
PHOSALONE JMPR 1972
IDENTITY
Chemical name
O,O-diethyl-S-[6-chloro-1,3 benzoxazol-2(3H)-onyl-methyl]
phosphorodithioate
Synonyms
11,974 R.P., R.P. 11,974, bensophos
Structural formula
 Other information on identity and properties
Physical state: non-hygroscopic crystalline solid
Colour: white
Odour: alliaceous
Melting point: 45 - 48°C
Solubility: in water at 20°C is about 0.01 g/l, in
methanol and ethanol about 200 g/l, in other
organic solvents (acetone, acetonitrile,
benzene, chloroform, cyclohexanone, dioxane,
ethyl acetate, methylene chloride, methyl-
thylketone, toluene, xylene) about 1 000 g/l
Stability: of phosalone and its formulations, good
Purity: the technical product contains 93% active
ingredient
Formulations: wettable powders and emulsifiable
concentrates.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Phosalone is rapidly absorbed and excreted by mice following oral
administration. Within 24 hours, less than 1% phosalone residues were
found in the body (Desmoras and Fournel, 1968).
Biotransformation
Phosalone, an organophosphorus ester, exhibiting a weak potential for
inhibiting cholinesterase, is converted to a more potent oxygen
analogue. This analogue is 2-3 times more active as an inhibitory
agent than phosalone, especially to serum cholinesterase
(Rhône-Poulenc, 1968b).
The metabolic fate of phosalone appears to follow a pattern typical of
phosphorodithioate esters, i.e., oxidation to the phosphate,
hydrolysis and conjugation of the leaving group and elimination, as
shown in Figure 1.
The major conjugate in plants has been described as the glucose
conjugate of the benzoxazol moiety (1-[6-chloro-1,3-benzoxazol-
(3H)-onyl] glucopyranose). This metabolite has an acute toxicity in
mice of greater than 4 gm/kg (Rhône-Poulenc, 1968a).
TOXICOLOGICAL STUDIES
Special studies on metabolites
Acute toxicity studies in rats and mice show the oxygen analogue of
phosalone to be 2-3 times more toxic than phosalone (see Table 1).
TABLE 1
Acute toxicity of the oxygen analogue of phosalone
LD50 (mg/kg)
Sex Rats Mice
M 36 35
F 20 40
(Rhône-Poulenc, 1968b; Desmoras et al., 1963)
Other information on identity and properties
Physical state: non-hygroscopic crystalline solid
Colour: white
Odour: alliaceous
Melting point: 45 - 48°C
Solubility: in water at 20°C is about 0.01 g/l, in
methanol and ethanol about 200 g/l, in other
organic solvents (acetone, acetonitrile,
benzene, chloroform, cyclohexanone, dioxane,
ethyl acetate, methylene chloride, methyl-
thylketone, toluene, xylene) about 1 000 g/l
Stability: of phosalone and its formulations, good
Purity: the technical product contains 93% active
ingredient
Formulations: wettable powders and emulsifiable
concentrates.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Phosalone is rapidly absorbed and excreted by mice following oral
administration. Within 24 hours, less than 1% phosalone residues were
found in the body (Desmoras and Fournel, 1968).
Biotransformation
Phosalone, an organophosphorus ester, exhibiting a weak potential for
inhibiting cholinesterase, is converted to a more potent oxygen
analogue. This analogue is 2-3 times more active as an inhibitory
agent than phosalone, especially to serum cholinesterase
(Rhône-Poulenc, 1968b).
The metabolic fate of phosalone appears to follow a pattern typical of
phosphorodithioate esters, i.e., oxidation to the phosphate,
hydrolysis and conjugation of the leaving group and elimination, as
shown in Figure 1.
The major conjugate in plants has been described as the glucose
conjugate of the benzoxazol moiety (1-[6-chloro-1,3-benzoxazol-
(3H)-onyl] glucopyranose). This metabolite has an acute toxicity in
mice of greater than 4 gm/kg (Rhône-Poulenc, 1968a).
TOXICOLOGICAL STUDIES
Special studies on metabolites
Acute toxicity studies in rats and mice show the oxygen analogue of
phosalone to be 2-3 times more toxic than phosalone (see Table 1).
TABLE 1
Acute toxicity of the oxygen analogue of phosalone
LD50 (mg/kg)
Sex Rats Mice
M 36 35
F 20 40
(Rhône-Poulenc, 1968b; Desmoras et al., 1963)
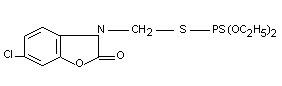 Special studies on neurotoxicity
Adult White Leghorn hens (ten per group) were fed phosalone at levels
of 0, 50, 163 and 500 ppm in the diet for 45 days. Another group was
fed tri-o-cresyl phosphate (TOCP) at 500 ppm. Phosalone at 163 and 500
ppm reduced egg production. No clinical or histological evidence of
paralysis or demyelination was observed. TOCP dietary exposure
resulted in signs of slight paralysis and evidence of minor central
and peripheral nerve degeneration, as determined histologically
(Woodard et al., 1966a).
A group of Rhode Island Red Hens (ten birds) was administered
phosalone subcutaneously at a dose of 350 mg/kg initially and after
three weeks. The birds were administered atropine and P2S to protect
against cholinergic stimulation. Mipafox (16 mg/kg) was administered
subcutaneously as a positive control to four hens. No clinical or
histological evidence in spinal cord or sciatic nerve of delayed
neurotoxicity was evident with phosalone. Mipafox paralyzed all hens
and induced demyelination in spinal cord or sciatic nerve (Heath
et al., 1967).
Special studies on potentiation
Phosalone, in combination with other anticholinesterase agents, was
examined for possible potentiation effects by administration of up to
one-half of the oral LD50 of each material. Disulfaton potentiated
the acute effects of phosalone. The toxicity of malathion and
coumaphos was potentiated to a lesser extent, while potentiation did
not occur with 15 other compounds, including: carbophenothion,
demeton, disyston, diazinon, trichlorfon, azinphos-methyl, dioxathion,
EPN, ethion, mevinphos, parathion-methyl, fenchlorfos, shradan,
merphos and carbaryl (Scott and Beliles, 1965b).
Special studies on reproduction
Three groups of rats (20 females and 10 males per group) were fed
phosalone at 0, 25 and 50 ppm in the diet and subjected to a standard
two-litter, three-generation reproduction study. There were no effects
on reproduction and lactation indices noted in this study which were
attributed to phosalone (Jones et al., 1967a).
Special studies on teratogenicity
Teratogenicity tests were conducted with phosalone in chick embryos
and rabbits. Doses of 0.2, 0.6 and 1.8 mg/egg were given to chick
embryos. Doses of 2, 6 and 18 mg/kg daily were given orally to
pregnant rabbits from day 6 to 16 of gestation. Thalidomide (100 mg/kg
p.o./day) was the positive control in the rabbit study. No
significantly greater incidence of total malformations due to
phosalone were noted in either chick embryos or rabbits (Julou, 1969).
Acute toxicity
The acute toxicity of phosalone has been studied in animals. A summary
of results of these studies is given in Table 2.
TABLE 2 Acute toxicity of phosalone in various animal species
LD50
Species Route (mg/kg) Reference
Dog oral >1 600 Cookrell et al., 1965
Rat (F) oral 153-207 Scott and Beliles, 1965a
(M) oral 125 Fournel et al., 1968
(F) oral 90 Ibid.
Mouse oral 320 Rivett and Davies, 1966
(M) oral 118 Fournel et al., 1968
(F) oral 93 Ibid.
Chicken SC 350 Heath et al., 1967
Signs of poisoning following acute administration of phosalone are the
same as for the other organophosphorus esters and include tremors,
lacrimation and eventually convulsions and death. Signs of poisoning
were evident within 10 to 20 minutes.
An LC50 value of 64 mg/l was calculated following inhalation exposure
of rats (male and female) to phosalone, for one hour at various doses,
having a 3µ particle size (Beliles, 1966). A single dermal application
to the shaved backs of rabbits at doses up to 2 000 mg/kg of a
formulation (equivalent to a dose of phosalone at approximately 800
mg/kg) for 24 hours resulted in no toxicity, but a mild erythema was
noted at 2 000 mg/kg (Horn et al., 1965).
Antidotal studies with rats and mice showed that both atropine and
P2S are effective antidotes to the acute toxic signs of poisoning
following oral administration of phosalone. P2S was apparently more
effective than atropine, although in both cases multiple applications
of antidotal material was suggested to be optimal (Rivett and Davies,
1966).
Short-term studies
Rabbit
Groups of rabbits (5 males and 5 females per group) were administered
phosalone dermally to intact or abraded skin for three weeks, five
days per week, at dosage levels of 1 ml of an emulsifiable
concentrate, 0.05 ml of the E.C. (a dilution of the concentrate) and 1
ml of a control E.C. with no phosalone. Weight loss, signs of toxicity
and death, as well as significant cholinesterase depression, were
apparent with both phosalone treatments to abraded skin. The rabbits
treated with 1 ml of emulsifiable concentrate had a lower liver
glycogen level, and both treated groups incurred renal congestion in
animals with abraded skin. Dermal irritation was evident in all
animals, and gross and histopathological examination showed no effects
on other tissues examined (Woodard et al., 1966b).
Dog
Four groups of dogs (four males and four females per group) were fed
phosalone in the diet for two years at levels of 0, 100, 200, and
1 000 ppm. Effects on cholinesterases were evident at all dose levels
throughout the study. Plasma cholinesterase was depressed at all
feeding levels; RBC cholinesterase was depressed at 200 and 1 000 ppm,
and brain cholinesterase was depressed at 1 000 ppm. One dog fed 1 000
ppm (female) died at 93 weeks, and several others at this level
exhibited fasciculations, nervousness and laboured respiration.
Reduced body-weight was noted in both sexes at 1 000 ppm and in males
at 200 ppm. Soft stools containing mucous and blood were observed for
each treated group, with considerably more frequency at 1 000 ppm.
This effect began to occur at the 100 ppm level after 53 weeks. ECG
changes included an increased T-wave amplitude at 1 000 ppm, which was
more apparent during the second year of study. Increased SGPT and SAP
activity were observed at 1 000 ppm.
Organ to body-weight ratios suggested that at 1 000 ppm the liver of
both sexes and adrenals and thyroid of females were enlarged.
Histological changes observed at all feeding levels were seen as
increased basophilic granules in the myofibrillar cytoplasm in smooth
muscle of the small intestine. These effects were less obvious as the
dose decreased. Vacuolation of the smooth muscle cells of the small
intestine was seen only at 1 000 ppm (Donoso et al., 1967).
Three groups of dogs (four males and four females per group) were fed
phosalone in the diet for 19 weeks at levels of 7.5, 15 and 25 ppm. In
addition, at weeks 14 -19 disyston at 1 ppm was added to the diet of
all animals. No effects on behaviour, growth, clinical chemistry,
urinalysis, biochemistry or cholinesterase activity were noted in this
study which were attributed to phosalone. Histological examination of
the small intestine revealed no abnormalities (Jones et al., 1967b).
Groups of dogs (four males and four females per group) were fed
phosalone in the diet, at levels of 0, 12.5, 25 and 37.5 ppm for one
month. Plasma cholinesterase activity was reduced at 37.5 ppm. In all
dogs at 25 and 37.5 ppm, and in one dog at 12.5 ppm, a pink-mauve
colouration of the mucosa of the bladder was noted although it was not
observed in any other experiment performed in dogs. No effects were
observed on growth, food consumption or RBC and brain cholinesterase
activity (Noel et al., 1970).
Five groups of calves were fed phosalone in the diet for ten weeks at
levels of O, 20, 50, 80 and 160 ppm. No significant effects
attributable to phosalone were observed on growth, behaviour, food
consumption, hematology and cholinesterase activity (Woodard et al.,
1967a).
Long-term studies
Rat
Four groups of rats (30 males and 30 females per group) were fed
phosalone in the diet for two years at levels of 0, 25, 50 and 250
ppm. Effects attributable to phosalone were observed only on plasma
cholinesterase, which was depressed at 50 ppm but not at 25 ppm. No
other effects of treatment, either gross, histopathological or
clinical, were noted at any of the dose levels studied (Woodard
et al., 1967b).
COMMENT
Phosalone is rapidly absorbed, metabolized and excreted by rodents.
Phosalone has been shown to have no effect on rat reproduction, is not
teratogenic in chick or rat and is not neurotoxic to chickens. The
acute toxic effect in rodents was potentiated with several
organo-phosphates, particularly disyston.
Two-year feeding studies in rats and dogs indicate no-effect levels
(based on cholinesterase depression) of 25 ppm for both species.
Bladder discolouration in dogs was reported in a short-term study, but
longer studies did not confirm this observation.
Observations in man are very limited.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 25 ppm in the diet, equivalent to 1.25 mg/kg
body-weight
Dog: 25 ppm in the diet, equivalent to 0.625 mg/kg
body-weight
ESTIMATE FOR ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.006 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phosalone is a non-systemic insecticide and acaricide active by
contact and ingestion. It is officially approved for use in many
countries on a wide variety of crops, and is practically nontoxic to
bees.
Pre-harvest treatments
Phosalone is recommended in a number of countries to control a wide
range of insects and mites on fruit, rape, sugar beet, potato,
alfalfa, cotton, vegetables, grapes, tea and ornamental crops.
Recommended rates of application vary in different countries from 0.4
to 1.2 kg a.i./ha (0.04-0.06% a.i. with high volume sprays at
1 000 - 2 000 l/ha). The recommended interval between two treatments
is, in most countries, two to three weeks and in Canada ten days. The
safety period in the treatment of orchard crops is in Canada, 1 - 7
days; in Great Britain, 3 weeks; in France, 2 weeks; in Switzerland, 3
weeks; in U.S.A., 1 week.
Other uses
Phosalone is used against ectoparasites of animals in some countries.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residue data from supervised trials are given in Table 3. These
are from treatments made in Australia, Canada, France, Great Britain,
New Zealand, South Africa and U.S.A. (Rhône-Poulenc, 1967-1971; May
and Baker Ltd., 1967-1969).
FATE OF RESIDUES
General comments
Studies on the behaviour of phosalone in animals, plants and soil have
shown that phosalone has good stability (generally active against
pests for 15 to 18 days) on plants, but in animals it is rapidly
degraded to nontoxic products; decomposition is also rapid in soil.
In animals
Phosalone is rapidly degraded in animals. One half of the dose
disappears after oral administration in four to six hours and the rest
in 24 hours.
Phosalone may be hydrolysed to nontoxic derivatives or oxidized to the
oxo-derivative, which is responsible for inhibition of
cholinesterases. The oxo-derivative, less stable than phosalone, is
practically never detected, except in the liver where it may be found
in minute quantities.
When beef cattle and sheep were administered levels of 10, 30 and 100
ppm in feed, traces of phosalone were found in fat and liver only at
the feeding level of 100 ppm (Rhodia Inc., 1967a). No phosalone
residues were found in milk of dairy cattle at any of the feeding
levels of 100, 50 or 25 ppm (Rhodia Inc., 1967b; Woodard Research
Corporation, 1967c).
