
MONOCROTOPHOS JMPR 1972
IDENTITY
Chemical name
Dimethyl-1-methyl-2-methyl carbamoyl-vinyl phosphate (E)-
Synonyms
"Azodrin", "Azodrin(R) Insecticide", "Nuvacron", SD9129
Structural formula
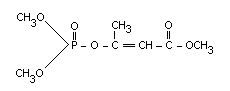 Physical and chemical properties of technical monocrotophos
Physical state: reddish-brown mixture of solid and liquid (at
25-30°C)
Odour: that of a mild ester
Melting point: 25-30°C (technical)
54-55°C (pure)
Vapour pressure: 7 x 10-5 mm Hg (at 20°C)
Solubility: soluble in water, acetone and alcohol; very
slightly soluble in mineral oils.
Stability: stable when stored in glass and polyethylene
containers. Technical grade has a half-life
of 2 500 days at 38°C. Rate of hydrolysis in
solution is slow.
Half-life in solution (2 ppm) at pH 7.0 and
38°C is 23 days; at pH 4.6 and 100°C,
half-life is 80 minutes.
Purity:
Analysis of a typical sample of technical monocrotophos gave the
following results:
% w
Monocrotophos 77
Dimethyl-1-methyl-2-methylcarbamoyl-vinyl phosphate (Z)- 9
N-methylacetoacetamide 2
Dimethyl phosphate 5
Others (less than 1% w each) 7
100
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Following an oral dose of 1 mg/kg to male and female rats,
monocrotophos was rapidly eliminated in the urine primarily as
hydrolysis products (70% of the urinary excretion within six hours),
unchanged monocrotophos (25%) and traces of the N-hydroxymethyl and
desmethyl metabolites. In 24 hours the rate of excretion had
diminished substantially, indicating rapid absorption and elimination
(Menzer and Casida, 1965). Monocrotophos administered orally to a goat
was found in the milk within one hour after treatment. Other
metabolites were found in lesser quantity than the parent
monocrotophos. Within 24 hours, the major quantity had cleared, and
only minute residues were found (Menzer and Casida, 1965).
Monocrotophos is a minor metabolite of dicrotophos (Bidrin(R)), its
N,N-dimethyl crotonamide analogue, and treatment of rats with
dicrotophos yielded small quantities of monocrotophos in the urine
within two hours (Bull and Lindquist, 1964).
Monocrotophos was fed to lactating dairy cows at a level of 45 ppm in
the diet. Monocrotophos and two metabolites were found in milk. In
urine further metabolites were observed (Potter, 1965).
Biotransformation
Monocrotophos injected into bean plants was metabolized to the
N-hydroxy-methylamide (SD 12657) and the amide (SD 11319).
Monocrotophos was more persistent in this plant study than
dicrotophos-Bidrin(R), its N,N dimethyl analogue (Menzer and Casida,
1965).
In cotton, monocrotophos has been shown to be metabolized to the
hydroxymethyl derivative (SD 12657) but not to the amide (SD 11319).
The oxidative route of metabolism yielding substituted crotonamides
was found to be slower than the metabolic route yielding substituted
phosphoric acid esters (Lindquist and Bull, 1967). A major
water-soluble metabolite observed in plants by these authors was
identified as a glycoside of the N-hydroxymethyl (SD 12657) derivative
(Shell Chemical Co., 1972).
The metabolic fate of monocrotophos in mammals has been well
documented (Menzer and Casida, 1965; Bull and Lindquist, 1964; 1967).
These data are summarized in Figure 1.
Effect on enzymes and other biochemical parameters
As with other organo-phosphorus esters, monocrotophos acts on the
organism as a direct cholinesterase inhibitor. The selectivity of
inhibition of cholinesterase from various sources in the body suggests
that blood cholinesterase activity is the most sensitive parameter for
exposure; brain cholinesterase activity is depressed only at higher
levels of exposure. Penetration into the brain may be the cause,
rather than a difference in sensitivity, although in vitro studies
have shown that monocrotophos has a somewhat greater affinity (k2) and
activity (I50) against rat RBC cholinesterase (Reiffy 1969).
Monocrotophos is metabolized to compounds which are less potent
inhibitors of human plasma cholinesterase, as evidenced by in vitro
pI50 values, i.e., monocrotophos (SD 9129) - 6.5; N-Methyl hydroxy
monocrotophos - 5.9; N-desmethyl monocrotophos - 5.6 (Menzer and
Casida, 1965).
Physical and chemical properties of technical monocrotophos
Physical state: reddish-brown mixture of solid and liquid (at
25-30°C)
Odour: that of a mild ester
Melting point: 25-30°C (technical)
54-55°C (pure)
Vapour pressure: 7 x 10-5 mm Hg (at 20°C)
Solubility: soluble in water, acetone and alcohol; very
slightly soluble in mineral oils.
Stability: stable when stored in glass and polyethylene
containers. Technical grade has a half-life
of 2 500 days at 38°C. Rate of hydrolysis in
solution is slow.
Half-life in solution (2 ppm) at pH 7.0 and
38°C is 23 days; at pH 4.6 and 100°C,
half-life is 80 minutes.
Purity:
Analysis of a typical sample of technical monocrotophos gave the
following results:
% w
Monocrotophos 77
Dimethyl-1-methyl-2-methylcarbamoyl-vinyl phosphate (Z)- 9
N-methylacetoacetamide 2
Dimethyl phosphate 5
Others (less than 1% w each) 7
100
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Following an oral dose of 1 mg/kg to male and female rats,
monocrotophos was rapidly eliminated in the urine primarily as
hydrolysis products (70% of the urinary excretion within six hours),
unchanged monocrotophos (25%) and traces of the N-hydroxymethyl and
desmethyl metabolites. In 24 hours the rate of excretion had
diminished substantially, indicating rapid absorption and elimination
(Menzer and Casida, 1965). Monocrotophos administered orally to a goat
was found in the milk within one hour after treatment. Other
metabolites were found in lesser quantity than the parent
monocrotophos. Within 24 hours, the major quantity had cleared, and
only minute residues were found (Menzer and Casida, 1965).
Monocrotophos is a minor metabolite of dicrotophos (Bidrin(R)), its
N,N-dimethyl crotonamide analogue, and treatment of rats with
dicrotophos yielded small quantities of monocrotophos in the urine
within two hours (Bull and Lindquist, 1964).
Monocrotophos was fed to lactating dairy cows at a level of 45 ppm in
the diet. Monocrotophos and two metabolites were found in milk. In
urine further metabolites were observed (Potter, 1965).
Biotransformation
Monocrotophos injected into bean plants was metabolized to the
N-hydroxy-methylamide (SD 12657) and the amide (SD 11319).
Monocrotophos was more persistent in this plant study than
dicrotophos-Bidrin(R), its N,N dimethyl analogue (Menzer and Casida,
1965).
In cotton, monocrotophos has been shown to be metabolized to the
hydroxymethyl derivative (SD 12657) but not to the amide (SD 11319).
The oxidative route of metabolism yielding substituted crotonamides
was found to be slower than the metabolic route yielding substituted
phosphoric acid esters (Lindquist and Bull, 1967). A major
water-soluble metabolite observed in plants by these authors was
identified as a glycoside of the N-hydroxymethyl (SD 12657) derivative
(Shell Chemical Co., 1972).
The metabolic fate of monocrotophos in mammals has been well
documented (Menzer and Casida, 1965; Bull and Lindquist, 1964; 1967).
These data are summarized in Figure 1.
Effect on enzymes and other biochemical parameters
As with other organo-phosphorus esters, monocrotophos acts on the
organism as a direct cholinesterase inhibitor. The selectivity of
inhibition of cholinesterase from various sources in the body suggests
that blood cholinesterase activity is the most sensitive parameter for
exposure; brain cholinesterase activity is depressed only at higher
levels of exposure. Penetration into the brain may be the cause,
rather than a difference in sensitivity, although in vitro studies
have shown that monocrotophos has a somewhat greater affinity (k2) and
activity (I50) against rat RBC cholinesterase (Reiffy 1969).
Monocrotophos is metabolized to compounds which are less potent
inhibitors of human plasma cholinesterase, as evidenced by in vitro
pI50 values, i.e., monocrotophos (SD 9129) - 6.5; N-Methyl hydroxy
monocrotophos - 5.9; N-desmethyl monocrotophos - 5.6 (Menzer and
Casida, 1965).
 TOXICOLOGICAL STUDIES
Special studies on metabolites
The acute toxicity of metabolites of monocrotophos in mouse and rat is
summarized in Table 1.
The acute toxicity of components of technical monocrotophos in rat is
summarized in Table 2.
TABLE 1 Acute toxicity of monocrotophos metabolites
Metabolite Reference
N-methyl hydroxy monocrotophos (SD = 12657)
Mouse ip LD50 = 12 mg/kg (Menzer and Casida, 1965)
Rat oral LD50 = 27 mg/kg (Shellenberger, 1966a)
N-desmethyl monocrotophos (SD 11319)
Mouse ip LD50 = 3 mg/kg (Menzer and Casida, 1965)
Mouse oral LD50 = 5.5 (Shellenberger, 1966a)
Glycoside of N-desmethyl monocrotophos (SD 13311)
Rat (M) oral LD50 = 168 mg/kg (Shell Chemical Co., 1966)
N-methyl acetoacetamide
Rat oral LD50 = >2 000 mg/kg (Shellenberger, 1966a)
TABLE 2 Acute toxicity of components of technical monocrotophos
Component Species Route LD50 (mg/kg)
Dimethyl phosphate of
3-hydroxy-N-methyl-trans-crotonamide Rat Oral 420
Dimethyl phosphate of 2-chloro-3
hydroxy-N-methyl-cis-crotonamide Rat Oral 14
Dimethyl phosphate of 2-chloro-3
hydroxy-N-methyl-trans-crotonamide Rat Oral 140
Short-term studies on the glycoside metabolite (SD 13311)
Groups of rats (42 males and 42 females per group, except for the
highest level which had 22 of each sex) were fed the glycoside
metabolite of monocrotophos (a glycoside of the dimethyl phosphate of
3-hydroxy-N-(hydroxymethyl)-cis-crotonamide) for 9 days at levels of
0, 1, 3, 9, 18 and 90 ppm (the 1 ppm was fed for seven weeks and the
dosage increased to 18 ppm for the final five weeks). Rats were fed a
normal diet for four weeks after the feeding study ended. Slight
growth effects were noted at 90 ppm from 7-12 weeks in both sexes.
Blood cholinesterase was depressed at 9 ppm in both sexes. At 18 ppm,
brain cholinesterase was depressed. Cholinesterase activity was not
affected at 3 ppm. No effects were observed on blood parameters or
gross and microscopic histology (Shell Chemical Co., 1966).
Special studies on neurotoxicity
Groups of adult White Leghorn hens (ten hens per group) were fed
monocrotophos in the diet at levels of 0, 1, 10 and 100 ppm for four
weeks. A single group of ten hens was fed TOCP in the diet at 1 000
ppm for four weeks. At the end of the feeding interval, five birds
from each group were sacrificed, and histological examinations of
brain, spinal cord and sciatic nerve were performed. Physiological
response, body-weight, food consumption and egg production data were
collected. Monocrotophos at all dose levels produced a weight loss in
hens. Egg production was not affected at 1 ppm but was inhibited at 10
and 100 ppm. Mortality occurred at 100 ppm, and although several birds
showed tremors 10-12 days after feeding started, all were able to
stand and walk normally. TOCP fed birds developed typical signs of
ataxia after 16-17 days. All survivors were unable to stand at the end
of the treatment period. Histological examination of TOCP-treated hens
showed severe demyelination in the posterior horns of the spinal cord.
Examination of nervous tissue from monocrotophos fed and control hens
showed slight evidence of demyelinated tracts, primarily in the
anterior horns. No differences were noted in controls and hens
undergoing monocrotophos treatments. Monocrotophos does not produce
delayed neurological signs of poisoning when compared to the response
induced by TOCP (Shellenberger, 1965b).
Special studies on potentiation
Male rats were given oral doses of a combination of monocrotophos and
the following anticholinesterase compounds, with no signs of
potentiation: dioxathion, dimethoate, carbaryl, disulfoton, EPN,
Folex, malathion, parathion, schradan, demeton, azinphos-methyl,
dicrotophos, crotoxyphos, coumaphos, dichlorvos, diazinon, naled,
trichlorphon, ethion, mevinphos, carbophenothion and phosphamidon.
Potentiation of the acute oral toxicity was observed with
monocrotophos in combination with fenchlorphos (Shellenberger, 1965a).
Special studies on reproduction
Groups of rats (10 males and 20 females per group) were fed
monocrotophos in the diet at 0, 2, 5, 12 and 30 ppm, and subsequently
mated. The progeny of this mating were maintained on this dietary
regime and mated. This was followed for three generations (Eisenlord
and Loquram, 1965). In a separate study groups of rats (10 males and
20 females per group) were fed monocrotophos at the same levels and
subjected to a three-generation, two-litter per generation study
(Eisenlord and Loquram, 1966).
Effects due to monocrotophos were noted in both studies at levels of 5
ppm and above. Appearance and behaviour was affected at 12 and 30 ppm,
as manifested in stunted growth and emaciation. Thinned or missing
hair on the flanks or head was seen in all monocrotophos groups among
adult females and pups, but not in adult males or controls. The level
of 30 ppm was lethal to pups and reduced pregnancies. Pup mortality
was evidenced at 5 ppm in both studies and absent at 2 ppm. Weight of
parents was adversely affected at 30 ppm. At 12 ppm, several parents
were affected (F2 and F3 males in one study and F2 females and F3
males in the second). No effects were noted on any parameter at 2 ppm.
Gross and histopathological examinations showed no differences from
the control values.
Special studies on teratogenicity
Groups of pregnant rabbits received oral daily doses of 0.7 and 2
mg/kg monocrotophos from day 6 to 18 of gestation. On day 28 of
pregnancy, the foetuses were removed and examined. No compound-related
teratogenic effects were observed (Thorpe and Dix, 1972).
Acute toxicity
The results of acute toxicity studies in animals are summarized in
Table 3.
TABLE 3 Acute toxicity of monocrotophos in animals
Species Sex Route LD50 Reference
(mg/kg)
Rat M IP 5 Bull & Lindquist, 1967
M Oral 14-23 Shellenberger & Newell, 1963
Shellenberger, 1965b; 1966a
F SC 7 Reiff, 1969
M Oral 18 Gaines, 1969
F Oral 20 Ibid.
Mouse M&F IP 8 Menzer & Casida, 1965
Oral 15 Shellenberger & Newell, 1963
Guinea Pig M&F SC app.60 Hunter, 1964
Signs of poisoning following monocrotophos are typical of other
anticholinesterase organo-phosphates. Toxic signs occur rapidly and in
animals include tremors, lacrimation, diarrhea, salivation, tonic and
clonic convulsions and other signs of cholinergic stimulation. Deaths
occur rapidly, usually within four hours, and animals surviving 24
hours generally recover and appear normal.
Atropine alone, but preferably in combination with reactivators (P2S
or Toxogonin), was shown to be an effective antidote to monocrotophos
poisoning in rodents (Hunter, 1964: Reiff, 1969).
Short-term studies
Japanese quail
Groups of Japanese quail (12 males and 12 females per group) were fed
monocrotophos at 0, 0.5, 5 and 50 ppm in the diet for three weeks.
Mortality and weight loss occurred at 50 ppm. Food consumption was
reduced at 5 ppm, while egg production was normal. Blood
cholinesterase activity was depressed at all feeding levels, while
brain cholinesterase activity was depressed at 5 ppm and above. At 5
ppm in the diet of adult females, monocrotophos had no effect on the
embryo heart beat or development of the chicks (Shellenberger, 1966b).
Rat
Groups of rats were fed monocrotophos in the diet at dosage levels of
0 (42 males and 42 females), 0.5, 1.5 (30 males and 30 females/group),
15 (42 males and 42 females), 45 and 135 ppm (12 males and 12
females/group) for twelve weeks. Growth was depressed at 45 ppm.
Cholinesterase depression was observed on haematology or gross and
microscopic pathology (Shellenberger and Newell, 1964).
Dog
Groups of Beagle dogs were fed monocrotophos in the dry diet at levels
of 0, 0.5, 1.5, 15 (four dogs of each sex/group), 45 and 135 ppm (two
dogs of each sex/group) for 13 weeks. After 8 weeks, the dogs fed 135
ppm were changed to 270 ppm for weeks 9-10, then to 540 ppm for weeks
11-12, and to 1 080 ppm for the final week.
Body-weights were reduced at dietary levels of 270 ppm. Organ weights
were also affected in this high feeding level group. Cholinesterase
depression was observed at 1.5 ppm in blood and brain. There were no
compound-related effects on haematological values, blood biochemistry,
organ weights or gross and microscopic pathology (Shellenberger and
Newell, 1964).
Groups of dogs were fed monocrotophos in the diet for two years at
levels of 0 (four males and four females) 0.16, 1.6, 16 ppm (three
males and three females/group) and for one year at 100 ppm (two males
and two females). Moderate cholinergic stimulation was observed at 100
ppm as tremors and salivation. Blood cholinesterase depression was
noted at 16 and 100 ppm in both males and females. Marginal brain
cholinesterase depression was also noted at a dose level of 1.6 ppm
after 93 and 104 weeks. No effects were noted on growth, mortality,
haematological parameters, urinalyses or physiological measurements.
Gross and histopathological examination showed no compound-related
effects (Johnston et al., 1967a).
Long-term studies
Rat
Groups of rats were fed monocrotophos in the diet for two years at
levels of 0 (40 males and 40 females), 1, 10 and 100 ppm (25 males and
25 females/group). Growth of males and females at 100 ppm was
depressed. Food consumption was also lower than controls at this
level. RBC and plasma cholinesterase depression was observed at 10 and
100 ppm in both males and females. Brain cholinesterase depression in
males and females was significantly inhibited at all levels. At 100
ppm, the weights of liver, gonad, thyroid and pituitary were reduced
in females. Thyroid and pituitary/body-weight ratios were depressed at
100 ppm. No effects were noted on survival, haematology or
histopathological examination of organs and tissues (Johnston
et al., 1967b).
COMMENT
Monocrotophos is rapidly absorbed, metabolized and eliminated by
mammals. Plant and animal metabolites are similar.
Potentiation was observed with fenchlorphos but not with other
cholinesterase-inhibiting compounds. No delayed neurotoxic effects
were observed.
Short-term studies in rats and dogs indicate no-effect levels at 0.5
ppm for cholinesterase depression.
In long-term studies a no-effect level has not been demonstrated in
the rat, the lowest dose tested (1 ppm) causing significant brain
cholinesterase depression.
In dog, two-year studies indicated a no-effect level of 0.16 ppm with
regard to brain cholinesterase depression. An effect was observed at
1.6 ppm.
In estimating an ADI for this compound, it was noted that in rat a
firm no-effect level was 0.5 ppm, with minimal effect level at 1 ppm.
In dog a no-effect level of 0.5 ppm was also demonstrated but the
minimal effect level was 1.5 ppm. Since the no-effect level is more
closely defined in rat, the latter species was used as a basis for
determining the ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.5 ppm in the diet, equivalent to 0.025 mg/kg/day
Dog: 0.5 ppm in the diet, equivalent to 0.0125 mg/kg/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0003 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Monocrotophos is an organo-phosphorus insecticide, which finds its
main use for foliar application to cotton (over 80% of monocrotophos
applied). It is also recommended for application against foliage pests
of maize, sugar cane, sugarbeet, vegetables, potatoes and certain
fruits. It is particularly effective against Lepidoptera, Homoptera
and certain Coleoptera, acting by both systemic and residual contact
properties.
Monocrotophos is known to be officially registered and/or approved for
use in the following countries:
Angola Yugoslavia
Argentina Mexico
Australia Mozambique
Austria Nicaragua
Brazil Pakistan
Bulgaria Peru
Chile Philippines
South Africa
Colombia Spain
Costa Rice Sudan
Ecuador Switzerland
El Salvador Thailand
Egypt Trinidad
France Turkey
Greece Uruguay
Guatemala U.S.A.
India Venezuela
Israel Viet-Nam
Italy Zambia
USE RECOMMENDATIONS
Typical maximum recommended application rates are: 1.0 kg a.i./ha for
cotton, potatoes and fruit; 1.5 kg a.i./ha for maize; 0.8 kg a.i./ha
for sugar cane; and 0.5 - 0.6 kg a.i./ha for vegetables and sugar
beet.
The recommended minimum period between treatment and harvest varies
from country to country, but is generally about 7 - 15 days for
vegetables and potatoes, 14 days for cotton, 21 days for tomatoes,
maize and citrus and 28 - 30 days for other crops.
Multiple applications of monocrotophos are often made, depending on
pest incidence, and use recommendations allow for these treatments.
The detailed use recommendations are given in Table 4.
TABLE 4 APPLICATIONS OF MONOCROTOPHOS TO CROP FOLIAGE
Crop Recommended Recommended period
application rates between treatment
(kg. a.i./ha) and harvest (days)1
Cotton 0.25 -1.0 Australia - 21
Mexico - 14
S. Africa - 30
Spain - 30
U.S.A. - 21
Maize 0.25 -1.5 Colombia - 30
Uruguay - 28
Sugarcane 0.25 -0.75 Uruguay - 28
Venezuela - 30
U.S.A. - 30
Carrots, turnips, onions 0.25 -0.5
Potatoes 0.25 -1.0 Colombia - 30
Italy - 21
S. Africa - 14
Venezuela - 14
U.S.A. - 7
Sugar beet 0.25 - 0.80 Italy - 21
TABLE 4 (Cont'd.)
Brussels sprouts, 0.2 -0.6 Yugoslavia - 28
cauliflower and cabbage Colombia - 30
Peru - 15
Venezuela - 21
Vietnam - 21
Tomatoes 0.25 -1.0
Hops 0.25 -0.5
Soybeans 0.25 -0.5 Colombia - 30
Vietnam - 21
Peas and beans 0.5 Colombia - 30
Pom - 15
Apples, pears2 0.01 - 0.04% a.i. Australia - 28
Italy - 30
Citrus 0.5 - 1.0 Venezuela - 21
Coffee 0.25 - 0.8
1 Monocrotophos is not recommended for post-harvest use on agricultural
commodities.
2 Monocrotophos is recommended for use on apples and pears in Australia,
and Italy only.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in 24
countries on food crops grown under various conditions, using various
rates of application and pre-harvest intervals.
In most trials, dosage rates, number of applications and pre-harvest
intervals were observed, in accordance with label recommendations, and
the data from these trials are summarized in Table 5. Residues of
monocrotophos in grain crops and root vegetables are usually below, or
close to, the limit of determination of the analytical method, while
leafy vegetables and fruit often contain detectable residues.
TABLE 5 Residues of monocrotophos in crops following recommended foliage treatments
Maximum Minimum
recommended preharvest Trials Results Range of
Crop rate interval residues (ppm)
(kg a.i./ha) (days) (no.) (no.)
Cotton (seed) 1.0 14 11 14 <0.02-0.08
Cotton (oil) 1.0 14 5 6 <0.02-0.04
Sugarcane 0.75 30 4 5 <0.05
Potatoes 1.0 7 14 27 <0.08-0.20
Maize grain 1.5 14 14 25 <0.01-0.05
Tomatoes 1.0 21 10 17 <0.01-0.51
Brussels sprouts 0.6 15 4 8 <0.05-0.21
Cauliflower 0.6 15 6 8 <0.01-0.14
Cabbage 0.6 21 9 23 <0.02-0.19
Carrots 0.5 14 2 5 <0.01-0.04
Turnips 0.5 10 1 3 <0.05
Onions 0.5 10 3 6 <0.02-0.09
Sugar beet 0.8 14 8 25 <0.01-<0.05
Peas 0.5 15 2 2 <0.07-0.60
Beans 0.5 21 3 6 <0.01-0.22
Soybeans 0.5 14 7 12 <0.01-0.03
Coffee 0.8 42 2 2 <0.01-0.08
Hops 0.5 28 1 2 0.3 - 0.41
Citrus fruit 1.0 21 11 26 <0.01-0.45
Apples 1.0 28 7 20 <0.05-1.0
Pears 1.0 28 5 10 <0.01-0.76
Cotton
Since the major outlet for monocrotophos is as a foliage insecticide
on cotton, numerous studies have been undertaken to examine the extent
of residues of monocrotophos in cottonseed and oil, if any, likely to
arise from recommended treatments.
Cottonseed
Monocrotophos is recommended for application to cotton foliage at
a maximum dosage rate of 1 kg a.i./ha, applications being made at
5-7 day intervals, where pest incidence dictates, with a final
treatment to harvest interval of 14 days. Trials in which these
recommendations were followed showed that residues of
monocrotophos in cottonseed were below the limit of determination
of the analytical method employed (<0.02 - <0.07 ppm) with the
exception of one sample which contained an apparent residue of
monocrotophos of 0.08 ppm. This sample had an unusually long
pre-harvest interval of 94 days, and the untreated corresponding
sample contained 0.05 ppm monocrotophos.
Cottonseed oil
Following recommended treatments of cotton with monocrotophos,
residues (max. 0.04 ppm) have only been detected occasionally in
the oil. A tendency was observed for residues to be lower in the
crude oil when they were detected in the corresponding seed.
Cotton foliage
On foliage, residue levels of monocrotophos were proportional to
the dosage rates at short pre-harvest intervals. The half-life on
foliage was between less than three days to six days. In U.S.A.,
where monocrotophos is widely used on cotton, no grazing of
livestock or feeding of treated trash is permitted since the
residue levels range from 8 to >100 ppm.
Potatoes
The data developed relative to the use of monocrotophos on potatoes
showed that, when treated at the recommended rate of 0.25 - 1.0 kg
a.i./ha, with a minimum pre-harvest interval of seven days, residues
of monocrotophos were consistently less than the limit of
determination of the analytical method employed (<0.01 - <0.08 ppm).
Sugarcane
Analyses of sugarcane which had been repeatedly treated at 0.5 - 1.0
kg a.i./ha, and products from the sugarcane, showed that the residue
levels of monocrotophos were consistently less than the limit of
determination of the analytical method (<0.05 ppm) when a pre-harvest
interval of 30 days was observed.
Maize
The results of a considerable number of studies, carried out to
determine the residues of monocrotophos in maize are given below:
Grain
No residues of monocrotophos (<0.01 - <0.05 ppm) have been
found in maize grain treated according to label recommendations
(max. dosage of 1.5 kg a.i./ha, pre-harvest interval of 14 days).
Residues in maize grain have been detected only in trials where
very short pre-harvest intervals have been used.
Husks and cobs
Extensive data show no significant residues of monocrotophos in
samples of husks and cobs from maize plants which had received
applications of monocrotophos. Residues of monocrotophos which
occur at short pre-harvest intervals (1-3 days) decrease rapidly.
At intervals of 14 days or longer they had decreased to an
average level of 0.04 ppm (range 0.005 - 0.40 ppm) from
treatments up to the maximum recommended dose rate of 1.5 kg
a.i./ha.
Silage
Following recommended treatments of monocrotophos to maize,
residues of monocrotophos in maize silage ranged from less than
0.01 ppm to 1.4 ppm (mean of 0.15 ppm).
Stover
Residues of monocrotophos in maize stover, following recommended
treatments, ranged from less than 0.01 ppm to 0.06 ppm (mean of
0.02 ppm). Levels of 13 - 17 ppm present in stover one day after
application rapidly decreased to 0.20 - 0.06 ppm 23 days later.
Tomatoes
Following recommended applications of monocrotophos (maximum rate of
1.0 kg a.i./ha, pre-harvest interval of 21 days) to tomatoes, residues
of monocrotophos ranged from less than 0.01 ppm to 0.51 ppm (mean of
0.11 ppm).
Brussels sprouts
Following recommended applications (dosage rate up to 0.6 kg a.i./ha,
pre-harvest interval of 15 days) of monocrotophos to Brussels sprouts,
residues of monocrotophos ranged from less than 0.05 ppm to 0.21 ppm,
with an arithmetic mean of 0.10 ppm.
Cauliflower
Residues of monocrotophos in samples of cauliflower, which had
received recommended applications of monocrotophos (up to 0.6 kg
a.i./ha, pre-harvest interval of 15 days) ranged from less than 0.01
ppm to 0.14 ppm (arithmetic mean of 0.03 ppm).
Cabbage
The results of studies carried out to determine the residues of
monocrotophos in cabbage, following recommended treatments (dosage
rate up to 0.6 kg a.i./ha, four applications and pre-harvest interval
of 21 days), were evaluated. Residues of monocrotophos in cabbage from
these trials ranged from less than 0.02 ppm to 0.19 ppm.
Carrots
Following recommended applications (maximum dosage rate of 0.5 kg
a.i./ha, pre-harvest interval of 14 days), maximum residues of
monocrotophos found in carrots were 0.04 ppm; generally no residues
(less than 0.01 ppm) were detected. Corresponding levels in the carrot
tops were 0.65 - 1.6 ppm (mean of 1.1. ppm).
Turnips
No residues of monocrotophos (less than 0.05 ppm) have been detected
in turnips, even when the turnips were harvested immediately after
application.
Onions
Onions, treated with monocrotophos at recommended applications
(maximum dosage rate of 0.5 kg a.i./ha, pre-harvest interval of ten
days) did not contain residues exceeding 0.09 ppm monocrotophos (mean
value of 0.03 ppm).
Sugar beet
Residues of monocrotophos in sugar beet roots did not exceed the limit
of determination of the analytical method employed (0.01 - 0.05 ppm),
following treatment at a maximum dosage rate of 0.8 kg a.i./ha. The
data cover applications made within 14 days of lifting, although in
practice applications as late as this are unusual. Corresponding
residues of monocrotophos in sugar beet leaves ranged from less than
0.01 ppm to 0.55 ppm.
