (24 male and 27 female/group) were fed fensulfothion in
the diet for 17 months at dietary levels of 0, 1, 5 and 20 ppm.
Mortality of males was increased at 5 and 20 ppm. Growth of males and
females was slightly impaired at 20 ppm. Cholinesterase depression was
observed to be dose dependent in serum (19%), RBC (21%), submaxillary
gland (18%) and brain (24%) in females at 1 ppm and above and in males
at 5 ppm and above. No effects were observed on gross and histological
examination of organs and tissues (Doull et al., 1966b).
COMMENT
Fensulfothion is acutely very toxic to mammals. The compound is
metabolized similarly in animals and plants to substances of greater
toxicity by oxidation of both the enolic leaving group and the
phosphorothionate moiety. No effects on reproduction in rats,
neurotoxicity in hens, mutagenicity or teratogenicity at low levels in
rodents, or potentiation with other organophosphate compounds have
been observed.
A no-effect level in long-term studies in rats has not been
established. In a 17-month study cholinesterase depression at 1 ppm in
plasma, RBC and brain was observed. By plotting effect versus dose, a
theoretical no-effect level of 0.5 ppm (0.025 mg/kg/day) may be
estimated. Somatic effects were not noted up to levels of about five
times this dose. In a 2-year study in dogs, no adverse effects at 1
ppm were observed.
No data are reported on the effects of fensulfothion in man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 1.0 ppm in the diet, equivalent to 0.025 mg/kg/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0003 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fensulfothion is a systemic organo-phosphorous nematicide and
insecticide, which is used against soil nematodes (free living, root
knot and cyst forming nematodes) and a broad spectrum of soil borne
insects in field crops, vegetables and fruit. It is also used against
nematodes in turf grasses, in flowers and in ornamentals. In most
cultures the material is applied before planting or sowing or at
planting; in some cultures fensulfothion is applied in the soil in
established cultures.
Fensulfothion is used a.o. in Canada, Germany, Japan, New Zealand and
the Philippines.
Pre-harvest treatments
Table 3 summarizes the recommendations in accordance with good
agricultural practice, including rates and methods of application
(applications to soil at or near sowing or planting time; broadcast
application or band treatment).
TABLE 3 Officially recommended and registered uses of fensulfothion
Country and Crop Pest Dosage
(a.i.) Application time Restrictions
or pre-harvest
interval
Canada1
Brassicas 15 g/100 m row at planting or
(broccoli, max. 2.5 kg/ha shortly after
Brussels sprouts, planting
cabbage, cauliflower,
rutabagas, turnips)
Maize corn root worm 6-12 g/100 m row at planting Do not feed or ensile
(=0.5-1 kg/ha) treated forage
Potatoes Colorado beetle 5.0 kg/ha pre-plant broadcast
wireworms 100 days interval
tuber flea beetle
larvae
Ctenicera spp.
Cuba, Ecuador
Banana 3-4.5 g/tree established tree
Germany (W)2
Sugarbeet beetfly 1.25-2.5 kg/ha at sowing
(Pegomya hyoscyami)
centipedes
Philippines
Rice (paddy)
in flooded fields 1 kg/ha 15 days after broadcast
planting
TABLE 3 (Cont'd.)
Country and Crop Pest Dosage
(a.i.) Application time Restrictions
or pre-harvest
interval
2 kg/ha (2x) 45 and 75 days broadcast, granular
after planting application to paddy
water
U.S.A.3
Field corn corn root worm 0.5.1 kg/ha at planting
40 inch row spacing at planting
Popcorn
Sweet corn 12 g/100 m row at planting
Onions onion maggot 1 kg/ha in furrow at Do not apply to green
(dry bulb) planting bunch onion.
Peanuts nematodes 2-4 kg/ha band, at planting Do not feed vines and
and/or at peggig hay to livestock.
southern corn
rootworm 2-4 g/100 m row Do not apply more than
7 kg/ha in one year
Pineapple nematodes 50 kg/ha broadcast
nematodes 50 kg/ha (drench) pre-plant
Potatoes 2-5.0 kg/ha pre-plant broadcast of granules
Sugarbeet 1-2 kg/ha at sowing
15 g/100 m row at sowing
TABLE 3 (Cont'd.)