In plants
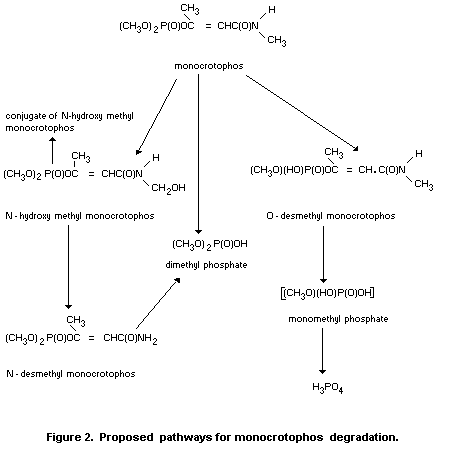
Degradation of phosalone in plants is shown in Figure 2 (Desmoras
et al., 1968a). The half-life of the residues in different plants is
shown in Table 4. Half-lives vary from 2 to 20 days with application
rates of 0.6 - 1.2 kg a.i./ha. The oxon derivative of phosalone is
found, but its amount has been less than 10% of the total amount of
the parent compound.
In soil
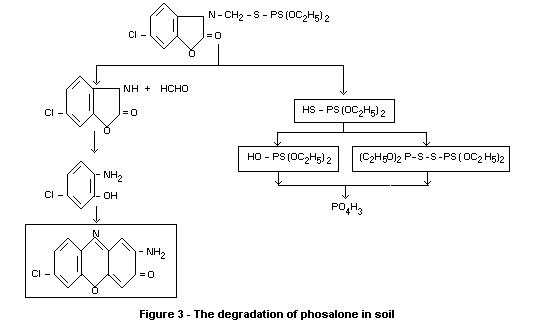
Degradation of phosalone in soils is shown in Figure 3. The half-life
of phosalone in soils was found to be about a week. No accumulation of
phosalone seems to occur in soils.
TABLE 3 Summary of phosalone residue, data from different trials
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
Alfalfa France 0.9 1 13 4 4 - 6 5
0.9 1 20 4 1.7 - 2.9 2.2
U.S.A. 1.7 1 20-27 10 1.4 - 11.6 5.2
1.7 1 11-14 4 24.5 - 37.0 30.6
0.8-1.1 1 14 10 2.2 - 12.6 7.8
0.8-1.1 1 20-23 20 0.8 - 7.6 3.3
Apple Australia 2.0 5-8 17-29 22 0.9 - 3.5 1.5
France 0.4- 0.6 4-7 16-28 20 0.75- z.6 1.5
0.4-0.8 7 28 35 0.9 - 3.8 2.3
1.3-1.5 3-4 14 24 0.9 - 2.9 1.8
0.6 1-8 14-33 24 c.4 - x.0 0.7
0.06% 7-8 21 8 2.1 - 3.6 2.9
" 7 36-64 20 1.3 - 2.4 1.7
" 7 75 4 0.9 - 1.0 1.0
England 0.46-0.69 2-3 59-70 3 0.05-0.49 0.2
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
New Zealand 0.056-0.09% 7-8 22-32 14 0.89-6.2 2.8
South Africa 0.03% 6-7 39-54 36 0.43-2.3 1.1
" 5-7 66-84 36 0.36-1.0 0.7
U.S.A. 0.06% 5 22-36 6 2.2-5.3 4.6
Beets France 0.4-0.6 1 1 8 0.19-1.67 0.6
" 1 3 8 0.20-1.35 0.4
Cabbage 0.4-0.6 1 1 4 4.1 -8.3 6.4
" 2 1 4 0.6 -2.8 1.7
" 6 1 4 0.3 -1.3 0.6
U.S.A. 0.06-0.12% 1 7-8 4 - <0.1
0.12% 5 26 2 - <0.1
Broccoli 0.06% 2 7 2 - 0.6
Brussels
sprouts 0.06% 6 14 2 - 0.7
Cherry Canada 0.03-0.04% 4 8 4 0.8 -4.9 2.8
U.S.A. 0.06% 4 21 2 - 0.1
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
France 0.06% 1 8-18 4 0.3 -0.4 0.4
Chestnut U.S.A. 2-3 1-2 14-78 6 - <0.1
Citrus fruit " 2.5 1 14 18 - <0.1
Cotton France 1 6-13 13-14 12 <0.1 -0.9 0.5
0.5-4 8-12 34-57 12 0.1 -1.6 0.8
Cucumber U.S.A. 0.06% 3 7-28 6 - 0.1
France 0.6-1.2 1 10 8 0.01-0.04 0.02
Grape U.S.A. 1 1-3 28-43 8 1.8 - 2.4 2.0
1.8-3.6 1 14 4 0.5 -0.9 0.7
1 3 59 2 - 0.9
7.2 1 35 2 - 1.3
South Africa 0.032-0.037% 3 41 12 0.4 -2.0 1.1
0.032-0.037% 3 61 12 0.08-1.0 0.3
0.032-0.037% 3 73 12 0.05-0.46 0.2
Canada 0.03-0.06% 5 14 12 1.8 - 4.7 3.0
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
France 0.06% 1 8 4 - 0.3
0.6 1 70 4 - 0.4
Hops U.S.A. 1-1.2 2 28 8 1.2 - 1.7 1.51
<0.12
Lettuce U.S.A. 0.12% 5 7 2 - 2.8
" 5 26 2 - 0.6
France 0.4-0.6 1 6 6 1.6 - 3.7 2.7
Pea U.S.A. 0.06% 1 49 2 - 0.1
France 0.4-0.6 1 1 8 1.3 - 2.8 2.1
" 1 2 8 0.5 -0.95 0.6
" 1 13 8 0.05-0.2 0.12
Peach Canada 0.03-0.045% 5 8 4 1.1 - 2.2 1.9
U.S.A. 0.06% 5 40 2 - 0.9
Australia 2.0 3-5 37-61 20 0.3 - 2.9 1.5
France 0.06% 1 7-14 4 0.7 - 1.9 1.4
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
Pear Australia 0.85 3-5 18-30 8 0.6 - 2.0 1.2
South Africa 0.03% 5-6 26-32 48 0.25- 1.8 0.7
France 0.06% 5 13 8 0.2 - 0.4 0.3
U.S.A. 0.06% 1-7 38-63 6 0.1 - 0.5 0.3
Pecan " 0.56 3-8 60-66 4 - <0.1
" 0.84 1 198 2 - <0.1
" 4.76 5 95 2 - <0.1
Plum Canada 0.03-0.045% 5 16 8 1.3 - 4.1 2.2
U.S.A. 0.06% 1 45-59 4 - 1.3
England 0.03% 1-3 11-26 16 0.22- 1.8 0.7
Potato U.S.A. 0.06% 1-7 7-54 6 - <0.1
Australia 0.06% 9 1 24 - <0.02
France 0.4-0.6 1 7-9 6 - <0.05
Rape seed " 1-2.4 3 77 8 - <0.1
Denmark 0.7-2.05 1 27 16 - <0.1
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
Sorghum U.S.A. 1 2 108 4 - 0.2
Strawberry " 0.06% 1 4-10 8 0.3 - 0.9 0.6
England 0.6 2 26 6 0.04-0.08 0.07
Tomato U.S.A. 0.6-0.12 1-4 14-15 6 <0.1 -0.6 0.3
France 0.4 -0.6 1 1 8 0.30-0.7 0.48
0.4 -0.6 1 3 8 0.15-0.7 0.23
Wheat " 0.6 1 25-44 6 <0.05-1 0.3
1 fresh
2 dried 3 days
Special studies on neurotoxicity
Adult White Leghorn hens (ten per group) were fed phosalone at levels
of 0, 50, 163 and 500 ppm in the diet for 45 days. Another group was
fed tri-o-cresyl phosphate (TOCP) at 500 ppm. Phosalone at 163 and 500
ppm reduced egg production. No clinical or histological evidence of
paralysis or demyelination was observed. TOCP dietary exposure
resulted in signs of slight paralysis and evidence of minor central
and peripheral nerve degeneration, as determined histologically
(Woodard et al., 1966a).
A group of Rhode Island Red Hens (ten birds) was administered
phosalone subcutaneously at a dose of 350 mg/kg initially and after
three weeks. The birds were administered atropine and P2S to protect
against cholinergic stimulation. Mipafox (16 mg/kg) was administered
subcutaneously as a positive control to four hens. No clinical or
histological evidence in spinal cord or sciatic nerve of delayed
neurotoxicity was evident with phosalone. Mipafox paralyzed all hens
and induced demyelination in spinal cord or sciatic nerve (Heath
et al., 1967).
Special studies on potentiation
Phosalone, in combination with other anticholinesterase agents, was
examined for possible potentiation effects by administration of up to
one-half of the oral LD50 of each material. Disulfaton potentiated
the acute effects of phosalone. The toxicity of malathion and
coumaphos was potentiated to a lesser extent, while potentiation did
not occur with 15 other compounds, including: carbophenothion,
demeton, disyston, diazinon, trichlorfon, azinphos-methyl, dioxathion,
EPN, ethion, mevinphos, parathion-methyl, fenchlorfos, shradan,
merphos and carbaryl (Scott and Beliles, 1965b).
Special studies on reproduction
Three groups of rats (20 females and 10 males per group) were fed
phosalone at 0, 25 and 50 ppm in the diet and subjected to a standard
two-litter, three-generation reproduction study. There were no effects
on reproduction and lactation indices noted in this study which were
attributed to phosalone (Jones et al., 1967a).
Special studies on teratogenicity
Teratogenicity tests were conducted with phosalone in chick embryos
and rabbits. Doses of 0.2, 0.6 and 1.8 mg/egg were given to chick
embryos. Doses of 2, 6 and 18 mg/kg daily were given orally to
pregnant rabbits from day 6 to 16 of gestation. Thalidomide (100 mg/kg
p.o./day) was the positive control in the rabbit study. No
significantly greater incidence of total malformations due to
phosalone were noted in either chick embryos or rabbits (Julou, 1969).
Acute toxicity
The acute toxicity of phosalone has been studied in animals. A summary
of results of these studies is given in Table 2.
TABLE 2 Acute toxicity of phosalone in various animal species
LD50
Species Route (mg/kg) Reference
Dog oral >1 600 Cookrell et al., 1965
Rat (F) oral 153-207 Scott and Beliles, 1965a
(M) oral 125 Fournel et al., 1968
(F) oral 90 Ibid.
Mouse oral 320 Rivett and Davies, 1966
(M) oral 118 Fournel et al., 1968
(F) oral 93 Ibid.
Chicken SC 350 Heath et al., 1967
Signs of poisoning following acute administration of phosalone are the
same as for the other organophosphorus esters and include tremors,
lacrimation and eventually convulsions and death. Signs of poisoning
were evident within 10 to 20 minutes.
An LC50 value of 64 mg/l was calculated following inhalation exposure
of rats (male and female) to phosalone, for one hour at various doses,
having a 3µ particle size (Beliles, 1966). A single dermal application
to the shaved backs of rabbits at doses up to 2 000 mg/kg of a
formulation (equivalent to a dose of phosalone at approximately 800
mg/kg) for 24 hours resulted in no toxicity, but a mild erythema was
noted at 2 000 mg/kg (Horn et al., 1965).
Antidotal studies with rats and mice showed that both atropine and
P2S are effective antidotes to the acute toxic signs of poisoning
following oral administration of phosalone. P2S was apparently more
effective than atropine, although in both cases multiple applications
of antidotal material was suggested to be optimal (Rivett and Davies,
1966).
Short-term studies
Rabbit
Groups of rabbits (5 males and 5 females per group) were administered
phosalone dermally to intact or abraded skin for three weeks, five
days per week, at dosage levels of 1 ml of an emulsifiable
concentrate, 0.05 ml of the E.C. (a dilution of the concentrate) and 1
ml of a control E.C. with no phosalone. Weight loss, signs of toxicity
and death, as well as significant cholinesterase depression, were
apparent with both phosalone treatments to abraded skin. The rabbits
treated with 1 ml of emulsifiable concentrate had a lower liver
glycogen level, and both treated groups incurred renal congestion in
animals with abraded skin. Dermal irritation was evident in all
animals, and gross and histopathological examination showed no effects
on other tissues examined (Woodard et al., 1966b).
Dog
Four groups of dogs (four males and four females per group) were fed
phosalone in the diet for two years at levels of 0, 100, 200, and
1 000 ppm. Effects on cholinesterases were evident at all dose levels
throughout the study. Plasma cholinesterase was depressed at all
feeding levels; RBC cholinesterase was depressed at 200 and 1 000 ppm,
and brain cholinesterase was depressed at 1 000 ppm. One dog fed 1 000
ppm (female) died at 93 weeks, and several others at this level
exhibited fasciculations, nervousness and laboured respiration.
Reduced body-weight was noted in both sexes at 1 000 ppm and in males
at 200 ppm. Soft stools containing mucous and blood were observed for
each treated group, with considerably more frequency at 1 000 ppm.
This effect began to occur at the 100 ppm level after 53 weeks. ECG
changes included an increased T-wave amplitude at 1 000 ppm, which was
more apparent during the second year of study. Increased SGPT and SAP
activity were observed at 1 000 ppm.
Organ to body-weight ratios suggested that at 1 000 ppm the liver of
both sexes and adrenals and thyroid of females were enlarged.
Histological changes observed at all feeding levels were seen as
increased basophilic granules in the myofibrillar cytoplasm in smooth
muscle of the small intestine. These effects were less obvious as the
dose decreased. Vacuolation of the smooth muscle cells of the small
intestine was seen only at 1 000 ppm (Donoso et al., 1967).
Three groups of dogs (four males and four females per group) were fed
phosalone in the diet for 19 weeks at levels of 7.5, 15 and 25 ppm. In
addition, at weeks 14 -19 disyston at 1 ppm was added to the diet of
all animals. No effects on behaviour, growth, clinical chemistry,
urinalysis, biochemistry or cholinesterase activity were noted in this
study which were attributed to phosalone. Histological examination of
the small intestine revealed no abnormalities (Jones et al., 1967b).
Groups of dogs (four males and four females per group) were fed
phosalone in the diet, at levels of 0, 12.5, 25 and 37.5 ppm for one
month. Plasma cholinesterase activity was reduced at 37.5 ppm. In all
dogs at 25 and 37.5 ppm, and in one dog at 12.5 ppm, a pink-mauve
colouration of the mucosa of the bladder was noted although it was not
observed in any other experiment performed in dogs. No effects were
observed on growth, food consumption or RBC and brain cholinesterase
activity (Noel et al., 1970).
Five groups of calves were fed phosalone in the diet for ten weeks at
levels of O, 20, 50, 80 and 160 ppm. No significant effects
attributable to phosalone were observed on growth, behaviour, food
consumption, hematology and cholinesterase activity (Woodard et al.,
1967a).
Long-term studies
Rat
Four groups of rats (30 males and 30 females per group) were fed
phosalone in the diet for two years at levels of 0, 25, 50 and 250
ppm. Effects attributable to phosalone were observed only on plasma
cholinesterase, which was depressed at 50 ppm but not at 25 ppm. No
other effects of treatment, either gross, histopathological or
clinical, were noted at any of the dose levels studied (Woodard
et al., 1967b).
COMMENT
Phosalone is rapidly absorbed, metabolized and excreted by rodents.
Phosalone has been shown to have no effect on rat reproduction, is not
teratogenic in chick or rat and is not neurotoxic to chickens. The
acute toxic effect in rodents was potentiated with several
organo-phosphates, particularly disyston.
Two-year feeding studies in rats and dogs indicate no-effect levels
(based on cholinesterase depression) of 25 ppm for both species.
Bladder discolouration in dogs was reported in a short-term study, but
longer studies did not confirm this observation.
