
MONOCROTOPHOS
First draft prepared by Dr. S. Caroldi,
University of Padua,
Padua, Italy
EXPLANATION
Monocrotophos was previously evaluated by the Joint Meeting in
1972 (Annex I, 18) when an ADI of 0.0003 mg/kg bw was allocated.
Additional data submitted in 1975 (Annex I, 24) with respect to
mutagenicity testing, biotransformation, and observations on man
allowed the Meeting to increase the ADI to 0.0006 mg/kg bw. Since
then, additional data have been generated, which have been evaluated
by the 1991 FAO/WHO Joint Meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Biotransformation
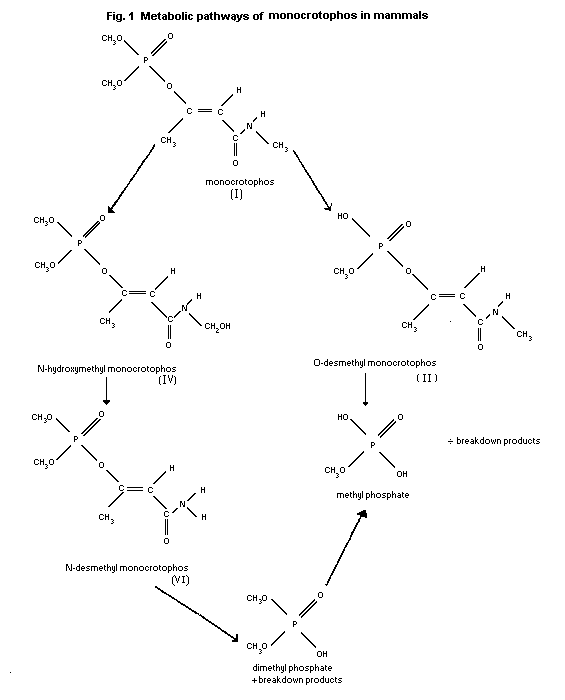
Metabolic pathways of monocrotophos in mammals are depicted in
Fig.1.
Effects on enzymes and other biochemical parameters
Thirty male and female (60 controls) Wistar-derived rats
(5 weeks old at the beginning of the study) were fed monocrotophos (E
isomer 78.7%) at dietary levels of 0, 0.1, 0.25, 0.5, 2.0 or 8.0 ppm
for 8 weeks. Ten rats/sex/dose level (20 controls) were sacrificed at
the end of the 8 week treatment. Ten rats/sex/dose level (20
controls) were fed control diet for 5 additional weeks (follow-up
group) and remaining 10 rats/sex/dose level (20 controls) were
continued on the same treatment as during the first 8 weeks for 5
additional weeks; these rats were killed after 13 weeks. No clinical
symptoms or deaths due to treatment with mono-crotophos were observed.
Trivial reduction of body weight occurred at 8 ppm in both sexes. A
dose-related decrease of plasma, erythrocyte and brain cholinesterase
activities was measured at all dose levels. Biologically significant
inhibition of brain cholinesterase activity was observed at 2.0 and
8.0 ppm monocrotophos. The level of inhibition was similar in both
sexes and after 8 or 13 weeks of treatment. Almost complete recovery
of both brain and erythrocyte cholinesterase activities, from the
biological point of view, was detected 5 weeks after the end of
treatment with the test substance. No obvious signs of cumulative
toxicity occurred nor that the observed changes were irreversible
(Hend & Brown 1981).
Toxicological studies
Long-term carcinogenicity studies
Mice
Male and female CD mice (5 weeks old at the beginning of the
study) were offered monocrotophos (E isomer 78.7%) at concentrations
of 1, 2, 5 or 10 ppm in powdered diet for either 55 (12
animals/sex/dose), 78 (15 animals/sex/dose) or 104 weeks (50 animals/
sex/dose). Control animals were 24, 30 and 100 for the 55, 78 and 104
week sections, respectively. The content of trimethyl phosphate was
below 0.5% and the content of the Z-isomer of monocrotophos was
approximately 1.5%. Monocrotophos was incorporated in a nutritionally
adequate diet; as significant decay was observed at room temperature
but not at -4 °C, diets were prepared and stored at -4 °C until used.
 No food was allowed to remain in food hopper for more than 4 days.
The actual content of monocrotophos in the diets was checked several
times throughout the duration of the study and it was always shown to
be within ± 10 % of nominal. The mice were observed daily for general
health and appearance, body weights and food intake were measured
weekly. Every three months groups of 10 mice/sex from the 0 and 10
ppm dose levels underwent ophthalmoscopic examination. At the
scheduled times animals were killed and blood samples were taken for
haematological examinations and cholinesterase activity
determinations. Full necropsies were performed on all animals, major
organs were weighed and tissues were examined histologically from all
animals in the 104 week section of the study and from mice fed 0, 5
and 10 ppm monocrotophos in the 55 and 78 week sections. Body weight
and food intake were not affected by treatment with monocrotophos.
Convulsions were observed in animals in all groups including controls.
The overall incidence of convulsions was increased in males from the
2 ppm dose level and in females from the 1 ppm dose level. No ocular
abnormalities related to monocrotophos feeding were detected. No
dose-related, biologically significant differences in hematological
tests were observed. Mean plasma, erythrocyte and brain
cholinesterase activities were significantly depressed in all
treatment groups at all times sampled. Inhibition was dose-related,
consistent throughout the duration of the study and no differences
between sexes were apparent. In brain after 104 weeks of treatment,
inhibition was approximately 20%, 30%, 50% and 65% at 1, 2, 5 and 10
ppm, respectively. At the end of the study the mortality rate was
60%, 56%, 60%, 62%, 62% in males and 51%, 50%, 58%, 46% and 54% in
females at 0, 1, 2, 5, 10 ppm, respectively. Pathology did not show
specific lesions attributable to treatment. Increased incidence of
pulmonary neoplasms was found in males after 18 months of treatment at
5 and 10 ppm but this observation was not confirmed at the end of the
study. There was no evidence of a treatment-related oncogenic effect
of monocrotophos up to 10 ppm, the NOAEL for cholinesterase inhibition
is below 1 ppm (Robinson & Brown 1982).
Rats
Male and female Wistar-derived rats (5 weeks old study) were
offered monocrotophos (E isomer 78.7%) at concentrations of 0.01,
0.03, 0.1, 1.0 or 10 ppm in powdered diet for either 6 months (8
animals/sex/dose), 12 months (8 animals/sex/dose), 18 months (19
animals/sex/dose) and 24 months (50 animals/sex/dose). Control rats
were 16, 16, 38 and 100 for the 6, 12, 18 and 24 month sections,
respectively. The preparation, storage and presentation of the diets
were the same as reported in the previous long-term study in mice.
The actual content of monocrotophos in the diets was checked several
times throughout the study and was always shown to be within ± 10 % of
nominal. The rats were observed twice daily (daily on week-ends) for
general health and appearance throughout the duration of the study.
Body weights and food intake were measured weekly. Every three months
groups of 10 rats/sex from the 0 and 10 ppm groups underwent
ophthalmoscopic examination. At 6, 12, 18 and 24 months blood samples
were taken and urine collected for haematological and clinical
chemistry examinations, cholinesterase activity determinations and
urinalysis. Full necropsies were performed on all animals, major
organs were weighed and tissues was examined histologically. Body
weight was significantly reduced between 5% and 10% in males fed 10
ppm monocrotophos. This difference was more apparent during the first
year of the study and corresponds to lower food intake. Body weight
and food intake were not affected by treatment with monocrotophos in
males up to 1 ppm nor in females at any dose levels. General health
and behaviour were not affected by monocrotophos. No ocular
abnormalities related to monocrotophos feeding were detected. Mean
plasma, erythrocyte and brain cholinesterase activities were
significantly depressed in the 1 and 10 ppm dose groups. Inhibition
was dose related (approximately 30-40% and 70-80% at 1 and 10 ppm,
respectively), consistent throughout the duration of the study and not
differences between sexes were detectable. The results of
haematology, clinical chemistry and urinalysis yielded no consistent
dose- nor time-related findings throughout the course of the study.
At the end of the study, the mortality rate was 24%, 20%, 28%, 30%,
20%, 32% in males and 49%, 60%, 52%, 56%, 44% and 64% in females at 0,
0.01, 0.03, 0.1, 1.0 and 10 ppm, respectively. Organ weight
variations among groups were of no toxicological relevance. Pathology
showed that the incidences of patchy alopecia and ulcerative
dermatitis of the tail were slightly higher in the 10 ppm groups of
both sexes. Pituitary neoplasm incidence was increased at the end of
the study in female but not in male rats fed 10 ppm monocrotophos.
Because of the high incidence of this type of neoplasm (88% of females
fed control diet for up to two years versus 96% of females fed 10 ppm
monocrotophos), this observation was of no biological relevance. The
NOAEL for cholinesterase inhibition was 0.1 ppm and there were no
specific macro- or microscopic lesions and there was no evidence of a
treatment-related oncogenic effect up to 10 ppm (Robinson et al.,
1983).
Reproduction studies
Rats
In a 1-litter 2-generation reproduction study, groups of 13 male
and 26 female Wistar rats approximately 5 weeks old received
monocrotophos (E isomer 78.7%) admixed in the diet at 0, 0.1, 1, 3 or
10 ppm. The preparation, storage and presentation of the diets was the
same as previously reported in long-term studies in mice and rats.
The actual content of monocrotophos in the diets was checked several
times throughout the duration of the study and was always shown to be
within ± 10 % of nominal. Rats were maintained on their respective
diets for at least 15 weeks, then each male rat was allocated to two
females of the same treatment group. The F1 generation was culled
to the same number of rats as in F0 and animals were exposed to the
appropriate test diet for 18 weeks before being bred to produce F2
generation. Body weights were recorded monthly for adult rats in the
pre-mating phase and at 1, 4, 7, 14 and 21 days of age for F1 and
F2 pups. Mating performance, fertility, litter size and viability
were recorded. Pathology was performed on F0 and F1 adults, on
selected F1 and F2 pups. Sperm head counts were made on the testes
of F0 and F1 adult rats. Significantly lower body weights (6-9%
reduction) were recorded in male but not in female rats fed 10 ppm
monocrotophos for both F0 and F1 generations. No treatment-related
toxic effects were observed in the F0 and F1 adults during the
pre-mating phase. Faecal pellets produced by F0 and F1 rats fed 10
ppm monocrotophos were smaller and darker than dose produced by
controls. There were no monocrotophos-related effects on sperm head
counts of the F0 or F1 parent rats. Mating performance, fertility
index and gestation index were not different among F0 groups. At 10
ppm the F1 male mating index was lower and fewer litters were
produced compared to controls. The gestation lengths of F0 and F1
females at 10 ppm dose level were significantly greater than those of
controls. Mean litter size, viability index and lactation index were
significantly reduced at 10 ppm for both F1 and F2 generations.
Viability index in the F2 generation was reduced also at 3 ppm. Mean
pup weights were lower at 10 ppm for both F1 and F2 generations and
at 3 ppm for the F2 generation. There were three total litter losses
at 10 ppm of the F1 and F2 generations and one litter loss of the
F2 generation at 3 ppm, probably due to lactation deficiency as
suggested by poor mammary development observed in dams. Higher kidney
and liver weights were observed for F2 female weanlings at 3 and 10
ppm. These finding were not related to any histopathological
abnormalities. No pathological changes of any tissues (excluding poor
mammary development mentioned above) could be related to monocrotophos
exposure. One ppm was the NOAEL in this reproduction study (Dix &
Thorpe, 1981).
Special studies on delayed neurotoxicity
Hens
Fourteen adult Warren Studdler laying hens were treated orally
with monocrotophos 60% w/v in acetone on two separate occasions 3
weeks apart. Monocrotophos was administered in gelatin capsules to
supply 6.7 mg/kg of monocrotophos (equivalent to LD50 in domestic
fowl). Hens were protected against cholinergic toxicity with atropine
sulphate (17.4 mg/kg i.m.) and pralidoxime chloride (50 mg/kg i.m.).
Positive controls received tri-O-tolyl phosphate (0.5 ml/kg
undiluted, p.o.), negative controls did not received any treatment.
Nine out of 14 birds died of acute cholinergic symptoms within 4 days
of the first or second monocrotophos dose; the five animals which
survived both monocrotophos doses did not develop clinical nor
histopathological signs of delayed neurotoxicity. Positive controls
gave the expected positive clinical and histopathological responses
(Owen et al., 1978).
Groups of 10 adult hens (COFAL/Marek) were dosed by gavage with
technical monocrotophos (containing 77.4% of the active E-isomer of
monocrot-ophos, 0.43% trimethyl phosphate) at concentrations of 0,
0.03, 0.1, 0.3 mg/kg for 96 days. A positive control group received
tri-orthocresol phosphate (TOCP) at 7.5 mg/kg p.o. Because of lack of
neurotoxic symptoms both the highest monocrotophos dose and TOCP dose
were increased on day 79 to 0.5 mg/kg and 10 mg/kg, respectively.
Monocrotophos was dissolved in acetone and pipetted into gelatin
capsules half-filled with feed; the acetone was allowed to evaporate
at room temperature. Stability of the test compound under these
conditions was confirmed by analytical tests. Plasma cholinesterase
activity was reduced in all groups, including the TOCP group. This
reduction was dose-related and at the highest dose level ranged
between 11.8% after the first dose and 46.9% after the final dose. No
changes in erythrocyte cholinesterase activity and no clinical signs
of the cholinergic type were observed. Body weights and egg-laying
performance were not consistently affected throughout the duration of
the study. Some of the hens dosed with TOCP showed clinical (3 out of
10 birds) and histopathological (7 out of 10 birds) signs of delayed
neuropathy after raising the TOCP dose to 10 mg/kg. None of the
monocrotophos-treated hens developed delayed neuropathy.