Country and Crop Pest Dosage
(a.i.) Application time Restrictions
or pre-harvest
interval
Sugarcane nematodes 2-5 kg/ha at planting band (40-45 cm)
Tobacco wireworms, 2-10 kg/ha pre-plant broadcast
nematodes
Tomatoes nematodes 3.2-6.4 kg/ha pre-plant band
10-20 kg/ha broadcast
1 Canada Dept. of Agriculture Plant products Division, Production & Marketing Branch
Use Claim for pesticides registered under the Pest Control Products Act May, 1971 & Jan. 1972, No. 834-128.
2 Pflanzenschutamittel-Verzeichnis, 23 Auflage, April 1972, Biologischen Bundesanstalt für Land - und
Forstwirtschaft Braunschweig.
3 USDA summary of Registered Agricultural Pesticide Chemical uses III D 22 5-31-69; 8-14-70, 3-24-72.
Post-harvest treatments
Fensulfothion is not recommended for post-harvest treatments.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in
different countries on food crops grown under various conditions. In
most cases dosage rates were applied in accordance with label
instructions. However, in some experiments higher dosages were also
included. Data from these trials are summarized in table 4.
TABLE 4 Residues in crops treated with fensulfothion
Crop Rate of application No. of Pre-harvest Net Residue
(a.i.) applications interval (ppm)
(days)
bananas 3.0 - 4.5 g/tree 1 1 - 90 <0.01 - 0.02
corn 1.2 - 3.2 kg/ha 2 21 - 67 <0.05
0.18 - 0.27 g/row )
plus 1.1 - 1.7 kg/ha ) 2 + 1 29 - 31 <0.01
cotton seed 0.55 - 0.82 g/m row 1 157 - 206 <0.01
onions 0.05 - 0.23 g/m row 1 80 - 191 <0.05
pineapple 60 - 200 kg/ha (drench) 1 561 - 973 <0.01
potatoes 7 - 8 kg/ha 1 102 - 150 <0.05
rutabagas 1.1 - 2.7 kg/ha 2 - 6 19 - 84 <0.01 - 0.05
soybeans 0.18 - 0.27 g/m row 1 71 - 119 <0.01
sugar beets 2 - 4 kg/ha 1 177 - 182 <0.01 - 0.10
0.12 - 2 g/m 1-3 58 - 196 <0.03
sugar cane 6 - 8 kg/ha (40 cm band) 1 228 - 343 <0.01 - 0.02
sweet potatoes 6.7 - 11.8 kg/ha 1 101 - 189 <0.01 - 0.05
tobacco 2 - 16 kg/ha 1 53 - 159 0.1 - 10.5
tomatoes 20 - 30 kg/ha 1 34 - 121 <0.05
The residues in these trials were predominantly determined by GLC
utilizing thermionic detection. Included in the analysis are total
toxic (cholinesterinase inhibiting) metabolites. Samples were taken at
harvest and immediately frozen until analysis. Bananas were stored
several days at room temperature before freezing.
In a ryegrass/white clover mixture the residue decreased from 90 ppm
(dry weight) immediately after application to 20 ppm after 13 days and
12, 4 and 1 ppm after 27, 55 and 90 days, respectively (Brewerton,
1971). Similar figures are given by Solly (1968) from grassland in New
Zealand.
FATE OF RESIDUES
General comments
The breakdown of fensulfothion in plants and animals seem identical
since the same metabolites have been identified in both.
In animals and animal products
In milk from cows grazing in pasture containing mainly residues of
fensulfothion sulfone, the oxygen analogue sulfone was detected. An
oxidative pathway in the ruminant appears most likely. Milk from dairy
cattle grazing for 10 days on pasture with an initial residue of 41
ppm fensulfothion contained 0.02 ppm oxygen analogue sulfone on the
third day, which decreased to 0.01 ppm on the 10th day (Solly
et al., 1971c).