Observations in man are very limited.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 25 ppm in the diet, equivalent to 1.25 mg/kg
body-weight
Dog: 25 ppm in the diet, equivalent to 0.625 mg/kg
body-weight
ESTIMATE FOR ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.006 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phosalone is a non-systemic insecticide and acaricide active by
contact and ingestion. It is officially approved for use in many
countries on a wide variety of crops, and is practically nontoxic to
bees.
Pre-harvest treatments
Phosalone is recommended in a number of countries to control a wide
range of insects and mites on fruit, rape, sugar beet, potato,
alfalfa, cotton, vegetables, grapes, tea and ornamental crops.
Recommended rates of application vary in different countries from 0.4
to 1.2 kg a.i./ha (0.04-0.06% a.i. with high volume sprays at
1 000 - 2 000 l/ha). The recommended interval between two treatments
is, in most countries, two to three weeks and in Canada ten days. The
safety period in the treatment of orchard crops is in Canada, 1 - 7
days; in Great Britain, 3 weeks; in France, 2 weeks; in Switzerland, 3
weeks; in U.S.A., 1 week.
Other uses
Phosalone is used against ectoparasites of animals in some countries.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residue data from supervised trials are given in Table 3. These
are from treatments made in Australia, Canada, France, Great Britain,
New Zealand, South Africa and U.S.A. (Rhône-Poulenc, 1967-1971; May
and Baker Ltd., 1967-1969).
FATE OF RESIDUES
General comments
Studies on the behaviour of phosalone in animals, plants and soil have
shown that phosalone has good stability (generally active against
pests for 15 to 18 days) on plants, but in animals it is rapidly
degraded to nontoxic products; decomposition is also rapid in soil.
In animals
Phosalone is rapidly degraded in animals. One half of the dose
disappears after oral administration in four to six hours and the rest
in 24 hours.
Phosalone may be hydrolysed to nontoxic derivatives or oxidized to the
oxo-derivative, which is responsible for inhibition of
cholinesterases. The oxo-derivative, less stable than phosalone, is
practically never detected, except in the liver where it may be found
in minute quantities.
When beef cattle and sheep were administered levels of 10, 30 and 100
ppm in feed, traces of phosalone were found in fat and liver only at
the feeding level of 100 ppm (Rhodia Inc., 1967a). No phosalone
residues were found in milk of dairy cattle at any of the feeding
levels of 100, 50 or 25 ppm (Rhodia Inc., 1967b; Woodard Research
Corporation, 1967c).
In plants
Degradation of phosalone in plants is shown in Figure 2 (Desmoras
et al., 1968a). The half-life of the residues in different plants is
shown in Table 4. Half-lives vary from 2 to 20 days with application
rates of 0.6 - 1.2 kg a.i./ha. The oxon derivative of phosalone is
found, but its amount has been less than 10% of the total amount of
the parent compound.
In soil
Degradation of phosalone in soils is shown in Figure 3. The half-life
of phosalone in soils was found to be about a week. No accumulation of
phosalone seems to occur in soils.
TABLE 3 Summary of phosalone residue, data from different trials
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
Alfalfa France 0.9 1 13 4 4 - 6 5
0.9 1 20 4 1.7 - 2.9 2.2
U.S.A. 1.7 1 20-27 10 1.4 - 11.6 5.2
1.7 1 11-14 4 24.5 - 37.0 30.6
0.8-1.1 1 14 10 2.2 - 12.6 7.8
0.8-1.1 1 20-23 20 0.8 - 7.6 3.3
Apple Australia 2.0 5-8 17-29 22 0.9 - 3.5 1.5
France 0.4- 0.6 4-7 16-28 20 0.75- z.6 1.5
0.4-0.8 7 28 35 0.9 - 3.8 2.3
1.3-1.5 3-4 14 24 0.9 - 2.9 1.8
0.6 1-8 14-33 24 c.4 - x.0 0.7
0.06% 7-8 21 8 2.1 - 3.6 2.9
" 7 36-64 20 1.3 - 2.4 1.7
" 7 75 4 0.9 - 1.0 1.0
England 0.46-0.69 2-3 59-70 3 0.05-0.49 0.2
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
New Zealand 0.056-0.09% 7-8 22-32 14 0.89-6.2 2.8
South Africa 0.03% 6-7 39-54 36 0.43-2.3 1.1
" 5-7 66-84 36 0.36-1.0 0.7
U.S.A. 0.06% 5 22-36 6 2.2-5.3 4.6
Beets France 0.4-0.6 1 1 8 0.19-1.67 0.6
" 1 3 8 0.20-1.35 0.4
Cabbage 0.4-0.6 1 1 4 4.1 -8.3 6.4
" 2 1 4 0.6 -2.8 1.7
" 6 1 4 0.3 -1.3 0.6
U.S.A. 0.06-0.12% 1 7-8 4 - <0.1
0.12% 5 26 2 - <0.1
Broccoli 0.06% 2 7 2 - 0.6
Brussels
sprouts 0.06% 6 14 2 - 0.7
Cherry Canada 0.03-0.04% 4 8 4 0.8 -4.9 2.8
U.S.A. 0.06% 4 21 2 - 0.1
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
France 0.06% 1 8-18 4 0.3 -0.4 0.4
Chestnut U.S.A. 2-3 1-2 14-78 6 - <0.1
Citrus fruit " 2.5 1 14 18 - <0.1
Cotton France 1 6-13 13-14 12 <0.1 -0.9 0.5
0.5-4 8-12 34-57 12 0.1 -1.6 0.8
Cucumber U.S.A. 0.06% 3 7-28 6 - 0.1
France 0.6-1.2 1 10 8 0.01-0.04 0.02
Grape U.S.A. 1 1-3 28-43 8 1.8 - 2.4 2.0
1.8-3.6 1 14 4 0.5 -0.9 0.7
1 3 59 2 - 0.9
7.2 1 35 2 - 1.3
South Africa 0.032-0.037% 3 41 12 0.4 -2.0 1.1
0.032-0.037% 3 61 12 0.08-1.0 0.3
0.032-0.037% 3 73 12 0.05-0.46 0.2
Canada 0.03-0.06% 5 14 12 1.8 - 4.7 3.0
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
France 0.06% 1 8 4 - 0.3
0.6 1 70 4 - 0.4
Hops U.S.A. 1-1.2 2 28 8 1.2 - 1.7 1.51
<0.12
Lettuce U.S.A. 0.12% 5 7 2 - 2.8
" 5 26 2 - 0.6
France 0.4-0.6 1 6 6 1.6 - 3.7 2.7
Pea U.S.A. 0.06% 1 49 2 - 0.1
France 0.4-0.6 1 1 8 1.3 - 2.8 2.1
" 1 2 8 0.5 -0.95 0.6
" 1 13 8 0.05-0.2 0.12
Peach Canada 0.03-0.045% 5 8 4 1.1 - 2.2 1.9
U.S.A. 0.06% 5 40 2 - 0.9
Australia 2.0 3-5 37-61 20 0.3 - 2.9 1.5
France 0.06% 1 7-14 4 0.7 - 1.9 1.4
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
Pear Australia 0.85 3-5 18-30 8 0.6 - 2.0 1.2
South Africa 0.03% 5-6 26-32 48 0.25- 1.8 0.7
France 0.06% 5 13 8 0.2 - 0.4 0.3
U.S.A. 0.06% 1-7 38-63 6 0.1 - 0.5 0.3
Pecan " 0.56 3-8 60-66 4 - <0.1
" 0.84 1 198 2 - <0.1
" 4.76 5 95 2 - <0.1
Plum Canada 0.03-0.045% 5 16 8 1.3 - 4.1 2.2
U.S.A. 0.06% 1 45-59 4 - 1.3
England 0.03% 1-3 11-26 16 0.22- 1.8 0.7
Potato U.S.A. 0.06% 1-7 7-54 6 - <0.1
Australia 0.06% 9 1 24 - <0.02
France 0.4-0.6 1 7-9 6 - <0.05
Rape seed " 1-2.4 3 77 8 - <0.1
Denmark 0.7-2.05 1 27 16 - <0.1
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
Sorghum U.S.A. 1 2 108 4 - 0.2
Strawberry " 0.06% 1 4-10 8 0.3 - 0.9 0.6
England 0.6 2 26 6 0.04-0.08 0.07
Tomato U.S.A. 0.6-0.12 1-4 14-15 6 <0.1 -0.6 0.3
France 0.4 -0.6 1 1 8 0.30-0.7 0.48
0.4 -0.6 1 3 8 0.15-0.7 0.23
Wheat " 0.6 1 25-44 6 <0.05-1 0.3
1 fresh
2 dried 3 days
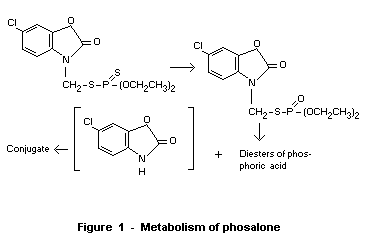
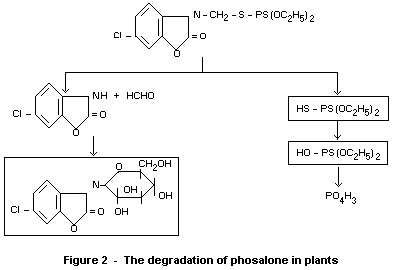 TABLE 4 Half-lives of phosalone residues in growing plants
Crop Application rate Half-life
(kg a.i./ha) (days)
Alfalfa 0.9 - 1.2 4 - 10
Apple 0.3 - 5.4 7 - 15
Cabbage 0.6 - 2.4 2 - 5
Cotton 7.2 - 13.8 4 - 6
Cherry 3.6 4 - 10
Grape 0.6 - 1.2 7 - 17
Lettuce 0.6 - 1.2 3 - 8
Peach 0.5 - 3.0 7 - 20
Pear 0.6 - 0.9 8 - 15
Plum 5.4 11 - 17
Strawberry 0.6 4 - 8
In storage and processing
There is no reliable data on the fate of phosalone in storage and
processing of foodstuffs.
METHODS OF RESIDUE ANALYSIS
Desmoras et al. (1968d) have described procedures for the analysis
of technical phosalone and its formulated products, and of residues in
crops, vegetable oils and soil. A colorimetric method is based on
hydrolysis to diethyldithiophosphoric acid followed by formation of
the cupric complex. The preferred procedures are gas chromatography
using catharometer detection and an ester as an internal standard for
macro-analysis and electron capture detection for residue
determinations; the use of a phosphorus sensitive thermionic detector
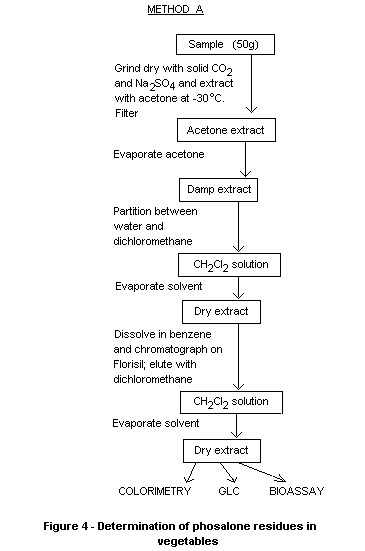
gave poorer sensitivity. The outlines of the schemes for vegetable
sample preparation are shown in Figures 4 and 5, the extracts being
suitable for colorimetry gas chromatography or for a bioassay
procedure using Daphnia or mosquito larvae. Variations are described
for analysis of olives, olive oil and rape oil, while a simple
extraction with acetone was suitable for soil samples. Method B with
gas chromatographic end determination should be suitable for
regulatory purposes.
Other similar multiresidue GLC procedures should also be suitable for
determining phosalone residues; that described by Abbott et al.
(1970) has been found to be applicable to fatty samples.
NATIONAL TOLERANCES
Examples of national tolerances as reported to the Meeting are given
in Table 5.
TABLE 5
Examples of national tolerances as reported to the meeting
Country Tolerance Commodity
ppm
Canada 10 apples, pears
4 peaches
6 cherries
South Africa 2 apples, pears
Switzerland 2 grapes, fruit
U.S.A. 10 apples, pears, grapes
20 raisins
Australia 3 peaches
2.5 apples, pears
1 fat of meat of sheep
APPRAISAL
Phosalone is a non-systemic dithiophosphate insecticide and acaricide
used for pre-harvest control of a wide range of pests in agriculture,
horticulture, ornamentals and vine-growing. It has limited use also
against ectoparasites of animals. Its pesticidal activity on plants
has a duration of two to three weeks, depending on the type of pest.
The residues of potential toxicity are composed of the parent compound
and, only to a minor extent, of the oxon derivative of phosalone. It
is readily decomposed in animals and soils. Practically no
contamination of animal tissues occurs as a result of feeding animals
with fodder crops containing phosalone residues. Residue data of
supervised trials on plants were available from many countries. No
data were available on residues resulting from control of
ectoparasites on animals, on fate of residues in food storage and
processing, on levels of residues in foodstuffs moving in commerce or
in total diets. Gas chromatographic procedures are the analytical
methods of choice for use in regulatory laboratories. The limit of
determination is at or about 0.1 ppm depending on the type of crop.
TABLE 4 Half-lives of phosalone residues in growing plants
Crop Application rate Half-life
(kg a.i./ha) (days)
Alfalfa 0.9 - 1.2 4 - 10
Apple 0.3 - 5.4 7 - 15
Cabbage 0.6 - 2.4 2 - 5
Cotton 7.2 - 13.8 4 - 6
Cherry 3.6 4 - 10
Grape 0.6 - 1.2 7 - 17
Lettuce 0.6 - 1.2 3 - 8
Peach 0.5 - 3.0 7 - 20
Pear 0.6 - 0.9 8 - 15
Plum 5.4 11 - 17
Strawberry 0.6 4 - 8
In storage and processing
There is no reliable data on the fate of phosalone in storage and
processing of foodstuffs.
METHODS OF RESIDUE ANALYSIS
Desmoras et al. (1968d) have described procedures for the analysis
of technical phosalone and its formulated products, and of residues in
crops, vegetable oils and soil. A colorimetric method is based on
hydrolysis to diethyldithiophosphoric acid followed by formation of
the cupric complex. The preferred procedures are gas chromatography
using catharometer detection and an ester as an internal standard for
macro-analysis and electron capture detection for residue
determinations; the use of a phosphorus sensitive thermionic detector
gave poorer sensitivity. The outlines of the schemes for vegetable
sample preparation are shown in Figures 4 and 5, the extracts being
suitable for colorimetry gas chromatography or for a bioassay
procedure using Daphnia or mosquito larvae. Variations are described
for analysis of olives, olive oil and rape oil, while a simple
extraction with acetone was suitable for soil samples. Method B with
gas chromatographic end determination should be suitable for
regulatory purposes.
Other similar multiresidue GLC procedures should also be suitable for
determining phosalone residues; that described by Abbott et al.
(1970) has been found to be applicable to fatty samples.
NATIONAL TOLERANCES
Examples of national tolerances as reported to the Meeting are given
in Table 5.
TABLE 5
Examples of national tolerances as reported to the meeting
Country Tolerance Commodity
ppm
Canada 10 apples, pears
4 peaches
6 cherries
South Africa 2 apples, pears
Switzerland 2 grapes, fruit
U.S.A. 10 apples, pears, grapes
20 raisins
Australia 3 peaches
2.5 apples, pears
1 fat of meat of sheep
APPRAISAL
Phosalone is a non-systemic dithiophosphate insecticide and acaricide
used for pre-harvest control of a wide range of pests in agriculture,
horticulture, ornamentals and vine-growing. It has limited use also
against ectoparasites of animals. Its pesticidal activity on plants
has a duration of two to three weeks, depending on the type of pest.