Mono-crotophos is devoid of neurotoxic potential under the conditions
of the study. However, the short-term study was performed at doses
too low at which neither acute cholinergic nor delayed neurotoxic
effects could have been detectable (Becci & Parent, 1981).
Special studies on embryotoxicity and teratogenicity
Rats
Twenty-six female Charles River Crl:CD (SD) BR albino rats were
treated with technical monocrotophos at concentrations of 0, 0.3, 1.0,
or 2.0 mg/kg/day orally by gavage (5 ml/kg, dissolved in distilled
water) on days 6 to 15 of gestation. The test chemical was supplied
by Shell Chemical Company which also performed characterization,
stability and chemical analysis of dosage formulations. The
concentrations of all samples before and after dosing were within ±
10% of the nominal concentrations. A single female rat dosed with 2.0
mg/kg died on gestation day 15, it had a diffuse, red-black crusted
exudate around both eyes and a thinned glandular stomach which lacked
mucosal convolutions. The remaining females survived up to the final
sacrifice. Mean body weights (from gestation day 12) and carcass
weights were significantly reduced in animals dosed at 1.0 and 2.0
mg/kg bw. Carcass weights were also reduced at 0.3 mg/kg. No
significant differences were noted for the gravid uterus weights.
Muscle tremors and/or twitchings, listlessness, salivation, urine
soaked fur and crusty eyes were observed in females dosed with 2.0
mg/kg, mainly within 4 hours of administration of the test substance.
Pathological examination at final sacrifice did not show abnormalities
which could be related to monocrotophos administration. There were no
statistically significant differences noted in the mean numbers of
corpora lutea, implantation sites, resorption sites, nor viable
fetuses in treated animals. Reproductive percentages calculated for
the treated dams were comparable to those of the control group. Mean
body weight and crown-rump length data obtained for the 2.0 mg/kg
fetuses were significantly lower than those of the control fetuses.
Mean percent of runt fetuses per litter was increased in 1.0 and 2.0
mg/kg groups. The percentage of fetuses with non-ossified
sternebra(e) was increased in litters of the 2.0 mg/kg group. The
incidence (58.4%) was approximately doubled in comparison with control
group and with the other groups at lower mono-crotophos dose levels
but within the range of previous historical data (4.7% to 82.1%).
Malformed and/or misshapen brain (malformed brain with subdural
haemorrhage/encephalocoele) was observed in 1, 3, 2 and 2 fetuses at
0, 0.3, 1, 2 mg monocrotophos/kg, respectively. This malformation is
uncommon in Sprague-Dawley rats; it appeared to be a progression of
the same type of lesion which differed only in the degree of severity.
These brain malformations may suggest a teratogenic effect of
monocrotophos in rats (Borders et al., 1983).
Table 1. Results of genotoxicity assays on monocrotophos
Test system Test object Concentration of Purity Results Reference
test substance
In vitro
Reversion assay (1) S. typhimurium 0-10 000 µg/plate Not given Positive with Moriya et al. (1983)
TA98, TA100, dissolved in DMSO TA100 and
TA1535, TA1537, WP2hcr (2)
TA1538
E. coli WP2hcr
Reversion assay (1) S. typhimurium 10-8000 µg/0.1 ml 78.4% Slight positive Hool & Arni (1986)
TA98, TA100, dissolved in acetone with TA100
TA102, TA1535, (3)
TA1538
L5178Y Tk +/- Mouse lymphoma 50-1200 µg/ml 58.4% Positive Jotz et al. (1980)
mutation assay (1) cells dissolved in DMSO (4)
Sister chromatid Human lymphoid 0-2 µg/ml in absolute Not given Positive Sobti et al. (1982)
exchange assay cells (LAZ-007) ethyl alcohol (5)
Sister chromatid Human lymphocytes 0.1-0.8 µg/8 ml 36% Positive Rupa et al. (1988)
exchange assay culture x 24, 48, (6)
72 hr dissolved
in DMSO
Sister chromatid Chinese hamster CHO: 25-400 µg/ml 78% Positive Wang et al. (1987)
exchange assay (1) ovary cells, RTE: 12.5-100 µg/ml (7)
Rat tracheal dissolved in DMSO
epithelial cells
Table 1 (contd).
Test system Test object Concentration of Purity Results Reference
test substance
Reverse mutation Saccharomyces 0.1-3% dissolved in 58.4% Positive Mortelmans et al. (1980)
mitotic recombination cerevisiae D7 DMSO (8)
gene conversion (1)
Chromosome Human lymphocytes 0.01 µg/ml x 24, 48, 36% Positive Rupa et al. (1988)
aberrations 72 hr dissolved in (6)
DMSO
Chromosome Human lymphocytes 10-4 - 10-9 M x 50 h 69% Positive Vaidya et al. (1982)
aberrations in distilled water (9)
Mitotic Saccharomyces 5% 55% Positive Simmon et al. (1977)
recombination (1) cerevisiae D3 (10)
DNA-repair Human fibroblast 0.0001-10 mM 55% Positive Simmon et al. (1977)
(UDS) (1) cells (WI-38) (11)
In vivo
Chromosome study Chinese hamster 1.4, 2.8, 5.6 mg/kg 78.4% Negative Strasser & Arni (1986)
(bone marrow) in distilled water (11)
administered orally
twice 24 h apart
Chromosome study Swiss mouse 1, 1.5, 2 mg/kg 69% Positive Vaidya et al. (1982)
(bone marrow) administered i.p. (8)
twice 24 h apart
in distilled water
Table 1 (contd).
Test system Test object Concentration of Purity Results Reference
test substance
Nucleus anomaly test Chinese hamster 1.4, 2.8, 5.6 mg/kg 78.4% Negative Strasser et al. (1986)
(bone marrow) in distilled water (12)
administered orally
twice 24 h apart
Micronucleus test Swiss mouse 2, 4, 8 mg/kg i.p. Not given Negative Kirkhart et al. (1980)
(bone marrow) administered twice (13)
24 h apart
Micronucleus test Swiss mouse 1, 1.5, 2 mg/kg i.p. 69% Positive Vaidya et al. (1982)
(bone marrow) administered twice (8)
24 h apart
Chromosome study Rat 0.5, 1, 2 mg/kg in Not given Equivocal Adhikari & Grover (1988)
(bone marrow) DMSO administered (15)
i.p. twice 24 h
apart
Dominant lethal Mouse 15, 30, 60 mg/kg of 55% Negative Simmon et al. (1977)
diet x 7 weeks (16)
Special studies on genotoxicity
(1) Both with and without metabolic activation
(2) Part of a study in which 228 pesticides were tested for
mutagenicity in bacterial reversion-assay systems
(3) The study was repeated three times at similar doses giving
similar results. Positive control without activation:
Daunorubicin HCl, 5-10 µg/0.1 ml; 4-nitroquinoline-N-oxide,
0.125-0.250 µg/0.1 ml; mitomycin-c, 0.5-1 µg/0.1 ml; sodium
azide, 2.5-5 µg/0.1 ml; aminoacridine hydrochloride, 50-100
µg/0.1 ml gave expected positive responses. Positive control with
activation: 2- aminoanthracene, 5-20 µg/0.1 ml; cyclophosphamide,
250 µg/0.1 ml gave the expected positive responses.
(4) Positive control without activation: ethylmethane sulfonate, 500
µg/ml gave expected positive response. Positive control with
metabolic activation: 3-methylcholantrene, 5 µg/ml gave expected
positive response. The test substance gave positive response at
200 µg/ml and above and at 840 µg/ml and above with and without
metabolic activation, respectively.
(5) Monocrotophos was tested without metabolic activation. A
dose-related statistically significant increase of sister
chromatid exchange frequency was observed.
(6) Positive control not run.
(7) Monochrotophos was positive for sister chromatid exchange
inductions in both CHO and RTE.
(8) Positive control with and without metabolic activation:
1,2,3,4-diepoxybutane, 0.013% gave expected positive response.
Monochrotophos induced mitotic crossing-over, gene conversion,
and reverse mutation, with and without metabolic activation.
(9) Positive control not run.
(10) Positive control: 1,2,3,4,-diepoxybutane, 0.1% gave expected
positive response.
(11) Positive control without metabolic activation:
4-nitroquinoline-N-oxide, 0.05 mM gave expected positive
response. Positive control with metabolic activation:
Dimethylnitrosoamine, 50 mM gave expected positive response.
(12) Positive control: cyclophosphamide, 64 mg/kg x 2 gave expected
positive response.
(13) Positive control: cyclophosphamide, 128 mg/kg x 2 gave expected
positive response.
(14) Positive control: trimethylphosphate, 1 g/kg x 2 gave expected
positive response.
(15) Positive control: ethyl methane sulfonate, 62.5-250 mg/kg x 2
gave expected positive response. Monochrotophos caused a
dose-related increase of the frequency of aberrant cells, but
the increase was significant only for the highest dose.
(16) Positive control: triethylenemelamine, 0.2 mg/kg i.p. gave
expected positive response.
COMMENTS
In a 13-week study in rats at dietary concentrations of 0, 0.1,
0.25, 0.5, 2, or 8 ppm, the NOAEL was 0.5 ppm, equivalent to 0.025
mg/kg bw/day, based on brain acetylcholinesterase inhibition at 2 ppm.
In a 2-year study in mice at dietary concentrations of 0, 1, 2,
5 or 10 ppm a NOAEL could not be established because brain
acetylcholinesterase inhibition was detected at the lowest dietary
concentration (approximately 20% inhibition). There was no evidence
of a treatment-related carcinogenic effect.
In a 2-year study in rats at dietary concentrations of 0, 0.01,
0.03, 0.1, 1.0 or 10 ppm the NOAEL was 0.1 ppm, equivalent to 0.005
mg/kg bw/day, based on brain acetylcholinesterase inhibition at the
next higher dose. Again, there was no evidence of carcinogenicity.
Monocrotophos did not cause delayed neuropathy in hens.
In a multigeneration reproduction study in rats at dietary
concentrations of 0, 0.1, 1, 3, or 10 ppm, the NOAEL was 1 ppm,
equivalent to 0.05 mg/kg bw/day, based on toxicity in pups seen in the
F2 generation at 3 ppm.
In a teratology study in rats at doses of 0, 0.3, 1 or 2 mg/kg
bw/day, the NOAEL was 0.3 mg/kg bw/day for both maternal toxicity and
teratogenicity. There was a slightly increased incidence of malformed
and/or misshapen brain at all dose levels (0.3 mg/kg bw/day included).
A dose-effect relationship for this uncommon malformation was lacking
and historical control data were not available.
Substantial variations in the purity of the test material used in
the genetic toxicology studies hampered thorough evaluation for
genotoxicity. When purity was at the level of technical grade
monocrotophos (78%) there were significant responses in tests for
mutagenicity in bacteria and sister chromatid exchange in Chinese
hamster ovary cells; in vivo studies for clastogenic activity were
negative.
The Meeting concluded that the significance of the brain
malformations observed in the teratology study in rats warranted
clarification, as did the genotoxic potential of monocrotophos.
Accordingly, the Meeting recommended that monocrotophos be reviewed
in 1994.
An ADI was allocated on the basis of the 2-year study in rats
using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: < 1 ppm in the diet, equivalent to < 0.15 mg/kg
bw/day
Rat: 0.1 ppm in the diet, equivalent to 0.005 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.00005 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
1. Genotoxicity studies, known to exist, with commercial and
purified monocrotophos.
2. Historical control data on the incidence of brain malformations
in rats at the relevant laboratory.
REFERENCES
Adhikari, N., Grover, I.S. (1988) Genotoxic effects of some systemic
pesticides: in vivo chromosomal aberrations in bone marrow cells
in rats. Environmental and Molecular Mutagenesis, 12: 235-242.
Becci, P.J., Parent, R.A. (1981) Neurotoxicity evaluation of Azodrin
insecticide: subchronic oral administration in hens. Unpublished
Report 6535-II by Food and Drug Research Laboratories, Inc. Waverly,
NY, USA. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Borders, C.K., Salamon, C.M., Mayhew, D.A. (1983) Technical Azodrin
(SD 9129) teratology study in SD CD rats. Unpublished Report 450-1248
by Toxi-Genics Inc. USA. Submitted to WHO by CIBA-GEIGY Ltd, Basle,
Switzerland.
Dix, K.M. & Thorpe, E. (1981) A reproduction study in rats fed
Azodrin. Unpublished Report 1752 by Sittingbourne Research Centre,
England. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Hend, R.W & Brown, V.K.H. (1981) A reversibility study of
cholinesterase activity in rats fed Azodrin for 8 weeks. Unpublished
Report 1720 by Sittingbourne Research Centre, Shell Toxicology
Laboratory (Tunstall), Sittingbourne, England. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Hool, G. & Arni, P. (1986) Salmonella/mammalian-microsome
mutagenicity test. C 1'414 tech. Unpublished Report 8508210 by
CIBA-GEIGY Ltd Basle, Switzerland. Submitted to WHO by CIBA-GEIGY Ltd,
Basle, Switzerland.
Kirkhart, B., Jones, D.C.L., Skinner, W.A. (1980) Micronucleus test
on monocrotophos. Unpublished Report LSU 7558-19 by SRI
International, Menlo Park, California, USA. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Jotz, M.M., Mitchell, A.D., Jones, D.C.L., Skinner, W.A. (1980) An
evaluation of mutagenic potential of monochrotophos employing the
L5178Y TK +/- mouse lymphoma assay. Unpublished Report LSU-7558 by
SRI International, Menlo Park, California, USA. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato K., Shirasu, Y.