Omental fat of sheep grazing for six days in pasture containing 43 ppm
initial residue of fensulfothion, showed a residue of 0.003 ppm,
although the erythrocyte cholinesterase depression was approximately
80% (Solly et al., 1971b).
In plants
The systemic action of fensulfothion was first demonstrated after
topical application stems of cotton plants (Benjamini et al.,
1959b). Katague and Anderson (1967) studied the fate of fensulfothion
after stem injection in cotton with 32P fensulfothion. They
identified the following metabolites by TLC with authentic standards:
O,O diethyl-O-[4-(methyl sulfinyl) phenyl)] phosphate (= oxygen
analogue) and O,O diethyl-[-4-(methylsulfonyl) phenyl]
phosphorothionate (sulfone).
After root uptake traces of O,O-diethyl-O-[-4 methyl sulfonyl
phenyl] phosphate (oxygen analogue sulfone) were found. The S-ethyl
analogue was not detected in either study.
After stem injection of 32P fensulfothion in maize, beans and cotton
the S-ethyl analogue could not be detected, but the previously
mentioned three metabolites were found (Everett and Gronberg, 1967).
Thornton (1967) analysed maize fodder from field samples by GLC for
fensulfothion and its metabolites. The interval for harvest was 40-121
days. The percent distribution of fensulfothion and metabolites was:
fensulfothion: 0-38% (average 16%), sulfone: 0-34% (average 12%),
oxygen analogue: 14-68% (average 45%), oxygen analogue sulfone: 8-49%
(average 27%).
Thornton (1968) showed the presence of 4-methylsulfonyl phenol in
maize fodder, but none in the maize kernels and cobs. Solly and
Harrison (1971a) found the sulfone as a major metabolite in pasture
grass, with only traces of the oxygen analogue and the oxygen analogue
sulfone. The same main metabolites were found in cured tobacco (Olson,
1972).
In soil
In soil the breakdown of fensulfothion and metabolites is rather
rapid. Duffy (1968) found experimentally that one half of the
fensulfothion in soil was degraded in 14 days. In other tests, in
which fensulfothion was incorporated in the top six inches of soil,
residues were determined over a period of one year. The time required
to decrease the residue to one half of the initial concentration was
generally less than 30 days; in another test it was slightly longer
than 180 days (Chemagro, 1968).
In storage and processing (cooking)
Thornton (1971) treated cotton seed oil fortified with 5 ppm
fensulfothion and the oxygen analogue with a laboratory steam
stripping similar to the process used commercially. The oil was heated
to 230 - 240°C and steam was passed through it for 3 hours. Analysis
of the processed oil showed that 48% of fensulfothion and 75% of the
oxygen analogue was swept away or degraded. The steam was scrubbed
with chloroform; 5 - 10% of the original 5 ppm were trapped and
identified as unchanged fensulfothion or oxygen analogue.
Katague (1968) simulated the refining of raw sugar beets to
concentrated syrup after fortifying the beets with 32P fensulfothion
and unlabelled metabolites. In the concentrated syrup the loss of
residue was 96 - 100% for fensulfothion, sulfone, oxygen analogue and
oxygen analogue sulfone. It was determined that the maximum total
concentration expected in the wet pulp would be no more than 5.5% of
the concentration in the raw beet.
In fresh harvested rutabagas stored 20 - 25 days at 4°C the total
residue of fensulfothion and metabolites decreased 50 - 92%. Cooking
reduced the residues by 48 - 68%. From these figures it may be
concluded that rutabagas, treated at recommended rates and stored for
a few weeks and then cooked, would contain no detectable residues.
Studies have been carried out to determine the extent of carry over of
residues in the tobacco smoke. Olson (1965) found 1.6% of
fensulfothion added to the tobacco in the smoke. After fortification
of cigarette tobacco with fensulfothion and subsequent analysis of the
solvent used for scrubbing the smoke, 7.9% recovery of unchanged
fensulfothion was shown in the smoke (Katague, 1966).
Olson (1968) fortified separate samples with fensulfothion and
metabolites. Recoveries in the smoke were 3.5, 3.0, 2.4 and 1.4%
respectively of fensulfothion, sulfone, oxygen analogue and oxygen
analogue sulfone.