Peas
Following application of monocrotophos to peas, at dosage rates up to
0.5 kg a.i./ha with pre-harvest intervals of 15 days or longer, the
only positive residue detected was 0.06 ppm, all the other samples
being below the limit of determination of the analytical methods
(<0.01 - <0.07 ppm).
Beans
Residues of monocrotophos in beans, following recommended treatments
(up to 0.5 kg a.i./ha, pre-harvest interval of 21 days), ranged from
less than 0.01 ppm to 0.22 ppm.
Soybeans
Residues of monocrotophos in soybeans did not exceed 0.03 ppm,
following treatment of monocrotophos according to label
recommendations (maximum dosage rate of 0.5 kg a.i./ha, pre-harvest
interval of 14 days). Maximum residue of monocrotophos in soybean
foliage was 0.87 ppm (mean of 0.22 ppm).
Coffee
Residues of 0.08 ppm in raw coffee beans, following a recommended
treatment of monocrotophos, were reduced to below the limit of
determination of the analytical method (less than 0.01 ppm) on
processing.
Hops
In a field trial carried out in Germany, with monocrotophos at 0.5 kg
a.i./ha and a minimum of 28 days pre-harvest interval, residues of
monocrotophos in dried hops ranged from 0.3 to 0.41 ppm.
Citrus fruit
Residues of monocrotophos in citrus fruit treated with monocrotophos
at label recommendations (up to 1.0 kg a.i./ha, pre-harvest interval
of 21 days) ranged from less than 0.01 ppm to 0.45 ppm (mean of 0.08
ppm).
Apples
Monocrotophos is recommended for use on apple trees in Australia and
Italy only, at dosage rates equivalent to 0.04% a.i. in the spray,
with a final treatment to harvest interval of four weeks. Data from
trials carried out in these countries were examined. Residues of
monocrotophos in apples treated according to this recommendation range
from <0.01 ppm to 1.5 ppm. These upper figures, however, derive from
experiments in which the spray was applied by hand, which usually
requires a somewhat higher volume; it is considered that in practical
conditions of use, 1.0 ppm would be the highest level reported.
Pears
Monocrotophos is only recommended for application to pears in
Australia and Italy. Maximum recommended dosage rate is 0.04% a.i. in
the spray with a final treatment to harvest interval of 28 days in
Australia and 30 days in Italy. Residues of monocrotophos treated
according to these recommendations ranged from 0.08 ppm to 0.76 ppm
(mean - 0.41 ppm) in pears from these two countries.
FATE OF RESIDUES
General comments
Residues of breakdown products do not occur in food commodities to a
significant extent.
Extensive studies with monocrotophos have shown that degradation can
occur by hydrolytic and/or oxidative mechanisms. From the experimental
work it appears that the primary metabolic pathway is hydrolysis,
yielding metabolic products which are non-cholinesterase inhibiting
and of low toxicity. Evidence of secondary metabolic pathways
involving oxidation reactions have been found in that small quantities
of the N-methylol analogue of monocrotophos, its glycoside and the
unsubstituted amide analogue were detected in the radiotracer studies.
Residues of cholinesterase inhibiting breakdown products have been
detected in crops only rarely, following recommended treatments, and
then at levels close to the limit of determination of the analytical
method employed. Likewise in meat and milk from animals fed
monocrotophos in their diet, at levels far exceeding those likely to
be encountered in practice, only extremely low levels of breakdown
products have been detected, if at all.
In animals
Studies have been conducted with monocrotophos fed to cattle and goats
to determine whether residues in feed give rise to residues in meat
and milk.
32P monocrotophos was fed continuously to two lactating cows at a
level equivalent to 45 ppm in their total diet (Shell Development Co.,
undated). This level is approximately 20 times that which would occur
in practice, assuming a maximum of 50% of monocrotophos-treated
feedstuff in the total diet. Levels of monocrotophos found in the milk
from the two cows were 0.01 ppm and 0.008 ppm. Average levels of
dimethyl phosphate and of N-hydroxy methyl monocrotophos were 0.002
ppm and 0.001 ppm, respectively (Ibid.). No residues of glycosides of
the N-hydroxy methyl compound were detected. After 14 days of this
feeding regime, the two cows were slaughtered and residues determined
on the red meat and liver. These results are given in Table 6.
TABLE 6 Residues of monocrotophos and its breakdown products
present in meat from cows fed monocrotophos
Residues present (ppm)
Sample dimethyl N-hydroxy methyl
analysed monocrotophos phosphate monocrotophos
Red meat 0.02, 0.04 0.005, 0.008 0.003, 0.0084
Liver 0.11, 0.13 0.05, 0.06 0.02, 0.04
32P monocrotophos was administered orally to goats (Menzer and
Casida, 1965) at a single dose of 1 mg/kg body-weight. Within 72 hours
of the application, only 1.4% of the applied radioactivity was present
in the goats' milk. The authors suggested that, in animals,
monocrotophos degradation took place by oxidative N-demethylation to
N-hydroxy methyl monocrotophos and unsubstituted amide. Residues of
these compounds and monocrotophos in samples of the milk from the
treated goats up to 24 hours after treatment are given in Table 7.
TABLE 7 Residues of monocrotophos and its breakdown products found
in milk from goats administered monocrotophos
Residues present in goats' milk (ppm)
Hours after N-hydroxy methyl unsubstituted total organo-
administration monocrotophos monocrotophos amide extractable
1 0.045 0.0001 <0.0001 0.067
2 0.032 0.0086 0.0007 0.061
4 0.034 0.0086 0.0015 0.074
6 0.023 0.011 0.0013 0.057
8 0.012 0.0099 0.0007 0.042
12 0.007 0.0044 0.0006 0.025
16 0.006 0.0020 0.0001 0.012
20 0.012 0.0009 0.0003 0.022
24 0.001 0.0007 <0.0001 0.007
As indicated in Table 7, residues of monocrotophos fell from a maximum
value of 0.0451 ppm one hour after administration to 0.0014 ppm 23
hours later. Maximum levels of N-hydroxy methyl monocrotophos, and the
unsubstituted amide, detected were 0.0105 ppm (after six hours) and
0.0015 ppm (after four hours), respectively. These levels had
decreased by a fifteen-fold factor 24 hours after application.
To determine whether residues in animal feeds could give residues of
the conjugate of N-hydroxy methyl monocrotophos in meat and milk, two
Guernsey cows were fed for ten days with the conjugate at a level of
20 ppm in the total diet (this level is more than 2 000 times that
which may be encountered in practice). The results showed that the
combined residues of the conjugate and its hydrolysis products,
N-hydroxy methyl monocrotophos and the unsubstituted amide, in milk,
were all below 0.01 ppm. At the end of the ten day study the cows were
slaughtered and their tissues analysed. The results are given in Table
8.
TABLE 8 Residues of monocrotophos breakdown products found in tissues
of cows fed monocrotophos conjugate
Tissues Residues present (ppm)
analysed
conjugate of N-hydroxy N-hydroxy methyl monocrotophos
methyl monocrotophos plus unsubstituted amide
fat <0.01 0.02, 0.04
kidney 0.02, 0.01 <0.01, 0.01
meat <0.01, <0.01 0.01, 0.01
liver <0.01, 0.01 <0.01, 0.01
In plants
In order to elucidate the mode of breakdown in leaves, 32P
monocrotophos was applied to cotton plants, both in the glasshouse and
in the field, either as a foliar spray, a stem banding treatment, a
seed dressing or by petiole injection (Lindquist and Bull, 1967). For
the foliar treatment, monocrotophos was applied at a rate of
40 mg/cotton leaf. In the field studies, 85% of the applied
radioactivity was lost during the first two days, presumably through
volatilization. Breakdown was shown to take place on both the surface
and the inside of the treated leaves. Principal breakdown products
found were O-desmethyl monocrotophos, dimethyl phosphate and N-hydroxy
methyl monocrotophos. When monocrotophos was applied as a stem bending
treatment, the recovered radioactivity after 21 days was unchanged
monocrotophos, probably because the lanolin in the formulation had a
stabilizing effect. Following the application of monocrotophos as a
seed treatment (not a recommended application) at a rate of 0.5 mg
monocrotophos/cotton seed, 50% of the radioactivity recovered after
one week was unchanged monocrotophos. O-desmethyl monocrotophos was
present in all samples, indicating the cleavage of a methyl-phosphate
bond. Dimethyl phosphate (formed by hydrolysis of the vinyl phosphate
bond) was recovered in increasing amounts with time. The N-hydroxy
methyl monocrotophos was only detected at 21 days after treatment,
while an unknown compound, later identified as a glycoside of
N-hydroxy methyl monocrotophos (Shell Development Co., undated), was
the major metabolite at 14 and 21 days.
Petiole injection of monocrotophos at 70 mg monocrotophos/cotton leaf
indicated half-lives of monocrotophos in cotton leaves of eight days
in the glasshouse and seven days in the field. Small amounts of the
N-hydroxy methyl monocrotophos and its conjugate were detected. No
residues of the unsubstituted amide (N-desmethyl monocrotophos) were
detected in cotton plants. The authors proposed the pathways shown in
Figure 2 for the degradation of monocrotophos.
Menzer and Casida (1965) injected 32P-monocrotophos into bean plants
at a level of 43.5 ppm and found trace amounts of the N-hydroxy methyl
monocrotophos (0.14% of monocrotophos applied) and the unsubstituted
amide (0.10% of monocrotophos applied) eight days after injection.
However, 20 and 32 days after application monocrotophos was the only
compound detected.
In studies with 14C-labelled monocrotophos (Shell Research Ltd.,
undated) on the foliage of maize, cabbage and apples, and on apple
fruit, the breakdown products present were the unsubstituted amide,
N-hydroxy methyl monocrotophos, (both in the free and conjugated
forms), O-desmethyl-monocrotophos acid and traces of
N-methylacetoacetamide and 3-hydroxy-N-methyl butyramide.
Since, of the breakdown products detected in plants following
application of monocrotophos, only the unsubstituted amide
(N-desmethyl monocrotophos) and the free and bound N-hydroxy methyl
monocrotophos can cause cholinesterase inhibition, analytical methods
(Ibid.) were developed for the determination of these compounds.
Samples from field trials were analysed using these methods.
Results from trials with sugar beet, potatoes, Brussels sprouts,
cauliflower, maize, pears, peas, onions and soybeans (Ibid.) showed
that residues of the unsubstituted amide and the N-hydroxyl methyl
monocrotophos (free and conjugated) could not be detected, nor could
monocrotophos.
TOXICOLOGICAL STUDIES
Special studies on metabolites
The acute toxicity of metabolites of monocrotophos in mouse and rat is
summarized in Table 1.
The acute toxicity of components of technical monocrotophos in rat is
summarized in Table 2.
TABLE 1 Acute toxicity of monocrotophos metabolites
Metabolite Reference
N-methyl hydroxy monocrotophos (SD = 12657)
Mouse ip LD50 = 12 mg/kg (Menzer and Casida, 1965)
Rat oral LD50 = 27 mg/kg (Shellenberger, 1966a)
N-desmethyl monocrotophos (SD 11319)
Mouse ip LD50 = 3 mg/kg (Menzer and Casida, 1965)
Mouse oral LD50 = 5.5 (Shellenberger, 1966a)
Glycoside of N-desmethyl monocrotophos (SD 13311)
Rat (M) oral LD50 = 168 mg/kg (Shell Chemical Co., 1966)
N-methyl acetoacetamide
Rat oral LD50 = >2 000 mg/kg (Shellenberger, 1966a)
TABLE 2 Acute toxicity of components of technical monocrotophos
Component Species Route LD50 (mg/kg)
Dimethyl phosphate of
3-hydroxy-N-methyl-trans-crotonamide Rat Oral 420
Dimethyl phosphate of 2-chloro-3
hydroxy-N-methyl-cis-crotonamide Rat Oral 14
Dimethyl phosphate of 2-chloro-3
hydroxy-N-methyl-trans-crotonamide Rat Oral 140
Short-term studies on the glycoside metabolite (SD 13311)
Groups of rats (42 males and 42 females per group, except for the
highest level which had 22 of each sex) were fed the glycoside
metabolite of monocrotophos (a glycoside of the dimethyl phosphate of
3-hydroxy-N-(hydroxymethyl)-cis-crotonamide) for 9 days at levels of
0, 1, 3, 9, 18 and 90 ppm (the 1 ppm was fed for seven weeks and the
dosage increased to 18 ppm for the final five weeks). Rats were fed a
normal diet for four weeks after the feeding study ended. Slight
growth effects were noted at 90 ppm from 7-12 weeks in both sexes.
Blood cholinesterase was depressed at 9 ppm in both sexes. At 18 ppm,
brain cholinesterase was depressed. Cholinesterase activity was not
affected at 3 ppm. No effects were observed on blood parameters or
gross and microscopic histology (Shell Chemical Co., 1966).
Special studies on neurotoxicity
Groups of adult White Leghorn hens (ten hens per group) were fed
monocrotophos in the diet at levels of 0, 1, 10 and 100 ppm for four
weeks. A single group of ten hens was fed TOCP in the diet at 1 000
ppm for four weeks. At the end of the feeding interval, five birds
from each group were sacrificed, and histological examinations of
brain, spinal cord and sciatic nerve were performed. Physiological
response, body-weight, food consumption and egg production data were
collected. Monocrotophos at all dose levels produced a weight loss in
hens. Egg production was not affected at 1 ppm but was inhibited at 10
and 100 ppm. Mortality occurred at 100 ppm, and although several birds
showed tremors 10-12 days after feeding started, all were able to
stand and walk normally. TOCP fed birds developed typical signs of
ataxia after 16-17 days. All survivors were unable to stand at the end
of the treatment period. Histological examination of TOCP-treated hens
showed severe demyelination in the posterior horns of the spinal cord.
Examination of nervous tissue from monocrotophos fed and control hens
showed slight evidence of demyelinated tracts, primarily in the
anterior horns. No differences were noted in controls and hens
undergoing monocrotophos treatments. Monocrotophos does not produce
delayed neurological signs of poisoning when compared to the response
induced by TOCP (Shellenberger, 1965b).
Special studies on potentiation
Male rats were given oral doses of a combination of monocrotophos and
the following anticholinesterase compounds, with no signs of
potentiation: dioxathion, dimethoate, carbaryl, disulfoton, EPN,
Folex, malathion, parathion, schradan, demeton, azinphos-methyl,
dicrotophos, crotoxyphos, coumaphos, dichlorvos, diazinon, naled,
trichlorphon, ethion, mevinphos, carbophenothion and phosphamidon.
Potentiation of the acute oral toxicity was observed with
monocrotophos in combination with fenchlorphos (Shellenberger, 1965a).
Special studies on reproduction
Groups of rats (10 males and 20 females per group) were fed
monocrotophos in the diet at 0, 2, 5, 12 and 30 ppm, and subsequently
mated. The progeny of this mating were maintained on this dietary
regime and mated. This was followed for three generations (Eisenlord
and Loquram, 1965). In a separate study groups of rats (10 males and
20 females per group) were fed monocrotophos at the same levels and
subjected to a three-generation, two-litter per generation study
(Eisenlord and Loquram, 1966).
Effects due to monocrotophos were noted in both studies at levels of 5
ppm and above. Appearance and behaviour was affected at 12 and 30 ppm,
as manifested in stunted growth and emaciation. Thinned or missing
hair on the flanks or head was seen in all monocrotophos groups among
adult females and pups, but not in adult males or controls. The level
of 30 ppm was lethal to pups and reduced pregnancies. Pup mortality
was evidenced at 5 ppm in both studies and absent at 2 ppm. Weight of
parents was adversely affected at 30 ppm. At 12 ppm, several parents
were affected (F2 and F3 males in one study and F2 females and F3
males in the second). No effects were noted on any parameter at 2 ppm.
Gross and histopathological examinations showed no differences from
the control values.
Special studies on teratogenicity
Groups of pregnant rabbits received oral daily doses of 0.7 and 2
mg/kg monocrotophos from day 6 to 18 of gestation. On day 28 of
pregnancy, the foetuses were removed and examined. No compound-related
teratogenic effects were observed (Thorpe and Dix, 1972).
Acute toxicity
The results of acute toxicity studies in animals are summarized in
Table 3.
TABLE 3 Acute toxicity of monocrotophos in animals
Species Sex Route LD50 Reference
(mg/kg)
Rat M IP 5 Bull & Lindquist, 1967
M Oral 14-23 Shellenberger & Newell, 1963
Shellenberger, 1965b; 1966a
F SC 7 Reiff, 1969
M Oral 18 Gaines, 1969
F Oral 20 Ibid.
Mouse M&F IP 8 Menzer & Casida, 1965
Oral 15 Shellenberger & Newell, 1963
Guinea Pig M&F SC app.60 Hunter, 1964
Signs of poisoning following monocrotophos are typical of other
anticholinesterase organo-phosphates. Toxic signs occur rapidly and in
animals include tremors, lacrimation, diarrhea, salivation, tonic and
clonic convulsions and other signs of cholinergic stimulation. Deaths
occur rapidly, usually within four hours, and animals surviving 24
hours generally recover and appear normal.
Atropine alone, but preferably in combination with reactivators (P2S
or Toxogonin), was shown to be an effective antidote to monocrotophos
poisoning in rodents (Hunter, 1964: Reiff, 1969).
Short-term studies
Japanese quail
Groups of Japanese quail (12 males and 12 females per group) were fed
monocrotophos at 0, 0.5, 5 and 50 ppm in the diet for three weeks.
Mortality and weight loss occurred at 50 ppm. Food consumption was
reduced at 5 ppm, while egg production was normal. Blood
cholinesterase activity was depressed at all feeding levels, while
brain cholinesterase activity was depressed at 5 ppm and above. At 5
ppm in the diet of adult females, monocrotophos had no effect on the
embryo heart beat or development of the chicks (Shellenberger, 1966b).
Rat
Groups of rats were fed monocrotophos in the diet at dosage levels of
0 (42 males and 42 females), 0.5, 1.5 (30 males and 30 females/group),
15 (42 males and 42 females), 45 and 135 ppm (12 males and 12
females/group) for twelve weeks. Growth was depressed at 45 ppm.
Cholinesterase depression was observed on haematology or gross and
microscopic pathology (Shellenberger and Newell, 1964).
Dog
Groups of Beagle dogs were fed monocrotophos in the dry diet at levels
of 0, 0.5, 1.5, 15 (four dogs of each sex/group), 45 and 135 ppm (two
dogs of each sex/group) for 13 weeks. After 8 weeks, the dogs fed 135
ppm were changed to 270 ppm for weeks 9-10, then to 540 ppm for weeks
11-12, and to 1 080 ppm for the final week.
Body-weights were reduced at dietary levels of 270 ppm. Organ weights
were also affected in this high feeding level group. Cholinesterase
depression was observed at 1.5 ppm in blood and brain. There were no
compound-related effects on haematological values, blood biochemistry,
organ weights or gross and microscopic pathology (Shellenberger and
Newell, 1964).
Groups of dogs were fed monocrotophos in the diet for two years at
levels of 0 (four males and four females) 0.16, 1.6, 16 ppm (three
males and three females/group) and for one year at 100 ppm (two males
and two females). Moderate cholinergic stimulation was observed at 100
ppm as tremors and salivation. Blood cholinesterase depression was
noted at 16 and 100 ppm in both males and females. Marginal brain
cholinesterase depression was also noted at a dose level of 1.6 ppm
after 93 and 104 weeks. No effects were noted on growth, mortality,
haematological parameters, urinalyses or physiological measurements.
Gross and histopathological examination showed no compound-related
effects (Johnston et al., 1967a).
Long-term studies
Rat
Groups of rats were fed monocrotophos in the diet for two years at
levels of 0 (40 males and 40 females), 1, 10 and 100 ppm (25 males and
25 females/group). Growth of males and females at 100 ppm was
depressed. Food consumption was also lower than controls at this
level. RBC and plasma cholinesterase depression was observed at 10 and
100 ppm in both males and females. Brain cholinesterase depression in
males and females was significantly inhibited at all levels. At 100
ppm, the weights of liver, gonad, thyroid and pituitary were reduced
in females. Thyroid and pituitary/body-weight ratios were depressed at
100 ppm. No effects were noted on survival, haematology or
histopathological examination of organs and tissues (Johnston
et al., 1967b).
COMMENT
Monocrotophos is rapidly absorbed, metabolized and eliminated by
mammals. Plant and animal metabolites are similar.
Potentiation was observed with fenchlorphos but not with other
cholinesterase-inhibiting compounds. No delayed neurotoxic effects
were observed.
Short-term studies in rats and dogs indicate no-effect levels at 0.5
ppm for cholinesterase depression.
In long-term studies a no-effect level has not been demonstrated in
the rat, the lowest dose tested (1 ppm) causing significant brain
cholinesterase depression.
In dog, two-year studies indicated a no-effect level of 0.16 ppm with
regard to brain cholinesterase depression. An effect was observed at
1.6 ppm.
In estimating an ADI for this compound, it was noted that in rat a
firm no-effect level was 0.5 ppm, with minimal effect level at 1 ppm.
In dog a no-effect level of 0.5 ppm was also demonstrated but the
minimal effect level was 1.5 ppm. Since the no-effect level is more
closely defined in rat, the latter species was used as a basis for
determining the ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.5 ppm in the diet, equivalent to 0.025 mg/kg/day
Dog: 0.5 ppm in the diet, equivalent to 0.0125 mg/kg/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0003 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Monocrotophos is an organo-phosphorus insecticide, which finds its
main use for foliar application to cotton (over 80% of monocrotophos
applied). It is also recommended for application against foliage pests
of maize, sugar cane, sugarbeet, vegetables, potatoes and certain
fruits. It is particularly effective against Lepidoptera, Homoptera
and certain Coleoptera, acting by both systemic and residual contact
properties.
Monocrotophos is known to be officially registered and/or approved for
use in the following countries:
Angola Yugoslavia
Argentina Mexico
Australia Mozambique
Austria Nicaragua
Brazil Pakistan
Bulgaria Peru
Chile Philippines
South Africa
Colombia Spain
Costa Rice Sudan
Ecuador Switzerland
El Salvador Thailand
Egypt Trinidad
France Turkey
Greece Uruguay
Guatemala U.S.A.
India Venezuela
Israel Viet-Nam
Italy Zambia
USE RECOMMENDATIONS
Typical maximum recommended application rates are: 1.0 kg a.i./ha for
cotton, potatoes and fruit; 1.5 kg a.i./ha for maize; 0.8 kg a.i./ha
for sugar cane; and 0.5 - 0.6 kg a.i./ha for vegetables and sugar
beet.
The recommended minimum period between treatment and harvest varies
from country to country, but is generally about 7 - 15 days for
vegetables and potatoes, 14 days for cotton, 21 days for tomatoes,
maize and citrus and 28 - 30 days for other crops.
Multiple applications of monocrotophos are often made, depending on
pest incidence, and use recommendations allow for these treatments.
The detailed use recommendations are given in Table 4.
TABLE 4 APPLICATIONS OF MONOCROTOPHOS TO CROP FOLIAGE
Crop Recommended Recommended period
application rates between treatment
(kg. a.i./ha) and harvest (days)1
Cotton 0.25 -1.0 Australia - 21
Mexico - 14
S. Africa - 30
Spain - 30
U.S.A. - 21
Maize 0.25 -1.5 Colombia - 30
Uruguay - 28
Sugarcane 0.25 -0.75 Uruguay - 28
Venezuela - 30
U.S.A. - 30
Carrots, turnips, onions 0.25 -0.5
Potatoes 0.25 -1.0 Colombia - 30
Italy - 21
S. Africa - 14
Venezuela - 14
U.S.A. - 7
Sugar beet 0.25 - 0.80 Italy - 21
TABLE 4 (Cont'd.)
Brussels sprouts, 0.2 -0.6 Yugoslavia - 28
cauliflower and cabbage Colombia - 30
Peru - 15
Venezuela - 21
Vietnam - 21
Tomatoes 0.25 -1.0
Hops 0.25 -0.5
Soybeans 0.25 -0.5 Colombia - 30
Vietnam - 21
Peas and beans 0.5 Colombia - 30
Pom - 15
Apples, pears2 0.01 - 0.04% a.i. Australia - 28
Italy - 30
Citrus 0.5 - 1.0 Venezuela - 21
Coffee 0.25 - 0.8
1 Monocrotophos is not recommended for post-harvest use on agricultural
commodities.
2 Monocrotophos is recommended for use on apples and pears in Australia,
and Italy only.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in 24
countries on food crops grown under various conditions, using various
rates of application and pre-harvest intervals.
In most trials, dosage rates, number of applications and pre-harvest
intervals were observed, in accordance with label recommendations, and
the data from these trials are summarized in Table 5. Residues of
monocrotophos in grain crops and root vegetables are usually below, or
close to, the limit of determination of the analytical method, while
leafy vegetables and fruit often contain detectable residues.
TABLE 5 Residues of monocrotophos in crops following recommended foliage treatments
Maximum Minimum
recommended preharvest Trials Results Range of
Crop rate interval residues (ppm)
(kg a.i./ha) (days) (no.) (no.)
Cotton (seed) 1.0 14 11 14 <0.02-0.08
Cotton (oil) 1.0 14 5 6 <0.02-0.04
Sugarcane 0.75 30 4 5 <0.05
Potatoes 1.0 7 14 27 <0.08-0.20
Maize grain 1.5 14 14 25 <0.01-0.05
Tomatoes 1.0 21 10 17 <0.01-0.51
Brussels sprouts 0.6 15 4 8 <0.05-0.21
Cauliflower 0.6 15 6 8 <0.01-0.14
Cabbage 0.6 21 9 23 <0.02-0.19
Carrots 0.5 14 2 5 <0.01-0.04
Turnips 0.5 10 1 3 <0.05
Onions 0.5 10 3 6 <0.02-0.09
Sugar beet 0.8 14 8 25 <0.01-<0.05
Peas 0.5 15 2 2 <0.07-0.60
Beans 0.5 21 3 6 <0.01-0.22
Soybeans 0.5 14 7 12 <0.01-0.03
Coffee 0.8 42 2 2 <0.01-0.08
Hops 0.5 28 1 2 0.3 - 0.41
Citrus fruit 1.0 21 11 26 <0.01-0.45
Apples 1.0 28 7 20 <0.05-1.0
Pears 1.0 28 5 10 <0.01-0.76
Cotton
Since the major outlet for monocrotophos is as a foliage insecticide
on cotton, numerous studies have been undertaken to examine the extent
of residues of monocrotophos in cottonseed and oil, if any, likely to
arise from recommended treatments.
Cottonseed
Monocrotophos is recommended for application to cotton foliage at
a maximum dosage rate of 1 kg a.i./ha, applications being made at
5-7 day intervals, where pest incidence dictates, with a final
treatment to harvest interval of 14 days. Trials in which these
recommendations were followed showed that residues of
monocrotophos in cottonseed were below the limit of determination
of the analytical method employed (<0.02 - <0.07 ppm) with the
exception of one sample which contained an apparent residue of
monocrotophos of 0.08 ppm. This sample had an unusually long
pre-harvest interval of 94 days, and the untreated corresponding
sample contained 0.05 ppm monocrotophos.
Cottonseed oil
Following recommended treatments of cotton with monocrotophos,
residues (max. 0.04 ppm) have only been detected occasionally in
the oil. A tendency was observed for residues to be lower in the
crude oil when they were detected in the corresponding seed.
Cotton foliage
On foliage, residue levels of monocrotophos were proportional to
the dosage rates at short pre-harvest intervals. The half-life on
foliage was between less than three days to six days. In U.S.A.,
where monocrotophos is widely used on cotton, no grazing of
livestock or feeding of treated trash is permitted since the
residue levels range from 8 to >100 ppm.
Potatoes
The data developed relative to the use of monocrotophos on potatoes
showed that, when treated at the recommended rate of 0.25 - 1.0 kg
a.i./ha, with a minimum pre-harvest interval of seven days, residues
of monocrotophos were consistently less than the limit of
determination of the analytical method employed (<0.01 - <0.08 ppm).
Sugarcane
Analyses of sugarcane which had been repeatedly treated at 0.5 - 1.0
kg a.i./ha, and products from the sugarcane, showed that the residue
levels of monocrotophos were consistently less than the limit of
determination of the analytical method (<0.05 ppm) when a pre-harvest
interval of 30 days was observed.
Maize
The results of a considerable number of studies, carried out to
determine the residues of monocrotophos in maize are given below:
Grain
No residues of monocrotophos (<0.01 - <0.05 ppm) have been
found in maize grain treated according to label recommendations
(max. dosage of 1.5 kg a.i./ha, pre-harvest interval of 14 days).
Residues in maize grain have been detected only in trials where
very short pre-harvest intervals have been used.
Husks and cobs
Extensive data show no significant residues of monocrotophos in
samples of husks and cobs from maize plants which had received
applications of monocrotophos. Residues of monocrotophos which
occur at short pre-harvest intervals (1-3 days) decrease rapidly.
At intervals of 14 days or longer they had decreased to an
average level of 0.04 ppm (range 0.005 - 0.40 ppm) from
treatments up to the maximum recommended dose rate of 1.5 kg
a.i./ha.
Silage
Following recommended treatments of monocrotophos to maize,
residues of monocrotophos in maize silage ranged from less than
0.01 ppm to 1.4 ppm (mean of 0.15 ppm).
Stover
Residues of monocrotophos in maize stover, following recommended
treatments, ranged from less than 0.01 ppm to 0.06 ppm (mean of
0.02 ppm). Levels of 13 - 17 ppm present in stover one day after
application rapidly decreased to 0.20 - 0.06 ppm 23 days later.