(1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutation Research, 116: 185-216.
Mortelmans, K.E., Shepherd, G.F., Jones, D.C.L., Skinner, W.A. (1980)
In vitro detection of mitotic crossing-over, mitotic gene conversion
and reverse mutation with Saccharomyces cerevisiae D7 for seven
pesticides. Unpublished Report LSU-7558-20 by SRI International,
Menlo Park, California, USA. Submitted to WHO by CIBA-GEIGY Ltd,
Basle, Switzerland.
Owen, D.E., Butterworth, S.T.G., Brown, V.K.H., Thorpe, E. (1978)
Toxicity of organophosphorus insecticide Azodrin: investigation of the
neurotoxicity of Azodrin-5 to adult domestic hens. Unpublished Report
TLGR 0066.78 by Shell Research Limited, Sittingbourne Res. Centre,
England. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Robinson, J. & Brown, V.K.H. (1982) A two-year oncogenicity study in
mice fed Azodrin. Unpublished Report 194/82 from Shell Research
Limited. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Robinson, J., Gellatly, J.B.M. & Brown, V.K.H. (1983) A long-term
feeding study with Azodrin in rats to investigate chronic toxicity and
oncogenicity (6, 12, 18 and 24 month necropsies). Unpublished Report
194/82 from Shell Research Limited. Submitted to WHO by CIBA-GEIGY
Ltd, Basle, Switzerland.
Rupa, D.S., Lakshaman, R.P.V., Reddy, P.P., Reddi, O.S. (1988)
In vitro effect of monochrotophos on human lymphocytes. Bull.
Environ. Contam. Toxicol., 41: 737-741
Simmon, V.F., Mitchell, A.D., Jorgenson, T.A., (1977) Evaluation of
selected pesticides as chemical mutagens in vitro and in vivo
studies. Unpublished Report EPA-600/1-77-028 by SRI International,
Menlo Park, California, USA. Submitted to WHO by CIBA-GEIGY Ltd,
Basle, Switzerland.
Sobti, R.C., Krishan, A., Pfaffenberger, C.D. (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human lymphoid
cells in vitro: organo-phosphates. Mutation Research, 102: 89-102.
Strasser, F., Arni, P. (1986) Chromosome studies on somatic cells of
Chinese hamster. Unpublished Report 850808 by CIBA-GEIGY Ltd Basle,
Switzerland. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Strasser, F., Langauer, M., Arni, P. (1986) Nucleus anomaly test in
somatic interphase nuclei of Chinese hamster. Unpublished Report
850809 by CIBA-GEIGY Ltd., Basle, Switzerland. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Vaidya, V.G., Patanakar, N. (1982) Mutagenic effect of monochrotophos
- an insecticide in mammalian test systems. Indian J. Med. Res., 76,
912-917.
Wang, T.C., Lee, T.C., Lin, M.F., Lin, S.Y. (1987) Induction of
sister chromatid exchange by pesticides in primary rat tracheal
epithelial cells and Chinese hamster ovary cells. Mutation
Research, 188, 311-321.
No food was allowed to remain in food hopper for more than 4 days.
The actual content of monocrotophos in the diets was checked several
times throughout the duration of the study and it was always shown to
be within ± 10 % of nominal. The mice were observed daily for general
health and appearance, body weights and food intake were measured
weekly. Every three months groups of 10 mice/sex from the 0 and 10
ppm dose levels underwent ophthalmoscopic examination. At the
scheduled times animals were killed and blood samples were taken for
haematological examinations and cholinesterase activity
determinations. Full necropsies were performed on all animals, major
organs were weighed and tissues were examined histologically from all
animals in the 104 week section of the study and from mice fed 0, 5
and 10 ppm monocrotophos in the 55 and 78 week sections. Body weight
and food intake were not affected by treatment with monocrotophos.
Convulsions were observed in animals in all groups including controls.
The overall incidence of convulsions was increased in males from the
2 ppm dose level and in females from the 1 ppm dose level. No ocular
abnormalities related to monocrotophos feeding were detected. No
dose-related, biologically significant differences in hematological
tests were observed. Mean plasma, erythrocyte and brain
cholinesterase activities were significantly depressed in all
treatment groups at all times sampled. Inhibition was dose-related,
consistent throughout the duration of the study and no differences
between sexes were apparent. In brain after 104 weeks of treatment,
inhibition was approximately 20%, 30%, 50% and 65% at 1, 2, 5 and 10
ppm, respectively. At the end of the study the mortality rate was
60%, 56%, 60%, 62%, 62% in males and 51%, 50%, 58%, 46% and 54% in
females at 0, 1, 2, 5, 10 ppm, respectively. Pathology did not show
specific lesions attributable to treatment. Increased incidence of
pulmonary neoplasms was found in males after 18 months of treatment at
5 and 10 ppm but this observation was not confirmed at the end of the
study. There was no evidence of a treatment-related oncogenic effect
of monocrotophos up to 10 ppm, the NOAEL for cholinesterase inhibition
is below 1 ppm (Robinson & Brown 1982).
Rats
Male and female Wistar-derived rats (5 weeks old study) were
offered monocrotophos (E isomer 78.7%) at concentrations of 0.01,
0.03, 0.1, 1.0 or 10 ppm in powdered diet for either 6 months (8
animals/sex/dose), 12 months (8 animals/sex/dose), 18 months (19
animals/sex/dose) and 24 months (50 animals/sex/dose). Control rats
were 16, 16, 38 and 100 for the 6, 12, 18 and 24 month sections,
respectively. The preparation, storage and presentation of the diets
were the same as reported in the previous long-term study in mice.
The actual content of monocrotophos in the diets was checked several
times throughout the study and was always shown to be within ± 10 % of
nominal. The rats were observed twice daily (daily on week-ends) for
general health and appearance throughout the duration of the study.
Body weights and food intake were measured weekly. Every three months
groups of 10 rats/sex from the 0 and 10 ppm groups underwent
ophthalmoscopic examination. At 6, 12, 18 and 24 months blood samples
were taken and urine collected for haematological and clinical
chemistry examinations, cholinesterase activity determinations and
urinalysis. Full necropsies were performed on all animals, major
organs were weighed and tissues was examined histologically. Body
weight was significantly reduced between 5% and 10% in males fed 10
ppm monocrotophos. This difference was more apparent during the first
year of the study and corresponds to lower food intake. Body weight
and food intake were not affected by treatment with monocrotophos in
males up to 1 ppm nor in females at any dose levels. General health
and behaviour were not affected by monocrotophos. No ocular
abnormalities related to monocrotophos feeding were detected. Mean
plasma, erythrocyte and brain cholinesterase activities were
significantly depressed in the 1 and 10 ppm dose groups. Inhibition
was dose related (approximately 30-40% and 70-80% at 1 and 10 ppm,
respectively), consistent throughout the duration of the study and not
differences between sexes were detectable. The results of
haematology, clinical chemistry and urinalysis yielded no consistent
dose- nor time-related findings throughout the course of the study.
At the end of the study, the mortality rate was 24%, 20%, 28%, 30%,
20%, 32% in males and 49%, 60%, 52%, 56%, 44% and 64% in females at 0,
0.01, 0.03, 0.1, 1.0 and 10 ppm, respectively. Organ weight
variations among groups were of no toxicological relevance. Pathology
showed that the incidences of patchy alopecia and ulcerative
dermatitis of the tail were slightly higher in the 10 ppm groups of
both sexes. Pituitary neoplasm incidence was increased at the end of
the study in female but not in male rats fed 10 ppm monocrotophos.
Because of the high incidence of this type of neoplasm (88% of females
fed control diet for up to two years versus 96% of females fed 10 ppm
monocrotophos), this observation was of no biological relevance. The
NOAEL for cholinesterase inhibition was 0.1 ppm and there were no
specific macro- or microscopic lesions and there was no evidence of a
treatment-related oncogenic effect up to 10 ppm (Robinson et al.,
1983).
Reproduction studies
Rats
In a 1-litter 2-generation reproduction study, groups of 13 male
and 26 female Wistar rats approximately 5 weeks old received
monocrotophos (E isomer 78.7%) admixed in the diet at 0, 0.1, 1, 3 or
10 ppm. The preparation, storage and presentation of the diets was the
same as previously reported in long-term studies in mice and rats.
The actual content of monocrotophos in the diets was checked several
times throughout the duration of the study and was always shown to be
within ± 10 % of nominal. Rats were maintained on their respective
diets for at least 15 weeks, then each male rat was allocated to two
females of the same treatment group. The F1 generation was culled
to the same number of rats as in F0 and animals were exposed to the
appropriate test diet for 18 weeks before being bred to produce F2
generation. Body weights were recorded monthly for adult rats in the
pre-mating phase and at 1, 4, 7, 14 and 21 days of age for F1 and
F2 pups. Mating performance, fertility, litter size and viability
were recorded. Pathology was performed on F0 and F1 adults, on
selected F1 and F2 pups. Sperm head counts were made on the testes
of F0 and F1 adult rats. Significantly lower body weights (6-9%
reduction) were recorded in male but not in female rats fed 10 ppm
monocrotophos for both F0 and F1 generations. No treatment-related
toxic effects were observed in the F0 and F1 adults during the
pre-mating phase. Faecal pellets produced by F0 and F1 rats fed 10
ppm monocrotophos were smaller and darker than dose produced by
controls. There were no monocrotophos-related effects on sperm head
counts of the F0 or F1 parent rats. Mating performance, fertility
index and gestation index were not different among F0 groups. At 10
ppm the F1 male mating index was lower and fewer litters were
produced compared to controls. The gestation lengths of F0 and F1
females at 10 ppm dose level were significantly greater than those of
controls. Mean litter size, viability index and lactation index were
significantly reduced at 10 ppm for both F1 and F2 generations.
Viability index in the F2 generation was reduced also at 3 ppm. Mean
pup weights were lower at 10 ppm for both F1 and F2 generations and
at 3 ppm for the F2 generation. There were three total litter losses
at 10 ppm of the F1 and F2 generations and one litter loss of the
F2 generation at 3 ppm, probably due to lactation deficiency as
suggested by poor mammary development observed in dams. Higher kidney
and liver weights were observed for F2 female weanlings at 3 and 10
ppm. These finding were not related to any histopathological
abnormalities. No pathological changes of any tissues (excluding poor
mammary development mentioned above) could be related to monocrotophos
exposure. One ppm was the NOAEL in this reproduction study (Dix &
Thorpe, 1981).
Special studies on delayed neurotoxicity
Hens
Fourteen adult Warren Studdler laying hens were treated orally
with monocrotophos 60% w/v in acetone on two separate occasions 3
weeks apart. Monocrotophos was administered in gelatin capsules to
supply 6.7 mg/kg of monocrotophos (equivalent to LD50 in domestic
fowl). Hens were protected against cholinergic toxicity with atropine
sulphate (17.4 mg/kg i.m.) and pralidoxime chloride (50 mg/kg i.m.).
Positive controls received tri-O-tolyl phosphate (0.5 ml/kg
undiluted, p.o.), negative controls did not received any treatment.
Nine out of 14 birds died of acute cholinergic symptoms within 4 days
of the first or second monocrotophos dose; the five animals which
survived both monocrotophos doses did not develop clinical nor
histopathological signs of delayed neurotoxicity. Positive controls
gave the expected positive clinical and histopathological responses
(Owen et al., 1978).
Groups of 10 adult hens (COFAL/Marek) were dosed by gavage with
technical monocrotophos (containing 77.4% of the active E-isomer of
monocrot-ophos, 0.43% trimethyl phosphate) at concentrations of 0,
0.03, 0.1, 0.3 mg/kg for 96 days. A positive control group received
tri-orthocresol phosphate (TOCP) at 7.5 mg/kg p.o. Because of lack of
neurotoxic symptoms both the highest monocrotophos dose and TOCP dose
were increased on day 79 to 0.5 mg/kg and 10 mg/kg, respectively.
Monocrotophos was dissolved in acetone and pipetted into gelatin
capsules half-filled with feed; the acetone was allowed to evaporate
at room temperature. Stability of the test compound under these
conditions was confirmed by analytical tests. Plasma cholinesterase
activity was reduced in all groups, including the TOCP group. This
reduction was dose-related and at the highest dose level ranged
between 11.8% after the first dose and 46.9% after the final dose. No
changes in erythrocyte cholinesterase activity and no clinical signs
of the cholinergic type were observed. Body weights and egg-laying
performance were not consistently affected throughout the duration of
the study. Some of the hens dosed with TOCP showed clinical (3 out of
10 birds) and histopathological (7 out of 10 birds) signs of delayed
neuropathy after raising the TOCP dose to 10 mg/kg. None of the
monocrotophos-treated hens developed delayed neuropathy.
Mono-crotophos is devoid of neurotoxic potential under the conditions
of the study. However, the short-term study was performed at doses
too low at which neither acute cholinergic nor delayed neurotoxic
effects could have been detectable (Becci & Parent, 1981).
Special studies on embryotoxicity and teratogenicity
Rats
Twenty-six female Charles River Crl:CD (SD) BR albino rats were
treated with technical monocrotophos at concentrations of 0, 0.3, 1.0,
or 2.0 mg/kg/day orally by gavage (5 ml/kg, dissolved in distilled
water) on days 6 to 15 of gestation. The test chemical was supplied
by Shell Chemical Company which also performed characterization,
stability and chemical analysis of dosage formulations. The
concentrations of all samples before and after dosing were within ±
10% of the nominal concentrations. A single female rat dosed with 2.0
mg/kg died on gestation day 15, it had a diffuse, red-black crusted
exudate around both eyes and a thinned glandular stomach which lacked
mucosal convolutions. The remaining females survived up to the final
sacrifice. Mean body weights (from gestation day 12) and carcass
weights were significantly reduced in animals dosed at 1.0 and 2.0
mg/kg bw. Carcass weights were also reduced at 0.3 mg/kg. No
significant differences were noted for the gravid uterus weights.