The residues of potential toxicity are composed of the parent compound
and, only to a minor extent, of the oxon derivative of phosalone. It
is readily decomposed in animals and soils. Practically no
contamination of animal tissues occurs as a result of feeding animals
with fodder crops containing phosalone residues. Residue data of
supervised trials on plants were available from many countries. No
data were available on residues resulting from control of
ectoparasites on animals, on fate of residues in food storage and
processing, on levels of residues in foodstuffs moving in commerce or
in total diets. Gas chromatographic procedures are the analytical
methods of choice for use in regulatory laboratories. The limit of
determination is at or about 0.1 ppm depending on the type of crop.
 RECOMMENDATIONS
TOLERANCES
Tolerances recommended apply to the parent compound. The time interval
between application and harvest which has been used in determining the
maximum residue limit is appropriate to the agricultural practices in
numerous countries.
Time interval
between application
Crop Ppm and harvest (weeks)
Apple, grape, peach, plum 5 2 - 3
Cherry, pear, beetroots 2 2
Hops (dried) 2 4
Citrus fruit, strawberry,
tomato, cabbage, broccoli,
Brussels sprouts, cucumber,
lettuce, peas 1 1 - 2
Chestnut (shelled), pecan
(shelled), potato, rapeseed 0.1* 2
* at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Studies on human exposure.
2. A study to determine dose levels causing no carboxylesterase
(aliesterase) activity depression.
3. Effect of food storage and processing on residues.
4. Data on residues occurring in food commodities moving in
commerce.
5. Nature and concentrations of impurities of the technical product.
6. Information on the use pattern of phosalone against ectoparasites
of domestic animals and data on residues in animal products
resulting from this use.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales 1966-67. III
- Organophosphorus pesticide residues in the total diet. Pestic. Sci.,
1: 10-13.
Beliles, R.P. (1966) Phosalone (R.P. 11 974) - Safety evaluation by
acute inhalation exposure of rats. Report Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Cockrell, K.O., Woodard, M.W. and Woodard, G. (1965) Phosalone R.P. 11
974 - Acute oral LD50 in dogs. Report Woodard Research Corporation,
submitted by Rhône-Poulenc. (unpublished)
Desmoras, J., LaCroix, L. and Metivier, J. (1963) Pesticides of the
benzoxazolone family. Phytiat.-Phytopharm., 12: 199-215.
Desmoras, J. and Fournel, J. (1968) Studies on degradation of
phosalone in mammals. Report Rhône-Poulenc. (unpublished)
Desmoras, J., Fournell, J., Laurent, M., Sauli, M. and Terlain, B.
(1968a) Etude de la vitesse de degradation de la phosalone dans de
sal, les plantes et les animaux. Report no. 12964 Rhône-Poulenc.
Desmoras, J., Laurent, M., Petrinko, P. and Buys, M. (1968b) Dosage de
residus dans les cultures maraicheres. Report no. 13322 Rhône-Poulenc.
Desmoras, J., Laurent, M., Sauli, M. and Terlain, B. (1968c) Etude du
metabolisme de la phosalone dans les plantes et les sals. Report no.
13093 Rhône-Poulenc.
Desmoras, J., Luborg, F., Laurent, M., Bales, I.W. and Guardigli, A.
(1968d) Application to the determination in the technical material.
Phytiat.-Phytopharm. 4: 277-292.
Desmoras, J., Laurent, M. and Buys, M. (1970a) Dosage de residus dans
des pommes. Report no. 14899 Rhône-Poulenc.
Desmoras, J., Laurent, M. and Buys, M. (1970b) Dosage de residus sur
pommès en prorenance des Pays-bas. Report no. 14986 Rhône-Poulenc.
Donoso, J., Woodard, M.W. and Woodard, G. (1967) Phosalone - Safety
evaluation by repeated administration to dogs for 107 weeks. Report by
Woodard Research Corporation, submitted by Rhône-Poulenc.
(unpublished)
Fournel, J., Julou, L. and Pasquet, J. (1968) Acute toxicity in mice
and rats and in vivo anticholinesterase activity in rats. Report
Rhône-Poulenc. (unpublished)
Heath, S.A.B., Rivett, K.F. and Woolf, N. (1967) Test for
neurotoxicity. Report May-Baker Ltd., submitted by Rhône-Poulenc.
(unpublished)
Horn, J.H., Woodard, M.W. and Woodard, G. (1965) Phosalone (R.P. 11
974) acute dermal toxicity for rabbits. Report by Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Jones, M.E., Post, K.F., Scott, W.J., Woodard, M.W. and Woodard, G.
(1967a) Three generation reproduction study in the rat. Report by
Woodard Research Corporation, submitted by Rhône-Poulenc.
(unpublished)
Jones, M.E., Imming, R.J., Woodard, M.W. and Woodard, G. (1967b)
Phosalone followed by a determination of potentiation activity with
the compound disyston.
Julou, L. (1969) Study of the teratogenic activity of phosalone on
chick embryo and rabbit. Report Rhône-Poulenc. (unpublished)
May and Baker Ltd. (1967a) Report no. C. 263. (unpublished)
May and Baker Ltd. (1967b) Report no. C. 274. (unpublished)
May and Baker Ltd. (1967c) Report no. C. 277. (unpublished)
May and Baker Ltd. (1968a) Report no. PRG./85. (unpublished)
May and Baker Ltd. (1968b) Report no. PRG./88. (unpublished)
May and Baker Ltd. (1968c) Report no. PRG./162. (unpublished)
May and Baker Ltd. (1968d) Report no. PRG./171. (unpublished)
May and Baker Ltd. (1968e) Report no. PRG./173. (unpublished)
May and Baker Ltd. (1968f) Report no. PRG./310. (unpublished)
May and Baker Ltd. (1969a) Report no. PRG./425. (unpublished)
May and Baker Ltd. (1969b) Report no. PRG./534. (unpublished)
Noel, P.R.B., Rivett, K.F., Osborne, G.E. and Street, A.E. (1970)
Phosalone dietary intake in Beagle dogs for 4 weeks. Report by
Huntingdon Research Centre, submitted by Rhône-Poulenc. (unpublished)
Rhodia Inc. (1967a) Results for phosalone residue in tissues of beef
cattle and sheep concerning blood plasma. (unpublished)
Rhodia Inc. (1967b) Results for phosalone in milk of dairy
cattle - Woodard Research Centre Project. (unpublished)
Rhône-Poulenc. (1967) Report no. 12433. (unpublished)
Rhône-Poulenc. (1968a) The final metabolite of phosalone in plants.
Synthesis, identification, toxicity, possible presence in plants.
(unpublished)
Rhône-Poulenc. (1968b) Oxygen analogue of phosalone. Acute oral
toxicity, and anticholinesterase activity in vitro. (unpublished)
Rhône-Poulenc. (1968c) Report no. 13018. (unpublished)
Rhône-Poulenc. (1969a) Report no. 13551. (unpublished)
Rhône-Poulenc. (1969b) Report no. 13553. (unpublished)
Rhône-Poulenc. (1970) Report no. 14356. (unpublished)
Rhône-Poulenc. (1971a) Report no. 15209. (unpublished)
Rhône-Poulenc. (1971b) Report no. 15210. (unpublished)
Rhône-Poulenc. (undated) Zolone, phosalone (with 27 references
concerning phosalone).
Rivett, K.F. and Davies, V. (1966) Effects of antidotes against
poisoning by phosalone. Report by May and Baker, submitted by
Rhône-Poulenc. (unpublished)
Scott, W.J. and Bililes, R.P. (1965a) Acute oral toxicity of phosalone
(R.P. 11 974) formulation to rats. Report by Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Scott, W.J. and Beliles, R.P. (1965b) Potentiation studies in the rat
with marketed pesticides. Report by Woodard Research Corporation,
submitted by Rhône-Poulenc. (unpublished)
Woodard, M.W., Cockrell, K.O. and Woodard, G. (1966a) Demyelination
study in chickens. Report by Woodard Research Corporation, submitted
by Rhône-Poulenc. (unpublished)
Woodard, M.W., Cockrell, K.O. and Woodard, G. (1966b) Sub-acute dermal
toxicity of phosalone for the rabbit. Report of Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Woodard, M.W., Imming, R.J., Thompson, W. and Woodard, G. (1967a)
Phosalone, safety evaluation by oral administration to calves for 10
weeks. Report by Woodard Research Corporation, submitted by
Rhône-Poulenc. (unpublished)
Woodard, M.W., Howard, D.J., Donoso, J. and Woodard, G. (1967b) Safety
evaluation by repeated oral administration to rats for 103 weeks.
Report by Woodard Research Corporation, submitted by Rhône-Poulenc.
(unpublished)
Woodard Research Corporation. (1967c) Phosalone analysis of residue in
milk of dairy cattle.
RECOMMENDATIONS
TOLERANCES
Tolerances recommended apply to the parent compound. The time interval
between application and harvest which has been used in determining the
maximum residue limit is appropriate to the agricultural practices in
numerous countries.
Time interval
between application
Crop Ppm and harvest (weeks)
Apple, grape, peach, plum 5 2 - 3
Cherry, pear, beetroots 2 2
Hops (dried) 2 4
Citrus fruit, strawberry,
tomato, cabbage, broccoli,
Brussels sprouts, cucumber,
lettuce, peas 1 1 - 2
Chestnut (shelled), pecan
(shelled), potato, rapeseed 0.1* 2
* at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Studies on human exposure.
2. A study to determine dose levels causing no carboxylesterase
(aliesterase) activity depression.
3. Effect of food storage and processing on residues.
4. Data on residues occurring in food commodities moving in
commerce.
5. Nature and concentrations of impurities of the technical product.
6. Information on the use pattern of phosalone against ectoparasites
of domestic animals and data on residues in animal products
resulting from this use.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales 1966-67. III
- Organophosphorus pesticide residues in the total diet. Pestic. Sci.,
1: 10-13.
Beliles, R.P. (1966) Phosalone (R.P. 11 974) - Safety evaluation by
acute inhalation exposure of rats. Report Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Cockrell, K.O., Woodard, M.W. and Woodard, G. (1965) Phosalone R.P. 11
974 - Acute oral LD50 in dogs. Report Woodard Research Corporation,
submitted by Rhône-Poulenc. (unpublished)
Desmoras, J., LaCroix, L. and Metivier, J. (1963) Pesticides of the
benzoxazolone family. Phytiat.-Phytopharm., 12: 199-215.
Desmoras, J. and Fournel, J. (1968) Studies on degradation of
phosalone in mammals. Report Rhône-Poulenc. (unpublished)
Desmoras, J., Fournell, J., Laurent, M., Sauli, M. and Terlain, B.
(1968a) Etude de la vitesse de degradation de la phosalone dans de
sal, les plantes et les animaux. Report no. 12964 Rhône-Poulenc.
Desmoras, J., Laurent, M., Petrinko, P. and Buys, M. (1968b) Dosage de
residus dans les cultures maraicheres. Report no. 13322 Rhône-Poulenc.
Desmoras, J., Laurent, M., Sauli, M. and Terlain, B. (1968c) Etude du
metabolisme de la phosalone dans les plantes et les sals. Report no.
13093 Rhône-Poulenc.
Desmoras, J., Luborg, F., Laurent, M., Bales, I.W. and Guardigli, A.
(1968d) Application to the determination in the technical material.
Phytiat.-Phytopharm. 4: 277-292.
Desmoras, J., Laurent, M. and Buys, M. (1970a) Dosage de residus dans
des pommes. Report no. 14899 Rhône-Poulenc.
Desmoras, J., Laurent, M. and Buys, M. (1970b) Dosage de residus sur
pommès en prorenance des Pays-bas. Report no. 14986 Rhône-Poulenc.
Donoso, J., Woodard, M.W. and Woodard, G. (1967) Phosalone - Safety
evaluation by repeated administration to dogs for 107 weeks. Report by
Woodard Research Corporation, submitted by Rhône-Poulenc.
(unpublished)
Fournel, J., Julou, L. and Pasquet, J. (1968) Acute toxicity in mice
and rats and in vivo anticholinesterase activity in rats. Report
Rhône-Poulenc. (unpublished)
Heath, S.A.B., Rivett, K.F. and Woolf, N. (1967) Test for
neurotoxicity. Report May-Baker Ltd., submitted by Rhône-Poulenc.
(unpublished)
Horn, J.H., Woodard, M.W. and Woodard, G. (1965) Phosalone (R.P. 11
974) acute dermal toxicity for rabbits. Report by Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Jones, M.E., Post, K.F., Scott, W.J., Woodard, M.W. and Woodard, G.
(1967a) Three generation reproduction study in the rat. Report by
Woodard Research Corporation, submitted by Rhône-Poulenc.
(unpublished)
Jones, M.E., Imming, R.J., Woodard, M.W. and Woodard, G. (1967b)
Phosalone followed by a determination of potentiation activity with
the compound disyston.
Julou, L. (1969) Study of the teratogenic activity of phosalone on
chick embryo and rabbit. Report Rhône-Poulenc. (unpublished)
May and Baker Ltd. (1967a) Report no. C. 263. (unpublished)
May and Baker Ltd. (1967b) Report no. C. 274. (unpublished)
May and Baker Ltd. (1967c) Report no. C. 277. (unpublished)
May and Baker Ltd. (1968a) Report no. PRG./85. (unpublished)
May and Baker Ltd. (1968b) Report no. PRG./88. (unpublished)
May and Baker Ltd. (1968c) Report no. PRG./162. (unpublished)
May and Baker Ltd. (1968d) Report no. PRG./171. (unpublished)
May and Baker Ltd. (1968e) Report no. PRG./173. (unpublished)
May and Baker Ltd. (1968f) Report no. PRG./310. (unpublished)
May and Baker Ltd. (1969a) Report no. PRG./425. (unpublished)
May and Baker Ltd. (1969b) Report no. PRG./534. (unpublished)
Noel, P.R.B., Rivett, K.F., Osborne, G.E. and Street, A.E. (1970)
Phosalone dietary intake in Beagle dogs for 4 weeks. Report by
Huntingdon Research Centre, submitted by Rhône-Poulenc. (unpublished)
Rhodia Inc. (1967a) Results for phosalone residue in tissues of beef
cattle and sheep concerning blood plasma. (unpublished)
Rhodia Inc. (1967b) Results for phosalone in milk of dairy
cattle - Woodard Research Centre Project. (unpublished)
Rhône-Poulenc. (1967) Report no. 12433. (unpublished)
Rhône-Poulenc. (1968a) The final metabolite of phosalone in plants.
Synthesis, identification, toxicity, possible presence in plants.
(unpublished)
Rhône-Poulenc. (1968b) Oxygen analogue of phosalone. Acute oral
toxicity, and anticholinesterase activity in vitro. (unpublished)
Rhône-Poulenc. (1968c) Report no. 13018. (unpublished)
Rhône-Poulenc. (1969a) Report no. 13551. (unpublished)
Rhône-Poulenc. (1969b) Report no. 13553. (unpublished)
Rhône-Poulenc. (1970) Report no. 14356. (unpublished)
Rhône-Poulenc. (1971a) Report no. 15209. (unpublished)
Rhône-Poulenc. (1971b) Report no. 15210. (unpublished)
Rhône-Poulenc. (undated) Zolone, phosalone (with 27 references
concerning phosalone).