Tomatoes
Following recommended applications of monocrotophos (maximum rate of
1.0 kg a.i./ha, pre-harvest interval of 21 days) to tomatoes, residues
of monocrotophos ranged from less than 0.01 ppm to 0.51 ppm (mean of
0.11 ppm).
Brussels sprouts
Following recommended applications (dosage rate up to 0.6 kg a.i./ha,
pre-harvest interval of 15 days) of monocrotophos to Brussels sprouts,
residues of monocrotophos ranged from less than 0.05 ppm to 0.21 ppm,
with an arithmetic mean of 0.10 ppm.
Cauliflower
Residues of monocrotophos in samples of cauliflower, which had
received recommended applications of monocrotophos (up to 0.6 kg
a.i./ha, pre-harvest interval of 15 days) ranged from less than 0.01
ppm to 0.14 ppm (arithmetic mean of 0.03 ppm).
Cabbage
The results of studies carried out to determine the residues of
monocrotophos in cabbage, following recommended treatments (dosage
rate up to 0.6 kg a.i./ha, four applications and pre-harvest interval
of 21 days), were evaluated. Residues of monocrotophos in cabbage from
these trials ranged from less than 0.02 ppm to 0.19 ppm.
Carrots
Following recommended applications (maximum dosage rate of 0.5 kg
a.i./ha, pre-harvest interval of 14 days), maximum residues of
monocrotophos found in carrots were 0.04 ppm; generally no residues
(less than 0.01 ppm) were detected. Corresponding levels in the carrot
tops were 0.65 - 1.6 ppm (mean of 1.1. ppm).
Turnips
No residues of monocrotophos (less than 0.05 ppm) have been detected
in turnips, even when the turnips were harvested immediately after
application.
Onions
Onions, treated with monocrotophos at recommended applications
(maximum dosage rate of 0.5 kg a.i./ha, pre-harvest interval of ten
days) did not contain residues exceeding 0.09 ppm monocrotophos (mean
value of 0.03 ppm).
Sugar beet
Residues of monocrotophos in sugar beet roots did not exceed the limit
of determination of the analytical method employed (0.01 - 0.05 ppm),
following treatment at a maximum dosage rate of 0.8 kg a.i./ha. The
data cover applications made within 14 days of lifting, although in
practice applications as late as this are unusual. Corresponding
residues of monocrotophos in sugar beet leaves ranged from less than
0.01 ppm to 0.55 ppm.
Peas
Following application of monocrotophos to peas, at dosage rates up to
0.5 kg a.i./ha with pre-harvest intervals of 15 days or longer, the
only positive residue detected was 0.06 ppm, all the other samples
being below the limit of determination of the analytical methods
(<0.01 - <0.07 ppm).
Beans
Residues of monocrotophos in beans, following recommended treatments
(up to 0.5 kg a.i./ha, pre-harvest interval of 21 days), ranged from
less than 0.01 ppm to 0.22 ppm.
Soybeans
Residues of monocrotophos in soybeans did not exceed 0.03 ppm,
following treatment of monocrotophos according to label
recommendations (maximum dosage rate of 0.5 kg a.i./ha, pre-harvest
interval of 14 days). Maximum residue of monocrotophos in soybean
foliage was 0.87 ppm (mean of 0.22 ppm).
Coffee
Residues of 0.08 ppm in raw coffee beans, following a recommended
treatment of monocrotophos, were reduced to below the limit of
determination of the analytical method (less than 0.01 ppm) on
processing.
Hops
In a field trial carried out in Germany, with monocrotophos at 0.5 kg
a.i./ha and a minimum of 28 days pre-harvest interval, residues of
monocrotophos in dried hops ranged from 0.3 to 0.41 ppm.
Citrus fruit
Residues of monocrotophos in citrus fruit treated with monocrotophos
at label recommendations (up to 1.0 kg a.i./ha, pre-harvest interval
of 21 days) ranged from less than 0.01 ppm to 0.45 ppm (mean of 0.08
ppm).
Apples
Monocrotophos is recommended for use on apple trees in Australia and
Italy only, at dosage rates equivalent to 0.04% a.i. in the spray,
with a final treatment to harvest interval of four weeks. Data from
trials carried out in these countries were examined. Residues of
monocrotophos in apples treated according to this recommendation range
from <0.01 ppm to 1.5 ppm. These upper figures, however, derive from
experiments in which the spray was applied by hand, which usually
requires a somewhat higher volume; it is considered that in practical
conditions of use, 1.0 ppm would be the highest level reported.
Pears
Monocrotophos is only recommended for application to pears in
Australia and Italy. Maximum recommended dosage rate is 0.04% a.i. in
the spray with a final treatment to harvest interval of 28 days in
Australia and 30 days in Italy. Residues of monocrotophos treated
according to these recommendations ranged from 0.08 ppm to 0.76 ppm
(mean - 0.41 ppm) in pears from these two countries.
FATE OF RESIDUES
General comments
Residues of breakdown products do not occur in food commodities to a
significant extent.
Extensive studies with monocrotophos have shown that degradation can
occur by hydrolytic and/or oxidative mechanisms. From the experimental
work it appears that the primary metabolic pathway is hydrolysis,
yielding metabolic products which are non-cholinesterase inhibiting
and of low toxicity. Evidence of secondary metabolic pathways
involving oxidation reactions have been found in that small quantities
of the N-methylol analogue of monocrotophos, its glycoside and the
unsubstituted amide analogue were detected in the radiotracer studies.
Residues of cholinesterase inhibiting breakdown products have been
detected in crops only rarely, following recommended treatments, and
then at levels close to the limit of determination of the analytical
method employed. Likewise in meat and milk from animals fed
monocrotophos in their diet, at levels far exceeding those likely to
be encountered in practice, only extremely low levels of breakdown
products have been detected, if at all.
In animals
Studies have been conducted with monocrotophos fed to cattle and goats
to determine whether residues in feed give rise to residues in meat
and milk.
32P monocrotophos was fed continuously to two lactating cows at a
level equivalent to 45 ppm in their total diet (Shell Development Co.,
undated). This level is approximately 20 times that which would occur
in practice, assuming a maximum of 50% of monocrotophos-treated
feedstuff in the total diet. Levels of monocrotophos found in the milk
from the two cows were 0.01 ppm and 0.008 ppm. Average levels of
dimethyl phosphate and of N-hydroxy methyl monocrotophos were 0.002
ppm and 0.001 ppm, respectively (Ibid.). No residues of glycosides of
the N-hydroxy methyl compound were detected. After 14 days of this
feeding regime, the two cows were slaughtered and residues determined
on the red meat and liver. These results are given in Table 6.
TABLE 6 Residues of monocrotophos and its breakdown products
present in meat from cows fed monocrotophos
Residues present (ppm)
Sample dimethyl N-hydroxy methyl
analysed monocrotophos phosphate monocrotophos
Red meat 0.02, 0.04 0.005, 0.008 0.003, 0.0084
Liver 0.11, 0.13 0.05, 0.06 0.02, 0.04
32P monocrotophos was administered orally to goats (Menzer and
Casida, 1965) at a single dose of 1 mg/kg body-weight. Within 72 hours
of the application, only 1.4% of the applied radioactivity was present
in the goats' milk. The authors suggested that, in animals,
monocrotophos degradation took place by oxidative N-demethylation to
N-hydroxy methyl monocrotophos and unsubstituted amide. Residues of
these compounds and monocrotophos in samples of the milk from the
treated goats up to 24 hours after treatment are given in Table 7.
TABLE 7 Residues of monocrotophos and its breakdown products found
in milk from goats administered monocrotophos
Residues present in goats' milk (ppm)
Hours after N-hydroxy methyl unsubstituted total organo-
administration monocrotophos monocrotophos amide extractable
1 0.045 0.0001 <0.0001 0.067
2 0.032 0.0086 0.0007 0.061
4 0.034 0.0086 0.0015 0.074
6 0.023 0.011 0.0013 0.057
8 0.012 0.0099 0.0007 0.042
12 0.007 0.0044 0.0006 0.025
16 0.006 0.0020 0.0001 0.012
20 0.012 0.0009 0.0003 0.022
24 0.001 0.0007 <0.0001 0.007
As indicated in Table 7, residues of monocrotophos fell from a maximum
value of 0.0451 ppm one hour after administration to 0.0014 ppm 23
hours later. Maximum levels of N-hydroxy methyl monocrotophos, and the
unsubstituted amide, detected were 0.0105 ppm (after six hours) and
0.0015 ppm (after four hours), respectively. These levels had
decreased by a fifteen-fold factor 24 hours after application.
To determine whether residues in animal feeds could give residues of
the conjugate of N-hydroxy methyl monocrotophos in meat and milk, two
Guernsey cows were fed for ten days with the conjugate at a level of
20 ppm in the total diet (this level is more than 2 000 times that
which may be encountered in practice). The results showed that the
combined residues of the conjugate and its hydrolysis products,
N-hydroxy methyl monocrotophos and the unsubstituted amide, in milk,
were all below 0.01 ppm. At the end of the ten day study the cows were
slaughtered and their tissues analysed. The results are given in Table
8.
TABLE 8 Residues of monocrotophos breakdown products found in tissues
of cows fed monocrotophos conjugate
Tissues Residues present (ppm)
analysed
conjugate of N-hydroxy N-hydroxy methyl monocrotophos
methyl monocrotophos plus unsubstituted amide
fat <0.01 0.02, 0.04
kidney 0.02, 0.01 <0.01, 0.01
meat <0.01, <0.01 0.01, 0.01
liver <0.01, 0.01 <0.01, 0.01
In plants
In order to elucidate the mode of breakdown in leaves, 32P
monocrotophos was applied to cotton plants, both in the glasshouse and
in the field, either as a foliar spray, a stem banding treatment, a
seed dressing or by petiole injection (Lindquist and Bull, 1967). For
the foliar treatment, monocrotophos was applied at a rate of
40 mg/cotton leaf. In the field studies, 85% of the applied
radioactivity was lost during the first two days, presumably through
volatilization. Breakdown was shown to take place on both the surface
and the inside of the treated leaves. Principal breakdown products
found were O-desmethyl monocrotophos, dimethyl phosphate and N-hydroxy
methyl monocrotophos. When monocrotophos was applied as a stem bending
treatment, the recovered radioactivity after 21 days was unchanged
monocrotophos, probably because the lanolin in the formulation had a
stabilizing effect. Following the application of monocrotophos as a
seed treatment (not a recommended application) at a rate of 0.5 mg
monocrotophos/cotton seed, 50% of the radioactivity recovered after
one week was unchanged monocrotophos. O-desmethyl monocrotophos was
present in all samples, indicating the cleavage of a methyl-phosphate
bond. Dimethyl phosphate (formed by hydrolysis of the vinyl phosphate
bond) was recovered in increasing amounts with time. The N-hydroxy
methyl monocrotophos was only detected at 21 days after treatment,
while an unknown compound, later identified as a glycoside of
N-hydroxy methyl monocrotophos (Shell Development Co., undated), was
the major metabolite at 14 and 21 days.
Petiole injection of monocrotophos at 70 mg monocrotophos/cotton leaf
indicated half-lives of monocrotophos in cotton leaves of eight days
in the glasshouse and seven days in the field. Small amounts of the
N-hydroxy methyl monocrotophos and its conjugate were detected. No
residues of the unsubstituted amide (N-desmethyl monocrotophos) were
detected in cotton plants. The authors proposed the pathways shown in
Figure 2 for the degradation of monocrotophos.
Menzer and Casida (1965) injected 32P-monocrotophos into bean plants
at a level of 43.5 ppm and found trace amounts of the N-hydroxy methyl
monocrotophos (0.14% of monocrotophos applied) and the unsubstituted
amide (0.10% of monocrotophos applied) eight days after injection.
However, 20 and 32 days after application monocrotophos was the only
compound detected.
In studies with 14C-labelled monocrotophos (Shell Research Ltd.,
undated) on the foliage of maize, cabbage and apples, and on apple
fruit, the breakdown products present were the unsubstituted amide,
N-hydroxy methyl monocrotophos, (both in the free and conjugated
forms), O-desmethyl-monocrotophos acid and traces of
N-methylacetoacetamide and 3-hydroxy-N-methyl butyramide.
Since, of the breakdown products detected in plants following
application of monocrotophos, only the unsubstituted amide
(N-desmethyl monocrotophos) and the free and bound N-hydroxy methyl
monocrotophos can cause cholinesterase inhibition, analytical methods
(Ibid.) were developed for the determination of these compounds.
Samples from field trials were analysed using these methods.
Results from trials with sugar beet, potatoes, Brussels sprouts,
cauliflower, maize, pears, peas, onions and soybeans (Ibid.) showed
that residues of the unsubstituted amide and the N-hydroxyl methyl
monocrotophos (free and conjugated) could not be detected, nor could
monocrotophos.
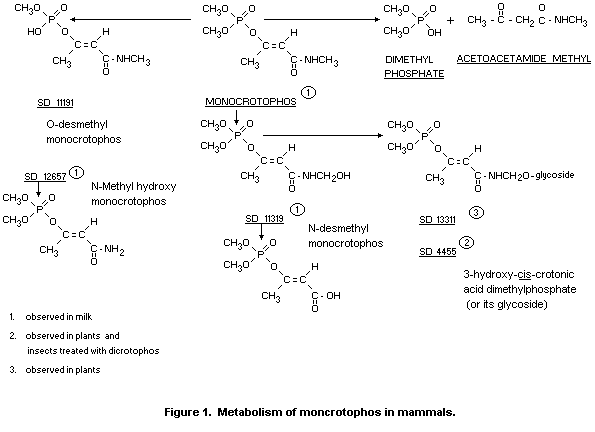 Further data, obtained in cases where monocrotophos residues were
above detectable levels, are shown in Table 9.
It will thus be seen that, in practice, where crops have been treated
with monocrotophos in the field, residues of cholinesterase inhibiting
degradation product in the edible parts did not reach detectable
levels. The only exception was a single sample of carrots in which a
residue of conjugated methylol at a level near to the level of
determination of the analytical method was reported.
In soils
Although monocrotophos is not used as a soil-applied pesticide, a
series of tests were conducted to determine the half-life of
monocrotophos in soil (Shell Chemical Co., undated). In Ripperdam
sandy loam and Sacramento clay loam, the half-lives of monocrotophos
were 18 and 6 days, respectively. In an early experiment (Shell
Research Ltd., undated), where monocrotophos was applied to soil at
rates of 0.25 or 0.5 kg a.i./ha, initial residues, immediately after
application, of 5.6 - 6.6 ppm monocrotophos had fallen to below the
limit of determination of the analytical method employed (less than
0.04 ppm) five months later.
In storage and processing
Studies to determine the effects of storage on residues of
monocrotophos in apples (Shell Research Ltd., undated) have shown that
these residues are not significantly reduced on storage at 15 - 20°C
for 24 days, or at 5 - 10°C for two to three months. Long-term
deep-freeze storage trials have shown that monocrotophos residues are
stable in broccoli and soybean foliage for 11-19 months, although
exceptionally 90% of monocrotophos residues were lost from cabbage
after 14 months deep-freeze storage (Shell Development Co., undated).
Numerous studies on the effect of domestic and commercial processing
on residues of monocrotophos in food crops have been carried out.
Domestic processes, such as washing, peeling and cooking, reduce the
levels of residues of monocrotophos in fruit and vegetables by between
35% and 95%. Commercial processes often have greater effects on
residues of monocrotophos. This canning can reduce typical levels by
over 90%, while the preparation of processed coffee beans, refined
cottonseed and soybean oil, and dried citrus pulp reduced typical
residues to below the limits of the analytical methods employed.
TABLE 9 Residues of monocrotophos breakdown products found in crops1
Preharvest Residues present (ppm)
Crop Country Interval Crop monocrotophos amide plus conjugated
part methylol methylol
21 roots <0.01 <0.01 <0.05
Sugar beet Italy 21 leaves 0.14 <0.01 -
31 roots <0.01 <0.03 <0.05
Sugar beet France 31 leaves 0.10 <0.03 -
31 roots <0.01 <0.03 -
31 leaves 0.04 0.06 -
Maize Italy 14 husk + 0.10 0.20 -
straw 0.10 0.30 -
13 grain <0.01 <0.01 <0.05
Maize S. Africa 13 husk 0.90 0.10 <0.10
13 straw 1.0 0.50 <0.20
3 whole 0.39 <0.05 -
Cabbage S. Africa 13 whole 0.22 <0.05 <0.10
3 whole 0.50 <0.05 -
13 whole 0.38 <0.05 <0.10
28 whole <0.02 <0.04 -
Onions U.K. 14 whole 0.14 <0.04 -
7 whole 0.17 <0.04 -
6 whole 0.06 <0.012 -
Tomatoes S. Africa 20 whole 0.08 <0.012 -
6 whole 0.34 <0.012 -
49 whole <0.01 <0.02 <0.05
Tomatoes S. Africa 28 whole 0.03 <0.02 -
49 whole 0.01 <0.02 <0.05
28 whole 0.10 <0.02 <0.05
TABLE 9 (Cont'd.)
Preharvest Residues present (ppm)
Crop Country Interval Crop monocrotophos amide plus conjugated
part methylol methylol
67 whole 0.18 <0.05 <0.05
Apples Australia 37 whole 0.51 <0.05 <0.05
8 whole 1.8 <0.05 0.06
Apples Italy 30 whole 1.0 <0.04 <0.03
Pears Italy 39 whole 0.15 <0.04 <0.05
14 roots 0.06 <0.02 0.10
Carrots S. Africa 21 roots 0.04 <0.02 <0.05
28 roots <0.01 <0.02 <0.05
1 Data from Shell Research Ltd.
2 Analysed only for unsubstituted amide.
Analyses of fruit with monocrotophos residues have demonstrated that
residues are mainly in the peel. Thus peeling removed 66% of residues
present in oranges, 35 - 70% in apples (Shell Research Ltd.; Shell
Chemical Co., undated) and 80 - 95% in grapefruit (Ibid.). In another
study to determine the levels of monocrotophos present in different
parts of apples, it was shown that 57 - 64% of the residues were
present in the pulp, 5% in the waxy surface layer, the remainder being
in the peel. The removal of the outer leaves of cabbages reduced the
monocrotophos levels by 95%, of cauliflower by 90% and of Brussels
sprouts by over 75% (Shell Research Ltd., undated).
Cold water washing can also reduce residues. Thus washing tomatoes
reduced residues of monocrotophos by up to 35% (Shell Development Co.;
Shell Chemical Co., undated). Domestic cooking reduced levels by
60 - 70% in green beans and 80 - 85% in Brussels sprouts (Shell
Research Ltd., undated).
The effect of the various steps in the commercial canning of beans
(Fahey et al., 1969) and tomatoes (Fahey et al., 1971) has been
studied. Cold water washing of beans effected a 27% reduction and
blanching a 40% reduction. Residues in the canned beans were only 2%
of the residues in the fresh beans. The processes of cold washing and
lye peeling reduced residues in tomatoes by 63% and 87%, respectively.
An 85% reduction in residues of monocrotophos was achieved in tomato
juice and a 91% reduction in whole tomatoes by canning. The process of
juicing oranges reduced the levels of monocrotophos from 0.8 ppm in
the pulp to 0.10 ppm and below (Shell Research Ltd., undated).
Commercial processing of raw coffee beans by removing the berry flesh
mechanically, followed by fermentation, and sun-drying to a moisture
level of 10%, reduced residues of 0.08 ppm monocrotophos in the raw
beans to below the limit of determination of the analytical method
(less than 0.01 ppm) in the processed beans (Ibid.).
The preparation of refined soybean oil reduced residues of 0.13 ppm
monocrotophos in beans to less than 0.01 ppm in the finished oil
(Shell Chemical Co., undated). Similarly, residues of 0.03 ppm in
kernels were reduced to less than 0.01 ppm in processed corn meal and
oil (Ibid.).
With cottonseed oil (Shell Research Ltd., unpublished) it has been
shown that 1 ppm monocrotophos added to the crude oil was reduced to
below the limit of determination of the analytical method (<0.01 ppm)
by the alkali refining step.
Experiments to determine the residues of monocrotophos likely to occur
in beer prepared from monocrotophos-treated hops have demonstrated
that residues of 1.8 ppm monocrotophos in hops were reduced to levels
at or below the limit of determination of the analytical method (0.02
ppm) in filtered and unfiltered beer (Ciba-Geigy Ltd., undated).
During the commercial preparation of citrus pulp cattle feed (Westlake
et al., 1970) from monocrotophos-treated oranges, residues were
reduced to below the limit of determination (less than 0.03 ppm), with
over 50% being lost during grinding and liming and the remainder
during drying.
The following sections summarize these effects in terms of individual
crops, in order to give an estimate of upper levels to be expected in
foods when they are ready to eat.
Cottonseed and soybean oils
Since the process of alkali refining destroys residues in crude
cottonseed oil at levels well above those in practice, refined
cottonseed oil products will not contain detectable residues of
monocrotophos. The same conclusion may be drawn for soybean oil.
Brassicas
In the case of Brussels sprouts, removal of outer leaves followed by
cooking reduced typical levels by 95%. Thus sprouts with initial
residues of 0.2 ppm (proposed tolerance levels) would not be expected
to contain more than 0.01 ppm when cooked and ready to be eaten.
In the case of cauliflower, removal of outer leaves alone reduced
residue levels some 10-fold so that even without cooking, levels in
trimmed cauliflower at an initial level of 0.5 ppm (proposed
tolerance) would be reduced to 0.05 ppm. In the case of cabbage, where
removal of outer leaves reduced residues 20-fold, cabbage with
residues at the proposed tolerance level of 0.05 ppm would not be
expected to contain more than 0.03 ppm when trimmed ready for cooking.
Fresh tomatoes
Fresh tomatoes with 0.5 ppm monocrotophos residues (proposed
tolerance) would lose, according to available data, an average 25% of
their residues, leading to a little less than 0.4 ppm at the point of
consumption.
Commercially processed tomatoes
The combined effects of washing and lye peeling reduced residues to
approximately 5% of their initial value so that residues in tomatoes
ready for canning would not exceed 0.01 ppm, a level which would be
further reduced by canning itself.
Similar considerations apply to canned tomato juice, and neither of
these finished products would contain detectable residues of
monocrotophos.
Beans
Domestic cooking of beans with monocrotophos residues at the tolerance
level of 0.2 ppm would reduce these to approximately 0.1 ppm.
Commercial canning would reduce these levels to below 0.01 ppm,
whereas freezing, which involves washing and blanching, would reduce
levels to 0.1 ppm.
Coffee
Coffee beans with initial residue at the tolerance level of 0.1 ppm
would be unlikely to contain detectable residues (< 0.01 ppm) after
fermentation and drying. For this reason, subsequent processes such as
roasting and brewing were not studied.
Hops
Hops with residues at the tolerance level do not give rise to
detectable residues in beer.
Citrus fruit
Residues in citrus fruit were reduced to an average of 30% of their
initial value on peeling, so that the raw fruit after peeling would
not contain residues above 0.15 ppm, where initial residues were at
the tolerance level of 0.5 ppm.
Juicing reduced levels of around 0.8 ppm in the pulp of oranges to
0.10 ppm in the juice.
Potatoes, maize grain and root vegetables
With the exception of carrots, where two samples out of five were
reported to contain low residues, these crops, treated as recommended,
have not contained measurable residues so that work to demonstrate the
effects of processing on reducing residues could not meaningfully be
undertaken.
Sugarcane and sugar beet
In the case of sugarcane the absence of detectable residues has been
demonstrated not only in the raw commodity but also in the
manufactured products. In neither crop were residues detected
following recommended treatments.
METHODS OF RESIDUE ANALYSIS
Residues of monocrotophos can be determined by specific methods
utilizing either gas-liquid chromatographic procedures or a
cholinesterase inhibition method.
Gas chromatographic methods
Gas chromatographic methods of analysis for monocrotophos are the
methods of choice, based on accuracy, specificity, sensitivity and
speed.
Monocrotophos can be determined by the general procedure using the
flame photometric detector (Beroza and Bowman, 1968; Brody and Chaney,
1966). Specific methods for monocrotophos using this detector have
been developed (Shell Research Ltd., undated; Bowman and Beroza, 1967)
and allow analyses of crops down to a limit of determination of 0.01
ppm. The following procedure (Shell Research, Ltd., undated) has
proved satisfactory in analysing crops: samples are extracted by
maceration with chloroform, low water content crops are first dampened
with water. Co-extracted natural products are removed using a column
adsorption chromatographic clean-up technique. The
monocrotophos-containing extract is analysed using the flame
photometric detector (FPD). Using this procedure, mean recoveries are
75 - 120% from crops at the 0.05 - 0.20 ppm level.
The thermionic detector has also been employed in the analyses of
crops for residues of monocrotophos (Shell Chemical Co., undated). In
this method, the sample is extracted with dichloromethane followed by
clean-up using a series of solvent exchanges and washings, prior to
injection of an aliquot of the extract onto the GLC instrument. Mean
recoveries with this method are 80 - 120% for crops, with a limit of
determination of 0.03 - 0.05 ppm.
Enzymatic inhibition
Specific enzymatic methods for the detection and determination of
monocrotophos in crops and milk have been developed (Shell Development
Co., undated). The samples for analysis are extracted with chloroform,
followed by a solvent exchange to hexane and concentration of the
extract. Column chromatography using a gradient column elution
technique with hexane/dichloromethane removes co-extractives. The
separated monocrotophos is transferred to water and determined by
enzyme inhibition spectrophotometric methods. Using this procedure,
the limit of determination of monocrotophos is about 0.10 ppm in crops
and 0.01 ppm in milk. Recoveries are in the range of 70 - 125%.
Methods for analyses of monocrotophos breakdown products
The analytical data for the N-methylol and for the unsubstituted amide
reported in this summary were developed using the method described for
monocrotophos (Shell Research Ltd., undated). The conjugate of the
N-methylol was determined using the method, which depends on
converting the conjugate to the sec-butyl thio-ether of the methylol
and subsequent determination by T.L.C. and enzyme inhibition (Ibid.).
A further method, using the thermionic detector (Griang and Beckman,
1968) has been developed for analysing crops for residues of
O-desmethyl monocrotophos, N-hydroxy methyl monocrotophos and its
conjugate.
NATIONAL TOLERANCES
Officially approved tolerances for monocrotophos have been established
in some countries and examples of these are given in Table 10.
TABLE 10
Examples of national tolerances as reported to the meeting
Country Crop(s) Tolerance
(ppm)
Australia Cottonseed, potatoes 0.1
Apples, pears 1.0
Italy Apples, pears 0.5
South Africa All treated crops 0.1
U.S.A. Cottonseed, potatoes,
sugarcane 0.1
Venezuela Cottonseed, rice, maize,
potatoes, sugarcane,
vegetables, citrus fruit 0.1
Yugoslavia All treated crops 0.1
APPRAISAL
Monocrotophos is an organophosphorus insecticide which finds its main
use for foliar application to cotton. It is also recommended for
application against foliage pests of maize, sugarcane, sugar beet,
vegetables, potatoes and certain fruits. It possesses both systemic
and residual contact properties.
Approved uses involve the application of from 0.25 - 1.0 kg a.i./ha.
The recommended pre-harvest interval varies according to crop and
country, ranging from seven days on potatoes to 30 days on fruit and
vegetables.
Extensive studies have shown that degradation of monocrotophos
residues on and in plants can occur by hydrolytic and/or oxidative
mechanisms. The products of hydrolysis are non-cholinesterase
inhibiting and of low toxicity. Only insignificant amounts of
secondary metabolites have been found during radiotracer studies.
Volatilization appears to be the major factor in the rapid loss of
residues following application. Monocrotophos itself is the principal
residue found on most plants even 7 - 28 days after application.
Metabolism studies suggest that ruminants receiving
monocrotophos-treated feedstuff metabolize the residue to metabolites
similar to those found on plants. There appears to be no tendency for
residues to accumulate in animals receiving contaminated rations. Only
low levels of monocrotophos and trace amounts of breakdown products
were detected in red meat, liver and milk of cattle receiving
feedstuffs containing monocrotophos at levels many times the maximum
likely to occur in practice.
Residue data, obtained from supervised trials in 24 different
countries clearly demonstrated the level of residues to be expected
under widely differing conditions on a wide range of crops. Except for
samples of leafy materials collected within one or two days of
application of heavy dosages of monocrotophos, residues in excess of
1.0 ppm were seldom encountered.
No substantial residue occurs in any fraction of the cotton plant used
for food, even when unprocessed.
As cotton foliage retains significant residues for more than three
weeks following application of approved rates of monocrotophos,
feeding of treated cotton foliage to cattle should be avoided.
Domestic processes, such as washing, peeling and cooking reduce the
levels of monocrotophos residues in fruit and vegetables by between
35% and 95%. Commercial processes often have greater effects on
reducing residues of monocrotophos. The peeling of citrus fruits
removes 60 - 73% of the total residue. Beer produced from treated hops
was shown to contain no detectable residues. The absence of residue
has been demonstrated in sugarcane and in sugar products manufactured
therefrom.
For determining monocrotophos residues, either gas-liquid
chromatography or cholinesterase inhibition methods are used, with the
former being the methods of choice from the aspect of accuracy,
specificity, sensitivity and speed. The limit of determination is 0.01
ppm. Using GLC methods recoveries of 75 - 120% were obtained from crop
material containing 0.05 - 0.2 ppm. Several of the available methods
appear suitable for regulatory purposes, but the method of Bowman and
Beroza (1967) is recommended.