Muscle tremors and/or twitchings, listlessness, salivation, urine
soaked fur and crusty eyes were observed in females dosed with 2.0
mg/kg, mainly within 4 hours of administration of the test substance.
Pathological examination at final sacrifice did not show abnormalities
which could be related to monocrotophos administration. There were no
statistically significant differences noted in the mean numbers of
corpora lutea, implantation sites, resorption sites, nor viable
fetuses in treated animals. Reproductive percentages calculated for
the treated dams were comparable to those of the control group. Mean
body weight and crown-rump length data obtained for the 2.0 mg/kg
fetuses were significantly lower than those of the control fetuses.
Mean percent of runt fetuses per litter was increased in 1.0 and 2.0
mg/kg groups. The percentage of fetuses with non-ossified
sternebra(e) was increased in litters of the 2.0 mg/kg group. The
incidence (58.4%) was approximately doubled in comparison with control
group and with the other groups at lower mono-crotophos dose levels
but within the range of previous historical data (4.7% to 82.1%).
Malformed and/or misshapen brain (malformed brain with subdural
haemorrhage/encephalocoele) was observed in 1, 3, 2 and 2 fetuses at
0, 0.3, 1, 2 mg monocrotophos/kg, respectively. This malformation is
uncommon in Sprague-Dawley rats; it appeared to be a progression of
the same type of lesion which differed only in the degree of severity.
These brain malformations may suggest a teratogenic effect of
monocrotophos in rats (Borders et al., 1983).
Table 1. Results of genotoxicity assays on monocrotophos
Test system Test object Concentration of Purity Results Reference
test substance
In vitro
Reversion assay (1) S. typhimurium 0-10 000 µg/plate Not given Positive with Moriya et al. (1983)
TA98, TA100, dissolved in DMSO TA100 and
TA1535, TA1537, WP2hcr (2)
TA1538
E. coli WP2hcr
Reversion assay (1) S. typhimurium 10-8000 µg/0.1 ml 78.4% Slight positive Hool & Arni (1986)
TA98, TA100, dissolved in acetone with TA100
TA102, TA1535, (3)
TA1538
L5178Y Tk +/- Mouse lymphoma 50-1200 µg/ml 58.4% Positive Jotz et al. (1980)
mutation assay (1) cells dissolved in DMSO (4)
Sister chromatid Human lymphoid 0-2 µg/ml in absolute Not given Positive Sobti et al. (1982)
exchange assay cells (LAZ-007) ethyl alcohol (5)
Sister chromatid Human lymphocytes 0.1-0.8 µg/8 ml 36% Positive Rupa et al. (1988)
exchange assay culture x 24, 48, (6)
72 hr dissolved
in DMSO
Sister chromatid Chinese hamster CHO: 25-400 µg/ml 78% Positive Wang et al. (1987)
exchange assay (1) ovary cells, RTE: 12.5-100 µg/ml (7)
Rat tracheal dissolved in DMSO
epithelial cells
Table 1 (contd).
Test system Test object Concentration of Purity Results Reference
test substance
Reverse mutation Saccharomyces 0.1-3% dissolved in 58.4% Positive Mortelmans et al. (1980)
mitotic recombination cerevisiae D7 DMSO (8)
gene conversion (1)
Chromosome Human lymphocytes 0.01 µg/ml x 24, 48, 36% Positive Rupa et al. (1988)
aberrations 72 hr dissolved in (6)
DMSO
Chromosome Human lymphocytes 10-4 - 10-9 M x 50 h 69% Positive Vaidya et al. (1982)
aberrations in distilled water (9)
Mitotic Saccharomyces 5% 55% Positive Simmon et al. (1977)
recombination (1) cerevisiae D3 (10)
DNA-repair Human fibroblast 0.0001-10 mM 55% Positive Simmon et al. (1977)
(UDS) (1) cells (WI-38) (11)
In vivo
Chromosome study Chinese hamster 1.4, 2.8, 5.6 mg/kg 78.4% Negative Strasser & Arni (1986)
(bone marrow) in distilled water (11)
administered orally
twice 24 h apart
Chromosome study Swiss mouse 1, 1.5, 2 mg/kg 69% Positive Vaidya et al. (1982)
(bone marrow) administered i.p. (8)
twice 24 h apart
in distilled water
Table 1 (contd).
Test system Test object Concentration of Purity Results Reference
test substance
Nucleus anomaly test Chinese hamster 1.4, 2.8, 5.6 mg/kg 78.4% Negative Strasser et al. (1986)
(bone marrow) in distilled water (12)
administered orally
twice 24 h apart
Micronucleus test Swiss mouse 2, 4, 8 mg/kg i.p. Not given Negative Kirkhart et al. (1980)
(bone marrow) administered twice (13)
24 h apart
Micronucleus test Swiss mouse 1, 1.5, 2 mg/kg i.p. 69% Positive Vaidya et al. (1982)
(bone marrow) administered twice (8)
24 h apart
Chromosome study Rat 0.5, 1, 2 mg/kg in Not given Equivocal Adhikari & Grover (1988)
(bone marrow) DMSO administered (15)
i.p. twice 24 h
apart
Dominant lethal Mouse 15, 30, 60 mg/kg of 55% Negative Simmon et al. (1977)
diet x 7 weeks (16)
Special studies on genotoxicity
(1) Both with and without metabolic activation
(2) Part of a study in which 228 pesticides were tested for
mutagenicity in bacterial reversion-assay systems
(3) The study was repeated three times at similar doses giving
similar results. Positive control without activation:
Daunorubicin HCl, 5-10 µg/0.1 ml; 4-nitroquinoline-N-oxide,
0.125-0.250 µg/0.1 ml; mitomycin-c, 0.5-1 µg/0.1 ml; sodium
azide, 2.5-5 µg/0.1 ml; aminoacridine hydrochloride, 50-100
µg/0.1 ml gave expected positive responses. Positive control with
activation: 2- aminoanthracene, 5-20 µg/0.1 ml; cyclophosphamide,
250 µg/0.1 ml gave the expected positive responses.
(4) Positive control without activation: ethylmethane sulfonate, 500
µg/ml gave expected positive response. Positive control with
metabolic activation: 3-methylcholantrene, 5 µg/ml gave expected
positive response. The test substance gave positive response at
200 µg/ml and above and at 840 µg/ml and above with and without
metabolic activation, respectively.
(5) Monocrotophos was tested without metabolic activation. A
dose-related statistically significant increase of sister
chromatid exchange frequency was observed.
(6) Positive control not run.
(7) Monochrotophos was positive for sister chromatid exchange
inductions in both CHO and RTE.
(8) Positive control with and without metabolic activation:
1,2,3,4-diepoxybutane, 0.013% gave expected positive response.
Monochrotophos induced mitotic crossing-over, gene conversion,
and reverse mutation, with and without metabolic activation.
(9) Positive control not run.
(10) Positive control: 1,2,3,4,-diepoxybutane, 0.1% gave expected
positive response.
(11) Positive control without metabolic activation:
4-nitroquinoline-N-oxide, 0.05 mM gave expected positive
response. Positive control with metabolic activation:
Dimethylnitrosoamine, 50 mM gave expected positive response.
(12) Positive control: cyclophosphamide, 64 mg/kg x 2 gave expected
positive response.
(13) Positive control: cyclophosphamide, 128 mg/kg x 2 gave expected
positive response.
(14) Positive control: trimethylphosphate, 1 g/kg x 2 gave expected
positive response.
(15) Positive control: ethyl methane sulfonate, 62.5-250 mg/kg x 2
gave expected positive response. Monochrotophos caused a
dose-related increase of the frequency of aberrant cells, but
the increase was significant only for the highest dose.
(16) Positive control: triethylenemelamine, 0.2 mg/kg i.p. gave
expected positive response.
COMMENTS
In a 13-week study in rats at dietary concentrations of 0, 0.1,
0.25, 0.5, 2, or 8 ppm, the NOAEL was 0.5 ppm, equivalent to 0.025
mg/kg bw/day, based on brain acetylcholinesterase inhibition at 2 ppm.
In a 2-year study in mice at dietary concentrations of 0, 1, 2,
5 or 10 ppm a NOAEL could not be established because brain
acetylcholinesterase inhibition was detected at the lowest dietary
concentration (approximately 20% inhibition). There was no evidence
of a treatment-related carcinogenic effect.
In a 2-year study in rats at dietary concentrations of 0, 0.01,
0.03, 0.1, 1.0 or 10 ppm the NOAEL was 0.1 ppm, equivalent to 0.005
mg/kg bw/day, based on brain acetylcholinesterase inhibition at the
next higher dose. Again, there was no evidence of carcinogenicity.
Monocrotophos did not cause delayed neuropathy in hens.
In a multigeneration reproduction study in rats at dietary
concentrations of 0, 0.1, 1, 3, or 10 ppm, the NOAEL was 1 ppm,
equivalent to 0.05 mg/kg bw/day, based on toxicity in pups seen in the
F2 generation at 3 ppm.
In a teratology study in rats at doses of 0, 0.3, 1 or 2 mg/kg
bw/day, the NOAEL was 0.3 mg/kg bw/day for both maternal toxicity and
teratogenicity. There was a slightly increased incidence of malformed
and/or misshapen brain at all dose levels (0.3 mg/kg bw/day included).
A dose-effect relationship for this uncommon malformation was lacking
and historical control data were not available.
Substantial variations in the purity of the test material used in
the genetic toxicology studies hampered thorough evaluation for
genotoxicity. When purity was at the level of technical grade
monocrotophos (78%) there were significant responses in tests for
mutagenicity in bacteria and sister chromatid exchange in Chinese
hamster ovary cells; in vivo studies for clastogenic activity were
negative.
The Meeting concluded that the significance of the brain
malformations observed in the teratology study in rats warranted
clarification, as did the genotoxic potential of monocrotophos.
Accordingly, the Meeting recommended that monocrotophos be reviewed
in 1994.
An ADI was allocated on the basis of the 2-year study in rats
using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: < 1 ppm in the diet, equivalent to < 0.15 mg/kg
bw/day
Rat: 0.1 ppm in the diet, equivalent to 0.005 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.00005 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
1. Genotoxicity studies, known to exist, with commercial and
purified monocrotophos.
2. Historical control data on the incidence of brain malformations
in rats at the relevant laboratory.
REFERENCES
Adhikari, N., Grover, I.S. (1988) Genotoxic effects of some systemic
pesticides: in vivo chromosomal aberrations in bone marrow cells
in rats. Environmental and Molecular Mutagenesis, 12: 235-242.
Becci, P.J., Parent, R.A. (1981) Neurotoxicity evaluation of Azodrin
insecticide: subchronic oral administration in hens. Unpublished
Report 6535-II by Food and Drug Research Laboratories, Inc. Waverly,
NY, USA. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Borders, C.K., Salamon, C.M., Mayhew, D.A. (1983) Technical Azodrin
(SD 9129) teratology study in SD CD rats. Unpublished Report 450-1248
by Toxi-Genics Inc. USA. Submitted to WHO by CIBA-GEIGY Ltd, Basle,
Switzerland.
Dix, K.M. & Thorpe, E. (1981) A reproduction study in rats fed
Azodrin. Unpublished Report 1752 by Sittingbourne Research Centre,
England. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Hend, R.W & Brown, V.K.H. (1981) A reversibility study of
cholinesterase activity in rats fed Azodrin for 8 weeks. Unpublished
Report 1720 by Sittingbourne Research Centre, Shell Toxicology
Laboratory (Tunstall), Sittingbourne, England. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Hool, G. & Arni, P. (1986) Salmonella/mammalian-microsome
mutagenicity test. C 1'414 tech. Unpublished Report 8508210 by
CIBA-GEIGY Ltd Basle, Switzerland. Submitted to WHO by CIBA-GEIGY Ltd,
Basle, Switzerland.
Kirkhart, B., Jones, D.C.L., Skinner, W.A. (1980) Micronucleus test
on monocrotophos. Unpublished Report LSU 7558-19 by SRI
International, Menlo Park, California, USA. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Jotz, M.M., Mitchell, A.D., Jones, D.C.L., Skinner, W.A. (1980) An
evaluation of mutagenic potential of monochrotophos employing the
L5178Y TK +/- mouse lymphoma assay. Unpublished Report LSU-7558 by
SRI International, Menlo Park, California, USA. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato K., Shirasu, Y.
(1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutation Research, 116: 185-216.
Mortelmans, K.E., Shepherd, G.F., Jones, D.C.L., Skinner, W.A. (1980)
In vitro detection of mitotic crossing-over, mitotic gene conversion
and reverse mutation with Saccharomyces cerevisiae D7 for seven
pesticides. Unpublished Report LSU-7558-20 by SRI International,
Menlo Park, California, USA. Submitted to WHO by CIBA-GEIGY Ltd,
Basle, Switzerland.
Owen, D.E., Butterworth, S.T.G., Brown, V.K.H., Thorpe, E. (1978)
Toxicity of organophosphorus insecticide Azodrin: investigation of the
neurotoxicity of Azodrin-5 to adult domestic hens. Unpublished Report
TLGR 0066.78 by Shell Research Limited, Sittingbourne Res. Centre,
England. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Robinson, J. & Brown, V.K.H. (1982) A two-year oncogenicity study in
mice fed Azodrin. Unpublished Report 194/82 from Shell Research
Limited. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Robinson, J., Gellatly, J.B.M. & Brown, V.K.H. (1983) A long-term
feeding study with Azodrin in rats to investigate chronic toxicity and
oncogenicity (6, 12, 18 and 24 month necropsies). Unpublished Report
194/82 from Shell Research Limited. Submitted to WHO by CIBA-GEIGY
Ltd, Basle, Switzerland.