Rivett, K.F. and Davies, V. (1966) Effects of antidotes against
poisoning by phosalone. Report by May and Baker, submitted by
Rhône-Poulenc. (unpublished)
Scott, W.J. and Bililes, R.P. (1965a) Acute oral toxicity of phosalone
(R.P. 11 974) formulation to rats. Report by Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Scott, W.J. and Beliles, R.P. (1965b) Potentiation studies in the rat
with marketed pesticides. Report by Woodard Research Corporation,
submitted by Rhône-Poulenc. (unpublished)
Woodard, M.W., Cockrell, K.O. and Woodard, G. (1966a) Demyelination
study in chickens. Report by Woodard Research Corporation, submitted
by Rhône-Poulenc. (unpublished)
Woodard, M.W., Cockrell, K.O. and Woodard, G. (1966b) Sub-acute dermal
toxicity of phosalone for the rabbit. Report of Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Woodard, M.W., Imming, R.J., Thompson, W. and Woodard, G. (1967a)
Phosalone, safety evaluation by oral administration to calves for 10
weeks. Report by Woodard Research Corporation, submitted by
Rhône-Poulenc. (unpublished)
Woodard, M.W., Howard, D.J., Donoso, J. and Woodard, G. (1967b) Safety
evaluation by repeated oral administration to rats for 103 weeks.
Report by Woodard Research Corporation, submitted by Rhône-Poulenc.
(unpublished)
Woodard Research Corporation. (1967c) Phosalone analysis of residue in
milk of dairy cattle.
 Other information on identity and properties
Physical state: non-hygroscopic crystalline solid
Colour: white
Odour: alliaceous
Melting point: 45 - 48°C
Solubility: in water at 20°C is about 0.01 g/l, in
methanol and ethanol about 200 g/l, in other
organic solvents (acetone, acetonitrile,
benzene, chloroform, cyclohexanone, dioxane,
ethyl acetate, methylene chloride, methyl-
thylketone, toluene, xylene) about 1 000 g/l
Stability: of phosalone and its formulations, good
Purity: the technical product contains 93% active
ingredient
Formulations: wettable powders and emulsifiable
concentrates.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Phosalone is rapidly absorbed and excreted by mice following oral
administration. Within 24 hours, less than 1% phosalone residues were
found in the body (Desmoras and Fournel, 1968).
Biotransformation
Phosalone, an organophosphorus ester, exhibiting a weak potential for
inhibiting cholinesterase, is converted to a more potent oxygen
analogue. This analogue is 2-3 times more active as an inhibitory
agent than phosalone, especially to serum cholinesterase
(Rhône-Poulenc, 1968b).
The metabolic fate of phosalone appears to follow a pattern typical of
phosphorodithioate esters, i.e., oxidation to the phosphate,
hydrolysis and conjugation of the leaving group and elimination, as
shown in Figure 1.
The major conjugate in plants has been described as the glucose
conjugate of the benzoxazol moiety (1-[6-chloro-1,3-benzoxazol-
(3H)-onyl] glucopyranose). This metabolite has an acute toxicity in
mice of greater than 4 gm/kg (Rhône-Poulenc, 1968a).
TOXICOLOGICAL STUDIES
Special studies on metabolites
Acute toxicity studies in rats and mice show the oxygen analogue of
phosalone to be 2-3 times more toxic than phosalone (see Table 1).
TABLE 1
Acute toxicity of the oxygen analogue of phosalone
LD50 (mg/kg)
Sex Rats Mice
M 36 35
F 20 40
(Rhône-Poulenc, 1968b; Desmoras et al., 1963)
Other information on identity and properties
Physical state: non-hygroscopic crystalline solid
Colour: white
Odour: alliaceous
Melting point: 45 - 48°C
Solubility: in water at 20°C is about 0.01 g/l, in
methanol and ethanol about 200 g/l, in other
organic solvents (acetone, acetonitrile,
benzene, chloroform, cyclohexanone, dioxane,
ethyl acetate, methylene chloride, methyl-
thylketone, toluene, xylene) about 1 000 g/l
Stability: of phosalone and its formulations, good
Purity: the technical product contains 93% active
ingredient
Formulations: wettable powders and emulsifiable
concentrates.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Phosalone is rapidly absorbed and excreted by mice following oral
administration. Within 24 hours, less than 1% phosalone residues were
found in the body (Desmoras and Fournel, 1968).
Biotransformation
Phosalone, an organophosphorus ester, exhibiting a weak potential for
inhibiting cholinesterase, is converted to a more potent oxygen
analogue. This analogue is 2-3 times more active as an inhibitory
agent than phosalone, especially to serum cholinesterase
(Rhône-Poulenc, 1968b).
The metabolic fate of phosalone appears to follow a pattern typical of
phosphorodithioate esters, i.e., oxidation to the phosphate,
hydrolysis and conjugation of the leaving group and elimination, as
shown in Figure 1.
The major conjugate in plants has been described as the glucose
conjugate of the benzoxazol moiety (1-[6-chloro-1,3-benzoxazol-
(3H)-onyl] glucopyranose). This metabolite has an acute toxicity in
mice of greater than 4 gm/kg (Rhône-Poulenc, 1968a).
TOXICOLOGICAL STUDIES
Special studies on metabolites
Acute toxicity studies in rats and mice show the oxygen analogue of
phosalone to be 2-3 times more toxic than phosalone (see Table 1).
TABLE 1
Acute toxicity of the oxygen analogue of phosalone
LD50 (mg/kg)
Sex Rats Mice
M 36 35
F 20 40
(Rhône-Poulenc, 1968b; Desmoras et al., 1963)
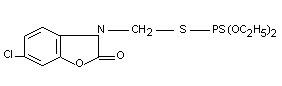 Special studies on neurotoxicity
Adult White Leghorn hens (ten per group) were fed phosalone at levels
of 0, 50, 163 and 500 ppm in the diet for 45 days. Another group was
fed tri-o-cresyl phosphate (TOCP) at 500 ppm. Phosalone at 163 and 500
ppm reduced egg production. No clinical or histological evidence of
paralysis or demyelination was observed. TOCP dietary exposure
resulted in signs of slight paralysis and evidence of minor central
and peripheral nerve degeneration, as determined histologically
(Woodard et al., 1966a).
A group of Rhode Island Red Hens (ten birds) was administered
phosalone subcutaneously at a dose of 350 mg/kg initially and after
three weeks. The birds were administered atropine and P2S to protect
against cholinergic stimulation. Mipafox (16 mg/kg) was administered
subcutaneously as a positive control to four hens. No clinical or
histological evidence in spinal cord or sciatic nerve of delayed
neurotoxicity was evident with phosalone. Mipafox paralyzed all hens
and induced demyelination in spinal cord or sciatic nerve (Heath
et al., 1967).
Special studies on potentiation
Phosalone, in combination with other anticholinesterase agents, was
examined for possible potentiation effects by administration of up to
one-half of the oral LD50 of each material. Disulfaton potentiated
the acute effects of phosalone. The toxicity of malathion and
coumaphos was potentiated to a lesser extent, while potentiation did
not occur with 15 other compounds, including: carbophenothion,
demeton, disyston, diazinon, trichlorfon, azinphos-methyl, dioxathion,
EPN, ethion, mevinphos, parathion-methyl, fenchlorfos, shradan,
merphos and carbaryl (Scott and Beliles, 1965b).
Special studies on reproduction
Three groups of rats (20 females and 10 males per group) were fed
phosalone at 0, 25 and 50 ppm in the diet and subjected to a standard
two-litter, three-generation reproduction study. There were no effects
on reproduction and lactation indices noted in this study which were
attributed to phosalone (Jones et al., 1967a).
Special studies on teratogenicity
Teratogenicity tests were conducted with phosalone in chick embryos
and rabbits. Doses of 0.2, 0.6 and 1.8 mg/egg were given to chick
embryos. Doses of 2, 6 and 18 mg/kg daily were given orally to
pregnant rabbits from day 6 to 16 of gestation. Thalidomide (100 mg/kg
p.o./day) was the positive control in the rabbit study. No
significantly greater incidence of total malformations due to
phosalone were noted in either chick embryos or rabbits (Julou, 1969).
Acute toxicity
The acute toxicity of phosalone has been studied in animals. A summary
of results of these studies is given in Table 2.
TABLE 2 Acute toxicity of phosalone in various animal species
LD50
Species Route (mg/kg) Reference
Dog oral >1 600 Cookrell et al., 1965
Rat (F) oral 153-207 Scott and Beliles, 1965a
(M) oral 125 Fournel et al., 1968
(F) oral 90 Ibid.
Mouse oral 320 Rivett and Davies, 1966
(M) oral 118 Fournel et al., 1968
(F) oral 93 Ibid.
Chicken SC 350 Heath et al., 1967
Signs of poisoning following acute administration of phosalone are the
same as for the other organophosphorus esters and include tremors,
lacrimation and eventually convulsions and death. Signs of poisoning
were evident within 10 to 20 minutes.
An LC50 value of 64 mg/l was calculated following inhalation exposure
of rats (male and female) to phosalone, for one hour at various doses,
having a 3µ particle size (Beliles, 1966). A single dermal application
to the shaved backs of rabbits at doses up to 2 000 mg/kg of a
formulation (equivalent to a dose of phosalone at approximately 800
mg/kg) for 24 hours resulted in no toxicity, but a mild erythema was
noted at 2 000 mg/kg (Horn et al., 1965).
Antidotal studies with rats and mice showed that both atropine and
P2S are effective antidotes to the acute toxic signs of poisoning
following oral administration of phosalone. P2S was apparently more
effective than atropine, although in both cases multiple applications
of antidotal material was suggested to be optimal (Rivett and Davies,
1966).
Short-term studies
Rabbit
Groups of rabbits (5 males and 5 females per group) were administered
phosalone dermally to intact or abraded skin for three weeks, five
days per week, at dosage levels of 1 ml of an emulsifiable
concentrate, 0.05 ml of the E.C. (a dilution of the concentrate) and 1
ml of a control E.C. with no phosalone. Weight loss, signs of toxicity
and death, as well as significant cholinesterase depression, were
apparent with both phosalone treatments to abraded skin. The rabbits
treated with 1 ml of emulsifiable concentrate had a lower liver
glycogen level, and both treated groups incurred renal congestion in
animals with abraded skin. Dermal irritation was evident in all
animals, and gross and histopathological examination showed no effects
on other tissues examined (Woodard et al., 1966b).
Dog
Four groups of dogs (four males and four females per group) were fed
phosalone in the diet for two years at levels of 0, 100, 200, and
1 000 ppm. Effects on cholinesterases were evident at all dose levels
throughout the study. Plasma cholinesterase was depressed at all
feeding levels; RBC cholinesterase was depressed at 200 and 1 000 ppm,
and brain cholinesterase was depressed at 1 000 ppm. One dog fed 1 000
ppm (female) died at 93 weeks, and several others at this level
exhibited fasciculations, nervousness and laboured respiration.
Reduced body-weight was noted in both sexes at 1 000 ppm and in males
at 200 ppm. Soft stools containing mucous and blood were observed for
each treated group, with considerably more frequency at 1 000 ppm.
This effect began to occur at the 100 ppm level after 53 weeks. ECG
changes included an increased T-wave amplitude at 1 000 ppm, which was
more apparent during the second year of study. Increased SGPT and SAP
activity were observed at 1 000 ppm.
Organ to body-weight ratios suggested that at 1 000 ppm the liver of
both sexes and adrenals and thyroid of females were enlarged.
Histological changes observed at all feeding levels were seen as
increased basophilic granules in the myofibrillar cytoplasm in smooth
muscle of the small intestine. These effects were less obvious as the
dose decreased. Vacuolation of the smooth muscle cells of the small
intestine was seen only at 1 000 ppm (Donoso et al., 1967).
Three groups of dogs (four males and four females per group) were fed
phosalone in the diet for 19 weeks at levels of 7.5, 15 and 25 ppm. In
addition, at weeks 14 -19 disyston at 1 ppm was added to the diet of
all animals. No effects on behaviour, growth, clinical chemistry,
urinalysis, biochemistry or cholinesterase activity were noted in this
study which were attributed to phosalone. Histological examination of
the small intestine revealed no abnormalities (Jones et al., 1967b).
Groups of dogs (four males and four females per group) were fed
phosalone in the diet, at levels of 0, 12.5, 25 and 37.5 ppm for one
month. Plasma cholinesterase activity was reduced at 37.5 ppm. In all
dogs at 25 and 37.5 ppm, and in one dog at 12.5 ppm, a pink-mauve
colouration of the mucosa of the bladder was noted although it was not
observed in any other experiment performed in dogs. No effects were
observed on growth, food consumption or RBC and brain cholinesterase
activity (Noel et al., 1970).
Five groups of calves were fed phosalone in the diet for ten weeks at
levels of O, 20, 50, 80 and 160 ppm. No significant effects
attributable to phosalone were observed on growth, behaviour, food
consumption, hematology and cholinesterase activity (Woodard et al.,
1967a).
Long-term studies
Rat
Four groups of rats (30 males and 30 females per group) were fed
phosalone in the diet for two years at levels of 0, 25, 50 and 250
ppm. Effects attributable to phosalone were observed only on plasma
cholinesterase, which was depressed at 50 ppm but not at 25 ppm. No
other effects of treatment, either gross, histopathological or
clinical, were noted at any of the dose levels studied (Woodard
et al., 1967b).
COMMENT
Phosalone is rapidly absorbed, metabolized and excreted by rodents.
Phosalone has been shown to have no effect on rat reproduction, is not
teratogenic in chick or rat and is not neurotoxic to chickens. The
acute toxic effect in rodents was potentiated with several
organo-phosphates, particularly disyston.
Two-year feeding studies in rats and dogs indicate no-effect levels
(based on cholinesterase depression) of 25 ppm for both species.
Bladder discolouration in dogs was reported in a short-term study, but
longer studies did not confirm this observation.
Observations in man are very limited.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 25 ppm in the diet, equivalent to 1.25 mg/kg
body-weight
Dog: 25 ppm in the diet, equivalent to 0.625 mg/kg
body-weight
ESTIMATE FOR ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.006 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phosalone is a non-systemic insecticide and acaricide active by
contact and ingestion. It is officially approved for use in many
countries on a wide variety of crops, and is practically nontoxic to
bees.
Pre-harvest treatments
Phosalone is recommended in a number of countries to control a wide
range of insects and mites on fruit, rape, sugar beet, potato,
alfalfa, cotton, vegetables, grapes, tea and ornamental crops.
Recommended rates of application vary in different countries from 0.4
to 1.2 kg a.i./ha (0.04-0.06% a.i. with high volume sprays at
1 000 - 2 000 l/ha). The recommended interval between two treatments
is, in most countries, two to three weeks and in Canada ten days. The
safety period in the treatment of orchard crops is in Canada, 1 - 7
days; in Great Britain, 3 weeks; in France, 2 weeks; in Switzerland, 3
weeks; in U.S.A., 1 week.
Other uses
Phosalone is used against ectoparasites of animals in some countries.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residue data from supervised trials are given in Table 3. These
are from treatments made in Australia, Canada, France, Great Britain,
New Zealand, South Africa and U.S.A. (Rhône-Poulenc, 1967-1971; May
and Baker Ltd., 1967-1969).
FATE OF RESIDUES
General comments
Studies on the behaviour of phosalone in animals, plants and soil have
shown that phosalone has good stability (generally active against
pests for 15 to 18 days) on plants, but in animals it is rapidly
degraded to nontoxic products; decomposition is also rapid in soil.
In animals
Phosalone is rapidly degraded in animals. One half of the dose
disappears after oral administration in four to six hours and the rest
in 24 hours.