RECOMMENDATIONS
The following recommended tolerances for animal products reflect a
value at or just above the limit of determination. The time interval
between application and harvest which has been used in determining the
maximum residue limits is appropriate to the agricultural practices in
numerous countries.
TOLERANCES
Interval between
Foodstuff Tolerance treatment and
(ppm) harvest (days)
Cottonseed 0.1 14
Cottonseed oil (raw) 0.05* 14
Potatoes 0.05* 7
Maize (grain) 0.05* 14
Tomatoes 0.5 21
Brussels sprouts 0.2 15
Cabbage, cauliflower 0.2 21
Carrots, turnips 0.05* 14
Onions 0.1 10
Sugar beet 0.05* 14
Peas 0.1 15
Beans 0.2 21
Soybeans 0.05* 14
Coffee (raw beans) 0.1 42
Hops (dried) 1 30
Citrus fruit 0.2 28
Apples and pears 1 (temporary 28
1975)
Meat and edible offal of
cattle, sheep, goats,
pigs and poultry 0.02* from feeding
Milk 0.002* treated plant
Milk products 0.02* products
Eggs (shell free) 0.02* "
* at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Studies on human exposure.
2. Information on the incidence of residues in apples and pears.
REFERENCES
Beroza, M. and Bowman, M.C. (1968) Chromatography of pesticide
residues containing phosphorus or sulphur with the flame photometric
detector. Environ. Sci., Technol., 2: 450.
Bowman, M.C. and Beroza, M. (1967) Chromatographic analysis of azodrin
and Bidrin. J. Agr. Fd. Chem., 15: 465
Brody, S.S. and Chaney, J.E. (1966) Flame photometric detector. J.
Gas. Chromat., 4: 42.
Bull, D.L. and Lindquist, D.A. (1964) Metabolism of 3-hydroxy-N,
N-dimethyl-crotonamide dimethylphosphate by cotton plants, insects and
rats. J. Agr. Fd. Chem., 12: 310-317.
Bull, D.L. and Lindquist, D.A. (1967) Metabolism of
3-hydroxy-N-methyl-cis-crotonamide dimethyl phosphate (azodrin) by
insects and rats. J. Agr. Fd. Chem., 14: 105-109.
Gaines, T.B. (1969) Acute toxicity of pesticides. Tox. Appl.
Pharmacol., 14: 515-534.
Ciba-Geigy Ltd. (undated) Reports on monocrotophos nos. 86 - 88,
90 - 93, 97, 111, 116 - 131. (unpublished)
Einsenlord, G. and Loquram, G.S. (1965) Results of short route
reproduction study of rats fed diets containing SD 9129 insecticide
over three generations. Data from the Hine Laboratory submitted by the
Shell Chemical Company. (unpublished)
Einsenlord, G. and Loquram, G.S. (1966) Results of long route
reproduction study of rats fed diets containing SD 9129 insecticide
over three generations. Data from the Hine laboratory submitted by the
Shell Chemical Co. (unpublished)
Fahey, J.E., Gould, G.E. and Nelson, P.E. (1969) Removal of gardona
and azodrin from vegetable crops by commercial preparative methods. J.
Agr. Fd. Chem., 17: 1204.
Fahey, J.E., Gould, G.E. and Nelson, P.E. (1971) Removal of azodrin
residues from tomatoes by commercial preparative methods. J. Agr. Fd.
Chem., 19: 81.
Griang, B.Y. and Beckman, H.F. (1968) Determination of bidrin, azodrin
and their metabolites with the thermionic detector. J. Agr. Fd. Chem.,
16: 899.
Hunter, C.G. (1964) The efficacy of atropine and oxime therapy as an
antidote to poisoning by SD 9129 in guinea pigs. Report Tunstall
Laboratory submitted by the Shell Chemical Co. (unpublished)
Johnston, C.D., Thompson, W.M. and Donoso, J. (1967a) Azodrin safety
evaluation by a chronic feeding study in the dog for two years. Data
from Woodard Research Corporation submitted by Shell Chemical Co.
(unpublished)
Johnston, C.D., Thompson, W.M. and Donoso, J. (1967b) Azodrin safety
evaluation by a chronic feeding study in the rat for two years. Data
from Woodard Research Corporation submitted by Shell Chemical Co.
(unpublished)
Lindquist, D.A. and Bull, D.L. (1967) Fate of
3-hydroxy-N- methyl-cis-crotonamide dimethyl phosphate in cotton
plants. J. Agr. Fd. Chem., 15: 267-272.
Menzer, R.L. and Casida, J.E. (1965) Nature of toxic metabolites
formed in mammals, insects and plants from 3-(dimethoxy
phosphinyloxy)-N,N-dimethyl-cis-crotonamide and its N-methyl
analogue. J. Agr. Fd. Chem., 13: 102-112.
Potter, J.C. (1965) Cattle feeding studies with 32P-labelled azodrin.
Data submitted by Shell Chemical Co. (unpublished)
Reiff, B. (1969) Pharmacological studies into the toxic actions of
cholinesterase inhibitors: (a) The effect of antidotes on the
subcutaneous toxicity of azodrin in the rat. Report of Tunstall
Laboratory submitted by Shell Chemical Co. (unpublished)
Shellenberger, T.E. (1965a) Potentiation studies - rats. Data from
Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. (1965b) Demyelination study - hens. Data from
Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. (1966a) Acute toxicity of azodrin and metabolites.
Data from Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. (1966b) Cholinesterase inhibition and
toxicological evaluations of two organophosphate pesticides in
Japanese quail. Tox. Appl. Pharm. 8: 22.
Shellenberger, T.E. and Newell, G.W. (1963) Acute oral toxicity. Data
from Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. and Newell, G.W. (1964) Subacute toxicity and
cholinesterase study of Shell compound SD 9129 - rat and dog. Data
from Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shell Chemical Co. (1966) Subacute toxicity and cholinesterase study
of Shell compound SD 13311 in rats. Data from Stanford Research
Institute submitted by Shell Chemical Co. (unpublished)
Shell Chemical Co. (1972) Identification of unknown 4. Report Shell
Chemical Co. (unpublished)
Shell Chemical Co. (undated) Reports on monocrotophos nos 50 - 67, 69
- 74, 76, 77, 79 - 81, 89, 101, 115, 132 - 136, 139 - 151, 153, 172,
176, 182 - 184. (unpublished)
Shell Development Co. (undated) Reports on monocrotophos nos. 68, 75,
78, 82 - 85, 100, 110, 112, 114, 137, 138, 152, 155, 171, 173 - 175,
177 - 181. (unpublished)
Shell Research Ltd. (undated) Reports on monocrotophos nos. 1 - 49,
94, 95, 98, 99, 154, 156 -170. (unpublished)
Thorpe, E. and Dix, M. (1972) Toxicity studies on azodrin:
Teratological studies in rabbits. Summary submitted by Shell Chemical
Co. (unpublished)
Westlake, W.E., Gunther, F.A. and Jeppson, L.R. (1970) Persistence of
azodrin residues on and in Valencia oranges and in
laboratory-processed citrus pulp cattle feed. J. Agr. Fd. Chem., 18:
864.
Young, J.R. and Bowman, M.C. (1967) Azodrin for corn earworm and fall
armyworm control and its persistence in sweet corn. J. Econ. Ent., 60:
1282.
Further data, obtained in cases where monocrotophos residues were
above detectable levels, are shown in Table 9.
It will thus be seen that, in practice, where crops have been treated
with monocrotophos in the field, residues of cholinesterase inhibiting
degradation product in the edible parts did not reach detectable
levels. The only exception was a single sample of carrots in which a
residue of conjugated methylol at a level near to the level of
determination of the analytical method was reported.
In soils
Although monocrotophos is not used as a soil-applied pesticide, a
series of tests were conducted to determine the half-life of
monocrotophos in soil (Shell Chemical Co., undated). In Ripperdam
sandy loam and Sacramento clay loam, the half-lives of monocrotophos
were 18 and 6 days, respectively. In an early experiment (Shell
Research Ltd., undated), where monocrotophos was applied to soil at
rates of 0.25 or 0.5 kg a.i./ha, initial residues, immediately after
application, of 5.6 - 6.6 ppm monocrotophos had fallen to below the
limit of determination of the analytical method employed (less than
0.04 ppm) five months later.
In storage and processing
Studies to determine the effects of storage on residues of
monocrotophos in apples (Shell Research Ltd., undated) have shown that
these residues are not significantly reduced on storage at 15 - 20°C
for 24 days, or at 5 - 10°C for two to three months. Long-term
deep-freeze storage trials have shown that monocrotophos residues are
stable in broccoli and soybean foliage for 11-19 months, although
exceptionally 90% of monocrotophos residues were lost from cabbage
after 14 months deep-freeze storage (Shell Development Co., undated).
Numerous studies on the effect of domestic and commercial processing
on residues of monocrotophos in food crops have been carried out.
Domestic processes, such as washing, peeling and cooking, reduce the
levels of residues of monocrotophos in fruit and vegetables by between
35% and 95%. Commercial processes often have greater effects on
residues of monocrotophos. This canning can reduce typical levels by
over 90%, while the preparation of processed coffee beans, refined
cottonseed and soybean oil, and dried citrus pulp reduced typical
residues to below the limits of the analytical methods employed.
TABLE 9 Residues of monocrotophos breakdown products found in crops1
Preharvest Residues present (ppm)
Crop Country Interval Crop monocrotophos amide plus conjugated
part methylol methylol
21 roots <0.01 <0.01 <0.05
Sugar beet Italy 21 leaves 0.14 <0.01 -
31 roots <0.01 <0.03 <0.05
Sugar beet France 31 leaves 0.10 <0.03 -
31 roots <0.01 <0.03 -
31 leaves 0.04 0.06 -
Maize Italy 14 husk + 0.10 0.20 -
straw 0.10 0.30 -
13 grain <0.01 <0.01 <0.05
Maize S. Africa 13 husk 0.90 0.10 <0.10
13 straw 1.0 0.50 <0.20
3 whole 0.39 <0.05 -
Cabbage S. Africa 13 whole 0.22 <0.05 <0.10
3 whole 0.50 <0.05 -
13 whole 0.38 <0.05 <0.10
28 whole <0.02 <0.04 -
Onions U.K. 14 whole 0.14 <0.04 -
7 whole 0.17 <0.04 -
6 whole 0.06 <0.012 -
Tomatoes S. Africa 20 whole 0.08 <0.012 -
6 whole 0.34 <0.012 -
49 whole <0.01 <0.02 <0.05
Tomatoes S. Africa 28 whole 0.03 <0.02 -
49 whole 0.01 <0.02 <0.05
28 whole 0.10 <0.02 <0.05
TABLE 9 (Cont'd.)
Preharvest Residues present (ppm)
Crop Country Interval Crop monocrotophos amide plus conjugated
part methylol methylol
67 whole 0.18 <0.05 <0.05
Apples Australia 37 whole 0.51 <0.05 <0.05
8 whole 1.8 <0.05 0.06
Apples Italy 30 whole 1.0 <0.04 <0.03
Pears Italy 39 whole 0.15 <0.04 <0.05
14 roots 0.06 <0.02 0.10
Carrots S. Africa 21 roots 0.04 <0.02 <0.05
28 roots <0.01 <0.02 <0.05
1 Data from Shell Research Ltd.
2 Analysed only for unsubstituted amide.
Analyses of fruit with monocrotophos residues have demonstrated that
residues are mainly in the peel. Thus peeling removed 66% of residues
present in oranges, 35 - 70% in apples (Shell Research Ltd.; Shell
Chemical Co., undated) and 80 - 95% in grapefruit (Ibid.). In another
study to determine the levels of monocrotophos present in different
parts of apples, it was shown that 57 - 64% of the residues were
present in the pulp, 5% in the waxy surface layer, the remainder being
in the peel. The removal of the outer leaves of cabbages reduced the
monocrotophos levels by 95%, of cauliflower by 90% and of Brussels
sprouts by over 75% (Shell Research Ltd., undated).
Cold water washing can also reduce residues. Thus washing tomatoes
reduced residues of monocrotophos by up to 35% (Shell Development Co.;
Shell Chemical Co., undated). Domestic cooking reduced levels by
60 - 70% in green beans and 80 - 85% in Brussels sprouts (Shell
Research Ltd., undated).
The effect of the various steps in the commercial canning of beans
(Fahey et al., 1969) and tomatoes (Fahey et al., 1971) has been
studied. Cold water washing of beans effected a 27% reduction and
blanching a 40% reduction. Residues in the canned beans were only 2%
of the residues in the fresh beans. The processes of cold washing and
lye peeling reduced residues in tomatoes by 63% and 87%, respectively.
An 85% reduction in residues of monocrotophos was achieved in tomato
juice and a 91% reduction in whole tomatoes by canning. The process of
juicing oranges reduced the levels of monocrotophos from 0.8 ppm in
the pulp to 0.10 ppm and below (Shell Research Ltd., undated).
Commercial processing of raw coffee beans by removing the berry flesh
mechanically, followed by fermentation, and sun-drying to a moisture
level of 10%, reduced residues of 0.08 ppm monocrotophos in the raw
beans to below the limit of determination of the analytical method
(less than 0.01 ppm) in the processed beans (Ibid.).
The preparation of refined soybean oil reduced residues of 0.13 ppm
monocrotophos in beans to less than 0.01 ppm in the finished oil
(Shell Chemical Co., undated). Similarly, residues of 0.03 ppm in
kernels were reduced to less than 0.01 ppm in processed corn meal and
oil (Ibid.).
With cottonseed oil (Shell Research Ltd., unpublished) it has been
shown that 1 ppm monocrotophos added to the crude oil was reduced to
below the limit of determination of the analytical method (<0.01 ppm)
by the alkali refining step.
Experiments to determine the residues of monocrotophos likely to occur
in beer prepared from monocrotophos-treated hops have demonstrated
that residues of 1.8 ppm monocrotophos in hops were reduced to levels
at or below the limit of determination of the analytical method (0.02
ppm) in filtered and unfiltered beer (Ciba-Geigy Ltd., undated).
During the commercial preparation of citrus pulp cattle feed (Westlake
et al., 1970) from monocrotophos-treated oranges, residues were
reduced to below the limit of determination (less than 0.03 ppm), with
over 50% being lost during grinding and liming and the remainder
during drying.
The following sections summarize these effects in terms of individual
crops, in order to give an estimate of upper levels to be expected in
foods when they are ready to eat.
Cottonseed and soybean oils
Since the process of alkali refining destroys residues in crude
cottonseed oil at levels well above those in practice, refined
cottonseed oil products will not contain detectable residues of
monocrotophos. The same conclusion may be drawn for soybean oil.
Brassicas
In the case of Brussels sprouts, removal of outer leaves followed by
cooking reduced typical levels by 95%. Thus sprouts with initial
residues of 0.2 ppm (proposed tolerance levels) would not be expected
to contain more than 0.01 ppm when cooked and ready to be eaten.
In the case of cauliflower, removal of outer leaves alone reduced
residue levels some 10-fold so that even without cooking, levels in
trimmed cauliflower at an initial level of 0.5 ppm (proposed
tolerance) would be reduced to 0.05 ppm. In the case of cabbage, where
removal of outer leaves reduced residues 20-fold, cabbage with
residues at the proposed tolerance level of 0.05 ppm would not be
expected to contain more than 0.03 ppm when trimmed ready for cooking.
Fresh tomatoes
Fresh tomatoes with 0.5 ppm monocrotophos residues (proposed
tolerance) would lose, according to available data, an average 25% of
their residues, leading to a little less than 0.4 ppm at the point of
consumption.
Commercially processed tomatoes
The combined effects of washing and lye peeling reduced residues to
approximately 5% of their initial value so that residues in tomatoes
ready for canning would not exceed 0.01 ppm, a level which would be
further reduced by canning itself.
Similar considerations apply to canned tomato juice, and neither of
these finished products would contain detectable residues of
monocrotophos.
Beans
Domestic cooking of beans with monocrotophos residues at the tolerance
level of 0.2 ppm would reduce these to approximately 0.1 ppm.
Commercial canning would reduce these levels to below 0.01 ppm,
whereas freezing, which involves washing and blanching, would reduce
levels to 0.1 ppm.
Coffee
Coffee beans with initial residue at the tolerance level of 0.1 ppm
would be unlikely to contain detectable residues (< 0.01 ppm) after
fermentation and drying. For this reason, subsequent processes such as
roasting and brewing were not studied.
Hops
Hops with residues at the tolerance level do not give rise to
detectable residues in beer.
Citrus fruit
Residues in citrus fruit were reduced to an average of 30% of their
initial value on peeling, so that the raw fruit after peeling would
not contain residues above 0.15 ppm, where initial residues were at
the tolerance level of 0.5 ppm.
Juicing reduced levels of around 0.8 ppm in the pulp of oranges to
0.10 ppm in the juice.
Potatoes, maize grain and root vegetables
With the exception of carrots, where two samples out of five were
reported to contain low residues, these crops, treated as recommended,
have not contained measurable residues so that work to demonstrate the
effects of processing on reducing residues could not meaningfully be
undertaken.
Sugarcane and sugar beet
In the case of sugarcane the absence of detectable residues has been
demonstrated not only in the raw commodity but also in the
manufactured products. In neither crop were residues detected
following recommended treatments.
METHODS OF RESIDUE ANALYSIS
Residues of monocrotophos can be determined by specific methods
utilizing either gas-liquid chromatographic procedures or a
cholinesterase inhibition method.
Gas chromatographic methods
Gas chromatographic methods of analysis for monocrotophos are the
methods of choice, based on accuracy, specificity, sensitivity and
speed.
Monocrotophos can be determined by the general procedure using the
flame photometric detector (Beroza and Bowman, 1968; Brody and Chaney,
1966). Specific methods for monocrotophos using this detector have
been developed (Shell Research Ltd., undated; Bowman and Beroza, 1967)
and allow analyses of crops down to a limit of determination of 0.01
ppm. The following procedure (Shell Research, Ltd., undated) has
proved satisfactory in analysing crops: samples are extracted by
maceration with chloroform, low water content crops are first dampened
with water. Co-extracted natural products are removed using a column
adsorption chromatographic clean-up technique. The
monocrotophos-containing extract is analysed using the flame
photometric detector (FPD). Using this procedure, mean recoveries are
75 - 120% from crops at the 0.05 - 0.20 ppm level.
The thermionic detector has also been employed in the analyses of
crops for residues of monocrotophos (Shell Chemical Co., undated). In
this method, the sample is extracted with dichloromethane followed by
clean-up using a series of solvent exchanges and washings, prior to
injection of an aliquot of the extract onto the GLC instrument. Mean
recoveries with this method are 80 - 120% for crops, with a limit of
determination of 0.03 - 0.05 ppm.
Enzymatic inhibition
Specific enzymatic methods for the detection and determination of
monocrotophos in crops and milk have been developed (Shell Development
Co., undated). The samples for analysis are extracted with chloroform,
followed by a solvent exchange to hexane and concentration of the
extract. Column chromatography using a gradient column elution
technique with hexane/dichloromethane removes co-extractives. The
separated monocrotophos is transferred to water and determined by
enzyme inhibition spectrophotometric methods. Using this procedure,
the limit of determination of monocrotophos is about 0.10 ppm in crops
and 0.01 ppm in milk. Recoveries are in the range of 70 - 125%.
Methods for analyses of monocrotophos breakdown products
The analytical data for the N-methylol and for the unsubstituted amide
reported in this summary were developed using the method described for
monocrotophos (Shell Research Ltd., undated). The conjugate of the
N-methylol was determined using the method, which depends on
converting the conjugate to the sec-butyl thio-ether of the methylol
and subsequent determination by T.L.C. and enzyme inhibition (Ibid.).
A further method, using the thermionic detector (Griang and Beckman,
1968) has been developed for analysing crops for residues of
O-desmethyl monocrotophos, N-hydroxy methyl monocrotophos and its
conjugate.
NATIONAL TOLERANCES
Officially approved tolerances for monocrotophos have been established
in some countries and examples of these are given in Table 10.
TABLE 10
Examples of national tolerances as reported to the meeting
Country Crop(s) Tolerance
(ppm)
Australia Cottonseed, potatoes 0.1
Apples, pears 1.0
Italy Apples, pears 0.5
South Africa All treated crops 0.1
U.S.A. Cottonseed, potatoes,
sugarcane 0.1
Venezuela Cottonseed, rice, maize,
potatoes, sugarcane,
vegetables, citrus fruit 0.1
Yugoslavia All treated crops 0.1
APPRAISAL
Monocrotophos is an organophosphorus insecticide which finds its main
use for foliar application to cotton. It is also recommended for
application against foliage pests of maize, sugarcane, sugar beet,
vegetables, potatoes and certain fruits. It possesses both systemic
and residual contact properties.
Approved uses involve the application of from 0.25 - 1.0 kg a.i./ha.
The recommended pre-harvest interval varies according to crop and
country, ranging from seven days on potatoes to 30 days on fruit and
vegetables.
Extensive studies have shown that degradation of monocrotophos
residues on and in plants can occur by hydrolytic and/or oxidative
mechanisms. The products of hydrolysis are non-cholinesterase
inhibiting and of low toxicity. Only insignificant amounts of
secondary metabolites have been found during radiotracer studies.
Volatilization appears to be the major factor in the rapid loss of
residues following application. Monocrotophos itself is the principal
residue found on most plants even 7 - 28 days after application.
Metabolism studies suggest that ruminants receiving
monocrotophos-treated feedstuff metabolize the residue to metabolites
similar to those found on plants. There appears to be no tendency for
residues to accumulate in animals receiving contaminated rations. Only
low levels of monocrotophos and trace amounts of breakdown products
were detected in red meat, liver and milk of cattle receiving
feedstuffs containing monocrotophos at levels many times the maximum
likely to occur in practice.
Residue data, obtained from supervised trials in 24 different
countries clearly demonstrated the level of residues to be expected
under widely differing conditions on a wide range of crops. Except for
samples of leafy materials collected within one or two days of
application of heavy dosages of monocrotophos, residues in excess of
1.0 ppm were seldom encountered.
No substantial residue occurs in any fraction of the cotton plant used
for food, even when unprocessed.
As cotton foliage retains significant residues for more than three
weeks following application of approved rates of monocrotophos,
feeding of treated cotton foliage to cattle should be avoided.
Domestic processes, such as washing, peeling and cooking reduce the
levels of monocrotophos residues in fruit and vegetables by between
35% and 95%. Commercial processes often have greater effects on
reducing residues of monocrotophos. The peeling of citrus fruits
removes 60 - 73% of the total residue. Beer produced from treated hops
was shown to contain no detectable residues. The absence of residue
has been demonstrated in sugarcane and in sugar products manufactured
therefrom.
For determining monocrotophos residues, either gas-liquid
chromatography or cholinesterase inhibition methods are used, with the
former being the methods of choice from the aspect of accuracy,
specificity, sensitivity and speed. The limit of determination is 0.01
ppm. Using GLC methods recoveries of 75 - 120% were obtained from crop
material containing 0.05 - 0.2 ppm. Several of the available methods
appear suitable for regulatory purposes, but the method of Bowman and
Beroza (1967) is recommended.
RECOMMENDATIONS
The following recommended tolerances for animal products reflect a
value at or just above the limit of determination. The time interval
between application and harvest which has been used in determining the
maximum residue limits is appropriate to the agricultural practices in
numerous countries.
TOLERANCES
Interval between
Foodstuff Tolerance treatment and
(ppm) harvest (days)
Cottonseed 0.1 14
Cottonseed oil (raw) 0.05* 14
Potatoes 0.05* 7
Maize (grain) 0.05* 14
Tomatoes 0.5 21
Brussels sprouts 0.2 15
Cabbage, cauliflower 0.2 21
Carrots, turnips 0.05* 14
Onions 0.1 10
Sugar beet 0.05* 14
Peas 0.1 15
Beans 0.2 21
Soybeans 0.05* 14
Coffee (raw beans) 0.1 42
Hops (dried) 1 30
Citrus fruit 0.2 28
Apples and pears 1 (temporary 28
1975)
Meat and edible offal of
cattle, sheep, goats,
pigs and poultry 0.02* from feeding
Milk 0.002* treated plant
Milk products 0.02* products
Eggs (shell free) 0.02* "
* at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Studies on human exposure.
2. Information on the incidence of residues in apples and pears.
REFERENCES
Beroza, M. and Bowman, M.C. (1968) Chromatography of pesticide
residues containing phosphorus or sulphur with the flame photometric
detector. Environ. Sci., Technol., 2: 450.
Bowman, M.C. and Beroza, M. (1967) Chromatographic analysis of azodrin
and Bidrin. J. Agr. Fd. Chem., 15: 465
Brody, S.S. and Chaney, J.E. (1966) Flame photometric detector. J.
Gas. Chromat., 4: 42.
Bull, D.L. and Lindquist, D.A. (1964) Metabolism of 3-hydroxy-N,
N-dimethyl-crotonamide dimethylphosphate by cotton plants, insects and
rats. J. Agr. Fd. Chem., 12: 310-317.
Bull, D.L. and Lindquist, D.A. (1967) Metabolism of
3-hydroxy-N-methyl-cis-crotonamide dimethyl phosphate (azodrin) by
insects and rats. J. Agr. Fd. Chem., 14: 105-109.
Gaines, T.B. (1969) Acute toxicity of pesticides. Tox. Appl.
Pharmacol., 14: 515-534.
Ciba-Geigy Ltd. (undated) Reports on monocrotophos nos. 86 - 88,
90 - 93, 97, 111, 116 - 131. (unpublished)
Einsenlord, G. and Loquram, G.S. (1965) Results of short route
reproduction study of rats fed diets containing SD 9129 insecticide
over three generations. Data from the Hine Laboratory submitted by the
Shell Chemical Company. (unpublished)
Einsenlord, G. and Loquram, G.S. (1966) Results of long route
reproduction study of rats fed diets containing SD 9129 insecticide
over three generations. Data from the Hine laboratory submitted by the
Shell Chemical Co. (unpublished)
Fahey, J.E., Gould, G.E. and Nelson, P.E. (1969) Removal of gardona
and azodrin from vegetable crops by commercial preparative methods. J.
Agr. Fd. Chem., 17: 1204.
Fahey, J.E., Gould, G.E. and Nelson, P.E. (1971) Removal of azodrin
residues from tomatoes by commercial preparative methods. J. Agr. Fd.
Chem., 19: 81.
Griang, B.Y. and Beckman, H.F. (1968) Determination of bidrin, azodrin
and their metabolites with the thermionic detector. J. Agr. Fd. Chem.,
16: 899.
Hunter, C.G. (1964) The efficacy of atropine and oxime therapy as an
antidote to poisoning by SD 9129 in guinea pigs. Report Tunstall
Laboratory submitted by the Shell Chemical Co. (unpublished)
Johnston, C.D., Thompson, W.M. and Donoso, J. (1967a) Azodrin safety
evaluation by a chronic feeding study in the dog for two years. Data
from Woodard Research Corporation submitted by Shell Chemical Co.
(unpublished)
Johnston, C.D., Thompson, W.M. and Donoso, J. (1967b) Azodrin safety
evaluation by a chronic feeding study in the rat for two years. Data
from Woodard Research Corporation submitted by Shell Chemical Co.
(unpublished)
Lindquist, D.A. and Bull, D.L. (1967) Fate of
3-hydroxy-N- methyl-cis-crotonamide dimethyl phosphate in cotton
plants. J. Agr. Fd. Chem., 15: 267-272.
Menzer, R.L. and Casida, J.E. (1965) Nature of toxic metabolites
formed in mammals, insects and plants from 3-(dimethoxy
phosphinyloxy)-N,N-dimethyl-cis-crotonamide and its N-methyl
analogue. J. Agr. Fd. Chem., 13: 102-112.
Potter, J.C. (1965) Cattle feeding studies with 32P-labelled azodrin.
Data submitted by Shell Chemical Co. (unpublished)
Reiff, B. (1969) Pharmacological studies into the toxic actions of
cholinesterase inhibitors: (a) The effect of antidotes on the
subcutaneous toxicity of azodrin in the rat. Report of Tunstall
Laboratory submitted by Shell Chemical Co. (unpublished)
Shellenberger, T.E. (1965a) Potentiation studies - rats. Data from
Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. (1965b) Demyelination study - hens. Data from
Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. (1966a) Acute toxicity of azodrin and metabolites.
Data from Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. (1966b) Cholinesterase inhibition and
toxicological evaluations of two organophosphate pesticides in
Japanese quail. Tox. Appl. Pharm. 8: 22.
Shellenberger, T.E. and Newell, G.W. (1963) Acute oral toxicity. Data
from Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. and Newell, G.W. (1964) Subacute toxicity and
cholinesterase study of Shell compound SD 9129 - rat and dog. Data
from Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shell Chemical Co. (1966) Subacute toxicity and cholinesterase study
of Shell compound SD 13311 in rats. Data from Stanford Research
Institute submitted by Shell Chemical Co. (unpublished)
Shell Chemical Co. (1972) Identification of unknown 4. Report Shell
Chemical Co. (unpublished)
Shell Chemical Co. (undated) Reports on monocrotophos nos 50 - 67, 69
- 74, 76, 77, 79 - 81, 89, 101, 115, 132 - 136, 139 - 151, 153, 172,
176, 182 - 184. (unpublished)
Shell Development Co. (undated) Reports on monocrotophos nos. 68, 75,
78, 82 - 85, 100, 110, 112, 114, 137, 138, 152, 155, 171, 173 - 175,
177 - 181. (unpublished)
Shell Research Ltd. (undated) Reports on monocrotophos nos. 1 - 49,
94, 95, 98, 99, 154, 156 -170. (unpublished)
Thorpe, E. and Dix, M. (1972) Toxicity studies on azodrin:
Teratological studies in rabbits. Summary submitted by Shell Chemical
Co. (unpublished)
Westlake, W.E., Gunther, F.A. and Jeppson, L.R. (1970) Persistence of
azodrin residues on and in Valencia oranges and in
laboratory-processed citrus pulp cattle feed. J. Agr. Fd. Chem., 18:
864.