Rupa, D.S., Lakshaman, R.P.V., Reddy, P.P., Reddi, O.S. (1988)
In vitro effect of monochrotophos on human lymphocytes. Bull.
Environ. Contam. Toxicol., 41: 737-741
Simmon, V.F., Mitchell, A.D., Jorgenson, T.A., (1977) Evaluation of
selected pesticides as chemical mutagens in vitro and in vivo
studies. Unpublished Report EPA-600/1-77-028 by SRI International,
Menlo Park, California, USA. Submitted to WHO by CIBA-GEIGY Ltd,
Basle, Switzerland.
Sobti, R.C., Krishan, A., Pfaffenberger, C.D. (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human lymphoid
cells in vitro: organo-phosphates. Mutation Research, 102: 89-102.
Strasser, F., Arni, P. (1986) Chromosome studies on somatic cells of
Chinese hamster. Unpublished Report 850808 by CIBA-GEIGY Ltd Basle,
Switzerland. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Strasser, F., Langauer, M., Arni, P. (1986) Nucleus anomaly test in
somatic interphase nuclei of Chinese hamster. Unpublished Report
850809 by CIBA-GEIGY Ltd., Basle, Switzerland. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Vaidya, V.G., Patanakar, N. (1982) Mutagenic effect of monochrotophos
- an insecticide in mammalian test systems. Indian J. Med. Res., 76,
912-917.
Wang, T.C., Lee, T.C., Lin, M.F., Lin, S.Y. (1987) Induction of
sister chromatid exchange by pesticides in primary rat tracheal
epithelial cells and Chinese hamster ovary cells. Mutation
Research, 188, 311-321.
 No food was allowed to remain in food hopper for more than 4 days.
The actual content of monocrotophos in the diets was checked several
times throughout the duration of the study and it was always shown to
be within ± 10 % of nominal. The mice were observed daily for general
health and appearance, body weights and food intake were measured
weekly. Every three months groups of 10 mice/sex from the 0 and 10
ppm dose levels underwent ophthalmoscopic examination. At the
scheduled times animals were killed and blood samples were taken for
haematological examinations and cholinesterase activity
determinations. Full necropsies were performed on all animals, major
organs were weighed and tissues were examined histologically from all
animals in the 104 week section of the study and from mice fed 0, 5
and 10 ppm monocrotophos in the 55 and 78 week sections. Body weight
and food intake were not affected by treatment with monocrotophos.
Convulsions were observed in animals in all groups including controls.
The overall incidence of convulsions was increased in males from the
2 ppm dose level and in females from the 1 ppm dose level. No ocular
abnormalities related to monocrotophos feeding were detected. No
dose-related, biologically significant differences in hematological
tests were observed. Mean plasma, erythrocyte and brain
cholinesterase activities were significantly depressed in all
treatment groups at all times sampled. Inhibition was dose-related,
consistent throughout the duration of the study and no differences
between sexes were apparent. In brain after 104 weeks of treatment,
inhibition was approximately 20%, 30%, 50% and 65% at 1, 2, 5 and 10
ppm, respectively. At the end of the study the mortality rate was
60%, 56%, 60%, 62%, 62% in males and 51%, 50%, 58%, 46% and 54% in
females at 0, 1, 2, 5, 10 ppm, respectively. Pathology did not show
specific lesions attributable to treatment. Increased incidence of
pulmonary neoplasms was found in males after 18 months of treatment at
5 and 10 ppm but this observation was not confirmed at the end of the
study. There was no evidence of a treatment-related oncogenic effect
of monocrotophos up to 10 ppm, the NOAEL for cholinesterase inhibition
is below 1 ppm (Robinson & Brown 1982).
Rats
Male and female Wistar-derived rats (5 weeks old study) were
offered monocrotophos (E isomer 78.7%) at concentrations of 0.01,
0.03, 0.1, 1.0 or 10 ppm in powdered diet for either 6 months (8
animals/sex/dose), 12 months (8 animals/sex/dose), 18 months (19
animals/sex/dose) and 24 months (50 animals/sex/dose). Control rats
were 16, 16, 38 and 100 for the 6, 12, 18 and 24 month sections,
respectively. The preparation, storage and presentation of the diets
were the same as reported in the previous long-term study in mice.
The actual content of monocrotophos in the diets was checked several
times throughout the study and was always shown to be within ± 10 % of
nominal. The rats were observed twice daily (daily on week-ends) for
general health and appearance throughout the duration of the study.
Body weights and food intake were measured weekly. Every three months
groups of 10 rats/sex from the 0 and 10 ppm groups underwent
ophthalmoscopic examination. At 6, 12, 18 and 24 months blood samples
were taken and urine collected for haematological and clinical
chemistry examinations, cholinesterase activity determinations and
urinalysis. Full necropsies were performed on all animals, major
organs were weighed and tissues was examined histologically. Body
weight was significantly reduced between 5% and 10% in males fed 10
ppm monocrotophos. This difference was more apparent during the first
year of the study and corresponds to lower food intake. Body weight
and food intake were not affected by treatment with monocrotophos in
males up to 1 ppm nor in females at any dose levels. General health
and behaviour were not affected by monocrotophos. No ocular
abnormalities related to monocrotophos feeding were detected. Mean
plasma, erythrocyte and brain cholinesterase activities were
significantly depressed in the 1 and 10 ppm dose groups. Inhibition
was dose related (approximately 30-40% and 70-80% at 1 and 10 ppm,
respectively), consistent throughout the duration of the study and not
differences between sexes were detectable. The results of
haematology, clinical chemistry and urinalysis yielded no consistent
dose- nor time-related findings throughout the course of the study.
At the end of the study, the mortality rate was 24%, 20%, 28%, 30%,
20%, 32% in males and 49%, 60%, 52%, 56%, 44% and 64% in females at 0,
0.01, 0.03, 0.1, 1.0 and 10 ppm, respectively. Organ weight
variations among groups were of no toxicological relevance. Pathology
showed that the incidences of patchy alopecia and ulcerative
dermatitis of the tail were slightly higher in the 10 ppm groups of
both sexes. Pituitary neoplasm incidence was increased at the end of
the study in female but not in male rats fed 10 ppm monocrotophos.
Because of the high incidence of this type of neoplasm (88% of females
fed control diet for up to two years versus 96% of females fed 10 ppm
monocrotophos), this observation was of no biological relevance. The
NOAEL for cholinesterase inhibition was 0.1 ppm and there were no
specific macro- or microscopic lesions and there was no evidence of a
treatment-related oncogenic effect up to 10 ppm (Robinson et al.,
1983).
Reproduction studies
Rats
In a 1-litter 2-generation reproduction study, groups of 13 male
and 26 female Wistar rats approximately 5 weeks old received
monocrotophos (E isomer 78.7%) admixed in the diet at 0, 0.1, 1, 3 or
10 ppm. The preparation, storage and presentation of the diets was the
same as previously reported in long-term studies in mice and rats.
The actual content of monocrotophos in the diets was checked several
times throughout the duration of the study and was always shown to be
within ± 10 % of nominal. Rats were maintained on their respective
diets for at least 15 weeks, then each male rat was allocated to two
females of the same treatment group. The F1 generation was culled
to the same number of rats as in F0 and animals were exposed to the
appropriate test diet for 18 weeks before being bred to produce F2
generation. Body weights were recorded monthly for adult rats in the
pre-mating phase and at 1, 4, 7, 14 and 21 days of age for F1 and
F2 pups. Mating performance, fertility, litter size and viability
were recorded. Pathology was performed on F0 and F1 adults, on
selected F1 and F2 pups. Sperm head counts were made on the testes
of F0 and F1 adult rats. Significantly lower body weights (6-9%
reduction) were recorded in male but not in female rats fed 10 ppm
monocrotophos for both F0 and F1 generations. No treatment-related
toxic effects were observed in the F0 and F1 adults during the
pre-mating phase. Faecal pellets produced by F0 and F1 rats fed 10
ppm monocrotophos were smaller and darker than dose produced by
controls. There were no monocrotophos-related effects on sperm head
counts of the F0 or F1 parent rats. Mating performance, fertility
index and gestation index were not different among F0 groups. At 10
ppm the F1 male mating index was lower and fewer litters were
produced compared to controls. The gestation lengths of F0 and F1
females at 10 ppm dose level were significantly greater than those of
controls. Mean litter size, viability index and lactation index were
significantly reduced at 10 ppm for both F1 and F2 generations.
Viability index in the F2 generation was reduced also at 3 ppm. Mean
pup weights were lower at 10 ppm for both F1 and F2 generations and
at 3 ppm for the F2 generation. There were three total litter losses
at 10 ppm of the F1 and F2 generations and one litter loss of the
F2 generation at 3 ppm, probably due to lactation deficiency as
suggested by poor mammary development observed in dams. Higher kidney
and liver weights were observed for F2 female weanlings at 3 and 10
ppm. These finding were not related to any histopathological
abnormalities. No pathological changes of any tissues (excluding poor
mammary development mentioned above) could be related to monocrotophos
exposure. One ppm was the NOAEL in this reproduction study (Dix &
Thorpe, 1981).
Special studies on delayed neurotoxicity
Hens
Fourteen adult Warren Studdler laying hens were treated orally
with monocrotophos 60% w/v in acetone on two separate occasions 3
weeks apart. Monocrotophos was administered in gelatin capsules to
supply 6.7 mg/kg of monocrotophos (equivalent to LD50 in domestic
fowl). Hens were protected against cholinergic toxicity with atropine
sulphate (17.4 mg/kg i.m.) and pralidoxime chloride (50 mg/kg i.m.).
Positive controls received tri-O-tolyl phosphate (0.5 ml/kg
undiluted, p.o.), negative controls did not received any treatment.
Nine out of 14 birds died of acute cholinergic symptoms within 4 days
of the first or second monocrotophos dose; the five animals which
survived both monocrotophos doses did not develop clinical nor
histopathological signs of delayed neurotoxicity. Positive controls
gave the expected positive clinical and histopathological responses
(Owen et al., 1978).
Groups of 10 adult hens (COFAL/Marek) were dosed by gavage with
technical monocrotophos (containing 77.4% of the active E-isomer of
monocrot-ophos, 0.43% trimethyl phosphate) at concentrations of 0,
0.03, 0.1, 0.3 mg/kg for 96 days. A positive control group received
tri-orthocresol phosphate (TOCP) at 7.5 mg/kg p.o. Because of lack of
neurotoxic symptoms both the highest monocrotophos dose and TOCP dose
were increased on day 79 to 0.5 mg/kg and 10 mg/kg, respectively.
Monocrotophos was dissolved in acetone and pipetted into gelatin
capsules half-filled with feed; the acetone was allowed to evaporate
at room temperature. Stability of the test compound under these
conditions was confirmed by analytical tests. Plasma cholinesterase
activity was reduced in all groups, including the TOCP group. This
reduction was dose-related and at the highest dose level ranged
between 11.8% after the first dose and 46.9% after the final dose. No
changes in erythrocyte cholinesterase activity and no clinical signs
of the cholinergic type were observed. Body weights and egg-laying
performance were not consistently affected throughout the duration of
the study. Some of the hens dosed with TOCP showed clinical (3 out of
10 birds) and histopathological (7 out of 10 birds) signs of delayed
neuropathy after raising the TOCP dose to 10 mg/kg. None of the
monocrotophos-treated hens developed delayed neuropathy.
Mono-crotophos is devoid of neurotoxic potential under the conditions
of the study. However, the short-term study was performed at doses
too low at which neither acute cholinergic nor delayed neurotoxic
effects could have been detectable (Becci & Parent, 1981).
Special studies on embryotoxicity and teratogenicity
Rats
Twenty-six female Charles River Crl:CD (SD) BR albino rats were
treated with technical monocrotophos at concentrations of 0, 0.3, 1.0,
or 2.0 mg/kg/day orally by gavage (5 ml/kg, dissolved in distilled
water) on days 6 to 15 of gestation. The test chemical was supplied
by Shell Chemical Company which also performed characterization,
stability and chemical analysis of dosage formulations. The
concentrations of all samples before and after dosing were within ±
10% of the nominal concentrations. A single female rat dosed with 2.0
mg/kg died on gestation day 15, it had a diffuse, red-black crusted
exudate around both eyes and a thinned glandular stomach which lacked
mucosal convolutions. The remaining females survived up to the final
sacrifice. Mean body weights (from gestation day 12) and carcass
weights were significantly reduced in animals dosed at 1.0 and 2.0
mg/kg bw. Carcass weights were also reduced at 0.3 mg/kg. No
significant differences were noted for the gravid uterus weights.
Muscle tremors and/or twitchings, listlessness, salivation, urine
soaked fur and crusty eyes were observed in females dosed with 2.0
mg/kg, mainly within 4 hours of administration of the test substance.
Pathological examination at final sacrifice did not show abnormalities
which could be related to monocrotophos administration. There were no
statistically significant differences noted in the mean numbers of
corpora lutea, implantation sites, resorption sites, nor viable
fetuses in treated animals. Reproductive percentages calculated for
the treated dams were comparable to those of the control group. Mean
body weight and crown-rump length data obtained for the 2.0 mg/kg
fetuses were significantly lower than those of the control fetuses.