Phosalone may be hydrolysed to nontoxic derivatives or oxidized to the
oxo-derivative, which is responsible for inhibition of
cholinesterases. The oxo-derivative, less stable than phosalone, is
practically never detected, except in the liver where it may be found
in minute quantities.
When beef cattle and sheep were administered levels of 10, 30 and 100
ppm in feed, traces of phosalone were found in fat and liver only at
the feeding level of 100 ppm (Rhodia Inc., 1967a). No phosalone
residues were found in milk of dairy cattle at any of the feeding
levels of 100, 50 or 25 ppm (Rhodia Inc., 1967b; Woodard Research
Corporation, 1967c).
In plants
Degradation of phosalone in plants is shown in Figure 2 (Desmoras
et al., 1968a). The half-life of the residues in different plants is
shown in Table 4. Half-lives vary from 2 to 20 days with application
rates of 0.6 - 1.2 kg a.i./ha. The oxon derivative of phosalone is
found, but its amount has been less than 10% of the total amount of
the parent compound.
In soil
Degradation of phosalone in soils is shown in Figure 3. The half-life
of phosalone in soils was found to be about a week. No accumulation of
phosalone seems to occur in soils.
TABLE 3 Summary of phosalone residue, data from different trials
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
Alfalfa France 0.9 1 13 4 4 - 6 5
0.9 1 20 4 1.7 - 2.9 2.2
U.S.A. 1.7 1 20-27 10 1.4 - 11.6 5.2
1.7 1 11-14 4 24.5 - 37.0 30.6
0.8-1.1 1 14 10 2.2 - 12.6 7.8
0.8-1.1 1 20-23 20 0.8 - 7.6 3.3
Apple Australia 2.0 5-8 17-29 22 0.9 - 3.5 1.5
France 0.4- 0.6 4-7 16-28 20 0.75- z.6 1.5
0.4-0.8 7 28 35 0.9 - 3.8 2.3
1.3-1.5 3-4 14 24 0.9 - 2.9 1.8
0.6 1-8 14-33 24 c.4 - x.0 0.7
0.06% 7-8 21 8 2.1 - 3.6 2.9
" 7 36-64 20 1.3 - 2.4 1.7
" 7 75 4 0.9 - 1.0 1.0
England 0.46-0.69 2-3 59-70 3 0.05-0.49 0.2
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
New Zealand 0.056-0.09% 7-8 22-32 14 0.89-6.2 2.8
South Africa 0.03% 6-7 39-54 36 0.43-2.3 1.1
" 5-7 66-84 36 0.36-1.0 0.7
U.S.A. 0.06% 5 22-36 6 2.2-5.3 4.6
Beets France 0.4-0.6 1 1 8 0.19-1.67 0.6
" 1 3 8 0.20-1.35 0.4
Cabbage 0.4-0.6 1 1 4 4.1 -8.3 6.4
" 2 1 4 0.6 -2.8 1.7
" 6 1 4 0.3 -1.3 0.6
U.S.A. 0.06-0.12% 1 7-8 4 - <0.1
0.12% 5 26 2 - <0.1
Broccoli 0.06% 2 7 2 - 0.6
Brussels
sprouts 0.06% 6 14 2 - 0.7
Cherry Canada 0.03-0.04% 4 8 4 0.8 -4.9 2.8
U.S.A. 0.06% 4 21 2 - 0.1
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
France 0.06% 1 8-18 4 0.3 -0.4 0.4
Chestnut U.S.A. 2-3 1-2 14-78 6 - <0.1
Citrus fruit " 2.5 1 14 18 - <0.1
Cotton France 1 6-13 13-14 12 <0.1 -0.9 0.5
0.5-4 8-12 34-57 12 0.1 -1.6 0.8
Cucumber U.S.A. 0.06% 3 7-28 6 - 0.1
France 0.6-1.2 1 10 8 0.01-0.04 0.02
Grape U.S.A. 1 1-3 28-43 8 1.8 - 2.4 2.0
1.8-3.6 1 14 4 0.5 -0.9 0.7
1 3 59 2 - 0.9
7.2 1 35 2 - 1.3
South Africa 0.032-0.037% 3 41 12 0.4 -2.0 1.1
0.032-0.037% 3 61 12 0.08-1.0 0.3
0.032-0.037% 3 73 12 0.05-0.46 0.2
Canada 0.03-0.06% 5 14 12 1.8 - 4.7 3.0
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
France 0.06% 1 8 4 - 0.3
0.6 1 70 4 - 0.4
Hops U.S.A. 1-1.2 2 28 8 1.2 - 1.7 1.51
<0.12
Lettuce U.S.A. 0.12% 5 7 2 - 2.8
" 5 26 2 - 0.6
France 0.4-0.6 1 6 6 1.6 - 3.7 2.7
Pea U.S.A. 0.06% 1 49 2 - 0.1
France 0.4-0.6 1 1 8 1.3 - 2.8 2.1
" 1 2 8 0.5 -0.95 0.6
" 1 13 8 0.05-0.2 0.12
Peach Canada 0.03-0.045% 5 8 4 1.1 - 2.2 1.9
U.S.A. 0.06% 5 40 2 - 0.9
Australia 2.0 3-5 37-61 20 0.3 - 2.9 1.5
France 0.06% 1 7-14 4 0.7 - 1.9 1.4
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
Pear Australia 0.85 3-5 18-30 8 0.6 - 2.0 1.2
South Africa 0.03% 5-6 26-32 48 0.25- 1.8 0.7
France 0.06% 5 13 8 0.2 - 0.4 0.3
U.S.A. 0.06% 1-7 38-63 6 0.1 - 0.5 0.3
Pecan " 0.56 3-8 60-66 4 - <0.1
" 0.84 1 198 2 - <0.1
" 4.76 5 95 2 - <0.1
Plum Canada 0.03-0.045% 5 16 8 1.3 - 4.1 2.2
U.S.A. 0.06% 1 45-59 4 - 1.3
England 0.03% 1-3 11-26 16 0.22- 1.8 0.7
Potato U.S.A. 0.06% 1-7 7-54 6 - <0.1
Australia 0.06% 9 1 24 - <0.02
France 0.4-0.6 1 7-9 6 - <0.05
Rape seed " 1-2.4 3 77 8 - <0.1
Denmark 0.7-2.05 1 27 16 - <0.1
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
Sorghum U.S.A. 1 2 108 4 - 0.2
Strawberry " 0.06% 1 4-10 8 0.3 - 0.9 0.6
England 0.6 2 26 6 0.04-0.08 0.07
Tomato U.S.A. 0.6-0.12 1-4 14-15 6 <0.1 -0.6 0.3
France 0.4 -0.6 1 1 8 0.30-0.7 0.48
0.4 -0.6 1 3 8 0.15-0.7 0.23
Wheat " 0.6 1 25-44 6 <0.05-1 0.3
1 fresh
2 dried 3 days
Special studies on neurotoxicity
Adult White Leghorn hens (ten per group) were fed phosalone at levels
of 0, 50, 163 and 500 ppm in the diet for 45 days. Another group was
fed tri-o-cresyl phosphate (TOCP) at 500 ppm. Phosalone at 163 and 500
ppm reduced egg production. No clinical or histological evidence of
paralysis or demyelination was observed. TOCP dietary exposure
resulted in signs of slight paralysis and evidence of minor central
and peripheral nerve degeneration, as determined histologically
(Woodard et al., 1966a).
A group of Rhode Island Red Hens (ten birds) was administered
phosalone subcutaneously at a dose of 350 mg/kg initially and after
three weeks. The birds were administered atropine and P2S to protect
against cholinergic stimulation. Mipafox (16 mg/kg) was administered
subcutaneously as a positive control to four hens. No clinical or
histological evidence in spinal cord or sciatic nerve of delayed
neurotoxicity was evident with phosalone. Mipafox paralyzed all hens
and induced demyelination in spinal cord or sciatic nerve (Heath
et al., 1967).
Special studies on potentiation
Phosalone, in combination with other anticholinesterase agents, was
examined for possible potentiation effects by administration of up to
one-half of the oral LD50 of each material. Disulfaton potentiated
the acute effects of phosalone. The toxicity of malathion and
coumaphos was potentiated to a lesser extent, while potentiation did
not occur with 15 other compounds, including: carbophenothion,
demeton, disyston, diazinon, trichlorfon, azinphos-methyl, dioxathion,
EPN, ethion, mevinphos, parathion-methyl, fenchlorfos, shradan,
merphos and carbaryl (Scott and Beliles, 1965b).
Special studies on reproduction
Three groups of rats (20 females and 10 males per group) were fed
phosalone at 0, 25 and 50 ppm in the diet and subjected to a standard
two-litter, three-generation reproduction study. There were no effects
on reproduction and lactation indices noted in this study which were
attributed to phosalone (Jones et al., 1967a).
Special studies on teratogenicity
Teratogenicity tests were conducted with phosalone in chick embryos
and rabbits. Doses of 0.2, 0.6 and 1.8 mg/egg were given to chick
embryos. Doses of 2, 6 and 18 mg/kg daily were given orally to
pregnant rabbits from day 6 to 16 of gestation. Thalidomide (100 mg/kg
p.o./day) was the positive control in the rabbit study. No
significantly greater incidence of total malformations due to
phosalone were noted in either chick embryos or rabbits (Julou, 1969).
Acute toxicity
The acute toxicity of phosalone has been studied in animals. A summary
of results of these studies is given in Table 2.
TABLE 2 Acute toxicity of phosalone in various animal species
LD50
Species Route (mg/kg) Reference
Dog oral >1 600 Cookrell et al., 1965
Rat (F) oral 153-207 Scott and Beliles, 1965a
(M) oral 125 Fournel et al., 1968
(F) oral 90 Ibid.
Mouse oral 320 Rivett and Davies, 1966
(M) oral 118 Fournel et al., 1968
(F) oral 93 Ibid.
Chicken SC 350 Heath et al., 1967
Signs of poisoning following acute administration of phosalone are the
same as for the other organophosphorus esters and include tremors,
lacrimation and eventually convulsions and death. Signs of poisoning
were evident within 10 to 20 minutes.
An LC50 value of 64 mg/l was calculated following inhalation exposure
of rats (male and female) to phosalone, for one hour at various doses,
having a 3µ particle size (Beliles, 1966). A single dermal application
to the shaved backs of rabbits at doses up to 2 000 mg/kg of a
formulation (equivalent to a dose of phosalone at approximately 800
mg/kg) for 24 hours resulted in no toxicity, but a mild erythema was
noted at 2 000 mg/kg (Horn et al., 1965).
Antidotal studies with rats and mice showed that both atropine and
P2S are effective antidotes to the acute toxic signs of poisoning
following oral administration of phosalone. P2S was apparently more
effective than atropine, although in both cases multiple applications
of antidotal material was suggested to be optimal (Rivett and Davies,
1966).
Short-term studies
Rabbit
Groups of rabbits (5 males and 5 females per group) were administered
phosalone dermally to intact or abraded skin for three weeks, five
days per week, at dosage levels of 1 ml of an emulsifiable
concentrate, 0.05 ml of the E.C. (a dilution of the concentrate) and 1
ml of a control E.C. with no phosalone. Weight loss, signs of toxicity
and death, as well as significant cholinesterase depression, were
apparent with both phosalone treatments to abraded skin. The rabbits
treated with 1 ml of emulsifiable concentrate had a lower liver
glycogen level, and both treated groups incurred renal congestion in
animals with abraded skin. Dermal irritation was evident in all
animals, and gross and histopathological examination showed no effects
on other tissues examined (Woodard et al., 1966b).
Dog
Four groups of dogs (four males and four females per group) were fed
phosalone in the diet for two years at levels of 0, 100, 200, and
1 000 ppm. Effects on cholinesterases were evident at all dose levels
throughout the study. Plasma cholinesterase was depressed at all
feeding levels; RBC cholinesterase was depressed at 200 and 1 000 ppm,
and brain cholinesterase was depressed at 1 000 ppm. One dog fed 1 000
ppm (female) died at 93 weeks, and several others at this level
exhibited fasciculations, nervousness and laboured respiration.
Reduced body-weight was noted in both sexes at 1 000 ppm and in males
at 200 ppm. Soft stools containing mucous and blood were observed for
each treated group, with considerably more frequency at 1 000 ppm.
This effect began to occur at the 100 ppm level after 53 weeks. ECG
changes included an increased T-wave amplitude at 1 000 ppm, which was
more apparent during the second year of study. Increased SGPT and SAP
activity were observed at 1 000 ppm.
Organ to body-weight ratios suggested that at 1 000 ppm the liver of
both sexes and adrenals and thyroid of females were enlarged.
Histological changes observed at all feeding levels were seen as
increased basophilic granules in the myofibrillar cytoplasm in smooth
muscle of the small intestine. These effects were less obvious as the
dose decreased. Vacuolation of the smooth muscle cells of the small
intestine was seen only at 1 000 ppm (Donoso et al., 1967).
Three groups of dogs (four males and four females per group) were fed
phosalone in the diet for 19 weeks at levels of 7.5, 15 and 25 ppm. In
addition, at weeks 14 -19 disyston at 1 ppm was added to the diet of
all animals. No effects on behaviour, growth, clinical chemistry,
urinalysis, biochemistry or cholinesterase activity were noted in this
study which were attributed to phosalone. Histological examination of
the small intestine revealed no abnormalities (Jones et al., 1967b).
Groups of dogs (four males and four females per group) were fed
phosalone in the diet, at levels of 0, 12.5, 25 and 37.5 ppm for one
month. Plasma cholinesterase activity was reduced at 37.5 ppm. In all
dogs at 25 and 37.5 ppm, and in one dog at 12.5 ppm, a pink-mauve
colouration of the mucosa of the bladder was noted although it was not
observed in any other experiment performed in dogs. No effects were
observed on growth, food consumption or RBC and brain cholinesterase
activity (Noel et al., 1970).
Five groups of calves were fed phosalone in the diet for ten weeks at
levels of O, 20, 50, 80 and 160 ppm. No significant effects
attributable to phosalone were observed on growth, behaviour, food
consumption, hematology and cholinesterase activity (Woodard et al.,
1967a).
Long-term studies
Rat
Four groups of rats (30 males and 30 females per group) were fed
phosalone in the diet for two years at levels of 0, 25, 50 and 250
ppm. Effects attributable to phosalone were observed only on plasma
cholinesterase, which was depressed at 50 ppm but not at 25 ppm. No
other effects of treatment, either gross, histopathological or
clinical, were noted at any of the dose levels studied (Woodard
et al., 1967b).
COMMENT
Phosalone is rapidly absorbed, metabolized and excreted by rodents.
Phosalone has been shown to have no effect on rat reproduction, is not
teratogenic in chick or rat and is not neurotoxic to chickens. The
acute toxic effect in rodents was potentiated with several
organo-phosphates, particularly disyston.
Two-year feeding studies in rats and dogs indicate no-effect levels
(based on cholinesterase depression) of 25 ppm for both species.
Bladder discolouration in dogs was reported in a short-term study, but
longer studies did not confirm this observation.
Observations in man are very limited.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 25 ppm in the diet, equivalent to 1.25 mg/kg
body-weight
Dog: 25 ppm in the diet, equivalent to 0.625 mg/kg
body-weight
ESTIMATE FOR ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.006 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phosalone is a non-systemic insecticide and acaricide active by
contact and ingestion. It is officially approved for use in many
countries on a wide variety of crops, and is practically nontoxic to
bees.