During frozen storage of onions, potatoes and turnips, residues were
stable for 770 days, in cowpea vines for at least 98 days (Chemagro,
1967, 1965).
Evidence of residues in food in commerce or at consumption
No data available.
METHODS OF RESIDUE ANALYSIS
Gas chromatographic methods for analysis of fensulfothion and
metabolites are the methods of choice for residue analysis of various
crops, animal tissue and milk. GLC methods utilizing a KCl thermionic
detector proved to be particularly suitable for regulatory purposes.
Methods have been developed and adapted for analysis of maize,
peanuts, vegetables, forage crops and oil crops (Katague and Olson,
1969; Olson, 1970). The course of the analysis may be summarized as
extraction of the crop with acetone followed by partitioning with
chloroform.
The dry residue from the extract is oxidized with m-chloroperbenzoic
acid, which converts fensulfothion, sulfone and oxygen analogue to
oxygen analogue sulfone. This is further purified by solvent
partitioning and injected into the gas chromatograph. Residues of
fensulfothion and its three metabolites are measured as a single peak
as oxygen analogue sulfone. The method is specific for fensulfothion
and its metabolites. Twenty-three insecticides and metabolites
containing phosphorus were shown not to interfere (Olson, 1971).
Limit of detection of the method is 0.05 ppm or lower for
fensultothion and metabolites. Recoveries in various crop samples, in
cattle and in milk, ranged respectively from 71 - 86%, 102 - 109% and
96 - 116%.
GLC methods have been developed for the determination of individual
metabolites (Williams et al., 1971; Bowman and Hill, 1971). These
methods are less suitable for regulatory purposes than the method
described above.
Multidetection GLC methods for determining residues of
organo-phosphorous compounds in various crop samples were investigated
by the U.S. Food and Drug Administration. Fensulfothion and its three
metabolites were included in the study.
Watts et al. (1969) described a new charcoal liquid chromatography
procedure for improved clean-up of 60 organo-phosphorous compounds
including fensulfothion. Bowman and Beroza (1971) determined retention
times for 146 organo-phosphorous or sulphur compounds, including
fensulfothion, using a column packed with Dexsil 300
(polycarborane-siloxane) on HCl washed chromosorb W. The column can
be purged at temperatures up to 400°C.
Gas chromatic methods for determination of fensulfothion and
metabolites in soil have been developed (Olson, 1968; Katague, 1966).
NATIONAL TOLERANCES
Examples of national tolerances of fensulfothion residues are reported
in Table 5.
APPRAISAL
Fensulfothion is a systemic organophosphorous nematicide and
insecticide which is used on a considerable scale in various countries
on a relatively wide range of crops. Main uses are as soil treatment,
either broadcast or band treatment, or as a drench, against soil borne
nematodes (free living, root knot and cyst forming nematodes) and a
considerable range of soil borne insects.
Technical fensulfothion contains 94-96% of the pure compound. The
impurities in the technical material are known. The main components
are: O,O-diethyl-O-[4-(methylthio)-phenyl]-phosphorothioate
(1-3%) and 4-(methylsulfinyl)-phenol (0.8-1.0%).
Fensulfothion is marketed in different formulations, i.e. granular 3,
5, 10 and 15% and emulsifiable liquid (720 g/l).
The concentration/rates of application vary depending on pest, crop
and method of application; "normal" application rates are 1-5 kg a.i.
per ha.
TABLE 5 Examples of national tolerances reported to the Meeting1
Country Commodity Tolerance
(ppm)
U.S.A. peanut hulls 5
maize forage and fodder of field corn,
pop corn and sweet corn 1
maize grain, including field corn and
pop corn (kernels); fresh maize
(including sweet corn), kernels plus
cobs, with husks removed; onions,
(dry); potatoes; rutabagas (roots) and
tomatoes 0.1
peanuts (shelled); pineapple, pineapple
forage, sugar beets (roots and tops) 0.05
bananas (whole); sugarcane; meat, fat and
meat by-products of cattle, goats and sheep 0.02
Canada Brassicas (broccoli, Brussels sprouts,
cabbage, cauliflower); rutabagas;
turnips, potatoes; maize no residue
Germany, No official tolerances established.