Young, J.R. and Bowman, M.C. (1967) Azodrin for corn earworm and fall
armyworm control and its persistence in sweet corn. J. Econ. Ent., 60:
1282.
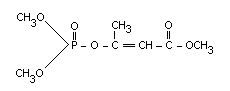 Physical and chemical properties of technical monocrotophos
Physical state: reddish-brown mixture of solid and liquid (at
25-30°C)
Odour: that of a mild ester
Melting point: 25-30°C (technical)
54-55°C (pure)
Vapour pressure: 7 x 10-5 mm Hg (at 20°C)
Solubility: soluble in water, acetone and alcohol; very
slightly soluble in mineral oils.
Stability: stable when stored in glass and polyethylene
containers. Technical grade has a half-life
of 2 500 days at 38°C. Rate of hydrolysis in
solution is slow.
Half-life in solution (2 ppm) at pH 7.0 and
38°C is 23 days; at pH 4.6 and 100°C,
half-life is 80 minutes.
Purity:
Analysis of a typical sample of technical monocrotophos gave the
following results:
% w
Monocrotophos 77
Dimethyl-1-methyl-2-methylcarbamoyl-vinyl phosphate (Z)- 9
N-methylacetoacetamide 2
Dimethyl phosphate 5
Others (less than 1% w each) 7
100
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Following an oral dose of 1 mg/kg to male and female rats,
monocrotophos was rapidly eliminated in the urine primarily as
hydrolysis products (70% of the urinary excretion within six hours),
unchanged monocrotophos (25%) and traces of the N-hydroxymethyl and
desmethyl metabolites. In 24 hours the rate of excretion had
diminished substantially, indicating rapid absorption and elimination
(Menzer and Casida, 1965). Monocrotophos administered orally to a goat
was found in the milk within one hour after treatment. Other
metabolites were found in lesser quantity than the parent
monocrotophos. Within 24 hours, the major quantity had cleared, and
only minute residues were found (Menzer and Casida, 1965).
Monocrotophos is a minor metabolite of dicrotophos (Bidrin(R)), its
N,N-dimethyl crotonamide analogue, and treatment of rats with
dicrotophos yielded small quantities of monocrotophos in the urine
within two hours (Bull and Lindquist, 1964).
Monocrotophos was fed to lactating dairy cows at a level of 45 ppm in
the diet. Monocrotophos and two metabolites were found in milk. In
urine further metabolites were observed (Potter, 1965).
Biotransformation
Monocrotophos injected into bean plants was metabolized to the
N-hydroxy-methylamide (SD 12657) and the amide (SD 11319).
Monocrotophos was more persistent in this plant study than
dicrotophos-Bidrin(R), its N,N dimethyl analogue (Menzer and Casida,
1965).
In cotton, monocrotophos has been shown to be metabolized to the
hydroxymethyl derivative (SD 12657) but not to the amide (SD 11319).
The oxidative route of metabolism yielding substituted crotonamides
was found to be slower than the metabolic route yielding substituted
phosphoric acid esters (Lindquist and Bull, 1967). A major
water-soluble metabolite observed in plants by these authors was
identified as a glycoside of the N-hydroxymethyl (SD 12657) derivative
(Shell Chemical Co., 1972).
The metabolic fate of monocrotophos in mammals has been well
documented (Menzer and Casida, 1965; Bull and Lindquist, 1964; 1967).
These data are summarized in Figure 1.
Effect on enzymes and other biochemical parameters
As with other organo-phosphorus esters, monocrotophos acts on the
organism as a direct cholinesterase inhibitor. The selectivity of
inhibition of cholinesterase from various sources in the body suggests
that blood cholinesterase activity is the most sensitive parameter for
exposure; brain cholinesterase activity is depressed only at higher
levels of exposure. Penetration into the brain may be the cause,
rather than a difference in sensitivity, although in vitro studies
have shown that monocrotophos has a somewhat greater affinity (k2) and
activity (I50) against rat RBC cholinesterase (Reiffy 1969).
Monocrotophos is metabolized to compounds which are less potent
inhibitors of human plasma cholinesterase, as evidenced by in vitro
pI50 values, i.e., monocrotophos (SD 9129) - 6.5; N-Methyl hydroxy
monocrotophos - 5.9; N-desmethyl monocrotophos - 5.6 (Menzer and
Casida, 1965).
Physical and chemical properties of technical monocrotophos
Physical state: reddish-brown mixture of solid and liquid (at
25-30°C)
Odour: that of a mild ester
Melting point: 25-30°C (technical)
54-55°C (pure)
Vapour pressure: 7 x 10-5 mm Hg (at 20°C)
Solubility: soluble in water, acetone and alcohol; very
slightly soluble in mineral oils.
Stability: stable when stored in glass and polyethylene
containers. Technical grade has a half-life
of 2 500 days at 38°C. Rate of hydrolysis in
solution is slow.
Half-life in solution (2 ppm) at pH 7.0 and
38°C is 23 days; at pH 4.6 and 100°C,
half-life is 80 minutes.
Purity:
Analysis of a typical sample of technical monocrotophos gave the
following results:
% w
Monocrotophos 77
Dimethyl-1-methyl-2-methylcarbamoyl-vinyl phosphate (Z)- 9
N-methylacetoacetamide 2
Dimethyl phosphate 5
Others (less than 1% w each) 7
100
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Following an oral dose of 1 mg/kg to male and female rats,
monocrotophos was rapidly eliminated in the urine primarily as
hydrolysis products (70% of the urinary excretion within six hours),
unchanged monocrotophos (25%) and traces of the N-hydroxymethyl and
desmethyl metabolites. In 24 hours the rate of excretion had
diminished substantially, indicating rapid absorption and elimination
(Menzer and Casida, 1965). Monocrotophos administered orally to a goat
was found in the milk within one hour after treatment. Other
metabolites were found in lesser quantity than the parent
monocrotophos. Within 24 hours, the major quantity had cleared, and
only minute residues were found (Menzer and Casida, 1965).
Monocrotophos is a minor metabolite of dicrotophos (Bidrin(R)), its
N,N-dimethyl crotonamide analogue, and treatment of rats with
dicrotophos yielded small quantities of monocrotophos in the urine
within two hours (Bull and Lindquist, 1964).
Monocrotophos was fed to lactating dairy cows at a level of 45 ppm in
the diet. Monocrotophos and two metabolites were found in milk. In
urine further metabolites were observed (Potter, 1965).
Biotransformation
Monocrotophos injected into bean plants was metabolized to the
N-hydroxy-methylamide (SD 12657) and the amide (SD 11319).
Monocrotophos was more persistent in this plant study than
dicrotophos-Bidrin(R), its N,N dimethyl analogue (Menzer and Casida,
1965).
In cotton, monocrotophos has been shown to be metabolized to the
hydroxymethyl derivative (SD 12657) but not to the amide (SD 11319).
The oxidative route of metabolism yielding substituted crotonamides
was found to be slower than the metabolic route yielding substituted
phosphoric acid esters (Lindquist and Bull, 1967). A major
water-soluble metabolite observed in plants by these authors was
identified as a glycoside of the N-hydroxymethyl (SD 12657) derivative
(Shell Chemical Co., 1972).
The metabolic fate of monocrotophos in mammals has been well
documented (Menzer and Casida, 1965; Bull and Lindquist, 1964; 1967).
These data are summarized in Figure 1.
Effect on enzymes and other biochemical parameters
As with other organo-phosphorus esters, monocrotophos acts on the
organism as a direct cholinesterase inhibitor. The selectivity of
inhibition of cholinesterase from various sources in the body suggests
that blood cholinesterase activity is the most sensitive parameter for
exposure; brain cholinesterase activity is depressed only at higher
levels of exposure. Penetration into the brain may be the cause,
rather than a difference in sensitivity, although in vitro studies
have shown that monocrotophos has a somewhat greater affinity (k2) and
activity (I50) against rat RBC cholinesterase (Reiffy 1969).
Monocrotophos is metabolized to compounds which are less potent
inhibitors of human plasma cholinesterase, as evidenced by in vitro
pI50 values, i.e., monocrotophos (SD 9129) - 6.5; N-Methyl hydroxy
monocrotophos - 5.9; N-desmethyl monocrotophos - 5.6 (Menzer and
Casida, 1965).
 TOXICOLOGICAL STUDIES
Special studies on metabolites
The acute toxicity of metabolites of monocrotophos in mouse and rat is
summarized in Table 1.
The acute toxicity of components of technical monocrotophos in rat is
summarized in Table 2.
TABLE 1 Acute toxicity of monocrotophos metabolites
Metabolite Reference
N-methyl hydroxy monocrotophos (SD = 12657)
Mouse ip LD50 = 12 mg/kg (Menzer and Casida, 1965)
Rat oral LD50 = 27 mg/kg (Shellenberger, 1966a)
N-desmethyl monocrotophos (SD 11319)
Mouse ip LD50 = 3 mg/kg (Menzer and Casida, 1965)
Mouse oral LD50 = 5.5 (Shellenberger, 1966a)
Glycoside of N-desmethyl monocrotophos (SD 13311)
Rat (M) oral LD50 = 168 mg/kg (Shell Chemical Co., 1966)
N-methyl acetoacetamide
Rat oral LD50 = >2 000 mg/kg (Shellenberger, 1966a)
TABLE 2 Acute toxicity of components of technical monocrotophos
Component Species Route LD50 (mg/kg)
Dimethyl phosphate of
3-hydroxy-N-methyl-trans-crotonamide Rat Oral 420
Dimethyl phosphate of 2-chloro-3
hydroxy-N-methyl-cis-crotonamide Rat Oral 14
Dimethyl phosphate of 2-chloro-3
hydroxy-N-methyl-trans-crotonamide Rat Oral 140
Short-term studies on the glycoside metabolite (SD 13311)
Groups of rats (42 males and 42 females per group, except for the
highest level which had 22 of each sex) were fed the glycoside
metabolite of monocrotophos (a glycoside of the dimethyl phosphate of
3-hydroxy-N-(hydroxymethyl)-cis-crotonamide) for 9 days at levels of
0, 1, 3, 9, 18 and 90 ppm (the 1 ppm was fed for seven weeks and the
dosage increased to 18 ppm for the final five weeks). Rats were fed a
normal diet for four weeks after the feeding study ended. Slight
growth effects were noted at 90 ppm from 7-12 weeks in both sexes.
Blood cholinesterase was depressed at 9 ppm in both sexes. At 18 ppm,
brain cholinesterase was depressed. Cholinesterase activity was not
affected at 3 ppm. No effects were observed on blood parameters or
gross and microscopic histology (Shell Chemical Co., 1966).
Special studies on neurotoxicity
Groups of adult White Leghorn hens (ten hens per group) were fed
monocrotophos in the diet at levels of 0, 1, 10 and 100 ppm for four
weeks. A single group of ten hens was fed TOCP in the diet at 1 000
ppm for four weeks. At the end of the feeding interval, five birds
from each group were sacrificed, and histological examinations of
brain, spinal cord and sciatic nerve were performed. Physiological
response, body-weight, food consumption and egg production data were
collected. Monocrotophos at all dose levels produced a weight loss in
hens. Egg production was not affected at 1 ppm but was inhibited at 10
and 100 ppm. Mortality occurred at 100 ppm, and although several birds
showed tremors 10-12 days after feeding started, all were able to
stand and walk normally. TOCP fed birds developed typical signs of
ataxia after 16-17 days. All survivors were unable to stand at the end
of the treatment period. Histological examination of TOCP-treated hens
showed severe demyelination in the posterior horns of the spinal cord.
Examination of nervous tissue from monocrotophos fed and control hens
showed slight evidence of demyelinated tracts, primarily in the
anterior horns. No differences were noted in controls and hens
undergoing monocrotophos treatments. Monocrotophos does not produce
delayed neurological signs of poisoning when compared to the response
induced by TOCP (Shellenberger, 1965b).
Special studies on potentiation
Male rats were given oral doses of a combination of monocrotophos and
the following anticholinesterase compounds, with no signs of
potentiation: dioxathion, dimethoate, carbaryl, disulfoton, EPN,
Folex, malathion, parathion, schradan, demeton, azinphos-methyl,
dicrotophos, crotoxyphos, coumaphos, dichlorvos, diazinon, naled,
trichlorphon, ethion, mevinphos, carbophenothion and phosphamidon.
Potentiation of the acute oral toxicity was observed with
monocrotophos in combination with fenchlorphos (Shellenberger, 1965a).
Special studies on reproduction
Groups of rats (10 males and 20 females per group) were fed
monocrotophos in the diet at 0, 2, 5, 12 and 30 ppm, and subsequently
mated. The progeny of this mating were maintained on this dietary
regime and mated. This was followed for three generations (Eisenlord
and Loquram, 1965). In a separate study groups of rats (10 males and
20 females per group) were fed monocrotophos at the same levels and
subjected to a three-generation, two-litter per generation study
(Eisenlord and Loquram, 1966).
Effects due to monocrotophos were noted in both studies at levels of 5
ppm and above. Appearance and behaviour was affected at 12 and 30 ppm,
as manifested in stunted growth and emaciation. Thinned or missing
hair on the flanks or head was seen in all monocrotophos groups among
adult females and pups, but not in adult males or controls. The level
of 30 ppm was lethal to pups and reduced pregnancies. Pup mortality
was evidenced at 5 ppm in both studies and absent at 2 ppm. Weight of
parents was adversely affected at 30 ppm. At 12 ppm, several parents
were affected (F2 and F3 males in one study and F2 females and F3
males in the second). No effects were noted on any parameter at 2 ppm.
Gross and histopathological examinations showed no differences from
the control values.
Special studies on teratogenicity
Groups of pregnant rabbits received oral daily doses of 0.7 and 2
mg/kg monocrotophos from day 6 to 18 of gestation. On day 28 of
pregnancy, the foetuses were removed and examined. No compound-related
teratogenic effects were observed (Thorpe and Dix, 1972).
Acute toxicity
The results of acute toxicity studies in animals are summarized in
Table 3.
TABLE 3 Acute toxicity of monocrotophos in animals
Species Sex Route LD50 Reference
(mg/kg)
Rat M IP 5 Bull & Lindquist, 1967
M Oral 14-23 Shellenberger & Newell, 1963
Shellenberger, 1965b; 1966a
F SC 7 Reiff, 1969
M Oral 18 Gaines, 1969
F Oral 20 Ibid.
Mouse M&F IP 8 Menzer & Casida, 1965
Oral 15 Shellenberger & Newell, 1963
Guinea Pig M&F SC app.60 Hunter, 1964
Signs of poisoning following monocrotophos are typical of other
anticholinesterase organo-phosphates. Toxic signs occur rapidly and in
animals include tremors, lacrimation, diarrhea, salivation, tonic and
clonic convulsions and other signs of cholinergic stimulation. Deaths
occur rapidly, usually within four hours, and animals surviving 24
hours generally recover and appear normal.
Atropine alone, but preferably in combination with reactivators (P2S
or Toxogonin), was shown to be an effective antidote to monocrotophos
poisoning in rodents (Hunter, 1964: Reiff, 1969).
Short-term studies
Japanese quail
Groups of Japanese quail (12 males and 12 females per group) were fed
monocrotophos at 0, 0.5, 5 and 50 ppm in the diet for three weeks.
Mortality and weight loss occurred at 50 ppm. Food consumption was
reduced at 5 ppm, while egg production was normal. Blood
cholinesterase activity was depressed at all feeding levels, while
brain cholinesterase activity was depressed at 5 ppm and above. At 5
ppm in the diet of adult females, monocrotophos had no effect on the
embryo heart beat or development of the chicks (Shellenberger, 1966b).
Rat
Groups of rats were fed monocrotophos in the diet at dosage levels of
0 (42 males and 42 females), 0.5, 1.5 (30 males and 30 females/group),
15 (42 males and 42 females), 45 and 135 ppm (12 males and 12
females/group) for twelve weeks. Growth was depressed at 45 ppm.
Cholinesterase depression was observed on haematology or gross and
microscopic pathology (Shellenberger and Newell, 1964).
Dog
Groups of Beagle dogs were fed monocrotophos in the dry diet at levels
of 0, 0.5, 1.5, 15 (four dogs of each sex/group), 45 and 135 ppm (two
dogs of each sex/group) for 13 weeks. After 8 weeks, the dogs fed 135
ppm were changed to 270 ppm for weeks 9-10, then to 540 ppm for weeks
11-12, and to 1 080 ppm for the final week.
Body-weights were reduced at dietary levels of 270 ppm. Organ weights
were also affected in this high feeding level group. Cholinesterase
depression was observed at 1.5 ppm in blood and brain. There were no
compound-related effects on haematological values, blood biochemistry,
organ weights or gross and microscopic pathology (Shellenberger and
Newell, 1964).
Groups of dogs were fed monocrotophos in the diet for two years at
levels of 0 (four males and four females) 0.16, 1.6, 16 ppm (three
males and three females/group) and for one year at 100 ppm (two males
and two females). Moderate cholinergic stimulation was observed at 100
ppm as tremors and salivation. Blood cholinesterase depression was
noted at 16 and 100 ppm in both males and females. Marginal brain
cholinesterase depression was also noted at a dose level of 1.6 ppm
after 93 and 104 weeks. No effects were noted on growth, mortality,
haematological parameters, urinalyses or physiological measurements.
Gross and histopathological examination showed no compound-related
effects (Johnston et al., 1967a).
Long-term studies
Rat
Groups of rats were fed monocrotophos in the diet for two years at
levels of 0 (40 males and 40 females), 1, 10 and 100 ppm (25 males and
25 females/group). Growth of males and females at 100 ppm was
depressed. Food consumption was also lower than controls at this
level. RBC and plasma cholinesterase depression was observed at 10 and
100 ppm in both males and females. Brain cholinesterase depression in
males and females was significantly inhibited at all levels. At 100
ppm, the weights of liver, gonad, thyroid and pituitary were reduced
in females. Thyroid and pituitary/body-weight ratios were depressed at
100 ppm. No effects were noted on survival, haematology or
histopathological examination of organs and tissues (Johnston
et al., 1967b).
COMMENT
Monocrotophos is rapidly absorbed, metabolized and eliminated by
mammals. Plant and animal metabolites are similar.
Potentiation was observed with fenchlorphos but not with other
cholinesterase-inhibiting compounds. No delayed neurotoxic effects
were observed.
Short-term studies in rats and dogs indicate no-effect levels at 0.5
ppm for cholinesterase depression.
In long-term studies a no-effect level has not been demonstrated in
the rat, the lowest dose tested (1 ppm) causing significant brain
cholinesterase depression.
In dog, two-year studies indicated a no-effect level of 0.16 ppm with
regard to brain cholinesterase depression. An effect was observed at
1.6 ppm.
In estimating an ADI for this compound, it was noted that in rat a
firm no-effect level was 0.5 ppm, with minimal effect level at 1 ppm.
In dog a no-effect level of 0.5 ppm was also demonstrated but the
minimal effect level was 1.5 ppm. Since the no-effect level is more
closely defined in rat, the latter species was used as a basis for
determining the ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.5 ppm in the diet, equivalent to 0.025 mg/kg/day
Dog: 0.5 ppm in the diet, equivalent to 0.0125 mg/kg/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0003 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Monocrotophos is an organo-phosphorus insecticide, which finds its
main use for foliar application to cotton (over 80% of monocrotophos
applied). It is also recommended for application against foliage pests
of maize, sugar cane, sugarbeet, vegetables, potatoes and certain
fruits. It is particularly effective against Lepidoptera, Homoptera
and certain Coleoptera, acting by both systemic and residual contact
properties.
Monocrotophos is known to be officially registered and/or approved for
use in the following countries:
Angola Yugoslavia
Argentina Mexico
Australia Mozambique
Austria Nicaragua
Brazil Pakistan
Bulgaria Peru
Chile Philippines
South Africa
Colombia Spain
Costa Rice Sudan
Ecuador Switzerland
El Salvador Thailand
Egypt Trinidad
France Turkey
Greece Uruguay
Guatemala U.S.A.
India Venezuela
Israel Viet-Nam
Italy Zambia
USE RECOMMENDATIONS
Typical maximum recommended application rates are: 1.0 kg a.i./ha for
cotton, potatoes and fruit; 1.5 kg a.i./ha for maize; 0.8 kg a.i./ha
for sugar cane; and 0.5 - 0.6 kg a.i./ha for vegetables and sugar
beet.
The recommended minimum period between treatment and harvest varies
from country to country, but is generally about 7 - 15 days for
vegetables and potatoes, 14 days for cotton, 21 days for tomatoes,
maize and citrus and 28 - 30 days for other crops.
Multiple applications of monocrotophos are often made, depending on
pest incidence, and use recommendations allow for these treatments.
The detailed use recommendations are given in Table 4.
TABLE 4 APPLICATIONS OF MONOCROTOPHOS TO CROP FOLIAGE
Crop Recommended Recommended period
application rates between treatment
(kg. a.i./ha) and harvest (days)1
Cotton 0.25 -1.0 Australia - 21
Mexico - 14
S. Africa - 30
Spain - 30
U.S.A. - 21
Maize 0.25 -1.5 Colombia - 30
Uruguay - 28
Sugarcane 0.25 -0.75 Uruguay - 28
Venezuela - 30
U.S.A. - 30
Carrots, turnips, onions 0.25 -0.5
Potatoes 0.25 -1.0 Colombia - 30
Italy - 21
S. Africa - 14
Venezuela - 14
U.S.A. - 7
Sugar beet 0.25 - 0.80 Italy - 21
TABLE 4 (Cont'd.)
Brussels sprouts, 0.2 -0.6 Yugoslavia - 28
cauliflower and cabbage Colombia - 30
Peru - 15
Venezuela - 21
Vietnam - 21
Tomatoes 0.25 -1.0
Hops 0.25 -0.5
Soybeans 0.25 -0.5 Colombia - 30
Vietnam - 21
Peas and beans 0.5 Colombia - 30
Pom - 15
Apples, pears2 0.01 - 0.04% a.i. Australia - 28
Italy - 30
Citrus 0.5 - 1.0 Venezuela - 21
Coffee 0.25 - 0.8
1 Monocrotophos is not recommended for post-harvest use on agricultural
commodities.
2 Monocrotophos is recommended for use on apples and pears in Australia,
and Italy only.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in 24
countries on food crops grown under various conditions, using various
rates of application and pre-harvest intervals.
In most trials, dosage rates, number of applications and pre-harvest
intervals were observed, in accordance with label recommendations, and
the data from these trials are summarized in Table 5. Residues of
monocrotophos in grain crops and root vegetables are usually below, or
close to, the limit of determination of the analytical method, while
leafy vegetables and fruit often contain detectable residues.
TABLE 5 Residues of monocrotophos in crops following recommended foliage treatments
Maximum Minimum
recommended preharvest Trials Results Range of
Crop rate interval residues (ppm)
(kg a.i./ha) (days) (no.) (no.)
Cotton (seed) 1.0 14 11 14 <0.02-0.08
Cotton (oil) 1.0 14 5 6 <0.02-0.04
Sugarcane 0.75 30 4 5 <0.05
Potatoes 1.0 7 14 27 <0.08-0.20
Maize grain 1.5 14 14 25 <0.01-0.05
Tomatoes 1.0 21 10 17 <0.01-0.51
Brussels sprouts 0.6 15 4 8 <0.05-0.21
Cauliflower 0.6 15 6 8 <0.01-0.14
Cabbage 0.6 21 9 23 <0.02-0.19
Carrots 0.5 14 2 5 <0.01-0.04
Turnips 0.5 10 1 3 <0.05
Onions 0.5 10 3 6 <0.02-0.09
Sugar beet 0.8 14 8 25 <0.01-<0.05
Peas 0.5 15 2 2 <0.07-0.60
Beans 0.5 21 3 6 <0.01-0.22
Soybeans 0.5 14 7 12 <0.01-0.03
Coffee 0.8 42 2 2 <0.01-0.08
Hops 0.5 28 1 2 0.3 - 0.41
Citrus fruit 1.0 21 11 26 <0.01-0.45
Apples 1.0 28 7 20 <0.05-1.0
Pears 1.0 28 5 10 <0.01-0.76
Cotton
Since the major outlet for monocrotophos is as a foliage insecticide
on cotton, numerous studies have been undertaken to examine the extent
of residues of monocrotophos in cottonseed and oil, if any, likely to
arise from recommended treatments.
Cottonseed
Monocrotophos is recommended for application to cotton foliage at
a maximum dosage rate of 1 kg a.i./ha, applications being made at
5-7 day intervals, where pest incidence dictates, with a final
treatment to harvest interval of 14 days. Trials in which these
recommendations were followed showed that residues of
monocrotophos in cottonseed were below the limit of determination
of the analytical method employed (<0.02 - <0.07 ppm) with the
exception of one sample which contained an apparent residue of
monocrotophos of 0.08 ppm. This sample had an unusually long
pre-harvest interval of 94 days, and the untreated corresponding
sample contained 0.05 ppm monocrotophos.
Cottonseed oil
Following recommended treatments of cotton with monocrotophos,
residues (max. 0.04 ppm) have only been detected occasionally in
the oil. A tendency was observed for residues to be lower in the
crude oil when they were detected in the corresponding seed.
Cotton foliage
On foliage, residue levels of monocrotophos were proportional to
the dosage rates at short pre-harvest intervals. The half-life on
foliage was between less than three days to six days. In U.S.A.,
where monocrotophos is widely used on cotton, no grazing of
livestock or feeding of treated trash is permitted since the
residue levels range from 8 to >100 ppm.
Potatoes
The data developed relative to the use of monocrotophos on potatoes
showed that, when treated at the recommended rate of 0.25 - 1.0 kg
a.i./ha, with a minimum pre-harvest interval of seven days, residues
of monocrotophos were consistently less than the limit of
determination of the analytical method employed (<0.01 - <0.08 ppm).
Sugarcane
Analyses of sugarcane which had been repeatedly treated at 0.5 - 1.0
kg a.i./ha, and products from the sugarcane, showed that the residue
levels of monocrotophos were consistently less than the limit of
determination of the analytical method (<0.05 ppm) when a pre-harvest
interval of 30 days was observed.
Maize
The results of a considerable number of studies, carried out to
determine the residues of monocrotophos in maize are given below:
Grain
No residues of monocrotophos (<0.01 - <0.05 ppm) have been
found in maize grain treated according to label recommendations
(max. dosage of 1.5 kg a.i./ha, pre-harvest interval of 14 days).
Residues in maize grain have been detected only in trials where
very short pre-harvest intervals have been used.
Husks and cobs
Extensive data show no significant residues of monocrotophos in
samples of husks and cobs from maize plants which had received
applications of monocrotophos. Residues of monocrotophos which
occur at short pre-harvest intervals (1-3 days) decrease rapidly.
At intervals of 14 days or longer they had decreased to an
average level of 0.04 ppm (range 0.005 - 0.40 ppm) from
treatments up to the maximum recommended dose rate of 1.5 kg
a.i./ha.
Silage
Following recommended treatments of monocrotophos to maize,
residues of monocrotophos in maize silage ranged from less than
0.01 ppm to 1.4 ppm (mean of 0.15 ppm).
Stover
Residues of monocrotophos in maize stover, following recommended
treatments, ranged from less than 0.01 ppm to 0.06 ppm (mean of
0.02 ppm). Levels of 13 - 17 ppm present in stover one day after
application rapidly decreased to 0.20 - 0.06 ppm 23 days later.
Tomatoes
Following recommended applications of monocrotophos (maximum rate of
1.0 kg a.i./ha, pre-harvest interval of 21 days) to tomatoes, residues
of monocrotophos ranged from less than 0.01 ppm to 0.51 ppm (mean of
0.11 ppm).
Brussels sprouts
Following recommended applications (dosage rate up to 0.6 kg a.i./ha,
pre-harvest interval of 15 days) of monocrotophos to Brussels sprouts,
residues of monocrotophos ranged from less than 0.05 ppm to 0.21 ppm,
with an arithmetic mean of 0.10 ppm.
Cauliflower
Residues of monocrotophos in samples of cauliflower, which had
received recommended applications of monocrotophos (up to 0.6 kg
a.i./ha, pre-harvest interval of 15 days) ranged from less than 0.01
ppm to 0.14 ppm (arithmetic mean of 0.03 ppm).
Cabbage
The results of studies carried out to determine the residues of
monocrotophos in cabbage, following recommended treatments (dosage
rate up to 0.6 kg a.i./ha, four applications and pre-harvest interval
of 21 days), were evaluated. Residues of monocrotophos in cabbage from
these trials ranged from less than 0.02 ppm to 0.19 ppm.
Carrots
Following recommended applications (maximum dosage rate of 0.5 kg
a.i./ha, pre-harvest interval of 14 days), maximum residues of
monocrotophos found in carrots were 0.04 ppm; generally no residues
(less than 0.01 ppm) were detected. Corresponding levels in the carrot
tops were 0.65 - 1.6 ppm (mean of 1.1. ppm).
Turnips
No residues of monocrotophos (less than 0.05 ppm) have been detected
in turnips, even when the turnips were harvested immediately after
application.
Onions
Onions, treated with monocrotophos at recommended applications
(maximum dosage rate of 0.5 kg a.i./ha, pre-harvest interval of ten
days) did not contain residues exceeding 0.09 ppm monocrotophos (mean
value of 0.03 ppm).