Mean percent of runt fetuses per litter was increased in 1.0 and 2.0
mg/kg groups. The percentage of fetuses with non-ossified
sternebra(e) was increased in litters of the 2.0 mg/kg group. The
incidence (58.4%) was approximately doubled in comparison with control
group and with the other groups at lower mono-crotophos dose levels
but within the range of previous historical data (4.7% to 82.1%).
Malformed and/or misshapen brain (malformed brain with subdural
haemorrhage/encephalocoele) was observed in 1, 3, 2 and 2 fetuses at
0, 0.3, 1, 2 mg monocrotophos/kg, respectively. This malformation is
uncommon in Sprague-Dawley rats; it appeared to be a progression of
the same type of lesion which differed only in the degree of severity.
These brain malformations may suggest a teratogenic effect of
monocrotophos in rats (Borders et al., 1983).
Table 1. Results of genotoxicity assays on monocrotophos
Test system Test object Concentration of Purity Results Reference
test substance
In vitro
Reversion assay (1) S. typhimurium 0-10 000 µg/plate Not given Positive with Moriya et al. (1983)
TA98, TA100, dissolved in DMSO TA100 and
TA1535, TA1537, WP2hcr (2)
TA1538
E. coli WP2hcr
Reversion assay (1) S. typhimurium 10-8000 µg/0.1 ml 78.4% Slight positive Hool & Arni (1986)
TA98, TA100, dissolved in acetone with TA100
TA102, TA1535, (3)
TA1538
L5178Y Tk +/- Mouse lymphoma 50-1200 µg/ml 58.4% Positive Jotz et al. (1980)
mutation assay (1) cells dissolved in DMSO (4)
Sister chromatid Human lymphoid 0-2 µg/ml in absolute Not given Positive Sobti et al. (1982)
exchange assay cells (LAZ-007) ethyl alcohol (5)
Sister chromatid Human lymphocytes 0.1-0.8 µg/8 ml 36% Positive Rupa et al. (1988)
exchange assay culture x 24, 48, (6)
72 hr dissolved
in DMSO
Sister chromatid Chinese hamster CHO: 25-400 µg/ml 78% Positive Wang et al. (1987)
exchange assay (1) ovary cells, RTE: 12.5-100 µg/ml (7)
Rat tracheal dissolved in DMSO
epithelial cells
Table 1 (contd).
Test system Test object Concentration of Purity Results Reference
test substance
Reverse mutation Saccharomyces 0.1-3% dissolved in 58.4% Positive Mortelmans et al. (1980)
mitotic recombination cerevisiae D7 DMSO (8)
gene conversion (1)
Chromosome Human lymphocytes 0.01 µg/ml x 24, 48, 36% Positive Rupa et al. (1988)
aberrations 72 hr dissolved in (6)
DMSO
Chromosome Human lymphocytes 10-4 - 10-9 M x 50 h 69% Positive Vaidya et al. (1982)
aberrations in distilled water (9)
Mitotic Saccharomyces 5% 55% Positive Simmon et al. (1977)
recombination (1) cerevisiae D3 (10)
DNA-repair Human fibroblast 0.0001-10 mM 55% Positive Simmon et al. (1977)
(UDS) (1) cells (WI-38) (11)
In vivo
Chromosome study Chinese hamster 1.4, 2.8, 5.6 mg/kg 78.4% Negative Strasser & Arni (1986)
(bone marrow) in distilled water (11)
administered orally
twice 24 h apart
Chromosome study Swiss mouse 1, 1.5, 2 mg/kg 69% Positive Vaidya et al. (1982)
(bone marrow) administered i.p. (8)
twice 24 h apart
in distilled water
Table 1 (contd).
Test system Test object Concentration of Purity Results Reference
test substance
Nucleus anomaly test Chinese hamster 1.4, 2.8, 5.6 mg/kg 78.4% Negative Strasser et al. (1986)
(bone marrow) in distilled water (12)
administered orally
twice 24 h apart
Micronucleus test Swiss mouse 2, 4, 8 mg/kg i.p. Not given Negative Kirkhart et al. (1980)
(bone marrow) administered twice (13)
24 h apart
Micronucleus test Swiss mouse 1, 1.5, 2 mg/kg i.p. 69% Positive Vaidya et al. (1982)
(bone marrow) administered twice (8)
24 h apart
Chromosome study Rat 0.5, 1, 2 mg/kg in Not given Equivocal Adhikari & Grover (1988)
(bone marrow) DMSO administered (15)
i.p. twice 24 h
apart
Dominant lethal Mouse 15, 30, 60 mg/kg of 55% Negative Simmon et al. (1977)
diet x 7 weeks (16)
Special studies on genotoxicity
(1) Both with and without metabolic activation
(2) Part of a study in which 228 pesticides were tested for
mutagenicity in bacterial reversion-assay systems
(3) The study was repeated three times at similar doses giving
similar results. Positive control without activation:
Daunorubicin HCl, 5-10 µg/0.1 ml; 4-nitroquinoline-N-oxide,
0.125-0.250 µg/0.1 ml; mitomycin-c, 0.5-1 µg/0.1 ml; sodium
azide, 2.5-5 µg/0.1 ml; aminoacridine hydrochloride, 50-100
µg/0.1 ml gave expected positive responses. Positive control with
activation: 2- aminoanthracene, 5-20 µg/0.1 ml; cyclophosphamide,
250 µg/0.1 ml gave the expected positive responses.
(4) Positive control without activation: ethylmethane sulfonate, 500
µg/ml gave expected positive response. Positive control with
metabolic activation: 3-methylcholantrene, 5 µg/ml gave expected
positive response. The test substance gave positive response at
200 µg/ml and above and at 840 µg/ml and above with and without
metabolic activation, respectively.
(5) Monocrotophos was tested without metabolic activation. A
dose-related statistically significant increase of sister
chromatid exchange frequency was observed.
(6) Positive control not run.
(7) Monochrotophos was positive for sister chromatid exchange
inductions in both CHO and RTE.
(8) Positive control with and without metabolic activation:
1,2,3,4-diepoxybutane, 0.013% gave expected positive response.
Monochrotophos induced mitotic crossing-over, gene conversion,
and reverse mutation, with and without metabolic activation.
(9) Positive control not run.
(10) Positive control: 1,2,3,4,-diepoxybutane, 0.1% gave expected
positive response.
(11) Positive control without metabolic activation:
4-nitroquinoline-N-oxide, 0.05 mM gave expected positive
response. Positive control with metabolic activation:
Dimethylnitrosoamine, 50 mM gave expected positive response.
(12) Positive control: cyclophosphamide, 64 mg/kg x 2 gave expected
positive response.
(13) Positive control: cyclophosphamide, 128 mg/kg x 2 gave expected
positive response.
(14) Positive control: trimethylphosphate, 1 g/kg x 2 gave expected
positive response.
(15) Positive control: ethyl methane sulfonate, 62.5-250 mg/kg x 2
gave expected positive response. Monochrotophos caused a
dose-related increase of the frequency of aberrant cells, but
the increase was significant only for the highest dose.
(16) Positive control: triethylenemelamine, 0.2 mg/kg i.p. gave
expected positive response.
COMMENTS
In a 13-week study in rats at dietary concentrations of 0, 0.1,
0.25, 0.5, 2, or 8 ppm, the NOAEL was 0.5 ppm, equivalent to 0.025
mg/kg bw/day, based on brain acetylcholinesterase inhibition at 2 ppm.
In a 2-year study in mice at dietary concentrations of 0, 1, 2,
5 or 10 ppm a NOAEL could not be established because brain
acetylcholinesterase inhibition was detected at the lowest dietary
concentration (approximately 20% inhibition). There was no evidence
of a treatment-related carcinogenic effect.
In a 2-year study in rats at dietary concentrations of 0, 0.01,
0.03, 0.1, 1.0 or 10 ppm the NOAEL was 0.1 ppm, equivalent to 0.005
mg/kg bw/day, based on brain acetylcholinesterase inhibition at the
next higher dose. Again, there was no evidence of carcinogenicity.
Monocrotophos did not cause delayed neuropathy in hens.
In a multigeneration reproduction study in rats at dietary
concentrations of 0, 0.1, 1, 3, or 10 ppm, the NOAEL was 1 ppm,
equivalent to 0.05 mg/kg bw/day, based on toxicity in pups seen in the
F2 generation at 3 ppm.
In a teratology study in rats at doses of 0, 0.3, 1 or 2 mg/kg
bw/day, the NOAEL was 0.3 mg/kg bw/day for both maternal toxicity and
teratogenicity. There was a slightly increased incidence of malformed
and/or misshapen brain at all dose levels (0.3 mg/kg bw/day included).
A dose-effect relationship for this uncommon malformation was lacking
and historical control data were not available.
Substantial variations in the purity of the test material used in
the genetic toxicology studies hampered thorough evaluation for
genotoxicity. When purity was at the level of technical grade
monocrotophos (78%) there were significant responses in tests for
mutagenicity in bacteria and sister chromatid exchange in Chinese
hamster ovary cells; in vivo studies for clastogenic activity were
negative.
The Meeting concluded that the significance of the brain
malformations observed in the teratology study in rats warranted
clarification, as did the genotoxic potential of monocrotophos.
Accordingly, the Meeting recommended that monocrotophos be reviewed
in 1994.
An ADI was allocated on the basis of the 2-year study in rats
using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: < 1 ppm in the diet, equivalent to < 0.15 mg/kg
bw/day
Rat: 0.1 ppm in the diet, equivalent to 0.005 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.00005 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
1. Genotoxicity studies, known to exist, with commercial and
purified monocrotophos.
2. Historical control data on the incidence of brain malformations
in rats at the relevant laboratory.
REFERENCES
Adhikari, N., Grover, I.S. (1988) Genotoxic effects of some systemic
pesticides: in vivo chromosomal aberrations in bone marrow cells
in rats. Environmental and Molecular Mutagenesis, 12: 235-242.
Becci, P.J., Parent, R.A. (1981) Neurotoxicity evaluation of Azodrin
insecticide: subchronic oral administration in hens. Unpublished
Report 6535-II by Food and Drug Research Laboratories, Inc. Waverly,
NY, USA. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Borders, C.K., Salamon, C.M., Mayhew, D.A. (1983) Technical Azodrin
(SD 9129) teratology study in SD CD rats. Unpublished Report 450-1248
by Toxi-Genics Inc. USA. Submitted to WHO by CIBA-GEIGY Ltd, Basle,
Switzerland.
Dix, K.M. & Thorpe, E. (1981) A reproduction study in rats fed
Azodrin. Unpublished Report 1752 by Sittingbourne Research Centre,
England. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Hend, R.W & Brown, V.K.H. (1981) A reversibility study of
cholinesterase activity in rats fed Azodrin for 8 weeks. Unpublished
Report 1720 by Sittingbourne Research Centre, Shell Toxicology
Laboratory (Tunstall), Sittingbourne, England. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Hool, G. & Arni, P. (1986) Salmonella/mammalian-microsome
mutagenicity test. C 1'414 tech. Unpublished Report 8508210 by
CIBA-GEIGY Ltd Basle, Switzerland. Submitted to WHO by CIBA-GEIGY Ltd,
Basle, Switzerland.
Kirkhart, B., Jones, D.C.L., Skinner, W.A. (1980) Micronucleus test
on monocrotophos. Unpublished Report LSU 7558-19 by SRI
International, Menlo Park, California, USA. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Jotz, M.M., Mitchell, A.D., Jones, D.C.L., Skinner, W.A. (1980) An
evaluation of mutagenic potential of monochrotophos employing the
L5178Y TK +/- mouse lymphoma assay. Unpublished Report LSU-7558 by
SRI International, Menlo Park, California, USA. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato K., Shirasu, Y.
(1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutation Research, 116: 185-216.
Mortelmans, K.E., Shepherd, G.F., Jones, D.C.L., Skinner, W.A. (1980)
In vitro detection of mitotic crossing-over, mitotic gene conversion
and reverse mutation with Saccharomyces cerevisiae D7 for seven
pesticides. Unpublished Report LSU-7558-20 by SRI International,
Menlo Park, California, USA. Submitted to WHO by CIBA-GEIGY Ltd,
Basle, Switzerland.
Owen, D.E., Butterworth, S.T.G., Brown, V.K.H., Thorpe, E. (1978)
Toxicity of organophosphorus insecticide Azodrin: investigation of the
neurotoxicity of Azodrin-5 to adult domestic hens. Unpublished Report
TLGR 0066.78 by Shell Research Limited, Sittingbourne Res. Centre,
England. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Robinson, J. & Brown, V.K.H. (1982) A two-year oncogenicity study in
mice fed Azodrin. Unpublished Report 194/82 from Shell Research
Limited. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Robinson, J., Gellatly, J.B.M. & Brown, V.K.H. (1983) A long-term
feeding study with Azodrin in rats to investigate chronic toxicity and
oncogenicity (6, 12, 18 and 24 month necropsies). Unpublished Report
194/82 from Shell Research Limited. Submitted to WHO by CIBA-GEIGY
Ltd, Basle, Switzerland.
Rupa, D.S., Lakshaman, R.P.V., Reddy, P.P., Reddi, O.S. (1988)
In vitro effect of monochrotophos on human lymphocytes. Bull.
Environ. Contam. Toxicol., 41: 737-741
Simmon, V.F., Mitchell, A.D., Jorgenson, T.A., (1977) Evaluation of
selected pesticides as chemical mutagens in vitro and in vivo
studies. Unpublished Report EPA-600/1-77-028 by SRI International,
Menlo Park, California, USA. Submitted to WHO by CIBA-GEIGY Ltd,
Basle, Switzerland.
Sobti, R.C., Krishan, A., Pfaffenberger, C.D. (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human lymphoid
cells in vitro: organo-phosphates. Mutation Research, 102: 89-102.