Pre-harvest treatments
Phosalone is recommended in a number of countries to control a wide
range of insects and mites on fruit, rape, sugar beet, potato,
alfalfa, cotton, vegetables, grapes, tea and ornamental crops.
Recommended rates of application vary in different countries from 0.4
to 1.2 kg a.i./ha (0.04-0.06% a.i. with high volume sprays at
1 000 - 2 000 l/ha). The recommended interval between two treatments
is, in most countries, two to three weeks and in Canada ten days. The
safety period in the treatment of orchard crops is in Canada, 1 - 7
days; in Great Britain, 3 weeks; in France, 2 weeks; in Switzerland, 3
weeks; in U.S.A., 1 week.
Other uses
Phosalone is used against ectoparasites of animals in some countries.
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residue data from supervised trials are given in Table 3. These
are from treatments made in Australia, Canada, France, Great Britain,
New Zealand, South Africa and U.S.A. (Rhône-Poulenc, 1967-1971; May
and Baker Ltd., 1967-1969).
FATE OF RESIDUES
General comments
Studies on the behaviour of phosalone in animals, plants and soil have
shown that phosalone has good stability (generally active against
pests for 15 to 18 days) on plants, but in animals it is rapidly
degraded to nontoxic products; decomposition is also rapid in soil.
In animals
Phosalone is rapidly degraded in animals. One half of the dose
disappears after oral administration in four to six hours and the rest
in 24 hours.
Phosalone may be hydrolysed to nontoxic derivatives or oxidized to the
oxo-derivative, which is responsible for inhibition of
cholinesterases. The oxo-derivative, less stable than phosalone, is
practically never detected, except in the liver where it may be found
in minute quantities.
When beef cattle and sheep were administered levels of 10, 30 and 100
ppm in feed, traces of phosalone were found in fat and liver only at
the feeding level of 100 ppm (Rhodia Inc., 1967a). No phosalone
residues were found in milk of dairy cattle at any of the feeding
levels of 100, 50 or 25 ppm (Rhodia Inc., 1967b; Woodard Research
Corporation, 1967c).
In plants
Degradation of phosalone in plants is shown in Figure 2 (Desmoras
et al., 1968a). The half-life of the residues in different plants is
shown in Table 4. Half-lives vary from 2 to 20 days with application
rates of 0.6 - 1.2 kg a.i./ha. The oxon derivative of phosalone is
found, but its amount has been less than 10% of the total amount of
the parent compound.
In soil
Degradation of phosalone in soils is shown in Figure 3. The half-life
of phosalone in soils was found to be about a week. No accumulation of
phosalone seems to occur in soils.
TABLE 3 Summary of phosalone residue, data from different trials
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
Alfalfa France 0.9 1 13 4 4 - 6 5
0.9 1 20 4 1.7 - 2.9 2.2
U.S.A. 1.7 1 20-27 10 1.4 - 11.6 5.2
1.7 1 11-14 4 24.5 - 37.0 30.6
0.8-1.1 1 14 10 2.2 - 12.6 7.8
0.8-1.1 1 20-23 20 0.8 - 7.6 3.3
Apple Australia 2.0 5-8 17-29 22 0.9 - 3.5 1.5
France 0.4- 0.6 4-7 16-28 20 0.75- z.6 1.5
0.4-0.8 7 28 35 0.9 - 3.8 2.3
1.3-1.5 3-4 14 24 0.9 - 2.9 1.8
0.6 1-8 14-33 24 c.4 - x.0 0.7
0.06% 7-8 21 8 2.1 - 3.6 2.9
" 7 36-64 20 1.3 - 2.4 1.7
" 7 75 4 0.9 - 1.0 1.0
England 0.46-0.69 2-3 59-70 3 0.05-0.49 0.2
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
New Zealand 0.056-0.09% 7-8 22-32 14 0.89-6.2 2.8
South Africa 0.03% 6-7 39-54 36 0.43-2.3 1.1
" 5-7 66-84 36 0.36-1.0 0.7
U.S.A. 0.06% 5 22-36 6 2.2-5.3 4.6
Beets France 0.4-0.6 1 1 8 0.19-1.67 0.6
" 1 3 8 0.20-1.35 0.4
Cabbage 0.4-0.6 1 1 4 4.1 -8.3 6.4
" 2 1 4 0.6 -2.8 1.7
" 6 1 4 0.3 -1.3 0.6
U.S.A. 0.06-0.12% 1 7-8 4 - <0.1
0.12% 5 26 2 - <0.1
Broccoli 0.06% 2 7 2 - 0.6
Brussels
sprouts 0.06% 6 14 2 - 0.7
Cherry Canada 0.03-0.04% 4 8 4 0.8 -4.9 2.8
U.S.A. 0.06% 4 21 2 - 0.1
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
France 0.06% 1 8-18 4 0.3 -0.4 0.4
Chestnut U.S.A. 2-3 1-2 14-78 6 - <0.1
Citrus fruit " 2.5 1 14 18 - <0.1
Cotton France 1 6-13 13-14 12 <0.1 -0.9 0.5
0.5-4 8-12 34-57 12 0.1 -1.6 0.8
Cucumber U.S.A. 0.06% 3 7-28 6 - 0.1
France 0.6-1.2 1 10 8 0.01-0.04 0.02
Grape U.S.A. 1 1-3 28-43 8 1.8 - 2.4 2.0
1.8-3.6 1 14 4 0.5 -0.9 0.7
1 3 59 2 - 0.9
7.2 1 35 2 - 1.3
South Africa 0.032-0.037% 3 41 12 0.4 -2.0 1.1
0.032-0.037% 3 61 12 0.08-1.0 0.3
0.032-0.037% 3 73 12 0.05-0.46 0.2
Canada 0.03-0.06% 5 14 12 1.8 - 4.7 3.0
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
France 0.06% 1 8 4 - 0.3
0.6 1 70 4 - 0.4
Hops U.S.A. 1-1.2 2 28 8 1.2 - 1.7 1.51
<0.12
Lettuce U.S.A. 0.12% 5 7 2 - 2.8
" 5 26 2 - 0.6
France 0.4-0.6 1 6 6 1.6 - 3.7 2.7
Pea U.S.A. 0.06% 1 49 2 - 0.1
France 0.4-0.6 1 1 8 1.3 - 2.8 2.1
" 1 2 8 0.5 -0.95 0.6
" 1 13 8 0.05-0.2 0.12
Peach Canada 0.03-0.045% 5 8 4 1.1 - 2.2 1.9
U.S.A. 0.06% 5 40 2 - 0.9
Australia 2.0 3-5 37-61 20 0.3 - 2.9 1.5
France 0.06% 1 7-14 4 0.7 - 1.9 1.4
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
Pear Australia 0.85 3-5 18-30 8 0.6 - 2.0 1.2
South Africa 0.03% 5-6 26-32 48 0.25- 1.8 0.7
France 0.06% 5 13 8 0.2 - 0.4 0.3
U.S.A. 0.06% 1-7 38-63 6 0.1 - 0.5 0.3
Pecan " 0.56 3-8 60-66 4 - <0.1
" 0.84 1 198 2 - <0.1
" 4.76 5 95 2 - <0.1
Plum Canada 0.03-0.045% 5 16 8 1.3 - 4.1 2.2
U.S.A. 0.06% 1 45-59 4 - 1.3
England 0.03% 1-3 11-26 16 0.22- 1.8 0.7
Potato U.S.A. 0.06% 1-7 7-54 6 - <0.1
Australia 0.06% 9 1 24 - <0.02
France 0.4-0.6 1 7-9 6 - <0.05
Rape seed " 1-2.4 3 77 8 - <0.1
Denmark 0.7-2.05 1 27 16 - <0.1
TABLE 3 (Cont'd.)
Residuee of phosalone
Crop Country Application Treatments Preharvest Number of
(kg/ha or (no.) interval analyses Range Mean
%) days (ppm) (ppm)
Sorghum U.S.A. 1 2 108 4 - 0.2
Strawberry " 0.06% 1 4-10 8 0.3 - 0.9 0.6
England 0.6 2 26 6 0.04-0.08 0.07
Tomato U.S.A. 0.6-0.12 1-4 14-15 6 <0.1 -0.6 0.3
France 0.4 -0.6 1 1 8 0.30-0.7 0.48
0.4 -0.6 1 3 8 0.15-0.7 0.23
Wheat " 0.6 1 25-44 6 <0.05-1 0.3
1 fresh
2 dried 3 days

 TABLE 4 Half-lives of phosalone residues in growing plants
Crop Application rate Half-life
(kg a.i./ha) (days)
Alfalfa 0.9 - 1.2 4 - 10
Apple 0.3 - 5.4 7 - 15
Cabbage 0.6 - 2.4 2 - 5
Cotton 7.2 - 13.8 4 - 6
Cherry 3.6 4 - 10
Grape 0.6 - 1.2 7 - 17
Lettuce 0.6 - 1.2 3 - 8
Peach 0.5 - 3.0 7 - 20
Pear 0.6 - 0.9 8 - 15
Plum 5.4 11 - 17
Strawberry 0.6 4 - 8
In storage and processing
There is no reliable data on the fate of phosalone in storage and
processing of foodstuffs.
METHODS OF RESIDUE ANALYSIS
Desmoras et al. (1968d) have described procedures for the analysis
of technical phosalone and its formulated products, and of residues in
crops, vegetable oils and soil. A colorimetric method is based on
hydrolysis to diethyldithiophosphoric acid followed by formation of
the cupric complex. The preferred procedures are gas chromatography
using catharometer detection and an ester as an internal standard for
macro-analysis and electron capture detection for residue
determinations; the use of a phosphorus sensitive thermionic detector
gave poorer sensitivity. The outlines of the schemes for vegetable
sample preparation are shown in Figures 4 and 5, the extracts being
suitable for colorimetry gas chromatography or for a bioassay
procedure using Daphnia or mosquito larvae. Variations are described
for analysis of olives, olive oil and rape oil, while a simple
extraction with acetone was suitable for soil samples. Method B with
gas chromatographic end determination should be suitable for
regulatory purposes.
Other similar multiresidue GLC procedures should also be suitable for
determining phosalone residues; that described by Abbott et al.
(1970) has been found to be applicable to fatty samples.
NATIONAL TOLERANCES
Examples of national tolerances as reported to the Meeting are given
in Table 5.
TABLE 5
Examples of national tolerances as reported to the meeting
Country Tolerance Commodity
ppm
Canada 10 apples, pears
4 peaches
6 cherries
South Africa 2 apples, pears
Switzerland 2 grapes, fruit
U.S.A. 10 apples, pears, grapes
20 raisins
Australia 3 peaches
2.5 apples, pears
1 fat of meat of sheep
APPRAISAL
Phosalone is a non-systemic dithiophosphate insecticide and acaricide
used for pre-harvest control of a wide range of pests in agriculture,
horticulture, ornamentals and vine-growing. It has limited use also
against ectoparasites of animals. Its pesticidal activity on plants
has a duration of two to three weeks, depending on the type of pest.
The residues of potential toxicity are composed of the parent compound
and, only to a minor extent, of the oxon derivative of phosalone. It
is readily decomposed in animals and soils. Practically no
contamination of animal tissues occurs as a result of feeding animals
with fodder crops containing phosalone residues. Residue data of
supervised trials on plants were available from many countries. No
data were available on residues resulting from control of
ectoparasites on animals, on fate of residues in food storage and
processing, on levels of residues in foodstuffs moving in commerce or
in total diets. Gas chromatographic procedures are the analytical
methods of choice for use in regulatory laboratories. The limit of
determination is at or about 0.1 ppm depending on the type of crop.
TABLE 4 Half-lives of phosalone residues in growing plants
Crop Application rate Half-life
(kg a.i./ha) (days)
Alfalfa 0.9 - 1.2 4 - 10
Apple 0.3 - 5.4 7 - 15
Cabbage 0.6 - 2.4 2 - 5
Cotton 7.2 - 13.8 4 - 6
Cherry 3.6 4 - 10
Grape 0.6 - 1.2 7 - 17
Lettuce 0.6 - 1.2 3 - 8
Peach 0.5 - 3.0 7 - 20
Pear 0.6 - 0.9 8 - 15
Plum 5.4 11 - 17
Strawberry 0.6 4 - 8
In storage and processing
There is no reliable data on the fate of phosalone in storage and
processing of foodstuffs.
METHODS OF RESIDUE ANALYSIS
Desmoras et al. (1968d) have described procedures for the analysis
of technical phosalone and its formulated products, and of residues in
crops, vegetable oils and soil. A colorimetric method is based on
hydrolysis to diethyldithiophosphoric acid followed by formation of
the cupric complex. The preferred procedures are gas chromatography
using catharometer detection and an ester as an internal standard for
macro-analysis and electron capture detection for residue
determinations; the use of a phosphorus sensitive thermionic detector
gave poorer sensitivity. The outlines of the schemes for vegetable
sample preparation are shown in Figures 4 and 5, the extracts being
suitable for colorimetry gas chromatography or for a bioassay
procedure using Daphnia or mosquito larvae. Variations are described
for analysis of olives, olive oil and rape oil, while a simple
extraction with acetone was suitable for soil samples. Method B with
gas chromatographic end determination should be suitable for
regulatory purposes.
Other similar multiresidue GLC procedures should also be suitable for
determining phosalone residues; that described by Abbott et al.
(1970) has been found to be applicable to fatty samples.
NATIONAL TOLERANCES
Examples of national tolerances as reported to the Meeting are given
in Table 5.
TABLE 5
Examples of national tolerances as reported to the meeting
Country Tolerance Commodity
ppm
Canada 10 apples, pears
4 peaches
6 cherries
South Africa 2 apples, pears
Switzerland 2 grapes, fruit
U.S.A. 10 apples, pears, grapes
20 raisins
Australia 3 peaches
2.5 apples, pears
1 fat of meat of sheep
APPRAISAL
Phosalone is a non-systemic dithiophosphate insecticide and acaricide
used for pre-harvest control of a wide range of pests in agriculture,
horticulture, ornamentals and vine-growing. It has limited use also
against ectoparasites of animals. Its pesticidal activity on plants
has a duration of two to three weeks, depending on the type of pest.
The residues of potential toxicity are composed of the parent compound
and, only to a minor extent, of the oxon derivative of phosalone. It
is readily decomposed in animals and soils. Practically no
contamination of animal tissues occurs as a result of feeding animals
with fodder crops containing phosalone residues. Residue data of
supervised trials on plants were available from many countries. No
data were available on residues resulting from control of
ectoparasites on animals, on fate of residues in food storage and
processing, on levels of residues in foodstuffs moving in commerce or
in total diets. Gas chromatographic procedures are the analytical
methods of choice for use in regulatory laboratories. The limit of
determination is at or about 0.1 ppm depending on the type of crop.

 RECOMMENDATIONS
TOLERANCES
Tolerances recommended apply to the parent compound. The time interval
between application and harvest which has been used in determining the
maximum residue limit is appropriate to the agricultural practices in
numerous countries.
Time interval
between application
Crop Ppm and harvest (weeks)
Apple, grape, peach, plum 5 2 - 3
Cherry, pear, beetroots 2 2
Hops (dried) 2 4
Citrus fruit, strawberry,
tomato, cabbage, broccoli,
Brussels sprouts, cucumber,
lettuce, peas 1 1 - 2
Chestnut (shelled), pecan
(shelled), potato, rapeseed 0.1* 2
* at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Studies on human exposure.
2. A study to determine dose levels causing no carboxylesterase
(aliesterase) activity depression.