Sugar beet
Residues of monocrotophos in sugar beet roots did not exceed the limit
of determination of the analytical method employed (0.01 - 0.05 ppm),
following treatment at a maximum dosage rate of 0.8 kg a.i./ha. The
data cover applications made within 14 days of lifting, although in
practice applications as late as this are unusual. Corresponding
residues of monocrotophos in sugar beet leaves ranged from less than
0.01 ppm to 0.55 ppm.
Peas
Following application of monocrotophos to peas, at dosage rates up to
0.5 kg a.i./ha with pre-harvest intervals of 15 days or longer, the
only positive residue detected was 0.06 ppm, all the other samples
being below the limit of determination of the analytical methods
(<0.01 - <0.07 ppm).
Beans
Residues of monocrotophos in beans, following recommended treatments
(up to 0.5 kg a.i./ha, pre-harvest interval of 21 days), ranged from
less than 0.01 ppm to 0.22 ppm.
Soybeans
Residues of monocrotophos in soybeans did not exceed 0.03 ppm,
following treatment of monocrotophos according to label
recommendations (maximum dosage rate of 0.5 kg a.i./ha, pre-harvest
interval of 14 days). Maximum residue of monocrotophos in soybean
foliage was 0.87 ppm (mean of 0.22 ppm).
Coffee
Residues of 0.08 ppm in raw coffee beans, following a recommended
treatment of monocrotophos, were reduced to below the limit of
determination of the analytical method (less than 0.01 ppm) on
processing.
Hops
In a field trial carried out in Germany, with monocrotophos at 0.5 kg
a.i./ha and a minimum of 28 days pre-harvest interval, residues of
monocrotophos in dried hops ranged from 0.3 to 0.41 ppm.
Citrus fruit
Residues of monocrotophos in citrus fruit treated with monocrotophos
at label recommendations (up to 1.0 kg a.i./ha, pre-harvest interval
of 21 days) ranged from less than 0.01 ppm to 0.45 ppm (mean of 0.08
ppm).
Apples
Monocrotophos is recommended for use on apple trees in Australia and
Italy only, at dosage rates equivalent to 0.04% a.i. in the spray,
with a final treatment to harvest interval of four weeks. Data from
trials carried out in these countries were examined. Residues of
monocrotophos in apples treated according to this recommendation range
from <0.01 ppm to 1.5 ppm. These upper figures, however, derive from
experiments in which the spray was applied by hand, which usually
requires a somewhat higher volume; it is considered that in practical
conditions of use, 1.0 ppm would be the highest level reported.
Pears
Monocrotophos is only recommended for application to pears in
Australia and Italy. Maximum recommended dosage rate is 0.04% a.i. in
the spray with a final treatment to harvest interval of 28 days in
Australia and 30 days in Italy. Residues of monocrotophos treated
according to these recommendations ranged from 0.08 ppm to 0.76 ppm
(mean - 0.41 ppm) in pears from these two countries.
FATE OF RESIDUES
General comments
Residues of breakdown products do not occur in food commodities to a
significant extent.
Extensive studies with monocrotophos have shown that degradation can
occur by hydrolytic and/or oxidative mechanisms. From the experimental
work it appears that the primary metabolic pathway is hydrolysis,
yielding metabolic products which are non-cholinesterase inhibiting
and of low toxicity. Evidence of secondary metabolic pathways
involving oxidation reactions have been found in that small quantities
of the N-methylol analogue of monocrotophos, its glycoside and the
unsubstituted amide analogue were detected in the radiotracer studies.
Residues of cholinesterase inhibiting breakdown products have been
detected in crops only rarely, following recommended treatments, and
then at levels close to the limit of determination of the analytical
method employed. Likewise in meat and milk from animals fed
monocrotophos in their diet, at levels far exceeding those likely to
be encountered in practice, only extremely low levels of breakdown
products have been detected, if at all.
In animals
Studies have been conducted with monocrotophos fed to cattle and goats
to determine whether residues in feed give rise to residues in meat
and milk.
32P monocrotophos was fed continuously to two lactating cows at a
level equivalent to 45 ppm in their total diet (Shell Development Co.,
undated). This level is approximately 20 times that which would occur
in practice, assuming a maximum of 50% of monocrotophos-treated
feedstuff in the total diet. Levels of monocrotophos found in the milk
from the two cows were 0.01 ppm and 0.008 ppm. Average levels of
dimethyl phosphate and of N-hydroxy methyl monocrotophos were 0.002
ppm and 0.001 ppm, respectively (Ibid.). No residues of glycosides of
the N-hydroxy methyl compound were detected. After 14 days of this
feeding regime, the two cows were slaughtered and residues determined
on the red meat and liver. These results are given in Table 6.
TABLE 6 Residues of monocrotophos and its breakdown products
present in meat from cows fed monocrotophos
Residues present (ppm)
Sample dimethyl N-hydroxy methyl
analysed monocrotophos phosphate monocrotophos
Red meat 0.02, 0.04 0.005, 0.008 0.003, 0.0084
Liver 0.11, 0.13 0.05, 0.06 0.02, 0.04
32P monocrotophos was administered orally to goats (Menzer and
Casida, 1965) at a single dose of 1 mg/kg body-weight. Within 72 hours
of the application, only 1.4% of the applied radioactivity was present
in the goats' milk. The authors suggested that, in animals,
monocrotophos degradation took place by oxidative N-demethylation to
N-hydroxy methyl monocrotophos and unsubstituted amide. Residues of
these compounds and monocrotophos in samples of the milk from the
treated goats up to 24 hours after treatment are given in Table 7.
TABLE 7 Residues of monocrotophos and its breakdown products found
in milk from goats administered monocrotophos
Residues present in goats' milk (ppm)
Hours after N-hydroxy methyl unsubstituted total organo-
administration monocrotophos monocrotophos amide extractable
1 0.045 0.0001 <0.0001 0.067
2 0.032 0.0086 0.0007 0.061
4 0.034 0.0086 0.0015 0.074
6 0.023 0.011 0.0013 0.057
8 0.012 0.0099 0.0007 0.042
12 0.007 0.0044 0.0006 0.025
16 0.006 0.0020 0.0001 0.012
20 0.012 0.0009 0.0003 0.022
24 0.001 0.0007 <0.0001 0.007
As indicated in Table 7, residues of monocrotophos fell from a maximum
value of 0.0451 ppm one hour after administration to 0.0014 ppm 23
hours later. Maximum levels of N-hydroxy methyl monocrotophos, and the
unsubstituted amide, detected were 0.0105 ppm (after six hours) and
0.0015 ppm (after four hours), respectively. These levels had
decreased by a fifteen-fold factor 24 hours after application.
To determine whether residues in animal feeds could give residues of
the conjugate of N-hydroxy methyl monocrotophos in meat and milk, two
Guernsey cows were fed for ten days with the conjugate at a level of
20 ppm in the total diet (this level is more than 2 000 times that
which may be encountered in practice). The results showed that the
combined residues of the conjugate and its hydrolysis products,
N-hydroxy methyl monocrotophos and the unsubstituted amide, in milk,
were all below 0.01 ppm. At the end of the ten day study the cows were
slaughtered and their tissues analysed. The results are given in Table
8.
TABLE 8 Residues of monocrotophos breakdown products found in tissues
of cows fed monocrotophos conjugate
Tissues Residues present (ppm)
analysed
conjugate of N-hydroxy N-hydroxy methyl monocrotophos
methyl monocrotophos plus unsubstituted amide
fat <0.01 0.02, 0.04
kidney 0.02, 0.01 <0.01, 0.01
meat <0.01, <0.01 0.01, 0.01
liver <0.01, 0.01 <0.01, 0.01
In plants
In order to elucidate the mode of breakdown in leaves, 32P
monocrotophos was applied to cotton plants, both in the glasshouse and
in the field, either as a foliar spray, a stem banding treatment, a
seed dressing or by petiole injection (Lindquist and Bull, 1967). For
the foliar treatment, monocrotophos was applied at a rate of
40 mg/cotton leaf. In the field studies, 85% of the applied
radioactivity was lost during the first two days, presumably through
volatilization. Breakdown was shown to take place on both the surface
and the inside of the treated leaves. Principal breakdown products
found were O-desmethyl monocrotophos, dimethyl phosphate and N-hydroxy
methyl monocrotophos. When monocrotophos was applied as a stem bending
treatment, the recovered radioactivity after 21 days was unchanged
monocrotophos, probably because the lanolin in the formulation had a
stabilizing effect. Following the application of monocrotophos as a
seed treatment (not a recommended application) at a rate of 0.5 mg
monocrotophos/cotton seed, 50% of the radioactivity recovered after
one week was unchanged monocrotophos. O-desmethyl monocrotophos was
present in all samples, indicating the cleavage of a methyl-phosphate
bond. Dimethyl phosphate (formed by hydrolysis of the vinyl phosphate
bond) was recovered in increasing amounts with time. The N-hydroxy
methyl monocrotophos was only detected at 21 days after treatment,
while an unknown compound, later identified as a glycoside of
N-hydroxy methyl monocrotophos (Shell Development Co., undated), was
the major metabolite at 14 and 21 days.
Petiole injection of monocrotophos at 70 mg monocrotophos/cotton leaf
indicated half-lives of monocrotophos in cotton leaves of eight days
in the glasshouse and seven days in the field. Small amounts of the
N-hydroxy methyl monocrotophos and its conjugate were detected. No
residues of the unsubstituted amide (N-desmethyl monocrotophos) were
detected in cotton plants. The authors proposed the pathways shown in
Figure 2 for the degradation of monocrotophos.
Menzer and Casida (1965) injected 32P-monocrotophos into bean plants
at a level of 43.5 ppm and found trace amounts of the N-hydroxy methyl
monocrotophos (0.14% of monocrotophos applied) and the unsubstituted
amide (0.10% of monocrotophos applied) eight days after injection.
However, 20 and 32 days after application monocrotophos was the only
compound detected.
In studies with 14C-labelled monocrotophos (Shell Research Ltd.,
undated) on the foliage of maize, cabbage and apples, and on apple
fruit, the breakdown products present were the unsubstituted amide,
N-hydroxy methyl monocrotophos, (both in the free and conjugated
forms), O-desmethyl-monocrotophos acid and traces of
N-methylacetoacetamide and 3-hydroxy-N-methyl butyramide.
Since, of the breakdown products detected in plants following
application of monocrotophos, only the unsubstituted amide
(N-desmethyl monocrotophos) and the free and bound N-hydroxy methyl
monocrotophos can cause cholinesterase inhibition, analytical methods
(Ibid.) were developed for the determination of these compounds.
Samples from field trials were analysed using these methods.
Results from trials with sugar beet, potatoes, Brussels sprouts,
cauliflower, maize, pears, peas, onions and soybeans (Ibid.) showed
that residues of the unsubstituted amide and the N-hydroxyl methyl
monocrotophos (free and conjugated) could not be detected, nor could
monocrotophos.
TOXICOLOGICAL STUDIES
Special studies on metabolites
The acute toxicity of metabolites of monocrotophos in mouse and rat is
summarized in Table 1.
The acute toxicity of components of technical monocrotophos in rat is
summarized in Table 2.
TABLE 1 Acute toxicity of monocrotophos metabolites
Metabolite Reference
N-methyl hydroxy monocrotophos (SD = 12657)
Mouse ip LD50 = 12 mg/kg (Menzer and Casida, 1965)
Rat oral LD50 = 27 mg/kg (Shellenberger, 1966a)
N-desmethyl monocrotophos (SD 11319)
Mouse ip LD50 = 3 mg/kg (Menzer and Casida, 1965)
Mouse oral LD50 = 5.5 (Shellenberger, 1966a)
Glycoside of N-desmethyl monocrotophos (SD 13311)
Rat (M) oral LD50 = 168 mg/kg (Shell Chemical Co., 1966)
N-methyl acetoacetamide
Rat oral LD50 = >2 000 mg/kg (Shellenberger, 1966a)
TABLE 2 Acute toxicity of components of technical monocrotophos
Component Species Route LD50 (mg/kg)
Dimethyl phosphate of
3-hydroxy-N-methyl-trans-crotonamide Rat Oral 420
Dimethyl phosphate of 2-chloro-3
hydroxy-N-methyl-cis-crotonamide Rat Oral 14
Dimethyl phosphate of 2-chloro-3
hydroxy-N-methyl-trans-crotonamide Rat Oral 140
Short-term studies on the glycoside metabolite (SD 13311)
Groups of rats (42 males and 42 females per group, except for the
highest level which had 22 of each sex) were fed the glycoside
metabolite of monocrotophos (a glycoside of the dimethyl phosphate of
3-hydroxy-N-(hydroxymethyl)-cis-crotonamide) for 9 days at levels of
0, 1, 3, 9, 18 and 90 ppm (the 1 ppm was fed for seven weeks and the
dosage increased to 18 ppm for the final five weeks). Rats were fed a
normal diet for four weeks after the feeding study ended. Slight
growth effects were noted at 90 ppm from 7-12 weeks in both sexes.
Blood cholinesterase was depressed at 9 ppm in both sexes. At 18 ppm,
brain cholinesterase was depressed. Cholinesterase activity was not
affected at 3 ppm. No effects were observed on blood parameters or
gross and microscopic histology (Shell Chemical Co., 1966).
Special studies on neurotoxicity
Groups of adult White Leghorn hens (ten hens per group) were fed
monocrotophos in the diet at levels of 0, 1, 10 and 100 ppm for four
weeks. A single group of ten hens was fed TOCP in the diet at 1 000
ppm for four weeks. At the end of the feeding interval, five birds
from each group were sacrificed, and histological examinations of
brain, spinal cord and sciatic nerve were performed. Physiological
response, body-weight, food consumption and egg production data were
collected. Monocrotophos at all dose levels produced a weight loss in
hens. Egg production was not affected at 1 ppm but was inhibited at 10
and 100 ppm. Mortality occurred at 100 ppm, and although several birds
showed tremors 10-12 days after feeding started, all were able to
stand and walk normally. TOCP fed birds developed typical signs of
ataxia after 16-17 days. All survivors were unable to stand at the end
of the treatment period. Histological examination of TOCP-treated hens
showed severe demyelination in the posterior horns of the spinal cord.
Examination of nervous tissue from monocrotophos fed and control hens
showed slight evidence of demyelinated tracts, primarily in the
anterior horns. No differences were noted in controls and hens
undergoing monocrotophos treatments. Monocrotophos does not produce
delayed neurological signs of poisoning when compared to the response
induced by TOCP (Shellenberger, 1965b).
Special studies on potentiation
Male rats were given oral doses of a combination of monocrotophos and
the following anticholinesterase compounds, with no signs of
potentiation: dioxathion, dimethoate, carbaryl, disulfoton, EPN,
Folex, malathion, parathion, schradan, demeton, azinphos-methyl,
dicrotophos, crotoxyphos, coumaphos, dichlorvos, diazinon, naled,
trichlorphon, ethion, mevinphos, carbophenothion and phosphamidon.
Potentiation of the acute oral toxicity was observed with
monocrotophos in combination with fenchlorphos (Shellenberger, 1965a).
Special studies on reproduction
Groups of rats (10 males and 20 females per group) were fed
monocrotophos in the diet at 0, 2, 5, 12 and 30 ppm, and subsequently
mated. The progeny of this mating were maintained on this dietary
regime and mated. This was followed for three generations (Eisenlord
and Loquram, 1965). In a separate study groups of rats (10 males and
20 females per group) were fed monocrotophos at the same levels and
subjected to a three-generation, two-litter per generation study
(Eisenlord and Loquram, 1966).
Effects due to monocrotophos were noted in both studies at levels of 5
ppm and above. Appearance and behaviour was affected at 12 and 30 ppm,
as manifested in stunted growth and emaciation. Thinned or missing
hair on the flanks or head was seen in all monocrotophos groups among
adult females and pups, but not in adult males or controls. The level
of 30 ppm was lethal to pups and reduced pregnancies. Pup mortality
was evidenced at 5 ppm in both studies and absent at 2 ppm. Weight of
parents was adversely affected at 30 ppm. At 12 ppm, several parents
were affected (F2 and F3 males in one study and F2 females and F3
males in the second). No effects were noted on any parameter at 2 ppm.
Gross and histopathological examinations showed no differences from
the control values.
Special studies on teratogenicity
Groups of pregnant rabbits received oral daily doses of 0.7 and 2
mg/kg monocrotophos from day 6 to 18 of gestation. On day 28 of
pregnancy, the foetuses were removed and examined. No compound-related
teratogenic effects were observed (Thorpe and Dix, 1972).
Acute toxicity
The results of acute toxicity studies in animals are summarized in
Table 3.
TABLE 3 Acute toxicity of monocrotophos in animals
Species Sex Route LD50 Reference
(mg/kg)
Rat M IP 5 Bull & Lindquist, 1967
M Oral 14-23 Shellenberger & Newell, 1963
Shellenberger, 1965b; 1966a
F SC 7 Reiff, 1969
M Oral 18 Gaines, 1969
F Oral 20 Ibid.
Mouse M&F IP 8 Menzer & Casida, 1965
Oral 15 Shellenberger & Newell, 1963
Guinea Pig M&F SC app.60 Hunter, 1964
Signs of poisoning following monocrotophos are typical of other
anticholinesterase organo-phosphates. Toxic signs occur rapidly and in
animals include tremors, lacrimation, diarrhea, salivation, tonic and
clonic convulsions and other signs of cholinergic stimulation. Deaths
occur rapidly, usually within four hours, and animals surviving 24
hours generally recover and appear normal.
Atropine alone, but preferably in combination with reactivators (P2S
or Toxogonin), was shown to be an effective antidote to monocrotophos
poisoning in rodents (Hunter, 1964: Reiff, 1969).
Short-term studies
Japanese quail
Groups of Japanese quail (12 males and 12 females per group) were fed
monocrotophos at 0, 0.5, 5 and 50 ppm in the diet for three weeks.
Mortality and weight loss occurred at 50 ppm. Food consumption was
reduced at 5 ppm, while egg production was normal. Blood
cholinesterase activity was depressed at all feeding levels, while
brain cholinesterase activity was depressed at 5 ppm and above. At 5
ppm in the diet of adult females, monocrotophos had no effect on the
embryo heart beat or development of the chicks (Shellenberger, 1966b).
Rat
Groups of rats were fed monocrotophos in the diet at dosage levels of
0 (42 males and 42 females), 0.5, 1.5 (30 males and 30 females/group),
15 (42 males and 42 females), 45 and 135 ppm (12 males and 12
females/group) for twelve weeks. Growth was depressed at 45 ppm.
Cholinesterase depression was observed on haematology or gross and
microscopic pathology (Shellenberger and Newell, 1964).
Dog
Groups of Beagle dogs were fed monocrotophos in the dry diet at levels
of 0, 0.5, 1.5, 15 (four dogs of each sex/group), 45 and 135 ppm (two
dogs of each sex/group) for 13 weeks. After 8 weeks, the dogs fed 135
ppm were changed to 270 ppm for weeks 9-10, then to 540 ppm for weeks
11-12, and to 1 080 ppm for the final week.
Body-weights were reduced at dietary levels of 270 ppm. Organ weights
were also affected in this high feeding level group. Cholinesterase
depression was observed at 1.5 ppm in blood and brain. There were no
compound-related effects on haematological values, blood biochemistry,
organ weights or gross and microscopic pathology (Shellenberger and
Newell, 1964).
Groups of dogs were fed monocrotophos in the diet for two years at
levels of 0 (four males and four females) 0.16, 1.6, 16 ppm (three
males and three females/group) and for one year at 100 ppm (two males
and two females). Moderate cholinergic stimulation was observed at 100
ppm as tremors and salivation. Blood cholinesterase depression was
noted at 16 and 100 ppm in both males and females. Marginal brain
cholinesterase depression was also noted at a dose level of 1.6 ppm
after 93 and 104 weeks. No effects were noted on growth, mortality,
haematological parameters, urinalyses or physiological measurements.
Gross and histopathological examination showed no compound-related
effects (Johnston et al., 1967a).
Long-term studies
Rat
Groups of rats were fed monocrotophos in the diet for two years at
levels of 0 (40 males and 40 females), 1, 10 and 100 ppm (25 males and
25 females/group). Growth of males and females at 100 ppm was
depressed. Food consumption was also lower than controls at this
level. RBC and plasma cholinesterase depression was observed at 10 and
100 ppm in both males and females. Brain cholinesterase depression in
males and females was significantly inhibited at all levels. At 100
ppm, the weights of liver, gonad, thyroid and pituitary were reduced
in females. Thyroid and pituitary/body-weight ratios were depressed at
100 ppm. No effects were noted on survival, haematology or
histopathological examination of organs and tissues (Johnston
et al., 1967b).
COMMENT
Monocrotophos is rapidly absorbed, metabolized and eliminated by
mammals. Plant and animal metabolites are similar.
Potentiation was observed with fenchlorphos but not with other
cholinesterase-inhibiting compounds. No delayed neurotoxic effects
were observed.
Short-term studies in rats and dogs indicate no-effect levels at 0.5
ppm for cholinesterase depression.
In long-term studies a no-effect level has not been demonstrated in
the rat, the lowest dose tested (1 ppm) causing significant brain
cholinesterase depression.
In dog, two-year studies indicated a no-effect level of 0.16 ppm with
regard to brain cholinesterase depression. An effect was observed at
1.6 ppm.
In estimating an ADI for this compound, it was noted that in rat a
firm no-effect level was 0.5 ppm, with minimal effect level at 1 ppm.
In dog a no-effect level of 0.5 ppm was also demonstrated but the
minimal effect level was 1.5 ppm. Since the no-effect level is more
closely defined in rat, the latter species was used as a basis for
determining the ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.5 ppm in the diet, equivalent to 0.025 mg/kg/day
Dog: 0.5 ppm in the diet, equivalent to 0.0125 mg/kg/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0003 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Monocrotophos is an organo-phosphorus insecticide, which finds its
main use for foliar application to cotton (over 80% of monocrotophos
applied). It is also recommended for application against foliage pests
of maize, sugar cane, sugarbeet, vegetables, potatoes and certain
fruits. It is particularly effective against Lepidoptera, Homoptera
and certain Coleoptera, acting by both systemic and residual contact
properties.
Monocrotophos is known to be officially registered and/or approved for
use in the following countries:
Angola Yugoslavia
Argentina Mexico
Australia Mozambique
Austria Nicaragua
Brazil Pakistan
Bulgaria Peru
Chile Philippines
South Africa
Colombia Spain
Costa Rice Sudan
Ecuador Switzerland
El Salvador Thailand
Egypt Trinidad
France Turkey
Greece Uruguay
Guatemala U.S.A.
India Venezuela
Israel Viet-Nam
Italy Zambia
USE RECOMMENDATIONS
Typical maximum recommended application rates are: 1.0 kg a.i./ha for
cotton, potatoes and fruit; 1.5 kg a.i./ha for maize; 0.8 kg a.i./ha
for sugar cane; and 0.5 - 0.6 kg a.i./ha for vegetables and sugar
beet.
The recommended minimum period between treatment and harvest varies
from country to country, but is generally about 7 - 15 days for
vegetables and potatoes, 14 days for cotton, 21 days for tomatoes,
maize and citrus and 28 - 30 days for other crops.
Multiple applications of monocrotophos are often made, depending on
pest incidence, and use recommendations allow for these treatments.
The detailed use recommendations are given in Table 4.
TABLE 4 APPLICATIONS OF MONOCROTOPHOS TO CROP FOLIAGE
Crop Recommended Recommended period
application rates between treatment
(kg. a.i./ha) and harvest (days)1
Cotton 0.25 -1.0 Australia - 21
Mexico - 14
S. Africa - 30
Spain - 30
U.S.A. - 21
Maize 0.25 -1.5 Colombia - 30
Uruguay - 28
Sugarcane 0.25 -0.75 Uruguay - 28
Venezuela - 30
U.S.A. - 30
Carrots, turnips, onions 0.25 -0.5
Potatoes 0.25 -1.0 Colombia - 30
Italy - 21
S. Africa - 14
Venezuela - 14
U.S.A. - 7
Sugar beet 0.25 - 0.80 Italy - 21
TABLE 4 (Cont'd.)
Brussels sprouts, 0.2 -0.6 Yugoslavia - 28
cauliflower and cabbage Colombia - 30
Peru - 15
Venezuela - 21
Vietnam - 21
Tomatoes 0.25 -1.0
Hops 0.25 -0.5
Soybeans 0.25 -0.5 Colombia - 30
Vietnam - 21
Peas and beans 0.5 Colombia - 30
Pom - 15
Apples, pears2 0.01 - 0.04% a.i. Australia - 28
Italy - 30
Citrus 0.5 - 1.0 Venezuela - 21
Coffee 0.25 - 0.8
1 Monocrotophos is not recommended for post-harvest use on agricultural
commodities.
2 Monocrotophos is recommended for use on apples and pears in Australia,
and Italy only.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data are available from supervised trials carried out in 24
countries on food crops grown under various conditions, using various
rates of application and pre-harvest intervals.
In most trials, dosage rates, number of applications and pre-harvest
intervals were observed, in accordance with label recommendations, and
the data from these trials are summarized in Table 5. Residues of
monocrotophos in grain crops and root vegetables are usually below, or
close to, the limit of determination of the analytical method, while
leafy vegetables and fruit often contain detectable residues.
TABLE 5 Residues of monocrotophos in crops following recommended foliage treatments
Maximum Minimum
recommended preharvest Trials Results Range of
Crop rate interval residues (ppm)
(kg a.i./ha) (days) (no.) (no.)
Cotton (seed) 1.0 14 11 14 <0.02-0.08
Cotton (oil) 1.0 14 5 6 <0.02-0.04
Sugarcane 0.75 30 4 5 <0.05
Potatoes 1.0 7 14 27 <0.08-0.20
Maize grain 1.5 14 14 25 <0.01-0.05
Tomatoes 1.0 21 10 17 <0.01-0.51
Brussels sprouts 0.6 15 4 8 <0.05-0.21
Cauliflower 0.6 15 6 8 <0.01-0.14
Cabbage 0.6 21 9 23 <0.02-0.19
Carrots 0.5 14 2 5 <0.01-0.04
Turnips 0.5 10 1 3 <0.05
Onions 0.5 10 3 6 <0.02-0.09
Sugar beet 0.8 14 8 25 <0.01-<0.05
Peas 0.5 15 2 2 <0.07-0.60
Beans 0.5 21 3 6 <0.01-0.22
Soybeans 0.5 14 7 12 <0.01-0.03
Coffee 0.8 42 2 2 <0.01-0.08
Hops 0.5 28 1 2 0.3 - 0.41
Citrus fruit 1.0 21 11 26 <0.01-0.45
Apples 1.0 28 7 20 <0.05-1.0
Pears 1.0 28 5 10 <0.01-0.76
Cotton
Since the major outlet for monocrotophos is as a foliage insecticide
on cotton, numerous studies have been undertaken to examine the extent
of residues of monocrotophos in cottonseed and oil, if any, likely to
arise from recommended treatments.
Cottonseed
Monocrotophos is recommended for application to cotton foliage at
a maximum dosage rate of 1 kg a.i./ha, applications being made at
5-7 day intervals, where pest incidence dictates, with a final
treatment to harvest interval of 14 days. Trials in which these
recommendations were followed showed that residues of
monocrotophos in cottonseed were below the limit of determination
of the analytical method employed (<0.02 - <0.07 ppm) with the
exception of one sample which contained an apparent residue of
monocrotophos of 0.08 ppm. This sample had an unusually long
pre-harvest interval of 94 days, and the untreated corresponding
sample contained 0.05 ppm monocrotophos.
Cottonseed oil
Following recommended treatments of cotton with monocrotophos,
residues (max. 0.04 ppm) have only been detected occasionally in
the oil. A tendency was observed for residues to be lower in the
crude oil when they were detected in the corresponding seed.
Cotton foliage
On foliage, residue levels of monocrotophos were proportional to
the dosage rates at short pre-harvest intervals. The half-life on
foliage was between less than three days to six days. In U.S.A.,
where monocrotophos is widely used on cotton, no grazing of
livestock or feeding of treated trash is permitted since the
residue levels range from 8 to >100 ppm.
Potatoes
The data developed relative to the use of monocrotophos on potatoes
showed that, when treated at the recommended rate of 0.25 - 1.0 kg
a.i./ha, with a minimum pre-harvest interval of seven days, residues
of monocrotophos were consistently less than the limit of
determination of the analytical method employed (<0.01 - <0.08 ppm).
Sugarcane
Analyses of sugarcane which had been repeatedly treated at 0.5 - 1.0
kg a.i./ha, and products from the sugarcane, showed that the residue
levels of monocrotophos were consistently less than the limit of
determination of the analytical method (<0.05 ppm) when a pre-harvest
interval of 30 days was observed.
Maize
The results of a considerable number of studies, carried out to
determine the residues of monocrotophos in maize are given below:
Grain
No residues of monocrotophos (<0.01 - <0.05 ppm) have been
found in maize grain treated according to label recommendations
(max. dosage of 1.5 kg a.i./ha, pre-harvest interval of 14 days).
Residues in maize grain have been detected only in trials where
very short pre-harvest intervals have been used.
Husks and cobs
Extensive data show no significant residues of monocrotophos in
samples of husks and cobs from maize plants which had received
applications of monocrotophos. Residues of monocrotophos which
occur at short pre-harvest intervals (1-3 days) decrease rapidly.
At intervals of 14 days or longer they had decreased to an
average level of 0.04 ppm (range 0.005 - 0.40 ppm) from
treatments up to the maximum recommended dose rate of 1.5 kg
a.i./ha.