Strasser, F., Arni, P. (1986) Chromosome studies on somatic cells of
Chinese hamster. Unpublished Report 850808 by CIBA-GEIGY Ltd Basle,
Switzerland. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Strasser, F., Langauer, M., Arni, P. (1986) Nucleus anomaly test in
somatic interphase nuclei of Chinese hamster. Unpublished Report
850809 by CIBA-GEIGY Ltd., Basle, Switzerland. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Vaidya, V.G., Patanakar, N. (1982) Mutagenic effect of monochrotophos
- an insecticide in mammalian test systems. Indian J. Med. Res., 76,
912-917.
Wang, T.C., Lee, T.C., Lin, M.F., Lin, S.Y. (1987) Induction of
sister chromatid exchange by pesticides in primary rat tracheal
epithelial cells and Chinese hamster ovary cells. Mutation
Research, 188, 311-321.
No food was allowed to remain in food hopper for more than 4 days.
The actual content of monocrotophos in the diets was checked several
times throughout the duration of the study and it was always shown to
be within ± 10 % of nominal. The mice were observed daily for general
health and appearance, body weights and food intake were measured
weekly. Every three months groups of 10 mice/sex from the 0 and 10
ppm dose levels underwent ophthalmoscopic examination. At the
scheduled times animals were killed and blood samples were taken for
haematological examinations and cholinesterase activity
determinations. Full necropsies were performed on all animals, major
organs were weighed and tissues were examined histologically from all
animals in the 104 week section of the study and from mice fed 0, 5
and 10 ppm monocrotophos in the 55 and 78 week sections. Body weight
and food intake were not affected by treatment with monocrotophos.
Convulsions were observed in animals in all groups including controls.
The overall incidence of convulsions was increased in males from the
2 ppm dose level and in females from the 1 ppm dose level. No ocular
abnormalities related to monocrotophos feeding were detected. No
dose-related, biologically significant differences in hematological
tests were observed. Mean plasma, erythrocyte and brain
cholinesterase activities were significantly depressed in all
treatment groups at all times sampled. Inhibition was dose-related,
consistent throughout the duration of the study and no differences
between sexes were apparent. In brain after 104 weeks of treatment,
inhibition was approximately 20%, 30%, 50% and 65% at 1, 2, 5 and 10
ppm, respectively. At the end of the study the mortality rate was
60%, 56%, 60%, 62%, 62% in males and 51%, 50%, 58%, 46% and 54% in
females at 0, 1, 2, 5, 10 ppm, respectively. Pathology did not show
specific lesions attributable to treatment. Increased incidence of
pulmonary neoplasms was found in males after 18 months of treatment at
5 and 10 ppm but this observation was not confirmed at the end of the
study. There was no evidence of a treatment-related oncogenic effect
of monocrotophos up to 10 ppm, the NOAEL for cholinesterase inhibition
is below 1 ppm (Robinson & Brown 1982).
Rats
Male and female Wistar-derived rats (5 weeks old study) were
offered monocrotophos (E isomer 78.7%) at concentrations of 0.01,
0.03, 0.1, 1.0 or 10 ppm in powdered diet for either 6 months (8
animals/sex/dose), 12 months (8 animals/sex/dose), 18 months (19
animals/sex/dose) and 24 months (50 animals/sex/dose). Control rats
were 16, 16, 38 and 100 for the 6, 12, 18 and 24 month sections,
respectively. The preparation, storage and presentation of the diets
were the same as reported in the previous long-term study in mice.
The actual content of monocrotophos in the diets was checked several
times throughout the study and was always shown to be within ± 10 % of
nominal. The rats were observed twice daily (daily on week-ends) for
general health and appearance throughout the duration of the study.
Body weights and food intake were measured weekly. Every three months
groups of 10 rats/sex from the 0 and 10 ppm groups underwent
ophthalmoscopic examination. At 6, 12, 18 and 24 months blood samples
were taken and urine collected for haematological and clinical
chemistry examinations, cholinesterase activity determinations and
urinalysis. Full necropsies were performed on all animals, major
organs were weighed and tissues was examined histologically. Body
weight was significantly reduced between 5% and 10% in males fed 10
ppm monocrotophos. This difference was more apparent during the first
year of the study and corresponds to lower food intake. Body weight
and food intake were not affected by treatment with monocrotophos in
males up to 1 ppm nor in females at any dose levels. General health
and behaviour were not affected by monocrotophos. No ocular
abnormalities related to monocrotophos feeding were detected. Mean
plasma, erythrocyte and brain cholinesterase activities were
significantly depressed in the 1 and 10 ppm dose groups. Inhibition
was dose related (approximately 30-40% and 70-80% at 1 and 10 ppm,
respectively), consistent throughout the duration of the study and not
differences between sexes were detectable. The results of
haematology, clinical chemistry and urinalysis yielded no consistent
dose- nor time-related findings throughout the course of the study.
At the end of the study, the mortality rate was 24%, 20%, 28%, 30%,
20%, 32% in males and 49%, 60%, 52%, 56%, 44% and 64% in females at 0,
0.01, 0.03, 0.1, 1.0 and 10 ppm, respectively. Organ weight
variations among groups were of no toxicological relevance. Pathology
showed that the incidences of patchy alopecia and ulcerative
dermatitis of the tail were slightly higher in the 10 ppm groups of
both sexes. Pituitary neoplasm incidence was increased at the end of
the study in female but not in male rats fed 10 ppm monocrotophos.
Because of the high incidence of this type of neoplasm (88% of females
fed control diet for up to two years versus 96% of females fed 10 ppm
monocrotophos), this observation was of no biological relevance. The
NOAEL for cholinesterase inhibition was 0.1 ppm and there were no
specific macro- or microscopic lesions and there was no evidence of a
treatment-related oncogenic effect up to 10 ppm (Robinson et al.,
1983).
Reproduction studies
Rats
In a 1-litter 2-generation reproduction study, groups of 13 male
and 26 female Wistar rats approximately 5 weeks old received
monocrotophos (E isomer 78.7%) admixed in the diet at 0, 0.1, 1, 3 or
10 ppm. The preparation, storage and presentation of the diets was the
same as previously reported in long-term studies in mice and rats.
The actual content of monocrotophos in the diets was checked several
times throughout the duration of the study and was always shown to be
within ± 10 % of nominal. Rats were maintained on their respective
diets for at least 15 weeks, then each male rat was allocated to two
females of the same treatment group. The F1 generation was culled
to the same number of rats as in F0 and animals were exposed to the
appropriate test diet for 18 weeks before being bred to produce F2
generation. Body weights were recorded monthly for adult rats in the
pre-mating phase and at 1, 4, 7, 14 and 21 days of age for F1 and
F2 pups. Mating performance, fertility, litter size and viability
were recorded. Pathology was performed on F0 and F1 adults, on
selected F1 and F2 pups. Sperm head counts were made on the testes
of F0 and F1 adult rats. Significantly lower body weights (6-9%
reduction) were recorded in male but not in female rats fed 10 ppm
monocrotophos for both F0 and F1 generations. No treatment-related
toxic effects were observed in the F0 and F1 adults during the
pre-mating phase. Faecal pellets produced by F0 and F1 rats fed 10
ppm monocrotophos were smaller and darker than dose produced by
controls. There were no monocrotophos-related effects on sperm head
counts of the F0 or F1 parent rats. Mating performance, fertility
index and gestation index were not different among F0 groups. At 10
ppm the F1 male mating index was lower and fewer litters were
produced compared to controls. The gestation lengths of F0 and F1
females at 10 ppm dose level were significantly greater than those of
controls. Mean litter size, viability index and lactation index were
significantly reduced at 10 ppm for both F1 and F2 generations.
Viability index in the F2 generation was reduced also at 3 ppm. Mean
pup weights were lower at 10 ppm for both F1 and F2 generations and
at 3 ppm for the F2 generation. There were three total litter losses
at 10 ppm of the F1 and F2 generations and one litter loss of the
F2 generation at 3 ppm, probably due to lactation deficiency as
suggested by poor mammary development observed in dams. Higher kidney
and liver weights were observed for F2 female weanlings at 3 and 10
ppm. These finding were not related to any histopathological
abnormalities. No pathological changes of any tissues (excluding poor
mammary development mentioned above) could be related to monocrotophos
exposure. One ppm was the NOAEL in this reproduction study (Dix &
Thorpe, 1981).
Special studies on delayed neurotoxicity
Hens
Fourteen adult Warren Studdler laying hens were treated orally
with monocrotophos 60% w/v in acetone on two separate occasions 3
weeks apart. Monocrotophos was administered in gelatin capsules to
supply 6.7 mg/kg of monocrotophos (equivalent to LD50 in domestic
fowl). Hens were protected against cholinergic toxicity with atropine
sulphate (17.4 mg/kg i.m.) and pralidoxime chloride (50 mg/kg i.m.).
Positive controls received tri-O-tolyl phosphate (0.5 ml/kg
undiluted, p.o.), negative controls did not received any treatment.
Nine out of 14 birds died of acute cholinergic symptoms within 4 days
of the first or second monocrotophos dose; the five animals which
survived both monocrotophos doses did not develop clinical nor
histopathological signs of delayed neurotoxicity. Positive controls
gave the expected positive clinical and histopathological responses
(Owen et al., 1978).
Groups of 10 adult hens (COFAL/Marek) were dosed by gavage with
technical monocrotophos (containing 77.4% of the active E-isomer of
monocrot-ophos, 0.43% trimethyl phosphate) at concentrations of 0,
0.03, 0.1, 0.3 mg/kg for 96 days. A positive control group received
tri-orthocresol phosphate (TOCP) at 7.5 mg/kg p.o. Because of lack of
neurotoxic symptoms both the highest monocrotophos dose and TOCP dose
were increased on day 79 to 0.5 mg/kg and 10 mg/kg, respectively.
Monocrotophos was dissolved in acetone and pipetted into gelatin
capsules half-filled with feed; the acetone was allowed to evaporate
at room temperature. Stability of the test compound under these
conditions was confirmed by analytical tests. Plasma cholinesterase
activity was reduced in all groups, including the TOCP group. This
reduction was dose-related and at the highest dose level ranged
between 11.8% after the first dose and 46.9% after the final dose. No
changes in erythrocyte cholinesterase activity and no clinical signs
of the cholinergic type were observed. Body weights and egg-laying
performance were not consistently affected throughout the duration of
the study. Some of the hens dosed with TOCP showed clinical (3 out of
10 birds) and histopathological (7 out of 10 birds) signs of delayed
neuropathy after raising the TOCP dose to 10 mg/kg. None of the
monocrotophos-treated hens developed delayed neuropathy.
Mono-crotophos is devoid of neurotoxic potential under the conditions
of the study. However, the short-term study was performed at doses
too low at which neither acute cholinergic nor delayed neurotoxic
effects could have been detectable (Becci & Parent, 1981).
Special studies on embryotoxicity and teratogenicity
Rats
Twenty-six female Charles River Crl:CD (SD) BR albino rats were
treated with technical monocrotophos at concentrations of 0, 0.3, 1.0,
or 2.0 mg/kg/day orally by gavage (5 ml/kg, dissolved in distilled
water) on days 6 to 15 of gestation. The test chemical was supplied
by Shell Chemical Company which also performed characterization,
stability and chemical analysis of dosage formulations. The
concentrations of all samples before and after dosing were within ±
10% of the nominal concentrations. A single female rat dosed with 2.0
mg/kg died on gestation day 15, it had a diffuse, red-black crusted
exudate around both eyes and a thinned glandular stomach which lacked
mucosal convolutions. The remaining females survived up to the final
sacrifice. Mean body weights (from gestation day 12) and carcass
weights were significantly reduced in animals dosed at 1.0 and 2.0
mg/kg bw. Carcass weights were also reduced at 0.3 mg/kg. No
significant differences were noted for the gravid uterus weights.
Muscle tremors and/or twitchings, listlessness, salivation, urine
soaked fur and crusty eyes were observed in females dosed with 2.0
mg/kg, mainly within 4 hours of administration of the test substance.
Pathological examination at final sacrifice did not show abnormalities
which could be related to monocrotophos administration. There were no
statistically significant differences noted in the mean numbers of
corpora lutea, implantation sites, resorption sites, nor viable
fetuses in treated animals. Reproductive percentages calculated for
the treated dams were comparable to those of the control group. Mean
body weight and crown-rump length data obtained for the 2.0 mg/kg
fetuses were significantly lower than those of the control fetuses.
Mean percent of runt fetuses per litter was increased in 1.0 and 2.0
mg/kg groups. The percentage of fetuses with non-ossified
sternebra(e) was increased in litters of the 2.0 mg/kg group. The
incidence (58.4%) was approximately doubled in comparison with control
group and with the other groups at lower mono-crotophos dose levels
but within the range of previous historical data (4.7% to 82.1%).
Malformed and/or misshapen brain (malformed brain with subdural
haemorrhage/encephalocoele) was observed in 1, 3, 2 and 2 fetuses at
0, 0.3, 1, 2 mg monocrotophos/kg, respectively. This malformation is
uncommon in Sprague-Dawley rats; it appeared to be a progression of
the same type of lesion which differed only in the degree of severity.
These brain malformations may suggest a teratogenic effect of
monocrotophos in rats (Borders et al., 1983).