3. Effect of food storage and processing on residues.
4. Data on residues occurring in food commodities moving in
commerce.
5. Nature and concentrations of impurities of the technical product.
6. Information on the use pattern of phosalone against ectoparasites
of domestic animals and data on residues in animal products
resulting from this use.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales 1966-67. III
- Organophosphorus pesticide residues in the total diet. Pestic. Sci.,
1: 10-13.
Beliles, R.P. (1966) Phosalone (R.P. 11 974) - Safety evaluation by
acute inhalation exposure of rats. Report Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Cockrell, K.O., Woodard, M.W. and Woodard, G. (1965) Phosalone R.P. 11
974 - Acute oral LD50 in dogs. Report Woodard Research Corporation,
submitted by Rhône-Poulenc. (unpublished)
Desmoras, J., LaCroix, L. and Metivier, J. (1963) Pesticides of the
benzoxazolone family. Phytiat.-Phytopharm., 12: 199-215.
Desmoras, J. and Fournel, J. (1968) Studies on degradation of
phosalone in mammals. Report Rhône-Poulenc. (unpublished)
Desmoras, J., Fournell, J., Laurent, M., Sauli, M. and Terlain, B.
(1968a) Etude de la vitesse de degradation de la phosalone dans de
sal, les plantes et les animaux. Report no. 12964 Rhône-Poulenc.
Desmoras, J., Laurent, M., Petrinko, P. and Buys, M. (1968b) Dosage de
residus dans les cultures maraicheres. Report no. 13322 Rhône-Poulenc.
Desmoras, J., Laurent, M., Sauli, M. and Terlain, B. (1968c) Etude du
metabolisme de la phosalone dans les plantes et les sals. Report no.
13093 Rhône-Poulenc.
Desmoras, J., Luborg, F., Laurent, M., Bales, I.W. and Guardigli, A.
(1968d) Application to the determination in the technical material.
Phytiat.-Phytopharm. 4: 277-292.
Desmoras, J., Laurent, M. and Buys, M. (1970a) Dosage de residus dans
des pommes. Report no. 14899 Rhône-Poulenc.
Desmoras, J., Laurent, M. and Buys, M. (1970b) Dosage de residus sur
pommès en prorenance des Pays-bas. Report no. 14986 Rhône-Poulenc.
Donoso, J., Woodard, M.W. and Woodard, G. (1967) Phosalone - Safety
evaluation by repeated administration to dogs for 107 weeks. Report by
Woodard Research Corporation, submitted by Rhône-Poulenc.
(unpublished)
Fournel, J., Julou, L. and Pasquet, J. (1968) Acute toxicity in mice
and rats and in vivo anticholinesterase activity in rats. Report
Rhône-Poulenc. (unpublished)
Heath, S.A.B., Rivett, K.F. and Woolf, N. (1967) Test for
neurotoxicity. Report May-Baker Ltd., submitted by Rhône-Poulenc.
(unpublished)
Horn, J.H., Woodard, M.W. and Woodard, G. (1965) Phosalone (R.P. 11
974) acute dermal toxicity for rabbits. Report by Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Jones, M.E., Post, K.F., Scott, W.J., Woodard, M.W. and Woodard, G.
(1967a) Three generation reproduction study in the rat. Report by
Woodard Research Corporation, submitted by Rhône-Poulenc.
(unpublished)
Jones, M.E., Imming, R.J., Woodard, M.W. and Woodard, G. (1967b)
Phosalone followed by a determination of potentiation activity with
the compound disyston.
Julou, L. (1969) Study of the teratogenic activity of phosalone on
chick embryo and rabbit. Report Rhône-Poulenc. (unpublished)
May and Baker Ltd. (1967a) Report no. C. 263. (unpublished)
May and Baker Ltd. (1967b) Report no. C. 274. (unpublished)
May and Baker Ltd. (1967c) Report no. C. 277. (unpublished)
May and Baker Ltd. (1968a) Report no. PRG./85. (unpublished)
May and Baker Ltd. (1968b) Report no. PRG./88. (unpublished)
May and Baker Ltd. (1968c) Report no. PRG./162. (unpublished)
May and Baker Ltd. (1968d) Report no. PRG./171. (unpublished)
May and Baker Ltd. (1968e) Report no. PRG./173. (unpublished)
May and Baker Ltd. (1968f) Report no. PRG./310. (unpublished)
May and Baker Ltd. (1969a) Report no. PRG./425. (unpublished)
May and Baker Ltd. (1969b) Report no. PRG./534. (unpublished)
Noel, P.R.B., Rivett, K.F., Osborne, G.E. and Street, A.E. (1970)
Phosalone dietary intake in Beagle dogs for 4 weeks. Report by
Huntingdon Research Centre, submitted by Rhône-Poulenc. (unpublished)
Rhodia Inc. (1967a) Results for phosalone residue in tissues of beef
cattle and sheep concerning blood plasma. (unpublished)
Rhodia Inc. (1967b) Results for phosalone in milk of dairy
cattle - Woodard Research Centre Project. (unpublished)
Rhône-Poulenc. (1967) Report no. 12433. (unpublished)
Rhône-Poulenc. (1968a) The final metabolite of phosalone in plants.
Synthesis, identification, toxicity, possible presence in plants.
(unpublished)
Rhône-Poulenc. (1968b) Oxygen analogue of phosalone. Acute oral
toxicity, and anticholinesterase activity in vitro. (unpublished)
Rhône-Poulenc. (1968c) Report no. 13018. (unpublished)
Rhône-Poulenc. (1969a) Report no. 13551. (unpublished)
Rhône-Poulenc. (1969b) Report no. 13553. (unpublished)
Rhône-Poulenc. (1970) Report no. 14356. (unpublished)
Rhône-Poulenc. (1971a) Report no. 15209. (unpublished)
Rhône-Poulenc. (1971b) Report no. 15210. (unpublished)
Rhône-Poulenc. (undated) Zolone, phosalone (with 27 references
concerning phosalone).
Rivett, K.F. and Davies, V. (1966) Effects of antidotes against
poisoning by phosalone. Report by May and Baker, submitted by
Rhône-Poulenc. (unpublished)
Scott, W.J. and Bililes, R.P. (1965a) Acute oral toxicity of phosalone
(R.P. 11 974) formulation to rats. Report by Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Scott, W.J. and Beliles, R.P. (1965b) Potentiation studies in the rat
with marketed pesticides. Report by Woodard Research Corporation,
submitted by Rhône-Poulenc. (unpublished)
Woodard, M.W., Cockrell, K.O. and Woodard, G. (1966a) Demyelination
study in chickens. Report by Woodard Research Corporation, submitted
by Rhône-Poulenc. (unpublished)
Woodard, M.W., Cockrell, K.O. and Woodard, G. (1966b) Sub-acute dermal
toxicity of phosalone for the rabbit. Report of Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Woodard, M.W., Imming, R.J., Thompson, W. and Woodard, G. (1967a)
Phosalone, safety evaluation by oral administration to calves for 10
weeks. Report by Woodard Research Corporation, submitted by
Rhône-Poulenc. (unpublished)
Woodard, M.W., Howard, D.J., Donoso, J. and Woodard, G. (1967b) Safety
evaluation by repeated oral administration to rats for 103 weeks.
Report by Woodard Research Corporation, submitted by Rhône-Poulenc.
(unpublished)
Woodard Research Corporation. (1967c) Phosalone analysis of residue in
milk of dairy cattle.
RECOMMENDATIONS
TOLERANCES
Tolerances recommended apply to the parent compound. The time interval
between application and harvest which has been used in determining the
maximum residue limit is appropriate to the agricultural practices in
numerous countries.
Time interval
between application
Crop Ppm and harvest (weeks)
Apple, grape, peach, plum 5 2 - 3
Cherry, pear, beetroots 2 2
Hops (dried) 2 4
Citrus fruit, strawberry,
tomato, cabbage, broccoli,
Brussels sprouts, cucumber,
lettuce, peas 1 1 - 2
Chestnut (shelled), pecan
(shelled), potato, rapeseed 0.1* 2
* at or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Studies on human exposure.
2. A study to determine dose levels causing no carboxylesterase
(aliesterase) activity depression.
3. Effect of food storage and processing on residues.
4. Data on residues occurring in food commodities moving in
commerce.
5. Nature and concentrations of impurities of the technical product.
6. Information on the use pattern of phosalone against ectoparasites
of domestic animals and data on residues in animal products
resulting from this use.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales 1966-67. III
- Organophosphorus pesticide residues in the total diet. Pestic. Sci.,
1: 10-13.
Beliles, R.P. (1966) Phosalone (R.P. 11 974) - Safety evaluation by
acute inhalation exposure of rats. Report Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Cockrell, K.O., Woodard, M.W. and Woodard, G. (1965) Phosalone R.P. 11
974 - Acute oral LD50 in dogs. Report Woodard Research Corporation,
submitted by Rhône-Poulenc. (unpublished)
Desmoras, J., LaCroix, L. and Metivier, J. (1963) Pesticides of the
benzoxazolone family. Phytiat.-Phytopharm., 12: 199-215.
Desmoras, J. and Fournel, J. (1968) Studies on degradation of
phosalone in mammals. Report Rhône-Poulenc. (unpublished)
Desmoras, J., Fournell, J., Laurent, M., Sauli, M. and Terlain, B.
(1968a) Etude de la vitesse de degradation de la phosalone dans de
sal, les plantes et les animaux. Report no. 12964 Rhône-Poulenc.
Desmoras, J., Laurent, M., Petrinko, P. and Buys, M. (1968b) Dosage de
residus dans les cultures maraicheres. Report no. 13322 Rhône-Poulenc.
Desmoras, J., Laurent, M., Sauli, M. and Terlain, B. (1968c) Etude du
metabolisme de la phosalone dans les plantes et les sals. Report no.
13093 Rhône-Poulenc.
Desmoras, J., Luborg, F., Laurent, M., Bales, I.W. and Guardigli, A.
(1968d) Application to the determination in the technical material.
Phytiat.-Phytopharm. 4: 277-292.
Desmoras, J., Laurent, M. and Buys, M. (1970a) Dosage de residus dans
des pommes. Report no. 14899 Rhône-Poulenc.
Desmoras, J., Laurent, M. and Buys, M. (1970b) Dosage de residus sur
pommès en prorenance des Pays-bas. Report no. 14986 Rhône-Poulenc.
Donoso, J., Woodard, M.W. and Woodard, G. (1967) Phosalone - Safety
evaluation by repeated administration to dogs for 107 weeks. Report by
Woodard Research Corporation, submitted by Rhône-Poulenc.
(unpublished)
Fournel, J., Julou, L. and Pasquet, J. (1968) Acute toxicity in mice
and rats and in vivo anticholinesterase activity in rats. Report
Rhône-Poulenc. (unpublished)
Heath, S.A.B., Rivett, K.F. and Woolf, N. (1967) Test for
neurotoxicity. Report May-Baker Ltd., submitted by Rhône-Poulenc.
(unpublished)
Horn, J.H., Woodard, M.W. and Woodard, G. (1965) Phosalone (R.P. 11
974) acute dermal toxicity for rabbits. Report by Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Jones, M.E., Post, K.F., Scott, W.J., Woodard, M.W. and Woodard, G.
(1967a) Three generation reproduction study in the rat. Report by
Woodard Research Corporation, submitted by Rhône-Poulenc.
(unpublished)
Jones, M.E., Imming, R.J., Woodard, M.W. and Woodard, G. (1967b)
Phosalone followed by a determination of potentiation activity with
the compound disyston.
Julou, L. (1969) Study of the teratogenic activity of phosalone on
chick embryo and rabbit. Report Rhône-Poulenc. (unpublished)
May and Baker Ltd. (1967a) Report no. C. 263. (unpublished)
May and Baker Ltd. (1967b) Report no. C. 274. (unpublished)
May and Baker Ltd. (1967c) Report no. C. 277. (unpublished)
May and Baker Ltd. (1968a) Report no. PRG./85. (unpublished)
May and Baker Ltd. (1968b) Report no. PRG./88. (unpublished)
May and Baker Ltd. (1968c) Report no. PRG./162. (unpublished)
May and Baker Ltd. (1968d) Report no. PRG./171. (unpublished)
May and Baker Ltd. (1968e) Report no. PRG./173. (unpublished)
May and Baker Ltd. (1968f) Report no. PRG./310. (unpublished)
May and Baker Ltd. (1969a) Report no. PRG./425. (unpublished)
May and Baker Ltd. (1969b) Report no. PRG./534. (unpublished)
Noel, P.R.B., Rivett, K.F., Osborne, G.E. and Street, A.E. (1970)
Phosalone dietary intake in Beagle dogs for 4 weeks. Report by
Huntingdon Research Centre, submitted by Rhône-Poulenc. (unpublished)
Rhodia Inc. (1967a) Results for phosalone residue in tissues of beef
cattle and sheep concerning blood plasma. (unpublished)
Rhodia Inc. (1967b) Results for phosalone in milk of dairy
cattle - Woodard Research Centre Project. (unpublished)
Rhône-Poulenc. (1967) Report no. 12433. (unpublished)
Rhône-Poulenc. (1968a) The final metabolite of phosalone in plants.
Synthesis, identification, toxicity, possible presence in plants.
(unpublished)
Rhône-Poulenc. (1968b) Oxygen analogue of phosalone. Acute oral
toxicity, and anticholinesterase activity in vitro. (unpublished)
Rhône-Poulenc. (1968c) Report no. 13018. (unpublished)
Rhône-Poulenc. (1969a) Report no. 13551. (unpublished)
Rhône-Poulenc. (1969b) Report no. 13553. (unpublished)
Rhône-Poulenc. (1970) Report no. 14356. (unpublished)
Rhône-Poulenc. (1971a) Report no. 15209. (unpublished)
Rhône-Poulenc. (1971b) Report no. 15210. (unpublished)
Rhône-Poulenc. (undated) Zolone, phosalone (with 27 references
concerning phosalone).
Rivett, K.F. and Davies, V. (1966) Effects of antidotes against
poisoning by phosalone. Report by May and Baker, submitted by
Rhône-Poulenc. (unpublished)
Scott, W.J. and Bililes, R.P. (1965a) Acute oral toxicity of phosalone
(R.P. 11 974) formulation to rats. Report by Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Scott, W.J. and Beliles, R.P. (1965b) Potentiation studies in the rat
with marketed pesticides. Report by Woodard Research Corporation,
submitted by Rhône-Poulenc. (unpublished)
Woodard, M.W., Cockrell, K.O. and Woodard, G. (1966a) Demyelination
study in chickens. Report by Woodard Research Corporation, submitted
by Rhône-Poulenc. (unpublished)
Woodard, M.W., Cockrell, K.O. and Woodard, G. (1966b) Sub-acute dermal
toxicity of phosalone for the rabbit. Report of Woodard Research
Corporation, submitted by Rhône-Poulenc. (unpublished)
Woodard, M.W., Imming, R.J., Thompson, W. and Woodard, G. (1967a)
Phosalone, safety evaluation by oral administration to calves for 10
weeks. Report by Woodard Research Corporation, submitted by
Rhône-Poulenc. (unpublished)
Woodard, M.W., Howard, D.J., Donoso, J. and Woodard, G. (1967b) Safety
evaluation by repeated oral administration to rats for 103 weeks.
Report by Woodard Research Corporation, submitted by Rhône-Poulenc.
(unpublished)
Woodard Research Corporation. (1967c) Phosalone analysis of residue in
milk of dairy cattle.