Federal For the following crops tolerances
Republic are being considered:
sugarbeet.
New Zealand all food crops 0.1
1 Fensulfothion and cholinesterase inhibiting metabolites in or on the
commodities.
The residue data available were obtained from different countries and
regions with different climatic and soil conditions. The residue data
presented for fensulfothion included the three metabolites, sulfone,
oxygen analogue and oxygen analogue sulfone, and are with a few
exceptions representative for those likely to occur in conditions of
good agricultural practice.
Information is available on the fate of fensulfothion residues in
soil, in plants and to a lesser extent in animal products after
feeding animals on treated pasture or with treated crops.
Approved uses on peanuts and pineapple give rise to residues in peanut
kernels and in pineapple not exceeding 0.05 ppm (the proposed
tolerances). Hulls of peanuts and pineapple forage from such treated
crops will not contain residues in excess of 5 ppm and 0.05 ppm,
respectively. Likewise, the residue in sugarcane will not exceed 0.02
ppm. A soil application of fensulfothion in maize (including pop corn
and sweet corn), giving rise to residues in the kernels not exceeding
the proposed tolerance of 0.1 ppm, will result in residues in the
forage of 1 ppm or lower. Feeding of the materials mentioned in this
paragraph as a part of the ration for cattle or sheep will not give
rise to residues in meat or milk above the limit of determination
(i.e. 0.01 ppm).
Residues which may occur in food either from plant or animal origin,
after observing the recommended directions of use and the recommended
harvest intervals, consist largely of the oxygen analogue and the
oxygen analogue sulfone and to a smaller extent the parent chemical
and the sulfone. The breakdown products mentioned above could be
identified in radio-labelled studies and confirmed with other relevant
methods of analysis, e.g. TLC.
The breakdown of fensulfothion in plants and animals follows an
identical pattern; the same metabolites have been identified in both.
Information is available on the rate of decrease of the residue of
fensulfothion and metabolites in some crops and commodities during
storage and processing, including household cooking. In addition,
information on the extent of carryover of residues in tobacco smoke
from residues initially applied on tobacco was presented.
Little information is available on fensulfothion residues in food in
commerce.
Gas chromatographic procedures are available for specific
determination of fensulfothion and its main metabolites, or all
compounds combined and measure as a single peak as the oxygen analogue
sulfone. These methods are suitable for regulatory purposes as
required. Recommendations are given for the most appropriate
extraction and clean up procedures in food products of plant and
animal origin. The limit of determination is 0.05 ppm for all crops
and animal tissue and 0.01 ppm for milk.
RECOMMENDATIONS
TOLERANCES
The following tolerances are recommended for fensulfothion, including
the metabolites (oxygen analogue, oxygen analogue sulfone and
sulfone).
ppm
Maize grain, including kernels of field
corn and pop corn, onions, potatoes,
rutabagas (roots), tomatoes 0.1
Peanuts (shelled), pineapple, sugarbeet 0.05
Bananas (whole fruit) 0.02
Fat of meat and edible offal of cattle,
goats and sheep 0.02
FURTHER WORK OR INFORMATION
DESIRABLE
1. Teratogenicity studies at higher dosage levels.
2. Studies on human exposure.
REFERENCES
Benjamini, E., Metcalf, R.L. and Fukuto, T.R. (1959a) The chemistry
and mode of action of the insecticide O,O-diethyl
O-p-methylsulfinyl-phenyl phosphorothionate and its analogues. J.
Econ. Ent., 52: 94-98.
Benjamini, E., Metcalf, R.L. and Fukuto, T.R. (1959b) Contact and
systemic insecticidal properties of O,O-diethyl O,O-diethyl
O-p-methylsulfinyl-phenyl phosphorothionate and its analogues. J.
Econ. Ent., 52: 99-102.