Silage
Following recommended treatments of monocrotophos to maize,
residues of monocrotophos in maize silage ranged from less than
0.01 ppm to 1.4 ppm (mean of 0.15 ppm).
Stover
Residues of monocrotophos in maize stover, following recommended
treatments, ranged from less than 0.01 ppm to 0.06 ppm (mean of
0.02 ppm). Levels of 13 - 17 ppm present in stover one day after
application rapidly decreased to 0.20 - 0.06 ppm 23 days later.
Tomatoes
Following recommended applications of monocrotophos (maximum rate of
1.0 kg a.i./ha, pre-harvest interval of 21 days) to tomatoes, residues
of monocrotophos ranged from less than 0.01 ppm to 0.51 ppm (mean of
0.11 ppm).
Brussels sprouts
Following recommended applications (dosage rate up to 0.6 kg a.i./ha,
pre-harvest interval of 15 days) of monocrotophos to Brussels sprouts,
residues of monocrotophos ranged from less than 0.05 ppm to 0.21 ppm,
with an arithmetic mean of 0.10 ppm.
Cauliflower
Residues of monocrotophos in samples of cauliflower, which had
received recommended applications of monocrotophos (up to 0.6 kg
a.i./ha, pre-harvest interval of 15 days) ranged from less than 0.01
ppm to 0.14 ppm (arithmetic mean of 0.03 ppm).
Cabbage
The results of studies carried out to determine the residues of
monocrotophos in cabbage, following recommended treatments (dosage
rate up to 0.6 kg a.i./ha, four applications and pre-harvest interval
of 21 days), were evaluated. Residues of monocrotophos in cabbage from
these trials ranged from less than 0.02 ppm to 0.19 ppm.
Carrots
Following recommended applications (maximum dosage rate of 0.5 kg
a.i./ha, pre-harvest interval of 14 days), maximum residues of
monocrotophos found in carrots were 0.04 ppm; generally no residues
(less than 0.01 ppm) were detected. Corresponding levels in the carrot
tops were 0.65 - 1.6 ppm (mean of 1.1. ppm).
Turnips
No residues of monocrotophos (less than 0.05 ppm) have been detected
in turnips, even when the turnips were harvested immediately after
application.
Onions
Onions, treated with monocrotophos at recommended applications
(maximum dosage rate of 0.5 kg a.i./ha, pre-harvest interval of ten
days) did not contain residues exceeding 0.09 ppm monocrotophos (mean
value of 0.03 ppm).
Sugar beet
Residues of monocrotophos in sugar beet roots did not exceed the limit
of determination of the analytical method employed (0.01 - 0.05 ppm),
following treatment at a maximum dosage rate of 0.8 kg a.i./ha. The
data cover applications made within 14 days of lifting, although in
practice applications as late as this are unusual. Corresponding
residues of monocrotophos in sugar beet leaves ranged from less than
0.01 ppm to 0.55 ppm.
Peas
Following application of monocrotophos to peas, at dosage rates up to
0.5 kg a.i./ha with pre-harvest intervals of 15 days or longer, the
only positive residue detected was 0.06 ppm, all the other samples
being below the limit of determination of the analytical methods
(<0.01 - <0.07 ppm).
Beans
Residues of monocrotophos in beans, following recommended treatments
(up to 0.5 kg a.i./ha, pre-harvest interval of 21 days), ranged from
less than 0.01 ppm to 0.22 ppm.
Soybeans
Residues of monocrotophos in soybeans did not exceed 0.03 ppm,
following treatment of monocrotophos according to label
recommendations (maximum dosage rate of 0.5 kg a.i./ha, pre-harvest
interval of 14 days). Maximum residue of monocrotophos in soybean
foliage was 0.87 ppm (mean of 0.22 ppm).
Coffee
Residues of 0.08 ppm in raw coffee beans, following a recommended
treatment of monocrotophos, were reduced to below the limit of
determination of the analytical method (less than 0.01 ppm) on
processing.
Hops
In a field trial carried out in Germany, with monocrotophos at 0.5 kg
a.i./ha and a minimum of 28 days pre-harvest interval, residues of
monocrotophos in dried hops ranged from 0.3 to 0.41 ppm.
Citrus fruit
Residues of monocrotophos in citrus fruit treated with monocrotophos
at label recommendations (up to 1.0 kg a.i./ha, pre-harvest interval
of 21 days) ranged from less than 0.01 ppm to 0.45 ppm (mean of 0.08
ppm).
Apples
Monocrotophos is recommended for use on apple trees in Australia and
Italy only, at dosage rates equivalent to 0.04% a.i. in the spray,
with a final treatment to harvest interval of four weeks. Data from
trials carried out in these countries were examined. Residues of
monocrotophos in apples treated according to this recommendation range
from <0.01 ppm to 1.5 ppm. These upper figures, however, derive from
experiments in which the spray was applied by hand, which usually
requires a somewhat higher volume; it is considered that in practical
conditions of use, 1.0 ppm would be the highest level reported.
Pears
Monocrotophos is only recommended for application to pears in
Australia and Italy. Maximum recommended dosage rate is 0.04% a.i. in
the spray with a final treatment to harvest interval of 28 days in
Australia and 30 days in Italy. Residues of monocrotophos treated
according to these recommendations ranged from 0.08 ppm to 0.76 ppm
(mean - 0.41 ppm) in pears from these two countries.
FATE OF RESIDUES
General comments
Residues of breakdown products do not occur in food commodities to a
significant extent.
Extensive studies with monocrotophos have shown that degradation can
occur by hydrolytic and/or oxidative mechanisms. From the experimental
work it appears that the primary metabolic pathway is hydrolysis,
yielding metabolic products which are non-cholinesterase inhibiting
and of low toxicity. Evidence of secondary metabolic pathways
involving oxidation reactions have been found in that small quantities
of the N-methylol analogue of monocrotophos, its glycoside and the
unsubstituted amide analogue were detected in the radiotracer studies.
Residues of cholinesterase inhibiting breakdown products have been
detected in crops only rarely, following recommended treatments, and
then at levels close to the limit of determination of the analytical
method employed. Likewise in meat and milk from animals fed
monocrotophos in their diet, at levels far exceeding those likely to
be encountered in practice, only extremely low levels of breakdown
products have been detected, if at all.
In animals
Studies have been conducted with monocrotophos fed to cattle and goats
to determine whether residues in feed give rise to residues in meat
and milk.
32P monocrotophos was fed continuously to two lactating cows at a
level equivalent to 45 ppm in their total diet (Shell Development Co.,
undated). This level is approximately 20 times that which would occur
in practice, assuming a maximum of 50% of monocrotophos-treated
feedstuff in the total diet. Levels of monocrotophos found in the milk
from the two cows were 0.01 ppm and 0.008 ppm. Average levels of
dimethyl phosphate and of N-hydroxy methyl monocrotophos were 0.002
ppm and 0.001 ppm, respectively (Ibid.). No residues of glycosides of
the N-hydroxy methyl compound were detected. After 14 days of this
feeding regime, the two cows were slaughtered and residues determined
on the red meat and liver. These results are given in Table 6.
TABLE 6 Residues of monocrotophos and its breakdown products
present in meat from cows fed monocrotophos
Residues present (ppm)
Sample dimethyl N-hydroxy methyl
analysed monocrotophos phosphate monocrotophos
Red meat 0.02, 0.04 0.005, 0.008 0.003, 0.0084
Liver 0.11, 0.13 0.05, 0.06 0.02, 0.04
32P monocrotophos was administered orally to goats (Menzer and
Casida, 1965) at a single dose of 1 mg/kg body-weight. Within 72 hours
of the application, only 1.4% of the applied radioactivity was present
in the goats' milk. The authors suggested that, in animals,
monocrotophos degradation took place by oxidative N-demethylation to
N-hydroxy methyl monocrotophos and unsubstituted amide. Residues of
these compounds and monocrotophos in samples of the milk from the
treated goats up to 24 hours after treatment are given in Table 7.
TABLE 7 Residues of monocrotophos and its breakdown products found
in milk from goats administered monocrotophos
Residues present in goats' milk (ppm)
Hours after N-hydroxy methyl unsubstituted total organo-
administration monocrotophos monocrotophos amide extractable
1 0.045 0.0001 <0.0001 0.067
2 0.032 0.0086 0.0007 0.061
4 0.034 0.0086 0.0015 0.074
6 0.023 0.011 0.0013 0.057
8 0.012 0.0099 0.0007 0.042
12 0.007 0.0044 0.0006 0.025
16 0.006 0.0020 0.0001 0.012
20 0.012 0.0009 0.0003 0.022
24 0.001 0.0007 <0.0001 0.007
As indicated in Table 7, residues of monocrotophos fell from a maximum
value of 0.0451 ppm one hour after administration to 0.0014 ppm 23
hours later. Maximum levels of N-hydroxy methyl monocrotophos, and the
unsubstituted amide, detected were 0.0105 ppm (after six hours) and
0.0015 ppm (after four hours), respectively. These levels had
decreased by a fifteen-fold factor 24 hours after application.
To determine whether residues in animal feeds could give residues of
the conjugate of N-hydroxy methyl monocrotophos in meat and milk, two
Guernsey cows were fed for ten days with the conjugate at a level of
20 ppm in the total diet (this level is more than 2 000 times that
which may be encountered in practice). The results showed that the
combined residues of the conjugate and its hydrolysis products,
N-hydroxy methyl monocrotophos and the unsubstituted amide, in milk,
were all below 0.01 ppm. At the end of the ten day study the cows were
slaughtered and their tissues analysed. The results are given in Table
8.
TABLE 8 Residues of monocrotophos breakdown products found in tissues
of cows fed monocrotophos conjugate
Tissues Residues present (ppm)
analysed
conjugate of N-hydroxy N-hydroxy methyl monocrotophos
methyl monocrotophos plus unsubstituted amide
fat <0.01 0.02, 0.04
kidney 0.02, 0.01 <0.01, 0.01
meat <0.01, <0.01 0.01, 0.01
liver <0.01, 0.01 <0.01, 0.01
In plants
In order to elucidate the mode of breakdown in leaves, 32P
monocrotophos was applied to cotton plants, both in the glasshouse and
in the field, either as a foliar spray, a stem banding treatment, a
seed dressing or by petiole injection (Lindquist and Bull, 1967). For
the foliar treatment, monocrotophos was applied at a rate of
40 mg/cotton leaf. In the field studies, 85% of the applied
radioactivity was lost during the first two days, presumably through
volatilization. Breakdown was shown to take place on both the surface
and the inside of the treated leaves. Principal breakdown products
found were O-desmethyl monocrotophos, dimethyl phosphate and N-hydroxy
methyl monocrotophos. When monocrotophos was applied as a stem bending
treatment, the recovered radioactivity after 21 days was unchanged
monocrotophos, probably because the lanolin in the formulation had a
stabilizing effect. Following the application of monocrotophos as a
seed treatment (not a recommended application) at a rate of 0.5 mg
monocrotophos/cotton seed, 50% of the radioactivity recovered after
one week was unchanged monocrotophos. O-desmethyl monocrotophos was
present in all samples, indicating the cleavage of a methyl-phosphate
bond. Dimethyl phosphate (formed by hydrolysis of the vinyl phosphate
bond) was recovered in increasing amounts with time. The N-hydroxy
methyl monocrotophos was only detected at 21 days after treatment,
while an unknown compound, later identified as a glycoside of
N-hydroxy methyl monocrotophos (Shell Development Co., undated), was
the major metabolite at 14 and 21 days.
Petiole injection of monocrotophos at 70 mg monocrotophos/cotton leaf
indicated half-lives of monocrotophos in cotton leaves of eight days
in the glasshouse and seven days in the field. Small amounts of the
N-hydroxy methyl monocrotophos and its conjugate were detected. No
residues of the unsubstituted amide (N-desmethyl monocrotophos) were
detected in cotton plants. The authors proposed the pathways shown in
Figure 2 for the degradation of monocrotophos.
Menzer and Casida (1965) injected 32P-monocrotophos into bean plants
at a level of 43.5 ppm and found trace amounts of the N-hydroxy methyl
monocrotophos (0.14% of monocrotophos applied) and the unsubstituted
amide (0.10% of monocrotophos applied) eight days after injection.
However, 20 and 32 days after application monocrotophos was the only
compound detected.
In studies with 14C-labelled monocrotophos (Shell Research Ltd.,
undated) on the foliage of maize, cabbage and apples, and on apple
fruit, the breakdown products present were the unsubstituted amide,
N-hydroxy methyl monocrotophos, (both in the free and conjugated
forms), O-desmethyl-monocrotophos acid and traces of
N-methylacetoacetamide and 3-hydroxy-N-methyl butyramide.
Since, of the breakdown products detected in plants following
application of monocrotophos, only the unsubstituted amide
(N-desmethyl monocrotophos) and the free and bound N-hydroxy methyl
monocrotophos can cause cholinesterase inhibition, analytical methods
(Ibid.) were developed for the determination of these compounds.
Samples from field trials were analysed using these methods.
Results from trials with sugar beet, potatoes, Brussels sprouts,
cauliflower, maize, pears, peas, onions and soybeans (Ibid.) showed
that residues of the unsubstituted amide and the N-hydroxyl methyl
monocrotophos (free and conjugated) could not be detected, nor could
monocrotophos.
 Further data, obtained in cases where monocrotophos residues were
above detectable levels, are shown in Table 9.
It will thus be seen that, in practice, where crops have been treated
with monocrotophos in the field, residues of cholinesterase inhibiting
degradation product in the edible parts did not reach detectable
levels. The only exception was a single sample of carrots in which a
residue of conjugated methylol at a level near to the level of
determination of the analytical method was reported.
In soils
Although monocrotophos is not used as a soil-applied pesticide, a
series of tests were conducted to determine the half-life of
monocrotophos in soil (Shell Chemical Co., undated). In Ripperdam
sandy loam and Sacramento clay loam, the half-lives of monocrotophos
were 18 and 6 days, respectively. In an early experiment (Shell
Research Ltd., undated), where monocrotophos was applied to soil at
rates of 0.25 or 0.5 kg a.i./ha, initial residues, immediately after
application, of 5.6 - 6.6 ppm monocrotophos had fallen to below the
limit of determination of the analytical method employed (less than
0.04 ppm) five months later.
In storage and processing
Studies to determine the effects of storage on residues of
monocrotophos in apples (Shell Research Ltd., undated) have shown that
these residues are not significantly reduced on storage at 15 - 20°C
for 24 days, or at 5 - 10°C for two to three months. Long-term
deep-freeze storage trials have shown that monocrotophos residues are
stable in broccoli and soybean foliage for 11-19 months, although
exceptionally 90% of monocrotophos residues were lost from cabbage
after 14 months deep-freeze storage (Shell Development Co., undated).
Numerous studies on the effect of domestic and commercial processing
on residues of monocrotophos in food crops have been carried out.
Domestic processes, such as washing, peeling and cooking, reduce the
levels of residues of monocrotophos in fruit and vegetables by between
35% and 95%. Commercial processes often have greater effects on
residues of monocrotophos. This canning can reduce typical levels by
over 90%, while the preparation of processed coffee beans, refined
cottonseed and soybean oil, and dried citrus pulp reduced typical
residues to below the limits of the analytical methods employed.
TABLE 9 Residues of monocrotophos breakdown products found in crops1
Preharvest Residues present (ppm)
Crop Country Interval Crop monocrotophos amide plus conjugated
part methylol methylol
21 roots <0.01 <0.01 <0.05
Sugar beet Italy 21 leaves 0.14 <0.01 -
31 roots <0.01 <0.03 <0.05
Sugar beet France 31 leaves 0.10 <0.03 -
31 roots <0.01 <0.03 -
31 leaves 0.04 0.06 -
Maize Italy 14 husk + 0.10 0.20 -
straw 0.10 0.30 -
13 grain <0.01 <0.01 <0.05
Maize S. Africa 13 husk 0.90 0.10 <0.10
13 straw 1.0 0.50 <0.20
3 whole 0.39 <0.05 -
Cabbage S. Africa 13 whole 0.22 <0.05 <0.10
3 whole 0.50 <0.05 -
13 whole 0.38 <0.05 <0.10
28 whole <0.02 <0.04 -
Onions U.K. 14 whole 0.14 <0.04 -
7 whole 0.17 <0.04 -
6 whole 0.06 <0.012 -
Tomatoes S. Africa 20 whole 0.08 <0.012 -
6 whole 0.34 <0.012 -
49 whole <0.01 <0.02 <0.05
Tomatoes S. Africa 28 whole 0.03 <0.02 -
49 whole 0.01 <0.02 <0.05
28 whole 0.10 <0.02 <0.05
TABLE 9 (Cont'd.)
Preharvest Residues present (ppm)
Crop Country Interval Crop monocrotophos amide plus conjugated
part methylol methylol
67 whole 0.18 <0.05 <0.05
Apples Australia 37 whole 0.51 <0.05 <0.05
8 whole 1.8 <0.05 0.06
Apples Italy 30 whole 1.0 <0.04 <0.03
Pears Italy 39 whole 0.15 <0.04 <0.05
14 roots 0.06 <0.02 0.10
Carrots S. Africa 21 roots 0.04 <0.02 <0.05
28 roots <0.01 <0.02 <0.05
1 Data from Shell Research Ltd.
2 Analysed only for unsubstituted amide.
Analyses of fruit with monocrotophos residues have demonstrated that
residues are mainly in the peel. Thus peeling removed 66% of residues
present in oranges, 35 - 70% in apples (Shell Research Ltd.; Shell
Chemical Co., undated) and 80 - 95% in grapefruit (Ibid.). In another
study to determine the levels of monocrotophos present in different
parts of apples, it was shown that 57 - 64% of the residues were
present in the pulp, 5% in the waxy surface layer, the remainder being
in the peel. The removal of the outer leaves of cabbages reduced the
monocrotophos levels by 95%, of cauliflower by 90% and of Brussels
sprouts by over 75% (Shell Research Ltd., undated).
Cold water washing can also reduce residues. Thus washing tomatoes
reduced residues of monocrotophos by up to 35% (Shell Development Co.;
Shell Chemical Co., undated). Domestic cooking reduced levels by
60 - 70% in green beans and 80 - 85% in Brussels sprouts (Shell
Research Ltd., undated).
The effect of the various steps in the commercial canning of beans
(Fahey et al., 1969) and tomatoes (Fahey et al., 1971) has been
studied. Cold water washing of beans effected a 27% reduction and
blanching a 40% reduction. Residues in the canned beans were only 2%
of the residues in the fresh beans. The processes of cold washing and
lye peeling reduced residues in tomatoes by 63% and 87%, respectively.
An 85% reduction in residues of monocrotophos was achieved in tomato
juice and a 91% reduction in whole tomatoes by canning. The process of
juicing oranges reduced the levels of monocrotophos from 0.8 ppm in
the pulp to 0.10 ppm and below (Shell Research Ltd., undated).
Commercial processing of raw coffee beans by removing the berry flesh
mechanically, followed by fermentation, and sun-drying to a moisture
level of 10%, reduced residues of 0.08 ppm monocrotophos in the raw
beans to below the limit of determination of the analytical method
(less than 0.01 ppm) in the processed beans (Ibid.).
The preparation of refined soybean oil reduced residues of 0.13 ppm
monocrotophos in beans to less than 0.01 ppm in the finished oil
(Shell Chemical Co., undated). Similarly, residues of 0.03 ppm in
kernels were reduced to less than 0.01 ppm in processed corn meal and
oil (Ibid.).
With cottonseed oil (Shell Research Ltd., unpublished) it has been
shown that 1 ppm monocrotophos added to the crude oil was reduced to
below the limit of determination of the analytical method (<0.01 ppm)
by the alkali refining step.
Experiments to determine the residues of monocrotophos likely to occur
in beer prepared from monocrotophos-treated hops have demonstrated
that residues of 1.8 ppm monocrotophos in hops were reduced to levels
at or below the limit of determination of the analytical method (0.02
ppm) in filtered and unfiltered beer (Ciba-Geigy Ltd., undated).
During the commercial preparation of citrus pulp cattle feed (Westlake
et al., 1970) from monocrotophos-treated oranges, residues were
reduced to below the limit of determination (less than 0.03 ppm), with
over 50% being lost during grinding and liming and the remainder
during drying.
The following sections summarize these effects in terms of individual
crops, in order to give an estimate of upper levels to be expected in
foods when they are ready to eat.
Cottonseed and soybean oils
Since the process of alkali refining destroys residues in crude
cottonseed oil at levels well above those in practice, refined
cottonseed oil products will not contain detectable residues of
monocrotophos. The same conclusion may be drawn for soybean oil.
Brassicas
In the case of Brussels sprouts, removal of outer leaves followed by
cooking reduced typical levels by 95%. Thus sprouts with initial
residues of 0.2 ppm (proposed tolerance levels) would not be expected
to contain more than 0.01 ppm when cooked and ready to be eaten.
In the case of cauliflower, removal of outer leaves alone reduced
residue levels some 10-fold so that even without cooking, levels in
trimmed cauliflower at an initial level of 0.5 ppm (proposed
tolerance) would be reduced to 0.05 ppm. In the case of cabbage, where
removal of outer leaves reduced residues 20-fold, cabbage with
residues at the proposed tolerance level of 0.05 ppm would not be
expected to contain more than 0.03 ppm when trimmed ready for cooking.
Fresh tomatoes
Fresh tomatoes with 0.5 ppm monocrotophos residues (proposed
tolerance) would lose, according to available data, an average 25% of
their residues, leading to a little less than 0.4 ppm at the point of
consumption.
Commercially processed tomatoes
The combined effects of washing and lye peeling reduced residues to
approximately 5% of their initial value so that residues in tomatoes
ready for canning would not exceed 0.01 ppm, a level which would be
further reduced by canning itself.
Similar considerations apply to canned tomato juice, and neither of
these finished products would contain detectable residues of
monocrotophos.
Beans
Domestic cooking of beans with monocrotophos residues at the tolerance
level of 0.2 ppm would reduce these to approximately 0.1 ppm.
Commercial canning would reduce these levels to below 0.01 ppm,
whereas freezing, which involves washing and blanching, would reduce
levels to 0.1 ppm.
Coffee
Coffee beans with initial residue at the tolerance level of 0.1 ppm
would be unlikely to contain detectable residues (< 0.01 ppm) after
fermentation and drying. For this reason, subsequent processes such as
roasting and brewing were not studied.
Hops
Hops with residues at the tolerance level do not give rise to
detectable residues in beer.
Citrus fruit
Residues in citrus fruit were reduced to an average of 30% of their
initial value on peeling, so that the raw fruit after peeling would
not contain residues above 0.15 ppm, where initial residues were at
the tolerance level of 0.5 ppm.
Juicing reduced levels of around 0.8 ppm in the pulp of oranges to
0.10 ppm in the juice.
Potatoes, maize grain and root vegetables
With the exception of carrots, where two samples out of five were
reported to contain low residues, these crops, treated as recommended,
have not contained measurable residues so that work to demonstrate the
effects of processing on reducing residues could not meaningfully be
undertaken.
Sugarcane and sugar beet
In the case of sugarcane the absence of detectable residues has been
demonstrated not only in the raw commodity but also in the
manufactured products. In neither crop were residues detected
following recommended treatments.
METHODS OF RESIDUE ANALYSIS
Residues of monocrotophos can be determined by specific methods
utilizing either gas-liquid chromatographic procedures or a
cholinesterase inhibition method.
Gas chromatographic methods
Gas chromatographic methods of analysis for monocrotophos are the
methods of choice, based on accuracy, specificity, sensitivity and
speed.
Monocrotophos can be determined by the general procedure using the
flame photometric detector (Beroza and Bowman, 1968; Brody and Chaney,
1966). Specific methods for monocrotophos using this detector have
been developed (Shell Research Ltd., undated; Bowman and Beroza, 1967)
and allow analyses of crops down to a limit of determination of 0.01
ppm. The following procedure (Shell Research, Ltd., undated) has
proved satisfactory in analysing crops: samples are extracted by
maceration with chloroform, low water content crops are first dampened
with water. Co-extracted natural products are removed using a column
adsorption chromatographic clean-up technique. The
monocrotophos-containing extract is analysed using the flame
photometric detector (FPD). Using this procedure, mean recoveries are
75 - 120% from crops at the 0.05 - 0.20 ppm level.
The thermionic detector has also been employed in the analyses of
crops for residues of monocrotophos (Shell Chemical Co., undated). In
this method, the sample is extracted with dichloromethane followed by
clean-up using a series of solvent exchanges and washings, prior to
injection of an aliquot of the extract onto the GLC instrument. Mean
recoveries with this method are 80 - 120% for crops, with a limit of
determination of 0.03 - 0.05 ppm.
Enzymatic inhibition
Specific enzymatic methods for the detection and determination of
monocrotophos in crops and milk have been developed (Shell Development
Co., undated). The samples for analysis are extracted with chloroform,
followed by a solvent exchange to hexane and concentration of the
extract. Column chromatography using a gradient column elution
technique with hexane/dichloromethane removes co-extractives. The
separated monocrotophos is transferred to water and determined by
enzyme inhibition spectrophotometric methods. Using this procedure,
the limit of determination of monocrotophos is about 0.10 ppm in crops
and 0.01 ppm in milk. Recoveries are in the range of 70 - 125%.
Methods for analyses of monocrotophos breakdown products
The analytical data for the N-methylol and for the unsubstituted amide
reported in this summary were developed using the method described for
monocrotophos (Shell Research Ltd., undated). The conjugate of the
N-methylol was determined using the method, which depends on
converting the conjugate to the sec-butyl thio-ether of the methylol
and subsequent determination by T.L.C. and enzyme inhibition (Ibid.).
A further method, using the thermionic detector (Griang and Beckman,
1968) has been developed for analysing crops for residues of
O-desmethyl monocrotophos, N-hydroxy methyl monocrotophos and its
conjugate.
NATIONAL TOLERANCES
Officially approved tolerances for monocrotophos have been established
in some countries and examples of these are given in Table 10.
TABLE 10
Examples of national tolerances as reported to the meeting
Country Crop(s) Tolerance
(ppm)
Australia Cottonseed, potatoes 0.1
Apples, pears 1.0
Italy Apples, pears 0.5
South Africa All treated crops 0.1
U.S.A. Cottonseed, potatoes,
sugarcane 0.1
Venezuela Cottonseed, rice, maize,
potatoes, sugarcane,
vegetables, citrus fruit 0.1
Yugoslavia All treated crops 0.1
APPRAISAL
Monocrotophos is an organophosphorus insecticide which finds its main
use for foliar application to cotton. It is also recommended for
application against foliage pests of maize, sugarcane, sugar beet,
vegetables, potatoes and certain fruits. It possesses both systemic
and residual contact properties.
Approved uses involve the application of from 0.25 - 1.0 kg a.i./ha.
The recommended pre-harvest interval varies according to crop and
country, ranging from seven days on potatoes to 30 days on fruit and
vegetables.
Extensive studies have shown that degradation of monocrotophos
residues on and in plants can occur by hydrolytic and/or oxidative
mechanisms. The products of hydrolysis are non-cholinesterase
inhibiting and of low toxicity. Only insignificant amounts of
secondary metabolites have been found during radiotracer studies.
Volatilization appears to be the major factor in the rapid loss of
residues following application. Monocrotophos itself is the principal
residue found on most plants even 7 - 28 days after application.
Metabolism studies suggest that ruminants receiving
monocrotophos-treated feedstuff metabolize the residue to metabolites
similar to those found on plants. There appears to be no tendency for
residues to accumulate in animals receiving contaminated rations. Only
low levels of monocrotophos and trace amounts of breakdown products
were detected in red meat, liver and milk of cattle receiving
feedstuffs containing monocrotophos at levels many times the maximum
likely to occur in practice.
Residue data, obtained from supervised trials in 24 different
countries clearly demonstrated the level of residues to be expected
under widely differing conditions on a wide range of crops. Except for
samples of leafy materials collected within one or two days of
application of heavy dosages of monocrotophos, residues in excess of
1.0 ppm were seldom encountered.
No substantial residue occurs in any fraction of the cotton plant used
for food, even when unprocessed.
As cotton foliage retains significant residues for more than three
weeks following application of approved rates of monocrotophos,
feeding of treated cotton foliage to cattle should be avoided.
Domestic processes, such as washing, peeling and cooking reduce the
levels of monocrotophos residues in fruit and vegetables by between
35% and 95%. Commercial processes often have greater effects on
reducing residues of monocrotophos. The peeling of citrus fruits
removes 60 - 73% of the total residue. Beer produced from treated hops
was shown to contain no detectable residues. The absence of residue
has been demonstrated in sugarcane and in sugar products manufactured
therefrom.
For determining monocrotophos residues, either gas-liquid
chromatography or cholinesterase inhibition methods are used, with the
former being the methods of choice from the aspect of accuracy,
specificity, sensitivity and speed. The limit of determination is 0.01
ppm. Using GLC methods recoveries of 75 - 120% were obtained from crop
material containing 0.05 - 0.2 ppm. Several of the available methods
appear suitable for regulatory purposes, but the method of Bowman and
Beroza (1967) is recommended.
RECOMMENDATIONS
The following recommended tolerances for animal products reflect a
value at or just above the limit of determination. The time interval
between application and harvest which has been used in determining the
maximum residue limits is appropriate to the agricultural practices in
numerous countries.