Table 1. Results of genotoxicity assays on monocrotophos
Test system Test object Concentration of Purity Results Reference
test substance
In vitro
Reversion assay (1) S. typhimurium 0-10 000 µg/plate Not given Positive with Moriya et al. (1983)
TA98, TA100, dissolved in DMSO TA100 and
TA1535, TA1537, WP2hcr (2)
TA1538
E. coli WP2hcr
Reversion assay (1) S. typhimurium 10-8000 µg/0.1 ml 78.4% Slight positive Hool & Arni (1986)
TA98, TA100, dissolved in acetone with TA100
TA102, TA1535, (3)
TA1538
L5178Y Tk +/- Mouse lymphoma 50-1200 µg/ml 58.4% Positive Jotz et al. (1980)
mutation assay (1) cells dissolved in DMSO (4)
Sister chromatid Human lymphoid 0-2 µg/ml in absolute Not given Positive Sobti et al. (1982)
exchange assay cells (LAZ-007) ethyl alcohol (5)
Sister chromatid Human lymphocytes 0.1-0.8 µg/8 ml 36% Positive Rupa et al. (1988)
exchange assay culture x 24, 48, (6)
72 hr dissolved
in DMSO
Sister chromatid Chinese hamster CHO: 25-400 µg/ml 78% Positive Wang et al. (1987)
exchange assay (1) ovary cells, RTE: 12.5-100 µg/ml (7)
Rat tracheal dissolved in DMSO
epithelial cells
Table 1 (contd).
Test system Test object Concentration of Purity Results Reference
test substance
Reverse mutation Saccharomyces 0.1-3% dissolved in 58.4% Positive Mortelmans et al. (1980)
mitotic recombination cerevisiae D7 DMSO (8)
gene conversion (1)
Chromosome Human lymphocytes 0.01 µg/ml x 24, 48, 36% Positive Rupa et al. (1988)
aberrations 72 hr dissolved in (6)
DMSO
Chromosome Human lymphocytes 10-4 - 10-9 M x 50 h 69% Positive Vaidya et al. (1982)
aberrations in distilled water (9)
Mitotic Saccharomyces 5% 55% Positive Simmon et al. (1977)
recombination (1) cerevisiae D3 (10)
DNA-repair Human fibroblast 0.0001-10 mM 55% Positive Simmon et al. (1977)
(UDS) (1) cells (WI-38) (11)
In vivo
Chromosome study Chinese hamster 1.4, 2.8, 5.6 mg/kg 78.4% Negative Strasser & Arni (1986)
(bone marrow) in distilled water (11)
administered orally
twice 24 h apart
Chromosome study Swiss mouse 1, 1.5, 2 mg/kg 69% Positive Vaidya et al. (1982)
(bone marrow) administered i.p. (8)
twice 24 h apart
in distilled water
Table 1 (contd).
Test system Test object Concentration of Purity Results Reference
test substance
Nucleus anomaly test Chinese hamster 1.4, 2.8, 5.6 mg/kg 78.4% Negative Strasser et al. (1986)
(bone marrow) in distilled water (12)
administered orally
twice 24 h apart
Micronucleus test Swiss mouse 2, 4, 8 mg/kg i.p. Not given Negative Kirkhart et al. (1980)
(bone marrow) administered twice (13)
24 h apart
Micronucleus test Swiss mouse 1, 1.5, 2 mg/kg i.p. 69% Positive Vaidya et al. (1982)
(bone marrow) administered twice (8)
24 h apart
Chromosome study Rat 0.5, 1, 2 mg/kg in Not given Equivocal Adhikari & Grover (1988)
(bone marrow) DMSO administered (15)
i.p. twice 24 h
apart
Dominant lethal Mouse 15, 30, 60 mg/kg of 55% Negative Simmon et al. (1977)
diet x 7 weeks (16)
Special studies on genotoxicity
(1) Both with and without metabolic activation
(2) Part of a study in which 228 pesticides were tested for
mutagenicity in bacterial reversion-assay systems
(3) The study was repeated three times at similar doses giving
similar results. Positive control without activation:
Daunorubicin HCl, 5-10 µg/0.1 ml; 4-nitroquinoline-N-oxide,
0.125-0.250 µg/0.1 ml; mitomycin-c, 0.5-1 µg/0.1 ml; sodium
azide, 2.5-5 µg/0.1 ml; aminoacridine hydrochloride, 50-100
µg/0.1 ml gave expected positive responses. Positive control with
activation: 2- aminoanthracene, 5-20 µg/0.1 ml; cyclophosphamide,
250 µg/0.1 ml gave the expected positive responses.
(4) Positive control without activation: ethylmethane sulfonate, 500
µg/ml gave expected positive response. Positive control with
metabolic activation: 3-methylcholantrene, 5 µg/ml gave expected
positive response. The test substance gave positive response at
200 µg/ml and above and at 840 µg/ml and above with and without
metabolic activation, respectively.
(5) Monocrotophos was tested without metabolic activation. A
dose-related statistically significant increase of sister
chromatid exchange frequency was observed.
(6) Positive control not run.
(7) Monochrotophos was positive for sister chromatid exchange
inductions in both CHO and RTE.
(8) Positive control with and without metabolic activation:
1,2,3,4-diepoxybutane, 0.013% gave expected positive response.
Monochrotophos induced mitotic crossing-over, gene conversion,
and reverse mutation, with and without metabolic activation.
(9) Positive control not run.
(10) Positive control: 1,2,3,4,-diepoxybutane, 0.1% gave expected
positive response.
(11) Positive control without metabolic activation:
4-nitroquinoline-N-oxide, 0.05 mM gave expected positive
response. Positive control with metabolic activation:
Dimethylnitrosoamine, 50 mM gave expected positive response.
(12) Positive control: cyclophosphamide, 64 mg/kg x 2 gave expected
positive response.
(13) Positive control: cyclophosphamide, 128 mg/kg x 2 gave expected
positive response.
(14) Positive control: trimethylphosphate, 1 g/kg x 2 gave expected
positive response.
(15) Positive control: ethyl methane sulfonate, 62.5-250 mg/kg x 2
gave expected positive response. Monochrotophos caused a
dose-related increase of the frequency of aberrant cells, but
the increase was significant only for the highest dose.
(16) Positive control: triethylenemelamine, 0.2 mg/kg i.p. gave
expected positive response.
COMMENTS
In a 13-week study in rats at dietary concentrations of 0, 0.1,
0.25, 0.5, 2, or 8 ppm, the NOAEL was 0.5 ppm, equivalent to 0.025
mg/kg bw/day, based on brain acetylcholinesterase inhibition at 2 ppm.
In a 2-year study in mice at dietary concentrations of 0, 1, 2,
5 or 10 ppm a NOAEL could not be established because brain
acetylcholinesterase inhibition was detected at the lowest dietary
concentration (approximately 20% inhibition). There was no evidence
of a treatment-related carcinogenic effect.
In a 2-year study in rats at dietary concentrations of 0, 0.01,
0.03, 0.1, 1.0 or 10 ppm the NOAEL was 0.1 ppm, equivalent to 0.005
mg/kg bw/day, based on brain acetylcholinesterase inhibition at the
next higher dose. Again, there was no evidence of carcinogenicity.
Monocrotophos did not cause delayed neuropathy in hens.
In a multigeneration reproduction study in rats at dietary
concentrations of 0, 0.1, 1, 3, or 10 ppm, the NOAEL was 1 ppm,
equivalent to 0.05 mg/kg bw/day, based on toxicity in pups seen in the
F2 generation at 3 ppm.
In a teratology study in rats at doses of 0, 0.3, 1 or 2 mg/kg
bw/day, the NOAEL was 0.3 mg/kg bw/day for both maternal toxicity and
teratogenicity. There was a slightly increased incidence of malformed
and/or misshapen brain at all dose levels (0.3 mg/kg bw/day included).
A dose-effect relationship for this uncommon malformation was lacking
and historical control data were not available.
Substantial variations in the purity of the test material used in
the genetic toxicology studies hampered thorough evaluation for
genotoxicity. When purity was at the level of technical grade
monocrotophos (78%) there were significant responses in tests for
mutagenicity in bacteria and sister chromatid exchange in Chinese
hamster ovary cells; in vivo studies for clastogenic activity were
negative.
The Meeting concluded that the significance of the brain
malformations observed in the teratology study in rats warranted
clarification, as did the genotoxic potential of monocrotophos.
Accordingly, the Meeting recommended that monocrotophos be reviewed
in 1994.
An ADI was allocated on the basis of the 2-year study in rats
using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: < 1 ppm in the diet, equivalent to < 0.15 mg/kg
bw/day
Rat: 0.1 ppm in the diet, equivalent to 0.005 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.00005 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
1. Genotoxicity studies, known to exist, with commercial and
purified monocrotophos.
2. Historical control data on the incidence of brain malformations
in rats at the relevant laboratory.
REFERENCES
Adhikari, N., Grover, I.S. (1988) Genotoxic effects of some systemic
pesticides: in vivo chromosomal aberrations in bone marrow cells
in rats. Environmental and Molecular Mutagenesis, 12: 235-242.
Becci, P.J., Parent, R.A. (1981) Neurotoxicity evaluation of Azodrin
insecticide: subchronic oral administration in hens. Unpublished
Report 6535-II by Food and Drug Research Laboratories, Inc. Waverly,
NY, USA. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Borders, C.K., Salamon, C.M., Mayhew, D.A. (1983) Technical Azodrin
(SD 9129) teratology study in SD CD rats. Unpublished Report 450-1248
by Toxi-Genics Inc. USA. Submitted to WHO by CIBA-GEIGY Ltd, Basle,
Switzerland.
Dix, K.M. & Thorpe, E. (1981) A reproduction study in rats fed
Azodrin. Unpublished Report 1752 by Sittingbourne Research Centre,
England. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Hend, R.W & Brown, V.K.H. (1981) A reversibility study of
cholinesterase activity in rats fed Azodrin for 8 weeks. Unpublished
Report 1720 by Sittingbourne Research Centre, Shell Toxicology
Laboratory (Tunstall), Sittingbourne, England. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Hool, G. & Arni, P. (1986) Salmonella/mammalian-microsome
mutagenicity test. C 1'414 tech. Unpublished Report 8508210 by
CIBA-GEIGY Ltd Basle, Switzerland. Submitted to WHO by CIBA-GEIGY Ltd,
Basle, Switzerland.
Kirkhart, B., Jones, D.C.L., Skinner, W.A. (1980) Micronucleus test
on monocrotophos. Unpublished Report LSU 7558-19 by SRI
International, Menlo Park, California, USA. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Jotz, M.M., Mitchell, A.D., Jones, D.C.L., Skinner, W.A. (1980) An
evaluation of mutagenic potential of monochrotophos employing the
L5178Y TK +/- mouse lymphoma assay. Unpublished Report LSU-7558 by
SRI International, Menlo Park, California, USA. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato K., Shirasu, Y.
(1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutation Research, 116: 185-216.
Mortelmans, K.E., Shepherd, G.F., Jones, D.C.L., Skinner, W.A. (1980)
In vitro detection of mitotic crossing-over, mitotic gene conversion
and reverse mutation with Saccharomyces cerevisiae D7 for seven
pesticides. Unpublished Report LSU-7558-20 by SRI International,
Menlo Park, California, USA. Submitted to WHO by CIBA-GEIGY Ltd,
Basle, Switzerland.
Owen, D.E., Butterworth, S.T.G., Brown, V.K.H., Thorpe, E. (1978)
Toxicity of organophosphorus insecticide Azodrin: investigation of the
neurotoxicity of Azodrin-5 to adult domestic hens. Unpublished Report
TLGR 0066.78 by Shell Research Limited, Sittingbourne Res. Centre,
England. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Robinson, J. & Brown, V.K.H. (1982) A two-year oncogenicity study in
mice fed Azodrin. Unpublished Report 194/82 from Shell Research
Limited. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Robinson, J., Gellatly, J.B.M. & Brown, V.K.H. (1983) A long-term
feeding study with Azodrin in rats to investigate chronic toxicity and
oncogenicity (6, 12, 18 and 24 month necropsies). Unpublished Report
194/82 from Shell Research Limited. Submitted to WHO by CIBA-GEIGY
Ltd, Basle, Switzerland.
Rupa, D.S., Lakshaman, R.P.V., Reddy, P.P., Reddi, O.S. (1988)
In vitro effect of monochrotophos on human lymphocytes. Bull.
Environ. Contam. Toxicol., 41: 737-741
Simmon, V.F., Mitchell, A.D., Jorgenson, T.A., (1977) Evaluation of
selected pesticides as chemical mutagens in vitro and in vivo
studies. Unpublished Report EPA-600/1-77-028 by SRI International,
Menlo Park, California, USA. Submitted to WHO by CIBA-GEIGY Ltd,
Basle, Switzerland.
Sobti, R.C., Krishan, A., Pfaffenberger, C.D. (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human lymphoid
cells in vitro: organo-phosphates. Mutation Research, 102: 89-102.
Strasser, F., Arni, P. (1986) Chromosome studies on somatic cells of
Chinese hamster. Unpublished Report 850808 by CIBA-GEIGY Ltd Basle,
Switzerland. Submitted to WHO by CIBA-GEIGY Ltd, Basle, Switzerland.
Strasser, F., Langauer, M., Arni, P. (1986) Nucleus anomaly test in
somatic interphase nuclei of Chinese hamster. Unpublished Report
850809 by CIBA-GEIGY Ltd., Basle, Switzerland. Submitted to WHO by
CIBA-GEIGY Ltd, Basle, Switzerland.
Vaidya, V.G., Patanakar, N. (1982) Mutagenic effect of monochrotophos
- an insecticide in mammalian test systems. Indian J. Med. Res., 76,
912-917.
Wang, T.C., Lee, T.C., Lin, M.F., Lin, S.Y. (1987) Induction of
sister chromatid exchange by pesticides in primary rat tracheal
epithelial cells and Chinese hamster ovary cells. Mutation
Research, 188, 311-321.