Bowman, M.C. and Beroza, M. (1971) Use of Dexsil 300 on a specially
washed Chromosorb W for multicomponent residue determinations of
phosphorus- and sulfur-containing pesticides by flame photometric GLC.
Ass. off. analyt. Chem., 54: 1086-1092.
Bowman, M.C. and Hill, K.R. (1971) Determination of Dasanit and three
of its metabolites in corn, grass and milk. J. Agr. Fd. Chem., 19:
342-345.
Brewerton, H.V., Gibbs, M.M. and Perrott, D.C.F. (1971) Fensulfothion
and BAY 37289 residues on pasture. N.Z.J. Agric. Res., 11: 303-312.
Chemagro Division of Baychem. Corp. (1965, 1967, 1968) Reports 21252,
23119-23122, 23124, 23125, 23136. (unpublished)
Doull, J., DiGiacomo, R., Root, M. and Meskauskas, J. (1966a) Chronic
oral toxicity of BAYER 25141 to dogs. Report by the University of
Chicago submitted by Farbenfabriken Bayer, AG. (unpublished)
Doull, J., DiGiacomo, R., Root, M. and Meskauskas, J. (1966b) Chronic
oral toxicity of Bayer 25141 to rats. Report by the University of
Chicago submitted by Farbenfabriken Bayer, AG. (unpublished)
Doull, J., Root, M. and DiGiacomo, R. (1967) Effect of BAYER 25141 in
the diet on the reproduction and lactation of mice. Report by the
University of Chicago submitted by Farbenfabriken Bayer, AG.
(unpublished)
Dubois, K.P. and Jackson, P. (1967) Comparison of the acute toxicity
and anticholinesterase action of BAYER 25141 and some possible
metabolites. Report by the University of Chicago submitted by
Farbenfabriken Bayer, AG. (unpublished)
Dubois, K.P. and Kinoshita, F. (1963) The acute toxicity of BAYER
25141 in combination with other anticholinesterase insecticides.
Report by the University of Chicago submitted by Farbenfabriken Bayer,
AG. (unpublished)
Dubois, K.P. and Kinoshita, F. (1964) Acute toxicity and
anticholinesterase action of O,O-diethyl-O-p-(methylsulfinyl)
phenyl phosphorothioate (DMSP) and some related compounds. Tox. Appl.
Pharmacol., 6: 78-85.
Duffy, J.R. (1968) The metabolism of zinophos and BAY 25141 in soils.
Report on extra-mural research project 167. (unpublished)
Everett, L.J. and Gronberg, R.R. (1967) Chemagro Division of Baychem.
Corp., Kansas City, Mo. Metabolism of P32-labelled Dasanit in beans,
corn and cotton. Chemagro Report no. 21513. (unpublished)
Everett, L.J. (1968) Rat metabolism of P32 labelled Dasanit. Report
of the Chemagro Corporation submitted by Farbenfabriken Bayer, AG.
(unpublished)
Gaines, T.B. (1969) Acute toxicity of pesticides. Tox. Appl.
Pharmacol., 14: 515-534.
Grundmann, E. (1965) Histologische Untersuckung/S767. Report by
Institut für Experimentelle pathologie, submitted by Farbenfabriken
Bayer, AG. (unpublished)
Homeyer, B. (1970) Zun gegenwärtigen Stand der Bekämpfung von
Bodeninsekten. Pflanzenschutz-Nachrichten-Bayer, 23/1970: 233-239.
Homeyer, B. (1971) Terracur(R), ein breit wirkendes Boden insektizid
und Nematizid. Pflanzenschutz-Nachrichten Bayer, 24/1971: 371-410.
Industrial Biotest Laboratory. (1971) Teratogenic study with Dasanit
technical in albino rabbits. Report by the Industrial Biotest
Laboratory submitted by Farbenfabriken Bayer, AG. (unpublished)
Industrial Biotest Laboratory. (1971b) Mutagenic study with Dasanit in
albino mice. Report by the Industrial Biotest Laboratory submitted by
Farbenfabriken Bayer, AG. (unpublished)
Jung, H.F. and Iwaya, K. (1971) Terracur P(R), ein Insektizid und
Nematizid mit besonderer Eignung für den Reisanbau.