TOLERANCES
Interval between
Foodstuff Tolerance treatment and
(ppm) harvest (days)
Cottonseed 0.1 14
Cottonseed oil (raw) 0.05* 14
Potatoes 0.05* 7
Maize (grain) 0.05* 14
Tomatoes 0.5 21
Brussels sprouts 0.2 15
Cabbage, cauliflower 0.2 21
Carrots, turnips 0.05* 14
Onions 0.1 10
Sugar beet 0.05* 14
Peas 0.1 15
Beans 0.2 21
Soybeans 0.05* 14
Coffee (raw beans) 0.1 42
Hops (dried) 1 30
Citrus fruit 0.2 28
Apples and pears 1 (temporary 28
1975)
Meat and edible offal of
cattle, sheep, goats,
pigs and poultry 0.02* from feeding
Milk 0.002* treated plant
Milk products 0.02* products
Eggs (shell free) 0.02* "
* at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Studies on human exposure.
2. Information on the incidence of residues in apples and pears.
REFERENCES
Beroza, M. and Bowman, M.C. (1968) Chromatography of pesticide
residues containing phosphorus or sulphur with the flame photometric
detector. Environ. Sci., Technol., 2: 450.
Bowman, M.C. and Beroza, M. (1967) Chromatographic analysis of azodrin
and Bidrin. J. Agr. Fd. Chem., 15: 465
Brody, S.S. and Chaney, J.E. (1966) Flame photometric detector. J.
Gas. Chromat., 4: 42.
Bull, D.L. and Lindquist, D.A. (1964) Metabolism of 3-hydroxy-N,
N-dimethyl-crotonamide dimethylphosphate by cotton plants, insects and
rats. J. Agr. Fd. Chem., 12: 310-317.
Bull, D.L. and Lindquist, D.A. (1967) Metabolism of
3-hydroxy-N-methyl-cis-crotonamide dimethyl phosphate (azodrin) by
insects and rats. J. Agr. Fd. Chem., 14: 105-109.
Gaines, T.B. (1969) Acute toxicity of pesticides. Tox. Appl.
Pharmacol., 14: 515-534.
Ciba-Geigy Ltd. (undated) Reports on monocrotophos nos. 86 - 88,
90 - 93, 97, 111, 116 - 131. (unpublished)
Einsenlord, G. and Loquram, G.S. (1965) Results of short route
reproduction study of rats fed diets containing SD 9129 insecticide
over three generations. Data from the Hine Laboratory submitted by the
Shell Chemical Company. (unpublished)
Einsenlord, G. and Loquram, G.S. (1966) Results of long route
reproduction study of rats fed diets containing SD 9129 insecticide
over three generations. Data from the Hine laboratory submitted by the
Shell Chemical Co. (unpublished)
Fahey, J.E., Gould, G.E. and Nelson, P.E. (1969) Removal of gardona
and azodrin from vegetable crops by commercial preparative methods. J.
Agr. Fd. Chem., 17: 1204.
Fahey, J.E., Gould, G.E. and Nelson, P.E. (1971) Removal of azodrin
residues from tomatoes by commercial preparative methods. J. Agr. Fd.
Chem., 19: 81.
Griang, B.Y. and Beckman, H.F. (1968) Determination of bidrin, azodrin
and their metabolites with the thermionic detector. J. Agr. Fd. Chem.,
16: 899.
Hunter, C.G. (1964) The efficacy of atropine and oxime therapy as an
antidote to poisoning by SD 9129 in guinea pigs. Report Tunstall
Laboratory submitted by the Shell Chemical Co. (unpublished)
Johnston, C.D., Thompson, W.M. and Donoso, J. (1967a) Azodrin safety
evaluation by a chronic feeding study in the dog for two years. Data
from Woodard Research Corporation submitted by Shell Chemical Co.
(unpublished)
Johnston, C.D., Thompson, W.M. and Donoso, J. (1967b) Azodrin safety
evaluation by a chronic feeding study in the rat for two years. Data
from Woodard Research Corporation submitted by Shell Chemical Co.
(unpublished)
Lindquist, D.A. and Bull, D.L. (1967) Fate of
3-hydroxy-N- methyl-cis-crotonamide dimethyl phosphate in cotton
plants. J. Agr. Fd. Chem., 15: 267-272.
Menzer, R.L. and Casida, J.E. (1965) Nature of toxic metabolites
formed in mammals, insects and plants from 3-(dimethoxy
phosphinyloxy)-N,N-dimethyl-cis-crotonamide and its N-methyl
analogue. J. Agr. Fd. Chem., 13: 102-112.
Potter, J.C. (1965) Cattle feeding studies with 32P-labelled azodrin.
Data submitted by Shell Chemical Co. (unpublished)
Reiff, B. (1969) Pharmacological studies into the toxic actions of
cholinesterase inhibitors: (a) The effect of antidotes on the
subcutaneous toxicity of azodrin in the rat. Report of Tunstall
Laboratory submitted by Shell Chemical Co. (unpublished)
Shellenberger, T.E. (1965a) Potentiation studies - rats. Data from
Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. (1965b) Demyelination study - hens. Data from
Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. (1966a) Acute toxicity of azodrin and metabolites.
Data from Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. (1966b) Cholinesterase inhibition and
toxicological evaluations of two organophosphate pesticides in
Japanese quail. Tox. Appl. Pharm. 8: 22.
Shellenberger, T.E. and Newell, G.W. (1963) Acute oral toxicity. Data
from Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. and Newell, G.W. (1964) Subacute toxicity and
cholinesterase study of Shell compound SD 9129 - rat and dog. Data
from Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shell Chemical Co. (1966) Subacute toxicity and cholinesterase study
of Shell compound SD 13311 in rats. Data from Stanford Research
Institute submitted by Shell Chemical Co. (unpublished)
Shell Chemical Co. (1972) Identification of unknown 4. Report Shell
Chemical Co. (unpublished)
Shell Chemical Co. (undated) Reports on monocrotophos nos 50 - 67, 69
- 74, 76, 77, 79 - 81, 89, 101, 115, 132 - 136, 139 - 151, 153, 172,
176, 182 - 184. (unpublished)
Shell Development Co. (undated) Reports on monocrotophos nos. 68, 75,
78, 82 - 85, 100, 110, 112, 114, 137, 138, 152, 155, 171, 173 - 175,
177 - 181. (unpublished)
Shell Research Ltd. (undated) Reports on monocrotophos nos. 1 - 49,
94, 95, 98, 99, 154, 156 -170. (unpublished)
Thorpe, E. and Dix, M. (1972) Toxicity studies on azodrin:
Teratological studies in rabbits. Summary submitted by Shell Chemical
Co. (unpublished)
Westlake, W.E., Gunther, F.A. and Jeppson, L.R. (1970) Persistence of
azodrin residues on and in Valencia oranges and in
laboratory-processed citrus pulp cattle feed. J. Agr. Fd. Chem., 18:
864.
Young, J.R. and Bowman, M.C. (1967) Azodrin for corn earworm and fall
armyworm control and its persistence in sweet corn. J. Econ. Ent., 60:
1282.
Further data, obtained in cases where monocrotophos residues were
above detectable levels, are shown in Table 9.
It will thus be seen that, in practice, where crops have been treated
with monocrotophos in the field, residues of cholinesterase inhibiting
degradation product in the edible parts did not reach detectable
levels. The only exception was a single sample of carrots in which a
residue of conjugated methylol at a level near to the level of
determination of the analytical method was reported.
In soils
Although monocrotophos is not used as a soil-applied pesticide, a
series of tests were conducted to determine the half-life of
monocrotophos in soil (Shell Chemical Co., undated). In Ripperdam
sandy loam and Sacramento clay loam, the half-lives of monocrotophos
were 18 and 6 days, respectively. In an early experiment (Shell
Research Ltd., undated), where monocrotophos was applied to soil at
rates of 0.25 or 0.5 kg a.i./ha, initial residues, immediately after
application, of 5.6 - 6.6 ppm monocrotophos had fallen to below the
limit of determination of the analytical method employed (less than
0.04 ppm) five months later.
In storage and processing
Studies to determine the effects of storage on residues of
monocrotophos in apples (Shell Research Ltd., undated) have shown that
these residues are not significantly reduced on storage at 15 - 20°C
for 24 days, or at 5 - 10°C for two to three months. Long-term
deep-freeze storage trials have shown that monocrotophos residues are
stable in broccoli and soybean foliage for 11-19 months, although
exceptionally 90% of monocrotophos residues were lost from cabbage
after 14 months deep-freeze storage (Shell Development Co., undated).
Numerous studies on the effect of domestic and commercial processing
on residues of monocrotophos in food crops have been carried out.
Domestic processes, such as washing, peeling and cooking, reduce the
levels of residues of monocrotophos in fruit and vegetables by between
35% and 95%. Commercial processes often have greater effects on
residues of monocrotophos. This canning can reduce typical levels by
over 90%, while the preparation of processed coffee beans, refined
cottonseed and soybean oil, and dried citrus pulp reduced typical
residues to below the limits of the analytical methods employed.
TABLE 9 Residues of monocrotophos breakdown products found in crops1
Preharvest Residues present (ppm)
Crop Country Interval Crop monocrotophos amide plus conjugated
part methylol methylol
21 roots <0.01 <0.01 <0.05
Sugar beet Italy 21 leaves 0.14 <0.01 -
31 roots <0.01 <0.03 <0.05
Sugar beet France 31 leaves 0.10 <0.03 -
31 roots <0.01 <0.03 -
31 leaves 0.04 0.06 -
Maize Italy 14 husk + 0.10 0.20 -
straw 0.10 0.30 -
13 grain <0.01 <0.01 <0.05
Maize S. Africa 13 husk 0.90 0.10 <0.10
13 straw 1.0 0.50 <0.20
3 whole 0.39 <0.05 -
Cabbage S. Africa 13 whole 0.22 <0.05 <0.10
3 whole 0.50 <0.05 -
13 whole 0.38 <0.05 <0.10
28 whole <0.02 <0.04 -
Onions U.K. 14 whole 0.14 <0.04 -
7 whole 0.17 <0.04 -
6 whole 0.06 <0.012 -
Tomatoes S. Africa 20 whole 0.08 <0.012 -
6 whole 0.34 <0.012 -
49 whole <0.01 <0.02 <0.05
Tomatoes S. Africa 28 whole 0.03 <0.02 -
49 whole 0.01 <0.02 <0.05
28 whole 0.10 <0.02 <0.05
TABLE 9 (Cont'd.)
Preharvest Residues present (ppm)
Crop Country Interval Crop monocrotophos amide plus conjugated
part methylol methylol
67 whole 0.18 <0.05 <0.05
Apples Australia 37 whole 0.51 <0.05 <0.05
8 whole 1.8 <0.05 0.06
Apples Italy 30 whole 1.0 <0.04 <0.03
Pears Italy 39 whole 0.15 <0.04 <0.05
14 roots 0.06 <0.02 0.10
Carrots S. Africa 21 roots 0.04 <0.02 <0.05
28 roots <0.01 <0.02 <0.05
1 Data from Shell Research Ltd.
2 Analysed only for unsubstituted amide.
Analyses of fruit with monocrotophos residues have demonstrated that
residues are mainly in the peel. Thus peeling removed 66% of residues
present in oranges, 35 - 70% in apples (Shell Research Ltd.; Shell
Chemical Co., undated) and 80 - 95% in grapefruit (Ibid.). In another
study to determine the levels of monocrotophos present in different
parts of apples, it was shown that 57 - 64% of the residues were
present in the pulp, 5% in the waxy surface layer, the remainder being
in the peel. The removal of the outer leaves of cabbages reduced the
monocrotophos levels by 95%, of cauliflower by 90% and of Brussels
sprouts by over 75% (Shell Research Ltd., undated).
Cold water washing can also reduce residues. Thus washing tomatoes
reduced residues of monocrotophos by up to 35% (Shell Development Co.;
Shell Chemical Co., undated). Domestic cooking reduced levels by
60 - 70% in green beans and 80 - 85% in Brussels sprouts (Shell
Research Ltd., undated).
The effect of the various steps in the commercial canning of beans
(Fahey et al., 1969) and tomatoes (Fahey et al., 1971) has been
studied. Cold water washing of beans effected a 27% reduction and
blanching a 40% reduction. Residues in the canned beans were only 2%
of the residues in the fresh beans. The processes of cold washing and
lye peeling reduced residues in tomatoes by 63% and 87%, respectively.
An 85% reduction in residues of monocrotophos was achieved in tomato
juice and a 91% reduction in whole tomatoes by canning. The process of
juicing oranges reduced the levels of monocrotophos from 0.8 ppm in
the pulp to 0.10 ppm and below (Shell Research Ltd., undated).
Commercial processing of raw coffee beans by removing the berry flesh
mechanically, followed by fermentation, and sun-drying to a moisture
level of 10%, reduced residues of 0.08 ppm monocrotophos in the raw
beans to below the limit of determination of the analytical method
(less than 0.01 ppm) in the processed beans (Ibid.).
The preparation of refined soybean oil reduced residues of 0.13 ppm
monocrotophos in beans to less than 0.01 ppm in the finished oil
(Shell Chemical Co., undated). Similarly, residues of 0.03 ppm in
kernels were reduced to less than 0.01 ppm in processed corn meal and
oil (Ibid.).
With cottonseed oil (Shell Research Ltd., unpublished) it has been
shown that 1 ppm monocrotophos added to the crude oil was reduced to
below the limit of determination of the analytical method (<0.01 ppm)
by the alkali refining step.
Experiments to determine the residues of monocrotophos likely to occur
in beer prepared from monocrotophos-treated hops have demonstrated
that residues of 1.8 ppm monocrotophos in hops were reduced to levels
at or below the limit of determination of the analytical method (0.02
ppm) in filtered and unfiltered beer (Ciba-Geigy Ltd., undated).
During the commercial preparation of citrus pulp cattle feed (Westlake
et al., 1970) from monocrotophos-treated oranges, residues were
reduced to below the limit of determination (less than 0.03 ppm), with
over 50% being lost during grinding and liming and the remainder
during drying.
The following sections summarize these effects in terms of individual
crops, in order to give an estimate of upper levels to be expected in
foods when they are ready to eat.
Cottonseed and soybean oils
Since the process of alkali refining destroys residues in crude
cottonseed oil at levels well above those in practice, refined
cottonseed oil products will not contain detectable residues of
monocrotophos. The same conclusion may be drawn for soybean oil.
Brassicas
In the case of Brussels sprouts, removal of outer leaves followed by
cooking reduced typical levels by 95%. Thus sprouts with initial
residues of 0.2 ppm (proposed tolerance levels) would not be expected
to contain more than 0.01 ppm when cooked and ready to be eaten.
In the case of cauliflower, removal of outer leaves alone reduced
residue levels some 10-fold so that even without cooking, levels in
trimmed cauliflower at an initial level of 0.5 ppm (proposed
tolerance) would be reduced to 0.05 ppm. In the case of cabbage, where
removal of outer leaves reduced residues 20-fold, cabbage with
residues at the proposed tolerance level of 0.05 ppm would not be
expected to contain more than 0.03 ppm when trimmed ready for cooking.
Fresh tomatoes
Fresh tomatoes with 0.5 ppm monocrotophos residues (proposed
tolerance) would lose, according to available data, an average 25% of
their residues, leading to a little less than 0.4 ppm at the point of
consumption.
Commercially processed tomatoes
The combined effects of washing and lye peeling reduced residues to
approximately 5% of their initial value so that residues in tomatoes
ready for canning would not exceed 0.01 ppm, a level which would be
further reduced by canning itself.
Similar considerations apply to canned tomato juice, and neither of
these finished products would contain detectable residues of
monocrotophos.
Beans
Domestic cooking of beans with monocrotophos residues at the tolerance
level of 0.2 ppm would reduce these to approximately 0.1 ppm.
Commercial canning would reduce these levels to below 0.01 ppm,
whereas freezing, which involves washing and blanching, would reduce
levels to 0.1 ppm.
Coffee
Coffee beans with initial residue at the tolerance level of 0.1 ppm
would be unlikely to contain detectable residues (< 0.01 ppm) after
fermentation and drying. For this reason, subsequent processes such as
roasting and brewing were not studied.
Hops
Hops with residues at the tolerance level do not give rise to
detectable residues in beer.
Citrus fruit
Residues in citrus fruit were reduced to an average of 30% of their
initial value on peeling, so that the raw fruit after peeling would
not contain residues above 0.15 ppm, where initial residues were at
the tolerance level of 0.5 ppm.
Juicing reduced levels of around 0.8 ppm in the pulp of oranges to
0.10 ppm in the juice.
Potatoes, maize grain and root vegetables
With the exception of carrots, where two samples out of five were
reported to contain low residues, these crops, treated as recommended,
have not contained measurable residues so that work to demonstrate the
effects of processing on reducing residues could not meaningfully be
undertaken.
Sugarcane and sugar beet
In the case of sugarcane the absence of detectable residues has been
demonstrated not only in the raw commodity but also in the
manufactured products. In neither crop were residues detected
following recommended treatments.
METHODS OF RESIDUE ANALYSIS
Residues of monocrotophos can be determined by specific methods
utilizing either gas-liquid chromatographic procedures or a
cholinesterase inhibition method.
Gas chromatographic methods
Gas chromatographic methods of analysis for monocrotophos are the
methods of choice, based on accuracy, specificity, sensitivity and
speed.
Monocrotophos can be determined by the general procedure using the
flame photometric detector (Beroza and Bowman, 1968; Brody and Chaney,
1966). Specific methods for monocrotophos using this detector have
been developed (Shell Research Ltd., undated; Bowman and Beroza, 1967)
and allow analyses of crops down to a limit of determination of 0.01
ppm. The following procedure (Shell Research, Ltd., undated) has
proved satisfactory in analysing crops: samples are extracted by
maceration with chloroform, low water content crops are first dampened
with water. Co-extracted natural products are removed using a column
adsorption chromatographic clean-up technique. The
monocrotophos-containing extract is analysed using the flame
photometric detector (FPD). Using this procedure, mean recoveries are
75 - 120% from crops at the 0.05 - 0.20 ppm level.
The thermionic detector has also been employed in the analyses of
crops for residues of monocrotophos (Shell Chemical Co., undated). In
this method, the sample is extracted with dichloromethane followed by
clean-up using a series of solvent exchanges and washings, prior to
injection of an aliquot of the extract onto the GLC instrument. Mean
recoveries with this method are 80 - 120% for crops, with a limit of
determination of 0.03 - 0.05 ppm.
Enzymatic inhibition
Specific enzymatic methods for the detection and determination of
monocrotophos in crops and milk have been developed (Shell Development
Co., undated). The samples for analysis are extracted with chloroform,
followed by a solvent exchange to hexane and concentration of the
extract. Column chromatography using a gradient column elution
technique with hexane/dichloromethane removes co-extractives. The
separated monocrotophos is transferred to water and determined by
enzyme inhibition spectrophotometric methods. Using this procedure,
the limit of determination of monocrotophos is about 0.10 ppm in crops
and 0.01 ppm in milk. Recoveries are in the range of 70 - 125%.
Methods for analyses of monocrotophos breakdown products
The analytical data for the N-methylol and for the unsubstituted amide
reported in this summary were developed using the method described for
monocrotophos (Shell Research Ltd., undated). The conjugate of the
N-methylol was determined using the method, which depends on
converting the conjugate to the sec-butyl thio-ether of the methylol
and subsequent determination by T.L.C. and enzyme inhibition (Ibid.).
A further method, using the thermionic detector (Griang and Beckman,
1968) has been developed for analysing crops for residues of
O-desmethyl monocrotophos, N-hydroxy methyl monocrotophos and its
conjugate.
NATIONAL TOLERANCES
Officially approved tolerances for monocrotophos have been established
in some countries and examples of these are given in Table 10.
TABLE 10
Examples of national tolerances as reported to the meeting
Country Crop(s) Tolerance
(ppm)
Australia Cottonseed, potatoes 0.1
Apples, pears 1.0
Italy Apples, pears 0.5
South Africa All treated crops 0.1
U.S.A. Cottonseed, potatoes,
sugarcane 0.1
Venezuela Cottonseed, rice, maize,
potatoes, sugarcane,
vegetables, citrus fruit 0.1
Yugoslavia All treated crops 0.1
APPRAISAL
Monocrotophos is an organophosphorus insecticide which finds its main
use for foliar application to cotton. It is also recommended for
application against foliage pests of maize, sugarcane, sugar beet,
vegetables, potatoes and certain fruits. It possesses both systemic
and residual contact properties.
Approved uses involve the application of from 0.25 - 1.0 kg a.i./ha.
The recommended pre-harvest interval varies according to crop and
country, ranging from seven days on potatoes to 30 days on fruit and
vegetables.
Extensive studies have shown that degradation of monocrotophos
residues on and in plants can occur by hydrolytic and/or oxidative
mechanisms. The products of hydrolysis are non-cholinesterase
inhibiting and of low toxicity. Only insignificant amounts of
secondary metabolites have been found during radiotracer studies.
Volatilization appears to be the major factor in the rapid loss of
residues following application. Monocrotophos itself is the principal
residue found on most plants even 7 - 28 days after application.
Metabolism studies suggest that ruminants receiving
monocrotophos-treated feedstuff metabolize the residue to metabolites
similar to those found on plants. There appears to be no tendency for
residues to accumulate in animals receiving contaminated rations. Only
low levels of monocrotophos and trace amounts of breakdown products
were detected in red meat, liver and milk of cattle receiving
feedstuffs containing monocrotophos at levels many times the maximum
likely to occur in practice.
Residue data, obtained from supervised trials in 24 different
countries clearly demonstrated the level of residues to be expected
under widely differing conditions on a wide range of crops. Except for
samples of leafy materials collected within one or two days of
application of heavy dosages of monocrotophos, residues in excess of
1.0 ppm were seldom encountered.
No substantial residue occurs in any fraction of the cotton plant used
for food, even when unprocessed.
As cotton foliage retains significant residues for more than three
weeks following application of approved rates of monocrotophos,
feeding of treated cotton foliage to cattle should be avoided.
Domestic processes, such as washing, peeling and cooking reduce the
levels of monocrotophos residues in fruit and vegetables by between
35% and 95%. Commercial processes often have greater effects on
reducing residues of monocrotophos. The peeling of citrus fruits
removes 60 - 73% of the total residue. Beer produced from treated hops
was shown to contain no detectable residues. The absence of residue
has been demonstrated in sugarcane and in sugar products manufactured
therefrom.
For determining monocrotophos residues, either gas-liquid
chromatography or cholinesterase inhibition methods are used, with the
former being the methods of choice from the aspect of accuracy,
specificity, sensitivity and speed. The limit of determination is 0.01
ppm. Using GLC methods recoveries of 75 - 120% were obtained from crop
material containing 0.05 - 0.2 ppm. Several of the available methods
appear suitable for regulatory purposes, but the method of Bowman and
Beroza (1967) is recommended.
RECOMMENDATIONS
The following recommended tolerances for animal products reflect a
value at or just above the limit of determination. The time interval
between application and harvest which has been used in determining the
maximum residue limits is appropriate to the agricultural practices in
numerous countries.
TOLERANCES
Interval between
Foodstuff Tolerance treatment and
(ppm) harvest (days)
Cottonseed 0.1 14
Cottonseed oil (raw) 0.05* 14
Potatoes 0.05* 7
Maize (grain) 0.05* 14
Tomatoes 0.5 21
Brussels sprouts 0.2 15
Cabbage, cauliflower 0.2 21
Carrots, turnips 0.05* 14
Onions 0.1 10
Sugar beet 0.05* 14
Peas 0.1 15
Beans 0.2 21
Soybeans 0.05* 14
Coffee (raw beans) 0.1 42
Hops (dried) 1 30
Citrus fruit 0.2 28
Apples and pears 1 (temporary 28
1975)
Meat and edible offal of
cattle, sheep, goats,
pigs and poultry 0.02* from feeding
Milk 0.002* treated plant
Milk products 0.02* products
Eggs (shell free) 0.02* "
* at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Studies on human exposure.
2. Information on the incidence of residues in apples and pears.
REFERENCES
Beroza, M. and Bowman, M.C. (1968) Chromatography of pesticide
residues containing phosphorus or sulphur with the flame photometric
detector. Environ. Sci., Technol., 2: 450.
Bowman, M.C. and Beroza, M. (1967) Chromatographic analysis of azodrin
and Bidrin. J. Agr. Fd. Chem., 15: 465
Brody, S.S. and Chaney, J.E. (1966) Flame photometric detector. J.
Gas. Chromat., 4: 42.
Bull, D.L. and Lindquist, D.A. (1964) Metabolism of 3-hydroxy-N,
N-dimethyl-crotonamide dimethylphosphate by cotton plants, insects and
rats. J. Agr. Fd. Chem., 12: 310-317.
Bull, D.L. and Lindquist, D.A. (1967) Metabolism of
3-hydroxy-N-methyl-cis-crotonamide dimethyl phosphate (azodrin) by
insects and rats. J. Agr. Fd. Chem., 14: 105-109.
Gaines, T.B. (1969) Acute toxicity of pesticides. Tox. Appl.
Pharmacol., 14: 515-534.
Ciba-Geigy Ltd. (undated) Reports on monocrotophos nos. 86 - 88,
90 - 93, 97, 111, 116 - 131. (unpublished)
Einsenlord, G. and Loquram, G.S. (1965) Results of short route
reproduction study of rats fed diets containing SD 9129 insecticide
over three generations. Data from the Hine Laboratory submitted by the
Shell Chemical Company. (unpublished)
Einsenlord, G. and Loquram, G.S. (1966) Results of long route
reproduction study of rats fed diets containing SD 9129 insecticide
over three generations. Data from the Hine laboratory submitted by the
Shell Chemical Co. (unpublished)
Fahey, J.E., Gould, G.E. and Nelson, P.E. (1969) Removal of gardona
and azodrin from vegetable crops by commercial preparative methods. J.
Agr. Fd. Chem., 17: 1204.
Fahey, J.E., Gould, G.E. and Nelson, P.E. (1971) Removal of azodrin
residues from tomatoes by commercial preparative methods. J. Agr. Fd.
Chem., 19: 81.
Griang, B.Y. and Beckman, H.F. (1968) Determination of bidrin, azodrin
and their metabolites with the thermionic detector. J. Agr. Fd. Chem.,
16: 899.
Hunter, C.G. (1964) The efficacy of atropine and oxime therapy as an
antidote to poisoning by SD 9129 in guinea pigs. Report Tunstall
Laboratory submitted by the Shell Chemical Co. (unpublished)
Johnston, C.D., Thompson, W.M. and Donoso, J. (1967a) Azodrin safety
evaluation by a chronic feeding study in the dog for two years. Data
from Woodard Research Corporation submitted by Shell Chemical Co.
(unpublished)
Johnston, C.D., Thompson, W.M. and Donoso, J. (1967b) Azodrin safety
evaluation by a chronic feeding study in the rat for two years. Data
from Woodard Research Corporation submitted by Shell Chemical Co.
(unpublished)
Lindquist, D.A. and Bull, D.L. (1967) Fate of
3-hydroxy-N- methyl-cis-crotonamide dimethyl phosphate in cotton
plants. J. Agr. Fd. Chem., 15: 267-272.
Menzer, R.L. and Casida, J.E. (1965) Nature of toxic metabolites
formed in mammals, insects and plants from 3-(dimethoxy
phosphinyloxy)-N,N-dimethyl-cis-crotonamide and its N-methyl
analogue. J. Agr. Fd. Chem., 13: 102-112.
Potter, J.C. (1965) Cattle feeding studies with 32P-labelled azodrin.
Data submitted by Shell Chemical Co. (unpublished)
Reiff, B. (1969) Pharmacological studies into the toxic actions of
cholinesterase inhibitors: (a) The effect of antidotes on the
subcutaneous toxicity of azodrin in the rat. Report of Tunstall
Laboratory submitted by Shell Chemical Co. (unpublished)
Shellenberger, T.E. (1965a) Potentiation studies - rats. Data from
Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. (1965b) Demyelination study - hens. Data from
Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. (1966a) Acute toxicity of azodrin and metabolites.
Data from Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. (1966b) Cholinesterase inhibition and
toxicological evaluations of two organophosphate pesticides in
Japanese quail. Tox. Appl. Pharm. 8: 22.
Shellenberger, T.E. and Newell, G.W. (1963) Acute oral toxicity. Data
from Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shellenberger, T.E. and Newell, G.W. (1964) Subacute toxicity and
cholinesterase study of Shell compound SD 9129 - rat and dog. Data
from Stanford Research Institute submitted by Shell Chemical Co.
(unpublished)
Shell Chemical Co. (1966) Subacute toxicity and cholinesterase study
of Shell compound SD 13311 in rats. Data from Stanford Research
Institute submitted by Shell Chemical Co. (unpublished)
Shell Chemical Co. (1972) Identification of unknown 4. Report Shell
Chemical Co. (unpublished)
Shell Chemical Co. (undated) Reports on monocrotophos nos 50 - 67, 69
- 74, 76, 77, 79 - 81, 89, 101, 115, 132 - 136, 139 - 151, 153, 172,
176, 182 - 184. (unpublished)
Shell Development Co. (undated) Reports on monocrotophos nos. 68, 75,
78, 82 - 85, 100, 110, 112, 114, 137, 138, 152, 155, 171, 173 - 175,
177 - 181. (unpublished)
Shell Research Ltd. (undated) Reports on monocrotophos nos. 1 - 49,
94, 95, 98, 99, 154, 156 -170. (unpublished)
Thorpe, E. and Dix, M. (1972) Toxicity studies on azodrin:
Teratological studies in rabbits. Summary submitted by Shell Chemical
Co. (unpublished)
Westlake, W.E., Gunther, F.A. and Jeppson, L.R. (1970) Persistence of
azodrin residues on and in Valencia oranges and in
laboratory-processed citrus pulp cattle feed. J. Agr. Fd. Chem., 18:
864.
Young, J.R. and Bowman, M.C. (1967) Azodrin for corn earworm and fall
armyworm control and its persistence in sweet corn. J. Econ. Ent., 60:
1282.