Pflanzenschutz-Nachrichten Bayer, 24/1971: 489-504.
Katague, D.B. (1966) Chemagro Division of Baychem. Corp. Reports nos.
17885, 17886, 18320, 18714. (unpublished)
Katague, D.B. and Anderson, C.A. (1967) Metabolism of P32-labelled
Dasanit in cotton plants. Bull. Env. Cont. Tox., 2: 228-235.
Katague, D.B. (1968) Chemagro Division of Baychem. Corp. Effect of
processing on Dasanit residues in sugar beets. Report no. 23015.
(unpublished)
Katague, D.B. and Olson, T.J. (1969) Chemagro Division of Baychem.
Corp. Determination of residues of Dasanit (BAY 25141) in corn by
thermionic emission gas chromatography. Report No. 20076.
(unpublished)
Kimmerle, G. (1965a) Neurotoxische Unkersuchungen Mit S-767-Wirkstoff.
Report by Institut für Toxikologie, Farbenfabriken Bayer, AG.
(unpublished)
Kimmerle, G. (1965b) Akute neurotoxizitatsuntersuchungen an huhnern.
Report by Institut für Toxikologie, Farbenfabriken Bayer, AG.
(unpublished)
Kimmerle, G. (1965c) S-767 (Production No. 3553) and Product 5121
(Production No. 3562). Report by Institut für Toxikologie,
Farbenfabriken Bayer, AG. (unpublished)
Kimmerle, G. (1966) S-767 Batch 1/64 Lo-Nr 214/antidotwirkung. Report
by Institut für Toxikologie, Farbenfabriken Bayer, AG. (unpublished)
Leuck, D.B. and Bowman, M.C. (1972) Persistence of residues of Dasanit
and its metabolites in coastal bermudagrass, forage corn and corn
silage. J. Econ. Ent., 65: 257-260.
Olson, T.J., (1965, 1968, 1969, 1970, 1971, 1972) Chemagro Division of
Baychem. Corp. Reports nos. 15188, 34051, 25464, 34164, 23055, 23037,
30278.
Proude, C.K. Ergebnisse sechsjähriger Arbeit mit Terracur P(R) in
Neuseeland. Pflanzenschutz-Nachrichten Bayer, 24/1971: 505-526.
Read, D.C. (1971) Bioactivity of Dasanit in a mineral soil, in
rutabagas in the field and in storage and effect of cooking on
toxicants in the roots. J. Econ. Ent., 64: 597-601.
Root, M., Taitel, C. and Doull, J. (1964) Subacute oral toxicity of
BAYER 25141 in male and female dogs. Report by The University of
Chicago, submitted by Farbenfabriken Bayer, AG. (unpublished)
Root, M., Kinoshita, F. and Flynn, M. (1969) Subacute oral toxicity of
Dasanit to female rats. Report by the University of Chicago, submitted
by Farbenfabriken Bayer, AG. (unpublished)
Thornton, J.S. and Olson, T.J. (1970) Chemagro Division of Baychem.
Corp. Determination of Dasanit residues in animal tissue by thermionic
emission flame gas chromatograhpy. Report no. 20750. (unpublished)
Thornton, J.S., (1971) Chemagro Division of Baychem Corp. Effect of
oil deodorization process on residues of Dasanit and Dasanit oxygen
analogue in cottonseed oil (simulated). Report no. 30299.
(unpublished)
Tucker, R.K. and Crabtree, D.G. (1970) Handbook of toxicity of
pesticides to wildlife. USDI. Resource Publication no. 84.
Watts, R.R., Storherr, R.W., Pardue, J.R. and Osgood, T. (1969)
Charcoal column clean-up method for many organo-phosphorus pesticide
residues in crop extracts. J. Ass. off. analyt. Chem., 52: 522-526.
Williams, I.H., Kore, R. and Finlayson, D.G. (1971) Determination of
residues of Dasanit and three metabolites by gas chromatography with
flame photometric detection. J. Agr. Fd. Chem., 19: 456-458.
